Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 14/19/09. The contractual start date was in June 2015. The final report began editorial review in September 2019 and was accepted for publication in April 2020. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2020. This work was produced by Noble et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2020 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
This project sought to develop and pilot seizure first aid training for adults with established epilepsy who frequently visit hospital emergency departments (EDs) and their significant others (SOs). It had two parts and used mixed methods. In part A, we developed the intervention: Seizure first Aid training For Epilepsy (SAFE). This was done by adapting a broader course that was being offered by the Epilepsy Society (ES) (www.epilepsysociety.org.uk; Chalfont St Peter, UK). Part B was a pilot randomised controlled trial (RCT) of the SAFE intervention with people with epilepsy (PWE) from the target population and their SOs, with the aim of assessing the feasibility and optimum design of a definitive RCT to test SAFE’s efficacy.
The primary and secondary objectives for the project are described here.
Primary objectives
Part A: intervention development –
-
optimise content, delivery and behaviour change potential of the ES’s course for PWE attending an ED, and their SOs.
Part B: pilot RCT –
-
conduct a pilot RCT of SAFE plus treatment as usual (TAU) versus TAU only to estimate probable recruitment, consent and follow-up rates in a definitive trial.
Secondary objectives
Part B: pilot RCT –
-
calculate estimates of the annual rate of ED visits in the TAU arm and dispersion parameter to inform the sample size calculation of a definitive RCT
-
test the acceptability of randomisation to participants
-
evaluate SAFE’s implementation fidelity in the pilot RCT
-
analyse the cost of implementing the SAFE programme.
In this chapter, the rationale for providing seizure first aid training is provided, we describe how the existing intervention was adapted and we outline why a pilot RCT, rather than proceeding straight to a definitive trial, was appropriate.
Background
Epilepsy and its epidemiology
With a prevalence of ≥ 1% in the UK,1–3 epilepsy is the UK’s second most common serious neurological condition. 4
The International League Against Epilepsy (Flower Mound, TX, USA) defines a person as having epilepsy if they experience two or more unprovoked (or reflex) seizures more than 24 hours apart or if they have experienced one such seizure and the probability of them experiencing another over the next 10 years is akin to that of a person who has experienced two (i.e. ≥ 60%). 5
Epilepsy’s aetiology is variable. It can, for example, arise as a consequence of cerebrovascular disease, head trauma, congenital abnormalities and neurodegenerative disease, or be idiopathic. 6 Mortality risk varies for PWE but, with a standardised mortality ratio of ≈ 2.2, it is higher than that in the general population. 7,8
Trial data indicate that most PWE (≈ 70%) can theoretically become ‘seizure free’ via treatment, typically using antiepileptic drugs. However, up to 48% of PWE in the UK continue to experience seizures. 9 However, epilepsy is more than ‘just’ seizures. This is reflected in the International League Against Epilepsy’s definition of epilepsy as ‘a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures, and by the neurobiological, cognitive, psychological, and social consequences of this condition’. 10 The diagnosis itself can be associated with significant psychological and social costs for the individual. Being labelled negatively as ‘epileptic’ can be accompanied by discrimination,11,12 with potential detrimental effects on education, unemployment and driving. Around 30% of PWE also meet diagnostic criteria for an anxiety and/or depressive disorder. All undermine well-being. 9,13,14
Use of emergency hospital services for epilepsy and societal implications
Epilepsy has societal impacts. One of these is the cost of providing health care. In the European Union, the total cost of epilepsy in 2004 was €15.5B,15 with a cost per case of €2000–11,500. One costly element is the provision of emergency care, which international evidence shows PWE frequently utilise.
In the UK, ≤ 20% of PWE visit a hospital ED for epilepsy each year. 16–18 Most (≈ 90%) are transported there by an emergency ambulance. 19–21 The 2015/16 cost of providing emergency care to PWE in England was estimated to be at least £70M (excluding indirect costs). 22
Costs are high in part because half of ED attendances by PWE result in hospital admission. 16,19,23 Indeed, 85% of all admissions for epilepsy occur on such an unplanned basis. 24 Among chronic ambulatory care sensitive conditions, epilepsy is the second most common cause of unplanned admissions (17%). 25–27
Reattendance also drives up costs. The exact distribution of ED use for epilepsy is unclear, but it is apparent that reattendance rates are high. The 2014 UK-wide National Audit of Seizure management in Hospitals (NASH)28 examined data from ≈ 4000 seizure-related ED attendances from 85% of acute hospitals. Among those with established epilepsy, 60% had attended the same ED as a result of a seizure in the previous 12 months. 20 Whitson et al. 29 found epilepsy to be the most frequent neurological reason for emergency readmission into UK hospitals. Dickson et al. ’s30 findings are also instructive: they examined data on unplanned admissions in hospitals in England for suspected seizures of any cause (not just epilepsy) in adults between 2007 and 2013. They found that 22.4% of patients had more than one admission per year and that there was a 34% chance of readmission within 6 years.
The most detailed evidence on ED use among PWE comes from a previous National Institute for Health Research (NIHR)-funded study (reference identifier 08/1808/247) by Ridsdale et al. 27,31,32 This study prospectively recruited 85 PWE from three London EDs and asked them to self-report on ED use in the prior 12 months; 60% reported multiple ED attendances,27 25% reported two attendances and 36% reported at least three attendances. The median number of visits for those who had made multiple visits was two [interquartile range (IQR) 2–5]. This pattern contrasts with that seen in the general ED population. Moore et al. 33 reported that only 24% of people from the general ED population reattend London EDs within 12 months.
Emergency department use for epilepsy is often clinically unnecessary but attendees often require more support
Seeking emergency care for epilepsy can be important, even life-saving. Reasons include a first seizure and status epilepticus. Some ED visits by PWE are for these reasons; most are not. The NASH,20,21,28 for example, found that most people attending an ED for a suspected seizure had diagnosed, rather than new, epilepsy. Moreover, most appear to have experienced an uncomplicated seizure. Dickson et al. 34 reviewed records for 178 seizure incidents presenting to one regional ambulance service over 1 month in 2012. Medical emergencies were uncommon and seizures had self-terminated before the emergency vehicle arrived in > 90% of cases. In only 8% of incidents were emergency drugs required to terminate the seizure. Uncomplicated seizures in someone with established epilepsy, although potentially frightening and distressing to experience and observe, do not typically require the full facilities of an ED and can be managed within the community by the person with epilepsy and their SOs. Indeed, going to an ED in these circumstances may result in iatrogenic harms caused by unnecessary investigations and interventions (e.g. unused intravenous cannulations, unnecessary head computerised tomography scans). 35–37
Although a high proportion of epilepsy-related ED visits do result in hospital admission, most appear unnecessary,38 with factors beyond the patient’s clinical need appearing to play a role in why the admission occurred (e.g. lack of access to senior medical review, need to avoid breaches of ED waiting-time targets). 39
Although the acute episodes leading PWE to visit an ED do not typically require the facilities of an ED, PWE attending an ED do often have poorer health than those in the wider epilepsy population. They report more seizures, poorer quality of life, less epilepsy knowledge, more anxiety and feeling more stigmatised because of epilepsy. 27,40–45 Despite this, most (≈ 65%) have not seen an epilepsy specialist in the prior 12 months. 28
There is also inequality in the use of EDs, with attendees being more likely to reside in areas where social deprivation is high and seizure control worse. 41,46,47 Indeed, ED admissions for seizures vary by more than fivefold between geographical regions in England42,44 and are most frequent in more socially deprived areas. 47,48
The National Institute for Health and Care Excellence (NICE)49 recommends that when seizures are not controlled a patient should be referred to specialist services. However, going to an ED because of an epileptic seizure does not typically lead to an increase in support. The NASH20,28 found that most (80%) PWE are not seen by a specialist during their attendance, usual care providers are often not informed of the attendance and most (60%) PWE are not referred to a specialist for follow-up. Among PWE attending an ED, those living in the most deprived areas are among those least likely to be referred to a specialist for follow-up. 50
Epilepsy as one area where opportunities exist to reduce demands
The NHS has been operating in a context of rising demand, slow funding growth and increasing operating costs. In 2015–16, this culminated in an aggregate funding deficit of £1.85B. 51,52 The NHS Plan,53 Five Year Forward View54,55 and related publications challenge the NHS to make substantial savings while working with patients and SOs to improve care experience and outcomes and reduce health inequalities. Epilepsy has been identified as one condition for which opportunities exist to improve patient outcome and experience, reduce demands and generate savings. 56
Although there is a drive to reduce ED visits for epilepsy and enhance patient outcomes, it has been challenging to identify how to achieve this,25,57 not least because the reason(s) for ED visits for epilepsy in publicly funded health-care systems has been unclear. The association between seizure frequency and ED use is, for example, only modest in size, and seizure type has not proved a robust predictor. 17,27,31,40
Emerging evidence highlights the potential importance of a person’s self-management skills in their use of EDs, specifically the confidence and skills that they and those around them have to manage seizures, with this potentially moderating the relationship between an uncomplicated seizure and the help sought at the time.
The importance of seizure management skills and confidence in emergency health-care service use
‘Self-management’ is a broad term. It refers to an ‘individual’s ability to manage their symptoms, treatment, physical and psychosocial consequences and lifestyle changes inherent in living with a chronic condition’. 58 In the context of epilepsy, self-management of adult epilepsy has been defined as ‘activities that an individual can perform alone that are known to either control frequency of seizures or promote well-being of the person with seizures’. 59
Much of the evidence regarding the potential importance of self-management skills in ED use comes from Ridsdale et al. ’s aforementioned NIHR study. 27,31,32 This study followed up its sample of 85 PWE for 12 months. A subgroup was interviewed about the reasons for their visits. Analyses indicated that participants fell into two groups. In the first were participants who reported high levels of confidence in managing their epilepsy. Their views closely aligned with seizure first aid guidelines. Explanations offered by these participants for their visits included having experienced an unusually long seizure or having sustained a significant injury. These persons had typically visited an ED only once in the previous 12 months.
By way of contrast, participants in the second group reported not feeling confident in managing seizures. They expressed a need for immediate access to urgent care when they had a seizure and had typically made two or more ED visits in the previous year. They explained that they and their family (to whom care decisions were often delegated when the patient was unconscious or lacked capacity) were fearful of seizures, including the possibility of death and brain damage, and were unsure about how to manage seizures and could not tell others about how to help should they have a seizure. 31,32 This, they reported, could lead them to call for an ambulance when they were about to have, or had had, a seizure. Despite having been diagnosed with epilepsy for an average of ≈ 10 years, these participants reported that they had not received sufficient information about epilepsy. Telling quotations from those interviewed included the following:31,32
[With] cancer, you’re awake. I know you can die, but you’re awake. I’d prefer something like that [. . .] Having epilepsy, you’re going into a fit. You don’t know if you’re going to wake up or die. That’s why I call [UK emergency services telephone number]!
Patient 23
[I was] just worried because I don’t know anything about epilepsy [. . .] I only know the bad things [. . .] I know you can die [. . .] I am so worried I decided just to ring an ambulance [. . .] Better safe than sorry.
SO 60
Quantitative results from the study reinforced what participants said at interview. There was, for example, evidence of less first aid knowledge in the ED sample than in the wider epilepsy population. One-third of the ED sample incorrectly stated that it was always necessary to call a doctor or ambulance if a person with epilepsy has a seizure, even if it occurs without complications. 27 Only 11% of the wider epilepsy population believe this. 60
Also important were the results of regression analyses to determine which baseline data could predict the number of ED visits participants reported 12 months later. A range of variables were examined for their association, including seizure frequency, seizure severity and medication management skills. It was participants’ level of confidence about managing epilepsy (as measured by the Wagner 6-item Mastery Scale)61 and the extent to which they felt stigmatised because of epilepsy (as measured by Jacoby’s Stigma Scale of Epilepsy)62 that were found to best predict ED use. These factors held similarly sized associations with ED use, with lower confidence (incidence rate ratio 0.86) and increased feelings of stigma (incidence rate ratio 1.32) being associated with more ED use.
‘Mastery’ refers to a state of confidence in which a person feels able to independently overcome the challenges with which they are faced. 63 The mastery measure captured the degree to which participants perceived themselves to have an internal or external locus of control, with example items from the scale including ‘I often feel helpless in dealing with my seizures’ and ‘Sometimes I feel that my epilepsy controls my life’. 61 Evidence suggests that when a person feels more confident in their ability to cope and manage their illness effectively they are more likely to put self-management behaviours into practice. 64
Epilepsy self-management and support currently offered to people with epilepsy and their significant others
The findings from Ridsdale et al. ’s study27,31,32 are in keeping with prior evidence: coping with life in the context of epilepsy requires an individual to accept their diagnosis and learn and adopt specific self-management behaviours to prevent seizures and manage consequences. These tasks together constitute what Corbin and Strauss65 labelled the ‘work’ of living with a chronic condition.
It is for these reasons that NICE49 recommends self-management support for PWE. Epilepsy specialist nurses form an important part of the way in which this support is delivered. However, it is known that current care models in the UK and beyond continue to fail some PWE and mean that these PWE have less than optimal levels of self-management confidence. In contrast to some other chronic conditions, there exists no routine course that PWE can go on to learn about their condition once diagnosed, and there is limited time in routine care appointments for advice on self-management. 66–68 Patients have previously summarised the lack of information and support that they were given following diagnosis as ‘I was left high and dry’. 69,70 It is PWE with lower levels of formal education who have been found to have the least epilepsy knowledge. 71,72
The role that Ridsdale et al. ’s study27,31,32 found a patient’s family and friends to play when a seizure occurs also accords with wider evidence: despite greater social isolation, up to 90% of PWE can still identify a SO who acts as an informal carer. 73 These SOs are not always trained in the management of seizures, including in the use of emergency medications.
One reason that SOs have been largely missing from discussions about the causes for ED visits for epilepsy is that it has widely been thought that such visits primarily occur because the person with epilepsy was alone in a public place, had a seizure and an ambulance was called by a bystander, and because a lack of information about the person’s medical history (such as from an epilepsy identification card) meant that paramedics transported the person to ED as a precaution. 74,75 Consequently, the focus has often been on how the public, rather than SOs, can be supported and educated. 76 Although ED visits for epilepsy certainly do occur via this route,77,78 this seems to be the minority.
Reuber et al. 23 examined attendances at a large ED in Leeds, UK. They found that, in this area of the UK, only 15% of ED visits by PWE occurred because the person was alone and had a seizure in a public place. Most seizures leading to an ED visit instead occurred in the patient’s home23 and most (70%) 999 calls for seizures are made by relatives or friends rather than by members of the public. 34
Given the indications that seizure management skills and confidence have an important role in emergency service use, a promising idea that has emerged is to offer PWE who frequently attend ED and their SOs a self-management intervention to improve their confidence and competence in managing seizures. 45,79 The objectives of self-management interventions are to facilitate patients taking an active role in their own health care by encouraging autonomy and providing accurate information on symptom management. 80 They are different from traditional educational approaches because, as well as educating, they seek to empower those living with chronic health-care conditions. 81 It is asserted that when people are supported to become more activated they benefit from better health outcomes and improved health-care experiences and make fewer unplanned health-care admissions. 80
What is the evidence that self-management skills, including seizure management, can be modified?
Evidence from adult and paediatric literature at the time of project design
Evidence from the literature on self-management interventions for people living with other chronic conditions such as diabetes,82,83 arthritis84–86 and asthma87–89 indicates that it is possible to elicit improvements in self-management. Consequently, in these fields self-management support has become well established. 90–96 In the UK, self-management courses have become freely accessible to people with diabetes. 97,98 The evidence base on improving self-management skills in PWE is more limited.
A Cochrane review by Bradley et al. 99 examined care delivery and self-management strategies for adults with epilepsy. It found four self-management studies focusing on adults with epilepsy. 100–103 None of the trialled programmes focused exclusively on seizure management or those attending EDs or systematically involved SOs, and none had been trialled in the UK. However, the review did conclude that there was tentative evidence that such interventions can improve epilepsy self-care skills.
Helgeson et al. ’s100 small US RCT is noteworthy because it evaluated an intervention that included some discussion of seizure first aid. The intervention, the Sepulveda Epilepsy Education programme, is a psychosocial 2-day group course. 100 At follow-up at 4 months, participants receiving the intervention demonstrated a statistically significant increase in their understanding of epilepsy, a decrease in seizure fear and a decrease in hazardous medical self-management practices compared with wait-list controls. However, health service utilisation was not measured and no significant changes were found in relation to anxiety or confidence managing epilepsy.
As the number of trials conducted with adults with epilepsy is small, Lindsay and Bradley’s104 Cochrane review of self-management interventions for children with epilepsy and their parents is instructive. Lindsay and Bradley identified two RCTs of interventions that contained modules on seizure first aid.
The first, conducted by Tieffenberg et al. ,87 evaluated a Spanish group-based programme, the Civil Association for Research and Development in Health (ACINDES in Spanish), for children aged 6–15 years and delivered by teachers to educate children and parents about epilepsy. It consists of five weekly 2-hour sessions, followed by a reinforcement session 2–6 months later. At follow-up at 12 months, children in the intervention arm showed statistically significant improvements in epilepsy knowledge compared with children in the control arm. There was also a significant reduction in ED visits. The mean number of ED visits made by children in the intervention arm in the 12 months prior to randomisation was 0.90. This reduced to 0.22 at follow-up at 12 months. For the TAU arm the mean number of ED visits made by children went from 0.83 to 0.46.
The second RCT was by Lewis et al.,105,106 and examined the effect of another Spanish intervention called the Children’s Epilepsy Programme. This intervention taught children and parents about seizures, living with epilepsy, and communication. Children and parents separately attended four 1.5-hour group sessions and met together at the end of each session to share learning. At follow-up at 5 months, children in the Children’s Epilepsy Programme arm had significantly improved epilepsy first aid knowledge compared with children in the control arm. 105 Their parents also showed significantly greater reductions in anxiety than parents in the control arm. 106
That training in self-care is associated with reduced service utilisation in the paediatric epilepsy population, without compromising patient outcome, concords with the larger, higher-quality evidence base on interventions to reduce ED use by children with asthma, which is another chronic, relapsing condition. 88,89,107 Boyd et al. ,89 for example, completed a Cochrane review of 17 RCTs of educational interventions for children (and their parents) at risk of asthma-related ED attendances. Data from > 3000 children who presented to an ED for acute exacerbations and were then followed for an average of 10 months were included. Educative interventions led to a 37% reduction in the relative risk of reattendance at an ED in the treatment arm compared with the control arm, and a 21% reduction in the relative risk of subsequent hospital admission in the treatment arm compared with the control arm.
Conclusions from trials regarding the ability of self-management interventions to elicit behaviour change in PWE do, however, need to be tempered, because most trials have been found to be of low methodological quality. 108–110 Such trials are at a higher risk of bias and potentially overestimate effects (see Savovic et al. ). 111 In Helgeson et al. ’s100 trial of the Seizures and Epilepsy Education programme, for instance, no information was provided regarding random sequence generation or allocation concealment, only 38% of those randomised to the intervention completed it and outcome assessments were not blinded. 110
Development of the SAFE intervention
On the basis of the evidence so far presented, we hypothesised that PWE who frequently visit ED for epilepsy (here operationalised as two or more visits in the previous year) might benefit from an intervention that improves their own and their SOs’ confidence and ability in managing seizures and empowers them to be able to tell others from their wider support networks about how to help if a seizure occurs. In the absence of an existing intervention it was necessary to develop one. This was done by adapting a broader intervention that was in existence and used by the ES for different purposes.
Offering such a programme to the target population is supported by the NHS policy of empowering and supporting people with long-term conditions to understand their own needs and self-manage them. 112–114 Indeed, the Keogh Urgent and Emergency Care Review115 identified that better supporting people to self-care for their condition is one way that EDs could become sustainable.
Background to the existing seizure management course and its development
The ES is an English charity with a 120-year history that has assumed an important role in the voluntary sector in producing informative materials for PWE and in offering epilepsy training for different audiences (see www.epilepsysociety.org.uk/training-courses-epilepsy#.XSR5AutKj3g; accessed 27 August, 2019). In 2012–13, > 2000 people attended ES training courses (Joanne Fox, Learning and Development Manager, ES, 2014, personal communication). Of relevance here was their training course entitled ‘Epilepsy awareness and seizure management’, which they had been offering since 1998 to a fee-paying audience. Recipients included teachers, health and social care staff, PWE and SOs. The course was developed iteratively, with the involvement of neurologists, psychologists and social workers.
The course was delivered by a single educational facilitator. To deliver a course, a facilitator needed to follow a training programme developed by the ES, and a quality assurance component of ongoing internal assessment promoted consistency in delivery. The professional background of facilitators was not fixed; although they did require experience of working with PWE. Facilitators were typically people with a nursing or social care background.
Although not formally evaluated, the course was considered of interest because one of its aims was to increase recipients’ confidence in seizure management, emphasising how most seizures are self-limiting, and providing a practical understanding of when seizures do and do not require emergency treatment.
Michie et al. ’s116 Affordability, Practicality, Effectiveness and cost-effectiveness, Acceptability, Side-effects/safety and Equity (APEASE) framework, which is intended to help guide choices about intervention development and selection for evaluation, emphasises the importance of considering, from the outset, issues such as the affordability and practicability of an intervention to ensure that the intervention is positioned to achieve its intended outcome once rolled out. With this in mind, what was also considered advantageous about the ES’s course was that its delivery did not depend solely on those with specialist training in epilepsy. The UK has fewer neurologists per head than other developed nations117,118 and only ≈ 55% of its acute trusts have access to an epilepsy nurse specialist. 119,120 Therefore, a care model that depends solely on such people may not be sustainable and generalisable.
The course in its original form
The ES’s course was delivered to groups of 10–20 people. It lasted ≈ 3 hours, with breaks included. Educational aims for course recipients are specified and outlined in Table 1. Materials for the course included slides, professionally produced video clips of seizure types and first aid and additional information booklets on topics such as risk management and emergency medication. An information pack provided participants with a permanent record of the material and included space for notes to promote active processing of material as well as participation.
| Scope | Content |
|---|---|
|
|
The intention was for learning to be elicited rather than taught, with the behaviour of the educational facilitator seeking to promote a non-didactic approach. Course participants were encouraged to share experiences and ask questions. To some extent there is therefore meant to be tailoring of the information that is presented so that it aligns with the needs of the group being taught (i.e. is patient centred). This, and that the course consists of a number of interacting components that may act both independently and interdependently, means that it is what the Medical Research Council refers to as a ‘complex intervention’. 121,122
Justification for developing seizure management training: a policy and service user response
The ES course is not informed by detailed theoretical modelling or a clear behaviour change model. Having reviewed and observed the intervention, our multidisciplinary research team nevertheless identified several ways by which receipt of the intervention (or a suitable adaption) could plausibly support PWE to make fewer ED attendances and improve patient and SO outcomes. These are detailed in full in Appendix 1. In brief, it was considered that it might increase patients’ and SOs’ practical understanding of seizure management, increase their knowledge of how to make appropriate care and lifestyle decisions (including the need for ED attendance) and reduce fears about risk, thereby increasing self-confidence and empowerment.
Importantly, the aims of the programme also broadly aligned with what PWE and their SOs had generally said they wanted and how they wanted to receive it. For example, studies consistently show that PWE and their SOs want more information about living with epilepsy. 27,31,123–129
Adapting SAFE
To maximise acceptability, benefit and behaviour change potential, it was recognised that the ES’s course would probably require adaptation for the target audience. To this end, the ES agreed for their course to form the basis of a new, adapted course, entitled SAFE, for PWE who frequently attend EDs and their SOs. To identify the changes required we utilised a collaborative framework underpinned by a philosophy of experience-based co-design. 130 This is an approach to improving health-care services that combines participatory and user experience design and processes to bring about quality improvements in health care. 130
The need for a pilot randomised controlled trial of the SAFE intervention
Having adapted the ES’s course, it would be necessary to evaluate SAFE’s efficacy to determine whether or not its use in routine practice was appropriate. A definitive RCT is the most reliable methodology for determining efficacy, including of complex interventions. However, RCTs can be costly and time-consuming, especially when the trial is evaluating change in health-care service contact and when the follow-up period needs to be reasonably long. Recruitment of participants for RCTs is often slower or more difficult than expected, thus jeopardising the ability of the trial to answer its intended research question. For example, only 56% of the trials funded between 2004 and 2016 by the NIHR’s Health Technology Assessment programme met their final recruitment target. 131 Before proceeding to a definitive RCT, the expenditure therefore needs to be justified, the trial deemed feasible and its design optimised. 132
Because the adapted intervention was new and the target population not studied to a great extent, important process, management, resource and scientific uncertainties existed concerning the feasibility and optimal design of a full RCT of SAFE. The uncertainties were as follows:
-
No estimates of likely recruitment, consent and follow-up rates for a definitive trial were available.
-
Acceptability of randomisation to participants was unknown.
-
Annual rate of ED visits in the TAU arm and the likely dispersion parameter were unknown.
-
It was unclear whether or not SAFE could be delivered as intended in a trial context (fidelity).
-
The resources/costs required to deliver SAFE in a trial context were unclear.
-
Estimates of the effect of SAFE on primary and secondary outcome measures and their precision were lacking.
For these reasons, and informed by Lancaster et al. ’s133 guidance, an external pilot RCT was considered appropriate. Resembling a full RCT in many respects, such a pilot would allow us to resolve the above uncertainties and permit an informed decision to be made about whether or not and how to proceed to a definitive trial. In advance of the pilot we specified two criteria against which we would primarily judge the pilot to help evaluate the feasibility of a definitive trial:
-
At least 20% of eligible patients need to agree to participate in the pilot RCT. This was based on results from previous evaluations of self-management interventions, including the diabetes programmes that have since been commissioned by the NHS. A participant uptake rate of < 20% was deemed to raise significant concerns regarding external validity and could have implications for trial duration, recruitment centres required and feasibility.
-
Twelve-month primary outcome data need to be secured for at least 75% of participants in the pilot RCT. This figure was based on results from previous evaluations of self-management interventions that reported rates of 70–80%. 109,110,134
Depending on the results from the pilot, one of the following judgements, based on Thabane et al. ’s132 decision framework, would be made regarding the feasibility of a definitive trial:
-
stop – the main study is not feasible
-
continue, but modify the protocol (feasible with modifications)
-
continue without modifications, but monitor closely (feasible with close monitoring)
-
continue without modification (feasible as it is).
Chapter 2 Part A: intervention development
Introduction
Part A sought to develop SAFE for both PWE who frequently visit hospital EDs and their SOs. To do this, the ES’s existing course ‘Epilepsy awareness and seizure management’ was adapted and its behaviour change potential optimised.
Many self-management interventions to date have been derived from limited expert opinion and have not involved PWE and other stakeholders in the planning process. 129 By contrast, and to help ensure maximum benefit and acceptability to users, we used an experience-based co-design approach. 130 Such an approach allows researchers to work collaboratively with people from the target population to identify their specific learning needs, clarify their delivery preferences and adapt the intervention to match these.
We were mindful of the need for SAFE to be viable for delivery within the NHS. 112–114 Examples abound of the importance of ensuring that any new intervention is supported by those who will be asked to ultimately refer their patients to it, deliver it or allocate resources to it, including in the context of epilepsy. 135 Therefore, representatives from different key professional bodies involved in the support of PWE were also consulted as part of our co-design approach.
Methods
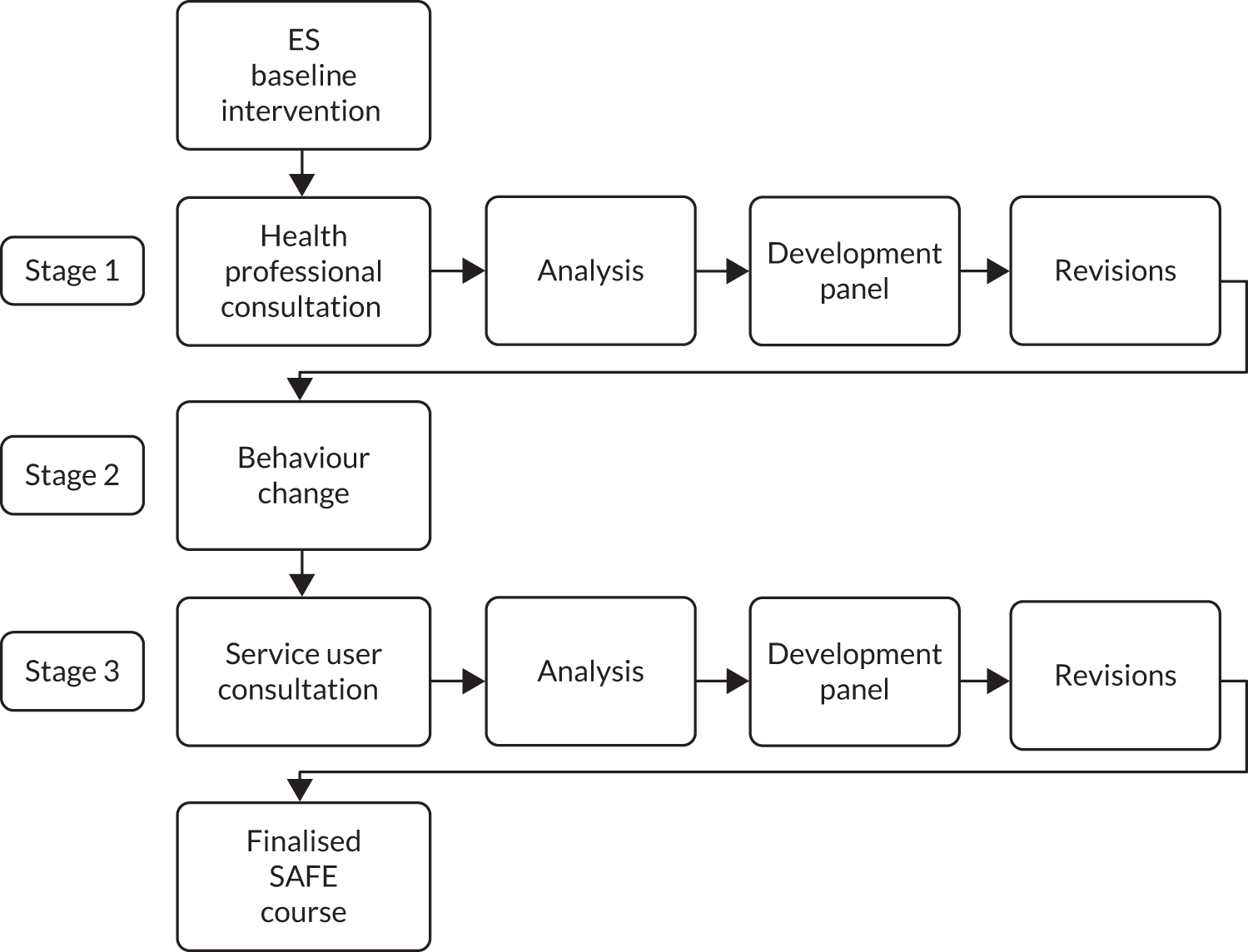
The co-design process used comprised three iterative stages, namely (1) qualitative interviews with health-care professionals about the existing intervention, (2) optimisation of its behaviour change potential and (3) focus group (FG) discussions with service users. The process is outlined in Figure 1.
FIGURE 1.
Intervention development process.
Participant settings and samples
Stage 1: recruitment of health-care professional representatives
Different health disciplines can be involved in the care of PWE. Some patients will identify a general practitioner (GP) as the main provider of their ambulatory care whereas others will identify a specialist, such as a neurologist or epilepsy. The voluntary sector is also an important support structure for many PWE. When someone seeks emergency care for a seizure, other parts of the health system come into contact with PWE, including paramedics and ED staff. All parties were considered as being positioned to be able to offer insights into the support needs of PWE who attend for seizures. We therefore chose to adopt purposeful sampling for the identification and selection of information-rich cases136 from the main parts of the care pathways encountered by PWE. This involved identifying and selecting individuals who were especially knowledgeable about or experienced with the phenomenon of interest. 137 In addition to knowledge and experience, participant availability and willingness to participate were key considerations. 138,139
Seven professional organisations (Table 2) identified a representative potentially interested in participating. Each representative was sent a participant information sheet (see Appendix 2), and those taking part signed a consent form. Interviews were conducted by a qualitative researcher (Adwoa Hughes-Morley; see Acknowledgements) and took place at a time and in a format convenient for the representative, be that via telephone, Skype™ (Microsoft Corporation, Redmond, WA, USA) or face to face at the representative’s office. Each representative was offered a consultancy fee of £200.
| Group | Details |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ultimately, a consultative group comprising nine health-care professionals from seven different disciplines was established to provide feedback on the content of the ES course. This group included two consultant neurologists (one with a specialist interest in epilepsy), two consultants in emergency medicine, a consultant paramedic, a epilepsy nurse specialist, a GP with a specialist interest in epilepsy, a service commissioner with a health-care background and an educational representative from an epilepsy charity other than the ES.
Stage 3: recruitment of service user representatives
Feedback on the intervention was obtained from a purposive sample of service user representatives. PWE were eligible to participate in SAFE training if they met the following inclusion criteria:
-
established diagnosis of epilepsy (≥ 1 year)
-
currently prescribed antiepileptic drug(s)
-
age ≥ 16 years (no upper age limit)
-
visited an ED for epilepsy at least once in the past 2 years (as reported by the patient)
-
living in the north-west area of England
-
able to provide informed consent and participate in SAFE course in English.
People with epilepsy were excluded from participating in SAFE training if they reported:
-
acute symptomatic seizures
-
severe current psychiatric disorders
-
life-threatening medical illness.
Patient participants could take part with or without a SO. SOs with the following characteristics were eligible to participate in SAFE training:
-
a SO to the patient (e.g. family member, friend) who the patient identifies as providing informal support
-
age ≥ 16 years (no upper age limit)
-
living in the north-west area of England
-
able to provide informed consent and participate in SAFE course in English.
Significant others were excluded from participating in SAFE training if they reported:
-
severe current psychiatric disorders (e.g. acute psychosis)
-
life-threatening medical illness.
The service user representatives were identified via adverts circulated (via newsletters and websites and at meetings) to the affiliates of user groups, including the Mersey Region Epilepsy Association (Liverpool, UK), the Brain & Spine Foundation (London, UK), NeuroSupport Services Ltd (Nottingham, UK) and the ES. This approach enabled the recruitment of ‘experts’, defined as informed individuals with knowledge or experience of a specific subject. 140,141 Eligible patients and SOs interested in taking part were sent a participant information sheet (see Appendix 3) and those taking part signed a consent form.
A total of 23 service user representatives were ultimately recruited, comprised 13 PWE (seven men and six women) and 10 SOs (four men and six women). Each received a £10 shopping voucher.
Intervention development panel members
The process was overseen by an intervention development panel. The panel considered and discussed the findings from the interviews and FGs and made required adaptations to the intervention. The panel included patient and SO representatives [Mike Perry (MP) and Linda Perry (LP)], a psychologist (AN), a neurologist (LR), a medical sociologist (MM), a research nurse with specialist qualitative research training (DS) and a representative from the ES’s training division. Patient and SO representatives were active in all aspects of the decision-making and were reimbursed in line with guidance. 142
Developing the intervention
Stage 1: consultation with health-care professional representatives
Consensus exists regarding what constitutes appropriate seizure first aid. 143–145 Thus, the purpose of this stage was to interview representatives from the main professional bodies caring for PWE to ascertain whether or not the medical information presented by the programme was correct and whether or not SAFE could be an intervention they could, in the future, support. It was considered important to seek feedback from professionals in the first instance to prevent PWE (and SOs) being exposed to possibly incorrect information. Moreover, it would allow us to identify from the start what sort of seizure first aid intervention was considered feasible for delivery in the context of the NHS. The health-care professional representatives each conducted a baseline document and audiovisual review of the course materials for the ES’s existing intervention.
In advance of their interviews, each representative was provided with the course materials. Approximately 2 weeks later, data from each of the nine representatives were collected via audio-recorded semistructured interviews. A topic guide was developed to reflect the intended purpose of the stage and on the basis of the literature (see Appendix 4). It was refined through the iterative process of the interviews. 146,147 The exact questions varied depending on the representative’s area of expertise, but all health-care professional interviews included key discussions on:
-
identifying inaccuracies in the content of the existing ES programme
-
likes/dislikes and the appropriateness of the current content and delivery
-
suggestions about how to make the programme more helpful
-
how SAFE might be best rolled out in the NHS if a future trial found it to be effective.
Stage 2: optimisation of the intervention’s behaviour change potential
A significant component of the ES’s intervention consisted of health-related information provision. It was anticipated that the provision of such information could reassure service users and increase seizure management confidence and competence. However, for some PWE the information might actually highlight that their prior use of ED conflicted with medical guidance. From a psychological perspective, this could be construed as a threat to self-integrity. As a consequence, these people might be at risk of rejecting or denigrating the information provided by the SAFE intervention, which may compromise its ability to elicit behaviour change. According to self-affirmation theory,148 people are fundamentally motivated to preserve a positive, moral and adaptive self-image and to maintain self-integrity. Consequently, health messages that threaten one’s sense of self-image can be subject to defensive processing (e.g. motivated scepticism, unrealistic optimism). 149
To mitigate against this and maximise the behaviour change potential of SAFE, the intervention development panel decided to introduce a self-affirmation exercise, namely Reed and Aspinwall’s150 kindness questionnaire, which individuals would complete at the start of SAFE training. The rationale was that evidence indicates that having a person complete an exercise such as recalling one’s acts of previous kindness prior to receipt of health risk messages reduces resistance to threatening or dissonant health risk information. This is because theory indicates that a person’s self-image can be maintained by self-affirming in one domain (e.g. recalling one’s acts of kindness) even if one is being threatened in another domain (e.g. health), because people can defend their global sense of self-worth rather than (for example) against the threat directly attributable to health risks. 151,152
The kindness questionnaire was considered ideal because it is brief (taking ≈ 5 minutes to complete) and effective150,153 and does not need to be delivered by specialists. It consists of 10 questions that participants work through by themselves, including ‘Have you ever been concerned with the happiness of another person?’ and ‘Have you ever forgiven another person when they have hurt you?’, with yes/no response options. The intention was that PWE and SOs would each complete it at the start of SAFE. Their questionnaires were not to be collected by any member of the intervention team and the participant would not be asked how they answered.
Stage 3: consultation with service user representatives
To allow service user representatives to critically feed back on SAFE, two practice courses were run in November 2015 using the intervention that resulted from the first two stages. These were followed by FG discussions.
The courses took place in a local hospital’s education centre and were delivered on weekdays to groups of ≈ 10 patient–SO dyads. A facilitator from the ES (Juliet Bransgrove; see Acknowledgements), who was an epilepsy nurse specialist with experience of delivering the ES’s course, delivered the courses. She underwent a period of familiarisation with the adapted intervention by reviewing the new materials and a trainers’ manual (see Report Supplementary Material 1) and meeting the intervention development panel.
The FGs, which lasted ≈ 60 minutes each, were conducted by a trained qualitative researcher (DS) to explore participant views. The researcher observed each course and recorded impressions of participants’ engagement with the materials, the group and the facilitator. A topic guide (see Appendix 5) reflecting the discrete sections of the course guided the FGs. Participants were asked about issues related to the course content and delivery as well as for views around its perceived strengths and barriers to its successful implementation.
Analytic process
The interviews, practice course events and FGs were recorded (with participants’ consent) and transcribed verbatim. Transcripts were checked for accuracy by the researcher (DS) who conducted the data collection. A comprehensive inductive and deductive approach was used, with NVivo version 10 (QSR International, Warrington, UK)154 being used to provide a transparent account of the work. Nodes (codes) were created to mark relevant concepts and topics in the text documents. Lower-level nodes were then grouped into themes. An account of the process of analysis was logged in the memos attached to categories and interview and FG documents, including the questions used to interrogate the data, and thoughts and decisions about what themes to focus on. These capabilities and the associated process fit with the iterative goals of the development stages of the training intervention. Direct quotations are provided in the text as a means to verify interpretation and illustrate themes.
Results
Health-care professionals
Analysis of health-care professionals’ responses highlighted three key themes, namely (1) initial impressions, (2) areas of intervention in need of revision to promote effective participation and (3) course delivery.
Initial impressions
There was consensus on the need for such an intervention and its potential for cost-effectiveness. As one representative noted:
[I]t will be a powerful tool to upskill and reassure [. . .] The clearer they are about what constitutes that person’s normal epileptic fit and what maybe a bit unusual, the more [. . .] appropriate the response the better their [. . .] overall experience, privacy, dignity and everything to follow [. . .] [I]t’s a fairly easy case to make for the commissioners [. . .] Resources directed by commissioners to pay for this really unnecessary attendance could be redirected [. . .] win–win.
ED consultant
The existing intervention was seen to provide a useful starting point for adaption and professionals liked the videos and associated information booklets:
There’s a solid foundation in there which you can build on to then create the course to make sure it reaches the kind of outcomes that you are after [. . .] It’s just making sure that it hits all those objectives along the way.
User group representative
The practical challenges of hosting a group-based course were highlighted by many; it was thought that ‘getting people together may be a difficulty’ (epilepsy specialist nurse) that would require careful consideration, especially in relation to ‘distance and timing’ (epilepsy specialist nurse). It was important, therefore, to ‘think about local delivery and minimising travel time’ (epilepsy nurse specialist).
It was also felt that substantial changes were needed to make the intervention appropriate for its new audience and to achieve the aim of attenuating unnecessary/avoidable ED use.
Areas of intervention in need of revision
It was deemed important to better emphasise the benefits of the course to PWE and SOs with a more focused and ‘clear message’ at the start on the need or not for ED attendance after a seizure. Language level was highlighted as an area that required revision in line with the average UK literacy level. There were also suggestions, as presented below, to revise the style of presentation so that it was less for people who might be involved in epilepsy because of their profession:
[I]t’s written in quite a wordy way and probably a few more pictures and a few less words [. . .] would be better. This is probably a very good presentation to new health-care professionals [. . .] but probably not great for people that [. . .] have no training whatsoever.
