Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as award number 16/116/43. The contractual start date was in March 2019. The draft manuscript began editorial review in March 2023 and was accepted for publication in March 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Walsh et al. This work was produced by Walsh et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Walsh et al.
Chapter 1 Introduction and overview
Frailty is a state of vulnerability to stressors that becomes increasingly common with age and is strongly associated with adverse outcomes, such as high service use, unplanned admissions and transfer to residential care. 1–4 Frailty is characterised by reduced physiological reserves and vulnerability to internal (e.g. infection) and external (e.g. changed environment) stressors. 4–6 Inflammatory processes driven by ageing and chronic disease lead to physiological and psychological decline (loss of strength, reduced cognitive function, atypical symptoms, sensory decline). In turn, as these pathological changes accumulate, functional decline and common health problems of old age, such as falls, mobility problems, reduced appetite, incontinence and depression/anxiety emerge. Frailty worsens as these deficits accumulate and is associated with higher mortality, dependency, residential care transition, high service use, emergency admissions and reduced quality of life. 1–3,7–9
Frailty will become more prevalent as populations age, with estimates of prevalence of frailty in the population aged over 60 at 14%, rising to 65% of those aged 90 and over. 5 It is also known that frailty is more prevalent in women and is associated with deprivation and multiple morbidity, but less is known about the impact of sociodemographic factors on frailty progression. Frail older people use considerable resources in the last 12 months of life,5,7 suggesting delayed identification and potential for earlier intervention, but also possible differences in frailty trajectories with age or multiple morbidity.
As populations age and prevalence of frailty increases, the impact of frailty on demand for and outcomes of care has become a significant issue for health services worldwide, including the NHS. The delivery of appropriate services to support people with frailty will be key to providing cost-effective, quality care for older people, especially in the context of reduced health service resources and rising demand for unplanned care. Identification of frailty in the primary care population has therefore been introduced into primary care, where it is likely to be a useful adjunct to existing risk stratification approaches. 1,2 There is evidence to support interventions, such as Comprehensive Geriatric Assessment (CGA), which have the potential to slow or prevent decline. 3,10 While consensus guidelines have emphasised the importance of identification and clinical management of frailty1,11 and delivery of effective interventions, such as CGA, resource restrictions in the NHS mean that capacity and workforce for delivery of frailty care are limited; recent data indicate 133,446 unfilled posts in health care and 165,000 in social care. 12,13 Capacity is further limited by the evidence gap in relation to the planning, commissioning and delivery of services for older people living with frailty. 3 There is a lack of evidence to support commissioners and service providers, who will need guidance on the future scale and mix of services required across the spectrum of frailty. Guidelines for management of frailty acknowledge a lack of evidence on which to base service design and commissioning. 2 There are unanswered questions about the incidence and prevalence of different levels of frailty at population or regional level and their consequences for health and care service use and costs. Little is known about the progression of frailty in the general older population and how this will impact on service demand as the population ages. There is also an evidence gap around frailty onset, with most frailty research in those aged 65 and over (often 75 and above) and little information on incidence in middle age. There is a need to better understand population trends in the development and impact of frailty, which can only be achieved by exploration of large-scale, routine healthcare data. A recent review3 noted that improved knowledge about prevalence could aid commissioners and service providers to plan more effectively for frail older people. Further evidence is needed around the dynamics of frailty within the population and the impact frailty has on demand. This research therefore addresses significant gaps in the evidence relating to the population burden of frailty and its impact on healthcare use and costs over time, informing recommendations for service providers and commissioners.
Aims and objectives
The overarching aim of this study was to explore trends in onset and progression of frailty, and the dynamics of frailty-related healthcare demand, outcomes and costs in the older general practice population, to inform the development of guidelines and tools to facilitate commissioning and service development for this patient group.
Specifically, we explored trends in development, prevalence and progression of frailty, in an ageing cohort of people aged 50 and over from primary care in England. Frailty-related healthcare demand and outcomes were explored through analysis of linked secondary care and mortality data. These analyses informed guidance for service planners and commissioners (in preparation). They also underpinned development of a simulation model of the progression and impact of frailty within the ageing population (see Chapter 6). The simulation model allows exploration of projected demand and costs into the future and exploration of ‘what-if’ scenarios for different demographic trends and service use and organisation patterns (see Chapter 7).
Study objectives were:
-
identification of incidence and prevalence of frailty states in an ageing population
-
identification of frailty trajectories and transitions in severity in the older population over time
-
exploration of drivers of progression of frailty, including clinical, socioeconomic and demographic factors
-
examination of the impact of frailty on service use, costs and pathways of care
-
exploration of the relationship between frailty status, socioeconomic factors, practice factors and service use and outcomes (mortality, unplanned admissions, residential care use)
-
prediction of trends in frailty, modelling of health and care demand and costs over time and in different service contexts.
Overarching design approach
We used a retrospective observational study design, with statistical and simulation modelling, using routine healthcare data from primary and secondary care. Population prevalence, incidence and trajectories of decline in frailty were determined within an ageing cohort using the electronic Frailty Index (eFI) tool and data from the Royal College of General Practitioners Research and Surveillance Centre (RCGP RSC) database, with additional linked data from Hospital Episode Statistics (HES) and Office for National Statistics (ONS) to provide information on hospital attendances and mortality. Relationships between demographic factors, practice characteristics, outcomes, service use and costs were explored for frailty (eFI score) strata [fit (< 0.12), mild (0.12 to < 0.24), moderate (0.24 to < 0.36) and severe (0.36 and above)]. These analyses informed the development of a population model for simulation of trends and exploration of ‘what-if’ scenarios. The population model was internally validated using the English RCGP RSC data set and externally validated using a comparable set of Welsh data from the Secure Anonymised Information Linkage (SAIL) Databank before being populated with population data for England.
Simulation modelling explored the impact of demographic factors on frailty prevalence and impact over time within the specified population. The impact of different demographic and service drivers was explored via modelling of ‘what-if’ scenarios developed with the Stakeholder Engagement Group (SEG) (see Chapter 5).
The project was broken down into the following workstreams (see Appendix 1, Figure 16):
-
Workstream 1: statistical modelling of population trends, incidence and prevalence of frailty, stratification of frailty and related outcomes, resource use and costs [Study Outcome (SO) 1–5].
-
Workstream 2: validation of the population model (SO 1,2,5).
-
Workstream 3: stakeholder engagement (SO 3,4,6).
-
Workstream 4: simulation modelling to explore impact of different service and demographic scenarios on population trends, service demand and costs in the future (SO 4,5,6).
A detailed account of the methods for Workstream 1 is presented in Chapter 3, with the results from this work presented in Chapter 4. Workstream 3, stakeholder engagement andpatient and public involvement (PPI), is presented in Chapter 5. Due to the iterative nature of the simulation modelling in Workstream 4, which used data emerging from Workstreams 1–3, an integrated account of the development and validation of the simulation model is presented in Chapter 6, with results of the modelling exercise in Chapter 7.
Ethics and governance
This study utilised routinely collected NHS patient data where explicit consent had not been gained from participants, provided by trusted third-party databanks. Following guidance from Health Research Authority (HRA) and Confidentiality Advisory Group (CAG), we determined that the use of non-identifiable, previously collected routine data extracts from the independent databank organisations (RCGP RSC, SAIL, NHS Digital, ONS) did not require formal ethical approval by an NHS REC or CAG. Although NHS ethical approvals were not required because this research used de-identified, routine data extracts from trusted third-party providers, ethical approval for these analyses was obtained from the Faculty of Environmental and Life Sciences Ethics Committee at the University of Southampton for access to routine, de-identified primary care data and de-identified primary, secondary and social care data from RCGP RCP, SAIL, NHS Digital and ONS (University of Southampton Ethics Committee ERGO II 46313).
We used approved information governance procedures for database access at each database organisation, including use of secure servers and independent data analysts for data extraction and linkage of de-identified data (see Chapters 3 and 6 for specific arrangements for each data source). Only anonymised, aggregate data extracts were exported from database secure servers.
Procedures for maintaining confidentiality were as per usual standard for data of this type; all databank organisations collate pseudonymised data with direct patient identifiers removed; data extracts and aggregate analyses were further de-identified as described; the research team did not seek individual patient identifiers; where required, data linkage was achieved through ‘hashing’ algorithms to generate non-identifiable, unique IDs from identifiable data; as a further protection, non-reversible, pseudonymised ID numbers held by database organisations were converted to unique study identifiers (IDs), the keys to which were not accessible to the research team; and, when using these data, we suppressed small numbers in reporting and avoided the presentation of data that could potentially be used to reveal identities. As this study was focused on the modelling of population flows and service use, a high level of data aggregation was used in the analyses and we did not encounter any issues in relation to potential breaches of confidentiality.
The study was registered on an appropriate register for observational studies – https://clinicaltrials.gov/, identifier NCT04139278.
This study was approved by the University of Southampton Research Ethics Committee (ERGO II 46313) on 6 February 2019, the RCGP RSC Information Governance Panel on 24 January 2019 and Data Access Request Service (DARS) IGARD panel on 19 April 2021. It was approved by the SAIL Information Governance Review Panel (IGRP) on 3 December 2020.
Patient and public involvement
Identification and management of older people with frailty in primary care are required under the general practitioner (GP) contract. 15 However, there is a lack of evidence to support service and workforce planning. At the same time, there are clear public concerns about the quality, appropriateness and accessibility of services for older people. Consumers of this research are likely to be at the commissioning and service planning level and patient and public engagement are vital in ensuring that both this study and future service development are guided by patient and carer priorities.
In developing this study, we were advised by the PPI Officer with Research Design Service (RDS) South Central. We recruited a lead PPI representative to the study who had experience in four previous projects about frailty. The PPI representative brought a range of personal and professional expertise relevant to this study: as a carer; as a parliamentary researcher, including on healthcare issues; as a parish and now a city councillor where she sat on her local Council Health and Well-being panel. This combination of experiences was particularly suited to this project with its service organisation perspective.
The PPI representative contributed to development of the study and funding application, confirming the importance of the study topic and assisting in focusing the development of the proposal, particularly in relation to study outputs and the role of the SEG. The PPI representative acted as a member of the SEG, led by co-applicant Abigail Barkham (Consultant Nurse for Frailty). Members of the Health Sciences Older People and Dementia Research Patient and Carer Panel also participated in stakeholder engagement events, alongside representatives from third sector and social care organisations. We benefitted from the PPI representative’s assistance in recruiting a wide network of patients, carers and professionals to our stakeholder events and activities, giving a broad insight into patient experience and challenges. Further details of the stakeholder engagement process can be found in Chapter 5.
The PPI representative also contributed to the dissemination strategy and helped guide the research team on formulating messages and media aimed at the wider public. In addition, the simulation modelling component of the proposed study drew on the feedback from the SEG events, the final one of which focused on emerging findings and identification of future research and practice priorities.
Study outcomes and outputs
The development of the eFI8 allowed routine primary care data to be used to identify the presence and severity of frailty in real-world populations. This study utilised the eFI to address a number of evidence gaps, through exploration of the dynamics of frailty at population level, stratifying the primary care population into robust, mild, moderate and severe frailty groups and comparing trajectories of decline and service use between these groups. We used the eFI to explore the dynamics of frailty and associated healthcare demand over time, using data from the nationally representative RCGP RSC databank, linked with supplementary data from HES and ONS, with additional data from the SAIL Databank (see Chapter 4). We examined the prevalence and progression of frailty within a cohort aged 50 and over, exploring the relationship between frailty, demographic, service and clinical factors, service demand and costs over time (see Chapter 4).
The analyses presented in this report have informed the development of a simulation model of the impact of frailty within an ageing population, informed by input from the SEG, with which it is possible to explore the impact of different demographic and service scenarios (see Chapter 5), and externally validated using population-level data from Wales (SAIL) (see Chapter 6). The simulation model has allowed exploration of ‘what-if’ scenarios and population trends for at least 10 years into the future for outcomes and resource use and up to 25 years for population trends (see Chapter 7).
In this study, the emphasis has been on a whole-system analysis of the dynamics and impact of frailty at a population level, including exploration of drivers of incidence and decline and association with outcomes and resources use. This report focuses on the research addressing the main study objectives and conducted via the above workstreams. Other study outputs relating to dissemination and implementation are not reported here. The following are key outputs of the study, with chapters indicated for those that are covered within this report:
-
Information gaps about the dynamics of frailty and service use in primary care populations have been addressed (see Chapter 4) and used to inform recommendations for service planning and commissioning (see Chapter 9).
-
Service providers and commissioners will be provided with new information in the form of guidance on population trends and key drivers relevant to service planning and prevention strategies.
-
A simulation model has been developed, which allows prediction of trends and exploration of the impact of different demographic and service configurations relevant to different contexts (see Chapters 6 and 7).
-
Future implementation and research priorities, including those relating to workforce planning, will be addressed using the simulation model as a basis for demand-led workforce planning (see Chapter 9).
-
Based on these analyses and output from the simulation model, a toolkit for commissioners is being prepared, comprising output from the prototype simulation model and commissioning guidelines that allow adjustment for specific demographic and service contexts
This study provides new evidence that has informed guidance and a toolkit for commissioners and providers and will, in future studies, inform development of workforce planning tools (see Chapter 8). This work will also form the basis of future research to develop simulation models of workforce needs related to frailty in the older population (HSDR NIHR134305).
Chapter 2 Background literature review
Identification of frailty
United Kingdom guidelines on the recognition and management of frailty1,12 recommend that older people should be assessed for frailty in encounters with health and social care professionals. Identification and stratification of frailty in primary care is intended to facilitate more effective clinical management, allowing patients with frailty to benefit from interventions, such as CGA and balance retraining. Until relatively recently, research into frailty, and its clinical management, in the population has been limited by the need for clinical assessment to identify frailty using phenotypic measures. The benefits of using routine data for risk prediction in primary care are well-established,1,2,16 and the use of frailty measures based on routine data has advantages for both clinical practice and research. The development and validation of the eFI tool used routine electronic health record (EHR) data from around 900,000 UK patients, demonstrating that the eFI could discriminate risk of adverse outcomes in an older population stratified into mild, moderate and severe frailty in a UK primary care population. 8 The eFI was found to have good predictive ability for important outcomes including mortality, hospitalisation and nursing home admission, with severity strata showing good discrimination of these outcomes. The eFI has subsequently been implemented into the SystmOne, EMISWeb, Vision and Microtest primary care EHR systems, where it is freely available to every general practice in England, and around 95% of all UK general practices. 15,17 The eFI is specified as a relevant tool for identification of frailty in the 2017–8 GMS contract, and use is supported in the 2016 NICE Multimorbidity Guideline. 11,15 Given the widespread use of the eFI within the NHS, and its suitability for large-scale analysis of routine health data for research purposes, this study used the eFI to identify and stratify frailty in the population.
Incidence, prevalence and progression of frailty
Frailty is a long-term condition where an individual demonstrates vulnerability to stressors, which may be internal (e.g. infection) or external (e.g. falls) stressors. As a consequence, their overall health is likely to decline over time and service use is expected to be high, particularly as the condition progresses. 4 Frailty can be identified following a physical assessment18 or by using a frailty score or ‘index’ based on an accumulation of conditions or disabilities. Index scores have the advantage that they can be derived from routinely collected data,8,19 allowing exploration of frailty at population level over time on a scale that is not possible using direct measures.
It has been estimated that 1.8 million people in the UK aged 60 and over are living with frailty, with prevalence higher in those aged 85 and above. 3 There is, however, some uncertainty about prevalence in the general population, with the majority of evidence coming from prospective cohort studies that might not be representative of the whole population. Frailty is known to be strongly associated with age, with prevalence estimates varying from 4% to 50% depending on the measure of frailty used and the age group studied. For example, using the Fried criteria,20 the prevalence varies from 4% in 65–69-year-olds to 26% in people aged ≥ 85 years, whereas with the eFI,8 50% of people aged 65–95 have been found to have some level of frailty. Little is known about the prevalence of frailty in those younger than age 65. International estimates also vary widely, from 3.9% to 51.4%, with a pooled prevalence ranging from 12% in pooled data from Europe21 to 17.4%22 in low- and middle-income countries. Risk factors for frailty onset are known to include female sex, deprivation, ethnicity and multiple morbidity. 23,24 Estimates of frailty incidence also show considerable variation, from 12 to 204 cases per 1000 person-years at risk (PYAR), with a pooled incidence of 43.4/1000 PYAR. 24
Although there is good evidence regarding prevalence from cross-sectional studies, less is known about the progression of frailty and expected transitions between frailty states over time. Planning and resourcing interventions for frail older people requires high-quality population-level data on incidence, prevalence and progression of frailty. In addition to improved clinical management, strategies for frailty prevention and slowing frailty progression are key in reducing the future burden on patients, health and social care services, and need to be applied at the population level. 13 However, there is an evidence gap in relation to population-level evidence across frailty severity strata to adequately inform service planning and commissioning.
It is known that more than half of those aged 70 and above with frailty will experience at least one frailty transition within a 4-year period and that the majority of these transitions represent worsening of frailty. 25 This work, however, utilised a criteria-based measure of frailty which is not directly comparable to the frailty index (FI) now being using within the NHS. The relationship between transitions in frailty indices and outcomes have been explored using multistate models. 26–28 However, these models were based in prospective cohorts of moderate size, included a limited number of covariates, and had few follow-up time points. A systematic review of studies using phenotypic assessment of frailty reported 29.1% of people progressed to a worsened frailty state over a mean period of 3.9 years, with 4.5% moving from robust to frail and 18.2% from pre-frail to frail. 29 As with incidence and prevalence, the studies cited in this review were diverse in design, with relatively small samples (generally ˂ 5000 participants) and relatively short periods of follow-up (4 years on average). These studies also used measures and cut-off scores that are not directly comparable to FI scores calculated using electronic health records (EHR), which limits their transferability to systems where FI scores are used. Heterogeneity in age ranges, follow-up duration and differing frailty measures also make it difficult to confidently synthesise results for the purpose of large-scale population planning.
Evidence using FI scores has emerged from the validation of the eFI, a 36-item frailty index developed using EHRs in England,8 which suggests progression of frailty accelerates over time. 30 Another study from the Netherlands using a 32-item frailty index31 described a doubling in deficits over an average of 12.6 years. 32 Previous studies suggest that a doubling in deficits (and therefore the frailty score) occurs over 12.6 years, although the small cohort size suggests further confirmation is needed. 32 There is also uncertainty as to the relationship between the rate of change of frailty deficit accumulation and death. 33,34 Although frailty is more common with increasing age, it is not synonymous with ageing; evidence suggests increased variability in frailty with ageing, but age only partly explains frailty trajectories. 35,36 Socio-demographic factors, specific long-term conditions, physical activity and level of education have been associated with frailty progression. 37 There remains limited evidence on the progression of frailty and factors predicting transitions to more severe frailty states, particularly in large-scale population studies and using FI measures, although this is essential for the prediction of future population trends and service needs. Further evidence on progression of frailty is needed from large-scale population studies designed for this purpose and which can provide more information on the incidence and progression of frailty in adults aged 50–64 (middle age), where prevention interventions are more likely to be targeted.
Impact of frailty on service use, costs and outcomes
Use of large-scale cohort data to explore the impact of frailty on service use is scarce, although recent analyses highlight additional healthcare utilisation and costs for people living with frailty, and increased costs with increased frailty severity. It should, however, be noted that these studies use a variety of frailty measures and studies have not all been designed to explore costs at the population level. Healthcare costs have also been described in different healthcare settings, with estimates of a doubling of costs in Spain38 and a fourfold increase in England. 39 Data on service use and costs appear to attribute the majority of increased service use and costs to increased numbers of hospital admissions and patient bed days, although the contribution of unplanned service use is not clear. Community health and care services, for example, community service referrals within 6 months, care plans and social care have also been shown to increase with the presence of frailty. 40,41 Data from a Finnish cohort assessing frailty using the Fried criteria found interesting indications of different types of service use with different frailty severity. In this study, frailty was associated with increased general practice on-site appointments and physiotherapy contacts, whereas pre-frailty predicted the use of GP remote consultations. 42 In Singapore, frailty was also associated with increased specialised outpatient clinic appointments, day surgery and emergency department visits. 43 A study of a cohort of ageing adults in Ireland found that frailty was a predictor of utilisation of most social and medical care services. This study noted that the majority of usage was medical, possibly reflecting unmet care needs for social care. 44 A longitudinal analysis of a small, ageing cohort in Ireland identified different classes of primary and secondary healthcare utilisation for community-dwelling older people which transitioned over time, reflecting changes in healthcare need, the drivers of which need further exploration, but for which frailty may be a key driver. 45 Evidence gaps remained in relation to service use and costs associated with frailty in ageing populations, particularly in relation to service use differences between frailty severity groups and patterns of service use as frailty progresses, which few studies were large enough to explore. It is important to note that service use patterns may also be influenced by other factors which impact on the incidence and progression of frailty, such as deprivation.
Although frailty is known to be associated with an individual risk of increased service use, analyses of the differences in utilisation and costs in large populations with known proportions of people living with different levels of frailty have not been performed. Understanding the population level impact of frailty dynamics on service use and costs will enable more informed planning of appropriate services, both to manage adverse outcomes and to target prevention of progression. It is important to note that analyses of service use in frailty are largely focused on its impact on general primary and secondary care services used by older people. As understanding of frailty has expanded, attention is moving to development of frailty-specific services aimed at preventing frailty, slowing progression or amelioration of adverse consequences of frailty. There is less information on the use of these frailty-specific services, many of which will be located within other more generic services. There is increasing evidence for specific services particularly within the community, for example case management of integrated care of people with frailty in the community, preventive integrated care interventions, and provision of physical exercise, protein/micronutrient supplementation and cognitive training. 46–48 However, reviews highlight that the existing evidence is still limited and conflicting, and includes heterogenous populations and design of interventions, with a limited impact on health outcomes apart from well-being and little evidence for cost-effectiveness as compared to standard care.
Simulation models in health services research and service planning
The simulation literature largely describes mathematical models that have been used to describe the development of frailty. Although frailty research has expanded over the last decade, there has been only limited modelling of the progression of frailty within an ageing population, particularly in the middle-aged and young-old age groups. The need for better planning and modelling of demand within the primary care system is well recognised,2 although no models have addressed population level or service level trends in frailty of older age, an evidence gap which limits service planning. Models in this area have focused on representation of the pathophysiology of frailty: Lipsitz49 considers a Boolean network to represent a series of neurons in the body’s neural network; and Varadhan et al. 50 describe a theoretical mathematical model that represents how the body’s stimulus-response mechanism may change following a stressor event. To date, the only study that uses SD in relation to frailty was conducted by West Kent Local Care, in connection with Whole Systems Partnerships, that looked at the care needed for patients with frailty and complex needs in Kent and Medway, UK. 51 This model considered the effect of interventions, such as multidisciplinary team (MDT) working in GP clusters and admission avoidance but does not address evidence gaps already noted in relation to frailty progression and whole-system impact in those aged 50 + and using data from a large population-based, longitudinal study.
Although there are relatively few simulation modelling studies relating to frailty, it is worth noting that studies related to modelling of dementia might provide useful learning for simulation of frailty, due to similarities in patient age and disease progression. A study predicting the prevalence of age-related dementia to support planning for the disease52,53 used SD modelling to show that, over time, the number of people with mild, moderate and severe dementia will increase and the prevalence of severe cases will increase more than the other categories. Another recent UK study54 used a hybrid simulation model to consider dementia in the over 65 population. SD was used to model the population level effects and agent-based simulation is used to represent the patient-level characteristics. Both dementia studies consider a progression/transition to higher state of illness and include mechanisms to age the patients in the population and both promote the usefulness of SD in looking at population level outcome measures and care planning under different ‘what-if’ scenarios, an approach that provided useful insights for this study.
Summary
The literature review revealed extensive evidence in relation to frailty incidence and progression, but largely from prospective cohort studies and with less evidence using frailty index measures, such as the eFI. Important gaps in the evidence were noted in relation to accurate estimates of prevalence and incidence within the ageing population over time, particularly the transition to frailty in middle age (50–64 years), for which there was no population-level evidence using the eFI. To inform service planning and commissioning, it was also noted that further evidence was needed on frailty progression in different age groups and which factors are associated with decline. Evidence was also limited on the prevalence of different frailty states at population level over time and its relationship to service use and costs. We found no simulation models that addressed how these population-level changes in frailty prevalence are likely to impact on the use of healthcare services, and their related costs, over time in the ageing population.
Chapter 3 Design and methods: dynamics and impact of frailty in the ageing population
Sections of this chapter have been reproduced with permission from Walsh et al.,55 Fogg et al. 14 and Fogg et al. 56 These are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC-BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Introduction
This chapter reports the work carried out in Workstream 1, exploration of the incidence and prevalence, development and impact of frailty within the population using retrospective data from the RCGP RSC database, which holds data for 2.7 million patients from 505 practices in England. 57 An open cohort of people aged 50 and over present within the database between 2006 and 2017 were identified, and the eFI tool8 was used to stratify the cohort into fit, mild, moderate and severe frailty groups. Data were extracted on factors contributing to the calculation of the frailty index, healthcare use, and outcomes over the 12-year period, calculating key service use costs from these data. Outcomes included mortality, unplanned hospital admission, Accident and Emergency (A&E) attendance and GP appointments. The RCGP RSC data set also included data on socioeconomic deprivation, practice size and location [specifically urban/rural and lower super output area (LSOA)] and residence in a care home during the study period. Frailty status was explored over time, determining incidence, prevalence and progression of frailty (measured through transitions between frailty strata) within the population. We examined the relationships between factors such as age, deprivation, ethnicity, location of individuals in relation to development of, and deterioration in, frailty status. The influence of frailty on outcomes, service use and costs was explored.
Design
This workstream used linked primary and secondary care data to explore the development and impact of frailty in the older population. The data extraction approach was designed to capture people aged 50 and over within the primary care population, including those turning 50 during the study period. To answer the study questions, people entering and leaving the cohort were identified, as were deaths, frailty transitions, age transitions and service use outcomes. An open cohort design was used, that is patients registered at a RCGP practice who had their 50th birthday between 2006 and 1 January 2017 were included in the cohort, in addition to any person aged ≥ 50 who joined the practice. Patients left the cohort through leaving a participating practice or death.
Population and data sources
The UK has a registration-based primary healthcare system, in which almost all of the population are registered with a general practice, with almost complete population coverage. Primary care patients are allocated a unique personal lifetime identifier, the NHS number. For research purposes, use of the NHS number to identify participants reduces the risk of duplicate records and facilitates the linkage of primary care to other healthcare data sets.
The primary data source for the study was the Oxford Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC) databank (see Appendix 1, Table 15), a pseudonymised EHR that collates routine primary care data from a population of more than 5 million people living in England, from more than 500 GP practices. This represents around 5% of primary care practices in England, contributing EHR data voluntarily. Practices registered with the RCGP RSC have been shown to be nationally representative in terms of the population served and health outcomes. 58–60
Sample size for the study was maximised to allow robust analysis of transitions over time by age and other subgroups of interest. This was achieved by using retrospective data from the most recent complete year at the point of data extraction and all preceding years with availability of study variables, a total of 12 years of available data.
The inclusion criteria were:
-
patients aged ≥ 50 years
-
registered at a general practice contributing to the Oxford-RCGP RSC network database
-
present on 1 January on any calendar year between January 2006 and January 2017 inclusive.
Potential duplicate and anomalous patient records were excluded, that is more than one sex present for a patient record, duplicated calendar years of data, differing birthdates in the patient record, and patients with missing or impossible birthdates. Yearly records were excluded as follows:
-
where a patient changed practice within a calendar year and had duplicate yearly records, the yearly record with the longest period was kept if continuous years of data were available
-
person-years of data following a gap of 1 year or more in the observation record, even if the patient re-registered with a RCGP RSC practice.
The unique NHS number was used as a basis for linking primary care data with secondary care and mortality data from additional data sources collated by NHS Digital – HES data, and ONS data. Following data linkage, patient records and follow-up years in the primary care data set were removed where these data were discrepant with ONS deaths that is:
-
whole patient records for patients who had died according to the ONS data prior to entry to the primary care data set (e.g. death in the ONS data in 2005, but present in the year 2006 in primary care)
-
person-years of primary care data following the year in which death was recorded in the ONS data (e.g. patient present in the primary care record from 2006 to 2009, but ONS death in 2008 had data from 2009 excluded).
Measurement of frailty
Frailty was identified and stratified using the eFI,8 a frailty identification tool utilising primary care data. This tool is specified as an appropriate tool for use in primary care11,15 and is now available to every general practice in England and around 95% of all UK general practices. 17 The eFI includes 36 deficits covering long-term health conditions, symptoms/signs, disabilities, abnormal laboratory test results and social conditions, which were identified according to standard methods for creating a frailty index. 61 Each deficit has a set of associated Read codes, and the calculation of the eFI scores ‘1’ for each deficit according to the occurrence of the related Read codes within the electronic general practice record for each patient. The deficits are totalled and divided by 36 to establish the score. The development and validation of the eFI categories found that categorisation of the eFI score into frailty categories of fit (0 to < 0.12), mild (0.12 to < 0.24), moderate (0.24 to < 0.36) and severe (0.36 and above) are predictive of an increased risk of hospital admission, mortality and nursing home admission and reflect cut-offs used in practice. 8,62,63 This study used the same cut-off scores and categories. The eFI, like other frailty index measures, is built on the cumulative deficit approach.
In this study, using the same Read codes (Clinical Terms Version 3 – CTV3) as in the original derivation of the score, variables for each deficit were created and flagged as ‘present’ if the Read codes were present in the patient EHR at any point in their prior medical history on 1 January and 1 July for each calendar year for each participant. As this method retrieves codes from the patient’s complete medical record, there is no missing data for any of the deficits. Although it is theoretically possible for scores to improve, this does not usually happen due to the way that patient data are recorded in routine clinical practice. It is uncommon for clinicians to actively remove codes from the record, so it was unlikely for the eFI to improve in our data set. Apparent reversals in the score were imputed to the most recent higher frailty category. Such reversals were either due to changes in polypharmacy score (reversing the frailty category in 3.9% of patients where polypharmacy is defined as five or more different medications prescribed in the previous 15 months) or coding omissions resulting from changing a GP practice, which may represent a ‘false’ reversal in the deficit.
Patient characteristic variables from primary care
Data for patient characteristics of interest in relation to frailty and service planning were extracted from primary care. These comprised:
-
Age (in years) at each calendar year of follow-up. Age was further categorised into four groups, reflecting groupings reported in literature relating to older adults’ health care, and cut-offs for services reported by the study SEG: 50–64, 65–74, 75–84 and ≥ 85.
-
Sex (male/female).
-
Ethnicity. Ethnicity data from routine healthcare records are often under-reported and are measured in different ways in different healthcare sectors and organisations. In order to maximise available ethnicity data in the primary care data set, a customised ontology was used and data were coded into broad categories (Asian, black, white, mixed/other). 64 The most recent ethnicity reported in the patient record was used as the baseline ethnicity value to reduce missing values in the year of entry to the cohort. Where ethnicity was missing from the primary care record, ethnicity data from linked secondary care data coded according to the 16 categories in the NHS data dictionary (www.datadictionary.nhs.uk/data_elements/ethnic_category.html) were used instead, following aggregation to Asian, Black, mixed/other and white categories. These data were retrieved from hospital admission records and from outpatient appointment records. Where there were conflicts, the most recent data from hospital admission records was used first, and then the most recent data from outpatient appointment data if still missing in the hospital records.
-
Indices of Multiple Deprivation (IMD) quintiles. The IMD is a small-area measure (LSOA) of socio-economic status, widely used as an indicator of deprivation, based on postcode, ranked nationally, which includes seven domains: income, employment, education/skills/training, health and disability, crime, barriers to housing and services, and living environment. 65 The LSOA with the highest level of deprivation is scored ‘1’, then other LSOAs are scored consecutively with the least deprived area having the highest score. The 2015 deprivation indices were related to the last known patient address in the data set or, where missing, were imputed using the IMD indices related to the GP practice address (3.6% of patients). For some analyses, the IMD quintiles were recategorised into two categories: the two most deprived quintiles versus the three least deprived quintiles.
-
Income Deprivation Affecting Older People Index (IDAOPI) quintiles. The IDAOPI is a subset of the Income Deprivation Domain from the IMD and focuses specifically on the percentage of the population aged 60 and over who receive income support, income-based job seeker’s allowance, pension credit or child tax credit and their partners aged ≥ 60.
-
Residential care. Receipt of residential care during the cohort period for each patient was coded ‘yes’ or ‘no’ by using a combination of Read codes30 and use of a household key (11 or more patients at the same address with a median age of 50 or above) for the patient’s last known address at the date of data extraction (May 2019).
-
Long-term conditions (LTCs). LTCs not included in the eFI (e.g. COPD, asthma, rheumatoid arthritis) and those present in the Quality Outcomes Framework (QOF) (e.g. dementia, depression, cancer, obesity) were indicated as present or absent in each calendar year and had dates of onset ascertained from the whole patient medical history.
-
Smoking status. This was defined as non-smoker, ex-smoker or active smoker.
-
Yearly influenza and pneumococcal vaccinations.
-
Body mass index (BMI). All measurements present in the patient record were provided. As these are not measured or recorded systematically, a baseline BMI value was defined as the first recording in a patient’s cohort entry year, or, where missing, the first value in the nearest previous year to cohort entry (up to a maximum of two years) or the nearest year afterwards (up to two years). This was further categorised using standard cut-offs as underweight (< 18.5), healthy (18/5–24.9), overweight (25–29.9) or obese (30 or over) (www.nhs.uk/common-health-questions/lifestyle/what-is-the-body-mass-index-bmi/).
General practice characteristic variables from primary care
Information on general practices was extracted, specifically the geographical region, urban/rural indicators based on the 2011 rural/urban classification (RUC11),66 IMD and IDAOPI for the practice postcode, number of patients registered in the practice, and total practice consultations per year. The total GP, nurse and overall practice staff full-time equivalent (FTE) for each general practice in 2013 (the first year this information is available to be linked on practice code) was included. 67 Each calendar year of participant data was linked to a general practice identifier and dates of the participant registering and leaving the RCGP RSC practices were provided.
Outcome variables from primary care
Death
The month and year of death were provided. Primary care death data in the RCGP data set have been shown to be accurate for this calendar period. 60,68 The primary care death data were used for initial description of the cohort, to allow interim assessment pending release of linked ONS mortality data. Although there were delays in approvals and data extraction due to the impact of the COVID-19 pandemic, the final analyses for the multistate modelling (MSM) and simulation modelling used ONS data, which was more likely to be complete and accurate.
Service use
The RCGP RSC provided the total number of primary care contacts (face-to-face appointments, home visits, telephone appointments and e-consultations) for each participant for each year they were present in the cohort (with a maximum of one of each type of record each day to account for double counting relating to data entry). The total number of medications per participant per year were summarised from RCGP RSC records, and the number of prescriptions overall and by British National Formulary (BNF) chapter for each calendar year. 69
Outcome variables from secondary care
Service use
Individual records of outpatient visits, emergency department (ED) attendances, hospital and critical care admissions from HES data were provided for each year that each patient was present in the cohort. These included dates of attendance or admission/discharge, reasons for admissions or attendance and the type of outpatient clinic, type of hospital admission, that is elective or unplanned, and outcome of hospital contact where relevant. Summary variables were generated, that is (1) total number of outpatient visits, total number of ED attendances, total number of hospital admissions (also stratified by elective/unplanned admissions), total number of critical care admissions for each cohort year for each patient; (2) total days in hospital for each cohort year for each patient (also stratified by elective/unplanned admissions), total days in critical care for each cohort year for each patient.
In addition, data from the SAIL Databank were used to explore the impact on specific services not available within the RCGP RSC data set, for example, ambulance services and residential care (see Chapter 6 for details of the methods for data extraction of these variables).
Generation of cost variables
The cost analyses used an NHS and personal social service perspective (PSS). Itemised primary and secondary care resource use was costed using corresponding unit costs based on NHS national reference costs and the Personal Social Services Research Unit (PSSRU) data. Reference costs from 2017,70 or 2016–7 (NHS national reference costs) were used when possible, and where unit costs from those years were not available, information from the closest possible calendar year relating to the end of the cohort (2017) was used. Costs were attributed as per Appendix 1, Table 16.
These costs were multiplied by the summary variables of service use counts to generate a cost per service item for each individual for each calendar year. The total costs for each component of service use and a summary for primary, secondary care and total care were then calculated for each individual for each calendar year. Summary variables of costs of elective admissions and unplanned admissions were also calculated. A discounting rate of 3.5% was then applied to the individual summary cost variables across the cohort calendar years to create a discounted cost variable for the five summary cost variables.
Data governance and data management
The RCGP RSC have a secure data and analytics hub at University of Oxford (from March 2021), and managed data governance, encryption and access. Their data were linked to HES secondary care data and ONS death registry data, which allowed treatment and care to be tracked across care settings and providers.
The RCGP RSC only use and store pseudonymised information extracted by an approved third-party provider, Apollo Medical Software Solutions. Identifiable personal data, including NHS numbers, were removed and individual pseudonymised IDs generated before transfer to the RCGP RSC. The research team worked with the RCGP clinical informatics team and analysts to define the data specification for the cohort. Variables were defined to minimise the risk of re-identification (e.g. by means of inference or ‘jigsaw’ attacks). Data collation was carried out by the RCGP RSC analysts. The research team had access only to the agreed data extracts via remote servers hosting a trusted research environment (TRE). Patients who had opted out of sharing their data were excluded from the cohort data extract. No patient-level data could be transferred from the secure servers. Data from all data sources was provided to the research team via the TRE in a pseudonymised, de-identified format, where the pseudonymised ID held by the RCGP was replaced with ID numbers that were unique to this study. Pseudonymisation for all data extracts was, therefore, non-reversible and the study team under no circumstances had access to the keys for creation of the unique study ID numbers. The research team and analysts at RCGP RSC did not have access to or use any patient identifiable information throughout this study. Only aggregate, anonymised results of analyses of data extracts were exported from the secure servers for use by members of the research team for use in simulation modelling. The research team applied for extraction of the specified data extract via the RCGP RSC Information Governance processes. Pseudonymised data extracts were supplied for analysis via secure, remote servers hosted by University of Surrey (up to 2021) and University of Oxford (from 2021 onward).
The primary care data provided by RCGP RSC was linked to HES secondary care data and ONS death registry data by NHS DARS. RCGP RSC data analysts, through a secure process, provided details of their non-reversible ‘one-way hashing’ algorithm to NHS Digital to enable data linkage between the primary care records and secondary care (HES) and death (ONS) data. This was the same hashing algorithm as provided by University of Oxford to Apollo Medical Software Systems for application at the point where data were extracted from GP systems. These organisations applied the code to ‘hash’ the patient identifiers, creating unique ID codes from the NHS number (the process is non-reversible). In this way, NHS number was ‘hashed’ in the same way in both data sets, which allowed linkage to the primary care data by matching of ‘hashed’ NHS numbers without requiring access to patient identifiable information. The data providers then provided the pseudonymised data to RCGP RSC, where the linked, pseudonymised data extract was prepared for the research team.
