Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 14/70/162. The contractual start date was in June 2016. The final report began editorial review in April 2019 and was accepted for publication in December 2019. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2021. This work was produced by Geary et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2021 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Urinary incontinence (UI), the involuntary loss of urine, affects between 25% and 45% of women at some point in their lives,1 which has a substantial impact on their quality of life. 2–5 Prevalence increases with age, affecting an estimated 17% of women aged > 20 years and 38% of women aged > 60 years. 6–8 However, despite this high prevalence and the impact on quality of life, there is evidence that UI is underdiagnosed and undertreated. Only 25% of women affected by UI seek care and, of those, fewer than half receive treatment, suggesting that there is a high unmet need for care. 9–11 If left untreated, UI is associated with falls and fractures, depression, sleep disturbance and urinary tract infections (UTIs). 12–14 Unplanned admissions for UTIs cost the NHS > £400M per year. 15
Types of urinary incontinence
Female urinary incontinence can be classified into subtypes. Stress urinary incontinence (SUI) is the involuntary loss of urine with increases in abdominal pressure, such as when exercising or coughing. Urgency urinary incontinence (UUI) is characterised by a sudden and compelling desire to pass urine that is difficult to defer. Overactive bladder syndrome, which can include UUI, is usually accompanied by frequency and nocturia. Many women experience coexisting stress and urgency UI symptoms, a subtype often called mixed urinary incontinence (MUI). However, women do not require extensive preliminary evaluation of the subtype of urinary incontinence before beginning initial non-invasive treatments as symptoms may commence without clear differentiation between the two most common subtypes: stress and urgency UI. Stress UI appears to be the most common subtype of UI. Epidemiological evidence suggests that about 50% of women with UI indicate that they have solely SUI symptoms and about 40% indicate symptoms suggesting that they have MUI. 16,17
Treatment
Non-invasive treatment
General practices are the gatekeepers of health care in the English NHS, acting as the first point of contact for non-emergency health issues, which may then be managed in primary care and/or referred to secondary care. In the UK, UI is initially managed at the primary care level. 11 Lifestyle changes may be recommended in primary care when women with UI also smoke cigarettes, report excessive fluid or caffeine consumption, or are overweight or obese. 18 Referral to a urinary incontinence specialist is available when non-surgical treatments do not improve symptoms or are not acceptable to women. Urgent referral (i.e. within 2 weeks) is recommended if there are concerns about conditions, such as cancer. 19
Surgical treatment
For women whose UI symptoms are predominantly SUI, the standard surgical treatments have been colposuspension (a major abdominal surgery) or a sling, with autologous fascia, or synthetic mesh. Women with SUI can also undergo urethral bulking injections to increase outflow resistance. Mid-urethral mesh tapes (MUTs) or slings were introduced in 1998 as a minimally invasive surgical treatment for female SUI. 2 The use of MUTs or slings rose precipitously in the decade following their introduction, with a corresponding decline in the use of colposuspension. 20 Their use has since rapidly declined with a change in patient choice and surgical practice that is likely to reflect growing publicity of concerns about longer-term complications after MUT procedures. 21–26 For women with UI symptoms that are predominantly UUI, invasive but predominantly outpatient/day case procedures include percutaneous tibial nerve stimulation (electrical stimulation via an acupuncture needle weekly for 3 months and monthly thereafter), OnabotulinumtoxinA (Botox, Allergan) injections and sacral neuromodulation (with an implanted electrode placed along the third sacral nerve root).
The project began in June 2016, immediately after discussions began about problems that some women experience after MUT insertion, such as pain, dyspareunia, persistent UI and mesh exposure or erosion. During the first 2 years of the project, the use of MUTs, then the most common surgical treatment for SUI, continued in the NHS England; however, the need for a multidisciplinary approach and better information for women considering surgery was highlighted. In response to this, we added an additional work package (WP) to the study to explore long-term removal and reoperation rates after MUT insertion. The routine use of MUTs for SUI was then ‘paused’ by NHS England in July 2018 following recommendations by the Independent Medicines and Medical Devices Safety Review27 that had engaged with patients and patient groups about complications. 28
In the UK, despite major national initiatives in the last decade, the quality and availability of continence services remains poor, variable and inequitable. The burden on secondary care resources is increasing because of demographic changes and higher referral rates. For women with SUI, rates of surgery have increased in the decade following the introduction of new procedures (i.e. MUTs) in 1998, but there is evidence of inequity in access and service provision, with concerns of underprovision in vulnerable groups. For example, although it has been shown that the minimally invasive procedures are as safe and effective in older women29 and can often be performed as a day-case procedure, these procedures are less frequently used in older patients than in younger patients. 30,31 It is likely that there is also suboptimal care for women from minority ethnic and different socioeconomic backgrounds. 32,33
There is considerable uncertainty about whether or not the level of provision for surgical services for women with SUI is uniform across regions and providers in England. Variations in care provision may also depend on the availability of UI services. A recent survey of the availability of specialist UI services has demonstrated that there is variable distribution of urogynaecologists with subspecialty training, dedicated teams to manage repeat surgery and availability of various surgical care treatments across the UK. 34 Information on variation in practice is important for examining relationships between policy decisions and clinical decisions and raises questions concerning the efficiency and effectiveness of health care. 35
There are also unanswered questions about assessment and treatment of women before referral, the duplication of treatment in each care environment and the appropriate delivery of secondary care interventions. 36 More than 50% of patients referred to secondary care are reported not to have received any treatment in primary care. 34
Patient perspectives
There is limited research on which treatments women with UI want themselves and which factors have an impact on their preferences. Moreover, to the best of our knowledge, there is no research to date on how general practitioners (GPs) decide when women need to be referred and how clinicians decide whether or not surgery is helpful. Although UI can have a substantial impact on the quality of life, there is evidence that many women with UI under-report or delay seeking treatment for several years after the problem has become bothersome,37 leading to high levels of unmet need for incontinence services. 38 For example, in the UK, only about one-quarter of women consult a doctor about their symptoms. 39 Delayed health-seeking behaviour and under-reporting might be caused by the belief that these symptoms are normal after childbirth or in old age and by a lack of awareness of available treatment options. 40,41
Clinical coding
It is recognised that UI and related procedures are poorly coded in electronic clinical records. Therefore, before existing administrative health-care databases can be used to study service provision in primary and secondary care, a detailed coding framework needs to be developed to overcome these deficiencies. This methodological work not only is an essential preparation for data analysis, but will also guide recommendations on how diagnoses and procedures related to UI can be better recorded in electronic databases in the future.
The structure of this report is as follows:
-
In Chapter 2, we outline the project’s aim and objectives and the corresponding six WPs.
-
In Chapter 3, we describe the data and methods used in each WP.
-
In Chapter 4, we summarise geographic variation in rates of surgery for SUI.
-
In Chapter 5, we describe the determinants of referral and surgery for UI.
-
In Chapter 6, we summarise long-term rates of mesh tape removal after MUT surgery for SUI.
-
In Chapter 7, we explore the impact of UI on women’s lives and when surgery is perceived to be a treatment option.
-
In Chapter 8, we explore gynaecologists’ approaches to recommending surgical treatment for women with UI.
-
In Chapter 9, we bring together the findings from each WP in terms of how they relate to the scientific literature and discuss their implications for future research.
Chapter 2 Aim and objectives
Overall aims
The aim of the project was to improve the delivery and organisation of surgical services for women with UI in England. We assessed the availability and use of surgical services for UI across NHS England and identified factors that explain observed variation in use, including the impact of administrative data issues, patients’ experiences and expectations, clinicians’ judgement, and organisational and contextual factors. During the first 2 years of the project, an additional objective was added to this project (i.e. WP 6) to explore the long-term removal and reoperation rates after MUT insertion for SUI.
Objectives
The objectives were captured in five WPs. We analysed existing primary and secondary care data sets and collected data from patients (using in-depth interviews) and clinicians (using case vignettes or ‘paper patients’).
-
Objective 1: methods development –
-
assesses the consistency, completeness and accuracy of diagnostic and procedure coding for UI in existing electronic data sets (WP 1)
-
develops a coding framework for UI allowing for divergent coding practices among providers (WP 1).
-
-
Objective 2: availability and delivery of services –
-
assesses variation between NHS Clinical Commissioning Groups (CCGs), Local Area Teams and Clinical Senates (or other relevant regional units) in rate of surgery for UI (WP 2)
-
examines the impact of supply-side factors (e.g. primary care characteristics and availability and delivery of secondary care services) on local surgical rates (WP 2).
-
-
Objective 3: understanding patients’ experiences and expectations –
-
explores the impact of UI on women’s lives and if and when it is perceived to be a medical problem (WP 3)
-
collects women’s own accounts of experiences and expectations of surgical and non-surgical treatments and outcomes, including the many different values that women draw on (WP 3).
-
-
Objective 4: understanding the determinants of referral and surgical treatment –
-
identifies determinants of outpatient referrals and surgery, using a linked primary–secondary care data set (WP 4)
-
explores the relative importance of specific patient characteristics for clinicians in their treatment decisions, using case vignettes (WP 5).
-
An additional objective was to explore the long-term removal and reoperation rates after MUT insertion for SUI (WP 6).
Within most WPs, further work was carried out to evaluate how findings vary according to age, economic deprivation, ethnicity and type of procedure.
Chapter 3 Overview of methods
A mixed-methods study was undertaken to assess the availability and use of surgical services for UI across England and to identify factors that could explain observed variation in practice, including the impact caused by data issues, patients’ experiences and expectations, clinicians’ judgement, and organisational and contextual factors.
Data sources
The Hospital Episode Statistics (HES) database contains information on each episode of admitted patient care (APC) in English NHS hospital trusts. These data are extracted from local patient administration systems as part of the Commissioning Data Set. This data set is submitted to NHS Digital for processing and made available for audit and research as the HES data set. 42 Each record contains data on patient demographics (e.g. age, sex, ethnicity and area of residence), the episode of care (e.g. hospital name, date of admission and discharge) and clinical information. Diagnoses are recorded using the International Classification of Diseases, Tenth Revision (ICD-10),43 and procedures using the Office of Population, Censuses and Surveys Classification of Interventions and Procedures, version 4 (OPCS-4). 44 Each patient is assigned a unique identifier, making it possible to study longitudinal patterns of care, including tracking any future admission or procedure in the same or a different NHS hospital.
The Clinical Practice Research Datalink (CPRD) (formerly the General Practice Research Database) collates routinely collected anonymised patient data from general practices that have agreed at a practice level to provide data. All patients registered with participating practices are included in the data set, unless they have individually requested to opt out of data sharing at their GP practice. CPRD uses Read codes (used to record symptoms, diagnoses, processes of care) and British National Formulary codes to record information on prescriptions. More than 600 GP practices contribute data to CPRD, covering 9% of the population. CPRD linkage data include patients from 411 practices, covering approximately 75% of contributing CPRD practices in England. 45 Compared with the 2011 UK census, CPRD patients are broadly representative of the UK population in terms of age, sex and ethnicity, and comparable for body mass index (BMI) distribution to the Health Survey for England. 46 CPRD is deemed ‘up to standard’ (UTS) for research purposes and widely used in epidemiological and health services research. CPRD data have been linked to HES APC and HES Outpatient data by the trusted third party, NHS Digital. Three distinct administrative health-care data sets were used in this project: HES APC, CPRD and CPRD-linked to HES APC and HES Outpatient. We indicate throughout this report which data were used by each WP.
In addition to using the administrative health-care databases HES and CPRD, we conducted primary data collection. This data collection comprised interviews with women who had been referred to secondary care and were considering surgical treatment, and an online survey of gynaecologists using clinical case vignettes (i.e. hypothetical patients). The primary data collection is described in detail in the sections below for WPs 3 and 5.
Methods for work package 1
Work package 1 aimed to assess the consistency, completeness and accuracy of diagnostic and procedure coding for UI in the HES and the CPRD databases, and to develop a coding framework for UI allowing for divergent coding practices among providers.
In the HES database, we first used a ‘forward and backward’ searching strategy. The forward searching step began with a list of ICD-10 and OPCS-4 codes that were compiled iteratively by the clinical and methodological members of the research team. The backwards strategy then explored whether or not records found on the basis of the prespecified ICD-10 codes contain additional relevant OPCS-4 codes that were not prespecified. A similar exercise considers additional ICD-10 codes in records found on the basis of the prespecified OPCS-4 codes. Second, we assessed the frequency with which all of these potentially relevant codes have been used and explore the consistency of diagnosis and procedure codes within the records of individual patients. Third, the variation in consistency of diagnosis and procedure codes among providers was explored to identify providers that have divergent coding practices. We have previously demonstrated that this strategy for using HES data has the potential to produce a coding framework creating groups of patients who are homogeneous with respect to diagnosis, prognosis and treatment. 47
In the CPRD database, we started with a previously published code list. 48 We tested this existing code list by evaluating the consistency between diagnostic and treatment codes and the temporal consistency of coding within records of the same patient. Next, additional relevant keywords, synonyms and possible codes were identified by searching additional published literature and code list repositories. Finally, the clinicians on the research team manually reviewed the code list (individually and collectively). Recommendations made by each clinician were collated and collectively discussed with the aim of reaching a consensus on the codes that would be regarded as UI diagnosis or treatment codes. The coding work conducted for WP 1 fed directly into the analyses conducted for WPs 2, 4 and 6.
Methods for work package 2
Work package 2 aimed to assess variation in rates of surgery for SUI using HES APC (inpatient) data. We examined variation in rates of SUI surgery between NHS CCGs and other relevant regional units, and the impact of supply-side factors (e.g. primary care characteristics and availability and organisation of secondary care services) on local rates. WP 2 focused on variation in surgery for SUI specifically because SUI symptoms are the most common subtype of UI symptoms; 50% of women with UI indicate solely SUI symptoms and approximately 40% indicate MUI symptoms, where SUI and UUI symptoms coexist. 16,17
The cohort comprised women aged ≥ 20 years who had received surgical treatment for SUI between 1 April 2013 and 31 March 2016 and had a SUI diagnosis recorded at the time of the procedure. SUI surgery was defined using UK OPCS-4 codes (Table 1) based on coding work conducted for WP 1. 44 SUI diagnosis was defined using the ICD-10 code N39.3 Stress urinary incontinence. 43
| OPSC-4 | Description |
|---|---|
| Mid-urethral mesh tape insertions | |
| M53.3 | Introduction of tension-free vaginal tape |
| M53.6 | Introduction of transobturator tape |
| Injection of urethral bulking agents | |
| M56.3 | Endoscopic injection of inert substance into the outlet of the female bladder |
| Other abdominal/vaginal operations | |
| M51.1 | Abdominoperineal suspension of the urethra |
| M51.2 | Endoscopic suspension of the neck of the bladder |
| M51.8 | Other specified combined abdominal and vaginal operations to support the outlet of the female bladder |
| M51.9 | Unspecified combined abdominal and vaginal operations to support the outlet of the female bladder |
| M52.1 | Suprapubic sling operation |
| M52.2 | Retropubic suspension of the neck of the bladder |
| M52.3 | Colposuspension of the neck of the bladder |
| M52.8 | Other specified abdominal operations to support the outlet of the female bladder |
| M52.9 | Unspecified abdominal operations to support the outlet of the female bladder |
| M53.1 | Vaginal buttressing of the urethra |
| M53.8 | Other specified vaginal operations to support the outlet of the female bladder |
| M53.9 | Unspecified vaginal operations to support the outlet of the female bladder |
| M55.2 | Implantation of artificial urinary sphincter into the outlet of the female bladder |
| M55.6 | Insertion of a retropubic device for female SUI NEC |
| M55.8 | Other specified – other open operations on the outlet of the female bladder |
| M55.9 | Unspecified other – open operations on the outlet of the female bladder |
| M58.8 | Other specified – other operations on the outlet of the female bladder |
| M58.9 | Unspecified other operations on the outlet of the female bladder |
The outcome measure was rate of surgery for SUI per 100,000 women per year at two geographic levels: 209 CCGs and 44 Sustainability and Transformation Partnership (STP) areas. CCGs are statutory NHS bodies responsible for the planning and commissioning of health care services in a local area (with an average population size of about 104,000 adult females). CCG areas are grouped into 44 STP areas (with an average population size of about 493,000 adult females), which were set up to co-ordinate improvements in the delivery of NHS services. 49 Reference denominator populations were derived by aggregating the 2011 census population counts for women aged ≥ 20 years in lower-layer super output areas (LSOAs) that are within the respective boundaries of the CCG and STP areas. There are 32,844 LSOAs (postcode-based geographic units) in England (with an average population of approximately 1700 people). 50 Women may have had repeat procedures in the study period, but only the first procedure was counted in calculating the surgery rate.
Sociodemographic factors may explain variations in the rates of surgery for SUI. We adjusted for age, socioeconomic deprivation, ethnicity and limiting long-term illness in our regression models. We handled age as a patient-level characteristic grouped into five categories (i.e. 20–39, 40–49, 50–59, 60–69 and ≥ 70 years). Socioeconomic deprivation, ethnicity and limiting long-term illness were CCG-level characteristics derived from 2011 census data. 50 For socioeconomic deprivation, we used the averages of the national ranking of the Index of Multiple Deprivation (IMD) of LSOAs within each CCG, and grouped the CCG averages into national quintiles ranging from 1 (most deprived CCGs) to 5 (least deprived CCGs). 51 For ethnicity, we used the percentage of the population reporting a black, Asian and minority ethnic (BAME) background, and for long-term illness we used the percentage of the population who reported that their day-to-day activities were limited because of a health problem or disability that had lasted, or was expected to last, at least 12 months. For each CCG, we took the averages of these percentages for LSOAs and grouped these CCG averages into national quintiles (range: 1 corresponds to CCGs with average percentages in the lowest quintile to 5 the highest quintile).
We calculated the number and the unadjusted and adjusted rates per 100,000 women per year of SUI procedures overall, and according to patient and regional characteristics. Incidence rate ratios (IRRs) were used to represent associations between the procedure rate and regional characteristics. Multilevel Poisson regression models were used to produce empirical Bayes’ estimates of the unadjusted and adjusted incidence rates for each CCG and STP area. In addition, risk-adjusted regression models were used to assess geographic variation in the rates of surgery by year. The empirical Bayes’ estimator produces more precise results by ‘pulling’ estimates for small outlier regions towards the mean. 52 For each geographic area level (CCG/STP), we illustrated the amount of variation in adjusted surgery rates using maps and range plots with 99.8% credibility intervals. CCGs and STPs were marked as ‘outliers’ where the national average rate of surgery was not within the 99.8% credibility interval of their rates. All statistical analyses were performed using Stata, version 15 (StataCorp LP, College Station, TX, USA).
Methods for work package 3
Work package 3 aimed to explore the impact of UI on women’s lives and if and when surgery is perceived to be a treatment option, collecting women’s own accounts of expectations of surgical and non-surgical treatments, experiences and outcomes through semistructured interviews. WP 3 used primary data collected from interviews with women. All women considering surgery for their UI could participate in the interviews. We did not restrict the interviews to women with a specific subtype of UI (e.g. SUI or UUI).
A broad narrative review of the patient experience social sciences literature was conducted to map out the issues female UI raises that are in common with other conditions, including those related to shame; embarrassment and stigma; gender, identity and sexuality; social relationships, work and mobility; experiences of care; and medicalisation of the female body. These higher-order topics informed the development of the semistructured interview topic guide and generated the analytical themes that were later used to complement the more descriptive codes derived from the content analysis of the interviews. The topic guide (see Appendix 6) was also informed by discussion with the research team, especially drawing on the input from the public and patient representatives. The topic guide was designed so that interviews would be sufficiently open and flexible to ensure that participants are able to talk at length about issues that most concern them.
Women were recruited from four urogynaecology outpatient clinics in different parts of England: Birmingham, Gillingham (in Kent), Leicester and Southampton. Recruitment was limited to women being treated in England for practical reasons. Between May and December 2017, women who had been referred to these clinics, were aged ≥ 18 years, had not previously had urology surgery (for UI or a related condition) and were now considering surgery for their UI were approached about the project by members of the clinic teams (which included physiotherapists, nurses, surgical consultants and specialist registrars) as part of the patient’s routine appointments. Women who were potentially interested in participating were given an information sheet and form to complete and return directly to the qualitative members of the research team at the London School of Hygiene & Tropical Medicine if they wished to participate. Those women who completed the form were contacted by telephone or e-mail (depending on their preference) and given further information about the study and what the interview would involve. Interviews were then scheduled and conducted either face to face or by telephone (also according to the women’s preferences). Women were given the opportunity to ask further questions about the research before agreeing to participate. Written consent was obtained from all interviewees and women were also informed that they could withdraw their consent at any time, during or after the interviews. Women were reassured that the interviewer was not part of the clinic staff and that their decision to participate or not would have no influence on their care. Ethics approval for the study was granted by the NHS Health Research Authority (Research Ethics Committee reference number 16/IEC08/0044).
Interviewees were initially purposively sampled by region (clinic) and age. The geographical location of those women interviewed did not appear to influence their responses, whereas ageing emerged as a strong theme through the interviews. Interviewees were therefore then sampled by age alone, to ensure that women of a wide range of ages were included. Interviews lasted between 40 and 130 minutes, were audio-recorded and transcribed verbatim. Transcription was undertaken by an external company experienced in dealing with confidential data. Transcripts were imported and coded in NVivo 10 (QSR International, Warrington, UK). Initial coding was undertaken by the interviewer while recruitment and interviews were under way. Following a constant comparative method53,54 emerging themes from the interviews were followed up in more depth in subsequent interviews such that the topic guide became more refined and focused on areas that were most important to the participants themselves.
The codes, drawn both deductively from the interview topic guide and literature review and inductively through early analysis, were augmented and refined through the process of analysis and through discussion with the WP 3 lead. Codes were then ordered into higher-level themes and hierarchies, with codes that were similar collapsed together. These codes were then discussed with the rest of the research team, which included clinicians, leading to further refinement.
Methods for work package 4
Work package 4 aimed to identify determinants of gynaecology outpatient referrals and surgery for women with UI.
Analysis cohorts
Initial non-invasive treatments can be started in primary care without extensive evaluation of the main subtype of UI indicated by a woman’s symptoms. Subtype-specific diagnoses may therefore not be present in primary care (CPRD) data. We therefore defined the cohorts for the analysis of both determinants of referral and determinants of surgery after referral on the basis of a diagnosis of UI, not restricted by UI subtype.
Diagnoses of UI were defined using Read codes and MedCODES identified in WP 1 (Table 2). Referral to a specialist was defined using a combination of Read codes and referral specialty codes developed in WP 1 (see Appendix 4, Tables 18 and 19). Surgery for UI was defined using OPCS-4 codes (see Appendix 1, Tables 13–15).
| Read code | Read code chapter | MedCODES | Read code definition |
|---|---|---|---|
| 1593 | 1 – history and symptoms | 15918 | H/O: stress incontinence |
| 1A23.00 | 1 – history and symptoms | 6161 | Incontinence of urine |
| 1A24.00 | 1 – history and symptoms | 1929 | Stress incontinence |
| 1A24.11 | 1 – history and symptoms | 5844 | Stress incontinence – symptom |
| 1A26.00 | 1 – history and symptoms | 3887 | Urge incontinence of urine |
| K198.00 | K – genitourinary system diseases | 3182 | Stress incontinence |
| K586.00 | K – genitourinary system diseases | 17620 | Stress incontinence – female |
| Kyu5A00 | K – genitourinary system diseases | 52763 | [X] Other specified urinary incontinence |
| R083.00 | R – symptoms, signs and ill-defined conditions | 3283 | [D] Incontinence of urine |
| R083000 | R – symptoms, signs and ill-defined conditions | 4375 | [D] Enuresis – NOS |
| R083100 | R – symptoms, signs and ill-defined conditions | 31220 | [D] Urethral sphincter incontinence |
| R083200 | R – symptoms, signs and ill-defined conditions | 17320 | [D] Urge incontinence |
| R083z00 | R – symptoms, signs and ill-defined conditions | 15400 | [D] Incontinence of urine – NOS |
Determinants of referrals analysis
The cohort for identifying determinants of referrals was derived from the CPRD data set and comprised women aged ≥ 18 years who had an index diagnosis of UI between 1 April 2004 and 31 March 2014. An index diagnosis of UI was defined among women who had no earlier record of a UI symptom/diagnosis (see Appendix 2, Table 16) or treatment (see Appendix 3, Table 17) within the 12 months prior to the date of first diagnosis in the study period. For the referral analyses, women were followed up until the date of the GP visit that they were referred to a UI specialist, transfer out of practice, death or to 1 April 2014. Women with < 12 months of UTS data prior to index diagnosis were excluded. Women were also excluded if the follow-up period was < 30 days.
Determinants of surgery analysis
The cohort for identifying determinants of surgery after referral comprised women aged ≥ 18 years who had an index UI diagnosis (defined as described above) and a referral to a urinary incontinence specialist in secondary care between 1 April 2004 and 31 March 2014. The cohort of women for surgery after referral was derived from the CPRD linked to HES APC data set and was therefore restricted to women registered in primary care practices that had linked CPRD–HES data. Women were followed up from the date of referral until the date of surgery, transfer out of practice, death or to 1 April 2014.
Outcome measures
Determinants of referrals analysis
For the determinants of referrals analysis, the outcome measure was referral to a UI specialist within 30 days of diagnosis.
Determinants of surgery analysis
For the determinants of surgery analysis, the primary outcome measure was risk of any UI surgery. (The codes for SUI surgery are shown in Table 1 and the codes for UUI surgery are shown in Appendix 1, Table 14.)
Potential determinants of referral and surgery
Potential determinants of both referral and surgery (defined a priori with the project team clinicians) were age at index diagnosis (analysed as 18–39, 40–49, 50–59, 60–69, 70–79, ≥ 80 years), BMI (< 20 kg/m2 = underweight, 20–24 kg/m2 = normal, 25–29 kg/m2 = overweight, 30–39 kg/m2 = obese, ≥ 40 kg/m2 = severely obese), smoking (non-, current or former smoker) and ethnic background (white, Asian/Asian-British, black/black-British, mixed or other ethnic group, or missing) and comorbidities. The comorbidities included were pelvic organ prolapse (POP), UTI, type 2 diabetes mellitus (T2DM), cardiovascular disease (CVD) (as defined as any cardiovascular or ischaemic heart disease, heart failure or hypertension), renal disease, respiratory disease [asthma or chronic obstructive pulmonary disease (COPD)], anxiety or depression and cancer. Comorbidities were defined using Read codes (from the clinical codes repository www.clinicalcodes.org; accessed 6 April 2020) in the 12 months before the start of follow-up (‘index date’), apart from UTI (defined as in the 30 days before). The ‘index date’ was the date of UI diagnosis for the referrals analysis and the referral date for the surgery analysis. A clinical code repository list was not available for POP, so this code list was developed for this project by the research team, including the clinicians (see Appendix 5, Table 20). BMI and smoking status were defined using the value recorded closest to the index date. To reduce the number of missing data, and given the more fixed nature of ethnic background, no time-restrictions were placed on ethnicity codes. Practice-level characteristics were IMD (quintiles: 1 = most deprived, 5 = least deprived), practice country for the referrals analysis (England, Northern Ireland, Scotland and Wales) and English region [for the surgery analysis, 10 strategic health authorities (SHAs): North East, North West, Yorkshire and The Humber, East Midlands, West Midlands, East of England, South West, South Central, London and South East Coast].
Statistical analyses
For the determinants of both referrals and surgery analyses, patient and practice characteristics were summarised using descriptive statistics. In both analyses, multiple imputation was used to impute missing values for BMI (missing for 5% of women) and smoking status (missing for 0.1% of women), with statistical coefficients obtained from imputed data sets, pooled using Rubin’s rules. 55 In the determinants of referral analysis, ethnicity data were available from the CPRD database only and were missing for 55% of women. A separate ‘missing’ category was therefore included in the models. In the determinants of surgery analysis, ethnicity data were available from both the CPRD and the HES databases and were missing for just 5% of women, allowing multiple imputation to be used to impute missing values. All statistical calculations were performed using Stata.
Determinants of referrals analysis
For the determinants of referrals analysis we used multivariate logistic regression, with cluster standard error estimands to account for clustering within general practices, to identify factors associated with referral within 30 days.
Determinants of surgery analysis
For the determinants of surgery analysis we used the cumulative incidence function to estimate surgery risk as a function of time from the initial referral to first surgical procedure. Death was considered as a competing event and patients reaching the end of the follow-up period were censored. 56 We used a multivariable Fine–Gray model to estimate subdistribution hazard ratios (sdHRs) to assess the association between patient and provider characteristics and the risk of surgery, with robust standard errors to account for within-hospital homogeneity. 57
Methods for work package 5
Work package 5 used primary data collected from an online survey. The survey used hypothetical simulated patients described by clinical case vignettes to measure variation in clinicians’ approaches to recommending surgical treatment for female SUI. This method has been used to measure variation in clinicians’ approaches to the diagnosis and treatment of patients with a range of similar health problems and is considered to be a cost-effective way of studying how clinicians respond to specific characteristics of their patients when making decisions rather than using medical records or standardised patients. 58–62
Survey
We conducted an online survey of all members of the British Society of Urogynaecology (BSUG) and members of the Royal College of Obstetricians and Gynaecologists (RCOG) who indicated that they had specialist interest in urogynaecology at the time of their RCOG registration (n = 1139). Data collection was carried out using the online survey platform, SurveyMonkey® (Palo Alto, CA, USA). In June 2017, a link to the online survey was e-mailed to the participants, with information about the survey and how to complete it. Three reminder e-mails were sent in the 1-month period following the initial e-mail. The survey included an information screen providing a brief description of the project, questions on the clinicians’ demographic characteristics, a page providing additional information and a number of clinical assumptions we asked the clinicians to make when responding to the survey (see Box 1). This was followed by the 18 case vignettes and response options.
Case vignette development
We used a three-stage approach to select the patient characteristics and their levels to be included in the clinical case vignettes. The first stage involved ‘item identification’. A targeted non-systematic literature search of English-language studies including female patients only was carried out to identify patient characteristics associated with surgical treatment for UI. We also drew on national guidelines and four senior clinical experts from the project team.
The second stage was ‘item reduction’. Each of the clinical experts reviewed the patient characteristics and selected those patients that they considered most likely to influence clinicians’ decisions about surgery for UI to be included in the vignettes. The recommendations made were collated and collectively discussed by the clinicians, reaching consensus on the most important characteristics for inclusion in the case vignette profiles. Seven potential characteristics were selected: age, BMI, type of SUI (pure SUI, stress predominant or MUI), previous SUI surgery, leakage, bother and physical status. Further discussions were held to decide on the relevant levels for these characteristics. Here the aim was to create maximum difference between the levels for each characteristic while ensuring that the clinical profiles captured in the vignettes were relevant and realistic. The seven patient characteristics and their levels (six characteristics with three levels and one with two) that were used in the case vignettes are presented in Table 3. Physical status was described according to categories recognised by the classification of the American Society of Anesthesiologists (ASA) at levels 2 (i.e. ‘a patient with mild systemic disease’) and 3 (i.e. ‘a patient with severe systemic disease’).
| Characteristic | Level |
|---|---|
| Age (years) | 55 |
| 68 | |
| 79 | |
| BMI (kg/m2) | 23 |
| 30 | |
| 36 | |
| Urinary incontinence conditions | Stress urinary incontinence |
| Stress-predominant mixed incontinence | |
| Mixed incontinence | |
| Previous SUI surgery | None |
| MIU (any route) | |
| Bladder neck injection | |
| Frequency of leakage | About two or three times a week |
| About once a day | |
| Several times a day | |
| Bother | A bit of a problem |
| Quite a problem | |
| A serious problem | |
| Physical status | ASA grade 2 |
| ASA grade 3 |
The third stage involved creating the ‘experimental design’. The total number of possible different cases using these seven patient characteristics is 1458 ( = 36 × 21) in a full factorial design. We used an orthogonal fractional factorial design [using SPSS software version 25 (SPSS Inc., Chicago, IL, USA)], which reduced the number of clinical case vignettes to 18. 63 This design reduces the number of vignettes while maximising the amount of information collected and retaining the absence of correlation between patient characteristics. 64 In addition to the 18 case profiles generated, we included two extra case profiles (‘holdout profiles’) to test our model specification.