Consultant paramedic
It was considered that a behavioural change focus (emergency medicine consultant 1) should be brought to the fore and that the benefits to the participant and SO of avoiding unnecessary ED visits should be emphasised. As explained by the GP representative:
It’s about helping people manage their epilepsy better. What will the course do for you? What can you get out of the course? [. . .] The course should focus on behavioural change, but the impression should not be given that the focus is about reducing A&E [accident and emergency department] admissions – that would be counterproductive. Rather, it is about highlighting the benefits, such as better management will reduce inconvenience in having to go to A&E.
GP
A recurring theme was the need to better elicit and address patient concerns and that patient and SO participation should be promoted through more interactive exercises, the inviting of questions and the discussing of fears, because this was when ‘true education happens’. This was summarised by one representative:
[S]eizure management, what you should do and what you wouldn’t do [. . .] I’d make that part interactive [. . .] get them to tell you, get their opinion and their views before and then you show them what they probably should do afterwards but find out. Because you’ll probably [. . .] challenge them [. . .] that’s the point where you really get them I think.
Consultant neurologist
A number of participants pointed out that, when seizures happen in public places, the decision to seek emergency care is not necessarily the patient’s or their SO’s. Therefore, it was recommended that the intervention should support patients to develop and carry with them personalised care plans on paper or on their smartphones, which could be used inform decision-making by paramedics:
[I]f we [the ambulance service] tip up and we don’t know anything about you we are taking you to hospital [. . .] We’re tipping up to what we consider to be a first-time fit until proven otherwise [. . .] Have a care plan written or something on you that says ‘I’m an epileptic and this is what happens to me normally’.
Consultant paramedic
Course delivery
Participants observed that, to make the course suitable for delivery in the NHS and to promote quality and consistency among trainers, the intervention should become fully standardised and a trainer’s manual, including recommended times for each topic/activity, developed.
With respect to attributes and skills of an ideal facilitator to deliver the course, some identified epilepsy nurse specialists. However, others felt that the epilepsy voluntary sector was well developed and, therefore, related commissioning organisations could help avoid shortfalls where specialist staff were not available. It was considered that the following should be true in any circumstance:
The facilitator is someone who should have knowledge of epilepsy and be good at leading groups. They need the ability to keep the course on track and to time, especially when participants may want to talk a lot!
Epilepsy nurse specialist
Changes made by intervention development panel to create version 1.1
There was agreement in the intervention development panel that the intervention’s content needed to be revised to be better directed towards the goal of attenuating unnecessary ED use. Therefore, the aims for the new intervention were specified as helping participants to:
-
feel more confident to manage their seizures/the seizures of someone they know
-
know how to tell others how to help
-
know some things that may reduce the chances of a seizure
-
know some things that may reduce the chances of injury from a seizure.
There was also agreement in the intervention development panel that more interactive activities were needed. Interactivity was considered important as it permits participants to share and learn from each other, can foster a sense of empowerment and means that participants ask questions and seek clarification to ensure that the intervention is tailored to their needs. As part of the discussion, evidence from the diabetes self-management literature was reflected on, which suggests that interaction between participants and the facilitator can be important in promoting behaviour change. Skinner et al. 155 calculated the ratio of facilitator talk to participant talk during a group-based education intervention for diabetes and found that lower facilitator talk ratios predicted greater improvements in participants’ metabolic control and beliefs about diabetes.
In line with these requirements, Adam Noble and Darlene Snape revised the intervention, generating new presentation materials, introducing new content and generating a training manual for facilitators. In doing this, attention was given to presenting information in an easy-to-understand style, with feedback from the ‘plain English’ section of the ES’s information department being obtained. Table 3 details the changes made to the ES’s intervention at this stage.
| Original course: version 1.0 | Post stage 1: version 1.1 (post professional consultation and discussion by intervention development panel; subsequently presented to users) | Post stage 2: version 1.2 (post pilot training sessions, user FGs and discussion by intervention development panel; delivered as the intervention in study phase B pilot RCT) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | |||||||||||
| Epilepsy awareness and seizure management | Epilepsy Seizure First Aid Training | Managing seizures: epilepsy first aid training, information and support | |||||||||
| Duration (hours) | |||||||||||
| 3 | 3 | 4 | |||||||||
| Materials | |||||||||||
| Slide projector, flipchart, video, information packs (including wallet-sized first aid instructions cards, paper epilepsy ID cards, contact details for further information) and certificates of attendance | Slide projector, flipchart, video, information packs (including wallet-sized first aid instructions cards, paper epilepsy ID cards, instructions for IDs on telephone, contact details for further information) and certificates of attendance | Slide projector, flipchart, video, information packs (including wallet-sized first aid instructions cards, paper epilepsy ID cards, instructions for IDs on telephone, contact details for further information, web address for copies of the course materials) and certificates of attendance | |||||||||
| Order | Learning topic | Learning activity | Minutes allotted | Order | Topics | Learning activity | Minutes allotted | Order | Topics | Learning activity | Minutes allotted |
| 1 | Aim of this session | Slide | – | 1 | Welcome | Slide | 5 | 1 | Welcome | Slide | 5 |
| 2 | Objectives | Slide | – | 2 | Taking on information (kindness questionnaire) | Interactive | 10 | 2 | Goals of this course | Slide | 2 |
| 3 | Session outline | Slide | – | 3 | Goals of this course | Slide | 2 | 3 | What would you like from today? | Interactive | 20 |
| 4 | Myth or truth? | Interactive | – | 4 | What would you like from today? | Interactive | 5 | 4 | True or false? | Interactive | 12 |
| 5 | What is epilepsy? | Slide | – | 5 | True or false? | Interactive | 8 | 5 | Taking on information (kindness questionnaire) | Interactive | 10 |
| 6 | The brain . . . | Slide | – | 6 | Epilepsy, seizures and how the brain works | Video | 10 | 6 | Epilepsy, seizures and how the brain works | Video | 10 |
| 7 | Lobes of the brain | Slide | – | 7 | First aid for convulsive seizures | Interactive | 10 | 7 | First aid for convulsive seizures | Interactive | 10 |
| 8 | How the brain works . . . | Slide | – | 8 | What can you do to help someone during a seizure? | Slide | 5 | 8 | What can you do to help someone during a seizure? | Slide | 5 |
| 9 | Seizures happen when . . . | Slide | – | 9 | What not to do during a seizure | Slide | 5 | 9 | What not to do during a seizure | Slide | 5 |
| 10 | Seizures are . . . | Slide | – | 10 | What to do after the seizure has stopped | Slide | 5 | 10 | What to do after the seizure has stopped | Slide | 5 |
| 11 | Possible causes of epilepsy | Slide | – | 11 | When to call an ambulance | Slide | 10 | 11 | Questions or comments? | Interactive | 10 |
| 12 | Diagnosis | Slide | – | 12 | Questions or comments? | Interactive | 5 | 12 | Post-seizure states | Slide | 15 |
| 13 | Triggers | Slide | – | 13 | Refreshment break | Networking | 10 | 13 | Injuries | Slide | 2 |
| 14 | Seizure types and first aid | Video | 15 | 14 | Recovery position | Slide | 2 | 14 | When to call an ambulance | Slide | 10 |
| 15 | Seizure management | Interactive | – | 15 | Recovery position | Video | 2 | 15 | Questions or comments? | Slide | 10 |
| 16 | Recovery position | Slide | – | 16 | Let’s practice the recovery position | Interactive | 8 | 16 | Refreshment break | Networking | 10 |
| 17 | Maintain airway | Slide | – | 17 | Questions or comments? | Interactive | 5 | 17 | Recovery position | Slide | 2 |
| 18 | When to call an ambulance? | Slide | – | 18 | Who needs to know how to help? | Interactive | 3 | 18 | Recovery position | Video | 2 |
| 19 | Most seizures are . . . | Slide | – | 19 | What they need to know and why | Slide | 5 | 19 | Let’s practise the recovery position | Interactive | 15 |
| 20 | Status epilepticus | Slide | – | 20 | How to get this information to them: family, friends and work colleagues | Slide | 5 | 20 | Questions or comments? | Interactive | 5 |
| 21 | Sudden unexpected death in epilepsy | Slide | – | 21 | How to get this information to them: members of the public and health workers | Slide | 5 | 21 | Who needs to know how to help? | Interactive | 5 |
| 22 | Treatment with drugs | Slide | – | 22 | Questions or comments? | Interactive | 5 | 22 | What they need to know and why | Slide | 5 |
| 23 | Medications | Slide | – | 23 | Refreshment break | Networking | 10 | 23 | How to get this information to them: family, friends and work | Slide | 5 |
| 24 | Medication | Slide | – | 24 | Personal stories: introduction | Slide | 2 | 24 | How to get this information to them: members of the public and health workers | Slide | 5 |
| 25 | Possible side effects of medications | Slide | – | 25 | Ben’s story | Slide | 5 | 25 | Questions or comments? | Interactive | 5 |
| 26 | Other possible treatments | Slide | – | 26 | How to change what happened to Ben (carrying medical ID; triggers) | Interactive | 5 | 26 | Refreshment break | Networking | 5 |
| 27 | Minimising risk | Slide | – | 27 | Triggers | Slide | 5 | 27 | Personal stories: introduction | Slide | 2 |
| 28 | Keeping safe | Slide | – | 28 | Knowing your triggers | Slide | 5 | 28 | Ben’s story | Slide | 6 |
| 29 | Supporting the ‘whole person’ | Slide | – | 29 | Some ways of dealing with triggers | Slide | 10 | 29 | How to change what happened to Ben (carrying medical ID; triggers) | Interactive | 5 |
| 30 | To the future . . . | Slide | – | 30 | Questions or comments? | Interactive | 5 | 30 | Triggers | Slide | 5 |
| 31 | Further sources of information | Slide | - | 31 | Sandra’s story | Slide | 6 | 31 | Knowing your triggers | Slide | 4 |
| 32 | Any questions? | Interactive | - | 32 | How to change what happened to Sandra (warning signs; home safety) | Interactive | 5 | 32 | Some ways of dealing with triggers | Slide | 4 |
| 33 | Certificates of attendance | Slide | - | 33 | Main points to remember if you have epilepsy | Slide | 5 | 33 | Sandra’s story | Slide | 6 |
| 34 | Main points to remember if you know someone with epilepsy | Slide | 5 | 34 | How to change what happened to Sandra (warning signs; home safety) | Interactive | 2 | ||||
To promote more interaction, four new activities were introduced. One involved practising the recovery position; another required subgroups to find answers to different questions concerning seizure first aid from among a group of acetates and to present these. This was designed to identify participant beliefs and fears and for these to be discussed. The final two activities centred on case studies. These involved participants being read illustrated stories of patients and asked to consider what things the patient in the story might have done to have achieved a better outcome. The carrying of epilepsy identification was one way in which the outcome of one of the stories could have been changed.
It was estimated that, in the revised intervention, 114 minutes (47.5%) was dedicated to interactional/networking elements, 114 minutes (47.5%) to slides and 12 minutes (5%) to video.
Service users
Having received version 1.1 of the adapted course, three key themes emerged from the analysis of service users’ responses. These included ‘the need to know’, ‘barriers to and drivers of effective participation in training’ and ‘course delivery’.
The need to know
All patient and SO participants identified the need for such a course, with lack of prior support in self-management as a recurring topic of discussion. For example, participants explained as follows:
It can be quite overwhelming I think for partners. It’s like ‘all on their shoulders’ what happens. I think your carer needs a lot of support too.
Patient 3, male (M), FG 1
The consultants they just presume that you know [about epilepsy] [. . .] but for all the years he got put on tablet or tablets [. . .] you didn’t see or hear any of this [information presented on the course]. So it’s suddenly all of an eye-opener and talking here you realise we are not on our own.
SO 1, female (F), FG 1
Similarly, another SO noted:
I always think of epilepsy as the poor relation [. . .] not much on TV or adverts about epilepsy support [. . .]. A course like this helps to develop that sense of support as well as improve knowledge.
SO 2, M, FG1
Concerns expressed by SOs centred on the ‘need to know I’m doing the right thing’ (SO 4, F, FG 1). PWE expressed concerns about disclosure and how best to tell others around them how they should help if a seizure happened; they wanted information and advice on how best to manage this. Overall, service user participants described three areas of perceived need: knowledge acquisition around epilepsy, emotional and/or practical support, and dealing with isolation and stigma.
Taken as a whole, the content of the revised course (version 1.1; see Table 3) was felt to be ‘excellent’ (patient 3, M, FG 2) and appropriate. Of particular importance to the users was the straightforward guidance that an ambulance was required when seizures lasted for 5 minutes or longer. This information alone was found to be helpful and reassuring, and some said that they would no longer always call immediately for an ambulance: ‘I think I will wait longer [to call an ambulance] than I did before picking up the phone’ (SO 3, F, FG 2); ‘I will wait [to call an ambulance] rather than when he has a seizure going for the phone straight away’ (SO 7, F, FG 2).
Concerns with regard to how to tell others about epilepsy and how to help if a seizure happens were expressed:
Like when you go somewhere you need to remember to like tell people that you have seizures.
Patient 1, M, FG 1
I need to know how best to share with others [family/friends/colleagues] the implications of having epilepsy.
Patient 2, F, FG 1
To this end there was consensus, for the most part, that the need to feel informed and reassured on what to do when seizures occurred had been met. Participants expressed how they had ‘learned a lot’ (SO 5, F, FG 1) from the session.
The balance between taught and interactive components was felt to be appropriate. The provision of information was viewed as reassuring and the opportunity to practise the recovery position was valued. As one patient asserted:
Watching a video would just go straight over me head, but actually putting [patient name] on the floor and putting him in the right position will help. For me that’s very useful [. . .] something like that I won’t forget.
Patient 5, F, FG 1
The training session was considered to cover more than implied by its title. Participants said that the ‘wider remit’ (patient 10, F, FG 2) was desirable but that a more accurate title was needed to engage future service users. ‘Managing seizures: epilepsy first aid training, information and support’ was identified as more suitable for the purposes of advertising.
Barriers to and drivers of effective participation in training
Service users’ perceptions of barriers to and drivers of successful training were explored. One was the self-affirmation kindness questionnaire. 149 Its positioning and purpose in the session were not understood by most participants: ‘[. . .] just coming into the session the questionnaire seemed inappropriate’ (SO 3, F, FG 1). It was also found by some to be threatening: ‘It felt like a test and a bit off-putting’ (SO 4, F, FG 1).
Some service users reported that another barrier was that there was ‘a lot of information to take in’ (patient 4, M, FG 1), and issues relating to memory difficulties were highlighted. Therefore, participants supported the use of handouts and requested an online copy of the materials that they could access and share with others.
With respect to content, important feedback from service users was that they appreciated that attention was given to the different types of seizures and how to manage them and that the focus was not simply on ‘grand mal seizures’ (SO 5, F, FG 1). However, they suggested that more time be given to exploring triggers and auras and to explain that not everyone has triggers, which in itself is a potential risk. It was also suggested that new sections should be included to discuss the risks associated with post-ictal states and how best to deal with them. Finally, some suggested that ‘dealing with an injury as well as dealing with the seizure can be difficult’ (SO 7, F, FG 2), and, therefore, information and advice on how to deal with common seizure injuries were needed.
Course delivery
The size of the group (up to 20 people) was considered to be appropriate and encouraged discussion. Indeed, peer support and a sense of feeling ‘less isolated’ was highlighted as a key training experience. Many patients had not previously discussed their epilepsy with people other than their immediate family members and/or health-care professionals. They valued the group format and described how they appreciated being able to meet others who were ‘in the same boat’ and ‘realising you are not on your own with it’ (patient 6, M, FG 1). One SO noted:
[R]eally glad I came. I think it’s just being around people who know exactly, exactly how you feel and that’s why this should have been put on a long, long time ago.
SO 1, F, FG 1
In terms of who would be best to facilitate the course, many felt that it should be a health-care professional because they believed that this would make the course ‘credible’ (patient 8, F, FG 2) and promote uptake. Others, however, argued that it could be facilitated by a representative from a user group because what was most important was that the trainer was knowledgeable and empathetic and had the skills to facilitate discussions. Either way, it was argued that standardised training for the facilitators was important.
Changes made by the intervention development panel to create version 1.2
The user feedback led to a refashioning of a number of details in the way that SAFE was to be delivered (as version 1.2; see Table 3). To increase the acceptability to users of the self-affirmation kindness questionnaire, it was agreed that this would not be introduced to participants until ≈ 30 minutes into the session and would follow the ‘icebreaker’ rather than being introduced immediately.
Given that the main aim of SAFE was to help patients and SOs manage uncomplicated seizures, the panel revised SAFE so that information on managing common post-ictal states was included. However, for the same reason, training PWE and SOs in dealing with seizure injuries as requested was deemed to be beyond SAFE’s scope. Therefore, SAFE was modified to simply acknowledge the possibility of injuries and direct participants to external resources on this.
The length of version 1.2 of SAFE was extended from ≈ 3 to ≈ 4 hours. The increased duration meant that more time could be allocated to the interactive elements of SAFE. Finally, a password-protected website (www.seizurefirstaid.org.uk/Intervention/; accessed 15 June 2020) that provided a copy of SAFE’s content was developed in an effort to mitigate potential memory difficulties in the target population. It also provided a means by which participants could share SAFE’s content with others in their social network.
Discussion
The focus of part A was to develop an epilepsy first aid training intervention that met the needs and preferences of both PWE who frequently visit hospital EDs and their SOs. To promote adequate development and piloting156 we worked collaboratively with service users and other key stakeholders. This activity was underpinned by the Medical Research Council’s complex intervention guidance. 156 The process enabled us to access the unique perspectives of service users and health-care professionals. Clear, tangible changes to the ES’s course were made in response to the feedback received; the developed SAFE intervention is substantially different in content and form. By making these changes, the acceptability of SAFE in the target population has been increased. Stakeholder collaboration has maximised SAFE’s potential benefit and positioned it well for sustained use in the NHS, should this ultimately prove warranted. We described the process we followed and detailed the content of the finalised intervention that will be used within the pilot trial. Such an account is rare, with outcome papers frequently being criticised for not providing readers with sufficient information to interpret trial results. 157
Chapter 3 Part B: pilot RCT – methods
Introduction
Part B of this project was an external pilot RCT of SAFE plus TAU versus TAU only. It sought to:
-
estimate the eligibility rate
-
estimate the consent rate
-
estimate the recruitment rate
-
estimate the retention rate
-
determine the acceptability of randomisation to participants
-
determine the speed of recruitment
-
estimate completion rates of study assessment tools
-
estimate rates of unblinding
-
estimate the annual rate of ED visits in the TAU arm and the likely dispersion parameter
-
generate summary statistics to measure the effect of SAFE on the proposed primary and secondary outcome measures for a future definitive trial and the precision of such estimates at the post-treatment time points
-
determine the feasibility of measuring the primary outcome measure (ED use) by means of routine data.
Design
The trial was as a multicentre, parallel-arm pilot RCT. Participants were PWE with or without a SO. PWE (and their SO if participating with one) were randomised at an intervention-to-control ratio of 1 : 1 and followed up for 12 months. The intervention arm received TAU and was offered the SAFE course and the control arm received TAU. To maximise recruitment, the TAU arm was offered the intervention once all scheduled 12-month follow-up assessments had been completed. The study also contained an evaluation of the fidelity with which SAFE was delivered (see Chapter 5) and an economic evaluation of the cost of delivering it (see Chapter 7). The trial’s design and the intended flow of participants are shown in Figure 2.
FIGURE 2.
Schematic of planned design for part B of the project: trial approval and monitoring.

The trial protocol received the favourable opinion of the National Research Ethics Service Committee North West — Liverpool East (reference: 15/NW/0225) and Health Research Authority (reference: 166241). Its sponsor was the University of Liverpool (reference: UoL001108). The trial was registered on an open access system (Current Controlled Trials ISRCTN13871327).
Trial progress and conduct was monitored by an independent Trial Steering Committee composed in line with National Institute for Health Research guidelines. In line with these oversight guidelines, a Data Monitoring and Ethics Committee was not required.
Study setting and population
Centres
Participants were retrospectively identified from the EDs of three NHS hospitals in Merseyside – namely, Aintree University Hospital, Arrowe Park Hospital and the Royal Liverpool University Hospital. Together, these EDs serve a local population of ≈ 827,000 people among whom the prevalence of adult epilepsy is 0.98%. 26 At each site, an ED consultant acted as a local principal investigator.
The EDs were selected as research sites because they would probably be similar to those sites that would be most appropriate for a definitive trial. Specifically, the EDs serve a population characterised by high levels of social deprivation,158,159 a level of epilepsy control that has been documented to be worse than the national average and rates of emergency admissions for epilepsy that are among the highest in England (Liverpool is ranked as the ninth highest and Wirral is ranked as the twelfth highest). 41 Epilepsy control is defined here – on the basis of the Quality and Outcomes Framework 2012–13160 domains – as the percentage of PWE prescribed one or more antiepileptic drugs in the local population who have been seizure free in the previous 12 months. When the trial was being designed, 70.9% of PWE from the Clinical Commissioning Group areas served by the hospitals were seizure free. The national average at the time was 75.4%. 41
Participant inclusion criteria
Patients with the following characteristics were eligible:
-
established diagnosis of epilepsy (for ≥ 1 year)
-
any epilepsy syndrome and any type of focal or generalised seizures
-
currently being prescribed one or more antiepileptic drugs
-
aged ≥ 16 years (no upper age limit)
-
visited an ED for epilepsy on two or more occasions in the previous 12 months (as reported by patient)
-
living within 25 miles of any of the three ED recruitment sites
-
able to provide informed consent, participate in SAFE and independently complete questionnaires in English.
Significant others with the following characteristics were eligible:
-
a SO to the patient (e.g. family member, friend) whom the patient identifies as providing informal support
-
aged ≥ 16 years (no upper age limit)
-
living in the north-west area of England
-
able to provide informed consent, participate in SAFE and independently complete questionnaires in English.
Participant exclusion criteria
Patients with the following characteristics were excluded:
-
actual or suspected psychogenic non-epileptic seizures alone or in combination with epilepsy
-
acute symptomatic seizures related to acute neurological illness or substance misuse (e.g. alcohol or drug induced)
-
severe current psychiatric disorders (e.g. acute psychosis) or life-threatening medical illness
-
enrolment in one or more other epilepsy-related non-pharmacological treatment studies.
Significant others with the following characteristics were excluded:
-
severe current psychiatric disorders (e.g. acute psychosis) or life-threatening medical illness
-
enrolment in one or more other epilepsy-related non-pharmacological treatment studies.
Screening
Stage 1
There is no central, ‘live’ system that can be accessed and searched to identify PWE who have made visits to NHS EDs in England. Well-documented challenges to information sharing between NHS services also mean that specialist services and GPs are not necessarily aware of ED attendances made by PWE for whom they care. 20,21 However, hospitals in the UK do maintain local electronic records of attendances at their EDs. For each attendance, a local record contains the patient’s contact details and a number of searchable fields, including date of attendance, patient age, home postcode, presenting complaint and, if provided, a discharge diagnosis. These attendance systems of the EDs were used to identify PWE who had attended the EDs in the last 12 months for invitation to the trial.
Searches of the local systems for PWE were completed by the business intelligence units of the hospitals. We had envisaged that an independent expert panel of neurologists and ED clinicians would determine the search criteria to be used (e.g. presentation and diagnosis codes). During study set-up it became apparent that the architecture of the systems and the coding processes at each of the sites were sufficiently different that local knowledge of the system was required to have confidence that the criteria used would allow for a sufficiently sensitive and specific search to occur. Therefore, we instead worked with the local principal investigators at each site to identify the optimal search strategy. The search terms used are detailed in Appendix 6.
Stage 2
The ‘triage cards’ of the patients captured by the searches during stage 1 were reviewed by the local principal investigator and their team to identify persons who were and were not eligible to invite. This level of detailed screening was necessary because (1) no symptom presentation is pathognomonic of epilepsy (e.g. a presentation coded as ‘blackout/faint’ or ‘fit/seizure’ could be attributable to syncope, diabetes, head injury or stroke rather than epilepsy) and (2) the discharge diagnosis field was often empty. In 2016/17, ≈ 35% of ED attendances in England were not allocated a valid primary diagnosis code. 161
Those patients who, following review of their triage card, were considered ostensibly eligible by the local principal investigator were sent an invitation pack, which included a covering letter from the ED consultant and a patient participant information sheet (see Appendix 7). The letter informed the patient that, unless they opted out of further contact within 3 weeks or sent notification that they were ineligible, they would be telephoned by the research team with more information about the study. Patients could opt out by e-mail, telephone or by returning a Freepost slip. Those opting out were encouraged to detail any reasons. Those interested in taking part were asked to await contact by the research team and consider whether or not they would like to take part with a SO.
Stage 3
Interested patients were telephoned to answer questions the patient had, confirm whether or not the patient wanted to participate and verify their eligibility (including that they had made two or more ED visits for epilepsy in the last 12 months). If a person was confirmed as eligible and wanted to participate, consent in principle was taken over the telephone and the research worker arranged a baseline/enrolment appointment at which informed signed consent was to be obtained from them and from their SO if they were taking part with one.
Multiple telephone calls were attempted with patients who had not opted out. Attempts to call participants were made on different days of the week and at different times. A total of three calls per unresponsive patient were attempted, typically one in the morning, one in the afternoon and one in the evening. It was important to do this to minimise the influence of work status on ability to consider participation in the study. If a person could not be contacted (e.g. a correct number was unavailable or they did not answer), they were posted a letter asking them to contact the team if they were interested in participating. The sending of a letter was not in the original protocol; this was a substantial amendment and, with funder and governance approval, incorporated into protocol 1.2 (9 June 2016).
Randomisation
Computer-generated randomisation was conducted remotely by the Liverpool Clinical Trials Centre [Clinical Trials Unit (CTU) registration number 12]. Following consent and completion of the baseline measures, the research worker entered the participant’s information into an online system and sent a request for randomisation to the CTU. This ensured that randomisation was performed independently of the trial’s research and statistical teams.
Patient participants were randomised to either the SAFE plus TAU or TAU only arm. If they were taking part with an SO, the patient and SO were randomised as a dyad. The unit of randomisation was the individual patient. Patients were randomised to intervention or control at a ratio of 1 : 1 and randomisation used a web-based minimisation program with a built-in random element utilising stratification factors that were not made known to the researcher (DS) involved in data collection to minimise any potential for predicting allocation.
Like most trials using stratification,162 we limited stratification to two factors. We selected factors based on evidence of their potential importance in influencing ED use and also pragmatic considerations. The factors chosen were recruitment site from which a patient participant was identified and whether or not the patient reported, at baseline, feeling stigmatised by epilepsy.
The reason for stratifying by recruitment site was that people who reside in more socially deprived areas are found to be more likely to use EDs46,47 and because the catchment areas of the three ED recruitment sites were not completely equal in deprivation. The reason for stratifying by felt stigma was because, as reported in Chapter 1, Ridsdale et al. 27,31,32 found felt stigma to be a possible predictor of ED use and that it held a similarly sized relationship with subsequent ED use as mastery/confidence managing epilepsy. 27,128 It was not considered ideal to stratify by mastery/confidence in epilepsy because mastery/confidence in epilepsy is typically measured on a quasi-continuous rather than categorical scale and so stratifying patients would have required transformation of scores prior to randomisation by the research worker (DS), whom we sought to keep blind to stratification factors.
Blinding and protection from bias
This was a single-blind (outcome assessors blinded) pilot trial. All participants were aware of their treatment allocation. The trial statistician (SN), senior trial statistician (CTS) and the research worker (DS) responsible for consent, data collection and the conducting of outcome assessments were not. Participants were asked not to inform the research worker of their treatment allocation and were reminded of this at the start of each data collection point.
Allocation concealment was maintained by e-mail confirmations being automatically generated each time a participant randomisation was requested by the research worker and these being sent to relevant staff with or without details of the treatment allocation included, depending on their role in the study. The research worker submitting the request received only confirmation of successful randomisation, whereas an administrator was notified of persons randomisation to SAFE. The administrator liaised with patients (and their SOs) to arrange attendance at a SAFE course. Participants’ usual care providers were informed of the patient’s involvement in the trial but not of treatment allocation.
To evaluate how the blinding process worked, the research worker completed a ‘research worker treatment guess’ form after the follow-up assessment at 12 months or after withdrawal, and reported the circumstances of any unblinding.
People with epilepsy attending SAFE courses did so outside their routine clinic appointments and so we did not expect transfer of SAFE-related knowledge (and therefore contamination of the TAU arm) between those in the SAFE plus TAU and TAU only arms at a single site.
Intervention delivery
The SAFE intervention has been fully described in Chapter 2. Materials for the course included Microsoft PowerPoint (Microsoft Corporation, Redmond, WA, USA) presentation slides, videos illustrating seizure types, the recovery position and appropriate first aid. Patients took copies of the slides and additional information booklets (such as on risk management and emergency medication) away with them and could access and share a website of the course’s content.
For the purposes of the pilot RCT, a single facilitator (Juliet Bransgrove; see Acknowledgements), recommended by the ES, was trained to deliver SAFE — She is a registered nurse with 30 years’ experience (18 months as an epilepsy nurse) who delivered the ES’s original course. Her training in the delivery of the revised intervention involved her familiarising herself with the trainer manual, delivering two practice courses with participants outside the trial and receiving feedback from the intervention development panel.
The SAFE courses were delivered in the education centre of a local teaching hospital, chosen because it was accessible and limited the distance participants needed to travel (i.e. it was near a major transport hub and centrally located), familiar to the patients and had access to emergency care services, if required.
To facilitate group interaction, chairs were arranged in a semicircle. A flip chart was set up for writing notes and discussion points. Workbooks, pens and name badges were ready for participants on arrival.
An administrator was present at each course to support its running. This included audio-recording the sessions and noting participant attendance. If a participant was present at the start and end of the course, it was considered that they had received the intervention in full. If a participant was unable to attend their scheduled course, the administrator attempted to contact them to identify the reason(s) and offer an alternative course date if appropriate and available.
All data on attendance and the communications between the administrator and participants to arrange attendance were inputted by them into a secure electronic database. These data were not accessible to the researcher responsible for participant recruitment and follow-up.
Data collection tools and follow-up visits
Primary
The proposed primary outcome measure for a definitive trial of SAFE would be subsequent ED use. This would require data on the number of ED visits each participant made over the 12 months following recruitment. In the pilot trial these data were sought from the NHS’s Hospital Episode Statistics (HES) system. This system is theoretically able to identify, among other things, the number of attendances a patient has made at all EDs in England. It is primarily an administrative system, but, with explicit patient consent, governance approvals and payment of a fee, it is possible to apply to NHS Digital for this HES system to be interrogated for research purposes. For the pilot RCT a request was thus submitted for the HES system to be searched for ED attendances within specified time periods by the patient participants (using their unique NHS numbers). Because evidence was available at the time that indicated that for ≈ 30% of ED visits no diagnosis is recorded,161 we requested data on the total number of ED visits made for any reason by individual patient participants during specified periods. Information on the number of visits each person had made over the 12 months prior to randomisation was also sought for the purpose of adjustment within the analyses and to allow comparison of self-reported ED use with routine data.
Secondary
The secondary outcome measures (Table 4) were based on participant self-report and collected using clinical research forms (CRFs). Baseline (T0) and follow-up at 12 months (T3) measures were completed during face-to-face sessions with a research worker (DS). During these appointments (and in line with completion guidelines), patient or SO participants completed their questionnaire by themselves, with the research worker offering assistance only if requested. An abbreviated questionnaire assessment occurred at follow-up at 6 months (T2); participants were posted a set of questionnaires for completion and instructed to return them in a pre-paid envelope.
| Outcome | Participants | Measure and derivation of outcome | Baseline (T0) | 3 months (T1) | 6 months (T2) | 12 months (T3) |
|---|---|---|---|---|---|---|
| ED visits | PWE |
At baseline (T0): At 6 months (T2): At 12 months (T3): |
✓ | – | ✓ | ✓ |
| Fear of seizures | PWE; SOs |
Epilepsy Knowledge and Management Questionnaire – fears subscale (five items)163 Total fear score was calculated as the sum of the scores on the scale’s five items, ranging from 5 to 30. Higher scores correspond to greater levels of fear |
✓ | – | – | ✓ |
| Knowledge of what to do when faced with a seizure | PWE; SOs |
Items from Thinking About Epilepsy questionnaire (13 questions)164 Knowledge score was calculated as the number of questions answered correctly (out of a total of 13). Only correctly answered questions were recorded. No down-weighting was applied for incorrectly answered questions or questions not answered |
✓ | – | – | ✓ |
| Confidence managing seizures/epilepsy | PWE; SOs |
PWE: Wagner Mastery Scale (six items)61 Total score was calculated as the sum of the scores on the scale’s six items (with reverse weighting for two of the items), ranging from 6 to 24. Higher scores correspond to higher levels of mastery SO: Parental Response to Child Illness Scale – condition management subscale (six items)165 Total score was calculated as the average of the scores, ranging from 1 to 5. A higher score corresponds to a higher level of confidence |
✓ | – | ✓ | ✓ |
| Quality of life | PWE |
QOLIE-31-P (31 items)166 Total QOLIE-31-P score was calculated, ranging from 0 to 100. Higher scores correspond to a better quality of life |
✓ | – | ✓ | ✓ |
| Distress | PWE; SOs |
Total anxiety score and total depression scores were calculated, ranging from 0 to 21. Higher scores correspond to higher levels of anxiety or depression, respectively. Anxiety and depression categories were defined based on the total anxiety or depression score as: |
✓ | – | – | ✓ |
| Seizure control | PWE |
At baseline (T0): Thapar’s seizure frequency scale169 for the prior 12 months At 6 months (T2) and 12 months (T3): patients were asked for number of seizures (any type) since the last assessment and dates of the first and most recent. (To assist, patients were offered a seizure diary at baseline) |
✓ | – | ✓ | ✓ |
| Felt stigma | PWE; SOs |
Stigma Scale of Epilepsy (9 items)62 Total stigma score calculated as sum scores on three items of scale, ranging from 0 to 9. Higher scores correspond to higher levels of stigma. Stigma categories were defined as: |
✓ | – | – | ✓ |
| Burden | SOs |
Zarit caregiver burden170 Total burden score calculated as sum of scores on the measure’s 22 items, ranging from 0 to 88. Higher scores correspond to a greater burden. Burden categories were defined as: |
✓ | – | ✓ | ✓ |
| Health economics | PWE | Client Service Receipt171 and EQ-5D (13 items)172 | ✓ | – | – | ✓ |
| Feedback on trial participation | PWE; SOs | Adapted from Magpie Trial (three items)173
|
– | – | – | ✓ |
The use of routine outcome data in clinical trials is advocated by the NIHR. However, limited evidence on its utility exists (see, for example, Powell et al. 174). The pilot provided an opportunity to explore the ease and costs of obtaining routine data, the time it took for routine data to be secured, the proportion of participants the data covered and, finally, how routinely collected administrative data on ED use compared with self-reported data.
Adverse events
As part of the trial, patient participants did not receive additional medical reviews. Therefore, the experience of adverse events was monitored by asking them to complete a standardised checklist as part of the CRF at 3 months (T1; by telephone), 6 months (T2; by telephone) and 12 months (T3; during a face-to-face appointment) post randomisation.
Defining the outcomes
Primary
The proposed primary outcome measure for a future definitive trial is the number of epilepsy-related ED visits patient participants made over the 12 months following randomisation.
Secondary
There is currently no core outcome set for epilepsy. 175 A number of measures described in Table 4 were used to assess secondary outcomes considered to be potentially important to capture in a definitive trial.
Participants in the pilot trial were assessed using the secondary outcome measures to permit the sample to be fully described according to them at baseline in order to accurately model potential burden of participation in a definitive trial and to allow us to describe completeness of data on these measures. A full description on these measures is provided in Appendix 8.
Adverse events
What constituted a serious adverse event (SAE) and how judgements regarding their relatedness to participation were made are described in Appendix 9.
Sample size
Because this was a pilot RCT, a formal power calculation to permit it to be able to detect a clinically meaningful difference in the primary outcome between SAFE plus TAU and TAU arms was not appropriate. Rather, the aim was to provide robust estimates of the likely rates of recruitment, consent and follow-up and to yield estimates of the ED event rate and dispersion parameter to accurately inform power calculations for a future definitive trial. To be able to do this, it was considered that 40 patients in each treatment arm would provide these estimates with adequate precision.
We estimated that ≥ 20% of those who have visited an ED on two or more occasions in the prior 12 months would agree to participate. It was anticipated that ≈ 400 eligible PWE would have visited the three ED recruitment sites over the 12 months preceding our recruitment phase. This was informed by NHS Digital data on the number of attendances at the sites in 2012/13176 and audit data that show that ≈ 1% of all ED visits are for epilepsy and ≈ 80% are by those with an established diagnosis. 23,177 We also factored in evidence on the proportion of ED visits within 1 year that had been made by the same individual20 and estimated, using data from Ridsdale et al. ,178 the number of PWE that would satisfy the inclusion/exclusion criteria.
With a sample size of 80, a participation rate of 20% could be estimated to within a 95% confidence interval (CI) of ± 4% and a drop-out rate of 25% to within a 95% CI of ± 10%. Assuming that data on the proposed primary outcome measure of ED visits at 12 months were not available for 25% of patients, outcome data from 60 patients would still allow robust estimation of the ED rate and dispersion parameter. We considered that one full-time researcher would be able to recruit the required sample within 8 months.
Completion of follow-ups
A number of evidence-based strategies were used to maximise retention of participants in the trial. 179 This included patient and SO participants receiving a £10 shopping voucher after each follow-up assessment that they completed and delayed access to SAFE for the TAU arm.
Unless a participant formally withdrew consent to participate in the trial, HES data on them were requested and up to three attempts were made to contact patients or SO participants each time a follow-up assessment was due. The research worker also contacted participants by telephone approximately 2 weeks after the 6-month questionnaires were posted.
When a patient participant did not complete a follow-up assessment, an attempt to monitor SAEs was made by sending their GP a letter asking them to inform us if the patient was no longer alive and the circumstances of death.
Data management
With the exception of the routine outcome data on ED use from NHS Digital, all data were collected on paper-based CRFs by the research worker. The CRFs were kept in locked filing cabinets in a central research office with restricted access at the University of Liverpool. The paper CRFs were sent to the CTU for entry into MACRO 4.0 (Elsevier, Amsterdam, the Netherlands) by a data manager. At the point of data entry, queries were raised. Participant contact information was kept on a secure central network server with access granted to study staff only.
Data analysis
All statistical analyses were performed with SAS® statistical software (version 9.4) (SAS Institute Inc., Cary, NC, USA) (by SN; overseen by CTS). A full statistical analysis plan was developed and approved prior to conducting the final analysis.
Rates of eligibility, consent, recruitment, retention and unblinding
Derivation and statistical analysis
The eligibility rate is defined as the percentage of patients screened that satisfy eligibility criteria (see Chapter 3). The consent rate is defined as the percentage of eligible patients who provided informed, written consent for randomisation. The recruitment rate is defined as the number of participants recruited per calendar month.
Eligibility and consent rates are presented as percentages with 95% CIs. Recruitment rates are presented as the actual number of participants recruited per calendar month presented alongside the expected number of participants recruited per calendar month on recruitment graphs.
The age, sex and social deprivation profiles (available from ED records) of eligible individuals who did and did not consent to take part in the trial are presented side by side in table columns for visual comparison to evaluate representativeness of the trial sample.
Retention rate is defined as the percentage of randomised patient and SO participants completing 3, 6 and 12 months of the study without withdrawing (i.e. without formally withdrawing from any further data collection). Reasons for withdrawal, where known, are presented.
The completion rate of study assessment tools (i.e. the percentage of patient and SO participants completing each measure outlined in Table 4) is presented as an indicator of retention and participation in the trial.
The unblinding rate is defined as the proportion of correct treatment allocation guesses made by the research worker at the end of the trial (12 months) or at withdrawal of the participant from the trial. The unblinding rate is presented with 95% CIs. A Cohen’s kappa statistic for agreement (i.e. the correct guess of allocation) and 95% CIs are also presented.
Demographic and baseline characteristics
Baseline characteristics are presented for PWE overall and by treatment arm with comparisons presented for a subset of characteristics for those individuals identified as eligible and invited to participate in the trial who did not agree to participate in the trial.
Relevant baseline characteristics are presented descriptively for SO participants.
Categorical data are summarised by numbers and percentages. Continuous data are summarised by mean, median, standard deviation (SD) and range (minimum and maximum values). Tests of statistical significance are not undertaken for baseline characteristics; rather, the potential clinical importance of any imbalance is noted.
Primary outcome: epilepsy-related emergency department visits
Derivation of outcome
The proposed primary outcome of a future trial is defined as the number of epilepsy-related ED visits made by patient participants over the 12 months following randomisation measured by HES data. As a secondary outcome, the number of self-reported epilepsy-related ED visits made by patient participants over the 12 months following randomisation is also presented.
Statistical analysis
Epilepsy-related ED visits are presented at baseline (T0) and at 12 months (T3), and the number of ED visits made by the end of the 12-month period compared with the number of ED visits made in the 12 months prior to baseline is also presented. Results are presented for all patient participants and by treatment arm as mean and SDs, in addition to the median, the minimum and the maximum number of epilepsy-related ED visits. Completeness of data for self-reported epilepsy-related ED visits is presented and no imputation of missing data was performed.