Additional data linkage
Publicly available data sets were imported into the RCGP RSC TRE and linked to the primary care data. These included the IDAOPI 2015,71 geographical information from the geography portal of the ONS72 linked by LSOA; and workforce data for GP practices linked by GP practice identifier. 73
Statistical analysis of population trends and service use
There were two main aims for this workstream: the identification of key variables that are capable of predicting frailty development and progression; and the assessment of the relationship between frailty status and key clinical outcomes (including mortality, unplanned admissions and costs). These analyses were then used to inform the simulation modelling being conducted in Workstream 4 (see Chapters 6 and 7).
Descriptive characteristics
The characteristics of RCGP RSC practices with participants in the cohort were described for the calendar year 2006 (first year of cohort). Participant characteristics were analysed at their year of entry into the cohort. Age category distributions for both the open and closed cohorts were analysed and presented graphically for the calendar years 2006–17. Analyses of transitions within the closed cohort were analysed for verification but are not presented here. The following analyses are based on the open cohort. The reasons for exit from the cohort were summarised. Patient sociodemographic and clinical characteristics at the year of cohort entry (i.e. for the open cohort) were described according to the four age groups, and missing data quantified.
Prevalence and incidence
The prevalence of frailty, overall and stratified by frailty severity category, was calculated as per the eFI value on 1 January for each calendar year, including all persons present in the cohort at the beginning of each year. Prevalence was also stratified by age group. Frailty incidence rates (new onset frailty of any severity) were calculated per 1000 person-years across the 12-year cohort period for the whole population, and according to sociodemographic and clinical characteristics.
Analysis of frailty transitions
We used continuous time multistate Markov (MSM) models to estimate the transition rates between frailty states and to identify determinants of frailty progression between states. 74–76 The variables used in the model were chosen to reflect factors associated with frailty, including socioeconomic and demographic variables that should be available to commissioners planning for their region. During each year of follow-up, the frailty category for each individual was treated as their current state, with a final absorbing state of death from any cause. 55
There were several assumptions within the MSM. The fitted models did not assume exact transition times to be observed and allowed for multiple transitions to occur between observation years, however the date of death was assumed to be recorded exactly. Where an individual ‘jumped’ between two or more frailty states within a 12-month period, the model assumed that intermediate states were passed through in that time. Reverse transitions were therefore excluded from the model design, although reversals due to polypharmacy were noted in only 3.9% of patients; these were imputed to the most recent higher frailty category.
An initial unadjusted MSM model was fitted to estimate the average annual transition probabilities. Multivariable models were then fitted to assess the impact of the key sociodemographic variables of age group, sex, ethnicity (categorised as black, Asian, white or other), deprivation (categorised into a binary variable grouping the two most deprived quintiles and the three least deprived) and rural/urban location on these transitions, in a forwards selection process. The Akaike information criterion (AIC) and likelihood ratio test were used to compare and choose between models. SAS version 9.4, R version 4.2.0 (SAS Institute Inc., Cary, NC, USA) and Stata version 16.0 (Stata Press, College Station, TX, USA) software were used for data manipulation and the statistical analyses. The R msm package version 1.6.9 (Cran R project multistate modelling package) was used for the MSM modelling. 76 p ˂ 0.05 were considered statistically significant and estimates are presented with 95% confidence intervals where appropriate.
Service use and cost analysis
Description of service use
Data were available on general primary, secondary and urgent care services used by older people, including those living with frailty. Total service use and summary statistics for each calendar year of the cohort (2006–17) were calculated. Summary statistics for annual service use [means, standard deviations (St.D), medians and interquartile ranges] were calculated for each aspect of service use in primary and secondary care, stratified by frailty category (fit, mild, moderate and severe) and by age group (50–64, 65–74, 75–84, 85 +). Aggregate data were used to produce appropriate graphics.
Description of costs
Total costs for each component of service use and a summary for primary, secondary care and total care for each calendar year of the cohort (2006–17) were calculated. Summary statistics for annual costs (means, standard deviations, medians and interquartile ranges) were stratified by frailty category (fit, mild, moderate and severe) and by age group (50–64, 65–74, 75–84, 85 +) combining all cohort years in each category. Aggregate data was used to produce appropriate graphics.
Cost modelling
Service use was aggregated into primary, secondary and total care, because some services had very low total use, for example virtual GP appointments. Generalised linear model (GLM) were used to explore the contribution of predictor variables including frailty and those shown to be associated with frailty in our previous analyses,55 that is age group, sex, ethnicity, deprivation and urban/rural location, were included in the model. Predictions of adjusted annual mean costs for primary, secondary and total care were produced for each frailty category with 95% confidence intervals.
Summary
Linked primary and secondary care data from RCGP RSC were extracted to allow estimation of frailty incidence, prevalence, and transition rates between frailty states in an ageing cohort aged 50 and over. Data were analysed using multistate models to determine what clinical, demographic and socio-economic variables predicted progression between different frailty states (fit, mild, moderate, severe) and death. Data were further analysed to explore the impact of frailty status on service use and costs. These analyses are presented in Chapter 4 The analyses, including identification of key demographic and socioeconomic drivers that predict frailty progression, were used to inform the development and validation of the SD model described in Chapters 6 and 7, including identification of ‘what-if’ scenarios for simulation.
Chapter 4 Analyses of frailty dynamics and impact at population level
Sections of this chapter have been reproduced with permission from Fogg et al.,14 Walsh et al. 55 and Fogg et al. 56 These are Open Access articles distributed in accordance with the terms of the Creative Commons Attribution (CC-BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
Cohort profile
In the initial description of the cohort using primary care data, 2,177,656 patients from 419 GP practices across England were included (see Appendix 1, Figure 17). 14 Full details of the cohort have been reported elsewhere. 55
Practice characteristics
The cohort drew data from 419 primary care practices distributed across England between 2006 and 2017 inclusive. Practice information has been reported elsewhere, but practices varied widely in their patient numbers and consequently their totals of yearly consultations (see Appendix 1, Table 17). Practices reflected population distributions throughout England, with 78% in urban areas and an even spread across IMD quintiles. Practices were predominantly urban, with only 21.8% in rural areas. Practice IMD scores indicated that 42.3% of practices were in the two most deprived categories.
Patient baseline characteristics
The sociodemographic baseline characteristics of participants in their year of entry to the primary care cohort are presented in Table 1. The mean age of participants was 61 years (St.D 12) and 52.1% were female. Demographic trends with increasing age were observed, including a higher proportion of female sex, lower ethnic diversity and rural residence in the older age groups. Ethnicity data were more likely to be missing with increasing age, decreasing deprivation, male sex, urban location and for people in residential care. Patterns of indices of deprivation appeared similar across age groups, with half the cohort located in the two least deprived quintiles.
| Age group | Total (%) | ||||
|---|---|---|---|---|---|
| 50–64 (%) | 65–74 (%) | 75–84 (%) | ≥ 85 (%) | ||
| Age groupa | 1,413,576 (64.9) | 385,474 (17.7) | 259,125 (11.9) | 119,481 (5.5) | 2,177,656 |
| Female | 698,158 (49.4) | 199,914 (51.9) | 151,462 (58.5) | 84,437 (70.7) | 1,133,971 (52.1) |
| Ethnicity b | |||||
| Asian | 52,703 (5.1) | 11,419 (4.1) | 4521 (2.7) | 916 (1.4) | 69,559 (4.5) |
| Black | 29,387 (2.8) | 5577 (2.0) | 2350 (1.4) | 440 (0.7) | 37,754 (2.4) |
| Mixed/other | 15,461 (1.5) | 2480 (0.9) | 1110 (0.6) | 277 (0.4) | 19,328 (1.3) |
| White | 937,135 (90.6) | 260,473 (93.0) | 160,063 (95.3) | 63,054 (97.5) | 1,420,725 (91.8) |
| Missingc | 378,890 (26.8) | 105,525 (27.4) | 91,081 (35.2) | 54,794 (45.9) | 630,290 (28.9) |
| Urban | 1,102,809 (78.0) | 294,247 (76.3) | 200,358 (77.3) | 91,492 (76.6) | 1,688,906 (77.6) |
| Residential care | 1019 (0.1) | 1708 (0.4) | 5371 (2.1) | 9121 (7.6) | 17,219 (0.8) |
| IMD quintile | |||||
| 1 (Most deprived) | 193,552 (13.7) | 49,320 (12.8) | 34,151 (13.2) | 14,894 (12.5) | 291,917 (13.4) |
| 2 | 220,674 (15.6) | 60,287 (15.6) | 41,887 (16.2) | 19,592 (16.4) | 342,440 (15.7) |
| 3 | 280,969 (19.9) | 79,288 (20.6) | 54,244 (20.9) | 25,806 (21.6) | 440,307 (20.2) |
| 4 | 340,796 (24.1) | 93,998 (24.4) | 62,573 (24.2) | 28.815 (24.1) | 526,182 (24.2) |
| 5 (Least deprived) | 377,585 (26.7) | 102,581 (26.6) | 66,270 (25.6) | 30,374 (25.4) | 576,810 (26.5) |
| IDAOPI quintile | |||||
| 1 (Most deprived) | 199,722 (14.1) | 50,167 (13.0) | 34,440 (13.3) | 15,493 (13.0) | 299,822 (13.8) |
| 2 | 217,183 (15.4) | 58,934 (15.3) | 42,894 (16.6) | 19,930 (16.7) | 338,941 (15.6) |
| 3 | 269,450 (19.1) | 76,828 (19.9) | 55,166 (21.3) | 27,233 (22.8) | 428,677 (19.7) |
| 4 | 336,857 (23.8) | 93,684 (24.3) | 62,160 (24.0) | 29,063 (24.3) | 521,764 (24.0) |
| 5 (Least deprived) | 390,364 (27.6) | 105,861 (27.5) | 64,465 (24.9) | 27,762 (23.2) | 588,452 (27.0) |
The clinical baseline characteristics of participants in their year of entry to the primary care cohort are presented in Table 2. The most common long-term conditions recorded at any time in the cohort participants were hypertension (28.7%), depression (17.6%), cardiovascular (13.1%), diabetes (8.8%), chronic kidney disease (8.8%), asthma (7.1%), malignancy (5.7%) and chronic obstructive pulmonary disease (3.6%). Long-term conditions were more prevalent in older age groups at baseline, with the exception of depression and obesity which were more common in younger age groups. The eFI score increased with age, as did the proportion of participants in the Mild, Moderate and Severe frailty categories. The proportion of people with frailty at cohort entry increased from 10% in the 50–64 age group to 69% in people aged ≥ 85. In total, at cohort entry, 78.2% were Fit and 17%, 3.4% and 0.8% were in the Mild, Moderate and Severe categories, respectively.
| Age group | Total (%) | ||||
|---|---|---|---|---|---|
| 50–64 | 65–74 | 75–84 | ≥ 85 | ||
| eFI score | |||||
| Median | 0.028 | 0.083 | 0.139 | 0.167 | 0.056 |
| Upper:lower quartile | (0:0.083) | (0.028:0.139) | (0.083:0.194) | (0.111:0.250) | (0.028:0.111) |
| Frailty category | |||||
| Fit | 1,273,304 (90.1%) | 272,694 (70.7%) | 120,357 (46.5%) | 37,243 (31.2%) | 1,703,598 (78.2%) |
| Mild | 127,029 (9.0%) | 94,558 (24.5%) | 99,154 (38.3%) | 49,192 (41.2%) | 369,933 (17.0%) |
| Moderate | 12,055 (0.9%) | 16,167 (4.2%) | 32,732 (12.6%) | 25,360 (21.2%) | 86,214 (3.4%) |
| Severe | 1188 (0.1%) | 2055 (0.5%) | 6882 (2.7%) | 7686 (6.4%) | 17,811 (0.8%) |
| Long-term conditions | |||||
| Atrial fibrillation | 11,359 (0.8%) | 15,381 (4.0%) | 23,978 (9.3%) | 17,553 (14.7%) | 68,271 (3.1%) |
| Coronary artery disease | 16,176 (1.1%) | 16,017 (4.2%) | 12,015 (4.6%) | 2419 (2.0%) | 46,627 (2.1%) |
| Dementia | 7705 (0.6%) | 7812 (2.0%) | 18,748 (7.2%) | 19,328 (16.2%) | 53,593 (2.5%) |
| Depression | 271,343 (19.2%) | 55,438 (14.4%) | 37,418 (14.4%) | 18,220 (15.3%) | 382,419 (17.6%) |
| Haemorrhagic stroke | 3938 (0.3%) | 1959 (0.5%) | 1733 (0.7%) | 867 (0.7%) | 8497 (0.4%) |
| Heart failure | 6219 (0.4%) | 8736 (2.3%) | 14,976 (5.8%) | 12,583 (10.5%) | 42,514 (2.0%) |
| Hypertension | 265,702 (18.8%) | 161,622 (41.9%) | 136,905 (52.8%) | 60,133 (50.3%) | 624,362 (28.7%) |
| Ischaemic stroke | 9833 (0.7%) | 11,097 (2.9%) | 15,836 (6.1%) | 10,617 (8.9%) | 47,383 (2.2%) |
| Malignancy | 48,115 (3.4%) | 32,230 (8.4%) | 29,796 (11.5%) | 14,646 (12.3%) | 124,787 (5.7%) |
| Peripheral arterial disease | 8144 (0.6%) | 9541 (2.5%) | 11,073 (4.3%) | 4992 (4.2%) | 33,750 (1.6%) |
| Rheumatoid arthritis | 11,149 (0.8%) | 6244 (1.6%) | 5236 (2.0%) | 2169 (1.8%) | 24,798 (1.1%) |
| Transient ischaemic attack | 8065 (0.6%) | 10,774 (2.8%) | 15,795 (6.1%) | 10,916 (9.1%) | 45,550 (2.1%) |
| Diabetes | 89,567 (6.3%) | 49,954 (13.0%) | 37,755 (14.6%) | 13,514 (11.3%) | 190,790 (8.8%) |
| Chronic obstructive pulmonary disease | 28,352 (2.0%) | 22,538 (5.9%) | 20,399 (7.9%) | 7395 (6.2%) | 78,684 (3.6%) |
| Chronic kidney disease | 83,821 (5.9%) | 42,059 (10.9%) | 36,783 (14.2%) | 19,404 (16.2%) | 182,067 (8.4%) |
| Asthma | 95,438 (6.8%) | 31,682 (8.2%) | 20,747 (8.0%) | 6365 (5.3%) | 154,232 (7.1%) |
| Osteoporosis | 26,939 (1.9%) | 21,884 (5.7%) | 24,155 (9.3%) | 14,096 (11.8%) | 87,074 (4.0%) |
| Morbid obesity risk group | 46,465 (3.3%) | 9516 (2.5%) | 3799 (1.5%) | 697 (0.6%) | 60,477 (2.8%) |
| BMI categorya | |||||
| Underweight | 10,660 (1.2%) | 4749 (1.6%) | 6520 (3.5%) | 5547 (8.8%) | 27,476 (1.9%) |
| Normal | 270,394 (29.3%) | 88,178 (29.9%) | 70,979 (37.9%) | 31,659 (50.4%) | 461,210 (31.4%) |
| Overweight | 350,099 (38.0%) | 119,969 (40.7%) | 72,079 (38.4%) | 18,858 (30.3%) | 561,005 (38.2%) |
| Obese | 290,704 (31.5%) | 82,017 (27.8%) | 37,970 (20.3%) | 6743 (10.7%) | 417,434 (28.5%) |
| Missingb | 491,719 (34.8%) | 90,561 (23.5%) | 71,577 (27.6%) | 56,674 (47.4%) | 710,531 (32.6%) |
| Vaccinations | |||||
| Flu vaccination | 248,157 (17.6%) | 269,364 (69.9%) | 187,976 (72.5%) | 71,906 (60.2%) | 777,403 (35.7%) |
| Pneumococcal vaccination | 119,926 (8.5%) | 231,908 (60.2%) | 184,638 (71.3%) | 77,218 (64.6%) | 613,690 (28.2%) |
| Smoking status a | |||||
| Non-smoker | 539,051 (40.7%) | 138,073 (37.9%) | 94,660 (39.7%) | 51,381 (52.4%) | 823,165 (40.6%) |
| Ex-smoker | 437,970 (33.0%) | 157,393 (43.2%) | 109,868 (46.1%) | 37,801 (38.5%) | 743,032 (36.7%) |
| Active smoker | 348,396 (26.3%) | 68,858 (18.9%) | 33,807 (14.2%) | 8904 (9.1%) | 459,965 (22.7%) |
| Missingb | 88,159 (6.2%) | 21,150 (5.5%) | 20,790 (8.0%) | 21,395 (17.9%) | 151,494 (7.0%) |
| Prescriptions (total count of prescription items) | |||||
| Median | 4 | 18 | 32 | 39 | 8 |
| Upper:lower quartile | (0:15) | (4:42) | (12:60) | (16:71) | (1:29) |
Entry to and exit from the cohort over the study period
There were 1,107,481 eligible patients in the first year of the cohort (2006), increasing to 1,491,954 at the beginning of 2017, with a total of 1,070,175 new participants joining the cohort between 2007 and 2017. Patients contributed a mean of 7 years of data, with 647,239 patients (58.4%) who were present in the first cohort year (2006) having the full 12 years of data. Patients present in 2006 comprised 50.9% of the cohort and contributed 67.0% of the total person-years. Between 2006 and 2017, 137,481 patients died (6.3% of cohort) and 635,400 patients moved out of a RCGP RSC practice (29.2% of the cohort). The full details of entry and exit to the cohort by calendar year according to age groups and frailty category at cohort entry can be found in Appendix 1, Tables 18 and 19. There was an inflow of new participants over the cohort period, across all age groups and frailty categories, which was more notable in younger age groups.
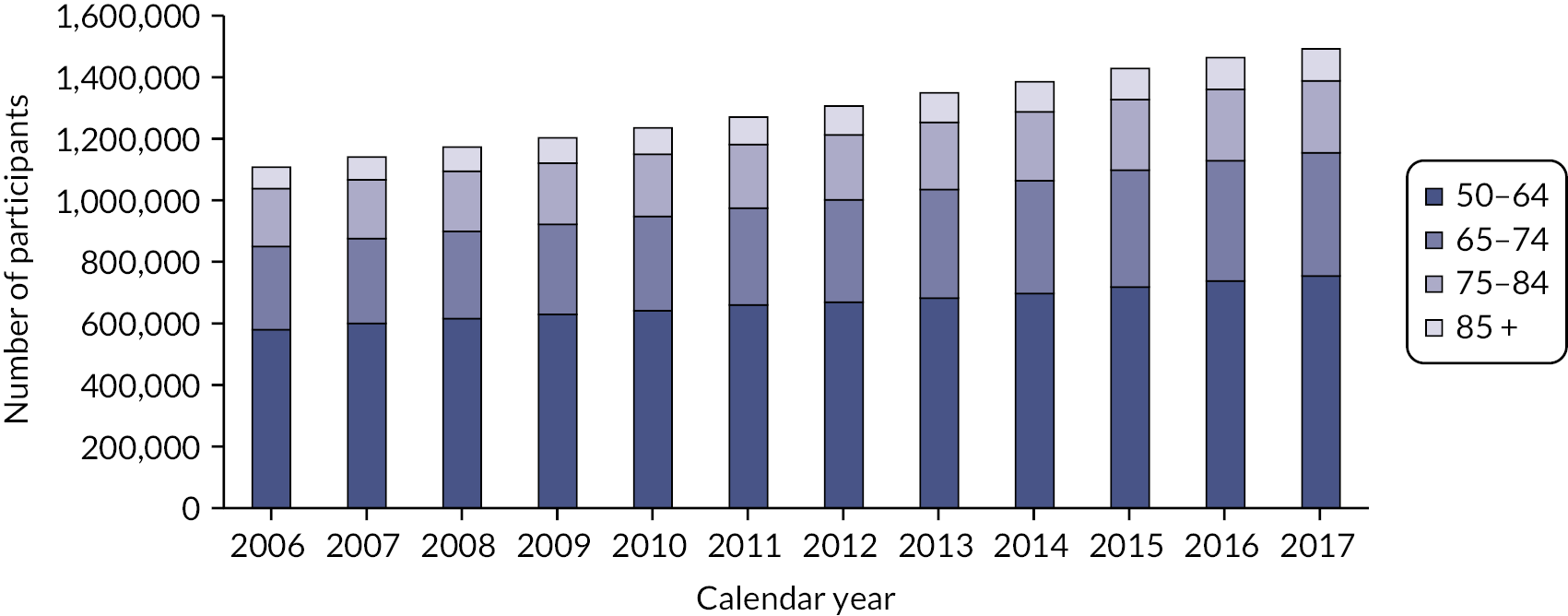
People aged 65–74 at cohort entry had the longest mean period of follow-up at 8 years, with the 85 + group having 4 years. The mean follow-up period according to frailty category ranged from 7.3 years in people categorised as fit at cohort entry to 4.1 years in people categorised as severely frail (see Appendix 1, Table 20). The age distribution over the cohort period for the closed cohort (participants who were present in 2006 onwards, showing attrition due to death and leaving RCGP RSC practices) is shown in Figure 1. The age distribution for the open cohort (participants present in 2006 plus those moving into a RCGP RSC practice and people turning 50) is given in Figure 2.
FIGURE 1.
Age group distribution over cohort period – closed cohort. Reproduced from Fogg et al. 14 This is an Open Access article distributed in accordance with the terms of the creative commons attribution (CC-BY 4.0) licence which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This figure includes minor additions and formatting changes to the original.
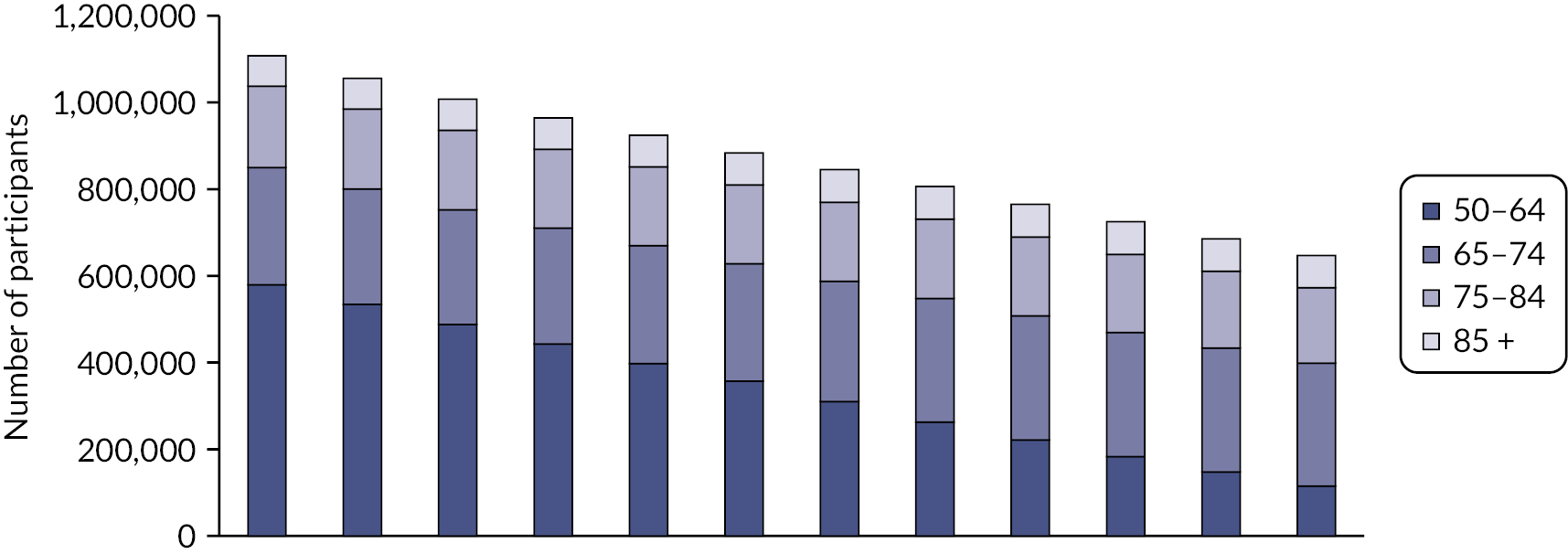
FIGURE 2.
Age group distribution over cohort period – open cohort. Reproduced from Fogg et al. 14 This is an Open Access article distributed in accordance with the terms of the creative commons attribution (CC-BY 4.0) licence which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This figure includes minor additions and formatting changes to the original.
Frailty incidence and transitions
Prior to analyses of prevalence, incidence and transitions, the data set was linked with the ONS mortality data as the most reliable data source for deaths; deaths occurring within any calendar year for which a patient was present in the cohort on 1 January were included. Following data linkage, patient records and follow-up years in the primary care data were removed where the data were discrepant with ONS deaths (6159 patients and 38,212 follow-up years). A total of 2,171,497 patients were analysed, with 1,104,135 patients in 2006 rising to 1,489,495 in 2017. Over the cohort period, 1,067,362 patients entered, 355,889 died (16.4%) and 411,378 (18.9%) deregistered from RCGP practices. These patients contributed 15,514,734 person-years of data, with a mean follow-up of 7 years (St.D 4 years).
Full results relating to incidence, transitions and prevalence of frailty have been reported elsewhere. 14 The average age of onset for frailty (any category) for patients who were fit at cohort entry was 69 years (SD 10 years). The overall frailty incidence rate was 47.1 cases per 1000 person-years (95% CI 47.0 to 47.2). Crude incidence was higher in older age groups, female sex, Asian ethnicity, more deprived quintiles, and people living in urban areas (see Appendix 1, Table 21). Incidence rates were 31.8 for the 50–65 age group, rising to 158.5 for the oldest. Rates remained stable in the 50–64 age group due to the open nature of the cohort, but gradually decreased in older age groups as prevalence increased and fewer non-frail people were present (see Appendix 1, Table 22).
The mean eFI score increased from 0.087 in 2006 to 0.120 in 2017. Analysis demonstrated at least one transition between frailty categories in 32.7% (n = 709,377) of the cohort over a median follow-up of 7 years. The average age of transition from fit to mild was 69 years (St.D 10 years), fit/mild to moderate was 77 years (St.D 10 years) and any category to severe was 81 years (St.D 9 years).
The multistate model included, in order of decreasing impact, the following statistically significant predictors of frailty transitions: age group, deprivation, sex, ethnicity and urban/rural location. The number of people transitioning to higher frailty category per 1000 in 1 year was greater with each increase in age group (Table 3).
| Sociodemographic variables | Hazard ratio (95% CI) for the listed transition | |||||||
|---|---|---|---|---|---|---|---|---|
| Fit to mild | Mild to moderate | Moderate to severe | Fit to death | Mild to death | Moderate to death | Severe to death | ||
| Age group | 50–64 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 65–74 | 2.44 (2.42 to 2.45) | 1.80 (1.78 to 1.83) | 1.55 (1.51 to 1.60) | 2.65 (2.60 to 2.71) | 1.81 (1.76 to 1.85) | 1.64 (1.58 to 1.71) | 1.56 (1.46 to 1.67) | |
| 75–84 | 4.90 (4.86 to 4.93) | 3.52 (3.48 to 3.56) | 2.60 (2.53 to 2.67) | 7.16 (7.00 to 7.31) | 3.84 (3.75 to 3.92) | 2.93 (2.83 to 3.04) | 2.45 (2.30 to 2.61) | |
| 85 + | 7.68 (7.59 to 7.77) | 5.50 (5.43 to 5.57) | 3.57 (3.48 to 3.67) | 27.53 (26.89 to 28.19) | 11.61 (11.37 to 11.87) | 6.98 (6.73 to 7.23) | 4.79 (4.50 to 5.11) | |
| Deprivation | Least deprived (3–5) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Most deprived (1 or 2) | 1.25 (1.25 to 1.26) | 1.23 (1.22 to 1.24) | 1.18 (1.16 to 1.19) | 1.49 (1.46 to 1.52) | 1.36 (1.34 to 1.38) | 1.17 (1.15 to 1.19) | 1.07 (1.05 to 1.09) | |
| Sex | Male | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Female | 1.13 (1.13 to 1.14) | 1.03 (1.02 to 1.03) | 1.02 (1.01 to 1.04) | 0.75 (0.74 to 0.77) | 0.71 (0.70 to 0.72) | 0.71 (0.70 to 0.72) | 0.71 (0.70 to 0.72) | |
| Ethnicity | White/other | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Asian | 1.28 (1.26 to 1.30) | 1.15 (1.13 to 1.18) | 1.01 (0.97 to 1.04) | 0.54 (0.50 to 0.58) | 0.54 (0.51 to 0.57) | 0.65 (0.61 to 0.68) | 0.74 (0.70 to 0.79) | |
| Black | 1.04 (1.02 to 1.07) | 0.97 (0.94 to 1.0) | 0.94 (0.89 to 1.00) | 0.74 (0.68 to 0.80) | 0.67 (0.62 to 0.72) | 0.77 (0.71 to 0.83) | 0.73 (0.66 to 0.81) | |
| Not stated | 0.21 (0.21 to 0.22) | 0.49 (0.48 to 0.51) | 0.59 (0.55 to 0.64) | 1.28 (1.25 to 1.31) | 3.28 (3.21 to 3.35) | 3.46 (3.36 to 3.56) | 3.01 (2.87 to 3.15) | |
| Urban status | Rural | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Urban | 1.06 (1.05 to 1.06) | 1.06 (1.05 to 1.07) | 1.07 (1.05 to 1.09) | 1.00 (0.98 to 1.02) | 0.98 (0.96 to 0.99) | 0.97 (0.95 to 0.99) | 0.96 (0.94 to 0.98) | |
The mean time spent within each frailty state decreased with age, indicating that frailty progresses more rapidly with older age, with the longest period in severe frailty at all ages (Table 4).
| Incidence of transition to a different frailty category after 1 year per 1000 person-years at risk (PYAR) | Died (per 1000 PYAR) | |||||
|---|---|---|---|---|---|---|
| Frailty category at the beginning of the year by age group | Number per 1000 remaining in category | Time in category (years), Mean (SEM) | Mild | Moderate | Severe | |
| Fit | ||||||
| 50–64 | 950 | 19.62 (0.171) | 47 | 1 | 0 | 2 |
| 65–74 | 880 | 7.82 (0.069) | 111 | 4 | 0 | 5 |
| 75–84 | 772 | 3.87 (0.035) | 198 | 15 | 1 | 14 |
| 85 + | 666 | 2.46 (0.025) | 250 | 29 | 1 | 53 |
| Mild | ||||||
| 50–64 | 954 | 21.01 (0.237) | – | 40 | 1 | 6 |
| 65–74 | 918 | 11.63 (0.130) | – | 70 | 2 | 11 |
| 75–84 | 844 | 5.91 (0.066) | – | 126 | 7 | 24 |
| 85 + | 743 | 3.36 (0.039) | – | 173 | 13 | 72 |
| Moderate | ||||||
| 50–64 | 947 | 18.47 (0.342) | – | – | 38 | 15 |
| 65–74 | 918 | 11.72 (0.199) | – | – | 57 | 25 |
| 75–84 | 864 | 6.86 (0.114) | – | – | 91 | 45 |
| 85 + | 781 | 4.05 (0.069) | – | – | 113 | 106 |
| Severe | ||||||
| 50–64 | 966 | 28.91 (1.299) | – | – | – | 34 |
| 65–74 | 948 | 18.53 (0.665) | – | – | – | 53 |
| 75–84 | 918 | 11.74 (0.396) | – | – | – | 82 |
| 85 + | 845 | 5.94 (0.200) | – | – | – | 155 |
The probabilities of each type of frailty transition over time for each age group from the fully adjusted model are displayed in Figure 3.
FIGURE 3.
Proportion transitioning into different frailty categories (states), by starting frailty categories and age groups (fully adjusted model). Reproduced from Walsh et al. 55 This is an Open Access article distributed in accordance with the terms of the creative commons attribution (CC-BY 4.0) licence which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This figure includes minor additions and formatting changes to the original.
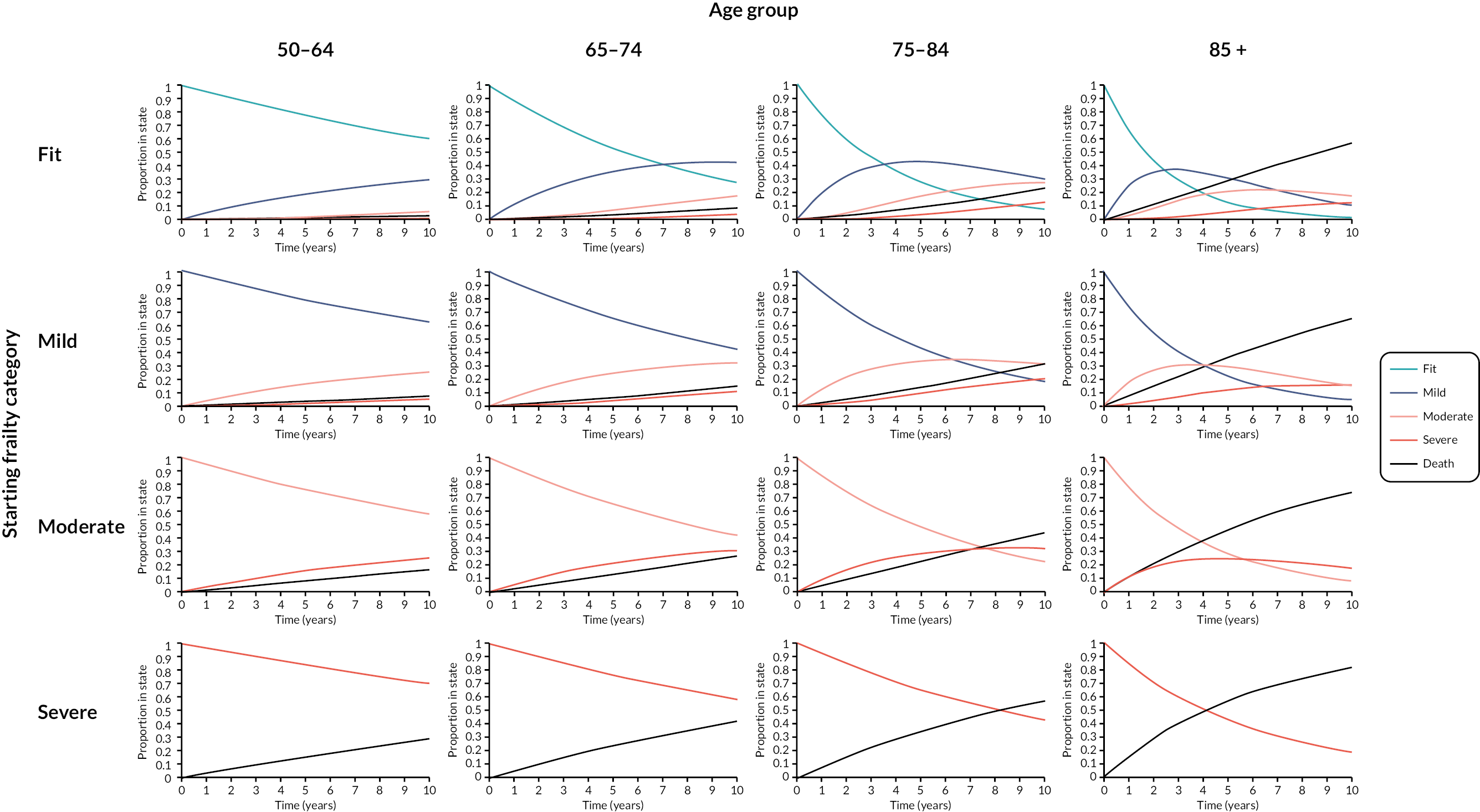
Prevalence of frailty
Findings on prevalence have been reported elsewhere. 55 Cohort age structure changed slightly over time, with 524,936 (47.5%) aged ≥ 65 in 2006 and 735,936 (49.4%) in 2017, for age 85 + this was 68,332 (6.2%) and 102,949 (6.9%), respectively. Over the same period, overall prevalence of frailty increased from 26.5% (95% CI 26.4 to 26.6) to 38.9% (95% CI 38.8 to 39.0). Frailty was already present in the 50–64 group, rising from 10.8% in 2006 to 19.6% in 2017 and prevalence increased with age (see Appendix 1, Table 23). Prevalence increased in all frailty categories, with the greatest proportion seen in mild and moderate frailty in all age groups (Figure 4). Total numbers with frailty increased from 292,751 to 579,828, with the greatest increase in numbers seen in 65–74 age and mild frailty categories.
FIGURE 4.
Prevalence of frailty categories 2006–17 by age group. Reproduced from Walsh et al. 55 This is an Open Access article distributed in accordance with the terms of the creative commons attribution (CC-BY 4.0) licence which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This figure includes minor additions and formatting changes to the original.