The case vignettes described the clinical profile of women referred to secondary care for further assessment and management of their SUI. Each case profile comprised a short patient description according to the seven characteristics followed by one question: ‘Would you recommend that this patient has surgical treatment now?’. The clinicians were asked to score their recommendation on a five-point Likert scale ranging from ‘certainly yes’ to ‘certainly not’. A pilot study was undertaken among eight clinicians to optimise the clarity of the vignettes. An example of one of the 18 clinical case vignettes is presented in Box 1.
A 55-year-old woman presents with symptoms of mixed incontinence.
She leaks several times a day. She says that her UI condition is affecting her daily activities and is a serious problem for her. Her BMI is 36 kg/m2.
Previous gynaecological history includes mid-urethral tape. She is ASA grade 2.
Would you recommend that this patient has surgical treatment now?
☐ Certainly yes
☐ Probably yes
☐ Not sure
☐ Probably not
☐ Certainly not
PLEASE ASSUME THAT: PATIENTS-
Have been referred by their GP for further assessment.
-
Have completed all conservative and behavioural treatments (e.g. frequency volume charts, pelvic floor exercises, etc.) without benefit.
-
Abdominal examination – normal.
-
Mid-stream urinalysis results – all negative.
-
Post-void residual volume of < 100 ml.
This survey focuses on the following conditions:
-
stress urinary incontinence
-
stress-predominant mixed urinary incontinence
-
mixed urinary incontinence (urodynamic stress incontinence with detrusor overactivity).
We describe physical status by the ASA grade classification.
Examples of patients with ASA grade 2-
Hypertension: well controlled with one type of antihypertensive medication.
-
Diabetes: well controlled with oral medication or insulin, without diabetic complication.
-
COPD/asthma: with productive cough and wheeze, well controlled by inhalers with rare episode of acute chest infection, not limiting lifestyle.
-
Hypertension: requiring multiple antihypertensive medications, or not well controlled.
-
Diabetes: diabetic complications, or not well controlled with oral medication or insulin.
-
COPD/asthma: not well controlled, limiting lifestyle, with high dose of inhaler or oral steroids, with frequent episodes of acute chest infections.
Statistical analyses
We used descriptive statistics to summarise the characteristics of the responding clinicians. We calculated the means of the response scores and the 25th and 75th percentiles to describe the recommendations of the clinicians for each of the 18 case vignettes.
To assess the relative influence of the patients’ characteristics (i.e. ‘weight’) on the clinicians’ recommendations, we calculated the means of the recommendation score by level of each of the characteristics. The weight of a patient characteristic was defined as the difference between the lowest and the highest mean recommendation score for that characteristic, divided by the sum of these differences for all seven clinical characteristics. 65 In other words, the weights express as a percentage the influence of each characteristic on the clinicians’ recommendations relative to the total overall weight of all patient characteristics. We used a mixed-effects analysis of variance model to test the statistical significance of differences in the clinicians’ recommendation scores according to level of the patient characteristics. This mixed-effects model recognised that the recommendation scores for the 18 case vignettes were nested within clinicians. The same mixed-effects model was used to analyse the impact of the clinicians’ own characteristics (subspecialty, gender and age group) on their recommendations. This was carried out by modelling the interaction between the patient characteristics and clinicians’ characteristics with a likelihood ratio test for composite models.
Latent class analysis was used to determine if mutually exclusive groups of clinicians (‘latent classes’) could be identified whose recommendations suggested a similar practice style. 66 Clinicians classed within the same group are expected to be more homogeneous with respect to their recommendation scores than gynaecologists classed in different groups. We used the Akaike information criterion (AIC) and the Bayesian information criterion (BIC) to determine the optimal number of latent classes. For both the AIC and BIC, a smaller value represents a better balance between the number of classes and the fit of the statistical model. The predicted posterior probabilities of latent class membership were used to assign each clinician to a group. Where a clinician did not have a posterior probability > 50% for one particular group, group membership was considered to be unknown. Statistical analyses were performed using Stata.
Methods for additional work package: work package 6
With growing concerns about the long-term outcomes of MUT procedures (of a subset of SUI procedures) changing the context of surgery for SUI, we also conducted additional research. The objective of this additional WP (WP 6) was to estimate the long-term rates of mesh tape removal and reoperation in women who had a MUT inserted for SUI.
Analysis cohort
The cohort for the additional work on mesh tape removal and reoperation comprised women aged ≥ 18 years who underwent a MUT insertion procedure for SUI for the first time between 1 April 2006 and 31 December 2015 in the HES APC data set. SUI was defined by the ICD-10 code N39.3. MUT insertions were defined with the OPCS-4 codes M53.3 (introduction of tension-free vaginal tape) and M53.6 (introduction of transobturator tape). The procedure was considered to be the ‘initial’ MUT insertion procedure in which there was no record of a MUT insertion in the preceding 3 years. Follow-up was from the date of the initial procedure to the date of a MUT removal, the date of reoperation or 31 March 2016, whichever was earliest. The minimum follow-up period was therefore 3 months and the maximum was 10 years.
Outcome measures
The outcomes were risk of MUT removal, reoperation for SUI and any reoperation (MUT removal and/or reoperation for SUI) (the codes are in Appendix 1, Table 13).
Potential determinants of mesh tape removal and reoperation
Potential determinants were age at initial procedure (analysed as 18–39, 40–49, 50–59, 60–69, ≥ 70 years); patient-level IMD, an area-based measure of economic deprivation (quintiles of the national distribution: 1 = most deprived, 5 = least deprived); ethnic background (white, Asian/Asian-British, black/black-British or other); number of comorbidities [as defined using the Royal College of Surgeons (RCS)’s Charlson Comorbidity Index, grouped as 0 or 1 or more]; route of MUT insertion (retropubic or transobturator); previous non-mesh SUI procedures in the 3 years prior to the initial MUT insertion; and concurrent prolapse repair procedures in the same episode of care as the initial MUT insertion. In a retropubic insertion, the mesh tape supporting the urethra is passed through two small incisions just above the pubic area. In a transobturator insertion it is passed through two small incisions on the inside of both thighs. The potential determinants at the organisational level that were related to the hospital where the initial procedure was performed were number of MUT insertions performed in the same year as the initial operation and the hospitals’ status as a specialist urogyneacology unit (according to accreditation by the BSUG unit) at any point in the study period.
Statistical analyses
The cumulative incidence function was used to estimate the risk of MUT removal and of reoperation as a function of time from the initial MUT insertion procedure. Death was considered as a competing event and patients reaching the end of the follow-up period were censored. 56 We used a multivariable Fine–Gray model to estimate sdHRs to assess the association between patient and provider characteristics and the risk of surgery, with robust standard errors to account for within-hospital homogeneity. 57 Multiple imputation was used to impute missing values for ethnic background (missing for 8.3% of women), with statistical coefficients obtained from 10 imputed data sets, pooled using Rubin’s rules. 55
Patient and public involvement
All WPs benefited from the input from two patient and public involvement (PPI) representatives who were members of the Project Advisory Group. They shared their experience of the NHS as patients, which guided the project’s overall design as well as the specific design of the qualitative and quantitative WPs. They also commented on all results and contributed to their interpretation.
Further PPI input was provided by the co-vice chairperson of the RCOG’s Women’s Network who was a member of the Project Steering Committee. In this capacity, she was able to comment on the results of the project from the perspective of the wider and diverse group of women represented by this network.
Chapter 4 Work package 2: geographic variation in surgery for female stress urinary incontinence
Parts of this chapter are reproduced with permission from Mamza et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
In this chapter, we present evidence from WP 2 on how the rates of surgery for female SUI vary across England. This addresses the second objective of the project that was to assess variation between NHS CCGs and other regional units in the rate of surgery for UI and to examine the impact of the supply-side factors on local surgical rates.
The methods are described in detail in Chapter 3. In summary, we used the HES database to identify women aged ≥ 20 years who had surgical treatment for SUI between 1 April 2013 and 31 March 2016 in NHS England. The outcome measure was the rate of surgery for SUI per 100,000 women per year at two geographic levels across 209 CCG areas with an average population size of 104,000 adult women and 44 STP areas with an average population size of about 493,000 adult women. Multilevel Poisson regression models were used to produce empirical Bayes’ estimates of the SUI surgery rates for each CCG and STP area, adjusted for age, socioeconomic deprivation, ethnicity and limiting long-term illness.
There were 33,708 inpatient episodes with a surgical procedure for SUI between April 2013 and March 2016. In total, 4996 episodes were excluded because, for example, they did not have a SUI diagnosis recorded at the time of the procedure. We focused on the first SUI procedure in the study period, which equated to 27,997 procedures, capturing > 97% of all SUI procedures in the study period. In total, 90% of procedures were MUT insertions and this did not vary between the first and later procedures.
The national annual rate of surgery was 40 procedures per 100,000 women. The adjusted SUI procedure rates for CCGs ranged from 20 to 106 procedures per 100,000 women per year [unadjusted rates ranged from 11 to 120 procedures per 100,000 women per year (Figure 1)]. Risk adjustment reduced the number of CCGs marked as ‘outliers’ (in which the national average was not within the 99.8% credibility interval of their rate) from 99 (47.4%) to 75 (36%), with the standard deviation (SD) of the CCG-level variation in adjusted rates [SD 0.27, 95% confidence interval (CI) 0.24 to 0.30] 16% lower than the SD of the unadjusted rates (SD 0.32, 95% CI 0.29 to 0.36).
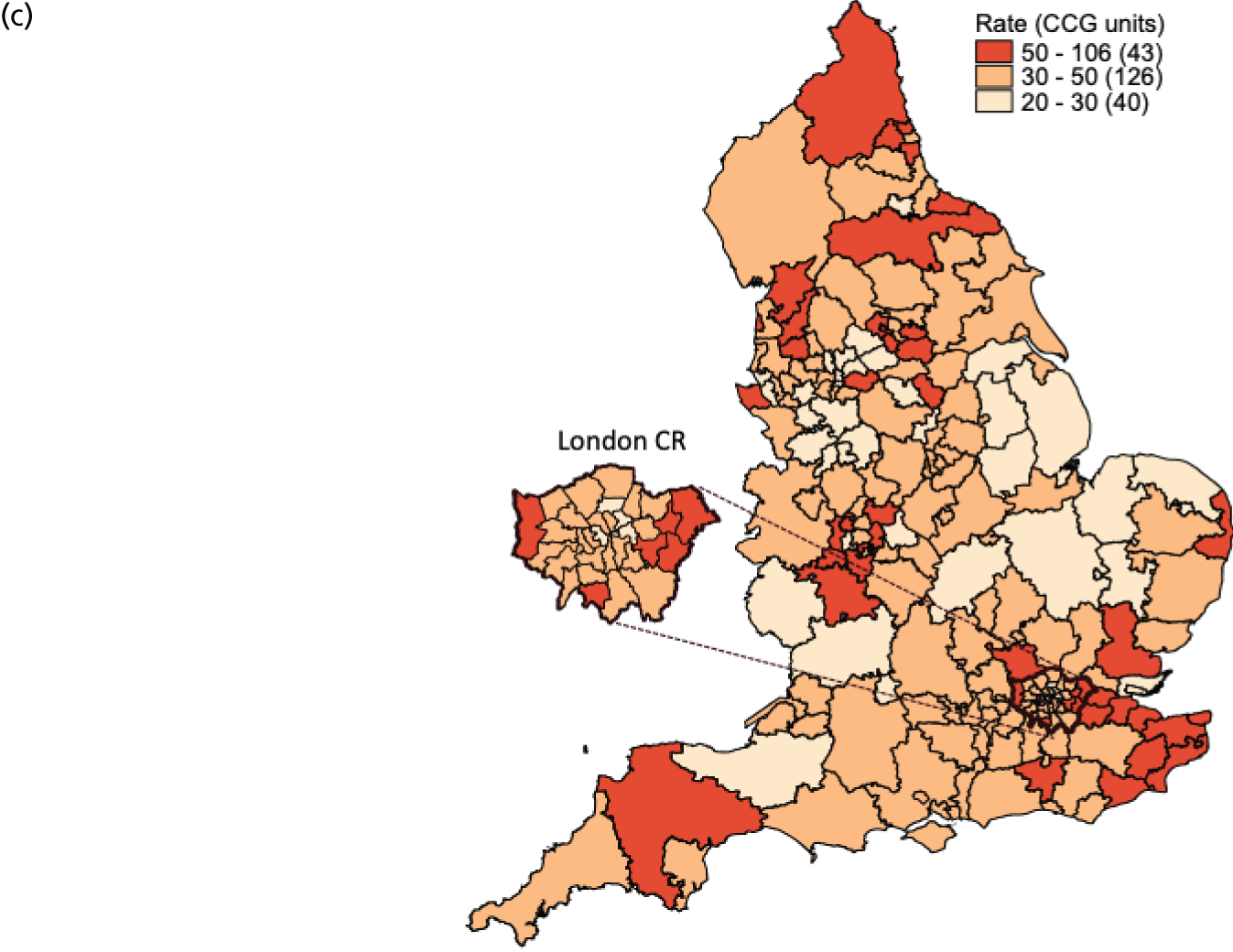
FIGURE 1.
Variation in SUI surgery rates by CCG. (a) Variation in the unadjusted empirical Bayes’ estimates of SUI procedure rates across CCGs; (b) adjusted for patients’ age and the CCG-level characteristics: IMD, percentage of the population reporting BAME background and percentage with a long-term illness; and (c) map of variation in SUI surgery rates by CCG. Reproduced with permission from Mamza et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
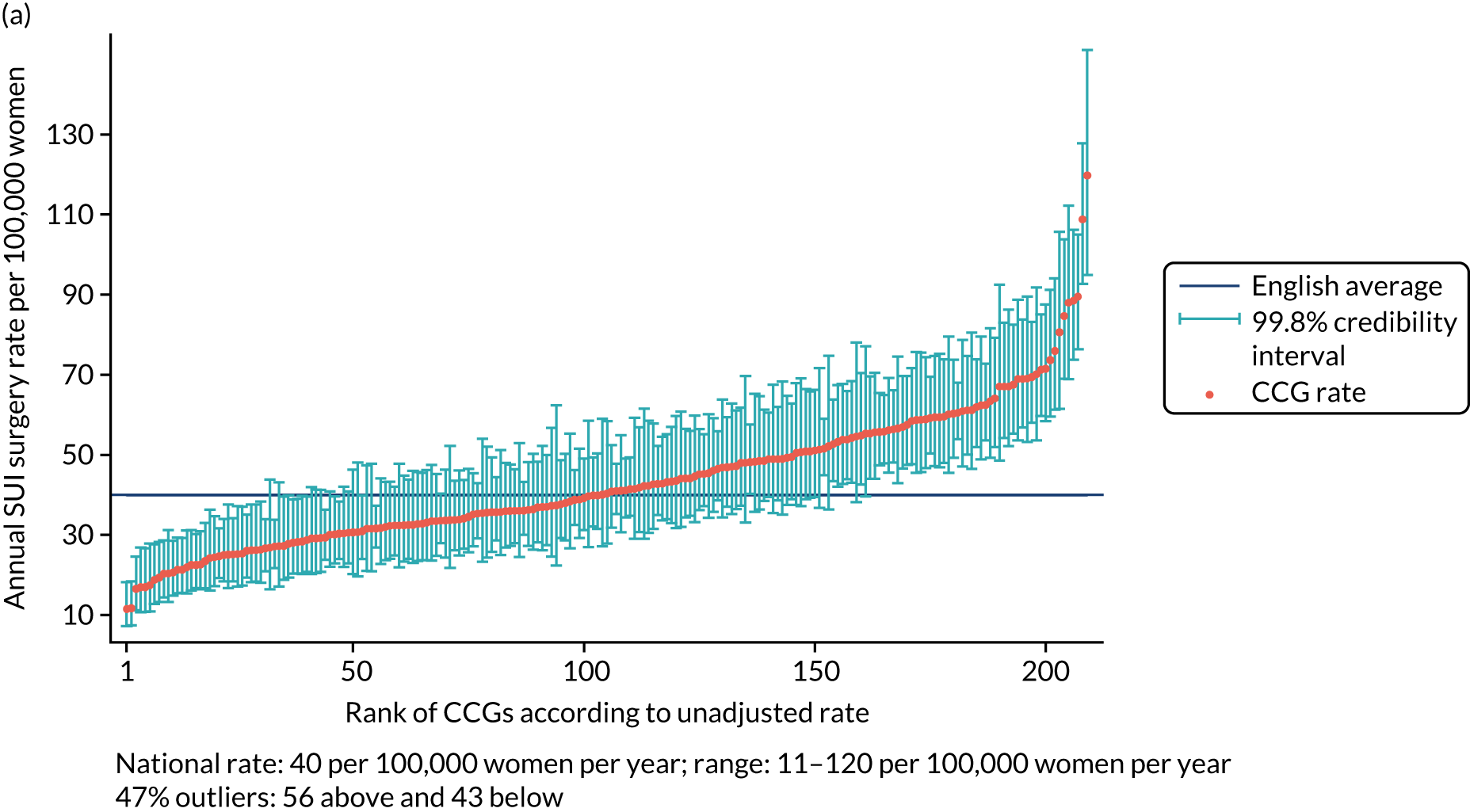
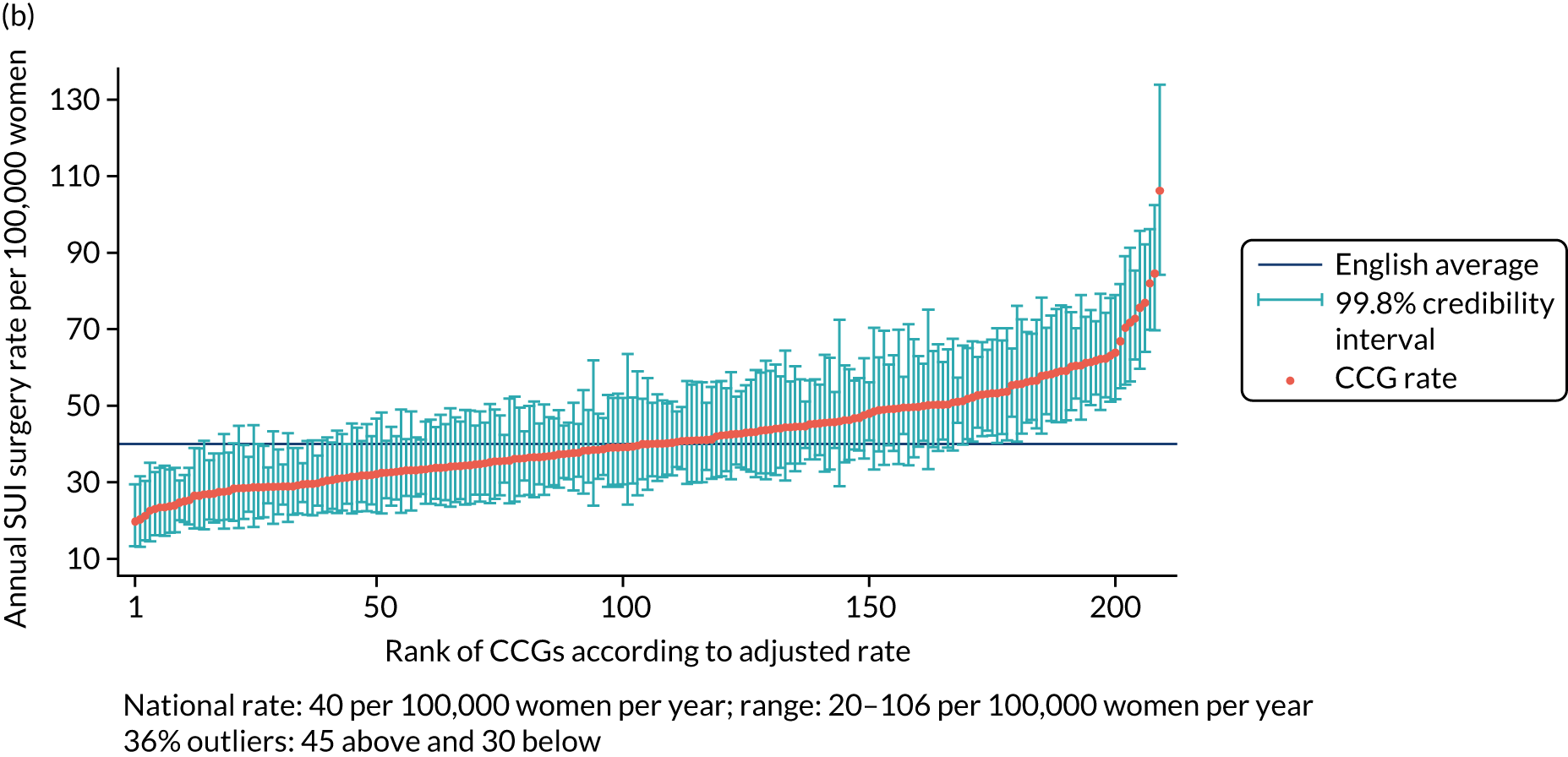
The adjusted SUI procedure rates for the STPs ranged from 24 to 69 procedures [unadjusted rates ranged from 20 to 77 procedures per 100,000 women per year (Figure 2)]. Risk adjustment reduced the number of STPs identified as outliers from 23 (52%) to 22 (50%). The amount of variation observed declined by 35% after risk adjustment, that is, unadjusted (SD 0.23, 95% CI 0.17 to 0.31) and adjusted (SD 0.15, 95% CI 0.11 to 0.22).
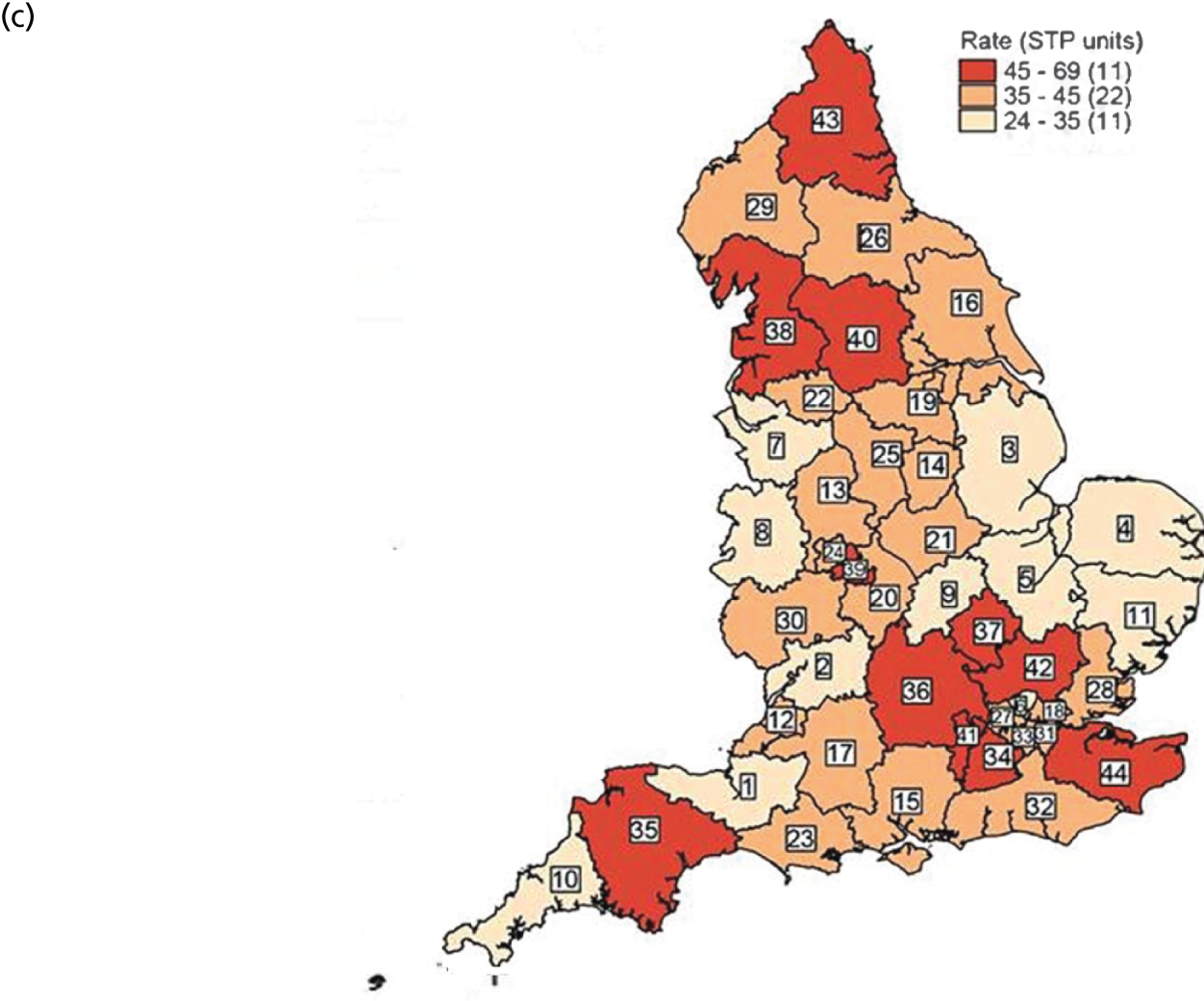
FIGURE 2.
Variation in SUI surgery rates by STP. (a) Variation in the unadjusted empirical Bayes’ estimates of SUI procedure rates across STPs; (b) adjusted for patients’ age and the CCG-level characteristics: IMD, percentage of the population reporting BAME background and percentage with a long-term illness; and (c) map of variation in SUI surgery rates by STP. 1, Somerset; 2, Gloucestershire; 3, Lincolnshire; 4, Norfolk and Waveney; 5, Cambridgeshire and Peterborough; 6, North Central London; 7, Cheshire and Merseyside; 8, Shropshire and Telford and Wrekin; 9, Northamptonshire; 10, Cornwall and the Isles of Scilly; 11, Suffolk and North East Essex; 12, Bristol, North Somerset and Sough Gloucestershire; 13, Staffordshire; 14, Nottinghamshire; 15, Hampshire and the Isle of Wight; 16, Coast, Humber and the Vale; 17, Bath, Swindon and Wiltshire; 18, North East London; 19, Sough Yorkshire and Bassetlaw; 20, Coventry and Warwickshire; 21, Leicester, Leicestershire and Rutland; 22, Greater Manchester; 23, Dorset; 24, The Black Country; 25, Derbyshire; 26, Durham, Darlington, Teesside, Hambleton, Richmondshire and Whitby; 27, North West London; 28, Mid and South Essex; 29, West, North and East Cumbria; 30, Herefordshire and Worcestershire; 31, South East London; 32, Sussex and East Surrey; 33, South West London; 34, Surrey Heartlands; 35, Devon; 36, Buckinghamshire, Oxfordshire and Berkshire West; 37, Milton Keynes, Bedfordshire and Luton; 38, Lancashire and South Cumbria; 39, Birmingham and Solihull; 40, West Yorkshire; 41, Frimley Health; 42, Hertfordshire and West Essex; 43, Northumberland, Tyne and Wear and North Durham; and 44, Kent and Medway. Reproduced with permission from Mamza et al. 67 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
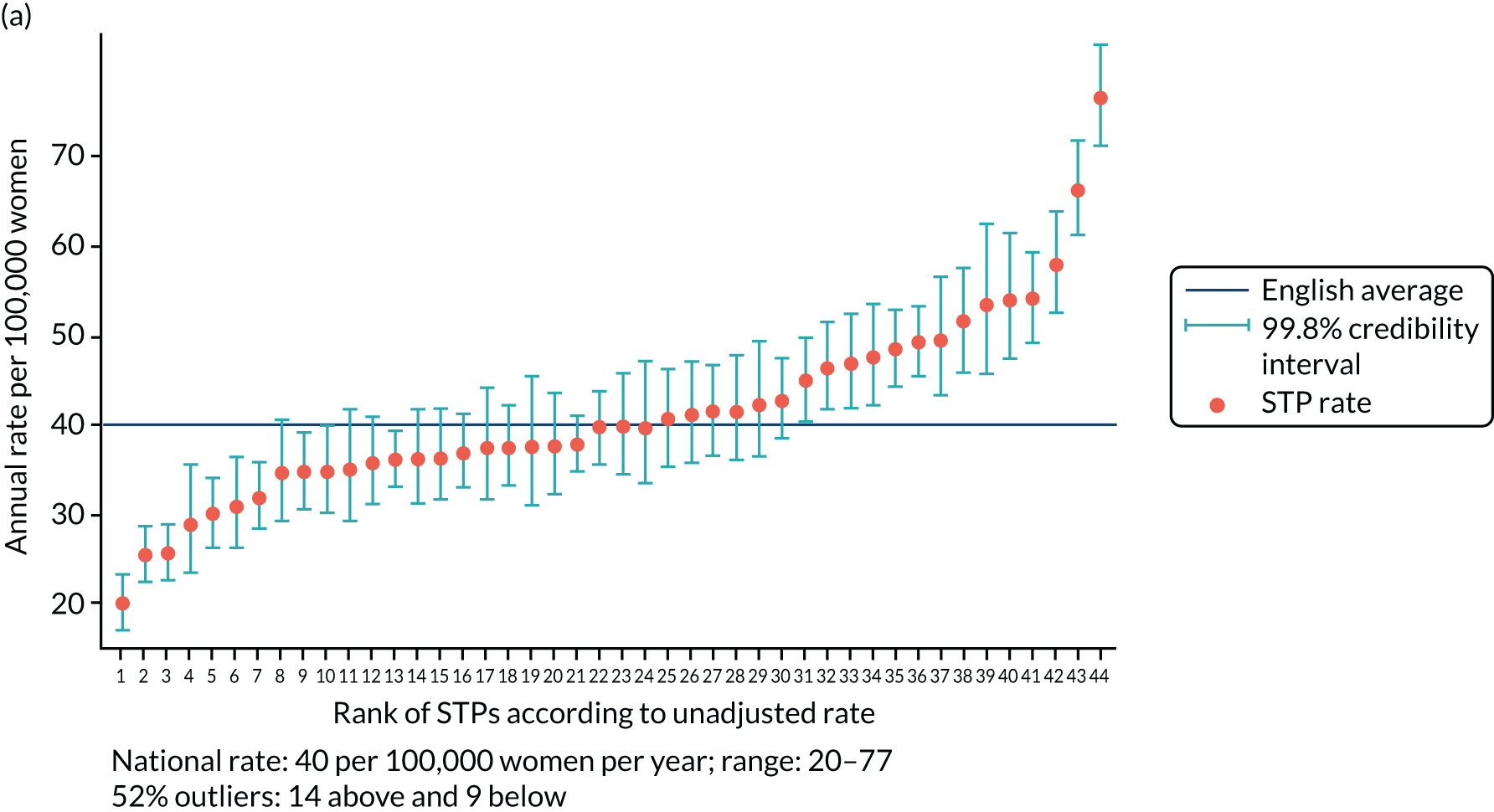
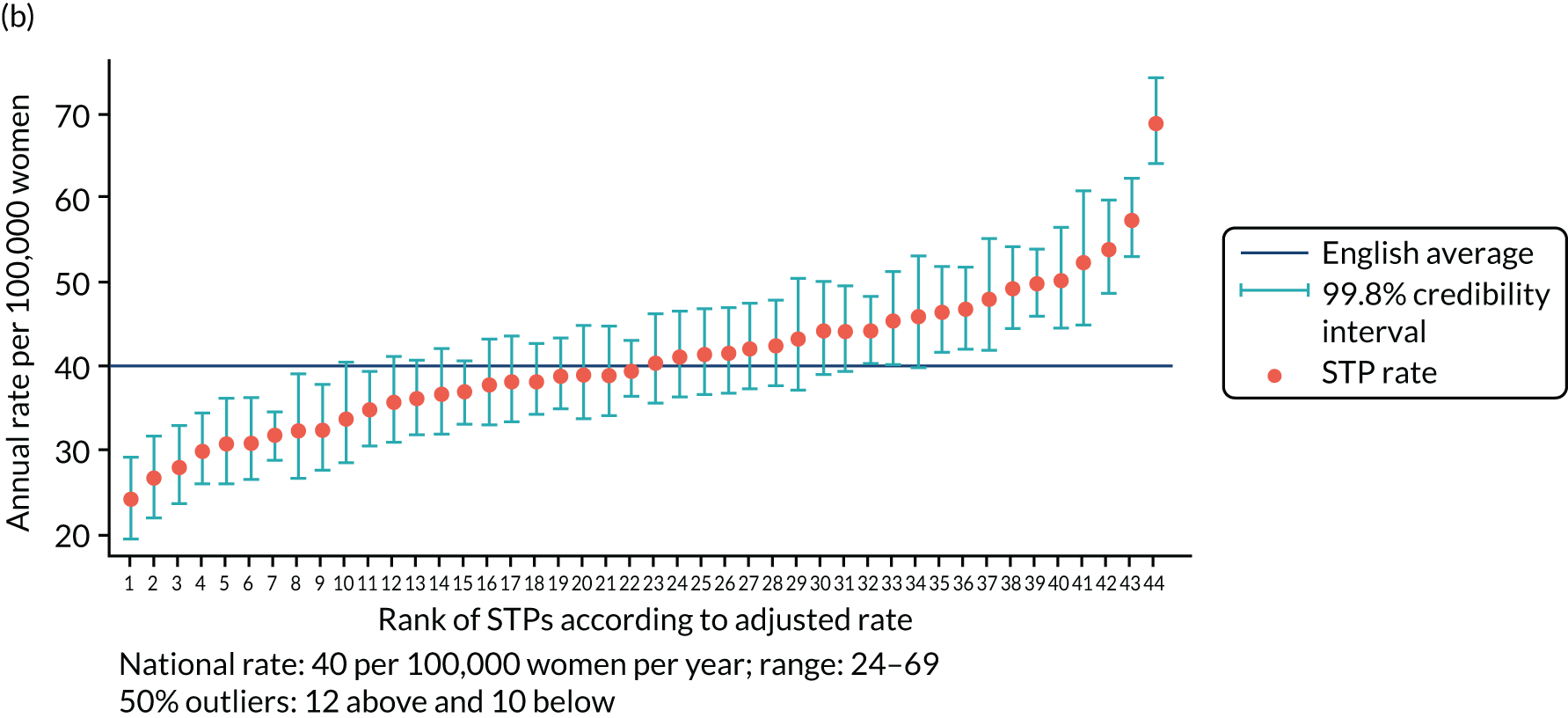
Annual SUI procedure rates declined over the study period from 52 per 100,000 women in 2013 to 36 per 100,000 women in 2015. However, there was no evidence that CCG- or STP-level variation changed over time. In separate (adjusted) regression models run by year, the SD of CCG-level variation was 0.26 (95% CI 0.23 to 0.30) in 2013, 0.27 (95% CI 0.23 to 0.31) in 2014 and 0.29 (95% CI 0.25 to 0.34) in 2015. For STP-level variation (adjusted) the SD was 0.13 (95% CI 0.08 to 0.20) in 2013, 0.17 (95% CI 0.11 to 0.25) in 2014 and 0.18 (95% CI 0.12 to 0.26) in 2015.