The difference between the SAFE plus TAU arm and TAU only arm is compared at 12 months with and without adjustment for baseline ED visits via negative binomial regression (NBR) models for count data. Overdispersion (i.e. variance larger than the mean) and an excess number of zeros for individuals who did not visit an ED in the last 12 months was anticipated; therefore, zero-inflated NBR models were also applied. The preferred model (negative binomial or zero-inflated negative binomial) was determined by Vuong’s test. 180
Between-arm differences are presented as rate ratios with 95% CIs and statistically tested according to a 5% level of significance. In addition, as per guidance for pilot trials,181 90% and 80% CIs are also presented to aid interpretation of between-arm differences.
Epilepsy-related ED visits, measured by HES data, were compared with self-reported epilepsy-related ED visits and Bland–Altman plots and limits of agreement statistics182 were calculated to determine the agreement of the two measurement methods. A Bland–Altman plot shows the mean number of ED visits in self-reported and HES data for each patient participant (x-axis) and the difference in the number of ED visits in self-reported and HES data for each patient participant (y-axis). Bland–Altman limits of agreement (mean ± 2 SDs) of the difference in the number of ED visits are also shown on the plot. These limits can be interpreted as the level of agreement that is ‘acceptable’ between the two measurements of recording ED visits.
Estimation of sample size of a future definitive trial
The average annual rate of ED visits in the SAFE plus TAU and TAU only arms and the likely dispersion parameter estimated from the preferred NBR model are used to estimate the sample size of a future definitive trial according to the methods outlined by Keene et al. 183 for a negative binomial regression. The number of patient participants required per arm in a definitive trial to detect the size of the effect shown in the pilot study is:
where Z1 – α/2 and Z1 – β are critical values of the normal distribution for specific values of α and β (typically, α = 0.05 for a 5% significance level and β = 0.2 or 0.1 for 80% or 90% power, respectively), µ1 and µ2 are the estimated ED rates from the two treatment arms and k is the negative binomial shape parameter from the associated gamma distribution, which explicitly represents variability between subjects.
Secondary outcome measures
Derivation of outcomes
Details of the secondary outcome measures are described in Table 4.
Total scores for Quality of Life in Epilepsy Scale-31 item-Patient-weighted (QOLIE-31-P) (PWE participants only), burden (SO participants only), confidence in managing seizures/epilepsy (SO participants only), mastery (PWE participants only) and fear of seizures (PWE and SO participants) are presented at baseline (T0), 6 months (T2) and 12 months (T3). The changes in total scores at 6 months (T2) and 12 months (T3) from baseline (T0) are presented. Burden categories (‘little or no burden’, ‘mild to moderate burden’, ‘moderate to severe burden’ and ‘severe burden’) are presented.
Total scores for stigma (PWE participants only) and distress (anxiety and depression scores; PWE and SO participants) are presented at baseline (T0) and at 12 months (T3). The changes in total scores at 12 months (T3) from baseline (T0) are also presented and stigma categories (‘no stigma’, ‘mildly to moderately stigmatised’ and ‘highly stigmatised’) and anxiety and depression categories (‘normal range’, ‘suggestive of anxiety/depression’ and ‘probable anxiety/depression’) are presented.
The ‘knowledge of what to do where faced with a seizure’ score is presented as the number of questions answered correctly (out of 13) at baseline (T0) and at 12 months (T3), in addition to the change in knowledge score at 12 months (T3) from baseline (T0).
Self-reported seizure frequency is presented at baseline (T0), at 6 months (T2) and at 12 months (T3) according to Thapar’s seizure frequency scale. 169 The total numbers of seizures between baseline (T0) and 6 months (T2) and between baseline (T0) and 12 months (T3) are also presented.
Statistical analysis
Total scores and the change from baseline scores of all measures, plus the total number of seizures, are presented as mean and SD in addition to the median, the minimum and the maximum scores for each scale. Burden, stigma, anxiety and depression categories are also presented as the number and percentage of participants in each category. Results are presented for all participants and by treatment arm and separately for PWE and SO participants where applicable.
Completeness of data for all self-reported scales is presented. Individual missing values in given scales of the QOLIE-31-P184 and the Hospital Anxiety and Depression Scale (HADS) scale were imputed according to recommended rules. 185 No imputation was performed for other scales. Total scores are presented separately for all individuals (including those with missing data) and for individuals with complete data for the scale.
Visual comparison of differences between treatment arms only are made for secondary outcome measures; no formal statistical testing is performed.
Chapter 4 Part B: pilot RCT – recruitment, retention, intervention delivery and participant baseline characteristics
Introduction
This chapter presents findings from the pilot trial relating to participant recruitment, retention and attendance at SAFE intervention sessions.
Participant flow through study
Search period
The stage 1 searches were originally set up to identify PWE who had made an ED visit between 1 January 2015 and 31 December 2015. However, recruitment proved more challenging than anticipated and, with funder and governance approval, the period in which people could be identified as having attended the EDs was extended by 7 months to 31 July 2016 (minor amendment, incorporated into protocol version 1.2 on 9 July 2016). A total of 417,381 attendances for any reason were recorded at the EDs between 1 January 2015 and 31 July 2016.
Eligibility
The stage 1 searches indicated that 6359 (1.5%) of the ED attendances were attributable to epilepsy or a suspected epileptic seizure. These visits had been made by 4016 individuals. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram is presented (Figure 3). Following stage 2 review of the triage card associated with these visits, 1220 individuals were considered to have visited for established epilepsy, with 555 of them being considered eligible for participation in the trial and sent an invitation.
FIGURE 3.
The CONSORT flow diagram of eligibility screening for the trial.
Having received the letter, no patient opted out, but 122 patients did send notification that they were ineligible (Tables 5–7). For nine patients the postal address was incorrect or the letter was not sent, in error. The remaining 424 patients were telephoned to determine their interest in the study and verify eligibility (stage 3). Based on these figures, the eligibility rate (424 eligible participants out of 4016 unique individuals screened) was 10.6% (95% CI 9.6% to 11.5%).
| Reason | Number of participants | |||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Total | |
| No confirmed diagnosis of epilepsy/not attending because of epilepsy | 737 | 483 | 715 | 1935 |
| Acute symptomatic seizures related to neurological illness or substance abuse | 238 | 240 | 191 | 669 |
| Psychogenic non-epileptic seizures | 19 | 55 | 29 | 103 |
| Medical record missing | 54 | 5 | 27 | 86 |
| Ineligible, no reason givena | 2 | 1 | 0 | 3 |
| Total | 1050 | 784 | 962 | 2796 |
| Reason | Number of participants | |||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Total | |
| Postcode outside catchment area | 148 | 94 | 60 | 302 |
| Learning disability likely to impede individuals’ capacity to provided signed, informed consent | 45 | 51 | 52 | 148 |
| Inability to converse in English and provide signed informed consent | 10 | 24 | 46 | 80 |
| Life-threatening medical illness | 35 | 26 | 10 | 71 |
| Severe psychiatric disorder | 11 | 3 | 18 | 32 |
| No fixed abode | 10 | 6 | 13 | 29 |
| Participating in another trial | 3 | 0 | 0 | 3 |
| Total | 262 | 204 | 199 | 665 |
| Reason | Number of participants | |||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Total | |
| Has not visited an ED for epilepsy on two or more occasions in previous 12 months (self-reported) | 9 | 15 | 24 | 48 |
| Not able to provide informed consent, participate in SAFE course if randomised or to independently complete questionnaires in English | 9 | 7 | 7 | 23 |
| Ineligible, no reason givena | 4 | 7 | 4 | 15 |
| No established diagnosis of epilepsy (< 1 year) | 3 | 2 | 5 | 10 |
| Moved out of area; postcode no longer within 25-mile catchment area | 4 | 1 | 3 | 8 |
| Actual or suspected psychogenic non-epileptic seizures alone or in combination with epilepsy | 2 | 1 | 4 | 7 |
| Severe current psychiatric disorders or life-threatening medical illness | 1 | 5 | 1 | 7 |
| Not currently being prescribed antiepileptic drug | 1 | 0 | 1 | 2 |
| Acute symptomatic seizures related to acute neurological illness or substance misuse | 0 | 1 | 0 | 1 |
| Participating in another trial | 1 | 0 | 0 | 1 |
| Total | 34 | 39 | 49 | 122 |
It took the local principal investigator at each site ≈ 3 full days to complete the stage 2 screening.
Recruitment rates and speed of recruitment
The process of enrolment (i.e. participant consent and baseline assessment, as opposed to the period within which the participants’ ED visits must have occurred) began in May 2016 and concluded at the end of December 2016. Among the 424 patients telephoned, 53 agreed to participate and provided informed consent, thus giving a consent rate of 12.5% (95% CI 9.3% to 15.6%). The reasons participants offered for not taking part are provided in Table 8.
| Reason | Number of participants | |||
|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Total | |
| Not interested | 30 | 11 | 23 | 64 |
| Too busy | 11 | 12 | 11 | 34 |
| No reason given | 9 | 10 | 8 | 27 |
| Too ill | 5 | 5 | 9 | 19 |
| Too well | 3 | 1 | 2 | 6 |
| Total | 58 | 39 | 53 | 150 |
Regarding consent, successful telephone contact could be made with only 203 out of the 424 (47.9%) eligible patients. When restricted to those who could be contacted, the consent rate is 26.1% (95% CI 20.0% to 32.2%).
The process of enrolment lasted 226 days (equivalent to 7.43 months), with an average randomisation rate of 6.9 patients per month (ranging from 4 to 11 patients per month). Actual rates and expected rates of recruitment each month are presented in Appendix 10. Because the target sample size of 80 patients was not reached, the speed of recruitment could not be calculated.
Randomisation
Among the 53 consenting patients, 51 were randomised (along with 37 SO participants with whom they took part). The first was randomised on 19 May 2016 and the last on 31 December 2016. Two patient participants were not randomised because they withdrew prior to randomisation.
Among the 51 patient participants randomised, 26 (with 18 SOs) were allocated to SAFE plus TAU and 25 (with 19 SOs) to TAU only. Participant flow through the SAFE trial is outlined in Figure 4.
FIGURE 4.
The CONSORT flow diagram for the SAFE trial. NA, not applicable.

Receipt of intervention
Seven SAFE intervention courses were run for the SAFE plus TAU arm between June 2016 and February 2017. No seizures were recorded as having occurred during the courses and there were no instances of participants in the TAU arm attending a SAFE session by mistake.
We anticipated that SAFE would be delivered to groups of 8–10 people in the pilot trial. In practice, the average group size was 5, with 20 (76.9%) out of the 26 patient participants and 13 (72.2%) out of the 18 SOs randomised to the intervention attending a course. No patients or SOs completed only part of a course.
A total of 33 course bookings were made for the 26 patient participants randomised to SAFE. Most (n = 18, 90%) of the 20 patient participants randomised to SAFE who actually attended a course attended the first course that they were booked on. The minority of patients who did not ultimately attend a course were associated with substantial administrative activity. Specifically, six patient participants who did not attend a course received, between them, 45 telephone calls from the study administrator and booked onto 11 course slots. This contrasts with the 34 telephone calls in total that were made to the 20 patient participants who attended a course. Reasons for non-attendance included poor health on the patients’ behalf and work commitments on behalf of the SO.
Withdrawals and completion of follow-ups
Formal withdrawals from trial
Among the 51 randomised patient participants, only three (5.9%) withdrew consent to participate and for routine data to be collected on them over the course of their 12 months in the trial; two withdrew from the TAU condition and one from the SAFE arm. This meant that consent remained in place for securing routine outcome ED data for 48 (94.1%) patient participants. The reasons for participant withdrawal are described in Appendix 11. No withdrawals were initiated on the patient’s behalf by a health-care provider or a member of the research team. Among the 37 randomised SO participants, five (13.5%) withdrew.
Participation in questionnaire-based follow-up assessments
A total of 37 (72.5%) of the patient participants and 21 (56.8%) SOs attended their scheduled post-randomisation primary outcome assessment at 12 months (T3). The 37 patient participants attended their post-randomisation follow-up visit at 12 months at a median of 286 days (range 252–365 days) post randomisation. Appointments often took place before 12 months because slower recruitment meant that time for follow-up within the life of the project was reduced.
With respect to the interim questionnaire assessments, 43 (84.3%) patients returned the 3-month (T1) follow-up questionnaire and 39 (76.5%) patients and 26 (70.3%) SOs returned their 6-month (T2) follow-up questionnaire.
Securing outcome data and their completeness
Primary outcome: emergency department data from Hospital Episode Statistics system
Completeness of data
Hospital Episode Statistics data on ED use were secured by the research team from NHS Digital for each of the 48 (94.1%) randomised patient participants for whom consent to participate and secure ED outcome data on them remained.
Process of securing the data
After delays in being granted permission to access NHS Digital’s application form, the research team submitted the application for the data on 16 February 2018. To minimise cost, a single application, rather than one relating to the baseline period and one for follow-up, was submitted. The data were ultimately received by the research team on 31 October 2018 at a direct cost of £6960.00 [inclusive of value-added tax (VAT)].
Prior to starting the trial and submitting the application, the research team communicated with NHS Digital and ensured that the participant information sheet and consent form were consistent with its requirements. Despite this, it took 7 months for the application to be reviewed and approved by NHS Digital and an additional 1.5 months for the data file to be produced and transferred.
During these periods, substantial effort was required by the research team to respond to queries raised by NHS Digital and to request progress updates once responses had been submitted. A log of all correspondence was kept by the research team. The team typically responded to queries raised by NHS Digital within 1 day; key milestones are outlined in Appendix 12. In one instance, the research team needed to successfully appeal – 5 months into the application process – against a decision by NHS Digital to reject it because of, among other things, apprehensions regarding the project’s scientific merit. The team was notified it was being rejected because of ‘concerns [over . . .] whether this pilot would yield findings that were statistically valuable to achieve the stated aims given the small numbers involved’.
Secondary outcome data
The extent to which the secondary outcome measures were fully completed by the patient participants who participated in the follow-up assessments varied by assessment point, by measure and by participant type (Tables 9 and 10). Self-reported ED use at 12 months post randomisation (T3) by patient participants was the secondary means by which ED use was captured and so, arguably, the most important of the secondary outcome measures. Self-reported ED use data at 12 months (T3) were available for 34 (66.7%) out of the 51 randomised patient participants.
| Study assessment tool | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | (N = 26) | (N = 25) | (N = 51) |
| ED self-report | 24 (92.3) | 21 (84.0) | 45 (88.2) |
| Thapar’s seizure frequency scale:169 seizure control | 26 (100.0) | 25 (100.0) | 51 (100.0) |
| QOLIE-31-P184 | 23 (88.5) | 16 (64.0) | 39 (76.5) |
| aHADS167,168 | 23 (88.5) | 24 (96.0) | 47 (92.2) |
| Stigma Scale of Epilepsy62 | 24 (92.3) | 24 (96.0) | 48 (94.1) |
| bClient Service Receipt Inventory171 | 19 (73.1) | 17 (68.0) | 36 (70.6) |
| EQ-5D172 | 21 (80.8) | 25 (100.0) | 46 (90.2) |
| Wagner 6-item Mastery Scale61 | 26 (100.0) | 21 (84.0) | 47 (92.2) |
| Epilepsy Knowledge and Management questionnaire:163 fear of seizures subscale | 16 (61.5) | 9 (36.0) | 25 (49.2) |
| 6 months (T2) | (N = 26) | (N = 23)c | (N = 49)c |
| QOLIE-31-P184 | 16 (61.5) | 12 (52.2) | 28 (57.1) |
| Wagner 6-item Mastery Scale61 | 21 (80.8) | 15 (65.2) | 36 (73.5) |
| Thapar’s seizure frequency scale:169 seizure control | 16 (61.5) | 15 (65.2) | 28 (57.1) |
| 12 months (T3) | (N = 25)d | (N = 22)d | (N = 47)d |
| ED self-report | 17 (68.0) | 17 (77.3) | 34 (72.3) |
| Thapar’s seizure frequency scale:169 seizure control | 20 (80.0) | 14 (60.9) | 34 (72.3) |
| QOLIE-31-P184 | 10 (40.0) | 8 (36.4) | 18 (38.3) |
| aHADS167,168 | 18 (72.0) | 17 (77.3) | 35 (74.4) |
| Stigma Scale of Epilepsy62 | 18 (72.0) | 17 (77.3) | 35 (74.4) |
| bClient Service Receipt Inventory171 | 13 (52.0) | 10 (45.5) | 23 (48.9) |
| EQ-5D172 | 18 (72.0) | 17 (77.3) | 35 (74.4) |
| Wagner 6-item Mastery Scale61 | 18 (72.0) | 16 (72.7) | 34 (72.3) |
| Epilepsy Knowledge and Management questionnaire:163 fear of seizures subscale | 8 (32.0) | 5 (22.7) | 13 (27.7) |
| Feedback on participation | 17 (68.0) | 15 (68.2) | 32 (68.1) |
| Study assessment tool | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | (N = 18) | (N = 19) | (N = 37) |
| Zarit caregiver burden170 | 17 (94.4) | 17 (89.5) | 34 (91.9) |
| aHADS167,168 | 17 (94.4) | 18 (94.7) | 35 (94.6) |
| Parent Response to Child Illness scale186 | 18 (100.0) | 19 (100.0) | 37 (100.0) |
| Epilepsy Knowledge and Management questionnaire:163 fear of seizures subscale | 6 (33.3) | 4 (21.1) | 10 (27.0) |
| 6 months (T2) | (N = 17)b | (N = 16)b | (N = 33)b |
| Zarit caregiver burden170 | 15 (88.2) | 8 (50.0) | 23 (69.7) |
| Parent Response to Child Illness scale186 | 16 (94.1) | 9 (56.3) | 25 (75.8) |
| 12 months (T3) | (N = 17)c | (N = 15)c | (N = 32)c |
| Zarit caregiver burden170 | 11 (64.7) | 10 (66.7) | 21 (65.6) |
| aHADS167,168 | 11 (64.7) | 10 (66.7) | 21 (65.6) |
| Parent Response to Child Illness scale186 | 11 (64.7) | 10 (66.7) | 21 (65.6) |
| Epilepsy Knowledge and Management questionnaire:163 fear of seizures subscale | 6 (35.3) | 2 (13.3) | 8 (25.0) |
| Feedback on participation | 11 (64.7) | 9 (60.0) | 20 (62.5) |
Baseline characteristics
Patient participants
Demographics
The mean age of the 51 randomised patient participants was 39.9 years (SD 15.6 years, range 16–71 years); 29 (56.9%) were female and 94.1% identified themselves as being of ‘white’ ethnicity (Table 11). The Index of Multiple Deprivation (IMD) (URL: www.gov.uk/government/statistics/english-indices-of-deprivation-2015; accessed 27 August 2019), measuring levels of deprivation according to participants’ home postcode, indicated that most (74.4%) patient participants lived in areas of high deprivation; 49% (n = 25) lived in areas in the top 10% most socially deprived in the country. Most (53%) patient participants had attained a basic level of formal education only [i.e. Ordinary levels (O levels)/General Certificate of Secondary Educations (GCSEs)/Level 1 or 2 National Vocational Qualification (NVQ)].
| Demographic characteristic | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Sex, n (%) | (N = 26) | (N = 25) | (N = 51) |
| Male | 10 (38.5) | 12 (48.0) | 22 (43.1) |
| Female | 16 (61.5) | 13 (52.0) | 29 (56.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Age (years) at presentation to the EDa | (N = 26) | (N = 25) | (N = 51) |
| Mean, years | 39.2 | 40.7 | 39.9 |
| SD, years | 13.96 | 17.52 | 15.66 |
| Minimum, years | 18.9 | 16.5 | 16.4 |
| Median, years | 37.1 | 41.4 | 38.8 |
| Maximum, years | 69.9 | 71.3 | 71.3 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| IMDb | (N = 26) | (N = 25) | (N = 51) |
| Decile 1 | |||
| n (%) | 11 (42.3) | 14 (56.0) | 25 (49.0) |
| Minimum rank | 44 | 48 | 44 |
| Median rank | 574.0 | 1231.5 | 673.0 |
| Maximum rank | 3202 | 2166 | 3202 |
| Deciles 2 and 3 | |||
| n (%) | 7 (26.9) | 6 (24.0) | 13 (25.4) |
| Minimum rank | 4649 | 3989 | 3989 |
| Median rank | 6785 | 6665 | 6785 |
| Maximum rank | 8281 | 7816 | 8281 |
| Deciles 4–6 | |||
| n (%) | 2 (7.7) | 4 (16.0) | 6 (11.8) |
| Minimum rank | 9881 | 11,480 | 9881 |
| Median rank | 12,836 | 11,924 | 11,924 |
| Maximum rank | 15,791 | 16,004 | 16,004 |
| Deciles 7–10 | |||
| n (%) | 5 (19.2) | 1 (4.0) | 6 (11.8) |
| Minimum rank | 24,971 | 32,724 | 24,971 |
| Median rank | 27,642 | 32,724 | 28,876 |
| Maximum rank | 31,002 | 32,724 | 32,724 |
| Decile missingc | |||
| n (%) | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| Ethnicity, n (%) | (N = 26) | (N = 25) | (N = 51) |
| White | 25 (96.2) | 23 (92.0) | 48 (94.1) |
| Asian/Asian British | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Black/African/Caribbean/black British | 1 (3.8) | 1 (4.0) | 2 (3.9) |
| Mixed/multiple | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 1 (4.0) | 1 (2.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other significant medical history, n (%) | (N = 26) | (N = 25) | (N = 51) |
| No, none | 13 (50.0) | 10 (40.0) | 23 (45.1) |
| Yes, another medical condition(s) | 10 (38.5) | 13 (52.0) | 23 (45.1) |
| Yes, a psychiatric condition | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| Yes, both medical and psychiatric conditions | 2 (7.7) | 2 (8.0) | 4 (7.8) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Education, n (%) | (N = 26) | (N = 25) | (N = 51) |
| O levels/GCSEs/Level 1 or 2 NVQ | 13 (50.0) | 14 (56.0) | 27 (53.0) |
| A levels/Level 3 NVQ | 5 (19.2) | 3 (12.0) | 8 (15.7) |
| University degree/graduate certificate or diploma | 5 (19.2) | 5 (20.0) | 10 (19.6) |
| Postgraduate university degree (e.g. PGCE, MSc, MA, PhD) | 2 (7.7) | 0 (0.0) | 2 (3.9) |
| Missing | 1 (3.9) | 3 (12.0) | 4 (7.8) |
Epilepsy characteristics
Patient participants had been diagnosed with epilepsy for a median of 21 years. Most participants (62.8%) reported having had ≥ 10 seizures in the previous year. Most participants (54.9%) reported having another health condition. The median time since their last seizure was 14 days. Most participants (n = 38; 74.5%) reported having seen a neurologist in the 12 months prior to their assessment (Table 12).
| Disease characteristic | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Time (years) since epilepsy diagnosis | |||
| n (%) | 26 (100.0) | 25 (100.0) | 51 (100.0) |
| Mean, years | 19.9 | 22.6 | 21.2 |
| SD, years | 14.85 | 18.38 | 16.57 |
| Minimum, years | 1.8 | 1.7 | 1.7 |
| Median, years | 16.8 | 19.3 | 17.3 |
| Maximum, years | 53.9 | 64.9 | 64.9 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Time (days) since last epileptic seizure | |||
| n (%) | 23 (88.5) | 22 (88.0) | 45 (88.2) |
| Mean, days | 53.6 | 40.1 | 47.0 |
| SD, days | 101.10 | 61.21 | 83.34 |
| Minimum, days | 1 | 0 | 0 |
| Median, days | 14.0 | 10.5 | 14.0 |
| Maximum, days | 340 | 235 | 340 |
| Missing, n (%) | 3 (11.5) | 3 (12.0) | 6 (11.8) |
| Number of epileptic seizures in the last 12 months | |||
| n (%) | 26 (100.0) | 25 (100.0) | 51 (100.0) |
| 0 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 1 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 2 | 0 (0.0) | 2 (8.0) | 2 (3.9) |
| 3 | 2 (7.7) | 1 (4.0) | 3 (5.9) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 2 (8.0) | 2 (3.9) |
| 6 | 1 (3.8) | 3 (12.0) | 4 (7.8) |
| 7 | 1 (3.8) | 1 (4.0) | 2 (3.9) |
| 8 | 2 (7.7) | 2 (8.0) | 4 (7.8) |
| 9 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ≥ 10 | 18 (69.2) | 14 (56.0) | 32 (62.8) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| HES health-care use in the last 12 months | |||
| ED attendance | |||
| n (%) | 25 (96.2) | 23 (92.0) | 48 (94.1) |
| At least one attendance, n (%)a | 23 (88.5) | 18 (72.0) | 41 (80.4) |
| Mean | 2.1 | 3 | 2.5 |
| SD | 2.22 | 2.76 | 2.51 |
| Minimum | 0 | 1 | 0 |
| Median | 1 | 2 | 2 |
| Maximum | 10 | 12 | 12 |
| Missing, n (%) | 1 (3.8) | 2 (8.0) | 3 (5.9) |
| Self-reported health-care use in the last 12 months for epilepsy | |||
| ED attendance | |||
| n (%) | 24 (92.2) | 21 (84.0) | 45 (88.2) |
| At least one attendance, n (%)a | 23 (88.5) | 18 (72.0) | 41 (80.4) |
| Mean | 4.3 | 9.8 | 6.7 |
| SD | 2.83 | 24.38 | 16.27 |
| Minimum | 1 | 1 | 1 |
| Median | 4 | 4 | 4 |
| Maximum | 12 | 107 | 107 |
| Missing, n (%) | 2 (7.8) | 4 (16.0) | 6 (11.8) |
| Neurology outpatient appointment | |||
| n (%) | 25 (96.2) | 22 (88.0) | 47 (92.1) |
| At least one attendance, n (%)a | 21 (80.8) | 16 (68.0) | 38 (74.5) |
| Mean | 2.6 | 3.1 | 2.8 |
| SD | 1.78 | 2.26 | 2.00 |
| Minimum | 1 | 1 | 1 |
| Median | 2 | 2 | 2 |
| Maximum | 8 | 10 | 10 |
| Missing, n (%) | 1 (3.8) | 3 (12.0) | 4 (7.8) |
| Emergency ambulance called (whether or not the ambulance took the patient to the ED) | |||
| n (%) | 21 (80.8) | 21 (84.0) | 42 (82.4) |
| At least one attendance, n (%)a | 18 (69.2) | 18 (72.0) | 36 (70.6) |
| Mean | 3.2 | 4.1 | 3.6 |
| SD | 2.87 | 2.86 | 2.86 |
| Minimum | 1 | 1 | 1 |
| Median | 2 | 3 | 3 |
| Maximum | 12 | 12 | 12 |
| Missing, n (%) | 5 (19.2) | 4 (16.0) | 9 (17.7) |
| GP contact | |||
| n (%) | 23 (88.5) | 23 (92.0) | 46 (90.2) |
| At least one contact, n (%)a | 16 (61.6) | 13 (52.0) | 29 (56.9) |
| Missing, n (%) | 3 (11.5) | 2 (8.0) | 5 (9.8) |
| Number of contacts (if used)a | |||
| Mean | 3.7 | 4.3 | 4.0 |
| SD | 2.02 | 3.03 | 2.50 |
| Minimum | 1 | 1 | 1 |
| Median | 3.5 | 4.0 | 4.0 |
| Maximum | 6 | 12 | 12 |
| Epilepsy nurse contact | |||
| n (%) | 22 (84.6) | 23 (92.0) | 45 (88.2) |
| At least one contact, n (%) | 12 (46.2) | 12 (48.0) | 24 (47.0) |
| Missing, n (%) | 4 (15.4) | 2 (8.0) | 6 (11.8) |
| Number of contacts (if used)a | |||
| Mean | 2.4 | 2.1 | 2.3 |
| SD | 1.37 | 1.56 | 1.45 |
| Minimum | 1 | 1 | 1 |
| Median | 2.0 | 1.5 | 2.0 |
| Maximum | 6 | 6 | 6 |
Emergency department use in 12 months prior to enrolment
Hospital Episode Statistics emergency department data
Hospital Episode Statistics ED data for the 48 patients for whom consent were maintained over the trial show that they together made 122 ED visits in the 12 months before randomisation. Frequency of ED use among them was positively skewed (Figure 5). The median number of visits was 2 (range 0–12) (see Table 12).
FIGURE 5.
Histogram of the number of ED attendances in the previous 12 months by patients according to HES system.
As described in Chapter 3, patient identification centred on the use of NHS ED attendance records and only patients who, when telephoned, said that they had made two or more ED visits for epilepsy in the prior 12 months were recruited. Despite this process, data from the HES system indicated that four (8.3%) patient participants had not made any ED visits during the 12 months prior to recruitment and a further 19 (39.6%) were noted to have made only one ED visit.
Self-reported emergency department data
Self-reported data were available for 45 (88.2%) patient participants. The median number of visits was four (range 0–107) (see Table 12). Again, despite the screening processes, four (8.9%) patient participants responded on the questionnaire completed subsequent to telephone screening that they had not made any visits during the prior 12 months and 3 (6.7%) reported that they had made only one visit.
Relationship between Hospital Episode Statistics and self-reported data
There were 42 patient participants who had self-reported ED data at baseline (T0) and for whom consent remained in place for obtaining their HES ED data. Bland–Altman plots of the agreement between self-reported and HES data on ED visits were produced.
Primary focus is given to the plot shown in Figure 6. This is based on data from 41 rather than 42 patient participants. This is because the distribution of self-reported ED use was particularly influenced by one patient participant who self-reported 107 visits in the prior 12 months. We can confirm that that report of 107 visits was checked against the participant’s answer on the questionnaire and did not reflect a data entry error. Given that only one ED visit in the 12 months was recorded for this patient participant in HES data, we considered the self-report of 107 ED visits to be an outlier and excluded this in the plot shown in Figure 6 (Appendix 14 presents a Bland–Altman plot without any exclusions).
FIGURE 6.
Bland–Altman plot of agreement between self-reported and HES data on ED visits at baseline (n = 41; excludes outlier).

Figure 6 shows that disagreement existed between self-reported and HES data for most patient participants. Most (76.2%) participants self-reported more ED visits than were indicated by the HES system. Only three (7.1%) participants reported the same number of ED visits as were recorded by HES. Figure 6 shows that the limit of agreement was –5.0 visits (i.e. five fewer visits in self-reported data than in HES data) to 7.7 visits (i.e. 7.7 visits more in self-reported data than in HES data).
Psychosocial measures
Compared with a maximum possible score of 100, the mean QOLIE-31-P score was 48.3 (SD 17.3), with a large range (17.1–79.5). Twenty-six (50.9%) participants had ‘probable’ anxiety and 11 (21.6%) had ‘probable’ depression. Assessment of self-stigma revealed that most (n = 42; 82.3%) felt at least some stigma because of epilepsy and 15 (29.4%) felt highly stigmatised.
Comparability of treatment arms, including in emergency department use
Considering the small sample size, the process of randomisation was largely successful in generating two treatment arms broadly similar in demographics and scores on the baseline assessment tools (see Tables 11 and 12). Some differences were apparent in their ED use prior to randomisation (as measured by HES and self-report); ED use was slightly higher in the TAU arm than in the SAFE plus TAU arm (see Table 12).
Representativeness
The age, sex and social deprivation profile of the randomised patient participant sample was compared with that of the patients who were eligible to participate but who declined to participate or were not contactable. This showed that the two groups were comparable in age and social deprivation profile but that females were slightly over-represented in the recruited sample (Table 13).
| Demographic characteristic | Agreed to participate | Did not agree to participate |
|---|---|---|
| Sex, n (%) | (N = 51) | (N = 379)a |
| Male | 22 (43.1) | 192 (50.7) |
| Female | 29 (56.9) | 187 (49.3) |
| Missing | 0 (0.0) | 0 (0.0) |
| Age (years) at presentation to the ED | ||
| n (%) | 51 (100.0) | 379 (100.0) |
| Mean, years | 39.9 | 40.6 |
| SD, years | 15.66 | 16.83 |
| Minimum, years | 16.4 | 16.2 |
| Median, years | 38.8 | 37.5 |
| Maximum, years | 71.3 | 84.4 |
| Missing, n (%) | 0 (0.0) | 0 (0) |
| IMDb | (N = 51) | (N = 379) |
| Decile 1 | ||
| n (%) | 25 (49.0) | 188 (49.6) |
| Minimum rank | 44 | 28 |
| Median rank | 673.0 | 924.5 |
| Maximum rank | 3202 | 3107 |
| Deciles 2 and 3 | ||
| n (%) | 13 (25.4) | 82 (21.6) |
| Minimum rank | 3989 | 3291 |
| Median rank | 6785 | 5309 |
| Maximum rank | 8281 | 9835 |
| Deciles 4–6 | ||
| n (%) | 6 (11.8) | 59 (15.6) |
| Minimum rank | 9881 | 10,277 |
| Median rank | 11,924 | 14,459 |
| Maximum rank | 16,004 | 19,659 |
| Deciles 7–10 | ||
| n (%) | 6 (11.8) | 50 (13.2) |
| Minimum rank | 24,971 | 19,826 |
| Median rank | 28,876 | 22,673 |
| Maximum rank | 32,724 | 32,396 |
| Decile missing | ||
| n (%) | 1 (2.0) | 0 (0) |
Significant other participants
Most (72.5%) patient participants took part in the trial with a SO. SOs were typically a partner or spouse (43.2%), a parent (21.6%), or a son or daughter (16.2%). Most (75.5%) SOs lived in the same household as the patient and had contact with them every day of the week (89.2%). Their mean age was 43.2 years (SD 17 years, range 17–79 years) and most (59.5%) were female. Among the SOs, 32.4% reported having a medical condition themselves.
Anxiety levels among SOs were high, with 15 (40.5%) of the SOs having a score indicating ‘probable’ clinical anxiety. Only three (8.1%) had a score indicating ‘probable’ clinical depression. The mean Zarit caregiver burden score170 was 18.9 (SD 12.51), with most being categorised as reporting either little or no burden (n = 23; 62.2%) or mild to moderate burden (n = 11; 29.7%). Further characteristics of the SOs, including by treatment arm, are provided in Appendix 15.
Researcher unblinding
The researcher correctly stated the treatment allocation of 35 out of the 51 patient participants, giving a rate of unblinding of 68.6% (95% CI 54.1% to 80.9%). The chance-corrected Cohen’s kappa statistic for this level of agreement is 0.37 (95% CI 0.12 to 0.63), indicating ‘fair’ agreement. For SO participants, the researcher correctly stated the treatment allocation of 24 out of the 36 SOs (estimate missing for one SO), giving a rate of unblinding of 66.7% (95% CI 49.0% to 81.4%) and a Cohen’s kappa statistic for agreement of 0.33 (95% CI 0.02 to 0.64). For only three (5.8%) patient participants and three (8.3%) SO participants did the researcher report that they strongly thought that they knew the patient participant’s treatment allocation because of comments participants had made.
Summary
A successful pilot trial was completed in terms of a largely representative sample being recruited and randomised and most patients randomised to SAFE receiving it. There were no protocol deviations and the research worker completing the outcome assessments remained blind to treatment allocation. However, only one of the two predetermined ‘stop/go’ criteria for a definitive trial was met. Specifically, the criterion of securing primary outcome data for ≥ 75% of participants was satisfied (in that it was secured for 94%), but the criterion regarding the percentage of eligible patients agreeing to participate was not: only 12.5% rather than ≥ 20% agreed to take part. The low consent rate, as well as fewer patients being found to be eligible to take part and contactable than anticipated, meant that the target sample size for the pilot RCT was not achieved. The pilot RCT also revealed that identifying patients from local ED records could be resource intensive and that securing routine outcome data on ED use via the HES system requires substantial time.
Chapter 5 Intervention fidelity
Introduction
Seizure first Aid training For Epilepsy is a group-based intervention comprising multiple interacting components and requires a number of behaviours on behalf of those delivering and receiving SAFE. These features create opportunity for variation in its delivery. To permit accurate interpretation of the estimates of its effect generated by the pilot RCT, as well as to help determine whether or not SAFE can be delivered as intended in a trial context, information on its implementation fidelity in the pilot RCT is required. Implementation fidelity refers to the extent to which the core content of SAFE was delivered (adherence) as intended and with what sort of skill (competency). Such monitoring is recommended by the Template for Intervention Description and Replication (TIDieR) extension to the CONSORT statement. 187
Assessing for both adherence and competence is important because the two are not necessarily equivalent (e.g. a facilitator can be highly adherent to treatment manual procedures but not be competent in deploying them). An aspect of competence that appears important when delivering group-based complex interventions155 is interactivity between facilitator and recipients – namely, the degree of ‘didacticism’. 155,188 Although some didacticism is needed to ensure that certain information is provided and participants remain oriented to the goals of the intervention, interaction means that participants are asking questions and obtaining clarification and so are ensuring that the intervention is tailored to their needs. It also permits participants to share and learn from one another.
In the light of the above information, we therefore report how we (1) developed a measure of adherence for SAFE and evaluated its reproducibility, (2) used an existing method for assessing didacticism and evaluated its reproducibility when applied to SAFE and (3), using audio-recordings of intervention sessions, determined the extent of adherence and didacticism demonstrated in the delivery of SAFE in the pilot RCT.
Methods
All seven courses run for the SAFE plus TAU arm of the pilot RCT were audio-recorded and assessed for implementation fidelity. Because SAFE is brief and it is not known which components are its key, active, behaviour-changing ingredients, all parts of SAFE were evaluated for their fidelity. The facilitator was aware that course recordings were to be rated.
Developing the intervention fidelity measurement instruments
Adherence
A checklist of SAFE’s intended content was developed on the basis of the facilitator’s manual (see Report Supplementary Material 1), which lists the 37 items that were meant to be delivered across SAFE’s six modules. The checklist asked a rater to report the extent to which each item was delivered (0 = not delivered; 1 = partially delivered; 2 = fully delivered).
The number of items in each module differed (range 4–10). To allow adherence in the different course modules to be compared, average adherence ratings were calculated.
Competence
The intention was that learning would be elicited rather than taught via SAFE, with the behaviour of the educational facilitator seeking to promote a non-didactic approach. It was not known what level of didacticism represented the optimum and would be associated with the greatest improvement in patient outcomes. It was nevertheless important to gauge what balance between adherence and didacticism was being achieved when SAFE was being delivered in the pilot.
Therefore, using a method previously developed by our team,189 didacticism was assessed using Eudico Linguistic Annotator 5.1 software (Max Planck Institute for Psycholinguistics, Language Archive, Nijmegen, Netherlands). 190 This software permitted a rater to listen to the audio-recording of a SAFE course and simultaneously code when the facilitator was speaking. The total amount of facilitator speech, as a proxy measure of how didactic the course was, was then calculated. This was divided by the duration of the course to generate the percentage of course time during which the facilitator was speaking. Filler words (e.g. ‘oh’, ‘OK’ and ‘yeah’) were not considered instances of facilitator speech.
Testing the measures
To assess reliability of the implementation fidelity measures, two independent raters individually evaluated each course using the fidelity measures. The raters were final-year students completing a Bachelor of Science degree in psychology. Their training consisted of completion of practice adherence and didacticism ratings on two courses not delivered as part of the trial.
Data analysis
Testing the measures
Inter-rater agreement was calculated using MedCalc 18.2.1 (MedCalc Software Ltd, Ostend, Belgium). The intraclass correlation coefficient191 evaluated agreement between the two raters’ didacticism ratings. For the adherence measure, the ratings from the two raters were tabulated and simple percentage agreement first calculated. Inter-rater reliability was then assessed using the chance-corrected weighted kappa statistic.
Because paradoxical values of kappa can occur owing to bias or skewed prevalence,192 the influence of these factors was considered by calculating a prevalence index and a bias index and by comparing the change in kappa when the prevalence-adjusted bias-adjusted kappa for ordinal scales (PABAK-OS) was calculated using a PABAK-OS calculator (URL: www.singlecaseresearch.org/calculators/pabak-os; accessed 27 August 2019). The prevalence index can range from −1 to 1 (0 indicates equal probability) and the bias index ranges from 0 to 1 (0 indicates equal marginal proportions and so no bias). 193
Course fidelity
The raters’ adherence and didacticism scores for each course were averaged and described using descriptive statistics.
Results
Testing the fidelity instruments
Adherence scale: inter-rater reliability
For 96% of adherence items, the raters made the same judgement about the extent to which the item was delivered but the weighted kappa statistic was only 0.66 (95% CI 0.50 to 0.83). This was a consequence of prevalence bias (–0.83; bias index 0.06), with 94.6% of the raters’ ratings using the category ‘fully delivered’. Given this, the PABAK-OS statistic of 0.91 (95% CI 0.85 to 0.97) (which equates to ‘substantial agreement’) provided a more accurate reflection of rater concordance.
Didacticism measure: inter-rater reliability
With a coefficient of 0.96 (95% CI 0.78 to 0.99), the intraclass correlation coefficient indicated that the two raters’ judgements regarding didacticism were highly correlated.
Course fidelity
Adherence
Adherence was high. Among the 259 items meant to be delivered across the seven courses, 228 (88.0%) were ‘fully delivered’. Only eight (3.1%) were ‘not delivered’ (Table 14). The average adherence rating (with a maximum of 2) given to the items across the SAFE courses was 1.88 (SD 0.11, range 1.65–1.97) (see Appendix 16).
| Course number | Adherence rating | Didacticism rating: percentage (SD) of time facilitator speaking | |
|---|---|---|---|
| Number (%) of items fully delivered | M rating (SD) across 37 items | ||
| 1 | 35 (94.6) | 1.97 (0.11) | 54.45 |
| 2 | 33 (89.2) | 1.91 (0.35) | 57.57 |
| 3 | 33 (89.2) | 1.92 (0.25) | 63.94 |
| 4 | 35 (94.6) | 1.95 (0.22) | 54.48 |
| 5 | 32 (86.5) | 1.86 (0.40) | 49.59 |
| 6 | 27 (73.0) | 1.65 (0.69) | 48.87 |
| 7 | 33 (89.2) | 1.88 (0.39) | 59.42 |
| Across all seven courses | 228 (88.0) | 1.88 (0.11) | 55.47 (5.35) |
The mean and range of adherence scores given to the individual intervention items showed that no item proved too challenging to be fully delivered at least once. The mean score of only one intervention item (namely, requiring the facilitator to inform the participants about when the demonstrated recovery position should and should not be delivered) fell below 1 (i.e. to 0.79).