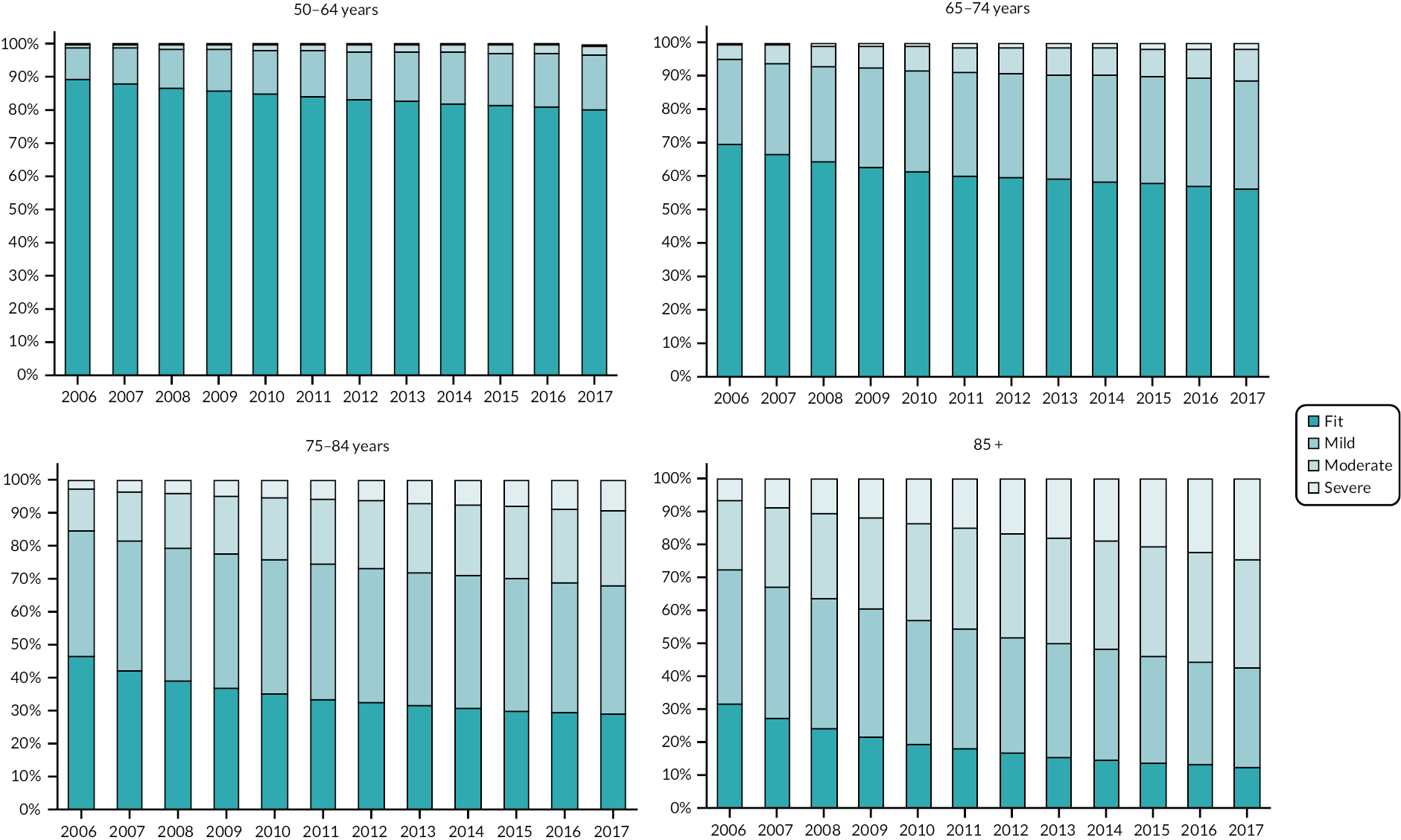
The prevalence of frailty according to the two characteristics most predictive of transitions to frailty in the multistate model, age group and deprivation, are displayed in Figure 5, for the year 2015 (the year for which the deprivation indicators relate to). The difference in prevalence between people in the most versus the least deprived quintiles rises from 11% in people aged 50–54 to 18% in people aged 65–69 years, showing the widest disparities in the retirement age population, but a significant difference in prevalence already evident in middle age. The difference in prevalence according to IMD quintile then decreases until the very oldest age groups.
FIGURE 5.
Prevalence of frailty by age and IMD quintile, 2015.
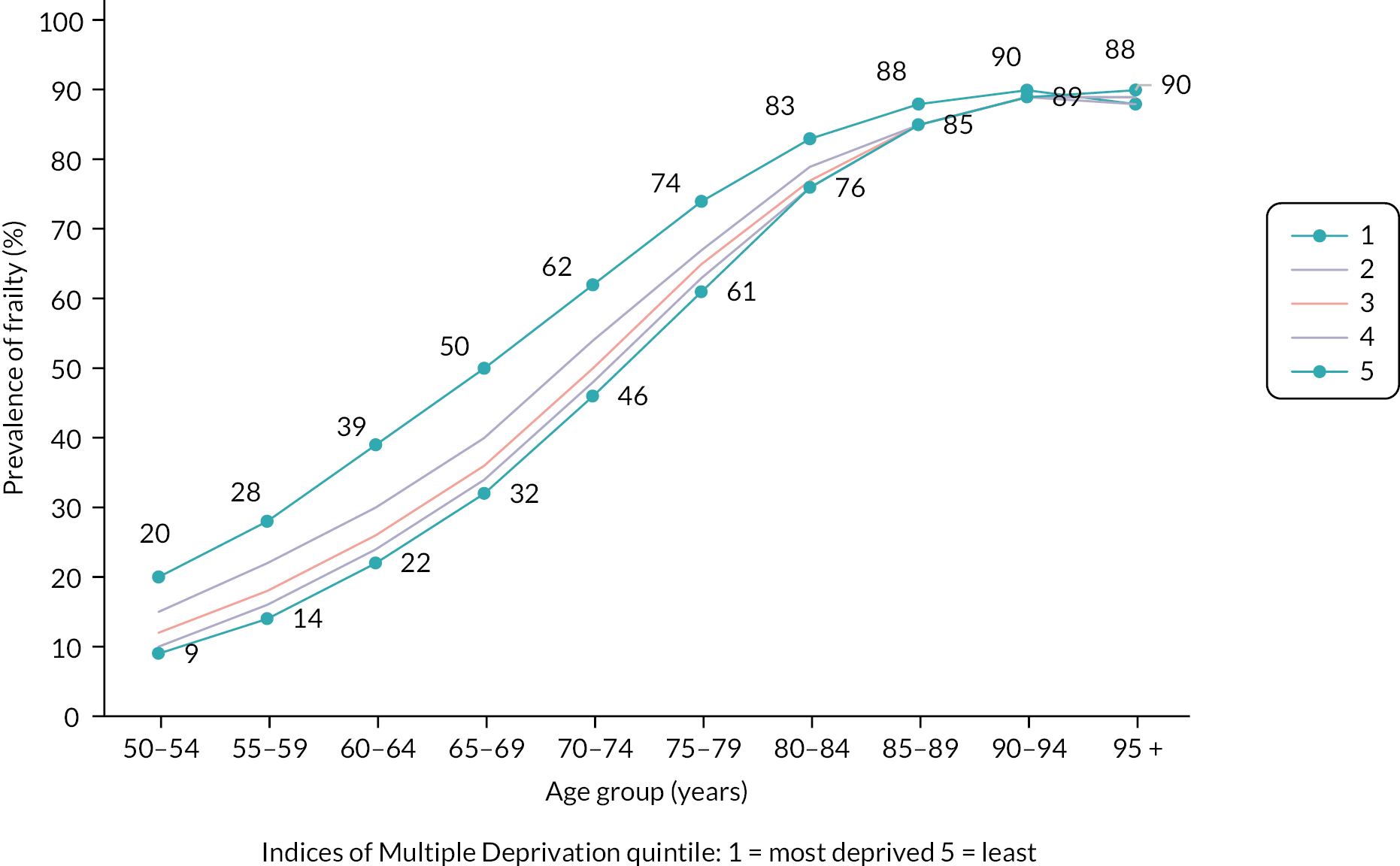
Primary care use
Overall, the mean annual use of each type of primary care service increased with increasing frailty (Table 5). However, different trends were observed when stratified by the four age categories. Mean face-to-face appointments were generally similar within frailty categories across age groups, but with a notable decrease in appointments with older age in the severe category. Telephone appointments followed the same pattern. Home visits increased with age in all frailty categories. E-consultations were still uncommon due to their more recent introduction and were more often used by adults aged 50–74. Prescriptions increased with increasing age in both fit and mild frailty categories but decreased in moderate and severe categories.
| Number of contributing calendar yearsa | Type of primary care service use | |||||
|---|---|---|---|---|---|---|
| Face-to-face appointments | Home visits | Telephone triage | E-consultations | Number of individual prescriptions for medicines | ||
| Mean (St.D) | Mean (St.D) | Mean (St.D) | Mean (St.D) | Mean (St.D) | ||
| Overall | ||||||
| Fit | 10,143,679 | 4.9 (6.9) | 0.066 (0.73) | 0.52 (1.4) | 0.0025 (0.095) | 13.9 (21.9) |
| Mild | 3,707,666 | 9.9 (10.9) | 0.38 (2.01) | 1.3 (2.6) | 0.0045 (0.14) | 52.0 (45.8) |
| Moderate | 1,254,796 | 12.2 (13.7) | 1.1 (3.7) | 2.3 (3.9) | 0.0056 (0.17) | 86.9 (73.2) |
| Severe | 408,593 | 13.2 (15.8) | 2.3 (5.6) | 3.7 (5.6) | 0.0078 (0.21) | 131.1 (109.5) |
| Frailty category, age group | ||||||
| Fit | ||||||
| 50–64 | 6,697,966 | 4.3 (6.2) | 0.029 (0.47) | 0.47 (1.3) | 0.0025 (0.095) | 10.9 (19.9) |
| 65–74 | 2,397,527 | 5.9 (7.5) | 0.064 (0.71) | 0.56 (1.5) | 0.0028 (0.10) | 18.6 (22.9) |
| 75–84 | 858,897 | 6.6 (8.4) | 0.20 (1.3) | 0.70 (1.8) | 0.0019 (0.086) | 22.2 (25.6) |
| 85 + | 189,289 | 5.9 (9.0) | 0.76 (2.5) | 0.92 (2.17) | 0.0013 (0.050) | 23.5 (30.6) |
| Mild | ||||||
| 50–64 | 1,108,641 | 9.5 (10.5) | 0.13 (1.2) | 1.3 (2.6) | 0.0058 (0.15) | 48.6 (49.5) |
| 65–74 | 1,210,346 | 10.1 (10.8) | 0.20 (1.5) | 1.2 (2.4) | 0.0051 (0.15) | 52.3 (41.9) |
| 75–84 | 1,011,372 | 10.4 (11.4) | 0.50 (2.3) | 1.4 (2.7) | 0.0032 (0.12) | 53.9 (43.5) |
| 85 + | 377,307 | 8.9 (11.5) | 1.4 (3.8) | 1.7 (3.1) | 0.0024 (0.11) | 56.1 (51.2) |
| Moderate | ||||||
| 50–64 | 152,409 | 13.2 (13.8) | 0.37 (2.3) | 2.3 (4.4) | 0.0091 (0.21) | 95.4 (90.1) |
| 65–74 | 296,118 | 13.3 (13.9) | 0.52 (3.0) | 2.1 (3.8) | 0.0075 (0.19) | 88.5 (71.6) |
| 75–84 | 487,797 | 12.7 (13.8) | 0.98 (3.5) | 2.2 (3.8) | 0.0047 (0.16) | 85.2 (69.1) |
| 85 + | 318,472 | 10.0 (13.0) | 2.0 (4.7) | 2.5 (4.0) | 0.0037 (0.13) | 84.1 (71.3) |
| Severe | ||||||
| 50–64 | 20,138 | 17.1 (18.1) | 0.97 (4.0) | 4.3 (7.3) | 0.022 (0.38) | 162.6 (143.7) |
| 65–74 | 55,727 | 16.3 (17.2) | 1.2 (4.4) | 3.8 (5.8) | 0.015 (0.32) | 142.8 (114.8) |
| 75–84 | 159,707 | 14.4 (16.1) | 2.0 (5.6) | 3.6 (5.5) | 0.0065 (0.19) | 131.7 (109.0) |
| 85 + | 173,021 | 10.6 (14.3) | 3.0 (6.0) | 3.7 (5.3) | 0.0050 (0.14) | 123.2 (102.3) |
As the cohort grew in population size over time, all aspects of the total primary care service use increased (see Appendix 1, Table 24).
Secondary care use
As with primary care, overall mean annual use of all secondary care services increased with increasing severity of frailty (Table 6). When stratified by age, mean annual outpatient appointments, A&E attendances and hospital admissions (both elective and unplanned) were similar across age groups within the fit and mild categories, and decreased with increasing age in moderate and severe categories. The total annual days of hospital stay were highest in the 85 + group across all frailty categories, although differences between age groups were less pronounced in the severe category.
| Frailty category, age group | Number of contributing yearsa | Type of secondary care service use | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outpatient appointments | Emergency department attendances | Hospital admissions | Days of hospital stay | |||||||
| Total | Elective | Unplanned | Critical care | Totalb | Electivec | Unplannedc | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Fit | 10,143,679 | 1.4 (3.5) | 0.15 (0.59) | 0.27 (1.4) | 0.20 (1.3) | 0.07 (0.35) | 0.0027 (0.054) | 4.6 (18.9) | 1.1 (9.3) | 3.1 (14.0) |
| Mild | 3,707,666 | 3.4 (5.5) | 0.32 (0.89) | 0.66 (3.1) | 0.45 (3.0) | 0.21 (0.66) | 0.0071 (0.088) | 7.5 (22.0) | 1.4 (10.5) | 5.5 (16.4) |
| Moderate | 1,254,796 | 4.8 (6.9) | 0.57 (1.2) | 1.1 (5.1) | 0.64 (4.9) | 0.42 (0.95) | 0.011 (0.11) | 11.5 (24.4) | 1.4 (9.6) | 9.1 (19.8) |
| Severe | 408,593 | 5.8 (9.2) | 0.92 (1.6) | 1.5 (6.0) | 0.71 (5.8) | 0.75 (1.3) | 0.013 (0.12) | 16.3 (27.8) | 1.3 (9.6) | 13.8 (23.5) |
| Fit | ||||||||||
| 50–64 | 6,697,966 | 1.3 (3.3) | 0.15 (0.59) | 0.22 (1.3) | 0.17 (1.2) | 0.05 (0.31) | 0.0020 (0.047) | 3.3 (17.3) | 0.96 (8.2) | 2.1 (12.7) |
| 65–74 | 2,397,527 | 1.7 (3.8) | 0.14 (0.54) | 0.33 (1.6) | 0.25 (1.5) | 0.07 (0.36) | 0.0037 (0.064) | 4.6 (18.3) | 1.3 (10.8) | 2.9 (12.8) |
| 75–84 | 858,897 | 2.0 (3.9) | 0.18 (0.60) | 0.40 (1.7) | 0.27 (1.5) | 0.12 (0.46) | 0.0045 (0.069) | 7.7 (22.4) | 1.4 (10.4) | 5.5 (17.6) |
| 85 + | 189,289 | 1.7 (3.5) | 0.28 (0.77) | 0.44 (1.6) | 0.18 (1.4) | 0.25 (0.64) | 0.0027 (0.052) | 14.4 (28.0) | 1.2 (9.8) | 11.6 (23.1) |
| Mild | ||||||||||
| 50–64 | 1,108,641 | 3.5 (5.8) | 0.33 (0.97) | 0.62 (3.5) | 0.46 (3.4) | 0.16 (0.65) | 0.0066 (0.086) | 4.9 (20.1) | 1.2 (10.8) | 3.3 (14.4) |
| 65–74 | 1,210,346 | 3.5 (5.5) | 0.28 (0.82) | 0.67 (3.1) | 0.49 (3.0) | 0.17 (0.60) | 0.0079 (0.093) | 5.8 (21.8) | 1.4 (12.1) | 4.0 (15.2) |
| 75–84 | 1,011,372 | 3.4 (5.2) | 0.32 (0.83) | 0.70 (3.0) | 0.46 (2.9) | 0.24 (0.66) | 0.0077 (0.091) | 8.7 (21.7) | 1.4 (8.8) | 6.5 (16.9) |
| 85 + | 377,307 | 2.7 (4.6) | 0.45 (0.99) | 0.67 (2.2) | 0.27 (2.0) | 0.38 (0.80) | 0.0041 (0.065) | 14.6 (25.2) | 1.2 (8.6) | 11.8 (20.6) |
| Moderate | ||||||||||
| 50–64 | 152,409 | 6.2 (8.6) | 0.62 (1.6) | 1.3 (7.3) | 0.94 (7.1) | 0.36 (1.0) | 0.015 (0.13) | 7.4 (21.8) | 1.4 (8.1) | 5.6 (17.6) |
| 65–74 | 296,118 | 5.5 (7.6) | 0.50 (1.2) | 1.2 (5.8) | 0.82 (5.6) | 0.35 (0.92) | 0.015 (0.13) | 8.5 (22.1) | 1.5 (9.0) | 6.3 (17.8) |
| 75–84 | 487,797 | 4.8 (6.5) | 0.54 (1.1) | 1.1 (4.8) | 0.65 (4.7) | 0.42 (0.92) | 0.012 (0.11) | 11.4 (24.8) | 1.5 (10.9) | 8.9 (19.7) |
| 85 + | 318,472 | 3.4 (5.8) | 0.64 (1.2) | 0.89 (2.9) | 0.32 (2.7) | 0.54 (0.98) | 0.0053 (0.074) | 16.2 (26.1) | 1.09 (8.6) | 13.5 (21.7) |
| Severe | ||||||||||
| 50–64 | 20,138 | 9.8 (11.6) | 1.1 (2.4) | 2.6 (11.9) | 1.8 (11.7) | 0.75 (1.7) | 0.028 (0.19) | 11.6 (26.8) | 1.7 (9.9) | 9.1 (21.9) |
| 65–74 | 55,727 | 8.2 (10.4) | 0.9 (1.7) | 1.9 (8.1) | 1.2 (7.9) | 0.71 (1.4) | 0.023 (0.16) | 12.9 (26.7) | 1.5 (8.5) | 10.5 (22.6) |
| 75–84 | 159,707 | 6.3 (9.4) | 0.90 (1.6) | 1.5 (6.1) | 0.79 (5.9) | 0.73 (1.3) | 0.015 (0.12) | 15.6 (28.1) | 1.4 (10.1) | 13.0 (23.8) |
| 85 + | 173,021 | 4.1 (7.8) | 0.92 (1.5) | 1.2 (3.7) | 0.36 (3.4) | 0.77 (1.2) | 0.0062 (0.080) | 18.8 (27.7) | 1.0 (9.4) | 16.2 (23.3) |
As with primary care, all aspects of the total secondary care service use increased as the cohort progressed (see Appendix 1, Table 25).
Costs associated with frailty
Annual care costs in primary care rose steeply from fit to mild categories, with a tripling of costs in the 50–64 age group and a doubling in the 85 + age group. Increases in costs by frailty category are also more pronounced in the 50–64 age group both to moderate and severe categories, which had the highest costs overall. For adults aged 65 and above, there is little difference in the annual primary care costs for moderate and severe frailty (Table 7). In contrast, annual secondary care costs were highest throughout in the 85 + age group at each level of frailty, and generally decreased with age within each frailty category, with the exception of the severely frail category which had similar costs across all ages (Table 8).
| Primary care costs £, mean (SD) | Secondary care costs £, mean (SD) | Elective costs £, mean (SD) | Unplanned costs £, mean (SD) | Total care costs £, mean (SD) | |
|---|---|---|---|---|---|
| Overall | |||||
| Fit | 324 (388) | 533 (3017) | 115 (1444) | 173 (2072) | 857 (3105) |
| Mild | 878 (665) | 1533 (5211) | 280 (2457) | 652 (3626) | 2411 (5355) |
| Moderate | 1339 (936) | 2861 (7076) | 409 (2952) | 1531 (5295) | 4200 (7275) |
| Severe | 1882 (1272) | 4592 (9072) | 468 (3356) | 2900 (7155) | 6475 (9344) |
| Frailty category, age group | |||||
| Fit | |||||
| 50–64 | 271 (349) | 407 (2570) | 90 (1199) | 105 (1724) | 678 (2649) |
| 65–74 | 402 (407) | 624 (3190) | 152 (1782) | 190 (2045) | 1027 (3280) |
| 75–84 | 476 (466) | 997 (4330) | 188 (1921) | 447 (3027) | 1473 (4423) |
| 85 + | 510 (561) | 1740 (6080) | 164 (1955) | 1137 (4867) | 2250 (6174) |
| Mild | |||||
| 50–64 | 813 (666) | 1190 (4598) | 256 (2469) | 360 (2955) | 2003 (4752) |
| 65–74 | 869 (624) | 1343 (5065) | 295 (2752) | 469 (3262) | 2212 (5198) |
| 75–84 | 925 (669) | 1765 (5372) | 306 (2181) | 828 (3901) | 2689 (5515) |
| 85 + | 966 (752) | 2531 (6595) | 236 (2087) | 1632 (5214) | 3498 (6724) |
| Moderate | |||||
| 50–64 | 1396 (1046) | 2446 (6628) | 485 (3127) | 917 (4496) | 3842 (6889) |
| 65–74 | 1347 (929) | 2494 (6523) | 480 (2976) | 1061 (4636) | 3841 (6750) |
| 75–84 | 1334 (910) | 2868 (7163) | 431 (3202) | 1520 (5295) | 4202 (7356) |
| 85 + | 1313 (923) | 3390 (7591) | 271 (2386) | 2278 (6078) | 4704 (7757) |
| Severe | |||||
| 50–64 | 2214 (1568) | 4656 (9909) | 908 (4950) | 2055 (6612) | 6870 (10,267) |
| 65–74 | 2021 (1323) | 4494 (9103) | 679 (3631) | 2333 (6858) | 6515 (9421) |
| 75–84 | 1910 (1282) | 4592 (9176) | 520 (3506) | 2800 (7265) | 6503 (9466) |
| 85 + | 1774 (1193) | 4617 (8862) | 301 (2844) | 3274 (7184) | 6390 (9089) |
| All frailty categories | |||||
| 50–64 | 372 (506) | 565 (3124) | 123 (1520) | 161 (2057) | 938 (3266) |
| 65–74 | 638 (650) | 1038 (4337) | 228 (2258) | 371 (2858) | 1677 (4522) |
| 75–84 | 913 (825) | 1896 (5848) | 303 (2438) | 957 (4333) | 2809 (6076) |
| 85 + | 1121 (950) | 2989 (7290) | 244 (2297) | 2006 (5828) | 4111 (7501) |
| Primary care | Secondary care | Total care | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | p-value | Ratio of mean costs (95% CI) | Coefficient | p-value | Ratio of mean costs (95% CI) | Coefficient | p-value | Ratio of mean costs (95% CI) | |
| Frailty category | |||||||||
| Fit | – | – | – | – | – | – | – | – | – |
| Mild | 0.850 | < 0.001 | 2.34 (2.34 to 2.34) | 0.79 | < 0.001 | 2.21 (2.20 to 2.23) | 0.813 | < 0.001 | 2.26 (2.25 to 2.26) |
| Moderate | 1.19 | < 0.001 | 3.28 (3.27 to 3.29) | 1.23 | < 0.001 | 3.42 (3.38 to 3.45) | 1.21 | < 0.001 | 3.36 (3.34 to 3.38) |
| Severe | 1.48 | < 0.001 | 4.38 (4.36 to 4.40) | 1.57 | < 0.001 | 4.81 (4.73 to 4.89) | 1.54 | < 0.001 | 4.66 (4.62 to 4.71) |
| Age group | |||||||||
| 50–64 | – | – | – | – | – | – | – | – | – |
| 65–74 | 0.312 | < 0.001 | 1.37 (1.36 to 1.37) | 0.378 | < 0.001 | 1.46 (1.45 to 1.47) | 0.349 | < 0.001 | 1.42 (1.41 to 1.42) |
| 75–84 | 0.387 | < 0.001 | 1.47 (1.47 to 1.48) | 0.734 | < 0.001 | 2.08 (2.07 to 2.10) | 0.600 | < 0.001 | 1.82 (1.81 to 1.82) |
| 85 + | 0.395 | < 0.001 | 1.48 (1.48 to 1.49) | 1.06 | < 0.001 | 2.88 (2.85 to 2.92) | 0.821 | < 0.001 | 2.27 (2.26 to 2.29) |
| Sex | |||||||||
| Male | – | – | – | – | – | – | – | – | – |
| Female | 0.084 | < 0.001 | 1.09 (1.09 to 1.09) | –0.065 | < 0.001 | 0.94 (0.93 to 0.94) | –0.009 | < 0.001 | 0.991 (0.988 to 0.995) |
| IMD quintile | |||||||||
| Least deprived | – | – | – | – | – | – | – | – | – |
| 4th quintile | 0.074 | < 0.001 | 1.08 (1.07 to 1.08) | 0.060 | < 0.001 | 1.06 (1.05 to 1.07) | 0.064 | < 0.001 | 1.07 (1.06 to 1.07) |
| 3rd quintile | 0.099 | < 0.001 | 1.10 (1.10 to 1.11) | 0.124 | < 0.001 | 1.13 (1.12 to 1.14) | 0.113 | < 0.001 | 1.12 (1.11 to 1.12) |
| 2nd quintile | 0.145 | < 0.001 | 1.16 (1.15 to 1.16) | 0.219 | < 0.001 | 1.24 (1.23 to 1.25) | 0.190 | < 0.001 | 1.21 (1.20 to 1.22) |
| Most deprived | 0.211 | < 0.001 | 1.24 (1.23 to 1.24) | 0.327 | < 0.001 | 1.39 (1.37 to 1.40) | 0.284 | < 0.001 | 1.33 (1.32 to 1.34) |
| Ethnicity | |||||||||
| White | – | – | – | – | – | – | – | – | – |
| Asian | 0.033 | < 0.001 | 1.03 (1.03 to 1.04) | –0.115 | < 0.001 | 0.891 (0.878 to 0.905) | –0.061 | < 0.001 | 0.94 (0.93 to 0.95) |
| Black | –0.086 | < 0.001 | 0.92 (0.91 to 0.92) | 0.127 | < 0.001 | 1.14 (1.11 to 1.16) | 0.051 | < 0.001 | 1.05 (1.04 to 1.07) |
| Mixed/other | –0.115 | < 0.001 | 0.89 (0.89 to 0.90) | 0.086 | < 0.001 | 1.09 (1.06 to 1.12) | 0.013 | 0.131 | 1.01 (1.00 to 1.03) |
| Missing | –0.872 | < 0.001 | 0.42 (0.42 to 0.42) | –1.85 | < 0.001 | 0.16 (0.16 to 0.16) | –1.36 | < 0.001 | 0.26 (0.25 to 0.26) |
| Rural/urban | |||||||||
| Rural | – | – | – | – | – | – | – | – | – |
| Urban | –0.112 | < 0.001 | 0.89 (0.89 to 0.90) | 0.045 | < 0.001 | 1.05 (1.04 to 1.05) | –0.017 | < 0.001 | 0.98 (0.98 to 0.99) |
The GLM provided estimates of the coefficients for the predictors for mean primary, secondary and total care costs as well as ratios of costs within each of the predictor categories as compared to the reference levels. Predicted mean annual costs for the four frailty categories groups, adjusted by age group, sex, deprivation, ethnicity and urban/rural location as per the GLM, are presented in Appendix 1, Table 26.
All covariates were statistically significantly associated with the three cost variables in the GLM (Table 9). The largest difference in cost ratios was seen in the frailty variable, with a doubling in cost between fit and mild categories, approximate trebling for moderate, and quadrupling for severe. Total care costs increased between 30% and 40% in all age groups as compared to the 50–64 group; primary care costs increased by 37% in the 65–74 group and around 50% for people aged 75 and above. Secondary care costs increased by 35% in the 75–84 age group as compared to the 50–64 group, with a 27% increase in the 65–74 group and only a 13% increase in the 85 +. There was little difference between male and female costs after adjustment, with a slight increase (4%) in the total care costs for females. A trend of increasing costs with increasing deprivation was observed compared to the least deprived quintile, with 24% increase in cost of primary care use and 14% increase in secondary care costs in the most deprived quintile. There was little difference in any of the cost variables between white and Asian recorded ethnicities. However, between 10% and 13% lower costs were seen in black and mixed/other groups in primary care as compared to the white group, with 10–17% higher costs in secondary care – 10–17%. Total care costs were similar across all groups. Small differences were seen according to location, with rural areas having around 10% lower primary care costs, 4% higher secondary care costs and 4% lower overall.
| Fit | Mild | Moderate | Severe | |
|---|---|---|---|---|
| 50–64 | 516,468 | 56,806 | 5397 | 528 |
| 65–74 | 187,162 | 69,189 | 11,979 | 1462 |
| 75–84 | 86,398 | 71,846 | 23,589 | 4979 |
| 85 + | 21,356 | 27,977 | 14,354 | 4645 |
The predicted mean costs adjusted for the factors included in the GLM for each frailty category show the increase in costs with increasing severity of frailty. Although confidence intervals are narrow due to the large sample size, it is to be noted that the means are influenced by the highly skewed nature of the data set towards a high number of lower costs and a smaller number of very high costs.
The estimated costs for the total cohort (based on the GLM mean estimates for each frailty category, multiplied by the people present in those calendar years) are presented in Figure 6.
FIGURE 6.
Proportion of costs of primary, secondary and total care by frailty category over the study period.
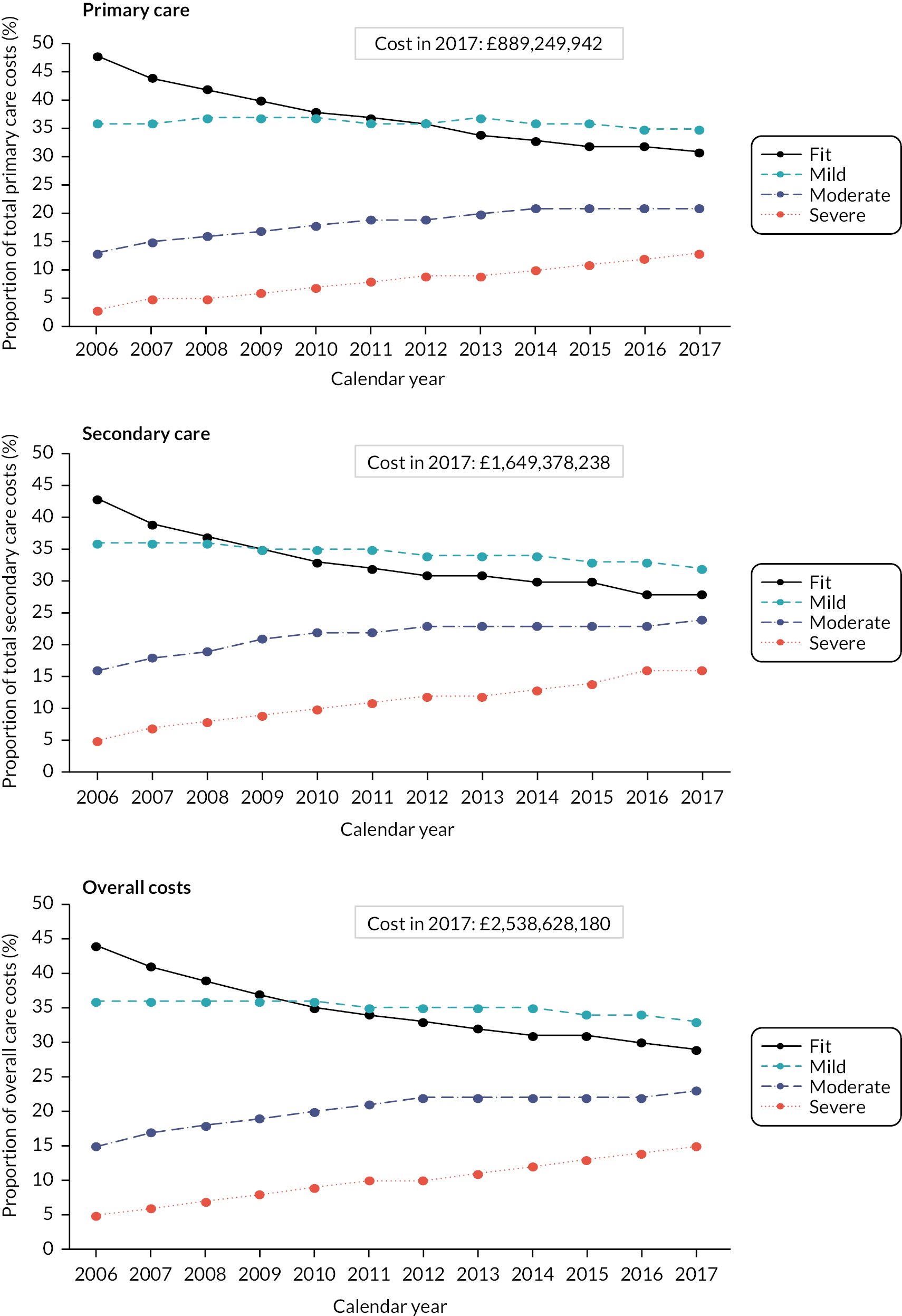
Costs for all frailty categories increased over the cohort period as the size of the cohort increased and the population aged and became more frail. Total estimated costs increased from £1,648,556,329 in 2006 to £2,538,628,180 in 2017. Although individual costs for people living with severe frailty are highest, the larger numbers of people living with mild and moderate frailty contribute most to the overall costs. For example, the proportion of primary care total costs in the mild plus moderate categories rose from 49% in 2006 to 56% in 2017, with costs attributed to patients living with severe frailty comprising only 4% of total primary care costs in 2006 and 13% in 2017. Conversely, as cohort participants aged and transitioned to higher categories of frailty, the proportion of primary care costs incurred by people in the fit category decreased from 48% to 31%. The proportions of costs attributed to the frailty categories were similar in secondary care and total care.
Summary
The large, retrospective cohort of primary care patients, combined with a long period of follow-up, allowed a robust analysis of incidence and prevalence of frailty, transitions between frailty states and service use and costs by frailty category in the population aged 50 and over. The practices and participants were reflective of the English population. A key finding was that 10% of people in the 50–64 age group were already frail at cohort entry. Additionally, the prevalence of mild and moderate frailty throughout the cohort but particularly in people aged 50–74 years was high in comparison to severe frailty, meaning that many more people are living in the community with mild/moderate frailty than with severe frailty. The average age of frailty onset was 69, with incidence of 47.1/1000 person-years (PY), however these figures mask wide variation between different age and socioeconomic subgroups. Risk of transition into, or between, frailty states was predicted by age, higher deprivation, female sex, Asian ethnicity and urban location, with the most marked increased risk associated with increased age.
Patterns of primary and secondary care use showed mean individual increases with the severity of frailty, although due to larger numbers of people with mild/moderate frailty, the absolute numbers of service use were higher in these groups. Primary care use increased with increased frailty severity, whereas prescriptions increased with age in both fit and mild frailty but decreased with age in moderate and severe frailty. Mean use of all secondary care services increased with increased severity of frailty, but total bed use was highest in the 85 + age group across all frailty categories. Annual care costs rose steeply from fit to mild frailty, tripling in the 50–64 age group and doubling in the 85 + age group. Cost increases between mild–moderate and moderate–severe were also higher in the 50–64 age group. Middle-aged adults (50–64 years) with moderate or severe frailty had the highest mean annual costs. However, as with the service use patterns, total primary and secondary costs at population level for each frailty category in the cohort were highest in the fit category, then mild, moderate and finally severe due to the larger numbers of people in the lower frailty categories.
Chapter 5 Stakeholder engagement
Introduction
Stakeholder involvement is considered crucial to the success of simulation studies, and it has been acknowledged that failure to involve stakeholders throughout the simulation model’s development can lead to the findings not being implemented. Use of a structured framework for stakeholder involvement, such as the PartiSim approach, is a valuable way to ensure appropriate contributions throughout the project to ensure model usefulness and acceptability. 77,78 As outlined in Chapter 1, Work Package 3 was dedicated to involving stakeholders at key stages in the study in order to align the progression of the project with stakeholder knowledge and expectations. The project therefore included significant input from contributors outside the core research team, with the aims of informing the planned analyses and simulation model structure, reviewing and considering the implications of both the statistical and simulation findings and informing scenario development.
The two main groups of contributors were: health and social care professionals and others involved in providing or commissioning frailty services; and members of the public with either direct or indirect (e.g. carer, relative) experience of using services designed to support and manage older people living with frailty. The inclusion of patients and the public within the stakeholder engagement sessions comprised the main component of the patient public involvement and engagement (PPIE) strategy for the project. Additionally, the PPIE co-applicant, Mrs Vivienne Windle, worked with the core research team to design, deliver and summarise the stakeholder sessions and also represented the public voice within the Study Steering Committee (as described in Chapter 1).
This chapter utilises the GRIPP2 checklist79 with modifications appropriate for the overall study design and adjusted in line with the concepts behind stakeholder engagement for development of simulation models.
Methods
Design
The SEG events were designed to inform the other work packages at strategic points within the project process and were planned using the methods below. Incorporation of the SEG meetings throughout the project ensured that patient/carer and professional experience in relation to service structures and care trajectories was central to model development. SEG meetings were organised at key points in the study with the intention to use a stakeholder engagement approach informed by that described by O’Haire et al. 80 for use in prioritising future healthcare research needs, with adjustments guided by the PartiSim framework specific for stakeholder engagement in simulation models. 79 The following data collection methods were planned:
-
conceptual mapping to inform simulation model structures (SEG 1)
-
focus group approach to inform recommendations and analysis (SEG 2)
-
nominal group processes to agree ‘what-if’ scenarios and future priorities (SEG 3).
These methods were broadly followed but had to be adapted to take account of the COVID-19 pandemic. The COVID-19 pandemic both delayed access to data and reduced our ability to conduct face-to-face SEG events as planned, due to restrictions on in-person meetings and intense workload pressure on health and social care staff. We were able to adapt our approach so that emerging findings from each stage of the data analysis were presented to key groups in virtual meetings, with participants then asked to reflect on implications for service delivery and organisation. At each stage, the SEG contributed to decision-making around data needs, model building, scenario development and future priorities (see Engagement outputs).
People involved
In this study, the stakeholders included both patients and carers and those who commission and provide healthcare services to older people living with frailty, using the framework developed with our PPI representative and leads for Workstream 2 (Figure 7).
FIGURE 7.
Stakeholder engagement framework.
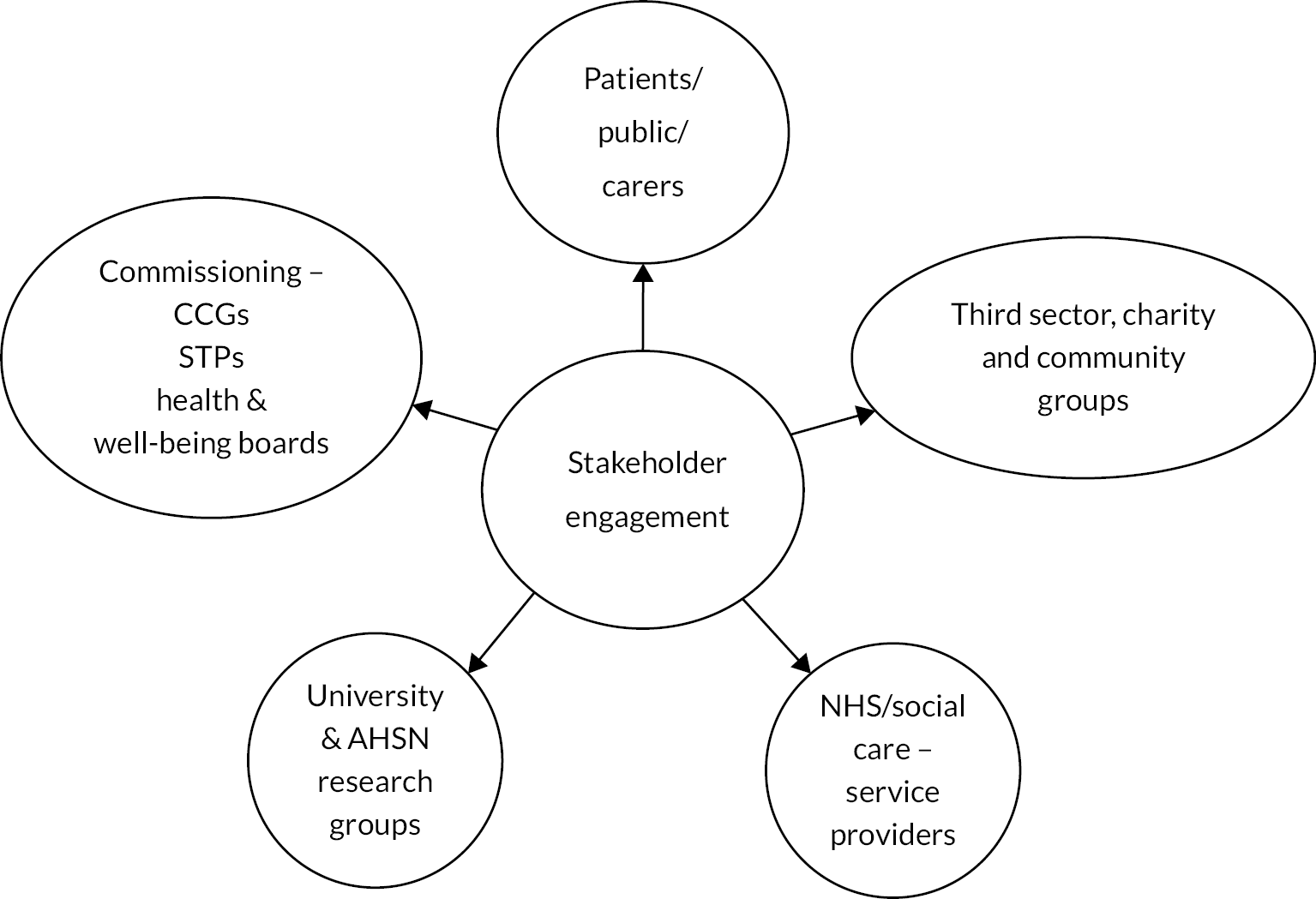
The stakeholder contributors were identified and invited to participate in the SEG in the following ways:
-
Health and social care professionals were invited via research groups, clinical and professional networks. Participants included primary, secondary, urgent and social care as well as third sector, commissioning and public health professionals in the Wessex region, that is Hampshire and the Isle of Wight, Berkshire, Cities of Southampton and Portsmouth. Organisations involved in local Sustainability and Transformation Plans (STPs)/Integrated Care Systems (which came into effect from July 2022) relevant to older people’s services were also represented.
