Notes
Article history
The research reported in this issue of the journal was funded by the HS&DR programme or one of its preceding programmes as project number 16/116/46. The contractual start date was in August 2018. The final report began editorial review in October 2020 and was accepted for publication in February 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HS&DR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© 2021 Gulliford et al. This work was produced by Gulliford et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2021 Gulliford et al.
Chapter 1 Introduction
The problem of antimicrobial drug resistance
The threat of antimicrobial drug resistance is attracting the concern of national governments and international organisations. 1 Antibiotic-resistant infections are now increasing in frequency and are more often being identified when cultures are performed. In England, in 2018, nearly one-third of urinary tract infections (UTIs) caused by Escherichia coli were resistant to trimethoprim2 and 43% of E. coli bacteraemia isolates were resistant to co-amoxiclav. 2 The emergence of antimicrobial resistance requires action from a range of sectors, including the pharmaceutical industry, as well as agriculture and food production, as outlined in the O’Neill review. 3 However, antimicrobial resistance has the most immediate relevance in the health-care sector, in which antibiotics are prescribed and in which patients with resistant infections are seen. 3 The UK Government has developed a 5-year antimicrobial resistance strategy that identifies optimising antibiotic prescribing practices as a key element of antimicrobial stewardship. 4
Antibiotic prescribing in primary care
In the UK, primary care accounts for nearly three-quarters of all antibiotic prescribing. Respiratory tract infections (RTIs) represent the largest single group of indications for antibiotic treatment. 2 General practitioners (GPs) prescribe antibiotics, on average, at 52% of consultations for ‘self-limiting’ RTIs, including common colds, acute cough and bronchitis, sore throat, otitis media and rhinosinusitis,5 with little change over the last two decades (Figure 1). 6,7
FIGURE 1.
Distribution of the proportion of the RTI consultations with antibiotics prescribed at 568 UK general practices. 6 Reproduced from Gulliford et al. 5 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.

The other main indications for antibiotic prescription include UTIs and skin infections, for which there may be less discretion concerning whether or not to use antibiotics, with greater emphasis given to appropriate antibiotic selection. 8,9 Since the inception of this research project, analysis of electronic health records has shown that between one-third and half of all antibiotic prescriptions in primary care in the UK may not be associated with specific diagnostic codes, possibly because GPs have recorded free-text information or recorded non-specific codes (such as ‘had a chat with the patient’). 8,10 This poor recording of consultations for common infections in primary care makes it difficult to evaluate the appropriateness of existing prescribing patterns. Consequently, Hay11 recommended that strategies should be adopted to ensure that all antibiotic prescriptions and all infection consultations should be documented through the recording of appropriate medical diagnostic codes.
Evidence to support no-prescribing strategies
Evidence from systematic reviews of randomised controlled trials shows that antibiotic treatment for self-limiting RTIs generally has little, if any, effect on the severity or duration of symptoms and is commonly associated with unwanted symptomatic side effects, including rashes and diarrhoea. 12,13 These side effects may not always be reported, but may lead to non-adherence. Prescribing antibiotics also has the effect of medicalising conditions that are generally self-limiting and should be amenable to self-care. Patients given antibiotics for sore throat are 69% more likely to consult again for the same condition. 14 Consequently, UK National Institute for Health and Care Excellence (NICE) guidelines recommend that a no antibiotic prescribing, or delayed antibiotic, strategy should be agreed with most patients presenting with self-limiting RTIs. 15 Respiratory conditions represent one of the most important opportunities to reduce antibiotic use. In 2018, NICE developed and disseminated guidance for managing a comprehensive range of common infections in primary care, which summarised the indications for prescribing antibiotics and appropriate drug selection. 16
Evidence that prescribing may be reduced
Several approaches are now being developed and tested to promote more effective antibiotic stewardship in primary care. Deferred or delayed prescribing, in which a prescription is given but used only if needed, gives patients more control and is sometimes advocated; however, this strategy may be less effective at reducing antibiotic use while offering similar patient satisfaction to a ‘no-prescribing’ strategy. 17 Algorithms are being developed to identify patients who may need antibiotics. 18,19 Near patient testing for biomarkers of bacterial infection is being developed to enable targeted prescribing of antibiotics, but this is not yet fully proven and may be difficult to integrate into usual clinical practice. 20 Behaviour change approaches are being tested. In one study in England, high-prescribing GPs were sent an individualised letter signed by England’s Chief Medical Officer, which resulted in a 3% reduction in antibiotic utilisation. 21 Finally, a contractual financial incentive, known as a ‘Quality Premium’, has been introduced into the English NHS for meeting indicative targets for year-on-year reductions in antibiotic utilisation. 22
Recently, attention has focused on evidence to support reducing antibiotic utilisation in primary care. Smieszek et al. 23 analysed electronic health record data for general practices in England and compared observed antibiotic prescribing practice with recommendations from guidelines and expert opinion. The study found that at different general practices between 6% and 44% of antibiotic prescriptions might be inappropriate, with the highest proportions of inappropriate prescription being for sore throat, cough, sinusitis and otitis media. 23 Across Europe, the number of antibiotic prescriptions (defined daily doses) per 1000 population per day ranges from 10 (in the Netherlands) to 36 (in Greece), with a value of 20 in the UK. 24 Based on these international comparisons, with both low25 and high26 antibiotic prescribing being observed across Europe without risks to patient safety, it appears that a substantial reduction of antibiotic prescribing in primary care might be reasonable.
Giving antibiotic treatment when needed
Strategies to reduce inappropriate use of antibiotics must ensure that antibiotics can be used when they are needed. 27 Reducing antibiotic use might potentially compromise patient safety by increasing the risk of serious bacterial infections following minor infections that are expected to be self-limiting. 28 This is recognised in the NHS, where reducing bloodstream infections is a key antimicrobial stewardship metric, alongside reducing inappropriate antibiotic prescriptions. 22 Bacterial infections, such as sepsis, are still of public health importance. 29 Early recognition and treatment of sepsis is being promoted. Most general practice systems are now incorporating alerts that flag at-risk consultations. 30
The safety of reduced antibiotic prescribing is a major concern for clinicians. One GP respondent commented:
It’s the fear of litigation or things going wrong, and if you have arbitrary targets like this . . . and I don’t want to prescribe, but if it’s needed, then pressure of some sort of appraisal and maybe being told off is not really needed.
Gulliford et al. 31
Parents are also concerned about safety issues, which are an important motivation for seeking active treatment for children. 32 Advice given by clinicians concerning ‘safety-netting’ may appear vague and unhelpful if patients are advised to re-consult ‘if they are worried’ or ‘if [the patient] doesn’t get better’. 32 Patients may be concerned that a repeat consultation may be difficult to obtain. A systematic review of qualitative studies found that clinicians commonly prescribe an antibiotic ‘just in case’ it might be needed. 33 There is a lack of research providing quantitative estimates of risk that might allow clinicians to provide more evidence-informed advice.
Trends in bacterial infections
Serious bacterial infections represent a growing concern for health systems. In the UK, sepsis is estimated to account for 36,900 deaths and 123,000 hospital admissions annually. 34 The Global Burden of Disease Study estimated that there were nearly 50 million incident cases of sepsis worldwide in 2017, with 11 million deaths, representing 19.7% of global deaths. 35 Sepsis is defined as a syndrome resulting from the interaction between an acute infection and the host response, leading to new organ dysfunction. 36 Sepsis is an intermediate state that links an infection or an infection-causing condition to adverse health outcomes. The term sepsis is now more commonly used than the term ‘septicaemia’, which refers to bloodstream infection. In the health-care systems of high-income countries, records of ‘sepsis’ have been increasing in both hospital and primary care settings. 37–39
There is also evidence that certain localised infections have been increasing in frequency in the UK (Table 1). Thornhill et al. 46 observed that the incidence of infective endocarditis was stable in England between 1998 and 2010, but between 2010 and 2019 there was an 86% increase in the frequency of the condition. This was contemporaneous with changes to the recommendations for antibiotic prophylaxis at dental procedures. Empyema is an infrequent complication of lung infections. A study from New Zealand44 found that the incidence of empyema increased from 1 per 100,000 children aged 0–14 years in 1998 to 13 per 100,000 in 2009. This increase has also been observed in Australia,52 the UK43 and Europe. 45 Some studies suggest that the introduction of a polyvalent pneumococcal vaccine may have been associated with an increase in infections with non-vaccine strains of pneumococci that may be associated with increased risk of empyema,45 despite that the introduction of the pneumococcal vaccine has been associated with a substantial reduction in pneumonia in children overall. 53 Other authors suggest that lower early initiation of antibiotic therapy for more serious respiratory infections might also be a contributory factor. 44 There is also more limited evidence for increasing trends in the occurrence of osteomyelitis and septic arthritis. 48–50
| Condition | Trend |
|---|---|
| Sepsis | Increasing diagnosis and recording40 |
| PTA | Unchanged or decreasing incidence28,41 |
| Mastoiditis | Stable incidence42 |
| Empyema | Increasing incidence43–45 |
| Infective endocarditis | Increasing incidence46,47 |
| Osteomyelitis | Increasing incidence48 |
| Septic arthritis | Increasing incidence49,50 |
| Kidney infections | Increasing trend in UTI hospital admissions51 |
The reasons for these apparent increases in serious bacterial infection are complex. There have been changes to case definitions, diagnostic criteria and disease labelling. This is particularly relevant for diagnoses of sepsis. A study from the Massachusetts General Hospital (Boston, MA, USA)54 found that recording of severe sepsis or septic shock increased by 706% in the decade between 2003 and 2012, whereas objective markers of severe infection, including positive blood cultures, remained stable or decreased. Alongside increasing use of the term sepsis, case definitions have expanded to include patients with evidence of both acute infection and acute organ dysfunction as having ‘implicit sepsis’, even when sepsis was not explicitly diagnosed. 35,55 Changes in disease labelling might be less relevant for localised bacterial infections. Thornhill et al. 46 and Quan et al. 47 concluded that the increased incidence of endocarditis might be accounted for by wider demographic, social and medical changes that increase susceptibility and risk. These include the effects of population ageing, the increase in obesity, possibly the more widespread use of intravenous drugs, the increasing prevalence of comorbidities (including diabetes mellitus), the more widespread use of invasive medical procedures and the increasing numbers of patients with immunosuppressive disorders or receiving immunosuppressive treatments. Nevertheless, more restrictive use of antibiotics for common infections cannot be excluded as a contributory cause of these increasing trends in serious bacterial infections. Recent research, therefore, has begun to investigate the safety of reduced antibiotic prescribing in primary care.
Previous studies of safety outcomes of antibiotic prescribing
Only a few existing research studies directly address the safety outcomes of reduced antibiotic prescribing. Petersen et al. 56 reported a cohort study in 162 General Practice Research Database general practices from 1991 to 2001, showing increased odds of pneumonia after ‘chest infection’, peritonsillar abscess (PTA) after sore throat and mastoiditis after otitis media. The absolute risks for these complications were generally low, with > 4000 antibiotic prescriptions being required to prevent one case. However, in people aged > 65 years, one case of pneumonia might be prevented for every 38 ‘chest infections’ treated with antibiotic.
Little et al. 57 reported on a clinical cohort of 14,610 patients presenting with sore throat. Fewer than 1% had complications (including PTA, otitis media, sinusitis, impetigo or cellulitis). It was generally difficult to predict when these complications might arise based on clinical features of the initial presentation. 58 In a cohort study of patients with acute lower RTI, Little et al. 59 found that hospital admissions and mortality were rare complications and that these did not appear to be prevented by initial prescription of antibiotics.
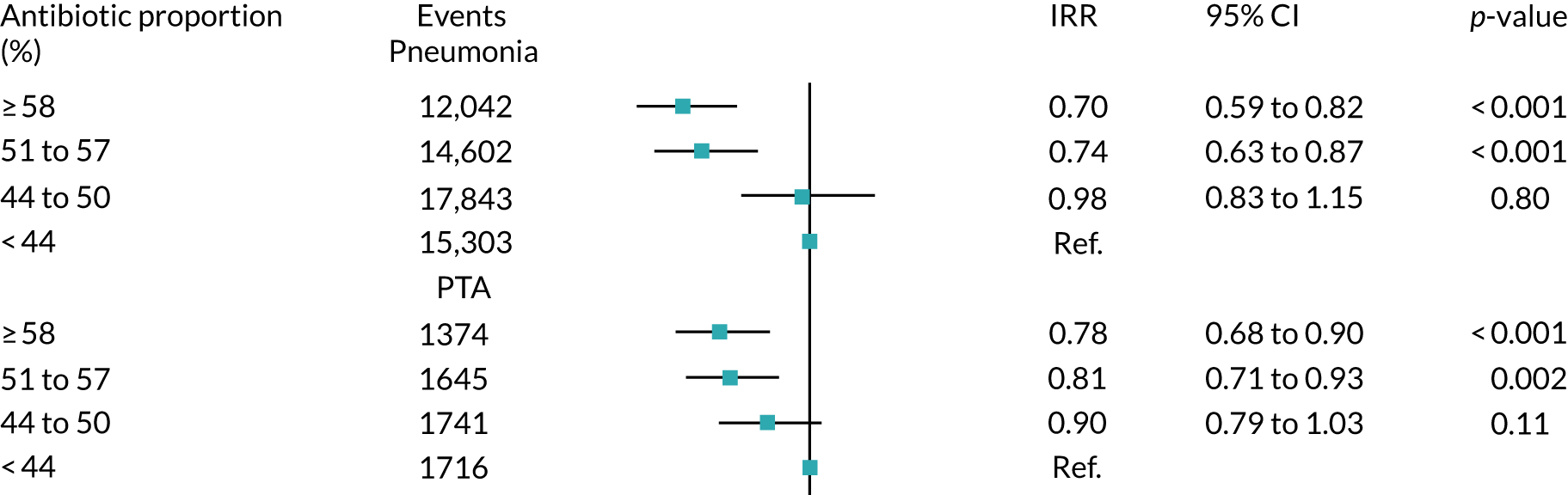
Our group reported a study using data for more than 600 Clinical Practice Research Datalink (CPRD) general practices from 2005 to 2015. 28 Of the seven outcomes studied, we found that pneumonia and PTA were more frequent at general practices that prescribed antibiotics less frequently at consultations for self-limiting RTI (Figure 2). Absolute risks were small, with an average general practice experiencing one more case of pneumonia per year and one more case of PTA per decade for a 10% reduction in antibiotic prescribing. We found no association of practice-level antibiotic prescribing for RTI with incidence of empyema, mastoiditis, intracranial abscess, bacterial meningitis or Lemierre’s syndrome (i.e. infective thrombophlebitis of the internal jugular vein). However, these were rare outcomes and even in this large data set it was not possible to exclude the possibility of small increases in risk. 28
FIGURE 2.
Association of the incidence of pneumonia and PTA with the quartile of antibiotic prescribing proportion. Antibiotic proportion is the proportion of RTI consultations with antibiotic prescribed at that general practice. 28 CI, confidence interval; IRR, incidence rate ratio. Reproduced from Gulliford et al. 28 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Since the present research project was initiated, several other groups have reported analyses of electronic health records to evaluate potential safety outcomes of reduced antibiotic prescribing. Cushen and Francis60 used data from the CPRD to evaluate the occurrence of brain abscess and mastoiditis after otitis media, and brain abscess and orbital cellulitis after acute sinusitis. Their analysis found that antibiotic prescription was associated with lower risk of acute mastoiditis following otitis media [odds ratio 0.54, 95% confidence interval (CI) 0.37 to 0.79] and of brain abscess following acute sinusitis (odds ratio 0.12, 95% CI 0.02 to 0.70). However, because of the low incidence of these conditions, the number of antibiotic prescriptions required to prevent one complication was > 2000 for otitis media and nearly 20,000 for acute sinusitis. 60
Gharbi et al. 61 conducted a cohort study, also using the CPRD, to evaluate the risk of bloodstream infection, hospital admission or mortality following UTI in adults aged ≥ 65 years. Their analysis suggested that patients with evidence of delayed initiation of antibiotics might have greater risk of these adverse outcomes. However, it is also possible that data-recording issues might have introduced bias (e.g. if more seriously ill patients are initially seen in urgent or out-of-hours settings and antibiotic prescriptions are not recorded into general practice records).
Mistry et al. 62 analysed CPRD data to develop a prediction model for infection-related hospital admission after a general practice consultation for RTIs and UTIs. The most important predictors were found to be age, Charlson Comorbidity Index and previous hospital admission in the last year. In this observational analysis, whether or not antibiotics were prescribed was not associated with hospital admission for infection-related complications.
The need for further research
The question of whether or not reducing antibiotic prescribing carries risks to patient safety is clearly important, but the evidence base is currently extremely limited. Previous research raises several questions about the safety of reducing antibiotic prescribing and requires more systematic and thorough study. Previous studies considered antibiotics prescribed for specific indications56 or for self-limiting RTIs,28 but antibiotic use for all indications should be evaluated. Previous studies relied on primary care records, but additional validation from hospital episode data is desirable because differential code selection might occur in primary care to justify an antibiotic prescription. 63 Different age groups require evaluation because these may have differing susceptibility to complications. With the rapid increase in numbers of older people, the effects of frailty64,65 and comorbidity on susceptibility to complications in the most vulnerable require evaluation. Universal compared with risk-stratified approaches to reduce antibiotic prescribing require evaluation.
The present use of targets for global reductions in antibiotic utilisation in the Quality Premium raises questions concerning the quality of the evidence available to inform target setting. Is a single target across all prescribing indications the optimal approach? Reducing antibiotic utilisation may be more readily achieved in some groups of patients and for some prescribing indications than others. This research aimed to provide the NHS with a more systematic understanding of potential safety outcomes of reducing antibiotic prescribing, therefore enabling the identification of safer strategies for reducing antibiotic utilisation. We aimed to quantify the risks of a comprehensive and systematically identified list of safety outcomes and distinguish population subgroups that may be at increased risk.
Chapter 2 Aims and objectives
This research asked whether or not it is safe to reduce antibiotic prescribing in primary care. Is there a risk that bacterial infections might be more frequent if antibiotics are prescribed less often? If so, what is the safest way for the NHS to promote reduction of antibiotic prescribing in primary care? The research specifically aimed to provide evidence concerning different prescribing indications and for different population groups based on risk stratification. The research aimed to develop new indicators of safe and appropriate antibiotic prescribing and to implement these into general practices.
The specific objectives were as follows:
-
Conduct an epidemiological analysis of electronic health records to estimate the relative and absolute risks of each outcome in association with lower antibiotic prescribing, based on both community- and individual patient-level associations.
-
Construct a decision-analysis model to identify, for each safety outcome, risk groups in whom the incidence of the outcome may be highest (and lowest) and to estimate absolute risks of antibiotic prescribing or non-prescribing in these groups.
-
Engage with members of the public, patients and clinicians to understand their views and values in developing candidate indicators of safe antibiotic prescribing reduction and implement these indicators into general practices.
Chapter 3 Methods
Summary
We conducted qualitative interview studies that included 31 patients who had recently consulted with infections in primary care and 30 primary care prescribers, and these informed the research of the perceptions and priorities of these groups. We conducted an epidemiological study, including data from CPRD GOLD (Vision® data) and CPRD Aurum (EMIS® data) to estimate secular trends and between-practice variation in antibiotic use. We also estimated whether or not overall antibiotic utilisation at general practices was associated with the incidence of 11 different serious bacterial infections. Drawing on epidemiological estimates, we developed a decision-analytic model that enabled us to estimate the probability of a serious bacterial infection following an infection consultation in primary care if antibiotics were prescribed or not. We initially developed and tested the mode using PTA as an outcome; we then evaluated the risk of sepsis and we finally evaluated a range of localised serious bacterial infections. We incorporated these modelling estimates into a Shiny application (app) (RStudio, Boston, MA, USA) so that they could be presented to primary care practitioners. We conducted qualitative interviews with practitioners to test the app and evaluate practitioners’ understanding of the estimates presented.
Qualitative research
Ethics statement
The proposal for the qualitative research study was reviewed and approved by the London – Hampstead NHS Research Ethics Committee (reference 18/LO/1874).
Study design
Semistructured interviews were conducted with patients and primary care prescribers, including GPs, nurses and pharmacists, in two English regions (one urban metropolitan area and one shire town with a high demand for primary care services). Metropolitan practices were invited to the study by the local Clinical Research Network that generated the expression of interest. A shire town practice was recruited through informal Clinical Research Network contact, which also helped to liase with potential respondents.
Patient interview study
Semistructured interviews were conducted with patients who consulted their general practice for an infection. Participants were invited to be interviewed if they had recently consulted and been diagnosed by a GP as having a bacterial infection. The bacterial infections were identified using Read codes for the relevant conditions, including RTIs, UTIs and skin infections as the major indications for antibiotic prescribing. An interview guide was developed to reflect expectational structures associated with antibiotics, as well as the experiences of illness and consultations. The items were informed by a review of the literature and included past and current experience of being prescribed antibiotics; knowledge, beliefs and attitudes towards taking antibiotics; and interactions with medical practitioners. The questions were discussed among the research team and piloted with a small number of patients before refining. The items in the topic guide were organised under six main headings (Box 1). All interviews were conducted by an experienced qualitative researcher (OB) to ensure consistent quality. The interviewer had a Doctor of Philosophy (PhD) in medical sociology and was an experienced qualitative researcher. Interviews were conducted in the period February–December 2019. Interviews lasted between 13 and 42 minutes.
To begin with, could you tell me about your recent experience of consulting the GP for infection?
What was the health issue and how it was dealt with? Did you have any expectations of specific treatment? Were you able to discuss them in the consultation? Was the risk associated with different treatment choices communicated and how? How was the issue resolved? Has seeing the doctor helped in infection management?
2. Knowledge of antibioticsOverall, what is your knowledge about different types of infections and associated treatment? Could you share with me what is your understanding of antibiotic treatment? How do the antibiotics work? What types of antibiotics are there? When should antibiotics be prescribed? What are the risks associated with non-prescribing of antibiotics? What are the potential complications and unwanted consequences of antibiotic treatment? Who should be making decision on antibiotic treatment? What was your previous experience of antibiotic treatment, if any? To what extent has your experience shaped your perception of antibiotics at present? Were there any changes in how you consider infections and their treatment? What has driven these changes?
3. Concerns about treatmentWould you say that you felt confident in managing the infection with/without treatment? Were you able to raise your concerns and have all your questions answered during the consultation? Have you experienced any difficulties in complying with the treatment plan?
4. Optimism regarding outcomesAre you hopeful for the antibiotic treatment to be the best possible course of action? If there was an uncertainty and anxiety around the treatment plan, how did you handle it? Have you been able to seize the impact of antibiotic treatment following the recent or previous consultations for infections?
5. Decision-making processesWhat would be your priorities in infection management? In consultations for infections, if a doctor’s advice differed from your interpretation, would you or have you challenged the decision? What would be/what were your actions following unresolved or repeated infection?
6. Social and environmental influencesSpeaking about the appropriate treatment for infections, what are the sources of information that are likely to influence your understanding? In your experience, are doctors consistent in their consultations for infections? What does your friends, family members and close networks believe with respect to antibiotic treatment and how does it compare with your beliefs? What are your perceptions of antimicrobial resistance?
What other information might be useful in making decisions on antibiotics treatment?
Recruitment of participants and data collection
The research invitations to participate generated expressions of interest from the general practices that agreed to purposively select patients who visited a primary care professional for infection in the last 6 months. Patient lists were approved by a GP acting as research gatekeeper in each practice and, initially, 927 patients were sent invitations to the study via the Docmail® postal system (Radstock, UK). The invitations contained a letter from the practice, inviting patients to participate, and an information sheet. Patients who agreed to take part either returned reply slips or contacted the researcher using the contact details provided. The researcher then communicated via e-mail or text message to establish the contact, followed by sending the consent form and confirmation of the interview meeting.
The sample size was determined using the pragmatic concept of ‘information power’,66 which proposes that the size of a sample with sufficient information power depends on the aim of the study, sample specificity, use of established theory, quality of dialogue and analysis strategy. Although our aim was broad, specificity was biased towards one group (almost half of the interviewees were older female patients consulted for UTI); therefore, we followed a theoretical model to explain the findings and the quality of the interviews was relatively high. As we aimed for a cross-case analysis, we decided to continue recruitment until the sample size reached 31 eligible patients.
Analysis
The interviews were digitally recorded, transcribed verbatim by a professional transcriber, imported to an NVivo 12 project (QSR International, Warrington, UK) and coded through an iterative six-phase process described in thematic analysis. 67 Data analysis occurred iteratively and involved familiarisation, coding, theme searching, theme reviewing, theme defining and naming, and producing the report. Repeated patterns in the data formed the basis for the codes, which were identified by the lead analyst (OB), and one single code for every different concept/idea was generated. To ensure that codes were applied consistently, a co-author (CB) independently coded a random sample of four interview transcripts. Coding was refined after discussion. Data identified by the same code were collated and all different codes were sorted into potential subthemes and themes using NVivo options of tree building. The potential themes were reassessed and reorganised to reflect major narratives and themes in the coded data. Finally, the analysts refined and named the five main themes and subthemes. Participants’ feedback on the transcripts or the summarised final findings was not sought because this was not feasible. However, the main themes were discussed at a patient and public involvement (PPI) meeting, as noted in Patient and public involvement.
Practitioner interview study
Interviews
The practitioner study investigated how primary care prescribers perceive risk and safety concerns associated with reduced antibiotic prescribing. Items for the interview were guided by the theoretical domains framework (TDF), which uses theories of behaviour change to understand factors influencing health-care practice. 68,69 The TDF comprises 14 domains, covering the main theoretical determinants of behaviour, and the interview was designed to reflect these domains (Box 2). 68 The interview was piloted with three GPs to ensure that the questions were appropriate, readily understood and covered relevant prescribing behaviours. All interviews were conducted by Olga Boiko to ensure consistent quality. All interviews except one telephone interview were conducted face to face on general practice (n = 26) and university (n = 4) premises in the period January–July 2019. The interviews lasted between 24 and 46 minutes. The participants were offered £60 to acknowledge their contribution.
What are the indications for antibiotic treatment?
To what extent do NICE (or local) guidelines influence your antibiotic prescribing?
What are the risks of antibiotic prescribing and non-prescribing?
How do you differentiate between infections and patients?
What are the common myths or stereotypes about antibiotics?
Can you give me an example that illustrates the inaccurate understanding of their purpose, mechanisms of action, risks and consequences?
In your view, is there the best way to elicit and manage patient expectations regarding antibiotics?
How would you communicate the risks associated with both prescribing and non-prescribing antibiotics?
How confident are you in decision-making around antibiotic prescribing?
Would you assess your approach to antibiotic prescribing as always adequate and, if so, what makes you think that?
Could you describe consequences of inappropriate treatment for infections?
What would be/what were your actions following unresolved or repeated infections?
What is your understanding of antimicrobial resistance?
What are your goals and priorities in infection management?
Are there any social norms or group pressures that affect your professional practice with regard to antibiotic prescribing and how?
Has your prescribing practice for antibiotics changed over the recent years?
Do you think patient expectations of antibiotic treatment have changed over the recent years?
Are you aware of the prescribing practice of other health-care professionals (your colleagues) in relation to antibiotics? Have you ever had to challenge their prescribing decisions?
Has anyone challenged your own decisions?
How hopeful are you usually that the antibiotic treatment is the best course of action?
Is it possible to assess both the short- and long-term impact of antibiotic treatment on the patients?
What is your decision-making strategy?
How anxious do you feel about the uncertainty around prescribing?
Which resources do you use to support your decisions on antibiotic prescribing?
Recruitment of participants
Contact details of primary care practitioners were obtained through the help of the local research facilitators and practice managers. Potential participants were then approached either directly by e-mail using the study information pack or indirectly via the practice manager or lead GP. The information pack included the invitation letter and study information sheet. A reminder was sent out 2 weeks after the initial approach to those who had not responded. A purposive sampling approach was followed. Forty-nine primary care providers from 10 general practices were invited and 30 agreed to take part. This number of participants was deemed sufficient, using the pragmatic concept of ‘information power’. 66 The uptake varied between practices (in five practices only a single primary care practitioner was interviewed). Interviews took place between January 2019 and July 2019.
Analysis
The interviews were digitally recorded, transcribed by a professional transcriber, imported to an NVivo 12 project and coded through an iterative six-phase process, as outlined above. 67 Repeated patterns in the data formed the basis for the codes, identified by the first author (OB), and one single code for every different concept/idea was generated. To ensure that codes were applied consistently, a co-author (CB) independently coded a random sample of four interview transcripts. Coding was refined after discussion. Data identified by the same code were collated and all different codes were sorted into potential subthemes and themes using NVivo options of tree building. Next, the potential themes were reassessed and reorganised to reflect major narratives and themes in the coded data. Finally, the themes and subthemes were refined and named (by OB, CB and MCG). The themes and subthemes were then mapped to the relevant domains of the TDF to assess the relative importance and salience of individual domains.
Epidemiological study
Ethics statement
The protocol for the epidemiological study was approved by the CPRD Independent Scientific Advisory Committee (protocols 18-041R and 19_110R). The CPRD holds overarching Multicentre Research Ethics Committee approval for the database and conduct of studies using fully anonymised data.
Data source
We carried out population-based cohort studies in the UK CPRD GOLD database, employing data for 2002–17. CPRD GOLD draws on general practices that use the Vision general practice software system. The Vision system has suffered from a declining market share in recent years. Consequently, CPRD has established the CPRD Aurum database, which draws data from general practices that use the EMIS general practice system.
The CPRD GOLD database collects data from the four countries of the UK, with about 30% of contributing practices located in England at the time of this study. The CPRD GOLD database has been well described70 and the high quality of the data collected has been documented in many studies. 71 CPRD records include details of consultations by general practice staff, as well as coded records of referrals to hospital or discharge letters from hospitals. The CPRD GOLD is a ‘live’ database, which is updated monthly. The October 2019 database release included data on 17.6 million patients, of whom 2.6 million were currently active. There were 320 general practices currently contributing to CPRD GOLD: 30% in England, 3% in Northern Ireland, 37% in Scotland and 30% in Wales. Data linkage in CPRD GOLD is restricted to volunteer general practices in England only. The CPRD Aurum database was more recently established and, at the time of this study (June 2019 release), was restricted to data collected from general practices in England. 72 The CPRD Aurum database included data on 883 general practices, from which patients were sampled, with 23.1 million patients, including 2.5 million currently active patients. This study was designed using the CPRD GOLD database. However, we conducted a substudy to compare antibiotic prescribing metrics between CPRD GOLD and CPRD Aurum.
The research aimed to evaluate safety outcomes of reduced antibiotic prescribing, including conditions such as sepsis, PTA and infective endocarditis. These are infrequent or rare events. Consequently, it was necessary to evaluate outcomes over a long period of time in the whole CPRD database to obtain sufficiently precise estimates of incidence rates. The period from 2002 to 2017 was selected for study. However, our licence agreement with CPRD places limits on the number of records that can be extracted for analysis. We were able to extract full CPRD data for the numerator, but for the denominator we were restricted to data included in the CPRD GOLD denominator file, which comprised age (year of birth), gender and study year. To address this, we employed sample data for the study denominator, as outlined below.
The research was further complicated by possible changes over time in the definition and recognition of outcomes. For example, definitions of sepsis have changed over time, and there have been substantial changes in professional and public awareness of sepsis as a complication of infection. There have also been changes in approach to antibiotic prescribing and antimicrobial stewardship during the period of study. This required analytical approaches that accounted for changes over time in key exposures or the incidence of outcomes.
As noted above, the CPRD GOLD is a ‘live’ database that is updated each month. The ‘last collection date’ for each general practice is updated for each release, as are the dates of death and the end of registration for each participant. In addition, the ‘up-to-standard’ date at which the general practice is judged to have been contributing research-quality data may be updated, even for historical data, based on an algorithm employed by CPRD. Patients have the possibility of ‘opting out’ of CPRD and the small number of opting-out patients may change over time. The present research was conducted over several years in the form of a series of related studies. The research is, therefore, based on data from several different releases of CPRD GOLD. In addition, we made ongoing revisions and updates to our medical code lists to enable updating consistent with our evolving understanding of conditions relevant for study. Consequently, the number of outcome events and person-years at risk may vary slightly when different analyses are compared, although relevant findings are expected to be consistent across different releases.
Antibiotic prescribing study
We evaluated rates for antibiotic prescription and infection consultations in the CPRD GOLD database between 2002 and 2017.
Selection of sample for antibiotic prescribing analysis
We estimated the infection consultation rates and the proportion of consultations with antibiotics prescribed from a sample of patients registered with CPRD GOLD. This was because it is not feasible to download and analyse data for the millions of records represented by all infection consultations and antibiotic prescriptions over 16 years. 39 A random sample of patients was drawn from the list of all registered patients from the November 2018 release of CPRD GOLD and was stratified by year between 2002 and 2017 and by general practice. The ‘sample’ command in the R program (The R Foundation for Statistical Computing, Vienna, Austria) was employed to provide a computer-generated random sequence. In each year of study, a sample of 10 patients was taken for each gender and age group, using 5-year age groups up to a maximum of 104 years. Each sampled patient contributed data for multiple years of follow-up. There was a total sample of 671,830 individual patients registered at 706 general practices who contributed person-time between 2002 and 2017. The sampling design enabled estimation of all age-specific rates with similar precision, while age standardisation provided weightings across age groups.
Main measures for antibiotic prescribing
Antibiotic prescriptions were evaluated using product codes for antibiotics that are listed in section 5.1 of the British National Formulary (BNF),73 excluding methenamine and drugs for tuberculosis and leprosy (see Report Supplementary Material 1 and 2). Different antibiotic classes and antibiotic doses were not considered further in this analysis. Multiple antibiotic prescription records on the same day were considered as a single antibiotic prescription. Medical codes recorded on the same date as the antibiotic prescription were used to classify the indication for prescription using categories of ‘respiratory’, ‘genitourinary’, ‘skin’ and ‘other specific’ indications (see Report Supplementary Material 1 and 2). All other codes were classified as ‘non-specific’ codes. A prescription was classified as ‘acute’ if it was the first prescription in a sequence or ‘repeat’ prescription otherwise, as reported previously. 10 Antibiotic prescriptions that were not associated with medical codes and were not repeat prescriptions were classified as ‘no codes recorded’.
For each participant in the antibiotic prescribing sample, we calculated the person-time at risk between the start and the end of the patient’s record. Person-time was grouped by gender, age group and comorbidity. Age groups were from 0 to 4 years, 5 to 9 years and 10 to 14 years, and then 10-year age groups up to ≥ 85 years. Comorbidity was evaluated as either present or absent in each person-year using the ‘seasonal flu at-risk codes’, which are used to identify individuals at higher risk of infection who may benefit from an influenza vaccination,74 as reported previously. 10 Seasonal flu at-risk Read codes include medical diagnostic codes for overweight and obesity, coronary heart disease, chronic kidney disease, chronic liver disease, chronic neurological disease, chronic respiratory disease, diabetes mellitus and disorders of the immune system, as well as drug product codes for asthma therapy, corticosteroid drugs and immunosuppressive drugs. Conditions were coded as present if they were ever diagnosed up to the end of the study year. Collectively, these provide a summary measure of potential susceptibility to infection complications.
Statistical analysis
In this stage of the analysis, we estimated general practice-specific estimates for antibiotic prescribing. We analysed antibiotic prescribing in primary care between 2002 and 2017. A hierarchical Poisson model was fitted using the ‘hglm’ package in the R program, with counts of antibiotic prescriptions as the outcome and the log of person-time as the offset (Equation 1):
Estimates were adjusted for the fixed effects of gender, age group, fifth of deprivation at the general practice level, comorbidity and region in the UK. Calendar year was included as a continuous predictor, together with quadratic and cubic terms to allow for non-linear trends. Random intercepts were estimated for each general practice and each estimate represented the adjusted log relative rate for antibiotic prescribing at that practice compared with the overall mean. The proportion of antibiotic prescriptions that were associated with specific medical codes was analysed in a similar framework, with coded prescriptions as the outcome and the log of antibiotic prescriptions as the offset. General practice-specific estimates for antibiotic prescription and infection consultation coding were, therefore, adjusted for calendar year, age group, gender, comorbidity, deprivation and region.
Comparison of antibiotic prescribing in Clinical Practice Research Datalink GOLD and Clinical Practice Research Datalink Aurum
To evaluate the transferability of our findings with respect to antibiotic prescribing between general practices using the Vision and EMIS practice systems, we conducted a substudy to compare estimates using sample data from the CPRD GOLD and CPRD Aurum databases. We evaluated antibiotic prescribing for the year 2017, as this was the most recent complete year for our study. 39
Data and participants
A sample of patients was drawn from the population of all patients registered in the CPRD Aurum database (June 2019 release) throughout 2017 by randomly selecting ‘n’ patients from each stratum of general practice, gender and age group. The value of n = 9 was selected to provide a total sample size of 158,305 patients. This sampling approach ensured that each general practice was equally represented in the analysis and that age-specific rates would be estimated with equal precision. Age was calculated as the difference between year of birth and 2017. Age groups were categorised as 0–4, 5–14, 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84 and ≥ 85 years. A comparison cohort of patients was extracted from the October 2019 release of CPRD GOLD using the online interface. In this release, there were 290 general practices contributing data to CPRD GOLD throughout 2017, including 112 in England. A sample of 160,394 patients was taken by randomly selecting 30 patients from each stratum of general practice, gender and age group. Patients were required to have at least 12 months of follow-up in the database, estimated as the difference between the latest of their registration start date and 1 January 2017, and the earliest of registration end, practice last collection date, CPRD derived death date and 31 December 2017. General practices that migrated from Vision to EMIS practice systems during 2017 were excluded.
Measures
We identified all antibiotic prescriptions issued in 2017, including all drugs from section 5.1 of the BNF,73 except antituberculous agents, antilepromatous agents and methenamine. The BNF includes the following categories of antibiotics: penicillins, cephalosporins (including carbapenems), tetracyclines, aminoglycosides, macrolides, clindamycin, sulfonamides (including combinations with trimethoprim), metronidazole and tinidazole, quinolones, drugs for UTI (nitrofurantoin) and other antibiotics. For CPRD GOLD, we employed a list of 2627 antibiotic drugs that were identified from searches of the CPRD GOLD product dictionary browser. Searches were made on the drug substance name, product name, BNF chapter and BNF codes. To identify the corresponding products in CPRD Aurum, dm+d codes (i.e. the prescribing codes from the NHS dictionary of medicines and devices75) associated with individual product codes in the CPRD GOLD dictionary browser were mapped to the corresponding dm+d codes in the CPRD Aurum product dictionary browser. A more complete search of the CPRD Aurum product dictionary browser was additionally undertaken on term, product name and drug substance. We also conducted searches using approximate string matching (‘fuzzy matching’) to match the CPRD Aurum product name to the CPRD GOLD product name or drug substance name from the CPRD GOLD antibiotic code list. The ‘agrep’ command was used in the R program, using the Levenshtein edit distance as a measure of approximateness. The resulting code list was edited manually, resulting in 896 CPRD Aurum product codes. CPRD Aurum product codes are up to 17 characters in length and the ‘bit64’ package in R was employed for data formatting and management. Although more product codes were identified for the CPRD GOLD database, only 195 CPRD GOLD product codes for antibiotics and 167 CPRD Aurum product codes were recorded in 2017.
We analysed medical codes recorded on the same date as antibiotic prescriptions. Medical diagnoses were identified by searching the CPRD GOLD medical dictionary browser for Read terms and inspecting the associated Read chapter hierarchy. As previously reported, all medical codes were subsequently classified as respiratory infections, genitourinary infections, skin infections, eye infections and ‘other codes’. 10 The CPRD Aurum medical dictionary includes Read terms, Read codes and SNOMED codes. To utilise the same codes, lists developed for CPRD GOLD were subsequently mapped to CPRD Aurum by matching Read codes. Evidence of infections was searched in the patient clinical and referral records in CPRD GOLD and in the observation tables in CPRD Aurum. We evaluated whether or not any medical code was recorded on the same date as an antibiotic prescription. We then classified medical codes into ‘respiratory infections’, ‘skin infections’, ‘genitourinary infections’ and ‘other codes’.
Analysis
Age-specific rates were estimated with 95% CIs from the Poisson distribution. Age- and sex-standardised rates, and associated 95% CIs, were calculated per 1000 person-years using the 2013 European Standard Population as reference. Estimates and 95% CIs were estimated to judge whether or not differences between the databases were of clinical or epidemiological importance. Potential differences between databases were evaluated using Bland–Altman plots and 95% CIs. 76 CPRD GOLD general practices in England were analysed as a subgroup.
General practice-level analysis of serious bacterial infections
Incident cases of serious bacterial infection were evaluated in the January 2019 release of CPRD for the years 2002–17, with the CPRD denominator providing the person-time at risk. The mean duration of follow-up was 6.9 years. Serious bacterial infections were selected for study from review of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10),77 from the Read code classification78 and through discussion with the research team. The final list of conditions is summarised in Table 2 and includes bacterial infections of the central nervous system (CNS), bacterial infections of the cardiovascular system (CVS), kidney infections, lung abscess and empyema, mastoiditis, osteomyelitis, PTA, resistant infections and Clostridium difficile (C. difficile), sepsis and septic arthritis. Further details of case definitions are provided in Report Supplementary Material 1 and 2. Incident events were the first record for each type of serious bacterial infection in a patient > 12 months after the start of the patient record. However, a single patient might have first episodes of more than one type of bacterial infection. Possible recurrent events in the same patient were not evaluated further because, in electronic health records, it may not be possible to distinguish new occurrences from reference to ongoing or previous problems.
| Group | Number of codes | Number of first events | Five most frequent conditions (number of first events 2002–17) |
|---|---|---|---|
| CNS infection | 30 | 576 | Epidural abscess (117), cerebral abscess (112), brain abscess (79), intraspinal abscess (49) and drainage of abscess of subdural space (44) |
| CVS infection | 24 | 1697 | Acute and subacute endocarditis (594), bacterial endocarditis (276), subacute bacterial endocarditis (270), acute endocarditis NOS (166) and acute bacterial endocarditis (114) |
| Kidney infection | 22 | 30,827 | Acute pyelonephritis (19,284), pyelonephritis unspecified (7115), infections of the kidney (1670), acute pyelitis (1008) and pyelitis unspecified (745) |
| Lung abscess/empyema | 24 | 2932 | Empyema (2314), abscess of lung (149), abscess of lung and mediastinum (139), thorax abscess NOS (68) and pleural empyema (56) |
| Mastoiditis | 10 | 1970 | Mastoiditis and related conditions (1293), mastoiditis NOS (487), acute mastoiditis (146), acute mastoiditis NOS (31) and abscess of mastoid (27) |
| Osteomyelitis | 65 | 4921 | Acute osteomyelitis (3297), unspecified osteomyelitis (678), unspecified osteomyelitis of unspecified site (284), osteomyelitis jaw (78) and unspecified osteomyelitis NOS (75) |
| PTA | 6 | 11,338 | Quinsy (8611), PTA – quinsy (1748), O/E quinsy present (654), drainage of PTA (232) and drainage of quinsy (226) |
| Resistant infections and C. difficile | 31 | 42,185 | C. difficile toxin detection (20,175), meticillin-resistant Staphylococcus aureus positive (9914), C. difficile infection (6397), meticillin-resistant S. aureus (4303) and meticillin-resistant S. aureus carrier (1017) |
| Sepsis | 100 | 39,059 | Sepsis (23,149), septicaemia (6204), urosepsis (4646), biliary sepsis (1233) and Clostridium infection (576) |
| Septic arthritis | 41 | 4254 | Septic arthritis (3649), pyogenic arthritis (184), arthropathy associated with infections (172), knee pyogenic arthritis (52) and staphylococcal arthritis and polyarthritis (39) |
Serious bacterial infections were analysed as the outcome (Equation 2):
The antibiotic prescribing level for each general practice was included as a predictor using the general practice-specific estimates from Equation 1. These estimates initially had a mean of zero and a standard deviation of 0.19, which is consistent with an adjusted relative rate of antibiotic prescribing of 1.21 for a general practice prescribing 1 standard deviation above the mean. Estimates were, therefore, standardised to give the change in serious bacterial infection for a 20% relative increase in antibiotic prescribing rate at a practice, as this represents a change of approximately 1 standard deviation. A 20% change generally represents a substantial change in antibiotic prescribing. We also estimated the change in serious bacterial infection for a 20% relative increase in the proportion of antibiotic prescriptions, with specific medical codes recorded at a general practice. Models were adjusted for age group, gender, region, deprivation fifth and calendar year (including quadratic and cubic terms for calendar year), with the log of person-time as offset (see Equation 2). The results were visualised using forest plots.
Sample size considerations
At the design stage, we envisaged that there might be 68 million person-years of follow-up divided into four quartiles. The incidence of outcomes might be < 1 per 100,000 per year for intracranial abscess. 23 Comparing the lowest with the highest quartiles, there would be 80% power to detect relative risks of 0.71 for the rare outcome of intracranial abscess, with higher relative risks detectable for more frequent outcomes.