Didacticism
The mean percentage of facilitator speech across the courses was 55% (SD 5.4%), with a range of 49–64% (see Table 14).
Summary
The SAFE intervention was found to have been delivered as intended across the pilot RCT. The adherence measure indicated that the facilitator delivered almost all the items prescribed by the treatment manual. Moreover, they were able to do this while maintaining a high degree of interactivity. In the light of these findings, we do not expect the estimate of intervention effect found in the pilot RCT to be influenced by poor implementation fidelity.
Chapter 6 Outcomes of the pilot randomised controlled trial and implications for a definitive trial
Introduction
Primary and secondary outcome measures were captured/completed to varying degrees at baseline and at 3-, 6- and 12-month follow-up. This chapter outlines the findings from these assessments.
Outcome measures
Proposed primary outcome: emergency department use measured by the Health Episode Statistics system
Routinely collected data on ED use from the HES system were secured for 48 (94.1%) of the randomised patient participants. The mean number of visits recorded over the 12 months following randomisation to the SAFE plus TAU arm was 1.8 (SD 3.14) and the median was 1 (range 0–12). For the TAU arm, the mean was 3.4 (SD 4.78) visits and the median was 2 (range 0–20) visits. Compared with the 12 months prior to randomisation, mean ED use over the follow-up period reduced for the SAFE plus TAU arm by 0.3 (28%) visits. For the TAU arm, it increased by 0.4 (11%) visits over the follow-up period (Table 15).
| Number of ED visits | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| During the 12 months prior to baseline | |||
| n (%) | 25 (96.2) | 23 (92.0) | 48 (94.1) |
| Mean | 2.1 | 3 | 2.5 |
| SD | 2.22 | 2.76 | 2.51 |
| Minimum | 0 | 1 | 0 |
| Median | 1 | 2 | 2 |
| Maximum | 10 | 12 | 12 |
| Missing, n (%) | 1 (3.8) | 2 (8.0) | 3 (5.9) |
| During the 12 months following randomisation | |||
| n (%) | 25 (96.2) | 23 (92.0) | 48 (94.1) |
| Mean | 1.8 | 3.4 | 2.6 |
| SD | 3.14 | 4.78 | 4.05 |
| Minimum | 0 | 0 | 0 |
| Median | 1 | 2 | 1 |
| Maximum | 12 | 20 | 20 |
| Missing, n (%) | 1 (3.8) | 2 (8.0) | 3 (5.9) |
| Change from baseline at 12 months | |||
| n (%) | 25 (96.2) | 23 (92.0) | 48 (94.1) |
| Mean | –0.3 | 0.4 | 0.1 |
| SD | 1.99 | 3.81 | 2.99 |
| Minimum | –4 | –5 | –5 |
| Median | 0 | 0 | 0 |
| Maximum | 5 | 16 | 16 |
| Missing, n (%) | 1 (3.8) | 2 (8.0) | 3 (5.9) |
Emergency department use was overdispersed. However, there was not an excess of zeros (Vuong’s test:180 z = –0.17; p = 0.87). For this reason, NBR compared the effect of treatment arm on ED use, which showed that the estimated rate of ED visits following randomisation was around 46% lower in the SAFE plus TAU arm than in the TAU arm (rate ratio = 0.54). This difference was not statistically significant at the 5% alpha level (95% CI 0.24 to 1.18), nor at the 10% level (90% CI 0.28 to 1.04). The difference was statistically significant at the 20% level (80% CI 0.32 to 0.90; p = 0.12) (Table 16).
| Model and parameter | Value | CI | p-value | ||
|---|---|---|---|---|---|
| 95% | 90% | 80% | |||
| 12 months following randomisationa | |||||
| Negative binomial | |||||
| SAFE plus TAU, rate ratio | 0.54 | 0.24 to 1.18 | 0.28 to 1.04 | 0.32 to 0.90 | 0.12 |
| Dispersion parameter | 1.53 | 0.67 to 2.39 | 0.80 to 2.25 | 0.96 to 2.09 | NA |
| Vuong’s testb | –0.17 | NA | NA | NA | 0.87 |
| 12 months following randomisation with adjustment for baseline ED visits | |||||
| Negative binomial | |||||
| SAFE plus TAU, rate ratio | 0.62 | 0.33 to 1.17 | 0.36 to 1.06 | 0.41 to 0.94 | 0.14 |
| Baseline ED visits, rate ratio | 1.33 | 1.18 to 1.52 | 1.20 to 1.49 | 1.23 to 1.45 | < 0.001 |
| Dispersion parameter | 0.69 | 0.17 to 1.21 | 0.26 to 1.13 | 0.35 to 1.03 | NA |
| Vuong’s testb | –0.13 | NA | NA | NA | 0.90 |
Owing to a slight imbalance between treatment arms in ED use in the 12 months prior to randomisation (i.e. ED visits at baseline), we also applied an adjusted NBR, including SAFE plus TAU arm and baseline ED visits. The results showed that an increased number of ED visits pre randomisation was associated with an increased number of post-randomisation ED attendances (rate ratio 1.33, 95% CI 1.18 to 1.52). With the adjustment, the estimated effect size for treatment arm reduced, with the rate of ED visits for the SAFE plus TAU arm being around 38% lower than that for the TAU arm (rate ratio 0.62). In this adjusted analysis, treatment arm was not significantly associated with ED use at the 5% (95% CI 0.33 to 1.17) or 10% (90% CI 0.36 to 1.06) level; the treatment arm was statistically significant at the 20% level (80% CI 0.41 to 0.94; p = 0.14).
Secondary outcome data
Emergency department use: self-reported
Self-reported data on ED use during both the 12 months prior to and the 12 months following randomisation were available for 34 (66.6%) of the randomised patient participants. The mean number of ED visits reported by patient participants in the SAFE plus TAU arm was 1.2 and the median was 0.0. The mean number of ED visits reported by patient participants in TAU arm was 2.9 and the median was 1.0. Compared with the 12 months prior to randomisation, mean ED use over the follow-up period reduced by 2.6 visits for participants in the SAFE plus TAU arm. For the TAU arm, this reduced by 0.3 visits (see Appendix 17).
Negative binomial regression with (rate ratio 0.34, 95% CI 0.11 to 1.07; p = 0.10) and without (rate ratio = 0.42, 95% CI 0.15 to 1.20; p = 0.06) adjustment for ED use over the 12 months prior to randomisation indicated that the estimated rate of ED visits following randomisation was around 66% and 58% lower in the SAFE plus TAU arm and in the TAU arm, respectively (Table 17).
| Model and parameter | Value | CI | p-value | ||
|---|---|---|---|---|---|
| 95% | 90% | 80% | |||
| 12 months following randomisation | |||||
| Negative binomial | |||||
| SAFE plus TAU, rate ratio | 0.42 | 0.15 to 1.20 | 0.17 to 1.01 | 0.21 to 0.83 | 0.10 |
| Dispersion parameter | 1.85 | 0.50 to 3.20 | 0.72 to 2.98 | 0.97 to 2.73 | NA |
| Vuong’s testa | 0.49 | NA | NA | NA | 0.62 |
| 12 months following randomisation with adjustment for baseline ED visits | |||||
| Negative binomial | |||||
| SAFE plus TAU, rate ratio | 0.34 | 0.11 to 1.07 | 0.13 to 0.88 | 0.16 to 0.72 | 0.06 |
| Baseline ED visits, rate ratio | 1.15 | 0.83 to 1.60 | 0.88 to 1.52 | 0.93 to 1.43 | 0.39 |
| Dispersion parameter | 1.87 | 0.38 to 3.36 | 0.62 to 3.12 | 0.89 to 2.84 | NA |
| Vuong’s Testa | –0.13 | NA | NA | NA | 0.90 |
Relationship between Hospital Episode Statistics and self-reported data
Among the 34 patient participants who had self-reported ED data at 12 months (T3) and for whom consent remained in place and HES data were secured, 11 (32.4%) had the same number of ED visits recorded in HES and self-reported data: seven (41.2%) from the TAU arm and four (23.5%) from the SAFE plus TAU arm. A total of 13 patient participants (38.27%) had more ED visits recorded in HES data than in self-reported data and 10 (29.4%) self-reported more ED visits than were recorded by HES data; proportions were similar across the two treatment arms (Table 18).
| HES-recorded vs. self-reported ED visits | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| More HES recorded than self-reported | 7 (41.2) | 6 (35.3) | 13 (38.2) |
| Equal | 4 (23.5) | 7 (41.2) | 11 (32.4) |
| More self-reported than HES recorded | 6 (35.3) | 4 (23.5) | 10 (29.4) |
Figure 7 shows a Bland–Altman plot of the agreement between the mean number of self-reported and HES-recorded ED visits for the 34 patient participants; the limits of agreement were –5.3 visits (i.e. 5.3 fewer visits recorded in self-reported data than in HES data) to 4.4 visits (i.e. 4.4 visits more recorded in self-reported data than in HES data). Compared with baseline (see Figure 6), this Bland–Altman plot demonstrates a relatively even spread of self-reported versus HES-recorded ED visits.
FIGURE 7.
Bland–Altman plot of agreement between self-reported and HES-recorded ED visits at 12 months (n = 34).
Other secondary outcome measures
As per the statistical analysis plan, differences between the two treatment arms on the remaining secondary outcome measures were not formally statistically tested. For completeness, summary statistics for the arms on these measures at 6 months’ (T2) and 12 months’ (T3) follow-up are reported in Appendix 18.
Safety and adverse events
A total of 18 (35.3%) patient participants reported adverse events over the course of their follow-up (see Appendix 19). In the SAFE plus TAU arm, six adverse events were reported by six participants; 13 adverse events were reported by 12 participants in the TAU arm. Only one SAE occurred during the study: a diagnosis of previous status epilepticus. This was reported by one SAFE plus TAU arm participant. On medical review, it was considered unlikely to be related to SAFE or participation in the trial because it arose from investigations that the participant had been having before they began participating in the trial.
Participant feedback
A total of 32 (68.1%) patient participants and 20 (62.5%) SOs completed the participant feedback questionnaire at 12 months (T3). All but one said that they would participate in the trial again (Tables 19 and 20).
| Feedback on participation in the SAFE trial | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| If time suddenly went backward, and you had to do it all over again, would you agree to participate in the trial? | (N = 26) | (N = 25) | (N = 51) |
| Definitely yes | 15 (57.7) | 10 (40.0) | 25 (49.0) |
| Probably yes | 2 (7.7) | 4 (16.0) | 6 (11.8) |
| Probably no | 0 (0.0) | 1 (4.0) | 1 (2.0) |
| Definitely no | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not sure | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 9 (34.6) | 10 (40.0) | 19 (37.2) |
| Feedback on participation in the SAFE trial | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| If time suddenly went backward, and you had to do it all over again, would you agree to participate in the trial? | (N = 18) | (N = 19) | (N = 37) |
| Definitely yes | 11 (61.1) | 9 (47.4) | 20 (54.1) |
| Probably yes | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Probably no | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Definitely no | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Not sure | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (38.9) | 10 (52.6) | 17 (45.9) |
When asked to explain why, the limited burden associated with participating was mentioned, as was a sense of duty to help improve things for others. Some SAFE plus TAU arm participants mentioned the benefit they perceived themselves to have derived from SAFE. Illustrative quotes included the following:
I found that the study was not an issue or an inconvenience and I was happy to participate and would happily take part in any future studies.
PWE participant
I’m more than happy to help in a study that helps towards epilepsy.
PWE participant
Before we done it [participated in the research] we didn’t have a clue [. . .] Sometimes we would have gone to the hospital if we needed [. . .] but now we don’t need to go.
SO
Some participants provided comments relating to recruitment. No concerns were raised regarding randomisation, indicating that the process was acceptable. A point made by a number of participants was that, although they valued the intervention, they felt the offer seemed somewhat reactive and that a more preventative approach to self-management support might be valuable. They said that taking part in SAFE had been their primary source of education since being diagnosed, with health-care encounters having previously focused on ‘medication and never about education or management’ (SO participant) and that there was a need to offer such an intervention to those who have been recently diagnosed with epilepsy.
In terms of continued participation in the trial, a number of participants reported that they had experienced challenges in completing the questionnaires during the course of the study owing to instances of being unwell. Several participants from the TAU arm noted that memory problems and the gap between data collection episodes meant that they had forgotten about their participation in the study and so their motivation to take part when contacted had reduced.
Sample size calculation for future trial
The proposed primary outcome measure for a definitive trial is ED use as measured by HES. The pilot RCT found a reduction of around 47% in ED use in the SAFE plus TAU arm in the 12 months following randomisation compared with the TAU arm.
Table 21 shows the number of patient participants that would be required per arm for a definitive trial. Depending on the estimate of the dispersion parameter from the pilot trial that is used and the statistical power required, the number of patient participants needed per arm in a definitive trial ranges from 123 to 451.
| Dispersion parameter (k) | ≈ 47% reduction (from 3.4 to 1.8 visits) in 12 months compared with TAU | |
|---|---|---|
| n per arm (α = 0.05; 80% power; β = 0.2) | n per arm (α = 0.05; 90% power; β = 0.1) | |
| 0.17 | 123 | 164 |
| 0.5 | 191 | 255 |
| 0.69 | 230 | 308 |
| 1 | 293 | 393 |
| 1.21 | 337 | 451 |
If the central value of the estimate range for the dispersion parameter (k) of 0.69 is used, 90% power stipulated and 9.4% of recruited patients eventually withdraw consent to access their HES data (as observed in the pilot), a sample of 674 patients [(308 × 2) + 58] would need to be recruited for a definitive trial of SAFE. Using the estimates for eligibility and consent from the pilot trial, it would be anticipated that the triage cards of > 51,000 patients would need to be screened and > 7000 patients invited to secure a sample of 674 patients.
For the pilot RCT, three type 1 EDs acted as recruitment sites. These EDs generated, on average, 17.6 patient accruals each from 19 months of attendances (i.e. ≈ 1 per month). The EDs were similar in size to EDs in England, with the mean number of attendances (for any reason) being in line with the average for English EDs. 194 It can therefore be estimated that a definitive trial would require around 39 average-sized ED sites to recruit the necessary sample. This would equate to about half of England’s type 1 EDs.
Summary
Using data from the 94% of patient participants on whom primary outcome data were secured, estimates for the effect of SAFE plus TAU on ED use were generated. Feedback from participants on trial participation was also secured. Participation in the trial was not associated with adverse effects and participants for the most part welcomed taking part and receiving the intervention. Using the estimate of effect and evidence from the pilot RCT on eligibility and uptake, it was calculated that a large definitive trial, in terms of recruitment sites and starting sample, would be needed to definitively judge the efficacy of the SAFE intervention.
Chapter 7 Economic evaluation
Introduction
When the feasibility of a definitive trial is being considered, an important part of the decision calculus is the cost of delivering the intervention to be trialled. Cost is important because it will affect the ease with which ‘excess treatment costs’ can be secured and would be fundamental to future cost-effectiveness analysis. It is also important because stakeholders will want to consider the budget impact and scalability of the intervention to be tested should it be found to be cost-effective. In contrast to health technologies, the costs of complex interventions such as SAFE are often less well characterised and may vary depending on the context of use. Microcosting is a method that provides detailed, bottom-up cost data based on each component of the delivery of an intervention. 195
We performed a microcosting to calculate the fixed and variable costs of delivering SAFE in the context of the pilot RCT. The cost of developing the SAFE intervention via the processes described in Chapter 2 was also determined. Such information is rarely provided but can be helpful for those planning similar endeavours and for research funders.
Methods
The microcosting adopted the perspective of an academic non-profit-making institution and was conducted in three stages: (1) identification of resources; (2) measurement; and (3) valuation. 196
Step 1: identification of resources
A health economics researcher (EH) met with staff central to the development of the SAFE intervention (academic staff AN and DS) and delivery (epilepsy nurse specialist and SAFE facilitator Juliet Bransgrove and project administrator Gail Moors; see Acknowledgements) to map out the courses run for the project. The work and resources required for each were attributed to one of three categories according to the primary purpose of the course: inform intervention design, participants in the SAFE plus TAU arm and participants in the TAU only arm (Figure 8).
FIGURE 8.
Workflow. Light blue shading signifies research and development.
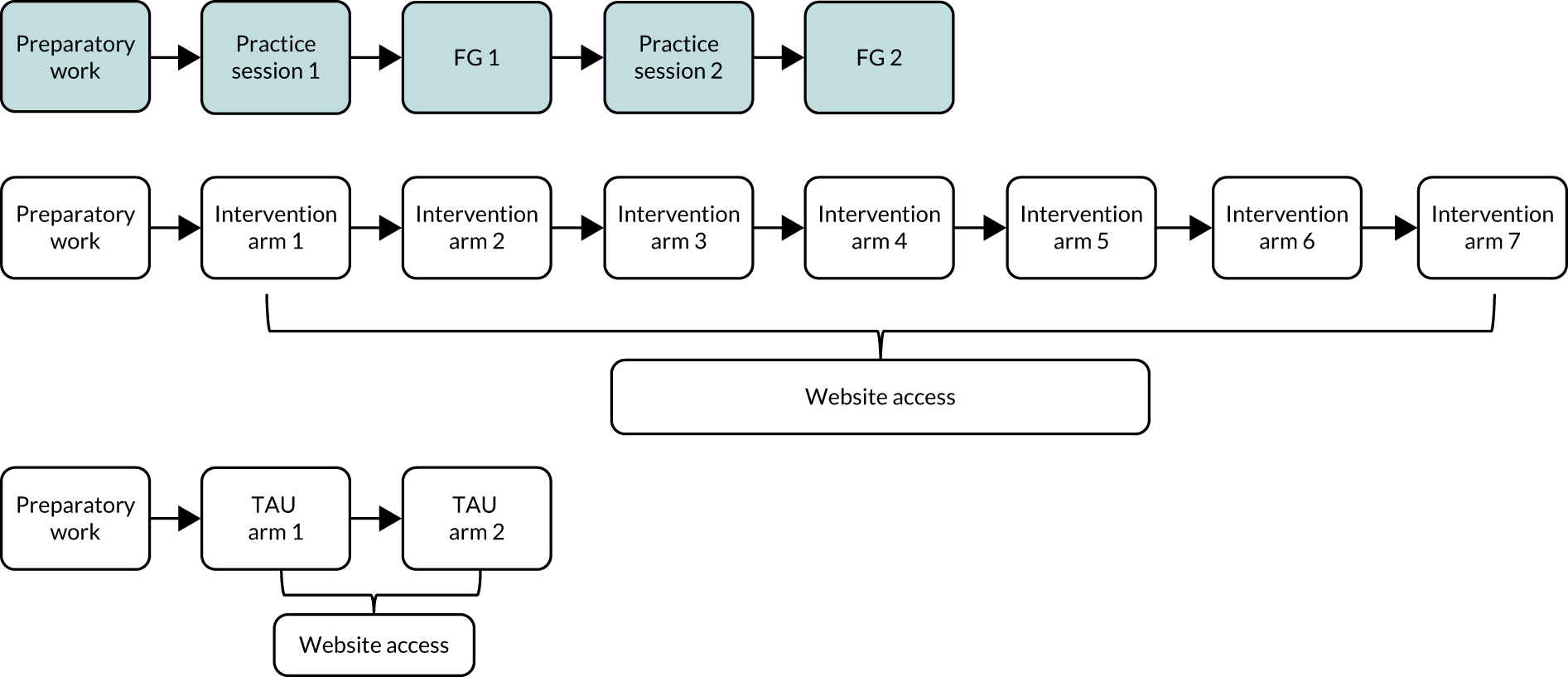
The resources and items required to deliver SAFE were also differentiated as fixed or variable. Fixed resources were constant regardless of the number of people attending the course (e.g. room hire, facilitator). Variable resources were those that changed depending on the number of attendees (e.g. patient/SO take-away information packs, travel expenses). Participant involvement was costed from the point at which they were invited to attend a SAFE session to more accurately reflect routine practice.
Step 2: measurement
The quantity of use of the resources identified in stage 1 was measured. To capture this information we used the ‘time and motion method’. 197 This involved the completion (by AN, DS, Juliet Bransgrove and Gail Moors; see Acknowledgements) of electronic data collection forms on which they self-reported the time that they dedicated to different activities. The research team (AN and DS) also recorded details of the number of participants invited and attending each of the SAFE sessions and non-staff resources.
Staff time and costs
Time was split into preparatory work (time in meetings, designing the SAFE intervention, administration of SAFE’s content, planning and organising practice sessions, attending practice sessions, reviewing and editing SAFE’s content) and programme delivery (time spent inviting participants, organising and attending SAFE sessions, packing up, and administration of participant expenses and the website). Staff recorded their time against each task for each SAFE session.
Travel time and costs
Staff involved in SAFE’s delivery recorded estimates of time spent travelling to sessions and any travel costs. Travel costs were recorded for participants and taken from the study finance records as the total claimed per group.
Other costs
The two academic staff central to adapting the intervention (AN and DS) recorded all resources and items required to complete the activities identified in stage 1. This included office space for staff involved in course preparation and administration, telephone calls, stationery, printing and postage, leaflets for patients/SOs, advertising, participant remuneration (practice sessions only), venue hire, refreshments, equipment, and website domain and administration costs.
Step 3: valuation
A monetary value was attached to the quantities of resources used that were measured in step 2. Unit costs and details of suppliers were obtained using local data from the finance office of the University of Liverpool or national sources [e.g. Royal Mail (Royal Mail Group Ltd, London, UK)] as applicable (Table 22). Costs were calculated using price year 2017/18 and as Great British pounds. VAT was applied to equipment and consumables at a rate of 20%.
| Category | Unit cost (£) | Source |
|---|---|---|
| Staff (per minute) | ||
| Lecturer (grade 8) | 0.46 | University of Liverpool198 |
| Administrative assistant | 0.23 | |
| Research fellow (grade 8) | 0.40 | |
| Epilepsy specialist nursea | 0.25 | |
| IT technician (grade 8) | 0.47 | |
| Delegate expenses (per group) | ||
| Practice group (travel) | 55.00 | Research study financial statement. Practice group remuneration: £10 shopping voucher per FG participant. Disaggregated travel expense data not available |
| Practice group (remuneration) | 135.00 | |
| SAFE plus TAU arm (travel) | 40.00 | |
| TAU arm (travel) | 46.40 | |
| Telephone (per minute, including VAT) | ||
| Office line to UK mobile | 0.06 | BT (London, UK)199 |
| Office line to UK landline | 0.05 | |
| Advertising (per advert) | ||
| Practice group recruitment | 45.00 | Liverpool Daily Echo (Liverpool Daily Post & Echo Ltd, Liverpool, UK) |
| Venue costs | ||
| Room hire (per course; 0.5 days, room capacity 20 delegates, includes PC and projector) | 80.00 | Royal Liverpool Academy200 |
| Refreshments (per head; tea and coffee) | 1.50 | Royal Liverpool Academy200 |
| Equipment (including VAT) | ||
| Dictaphone (DM-650; Olympus, Tokyo, Japan) and boundary microphone (ME33; Olympus) | 355.98 | Research study financial statement |
| Study laptop | 592.87 | Desk Top Publishing Microsystems Ltd (Leeds, UK); purchased via University of Liverpool |
| Annual fees (including VAT) | ||
| Website domain | 20.94 | Fasthosts Internet Ltd (Gloucester, UK) |
| Freepost licence fee | 116.40 | Royal Mail201 |
| Delegate packs (including VAT) | ||
| Invite pack (1 black and white, single-sided A4 page; envelope; second-class post; Freepost reply envelope) | 1.12 | Lyreco (Marly, France); University of Liverpool printing rates; Royal Mail |
| Reminder pack (1 black and white, single-sided A4 page; envelope; second-class post; Freepost reply envelope) | 1.12 | |
| FG consent form (1 black and white, single-sided A4 page) | 0.04 | |
| Delegate booking confirmation (1 colour, double-sided A4 page; envelope; first-class) | 0.80 | |
| Delegate course pack (SAFE plus TAU) (slide handouts: 10 colour, single-sided A4 pages; certificate; folder) | 0.89 | |
| Delegate course pack (TAU) (slide handouts: 20 black and white, single-sided A4 pages; certificate; folder) | 0.89 | |
| Name badge | 0.60 | |
| Leaflet | 1.00 | ES |
| Convener packb (4 acetates; 4 answer cards; 10 pens; flip chart; trainer manual;c clipboard;c 2 whiteboard markers;c 12 coloured marker pensc) | 4.96 | Lyreco |
Details of staff job titles, employer and salary bands were obtained. Academic staff costs were calculated using University of Liverpool salary scales 2017/18 (hourly rates). Salary costs for the epilepsy nurse specialist delivering the training and the administrative assistant were calculated using hourly rates for casual workers and included 12.07% holiday pay. The epilepsy nurse specialist completed her role on the SAFE pilot study on an honorary contract with University of Liverpool at an hourly rate equivalent to band 6 in NHS England or Wales (Royal College of Nursing 2018). All salary costs were converted into cost per minute for the purpose of valuing time on activities. The staff costs per minute were multiplied by the time spent on each activity, then summed to obtain total staff costs.
Analysis
Resource use and costs were analysed to calculate the cost of developing the intervention, the fixed and variable costs of delivering the training sessions (SAFE plus TAU or TAU) and the mean cost per participant or patient (which includes the cost of attending with or without an SO). The cost of developing SAFE was calculated as the sunk cost of designing the intervention (incurred and independent of future training courses) plus the total fixed annual set-up cost. Calculations were based on an assumption of 11 groups per year and an equipment life of 1 year.
The epilepsy nurse specialist who facilitated the SAFE courses was not based locally but located in the region of the ES offices. This meant that she travelled to Liverpool to deliver the course. For this reason, a scenario analysis that excluded the travel time and travel expenses of the epilepsy nurse specialist facilitating the groups was conducted to explore a more realistic scenario of employing a local nurse.
The means and SDs of observed data were calculated. The 95% central range (CR) for costs and differences was generated using 10,000 bootstrap replications. All data were managed and analysed in Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA).
Results
Over the course of the project, 11 SAFE intervention courses were run. Two were run to inform intervention design, seven were run for participants in the SAFE plus TAU arm and two were run after the trial was completed for the TAU arm. Data were collected from the four noted members of staff, with all data collection forms being complete and no missing data.
The total cost of developing the training was £9947, which included two practice SAFE courses attended by 27 people (Table 23); 88.72% of the development cost was a sunk cost independent of future training courses.
| Description | Design and set-up | SAFE delivery course | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Practice session | Treatment arm | |||||||||||
| SAFE plus TAU | TAU | |||||||||||
| 1 | 2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 1 | 2 | ||
| Fixed costs (£) | ||||||||||||
| Equipment | 928.85 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Website | 77.12 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Freepost licence | 116.40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Venue | 0.00 | 80.00 | 80.00 | 80.00 | 80.00 | 80.00 | 80.00 | 80.00 | 80.00 | 80.00 | 80.00 | 80.00 |
| Facilitator (staff cost) | – | 192.79 | 192.79 | 177.96 | 177.96 | 177.96 | 177.96 | 177.96 | 177.96 | 177.96 | 177.96 | 177.96 |
| Facilitation resources | – | 4.96 | 4.96 | 4.96 | 4.96 | 4.96 | 4.96 | 4.96 | 4.96 | 4.96 | 4.96 | 4.96 |
| Total (fixed) | 1122.37 | 277.75 | 277.75 | 262.92 | 262.92 | 262.92 | 262.92 | 262.92 | 262.92 | 262.92 | 262.92 | 262.92 |
| Variable costs (£) | ||||||||||||
| Staff costs | 4243.41 | 612.88 | 1158.56 | 591.19 | 521.84 | 429.37 | 429.37 | 318.41 | 318.41 | 318.41 | 299.96 | 416.03 |
| Staff travel expenses | 44.10 | 279.87 | 279.87 | 274.67 | 274.67 | 265.43 | 265.43 | 265.43 | 265.43 | 265.43 | 233.63 | 265.41 |
| Participant expenses | 0.00 | 180.00 | 200.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 40.00 | 92.80 | 0.00 |
| Office costs (including telephone) | 589.33 | 33.00 | 105.50 | 81.63 | 85.23 | 92.60 | 88.70 | 89.00 | 86.60 | 89.00 | 57.17 | 70.13 |
| Stationery/print/postage | 0.00 | 156.35 | 196.96 | 57.44 | 93.68 | 68.93 | 68.93 | 34.46 | 22.98 | 45.95 | 182.73 | 298.44 |
| Refreshments | 0.00 | 72.25 | 72.25 | 41.80 | 41.80 | 32.81 | 32.00 | 41.00 | 26.60 | 18.50 | 21.00 | 34.50 |
| Advertising | 45.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total (variable) | 4921.84 | 1334.34 | 2013.13 | 1086.73 | 1057.22 | 929.13 | 924.42 | 788.30 | 760.01 | 777.29 | 887.28 | 1084.51 |
| Fixed and variable costs (£) | ||||||||||||
| Total | 6044.21 | 1612.09 | 2290.88 | 1349.65 | 1320.14 | 1192.05 | 1187.34 | 1051.22 | 1022.93 | 1040.21 | 1150.20 | 1347.43 |
| Delegates and arms | ||||||||||||
| Delegates (patient : SO ratio) | – | 12 | 15 | 2 : 3 | 4 : 4 | 3 : 3 | 4 : 2 | 3 : 0 | 1 : 1 | 2 : 2 | 4 : 4 | 8 : 5 |
| Mean cost (£) per delegate | – | 134.34 | 152.73 | 269.93 | 165.02 | 198.68 | 197.89 | 350.41 | 511.47 | 260.05 | 143.78 | 103.65 |
| Mean cost per arm, £ (SD; 95% CR)a | 1166 (134.22; 1023 to 1350) | 1249 (139.46; 1150 to 1347) | ||||||||||
| Mean cost per delegate, £ (95% CR)a | 240 (211 to 278) | 119 (110 to 128) | ||||||||||
| Mean cost per patient (with or without SO), £ (95% CR)a | 408 (358 to 472) | 208 (192 to 225) | ||||||||||
To deliver SAFE in the pilot RCT, the fixed cost (SAFE plus TAU arm or TAU arm) was £263 per group, which represented the cost of course facilitation and venue hire. The mean variable cost per group (n = 9) was £922 (SD £131, 95% CR £887 to £1085). There were no statistically significant differences in the mean cost per group between SAFE plus TAU and TAU only (SAFE plus TAU arm: mean £903, SD £134; 95% CR £760 to £1087; TAU arm: mean £986, SD £140, 95% CR £887 to £1085; difference in CR –£324 to £119).
On average, the TAU arms involved six more participants than the SAFE plus TAU arms. The mean cost per participant was £119 (95% CR £110 to £128) in the TAU arm; this was significantly lower than the cost per participant in the SAFE plus TAU arm, which was £240 (95% CR £211 to £278; difference in CR –£1137 to –£872); both estimates exclude fixed annual set-up costs of £1122.
The average ratio of patients to SOs was 3 : 2. The mean cost per patient (with or without SO) was £208 (95% CR £192 to £225) in the TAU arm; this was significantly lower than the cost per patient in the SAFE plus TAU arm, which was £408 (95% CR £358 to £472).
When including the cost of developing SAFE (£181 per person), the mean cost per participant, based on all 55 participants in SAFE plus TAU or TAU arms, was £375 (95% CR £348 to £402). This reduced to £214 (95% CR £188 to £241) when excluding sunk costs that would not be incurred again in the future.
Staff time accounted for 50.01% of the cost of the delivering SAFE and almost 70.21% of the development cost. Staff travel expenses accounted for an additional 21.10%. Travel expenses were high as a consequence of the specialist nurse delivering SAFE not being based locally.
Office, stationery, printing, postage and venue hire costs were relatively low. A scenario analysis of facilitator costs without travel expenses (time and cost) reduced the mean cost by 21.08%, from £194 (95% CR £167 to £221) to £152 (95% CR £124 to £179) per delegate or from £333 (95% CR £288 to £380) to £261 per patient.
Discussion
Delivering SAFE was estimated to cost £333 per patient (with or without a SO) and it is plausible that it could be delivered for as little as £261 per patient or £152 per delegate. The main cost contributors are staff costs and associated travel expenses and office costs. The annual fixed cost of setting up and running SAFE is £1122 (plus £35.07 per patient, based on 32 patients per year attending with or without a SO).
Chapter 8 Discussion
Principal findings
Part A: intervention development
A novel co-production approach enabled us to achieve our objectives of (1) optimising the content, delivery and behaviour change potential of the ES’s existing course for PWE attending ED and their SOs and (2) developing a seizure management intervention that had support from representatives from the target service user population and from professional groups involved in their care.
The finalised intervention – SAFE – was brief, manualised, supported by the inclusion of a theory of behaviour change and based on a delivery method considered by stakeholders to be sustainable for the NHS. Feedback from service users who received the test version of the intervention was positive. This is despite it focusing on sensitive areas, such as potentially unnecessary hospital service by them and their own beliefs about epilepsy. Participants valued the straightforward guidance offered by SAFE and liked its group format. This concurs with previous observations, with PWE previously reporting that the format helps them ‘feel less alone’ and able to share and learn from others. 127 Our participants did identify potential barriers to intervention effect (e.g. memory difficulties) and we sought to ameliorate these (e.g. by providing participants with access to hard copies and online copies of the course materials).
Triallists have often been criticised for not describing complex interventions sufficiently for replication. 202 This is not the case for SAFE. Its content and the origin of each of its elements are clearly documented. A TIDieR checklist187 is also available (see Report Supplementary Material 2).
The microcosting of intervention development activity is rare. At just under £10,000, the cost of developing SAFE is arguably low. This was, in part, because SAFE was adapted from an existing intervention and because the owners of the original course (the ES) did not charge for access to it or its materials. Our findings are important to disseminate because they could help those considering similar endeavours to accurately forecast activity, timelines and costs. They also provide funders with a benchmark against which to judge applications.
Part B: pilot randomised controlled trial
A pilot RCT comparing SAFE plus TAU with TAU only was successfully completed. As planned, it provided estimates of key parameters, including recruitment and retention, and allows an informed decision regarding the feasibility and optimal design of a definitive trial of SAFE to be made. In this chapter we discuss the estimates, their relation to the two progression criteria established at project outset and the implications.
Positives for feasibility of a definitive trial
The pilot demonstrated that it is possible to identify, consent, randomise and largely retain people from the target population and their SOs in a RCT. This was not a given at the outset because, to our knowledge, no previous trial had focused recruitment on this population and because this population is potentially vulnerable.
No safety concerns arose from the trial’s conduct and no protocol deviations occurred. Strategies in the trial to conceal treatment allocation worked. It was possible to get most (76.9%) patient participants who were randomised to the SAFE plus TAU arm to a SAFE session and for it to be delivered as intended (i.e. with high fidelity). This is in line with previous RCTs of complex interventions with the epilepsy population. 71,72,203–205
Feedback from participants indicated that they valued SAFE and the perceived benefits from receiving it. The SAFE intervention was perceived as being outside current epilepsy service provision. Most said that they would participate in such a trial again if invited.
Importantly, by using routine outcome data on ED use as the basis for the proposed primary outcome measure, it was possible to secure 12-month outcome data for 94% of the randomised patient participants. This means that the second progression criterion – that 12-month follow-up data could be obtained for at least 75% of patient participants – was satisfied. The direct cost for these data was ≈ £10,000. The progression criterion would not have been met had the trial relied on patients self-reporting on ED use because only 67.7% of participants were available and/or able to provide this information. Routine data would thus make a definitive trial less vulnerable to the effect of attrition. By way of context, the proportion of randomised patient participants with primary outcome data in recent NIHR Health Technology Assessment programme trials (conducted between 2005 and 2017) was 89% (IQR 79–97%), but these typically had shorter follow-up periods (median 6 months post randomisation). 131
Our pilot also revealed that relying on self-reported data would expose a definitive trial to what appears to be recall bias by patients relating to their use of ED. At baseline, 76.2% of patients reported more ED visits during the 12 months prior to recruitment than indicated by routine data for them (on average by 3.8 visits). It has often been claimed that recall bias is not such an issue for ‘big ticket’ items such as hospitalisations as it is for more routine lower-cost service items (e.g. ambulatory outpatient clinic visits). 206,207 Our findings indicate that caution should be exercised in using self-reported data on ED use.
The proportion of patients who self-reported a different number of ED visits to that recorded by HES was slightly smaller at 12-month follow-up (i.e. 29.4% reported more visits and 38.2% reported fewer). The reason for the greater congruence between the two data sources at this assessment point is not clear. The provision to patient participants at recruitment of seizure diaries by the trial team in which they were encouraged to record seizures and associated events might have attenuated bias.
The apparent bias and the smaller proportion of patients with self-reported ED outcome data meant that the effect size estimate for SAFE differed substantially depending on whether the calculation used self-reported or HES data. When looking at the unadjusted NBR models, the self-reported data indicate that the estimated rate of ED use in the SAFE plus TAU arm was 58% lower than for the TAU arm. HES data indicate that the rate of ED use was around 46% lower.
A microcosting exercise indicated that the cost per attendee (when excluding facilitator travel costs) of attending a SAFE course was ≈ £152. Accurately identifying the cost of an intervention’s delivery allows excess treatment costs for a definitive trial to be forecast and helps with assessments regarding the potential for an intervention to be offered in clinical practice. The figure also allows for a simple and preliminary evaluation to be made of the potential for savings to be generated by comparing the cost of the intervention to the direct cost of the health service contact that the intervention seeks to reduce. The average cost of an ED attendance for a suspected seizure in 2007–13 was £123, with an additional £1651 being incurred if the visit resulted in hospital admission (which it did in around half of cases). 34
Microcosting of complex interventions remains rare. However, it is helpful to note that the NIHR-funded Self-Management education for adults with poorly controlled epILEpsy (SMILE) UK trial did include a microcosting of a self-management education for adults with poorly controlled epilepsy,208 where the 2014/15 price was estimated to be £56.05 per session. However, the SMILE intervention required multiple sessions, bringing the mean cost associated with the intervention to £180 (inflated to 2017/18 prices). By contrast, the SAFE intervention requires a single session at a comparable cost to the SMILE intervention (between £152 and £194 per delegate). The SAFE cost could be reduced if course attendance could be maximised to the intended level of ≈ 10 patient–SO dyads per course.
Negatives for feasibility of a definitive trial
The pilot revealed that only a small proportion of patients attending ED for a suspected seizure were eligible (10.6%) and willing (12.5%) to participate in a trial. A key reason for ineligibility was intellectual impairment. The leading reason for not wanting to take part was lack of interest. The low rate of uptake means that the other progression criterion stated at the project’s outset – that at least 20% of eligible patients agree to participate – was not satisfied.
Low participant uptake does not preclude a definitive trial from happening. It also may not reflect the acceptability of the intervention outside the trial context. In the RCT of the self-management intervention Dose Adjustment for Normal Eating for Type 1 diabetes, which is now offered by the NHS as part of usual care, only 17% of those eligible participated. 97 If the low recruitment rate seen in our pilot trial did reflect patient support or interest in the intervention outside a trial context, this would pose challenges to the intervention’s delivery and commission within the NHS since, on average, a single ED was generating only one eligible patient who was willing to take part for every month of attendances.
One reason we set the consent rate criterion was that, with such low participation, there is an increased likelihood that those who do and do not agree to take part may differ in important ways, thus limiting the generalisability of the trial’s findings. We compared eligible patients who did and did not agree to take part in the pilot RCT by age, sex and deprivation status. We found no obvious differences in age or deprivation. Females were slightly over-represented. The reason for this is not known (trials generally tend to under-represent females). When telephoning patients about participation in the pilot trial we intentionally attempted telephone calls at different times of the day to minimise the influence of work status on participation.
We do not know if differences existed between participants and non-participants on other indices because access to the wider medical records of non-participants was not ethically permissible. One indication that the pilot trial might not have been recruiting a representative sample was that a high proportion (75.5%) of the recruited patient participants reported having seen an epilepsy specialist within the 12 months prior to the ED attendance that led to their identification. National audit data28 indicate that most (≈ 65%) PWE attending ED have not done this.
The low uptake seen in the pilot RCT has implication for the likely cost of a definitive trial. In Chapter 6 we calculated that the sample size required for a definitive trial could be ≈ 680 PWE. This was informed by the pilot trial estimate that SAFE may have only a modest effect on participants’ subsequent ED use, reducing it on average by 0.3 ED visits over 12 months (compared with an increase of 0.4 ED visits for the TAU only arm). Based on the participant uptake rate seen in the pilot, 39 recruitment sites could be needed to secure the required sample. The mean cost of NIHR-funded (Health Technology Assessment programme) RCT trials is ≈ £1.3M. 209 The median number of recruitment sites in these trials was only 15. 131 In requiring 2.5 times more sites, a definitive trial of SAFE would be more expensive. This, and the potential unrepresentativeness of the sample likely to take part, could mean that a definitive RCT of SAFE would not represent value for money from a funder’s perspective.
It might not even be possible for a definitive trial to secure 39 EDs as recruitment sites. The requirement for them to release clinical personnel to the extent required in the pilot RCT to complete the screening work would not seem realistic considering how stretched most EDs already are in meeting their clinical priorities (e.g. Hassan et al. 210). Thirty-nine EDs equates to about half of type 1 EDs in England. A recent survey210 highlighted that 62% of ED consultants in the UK viewed their current job as unsustainable and 94% regularly worked in excess of their planned activities.