-
Patients and the public were recruited via the existing networks of the PPIE co-applicant (Vivienne Windle) and the PPIE research lead (Francesca Lambert), through the School of Health Sciences Ageing and Dementia Panel, which maintains a database of older people who are willing to provide patient and carer input to the design and implementation of research studies. Opportunities were also advertised via the NIHR Applied Research Collaboration (ARC) Wessex, and via adverts through carers’ networks and advertisements on social media (Twitter), in the later stages of the project where meetings were virtual only due to the COVID-19 pandemic.
The first SEG event had 21 attendees, including representatives from primary care medicine, geriatrics, nursing, social care, health and social care commissioning, third sector (charities for older people) and two members of the public (PPIE contributors). A further four people were unable to attend on the day but were included in the dissemination and consultation on the summaries.
Due to COVID-19 and the feedback from patient and carer representatives at SEG 1, further health and social care professional SEGs were held separately to those with the public members, and presentation materials and discussion topics were adjusted accordingly. A total of 10 people were invited to subsequent patient and carer events (two male, three patients, four carers, both patients and carers, and one had unknown status). At the first patient and carer event for SEG 2, eight of these attended (two male, five carers, two patients, one both carer/patient). At the second patient and carer event for SEG 2, six attended (one male, five females; two carers, three patients, one carer/patient). At the final patient and carer event for SEG 3, two attended (one male, both carers). Meeting summaries and questions were circulated to all the PPIE group for comment, even if they had not attended a specific event. The declining number of patient and carer participants as the PPIE events progressed reflects increasing health and caring demands due to ageing and the pandemic, as well as the challenges of moving to virtual events. The pandemic compounded the known difficulty in a study of this type, where there is inevitable loss of PPIE participants over time, but it was not possible to invite new members due to the iterative nature of the work, because new people would have found difficulty in contributing without having been in the project from the outset.
One of the difficulties associated with running stakeholder events is that it is challenging to include all the relevant people. Despite our best efforts, including attempting to source additional funding to expand the diversity of members in our public involvement group, we were unable to involve people from under-represented communities in the PPIE group. To address this, our results will be disseminated via a local South Asian radio station and other local ethnic minority networks and groups to gain depth of understanding of the particular issues that these communities face. We hope to reach and involve older people from more different communities in our follow-up study, particularly as the results of this study have highlighted the role of ethnicity and deprivation in the earlier onset and faster progression of frailty.
Stages of involvement
The two groups of stakeholders were involved in the three SEG sessions and will be invited to the forthcoming dissemination event. Mrs Windle was involved in the original grant application and design of the project, the design and delivery of SEG sessions and steering of the overall project, and in the forthcoming event/ideas planning and production of dissemination materials.
Measurement of impact
Following each SEG, detailed minutes were written and themes for each topic of discussion drawn out and presented in a summary leaflet. These included contributions from the SEGs which resulted in direct impact to the project, for example discussion around the key messages from the initial cohort data analysis and how this related to the experience of the stakeholders, clarification of the structure of the simulation model, suggestions for simulation model parameterisation and prioritisation of scenarios, as well as guidance on recommendations and dissemination. Additionally, there were aspects which could not be addressed within this project, but which have informed subsequent grant applications, for example around having an adequate workforce with the appropriate skills to provide the care needed for older people with frailty.
Overview of stakeholder sessions
The SEG meetings were facilitated by core study team members (CF, BW, FL, TE) and a member of the research team (AB), a Frailty Nurse Consultant with experience of interviewing, focus groups and stakeholder engagement. Other clinical members of the research team joined the SEG (HP, SF), and the PPIE co-applicant (VW).
Stakeholder Engagement Group Event 1
The focus of this session was conceptual mapping to inform simulation model structures and this session was run as originally planned, in-person, with both patient/public and professional stakeholders involved. Following preliminary discussions with the School of Health Sciences PPIE panel, the first stakeholder session was held in a local, accessible garden centre in January 2020, and 21 people attended. Patient/carer participants were particularly positive about the use of a less intimidating and more accessible venue.
The session included presentations on what frailty is, why it is important to consider in clinical practice and service planning, as well as an overview of the study and the purpose of the stakeholder sessions. Following presentation of the study overview and definitions of frailty, participants were allocated to three groups to include representatives from a balance of different organisations in each, with a facilitator from the study team. Two discussion sessions with plenaries designed to inform the project then followed, with the availability of vignettes, prepared by co-applicant HP, to act as prompts for discussion. Each group was provided with an A3 piece of paper with a diagrammatic representation of the simulation model structure (see Appendix 2, Figure 18) and asked to consider the following questions:
How do we identify and categorise frailty in different age groups/different services?
Consider:
-
Where and how frailty is identified in these age groups?
-
How are patients categorised in the different service settings?
-
What age cut-offs are used?
-
What frailty cut-offs are used?
-
What caseload/proportions in each age group would you expect to see?
Participants were asked to consider the following questions in relation to the frailty trajectory and services (see Appendix 2, Figure 19):
-
How are patients managed in different services, and how do they move between services that they need?
-
What works well?
-
Where are the gaps in provision, what would you like to see on offer?
-
What are the issues with access/capacity?
-
Which types of interventions lead to movement between more to less frail categories?
Detailed notes were made of the whole session, in addition to flip charts with responses to vignettes.
Stakeholder Engagement Group Event 2
The original aim of this session was to explore findings from the statistical analyses and use a focus group approach to inform recommendations for service providers and commissioners emerging from these. Unfortunately, due to the pandemic, data delivery was substantially delayed, and only partial data were available for discussion. The focus therefore shifted to more general considerations of the implications of frailty for service provision and service priorities. Although the intention had been to present the results from the initial data analysis and development of the simulation model in a joint event including all stakeholders, the necessity of carrying out the sessions through virtual media led to the decision to hold separate sessions for professionals and patients and carers. The study PPIE representative also felt that, in contrast to the first SEG session in which the study was introduced, presentation of data in the second event might result in professional participants dominating discussion, especially given their greater familiarity with both data interpretation and the virtual format. In both sessions, participants were presented with summaries of the numbers of people with frailty in different age groups over the 12-year study period in graphical and tabular format, and its potential implications for current and future service provision were highlighted for discussion.
Professionals (seven attendees) were asked:
-
Are there any surprising findings?
-
What are the most important findings to you?
-
How relevant are these finding to your role/organisation?
-
Would this information change anything you do/are planning?
An outline of the simulation model structure as well as preliminary graphical outputs for the population model, demonstrating changes in ageing and the development of frailty over time, was then presented. Professionals were then asked:
-
Are the model frailty thresholds appropriate for your purposes?
Fit (0–0.12)/Mild (0.13–0.24)/Moderate (0.25–0.36)/Severe (> 0.36)
-
Which parameters would you like to be able to adjust for your organisation/area?
-
Where is it possible to intervene and how would that impact on parameters?
-
Which scenario models are most important for your role/organisation?
-
Preventing/delaying frailty?
-
Slowing progression?
-
Reducing the number of GP consultations by a certain number each year?
-
Using more telephone and virtual consultations?
-
Reducing the number of prescriptions.
The professionals were also asked to consider:
-
The model structure
-
How robust the model needs to be?
-
What scenarios to consider?
-
How to use the model?
In the public/patient sessions, which were split over two sessions (eight and six attendees), a recap of the findings from the first session and a more detailed overview of the project was provided, with opportunities for the attendees to ask AB (a consultant frailty nurse) questions about frailty and associated services. The research team firstly introduced the study and its purpose, and the session then focused on eliciting information about services important to older people, including those living with frailty, using broad questions and drawing out people’s priorities for care, identifying what works well and where there are gaps. This was achieved using the concept of what a ‘good day’ looks like for them, enabling discussion of barriers to achieving this goal when living with frailty.
-
Tell us about a day that goes well for you. What do you need, or what do you have to do to have a ‘good day’?
For example – tell us:
-
What are the day-to-day challenges for you or the person you assist, and how do you get around them?
-
What adaptations have you made/would you like to make?
-
What would help make it easier? (This could be people, health or care services, local groups, physical equipment, technology etc.)
-
Good versus bad days – what makes the difference? ‘Day in the life’ – normal day. Doing routine things … Giving an example …
A word cloud (see Appendix 2, Figures 20 and 21) was made in real time from the discussions, and, following a break, the word cloud was put on the screen and the following questions asked for further reflection:
-
Is there anything else anyone would like to add or comment on?
-
What works well?
-
What could be done differently?
-
What would be your priorities for services and why?
In the second session, we recapped using the word cloud and data were then presented as for the professionals, and a general discussion was facilitated, covering how frailty progressed, factors which might affect frailty and the organisation of services. In addition, the study team asked the participants about different ways of presenting the study findings and recommendations and how this could be best done for a public audience.
Stakeholder Engagement Group Event 3
The original aim of this session was to use a nominal group process to agree ‘what-if’ scenarios and future priorities. The third SEG session with professionals was held as a virtual event due as per the preference of the attendees, largely due to COVID-related work pressures (six attendees, although we did offer the opportunity for contribution by e-mail). This, combined with delays to the service use and cost analyses, meant that the focus had to be on gaining agreement on ‘what-if’ scenarios. The research team organised a hybrid event, with the public members attending in person, in part because this was their preference and as an opportunity to thank them for their input (three attendees).
As new elements of analysis were available following delays in data provision and analysis due to the COVID-19 pandemic, which included service use and associated costs for age/frailty categories, these were presented to both groups, prior to presentation of the updated simulation model and its results. The discussion for both groups of stakeholders then focused on identifying scenarios to consider for the simulation model, including the following:
-
What would happen if we did nothing?
-
What would happen if we could slow down the progression of frailty?
-
What would happen if we improved services/outcomes?
-
What would happen if frailty onset could happen later?
-
Any others that should be considered?
Engagement outputs
The SEG events ensured that outputs from the workstreams were reviewed by key stakeholders and that outputs from the SEG events were fed back directly to the Study Management Group at key points in the data analysis, development of the model and preparation of outputs. The SEG leads collated the information gathered from the events in the form of contemporaneous notes and graphics. These were summarised in leaflets created in consultation with participants. The information gathered was shared with SEG participants in summary format to ensure a sense check and trustworthiness of the information gathered prior to sharing with the full study team.
Stakeholder Engagement Group Event 1 outputs
The summary of key points from the professionals’ session is given in Figure 8 as an example of the type of summary document produced for participants throughout.
FIGURE 8.
Summary poster for SEG Event 1.

Stakeholder Engagement Group Event 2 outputs
The summary pamphlets for the two patient/carer sessions are shown in Appendix 2, Figures 22 and 23.
The summary of the third SEG 2 event for health and social care professionals is in Appendix 2, Figure 24.
Stakeholder Engagement Group Event 3 outputs
A summary of the discussion points from the professionals’ session is given in Appendix 2, Figure 25.
A summary of the discussion from the public/patient session is given in Appendix 2, Figure 26.
Summary of impact
The SEG meetings were essential and integral to the study, providing opportunities to sense check results from descriptive analyses, think about how the simulation model would be useful in practice and to consider the best way of designing the simulation to be useful to different groups of health and care professionals and identify future needs or modifications.
The meetings with professionals provided a forum for communication across sectors and demonstrated the variety of practice in how frailty is measured and managed. The complexity of pathways used for older people with frailty highlighted a gap in the sources of data available to us for analysis and informing the simulation model, which we will attempt to address in future work. The importance of being able to use locally adapted data in the simulation model was also emphasised and could be considered in future. Overall, the sessions with professionals provided substantial direction for the model design, and feedback confirmed that this method of estimating future care needs is sorely needed to promote proactive planning and organisation of services across sectors.
The meetings with the public members showed a stark contrast between the focus on services for which we had data and could include in the simulation model (primary, secondary and urgent care) and a group of care needs that patients and carers considered essential to be able to ‘live well’ with frailty and reduce the risk of deterioration and service use. The latter included timely access to physiotherapy, occupational therapy and social care services in addition to community care, social prescribing and mental health services. The contribution of the voluntary sector, and potentially the role of workplace support services for early onset frailty, featured as extremely important to consider in future service planning.
It is important to note that the aim of the stakeholder engagement was not to achieve consensus, but to explore a range of perspectives. However, within the professional and service user groups respectively, there was a high degree of agreement, but there was more of a contrast between the two groups of stakeholders. Professionals’ contributions were influenced by their knowledge and experience of service provision and organisation, but service users were more concerned with the support they needed to enable them to live positively and were less concerned with sector, service or professional boundaries. The contributions of both stakeholder groups were more complementary than conflicting. They did, however, highlight the ways in which professional and organisational boundaries might limit thinking about service development and planning. Ensuring good engagement from service users will therefore be important in future service planning. Interestingly, in line with the statistical analyses, both groups highlighted the need for further work to understand and deal with the links between higher deprivation levels and higher incidence and progression of frailty, and the role of health, education and income inequalities in being able to self-manage or organise appropriate care, either formal or informal, was a significant concern.
Given the extremely challenging circumstances of the project duration being carried out within the COVID-19 pandemic, we are incredibly grateful to the SEG members for their engagement, and for their sustained interest in the project given issues with declining health, increased caring responsibilities and bereavement experienced by some of the participants, as well as significant work pressure and uncertainty for the professionals. Readvertising from our pool of interested people for each SEG helped to sustain membership to the meetings, but continued and consistent involvement is still a challenge in this type of research.
Considerations for further research
In both professional and public stakeholder groups, the sessions which presented results from the data using graphs and tables and the simulation outputs provoked discussion about the best ways of presenting this information and how to explain it to different audiences. This is an area which rarely has much attention in large projects, which are expected to disseminate work mainly with scientific publications, leaving little time for development of impactful summaries for different audiences. The team will explore this further in the planned dissemination event and consider it as a separate line of enquiry within future research projects.
The SEGs also highlighted different clinical and care roles within services which provide support to people with frailty, emphasising the key role of considering the workforce in terms of future demand for frailty prevention and management. As mentioned above, consideration of a wider scope of services, for example community nursing and mental health services, is important to have a 360° view of the interconnectivity of services and how service reorganisation and substitution may impact on patient outcomes and costs. We have already taken these considerations forward to a successful grant application to NIHR HSDR to explore the optimal workforce for delivering services related to frailty and expanding the simulation model to inform current and future workforce needs.
Participation was difficult to maintain for the duration of the study and, by the final event, there was limited patient/carer involvement. Bringing in new participants as the project progressed was not possible due to the iterative nature of the work and would have meant that each round of engagement could not build on the previous one. This, combined with pandemic limitations, restricted our ability to expand engagement during the study. However, those patient/carer participants still involved at the final SEG were highly engaged and were able to make useful contributions based on their prior knowledge of the study and its findings. In planning studies such as this, where PPIE is iterative, we recommend consideration of how to maintain engagement of older and frail people over the course of a long study. Finally, we intend to address ensuring representation from a wider diversity of communities in our follow-up study, FLOWS, by working together with representatives of varied ethnicities and from more deprived communities to plan analyses and interpret findings more relevant to their experiences of frailty and care needs.
Chapter 6 Development and validation of the simulation model
Introduction
This chapter gives an overview of the development and the validation of the system dynamics (SD) simulation model, the aim of which was to describe the dynamics of frailty in the adult population (aged 50 and over) and its impact on healthcare services. The model development was informed by analysis of a large, routine primary care data set from RCGP RSC (see Chapter 4) and stakeholder engagement events (see Chapter 5). These information sources were used to determine the model structure and the population flow parameters required to estimate incidence and prevalence of frailty and transitions between frailty states, in an ageing population over time. In this chapter, the development of the simulation is described, including the approach to verification and validation of the model, along with the ‘what-if’ scenarios identified following the stakeholder events. The baseline results, along with three ‘what-if’ scenarios are presented in Chapter 7. The benefits and limitations of the modelling approach are discussed in Chapter 8 along with potential future research. Technical aspects of the model such as the underlying differential equations are described in Appendix 3 and Appendix 4.
Simulation model design and aims
System dynamics is a computer simulation modelling approach whose purpose is to analyse changes over time in complex, interacting systems. SD has been used for decades in many different application areas and is ideally suited for health and care systems. 81,82 SD was selected for use in this study because a ‘big picture’, population-level approach was required to provide information on projected changes to demand associated with frailty over time in an ageing population. The model also needed to be useful to a wide range of potential users across many different healthcare settings while minimising data requirements, because we were reliant on retrospective, routine data. In such a complex system, it was also important to capture any feedback effects or unintended consequences as well as the immediate outcomes of the interventions examined in the scenario analysis. The SD approach lends itself particularly well to these requirements and also remains sufficiently flexible to allow it to be adapted to different contexts. The SD approach also allowed us to develop a model that was fairly simple to visualise and describe due to its easily understood organisational-based structure. In this study, we used a SD approach to model population dynamics and predict future frailty-related healthcare demand in the UK. Together with cost analyses, the model allowed us to explore the impact of frailty on future service use and costs. A SD model consists of stocks (accumulations) of material, and flows between them, analogous to a series of water tanks connected by pipes. The rate of flow along each pipe is governed by valves that can be turned up or down. We developed a stock-flow model depicting patient transitions between different states. In our case, the ‘material’ was frail patients, and the stocks were the numbers of patients in different age groups in different health and social care states as identified in Workstream 1 (see Chapters 3 and 4).
The aim of this study was to develop a simulation model of frailty incidence, prevalence and transitions in an ageing population aged 50 and over. SD modelling has frequently been used to model healthcare systems, particularly when a more high-level, aggregated, strategic view is needed, as in this study. SD models are powerful tools that can be used to represent transitions between different stages of illness progression. 83 SD models are effective as they help illustrate how an illness or disease develops in populations over time. This is in line with the overarching aims of the wider project and its focus on frailty trajectories over time in a large population.
We considered the population aged 50 and over, divided into four age groups reflecting the groupings reported in literature relating to older adults’ health care, and cut-offs for services: 50–64, 65–74, 75–84 and ≥ 85. Each age group was further divided according to the frailty severity state measured by the eFI. 8 The model therefore had 16 subgroups, with four frailty categories (fit, mild, moderate and severe) within each age group. A schematic of the conceptual underlying SD model is shown in Appendix 3, Figure 27.
As well as the 16 population subgroups, the model considered ageing progression from one age category to the next (i.e. patients turning 65, 75 or 85), the transition of people from one frailty state to another (i.e. Fit to Mild, Mild to Moderate, Moderate to Severe), deaths and those leaving a participating GP practice. As the data used to inform the simulation model (RCGP RSC data set) represents 8% of all GP practices,58 and patients may therefore leave a RCGP registered GP practice for one that is not included in the cohort data, this had to be considered within the initial simulation structure. The model also allowed for new patients entering the population in any of the 16 subgroups over the study period (January 2006–December 2017), largely due to entering the 50–64 age group.
The model structure was informed by the SEG (see Chapter 5) and the statistical analyses from Workstream 1 (see Chapter 4), which then informed the development of a prototype simulation model. The SD model for population trends was internally validated using the RCGP RSC data set analyses and comparable data from Wales was used for external validation. The Welsh data were provided by the SAIL Databank, which is a pseudonymised database containing linked secondary and primary care data, including the same key variables as the primary cohort from RCGP RSC, but which has 80% population coverage.
Following validation, the simulation model was further developed using ONS population and mortality estimates for England to provide a national level estimate of the frailty incidence, prevalence and transitions in an ageing population aged 50 and over.
Data sources for development of the simulation model
Data extracts and analyses of the RCGP RSC data set, including linked HES and ONS data, from Workstream 1 were used in the development and internal validation of the simulation model. Details of the RCGP RSC data set and its data government and management are provided in Chapter 3. The description of the RCGP RSC cohort is provided in Chapter 4. We used a similar data extract from the SAIL Databank for the external validation of the simulation model. Details of the SAIL data and research governance and management are found in Chapter 3. The data sources are summarised in Appendix 3, Table 27.
Description of primary data analysis to inform simulation model development and validation
Data analyses from Workstreams 1 and 2 were used to populate and then validate the simulation model to enable simulation of population trends, service use and costs. The simulation model does not follow individual patients, but uses the results obtained from the statistical analysis to calculate monthly transition probabilities between states (stocks). The simulation model used data from Workstreams 1 and 2 to capture the key clinical and demographic differences that influence these transitions, as well as information about the costs and outcomes associated with each state.
The RCGP RSC data set, with exclusions applied after linkage to ONS mortality statistics (e.g. data conflicts, see Chapter 3), was analysed to provide data for the stocks and parameterisation of the prototype simulation model. The data set included data on 2,171,497 patients and 15,514,734 person-years of observation. There were 1,104,135 patients in 2006 rising to 1,489,495 in 2017. Over the cohort period, 1,067,362 patients entered, 355,889 died (16.4%) and 411,378 (18.9%) deregistered from RCGP practices.
Variables generated from the raw data set included age group with four categories (50–64 years, 65–74 years, 75–84 years and 85 +), frailty category (fit, mild, moderate, severe), enabling data aggregation by the 16 distinct age/frailty groups. The year of cohort entry and exit was generated for each patient from the minimum and maximum year of data available between 2006 and 2017. Entry and exit to the cohort reflect a patient entering a GP practice or turning 50, or leaving a GP practice – that is, meeting the eligibility criteria for the study (outlined in Chapter 3) and depicted in Figure 27 as vertical arrows labelled ‘Entering’ and ‘Leaving’. Patient deaths are also captured in the model and are represented in Figure 27 by the arrows labelled ‘Deaths’.
Analyses from Workstream 1 were summarised to inform stocks, entry, and exit, age and frailty flows required for simulation of frailty progression in the ageing population. Descriptive analyses can be found in Chapter 4. For the purposes of the simulation, additional descriptive analyses were carried out to generate information in the format required for the simulation. The full list of data requirements for the simulation is given in Appendix 3, Table 28.
The same approach was used for the SAIL data extract and analysis as for the RCGP RSC data in terms of the variables and descriptive tables produced. In the SAIL data, there were differences from the RCGP data set in that all GP contacts were summarised in a single variable, and that ambulance attendances and residential care data was also available. Residence data from RCGP RSC were supplemented by data on residential care transitions and ambulance use by frailty status from SAIL Databank, to inform simulation of impacts and costs beyond the healthcare setting. Initial comparison of the descriptive data from both data sources suggested that the SAIL population differed in proportions of patients with frailty by age group and in mortality rates. Additional descriptive analyses of frequencies and proportions of patient characteristics such as deprivation were therefore produced to establish potential causes and inform the simulation model validation phase.
Structure and principles of the frailty dynamics population model
The structure of the conceptual model was informed by the SEG (see Chapter 5) and by the statistical analyses in Workstream 1 (see Chapter 4). We also used these analyses to inform the development of a prototype simulation model, using a SD based approach to explore the development and impact of frailty in the population and likely future scenarios over a 10-year+ time frame. The simulation model population projections were internally validated against retrospective data from the RCGP RSC data set and externally validated against comparable data from the SAIL Databank following validation of the simulation model, and ‘what-if’ scenarios were developed with the SEG. To explore the future impact of the ‘what-if’ scenarios at a national level, the validated simulation model was adapted further to include ONS population and mortality estimates. The exploration of the ‘what-if’ scenarios via simulation is ongoing but will be able to inform recommendations to commissioners and service leaders; some illustrative results are provided in Chapter 7. A flow diagram of the processes involved in developing, validating and communicating the simulation model is shown in Figure 9.
FIGURE 9.
Steps involved in the development and validation of the frailty dynamics SD model.
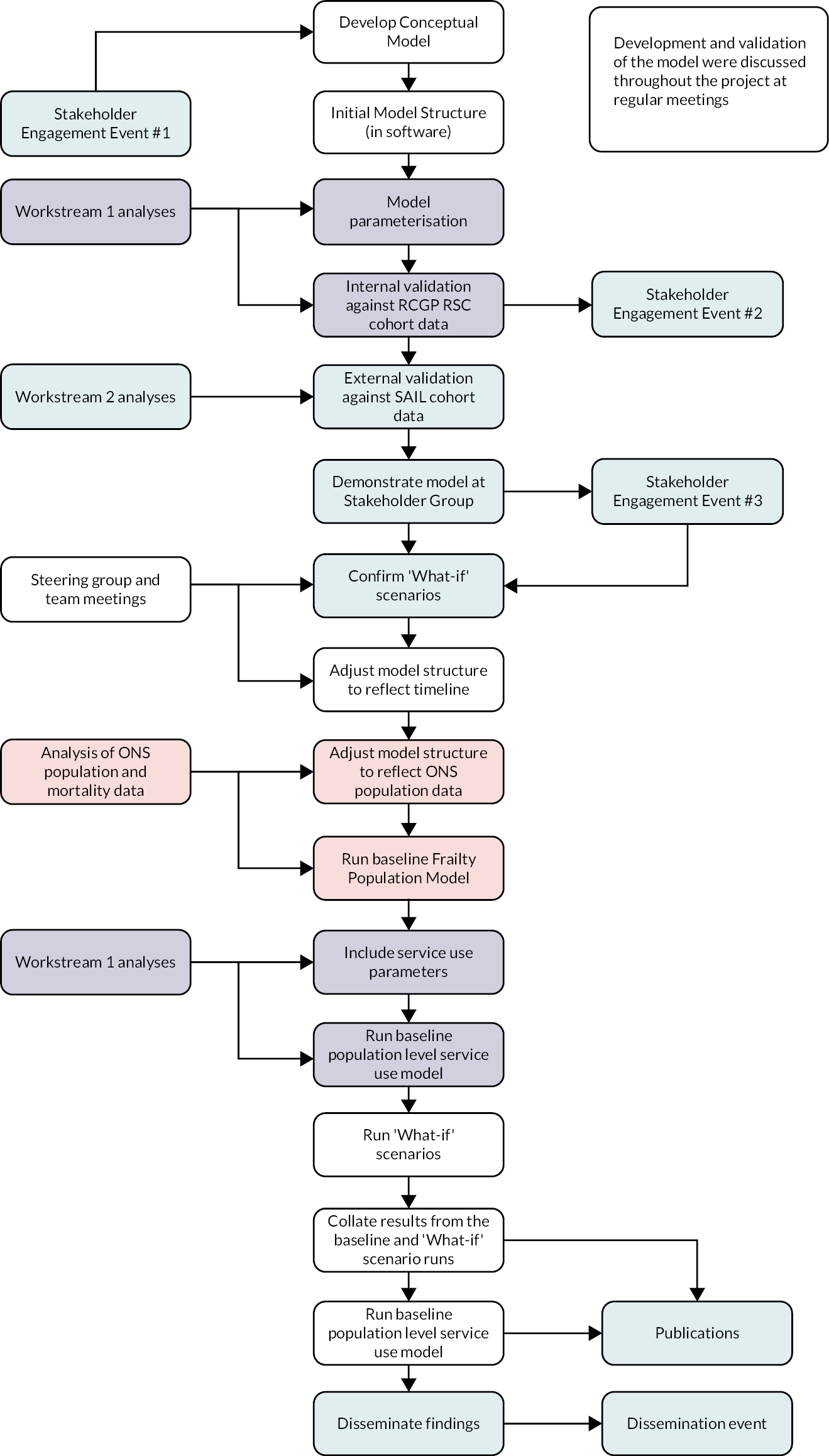
An initial model structure comprising an ageing population flow and frailty severity states was developed by the research team and presented in the first SEG event (see Appendix 3, Figure 28).
Analysis from the RCGP RSC data set indicated that there was unexpectedly high movement in and out of participating practices in that sample, so practice deregistrations was added to the model structure, but otherwise stakeholders agreed that the diagram captured relevant transitions. After further rounds of discussion with both the research team and SEG participants, it was agreed that the population flow, ageing progression and frailty progression would form the underpinning structure for the simulation model (see Appendix 3, Figure 27).
The frailty progression system dynamics model
The SD model was developed to reflect the structure described above and to mirror the same time period as the cohort study (2006–17)55 to allow for internal validation against real world data. The model was implemented in AnyLogic (8.7.3).
The RCGP RSC data were used to identify the transition states (Fit, Mild, Moderate and Severe) and to estimate the transition rates between the frailty categories (e.g. Fit to Mild, Mild to Moderate and Moderate to Severe). The RCGP RSC data were also used to examine the ageing progression (turning 50, 65, 75 or 85) as well as the entry and exit components.
The initial SD model (screenshot of the AnyLogic model is provided in Appendix 3, Figure 29) consisted of 48 stocks (the number of patients alive, who have died or who have left their GP practice; in each of the 16 age/frailty groups) and 72 flows (entering the cohort, frailty transitions, ageing, dying, and leaving their GP practice). The number of patients in each stock are calculated every month from a set of differential equations (see Appendix 4) where the movement between the stocks is governed by the flow equations. Each month a patient can remain in the same stock or move to (1) the next frailty state (e.g. from Fit to Mild), (2) the next age group, (3) die or (4) leave the cohort through deregistration. The model allows for a patient moving to the next frailty category and not jumping more than one frailty state as this was only observed in a very small number of situations in the data. This also reflects the structure of the MSM used to analyse the cohort data (see Chapter 4).
For example, considering the number of fit, 50–64-year-olds, the change in the stock level is governed by the number of new patients that (1) enter the population, (2) die, (3) leave a registered GP, (4) become 1 year older and move into the next age group, or (5) become mildly frail.
In the mathematical notation, the differential equation representing the monthly change in the number of patients that are fit and 50–64-year-olds is governed by Equation (1) where each of the terms on the right-hand side of the equation are flows into or out of the subgroup. Each of the 16 age/frailty subgroups has a similar equation, although the flows are different. Key equations are given in Appendix 4.
Equation 1: Monthly change in patients that are fit, aged 50–64:
Modelling assumptions
In the final Frailty Dynamics Simulation Model, population ageing, and mortality are assumed to reflect projections from the ONS. These take into account historical population trends and known mortality statistics. More recent short-term trends in longevity were not incorporated into the model.
Several of the flows are dependent on the number of patients in an age/frailty group in a month. Before the next month, a proportion of those will move out from the group. In estimating several of the flows, the proportion is based on a fixed parameter while others are time dependent, reflecting how the dynamics of a population subgroup changes over time. For example, the number of patients in a given population subgroup that die depends on a constant death rate, whereas the number transitioning to a new frailty category or age group depends on quadratic, cubic or quartic equations in time. The exact form of the flow equation was determined after using the RCGP RSC data to examine the proportion of patients that transitioned to the next category over the observed 12-year time period and using multiple least squares regression (with time, time,2 time3 and time4 as the explanatory variables) to best reflect the time dependent nature in the data.
Parameterisation of simulation model stocks
Stocks were populated for the first year of the model, based on data from Workstream 1. This required starting population (Table 10) data for each age group and frailty state, initially in line with the numbers within the RCGP RSC cohort to provide a simulation for that English cohort.
| Year | 50–64 | 65–74 | 75–84 | 85 + | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fit | Mild | Moderate | Severe | Fit | Mild | Moderate | Severe | Fit | Mild | Moderate | Severe | Fit | Mild | Moderate | Severe | ||
| 2017 | 76.9 | 18.9 | 3.88 | 0.63 | 28.1 | 18.7 | 5.22 | 1.48 | 10.0 | 11.8 | 6.86 | 3.23 | 2.18 | 4.76 | 4.61 | 3.18 | 200.4 |
| 2018 | 77.4 | 19.7 | 4.18 | 0.71 | 28.1 | 19.4 | 5.41 | 1.60 | 9.76 | 11.6 | 6.88 | 3.46 | 2.10 | 4.70 | 4.64 | 3.47 | 203.1 |
| 2019 | 77.9 | 20.4 | 4.48 | 0.78 | 27.9 | 20.0 | 5.54 | 1.71 | 9.66 | 11.5 | 6.94 | 3.71 | 1.99 | 4.60 | 4.65 | 3.78 | 205.6 |
| 2020 | 78.2 | 21.1 | 4.78 | 0.86 | 27.6 | 20.5 | 5.61 | 1.80 | 9.71 | 11.7 | 7.07 | 4.02 | 1.85 | 4.45 | 4.61 | 4.11 | 207.9 |
| 2021 | 78.6 | 21.9 | 5.07 | 0.94 | 27.1 | 20.8 | 5.62 | 1.88 | 9.79 | 11.9 | 7.24 | 4.38 | 1.71 | 4.27 | 4.56 | 4.46 | 210.1 |
| 2022 | 78.6 | 22.5 | 5.36 | 1.02 | 26.8 | 21.2 | 5.63 | 1.94 | 9.71 | 12.0 | 7.41 | 4.77 | 1.58 | 4.11 | 4.49 | 4.84 | 212.0 |
| 2023 | 78.7 | 23.1 | 5.64 | 1.10 | 26.6 | 21.6 | 5.61 | 2.00 | 9.60 | 12.1 | 7.59 | 5.21 | 1.45 | 3.92 | 4.41 | 5.25 | 213.9 |
| 2024 | 78.4 | 23.6 | 5.90 | 1.18 | 26.0 | 21.5 | 5.45 | 2.01 | 9.96 | 12.7 | 7.95 | 5.78 | 1.32 | 3.72 | 4.30 | 5.68 | 215.4 |
| 2025 | 77.8 | 24.0 | 6.15 | 1.26 | 25.7 | 21.6 | 5.34 | 2.02 | 9.86 | 13.0 | 8.26 | 6.40 | 1.19 | 3.51 | 4.18 | 6.12 | 216.5 |
| 2026 | 76.9 | 24.4 | 6.37 | 1.33 | 25.7 | 22.0 | 5.27 | 2.05 | 9.5 | 13.0 | 8.53 | 7.09 | 1.07 | 3.28 | 4.03 | 6.58 | 217.2 |
| 2027 | 75.8 | 24.6 | 6.57 | 1.40 | 25.9 | 22.5 | 5.22 | 2.08 | 9.03 | 12.9 | 8.75 | 7.86 | 0.92 | 3.02 | 3.85 | 7.04 | 217.6 |
Following internal and external validation, each age category was then populated with numbers from ONS population estimates for 2006, producing a simulation for the entire English primary care population.
Parameterisation of simulation transition rates and flows
Each of the stocks in the simulation were subject to gains and losses from the following flows:
-
cohort entry at age 50
-
age progression
-
deaths
-
cohort entry on entering participating practices
-
cohort exit on leaving participating practices
-
frailty transitions – to mild, moderate or severe states.
For example, the number of patients that are fit and 50–64-year-old is subject to gains from those patients who turn 50 and are eligible for inclusion into the cohort as well as patients aged 50–64-year-old that enter participating practices. The group is also subject to losses from those patients that leave the cohort through death or leaving their registered GP practice. The number of patients in the fit, 50–64-year-old subgroup is also affected by those that turn 65 and move into the next age group but maintain their fitness levels and those that have not aged but have become mildly frail. The parameters associated with each of the flows are given in Appendix 3, Tables 29 and 30.
The baseline prevalence of frailty (for each combination of stratification variables and for the overall SAIL population) was estimated from the SD model (having been informed from the RCGP RSC data set). These model estimates were initially compared against those seen in the RCGP RSC data set before being compared against those seen in the SAIL data set in both a graphical and tabular fashion. The model output from all the stocks and flows were compared against those seen in the RCGP RSC and SAIL data sets. Comparisons were performed using standard goodness-of-fit tests where comparable data were available and by plotting selected outputs as time series for visual comparison. Frailty transition rates and predicted frailty status levels at yearly time interval were also compared between the SD model and the observed data in a similar way.
Validation and verification of the population dynamics model
Validation and verification are challenging in SD modelling. 84–86 The aim of verification and validation is to build confidence in the model, determining whether it is useful and fit for purpose, often using a mixture of qualitative and quantitative tests throughout the model’s development. One robust approach is to compare the model output with historic data, but this relies on the availability of accurate data for all the model variables. A major contribution of this study is that we have been able to validate the SD model using retrospective data from two large data sets (RCGP RSC for internal validation and SAIL for external validation), which cover all the model’s population subgroups and variables.
Validation cohort description and comparison with development cohort
The data set used for simulation validation was extracted from the SAIL Databank. 87–90 The data extraction was designed to mirror that from the RCGP RSC used for model development and included patients registered at a Welsh GP, aged 50 and above, at any time between 2006 and 2017 inclusive. Following exclusions, the final data set included 1,380,959 patients contributing 11,090,653 follow-up years. As the data set includes all patients registered in Wales, this databank provides Welsh population data. The demographic structure of the Welsh population is broadly similar to that of England, making it a suitable data source for validation of the development model based on English primary care data. There are, however, some differences in demographic and socioeconomic structure that were reflected in the cohort data sets and needed to be considered in the validation and development process.
The two cohorts (RCGP RSC and SAIL) were very similar in age structure at the start of 2006 (Figure 10), with the proportion in each age group as follows: age 50–64: 52.5% and 52.8%; age 65 to 74: 24.4% and 24.9%; age 75 to 84: 16.9% and 16.6%; age 85 and over: 6.2% and 5.7% respectively. However, there were differences in the prevalence of frailty across the two cohorts at the same time, with 73.5% against 66.0% fit, 20.5% and 26.3% mild, 5.0% and 6.5% moderate and 1.1% versus 1.3% severe in RCGP RSC and SAIL, respectively. 91 In terms of the median eFI score recorded for each cohort, the value for RCGP RSC is lower [median: 0.056 (0.028–0.111)] compared with that of SAIL [median: 0.0833 (0.0277–0.1388)] suggesting a fitter population.