Modelling study
Decision tree
To evaluate the probability of a serious bacterial infection following an infection consultation in primary care we constructed a decision tree (Figure 3). 79 An individual developing an infection may decide to consult their general practice or not. If they consult, they then may be prescribed antibiotics and subsequently they may develop a serious bacterial infection. We used estimates from CPRD data analysis to populate the decision tree with empirical estimates for probabilities, as outlined in Table 3. We used Bayes’ theorem to estimate the probability of a serious bacterial infection following an infection consultation if antibiotics were prescribed or if antibiotics were not prescribed. We estimated the number needed to treat (NNT) (i.e. the number of antibiotic prescriptions required to prevent one serious bacterial infection) as the reciprocal of the difference in probability of sepsis with and without antibiotics. We obtained central estimates and 95% uncertainty intervals (UIs) from 10,000 random draws from the beta distribution. 80 All estimates were stratified by gender and 10-year age group. For the population aged ≥ 55 years, we also stratified by frailty category. In addition, we evaluated subgroups of common infections, including RTIs, skin infections and UTIs.
FIGURE 3.
Decision tree showing the probability of a patient consulting for an infection, being prescribed an antibiotic at that consultation and developing serious bacterial infection (see Table 3 for explanation of abbreviations). AB, antibiotic; SBI, serious bacterial infection.
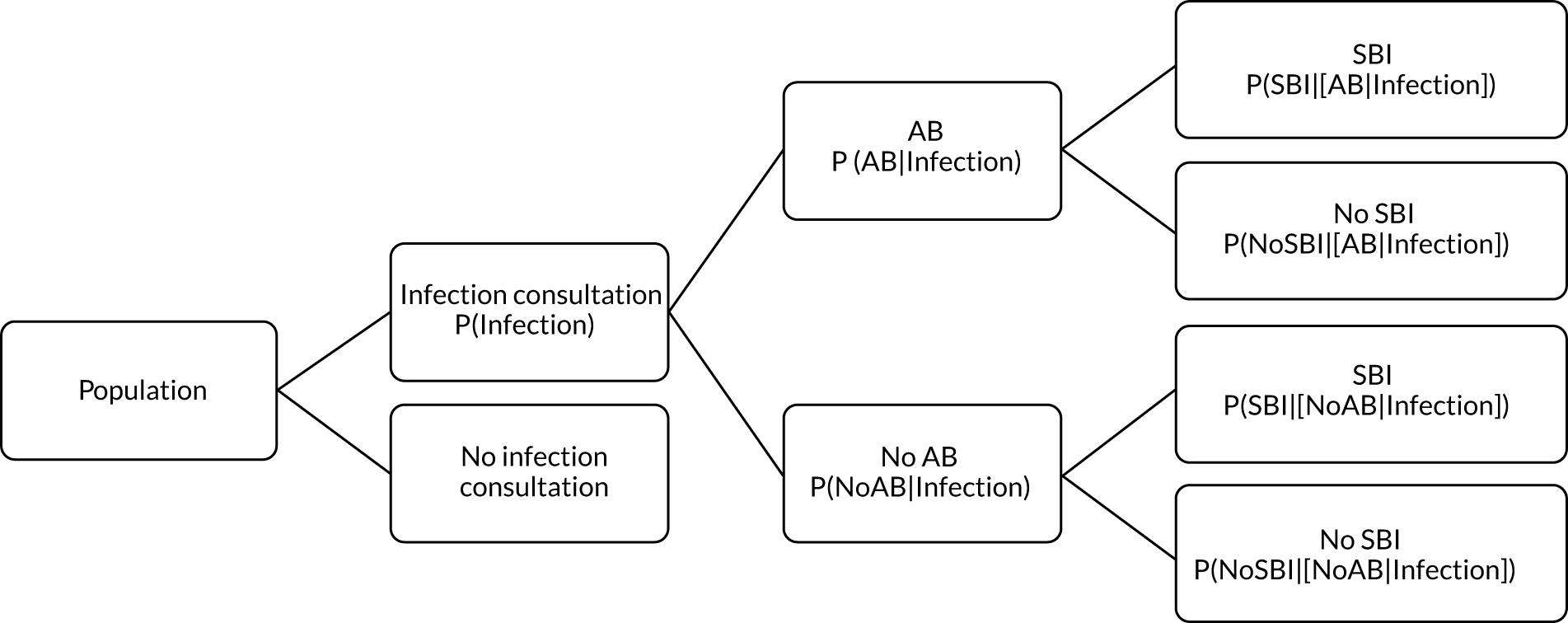
| Term | Explanation | Data source |
|---|---|---|
| P(Infection) | Probability of a person consulting with infection in a 30-day period | From infection consultation rate per 30 days in sampled data set from CPRD |
| P(AB|Infection) | Probability of receiving an AB prescription on the same date as an infection consultation | From proportion of infection consultations with ABs prescribed in sampled data set from CPRD |
| P(SBI) | Probability of SBI per 30 days | From incidence of SBI from entire registered CPRD population |
| P(Infection|SBI) | Probability of patients with SBI, having consulted for an infection in 30 days preceding their sepsis diagnosis | Proportion of SBI cases with previous infection consultation, calculated from entire registered CPRD population |
| P(SBI|Infection) | Probability of SBI in the 30 days following an infection consultation | P(Infection|SBI)P(SBI)P(Infection) |
| P(SBI|[AB|Infection]) | Probability of SBI, having consulted for an infection and received an AB prescription | P([AB|Infection]|SBI)P([SBI|Infection])P(AB|Infection) |
| P(SBI|[NoAB|Infection]) | Probability of SBI, having consulted for an infection and not received an AB prescription | P([NoAB|Infection]|SBI)P([SBI|Infection])P(NoAB|Infection) |
| NNT | The number of additional AB prescriptions required to prevent one SBI | 1P(SBI|[AB|Infection])−P(SBI|[NoAB|Infection]) |
Data sources for decision model
Case definitions
We employed the same case definitions as outlined above. However, we refined our definition of sepsis to include only those conditions considered relevant as complications of infection consultations in primary care. This led to the omission of 23 Read codes, with 77 Read codes remaining to define sepsis (as outlined in Report Supplementary Material 1 and 2). In individual patient analyses, we also adopted a less inclusive approach to the definition of infection consultations, including ‘UTIs’ rather than ‘genitourinary tract infections’ (GUTIs), with further details provided in Report Supplementary Material 1 and 2. For the estimation of person-time, the current registration date was advanced by 12 months because only incident events 12 months after the patient’s start date were included in analyses.
Evaluation of frailty
We used Clegg et al. ’s65 electronic frailty index (eFI) to evaluate frailty level. The eFI includes 36 deficits that are evaluated as present or absent based on Read-coded electronic health records. Patients were classified as being ‘non-frail’ or having ‘mild’, ‘moderate’ or ‘severe’ frailty based on the number of deficits recorded. We evaluated frailty for each patient in each calendar year of the study81 to provide a frailty estimate for the index year of each sepsis episode. We also estimated consultation rates and antibiotic prescribing proportions by frailty category for the antibiotic prescribing sample. Given that full electronic health record data were not available for the entire CPRD GOLD denominator, we allocated person-time to frailty categories, using the proportion in each frailty category that we observed in the antibiotic prescribing sample. Although the concept of frailty may be applied at any age, frailty was evaluated from only age ≥ 55 years because most patients under the age of 55 years were classed as ‘non-frail’ or as having only ‘mild’ frailty (see Report Supplementary Material 3, Supplementary Table 2).
Outcome events
We ascertained serious bacterial infection events from the entire registered population of CPRD GOLD because these are generally rare events. Incident cases of sepsis were obtained from CPRD GOLD for the years 2002–17, with person-time at risk providing the denominator. The start of the patient record was the latest of 1 year after the patient’s current registration date, the date that the general practice began contributing up-to-standard data to CPRD GOLD or 1 January 2002. The end of the patient’s record was defined as the earliest of the end of registration, the patient’s death date or 31 December 2017. The mean duration of follow-up was 6.9 years. Serious bacterial infection events were evaluated using Read codes recorded in patients’ clinical and referral records. 39 There were 77 Read codes for sepsis and septicaemia but the four most frequent codes accounted for 92% of events, including ‘sepsis’ (two codes), ‘septicaemia’ and ‘urosepsis’ (see Report Supplementary Material 1 and 2). We included the incident first events in further analyses. Recurrent events in the same patient were not evaluated further because it may not always be possible to distinguish in electronic health records new occurrences from reference to ongoing or previous problems.
For each serious bacterial infection, we evaluated whether or not a consultation for a common infection was recorded within the preceding 30 days. We employed a 30-day time window with the intention of capturing data for acute infections and their short-term outcomes. We identified consultations for RTIs (including upper and lower RTIs), skin infections and UTIs (including ‘cystitis’ and uncomplicated ‘UTIs’ only) because these are the most important groups of conditions for which antibiotics are prescribed in primary care (see Report Supplementary Material 1 and 2). 10 We evaluated Read codes in patients’ clinical and referral records to identify consultations associated with common infections. We also evaluated whether or not an antibiotic prescription was issued during the 30 days preceding a sepsis event, either on the same date as an infection consultation or on a different date (see Report Supplementary Material 1 and 2). 10,39
Sensitivity analyses
In sensitivity analyses for the outcome of sepsis, we evaluated whether or not use of a 60-day time window gave different results from a 30-day time window. The primary analysis reported data for a 16-year period, but the incidence of sepsis has been increasing. 24 We repeated the analysis using only data for 4-year periods from 2002–5 to 2014–17, to evaluate whether or not estimates differed from the whole period from 2002 to 2017. We also investigated whether estimates differed if sepsis diagnoses recorded in Hospital Episode Statistics (HES) or as causes of death on mortality certificates. The sample for linkage was obtained from CPRD (Linkage Set 16). The linked sample included data for 378 English general practices, with 5,524,983 patients providing primary care electronic record data linked to HES and mortality statistics. We searched for ICD-10 codes for sepsis and septicaemia. We included primary diagnoses from HES admitted patient care records and all mentions of sepsis in mortality statistics data. We repeated analyses using primary care electronic health records alone, primary care electronic health records with linked HES data or primary care electronic health records with linked HES and mortality data.
Data linkage
Study population and data sources
The study employed the UK CPRD GOLD database. The CPRD GOLD is a primary care database of anonymised electronic health records for general practices in the UK. The high quality of CPRD GOLD data is well established. 71 CPRD GOLD has a coverage of some 11.3 million patients, including approximately 7% of the UK population, of which it is broadly representative in terms of age and sex. 70 Consenting practices in England participate in a data linkage scheme. 82 Approximately 74% of all CPRD GOLD practices in England are eligible for linkage. Linkages are available for the HES and mortality registration data from the Office for National Statistics (ONS). HES admitted patient care data include admission and discharge dates and diagnostic data coded using the ICD-10. Mortality registration data include information on the date and causes of death coded using ICD-10. ONS identifies one underlying cause of death and secondary causes of death, including up to 15 additional causes of death. Linked area-based measures of deprivation include the Index of Multiple Deprivation (IMD) and are based on a weighted profile of indicators. 83 We employed deprivation for the general practice postcode for this study because of the low proportion of missing values. The protocol was approved by the CPRD Independent Scientific Advisory Committee (protocol 18-041R).
Main measures
We included patient records between 1 January 2002 and 31 December 2017. The start of the patient record was the later of the patient registration date or the date that the general practice joined CPRD. The end of the patient record was the earliest of the last data collection date, the end of registration or the date of death. We evaluated the first records of sepsis > 12 months after the start of registration in primary care electronic health records as a primary diagnosis in HES or sepsis as any mentioned cause of death in mortality records. In UK primary care records, diagnoses recorded at consultations or referrals to or from hospitals were coded, at the time of this study, using Read codes. We identified sepsis records using a list of 77 eligible Read codes. Incident episodes of sepsis in CPRD were recorded using 55 Read codes, with four codes accounting for 92% of events, including ‘sepsis’ (two codes) (64%), ‘septicaemia’ (18%) and ‘urosepsis’ (10%). In HES and death registry records, sepsis diagnoses and sepsis deaths were defined using 23 ICD-10 codes for sepsis. In HES records, we evaluated the primary diagnosis, which accounts for the majority of the length of stay of the episode, with other diagnoses being referred to as comorbidities. 84 Incident diagnoses of sepsis in HES were coded with 20 ICD-10 codes, with three codes accounting for 89% of events, including ‘sepsis, unspecified’ (72%), ‘sepsis due to other Gram-negative organisms’ (13%) and ‘sepsis due to Staphylococcus aureus’ (5%). In mortality data, we included all mentioned causes of death because sepsis may be part of a sequence of morbid events and not always be an underlying cause of death. 24 ‘Sepsis, unspecified’ accounted for 93% of causes of death among those in the ONS death registry with sepsis as any mentioned cause of death.
Analysis
Incident sepsis events were identified for each data source. We calculated person-time at risk from the start to the end of the patient record. Person-time was grouped by gender and age group from 0 to 4 years, 5 to 9 years and 10 to 14 years, and then 10-year age groups up to ≥ 85 years. Incidence and mortality rates were age standardised using the European Standard Population for reference. We searched for concurrent events across data sources using a 30-day time window. We calculated age-specific incidence rates using primary care electronic health records and then adding HES records, mortality records or both. We fitted a logistic regression model to evaluate associations of gender, age group, fifth of deprivation and period of diagnosis with concurrent sepsis recording. All data were analysed in R.
Sensitivity analyses
To consider recurrent sepsis events, we conducted a sensitivity analysis using CPRD and HES where incident events were first sepsis records during each calendar year during the study period. We also evaluated the effect of extending the time window for concurrent events from 30 to 90 days.
Development and testing of the Shiny app
Design of study
The study used a qualitative design involving both semistructured and ‘think-aloud’ interviews with six GPs. The study was designed and conducted by LM in consultation with the study team. Face-to-face interviews were conducted online using Zoom (Zoom Video Communications, Inc., San Jose, CA, USA). Interviews lasted approximately 40 minutes. All interviews were recorded using Zoom and fully transcribed. The evaluation was constrained by the circumstances of the research during the COVID-19 pandemic and it was possible to conduct a preliminary study only.
Participants
Participants for early user testing were six GPs from practices across London, Southampton and Oxfordshire. Participants included GPs who were also academic researchers and GPs who worked in a practice setting only. Participants were recruited initially from members of the study team who were also practising GPs. In addition, two GPs who were colleagues of study members also agreed to take part following an invitation from the study team. This part of the research was conducted during January–July 2020 and its scope was limited by the circumstances of the research.
Procedure
General practitioners were first shown the web pages using the screen share function on Zoom. Think-aloud interviews (Box 3) were conducted at this point to study the reactions to the various features of the web pages. GPs were encouraged to discuss their views, thoughts and perceptions of each section as it was demonstrated by the interviewer. This technique allowed GPs to explore the various drop-down menu options and openly discuss the tool as they wished, but also ensured that opinions were obtained for all sections of the pages. Following this, a semistructured interview (Box 4) was conducted, drawing on our previous experience of developing electronic interventions. 31,85 The interview was designed to identify factors likely to influence successful implementation of the tool and discover likely responses to the proposed sections to further inform development and aid refinement of the web pages. GPs were asked questions regarding their views, expectations, acceptability and feasibility of pages. The semistructured interviews were used to explore and discover issues that may be related to the web page content and usage.
(Web pages presented on screen.)
Each section is displayed and explained.
Tell the participant that they can go back to view or explore any feature again.
Ask the participant to say aloud what they are thinking and feeling about each feature (content and functions).
To ask during interview-
Question why certain choices or comments are being made if a full description is not given.
-
Ask for comments on any features that were not discussed.
-
How would you feel about using this tool in practice?
-
How do you think these any of features could be improved?
(Prompts: title, list of problems, order of conditions, clear to use and order of presentation.)
Graphs-
How would you feel about using these graphs during a consultation?
-
How would you improve the presentation of these graphs?
(Prompts: clear to see how to access these/design.)
Design features-
Overall, how do you feel about the design of these pages?
-
How would you improve the design of these pages?
(Prompts: colour, areas, titles, font and functions clear.)
-
What would be the best way to access these pages during a consultation?
-
Would you be happy to share these with pages with patients? (Which parts?)
-
Do you feel that information presented is easy to interpret? (If not, which parts?)
-
Do you think that the information would be easy for other GPs to interpret?
-
Do you think that these pages could be useful in practice (could they be used during a consultation?)
-
Do you think that the pages could help to inform decision-making?
-
Do you have any further comments on the pages?
Analysis
Inductive thematic analysis was conducted on all transcripts to determine likely responses to the web pages and identify factors involved in the decision to use the tool. Analysis began after the first interview had been conducted and continued throughout data collection for all interviews conducted. Interviews were read in detail and re-read, and then following this immersion in the transcripts commonly occurring patterns and prominent themes were identified in the data and labelled with codes. Each code label referred to the operationalisation of the theme content. A coding manual was developed containing the label, a definition of each theme, positive examples from the interview transcripts and possible exclusions for each code. The coding manual was refined as more data became available and transcripts were re-read. The continuing process involved themes being linked, grouped, moved, re-labelled, added and removed to produce a set of themes and coding manual that adequately fit and thoroughly explained the data.
Public and patient involvement
The purpose of PPI in the project was to inform all stages of the research of patient and service user perspectives and concerns. A PPI group was formed that included patients and service users recruited from the NIHR Biomedical Research Centre at Guy’s and St Thomas’ Hospitals and from general practices in South London. The group included seven PPI members: five women and two men of diverse ages and ethnic origins. Most PPI members had experience of consulting with infections and some also had experience of antibiotic-resistant infections. Meetings were held at intervals during the project. Preliminary findings from the research were presented, and members were invited to discuss emerging findings and themes and comment on their relevance. The following chapters report discussion and reflection on the feedback received at the PPI group meetings.
Chapter 4 Patient expectations and experiences of antibiotics in the context of antimicrobial resistance
Adapted with permission from Boiko et al. 86 This is an open access article distributed in accordance with the Creative Commons Attribution (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Summary
This part of the research investigated contemporary patient expectations and experiences of antibiotic prescribing through semistructured interviews with patients who recently consulted for infections in two English regions. The patient accounts reflected improved public knowledge. Although antibiotics were perceived to be much-needed medicines that should be prescribed when appropriate, patient experiences were nuanced and detailed with knowledge of antimicrobial resistance and the side effects of antibiotics. The research found that patients are seeking care and antibiotic treatment in reflexive, informed ways, with dependency between patient and practitioner expectations. Ensuring that present and future patients are informed about potential benefits and harms of antibiotic use will contribute to future antimicrobial stewardship.
Background
Public awareness of antibiotic resistance and the need for more judicious use of antibiotics is increasing, but inappropriate use of antibiotics remains widespread. 5,23 Older studies have ascribed a prominent role of patient influences on antibiotic prescribing, with many studies stressing the view that prescribers may be responsive to patient expectations for antibiotic treatment. 87 This ‘patient influence’ factor has been identified in most systematic reviews. 87 Estimates from patient surveys suggest that patients’ positive expectations for antibiotics are substantial, but have varied between studies. 88–94 Family physicians may assume that patients consulting for infections want antibiotics,95 but primary care clinicians can overestimate the extent to which patients are seeking and expecting antibiotic prescriptions,95,96 especially for parents of young children. 32 There is consistent evidence that GPs are more likely to prescribe antibiotics when their patients are perceived to be expecting them. 92,97–99 A systematic review found a generally positive association between physician perceptions of patient expectation and antibiotic prescription,100 but some studies find evidence of a negative association between expectation and prescription,88 with evidence of inconsistency between physicians’ perceptions and patients’ desire for antibiotics. It is also well established that prescribing antibiotics increases the likelihood that patients will consult in future illness episodes,14 raising the possibility that expectations are a consequence and not a cause of antibiotic prescribing.
Relationships between patients and primary care providers play a major part in antibiotic-seeking and antibiotic prescribing behaviours. A qualitative study in the UK found that doctors prescribed antibiotics to maintain good relationships with patients, with potential patient benefits outweighing the less tangible community risks from antimicrobial resistance. 98 However, patient expectations are seldom made explicit during consultations. Although a high proportion of patients may want antibiotics and expect to be given a prescription, only a minority ask directly for antibiotics. 97 Some studies confirm that meeting patient expectations is associated with greater patient satisfaction, but other research suggests a more nuanced interpretation. A mixed-methods study in Australia demonstrated that, even though parents consulting with their children wanted antibiotics, satisfaction with their GP visit was not dependent on solely receiving antibiotics. 94 In a qualitative study of parents consulting with GPs in four European countries, parents’ accounts revealed that a trusting and open relationship with the clinician, in which parents felt comfortable to ask questions, challenge and discuss decisions, led them to feel generally satisfied with consultations and accept clinicians’ decisions of whether or not to prescribe antibiotics. 101
In recent years, there have been concerted efforts from scientists, clinicians and policy-makers to publicise and address the growing threat of antimicrobial resistance both in the UK and worldwide. 3,102,103 In this context, it is timely to revisit patient beliefs, expectations and experiences of antibiotics and of antimicrobial resistance. Recent systematic reviews have included studies that may antedate current increased concerns for antimicrobial resistance. 97,98 This research aimed to address a need for additional qualitative investigation to understand contemporary patient perspectives on antibiotic prescribing in this era of antimicrobial resistance.
Results
In total, 33 patients agreed to participate. The interviews with two patients were discarded (one involving a parent interview and one in which the patient did not consult for an infection). Out of the 31 patients who constituted the final sample, 26 patients were interviewed face to face in patients’ homes and five patients were interviewed over the telephone (Table 4). We summarise the results under the headings of the five main themes that were identified in the thematic analysis. Analysis did not identify systematic differences according to metropolitan or rural location, nor according to mode of interview completion.
| Characteristic | Frequency (n) |
|---|---|
| Age (years) | |
| 20–29 | 2 |
| 30–39 | 3 |
| 40–49 | 4 |
| 50–59 | 3 |
| 60–69 | 4 |
| 70–79 | 9 |
| 80–89 | 5 |
| 90–99 | 1 |
| Gender | |
| Female | 24 |
| Male | 7 |
| Ethnicity | |
| White (British) | 25 |
| White (other) | 3 |
| Black | 2 |
| Asian | 1 |
| IMD | |
| High | 12 |
| Medium | 9 |
| Low | 8 |
| Region | |
| Urban | 22 |
| Shire town | 9 |
| Type of infection | |
| Respiratory | 16 |
| Urinary | 11 |
| Skin | 4 |
Beliefs about antibiotics and antimicrobial resistance
Antibiotics emerged as trusted medicines that had widespread use. The descriptors used by the interviewed patients ranged from ‘magic answer’ to ‘sledgehammer’ treatment. Those who referred to antibiotics as a ‘magic’ pill were often older females with recurrent UTIs and with expectations for apparently appropriate prescribing. On a whole, the most common belief among the interviewees was that antibiotics should be prescribed and taken when necessary. The patients’ concerns were rather about ‘finding the right one for what infection you have at the time and making sure that you’re going to be safe’ (interview 13, female, chest infection). There was also recognition of the need for better scrutiny in prescribing, which highlighted the complexity of decision-making concerning safe antibiotic treatment.
The interviewees spoke about different thresholds of illness and about reducing the use of antibiotics. There were accounts of the decreased impact of antibiotics and of antimicrobial resistance by informed patients, especially by younger and more educated patients:
Tell me more about antibiotics. What do you know about antibiotics?
. . . the effectiveness of some of our standard treatments is decreasing rapidly. And I believe that a lot of that is to do with inappropriate usage, both inappropriate prescribing which I have witnessed myself. But then also inappropriate use by patients of antibiotics.
Or in another example:
Do you want to add anything else in terms of your experience with antibiotics?
I find it alarming that we may be getting towards the end of the road with antibiotics . . . They’re finding ways of compensating for the overuse of antibiotics. And clearly there are issues with the pharmaceutical industry as to how much they’re prepared to invest in developing new antibiotics . . . And I don’t want to get political about it but there needs to be some sort of disentanglement of the profit motive, which I understand and the service to the general public.
Expectations of antibiotics
Interviewees’ expectations varied. Approximately half of the interviewees expressed a wish for an antibiotic, but the other half had undifferentiated expectations of help in getting better when attending for the consultation. Those with recurrent UTIs wanted antibiotics based on their previous experiences:
Did you have expectations of specific treatments when you went to see the GP?
While I was waiting for the antibiotics, I also tried stuff myself from the chemist. And also, drunk the cranberry juice, which was no good. I knew that I needed antibiotics. It seems for me if I get cystitis it starts, and it comes on really quickly.
Those with chest infections had thought about the need for antibiotics, but preferred to leave the decision concerning antibiotic treatment to health-care professionals:
When you went to see the doctor, did you expect a particular treatment or prescriptions?
I did expect that if it is something on the lungs, that I would be given antibiotics. But I didn’t push for them or anything. I really went there to, to see what it is. But I wasn’t particularly surprised that they heard the noise on the lungs.
Patients interviewed often referred to antibiotics as something that would ‘shift’ their illness, but also as a symptomatic cure and something ‘to boost the immune system with’. This female patient held a radically different view:
OK, so, you didn’t expect any particular treatments or antibiotics in particular?
I feel bad to say this to the doctor, but I’m quite anti the use of antibiotics. I think I’ve read too many horror stories about overuse of antibiotics. So, I avoid them at all costs. And I definitely wouldn’t have even considered antibiotics unless the doctor had mentioned them.
Approximately half of the interviewees, and those who were not consulting for UTI, had less differentiated expectations:
Did you have any expectations of specific treatment when you came with the symptoms?
I didn’t have any expectation of treatment. And, in actual fact, I remember thinking, ‘Oh do I really need antibiotics?’. But I don’t know, I guess with kind of almost pre-programmed to say, ‘OK, if that’s what you want to give me, that’s what I’ll take’. So, I didn’t really have any expectations. To be honest, I hadn’t even considered that I may have tonsillitis.
In terms of how interviewees perceived prescribers’ expectations, there was a difference in understanding of the prescribers’ mindset. Several interviewees held a view that prescribers’ expectations were contingent on patient expectations and so that they brought up an association with patient pressure: ‘. . . they don’t want to prescribe antibiotics till they see that you are really, really desperate or you need it’ (interview 6, female, tonsillitis). Prescribers’ expectations were in these cases dependent on patients’ wishes as well as on symptoms. Others believed that patient expectations might not influence the professional’s decision, which might depend only on clinical findings. Such views were based on critical reflection:
What do you think doctor thought of, about your expectations?
I don’t think a doctor, any GP in the UK takes into consideration a person’s expectations. They give, they prescribe based on the symptoms that you present. Not necessarily your expectations.
Experience of taking antibiotics
The interviewees had different exposure to antibiotics. Some interviewees were prone to recurrent infections and had been prescribed antibiotics more than once in recent months; whereas others had received an occasional prescription for antibiotics. Most interviewees described days and weeks of experiencing illness before they consulted with a clinician. With the exception of one participant, there had been a sense of welcoming antibiotic treatment; for example, according to a patient with chest infection, ‘antibiotics, without being dramatic, saved my life’ (interview 1). At the same time, many patients were reflexive about the role of antibiotics in coping with the ailments. For example, a patient with tonsillitis questioned the appropriateness of prescribing:
So, it seems like it cleared everything off? Was it efficient?
To be honest, I remember starting to feel like my throat was feeling better within 12 hours of taking the antibiotics. And, at that stage, I started to think, OK, actually maybe I didn’t need antibiotics because this has cleared up very quickly and, in hindsight, kind of thinking about it, thinking, well there’s no way the antibiotics would work that quickly.
The interviewees had a range of various experiences of past antibiotic treatment. Patients with a history of infectious diseases shared positive accounts of antibiotics in general:
So, obviously were you hopeful for the antibiotic treatment to be the best possible course of action when you came with chest infection?
I’ve had chest infections before. They’ve always cleared up and you know I’ve never sort of thought it could lead to anything significantly worse. I’ve always been confident that whatever antibiotic I was given would do the job, you know.
Experience of antimicrobial resistance and side effects
Approximately one-quarter of the patients spoke about their experience of antimicrobial resistance. The accounts were full of frustration and confusion because either the antibiotics had not worked or the first-line treatment had not worked. This was especially true for patients with UTIs, but also for patients with tonsillitis. Several patients experienced up to three to five episodes of UTI per year and found it hard to tolerate its recurrent nature:
Did you mention you had a bad reaction to that as well?
I felt really ill in the morning. I didn’t know why, and I had to drive somewhere. And oh, and really, really had to go to bed. I felt so bad. And then on the Monday as I say, I had a call from the surgery to say they’d discovered that it was resistant to that and they changed it . . . I have heard antibiotics can turn toxic in you if they’re not the right one, can’t they?
Antibiotics were sometimes associated with mild to serious side effects; for example, an anaphylactic allergic reaction was coupled with resistance in the following account:
Did you say you were allergic to one particular antibiotic?
I was allergic, yes.
How did you know that?
I was given penicillin that was in 2000 and I had an anaphylactic shock. I was rushed into hospital and I had to spend a night in the emergency room and given steroids for 5 days because of that. So, then I couldn’t take penicillin, I couldn’t take erythromycin and I became resistant to doxycycline and there were other ones that I started to become resistant to, that didn’t work.
Several interviewees confirmed that the most common side effects were nausea, stomach upset, vomiting and thrush. The interviewees spoke about compensating with probiotics to resolve digestive side effects, but continued with antibiotics. Where discontinued or prescribed different antibiotics, this was in the case of more significant side effects, such as liver derangement or shortness:
So, have you been, having any side effects because of antibiotics?
But when I was prescribed it a second time, within 24 hours I was getting very short of breath . . . it was clear to me that this was having an effect on my breathing. So, I went straight back to the GP and then they put me on, on a different antibiotic.
Experience of consultation
The patients who were interviewed reported predominantly positive experiences of consultation with a prescriber who they had recently seen for infection. With the exception of a very few rushed encounters, when there had not been time for asking/answering questions, most consultations were described as patient centred:
And how did the consultation go?
It was good. She [prescriber] was very thorough. She understood that 3 weeks of suffering was quite a long time, so she was understanding. So, yes, she was thorough, she was very sympathetic, and I felt silly going about something that was really, I thought just a cold. But she said she could tell that I was in discomfort and she listened to my symptoms.
Meanwhile, the patients were concerned with the issue of the appropriateness of antibiotics use and understood the uncertainty and complexity associated with it. They appreciated both diagnostic uncertainty and how diagnostic uncertainty has been resolved in real-time consultations. A patient perceived the complexity of weighing clinical decision-making against the risks to patients’ health:
Who should be making decisions on antibiotics, the doctors or the doctors and patients or maybe patients?
. . . and the doctor would have to be able to take a closer look and to really be able to assess whether he can take the risk of not giving the antibiotics or not, because maybe it’s, it’s just finding a balance of hitting the riskiest bit of this illness first and then dealing with the side effects as you go along.
It was also apparent that the patients who were interviewed reflected on patients’ collective state of mind and role in pressurising a prescriber:
I’m sure many people lie just to get antibiotics.
Interview 19, female, tonsillitis
There were, however, consultations in which shared decision-making took the form of expectation elicitation. Reflexivity emerged in these cases where prescribers directly elicited patient expectations. Two patients admitted being overtly asked about their agreement to use antibiotics:
So, to begin with can I ask you what’s your recent consultation with GP? What was the problem?
. . . she [prescriber] said to me would I mind taking some antibiotics for that. And I said if she felt that they would help then yes, I did. It was a 5-day course . . . Yes, that was the first time actually that I’ve sort of been asked rather than told.
Patient and public involvement
The process of developing subthemes and themes was discussed at a PPI meeting. The research team presented an overview of the interview study, including the objectives and design. The main findings with respect to patient understanding of antibiotics, patient expectations and patient experience of side effects were presented using illustrations from the interview extracts. The meeting discussed selected quotes from patient interviews and the PPI members were asked for their interpretation. The feedback received was included in the final interpretation. The discussion focused on patient pressures for antibiotic prescriptions, trust and communication with GPs. There was discussion of patient education and implied criticism of patient-led consultation if wants and expectations are inconsistent with clinical need. There was also discussion of how dissemination of the findings might contribute to patient education with respect to antibiotic-seeking behaviour.
Discussion
Main findings and comparison with the literature
Participants perceived antibiotics to be much-needed medicines that should be prescribed when appropriate. Expectations for antibiotic treatment were often conditioned on previous experiences. However, past experiences were not restricted to successful treatment of infections, but also included experience of antibiotic-resistant infections, antibiotic drug side effects and inappropriate prescribing. Patients’ views were also informed by genuine concern about antimicrobial resistance. These lay accounts appeared to reflect the contemporary medical ideas of ‘precision’ or ‘personalised’ medicine, which are represented in the slogan of the ‘right drug for the right patient at the right time’. 104
Consistent with other studies,93 participants’ knowledge about side effects was not associated with their expectations of antibiotics. The concern about and experience of antimicrobial resistance found in our sample contradicts evidence from a review of general public attitudes, which reported low awareness of antimicrobial resistance. 105 We found that around one-quarter of the sample experienced antimicrobial resistance in one form or another, and these participants were more sceptical about antibiotics. The accounts of those who experienced antimicrobial resistance were full of frustration and confusion because either the antibiotics had not worked or the first-line treatment had not worked. Likewise, in the qualitative arm of their study, Gaarslev et al. 92 established a growing number of patients who knew that antibiotics did not kill viruses and who agreed that taking antibiotics when not needed means that they may not work in the future. Although compliant with antibiotic treatment, participants in our study raised important questions of the right antibiotics being prescribed at the right time. Their accounts of illness suggested explicit and informed choices behind the experiences of both treatment and consultation for infections.
Participants tried to justify the prescription by relating to prescribers’ decision-making processes and their own and prescribers’ expectations. Some interviewees believed that prescribers seek to meet patient expectations who, in their turn, acted on an adherence principle. We suggest that adherence is often determined by complementarity of expectations. Although we did not attempt to quantify findings, we established that the rate of patients expecting antibiotics was relatively high (half of the participants). This finding, however, can be explained by a high proportion of patients who consulted for UTIs: an often recurrent health condition requiring appropriate antibiotic treatment. Our data also showed that patients could form expectations of expectations, trying to read the prescribers’ intentions and reflect on the dependency between what prescribers and patients wanted. Patients were also aware of the possibility of patient pressure. A patient acknowledged that other patients’ intentions may be based on exaggeration to receive a prescription. These accounts evidenced the observation that practitioners’ perceptions of patient expectations matter, rather than patient expectations per se.
Where participants were debating the appropriateness of prescribing, it was associated with informed choice and shared decision-making. Information sharing is a prerequisite to shared decision-making and it appears that patients want information about their medical condition and treatment options without necessarily being responsible for making treatment decisions. 106,107 In the medical sociological literature on late modernity, it is argued that individuals experience self, the body and the social and physical worlds with a high degree of reflection, questioning, evaluation and uncertainty. 108 It is assumed that the ‘consumerist’ patient and the ‘reflexive’ actor ‘both are understood as actively calculating, assessing and, if necessary, countering expert knowledge and autonomy with the objective of maximizing the value of services such as health care’. 108 Recently, it has been shown that when informed about individual and social consequences of antibiotic overuse, patients may be more receptive to antibiotic prescription limits. 109 This evidence suggested that the patient role involves staying informed about the issue of antibiotic use and considering potential benefits and harms when making decisions about antibiotic use. We found that informed patients (of antibiotics and the associated risks) displayed more satisfaction with the consultation. An interview study in Australia110 also established that most consumers would accept the GP’s decision not to prescribe an antibiotic if it was clearly explained. Therefore, the re-emergence of the informed patient is inevitable in the era of antimicrobial resistance, in which expert knowledge of antibiotics is broadcast through the media and public health campaigns.
The recent research demonstrated that patients were unwilling to follow the prescriber’s recommendations blindly and wanted to know about the appropriateness of prescribing,96 and our study of expectations and experiences lent support to this. Patients seemed to have been more prepared to openly deliberate on prescribing decisions and their expectations were more explicit than they were previously, despite that trust in the clinician still had a major role to play. 96,101,111 Those participants who emerged as informed patients rejected a blind compliance. Indeed, patient expectations were because of disclosure [e.g. it was manifested in the consultations that used elicitation (of expectations) technique]. Expectation elicitation by clinicians, directly or indirectly (i.e. by running commentary), and their open communication appeared important for an ongoing clinician–patient relationship. 95,101,112,113 Instead of trying to read the patient’s mind, prescribers were, and must be, making the expectations apparent by asking about them. 114
Strengths and limitations
We established the variety of patients’ expectations, which, in some cases, attested to an unquestionable compliance and, in other cases, to reflexive accounts of expectations. A wish for the right antibiotic with no resistance and no side effects prescribed at the right time (for bacterial infection) confirmed the patients’ expectations of appropriate treatment. This paramount expectation, according to the patients interviewed, was actualised in consultations with prescribers. Moreover, the patient experiences appeared more nuanced, and patients had more knowledge of antimicrobial resistance and side effects of antibiotics than might be assumed. Prescribers might be reassured that their patients may be knowledgeable and accepting of the limitations of antibiotics.
Although we investigated the accounts of patients, we were unable to interview the health-care professionals who treated our patient participants; however, we believe that the credibility of the research was enhanced by conducting interviews with a group of prescribers (reported in the next chapter). Similarly, our aim was not to observe actual encounters, but rather to examine the expectational structures of patients. Some qualitative research endeavoured to compare the perspectives of professionals and patients (e.g. Courtenay et al. 88), and questioned the pressure originating from patient expectations. Our analysis demonstrated that the participants believed that patient expectations did not influence prescription [in half of the encounters because the antibiotic treatment was evident (UTIs) and in the other half because expectations were undifferentiated]. However, because we did not have the prescribers’ accounts, we could not draw conclusions about the actual impact of patient expectations. Ideally, expectations on both sides should be studied to answer the validity of the notion of expectations of expectations. The study was conducted in the context of general practices in England. Although attempts were made to recruit patients based on purposive sampling, the sample of our study was skewed towards white British female patients who consulted for UTIs. The perspective may differ with gender, age, ethnicity or socioeconomic differences. This affects the results in the form of patients explicitly expecting antibiotics and their appropriate prescribing. Follow-up studies should attempt to diversify the sampling by purposefully including patients of different backgrounds who consult for other bacterial infections. For example, interpretation of our data suggested that education level might be influential. Future studies should also aim to include patients with infections who were not prescribed antibiotics to evaluate their experiences of illness and consultation.
Chapter 5 Prescribers’ views of the risks of use and non-use of antibiotics in primary care
Adapted with permission from Boiko et al. 115 This is an open access article distributed in accordance with the Creative Commons Attribution (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Summary
This part of the research investigated how primary care prescribers perceive risk and safety concerns associated with reduced antibiotic prescribing through semistructured interviews conducted with 23 GPs, five nurses and two pharmacists. Respondents indicated that their decisions were grounded in clinical risk assessment; however, this was informed by different approaches to antibiotic use, with most leaning towards reduced prescribing. Prescribers’ perceptions of risk included the consequences of both inappropriate prescribing and inappropriate withholding of antibiotics. Sepsis was viewed as the most concerning potential outcome of non-prescribing, leading to possible patient harm and potential litigation. Risks of antibiotic prescribing included antibiotic-resistant and C. difficile infections, as well as side effects, such as rashes, that might be mislabeled as an antibiotic allergy. Reduced antibiotic prescribing is now being approached more systematically, but the safety trade-offs associated with either use or non-use of antibiotics present difficulties, especially when prescribing decisions are inconsistent with patients’ expectations.
Introduction
Inappropriate antibiotic prescribing is widespread, but may bring risks for individual116 and population health from drug side effects as well as from growing antimicrobial resistance. 117 Conversely, antibiotic avoidance may be associated with risks from serious bacterial infections that could be avoided through earlier treatment of infection episodes. 28 Many studies have provided insights into the reasons for inappropriate antibiotic prescribing and several syntheses have been published,118–120 but the safety gradient associated with reducing antibiotic prescribing has developed as a new and highly relevant area of research. In this paper, patient safety is understood as ‘the avoidance, prevention and amelioration of adverse outcomes or injuries stemming from the process of healthcare’. 121 The risks associated with antibiotic prescribing decisions are a key element of patient safety and require in-depth analysis. This paper addresses the gap in knowledge about prescribers’ perceptions of potential adverse outcomes associated with reduced antibiotic prescribing.
In the UK, primary care services account for nearly 80% of all medical antibiotic use, but antibiotic utilisation in primary care has been declining in recent years and the choice of antimicrobial agents has become more selective. 2,10 A national target proposes a further reduction in antimicrobial use of 15% by 2024, with antimicrobial resistance providing the rationale for the reduction in antibiotic prescribing. 4 There were an estimated 60,788 antibiotic-resistant infections in England in 2018,2 resulting from infection with diverse bacterial pathogens. In addition, superinfection with C. difficile may cause illness. 122 The scale of antimicrobial resistance is increasing, especially across middle- and low-income countries. Unnecessary exposure to antibiotics may also be associated with more immediate harms. As a result of prescribing in the community, antibiotic-associated adverse events, including allergic reactions, lead to many emergency visits, with antibiotics accounting for up to 20% of hospital admissions from drug reactions in the USA. 123,124 On the other hand, withholding antibiotics might potentially carry risks, and reduced antibiotic prescribing in general practice is associated with a small increase in complications, such as treatable pneumonia and PTA. 28,56
The perceived priority of risks from either prescribing or not prescribing antibiotics requires a nuanced explanation within the broader realm of professionals’ perceptions of safety and associated risk management. Fear of the risk of bacterial complications119,125 and prognostic uncertainty about potential outcomes when not prescribing118,126 are reportedly among key factors that influence the prescription of antibiotics. Among hospital doctors, there is evidence that overtreatment is preferred to the potential for adverse patient outcomes from not prescribing. 127,128 Klein et al. 129 and Broniatowski et al. ,130 for example, demonstrate that medical decision-making tends to favour views that favour prescription (i.e. ‘why take risks’), rather than on prescription avoidance (i.e. ‘antibiotics can be harmful’). In primary care, GPs and other prescribers deal with safety concerns in their decision-making and a better understanding needs to be developed concerning the balance of risk between prescribing or non-prescribing of antibiotics.
Patient factors influencing decision-making on antibiotic prescribing include compliance with patient expectations and pressures. 112,125,131,132 Reducing antibiotic prescribing in primary care is, therefore, highly dependent on successful management of patient expectations95,133,134 and on shared decision-making. 107,113,135,136 It is known that clinicians weigh individual best practice against perceived patient satisfaction so that complex trade-offs are enacted. 137 Therefore, of research interest is how the issues of safety and risk information are communicated to patients.
In the present study we investigate how primary care prescribers perceive risk and safety concerns associated with reduced antibiotic prescribing.
Results
We recruited 30 participants from 10 general practices (Table 5): comprising 23 GPs, five nurses and two pharmacists. The interviews lasted between 24 and 46 minutes. GPs’, nurses’ and pharmacists’ responses were analysed as a single group because of the many commonalities and the smaller number of non-medical respondents. We found that there were no discernible differences in participants’ accounts between the shire town and the metropolitan settings. Three participants expressed an overt avoidance of antibiotics and three others acknowledged overprescribing, whereas most prescribers leaned towards reduced prescribing. We distinguished three major themes from the data: (1) risk assessment, (2) balancing treatment risks and (3) negotiating decisions and risks (Table 6).
| Characteristic | Frequency (n) |
|---|---|
| Gender | |
| Male | 8 |
| Female | 22 |
| Location | |
| Metropolitan | 21 |
| Shire town | 9 |
| Occupation | |
| GP | 23 |
| Nurse prescriber | 5 |
| Pharmacist | 2 |
| Years of practice | |
| < 10 | 16 |
| 10–20 | 10 |
| > 20 | 4 |
| Theme | Subtheme |
|---|---|
| Theme 1: risk assessment | Identifying treatment thresholds |
| Confidence in prescribing | |
| Theme 2: balancing treatment risks | Risks of prescribing and non-prescribing |
| Facing antimicrobial resistance | |
| Theme 3: negotiating decisions and risks | Managing patient expectations |
| Communicating risks |
Theme 1: risk assessment
Identifying treatment thresholds
The primary focus of diagnostic decision-making for participants was concerned with identifying major indications for antibiotic treatment. These were judged to include the nature and severity of illness based on presentation of symptoms and signs in the context of the patient’s medical history. The majority of participants adopted a risk stratification approach in undertaking clinical assessment:
It’s a combination of things . . . For example, for an upper respiratory tract infection, tonsillitis, pharyngitis, you know, there’s a Centor guidance. So that’s where you have a checklist of things. Does this person have cervical lymphadenopathy? Do they have a fever? Do they have like absence of a cough, you know? Do they have exudate on their tonsil? So, then if you have a score of 3 or more then they have antibiotics.
Interview 1, GP
Risk stratification approaches included additional patient factors, such as patient age and the presence of comorbidities including chronic obstructive pulmonary disease, asthma, diabetes, cancer or a history of pneumonia. Although many followed risk assessment protocols based explicitly on local or national clinical guidelines, some participants stressed the importance of clinical judgement in making safety-driven decisions:
You don’t want to miss something very serious. So, that’s where your clinical judgement and decision-making skills play a major role. And experience, obviously, because these are things I deal with every day.