A final negative to emerge from the pilot trial was that securing the routine outcome data on ED use was not straightforward. For data applications, such as ours, that involve identifiable data and require linkage across a series of data sets, NHS Digital’s Service Level Agreements state that these should be processed within ≈ 2 months. 211 In practice, for our pilot RCT it was not until 9 months after the last participant follow-up assessment at 12 months had been completed that the raw outcome data were secured. This is despite our research team having communicated with the data provider before and during the pilot trial to ensure recruitment and consent approaches were consistent with their requirements. Substantial time from the research team was also required following application submission to respond to queries from the data provider. In addition, despite the current project being funded on the basis of external scientific reviews and its potential significance, the research team in one instance needed to appeal against a decision by the data provider midway through the application process to reject our data request, primarily because of unfounded concerns regarding the project’s scientific merit.
Possible modifications to address negatives for progression to definitive trial
Participant uptake
Recruitment of people with uncontrolled epilepsy into evaluative studies has previously been reported to be challenging. In the SMILE (UK) trial,208 PWE living in the London area who had experienced at least two seizures in the prior year were invited to a trial of self-management that had a 12-month follow-up period;212 37% of those invited took part. In a non-randomised trial of self-management for PWE who had attended ED on one or more occasions in the prior 12 months, the uptake rate was 26%. 128 Challenges can arise owing to the nature of the condition itself (e.g. unpredictable but frequent seizures) and its consequences (e.g. anxiety about travelling alone, inability to drive, memory impairment). A range of evidenced-based strategies were therefore used in the pilot RCT to try to maximise recruitment. These included participant vouchers, first-class postage for invitations, flexibility regarding when and where research appointments took place, the use of an ‘opt-out’ approach to contacting patients about the study, multiple contact attempts and all patient-facing documents being developed collaboratively with a patient and public involvement group. Despite this, a low recruitment rate was experienced.
The characteristics of the recruited patients offers one potential suggestion for the particularly low participant rate seen in the pilot RCT. Specifically, 82% of participants felt stigmatised by their epilepsy, 21.6% highly stigmatised. This is higher than among those with epilepsy generally, including those with uncontrolled seizures (63%). 212 Epilepsy, for reasons rooted deep in its history, has been called a ‘stigmatising condition par excellence,’213 and negative beliefs and lack of knowledge about the condition continue to be present. Consequently, PWE can feel ashamed of their diagnosis, guilty and reluctant to talk about it. 214 Those attending ED appear to be particularly at risk of this. This could be a barrier to participating in research. It is currently unclear how recruitment could be altered to mitigate the feeling of stigmatisation.
It is also worth considering what changes might be possible to who approaches patients regarding participation. Local ED clinicians invited PWE to participate in the pilot RCT. In trials it is usually preferable for a usual care provider with whom a patient has an established relationship to do the inviting. This was not considered ideal for the pilot RCT because national data indicated that most from the target population do not have a specialist usual care provider for their epilepsy (at least not one they have seen recently). Moreover, it was apparent that GPs were often not informed of ED visits made by PWE on their practice registers. Therefore, neither specialists nor GPs are positioned to reliably identify PWE for invite. Given the low participant uptake rate, a modification that a definitive trial could consider making would be for the EDs to identify ostensibly eligible patients from their records and then request that the identified patients’ GPs do the inviting. What impact this would have on uptake is not clear. It would require additional NHS support costs and additional approvals to permit EDs to communicate with general practices on what would be a research matter.
One of the leading reasons that patients gave for not wanting to take part was lack of interest. Some of this lack of interest might have arisen because of the time that had elapsed between the ED visit that led to their identification and them receiving the study invite (the median was 9 months). This could be addressed by shortening the time period between identification and invite, or, even more drastically, recruiting patients prospectively instead. The latter would have significant implications for the resources required by a definitive trial. In addition, the pragmatics of recruiting PWE directly from the ED requires clarification. For example, patients may often be in a post-ictal state and, furthermore, a stay in the ED is typically far shorter than a stay on a hospital ward.
We note that it is possible that the serial assessment of participants and the need for them to complete multiple secondary outcome questionnaires at the assessments might have been seen as overly burdensome by people considering the invitation to participate in the trial. It might also have negatively affected retention of the participants who were recruited. If a definitive trial committed itself to using routine ED data as the source of data for the primary outcome measure, that trial could theoretically reduce or even totally drop the use of the secondary assessment measures. This might increase uptake and improve retention. In considering how dramatic the improvements might be, it is perhaps worth noting that none of the participants who did take part in the trial and who completed the trial participation feedback questionnaire identified the questionnaire packs as being burdensome or as an area of the trial that required improvement.
Identifying eligible patients
Identifying eligible patients for the pilot RCT from ED attendance systems required substantial time and effort on behalf of the local principal investigators at the EDs. This was because of the nature of the condition, because the ED administrative systems did not code visits in a sufficiently granular way and because many of the data on patients that was needed to determine eligibility (e.g. presence of certain comorbidities) was recorded in an unstandardised way in non-searchable, free-text fields. Consequently, searches of the attendances systems were limited to simply identifying persons who had attended for a ‘suspected seizure’. The triage cards of these identified persons needed to be accessed and screened by local clinicians to find people suitable for invitation. This activity could not have been delegated to research staff, including research nurses, since it is not ethically permissible for a person outside the patient’s care team to access information on their medical history for the purposes of research without prior consent. 215
Since our pilot RCT took place, a new coding system for EDs – the Emergency Care Data Set216 – has been phased in. From the perspective of a definitive trial, it brings some advantages because it seeks to standardise and increase the granularity with which presentations and comorbidities are recorded. For example, a specific presentation code for seizure caused by a neurological condition now exists. 216 The system also asks for diagnosis to be recorded using detailed Systematised Nomenclature of Medicine (SNOMED) clinical terms; however, the fields ‘discharge diagnosis’ and ‘comorbidity’ in the Emergency Care Data Set are not mandatory. 217,218 Therefore, it remains to be seen how well completed these fields are before it is possible to conclude that the presence of the new system means that PWE can be more readily identified for a definitive trial, and demands on local sites reduced to a reasonable level.
Securing routine outcome data on emergency department use
For a definitive trial of SAFE to be well positioned to be funded and for it to be designed optimally, stakeholders would want to be confident that primary outcome data could be secured and a fairly precise estimate would be needed as to when the data would be available. Our pilot demonstrated that the provision of such data is not a given and that the timeline is hard to estimate. Bodies such as the NIHR have endorsed the use of routine data sources in clinical research. It is possible that, as routine data are requested more often for trials, NHS Digital’s application processes will be revised and become more suitable for the research environment. It is notable that our experience is not unique. The challenges in securing routine outcome data from NHS Digital were described in detail by Powell et al. 174 in 2017, and recommendations for improvements with the system were made.
Effect of the SAFE intervention
Efficacy was not the primary focus of our pilot RCT. No power calculation was completed and, with such a small sample, imbalance in pre-randomisation covariates between the treatment arms is possible. The estimated modest effect of SAFE on subsequent ED use – namely, reducing it from 2.1 visits over a 12-month period to 1.8 (compared with a slight increase in the TAU only arm from 3.0 to 3.4 visits) – was used to inform the sample size calculations for a definitive trial. Therefore, it is important to consider how realistic this estimate is because a larger effect would probably make a definitive trial cheaper (i.e. because fewer patient participants and thus recruitment sites would be required).
When the pilot RCT was being designed the empirical evidence on the ability of self-management interventions to change behaviour in PWE was positive but inconclusive. An updated systematic review by Luedke et al. 219 has since been published. It considered evidence from 13 randomised trials and two non-randomised trials of self-management interventions for epilepsy published before April 2018. The review concluded that interventions showed clinically important benefit on only a limited number of outcome measures. Interventions that primarily sought to increase knowledge changed self-management behaviours moderately, whereas interventions that included techniques such as cognitive behavioural therapy or problem-solving treatment improved quality of life only modestly. Overall, self-management interventions were not found to decrease seizure rates.
One RCT from the USA220 identified by Luedke et al. ’s review219 is particularly instructive because it is the only one to have also considered change in ED use. It focused on what its authors, Sajatovic et al. ,220 labelled an ‘at-risk’ population, defined as adults with established epilepsy who had had at least one negative health event within the past 6 months, be it a seizure, an accident, a self-harm attempt or an ED visit. The RCT compared the effects of an educational intervention, called ‘self-management for people with epilepsy and a history of negative health events’, to a wait list control. It comprised eight sessions, addressed myths surrounding epilepsy and introduced participants to key self-management tasks. A total of 120 patients were recruited and followed up for 6 months. The trial found no statistically significant differences between arms in terms of subsequent ED/hospitalisation use. For the intervention arm it reduced by 0.44 visits from 1.22 visits. For the wait list control arm it reduced by –1.26 visits from 2.4 visits.
The findings of the updated review suggest that the relatively small estimated effect of SAFE is in the region of what might be expected of a self-management intervention and thus the estimate from our pilot trial constitutes a reasonable basis for the sample size calculations for a definitive trial. That a self-management intervention reduces ED visits by a only small amount also makes sense from a clinical perspective because we now know that factors beyond low seizure confidence can influence whether or not an ED visit occurs. One is that studies with the ambulance service show that peripheral issues, such as performance targets and difficulties accessing information on patient medical history to determine normality or otherwise of the seizure presentation, can influence whether or not a person is conveyed to ED following an emergency call. 77,78
Progression, feasibility and optimal design of a definitive trial
Thabane et al. 132 provide a framework for judging whether or not to proceed to a definitive trial following a pilot. They describe the options as (1) ‘continue without modification’ (feasible as it is), (2) ‘continue without modifications, but monitor closely’ (feasible with close monitoring), (3) ‘continue, but modify the protocol’ (feasible with modifications) and (4) ‘stop’ (main study is not feasible). In satisfying only one of the pre-specified progression criteria, a definitive trial based on our pilot trial’s design is not feasible. On the basis of the discussion above, we do not consider that any options are currently available to modify the protocol in such a way for it to become feasible. Therefore, we recommend not proceeding to a definitive trial.
Alternative steps to the diffusion of the SAFE intervention
A definitive trial of SAFE is needed to determine its efficacy. This information would help commissioners understand with confidence whether or not SAFE should be scaled up and rolled out. As noted, our pilot indicates that such a trial is not currently feasible, although it might be in the future. In the meantime, the needs of many PWE who visit ED with respect to seizure first aid will continue to be unmet, with all the consequences that this has. Because it is not known when any other intervention will become available, an argument can be made for making SAFE available in some form to the epilepsy population now.
In support of this approach is that SAFE was rigorously produced, including the key information that health-care professional representatives reported that the target population needs to routinely receive (but for various reasons often do not). It received positive support from service users, with most who received it welcoming it. There were also no indications that SAFE had any serious adverse effects and it might reduce some ED use.
One way of making SAFE available now would be to convert its materials into a free online resource to which health-care professionals, such as epilepsy nurses and neurologists, and charities could direct interested people. The conversion could be in the form of a professionally produced video of a SAFE course that could be run with agreeable service users. To engage those viewing the online resource, interactive questions could be integrated into recordings.
The conversion would be a relatively straightforward process and allow the investment already made via this project to be capitalised on. To enable the conversion, only modest funding would be required to produce the website and maintain and update it over time. For the pilot RCT, a more basic website was developed for trial participants to allow them to download (rather than interact with) the course slides and videos of the recovery position. The costs of the former activity provide some guidance as to the sort of costs required. In 2017–18 the website domain cost the project £20.94 for 5 years and the hourly rate for an information technology (IT) technician to maintain the site was only £28.09.
It is likely that a full conversion of SAFE would be welcomed by many health-care professionals because specialist services are currently being asked to innovate, identify and care for those currently underserved, such as PWE who visit EDs. However, the workforce is small and can already struggle to achieve waiting time targets and respond to requests for help.
Sharing SAFE in the way described would also present new opportunities to evaluate SAFE’s effect with a potentially large sample over time, albeit in a less rigorous way than by a full RCT. For instance, by using a pre–post design, those viewing the materials could be asked to fill in a brief online questionnaire immediately before and after the intervention to provide a measure of the intervention’s effect on seizure first aid knowledge and confidence. The same people could be asked for consent for access to be given to routine data from NHS Digital on its use of ED during the 12 months before and after completion of the course.
Strengths and limitations
Intervention development
The intervention was co-produced. The process enabled us to access the unique perspectives of health-care professionals and service users in a non-tokenistic way. Clear, tangible changes to the intervention were made in response to their views. The APEASE framework116 provides criteria that need to be considered when considering the utility and potential of interventions. Our project has provided positive evidence for the SAFE intervention on a number of these criteria, namely acceptability, practicability, affordability and safety. SAFE is well positioned to be implemented in the NHS should this ultimately prove appropriate.
We anticipate that the description provided of the co-production process we adopted could also provide a useful template for the development of interventions by other groups. A concern of some researchers is that the process of engaging service users and other stakeholders will be unwieldy and it will be challenging to engage stakeholders. 221 To this point we note that this process is not one that should be entered into lightly because it requires careful planning, expertise, resources and ethics approval. It took 9 months to complete, with a team including four experienced academics, two at the professorial level. However, we would argue that the length of the feedback sessions and the quality of consideration given by the stakeholders goes some way to demonstrating how willing stakeholders can be to contribute to such processes. In terms of recruitment, we note that those with epilepsy can often have low self-esteem and confidence. 186,213 This meant that we needed to be mindful from the start of the need, when bringing people into this process, to clearly emphasise to participants what we were doing, why we wanted them involved and that it was their views, however critical, that we needed to hear.
Pilot trial
The pilot trial was completed in a rigorous manner in accordance with best practice guidelines and reported according to the CONSORT extension for randomised pilot and feasibility trial222 (see Report Supplementary Material 3). Randomisation was done remotely by computer and stratification factors and allocations concealed from those collecting baseline and follow-up data and analysing them. Patients were also recruited from NHS sites. Some RCTs, including Sajatovic et al. ’s trial,220 have focused recruitment on user group participants. People in such groups might differ in important ways from those who are not. We also used an opt-out method of recruitment whereby we contacted patients about the study unless they told us otherwise. The opt-in method, where the onus is on the patient to contact the research team to express their interest, was not considered ideal for a patient population that frequently reports memory problems and whose lives can be disrupted by episodic relapses of their condition. Another strength is that recruitment sites were in areas where social deprivation was high and epilepsy control poor and so similar to those where a definitive trial would probably need to focus recruitment.
We completed one of the few microcostings of a self-management intervention for epilepsy. We also described the content of the SAFE intervention in detail and independently monitored its fidelity and reported the results. A recent review of trials of psychosocial interventions for epilepsy found that only ≈ 5% of trials did this for ‘adherence’ and ≈ 2% for ‘competence’. 223 Indeed, we have also provided a rare worked example of how to conduct a fidelity assessment and the resources required. Medical Research Council publications224 note the importance of evaluating fidelity; however, minimal guidance is provided regarding how to do it. Intervention developers report that this is one reason why they fail to assess fidelity. 225
The pilot trial is not without potential weaknesses. First, despite the trial team identifying PWE for inclusion on the basis of them having at least one attendance at ED for epilepsy recorded and the patient, when questioned, reporting at least two visits in the 12 months prior to recruitment, ≈ 40% of these patients might not have met the inclusion criteria (according to the routine health data subsequently obtained). The inclusion of these people could affect the external validity of the trial and attenuate the estimated effect of SAFE because they had less opportunity for a reduction in ED use.
In addition, while 18 (69%) of the 26 patient participants randomised to SAFE took part with an informal carer, five (28%) of these did not ultimately attend an intervention session. This is comparable to experience in the SMILE (UK) trial. 208 Their non-attendance may have limited the effect of SAFE because carers can be key to decision-making when a seizure occurs.
Because a diagnostic code is often not recorded for ED visits, we asked NHS Digital to inform us how many ED visits, regardless of diagnosis, each patient had made during specific periods of time. Therefore, it is possible that not all of the visits reported for the participants were associated with epilepsy. UK data on how many ED visits a person with epilepsy has made for reasons other than epilepsy in a set period is not known. PWE are certainly more likely to have a comorbidity than the general population and so it is possible that they attend for other reasons.
When calculating the size of sample probably required for a definitive trial of SAFE, the pilot trial’s estimate of SAFE’s effect on ED use was used. We used the estimate because there is no consensus about what constitutes a minimally important clinical reduction in ED visits. We acknowledge that the estimate from the pilot trial might be lacking in precision133 and that basing the sample size calculations on a smaller or larger effect size would substantially change the required sample size for a definitive trial.
Implications for NHS service commissioning, policy and practice
-
The trial was not designed to determine SAFE’s efficacy. There remains limited evidence to justify the commissioning of such a service.
-
Some PWE are unknown to ambulatory care services and cannot be readily identified. Increasing the granularity with which attendances at EDs are coded could enable them to be readily identified, supported and involved in research.
-
Using routine data on ED could make trials less vulnerable to losses to follow-up and mean that they are not exposed to apparent recall bias. Stakeholders need to be given more assurances from those holding the data that it is likely to be provided and done so in a timely manner.
-
Given that SAFE is liked by service users, that it might reduce ED use in a small way and that it does not appear to have serious adverse effects, there is a case for converting it into an free online resource that people could be directed to in the short term to go some way to address the otherwise unmet seizure first aid training needs of PWE who visit EDs and their SOs.
Recommendations for research
-
A definitive SAFE trial, with its current design, should not be conducted because it is not feasible for the reasons given above. We cannot envisage any immediate changes to trial design that could make it feasible.
-
Research is required to understand how people from the target population can be better recruited. It is possible that the feelings of stigma apparent in the target population might be an important barrier to recruitment. If this is the case, it is important that recruitment methods are identified or developed to ensure that participants’ feelings of stigma do not compound the difficulties that they may already be facing by preventing high-quality research into their condition from being conducted.
-
Converting SAFE into a free online resource that people could be directed to could provide an opportunity for an alternative method of evaluating its effect on some of the primary and secondary outcome measures used in the pilot RCT. Via a pre–post design, people accessing the online resource could complete brief measures to assess change in seizure first aid confidence and skills. They could also be asked for consent to access routine data on their use of EDs in the 12 months before and after their viewing of the resource.
Acknowledgements
We thank the people who participated in this study and the local principal investigators (Dr Mark Buchanan, Dr Elizabeth MacCallum and Dr Jane McVicar) who helped identify potential participants for the trial. Other people who contributed to this work are Mr Mike Perry and Mrs Linda Perry (service user representatives), Mrs Jayne and Mr Sam Burton (service user representatives), Dr Adwoa Hughes-Morley (researcher), Mrs Juliet Bransgrove (ES), Dr Duncan Appelbe (website development/management) and Mrs Gail Moors (administrator). We also acknowledge the support we received from our study’s steering committee [Professor Alasdair Gray (chairperson), Professor Pete Bower, Dr Paul Cooper, Mr Mike Jackson, Ms Helen Coyle] and from the Mersey Region Epilepsy Association, Epilepsy Research UK, NeuroSupport, the Brain and Spine Foundation and the ES, all of which assisted with recruitment.
Contributions of authors
Adam Noble (https://orcid.org/0000-0002-8070-4352) (Senior Lecturer in Health Services Research) was chief investigator and led in the conception and design of the study, the supervision and co-ordination of the study, the interpretation of the data and, with Darlene Snape and Sarah Nevitt, the writing of the final report.
Sarah Nevitt (https://orcid.org/0000-0001-9988-2709) (Research Associate, Biostatistics) contributed to conducting the study, developed and conducted the statistical analysis of the quantitative data and helped write the final report.
Emily Holmes (https://orcid.org/0000-0002-0479-5336) (Research Fellow, Pharmacoeconomics) contributed to the design of the study, developed and conducted the statistical analysis of the health economic data and wrote up the health economics results for the final report.
Leone Ridsdale (https://orcid.org/0000-0002-2234-2859) (Professor of Neurology and General Practice) supported Adam Noble in the conception and design of the study, contributed to its conduct and reviewed the final report.
Myfanwy Morgan (https://orcid.org/0000-0001-5532-8941) (Professor of Medical Sociology) contributed to the design of study and its conduct, oversaw its qualitative aspects and reviewed the final report.
Catrin Tudur-Smith (https://orcid.org/0000-0003-3051-1445) (Professor in Medical Statistics) contributed to the design of study, supervised the statistical analysis of the quantitative data and reviewed the report.
Dyfrig Hughes (https://orcid.org/0000-0001-8247-7459) (Professor in Pharmacoeconomics) contributed to the design of the study, supervised the collection of health economic data and its analysis and reviewed the final report.
Steve Goodacre (https://orcid.org/0000-0003-0803-8444) (Professor of Emergency Medicine) contributed to the design of the research and its conduct and reviewed the final report.
Tony Marson (https://orcid.org/0000-0002-6861-8806) (Professor of Neurology) contributed to the design of the research and its conduct and reviewed the final report.
Darlene Snape (https://orcid.org/0000-0002-0787-3299) (Research Fellow, Health Services Research) was responsible for setting up the study, for participant recruitment and assessment and for analysis and interpretation of the qualitative findings and helped write the final report.
Publications
Noble AJ, Marson AG, Tudur-Smith C, Morgan M, Hughes DA, Goodacre S, et al. ‘Seizure First Aid Training’ for people with epilepsy who attend emergency departments, and their family and friends: study protocol for intervention development and a pilot randomised controlled trial. BMJ Open 2015;5:e009040.
Noble AJ, Snape D, Morgan M, Goodacre S, Marson A, Ridsdale L. Seizure first aid training for people with epilepsy attending emergency departments, and SOs. J Neurol Neurosurg Psychiat 2017;88:A25.
Snape DA, Morgan M, Ridsdale L, Goodacre S, Marson AG, Noble AJ. Developing and assessing the acceptability of an epilepsy first aid training intervention for patients who visit UK emergency departments: a multi-method study of patients and professionals. Epilepsy Behav 2017;68:177–85.
Snape D, Nevitt S, Tudur-Smith C, Goodacre S, Hughes DA, Morgan M, et al. Seizure first aid training for people with epilepsy who attend emergency departments and their family and friends: results from a pilot randomised controlled trial. Epilepsia 2018;59:S140.
Noble A, Snape D, Ridsdale L, Morgan M, Nevitt SJ, Goodacre S, Marson A. Assessing treatment fidelity within an epilepsy randomized controlled trial – seizure first aid training for people with epilepsy who visit emergency departments. Behav Neurol 2019;3:5048794.
Noble AJ, Snape D, Nevitt S, Holmes EAF, Morgan M, Tudur Smith C, et al. Seizure First Aid Training For people with Epilepsy (SAFE) frequently attending emergency departments and their significant others: results of a UK multi-centre randomised controlled pilot trial. BMJ Open 2020;10:e035516.
Presentations
Snape D, Morgan M, Ridsdale L, Goodacre S, Marson AG, Noble AJ. Developing and Assessing Acceptability of Seizure First-Aid Training for Emergency Department Users with Epilepsy. Presented at the Association of British Neurologists Conference, Liverpool, 3–5 May 2017.
Snape D, Nevitt S, Tudur-Smith C, Goodacre S, Hughes DA, Morgan M, et al. Seizure First Aid Training for People with Epilepsy who Attend Emergency Departments and their Family and Friends: Results from a Pilot Randomised Controlled Trial. Presented at the 13th European Conference on Epileptology, Vienna, 26–30 August 2018.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Longmore M, Wilkinson I, Baldwin A, Wallin E. Oxford Handbook of Clinical Medicine. Oxford: Oxford University Press; 2014.
- Smithson WH, Walker MC. ABC of Epilepsy. Chichester: Wiley-Blackwell; 2012.
- Ridsdale L, Charlton J, Ashworth M, Richardson MP, Gulliford MC. Epilepsy mortality and risk factors for death in epilepsy: a population-based study. Br J Gen Pract 2011;61:e271-8. https://doi.org/10.3399/bjgp11X572463.
- MacDonald BK, Cockerell OC, Sander JW, Shorvon SD. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain 2000;123:665-76. https://doi.org/10.1093/brain/123.4.665.
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475-82. https://doi.org/10.1111/epi.12550.
- Forsgren L, Beghi E, Oun A, Sillanpää M. The epidemiology of epilepsy in Europe – a systematic review. Eur J Neurol 2005;12:245-53. https://doi.org/10.1111/j.1468-1331.2004.00992.x.
- Aidan NGS, Bell GS, Johnson AL, Goodridge DM, Shorvon SD, Sander JW. The long-term risk of premature mortality in people with epilepsy. Brain 2011;134:388-95. https://doi.org/10.1093/brain/awq378.
- Ridsdale L. Avoiding premature death in epilepsy. BMJ 2015;350. https://doi.org/10.1136/bmj.h718.
- Moran NF, Poole K, Bell G, Solomon J, Kendall S, McCarthy M, et al. Epilepsy in the United Kingdom: seizure frequency and severity, anti-epileptic drug utilization and impact on life in 1652 people with epilepsy. Seizure 2004;13:425-33. https://doi.org/10.1016/j.seizure.2003.10.002.
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46:470-2. https://doi.org/10.1111/j.0013-9580.2005.66104.x.
- Räty LK, Wilde-Larsson BM. Patients’ perceptions of living with epilepsy: a phenomenographic study. J Clin Nurs 2011;20:1993-2002. https://doi.org/10.1111/j.1365-2702.2010.03572.x.
- Jacoby A, Snape D, Baker GA. Epilepsy and social identity: the stigma of a chronic neurological disorder. Lancet Neurol 2005;4:171-8. https://doi.org/10.1016/S1474-4422(05)70020-X.
- McLaughlin DP, Pachana NA, Mcfarland K. Stigma, seizure frequency and quality of life: the impact of epilepsy in late adulthood. Seizure 2008;17:281-7. https://doi.org/10.1016/j.seizure.2007.09.001.
- Taylor J, Baker GA, Jacoby A. Levels of epilepsy stigma in an incident population and associated factors. Epilepsy Behav 2011;21:255-60. https://doi.org/10.1016/j.yebeh.2011.04.002.
- Pugliatti M, Beghi E, Forsgren L, Ekman M, Sobocki P. Estimating the cost of epilepsy in Europe: a review with economic modeling. Epilepsia 2007;48:2224-33. https://doi.org/10.1111/j.1528-1167.2007.01251.x.
- Kitson A, Shorvon S. Clinical Standards Advisory Group . Services for Patients With Epilepsy: A Report of a CSAG Committee 2000.
- Jacoby A, Buck D, Baker G, McNamee P, Graham-Jones S, Chadwick D. Uptake and costs of care for epilepsy: findings from a U.K. regional study. Epilepsia 1998;39:776-86. https://doi.org/10.1111/j.1528-1157.1998.tb01164.x.
- Hart YM, Shorvon SD. The nature of epilepsy in the general population. II. Medical care. Epilepsy Res 1995;21:51-8. https://doi.org/10.1016/0920-1211(95)00008-X.
- Ryan J, Nash S, Lyndon J. Epilepsy in the accident and emergency department – developing a code of safe practice for adult patients. South East and South West Thames Accident and Emergency Specialty Sub-committees. J Accid Emerg Med 1998;15:237-43. https://doi.org/10.1136/emj.15.4.237.
- Pearson M, Marson T, Dixon P, Scott K. National Audit of Seizure Management in Hospitals: St. Elsewhere’s Hospital – Clinical Report: April 2014 2014. www.nashstudy.org.uk/Newsletters/St%20Elsewhere%27s%20Clinical%20Report%20NASH%202.pdf (accessed 24 April 2018).
- Pearson M, Marson T, Dixon P, Billington K. National Audit of Seizure Management in Hospitals: St. Elsewhere’s Hospital – Clinical Report: April 2012 2012. www.nashstudy.org.uk/Newsletters/St%20Elsewhere%27s%20NASH%20Report.pdf (accessed 24 April 2018).
- House of Commons Committee of Public Accounts . Services to People With Neurological Conditions: Progress Review 2016. https://publications.parliament.uk/pa/cm201516/cmselect/cmpubacc/502/502.pdf (accessed 24 April 2018).
- Reuber M, Hattingh L, Goulding PJ. Epileptological emergencies in accident and emergency: a survey at St James’s university hospital, Leeds. Seizure 2000;9:216-20. https://doi.org/10.1053/seiz.2000.0386.
- Bruce M, Griffiths C, Brock A, Majeed A. Trends in mortality and hospital admissions associated with epilepsy in England and Wales during the 1990s. Health Stat Q 2004;21:23-9.
- Bardsley M, Blunt I, Davies S, Dixon J. Is secondary preventive care improving? Observational study of 10-year trends in emergency admissions for conditions amenable to ambulatory care. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2012-002007.
- NHS Digital . CCG Outcomes Indicator Set – June 2013: Emergency Admissions 2013. https://digital.nhs.uk/data-and-information/publications/statistical/ccg-outcomes-indicator-set/june-2013/ccg-outcomes-indicator-set---june-2013 (accessed 4 August 2017).
- Noble AJ, Goldstein LH, Seed P, Glucksman E, Ridsdale L. Characteristics of people with epilepsy who attend emergency departments: prospective study of metropolitan hospital attendees. Epilepsia 2012;53:1820-8. https://doi.org/10.1111/j.1528-1167.2012.03586.x.
- Dixon PA, Kirkham JJ, Marson AG, Pearson MG. National Audit of Seizure management in Hospitals (NASH): results of the national audit of adult epilepsy in the UK. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2014-007325.
- Whiston S, Coyle B, Chappel D. Health Needs Assessment for Long Term Neurological Conditions in North East England. Stockton on Tees: North East Public Health Observatory; 2009.
- Dickson JM, Jacques R, Reuber M, Hick J, Campbell MJ, Morley R, et al. Emergency hospital care for adults with suspected seizures in the NHS in England 2007–2013: a cross-sectional study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-023352.
- Ridsdale L, Virdi C, Noble A, Morgan M. Explanations given by people with epilepsy for using emergency medical services: a qualitative study. Epilepsy Behav 2012;25:529-33. https://doi.org/10.1016/j.yebeh.2012.09.034.
- Noble AJ, Morgan M, Virdi C, Ridsdale L. A nurse-led self-management intervention for people who attend emergency departments with epilepsy: the patients’ view. J Neurol 2013;260:1022-30. https://doi.org/10.1007/s00415-012-6749-2.
- Moore L, Deehan A, Seed P, Jones R. Characteristics of frequent attenders in an emergency department: analysis of 1-year attendance data. Emerg Med J 2009;26:263-7. https://doi.org/10.1136/emj.2008.059428.
- Dickson JM, Taylor LH, Shewan J, Baldwin T, Grünewald RA, Reuber M. Cross-sectional study of the prehospital management of adult patients with a suspected seizure (EPIC1). BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010573.
- Allen L, Jones CT. Emergency department use of computed tomography in children with epilepsy and breakthrough seizure activity. J Child Neurol 2007;22:1099-101. https://doi.org/10.1177/0883073807306249.
- Limm EI, Fang X, Dendle C, Stuart RL, Egerton Warburton D. Half of all peripheral intravenous lines in an Australian tertiary emergency department are unused: pain with no gain?. Ann Emerg Med 2013;62:521-5. https://doi.org/10.1016/j.annemergmed.2013.02.022.
- Peterson CL, Walker C, Coleman H. ‘I hate wasting the hospital’s time’: experiences of emergency department admissions of Australian people with epilepsy. Epilepsy Behav 2019;90:228-32. https://doi.org/10.1016/j.yebeh.2018.11.018.
- Craig J, Patterson V, Rocke L, Jamison J. Accident and emergency neurology: time for a reappraisal?. Health Trends 1997;29:89-91.
- O’Cathain A, Knowles E, Turner J, Maheswaran R, Goodacre S, Hirst E, et al. Explaining variation in emergency admissions: a mixed-methods study of emergency and urgent care systems. Health Serv Deliv Res n.d.;2. https://doi.org/10.3310/hsdr02480.
- Bautista RE, Glen ET, Wludyka PS, Shetty NK. Factors associated with utilization of healthcare resources among epilepsy patients. Epilepsy Res 2008;79:120-9. https://doi.org/10.1016/j.eplepsyres.2008.01.003.
- Public Health England . The NHS Atlas of Variation in Healthcare: November 2010 2010. https://fingertips.phe.org.uk/documents/Atlas_2010%20Compendium.pdf (accessed 25 April 2018).
- Public Health England . The NHS Atlas of Variation in Healthcare: November 2011 2011. https://fingertips.phe.org.uk/documents/Atlas_2011%20Compendium.pdf (accessed 25 April 2018).
- Sajatovic M, Welter E, Tatsuoka C, Perzynski AT, Einstadter D. Electronic medical record analysis of emergency room visits and hospitalizations in individuals with epilepsy and mental illness comorbidity. Epilepsy Behav 2015;50:55-60. https://doi.org/10.1016/j.yebeh.2015.05.016.
- Public Health England . The NHS Atlas of Variation in Healthcare: November 2015 2015. https://fingertips.phe.org.uk/documents/Atlas_2015%20Compendium.pdf (accessed 25 April 2018).
- Allard J, Shankar R, Henley W, Brown A, McLean B, Jadav M, et al. Frequency and factors associated with emergency department attendance for people with epilepsy in a rural UK population. Epilepsy Behav 2017;68:192-5. https://doi.org/10.1016/j.yebeh.2017.01.017.
- Ashworth M, Seed P, Armstrong D, Durbaba S, Jones R. The relationship between social deprivation and the quality of primary care: a national survey using indicators from the UK Quality and Outcomes Framework. Br J Gen Pract 2007;57:441-8.
- Shohet C, Yelloly J, Bingham P, Lyratzopoulos G. The association between the quality of epilepsy management in primary care, general practice population deprivation status and epilepsy-related emergency hospitalisations. Seizure 2007;16:351-5. https://doi.org/10.1016/j.seizure.2007.02.005.
- Morgan CL, Ahmed Z, Kerr MP. Social deprivation and prevalence of epilepsy and associated health usage. J Neurol Neurosurg Psychiatry 2000;69:13-7. https://doi.org/10.1136/jnnp.69.1.13.
- National Institute for Health and Care Excellence (NICE) . Epilepsies: Diagnosis and Management. Clinical Guideline [CG137] 2012. www.nice.org.uk/guidance/cg137 (accessed 21 August 2019).
- Grainger R, Pearson M, Dixon P, Devonport E, Timoney M, Bodger K, et al. Referral patterns after a seizure admission in an English region: an opportunity for effective intervention? An observational study of routine hospital data. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2015-010100.
- House of Commons Committee of Public Accounts . Financial Sustainability of the NHS 2017. http://publications.parliament.uk/pa/cm201617/cmselect/cmpubacc/887/887.pdf (accessed 24 April 2018).
- Anandaciva S. Nine Things We Learnt About Provider Finances in 2016 17 2017. www.kingsfund.org.uk/publications/nine-things-NHS-provider-finances-2016-17 (accessed 24 April 2018).
- Department of Health and Social Care (DHSC) . The NHS Plan: A Plan for Investment, a Plan for Reform 2000.
- NHS England . Five Year Forward View 2014. www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 8 August 2017).
- NHS England . Next Step on the NHS Five Year Forward View 2017. www.england.nhs.uk/wp-content/uploads/2017/03/next-steps-on-the-nhs-five-year-forward-view.pdf (accessed 8 August 2017).
- NHS Digital . NHS Outcomes Framework Indicators – February 2017 Release 2017. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-outcomes-framework/february-2017/nhs-outcomes-framework-indicators---february-2017-release (accessed 8 August 2017).
- National Institute for Health Research (NIHR) . Glossary 2012. www.nihr.ac.uk/glossary (accessed 21 August 2017).
- Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns 2002;48:177-87. https://doi.org/10.1016/S0738-3991(02)00032-0.
- Dilorio C, Faherty B, Manteuffel B. The development and testing of an instrument to measure self-efficacy in individuals with epilepsy. J Neurosci Nurs 1992;24:9-13. https://doi.org/10.1097/01376517-199202000-00004.
- Jarvie S. Self Perception and Psychosocial Functioning in People With Intractable Epilepsy 1993.
- Wagner AK, Keller SD, Kosinski M, Baker GA, Jacoby A, Hsu MA, et al. Advances in methods for assessing the impact of epilepsy and antiepileptic drug therapy on patients’ health-related quality of life. Qual Life Res 1995;4:115-34. https://doi.org/10.1007/BF01833606.
- Jacoby A. Felt versus enacted stigma: a concept revisited. Evidence from a study of people with epilepsy in remission. Soc Sci Med 1994;38:269-74. https://doi.org/10.1016/0277-9536(94)90396-4.
- Morgan GA, Harmon RJ, Maslin-Cole CA. Mastery motivation: definition and measurement. Early Edu Dev 1990;1:318-39. https://doi.org/10.1207/s15566935eed0105_1.
- Au A, Chan F, Li K, Leung P, Li P, Chan J. Cognitive-behavioral group treatment program for adults with epilepsy in Hong Kong. Epilepsy Behav 2003;4:441-6. https://doi.org/10.1016/S1525-5050(03)00149-5.
- Corbin JM, Strauss A. Unending Work and Care: Managing Chronic Illness at Home. San Francisco, CA: Jossey-Bass; 1988.
- Deveugele M, Derese A, van den Brink-Muinen A, Bensing J, De Maeseneer J. Consultation length in general practice: cross sectional study in six European countries. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7362.472.
- Royal College of Physicians (RCP) . Consultant Physicians Working With Patients – Revised 5th Edition 2013. www.rcplondon.ac.uk/projects/outputs/consultant-physicians-working-patients-revised-5th-edition (accessed 29 August 2018).
- Gilliam F, Penovich PE, Eagan CA, Stern JM, Labiner DM, Onofrey M, et al. Conversations between community-based neurologists and patients with epilepsy: results of an observational linguistic study. Epilepsy Behav 2009;16:315-20. https://doi.org/10.1016/j.yebeh.2009.07.039.
- Ridsdale L, Morgan M, O’Connor C. Promoting self-care in epilepsy: the views of patients on the advice they had received from specialists, family doctors and an epilepsy nurse. Patient Educ Couns 1999;37:43-7. https://doi.org/10.1016/S0738-3991(98)00094-9.
- Long L, Reeves AL, Moore JL, Roach J, Pickering CT. An assessment of epilepsy patients’ knowledge of their disorder. Epilepsia 2000;41:727-31. https://doi.org/10.1111/j.1528-1157.2000.tb00235.x.
- Ridsdale L, Kwan I, Cryer C. The effect of a special nurse on patients’ knowledge of epilepsy and their emotional state. Epilepsy Evaluation Care Group. Br J Gen Pract 1999;49:285-9.
- Ridsdale L, Kwan I, Cryer C. Newly diagnosed epilepsy: can nurse specialists help? A randomized controlled trial. Epilepsy Care Evaluation Group. Epilepsia 2000;41:1014-19. https://doi.org/10.1111/j.1528-1157.2000.tb00287.x.
- Walker ER, Bamps Y, Burdett A, Rothkopf J, Diiorio C. Social support for self-management behaviors among people with epilepsy: a content analysis of the WebEase program. Epilepsy Behav 2012;23:285-90. https://doi.org/10.1016/j.yebeh.2012.01.006.
- Swanborough N. Not Always A&Amp;E 2014. www.epilepsysociety.org.uk/not-always-a%26e-07-02-2014#.Xt9Vvi2ZMkg (accessed 27 August 2019).
- Hunt N, Touquet VL. Known epileptic patients brought to the accident and emergency department. J R Coll Gen Pract 1986;36:224-5.
- BBC News . Health: public ‘ignorant’ about epilepsy. BBC News 1999. http://news.bbc.co.uk/1/hi/health/339872.stm (accessed 27 August 2019).
- Noble AJ, Snape D, Goodacre S, Jackson M, Sherratt FC, Pearson M, et al. Qualitative study of paramedics’ experiences of managing seizures: a national perspective from England. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-014022.
- Burrell L, Noble A, Ridsdale L. Decision-making by ambulance clinicians in London when managing patients with epilepsy: a qualitative study. Emerg Med J 2013;30:236-40. https://doi.org/10.1136/emermed-2011-200388.
- Ridsdale L. The social causes of inequality in epilepsy and developing a rehabilitation strategy: a UK-based analysis. Epilepsia 2009;50:2175-9. https://doi.org/10.1111/j.1528-1167.2009.02150.x.
- NHS England . Patient Activation: People’s Ability to Manage Their Own Health and Wellbeing 2018.
- Foster G, Taylor SJ, Eldridge SE, Ramsay J, Griffiths CJ. Self-management education programmes by lay leaders for people with chronic conditions. Cochrane Database Syst Rev 2007;4. https://doi.org/10.1002/14651858.CD005108.pub2.
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trials. BMJ 2008;336:491-5. https://doi.org/10.1136/bmj.39474.922025.BE.
- Deakin TA, Cade JE, Williams R, Greenwood DC. Structured patient education: the diabetes X-PERT programme makes a difference. Diabet Med 2006;23:944-54. https://doi.org/10.1111/j.1464-5491.2006.01906.x.
- Barlow JH, Turner AP, Wright CC. A randomized controlled study of the Arthritis Self-Management Programme in the UK. Health Educ Res 2000;15:665-80. https://doi.org/10.1093/her/15.6.665.
- Buszewicz M, Rait G, Griffin M, Nazareth I, Patel A, Atkinson A, et al. Self management of arthritis in primary care: randomised controlled trial. BMJ 2006;333. https://doi.org/10.1136/bmj.38965.375718.80.
- Willis E. The making of expert patients: the role of online health communities in arthritis self-management. J Health Psychol 2014;19:1613-25. https://doi.org/10.1177/1359105313496446.
- Tieffenberg JA, Wood EI, Alonso A, Tossutti MS, Vicente MF. A randomized field trial of ACINDES: a child-centered training model for children with chronic illnesses (asthma and epilepsy). J Urban Health 2000;77:280-97. https://doi.org/10.1007/BF02390539.
- Tapp S, Lasserson TJ, Rowe B. Education interventions for adults who attend the emergency room for acute asthma. Cochrane Database Syst Rev 2007;3. https://doi.org/10.1002/14651858.CD003000.pub2.