FIGURE 10.
Age and frailty distribution in RCGP RSC and SAIL data sets in 2006–17.
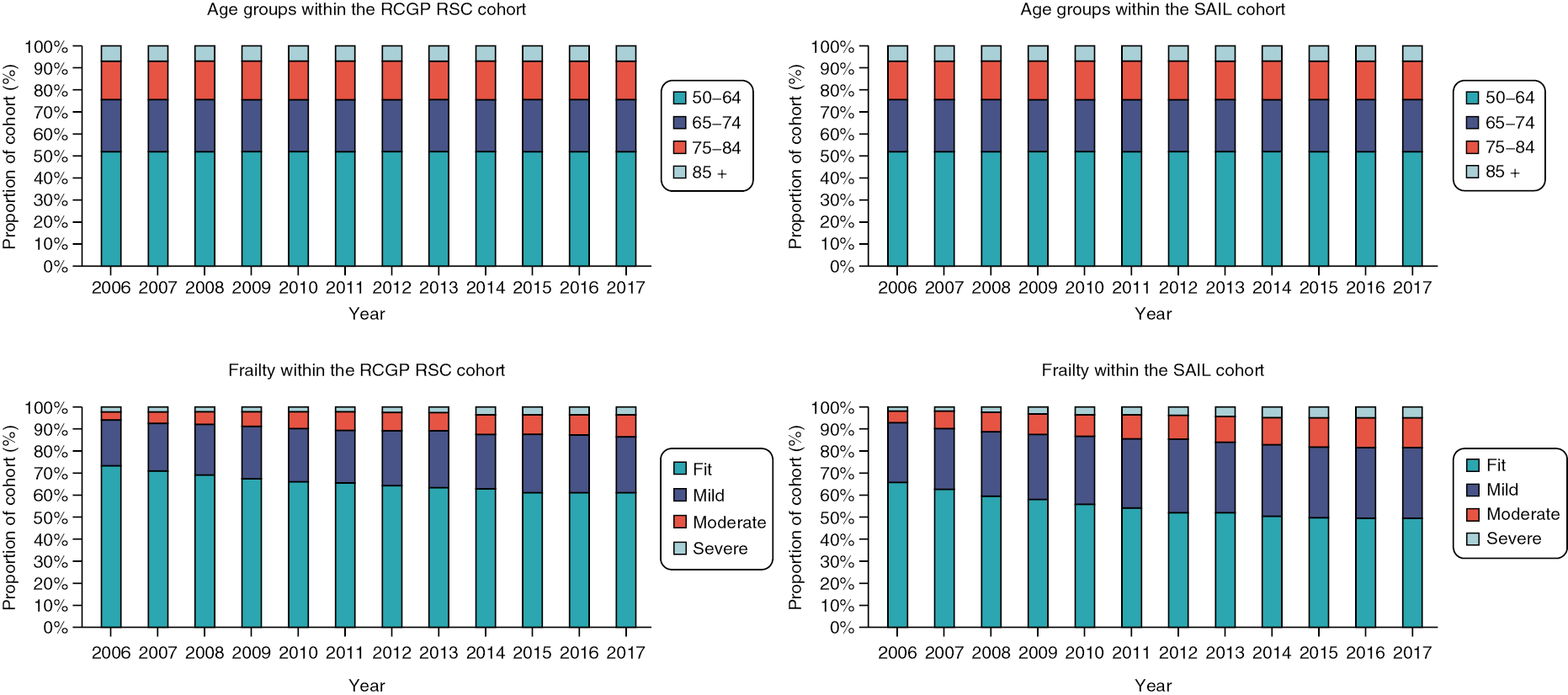
The RCGP RSC cohort had a slightly higher proportion of females (52.1%) compared with SAIL (51.8%). There were fewer missing records on ethnicity in RCGP RSC (28.9%) compared with SAIL (63.4%). Of patients whose ethnicity information had been captured, RCGP RSC appeared to have a more diverse structure (Asian: 4.5%; black: 2.4%; mixed/other: 1.3%; white: 91.8%) compared with (Asian: 0.8%; black: 0.55%; mixed/other: 0.18%; white: 98.5%). As expected, a smaller proportion of the SAIL cohort (64.4%) were living in urban locations than in the RCGP RSC cohort (77.6%). It was not possible to compare the data sets on residential care; in SAIL, 17,000 people were in residential care in the year that they entered the cohort, whereas this variable was not captured in the RCGP RSC data set. Long-term conditions were generally similar across the two populations with a few exceptions. The levels of depression, hypertension and diabetes appear higher in SAIL while dementia, malignancy and osteoporosis are slightly higher in RCGP RSC.
While deprivation is recorded in each of the data sets, they are calculated slightly differently, with SAIL based on the Welsh Index of Multiple Deprivation92 and RCGP RSC on the 2015 version of the Index of Multiple Deprivation. Comparing the quintiles (with 1 representing the most deprived and 5 representing the least) suggests a more deprived population is represented using the SAIL data. 91 Comparing the IDAOPI across the two data sets also suggests that the SAIL cohort represents a more deprived population in terms of older people’s income. The analyses presented in Chapter 4 indicated that both age and deprivation are independent predictors of frailty onset and progression. Given the similarity in age structure in the two cohorts, differences in deprivation are the likely explanation for differences in frailty prevalence between the two cohorts. 91
Figure 11 shows that, while the number of patients differ in each age and frailty categories between the two populations, the progression over time follows similar trajectories; however, the Welsh population (in SAIL) demonstrate earlier transition to frailty. This is consistent with the pattern of long-term conditions in the SAIL data set. Due to the different starting conditions and population sizes, model parameters, including transition rates, were adjusted to reflect the Welsh population structure to allow for validation against the Welsh data.
FIGURE 11.
Comparison of the 50–64 age groups in the RCGP RSC and SAIL data sets.
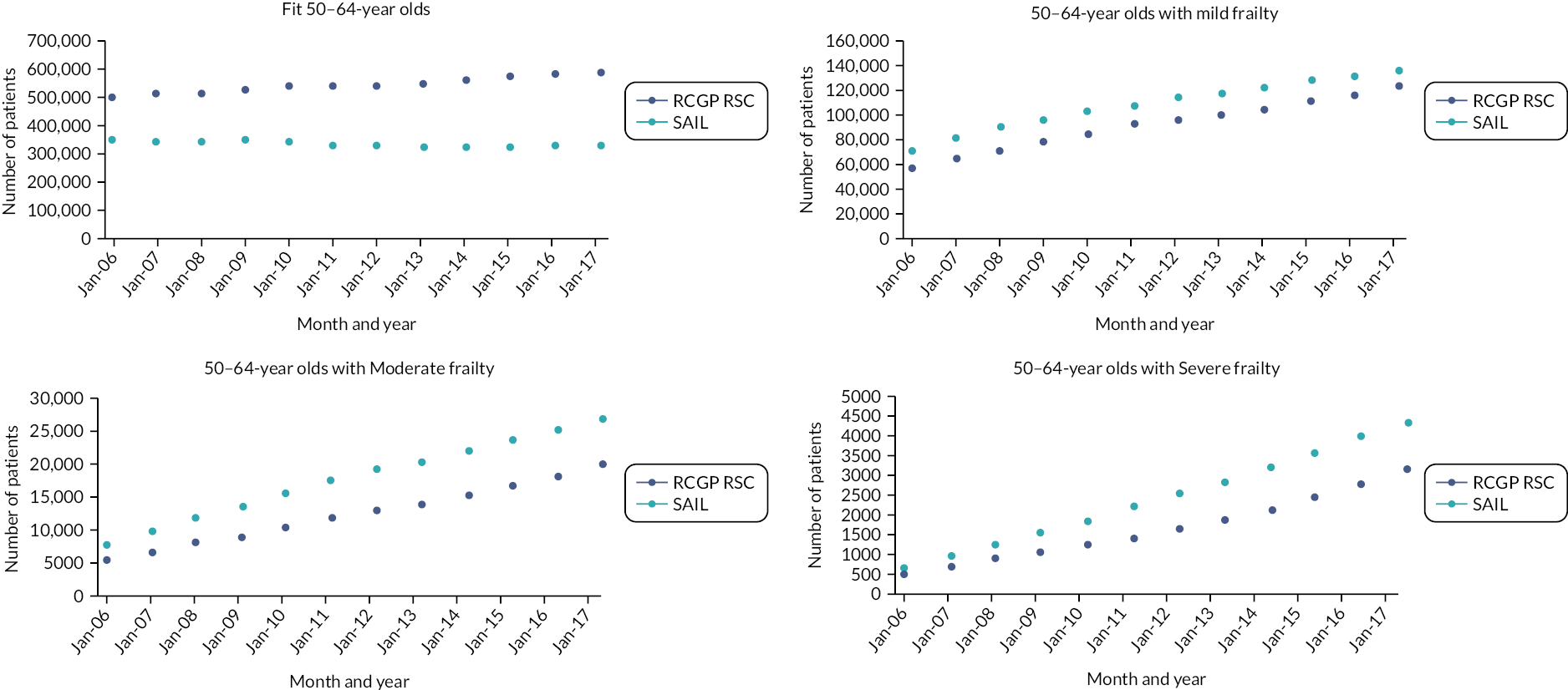
Internal validation
The simulation model was run for a simulated 12-year period to match the cohort study with the initial population levels matching those in the cohort (see Appendix 3, Figure 27). The outputs from the model were then compared against the observed data from the RCGP RSC cohort.
The estimated number of patients in each of the model’s 48 stocks and 72 flows were compared against the observed data from the RCGP RSC database at the start of each of the 12 years in the cohort study. Time plots were used for graphical comparison of the observed and estimated data. For example, Figure 12 shows one of the comparison plots for the number of fit patients aged 85 + over time. The observed data are shown by the line with circular markers (grey) and the model output by a solid black line. There is close agreement between the observed data and the model outputs, even in the smallest age subcategory.
FIGURE 12.
Comparison of the observed and model values for the number of fit over-85s.
Error statistics, using the mean absolute percentage error, were also used to assess agreement between the observed data and model output (values closer to 0% indicate a better fit) for each of the stocks and flows in the model. The mean absolute percentage error (MAPE) was calculated for 12 years of data. The MAPE is the average of the absolute percentage error (APE) over the 12-year period. The APE is calculated as:
Equation 2: Percentage error between observed data and model output:
Number of patients in each age/frailty subgroup
The MAPEs for each of the age/frailty subgroups range in size from 0.45% (fit, 50–64-year-olds) to 10.44% (severe, 85 and over) with the larger errors associated with the smaller population subgroups. Appendix 3, Table 31 shows the MAPEs for each of the 16 population subgroups. As the majority are close to or under 5%, it shows that the model provided a close approximation to the number of patients in a given population subgroup throughout the 12-year period of the RCGP RSC cohort.
Number of patients dying or leaving their general practitioner practice (from each age/frailty subgroup)
Similar comparisons were performed on the number of deaths and people leaving their GP practice in each subgroup. Appendix 3, Table 32 shows the error statistics for the number of patients dying/ leaving their GP practice. Error statistics associated with the number of patients dying range from 2.51% (moderate 85 and over) to 8.16% (fit 75–84-year-olds) with most groups around 5%. In comparison with the error statistics in Appendix 3, Table 31, these values are slightly worse, which is to be expected due to the smaller subgroup size for this variable.
The error statistics for the numbers leaving the GP practices range from 1.83% (fit 75–84) to 11.87% (severe 85 and over) with the larger errors associated with deregistrations in the smaller subgroups.
Number of patients in each age subgroup where a frailty transition occurs
The corresponding results for the frailty progressions are given in Appendix 3, Table 31.
The error statistics are slightly larger for the frailty transitions, particularly in the fit to mild transition. This may be due to the uncertainty associated with the event; the eFI was only calculated once (January) a year whereas the model is running with a monthly timestep.
Number of patients in each age that move to the next age subgroup (divided by frailty)
The error statistics in Appendix 3, Table 31 are larger in the patients that are turning 65. Figure 13 shows how the model underestimates the numbers of mildly frail patients turning 65 particularly in 2012–3.
FIGURE 13.
Validation of the ageing transition for mildly frail 50–64-year-olds.
Number of patients entering the cohort
The error statistics in Appendix 3, Table 33 range between 1.96% and 22.48% with the larger errors associated with the numbers of patients entering the severe categories of each age group.
Assessing the overall performance of the model against the Royal College of General Practitioners Research and Surveillance Centre data set
The overall average percentage error (which equally weights the contribution from all the error statistics) for the model was 7.09%, indicating that the SD model provided a close approximation to the RCGP RSC cohort data, with most population subgroups and transitions represented accurately, but slightly diminished accuracy for subgroups with small numbers. The SD frailty transitions model performed well in estimating the number of patients in each age/frailty subgroup over the 2006–17 period of the cohort study. However, it underestimated the number entering the cohort particularly in the older or more severely frail groups. This was examined further during the external validation process and before we considered extending the model’s time horizon.
External validation
Data sources for external validation
It was originally intended to use Leeds Data Model for the validation data set and Welsh data from SAIL for additional social care and residential care data that was not available in the other two data sources. At data specification, the Leeds data set was found to be unable to meet the data needs of the study, due to deceased patients’ data being removed from the available data. Exploration of the data available from SAIL revealed that they could provide a comparable data set for the purposes of external validation of the SD model, plus additional data on residential care. The SAIL Databank provides a fully linked database. An amendment was made to the study approvals and protocol to allow use of the SAIL Databank for both these purposes. Unfortunately, no data provider could provide any data on social or community care use at the time of this study.
Secure anonymised information linkage data governance and management
The SAIL Databank contains linked, pseudonymised primary, secondary and social care records, from which de-identified data were extracted. A summary of the research ethics and governance approvals for the study is in Chapter 1. The research team applied for extraction of the specified data via SAIL information governance processes. The research team and analysts at SAIL did not have access to or use any patient identifiable information throughout this study. Unique study IDs were applied to the data extract and the study team had no access to the key. The research team could access only the SAIL research data extract via remote access to secure servers and could export only aggregated data analyses. Access to the pseudonymised database was limited to databank analysts. The research team had access only to the de-identified data extract provided by SAIL.
The external validation took a stepwise approach starting by parameterising the model with Welsh population data extracted from the SAIL Databank. The SD model was run and the outputs (number of patients living, deaths, leaving GP practices, ageing, and transitioning to the next frailty state) compared against the observed data (extracted from the SAIL Databank) for the same categories from the RCGP RSC cohort. As with the internal validation, comparison plots and MAPEs were produced for each age/frailty category. As the two population data sets differed slightly in terms of frailty onset, the outputs of the two model runs were expected to differ. This was the case, with the error statistics worsened in almost every category, but with the variables associated with patients either entering the cohort or leaving the GP practices suffering the most. It was therefore evident that the simulation required some adjustment to reflect the differences in the Welsh population before it could be used for external validation.
After observing that the numbers entering the Welsh cohort differed from those in the development model, the parameters associated with the entry rates into each of the 16 population subgroups were adjusted accordingly. The adjustment factors applied for these parameters and others are given in Appendix 3, Table 33.
Number of patients entering the cohort
Adjusting the model parameters to reflect the different population structure in the SAIL data improved some of the error statistics (see Appendix 3, Table 33); however, due to increases in the Welsh cohort numbers in 2008 and 2016, the error statistics for the entry rates varied between 5.1% and 49% with the older age groups and more frail patient groups being the most affected due to their smaller group sizes. The increase in the cohort size might have been due to additional data being added into the study or increases in the underlying Welsh population through patients born in known ‘baby boom’ periods (e.g. post-World War II).
Number of patients dying or leaving their general practitioner practice (from each age/frailty subgroup)
The next model outputs considered were the patients leaving each of the frailty categories (through either death or leaving a GP practice). The differences in deregistrations between the two data sets were because the data from SAIL includes almost the whole Welsh GP population, whereas the RCGP RSC data set represents approximately 8% of the English GP population. There is therefore more likelihood that patients would leave participating practices in RCGP RSC than in SAIL and this was borne out in the data. Scaling the proportion of patients that leave through either death or deregistration (see Appendix 3, Table 33) provided a better fit to the number of patients that die or leave their GP practice, which in turn resulted in improvements to the error statistics for some of the other categories.
Number of patients in each age that move to the next age subgroup (divided by frailty)
A similar process was conducted on the ageing process and the error statistics are shown in Appendix 3, Table 33. Several of the subgroups saw an improved fit compared to that observed during the internal validation process. In particular, the ageing behaviour for those turning 85 had smaller error statistics.
Number of patients in each age subgroup where a frailty transition occurs
The error statistics associated with the frailty transitions for each of the age groups are shown in Appendix 3, Table 33. There is a marked improvement in the error associated with the Fit to Mild transition in the youngest age group and other subgroups saw similar or smaller errors when compared with the results from the internal validation.
Assessing the overall performance of the model against the Royal College of General Practitioners Research and Surveillance Centre data set
The overall error statistic following the external validation was 9.53%, approximately 2.5% worse than with the original model, but still demonstrating a good level of accuracy. The aim of simulation modelling is to provide projections rather than exact figures, with the emphasis on consideration of general trends and comparison of different subgroups and scenarios. Although errors of ˂ 10% would generally be considered to be good, in SD modelling there are no generally accepted limits on acceptable errors. The aim is obviously to reduce error as far as possible and to consider where the model offers a good fit or to identify where the errors are larger than expected and for what reasons. Despite the differences between the two populations, the external validation shows that the underlying model structure is robust and that it can be used for different demographic populations following appropriate adjustment of the model’s input parameters. In particular, the flow in and out of the population under consideration needs to be adjusted accordingly. 91
Examining the projection capabilities of the model – extending the time horizon
We developed a model that has been validated against RCGP RSC cohort data and a second comparable cohort data set (from SAIL) for the same period of time, 2006–17. The internal and external validation shows that the model structure (see Appendix 3, Figure 27) provides a simplified yet robust representation of frailty progression in people aged 50 and over. However, the true test of the simulation is how well it can project into the future. The next stage in the model’s development was to extend the time horizon and to see whether the model’s stocks and flows remain stable; we considered a further 10 years following the end of the RCGP RSC cohort, up until the end of 2027. To meet the study objectives, the simulation model also needed to be adapted to reflect the population for England.
The initial model based on the RCGP RSC data from England was used for preliminary model projections but became unstable over short time periods. In particular, the number of patients in several of the age/frailty subgroups rapidly declined with some dropping below zero and becoming negative (see Appendix 3, Figure 30 for an example). We also observed that several of the entry flows displayed a similar behaviour pattern, as did the flows associated with the frailty transitions. We also noted that these issues were compounded as numbers of patients moving into the next age group declined too far, particularly in the 50–64-year-old group.
These instabilities suggested that the number of patients entering the population (aged 50 and over) should be examined, and it was anticipated that these issues might also be resolved by scaling up the starting population stocks from our RCGP RSC cohort to the population of England. However, Appendix 3, Figure 31 shows that, while the model became more stable, there were still some problems with flows into and between some subgroups – in particular, the entry flow into the youngest age group and movement of patients turning 75 and classified as severe. To stabilise the model projections, further work was required to ensure that entry and exit flows reflected projected population trends for England (see Parameterisation with national population data).
Parameterisation with national population data
To be able to use the SD model to represent frailty progression at a national level for the specified amount of time (until the end of 2027), several adjustments were made to the model developed and validated with data from RCGP RSC and SAIL described thus far. The first was to use ONS mid-year population estimates93,94 to provide an estimate of the number of people in each age group on 1 January 2006 to coincide with the time frame used in both the RCGP RSC and SAIL cohorts. Next, we adjusted the entry and exit flows, simplifying the model further. We removed the deregistrations, assuming that the majority of people once in the 50 and over population would not leave the GP registered population, as was the case in the Welsh population data from SAIL. We also adjusted the entry flow; rather than having flows entering each of the four age groups, there is one entry point – people becoming 50. The model therefore assumes that once a patient is in the population, they will remain in it and age into the next age groups unless they die. We have also assumed negligible migration effect at the national level. 95 Appendix 3, Figure 32 shows the revised conceptual model structure for national level data used for the final version of the Frailty Dynamics Simulation Model. 91
Appendix 3, Table 34 provides the ONS mid-year population estimates93,96 for people aged 50 and over in England. The population estimates and ONS projections have been divided into the same four age groups as in our cohort study. The midpoint estimate (mid-year 2005 and mid-year 2006) for each age group was used to represent the initial population size in the SD model. The remaining rows of data in Appendix 3, Table 34 were used as a way of sense-checking the population model for England.
The population estimates for the three younger groups (50–64; 65–74; 75–84) were also used with the ONS estimates of people turning 65, 75 and 85 (see Appendix 3, Table 35) to determine the proportion of people that leave a specific age group and age into the next one within the simulation. These percentages are used in the SD model to provide an estimate of the ageing behaviour in the population as this was identified as an area of instability.
As well as providing the percentage of each of the younger age groups that leave their current age/frailty subgroup in each timestep, Appendix 3, Table 35 also provided the ONS mid-year population estimates for people becoming 50 in England (second column). The estimated number that become 50 in a given year was combined with the frailty transition behaviour identified in the RCGP RSC cohort to provide an estimate of the number of people that turn 50 and are categorised as fit, mild, moderate or severe.
Validation of the population level model
Further model validation was carried out using the SD population model with a comparison of the model output against available population and mortality estimates for ONS England. For example, the ONS population estimates for those turning 65, 75 and 85 in Appendix 3, Table 35 were also used in validating the model output to check that the model remained stable and was producing plausible results in relation to the ageing population. Figure 14 shows the estimated number of people in each of the four age groups (from the SD population model) compared with the ONS population estimates where available. Although there is close approximation between the model output and the ONS population data, this is closer in the younger age groups, but the simulation output deviated slightly more for those aged 75 and over and in the later years of the simulation. These differences are likely to be due to the real world data fluctuating in line with demographic trends in those years and age groups, whereas the simulation used average mortality data based on the analysis from Workstream 1. Following these adjustments, the simulation projections remained stable over the required time period, simulation outputs remained a good fit and trends were as expected (Figure 14), so no further adjustment was necessary and this final version of the simulation model was used for baseline and scenario analyses.
FIGURE 14.
Comparison of the model output for population numbers with ONS population estimates, by age group.
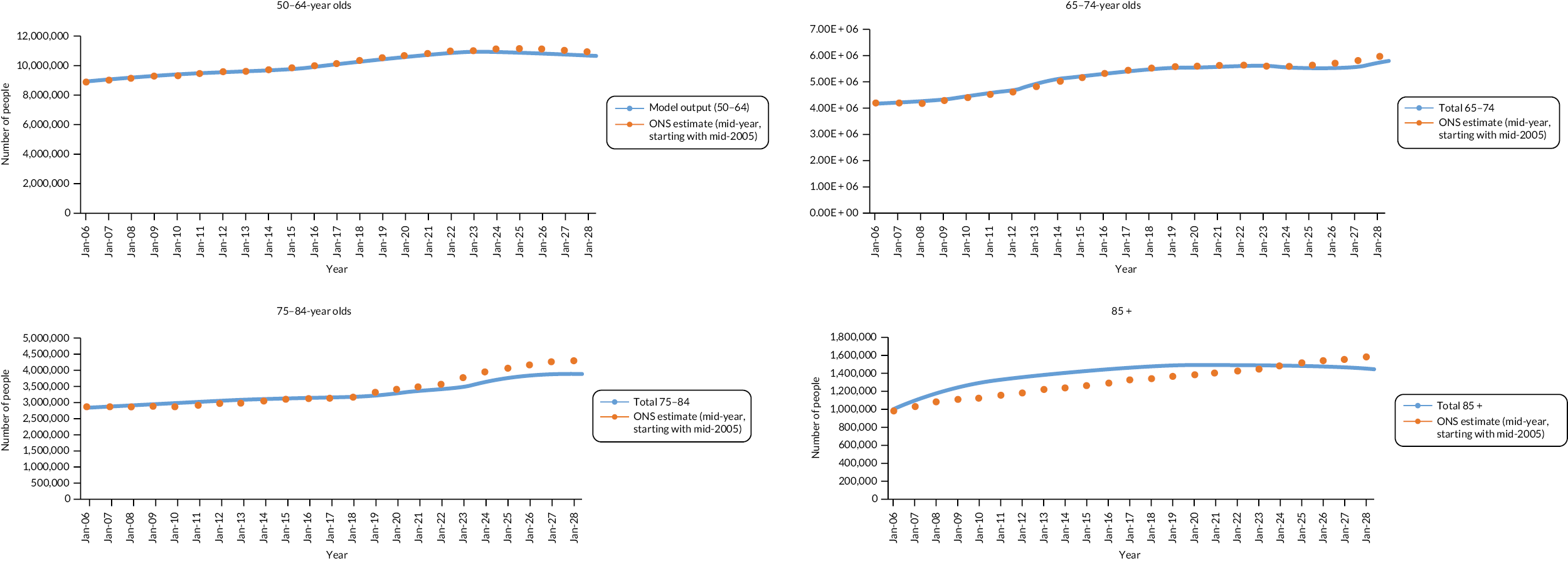
Population level model estimates (2006–17)
Figure 15 shows the estimated number of people in each of the 16 age/frailty strata (from the SD population model) with Fit (teal), Mild (navy), Moderate (cyan) and Severe (orange). As age increases, the model demonstrates how the prevalence of frailty increases (a larger share of yellow, blue and purple in the older age groups). A similar pattern of behaviour was observed in the RCGP RSC cohort analysis. It should be noted that the scale of the population size in each age group differs; approximately 10 million in the 50–64 age group in 2017 compared with close to 1.5 million in the 85 and over group. The figures are useful in showing relative prevalence of frailty strata, but actual numbers are smaller in the oldest age group. When scaled up to whole population level, the simulation projections were stable for the 10-year period. Further work will be required to extend the projection period.
FIGURE 15.
Population level estimates for numbers in each frailty state, by age group (2006–17).
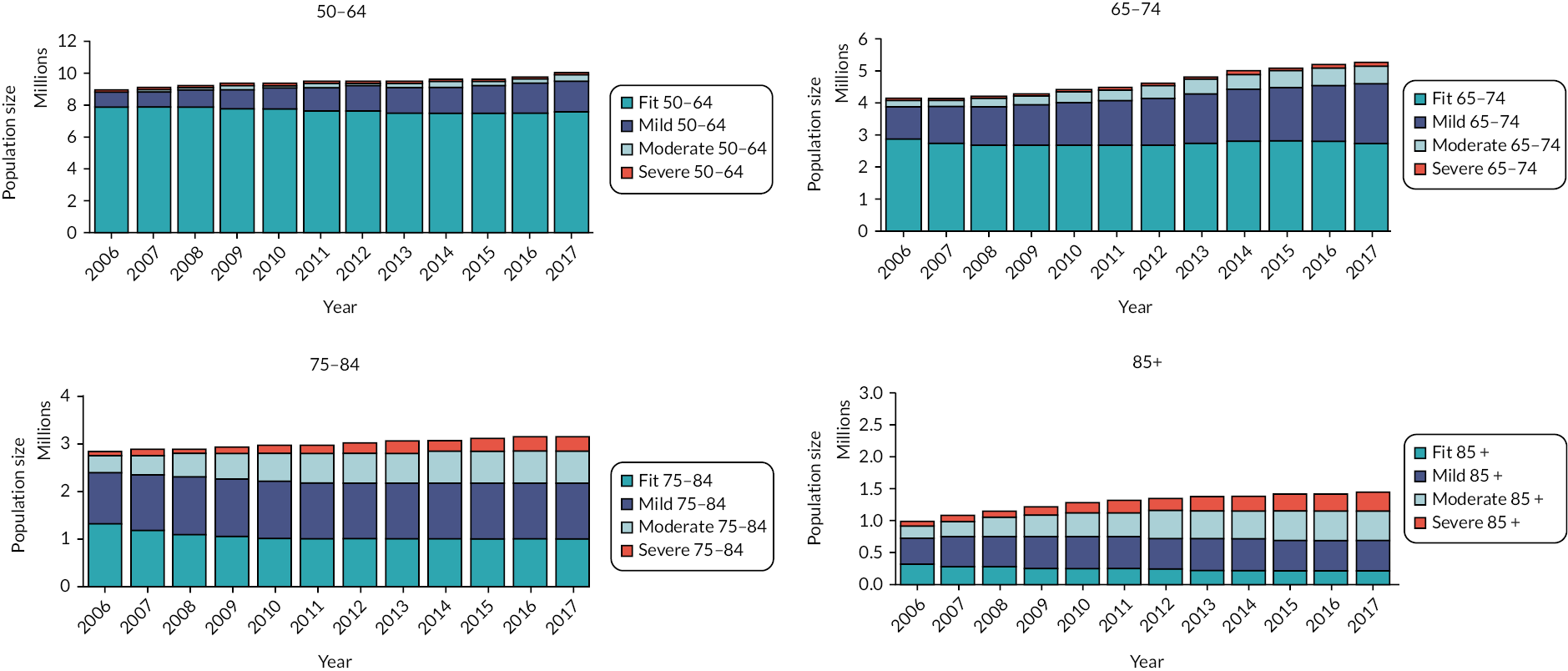
Summary
We developed a conceptual model to reflect the progression of frailty in the population (aged 50 and over) before implementing an initial system dynamics model in AnyLogic (version 8.7.3) software (The AnyLogic Company). The initial model structure consisted of 16 connected subgroups (our stocks) with flows in and out of the groups, representing the movement of patients in and out of our cohort. We initially parameterised the SD model using information from Workstream 1 (see Chapter 4) before validating the model both internally (against summary descriptive data from the RCGP RSC cohort) and externally (against a similar data set from SAIL). Following validation, we sought to expand the model’s use to consider how frailty incidence and prevalence at a national population level could be represented over the period of the cohort study (2006–17) and 10 years into the future. This involved adapting the model structure to use ONS population estimates for the number of people entering the 50 + population and those turning 65, 75 or 85 in a given year. We also extended the time horizon of the SD model 10 years into the future and were able to demonstrate stable model projections over this time period. The final version of the SD model with the ONS population estimates was used to produce the baseline and scenario results reported in Chapter 7, which considers the estimated frailty dynamics for England as well as the associated service use and costs (see Chapter 4) in both the baseline and ‘what-if’ scenarios identified during the stakeholder engagement events (see Chapter 5).
Chapter 7 Simulation of future frailty dynamics and service demand in an ageing population
Introduction
The key benefit of using simulation is that, in addition to prediction of future trends, a range of ‘what-if’ scenarios can be tested and compared. In this study, we have explored the impact of demographic trends and changes to incidence and progression rates, in addition to the impact of service delivery scenarios developed with the SEG in Workstream 3. The scenario model outputs include:
-
the number of patients in each frailty state as the population ages over time
-
demand for primary and secondary care services over time
-
mortality costs of service use.
Rationale for simulation scenarios
Through consultation with the SEG, a number of scenarios were identified which had the potential to be useful to service planners and commissioners. Potential scenarios were prioritised at the final SEG event, and the agreed scenarios were as follows:
Scenario 1: baseline (no change to services)
In this scenario, the numbers of people living within different frailty states over a 10-year period was determined. Average annual population growth, mortality and frailty transition rates were assumed to continue current trends. Service use and cost parameters applied to each age and frailty subgroup used the mean values determined from analysis of the cohort data (see Chapter 4). Services intended for management or prevention of frailty were assumed to remain at 2017 levels. This scenario provided the baseline comparison for considering the impact of service changes in the other scenarios. These results also provide useful information on the likely growth in numbers in different frailty states, and associated service use and costs, without any changes to provision for frail older people.
Scenario 2: reduced incidence and prevalence of frailty
This scenario aimed to explore the impact of interventions to prevent onset of frailty. These might comprise general public health measures or the introduction of services targeted to those who are pre-frail or at risk of developing frailty. Population growth and mortality rates were assumed to continue current trends. Service use and cost parameters remained unchanged from the baseline scenario. The incidence rate for frailty, that is the rate of transitions from fit to mild frailty, were reduced by 5% in all age groups. Cohort studies have demonstrated differences in frailty incidence associated with lifestyle factors, such as physical activity, diet and smoking cessation, of up to 20%. 97–100 There is little evidence on the impact of frailty prevention interventions, but one small trial of a pre-frailty lifestyle intervention programme reported 15.1% lower transition to frailty. 101 In this scenario, we took a conservative approach and assumed that a 5% lower transition rate was feasible at population level, taking into account the challenges of implementing lifestyle changes at scale in practice. This scenario was considered by SEG participants to be particularly important for those involved in the public health sector.
Scenario 3: reduced progression of frailty severity
In this scenario, the aim was to consider the impact of a slowing in progression of frailty. The stakeholders agreed that, however achieved, this is likely to focus on clinical activity within services for older people. Evidence suggests that modest reductions in frailty progression are possible with a range of interventions, especially physical activity and nutritional interventions. 48,102,103 We have attempted to illustrate the impact of such interventions through reduced transition to more severe frailty states. Current evidence provides limited information on overall reduction in progression rates, with many reviews reporting on reduction in scores only within specific frailty domains. Following discussion with the professional stakeholders, this scenario explored the impact of a 10% reduction in mild to moderate and 5% reduction in moderate to severe transitions per year, thought to be realistic in practice with specific interventions in place. Liu et al. 102 note that adherence to interventions was generally low and more work is needed on translation to community settings, so a conservative estimate of reduction in progression was used for this scenario. It should be noted that, in contrast to other scenarios, which alter only one parameter at a time, this scenario required changes to two different transition rates applied to all age groups in the usual way. The results represent the combined effect of these two changes in transition rates.
Scenario 4: reduced impact of frailty on service use (unplanned admissions)
The SEG participants noted that many services and interventions would not act directly on onset or progression of frailty but would focus on reducing negative health consequences for those living with frailty. Following discussion with the stakeholders, we focused this scenario on the impact of interventions to reduced unplanned hospital admissions in older people with frailty. This was felt to represent the focus of clinical efforts in this area and to reflect both patient and service priorities. Based on the evidence around admission reduction in older people, we have modelled a 2.5% reduction in unplanned admissions per year across all frailty groups and ages. A number of reviews of admission prevention interventions has noted that the overall quality of evidence is poor, there is wide geographic variation, translation to practice also varies and that evidence is weak. 104–107 Wallace et al. 105 report, however, that 2% of emergency admissions are preventable. A recent evaluation of a pharmacist intervention to reduce readmissions showed only a 2% reduction in emergency admissions at seven days. There is possibly more scope for admission reduction through application of system level interventions and integrated care,106–108 but improvements are likely to be modest. The reduction proposed for this scenario was considered achievable while remaining sufficiently large to have meaningful impact.
Within the SEG events, other potential scenarios were discussed. There was particular interest in exploring the impact of deprivation (including austerity policies) on frailty prevalence, changes to mortality resulting from both austerity and the COVID-19 pandemic and changes to frailty prevalence as a consequence of COVID-19 and long Covid. It was decided that, while these will be important areas for future research, we currently lack sufficient evidence to inform model parameters relevant to these issues.
The scenarios allowed comparison of the number of patients in each frailty subgroup, and service use and costs, against the baseline results.
Model adjustments for simulation scenarios
In each of the scenario experiments, the size of each of the 16 age/frailty population subgroups was obtained from the model and combined with the mean service use and cost parameters applied in the baseline scenario experiment to examine the impact of the scenario on both the population and the associated primary and secondary care use and costs.
In the baseline scenario, no changes were made to the projected trends in ageing or frailty transitions. It was assumed that no service or demographic changes would occur that might alter the underlying model assumptions around the key transition rate which would require additional changes to the underlying model parameters.
In Scenario 2, the fit to mild transitions rates were reduced by 5% per year, from the start of 2017. The same level of reduction was applied to all age groups. It was assumed that no service or demographic changes would occur that might alter the model assumptions around the other frailty transition and mortality rates.
In Scenario 3, the Fit to Mild transition parameters were reset to their original values (used in the baseline scenario) and the Mild to Moderate and Moderate to Severe parameters were adjusted for all four age groups. The flow rate parameters associated with the Mild to Moderate transition were reduced by 10% and those for the Moderate to Severe saw a smaller 5% reduction. All other model parameters remained the same as in the baseline scenario experiment.
Finally, in Scenario 4, all frailty transition scenarios were reset to their original values (as per the baseline scenario) and unplanned admission rates were adjusted by 2.5% in all age and frailty groups.
Baseline scenario: trends in frailty and service use in an ageing population
While the simulation model represents the time horizon from 2006 through to the end of 2027, the projections needed in the scenario analysis are the 10 years that follow the RCGP RSC cohort study; 2017–27.This length of time is required to capture fully the population dynamics and the evolution of frailty. While the demographic predictions thus derived are robust, we recognised that any cost calculations more than 2 or 3 years into the future can only be indicative, given that service delivery modalities and health and social care organisational structures are unlikely to remain fixed for the whole period, hence the focus of these results is on comparison of the relative differences between groups and impact across scenarios.
Baseline scenario: parameterisation of service use
The primary and secondary healthcare service use analysis which informed the model development is provided in Chapter 4 Primary care service use focuses on GP activities (face-to-face, telephone and electronic consultations as well as home visits) and individual medicines prescribed. Secondary care includes outpatient visits, A&E attendances, hospital admissions and admissions to critical care. Ambulance calls are considered separately as is residential care use. The service use parameters for primary and secondary care, ambulance calls and residential care derived from the statistical analyses of RCGP and SAIL data (see Chapter 4) were applied to the baseline population SD model. The average (mean) service use parameters (see Chapter 4) were applied to each of the age/frailty strata of the population for each complete year of data. The service use information (from the RCGP RSC data set) for primary care (GP face-to-face, home visits, telephone and e-consultations, and individual medicine prescriptions) was complete for 2006–17. In the secondary care data, information on A&E attendances started in 2007 and critical care admissions in 2008. The service use relating to ambulance attendances and residential care was provided in the SAIL analysis. The residential care data covered 2006–17 while the ambulance data covered 2011– 17. The service use parameters for primary and secondary care, ambulance and residential care were applied to the baseline population SD model which was extended to run until 2027. This allowed estimation of service use demand associated with the projected incidence and prevalence of frailty for 10 years after the end of the cohort study if no other changes were made to services and interventions.
Baseline scenario: service use costs applied
The descriptive mean costs summarising all the observational data (see Chapter 4) were applied to the baseline SD service use model (see Baseline scenario: trends in frailty and service use in an ageing population) to provide an estimate of the projected healthcare costs for people aged 50 and over in England during (2017–27). The cost projections are presented at the end of Scenario 2.