Interview 14, nurse
Threshold-guided decision-making spanned the continuum from ‘I am prescribing’ to ‘I am not prescribing’. Diagnostic uncertainty was part and parcel of the threshold-guided decision-making. Prescribers pointed to the difference between more and less obvious cases, characterised by equivocal, ambiguous and non-convincing evidence:
. . . a patient with COPD [chronic obstructive pulmonary disease], bronchiectasis, I may have a lower threshold for treating than a very fit and well 20 year old, even if that 20 year old had a productive cough with green sputum, their chest is clear, I’m not likely to give them antibiotics. Well they’re not feverish, whereas if they’re an 80-something with a history of COPD then I’d have a lower threshold for starting antibiotics because they’re likely to have less reserve and more likely to have complications from an infection.
Interview 20, GP
Confidence in prescribing
Appropriate prescribing and not just a reduction in antibiotics emerged as a priority for participants who reflected on their own performance from different perspectives. In general, participants reported a high level of confidence in prescribing, but also noted occasional limitations:
I feel confident but that doesn’t mean necessarily that I think I’m making the right decision in every case. Sometimes when I’m making perhaps the wrong decision, I’m making that maybe because of patient pressure or because of my unwillingness to tolerate risk.
Interview 22, GP
Many participants acknowledged changes towards less prescribing over the last few years:
I prescribe less because I guess we’re more aware now of drug resistance than we were 5 years ago. It’s much more talked about and we’re seeing it more. But also, I’m now more confident in having that difficult discussion with the patient.
Interview 5, GP
Theme 2: balancing treatment risks
Risks of prescribing and non-prescribing
Seven participants explicitly identified safety as a priority in infection management. All participants demonstrated vigilance to risks arising from both prescribing antibiotics and from not prescribing antibiotics. The fear was expressed of ‘missing something’ that could cause deterioration and, consequently, participants admitted ‘being cautious’ and favoured prescribing antibiotics. At the same time, the common concern was also the avoidance of prescribing unnecessarily. Among the risks of prescribing, several side effects were reported, most commonly gastrointestinal upsets, nausea, C. difficile infection and thrush, but also allergic reactions, anaphylactic reactions, antibiotic resistance and less common side effects, such as liver problems (failure). Participants also observed long-term adverse consequences of inappropriate prescribing:
I think, certainly for children, I think if you prescribe antibiotics and they don’t need them and then they have a rash because they’ve got a virus and then a penicillin allergy on their notes for the rest of their lives . . . I think another consequence is that if you prescribe inappropriately, it’s very difficult for another health-care professional, down the line, to explain to that patient, you’re almost saying the other person was wrong.
Interview 15, nurse
Risks of non-prescribing generated a shorter list, with sepsis being the most concerning consequence:
Sepsis . . . that’s one thing I do worry about. If I see someone who’s got a high temperature and a high heart rate . . . then I think about those factors and I think actually if this was in my clinical judgement – if I left this for 2 days, then I think they would be crossing that line.
Interview 26, GP
Three prescribers who acknowledged the tendency to overprescribe did so in one case because they assessed the benefits of antibiotics to exceed the harms, and in two cases because of potential litigation following a missed serious bacterial infection:
Because medicolegally you’re much more likely to be brought up on missing something and not prescribing antibiotics than giving antibiotics when it wasn’t necessary . . . if there’s any uncertainty about prescribing antibiotics I would always err on the side of giving them because the risk, however small, of missing an infection that then gets worse would be enough for me to give antibiotics.
Interview 19, GP
Facing antimicrobial resistance
Participants shared concern for the global rise in antimicrobial resistance. At the same time, they acknowledged lacking in-depth microbiological knowledge:
. . . we talk more about not prescribing and prescribing correctly than resistance itself.
Interview 9, pharmacist
Meanwhile, they had to deal with the consequences of the antimicrobial resistance in their daily practice:
I’ve had a few patients that have had MRSA [meticillin-resistant S. aureus]. I’ve had a few people who have had PVL [Panton–Valentine leukocidin form of MRSA] infections, skin infections with multiple resistance . . . So we can sometimes struggle to find an antibiotic that’s oral, that’s then suitable. I’ve got a type 1 diabetic, young lady, who has very poorly controlled diabetes and recurrent boils and abscesses on her back. And we did a swab of that and yes, there was only one oral antibiotic that was sensitive – everything else was resistant.
Interview 23, GP
Antimicrobial resistance was most commonly encountered in older women with UTIs:
I think sometimes you do see, for example, in the UTI breakdown, some people have quite resistant UTIs and that becomes difficult.
Interview 15, nurse
I’ve been a GP for about 10 years and you’ve already seen that certain antibiotics just aren’t working anymore, and we need to change the way that we’re doing things and you know we used to give trimethoprim locally first line for UTIs. Resistant in the majority of cases. So, we’re giving nitrofurantoin.
Interview 10, GP
There was mention of difficulties in conveying information about resistance to patients, that is discussing it in the encounters and emphasising that community impact may have been less efficient than focusing on individual risks. There was also a worry that primary care is running out of antibiotics, despite the strategies of second- and third-line antibiotics:
They [patients] literally cannot have any, they’ve got an E. coli infection that’s not sensitive to amoxicillin or nitrofurantoin or trimethoprim or even cefalexin or the cipro. It’s just like literally multiply resistant. And there’s some quite virulent, my understanding is it is strains of bacteria where antibiotics will not work. And then you kind of get to the hardcore ones.
Interview 11, GP
In such cases of failure of several courses of antibiotics, referral to secondary care, possibly for intravenous therapy, was reported as the only option. Alternatively, if the resistant organism could be tackled in primary care, the last resort was a longer course or long-term prophylactic antibiotics. More investigations and consultations with microbiologists about unresolved infections appeared to precede these decisions.
Theme 3: negotiating decisions and risks
Managing patient expectations
Participants identified patient pressure as a factor in their decision-making, but they shared the view that patients differ in terms of their expectations regarding antibiotics. On the one hand, increased knowledge of the appropriate indications for antibiotic therapy (not for viruses) and understanding of antimicrobial resistance from public health and media campaigns was noted. On the other hand, patient pressure in a form of implicit expectations or explicit demands remained frequent (i.e. readily prescribed in the past, antibiotics had a profile of immediate cure in large parts of patient population):
. . . so many people have been mis-prescribed antibiotics in the past that I think they just won’t believe you that they don’t need them.
Interview 25
A GP summarised this ambivalence:
There’s a reasonable cohort now who come in and say they don’t want them [antibiotics]. They’ve read, they’re educated, they know that they’re contributing potentially to resistance and they don’t want to risk the side effects. But there’s also a large cohort still who come in and say, ‘My cough’s gone to my chest, I need antibiotics’. So, it’s trying to often you know, get through those barriers and explain to them that their chest is clear.
Interview 10, GP
Eliciting expectations, educating patients and delayed prescription were the key strategies for managing patient expectations. Explaining assessment results and positive language were deemed important for the success of the consultation. Several participants preferred the time-saving mode of giving out written information about the expected length of illness (e.g. about the duration of sinusitis with and without antibiotics) and about the side effects of antibiotics. Elicitation of expectations included asking patients ‘What were you hoping for when you came in today?’ (interview 26, GP). Delayed prescriptions were used by all but three of the participants interviewed. This was considered as a form of partnership and of shared decision-making between the clinician and the patient:
. . . that helps patients because at least psychologically they have got an antibiotic, but they know they can’t use it straight away.
Interview 25, GP
Communicating risks
As above, participants demonstrated that the commitment to reduced prescribing was dependent on patient understanding of the need for antibiotics. This meant that, at times, building and maintaining relationships were prioritised and led to prescribing decisions:
Much of my job is trying to build a rapport with someone and build a rapport so that we can have a conversation that’s therapeutic. If someone has come in adamant that they want antibiotics there is some conversation to be had there. Why did you get this idea from? What is it that you believed this would do? And what is your previous experience? Now, if they’re not willing to go into that today, I may actually give them a short course of antibiotics with the understanding that we have another conversation. This is a way of building some trust.
Interview 29, GP
The participants differed in terms of how they dealt with risk in encounters with patients. Some were liberal prescribers who tended to avoid complaints and patient frustration; whereas others preferred having difficult conversations about non-antibiotic course of actions. Among liberal prescribers, there was the notion of offering antibiotics to be safe. In the case of non-prescribing, prescribers sometimes delved into lengthy explanations to secure patient adherence:
When I’m explaining that there’s no sign of bacterial infection and we don’t want to give you antibiotics if we don’t need to. Most people go, ‘Oh yes, yes, no, of course not’. But some people might say, ‘Oh, well, you know’. Then I will go into the reasons why, you know. ‘Well actually you might get side effects, you know, it can make you, give you diarrhoea, it can give you thrush. And things can become resistant to it and it won’t be helpful for you in the future’.
Interview 30, GP
Advice on possible warning signs (i.e. ‘safety-netting’) emerged as a dominant risk reduction strategy:
I will give them [patients] an awful lot of safety-netting, and tell them what, ‘If this doesn’t get better, this is when you come back’. You know, or ‘These are the signs of you getting worse’, or what they do if they are getting worse.
Interview 16, GP
Patient and public involvement
The process of developing subthemes and themes was discussed at a PPI meeting. The preliminary findings were presented and members were invited to discuss emerging themes and to review selected quotes from the interview transcripts for relevance. Feedback included comments on patient expectations, patient pressure for antibiotics, and trust and communication with GPs leading to additional interpretation.
Discussion
Main findings in comparison with previous research
The study describes primary care prescribers’ perceptions of safety and associated trade-offs in the context of reduced antibiotic prescribing. We identify three key themes with relevance to safety: (1) risk assessment, (2) balancing treatment risks and (3) negotiating decisions and risks. These accounts from primary care demonstrated variations in prescribers’ approaches to decision-making behaviour, including perceptions of risks associated with prescribing or not prescribing antibiotics and in the communication of these decisions and risks to patients.
Decision-making for appropriate antibiotic prescribing was informed by safety considerations. Guideline-concordant risk assessment was generally preferred to tacit clinical judgement based on informal heuristics, in line with previous research. 138 Confidence in prescribing can be contrasted with views that accentuate diagnostic uncertainty. 118,126 In complex or uncertain cases, resolution was usually in favour of antibiotic prescribing, but this was in the context of a secular shift to generally more restrictive antibiotic prescribing behaviour. The reduction imperative co-exists with liberal prescribing, which was influenced by low tolerance of risks and patient pressures. This corresponds with extant literature that identifies the co-existence of different prescribing behaviours, including antibiotic compromising, antibiotic delaying and antibiotic withholding. 132
Safety trade-offs emerged from the respondents’ perceptions of risk by lending support to recent qualitative research that reported the complexity of balancing risks of antibiotic prescribing in hospitals. 139 In addition to the anticipated benefits, respondents identified multiple risks associated with either prescribing or not prescribing antibiotics, so that the immediate and long-term adverse effects of prescribing, including antimicrobial resistance, were weighed against potential complications of non-prescribing, such as sepsis. These untoward consequences rendered risk a double-edge sword. In the theory of social systems, such a conundrum can be described by the distinction risk/danger rather than risk/safety, as there is no absolute safety in prescribing decisions and hence the other side of risk remains danger, not safety. 140,141 From Luhmann’s140 perspective, some distinctions are two-sided forms of ‘second-order’ observations where one side is actualised at any given moment, but both sides can be seen as equally relevant in the situation. Risk/danger represents such a form, which exemplifies the contingency associated with seemingly binary choices, but which in itself is actuality versus potentiality. Safety experts, according to this perspective, are ‘first-order’ observers who may not account for the mutuality of contingency (i.e. the other side is always present in the background). Boiko et al. 141 applied this understanding to the analysis of clinical risks associated with anticoagulant prophylaxis, where risks of thrombosis were complemented by dangers of contraindications (e.g. bleeding). In our situation of antibiotic prescribing, the ‘risk’ element is associated with antibiotic prescribing potentially resulting in antimicrobial resistance and drug side effects, whereas the other side (danger) can be actualised if non-prescribing is chosen and can become the actual risk through the complications, such as sepsis. We found variation in how the prescribers perceived this duality, with the safety argument contributing in both directions (i.e. prescribing and non-prescribing). In other words, professionals acting on ‘doing something’ were juxtaposed against ‘doing no harm’ concerns. The participants were able to distinguish between short- (e.g. side effects) and long-term (e.g. antimicrobial resistance and effect on doctor–patient relationship) trade-offs of prescribing. Antimicrobial resistance was generally viewed as a stand-alone long-term adversity now being encountered in daily practice. It is gaining in prominence in contrast to findings from the earlier qualitative studies,142,143 and now has a more personalised relevance and clinical significance than some recent reviews have suggested. 144
Respondents negotiated safety in dealing with patients by rendering medical decision-making more explicitly during consultations. Patient expectations were found to be changing, as were the strategies employed to manage them. There was an emerging consensus on strategies to reduce antibiotic prescribing, including patient education, improved self-management advice and delayed prescribing, supported by patient-centred communication emphasised in the other literature too. 145 At the same time, our study showed that communication was primarily centred on warning signs and on maintaining a clinician–patient relationship, rather than on the discussion of risks and benefits with patients. This is consistent with previous findings that explicit analysis of trade-offs is most often undertaken by physicians alone, rather than as part of a dialogue with patients. 146 More explicit risk communication might become a focus of the consultations for (bacterial) infections. Systematic review evidence suggests that shared decision-making reduces prescribing,147 and our study also found that both delayed prescribing148–151 and safety-netting appeared to be effective strategies of shared decision-making.
Strengths and limitations
The study provided a coherent analysis of the views of primary care prescribers. It drew on participants working in rural and urban settings and included a sample that was diverse with respect to professional training and years of experience. The size of the sample may not have been sufficient to distinguish differences in approach between groups with different professional training, but this could be explored further in future studies. However, the study may have reduced transferability to other settings beyond UK primary care or beyond high-income countries. The study is based on interviews with prescribers and may be prone to the limitations associated with qualitative studies. Participants were necessarily informed of the nature and purpose of the research; consequently, both their participation in the interview and the interview responses might have been influenced by research participation. It is possible that respondents who were less inclined to reduce antibiotic prescribing might have been less prepared to participate. Interviewees might have been inclined to give what they perceived as ‘socially acceptable’ responses. We employed a thematic analysis because this enables a flexible investigation of a complex topic without drawing on pre-existing theory. To reduce the possibility of inconsistency, we employed a systematic, staged approach to analysis and a sample of transcripts was repeat coded by a second analyst.
Implications for further research
This study explored and characterised primary care prescribers’ perceptions of safety issues and risk management strategies relevant to reduced antibiotic prescribing. The study is a valuable investigation of primary care prescribers’ perceptions and, as such, it emphasises the safety perspective within the current debate on antibiotic prescribing and antimicrobial stewardship. The study identified dilemmas that are recognisable in the course of daily primary care practice and can form the basis for future improvement and antimicrobial stewardship programmes. Our research paves the way for a cross-sectional survey of risk perceptions. It highlights the need for further development of risk stratification and risk communication tools, such as decision-making checklists and evidence-based support tools. It also stresses the need for adequate training on antimicrobial resistance and reducing antibiotic prescribing (e.g. GRACE-INTRO and REDUCE). 152,153 Safety-netting had a strong presence in the interviews; however, this is under-researched and requires further exploration. Our findings support the argument136 that prescribers need more time to discuss the benefit–harm trade-off in shared decision-making, as this may help to reduce antibiotic prescribing in primary care.
Chapter 6 Patterns of antibiotic prescribing in UK primary care from 2002 to 2017
Adapted with permission from Gulliford et al. 39,154,155 These are open access articles distributed in accordance with the Creative Commons Attribution (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Summary
This part of the research evaluated antibiotic prescribing and coding of the reasons for antibiotic prescribing at UK general practices from 2002 to 2017. A cohort study was conducted using data from 706 UK general practices in the CPRD GOLD database. The age-standardised antibiotic prescribing rate per 1000 patient-years increased from 2002 (male, n = 423; female, n = 621) to 2012 (male, n = 530; female, n = 842) before declining in 2017 (male, n = 449; female, n = 753). The median general practice had an antibiotic prescribing rate of 648 per 1000 patient-years, and the 95% range for different practices was 430–1038 antibiotic prescriptions per 1000 patient-years. Specific coded indications were recorded for 58% of antibiotic prescriptions at the median general practice and the 95% range at different general practices was from 10% to 75%. In a substudy, we compared estimates for 2017 from CPRD GOLD (Vision data) and CPRD Aurum (EMIS data). Estimates for antibiotic prescribing and infection recording were broadly similar in both databases, suggesting similar recording across EMIS and Vision systems.
Introduction
This chapter presents results for antibiotic utilisation at 706 general practices in the CPRD GOLD database. Analyses explored trends in total antibiotic utilisation over time, recording of indications for antibiotic prescriptions and variation among general practices in antibiotic prescription and infection recording. The analyses also aimed to determine whether or not analysis of data from CPRD Aurum and CPRD GOLD provides similar estimates for antibiotic prescription and recording.
Results
In the sample analysed for antibiotic prescribing, there were 706 general practices with 671,830 registered patients and 6,541,195 person-years of follow-up (Table 7 and Figure 4). There was a total of 4,371,715 antibiotic prescriptions between 2002 and 2017. This included 2,368,551 (54%) prescriptions with coded indications, including 1,531,645 (35%) associated with respiratory infections, 369,389 (8%) with GUTIs, 414,680 (10%) with skin infections and 52,837 (1%) with other specific indications. There were 2,003,164 (46%) antibiotic prescriptions without specific coded indications, consisting of 479,421 (11%) repeat prescriptions, 1,154,789 (26%) with non-specific medical codes recorded and 368,954 (8%) with no medical codes recorded (see Figure 4).
| Characteristic | Time period | ||
|---|---|---|---|
| 2002–6 | 2007–12 | 2013–17 | |
| Number of general practices | 652 | 672 | 589 |
| Number of patients contributing person-timea | 548,558 | 576,985 | 439,627 |
| Number of person-years | 2,253,436 | 2,768,176 | 1,519,582 |
| Age 0–4 years | 275,539 | 313,806 | 104,688 |
| Age 5–14 years | 371,352 | 611,610 | 393,224 |
| Age ≥ 85 years | 169,709 | 216,966 | 111,606 |
| Comorbidity presentb | 835,565 | 1,147,828 | 686,777 |
| Number of antibiotic prescriptions | 1,422,009 | 1,941,102 | 1,008,604 |
| Acute antibiotic prescriptions | 1,289,615 (91) | 1,739,666 (90) | 863,013 (86) |
| For RTI | 534,535 (38) | 705,262 (36) | 291,848 (29) |
| For GUTI | 115,928 (8) | 166,336 (9) | 87,125 (9) |
| For skin infection | 137,936 (10) | 184,420 (10) | 92,324 (9) |
| Other specific codes recorded | 18,277 (1) | 24,849 (1) | 9711 (1) |
| Non-specific codes recorded | 290,472 (20) | 537,110 (28) | 327,207 (32) |
| No codes recorded | 192,467 (14) | 121,689 (6) | 54,798 (5) |
| Repeat antibiotic prescriptions | 132,394 (9) | 201,436 (10) | 145,591 (15) |
FIGURE 4.
Flow chart showing the classification of antibiotic prescriptions from 2002 to 2017. Figures are frequencies (per cent of total number of antibiotic prescriptions).
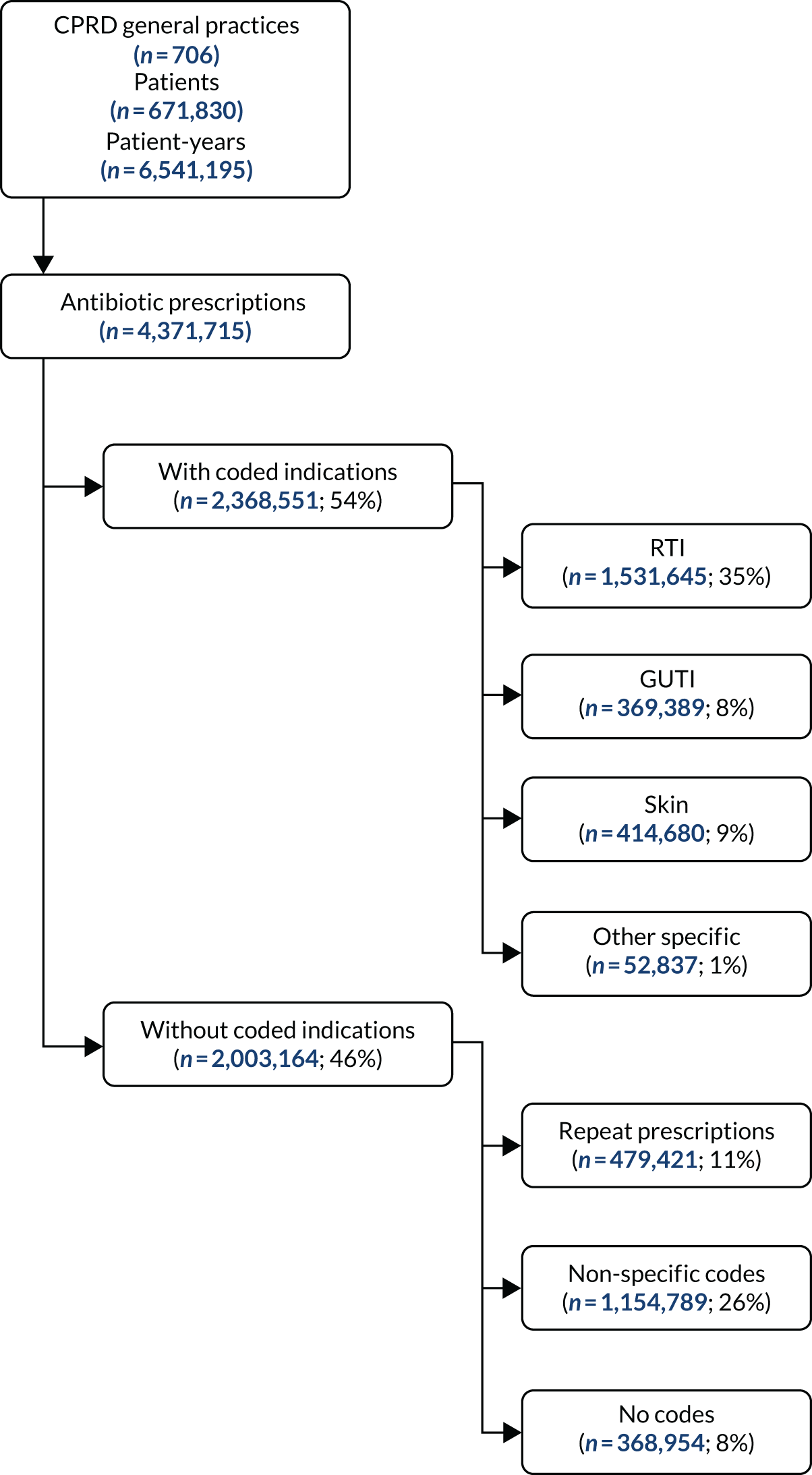
Overall trends in antibiotic prescribing and infection recording
Figure 5 shows changes over time in age-standardised antibiotic prescribing rates per 1000 patient-years for coded and not coded indications. During the initial period of the study, from 2002 to 2012, the age-standardised total antibiotic prescribing rate per 1000 patient-years increased from 2002 (male, n = 423; female, n = 621) to 2012 (male, n = 530; female, n = 842), before declining in 2017 (male, n = 449; female, n = 753). The recent decrease in total antibiotic prescribing was accompanied by a decline in antibiotic prescribing for coded indications, but antibiotic prescriptions that were not associated with specific coded indications continued to increase. There was evidence of a decline in antibiotic prescribing for respiratory illness from 2008 onwards (Figure 6), and after 2012 there was evidence of decreasing prescribing for genitourinary and skin infections, as well as other specific indications. From 2002 to 2017, antibiotic prescriptions associated with non-specific codes increased, as did repeat prescriptions. Antibiotic prescriptions that were not associated with medical codes declined initially, but then remained constant (see Figure 6).
FIGURE 5.
Age-standardised antibiotic prescribing rates per 1000 patient-years for males and females from 2002 to 2017. (a) Male; and (b) female.
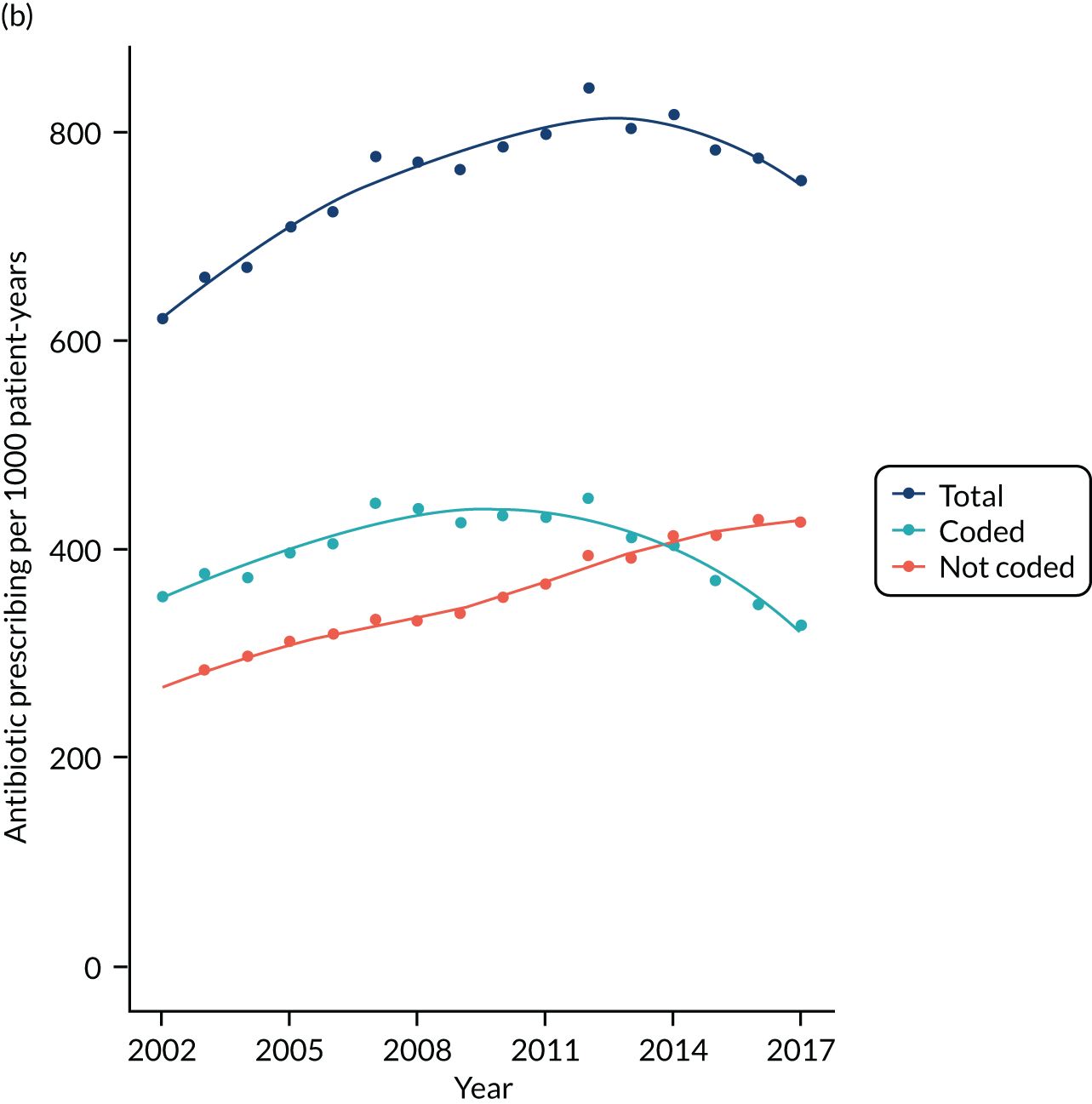
FIGURE 6.
Age- and sex-standardised antibiotic prescribing rates per 1000 patient-years for coded and not coded indications from 2002 to 2017. (a) Coded; and (b) not coded.
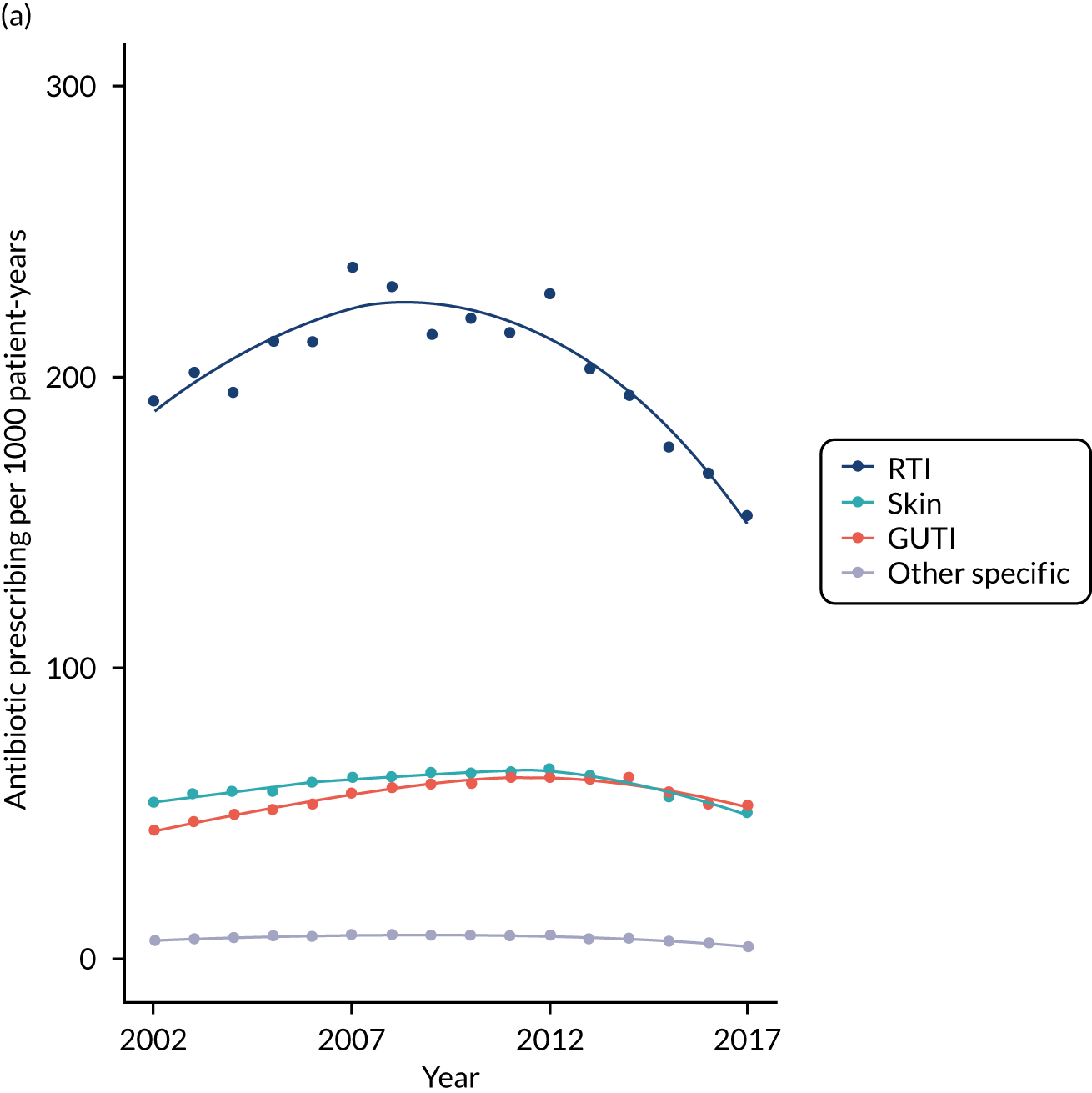

Variation in antibiotic prescription and infection recording among general practices
Table 8 summarises variation in antibiotic prescribing metrics between general practices in the sample. The 95% range for general practice-specific antibiotic prescribing rates was from 430 to 1038 antibiotic prescriptions per 1000 person-years, with a median of 648 antibiotic prescriptions per 1000 patient-years. The 95% range for the proportion of repeat prescriptions was from 3% to 24%. The 95% range for the proportion of antibiotic prescriptions with specific coded indications recorded ranged from 10% to 75%. Figure 7 presents a correlation matrix of the estimated general practice-specific random intercepts for different types of antibiotic prescribing. General practices with more antibiotic prescribing for RTI also tended to be higher prescribers for skin infections, genitourinary infections and other specific infections. Antibiotic prescribing for these conditions was, as expected, negatively correlated with prescribing for non-specific and non-coded indications, which were in turn correlated with each other.
| Measure | Centiles of general practices | ||||
|---|---|---|---|---|---|
| 2.5th | 25th | Median | 75th | 97.5th | |
| Antibiotic prescribing rate per 1000 patient-years | 430 | 563 | 648 | 748 | 1038 |
| Acute prescriptions (% of all antibiotic prescriptions) | 76 | 86 | 90 | 93 | 97 |
| Repeat prescriptions (% of all antibiotic prescriptions) | 3 | 7 | 10 | 14 | 24 |
| Coded indication (% of all antibiotic prescriptions) | 10 | 48 | 58 | 65 | 75 |
| Respiratory (% of all antibiotic prescriptions) | 6 | 31 | 36 | 42 | 52 |
| Genitourinary (% of all antibiotic prescriptions) | 1 | 7 | 8 | 11 | 16 |
| Skin (% of all antibiotic prescriptions) | 2 | 8 | 10 | 12 | 16 |
| Other specific (% of all antibiotic prescriptions) | 0 | 1 | 1 | 2 | 3 |
| Non-coded indications (% of all antibiotic prescriptions) | 24 | 35 | 42 | 51 | 90 |
| No codes recorded (% of all antibiotic prescriptions) | 1 | 3 | 6 | 11 | 28 |
| Non-specific codes recorded (% of all antibiotic prescriptions) | 12 | 19 | 24 | 29 | 59 |
FIGURE 7.
Correlation matrix of general practice-specific random effects for different measures of antibiotic prescribing. Red indicates positive correlation and blue indicates negative correlation.
Comparison of antibiotic prescribing and prescription recording in Vision and EMIS general practices
We evaluated whether or not similar results would be obtained in EMIS data from the CPRD Aurum database. In the CPRD Aurum sample, there were 158,305 participants from 883 general practices with 101,360 antibiotic prescriptions during 2017. In the CPRD GOLD sample, there were 160,394 patients from 290 general practices with 112,931 antibiotic prescriptions during 2017. This included 112 general practices in England and 178 in Scotland, Wales and Northern Ireland. The age- and sex-standardised antibiotic prescribing rate was 512.6 (95% CI 510.4 to 514.9) per 1000 person-years in CPRD Aurum and 584.3 (95% CI 582.1 to 586.5) per 1000 person-years in CPRD GOLD. The rate for CPRD GOLD practices in England was 505.2 (95% CI 501.6 to 508.9) per 1000 person-years, which is similar to the rate observed in CPRD Aurum.
Figure 8 presents age- and sex-specific antibiotic prescribing rates for 2017. Antibiotic prescribing was higher in children aged < 5 years, it decreased until the teenage years, and increased again (especially in women), before increasing steadily into older ages. This pattern of association was observed in both CPRD Aurum and CPRD GOLD, but estimates for CPRD GOLD were slightly higher than those for CPRD Aurum, but broadly similar when restricted to CPRD GOLD general practices in England. The lower panels of Figure 8 (see Figures 8b and 8d) provide Bland–Altman plots that presents the difference (95% CI) between all CPRD GOLD and CPRD Aurum practices (blue) and CPRD GOLD practices in England only (orange). For men and women in all age groups, CPRD GOLD general practices generally had slightly higher antibiotic prescribing rates than CPRD Aurum, whereas CPRD GOLD general practices in England had broadly similar antibiotic prescribing rates to CPRD Aurum. CIs were compatible, with no difference in any except the oldest age group (i.e. ≥ 85 years), for whom data are more sparse.
FIGURE 8.
Antibiotic prescribing rates in CPRD Aurum and CPRD GOLD, by age group and sex. Upper figure shows antibiotic prescribing rate per 1000 patient-years and lower figure shows difference in antibiotic prescribing rate per 1000 patient-years. (a) Antibiotic prescribing rate by age in CPRD Aurum and CPRD GOLD (male); (b) difference between all CPRD GOLD and CPRD Aurum practices (male); (c) antibiotic prescribing rate by age in CPRD Aurum and CPRD GOLD (female); and (d) difference between all CPRD GOLD and CPRD Aurum practices (female).
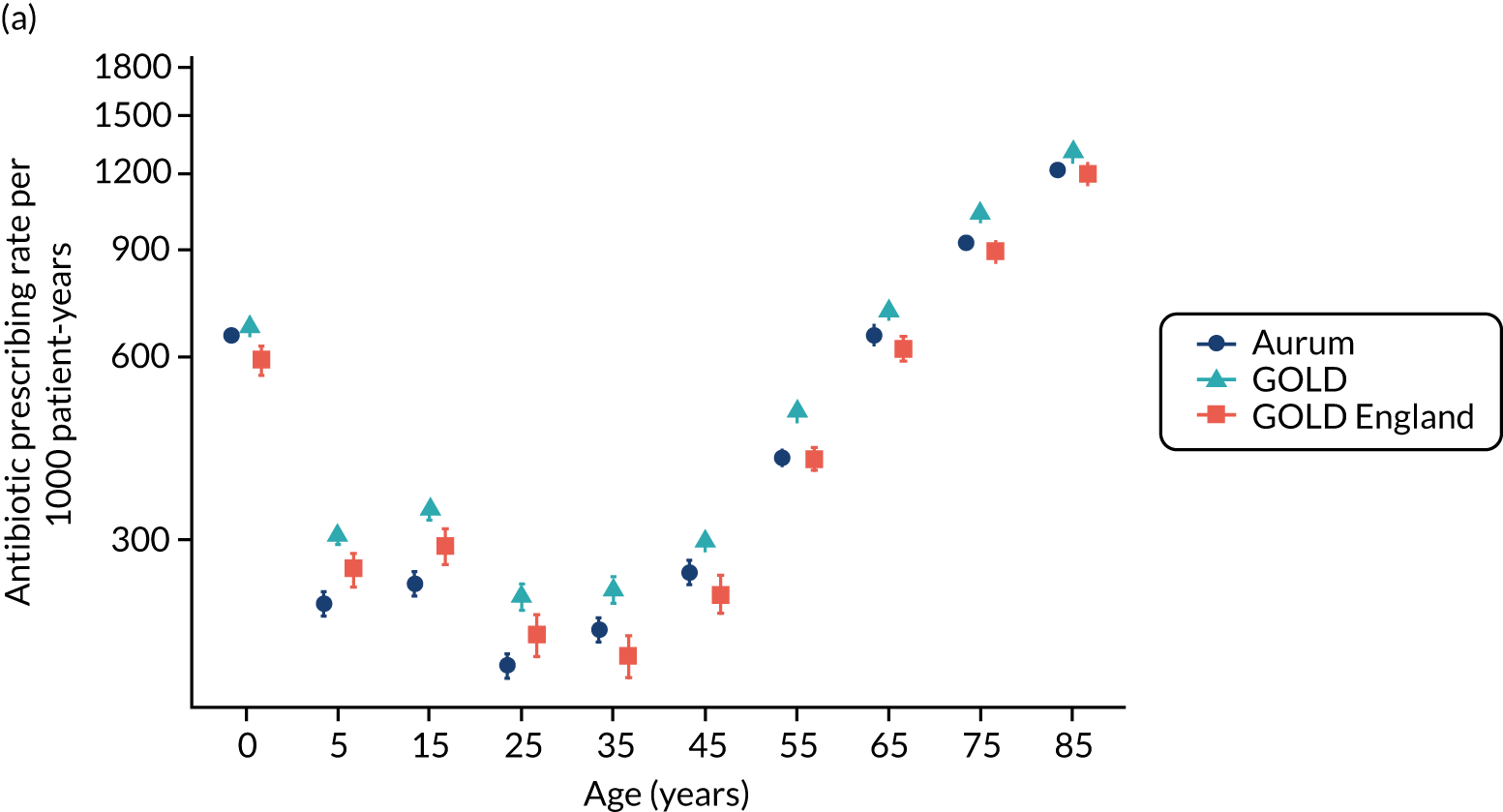
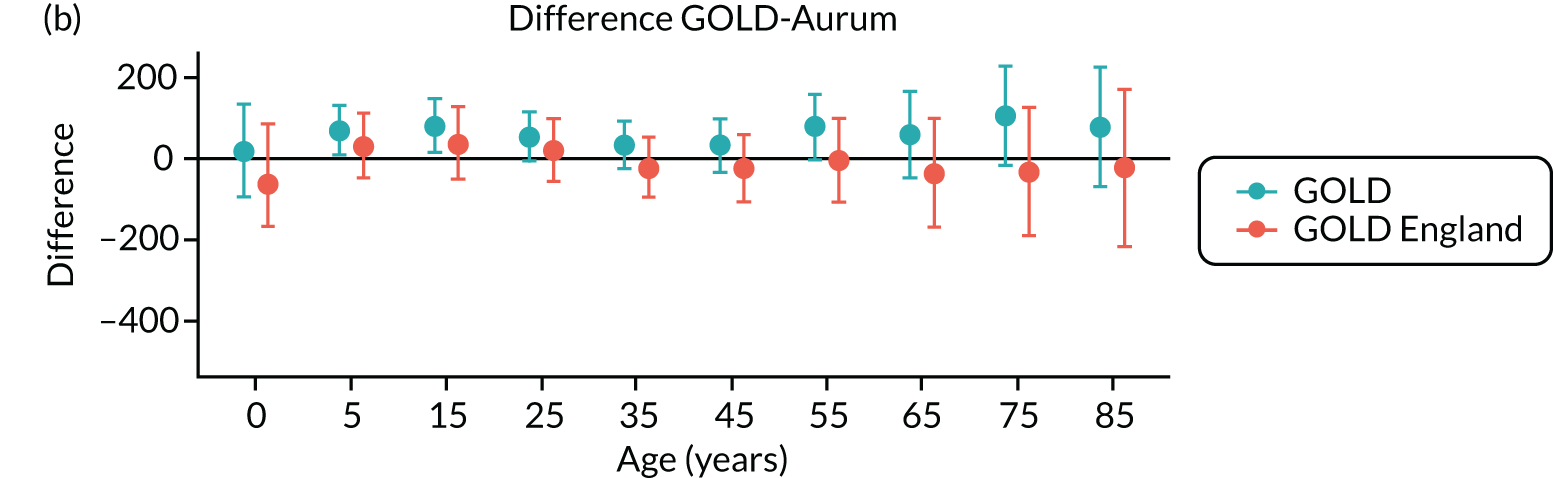
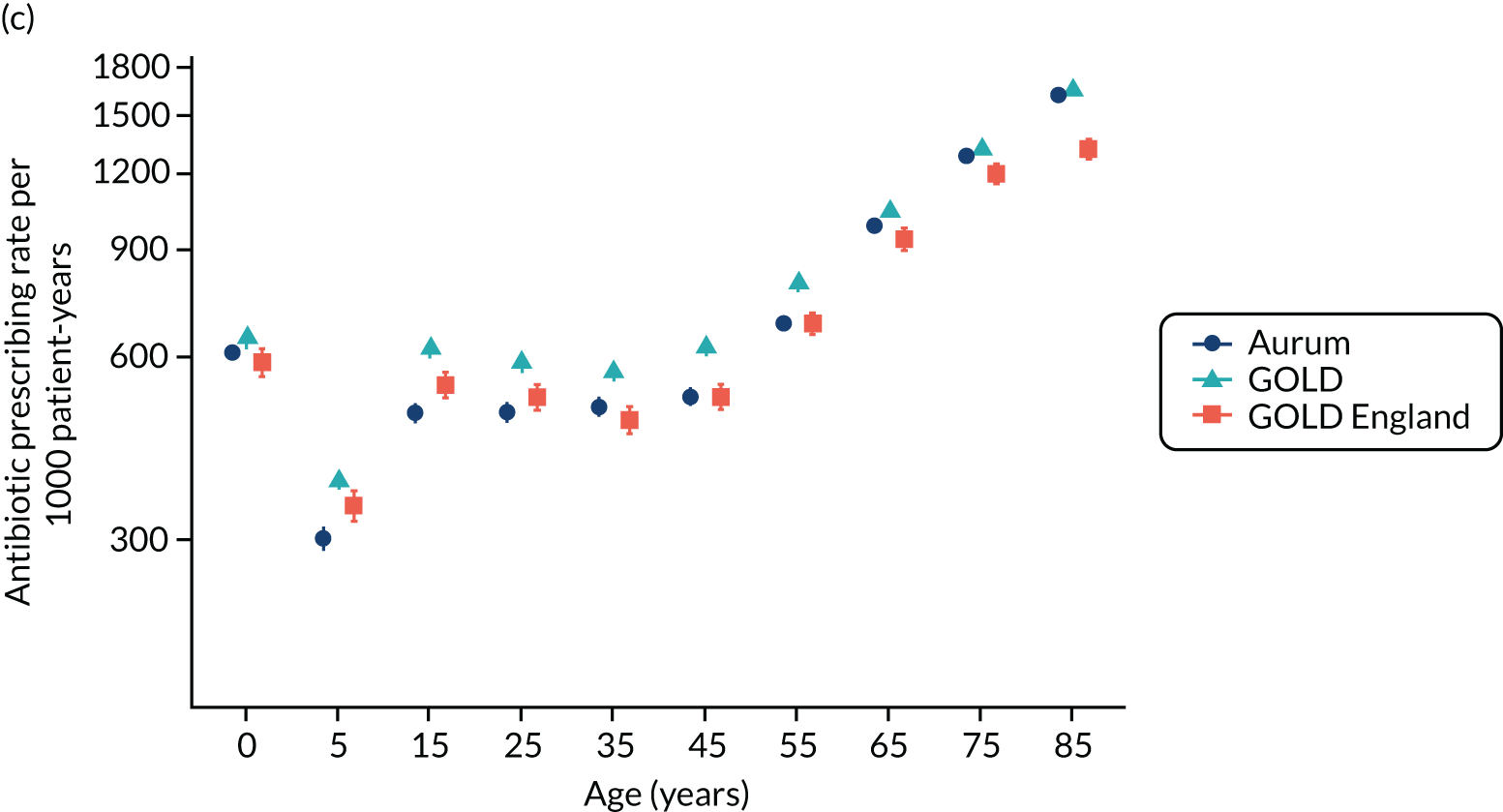

Most frequently prescribed products
Report Supplementary Material 3, Supplementary Table 1, presents data for the 25 most frequently prescribed antibiotic products. In CPRD Aurum, amoxicillin 500-mg capsules, doxycycline 100-mg capsules, flucloxacillin 500-mg capsules, trimethoprim 200-mg tablets and nitrofurantoin 100-mg modified-release capsules were the five most frequently prescribed products, accounting for 45% of all antibiotic prescriptions. In CPRD GOLD, there were more prescriptions for trimethoprim (8%) and fewer prescriptions for nitrofurantoin (3%); consequently, clarithromycin 500-mg tablets and not nitrofurantoin appeared as the fifth ranked product. The same pattern was observed for CPRD GOLD practices in England, although trimethoprim constituted a smaller proportion of all prescriptions than in CPRD GOLD as a whole. Twenty-three of the 25 most frequently prescribed drugs in CPRD Aurum were also in the top 25-ranked prescriptions in CPRD GOLD general practices.
