Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number 14/21/21. The contractual start date was in February 2016. The final report began editorial review in December 2021 and was accepted for publication in March 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Knapp et al. This work was produced by Knapp et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Knapp et al.
Chapter 1 Background
There is insufficient evidence on how we should treat children and young people (CYP) across a range of health conditions. Unfortunately, adult research findings are not transferrable to CYP. Safety, dosage and effectiveness data are lacking for paediatric populations, meaning that most medical treatments for CYP are used off-label. 1
The effectiveness and safety of healthcare interventions are best determined by evidence from randomised controlled trials (RCTs); when they are well-designed and conducted, trials have greater validity than other study designs through reduced bias. High-quality trials involving CYP are essential to ensure that medication and treatments used in CYP are effective and safe. 2–4 However, it has been reported that in the UK only 6% of registered trials involve children. 5 While the publication rate of trials in adults almost doubled between 1990 and 2010, the rate increase over the same period was just 20% in paediatric trials. 6 In 2013, the UK Chief Medical Officer7 stressed the need to increase the size and rigour of the evidence base for treating CYP, as well as to actively involve CYP in that process: ‘The National Institute for Health and Care Research (NIHR) Clinical Research Network … should work with CYP to input to the design of clinical studies … to facilitate (their) increased participation in trials’. There is now international recognition of the importance of paediatric clinical trials to inform healthcare decisions for CYP. 2–4,8
In the past, the scarcity of paediatric trials was mainly thought to be due to a concern for the vulnerability of CYP, leading to a reluctance of clinicians to undertake trials. 5,9 However, it is apparent that there are many reasons for a lack of CYP trial evidence, including low recruitment, such that a third of trials have delayed completion and about a fifth are discontinued due to inadequate recruitments,10,11 and high levels of participant attrition. 12,13 In any patient population, poor recruitment and retention of trial participants are costly14 and contribute to research waste. 15,16
Challenges in recruitment and retention in paediatric trials arise partly from difficulties with informed consent or assent, which, in legal terms, is more complex than with adults. In the UK, parental consent is needed when a child aged under 16 is immature (‘not competent’), or when a mature (‘competent’) child is being enrolled into a Clinical Trial of a Medicinal Product (CTIMP). In non-CTIMP trials consent may be given by the mature child or through a parent or guardian, although legal uncertainty means that dual consent may be preferable. 17,18 However, anecdotal reports are that parents are often preferentially approached, even when a child’s consent would be equally valid. The child may often be in a better position than their parent to envisage, and prepare for, what participation will mean19,20 and so excluding the child from decision-making may increase the chance of misunderstanding and subsequent withdrawal. Furthermore, when parents are consenting for a child, the perceived threshold for consent is higher than for parents themselves;21 that is, parents and clinicians tend to be risk-averse and will opt for standard care rather than a trial for a child, more often than would be the case for an adult. 22
The information provided to potential participants plays a crucial role in decision-making about trial recruitment in all age groups, and may facilitate participation. 23 In the UK, as in many countries, written information to inform a research consent decision is legally required, and its content and format must be approved by a Research Ethics Committee (REC). In most cases the mandated written information is provided to patients in printed form, although this form of information has received prolonged criticism for being too long and technical, unappealing and hard to navigate. 24–27 Patients with lower levels of literacy are most likely to be affected by these deficiencies. 28 The UK Health Research Authority (HRA) has responded to these criticisms by encouraging researchers to provide shorter participant information sheets for low-risk research, as part of its proportionate review initiatives, and recommending exploration of the use of non-print media. 29 However, the provision of shorter or condensed participant information sheet (PIS) has been shown to have no impact on trial recruitment rates in adults. 30
It is uncertain if length of recruitment information is the only feature determining its utility. For example, in a series of studies in adults, printed trial participant information sheets were revised through information design, plain English and user testing, although the amount of written content was not reduced. The utility of the sheets was significantly improved, with participants preferring the revised versions and finding them easier to navigate and understand. 31–33 This finding illustrates the importance of theory and evidence in the development of patient information, as well as the crucial role played by performance testing with potential end-users. 34 Unfortunately, revising sheets in this way has subsequently been found to have no effect on trial recruitment rates in adult patients35 although the studies did not assess the ease of understanding, people’s preferences or the quality of decision-making, and none were undertaken with CYP.
An alternative to printed information is to provide information digitally via PC, laptop, tablet computer or smartphone. Digital provision could enable the use of multimedia information in research recruitment, that is, through a combination of animations, talking-head videos, diagrams and photos, as well as written text. 28,36,37 The user can receive the information aurally and visually. Furthermore, users can select the order in which they access the information, according to personal preference, to use the resource most effectively. People’s increasing familiarity with websites and with obtaining mandated and health information online, means that digital multimedia information may now be used intuitively by most people.
In a range of health settings, multimedia information resources (MMIs) have been found in most cases to be more effective in informing people than printed information. 38–41 Informational MMIs about medical procedures can improve patient knowledge,42–48 but their effect on patient understanding is variable. 49 However, few studies have included children or young people. One trial of CYP undergoing endoscopy reported that presenting the information digitally resulted in more certain decisions by CYP about consent to the procedure, compared to printed information. 50
A number of studies involving CYP, some of them using controlled designs, have tested the effect of video animations as informational tools. Video animation is multimedia, featuring sound and visual content, although animations themselves may be just one component of a larger MMI. A systematic review of video animations showed them to have consistently positive effects on patient knowledge, and some evidence of positive effects on patient cognitions and health-related behaviours51 in a range of health conditions when compared to spoken information from a clinician, printed information or static images. The review included several studies involving CYP. For example, in CYP with epilepsy, the addition of an animated video to usual clinician interaction increased medication adherence and patient knowledge,52 and two trials in respiratory conditions reported that video animations increased the length of young children’s ‘co-operative time’ with a spacer device53 and the proportion of children able to use an intranasal device. 54
There is limited evidence on the effects of multimedia patient information on research recruitment rates,36,37 although several recent studies have examined the use of MMIs in trials involving adults. For example, in a study involving adults with cancer, multimedia information about a trial resulted in more positive attitudes to the research and a greater stated willingness to be involved, than printed information. 55 Another study reported that multimedia produced greater comprehension of the trial, compared to printed information, and that this knowledge was more likely to be retained. 56 A small study of adults with osteoporosis compared multimedia information on tablet computer with traditional printed information, and reported no effect on comprehension. 57 However, those recruiting the participants preferred the multimedia information, despite it taking longer to administer. In Hispanic adults with cancer in the USA, multimedia about a trial was positively evaluated by participants, although the study revealed mixed success with cultural adaptation of the information. 58 Finally, in the UK, one study reported that multimedia and PISs resulted in similar trial recruitment rates. 33,59
For CYP one study has reported greater understanding of trials from multimedia information when compared with traditional paper-based information,28 but otherwise there is a lack of evidence in this population. Several studies have used video for informed consent, as a limited form of multimedia. 60 However, the video presentation used has been restrictive for end-users, requiring them to view the video from start to finish, and offering less user flexibility than in other MMIs.
MMIs have the potential to inform and engage potential trial participants in ways that printed information can struggle to do. MMIs have several potential advantages:
-
information presentation is non-linear, enabling people to choose what they view and in what order
-
increased choice of content effectively allows users to personalise content
-
concurrent delivery in sound and vision, which, according to dual channel theory make multimedia more effective at holding attention and increasing understanding30
-
concurrent delivery may make more efficient demands on cognitive load61
-
possible benefits through ‘signalling’ (the provision of cues within MMIs)62
-
possible benefits through a lack of ‘information redundancy’, ensuring that content is not duplicated. 62
However, multimedia also has potential disadvantages. Producing an MMI for a trial may be more time-consuming and expensive than producing participant information sheets, and there may be connectivity problems when using online resources in some hospitals. Furthermore, among potential users easy internet access is commonplace but not universal. Consequently, health inequalities may be widened if information is exclusively provided on MMIs, or if MMIs and printed information are provided as alternatives, but the MMI content is of higher quality. MMIs may also have negative connotations in some users: video and animations have potential to appeal and engage, although it is vital this is not achieved through superficiality or imbalance. Finally, a recent scoping review identified that CYP with long-term health conditions may associate digital health technologies with concerns about privacy, trust and confidentiality. 63
There is a research evidence gap for the use of multimedia participant information for trials involving CYP. In particular, there is a lack of knowledge about the preferred format and content of MMIs, and their effectiveness and acceptability for informing consent decisions in young patients and their parents. More generally, in CYP there is scant evidence into the science of recruitment, which contrasts with a recent acceleration in the number of such studies in adults. The most recent update of the Cochrane systematic review into recruitment interventions includes 68 trials and 72 intervention comparisons. However, only one of the included studies involved CYP, which is noted as a key research gap. 64 Similarly, the Cochrane systematic review on interventions aimed at increasing trial retention also features a dearth of evidence in CYP among its 70 included studies. 65
The science of trial recruitment and retention is being developed through the Study Within A Trial (SWAT) method; these are ‘self-contained studies that have been embedded within a host trial with the aim of evaluating or exploring alternative ways of delivering or organising a particular trial process’. 64,66,67 SWATs provide evidence on improving different aspects of trial processes and outcomes, including the recruitment and retention of patients, maximising questionnaire returns and the effective reporting of trial results. The quality of SWATs is being enhanced through a repository, currently containing almost 200 studies, to encourage pre-SWAT protocol registration and the sharing of findings. 68 Furthermore, the volume of SWATs in the UK is increasing as a result of dedicated funding, which encourages researchers to incorporate a SWAT within a funded host trial. 67 However, the majority of SWATs are still being undertaken in RCTs involving adult patients and there remains a lack of SWATs and a consequent lack of evidence for trial recruitment and retention strategies in the CYP population.
Study aims and objectives
The immediate aims of the TRials Engagement in Children and Adolescents (TRECA) study were to evaluate the potential for MMIs to improve rates of recruitment to trials involving CYP, the quality of decision-making about participation in trials and the impact on trial retention.
The long-term aims of the project are to increase the available research evidence base for the treatment of CYP, including those with long-term health conditions.
The objectives were as follows:
-
to develop template MMIs through participatory design, for use when recruiting CYP to clinical trials
-
to obtain and analyse qualitative data from interviews and focus groups with members of key stakeholder groups (i.e. young patients; parents or guardians; and clinicians) to ensure that the content, format and delivery of the MMIs reflect their preferences
-
to user-test the MMIs with CYP (and their parents or guardians), to test the ability of the MMIs to inform potential users
-
to establish a Patient and Parent Advisory Group (PPAG) and actively liaise with them in the development and evaluation of the MMIs
-
to evaluate the developed MMIs in a series of trials nested within trials (aka SWATs), to test their effects on recruitment and retention rates, and patient decision-making, by comparing the provision of MMIs instead of standard printed participant information (as the primary research question), and the provision of MMIs in addition to standard printed participant information (as the secondary research question).
The projected effects of the MMIs on recruitment, retention and decision-making were anticipated to happen through positive effects on use of the information, leading to benefits to knowledge, attitudes to trials and decisional-confidence (Figure 1).
FIGURE 1.
Logic model for potential benefits of MMI provision.

The TRECA objectives were met through a two-phase study:
-
Phase 1 of TRECA involved the development of template MMIs through two empirical studies (a qualitative study with stakeholders; a user testing study with potential MMI users) and by drawing on a range of relevant expertise (in published research; in plain English writing and readability metrics; and through an active PPAG).
-
Phase 2 of TRECA comprised a series of six SWATs within host clinical trials involving CYP, assessing the effects of information format on recruitment rates, retention rates and participant decision-making, followed by a pre-planned meta-analysis of these data from the SWATs.
Chapter 2 Methods
In order to achieve the objectives of the TRECA study, a two-phase study design was used:
-
Phase 1, in which two templates for the MMIs were developed.
-
Phase 2, in which the developed MMIs were evaluated for effectiveness within a series of SWATs, to assess their effects on trial recruitment and retention, and decision-making among CYP and parents being asked to participate in a trial.
Phase 1 studies
The overarching objective of Phase 1 was development of the MMIs, and we decided to create two MMI templates, for use in the recruitment of the following: (1) young children (aged 6–11) and (2) older children (12–18) and parents/guardians. When we undertook the Phase 1 study, we had yet to recruit the six host trials for Phase 2, and so did not know the target ages of the host trials, nor how many different age-group versions of the PIS there would be. Therefore, although two templates were developed, there was an understanding of the possible need for their adaptation according to the sample age ranges of the host trials.
Templates for the MMIs were developed for three reasons:
-
to ensure the MMIs being evaluated in Phase 2 had sufficient similarity to enable comparisons between them, and a valid statistical meta-analysis
-
to increase the efficiency of Phase 2, so that MMIs for individual host trials could be developed quickly
-
to allow the templates to be used in trials involving CYP beyond the TRECA study, if using the MMIs was shown to be feasible, effective and acceptable.
Phase 1 was driven by participatory design (aka co-design), that is, the involvement of end-users of the MMIs, including CYP, parents and clinicians and researchers involved in trial recruitment. This decision was taken to ensure that the MMIs met the needs and preferences of end-users, particularly CYP, and to ensure they functioned as effective information resources, in being understandable, easily navigable and appealing.
Participatory design (aka co-design) has a variety of forms,69 varying in the degree of control that potential end-users have over the process and the resultant product. In TRECA the participatory design process was researcher-led and end-user-informed, largely because there were some constraints on the design and content of the MMIs we were developing. That is, they would be the digital equivalents of the printed participant information sheets and so would need to be acceptable both to trial researchers and RECs. Furthermore, their development was being undertaken within a commercial contract with a production company, which placed some limits on what was possible.
Phase 1 was a multicomponent study, comprising four main sources of data:
-
a qualitative study
-
a user testing study
-
rewriting of the written content, including readability testing
-
enhanced patient and parent involvement.
More details of the individual Phase 1 components are provided in Chapters 3, 4 and 5 but are summarised briefly below.
The qualitative study with potential end-users of the MMIs involved two stages, followed by iterative, thematic analysis: (1) to ascertain people’s priorities for information about trials to inform consent decisions, and their preferences for MMI appearance and design features; (2) to receive their feedback on two age-group versions of a prototype MMI (about a recently completed trial), so we could adapt it accordingly.
The user testing study involved a form of performance-based testing, in which small samples of potential end-users were observed using a prototype MMI in order to answer a series of factual questions based on its content. User testing gives an indication of two essential features of an information resource: whether specific content can be found and once found, whether it can be understood. User testing used the same prototype MMI that was used in the qualitative study.
The written content of the MMIs would need to be the equivalent of the printed participant information sheets, to meet ethical and governance requirements. The MMIs for older children and parents would contain all the content of the PIS; the MMIs for younger children (6–11) would contain less content, but be sufficient to inform assent decisions. Rewriting of the content was guided by the principles of plain English (e.g. use of short sentences, short words and explanations for technical terms) and age-appropriateness, and the use of a range of readability metrics (through ReadabilityFormulas.com). Finally, the written content was structured to enhance navigation, including the use of a small number of information subsections, which has previously been shown to enhance patient understanding of information about trials. 33 This process of rewriting and restructuring was applied to written text included in the prototype MMIs; it was also applied to the actual MMIs that were evaluated in Phase 2 of the TRECA study.
We formed a PPAG at the start of the TRECA study, which met regularly and provided feedback on written documents and animation images, to ensure the aims and objectives of the study were being met with due consideration of the needs and preferences of patients and parents. The PPAG was active throughout Phase 1 of the study and continued to meet and provide feedback during the development of the six MMIs that were tested in the Phase 2 SWATs.
Phase 2 studies
The aim of Phase 2 was to evaluate the developed MMIs when used within six trials involving CYP.
The primary outcome was trial recruitment rates, evaluating the effect of MMIs as an alternative to participant information sheets (i.e. MMI-only vs. PIS-only).
Secondary outcomes were as follows:
-
Trial recruitment rates, evaluating the effect of MMIs in addition to participant information sheets, compared to participant information sheets alone (i.e. combined MMI and PIS vs. PIS-only).
-
Participant decision-making scores, evaluating the effect of MMIs as an alternative to participant information sheets (i.e. MMI-only vs. PIS-only).
-
Participant decision-making scores, evaluating the effect of MMIs in addition to participant information sheets, compared to participant information sheets alone (i.e. combined MMI and PIS vs. PIS-only).
-
Trial retention rates, evaluating the effect of MMIs as an alternative to participant information sheets (i.e. MMI-only vs. PIS-only).
-
Trial retention rates, evaluating the effect of MMIs in addition to participant information sheets, compared to participant information sheets alone (i.e. combined MMI and PIS vs. PIS-only).
The MMIs were evaluated within a series of SWATs, aka embedded or nested trials. SWATs involve a second randomisation process in addition to the random allocation of participants within the host trial. In TRECA, the SWATs were used to evaluate the effects of information format on participant behaviours (recruitment and retention) and decision-making (at the point of recruitment).
Host trials involved in the six SWATs were able to choose between a two-arm and a three-arm SWAT. The primary question for TRECA was whether MMIs could be used effectively as an alternative to (or replacement for) the PIS; in the development of the study outline and its methods, this question was the primary interest of staff from the clinical trial units that were engaged in discussion. However, the combined MMI and PIS information arm was included for two reasons. First, to permit evaluation of the effectiveness of the MMI in addition to the PIS; given that providing printed information has become the conventional approach over past decades, it was thought useful to evaluate the effects of adding another format. Secondly, because it would give an option in case either the host trial personnel or the REC were unwilling to accept potential trial participants not being provided with participant information sheets; in that case, a two-arm SWAT would be possible, comparing combined MMI and PIS versus PIS-only.
The results of the six SWATs were combined and analysed within preplanned meta-analyses, which were undertaken in the assessment of the primary outcome and all secondary outcomes. Preplanned meta-analyses make the same statistical and methodological assumptions as retrospective meta-analyses following systematic review. The advantages of a preplanned meta-analysis are that it can ensure that individual included studies are sufficiently similar to permit meta-analysis, and it ensures that the meta-analysis has sufficient statistical power to avoid type II statistical error.
Statistical analysis plan
Design
Host trials
Host trials used individual or cluster randomisation as deemed practical and appropriate.
Trial objectives
The immediate aims of TRECA were to evaluate the potential for MMIs to improve the quality of decision-making about participation in healthcare trials involving CYP, and to assess the impact on trial recruitment and retention.
The long-term aim of the project is to increase the available clinical evidence base for the treatment of children and adolescents, including those with long-term health conditions.
The aim of Phase 2 of TRECA was to evaluate the MMIs in a series of SWATs, and test their effects on recruitment and retention rates, and decision-making, by comparing the effects of providing standard written participant information with provision of the MMIs either alone or in addition to the standard written participant information.
Hence, the two pairwise comparisons of the three TRECA arms of interest are whether the MMI provision could replace (MMI-only vs. PIS-only) or supplement (MMI and PIS vs. PIS-only) the standard written participant information. The results of the individual trials were combined statistically in a meta-analysis.
Sample size
Overall
The sample size for each SWAT was ultimately determined, and constrained, by the number of people approached to take part in the host trial.
An example sample size calculation based on the expected baseline recruitment rate of the host trials (i.e. their recruitment rate without the intervention) is provided. Baseline recruitment rates in trials vary greatly, often ranging from 20% to 80%. A baseline rate of 80% was assumed.
We estimated the sample size based on the relative effect of MMI-only (when compared to PIS-only). Further, we assumed 80% power at standard 5% type I error (α rate) to detect the specified effect and we characterised the effect size as the relative increase in recruitment rate (i.e. a relative risk increase).
Assuming the baseline recruitment rate (in the PIS-only arm) was 80%, to detect an increase to 88% in the MMI arm in a single RCT (with 1 : 1 randomisation between MMI and PIS arms), a sample size of 329 per group was needed. This figure was multiplied × 3 to account for the three-arm randomisation in the SWATs (n = 987).
Results from each SWAT were ultimately combined in a meta-analysis. Given that the Phase 2 host trials had variation in the age and health condition of participants, the baseline recruitment rates and the evaluated interventions, it was thought plausible that MMI effectiveness would vary; that is, there would be heterogeneity in the observed effect across trials. Adjusting for this was approximate (particularly as the heterogeneity was unknown before the host trials were undertaken). However, a rule of thumb for the I2 statistic of 50% in the meta-analysis was applied, having the effect of doubling the sample size, deriving an overall sample of 1974 across the six SWATs.
Therefore, the six SWATs in TRECA should (on average) each be approaching 329 people, assuming a baseline trial recruitment rate of 80% of those approached.
This calculation has been updated from that included in the initial published protocol, due to reproducibility issues. However, this does not impact on the validity of the results as the sample size of the overall project was driven by the individual SWATs that were undertaken and could not be pre-determined.
Bone Anchored Maxillary Protraction trial hypothetical setting substudy
The primary outcome of this hypothetical setting substudy was the mean Decision-Making Questionnaire (DMQ) total score. We estimated that a sample size of 109 would give 90% power (alpha 0.05) to detect a statistically significant difference between the groups. A sample of 109 participants (54 and 55 in the two trial arms), allowed for 10% of those randomised not being able to complete the questionnaires. We assumed that a mean between-groups difference of 4.5 on the total DMQ score would be meaningful and estimated that the standard deviation (SD) of the pooled scores would be 6.75 (assuming that 95% scores would fall between 4.5 and 31.5). 70
Randomisation
Allocation to groups in the SWATs was achieved either by random number generator or by another randomisation method that suited the practicalities of the host trial.
Masking of the allocation at outcome measurement was not possible but was also irrelevant: the patient could not be masked to the information format they received but, as they were unaware of the SWAT (the information trial), a lack of masking did not affect their responses on the self-completion measures or have any biasing effect on their decisions on trial participation or continuation.
Outcomes
Primary outcome
Recruitment – MMI-only versus PIS-only
The primary outcome was recruitment to each host trial between the MMI-only arm and the PIS-only arm. For recruitment we calculated the proportion of patients who agreed to participate from the total approached, for each arm of the SWAT. We assumed that patient eligibility for host trial participation would have been assessed before an approach was made.
Data on recruitment to the host trial were recorded automatically within the host trial data set.
Secondary outcomes
Recruitment – MMI and PIS versus PIS-only
The secondary recruitment outcome was recruitment to each host trial between the combined MMI and PIS arm and the PIS-only arm.
Retention
For the retention outcome, we obtained data on the number and timing of dropouts from each host trial. A single time point was specified for use in the analyses. Data on trial retention were recorded automatically within the host trial data set.
Quality of decision-making
We also measured the quality of decision-making by potential host trial participants. Children and adolescents were asked to complete a brief decisional scale, adapted from one used within the REFORM trial71 and drawing conceptually on the SURE72,73 and DelibeRATE scales. 74 When a parent/guardian was involved in the participation decision, we also asked them to complete the scale. The scale was adapted to facilitate completion by young children (see Web Documents 1 and 2). We aimed to obtain decision quality scores both from individuals who decided to participate in the host trial and those who declined. In patients who decided to take part, the CYP and/or parent/guardian were asked to complete the decisional scale once the host trial participation documentation had been completed. In patients who declined participation, they were asked to complete the measure in the clinic or have them posted at home or e-mailed, as appropriate.
The older child and parent/family versions of the questionnaire contained the same number of questions, with slight changes in the phrasing of questions. The decisional scale contained nine Likert questions (with five possible answer options) and there were a further three free text questions, which gave participants the opportunity to give further opinions. The younger patient version of the questionnaire was of a similar format. The decisional scale contained three Likert questions (each with five possible answer options) and the further three ‘free text’ questions (see Web Document 1).
The decision-making scales were scored for both the three- and nine-question versions. Answers to each Likert question were allocated a value of 0–4. The values for each question were summed to create an overall score, out of 12 and 36, for the two scale versions. Up to three missing responses were allowed on the nine-question scale. One missing value was allowed on the three-question version of the scale. A total score was calculated by replacing missing values with the mean score from the completed responses given by the participant. Any questionnaires with more than three (older child/parent/family version) or more than one (younger patient version) missing values, were not scored.
Other important information
To assess any potential moderating influences of other variables on the effectiveness of the MMIs, we aimed to obtain data within each host trial of CYP age, gender and deprivation score, according to allocation in the embedded trial and to host trial participation decision.
Analysis
A Consolidated Standards of Reporting Trials (CONSORT) diagram displaying the flow of participants through each SWAT has been reported.
All analyses were conducted in STATA® v16 (StataCorp LLC, College Station, TX, USA),75 following the principles of intention-to-treat (ITT) with participants’ outcomes analysed according to their original, randomised group. The analysis, outcomes and significance levels were pre-specified in a statistical analysis plan (SAP) before the analysis was conducted.
Analysis of the primary outcome was checked independently by a second statistician after the analyses had been conducted. The data relating to the main Bone Anchored Maxillary Protraction (BAMP) trial and the BAMP substudy were analysed separately. Appropriate model diagnostics were assessed when models were fitted, including checking normality (for continuous outcomes), checking normality of random effects and checking the homoscedasticity of residuals.
As all the analyses include the PIS-only arm, it was important to consider the effect on the type 1 error rate. However, we did not adjust for multiplicity as we only had a single primary outcome comparison.
Statistical analysis has firstly used a modified ITT approach, with participants analysed according to their randomly allocated arm but after the removal of any participants who were subsequently found to be ineligible for the host trial. We have also undertaken a per-protocol analysis, in which participants have been analysed only if they received the information in the format as randomly allocated.
Baseline data
All participant baseline data have been summarised descriptively by TRECA trial arm. No formal statistical comparisons were undertaken. Continuous measures have been reported as means and standard deviations (normality was checked and if non-normal, medians and interquartile ranges were reported) and categorical data were reported as counts and percentages. Baseline data for the host trials varied due to different trial data collection; all data that were collected have been reported. Baseline data were only available for participants who were randomised into each host trial.
Primary analysis
Recruitment – MMI-only versus PIS-only
The proportion of eligible patients entering the trial, which was defined as the number randomised over the number of eligible participants approached have been reported by the SWAT trial arm. Recruitment rates were compared using logistic regression, with TRECA allocation included as a covariate. Clustering was accounted for in the analysis of FORCE, by including the cluster variable as a random effect. The primary comparison was between the MMI-only arm and the PIS-only arm, hence this pairwise comparison was extracted from the model. The results from the regression have been presented as odds ratios, with associated 95% confidence intervals and p-values.
Secondary analyses
Recruitment – MMI and PIS versus PIS-only
The secondary outcome is looking at the effect of the addition of MMI to PIS. This pairwise comparison was extracted from the same model as for the primary analysis. This outcome was not applicable for FORCE as that trial only used the MMI-only and PIS-only arms.
Retention
The time point for assessing retention in each trial is given in Table 8. The proportion of participants who were retained at the specified time point have been reported. This is defined as the number of participants who reached that time point, over the number of participants randomised in the host trial.
The retention rate was compared using logistic regression for each host trial, with host trial allocation (except for the Thermic-3 trial, in which it was not available) and TRECA allocation included as covariates. Where a host trial used stratification variables (Table 1) in the randomisation, these were included as covariates. Clustering was accounted for in the analysis of FORCE, by including the cluster variable as a random effect. Similar to the recruitment analyses, two pairwise comparisons were used: MMI-only versus PIS-only, and MMI and PIS versus PIS-only. The results have been presented as odds ratios, with associated 95% confidence intervals and p-values.
| Trial | Stratification factors |
|---|---|
| BALANCE | Type of amblyopia and centre by stratification |
| BAMP | Gender by stratification |
| CHAMP-UK | Centre, ethnicity, severity of myopia. The unit of randomisation will be the participant (not the eye) using a minimisation algorithm |
| FORCE | Age and centre by stratification |
| THERMIC-3 | Risk adjustment for congenital heart surgery (RACH scores) by stratification |
| UKALL-2011 | Cytogenetic risk and minimal residual disease (MRD) level, National Cancer Institute (NCI) risk, early morphologic response by stratification |
Quality of decision-making
The responses to each question (including the number of missing responses) and the calculated total scores of the decisional questionnaire were summarised descriptively overall and broken down by host trial, TRECA allocation and type of questionnaire (younger patient, adolescent or parent/family).
As a patient and their parent/carer may have both completed a questionnaire, all the data from all three questionnaires were not combined, due to lack of independence. Hence, scores for patients (younger or older) and parents/family questionnaires were analysed separately using a linear regression, with TRECA allocation and host trial status (whether the participant entered the trial) included as covariates. Clustering was accounted for by using the cluster variable as a random effect. Mean difference has been presented with 95% confidence intervals.
If both the younger and older patient questionnaires were used in a host trial, the questionnaire data from the younger and older patient questionnaires were combined. The scores for these were standardised within the trial by subtracting the sample mean score from the observed score and dividing the result by the sample standard deviation, where the ‘sample’ refers to the subset of participants that used that questionnaire. Then the standard scores for all participants were analysed as described above.
The results were compared using a regression model, adjusted for SWAT allocation, and whether the patient consented to participate in the trial. To assess the robustness of the method used to replace the missing values, a sensitivity analysis was conducted, where the analysis was repeated using only the questionnaires in which all nine questions were answered.
The analysis including missing values was repeated but for only those participants who went on to consent to the host trial.
Meta-analyses
Results from each SWAT were combined in meta-analyses. A two-stage random-effects meta-analysis was used in each case, where the results from each model were combined using an inverse-variance approach. No further adjustments were made to these results. The BAMP substudy was not included due to its hypothetical trial setting.
Recruitment
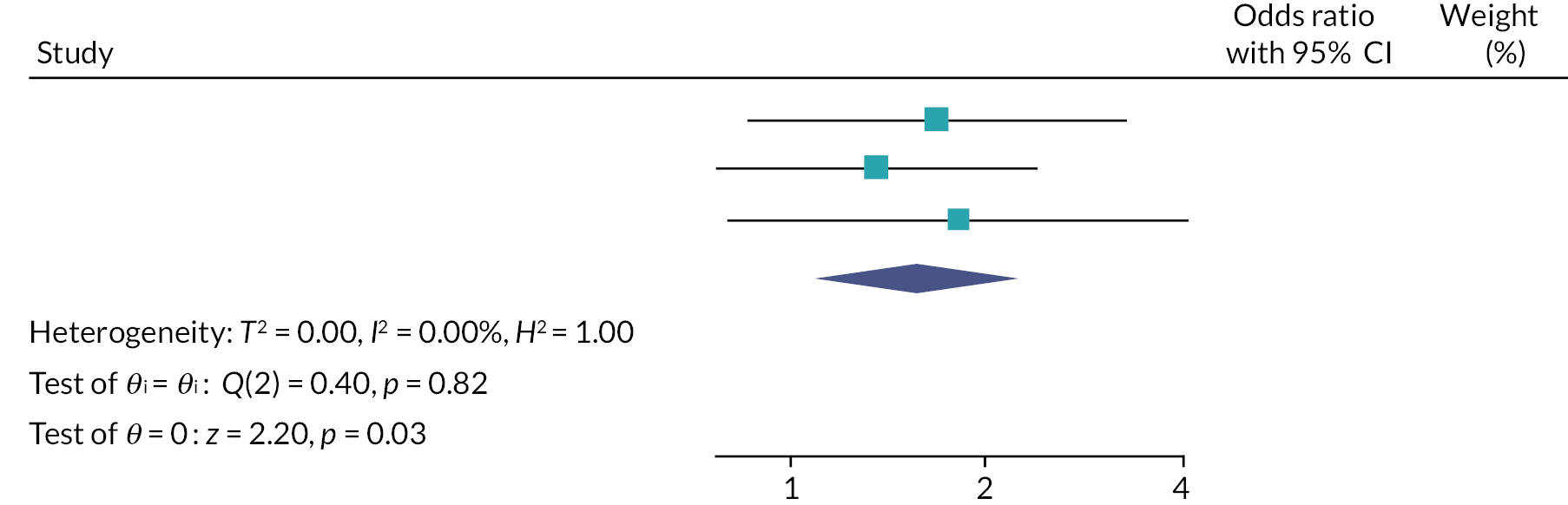
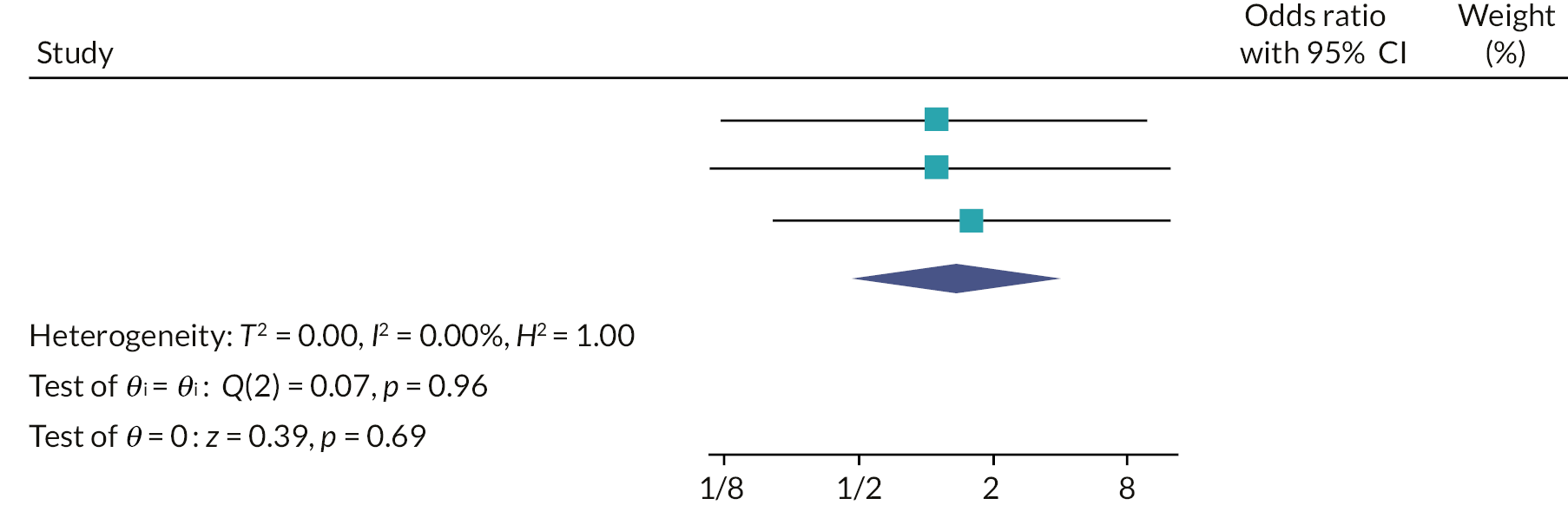
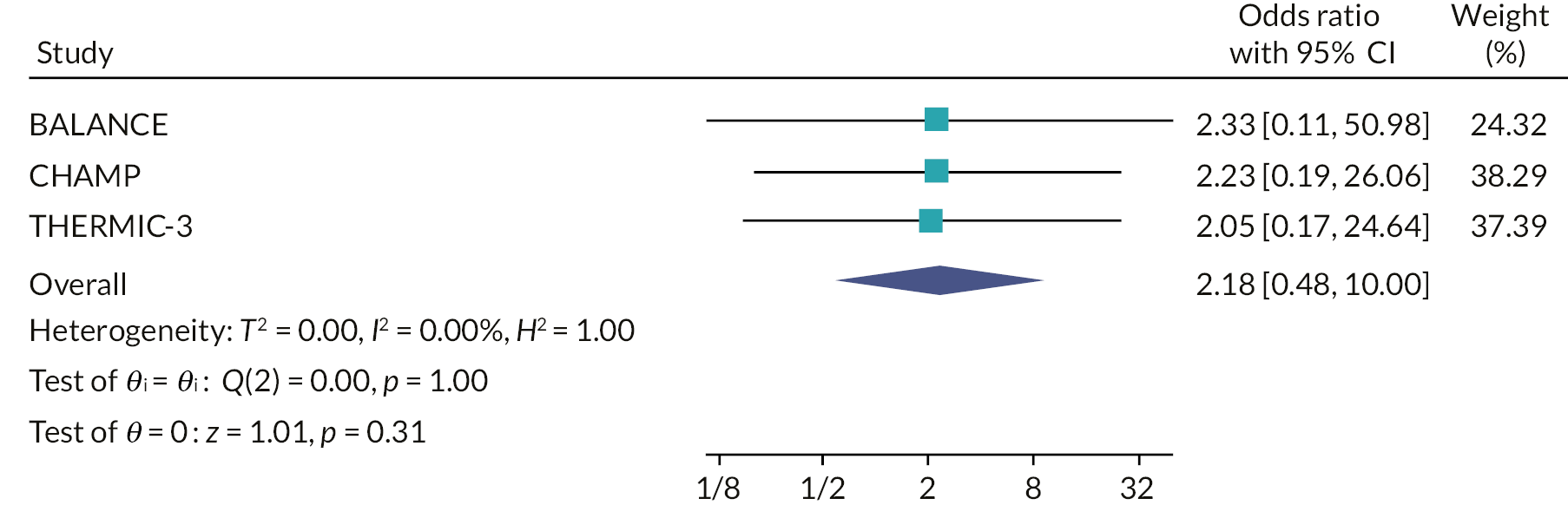
Two random-effects meta-analyses were conducted using the odds ratio for each of the host trials which were calculated in the recruitment primary analysis (PIS-only vs. MMI-only) and the recruitment secondary analysis (MMI and PIS vs. PIS-only). FORCE could not be included in the second meta-analysis as only two arms were used in the trial. The I2 statistic was used to assess the heterogeneity between the trials.