- Boyd M, Lasserson TJ, McKean MC, Gibson PG, Ducharme FM, Haby M. Interventions for educating children who are at risk of asthma-related emergency department attendance. Cochrane Database Syst Rev 2009;2. https://doi.org/10.1002/14651858.CD001290.pub2.
- Institute of Medicine . Priority Areas for National Action: Transforming Health Care Quality 2003.
- Brady TJ, Murphy L, O’Colmain BJ, Beauchesne D, Daniels B, Greenberg M, et al. A meta-analysis of health status, health behaviors, and health care utilization outcomes of the Chronic Disease Self-Management Program. Prev Chronic Dis 2013;10. https://doi.org/10.5888/pcd10.120112.
- Centers for Disease Control and Prevention (CDC) . Asthma Facts: CDC’s National Asthma Control Program Grantees 2013. austinpureair.com/uploadimage/139828838266969.pdf (accessed 29 June 2019).
- National Association of County and City Health Officials . Diabetes Self-Management Education and Training: Fact Sheet – 2013 2013. http://archived.naccho.org/topics/HPDP/diabetes/resources/upload/factsheet_diabetes_aug2013_v2.pdf (accessed 29 June 2019).
- Brady TJ, Anderson LA, Kobau R. Chronic disease self-management support: public health perspectives. Front Public Health 2014;2. https://doi.org/10.3389/fpubh.2014.00234.
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) . National Diabetes Education Program: National Diabetes Survey 2016 2016. www.niddk.nih.gov/health-information/health-statistics/diabetes-statistics/ndep-national-diabetes-survey (accessed 29 June 2019).
- Centers for Disease Control and Prevention (CDC) . Self-Management Education Workshops 2019. www.cdc.gov/arthritis/interventions/self_manage.htm (accessed 29 June 2019).
- DAFNE Study Group . Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002;325. https://doi.org/10.1136/bmj.325.7367.746.
- Harris SM, Shah P, Mulnier H, Healey A, Thomas SM, Amiel SA, et al. Factors influencing attendance at structured education for type 1 diabetes in south London. Diabet Med 2017;34:828-33. https://doi.org/10.1111/dme.13333.
- Bradley PM, Lindsay B, Fleeman N. Care delivery and self management strategies for adults with epilepsy. Cochrane Database Syst Rev 2016;2. https://doi.org/10.1002/14651858.CD006244.pub3.
- Helgeson DC, Mittan R, Tan SY, Chayasirisobhon S. Sepulveda Epilepsy Education: the efficacy of a psychoeducational treatment program in treating medical and psychosocial aspects of epilepsy. Epilepsia 1990;31:75-82. https://doi.org/10.1111/j.1528-1157.1990.tb05363.x.
- May TW, Pfäfflin M. The efficacy of an educational treatment program for patients with epilepsy (MOSES): results of a controlled, randomized study. Modular Service Package Epilepsy. Epilepsia 2002;43:539-49. https://doi.org/10.1046/j.1528-1157.2002.23801.x.
- DiIorio C, Bamps Y, Walker ER, Escoffery C. Results of a research study evaluating WebEase, an online epilepsy self-management program. Epilepsy Behav 2011;22:469-74. https://doi.org/10.1016/j.yebeh.2011.07.030.
- Aliasgharpour M, Dehgahn Nayeri N, Yadegary MA, Haghani H. Effects of an educational program on self-management in patients with epilepsy. Seizure 2013;22:48-52. https://doi.org/10.1016/j.seizure.2012.10.005.
- Lindsay B, Bradley PM. Care delivery and self-management strategies for children with epilepsy. Cochrane Database Syst Rev 2010;12. https://doi.org/10.1002/14651858.CD006245.pub2.
- Lewis MA, Salas I, de la Sota A, Chiofalo N, Leake B. Randomized trial of a program to enhance the competencies of children with epilepsy. Epilepsia 1990;31:101-9. https://doi.org/10.1111/j.1528-1157.1990.tb05367.x.
- Lewis MA, Hatton CL, Salas I, Leake B, Chiofalo N. Impact of the Children’s Epilepsy Program on parents. Epilepsia 1991;32:365-74. https://doi.org/10.1111/j.1528-1157.1991.tb04665.x.
- Gibson PG, Powell H, Coughlan J, Wilson AJ, Hensley MJ, Abramson M, et al. Limited (information only) patient education programs for adults with asthma. Cochrane Database Syst Rev 2002;2. https://doi.org/10.1002/14651858.CD001005.
- Michaelis R, Tang V, Wagner JL, Modi AC, LaFrance WC, Goldstein LH, et al. Psychological treatments for people with epilepsy. Cochrane Database Syst Rev 2017;10. https://doi.org/10.1002/14651858.CD012081.pub2.
- Ramaratnam S, Baker GA, Goldstein LH. Psychological treatments for epilepsy. Cochrane Database Syst Rev 2008;3. https://doi.org/10.1002/14651858.CD002029.pub3.
- Bradley PM, Lindsay B. Care delivery and self-management strategies for adults with epilepsy. Cochrane Database Syst Rev 2008;1. https://doi.org/10.1002/14651858.CD006244.pub2.
- Savovic J, Turner RM, Mawdsley D, Jones HE, Beynon R, Higgins JPT, et al. Association between risk-of-bias assessments and results of randomized trials in Cochrane reviews: the ROBES meta-epidemiologic study. Am J Epidemiol 2018;187:1113-22. https://doi.org/10.1093/aje/kwx344.
- Department of Health and Social Care (DHSC) . The Expert Patient: A New Approach to Chronic Disease Management for the 21st Century 2001.
- National Clinical Guideline Centre . The Epilepsies: The Diagnosis and Management of the Epilepsies in Adults and Children in Primary and Secondary Care 2011.
- NHS England . Living With Epilepsy 2017. www.nhs.uk/conditions/epilepsy/pages/living-with.aspx (accessed 14 September 2017).
- NHS England . Transforming Urgent and Emergency Care Services in England: Urgent and Emergency Care Review – End of Phase 1 Report 2031. www.nhs.uk/NHSEngland/keogh-review/Documents/UECR.Ph1Report.FV.pdf (accessed 23 August 2019).
- Michie S, West R, Campbell R, Brown J, Gainforth H. ABC of Behaviour Change Theories: An Essential Resource for Researchers, Policy Makers and Practitioners. London: Silverback Publishing; 2014.
- Morrish PK. Inadequate neurology services undermine patient care in the UK. BMJ 2015;350. https://doi.org/10.1136/bmj.h3284.
- Royal College of Physicians (RCP) . Local Adult Neurology Services for the Next Decade: Report of a Working Party 2011. www.mstrust.org.uk/sites/default/files/files/Local%20adult%20neurology%20services%20for%20the%20next%20decade.pdf (accessed 29 June 2019).
- Epilepsy Action . Best Care: The Value of Epilepsy Specialist Nurses 2010.
- Epilepsy Action . A Critical Time for Epilepsy in England: A Study of Epilepsy Service Provision in England 2013.
- Medical Research Council (MRC) . A Framework for the Development and Evaluation of RCTs for Complex Interventions to Improve Health 2000.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. https://doi.org/10.1136/bmj.321.7262.694.
- Jain P, Patterson VH, Morrow JI. What people with epilepsy want from a hospital clinic. Seizure 1993;2:75-8. https://doi.org/10.1016/S1059-1311(05)80106-2.
- Davis KL, Candrilli SD, Edin HM. Prevalence and cost of nonadherence with antiepileptic drugs in an adult managed care population. Epilepsia 2008;49:446-54. https://doi.org/10.1111/j.1528-1167.2007.01414.x.
- Moran N, Poole K, Bell G, Solomon J, Kendall S, McCarthy M, et al. NHS services for epilepsy from the patient’s perspective: a survey of primary, secondary and tertiary care access throughout the UK. Seizure 2000;9:559-65. https://doi.org/10.1053/seiz.2000.0451.
- Fraser RT, Johnson EK, Lashley S, Barber J, Chaytor N, Miller JW, et al. PACES in epilepsy: results of a self-management randomized controlled trial. Epilepsia 2015;56:1264-74. https://doi.org/10.1111/epi.13052.
- Laybourne AH, Morgan M, Watkins SH, Goldstein LH. Self-management for people with poorly control-led epilepsy: participants’ views of the UK self-management in epilepsy (SMILE) program. Epil 2015;52:159-64. https://doi.org/10.1016/j.yebeh.2015.08.023.
- Noble AJ, McCrone P, Seed PT, Goldstein LH, Ridsdale L. Clinical- and cost-effectiveness of a nurse led self-management intervention to reduce emergency visits by people with epilepsy. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0090789.
- Fraser RT, Johnson EK, Miller JW, Temkin N, Barber J, Caylor L, et al. Managing epilepsy well: self-management needs assessment. Epilepsy Behav 2011;20:291-8. https://doi.org/10.1016/j.yebeh.2010.10.010.
- Donetto S, Pierri P, Tsianakas V, Robert G. Experience-based co-design and healthcare improvement: realising participatory design in the public sector. Design J 2015;18:227-48. https://doi.org/10.2752/175630615X14212498964312.
- Walters SJ, Bonacho Dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment programme. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015276.
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10. https://doi.org/10.1186/1471-2288-10-1.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. https://doi.org/10.1111/j.2002.384.doc.x.
- Kralj-Hans I, Goldstein LH, Noble AJ, Landau S, Magill N, McCrone P, et al. Self-Management education for adults with poorly controlled epILEpsy [SMILE (UK)]: a randomised controlled trial protocol. BMC Neurol 2014;14. https://doi.org/10.1186/1471-2377-14-69.
- Williams B, Skinner J, Dowell J, Roberts R, Crombie I, Davis J. General practitioners’ reasons for the failure of a randomized controlled trial (the TIGER trial) to implement epilepsy guidelines in primary care. Epilepsia 2007;48:1275-82. https://doi.org/10.1111/j.1528-1167.2007.01057.x.
- Patton MQ. Qualitative Research and Evaluation Methods. Thousand Oaks, CA: SAGE Publications Ltd; 2002.
- Cresswell JW, Plano Clark VL. Designing and Conducting Mixed Method Research. Thousand Oaks, CA: SAGE Publications Ltd; 2011.
- Bernard HR. Research Methods in Anthropology: Qualitative and Quantitative Approaches. Walnut Creek, CA: Alta Mira Press; 2002.
- Spradley JP. The Ethnographic Interview. New York, NY: Holt, Rinehart & Winston; 1979.
- Duffield C. The Delphi technique: a comparison of results obtained using two expert panels. Int J Nurs Stud 1993;30:227-37. https://doi.org/10.1016/0020-7489(93)90033-q.
- Lemmer B. Successive surveys of an expert panel: research in decision making with health visitors. J Adv Nurs 1998;27:538-45. https://doi.org/10.1046/j.1365-2648.1998.00544.x.
- INVOLVE . Briefing Notes for Researchers: Involving the Public in NHS, Public Health and Social Care Research 2012.
- NHS England . What To Do If Someone Has a Seizure (Fit) 2014. www.nhs.uk/Livewell/Epilepsy/Pages/Ifyouseeaseizure.aspx (accessed 19 September 2017).
- Epilepsy Action . What To Do When Someone Has a Seizure 2016. www.epilepsy.org.uk/info/firstaid (accessed 7 August 2016).
- Epilepsy Society . When To Dial 999 2012. www.epilepsysociety.org.uk/AboutEpilepsy/Firstaid/Whentodial999 (accessed 8 January 2016).
- Britten N. Qualitative interviews in medical research. BMJ 1995;311:251-3. https://doi.org/10.1136/bmj.311.6999.251.
- Mishler EG. Research Interviewing Context and Narrative. London: Harvard University Press; 1986.
- Steele CM, Berkowitz L. Advances in Experimental Social Psychology. New York, NY: Academic Press; 1988.
- Epton T, Harris PR. Self-affirmation promotes health behavior change. Health Psychol 2008;27:746-52. https://doi.org/10.1037/0278-6133.27.6.746.
- Reed MB, Aspinwall MG. Self-affirmation reduces biased processing of health-risk information. Motiv 1998;22:99-132. https://doi.org/10.1023/A:1021463221281.
- Armitage CJ, Rowe R. Testing multiple means of self-affirmation. Br J Psychol 2011;102:535-45. https://doi.org/10.1111/j.2044-8295.2010.02014.x.
- Schüz N, Schüz B, Eid M. When risk communication backfires: randomized controlled trial on self-affirmation and reactance to personalized risk feedback in high-risk individuals. Health Psychol 2013;32:561-70. https://doi.org/10.1037/a0029887.
- Cohen GL, Sherman DK. The psychology of change: self-affirmation and social psychological intervention. Annu Rev Psychol 2014;65:333-71. https://doi.org/10.1146/annurev-psych-010213-115137.
- Bazeley P, Richards L. The NVivo Qualitative Project Book. London: SAGE Publications Ltd; 2000.
- Skinner TC, Carey ME, Cradock S, Dallosso HM, Daly H, Davies MJ, et al. ‘Educator talk’ and patient change: some insights from the DESMOND (Diabetes Education and Self Management for Ongoing and Newly Diagnosed) randomized controlled trial. Diabet Med 2008;25:1117-20. https://doi.org/10.1111/j.1464-5491.2008.02492.x.
- Medical Research Council (MRC) . Developing and Evaluating Complex Interventions n.d. https://mrc.ukri.org/documents/pdf/complex-interventions-guidance (accessed 6 January 2016).
- Möhler R, Köpke S, Meyer G. Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2). Trials 2015;16. https://doi.org/10.1186/s13063-015-0709-y.
- Public Health England (PHE) . Local Health Profiles 2015: Liverpool 2015. https://fingertips.phe.org.uk/profile/health-profiles/data#page/1/gid/1938133217/pat/6/par/E12000002/ati/202/are/E08000012/iid/91872/age/1/sex/4/cid/4/page-options/cin-ci-4_ine-vo-0_ine-ct-9_ine-yo-3: 2010:-1:-1_ine-pt-0_eng-vo-0_eng-do-0_car-do-0_ovw-do-0 (accessed 10 August 2020).
- Public Health England (PHE) . Local Health Profiles 2015: Wirral 2015. https://fingertips.phe.org.uk/profile/health-profiles/data#page/1/gid/1938133217/pat/6/par/E12000002/ati/202/are/E08000015/iid/91872/age/1/sex/4/cid/4/page-options/cin-ci-4_ine-vo-0_ine-ct-9_ine-yo-3: 2010:-1:-1_ine-pt-0_eng-vo-0_eng-do-0_car-do-0_ovw-do-0 (accessed 10 August 2020).
- NHS Digital . Quality and Outcomes Framework (QOF) 2012–13 2013. https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/quality-and-outcomes-framework-2012-13 (accessed 10 August 2020).
- NHS Digital . Hospital Accident and Emergency Activity: Supporting Information 2017-18 2018. https://files.digital.nhs.uk/8B/94BB9E/AE1718_supporting_information.pdf (accessed 27 August 2019).
- Kahan BC, Morris TP. Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis. BMJ 2012;345. https://doi.org/10.1136/bmj.e5840.
- Mittan R, Whitman S, Hermann B. Psychopathology in Epilepsy: Social Dimensions. New York, NY: Oxford University Press; 1986.
- Martiniuk AL, Speechley KN, Secco M, Karen Campbell M. Development and psychometric properties of the Thinking about Epilepsy questionnaire assessing children’s knowledge and attitudes about epilepsy. Epilepsy Behav 2007;10:595-603. https://doi.org/10.1016/j.yebeh.2007.01.011.
- Shore CP, Austin JK, Dunn DW. Maternal adaptation to a child’s epilepsy. Epilepsy Behav 2004;5:557-68. https://doi.org/10.1016/j.yebeh.2004.04.015.
- Cramer JA, Perrine K, Devinsky O, Bryant-Comstock L, Meador K, Hermann B. Development and cross-cultural translations of a 31-item quality of life in epilepsy inventory. Epilepsia 1998;39:81-8. https://doi.org/10.1111/j.1528-1157.1998.tb01278.x.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. https://doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Snaith P. What do depression rating scales measure?. Br J Psychiatry 1993;163:293-8. https://doi.org/10.1192/bjp.163.3.293.
- Thapar A, Kerr M, Harold G. Stress, anxiety, depression, and epilepsy: investigating the relationship between psychological factors and seizures. Epilepsy Behav 2009;14:134-40. https://doi.org/10.1016/j.yebeh.2008.09.004.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55. https://doi.org/10.1093/geront/20.6.649.
- Beecham J, Knapp M, Thornicroft G, Brewin C, Wing J. Measuring Mental Health Needs. London: Gaskell; 1992.
- Williams A. The Role of the EUROQOL Instrument in QALY Calculations. York: Centre for Health Economics, University of York; 1995.
- Smyth RM, Duley L, Jacoby A, Elbourne D. Women’s experiences of participating in the Magpie Trial: a postal survey in the United Kingdom. Birth 2009;36:220-9. https://doi.org/10.1111/j.1523-536X.2009.00326.x.
- Powell GA, Bonnett LJ, Tudur-Smith C, Hughes DA, Williamson PR, Marson AG. Using routinely recorded data in the UK to assess outcomes in a randomised controlled trial: The Trials of Access. Trials 2017;18. https://doi.org/10.1186/s13063-017-2135-9.
- Noble AJ, Marson AG. Which outcomes should we measure in adult epilepsy trials? The views of people with epilepsy and informal carers. Epilepsy Behav 2016;59:105-10. https://doi.org/10.1016/j.yebeh.2016.01.036.
- NHS Digital . Accident and Emergency Attendances in England 2012–13 2014. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-accident--emergency-activity/accident-and-emergency-attendances-in-england-2012-13 (accessed 4 August 2019).
- Bhatt H, Matharu MS, Henderson K, Greenwood R. An audit of first seizures presenting to an accident and emergency department. Seizure 2005;14:58-61. https://doi.org/10.1016/j.seizure.2004.10.003.
- Ridsdale L, McCrone P, Morgan M, Goldstein L, Seed P, Noble A. Can an epilepsy nurse specialist-led self-management intervention reduce attendance at emergency departments and promote well-being for people with severe epilepsy? A non-randomised trial with a nested qualitative phase. Health Serv Deliv Res 2013;1. https://doi.org/10.3310/hsdr01090.
- Brueton VC, Tierney JF, Stenning S, Meredith S, Harding S, Nazareth I, et al. Strategies to improve retention in randomised trials: a Cochrane systematic review and meta-analysis. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-003821.
- Vuong Q. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica 1989;57:307-33. https://doi.org/10.2307/1912557.
- Lee EC, Whitehead AL, Jacques RM, Julious SA. The statistical interpretation of pilot trials: should significance thresholds be reconsidered?. BMC Med Res Methodol 2014;14. https://doi.org/10.1186/1471-2288-14-41.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10. https://doi.org/10.1016/S0140-6736(86)90837-8.
- Keene ON, Jones MR, Lane PW, Anderson J. Analysis of exacerbation rates in asthma and chronic obstructive pulmonary disease: example from the TRISTAN study. Pharm Stat 2007;6:89-97. https://doi.org/10.1002/pst.250.
- Cramer JA, Van Hammée G. N132 Study Group . Maintenance of improvement in health-related quality of life during long-term treatment with levetiracetam. Epilepsy Behav 2003;4:118-23. https://doi.org/10.1016/S1525-5050(03)00004-0.
- Bell ML, Fairclough DL, Fiero MH, Butow PN. Handling missing items in the Hospital Anxiety and Depression Scale (HADS): a simulation study. BMC Res Notes 2016;9. https://doi.org/10.1186/s13104-016-2284-z.
- Austin JK, Dunn DW, Johnson CS, Perkins SM. Behavioral issues involving children and adolescents with epilepsy and the impact of their families: recent research data. Epilepsy Behav 2004;5:33-41. https://doi.org/10.1016/j.yebeh.2004.06.014.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Coffman JM, Cabana MD, Halpin HA, Yelin EH. Effects of asthma education on children’s use of acute care services: a meta-analysis. Pediatrics 2008;121:575-86. https://doi.org/10.1542/peds.2007-0113.
- Wojewodka G, Hurley S, Taylor SJC, Noble AJ, Ridsdale L, Goldstein LH. Implementation fidelity of a self-management course for epilepsy: method and assessment. BMC Med Res Methodol 2017;17. https://doi.org/10.1186/s12874-017-0373-x.
- Lausberg H, Sloetjes H. Coding gestural behavior with the NEUROGES – ELAN system. Behav Res Methods 2009;41:841-9. https://doi.org/10.3758/BRM.41.3.841.
- Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15:155-63. https://doi.org/10.1016/j.jcm.2016.02.012.
- Feinstein AR, Cicchetti DV. High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol 1990;43:543-9. https://doi.org/10.1016/0895-4356(90)90158-L.
- Byrt T, Bishop J, Carlin JB. Bias, prevalence and kappa. J Clin Epidemiol 1993;46:423-9. https://doi.org/10.1016/0895-4356(93)90018-V.
- NHS England . AE Waiting Times and Activities 2015. www.england.nhs.uk/statistics/statistical-work-areas/ae-waiting-times-and-activity/statistical-work-areasae-waiting-times-and-activityae-attendances-and-emergency-admissions-2015-16-monthly-3/ (accessed 27 August 2019).
- Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford: Oxford University Press; 2014.
- Sach TH, Desborough J, Houghton J, Holland R. CAREMED study team . Applying micro-costing methods to estimate the costs of pharmacy interventions: an illustration using multi-professional clinical medication reviews in care homes for older people. Int J Pharm Pract 2015;23:237-47. https://doi.org/10.1111/ijpp.12162.
- Lopetegui M, Yen PY, Lai A, Jeffries J, Embi P, Payne P. Time motion studies in healthcare: what are we talking about?. J Biomed Informat 2014;49:292-99. https://doi.org/10.1016/j.jbi.2014.02.017.
- University of Liverpool . Salary, Scales and Hourly Rates 2017–18 2018. www.liverpool.ac.uk/media/livacuk/hr/working/Salary,Scales,and,Hourly,Rates,2017–18.pdf (accessed 30 April 2018).
- BT . BT Business Phone Line Call Charges n.d. https://business.bt.com/content/dam/bt/business/PDFs/phonelines/BT-Business-Call-Charges.pdf (accessed 30 August 2018).
- Royal Liverpool Academy . Room Capacity &Amp; Charges n.d. www.royalliverpoolacademy.nhs.uk/media/2777/room-charges-0417-pdf.pdf (accessed 30 August 2018).
- Royal Mail . Response Rate Card n.d. www.royalmail.com/business/system/files/Response-Services-rate-card-March-2018–138.pdf (accessed 30 August 2018).
- Ioannidis JP. How to make more published research true. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001747.
- Ridsdale L, Robins D, Cryer C, Williams H. Feasibility and effects of nurse run clinics for patients with epilepsy in general practice: randomised controlled trial. Epilepsy Care Evaluation Group. BMJ 1997;314:120-2. https://doi.org/10.1136/bmj.314.7074.120.
- Warren E, Baker GA, Campion P, Chadwick DR, Jacoby A, Luker K. An Evaluation of a Nurse Specialist Case Manager Intervention in the Management of Epilepsy. Report for the North West Regional Health Authority, R&D Directorate; 1998.
- Helde G, Bovim G, Bråthen G, Brodtkorb E. A structured, nurse-led intervention program improves quality of life in patients with epilepsy: a randomized, controlled trial. Epilepsy Behav 2005;7:451-7. https://doi.org/10.1016/j.yebeh.2005.06.008.
- Monfared AA, Lelorier J. Accuracy and validity of using medical claims data to identify episodes of hospitalizations in patients with COPD. Pharmacoepidemiol Drug Saf 2006;15:19-2. https://doi.org/10.1002/pds.1131.
- Roberts RO, Bergstralh EJ, Schmidt L, Jacobsen SJ. Comparison of self-reported and medical record health care utilization measures. J Clin Epidemiol 1996;49:989-95. https://doi.org/10.1016/0895-4356(96)00143-6.
- Ridsdale L, Wojewodka G, Robinson EJ, Noble AJ, Morgan M, Taylor SJC, et al. The effectiveness of a group self-management education course for adults with poorly controlled epilepsy, SMILE (UK): a randomized controlled trial. Epilepsia 2018;59:1048-61. https://doi.org/10.1111/epi.14073.
- Raftery J, Young A, Stanton L, Milne R, Cook A, Turner D, et al. Clinical trial metadata: defining and extracting metadata on the design, conduct, results and costs of 125 randomised clinical trials funded by the National Institute for Health Research Health Technology Assessment programme. Health Technol Assess 2015;19. https://doi.org/10.3310/hta19110.
- Hassan TB, Walker B, Harrison M, Rae F. Stretched to the Limit: A Survey of Emergency Medicine Consultants in the UK. London: College of Emergency Medicine; 2013.
- Medical Research Council (MRC) . Obtaining Data from NHS Digital n.d. https://mrc.ukri.org/documents/pdf/obtaining-nhs-digital-data-guidance-v300317/ (accessed 3 September 2020).
- Ridsdale L, McKinlay A, Wojewodka G, Robinson EJ, Mosweu I, Feehan SJ, et al. Self-Management education for adults with poorly controlled epILEpsy [SMILE (UK)]: a randomised controlled trial. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22210.
- Baker GA, Jacoby A, Buck D, Stalgis C, Monnet D. Quality of life of people with epilepsy: a European study. Epilepsia 1997;38:353-62. https://doi.org/10.1111/j.1528-1157.1997.tb01128.x.
- Scambler G, Hopkins A. Becoming epileptic: coming to terms with stigma. Social Health Ill 1986;8:22-43. https://doi.org/10.1111/1467-9566.ep11346455.
- Department of Health and Social Care (DHSC) . The NHS Constitution for England 2015. www.gov.uk/government/publications/the-nhs-constitution-for-england/the-nhs-constitution-for-england (accessed 27 August 2019).
- NHS England . Emergency Care Data Set 2015. www.england.nhs.uk/wp-content/uploads/2015/08/em-care-data-set.pdf (accessed 27 August 2019).
- NHS Digital . CDS Type 011: Emergency Care Data Set (ECDS) User Guidance 2017. https://digital.nhs.uk/data-and-information/data-collections-and-data-sets/data-sets/emergency-care-data-set-ecds/ecds-guidance (accessed 15 July 2019).
- NHS Digital . ECDS FAQ: May 2017 Emergency Care Data Set Frequently Asked Questions 2017. https://digital.nhs.uk/binaries/content/assets/legacy/pdf/j/1/ecds_implementation_faqs_v1.0.pdf (accessed 15 July 2019).
- Luedke MW, Blalock DV, Lewinski AA, Shapiro A, Drake C, Lewis JD, et al. Self-management of Epilepsy: A Systematic Review. Washington, DC: US Department of Veteran Affairs; 2019.
- Sajatovic M, Colon-Zimmermann K, Kahriman M, Fuentes-Casiano E, Liu H, Tatsuoka C, et al. A 6-month prospective randomized controlled trial of remotely delivered group format epilepsy self-management versus waitlist control for high-risk people with epilepsy. Epilepsia 2018;59:1684-95. https://doi.org/10.1111/epi.14527.
- Snape D, Kirkham J, Britten N, Froggatt K, Gradinger F, Lobban F, et al. Exploring perceived barriers, drivers, impacts and the need for evaluation of public involvement in health and social care research: a modified Delphi study. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-004943.
- Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. PAFS consensus group . CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355. https://doi.org/10.1136/bmj.i5239.
- Noble AJ, Marson AG, Blower SL. Psychological interventions for epilepsy: How good are trialists at assessing their implementation fidelity, what are the barriers, and what are journals doing to encourage it? A mixed methods study. Epilepsy Behav 2019;97:174-81. https://doi.org/10.1016/j.yebeh.2019.05.041.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Perepletchikova F, Hilt LM, Chereji E, Kazdin AE. Barriers to implementing treatment integrity procedures: survey of treatment outcome researchers. J Consult Clin Psychol 2009;77:212-18. https://doi.org/10.1037/a0015232.
- Kobau R, Price P. Knowledge of epilepsy and familiarity with this disorder in the US population: results from the 2002 HealthStyles Survey. Epilepsia 2003;44:1449-54. https://doi.org/10.1046/j.1528-1157.2003.17603.x.
- Faught E, Duh MS, Weiner JR, Guérin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM Study. Neurology 2008;71:1572-8. https://doi.org/10.1212/01.wnl.0000319693.10338.b9.
- Tan JH, Wilder-Smith E, Lim EC, Ong BK. Frequency of provocative factors in epileptic patients admitted for seizures: a prospective study in Singapore. Seizure 2005;14:464-9. https://doi.org/10.1016/j.seizure.2005.07.010.
- Mitchell WG, Conry JA, Crumrine PK, Kriel RL, Cereghino JJ, Groves L, et al. An open-label study of repeated use of diazepam rectal gel (Diastat) for episodes of acute breakthrough seizures and clusters: safety, efficacy, and tolerance. North American Diastat Group. Epilepsia 1999;40:1610-17. https://doi.org/10.1111/j.1528-1157.1999.tb02047.x.
- O’Dell C, Shinnar S, Ballaban-Gil KR, Hornick M, Sigalova M, Kang H, et al. Rectal diazepam gel in the home management of seizures in children. Pediatr Neurol 2005;33:166-72. https://doi.org/10.1016/j.pediatrneurol.2005.03.005.
- Stavem K, Bjørnaes H, Lossius MI. Properties of the 15D and EQ-5D utility measures in a community sample of people with epilepsy. Epilepsy Res 2001;44:179-89. https://doi.org/10.1016/S0920-1211(01)00201-7.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. https://doi.org/10.1016/S0022-3999(01)00296-3.
- Taylor SJC, Pinnock H, Epiphaniou E, Pearce G, Parke HL, Schwappach A, et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS – Practical systematic RevIew of Self-Management Support for long-term conditions. Health Serv Deliv Res 2014;2. https://doi.org/10.3310/hsdr02530.
- Shore CP, Perkins SM, Austin JK. The Seizures and Epilepsy Education (SEE) program for families of children with epilepsy: a preliminary study. Epilepsy Behav 2008;12:157-64. https://doi.org/10.1016/j.yebeh.2007.10.001.
- Baumeister RF, Gollwitzer PM, Bargh JA. The Psychology of Action: Linking Cognition and Motivation to Behavior. New York, NY: Guilford Press; 1996.
- Morgan SR, Chang AM, Alqatari M, Pines JM. Non-emergency department interventions to reduce ED utilization: a systematic review. Acad Emerg Med 2013;20:969-85. https://doi.org/10.1111/acem.12219.
- Great Britain . Medicines for Human Use (Clinical Trials) Regulations 2004 2004. www.legislation.gov.uk/uksi/2004/1031/pdfs/uksi_20041031_en.pdf (accessed 10 August 2020).
Appendix 1 Possible ways that the SAFE course could reduce unnecessary/avoidable emergency department use
| Primary putative mechanisms | |
|---|---|
| Low seizure management confidence and skills | How might the course address and reduce ED use? |
|
PWE can have low levels of knowledge of epilepsy and seizures, have incorrect beliefs concerning first aid, lack confidence managing seizures and be disproportionately fearful (e.g. Long et al. 70). Consequently, some patients can routinely call for emergency medical assistance when they are about to have or have had a seizure, regardless of whether or not it is medically required Responsibility for managing epileptic seizures is often delegated to SOs (Walker et al. 73). Some have low levels of epilepsy knowledge, have incorrect beliefs concerning seizure first aid, lack of confidence in managing seizures and can be fearful of seizures and their threat to a patient’s life (Kobau et al. 226). Consequently, some SOs call for emergency medical attention when the patient has or has had a seizure, regardless of whether or not it is medically required |
Topics covered in the ES course could increase patients’ knowledge of what seizures are, what seizures’ effects are and help patients to know when it is necessary to seek emergency assistance. This could serve to increase patients’ confidence in managing seizures, including post-ictal states, and help them to delineate the circumstances that require emergency services. The course could also help allay disproportionate fears The ES course could help increase SOs’ knowledge of what seizures are, what seizures’ effects are and help them to know when it is necessary to seek emergency medical attention and identify alternative pathways of support. It could also help allay disproportionate fears held by the SO The topics covered in the ES course could also help PWE feel more informed and comfortable with their diagnosis. This could empower them to have a discussion with others in their caring network about epilepsy, relay correct information about how they can be helped if they have a seizure and what their preferences are regarding transportation to ED. To help PWE to do this, the ES has a variety of resources that participants are given or have their attention drawn to. For example, in the participant take-away information pack there are a number of free wallet-sized ‘seizure first aid cards’ that can be given to friends and family members |
| Secondary putative mechanisms | |
| Suboptimal medication or risk management | How might the course address and reduce ED use? |
| Evidence that in some cases ED attendance by PWE may have been precipitated by the person having experienced a seizure or seizure-related injury because they have not managed their medication (e.g. have skipped doses) or epilepsy in an optimum manner or managed risk (e.g. not taken precaution to avoid a seizure trigger) (Faught et al.;227 Tan et al.228) |
The ES course describes what antiepileptic medications do, explains why adhering to prescribed regimes is important and outlines potential seizure triggers. This could promote better medication and risk management by PWE and so reduce avoidable seizures and associated complications. Imparting this information to SOs could also be helpful and allow them to become powerful supporters of the development of self-management responsibility in the PWE they know (e.g. helping PWE with reminders) and risk management (e.g. helping the PWE to identify and avoid triggers, such as sleep deprivation and stress) The ES course also briefly covers issues to do with the commonality of epilepsy, whom it affects and its emotional impact, provides participants with the contact details of support agencies and helps to dispel some misconceptions and myths about epilepsy (e.g. its causes). This, along with meeting other PWE, may help reduce feelings of stigma and shame about the diagnosis. Stigma can be associated with willingness to accept one’s diagnosis and antiepileptic treatments |
| Lack of emergency seizure medication | How might the course address and reduce ED use? |
| Portable emergency seizure medications (e.g. buccal midazolam) are suitable for some PWE. They can be prescribed to PWE who have had a previous episode of prolonged or serial convulsive seizures (NICE).49 They can empower patients and families to manage some seizures, without the need for medical assistance (Mitchell et al.;229 O’Dell et al.230). However, patients and SOs might not be aware of them or their utility. They may also have incorrect beliefs about them (e.g. that all need to be rectally administered), which could act as a barrier to their use | The ES course covers emergency medication. Sources of further information on this topic are also provided. The information could enable PWE to start a discussion with usual care providers about whether or not such treatment is suitable for them |
| Lack of epilepsy identification at time of seizure | How might the course address and reduce ED use? |
| In a minority of cases (≈ 15%; Reuber et al.23), ED visits by PWE occur because the person is alone, has an uncomplicated seizure in a public place and a bystander calls for an ambulance. Evidence indicates that it can be challenging for ambulance staff to know whether or not it is safe to discharge the patient at the scene because they do not know the patient’s medical history. The patient may also be post-ictal; although it is not necessary to transport them to hospital, they do not have an alternative way of ensuring patient safety. Some evidence indicates that low confidence in managing seizures results in some ambulance staff being insistent about transporting the patient to ED (Burrell et al.78) | The ES course does not currently cover this topic. However, with the help of experts and users consulted during the development phase, it might be feasible to introduce this as a new topic. This could involve briefly discussing with PWE and SOs the challenges that face ambulance crews when managing seizures, the benefits of patients carrying easily accessible epilepsy identification cards and the benefits of patients carrying the contact details of a SO who could be contacted to look after the patient and explain the patient’s medical history. Patients could also be advised on the rules and regulations concerning transportation to ED by paramedics |
Appendix 2 Participant information sheet for health-care professional representatives (part A)
Appendix 3 Participant information sheet for service user representatives (part A)
Appendix 4 Topic guide for interviews with health-care professional representatives (part A)
Appendix 5 Topic guide for focus groups with service user representatives (part A)
Appendix 6 Search criteria employed at the different recruitment sites to identify potentially suitable persons attending emergency departments for epilepsy
| ED site | ||
|---|---|---|
| 1 | 2 | 3 |
Identify those people who in the free-text box ‘presenting complaint’ are recorded as having visited the ED for a:
|
Identify those people who in the free-text box ‘reason for visit’ or ‘presenting complaint’ are recorded as having visited the ED for:
|
Identify those people who attended the ED and were given any of the following presenting complaint/discharge diagnosis profiles:
|
Appendix 7 Participant information sheet for patients in the pilot randomised controlled trial (project part B)
Appendix 8 Full details of secondary outcome measures
Quality of life (patients only; baseline, 6 months and 12 months)
Quality of life was measured using the epilepsy-specific quality of life measure QOLIE-31-P. 184 This asks participants to reflect on how they felt over the past 4 weeks. It has seven subscales that reflect the aspects of living that can be affected by epilepsy (emotional well-being, energy fatigue, cognitive functioning, seizure worry, medication effects, social functioning and overall quality of life). Scored according to guidelines, a participant’s total score ranges from 0 to 100, with higher scores indicating better overall perceived quality of life. The QOLIE-31-P varies from the original version of the tool166 in that at the end of each subscale there is one additional item that asks the participant to rate the degree of ‘distress’ caused by that particular topic.
Burden (significant others only; baseline, 6 months and 12 months)
No epilepsy-specific measure is available to measure so-called ‘caregiver burden’ in the target population. Therefore, Zarit caregiver burden scores170 were used to capture the impact of informal caring on the patient participant’s designated SO. Its 22 items evaluate the effect of a condition on quality of life, difficulty in social and family relationships, psychological suffering, shame, guilt and financial difficulty. It is the most widely used, standardised, validated scale and has previously been used in epilepsy (Stavem et al. 231).
Psychological distress (patients and significant others; baseline and 12 months)
The 14-item HADS167,168 was used to measure self-reported distress in patients and SOs. It is a reliable, valid scale widely used in UK epilepsy research. Anxiety and depression scores are grouped into symptom categories: ‘normal’ (0–7), ‘suggestive of anxiety/depression’ (8–10) and ‘probable anxiety/depression’ (11–21). 232
Felt stigma (patients only; baseline and 12 months)
Jacoby’s 3-item Stigma Scale of Epilepsy with revised 4-point scoring14,62,233 measured the extent to which patient participants felt that they are stigmatised because of their epilepsy. Response options include a four-point Likert scale: ‘not at all’ (0), ‘yes, maybe’ (1), ‘yes, probably’ (2) and ‘yes, definitely’ (3). A total score of 0 is classified as ‘does not feel stigmatised’ while total scores of 1–6 are classified as ‘mildly to moderately stigmatised’ and total scores of 7–9 are classified as ‘highly stigmatised’. The scale’s internal consistency (Cronbach’s α = 0.85) is good. 14
Fear of seizures (patients and significant others; baseline and 12 months)
Both patients and SOs completed five items from the fears subscale of the 60-item Epilepsy Knowledge and Management questionnaire. 163 They focus on knowledge about seizures and on fears of death or brain damage. The five items have previously been used in isolation. 234
Confidence managing seizures/epilepsy (patients and significant others; baseline, 6 months and 12 months)
The epilepsy-specific Wagner 6-item Mastery Scale61 was used to measure patient participants’ perception of epilepsy and its treatment and the extent to which they felt able to control these. This measure is able to distinguish between groups of PWE with differing levels of severity. It has adequate internal consistency (Cronbach’s α = 0.7) and test–retest reliability. 235 SOs completed the 6-item Condition Management subscale from the Parents Response to Child Illness Scale,186 which has been shown to have good internal consistency. 165 SOs respond to each item using a 5-point scale.
Knowledge of what to do when faced with a seizure (patient and significant others; baseline and 12 months)
Both patients and SOs completed a measure assessing their knowledge of what to do when faced with a seizure. The standardised questions come from Martiniuk et al. ’s Thinking About Epilepsy questionnaire. 164
Seizure control (patients only; baseline, 6 months and 12 months)
At baseline (T0), patients were asked to complete Thapar’s seizure frequency scale169 for the prior 12 months. At 6- (T2) and 12-month (T3) follow-up, patient participants were asked for the number of seizures (of any type) that they had experienced since the last assessment and the date of the first and most recent seizure (if applicable) since last assessment. To assist patients to be able to provide this information they were offered a seizure diary at baseline (T0) and instructed on how to complete it.
Health economics (patients only; baseline and 12 months)
The Client Service Receipt Inventory171 enabled measurement of patient participants’ health service use (including use of ambulance services, regardless of whether or not transfer to ED happened), informal care (including work time lost by SOs), benefits received and employment status during the 12 months prior to baseline (T0) and at 12 months (T3). The 5-item EQ-5D,172 already shown to be valid in PWE,153 was also used.
Feedback on participation (patients and significant others and 12 months)
To capture patient and SOs feedback on their experience of taking part in the trial, including randomisation, we asked both parties to complete three questions at their final assessment. These were (1) ‘If time suddenly went backward, and you had to do it all over again, would you agree to participate in the Seizure First Aid Training trial?’ (options: ‘definitely yes’, ‘probably yes’, ‘probably no’, ‘definitely no’ and ‘not sure’, plus a free-text field to explain the response), (2) ‘Please tell us if there was anything about the Seizure First Aid Training Trial that you think could have been done better’ (options: free-text response) and (3) ‘Please tell us if there was anything about the Seizure First Aid Training Trial, or your experience of joining the trial, that you think was particularly good’ (options: free-text response). The questions are based on those used to explore participants’ experience of participating in Morgan et al. ’s Magpie trial. 236
Appendix 9 Serious adverse event protocol
Event
We defined a SAE – adapting the definition in Medicines for Human Use (Clinical Trials) Regulations 2004237 – as an adverse event that:
-
resulted in death
-
was life-threatening (subject at immediate risk of death, e.g. status epilepticus)
-
resulted in a seizure that led to hospital admission for ≥ 24 hours
-
resulted in emergency attendance or hospital admission for reason other than seizure
-
resulted in persistent or significant disability or incapacity
-
was otherwise considered medically significant.