Recording of medical terms associated with prescriptions
Table 9 summarises data for recording of medical diagnostic codes on the same date as antibiotic prescriptions. Medical codes were recorded on the same date for 72,989 (74%) antibiotic prescriptions in CPRD Aurum, 84,756 (78%) antibiotic prescriptions in CPRD GOLD and 28,471 (78%) in CPRD GOLD general practices in England. Infections of the skin, respiratory tract and genitourinary tracts accounted for 39,035 (40%) CPRD Aurum prescriptions, 41,326 (38%) CPRD GOLD prescriptions and 15,481 (42%) CPRD GOLD prescriptions in general practices in England. All other medical codes accounted for 33,954 (34%) antibiotic prescriptions in CPRD Aurum, 43,430 (40%) antibiotic prescriptions in CPRD GOLD and 12,990 (36%) antibiotic prescriptions in CPRD GOLD in general practices in England.
| Medical coding of antibiotic prescriptions | CPRD Aurum | CPRD GOLD | CPRD GOLD England |
|---|---|---|---|
| Number of prescription items | 101,360 | 112,931 | 37,551 |
| Number of prescriptions with unique date | 98,727 | 108,397 | 36,617 |
| Medical code recorded on same date | 72,989 (74.0) | 84,756 (78.2) | 28,471 (77.8) |
| No medical code recorded on same date | 25,738 (26.0) | 23,641 (21.8) | 8146 (22.2) |
| RTI | 21,350 (21.6) | 26,005 (24.0) | 9549 (26.1) |
| GUIT | 11,126 (11.3) | 8762 (8.1) | 3315 (9.1) |
| Skin infection | 6559 (6.6) | 6559 (6.1) | 2617 (7.1) |
| Other codes | 33,954 (34.4) | 43,430 (40.1) | 12,990 (35.5) |
Patient and public involvement
The main findings from these analyses were discussed at a PPI meeting. Discussion focused on the reason for the increase in antibiotic prescribing between 2002 and 2012, whether or not further reductions are achievable, whether there are international differences or consensus in terms of antibiotic prescribing, and how the outcomes of antibiotic prescribing compared with no antibiotic prescribing could be compared.
Discussion
Principal findings
This study found that antibiotic prescribing increased from 2002 to 2012, but declined subsequently, with changes over time being of larger magnitude for women than for men. The decline in antibiotic prescribing was earliest and most pronounced for RTIs, followed by other specific coded indications. We did not find evidence for a decline in antibiotic prescriptions, with poorly documented reasons for prescription.
We observed that nearly half of antibiotic prescriptions were not associated with specific coded indications, which is consistent with previous studies. 8,10 This suggests that total antibiotic prescribing is the most appropriate exposure measure for consideration in the following studies, because indication-specific antibiotic prescribing may be associated with considerable misclassification. Measures are needed to improve the recording of infection episodes in primary care both when antibiotics are prescribed and when antibiotics are not prescribed. Repeat prescriptions account for a significant proportion of uncoded prescriptions and repeat prescriptions might be indicated for prolonged or serious infections. Certain conditions may be associated with a higher rate of repeat antibiotic prescribing if there is initial treatment failure. For example, surgical intervention may eventually be required for treatment of empyema, osteomyelitis or infective endocarditis.
Comparison of Vision and EMIS data
Electronic health record systems may offer users discretion over the recording of data items. We found that EMIS data included similar proportions of antibiotic prescriptions being associated with no codes, non-specific codes and codes for infection episodes. This analysis suggests that antibiotic prescribing estimates from EMIS-derived data in CPRD Aurum are broadly similar to those obtained through analysis of Vision-derived data in CPRD GOLD. This similarity includes the rates of antibiotic prescriptions for subgroups of age and gender, the drug name and strength of antibiotic products prescribed, and the recording of medical diagnoses on the same day as the antibiotic prescription. We noted that antibiotics were more frequently prescribed in CPRD GOLD than in CPRD Aurum, but this was not the case when the CPRD GOLD sample was restricted to general practices in England. This suggests that antibiotic prescribing may be higher in Wales, Northern Ireland and Scotland, where prescription charges have been abolished since 2007, 2010 and 2011, respectively,156,157 although our study did not investigate the reasons for this difference. As well as slight differences in overall rates, we noted that drug choice might vary between databases. Trimethoprim prescribing was higher in CPRD GOLD than in CPRD Aurum. Nitrofurantoin has been recommended by Public Health England as the drug of first choice for UTIs in adults2 because of increasing antimicrobial resistance to trimethoprim, but this guidance may not apply in the devolved administrations. CPRD GOLD general practices in England were more similar to CPRD Aurum general practices with respect to prescribing of trimethoprim and nitrofurantoin. It is likely that differences in clinical practice between England and the devolved administrations (i.e. Scotland, Wales and Northern Ireland) may be greater than the differences between EMIS and Vision practices within England.
A strength of our study is that we used real-world data from primary care to estimate the rates of antibiotic prescribing. Using data from primary care is likely to provide a reliable picture of prescribing patterns, given that about 80% of all antibiotic prescribing in the UK’s NHS take place in primary care. 2 We estimated the difference between CPRD Aurum and CPRD GOLD, as well as the difference between CPRD Aurum and CPRD GOLD in England. A comparison between CPRD GOLD practices in England and CPRD Aurum practices was essential to benchmark recording in the CPRD Aurum database, which at the time of this study comprised contributing practices from England only. We recommend that future comparative studies of antibiotic prescribing in the UK should separately evaluate prescribing in CPRD Aurum compared with CPRD GOLD practices in England, and CPRD GOLD practices in Wales, Scotland and Northern Ireland, given that factors such as socioeconomic differences, as well as abolished prescription charges, in the devolved nations may have an impact on prescribing in these countries. There were generally only small differences between CPRD Aurum and CPRD GOLD in England. We acknowledge that we could have obtained greater precision with larger samples, but the present approach was pragmatic and provided sufficiently precise estimates for age-specific rates. We did not employ null-hypothesis significance testing, but elected to present CIs so that readers could reflect on the substantive importance of any estimated differences for their proposed studies. The community of CPRD researchers collectively has wide experience of compiling code lists for research in the CPRD GOLD database. Less experience is available for the CPRD Aurum database. We noted that CPRD Aurum product codes may be up to 17 characters in length, and the use of special programming features, such as the ‘bit64’ package in R, is required to maintain data integrity. We completed extensive searches for product codes to identify antibiotic products. We identified a larger number of potential products from the CPRD GOLD data dictionary, but a generally similar number of antibiotic product codes were actually recorded in the two data sets during 2017. Searches in the CPRD Aurum product dictionary should be based on term, drug substance and product names, as the BNF classification is less widely available in the CPRD Aurum product dictionary than in the CPRD GOLD. It may also be possible to compare ‘dm+d’ codes from the dictionary of medicines and devices, which are now employed in both CPRD GOLD and CPRD Aurum product dictionaries. We mapped medical code sets between CPRD GOLD and CPRD Aurum by matching on Read codes to make a like-for-like comparison. The analysis shows that, for these conditions, the use of the same Read codes gives similar results in CPRD Aurum and CPRD GOLD. There are some medical codes that are employed in EMIS only, which might be omitted through this process, and this merits further evaluation. Experience shows that, in Read-coded data, the majority of events are associated with a small number of codes; consequently, the omission of infrequently used codes is seldom important. Our main findings with respect to medical codes were consistent between databases. We also note that records of antibiotic prescribing do not indicate whether or not medicines were dispensed, whether or not they were taken, or whether they were taken by the patient they were prescribed to or by someone else. It is also possible that prescriptions recorded at out-of-hours visits, home visits or during attendance at residential care homes may be missing from the patient electronic record. Finally, the analysis undertaken was cross-sectional in nature and does not provide evidence about trends in antibiotic recording over time between CPRD GOLD and CPRD Aurum. We used data from the July and October 2019 releases of CPRD Aurum and CPRD GOLD, respectively; however, it may be preferable to compare the same month’s releases, and data for 2017 should be complete by 2019.
This study found that the analysis of EMIS-derived data in CPRD Aurum gives broadly similar estimates for antibiotic prescribing and infection recording to those reported for Vision-derived data in CPRD GOLD. CPRD GOLD includes general practices in Scotland, Wales and Northern Ireland, which have slightly higher antibiotic prescribing than either EMIS or Vision general practices in England. Based on these results, we believe that future research studies can be conducted in CPRD Aurum, informed by previous results from CPRD GOLD or The Health Improvement Network. It may also be possible to combine data from CPRD GOLD English practices with CPRD Aurum data for research on antibiotic prescribing. As CPRD Aurum includes an increasing number of general practices, this database will become increasingly important for public health research. However, further work is required to better understand the quality and completeness of information recorded in areas such as dosing regimen and treatment duration, which are important in estimating treatment exposure in pharmacoepidemiology and pharmacovigilance research.
Chapter 7 Serious bacterial infections and antibiotic prescribing in primary care: general practice-level analysis
Adapted with permission from Gulliford et al. 39 This is an open access article distributed in accordance with the Creative Commons Attribution (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/ licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Summary
This part of the research evaluated whether or not serious bacterial infections are more frequent at general practices with lower antibiotic prescribing rates. Exposures were all antibiotic prescriptions, subgroups of acute and repeat antibiotic prescriptions, and the proportion of antibiotic prescriptions associated with specific-coded indications. Outcomes were first episodes of serious bacterial infections. Poisson models were fitted, adjusting for age group, gender, comorbidity, deprivation, region and calendar year, with random intercepts representing general practice-specific estimates. After adjusting for covariates, there was no evidence that serious bacterial infections were lower at general practices with higher total antibiotic prescribing. The adjusted rate ratio (RR) for 20% higher total antibiotic prescribing was 1.03 (95% CI 1.00 to 1.06; p = 0.074). This research did not find population-level evidence that general practices with lower total antibiotic prescribing might have more frequent occurrence of serious bacterial infections overall.
Introduction
It is possible that reducing antibiotic prescribing might be associated with a greater risk of serious bacterial infections. Previous research investigated infection risk and antibiotic prescribing for respiratory illnesses. 8,10 In a cohort study, Petersen et al. 56 found that antibiotic treatment reduced risks of mastoiditis after otitis media, PTA after sore throat and pneumonia after respiratory infection. An analysis of electronic health records28 found that general practices that prescribed antibiotics more frequently to patients with self-limiting respiratory illnesses might have a lower risk of pneumonia and PTA, but there were no associations with risk of mastoiditis, empyema, meningitis, intracranial abscess or Lemierre’s syndrome. A cluster randomised trial of an antimicrobial stewardship intervention for respiratory prescribing,152 as well as an interrupted time series analysis, found no clear evidence that antimicrobial stewardship policies might be associated with increased bacterial infections overall. 158 However, Gharbi et al. 61 found that apparent non-use of antibiotics for UTIs might be associated with a higher risk of sepsis. It is important to extend these investigations to include antibiotic prescribing for all indications because the reasons for antibiotic prescribing may not always be well documented, with up to half of antibiotic prescriptions in UK primary care not associated with any record of specific diagnostic medical codes. 8 When analyses are restricted to antibiotic prescriptions for clearly recorded indications, the true extent of antibiotic prescribing may be underestimated. It is also important to assess repeat antibiotic prescriptions that may be given for prevention of recurrent infections or treatment of serious or chronic infections. 10 The present study aimed to test the hypothesis that greater use of antibiotics for all indications might be associated with a lower risk of serious bacterial infection. We also investigated whether or not patterns of medical coding were associated with the apparent occurrence of serious bacterial infection.
Results
There were 706 general practices included in the analysis, with 10.1 million registered patients. In the subsample analysed for antibiotic prescribing, there were 706 general practices with 671,830 patients. There were 139,759 first episodes of serious bacterial infections (Table 10). Figure 9 shows trends in the age-standardised incidence of serious bacterial infections from 2002 to 2017. The total incidence of serious bacterial infections increased during the period. This increase was largely accounted for by increases in sepsis, antibiotic-resistant and C. difficile infections, kidney infections and osteomyelitis. The remaining conditions showed either stable incidence or slight declines in incidence. Table 11 presents age- and sex-standardised incidence rates per 1000 patient-years for serious bacterial infections for the highest and lowest fourths of antibiotic prescribing. There was no evidence that serious bacterial infections might be more frequent at general practices in the lowest fourth of antibiotic prescribing. In general, age- and sex-standardised incidence rates tended to be the highest at general practices that were higher prescribers of antibiotics. Table 11 also compares the incidence of serious bacterial infection for the lowest and highest fourths of medical coding. In the lowest quartile of general practices, a median of 38% of antibiotic prescriptions were coded compared with 70% for practices in the highest quartile. General practices in the highest fourth of medical coding had an incidence of serious bacterial infection of 2.39 per 1000 patient-years (95% CI 2.37 to 2.42 per 1000 patient-years) compared with an incidence of 1.94 per 1000 patient-years (95% CI 1.91 to 1.96 per 1000 patient-years) in the lowest fourth of medical coding.
| Variable | CNS infection | CVS infection | Kidney infection | Lung abscess/empyema | Mastoiditis | Osteomyelitis | PTA | Antibiotic-resistant infections | Sepsis | Septic arthritis |
|---|---|---|---|---|---|---|---|---|---|---|
| All | 576 | 1697 | 30,827 | 2932 | 1970 | 4921 | 11,338 | 42,185 | 39,059 | 4254 |
| Male | 352 | 1144 | 4997 | 1903 | 814 | 3055 | 6021 | 18,312 | 18,999 | 2496 |
| Female | 224 | 553 | 25,830 | 1029 | 1156 | 1866 | 5317 | 23,873 | 20,060 | 1758 |
| Age group (years) | ||||||||||
| 0–4 | 11 | 20 | 198 | 138 | 178 | 138 | 73 | 576 | 469 | 147 |
| 5–9 | 17 | 18 | 386 | 106 | 153 | 118 | 232 | 409 | 334 | 104 |
| 10–14 | 17 | 17 | 474 | 60 | 111 | 167 | 465 | 308 | 244 | 93 |
| 15–24 | 47 | 42 | 6140 | 106 | 167 | 152 | 3428 | 1528 | 970 | 129 |
| 25–34 | 38 | 92 | 5523 | 149 | 203 | 160 | 2621 | 2444 | 1474 | 243 |
| 35–44 | 65 | 146 | 5176 | 294 | 280 | 392 | 2483 | 3089 | 2164 | 392 |
| 45–54 | 115 | 189 | 4519 | 438 | 270 | 635 | 1079 | 4001 | 3345 | 555 |
| 55–64 | 105 | 274 | 3725 | 561 | 255 | 865 | 553 | 5045 | 5385 | 678 |
| 65–74 | 90 | 407 | 2562 | 525 | 210 | 937 | 285 | 8252 | 7817 | 775 |
| 75–84 | 58 | 365 | 1548 | 423 | 109 | 924 | 94 | 9469 | 9646 | 727 |
| ≥ 85 | 13 | 127 | 576 | 132 | 34 | 433 | 24 | 7064 | 7211 | 411 |
FIGURE 9.
Age-standardised rates of serious bacterial infections per 1000 patient-years from 2002 to 2017. Shaded areas are 95% CIs.
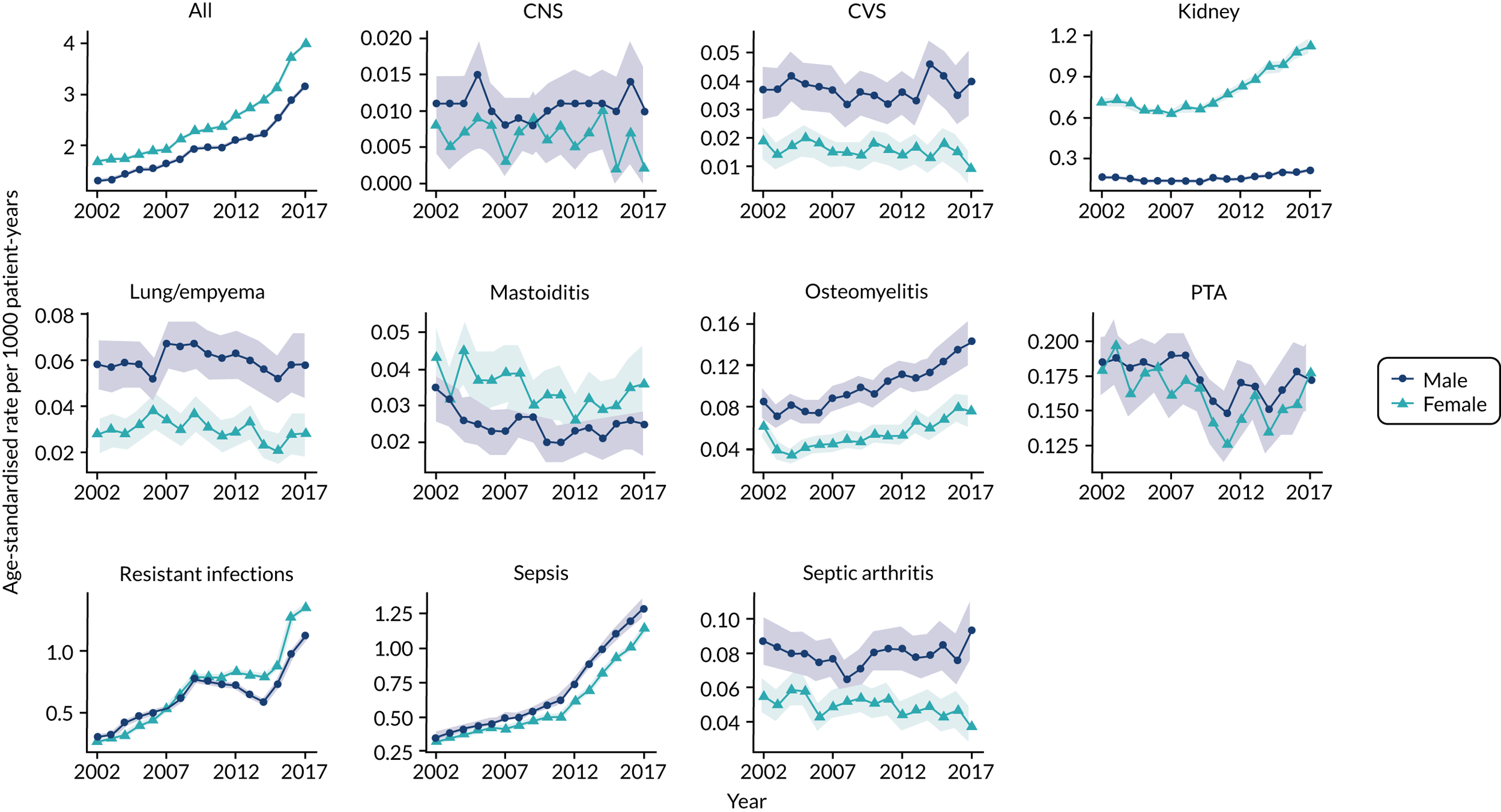
| Group | General practice antibiotic prescribinga | General practice medical codinga | ||
|---|---|---|---|---|
| Lowest fourth of general practices | Highest fourth of general practices | Lowest fourth of general practices | Highest fourth of general practices | |
| All | 1.86 (1.83 to 1.88) | 2.23 (2.20 to 2.25) | 1.94 (1.91 to 1.96) | 2.39 (2.37 to 2.42) |
| CNS infection | 0.008 (0.007 to 0.010) | 0.009 (0.008 to 0.011) | 0.008 (0.007 to 0.009) | 0.010 (0.009 to 0.012) |
| CVS infection | 0.024 (0.021 to 0.027) | 0.026 (0.023 to 0.028) | 0.026 (0.024 to 0.029) | 0.027 (0.025 to 0.030) |
| Kidney infection | 0.40 (0.39 to 0.41) | 0.49 (0.48 to 0.50) | 0.37 (0.37 to 0.38) | 0.55 (0.53 to 0.56) |
| Lung abscess/empyema | 0.042 (0.039 to 0.045) | 0.045 (0.042 to 0.049) | 0.044 (0.041 to 0.047) | 0.049 (0.046 to 0.053) |
| Mastoiditis | 0.025 (0.022 to 0.027) | 0.033 (0.030 to 0.036) | 0.021 (0.019 to 0.023) | 0.036 (0.033 to 0.039) |
| Osteomyelitis | 0.071 (0.067 to 0.075) | 0.073 (0.069 to 0.077) | 0.071 (0.067 to 0.075) | 0.081 (0.077 to 0.086) |
| PTA | 0.16 (0.15 to 0.17) | 0.16 (0.16 to 0.17) | 0.14 (0.14 to 0.15) | 0.17 (0.17 to 0.18) |
| Resistant infections and C. difficile | 0.50 (0.49 to 0.51) | 0.68 (0.67 to 0.69) | 0.63 (0.62 to 0.64) | 0.73 (0.72 to 0.74) |
| Sepsis | 0.57 (0.56 to 0.58) | 0.65 (0.63 to 0.66) | 0.56 (0.55 to 0.57) | 0.67 (0.66 to 0.68) |
| Septic arthritis | 0.064 (0.059 to 0.068) | 0.064 (0.060 to 0.068) | 0.057 (0.053 to 0.061) | 0.068 (0.064 to 0.072) |
Figure 10 presents a forest plot for the association of each serious bacterial infection with 20% higher total antibiotic prescribing at a general practice. The combined estimate revealed that there was no evidence that higher total antibiotic prescribing was associated with lower incidence of serious bacterial infections (adjusted RR 1.03, 95% CI 1.00 to 1.06; p = 0.074). When the 10 classes of serious bacterial infection were considered individually, there was no evidence that higher antibiotic prescribing might be associated with a lower incidence of infections. However, there was weak evidence that incidence of lung abscess and empyema (RR 0.94, 0.88 to 1.00; p = 0.038) might be lower at higher prescribing general practices. There was strong evidence that the recorded incidence of serious bacterial infections was associated with the coding of specific indications for antibiotic prescriptions (adjusted RR for a 20% increase in coding proportion 1.24, 95% CI 1.18 to 1.29; p < 0.001). This association held for each of the 10 classes of serious bacterial infections considered individually.
FIGURE 10.
Forest plot showing the adjusted RR for each type of serious bacterial infection for 20% higher total antibiotic prescribing (dark blue) or 20% higher proportion of antibiotic prescriptions with specific coded indications recorded (light blue). Estimates were adjusted for each variable shown and gender, age group, comorbidity, deprivation fifth, region and year (including quadratic and cubic terms). LL, lower limit; UL, upper limit.
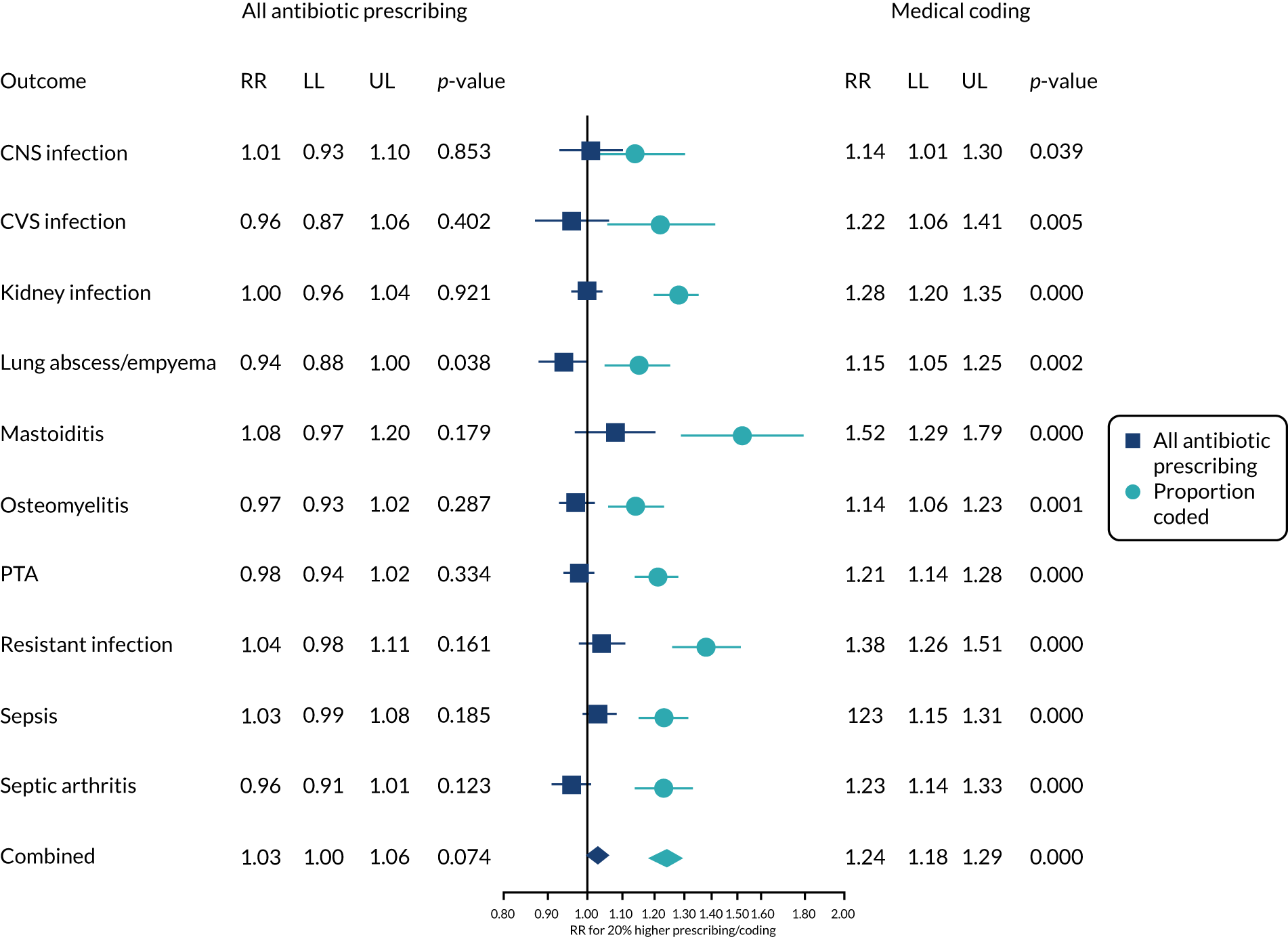
We conducted a sensitivity analysis by excluding repeat prescriptions that might not have been for acute infection episodes. There was no evidence that higher acute (non-repeat) antibiotic prescribing was associated with serious bacterial infections overall (RR 1.02, 95% CI 0.99 to 1.05; p = 0.227) (Figure 11). There was evidence that higher acute antibiotic prescribing might be associated with lower incidence of lung abscess and empyema and septic arthritis. Osteomyelitis and PTA were not judged to be associated with acute antibiotic prescribing after controlling the false discovery rate. There was weak evidence that higher repeat antibiotic prescribing might be associated with higher incidence of serious bacterial infections overall (RR 1.01, 95% CI 1.00 to 1.02; p = 0.054), with evidence of this association for kidney infections, osteomyelitis, PTA and septic arthritis considered separately.
FIGURE 11.
Forest plot showing the adjusted RR for each type of serious bacterial infection for 20% higher acute antibiotic prescribing (dark blue) or repeat antibiotic prescribing (light blue). Estimates were adjusted for each variable shown and gender, age group, comorbidity, deprivation fifth, region and year (including quadratic and cubic terms). LL, lower limit; UL, upper limit.
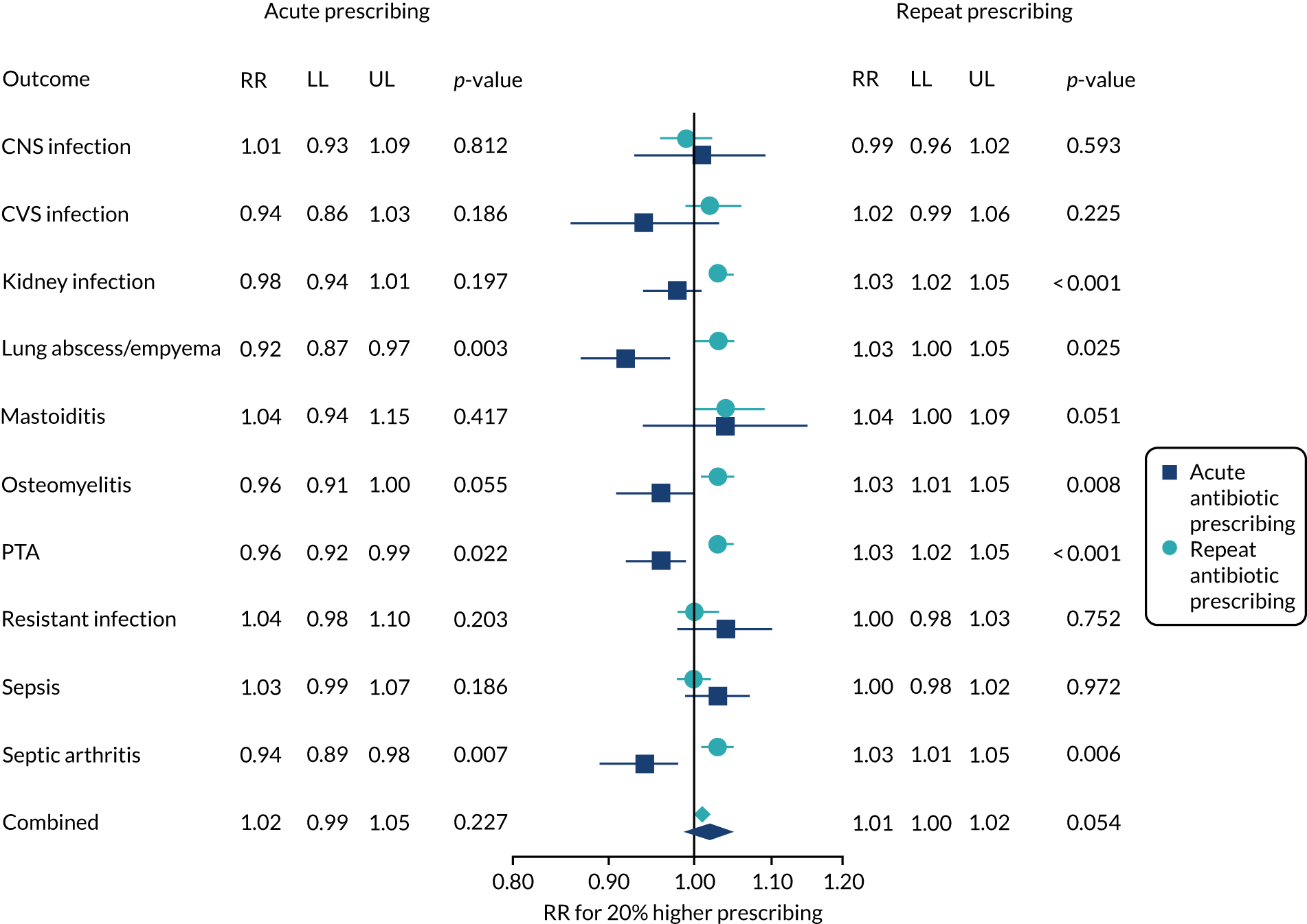
Discussion
Principal findings
The incidence of serious bacterial infections in men and women rose steadily between 2002 and 2017, particularly for sepsis (men and women), osteomyelitis (mainly in men) and kidney infections (mainly in women). The research aimed to test the hypothesis that patients from general practices with lower utilisation of antibiotics might have a greater risk of serious bacterial infections. We evaluated the incidence of serious bacterial infections, including 10 groups of infections that affect different systems of the body, as well as sepsis (including septicaemia). We did not find evidence that general practices that prescribe antibiotics less frequently might have a higher incidence of serious bacterial infections. We found evidence that each type of serious bacterial infection was recorded more frequently at general practices that record diagnostic codes for a high proportion of antibiotic prescriptions, suggesting that variation in the incidence of serious bacterial infection among general practices may be partly an artefact of data recording.
We conducted analyses after excluding repeat prescriptions, and these analyses raised the possibility that general practices with lower acute (non-repeat) antibiotic prescribing might have higher incidence of lung abscess and empyema and septic arthritis. However, these analyses were not pre-planned and should be considered as hypothesis generating, requiring confirmation in future studies. The incidence of these two conditions is less than 1 per 10,000 patients per year, and a relative rate of 0.9 for a 20% increase in prescribing implies that, at most, one additional case might arise every 10 years from a 20% reduction in prescribing at a general practice with 10,000 registered patients.
Strengths and weaknesses of the study
The study drew on data for a large population, comprising data for about 7% of the UK general population. In view of sample size constraints, antibiotic utilisation was estimated through analysis of data for a sample of patients, using hierarchical (multilevel) regression models to obtain general practice-specific antibiotic prescribing estimates. This contrasts with our previous study28 in which age- and sex-standardised rates were calculated from the data for each practice. Use of a regression modelling approach enabled us to make optimal use of the data, as well as adjusting for covariates that are associated with variations in antibiotic prescribing,159 including comorbidity, deprivation, region and calendar year, in addition to age and sex. 160 Consistent with previous studies,8,10 we observed that nearly half of antibiotic prescriptions were not associated with specific coded indications. This suggests that total antibiotic prescribing is the most appropriate exposure measure for consideration, as indication-specific antibiotic prescribing may be associated with considerable misclassification. Serious bacterial infections were identified from medical diagnostic codes recorded into primary care electronic health records, which include general practice records of consultations, hospital referrals and discharges. Many studies have shown that these records have a high predictive value for a range of diagnoses,161 but relying on a single data source can lead to underestimation of the total number of events. 162 CPRD records are linked to HES, but for only a subset of general practices in England, leading to a reduced sample size. There may be changes over time in the use of diagnostic categories, which might, in part, account for increasing diagnoses of ‘sepsis’. A study of US hospitals’ data found that there was a 706% increase in sepsis between 2003 and 2012, without any corresponding increase in positive blood cultures. 54 There was also an apparent increase in resistant infections, but this might also be due in part to data-recording changes and growing awareness of the problem of antimicrobial resistance, as well as true increases in resistant infections. An interrupted time series analysis158 offers an alternative approach to analysis, but this might be susceptible to changes over time in unmeasured confounders, such as code selection. The results of our study draw attention to the problem of poor coding quality in the context of infection management in primary care. Evidence from other studies suggests that missing values are typically missing not at random and the act of data recording may introduce a form of confounding by indication that may bias results. 163 To allow for this, we explicitly evaluated the extent to which differences in data recording between practices might account for variations in the incidence of serious bacterial infections. It is likely that misclassification of exposure and outcome variables from incomplete data recording might lead to underestimation of associations, although the direction of bias cannot always be anticipated. 164 We adjusted for a summary measure of comorbidity. Our analyses do not exclude the possibility that there may be vulnerable subgroups of patients, such as those with immunosuppression, who may be at increased risk if antibiotics are withheld.
Comparison with other studies
Consistent with our findings, Balinskaite et al. 158 reported increasing rates of infection in English primary care and hospital admissions data from 2010 to 2017. Their time series analysis suggested that antimicrobial stewardship intervention in 2015 had no impact on bacterial infections overall, but there was some evidence for increasing hospital admissions for peritonsillar abscess, decreasing hospital admissions for pyelonephritis and decreasing GP consultation rates for empyema. In a previous study, we found that PTA and pneumonia might be more frequent when general practices prescribe antibiotics less frequently for RTIs. 28 We did not include pneumonia in this study because we found that syndromes of ‘chest infection’ and ‘pneumonia’ may be difficult to distinguish in primary care records, with evidence of code shifting between the two categories. 165 In the present study, the incidence of PTA was not associated with total antibiotic prescribing. Randomised trials suggest that antibiotics protect against PTA166 and so it is plausible that this condition might be associated with respiratory antibiotic prescribing, but not with total antibiotic prescribing.
Chapter 8 Peritonsillar abscess and antibiotic prescribing for respiratory infection in primary care
Adapted with permission from Winter et al. 79 © 2020 Annals of Family Medicine, Inc.
Summary
This part of the research aimed to quantify the risk of PTA following consultation for RTI if antibiotics were prescribed or not. From a decision tree, we estimated the probability of PTA following a RTI consultation if antibiotics were prescribed or not. The incidence of new episodes of PTA was 17.2 per 100,000 patient-years for men and 16.1 per 100,000 patient-years for women. A total of 6996 (64%) patients consulted their GP in the 30 days preceding PTA diagnosis, including 4243 (39%) patients consulting for RTI. The probability of PTA following a RTI consultation was greatest in men aged 15–24 years, with one PTA in 565 (95% UI 527 to 605) RTI consultations without antibiotics prescribed, but 1 in 1139 RTI consultations (95% UI 1044 to 1242) if antibiotics were prescribed. One PTA might be avoided for every 1121 (95% UI 975 to 1310) additional antibiotic prescriptions for men aged 15–24 years and for every 926 (95% UI 814 to 1063) additional antibiotic prescriptions for men aged 25–34 years. The risk of PTA following RTI consultation was lower at other ages and lower in women than in men. The risk of PTA may be lower if antibiotics are prescribed for RTI, but even in young men nearly 1000 antibiotic prescriptions may be required to prevent one PTA.
Introduction
Reducing antibiotic prescribing raises a concern that lower antibiotic prescribing might increase the risk of serious bacterial infections. In a recent study, we found evidence that PTA, also known as quinsy, may be more frequent at general practices that prescribe fewer antibiotics for respiratory infections. 28 This is plausible because a meta-analysis of eight clinical trials found that antibiotic treatment for sore throat reduced the risk of PTA, but the review included only 25 cases of PTA, of which 16 were from a trial reported in 1951 when quinsy was more frequent. 166 PTA is less frequent now. 167 There is often evidence of infection with multiple micro-organisms,168 but group A streptococci and Fusobacterium necrophorum infections may be the most common pathogens. 169 The present study aimed to quantify the risks of PTA following a RTI consultation according to whether or not antibiotics were prescribed. We determined how often cases of PTA arise in patients who have previously consulted for RTI and quantified the effect of antibiotic treatment on the risks of PTA. We aimed to integrate these empirical estimates into a model to contribute to informing clinical decision-making.
Results
Incidence of peritonsillar abscess
There were 11,007 patients from 718 general practices in the May 2018 release of CPRD GOLD, with first episodes of PTA from 2002 to 2017. Fifty-three per cent of patients were male and 75% were aged 15–44 years (Table 12). The age-standardised incidence of new episodes of PTA was 17.2 per 100,000 patient-years for men and 16.1 per 100,000 patient-years for women. Between 2002 and 2017, annual age-standardised incidence rates ranged between 12.7 and 19.5 per 100,000 patient-years for both men and women, with no consistent trend. Age-specific incidence rates revealed that the incidence of PTA increased up to 15–19 years (men: 45.3 per 100,000 patient-years, 95% CI 44.7 to 45.8; women: 51.8 per 100,000 patient-years, 95% CI 51.3 to 52.4) and then decreased with increasing age (Figure 12).
| Characteristic | Male | Female | Total |
|---|---|---|---|
| Total | 5817 (53) | 5190 (47) | 11,007 |
| Age group (years) | |||
| 0–4 | 42 (1) | 30 (1) | 72 (1) |
| 5–14 | 316 (5) | 365 (7) | 681 (6) |
| 15–24 | 1737 (30) | 1593 (31) | 3330 (30) |
| 25–34 | 1255 (22) | 1276 (25) | 2531 (23) |
| 35–44 | 1330 (23) | 1085 (21) | 2415 (22) |
| 45–54 | 611 (11) | 440 (8) | 1051 (10) |
| 55–64 | 311 (5) | 224 (4) | 535 (5) |
| 65–74 | 153 (3) | 125 (2) | 278 (3) |
| 75–84 | 54 (1) | 35 (1) | 89 (1) |
| ≥ 85 | 8 (0) | 17 (0) | 25 (0) |
| Period of diagnosis | |||
| 2002–6 | 2042 (35) | 1877 (36) | 3919 (36) |
| 2007–11 | 2039 (35) | 1743 (34) | 3782 (34) |
| 2012–17 | 1736 (30) | 1570 (30) | 3306 (30) |
| Comorbidity | |||
| Absent | 4268 (73) | 3596 (69) | 7864 (71) |
| Present | 1549 (27) | 1594 (31) | 3143 (29) |
| Smoking status | |||
| Current smoker | 2078 (36) | 1680 (32) | 3758 (34) |
| Ex-smoker | 673 (12) | 619 (12) | 1292 (12) |
| Non-smoker | 3066 (53) | 2891 (56) | 5957 (54) |
| Season | |||
| Winter (December–February) | 1535 (26) | 1411 (27) | 2946 (27) |
| Spring (March–May) | 1530 (26) | 1367 (26) | 2897 (26) |
| Summer (June–August) | 1397 (24) | 1227 (24) | 2624 (24) |
| Fall (September–November) | 1355 (23) | 1185 (23) | 2540 (23) |
FIGURE 12.
Age- and gender-specific 30-day probability of PTA after RTI consultation in patients who consulted for self-limiting RTI, with and without antibiotic prescription. Vertical lines represent 95% UIs. (a) Female; and (b) male.

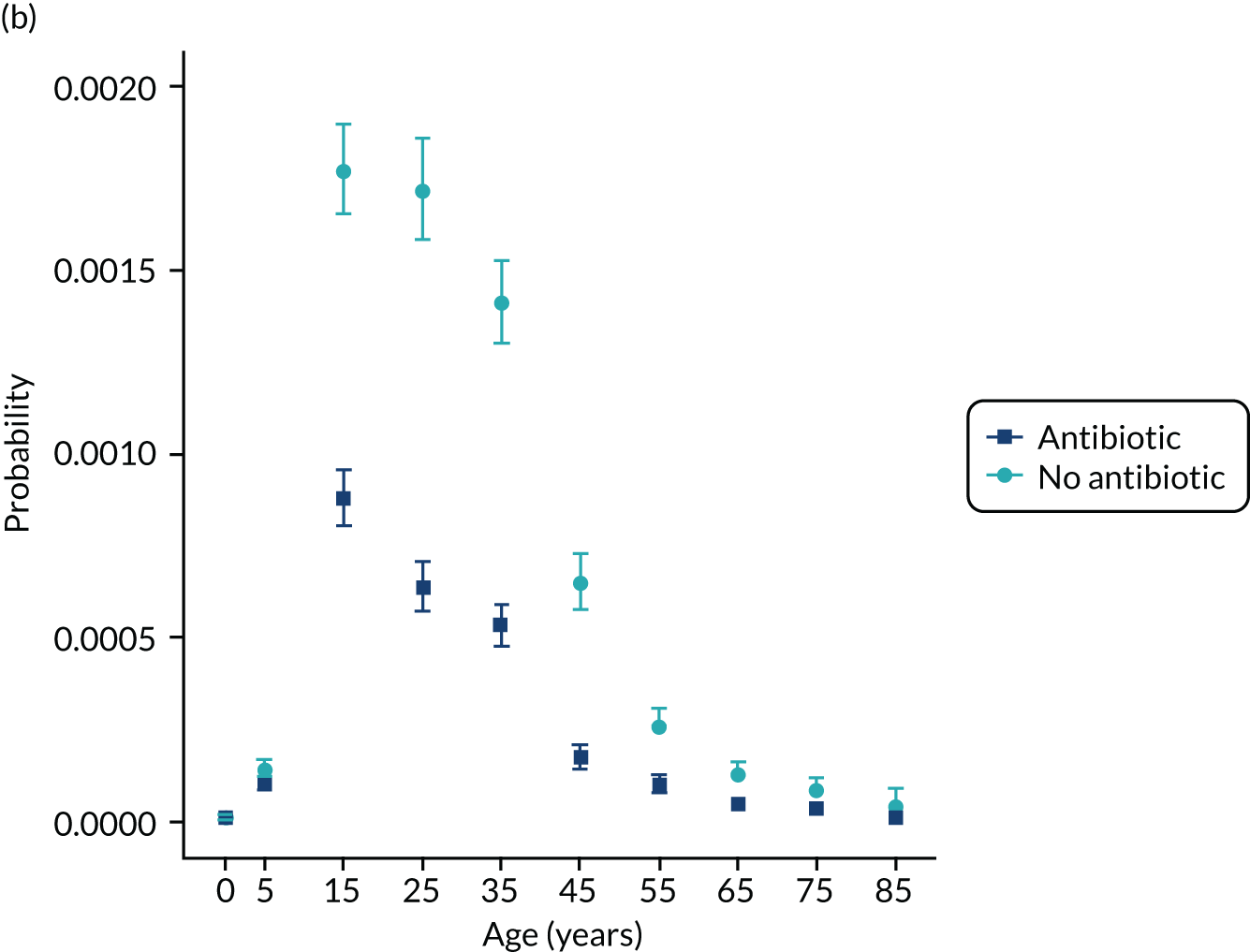
Patients with PTA were more likely to be smokers (34% of PTA patients were current smokers vs. 16% of person-time in a sample of the general population), whereas 12% of both groups had previously smoked. This effect was consistent when stratifying by gender and remained consistent over time. The prevalence of comorbidity was lower among PTA cases (28.6%) than in the general population (40.8% of all follow-up time was in patients with comorbidities). This pattern persisted when stratifying by gender. When stratifying by age, comorbidity prevalence was lower in PTA patients than in the general population in people aged 15–64 years, but not in people aged < 15 years or ≥ 65 years. PTA was evenly distributed across general practice IMD deciles (see Table 12). There were slightly more cases in winter and spring than in summer and autumn.