Retention
Two random-effects meta-analyses were conducted using the odds ratio for each of the host trials, which were calculated in the retention secondary analyses. The I2 statistic was used to assess the heterogeneity between the trials.
Quality of decision-making
The decision-making data from all six SWATs (and not the BAMP substudy) were also combined in four meta-analyses. Mean difference scores from each of the trials were combined in a random-effects meta-analysis.
Research ethics and registration
The TRECA study received approval from the NHS Yorkshire & the Humber – Bradford Leeds Research Ethics Committee (17/YH/0082) and the HRA (IRAS ID 212761) on 14 July 2017.
Trial and SWAT registration: TRECA ISRCTN73136092 and Northern Ireland Hub for Trials Methodology Research SWAT Repository (SWAT 97). The SWATs were undertaken through amendments to REC approvals obtained by the host trial research teams.
Chapter 3 Phase 1 – qualitative study
Sections of this chapter have been reproduced from Martin-Kerry et al. 76 and Martin-Kerry et al. 77 These articles are distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text below includes minor additions and formatting changes to the original text.
This chapter reports the qualitative interview and focus group study with children, young people, parents and professionals, which helped inform the development of the MMIs. The qualitative study drew on the principles of participatory design (aka co-design) to ensure that the newly developed MMIs would meet the preferences and needs of CYP being approached about trial participation, while also being acceptable to professionals and parents. The role of professionals and parents as gatekeepers to children’s access to information about trials or treatments means that their acceptance of a MMI template for information content and format was crucial. 78 Here we describe the qualitative study, including the use of a novel interactive ranking exercise, and how we used the findings to inform the development of the MMIs.
Methods
The qualitative study involved two rounds of interviews (individual, joint or focus group discussions). The focus of the first round was on the identification of participants’ preferences and needs for information about trials, while the second round sought participants’ views on prototype versions of the MMIs that had been developed based on the first round of data collection. We aimed to include the same participants in both data collection rounds, to maximise the iterative development of the resources, but with replacement of participants for the second round, as required.
Participant sampling and recruitment
We used two routes to recruit children, young people and parents: a paediatric hospital in north-west England, and a Generation R Young Persons’ Advisory Group (YPAG) based in the English Midlands, whose members are CYP and which advises on various aspects of research involving CYP in the NHS, including research priorities, design and materials. Recruitment via the hospital route involved nurses contacting families of children receiving specialist care for long-term conditions; the YPAG route involved the co-ordinator contacting members and parents or guardians by text message or phone. The interviewer then telephoned those who agreed to contact, to explain the study and arrange a suitable interview time.
Sampling of children, young people and parents was intended to achieve diversity in a number of characteristics, including gender, age, ethnicity, long-term condition and trial experience. Professionals were initially contacted by e-mail by an academic paediatrician working in the study hospital. Those expressing an interest were contacted by the interviewer by e-mail to arrange the interview date. Sampling of professionals was intended to achieve diversity in paediatric specialities and roles within trials.
We provided participants with printed study information sheets with different versions for children, young people, parents and professionals. Participants aged under 16 years gave assent and their parents provided consent for participation. Participants aged 16 years and over provided consent. After the interview each participant received a gift voucher (£10).
Data collection
Topic-guided, semistructured interviews were undertaken with CYP with long-term health conditions, their parents and professionals. The same interviewer (Martin-Kerry) conducted the interviews over the two rounds (held July–October 2016 and November 2016–January 2017). At the start of the interviews, it was explained that all comments were welcome and that the primary aim of the interviews was to inform the development of MMIs (described as ‘websites about trials’) that would be suitable for potential users. Interviews and focus groups were audio-recorded and fully transcribed. Transcripts were then checked for accuracy and pseudo-anonymised before analysis.
First round of interviews
The interviews began with participants being asked about their experiences of health research. Following discussion, all participants were shown 20 cards, each naming a topic that adults had previously identified as being important when deciding to take part in a trial. 79 Participants were asked to rank the topics (Box 1) into three categories: ‘important’, ‘somewhat important’ or ‘not important’,80,81 using an interactive ‘target game’ ranking exercise. 77 While they were undertaking the ranking, participants were asked to comment on the decisions and also suggest any other information not included on the 20 cards that they thought would be important for someone deciding whether or not to take part in a trial.
-
Why is the study happening?
-
Why have I been invited?
-
Do I have to take part?
-
What will happen if I don’t want to carry on with the study?
-
What will happen to me if I take part?
-
Will it cost me any money to take part?
-
Will I be paid for taking part?
-
What is being tested in the study (e.g. what drug or device)?
-
What are the different treatments or types of health care being provided in the study?
-
What are the risks of taking part?
-
What are the possible benefits of taking part?
-
What happens when the study ends?
-
What would happen if a problem happens in the study/could I make a complaint?
-
Will my taking part be kept confidential (private)?
-
Will my general practitioner (GP)/family doctor be told about my taking part?
-
What will happen to any blood tests or other samples that I have as part of the study?
-
What will happen to the results of the study?
-
Who is running the research?
-
Who is paying for the research?
-
Who has reviewed the study?
We had previously reviewed ranking methods and identified the diamond exercise82,83 as promising. However, this needed adaptation for our purpose as it had a maximum number of topics (usually 9), whereas we needed to include 20 topics. Therefore, we used a ranking exercise that involved an A0 size mock target and laminated brightly coloured cards, which named each topic. The activity combined the ‘ranking’ and ‘sorting’ features recommended by Colucci,84 with participants being asked to place each card on an area of the target according to its importance: ‘important’ (yellow; centre circle of target); ‘somewhat important’ (red; intermediate circle of target); or ‘not important’ (blue; outer circle of target). Research can be quite an abstract and challenging topic particularly for younger participants, and this exercise helped to facilitate discussion and support participants in identifying what was important to them. 85
Participants were then asked to discuss their preferences for different designs of multimedia websites. The interviewer showed participants examples of several existing websites, animations and a video (Hi-Light Trial video, see Table 2 for details), as well as examples of character designs for animations, which had been developed by the website creation company; participants were asked to comment on these. The website and character examples represented a range of presentation and design styles.
| Resource | Example of | Link |
|---|---|---|
| HeadSpace | Website | https://www.headspace.com/ |
| Toca | Website | https://tocaboca.com/ |
| Health Research: making the right decision for me (Nuffield Council on Bioethics) | Animation | https://www.youtube.com/watch?v=6yaKwLG_vlE |
| What is a randomised trial? (Cancer Research UK) | Animation | https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/what-clinical-trials-are/randomised-trials |
| Can we tell which children with febrile neutropenia have a bad bug or infection? (Dr Bob Phillips) | Animation (Lego) | https://www.youtube.com/watch?v=Z1AXzJqatds |
| Hi-Light Trial video | Video of child and parent talking about participating in the trial | Video not publicly available. Hi-Light protocol is available87 |
Finally, participants were asked to discuss how the multimedia websites should function and look by asking them to rank six cards, each of which included a criterion for assessing websites. 86 These cards covered content, structure and navigation, visual design, interactivity, functionality and credibility. An initial analysis of the first round of interviews informed the design of the prototype MMIs.
Second round of interviews
The second round of interviews explored participants’ views of the prototype multimedia websites. The second round topic guides were developed with contributions from a qualitative methodologist (BY) with extensive relevant experience, and an expert in children’s education (SH). The prototype MMIs concerned a trial involving children with type 1 diabetes,88 with one MMI version aimed at 6–11-year-olds and parents/guardians, and the other intended for young people aged 12 years upwards and parents/guardians. [A summary of the subcutaneous insulin: pumps or injections (SCIPI) MMIs can be accessed at: https://www.york.ac.uk/healthsciences/research/health-policy/research/scipi.] The text was checked and edited for readability for the different age groups. The multimedia websites included two age-generic animations: one that functioned as a trial-specific ‘explainer’ animation, summarising the trial; and a trial-generic animation ‘why do we do trials’, which outlined the rationale for conducting trials. We showed participants the animations and the multimedia website that was suited to their age (and the other website version if they wanted) and asked them for their views (Box 2). We also invited their suggestions for improving the animations and MMIs.
Can we talk about what your first impressions of the MMI are?
Now I would like us to discuss different aspects of the MMI:
Visual design-
What do you think about how the MMI looks?
-
What parts of the MMI appearance do you like? Which do you not like?
-
How did you find moving through the different parts of the MMI?
-
How was it to find and understand information?
-
What do you think about the mix of video, text, pictures and animation? Should anything be added or changed?
-
How did the MMI pages load?
-
Were there any problems accessing the different parts of the MMI?
-
What do you think about the information content of the MMI?
-
Do you feel it gives you enough information about what is involved in participating in a clinical trial?
-
Can you tell me about anything that you think is missing from the MMI?
Is there anything else that you like about the MMI that we haven’t covered?
Is there anything you particularly liked about the MMI?
Is there anything that you didn’t like about the MMI?
What should we focus on, if anything, to improve the MMI?
Can you tell me how you think you would use the MMI?
Data collection with the youngest study participants (aged 6–8 years) and their parents was not undertaken until the second round, as we thought that the content of the first interview round would be too abstract to engage young children. More concrete information materials (the prototypes) were available for the second round of interviews.
Data analysis to inform the development of the multimedia websites
The initial data analysis was largely deductive descriptive, drawing on rapid ethnographic methods89 in order to provide feedback to the web development company on the MMIs. This analysis focused on the design aspects and content of the MMIs. Subsequent analyses were iterative and thematic, including both inductive and deductive aspects. 90,91 This process involved repeated reading of transcripts to aid familiarisation, followed by line-by-line coding to identify recurrent ideas, which were then organised into themes and subthemes. Interviews were also compared across participant groups in order to identify differences and similarities. Analysis was undertaken by one researcher (Martin-Kerry) with regular guidance and advice provided by two experienced qualitative researchers. Findings were also discussed with the TRECA PPAG, in order to incorporate their views on the development of the multimedia websites. Data coding and indexing was assisted by Microsoft Excel 2010 software.
Results
Participants
A total of 87 people were invited to take part across the two rounds, of whom 62 were interviewed. Among the 87 people invited:
-
35 were professionals of whom 17 were interviewed; those who declined either were unavailable for interview or did not respond
-
28 were parents of whom 24 were interviewed
-
24 were young people of whom 21 were interviewed (see Appendix 8, Table 48).
Those parents or young people who declined did so either due to other commitments or because they did not want to participate.
Among the 62 participants, 15 participated only in round 1 (6 CYP, 6 parents, 3 professionals), 22 only in round 2 (10 CYP, 10 parents, 2 professionals) and 25 participated in both rounds (5 CYP, 8 parents, 12 professionals).
Table 3 provides a summary of participant characteristics and Appendix 8, Table 48 shows detailed participant characteristics.
| Participant group | n | Age, years, mean (range) | Gender | Experience of being approached about a children’s trial | Had taken part in a trial |
|---|---|---|---|---|---|
| CYP | 21 | 12 (6–19) | 8 males 13 females |
10 | 8 |
| Parents | 24 | – | 8 males 16 females | 13 | 12 |
| Health professionals | 17 | – | 4 males 13 females | n/a | n/a |
Among the 21 CYP interviewees, 16 preferred to be interviewed with a parent(s). The five young people who were members of the YPAG preferred to take part in focus group interviews, as did all of the professionals except three. Focus groups contained four to nine participants. One parent participant was interviewed individually via Skype; the remaining interviews were held face-to-face in a room at the hospital or at a YPAG meeting. The child and young person participants had a range of long-term health conditions including asthma, cancer, arthritis and neurological and muscular conditions.
Findings
The findings of the two rounds of interviews are reported together, including participants’ views on important aspects when considering trial participation, plus their preferences for the design, wording and tone of the MMIs, and how these views informed the MMI development.
One notable overarching finding is that most participants spoke about the need for the preferences and views of CYP to be central to the design of the MMIs. One parent commented:
It’s more important to get across to the teenagers because it’s the teenagers who are going through it, you know, the parents are just a by product of what is happening (Parent/33, male, no experience of being approached about a paediatric trial).
While the aspiration to prioritise the views of CYP may have shaped people’s accounts, there were also areas in which the participant groups (children, young people, parents, healthcare professionals) differed in their views. Taking account of such differences is important, particularly given the role that parents and professionals have in guiding and sometimes limiting children’s and young people’s access to the internet and to health information more generally.
Content of the multimedia websites
The following sections outline the aspects of information content and provision that participants prioritised when discussing the developing MMIs.
That you can leave a trial
This piece of information was particularly important to CYP. CYP with trial participation experience emphasised the importance of knowing that leaving the trial was ‘okay’. For example, knowing that they would be free to leave if the treatment caused significant side effects, helped them to say ‘yes’ to a trial. One young person said this information was her only priority. Professionals also ranked this as an important aspect for families; they reported emphasising it during initial discussions with families about trials.
Knowing that you can stop at any time is also good because say you just didn’t want it anymore and you were getting bad side effects and you didn’t like it, then they could stop it just then and there.
(CYP/16, male, 10 years old, experience of being approached about a trial)
If I don’t feel comfortable in the trial anymore or if I didn’t want to take part because I didn’t think it was working for me, could I just stop it – and they were really good at explaining that you could just stop at any time that you wanted and you just have to let them know … so that was one of the only questions that I had really.
(CYP/23, female, 16 years, experience of being approached about a trial)
Therefore, information that participants could leave the trial at any time and without a reason was given prominence within the MMIs.
What is involved if I participate?
Children, young people and parents wanted to understand what trial participation involved. Several with prior experience of being approached about a trial commented that they would have liked to have known more about what was going to be required. In particular, young people wanted detailed information about the frequency of hospital or clinic visits, whether they would need to take time off school, and what the treatments involved, including what they might experience during treatment and beyond. One parent said that participant information sheets never gave details on how participation would affect the day-to-day life of their child.
Some CYP had a pronounced fear of needles and wanted to avoid pictorial or animated portrayals of injections in the MMIs that could be ‘aggressive’ or ‘scary’. This fear was not only expressed by younger participants. CYP also wanted information about the taste of oral medicines, although professionals noted it can be difficult to provide this information in advance for new drug treatments. Parents often spoke of the need to know how participation would affect their child emotionally and whether it would cause ‘burden’ or ‘undue stress’. Some young people and parents talked about the importance of stress, and that it was not often covered in written trial information. Professionals echoed this, agreeing that most families wanted to know about the potential impact of trial participation.
For young people what’s often overlooked is missing out on things like school and social, that’s often not mentioned.
(CYP/18, female, 18 years, experience of being approached about a trial)
How much time is this going to take? That’s by far the biggest anxiety that our families have that they’re going to have to make extra visits to the hospital or extra tests to do, extra diaries to fill in at home. The burden to them is really important and then safety.
(Professional/15, male)
Detailed information about what trial participation would involve was included in the MMIs. The potential for MMIs to include diagrams, photos and animations also make it much easier to convey this detail.
What is the trial testing?
Children and young people with trial experience often described the science behind their condition and treatment and indicated an interest in knowing how the treatment being tested was thought to work. Parents did not always prioritise such information but some wanted to know whether treatments had been tested previously. Professionals talked about the need to explain the science in simple terms, in order to convey the trial rationale. They stressed the need to be clear that professionals not knowing the best treatment for a condition is the rationale for a trial. Therefore, we ensured that this content was prominent in the MMIs.
Risks associated with the trial – and how to inform about them
Most participants talked about the importance of having risks explained in a ‘balanced’ way. Parents specifically wanted to know about possible side effects of treatments. However, they also thought there was sometimes ‘too much information’ about risks, and that some risks were ‘tiny’ but the explanation of them could be ‘scary’ for young people. Young people and some children wanted to know what they were consenting to in terms of risks and side effects but preferred the focus to be on ‘likely’ risks. A list of every ‘potential’ risk could result in being ‘overwhelmed’. Professionals noted that families often wanted to understand risks and safety issues associated with a trial.
Well risks is mostly for, I’d say mostly for the person taking part because it’s going to happen to you if there is any possible risks. Like I know that my drug doesn’t always work because I’ll have a very, very bad flare up and that’s kind of a risk. It can’t always work but I think it’s really important to know that what could happen to you, what you’re getting yourself into. That just makes you feel, I don’t want to do this or I’m willing to do this. I think that’s really important.
(CYP/23, female, 16 years, experience of being approached about a trial)
The risk factor thing is a huge thing, you know, to me some of the information sheets I’ve read I wouldn’t do it because I mean potentially some of those side-effects are tiny but yet the way it’s put across is so scary that you just wouldn’t do it. I know they have to do it, but is there a better way of doing that?
(Parent/24, female, experience about being approached about a paediatric trial)
Therefore, information about the risks were provided within the key section ‘taking part in the trial’.
Possible benefits of trial participation
Parents and young people wanted to know how trial participation might benefit others, as well as any possible personal benefit for themselves or their child. One parent stressed that clinicians should convey this information, because it is ‘important’ to future patients.
But one thing I do like about trials is that I like to know that the trial is going to benefit somebody in the future.
(Parent/36, female, no experience about being approached about a paediatric trial)
That it’s just going to help other people if they’ve got problems.
(CYP/16, male, 10 years, experience of being approached about a trial)
Sometimes families thought that their child may get better treatment in a trial, which led to a concern for some that non-participation, or quitting the trial, could lead to reduced quality of care. This indicated the importance of emphasising that children will receive good care regardless of their decision on trial participation. The issue of possible differences in care quality between trial participants and non-participants, was difficult to address in the MMI template, given the trial specificity of the issue. However, the MMI section on potential benefits of the trial was made prominent, including its potential to inform future treatments to the benefit of other patients.
Confidentiality
Confidentiality was interpreted differently by different participants. Some parents expected that their child’s data would be shared amongst care providers and NHS organisations, including the General Medical Practitioner, to enable continuity of care. However, others thought it was not important for the general practitioner (GP) to be informed of their child’s trial participation. One parent said GPs are ‘copied into everything’, implying an automatic and sometimes unnecessary sharing of information. Young people and parents said that confidentiality was important but they ‘assumed’ it would be observed; therefore, there was not a need to emphasise this information on the MMIs. Professionals did not see information about confidentiality as a priority for families, particularly for CYP, when considering trial participation.
Well you kind of assume your information is confidential anyway so it’s not an issue that you would encounter getting normal health care so you’d assumed the rules wouldn’t be different in a research study (CYP/18) … But it’s nice to be reassured about it.
(CYP/20, male, 20 years, no experience of being approached about a trial)
In the light of these differing interpretations, we decided to include information on confidentiality in the MMIs, but as a topic within the ‘Questions and Answers’ section, rather than giving it prominence.
Who has reviewed or funded the trial
Most children, young people and parents similarly assumed that quality control of trials was undertaken as a matter of course, and that they trusted the organisations running studies to provide scrutiny. However, some young people and parents talked about preferring hospital-led over commerciallyfunded trials, implying that funding sources was of interest to them. Indeed, some professionals thought that families were more willing to join studies being led by doctors they knew. Therefore, there were mixed views on its prominence within the MMIs. Parents also thought that the inclusion of a relevant logo on the MMIs was important:
If I saw that NHS logo, for me, it gives it more credibility I think.
(Parent/60, male, no experience of being approached about a paediatric trial)
Parents further emphasised the need to know that a website their child was accessing was credible, and that a credible, reputable logo would reassure them. Young people were generally less concerned about website credibility but liked the inclusion of a logo. Therefore, given the range of views, we included information on trial scrutiny and funding but did not make this prominent, and we included logos within the MMIs.
Information about payments
Children and young people did not think that information about incentive payments would influence the decision to take part in a trial. However, they worried that such payments could lead others to overlook the risks, and that payment to trial participants could be ‘dodgy’. Professionals noted that incentive payments to participants in UK paediatric trials were rare. In contrast, professionals and young people agreed that for studies involving healthy volunteers undertaking multiple blood tests with no personal benefit, information about payments would be necessary to achieve recruitment. Most participants did not think that providing information about payments for patient participation was advisable. As a compromise, information on payment was included but in the less prominent ‘Questions and Answers’ section.
Design and tone of the multimedia websites
Colour
Young people thought that colour in multimedia websites was important for engagement but noted a need for balance to ensure a ‘professional’ appearance. Young children (aged 6–11 years) and their parents preferred brighter colours whereas young people preferred more muted colours. Some parents and professionals advised avoiding a particular shade of green, due to associations with illness. Participants liked that the animation characters in the prototype websites represented a range of ethnicities, but there was concern among some parents to ensure that skin tones were realistic. In response to these comments, we changed the skin tone for several of the characters after the second interviews. The colours of the MMIs were also selected to vary according to target age, using bright colours (predominantly orange) for children and more muted colours (predominantly teal) for young people.
Layout
The need for the MMIs to be well-structured and easily navigable was stressed by young people, with main points ‘staring right in your face’ and ‘simple’ headings. They gave examples of websites that they liked, which were clearly laid out and easy to use, despite having a lot of content. They liked the relative ‘formality’ and ‘professionalism’ of the prototype MMIs and wanted the inclusion of characters and other images to balance out the text; this would make them more interesting. We ensured these aspects were included in the MMIs.
Font, characters, quirkiness and details
Young people had preferences for the font to be easy to read. Indeed, they had little tolerance for decorative fonts that were difficult to read and said this could stop them from using a website. Large font size was particularly important for young children; following their comments we further increased the font size after the second round of interviews.
Something else that is really important is the type and size of font because it’s obvious that people don’t think about. If you can’t read the font then there is no point to making a website.
(CYP/18, female, 18 years, experience of being approached about a trial)
In the first round of interviews participants stated that they wanted the drawn characters to resemble people rather than more abstract ‘blobs’. Both CYP preferred characters that they could ‘relate to’ and easily recognise the role a character was representing:
Maybe you need a nurse that suits a teenager because they [nurse characters in prototype website] seem like they’re a children’s one with the bear. You need one that you can relate to.
(CYP/35, female, 16 years, no experience of being approached about a trial)
Some of the characters in the websites were revised to ensure that portrayed roles were easily identifiable.
Overall, CYP were drawn to characters with brighter clothing and details (e.g. red dress with white spots) and we incorporated these details into the final character set. Quirky or slightly unexpected details often received favourable responses. For example, the initial ‘why do we do trials’ animation often prompted families of young children to begin talking together about the animation:
It was funny wasn’t it the guy with the pan on his head, it’s quite funny.
(Parent/41, female, experience of being approached about a paediatric trial)
Funny. That was funny with the pan on his head [laugh].
(CYP/43, male, 9 years, experience of being approached about a trial)
Consequently, we included characters in the MMIs with interesting details and quirky elements within the animations, to increase engagement.
Animations
Participants stressed the need for animations to be concise, with most saying they would watch animations for up to 90 seconds, implying that longer animations would not sustain their attention. Young people were particularly critical of repetition and some parents also found this distracting. Several animations were shown to participants in the first round of data collection. One animation about a health condition, depicted using Lego characters and narrated by a child, was popular particularly with children (9–11 years) and their parents. Hearing the child’s voice engaged children. However, young people often said that this animation style and narration was too ‘young’ for them. Another animation, ‘Making the right decision for me’, was narrated by a young person and used sounds to accompany actions and was popular with most children, young people and parents.
It was so intriguing just like the video and what she was saying all went together really nicely. You could put yourself physically in it and just imagine it (CYP/20, male, 18 years, no experience of being approached about a trial).
However, one group of professionals responded very negatively to this particular animation commenting that it was ‘absolutely horrendous’ (Professional/3). The animation depicted a young person arguing with her parents when deciding whether to participate in research. These professionals felt the tone was negative and the content condescending as it implied that assent was less important than consent.
Despite differing views on this animation, all participants agreed that animations were a suitable means of providing information, regardless of patients’ age. Parents often talked about the benefits of seeing and hearing an animation to convey complex information. Consequently, we developed four trial-generic animations and a fifth trial-specific ‘explainer’ animation, which would succinctly summarise the key features of a particular trial.
I was thinking just then of watching it as an adult that even though it was an animation, you think animations are for children but actually when you look at it, sometimes when you just read something on a page or you’ve just got somebody speaking at you, it doesn’t necessarily go in easily and I would say it doesn’t matter that it’s an animation like that for an adult watching it even, because you tend to think of it as just for children don’t you? But they’ve made it [Nuffield animation] nice and simple (Parent/32, female, no experience of being approached about a paediatric trial).
Narration
Young people were sensitive to the tone and sound of the narrator’s voice. As previously noted, children and parents liked the example animation narrated by a child:
I really like it because of the Lego and the child’s voice. That catches your attention doesn’t it? (Parent/27, male, experience of being approached about a paediatric trial).
However, young people and parents thought that a child’s voiceover would make relating to the animation harder, and several found imperfect pronunciation distracting. Therefore, we decided to use an adult’s voice to narrate the prototype animations. However, when the prototype was shown, most young people reacted negatively to it, commenting that they found it ‘boring’, ‘monotonous’ or ‘robotic’ and that this could stop them from focusing on the animation. Parents did not generally comment on the voiceovers. In order to address young people’s concerns, we asked the narrator to rerecord the narrations with greater tonal variation.
Hearing from other families about their experience of trials
We showed participants a short example video in the round one data collection of a parent and young child talking about their trial participation. 87 Most parents, young people and children responded favourably, saying that they found it ‘reassuring’ to hear from those actually involved in the trial or treatment. Professionals echoed this, saying that children would want to hear from and see other children in trials, rather than parents or other adults.
It needs to be people who are actually in trials so then it will put people of my age and younger under reassurance that other people have had a trial before (CYP/51, female, 15 years, experience of being approached about a trial).
Therefore, we created and included short video clips of trial participants and parents talking about being in a trial for the MMIs.
Interactivity
Participants differed in the value they placed on interactivity in MMIs, and they varied in how they defined interactivity (which could explain the differences in opinion). For some, interactivity meant including games, while others felt it referred to being able to ask questions and receive a response, and others thought it could mean following a pathway through the website. One young patient interpreted websites with multimedia to be interactive because you can ‘hear and see things’ rather than just read them.
Some parents of the youngest children wanted games, interactive characters and music included in the MMIs and thought that without this their child would not engage. Some professionals agreed that children would like interactivity and this could include ‘quizzes’, ‘games’ or ‘being able to follow a character along a journey’. However, most young people felt that interactivity could distract from the purpose of the MMI unless the interactive features were relevant. Furthermore, some parents thought that interactive elements such as blogs and forums could be ‘negative’ and ‘unhelpful’ unless managed carefully.
It goes one way or the other I think, either people just ignore it [interactivity] entirely or it’s the only thing they look at, neither of which are particularly useful (CYP/20, male, 18 years, no experience of being approached about a trial).
On reflection we decided against interactivity in the MMIs, in part due to the demand it would place on host trials to provide responses, and due to the mixed views on its value.
The importance of wording
The careful choice of words was important to prevent scaring children and families. For example, terms such as ‘risk’ and ‘dangerous’ were seen by parents, some young people and professionals as possible sources of worry. Therefore, in the MMIs for younger children we used an ‘Is taking part safe?’ heading rather than the intended ‘Is taking part dangerous?’ heading.
Children and young people also indicated the importance of familiar terms. For example, ‘FAQs’ (i.e. frequently asked questions, included in the prototype MMIs) meant little to CYP, who preferred ‘Q&A’ or ‘Questions and Answers’. Young people often spoke positively about the text in the MMIs and liked its simplicity.
I like how there’s no big fancy medical words [in the website] because I kind of feel like that’s kind of really difficult to understand when you’re trying to read about what’s going to happen and they’ve got these massive words. You kind of feel like, for people my age and younger, it needs to be easy for us to understand so you actually know what’s going to happen to us (CYP/51).
Therefore, we revised the MMI wording after the second round of data collection to ensure that wording was meaningful to CYP.
Participating in the qualitative study
Most CYP were engaged and interested in talking about their experiences of research during the more traditional part of the interview when the researcher asked questions. However, the energy within the room changed when the ranking exercise was used in the first round of interviews. Some children jumped up in excitement at the opportunity to do an activity; often keen to be able to choose the colour of the laminated topic cards. When the researcher explained how the exercise would work, some children were very animated.
For example,
interviewer: ‘ … but what I want to find out from you is whether you would be able to put them in terms of what’s important to you. So if you think something is really important put it in the middle ….’
Yeah target, where you want the hit! (CYP/16, male aged 10 years, experience of being approached about a trial).
However, two members of a young person’s focus group initially looked hesitant about the ranking exercise. They seemed concerned about having to proactively contribute rather than respond to questions. Any concern about the exercise seemed to cease once the young people began ranking cards and they were actively chatting as each card was picked out. The process was described as ‘fun’ and ‘interesting’ and they challenged each other’s initial response in a friendly way, often with laughter or enthusiasm. This enabled group discussion and a joint decision on final rankings. For example, one focus group of young people was particularly interactive:
‘Why have I been invited?’ [reading card] [pause, laugh] (CYP/18, female, aged 18 years, experience of being approached about a trial).
I assume that’s to the research as in why they’ve selected you to actually participate, I suppose (CYP/20, male, 18 years, no experience of being approached about a trial).
Isn’t it kind of obvious … explanatory? (CYP/18 female, aged 18 years).
I guess sometimes it wouldn’t be … especially in randomised trials, it’s not (CYP/20, male, 18 years).
So I think, there’s like, there’s a lot of leeway in that question (CYP/18, female, aged 18 years).
I don’t think it might be that important (CYP/21, female, 15 years).
It’s not particularly important [laugh] (CYP/20, male, 18 years).
Yeah it’s like if I saw that on a website as I was like scrolling through, I think I’d skim it but I wouldn’t like, it wouldn’t be the one that I’m there like, okay this is the most important question (CYP/19, female, 16 years).
I think like sometimes, like especially in specific conditions really of trials, it’s kind of self-explanatory (CYP/18, female, aged 18 years).
‘Red!’ ‘Yes, I’d say red’ (CYP/18, female, aged 18 years).
Yeah (All).