Stress UI surgery rates were lowest for those women aged 20–39 years (16 per 100,000 women per year) and highest for those women aged 40–49 years (84 per 100,000 women per year), declining with age (beyond 50 years). Compared with the rate among women aged 40–49 years, the surgery rate for women aged 50–59 years was 20% lower (IRR 0.80, 95% CI 0.78 to 0.83) and 46% lower for women aged 60–69 years (IRR 0.54, 95% CI 0.52 to 0.56). Rates were lower in areas with higher proportions of BAME populations (highest vs. lowest quintile IRR 0.63, 95% CI 0.49 to 0.81). There were no differences in surgery rates according to the proportion of people with long-term limiting illness or CCG-level socioeconomic deprivation.
Key findings
-
The rate of surgery for SUI was 40 procedures per 100,000 women per year.
-
Risk-adjusted rates ranged from 20 to 106 procedures per 100,000 women per year across CCGs and from 24 to 69 procedures per 100,000 women per year across the STP areas.
-
These regional differences were only partially explained by demographic characteristics, as adjustment reduced variance of surgery rates by 16% among the CCGs and 35% among the STPs.
Table 4 describes the distribution of regional characteristics and the association between these factors and SUI procedure rates.
| Regional factor | Scale of factor (1 unit) | Procedures, n (%) | Crude rate per 100,000 women per year | Procedure rate ratio (95% CI) | p-valuea |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 20–39 | Age group in years | 3253 (11.6) | 15.9 | 0.18 (0.17 to 0.19) | |
| 40–49 | 9761 (34.9) | 84.4 | Reference | < 0.001 | |
| 50–59 | 7496 (26.8) | 67.5 | 0.80 (0.78 to 0.83) | ||
| 60–69 | 4352 (15.5) | 46.2 | 0.54 (0.52 to 0.56) | ||
| ≥ 70 | 3135 (11.2) | 26.8 | 0.31 (0.30 to 0.33) | ||
| Socioeconomic status | |||||
| 1: most deprived | Quintile category of IMD ranking | 5838 (20.9) | 43.0 | Reference | 0.84 |
| 2: more deprived | 6315 (22.6) | 47.5 | 1.08 (0.93 to 1.25) | ||
| 3: average | 6371 (22.8) | 47.9 | 1.05 (0.89 to 1.25) | ||
| 4: less deprived | 5001 (17.9) | 39.9 | 1.02 (0.85 to 1.21) | ||
| 5: least deprived | 4472 (15.1) | 36.3 | 1.05 (0.85 to 1.29) | ||
| BAME population | |||||
| 1: CCGs with lowest proportion | Ranked category of proportion of BAME population | 5579 (19.9) | 48.8 | Reference | 0.001 |
| 2 | 6867 (24.5) | 49.8 | 1.02 (0.89 to 1.17) | ||
| 3 | 6326 (22.6) | 45.7 | 1.00 (0.86 to 1.17) | ||
| 4 | 5725 (20.4) | 41.5 | 0.89 (0.75 to 1.06) | ||
| 5: CCGs with highest proportion | 3500 (12.5) | 27.2 | 0.63 (0.49 to 0.81) | ||
| Limiting long-term illness | |||||
| 1: CCGs with lowest proportion | Ranked category of proportion of people with limiting illness | 4433 (15.8) | 32.8 | Reference | 0.46 |
| 2 | 6328 (22.6) | 44.4 | 1.16 (0.99 to 1.36) | ||
| 3 | 4882 (17.4) | 43.7 | 1.11 (0.91 to 1.34) | ||
| 4 | 6896 (24.6) | 46.1 | 1.12 (0.91 to 1.39) | ||
| 5: CCGs with highest proportion | 5458 (19.5) | 48.9 | 1.16 (0.91 to 1.49) | ||
| Random-effects estimates | SD (95% CI)b | SD (95% CI)c | |||
| STP-level variation (level 2) | 0.23 (0.17 to 0.31) | 0.15 (0.11 to 0.22) | |||
| CCG-level variation (level 1) | 0.32 (0.29 to 0.36) | 0.27 (0.24 to 0.30) | |||
Chapter 5 Work package 4: determinants of referral and surgery for female urinary incontinence
In this chapter we present findings from WP 4, addressing the first element of the fourth project objective, which was to identify determinants of referrals and surgical treatment for UI.
The methods are described in detail in Chapter 3. Briefly, the cohort for identifying determinants of referral to a UI specialist was derived from the CPRD data set45 and comprised women aged ≥ 18 years who had an index diagnosis of UI between 1 April 2004 and 31 March 2014. An index diagnosis of UI was defined among women who had no earlier record of UI diagnosis or treatment within the 12 months prior to the date of their first diagnosis in the study period. Women with < 12 months of UTS data prior to index diagnosis or with a follow-up period of < 30 days were excluded. Women were followed up until the date of a referral to a UI specialist, transfer out of the practice, death or 1 April 2014, whichever was earliest. The primary outcome measure was referral to a UI specialist within 30 days of diagnosis.
The cohort for identifying determinants of surgery comprised women aged ≥ 18 years who had an index UI diagnosis (defined as above) and a referral to a UI specialist in secondary care between 1 April 2004 and 31 March 2014. This cohort for surgery after referral was derived from the CPRD linked to HES (APC and Outpatient) data set and was therefore restricted to women registered in primary care practices that had linked CPRD–HES data (England only). Women were followed up until the date of surgery, transfer out of the practice, death or 1 April 2014, whichever was earliest. The primary outcome measure was time to first UI surgery after referral.
Diagnoses of UI were defined using Read codes (see Appendix 2, Table 16). Referral to a UI specialist was defined using a combination of Read codes and referral specialty codes (see Appendix 4, Tables 18 and 19). UI surgery was defined using OPCS-4 codes (see Appendix 1, Tables 13–15).
Referrals
Parts of this text have been reproduced with permission from Gurol-Urganci et al. 68 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data. The text includes minor additions and formatting changes to the original text.
Between April 2004 and March 2014, 104,466 women had at least one UI diagnosis code and met the cohort criteria. The median age of women in the cohort was 58 years [interquartile range (IQR) 45–73 years]. Almost one-third of women (32%) were overweight (i.e. with a BMI of 25–29 kg/m2) and 29% were obese (i.e. with a BMI of 30–39 kg/m2). Ethnicity data were missing for over half (55%) of the referrals cohort; 92% were white, 4% were Asian/Asian British and 2% were black/black British. Of the comorbidities considered, CVD and anxiety or depression were the most common, each recorded for approximately 12% of women (Table 5).
| Total, n (%) | Referred, n (%) | 30-day referral rate (%) | Unadjusted OR (95% CI) | p-value | aOR (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|
| Overall | 104,466 | 28,476 | 27.3 | ||||
| Patient-level characteristics | |||||||
| Age group (years) | |||||||
| 18–39 | 14,599 (14) | 4696 (16.5) | 32.2 | 0.91 (0.87 to 0.96) | 0.91 (0.87 to 0.96) | ||
| 40–49 | 21,642 (20.7) | 7411 (26) | 34.2 | Reference | < 0.001 | Reference | < 0.001 |
| 50–59 | 19,654 (18.8) | 5964 (20.9) | 30.3 | 0.84 (0.80 to 0.87) | 0.84 (0.80 to 0.88) | ||
| 60–69 | 17,468 (16.7) | 4687 (16.5) | 26.8 | 0.70 (0.67 to 0.74) | 0.70 (0.66 to 0.73) | ||
| 70–79 | 15,834 (15.2) | 3372 (11.8) | 21.3 | 0.52 (0.49 to 0.55) | 0.51 (0.49 to 0.54) | ||
| ≥ 80 | 15,269 (14.6) | 2346 (8.2) | 15.4 | 0.35 (0.32 to 0.38) | 0.34 (0.31 to 0.37) | ||
| BMI (kg/m2) | |||||||
| Underweight (< 20) | 5224 (5.3) | 1190 (4.4) | 22.8 | 0.75 (0.70 to 0.81) | 0.85 (0.79 to 0.91) | ||
| Normal (20–24) | 28,044 (28.3) | 7966 (29.2) | 28.4 | Reference | < 0.001 | Reference | < 0.001 |
| Overweight (25–29) | 31,580 (31.8) | 8748 (32.1) | 27.7 | 0.98 (0.94 to 1.01) | 0.99 (0.95 to 1.03) | ||
| Obese (30–39) | 28,873 (29.1) | 7922 (29.1) | 27.4 | 0.97 (0.93 to 1.01) | 0.95 (0.91 to 0.99) | ||
| Severely obese (≥ 40) | 5474 (5.5) | 1439 (5.3) | 26.3 | 0.91 (0.85 to 0.98) | 0.84 (0.78 to 0.90) | ||
| Missing (imputed, n = 5271, 5.0%) | |||||||
| Smoking status | |||||||
| Non-smoker | 61,109 (58.6) | 16,471 (57.9) | 27.0 | Reference | 0.02 | Reference | < 0.001 |
| Current | 18,827 (18) | 5350 (18.8) | 28.4 | 1.08 (1.02 to 1.13) | 0.94 (0.90 to 0.98) | ||
| Ex-smoker | 24,395 (23.4) | 6632 (23.3) | 27.2 | 1.01 (0.98 to 1.05) | 1.04 (1.01 to 1.08) | ||
| Missing (imputed, n = 135, 0.1%) | |||||||
| Ethnicity | |||||||
| White | 43,015 (92.4) | 11,398 (92.9) | 26.5 | Reference | 0.04 | Reference | 0.001 |
| Asian/Asian British | 1722 (3.7) | 416 (3.4) | 24.2 | 0.88 (0.76 to 1.03) | 0.76 (0.65 to 0.89) | ||
| Black/black British | 930 (2) | 221 (1.8) | 23.8 | 0.86 (0.71 to 1.05) | 0.76 (0.62 to 0.92) | ||
| Mixed/other | 888 (1.9) | 233 (1.9) | 26.2 | 0.99 (0.80 to 1.22) | 0.85 (0.69 to 1.05) | ||
| Missing (category, n = 57,991, 55.4%) | – | – | 28.0 | 1.08 (1.00 to 1.16) | 1.04 (0.97 to 1.11) | ||
| Comorbidities | |||||||
| UTI | 2503 (2.4) | 659 (2.3) | 26.3 | 0.95 (0.86 to 1.05) | 0.33 | 1.10 (1.00 to 1.21) | 0.06 |
| POP | 3230 (3.1) | 720 (2.5) | 22.3 | 0.76 (0.67 to 0.85) | < 0.001 | 0.77 (0.68 to 0.87) | 0.00 |
| T2DM | 5639 (5.4) | 1221 (4.3) | 21.7 | 0.73 (0.67 to 0.78) | < 0.001 | 0.92 (0.85 to 0.99) | 0.02 |
| CVD | 12,034 (11.5) | 2632 (9.2) | 21.9 | 0.72 (0.68 to 0.76) | < 0.001 | 0.95 (0.90 to 1.00) | 0.07 |
| Renal disease | 2507 (2.4) | 491 (1.7) | 19.6 | 0.64 (0.57 to 0.73) | < 0.001 | 0.97 (0.86 to 1.09) | 0.59 |
| Respiratory disease | 9396 (9) | 2590 (9.1) | 27.6 | 1.02 (0.97 to 1.07) | 0.51 | 1.01 (0.96 to 1.06) | 0.68 |
| Anxiety or depression | 12,101 (11.6) | 3358 (11.8) | 27.7 | 1.03 (0.97 to 1.09) | 0.33 | 0.95 (0.90 to 1.00) | 0.05 |
| Cancer | 1785 (1.7) | 365 (1.3) | 27.3 | 0.68 (0.61 to 0.77) | < 0.001 | 0.84 (0.75 to 0.94) | 0.00 |
| Practice-level characteristics | |||||||
| Country | |||||||
| England | 80,751 (77.3) | 22,189 (77.9) | 27.5 | Reference | < 0.001 | Reference | < 0.001 |
| Northern Ireland | 4187 (4.0) | 1774 (6.2) | 42.4 | 1.94 (1.50 to 2.52) | 1.83 (1.40 to 2.39) | ||
| Scotland | 10,908 (10.4) | 2049 (7.2) | 18.8 | 0.61 (0.47 to 0.79) | 0.60 (0.46 to 0.78) | ||
| Wales | 8620 (8.3) | 2464 (8.7) | 28.6 | 1.06 (0.90 to 1.23) | 1.05 (0.89 to 1.24) | ||
| IMD (quintiles) | |||||||
| 1 (most deprived) | 19,485 (18.7) | 5486 (19.3) | 28.2 | 0.19 | Reference | 0.16 | |
| 2 | 20,782 (19.9) | 6024 (21.2) | 29.0 | 1.04 (0.89 to 1.22) | 1.02 (0.88 to 1.19) | ||
| 3 | 20,576 (19.7) | 5706 (20) | 27.7 | 0.98 (0.84 to 1.14) | 1.00 (0.86 to 1.16) | ||
| 4 | 21,960 (21) | 5750 (20.2) | 26.2 | 0.91 (0.78 to 1.05) | 0.90 (0.77 to 1.04) | ||
| 5 (least deprived) | 21,663 (20.7) | 5510 (19.3) | 25.4 | 0.87 (0.73 to 1.03) | 0.88 (0.74 to 1.05) | ||
Of the 104,466 women with UI, 47,838 (45.8%) had a referral to a UI specialist (Figure 3). Of these, 28,476 women (27.3% of the 104,466 women with UI, 59.5% of the 47,838 women referred) were referred within 30 days of their index UI diagnosis. The cumulative incidence of referral (with death as a competing risk) at 30 days, 1 year and 9 years was 25.5% (95% CI 25.3% to 25.8%), 34.0% (95% CI 33.7% to 34.3%) and 54.5% (95% CI 53.9% to 55.2%), respectively (Figure 4).
FIGURE 3.
Referrals analysis cohort. Parts of this figure have been reproduced with permission from Gurol-Urganci et al. 68 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data. The figure includes minor additions and formatting changes to the original figure.
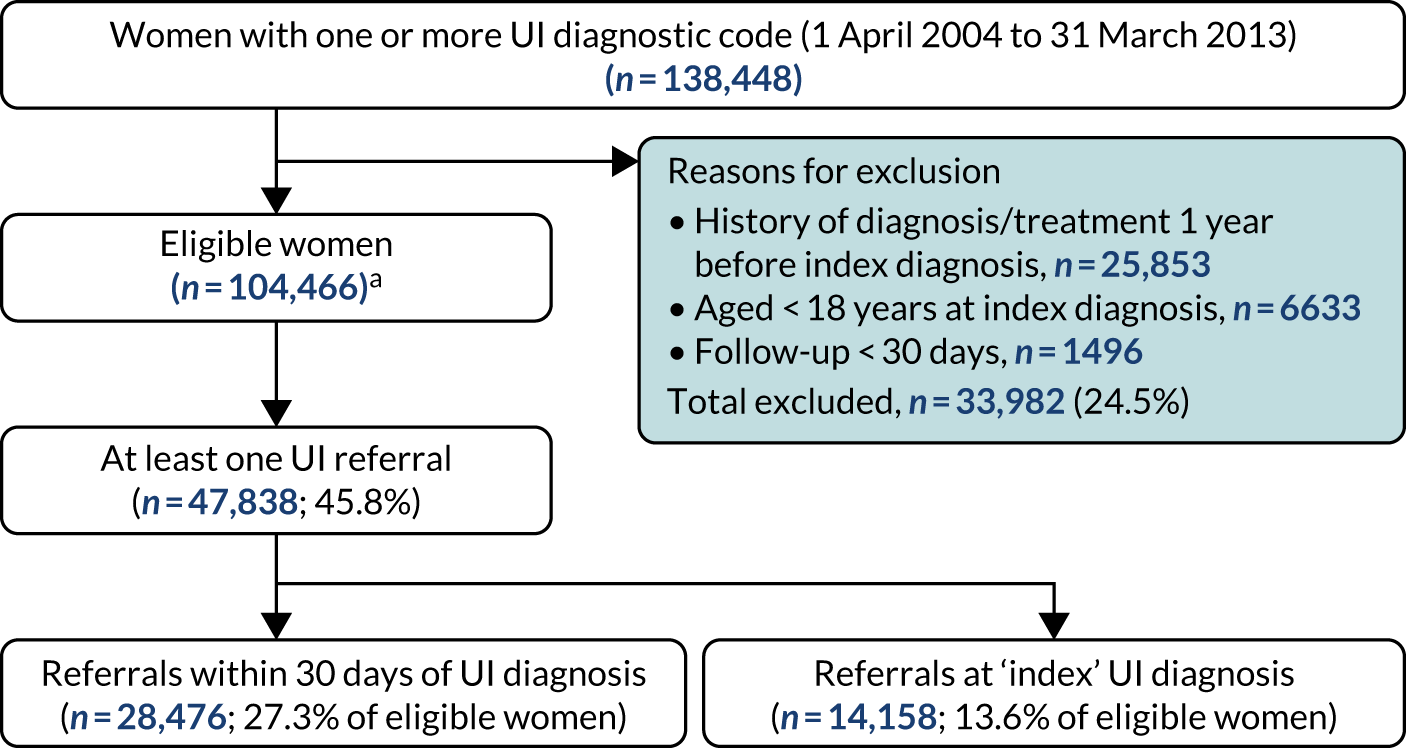
FIGURE 4.
Cumulative incidence of any referral within the study period.

Patient/practice characteristics associated with referral within 30 days
The likelihood of being referred within 30 days declined with increasing age. Women in all age groups ≥ 50 years (i.e. 50–59, 60–69, 70–79 and ≥ 80 years) were less likely to have been referred than those aged 40–49 years. Compared with those women aged 40–49 years, women aged ≥ 80 years were 66% less likely to have been referred within 30 days [adjusted odds ratio (aOR) 0.34, 95% CI 0.31 to 0.37] and women aged 70–79 years were 49% less likely to have been referred within 30 days (aOR 0.51, 95% CI 0.49 to 0.54). Women from an Asian/Asian British and black/black British minority ethnic background were less likely to have been referred than white women (aOR 0.76, 95% CI 0.65 to 0.89 for Asian vs. white women; aOR 0.76, 95% CI 0.62 to 0.92 for black vs. white women).
Women with a BMI indicating that they were underweight (aOR 0.85, 95% CI 0.79 to 0.91) or severely obese (aOR 0.84, 95% CI 0.78 to 0.90) were less likely to have been referred than women with a normal range BMI. Current smokers were less likely to have been referred than non-smokers (aOR 0.94, 95% CI 0.90 to 0.98). Three comorbidities were associated with the likelihood of referral within 30 days. Women with a POP diagnosis were 23% less likely to have been referred for UI than women without a diagnosis of POP (aOR 0.77, 95% CI 0.68 to 0.87). Women with T2DM were slightly less likely to have been referred than those without (aOR 0.92, 95% CI 0.85 to 0.99). Finally, women with any type of cancer recorded in the previous 12 months were less likely to have been referred within 30 days (aOR 0.84, 95% CI 0.75 to 0.94). Other comorbidities were not associated with referral.
The country in which women accessed primary care for their ‘index’ UI diagnosis was also associated with the likelihood of referral within 30 days. Women in Scotland were 40% less likely to be referred than those accessing care in England (aOR 0.60, 95% CI 0.46 to 0.78), whereas women in Northern Ireland were 83% more likely to have been referred than those in England (aOR 1.83, 95% CI 1.40 to 2.39).
Surgical treatment
A total of 30,312 women in the linked CPRD–HES data set were identified as having been referred for UI between 1 April 2004 and 31 March 2014 (see Box 1). The median follow-up time was 4.6 years for women alive at the end of follow-up (IQR 2.4–6.9 years). The median age of women in the ‘determinants of surgery’ cohort was 53.6 years (IQR 43.4–67.6 years) and > 90% had a white ethnicity recorded. As in the referrals cohort, two-thirds of women (66.4%) were overweight, obese or severely obese and less than one-fifth (17%) were current smokers. Of the comorbidities considered, anxiety or depression and CVD were the most common, recorded for approximately 8.4% and 6.8% of women, respectively (Table 6).
| Total, n (%) | Had a UI operation, n (%) | 9-year cumulative incidence of surgery (95% CI) | Unadjusted sdHR (95% CI) | p-value | Adjusted sdHR (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|
| Overall | 30,312 | 4307 (14.2) | 18.1 (17.5 to 18.7) | ||||
| Patient-level characteristics | |||||||
| Age group (years) | |||||||
| 18–39 | 4505 (14.9) | 592 (13.7) | 19 (17.4 to 20.8) | 0.6 (0.54 to 0.66) | 0.63 (0.57 to 0.69) | ||
| 40–49 | 7360 (24.3) | 1541 (35.8) | 26.7 (25.3 to 28.2) | Reference | < 0.001 | Reference | < 0.001 |
| 50–59 | 6251 (20.6) | 973 (22.6) | 19.3 (18.1 to 20.6) | 0.71 (0.66 to 0.78) | 0.70 (0.64 to 0.76) | ||
| 60–69 | 5071 (16.7) | 701 (16.3) | 17 (15.7 to 18.3) | 0.62 (0.56 to 0.68) | 0.59 (0.54 to 0.65) | ||
| 70–79 | 4098 (13.5) | 409 (9.5) | 11.7 (10.6 to 13) | 0.43 (0.38 to 0.49) | 0.42 (0.37 to 0.48) | ||
| ≥ 80 | 3027 (10) | 91 (2.1) | 3.4 (2.6 to 4.3) | 0.12 (0.09 to 0.15) | 0.12 (0.10 to 0.16) | ||
| BMI (kg/m2) | |||||||
| Underweight (< 20) | 1286 (4.4) | 108 (2.6) | 11.1 (9 to 13.4) | 0.56 (0.46 to 0.69) | 0.63 (0.51 to 0.78) | ||
| Normal (20–24) | 8507 (29.2) | 1214 (29.1) | 18.5 (17.4 to 19.6) | Reference | < 0.001 | Reference | < 0.001 |
| Overweight (25–29) | 9277 (31.9) | 1453 (34.9) | 20.1 (18.9 to 21.2) | 1.1 (1.02 to 1.19) | 1.13 (1.05 to 1.22) | ||
| Obese (30–39) | 8484 (29.2) | 1248 (29.9) | 18.2 (17.1 to 19.2) | 1.04 (0.96 to 1.13) | 1.05 (0.97 to 1.14) | ||
| Severely obese (≥ 40) | 1540 (5.3) | 145 (3.5) | 12.4 (10.4 to 14.5) | 0.67 (0.55 to 0.8) | 0.64 (0.53 to 0.77) | ||
| Missing (imputed, n = 1218, 4.0%) | |||||||
| Smoking status | |||||||
| Non-smoker | 17,746 (58.6) | 2416 (56.1) | 17.7 (16.9 to 18.4) | Reference | 0.004 | Reference | 0.001 |
| Current | 5143 (17) | 748 (17.4) | 18.1 (16.8 to 19.5) | 1.06 (0.97 to 1.15) | 0.91 (0.83 to 1.00) | ||
| Ex-smoker | 7405 (24.4) | 1142 (26.5) | 19.3 (18.1 to 20.5) | 1.14 (1.05 to 1.23) | 1.10 (1.02 to 1.19) | ||
| Missing (imputed, n = 18, 0.1%) | |||||||
| Ethnicity | |||||||
| White | 26,598 (92.5) | 4007 (95.4) | 19.2 (18.6 to 19.8) | Reference | < 0.001 | Reference | < 0.001 |
| Asian/Asian British | 842 (2.9) | 60 (1.4) | 9.4 (7.2 to 11.9) | 0.48 (0.36 to 0.63) | 0.50 (0.38 to 0.67) | ||
| Black/Black British | 430 (1.5) | 35 (0.8) | 11.5 (7.6 to 16.3) | 0.53 (0.4 to 0.7) | 0.57 (0.43 to 0.76) | ||
| Mixed/Other | 876 (3) | 98 (2.3) | 17.1 (13.5 to 21) | 0.78 (0.65 to 0.95) | 0.76 (0.63 to 0.92) | ||
| Missing (imputed, n = 1566, 5.2%) | |||||||
| Comorbidities | |||||||
| UTI | 946 (3.1) | 90 (2.1) | 12.7 (10.1 to 15.6) | 0.66 (0.54 to 0.81) | < 0.001 | 0.82 (0.67 to 1.01) | 0.07 |
| POP | 1063 (3.5) | 185 (4.3) | 18.7 (16.2 to 21.4) | 1.26 (1.08 to 1.48) | 0.003 | 1.30 (1.11 to 1.52) | 0.001 |
| T2DM | 1027 (3.4) | 81 (1.9) | 9.9 (7.7 to 12.5) | 0.5 (0.39 to 0.63) | < 0.001 | 0.62 (0.50 to 0.79) | < 0.001 |
| CVD | 2078 (6.9) | 243 (5.6) | 12.7 (11.2 to 14.3) | 0.75 (0.66 to 0.86) | < 0.001 | 0.95 (0.83 to 1.09) | 0.49 |
| Renal disease | 431 (1.4) | 40 (0.9) | 9.7 (7.1 to 12.9) | 0.57 (0.41 to 0.79) | 0.001 | 0.89 (0.66 to 1.22) | 0.48 |
| Respiratory disease | 2058 (6.8) | 297 (6.9) | 19 (16.6 to 21.6) | 0.98 (0.87 to 1.11) | 0.74 | 0.93 (0.83 to 1.06) | 0.29 |
| Anxiety or depression | 2561 (8.4) | 372 (8.6) | 17.7 (15.8 to 19.6) | 0.95 (0.85 to 1.06) | 0.36 | 0.84 (0.76 to 0.94) | 0.003 |
| Cancer | 293 (1) | 28 (0.7) | 14.9 (8 to 23.9) | 0.62 (0.43 to 0.9) | 0.01 | 0.71 (0.49 to 1.04) | 0.08 |
| IMD (quintiles) | |||||||
| 1 (most deprived) | 7260 (24.6) | 1069 (24.8) | 18.6 (17.4 to 19.8) | Reference | 0.08 | Reference | 0.20 |
| 2 | 6906 (23.4) | 1057 (24.6) | 19.9 (18.6 to 21.3) | 1.05 (0.95 to 1.16) | 1.11 (1.01 to 1.22) | ||
| 3 | 5685 (19.2) | 854 (19.8) | 18.8 (17.4 to 20.2) | 1.03 (0.93 to 1.14) | 1.09 (0.99 to 1.21) | ||
| 4 | 5388 (18.2) | 774 (18) | 18.6 (17.3 to 20.1) | 0.99 (0.88 to 1.11) | 1.09 (0.98 to 1.22) | ||
| 5 (least deprived) | 4307 (14.6) | 549 (12.8) | 16.9 (15.4 to 18.5) | 0.87 (0.76 to 0.99) | 1.03 (0.90 to 1.16) | ||
| Missing (imputed, n = 776, 2.5%) | |||||||
| Region | |||||||
| North East | 712 (2.3) | 90 (2.1) | 15.1 (12.2 to 18.3) | Reference | < 0.001 | Reference | < 0.001 |
| North West | 5133 (16.9) | 629 (14.6) | 16.4 (15.1 to 17.9) | 0.96 (0.72 to 1.27) | 0.95 (0.72 to 1.25) | ||
| Yorkshire and The Humber | 1213 (4) | 200 (4.6) | 20.7 (17.8 to 23.8) | 1.22 (0.90 to 1.67) | 1.17 (0.87 to 1.58) | ||
| East Midlands | 808 (2.7) | 138 (3.2) | 18.6 (15.8 to 21.6) | 1.23 (0.85 to 1.77) | 1.17 (0.79 to 1.72) | ||
| West Midlands | 3478 (11.5) | 455 (10.6) | 17.4 (15.8 to 19.2) | 1.04 (0.79 to 1.38) | 1.07 (0.82 to 1.41) | ||
| East of England | 4042 (13.3) | 555 (12.9) | 16.8 (15.4 to 18.3) | 1.04 (0.78 to 1.39) | 1.05 (0.79 to 1.39) | ||
| South West | 3574 (11.8) | 542 (12.6) | 19.2 (17.4 to 21.1) | 1.17 (0.89 to 1.53) | 1.18 (0.91 to 1.54) | ||
| South Central | 3586 (11.8) | 611 (14.2) | 21.1 (19.4 to 22.9) | 1.33 (1.01 to 1.75) | 1.28 (0.98 to 1.67) | ||
| London | 3836 (12.7) | 397 (9.2) | 14.4 (12.8 to 16) | 0.82 (0.62 to 1.09) | 0.86 (0.65 to 1.14) | ||
| South East Coast | 3930 (13) | 690 (16) | 21.7 (20 to 23.5) | 1.42 (1.08 to 1.87) | 1.35 (1.04 to 1.75) | ||
Of the 30,312 women in the CPRD–HES ‘determinants of surgery’ cohort, 4307 (14.2%) underwent a UI procedure (Figure 5), of which 4050 (94.5%) were SUI procedures and 257 (5.5%) were UUI procedures. Of the SUI procedures, 89% (n = 3606) were MUT insertions.