Consultation and prescription patterns before peritonsillar abscess diagnosis
Among 11,007 incident cases of PTA, 6996 patients (64%) had consulted the general practice in the 30 days preceding diagnosis of PTA, including 4243 (39%) consulting with any self-limiting RTI (Table 13). The majority of consultations were in the 7 days before PTA diagnosis. Similar patterns of associations were observed when considering 14- and 60-day thresholds. There were 3782 (34%) patients who received a prescription for antibiotics in the 30 days before PTA diagnosis.
| Days preceding PTA diagnosis | Number (%) of PTA cases consulting for | Number (%) of PTA cases prescribed antibiotics in the same period | |
|---|---|---|---|
| RTI | Any reason | ||
| 7 | 3406 (30.9) | 5358 (48.7) | 2826 (25.7) |
| 14 | 3874 (35.2) | 6215 (56.5) | 3305 (30.0) |
| 30 | 4243 (38.5) | 6996 (63.6) | 3782 (34.4) |
| 60 | 4556 (41.4) | 7903 (71.8) | 4185 (38.0) |
Decision tree
A decision tree was constructed to calculate the 30-day probability of RTI consultation, antibiotic prescription and PTA. For the whole population, the probability of consultation for self-limiting RTI in a 30-day period was 0.0275 (95% CI 0.0275 to 0.0276). The probability of antibiotic prescription at a self-limiting RTI consultation was 0.483 (95% CI 0.483 to 0.484). Age- and gender-specific probabilities of PTA, given that patients had consulted for RTI, were also calculated (Table 14). The probability of PTA was higher in men than in women and was the greatest between 15 and 34 years of age (see Figure 12). In both men and women and at all ages, the probability of PTA following a RTI was lower if an antibiotic was prescribed. The NNT with an antibiotic to prevent one case of PTA was also calculated for each age group and gender. The NNT was lower for patients with the highest probability of PTA. At aged 25–34 years, the number of patients with RTI who would need to be prescribed an antibiotic to prevent one PTA case was 926 (95% CI 814 to 1063) for men and 1984 (95% CI 1756 to 2263) for women. The numbers were similar in men and women aged 15–24 and 35–44 years, but were substantially greater for either younger or older patients (Table 15).
| Age group (years) | Probability of PTA (× 100,000) (95% UI) | |||
|---|---|---|---|---|
| Male | Female | |||
| Without antibiotics | With antibiotics | Without antibiotics | With antibiotics | |
| 0–4 | 1.1 (0.6 to 1.7) | 0.9 (0.4 to 1.7) | 0.9 (0.4 to 1.4) | 0.8 (0.3 to 1.5) |
| 5–14 | 14.3 (12.2 to 16.6) | 10.3 (8.5 to 12.3) | 16.4 (14.1 to 18.8) | 10.8 (8.9 to 12.9) |
| 15–24 | 177.1 (165.3 to 189.6) | 87.8 (80.5 to 95.7) | 97.4 (90.9 to 104.4) | 48.1 (44.0 to 52.5) |
| 25–34 | 171.7 (158.3 to 185.8) | 63.6 (57.1 to 70.6) | 79.5 (73.4 to 85.7) | 29.0 (26.0 to 32.2) |
| 35–44 | 141.2 (130.2 to 152.7) | 53.3 (47.7 to 59.0) | 62.3 (57.3 to 67.7) | 21.2 (18.9 to 23.6) |
| 45–54 | 65.0 (57.8 to 72.9) | 17.4 (14.3 to 20.9) | 27.6 (24.3 to 31.2) | 6.8 (5.5 to 8.2) |
| 55–64 | 25.8 (21.4 to 30.6) | 10.1 (7.8 to 12.6) | 12.4 (10.2 to 14.9) | 4.4 (3.4 to 5.6) |
| 65–74 | 12.7 (9.7 to 16.1) | 4.3 (2.9 to 6.1) | 7.7 (5.8 to 9.8) | 2.6 (1.7 to 3.6) |
| 75–84 | 8.3 (5.3 to 12.0) | 3.1 (1.6 to 5.1) | 3.7 (2.2 to 5.6) | 1.5 (0.7 to 2.5) |
| ≥ 85 | 4.1 (1.4 to 8.9) | 0.8 (0.1 to 2.6) | 4.1 (1.6 to 7.9) | 1.0 (0.1 to 2.8) |
| Age group (years) | Antibiotic prescriptions to prevent one PTA (95% UI) | |
|---|---|---|
| Male | Female | |
| 0–4 | 170,895 (–3,454,195 to 3,610,347)a | 195,903 (–4,477,555 to 4,509,199)a |
| 5–14 | 25,042 (15,160 to 63,304) | 17,982 (11,927 to 35,987) |
| 15–24 | 1121 (975 to 1310) | 2032 (1770 to 2366) |
| 25–34 | 926 (814 to 1063) | 1984 (1756 to 2263) |
| 35–44 | 1139 (1002 to 1314) | 2440 (2154 to 2780) |
| 45–54 | 2107 (1798 to 2512) | 4805 (4093 to 5684) |
| 55–64 | 6386 (4818 to 9074) | 12,559 (9591 to 17,435) |
| 65–74 | 12,076 (8410 to 19,391) | 19,876 (13,857 to 32,275) |
| 75–84 | 19,386 (10,941 to 53,619) | 45,266 (23,961 to 139,012) |
| ≥ 85 | 33,156 (11,691 to 164,491) | 33,298 (12,465 to 178,502) |
Linked hospital episodes data
In the subset of patients with linked hospital records, the age-standardised incidence of PTA was 20.6 per 100,000 patient-years for men and 19.0 per 100,000 patient-years for women. The distribution of cases over time and between age groups was similar between CPRD and HES data (see Report Supplementary Material 3, Supplementary Figure 1). Estimates of the risk of PTA with and without antibiotics were consistent with data from CPRD. The protective effect of antibiotics was also evident when considering only PTA cases resulting in hospitalisation (see Report Supplementary Material 3, Supplementary Figure 2).
Patient and public involvement
The findings from these analyses were presented at a PPI meeting. Patients observed that it would be useful to understand the experience of having PTA and this would help to clarify the nature of any benefit from antibiotic prescribing. However, benefits from prescribing were at the individual level, whereas from the population perspective the risks from antimicrobial resistance might be more concerning. In the worst-case scenario, reduced use of antibiotics would have to be tolerated to ensure that antibiotics did not stop working. Patients commented that the results were an ‘eye opener’, with GPs needing to see these numbers before thinking about prescribing. The results could also be communicated to patients to inform their understanding of when antibiotics might be needed.
Discussion
Principal findings
There have been few large population-based studies of PTA. 167 This study provides precise estimates for disease incidence and showed that PTA is most frequent in young people aged 15–24 years and is more frequent in men. Nearly two-thirds of patients consulted at their general practice during the 30 days before PTA diagnosis, with about half of these consultations being recorded as self-limiting RTIs. The analyses demonstrate a protective effect of antibiotic prescribing against PTA following a RTI consultation for both men and women and in all age groups. However, the overall risk of PTA was low, and ≥ 1000 antibiotic prescriptions would be required to prevent one PTA, even in the age group at the highest risk. Consultation rates for respiratory illness are generally considerably higher in women than in men, and this may account for the generally lower risk of PTA following a consultation for RTI in women than men.
Strengths and weaknesses of the study
The study benefited from a very large sample size drawn from all parts of the UK. The study population comprised just under 10 million patients, with 11,000 PTA cases over 16 years, providing precise estimates for a rare outcome. A key limitation of this study is that its non-randomised design meant that patients were not randomly assigned to antibiotic treatment. It is likely that patients who received antibiotics had more severe illness and a greater risk of PTA than patients who did not receive antibiotics. Consequently, the risk of PTA in antibiotic-treated patients may have been overestimated, and that in untreated patients underestimated, in comparison with possible random allocation. As a result, the protective effect of antibiotic treatment may have been underestimated and the true number of antibiotic prescriptions needed to prevent one PTA may be smaller than we estimate. It was not possible to include data on symptoms or severity of RTIs. We searched patients’ records for markers of illness severity, including fever, lymphadenitis or mention of Centor criteria,170 but these were recorded in only a very small number of cases. As noted by others, not all consultations in primary care electronic records have a clinical code assigned to them. 8 Almost one-third of PTA patients consulted their general practice with a self-limiting RTI in the 30 days preceding their PTA diagnosis, but this figure may be an underestimate because it is known that acute illness presentations are not always well coded in primary care. 8 The data for the study were recorded prospectively by family physicians and other primary care professionals, but the data were collected for clinical practice and not research. Standardised recording procedures were not used, leading to variability in data recording. The estimates represent values expected at an average practice, but estimates might vary depending on the consultation and prescribing rates or data recording procedures used at different practices. Previous studies have shown that diagnoses recorded into electronic health records have high predictive validity,161 but there is evidence of differential recording in alternative data sources. 162 Our analyses showed that analysis of hospital records, in addition to primary care records, did not change conclusions overall. In a clinical trial, an overall effect is usually estimated before subgroup analyses are performed, but this approach is not always desirable in epidemiological analysis of population data, especially when rates vary considerably by age. 171,172 In view of the age-related variations in incidence of PTA, we analysed data by subgroup of age and gender, but we note that small differences across subgroups should be interpreted with caution.
Comparison with previous studies
In a previous study, there was evidence that general practices with lower rates of antibiotic prescription for RTI had a higher incidence of PTA,28 it being estimated that there might be one additional case of PTA over 10 years for a general practice that reduced antibiotic prescribing for RTI by 10%. 28 The present individual patient-level analyses show that antibiotic prescription at a RTI consultation is associated with reduced risk of PTA, consistent with the more limited evidence from randomised clinical trials. In a review of randomised controlled trials,166 the relative risk of PTA within 60 days of a sore throat was 0.15 (95% CI 0.05 to 0.47) after antibiotic treatment compared with no antibiotic. However, most evidence was provided by one trial published in 1951 with a relatively small sample size. 166,173 Previous non-randomised studies also suggested a protective effect of antibiotics against PTA, but did not quantify age- and gender-specific risks following a primary care consultation for RTI. 56,58,174
Our estimates of PTA incidence (16–17/100,000 person-years) from CPRD data were consistent with those of previous studies,28 although our study spanned a wider time period. Previous studies have been limited by not including data from hospital discharge records, but we found that the associations between PTA incidence and antibiotic use were consistent whether we analysed CPRD data only or also included HES data. Consistent with previous studies, our data show an excess of current smokers compared with population reference data for smoking prevalence. 175 Comorbidity is not usually considered a risk factor for PTA. 167 Consistent with Klug’s review,167 there was only weak evidence of seasonality.
The risk of PTA might be higher following presentations for sore throat or tonsillitis. However, respiratory infections often have mixed presentation, beginning with a sore throat, followed by symptoms of coryza and later cough. 176 RTI presentations are also often indistinctly coded, making analysis of subgroups challenging. 8 Clinical guidelines for the management of sore throat in the UK advocate the use of clinical risk scoring with either Centor170 or FeverPAIN177 scores. Bacteriological testing is discouraged and is infrequent in the UK, possibly in contrast to the USA, where this is endorsed in some clinical guidelines. 178
Chapter 9 Probability of sepsis after infection consultations in primary care
Adapted with permission from Gulliford et al. 179 This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Summary
This part of the research aimed to estimate, through a cohort study, the probability of sepsis following infection consultations in primary care when antibiotics were or were not prescribed. The probability of sepsis was found to be lower if an antibiotic was prescribed, but the number of antibiotic prescriptions required to prevent one episode of sepsis (i.e. NNT) decreased with age. At age 0–4 years, the NNT was 29,773 (95% UI 18,458 to 71,091) in boys and 27,014 (95% UI 16,739 to 65,709) in girls. At the age of ≥ 85 years, the NNT was 262 (95% UI 236 to 293) in men and 385 (95% UI 352 to 421) in women. Frailty was associated with a greater risk of sepsis and a lower NNT. For severely frail patients aged 55–64 years, the NNT was 247 (95% UI 156 to 459) in men and 343 (95% UI 234 to 556) in women. At all ages, the probability of sepsis was greatest for UTI, followed by skin infection, followed by RTI. At the age of 65–74 years, the NNT following RTI was 1257 (95% UI 1112 to 1434) in men and 2278 (95% UI 1966 to 2686) in women. The NNT following skin infection was 503 (95% UI 398 to 646) in men and 784 (95% UI 602 to 1051) in women. Following UTI, the NNT was 121 (95% UI 102 to 145) in men and 284 (95% UI 241 to 342) in women. NNT values were generally smaller for the period from 2014 to 2017, when sepsis was diagnosed more frequently. These stratified estimates of risk help to identify groups in whom antibiotic prescribing may be more safely reduced. Risks of sepsis and benefits of antibiotics are more substantial among older adults, persons with more advanced frailty or following UTIs.
Introduction
Strategies to reduce inappropriate use of antibiotics must ensure that antibiotics can be used when they are needed. 22,27 Bacterial infections are still of public health importance and there has been growing recognition of the importance of sepsis, with > 200,000 hospital admissions for sepsis each year in England, with up to 59,000 deaths. 29 Early recognition and treatment of sepsis is being promoted by health services and professional organisations through assessment of risk for individual patients. 34 In the UK, a National Early Warning Score based on six physiological parameters has been promoted to identify individual patients who may be at risk of sepsis. 30 However, this approach has also been criticised because early warnings signs of sepsis are often non-specific and alerting systems may result in false-positive signals at many consultations. 180
Research is required to provide quantitative estimates of risk that might provide clinicians and patients with evidence to inform antibiotic prescribing decisions. This study aimed to estimate the probability of sepsis if antibiotics were prescribed or not and to estimate the number of antibiotic prescriptions required to prevent one episode of sepsis. We estimated the probability of sepsis for groups of patients characterised by age, gender and frailty, as well as reason for consultation.
Results
Main results
The study included 706 general practices with a total of 66.2 million person-years of follow-up (Figure 13). Data for the distribution of sepsis patients by age and gender are shown in Table 16. The probability of a consultation with a common infection of the skin, respiratory tract or urinary tract in any 30-day period ranged between 0.02 (1 in 50) and 0.08 (1 in 12). This probability of an infection consultation was greater in children and older people and higher in women than men during mid-life (Table 17). The probability of an antibiotic being prescribed at an infection consultation ranged from 0.43 to 0.67, being lowest for young children, in whom consultation rates are highest (see Table 17).
FIGURE 13.
Flow chart showing participant selection for the main and linked samples.

| Gender | Age group (years) | Sepsis events (n) | Infection consultations in previous 30 days (n) | Proportion (%) of sepsis events preceded by infection consultations | Antibiotic prescriptions on same date (n) | Proportion (%) of infection consultations with antibiotics prescribed |
|---|---|---|---|---|---|---|
| Male | 0–4 | 224 | 51 | 22.8 | 11 | 21.6 |
| 5–14 | 303 | 48 | 15.8 | 6 | 12.5 | |
| 15–24 | 360 | 59 | 16.4 | 21 | 35.6 | |
| 25–34 | 449 | 78 | 17.4 | 18 | 23.1 | |
| 35–44 | 791 | 117 | 14.8 | 24 | 20.5 | |
| 45–54 | 1342 | 241 | 18.0 | 47 | 19.5 | |
| 55–64 | 2466 | 472 | 19.1 | 102 | 21.6 | |
| 65–74 | 3933 | 724 | 18.4 | 155 | 21.4 | |
| 75–84 | 4752 | 1089 | 22.9 | 256 | 23.5 | |
| ≥ 85 | 2738 | 713 | 26.0 | 158 | 22.2 | |
| Female | 0–4 | 204 | 55 | 27.0 | 12 | 21.8 |
| 5–14 | 238 | 32 | 13.4 | 9 | 28.1 | |
| 15–24 | 500 | 76 | 15.2 | 24 | 31.6 | |
| 25–34 | 806 | 110 | 13.6 | 38 | 34.5 | |
| 35–44 | 1095 | 175 | 16.0 | 41 | 23.4 | |
| 45–54 | 1631 | 267 | 16.4 | 72 | 27.0 | |
| 55–64 | 2443 | 445 | 18.2 | 119 | 26.7 | |
| 65–74 | 3215 | 646 | 20.1 | 180 | 27.9 | |
| 75–84 | 3982 | 890 | 22.4 | 204 | 22.9 | |
| ≥ 85 | 3772 | 984 | 26.1 | 222 | 22.6 |
| Gender | Age group (years) | Probability of . . . | NNT (95% UI) | |||||
|---|---|---|---|---|---|---|---|---|
| Infection consultation per 30 days, P(Infection) | First sepsis event in any 30-day period, P(Sepsis) | Infection consultation in 30 days before sepsis event, P(Infection | Sepsis) | Antibiotics at infection consultation, P(AB | Infection) | Sepsis after infection consultation, no antibiotics, P(Sepsis|[No AB|Infection] | Sepsis after infection consultation, antibiotics, P(Sepsis| [AB|Infection] | |||
| Male | 0–4 | 0.08 | 0.000014 | 0.23 | 0.43 | 0.000054 | 0.000020 | 29,773 (18,458 to 71,091) |
| 5–14 | 0.04 | 0.000006 | 0.16 | 0.48 | 0.000047 | 0.000008 | 25,606 (17,962 to 40,817) | |
| 15–24 | 0.02 | 0.000008 | 0.17 | 0.58 | 0.000101 | 0.000041 | 16,921 (10,285 to 39,551) | |
| 25–34 | 0.02 | 0.000009 | 0.17 | 0.60 | 0.000193 | 0.000039 | 6517 (4779 to 9522) | |
| 35–44 | 0.02 | 0.000013 | 0.15 | 0.62 | 0.000239 | 0.000039 | 5035 (3980 to 6610) | |
| 45–54 | 0.02 | 0.000022 | 0.18 | 0.62 | 0.000472 | 0.000071 | 2497 (2121 to 2999) | |
| 55–64 | 0.02 | 0.000048 | 0.19 | 0.63 | 0.000825 | 0.000135 | 1449 (1282 to 1652) | |
| 65–74 | 0.03 | 0.000105 | 0.18 | 0.64 | 0.001305 | 0.000202 | 907 (823 to 1007) | |
| 75–84 | 0.04 | 0.000219 | 0.23 | 0.63 | 0.002700 | 0.000478 | 450 (413 to 492) | |
| ≥ 85 | 0.05 | 0.000416 | 0.26 | 0.61 | 0.004647 | 0.000833 | 262 (236 to 293) | |
| Female | 0–4 | 0.08 | 0.000014 | 0.27 | 0.43 | 0.000060 | 0.000023 | 27,014 (16,739 to 65,709) |
| 5–14 | 0.04 | 0.000005 | 0.14 | 0.51 | 0.000025 | 0.000010 | 65,522 (35,239 to 240,067) | |
| 15–24 | 0.04 | 0.000012 | 0.15 | 0.61 | 0.000080 | 0.000024 | 18,120 (12,472 to 30,241) | |
| 25–34 | 0.04 | 0.000016 | 0.14 | 0.63 | 0.000105 | 0.000033 | 13,926 (10,044 to 21,273) | |
| 35–44 | 0.04 | 0.000018 | 0.16 | 0.66 | 0.000184 | 0.000030 | 6513 (5349 to 8194) | |
| 45–54 | 0.03 | 0.000028 | 0.16 | 0.66 | 0.000278 | 0.000054 | 4463 (3756 to 5421) | |
| 55–64 | 0.04 | 0.000048 | 0.18 | 0.67 | 0.000490 | 0.000088 | 2486 (2179 to 2876) | |
| 65–74 | 0.04 | 0.000080 | 0.20 | 0.67 | 0.000793 | 0.000151 | 1557 (1388 to 1758) | |
| 75–84 | 0.05 | 0.000138 | 0.22 | 0.66 | 0.001525 | 0.000231 | 773 (705 to 847) | |
| ≥ 85 | 0.05 | 0.000271 | 0.26 | 0.64 | 0.003110 | 0.000509 | 385 (352 to 421) | |
There were 35,244 first episodes of sepsis between 2002 and 2017. The probability of an infection consultation within 30 days before a sepsis event ranged between 0.14 (one in seven) and 0.26 (one in four), with larger values at the extremes of age (see Table 17). If no antibiotic was prescribed, the probability of sepsis at age 0–4 years was 0.000054 (1 in 18,519 consultations) in males and 0.000060 (1 in 16,667 consultations) in females. The probability of sepsis following an infection consultation without antibiotics increased linearly with age on a log-scale (Figures 14a and b), reaching 0.004647 (1 in 215 consultations) in males and 0.003110 (1 in 321 consultations) in females aged ≥ 85 years (see Table 17). If antibiotics were prescribed at an infection consultation, the estimated probability of sepsis was lower, ranging from 0.000020 (1 in 50,000 consultations) in males to 0.000023 (1 in 43,478 consultations) in females aged 0–4 years to 0.000833 (1 in 1200 consultations) in males and 0.000509 (1 in 1965 consultations) in females aged ≥ 85 years. The number of antibiotic prescriptions required to prevent one sepsis event was highly age dependent (see Figures 14c and d). Among children aged 0–4 years, the NNT was 29,773 (95% UI 18,458 to 71,091) in males and 27,014 (95% UI 16,739 to 65,709) in females. However, at the age of ≥ 85 years, the NNT was 262 (95% UI 236 to 293) in males and 385 (95% UI 352 to 421) in females.
FIGURE 14.
Probability of sepsis following infection consultations in primary care if antibiotics are prescribed or not for (a) males and (b) females. Number of antibiotic prescriptions required to prevent one sepsis event (i.e. NNT) for (c) males and (d) females. Figures are median probability (95% UI).
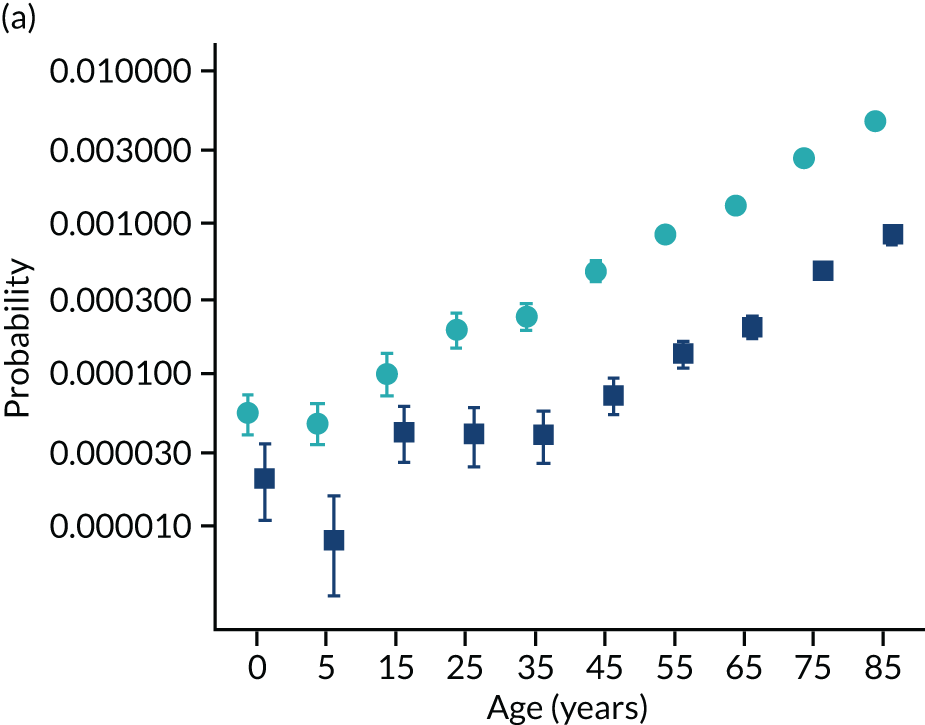
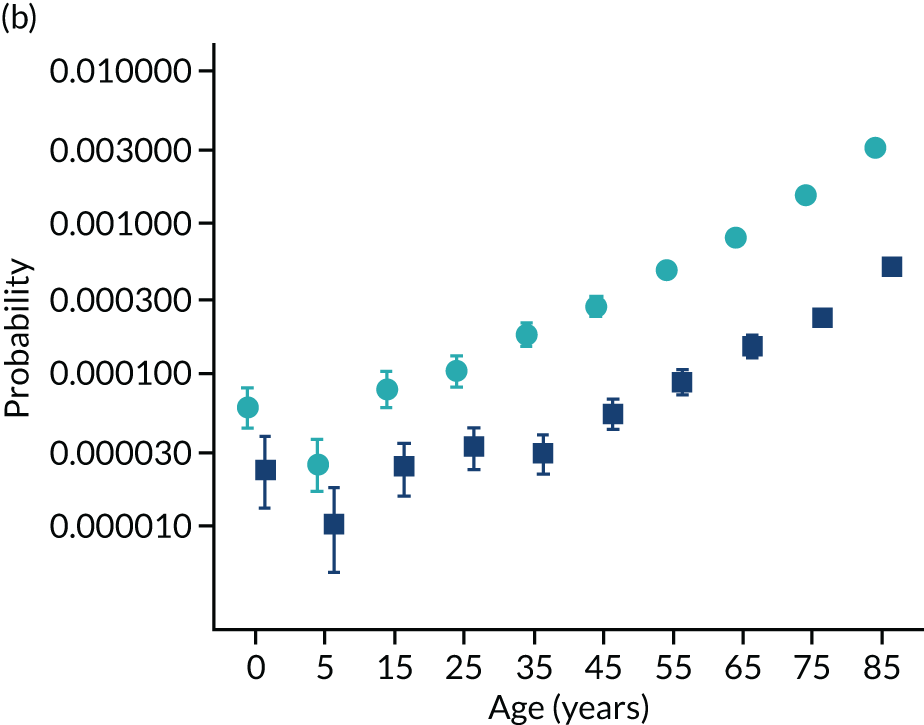
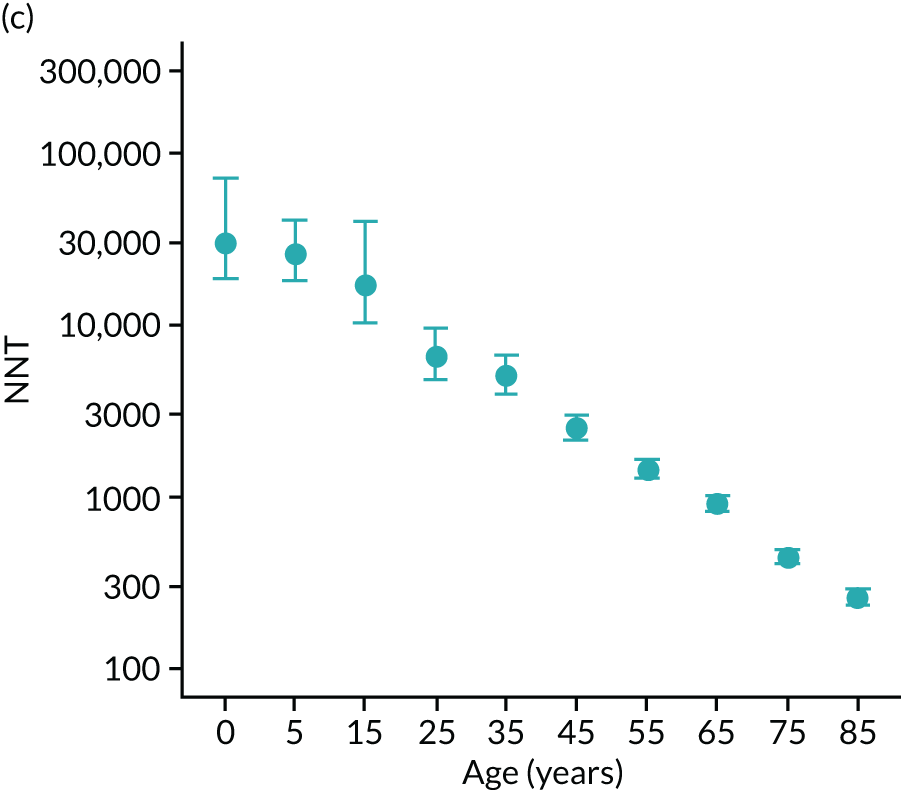
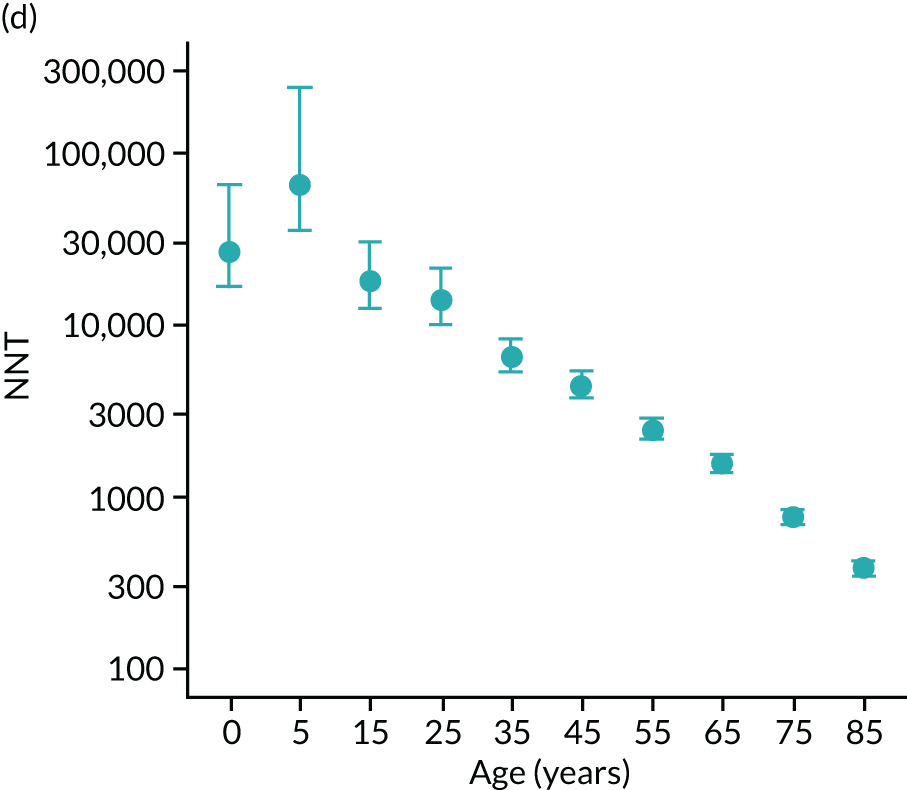
In the population aged ≥ 55 years, estimates were obtained separately by frailty category (Figure 15). The probability of sepsis was greater and the NNT smaller for patients with more advanced frailty. For ‘non-frail’ patients aged 65–74 years, the NNT was 1680 (95% UI 1354 to 2133) for men and 2718 (95% UI 2089 to 3697) for women. However, for patients of the same age with severe frailty, the NNT was 259 (95% UI 196 to 360) for men and 438 (95% UI 329 to 624) for women. For patients with severe frailty, the NNT was < 250 in men and < 400 in women for all age groups over 55 years. For non-frail patients, the probability of sepsis increased and the NNT decreased with increasing age (see Figure 15). In ‘non-frail’ patients, the NNT declined from 2309 (95% UI 1890 to 2879) in men and 3782 (95% UI 3001 to 4907) in women at 55–64 years to 407 (95% UI 274 to 677) in men and 499 (95% UI 346 to 780) in women aged ≥ 85 years. Estimates for patients with ‘mild’ or ‘moderate’ frailty exhibited an intermediate pattern (see Figure 15).
FIGURE 15.
Number of antibiotic prescriptions required to prevent one sepsis event (i.e. NNT) following infection consultations in primary care, by frailty level, gender and age group. Figures are median estimate (95% UI). (a) Male; and (b) female.
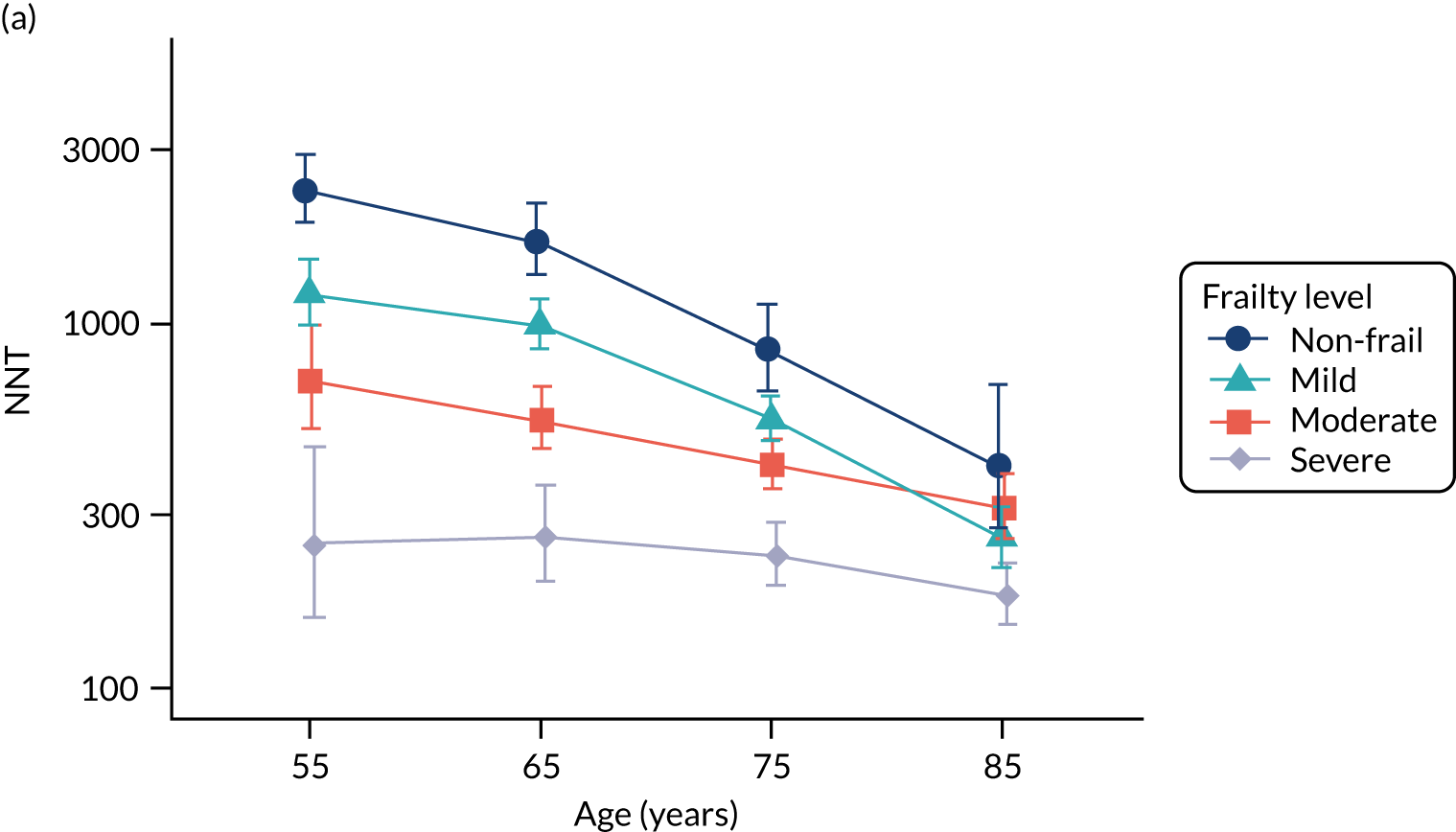
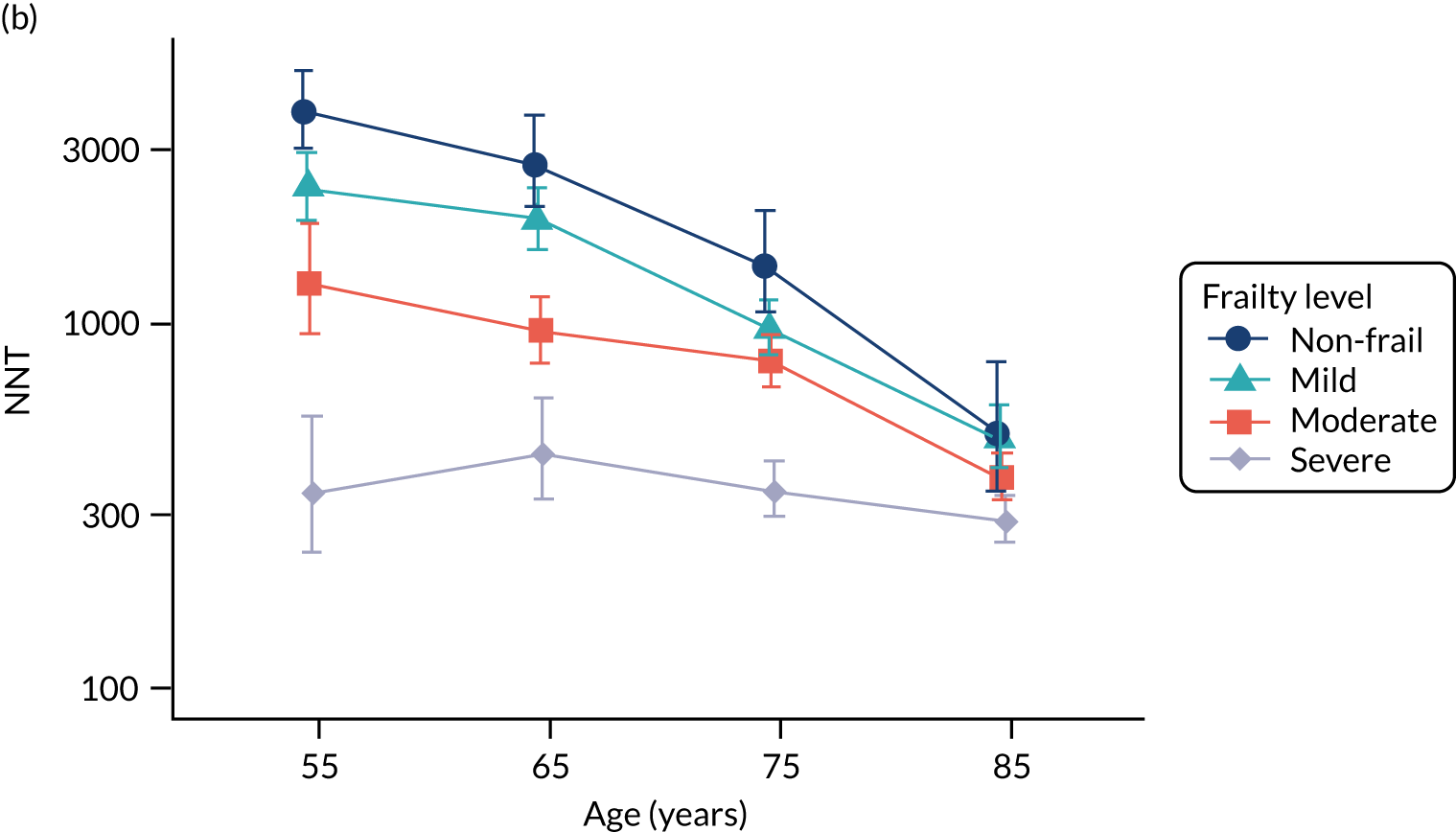
The probability of sepsis was higher following consultations for UTI than for skin infections or RTI; a pattern of association that was observed across all age groups and in both men and women (Figure 16). For patients aged 65 years without antibiotic treatment, the probability of sepsis following a RTI consultation was 0.00090 (1 in 1111 consultations) in men and 0.00053 (1 in 1887 consultations) in women. Following a skin infection consultation, the probability of sepsis following a RTI consultation was 0.00224 (1 in 446 consultations) in men and 0.00150 (1 in 667 consultations) in women. Following a UTI consultation, the probability was 0.009227 (1 in 108) in men and 0.003787 (1 in 264) in women. At the same age, the corresponding NNTs for RTI were 1257 (95% UI 1112 to 1434) for men and 2278 (95% UI 1965 to 2686) for women. For skin infection, this was 502 (95% UI 398 to 646) for men and 784 (95% UI 602 to 1051) for women. Finally, for UTI consultations, this was 120 (95% UI 102 to 145) for men and 284 (95% UI 241 to 342) for women (see Figure 16).
FIGURE 16.
Number of antibiotic prescriptions required to prevent one sepsis event (i.e. NNT), by age group, gender and type of infection consultation. Figures are median estimate (95% UI). UIs were omitted for age 0–4 and 5–9 years if data were too sparse to give reliable estimates. (a) Male; and (b) female.
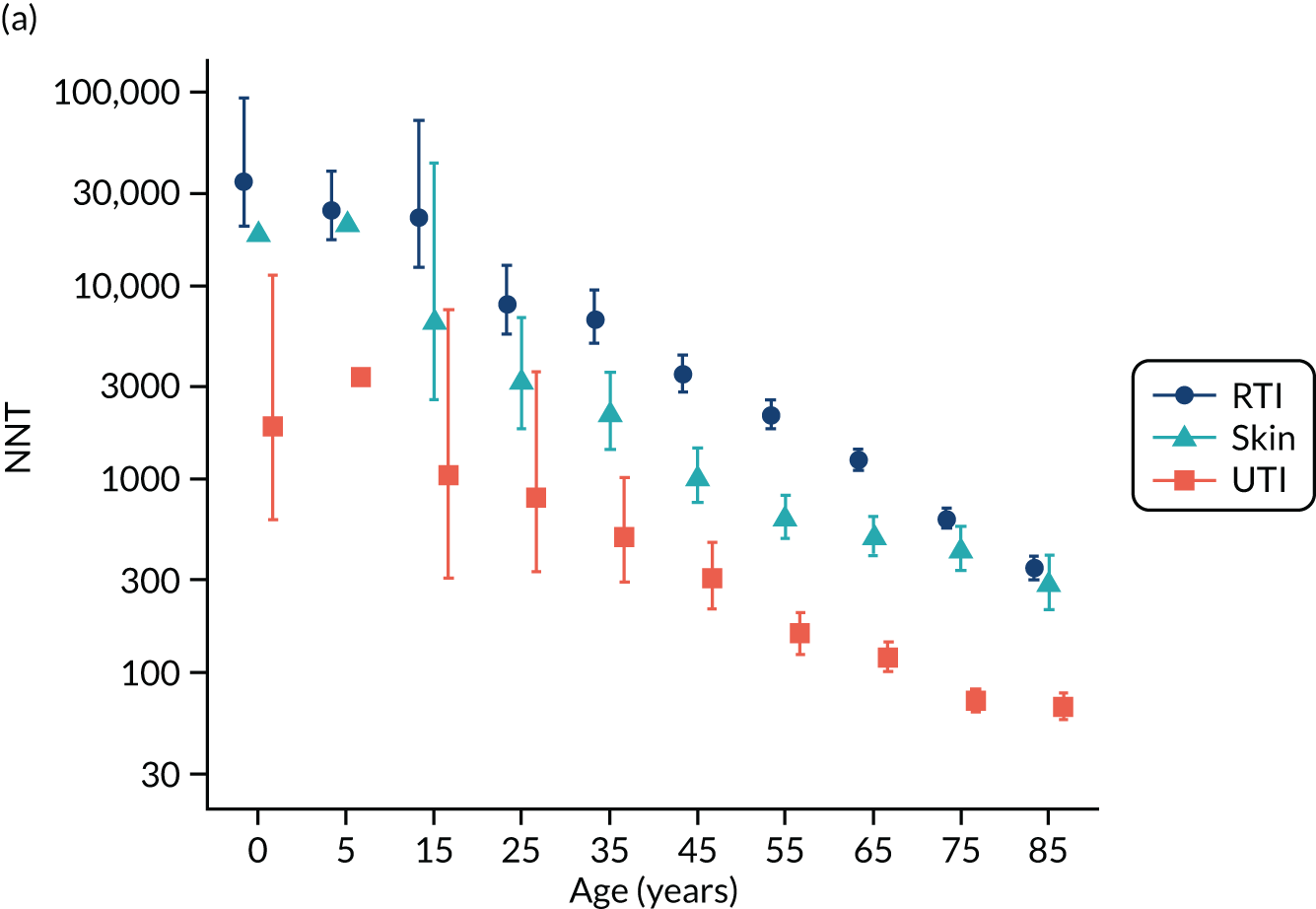

Sensitivity analyses
An analysis employing a 60-day time window to evaluate exposure gave generally similar results to those using a 30-day time window. In men aged ≥ 85 years, the NNT for all infections was 262 (95% UI 236 to 293) with a 30-day time window, but 313 (95% UI 276 to 359) with a 60-day time window. For women of the same age, the NNTs were 385 (95% UI 352 to 421) and 466 (95% UI 419 to 523), respectively. When the analysis results were compared for the 4-year periods from 2002 to 2005 with from 2014 to 2017, estimates for the probability of sepsis were slightly higher and for NNT were slightly lower for the more recent period (Figure 17), consistent with the higher reported incidence of sepsis in this period. In the oldest age group (i.e. aged ≥ 85 years), in 2014–17, the probability of sepsis without antibiotics was 0.007287 in men and 0.004775 in women. The probability of sepsis with antibiotics was 0.001290 in men and 0.000839 in women. The NNT was 167 (95% UI 141 to 202) for men and 254 (95% UI 216 to 302) for women.