In joint interviews, participants could choose how they wanted to undertake the activity. Some parents watched and then added their thoughts after their child had completed it, which enabled parents’ views to be captured separately and contrasted with children’s responses, without either party’s views being dominant. Parents sometimes talked to their child about the meaning of the topic on the card, helping them decide its placement. The activity also allowed the researcher to ask questions about that topic as the card was placed. In some joint interviews with parents/guardians and children one participant would rank the card and then the other would say where they would rank it and why. This enabled a discussion on any disagreements about a topic’s ranking. A joint interview between a young person (who was in hospital for treatment of a long-term health condition) and accompanied by a grandfather, showed how the young person could challenge the grandfather’s initial ranking:
Do I have to take part? [reading card] (CYP/29, female, 12 years, no experience of being approached about a trial).
I don’t think that’s important (Grandfather, Parent/29, male).
It’s up to you [taking part]. That’s very important, it’s your decision. And then this one, ‘what are the possible benefits of taking part? [reading card] (CYP/29, female, 12 years, no experience of being approached about a trial).
Do you think that goes in the red or would you put that in the blue? (Grandfather, Parent/29, male).
Benefits are the good things about taking part. I don’t know…I think the red, you want to get something out of it don’t you (CYP/29, female, 12 years).
Participants talked to each other more during the ranking exercise, than they did for the initial part of the data collection. This is illustrated by this conversation in the young people’s focus group:
What will happen to the results of the study? [reading card] (CYP/20, male, 18 years, no experience of being approached about a trial).
We like that (All speaking).
We do (CYP/20, male, 18 years).
That goes in the yellow. Everyone agreed? (CYP/18, female, aged 18 years, experience of being approached about a trial).
Do I have to take part? [reading card] (CYP/19, female, 16 years, no experience of being approached about a trial).
‘Yeah that’s important. Yeah’ (All)
I’m going to put that right in the middle. Does everyone agree? (CYP/19, female, 16 years, no experience of being approached about a trial).
Yeah. Yes. (All).
I’m really enjoying this! [laughs] (CYP/18, female, aged 18 years).
The exercise also worked when interviewing individuals, as the discussion continued between the participant and researcher. Most CYP were very animated with clapping and spontaneous chatting about the importance of each card. Children particularly enjoyed reading aloud and then describing where each card should be placed.
Some young people and parents ranked within each category. For example, the ‘somewhat important’ category had two layers or circles of red and participants would sometimes say something was ‘inner red’ or ‘outer red’, which had not been anticipated.
Professionals also enjoyed and engaged with the activity. There was often laughter within groups about who would take on the role of reading out the cards and who would place them. At the end of the exercise, professional participants compared their rankings with other groups; with one professional remarking:
They [the rankings] are quite similar, it’s surprising (Professional/1, female).
One young person commented that the card topics were ‘all valid’ but individually they would ‘not have been able to recall and discuss all of these topics’ without the cards. It also ensured that all topics were consistently covered with each participant so that we could get an overall view on the importance of each topic ahead of developing the MMIs. It is possible that the use of cards constrained the discussion to only cover the information on the cards. However, allowing participants the chance to offer any topics not covered on the cards at the end of the activity also elicited a few additional topics and rankings.
Discussion
Main findings
This was the first UK study of stakeholders’ views when developing MMIs to help CYP decide about clinical trial participation. Children, young people and parents prioritised information about what would happen to the participant, the time commitment, and potential harms and benefits of participation. We incorporated this information in the multimedia website and made the prioritised information prominent. Participants wanted multimedia websites that were easy to use and engaging with plain language. They also valued learning from other young patients and their families about what it was like to be in a trial and we therefore developed and incorporated video clips with other families talking about their experiences.
Participants suggested how the websites should look, and despite some variation in opinion, had strong preferences for character styles and colour preferences, which we incorporated within the MMIs. However, we were unable to incorporate interactivity, despite this being important to parents of younger children. Furthermore, when the use of animations was first suggested, some parents were concerned that young people might regard these as ‘too childish’. These concerns were allayed when the prototypes were viewed, with several commenting that they were engaging and provided information in ways that other methods struggled to achieve.
Findings in context
There is little previous research about the development of healthcare MMIs, websites or apps for CYP. 92–96 Our findings largely agree with the information content priorities identified in a previous survey of CYP96 although that survey did not explore priorities for design. Findings from previous studies97–103 of trial participation have shown that personal benefit and helping others are important for young people and parents in deciding on trial participation; our findings concur with that view. However, children, young people and parents in our study also identified information that was of lesser priority to them when deciding about trial participation, including regulatory information and participant payments, consistent with a previous study. 102 Privacy was not reported as an important consideration for CYP, contrasting with a previous survey. 96 Participants’ priorities on website design, font and colour are similar to another study of young people in the development of a self-management app for asthma;94 while other studies have shown that adults37 and young people value clarity in trial information. 104
We did find strong divergences between participants’ information priorities and current UK HRA guidance. Confidentiality was the area where participants’ priorities diverged most from the HRA guidance, which states that confidentiality should be included in study information. Participants regarded confidentiality as important but assumed it would be observed by researchers as a matter of course. We resolved this divergence by including information about confidentiality but not making it prominent. We acknowledge that stakeholders’ views may not always be reconciled with guidance, which would need a case-by-case resolution.
This study adds to the growing number of studies describing child-friendly participatory research methods. 84,85,105,106 In particular, it demonstrates the benefits of using participatory visual ranking activities with CYP, a relatively simple and inexpensive technique. Importantly, the activity helps to avoid participants thinking the researchers want a ‘particular’, ‘right’ or ‘correct’ answer as the activity provides a shared focus. By using movable cards, participants can consider the best placement; the activity also facilitates discussion between participants. In value-based research there is a risk that participants will try to second-guess what the researcher is expecting; the ranking exercise helped to reduce that risk.
While there is limited research on the use of interactive or novel ranking methods to engage CYP in research, there are studies demonstrating their benefits in qualitative research. For example, visual and spatial methods such as drawings and photography can support and engage children in discussion. 107–110 Methods to access the views of CYP with impaired communication or cognition can promote inclusivity. 111,112 The value of using diagrams and graphs to complement conventional interview structures and support participants has also been reported. 108 Similar to our observations, Fängström advocated the use of interactive activities in interviews to shift the focus to the activity,113 thereby reducing the social demands of traditional interviews, when participants may worry about answering correctly.
Strengths and limitations
The design of the MMIs was strengthened by interviewing children, young people, parents and professionals to identify their needs and preferences. We used activities and showed examples of animations, video, websites and character styles to facilitate children’s and young people’s engagement in the interviews and enable them to say what was important to them. We conducted two rounds of interviews to ensure the MMIs could be adapted and responsively refined. Young people gave feedback that they enjoyed participating in the interviews and development of the MMIs. They said they could see where their input had been incorporated, and appreciated being given reasons when their feedback could not be incorporated. A potential weakness is that some young children involved in the second round of interviews had difficulty explaining what they liked or disliked about the prototype MMIs, and so their preferences may have been lost to the final designs. We combined data from individual interviews, joint interviews and focus group, and did this in order to be pragmatic and accommodate participants’ preferences for participating in the study and we note that research does report benefits from combining interviews and focus groups as a means of increasing data richness. 114–116 The study sampling was limited to two UK regions, and it is unclear how far our findings are transferable. However, we have tried to describe our methods in sufficient detail so that other researchers can consider the transferability of the findings to their own setting or implement a similar study.
Use of the ranking exercise facilitated discussion and provided a record of the discussion and final decisions (which were photographed). Asking ‘why’ questions in interviews can be challenging, with participants feeling they must provide justification. Having the shared visual focus of the cards made it easier to explore why participants ranked a particular card: the focus was on the card placement, not just the participant. Sometimes within a focus group, a participant would quickly announce a ranking (important, not important) and then others would query this, giving an example of why they felt it should be ranked differently. The activity seemed to support participants, particularly CYP, to express their views but also avoided them being able to guess what the ‘expected response’ was. The ranking exercise enabled young people to place the cards and consider their relative placement.
Chapter 4 Phase 1 – user testing study
Sections of this chapter have been reproduced from Sheridan et al. 117 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text below includes minor additions and formatting changes to the original text.
This chapter reports the second part of Phase 1 of the project, which involved the user testing of the prototype MMIs. User testing determines how well a resource performs using a stepwise process where information materials are changed based on data provided by participants. 34,118 Issues are highlighted by asking participants to read, or use, the materials and then answer a set of fixed questions to determine how easy items of information are to both find and understand. 34,118
Recent research has questioned whether user testing can improve recruitment to clinical trials. 35,64 However, studies have shown that it can lead to increased comprehension of, and satisfaction with patient information about trials31–33,119 and medicines. 34,118,120,121 Furthermore, user testing is recommended in recently developed guidelines to improve the comprehensibility of participant recruitment materials. 122 While the prototype MMIs had been developed with significant input from stakeholders, including potential users, this did not ensure that they were comprehensible and easy to use. Therefore, this study evaluated the prototype MMIs for ease of use and comprehension, and indicated any amendments that were required.
Method
Participants
Participants were recruited through secondary schools in northern England, investigator networks and publicity flyers. To approximately match the target age groups of the MMIs, participants user testing prototype MMI-1 were 7–11 years old (accompanied by a parent); for prototype MMI-2 participants were young people aged 12–17 years, and parents of young people. Young people and parents tested prototype MMI-2 separately, acknowledging their greater independence in decision-making.
User testing produces its most valid, insightful data when participants are potential information users without significant relevant prior knowledge or experience. 31 Thus, participants did not have type 1 diabetes (the subject of the SCIPI trial featured in the prototype MMIs) or any recent trial experience. Similarly, new participants were recruited for each user testing round. Participants were purposively sampled (i.e. targeted for recruitment in response to the characteristics of participants already recruited) to ensure maximum variation in age, sex and educational attainment (reading age was not assessed). Participants aged 7–15 years old gave assent; a parent provided consent. Other participants provided consent. Each participant received a £10 voucher to compensate their time.
Tested materials
The prototype MMIs were intended for younger children and their parents (MMI-1) and adolescents and parents (MMI-2). Both prototype MMIs contained trial-generic content (e.g. explanations of randomisation) and trial-specific content (e.g. procedures, risks). Example trial-specific content was developed for the prototypes based on SCIPI, a recently completed insulin delivery trial recruiting CYP with type 1 diabetes. 88 All information from the SCIPI trial PIS was incorporated into the MMIs. Short video clips were developed from interviews with four people from the SCIPI trial (child and adolescent participants, a participant’s parent and a clinician).
Both prototype MMIs comprised a home page and five additional tabs: ‘About the trial’, ‘Taking part’, ‘After the trial’, ‘Questions’ and ‘Contact’. The first three tabs had subtabs for additional information. Both MMIs contained the same information; MMI-1 used simpler text and brighter colours to appeal to younger children. A summary of the prototype MMIs can be accessed at: https://www.york.ac.uk/healthsciences/research/health-policy/research/scipi.
During user testing, the MMIs were displayed on a laptop or desktop computer.
Procedure
The researcher (Sheridan) first communicated with participants to build rapport, before explaining that after interacting with the prototype MMI, they would be asked about its content. They were reassured that user testing assesses the information, not the participants. Sessions took place at a time and location convenient to the participant (either at participants’ home or school, or at the University of York).
Testing of the original MMIs
Participants were allowed up to 15 minutes (timed by the researcher) to interact with the MMI before testing; participants informed the researcher when they had finished (if sooner). The researcher then asked each participant 10 questions in a fixed order (Table 4) based on key information items in the MMIs; the question order deliberately did not reflect the order of information presentation in the MMIs.
| Round 1 | Original prototype MMIs | N = 20 | Understanding | |||
|---|---|---|---|---|---|---|
| Finding | ||||||
| NF | D | F | NU | UD | U | |
| Q1. Imagine you decided to take part in the SCIPI trial. How long would you take part in the trial for? | 6 | 4 | 10 | 0 | 2 | 18 |
| Q2. If you took part in the SCIPI trial, what extra things would you need to do at your diabetes clinic visit? | 0 | 2 | 18 | 0 | 1 | 19 |
| Q3. What is the SCIPI trial testing? | 1 | 1 | 18 | 1 | 0 | 19 |
| Q4. Imagine you are in the group that uses the pump, when should you wear it? | 6 | 4 | 10 | 1 | 1 | 18 |
| Q5. Imagine you are in the SCIPI trial. What will happen when the SCIPI trial ends? | 0 | 0 | 20 | 0 | 0 | 20 |
| Q6. How often would you visit the diabetes clinic as part of the SCIPI trial? | 0 | 0 | 20 | 0 | 0 | 20 |
| Q7. Are there any risks to taking part in the SCIPI trial – is it safe? | 0 | 1 | 19 | 0 | 0 | 20 |
| Q8. Who can take part in the SCIPI trial? | 3 | 1 | 16 | 0 | 1 | 19 |
| Q9. If you decided you didn’t want to take part in the SCIPI trial anymore, what would you need to do? | 0 | 0 | 20 | 0 | 0 | 20 |
| Q10. Why do people with type 1 diabetes need to take/inject insulin? | 0 | 2 | 18 | 0 | 1 | 19 |
| Total (%) | 16/200 (8.0) | 15/200 (7.5) | 169/200 (84.5) | 2/200 (1.0) | 6/200 (3.0) | 192/200 (96.0) |
For each question, participants located the required information in the MMI, then explained what it meant using their own words. If participants struggled to find or understand information, the researcher repeated the question and prompted gently for an answer. A 2-minute cut-off per question ensured that participants did not become unnecessarily distressed if they could not find or understand information and ensured consistency across questions and participants. If participants could not locate the information, the researcher directed them to the relevant content and asked them to explain the information.
Finally, participants were asked for their general opinions of the MMI to determine preferences for style, layout and overall design. Interviews were audio-recorded and transcribed verbatim.
Revision and testing of the revised MMIs
Revisions made between user testing rounds were based on scores and participants’ observed behaviour and feedback. After revisions were made, a second user testing round (as above) was completed.
Data analysis
User testing data are indicative and not analysed statistically. Each question was scored by the researcher (Sheridan) for finding (categorised as one of the following: found; found with difficulty, e.g. found with prompting; not found) and understanding (one of the following: understood; understood with difficulty, e.g. understood with prompting; not understood). The number of participants able to find and understand each target information item was calculated.
Viewing time, response time and any further behaviour indicating the ease or difficulty of finding or understanding information were recorded by the researcher to inform revisions. Quotations from participants provided a rationale for MMI revision, and are reported for illustration; they have not been analysed or developed thematically.
Results
Pilot testing
Four participants (one parent, one adolescent and one parent and child pair) took part in pilot user testing to assess questionnaire suitability and timings. No significant issues were identified.
Round one
Participants
The prototype MMIs were tested by 26 participants, comprising seven children aged 7–11 years (mean 8.4 years) tested prototype MMI-1 alongside a parent (n = 6; one parent took part with two of their children); six adolescents aged 12–17 years (mean 14.2 years) and seven parents of adolescents tested prototype MMI-2. As such 20 user testing sessions were completed. Fourteen participants were male (53%). Of the parent participants, 10 (76%) had higher education qualifications. All participants spoke English as their first language.
Findings
Participants spent an average of 11.5 minutes (range 5.0–15.0) looking at the MMI before testing and 17.9 minutes (range 6.9–26.5) completing the user testing questions and providing feedback. Child and parent pairs spent longer looking at the MMI (mean 14.1 minutes, range 12.0–14.1) than adolescents (mean 8.6 minutes, range 5.0–13.5) or parents of adolescents (mean 11.5 minutes, range 7.1–14.5).
Ninety-two per cent of requested information was found (see Table 4). However, 7.5% of this was found with difficulty and only 3 of 20 participants (15%) scored a ‘clear round’ in locating all answers without difficulty. In particular, participants had difficulty locating information about trial length (Q1) and the insulin pump (Q4). Comprehension was high: 17 of 20 participants (85%) scored a ‘clear round’ for understanding.
MMI revisions
In response to user testing scores, observed behaviour and feedback, some changes were made. Data suggested the MMI layout was suboptimal; participants felt they had to scroll too much, causing frustration and resulting in participants missing information at the bottom of pages:
I scrolled down too fast I think, there’s lots of this, scroll, scroll, scroll sort of thing [P11, parent of adolescent (14 years)].
You’re having to scroll up and down to see everything and a lot of kids, people won’t bother they’ll just look at the front page [P23, parent of adolescent (12 years)].
Some participants did not notice the two subtabs under ‘About the trial’ (‘Why is it happening?’, ‘What is being tested?’), possibly due to the first subtab opening automatically. The tabs and subtabs also disappeared from the top of the page when scrolling:
There is a problem with the tabs that first one you read and then, even the first time, [child’s name] and I didn’t spot there was a second tab–[P27, parent of two children (7 and 9 years)].
I didn’t see those [tabs], I think maybe because they’re not quite as bright as the tabs at the bottom … You go down don’t you in order to read the bottom, so you lose the top–[P25, parent of adolescent (17 years)].
This produced specific difficulties in locating information about the insulin pen and pump, exacerbated by the video layout on the page; a line of three videos taking up the width of the screen was located underneath information about the pen. Participants assumed this was the end of the web page and did not scroll down for information about the pump:
Because you said about the difference between the pump and the pen, I’d have done a separate page … because it’s confusing because you had videos so you think it stops there [P34, parent of two adolescents (14 and 17 years)].
Following this feedback, edits included changing subtab colour and size, fixing the tabs and subtabs panel to the top of the web page, separating information about the pen and pump onto different subtabs and rearranging the video layout.
Participants highlighted that important information should not be solely within video as connectivity issues may mean they do not play, or participants may prefer text. This issue caused difficulties in finding out the trial length (Q1) as it was only located in video. Furthermore, this video was located at the foot of a web page, but participants considered this information important and expected it to be more prominent. Finally, participants reported being confused and discouraged by some video content, due to language complexity and regional accents:
There was one thing which I found quite hard, it was one she [the clinician] just said stuff a bit fast [P14, child (8 years)].
Some of the videos were quite hard to understand, certainly the professional, the doctor talking [P17, parent of two children (7 and 8 years)].
Consequently, additional text was included to ensure that important information was always available as text; for example, the length of the trial was written within the ‘Taking part’ tab. While it was not possible to refilm video content, comments on complexity were noted for developing the MMIs for the SWATs in Phase 2.
Round two
Participants
The revised prototype MMIs were tested with 26 new participants: seven children aged 7–11 years (mean 8.9 years) tested prototype MMI-1 alongside a parent (n = 6; one parent took part with two children); six adolescents aged 12–17 years (mean 15.0 years), and seven parents of adolescents tested MMI-2. As such, a total of 20 user testing sessions were completed. Twelve participants (46%) were male. Of the parent participants, 12 (92%) had higher education qualifications. All participants spoke English as their first language.
Findings
Participants spent an average of 9.2 minutes (range 4.4–14.5) looking at the MMI and 15.4 minutes (range 6.8–31.2) completing user testing questions and providing feedback. Once again, younger children and their parents spent longer looking at the MMI (mean 12.4 minutes, range 8.4–14.5), compared to adolescents (mean 7.3 minutes, range 5.5–11.1) and parents of adolescents (mean 9.1 minutes, range 4.4–12.6). Second round participants spent less time looking at the MMI and answering questions than first round participants.
The MMI revisions appeared successful; 93.0% of information was found without difficulty compared to 84.5% in round one (Table 5). Overall, 14 of 20 participants (70%) scored a ‘clear round’ for finding information. Persistent difficulties with locating information were largely due to participants reaching the 2-minute limit allocated per question. Finally, 99% of the information was understood, and 19 of 20 participants (95%) scored a ‘clear round’ for understanding.
| Round 2 | Revised prototype MMIs | N = 20 | Understanding | |||
|---|---|---|---|---|---|---|
| Finding | ||||||
| NF | D | F | NU | UD | U | |
| Q1. Imagine you decided to take part in the SCIPI trial. How long would you take part in the trial for? | 0 | 2 | 18 | 0 | 0 | 20 |
| Q2. If you took part in the SCIPI trial, what extra things would you need to do at your diabetes clinic visit? | 1 | 2 | 17 | 1 | 0 | 19 |
| Q3. What is the SCIPI trial testing? | 0 | 1 | 19 | 0 | 0 | 20 |
| Q4. Imagine you are in the group that uses the pump, when should you wear it? | 0 | 2 | 18 | 0 | 0 | 20 |
| Q5. Imagine you are in the SCIPI trial. What will happen when the SCIPI trial ends? | 0 | 0 | 20 | 0 | 0 | 20 |
| Q6. How often would you visit the diabetes clinic as part of the SCIPI trial? | 0 | 1 | 19 | 0 | 0 | 20 |
| Q7. Are there any risks to taking part in the SCIPI trial – is it safe? | 0 | 0 | 20 | 0 | 0 | 20 |
| Q8. Who can take part in the SCIPI trial? | 1 | 1 | 18 | 0 | 0 | 20 |
| Q9. If you decided you didn’t want to take part in the SCIPI trial anymore, what would you need to do? | 0 | 1 | 19 | 0 | 0 | 20 |
| Q10. Why do people with type 1 diabetes need to take/inject insulin? | 2 | 0 | 18 | 1 | 0 | 19 |
| Total (%) | 4/200 (2.0) | 10/200 (5.0) | 186/200 (93.0) | 2/200 (1.0) | 0/200 (0) | 198/200 (99.0) |
MMI revisions
The revised MMIs in round two performed well and required no further revisions. Previously identified problems with the subtab layout were resolved with round two participants noting the ease of navigation:
I think the website is very good, you know, it flows really well and you can clearly see where everything is [P47, adolescent (15 years)].
The fact that [child’s name] can navigate his way round it, it’s pitched at the right level [P56, parent of child (9 years)].
Further recommendations were identified for consideration when developing the Phase 2 SWAT MMIs, including adding information to the ‘Questions’ page about (1) potential risks and (2) how to contact trial staff with unanswered questions. It was suggested that hyperlinks could be used to provide links to relevant MMI pages.
Feedback from both testing rounds was combined to determine the overall acceptability of the approach. Feedback was largely positive; most participants liked the appearance of the MMIs and found them reassuring and child-friendly:
I like how there’s all the little pictures and, there’s on the videos, they look very easy to look at, there’s nothing complicated [P57, child (9 years)].
I think it’s really good, like the website looks a lot better than even usual websites. It’s very clean, it’s clear navigation as well [P20, adolescent (16 years)].
The videos were generally received as informative, engaging and reassuring, although not all were found relevant, such as the SCIPI trial participant’s brief video description of the pen and pump, which was also written in text:
There are real people speaking, like, which is a good thing ‘cause it kind of shows evidence that people are actually safe [P12, child (10 years)].
The sound bites are short enough in length so you don’t lose concentration [P59, parent of adolescent (15 years)].
There’s a lot of information, and I’m not sure to be honest whether some of these videos were relevant [P11, parent of adolescent (14 years)].
Participants generally liked the different media, although individual preferences varied:
Some of the cartoony videos were kind of fun to watch and they were also very simple and easy to understand and I think, well I do know a lot more now [P57, child (10 years)].
It’s quite well balanced in terms of pictures and I suppose it’s those kind of learning styles stuff, some people are quite visual so pictures work and then you’ve got your detail of the wording [P25, parent of adolescent (17 years)].
Videos is not for me, so I’d just prefer to see it as text so more easily found, so what will happen and it’s there rather than having to click [P32, parent of two adolescents (14 and 17 years)].
Participants liked the diagrams, noting their role as visual cues to information. For example, they remembered the calendar alongside information about clinic visit frequency on the SCIPI trial. However, it is important that the appropriate format is selected; the insulin pen and pump were noted as abstract and that photos would be preferred. However, participants did not have the condition-specific knowledge that most patients with diabetes would have.
Participants found most of the information understandable (with the exception of some video content), although they appreciated that the topic necessitated some technical language. Views on the amount of included information differed: some found it succinct, while others noted the large quantity:
It was the right balance of not patronising but understandable. You feel you’ve come away, well I felt I came away understanding what the thing was [P10, parent of child (9 years)].
There were certainly bits that I thought were really quite complicated but that she remembered, you know like whether it’s safe to take part [P17, parent of two children (7 and 8 years)].
There’s quite a lot of information but it doesn’t feel unmanageable [P43, parent of adolescent (13 years)].
They’re all simple [words] or if they are big words they’ve been explained to what they mean, which is good [P54, adolescent (17 years)].
Discussion
Summary of results
User testing highlighted that while most information within the prototype MMIs was understood without difficulty (96% in round one and 99% in round two), there were issues with locating information in the first round, with 15.5% of information not being found or being found with difficulty. This was largely due to the layout; participants reported having to scroll too much and not being able to see the tabs and subtabs when moving down the page. The revisions were successful; in round two 70% of participants were able to locate all requested information, compared to 15% in round one. Second round participants also took less time to review the MMIs and answer user testing questions. Participant feedback was mostly positive with particular reference to the appearance of the MMIs as engaging and child-friendly, and the variety of media used (e.g. videos, diagrams and text) meaning that different preferences for information provision were acknowledged. That user testing was able to identify issues not identified by stakeholders in the prior qualitative work demonstrates the value of performance-based analysis.
Previous research
There is little published user testing research involving medical information for children and adolescents. Nevertheless, usability testing, which also involves potential users in development, has been used with adolescents and young people to improve digital medical self-management and intervention programmes. 93,94,123,124 Usability testing of other media (e.g. video games) has highlighted that adolescents identify different issues to adults,125 demonstrating the importance of involving all target groups. While younger children’s feedback in the present study was not often detailed, observations of their behaviour, and the reflections provided by parents, were informative.
High levels of comprehension were observed in the present study, which may be due to the extensive development of the MMIs, including the use of readability metrics and the qualitative study. The use of different media in the MMIs may also have influenced comprehension, given previous evidence of positive evaluations and comprehension. 49,126–128
Limitations
The inclusion of a 2-minute time limit for locating information may have negatively affected results; most remaining difficulties were related to finding information in time. While this time limit is lower than in other user testing studies,31,32,121 it was thought that children may become bored or agitated with a longer time interval. This is somewhat supported by our observation that the youngest participants struggled to maintain focus for the whole session. The time limit may also reduce the external validity of findings as real-world potential participants would be able to study materials for longer. Nevertheless, it does suggest that any further issues with locating information would be resolved in a real setting, without such strict time limits. The experimental setting may also have influenced the amount of parental assistance provided to children. This varied, with some parent and child pairs completing the testing together and others relying more on the child. Again, it is not anticipated that this would be an issue during real trial recruitment where parents would presumably provide as much assistance as needed.
Study results may have been inflated by the over-representation of highly educated parents. However, children and adolescents were also included, which should ensure that the MMIs are usable by a wide variety of people. Participants were generally able to show good understanding of information contained in the prototype MMIs, but the subject trial (SCIPI) is relatively simple with no known intervention risks and two trial arms. It is therefore not known how well the MMIs would perform with more complex interventions and trials. We limited participation to individuals having English as their first language, to allow more accurate comparisons across the rounds of testing. We acknowledge that this restriction may limit the real-world application of the findings. Testing took place either at the university or in participants’ homes, according to their preference, and while this decision had potential to cause some variation in performance during testing, it did not seem that it did. Finally, we acknowledge the study’s relatively small sample size but note that this is consistent with previous user testing work using 10 participants per round, and the view that user testing with small samples is sufficient to identify all or almost all important information deficiencies. 118 Our study included more participants due to there being three different target groups (children, adolescents and parents).
Conclusion
User testing highlighted issues not identified during the initial development of the MMIs and guided further development through performance-led revisions.
Chapter 5 Phase 1 – the role of the Patient and Parent Advisory Group
Sections of this chapter have been reproduced from Sheridan et al. 129 This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (https://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. The text below includes minor additions and formatting changes to the original text.
The model of patient and public involvement (PPI) chosen was a combination of ‘managerial’ and ‘responsive’ involvement, as described in the Evidence base for Patient and public Involvement in Clinical trials (EPIC) study. 130 Two PPI approaches were adopted:
-
a responsive approach: seeking input as and when required from the Liverpool Generation R Young People’s Advisory Group (YPAG; https://generationr.org.uk/liverpool/) and
-
a mixed managerial and responsive approach: establishing a dedicated, study-specific PPAG to inform decision-making throughout the study, which reviewed study documentation and tools.
We then adapted these models to suit the specific nature of the study, given that it also included an extensive participatory design component in which potential users of the resources contributed to their development as research participants.
TRECA Patient and Parent Advisory Group
The PPAG was established at the beginning of the study, after funding had been secured, with members recruited via investigator networks. Potential members were asked to provide an expression of interest indicating why they would like to join. No formal interview was used to select members, and no formal training was provided. Informal training included small presentations, workshops and group discussions. Terms of reference were also developed in collaboration with the PPAG, detailing the study aims, the group’s remit and membership and information regarding payment and expenses, accountability and confidentiality.
The PPAG included three people aged 19–24 years with long-term health conditions (two female, one male), and three parents (all female) of young people with long-term health conditions. All members had prior experience of PPI. One parent member withdrew from the group during the second year of the study due to personal commitments. The group chairperson (Preston) was a TRECA coinvestigator with extensive experience of involving children, young people and families in research through her role as a senior PPI manager with numerous organisations. A TRECA researcher (Sheridan) acted as group co-ordinator and facilitated contact between the group and the researchers, organised meetings and co-ordinated requests for input.
The group’s role was to: (1) review and provide input into study documentation; (2) review the prototype MMI and the various ‘host’ trial MMI content; (3) pilot questionnaires to ensure wording and length were appropriate; (4) advertise the study to relevant audiences; and (5) assist with reporting and disseminating TRECA findings, including contributing to publications and conference proceedings. Two members of the PPAG sat on the TRECA Study Advisory Group (SAG), and attended its meetings.
The PPAG met regularly throughout the study, typically every 3 to 4 months. All members were also regularly asked for their opinions on various study design and conduct queries by e-mail and/or telephone, and the group members were seen as valued and equal partners in the research; their views were considered with the same weight as those of the academic members of the study team. An hourly rate of payment for members for all contributions (such as attending meetings and reviewing documentation) was used, as opposed to daily rates, due to the varied time commitments and opportunities for involvement.
Evaluation of the impact of the Patient and Parent Advisory Group
The impact of the PPAG on the study was evaluated for discussion in a publication, to which all members were invited to contribute. 129 Data regarding the impact, alongside the strengths and difficulties of involvement in TRECA were generated during three writing workshops involving members of the PPAG, the group co-ordinator and the chairperson. Themes and quotes were identified during these workshops and further developed using recorded minutes. Additionally, all feedback on the study received from members was recorded in full and summarised in a dedicated document, to keep track of member contributions and changes to the study.
Findings
Impact on the TRECA study
Group members fulfilled all aspects of their agreed roles, with most of the feedback being requested and received via e-mail. The feedback provided by members often improved the clarity of study documentation. For example, members edited text, alongside both an education expert coinvestigator (SH), and the research team, to ensure the content was appropriate for children, young people and families without compromising scientific accuracy. This collaborative approach was beneficial as people identified different aspects of the materials that could be improved. They also suggested modifications to participant information sheets to make them more visually appealing. The group was actively involved in reviewing animation storyboards and the written content for use in the prototype and ‘host’ trial MMIs, ensuring the language used was easily understood. They also contributed to discussions regarding the voiceovers for the MMI animations. When reviewing the MMIs, members were often able to highlight where concepts needed further explanation and suggest rewording or identify where images could be improved. For example, one of the animated characters in the prototype MMI wore a t-shirt with a skull and crossbones. This was changed after members expressed concern about including this image in information about a healthcare trial involving unwell CYP. Other examples included adding eyelashes to an image of an eyeball to make it more recognisable and editing text regarding blood samples to include an easier measure to visualise (e.g. a teaspoon of blood rather than 5 ml).
From a strategic point of view, young person and parent involvement in the running and conduct of the study was mostly achieved during SAG meetings. Although demonstrating impact from these meetings was more challenging, minutes showed that members made many insightful contributions. PPAG members also provided a letter of support for a study extension request to the funder, and were consulted on important study decisions via e-mail, such as which trials to accept as ‘host’ trials in the study. Regarding dissemination, members actively promoted the study at a variety of regional, national and international research and patient events, through their existing roles as patient research partners. Members also suggested suitable conferences at which to present TRECA work and have subsequently coauthored conference proceedings and a publication129 (with more planned). Members were particularly vocal about using social media to enhance the way in which the study engaged with people more broadly, for example, to open a study-specific Twitter account. While this was initially not a priority for the researchers, listening and proactively responding to members was an important step in increasing the study visibility, which made members feel that they had made a positive impact:
The main impact the group has had on the study is challenging the view of the researchers and an example of this was when the group was in agreement that the study should have a Twitter Page as this was a multimedia based study but couldn’t use it to promote or advertise the study (Young person 02).
The impact of the group on the TRECA study is further summarised in Table 6, alongside any relevant barriers to impact which were identified by the study team.
| Impact of PPI on the TRECA study | Relevant barriers |
|---|---|
| Improving the clarity of printed documentation | Difficulty incorporating suggestions when ‘host’ trial materials were already approved by RECs. |
| Reviewing multimedia and written MMI content | Based on the design of TRECA, contributions for the development of the MMIs in Phase 1 were focused on the study participants. If PPI members had different opinions, these were noted but not implemented. |
| Contributing to study design and governance decisions | No relevant barriers identified. |
| Promoting the study via social media or conferences | Due to the PPAG members’ other commitments, it was not possible for members to attend conferences at which the TRECA was being presented. |
| Contributing to the writing of presentations and publications from the study | No relevant barriers were identified. |
Strengths and challenges of the PPAG process
While reflecting on their role within the PPAG, members also highlighted aspects that they thought were strengths of the model of involvement employed during the TRECA study, as well as challenges that were encountered.