FIGURE 5.
Surgery analysis cohort.
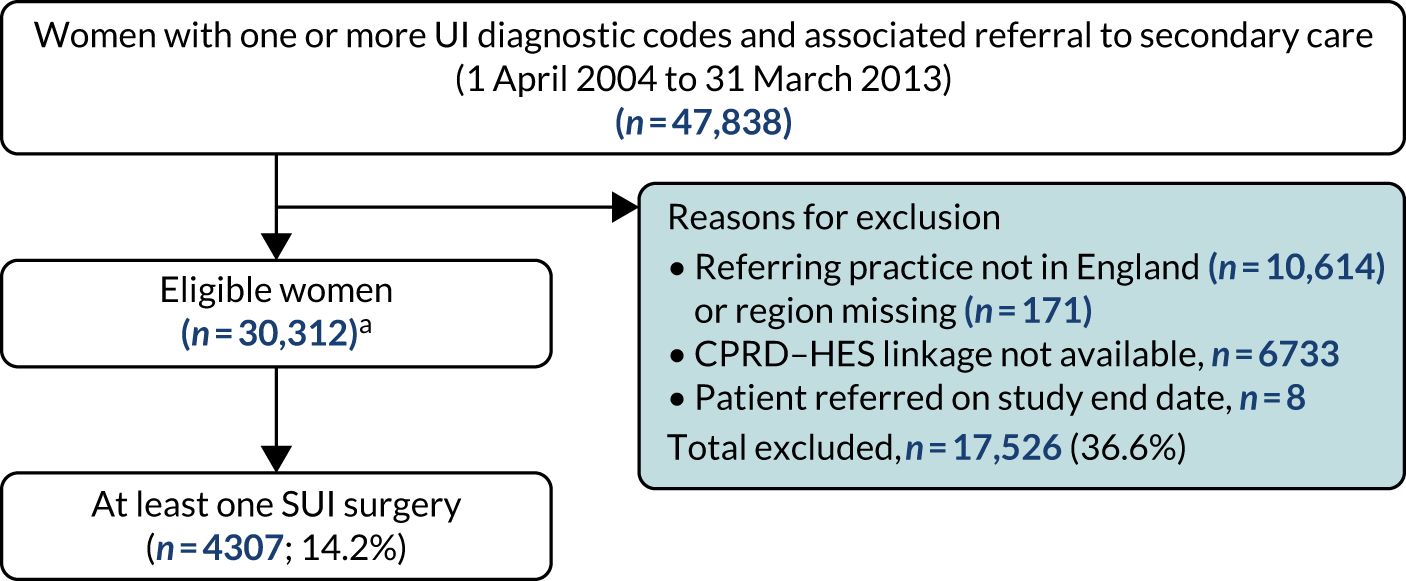
The rate of UI surgery was 7.3% (95% CI 7.0% to 7.6%) at 1 year, 13.4% (95% CI 13.0% to 13.8%) at 3 years, 15.5% (95% CI 15.0% to 15.9%) at 5 years, and 18.1% of the women (95% CI 17.5% to 18.7%) at 9 years after the initial referral (with death as a competing risk; Figure 6, see Table 6).
FIGURE 6.
Cumulative incidence of any UI operation after referral within the study period.
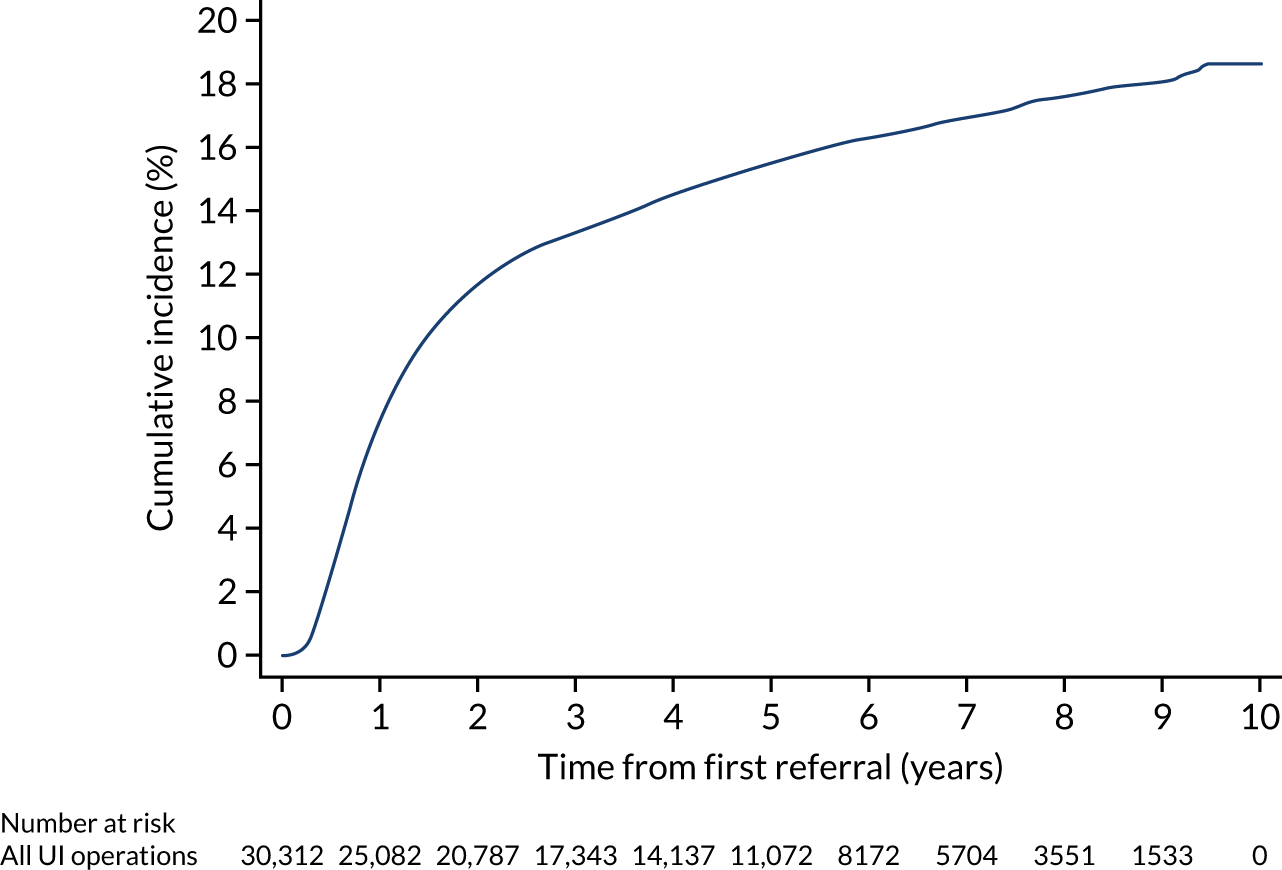
Patient/practice characteristics associated with surgery
As in the findings with respect to referrals, age and ethnicity were associated with being less likely to have received surgical treatment. The rate of surgery was lower among older women (aged ≥ 50 years) than among those aged 40–49 years. The rate of surgery was lowest among those women aged 70–79 years [11.7% at 9 years after referral compared with 26.7% among women aged 40–49 years (sdHR 0.42, 95% CI 0.37 to 0.48); see Table 6] and ≥ 80 years [3.4% at 9 years after referral compared with 26.7% among women aged 40–49 years (sdHR 0.12, 95% CI 0.10 to 0.16); see Table 6]. Asian/Asian British and black/black British women had a lower rate of surgery than white women [Asian/Asian British women: 9.4% at 9 years after referral compared with 19.2% for white women (sdHR 0.50, 95% CI 0.38 to 0.67); black/black British women: 11.5% at 9 years compared with 17.8% for white women (sdHR 0.57, 95% CI 0.43 to 0.76)].
Similar associations between BMI and surgery were observed as between BMI and referral. Women whose BMI placed them in the severely obese group had a lower rate of surgical treatment than women with a BMI in the normal range [12.4% for severely obese women 9 years after referral compared with 18.5% for women with a normal BMI (sdHR 0.64, 95% CI 0.53 to 0.77)]. Women whose BMI indicated that they were underweight also had a lower rate of surgery than women with a normal range BMI [11.1% for underweight women at 9 years compared with 18.5% for women with a normal BMI (sdHR 0.63, 95% CI 0.51 to 0.78)]. Three comorbidities were associated with the rate of surgical treatment. Women with a diagnosis of POP had a higher rate of surgery than women without a POP diagnosis (sdHR 1.30, 95% CI 1.11 to 1.52). Women with T2DM or anxiety/depression had a lower rate of surgery than women without a diagnosis of these conditions (sdHR 0.62, 95% CI 0.50 to 0.79 and sdHR 0.84, 95% CI 0.76 to 0.94, respectively). There was substantial variation in the rate of surgery by region of referring general practice, ranging from 14.4% in London at 9 years after referral to 21.7% in the South East Coast region (Figure 7).
FIGURE 7.
Geographical variation in rate of referral and UI surgery in England. (a) Referral after a new diagnosis; and (b) UI surgery after referral.
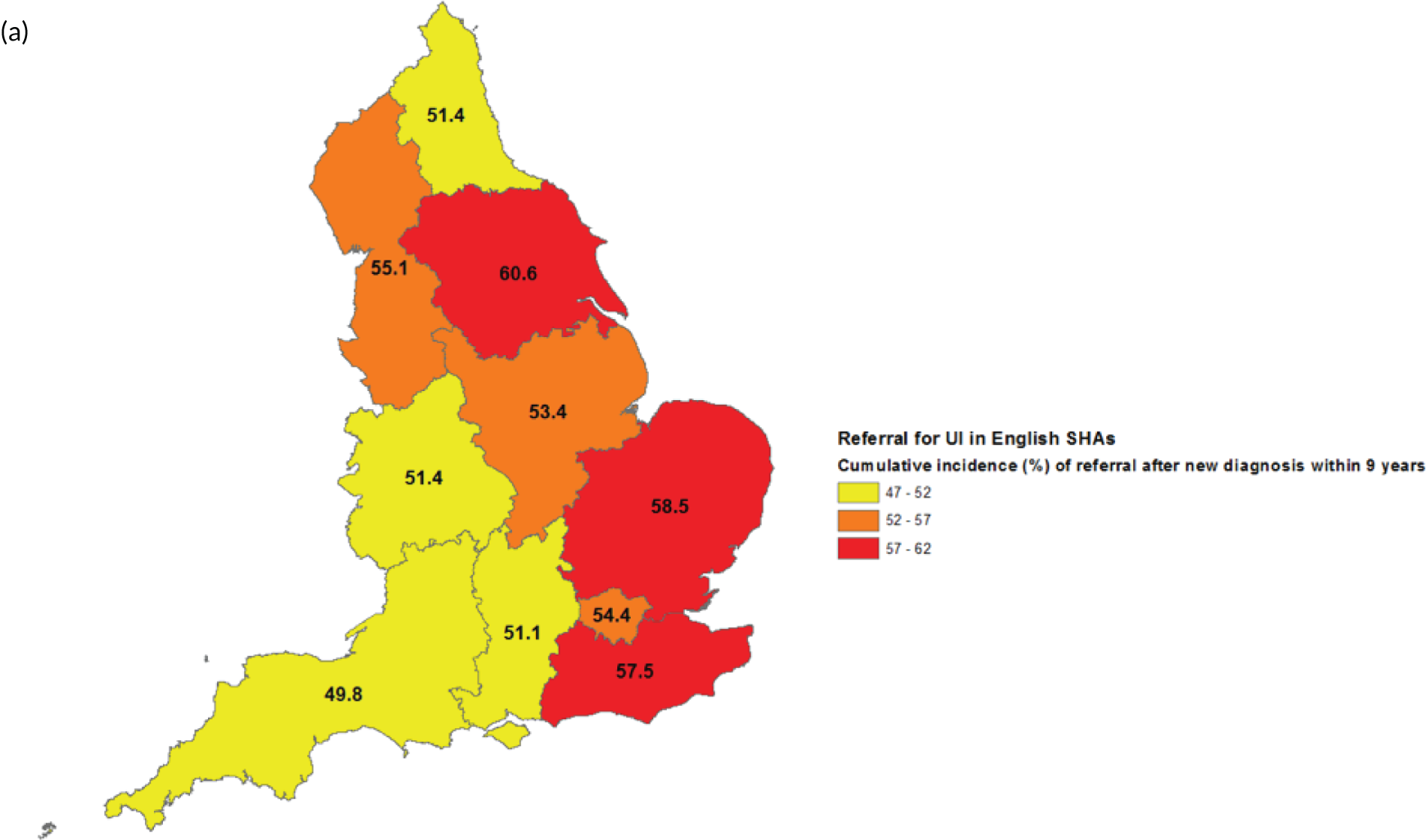
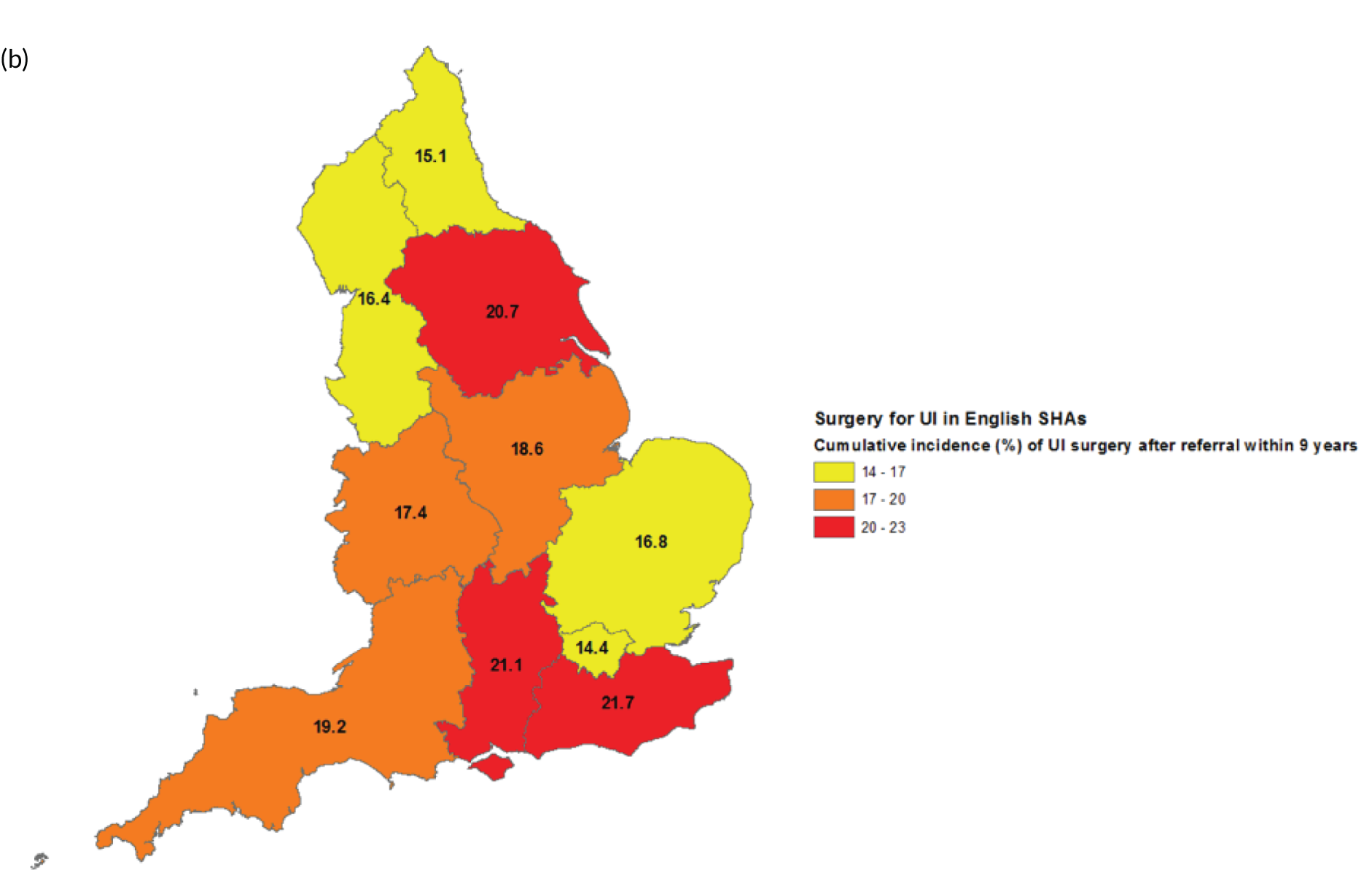
Figure 7 illustrates geographical variation in the rate of referral and SUI surgery in England. For this figure, we have restricted the referrals cohort to England only for comparability with the surgery cohort, which is England only as a result of the linkage of CPRD data (UK wide) with HES (English hospital data). Figure 7 demonstrates that two regions, the South East and Yorkshire and The Humber, have high rates of both referrals and surgical treatment, whereas, in the North East, both referral and surgery rates are relatively low. Figure 7 suggests that geographical variation in the overall rate of surgery (as demonstrated in WP 2, Chapter 4) is likely to arise from differences in both primary care (in terms of referrals) and secondary care.
Key findings
-
Almost half of women newly diagnosed with UI in primary care in the UK between April 2004 and March 2014 were referred to secondary care within 9 years. Of those women, 59.5% were referred within 30 days.
-
Referral rates were lower for older women, women from a minority ethnic background, women who were underweight (BMI of < 20 kg/m2) and women who were severely obese (BMI of ≥ 40 kg/m2).
-
Of the women who had been referred for UI, 7.3% underwent a UI procedure within 1 year of referral, 15.5% within 5 years and 18.1% within 9 years.
-
Surgery rates were lower in older women, women from a minority ethnic background and women who were underweight or severely obese.
Chapter 6 Work package 6: long-term rates of mesh tape removal following mid-urethral mesh tape insertion for female stress urinary incontinence
In this chapter, we present evidence on rates of mesh tape removal after MUT insertion for female SUI in England.
The methods are described in detail in Chapter 3. Briefly, the cohort for identifying long-term rates of MUT removal following MUT insertion was derived from the HES APC (inpatient) data set and comprised women aged ≥ 18 years who underwent an initial MUT insertion procedure for SUI between 1 April 2006 and 31 December 2015. The procedure was considered to be the ‘initial’ insertion procedure in which there was no record of a MUT insertion in the preceding 3 years. Follow-up was from date of initial procedure to date of a MUT removal or reoperation or to 31 March 2016, whichever was earlier. The minimum follow-up period was, therefore, 3 months and the maximum was 10 years. SUI was defined using the ICD-10 code N39.3. MUT insertions were defined using OPCS-4 codes M53.3 and M53.6. The outcomes were risk of MUT removal, reoperation for SUI and any reoperation (MUT removal and/or reoperation for SUI) (the codes are in Appendix 1, Table 13).
Mid-urethral mesh tape insertions
A total of 95,057 women resident in England had a first MUT insertion between 1 April 2006 and 31 December 2015 and a diagnosis of SUI. Of these, 60,194 women (63.3%) had a retropubic and 34,863 (36.7%) had a transobturator insertion. The median follow-up time was 5.5 years for women who were alive at the end of follow-up (IQR 3.2–7.5 years). The women’s median age was 51 years (IQR 44–61 years) and 19.8% had one or more comorbidity. A total of 18.1% had a concurrent prolapse operation in the same episode as their MUT insertion (Table 7).
| Number (%) | Risk of removala (%) | sdHRb (95% CI) | p-valuec | |||
|---|---|---|---|---|---|---|
| 1 year (95% CI) | 5 year (95% CI) | 9 year (95% CI) | ||||
| All: crude risk, n/N (%) | 1275/90,215 (1.4) | 1508/52,715 (2.9) | 240/6981 (3.4) | |||
| All: adjusted risk | 95,057 (100) | 1.4 (1.3 to 1.4) | 2.7 (2.6 to 2.8) | 3.3 (3.2 to 3.4) | ||
| Age at initial surgery (years) | ||||||
| 18–39 | 10,292 (10.8) | 2.0 (1.7 to 2.2) | 3.6 (3.2 to 4.0) | 4.4 (3.9 to 4.9) | Reference | < 0.001 |
| 40–49 | 33,094 (34.8) | 1.5 (1.4 to 1.6) | 2.9 (2.8 to 3.1) | 3.7 (3.4 to 4.0) | 0.83 (0.74 to 0.93) | |
| 50–59 | 24,664 (26.0) | 1.4 (1.2 to 1.5) | 2.8 (2.6 to 3.1) | 3.4 (3.1 to 3.6) | 0.77 (0.68 to 0.87) | |
| 60–69 | 16,877 (17.8) | 1.0 (0.8 to 1.1) | 2.1 (1.9 to 2.3) | 2.5 (2.2 to 2.8) | 0.56 (0.48 to 0.66) | |
| ≥ 70 | 10,130 (10.7) | 0.9 (0.8 to 1.1) | 1.7 (1.5 to 2.0) | 2.1 (1.7 to 2.5) | 0.46 (0.38 to 0.56) | |
| IMD (quintiles) | ||||||
| 1 (most deprived) | 16,136 (17.0) | 1.3 (1.1 to 1.5) | 2.7 (2.4 to 2.9) | 3.2 (2.9 to 3.5) | Reference | 0.12 |
| 2 | 18,277 (19.2) | 1.5 (1.3 to 1.7) | 2.9 (2.7 to 3.2) | 3.5 (3.2 to 3.8) | 1.12 (0.97 to 1.30) | |
| 3 | 20,468 (21.5) | 1.3 (1.1 to 1.5) | 2.5 (2.3 to 2.8) | 3.0 (2.7 to 3.3) | 0.96 (0.83 to 1.12) | |
| 4 | 20,779 (21.9) | 1.3 (1.1 to 1.4) | 2.6 (2.4 to 2.9) | 3.2 (2.9 to 3.6) | 1.01 (0.87 to 1.18) | |
| 5 (least deprived) | 19,397 (20.4) | 1.5 (1.3 to 1.7) | 2.8 (2.5 to 3) | 3.5 (3.2 to 3.9) | 1.08 (0.92 to 1.25) | |
| Ethnic backgroundd | ||||||
| White | 83,451 (95.8) | 1.4 (1.3 to 1.5) | 2.7 (2.6 to 2.8) | 3.3 (3.2 to 3.4) | Reference | 0.08 |
| Asian/Asian-British | 2049 (2.4) | 0.9 (0.5 to 1.3) | 2.1 (1.6 to 2.9) | 2.9 (2.0 to 4.1) | 0.73 (0.51 to 1.05) | |
| Black/black-British | 576 (0.6) | 1.7 (0.9 to 3.0) | 2.3 (1.3 to 3.7) | 2.3 (1.3 to 3.7) | 0.71 (0.42 to 1.19) | |
| Other | 1057 (1.2) | 0.9 (0.5 to 1.6) | 1.9 (1.2 to 2.9) | 2.8 (1.6 to 4.5) | 0.73 (0.49 to 1.09) | |
| Missing (n = 7924, 8.3%) | ||||||
| Route of mesh tape insertion | ||||||
| Retropubic | 60,194 (63.3) | 1.6 (1.5 to 1.7) | 3.0 (2.9 to 3.2) | 3.6 (3.5 to 3.8) | Reference | < 0.001 |
| Transobturator | 34,863 (36.7) | 0.9 (0.8 to 1.0) | 2.2 (2.0 to 2.3) | 2.7 (2.4 to 2.9) | 0.72 (0.62 to 0.84) | |
| Comorbiditiese | ||||||
| None | 76,252 (80.2) | 1.4 (1.3 to 1.5) | 2.7 (2.6 to 2.8) | 3.3 (3.2 to 3.5) | Reference | 0.37 |
| 1 or more | 18,805 (19.8) | 1.3 (1.1 to 1.5) | 2.7 (2.4 to 2.9) | 3.0 (2.7 to 3.3) | 1.05 (0.94 to 1.17) | |
| Previous bulking injection | ||||||
| No | 94,349 (99.2) | 1.4 (1.3 to 1.5) | 2.7 (2.6 to 2.8) | 3.3 (3.1 to 3.4) | Reference | 0.36 |
| Yes | 709 (0.8) | 0.7 (0.3 to 1.6) | 3.0 (1.9 to 4.6) | 3.3 (2.1 to 5.0) | 1.21 (0.80 to 1.83) | |
| Previous other SUI procedure | ||||||
| No | 94,710 (99.6) | 1.4 (1.3 to 1.4) | 2.7 (2.6 to 2.8) | 3.3 (3.1 to 3.4) | Reference | 0.13 |
| Yes | 347 (0.4) | 2.6 (1.3 to 4.7) | 4.2 (2.4 to 6.8) | 4.2 (2.4 to 6.8) | 1.50 (0.89 to 2.52) | |
| Concurrent prolapse repair | ||||||
| No | 77,932 (82.0) | 1.4 (1.3 to 1.4) | 2.7 (2.6 to 2.8) | 3.3 (3.1 to 3.4) | Reference | 0.09 |
| Repair with mesh | 817 (0.9) | 1.7 (1.0 to 2.8) | 3.4 (2.3 to 4.9) | 3.9 (2.6 to 5.6) | 1.43 (0.98 to 2.08) | |
| Repair without mesh | 16,308 (17.2) | 1.4 (1.2 to 1.6) | 2.7 (2.5 to 3.0) | 3.3 (2.9 to 3.6) | 1.07 (0.93 to 1.22) | |
| Specialist urogynecology unit | ||||||
| No | 75,695 (79.6) | 1.3 (1.2 to 1.4) | 2.6 (2.5 to 2.7) | 3.1 (3.0 to 3.3) | Reference | 0.17 |
| Yes | 19,362 (20.4) | 1.7 (1.6 to 1.9) | 3.2 (2.9 to 3.4) | 3.8 (3.5 to 4.2) | 1.17 (0.94 to 1.47) | |
| Annual volume of mesh tape insertions in the unit per year | ||||||
| < 60 | 28,939 (30.3) | 1.3 (1.2 to 1.4) | 2.5 (2.3 to 2.7) | 3.0 (2.8 to 3.3) | Reference | 0.21 |
| 60–119 | 44,228 (46.5) | 1.4 (1.3 to 1.5) | 2.7 (2.6 to 2.9) | 3.4 (3.2 to 3.6) | 1.07 (0.92 to 1.24) | |
| ≥ 120 | 21,990 (23.1) | 1.5 (1.3 to 1.6) | 2.8 (2.6 to 3.0) | 3.4 (3.1 to 3.7) | 1.02 (0.82 to 1.28) | |
Mid-urethral mesh tape removal
Mid-urethral mesh tape was removed in 1.4% (95% CI 1.3% to 1.4%) of the women at 1 year, in 2.7% (95% CI 2.6% to 2.8%) at 5 years and in 3.3% (95% CI 3.2% to 3.4%) at 9 years after the initial insertion, accounting for the competing risk of death (see Table 7 and Figure 8). The risk of removal was higher (at all time points) in women who had a retropubic insertion than in those who had a transobturator insertion (3.6% compared with 2.7% at 9 years after insertion; see Table 7). This difference remained after adjusting for other risk factors (sdHR for transobturator insertion: 0.72, 95% CI 0.62 to 0.84). The risk of MUT removal decreased with age (4.4% for women aged 18–39 years compared with 2.1% for women aged ≥ 70 years at 9 years after insertion; sdHR 0.46, 95% CI 0.38 to 0.56; see Table 7).
FIGURE 8.
Mid-urethral mesh tape removal, reoperation for SUI and any reoperation by time from insertion. Reproduced with permission from Gurol-Urganci et al. 70 JAMA 2018;320(16):1659–69. Copyright © 2018, American Medical Association. All rights reserved.