Baseline simulation of frailty incidence and progression in England
Table 11 describes the estimated number of people in each of the age/frailty strata in England between 2017 and 2027.
| Year | Primary care use (face-face, telephone and e-consultations and home visits) | Secondary care use (A&E attendances, critical care admissions, hospital admissions, outpatient appointments) | Total service use | Costs (£ billions) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fit | Mild | Mod | Severe | Fit | Mild | Mod | Severe | |||
| 2017 | 64.4 | 62.4 | 32.1 | 16.6 | 22.1 | 23.8 | 13.6 | 7.3 | 242.2 | 36.9 |
| 2018 | 64.4 | 63.8 | 32.9 | 17.9 | 22.1 | 24.4 | 14.0 | 7.9 | 247.4 | 37.8 |
| 2019 | 64.4 | 65.1 | 33.7 | 19.4 | 22.1 | 24.9 | 14.4 | 8.5 | 252.5 | 38.7 |
| 2020 | 64.2 | 66.4 | 34.4 | 20.9 | 22.0 | 25.4 | 14.8 | 9.2 | 257.4 | 39.6 |
| 2021 | 64.1 | 67.6 | 35.1 | 22.6 | 22.0 | 25.9 | 15.1 | 9.9 | 262.2 | 40.5 |
| 2022 | 63.8 | 68.7 | 35.8 | 24.3 | 21.9 | 26.3 | 15.4 | 10.7 | 266.8 | 41.3 |
| 2023 | 63.5 | 69.7 | 36.4 | 26.2 | 21.8 | 26.8 | 15.7 | 11.5 | 271.5 | 42.2 |
| 2024 | 63.1 | 70.6 | 36.9 | 28.3 | 21.7 | 27.1 | 16.0 | 12.4 | 276.1 | 43.2 |
| 2025 | 62.5 | 71.4 | 37.5 | 30.5 | 21.4 | 27.4 | 16.2 | 13.3 | 280.3 | 44.1 |
| 2026 | 61.7 | 71.9 | 37.9 | 32.9 | 21.2 | 27.7 | 16.5 | 14.3 | 284.1 | 44.9 |
| 2027 | 60.8 | 72.4 | 38.2 | 35.5 | 20.9 | 27.9 | 16.7 | 15.4 | 287.7 | 45.7 |
In 2017, the population projections (see Table 42) suggest 20,045,766 people are aged 50 and over at the start of 2017 rising to 21,755,097 at the start of 2027. As time progresses, the proportion with frailty increases, especially in the oldest age group.
Projected service use and costs: baseline simulation
Table 12 summarises the projected changes in frailty prevalence, service use and associated costs over a 10-year period. The detailed breakdown of the projections is provided in Appendix 3, Tables 36–45.
| Year | Change in primary care use | Change in secondary care use | Net change in total service use | Net change in costs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fit | Mild | Mod | Severe | Fit | Mild | Mod | Severe | |||
| 2017 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2018 | + 1.70 | –2.98 | –0.14 | –0.006 | + 0.57 | –1.14 | –0.057 | –0.002 | –2.05 | –0.034 |
| 2019 | + 3.29 | –5.55 | –0.50 | –0.042 | + 1.11 | –2.12 | –0.21 | –0.018 | –4.03 | –0.0687 |
| 2020 | + 4.73 | –7.65 | –1.00 | –0.133 | + 1.60 | –2.93 | –0.42 | –0.057 | –5.86 | –0.101 |
| 2021 | + 6.02 | –9.34 | –1.57 | –0.288 | + 2.04 | –3.59 | –0.66 | –0.123 | –7.53 | –0.132 |
| 2022 | + 7.17 | –10.69 | –2.16 | –0.514 | + 2.43 | –4.12 | –0.92 | –0.220 | –9.04 | –0.160 |
| 2023 | + 8.19 | –11.75 | –2.72 | –0.808 | + 2.78 | –4.55 | –1.17 | –0.346 | –10.38 | –0.187 |
| 2024 | + 9.10 | –12.57 | –3.24 | –1.16 | + 3.09 | –4.87 | –1.40 | –0.50 | –11.56 | –0.211 |
| 2025 | + 9.90 | –13.16 | –3.70 | –1.57 | + 3.36 | –5.12 | –1.61 | –0.68 | –12.58 | –0.232 |
| 2026 | + 10.57 | –13.55 | –4.07 | –2.02 | + 3.59 | –5.29 | –1.78 | –0.87 | –13.43 | –0.251 |
| 2027 | + 11.12 | –13.79 | –4.34 | –2.48 | + 3.78 | –5.40 | –1.91 | –1.08 | –14.10 | –0.266 |
| Total over projected period | + 71.77 | –101.04 | –23.45 | –9.03 | + 24.35 | –39.12 | –10.13 | –3.89 | –90.55 | –1.64 |
The estimated projections for primary care use (GP face-to-face, telephone and e-consultations and home visits) are given in Appendix 3, Table 36 and the estimated number of individual prescriptions is given in Appendix 3, Table 37. In 2017, the population projections suggest 20,045,766 people are aged 50 and over. The simulation model combined with the primary care service use indicates this would generate 175,440,543 GP activities (face-to-face, telephone, home visits and e-consultations). Of these, 37.1% would be among the 50–64 age group; 29.3% in the 65–4 group; 22.4% in the 75–84 group and 11.2% in the oldest group. By 2027, the population estimate increases to 21,755,097 people, generating 206,870,092 GP activities of which the percentage share changes to 37.2%, 26.9%, 25.3% and 10.7% in those age groups, respectively.
For frailty in the population, simulation projections indicate that, in 2017, the share of the GP services is 36.7% for Fit, 35.6% for Mild, 18.3% for Moderate and 9.4% for Severe. By 2027, those with frailty make more use of primary care GP services, accounting for 29.4%, 35.0%, 18.5% and 17.2% of service use in those groups, respectively. GP service use for those aged 50 and over is projected to rise from 175,440,543 consultations in 2017 to 206,870,092 in 2027: a potential increase of 17.9%.
In relation to the number of individual medicines prescribed during 2017, the simulation model predicts 736,987,280, rising to 935,672,469 in 2027 (see Appendix 3, Table 37); a potential increase of 27.0%. As with the GP activity, the proportion of medicines used by each age group changes over time, with the share in 2017 being 30.3% for 50–64, 29.5% for 65–74, 25.3% for 75–84 and 14.9% for those aged 85 +. By 2027 the share of medications is 30.7%, 25.9%, 28.6% and 14.8% in those age groups, respectively. The medicine share by frailty category sees a more dramatic shift between 2017 and 2027 as a smaller proportion is issued to the Fit group and more to the Severe. In 2017, the Fit group accounts for 22.2% of medications, with 38.0% in Mild, 24.5% in Moderate and 15.4% in Severe groups. By 2027, the proportions are 16.4%, 34.6%, 23.1% and 25.9% in those groups, respectively.
In 2017, the projected number of attendances at A&E departments in England is 5,480,604, rising to 6,764,742 in 2027; a potential increase of 23% (see Appendix 3, Table 38).
The percentage share of service use by those in the Fit groups reduces across all age groups (50–64: from 21.0% to 16.8%; 65–74: from 7.2% to 5.4%; 75–85: from 3.3 to 2.4; 85 +: from 1.1% to 0.4%). The difference in A&E attendance is more mixed in those in the Mild groups with a slight increase in the youngest age group and reductions in the others. The Moderate groups also see an increased share in attendances in the 50–64 and 75–84 age groups while the other two see a reduction. The Severe groups see an increased share of attendances in all four age groups.
The projected service use attributed to critical care admissions (see Appendix 3, Table 39) rises from 100,089 admissions in 2017 to 121,749 in 2027: a potential increase of 21.6%. Comparing the critical care service use over the 2 years sees a reduction in the percentage share from the Fit and Mild population subgroups in most age groups. The picture is more mixed in the Moderate groups with an increase in the 50–64 and 75–84-year-olds and a reduction in the others. All the Severe groups see an increased share of use in critical care admissions.
The projected service use attributed to outpatient appointments (see Appendix 3, Table 40) rises from 50,914,623 appointments in 2017 to 61,305,715 in 2027: a potential increase of 20.4%.
The projected number of hospital admissions through elective and unplanned stays are given in Appendix 3, Tables 41 and 42, respectively. The projected service use attributed to elective hospital admissions rises from 6,823,836 in 2017 to 8,223,292 in 2027; a potential increase of 20.5%. In comparison, the unplanned admissions increase from 3,332,944 in 2017 to 4,257,107 in 2027; an increase of 27.7%.
The projected ambulance service use (see Appendix 3, Table 43) rises from 1,769,556 calls in 2017 to 2,324,497 in 2027: a potential increase of 31.4%. The projected number of people located in residential care (see Appendix 3, Table 44) rises from 269,338 in 2017 to 364,617 in 2027; a potential increase of 35.4%.
A detailed breakdown of total costs can be found in Appendix 3, Table 45. In 2017, the cost for providing GP services and individual medicines in England is projected to be approximately £142.49 billion, of which £9.1 billion is attributed to those with frailty (71%). By 2027, this is estimated to increase to £15.8 billion, with £12.2 billion required for those with frailty (77%).
In 2017, the projected secondary care cost is estimated to be almost £24 billion, of which approximately £17.7 billion is attributable to patients with frailty (74%). By 2027, the costs are projected to increase to £29.49 billion, with almost £24.2 billion required for those with frailty (81%).
Appendix 3, Table 45 shows the projected combined costs associated with primary and secondary care for 2017–27 by frailty category. In 2017, the projected combined costs are estimated to be £36.9 billion, of which £26.8 billion is accounted for by those with frailty (73%). By 2027, this is projected to increase to £45.7 billion, with £36.4 billion for those with frailty (80%).
Scenario 2
Model adjustments for Scenario 2
In order to conduct the second scenario experiment that considers the impact of reducing the chance of becoming frail by 5%, the transition parameters in the model associated with the Fit to Mild transition were reduced by 5% in all four age categories starting in January 2017. All other model parameters remained the same.
Summary results from Scenario 2
In the second scenario, the Fit to Mild transition parameters were reset to their original values (used in the baseline scenario) and the Mild to Moderate and Moderate to Severe parameters were adjusted for all four age groups. The parameters associated with the Mild to Moderate transition were reduced by 10% and those for the Moderate to Severe saw a smaller 5% reduction. All other model parameters remained the same as in the baseline scenario experiment.
Results from Scenario 2 experiment suggest that reducing the chance of becoming mildly frail by 5% could see a subtle change in the frailty prevalence among those aged 50 and over (Table 13). If we consider 10 years into the future, where the baseline simulation model estimates a population of almost 22 million, reducing incidence of frailty by 5% could see almost 180,000 people remain fit each year rather than becoming frail (121,000 fewer mildly frail people, 27,500 fewer moderately frail and 12,800 fewer severely frail). From Table 13, it can be seen that in 2018 almost 300,000 fewer GP consultations would be needed by those that are described as mildly frail, but with a subsequent increase over the baseline scenario of almost 170,000 events by those described as Fit. There would also be a slight reduction (approximately 14,000) in the primary care services used by moderate and severely frail.
| Year | Change in primary care use | Change in secondary care use | Change in total service use | Change in costs | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fit | Mild | Mod | Severe | Fit | Mild | Mod | Severe | |||
| 2017 | – | – | – | – | – | – | – | – | – | – |
| 2018 | 0 | + 4.10 | –3.81 | –1.89 | 0 | + 1.49 | –1.64 | –0.83 | –2.56 | –0.071 |
| 2019 | 0 | + 7.91 | –6.79 | –4.08 | 0 | + 2.87 | –2.94 | –1.79 | –4.81 | –0.137 |
| 2020 | 0 | + 11.28 | –8.93 | –6.41 | 0 | + 4.11 | –3.91 | –2.81 | –6.67 | –0.194 |
| 2021 | 0 | + 14.27 | –10.40 | –8.79 | 0 | + 5.20 | –4.61 | –3.86 | –8.18 | –0.243 |
| 2022 | 0 | + 16.93 | –11.33 | –11.16 | 0 | + 6.18 | –5.08 | –4.91 | –9.36 | –0.282 |
| 2023 | 0 | + 19.27 | –11.81 | –13.46 | 0 | + 7.05 | –5.37 | –5.93 | –10.25 | –0.319 |
| 2024 | 0 | + 21.38 | –11.97 | –15.67 | 0 | + 7.82 | –5.52 | –6.91 | –10.87 | –0.347 |
| 2025 | 0 | + 23.25 | –11.87 | –17.77 | 0 | + 8.52 | –5.56 | –7.86 | –11.29 | –0.370 |
| 2026 | 0 | + 24.88 | –11.53 | –19.73 | 0 | + 9.13 | –5.49 | –8.75 | –11.49 | –0.387 |
| 2027 | 0 | + 26.23 | –10.98 | –21.49 | 0 | + 9.64 | –5.33 | –9.57 | –11.49 | –0.399 |
| Total over projected period | 0 | + 169.5 | –99.41 | –120.4 | 0 | + 62.02 | –45.46 | –53.21 | –86.98 | –2.75 |
For secondary care, there would be 114,000 fewer events among those that are mildly frail and an extra 57,000 among those that are fit. Under this scenario, there would be the potential for 5.3 million fewer secondary care service events in those with some degree of frailty over the projected 10-year period. In terms of hospital admissions, a reduction in the frailty transition under Scenario 2 could see 26,418 fewer unplanned admissions and 47,506 fewer elective admissions.
Overall, approximately 9.1 million fewer primary and secondary care services would be used by the 50 and over population in England following a 5% reduction in frailty incidence. The cost-savings from all these service use changes could amount to £266.3 million per annum by 2027. It should be noted that the cost saving of £2.9 billion is somewhat offset by service use by those remaining in the Fit group for longer, with associated costs of £1.26 billion.
Scenario 3
Model parameter adjustments for Scenario 3
In the third scenario, the Fit to Mild transition parameters were reset to their original values (used in the baseline scenario) and the Mild to Moderate and Moderate to Severe parameters were adjusted for all four age groups. The parameters associated with the Mild to Moderate transition were reduced by 10% and those for the Moderate to Severe saw a smaller 5% reduction. All other model parameters remained the same as in the baseline scenario experiment.
Scenario 3 summary results
Results from Scenario 3 experiment suggest that reducing the chance of becoming moderately frail by 10% and severely frail by 5% could see a subtle change in the frailty prevalence among those aged 50 and over (Table 14). Over 10 years, where the baseline simulation model estimates a population of almost 22 million, reducing the chance of progressing through moderate and severe frailty could see almost 222,000 people remain mildly frail each year rather than becoming moderately or severely frail (68,200 fewer moderately frail and 110,000 fewer severely frail). The corresponding impact on primary care service use is projected to be 623,700 fewer GP consultations (face-to-face, telephone, home visits and e-consultations) and 9.1 million fewer medicines issued per annum. In relation to secondary care services, there could be 64,111 fewer A&E attendances, 1540 fewer admissions to critical care and 350,762 fewer outpatient appointments. In terms of hospital admissions, a reduction in the frailty transition under Scenario 3 could see 53,162 fewer unplanned admissions and 54,283 fewer elective admissions. The cost-savings from all these service use changes could amount to £298.9 million per annum by 2027.
| Year | Change in secondary care use | Change in total service use | Change in costs | |||
|---|---|---|---|---|---|---|
| Fit | Mild | Mod | Severe | |||
| 2017 | – | – | – | – | – | – |
| 2018 | – | –0.276 | –0.220 | –0.172 | –0.667 | –0.102 |
| 2019 | – | –0.280 | –0.224 | –0.186 | –0.690 | –0.107 |
| 2020 | – | –0.284 | –0.229 | –0.201 | –0.713 | –0.112 |
| 2021 | – | –0.288 | –0.232 | –0.217 | –0.737 | –0.117 |
| 2022 | – | –0.291 | –0.236 | –0.234 | –0.761 | –0.122 |
| 2023 | – | –0.294 | –0.239 | –0.252 | –0.786 | –0.128 |
| 2024 | – | –0.297 | –0.242 | –0.272 | –0.812 | –0.134 |
| 2025 | – | –0.299 | –0.245 | –0.294 | –0.839 | –0.140 |
| 2026 | – | –0.300 | –0.247 | –0.317 | –0.865 | –0.147 |
| 2027 | – | –0.300 | –0.249 | –0.342 | –0.891 | –0.154 |
| Total over projected period | – | –2.91 | –2.36 | –2.49 | –7.76 | –1.26 |
Scenario 4
Model parameter adjustments for Scenario 4
In the fourth scenario, the Mild to Moderate and Moderate to Severe parameters were reset to their original values (used in the baseline scenario). The parameters associated with the mean service use for unplanned hospital admissions were reduced by 2.5% in each of the Mild, Moderate and Severe groups in all four age groups. The corresponding service use parameter for those described as Fit remained unchanged.
Scenario 4 summary results
Results from the Scenario 4 experiment suggest that reducing the number of unplanned hospital admissions among patients with frailty by 5% could result in a modest change in the service use among those aged 50 and over (Table 14). Over 10 years, where the baseline simulation model estimates a population of almost 22 million, reducing unplanned admissions could result in 89,000 fewer hospital admissions. The cost-savings from this service use change could amount to £153.8 million per annum by 2027. The saving is less than in scenarios 2 and 3, but this scenario only addresses the relatively uncommon unplanned admission events within the mild, moderate and severely frail patients, whereas the other scenarios address multiple services across all frailty categories. This scenario assumes that there is no impact on unplanned hospital admissions within the Fit group, but in reality, any release of capacity through a reduction in unplanned hospital admissions from those with frailty would potentially lead to increased elective admissions and associated costs in this group. In the simulation, it is also assumed that reductions in unplanned hospital admissions have no impact on any of the other secondary care service use (A&E, critical care, outpatients, elective). In reality, there could be increased use of these other services by frail people or due to capacity being released for elective admissions.
Summary
A baseline scenario experiment, in which it was assumed that no changes were made to services, but demographic trends and frailty transitions continued, was conducted in order to understand how frailty incidence and prevalence might develop in the population over 10 years, starting at the end of the cohort study period. The baseline scenario provides an indication of the impact of frailty on the ageing population, and associated service use and costs, if there is no change to current service provision and interventions. Between 2017 and 2027, the population aged 50 and over will increase from 20,045,766 to 21,755,097 and the baseline simulation presented here illustrates how the prevalence of frailty changes over the 10-year period, with the proportion of those with some level of frailty increasing over the period. Over the same time period, the baseline simulation indicates a projected increase in primary care service use and medication use. However, it is worth commenting that the percentage share of service use varies within age and frailty subgroups, and it is therefore important to consider specific services and frailty strata to fully understand the impact of frailty on service use and costs. Initial cost projections for primary and secondary care services suggest that the cost for providing GP services and individual medicines in England will rise by £3.1 billion for people with frailty, from £9.1 billion to £12.2 billion. For secondary care, the rise in costs is estimated to be £6.4 billion for patients with frailty. A scenario experiment exploring reducing the chance of frailty incidence by 5% indicates a potential cost-saving of £1.64 billion over a 10-year period. A scenario exploring slowing down progression of frailty through moderate and severe states indicates a potential cost saving of £2.75 billion over a similar period. A targeted approach to reduce unplanned admissions among those with frailty indicates a potential saving of £1.26 billion in the same period. Further scenario analyses are continuing and will be used to inform guidelines and recommendations for service providers and commissioners.
Chapter 8 Discussion
Introduction
This study provides new evidence about the impact of frailty on health service use and costs within an ageing population. It has combined observational data from primary and secondary care, statistical analyses and simulation modelling to enhance understanding of likely future changes in frailty prevalence and impact and to explore the effect of service-level changes on future service use and costs associated with frailty. In this chapter, the strengths and limitations of the study will be discussed. The findings from different study components will be considered in an integrated discussion in relation to key issues of frailty prevalence, frailty incidence and progression, and finally, service use and costs associated with frailty.
Study strengths and limitations
Strengths of the study
This study analyses the largest known data set providing longitudinal data on frailty transitions and outcomes in those aged 50 and over and provides unique information both on frailty within middle-aged to young-old populations and progression within the ageing population. The scale of the data set allowed exploration of the impact of socioeconomic and demographic factors on frailty onset, progression and outcomes over time. The data set included a wide range of covariates which have been identified in other studies as being associated with either frailty onset or outcomes following frailty occurrence and this study provides confirmatory data on key predictors of frailty onset in addition to new information on frailty transitions over time. GP registration coverage in England is high,96 therefore using data from the primary care population ensures that findings are representative of the overall population. Further strengths of this study are the use of data linkage to secondary care use and healthcare costs, and a cohort representing a dynamically ageing population with lengthy follow-up, enabling novel insights into the onset and progression of frailty. The multistate model developed in this study also uses commonly available variables that are applicable to real world planning and service delivery, particularly population-level factors that are not clinically modifiable and are therefore particularly important to account for when considering service demand. The development and validation of a simulation model based on the linked data have enabled an examination of projected impacts of frailty if current trends continue.
Adherence to the study objectives
The study objectives were:
-
identification of incidence and prevalence of frailty states in an ageing population
-
identification of frailty trajectories and transitions in severity in the older population over time
-
exploration of drivers of progression of frailty, including clinical, socioeconomic and demographic factors
-
examination of the impact of frailty on service use, costs and pathways of care
-
exploration of the relationship between frailty status, socioeconomic factors, practice factors and service use and outcomes (mortality, unplanned admissions, residential care use)
-
prediction of trends in frailty, modelling of health and care demand and costs over time and in different service contexts.
All the study objectives were met, and this study has demonstrated that it is possible to identify frailty (with the eFI approach) in people in middle age and older using routinely collected primary care data in both England and Wales. The analyses presented here provide new evidence on incidence and prevalence of frailty in the ageing population, particularly in relation to onset and progression of frailty in people aged 50–64. These findings are consistent with previous population studies, but this study expands on current knowledge through its use of a large, nationally representative data set, analysis of data on those in middle age and its use of longitudinal data. These analyses allowed us to explore expected frailty transitions, severity and prevalence in the ageing population and to explore the impact of frailty on service use and costs over time. The multistate model enabled us to develop precise estimates of frailty transitions adjusted for key socioeconomic and practice factors. The impact of frailty severity on service use and costs were determined through generalised linear models adjusted for socioeconomic and practice factors, which demonstrate that frailty is the most important predictor of service use and costs. These analyses further informed the simulation model, which provides projections of trends in frailty and associated demand over time, with scenario modelling allowing for different contextual factors to be explored.
There were some minor deviations from the original detailed research plan, specifically in relation to analysis of clinical drivers of frailty progression. Analysis for Objective 3 focused on socioeconomic and demographic factors that were essential for development of the simulation model. Clinical features of the cohort were presented via descriptive analyses, but further analysis in the MSM was not possible due to collinearity, as many of the clinical conditions are also components of the eFI score.
Due to the problems with data provision during the pandemic, service use and cost analyses, guideline development and dissemination were delayed. Work continues with our SEG to incorporate findings into the guidance and toolkit for commissioners.
Equality, diversity and inclusion
We were able to ensure that the study population was broadly representative of the English population by using large-scale routine data. As is often the case with routine healthcare data, ethnicity data from primary care were subject to a large amount of missing data. We addressed this by supplementing the missing ethnicity data with information from linked secondary care records, allowing us to have more complete data and to include broad ethnicity categories within the analyses. The impact of ethnicity on development and progression of frailty is a key finding and we have noted the importance of further research on this particular issue elsewhere in this discussion. In Chapter 5, we have highlighted our difficulties in recruiting more diverse PPIE representatives to the stakeholder engagement events. The FLOWS study, which follows on from this work, has been designed to allow us to increase data collection and PPIE with more ethnically diverse populations in London. We have also reported the efforts to ensure inclusivity in the stakeholder engagement process and recommendations for future studies in Chapter 5. This included hosting in-person events at accessible venues and facilitating virtual participation during the pandemic. We ensured that text and pictures were accessible to our PPIE participants and included discussion of presentation of data, offering alternative examples, in the stakeholder engagement events. In addition to our PPIE representative and patient/carer panel, the research team includes carers and people with disabilities, providing a useful perspective as both service experts and users.
Patient and public involvement: impact of the pandemic on data collection and stakeholder engagement
A full discussion of the role of PPIE in this study is provided in Chapter 5, in line with GRIPP2 guidelines. It is important to note that the study was subject to considerable delays in stakeholder engagement and data extraction and delivery as a result of the COVID-19 pandemic. Planned stakeholder events could not take place during the lockdown periods and were not feasible even outside these; many participants were healthcare professionals, who were prioritising clinical activity, and patient and carer participants were likely to be clinically vulnerable or shielding, so could not attend in-person events. The pandemic also placed considerable pressure on the organisations providing our data, which had to prioritise COVID-19-specific studies and were also experiencing the challenges of remote working and staff absence. We were able to adjust our approach to stakeholder engagement and make use of virtual events. Data extraction and delivery was, however, beyond our control and necessitated a project extension.
Study limitations
This study relies on analysis of large-scale routine healthcare data, which is gathered for clinical and administrative purposes and inevitably has some limitations in comparison to data collected primarily for research purposes. Large EHR data sets of this type will necessarily be subject to local differences in data entry and coding procedures. The accuracy of the eFI score will also depend on attendance of patients to healthcare services to enable assessment and diagnosis to be recorded. It is feasible that more frail patients and those who are more vulnerable due to deprivation would be less likely to attend a GP and be incorrectly coded as fit based on eFI scores. The very high GP coverage in the UK, plus the cumulative nature of the eFI, where any previous diagnoses from any GP attendance are likely to remain on the record, suggest this is unlikely to be a major issue, but any effect would be likely to lead to underestimation of frailty and associated service use. The clear trends in service use associated with increased frailty do not suggest unexpectedly high service use in people designated as fit, which might be the case if there was systematic underestimation of the eFI score. Nevertheless, some populations, such as the homeless and those in prison, could be missed and this was raised in stakeholder engagement events; further study of these high-risk groups would be useful. Accurate transfer of information between different healthcare services will also be important to the accuracy of the data. The eFI has been validated in large routine healthcare data sets from different countries,40,109,110 demonstrating that the eFI is capable of identifying frailty as a state of vulnerability and variability in coding accuracy or completeness does not have a significant impact on the ability of the eFI to identify changes in frailty status in ageing populations. In this study, the 36 eFI deficits and other long-term conditions were defined using Read codes. For future data extractions, migration of clinical term definitions from Read codes to the Systemised Nomenclature of Medicine – Clinical Terms (SNOMED CT® – SNOMED International) will be necessary to reflect national harmonisation of coding tools across the healthcare pathway. 111
Although missing data were not a problem for most of the study variables, we noted that approximately one third of participants had no ethnicity data recorded. This is presumably because ethnicity is self-reported administrative data and is therefore more prone to be missing than clinical data. There appears to be under-reporting of non-white ethnicity, with participants identifying as ‘white’ comprising 92% of the given values, as compared with 86% in the 2011 Census (data for England and Wales). 112 It is, however, possible that the under-representation of people from ethnic minorities could also be due to recognised issues with lower primary healthcare usage, rather than practices not reflecting their catchment populations. 113 We were able to address this issue by inclusion of supplementary ethnicity data from linked HES records, enabling missing ethnicity data to be reduced. Despite the missing ethnicity data, our analyses showed that Asian ethnicity was related to frailty onset and progression.
It should be noted that the study data set does not include information from private health care purchased by the patient, which is most commonly available via private medical insurance and accessed by around 11% of the UK population, although schemes have limited cover for general practice. 114 This could result in slight underestimation of service use, particularly in relation to elective care. Information on adult social care, which is means-tested, was not available as it is organised via a mix of state, private and voluntary providers. This is likely to be more relevant for those living with moderate and severe frailty who are more likely to require social care. The data on service use did suggest a reduction in some service use in severe frailty. Current research (NIHR134305 FLOWS) will provide additional data on community and social care use to inform refinements to the simulation model to address this gap. Covariates such as social factors (e.g. loneliness, living situation) and contemporaneous information on residential care status were also not available from the primary data, although additional information on residential care was provided by the validation data set for the development of the simulation model. Older people are more likely to have additional community health and social care services which are not reflected in our analyses, but which may explain the reduction in GP and hospital outpatient and emergency department visits seen in our analyses at the oldest ages within all frailty categories. Future research will access additional data on social and community care to address this gap in the evidence (NIHR134305 FLOWS). It should be noted that, whereas the average service use and costs in the UK reflect a fairly standardised system of care, application to other settings will depend on the health system involved and factors such as extent of private/public care provision. In addition, our analyses reflect the actual usage of services over the study period, but do not reflect unmet need, for example appointments and admissions which are on waiting lists, or difficulties in accessing services by particular sociodemographic groups.
Analysis of the RCGP RSC cohort indicated that there was significant movement of participants both into and out of the cohort during the study period, reflecting real-life population flows in a sample drawn from 5% of total GP practices. Frailty data and patient and service use outcomes were collected for each year that the patient was registered in participating practices, thus providing full outcome ascertainment for each year of participation. The 65–74 group had the lowest number of exits, and higher numbers in other age groups could be a consequence of greater mobility in the working age population or moves related to higher levels of support in older age groups, for example following a health or social care crisis. 115 This could have led to an underestimation of incidence and progression of frailty in this study. Participants of older age and greater frailty severity also had the longest follow-up periods, so under-estimation of frailty incidence is more likely to affect the 50–64 age group. In the validation of the simulation model, it was noted that the Welsh data (SAIL) was not subject to the same level of practice deregistrations, presumably because this data set had much higher population coverage. Losses due to deregistration were ultimately removed from the final population model, although frailty transitions rates were based on the original analyses.
There are some disadvantages to the use of large-scale EHR to calculate the eFI as a measure of frailty in the study population. The eFI has not been formally validated in people aged 50–64 and, therefore, estimates of prevalence and incidence in this age group are less certain than those for older ages. However, there is no reason to assume that a cumulative frailty index approach would not be valid in this age group. This study suggests a higher prevalence in this age group than reported elsewhere, albeit utilising different frailty indices and data sources,116 but patterns of frailty onset and progression are consistent with overall trends. As noted above, the way in which the cohort was constructed would be more likely to have underestimated frailty in this younger age group than overestimated it. Although deficits-based frailty indices (FI) produce higher overall frailty prevalence estimates than phenotypic scores, they are known to have better discrimination in patients with mild frailty, which is still related to poorer outcomes and therefore useful for service planning. 117 Overall, these limitations were outweighed by the advantages of using an FI measure that can be calculated from routine healthcare data, which allowed longitudinal analysis of a large data set. Future development of the eFI will place time restrictions on data included in the score, which could reduce the risk of overidentification (clinicaltrials.gov ID NCT04113174).
It should also be noted that the calculation of the eFI score depends on the quality and completeness of the EHR data. Although increasing prevalence could be influenced by more complete coding of eFI deficits since the introduction of the eFI, this is unlikely within this study. Methods of calculating the eFI in English general practice were not introduced until 2016, so would not have affected coding during most of the study period, which was 2006–17. We observed no sharp change in prevalence that might indicate a change in recording practices in that final year of the cohort. Policy initiatives that attract funding, such as the Quality and Outcomes Framework (QOF), might also influence coding, but their impact is likely to be seen more rapidly. Again, no clear changes in rates were observed that might correspond to changes in coding practices following these initiatives. A further issue is that the eFI is a cumulative measure based on conditions recorded in the EHR and, although it is theoretically possible for improvement to occur (reversal of scores), recording of conditions within GP practice is unlikely to be reversed. The presence of a deficit at any time within the patient’s medical history stands in all future eFI measurements, which could mean that frailty is overestimated. We noted few score reversals and analysis of these indicated that they were due to changes to the polypharmacy score rather than underlying conditions. The eFI has also been shown to demonstrate progression in frailty in longitudinal studies,8,30 a property required to achieve the aims of this study. Planned revisions to the eFI should address this issue and provide data on the rate of reversals, which could be incorporated into the simulation model in the future.
As with all simulation models, simplifications have been made in the development of the system dynamics model. The model assumes that when a patient moves from one frailty state to another, they move through one state at a time and do not miss out states. It also assumes that a patient’s frailty status deteriorates or remains static over time and does not improve. While the data suggested frailty score could improve over time for a very small percentage of the population, reversals were not included in the simulation. As noted above, reversals were in most cases due to a change in polypharmacy score, presumably following a review of the patient’s pharmacy requirements118 rather than the true underlying frailty state of the patient. Although reversal in frailty state was uncommon in this analysis, this event could become a key goal of future frailty-specific services. Their potential impact could be addressed in the simulation model through altering transition rates in scenario models, but in future work there could be benefits to developing the model to reflect reversals in frailty state.
Simulation model development
In this study, we were able to develop and validate a functioning SD simulation model of the development and progression of frailty in an ageing population. Farrell et al. 119 comment that for models to be useful, they should be predictive, and be parameterised by data from large populations of ageing individuals, as is the case in this study, where we have used two large population data sets (RCGP RSC and SAIL) to fit and validate our model. The data-driven approach taken in this research has enabled us to use system dynamics to provide an accurate representation of frailty over time in a population aged 50 and over, allowing projections of population ageing, frailty incidence, progression and health service impact over time. The simulation model has undergone extensive internal and external validation against real world data and has been shown to be both robust and accurate. This underlying simulation of population ageing, and frailty progression provided a robust structure on which we have overlaid primary and secondary care usage for the over 50 patient population. This allowed us to examine the service use associated with frailty at different ages and to begin to explore the effect of different scenarios on frailty progression and service use. The final population-level model is able to estimate future primary and secondary care needs associated with different frailty strata in the ageing population, thus providing a useful evidence base for commissioners and health and social care providers. The development and validation of the system dynamics simulation was further strengthened by the extensive consultation with patients, carers and health and social care professionals at key points in the study. An important learning point from the validation of the simulation model was that, even though the data set was large and comprehensive, it was not sufficient on its own to estimate transition rates that would be valid for subsequent years. It was also important to take account of broader population changes over the study period.
Integrated study findings
Frailty prevalence
Literature estimates of frailty prevalence range from 3.9% to 51.4%, with a pooled prevalence of 12–17.4%, and wide variation with age. Our analysis of large-scale longitudinal data suggests a much higher population prevalence (26.5% in 2006), with increasing prevalence over time evident within each age group as the cohort aged. The finding that at least 1 in 10 people aged 50–64 are already frail is noteworthy and has important implications for future service development,120 as is the scale of change in prevalence within the study period, with substantial increases in moderate to severe frailty in all age groups over time, tripling in the 50–64 age group and approximately doubling in all others. The simulation projections indicate that these trends will continue in the future, with increases in frailty prevalence in all age groups and a corresponding decline in the fit population over time. The scenario analyses presented in Chapter 7 indicated that both reducing incidence of frailty and slowing progression have the potential to reduce prevalence of frailty (or of more severe frailty) over time, with benefits for reducing associated service use and costs associated with frailty. Moving towards targeted prevention and management earlier in the frailty journey would necessitate a fundamental shift in policy and practice. Further work is needed to determine the most cost-effective approaches when taking into account costs associated with both general and frailty-specific services. There are also clearly workforce implications to any such shift in focus. 46,47
Incidence and progression of frailty
Our analysis of longitudinal data from primary care in England has provided new evidence on frailty incidence and transitions in an ageing population, consistent with current knowledge and has strengthened the case for the important role of deprivation in relation to frailty onset and progression. 38,116,121–123 Our overall incidence rate of 47.1 per 1000 PYAR is in line with pooled estimates of 43.4 per 1000 PYAR in the literature. As with prevalence, the population figure masks wide variation within age and other subgroups, with rates higher at older ages and in females, as expected. Estimated incidence in the younger age group was also higher than expected, at 31.8 per 1000 PYAR.
The average age of onset for the development of frailty (of any category) for patients who were fit in the year they entered the cohort was 69 years (SD 10 years). The overall incidence rate of frailty was 47.1 cases per 1000 person-years (95% CI 47.0 to 47.2). Incidence was higher in older age groups, female sex, Asian ethnicity, more deprived quintiles and those living in urban areas. Incidence rates were 31.8 for the 50–65 year age group, rising to 158.5 for the oldest, but rates within age groups were stable across years. Ex-smokers and people who were underweight or obese had a higher incidence of frailty.
The multistate model demonstrated increasing speed of transitions with increasing age, as shown in other studies predominantly using phenotypic frailty assessments. 29,124 Within each age group, the longer time spent within each frailty category as the severity of frailty increases may be explained by a combination of a saturation effect of deficits for each individual, that is a slowing in the accumulation of deficits with time, and increased death rates at higher levels of frailty acting as a competing risk to progression. The multistate model established that in addition to recognised risk factors for frailty (i.e. age and female sex), deprivation, Asian ethnicity and urban residence were independently associated with an increased risk of frailty transitions. Deprivation was the most important factor after age, with people living in the two most deprived IMD quintiles having earlier onset of frailty and faster progression. This concurs with previous studies in which individual domains associated with deprivation, such as education and wealth, have been considered, and supports the suggestion that poorer older people spend additional years of life in a frail state. 125–131
Service use and costs
One of the most important findings from this study is that the increases in service use and associated costs with increasing frailty at an individual level need to be viewed within the context of the wider population structure. For example, whereas a 65–74-year-old person in the mild frailty category may have an average total cost of £1214 per year, which is lower than those of the same age with severe frailty, the large numbers of people with these characteristics in the overall population result in the cumulative costs for this group being very high. It is worth noting that more severe frailty is associated with higher costs at all ages, so reducing incidence and progression is likely to be key to reducing costs. Findings from the simulation predict substantial increases in service use associated with frail older people over a 10-year period, with primary care use increasing by 18%, A&E attendances by 23%, ambulance calls by 35% and hospital admissions by up to 13%. Costs associated with frail older people will also rise over this period, with costs attributable to frail older people taking up an increasing proportion of total costs (from 65% to 72%). It is important to be able to consider both the population structure as well as the degree of frailty of patients within it when planning services. It should be noted that the analyses presented here are focused on the impact of frailty on use of general primary and secondary care services that are used by all older people, including those living with frailty. In recent years, there has been increased development of services specifically addressing frailty, but these have not yet been researched or implemented on a large scale. Although the scenario analyses suggest that frailty-specific interventions aimed at key transition points could have potential to reduce general service use, such interventions will incur their own costs and workforce needs.
The relationships between sociodemographic characteristics and costs raised some interesting issues around differentials in healthcare use. The largest impact on healthcare use after adjustment for age and other characteristics was presence of frailty, with a fivefold increase in primary care costs in people with severe frailty as compared to fit. A trend in increased primary care use with increasing deprivation quintile was seen, with an additional 15% of costs seen in the most deprived quintile. Differences in ethnicity were also observed, with patients of Asian or black ethnicity having higher primary care costs than white. It is known that inequalities have a significant impact on frailty even in middle age, leading to large differences in frailty prevalence around UK retirement age, but reducing in impact in the oldest age group, as was also found in the Newcastle 85 + cohort. 39,132 This study demonstrates that the pattern of frailty prevalence and associated service use is underpinned by patterns in health inequalities, reflected in our cohort data by the influence of sex, deprivation, ethnicity and rural location on service use and costs. The higher levels of frailty onset and progression in people of Asian ethnicity is consistent with differences in the prevalence of frailty with ethnicity observed in a London cohort,133 and suggests tailored approaches for different communities may be important. Further investigation of these variations in risk, particularly the effect of deprivation and ethnicity, is needed to inform the development and targeting of frailty-specific services aimed at prevention of frailty onset and slowing of its progression. The higher transition rate in people living in urban areas suggests that geographical considerations may also be important, in line with findings from a small English cohort, which also suggests coastal communities may be at higher risk. 117 Understanding frailty and its impact needs to be better contextualised within the sociodemographic variation contributing to frailty to be able to better plan services for prevention and management. Current research on workforce and frailty services (NIHR134305 FLOWS) has provided an opportunity to expand community engagement to more diverse communities.