Strengths
Researchers’ attitude to patient and parent involvement
The PPAG thought the study team appreciated the importance of PPI, which enabled them to express opinions openly and honestly. They also noted the importance of being treated with the same courtesies as the academic team members to ensure they felt valued. For example, those members of the PPAG who sat on the SAG appreciated the use of alphabetical ordering of meeting attendees on agendas as they felt this was more inclusive than including all academic members followed by PPAG members.
We really felt like part of a team, as we began our work on the study. To me [group co-ordinator]’s leadership of the group was an example of good PPI [Patient and Public Involvement] as she made us feel valued and an equal with a common aim (Parent 01).
PPI group felt very enthusiastic and cohesive, our contributions felt valued, so we were able to input into design and content, as well as the on the SAG [Study Advisory Group]. There was a feeling of egalitarianism so that even on the SAG, our opinions were listened to and taken on board (Parent 02).
Having a ‘PPI champion’ [PPI chairperson], who is a coapplicant of the project, who then champions and supports a wider group of PPI members [is important] (Young person 03).
Motivation of members
While PPAG members were not formally involved in the development of the research question or protocol, all members felt the topic was important and were therefore committed to being involved.
From the outset I was excited to be part of the study. … I knew as soon as [child’s name] was diagnosed that I wanted to try and change things for others in a similar position to us. I wanted to make a direct impact, help to make a change for the better and it was important that this was not just tokenistic.
(Parent 01)
Without doubt, my motivation for joining the group was inspired by the aim of the TRECA project, as I had been complaining quite vocally about how much I disliked how we provide information to patients about research – particularly for children, young people and their families.
(Young person 03)
Members also felt that it was positive that all members of the group were motivated to be involved for the ‘right reasons’, meaning that they were there to contribute to the project. While members acknowledged the personal benefits of being involved, they thought this was secondary to their desire to influence the TRECA study.
Communication
The inclusion of a PPAG co-ordinator who took time to get to know members was highlighted as a strength. The role of the co-ordinator was particularly important in communicating to the group when their feedback had been incorporated, but also when it had not, and why:
I think that the TRECA team in York listened to us and made our opinions and suggestions real and valid. Interestingly, we did not always agree but I still felt that my views were being respected.
(Parent 01)
Communicating roles at the beginning of the project was also viewed as an important step in enabling people to assess whether they have the capacity and capability to get involved:
Receiving all of this information upfront [e.g. Terms of Reference] was really important in helping me to decide whether or not to join the group. Given that I was about to commence the final few months of my undergraduate degree, without this information notably on the frequency of meetings and time commitment, I would have probably said no – and I would have missed out on so many wonderful opportunities to influence the project.
(Young person 03)
The co-ordinator aimed to maintain communication throughout the study, with updates provided between meetings where possible. Members felt that continued contact was important to keep momentum going, particularly during quieter periods in the study.
Varied opportunities
Members appreciated the variety of ways they could be involved, including more traditional PPI (e.g. reviewing participant information) and wider opportunities (e.g. contributing to presentations and publications). In addition, the level at which members could be involved was flexible around individual needs and commitments. Everyone could be involved in different activities, but there was no pressure to do so.
By getting involved in TRECA, I have been able to learn new approaches, methods and opinions which have been an invaluable learning experience. I have also been privileged to be able to contribute to writing and editing different abstracts and papers, which have been incredibly useful, both from the preparation aspect of things, and also from having publications.
(Young person 03)
Expenses
It was important for inconveniences and out-of-pocket expenses to be minimised, for example, by printing meeting documentation for members, booking travel in advance and providing return stamped addressed envelopes when information was requested via post:
The PPI lead [coordinator] was wonderful in always thinking ahead so we never had to worry about booking trains, getting expense forms – everything was anticipated.
(Parent 02)
Challenges
Logistics
Difficulties encountered during the process were largely logistical. Despite the flexibility of the PPAG with regards to scheduling, it was sometimes not possible to arrange a meeting due to other commitments including work, education, care or health-related factors. Occasionally, teleconferencing was used at meetings to reduce travel demands, but face-to-face meetings were preferred:
Being able to meet face-to-face was a huge benefit as you can react to people’s body language and pick up on thoughts and feelings of different people in the room.
(Parent 01)
Members would also have liked to meet the wider SAG but due to competing work commitments this was difficult to achieve, especially as most members of the SAG joined meetings by phone.
While this is a logistical nightmare, it would have been beneficial for the entire TRECA PPI group to meet in person with the entire TRECA study team for a kick-off meeting at the start of the project.
(Young person 03)
Payment
While transparency regarding expenses and payment was highlighted as a strength, there were some difficulties. This was largely due to the requirement for members to be officially employed on a casual basis by the University of York, to allow cash payment, which members preferred to vouchers. This had not been anticipated, and therefore not communicated to members at the outset. This had some negative impact upon members, as all payments were taxed. This may be particularly problematic for members who may be receiving carer or health-related state benefits, as well as members in full-time employment.
The way we were compensated for our time was complicated and affected my salary from my job. This is something that would in future make me say I’d rather not accept any money for my time.
(Young person 02)
Appropriate membership
As the focus of the TRECA study was improving information for children, young people and their families, it was important to include members from each of these groups. While a younger child representative was invited to the group, there was no capacity to adapt materials and meetings to be suitable for them. It was discussed that when younger children’s input is needed, it may be preferable to involve a separate group of children (supported by their parents if necessary) to appropriately engage them. Members of the group also highlighted the need to be mindful of the inclusion of members with differing levels of experience of PPI and acknowledged that there is a need to approach hard-to-reach groups to ensure diversity.
Researcher reflections on the public involvement process
The TRECA research team thought the involvement of the PPAG was a highly positive addition to the study. The PPAG co-ordinator felt that being involved with the group also allowed them to develop as a researcher by improving their communication with patients and families and generating an understanding of what factors are important to them and why. Nevertheless, engagement was not always straightforward and study-specific difficulties were identified. For example, due to the extensive use of participatory design in Phase 1 of the TRECA study, researchers occasionally found that incorporating feedback was difficult when Phase 1 study participants and PPAG members had differing opinions. Further, due to the embedded SWAT design of TRECA, MMIs for use in the ‘host’ trials were developed based on the existing participant information in the ‘host’ trials, many of which had already been approved by RECs and may have had prior PPI. Consequently, valid feedback from the PPAG could not always be incorporated into the MMIs. Sensitivity and honest dialogue were required to explain to members why it was not always possible to incorporate feedback. In turn, the researchers appreciated PPAG members’ attention to detail and the confidence with which they articulated their feedback. PPAG members were often able to highlight aspects that the research team may not have noticed or considered, especially on visual aspects or subtle but beneficial wording changes. For example, ‘once daily’ was changed to ‘once a day’.
From a practical perspective time and resource constraints and ongoing study developments meant that some of the researchers’ objectives for PPI could not be met. For example, it was not possible to include younger children on the PPAG and consequently the majority of PPAG members were older than the TRECA study target demographic (CYP aged 6–18 years). The research team had also planned to involve members as cofacilitators in focus groups within Phase 1. However, this was not possible mainly because study participants’ preferences were to take part in individual or joint interviews instead of focus groups. There were also some research governance issues related to whether PPAG members would need special permissions (such as an enhanced Disclosure and Barring Service check and a research passport) to assist with interviews. When focus groups did take place, their location or time was not convenient to PPAG members. It was hoped that members would be able to assist with presenting the work at conferences, but while they were invited to attend it was not possible due to conflicting schedules. These resource constraints were experienced despite allocating a dedicated group co-ordinator to liaise with PPAG members (though the co-ordinator also worked on other projects alongside this role).
Conclusion
Achieving successful patient and public (or parent) involvement was an important aim of the TRECA study. The work of the PPAG informed the content of the MMIs and enhanced their quality. While we have highlighted the difficulties encountered and discussed considerations for future PPI, we feel the level of involvement of the PPAG is a strength of the study and are grateful to members for the unique insight they have provided.
Chapter 6 Phase 2 – summary features of the Phase 2 SWATS
Forearm Fracture Recovery in Children Evaluation (FORCE)
The Forearm Fracture Recovery in Children Evaluation (FORCE) trial was a NIHR Health Technology Assessment funded, multicentre trial seeking to improve the treatment of children (aged 4–16) who have a minor injury to their wrist, called a torus (or buckle) fracture. 131 There are several options for the treatment of torus fractures that is, removable rigid splint, plaster cast immobilisation or flexible splints.
The aim of the FORCE trial was to evaluate the clinical- and cost-effectiveness of soft bandage immobilisation and immediate discharge compared to splint immobilisation. All children identified as potentially eligible for FORCE were eligible for TRECA. Individuals were randomised into FORCE on a 1 : 1 basis, with age and centre as stratification variables (Tables 7–14).
| Trial | Target sample size | Expected TRECA sample size | Type of randomisation in trial | Type of randomisation in TRECA, generation method | TRECA arms being used | DMQs used |
|---|---|---|---|---|---|---|
| FORCE | 696 | 1071 | Individual | Cluster, random number generator153 | PIS or MMI | P/F |
| CHAMP-UK | 289 | 413 | Individual | Individual, random number generator153 | PIS, MMI or both | Y, P/F |
| THERMIC-3 | 94 | 118 | Individual | Individual, random number generator153 | PIS, MMI or both | Y, O, P/F |
| BALANCE | 66 | 100 | Individual | Individual, random number generator153 | PIS, MMI or both | Y, P/F |
| BAMP | 60 | 10 | Individual | Individual, random number generator153 | PIS, MMI or both | O, P/F |
| UKALL-2011 | 40 | 50 | Individual | Individual, random number generator153 | PIS, MMI or both | Y, O, P/F |
| Trial | Follow-up time points | Time point for assessing retention |
|---|---|---|
| FORCE | 1 day, 3 days, 7 days, 21 days, 6 weeks | 6 weeks |
| CHAMP-UK | 6, 12, 18, 24 months (primary outcomes); 5 years post randomisation | 6 months |
| Thermic-3 | 3 months | 3 months |
| BALANCE | 16 weeks follow-up time point | 16 weeks |
| BAMP | 18 months and 3 years | 18 months |
| UKALL-2011 | After each course of intensive therapy and 3 monthly while on therapy | 3 months |
| Trial | Baseline data collected |
|---|---|
| FORCE | Site, main trial allocation, age, gender, ethnicity, whether English is first language, deprivation index for home address |
| CHAMP-UK | Site, main trial allocation, age, gender, ethnicity, parent/family who provides consent gender, deprivation index for home address |
| THERMIC-3 | Main trial allocation, age, gender, deprivation index for home address |
| BALANCE | Site, main trial allocation, age, gender, ethnicity, parent/family who provides consent gender, parent/family who provides consent age, whether English is first language of parent/family who provides consent, deprivation index for home address |
| BAMP | Main trial allocation, age, deprivation index for home address |
| UKALL-2011 | Site, main trial allocation, age, gender, ethnicity, parent/family who provides consent gender, deprivation index for home address |
| Trial | Swat design |
|---|---|
| FORCE | The FORCE SWAT used a two-arm (PIS or MMI), parallel-group, cluster RCT. Clusters were 23 UK hospital Emergency Departments. According to cluster, participants received either a PIS or MMI – that is, each centre would either provide all their participants with PIS-only or MMI-only, depending on allocation |
| CHAMP-UK | The SWAT within the CHAMP-UK trial had three arms, with individuals being randomised 1 : 1 : 1 to PIS-only, MMI-only, or both MMI and PIS |
| THERMIC-3 | The SWAT within the Thermic-3 trial had three arms, with individuals being randomised 1 : 1 : 1 to PIS-only, MMI-only, or both PIS and MMI |
| BALANCE | The SWAT used a three-arm, parallel-group, RCT. Participants were randomised 1 : 1 : 1 to the three TRECA arms: PIS-only, MMI-only, or both MMI and PIS |
| BAMP | The SWAT within the BAMP trial had three arms, with individuals being randomised 1 : 1 : 1 to PIS-only, MMI-only, or both PIS and MMI |
| UKALL-2011 | The SWAT used a three-arm, parallel-group, RCT. The eligible patients were randomised 1 : 1 : 1 to PIS-only, MMI-only, or both MMI and PIS |
| Trial | Procedure |
|---|---|
| FORCE | Children attending the hospital who met the inclusion criteria for FORCE were invited to take part. They were given the printed PIS or MMI, according to allocation. All patients/parents who were approached for participation were given the printed decision-making questionnaire (DMQ). Families were asked to return the questionnaires by FREEPOST envelope to the TRECA study team. Recruitment to the FORCE SWAT was between February 2019 and July 2020. |
| CHAMP-UK | Parents of children with myopia were recruited by community optometrists and paediatric ophthalmologists. All study materials for CHAMP-UK were created to accommodate children aged 6–12 years and their parents. The parents and patients received a hard copy (PIS) or URL link (MMI) on a laminated card or, if preferred, were sent them by e-mail (including the URL for the MMI or as e-mail attachment for the PIS). DMQs were also sent either by post or e-mail, as preferred. Families returned completed DMQs by post (in Freepost envelopes to the study team) or digitally via Qualtrics survey tool to the University of York.61 Recruitment to the CHAMP-UK SWAT was between October 2019 and March 2021. |
| THERMIC-3 | Potential participants were identified from operating theatre and clinic lists. Eligible patients from the Thermic-3 were randomised to one of the three SWAT arms (PIS, MMI, or MMI and PIS). Patients and/or parents were given at least 24 hours to decide whether to participate in the Thermic-3 trial. Approached patients and families were given a printed DMQ (and Freepost envelope to the TRECA study team) for completion. Recruitment to the Thermic-3 SWAT was between September 2018 and March 2020. |
| BALANCE | The research orthoptists pre-screened medical notes for potential eligible children. When patching or blurring treatment was indicated, the research team asked families whether they might be interested in taking part. Interested patients were randomised in the TRECA SWAT. Participants (or their family) were given a printed DMQ for return in FREEPOST envelopes. Recruitment to the BALANCE SWAT was between October 2019 and March 2021. |
| BAMP | Patients were approached via joint orthodontic clinics at Tameside and Glossop Integrated Care NHS Foundation Trust located in Manchester. The orthodontist or a research nurse spoke to eligible participants for BAMP during an orthodontic consultation. Those who were willing to consider randomisation within BAMP received, according to allocation, the MMI or the PIS or the MMI and PIS. The PIS was provided as printed sheets, which individuals could read during the consultation and take home. The MMI was provided on a tablet computer, which individuals could view during the consultation; they were also given a laminated card containing the URL of the MMI, to allow them to access it at home. All participants were also given a printed copy of the DMQ plus a Freepost envelope (sent to the TRECA study at the University of York). Patients were given at least 1 week to decide to participate. Recruitment to the BAMP SWAT was between March 2018 and May 2019. |
| UKALL-2011 | After being given information about UKALL, patients had approximately 24 hours to decide whether to take part. Parents or patients were given the hard copy DMQ with a FREEPOST envelope, for return to the TRECA study team. Recruitment to the UKALL-2011 SWAT was between January 2019 and April 2019. |
| Trial | PIS summary |
|---|---|
| FORCE | The printed PIS had three versions, one for parents and two for children (younger and older). It was developed with PPI representatives. The PIS for parents was four pages and the child versions were two pages and one page, respectively. |
| CHAMP-UK | The printed PIS had three versions, one for parents and two for children (of which one was a two pages picture booklet for those aged 6–7 years). It was developed with PPI representatives. The PIS for parents was seven pages. |
| THERMIC-3 | The PIS was developed by the Thermic-3 trial team with feedback from the Generation R YPAG and approved by the NHS REC. Several versions were developed, including one for parents and three versions for children (7–10, 11–15 and 16–17 years). The length of the PIS ranged from 2 to 7 pages depending on the age group. |
| BALANCE | The PIS was the standard participant information sheet, which had three versions (< 6 years, 6–8 years, parents). The PIS pages ranged from 1 to 9 pages according to age group. |
| BAMP | The printed PIS was the standard participant information sheet, which had two versions, one for parents and one for children. It was developed with PPI representatives and approved by NHS REC. The PIS for parents was 7 pages and the children’s PIS was eight pages (one page for Patients’ Assent Form). |
| UKALL-2011 | The PIS in the UKALL-2011 trial was the standard participant information sheet comprising age-appropriate information for children and young adults with ALL and LBL (under 8, 8–12 years, 13–15 years, 16 plus and parents). The PIS ranged from 4–9 pages according to age group. |
| Trial | MMI summary |
|---|---|
| FORCE | Two versions of the MMI were developed for the FORCE trial (one for children 6–11 years, and another for adolescents and parents). The MMIs contained all written content of the PIS, with text amended to improve clarity when required. The MMIs were viewed on a tablet computer at the hospital. The resource included five short video animations, each lasting 45–60 seconds and 12 short ‘talking head’ videos: five with a study investigator; three with a research nurse; plus one with an adolescent and three with parents of children who had taken part in similar studies, each lasting 15–50 seconds and describing different aspects of the trial. The MMI content was organised on six main web pages. |
| CHAMP-UK | Two MMI versions (child and parents) were developed and included five short video animations, each lasting 45–80 seconds. The parents’ MMI contents were organised on six main web pages: ‘Home page’; ‘About the Study’; ‘Taking part’; ‘After the trial’; ‘Questions’; ‘Contacts’. The MMI for children had similar but less content under the six headings. The MMI for children included 19 ‘talking head’ videos: from the study investigator (13), children (5) and a parent from a similar study (1). The parents’ MMI included 19 ‘talking head’ videos: from the study investigator (14), parents (3) and children from a similar study (2). All clips lasted under 1 minute and described different aspects of the trial. |
| THERMIC-3 | Three versions of the MMI for different ages of children and adolescents were developed. The multimedia resources were provided as a link (on laminated card), which could be viewed either at the hospital or at home. The resource included five short video animations, each lasting 45–60 seconds and 34 short ‘talking head’ videos. The number of talking head videos varied by age of intended user. The ‘talking head’ video clips were recorded with four individuals: a trial investigator, a research nurse, a parent, and a patient involved in a similar cardiac surgery study. Each clip lasted 10–80 seconds, describing different aspects of the trial. |
| BALANCE | Two versions of the MMIs were created for children and parents. The MMIs included five short animation videos, each lasting 45–60 seconds. The parents’ MMI was organised on six main pages: ‘Home page’; ‘About the Study’; ‘Taking part’; ‘After the trial’; ‘Questions’; ‘Contacts’. The MMI for younger children had similar contents as the parents. Both MMIs included 16 ‘talking head’ videos: study investigator (13), plus a young adult (1) and parent (2) from previous study. Each clip lasted 20 seconds to 4 minutes, describing different aspects of the trial. |
| BAMP | The MMI text addressed the patient rather than the parent (e.g. ‘your treatment’ rather than ‘their treatment’). The resource also included five short animation videos, each lasting 45–60 seconds (‘Summary of the key aspects of the BAMP trial’; ‘Why do we do trials?’; ‘What are trials?’; ‘Who’s in a trial team’; ‘Assent and consent’), and 17 short ‘talking head’ videos, featuring three individuals (10 with the trial principal investigator; 4 with an adolescent who had received bone anchored maxillary protraction; 3 with a parent of a child who had received bone anchored maxillary protraction), each lasting 15–50 seconds and describing different aspects of the trial and clinical procedures. The MMI content was organised on six main web pages with the following headings: ‘Home page (including summary animation)’; ‘About the trial’; ‘Taking part’; ‘After the trial’; ‘Questions’; ‘Contacts’. |
| UKALL-2011 | The different versions of the MMIs were created for younger and older patients/parents and according to type of leukaemia or lymphoma. The MMIs also included five short animation videos, each lasting 45–80 seconds. The parents’ MMI contents were organised on six main web pages: ‘Home page’; ‘About the Study’; ‘Taking part’; ‘After the trial’; ‘Questions’; and ‘Contacts’. The younger age group MMI had less content under each heading. The MMIs for younger patients included 17 ‘talking head’ videos: with the study investigator (16) and a parent of a child from a similar study (1). The MMIs for older patients and parents included 22 ‘talking head’ videos: from the study investigator (18) parents of a child from a similar study (4). Each clip lasted 20 seconds to 3 minutes and described different aspects of the trial. |
Low-dose atropine eye drops to reduce progression of myopia in children (CHAMP-UK)
This host trial (CHAMP-UK) was a multicentre, randomised, double-blind, placebo-controlled trial with 2 : 1 allocation to atropine versus placebo, investigating whether low-dose atropine reduces progression of myopia in UK children. 132 Randomisation into this trial was done using a minimisation algorithm, where the unit of randomisation was the patient, not eye, and centre, ethnicity and severity of myopia were used.
Myopia (also known as short-sightedness) is a very common eye condition that causes poor vision when looking at distant objects, but close objects can be seen clearly. It can be corrected with glasses, contact lenses or laser eye surgery but myopic eyes have an increased risk of developing comorbidities, such as glaucoma,133 retinal detachment and blindness. 134
Cycloplegic agents, such as atropine, significantly reduce myopic progression135 and are widely used in some East Asian countries for treating children with myopia, however, the mechanism by which they act is unknown. In several countries (especially China) children with myopia are now treated routinely with low-dose atropine eye drops. However, in the UK, these drops are not available and there have been no trials to determine their efficacy especially in white populations. Besides, myopia onset and progression are known to be influenced by ethnicity and environment, it is not clear how effective this atropine treatment would be for slowing myopic progression in UK children.
This trial recruited children aged 6–12 years with myopia of –0.50 diopters (D) or greater in both eyes. The TRECA CHAMP-UK SWAT was run in four NHS trusts (Belfast, Glasgow, Cambridge, Moorfields Eye Hospital). The CHAMP-UK trial was funded by the NIHR Efficacy and Mechanism Evaluation Programme (ISRCTN 99883695; Eudract Number: 2017-004108-23; NCT03690089)136 (see Tables 7–14).
Intermittent Antegrade Warm Blood versus Cold Blood Cardioplegia in Children Undergoing Open Heart Surgery (Thermic-3)
The Thermic-3 trial was funded through the British Heart Foundation (CH/1992027/7163 and CH/17/1/32804) the NIHR Bristol Biomedical Research Centre. 137 Thermic-3 was a single-centre (Bristol Royal Hospital for Children, UK) RCT seeking to improve the outcomes of children undergoing surgical repair of congenital heart defects, involving cardiopulmonary bypass (CPB). Surgical repair of congenital heart defects can require the use of CPB and stopping the heart. Cardioplegia is used to stop the heart and provide myocardial protection during surgery. 138–141 Hypothermic cardioplegia solution is usually used in paediatric patients, however studies have confirmed the effectiveness of intermittent warm-blood cardioplegia (IWBC) in adults. 142,143
The Thermic-3 trial investigated the effects of IWBC versus cold-blood cardioplegia (CBC) on clinical and biochemical outcomes after paediatric congenital heart surgery. CYP (aged < 1 to 18 years) about to undergo surgery for the correction of a congenital heart problem were approached about the trial. Participants were randomly allocated 1 : 1 to cold-blood or warm-blood cardioplegia, where randomisation was stratified by the risk adjustment for congenital heart surgery (RACH) score (see Tables 7–14).
Randomised Controlled Trial to Determine Safety of and Adherence With a New ‘Binocularly Balanced Viewing’ Treatment for Unilateral Amblyopia Compared With Standard Treatment (BALANCE)
BALANCE was funded by the charity Action for Medical Research. 144 Lazy eye or amblyopia is the most common eye disorder in children, which occurs when one eye becomes weaker than the other, resulting in poor vision. The condition affects 2–4% of children and is the most common cause of vision loss. 145,146
Current treatment is glasses, followed by either occlusion or blurring with eye drops of the better-seeing eye. However, only two-thirds of children achieve near-normal vision in the amblyopic eye with these treatments. 147,148 In this trial, the children with unilateral amblyopia aged 3–8 years were randomly allocated to either a Nintendo 3DSXL console with blurred movies, or standard patching/blurring eye-drop. The BALANCE trial ran in two NHS Trusts: Moorfields Eye Hospital NHS Foundation Trust (City Road, St Georges and Northwick Park Hospital), and Bedfordshire Community Eye Clinics (Essex Partnership University Trust). Participants were randomised on a 1 : 1 basis to the two treatments, with randomisation stratified by type of amblyopia and centre (see Tables 7–14).
Bone Anchored Maxillary Protraction (BAMP) trial
The BAMP trial was a single-centre trial funded by DB Orthodontics (UK) (ISRCTN93900866). 149,150 BAMP recruited children and adolescents aged 11–14 years with a condition called ‘reverse bite’, when the child’s lower jaw projects further forwards than is usual. An operation to correct this problem is usually undertaken after the age of 17. The BAMP procedure, which is not currently in routine use in the UK, involves day-case surgery under general anaesthetic and the insertion of mini-plates and elastics, to correct the jaw position. The elastics remain in place for 6–8 months. The BAMP trial had two arms: random allocated on a 1 : 1 basis either to having the surgery at age 11–14 or no treatment (with the potential to have surgery at the age of 17+). Gender was used as a stratification variable in the randomisation. Potential participants were referred to the specialist centre by orthodontic practitioners (see Tables 7–14).
United Kingdom National Randomised Trial For Children and Young Adults With Acute Lymphoblastic Leukaemia and Lymphoma 2011 (UKALL)
UKALL-2011 was a multicentre RCT for children (under 8 years) and young adults (< 25 years) with acute lymphoblastic leukaemia (ALL) and lymphoblastic lymphoma (LBL). 151 UKALL-2011 was a two-part study (induction and maintenance). TRECA was involved in the second part, interim maintenance and a maintenance phase, which contained two randomisations. Children in this phase had already taken part in the first induction phase trial. 152
In the interim maintenance phase, patients received either standard interim maintenance or high-dose methotrexate, to evaluate whether a higher dose methotrexate is better at preventing relapse. Following the 9-weeks interim maintenance phase, they moved on to the maintenance phase (12 weeks), in which they were randomised again to have standard maintenance with or without pulses, or high-dose methotrexate maintenance with or without pulses. UKALL-2011 was funded by Leukaemia and Lymphoma Research (ISRCTN64515327; Eudract Number: 2010-020924-22). 151 In total, 7 of the 11 UK hospitals that were recruiting patients to UKALL-2011, were included in the TRECA SWAT, although TRECA randomisation happened at only two hospitals (Glasgow and Manchester). Cytogenetic risk and minimal residual disease level, National Cancer Institute Risk, and early morphologic response were used as stratification variables (see Tables 7–14).
The BAMP substudy
The BAMP substudy used the setting and materials of the BAMP trial. However, these were applied in a hypothetical trial setting, with adolescents who were awaiting orthodontic treatment being asked to imagine themselves considering participation in the BAMP trial (as above). They were randomly allocated (1 : 1) to receive either printed or MMI information (Table 15).
| Trial | BAMP substudy summary |
|---|---|
| SWAT design | The study used a two-arm, parallel-group, individually RCT design. Participants were asked to imagine being approached about participation in the BAMP trial of orthodontic treatment. Unlike the BAMP trial, the substudy used 1 : 1 allocation to PIS-only or MMI-only and did not evaluate MMI and PIS. |
| Procedure | Adolescents attending for orthodontic treatment were asked by the orthodontist or nurse to take part while they were in the waiting area. After giving consent they were randomly allocated. They were given a copy of the printed PIS or the MMI on a tablet computer. They were given a printed copy of the DMQ about the information they received. Recruitment to the BAMP substudy SWAT was between June 2019 and March 2020. |
| Summary (URL for MMI link) | https://www.york.ac.uk/healthsciences/research/health-policy/research/decision-making-projects/trials-engagement-in-children/bampstudy/ |
| PIS and MMI summary | The PIS and MMI were the same as for the BAMP SWAT (see Tables 13 and 14). |
Chapter 7 Phase 2 – results of the individual SWATs
Chapters 7 and 8 report the results of the SWATs undertaken in Phase 2 of the study. Chapter 7 reports the results of the seven individual studies undertaken within Phase 2 of TRECA: the six SWATs plus the BAMP substudy. Chapter 8 reports the meta-analyses of the SWATs, which are the TRECA study’s main results.
For each SWAT we have reported a CONSORT diagram showing recruitment to the host trial and retention to the selected time point. Baseline characteristics of age, gender and deprivation [based on national Index of Multiple Deprivation (IMD) decile for the patient’s home address] have also been reported for patients recruited to the host trial.
The effect of information format has been evaluated by comparing MMI-only with PIS-only (to assess the effect of replacement provision), which is the primary analysis, using a modified intention-to-treat (ITT) analysis.
Secondary analyses are also reported:
-
MMI and PIS versus PIS-only on trial recruitment
-
MMI-only versus PIS-only on trial retention
-
MMI and PIS versus PIS-only on trial retention.
The DMQ total scores have been analysed according to TRECA allocation in three ways: first, all DMQs per arm; second, only fully completed DMQs per arm; third, only DMQs for participants who were randomised within the host trial. Individual item analysis of the DMQs has also been undertaken when sufficient data were available. Finally, free text comments on the DMQs have been reported, according to the allocated SWAT arm.
Statistical analysis has firstly used a modified ITT approach, with participants analysed according to their randomly allocated arm but after the removal of any participants who were subsequently found to be ineligible for the host trial.
We have also undertaken a per-protocol analysis, in which participants have been analysed only if they received the information in the format as randomly allocated.
The FORCE SWAT
Trial recruitment
Twenty-three hospital sites recruited patients within the TRECA SWAT for FORCE. This SWAT used cluster randomisation and a two-arm allocation of information (PIS-only and MMI-only). Eleven of the sites were randomly allocated to PIS and 12 sites to MMI.
A total of 1410 participants met the inclusion criteria for FORCE (728 at PIS sites and 681 at MMI sites), and 959 (68.0%) patients consented to the FORCE trial (see Figure 2 CONSORT diagram). It was not possible for a participant to receive a different format to their allocation, and so no per-protocol analysis was undertaken. Furthermore, there was no MMI and PIS arm in this SWAT, and so the effect of MMI and PIS versus PIS-only was not possible.
FIGURE 2.
CONSORT diagram for the FORCE SWAT.
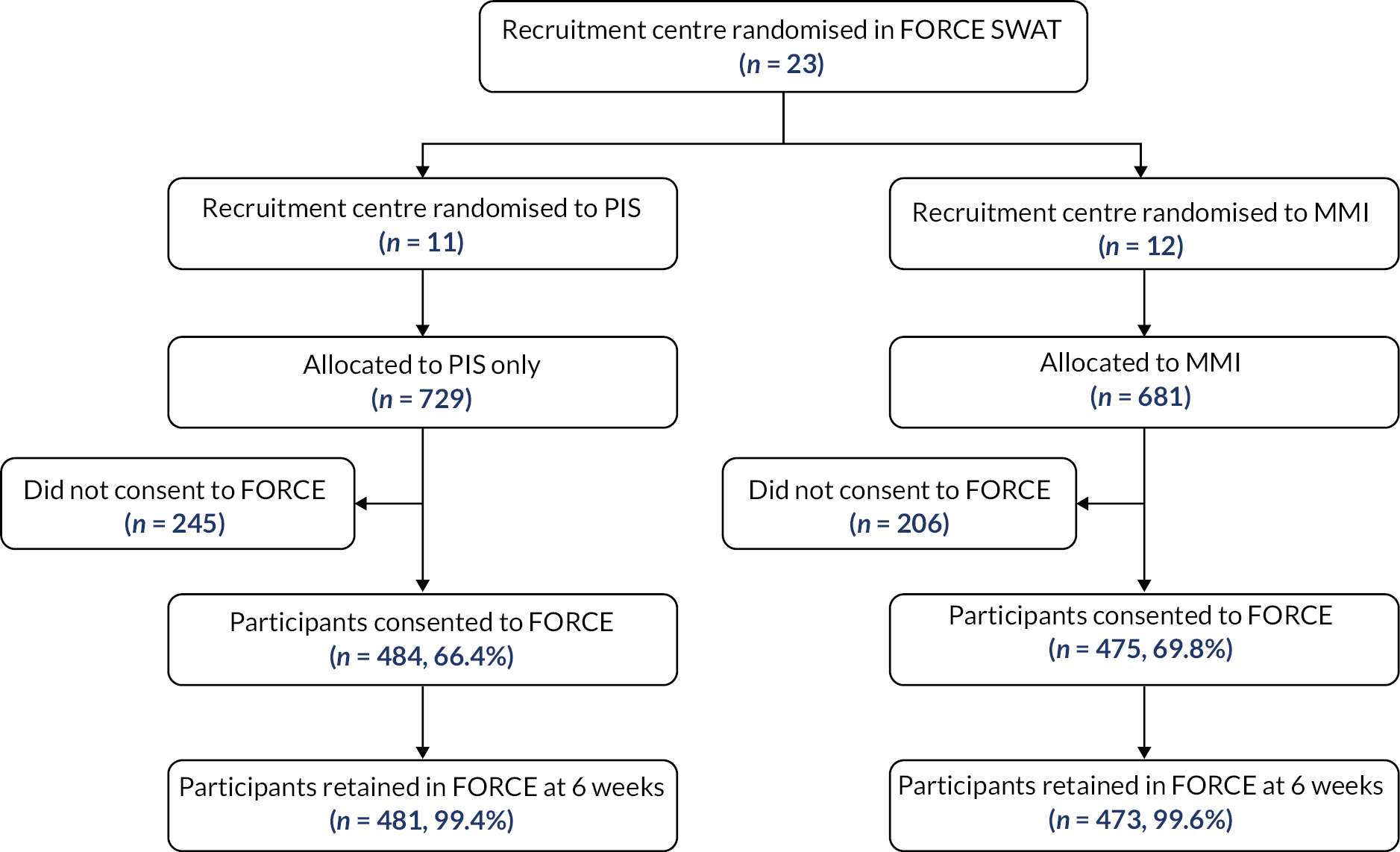
Primary analysis (MMI-only, PIS-only: intention to treat)
Of the 728 participants in the PIS arm, 484 consented to the trial (66.4%), compared to 475 of the 681 participants in the MMI arm (69.7%). A mixed-effects logistic regression model gave an OR = 1.35 (95% CI 0.76 to 2.40; p = 0.30), suggesting a small increase in recruitment in the MMI arm, but which is not statistically significant.