‘Life-threatening’ in the context of ‘serious’ refers to an event in which the patient is at risk of death at the time of the event; it does not refer to an event that hypothetically might have caused death if it were more severe. Hospitalisations for a pre-existing condition, including elective procedures that had not worsened, do not constitute a SAE. Prolongation of hospital stay owing to social factors, for example geographical location of the participant’s home, which prevented discharge is also not considered a SAE.
Given the characteristics of the subject population being studied, the following events are expected in this study population and will not be recorded as part of the SAE monitoring process:
-
Epileptic seizures with or without injury.
-
Emergency or urgent medical attention. This includes a visit to a hospital ED lasting < 24 hours, attending an NHS out-of-hours primary care service, telephoning for an ambulance, telephoning NHS 111, seeking/having an urgent/fast-tracked appointment with a usual care provider (GP or specialist) or other registered health-care professional (e.g. a pharmacist).
-
Side effects of antiepileptic drug.
-
Diagnosis of a comorbid psychiatric condition.
A delegated medically qualified person in the team (AM or LR) will assess each unexpected SAE. This person will consider information on the temporal and physical relationship between the event and possible causes and assess whether the event was related or unrelated to the patient’s participation in the study. Further details on the process follow.
Monitoring
As part of this trial, patient participants will not receive additional medical reviews. There is also no ‘live’ system that can be used to track SAEs such as emergency admissions, and usual care providers are not systematically informed of them. Therefore, to monitor SAEs the research team will liaise with patients themselves. A standardised form will be completed as part of the CRF at 3 months (T1, by telephone), 6 months (T2, by telephone) and 12 months (T3, during a face-to-face appointment) post randomisation to collect information on patient participants’ experience of unexpected SAEs.
In each instance, a maximum of three attempts will be made to contact the patient participant by telephone (including trying to contact them via their informal carer if they are taking part with one). Should the patient not be contactable a letter will be sent the patient’s GP asking them to inform the research team if the patient is no longer alive and of the circumstances of their death.
Given the characteristics of the subject population being studied the potential adverse events expected in this study population are as follows:
-
Epileptic seizures with or without injury.
-
Emergency or urgent medical attention. This includes a visit to a hospital ED lasting < 24 hours, attending an NHS out-of-hours primary care service, telephoning for a ambulance, telephoning NHS 111, seeking/having an urgent/fast-tracked appointment with a usual care provider (GP or specialist) or other registered health-care professional (e.g. a pharmacist).
-
Side effects of antiepileptic drug.
-
Diagnosis of a comorbid psychiatric condition.
Although they will be captured as outcomes of the trial, they will not be recorded as part of the SAE monitoring process.
Causality
A delegated, medically qualified person in the team will assess each unexpected SAE. This person will consider information on the temporal and physical relationship between the event and possible causes and assess whether the event was related or unrelated to the patient’s participation in the study. In doing this, they will use the definitions in the table below.
To complete their assessment, the research team may need to obtain medical records, such as contacting a hospital where a patient was admitted as an emergency. For the following reasons a window of 10 days will be allowed for a SAE to be reviewed by the medic: information on adverse events will have been collected by research workers during concentrated follow-up periods, there will be only one delegated medical assessor and assessment may depend on the timeliness of response from hospitals for historically distant admissions.
Definitions of causality for serious adverse event
| Term | Description |
|---|---|
| Unrelated | There is no evidence of any causal relationship. There is an alternative cause for the SAE |
| Unlikely | There is little evidence to suggest that there is a causal relationship (e.g. the event did not occur within a reasonable time after receipt of the intervention). There is another reasonable explanation for the event (e.g. the participant’s clinical condition, other concomitant treatment) |
| Possibly | There is some evidence to suggest a causal relationship (e.g. because the event occurs within a reasonable time after receipt of the intervention). However, the influence of other factors may have contributed to the event (e.g. the participants clinical condition, other concomitant treatment) |
| Probably | There is evidence to suggest a causal relationship and the influence of other factors is unlikely |
| Almost certainly | There is clear evidence to suggest a causal relationship and other possible contributing factors can be ruled out |
Reporting
The main research ethics committee (REC) approving the study and the sponsor will be informed within 15 days of the team becoming aware of any SAE that in the opinion of the medical reviewers is both unexpected (that is, the type of event is not listed in the protocol as an expected occurrence) and judged to be ‘possibly’, ‘probably’ or ‘almost certainly’ related to participation in the study (that is, it resulted from administration of any of the research procedures, including the intervention).
Notifications will include the following details: date of the SAE, location, a description of the circumstances of the event and an assessment of the causal relationship to the SAFE intervention and the implications, if any, for the safety of study participants and how these will be addressed. Notifications made to the main REC shall be made using the National Research Ethics Service SAE reporting form for non-Clinical Trials of an Investigational Medicinal Product. A flowchart is given below to aid the determination of reporting requirements.
A log of all SAEs that are unexpected and which were judged to be related to participation in the study will also be reviewed by the Independent Trial Steering Committee and the implications for the study considered. This information will also be sent to the funder as part of the progress reports and the chief investigator will include details of the event in the annual progress report to the REC and a copy sent to the sponsor.
FIGURE Flowchart for determining of reporting requirements for SAEs.
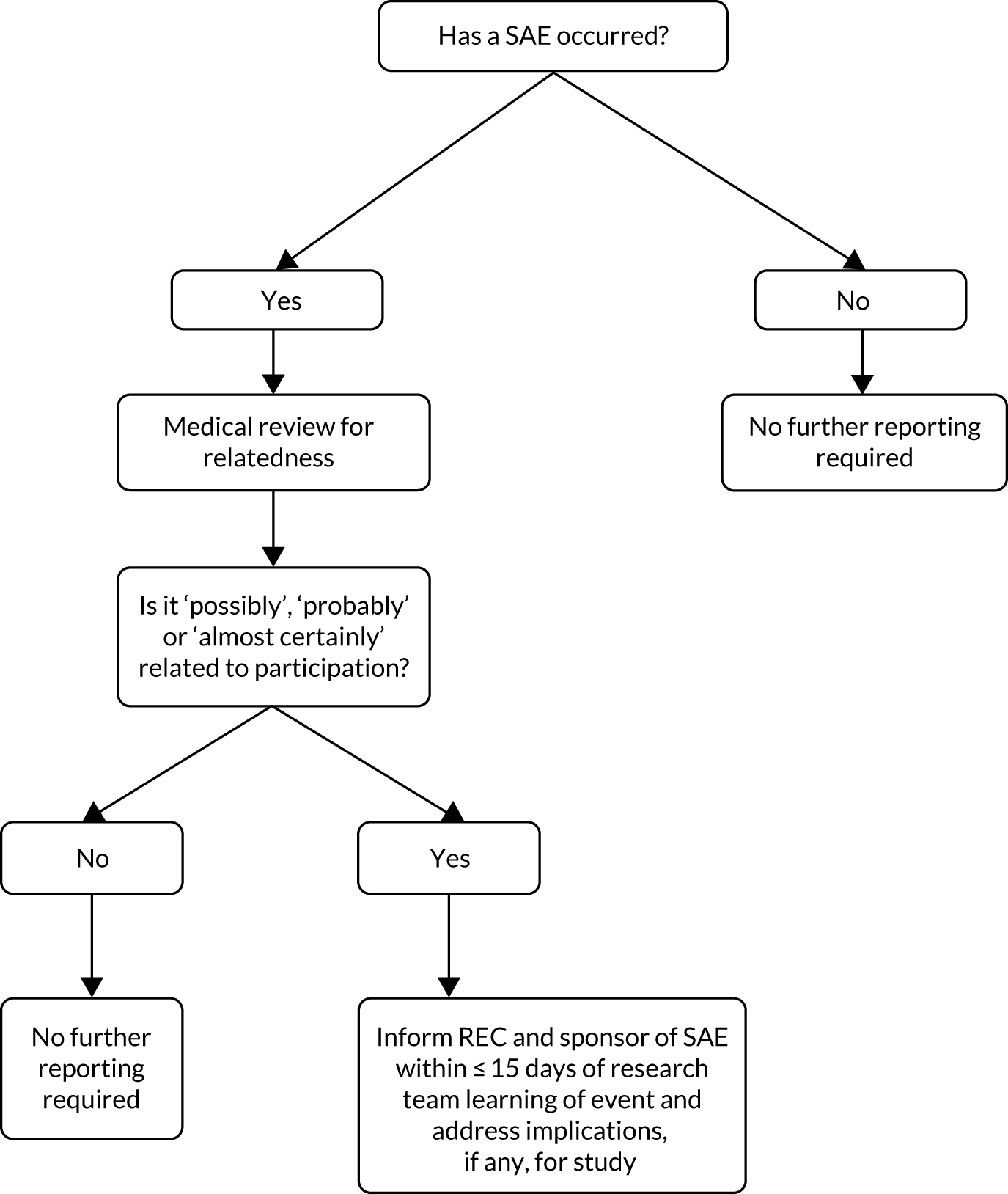
Appendix 10 Cumulative, actual and expected recruitment (consented and randomised) to SAFE trial
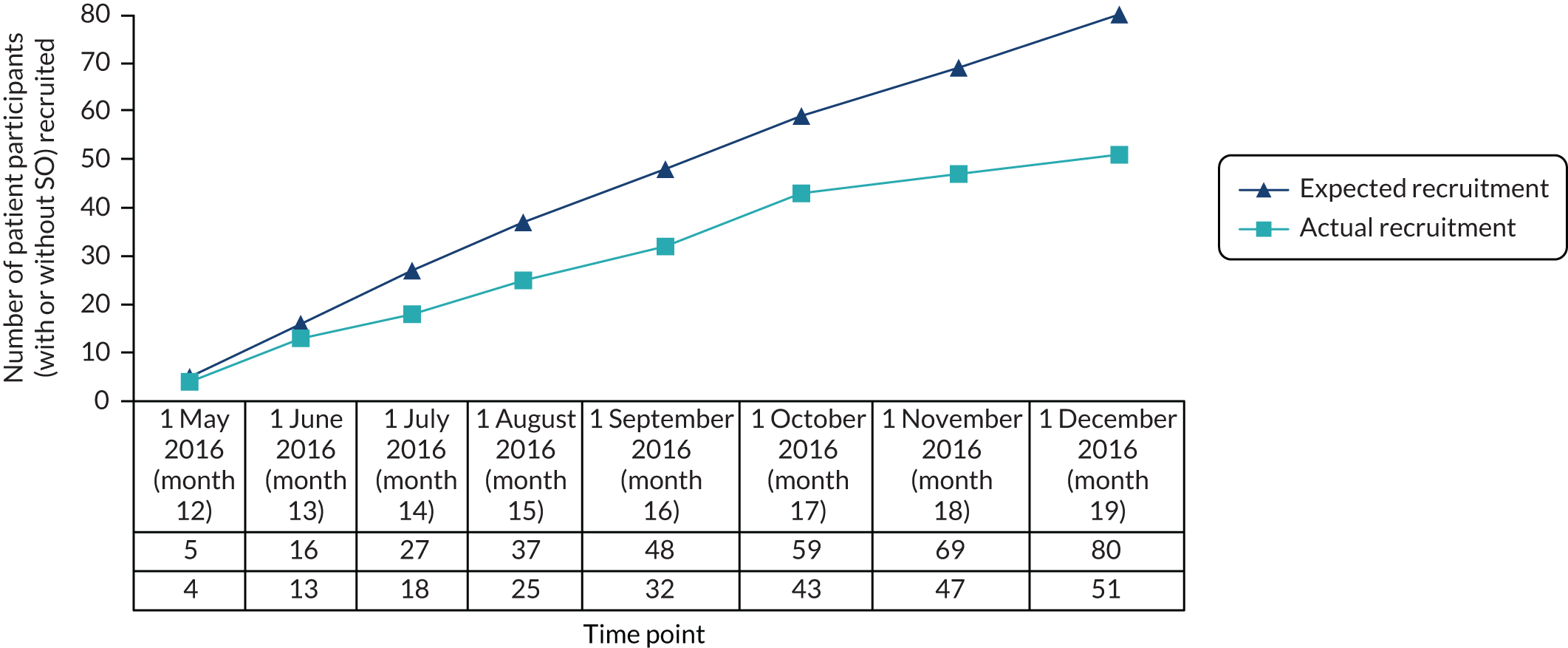
Appendix 11 Reasons for withdrawal from the pilot randomised controlled trial
| Reasons for withdrawal | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Patient participants withdrawing from the study | (N = 1) | (N = 3) | (N = 4) |
| Too busy | 1 (100.0) | 0 (0.0) | 1 (25.0) |
| Not interested any more | 0 (0.0) | 1 (33.3) | 1 (25.0) |
| Other: moved away to work | 0 (0.0) | 1 (33.3) | 1 (25.0) |
| Other: wife has cancer | 0 (0.0) | 1 (33.3) | 1 (25.0) |
| SO participants withdrawing from the study | (N = 1) | (N = 4) | (N = 5) |
| Too ill | 0 (0.0) | 1 (25.0) | 1 (20.0) |
| Not interested any more | 0 (0.0) | 1 (25.0) | 1 (20.0) |
| Other: moved away to work | 0 (0.0) | 1 (25.0) | 1 (20.0) |
| Other: no longer friends with person with epilepsy | 0 (0.0) | 1 (25.0) | 1 (20.0) |
| Reason missing | 1 (100.0) | 0 (0.0) | 1 (20.0) |
Appendix 12 Milestones in securing data from NHS Digital
| Period | Project milestone | Milestone in obtaining data from NHS Digital | ||
|---|---|---|---|---|
| Date | Note | Date | Note | |
| Pre trial | 1 July 2015 | Project officially starts | ||
| 1 August 2015 | Intervention development starts | |||
| 28 September 2015 | Received feedback on draft participant information sheets and consent form for trial to ensure compliance | |||
| Trial period | 19 May 2016 | First trial participant randomised | ||
| 11 October 2017 | Participated in NHS Digital training | |||
| 18 October 2017 | Registered as new user of application system | |||
| 7 November 2017 | Delays experienced. Access to application form granted after chasing | |||
| December 2017 | Final 12-month follow-up completed | |||
| Post trial | 16 February 2018 | Application submitted to NHS Digital | ||
| 28 February 2018 | Teleconference with NHS Digital. Provisional opinion given that only minor changes required and likely time frame for receipt of data 1.5 months | |||
| 7 March 2018 | Received written feedback from NHS Digital after chasing on minor changes required | |||
| 22 March 2018 | Revised application submitted | |||
| 3 April 2018 | NHS Digital submitted new query (1) to applicants requesting additional information (applicants respond 3 April 2018) | |||
| 26 April 2018 | NHS Digital submitted new query (2) to applicants requesting additional information (applicants respond 26 April 2018) | |||
| 30 April 2018 | NHS Digital submitted new query (3) to applicants requesting additional information (applicants respond 30 April 2018) | |||
| 5 May 2018 | NHS Digital submitted new query (4) to applicants requesting additional information (applicants respond 5 May 2018) | |||
| 10 May 2018 | NHS Digital submitted new query (5) to applicants requesting additional information (in the light of General Data Protection Regulation introduced in May 2018) (applicants respond 11 May 2018) | |||
| 30 May 2018 | NHS Digital submitted new query (6) to applicants requesting additional information (in the light of General Data Protection Regulation introduced in May 2018) (applicants respond 30 May 2018) | |||
| 30 May 2018 | NHS Digital submitted new query (7) to applicants requesting additional information (applicants respond 30 May 2018) | |||
| 15 June 2018 | NHS Digital confirmed that data approvals owner will review revised application | |||
| 27 June 2018 | NHS Digital submitted new query (8) to applicants requesting additional information and request revisions to trial website (in the light of General Data Protection Regulation introduced in May 2018) (applicants respond 28 June 2018) | |||
| 2 July 2018 | NHS Digital submitted new query (9) to applicants requesting additional information (applicants 2 July 2018) | |||
| 6 July 2018 | NHS Digital notified applicants that data approval owner has rejected application primarily because of ‘concerns [over] whether this pilot would yield findings that were statistically valuable to achieve the stated aims given the small numbers’ | |||
| 10 July 2018 | Secured confirmation of right to appeal and process | |||
| 20 July 2018 | Applicants submitted letter of appeal | |||
| 25 July 2018 | Appeal accepted by NHS Digital | |||
| 15 August 2018 | NHS Digital submitted new query (10) to applicants following IGARD committee’s review of application (applicants respond 16 August 2018) | |||
| 16 August 2018 | Teleconference with NHS Digital data production team | |||
| 04 September 2018 | NHS Digital sent data-sharing agreement to applicants | |||
| 10 September 2018 | NHS Digital confirmed receipt of completed data-sharing agreement | |||
| 25 September 2018 | Applicants securely transferred patient participants’ details to NHS Digital | |||
| 31 September 2018 | NHS Digital released data to applicants | |||
Appendix 13 Completeness of secondary outcome measures by assessment point, tool and participant type
Number of questions of study assessment tool completed by patient participants at each time point
| Number of questions of study assessment tool completed at each time point | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | (N = 26) | (N = 25) | (N = 51) |
| QOLIE-31-P184 | |||
| 33 | 0 (0.0) | 1 (4.0) | 1 (2.0) |
| 35 | 2 (7.7) | 2 (8.0) | 4 (7.8) |
| 36 | 1 (3.8) | 6 (24.0) | 7 (13.7) |
| 37 (maximum) | 23 (88.5) | 16 (64.0) | 39 (76.5) |
| aHADS167,168 | |||
| 7 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 13 | 2 (7.7) | 1 (4.0) | 3 (5.9) |
| 14 (maximum) | 23 (88.5) | 24 (96.0) | 47 (92.2) |
| Stigma Scale of Epilepsy62 | |||
| 0 | 1 (3.8) | 1 (4.0) | 2 (3.9) |
| 2 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 3 (maximum) | 24 (92.4) | 24 (96.0) | 48 (94.1) |
| bClient Service Receipt Inventory171 | |||
| 2 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 4 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 5 | 5 (19.2) | 8 (32.0) | 13 (25.5) |
| 6 (maximum) | 19 (73.2) | 17 (68.0) | 36 (70.5) |
| EQ-5D172 | |||
| 5 | 5 (19.2) | 0 (0.0) | 5 (9.8) |
| 6 (maximum) | 21 (80.8) | 25 (100.0) | 46 (90.2) |
| Wagner 6-item Mastery Scale61 | |||
| 1 | 0 (0.0) | 1 (4.0) | 1 (2.0) |
| 3 | 0 (0.0) | 1 (4.0.) | 1 (2.0) |
| 5 | 0 (0.0) | 2 (8.0) | 2 (3.9) |
| 6 (maximum) | 26 (100.0) | 21 (84.0) | 47 (92.1) |
| Epilepsy Knowledge and Management Questionnaire:163 fear of seizures subscale163 | |||
| 0 | 3 (11.6) | 2 (8.0) | 5 (9.8) |
| 1 | 1 (3.8) | 1 (4.0) | 2 (3.9) |
| 2 | 1 (3.8) | 5 (20.0) | 6 (11.8) |
| 3 | 1 (3.8) | 4 (16.0) | 5 (9.8) |
| 4 | 4 (15.4) | 4 (16.0) | 8 (15.7) |
| 5 (maximum) | 16 (61.6) | 9 (36.0) | 25 (49.0) |
| 6 months (T2) | (N = 26) | (N = 25) | (N = 51) |
| QOLIE-31-P184 | |||
| 0 | 4 (15.4) | 8 (32.0)c | 12 (23.5)c |
| 16 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 34 | 0 (0.0) | 1 (4.0) | 1 (2.0) |
| 35 | 0 (0.0) | 2 (8.0) | 2 (3.9) |
| 36 | 5 (19.2) | 2 (8.0) | 7 (13.7) |
| 37 (maximum) | 16 (61.6) | 12 (48.0) | 28 (54.9) |
| Wagner 6-item Mastery Scale61 | |||
| 0 | 4 (15.4) | 8 (32.0)c | 12 (23.5)c |
| 2 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 5 | 0 (0.0) | 2 (4.0) | 2 (3.9) |
| 6 (maximum) | 21 (80.8) | 15 (60.0) | 36 (70.6) |
| 12 months (T3) | (N = 26) | (N = 25) | (N = 51) |
| QOLIE-31-P184 | |||
| 0 | 7 (26.9)d | 8 (32.0)d | 15 (29.4)d |
| 1 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 31 | 0 (0.0) | 1 (4.0) | 1 (2.0) |
| 35 | 1 (3.8) | 4 (16.0) | 5 (9.8) |
| 36 | 7 (26.9) | 4 (16.0) | 11 (21.6) |
| 37 (maximum) | 10 (38.5) | 8 (32.0) | 18 (35.3) |
| aHADS167,168 | |||
| 0 | 8 (30.8)d | 8 (32.0)d | 16 (31.4)d |
| 14 (maximum) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Stigma Scale of Epilepsy62 | |||
| 0 | 8 (30.8)d | 8 (32.0)d | 16 (31.4)d |
| 3 (maximum) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| bClient Service Receipt Inventory171 | |||
| 0 | 8 (30.8)d | 8 (32.0)d | 16 (31.4)d |
| 3 | 0 (0.0) | 1 (4.0) | 1 (2.0) |
| 4 | 1 (3.8) | 1 (4.0) | 2 (3.9) |
| 5 | 4 (15.4) | 5 (20.0) | 9 (17.7) |
| 6 (maximum) | 13 (50.0) | 10 (40.0) | 23 (45.0) |
| EQ-5D172 | |||
| 0 | 8 (30.8)d | 8 (32.0)d | 16 (31.4)d |
| 6 | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Wagner 6-item Mastery Scale61 | |||
| 0 | 8 (30.8)d | 8 (32.0)d | 16 (31.4)d |
| 5 | 0 (0.0) | 1 (4.0) | 1 (2.0) |
| 6 | 18 (69.2) | 16 (64.0) | 34 (66.6) |
| Epilepsy Knowledge and Management Questionnaire:163 fear of seizures subscale163 | |||
| 0 | 10 (38.5)d | 8 (32.0)d | 18 (35.3)d |
| 1 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 2 | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 3 | 1 (3.8) | 7 (28.0) | 8 (15.7) |
| 4 | 5 (19.2) | 5 (20.0) | 10 (19.6) |
| 5 (maximum) | 8 (30.9) | 5 (20.0) | 13 (25.5) |
Number of questions of study assessment tool completed by significant other participants at each time point
| Number of questions of study assessment tool completed at each time point | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | (N = 18) | (N = 19) | (N = 37) |
| Zarit caregiver burden170 | |||
| 16 | 0 (0.0) | 1 (5.3) | 1 (2.7) |
| 21 | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| 22 (maximum) | 17 (94.4) | 17 (89.5) | 34 (91.9) |
| aHADS167,168 | |||
| 13 | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| 14 (maximum) | 17 (94.4) | 18 (94.7) | 35 (94.6) |
| Parent Response to Child Illness scale186 | |||
| 6 (maximum) | 18 (100.0) | 19 (100.0) | 37 (100.0) |
| Epilepsy Knowledge and Management Questionnaire:163 fear of seizures subscale163 | |||
| 0 | 2 (11.1) | 2 (10.5) | 4 (10.8) |
| 1 | 2 (11.1) | 2 (10.5) | 4 (10.8) |
| 2 | 1 (5.6) | 3 (15.8) | 4 (10.8) |
| 3 | 1 (5.6) | 2 (10.5) | 3 (8.1) |
| 4 | 6 (33.3) | 6 (31.6) | 12 (32.4) |
| 5 (maximum) | 6 (33.3) | 4 (21.1) | 10 (27.0) |
| 6 months (T2) | (N = 18) | (N = 19) | (N = 37) |
| Zarit caregiver burden170 | |||
| 0 | 2 (11.1)b | 9 (47.4)b | 11 (29.7)b |
| 10 | 0 (0.0) | 1 (5.3) | 1 (2.7) |
| 21 | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| 22 (maximum) | 15 (83.4) | 8 (42.0) | 23 (62.2) |
| Parent Response to Child Illness scale186 | |||
| 0 | 2 (11.1)b | 10 (52.6)b | 12 (32.4)b |
| 6 (maximum) | 16 (88.9) | 9 (47.4) | 25 (67.6) |
| 12 months (T3) | (N = 18) | (N = 19) | (N = 37) |
| Zarit caregiver burden170 | |||
| 0 | 7 (38.9)c | 9 (47.4)c | 16 (43.2)c |
| 22 (maximum) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| aHADS167,168 | |||
| 0 | 7 (38.9)c | 9 (47.4)c | 16 (43.2)c |
| 14 (maximum) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Parent Response to Child Illness scale186 | |||
| 0 | 7 (38.9)c | 9 (47.4)c | 16 (43.2)c |
| 6 (maximum) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Epilepsy Knowledge and Management Questionnaire:163 fear of seizures subscale163 | |||
| 0 | 7 (38.9)c | 10 (52.7)c | 17 (46.0)c |
| 1 | 1 (5.6) | 0 (0.0) | 1 (2.7) |
| 2 | 2 (11.1) | 2 (10.5) | 4 (10.8) |
| 3 | 2 (11.1) | 3 (15.8) | 5 (13.5) |
| 4 | 0 (0.0) | 2 (10.5) | 2 (5.4) |
| 5 (maximum) | 6 (33.3) | 2 (10.5) | 8 (21.6) |
Appendix 14 Bland–Altman plot of agreement between self-reported and Hospital Episode Statistics data on emergency department visits at baseline, without any exclusions
Appendix 15 Demographic characteristics of significant other participants
| Demographic characteristic | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Relationship with patient participant, n (%) | (N = 18) | (N = 19) | (N = 37) |
| Parent | 4 (22.2) | 4 (21.1) | 8 (21.6) |
| Son/daughter | 2 (11.1) | 4 (21.1) | 6 (16.2) |
| Grandparent | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Spouse/partner | 9 (50.0) | 7 (36.8) | 16 (43.2) |
| Sibling | 1 (5.6) | 0 (0.0) | 1 (2.7) |
| Cousin, aunt/uncle | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Niece/nephew | 0 (0.0) | 1 (5.3) | 1 (2.7) |
| Friend | 2 (11.1) | 3 (15.8) | 5 (13.5) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Co-habitation with patient participant, n (%) | (N = 18) | (N = 19) | (N = 37) |
| Yes | 14 (77.8) | 14 (73.4) | 28 (75.7) |
| No | 4 (22.2) | 5 (26.3) | 9 (24.3) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Contact with patient participant (days per week), n (%) | (N = 18) | (N = 19) | (N = 37) |
| 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1 | 1 (5.6) | 0 (0.0) | 1 (2.7) |
| 2 | 0 (0.0) | 1 (5.3) | 1 (2.7) |
| 3 | 0 (0.0) | 2 (10.5) | 2 (5.4) |
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 7 (every day) | 17 (94.4) | 16 (84.2) | 33 (89.2) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sex, n (%) | (N = 18) | (N = 19) | (N = 37) |
| Male | 6 (33.3) | 9 (47.4) | 15 (40.5) |
| Female | 12 (67.7) | 10 (52.6) | 22 (59.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Age (years) at consent into the trial | |||
| n (%) | 17 (94.4) | 19 (100.0) | 36 (94.6) |
| Mean, years | 41.3 | 44.9 | 43.2 |
| SD, years | 18.66 | 15.69 | 17.01 |
| Minimum, years | 17.8 | 18.1 | 17.8 |
| Median, years | 43.5 | 49.7 | 48.0 |
| Maximum, years | 79.7 | 71.5 | 79.7 |
| Missing, n (%) | 1 (5.6) | 0 (0.0) | 1 (2.7) |
| Ethnicity, n (%) | (N = 18) | (N = 19) | (N = 37) |
| White | 18 (100.0) | 18 (94.5) | 36 (97.3) |
| Asian/Asian British | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Black/African/Caribbean/black British | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mixed/multiple | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 1 (5.3) | 1 (2.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Significant medical history, n (%) | (N = 18) | (N = 19) | (N = 37) |
| No, none | 13 (72.2) | 12 (63.2) | 25 (67.6) |
| Yes, a medical condition | 5 (27.8) | 6 (31.6) | 11 (29.7) |
| Yes, a psychiatric condition | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Yes, both medical and psychiatric conditions | 0 (0.0) | 1 (5.3) | 1 (2.7) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Education, n (%) | (N = 18) | (N = 19) | (N = 37) |
| O levels/GCSEs/Level 1 or 2 NVQ | 9 (50.0) | 14 (73.7) | 23 (62.2) |
| A levels/Level 3 NVQ | 4 (22.2) | 2 (10.5) | 6 (16.2) |
| University degree/graduate certificate or diploma | 5 (27.8) | 3 (15.8) | 8 (21.6) |
| Postgraduate university degree (e.g. PGCE, MSc, MA, PhD) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Appendix 16 Adherence ratings for each checklist item and module
| Module | Mean adherence rating for module across courses (SD; range) | Item | Mean adherence rating for item across courses (range) |
|---|---|---|---|
| Orientation and behaviour change optimisation | 1.98 (0.08; 1.50–2.00) | 1. Welcome | 1.92 (1.50–2.00) |
| 2. Goals of this course | 2.00 (2.00–2.00) | ||
| 3. What would you like from today? | 2.00 (2.00–2.00) | ||
| 4. True or false? | 2.00 (2.00–2.00) | ||
| 5. Taking on information (kindness questionnaire) | 2.00 (2.00–2.00) | ||
| Basic epilepsy and first aid knowledge | 1.86 (0.37; 0.00–2.00) | 6. Epilepsy, seizures and how the brain works | 2.00 (2.00–2.00) |
| 7. First aid for convulsive seizures exercise | 2.00 (2.00–2.00) | ||
| 8. What can you do to help someone during a seizure? | 2.00 (2.00–2.00) | ||
| 9. What not to do during a seizure | 2.00 (2.00–2.00) | ||
| 10. What to do after the seizure has stopped | 2.00 (2.00–2.00) | ||
| 11. Questions or comments | 2.00 (2.00–2.00) | ||
| 12. Post-seizure states | 1.64 (1.00–2.00) | ||
| 13. Injuries | 1.07 (0.00–2.00) | ||
| 14. When to call an ambulance? | 1.92 (1.50–2.00) | ||
| 15. Questions or comments | 2.00 (2.00–2.00) | ||
| Recovery position | 1.55 (0.78; 0.00–2.00) | 16. Recovery position I | 0.79 (0.00–2.00) |
| 17. Recovery position II | 2.00 (2.00–2.00) | ||
| 18. Let’s practise the recovery position | 1.71 (0.00–2.00) | ||
| 19. Questions or comments | 1.71 (0.00–2.00) | ||
| Informing others about epilepsy and how to help if seizures occur | 1.95 (0.14; 1.50–2.00) | 1. Who needs to know how to help? | 1.93 (1.50–2.00) |
| 2. What they need to know and why | 2.00 (2.00–2.00) | ||
| 3. How to get this information to them: family, friends and work colleagues | 2.00 (2.00–2.00) | ||
| 4. How to get this information to them: members of the public and health workers | 2.00 (2.00–2.00) | ||
| 5. Questions or comments | 1.86 (1.50–2.00) | ||
| Medical identification, seizure triggers and home safety | 2.00 (0.00; 2.00–2.00) | 6. Personal stories: introduction | 2.00 (2.00–2.00) |
| 7. Ben’s story | 2.00 (2.00–2.00) | ||
| 8. How to change what happened to Ben | 2.00 (2.00–2.00) | ||
| 9. Triggers | 2.00 (2.00–2.00) | ||
| 10. Knowing your triggers | 2.00 (2.00–2.00) | ||
| 11. Some ways of dealing with triggers | 2.00 (2.00–2.00) | ||
| 12. Sandra’s story | 2.00 (2.00–2.00) | ||
| 13. How to change what happened to Sandra (warning signs; home safety) | 2.00 (2.00–2.00) | ||
| Summary and consolidating learning | 1.77 (0.50; 0.00–2.00) | 14. Main points to remember if you have epilepsy | 1.93 (1.50–2.00) |
| 15. Main points to remember if you know someone with epilepsy | 2.00 (2.00–2.00) | ||
| 16. Sources of further information | 1.57 (0.00–2.00) | ||
| 17. What’s on the back table and accessing the study website | 1.50 (0.00–2.00) | ||
| 18. Questions or comments | 1.86 (1.00–2.00) |
Appendix 17 Number of self-reported epilepsy-related emergency department visits
| Number of epilepsy-related ED visits | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| During the 12 months prior to baseline (T0) | |||
| n (%) | 24 (92.3) | 21 (84.0) | 45 (88.2) |
| Mean | 4.2 | 8.4 | 6.2 |
| SD | 2.91 | 22.75 | 15.63 |
| Minimuma | 0 | 0 | 0 |
| Median | 3.5 | 4.0 | 4.0 |
| Maximum | 12 | 107 | 107 |
| Missing | 2 (7.7) | 4 (16.0) | 6 (11.8) |
| During the 12 months following randomisation according to participant self-report | |||
| n (%) | 17 (65.4) | 17 (68.0) | 34 (67.7) |
| Mean | 1.2 | 2.9 | 2.1 |
| SD | 1.60 | 5.55 | 4.11 |
| Minimum | 0 | 0 | 0 |
| Median | 0 | 1 | 1 |
| Maximum | 4 | 23 | 23 |
| Missing | 9 (34.6) | 8 (32.0) | 17 (33.3) |
| Change from baseline over 12 months following randomisation according to participant self-reportb | |||
| n (%) | 16 (61.5) | 14 (56.0) | 30 (58.8) |
| Mean | –2.6 | –0.3 | –1.5 |
| SD | 2.83 | 6.17 | 4.75 |
| Minimum | –10 | –10 | –10 |
| Median | –2 | –2 | –2 |
| Maximum | 1 | 18 | 18 |
| Missing | 10 (38.5) | 11 (44.0) | 21 (41.2) |
Appendix 18 Baseline scores of patient participants and significant other participants and change over follow-up period on secondary outcome measures
Quality of life
Total QOLIE-31-P score (patient participants with complete clinical research form data)
| Total QOLIE-31-P scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 23 (88.5) | 16 (64.0) | 39 (76.5) |
| Mean | 51.2 | 44.1 | 48.3 |
| SD | 18.4 | 14.92 | 17.25 |
| Minimum | 17.7 | 17.1 | 17.1 |
| Median | 48.8 | 42.6 | 46.3 |
| Maximum | 78.7 | 79.5 | 79.5 |
| Missing, n (%) | 3 (11.5) | 9 (36.0) | 12 (23.5) |
| 6 months (T2) | |||
| n (%) | 16 (61.5) | 12 (48.0) | 28 (54.9) |
| Mean | 49.5 | 43.0 | 46.7 |
| SD | 21.86 | 10.46 | 17.92 |
| Minimum | 10.4 | 20.4 | 10.4 |
| Median | 50.2 | 45.1 | 47.7 |
| Maximum | 85.7 | 59.8 | 85.7 |
| Missing, n (%) | 10 (38.5) | 13 (52.0) | 23 (45.1) |
| 12 months (T3) | |||
| n (%) | 10 (38.5) | 8 (32.0) | 18 (35.3) |
| Mean | 47.6 | 42.9 | 45.5 |
| SD | 20.35 | 12.23 | 16.93 |
| Minimum | 9.7 | 23.8 | 9.7 |
| Median | 48.6 | 48.6 | 47.0 |
| Maximum | 72.9 | 57.0 | 72.9 |
| Missing, n (%) | 16 (61.5) | 17 (68.0) | 33 (64.7) |
| Change from baseline (T0) at 6 months (T2)b | |||
| n (%) | 14 (53.8) | 8 (32.0) | 22 (43.1) |
| Mean | –5.9 | –0.7 | –4.0 |
| SD | 13.81 | 8.57 | 12.20 |
| Minimum | –33.7 | –12.0 | –33.7 |
| Median | –6.5 | –1.8 | –3.2 |
| Maximum | 18.7 | 12.9 | 18.7 |
| Missing, n (%) | 12 (46.2) | 17 (68.0) | 29 (56.9) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 10 (38.5) | 6 (24.0) | 16 (31.4) |
| Mean | 0.8 | –2.18 | –0.3 |
| SD | 10.72 | 10.48 | 10.38 |
| Minimum | –14.9 | –19.5 | –19.5 |
| Median | 1.0 | –0.9 | 0.2 |
| Maximum | 20.5 | 12.9 | 20.5 |
| Missing, n (%) | 16 (61.5) | 19 (76.0) | 35 (68.6) |
Total QOLIE-31-P score (patient participants with complete data following data imputation)
| Total QOLIE-31-P scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 25 (96.2) | 24 (96.0) | 49 (96.1) |
| Mean | 51.6 | 44.3 | 48.0 |
| SD | 18.63 | 16.38 | 17.77 |
| Minimum | 17.7 | 12.3 | 12.3 |
| Median | 48.8 | 42.6 | 46.3 |
| Maximum | 78.7 | 79.5 | 79.5 |
| Missing, n (%) | 1 (3.8) | 1 (4.0) | 2 (3.9) |
| 6 months (T2) | |||
| n (%) | 21 (80.8) | 14 (56.0) | 35 (68.6) |
| Mean | 47.8 | 42.6 | 45.7 |
| SD | 21.67 | 9.76 | 17.86 |
| Minimum | 10.4 | 20.4 | 10.4 |
| Median | 49.7 | 43.6 | 44.4 |
| Maximum | 85.7 | 59.8 | 85.7 |
| Missing, n (%) | 5 (19.2) | 11 (44.0) | 16 (31.4) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 12 (48.0) | 30 (58.8) |
| Mean | 51.0 | 44.0 | 48.2 |
| SD | 20.43 | 11.72 | 17.58 |
| Minimum | 9.7 | 23.8 | 9.7 |
| Median | 48.6 | 47.3 | 47.3 |
| Maximum | 82.8 | 57.0 | 82.8 |
| Missing, n (%) | 8 (30.8) | 13 (52.0) | 21 (41.2) |
| Change from baseline (T0) at 6 months (T2)b | |||
| n (%) | 20 (76.9) | 13 (52.0) | 33 (64.7) |
| Mean | –3.0 | –0.8 | –2.2 |
| SD | 13.16 | 9.07 | 11.61 |
| Minimum | –33.7 | –12.3 | –33.7 |
| Median | –2.4 | –1.6 | –1.6 |
| Maximum | 18.7 | 15.7 | 18.7 |
| Missing, n (%) | 6 (23.1) | 12 (48.0) | 18 (35.3) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 18 (69.2) | 12 (48.0) | 30 (58.9) |
| Mean | 2.2 | –1.9 | 0.6 |
| SD | 11.27 | 12.13 | 11.59 |
| Minimum | –20.5 | –22.7 | –22.7 |
| Median | 2.1 | –0.9 | 1.0 |
| Maximum | 20.5 | 18.6 | 20.5 |
| Missing, n (%) | 8 (30.8) | 13 (52.0) | 21 (41.2) |
Caregiver burden
Total burden score (significant other participants with complete clinical research form data)
| Total burden scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 17 (94.4) | 17 (89.5) | 34 (91.9) |
| Mean | 15.8 | 20.6 | 18.2 |
| SD | 11.01 | 13.59 | 12.42 |
| Minimum | 0 | 0 | 0 |
| Median | 14.0 | 19.0 | 15.5 |
| Maximum | 45 | 45 | 45 |
| Missing, n (%) | 1 (5.6) | 2 (10.5) | 3 (8.1) |
| 6 months (T2) | |||
| n (%) | 15 (83.3) | 8 (42.1) | 23 (62.2) |
| Mean | 19.7 | 22.7 | 20.7 |
| SD | 12.13 | 11.3 | 11.68 |
| Minimum | 0 | 13 | 0 |
| Median | 19 | 20 | 19 |
| Maximum | 42 | 48 | 48 |
| Missing, n (%) | 3 (16.7) | 11 (57.9) | 14 (37.8) |
| 12 months (T3) | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 19.5 | 24.9 | 22.1 |
| SD | 16.24 | 15.83 | 15.88 |
| Minimum | 0 | 7 | 0 |
| Median | 15 | 20 | 18 |
| Maximum | 48 | 54 | 54 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
| Change from baseline (T0) at 6 months (T2)b | |||
| n (%) | 14 (77.8) | 7 (36.8) | 21 (56.8) |
| Mean | 2.7 | 1.6 | 2.3 |
| SD | 7.01 | 6.21 | 6.62 |
| Minimum | –9 | –5 | –9 |
| Median | 1 | 1 | 1 |
| Maximum | 20 | 12 | 20 |
| Missing, n (%) | 4 (22.2) | 12 (63.2) | 16 (43.2) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 10 (55.6) | 9 (47.4) | 19 (51.4) |
| Mean | 1.4 | 0.8 | 1.1 |
| SD | 10.04 | 8.38 | 9.04 |
| Minimum | –20 | –12 | –20 |
| Median | 0.5 | 2.0 | 1.0 |
| Maximum | 15 | 14 | 15 |
| Missing, n (%) | 8 (44.4) | 10 (52.6) | 18 (48.6) |
Burden categories (significant other participants with complete clinical research form data)
| Burden category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Little or no burden | 12 (66.7) | 10 (52.7) | 22 (59.5) |
| Mild to moderate burden | 4 (22.2) | 5 (26.3) | 9 (24.3) |
| Moderate to severe burden | 1 (5.6) | 2 (10.5) | 3 (8.1) |
| Severe burden | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 1 (5.6) | 2 (10.5) | 3 (8.1) |
| 6 months (T2) | |||
| Little or no burden | 9 (50.0) | 4 (21.1) | 13 (35.1) |
| Mild to moderate burden | 5 (27.7) | 3 (15.8) | 8 (21.6) |
| Moderate to severe burden | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| Severe burden | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 3 (16.7) | 11 (57.8) | 14 (37.8) |
| 12 months (T3) | |||
| Little or no burden | 6 (33.3) | 5 (26.3) | 11 (29.7) |
| Mild to moderate burden | 4 (22.2) | 3 (15.8) | 7 (18.9) |
| Moderate to severe burden | 1 (5.6) | 2 (10.5) | 3 (8.1) |
| Severe burden | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Total burden score (all significant other participants, including those with missing clinical research form data)
| Total burden scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 18 (100.0) | 19 (100.0) | 37 (100.0) |
| Mean | 17.1 | 20.7 | 18.9 |
| SD | 12.00 | 13.03 | 12.51 |
| Minimum | 0 | 0 | 0 |
| Median | 14.5 | 19.0 | 16.0 |
| Maximum | 45 | 45 | 45 |
| Missing, n (%)b | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months (T2) | |||
| n (%) | 16 (88.9) | 10 (52.6) | 26 (70.3) |
| Mean | 19.4 | 22.7 | 20.7 |
| SD | 11.78 | 9.98 | 11.04 |
| Minimum | 0 | 13 | 0 |
| Median | 19.0 | 22.0 | 19.5 |
| Maximum | 42 | 48 | 48 |
| Missing, n (%)b | 2 (11.1) | 9 (47.4) | 11 (29.7) |
| 12 months (T3) | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 19.5 | 24.9 | 22.1 |
| SD | 16.24 | 15.83 | 15.88 |
| Minimum | 0 | 7 | 0 |
| Median | 15 | 20 | 18 |
| Maximum | 48 | 54 | 54 |