FIGURE 17.
Estimates for number of antibiotic prescriptions needed to prevent one sepsis episode (i.e. NNT) for four periods: 2002–5, 2006–9, 2010–13 and 2014–17. (a) Male; and (b) female.

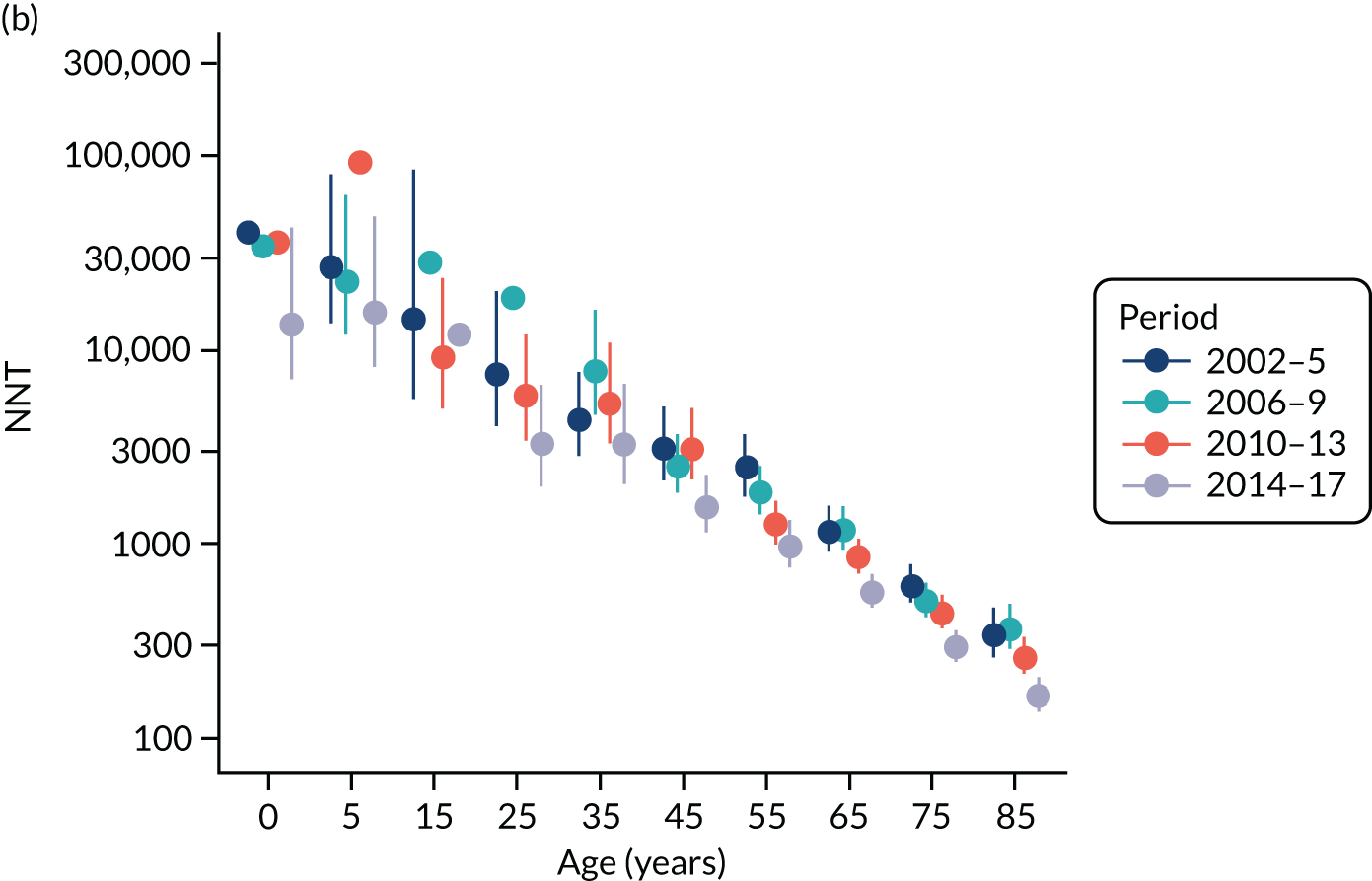
In the linked sample, there were 42,785 first sepsis events, considering the first sepsis event in each patient across all three data sources: 17,341 from primary care records, 17,363 from HES Admitted Patient Care primary diagnoses and 8081 from ONS mortality records during 36.2 million patient-years’ follow-up. Accordingly, the underlying probability of sepsis was greater when linked records were employed. However, sepsis events in HES and ONS mortality statistics were less frequently associated with preceding infection consultations in general practice (see Report Supplementary Material 3, Supplementary Figure 3). Consequently, the probability of sepsis following an infection consultation was only slightly higher if linked data were included in the analysis (see Report Supplementary Material 3, Supplementary Figure 4) and the estimated NNT was only slightly lower (see Report Supplementary Material 3, Supplementary Figure 5).
Patient and public involvement
The results from these analyses were discussed at a PPI meeting. The results were considered to be informative. However, a wider range of influences, such as the patient’s past medical history, the severity of their symptoms and their expected tolerance of a condition, might also be important. However, general practice record-keeping is often very poor in respect of not only infection consultations and antibiotic prescribing, but also in other areas of medical practice. This could result from limitations of general practice information systems, as well as limitations of staffing, with poor practice often being attributed to locums. This might mean that the required information was not available for analysis. Furthermore, there is often wide variation between general practices so that average findings might not always be transferable to specific contexts.
Discussion
Main findings
This study analysed primary care electronic health record data for a large population followed for 16 years with 35,244 new sepsis events. We found that the probability of sepsis following consultation for common infection episodes in primary care is highly age dependent. Without antibiotic treatment, sepsis may follow < 1 in 10,000 infection consultations for those aged < 25 years and < 1 in 1000 consultations for those aged < 65 years. The probability of sepsis increases at older ages, and sepsis may follow approximately 1 in 200 (men) or 1 in 300 (women) consultations at the age of ≥ 85 years. At older ages, the probability of sepsis is also highly dependent on frailty level (i.e. 55 year olds with severe frailty have a similar probability of sepsis as non-frail 85 year olds). The probability of sepsis is related to infection type, being greatest following consultations for UTI and least for RTI, and consultations for skin infections being in an intermediate position. Risks were generally slightly higher for men, which might be accounted for by their generally lower consultation rates.
The incidence of recorded sepsis has been increasing over time, with more inclusive case definitions and increasing awareness of the condition. 39,54 When we estimated the main results for the period 2014–17, the probability of sepsis was higher and the NNT was lower than for the period 2002–17. Although we caution that the absolute values of estimates may vary depending on the temporal or geographical context, we expect that in relative terms estimates will continue to identify older age, frailty and UTI consultations as being associated with greatest risks of sepsis.
Sepsis is an uncommon but concerning outcome of common infection episodes in primary care. Appropriate antibiotic therapy may have immediate benefits that are not restricted to reduction in risk of sepsis, but antibiotic prescriptions are also often associated with immediate harms in the form of drug side effects. The potential risk of antimicrobial resistance has a significance that extends beyond the context of an individual consultation. Prescribing decisions must, therefore, be informed by the balance of all of the benefits and harms of either prescribing or not prescribing antibiotics. Quantification of the possible risks of sepsis contributes to informing these decisions.
Strengths and limitations
The study drew on a large population-based cohort, enabling us to analyse representative data and obtain precise estimates that may be widely applicable. However, electronic health records comprise clinical data with several limitations and potential for bias. We analysed the outcomes of clinical decisions on whether or not to prescribe antibiotics. In the absence of randomisation, it may be expected that antibiotics were prescribed to individuals at higher risk, whereas lower-risk patients may be less likely to be prescribed antibiotics. Consequently, the probability of sepsis may be underestimated (in comparison with a study employing random allocation) in the absence of antibiotics and overestimated for patients receiving antibiotics, with the NNT being overestimated. However, the analysis depended on general practice electronic health records of antibiotic prescriptions, which account for about 85% of community antibiotic prescribing,2 but we cannot exclude the possibility that patients might have obtained antibiotic prescriptions from alternative sources, including out-of-hours services. Measures of illness severity are rarely recorded for common infection consultations in primary care and so it was not possible to adjust for illness severity in analyses. It is also established that not all infection consultations in primary care are correctly coded, leading to underestimation of consultation rates. 10 We included data from 706 general practices over a 16-year period. Infection consultation and antibiotic prescribing rates were estimated from sample data. The estimates in this paper represent average values for this population of general practices and period of time. However, we conducted a sensitivity analysis with data from 2014 to 2017 only. We also acknowledge that, in addition to changes in overall antibiotic utilisation, there have been changes in the proportion of prescriptions for broad-spectrum antibiotics. Future studies might be designed to compare the probability of sepsis if broad- or narrow-spectrum antibiotics are prescribed. The sample design used to estimate infection consultation rates and antibiotic prescribing proportions gave each practice and each study year equal weight, but we could have weighted the sample by practice size.
We analysed data for infection consultations in primary care and compared outcomes if antibiotics were or were not prescribed. However, previous studies have shown that antibiotics may be prescribed at consultations with no definite diagnosis recorded. 8,10 We did not include these prescriptions because there was no valid comparator in terms of consultations without antibiotic prescriptions, but conclusions might have differed if missing diagnosis information had been complete. We caution that the precise values of these estimates may be expected to vary in different local contexts and according to the types of infection circulating in a community at a given time. We did not employ the approach of null hypothesis significance testing and do not report p-values. We evaluated association modification by age, gender, frailty level and consultation type. We employed the eFI, which is a well-described measure based on 36 deficits,65 although we also applied it in the age range 55–64 years, in which it is less well documented. We estimated stratified values for broad groups of patients defined in terms of age, gender and frailty. We did not estimate personalised risks for individual patients and the clinical circumstances in each specific consultation should be used to inform estimates of sepsis risk for individuals. We relied on clinical records of sepsis from general practice, but we cannot be sure that all sepsis events were community rather than hospital acquired. In the UK, patients register with a general practice for continuing care, but patients may utilise emergency and out-of-hours services for acute problems, such as sepsis, and these events might not be captured in primary care records. Providers may vary in their use of the term ‘sepsis’, which is an intermediate condition linking an infection and organ damage consequent on infection. The selection of clinical terms and medical codes is at the discretion of clinical staff, leading to lack of data standardisation. The conditions identified as ‘sepsis’ may represent a range of disease severity, and probability estimates might be proportionately lower if only severe sepsis was included. However, by using linked data, we showed that inclusion of hospital episodes and mortality records did not lead to any important changes in conclusions. Further research is needed to refine, update and improve the accuracy of these initial estimates.
Comparison with other studies
There has been a trend towards more frequent recording of sepsis in recent years, and this has not always been accompanied by evidence of increased bloodstream infections. In an interrupted time series analysis, Balinskaite et al. 174 found no evidence that antimicrobial stewardship interventions in the UK might be associated with increased rates of sepsis. In an ecological analysis,39 we did not find evidence that general practices with lower antibiotic prescribing might have greater risk of sepsis over the same period of time and in the same practices as were included in the present study. Gharbi et al. 61 found that in older adults presenting with UTI, there was an increased risk of sepsis if antibiotic prescriptions were not given or were delayed. The present results extend these findings by estimating risks across all ages, different levels of frailty and different types of infection consultations. The lack of consistency between estimates from ecological- and individual-level analyses is likely to be explained by the substantial proportion of patients with sepsis who present without preceding infection consultations in primary care, as well as the small proportion of higher-risk consultations that are not associated with antibiotic prescriptions. RTI consultations are extremely frequent, which may account for the lower probability of associated sepsis. Respiratory infections are often caused by viruses, and clinicians may tend to reserve the term ‘sepsis’ for bacterial infections. We evaluated uncomplicated lower UTIs, but estimates for the probability of sepsis might have been higher if kidney infections had been included.
Chapter 10 Sepsis recording in primary care electronic health records, linked hospital episodes and mortality records
Adapted with permission from Rezel-Potts et al. 181,182 These are open access articles distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Summary
This part of the research evaluated consistency of sepsis recording across primary care electronic records, hospital episodes and mortality registrations. We analysed data from 378 general practices in England from CPRD GOLD database from 2002 to 2017 with 36,209,676 patient-years of follow-up with linked HES and ONS mortality registrations. Incident sepsis episodes were identified for each source. Concurrent records from different sources were identified using 30- and 90-day time windows. Age-standardised and age-specific incidence rates were compared. There were 20,206 first episodes of sepsis from primary care records, 20,278 from HES and 13,972 from ONS. There were 4117 (20%) first HES sepsis events and 2438 (17%) mortality records concurrent with incident primary care sepsis records. Including first and subsequent sepsis records in a 90-day time window gave 4770 (24%) HES records and 2635 (19%) mortality records concurrent with an incident primary care sepsis record. Explicit recording of the term ‘sepsis’ is inconsistent across primary care, secondary care and mortality registration, with a high proportion of non-concurrent records.
Introduction
Sepsis is a growing concern for health systems. In the UK, sepsis is estimated to account for 36,900 deaths and 123,000 hospital admissions annually. 34 The Global Burden of Disease Study estimated that there were nearly 50 million incident cases of sepsis worldwide in 2017, with 11 million deaths, representing 19.7% of global deaths. 35 The term sepsis was introduced by ancient Greek physicians, but in only recent years has sepsis come to be defined as a syndrome resulting from the interaction between an acute infection and host response, leading to new organ dysfunction. 36 Sepsis is an intermediate state that links an infection, or an infection-causing condition, to adverse health outcomes. The term sepsis is now more commonly used than the term ‘septicaemia’, which refers to bloodstream infection. In the health-care systems of high-income countries, records of ‘sepsis’ have been increasing in both hospital and primary care settings. 37–39 A study from the US Massachusetts General Hospital54 found that recording of severe sepsis or septic shock increased by 706% in the decade between 2003 and 2012, whereas objective markers of severe infection, including positive blood cultures, remained stable or decreased. In a large study from UK primary care,39 the incidence of sepsis diagnoses increased throughout the period from 2002 to 2017, with an especially rapid increase after 2011. Alongside the increasing use of the term sepsis, case definitions have expanded to include patients with evidence of both acute infection and acute organ dysfunction as having ‘implicit sepsis’, even when sepsis was not explicitly diagnosed. 35,55
Accurate surveillance of sepsis and other bacterial infections is of importance in the context of antimicrobial resistance, which can limit options for effective treatment. 183 Antimicrobial stewardship is a strategy to optimise antibiotic prescribing practices to prevent increasing antimicrobial resistance, aiming to strike a balance between effective management of suspected infection and avoidance of inappropriate or unnecessary antibiotic use. 184 The recent increase in sepsis has been accompanied by heightened awareness of antimicrobial resistance and antimicrobial stewardship, raising safety concerns about the potential for increased rates of serious bacterial infections if antibiotics are not used when needed. 28
Electronic health records provide an important data resource for epidemiological research and health surveillance, especially in health-care settings. Data linkage provides opportunities to enhance the completeness of ascertainment of health events across health service sectors and population health registries. The advantages of linked records for case ascertainment have been demonstrated for long-term conditions,162,185–187 but research into the use of linked records for the evaluation of infectious diseases has been limited. However, studies investigating the incidence of community-acquired pneumonia indicate that primary care data alone may lead to underestimation of the burden of infections. 188,189
This part of the research aimed to exploit data linkage to evaluate the recording of sepsis across primary care electronic health records, hospital episodes and mortality registrations for individual patients registered at general practices in England. We conducted a population-based cohort study to compare simultaneous recording of sepsis in primary care, hospital episodes and mortality data, and to estimate the incidence of sepsis from different data sources.
Results
Incidence of sepsis in each data source
There were 4,081,214 registered patients from 378 general practices who were eligible for linkage and contributed 36,209,676 patient-years of follow-up. There were 20,206 patients with a first episode of sepsis recorded in primary care electronic health records, 20,278 in HES and 13,972 in ONS. The characteristics of patients with sepsis from each data source are presented in Table 18. Each data source showed a slightly higher proportion of female patients than male patients (CPRD, 51%; HES, 52%; ONS, 55%). The frequency of sepsis increased with age, with a maximum over 75 years of age. The most deprived IMD quintile had the highest proportion of sepsis cases for each data source for men and women (25–26%) and the least deprived quintile consistently had the lowest (15–17%). The number of first sepsis events increased over time for primary care and hospital records, with more first episode sepsis cases in the period 2012–17 for both males and females. In CPRD, the period 2012–17 accounted for 44% of sepsis episodes for men and 42% for women. In HES, the latest period accounted for 50% of cases for both genders. This increasing trend was not apparent in mortality records. The period with the largest number of sepsis deaths in the ONS file was 2007–11.
| Characteristic | Primary care (CPRD) | Hospital episodes (HES) | Mortality data (ONS) | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Total | 9893 (49) | 10,313 (51) | 9796 (48) | 10,482 (52) | 6245 (45) | 7727 (55) |
| Age group (years) | ||||||
| 0–4 | 137 (1) | 138 (1) | 122 (1) | 142 (1) | 5 (0) | 5 (0) |
| 5–14 | 194 (2) | 181 (2 | 131 (1) | 82 (1) | 13 (0) | 6 (0) |
| 15–24 | 230 (2) | 293 (3) | 114 (1) | 121 (2) | 19 (0) | 17 (0) |
| 25–34 | 257 (3) | 464 (4) | 191 (2) | 238 (2) | 33 (0) | 19 (0) |
| 35–44 | 461 (5) | 667 (6) | 353 (4) | 430 (4) | 91 (1) | 83 (1) |
| 45–54 | 786 (8) | 911 (9) | 651 (7) | 840 (8) | 219 (4) | 219 (3) |
| 55–64 | 1410 (14) | 1351 (13) | 1298 (13) | 1180 (11) | 520 (8) | 468 (6) |
| 65–74 | 2186 (22) | 1756 (17) | 2178 (22) | 1809 (17) | 1172 (19) | 962 (12) |
| 75–84 | 2639 (27) | 2281 (22) | 2855 (29) | 2661 (25) | 2169 (35) | 2423 (31) |
| ≥ 85 | 1593 (16) | 2271 (22) | 1903 (19) | 2979 (28) | 2004 (32) | 3525 (46) |
| Deprivation fifth | ||||||
| Least deprived | 1570 (16) | 1502 (15) | 1630 (17) | 1662 (16) | 923 (15) | 1123 (15) |
| Second | 1570 (16) | 1502 (15) | 1630 (17) | 1662 (16) | 923 (15) | 1123 (15) |
| Third | 2126 (21) | 2403 (23) | 1892 (19) | 2004 (19) | 1258 (20) | 1525 (20) |
| Fourth | 1852 (19) | 1817 (18) | 1892 (19) | 1960 (19) | 1150 (18) | 1408 (18) |
| Most deprived | 1889 (19) | 1914 (19) | 1944 (20) | 2178 (21) | 1308 (21) | 1657 (21) |
| Data missing | 1 (0) | 2 (0) | 1 (0) | 4 (0) | 6 (0) | 4 (0) |
| Period | ||||||
| 2002–6 | 2271 (23) | 2604 (25) | 1911 (19) | 2133 (20) | 2077 (33) | 2550 (33) |
| 2007–11 | 3234 (33) | 3422 (33) | 2964 (30) | 3155 (30) | 2398 (38) | 3086 (40) |
| 2012–17 | 4388 (44) | 4287 (42) | 4921 (50) | 5194 (50) | 1770 (28) | 2091 (27) |
Annual age- and gender-standardised incidence of sepsis is shown for each data source in Report Supplementary Material 3, Supplementary Figure 6. Primary care electronic records showed a steady increase in sepsis cases over the study period, with an acceleration from 2012 to 2017. In the CPRD, in 2002, the age-standardised incidence was 0.35 (95% CI 0.32 to 0.39) per 1000 person-years in male patients and 0.34 (95% CI 0.30 to 0.37) per 1000 person-years in female patients. By 2017, this had increased to 1.15 (95% CI 1.04 to 1.26) per 1000 person-years among male patients and 1.10 (95% CI 95% CI 1.00 to 1.19) per 1000 person-years among female patients. Consistent with primary care data, HES data showed a steep increase in sepsis cases over the study period, particularly from 2012 to 2017. In HES records, for 2002, the age-standardised incidence was 0.29 (95% CI 0.25 to 0.32) per 1000 person-years among male patients and 0.25 (95% CI 0.22 to 0.28) per 1000 person-years among female patients. By 2017, this had increased to 2.52 (95% CI 2.35 to 2.68) per 1000 person-years among male patients and 2.10 (95% CI 1.96 to 2.23) per 1000 person-years among female patients. In contrast to primary care and hospital records, there was no consistent trend in the recording of sepsis as a cause of death during the study period. In 2002, sepsis mortality was 0.34 (95% CI 0.31 to 0.38) per 1000 person-years among male patients and 0.28 (95% CI 0.26 to 0.31) per 1000 person-years among female patients. By 2017, this had risen to 0.42 (95% CI 0.35 to 0.49) per 1000 person-years among male patients and 0.30 (95% CI 0.25 to 0.35) per 1000 person-years among female patients, although the highest peak in mortality rate was in 2006 for female patients and 2007 for male patients.
Concurrent recording of sepsis events across data sources
There were 4117 (20%) first HES sepsis events and 2438 (17%) mortality records that were concurrent with incident primary care sepsis records, based on a 30-day time window (Figure 18). Among the 13,972 deceased patients with sepsis listed as any cause of death, 2438 (17%) had an incident sepsis event recorded in primary care electronic health records and 3397 (24%) had incident sepsis events recorded in HES in the same period. Only 614 patients had index sepsis events recorded across all three data sources within 30 days of the date of event or date of death. We evaluated whether or not extending the time window influenced conclusions. Including both first and subsequent sepsis records and a 90-day time window gave 4770 (24%) HES records and 2635 (19%) mortality records concurrent with an incident primary care sepsis record.
FIGURE 18.
Incident first sepsis events in CPRD, HES and ONS using a 30-day time window to evaluate concurrence.
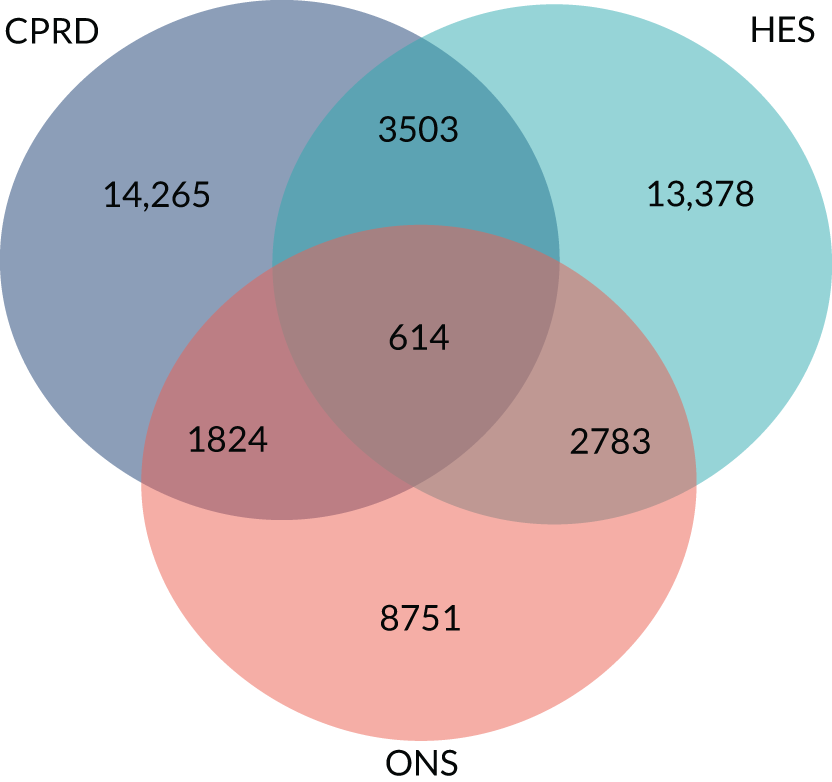
Variables associated with concurrent records
Report Supplementary Material 3, Supplementary Figure 7, shows the results of a logistic regression analysis of variables associated with concurrent recording in more than one data source for patients with sepsis. Among patients with sepsis events recorded in primary care, concurrent HES records of sepsis within 30 days before or after first diagnosis were lowest in the age range 5–34 years, but higher at younger or older ages. There was no consistent association with deprivation overall. There were higher odds of concurrent recording in the most recent period of diagnosis. For index sepsis events recorded in HES, concurrent recording in primary care electronic health records was not consistently associated with age, deprivation or period. Among patients with sepsis events recorded in ONS, there were lower odds of a concurrent CPRD record of sepsis for the age range 35–54 years, being registered with a practice in the least deprived quintiles and the most recent period of diagnosis.
Incidence of sepsis from linked data sources
Table 19 shows age-specific sepsis incidence rate per 1000 patient-years from primary care electronic health records and for primary care electronic health records combined with HES and ONS, stratified. With primary care electronic health records alone, sepsis incidence rates increased from 0.19 in male patients and 0.20 in female patients per 1000 person-years for those aged < 5 years to 5.22 in male patients and 3.55 in female patients aged ≥ 85 years. Estimated incidence rates were substantially higher when either HES or mortality records were included. When all three data sources were combined, the incidence of sepsis was 0.32 per 1000 person-years in male patients and 0.36 per 1000 person-years in female patients aged < 5 years, increasing to 10.09 per 1000 person-years in male patients and 7.22 per 1000 person-years in female patients at age ≥ 85 years. Report Supplementary Material 3, Supplementary Figure 2, shows equivalent age-specific incidence when first sepsis records in each calendar year, rather than the first in the study period, were included.
| Age group (years) | CPRD | CPRD + HES | CPRD + ONS | CPRD + ONS + HES | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| 0–4 | 0.19 (0.00 to 4.08) | 0.20 (0.00 to 4.10) | 0.32 (0.00 to 4.33) | 0.36 (0.00 to 4.40) | 0.20 (0.00 to 4.09) | 0.21 (0.00 to 4.11) | 0.32 (0.00 to 4.33) | 0.36 (0.00 to 4.40) |
| 5–14 | 0.09 (0.00 to 3.87) | 0.09 (0.00 to 3.87) | 0.13 (0.00 to 3.96) | 0.12 (0.00 to 3.93) | 0.09 (0.00 to 3.88) | 0.09 (0.00 to 3.88) | 0.13 (0.00 to 3.96) | 0.12 (0.00 to 3.93) |
| 15–24 | 0.11 (0.00 to 3.92) | 0.16 (0.00 to 4.02) | 0.14 (0.00 to 3.99) | 0.21 (0.00 to 4.11) | 0.12 (0.00 to 3.94) | 0.17 (0.00 to 4.03) | 0.14 (0.00 to 3.99) | 0.21 (0.00 to 4.11) |
| 25–34 | 0.12 (0.00 to 3.93) | 0.21 (0.00 to 4.12) | 0.18 (0.00 to 4.05) | 0.29 (0.00 to 4.28) | 0.13 (0.00 to 3.95) | 0.22 (0.00 to 4.13) | 0.18 (0.00 to 4.05) | 0.29 (0.00 to 4.28) |
| 35–44 | 0.17 (0.00 to 4.03) | 0.25 (0.00 to 4.19) | 0.26 (0.00 to 4.21) | 0.36 (0.00 to 4.41) | 0.19 (0.00 to 4.08) | 0.27 (0.00 to 4.24) | 0.26 (0.00 to 4.21) | 0.36 (0.00 to 4.41) |
| 45–54 | 0.29 (0.00 to 4.27) | 0.35 (0.00 to 4.38) | 0.46 (0.00 to 4.60) | 0.57 (0.00 to 4.81) | 0.35 (0.00 to 4.39) | 0.42 (0.00 to 4.52) | 0.46 (0.00 to 4.60) | 0.57 (0.00 to 4.81) |
| 55–64 | 0.61 (0.00 to 4.87) | 0.59 (0.00 to 4.84) | 0.99 (0.02 to 5.55) | 0.93 (0.02 to 5.45) | 0.78 (0.01 to 5.19) | 0.74 (0.01 to 5.11) | 0.99 (0.02 to 5.55) | 0.93 (0.02 to 5.45) |
| 65–74 | 1.30 (0.07 to 6.09) | 0.98 (0.02 to 5.53) | 2.19 (0.31 to 7.53) | 1.68 (0.15 to 6.72) | 1.83 (0.19 to 6.95) | 1.39 (0.08 to 6.24) | 2.19 (0.31 to 7.53) | 1.68 (0.15 to 6.72) |
| 75–84 | 2.65 (0.47 to 8.23) | 1.73 (0.16 to 6.79) | 4.74 (1.86 to 11.29) | 3.23 (0.72 to 9.12) | 4.35 (1.27 to 10.75) | 3.17 (0.69 to 9.02) | 4.74 (1.48 to 11.29) | 3.23 (0.72 to 9.12) |
| ≥ 85 | 5.22 (1.75 to 11.97) | 3.55 (0.87 to 9.58) | 10.09 (4.86 to 18.51) | 7.22 (2.96 to 14.72) | 10.42 (5.09 to 18.93) | 7.93 (3.41 to 15.67) | 10.09 (4.86 to 18.51) | 7.22 (2.96 to 14.72) |
Discussion
This study evaluated sepsis recording in primary care electronic records, hospital episodes and mortality records for a large population registered with general practices in England. We found that, over a 16-year period, a similar number of incident sepsis events were recorded in primary care and hospital records. However, a large proportion of these records were not concurrent across data sources. The majority of sepsis events recorded in primary care were not recorded in hospital episodes, and the majority of hospital episodes were not recorded in primary care. This conclusion held even when events were evaluated over a longer time window or if recurrent as well as incident events were included. There were a smaller number of records of sepsis from mortality registration, but a majority of these were not associated with concurrent primary care or hospital records for sepsis. Patients with sepsis recorded in primary care were more likely to have concurrent hospital episode records if they were either very young or very old, or if the episode was in a more recent period. However, these associations were not consistent across the other linkages, suggesting that coding variations were largely unexplained. Estimates for age-specific incidence rates may be up to twice as high if linked data sources are employed.
Current estimates suggest that about 50–70% of all sepsis events are community acquired. 190,191 Clinical guidelines recommend that patients with suspected sepsis should be referred for management in hospital, which suggests that most patients seen in primary care may be admitted to hospital. 34 However, some patients with sepsis may access hospital services directly without first presenting in primary care. Investigations in hospital may identify underlying causes for sepsis, which might be coded as the reason for admission. Just under half of severe sepsis cases admitted to intensive care in England and Wales are associated with a fatal outcome. 192 It may be expected that a sepsis diagnosis will be communicated to the patient’s general practice or, in the event of a fatal outcome, recorded in mortality records. Our results indicate that the process of recording sepsis episodes across different health information systems is highly inconsistent. Health professionals may make varying use of the concept of sepsis in the clinical recording of patients’ conditions. ‘Sepsis’ may sometimes form an element of the clinical narrative but, on other occasions, an underlying cause may be given greater prominence, as in the COVID-19 pandemic. The NHS in England produced a cross-sectoral plan to improve outcomes for patients with sepsis. 193 This aimed to improve training between different health-care professional groups and reduce differences in coding practices between organisations. A National Early Warning Score has been rolled out to improve early recognition of signs of sepsis, but this has been accompanied by concerns for false-positive alerts and possible overdiagnosis of sepsis. 180
The present results derive from a large, representative population. However, a disadvantage of using linked rather than stand-alone CPRD records is that linkage eligibility restrictions reduce the sample size and possibly representativeness. GPs must be eligible for linkage, which requires meeting standards of data completeness and, therefore, biasing the practices within the linked sample towards those that record disease events with greater accuracy. It is also possible that there are some inaccuracies with linkage across sources. Discrepancies between the CPRD GOLD and the ONS death data have been highlighted, particularly in the years prior to 2013. 194 This study does not differentiate between community- and hospital-acquired sepsis. Further research is required to understand how sepsis incidence in the combined sources can reveal more about the impact of antimicrobial stewardship strategies.
This study is broadly consistent with the growing body of literature that advocates the use of linked data sources. 185–188 However, it also indicates that stand-alone CPRD data may provide accurate estimates of changes in the burden of sepsis. Millet et al. 188 found that population-averaged community-acquired pneumonia incidence was 39% higher using the linked data than using the stand-alone data. It may be that increased awareness and standardisation of detection and recording have prevented such discrepancies being observed for sepsis.
Chapter 11 Probability of localised serious bacterial infections and antibiotic prescriptions
Abstract
In this part of the research, we calculated the probability of 10 different serious bacterial infections following general practice consultations for RTIs, skin infections or UTIs. We estimated the number of antibiotic prescriptions required to prevent one event of each type. Risks of serious bacterial infections were generally low, except for kidney infection following UTI in young women, PTA following respiratory infections in young adults and sepsis in older adults.
Introduction
As noted in Chapter 1, there is growing evidence that several localised bacterial infections, including empyema, infective endocarditis, osteomyelitis, septic arthritis and kidney infections, may be increasing in frequency (see Table 1). In the CPRD data set, we found evidence of increases in osteomyelitis and kidney infections, the latter mainly in women, as well as sepsis (see Figure 10). The underlying reasons for these changes may be complex and multifactorial, as noted in Chapter 1. This part of the research aimed to evaluate whether or not these localised serious bacterial infections were more likely after general practice consultations when antibiotics were not prescribed.
Results
We employed the methods outlined in Chapters 8 and 9, conducting analyses for nine conditions: (1) infections of the CNS, (2) infections of the CVS, (3) empyema and lung abscess, (4) kidney infections, (5) mastoiditis, (6) osteomyelitis, (7) PTA, (8) sepsis and (9) septic arthritis. Results for sepsis and PTA are included to illustrate the complete range of safety outcomes following consultations for RTIs, skin infections and UTIs. Estimates were obtained for the probability of each outcome, that is, whether or not antibiotics were prescribed following general practice consultations for RTIs, UTIs, skin infections or any infection consultation. Initially, estimates were obtained by age group and gender. Subsequently, the population aged ≥ 55 years was stratified by frailty category. Infections of the CNS and CVS were omitted from the analysis by frailty level because data were too sparse. Report Supplementary Material 3, Supplementary Figure 8, presents estimates for the probability of each of seven localised serious bacterial infections not previously considered. Data are presented for the probability of each outcome within 30 days of any infection consultation. Figure 19 presents estimates for the number of antibiotic prescriptions required to prevent one outcome occurrence (i.e. the NNT).
FIGURE 19.
Number of antibiotic prescriptions required to prevent one serious bacterial infection after general practice consultations for any infection. Note that when UIs are not shown; this is because the data set did not include sufficient information to provide an estimate.
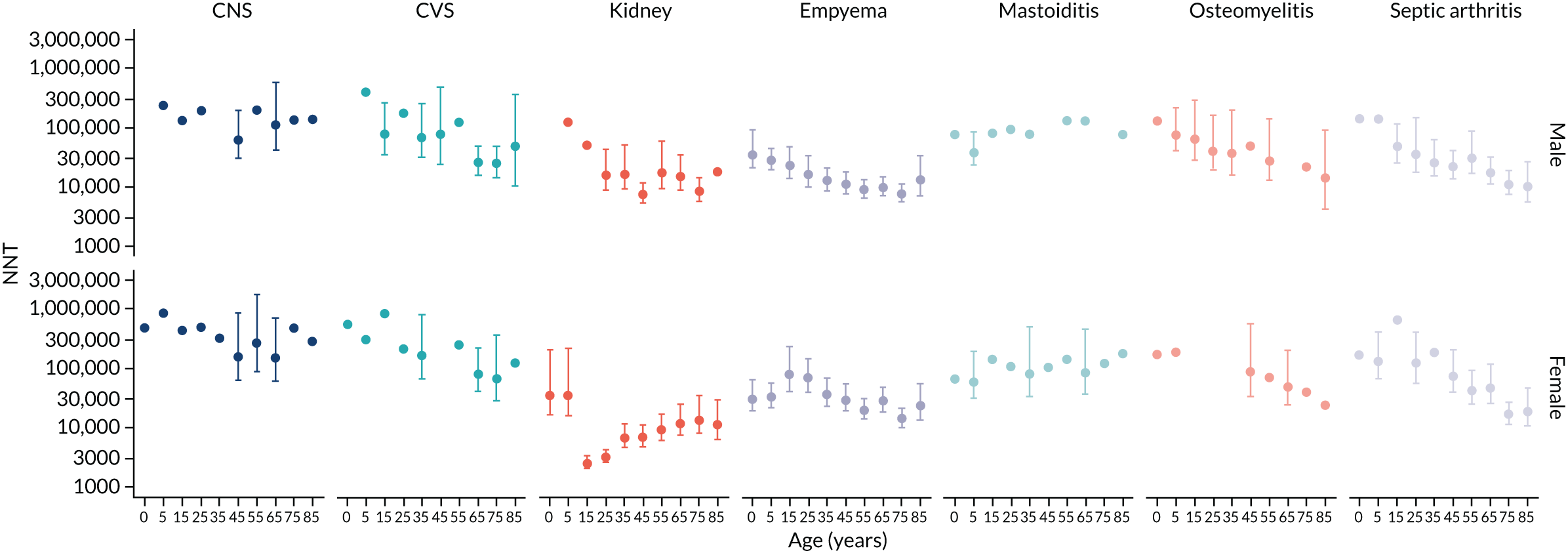
Infections of the CNS and CVS were generally rare and were estimated to follow between 1 in 100,000 and 1 in 1,000,000 consultations. Estimates were generally lower for consultations with antibiotics prescribed. There was evidence that for older adults, in the absence of antibiotic prescriptions, these outcomes might be associated with between 1 in 10,000 and 1 in 100,000 consultations. Note that, in Figure 19 UIs have been omitted where the lower bound had a negative value. This indicates that the data included in CPRD over a 16-year period were not sufficient to enable estimation of the likely value of the parameter.
Kidney infections following any infection consultation (see Report Supplementary Material 3, Supplementary Figure 8) or UTI consultation (see Report Supplementary Material 3, Supplementary Figure 9) were patterned by age and gender. In women, the probability of kidney infections following general practice infection consultations showed a peak in the 15–24 year age group before declining. In men, the probability of kidney infections tended to increase up to middle age. The estimated NNT was, as expected, lower following UTI consultations (Figure 20) than after any infection consultation (see Figure 19). In women aged 15–24 years, the probability of a kidney infection following a UTI consultation without antibiotics prescribed was estimated to be 0.0146 (95% UI 0.0134 to 0.0161) or 1 in 68.5 consultations. The number of antibiotic prescriptions estimated to prevent one kidney infection was 81 (95% UI 72 to 90). In men, the probability of a kidney infection following a UTI consultation was greatest at age 45–54 years (0.0066, 95% UI 0.0051 to 0.0085), with a NNT of 186 (95% UI 136 to 267).
FIGURE 20.
Number of antibiotic prescriptions required to prevent one serious bacterial infection after general practice consultations for UTIs. Note that when UIs are not shown this is because the data set did not include sufficient information to provide an estimate.
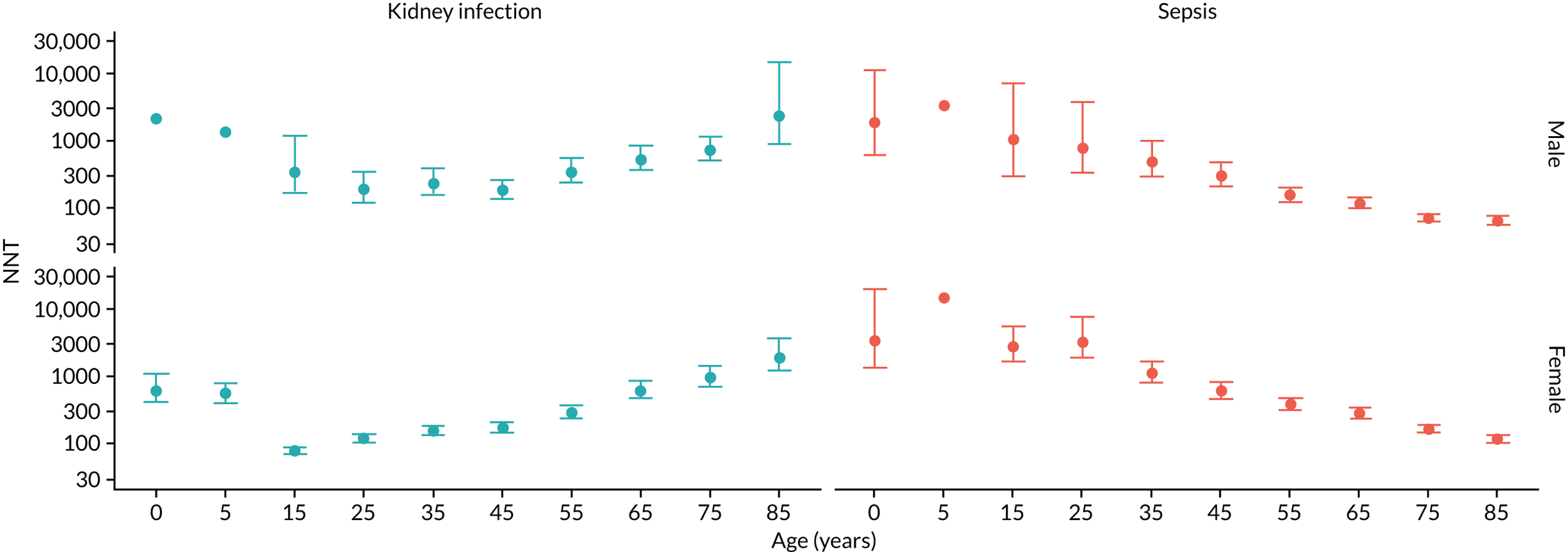
Empyema and lung abscess were infrequent outcomes that were associated with low probability of occurrence following a general practice consultation for any infection (see Report Supplementary Material 3, Supplementary Figure 8) or for RTI (see Report Supplementary Material 3, Supplementary Figure 10). Without antibiotic treatment, the probability of empyema or lung abscess following a RTI consultation was 0.000169 (95% UI 0.000131 to 0.000213) in men aged 55–64 years or 1 in 5917 consultations. However, the probability of lung abscess or empyema was consistently lower following consultations associated with antibiotic treatment, being 0.000047 (95% UI 0.000032 to 0.000066) or 1 in 21,277 in men of the same age when antibiotics were prescribed for a RTI. The number of antibiotic prescriptions required to prevent one episode of lung abscess or empyema was generally > 10,000 (see Figures 19 and 21), except in older men among whom the NNT was 8208 (95% UI 5955 to 12,506) at age 55–64 years and 7588 (95% UI 5419 to 11,763) at age 75–84 years.
FIGURE 21.
Number of antibiotic prescriptions required to prevent one serious bacterial infection after general practice consultations for RTIs. Note that when UIs are not shown this is because the data set did not include sufficient information to provide an estimate.