The baseline characteristics for participants who consented to the FORCE trial are reported in Table 16.
| PIS-only (n = 484) | MMI-only (n = 475) | Overall (n = 959) | |
|---|---|---|---|
| Age (child) | |||
| n (n missing) | 484 (0) | 475 (0) | 959 (0) |
| Mean (SD) | 9.3 (2.8) | 9.0 (3.0) | 9.1 (2.9) |
| Gender (child), n (%) | |||
| Male | 302 (62.4) | 280 (59.0) | 582 (60.7) |
| Female | 182 (37.6) | 195 (41.1) | 377 (39.3) |
| Ethnicity, n (%) | |||
| Asian/Asian British | 66 (13.6) | 31 (6.5) | 97 (10.1) |
| Black/African/Caribbean/Black British | 28 (5.8) | 20 (4.2) | 48 (5.0) |
| White | 361 (74.6) | 408 (85.9) | 769 (80.2) |
| Mixed/multiple ethnic groups | 10 (2.1) | 9 (1.9) | 19 (2.0) |
| Other ethnic group | 15 (3.1) | 6 (1.3) | 21 (2.2) |
| Not stated | 4 (0.8) | 1 (0.2) | 5 (0.5) |
| English as first language, n (%) | |||
| Yes | 439 (90.7) | 452 (95.2) | 891 (92.9) |
| No | 43 (8.9) | 23 (4.8) | 66 (6.9) |
| Missing | 2 (0.4) | 0 (0.0) | 2 (0.2) |
| Deprivation index for home address decile | |||
| n (missing) | 484 (0) | 474 (1) | 958 (1) |
| Median (p25, p75) | 5 (2, 7) | 4 (2, 7) | 4 (2, 7) |
Trial retention
Of the 959 participants who were randomised into FORCE, 954 (99.5%) reached the 6 weeks outcome follow-up time point [PIS: n = 481 (99.4%); MMI: n = 473 (99.6%)]. The logistic regression analysis model was adjusted for TRECA allocation, FORCE allocation and age. The logistic regression gave an OR of 1.14 (95% CI 0.11 to 12.32; p = 0.91), showing a very small benefit favouring the MMI arm but a difference that was not statistically significant.
Decision-Making Questionnaires
Decision-Making Questionnaires were received from 308 participants: 259 from those who consented to the FORCE trial and 49 from those who declined consent. The DMQ return rate was 27.0% among consenters and 10.9% among decliners.
Mean scores were almost identical in the MMI and PIS arms and the adjusted mean difference (AMD) was not statistically significant: AMD = 0.07 (95% CI –1.08 to 1.22; p = 0.91).
When the DMQs were analysed for only those participants who completed the measure, the means remained the same and the AMD was similarly not statistically significant: AMD = 0.11 (95% CI –0.95 to 1.19).
The DMQs were received from 259 participants who consented to the FORCE trial. These showed a very small difference favouring the PIS arm but the difference was not statistically significant: AMD: –0.10 (95% CI –1.30 to 1.11; p = 0.88). See Appendix 1, Tables 27–30 for analyses.
Individual DMQ item analysis
The nine individual DMQ questions were analysed post hoc. A difference was found on one of the nine items: on Q1 ‘the information I saw about the FORCE trial was easy to understand’ was given higher ratings by participants who received the MMI information (median MMI score 4, median PIS score 3; Z-statistic 2.60; p = 0.010).
Scores on the remaining eight DMQ questions showed no difference between the MMI and PIS arms. See Appendix 3 for analysis table.
Decision-Making Questionnaires ‘free text’ comments
There were 32 responses to Question 10 (‘any additional information they would have wanted’): 22/154 (14.3%) in the MMI group and 10/170 (5.9%) in the PIS group, although seven of the responses (PIS n = 1; MMI n = 6) related to the FORCE trial itself rather than the trial information. Responses about the information were highly varied and included: possible disadvantages of taking part (four respondents); questionnaire follow-up timing and frequency (two respondents); washing the bandage (two respondents); current standard practice for this fracture; as well as more general evaluations (‘no, it was all explained really well’).
Question 11 (‘identify aspects of information that were explained well’) was answered by 167 participants [96/154 (62.3%)] in the MMI group and [71/170 (41.8%)] in the PIS group. However, four participants used Q11 to fault rather than praise the information (PIS n = 1; MMI n = 3).
Approximately 1 in 8 (12.4%) of those answering Question 11 stated that ‘all’ or ‘everything’ was explained well (18 in the PIS group and 19 in the MMI group). Of the remaining respondents, Q11 comments fell into eight categories: ‘the FORCE trial’; relationship with clinical staff; treatment preference; randomisation/opt out; advantages and disadvantages; future benefits of the FORCE trial; and the rationale for the FORCE trial. Comments from some participants fell into more than one category.
For Question 12 (‘do you have any other comments?’) there were responses from 17/158 (10.8%) participants in the PIS group and 27/152 (17.8%) participants in the MMI group. Comments varied but in several cases, the response was used to explain their decision whether or not to take part in the FORCE trial.
There were two notable post hoc findings. Firstly, 13 (4.0%) ‘free text’ respondents mentioned the age-appropriateness or age-suitability of the trial information. Among those allocated to the MMI there were 10 comments, all of them positive. In those allocated to the PIS there were three comments on age-suitability (one negative and two positive).
Secondly, among participants allocated to the MMI information, 13 mentioned the use of video in the ‘free text’ comments. Video animations and talking head videos were a key element of the MMIs. Eight evaluations were positive: for example, ‘helpful video’; ‘I liked … video showing what RCTs are’; ‘the video was … clear about the different types of treatment’; and ‘involving kids in watching the videos makes them feel more involved’. However, two comments were negative: ‘the videos didn’t have subtitles and it was hard to hear in the hospital’; and ‘the videos were harder to access due to slow wi-fi and no service at (the hospital)’. A further two comments were mixed or neutral: ‘video was a good visual tool, but very minimalistic and not a great deal of detail or content’ and ‘the video could include what paperwork and questionnaire will need to be undertaken’.
The CHAMP-UK SWAT
Trial recruitment
Participants were recruited at four sites for TRECA within the CHAMP-UK SWAT. In total, 208 participants were randomised within TRECA: 68 PIS, 69 MMI and 71 both MMI and PIS.
Seven participants (3.4%) were subsequently found to be ineligible for CHAMP-UK, and so the analysis comprises 201 participants: 65 PIS, 68 MMI and 68 both MMI and PIS. Ninety-six (47.8%) of the 201 participants were consented to the CHAMP-UK trial (see Figure 3 for CONSORT diagram).
FIGURE 3.
CONSORT diagram for the CHAMP-UK SWAT.
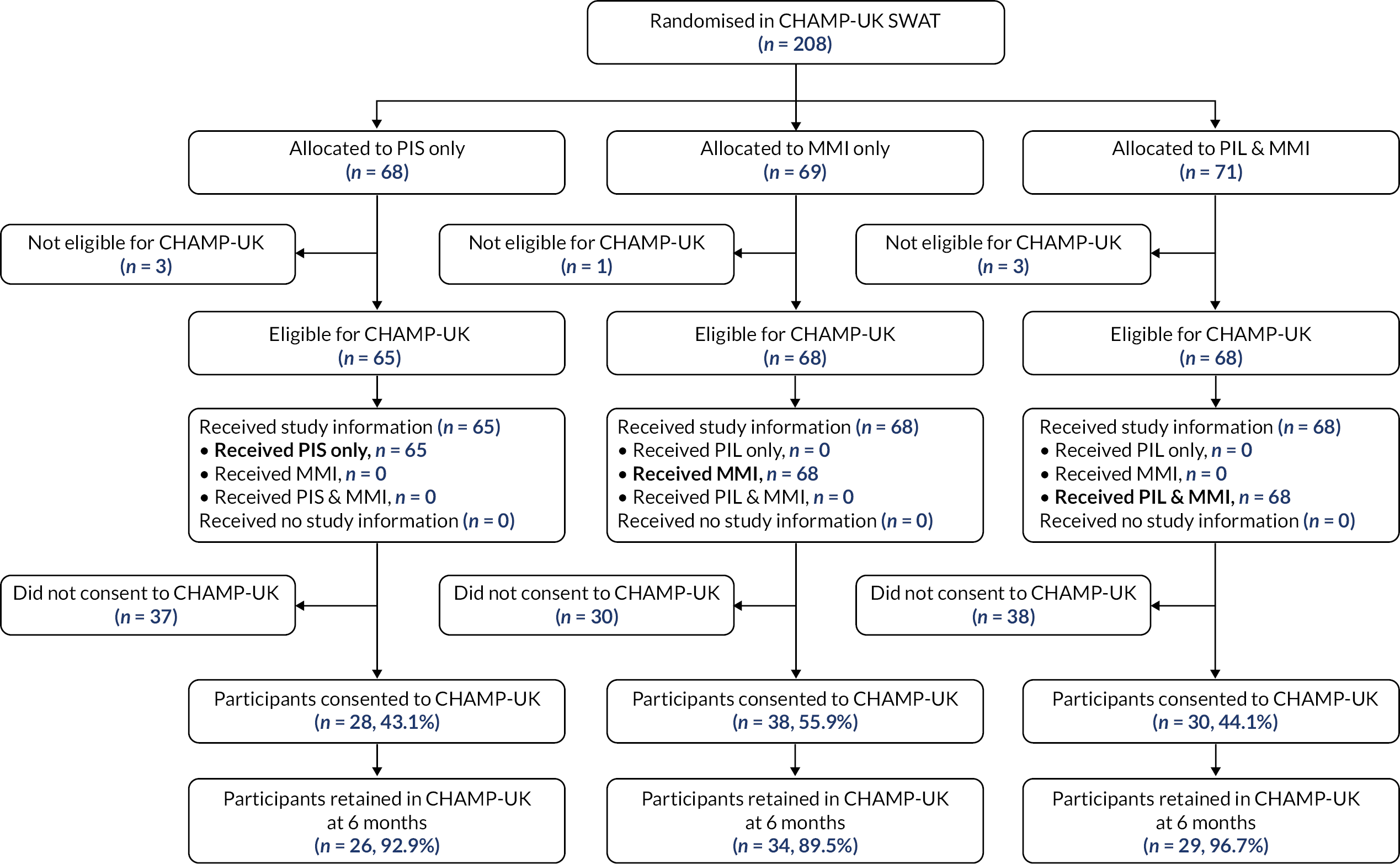
Given the procedure for participants to access information in this SWAT, it was not possible for a participant to receive a different format of information to their allocation, and so no per-protocol analysis was done.
Primary analysis (MMI-only, PIS-only: modified ITT)
In the MMI arm 38 out of 68 participants consented to CHAMP-UK (55.9%), compared to 28 out of 65 (43.1%) in the PIS arm. A logistic regression model produced an OR = 1.67 (95% CI 0.84 to 3.32; p = 0.14), suggesting that those in the MMI arm were more likely to be recruited, but this result was not statistically significant.
The baseline characteristics for participants who consented to the CHAMP-UK trial are reported in Table 17.
| PIS (n = 29) | MMI (n = 38) | MMI and PIS (n = 29) | Overall (n = 96) | |
|---|---|---|---|---|
| Age (child) | ||||
| n (n missing) | 29 (0) | 37 (1) | 29 (0) | 95 (1) |
| Mean (SD) | 8.8 (1.5) | 9.2 (1.8) | 9.4 (1.5) | 9.2 (1.6) |
| Gender (child), n (%) | ||||
| Male | 15 (51.7) | 18 (47.4) | 18 (62.1) | 51 (53.1) |
| Female | 14 (48.3) | 19 (50.0) | 11 (37.9) | 44 (45.8) |
| Missing | 0 (0.0) | 1 (2.6) | 0 (0.0) | 1 (1.0) |
| Ethnicity, n (%) | ||||
| Asian/Asian British | 6 (20.7) | 4 (10.4) | 1 (3.5) | 11 (11.5) |
| Black/African/Caribbean/Black British | 2 (6.9) | 1 (2.6) | 1 (3.5) | 4 (4.2) |
| White | 14 (48.3) | 25 (65.8) | 23 (79.3) | 62 (64.6) |
| Mixed/multiple ethnic groups | 1 (3.5) | 6 (15.8) | 0 (0.0) | 7 (7.3) |
| Other ethnic group | 5 (17.2) | 0 (0.0) | 4 (13.8) | 9 (9.4) |
| Not stated/missing | 1 (3.5) | 2 (5.2) | 0 (0.0) | 3 (3.1) |
| Gender (parent), n (%) | ||||
| Male | 12 (41.4) | 8 (21.1) | 12 (41.4) | 32 (33.3) |
| Female | 16 (55.2) | 29 (76.3) | 17 (58.6) | 62 (64.6) |
| Missing | 0 (0.0) | 1 (2.6) | 0 (0.0) | 1 (1.0) |
Secondary analysis (MMI and PIS, PIS-only: modified ITT)
There were similar recruitment rates for the combined MMI and PIS (30/68; 44.1%) and the PIS-only arms (28/65; 43.1%). A logistic regression model produced an OR = 1.04 (95% CI 0.53 to 2.07; p = 0.90), suggesting a very small increase in the odds of being recruited for those receiving both MMI and PIS, but the result was not statistically significant.
Trial retention
Of the 96 participants who consented to CHAMP-UK through TRECA, 89 (92.7%) completed their follow-up: 26 PIS (92.9%, of 28), 34 MMI (98.5%, of 38) and 29 MMI and PIS (96.7%, of 30).
The retention analysis for PIS versus MMI was adjusted for TRECA allocation (PIS or MMI), severity of myopia (≥ 3D, or < 3D) and ethnicity (white or non-white), as these were used in the minimisation for the host trial randomisation. Trial site was also used in the minimisation but was omitted in this analysis because it allowed the inclusion of more participants in the model. It was not possible to adjust for host trial allocation, as this was not available at the time of analysis (CHAMP-UK was a double-blind trial and had not been analysed when the SWAT data were needed). The results were an OR of 1.11 (95% CI 0.12 to 10.27; p = 0.92), which suggest a small positive benefit to retention for those who received MMI compared to the PIS, however, the results were not statistically significant.
When analysing the retention rate for PIS-only compared to combined MMI and PIS, the model was only adjusted for TRECA allocation, to improve accuracy of results, by increasing the number of participants. An OR of 2.23 was found (95% CI 0.19 to 26.06; p = 0.52), which suggests a benefit to retention when the MMI was used alongside the PIS, compared to PIS alone, but again the result is not statistically significant.
Decision-Making Questionnaires
Decision-Making Questionnaires were received from 81 participants: 55 from those who consented to the CHAMP-UK trial and 16 from those who declined consent. The DMQ return rate was 27.4% among consenters and 15.2% among decliners.
The results indicate that mean DMQ scores were 2.5 points higher in the PIS arm when compared to the MMI arm, with the results statistically significant (n = 57; AMD: –2.43; 95% CI –4.61 to –0.24; p = 0.03). When the combined MMI and PIS arm was compared with the PIS-only arm, mean DMQ scores were 2.2 points higher in the PIS arm, and the difference is statistically significant (n = 50; AMD: –2.11; 95% CI –4.23 to 0.01; p = 0.05).
Analysing only those participants who had fully completed the DMQ measure, the same pattern of results is evident: scores were 2.6 points higher in the PIS arm than in the MMI arm (n = 56; AMD: –2.53; 95% CI –4.73 to –0.32; p = 0.03) and scores were 2.5 points higher in the combined MMI and PIS arm than in the PIS-only arm (n = 48; AMD: –2.38; 95% CI –4.52 to –0.23; p = 0.03). Both differences are statistically significant.
When analysing only those participants who consented to the host trial, the same pattern of differences is maintained. However, the differences are not statistically significant. MMI-only versus PIS-only: n = 41; AMD: –1.12; 95% CI –3.58 to 1.34; p = 0.36. Combined MMI and PIS versus PIS-only: n = 33; AMD: –1.26; 95% CI –3.81 to 1.29; p = 0.32. See Appendix 2, Tables 31–34 for analyses.
Individual DMQ item analysis
When comparing the MMI-only with the PIS-only arms of this trial, statistically significant differences were found for three questions.
Participants who received the PIS information were more likely to give higher ratings to Q5 ‘The possible disadvantages of taking part in the CHAP-UK trial were made clear in the information’ (Z-statistic = 2.81; p = 0.005).
The PIS recipients were also more likely to give higher ratings to Q8 ‘I am confident that I have made the right decision about whether or not my son or daughter should take part in the CHAMP-UK study’ (Z-statistic = 2.69; p = 0.008).
Participants who received the PIS were also more likely to give higher ratings to Q9 ‘In all, the information about the CHAMP-UK trial helped me make my decision about whether or not my son or daughter should take part’ (Z-statistic = 2.68; p = 0.012).
The ratings on the remaining six question items showed no differences between the information arms. See Appendix 6 for analysis tables.
When the individual DMQ items were compared for the combined MMI and PIS arm compared with the PIS-only arm, a statistically significant difference was noted for one of the nine questions. On item nine ‘In all, the information about the CHAMP-UK trial helped me make my decision about whether or not my son or daughter should take part’ the median scores were four in the PIS-only arm and three in the combined MMI and PIS arm (Z-statistic 2.24; p = 0.048). There were no statistically significant differences found for the other eight DMQ items.
The Thermic-3 SWAT
Of the 153 participants who were randomised within the Thermic-3 SWAT, 147 were subsequently found to be eligible. Of these, 78 (53.1%) went on to consent to the Thermic-3 trial. Broken down by randomised TRECA allocation we have the following: PIS 24 out of 47 (51.1%); MMI 32 out of 49 (65.3%); MMI and PIS 22 out of 51 (43.1%).
A number of participants did not receive their allocated information (Table 18):
| PIS-only (n = 47) | MMI-only (n = 49) | MMI and PIS (n = 51) | Overall (n = 147) | |
|---|---|---|---|---|
| Age (child) | ||||
| n (missing) | 47 (0) | 49 (0) | 51 (0) | 147 (0) |
| Median (p25, p75) | 0.75 (0.25, 4.33) | 0.5 (0.33, 3.33) | 0.91 (0.33, 3.75) | 0.67 (0.33, 3.75) |
| Gender (child), n (%) | ||||
| Male | 30 (64) | 28 (57) | 33 (65) | 91 (62) |
| Female | 17 (36) | 21 (43) | 18 (35) | 56 (38) |
| Deprivation index for home address decile | ||||
| n (missing) | 47 (0) | 49 (0) | 51 (0) | 147 (0) |
| Median (p25, p75) | 4 (1, 8) | 5 (2, 6) | 2 (1, 6) | 4 (1, 7) |
-
of the 49 participants randomised to MMI-only, three received the PIS-only, four received the MMI and PIS and eight received no information
-
of the 47 participants randomised to PIS-only, five received no information
-
of the 51 participants randomised to MMI and PIS, four received PIS-only and eight received no information.
The flow of participants through the Thermic-3 SWAT are reported in the CONSORT diagram (Figure 4).
FIGURE 4.
CONSORT diagram for recruitment and retention in the Thermic-3 SWAT.
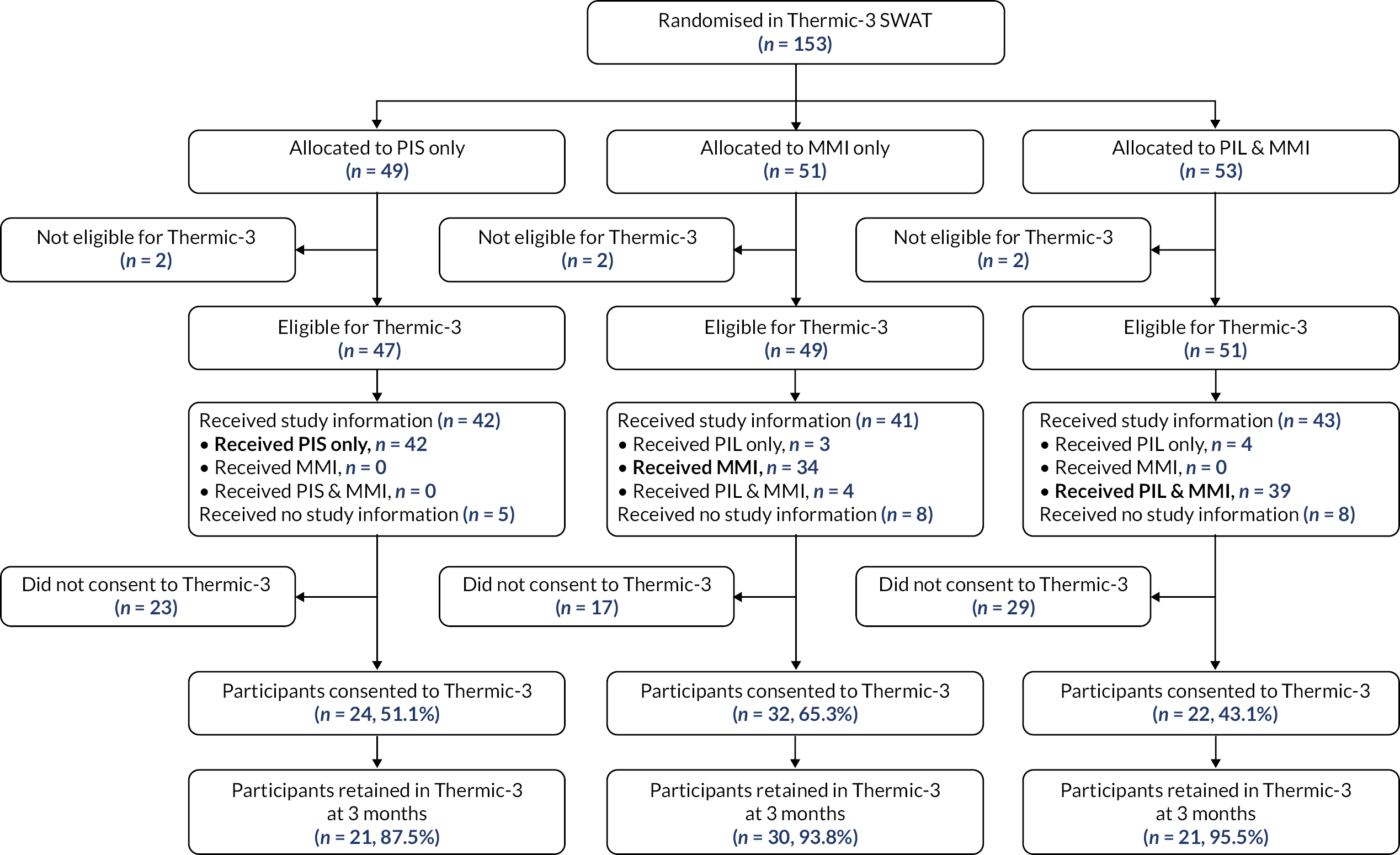
Trial recruitment
Primary analysis (MMI-only, PIS-only: modified intention to treat)
The recruitment rate was 51.1% in the PIS arm (24/47), compared to 65.3% in the MMI group (32/49). The modified ITT primary analysis gave an OR of 1.80 (95% CI 0.79 to 4.10; p = 0.16), showing that the MMI produced an increase in the recruitment rate, but this did not reach statistical significance.
Baseline characteristics for those participants who consented to the Thermic-3 trial are reported in Table 18.
Secondary analysis (MMI-only, PIS-only: per protocol)
The recruitment rates when looking at per-protocol analysis was 57.1% in the PIS arm (24/42), and 79.4% in the MMI arm (27/34). The per-protocol analysis gave an OR of 2.89 (95 CI% 1.03 to 8.11; p = 0.04), showing that the MMI produced an increase in the rate of recruitment, which reached statistical significance.
Secondary analysis (MMI and PIS, PIS-only: modified ITT)
In the combined MMI and PIS arm the recruitment rate was 43.1% (22/51), and the rate in the PIS-only arm was 51.1% (24/47). The ITT secondary analysis yielded an OR of 0.73 (95% CI 0.33 to 1.61; p = 0.43), showing that the combination of MMI and PIS did not produce any increase in the rate of recruitment.
Secondary analysis (MMI and PIS, PIS-only: per protocol)
When considering only those who received their allocated intervention, the recruitment rates were 53.9% in the MMI and PIS group (21/39) and 57.1% in the PIS-only arm (24/42). The per-protocol analysis had an OR of 0.88 (95% CI 0.36 to 2.10; p = 0.77), showing that the combination of MMI and PIS did not produce any increase in the rate of recruitment.
Trial retention
Of the 78 participants who consented to Thermic-3, 72 (93.5%) reached the 3-month time point: PIS: n = 21 (87.5%); MMI: n = 30 (93.8%); MMI and MMI and PIS: n = 21 (95.5%). The analysis models for Thermic-3 are adjusted for RACH and TRECA allocation only, as host trial allocation was not available at the time of analysis.
The logistic regression gave an OR of 1.62 (95% CI 0.20 to 12.98; p = 0.65), showing a higher retention rate in the MMI arm but the difference did not reach statistical significance.
The retention rate for combined MMI and PIS was 95.5% (21/22), compared to that of PIS-only 87.5% (21/24), showing a higher retention rate in the MMI and PIS arm but the difference did not reach statistical significance: OR = 2.05 (95% CI 0.17 to 24.64; p = 0.57).
Decision-Making Questionnaires
Decision-Making Questionnaires were received from 17 participants: 12 from those who consented to the Thermic-3 trial and five from those who declined consent. The DMQ return rate was 21.8% among consenters and 7.2% among decliners.
Mean scores were 2.3 points higher in the MMI-only than PIS-only arm but the difference did not reach statistical significance: AMD = 0.73 (95% CI –5.34 to 6.80; p = 0.80). When comparing the MMI and PIS arm with the PIS-only arm, mean scores were 1.2 points lower in the combined arm, but the difference was not statistically significant: AMD = 0.72 (95% CI –10.43 to 7.52; p = 0.72).
Comparing the scores for those who fully completed the DMQ measure, mean scores were 0.9 points higher in the MMI-only than PIS-only arm but the difference did not reach statistical significance: AMD = 1.35 (95% CI –5.06 to 7.75; p = 0.65). When comparing the MMI and PIS arm with the PIS-only arm, mean scores were 0.5 points lower in the combined arm, but the difference was not statistically significant: AMD = 0.50 (95% CI –10.72 to 9.72; p = 0.91).
Comparing the scores for those who consented to the Thermic-3 trial, mean scores were 0.9 points higher in the MMI-only than PIS-only arm but the difference did not reach statistical significance: AMD = 0.90 (95% CI –3.11 to 4.91; p = 0.62). When comparing the MMI and PIS arm with the PIS-only arm, mean scores were 3.2 points higher in the combined arm but the difference was not statistically significant: AMD = –2.30 (95% CI –9.09 to 4.49; p = 0.42). See Appendix 3, Tables 35–37 for analyses.
Decision-Making Questionnaires ‘free text’ comments
All participants’ responses have been categorised according to the information format actually received.
There were three responses to Question 10 (‘any additional information they would have wanted’), requesting the inclusion of an internet link to the completed study in the adult population, and the standard approach. Eleven participants provided comments for Question 11 (‘which aspects were explained well?’): many stating that everything was explained well (n = 5). In response to Question 12 (‘do you have any other comments?’) three participants made comments, including one referring to the risk of harm in the trial (from a participant who did consent to the Thermic-3 trial).
The BALANCE SWAT
Trial recruitment
Three sites were involved in the TRECA recruitment for the BALANCE SWAT, and they assessed a total of 25 patients as eligible for randomisation in TRECA: 9 to MMI, 6 to PIS, and 10 to both MMI and PIS.
Subsequently four participants were found to be ineligible for BALANCE, and so the recruitment analysis has been conducted on 21 participants: 8 in the MMI arm, 4 in the PIS arm, and 9 in the MMI and PIS arm. A regression model was not fitted to any of the recruitment data due to a small sample size, and lack of variation between the outcomes. See Appendix 4 for analysis tables.
The flow of participants through the Thermic-3 SWAT are reported in the CONSORT diagram (Figure 5).
FIGURE 5.
CONSORT diagram for recruitment and retention in the BALANCE SWAT.
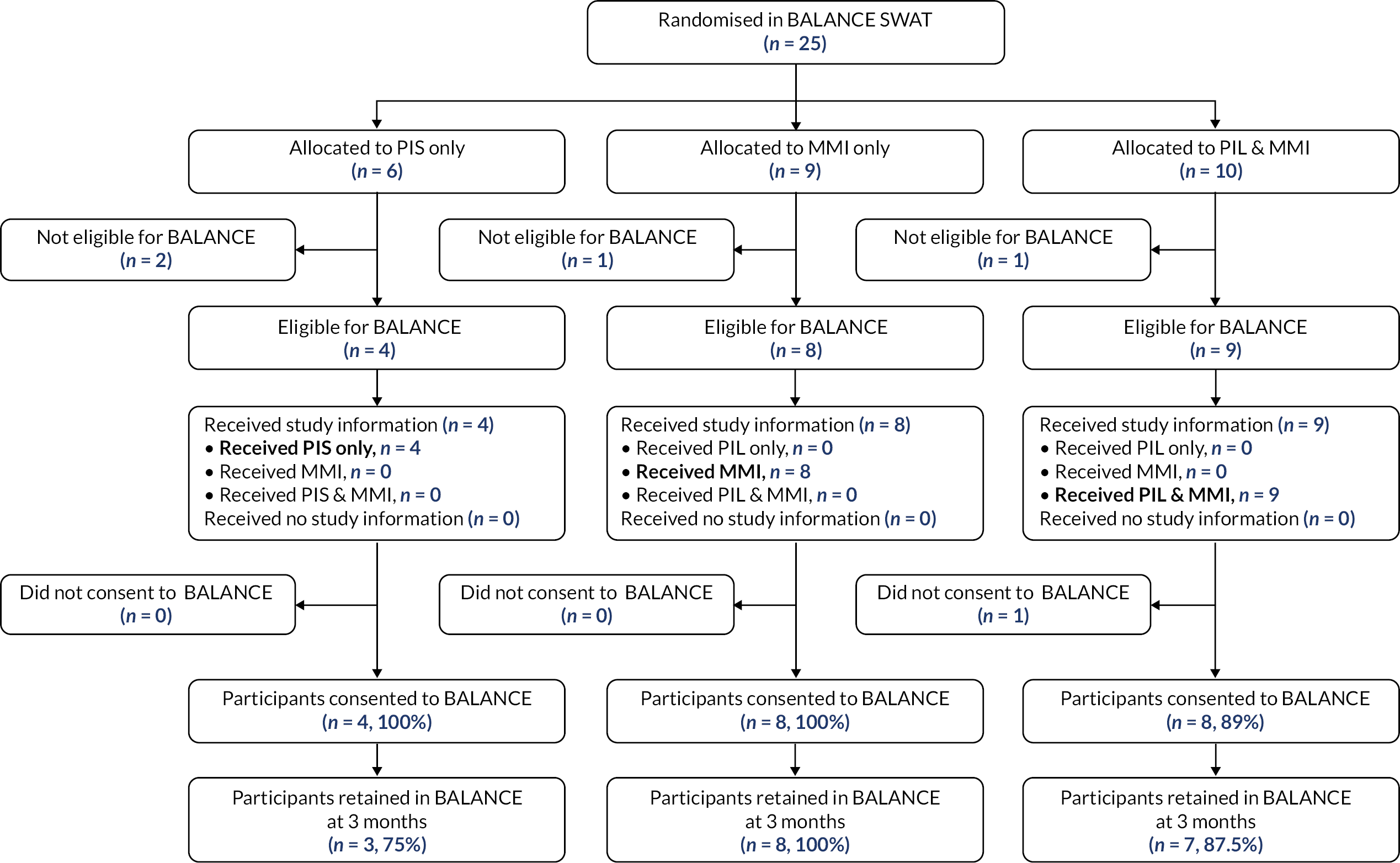
The baseline characteristics for participants who consented to the BALANCE trial are reported in Table 19.
| PIS-only (n = 4) | MMI-only (n = 8) | MMI and PIS (n = 9) | Overall (n = 21) | |
|---|---|---|---|---|
| Age (child) | ||||
| n (n missing) | 4 (0) | 8 (0) | 8 (1) | 20 (1) |
| Median (p25, p75) | 5 (4, 5) | 6 (5.5, 6) | 5.5 (5, 6) | 6 (5, 6) |
| Gender (child), n (%) | ||||
| Male | 1 (25.0) | 5 (62.5) | 4 (44.4) | 10 (47.6) |
| Female | 3 (75.0) | 3 (37.5) | 4 (44.4) | 10 (47.6) |
| Missing | 0 (0.0) | 0 (0) | 1 (11.1) | 1 (4.8) |
| Gender (parent), n (%) | ||||
| Male | 0 (0.0) | 2 (25.0) | 2 (22.2) | 4 (19.1) |
| Female | 4 (100.0) | 6 (75.0) | 6 (66.7) | 16 (76.2) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (11.1) | 1 (4.8) |
| Ethnicity, n (%) | ||||
| White | 3 (75.0) | 5 (62.5) | 1 (11.1) | 9 (42.9) |
| Non-white | 1 (25.0) | 3 (37.5) | 7 (77.8) | 10 (47.6) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (11.1) | 1 (4.8) |
| English as first language, n (%) | ||||
| Yes | 3 (75.0) | 7 (87.50) | 7 (77.8) | 17 (81.0) |
| No | 1 (25.0) | 1 (12.5) | 1 (11.1) | 3 (14.3) |
| Missing | 0 (0.0) | 0 (0.0) | 1 (11.1) | 1 (4.8) |
| Deprivation index for home address decile | ||||
| n (n missing) | 3 (1) | 7 (1) | 8 (1) | 18 (3) |
| Median (p25, p75) | 5 (5, 9) | 3 (2, 4) | 3 (3, 5.5) | 3.5 (3, 5) |
Trial retention
Of the 20 participants who consented to the BALANCE trial via TRECA, 18 were still involved at their follow-up (90%). This was 75% of those in the PIS arm (3/4), 100% in the MMI arm (8/8), and 88% in the MMI and PIS arm (7/8).