| Missing, n (%)b | 7 (38.9) | 9 (47.4) | 16 (43.2) |
| Change from baseline (T0) at 6 months (T2)c | |||
| n (%) | 16 (88.9) | 10 (52.6) | 26 (70.3) |
| Mean | 1.4 | 2.0 | 1.7 |
| SD | 7.89 | 6.96 | 7.41 |
| Minimum | –15 | –7 | –15 |
| Median | 0.0 | 1.5 | 0.5 |
| Maximum | 20 | 13 | 20 |
| Missing, n (%) | 2 (11.1) | 9 (47.4) | 11 (29.7) |
| Change from baseline (T0) at 12 months (T3)c | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 2.1 | 0.9 | 1.5 |
| SD | 9.79 | 7.91 | 8.74 |
| Minimum | –20 | –12 | –20 |
| Median | 1 | 2 | 1 |
| Maximum | 15 | 14 | 15 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Burden categories (all significant other participants, including those with missing clinical research form data)
| Burden category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Little or no burden | 12 (67.7) | 11 (57.9) | 23 (62.2) |
| Mild to moderate burden | 5 (27.8) | 6 (31.6) | 11 (29.7) |
| Moderate to severe burden | 1 (5.6) | 2 (10.5) | 3 (8.1) |
| Severe burden | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months (T2) | |||
| Little or no burden | 10 (55.6) | 4 (21.1) | 14 (37.8) |
| Mild to moderate burden | 5 (27.8) | 5 (26.3) | 10 (27.0) |
| Moderate to severe burden | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| Severe burden | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 2 (11.1) | 9 (47.4) | 11 (29.7) |
| 12 months (T3) | |||
| Little or no burden | 6 (33.3) | 5 (26.3) | 11 (29.7) |
| Mild to moderate burden | 4 (22.2) | 3 (15.8) | 7 (18.9) |
| Moderate to severe burden | 1 (5.6) | 2 (10.5) | 3 (8.1) |
| Severe burden | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Distress
Total anxiety score (patient participants with complete clinical research form data)
| Total anxiety scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 25 (96.2) | 25 (100.0) | 50 (98.0) |
| Mean | 10.2 | 10.4 | 10.3 |
| SD | 5.22 | 4.54 | 4.84 |
| Minimum | 2 | 0 | 0 |
| Median | 11 | 11 | 11 |
| Maximum | 20 | 18 | 20 |
| Missing, n (%) | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 10.8 | 10.6 | 10.7 |
| SD | 5.49 | 3.18 | 4.46 |
| Minimum | 3 | 5 | 3 |
| Median | 10.5 | 10.0 | 10.0 |
| Maximum | 19 | 16 | 19 |
| Missing, n (%) | 8 (30.8) | 8 (32.0) | 16 (31.4) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 0.2 | –0.6 | –0.2 |
| SD | 3.91 | 3.26 | 3.58 |
| Minimum | –7 | –7 | –7 |
| Median | –0.5 | –1 | –1 |
| Maximum | 13 | 6 | 13.0 |
| Missing, n (%) | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Anxiety categories (patient participants with complete clinical research form data)
| Anxiety category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Normal range | 9 (34.6) | 5 (20.0) | 14 (27.5) |
| Suggestive of anxiety | 3 (11.5) | 7 (28.0) | 10 (19.6) |
| Probable anxiety | 13 (50.0) | 13 (52.0) | 26 (50.9) |
| Missing | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 12 months (T3) | |||
| Normal range | 6 (33.3) | 2 (8.0) | 8 (15.6) |
| Suggestive of anxiety | 3 (16.7) | 8 (32.0) | 11 (21.6) |
| Probable anxiety | 9 (50.0) | 7 (28.0) | 16 (31.4) |
| Missing | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Total anxiety score (patient participants with complete data following data imputation)
| Total anxiety scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 25 (96.2) | 25 (100.0) | 50 (98.0) |
| Mean | 10.2 | 10.4 | 10.3 |
| SD | 5.22 | 4.54 | 4.84 |
| Minimum | 2 | 0 | 0 |
| Median | 11 | 11 | 11 |
| Maximum | 20 | 18 | 20 |
| Missing, n (%) | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 10.8 | 10.6 | 10.7 |
| SD | 5.49 | 3.18 | 4.46 |
| Minimum | 3 | 5 | 3 |
| Median | 10.5 | 10.0 | 10.0 |
| Maximum | 19 | 16 | 19 |
| Missing, n (%) | 8 (30.8) | 8 (40.0) | 16 (31.4) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 0.2 | –0.6 | –0.2 |
| SD | 3.91 | 3.26 | 3.58 |
| Minimum | –7 | –7 | –7 |
| Median | –0.5 | –1 | –1 |
| Maximum | 13 | 6 | 13.0 |
| Missing, n (%) | 8 (30.8) | 8 (40.0) | 16 (31.4) |
Anxiety categories (patient participants with complete data following data imputation)
| Anxiety category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Normal range | 9 (34.6) | 5 (20.0) | 14 (27.5) |
| Suggestive of anxiety | 3 (11.5) | 7 (28.0) | 10 (19.6) |
| Probable anxiety | 13 (50.0) | 13 (52.0) | 26 (50.9) |
| Missing | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 12 months (T3) | |||
| Normal range | 6 (33.3) | 2 (8.0) | 8 (15.6) |
| Suggestive of anxiety | 3 (16.7) | 8 (32.0) | 11 (21.6) |
| Probable anxiety | 9 (50.0) | 7 (28.0) | 16 (31.4) |
| Missing | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Total depression score (patient participants with complete clinical research form data)
| Total depression scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 23 (88.5) | 24 (96.0) | 47 (92.2) |
| Mean | 6.6 | 8.7 | 7.7 |
| SD | 4.51 | 4.67 | 4.66 |
| Minimum | 0 | 0 | 0 |
| Median | 7.0 | 8.5 | 7 |
| Maximum | 17 | 19 | 19 |
| Missing, n (%) | 3 (11.5) | 1 (4.0) | 4 (7.8) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 7.2 | 8.6 | 7.9 |
| SD | 4.07 | 3.46 | 3.80 |
| Minimum | 1 | 4 | 1 |
| Median | 7 | 9 | 8 |
| Maximum | 14 | 16 | 16 |
| Missing, n (%) | 8 (30.8) | 8 (32.0) | 16 (31.4) |
| 12 months (T3)b | |||
| n (%) | 16 (61.5) | 16 (64.0) | 32 (62.7) |
| Mean | –0.5 | 0.1 | –0.2 |
| SD | 1.97 | 2.90 | 2.46 |
| Minimum | –3 | –5 | –5 |
| Median | –0.5 | 1.0 | 0.0 |
| Maximum | 3 | 5 | 5 |
| Missing, n (%) | 10 (38.5) | 9 (36.0) | 19 (37.3) |
Depression categories (patient participants with complete clinical research form data)
| Depression category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Normal range | 14 (53.9) | 10 (40.0) | 24 (47.1) |
| Suggestive of depression | 5 (19.2) | 7 (28.0) | 12 (23.5) |
| Probable depression | 4 (15.4) | 7 (28.0) | 11 (21.6) |
| Missing | 3 (11.5) | 1 (4.0) | 4 (7.8) |
| 12 months (T3) | |||
| Normal range | 10 (38.4) | 7 (28.0) | 17 (33.3) |
| Suggestive of depression | 4 (15.4) | 4 (16.0) | 8 (15.7) |
| Probable depression | 4 (15.4) | 6 (24.0) | 10 (19.6) |
| Missing | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Total depression score (patient participants with complete data following data imputation)
| Total depression scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 25 (96.2) | 25 (100.0) | 50 (98.0) |
| Mean | 6.7 | 8.6 | 7.6 |
| SD | 4.36 | 4.61 | 4.53 |
| Minimum | 0 | 0 | 0 |
| Median | 7 | 8 | 7.5 |
| Maximum | 17 | 19 | 19 |
| Missing, n (%) | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 7.2 | 8.6 | 7.9 |
| SD | 4.07 | 3.46 | 3.80 |
| Minimum | 1 | 4 | 1 |
| Median | 7 | 9 | 8 |
| Maximum | 14 | 16 | 16 |
| Missing, n (%) | 8 (30.8) | 8 (32.0) | 16 (31.4) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | –0.2 | 0.5 | 0.1 |
| SD | 2.39 | 3.16 | 2.77 |
| Minimum | –3.0 | –5.0 | –5.0 |
| Median | –0.5 | 1.0 | 0.0 |
| Maximum | 5.8 | 6.2 | 6.2 |
| Missing, n (%) | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Depression categories (patient participants with complete data following data imputation)
| Depression category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Normal range | 14 (53.8) | 11 (44.0) | 25 (49.0) |
| Suggestive of depression | 7 (26.9) | 7 (28.0) | 14 (27.5) |
| Probable depression | 4 (15.3) | 7 (28.0) | 11 (21.6) |
| Missing | 1 (3.8) | 0 (0.0) | 1 (2.0) |
| 12 months (T3) | |||
| Normal range | 10 (38.4) | 7 (28.0) | 17 (33.3) |
| Suggestive of depression | 4 (15.3) | 4 (16.0) | 8 (15.7) |
| Probable depression | 4 (15.3) | 6 (24.0) | 10 (19.6) |
| Missing | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Total anxiety score (significant other participants with complete clinical research form data)
| Total anxiety scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 17 (94.4) | 18 (94.7) | 35 (94.6) |
| Mean | 8.4 | 7.9 | 8.1 |
| SD | 4.21 | 4.37 | 4.24 |
| Minimum | 2 | 0 | 0 |
| Median | 9 | 10 | 9 |
| Maximum | 15 | 14 | 15 |
| Missing, n (%) | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| 12 months (T3) | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 9.2 | 8.2 | 8.7 |
| SD | 5.15 | 2.39 | 4.01 |
| Minimum | 1 | 4 | 1 |
| Median | 9 | 9 | 9 |
| Maximum | 16 | 12 | 16 |
| Missing, n (%) | 7 (38.9) | 9 (47.8) | 16 (43.2) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 0.5 | –0.5 | 0.0 |
| SD | 2.70 | 3.13 | 2.88 |
| Minimum | –3 | –5 | –5 |
| Median | 1 | –1.5 | –1 |
| Maximum | 3 | 5 | 5 |
| Missing, n (%) | 7 (38.9) | 9 (47.8) | 16 (43.2) |
Anxiety categories (significant other participants with complete clinical research form data)
| Anxiety category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Normal range | 7 (38.9) | 7 (36.8) | 14 (37.8) |
| Suggestive of anxiety | 4 (22.2) | 3 (15.8) | 7 (18.8) |
| Probable anxiety | 6 (33.3) | 8 (42.1) | 14 (37.8) |
| Missing | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| 12 months (T3) | |||
| Normal range | 4 (22.2) | 4 (21.1) | 8 (21.7) |
| Suggestive of anxiety | 2 (11.1) | 5 (26.3) | 7 (18.9) |
| Probable anxiety | 5 (27.8) | 1 (5.3) | 6 (16.2) |
| Missing | 7 (38.9) | 9 (47.8) | 16 (43.2) |
Total anxiety score (significant other participants with complete data following data imputation)
| Total anxiety scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 18 (100.0) | 19 (100.0) | 37 (100.0) |
| Mean | 8.4 | 8.3 | 8.3 |
| SD | 4.09 | 4.47 | 4.23 |
| Minimum | 2 | 0.0 | 0.0 |
| Median | 9.0 | 10.0 | 9.3 |
| Maximum | 15 | 14 | 15 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 months (T3) | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 9.2 | 8.2 | 8.7 |
| SD | 5.15 | 2.39 | 4.01 |
| Minimum | 1 | 4 | 1 |
| Median | 9 | 9 | 9 |
| Maximum | 16 | 12 | 16 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 0.5 | –0.5 | 0.0 |
| SD | 2.70 | 3.13 | 2.88 |
| Minimum | –3 | –5 | –5 |
| Median | 1.0 | –1.5 | –1.0 |
| Maximum | 3 | 5 | 5 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Anxiety categories (significant other participants with complete data following data imputation)
| Anxiety category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Normal range | 7 (38.9) | 7 (36.8) | 14 (37.8) |
| Suggestive of anxiety | 5 (27.8) | 3 (15.8) | 8 (21.7) |
| Probable anxiety | 6 (33.3) | 9 (47.4) | 15 (40.5) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 months (T3) | |||
| Normal range | 4 (22.2) | 4 (21.1) | 8 (21.7) |
| Suggestive of anxiety | 2 (11.1) | 5 (26.3) | 7 (18.9) |
| Probable anxiety | 5 (27.8) | 1 (5.3) | 6 (16.2) |
| Missing | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Total depression score (significant other participants with complete clinical research form data)
| Total depression scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 18 (100.0) | 19 (100.0) | 37 (100.0) |
| Mean | 4.3 | 3.4 | 3.8 |
| SD | 4.40 | 2.65 | 3.59 |
| Minimum | 0 | 0 | 0 |
| Median | 2.5 | 3.0 | 3.0 |
| Maximum | 12 | 8 | 12 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 months (T3) | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 5.2 | 5.1 | 5.1 |
| SD | 3.60 | 3.60 | 3.51 |
| Minimum | 0 | 0 | 0 |
| Median | 5 | 5 | 5 |
| Maximum | 12 | 11 | 12 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
| Change from baseline at 12 months (T3)b | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | –0.4 | 1.3 | 0.4 |
| SD | 3.14 | 1.83 | 2.68 |
| Minimum | –9 | –1 | –9 |
| Median | 0 | 1 | 1 |
| Maximum | 3 | 4 | 4 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Depression categories (significant other participants with complete clinical research form data)
| Depression category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Normal range | 14 (77.8) | 18 (94.7) | 32 (86.5) |
| Suggestive of depression | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| Probable depression | 3 (16.7) | 0 (0.0) | 3 (8.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 months (T3) | |||
| Normal range | 7 (38.9) | 8 (42.1) | 15 (40.6) |
| Suggestive of depression | 3 (16.7) | 1 (5.3) | 4 (10.8) |
| Probable depression | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| Missing | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Total depression score (significant other participants with complete data following data imputation)
| Total depression scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 18 (100.0) | 19 (100.0) | 37 (100.0) |
| Mean | 4.3 | 3.4 | 3.8 |
| SD | 4.40 | 2.65 | 3.59 |
| Minimum | 0 | 0 | 0 |
| Median | 2.5 | 3.0 | 3 |
| Maximum | 12 | 8 | 12 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 months (T3) | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 5.2 | 5.1 | 5.1 |
| SD | 3.60 | 3.60 | 3.51 |
| Minimum | 0 | 0 | 0 |
| Median | 5 | 5 | 5 |
| Maximum | 12 | 11 | 12 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | –0.4 | 1.3 | 0.4 |
| SD | 3.14 | 1.83 | 2.68 |
| Minimum | –9 | –1 | –9 |
| Median | 0 | 1 | 1 |
| Maximum | 3 | 4 | 4 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Depression categories (significant other participants with complete data following data imputation)
| Depression category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| Normal range | 14 (77.8) | 18 (94.7) | 32 (86.5) |
| Suggestive of depression | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| Probable depression | 3 (16.7) | 0 (0.0) | 3 (8.1) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 months (T3) | |||
| Normal range | 7 (38.9) | 8 (42.1) | 15 (40.6) |
| Suggestive of depression | 3 (16.7) | 1 (5.3) | 4 (10.8) |
| Probable depression | 1 (5.6) | 1 (5.3) | 2 (5.4) |
| Missing | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Stigma
Total stigma score (patient participants with complete clinical research form data)
| Total stigma scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 24 (92.3) | 24 (96.0) | 48 (94.1) |
| Mean | 3.2 | 4.1 | 3.6 |
| SD | 2.45 | 3.26 | 2.89 |
| Minimum | 0 | 0 | 0 |
| Median | 3 | 3 | 3 |
| Maximum | 7 | 9 | 9 |
| Missing, n (%) | 2 (7.7) | 1 (4.0) | 3 (5.9) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 3.2 | 3.9 | 3.5 |
| SD | 2.57 | 3.03 | 2.79 |
| Minimum | 0 | 0 | 0 |
| Median | 3.5 | 3.0 | 3.0 |
| Maximum | 8 | 9 | 9 |
| Missing, n (%) | 8 (30.8) | 8 (32.0) | 16 (31.4) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 17 (65.4) | 17 (68.0) | 34 (66.7) |
| Mean | –0.1 | 0.3 | 0.1 |
| SD | 2.30 | 1.79 | 2.04 |
| Minimum | –6 | –2 | –6 |
| Median | 0 | 0 | 0 |
| Maximum | 4 | 4 | 4 |
| Missing, n (%) | 9 (34.6) | 8 (32.0) | 17 (33.3) |
Stigma categories (patient participants with complete clinical research form data)
| Stigma category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| No stigma | 4 (15.4) | 3 (12.0) | 7 (13.7) |
| Mildly to moderately stigmatised | 13 (50.0) | 13 (52.0) | 26 (51.0) |
| Highly stigmatised | 7 (26.9) | 8 (32.0) | 15 (29.4) |
| Missing | 2 (7.7) | 1 (4.0) | 3 (5.9) |
| 12 months (T3) | |||
| No stigma | 4 (15.4) | 3 (12.0) | 7 (13.7) |
| Mildly to moderately stigmatised | 10 (38.4) | 8 (32.0) | 18 (35.3) |
| Highly stigmatised | 4 (15.4) | 6 (24.0) | 10 (19.6) |
| Missing | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Total stigma score (all patient participants, including those with missing clinical research form data)
| Total stigma scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 25 (96.2) | 24 (96.0) | 49 (96.8) |
| Mean | 3.2 | 4.1 | 3.6 |
| SD | 2.41 | 3.26 | 2.87 |
| Minimum | 0 | 0 | 0 |
| Median | 3 | 3 | 3 |
| Maximum | 7 | 9 | 9 |
| Missing, n (%)b | 1 (3.8) | 1 (4.0) | 2 (3.9) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 3.2 | 3.9 | 3.5 |
| SD | 2.57 | 3.03 | 2.79 |
| Minimum | 0 | 0 | 0 |
| Median | 3.5 | 3.0 | 3.0 |
| Maximum | 8 | 9 | 9 |
| Missing, n (%)b | 8 (30.8) | 8 (32.0) | 16 (31.4) |
| Change from baseline (T0) at 12 months (T3)c | |||
| n (%) | 17 (65.4) | 17 (68.0) | 34 (66.7) |
| Mean | –0.1 | 0.3 | 0.1 |
| SD | 2.30 | 1.79 | 2.04 |
| Minimum | –6 | –2 | –6 |
| Median | 0 | 0 | 0 |
| Maximum | 4 | 4 | 4 |
| Missing, n (%) | 9 (34.6) | 8 (32.0) | 17 (33.3) |
Stigma categories (all patient participants, including those with missing clinical research form data)
| Stigma category | Treatment arm, n (%) | Total, n (%) | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| No stigma | 4 (15.4) | 3 (12.0) | 7 (13.7) |
| Mildly to moderately stigmatised | 14 (53.9) | 13 (52.0) | 27 (52.9) |
| Highly stigmatised | 7 (26.9) | 8 (32.0) | 15 (29.4) |
| Missing | 1 (3.8) | 1 (4.0) | 2 (3.9) |
| 12 months (T3) | |||
| No stigma | 4 (15.4) | 3 (12.0) | 7 (13.7) |
| Mildly to moderately stigmatised | 10 (38.4) | 8 (32.0) | 18 (35.3) |
| Highly stigmatised | 4 (15.4) | 6 (24.0) | 10 (19.6) |
| Missing | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Fear of seizures
Total fear score (patient participants with complete clinical research form data)
| Total fear scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 16 (61.5) | 9 (36.0) | 25 (49.0) |
| Mean | 16.4 | 17.6 | 16.8 |
| SD | 5.48 | 4.77 | 5.16 |
| Minimum | 6 | 8 | 6 |
| Median | 17 | 18 | 17 |
| Maximum | 24 | 25 | 25 |
| Missing, n (%) | 10 (38.5) | 16 (64.0) | 26 (51.0) |
| 12 months (T3) | |||
| n (%) | 8 (30.8) | 5 (20.0) | 13 (25.5) |
| Mean | 15.5 | 20.8 | 17.5 |
| SD | 5.32 | 2.49 | 5.07 |
| Minimum | 8 | 17 | 8 |
| Median | 16 | 21 | 18 |
| Maximum | 23 | 23 | 23 |
| Missing, n (%) | 18 (69.2) | 20 (80.0) | 38 (74.5) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 6 (23.1) | 3 (12.0) | 9 (17.6) |
| Mean | –1.5 | 2.0 | –0.3 |
| SD | 2.66 | 1.00 | 2.78 |
| Minimum | –6 | 1 | –6 |
| Median | –1.5 | 2.0 | 0.0 |
| Maximum | 2 | 3 | 3 |
| Missing, n (%) | 20 (76.9) | 22 (88.0) | 42 (82.4) |
Total fear score (all patient participants, including those with missing clinical research form data)
| Total fear scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 23 (88.5) | 23 (92.0) | 46 (90.2) |
| Mean | 15.2 | 13.6 | 14.4 |
| SD | 5.32 | 5.80 | 5.56 |
| Minimum | 5 | 3b | 3b |
| Median | 16 | 14 | 14 |
| Maximum | 24 | 25 | 25 |
| Missing, n (%)c | 3 (11.5) | 2 (8.0) | 5 (9.8) |
| 12 months (T3) | |||
| n (%) | 16 (61.5) | 17 (68.0) | 33 (64.7) |
| Mean | 14.4 | 16.0 | 15.2 |
| SD | 4.88 | 4.15 | 4.52 |
| Minimum | 5 | 10 | 5 |
| Median | 15 | 16 | 16 |
| Maximum | 23 | 23 | 23 |
| Missing, n (%)c | 10 (38.5) | 8 (32.0) | 18 (35.3) |
| Change from baseline (T0) at 12 months (T3)d | |||
| n (%) | 15 (57.7) | 17 (68.0) | 32 (62.8) |
| Mean | 0.6 | 1.5 | 1.1 |
| SD | 4.03 | 7.25 | 5.89 |
| Minimum | –6 | –10 | –10 |
| Median | 0 | 1 | 0 |
| Maximum | 9 | 18 | 18 |
| Missing, n (%) | 11 (42.3) | 8 (32.0) | 19 (37.2) |
Total fear score (significant other participants with complete clinical research form data)
| Total fear scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 6 (33.3) | 4 (21.1) | 10 (27.0) |
| Mean | 15.5 | 16.0 | 15.7 |
| SD | 3.45 | 3.92 | 3.43 |
| Minimum | 10 | 11 | 10 |
| Median | 15.5 | 16.5 | 15.5 |
| Maximum | 20 | 20 | 20 |
| Missing, n (%) | 12 (66.7) | 21 (78.9) | 27 (73.0) |
| 12 months (T3) | |||
| n (%) | 6 (33.3) | 2 (10.5) | 8 (21.6) |
| Mean | 20.7 | 22.5 | 21.1 |
| SD | 3.78 | 0.71 | 3.31 |
| Minimum | 16 | 22 | 16 |
| Median | 22.0 | 22.5 | 22.5 |
| Maximum | 24 | 23 | 24 |
| Missing, n (%) | 12 (67.7) | 17 (89.5) | 29 (78.4) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 3 (16.7) | 0 | 3 (8.8) |
| Mean | 6.7 | NAc | 6.7 |
| SD | 7.02 | NAc | 7.02 |
| Minimum | 0 | NAc | 0 |
| Median | 6 | NAc | 6.0 |
| Maximum | 14 | NAc | 14 |
| Missing, n (%) | 15 (83.3) | 19 (100.0) | 34 (91.2) |
Total fear score (all significant other participants, including those with missing clinical research form data)
| Total fear scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 16 (88.9) | 17 (89.5) | 33 (89.2) |
| Mean | 13.8 | 12.4 | 13.1 |
| SD | 4.83 | 5.10 | 4.95 |
| Minimum | 4b | 1b | 1b |
| Median | 14.5 | 13.0 | 14.0 |
| Maximum | 20 | 20 | 20 |
| Missing, n (%)c | 2 (11.1) | 2 (10.5) | 4 (10.8) |
| 12 months (T3) | |||
| n (%) | 11 (61.1) | 9 (47.4) | 20 (54.0) |
| Mean | 15.6 | 16.0 | 15.8 |
| SD | 6.59 | 5.00 | 5.78 |
| Minimum | 5 | 9 | 5 |
| Median | 16 | 16 | 16 |
| Maximum | 24 | 23 | 24 |
| Missing, n (%)c | 7 (38.9) | 10 (52.6) | 17 (46.0) |
| Change from baseline (T0) at 12 months (T3)d | |||
| n (%) | 9 (50.0) | 8 (42.1) | 17 (45.9) |
| Mean | 2.2 | 1.9 | 2.1 |
| SD | 5.40 | 4.76 | 4.96 |
| Minimum | –4 | –5 | –5 |
| Median | 0.0 | 2.5 | 0.0 |
| Maximum | 14 | 7 | 14 |
| Missing, n (%) | 9 (50.0) | 11 (57.9) | 20 (54.1) |
Confidence in managing seizures/epilepsy
Total mastery score (patient participants with complete clinical research form data)
| Total mastery scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 26 (100.0) | 21 (84.0) | 47 (92.3) |
| Mean | 13.9 | 12.1 | 13.1 |
| SD | 2.86 | 2.95 | 3.00 |
| Minimum | 8 | 7 | 7 |
| Median | 14 | 12 | 13 |
| Maximum | 20 | 19 | 20 |
| Missing, n (%) | 0 (0.0) | 4 (16.0) | 4 (7.8) |
| 6 months (T2) | |||
| n (%) | 21 (84.6) | 15 (60.0) | 36 (70.6) |
| Mean | 12.7 | 11.7 | 12.3 |
| SD | 3.89 | 2.97 | 3.53 |
| Minimum | 7 | 6 | 6 |
| Median | 11 | 12 | 11 |
| Maximum | 21 | 17 | 21 |
| Missing, n (%) | 4 (15.4) | 10 (40.0) | 15 (29.4) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 16 (64.0) | 34 (66.7) |
| Mean | 13.8 | 11.3 | 12.6 |
| SD | 3.47 | 2.60 | 3.29 |
| Minimum | 9 | 6 | 6 |
| Median | 13.5 | 12.0 | 12.0 |
| Maximum | 21 | 16 | 21 |
| Missing, n (%) | 8 (30.8) | 9 (36.0) | 17 (33.3) |
| Change from baseline (T0) at 6 months (T2)b | |||
| n (%) | 21 (80.8) | 12 (48.0) | 33 (64.7) |
| Mean | –0.7 | –1.4 | –0.9 |
| SD | 3.71 | 3.34 | 3.54 |
| Minimum | –8 | –8 | –8 |
| Median | –1 | –2 | –1 |
| Maximum | 8 | 4 | 8 |
| Missing, n (%) | 5 (19.2) | 13 (52.0) | 18 (35.3) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 18 (69.2) | 14 (56.0) | 32 (62.7) |
| Mean | 0.2 | –1.1 | –0.4 |
| SD | 2.96 | 3.41 | 3.17 |
| Minimum | –5 | –9 | –9 |
| Median | 0.5 | –1.0 | 0.0 |
| Maximum | 6 | 3 | 6 |
| Missing, n (%) | 8 (30.8) | 11 (44.0) | 19 (37.3) |
Total mastery score (all patient participants, including those with missing clinical research form data)
| Total mastery scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 26 (100.0) | 25 (100.0) | 51 (100) |
| Mean | 13.9 | 11.4 | 12.7 |
| SD | 2.86 | 3.50 | 3.40 |
| Minimum | 8 | 3b | 3b |
| Median | 14 | 12 | 13 |
| Maximum | 20 | 19 | 20 |
| Missing, n (%)c | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months (T2) | |||
| n (%) | 22 (84.6) | 17 (68.0) | 39 (76.5) |
| Mean | 12.3 | 11.4 | 11.9 |
| SD | 4.32 | 2.91 | 3.76 |
| Minimum | 3d | 6 | 3d |
| Median | 11 | 11 | 11 |
| Maximum | 21 | 17 | 21 |
| Missing, n (%)c | 4 (15.4) | 8 (32.0) | 12 (23.5) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 13.8 | 11.5 | 12.7 |
| SD | 3.47 | 2.60 | 3.25 |
| Minimum | 9 | 6 | 6 |
| Median | 13.5 | 12.0 | 12.0 |
| Maximum | 21 | 16 | 21 |
| Missing, n (%)c | 8 (30.8) | 8 (32.0) | 16 (31.4) |
| Change from baseline (T0) at 6 months (T2)c | |||
| n (%) | 22 (84.6) | 17 (68.0) | 39 (76.5) |
| Mean | –1.2 | –0.2 | –0.8 |
| SD | 4.35 | 4.25 | 4.28 |
| Minimum | –12 | –8 | –12 |
| Median | –1 | –1 | –1 |
| Maximum | 8 | 11 | 11 |
| Missing, n (%) | 4 (15.4) | 8 (32.0) | 12 (23.5) |
| Change from baseline (T0) at 12 months (T3)c | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 0.2 | –0.5 | –0.2 |
| SD | 2.96 | 3.36 | 3.13 |
| Minimum | –5 | –9 | –9 |
| Median | 0.5 | 0.0 | 0.0 |
| Maximum | 6 | 3 | 6 |
| Missing, n (%) | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Total confidence score (significant other participants with complete clinical research form data)
| Total confidence scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 18 (100.0) | 19 (100.0) | 37 (100.0) |
| Mean | 3.5 | 3.8 | 3.6 |
| SD | 0.62 | 0.72 | 0.69 |
| Minimum | 2.0 | 2.3 | 2.0 |
| Median | 3.5 | 3.8 | 3.7 |
| Maximum | 4.5 | 5.0 | 5.0 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months (T2) | |||
| n (%) | 16 (88.9) | 9 (47.4) | 25 (67.6) |
| Mean | 4.2 | 3.9 | 4.1 |
| SD | 0.75 | 0.71 | 0.73 |
| Minimum | 2.7 | 2.8 | 2.7 |
| Median | 4.1 | 4.0 | 4.0 |
| Maximum | 5.0 | 4.8 | 5.0 |
| Missing, n (%) | 2 (11.1) | 10 (52.6) | 12 (32.4) |
| 12 months (T3) | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 4.3 | 4.1 | 4.2 |
| SD | 0.57 | 0.99 | 0.78 |
| Minimum | 3.5 | 2.2 | 2.2 |
| Median | 4.2 | 4.7 | 4.3 |
| Maximum | 5.0 | 5.0 | 5.0 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
| Change from baseline (T0) at 6 months (T2)b | |||
| n (%) | 16 (88.9) | 9 (47.4) | 25 (67.6) |
| Mean | 0.6 | 0.2 | 0.5 |
| SD | 0.70 | 0.32 | 0.61 |
| Minimum | –0.8 | –0.2 | –0.8 |
| Median | 0.6 | 0.2 | 0.2 |
| Maximum | 1.7 | 0.8 | 1.7 |
| Missing, n (%) | 3 (11.1) | 10 (52.6) | 12 (32.4) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 0.7 | 0.3 | 0.5 |
| SD | 0.49 | 0.43 | 0.50 |
| Minimum | 0.0 | –0.2 | –0.2 |
| Median | 0.7 | 0.3 | 0.5 |
| Maximum | 1.7 | 1.0 | 1.7 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Total confidence score (all significant other participants, including those with missing clinical research form data)
| Total confidence scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 18 (100.0) | 19 (100.0) | 37 (100.0) |
| Mean | 3.5 | 3.8 | 3.6 |
| SD | 0.62 | 0.72 | 0.69 |
| Minimum | 2.0 | 2.3 | 2.0 |
| Median | 3.5 | 3.8 | 3.7 |
| Maximum | 4.5 | 5.0 | 5.0 |
| Missing, n (%)b | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 6 months (T2) | |||
| n (%) | 16 (88.9) | 9 (47.4) | 25 (67.6) |
| Mean | 4.2 | 3.9 | 4.1 |
| SD | 0.75 | 0.71 | 0.73 |
| Minimum | 2.7 | 2.8 | 2.7 |
| Median | 4.1 | 4.0 | 4.0 |
| Maximum | 5.0 | 4.8 | 5.0 |
| Missing, n (%)b | 2 (11.1) | 10 (52.6) | 12 (32.4) |
| 12 months (T3) | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.6) |
| Mean | 4.3 | 4.1 | 4.2 |
| SD | 0.57 | 0.99 | 0.78 |
| Minimum | 3.5 | 2.2 | 2.2 |
| Median | 4.2 | 4.7 | 4.3 |
| Maximum | 5.0 | 5.0 | 5.0 |
| Missing, n (%)b | 7 (38.9) | 9 (47.4) | 16 (43.24) |
| Change from baseline (T0) at 6 months (T2)c | |||
| n (%) | 16 (88.9) | 9 (47.4) | 25 (67.6) |
| Mean | 0.6 | 0.2 | 0.5 |
| SD | 0.70 | 0.32 | 0.61 |
| Minimum | –0.8 | –0.2 | –0.8 |
| Median | 0.6 | 0.2 | 0.2 |
| Maximum | 1.7 | 0.8 | 1.7 |
| Missing, n (%) | 3 (11.1) | 10 (52.6) | 12 (32.4) |
| Change from baseline (T0) at 12 months (T3)c | |||
| n (%) | 11 (61.1) | 10 (52.6) | 21 (56.8) |
| Mean | 0.7 | 0.3 | 0.5 |
| SD | 0.49 | 0.43 | 0.50 |
| Minimum | 0.0 | –0.2 | –0.2 |
| Median | 0.7 | 0.3 | 1.0 |
| Maximum | 1.7 | 1.0 | 1.7 |
| Missing, n (%) | 7 (38.9) | 9 (47.4) | 16 (43.2) |
Knowledge of what to do when faced with a seizure
Total knowledge score (patient participants)
| Total knowledge scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 26 (100.0) | 25 (100.0) | 51 (100) |
| Mean | 9.0 | 9.0 | 9.0 |
| SD | 4.05 | 4.19 | 4.08 |
| Minimum | 0 | 0 | 0 |
| Median | 10 | 11 | 11 |
| Maximum | 13 | 13 | 13 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 months (T3) | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | 9.4 | 9.4 | 9.4 |
| SD | 3.68 | 3.69 | 3.63 |
| Minimum | 1 | 3 | 1 |
| Median | 10.5 | 11.0 | 6.0 |
| Maximum | 13 | 13 | 13 |
| Missing, n (%) | 8 (30.8) | 8 (32.0) | 16 (31.4) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 18 (69.2) | 17 (68.0) | 35 (68.6) |
| Mean | –0.4 | 0.8 | 0.1 |
| SD | 3.62 | 3.15 | 3.41 |
| Minimum | –8 | –8 | –8 |
| Median | 0 | 1 | 0 |
| Maximum | 7 | 5 | 7 |
| Missing, n (%) | 8 (30.8) | 8 (32.0) | 16 (31.4) |
Total knowledge score (significant other participants)
| Total knowledge scorea | Treatment arm | Total | |
|---|---|---|---|
| SAFE plus TAU | TAU | ||
| Baseline (T0) | |||
| n (%) | 18 (100.0) | 19 (100.0) | 37 (100.0) |
| Mean | 9.9 | 7.7 | 8.8 |
| SD | 3.07 | 4.19 | 3.81 |
| Minimum | 4 | 1 | 1 |
| Median | 11 | 7 | 9 |
| Maximum | 13 | 13 | 13 |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 12 months (T3) | |||
| n (%) | 15 (83.3) | 13 (68.4) | 28 (75.7) |
| Mean | 7.4 | 8.15 | 7.8 |
| SD | 5.36 | 5.40 | 5.29 |
| Minimum | 0 | 0 | 0 |
| Median | 9 | 11 | 9.5 |
| Maximum | 13 | 13 | 13 |
| Missing, n (%) | 3 (16.7) | 6 (31.6) | 9 (24.3) |
| Change from baseline (T0) at 12 months (T3)b | |||
| n (%) | 15 (83.3) | 13 (68.4) | 28 (75.7) |
| Mean | –2.0 | –0.8 | –1.5 |
| SD | 5.03 | 5.01 | 4.96 |
| Minimum | –12 | –12 | –12 |
| Median | 0 | 0 | 0 |
| Maximum | 4 | 8 | 8 |
| Missing, n (%) | 3 (16.7) | 6 (31.6) | 9 (24.3) |
Appendix 19 Adverse events occurring during pilot trial in descending order, according to frequency overall
| Adverse event | Treatment arm | Total | |||||
|---|---|---|---|---|---|---|---|
| SAFE plus TAU | TAU | ||||||
| Category of event (e.g. body system) | Event | Events, n | Patients, n (%) | Events, n | Patients, n (%) | Events, n | Patients, n (%) |
| Eyes, ear, nose and throat | Problem with eyes and sinuses | 0 | 0 (0.0) | 1 | 1 (9.1) | 1 | 1 (5.6) |
| Genitourinary | Overnight hospital admission required owing to urinary tract infection (pre-existing) | 0 | 0 (0.0) | 1 | 1 (9.1) | 1 | 1 (5.6) |
| Haematological | Dislocated shoulder | 0 | 0 (0.0) | 1 | 1 (9.1) | 1 | 1 (5.6) |
| Neoplasiaa | Change in seizure pattern | 1 | 1 (14.3) | 0 | 0 (0.0) | 1 | 1 (5.6) |
| Increase in seizure frequency | 1 | 1 (14.3) | 0 | 0 (0.0) | 1 | 1 (5.6) | |
| Neurological | Increased seizures; medications changed | 1 | 1 (14.3) | 3 | 3 (27.3) | 4 | 4 (22.2) |
| Increased seizures (shift patterns at work) | 1 | 1 (14.3) | 0 | 0 (0.0) | 1 | 1 (5.6) | |
| New seizure type; frequent absence seizures as well as usual seizure types | 1 | 1 (14.3) | 0 | 0 (0.0) | 1 | 1 (5.6) | |
| Seizure frequency; sodium levels requiring in patient monitoring | 0 | 0 (0.0) | 1 | 1 (9.1) | 1 | 1 (5.6) | |
| Seizures more severe (tonic–clonic) | 0 | 0 (0.0) | 1 | 1 (9.1) | 1 | 1 (5.6) | |
| Vagus nerve stimulation not working properly; going to hospital for observation | 0 | 0 (0.0) | 1 | 1 (9.1) | 1 | 1 (5.6) | |
| Respiratory | Increases number of seizures owing to chest infection and antibiotics | 0 | 0 (0.0) | 1 | 1 (9.1) | 1 | 1 (5.6) |
| Other | Adverse events occurring during pilot trial increase in the number of fits owing to being pregnant/just given birth | 0 | 0 (0.0) | 2 | 1 (9.1) | 2 | 1 (5.6) |
| Diagnosis of status epilepticus | 1 | 1 (14.3) | 0 | 0 (0.0) | 1 | 1 (5.6) | |
| Vertigo | 0 | 0 (0.0) | 1 | 1 (9.1) | 1 | 1 (5.6) | |
| Total | 6 | 6 (100.0) | 13 | 11 (100.0) | 19 | 18 (100.0) | |
List of abbreviations
- A&E
- accident and emergency department
- APEASE
- Affordability, Practicality, Effectiveness and cost-effectiveness, Acceptability, Side-effects/safety and Equity
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CR
- central range
- CRF
- clinical research form
- CTU
- Clinical Trials Unit
- ED
- emergency department
- ES
- Epilepsy Society
- FG
- focus group
- GP
- general practitioner
- GCSE
- General Certificate of Secondary Education
- HADS
- Hospital Anxiety and Depression Scale
- HES
- Hospital Episode Statistics
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- NASH
- National Audit of Seizure management in Hospitals
- NBR
- negative binomial regression
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NVQ
- National Vocational Qualification
- O level
- Ordinary level
- PABAK-OS
- prevalence-adjusted bias-adjusted kappa for ordinal scales
- PWE
- people with epilepsy
- QOLIE-31-P
- Quality of Life in Epilepsy Scale-31 item-Patient-weighted
- RCT
- randomised controlled trial
- REC
- research ethics committee
- SAE
- serious adverse event
- SAFE
- Seizure first Aid training For Epilepsy
- SD
- standard deviation
- SMILE
- Self-Management education for adults with poorly controlled epILEpsy
- SO
- significant other
- TAU
- treatment as usual
- TIDieR
- Template for Intervention Description and Replication
- VAT
- value-added tax
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hsdr08390).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