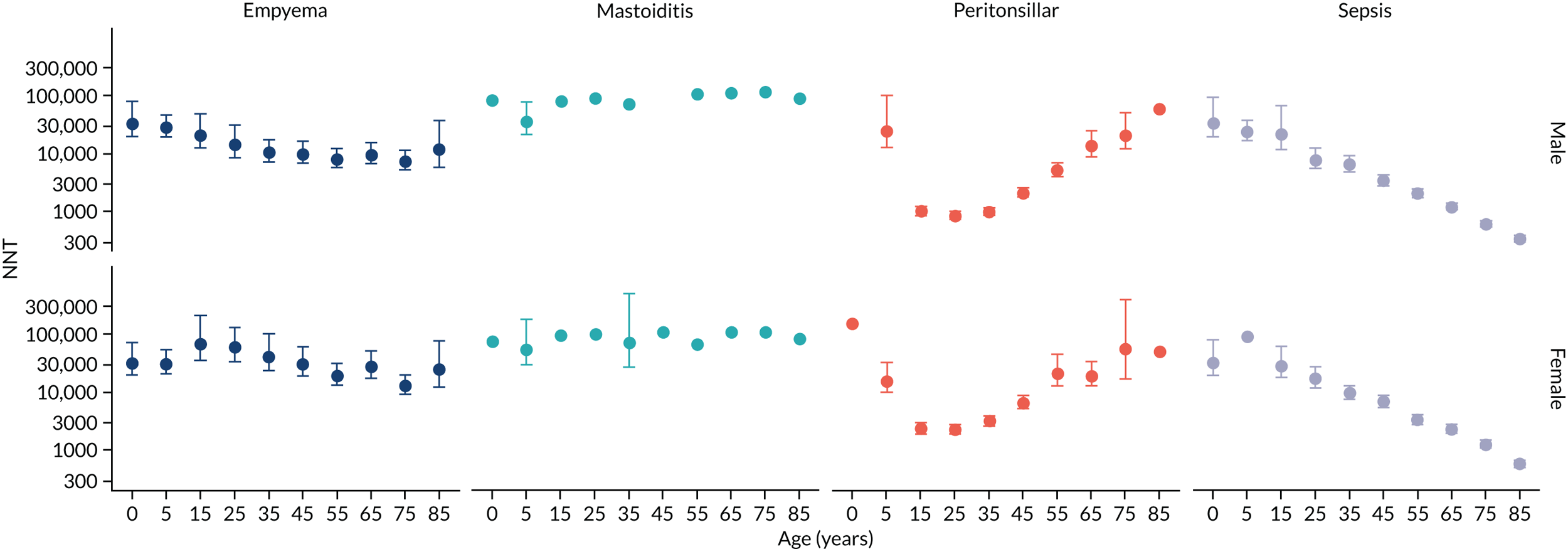
Mastoiditis was an infrequent outcome for which probability declined with age (see Report Supplementary Material 3, Supplementary Figures 8 and 10). The greatest probability was observed in boys aged 5–14 years following RTIs, with a probability of 0.0000415 (95% UI 0.0000289 to 0.0000574) or 1 in 24,096 consultations. At age 5–14 years, the number of RTI consultations requiring antibiotic treatment to prevent one case of mastoiditis was estimated to be 35,710 (95% UI 22,054 to 80,236) for boys and 52,500 (95% UI 29,003 to 175,161) for girls (Figure 21). Note that the number of cases analysed was insufficient to estimate UIs for the majority of the remaining age groups.
Osteomyelitis was an uncommon outcome that generally increased in frequency with age. The outcome was found to be the most strongly associated with skin infection consultations (see Report Supplementary Material 3, Supplementary Figure 11). The highest probability without antibiotic treatment was observed following skin infections in men age 74–84 years, being 0.000968 (95% UI 0.000663 to 0.00135) or 1 in 1033 consultations. The corresponding NNT (Figure 22) was estimated as 2574 (95% UI 1102 to 15,373). Similarly, for septic arthritis, the highest frequency was observed following skin infections in men aged 75–84 years. Here, without antibiotics, the probability of septic arthritis was 0.000541 (95% UI 0.000324 to 0.000834) or 1 in 1848 consultations, with a NNT of 2204 (95% UI 1329 to 4499).
FIGURE 22.
Number of antibiotic prescriptions required to prevent one serious bacterial infection after general practice consultations for skin infections. Note that when UIs are not shown this is because the data set did not include sufficient information to provide an estimate.
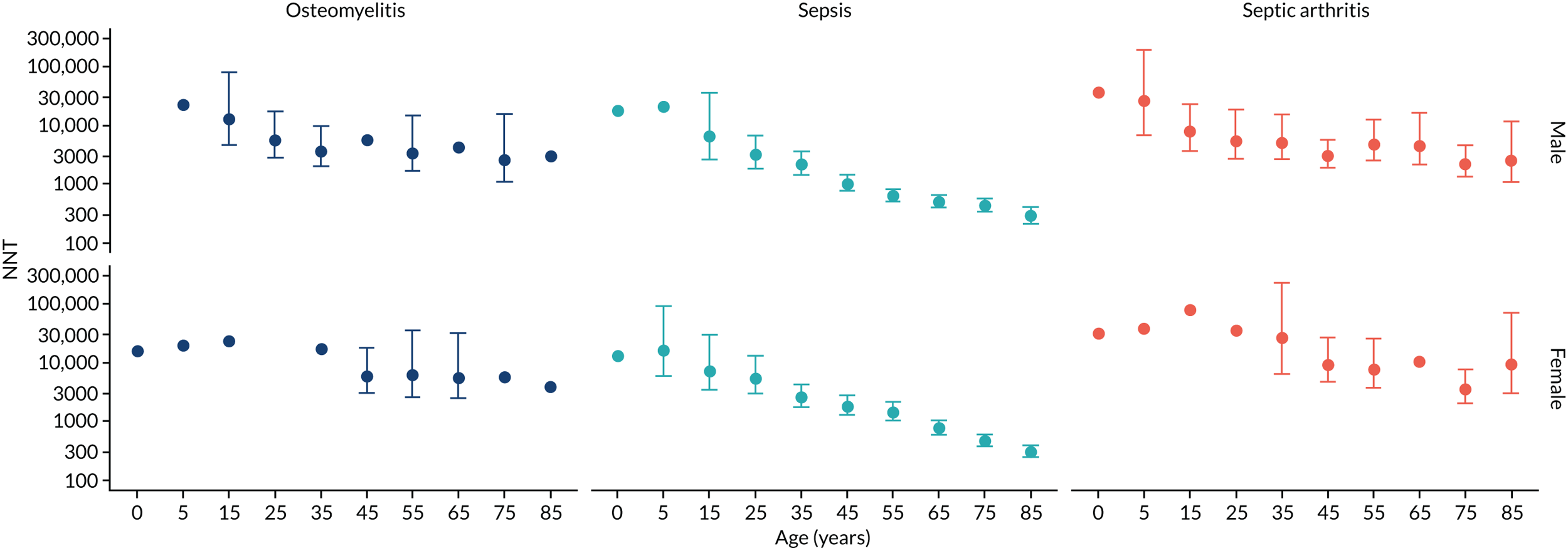
Chapter 12 Development and testing of a Shiny app to communicate results to primary care prescribers
Summary
We used Shiny software to incorporate estimates into an app that presented data to GPs through web pages that could be viewed during consultations. A qualitative study was conducted to obtain end-user feedback to inform the design of the app.
Introduction
We developed a Shiny app that would enable us to present the data in the form of accessible web pages to primary care prescribers. The app comprises two pages that present selected data in tabular and graphical form, respectively. On entering the app, the user is asked to enter the details relevant to their consultation, including the type of infection consultation (i.e. any infection, respiratory, urinary or skin infection), the nature of serious infections that are of concern, the age group and gender of the patient, and the frailty level based on the eFI (Figure 23). Based on the entered values, a tabulation is then provided of the probability of the selected outcome, with and without antibiotic treatment, together with the estimated NNT (Figure 24). The app also presents, in a separate tab, a graphic presentation of the data for each of the outcomes relevant to the consultation type and age group (Figure 25). For ease of presentation, each figure is presented with a horizontal y-axis, with the scale reversed for the NNT to enhance clarity of communication.
FIGURE 23.
Data selection page of Shiny app.
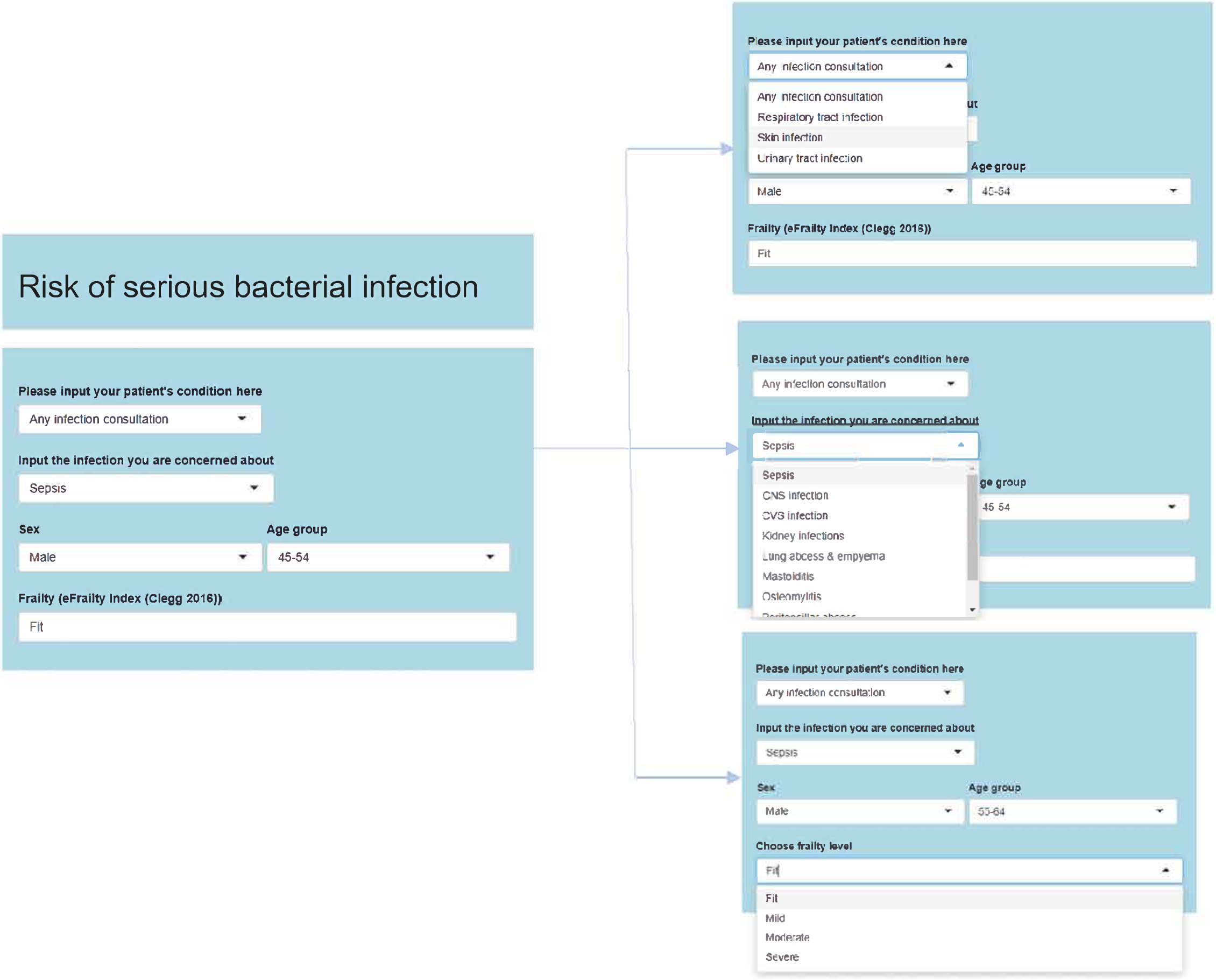
FIGURE 24.
Illustration of data table page of the Shiny app.
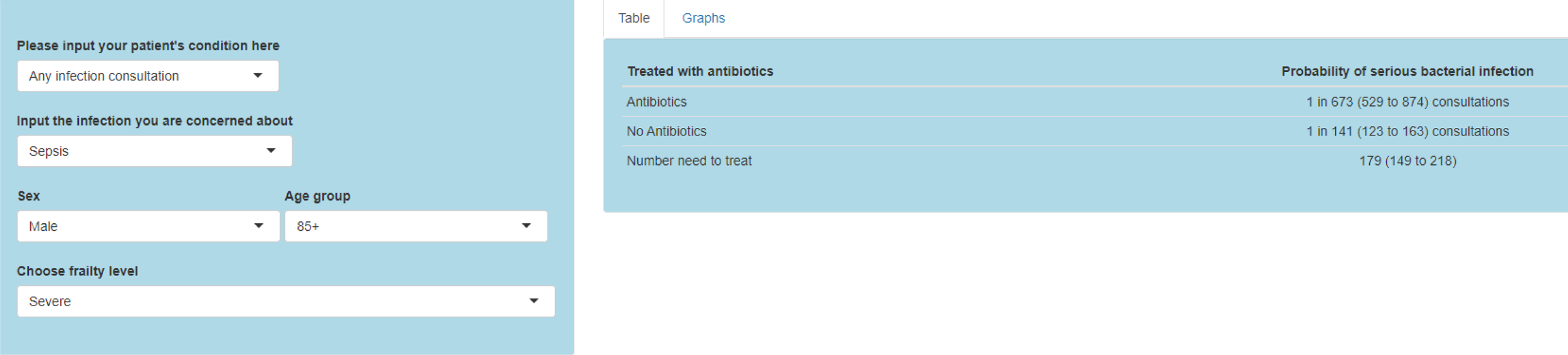
FIGURE 25.
Illustrations of the graphical outputs of the Shiny app. (a) Results for a woman with a UTI aged 25–34 years; and (b) results for a non-frail woman with a UTI aged 75–84 years.
Results
Participants were shown the Shiny app, which consisted of two web pages. The ‘home page’ (see Figure 23) asked GPs to enter information about their patient and then displayed the risk of serious bacterial infection specific to the characteristics that they had input (see Figure 24). The ‘graphs’ page (see Figure 25) displayed two graphs also showing the risk of bacterial infection statistics based on the information that the GP had input.
Description of themes
Four themes emerged from the interviews, relating to the decision to use the web pages. Subthemes were identified within each theme, which are presented in Table 20.
| Theme | Subtheme |
|---|---|
| A decision-making tool |
|
| Use with patients |
|
| Design of tool |
|
| Accessing pages during consultation |
|
A decision-making tool
General practitioners discussed the use of the web pages as a decision-making tool. Most GPs considered that the pages would be useful for decision-making in practice. Some GPs thought that the pages would be useful for training and some discussed their use for less experienced staff.
Useful for decision-making in practice
Most GPs felt that the web pages would be useful for decision-making in practice. GPs reported that they might discuss the results with patients during a consultation to support their prescribing decisions. General practitioners reported that the tool could also help to support a decision that they had already made and give them more confidence in delivering this:
I do think it would help decision-making.
P1
I think it could [inform decision-making] because the . . . risk factors for sepsis are so clearly age and . . . frailty so . . . you know, the worry for younger people is really for sepsis.
P4
Useful for training
Some GPs felt that the web pages could be useful for training. GPs suggested that the web pages could be used during a practice meeting to update all GPs on the risks of bacterial infections. Some GPs could work through the options on each page to refresh their knowledge of bacterial infection risk:
I can see it as a very good learning tool in a clinical meeting.
P3
I think it would be really useful for clinical meetings, for an education event.
P3
Most useful for inexperienced staff
Some GPs considered that less experienced GPs or nurse practitioners might benefit the most from using the pages as a decision-making tool. GPs suggested that these groups would find it useful to be presented with strong evidence during a consultation to help guide them towards a prescribing decision that they may find more difficult than experienced GPs:
And I think [it would be useful] though definitely for the out-of-hours work and maybe for GPs who work in A&E [accident and emergency]. Or, like the nurse practitioners, for example . . . So, for those people I think it would probably be used a little bit more.
P5
Use with patients
General practitioners discussed using the web pages with a patient and how they would show these to a patient. However, some GPs felt that they would not use the pages with a patient at present.
A tool to share with patients
Most GPs reported that they would share the web pages with patients during a consultation. Some GPs felt that they would show patients the full screen of probability results, whereas others felt that they would share the graphs pages. GPs reported that sharing the pages could help support their prescribing decision:
OK, we’ve got the decision to make about antibiotics. In your particular circumstances, with your infection, your age, your level of frailty, shall we have a look at what there is to be gained from prescribing an antibiotic for you. One click. Here you go . . . And then, and then you’re into a kind of shared decision-making conversation with the patient. So, I can see that working.
P2
So, I think it might be very good to have something not just that you look at yourself to support your own decision but to help show a patient and I could see that screen being useful, pointed towards a patient.
P3
Pages too complex for patient use
Some GPs reported that they would not show patients the web pages during a consultation, as they felt that, at present, the information was too complex for patients to readily understand. However, GPs suggested that if the information and graphs were simplified then they would be happy to show the pages to patients:
[The tool] it’s more academic for you know GP medical students or something like that.
P5
I quite like sharing stuff with patients really. So, but it’s got to be pretty, I’m not sure that people would understand these really.
P4
Design of tool
General practitioners discussed the way in which the design of the pages could help them during a consultation and also how these could be improved. Overall, GPs felt that the pages provided a useful selection of data. However, GPs felt that clearer descriptions were needed and that the data should be simplified further.
A useful selection of data
All GPs felt that the data presented in the web pages were useful and would contribute to making prescribing decisions. In particular, GPs reported that they would use the NNT and probability of contracting a serious bacterial infection if no antibiotics were prescribed:
Now, if this is a sort of within consultation tool, I would say the number data, not the graphical data that you just showed me was much more powerful.
P2
Clearer descriptions needed
All GPs reported the importance of being able to use the pages as quickly as possible during a consultation. GPs felt that clearer descriptions for each item on the web pages were needed. In particular, GPs requested more headings and a clear title for each item and graph. GPs also requested a reduction in the use of abbreviations to make conditions easier to identify and read as quickly as possible:
. . . the word ‘consultations’ doesn’t mean anything . . . What we’re actually doing is, saying is, what’s the chance of a consultation following this consultation of sepsis happening?
P1
. . . the reason I asked what ‘play’ was is because . . . it’s not immediately obvious. And I think . . . there’s no labelling of the x-axis obviously. But the, the x-axis should be labelled adjacent to the left of 55.
P1
I’m thinking what are you saying about antibiotics and no antibiotics? What exactly and what does the heading AB [antibiotic] mean, anyway, above that? But AB isn’t immediately obvious. But it’s just not quite clear from that. And I think that should, could be a bit clearer.
P3
Simplify data
To assist GPs in understanding and reading the information during a busy practice, most GPs requested that the data be simplified as much as possible. GPs felt that reducing the information on each graph to make it more specific to the patient data that they had entered would help them explain and deliver this during a consultation. In addition, GPs requested that only bacterial infections most likely to be linked to each patient condition appear in the drop-down box. GPs felt that minimising options here and making it more specific to each patient would help to reduce time using the pages:
I think the graph is too complicated. And . . . it would help me would be if in the big box at the top it would tell me what I’ve got below. So, we’ve got somebody we think is an RTI. What’s the risk of complications in each of these areas? Which are completely, apart from the lung, the rest are completely unconnected with an RTI . . . it’s just, it’s not relevant really. You’re asking me to, you’re asking me to consider complications which are irrelevant.
P6
Accessing pages during consultation
General practitioners discussed the ways in which the pages could be accessed during a consultation. Some GPs felt that the pages should pop up automatically, whereas others said that they would prefer it if pages popped up on their screen only if they had selected them.
Pages should pop up automatically
Most GPs considered that it would be useful if the web pages could appear automatically during a consultation. GPs reported that automatic pop-ups would remind them that the function was there to use. In addition, some GPs felt that if the computer system could automatically enter data for an individual patient it would encourage them to use it:
If you can get something within a click. I think, you know, it will get used. Much more if it’s got the navigator on the website.
P2
I mean the best thing is if these things, if you can click on a link and it self-completed so that you, so that you were in the patient and you thought well I’m just making this decision, I’ll click on this tool and it’s already put the gender and the age in.
P4
Pages should not pop up on screen
Some GPs felt that the web pages should not pop up on the screen as this would hinder their consultation. These GPs felt that the option to view the pages should be clear for them to select if needed, but not require them to close it if it was not needed. One GP suggested that a link to a PDF (Portable Document Format) with all possible combinations of infection would be useful to view, if required:
. . . in order to cope with prompts, GPs have to ignore them.
P6
So, what I personally sometimes do is I sometimes copy a web link in. And I paste that in as a piece of evidence. But the problem is you can’t do that here because you’ve had to, you’ve had to put the specific details in. So, therefore . . . you need to turn it into a static document so it would have to be downloadable . . . would be most useful if it was embedded in the system.
P1
Development of tool
The web pages were refined throughout the interview process based on continuing feedback. Early interviews provided many constructive criticisms and suggestions for change. The themes informed areas for change and extensive analysis of transcripts informed the exact amendments to be made to the pages to increase acceptability and aid usage. Key changes and adaptations made to the prompts and the main themes that informed these can be seen in Table 21.
| Page | Section | Feature | Amendment |
|---|---|---|---|
| Home | Input the values for your patient section | Type of problem | Remove ‘RSU’ option on drop-down list |
| Type of problem | Reword title ‘type of problem’ to ‘please input your patient’s condition here’ | ||
| Serious bacterial infection | Add new option of ‘all bacterial infections’ in drop-down list (this should be the first option) | ||
| Serious bacterial infection | Allow only relevant infections to appear for each ‘type of problem’ (e.g. remove ‘lung abscess’ from UTI problem) | ||
| Serious bacterial infection | Define MAS (or make clearer) | ||
| Serious bacterial infection | Define PTA (or make clearer) | ||
| Serious bacterial infection | Reword title ‘serious bacterial infection’ to ‘input the infection you are concerned about’, etc. | ||
| Frailty | Reference the frailty measure used here | ||
| Statistics output section | Change ‘probability’ to ‘probability of infection’ | ||
| Reword AB to ‘treated with antibiotics’, etc. | |||
| Add a reference and/or weblink to the source of these statistics | |||
| Reword the output where it says ‘1 in . . . consultations’ to make this as clear as possible | |||
| Move output to the top of page | |||
| Graphs | Move the graphs button to the output section on the home page and make this large and easily visible | ||
| Reduce the number of decimal places that appear when an option is clicked on the graph | |||
| Make ‘age group’ clearer and more visible | |||
| Remove play button |
Patient and public involvement
The findings were discussed at a PPI meeting and the results were thought to be useful. However, GPs and other members of primary care staff may vary in terms of how risk averse they are. Less experienced practitioners may be more risk averse and less inclined to reduce their prescribing. There is also a need to use this information to communicate to patients the need to reduce antibiotic prescribing. However, if patients see doctors ‘looking things up’ they might lose confidence in them. This would need careful handling if the tool is to be used effectively.
Discussion
Analysis of data from interviews with GPs identified four key themes that GPs reported as likely to influence willingness to use the web pages. The themes identified were (1) use of the pages as ‘a decision-making tool’, (2) ‘use with patients’, (3) ‘design of tool’ and (4) ‘accessing pages during consultation’.
The themes were used to refine and adapt the original pages and led to the amendment of features, such as clearer and more explanatory titles on each section, a reduction in the number of bacterial infections available for each condition (only options likely to be used now appear), addition of references for frailty measure and data, and the simplification of graphs. GPs expressed generally positive views of the pages following these changes being made.
This research provided only preliminary testing of the app with a selected group of GPs. More extensive iterative testing would be required before further implementation and evaluation of the app could be considered.
Chapter 13 Discussion
Main findings of this research
The initial qualitative research for this project found that primary care practitioners were generally concerned with the consequences of both inappropriate prescribing and inappropriate withholding of antibiotics (see Chapter 5). The possibility of sepsis and other bacterial infection complications occurring in patients who were not prescribed antibiotics was a significant worry. Practitioners were also sometimes concerned about meeting the perceived expectations of patients for antibiotic treatment. However, prescribers’ attitudes towards antibiotic prescribing are changing and becoming more nuanced. There is growing confidence in the capacity to reduce the rate of prescribing and to manage patient expectations, which are themselves undergoing change. There is growing recognition that there may be safety trade-offs associated with antimicrobial stewardship, linked to concerns about sepsis and other serious bacterial infections. There is a need to develop better-quantified estimates of risk that can be used to inform clinical decision-making and ‘safety-netting’ advice given to patients.
For their part, patients were concerned to receive the required treatment; however, they also wished to avoid unnecessary antibiotic use and were concerned about the immediate side effects of antibiotics and the possibility of antibiotic-resistant infections, which some had experienced (see Chapter 4). Patients’ expectations could sometimes depend on what they perceived their care provider wanted. Participants in our study, although compliant with antibiotic treatment, raised important questions concerning the right antibiotics being prescribed at the right time. Their accounts of illness suggested explicit and informed choices behind the experiences of both antibiotic treatment and consultation for infections. Patient experiences featured as nuanced and detailed with knowledge of antimicrobial resistance and side effects of antibiotics. Our study highlighted complex interplays between adherence to antibiotics and consuming antibiotics in reflexive, informed ways. These findings offer an important message to practitioners who may be involved in prescribing antibiotics in primary care. Patients seeking advice for common infections in primary care may benefit from explanation and information concerning appropriate treatment options, accounting for risks from both prescribing and withholding antibiotics. Inappropriate or unnecessary antibiotic prescribing is commonplace in primary care, but this no longer appears justifiable in terms of patients’ expectations and knowledge of drug side effects and antimicrobial resistance. From a public health perspective, efforts to inform the public and potential patients of the risks of inappropriate antibiotic treatment, as well as the conditions in which timely treatment is required, should be a key element of continuing antimicrobial stewardship efforts.
Analysis of primary care electronic health records showed that infection consultations in primary care were often poorly coded, with > 40% of antibiotic prescriptions not being associated with informative diagnostic codes across both EMIS and Vision practice systems (see Chapter 6). This is consistent with other recent studies. 8,10 Measures are needed to improve the recording of infection episodes in primary care both when antibiotics are prescribed and when antibiotics are not prescribed. This study found that antibiotic prescribing increased from 2002 to 2012, but declined subsequently, with changes over time being of larger magnitude for women than for men. The decline in antibiotic prescribing was the earliest and most pronounced for RTIs, followed by other specific coded indications. We did not find evidence for a decline in antibiotic prescriptions with poorly documented reasons for prescription. Although there has been a decline in antibiotic prescription in recent years, this is confined to informatively coded prescriptions. There has been no reduction in poorly coded antibiotic prescriptions. This suggests that total antibiotic prescribing is the most appropriate exposure measure for consideration, given that indication-specific antibiotic prescribing may be associated with considerable misclassification. As with most aspects of primary care, there is wide variation in rates of prescriptions for antibiotics at different general practices, as well as variation in the use of informative codes at infection consultations.
The research found that analysis of EMIS-derived data in CPRD Aurum gives broadly similar estimates for antibiotic prescribing and infection recording to those reported for Vision-derived data in CPRD GOLD (see Chapter 6). CPRD GOLD includes general practices in Scotland, Wales and Northern Ireland, which have slightly higher antibiotic prescribing rates than either EMIS or Vision general practices in England. Based on these results, we believe that future research studies can be conducted in CPRD Aurum, informed by previous results from CPRD GOLD or The Health Improvement Network. It may also be possible to combine data from CPRD GOLD English practices with CPRD Aurum data in research on antibiotic prescribing. As CPRD Aurum includes an increasing number of general practices, this database will become increasingly important for public health research. However, further work is needed to better understand the quality and completeness of information recorded in areas such as dosing regimen and treatment duration, which are important in estimating treatment exposure in pharmacoepidemiology and pharmacovigilance research.
We evaluated the incidence of serious bacterial infections, including sepsis and localised bacterial infections (see Chapter 7). Evidence from primary care electronic health records showed that these conditions are generally infrequent, especially in the context of the large number of consultations for common infections, including RTIs, skin infections and UTIs. The incidence of serious bacterial infections in men and women rose steadily between 2002 and 2017, particularly for sepsis (men and women), osteomyelitis (mainly in men) and kidney infections (mainly in women). Antibiotic-resistant infections and C. difficile also increased. We found no evidence that serious bacterial infections might be more frequent at general practices with lower rates of antibiotic prescribing. However, general practices that used informative diagnostic codes more frequently were more likely to record serious bacterial infections.
Although general practices that reduce the amount of antibiotics prescribed appear not to risk any increase in serious bacterial infections overall, this finding does not exclude the possibility that antibiotic prescribing patterns may be associated with serious bacterial infection at the individual patient level. Estimation of associations at the individual patient level, rather than the general practice level, presented challenges because of the rarity of most of the conditions and the limitations of our data access. We employed a decision-analytic modelling approach that enabled us to estimate the probability of a serious bacterial infection following an infection consultation when antibiotics were prescribed or not. We used the concept of the NNT to summarise the potential benefit of an antibiotic prescription for a given consultation context for each outcome of concern. In the context of clinical trials and meta-analyses, use of the NNT has been criticised because of the difficulties of CI estimation. 195 In the context of our decision model, UIs were readily derived through the probabilistic approach to model estimation. Given the low frequency of most of the serious bacterial infections studied, the probability of an event following an infection was often low and the NNT was often very high.
We found evidence that antibiotic prescriptions were protective against PTA following RTIs across all age groups studied (see Chapter 8). However, the absolute risk of PTA was very low and the number of antibiotic prescriptions needed to prevent one case was very large. Even among young adults, the group at the highest risk for PTA, 1000 or more patients consulting with RTI would need to be prescribed antibiotics to prevent a single PTA event. Furthermore, a considerable proportion of PTA patients had not presented to their family physician previously, with the implication that their complication might not have been preventable through medical treatment. This adds further weight to the growing body of evidence that reducing antibiotic prescribing further will not result in a substantial increase in this complication, particularly as only around one-third of PTA patients consulted with a RTI prior to their PTA diagnosis, one-third had non-specific consultation codes recorded and a further one-third of patients did not consult at all. The risk of PTA is greater in smokers and sore throat consultations may be an opportunity to discuss smoking cessation.
We found that the probability of sepsis following consultation for common infection episodes in primary care is highly age dependent (see Chapter 9). Without antibiotic treatment, sepsis may follow less than 1 in 10,000 infection consultations among patients under 25 years of age and less than 1 in 1000 infection consultations under 65 years of age. The probability of sepsis increases at older ages, and sepsis may follow approximately 1 in 200 (men) or 1 in 300 (women) consultations at age ≥ 85 years. At older ages, the probability of sepsis is also highly dependent on frailty level (i.e. a 55 year old with severe frailty has a similar probability of sepsis as a non-frail 85 year old). The probability of sepsis is also related to infection type, with the risk being greatest following consultations for UTI and least following consultations for RTI, with consultations for skin infections being in an intermediate position. Risks were generally slightly higher for men, which might be accounted for by their generally lower consultation rates. These quantified estimates of the risk of sepsis following common infection consultations in primary care may be used in antimicrobial stewardship to identify groups of consultations at which reduction of antibiotic prescribing can be pursued more safely. The estimates show that risks of sepsis and benefits of antibiotics are generally more substantial among older adults, people with more advanced frailty and following UTIs.
Analysing linked data enhances the completeness of ascertainment of health events across health service sectors and population health registries (see Chapter 10). However, we found that recording of sepsis diagnoses was inconsistent across primary and secondary care, as well as in mortality statistics. Further standardisation of case definitions and coding practices across linked sources, in addition to more timely and accurate recording of secondary care and mortality events into GP records, would help to improve comparability. However, serious bacterial infections may sometimes be hospital rather than community acquired, and this distinction may not be easy to make from analysing electronic health records data. Further research is required to investigate the reasons for any divergent trends across the data sources and to differentiate trends in community- compared with hospital-acquired sepsis.
We analysed a range of other localised serious bacterial infections (see Chapter 11), but even this large data set did not provide sufficient information to enable estimates of the probability of some rare events, including mastoiditis and infections of the CVS or CNS. In younger adults, the NNT was estimated to be low for the prevention of kidney infections following UTIs in young women. We found that septic arthritis and osteomyelitis appeared to be more likely to be encountered after skin infections, but the probability of these outcomes was low and the NNT was high.
Strengths and limitations
The research aimed to evaluate safety outcomes of reduced antibiotic prescribing, including conditions such as sepsis, PTA and infective endocarditis; these are infrequent or rare events. Consequently, it was necessary to evaluate outcomes over a long period of time in the whole CPRD database to obtain sufficiently precise estimates of incidence rates. The period from 2002 to 2017 was selected for study. However, we found that there were important secular trends in several of the outcomes studied and we cannot assure that our estimates will be fully transferable to future periods. The research was further complicated by possible changes over time in the definition and recognition of outcomes. For example, definitions of sepsis have changed over time, and there have been substantial changes in professional and public awareness of sepsis as a complication of infection. There have also been changes in approach to antibiotic prescribing and antimicrobial stewardship during the period of study. This required analytical approaches that accounted for changes over time in key exposures or the incidence of outcomes.
Our licence agreement with CPRD placed limits on the number of records that could be extracted for analysis. We were able to extract full CPRD data for the numerator, but for the denominator we were restricted to data included in the CPRD GOLD denominator file, which comprised age (year of birth), gender and study year. To address this, we employed sample data for the study denominator as outlined in earlier chapters. As noted above, the CPRD GOLD is a ‘live’ database that is updated each month. The ‘last collection date’ for each general practice is updated for each release, as are dates of death and end of registration for each participant. In addition, the ‘up-to-standard’ date at which the general practice is judged to have been contributing research quality data may be updated even for historical data, based on an algorithm employed by CPRD. Patients have the possibility of ‘opting out’ of CPRD and the small number of opting-out patients may change over time. The present research was conducted over several years in the form of a series of related studies. It drew on data from several different releases of CPRD GOLD. Consequently, there may be slight numerical inconsistencies when different analyses are compared, although relevant findings are expected to be consistent across different releases. In addition, we made a number of modifications to our medical and drug code lists to enable updating consistent with our evolving understanding of conditions relevant for study. We also note that CPRD is UK wide, but linked data are available for only England and there may be appreciable differences in measures of interest among the UK nations. Future studies might consider linked data resources from Wales, Scotland and Northern Ireland.
We studied generally rare infection complications. In the age of big data, it may be less problematic to evaluate rare outcomes, but this does require satisfactory data coverage and data quality to ensure meaningful results. When outcomes are rare, the number of antibiotic prescriptions to prevent one event will necessarily be large. However, from a clinical perspective, there is no consensus on what value of the NNT might be ‘too high’. Another approach may be to identify clinical features that provide improved sensitivity and specificity for prediction of complications. Although this approach holds promise,19 the poor quality of recording of infection episodes in primary care makes it difficult to fully implement at present.
We analysed only coded electronic health records data. It is possible that further relevant information might have been recorded during consultations in the form of free text. However, free-text data are not available for analysis at present.
Our qualitative research reached acceptable sample sizes based on the information power approach. We also included general practices from two sites: one in inner London and one in Oxfordshire. However, the responses analysed may have been influenced by the context of the research and might not be entirely representative of all areas of the UK. The sample of patients included a high proportion of older women whose experience was often with UTIs. The views of younger patients with other types of infections might have been under-represented.
The translational part of the research was conducted during the COVID-19 pandemic and was constrained by the circumstances. Only a preliminary evaluation of the Shiny app was possible, including a small number of GP respondents, most of whom were members of the study team.
Comparison with other studies
This research has addressed the safety outcomes of reduced antibiotic prescribing more systematically than previous studies. Earlier studies have addressed antibiotic prescribing for either RTIs28,56 or UTIs. 61 The recent recognition that many infection episodes in primary care are poorly coded makes it important to evaluate antibiotic prescribing for all indications, as well as all infection consultations. We included a systematic range of serious bacterial infections, including sepsis and localised suppurative infections, in contrast to previous papers that have generally evaluated a more limited range of potential complications. 56,61 We did not include pneumonia because a previous study led by one of the authors suggested that there may be considerable misclassification in primary care between diagnoses of ‘pneumonia’ and ‘chest infection’. 165 Use of the term ‘chest infection’ is very much more frequent than diagnoses of ‘pneumonia’, but the term ‘chest infection’ may refer to either bronchitis or pneumonia. Although ‘chest infection’ records have been decreasing substantially in recent years, pneumonia records have been increasing. 165 This may result from ‘code-shifting’: a term that justifies antibiotic prescription is selected more frequently as antibiotic prescriptions begin to decrease overall. Further predictive modelling of pneumonia after respiratory consultations is presented in the PhD thesis of Xiaohui Sun. 196
Most previous studies have presented relative measures of association, including odds ratios, RRs and hazard ratios. 28,60,61 This research focused on estimating absolute measures of association, including the probability of a serious bacterial infection after a primary care consultation if antibiotics were prescribed or not and the number of antibiotic prescriptions required to prevent one adverse outcome. We adopted a Bayesian approach that facilitated estimation of 95% UIs for probabilities and NNT, thereby overcoming one of the criticisms of the NNT concept. 197 We provide stratified estimates of risk, focusing on age, gender, frailty and type of infection consultation. This is in contrast to previous studies that have generally estimated measures of association for the whole sample, adjusting for covariates. 56 We conducted preliminary work to show how these estimates could be translated into primary care to inform antibiotic prescribing at routine consultations. Further research is needed to produce a tool that can influence primary care prescribing behaviour and achieve safe reduction in antibiotic prescribing.
Patient and public involvement
Patient and public involvement was included at each stage of the project, but this was necessarily more limited in the final year of the project because of restrictions associated with the COVID-19 pandemic. PPI contributed to the interpretation of study findings, as outlined in the preceding chapters. However, we acknowledge that the patient involvement contribution to the research must be managed carefully to avoid introducing bias. For example, in the qualitative research, PPI input did not, in our case, lead to any modification of themes that were identified from qualitative data analysis.
The PPI group observed that the research identified issues that have wider application than antimicrobial stewardship. These include the poor quality of record-keeping in primary care and the wide variations in clinical practice across different general practices. These issues also apply in other clinical areas, such as mental health, and in other areas of problematic prescribing, such as drugs associated with dependence. Poor record-keeping and variations in quality of care need to be addressed by more broadly based health services research and quality improvement initiatives.
With respect to antimicrobial stewardship, the PPI group observed that the research might be considered inconclusive. On the one hand, the research showed that low antibiotic prescribing general practices do not appear to risk any increase in serious bacterial infections. On the other hand, the research showed that not prescribing antibiotics is associated with greater risk of serious infection episodes, including sepsis. The research provided quantified estimates of risk, but it did not show what level of risk is acceptable. Practitioners may vary widely if the level of risk that they are prepared to accept. This raises the question of ‘what should be happening’? How can the results be used to inform better infection management in primary care? Should there be a greater role for near-patient testing? How can patient expectations be managed and changed? For how long, as well as how often, should antibiotic courses be prescribed?
Conclusions and recommendations
Implications for health care
-
The research found that antibiotic prescribing in primary care is decreasing, but the decline is most evident for prescriptions with clearly defined indications recorded. Incompletely coded prescriptions have not decreased. Improving the recording of infection episodes is important for informing antimicrobial stewardship in primary care.
-
Both antibiotic prescribing and the coding of prescriptions vary widely between general practices. The research did not find evidence that general practices with lower total antibiotic prescribing might have more frequent occurrence of serious bacterial infections. Serious bacterial infections were more frequently recorded at general practices with higher proportions of informatively coded infection consultations.
-
The research provided stratified estimates of risk that identify groups of patients in whom, and types of consultations in which, antibiotic prescribing can be more safely reduced. We developed an interactive app that can be used to communicate these estimates to primary care prescribers. We found evidence that serious bacterial infection complications were generally less frequent if antibiotics were prescribed, but the possibility of benefit depended on the underlying frequency of the complication in the context of a particular patient’s characteristics.
-
The safety trade-offs associated with either use or non-use of antibiotics present difficulties, especially when prescribing decisions are inconsistent with patients’ expectations. The research highlighted how patients’ expectations are now more complex than earlier research reported and exhibit tensions between adherence to antibiotics and consuming antibiotics in more reflexive, informed ways. Ensuring that present and future patients are better informed about both the potential benefits and the potential harms of antibiotic use will contribute to future antimicrobial stewardship.
Recommendations for research
-
Measures are needed to improve the recording of infection episodes in primary care both when antibiotics are prescribed and when antibiotics are not prescribed. Interventions should be developed and tested to improve the quality of infection recording in primary care electronic health records and ensure consistency of terminology and coding across primary and secondary care.
-
Estimates for antibiotic prescribing and infection recording were broadly similar in both CPRD GOLD and CPRD Aurum databases, suggesting that future research on antimicrobial stewardship may be conducted using primary care data in CPRD Aurum.
-
The conditions identified as ‘sepsis’ may represent a range of disease severity and further research is needed to refine the predictive accuracy of models of sepsis following primary care infection consultations.
-
The app developed for this research should undergo further iterative development to incorporate antibiotic prescribing and coding information, drawn from individual patient data rather than the aggregate data presently utilised in existing information feedback strategies. This can then be employed as an antimicrobial stewardship tool and tested in a randomised controlled trial.
-
Previous research into antibiotic prescribing practices in primary care may need to be updated to include the need to understand more about prescribing behaviour by professional background (e.g. GP/nurse/pharmacist), risk perceptions and further research on the quality of prescribing information and safety-netting by clinicians.
Acknowledgements
Patient and public involvement
We thank the patients and practitioners who took part in the study. We also thank the members of our PPI group, including Priscilla Baines, Jane Harris, Shaila Hussain, Gavin Jackson, Sandra Jayacodi, Dolapo Ogunleye, Kamran Shafiq and Ellen Thomas.
Data sources
The study is based, in part, on data from the CPRD obtained under license from the UK Medicines and Healthcare products Regulatory Agency. However, the interpretation and conclusions contained in this report are those of the authors alone.
Contributions of authors
Martin C Gulliford (https://orcid.org/0000-0003-1898-9075) (Professor, Public Health) contributed to the design, conduct, analysis and reporting of the study.
Judith Charlton (https://orcid.org/0000-0001-5922-914X) (Research Associate, Computer Programming) contributed to quantitative study design and data analysis.
Olga Boiko (https://orcid.org/0000-0002-1310-1949) (Research Associate, Sociology) contributed to the design, conduct, analysis and reporting of the qualitative research studies.
Joanne R Winter (https://orcid.org/0000-0002-0289-4648) (Research Associate, Epidemiology) contributed to the design, conduct and analysis of the quantitative studies.
Emma Rezel-Potts (https://orcid.org/0000-0003-2986-792X) (Research Associate, Epidemiology) contributed to the design, conduct and analysis of the quantitative studies.
Xiaohui Sun (https://orcid.org/0000-0003-1576-4903) (PhD student, Epidemiology) contributed to the design, conduct and analysis of the quantitative studies.
Caroline Burgess (https://orcid.org/0000-0003-2691-9667) (Research Fellow, Health Psychology) contributed to the design, analysis and reporting of the qualitative research studies.
Lisa McDermott (https://orcid.org/0000-0001-9419-0666) (Research Associate, Health Psychology) contributed to the design, analysis and reporting of the qualitative research studies.
Catey Bunce (https://orcid.org/0000-0002-0935-3713) (Reader, Statistics) contributed to the design, conduct and analysis of the quantitative studies.
James Shearer (https://orcid.org/0000-0002-1658-9767) (Lecturer, Health Economics) contributed to the design, conduct and analysis of the modelling studies.
Vasa Curcin (https://orcid.org/0000-0002-8308-2886) (Senior Lecturer, Health Informatics) contributed to the design and conduct of the translational studies.
Robin Fox (https://orcid.org/0000-0003-1131-5545) (Principal, General Practice) contributed clinical primary care advice to the design, conduct, analysis and reporting of the study.
Alastair D Hay (https://orcid.org/0000-0003-3012-375X) (Professor and GP, Primary Care) contributed primary care research advice to the design, conduct, analysis and reporting of the study.
Paul Little (https://orcid.org/0000-0003-3664-1873) (Professor and GP, Primary Care) contributed primary care research advice to the design, conduct, analysis and reporting of the study.
Michael V Moore (https://orcid.org/0000-0002-5127-4509) (Professor and GP, Primary Care) contributed primary care research advice to the design, conduct, analysis and reporting of the study.
Mark Ashworth (https://orcid.org/0000-0001-6514-9904) (Reader in Primary Care Research) contributed clinical primary care advice to the design, conduct, analysis and reporting of the study.
All authors commented on and approved the final report.
Publications
Sepsis recording in primary care electronic health records, linked hospital episodes and mortality records: population-based cohort Study in England. JCE.
Risks of use and non-use of antibiotics in primary care. Qualitative study of prescribers’ views. BMJ.
Boiko O, Burgess C, Fox R, Ashworth M, Gulliford MC. Risks of use and non-use of antibiotics in primary care: qualitative study of prescribers’ views. BMJ Open 2020;10:e038851.
Boiko O, Gulliford MC, Burgess C. Revisiting patient expectations and experiences of antibiotics in an era of antimicrobial resistance: qualitative study. Health Expec 2020;23:1250–8.
Gulliford MC, Charlton J, Winter JR, Sun X, Rezel-Potts E, Bunce C, et al. Probability of sepsis after infection consultations in primary care in the United Kingdom in 2002–2017: population-based cohort study and decision analytic model. PLOS Med 2020;17:e1003202.