Due to the limited sample size, it was not possible to run the regression model adjusting for type of amblyopia (anisometropic, strabismic, or both) which was a stratification variable in the host trial randomisation, and as such the models have been run adjusting only for TRECA allocation.
When comparing PIS-only with combined MMI and PIS there is an OR of 2.33 (95% CI 0.11 to 50.98; p = 0.59), suggesting that those who received both MMI and PIS were more likely to still be in the trial than those who only received PIS, although this difference is not statistically significant.
Due to the 100% retention rate in the MMI arm, and the limited sample size, it was not possible to fit a regression model for the comparison of MMI with PIS.
Decision-Making Questionnaires
It was not possible to link the DMQ and recruitment data for one site in the BALANCE SWAT, and due to the low numbers in the SWAT arms, a full table has not been generated. Overall, 13 DMQs were received, 4 from younger participants and 9 from those who completed the ‘parents/ family’ version of the questionnaire. See Appendix 4, Tables 38 and 39 for analyses.
The BAMP SWAT
Trial recruitment
Participants were recruited through one site for TRECA in the BAMP SWAT, and 10 patients were randomised. They were allocated to receive a PIS (n = 3), MMI (n = 3) or both MMI and PIS (n = 4).
Nine of the 10 patients received the allocation that they were randomised to receive, although one participant randomised to the PIS-only arm stated that they had received MMI and PIS.
Of the 10 patients, 8 (80%) went on to be consented to the BAMP trial. Due to limited sample size, no regression models were fitted to these data.
The flow of participants through the BAMP SWAT are reported in the CONSORT diagram (Figure 6).
FIGURE 6.
CONSORT diagram for recruitment and retention in the BAMP SWAT.
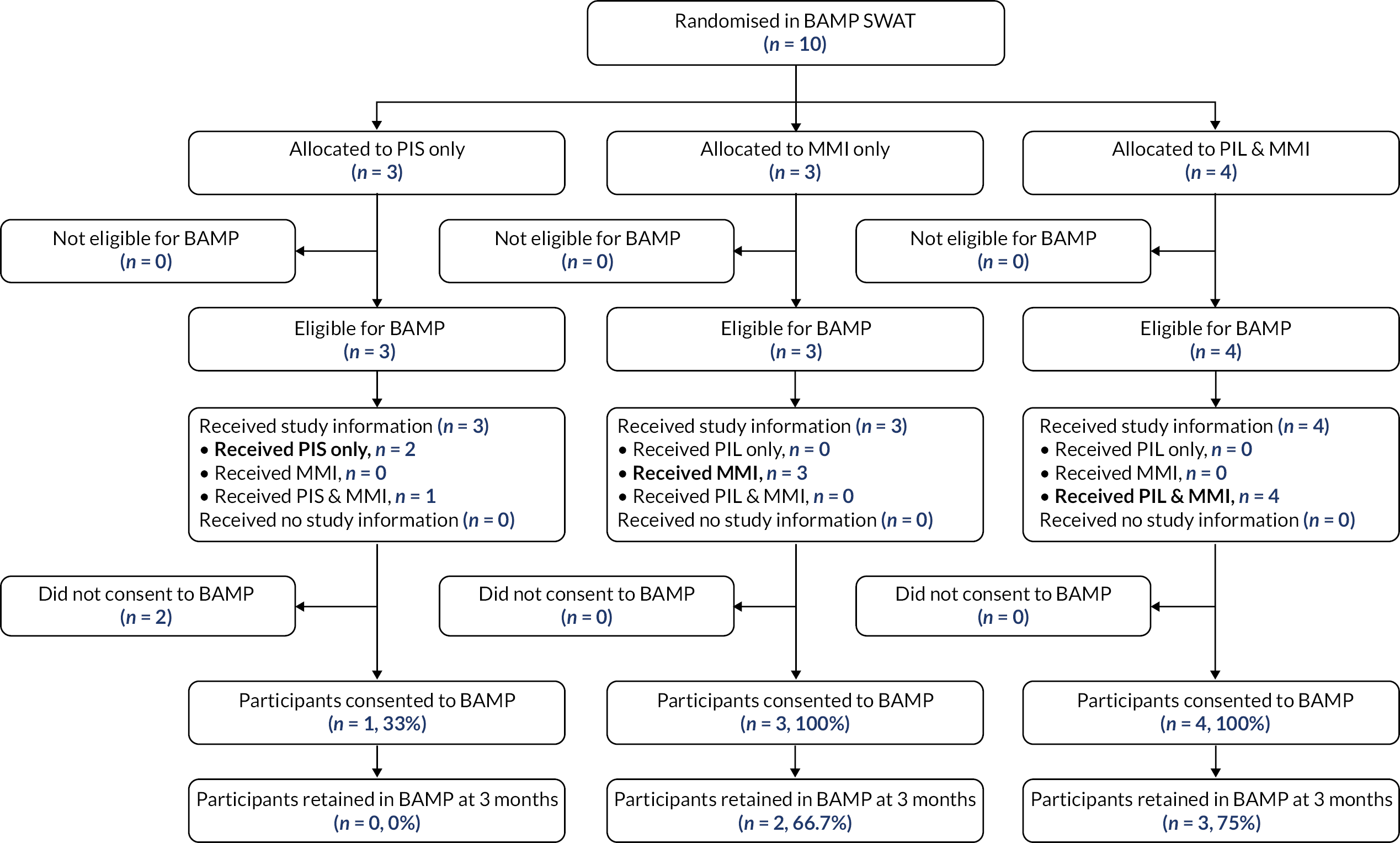
The baseline characteristics for participants who consented to the BAMP trial are reported in Table 20.
| PIS-only (n = 1) | MMI-only (n = 3) | MMI and PIS (n = 4) | Overall (n = 8) | |
|---|---|---|---|---|
| Age (child) | ||||
| n (n missing) | 1 (0) | 3 (0) | 4 (0) | 8 (0) |
| Median (p25, p75) | – (–,–) | 12 (11, 12) | 12.5 (12, 13) | 12 (11.5, 12.5) |
| Gender (child), n (%) | ||||
| Male | – (–) | 1 (33.3) | 2 (50.0) | 3 (37.5) |
| Female | – (–) | 2 (66.7) | 2 (50.0) | 5(62.5) |
| Deprivation index for home address decile | ||||
| n (n missing) | 1 (0) | 3 (0) | 4 (0) | 8 (0) |
| Median (p25, p75) | – (–,–) | 6 (6, 8) | 2.5 (1.5, 6) | 4.5 (1.5, 7) |
Primary analysis (MMI-only, PIS-only: modified ITT)
All of the MMI patients were randomised into the BAMP host trial (100.0%), compared to only one out of three in the PIS arm (33.3%).
Secondary analysis (MMI-only, PIS-only: per protocol)
All three MMI patients received their allocated intervention, and were randomised into BAMP (100.0%). Two of the three PIS patients received only PIS, and one of the two was randomised into BAMP (50.0%).
Secondary analysis (MMI and PIS, PIS-only: modified ITT)
Similarly to the primary analysis, all those in the MMI and PIS group were randomised (100.0%), but only one of in the PIS-only group (33.3%).
Secondary analysis (MMI and PIS, PIS-only: per protocol)
Only two of the three PIS-only patients received this intervention, and one was randomised into BAMP (50.0%). Of the four patients allocated to receive PIS + MMI, they all received that intervention, and were all randomised into BAMP (100.0%).
Trial retention
Of the eight participants who were randomised into BAMP, seven (87.5%) were retained at 3 months [PIS: n = 1 (100%, n = 1); MMI: n = 3 (100.0%, n = 3); MM and PIS: n = 3 (75.0%, n = 4)]. It was not possible to fit a logistic regression model to this data due to the small sample.
Decision-Making Questionnaires analysis
There was no difference in DMQ means between the MMI and PIS, or between the combined MMI and PIS and the PIS-alone arms.
All DMQ respondents fully completed the measure. See Appendix 5, Tables 40 and 41 for analyses.
The UKALL-2011 SWAT
Four patients were included in the TRECA SWAT in UKALL-2011; one in the MMI arm, two in the PIS arm and one in the MMI and PIS arm. The one patient in the MMI group did not receive this allocation and was found post randomisation not to be eligible for UKALL due to a clinical decision. Thus, the included patients are: two PIS and one MMI and PIS. It was not possible to fit a regression model for any of the recruitment date for this trial.
Trial recruitment
The flow of participants through the UKALL-2011 SWAT is indicated in Figure 7.
FIGURE 7.
CONSORT diagram for the UKALL-2011 SWAT.

Primary analysis (MMI-only, PIS-only: modified ITT)
There were no patients in the MMI-only arm. Both of those in the PIS-only group were randomised into UKALL-2011 (100.0%).
Secondary analysis (MMI-only, PIS-only: per protocol)
There were no patients in the MMI-only arm. Both of those in the PIS-only group received the correct intervention and randomised into UKALL-2011 (100.0%).
Secondary analysis (MMI and PIS, PIS-only: modified ITT)
Both of the patients who were allocated to PIS-only were randomised into the trial (100.0%), as was the one patient who received the MMI and PIS (100.0%).
Trial retention
All participants that were recruited into the UKALL SWAT were retained at three months (2/2 PIS, 1/1 MMI and PIS). It was not possible to fit a model to this data.
Decision-Making Questionnaires
Questionnaires were received from all three participants who were correctly consented to the UKALL-2011 trial. Given the small volume of data, no statistical comparisons were undertaken. See Appendix 6, Tables 42–44 for analyses.
The BAMP substudy
Decision-Making Questionnaires
Decision-Making Questionnaires were received from all 104 participants, of whom 103 had fully completed the measure. Mean scores were 1.1 points higher in the MMI arm, although the difference was not statistically significant: AMD = 1.08 (95% CI –0.59 to 2.75; p = 0.20).
When analysing only those participants who completed all items of the measure, mean scores remained 1.1 points higher in the MMI arm, and the difference was similar not statistically significant: AMD = 1.06 (95% CI –0.63 to 2.74; p = 0.22). See Appendix 7 for analysis tables.
Individual DMQ item analysis
Analysis of the nine individual questions on the DMQ indicated that participants who received the MMI were more likely to give higher ratings on Q1 ‘The information I saw about the BAMP trial was easy to understand’: Z-statistic = –3.03, p = 0.002. Participants in the MMI group were also more likely to give higher ratings on Q8 ‘I am confident that I have made the right decision about whether or not to take part in the BAMP trial’: Z-statistic = –2.00, p = 0.044. The scores on the remaining seven DMQ items showed no statistical differences between the two groups. See Appendix 7, Tables 45–47 for analyses.
Decision-Making Questionnaires ‘free text’ comments
In total, 63 of the 104 respondents (60.6%) made at least one positive comment about the information (34 in the MMI group; 29 in the PIS group). Three respondents made negative comments about the information (one in the MMI group and two in the PIS group).
In answer to Question 10, ‘Was there anything you wanted to know about the BAMP trial but which wasn’t included in the information you saw?’ 15 participants (14.4%) replied ‘yes’ (eight in the MMI group; seven in the PIS group), although 17 participants provided responses (nine MMI; eight PIS). In the PIS group, six respondents would have liked more information on a variety of aspects, including three who wanted to know more about possible harms of treatment. One would have liked the inclusion of images to aid understanding. In the MMI group, the responses were similarly varied.
To Question 11, ‘Can you tell us which aspect(s) about BAMP was explained well in the information you saw?’ 64 (61.5%) participants responded: 34 in the MMI group and 30 in the PIS group. Responses were highly varied. In the PIS group, five participants mentioned the benefits and disadvantages of the surgery, while another three mentioned its potential benefits. Five participants commented on the description of the process; that is, what would happen. One made a very negative comment about the PIS (‘It is a lot of writing and not attractive to read and it is boring’). In the MMI group, 12 participants mentioned the description of the process, while four mentioned the advantages and disadvantages. Five participants mentioned the videos as being helpful, and one praised the MMI ‘interface’.
To Question 12 (‘any other comments?’), there were two responses in the PIS group (both negative: ‘I think it isn’t very attractive’; ‘As a mother I feel this is a lot of information for his age group’) and there were eight responses in the MMI group, of which six made positive comments (‘the information was explained clearly …’; ‘very understandable …’; ‘helps me understand the BAMP trial’; ‘the vodcast/cartoons were useful’; ‘easy to navigate and understand’; ‘helpful when trying to understand how the trial helps’), with one negative comment (‘the main reason of the trial was hard to understand’) and one question.
Chapter 8 Phase 2 – meta-analysis of SWAT data
Chapter 8 reports the meta-analysis of data from the TRECA SWATs, including data on recruitment, retention and decision-making.
Phase 2 of TRECA included six SWATs within host trials. Unfortunately, it was not possible to run logistic regression models for three of the SWATs, due to a lack of sample size (for BAMP and UKALL-2011) and a lack of variation in outcome data (for BALANCE). Consequently, the meta-analysis comprises data from three SWATs: FORCE, Thermic-3 and CHAMP-UK (Table 21).
| Trial | PIS | MMI | MMI and PIS | Overall | MMI vs. PISa | MMI and PIS vs. PISa |
|---|---|---|---|---|---|---|
| BAMP | 1/3 (33.3%) |
3/3 (100.0%) |
4/4 (100.0%) |
8/10 (80.0%) |
– | – |
| BALANCE | 4/4 (100.0%) |
8/8 (10.00%) |
8/9 (88.9%) |
20/21 (95.2%) |
– | |
| CHAMP | 28/65 (43.1%) |
38/68 (55.9%) |
30/68 (44.1%) |
96/201 (47.8%) |
OR: 1.67 95% CI (0.84 to 3.32) p = 0.14 |
OR: 1.04 95% CI (0.53 to 2.07) p = 0.90 |
| FORCE | 484/729 (66.4%) |
475/681 (69.8%) |
– | 959/1410 (68.0%) |
OR: 1.35 95% CI (0.76 to 2.40) p = 0.30 |
– |
| THERMIC-3 | 24/47 (51.1%) |
32/49 (65.3%) |
22/51 (43.1%) |
77/139 (55.4%) |
OR: 1.80 95% CI (0.79 to 4.10) p = 0.16 |
OR: 0.73 95% CI (0.33 to 1.61) p = 0.43 |
| UKALL | 2/2 (100.0%) |
– | 1/1 (100.0%) |
3/3 (100.0%) |
– | – |
| Pooled |
543/850
(63.9%) |
556/809 (68.7%) | 65/133 (48.9%) | 1164/1792 (65.0%) |
OR: 1.54
95% CI (1.05 to 2.28) p = 0.03 |
OR: 0.89
95% CI (0.53 to 1.50) p = 0.67 |
Trial recruitment
Multimedia information resource-only versus PIS-only
The pooled results of the modified ITT data show that those CYP who received MMI-only information were more likely to be recruited into a trial than those who received PIS-only information: pooled OR = 1.54 (95% CI 1.05 to 2.28; p = 0.03), which is statistically significant. The width of the 95% confidence interval indicates considerable uncertainty about the true effect of the intervention. The statistical heterogeneity in the meta-analysis (I2) was 0% (see Figure 8 for a forest plot of the meta-analysis).
Combined MMI and PIS versus PIS-only
Those participants randomly allocated to the combined MMI and PIS arms were less likely to be recruited than those in the PIS-only arms: the pooled OR = 0.89 (95% CI 0.53 to 1.50; p = 0.67), but the difference is not statistically significant. The meta-analysis included data from only two SWATs (CHAMP-UK and Thermic-370) because the FORCE SWAT did not include a combined MMI and PIS arm (see data in Table 21 and forest plot in Figure 9).
Per-protocol analysis of MMI-only versus PIS-only
It was not possible to undertake a meta-analysis of per-protocol analyses as the only available data were from the Thermic-3 SWAT. Participants in the FORCE and CHAMP-UK SWATs could not receive an information intervention different to the one they had been allocated (Table 22).
| Trial | PIS | MMI | MMI and PIS | Overall | MMI vs. PIS | MMI and PIS vs. PIS |
|---|---|---|---|---|---|---|
| BAMP | 1/2 (50.0%) |
3/3 (100.0%) |
4/4 (100.0%) |
8/9 (88.9%) |
– | – |
| BALANCE | 4/4 (100.0%) |
8/8 (100.0%) |
8/9 (88.9%) |
20/21 (95.2%) |
– | – |
| THERMIC-3 | 24/42 (57.1%) | 27/34 (79.4%) | 21/39 (53.9%) | 72/115 (62.6%) | OR: 2.89 95% CI (1.03 to 8.11) p = 0.04 |
OR 0.88 95% CI (0.36 to 2.10) p = 0.77 |
| UKALL | 2/2 (100.0%) |
– | 1/1 (100.0%) |
3/3 (100.0%) |
– | – |
| Pooled | 31/50 (62.0%) | 38/45 (84.4%) | 34/53 (64.2%) | 103/148 (69.6%) | – | – |
Trial retention
Multimedia information resource-only versus PIS-only
The pooled results of the trial retention analysis suggest that retention rates were higher in the MMI-only arm compared to PIS-only, although the difference was not statistically significant: OR = 1.29 (95% CI 0.36 to 4.65; p = 0.70). Statistical heterogeneity (I2) was zero (see Table 23 and Figure 10).
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| BAMP | 0/1 (0%) |
2/3 (66.7%) |
3/4 (75.0%) |
5/8 (63%) |
– | – |
| BALANCE | 3/4 (75.0%) |
8/8 (100%) |
7/8 (87.5%) |
18/20 (90%) |
– | OR: 2.33 95% CI (0.11 to 50.98) p = 0.59 |
| CHAMP | 26/28 (92.9%) | 34/38 (89.5%) |
29/30 (96.7%) |
89/96 (93%) |
OR: 1.11 95% CI (0.12 to 10.27) p = 0.92 |
OR: 2.23 95% CI (0.19 to 26.06) p = 0.52 |
| FORCE | 481/484 (99.4%) |
473/475 (99.6%) |
– | 954/959 (99.5%) |
OR: 1.14 95% CI (0.11 to 12.32) p = 0.91 |
– |
| THERMIC-3 | 21/24 (87.5%) |
30/32 (93.8%) |
21/22 (95.5%) |
72/77 (94%) |
OR: 1.62 95% CI (0.20 to 12.98) p = 0.65 |
OR: 2.05 95% CI (0.17 to 24.64) p = 0.57 |
| UKALL | 2/2 (100.0%) |
– | 1/1 (100.0%) |
3/3 (100.0%) |
– | – |
| Pooled | 533/543 (98.2%) | 574/556 (98.4%) | 61/65 (93.8%) | 1141/1164 (98.0%) |
OR: 1.29
95% CI (0.36 to 4.65) p = 0.70 |
OR: 2.18
95% CI (0.48 to 10.00) p = 0.31 |
Combined MMI and PIS versus PIS-only
Participants randomly allocated to the combined MMI and PIS arms were more likely to be retained in the trials than those in the PIS-only arms but the difference was not statistically significant (pooled OR = 2.18; 95% CI 0.48 to 10.00; p = 0.31). Heterogeneity (I2) was 0% (see Figure 11 for forest plot).
Decision-Making Questionnaires
The meta-analysis of the DMQs is limited to the parent/family version of the questionnaire because there were insufficient data for meta-analyses of the younger child and older child versions.
Table 24 presents the results of the DMQs for those participants from whom a score could be calculated. Group summary data have been omitted when the number of participants is small. Meta-analyses have been undertaken when this was possible. The results for the BAMP substudy have been included in Tables 24 and 25, but these data have not been included in meta-analyses because they were derived from a hypothetical trial setting rather than a SWAT.
| Trial | PIS | MMI | Both | Overall | MMI vs. PISa | Both vs. PISa |
|---|---|---|---|---|---|---|
| BAMP substudy (Older) |
Older: N = 52 27.0 (4.3) |
Older: N = 52 28.1 (4.2) |
– | Older: N = 104 27.5 (4.3) |
Older: N = 104 AMD: 1.08 (–0.59, 2.75) p = 0.20 |
– |
| BAMP (Older) |
Older: N = 3 29.0 (8.9) |
Older: N = 3 33.7 (2.1) |
Older: N = 4 31.3 (4.4) |
Older: N = 10 31.3 (5.4) |
Older: N = 6 AMD: 1.67 95% CI (–24.59 to 27.92) p = 0.85 |
Older: N = 7 AMD: –0.75 95% CI (–22.88 to 21.37) p = 0.93 |
|
BALANCE
(Young and P/F) |
Young: – P/F: – |
Young: – P/F: – |
Young: – P/F: – |
Young: N = 4 8.8 (2.2) P/F: N = 9 30.1 (4.0) |
Young: – P/F: – |
Young: – P/F: – |
|
CHAMP
(Young and P/F) |
Young: – P/F: N = 26 32.2 (3.4) |
Young: – P/F: N = 31 29.7 (4.6) |
Young: – P/F: N = 24 30.0 (3.8) |
Young: N = 1 – P/F: N = 81 30.6 (4.1) |
Young: – P/F: N = 57 AMD: –2.43 95% CI (–4.61 to –0.24) p = 0.03 |
Young: – P/F: N = 50 AMD: –2.11 95% CI (–4.23 to 0.01) p = 0.05 |
|
FORCE
(P/F) |
P/F: N = 157 31.2 (4.9) |
P/F: N = 151 31.3 (4.5) |
– | P/F: N = 308 31.3 (4.7) |
P/F: N = 308 AMD: 0.07 95% CI (–1.08 to 1.22) p = 0.91 |
– |
|
THERMIC-3
(all three) |
Young: – Older: – P/F: N = 8 29.5 (6.9) |
Young: – Older: – P/F: N = 6 31.8 (4.4) |
Young: – Older: – P/F: N = 3 28.3 (4.9) |
Young: – Older: – P/F: N = 17 30.1 (5.7) |
Young: – Older: – P/F: N = 14 AMD: 0.73 (–5.34 to 6.80) p = 0.80 |
Young: – Older: – P/F: N = 11 AMD: 0.72 (–10.43 to 7.52) p = 0.72 |
|
UKALL
(all three) |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: – Older: N = 2 19.5 (2.1) P/F: N = 3 21.3 (14.6) |
– | – |
| Pooled |
Young:
– Older: N = 4 26.3 (9.1) P/F: N = 217 31.0 (4.9) |
Young:
– Older: N = 3 33.7 (2.1) P/F: N = 166 31.0 (5.6) |
Young:
– Older: N = 5 29.2 (6.0) P/F: N = 28 30.0 (4.0) |
Young: N = 5 9.4 (2.4) Older: N = 12 29.3 (6.7 P/F: N = 411 30.9 (5.1) |
Young: – Older: – P/F AMD: –0.79 95% CI (–2.80, 1.22) p = 0.44 |
Young: – Older: – P/F AMD: –2.07 95% CI (–4.13 to –0.01) p = 0.05 |
| Trial | PIS | MMI | Both | Overall | MMI vs. PISa | Both vs. PISa |
|---|---|---|---|---|---|---|
| BAMP substudy (Older) |
Older: N = 51 27.0 (4.4) |
Older: N = 52 28.1 (4.2) |
– | Older: N = 104 27.5 (4.3) |
Older: N = 103 AMD: 1.06 (–0.63 to 2.74) p = 0.22 |
– |
| BAMP (Older) |
Older: N = 3 29.0 (8.9) |
Older: N = 3 33.7 (2.1) |
Older: N = 4 31.3 (4.4) |
Older: N = 10 31.3 (5.4) |
Older: N = 6 AMD: 1.67 95% CI (–24.59 to 27.92) p = 0.85 |
Older: N = 7 AMD: –0.75 95% CI (–22.88 to 21.37) p = 0.93 |
|
BALANCE
(Y and P/F) |
Young: – P/F: – |
Young: – P/F: – |
Young: – P/F: – |
Young: N = 4 8.8 (2.2) P/F: N = 9 30.1 (4.0) |
Young: – P/F: – |
Young: – P/F: – |
|
CHAMP
(Young and P/F) |
Young: – P/F: N = 26 32.2 (3.4) |
Young: – P/F: N = 30 29.6 (4.7) |
Young: – P/F: N = 22 29.7 (3.8) |
Young: N = 1 – P/F: N = 78 30.5 (4.2) |
Young: – P/F: N = 56 AMD: –2.53 95% CI (–4.73 to –0.32) p = 0.03 |
Young: – P/F: N = 48 AMD: –2.38 95% CI (–4.52 to –0.23) p = 0.03 |
|
FORCE
(P/F) |
P/F: N = 156 31.2 (4.9) |
P/F: N = 146 31.3 (4.5) |
– | P/F: N = 302 31.3 (4.7) |
P/F: N = 302 AMD: 0.11 95% CI (–0.95 to 1.19) p = 0.83 |
– |
|
THERMIC-3
(all three) |
Young: – Older: – P/F: N = 8 29.5 (6.9) |
Young: – Older: – P/F: N = 6 31.8 (4.4) |
Young: – Older: – P/F: N = 3 28.3 (4.9) |
Young: – Older: – P/F: N = 17 30.1 (5.7) |
Young: – Older: – P/F: N = 12 AMD: 1.35 (–5.06 to 7.75) p = 0.65 |
Young: – Older: – P/F: N = 9 AMD: –0.50 (–10.72 to 9.72) p = 0.91 |
|
UKALL
(all three) |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: Older: N = 2 19.5 (2.1) P/F: N = 2 21.0 (10.6) |
– | – |
| Pooled |
Young:
– Older: N = 4 26.3 (9.1) P/F: N = 209 31.2 (4.5) |
Young:
– Older: N = 3 33.7 (2.1) P/F: N = 162 31.1 (5.1) |
Young:
– Older: N = 5 29.2 (6.0) P/F: N = 26 29.8 (4.0) |
Young:
N = 5 9.4 (2.4) Older: N = 12 29.3 (6.7) P/F: N = 397 31.1 (4.7) |
Young: – Older: – P/F: AMD: –1.11 (–3.36 to 1.13) p = 0.33 |
Young: – Older: – P/F: AMD: –2.30 (–7.39 to –0.20) p = 0.03 |
Overall, for those participants who completed the parent/family version, those in the MMI-only arms were found to have lower total DMQ scores than those in the PIS-only arms: pooled AMD = –0.79 (95% CI –2.80 to 1.22; p = 0.44), although the difference is not statistically significant. Statistical heterogeneity was moderately high (I2 = 53.6%).
Similarly, participants in the combined MMI and PIS arms had lower total DMQ scores than those in the PIS-only arms when completing the parent/family version: pooled AMD = –2.07 (95% CI –4.13 to 0.01; p = 0.05), which is borderline statistically significant. Statistical heterogeneity was zero (I2 = 0%).
Forest plots for both the parent/family DMQ comparisons for all patients who completed it can be seen in Figures 12 and 13.
FIGURE 13.
Forest plot of the DMQ results compared combined MMI and PIS with PIS-only for the parent/family version. (The figure includes data from a published study. 154) Table 25 presents the analysis of DMQ total scores, including only those participants who fully completed the measure (i.e. when no imputation was used). When participants completed the parent/family version of the DMQ, meta-analysis indicates that the mean scores were lower in the MMI-only arms compared with the PIS-only arms: AMD = –1.11 (95% CI –3.36 to 1.13; p = 0.33), although the difference was not statistically significant.

When comparing the combined MMI and PIS arms with the PIS-only arms for the parent/family version of the DMQ, meta-analysis indicates that the mean scores were lower in the combined MMI and PIS arms: AMD = –2.30 (95% CI –7.39 to –0.20; p = 0.03) and the difference was statistically significant.
Table 26 shows the findings for participants who completed the parent/family version of the DMQ, including only those participants who consented to their host trial. When comparing the MMI-only with the PIS-only arms it indicates that mean DMQ scores were lower in participants provided with MMI information: AMD = –0.21 (95% CI –1.26 to 0.83; p = 0.69), although the difference was not statistically significant.
| Trial | PIS | MMI | Both | Overall | MMI vs. PISa | Both vs. PISa |
|---|---|---|---|---|---|---|
| BAMP (Older) |
Older: N = 1 – |
Older: N = 3 33.7 (2.1) |
Older: N = 4 31.3 (4.4) |
Older: N = 8 32.3 (3.3) |
Older: N = 4 AMD: 1.67 95% CI (–8.68 to 12.01) p = 0.56 |
Older: N = 5 AMD: –0.75 95% CI (–16.5 to 15.0) p = 0.89 |
|
CHAMP
(Young and P/F) |
Young: – P/F: N = 19 31.9 (3.5) |
Young: – P/F: N = 22 30.8 (4.2) |
Young: – P/F: N = 14 30.6 (3.7) |
Young: N = 1 – P/F: N = 55 31.1 (3.8) |
Young: – P/F: N = 41 AMD: –1.12 95% CI (–3.58 to 1.34) p = 0.36 |
Young: – P/F: N = 33 AMD: –1.26 95% CI (–3.81 to 1.29) p = 0.32 |
|
FORCE
(P/F) |
P/F: N = 143 31.2 (4.8) |
P/F: N = 116 31.1 (4.8) |
– | P/F: N = 259 31.2 (4.8) |
P/F: N = 259 AMD: –0.10 95% CI (–1.30 to 1.11) p = 0.88 |
– |
|
THERMIC-3
(all three) |
Young: – Older: – P/F N = 5 32.3 (2.1) |
Young: – Older: – P/F N = 5 33.2 (3.3) |
Young: – Older: – P/F N = 2 30.0 (5.7) |
Young: – Older: – P/F N = 12 32.3 (3.1) |
Young: – Older: – P/F N = 10 AMD: 0.90 95% CI (–3.11 to 4.91) p = 0.62 |
Young: – Older: – P/F N = 7 AMD: –2.30 95% CI (–9.09 to 4.49) p = 0.42 |
|
UKALL
(all three) |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: N = 0 Older: N = 2 19.5 (2.1) P/F: N = 3 21.3 (14.6) |
– | – |
| Pooled |
Young:
– Older: N = 2 25.0 (9.9) P/F: N = 149 31.1 (5.4) |
Young:
– Older: N = 3 33.7 (2.1) P/F: N = 165 31.0 (5.0) |
Young:
– Older: N = 5 29.2 (6.0) P/F: N = 17 30.9 (3.9) |
Young:
N = 1 – Older: N = 10 29.7 (6.2) P/F: N = 331 30.0 (5.1) |
Young: – Older: – P/F AMD: –0.21 95% CI (–1.26 to 0.83) p = 0.69 |
Young: – Older: – P/F AMD: –1.39 95% CI (–3.78 to 1.00) p = 0.25 |
When comparing the combined MMI and PIS arms with the PIS-only arms, findings indicate that lower DMQ scores were obtained from those in the combined MMI and PIS arms: AMD = –1.39 (95% CI –3.78 to 1.00; p = 0.25), although the difference was not statistically significant.
Chapter 9 Discussion
Brief summary of findings
The TRECA study was undertaken in two phases. The MMIs were developed in the first phase, through extensive participatory design (including qualitative and user testing studies, readability testing and sustained patient and parent involvement). Consequently, two templates for MMIs for use in CYP trials were developed (one for younger children aged 6–11 years, and the other for young people aged 12–18 years and parents), which included short animations (both trial-specific and trial-generic), short ‘talking heads’ videos, and written information revised for age-appropriateness and organised under a clear structure of six pages within the MMI.
During the TRECA second phase a set of six SWATs was undertaken, and their data were combined within a pre-planned meta-analysis. Meta-analysis showed that provision of MMI rather than standard printed information led to higher recruitment rates of CYP to trials. There was no effect on trial retention. The provision of combined multimedia and printed information resulted in no benefit on trial recruitment or retention rates compared to printed information alone.
Provision of MMI rather than printed information produced no effect on DMQ scores. However, the provision of combined MMI and printed information resulted in less favourable evaluations than from printed information alone.
Strengths and limitations
The MMIs were developed by drawing on extensive empirical work in Phase 1, to ensure that their content, layout and appearance would best meet the preferences and needs of several stakeholder groups, including CYP, parents and researchers. Although this co-design phase of the study lasted more than 12 months, it did mean there was an evidential basis to guide the creation of the information resources. The MMIs went through several iterations in response to obtained data, and the success of this development process depended on being able to work collaboratively with a website and video production company that was willing and able to respond to requests for changes. The result of this careful development process is that multiple MMI iterations are unlikely to be required if the developed MMI templates are adapted for future trials.
The six SWATs were run with host trials recruiting CYP with a range of ages and health conditions, increasing the external validity of the study findings. Furthermore, the pre-planned meta-analysis, in which a number of SWATs evaluated a similar intervention, built on the approach undertaken in the UK MRC-funded Systematic Techniques for Assisting Recruitment to Trial (START) study35 and had four main strengths:
-
It increased the total sample size and the consequent certainty of statistical findings; it is notable in TRECA that individually none of the three SWATs produced statistically significant effects on recruitment, although the pooled data set did.