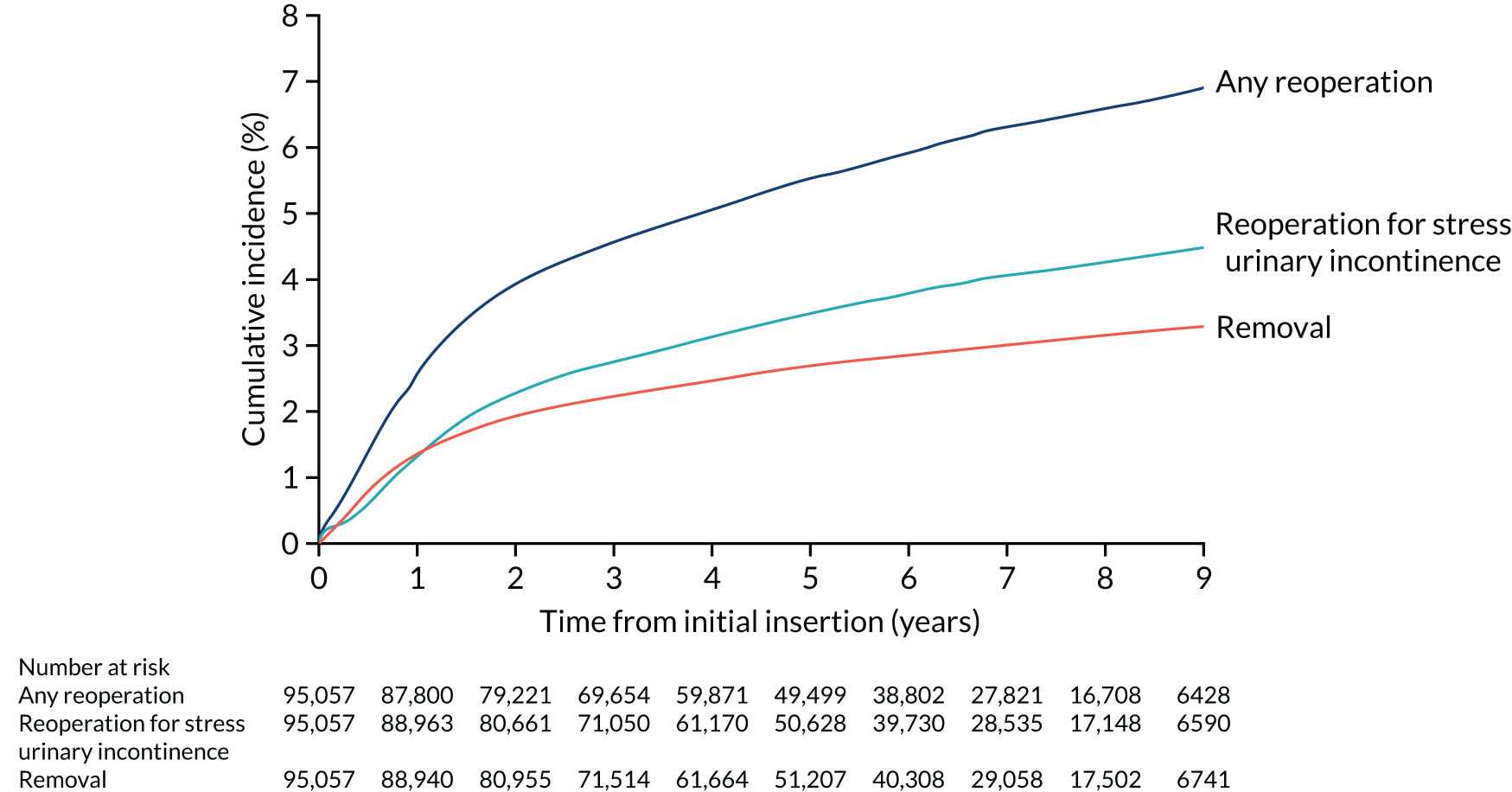
Reoperation for stress urinary incontinence
Risk of reoperation for SUI was 1.3% (95% CI 1.3% to 1.4%) of the women at 1 year, 3.5% (95% CI 3.4% to 3.6%) at 5 years and 4.5% (95% CI 4.3% to 4.7%) at 9 years after the initial insertion, accounting for the competing risk of death (Table 8 and Figure 8). The risk of reoperation for SUI was higher (at all time points) in women who had a transobturator insertion than in those who had a retropubic insertion (5.3% compared with 4.1% at 9 years after insertion; see Table 8), which remained after adjusting for other risk factors (sdHR for transobturator insertion, 1.31, 95% CI 1.14 to 1.51). A higher risk of reoperation for SUI was associated with having undergone a non-mesh continence procedure prior to the initial MUT insertion in this study [8.1% for women who had a bulking injection and 4.5% for women who did not (sdHR 1.74, 95% CI 1.32 to 2.29); 11.1% for women who had another non-mesh SUI continence procedure and 4.5% for women who did not (sdHR 2.60, 95% CI 1.85 to 3.65); see Table 8].
| Number (%) | Risk of reoperation for stress urinary incontinencea (%) | 9 year (95% CI) | sdHR (95%CI)b | p-valuec | ||
|---|---|---|---|---|---|---|
| 1 year (95% CI) | 5 year (95% CI) | |||||
| All: crude risk, n/N (%) | 1252/90,215 (1.4) | 2087/52,715 (3.9) | 391/6981 (5.6) | |||
| All: adjusted risk | 95,057 (100) | 1.3 (1.3 to 1.4) | 3.5 (3.4 to 3.6) | 4.5 (4.3 to 4.7) | ||
| Age at initial surgery (years) | ||||||
| 18–39 | 10,292 (10.8) | 1.1 (1.0 to 1.3) | 3.3 (3.1 to 3.5) | 4.5 (4.2 to 4.8) | Reference | 0.29 |
| 40–49 | 33,094 (34.8) | 1.3 (1.1 to 1.4) | 3.4 (3.2 to 3.7) | 4.2 (3.9 to 4.6) | 0.91 (0.80 to 1.03) | |
| 50–59 | 24,664 (26.0) | 1.6 (1.4 to 1.8) | 3.7 (3.4 to 4.1) | 4.6 (4.2 to 5.0) | 0.92 (0.81 to 1.05) | |
| 60–69 | 16,877 (17.8) | 1.7 (1.4 to 1.9) | 3.8 (3.4 to 4.2) | 4.3 (3.9 to 4.8) | 1.00 (0.86 to 1.16) | |
| 70 + | 10,130 (10.7) | 1.1 (1.0 to 1.3) | 3.3 (3.1 to 3.5) | 4.5 (4.2 to 4.8) | 0.99 (0.83 to 1.18) | |
| IMD (quintiles) | ||||||
| 1 (most deprived) | 16,136 (17.0) | 1.3 (1.1 to 1.5) | 3.5 (3.2 to 3.8) | 4.2 (3.9 to 4.6) | Reference | |
| 2 | 18,277 (19.2) | 1.4 (1.2 to 1.5) | 3.7 (3.4 to 4.0) | 4.9 (4.5 to 5.4) | 1.08 (0.96 to 1.23) | |
| 3 | 20,468 (21.5) | 1.3 (1.2 to 1.5) | 3.5 (3.2 to 3.7) | 4.3 (4.0 to 4.7) | 0.99 (0.87 to 1.13) | |
| 4 | 20,779 (21.9) | 1.4 (1.2 to 1.5) | 3.8 (3.5 to 4.1) | 4.9 (4.5 to 5.3) | 1.09 (0.94 to 1.26) | |
| 5 (least deprived) | 19,397 (20.4) | 1.3 (1.1 to 1.4) | 3.1 (2.8 to 3.3) | 4.1 (3.8 to 4.5) | 0.92 (0.78 to 1.09) | |
| Ethnic backgroundd | ||||||
| White | 83,451 (95.8) | 1.4 (1.3 to 1.4) | 3.5 (3.4 to 3.7) | 4.6 (4.4 to 4.8) | Reference | |
| Asian/Asian-British | 2049 (2.4) | 0.8 (0.5 to 1.2) | 2.5 (1.9 to 3.3) | 3.1 (2.3 to 4.0) | 0.74 (0.54 to 1.01) | |
| Black/black-British | 576 (0.6) | 1.0 (0.4 to 2.1) | 3.1 (1.8 to 4.9) | 3.4 (2.0 to 5.3) | 0.71 (0.46 to 1.10) | |
| Other | 1057 (1.2) | 0.8 (0.4 to 1.5) | 2.4 (1.5 to 3.5) | 2.8 (1.7 to 4.2) | 0.70 (0.45 to 1.07) | |
| Missing (n = 7924, 8.3%) | ||||||
| Route of mesh tape insertion | ||||||
| Retropubic | 60,194 (63.3) | 1.2 (1.1 to 1.3) | 3.1 (3.0 to 3.3) | 4.1 (3.8 to 4.3) | Reference | < 0.001 |
| Transobturator | 34,863 (36.7) | 1.5 (1.4 to 1.6) | 4.1 (3.9 to 4.4) | 5.3 (5.0 to 5.7) | 1.31 (1.14 to 1.51) | |
| Comorbiditiese | ||||||
| None | 76,252 (80.2) | 1.3 (1.2 to 1.4) | 3.4 (3.3 to 3.6) | 4.5 (4.3 to 4.7) | Reference | 0.35 |
| 1 or more | 18,805 (19.8) | 1.5 (1.3 to 1.7) | 3.8 (3.5 to 4.1) | 4.4 (4.0 to 4.8) | 1.04 (0.95 to 1.14) | |
| Previous bulking injection | ||||||
| No | 94,349 (99.2) | 1.3 (1.2 to 1.4) | 3.5 (3.3 to 3.6) | 4.5 (4.3 to 4.7) | Reference | < 0.001 |
| Yes | 709 (0.8) | 2.9 (1.8 to 4.3) | 6.9 (5.1 to 9.1) | 8.1 (5.9 to 10.6) | 1.74 (1.32 to 2.29) | |
| Previous other SUI procedure | ||||||
| No | 94,710 (99.6) | 1.3 (1.2 to 1.4) | 3.5 (3.3 to 3.6) | 4.5 (4.3 to 4.7) | Reference | < 0.001 |
| Yes | 347 (0.4) | 4.9 (3.0 to 7.6) | 9.1 (6.3 to 12.6) | 11.1 (7.8 to 15.1) | 2.60 (1.85 to 3.65) | |
| Concurrent prolapse repair | ||||||
| No | 77,932 (82.0) | 1.4 (1.3 to 1.4) | 3.6 (3.5 to 3.7) | 4.7 (4.5 to 4.9) | Reference | 0.001 |
| Repair with mesh | 817 (0.9) | 2.8 (1.9 to 4.2) | 4.9 (3.5 to 6.6) | 6.2 (3.9 to 9.3) | 1.32 (0.94 to 1.84) | |
| Repair without mesh | 16,308 (17.2) | 1.1 (0.9 to 1.3) | 3.0 (2.7 to 3.3) | 3.7 (3.3 to 4.1) | 0.80 (0.69 to 0.93) | |
| Specialist urogynecology unit | ||||||
| No | 75,695 (79.6) | 1.3 (1.2 to 1.4) | 3.5 (3.4 to 3.6) | 4.5 (4.3 to 4.8) | Reference | 0.84 |
| Yes | 19,362 (20.4) | 1.4 (1.2 to 1.5) | 3.5 (3.2 to 3.8) | 4.4 (4.0 to 4.8) | 1.02 (0.81 to 1.29) | |
| Annual volume of mesh tape insertions | ||||||
| < 60 | 28,939 (30.3) | 1.2 (1.1 to 1.4) | 3.4 (3.2 to 3.6) | 4.4 (4.1 to 4.8) | Reference | 0.37 |
| 60–119 | 44,228 (46.5) | 1.4 (1.3 to 1.5) | 3.6 (3.5 to 3.8) | 4.7 (4.4 to 4.9) | 1.09 (0.96 to 1.24) | |
| ≥ 120 | 21,990 (23.1) | 1.3 (1.1 to 1.4) | 3.4 (3.1 to 3.6) | 4.3 (4.0 to 4.7) | 1.03 (0.83 to 1.28) | |
Any reoperation
The risk of any reoperation (i.e. mesh tape removal and/or reoperation for SUI) following the initial MUT insertion was 2.6% (95% CI 2.5% to 2.7%) at 1 year, 5.5% (95% CI 5.4% to 5.7%) at 5 years and 6.9% (95% CI 6.7% to 7.1%) at 9 years (Table 9). The risk of any reoperation was not statistically significantly different between initial retropubic or transobturator insertions (see Table 9).
| Number (%) | Risk of any reoperationa (%) | sdHR (95%CI)b | p-valuec | |||
|---|---|---|---|---|---|---|
| 1 year (95% CI) | 5 year (95% CI) | 9 year (95% CI) | ||||
| All: crude risk, n/N (%) | 2415//90,215 (2.7) | 3216/52,715 (6.1) | 553/6981 (7.9) | |||
| All: adjusted risk | 2.6 (2.5 to 2.7) | 5.5 (5.4 to 5.7) | 6.9 (6.7 to 7.1) | |||
| Age at initial surgery (years) | ||||||
| 18–39 | 10,292 (10.8) | 3.1 (2.8 to 3.4) | 6.3 (5.8 to 6.9) | 8.3 (7.6 to 9.2) | Reference | 0.01 |
| 40–49 | 33,094 (34.8) | 2.5 (2.3 to 2.7) | 5.4 (5.2 to 5.7) | 7.1 (6.7 to 7.4) | 0.86 (0.79 to 0.94) | |
| 50–59 | 24,664 (26.0) | 2.5 (2.3 to 2.7) | 5.6 (5.3 to 5.9) | 6.8 (6.4 to 7.2) | 0.86 (0.78 to 0.95) | |
| 60–69 | 16,877 (17.8) | 2.5 (2.3 to 2.7) | 5.4 (5.1 to 5.8) | 6.6 (6.1 to 7.1) | 0.83 (0.74 to 0.94) | |
| 70 + | 10,130 (10.7) | 2.5 (2.2 to 2.9) | 5.3 (4.9 to 5.8) | 6.1 (5.5 to 6.7) | 0.79 (0.68 to 0.91) | |
| IMD (quintiles) | ||||||
| 1 (most deprived) | 16,136 (17.0) | 2.5 (2.2 to 2.7) | 5.5 (5.1 to 5.9) | 6.6 (6.1 to 7.1) | Reference | 0.13 |
| 2 | 18,277 (19.2) | 2.7 (2.4 to 2.9) | 5.8 (5.5 to 6.2) | 7.3 (6.8 to 7.8) | 1.08 (0.97 to 1.20) | |
| 3 | 20,468 (21.5) | 2.5 (2.3 to 2.7) | 5.4 (5.0 to 5.7) | 6.5 (6.1 to 7.0) | 0.98 (0.88 to 1.08) | |
| 4 | 20,779 (21.9) | 2.6 (2.3 to 2.8) | 5.7 (5.3 to 6.0) | 7.2 (6.8 to 7.7) | 1.05 (0.94 to 1.17) | |
| 5 (least deprived) | 19,397 (20.4) | 2.6 (2.4 to 2.9) | 5.3 (5.0 to 5.7) | 6.9 (6.4 to 7.4) | 1.00 (0.88 to 1.13) | |
| Ethnic backgroundd | ||||||
| White | 83,451 (95.8) | 2.6 (2.5 to 2.7) | 5.6 (5.4 to 5.8) | 7.0 (6.8 to 7.2) | Reference | 0.01 |
| Asian/Asian-British | 2049 (2.4) | 1.5 (1.1 to 2.1) | 4.3 (3.5 to 5.3) | 5.4 (4.2 to 6.8) | 0.75 (0.59 to 0.96) | |
| Black/black-British | 576 (0.6) | 2.7 (1.6 to 4.2) | 5.0 (3.4 to 7.1) | 5.0 (3.4 to 7.1) | 0.73 (0.51 to 1.05) | |
| Other | 1057 (1.2) | 1.7 (1.1 to 2.6) | 3.9 (2.8 to 5.2) | 5.2 (3.6 to 7.3) | 0.74 (0.54 to 1.01) | |
| Missing (n = 7924, 8.3%) | ||||||
| Route of mesh tape insertion | ||||||
| Retropubic | 60,194 (63.3) | 2.7 (2.6 to 2.9) | 5.5 (5.3 to 5.7) | 6.8 (6.5 to 7.0) | Reference | 0.61 |
| Transobturator | 34,863 (36.7) | 2.3 (2.1 to 2.4) | 5.7 (5.4 to 5.9) | 7.2 (6.8 to 7.5) | 1.03 (0.92 to 1.16) | |
| Comorbiditiese | ||||||
| None | 76,252 (80.2) | 2.5 (2.4 to 2.6) | 5.5 (5.3 to 5.7) | 7.0 (6.7 to 7.2) | Reference | 0.22 |
| 1 or more | 18,805 (19.8) | 2.7 (2.5 to 2.9) | 5.8 (5.4 to 6.1) | 6.6 (6.2 to 7.1) | 1.05 (0.97 to 1.13) | |
| Previous bulking injection | ||||||
| No | 94,349 (99.2) | 2.6 (2.5 to 2.7) | 5.5 (5.4 to 5.7) | 6.9 (6.7 to 7.1) | Reference | 0.001 |
| Yes | 709 (0.8) | 3.6 (2.4 to 5.2) | 8.9 (6.8 to 11.4) | 10.3 (7.9 to 13.2) | 1.55 (1.20 to 1.99) | |
| Previous other SUi procedure | ||||||
| No | 94,710 (99.6) | 2.6 (2.5 to 2.7) | 5.5 (5.4 to 5.7) | 6.9 (6.7 to 7.1) | Reference | < 0.001 |
| Yes | 347 (0.4) | 7.0 (4.6 to 10.0) | 12.5 (9.2 to 16.3) | 14.5 (10.7 to 18.8) | 2.29 (1.66 to 3.14) | |
| Concurrent prolapse repair | ||||||
| No | 77,932 (82.0) | 2.6 (2.5 to 2.7) | 5.6 (5.4 to 5.8) | 7.0 (6.8 to 7.3) | Reference | 0.001 |
| Repair with mesh | 817 (0.9) | 4.3 (3.1 to 5.9) | 7.8 (6.0 to 9.8) | 9.6 (6.9 to 12.8) | 1.43 (1.09 to 1.87) | |
| Repair without mesh | 16,308 (17.2) | 2.4 (2.2 to 2.7) | 5.2 (4.8 to 5.6) | 6.3 (5.8 to 6.8) | 0.92 (0.83 to 1.01) | |
| Specialist urogynecology unit | ||||||
| No | 75,695 (79.6) | 2.5 (2.4 to 2.6) | 5.5 (5.3 to 5.6) | 6.8 (6.6 to 7.1) | Reference | 0.37 |
| Yes | 19,362 (20.4) | 3.0 (2.8 to 3.2) | 5.9 (5.6 to 6.3) | 7.2 (6.7 to 7.7) | 1.08 (0.91 to 1.29) | |
| Annual volume of mesh tape insertions | ||||||
| < 60 | 28,939 (30.3) | 2.4 (2.2 to 2.6) | 5.3 (5.0 to 5.6) | 6.7 (6.3 to 7.1) | Reference | 0.4 |
| 60–119 | 44,228 (46.5) | 2.7 (2.5 to 2.8) | 5.7 (5.5 to 6.0) | 7.1 (6.8 to 7.4) | 1.07 (0.96 to 1.20) | |
| ≥ 120 | 21,990 (23.1) | 2.6 (2.4 to 2.8) | 5.5 (5.2 to 5.8) | 6.8 (6.4 to 7.3) | 1.02 (0.86 to 1.20) | |
Key findings
-
The rate of MUT removal was 1.4% at 1 year, 2.7% at 5 years and 3.3% at 9 years.
-
The 9-year removal risk after transobturator insertion (2.7%) was lower than the risk after retropubic insertion (3.6%).
-
The rate of any reoperation, including mesh tape removal, was 2.6% at 1 year, 5.5% at 5 years and 6.9% at 9 years.
-
The 9-year risk of any reoperation was not statistically significantly different after transobturator insertion (7.2%) or retropubic insertion (6.8%).
Chapter 7 Work package 3: women’s perspectives on urinary incontinence and its treatment
Parts of this text have been reproduced with permission from Lynch et al. 71 This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
In this chapter, we explore the impact that UI has on women’s lives and their expectations when surgery is perceived to be a treatment option.
The methods are described in detail in Chapter 3. Briefly, we collected women’s own accounts of their experiences of UI and their expectation of surgical and non-surgical treatments in semistructured interviews. Women who were aged ≥ 18 years and had not had previous urological surgery were purposively sampled from four English urogynaecology outpatient clinics. We did not restrict the interviews to women with a specific subtype of UI. The semistructured interviews lasted approximately between 60 and 90 minutes. Transcripts of the interviews were analysed following a constant comparative method. Emerging themes from the interviews were followed up in more depth in subsequent interviews. Themes were then ordered into higher-level themes and hierarchies.
Twenty-eight women were interviewed from four urogynaecology outpatient clinics in different parts of England. They were categorised by age group and the overall age range was between 26 and 74 years (Table 10).
| Characteristic | Number of women |
|---|---|
| Age group (years) | |
| 20–39 | 6 |
| 40–49 | 4 |
| 50–59 | 7 |
| 60–69 | 6 |
| ≥ 70 | 5 |
| Clinic attended | |
| Birmingham | 14 |
| Gillingham (Kent) | 7 |
| Leicester | 7 |
| Southampton | 1 |
Women of similar ages drew on similar ideas and understanding of their bodies, their UI and the possibilities that surgery may bring. Women’s decision-making around surgery at the time we interviewed them revolved around two key areas: their perceptions of the severity of their condition and their perception of the seriousness, or risk, of surgery. Both of these areas drew on common themes that spoke to women’s wider experiences, values and concerns around their bodies: first, how their UI competed with other demands they needed to deal with in their everyday lives and, second, that surgery was regarded as a major intervention and perhaps unnecessary. Issues of age and ageing ran through these subthemes, cutting across the two fields of concerns around seriousness and resulting in arguments for and against surgical intervention in both fields.
How serious is my condition?
Women placed their management of UI symptoms and their contemplation of having surgery in relation to the competing demands on their time as mothers, daughters, partners, siblings and/or employees. To continue these roles and responsibilities as far as possible, all of the women interviewed had made substantial changes to their lives such as taking (and hiding) incontinence pads as they went about their day (sometimes having supplies at the houses of other family members); keeping changes of clothes at work or in their car; wearing loose, dark trousers or flowing skirts to hide any leaking; and avoiding activities that might trigger urine loss (e.g. running or going on the trampoline with their children). As the circumstances of daily life changed, UI symptoms could also become more or less obvious:
I feel like it’s got worse, I feel like my bladder has got weaker, that I haven’t been able to retain it as much. But I don’t know whether that’s because I’m more aware of it now because [name of daughter]’s that much older . . . Whereas when they’re younger you’re at home, you can go to the toilet that much easier.
Interviewee 26, 32 years old
The management of UI in relation to everyday life also had an impact on how women regarded the pragmatics of having a potential operation. Timing both the surgery and the recovery time around childcare, caring for others and work (along with their sick leave options) were key concerns. Although taking time out of these roles might be seen as an investment in their future bodies, this was often hard to negotiate:
I don’t really want it [the operation]. But if I delay it, I’m getting older, whereas obviously, 40, almost 41, my youngest is 13 [years old], my oldest is 17 [years old], so at some point they’re going to leave. I might as well enjoy the time now and be able to do more. Because we like to put the badminton net up in the garden. I can’t play badminton, or go running, you know . . .
Interviewee 23, 40 years old
Changes in life circumstances for some older women meant that they experienced reduced demands on their time and could now consider having surgery, which in the past they could not have done. One woman in her 60s talked about how, now that her husband’s ill health had got better, she could attend to her own issues, whereas another said:
. . . I did give up work, so it wasn’t an issue to think, oh, crikey, I’ve got to get back to work. So, I felt that, all in all, I could cope with the operation.
Interviewee 5, 62 years old
Finding time for surgery in relation to competing demands also depended on the extent to which women prioritised their UI and saw it as something that should be dealt with. For many women whose symptoms were very pronounced and had an impact on their lives in a dramatic way, it was easy to decide that something more intrusive than conservative treatments should be undertaken. However, for others, this step was less clear:
. . . my problem doesn’t feel bad enough to go for surgery . . . I really wanted to keep going with the physio[therapy] and try and sort it out that way. And I’ve been signed off physio[therapy] now because it just wasn’t getting any better . . . they gave me this sheet explaining the surgery and the risks involved and things, and it feels like quite a hard decision to make, because I think if I had a really bad problem the risks would be worth taking . . . I feel the problem I’ve got, I’ve made some improvement but it’s only going to get worse as I get older, and why not sort it out now rather than wait for another 10 years where it’s really bad. It feels quite a difficult decision . . .
Interviewee 7, 51 years old
Women across the age groups compared themselves with others they knew with similar symptoms, to try and contextualise the severity of their own condition. Although some women were not aware of others with similar problems, others talked about particular discussions and comparisons. Many women put their symptoms worsening as down to ageing, and comparisons were often drawn with others of a similar age:
I’ve had the same peer group since I was a young girl, and my friends will go out and they don’t have to take spare knickers in their bag in case they laugh on the dance floor or they trip over, or a sudden movement happens that they have an issue with incontinence. I run with my friends, and they don’t have a problem, they can run for ages and ages.
Interviewee 11, 38 years old
The cost of such surgery, not only in terms of their own bodies and time but also in terms of NHS resources, was also a concern expressed spontaneously, particularly by some older women. This led these women to question whether or not their current symptoms were worth such a major intervention, especially when this might just be part of ‘normal’ ageing:
I suppose one of my issues around do I take any action around this is, is this a normal part of ageing, do I accept it, do other people accept it, is the problem I’ve got worse, or not as bad as other people? You don’t know how to compare yourself with other people because it’s not really talked about . . . I mentioned it to two people and they’ve both said, well they implied, that it’s something you just accept with age.
Interviewee 6, 66 years old
Drawing on the lives and experiences of others to find out what was a ‘normal’ amount and frequency of urine loss allowed women of different ages to make a judgement about how severe their symptoms were and, therefore, whether or not they should consider surgical interventions. However, combining this with the other demands on their time, and the extent to which they were able to deal with their symptoms in everyday life, made different types of surgery more or less possible.
How serious is surgery?
In addition to drawing on the experiences of others and their own life circumstances to evaluate the seriousness of their UI condition, women’s considerations about surgery were also shaped by hearing about other people’s experiences of having the procedure for the same symptoms:
My sister and one of my best friends who I worked with, they’ve both had their bladders done . . . my sister now, she’s back out there and doing things. And I go, I want to be like my sister . . . My mate, she paid private rather than wait. ’Cause we didn’t know about this. We were all just taking tablets, didn’t know you could have an operation for it.
Interviewee 16, 50 years old
Without having experienced UI surgery themselves, women across the age groups drew on a range of different sources of information to try to anticipate the impact that such surgery would have on their bodies, on their time and in relation to their other roles and responsibilities. Mentioning the stigma associated with UI, a number of women of all ages referred to the fact that the symptoms of, and surgery for, UI were not often talked about and so because they did not know other women with the same issue directly, it was difficult to understand what was ‘normal’ and what surgery might be like. A few women had researched online for reports of what UI surgery might be like, or looked for news articles or radio or TV programmes that might mention this, although others made a conscious decision not to do this:
I don’t want to fill my head with what might happen . . . I don’t want to be like, oh, so, if mine is different, what have I got then? I try not to, I just get the leaflets from the physio lady or from my doctors and I really try not to go on Google or research it myself.
Interviewee 10, 26 years old
In relation to the procedure itself, it was more common for women to draw on their own previous experiences of surgery for other conditions to guess what surgery for UI might be like; some were concerned about repeating negative experiences, whereas for others recalling past surgical experiences was reassuring:
. . . it doesn’t bother me at all because I know what I’m going in to . . . it’s just like when I came out of my hysterectomy they said, right, okay, you can’t do anything for 6 weeks, and I’m like, what does that mean, you know? . . . from that point of view I feel more prepared almost, so, not that you ever are for surgery, but I do feel more prepared.
Interviewee 15, 36 years old
Embedded in many of these accounts was the idea that the surgery was always a significant intervention that not only required time to recover from, but also might put the body under great strain. A number of women talked about how their body found it hard to recover from general anaesthetic or being sutured. Such thinking focused on the fact that an operation always puts the body at risk, rather than the possible longer-term outcomes or chances of success. Nonetheless, the specifics of how this particular surgery would be performed on an intimate area of their anatomy was a particular concern for some women, as one woman explained:
I thought they will [use] keyhole [surgery] or [go] through the stomach or something. I didn’t think they would actually be doing it through the vagina. So, obviously anything to do with your private area you’re going to be a bit nervous about. It’s something completely different that, the only person that’s ever been there is when I’ve had children.
Interviewee 10, 26 years old
The overall point is that risks and fears around surgery, and particularly surgery around such intimate areas, were already present for women outside any discussion around the use of mesh. As the study progressed, issues around the mesh controversy were sometimes spontaneously raised, but they never dominated the interviews. In some of the later interviews, women talked about how they, or others in their family, had heard about the mesh controversy. However, the women were rarely clear whether or not this might relate to the surgery that they were considering, and there were always many other factors to take into account:
. . . I’ve got an 8-year-old, I’m busy, and I didn’t want, and also I had read a bit about the controversy about the sling that’s been in the papers and stuff in the last year or so, so I opted just to get the injections.
Interviewee 21, 46 years old
Another woman balanced if she might have problems with the mesh with the necessity of the operation, and had decided not to proceed:
. . . after reading all that in the Daily Mail [a British tabloid newspaper, London], I’m not sure how much of it is true, and whether they don’t know until you have the operation if you’re going to be allergic to that mesh . . . it’s not life-threatening this. If it was life-threatening I wouldn’t have a choice, but I have got a choice.
Interviewee 12, 74 years old
The perception of surgery as a serious and dramatic intervention led a number of women to describe trying to find ways to avoid surgery; despite surgery being raised as a possibility, one woman wondered:
Perhaps if I carry on with my exercises I might . . . you know, I might be OK.
Interviewee 8, 71 years old
Given the nature of our interview cohort of women, conservative treatments, by definition, had largely not been successful. Some women were nevertheless hopeful that continuing with these approaches might eventually prove effective. This subgroup of women, therefore, found it particularly difficult to decide whether or not to have surgery, because the decision had to be made not merely on the clinical information they were given, but also the hope that they held on to that a non-surgical intervention might work in the future. Some women talked about the need to encourage younger women to start pelvic floor exercises earlier and a couple of women had mentioned this to their daughters. The idea behind this was both that muscle strength might be maintained and that early issues could be stopped from developing, particularly if they came after childbirth:
I think I’d let it go too far . . . I think perhaps the pelvic floor exercises would have worked but I’d left it too long.
Interviewee 19, 54 years old
Ageing, and the changes to the body and everyday life that this might bring, was a key aspect that crossed different themes from the interviews. This related not only to the ideas of the ageing body across women’s accounts and where the body should be at particular ages, but also to different experiences that women of different ages had and how UI symptoms and surgery fitted into their daily lives. This resulted in greater clarity about whether or not to have surgery and what type to have for some women. However, many of the women interviewed were as yet undecided about surgery.
Key findings
-
Women have two key concerns when they make decisions about whether or not to have surgical treatment for their incontinence: perceptions of the severity of their condition and perceptions of the seriousness, or risk, of surgery.
-
Women assessed the severity of their UI according to their individual circumstances rather than criteria such as frequency or quantity of leakage. Women moved the concept of ‘severity’ beyond a medical definition of UI to include what is important to them as individuals.
-
For women with UI, decision-making about surgical treatment is a distributed process based on multivariate criteria, which often shift in priority over time. As a consequence, decisions are rarely made conclusively.
Chapter 8 Work package 5: clinicians’ decision-making for recommending surgical treatment for female stress urinary incontinence
Parts of this chapter are reproduced with permission from Mamza et al. 72 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/. The text includes minor additions and formatting changes to the original text.
In this chapter, we present the findings from WP 5, which addresses objective 4 and explores variation in treatment advice by clinicians for women with SUI using case vignettes.
The methods are described in detail in Chapter 3. Briefly, we conducted an online survey of all members of the BSUG and members of the RCOG who indicated that they had specialist interest in urogynaecology at the time of their RCOG registration (n = 1139). The survey comprised 18 case vignettes that described the clinical profile of hypothetical women referred to secondary care for further assessment and management of their SUI. Each case profile comprised a short patient description according to seven patient characteristics. Clinicians were then asked to score their recommendation on whether or not they would recommend surgery for this patient on a five-point Likert scale ranging from ‘certainly yes’ to ‘certainly not’. We used descriptive statistics to summarise the characteristics of the responding clinicians. We calculated the means of the response scores and the 25th and 75th percentiles to describe the recommendations of the clinicians for each of the 18 case vignettes. We used a mixed-effects analysis of variance model to test the statistical significance of differences in the clinicians’ recommendation scores according to level of the patient characteristics. Latent class analysis was used to determine if mutually exclusive groups of clinicians (‘latent classes’) could be identified whose recommendations suggested a similar practice style.
In total, 334 gynaecologists participated in the survey, of whom 245 (73.4%) fully completed the questionnaire. Of the 245 gynaecologists, 56.7% were male and 55.9% indicated that urogynaecology was their main specialty area (Table 11).
| Characteristic | Number of clinicians responding | % of total | Mean recommendation score (SD)a |
|---|---|---|---|
| Total | 245 | 100 | 2.87 (1.28) |
| Gender | |||
| Female | 106 | 43 | 2.88 (1.26) |
| Male | 139 | 57 | 2.86 (1.30) |
| Age category (years) | |||
| < 45 | 85 | 35 | 2.88 (1.27) |
| 45–54 | 84 | 34 | 2.87 (1.30) |
| ≥ 55 | 76 | 31 | 2.85 (1.27) |
| Clinical specialty | |||
| Gynaecology | 108 | 44 | 2.83 (1.27) |
| Urogynaecology | 137 | 56 | 2.90 (1.29) |
| Trainee | |||
| No | 212 | 87 | 2.86 (1.29) |
| Yes | 33 | 13 | 2.91 (1.24) |
| Country of practice | |||
| England | 212 | 87 | 2.86 (1.28) |
| Northern Ireland | 11 | 4 | 2.75 (1.13) |
| Scotland | 15 | 6 | 3.03 (1.34) |
| Wales | 7 | 3 | 2.90 (1.34) |
Recommendations for surgery
Figure 9 shows the variation in the recommendation scores of all clinicians for the 18 case profiles. The IQR was between 2 and 4 on the 5-point scale for 11 of the 18 vignettes. The scores were most strongly in favour of recommending surgery for case 5 (a 68-year-old woman with a BMI of 30 kg/m2, pure SUI and no previous SUI surgery, with leakage several times a day, for whom her incontinence is quite a problem, and ASA grade 2) and most strongly against recommending surgery for case 6 (a 68-year-old woman with a BMI of 23 kg/m2, with MUI, previous SUI surgery, leakage once a day, for whom her incontinence is a bit of a problem, and ASA grade 3).