Gulliford MC, Sun X, Anjuman T, Yelland E, Murray-Thomas T. Comparison of antibiotic prescribing records in two UK primary care electronic health record systems: cohort study using CPRD GOLD and CPRD Aurum databases. BMJ Open 2020;10:e038767.
Gulliford MC, Sun X, Charlton J, Winter JR, Bunce C, Boiko O, et al. Serious bacterial infections and antibiotic prescribing in primary care: cohort study using electronic health records in the UK. BMJ Open 2020;10:e036975.
Rezel-Potts E, Gulliford MC, Safe AB Study Group. Sepsis recording in primary care electronic health records, linked hospital episodes and mortality records: population-based cohort study in England. PLOS ONE 2020;15:e0244764.
Winter JR, Charlton J, Ashworth M, Bunce C, Gulliford MC. Peritonsillar abscess and antibiotic prescribing for respiratory infection in primary care: a population-based cohort study and decision-analytic model. Ann Fam Med 2020;18:390–6.
Preprints
Boiko O, Burgess C, Fox R, Ashworth M, Gulliford MC. Risks of use and non-use of antibiotics in primary care. Qualitative study of prescribers’ views. BMJ Open 2020;10:e038851. https://doi.org/10.1136/bmjopen-2020-038851
Gulliford MC, Sun X, Anjuman T, Yelland E, Murray-Thomas T. Comparison of antibiotic prescribing records in two UK primary care electronic health record systems: cohort using the CPRD GOLD and CPRD Aurum databases. BMJ Open 2020;10:e038767. https://doi.org/10.1136/bmjopen-2020-038767
Rezel-Potts E, Gulliford MC, Safe AB Study Group. Sepsis recording in primary care electronic health records, linked hospital episodes and mortality records: population-based cohort study in England. PLOS ONE 2020;15:e0244764. https://doi.org/10.1371/journal.pone.0244764
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HS&DR programme or the Department of Health and Social Care.
References
- Chan M. WHO Director-General Addresses Ministerial Conference on Antimicrobial Resistance. Geneva: World Health Organization; 2016.
- Public Health England (PHE) . English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR). Report 2019 to 2020 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/843129/English_Surveillance_Programme_for_Antimicrobial_Utilisation_and_Resistance_2019.pdf (accessed 8 October 2020).
- O’Neill J. Review on Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations 2016. https://amr-review.org/ (accessed 8 October 2020).
- Department of Health and Social Care . UK 5 Year Antimicrobial Resistance Strategy 2013 to 2018 2013. www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018 (accessed 8 October 2020).
- Gulliford MC, Dregan A, Moore MV, Ashworth M, Staa TV, McCann G, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-006245.
- Ashworth M, Latinovic R, Charlton J, Cox K, Rowlands G, Gulliford M. Why has antibiotic prescribing for respiratory illness declined in primary care? A longitudinal study using the General Practice Research Database. J Public Health 2004;26:268-74. https://doi.org/10.1093/pubmed/fdh160.
- Gulliford M, Latinovic R, Charlton J, Little P, van Staa T, Ashworth M. Selective decrease in consultations and antibiotic prescribing for acute respiratory tract infections in UK primary care up to 2006. J Public Health 2009;31:512-20. https://doi.org/10.1093/pubmed/fdp081.
- Dolk FCK, Pouwels KB, Smith DRM, Robotham JV, Smieszek T. Antibiotics in primary care in England: which antibiotics are prescribed and for which conditions?. J Antimicrobial Chemother 2018;73:ii2-10. https://doi.org/10.1093/jac/dkx504.
- Aabenhus R, Hansen MP, Siersma V, Bjerrum L. Clinical indications for antibiotic use in Danish general practice: results from a nationwide electronic prescription database. Scand J Prim Health Care 2017;35:162-9. https://doi.org/10.1080/02813432.2017.1333321.
- Sun X, Gulliford MC. Reducing antibiotic prescribing in primary care in England from 2014 to 2017: population-based cohort study. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-023989.
- Hay AD. Coding infections in primary care. BMJ 2019;367. https://doi.org/10.1136/bmj.l6816.
- Del Mar CB, Glasziou PP, Spinks AB. Antibiotics for sore throat. Cochrane Database Syst Rev 2000;2. https://doi.org/10.1002/14651858.CD000023.
- Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2015;6. https://doi.org/10.1002/14651858.CD000219.pub4.
- Little P, Gould C, Williamson I, Warner G, Gantley M, Kinmonth AL. Reattendance and complications in a randomised trial of prescribing strategies for sore throat: the medicalising effect of prescribing antibiotics. BMJ 1997;315:350-2. https://doi.org/10.1136/bmj.315.7104.350.
- National Institute for Health and Care Excellence (NICE) . Prescribing of Antibiotics for Self-Limiting Respiratory Tract Infections in Adults and Children in Primary Care 2008. www.nice.org.uk/guidance/cg69/evidence/full-guideline-pdf-196853293 (accessed 8 October 2020).
- National Institute for Health and Care Excellence (NICE) . Summary of Antimicrobial Prescribing Guidance – Managing Common Infections 2018. www.nice.org.uk/Media/Default/About/what-we-do/NICE-guidance/antimicrobial%20guidance/summary-antimicrobial-prescribing-guidance.pdf (accessed 8 October 2020).
- Spurling GK, Del Mar CB, Dooley L, Foxlee R, Farley R. Delayed antibiotics for respiratory infections. Cochrane Database Syst Rev 2013;4. https://doi.org/10.1002/14651858.CD004417.pub4.
- Hay AD, Sterne JA, Hood K, Little P, Delaney B, Hollingworth W, et al. Improving the diagnosis and treatment of urinary tract infection in young children in primary care: results from the DUTY prospective diagnostic cohort study. Ann Fam Med 2016;14:325-36. https://doi.org/10.1370/afm.1954.
- Hay AD, Redmond NM, Turnbull S, Christensen H, Thornton H, Little P, et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med 2016;4:902-10. https://doi.org/10.1016/S2213-2600(16)30223-5.
- Aabenhus R, Jensen JU, Jørgensen KJ, Hróbjartsson A, Bjerrum L. Biomarkers as point-of-care tests to guide prescription of antibiotics in patients with acute respiratory infections in primary care. Cochrane Database Syst Rev 2014;11. https://doi.org/10.1002/14651858.CD010130.pub2.
- Hallsworth M, Chadborn T, Sallis A, Sanders M, Berry D, Greaves F, et al. Provision of social norm feedback to high prescribers of antibiotics in general practice: a pragmatic national randomised controlled trial. Lancet 2016;387:1743-52. https://doi.org/10.1016/S0140-6736(16)00215-4.
- NHS England . Quality Premium: 2016 17 Guidance for CCGs 2016. www.england.nhs.uk/ccg-out-tool/qual-prem/ (accessed 8 October 2020).
- Smieszek T, Pouwels KB, Dolk FCK, Smith DRM, Hopkins S, Sharland M, et al. Potential for reducing inappropriate antibiotic prescribing in English primary care. J Antimicrob Chemother 2018;73:ii36-ii43. https://doi.org/10.1093/jac/dkx500.
- National Institute for Health and Care Excellence (NICE) . NICE Impact: Antimicrobial Resistance 2018. www.nice.org.uk/media/default/about/what-we-do/into-practice/measuring-uptake/niceimpact-antimicrobial-resistance.pdf (accessed 8 October 2020).
- van den Broek d’Obrenan J, Verheij TJ, Numans ME, van der Velden AW. Antibiotic use in Dutch primary care: relation between diagnosis, consultation and treatment. J Antimicrob Chemother 2014;69:1701-7. https://doi.org/10.1093/jac/dku005.
- Lusini G, Lapi F, Sara B, Vannacci A, Mugelli A, Kragstrup J, et al. Antibiotic prescribing in paediatric populations: a comparison between Viareggio, Italy and Funen, Denmark. Eur J Public Health 2009;19:434-8. https://doi.org/10.1093/eurpub/ckp040.
- Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet 2016;387:168-75. https://doi.org/10.1016/S0140-6736(15)00474-2.
- Gulliford MC, Moore MV, Little P, Hay AD, Fox R, Prevost AT, et al. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ 2016;354. https://doi.org/10.1136/bmj.i3410.
- NHS Choices . Sepsis 2017. www.nhs.uk/Conditions/Blood-poisoning/Pages/Introduction.aspx (accessed 8 October 2020).
- Royal College of Physicians . National Early Warning Score (NEWS) 2 n.d. www.rcplondon.ac.uk/projects/outputs/national-early-warning-score-news-2 (accessed 8 October 2020).
- Gulliford MC, Juszczyk D, Prevost AT, Soames J, McDermott L, Sultana K, et al. Electronically-delivered, multi-component intervention for antimicrobial stewardship in primary care. Cluster randomised controlled trial (REDUCE trial) and cohort study of safety outcomes. Health Technol Assess 2019;23. https://doi.org/10.3310/hta23110.
- Cabral C, Lucas PJ, Ingram J, Hay AD, Horwood J. ‘It’s safer to . . . ’ parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: an analysis across four qualitative studies. Soc Sci Med 2015;136-137:156-64. https://doi.org/10.1016/j.socscimed.2015.05.027.
- Lucas PJ, Cabral C, Hay AD, Horwood J. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand J Prim Health Care 2015;33:11-20. https://doi.org/10.3109/02813432.2015.1001942.
- National Institute for Health and Care Excellence (NICE) . Sepsis: Recognition, Diagnosis and Early Management 2017. www.nice.org.uk/guidance/ng51 (accessed 8 October 2020).
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet 2020;395:200-11. https://doi.org/10.1016/S0140-6736(19)32989-7.
- Funk DJ, Parrillo JE, Kumar A. Sepsis and septic shock: a history. Crit Care Clin 2009;25:83-101. https://doi.org/10.1016/j.ccc.2008.12.003.
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003;348:1546-54. https://doi.org/10.1056/NEJMoa022139.
- Burki TK. Sharp rise in sepsis deaths in the UK. Lancet Respir Med 2018;6. https://doi.org/10.1016/S2213-2600(18)30382-5.
- Gulliford MC, Sun X, Charlton J, Winter JR, Bunce C, Boiko O, et al. Serious bacterial infections and antibiotic prescribing in primary care: cohort study using electronic health records in the UK. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-036975.
- Shappell CN, Klompas M, Rhee C. Surveillance strategies for tracking sepsis incidence and outcomes. J Infect Dis 2020;222:S74-S83. https://doi.org/10.1093/infdis/jiaa102.
- Sharland M, Kendall H, Yeates D, Randall A, Hughes G, Glasziou P, et al. Antibiotic prescribing in general practice and hospital admissions for peritonsillar abscess, mastoiditis, and rheumatic fever in children: time trend analysis. BMJ 2005;331:328-9. https://doi.org/10.1136/bmj.38503.706887.AE1.
- Thompson PL, Gilbert RE, Long PF, Saxena S, Sharland M, Wong IC. Effect of antibiotics for otitis media on mastoiditis in children: a retrospective cohort study using the United kingdom general practice research database. Pediatrics 2009;123:424-30. https://doi.org/10.1542/peds.2007-3349.
- Rees JH, Spencer DA, Parikh D, Weller P. Increase in incidence of childhood empyema in West Midlands, UK. Lancet 1997;349. https://doi.org/10.1016/S0140-6736(97)80022-0.
- Mahon C, Walker W, Drage A, Best E. Incidence, aetiology and outcome of pleural empyema and parapneumonic effusion from 1998 to 2012 in a population of New Zealand children. J Paediatr Child Health 2016;52:662-8. https://doi.org/10.1111/jpc.13172.
- Obando I, Muñoz-Almagro C, Arroyo LA, Tarrago D, Sanchez-Tatay D, Moreno-Perez D, et al. Pediatric parapneumonic empyema, Spain. Emerg Infect Dis 2008;14:1390-7. https://doi.org/10.3201/eid1409.071094.
- Thornhill MH, Dayer MJ, Nicholl J, Prendergast BD, Lockhart PB, Baddour LM. An alarming rise in incidence of infective endocarditis in England since 2009: why?. Lancet 2020;395:1325-7. https://doi.org/10.1016/S0140-6736(20)30530-4.
- Quan TP, Muller-Pebody B, Fawcett N, Young BC, Minaji M, Sandoe J, et al. Investigation of the impact of the NICE guidelines regarding antibiotic prophylaxis during invasive dental procedures on the incidence of infective endocarditis in England: an electronic health records study. BMC Med 2020;18. https://doi.org/10.1186/s12916-020-01531-y.
- Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ, Huddleston PM. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am 2015;97:837-45. https://doi.org/10.2106/JBJS.N.01350.
- Geirsson AJ, Statkevicius S, Víkingsson A. Septic arthritis in Iceland 1990–2002: increasing incidence due to iatrogenic infections. Ann Rheum Dis 2008;67:638-43. https://doi.org/10.1136/ard.2007.077131.
- Kalagate R, Rivera A, Pritchard CH, Brent LH. Septic arthritis: changing trends in epidemiology over two decades. Annals Rheumatic Dis 2013;71. https://doi.org/10.1136/annrheumdis-2012-eular.2334.
- Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998–2011. Open Forum Infect Dis 2017;4. https://doi.org/10.1093/ofid/ofw281.
- Strachan RE, Snelling TL, Jaffé A. Increased paediatric hospitalizations for empyema in Australia after introduction of the 7-valent pneumococcal conjugate vaccine. Bull World Health Organ 2013;91:167-73. https://doi.org/10.2471/BLT.12.109231.
- Shiri T, McCarthy ND, Petrou S. The impact of childhood pneumococcal vaccination on hospital admissions in England: a whole population observational study. BMC Infect Dis 2019;19. https://doi.org/10.1186/s12879-019-4119-8.
- Rhee C, Murphy MV, Li L, Platt R, Klompas M. Centers for Disease Control and Prevention Epicenters Program . Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin Infect Dis 2015;60:88-95. https://doi.org/10.1093/cid/ciu750.
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303-10. https://doi.org/10.1097/00003246-200107000-00002.
- Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ 2007;335. https://doi.org/10.1136/bmj.39345.405243.BE.
- Little P, Stuart B, Hobbs FD, Butler CC, Hay AD, Campbell J, et al. Predictors of suppurative complications for acute sore throat in primary care: prospective clinical cohort study. BMJ 2013;347. https://doi.org/10.1136/bmj.f6867.
- Little P, Stuart B, Hobbs FD, Butler CC, Hay AD, Delaney B, et al. Antibiotic prescription strategies for acute sore throat: a prospective observational cohort study. Lancet Infect Dis 2014;14:213-19. https://doi.org/10.1016/S1473-3099(13)70294-9.
- Little P, Stuart B, Smith S, Thompson MJ, Knox K, van den Bruel A, et al. Antibiotic prescription strategies and adverse outcome for uncomplicated lower respiratory tract infections: prospective cough complication cohort (3C) study. BMJ 2017;357. https://doi.org/10.1136/bmj.j2148.
- Cushen R, Francis NA. Antibiotic use and serious complications following acute otitis media and acute sinusitis: a retrospective cohort study. Br J Gen Pract 2020;70:e255-e263. https://doi.org/10.3399/bjgp20X708821.
- Gharbi M, Drysdale JH, Lishman H, Goudie R, Molokhia M, Johnson AP, et al. Antibiotic management of urinary tract infection in elderly patients in primary care and its association with bloodstream infections and all cause mortality: population based cohort study. BMJ 2019;364. https://doi.org/10.1136/bmj.l525.
- Mistry C, Palin V, Li Y, Martin GP, Jenkins D, Welfare W, et al. Development and validation of a multivariable prediction model for infection-related complications in patients with common infections in UK primary care and the extent of risk-based prescribing of antibiotics. BMC Med 2020;18. https://doi.org/10.1186/s12916-020-01581-2.
- Stocks N, Fahey T. Labelling of acute respiratory illness: evidence of between-practitioner variation in the UK. Fam Pract 2002;19:375-7. https://doi.org/10.1093/fampra/19.4.375.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752-62. https://doi.org/10.1016/S0140-6736(12)62167-9.
- Clegg A, Bates C, Young J, Ryan R, Nichols L, Ann Teale E, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016;45:353-60. https://doi.org/10.1093/ageing/afw039.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60. https://doi.org/10.1177/1049732315617444.
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Atkins L, Francis J, Islam R, O’Connor D, Patey A, Ivers N, et al. A guide to using the theoretical domains framework of behaviour change to investigate implementation problems. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0605-9.
- Lorencatto F, Charani E, Sevdalis N, Tarrant C, Davey P. Driving sustainable change in antimicrobial prescribing practice: how can social and behavioural sciences help?. J Antimicrob Chemother 2018;73:2613-24. https://doi.org/10.1093/jac/dky222.
- Herrett E, Gallagher AM, Bhaskaran K, Forbes H, Mathur R, van Staa T, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. https://doi.org/10.1093/ije/dyv098.
- Herrett EL, Thomas SL, Smeeth L. Validity of diagnoses in the general practice research database. Br J Gen Pract 2011;61:438-9. https://doi.org/10.3399/bjgp11X583092.
- Wolf A, Dedman D, Campbell J, Booth H, Lunn D, Chapman J, et al. Data resource profile: Clinical Practice Research Datalink (CPRD) Aurum. Int J Epidemiol 2019;48:1740-1740g. https://doi.org/10.1093/ije/dyz034.
- Joint Formulary Committee . British National Formulary (online) n.d. www.medicinescomplete.com (accessed 8 October 2020).
- NHS Employers . Seasonal Flu At Risk Read Codes 2015–2016 2016.
- NHS Business and Authority . Dictionary of Medicines and Devices (dm+d) n.d. www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/dictionary-medicines-and-devices-dmd (accessed 27 April 2021).
- Bland JM, Altman DG. Statistical methods for measuring agreement between two methods of clinical measurement. Lancet 1986;1:307-10. https://doi.org/10.1016/S0140-6736(86)90837-8.
- World Health Organization . International Statistical Classification of Diseases and Related Health Problems 10th Revision n.d. http://apps.who.int/classifications/icd10/browse/2010/en (accessed 8 October 2020).
- NHS Information Authority . Clinical Terms (Read Codes). Summarised Product Description 2004.
- Winter JR, Charlton J, Ashworth M, Bunce C, Gulliford MC. Peritonsillar abscess and antibiotic prescribing for respiratory infection in primary care: a population-based cohort study and decision-analytic model. Ann Fam Med 2020;18:390-6. https://doi.org/10.1370/afm.2570.
- Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis. Boca Raton, FL: Chapman and Hall/CRC; 2013.
- Gafoor R, Charlton J, Ravindrarajah R, Gulliford MC. Importance of frailty for association of antipsychotic drug use with risk of fracture: cohort study using electronic health records. J Am Med Dir Assoc 2019;20:1495-501.e1. https://doi.org/10.1016/j.jamda.2019.05.009.
- Medicines and Healthcare products Regulatory Agency . CPRD Linked Data n.d. https://cprd.com/linked-data (accessed 27 March 2020).
- Ministry of Housing Communities and Local Government . The English Index of Multiple Deprivation 2015.
- Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data resource profile: Hospital Episode Statistics admitted patient care (HES APC). Int J Epidemiol 2017;46:1093-1093i. https://doi.org/10.1093/ije/dyx015.
- McDermott L, Yardley L, Little P, Ashworth M, Gulliford M. eCRT Research Team. Developing a computer delivered, theory based intervention for guideline implementation in general practice. BMC Fam Pract 2010;11. https://doi.org/10.1186/1471-2296-11-90.
- Boiko O, Gulliford MC, Burgess C. Revisiting patient expectations and experiences of antibiotics in an era of antimicrobial resistance: qualitative study. Health Expec 2020;23:1250-8. https://dx.doi.org/10.1111/hex.13102.
- Thompson W, Tonkin-Crine S, Pavitt SH, McEachan RRC, Douglas GVA, Aggarwal VR, et al. Factors associated with antibiotic prescribing for adults with acute conditions: an umbrella review across primary care and a systematic review focusing on primary dental care. J Antimicrob Chemother 2019;74:2139-52. https://doi.org/10.1093/jac/dkz152.
- Courtenay M, Rowbotham S, Lim R, Deslandes R, Hodson K, MacLure K, et al. Antibiotics for acute respiratory tract infections: a mixed-methods study of patient experiences of non-medical prescriber management. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013515.
- Linder JA, Singer DE. Desire for antibiotics and antibiotic prescribing for adults with upper respiratory tract infections. J Gen Intern Med 2003;18:795-801. https://doi.org/10.1046/j.1525-1497.2003.21101.x.
- van Driel ML, Coenen S, Dirven K, Lobbestael J, Janssens I, Van Royen P, et al. What is the role of quality circles in strategies to optimise antibiotic prescribing? A pragmatic cluster-randomised controlled trial in primary care. Qual Saf Health Care 2007;16:197-202. https://doi.org/10.1136/qshc.2006.018663.
- O’Connor R, O’Doherty J, O’Regan A, O’Neill A, McMahon C, Dunne CP. Medical management of acute upper respiratory infections in an urban primary care out-of-hours facility: cross-sectional study of patient presentations and expectations. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-025396.
- Gaarslev C, Yee M, Chan G, Fletcher-Lartey S, Khan R. A mixed methods study to understand patient expectations for antibiotics for an upper respiratory tract infection. Antimicrob Resist Infect Control 2016;5. https://doi.org/10.1186/s13756-016-0134-3.
- McNulty CA, Nichols T, French DP, Joshi P, Butler CC. Expectations for consultations and antibiotics for respiratory tract infection in primary care: the RTI clinical iceberg. Br J Gen Pract 2013;63:e429-36. https://doi.org/10.3399/bjgp13X669149.
- Biezen R, Grando D, Mazza D, Brijnath B. Dissonant views – GPs’ and parents’ perspectives on antibiotic prescribing for young children with respiratory tract infections. BMC Fam Pract 2019;20. https://doi.org/10.1186/s12875-019-0936-5.
- Mustafa M, Wood F, Butler CC, Elwyn G. Managing expectations of antibiotics for upper respiratory tract infections: a qualitative study. Ann Fam Med 2014;12:29-36. https://doi.org/10.1370/afm.1583.
- Bagnulo A, Muñoz Sastre MT, Kpanake L, Sorum PC, Mullet E. Why patients want to take or refuse to take antibiotics: an inventory of motives. BMC Public Health 2019;19. https://doi.org/10.1186/s12889-019-6834-x.
- Macfarlane J, Holmes W, Macfarlane R, Britten N. Influence of patients’ expectations on antibiotic management of acute lower respiratory tract illness in general practice: questionnaire study. BMJ 1997;315:1211-14. https://doi.org/10.1136/bmj.315.7117.1211.
- Butler CC, Rollnick S, Pill R, Maggs-Rapport F, Stott N. Understanding the culture of prescribing: qualitative study of general practitioners’ and patients’ perceptions of antibiotics for sore throats. BMJ 1998;317:637-42. https://doi.org/10.1136/bmj.317.7159.637.
- Faber MS, Heckenbach K, Velasco E, Eckmanns T. Antibiotics for the common cold: expectations of Germany’s general population. Euro Surveill 2010;15.
- Kianmehr H, Sabounchi NS, Seyedzadeh Sabounchi S, Cosler LE. Patient expectation trends on receiving antibiotic prescriptions for respiratory tract infections: a systematic review and meta-regression analysis. Int J Clin Pract 2019;73. https://doi.org/10.1111/ijcp.13360.
- Brookes-Howell L, Wood F, Verheij T, Prout H, Cooper L, Hood K, et al. Trust, openness and continuity of care influence acceptance of antibiotics for children with respiratory tract infections: a four country qualitative study. Fam Pract 2014;31:102-10. https://doi.org/10.1093/fampra/cmt052.
- Chief Medical Officer . Annual Report of the Chief Medical Officer. Volume 2, 2011. Infections and the Rise of Antimicrobial Resistance 2013.
- House of Commons Health Select Committee . Antimicrobial Resistance. Eleventh Report of Session 2017–2019 2018.
- Abrahams E. Right drug-right patient-right time: personalized medicine coalition. Clin Transl Sci 2008;1:11-2. https://doi.org/10.1111/j.1752-8062.2008.00003.x.
- McCullough AR, Parekh S, Rathbone J, Del Mar CB, Hoffmann TC. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J Antimicrobial Chemother 2015;71:27-33. https://doi.org/10.1093/jac/dkv310.
- Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med 1997;44:681-92. https://doi.org/10.1016/S0277-9536(96)00221-3.
- Butler CC, Kinnersley P, Prout H, Rollnick S, Edwards A, Elwyn G. Antibiotics and shared decision-making in primary care. J Antimicrob Chemother 2001;48:435-40. https://doi.org/10.1093/jac/48.3.435.
- Lupton D. Consumerism, reflexivity and the medical encounter. Soc Sci Med 1997;45:373-81. https://doi.org/10.1016/S0277-9536(96)00353-X.
- Richmond J, Mangrum R, Wang G, Maurer M, Sofaer S, Yang M. An informed public’s views on reducing antibiotic overuse. Health Serv Res 2019;54:1283-94. https://doi.org/10.1111/1475-6773.13175.
- Lum EPM, Page K, Nissen L, Doust J, Graves N. Australian consumer perspectives, attitudes and behaviours on antibiotic use and antibiotic resistance: a qualitative study with implications for public health policy and practice. BMC Public Health 2017;17. https://doi.org/10.1186/s12889-017-4813-7.
- Dekker AR, de Groot E, Sebalj T, Yardley L, Cals JW, Verheij TJ, et al. Parents’ attitudes and views regarding antibiotics in the management of respiratory tract infections in children: a qualitative study of the influence of an information booklet. BJGP Open 2018;2. https://doi.org/10.3399/bjgpopen18X101553.
- Coenen S, Francis N, Kelly M, Hood K, Nuttall J, Little P, et al. Are patient views about antibiotics related to clinician perceptions, management and outcome? A multi-country study in outpatients with acute cough. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0076691.
- Welschen I, Kuyvenhoven M, Hoes A, Verheij T. Antibiotics for acute respiratory tract symptoms: patients’ expectations, GPs’ management and patient satisfaction. Fam Pract 2004;21:234-7. https://doi.org/10.1093/fampra/cmh303.
- Britten N. Prescribing and the defence of clinical autonomy. Sociol Health Illn 2001;23:478-96. https://doi.org/10.1111/1467-9566.00261.
- Boiko O, Burgess C, Fox R, Ashworth M, Gulliford MC. Risks of use and non-use of antibiotics in primary care: qualitative study of prescribers’ views. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-038851.
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340. https://doi.org/10.1136/bmj.c2096.
- Shallcross LJ, Davies SC. The World Health Assembly resolution on antimicrobial resistance. J Antimicrob Chemother 2014;69:2883-5. https://doi.org/10.1093/jac/dku346.
- Tonkin-Crine S, Yardley L, Little P. Antibiotic prescribing for acute respiratory tract infections in primary care: a systematic review and meta-ethnography. J Antimicrob Chemother 2011;66:2215-23. https://doi.org/10.1093/jac/dkr279.
- Teixeira Rodrigues A, Roque F, Falcão A, Figueiras A, Herdeiro MT. Understanding physician antibiotic prescribing behaviour: a systematic review of qualitative studies. Int J Antimicrob Agents 2013;41:203-12. https://doi.org/10.1016/j.ijantimicag.2012.09.003.
- Krockow EM, Colman AM, Chattoe-Brown E, Jenkins DR, Perera N, Mehtar S, et al. Balancing the risks to individual and society: a systematic review and synthesis of qualitative research on antibiotic prescribing behaviour in hospitals. J Hosp Infect 2019;101:428-39. https://doi.org/10.1016/j.jhin.2018.08.007.
- Vincent C. Patient Safety. London: Churchill Livingstone Elsevier; 2006.
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014;5:229-41. https://doi.org/10.1177/2042098614554919.
- Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis 2008;47:735-43. https://doi.org/10.1086/591126.
- Roehr B. Antibiotics account for 19% of emergency department visits in US for adverse events. BMJ 2008;337. https://doi.org/10.1136/bmj.a1324.
- Vazquez-Lago JM, Lopez-Vazquez P, López-Durán A, Taracido-Trunk M, Figueiras A. Attitudes of primary care physicians to the prescribing of antibiotics and antimicrobial resistance: a qualitative study from Spain. Fam Pract 2012;29:352-60. https://doi.org/10.1093/fampra/cmr084.
- Horwood J, Cabral C, Hay AD, Ingram J. Primary care clinician antibiotic prescribing decisions in consultations for children with RTIs: a qualitative interview study. Br J Gen Pract 2016;66:e207-13. https://doi.org/10.3399/bjgp16X683821.
- Broom A, Broom J, Kirby E. Cultures of resistance? A Bourdieusian analysis of doctors’ antibiotic prescribing. Soc Sci Med 2014;110:81-8. https://doi.org/10.1016/j.socscimed.2014.03.030.
- Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol 2015;36:1065-72. https://doi.org/10.1017/ice.2015.136.
- Klein EY, Martinez EM, May L, Saheed M, Reyna V, Broniatowski DA. Categorical risk perception drives variability in antibiotic prescribing in the emergency department: a mixed methods observational study. J Gen Intern Med 2017;32:1083-9. https://doi.org/10.1007/s11606-017-4099-6.
- Broniatowski DA, Klein EY, May L, Martinez EM, Ware C, Reyna VF. Patients’ and clinicians’ perceptions of antibiotic prescribing for upper respiratory infections in the acute care setting. Med Decis Making 2018;38:547-61. https://doi.org/10.1177/0272989X18770664.
- Yates TD, Davis ME, Taylor YJ, Davidson L, Connor CD, Buehler K, et al. Not a magic pill: a qualitative exploration of provider perspectives on antibiotic prescribing in the outpatient setting. BMC Fam Pract 2018;19. https://doi.org/10.1186/s12875-018-0788-4.
- Fletcher-Lartey S, Yee M, Gaarslev C, Khan R. Why do general practitioners prescribe antibiotics for upper respiratory tract infections to meet patient expectations: a mixed methods study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012244.
- Lum EPM, Page K, Whitty JA, Doust J, Graves N. Antibiotic prescribing in primary healthcare: dominant factors and trade-offs in decision-making. Infect Dis Health 2018;23:74-86. https://doi.org/10.1016/j.idh.2017.12.002.
- Altiner A, Knauf A, Moebes J, Sielk M, Wilm S. Acute cough: a qualitative analysis of how GPs manage the consultation when patients explicitly or implicitly expect antibiotic prescriptions. Fam Pract 2004;21:500-6. https://doi.org/10.1093/fampra/cmh505.
- Légaré F, Labrecque M, Cauchon M, Castel J, Turcotte S, Grimshaw J. Training family physicians in shared decision-making to reduce the overuse of antibiotics in acute respiratory infections: a cluster randomized trial. CMAJ 2012;184:E726-34. https://doi.org/10.1503/cmaj.120568.
- Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev 2015;11. https://doi.org/10.1002/14651858.CD010907.pub2.
- Hart AM, Pepper GA, Gonzales R. Balancing acts: deciding for or against antibiotics in acute respiratory infections. J Fam Pract 2006;55:320-5.
- Biezen R, Roberts C, Buising K, Thursky K, Boyle D, Lau P, et al. How do general practitioners access guidelines and utilise electronic medical records to make clinical decisions on antibiotic use? Results from an Australian qualitative study. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-028329.
- Broom J, Broom A, Kirby E. The drivers of antimicrobial use across institutions, stakeholders and economic settings: a paradigm shift is required for effective optimization. J Antimicrob Chemother 2019;74:2803-9. https://doi.org/10.1093/jac/dkz233.
- Luhmann N. Risk: A Sociological Theory. New York, NY: Routledge; 2002.
- Boiko O, Sheaff R, Child S, Gericke CA. Risks, dangers and competing clinical decisions on venous thromboembolism prophylaxis in hospital care. Sociol Health Illn 2014;36:932-47. https://doi.org/10.1111/1467-9566.12127.
- Simpson SA, Wood F, Butler CC. General practitioners’ perceptions of antimicrobial resistance: a qualitative study. J Antimicrob Chemother 2007;59:292-6. https://doi.org/10.1093/jac/dkl467.
- Wood F, Phillips C, Brookes-Howell L, Hood K, Verheij T, Coenen S, et al. Primary care clinicians’ perceptions of antibiotic resistance: a multi-country qualitative interview study. J Antimicrob Chemother 2013;68:237-43. https://doi.org/10.1093/jac/dks338.
- McCullough AR, Rathbone J, Parekh S, Hoffmann TC, Del Mar CB. Not in my backyard: a systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother 2015;70:2465-73. https://doi.org/10.1093/jac/dkv164.
- O’Connor R, O’Doherty J, O’Regan A, Dunne C. Antibiotic use for acute respiratory tract infections (ARTI) in primary care; what factors affect prescribing and why is it important? A narrative review. Ir J Med Sci 2018;187:969-86. https://doi.org/10.1007/s11845-018-1774-5.
- Gregory R, Peters E, Slovic P. Making decisions about prescription drugs: a study of doctor–patient communication. Health Risk Soc 2011;13:347-71. https://doi.org/10.1080/13698575.2011.575455.
- Tonkin-Crine SK, Tan PS, van Hecke O, Wang K, Roberts NW, McCullough A, et al. Clinician-targeted interventions to influence antibiotic prescribing behaviour for acute respiratory infections in primary care: an overview of systematic reviews. Cochrane Database Syst Rev 2017;9. https://doi.org/10.1002/14651858.CD012252.pub2.
- Arroll B, Kenealy T, Kerse N. Do delayed prescriptions reduce antibiotic use in respiratory tract infections? A systematic review. Br J Gen Pract 2003;53:871-7. https://doi.org/10.1002/14651858.CD004558.
- Marchetti F, Ronfani L, Nibali SC, Tamburlini G. Italian Study Group on Acute Otitis Media . Delayed prescription may reduce the use of antibiotics for acute otitis media: a prospective observational study in primary care. Arch Pediatr Adolesc Med 2005;159:679-84. https://doi.org/10.1001/archpedi.159.7.679.
- Little P, Moore M, Kelly J, Williamson I, Leydon G, McDermott L, et al. Delayed antibiotic prescribing strategies for respiratory tract infections in primary care: pragmatic, factorial, randomised controlled trial. BMJ 2014;348. https://doi.org/10.1136/bmj.g1606.
- Ryves R, Eyles C, Moore M, McDermott L, Little P, Leydon GM. Understanding the delayed prescribing of antibiotics for respiratory tract infection in primary care: a qualitative analysis. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011882.
- Gulliford MC, Prevost AT, Charlton J, Juszczyk D, Soames J, McDermott L, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ 2019;364. https://doi.org/10.1136/bmj.l236.
- Yardley L, Douglas E, Anthierens S, Tonkin-Crine S, O’Reilly G, Stuart B, et al. Evaluation of a web-based intervention to reduce antibiotic prescribing for LRTI in six European countries: quantitative process analysis of the GRACE/INTRO randomised controlled trial. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-134.
- Gulliford MC, Sun X, Anjuman T, Yelland E, Murray-Thomas T. Comparison of antibiotic prescribing records in two UK primary care electronic health record systems: cohort study using CPRD GOLD and CPRD Aurum databases. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-038767.
- Gulliford MC, Sun X, Anjuman T, Yelland E, Murray-Thomas T. Antibiotic prescribing records in two UK primary care electronic health record systems. Comparison of the CPRD GOLD and CPRD Aurum databases. MedRxiv 2020. https://doi.org/10.1101/2020.03.07.20028290.
- Cohen D, Alam MF, Dunstan FD, Myles S, Hughes DA, Routledge PA. Abolition of prescription copayments in Wales: an observational study on dispensing rates. Value Health 2010;13:675-80. https://doi.org/10.1111/j.1524-4733.2010.00717.x.
- Williams AJ, Henley W, Frank J. Impact of abolishing prescription fees in Scotland on hospital admissions and prescribed medicines: an interrupted time series evaluation. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-021318.
- Balinskaite V, Johnson AP, Holmes A, Aylin P. The impact of a national antimicrobial stewardship program on antibiotic prescribing in primary care: an interrupted time series analysis. Clin Infect Dis 2019;69:227-32. https://doi.org/10.1093/cid/ciy902.
- Pouwels KB, Dolk FCK, Smith DRM, Smieszek T, Robotham JV. Explaining variation in antibiotic prescribing between general practices in the UK. J Antimicrob Chemother 2018;73:ii27-ii35. https://doi.org/10.1093/jac/dkx501.
- Goldstein H, Spiegelhalter DJ. League tables and their limitations: statistical issues in comparisons of institutional performance. J R Stat Soc Ser Stat Soc A 1996;159:385-443. https://doi.org/10.2307/2983325.
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010;69:4-14. https://doi.org/10.1111/j.1365-2125.2009.03537.x.
- Herrett E, Shah AD, Boggon R, Denaxas S, Smeeth L, van Staa T, et al. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ 2013;346. https://doi.org/10.1136/bmj.f2350.
- Agniel D, Kohane IS, Weber GM. Biases in electronic health record data due to processes within the healthcare system: retrospective observational study. BMJ 2018;361. https://doi.org/10.1136/bmj.k1479.
- Greenland S, Robins JM. Confounding and misclassification. Am J Epidemiol 1985;122:495-506. https://doi.org/10.1093/oxfordjournals.aje.a114131.
- Sun X, Douiri A, Gulliford M. Pneumonia incidence trends in UK primary care from 2002 to 2017: population-based cohort study. Epidemiol Infect 2019;147. https://doi.org/10.1017/S0950268819001559.
- Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev 2013;11. https://doi.org/10.1002/14651858.CD000023.pub4.
- Klug TE. Peritonsillar abscess: clinical aspects of microbiology, risk factors, and the association with parapharyngeal abscess. Dan Med J 2017;64.
- Powell EL, Powell J, Samuel JR, Wilson JA. A review of the pathogenesis of adult peritonsillar abscess: time for a re-evaluation. J Antimicrob Chemother 2013;68:1941-50. https://doi.org/10.1093/jac/dkt128.
- Klug TE, Henriksen JJ, Fuursted K, Ovesen T. Significant pathogens in peritonsillar abscesses. Eur J Clin Microbiol Infect Dis 2011;30:619-27. https://doi.org/10.1007/s10096-010-1130-9.
- Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Med Decis Making 1981;1:239-46. https://doi.org/10.1177/0272989X8100100304.
- Choi BCK, de Guia NA, Walsh P. Look before you leap: stratify before you standardize. Am J Epidemiol 1999;149:1087-96. https://doi.org/10.1093/oxfordjournals.aje.a009762.
- Rothman K, Greenland S, Lash T. Modern Epidemiology. Philadelphia, PA: Lippincott, Williams and Wilkins; 2008.
- Bennike TBMK, Kjaer E, Skadhauge K, Trolle E. Penicillin therapy in acute tonsillitis, phlegmonous tonsillitis and ulcerative tonsillitis. Acta Medica Scandinavica 1951;139:253-74. https://doi.org/10.1111/j.0954-6820.1951.tb17166.x.
- Balinskaite V, Bou-Antoun S, Johnson AP, Holmes A, Aylin P. An assessment of potential unintended consequences following a national antimicrobial stewardship program in England: an interrupted time series analysis. Clin Infect Dis 2019;69:233-42. https://doi.org/10.1093/cid/ciy904.
- Office for National Statistics . Adult Smoking Habits in the UK: 2018 n.d. www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/bulletins/adultsmokinghabitsingreatbritain/2018 (accessed 8 October 2020).
- Witek TJ, Ramsey DL, Carr AN, Riker DK. The natural history of community-acquired common colds symptoms assessed over 4-years. Rhinology 2015;53:81-8. https://doi.org/10.4193/Rhin14.149.
- Little P, Hobbs FD, Moore M, Mant D, Williamson I, McNulty C, et al. Clinical score and rapid antigen detection test to guide antibiotic use for sore throats: randomised controlled trial of PRISM (primary care streptococcal management). BMJ 2013;347. https://doi.org/10.1136/bmj.f5806.
- Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012;55:e86-102. https://doi.org/10.1093/cid/cis629.
- Gulliford MC, Charlton J, Winter JR, Sun X, Rezel-Potts E, Bunce C, et al. Probability of sepsis after infection consultations in primary care in the United Kingdom in 2002–17: population-based cohort study and decision analytic model. PLOS Med 2020;17. https://doi.org/10.1371/journal.pmed.1003202.
- Editorial . Crying wolf: the growing fatigue around sepsis alerts. Lancet Respir Med 2018;6. https://doi.org/10.1016/S2213-2600(18)30072-9.
- Rezel-Potts E, Gulliford MC. Safe AB Study Group . Sepsis recording in primary care electronic health records, linked hospital episodes and mortality records: population-based cohort study in England. PLOS ONE 2020;15. https://doi.org/10.1371/journal.pone.0244764.
- Rezel-Potts E, Gulliford MC. Safe AB Study Group . Sepsis recording in primary care electronic health records, linked hospital episodes and mortality records: population-based cohort study in England. MedRxiv 2020. https://doi.org/10.1101/2020.10.12.20211326.
- HM Government . Tackling Antimicrobial Resistance 2019–2024: The UK’s Five Year National Action Plan 2019.
- Fitzpatrick F, Tarrant C, Hamilton V, Kiernan FM, Jenkins D, Krockow EM. Sepsis and antimicrobial stewardship: two sides of the same coin. BMJ Qual Saf 2019;28:758-61. https://doi.org/10.1136/bmjqs-2019-009445.
- Crooks CJ, Card TR, West J. Defining upper gastrointestinal bleeding from linked primary and secondary care data and the effect on occurrence and 28 day mortality. BMC Health Serv Res 2012;12. https://doi.org/10.1186/1472-6963-12-392.
- Vollmer WM, O’Connor EA, Heumann M, Frazier EA, Breen V, Villnave J, et al. Searching multiple clinical information systems for longer time periods found more prevalent cases of asthma. J Clin Epidemiol 2004;57:392-7. https://doi.org/10.1016/j.jclinepi.2003.08.014.
- Morgan CL, Currie CJ, Stott NC, Smithers M, Butler CC, Peters JR. Estimating the prevalence of diagnosed diabetes in a health district of Wales: the importance of using primary and secondary care sources of ascertainment with adjustment for death and migration. Diabet Med 2000;17:141-5. https://doi.org/10.1046/j.1464-5491.2000.00221.x.
- Millett ER, Quint JK, De Stavola BL, Smeeth L, Thomas SL. Improved incidence estimates from linked vs. stand-alone electronic health records. J Clin Epidemiol 2016;75:66-9. https://doi.org/10.1016/j.jclinepi.2016.01.005.
- Williams NP, Coombs NA, Johnson MJ, Josephs LK, Rigge LA, Staples KJ, et al. Seasonality, risk factors and burden of community-acquired pneumonia in COPD patients: a population database study using linked health care records. Int J Chron Obstruct Pulmon Dis 2017;12:313-22. https://doi.org/10.2147/COPD.S121389.
- Page DB, Donnelly JP, Wang HE. Community-, healthcare-, and hospital-acquired severe sepsis hospitalizations in the university healthsystem consortium. Crit Care Med 2015;43:1945-51. https://doi.org/10.1097/CCM.0000000000001164.
- Adrie C, Alberti C, Chaix-Couturier C, Azoulay E, De Lassence A, Cohen Y, et al. Epidemiology and economic evaluation of severe sepsis in France: age, severity, infection site, and place of acquisition (community, hospital, or intensive care unit) as determinants of workload and cost. J Crit Care 2005;20:46-58. https://doi.org/10.1016/j.jcrc.2004.10.005.
- Padkin A, Goldfrad C, Brady AR, Young D, Black N, Rowan K. Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 2003;31:2332-8. https://doi.org/10.1097/01.CCM.0000085141.75513.2B.
- NHS England . Improving Outcomes For Patients With Sepsis: A Cross System Action Plan 2015.
- Gallagher AM, Dedman D, Padmanabhan S, Leufkens HGM, de Vries F. The accuracy of date of death recording in the Clinical Practice Research Datalink GOLD database in England compared with the Office for National Statistics death registrations. Pharmacoepidemiol Drug Saf 2019;28:563-9. https://doi.org/10.1002/pds.4747.
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. Br J Haematol 2009;146:27-30. https://doi.org/10.1111/j.1365-2141.2009.07707.x.
- Sun X. Epidemiological Study of Antibiotic Utilisation and Pneumonia Using Electronic Health Records 2021.
- Hutton JL. Number needed to treat: properties and problems. J R Stat Soc Ser A 2000;163:381-402. https://doi.org/10.1111/1467-985x.00175.
List of abbreviations
- app
- application
- BNF
- British National Formulary
- CI
- confidence interval
- CNS
- central nervous system
- CPRD
- Clinical Practice Research Datalink
- CVS
- cardiovascular system
- eFI
- electronic frailty index
- GP
- general practitioner
- GUTI
- genitourinary tract infection
- HES
- Hospital Episode Statistics
- ICD-10
- International Statistical Classification of Diseases and Related Health Problems, Tenth Revision
- IMD
- Index of Multiple Deprivation
- NICE
- National Institute for Health and Care Excellence
- NNT
- number needed to treat
- ONS
- Office for National Statistics
- PhD
- Doctor of Philosophy
- PPI
- patient and public involvement
- PTA
- peritonsillar abscess
- RR
- rate ratio
- RTI
- respiratory tract infection
- TDF
- theoretical domains framework
- UI
- uncertainty interval
- UTI
- urinary tract infection
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/hsdr09090).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