Further understanding of the role of deprivation and the interaction and impact with different characteristics, for example, sex and ethnicity, requires more research to be able to identify appropriate care models. For example, a life-course approach including multiple measurements of neighbourhood social deprivation (NSD) in Scotland identified sex differences in the relationships between deprivation, with accumulated deprivation across life-course being more related to frailty onset in men, whereas deprivation in mid-late adulthood was more important for women, both for frailty onset and for progression. 29,134 It is likely that socioeconomic and health inequalities such as those which have been exacerbated by the COVID-19 pandemic will further impact on illness (and frailty) trajectories and service use over the coming years, including a further acceleration in frailty onset and decline for middle-aged adults. 135 Further longitudinal studies of frailty at the population level are needed to monitor these effects and pre-empt service configurations and capacity for the future.
Higher service use in people with frailty may also be influenced by suboptimal design of care provision. An example is given from a US stroke cohort which identified an increase in gaps in care co-ordination (e.g. between care providers for multimorbidity) in people aged ≥ 65 with frailty and a related increase in preventable adverse events (ED attendance, hospital admission, drug interaction). 136 Effective primary care (timely access, seeing the same practitioner with knowledge about the patient, communication, and co-ordination with specialist services) has been associated with reduced hospital admissions in people aged ≥ 50 with frailty in Brazil. 137 These studies have implications not only for service design but also for the workforce required to deliver and co-ordinate care services to achieve the optimal balance between efficiency and effective care. Commissioning for services to meet the needs of people with frailty is going to require up-to-date knowledge of the local population age structure and also information regarding patterns of deprivation in the community.
Given the increasing number of people living with frailty, understanding of service use and planning ahead to commission targeted services which may decrease costs are essential. As noted above, existing services in primary and secondary care, especially those serving older people, are already dealing with large numbers of older people living with frailty. This study provides useful analyses of the likely impact of frailty on these services in the future, particularly the large numbers of older people living with mild and moderate frailty. The scenario analyses also indicate some avenues for targeting of frailty-specific services to reduce downstream demand, but there is a lack of evidence on the most effective approaches for implementation at scale, or their costs and workforce implications.
There is some evidence that both public health and primary care interventions can prevent frailty onset or slow progression, but if these are widely implemented there is likely to be some substitution between general services meeting the needs of those who are frail and the frailty-specific services. Although intervention components such as nutrition and exercise, public health measures (i.e. reducing obesity, stopping smoking and reducing alcohol intake) and management of comorbidities contributing to frailty may reduce progression and/or delay frailty onset, there is less evidence for methods of implementing these components into integrated care models and current health and care pathways. 46,137,138,139 This is a key area for further research targeting higher risk groups for frailty onset and faster progression. The scenario modelling presented here can inform consideration of the impact of different approaches to services for frailty, but further information is needed to understand the service and workforce requirements of moving towards more targeted provision of frailty-specific services.
The large variation in costs associated with frail older people, particularly in the 85 + age group, may be influenced by comorbidities, impairments and proximity to death. 37,140 The lack of information on alternative services and their costs, such as end-of-life care, domiciliary care packages or nursing home placements, in this and other analyses may be providing an incomplete view of typical costs for the oldest patients, with a bias towards the more expensive hospital costs. More complete care usage data, or use of a combination of data sources, is urgently needed to have an overall understanding of all care provided to inform future commissioning of appropriate services for frail older people. Central to future planning is the estimation of the required workforce to deliver services, informed by assessment of demands. Demand-led service planning for frailty-specific services is as yet uncommon, and we aim to use this data on service use within our frailty dynamics population simulation model and extend it to model workforce requirements in different service configurations (NIHR134305 FLOWS).
Findings on costs are higher than those previously reported,39 which estimated the potential cost of service use due to frailty to be £5.8 billion in 2014. However, that study included a sample of those aged 65 and over, whereas the analyses presented here include those aged 50 and over and therefore provides costs for a larger subset of the population. While Han et al. 39 acknowledge that their findings are likely to be an underestimate of the true picture, some of the difference between these two studies is also due to the fact that this study uses a later version of unit costs (2017 compared with 2014) as well as considering different aspects of secondary care usage. Han et al. 39 consider emergency and elective hospital admissions while we consider short stay (< 24 hours) and typical stay (24 hours and over) as well as the cost of outpatient appointments, A&E attendances and admissions to critical care. For primary care, Han et al. 39 include both GP and nurse appointments as well as the prescriptions relating to those appointments. In this study, individual medicines are considered rather than the number of prescriptions issued. We therefore believe that we can account for the differences between the two cost estimates across the two studies. However, the strength of these findings is not in precise cost estimates, but the ability to compare costs by frailty group over time. The most important findings in relation to cost are therefore the projected increases in costs and the increased proportion associated with more severe frailty over time. The scenario analysis indicates that a 5% reduction in incidence would result in substantial decreases in frailty-associated costs over a 10-year period.
Summary
This study was unique in its use of a large, population-level, linked data set with a long period of follow-up to inform development of a simulation model for prediction of the impact of frailty in an ageing population. The analysis of this data set, using an advanced statistical modelling approach, has allowed precise estimates of incidence and prevalence at whole population level and also within subgroups of interest, features which were vital in the development of a robust simulation model. The large cohort size and long period of follow-up also enabled us to describe transitions between frailty categories over time and assess the impact of key variables associated with frailty on these transitions. This study was therefore able to provide robust analysis of frailty transitions in an ageing population, with new evidence on the rate of decline within an ageing cohort. It should also be noted that this study was unique in allowing exploration of frailty transitions in people aged ≥ 50 and, importantly, the retrospective analysis of primary care demonstrated that frailty is already prevalent before age 65. The use of the eFI as a measure of frailty, and its application with routinely available data sets, ensures that these analyses are applicable to real world service planning and commissioning and are key in enabling replicability of these analyses in other settings. The development of a population-level simulation model informed by data from a large, population-level, linked data set and complemented with ONS population estimates has enabled the initial steps towards a demand-led population and service use tool for frailty to be undertaken. While the value in the model associated with carrying out the scenario experiments (currently underway) is yet to be fully realised, the baseline projections have provided some useful insight into how frailty among those aged 50 and over and what the associated service use and costs might look in the next few years.
Chapter 9 Conclusions and recommendations
Implications for service planning and provision
The findings from this study are likely to be of particular importance when planning and commissioning services, particularly for those aged 50–64. Given that the majority of frailty-specific services are currently targeted at those aged 65 and over and high prevalence rates in older age groups are the focus of considerable policy and practice attention in terms of individual management, our analyses demonstrate that absolute numbers of younger old people with mild to moderate frailty exceed those of older people with severe frailty due to the greater population numbers within the youngest age group. This study, particularly the simulation modelling of future demand associated with frailty, demonstrates that older people living with frailty have a considerable and growing impact on use of healthcare services and suggests that population-level preventative strategies are needed and could have more impact on service use associated with frailty than the current focus on severe frailty. A population-level approach to early prevention of frailty, or slowing of frailty progression, is therefore likely to be a key strategy for long-term reduction of population morbidity and disability and the impact of frailty on services. The evidence to support development and wide-scale implementation of frailty-specific services, whether for prevention or slowing of progression, is limited. It is also important to note that our multistate model analysis demonstrated that older age groups transition to higher levels of frailty faster than middle-aged adults and interventions to slow progression and support those with high care needs will also be important.
This study also adds to the evidence in relation to the impact of frailty on general health service use and costs, including those from age 50 onwards. Further work is needed to explore the impact on community-based health and social care services, including residential care, and the potential workforce implications of providing specific and targeted services for frailty. The simulation model developed in this study allows predictions of prevalence in different age groups and associated service use and costs in an ageing population if no changes are made to services and if services are targeted at frailty at key points in the frailty trajectory. A strength of this study is that it provides new knowledge about projected numbers of people living with frailty, including in middle age, and associated demand for primary and secondary care services. This analysis confirmed the impact of inequality on development and progression of frailty, suggesting that service planners will also need to consider targeted prevention and management that address intersectionality in relation to the risk and impact of frailty. Existing initiatives, such as the Women’s Health Strategy,141 could have an impact on frailty in the population in this way. Scenario models also provide projections of the potential impact of interventions at key points in the frailty trajectory. The information on both these aspects is essential to allow service providers and commissioners to balance the impact of preventive and supportive services and to effectively plan appropriate service configurations. However, development of these specific services for frail older people, or those at risk of developing frailty, will require more evidence on cost-effective interventions to inform service changes to manage the impact of frailty as the population ages.
The clinical management of frailty will become increasingly important as the population ages, with our analyses showing the prevalence of frailty rising from 10% of people aged 50–64, up to 88% in those aged over 85. Despite the scale of this patient group, research indicates that half of patients with frailty are not receiving effective healthcare interventions. In Fit for Frailty Part 2,2 it is noted that there is potential for significant harm to frail patients if they receive inappropriate interventions. However, many services across the health and care system do not take adequate account of individuals’ frailty and so opportunities to improve quality of care are missed. At the individual patient level, guidance for patient management exists and there is general agreement about the features of good quality care. Importantly, this research adds to our understanding of service-level impact of frailty, indicating that, in addition to planning for increasing numbers of older people with frailty, targeting of interventions earlier in the frailty journey, focusing on those at higher risk of developing frailty and reducing progression to more severe frailty states will be vital in managing demand and costs associated with frailty. This would necessitate a shift from the current policy and practice focus on moderate to severe frailty.
The analyses presented here, including the simulation projections, provide robust estimates of the likely demand associated with frailty in the population over a 10–20-year period. These findings will be incorporated into guidance for service providers and commissioners. The improved understanding of population needs offered by this study will inform appropriate service planning and delivery, giving direct benefit for patients through provision of timely and appropriate care.
This study, with its emphasis on whole-system population dynamics of frailty, explores issues around population need, service configurations and clinical interventions highlighted above. A strength of the simulation modelling approach is that it allows for identification of key transition points, projection of future demand and rapid testing of ‘what-if’ scenarios to aid decision-making. The findings of this study, by identifying population drivers of frailty onset and progression and allowing prediction of service needs associated with frailty, inform targeted prevention and intervention. In addition to the results and discussion presented in this report, work continues on distilling these findings into guidance for commissioners and service providers.
Future research
The focus of this research was to develop recommendations and a decision support tool for commissioners and social and healthcare providers. While we have considered the demands on primary and secondary care posed by frailty, we have not provided an estimate of the workforce needed to support provision to meet the demand associated with frailty. Future research will consider the health and social care workforce need associated with frailty in the population aged 50 and over (NIHR134305 FLOWS). We will consider capacity constraints in relation to the workforce model being developed in FLOWS.
This study has highlighted the need for further research into the relationship between ethnicity, deprivation and incidence and progression of frailty. Further analysis of clinical characteristics associated with frailty and different care pathways in subgroups of frail older people is also recommended. The descriptive analysis of clinical features indicates the large number and type of conditions recorded for the participants. As a cumulative score, there are clearly numerous different routes to frailty and further analysis of the clinical diagnoses to explore clustering would be useful in determining any important differences in the onset of frailty in subgroups of interest, particularly those developing frailty at a younger age or with particularly rapid progression. This would provide an opportunity to explore the issue of intersectionality through the relationships between sociodemographic factors, clinical conditions and onset of frailty. We propose to seek funding to continue this aspect of our work.
The study presented here focuses primarily on frailty within England as the model has been informed by data collected in the RCGP RSC database. However, in externally validating the model, data from Wales has been analysed. In comparing these two large data sets, differences in the population structure and frailty transitions have been observed and poses the question as to whether the simulation model could be adapted to other countries. Validation of the model presented here indicates that population structure and changes over time need to be taken account of in model development. In conducting this research, data from England was used to build the simulation model, which was then externally validated using data from Wales. During the validation process, differences in the population structure and frailty transitions were observed between the two countries. The model has been constructed in a way that allows it to be adapted for use in different countries or regions, with appropriate adjustment of the model parameters and starting stocks. This work raises the question of how far the simulation model would be transferable to other countries and healthcare systems; international validation work is recommended.
Dissemination and implementation
Planned dissemination and implementation activities were delayed by the COVID-19 pandemic, but work continues on these. Given the above point about adapting the model for use in other countries, we will engage with our collaborators in SAIL to identify opportunities for dissemination planning with colleagues in Wales. We will produce an executive summary of this study, suitable for use as a briefing paper for NHS managers and commissioners. In addition, we will prepare a short PowerPoint presentation to present the main findings to NHS organisations. We will collate the outputs of the study into a commissioning toolkit, comprising guidance on drivers of frailty-related demand and summary simulation model outputs that can be used for prediction of future demand in a typical population, with adjustments for specific scenarios. Our dissemination strategy will be guided by our PPI representatives and other key stakeholders on the SEG, including those commissioning frailty services. The planning stage of the commissioning cycle is often limited by a lack of reliable data on demand, particularly data which allows for forward projections; findings from this study address this need in relation to older people with frailty. Findings will be collated to provide new guidance on the impact of frailty at population level, specifically in relation to trends in service use as the population ages.
Summary of implications for practice
In summary, the combined results of the statistical and simulation modelling presented here demonstrate that demographic and socioeconomic features of local populations are the most significant aspects to consider in planning both services for frailty and general services used by older people living with frailty, given their impact on the onset and progression of frailty, and therefore on health and care service needs and costs. Service providers also need to consider that frailty is already present in the population before age 65 and the average age of onset of any frailty is lower than expected. Multisectoral strategies to reduce the incidence of frailty in middle age need to focus on specific risk groups, such as people living in areas of higher deprivation. Simulation modelling scenarios indicated that delaying onset of frailty will be important in reducing downstream service use by people with frailty and associated costs; targeted prevention at national population level has the potential to result in substantial cost-savings but requires development and evaluation of services specific to older people at risk of frailty. In addition, health and social care services will need to take into account that the speed of frailty progression increases with age, requiring appropriately tailored and evidenced interventions to manage patients to delay further progression, as well as properly resourced health and care systems to cope with the increased service use expected in our ageing population. Service planners should also note that scenario modelling in this study revealed that reductions in service use and costs achieved through reduced frailty incidence and progression are, to some extent, offset by service use and costs in less frail states.
Additional information
Contributions of authors
Bronagh Walsh (https://orcid.org/0000-0003-1008-0545) is Principal Investigator for the study.
Carole Fogg (https://orcid.org/0000-0002-3000-6185) is Senior Research Fellow providing expertise in epidemiology, data management and data analysis across all the workstreams.
Tracey England (https://orcid.org/0000-0001-7565-4189) is Senior Research Fellow and worked on the operational research and simulation modelling.
Sally Brailsford (https://orcid.org/0000-0002-6665-8230) is Professor of Management Science and works on healthcare operational research (OR). Sally has worked on the Operational Research and simulation modelling and was lead for Workstream 4.
Paul Roderick (https://orcid.org/0000-0001-9475-6850) is Professor of Public Health (now retired). Paul provided co-leadership for Workstream 1 and guidance for Workstream 2.
Scott Harris (https://orcid.org/0000-0001-5774-1537) is Associate Professor of Medical Statistics. His role for the study was to provide statistical analysis expertise and support.
Simon Fraser (https://orcid.org/0000-0002-4172-4406) is Associate Professor of Public Health. Simon provided co-leadership for Workstream 1 and guidance for Workstream 2 and primary care expertise.
Andrew Clegg (https://orcid.org/0000-0001-5972-1097) is Professor of Geriatric Medicine. Andrew provided expertise on care of older people and the eFI across all the workstreams.
Simon de Lusignan (https://orcid.org/0000-0002-8553-2641) is a Senior Academic GP and Director of the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC). Simon provided primary care expertise and guidance on data extraction, management, linkage and analysis for Workstream 1.
Shihua Zhu (https://orcid.org/0000-0002-1430-713X) is Senior Research Fellow in Health Economics. His role in the study was to advise on service use cost attribution and analysis for Workstream 1.
Francesca Lambert (https://orcid.org/0000-0003-0327-4325) is Research and PPIE co-ordinator for the study. Her role has been to plan and facilitate our Stakeholder Engagement Groups, writing up of SEGs, and be the contact for our PPIE contributors.
Abigail Barkham (https://orcid.org/0000-0001-6267-6370) is Divisional Clinical Lead for Physical Health and her role for this study was to facilitate, advise and support the public/patient/care stakeholder groups and lead on Workstream 3.
Harnish Patel (https://orcid.org/0000-0002-0081-1802) is Consultant in Medicine for Older People who has a specialist interest in managing frailty. His role in the study was to advise and support on stakeholder engagement in Workstream 3.
Vivienne Windle (https://orcid.org/0009-0005-4783-7531) is a retired Parliamentary Researcher and City Councillor. She is the Public and Patient Involvement representative for the study. She helped us plan and participated in our Stakeholder Engagement Groups and advised on recruitment to the study PPIE events.
Acknowledgements
Study contributors and collaborators
We would like to thank the following individuals and groups for their participation and assistance in this study:
-
School of Health Sciences Older People & Dementia PPIE Panel and Contributors.
-
Health and social care professionals from the Frailty Dynamics Stakeholder Engagement Group.
-
SAIL Databank staff, in particular Dr Helen Daniels and Dr Ashley Akbari.
-
RCGP Databank staff, in particular Mr Julian Sherlock, Dr John Williams, Dr Jeremy van Vlymen and Dr Filipa Ferreira.
-
DARS (NHS Digital) staff.
We would also like to give our grateful thanks to the members of our Steering Committee:
-
Professor Simon Conroy (Chair) is Consultant Geriatrician and Honorary Professor of Geriatric Medicine in the MRC Lifelong Health & Ageing unit at University College London.
-
Professor Kathy Kotadis is Professor of Management Science/Operational Research at the University of Kent Business School.
-
Dr Helen Cruikshank is Consultant in Public Health at Hampshire County Council.
-
Dr Sue Green is Professor of Nursing Science at Bournemouth University.
-
Professor Martin Vernon is Consultant Geriatrician and Clinical Director for Integration at Tameside and Glossop Integrated Care NHS Foundation Trust.
-
Mrs Vivienne Windle is a retired Parliamentary Researcher and City Councillor in Southampton. She is a Public and Patient Involvement representative for the study.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Data-sharing statement
The data used in the study are secondary data and are retained by the data providers; therefore there are no data available for further access or sharing. All queries should be submitted to the corresponding author.
Ethics statement
This study was approved by the University of Southampton Research Ethics Committee (ERGO II 46313) on 6 February 2019, the RCGP RSC Information Governance Panel on 24 January 2019 and DARS IGARD panel on 19 April 2021. It was approved by the SAIL Information Governance Review Panel (IGRP) on 3 December 2020.
Information governance statement
The University of Southampton is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation University of Southampton is the Data Processor; RCGP RSC and SAIL are the Data Controllers, and we process personal data in accordance with their instructions. You can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for RCGP RSC Data Protection Officer here: https://orchid.phc.ox.ac.uk/index.php/information-governance/ and SAIL here: https://saildatabank.com/governance/approvals-public-engagement/information-governance/.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/LKJF3976.
Primary conflicts of interest: Bronagh Walsh reports other NIHR grants – NIHR HSDR 134305. Carole Fogg reports other NIHR grants – NIHR HSDR 134305. Tracey England reports other NIHR grants – NIHR HSDR 17/05/96 and NIHR HSDR 134305. Sally Brailsford reports other NIHR grants – NIHR HSDR 17/05/96 and NIHR HSDR 134305. Simon Fraser reports other NIHR grants – NIHR ARC Wessex; NIHR CCF 203988; NIHR SPCR 564; NIHR HSDR 131948; NIHR CCF 202644. Francesca Lambert reports other NIHR grants – NIHR HSDR 128056 and NIHR HSDR 134305. Abigail Barkham reports other NIHR grants – NIHR HSDR 134305.
Publications
Fogg C, Fraser SDS, Roderick P, de Lusignan S, Clegg A, Brailsford S, et al. The dynamics of frailty development and progression in older adults in primary care in England (2006–2017): a retrospective cohort profile. BMC Geriatr 2022;22(1):30. https://doi.org/10.1186/s12877-021-02684-y
Walsh B, Fogg C, Harris S, Roderick P, de Lusignan S, England T, et al. Frailty transitions and prevalence in an ageing population: longitudinal analysis of primary care data from an open cohort of adults aged 50 and over in England, 2006–2017. Age Ageing 2023;52(5):afad058. https://doi.org/10.1093/ageing/afad058
Fogg C, England T, Zhu S, Jones J, de Lusignan S, et al. Primary and secondary care service use and costs associated with frailty in an ageing population: longitudinal analysis of an English primary care cohort of adults aged 50 and over, 2006–2017. Age Ageing 2024;53(2):afae010. https://doi.org/10.1093/ageing/afae010
England T, Walsh B, Brailsford S, Fogg C, de Lusignan S, Fraser SDS, et al. Using routine healthcare data to develop and validate a system dynamics simulation model of frailty trajectories in an ageing population. Health Syst, under review, October 2023.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- British Geriatrics Society . Fit for Frailty Part I: Consensus Best Practice Guidance for the Care of Older People Living With Frailty in Community and Outpatient Settings 2014.
- British Geriatrics Society . Fit for Frailty Part 2: Developing, Commissioning and Managing Services for People Living With Frailty in Community Settings 2015.
- NIHR Dissemination Centre . Themed Review: Comprehensive Care – Older People Living With Frailty in Hospitals 2017.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752-62.
- Gale CR, Cooper C, Sayer AA. Prevalence of frailty and disability: findings from the English Longitudinal Study of Ageing. Age Ageing 2015;44:162-5.
- Campbell AJ, Buchner DM. Unstable disability and the fluctuations of frailty. Age Ageing 1997;26:315-8.
- NHS England . Using Case Finding and Risk Stratification: A Key Service Component for Personalised Care and Support Planning 2015.
- Clegg A, Bates C, Ryan R, Nichols L, Ann Teale E, Mohammed MA, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing 2016;45:353-60.
- Theou O, Tan ECK, Bell S, Emery T, Robson L, Morley JE, et al. Frailty levels in residential aged care facilities measured using the Frailty Index and FRAIL-NH scale. J Am Geriatr Soc 2016;64:e207-12.
- Pilotto A, Cella A, Pilotto A, Davagjati J, Veronese N, Musacchio C, et al. Three decades of comprehensive geriatric assessment: evidence coming from different healthcare settings and specific clinical conditions. J Am Med Dir Assoc 2017;18:192.e1-11.
- National Institute for Health and Care Excellence . Multimorbidity: Clinical Assessment and Management 2016.
- NHS Digital . NHS Vacancy Statistics England April 2015–June 2022 Experimental Statistics 2022. https://digital.nhs.uk/data-and-information/publications/statistical/nhs-vacancies-survey/april-2015-june-2022-experimental-statistics (accessed 3 March 2023).
- Skills for Care . The State of the Adult Social Care Sector and Workforce in England 2022.
- Fogg C, Fraser SDS, Roderick P, de Lusignan S, Clegg A, Brailsford S, et al. Frailty Dynamics Study Team . The dynamics of frailty development and progression in older adults in primary care in England (2006–2017): a retrospective cohort profile. BMC Geriatr 2022;22.
- NHS England . Standard General Medical Services Contract 2017 18 2017.
- Dixon J, Bardsley M. Predictive risk modelling using routine data: underexploited potential to benefit patients. J Health Serv Res Policy 2012;17:131-2.
- Improvement Academy . Electronic Fidelity Index (eFI) 2017. https://improvementacademy.org/our-impact/electronic-frailty-index-efi-tool/ (accessed 4 December 2023).
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Cardiovascular Health Study Collaborative Research Group . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146-56.
- Aguayo GA, Donneau AF, Vaillant MT, Schritz A, Franco OH, Stranges S, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol 2017;186:420-34.
- Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487-92.
- O’Caoimh R, Galluzzo L, Rodriguez-Laso A, Van der Heyden J, Ranhoff AH, Lamprini-Koula M, et al. Work Package 5 of the Joint Action ADVANTAGE . Prevalence of frailty at population level in European Advantage joint action member states: a systematic review and meta-analysis. Ann Ist Super Sanita 2018;54:226-38.
- Siriwardhana DD, Hardoon S, Rait G, Weerasinghe MC, Walters KR. Prevalence of frailty and prefrailty among community-dwelling older adults in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Open 2018;8. https://bmjopen.bmj.com/content/8/3/e018195 (accessed 21 May 2024).
- Pradhananga S, Regmi K, Razzaq N, Ettefaghian A, Dey AB, Hewson D. Ethnic differences in the prevalence of frailty in the United Kingdom assessed using the electronic Frailty Index. Aging Med (Milton) 2019;2:168-73.
- Ofori-Asenso R, Chin KL, Mazidi M, Zomer E, Ilomaki J, Zullo AR, et al. Global incidence of frailty and prefrailty among community-dwelling older adults: a systematic review and meta-analysis. JAMA Netw Open 2019;2.
- Gill TM, Gahbauer EA, Allore HG, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med 2006;166:418-23.
- Mitnitski A, Bao L, Skoog I, Rockwood K. A cross-national study of transitions in deficit counts in two birth cohorts: implications for modeling ageing. Exp Gerontol 2007;42:241-6.
- Fallah N, Mitnitski A, Searle SD, Gahbauer EA, Gill TM, Rockwood K. Transitions in frailty status in older adults in relation to mobility: a multistate modeling approach employing a deficit count. J Am Geriatr Soc 2011;59:524-9.
- Wang C, Song X, Mitnitski A, Fang X, Tang Z, Yu P, et al. Effect of health protective factors on health deficit accumulation and mortality risk in older adults in the Beijing Longitudinal Study of Aging. J Am Geriatr Soc 2014;62:821-8.
- Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community-dwelling older people: a systematic review and meta-analysis. Ageing Res Rev 2019;50:81-8.
- Hollinghurst J, Fry R, Akbari A, Clegg A, Lyons RA, Watkins A, et al. External validation of the electronic Frailty Index using the population of Wales within the Secure Anonymised Information Linkage Databank. Age Ageing 2019;48:922-6.
- Hoogendijk EO, Theou O, Rockwood K, Onwuteaka-Philipsen BD, Deeg DJH, Huisman M. Development and validation of a frailty index in the Longitudinal Aging Study Amsterdam. Aging Clin Exp Res 2017;29:927-33.
- Hoogendijk EO, Rockwood K, Theou O, Armstrong JJ, Onwuteaka-Philipsen BD, Deeg DJH, et al. Tracking changes in frailty throughout later life: results from a 17-year longitudinal study in the Netherlands. Age Ageing 2018;47:727-33.
- Stolz E, Mayerl H, Hoogendijk EO, Armstrong JJ, Roller-Wirnsberger R, Freidl W. Acceleration of health deficit accumulation in late-life: evidence of terminal decline in frailty index three years before death in the US Health and Retirement Study. Ann Epidemiol 2021;58:156-61.
- Bai G, Szwajda A, Wang Y, Li X, Bower H, Karlsson IK, et al. Frailty trajectories in three longitudinal studies of aging: is the level or the rate of change more predictive of mortality?. Age Ageing 2021;50:2174-82.
- Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Exp Aging Res 2009;35:61-82.
- Stolz E, Mayerl H, Freidl W. Fluctuations in frailty among older adults. Age Ageing 2019;48:547-52.
- Welstead M, Jenkins ND, Russ TC, Luciano M, Muniz-Terrera G. A systematic review of frailty trajectories: their shape and influencing factors. Gerontologist 2021;61:e463-75.
- García-Nogueras I, Aranda-Reneo I, Peña-Longobardo LM, Oliva-Moreno J, Abizanda P. Use of health resources and healthcare costs associated with frailty: the FRADEA study. J Nutr Health Aging 2017;2:207-14.
- Han L, Clegg A, Doran T, Fraser L. The impact of frailty on healthcare resource use: a longitudinal analysis using the Clinical Practice Research Datalink in England. Age Ageing 2019;48:665-71.
- Boyd PJ, Nevard M, Ford JA, Khondoker M, Cross JL, Fox C. The electronic frailty index as an indicator of community healthcare service utilisation in the older population. Age Ageing 2019;48:273-7.
- O’Halloran AM, Hartley P, Moloney D, McGarrigle C, Kenny RA, Romero-Ortuno R. Informing patterns of health and social care utilisation in Irish older people according to the Clinical Frailty Scale. HRB Open Res 2021;4.
- Ikonen JN, Eriksson JG, von Bonsdorff MB, Kajantie E, Arponen O, Haapanen MJ. The utilization of primary healthcare services among frail older adults: findings from the Helsinki Birth Cohort Study. BMC Geriatr 2022;22.
- Ge L, Yap CW, Heng BH, Tan WS. Frailty and healthcare utilisation across care settings among community-dwelling older adults in Singapore. BMC Geriatr 2020;20.
- Roe L, Normand C, Wren MA, Browne J, O’Halloran AM. The impact of frailty on healthcare utilisation in Ireland: evidence from the Irish longitudinal study on ageing. BMC Geriatr 2017;17.
- Doherty AS, Miller R, Mallett J, Adamson G. Heterogeneity in longitudinal healthcare utilisation by older adults: a latent transition analysis of the Irish Longitudinal Study on Ageing. J Aging Health 2022;34:253-65.
- Sadler E, Khadjesari Z, Ziemann A, Sheehan KJ, Whitney J, Wilson D, et al. Case management for integrated care of older people with frailty in community settings. Cochrane Database Syst Rev 2023;5.
- Looman WM, Huijsman R, Fabbricotti IN. The (cost-)effectiveness of preventive, integrated care for community-dwelling frail older people: a systematic review. Health Soc Care Community 2019;27:1-30.
- Apóstolo J, Cooke R, Bobrowicz-Campos E, Santana S, Marcucci M, Cano A, et al. Effectiveness of the interventions in preventing the progression of pre-frailty and frailty in older adults: a systematic review protocol. JBI Database System Rev Implement Rep 2016;14:4-19.
- Lipsitz LA. Dynamic models for the study of frailty. Mech Ageing Dev 2008;129:675-6.
- Varadhan R, Seplaki CS, Xue QL, Bandeen-Roche K, Fried LP. Stimulus-response paradigm for characterizing the loss of resilience in homeostatic regulation associated with frailty. Mech Ageing Dev 2008;129:666-70.
- Whole Systems Partnership . System Dynamics in Healthcare 2018. www.thewholesystem.co.uk/wp-content/uploads/2019/01/SCIO-talk.pdf (accessed 14 February 2024).
- Thompson JP, Riley CM, Eberlein RL. Modelling for insight: the case of dementia in Singapore. Syst Res Behav Sci 2014;31:227-35.
- Thompson JP, Riley CM, Eberlein RM, Matchar DB. Future living arrangements of Singaporeans with age-related dementia. Int Psychogeriatr 2012;24:1592-9.
- Evenden DC, Brailsford SC, Kipps CM, Roderick PJ, Walsh B. Hybrid simulation modelling for dementia care services planning. J Oper Res Soc 2021;72:2147-59.
- Walsh B, Fogg C, Harris S, Roderick P, de Lusignan S, England T, et al. Frailty transitions and prevalence in an ageing population: longitudinal analysis of primary care data from an open cohort of adults aged 50 and over in England, 2006–2017. Age Ageing 2023;52.
- Fogg C, England T, Zhu S, Jones J, de Lusignan S, Fraser S, et al. Primary and secondary care service use and costs associated with frailty in an ageing population: longitudinal analysis of an English primary care cohort of adults aged 50 and over, 2006–2017. Age Ageing 2024;53. https://doi.org/10.1093/ageing/afae010.
- Royal College of General Practitioners . Annual Report 2018 19 n.d. www.rcgp.org.uk/getmedia/14b117d3-7108-46da-93bd-c5973ee5ba09/RCGP-annual-report-and-accounts-2018-2019.pdf (accessed 21 May 2024).
- Correa A, Hinton W, McGovern A, van Vlymen J, Yonova I, Jones S, et al. Royal College of General Practitioners Research and Surveillance Centre (RCGP RSC) sentinel network: a cohort profile. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011092.
- Hinton W, McGovern A, Coyle R, Han TS, Sharma P, Correa A, et al. Incidence and prevalence of cardiovascular disease in English primary care: a cross-sectional and follow-up study of the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC). BMJ Open 2018;8.
- Joy M, Hobbs FDR, McGagh D, Akinyemi O, de Lusignan S. Excess mortality from COVID-19 in an English sentinel network population. Lancet Infect Dis 2021;21. https://doi.org/10.1016/S1473-3099(20)30632-0.
- Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008;8.
- Lansbury LN, Roberts HC, Clift E, Herklots A, Robinson N, Sayer AA. Use of the electronic Frailty Index to identify vulnerable patients: a pilot study in primary care. Br J Gen Pract 2017;67:e751-6.
- Healthcare Improvement Scotland . Electronic Frailty Index: Briefing and How to Guide n.d. https://ihub.scot/media/5948/e-frailty-index-brief-guide.pdf (accessed 14 February 2024).
- Tippu Z, Correa A, Liyanage H, Van Vlymen J, Burleigh D, McGovern A, et al. Ethnicity recording in primary care computerised medical record systems: an ontological approach. BMJ Health Care Inform 2016;23.
- Department for Communities and Local Government . The English Index of Multiple Deprivation (IMD) 2015. www.gov.uk/government/collections/english-indices-of-deprivation (accessed 14 February 2024).
- Office for National Statistics . 2011 Rural Urban Classification n.d. www.ons.gov.uk/methodology/geography/geographicalproducts/ruralurbanclassifications/2011ruralurbanclassification (accessed 14 February 2024).
- NHS Digital . General and Personal Medical Services, Practice Level Dataset 2013. https://digital.nhs.uk/data-and-information/publications/statistical/general-and-personal-medical-services/2003-2013-as-at-30-september (accessed 14 February 2024).
- Maguire A, Blak BT, Thompson M. The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf 2009;18:76-83.
- Joint Formulary Committee . British National Formulary 2020.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017. Canterbury: Personal Social Services Research Unit, University of Kent; 2017.
- HM Government . File 3: Supplementary Indices – Income Deprivation Affecting Children Index and Income Deprivation Affected Older People Index 2015.
- Office for National Statistics . Output Area to LSOA to MSOA to Local Authority District (December 2017) Lookup With Area Classifications in Great Britain 2017.
- NHS Digital . General Practice Workforce 2006. https://digital.nhs.uk/data-and-information/publications/statistical/general-and-personal-medical-services (accessed 14 February 2023).
- Cook RJ, Lawless JF. Multistate Models for the Analysis of Life History Data. London: Routledge; 2018.
- Geskus RB. Data Analysis with Competing Risks and Intermediate States. London: Routledge; 2016.
- Jackson C. Multistate Modelling with R: The msm Package. Cambridge: MRC Biostatistics Unit; 2016.
- Kotiadis K, Tako AA. A Tutorial on Involving Stakeholders in Facilitated Simulation Studies 2021. https://kar.kent.ac.uk/91450/#:~:text=This%20tutorial%20introduces%20the%20PartiSim,(clients)%20in%20simulation%20projects (accessed 5 August 2024).
- Tako AA, Kotiadis K. Partisim: a multi-methodology framework to support facilitated simulation modelling in healthcare. Eur J Oper Res 2015;244:555-64.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Res Involv Engagem 2017;3.
- O’Haire C, McPheeters M, Nakamoto E, LaBrant L, Most C, Lee K, et al. Rockville, MD: Agency for Healthcare Research & Quality; 2011.
- Brailsford SC, Lattimer VA, Tarnaras P, Turnbull JC. Emergency and on-demand health care: modelling a large complex system. J Oper Res Soc 2004;55:34-42.
- Northridge ME, Metcalf SS. Enhancing implementation science by applying best principles of systems science. Health Res Policy Syst 2016;14.
- Darabi N, Hosseinichimeh N. System dynamics modeling in health and medicine: a systematic literature review. Syst Dyn Rev 2020;36:29-73.
- McLucas AC, Ryan MJ, Chan KM. Camberra; 2012.
- Sterman JD. Business Dynamics: Systems Thinking and Modelling for a Complex World. New York, NY: McGraw-Hill; 2000.
- Lane D. You Just Don’t Understand Me: Modes of Failure and Success in the Discourse between System Dynamics and Discrete Event Simulation (Working Paper LSEOR 00-34). London: LSE OR Department; 2000.
- Ford DV, Jones KH, Verplancke JP, Lyons RA, John G, Brown G, et al. The SAIL Databank: building a national architecture for e-health research and evaluation. BMC Health Serv Res 2009;9.
- Jones KH, Ford DV, Thompson S, Lyons RA. A profile of the SAIL Databank on the UK Secure Research Platform. Int J Popul Data Sci 2019;4.
- Lyons R, Jones KH, John G, Brooks CJ, Verplancke JP, Ford DV, et al. The SAIL Databank: linking multiple health and social care datasets. BMC Med Inform Decis Mak 2009;9.
- Sail Databank . Secure Anonymised Information Linkage (SAIL) Databank 2021. https://saildatabank.com/ (accessed 14 February 2024).
- England T, Walsh B, Brailsford S, Fogg C, de Lusignan S, Fraser SDS, et al. Health Syst. n.d.
- Stats Wales . Welsh Index of Multiple Deprivation 2019. https://statswales.gov.wales/Catalogue/Community-Safety-and-Social-Inclusion/Welsh-Index-of-Multiple-Deprivation (accessed 14 February 2024).
- Office for National Statistics . Population Estimates for the UK, England, Wales, Scotland and Northern Ireland: Mid-2021 2022. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/latest#population-change-for-uk-countries (accessed 2 March 2023).
- Office for National Statistics . Population Estimates for the UK, England and Wales, Scotland and Northern Ireland: Mid-2020 2021. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2020#age-structure-of-the-uk-population (accessed 7 February 2023).
- Office for National Statistics . Dataset Zipped Population Projections Data Files, England 2022. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationprojections/datasets/z3zippedpopulationprojectionsdatafilesengland (accessed 26 January 2023).
- Burch P, Doran T, Kontopantelis E. Regional variation and predictors of over-registration in English primary care in 2014: a spatial analysis. J Epidemiol Community Health 2018;72:532-8.
- Borda MG, Pérez-Zepeda MU, Samper-Ternent R, Gómez RC, Avila-Funes JA, Cano-Gutierrez CA. The influence of lifestyle behaviors on the incidence of frailty. J Frailty Aging 2020;9:144-9.