FIGURE 9.
Surgical treatment recommendations of the clinicians for the 18 clinical case profiles. The recommendations are scored on a five-point Likert scale ranging from ‘certainly not’ to ‘certainly yes’. The range plots (horizontal bars) represent the 25th and the 75th percentile of the mean recommendation score. BNI, bladder neck injection. Reproduced with permission from Mamza et al. 72 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
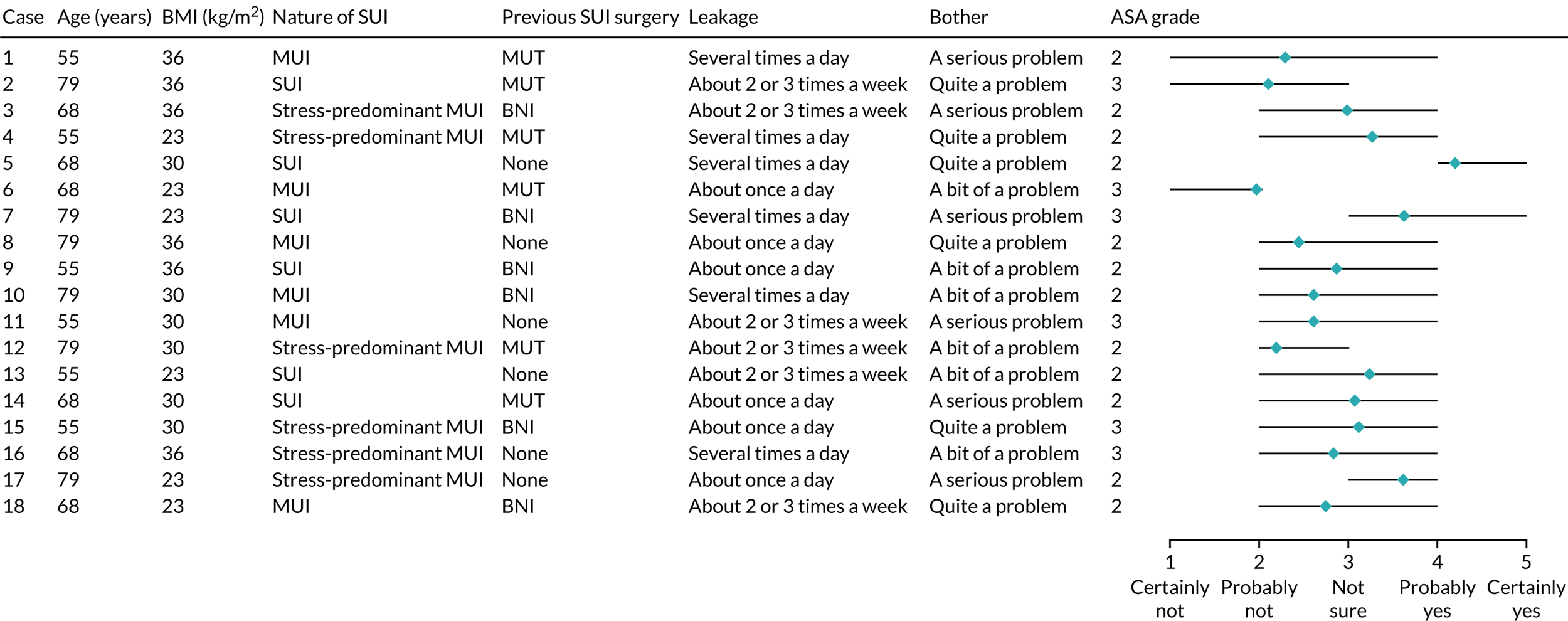
Impact of women’s characteristics on gynaecologists’ recommendations
Figure 10 demonstrates the impact that the patient characteristics have on the clinicians’ recommendations. Overall, the impact that the patient characteristics have was relatively small. UI type had the greatest impact (i.e. a mean difference in recommendation score for women with SUI and MUI of 0.8, weight = 23%; see Chapter 3), closely followed by the impact of previous SUI surgery (weight = 21%) and, to a lesser extent, the frequency of leakage (weight = 15%) and BMI (weight = 15%). Extent of bother (weight = 13%), physical status (as assessed via the ASA grade) (weight = 8%) and age (weight = 6%) had the least influence on the clinicians’ recommendations. The mixed-effects analysis of variance indicated that all patient characteristics captured in the vignettes significantly influenced the recommendation score (i.e. the p-value was always < 0.001).
FIGURE 10.
Influence of patient characteristics on clinicians’ recommendation for surgical treatment. The recommendations are scored on a five-point Likert scale ranging from ‘certainly not’ to ‘certainly yes’. Average score based on responses to a 5-point scale ranging from 1 ‘certainly not’ to 5 ‘certainly yes’. Reproduced with permission from Mamza et al. 72 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.
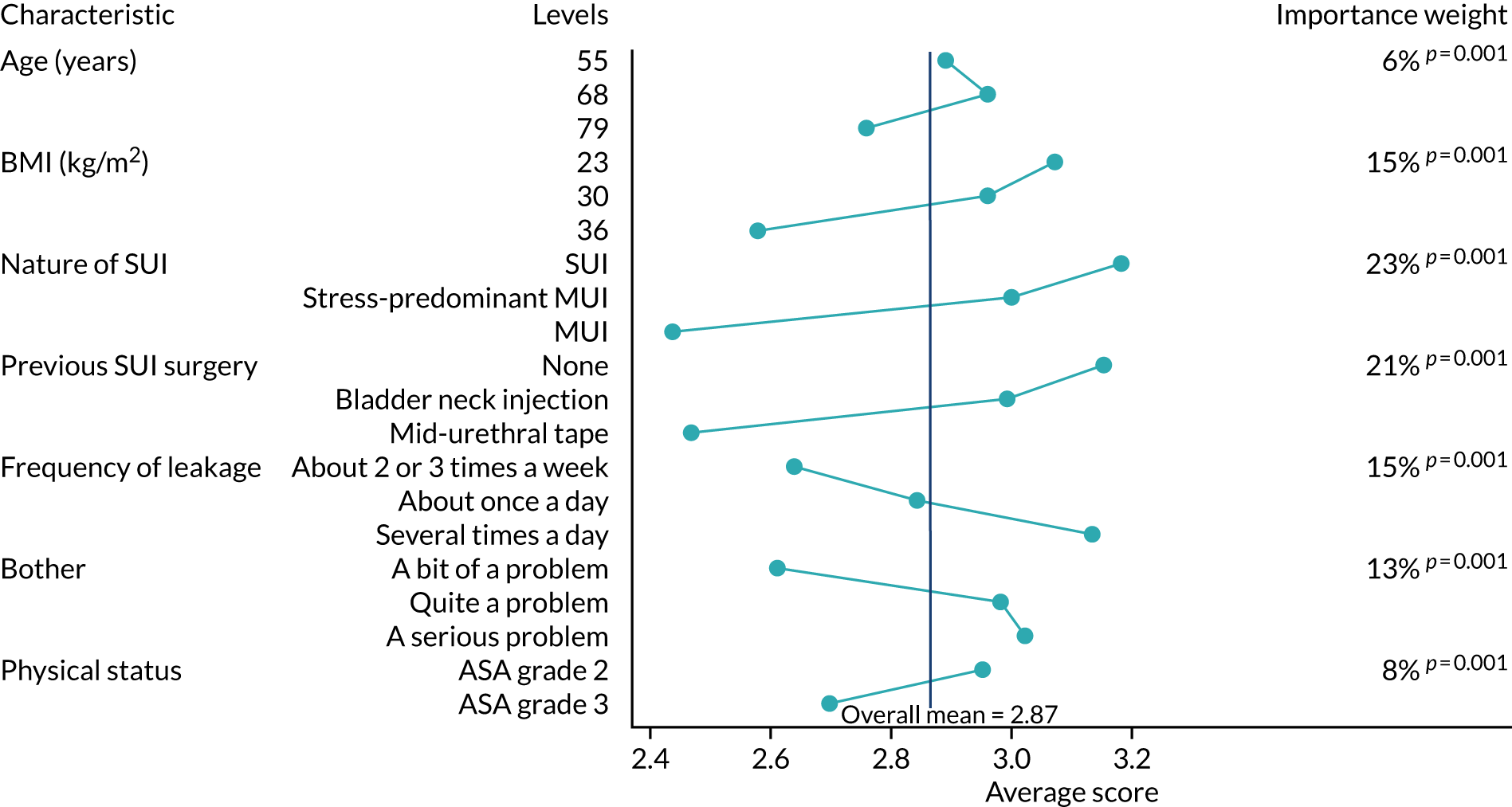
Impact of clinicians’ characteristics on their recommendations
The average recommendation scores did not differ significantly by clinicians’ gender, age, specialty or trainee status (Table 12). In addition, there were no statistically significant differences between the scores of clinicians in England, Scotland, Wales and Northern Ireland. Using the mixed-effects model, we did not find any evidence that the relative influence of the patient characteristics on the recommendation scores varied according to the clinician’s characteristics (i.e. the p-value of the interaction test for specialty, gender and age was always > 0.05).
| Characteristic | All | Group | p-value | ||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| Number of clinicians | 244 | 17 | 85 | 52 | 69 | 21 | |
| Mean recommendation scorea | 2.87 | 1.25 | 2.47 | 3.10 | 3.24 | 4.04 | < 0.001 |
| Gender, n (%) | 0.77 | ||||||
| Female | 105 (43.0) | 6 (35.3) | 41 (48.2) | 22 (42.3) | 27 (39.1) | 9 (42.9) | |
| Male | 139 (57.0) | 11 (64.7) | 44 (51.8) | 30 (57.7) | 42 (60.9) | 12 (57.1) | |
| Age category (years), n (%) | 0.60 | ||||||
| < 45 | 71 (29.1) | 4 (23.5) | 28 (32.9) | 11 (21.2) | 23 (33.3) | 5 (23.8) | |
| 45–54 | 97 (39.8) | 10 (58.8) | 30 (35.3) | 24 (46.2) | 24 (34.8) | 9 (42.9) | |
| ≥ 55 | 76 (31.1) | 3 (17.7) | 27 (31.8) | 17 (32.7) | 22 (31.9) | 7 (33.3) | |
| Specialty, n (%) | 0.14 | ||||||
| Gynaecology | 107 (43.9) | 5 (29.4) | 43 (50.6) | 19 (36.5) | 34 (49.3) | 6 (28.6) | |
| Urogynaecology | 137 (56.1) | 12 (70.6) | 42 (49.4) | 33 (63.5) | 35 (50.7) | 15 (71.4) | |
| Trainee, n (%) | 0.55 | ||||||
| No | 211 (86.5) | 16 (94.1) | 75 (88.2) | 45 (86.5) | 56 (81.2) | 19 (90.5) | |
| Yes | 33 (13.5) | 1 (88.2) | 10 (11.8) | 7 (13.5) | 13 (18.8) | 2 (9.5) | |
Additional analyses found that six clinicians gave a recommendation score of 1 (‘certainly not’) to all 18 vignettes. However, excluding the responses of these six clinicians had only a slight impact on the mean recommendation scores and weights associated with the patient characteristics.
Gynaecologists’ practice styles
The latent class analysis identified five mutually exclusive groups of clinicians with a different practice style. The AIC and BIC decreased as more latent classes were added, but levelled off after five latent classes. In total, 244 of the 245 respondents could be assigned to a practice style group (see Chapter 3). The mean recommendation scores for the five practice style groups ranged from 1.25 to 4.04 (p < 0.001), illustrating wide variation in clinicians’ inclination to recommend surgical treatment (see Table 12). There were no statistically significant differences in clinicians’ characteristics between the five groups.
Compared with the differences in overall inclination to recommend surgical treatment between the practice style groups, the impact of the women’s characteristics on clinicians’ recommendation scores is small (Figure 11). The largest differences are observed for practice style groups 2 and 3, which appeared to give a greater weight to BMI, type of SUI and previous SUI surgery than the clinicians in the other practice style groups.
FIGURE 11.
Influence of patient characteristics on clinicians’ recommendations for surgical treatment according to practice style group. The recommendations are scored on a five-point Likert scale ranging from ‘certainly not’ to ‘certainly yes’. Reproduced with permission from Mamza et al. 72 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: http://creativecommons.org/licenses/by/4.0/.

Key findings
-
The type of UI (SUI, stress-predominant MUI or mixed UI) was the most important determinant of gynaecologists’ decisions to recommend surgical treatment, followed by previous SUI surgery (none, bladder neck injection, MUT).
-
Five groups of gynaecologists whose practice style differed mainly with respect to their mean recommendation score could be distinguished.
Chapter 9 Discussion
In this project, we set out to understand the drivers of variation in the use of surgery for women with UI in England. We combined patient-level administrative health-care databases with general practice and hospital-level information to explore women’s UI care pathway. We also used information collected from women with UI and gynaecologists to understand their decision-making in relation to surgery for UI. In this chapter, we first discuss the results of each of our objectives in the context of the literature. We then describe the main limitations and suggest further research.
The aim of this project was to improve the delivery of surgical care for women with UI. Although surgical care refers to all surgical approaches and invasive procedures for all subtypes of UI, there is a focus, in some WPs, on SUI and surgical treatments with MUT insertion for this type of UI because SUI is the most common form of UI and > 95% of UI procedures conducted are SUI procedures. In our summary of the results of each WP below, we define which patient group the results relate to.
Changing context
The data used in this project capture a period of substantial change in surgical treatment for UI. MUTs were introduced in 1998 as a new, minimally invasive surgical treatment for SUI. 23 The use of MUTs rose precipitously and reached a maximum of 11,365 procedures in 2009 in England, becoming almost the sole surgical treatment conducted for SUI. At the same time, the use of the previous standard treatment for SUI, colposuspension (a major abdominal surgery), declined from > 3500 procedures per year to just 200. 20 However, the use of MUTs for SUI has since rapidly declined in the England (and elsewhere), with a 50% reduction between 2008 and 2017 (to just 6227 procedures in 2016–17). 21 This highlights a change in surgical practice that is likely to reflect growing public concerns about longer-term complications, outcomes and risk of further surgery after these MUT procedures. 21–26 In 2018, the use of MUTs as a treatment for female SUI was ‘paused’ in the NHS in England, following an interim recommendation of the Independent Medicines and Medical Devices Safety Review. 73,74
Project overview
This project used five different approaches:
-
We analysed existing NHS data sets from primary care (CPRD) and secondary care (HES) to understand the determinants of referral of women with UI to secondary care as well as the determinants of the use of surgical treatment in women with UI, which were mainly SUI procedures (predominantly MUT insertions) (WPs 1 and 4).
-
We analysed an existing NHS data set from secondary care (HES) to understand geographical variation in the use of surgical treatment in women with SUI (WP 2).
-
We collected accounts from women who had been referred to an outpatient clinic in secondary care about the impact of UI on their lives and their expectations of surgical and non-surgical treatments (WP 3).
-
We carried out a national survey of gynaecologists in the UK with a special interest in urogynaecology to understand better how their decisions to recommend surgical treatment are influenced by the characteristics of women with UI (WP 5).
-
In response to the discussions about the safety of MUT insertions, we conducted supplementary work to assess long-term removal and reoperation rates after MUT insertion for SUI using the HES data set (additional WP – WP 6).
Brief overview of findings
There were a number of key findings.
With respect to WPs 2 and 4, which aimed to assess the determinants of referral to a UI specialist and surgery for women with UI (WP 4) and variation in the rate of surgery for SUI and determinants of this variation between NHS CCGs and other regional units (WP 2):
-
Our analyses of primary care data demonstrated that almost 50% of women who were diagnosed with UI by their GP between 2004 and 2014 were referred to a UI specialist in the 9 years after the UI was first recorded. Almost 60% of those women who were referred were referred within the first 30 days. The referral rate was lower in older women, in women from a minority ethnic background and in women who were either underweight or severely obese. We also found considerable geographical variation.
-
In addition, using primary care data linked to secondary care data we found that 7.3% of women who were referred to a UI specialist between 2004 and 2014 had surgical treatment for UI within 1 year and 18.1% within 9 years. Of the 4307 UI procedures conducted, 4050 (94.5%) were SUI procedures and 257 (5.5%) were UUI procedures. Of the SUI procedures, 89% (n = 3606) were MUT insertions. Again, we found that UI surgery rates were lower in older women, in women from a minority ethnic background, and in women who were either underweight or severely obese. There was also considerable regional variation in the use of surgery.
-
Between 2013 and 2016, the overall rate of SUI surgery was 40 per 100,000 women per year in the NHS England (WP 2). There was substantial variation in rates between geographic regions with adjusted surgical rates ranging from 20 to 106 procedures per 100,000 women per year between CCGs and from 24 to 69 procedures per 100,000 women per year between STPs. Annual SUI surgery rates per 100,000 women per year declined over the study period from 52 in 2013 to 36 in 2015. Regional age and ethnicity distributions were associated with surgery rates.
With respect to WP 3, which aimed to explore women’s experiences of UI and their expectations of surgical and non-surgical treatments and outcomes:
-
The interviews demonstrated that women’s decision-making centred on perceptions of the severity of their UI and the seriousness, or risk, of surgery.
-
Women judged the severity of their UI according to their individual circumstances rather than criteria such as quantity and frequency of leakage, which are often used clinically. The women’s accounts moved the concept of ‘severity’ beyond the commonly used medical definitions of UI severity to include what is important to women themselves.
-
Decisions about surgery were rarely made conclusively. Decision-making about surgery is a distributed process based on multivariate criteria, which often change in priority over time.
With respect to WP 5, which aimed to explore the relative importance of specific patient characteristics for clinicians in their treatment recommendations:
-
The survey of gynaecologists in the UK, asking them whether or not they would recommend surgery for women with UI described in a series of clinical case vignettes, demonstrated that the decisions of the 245 gynaecologists were most strongly determined by the subtype of UI (stress, stress-predominant or mixed UI) and whether or not a patient had undergone previous SUI surgery (none, bladder neck injection or MUT).
-
Five distinct groups of gynaecologists could be distinguished whose practice style differed mainly with respect to their average inclination to recommend surgery.
With respect to the additional WP on long-term removal and reoperation rates after MUT insertion:
-
The overall 9-year mesh tape removal rate was 3.3% and the reoperation rate, including mesh tape removal, was 6.9%.
-
The 9-year removal rates were 3.6% after a retropubic insertion and 2.7% after a transobturator insertion.
-
The 9-year reoperation rates were 6.8% after a retropubic insertion and 7.2% after a transobturator insertion.
Discussion
Variation in practice
There is considerable geographical variation in the rate of surgery for women with SUI. This variation suggests that women in some areas are more likely to be treated than women with the same condition in other areas. We found that adjusting for regional characteristics that may influence demand for treatment reduced only the observed geographical variation in rates of surgery for SUI slightly.
The rates of surgical treatment for SUI were lower in areas with older populations. This agrees with findings for other aspects of continence care that the management of UI is different in older people. 75 Although surgical treatments for SUI are considered to be safe and effective in older women,76 these procedures are performed less frequently than in younger women. 30,77
The overall rate of SUI surgery dropped by one-third over the 3-year study period, whereas the extent of geographic variation remained stable. The variation is likely, in part, to reflect differences in professional opinion about the appropriateness of these surgical treatments for SUI, in the context of the debate about the safety of MUT surgery, before the ‘pause’ in their use in 2018.
This variation in the use of surgery may partly originate in the limited involvement of primary care for some women. We found that a relatively large proportion of women, about one in two, who have a diagnosis of UI recorded in primary care were referred to secondary care and about 60% of these referrals occurred within 1 month of the diagnosis first being recorded. This demonstrates that much of the care for women with UI is provided in secondary care.
Some of the variation may reflect how physiotherapy for UI, in particular pelvic floor muscle training, is accessed in different regions, which may be through referral to urogynaecology in secondary care, or through direct referral from a GP to a physiotherapist. Updated guidelines from the National Institute for Health and Care Excellence19 (NICE) recommend that women are offered:
a trial of supervised pelvic floor muscle training of at least 3 months’ duration as first-line treatment . . . for stress or mixed urinary incontinence.
This recent guideline may change referral patterns in the future because pelvic floor muscle training can be provided in primary care.
Age and ethnicity were strongly associated with both referral to a UI specialist and surgical treatment after referral. Of women seeking primary care for their UI, older women and those from minority ethnic backgrounds were much less likely to be referred to a specialist than younger women and white women. Referral rates were also lower in women who had a POP diagnosis, possibly because they had already been referred in the past for this diagnosis.
Among women referred, older women were also much less likely to have received surgical treatment. This adds to a body of evidence that older women are less likely to receive continence care than younger women. 30,31,78 Taken together, our findings suggest that potential deficiencies in continence care occur along the whole care pathway (from primary to secondary care) for older women, the group among whom UI is most prevalent.
Lifestyle changes may be recommended in primary care for women with UI who also smoke or are obese and we observed referral rates that were correspondingly lower among these groups than for non-smokers and those with a BMI in the normal range. 19 Our findings indicate that even among those women referred, differences in surgical treatment according to smoking status and BMI persist. This may reflect lifestyle changes also being recommended in secondary care, which for some women may result in possible delays in surgical treatment.
Surgery rates varied substantially by region and these variations remained after adjustment for individual-level factors likely to affect women’s preferences. However, women’s preferences will also be strongly guided by the advice they receive from their clinicians, which may have varied by region, particularly in the context of the debate around the safety of MUT procedures that was ongoing at the time from which the data are drawn. Differences in referral rates between England, Scotland, Wales and North Ireland may reflect the differences in how care is being delivered, especially with respect to the threshold for referral to secondary care.
Women presenting in primary care represent the ‘tip of the iceberg’ in terms of those affected by UI, as it has been found that only 25% of women with UI seek care. 9 However, the women included in all our analyses had all sought care in primary care. Once they have sought primary care, these same groups of women experience differences in rates of referral and of UI surgery that may indicate clinical uncertainty among GPs and secondary care UI specialists about the appropriateness of referral or surgery, differences in women’s preferences for more or less conservative treatment and expectations for improvement or cure, or inequities in the use of referral or surgery. An ageing population coupled with rising prevalence of UI with age means that the number of women who experience UI and could benefit from care is likely to increase over time, increasing the importance of addressing inequalities in continence care.
Women’s own accounts
Our results demonstrate that women’s decision-making in relation to surgery centred on their individual perceptions of the severity of their UI and the risk of surgery. This reflects findings of previous research; for example, it has been suggested that only around half of older people seek help for UI, commonly because of the belief that it is a ‘normal’ part of ageing. 40,79 Some women questioned whether or not their symptoms justified the cost of surgical treatment to the NHS.
In addition, differences in the treatment of UI received by older women and women from minority ethnic backgrounds may reflect differences their incontinence-related health beliefs, preferences and care-seeking behaviour. 33,41,80–82 It has also been reported that women from Asian backgrounds in particular are less likely to seek care because of sociocultural norms and related embarrassment, particularly in relation to discussing sensitive problems with male health-care providers. 83,84
Clinical recommendations about treatments for UI frequently involve considering severity in terms of the quantity and frequency of leakage. However, we found that women judge the severity and impact of their UI from a much broader set of criteria, ranging from personal bodily experiences to how extensively the condition disrupts their social roles and daily lives, as well as the ideas, opinions, and experiences of others. Two different women with the same quantity and frequency of leakage can experience UI as having very different levels of severity and impact on their daily lives. Acknowledging this in the process of shared decision-making around treatment, including surgery, provides an important opportunity to ensure that discussions and treatment decisions reflect women’s own lives and experiences in personalising medicine.
Women’s perceptions and decisions were open to new information and changing circumstances. The ideas, opinions and experiences of others were important to women’s treatment decision-making process. The ‘unfinished’, flexible nature of this decision-making and the growing publicity of safety concerns relating to MUT procedures had the potential to increase uncertainty in women’s decisions regarding surgery.
Recommendations of gynaecologists on surgical treatment
The study using clinical case vignettes showed that five groups of clinicians could be distinguished who differed in ‘practice style’, especially in their average inclination to recommend surgical treatment. These results reflect that some gynaecologists recommend surgery to many of their patients, whereas others would hardly recommend it to anyone. The recommendations for surgical treatment were only minimally influenced by patient characteristics.
In our clinical case vignettes, we included patient characteristics that can be derived only from patient-reported information (frequency of leakage and bother) and those that are derived from information determined by the clinicians (type and severity of UI, previous surgery). We found that the weights assigned to the clinician-derived characteristics had a stronger combined influence on the gynaecologists’ recommendations than the patient-reported characteristics. It is important to note that the clinician-derived characteristics describe the severity and type of the UI that have a recognised impact on the effectiveness of specific surgical treatments. 85
We found that a woman’s age and BMI had relatively little impact on clinicians’ recommendations for surgical treatment, which is in line with evidence that surgical treatments are effective in obese and elderly patients. 76,86–89 This finding contrasts with that from our linked primary and secondary care data study, where we observed that older women and those who were obese were less likely to have received surgical treatment. However, it is also generally accepted that clinical decision-making is more complex for these groups given that their overall health and functional status may be poorer. 89
Removal and reoperation rates after mid-urethral mesh tape insertions
More than 95,000 women had a MUT insertion for SUI between 2006 and 2016. Within 9 years of MUT insertion, 1 in 30 women had undergone a removal procedure and 1 in 14 had undergone a reoperation, including mesh tape removal.
Risks of removal and any reoperation (mesh tape removal and/or reoperation for SUI) were lower among older women and among women from a minority ethnic background. This suggests that removal and reoperation risks may be associated with women’s background. However, it is not possible to disentangle potential explanations for these differences, which could include higher morbidity, differences in the severity of the underlying UI that led to the initial surgery and women’s choices about seeking further clinical advice and treatment.
The risk of a removal was about 30% lower following transobturator MUT insertions than following retropubic MUT insertions, which is in line with earlier findings from Scotland and England. 90,91 This may be explained by the removal of transobturator sling being a more complicated procedure. 19 Recently issued NICE guidelines on the management of urinary incontinence in women19 therefore recommend that the transobturator approach should no longer be offered:
unless there are specific clinical circumstances (for example, previous pelvic procedures) in which the retropubic approach should be avoided.
It is important to note that the risk of any reoperation, also including mesh tape removal, was not associated with the route of insertion, which confirms that the lower rate of removal after a transobturator MUT insertion is not the result of a lower complication rate.
Study strengths and limitations
A first strength of this project was the availability of comprehensive national data sets with records of referral from primary to secondary care and of the use of surgery for female UI. Second, we complemented analyses of these existing national data sets with primary data collected from women and clinicians themselves. Our interviews with women added considerably to our quantitative findings, indicating the importance of the broader values and understandings women drew on in making treatment decisions and the importance of the wider context of their lives. Our survey of gynaecologists provided an efficient and explicit approach to study the differences in the gynaecologists’ practice style in terms of their average inclination to recommend surgery and the impact that patient characteristics have on their recommendations.
The main limitations of this project concern the primary and secondary care data, particularly the quality and scope of some of their data fields. Although primary diagnosis and primary procedure fields are established as highly accurate in many routinely collected data sets, the recording of comorbidities is known to be more variable, potentially introducing an unknown amount of bias. 92 Despite this, studies comparing administrative data with case note reviews have shown that administrative data are sufficiently robust to support their use for research. 93 Routinely collected administrative data, primarily collected for management and reimbursement purposes, such as HES, lack information on relevant case mix factors, in particular those relating to symptom severity. This is likely to have contributed to some of the observed variation in the referral and surgery rates between geographical regions and between specific groups of women. However, other studies81–84,94 have also demonstrated variation in other aspects of continence care geographically and by age and ethnicity, so residual confounding is unlikely to fully explain the variation in referral and surgery rates observed in our study.
In the context of growing safety concerns about some of the surgical treatments for SUI, it is also likely that at least some of the variation observed in our project was related to this debate leading to differences in professional opinion about the appropriateness of these surgical treatments. However, although rates of SUI surgery dropped substantially over the study period, the extent of geographic variation remained stable. This, and the level of variation remaining after adjusting for regional and patient-level characteristics, suggests that residual confounding is unlikely to explain all of the geographical variation and that women in some areas and from some groups were less likely to be referred or treated surgically than women with the same condition in other areas or in other groups.
An additional complexity of using routinely collected administrative or health-care data for research is that the absence of a code for a condition in the records relating to a particular patient must be interpreted as absence of that condition in that patient. Potential misclassification may arise from patients not presenting to the health-care provider with the condition and from variation in coding practice among health-care providers. It is also likely that the extent of such misclassification varies between conditions. 46
Furthermore, there are often no standardised definitions for diagnoses, so we developed code lists to identify key exposures and outcomes. To avoid the use of inconsistent definitions between studies, we used published code lists as far as possible.
A further limitation inherent to studies using routinely collected data is that reasons for clinical decisions are often not available or are unspecific. For example, the reasons why MUT removals or reoperations were conducted were not available. The most common diagnostic code recorded in HES for removals was ‘complications of genitourinary prosthetic devices, implants, and grafts’, which cannot capture common specific problems following MUT insertion, such as mesh exposure, pain, dyspareunia and voiding difficulties. One approach to obtain better data for the reasons for removal surgery would be to collect information reported by women themselves. Patient-reported outcome measures could be included in the registry that will be established to monitor surgical procedures for UI in England, especially if these patient-reported outcome measures are collected before and after mesh and non-mesh treatments.
Where routinely collected clinical and administrative data sets are well-established, as in England, they allow consideration of outcomes in the longer term that would be prohibitively expensive and subject to loss to follow-up in prospective studies. However, those using these data to look at longer-term outcomes must consider changes in coding practices over time. For example, in our project, new OPCS-4 codes (used in the HES database) for retropubic and transobturator MUT insertions were introduced in April 2006. Before April 2006, these procedures were recorded with other non-classified procedures. We ensured that our procedure classifications took these coding standard changes into account. In addition, for the ‘removals’ analyses, which explicitly distinguished between route of MUT insertion, we conducted sensitivity analyses to examine the potential impact that some hospitals continuing to use the old coding standards had beyond April 2006. Our findings were robust to sensitivity analyses starting the study period 1 year later and to including the previous coding standards.
Routinely collected data provide large sample sizes of nationally representative data. In this project, this enabled us to capture the care that women received in primary and secondary care. For example, CPRD, although it covers only 7% of the UK population, has been shown to be a representative sample of primary care patients in the UK. Furthermore, HES has nearly 100% coverage of patients treated by NHS England. Therefore, our findings from these data sets can be considered generalisable to the national population of women with UI who seek care. 46,95 However, previous research has indicated that as many as 75% of women with UI do not seek care and some of those who experience complications after surgery may not seek further care. We were unable to capture the experiences of these women using these data sources.
The focus of this project was on surgical treatments for women with UI, which the routinely collected administrative data are well placed to capture. The interviews were also designed to capture women’s decision-making processes around surgical treatment, recruiting women who had been referred to secondary care and were considering surgery. However, because of this we do not capture those women whose experiences of UI, and perhaps thoughts around surgery, meant that they were not referred to secondary care or were not using NHS services at all. There is also the possibility that women who were approached but did not consent to be interviewed may share characteristics that were not captured in the interviews. This may be particularly relevant given that we were unable to purposively sample for the interviews according to ethnicity.