-
It meant that there was greater researcher control over participant sampling, outcomes and (especially) interventions than would be the case in a conventional retrospective meta-analysis after systematic review.
-
This approach is likely to have been cost-efficient compared with running individual SWATs, particularly because the intervention evaluated in the TRECA SWATs required significant development time.
-
A co-ordinated approach to the development of ‘recruitment science’ is likely to accelerate the generation of evidence. 64,66
The co-ordinated, pre-planned approach to SWATs used in TRECA is also being undertaken by the PROMETHEUS study (funded jointly by the UK MRC and NIHR). 70
The study faced several challenges,77 the main effect of which was to reduce the size of the data set for the meta-analysis. There was a very good response to publicity about TRECA, seeking expressions of interest in being involved as a host trial; unfortunately, some potential host trials did not meet our criteria, most often due to recruitment dates not matching TRECA timelines, or trials already planning to use a video within recruitment or have an active study website once the trial was underway. The TRECA meta-analysis was intended to include six SWATs rather than the three that were achieved. All three of the TRECA SWATs that could not be included in the meta-analysis were affected by unanticipated circumstances: for example, one closed early due to funding constraints; one faced significant delays in research ethics and governance approvals; and one was affected by COVID-19 recruitment postponement. Indeed, COVID-19 recruitment postponements affected three SWATs in all, including two that contributed to the meta-analysis, which reduced the size of the data set and delayed the production of findings. One risk associated with undertaking any SWAT is that adverse circumstances affecting the running of the host trial, will also adversely affect the SWAT.
TRECA questionnaire return rates were lower than anticipated, particularly in some SWATs. Consequently, the DMQ findings had lower levels of statistical precision, which has reduced certainty in the findings. The return rate of questionnaires was much higher in those recruited to host trials than in those who declined participation. While this differential is not surprising, given its occurrence in previous studies of research recruitment interventions, it constitutes a significant limit to the study findings; potentially, it was also a source of bias in the DMQ data set. This issue could have been addressed by adopting a different approach to obtaining questionnaire responses, or by including other sources of data, such as a smaller sample qualitative study, to look at the question with a different approach. For example, qualitative research in Phase 2 of the study could have interviewed consenters and decliners in host trials, or clinical and research staff responsible for trial recruitment, although this approach would have had challenges of its own.
Finally, a limitation of this study is one that affects many SWATs addressing trial recruitment questions, which is the application of host trial entry criteria to patient eligibility after the SWAT random allocation of individuals has happened. Possible effects are a loss of SWAT participants, as well as a reduction in the fidelity of the SWAT randomisation process; consequently, a modified ITT analysis was required in this study.
What this study adds
This study will contribute to the growing evidence base of RCT-level evidence for interventions targeted at trial recruitment and retention. 64,65 In particular, it will contribute to the evidence around these processes in CYP patients, which is currently lacking.
Although there are now a substantial number of SWATs to have evaluated trial recruitment interventions, not many have shown the increase in recruitment rates that is reported here. The fact that participants were retained at follow-up in the trials is reassuring, indicating that increased recruitment in the MMI arms was not achieved at the expense of participant understanding of the host trial when being recruited.
One aim of the TRECA study was to produce a template for MMIs for trial recruitment, in part to ensure that all the TRECA SWATs would be testing very similar multimedia information, but also to allow future researchers to use the template in trials if the MMIs were shown to be effective and/or acceptable. That opportunity is now available.
There is a growing evidence base on the use of MMIs in research recruitment and in healthcare practice, although not many interventions have been evaluated using a SWAT approach (with the benefits of random allocation). Consequently, this study will make a meaningful contribution to the evidence.
Finally, this study also adds to the knowledge that using multimedia information in research recruitment is achievable and acceptable. Most UK trials and trial centres currently use printed information. Our perception is that this is not due to a resistance to digital or multimedia information, but that it stems from two concerns among trial researchers:
-
That RECs, which must approve research before it is undertaken, are resistant or hesitant about the provision of participant information in a non-printed form. Notably this study did not encounter any resistance of that type.
-
A view that multimedia is too difficult, expensive or time-consuming to produce. This study provides some evidence to counter that view. Depending on their complexity, an MMI for a trial currently (in 2023) costs £10,000–15,000. The evidence from TRECA in CYP populations is that an MMI could reduce the recruitment period of a trial by 5–8%, that is, potentially reducing a 12-month trial recruitment period by 1 month. In larger trials or those with longer recruitment periods, use of MMIs could be cost-neutral or potentially provide a cost saving. However, no formal health economic analysis was undertaken within this study. The written text in the TRECA MMIs was taken from the PIS; hence, the additional researcher time associated with MMI-development for a trial would largely be limited to generating the non-text elements (i.e. writing scripts for animations and other video, and liaising with the MMI developer).
Implications of the study
This study demonstrated a benefit of MMI on trial recruitment in CYP, although the relevant evidence base remains small and there is a need for further studies. Furthermore, the effect of MMIs on recruitment generated an odds ratio statistic with the lower end of the confidence interval not far above one (with an associated probability value of 0.03) and so there remains considerable uncertainty around the true effect of multimedia information on trial recruitment. That is particularly the case for CYP patients, but it also applies to adult patients. There have been recent initiatives to create a set of criteria for assessing the need for further SWATs of particular interventions before they are undertaken, and it would be beneficial to apply those to MMIs in trials involving CYP. 153
The provision of combined MMI and printed information resulted in no benefits to recruitment or retention, and possibly in less positive questionnaire evaluations than for printed information alone. However, the small available data set for this question greatly lessens the certainty of this finding. While the small scope and size of this study limits its generalisability, given the time-demand and complexity of providing information to participants in two formats, this study does not suggest that providing both multimedia and printed information is a better option than printed information alone.
One of the included TRECA SWATs was unable to provide participants with access to the MMIs as planned (on tablet computers during clinic visits) due to internet connectivity problems within the hospital, which is not uncommon in UK NHS hospitals. Consequently, in that particular SWAT participants allocated to MMI were given the MMI URL (i.e. internet address) on a laminated card, for access at home. Clearly this increased the risk of participants not accessing the MMI. The implications for future MMI studies are to find a solution to this problem. This could be achieved through digital or e-mail communication between the healthcare provider and patients, although there are several barriers (including confidentiality and practicalities), which would need to be overcome.
One study aim was to include data on participant deprivation within the analysis, to assess the relative impacts of multimedia and printed information on more deprived and/or less health literate populations. In part that was motivated by previous evidence that research and trial participation rates are socially structured; and in part by a knowledge that suboptimal or complex patient information tends to have a disproportionately negative effect on people with low literacy or low health literacy. Unfortunately, because of the data set challenges experienced in this study, we were unable to examine these aspects. The small size of some of the SWATs in this study meant that there was a lack of reliable deprivation data. However, the problem was not specific to the TRECA study: demographic baseline data are generally only available for those who have consented to the host trial, due to research ethics restrictions. Data access is rarely permitted on those people who have declined trial participation, and this places a significant limit on the ability to answer important questions of equity within ‘recruitment science’. There remains a need to assess whether the provision of carefully and responsively developed multimedia information (or indeed printed trial information) has benefits on participant understanding and decision-confidence across all groups in the population.
Participant information plays an essential role in consent to research. While the focus of this study has been on rates of trial recruitment, it is self-evident that trial rates are made up of individual patient decisions to give or withhold consent. For individual participation decisions (whether positive or negative) to be valid they must be informed, and the ethical duty rests with the person taking consent to be assured that the participant is sufficiently informed to make an informed decision. The role of multimedia information in informing individual consent decisions, and in allowing recruiters to fulfil their ethical duty, is an area in need of research. This study has generated some relevant findings from the use of participant DMQs, although the low return rates (particularly from host trial decliners) means that there remains considerable uncertainty about the effects of multimedia information on participant appraisals. There is also currently a lack of research in both adults and CYP around the effects of multimedia information (rather than print) on communication between potential research participants and the people who are recruiting them.
Furthermore, we need to know more about people’s use of MMIs, when using them to inform a consent decision, to be sure that participants are being informed about the research and not just entertained. It is unclear whether all the components of the multimedia information, and the development process used in this study, are required to achieve a beneficial effect. There is a need for further research into the effects of different formats of multimedia information, and the ways that research participants may use them to inform a decision on consent and beyond. There are two main concerns about the use of animations and ‘talking head’ video clips:
-
they are relatively expensive components of MMIs, which may create a barrier to use in less well-funded trials
-
the inclusion within MMIs of all the written text of a PIS, plus information content within the animations and videos, generates user choice but may also create information duplication or redundancy, considered to be a key aspect in the quality appraisal of multimedia provision. 62
The TRECA study evaluated digital information that was multimedia, comprising animations, ‘talking head’ videos, and written information that had been revised for readability and age-appropriateness. Furthermore, the design and content of the multimedia information was greatly informed by the Phase 1 development work, including enhanced patient and parent involvement. The overall effect was to increase recruitment in three trials (indicated by a meta-analysis of one SWAT with negligible statistical heterogeneity), but there remains a need for further research on the effects of MMIs on recruitment. Cautiously, there is a need for more SWAT research with MMIs before recommending their use routinely in CYP trials.
Acknowledgements
This project was funded by the National Institute for Health and Care Research (NIHR) Health Services and Delivery Research Programme (Award ID: 14/21/21). TRECA received approval from the NHS Yorkshire & the Humber – Bradford Leeds Research Ethics Committee (17/YH/0082) and the HRA (IRAS ID 212761). Trial and SWAT registration: TRECA ISRCTN73136092 and Northern Ireland Hub for Trials Methodology Research SWAT Repository (SWAT 97). We are grateful to the NIHR HS&DR programme for funding TRECA and providing the opportunity to undertake this work. We wish to thank the following people and organisations for their significant contributions to the TRECA study:
-
Members of the TRECA Patient and Parent Advisory Group [Danielle Horton Taylor; Robyn Challinor; Simon Stones; Sophie Ainsworth; Jenny (Sammy) Ainsworth].
-
Members of the TRECA NIHR Study Steering Committee [Professor Faith Gibson (Chair); Professor Louise Locock; Professor Matt Sydes; Dr Louca-Mai Brady; Dr Bob Phillips].
-
Participants in the Phase 1 studies and Phase 2 SWATs.
-
Researchers and recruiting staff working on Phase 2 SWATs, particularly those willing to be filmed for the ‘talking head’ videos for the MMIs.
-
Researchers on the SCIPI trial.
-
Morph (particularly Tom Urry; Gareth Dennison; Matthew Brook).
-
Past members of the TRECA study advisory group (Dr Jonathan Graffy; Dr Paul Baines).
-
Colleagues at the University of York for their support, advice and encouragement (Sandi Newby; Oliver Short; Gavin MacMillan; Michael Barber; Charlotte Boyce; Richard Scott; Professor Ian Watt; Professor Patrick Doherty; Professor Karen Bloor).
-
NIHR programme managers assigned to TRECA, for their support throughout the study (Martin Simpson-Scott; Avril Lloyd).
We also thank the three peer reviewers, nominated by NIHR, whose suggestions and comments (both positive and negative) allowed us to improve the reporting of the TRECA study.
Contributions of authors
Peter Knapp (https://orcid.org/0000-0001-5904-8699) (Reader in Health Sciences) was the principal investigator, led the design of the study, contributed to design of the multimedia information, and is the lead author of the report.
Jacqueline Martin-Kerry (https://orcid.org/0000-0002-9299-1360) (Research Fellow) was responsible for day-to-day management of the project (until 2019), undertook data collection, led on the qualitative study, and was involved in the preparation of this report.
Thirimon Moe-Byrne (https://orcid.org/0000-0002-2827-9715) (Research Fellow) was responsible for day-to-day management of the project (from 2019), undertook data collection and was involved in the analysis and preparation of this report.
Rebecca Sheridan (https://orcid.org/0000-0002-7715-1224) (Research Fellow) undertook data collection, chaired the Patient and Parent Advisory Group, led on the user testing study and was involved in the preparation of this report.
Elizabeth Coleman (https://orcid.org/0000-0003-4210-1865) (Research Fellow – Statistician) led on the data analysis and was involved in the preparation of this report.
Jenny Roche (https://orcid.org/0000-0003-1822-9519) (Research Fellow – Statistician) led on the data analysis and was involved in the preparation of this report.
Bridget Young (https://orcid.org/0000-0001-6041-9901) (Professor of Psychology) contributed to the study design, advised on the study throughout, particularly the qualitative study and was involved in the preparation of this report.
Steven Higgins (https://orcid.org/0000-0003-0314-4846) (Professor of Education) contributed to the study design, led on the readability revisions of text in the MMIs, advised on the study throughout, and was involved in the preparation of this report.
Jennifer Preston (https://orcid.org/0000-0003-4800-234X) (Senior Patient and Public Involvement Lead) contributed to the study design, advised on the PPI aspects of this study and contributed to PPI data collection and was involved in the preparation of this report.
Peter Bower (https://orcid.org/0000-0001-9558-3349) (Professor of Health Services Research) contributed to the study design, advised on the study throughout, particularly the Phase 2 study, and was involved in the analysis and preparation of this report.
Carrol Gamble (https://orcid.org/0000-0002-3021-1955) (Professor of Medical Statistics) contributed to the study design, advised on the data analysis and was involved in the preparation of this report.
Catherine Stones (https://orcid.org/0000-0002-9797-8587) (Associate Professor – Graphic Design) contributed to the study design, advised on the MMI design and was involved in the preparation of this report.
Data-sharing statement
Data are available upon reasonable request. All data requests should be submitted to the corresponding author. Access to anonymised data may be granted following review. If the request is approved, data will be shared via encrypted third-party transfer. The TRECA statistical analysis plan has not been published but can be provided on request.
Publications from the TRECA study
-
Martin-Kerry JM, Bower P, Young B, Graffy J, Sheridan R, Watt I, et al. Developing and evaluating multimedia information resources to improve engagement of children, adolescents, and their parents with trials (TRECA study): study protocol for a series of linked randomised controlled trials. Trials 2017;18:265. https://doi.org/10.1186/s13063-017-1962-z
-
Sheridan R, Martin-Kerry JM, Watt I, Higgins SE, Stones S, Horton-Taylor D, Knapp P. User testing digital, multimedia information to inform children, adolescents and their parents about healthcare trials. J Child Health Care 2018;23:468. https://doi.org/10.1177/1367493518807325
-
Martin-Kerry JM, Knapp P, Atkin K, Bower P, Watt I, Stones C, et al. Supporting children and young people when making decisions about joining clinical trials: qualitative study to inform multimedia website development. BMJ Open 2019;9:e023984.
-
Martin-Kerry JM, Parker A, Arundel C, Torgerson D, Tilbrook H, Watt I, et al. SWATted away: the challenging experience of setting up a SWAT in paediatric trials. Trials 2019;20:141. https://doi.org/10.1186/s13063-019-3236-4
-
Sheridan R, Preston J, Stones S, Ainsworth S, Horton Taylor D, Challinor R, et al. Public involvement – why bother? Reflections from a study informing the design of multimedia information resources to improve engagement of children, young people and their families with trials. Res All 2020;4:47–65. https://doi.org/10.18546/RFA.04.1.05
-
Knapp P, Mandall N, Hulse W, Roche J, Moe-Byrne T, Martin-Kerry JM, et al.; for the TRECA Study Group. Evaluating the use of multimedia information when recruiting adolescents to orthodontics research: a randomised controlled trial. J Orthodont 2021;48(4). https://doi.org/10.1177/14653125211024250
-
Knapp P, Dabner L, Heys R, Sheehan K, Smartt H, Walker-Smith T, et al.; for the TRECA Study Group. The effects of multimedia information on recruitment to a children’s cardiac surgery trial: a SWAT (Study Within A Trial). F1000Res 2022;11:340. https://f1000research.com/articles/11-340/v1
-
Martin-Kerry J M, Higgins S E, Knapp P, Liabo K, Young B. Engaging children, young people, parents and health professionals in interviews: using an interactive ranking exercise within the co-design of multimedia websites. Journal of Child Health Care 2022. https://doi.org/10.1177/13674935221109684
-
Moe-Byrne T, Knapp P, Perry D, Achten J, Spoors L, Appelbe D, et al. Does digital, multimedia information increase recruitment to a children’s wrist fracture treatment trial, and what do people think of it? A SWAT (Study Within A Trial). BMJ Open 2022;12:e057508. https://doi.org/10.1136/bmjopen-2021-057508
-
Knapp P, Moe-Byrne T, Martin-Kerry J, Sheridan R, Roche J, Coleman E, et al.Providing multimedia information to children and young people increases recruitment to trials: pre-planned meta-analysis of SWATs. BMC Medicine 2023;21:244. https://doi.org/10.1186/s12916-023-02936-1
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- Schrier L, Hadjipanayis A, Stiris T, Ross-Russell RI, Valiulis A, Turner MA, et al. Off-label use of medicines in neonates, infants, children, and adolescents: a joint policy statement by the European Academy of Paediatrics and the European society for Developmental Perinatal and Pediatric Pharmacology. Eur J Pediatr 2020;179:839-47. https://doi.org/10.1007/s00431-019-03556-9.
- Joseph PD, Craig JC, Caldwell PH. Clinical trials in children. Br J Clin Pharmacol 2013;79:357-69. https://doi.org/10.1111/bcp.12305.
- Rocchi F, Tomasi P. The development of medicines for children: part of a series on Pediatric Pharmacology, guest edited by Gianvincenzo Zuccotti, Emilio Clementi, and Massimo Molteni. Pharmacol Res 2011;64:169-75. https://doi.org/10.1016/j.phrs.2011.01.016.
- Klassen TP, Hartling L, Craig JC, Offringa M. Children are not just small adults: the urgent need for high-quality trial evidence in children. PLOS Med 2008;5. https://doi.org/10.1371/journal.pmed.005017.
- Modi N, Clark H, Wolfe I, Costello A, Budge H, Goodier R, et al. Writing Group of the Royal College of Paediatrics and Child Health Commission on Child Health Research . A healthy nation: strengthening child health research in the UK. Lancet 2013;381:73-87. https://doi.org/10.1016/s0140-6736(12)61818-2.
- Cohen E, Goldman RD, Ragone A, Uleryk E, Atenafu EG, Siddiqui U, et al. Child vs. adult randomized controlled trials in specialist journals: a citation analysis of trends, 1985–2005. Arch Pediatr Adolesc Med 2010;164:283-8. https://doi.org/10.1001/archpediatrics.2009.291.
- Health Do . Chief Medical Officer’s Annual Report 2012: Our Children Deserve Better: Prevention Pays 2013.
- Chappuy H, Doz F, Blanche S, Gentet JC, Tréluyer JM. Children’s views on their involvement in clinical research. Pediatr Blood Cancer 2008;50:1043-6.
- Caldwell PH, Butow PN, Craig JC. Pediatricians’ attitudes toward randomized controlled trials involving children. J Pediatr 2002;141:798-803. https://doi.org/10.1067/mpd.2002.129173.
- Srivastava A, Bourgeois FT. Evaluation of publication of pediatric drug trials. JAMA Netw Open 2021;4. https://doi.org/10.1001/jamanetworkopen.2021.5829.
- Pica N, Bourgeois F. Discontinuation and nonpublication of randomized clinical trials conducted in children. Pediatrics 2016;138. https://doi.org/10.1542/peds.2016-0223.
- Walters SJ, Bonacho dos Anjos Henriques-Cadby I, Bortolami O, Flight L, Hind D, Jacques RM, et al. Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-015276.
- Bruzzese JM, Gallagher R, McCann-Doyle S, Reiss PT, Wijetunga NA. Effective methods to improve recruitment and retention in school-based substance use prevention studies. J Sch Health 2009;79:400-7. https://doi.org/10.1111/j.1746-1561.2009.00427.x.
- Kitterman DR, Cheng SK, Dilts DM, Orwoll ES. The prevalence and economic impact of low-enrolling clinical studies at an academic medical center. Acad Med 2011;86:1360-6. https://doi.org/10.1097/ACM.0b013e3182306440.
- Moher D, Glasziou P, Chalmers I, Nasser M, Bossuyt PM, Korevaar DA, et al. Increasing value and reducing waste in biomedical research: who’s listening?. Lancet 2016;387:1573-86. https://doi.org/10.1016/s0140-6736(15)00307-4.
- Ioannidis JP, Greenland S, Hlatky MA, Khoury MJ, Macleod MR, Moher D, et al. Increasing value and reducing waste in research design, conduct, and analysis. Lancet 2014;383:166-75. https://doi.org/10.1016/s0140-6736(13)62227-8.
- The General Medical Council . 0–18 Years: Guidance for All Doctors 2007. https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/0-18-years (accessed 4 October 2021).
- UK Medical Research Council . MRC Ethics Guide: Medical Research Involving Children 2004. https://mrc.ukri.org/documents/pdf/medical-research-involving-children/ (accessed 5 December 2021).
- Coyne I. Research with children and young people: the issue of parental (proxy) consent. Child Soc 2010;24:227-37.
- Luchtenberg M, Maeckelberghe E, Locock L, Powell L, Verhagen AA. Young people’s experiences of participation in clinical trials: reasons for taking part. Am J Bioeth 2015;15:3-13. https://doi.org/10.1080/15265161.2015.1088974.
- Caldwell PH, Butow PN, Craig JC. Parents’ attitudes to children’s participation in randomized controlled trials. J Pediatr 2003;142:554-9. https://doi.org/10.1067/mpd.2003.192.
- Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet 2004;364:803-11. https://doi.org/10.1016/s0140-6736(04)16942-0.
- Sheridan R, Martin-Kerry J, Hudson J, Parker A, Bower P, Knapp P. Why do patients take part in research? An overview of systematic reviews of psychosocial barriers and facilitators. Trials 2020;21. https://doi.org/10.1186/s13063-020-4197-3.
- Tarnowski KJ, Allen DM, Mayhall C, Kelly PA. Readability of pediatric biomedical research informed consent forms. Pediatrics 1990;85:58-62.
- Ogloff JR, Otto RK. Are research participants truly informed? Readability of informed consent forms used in research. Ethics Behav 1991;1:239-52. https://doi.org/10.1207/s15327019eb0104_2.
- Eder ML, Yamokoski AD, Wittmann PW, Kodish ED. Improving informed consent: suggestions from parents of children with leukemia. Pediatrics 2007;119:e849-59. https://doi.org/10.1542/peds.2006-2208.
- Caldwell PH, Dans L, de Vries MC, Newman Ba Hons J, Sammons H, Spriggs MBM, et al. Standard 1: consent and recruitment. Pediatrics 2012;129:S118-23. https://doi.org/10.1542/peds.2012-0055D.
- Tait AR, Voepel-Lewis T. Digital multimedia: a new approach for informed consent?. JAMA 2015;313:463-4. https://doi.org/10.1001/jama.2014.17122.
- Health Research Authority . Applying a Proportionate Approach to the Process of Seeking Consent: HRA Guidance 2016.
- Hermann M. 3-dimensional computer animation – a new medium for supporting patient education before surgery: acceptance and assessment of patients based on a prospective randomized study – picture versus text. Chirurg 2002;73:500-7. https://doi.org/10.1007/s00104-001-0416-y.
- Knapp P, Raynor D, Silcock J, Parkinson B. Performance-based readability testing of participant information for a Phase 3 IVF trial. Trials 2009;10.
- Knapp P, Raynor DK, Silcock J, Parkinson B. Performance-based readability testing of participant materials for a phase I trial: TGN1412. J Med Ethics 2009;35:573-8. https://doi.org/10.1136/jme.2008.026708.
- Knapp P, Raynor DK, Silcock J, Parkinson B. Can user testing of a clinical trial patient information sheet make it fit-for-purpose? A randomized controlled trial. BMC Med 2011;9. https://doi.org/10.1186/1741-7015-9-89.
- Raynor D, Knapp P, Silcock J, Parkinson B, Feeney K. ‘User-testing’ as a method for testing the fitness-for-purpose of written medicine information. Patient Educ Couns 2011;83:404-10. https://doi.org/10.1016/j.pec.2011.03.016.
- Madurasinghe VW, Bower P, Eldridge S, Collier D, Graffy J, Treweek S, et al. Can we achieve better recruitment by providing better information? Meta-analysis of ‘studies within a trial’ (SWATs) of optimised participant information sheets. BMC Med 2021;19:1-8.
- Hutchison C, Cowan C, McMahon T, Paul J. A randomised controlled study of an audiovisual patient information intervention on informed consent and recruitment to cancer clinical trials. Br J Cancer 2007;97:705-11. https://doi.org/10.1038/sj.bjc.6603943.
- Shneerson C, Windle R, Cox K. Innovating information-delivery for potential clinical trials participants: what do patients want from multi-media resources?. Patient Educ Couns 2013;90:111-7. https://doi.org/10.1016/j.pec.2012.06.031.
- Savage I, Goodyer L. Providing information on metered dose inhaler technique: is multimedia as effective as print?. Fam Pract 2003;20:552-7.
- Hopper KD, Zajdel M, Hulse SF, Yoanidis NR, TenHave TR, Labuski MR, et al. Interactive method of informing patients of the risks of intravenous contrast media. Radiology 1994;192:67-71. https://doi.org/10.1148/radiology.192.1.8208968.
- Krishna S, Francisco BD, Balas EA, Konig P, Graff GR, Madsen RW. Randomized Trial . Internet-enabled interactive multimedia asthma education program: a randomized trial. Pediatrics 2003;111:503-10.
- Wilson EA, Makoul G, Bojarski EA, Bailey SC, Waite KR, Rapp DN, et al. Comparative analysis of print and multimedia health materials: a review of the literature. Patient Educ Couns 2012;89:7-14. https://doi.org/10.1016/j.pec.2012.06.007.
- Schenker Y, Fernandez A, Sudore R, Schillinger D. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review. Med Decis Making 2011;31:151-73. https://doi.org/10.1177/0272989x10364247.
- Kinnersley P, Phillips K, Savage K, Kelly MJ, Farrell E, Morgan B, et al. Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database Syst Rev 2013;7. https://doi.org/10.1002/14651858.CD009445.pub2.
- Tuong W, Larsen ER, Armstrong AW. Videos to influence: a systematic review of effectiveness of video-based education in modifying health behaviors. J Behav Med 2014;37:218-33. https://doi.org/10.1007/s10865-012-9480-7.
- Dahodwala M, Geransar R, Babion J, de Grood J, Sargious P. The impact of the use of video-based educational interventions on patient outcomes in hospital settings: a scoping review. Patient Educ Couns 2018;101:2116-24. https://doi.org/10.1016/j.pec.2018.06.018.
- Dekkers T, Melles M, Groeneveld BS, de Ridder H. Web-based patient education in orthopedics: systematic review. J Med Internet Res 2018;20. https://doi.org/10.2196/jmir.9013.
- Knox ECL, Quirk H, Glazebrook C, Randell T, Blake H. Impact of technology-based interventions for children and young people with type 1 diabetes on key diabetes self-management behaviours and prerequisites: a systematic review. BMC Endocr Disord 2019;19. https://doi.org/10.1186/s12902-018-0331-6.
- Ciciriello S, Johnston RV, Osborne RH, Wicks I, deKroo T, Clerehan R, et al. Multimedia educational interventions for consumers about prescribed and over-the-counter medications. Cochrane Database Syst Rev 2013;4. https://doi.org/10.1002/14651858.CD008416.pub2.
- Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, McCormick JB. Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics 2013;14. https://doi.org/10.1186/1472-6939-14-28.
- Friedlander JA, Loeben GS, Finnegan PK, Puma AE, Zhang X, de Zoeten EF, et al. A novel method to enhance informed consent: a prospective and randomised trial of form-based versus electronic assisted informed consent in paediatric endoscopy. J Med Ethics 2011;37:194-200. https://doi.org/10.1136/jme.2010.037622.
- Moe-Byrne T, Evans E, Benhebil N, Knapp P. The effectiveness of video animations as information tools for patients and the general public: a systematic review. Front Dig Health 2022;4. https://doi.org/10.3389/fdgth.2022.1010779.
- Saengow VE, Chancharoenchai P, Saartying W, Pimpa W, Chotichanon N, Lewsirirat T, et al. Epilepsy video animation: impact on knowledge and drug adherence in pediatric epilepsy patients and caregivers. Clin Neurol Neurosurg 2018;172:59-61. https://doi.org/10.1016/j.clineuro.2018.06.031.
- Frémont A, Abou Taam R, Wanin S, Lebras MN, Ollier V, Nathanson S, et al. Cartoons to improve young children’s cooperation with inhaled corticosteroids: a preliminary study. Pediatr Pulmonol 2018;53:1193-9. https://doi.org/10.1002/ppul.24070.
- Indradat S, Jirapongsananuruk O, Visitsunthorn N. Evaluation of animated cartoon-aided teaching of intranasal corticosteroid administration technique among Thai children with allergic rhinitis. Asian Pac J Allergy Immunol 2014;32:166-70. https://doi.org/10.12932/ap0339.32.2.2013.
- Jacobsen PB, Wells KJ, Meade CD, Quinn GP, Lee J-H, Fulp WJ, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol 2012;30:2516-21.
- Afolabi MO, Bojang K, D’Alessandro U, Imoukhuede EB, Ravinetto RM, Larson HJ, et al. Multimedia informed consent tool for a low literacy African research population: development and pilot-testing. J Clin Res Bioethics 2014;5. https://doi.org/10.4172/2155-9627.1000178.
- Warriner AH, Foster PJ, Mudano A, Wright NC, Melton ME, Sattui SE, et al. A pragmatic randomized trial comparing tablet computer informed consent to traditional paper-based methods for an osteoporosis study. Contemp Clin Trials Commun 2016;3:32-8. https://doi.org/https://doi.org/10.1016/j.conctc.2016.02.003.
- McFarlane SJ, Morgan SE, Occa A, Peng W. An evaluation of clinical trial multimedia to support Hispanic cancer patients’ informational and decision-making needs. J Cancer Educ 2021;36:110-7. https://doi.org/10.1007/s13187-019-01606-2.
- Jolly K, Sidhu M, Bower P, Madurasinghe V, Eldridge S, Graffy J, et al. Improving recruitment to a study of telehealth management for COPD: a cluster randomised controlled ‘study within a trial’ (SWAT) of a multimedia information resource. Trials 2019;20. https://doi.org/10.1186/s13063-019-3496-z.
- Blake K, Holbrook JT, Antal H, Shade D, Bunnell HT, McCahan SM, et al. Use of mobile devices and the internet for multimedia informed consent delivery and data entry in a pediatric asthma trial: study design and rationale. Contemp Clin Trials 2015;42:105-18.
- Qualtrics 2019. https://www.qualtrics.com (accessed September 2019).
- Antal H, Bunnell HT, McCahan SM, Pennington C, Wysocki T, Blake KV. A cognitive approach for design of a multimedia informed consent video and website in pediatric research. J Biomed Inform 2017;66:248-58.
- Blower S, Swallow V, Maturana C, Stones S, Phillips R, Dimitri P, et al. Children and young people’s concerns and needs relating to their use of health technology to self-manage long-term conditions: a scoping review. Arch Dis Child 2020;105:1093-104. https://doi.org/10.1136/archdischild-2020-319103.
- Treweek S, Pitkethly M, Cook J, Fraser C, Mitchell E, Sullivan F, et al. Strategies to improve recruitment to randomised trials. Cochrane Database Syst Rev 2018;2. https://doi.org/10.1002/14651858.MR000013.pub6.
- Gillies K, Kearney A, Keenan C, Treweek S, Hudson J, Brueton VC, et al. Strategies to improve retention in randomised trials. Cochrane Database Syst Rev 2021;12. https://doi.org/10.1002/14651858.MR000032.pub3.
- Treweek S, Bevan S, Bower P, Campbell M, Christie J, Clarke M, et al. Trial Forge Guidance 1: what is a study within a trial (SWAT)?. Trials 2018;19. https://doi.org/10.1186/s13063-018-2535-5.
- National Institute for Health and Care Research (NIHR) . Why the NIHR’s New Funding Stream for ‘Studies Within A Trial (SWATs) Is Potentially Game-Changing 2018.
- MRC Hubs for Trials and Methodology Research . SWAT Store 2021. https://www.qub.ac.uk/sites/TheNorthernIrelandNetworkforTrialsMethodologyResearch/SWATSWARInformation/Repositories/SWATStore/ (accessed 30 November 2021).
- Robertson T, Simonsen J. Routledge International Handbook of Participatory Design. London: Routledge; 2012.
- Clark L, Arundel C, Coleman E, . The PROMoting the USE of SWATs (PROMETHEUS) programme: Lessons learnt and future developments for SWATs. Res Methods Med Health Sci 2022;3:100-6. https://doi.org/10.1177/26320843221089632.
- Cockayne S, Fairhurst C, Adamson J, Hewitt C, Hull R, Hicks K, et al. An optimised patient information sheet did not significantly increase recruitment or retention in a falls prevention study: an embedded randomised recruitment trial. Trials 2017;18.
- Ferron Parayre A, Labrecque M, Rousseau M, Turcotte S, Legare F. Validation of SURE, a four-item clinical checklist for detecting decisional conflict in patients. Med Decis Making 2014;34:54-62.
- Légaré F, Kearing S, Clay K, Gagnon S, D’Amours D, Rousseau M, et al. Are you SURE? Assessing patient decisional conflict with a 4-item screening test. Can Fam Physician 2010;56:e308-14.
- Gillies K, Elwyn G, Cook J. Making a decision about trial participation: the feasibility of measuring deliberation during the informed consent process for clinical trials. Trials 2014;15:1-12.
- StataCorp . Stata Statistical Software: Release 16 2019.