A further limitation of administrative health-care data is that they do not capture care provided in the private sector. Although precise figures are lacking, it is likely that at least 90% of all UI procedures in England are provided by the NHS, as the total annual spending on private health care in England is about 5% of the total annual NHS spend. 96 Primary data collection also has some limitations. For example, for the case vignettes survey we invited all members of two professional bodies practising in the UK with a specialist interest in UI. However, urologists were not included, and their practice and views may be different. As a result, our case vignette findings are applicable only to gynaecological practice.
Moreover, we could include only gynaecologists in our study, using case vignettes to explore the impact of patient characteristics on recommendations about surgery. The contact details of urologists involved in treatment of female UI were simply not available. We can only speculate to what extent the results observed in gynaecologists reflect those that would have been obtained in urologists. In addition, the response of only 245 gynaecologists could be included, out of the 1139 members of the RCOG with special interest in urogynaecology. We could not explore the differences between responders and non-responders because we did not have access to the characteristics of the non-responders.
Interpretation of findings
Information on variation in practice is important for examining relationships between policy decisions and clinical decisions, and this information raises questions concerning the efficiency and effectiveness of health care. 35 In evaluating practice variation, surgical care can be considered to be ‘preference sensitive’ where more than one generally accepted treatment option is available. In this situation, the ‘correct’ rate of treatment should depend on informed patient choice, but rates may vary substantially because of the differences in the professional opinion of clinicians. International data illustrate extensive variation in elective surgery rates in many areas of practice that is much greater than can reasonably be explained by differences in condition severity. 92,97,98 Our findings suggest that this is also the case for surgery for female UI.
It is important to remember that patients in geographical areas with low rates of elective surgery, such as for UI, are not necessarily untreated; they may be treated differently. Patients’ decisions regarding their treatment are understandably strongly influenced by the opinions of their clinicians. Treating patients according to their preferences requires shared decision-making and the active engagement of patients in their choice of treatment. In the context of UI surgery, it is likely that at least some of the observed variation in the use of surgery will have been related to the debate about the safety of MUTs for SUI, which would have led to differences in professional opinion about the appropriateness of these treatments for female SUI. Surgical care for UI may also be considered ‘supply sensitive’, with the frequency of use relating to capacity in the local health-care system. The geographic areas used in the analyses of geographical variation in SUI surgery rates (CCGs and STPs) are defined by NHS bodies that commission local hospital services. For this reason, differences in capacity of the local health-care system may have contributed to the observed variation.
The data used in this project (from the routinely collected clinical and administrative data sets and obtained from primary data collection in women and clinicians) were collected before the national ‘pause’ in the use of MUTs for SUI. However, the growing controversy over the use of MUTs for UI over the course of the period from which our data are drawn is likely to have played a role in our findings. Growing safety concerns around these most common surgical treatments for SUI over the study period,99–101 with some women experiencing pain, painful sex and mesh exposure,99,102 are likely to have introduced more uncertainty to women’s and clinician’s decision-making in relation to treatment and referral for female UI. This is reflected in our findings of substantial variation in rates of referrals, surgery and mesh tape removals both geographically and between groups (i.e. older women and those from minority ethnic backgrounds), which persisted after adjustment for individual-level factors likely to affect women’s preferences.
Implications and recommendations for future research
Collecting and reporting patient-reported outcome measures
In April 2019, NICE published updated guidelines on the management of urinary incontinence in women. 19 This included that providers must ensure that data on surgery for SUI and surgical complications are recorded in a national registry,103 including data for mesh and non-mesh procedures. Confirmed mesh complications must also be reported to the Medicines and Healthcare products Regulatory Agency. However, it is important that the focus on complications following surgical treatment for UI reflects women’s concerns. Therefore, research in this area could benefit from a future national registry that contains patient-reported outcome measures. The current guidelines recommend that follow-up data include ‘validated relevant outcome measures’, ‘adverse events including pain’ and ‘suspected and confirmed mesh-related complications’. However, it is important that outcomes are collected not only for women who have surgical treatment but also for those women who are referred back to secondary care with complications after surgical treatment. This could be achieved by conducting a national survey of women reporting on their UI, symptoms and quality of life before and after mesh and non-mesh treatments.
Future research could explore patient-reported symptom severity to examine long-term outcomes, including those that do not lead women to seek further health care or surgery, after mesh and non-mesh treatments to inform treatment decisions. Using patient-reported outcome measures, future research could also explore the extent to which patient-reported symptom severity explains observed variation in referrals and surgery. Future research could also explore barriers to seeking care among women who do not seek care for their UI, who delay seeking care or who do not seek care for complications following surgical treatment. Future research could also investigate the experiences of women who are considering a further continence procedure or mesh tape removal, whose decisions may be influenced by factors different from those that influence women considering surgery for the first time.
Utilising updates in procedure coding
During the study period, HES coded procedures using OPCS release 4.7 and it was not possible to distinguish between partial or total mesh tape removals after transobturator MUT insertions. From April 2017, procedures will be recorded using OPCS-4.8, which will distinguish between these partial and total mesh tape removals. We were not able to obtain these more recent data during the project, but future research should compare total and partial removal rates following transobturator and retropubic mesh MUT insertions to inform the use of these procedures.
Identifying best practice
This research, added to existing knowledge, has identified that there may be opportunities for GPs to initiate discussions to detect UI and to identify and treat modifiable factors such as obesity. Future research could identify best practice and promising interventions to support clinicians working in primary care to initiate discussions on these sensitive topics, particularly with women experiencing additional barriers, such as those from older age groups and minority ethnic backgrounds.
At the same time, we found that a relatively large proportion of women were referred to secondary care shortly after their UI problems were first recorded in primary care. Future research could focus on management within primary care prior to referral and for women who were not referred, especially relating to questions about how better assessment of UI (including history taking, symptom scoring and quality-of-life assessment, and physical examination), as well as conservative management (including lifestyle interventions and pelvic floor muscle training), may reduce the number of women referred to secondary care without negatively affecting outcomes.
There was wide geographical variation in the use of SUI surgery, which is also reflected in the large differences between gynaecologists in their average inclination to recommend surgery to a series of hypothetical patients. This demonstrates the need to get a better understanding of the determinants of variation and to be able to identify for whom the benefits of surgical treatment outweigh the risks. A national registry, as mentioned above, that collects data reported by women themselves is being established. This registry will provide crucial data that could provide input parameters for a detailed modelling exercise of quality-adjusted life-years following the available treatment options for women with specific clinical profiles.
Patient decision aids on surgery for stress urinary incontinence
A patient decision aid for women thinking about a surgical procedure for SUI has recently been produced by NICE. 19 This decision aid provides information about the risks and benefits of all surgical options, including of long-term complications. The aid includes three key components: descriptions of the surgical options available, a summary of the short- and long-term outcomes with graphical representations of risk, and a chart that women can use to explore how they feel about the options. More research is needed about how these types of decisions can be best supported, acknowledging that these decisions are rarely conclusive and that the women’s own prioritisation of specific criteria, which often go beyond objective measures of urine leakage, can shift over time in the light of their own experiences.
Summary of the research agenda
In summary, the proposed research agenda includes:
-
a stronger emphasis on using data based on experiences and outcomes reported by women themselves
-
ongoing refinement of procedure codes in HES data to ensure that partial and total removal of mesh tape can be distinguished
-
initiation of studies that specifically aim to get a better understanding of the characteristics of patients for whom the benefits of surgical treatment outweigh the risks
-
the development of approaches, including patient decision aids, which support women in making decisions about surgical treatment for SUI.
Acknowledgements
We thank Lynn Copley and Natalie Eugene of the Clinical Effectiveness Unit of the RCS of England for their work extracting the HES data and Masao Iwagami for his help extracting the CPRD data. We also thank our Project Advisory Group and the Trial Steering Committee for their input and guidance throughout the project, including their comments on this report.
We thank our two project PPI representatives, Jacqueline Emkes and Margaret Buchanan-Geddes, for sharing their experiences of the NHS as patients with UI, for their input in the design of quantitative analyses, and for their invaluable help with the design of the qualitative interviews. Finally, we thank all the women who took part in interviews and generously shared their time and experiences.
Project team and roles
| Team member | Position | Institution | Role |
|---|---|---|---|
| Jan van der Meulen | Professor | LSHTM | Principal investigator |
| Ipek Gurol-Urganci | Assistant professor | LSHTM | Co-PI; WPs 1, 2, 4 and 6 |
| Rebecca Geary | Assistant professor | LSHTM | WPs 1, 2, 4 and 6 |
| Jil Mamza | Research fellow | LSHTM | WPs 1, 2 and 4–6 |
| Simon Cohn | Professor | LSHTM | WP 3 |
| Rebecca Lynch | Assistant professor | LSHTM | WP 3 |
| Douglas Tincello | Professor | University of Leicester | Co-PI; clinical input, urogynaecology |
| Andrew Wilson | Professor | University of Leicester | Clinical input, primary care |
| Dina el-Hamamsy | Research fellow | University of Leicester | Clinical input, urogynaecology |
Project Advisory Group and roles
Jonathan Duckett (BSUG and Medway NHS Foundation Trust; clinical input), Philip Toozs-Hobson (BSUG and Birmingham Women’s NHS Foundation Trust; clinical input), Ash Monga (BSUG and University Hospitals Southampton; clinical input), Tahir Mahmood (RCOG and Victoria Hospital; clinical input), Margaret Buchanan-Geddes (RCOG Women’s Voices; lay member), Jacqueline Emkes (independent; lay member), David Cromwell [London School of Hygiene & Tropical Medicine (LSHTM); methodological input] and Mylene Lagarde (LSHTM and London School of Economics; methodological input).
Project Steering Committee
Simon de Lusignan (Professor of Primary Care and Clinical Informatics, University of Surrey), Jenni Burt (Senior Research Associate, University of Cambridge), Lucia Dolan (Consultant Urogynaecologist, Belfast City Hospital), Marcus Drake (Consultant Urologist, Senior Lecturer in Urology, North Bristol NHS Trust, University of Bristol), Pallavi Latthe (Consultant Obstetrician and Gynaecologist, Birmingham Women’s NHS Foundation Trust), Karen Ward (Consultant Urogynaecologist, Manchester University Hospital NHS Trust) and Catherine Nestor (lay representative, co-vice chairperson, RCOG Women’s Network).
Contributions of authors
Rebecca S Geary (https://orcid.org/0000-0003-1417-1057) (Assistant Professor, Health Services Research) was the co-lead for WPs 1, 2 and 4, she contributed to writing up WP 6, wrote the first draft of the report and managed all processes in preparation for the final report.
Ipek Gurol-Urganci (https://orcid.org/0000-0002-6517-3485) (Assistant Professor, Health Services Research) was co-principal investigator, the co-lead for WPs 1, 2, 4 and 6, she conducted all quantitative analyses, prepared the results for the report and contributed to writing up the report.
Jil B Mamza (https://orcid.org/0000-0001-6240-7525) (Research Fellow, Epidemiology) was the co-lead for WP 5 and supported data management and analyses for WPs 2, 4 and 6.
Rebecca Lynch (https://orcid.org/0000-0002-6890-698X) (Assistant Professor, Medical Anthropology) was the co-lead for WP 3, conducted all qualitative data collection and analyses, and prepared the qualitative results for the report.
Dina El-Hamamsy (https://orcid.org/0000-0002-0257-533X) (Clinical Research Fellow, Urogynaecology) provided clinical input for the interpretation of results for all WPs and commented on the draft report, focusing on key findings, discussion and recommendations.
Andrew Wilson (https://orcid.org/0000-0002-9814-5966) (Professor, Primary Care) provided clinical input for the design and conduct of all WPs and interpretation of the results, and commented on the draft report, focusing on key findings, discussion and recommendations.
Simon Cohn (https://orcid.org/0000-0003-4660-2800) (Professor, Medical Anthropology) was the co-lead for WP 3, advised on the design and conduct of WP 3 and commented on the results and key findings for WP 3.
Douglas Tincello (https://orcid.org/0000-0002-6385-851X) (Professor, Urogynaecology) was co-principal investigator, provided clinical input for the design and conduct of all WPs and interpretation of the results. He also commented on the draft report, focusing on key findings, discussion and recommendations.
Jan van der Meulen (https://orcid.org/0000-0002-9451-2335) (Professor, Clinical Epidemiology) was the principal investigator for the project. He advised on the design, conduct and analyses for all WPs, was the co-lead for WPs 5 and 6, provided key input for interpretation of the results and revised all sections of the report critically.
Publications
Gurol-Urganci I, Geary RS, Mamza JB, Duckett J, El-Hamamsy D, Dolan L, et al. Long-term rate of mesh sling removal following midurethral mesh sling insertion among women with stress urinary incontinence. JAMA 2018;320:1659–69.
Lynch R. Of Flesh and Mesh: Time, Materiality, and Health in Surgical Recovery. In Parkhurst A, Carroll T, editors. Medical Materialities Towards a Material Culture of Medical Anthropology. London: Routledge; 2019. pp. 23–35.
Mamza JB, Geary RS, El-Hamamsy D, Cromwell DA, Duckett J, Monga A, et al. Geographical variation in rates of surgical treatment for female stress urinary incontinence in England: a national cohort study. BMJ Open 2019;9:e029878.
Gurol-Urganci I, Geary RS, Mamza JB, Iwagami M, El-Hamamsy D, Duckett J, et al. Determinants of referral of women with urinary incontinence to specialist services: a national cohort study using primary care data from the UK. BMC Fam Pract 2020;21:211.
Lynch R, Toozs-Hobson P, Duckett J, Tincello D, Cohn S. Making a decision about surgery for female urinary incontinence: a qualitative study of women’s views. Int Urogynecol J 2021;32:127–33.
Mamza JB, Geary R, El-Hamamsy D, Gurol I, Duckett J, Mahmood T, et al. Variation in surgical treatment advice for women with stress urinary incontinence: a study using clinical case vignettes. Int Urogynecol J 2020;31:1153–61.
Data-sharing statement
The HES data were made available by NHS Digital and reused with the permission of NHS Digital. The CPRD data (including HES-linked data) were made available by the Medicines and Healthcare products Regulatory Agency and reused with the permission of the Medicines and Healthcare products Regulatory Agency. The HES and CPRD data are available on application to NHS Digital and the Medicines and Healthcare products Regulatory Agency. The data generated from the interviews with women and the survey of gynaecologists are not suitable for sharing beyond those contained within the report. Further information can be obtained from the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Hunskaar S, Burgio K, Clark A, Lapitan M, Nelson R, Sillen U, et al. WHO-ICS International Consultation on Incontinence. Paris: Health Publications Ltd; 2005.
- Ford AA, Rogerson L, Cody JD, Aluko P, Ogah JA. Mid-urethral sling operations for stress urinary incontinence in women. Cochrane Database Syst Rev 2017;7. https://doi.org/10.1002/14651858.CD006375.pub4.
- Coyne KS, Kvasz M, Ireland AM, Milsom I, Kopp ZS, Chapple CR. Urinary incontinence and its relationship to mental health and health-related quality of life in men and women in Sweden, the United Kingdom, and the United States. Eur Urol 2012;61:88-95. https://doi.org/10.1016/j.eururo.2011.07.049.
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997;89:501-6. https://doi.org/10.1016/S0029-7844(97)00058-6.
- Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J 2010;21:5-26. https://doi.org/10.1007/s00192-009-0976-9.
- Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, et al. Prevalence and trends of symptomatic pelvic floor disorders in US women. Obstet Gynecol 2014;123:141-8. https://doi.org/10.1097/AOG.0000000000000057.
- Offermans MP, Du Moulin MF, Hamers JP, Dassen T, Halfens RJ. Prevalence of urinary incontinence and associated risk factors in nursing home residents: a systematic review. Neurourol Urodyn 2009;28:288-94. https://doi.org/10.1002/nau.20668.
- Anger JT, Saigal CS, Litwin MS. Urologic Diseases of America Project . The prevalence of urinary incontinence among community dwelling adult women: results from the National Health and Nutrition Examination Survey. J Urol 2006;175:601-4. https://doi.org/10.1016/S0022-5347(05)00242-9.
- Minassian VA, Yan X, Lichtenfeld MJ, Sun H, Stewart WF. The iceberg of health care utilization in women with urinary incontinence. Int Urogynecol J 2012;23:1087-93. https://doi.org/10.1007/s00192-012-1743-x.
- Seim A, Sandvik H, Hermstad R, Hunskaar S. Female urinary incontinence – consultation behaviour and patient experiences: an epidemiological survey in a Norwegian community. Fam Pract 1995;12:18-21. https://doi.org/10.1093/fampra/12.1.18.
- Monz B, Hampel C, Porkess S, Wagg A, Pons ME, Samsioe G, et al. A description of health care provision and access to treatment for women with urinary incontinence in Europe – a five-country comparison. Maturitas 2005;52:3-12. https://doi.org/10.1016/j.maturitas.2005.09.007.
- Brown JS, McGhan WF, Chokroverty S. Comorbidities associated with overactive bladder. Am J Manag Care 2000;6:574-9.
- Wagner TH, Hu TW, Bentkover J, LeBlanc K, Stewart W, Corey R, et al. Health-related consequences of overactive bladder. Am J Manag Care 2002;8:598-607.
- Gibson W, Hunter KF, Camicioli R, Booth J, Skelton DA, Dumoulin C, et al. The association between lower urinary tract symptoms and falls: forming a theoretical model for a research agenda. Neurourol Urodyn 2018;37:501-9. https://doi.org/10.1002/nau.23295.
- NHS England . Excellence in Continence Care. Practical Guidance for Commissioners, and Leaders in Health and Social Care 2018.
- Minassian VA, Drutz HP, Al-Badr A. Urinary incontinence as a worldwide problem. Int J Gynaecol Obstet 2003;82:327-38. https://doi.org/10.1016/S0020-7292(03)00220-0.
- Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. Norwegian EPINCONT study . Epidemiology of incontinence in the county of Nord-Trøndelag. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. J Clin Epidemiol 2000;53:1150-7. https://doi.org/10.1016/S0895-4356(00)00232-8.
- Lukacz ES, Santiago-Lastra Y, Albo ME, Brubaker L. Urinary incontinence in women: a review. JAMA 2017;318:1592-604. https://doi.org/10.1001/jama.2017.12137.
- National Institute for Health and Care Excellence (NICE) . Urinary Incontinence and Pelvic Organ Prolapse in Women: Management (NG123) 2019. https://doi.org/10.1111/bju.14763.
- Withington J, Hirji S, Sahai A. The changing face of urinary continence surgery in England: a perspective from the Hospital Episode Statistics database. BJU Int 2014;114:268-77. https://doi.org/10.1111/bju.12650.
- NHS Digital . Retrospective Review of Surgery for Urogynaecological Prolapse and Stress Urinary Incontinence Using Tape or Mesh: Hospital Episode Statistics (HES), Experimental Statistics, April 2008 – March 2017 [PAS] 2018. https://digital.nhs.uk/data-and-information/publications/statistical/mesh/apr08-mar17/retrospective-review-of-surgery-for-vaginal-prolapse-and-stress-urinary-incontinence-using-tape-or-mesh-copy (accessed 31 October 2019).
- NHS Digital . Hospital Episode Statistics. Patient Admitted Care, England – 2017–18 2018. https://digital.nhs.uk/data-and-information/publications/statistical/hospital-admitted-patient-care-activity (accessed 31 October 2019).
- Gornall J. How mesh became a four letter word. BMJ 2018;363. https://doi.org/10.1136/bmj.k4137.
- Schultz DG. FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. Silver Spring, MD: US Food and Drug Administration; 2008.
- NHS England . Mesh Oversight Group Report 2017.
- Scottish Government . Scottish Independent Review of the Use, Safety and Efficacy of Transvaginal Mesh Implants in the Treatment of Stress Urinary Incontinence and Pelvic Organ Prolapse in Women 2017. www.gov.scot/Resource/0051/00515856.pdf (accessed 16 September 2017).
- The Independent Medicines and Medical Devices Safety Review 2018. www.immdsreview.org.uk (accessed 19 May 2020).
- NHS England . Provider Bulletin: 11 July 2018 2018. www.england.nhs.uk/2018/07/provider-bulletin-11-july-2018/ (accessed 19 May 2020).
- Sung VW, Joo K, Marques F, Myers DL. Patient-reported outcomes after combined surgery for pelvic floor disorders in older compared to younger women. Am J Obstet Gynecol 2009;201:534.e1-5. https://doi.org/10.1016/j.ajog.2009.07.024.
- Wagg A, Duckett J, McClurg D, Harari D, Lowe D. To what extent are national guidelines for the management of urinary incontinence in women adhered? Data from a national audit. BJOG 2011;118:1592-600. https://doi.org/10.1111/j.1471-0528.2011.03100.x.
- Gibson W, Wagg A. New horizons: urinary incontinence in older people. Age Ageing 2014;43:157-63. https://doi.org/10.1093/ageing/aft214.
- Brown JS, Nyberg LM, Kusek JW, Burgio KL, Diokno AC, Foldspang A, et al. Proceedings of the National Institute of Diabetes and Digestive and Kidney Diseases International Symposium on Epidemiologic Issues in Urinary Incontinence in Women. Am J Obstet Gynecol 2003;188:S77-88. https://doi.org/10.1067/mob.2003.353.
- Siddiqui NY, Levin PJ, Phadtare A, Pietrobon R, Ammarell N. Perceptions about female urinary incontinence: a systematic review. Int Urogynecol J 2014;25:863-71. https://doi.org/10.1007/s00192-013-2276-7.
- Royal College of Obstetricians and Gynaecologists . National Urinary Incontinence Organisational Survey 2013. www.rcog.org.uk/en/guidelines-research-services/audit-quality-improvement/completed-projects/national-urinary-incontinence-organisational-survey/ (accessed 31 October 2019).
- Wennberg JE. Time to tackle unwarranted variations in practice. BMJ 2011;342. https://doi.org/10.1136/bmj.d1513.
- Wagg A, Das Gupta R, Assassa P, Shaw C, Mayne C, Martin M. Secondary-care treatment patterns in the UK for women with urinary incontinence. BJU Int 2005;96:839-42. https://doi.org/10.1111/j.1464-410X.2005.05723.x.
- Hägglund D, Walker-Engström ML, Larsson G, Leppert J. Reasons why women with long-term urinary incontinence do not seek professional help: a cross-sectional population-based cohort study. Int Urogynecol J Pelvic Floor Dysfunct 2003;14:296-304. https://doi.org/10.1007/s00192-003-1077-9.
- McGrother CW, Donaldson MM, Shaw C, Matthews RJ, Hayward TA, Dallosso HM, et al. Storage symptoms of the bladder: prevalence, incidence and need for services in the UK. BJU Int 2004;93:763-9. https://doi.org/10.1111/j.1464-410X.2003.04721.x.
- Hunskaar S, Lose G, Sykes D, Voss S. The prevalence of urinary incontinence in women in four European countries. BJU Int 2004;93:324-30. https://doi.org/10.1111/j.1464-410X.2003.04609.x.
- Shaw C, Tansey R, Jackson C, Hyde C, Allan R. Barriers to help seeking in people with urinary symptoms. Fam Pract 2001;18:48-52. https://doi.org/10.1093/fampra/18.1.48.
- Strickland R. Reasons for not seeking care for urinary incontinence in older community-dwelling women: a contemporary review. Urol Nurs 2014;34. https://doi.org/10.7257/1053-816X.2014.34.2.63.
- NHS Digital . Hospital Episode Statistics (HES) 2018. https://digital.nhs.uk/data-and-information/data-tools-and-services/data-services/hospital-episode-statistics (accessed 1 April 2019).
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems 2018. https://icd.who.int/browse10/2016/en (accessed 1 December 2018).
- NHS Digital . OPCS Classification of Interventions and Procedures 2018. www.datadictionary.nhs.uk/web_site_content/supporting_information/clinical_coding/opcs_classification_of_interventions_and_procedures.asp (accessed 1 April 2019).
- Clinical Practice Research Datalink . CPRD Linked Data 2019. www.cprd.com/linked-data (accessed 1 April 2019).
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- Johal A, Mitchell D, Lees T, Cromwell D, van der Meulen J. Use of Hospital Episode Statistics to investigate abdominal aortic aneurysm surgery. Br J Surg 2012;99:66-72. https://doi.org/10.1002/bjs.7772.
- Grant RL, Drennan VM, Rait G, Petersen I, Iliffe S. First diagnosis and management of incontinence in older people with and without dementia in primary care: a cohort study using The Health Improvement Network primary care database. PLOS Med 2013;10. https://doi.org/10.1371/journal.pmed.1001505.
- NHS England . Sustainability and Transformation Plans (STPs): Local Plans to Improve Health and Care n.d. www.england.nhs.uk/integratedcare/stps/ (accessed 9 May 2020).
- Office for National Statistics . Table P01UK2011 Census: Usual Resident Population by Five-Year Age Group 2013. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/2011censuspopulationestimatesbyfiveyearagebandsandhouseholdestimatesforlocalauthoritiesintheunitedkingdom (accessed 31 October 2019).
- Ministry of Housing, Communities and Local Government . The English Indices of Deprivation 2015 n.d. www.gov.uk/government/collections/english-indices-of-deprivation (accessed 31 Oct 2019).
- Greenland S. Principles of multilevel modelling. Int J Epidemiol 2000;29:158-67. https://doi.org/10.1093/ije/29.1.158.
- Glaser BG, Strauss AL. The discovery of grounded theory: strategies for qualitative research. Chicago, IL: Aldine Publishing; 1967.
- Charmaz K. Constructing Grounded Theory. London: SAGE Publications Ltd; 2014.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med 2007;26:2389-430. https://doi.org/10.1002/sim.2712.
- Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev 2017;85:185-203. https://doi.org/10.1111/insr.12214.
- Bachmann LM, Mühleisen A, Bock A, ter Riet G, Held U, Kessels AG. Vignette studies of medical choice and judgement to study caregivers’ medical decision behaviour: systematic review. BMC Med Res Methodol 2008;8. https://doi.org/10.1186/1471-2288-8-50.
- Veloski J, Tai S, Evans AS, Nash DB. Clinical vignette-based surveys: a tool for assessing physician practice variation. Am J Med Qual 2005;20:151-7. https://doi.org/10.1177/1062860605274520.
- Peabody JW, Luck J, Glassman P, Dresselhaus TR, Lee M. Comparison of vignettes, standardized patients, and chart abstraction: a prospective validation study of 3 methods for measuring quality. JAMA 2000;283:1715-22. https://doi.org/10.1001/jama.283.13.1715.
- Peabody JW, Luck J, Glassman P, Jain S, Hansen J, Spell M, et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med 2004;141:771-80. https://doi.org/10.7326/0003-4819-141-10-200411160-00008.
- Dresselhaus TR, Peabody JW, Lee M, Wang MM, Luck J. Measuring compliance with preventive care guidelines: standardized patients, clinical vignettes, and the medical record. J Gen Intern Med 2000;15:782-8. https://doi.org/10.1046/j.1525-1497.2000.91007.x.
- Louviere JJ. A SAGE University Paper Series on Quantitative Applications in the Social Sciences. Newbury Park, CA: SAGE Publications Ltd; 1988.
- Gustafsson A, Herrmann A, Huber F. Conjoint Measurement: Methods and Applications. New York, NY: Springer Publishing; 2007.
- Green P, Srinivasan V. Conjoint analysis in consumer research: issues and outlook. J Consum Res 1978;5:103-23. https://doi.org/10.1086/208721.
- Collins LM, Lanza ST. Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences. Hoboken, NJ: John Wiley & Sons; 2010.
- Mamza JB, Geary RS, El-Hamamsy D, Cromwell DA, Duckett J, Monga A, et al. Geographical variation in rates of surgical treatment for female stress urinary incontinence in England: a national cohort study. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019-029878.
- Gurol-Urganci I, Geary RS, Mamza JB, Iwagami M, El-Hamamsy D, Duckett J, et al. Determinants of referral of women with urinary incontinence to specialist services: a national cohort study using primary care data from the UK. BMC Fam Pract 2020;21. https://doi.org/10.1186/s12875-020-01282-y.
- Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. https://doi.org/10.1080/01621459.1999.10474144.
- Gurol-Urganci I, Geary RS, Mamza JB, Duckett J, El-Hamamsy D, Dolan L, et al. Long-term rate of mesh sling removal following midurethral mesh sling insertion among women with stress urinary incontinence. JAMA 2018;320:1659-69. https://doi.org/10.1001/jama.2018.14997.
- Lynch R, Toozs-Hobson P, Duckett J, Tincello D, Cohn S. Making a decision about surgery for female urinary incontinence: a qualitative study of women’s views. Int Urogynecol J 2021;32:127-33. https://doi.org/10.1007/s00192-020-04383-5.
- Mamza JB, Geary R, El-Hamamsy D, Gurol I, Duckett J, Mahmood T, et al. Variation in surgical treatment advice for women with stress urinary incontinence: a study using clinical case vignettes. Int Urogynecol J 2020;31:1153-61. https://doi.org/10.1007/s00192-020-04295-4.
- Department of Health and Social Care (DHSC) . Review Launched to Respond to Patient Concerns About NHS Treatments 2018. www.gov.uk/government/news/review-launched-to-respond-to-patient-concerns-about-nhs-treatments (accessed 31 October 2019).
- Department of Health and Social Care (DHSC) . Government Announces Strict Rules for the Use of Vaginal Mesh 2018. www.gov.uk/government/news/government-announces-strict-rules-for-the-use-of-vaginal-mesh (accessed 31 October 2019).
- Wagg A, Lowe D, Peel P, Potter J. Continence care for older people in England and Wales: data from a national audit. J Wound Ostomy Continence Nurs 2008;35:215-20. https://doi.org/10.1097/01.WON.0000313646.44870.d3.
- Franzen K, Andersson G, Odeberg J, Midlöv P, Samuelsson E, Stenzelius K, et al. Surgery for urinary incontinence in women 65 years and older: a systematic review. Int Urogynecol J 2015;26:1095-102. https://doi.org/10.1007/s00192-014-2573-9.
- Gibson W, Wagg A. Are older women more likely to receive surgical treatment for stress urinary incontinence since the introduction of the mid-urethral sling? An examination of Hospital Episode Statistics data. BJOG 2016;123:1386-92. https://doi.org/10.1111/1471-0528.13338.
- Wagg A, Mian S, Lowe D, Potter J, Pearson M. Continence Programme Working Party. National audit of continence care for older people: results of a pilot study*. J Eval Clin Pract 2005;11:525-32. https://doi.org/10.1111/j.1365-2753.2005.00570.x.
- Horrocks S, Somerset M, Stoddart H, Peters TJ. What prevents older people from seeking treatment for urinary incontinence? A qualitative exploration of barriers to the use of community continence services. Fam Pract 2004;21:689-96. https://doi.org/10.1093/fampra/cmh622.
- Teunissen D, van Weel C, Lagro-Janssen T. Urinary incontinence in older people living in the community: examining help-seeking behaviour. Br J Gen Pract 2005;55:776-82.
- Andersson G, Johansson JE, Nilsson K, Sahlberg-Blom E. Perceptions of urinary incontinence among Syrian Christian women living in Sweden. J Transcult Nurs 2009;20:296-303. https://doi.org/10.1177/1043659609334850.
- Welch LC, Botelho EM, Tennstedt SL. Race and ethnic differences in health beliefs about lower urinary tract symptoms. Nurs Res 2011;60:165-72. https://doi.org/10.1097/NNR.0b013e3182159cac.
- Wells M, Wagg A. Integrated continence services and the female Bangladeshi population. Br J Nurs 2007;16:516-19. https://doi.org/10.12968/bjon.2007.16.9.23427.
- Doshani A, Pitchforth E, Mayne CJ, Tincello DG. Culturally sensitive continence care: a qualitative study among South Asian Indian women in Leicester. Fam Pract 2007;24:585-93. https://doi.org/10.1093/fampra/cmm058.
- Giarenis I, Thiagamoorthy G, Zacchè M, Robinson D, Cardozo L. Management of recurrent stress urinary incontinence after failed midurethral sling: a survey of members of the International Urogynecological Association (IUGA). Int Urogynecol J 2015;26:1285-91. https://doi.org/10.1007/s00192-015-2696-7.
- Rogers R, Lebkuchner U, Kammer-Doak D, Thompson P, Walters M, Nygaard I. Obesity and retropubic surgery for stress incontinence: is there really an increased risk of intraoperative complications?. Am J Obstet Gynecol 2006;195:1794-8. https://doi.org/10.1016/j.ajog.2006.07.012.
- Rechberger T, Futyma K, Jankiewicz K, Adamiak A, Bogusiewicz M, Skorupski P. Body mass index does not influence the outcome of anti-incontinence surgery among women whereas menopausal status and ageing do: a randomised trial. Int Urogynecol J 2010;21:801-6. https://doi.org/10.1007/s00192-010-1116-2.
- Ellington DR, Erekson EA, Richter HE. Outcomes of surgery for stress urinary incontinence in the older woman. Clin Geriatr Med 2015;31:487-505. https://doi.org/10.1016/j.cger.2015.06.006.
- Ellington D, Ballard A. Surgical treatment and outcomes for the management of stress urinary incontinence in the older woman. Curr Geriatr Rep 2017;6:90-7. https://doi.org/10.1007/s13670-017-0204-2.
- Morling JR, McAllister DA, Agur W, Fischbacher CM, Glazener CM, Guerrero K, et al. Adverse events after first, single, mesh and non-mesh surgical procedures for stress urinary incontinence and pelvic organ prolapse in Scotland, 1997-2016: a population-based cohort study. Lancet 2017;389:629-40. https://doi.org/10.1016/S0140-6736(16)32572-7.
- Keltie K, Elneil S, Monga A, Patrick H, Powell J, Campbell B, et al. Complications following vaginal mesh procedures for stress urinary incontinence: an 8 year study of 92,246 women. Sci Rep 2017;7. https://doi.org/10.1038/s41598-017-11821-w.
- Mohammed MA, Deeks JJ, Girling A, Rudge G, Carmalt M, Stevens AJ, et al. Evidence of methodological bias in hospital standardised mortality ratios: retrospective database study of English hospitals. BMJ 2009;338. https://doi.org/10.1136/bmj.b780.
- Burns EM, Rigby E, Mamidanna R, Bottle A, Aylin P, Ziprin P, et al. Systematic review of discharge coding accuracy. J Public Health 2012;34:138-48. https://doi.org/10.1093/pubmed/fdr054.
- Teunissen TA, de Jonge A, van Weel C, Lagro-Janssen AL. Treating urinary incontinence in the elderly – conservative therapies that work: a systematic review. J Fam Pract 2004;53:25-30.
- Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012;3:89-9. https://doi.org/10.1177/2042098611435911.
- The King’s Fund . The UK Private Health Market 2014. www.kingsfund.org.uk/sites/default/files/media/commission-appendix-uk-private-health-market.pdf (accessed 9 May 2020).
- Wennberg A. Tracking Medicine: A Researcher’s Quest to Understand Health Care. Oxford: Oxford University Press; 2010.
- Public Health England (PHE), NHS RightCare . The NHS Atlas of Variation in Healthcare: Reducing Unwarranted Variation to Increase Value and Improve Quality 2015.
- NHS England . Mesh Complications 2017. www.england.nhs.uk/wp-content/uploads/2017/07/mesh-complications.pdf (accessed 9 May 2020).
- US Food and Drug Administration . Considerations About Surgical Mesh for SUI 2018. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/UroGynSurgicalMesh/ucm345219.htm (accessed 31 October 2019).
- American Urogynecologic Society (AUGS), Society for Urodynamics (SUFU) . Position Statement on Mesh Midurethral Slings for Stress Urinary Incontinence 2018. www.augs.org/assets/1/6/AUGS-SUFU_MUS_Position_Statement.pdf (accessed 9 May 2020).
- Leone Roberti Maggiore U, Finazzi Agrò E, Soligo M, Li Marzi V, Digesu A, Serati M. Long-term outcomes of TOT and TVT procedures for the treatment of female stress urinary incontinence: a systematic review and meta-analysis. Int Urogynecol J 2017;28:1119-30. https://doi.org/10.1007/s00192-017-3275-x.
- NICE . Collecting Data on Surgery and Surgical Complications 2019. www.nice.org.uk/guidance/ng123/chapter/Recommendations#collecting-data-on-surgery-and-surgical-complications (accessed 19 May 2020).
- Wright AK, Kontopantelis E, Emsley R, Buchan I, Sattar N, Rutter MK, et al. Life expectancy and cause-specific mortality in type 2 diabetes: a population-based cohort study quantifying relationships in ethnic subgroups. Diabetes Care 2017;40:338-45. https://doi.org/10.2337/dc16-1616.
- Stocks SJ, Kontopantelis E, Akbarov A, Rodgers S, Avery AJ. Examining variations in prescribing safety in UK general practice: cross sectional study using the Clinical Practice Research Datalink. BMJ 2015;351. https://doi.org/10.1136/bmj.h5501.
- Joseph RM, Movahedi M, Dixon WG, Symmons DPM. Risks of smoking and benefits of smoking cessation on hospitalisations for cardiovascular events and respiratory infection in patients with rheumatoid arthritis: a retrospective cohort study using the Clinical Practice Research Datalink. RMD Open 2017;3. https://doi.org/10.1136/rmdopen-2017-000506.
Appendix 1 Office of Population, Census and Surveys Classification of Interventions and Procedures, version 4, codes to identify surgical procedures (Hospital Episode Statistics data)
| OPCS-4 | Description |
|---|---|
| MUT insertions | |
| M53.3 | Introduction of tension-free vaginal tape |
| M53.6 | Introduction of transobturator tape |
| MUT removals | |
| M53.4 | Total removal of tension-free vaginal tape |
| M53.5 | Partial removal of tension-free vaginal tape |
| M53.7 | Removal of transobturator tape |
| Injection of urethral bulking agents | |
| M56.3 | Endoscopic injection of inert substance into outlet of the female bladder |
| Other abdominal/vaginal operations | |
| M51.1 | Abdominoperineal suspension of the urethra |
| M51.2 | Endoscopic suspension of the neck of the bladder |
| M51.8 | Other specified combined abdominal and vaginal operations to support the outlet of the female bladder |
| M51.9 | Unspecified combined abdominal and vaginal operations to support the outlet of the female bladder |
| M52.1 | Suprapubic sling operation |
| M52.2 | Retropubic suspension of the neck of the bladder |
| M52.3 | Colposuspension of the neck of the bladder |
| M52.8 | Other specified abdominal operations to support the outlet of the female bladder |
| M52.9 | Unspecified abdominal operations to support the outlet of the female bladder |
| M53.1 | Vaginal buttressing of the urethra |
| M53.8 | Other specified vaginal operations to support the outlet of the female bladder |
| M53.9 | Unspecified vaginal operations to support the outlet of the female bladder |
| M55.2 | Implantation of artificial urinary sphincter into the outlet of the female bladder |
| M55.6 | Insertion of retropubic device for female SUI – NEC |
| M55.8 | Other specified other open operations on the outlet of the female bladder |
| M55.9 | Unspecified other open operations on the outlet of the female bladder |
| M58.8 | Other specified other operations on the outlet of the female bladder |
| M58.9 | Unspecified other operations on the outlet of the female bladder |
| OPCS-4 | Description |
|---|---|
| Enlargement of the bladder | |
| M36.2 | Ileocystoplasty |
| Botulinum toxin A injection | |
| M43.4 | Endoscopic injection of neurolytic substance into the nerve of the bladder |
| M49.5 | Injection of therapeutic substance into the bladder wall |
| Neuromodulation | |
| A70.1 | Implantation of neurostimulator into the peripheral nerve |
| A70.2 | Maintenance of neurostimulator in the peripheral nerve |
| A70.3 | Removal of neurostimulator from the peripheral nerve |
| A70.4 | Insertion of neurostimulator electrodes into the peripheral nerve |
| ICD-10 | Description |
|---|---|
| N39.3 | SUI |
| N39.4 | UUI |
| R32 | Other specified urinary incontinence |
| N32.8 | Unspecified urinary incontinence |
| Other specified disorders of the bladder |
Appendix 2 Read codes to identify urinary incontinence symptoms/diagnoses (Clinical Practice Research Datalink data)
| Read code category | Read code | CPRD MedCODES | Read code description |
|---|---|---|---|
| 1 – history and symptoms | 1593. | 15918 | H/O: stress incontinence |
| 1 – history and symptoms | 1A23.00 | 6161 | Incontinence of urine |
| 1 – history and symptoms | 1A23000 | 110001 | Functional urinary incontinence |
| 1 – history and symptoms | 1A24.00 | 1929 | Stress incontinence |
| 1 – history and symptoms | 1A24.11 | 5844 | Stress incontinence – symptom |
| 1 – history and symptoms | 1A26.00 | 3887 | Urge incontinence of urine |
| K – genitourinary system diseases | K198.00 | 3182 | Stress incontinence |
| K – genitourinary system diseases | K586.00 | 17620 | Stress incontinence – female |
| K – genitourinary system diseases | Kyu5A00 | 52763 | [X] Other specified urinary incontinence |
| R – symptoms, signs and ill-defined conditions | R083.00 | 3283 | [D] Incontinence of urine |
| R – symptoms, signs and ill-defined conditions | R083000 | 4375 | [D] Enuresis – NOS |
| R – symptoms, signs and ill-defined conditions | R083100 | 31220 | [D] Urethral sphincter incontinence |
| R – symptoms, signs and ill-defined conditions | R083200 | 17320 | [D] Urge incontinence |
| R – symptoms, signs and ill-defined conditions | R083z00 | 15400 | [D] Incontinence of urine – NOS |
Appendix 3 Read codes to identify the history of urinary incontinence procedures/treatments (Clinical Practice Research Datalink data)
| Read code chapter | Read code | CPRD MedCODES | Read code description |
|---|---|---|---|
| 3 – diagnostic procedures | 3940 | 13421 | Bladder: incontinent |
| 3 – diagnostic procedures | 3941 | 13422 | Bladder: occasional accident |
| 7 – operations, procedures and sites | 7B3..11 | 2498 | Bladder neck operations |
| 7 – operations, procedures and sites | 7B30.00 | 40515 | Combined abdominal and vaginal operations to support the outlet of the female bladder |
| 7 – operations, procedures and sites | 7B30000 | 26236 | Abdominoperineal suspension of the urethra |
| 7 – operations, procedures and sites | 7B30100 | 34857 | Unspecified endoscopic suspension of the bladder neck |
| 7 – operations, procedures and sites | 7B30200 | 36712 | Stamey endoscopic bladder neck suspension |
| 7 – operations, procedures and sites | 7B30300 | 67945 | Pereyra–Raz endoscopic bladder neck suspension |
| 7 – operations, procedures and sites | 7B30400 | 29989 | Gittes’ endoscopic bladder neck suspension |
| 7 – operations, procedures and sites | 7B30y00 | 35658 | Combined abdominal and vaginal operation to support the outlet of the female bladder – OS |
| 7 – operations, procedures and sites | 7B30z00 | 35662 | Combined abdominal and vaginal operation to support the outlet of the female bladder – NOS |
| 7 – operations, procedures and sites | 7B31000 | 15696 | Suprapubic sling operation |
| 7 – operations, procedures and sites | 7B31011 | 21250 | Aldridge suprapubic sling |
| 7 – operations, procedures and sites | 7B31014 | 64217 | Suprapubic urethrovesical suspension |
| 7 – operations, procedures and sites | 7B31100 | 12737 | Retropubic suspension of the bladder neck |
| 7 – operations, procedures and sites | 7B31112 | 4313 | Marshall–Marchetti suspension |
| 7 – operations, procedures and sites | 7B31113 | 34104 | Marshall–Marchetti–Krantz retropubic suspension of the urethra |
| 7 – operations, procedures and sites | 7B31200 | 4202 | Colposuspension of the bladder neck |
| 7 – operations, procedures and sites | 7B31211 | 17771 | Burch colposuspension |
| 7 – operations, procedures and sites | 7B31z00 | 71003 | Abdominal operation to support the outlet of the female bladder – NOS |
| 7 – operations, procedures and sites | 7B32000 | 31461 | Vaginal buttressing of the urethra |
| 7 – operations, procedures and sites | 7B32011 | 45544 | Kelly urethrovesical plication |
| 7 – operations, procedures and sites | 7B32012 | 98338 | Kennedy urethrovesical plication |
| 7 – operations, procedures and sites | 7B32200 | 11197 | Introduction of tension-free vaginal tape |
| 7 – operations, procedures and sites | 7B32500 | 57283 | Introduction of transobturator tape |
| 7 – operations, procedures and sites | 7B32y00 | 49097 | Vaginal operation to support the outlet of the female bladder – OS |
| 7 – operations, procedures and sites | 7B32z00 | 48900 | Vaginal operation to support the outlet of the female bladder – NOS |
| 7 – operations, procedures and sites | 7B33400 | 46819 | Insertion of sphincter around the female bladder neck |
| 7 – operations, procedures and sites | 7B33411 | 42425 | Implantation of sphincter around the female bladder neck |
| 7 – operations, procedures and sites | 7B33412 | 52000 | Insertion of artificial urinary sphincter in the outlet of the female bladder |
| 7 – operations, procedures and sites | 7B33600 | 49520 | Maintenance of the bladder neck sphincter in female |
| 7 – operations, procedures and sites | 7B33800 | 98767 | Insertion of retropubic device SUI – NEC |
| 7 – operations, procedures and sites | 7B33B00 | 89908 | Reconstruction of the neck of female bladder – NEC |
| 7 – operations, procedures and sites | 7B34200 | 36539 | Endoscopic suburethral injection of inert substance in female |
| 7 – operations, procedures and sites | 7B34211 | 18434 | Endoscopic suburethral injection of collagen in female |
| 7 – operations, procedures and sites | 7B34212 | 46542 | Endoscopic suburethral teflon injection in female |
| 7 – operations, procedures and sites | 7B34300 | 40010 | Endoscopic uroplastique injection in the outlet of female bladder |
| 7 – operations, procedures and sites | 7B38900 | 97037 | Introduction of transobturator sling |
| 8 – other therapeutic procedures | 8C14.00 | 2739 | Incontinence care |
| 8 – other therapeutic procedures | 8D7..12 | 17637 | Incontinence control |
| 8 – other therapeutic procedures | 8D71.00 | 48601 | Incontinence control |
| Z – unspecified conditions | Z9EA.00 | 45495 | Provision of incontinence appliance |
Appendix 4 Read codes to identify referrals to secondary care (Clinical Practice Research Datalink referrals data)
| CPRD MedCODES | Read code | Read code description |
|---|---|---|
| Physiotherapya | ||
| 2407 | 8E. . .00 | Physiotherapy/remedial therapy |
| 213 | 8E. . .11 | Physiotherapy |
| 24338 | 8EZ..00 | Other physiotherapy |
| 69779 | 8E74.11 | Pelvic floor exercises |
| 17237 | 8E77.00 | Pelvic floor exercises |
| 9020 | 8E97.00 | Bladder training |
| 33268 | 8E97000 | Bladder drill |
| 8437 | 9NJ3.00 | In-house physiotherapy |
| 6010 | 9NJ4.00 | In-house physiotherapy – domiciliary visit |
| 10089 | ZL85.00 | Referral to physiotherapist |
| 8164 | ZL85.11 | Referral to physiotherapist |
| 11894 | ZL85100 | Referral to community-based physiotherapist |
| 13681 | ZL85111 | Referral to community-based physiotherapist |
| 32769 | ZL85200 | Referral to hospital-based physiotherapist |
| 12282 | ZL85211 | Referral to hospital-based physiotherapist |
| 8543 | 8HH5.00 | Referral to domiciliary physiotherapy |
| 31147 | 8HHA.00 | Referral to community-based physiotherapist |
| 1116 | 8H77.00 | Referral to physiotherapist |
| 13671 | 8HVb.00 | Private referral to physiotherapist |
| Continence care/assessment (including urodynamics) | ||
| 7650 | 394..00 | Bladder – assessment |
| 13424 | 394..11 | Bladder – incontinence assessment |
| 13423 | 394..12 | Bladder – continence assessment |
| 13421 | 3940 | Bladder – incontinent |
| 13422 | 3941 | Bladder – occasional accident |
| 13420 | 3942 | Bladder – fully continent |
| 12424 | ZQ3H.00 | Bladder assessment |
| 40789 | 39H..00 | Continence assessment |
| 49417 | 39H0.00 | Continence reassessment |
| 5269 | 317..00 | Special urinary procedures |
| 15290 | 317..11 | Urinary – special tests |
| 2916 | 317..12 | Urodynamic studies |
| 20140 | 3174 | Special urinary test abnormal |
| 41472 | 3174.11 | Urodynamic studies abnormal |
| 40731 | 3174000 | Cystometry abnormal |
| 12169 | 3175 | Detrusor reflex testing |
| 10876 | 3176 | Residual urinary volume |
| 103500 | 3177 | Uroflowmetry |
| 103851 | 3178 | Voided urinary volume |
| 103674 | 3179 | Average urinary flow rate |
| 20728 | 317 A.00 | Pad test for incontinence |
| 6716 | 317B.00 | Other urodynamic tests |
| 18036 | 317C.00 | Urinary flow rate |
| 104865 | 317D.00 | Time to maximum urinary flow |
| 105951 | 317D.11 | TQmax –time to maximum urinary flow rate |
| 103615 | 317E.00 | Urinary voiding total flow time |
| 103913 | 317F.00 | Urinary flow time |
| 14962 | 317Z.00 | Special urinary procedure – NOS |
| 64138 | 7P14300 | Urodynamics – NEC |
| 2739 | 8C14.00 | Incontinence care |
| 12138 | 8C14.11 | Continence care |
| 22095 | ZLA2400 | Seen by continence nurse |
| 18998 | 8HR6.00 | Referral to urodynamic studies |
| 29192 | 8H7w.00 | Referral to continence nurse |
| 25899 | 8HTX.00 | Referral to incontinence clinic |
| 25901 | ZL62400 | Referral to continence nurse |
| Gynaecology/urology/GUM referral | ||
| 48014 | 8H4 V.00 | Referral to gynaecology special-interest GP |
| 2116 | 8H58.00 | Gynaecological referral |
| 103854 | 8Hku.00 | Referral to community gynaecology service |
| 31873 | 8HMO.00 | Listed for gynaecological admission |
| 13647 | 8HV7.00 | Private referral to gynaecologist |
| 9966 | ZL5D.00 | Referral to obstetrician and gynaecologist |
| 10663 | ZL5D200 | Referral to gynaecologist |
| 6589 | 8H4 A.11 | Referred to genitourinary physician |
| 30868 | 8H4 W.00 | Referral to urology special-interest GP |
| 2568 | 8H5B.00 | Referred to urologist |
| 13704 | 8HTa.00 | Referral to genitourinary clinic |
| 13644 | 8HVA.00 | Private referral to urologist |
| 23104 | ZL5AJ00 | Referral to genitourinary physician |
| 10313 | ZL5GP00 | Referral to urologist |
| Further care/general medicine/general surgeon referrala | ||
| 91 | 8H...00 | Referral for further care |
| 13674 | 8H4..00 | Referral to physician |
| 1861 | 8H4..11 | Medical referral |
| 11219 | 8H4..12 | Refer to physician |
| 7124 | 8H41.00 | General medical referral |
| 20251 | 8H4Z.00 | Referral to physician – NOS |
| 43014 | ZL5 A.00 | Referral to physician |
| 10449 | ZL5AE00 | Referral to general physician |
| 22670 | 8H5..00 | Referral to surgeon |
| 3016 | 8H5..11 | Surgical referral |
| 5156 | 8H51.00 | General surgical referral |
| 21020 | 8H5Z.00 | Referral to surgeon – NOS |
| 10214 | ZL5G500 | Referral to general surgeon |
| 15812 | 8H7..00 | Other referral |
| 6535 | 8H72.00 | Referral to district nurse |
| 3975 | 8H7a.00 | Referral to hospital |
| 2558 | 8HD..00 | Referral to hospital – OPD |
| 32882 | 8He..00 | Referral to intermediate care |
| 39479 | 8HH..00 | Referred – other care |
| 19171 | 8HT..00 | Referral to clinic |
| 30263 | ZL6..00 | Referral to nurse |
| 25924 | ZL62.00 | Referral to clinical nurse specialist |
| 11495 | ZL63211 | Refer to district nurse |
| 56102 | ZL65.00 | Referral to nurse practitioner |
| 20965 | 8H61.00 | Referral to private doctor |
| 7820 | 8H61.11 | Private referral |
| 17946 | 8HV..00 | Private referral |
| 13634 | 8HV0.00 | Private referral to general surgeon |
| Specialty code | Description | Data field |
|---|---|---|
| Physiotherapy referral – specialty codesa | ||
| 77 | Physiotherapy | NHSSPEC |
| Gynaecology/urology/GUM referral – specialty codes | ||
| 2 | Urology | NHSSPEC |
| 32 | Genitourinary medicine | NHSSPEC |
| 44 | Gynaecology | NHSSPEC |
| 81 | Obstetrics and gynaecology | NHSSPEC |
| 6 | Gynaecology | FSHASPEC |
| 14 | Genitourinary medicine | FSHASPEC |
| Further care referral – specialty codesa | ||
| 1 | General surgery | NHSSPEC |
| 16 | General medicine | NHSSPEC |
| 1 | General surgical | FSHASPEC |
| 2 | General medical | FSHASPEC |
Appendix 5 Read codes to identify patients’ ethnicity, smoking status and comorbidities
Ethnicity
Ethnicity information was captured based on Read codes reported in Wright et al. 104
Smoking status
Information on smoking was captured based on Read codes reported in Stocks et al. 105 and Joseph et al. 106
Comorbidities
All comorbidities were captured using Read code repositories reported at https://clinicalcodes.rss.mhs.man.ac.uk/medcodes/articles/ (accessed 9 May 2020), with the exception of those listed below.
| Comorbidity | Method |
|---|---|
| POP | Keyword searches of Read code directories, reviewed and finalised by clinical advisory team (table 5a) |
| Asthma | Keyword searches of Read code directories, reviewed and finalised by clinical advisory team |
| Cancer | Repository and keyword searches of Read code directories, reviewed and finalised by clinical advisory team |
| Read code chapter | Read code | CPRD MedCODES | Read code description |
|---|---|---|---|
| 7 – operations, procedures and sites | 7D19.00 | 17020 | Repair of the vault of the vagina |
| 7 – operations, procedures and sites | 7D19000 | 66379 | Repair of the vaginal vault combined abdominal and vaginal approach |
| 7 – operations, procedures and sites | 7D19100 | 52417 | Repair of the vault of the vagina using an abdominal approach – NEC |
| 7 – operations, procedures and sites | 7D19200 | 57237 | Repair of the vault of the vagina using a vaginal approach – NEC |
| 7 – operations, procedures and sites | 7D19300 | 16175 | Sacrocolpopexy |
| 7 – operations, procedures and sites | 7D19400 | 1652 | Suspension of the vagina – NEC |
| 7 – operations, procedures and sites | 7D19500 | 18931 | Sacrospinous fixation of the vaginal vault |
| 7 – operations, procedures and sites | 7D19600 | 96345 | Repair of the vault of the vagina with mesh using an abdominal approach |
| 7 – operations, procedures and sites | 7D19700 | 46339 | Repair of the vault of the vagina with mesh using a vaginal approach |
| 7 – operations, procedures and sites | 7D19y00 | 27604 | Other specified repair of the vault of the vagina |
| 7 – operations, procedures and sites | 7D19z00 | 48218 | Repair of the vault of the vagina – NOS |
| K – genitourinary system diseases | K195.11 | 16981 | Urethrocele |
| K – genitourinary system diseases | K51..00 | 6819 | Genital prolapse |
| K – genitourinary system diseases | K510000 | 211 | Cystocele without uterine prolapse |
| K – genitourinary system diseases | K510100 | 25278 | Cystourethrocele without uterine prolapse |
| K – genitourinary system diseases | K510200 | 2285 | Rectocele without uterine prolapse |
| K – genitourinary system diseases | K510211 | 37918 | Proctocele without uterine prolapse |
| K – genitourinary system diseases | K510300 | 4575 | Urethrocele without uterine prolapse |
| K – genitourinary system diseases | K512.00 | 7870 | Uterovaginal prolapse, incomplete |
| K – genitourinary system diseases | K512000 | 30419 | Cystocele with first-degree uterine prolapse |
| K – genitourinary system diseases | K512100 | 12359 | Cystocele with second-degree uterine prolapse |
| K – genitourinary system diseases | K513.00 | 9356 | Uterovaginal prolapse, complete |
| K – genitourinary system diseases | K513000 | 25974 | Cystocele with third-degree uterine prolapse |
| K – genitourinary system diseases | K514.00 | 1057 | Uterovaginal prolapse, unspecified |
| K – genitourinary system diseases | K514000 | 12845 | Cystocele with unspecified uterine prolapse |
| K – genitourinary system diseases | K515.00 | 10888 | Post-hysterectomy vaginal vault prolapse |
| K – genitourinary system diseases | K516.00 | 2846 | Vaginal enterocele |
| K – genitourinary system diseases | K516100 | 41136 | Acquired vaginal enterocele |
| K – genitourinary system diseases | K516z00 | 42057 | Vaginal enterocele – NOS |
| K – genitourinary system diseases | K518.00 | 96896 | Female rectocele |
| K – genitourinary system diseases | K51y.00 | 23941 | Other genital prolapse |
| K – genitourinary system diseases | K51yz00 | 41895 | Other genital prolapse – NOS |
| K – genitourinary system diseases | K51z.00 | 33440 | Genital prolapse – NOS |
| K – genitourinary system diseases | Kyu9100 | 97649 | [X] Other female genital prolapse |
| L – Pregnancy/childbirth/puerperium | L244.11 | 20850 | Cystocele in pregnancy, childbirth and the puerperium |
| L – Pregnancy/childbirth/puerperium | L244.13 | 39492 | Rectocele in pregnancy, childbirth and the puerperium |
| L – Pregnancy/childbirth/puerperium | L244011 | 20907 | Cystocele affecting obstetric care |
| L – Pregnancy/childbirth/puerperium | L244012 | 58517 | Rectocele affecting obstetric care |
| L – Pregnancy/childbirth/puerperium | L244111 | 32286 | Cystocele – baby delivered |
| L – Pregnancy/childbirth/puerperium | L244112 | 32287 | Rectocele – baby delivered |
| L – Pregnancy/childbirth/puerperium | L244211 | 66127 | Cystocele – delivered with postpartum complication |
| L – Pregnancy/childbirth/puerperium | L244212 | 57581 | Rectocele – delivered with postpartum complication |
| L – Pregnancy/childbirth/puerperium | L244311 | 101354 | Cystocele complicating antenatal care – baby not delivered |
| L – Pregnancy/childbirth/puerperium | L244312 | 30378 | Rectocele complicating antenatal care – baby not delivered |
| L – Pregnancy/childbirth/puerperium | L244411 | 38439 | Cystocele complicating postpartum care – baby delivered during previous episode of care |
| L – Pregnancy/childbirth/puerperium | L244412 | 51111 | Rectocele complicating postpartum care – baby delivered during previous episode of care |
| L – Pregnancy/childbirth/puerperium | L244z11 | 39550 | Cystocele in pregnancy, childbirth or the puerperium – NOS |
| L – Pregnancy/childbirth/puerperium | L244z12 | 64147 | Rectocele in pregnancy, childbirth or the puerperium – NOS |
Appendix 6 Interview topic guide and sample questions
Preamble to interview
My name is Rebecca Lynch and I’m a researcher based at the London School of Hygiene and Tropical Medicine (a university based in London). I’m interviewing women in different parts of the country about their experiences of urinary incontinence and incontinence care for a project that looks to improve the services that women are offered.
I am not directly connected to the clinic where you are a patient. This study is separate to the services offered at the hospital. What we talk about will not affect your treatment and care at the clinic and our conversation won’t be disclosed to people working there.
If you’re happy for me to do so, I’d like to record our conversation so that it can be written up for analysis. All recordings and access to what we’ve talked about will be kept confidential, including from those working at the clinic, unless you tell me something that indicates that you or someone else is at risk of harm. I would discuss this with you before telling anyone else. When I write about what patients have told me, this will be anonymised and I will be careful that you cannot be identified in anything I write up or present.
We are planning to write and present our findings in academic journals and at academic conferences, links to papers will be available through our project website. We are also planning to write a summary of our findings that might be easier for people to look through, this will also be available for you to read from our project website.
You have already had a chance to look at the participant information sheet and consent form – do you have any questions about either of these?
Can I talk you through the statements on the consent form to check that these are clear?
Are you happy to participate? If you are happy to be involved, can I remind you again that you are able to leave at any time during the interview if you do not wish to continue, and if you would like to withdraw from the study afterwards, you can ask me to withdraw your data any time up to when we publish, without giving a reason for doing this.
If you are happy to do so, can I then ask you to complete the consent form, including whether you would be happy for me to record the interview?
Interview topic guide and sample questions
-
Experience of urinary incontinence
-
When did your urinary incontinence start?
-
How has this changed over time?
-
How has the experience of UI impacted on your everyday life? (Further prompts will be on effects on their personal relationships, social life and employment.)
-
What are the most significant aspects of these experiences for you personally?
-
-
Care for urinary incontinence
-
What medical, or non-medical, forms of care have you drawn on to treat your incontinence?
-
What are the most significant aspects of this for you?
-
What are the symptoms/experiences you’d most like to be addressed?
-
What sorts of options have you been presented with?
-
How did you come to be referred to the hospital clinic?
-
What has your experience been with investigations and assessments of your UI?
-
What have you heard about the surgical options available to you?
-
What do you think about having surgical treatment?
-
What factors are you thinking about in making these decisions?
-
Who, if anyone, have you talked about this with or gained ideas from?
-
-
Expectations of surgical/non-surgical care and future outcomes
-
How do you hope treatment might improve your current circumstances?
-
How do you hope treatment might impact on longer term outcomes?
-
What is your understanding of how your treatment will change your body?
-
List of abbreviations
- AIC
- Akaike information criterion
- aOR
- adjusted odds ratio
- APC
- admitted patient care
- ASA
- American Society of Anesthesiologists
- BAME
- black, Asian and minority ethnic
- BIC
- Bayesian information criterion
- BMI
- body mass index
- BSUG
- British Society of Urogynaecology
- CCG
- Clinical Commissioning Group
- CI
- confidence interval
- COPD
- chronic obstructive pulmonary disease
- CPRD
- Clinical Practice Research Datalink
- CVD
- cardiovascular disease
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- ICD-10
- International Classification of Diseases, Tenth Revision
- IMD
- Index of Multiple Deprivation
- IQR
- interquartile range
- IRR
- incidence rate ratio
- LSHTM
- London School of Hygiene & Tropical Medicine
- LSOA
- lower-layer super output area
- MUI
- mixed urinary incontinence
- MUT
- mid-urethral mesh tape
- NICE
- National Institute for Health and Care Excellence
- OPCS-4
- Office of Population, Censuses and Surveys Classification of Interventions and Procedures, version 4
- POP
- pelvic organ prolapse
- PPI
- patient and public involvement
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCS
- Royal College of Surgeons
- SD
- standard deviation
- sdHR
- subdistribution hazard ratio
- STP
- Sustainability and Transformation Partnership
- SUI
- stress urinary incontinence
- T2DM
- type 2 diabetes mellitus
- UI
- urinary incontinence
- UTI
- urinary tract infection
- UTS
- up to standard
- UUI
- urgency urinary incontinence
- WP
- work package