- Martin-Kerry JM, Knapp P, Atkin K, Bower P, Watt I, Stones C, et al. Supporting children and young people when making decisions about joining clinical trials: qualitative study to inform multimedia website development. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-023984.
- Martin-Kerry J, Parker A, Bower P, Watt I, Treweek S, Torgerson D, et al. SWATted away: the challenging experience of setting up a programme of SWATs in paediatric trials. Trials 2019;20. https://doi.org/10.1186/s13063-019-3236-4.
- Coyne I. Accessing children as research participants: examining the role of gatekeepers. Child Care Health Dev 2010;36:452-4. https://doi.org/10.1111/j.1365-2214.2009.01012.x.
- Kirkby HM, Calvert M, Draper H, Keeley T, Wilson S. What potential research participants want to know about research: a systematic review. BMJ Open 2012;2.
- Fargas-Malet M, McSherry D, Larkin E, Robinson C. Research with children: methodological issues and innovative techniques. J Early Childh Res 2010;8:175-92.
- Greig AD, Taylor J, MacKay T. Doing Research with Children: A Practical Guide. London: Sage; 2012.
- Thomas N, O’Kane C. The ethics of participatory research with children. Child Soc 1998;12:336-48. https://doi.org/10.1111/j.1099-0860.1998.tb00090.x.
- Rockett M, Percival S. Thinking for Learning. Stafford, CA: Network Educational Press; 2002.
- Colucci E. “Focus groups can be fun”: the use of activity-oriented questions in focus group discussions. Qual Health Res 2007;17:1422-33.
- Flanagan SM, Greenfield S, Coad J, Neilson S. An exploration of the data collection methods utilised with children, teenagers and young people (CTYPs). BMC Res Notes 2015;8. https://doi.org/10.1186/s13104-015-1018-y.
- The Webby Awards . Webby Awards Judging Criteria 2015. http://webbyawards.com/about/webbyfact/ (accessed 12 April 2018).
- Eleftheriadou V, Thomas K, Ravenscroft J, Whitton M, Batchelor J, Williams H. Feasibility, double-blind, randomised, placebo-controlled, multi-centre trial of hand-held NB-UVB phototherapy for the treatment of vitiligo at home (HI-Light trial: Home Intervention of Light therapy). Trials 2014;15. https://doi.org/10.1186/1745-6215-15-51.
- Blair J, Gregory JW, Hughes D, Ridyard CH, Gamble C, McKay A, et al. Study protocol for a randomised controlled trial of insulin delivery by continuous subcutaneous infusion compared to multiple daily injections. Trials 2015;16.
- Miles MB, Huberman AM, Saldana J. Qualitative Data Analysis. London: Sage Publishing; 2013.
- Braun V, Clarke V. What can “thematic analysis” offer health and wellbeing researchers?. Int J Qual Stud Health Well-Being 2014;9. https://doi.org/10.3402/qhw.v9.26152.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Huby K, Swallow V, Smith T, Carolan I. Children and young people’s views on access to a web-based application to support personal management of long-term conditions: a qualitative study. Child Care Health Dev 2017;43:126-32. https://doi.org/10.1111/cch.12394.
- Breakey VR, Warias AV, Ignas DM, White M, Blanchette VS, Stinson JN. The value of usability testing for Internet-based adolescent self-management interventions: “managing hemophilia online”. BMC Med Inform Decis Mak 2013;13. https://doi.org/10.1186/1472-6947-13-113.
- Davis SR, Peters D, Calvo RA, Sawyer SM, Foster JM, Smith L. “Kiss myAsthma”: Using a participatory design approach to develop a self-management app with young people with asthma. J Asthma 2017;55:1018-27. https://doi.org/10.1080/02770903.2017.1388391.
- Cai RA, Beste D, Chaplin H, Varakliotis S, Suffield L, Josephs F, et al. Developing and Evaluating JIApp: acceptability and usability of a smartphone app system to improve self-management in young people with juvenile idiopathic arthritis. JMIR MHealth UHealth 2017;5. https://doi.org/10.2196/mhealth.7229.
- Tait AR, Geisser ME, Ray L, Hutchinson RJ, Voepel-Lewis T. Disclosing study information to children and adolescents: Is what they want, what their parents think they want?. Acad Pediat 2017;18:370-5. https://doi.org/10.1016/j.acap.2017.06.005.
- Vanhelst J, Hardy L, Bert D, Duhem S, Coopman S, Libersa C, et al. Effect of child health status on parents’ allowing children to participate in pediatric research. BMC Med Ethics 2013;14. https://doi.org/10.1186/1472-6939-14-7.
- Tait AR, Voepel-Lewis T, Malviya S. Factors that influence parents’ assessments of the risks and benefits of research involving their children. Pediatrics 2004;113:727-32.
- Grady C, Wiener L, Abdoler E, Trauernicht E, Zadeh S, Diekema DS, et al. Assent in research: the voices of adolescents. J Adolesc Health 2014;54:515-20. https://doi.org/10.1016/j.jadohealth.2014.02.005.
- Harvey M, Nongena P, Edwards D, Redshaw M. ‘We knew it was a totally at random thing’: parents’ experiences of being part of a neonatal trial. Trials 2017;18. https://doi.org/10.1186/s13063-017-2112-3.
- Woolfall K, Shilling V, Hickey H, Smyth RL, Sowden E, Williamson PR, et al. Parents’ agendas in paediatric clinical trial recruitment are different from researchers’ and often remain unvoiced: a qualitative study. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0067352.
- Luchtenberg M, Maeckelberghe E, Locock L, Powell L, Verhagen AE. Young people’s experiences of participation in clinical trials: reasons for taking part. Am J Bioethics 2015;15:3-13.
- Ingersgaard MV, Tulstrup M, Schmiegelow K, Bækgaard Larsen H. A qualitative study of decision‐making on phase iii randomized clinical trial participation in pediatric oncology: adolescents’ and parents’ perspectives and preferences. J Adv Nurs 2018;74:110-8.
- Blake DR, Lemay CA, Maranda LS, Fortenberry JD, Kearney MH, Mazor KM. Development and evaluation of a web-based assent for adolescents considering an HIV vaccine trial. AIDS Care 2015;27:1005-13.
- Niemi R, Kumpulainen K, Lipponen L. Pupils as active participants: diamond ranking as a tool to investigate pupils’ experiences of classroom practices. Eur Educ Res J 2015;14:138-50.
- Gibson F, Aldiss S, Horstman M, Kumpunen S, Richardson A. Children and young people’s experiences of cancer care: a qualitative research study using participatory methods. Int J Nurs Stud 2010;47:1397-407. https://doi.org/10.1016/j.ijnurstu.2010.03.019.
- White A, Bushin N, Carpena-Méndez F, Ní Laoire C. Using visual methodologies to explore contemporary Irish childhoods. Qual Res 2010;10:143-58. https://doi.org/10.1177/1468794109356735.
- Crilly N, Blackwell AF, Clarkson PJ. Graphic elicitation: using research diagrams as interview stimuli. Qual Res 2006;6:341-66. https://doi.org/10.1177/1468794106065007.
- Lobinger K, Brantner C. The Sage Handbook of Visual Research Methods. London: Sage; 2020.
- Woolner P, Clark J, Hall E, Tiplady L, Thomas U, Wall K. Pictures are necessary but not sufficient: using a range of visual methods to engage users about school design. Learn Env Res 2010;13:1-22.
- Morris J. Including all children: finding out about the experiences of children with communication and/or cognitive impairments. Child Soc 2003;17:337-48. https://doi.org/10.1002/chi.754.
- Boggis A. Deafening silences: researching with inarticulate children. Disab Stud Quart 2011;31.
- Fängström K, Salari R, Eriksson M, Sarkadi A. The computer-assisted interview in my shoes can benefit shy preschool children’s communication. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0182978.
- Lewis J, McNaughton Nicholls C. Qualitative Research Practice. London: Sage; 2014.
- Michel L. Developing Focus Group Research. London: Sage; 2011.
- Lambert SD, Loiselle CG. Combining individual interviews and focus groups to enhance data richness. J Adv Nurs 2008;62:228-37. https://doi.org/10.1111/j.1365-2648.2007.04559.x.
- Sheridan R, Martin-Kerry J, Watt I, Higgins S, Stones SR, Taylor DH, et al. User testing digital, multimedia information to inform children, adolescents and their parents about healthcare trials. J Child Health Care: Prof Work Child Hosp Commun 2018;23:468-82. https://doi.org/10.1177/1367493518807325.
- Raynor DK. User testing in developing patient medication information in Europe. Res Social Adm Pharm 2013;9:640-5. https://doi.org/10.1016/j.sapharm.2013.02.007.
- Raynor D, Myers L, Blackwell K, Kress B, Dubost A, Joos A. Clinical trial results summary for laypersons: a user testing study. Therap Innov Reg Sci 2018;52:606-28.
- Yamamoto M, Doi H, Yamamoto K, Watanabe K, Sato T, Suka M, et al. Adaptation of the European Commission-recommended user testing method to patient medication information leaflets in Japan. Drug Healthc Patient Saf 2017;9:39-63.
- Knapp P, Wanklyn P, Raynor D, Waxman R. Developing and testing a patient information booklet for thrombolysis used in acute stroke. Int J Pharm Pract 2010;18:362-9.
- Coleman E, O’Sullivan L, Crowley R, Hanbidge M, Driver S, Kroll T, et al. Preparing accessible and understandable clinical research participant information leaflets and consent forms: a set of guidelines from an expert consensus conference. Research Involvement and Engagement 2021;7. https://doi.org/10.1186/s40900-021-00265-2.
- Korus M, Cruchley E, Stinson J, Gold A, Anthony S. Usability testing of the internet program: “teens taking charge: managing my transplant online”. Pediatr Transplant 2015;19:107-17.
- Nitsch M, Dimopoulos CN, Flaschberger E, Saffran K, Kruger JF, Garlock L, et al. A guided online and mobile self-help program for individuals with eating disorders: an iterative engagement and usability study. J Med Internet Res 2016;18.
- Andersen MH, Khalid MS, Brooks EI. Considerations and Methods for Usability Testing with Children n.d.:228-38.
- O’Lonergan TA, Forster-Harwood JE. Novel approach to parental permission and child assent for research: improving comprehension. Pediatrics 2011;127:917-24.
- Baker JN, Leek AC, Salas HS, Drotar D, Noll R, Rheingold SR, et al. Suggestions from adolescents, young adults, and parents for improving informed consent in phase 1 pediatric oncology trials. Cancer 2013;119:4154-61.
- Tait AR, Voepel-Lewis T, Levine R. Using digital multimedia to improve parents’ and children’s understanding of clinical trials. Arch Dis Child 2015;100:589-93. https://doi.org/10.1136/archdischild-2014-308021.
- Sheridan R, Preston J, Stones S, Ainsworth S, Horton-Taylor D, Challinor R, et al. Patient and public involvement in a study of multimedia clinical trial information for children, young people and families. Research All 2020;4:47-65. https://doi.org/10.18546/RFA.04.1.05.
- Buck D, Gamble C, Dudley L, Preston J, Hanley B, Williamson PR, et al. EPIC Patient Advisory Group . From plans to actions in patient and public involvement: qualitative study of documented plans and the accounts of researchers and patients sampled from a cohort of clinical trials. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014-006400.
- Achten J, Knight R, Dutton SJ, Costa ML, Mason J, Dritsaki M, et al. A multicentre prospective randomized equivalence trial of a soft bandage and immediate discharge versus current treatment with rigid immobilization for torus fractures of the distal radius in children. Bone Joint Open 2020;1:214-21. https://doi.org/10.1302/2633-1462.16.Bjo-2020-0014.R1.
- Azuara-Blanco A, Logan N, Strang N, Saunders K, Allen PM, Weir R, et al. Low-dose (0.01%) atropine eye-drops to reduce progression of myopia in children: a multicentre placebo-controlled randomised trial in the UK (CHAMP-UK) – study protocol. Br J Ophthalmol 2020;104:950-5. https://doi.org/10.1136/bjophthalmol-2019-314819.
- Marcus MW, de Vries MM, Montolio FGJ, Jansonius NM. Myopia as a risk factor for open-angle glaucoma: a systematic review and meta-analysis. Ophthalmology 2011;118:1989.e-94.e2.
- Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res 2012;31:622-60. https://doi.org/10.1016/j.preteyeres.2012.06.004.
- Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev 2011;12. https://doi.org/10.1002/14651858.CD004916.pub3.
- CHAMP UK . Low-Dose Atropine Eye Drops to Reduce Progression of Short-Sightedness (Myopia) in Children in the United Kingdom 2018. https://doi.org/10.1186/ISRCTN99883695.
- Heys R, Stoica S, Angelini G, Beringer R, Evans R, Ghorbel M, et al. Intermittent antegrade warm-blood versus cold-blood cardioplegia in children undergoing open heart surgery: a protocol for a randomised controlled study (Thermic-3). BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2020-036974.
- Baue AE, Geha AS. Glenn’s Thoracic and Cardiovascular Surgery. New York: Appleton & Lange; 1996.
- Castaneda AR, Jonas RA, Mayer JE. Cardiac Surgery of the Neonate And Infant. St. Louis, MO: WB Saunders Company; 1994.
- Hammon JW. Myocardial protection in the immature heart. Ann Thorac Surg 1995;60:839-42.
- Gaies M, Pasquali SK, Donohue JE, Dimick JB, Limbach S, Burnham N, et al. Seminal postoperative complications and mode of death after pediatric cardiac surgical procedures. Ann Thorac Surg 2016;102:628-35.
- Pelletier LC, Carrier M, Leclerc Y, Cartier R, Wesolowska E, Solymoss BC. Intermittent antegrade warm versus cold blood cardioplegia: a prospective, randomized study. Ann Thorac Surg 1994;58:41-8.
- Caputo M, Bryan A, Calafiore A, Suleiman M-S, Angelini G. Intermittent antegrade hyperkalaemic warm blood cardioplegia supplemented with magnesium prevents myocardial substrate derangement in patients undergoing coronary artery bypass surgery. Eur J Cardiothorac Surg 1998;14:596-601.
- NCT . Binocularly Balanced Viewing Study 2018. https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01918476/full (accessed 20 November 2021).
- Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J. The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4-5 years: a systematic review and economic evaluation. Health Technol Assess 2008;12:iii, xi-194. https://doi.org/10.3310/hta12250.
- Powell C, Hatt SR. Vision screening for amblyopia in childhood. Cochrane Database Syst Rev 2009. https://doi.org/10.1002/14651858.CD005020.pub3.
- Awan M, Proudlock FA, Grosvenor D, Choudhuri I, Sarvanananthan N, Gottlob I. An audit of the outcome of amblyopia treatment: a retrospective analysis of 322 children. Br J Ophthalmol 2010;94:1007-11. https://doi.org/10.1136/bjo.2008.154674.
- Stewart CE, Fielder AR, Stephens DA, Moseley MJ. Treatment of unilateral amblyopia: factors influencing visual outcome. Invest Ophthalmol Vis Sci 2005;46:3152-60. https://doi.org/10.1167/iovs.05-0357.
- Health Research Authority . Bone Anchored Maxillary Protraction (BAMP)RCT 2015. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/bone-anchored-maxillary-protraction-bamprct/ (accessed 25 November 2021).
- Mandall N. A Clinical Trial to Assess the Effectiveness of Miniplate Surgical Treatment to Bring the Upper Jaw Forwards in 11–14 Years Old Children 2014. https://doi.org/10.1186/ISRCTN93900866.
- UKALL-2011 . United Kingdom National Randomised Trial for Children and Young Adults With Acute Lymphoblastic Leukaemia and Lymphoma 2011. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/ukall-2011/ (accessed 12 October 2021).
- Jackson RK, Liebich M, Berry P, Errington J, Liu J, Parker C, et al. Impact of dose and duration of therapy on dexamethasone pharmacokinetics in childhood acute lymphoblastic leukaemia: a report from the UKALL-2011 trial. Eur J Cancer 2019;120:75-8. https://doi.org/10.1016/j.ejca.2019.07.026.
- Treweek S, Bevan S, Bower P, Briel M, Campbell M, Christie J, et al. Trial Forge Guidance 2: how to decide if a further Study Within A Trial (SWAT) is needed. Trials 2020;21. https://doi.org/10.1186/s13063-019-3980-5.
- Knapp P, Heys R, Dabner L, Sheehan K, Smartt H, Walker-Smith T, et al. The effects of multimedia information on recruitment and retention in a children's cardiac surgery trial: a randomised controlled SWAT (study within a trial) [version 1; peer review: awaiting peer review]. F1000Research 2022;11. https://doi.org/10.12688/f1000research.110083.1.
- Moe-Byrne T, Knapp P, Perry D, Achten J, Spoors L, Appelbe D, et al. Does digital, multimedia information increase recruitment and retention in a children’s wrist fracture treatment trial, and what do people think of it? A randomised controlled Study Within A Trial (SWAT). BMJ Open 2022;12. https://doi.org/10.1136/bmjopen-2021-057508.
Appendix 1 FORCE SWAT DMQ analysis tables
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| FORCE (P/F) |
P/F: N = 157 31.2 (4.9) |
P/F: N = 151 31.3 (4.5) |
– | P/F: N = 308 31.3 (4.7) |
P/F: N = 308 AMD: 0.07 95% CI (–1.08 to 1.22) p = 0.91 |
– |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| FORCE (P/F) |
P/F: N = 156 31.2 (4.9) |
P/F: N = 146 31.3 (4.5) |
– | P/F: N = 302 31.3 (4.7) |
P/F: N = 302 AMD: 0.11 95% CI (–0.95 to 1.19) p = 0.83 |
– |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| FORCE (P/F) |
P/F: N = 143 31.2 (4.8) |
P/F: N = 116 31.1 (4.8) |
– | P/F: N = 259 31.2 (4.8) |
P/F: N = 259 AMD: –0.10 95% CI (–1.30 to 1.11) p = 0.88 |
– |
| Question | Allocation | N | Median (IQR) | Z-statistic | p-value |
|---|---|---|---|---|---|
| 1. The information I saw about the FORCE trial was easy to understand. | PIS | 157 | 3 (1) | 2.60 | 0.010 |
| MMI | 151 | 4 (1) | |||
| 2. The information helped me understand what it would be like for my son or daughter to take part in the FORCE study. | PIS | 157 | 4 (1) | 0.79 | 0.446 |
| MMI | 152 | 4 (1) | |||
| 3. The information helped me understand how my son’s or daughter’s treatment or care might change if s/he took part in the FORCE study. | PIS | 157 | 4 (1) | 0.87 | 0.387 |
| MMI | 152 | 4 (1) | |||
| 4. The possible benefits of taking part in the FORCE trial were made clear in the information. | PIS | 157 | 4 (1) | –0.37 | 0.714 |
| MMI | 151 | 4 (1) | |||
| 5. The possible disadvantages of taking part in the FORCE trial were made clear in the information. | PIS | 157 | 3 (2) | –1.34 | 0.180 |
| MMI | 151 | 3 (2) | |||
| 6. The information about the FORCE trial helped me discuss the trial with the person who asked my son or daughter to take part (usually a doctor, nurse or researcher). | PIS | 157 | 4 (1) | 0.53 | 0.603 |
| MMI | 151 | 4 (1) | |||
| 7. The information about the FORCE study helped me discuss taking part with my son or daughter. | PIS | 157 | 4 (1) | –0.13 | 0.909 |
| MMI | 151 | 4 (1) | |||
| 8. I am confident that I have made the right decision about whether or not my son or daughter should take part in the FORCE study. | PIS | 157 | 4 (1) | –0.34 | 0.733 |
| MMI | 151 | 4 (1) | |||
| 9. In all, the information about the FORCE trial helped me make my decision about whether or not my son or daughter should take part. | PIS | 157 | 4 (1) | –0.39 | 0.700 |
Appendix 2 CHAMP-UK DMQ analysis
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| CHAMP (young and P/F) |
Young: – P/F: N = 26 32.2 (3.4) |
Young: – P/F: N = 31 29.7 (4.6) |
Young: – P/F: N = 24 30.0 (3.8) |
Young: N = 1 – P/F: N = 81 30.6 (4.1) |
Young: – P/F: N = 57 AMD: –2.43 95% CI (–4.61 to –0.24) p = 0.03 |
Young: – P/F: N = 50 AMD: –2.11 95% CI (–4.23 to 0.01) p = 0.05 |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| CHAMP (young and P/F) |
Young: – P/F: N = 26 32.2 (3.4) |
Young: – P/F: N = 30 29.6 (4.7) |
Young: – P/F: N = 22 29.7 (3.8) |
Young: N = 1 – P/F: N = 78 30.5 (4.2) |
Young: – P/F: N = 56 AMD: –2.53 95% CI (–4.73 to –0.32) p = 0.03 |
Young: – P/F: N = 48 AMD: –2.38 95% CI (–4.52 to –0.23) p = 0.03 |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| CHAMP (young and P/F) |
Young: – P/F: N = 19 31.9 (3.5) |
Young: – P/F: N = 22 30.8 (4.2) |
Young: – P/F: N = 14 30.6 (3.7) |
Young: N = 1 – P/F: N = 55 31.1 (3.8) |
Young: – P/F: N = 41 AMD: –1.12 95% CI (–3.58 to 1.34) p = 0.36 |
Young: – P/F: N = 33 AMD: –1.26 95% CI (–3.81 to 1.29) p = 0.32 |
| Question | Allocation | N | Median (IQR) | Z-statistic | p-value |
|---|---|---|---|---|---|
| 1. The information I saw about the CHAMP-UK trial was easy to understand. | PIS | 26 | 4 (1) | –0.24 | 0.904 |
| MMI | 31 | 4 (1) | |||
| 2. The information helped me understand what it would be like for my son or daughter to take part in the CHAMP-UK study. | PIS | 26 | 4 (1) | –0.56 | 0.765 |
| MMI | 31 | 4 (1) | |||
| 3. The information helped me understand how my son’s or daughter’s treatment or care might change if s/he took part in the CHAMP-UK study. | PIS | 26 | 4 (1) | 1.62 | 0.134 |
| MMI | 31 | 3 (1) | |||
| 4. The possible benefits of taking part in the CHAMP-UK trial were made clear in the information. | PIS | 26 | 4 (1) | 0.48 | 0.684 |
| MMI | 31 | 4 (1) | |||
| 5. The possible disadvantages of taking part in the CHAMP-UK trial were made clear in the information. | PIS | 26 | 3 (1) | 2.81 | 0.005 |
| MMI | 31 | 3 (2) | |||
| 6. The information about the CHAMP-UK trial helped me discuss the trial with the person who asked my son or daughter to take part (usually a doctor, nurse or researcher). | PIS | 26 | 3 (1) | 1.08 | 0.287 |
| MMI | 31 | 3 (2) | |||
| 7. The information about the CHAMP-UK study helped me discuss taking part with my son or daughter. | PIS | 26 | 4 (1) | 1.01 | 0.364 |
| MMI | 31 | 4 (1) | |||
| 8. I am confident that I have made the right decision about whether or not my son or daughter should take part in the CHAMP-UK study. | PIS | 26 | 4 (0) | 2.69 | 0.008 |
| MMI | 31 | 3 (1) | |||
| 9. In all, the information about the CHAMP-UK trial helped me make my decision about whether or not my son or daughter should take part. | PIS | 26 | 4 (0) | 2.68 | 0.012 |
Appendix 3 Thermic-3 SWAT DMQ analysis
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| THERMIC-3 (all three) |
Young: – Older: – P/F: N = 8 29.5 (6.9) |
Young: – Older: – P/F: N = 6 31.8 (4.4) |
Young: – Older: – P/F: N = 3 28.3 (4.9) |
Young: – Older: – P/F: N = 17 30.1 (5.7) |
Young: – Older: – P/F: N = 14 AMD: 0.73 (–5.34 to 6.80) p = 0.80 |
Young: – Older: – P/F: N = 11 AMD: 0.72 (–10.43, 7.52) p = 0.72 |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| THERMIC-3 (all three) |
Young: – Older: – P/F: N = 6 28.8 (8.0) |
Young: – Older: – P/F: N = 6 31.8 (4.4) |
Young: – Older: – P/F: N = 3 28.3 (4.9) |
Young: – Older: – P/F: N = 15 29.9 (6.0) |
Young: – Older: – P/F: N = 12 AMD: 1.35 (–5.06 to 7.75) p = 0.65 |
Young: – Older: – P/F: N = 9 AMD: –0.50 (–10.72 to 9.72) p = 0.91 |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| THERMIC-3 (all three) |
Young: – Older: – P/F N = 5 32.3 (2.1) |
Young: – Older: – P/F N = 5 33.2 (3.3) |
Young: – Older: – P/F N = 2 30.0 (5.7) |
Young: – Older: – P/F N = 12 32.3 (3.1) |
Young: - Older: – P/F N = 10 AMD: 0.90 95% CI (–3.11 to 4.91) p = 0.62 |
Young: – Older: – P/F N = 7 AMD: –2.30 95% CI (–9.09 to 4.49) p = 0.42 |
Appendix 4 BALANCE SWAT DMQ analysis
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| BALANCE (young and P/F) |
Young: – P/F: – |
Young: – P/F: – |
Young: – P/F: – |
Young: N = 4 8.8 (2.2) P/F: N = 9 30.1 (4.0) |
Young: – P/F: – |
Young: – P/F: – |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| BALANCE (young and P/F) |
Young: – P/F: – |
Young: – P/F: – |
Young: – P/F: – |
Young: N = 4 8.8 (2.2) P/F: N = 9 30.1 (4.0) |
Young: – P/F: – |
Young: – P/F: – |
Appendix 5 BAMP DMQ analysis
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| BAMP (older) |
Older: N = 3 29.0 (8.9) |
Older: N = 3 33.7 (2.1) |
Older: N = 4 31.3 (4.4) |
Older: N = 10 31.3 (5.4) |
Older: N = 6 AMD: 1.67 95% CI (–24.59 to 27.92) p = 0.85 |
Older: N = 7 AMD: –0.75 95% CI (–22.88 to 21.37) p = 0.93 |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| BAMP (older) |
Older: N = 1 – |
Older: N = 3 33.7 (2.1) |
Older: N = 4 31.3 (4.4) |
Older: N = 8 32.3 (3.3) |
Older: N = 4 AMD: 1.67 95% CI (–8.68 to 12.01) p = 0.56 |
Older: N = 5 AMD: –0.75 95% CI (–16.5 to 15.0) p = 0.89 |
Appendix 6 UKALL-2011 SWAT DMQ analysis
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| UKALL (all three) |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: N = 0 Older: N = 2 19.5 (2.1) P/F: N = 3 21.3 (14.6) |
– | – |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| UKALL (all three) |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: N = 0 Older: N = 2 19.5 (2.1) P/F: N = 2 21.0 (10.6) |
– | – |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| UKALL (all three) |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: – Older: – P/F: – |
Young: N = 0 Older: N = 2 19.5 (2.1) P/F: N = 3 21.3 (14.6) |
– | – |
Appendix 7 BAMP substudy DMQ analysis
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| BAMP substudy (older) |
Older: N = 52 27.0 (4.3) |
Older: N = 52 28.1 (4.2) |
– | Older: N = 104 27.5 (4.3) |
Older: N = 104 AMD: 1.08 (–0.59 to 2.75) p = 0.20 |
– |
| Trial | PIS | MMI | Both | Overall | MMI vs. PIS | Both vs. PIS |
|---|---|---|---|---|---|---|
| BAMP substudy (older) |
Older: N = 51 27.0 (4.4) |
Older: N = 52 28.1 (4.2) |
– | Older: N = 104 27.5 (4.3) |
Older: N = 103 AMD: 1.06 (–0.63 to 2.74) p = 0.22 |
– |
| Question | Allocation | N | Median (IQR) | Z-statistic | p-value |
|---|---|---|---|---|---|
| Q1 The information I saw about the BAMP trial was easy to understand. | ISP | 52 | 3 (1) | –3.03 | 0.002 |
| Multimedia | 52 | 3 (0.5) | |||
| Q2 After seeing the information about the BAMP trial I knew what taking part would be like. | ISP | 52 | 3 (1) | –0.13 | 0.940 |
| Multimedia | 52 | 3 (1) | |||
| Q3 The information helped me understand how my treatment or care might change if I took part in the BAMP trial. | ISP | 51 | 3 (0) | –0.52 | 0.601 |
| Multimedia | 52 | 3 (1) | |||
| Q4 The possible benefits of taking part in the BAMP trial were made clear in the information. | ISP | 52 | 3 (1) | –0.53 | 0.602 |
| Multimedia | 52 | 3 (1) | |||
| Q5 The possible disadvantages of taking part in the BAMP trial were made clear in the information. | ISP | 52 | 3 (1.5) | –0.92 | 0.362 |
| Multimedia | 52 | 3 (1) | |||
| Q6 The information about the BAMP trial helped me discuss the trial with the person who asked me to take part (usually a doctor, nurse or researcher). | ISP | 52 | 3 (0) | –0.04 | 0.981 |
| Multimedia | 52 | 3 (2) | |||
| Q7 The information about the BAMP trial helped me discuss taking part with my parent(s) or family. | ISP | 52 | 3 (1) | –0.50 | 0.647 |
| Multimedia | 52 | 3 (0) | |||
| Q8 I am confident that I have made the right decision about whether or not to take part in the BAMP trial. | ISP | 52 | 3 (0.5) | –2.00 | 0.044 |
| Multimedia | 52 | 3 (1) | |||
| Q9 In all, the information about the BAMP trial helped me make my decision about whether or not to take part. | ISP | 52 | 3 (1) | –1.41 | 0.160 |
Appendix 8 Qualitative study participants
| CYP | |||
|---|---|---|---|
| Participant ID | Gender | Age (years) | Experience of being approached about a children’s trial |
| Parents/carers | |||
| Participant ID | Gender | Experience of being approached about a children’s trial | |
| Professionals | |||
| Participant ID | Gender | Current role | |
| CYP/16 | Male | 10 | Yes |
| CYP/18 | Female | 18 | Yes |
| CYP/19 | Female | 16 | No |
| CYP/20 | Male | 18 | No |
| CYP/21 | Female | 15 | No |
| CYP/23 | Female | 16 | Yes |
| CYP/25 | Male | 9 | Yes |
| CYP/29 | Female | 12 | No |
| CYP/31 | Female | 19 | Yes |
| CYP/35 | Female | 16 | No |
| CYP/39 | Female | 14 | No |
| CYP/43 | Male | 9 | Yes |
| CYP/44 | Male | 6 | No |
| CYP/47 | Male | 6 | No |
| CYP/49 | Male | 7 | Yes |
| CYP/51 | Female | 15 | Yes |
| CYP/53 | Female | 16 | No |
| CYP/55 | Female | 6 | No |
| CYP/57 | Female | 6 | Yes |
| CYP/59 | Male | 13 | No |
| CYP/62 | Female | 8 | Yes |
| Parent/17 | Female | Yes | |
| Parent/22 | Female | Yes | |
| Parent/24 | Female | Yes | |
| Parent/26 | Female | Yes | |
| Parent/27 | Male | Yes | |
| Parent/28 | Male | No | |
| Parent/30 | Female | No | |
| Parent/32 | Female | No | |
| Parent/33 | Male | No | |
| Parent/34 | Female | No | |
| Parent/36 | Female | No | |
| Parent/37 | Female | Yes | |
| Parent/38 | Female | No | |
| Parent/41 | Female | Yes | |
| Parent/42 | Male | Yes | |
| Parent/46 | Female | Yes | |
| Parent/48 | Male | Yes | |
| Parent/50 | Female | Yes | |
| Parent/52 | Male | No | |
| Parent/54 | Female | No | |
| Parent/56 | Female | No | |
| Parent/58 | Male | Yes | |
| Parent/60 | Male | No | |
| Parent/61 | Female | Yes | |
| Professional/1 | Female | Paediatric rheumatology consultant | |
| Professional/2 | Female | Paediatric rheumatology consultant | |
| Professional/3 | Female | Paediatric renal consultant | |
| Professional/4 | Female | Paediatric intensive care nurse | |
| Professional/5 | Female | Operations manager | |
| Professional/6 | Female | Paediatric research nurse | |
| Professional/7 | Female | Research governance (previous researcher) | |
| Professional/8 | Female | Research pharmacist | |
| Professional/9 | Female | Research nurse | |
| Professional/10 | Female | Research nurse | |
| Professional/11 | Male | Research nurse | |
| Professional/12 | Female | Research nurse | |
| Professional/13 | Female | Data manager (involved in recruitment) | |
| Professional/14 | Male | Paediatric respiratory consultant | |
| Professional/15 | Male | Paediatric respiratory consultant | |
| Professional/40 | Female | Play specialist | |
| Professional/45 | Male | Paediatric pharmacist | |
List of abbreviations
- AMD
- adjusted mean difference
- ALL
- acute lymphoblastic leukaemia
- BAMP
- Bone Anchored Maxillary Protraction
- CONSORT
- Consolidated Standards of Reporting Trials
- CTIMP
- Clinical Trial of a Medicinal Product
- CYP
- children and young people
- DMQ
- Decision-Making Questionnaire
- GP
- general practitioner
- HRA
- Health Research Authority
- ITT
- intention-to-treat
- LBL
- lymphoblastic lymphoma
- MMI
- multimedia information resource
- NIHR
- National Institute for Health and Care Research
- PIS
- printed participant information sheet
- PPAG
- Patient and Parent Advisory Group
- PPI
- patient and public involvement
- RACH
- risk adjustment for congenital heart surgery
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAG
- Study Advisory Group
- SCIPI
- subcutaneous insulin: pumps or injections
- SWAT
- Study Within A Trial
- TRECA
- TRials Engagement in Children and Adolescents
- YPAG
- Young Persons’ Advisory Group