- Maroto-Rodriguez J, Delgado-Velandia M, Ortolá R, García-Esquinas E, Martinez-Gomez D, Struijk EA, . A Mediterranean lifestyle and frailty incidence in older adults: the seniors-ENRICA-1 cohort. J Gerontol A Biol Sci Med Sci 2022;77:1845-52.
- Osuka Y, Kojima N, Yoshida Y, Kim M, Won CW, Suzuki T, et al. Exercise and/or dietary varieties and incidence of frailty in community-dwelling older women: a 2-year cohort study. J Nutr Health Aging 2019;23:425-30.
- Oliveira JS, Pinheiro MB, Fairhall N, Walsh S, Chesterfield Franks T, Kwok W, et al. Evidence on physical activity and the prevention of frailty and sarcopenia among older people: a systematic review to inform the World Health Organization physical activity guidelines. J Phys Act Health 2020;17:1247-58.
- Gené Huguet L, Navarro González M, Kostov B, Ortega Carmona M, Colungo Francia C, Carpallo Nieto M, et al. Pre frail 80: multifactorial intervention to prevent progression of pre-frailty to frailty in the elderly. J Nutr Health Aging 2018;22:1266-74.
- Liu X, Ng DHM, Seah JWT, Munro YL, Wee SL. Update on interventions to prevent or reduce frailty in community-dwelling older adults: a scoping review and community translation. Curr Geriatr Rep 2019;8:72-86.
- Puts MTE, Toubasi S, Andrew MK, Ashe MC, Ploeg J, Atkinson E, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age Ageing 2017;46:383-92.
- Walsh B. Unplanned admissions and readmission in older people: a review of recent evidence on identifying and managing high-risk individuals. Rev Clin Gerontol 2014;24:228-37.
- Wallace E, Smith SM, Fahey T, Roland M. Reducing emergency admissions through community based interventions. BMJ 2016;352.
- Wilson A, Baker R, Bankart J, Banerjee J, Bhamra R, Conroy S, et al. Understanding variation in unplanned admissions of people aged 85 and over: a systems-based approach. BMJ Open 2019;9.
- Busby J, Purdy S, Hollingworth W. How do population, general practice and hospital factors influence ambulatory care sensitive admissions: a cross sectional study. BMC Fam Pract 2017;18.
- Abel J, Kingston H, Scally A, Hartnoll J, Hannam G, Thomson-Moore A, et al. Reducing emergency hospital admissions: a population health complex intervention of an enhanced model of primary care and compassionate communities. Br J Gen Pract 2018;68:e803-e81.
- Millares-Martin P. Large retrospective analysis on frailty assessment in primary care: electronic Frailty Index versus frailty coding. BMJ Health Care Inform 2019;26.
- Devereux N, Ellis G, Dobie L, Baughan P, Monaghan T. Testing a proactive approach to frailty identification: the electronic frailty index. BMJ Open Qual 2019;8.
- HM Government . Personalised Health and Care 2020: Using Data and Technology to Transform Outcomes for Patients and Citizens. A Framework for Action 2014.
- HM Government . Ethnicity Facts and Figures 2022. www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/national-and-regional-populations/population-of-england-and-wales/latest (accessed 14 February 2024).
- Department of Health . No Patient Left Behind: How Can We Ensure World Class Primary Care for Black and Minority Ethnic People? 2008.
- The King’s Fund . The UK Private Health Market 2014.
- Scheibl F, Farquhar M, Buck J, Barclay S, Brayne C, Fleming J. When frail older people relocate in very old age, who makes the decision?. Innov Aging 2019;3.
- Sinclair DR, Maharani A, Chandola T, Bower P, Hanratty B, Nazroo J, et al. Frailty among older adults and its distribution in England. J Frailty Aging 2022;11:163-8.
- Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: comparing the frailty index and phenotype. Arch Gerontol Geriatr 2015;60:464-70.
- Uchmanowicz I, Jankowska-Polanska B, Wleklik M, Lisiak M, Gobbens R. Frailty syndrome: nursing interventions. SAGE Open Nurs 2018;4.
- Farrell S, Stubbings G, Rockwood K, Mitnitski A, Rutenberg A. The potential for complex computational models of aging. Mech Ageing Dev 2021;193.
- Drennan V, Walters K, Avgerinou C, Gardner B, Goodman C, Frost R, et al. Moving upstream in health promoting policies for older people with early frailty in England? A policy analysis. J Health Serv Res Policy 2018;23:168-75.
- Stow D, Hanratty B, Matthews FE. The relationship between deprivation and frailty trajectories over 1 year and at the end of life: a case-control study. J Public Health (Oxf) 2022;44:844-50.
- Espinoza SE, Jung I, Hazuda H. Frailty transitions in the San Antonio Longitudinal Study of Aging. J Am Geriatr Soc 2012;60:652-60.
- Reeves D, Pye S, Ashcroft DM, Clegg A, Kontopantelis E, Blakeman T, et al. The challenge of ageing populations and patient frailty: can primary care adapt?. BMJ 2018;362. https://doi.org/10.1136/bmj.k3349.
- Ho LYW, Cheung DSK, Kwan RYC, Wong ASW, Lai CKY. Factors associated with frailty transition at different follow-up intervals: a scoping review. Geriatr Nurs 2021;42:555-65.
- Chen F, Mair CA, Bao L, Yang YC. Race/ethnic differentials in the health consequences of caring for grandchildren for grandparents. J Gerontol B Psychol Sci Soc Sci 2015;70:793-80.
- Peek MK, Howrey BT, Ternent RS, Ray LA, Ottenbacher KJ. Social support, stressors, and frailty among older Mexican American adults. J Gerontol B Psychol Sci Soc Sci 2012;67:755-64.
- Marshall A, Nazroo J, Tampubolon G, Vanhoutte B. Cohort differences in the levels and trajectories of frailty among older people in England. J Epidemiol Community Health 2015;69:316-21.
- Woo J, Goggins W, Sham A, Ho SC. Social determinants of frailty. Gerontology 2005;51:402-8.
- Ye B, Chen H, Huang L, Ruan Y, Qi S, Guo Y, et al. Changes in frailty among community-dwelling Chinese older adults and its predictors: evidence from a two-year longitudinal study. BMC Geriatr 2020;20.
- Stolz E, Mayerl H, Waxenegger A, Freidl W. Explaining the impact of poverty on old-age frailty in Europe: material, psychosocial and behavioural factors. Eur J Public Health 2017;27:1003-9.
- Stolz E, Mayerl H, Waxenegger A, Rásky E, Freidl W. Impact of socioeconomic position on frailty trajectories in 10 European countries: evidence from the Survey of Health, Ageing and Retirement in Europe (2004–2013). J Epidemiol Community Health 2017;71:73-80.
- Mendonça N, Kingston A, Yadegarfar M, Hanson H, Duncan R, Jagger C, et al. Transitions between frailty states in the very old: the influence of socioeconomic status and multi-morbidity in the Newcastle 85+ cohort study. Age Ageing 2020;49:974-81.
- Niederstrasser NG, Rogers NT, Bandelow S. Determinants of frailty development and progression using a multidimensional frailty index: evidence from the English Longitudinal Study of Ageing. PLOS ONE 2019;14.
- Baranyi G, Welstead M, Corley J, Deary IJ, Muniz-Terrera G, Redmond P, et al. Association of life-course neighborhood deprivation with frailty and frailty progression from ages 70 to 82 years in the Lothian birth cohort 1936. Am J Epidemiol 2022;191:1856-66.
- Finch D, Tinson A. The Continuing Impact of COVID-19 on Health and Inequalities. London: The Health Foundation; 2022.
- Akinyelure OP, Colvin CL, Sterling MR, Safford MM, Muntner P, Colantonio LD, et al. Frailty, gaps in care coordination, and preventable adverse events. BMC Geriatr 2022;22.
- Silva SLA, Macinko J, Lima-Costa MF, Torres JL. Effective primary care attenuates the association between frailty and hospital admission in old age: the ELSI-Brazil. Fam Pract 2023;40:47-54.
- National Institute for Health and Care Excellence . Dementia, Disability and Frailty in Later Life – Mid-Life Approaches to Delay or Prevent Onset 2015.
- Woolford SJ, Sohan O, Dennison EM, Cooper C, Patel HP. Approaches to the diagnosis and prevention of frailty. Aging Clin Exp Res 2020;32:1629-37.
- Abel J, Kingston H, Scally A, Hartnoll J, Hannam G, Thomson-Moore A, et al. Reducing emergency hospital admissions: a population health complex intervention of an enhanced model of primary care and compassionate communities. Br J Gen Pract 2018;68:e803-10. https://doi.org/10.3399/bjgp18X699437.
- Gov.UK . Women’s Health Strategy for England n.d. www.gov.uk/government/publications/womens-health-strategy-for-england/womens-health-strategy-for-england#healthy-ageing-and-long-term-conditions (accessed 4 December 2023).
Appendix 1 Methods and Workstream 1 analyses
FIGURE 16.
Content of, and information flows between, study workstreams.
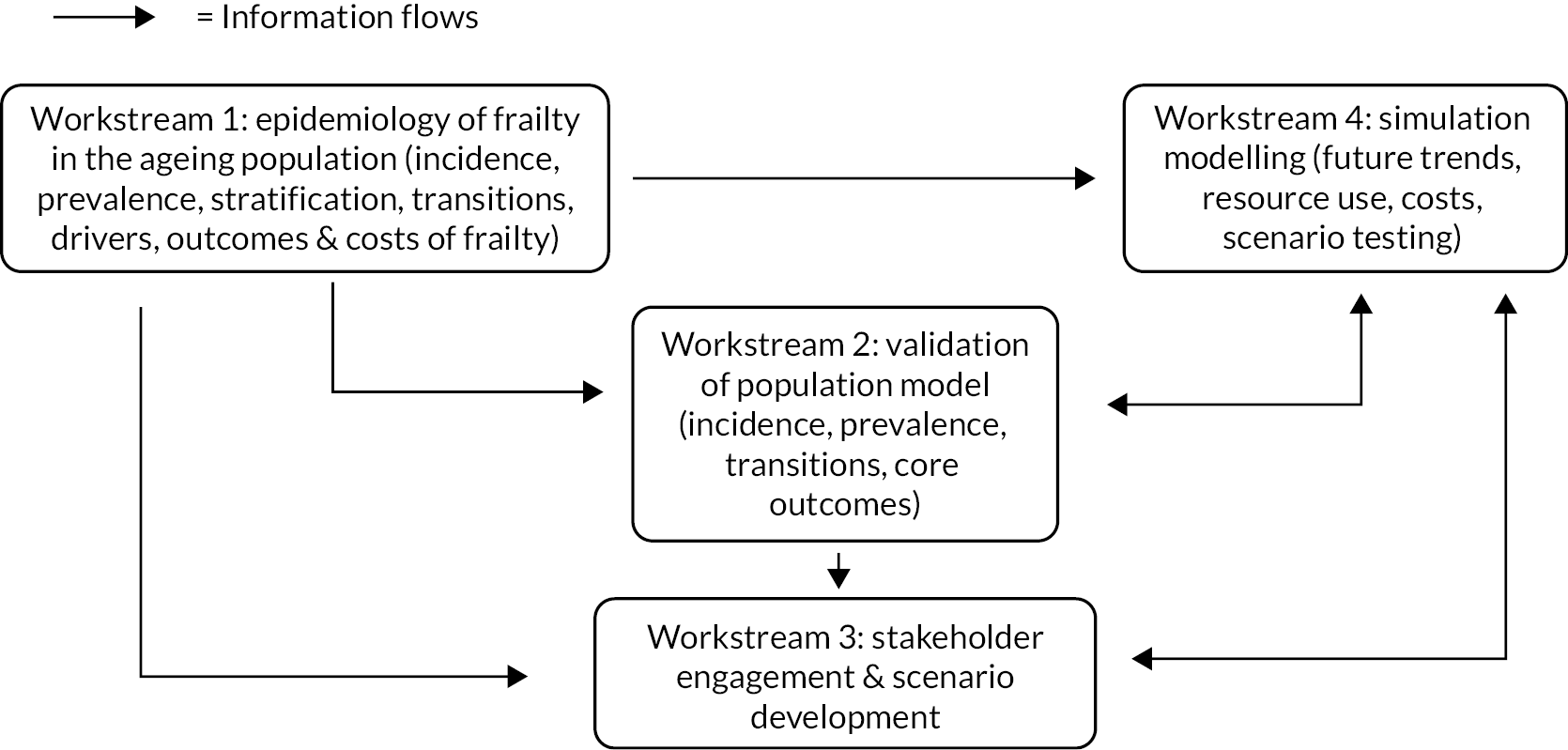
| Data set | Data items | Study phase | Access |
|---|---|---|---|
| RCGP RSC 1.8 m patients 1.1 million patients aged 50 and over 230 practices National – England Over 50 |
Age Gender IMD LTC diagnoses Ethnicity Smoking BMI Prescriptions Urban/rural Practice size eFI Mortality |
Workstream 1 | Host: University of Oxford (previously University of Surrey) Analysis: on site/secure remote access via University of Surrey and University of Oxford servers Approvals: Unlinked primary care: UoS Ethics Committee and RCGP RSC IG approvals |
| HES | ED attendances Hospital Admissions |
Workstream 1 | Linkage: via NHS DigitalDARS and RCGP RSC IG approvals and UoS Ethics Committee |
| ONS | Mortality | Workstream 1 | Linkage: via NHS Digital DARS and RCGP RSC IG approvals and UoS Ethics Committee |
| Resource | Cost | Reference |
|---|---|---|
| Primary care a | ||
| GP face-to-face visit | £38 | PSSRU 2017 |
| GP home visit estimated at 12.8 minutes including travel time | £74 | PSSRU 2015 |
| Telephone triage | £14.60 | PSSRU 2017 |
| E-consultation | £37.70 | PSSRU 2018 |
| Cost per medication prescribed | £8.20 | NHS Prescription Chargeb 2015–6 |
| Secondary care | ||
| Outpatient appointment | £138 | PSSRU 2017 |
| Accident and Emergency department visit | £148.36 | National reference costs, 2016–7 |
| Hospital admission | < 24 hours £322 > 24 hours £384 per day |
National reference costs, 2016–7 |
| Critical care admission | £1082 | National reference costs, 2016–7 |
FIGURE 17.
Cohort identification. Reproduced from Fogg et al. 14 This is an Open Access article distributed in accordance with the terms of the creative commons attribution (CC-BY 4.0) licence which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. This figure includes minor additions, and formatting changes to the original.
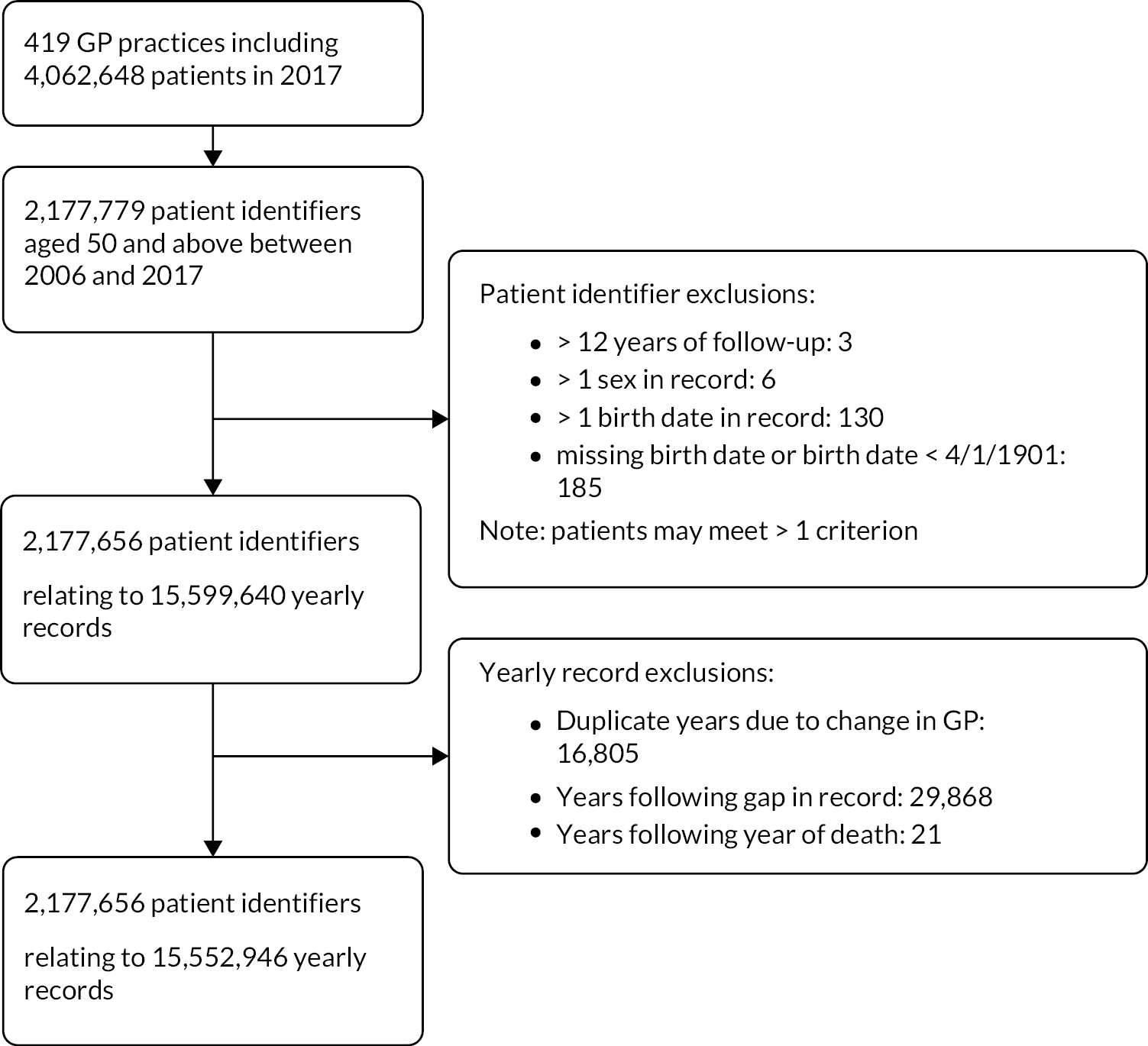
| Practice characteristic | n (%) |
|---|---|
| Geographic region (n, %) | |
| London | 64 (15.3%) |
| Midlands and East | 90 (21.5%) |
| North | 128 (30.6%) |
| South | 137 (32.7%) |
| Rural/urban classification | |
| Urban: major conurbation | 150 (35.8%) |
| Urban: minor conurbation | 15 (3.6%) |
| Urban: city and town | 163 (38.9%) |
| Rural: town and fringe | 69 (16.5%) |
| Rural: village and dispersed | 22 (5.3%) |
| Practice size (patients) | |
| Median | 6858 |
| Upper:lower quartile | 4110:9819 |
| Practice size (staff – FTEs) a | |
| GPs (mean, SD) | 6 (3) |
| Nursesb (mean, SD) | 2 (2) |
| Total staffb (mean, SD) | 14 (8) |
| Consultations (median, upper:lower quartile) | |
| Face to face | 42,661 (22,426:72,362) |
| Clinical administration | 20,474 (5811:73,050) |
| E-consultations (data from 2017) | 4 (0:43) |
| Telephone | 1713 (261:4383) |
| Home visits | 669 (50:1992) |
| Practice IMD quintile | |
| Most deprived | 93 (22.2%) |
| 2nd quintile | 84 (20.1%) |
| 3rd quintile | 83 (19.8%) |
| 4th quintile | 85 (20.3%) |
| Least deprived | 74 (17.7%) |
| Calendar year | Age groupa | |||||||
|---|---|---|---|---|---|---|---|---|
| 50–64 | 65–74 | 75–84 | 85 + | |||||
| Entry | Exit | Entry | Exit | Entry | Exit | Entry | Exit | |
| 2006b | 579,593 | 17,176 | 270,326 | 9538 | 187,870 | 12,951 | 69,692 | 12,071 |
| 2007 | 65,363 | 18,432 | 9550 | 9741 | 6226 | 12,755 | 3875 | 12,484 |
| 2008 | 66,678 | 17,474 | 8749 | 9158 | 5977 | 12,567 | 3864 | 13,255 |
| 2009 | 65,529 | 16,868 | 7629 | 9224 | 5393 | 12,495 | 3951 | 13,766 |
| 2010 | 66,949 | 18,866 | 8237 | 10,285 | 5410 | 12,644 | 4003 | 13,875 |
| 2011 | 71,990 | 20,515 | 8792 | 10,644 | 5924 | 12,619 | 4192 | 14,326 |
| 2012 | 74,235 | 21,278 | 9413 | 11,248 | 5958 | 13,211 | 4330 | 15,562 |
| 2013 | 81,143 | 24,506 | 11,940 | 12,974 | 6877 | 14,765 | 4788 | 17,518 |
| 2014 | 81,922 | 27,413 | 11,598 | 14,034 | 6887 | 15,200 | 5039 | 17,475 |
| 2015 | 88,893 | 27,446 | 14,398 | 14,589 | 8322 | 15,637 | 5586 | 18,953 |
| 2016 | 86,650 | 29,270 | 12,638 | 15,587 | 7499 | 15,966 | 5425 | 19,344 |
| 2017 | 84,631 | 31,297 | 12,204 | 17,196 | 6782 | 16,856 | 4736 | 20,477 |
| Total | 1,413,576 | 270,541 | 385,474 | 144,218 | 259,125 | 167,666 | 119,481 | 189,106 |
| Calendar year | Frailty categorya | |||||||
|---|---|---|---|---|---|---|---|---|
| Fit | Mild | Moderate | Severe | |||||
| Entry | Exit | Entry | Exit | Entry | Exit | Entry | Exit | |
| 2006b | 812,788 | 28,259 | 226,987 | 14,630 | 55,885 | 6604 | 11,821 | 2243 |
| 2007 | 73,340 | 27,796 | 9842 | 14,898 | 1639 | 7701 | 193 | 3017 |
| 2008 | 73,357 | 25,289 | 9985 | 14,738 | 1707 | 8641 | 219 | 3786 |
| 2009 | 70,460 | 24,460 | 9875 | 14,604 | 1906 | 9068 | 261 | 4221 |
| 2010 | 71,270 | 25,971 | 10,892 | 15,183 | 2152 | 9647 | 285 | 4869 |
| 2011 | 76,277 | 27,151 | 11,880 | 15,404 | 2388 | 10,319 | 353 | 5230 |
| 2012 | 78,432 | 27,492 | 12,471 | 16,298 | 2580 | 11,268 | 453 | 6241 |
| 2013 | 86,412 | 31,918 | 14,585 | 18,049 | 3164 | 12,533 | 587 | 7263 |
| 2014 | 87,092 | 34,722 | 14,701 | 18,614 | 3104 | 12,873 | 549 | 7913 |
| 2015 | 95,372 | 33,935 | 17,053 | 19,488 | 3961 | 14,011 | 813 | 9191 |
| 2016 | 91,287 | 36,417 | 16,207 | 19,645 | 3823 | 14,226 | 895 | 9879 |
| 2017 | 87,511 | 37,542 | 15,455 | 21,268 | 4005 | 15,374 | 1382 | 11,642 |
| Total | 1,703,598 | 360,952 | 369,933 | 202,819 | 86,314 | 132,265 | 17,811 | 75,495 |
| Number of participants | Mean years of follow-up (St.D) | |
|---|---|---|
| Age group | ||
| 50–64 | 1,413,576 | 7.2 (4.1) |
| 65–74 | 385,474 | 8.2 (4.1) |
| 75–84 | 259,125 | 6.7 (4.0) |
| 85 + | 119,481 | 4 (3.0) |
| Frailty category | ||
| Fit | 1,703,598 | 7.3 (4.1) |
| Mild | 369,933 | 6.8 (4.1) |
| Moderate | 86,214 | 5.4 (3.9) |
| Severe | 17,811 | 4.1 (3.3) |
| Characteristic | Category | n (%)a | n, fit at cohort entry | Incidence rate per 1000 person-years at risk (95% CI) |
|---|---|---|---|---|
| Age at cohort entry | 50–64 | 1,412,823 (65.1) | 1,272,762 | 31.8 (31.7 to 32.0) |
| 65–74 | 384,640 (17.1) | 272,232 | 85.2 (84.7 to 85.6) | |
| 75–84 | 257,276 (11.9) | 119,597 | 136.9 (135.9 to 137.9) | |
| ≥ 85 | 116,758 (5.4) | 36,133 | 158.5 (156.2 to 160.8) | |
| Sex | Male | 1,040,906 (48.0) | 855,015 | 42.2 (42.0 to 42.4) |
| Female | 1,130,591 (52.1) | 845,709 | 52.1 (51.9 to 52.3) | |
| Ethnicityb | Asian | 73,932 (3.8) | 56,482 | 57.3 (56.4 to 58.2) |
| Black | 40,122 (2.1) | 32,761 | 49.1 (48.0 to 50.3) | |
| Mixed/other | 24,235 (1.3) | 20,292 | 42.8 (41.6 to 44.1) | |
| White | 1,807,038 (92.9) | 1,392,050 | 50.9 (50.7 to 51.0) | |
| Location | Urban | 1,684,020 (77.6) | 1,311,431 | 47.8 (47.6 to 47.9) |
| Rural | 487,477 (22.5) | 389,293 | 45.0 (44.8 to 45.3) | |
| Residential carec | Yes | 16,647 (0.77) | 3317 | 307.8 (298.4 to 317.5) |
| No | 2,154,850 (99.2) | 1,697,407 | 46.8 (46.6 to 46.9) | |
| IMD | Most deprived | 290,760 (13.4) | 212,867 | 57.9 (57.4 to 58.3) |
| 2nd quintile | 341,323 (15.7) | 261,520 | 51.1 (50.8 to 51.5) | |
| 3rd quintile | 439,069 (20.2) | 343,472 | 47.6 (47.3 to 47.9) | |
| 4th quintile | 524,849 (24.2) | 417,448 | 44.8 (44.5 to 45.0) | |
| Least deprived | 575,496 (26.5) | 465,417 | 42.7 (42.4 to 42.9) | |
| IDAOPI | Most deprived | 298,519 (13.8) | 220,689 | 57.5 (57.1 to 58.0) |
| 2nd quintile | 337,977 (15.6) | 254,043 | 52.3 (51.9 to 52.6) | |
| 3rd quintile | 427,344 (19.7) | 331,038 | 48.6 (48.3 to 48.9) | |
| 4th quintile | 520,409 (24.0) | 413,922 | 44.9 (44.7 to 45.2) | |
| Least deprived | 587,248 (27.0) | 481,032 | 41.6 (41.4 to 41.9) | |
| Smoking status at cohort entryd | Non-smoker | 821,284 (40.6) | 663,397 | 43.2 (43.0 to 43.4) |
| Ex-smoker | 741,531 (36.7) | 529,389 | 57.5 (57.3 to 57.8) | |
| Active smoker | 459,084 (22.7) | 376,941 | 47.4 (47.1 to 47.6) | |
| BMI at cohort entrye | Underweight | 27,242 (1.9) | 15,928 | 79.7 (77.8 to 81.7) |
| Normal | 460,420 (31.4) | 351,406 | 52.0 (51.7 to 52.3) | |
| Overweight | 560,512 (38.3) | 429,637 | 56.0 (55.7 to 56.3) | |
| Obese | 417,190 (28.5) | 295,071 | 68.2 (67.8 to 68.5) |
| Calendar year | Age group | |||
|---|---|---|---|---|
| 50–64 | 65–74 | 75–84 | ≥ 85 | |
| 2007 | 28.6 (28.1 to 29.0) | 84.8 (83.5 to 86.1) | 165.3 (162.7 to 167.9) | 217.6 (211.9 to 223.5) |
| 2008 | 27.1 (26.7 to 27.5) | 76.4 (75.2 to 77.6) | 148.8 (146.3 to 151.4) | 201.9 (196.0 to 207.9) |
| 2009 | 27.2 (26.8 to 27.6) | 74.0 (72.8 to 75.2) | 138.0 (135.4 to 140.5) | 194.5 (188.5 to 200.6) |
| 2010 | 27.5 (27.1 to 28.0) | 74.1 (72.9 to 75.3) | 138.0 (135.0 to 140.2) | 191.0 (184.8 to 197.3) |
| 2011 | 27.1 (26.7 to 27.5) | 70.4 (69.3 to 71.6) | 131.9 (129.4 to 134.5) | 183.4 (177.2 to 189.8) |
| 2012 | 26.8 (26.4 to 27.3) | 67.1 (66.0 to 68.3) | 126.0 (123.5 to 128.6) | 174.8 (168.7 to 181.2) |
| 2013 | 27.7 (27.3 to 28.2) | 67.3 (66.2 to 68.4) | 126.7 (124.2 to 129.3) | 183.1 (176.7 to 189.7) |
| 2014 | 28.4 (28.0 to 28.8) | 67.6 (64.6 to 66.7) | 129.9 (127.3 to 132.5) | 195.7 (189.0 to 202.7) |
| 2015 | 27.6 (27.2 to 28.0) | 65.6 (64.6 to 66.7) | 127.3 (124.8 to 129.9) | 193.4 (186.6 to 200.5) |
| 2016 | 27.3 (26.9 to 27.7) | 65.3 (64.3 to 66.4) | 130.6 (128.0 to 133.3) | 195.5 (188.6 to 202.7) |
| 2017 | 28.5 (28.1 to 29.0) | 65.8 (64.7 to 66.8) | 128.3 (125.8 to 131.0) | 199.0 (191.9 to 206.5) |
| Calendar year | Age group 50–64 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fit | (n, %) | Mild | (n, %) | Moderate | (n, %) | Severe | (n, %) | Total | |
| 2006 | 516,468 | 89.2 | 56,806 | 9.8 | 5397 | 0.9 | 528 | 0.1 | 579,199 |
| 2007 | 526,828 | 87.9 | 64,958 | 10.8 | 6833 | 1.1 | 710 | 0.1 | 599,329 |
| 2008 | 534,114 | 86.8 | 72,112 | 11.7 | 8077 | 1.3 | 891 | 0.1 | 615,194 |
| 2009 | 539,746 | 85.8 | 78,808 | 12.5 | 9366 | 1.5 | 1057 | 0.2 | 628,977 |
| 2010 | 543,754 | 84.9 | 85,046 | 13.3 | 10,793 | 1.7 | 1240 | 0.2 | 640,833 |
| 2011 | 553,787 | 84.0 | 91,806 | 13.9 | 12,217 | 1.9 | 1453 | 0.2 | 659,263 |
| 2012 | 557,393 | 83.3 | 96,358 | 14.4 | 13,310 | 2.0 | 1682 | 0.3 | 668,743 |
| 2013 | 564,539 | 82.8 | 101,212 | 14.8 | 14,422 | 2.1 | 1913 | 0.3 | 682,086 |
| 2014 | 572,356 | 82.1 | 106,641 | 15.3 | 15,817 | 2.3 | 2149 | 0.3 | 696,963 |
| 2015 | 585,640 | 81.6 | 112,484 | 15.7 | 17,215 | 2.4 | 2490 | 0.3 | 717,829 |
| 2016 | 597,759 | 81.1 | 118,080 | 16.0 | 18,533 | 2.5 | 2807 | 0.4 | 737,179 |
| 2017 | 605,582 | 80.4 | 124,330 | 16.5 | 20,429 | 2.7 | 3218 | 0.4 | 753,559 |
| Age group 65–74 | |||||||||
| Fit | (n, %) | Mild | (n, %) | Moderate | (n, %) | Severe | (n, %) | Total | |
| 2006 | 187,162 | 69.4 | 69,189 | 25.6 | 11,979 | 4.4 | 1462 | 0.5 | 269,792 |
| 2007 | 182,478 | 66.4 | 75,536 | 27.5 | 14,785 | 5.4 | 2102 | 0.8 | 274,901 |
| Calendar year | Age group 65–74 | ||||||||
| Fit | (n, %) | Mild | (n, %) | Moderate | (n, %) | Severe | (n, %) | Total | |
| 2008 | 181,870 | 64.4 | 80,838 | 28.6 | 17,219 | 6.1 | 2602 | 0.9 | 282,529 |
| 2009 | 183,204 | 62.6 | 86,749 | 29.7 | 19,314 | 6.6 | 3169 | 1.1 | 292,436 |
| 2010 | 187,369 | 61.3 | 93,107 | 30.5 | 21,575 | 7.1 | 3658 | 1.2 | 305,709 |
| 2011 | 188,626 | 60.1 | 97,515 | 31.1 | 23,654 | 7.5 | 4127 | 1.3 | 313,922 |
| 2012 | 197,398 | 59.6 | 103,626 | 31.3 | 25,616 | 7.7 | 4682 | 1.4 | 331,322 |
| 2013 | 208,023 | 59.1 | 110,766 | 31.5 | 28,097 | 8.0 | 5242 | 1.5 | 352,128 |
| 2014 | 213,851 | 58.4 | 116,015 | 31.7 | 30,110 | 8.2 | 5974 | 1.6 | 365,950 |
| 2015 | 219,306 | 57.8 | 121,458 | 32.0 | 32,260 | 8.5 | 6712 | 1.8 | 379,736 |
| 2016 | 223,302 | 57.1 | 125,816 | 32.2 | 34,489 | 8.8 | 7570 | 1.9 | 391,177 |
| 2017 | 224,938 | 56.2 | 129,731 | 32.4 | 37,020 | 9.3 | 8427 | 2.1 | 400,116 |
| Calendar year | Age group 75–84 | ||||||||
| Fit | (n, %) | Mild | (n, %) | Moderate | (n, %) | Severe | (n, %) | Total | |
| 2006 | 86,398 | 46.2 | 71,846 | 38.5 | 23,589 | 12.6 | 4979 | 2.7 | 186,812 |
| 2007 | 79,804 | 42.0 | 75,347 | 39.6 | 28,262 | 14.9 | 6820 | 3.6 | 190,233 |
| 2008 | 75,712 | 38.9 | 78,478 | 40.4 | 31,950 | 16.4 | 8247 | 4.2 | 194,387 |
| 2009 | 72,473 | 36.8 | 80,114 | 40.7 | 34,828 | 17.7 | 9534 | 4.8 | 196,949 |
| 2010 | 69,897 | 34.8 | 82,174 | 40.9 | 38,014 | 18.9 | 10,986 | 5.5 | 201,071 |
| 2011 | 68,526 | 33.3 | 84,539 | 41.0 | 40,672 | 19.7 | 12,261 | 6.0 | 205,998 |
| 2012 | 68,123 | 32.3 | 86,253 | 40.9 | 43,047 | 20.4 | 13,672 | 6.5 | 211,095 |
| 2013 | 68,009 | 31.3 | 88,380 | 40.7 | 45,548 | 21.0 | 15,282 | 7.0 | 217,219 |
| 2014 | 67,691 | 30.5 | 89,981 | 40.5 | 47,724 | 21.5 | 16,904 | 7.6 | 222,300 |
| 2015 | 67,962 | 29.8 | 91,430 | 40.1 | 49,864 | 21.9 | 18,697 | 8.2 | 227,953 |
| 2016 | 67,487 | 29.2 | 91,509 | 39.6 | 51,594 | 22.3 | 20,295 | 8.8 | 230,885 |
| 2017 | 66,815 | 28.7 | 91,321 | 39.2 | 52,705 | 22.6 | 22,030 | 9.5 | 232,871 |
| Calender year | Age group 85 and above | ||||||||
| Fit | (n, %) | Mild | (n, %) | Moderate | (n, %) | Severe | (n, %) | Total | |
| 2006 | 21,356 | 31.3 | 27,977 | 40.9 | 14,354 | 21.0 | 4645 | 6.8 | 68,332 |
| 2007 | 19,596 | 26.8 | 29,372 | 40.2 | 17,621 | 24.1 | 6531 | 8.9 | 73,120 |
| 2008 | 18,239 | 23.7 | 30,401 | 39.6 | 20,027 | 26.1 | 8178 | 10.6 | 76,845 |
| 2009 | 17,121 | 21.3 | 31,323 | 38.9 | 22,428 | 27.9 | 9555 | 11.9 | 80,427 |
| 2010 | 16,042 | 19.1 | 31,647 | 37.7 | 24,926 | 29.7 | 11,378 | 13.5 | 83,993 |
| 2011 | 15,368 | 17.6 | 32,204 | 36.8 | 26,786 | 30.6 | 13,206 | 15.1 | 87,564 |
| 2012 | 14,946 | 16.3 | 32,549 | 35.5 | 28,957 | 31.6 | 15,203 | 16.6 | 91,655 |
| 2013 | 14,387 | 15.2 | 32,900 | 34.8 | 30,369 | 32.1 | 16,838 | 17.8 | 94,494 |
| 2014 | 13,681 | 14.1 | 32,855 | 33.9 | 31,716 | 32.8 | 18,555 | 19.2 | 96,807 |
| Calendar year | Age group 85 and above | ||||||||
| Fit | (n, %) | Mild | (n, %) | Moderate | (n, %) | Severe | (n, %) | Total | |
| 2015 | 13,257 | 13.3 | 32,692 | 32.8 | 33,036 | 33.1 | 20,772 | 20.8 | 99,757 |
| 2016 | 12,964 | 12.7 | 32,206 | 31.5 | 33,987 | 33.3 | 22,989 | 22.5 | 102,146 |
| 2017 | 12,332 | 12.0 | 31,181 | 30.3 | 34,265 | 33.3 | 25,171 | 24.4 | 102,949 |
| Year | Population (n)a | Type of primary care service use | ||||
|---|---|---|---|---|---|---|
| Face-to-face appointments | Home visits | Telephone triage | E-consultations | Number of individual prescriptions for medicines | ||
| 2006 | 1,104,135 | 7,370,637 | 384,918 | 587,971 | 55 | 28,891,568 |
| 2007 | 1,137,583 | 7,745,118 | 396,894 | 674,454 | 470 | 31,139,738 |
| 2008 | 1,168,955 | 8,147,716 | 401,526 | 800,575 | 763 | 33,695,233 |
| 2009 | 1,198,789 | 8,829,294 | 399,355 | 892,740 | 960 | 36,128,665 |
| 2010 | 1,231,606 | 9,026,016 | 392,703 | 944,429 | 1360 | 38,433,223 |
| 2011 | 1,266,747 | 9,301,347 | 371,122 | 1,022,779 | 1667 | 40,600,594 |
| 2012 | 1,302,815 | 9,488,829 | 351,795 | 1,139,451 | 2701 | 42,665,590 |
| 2013 | 1,345,927 | 9,392,535 | 330,637 | 1,329,383 | 3749 | 45,067,424 |
| 2014 | 1,382,020 | 9,342,733 | 330,592 | 1,549,521 | 5261 | 47,226,025 |
| 2015 | 1,425,275 | 9,310,318 | 333,296 | 1,700,580 | 5728 | 49,432,669 |
| 2016 | 1,461,387 | 9,462,405 | 331,005 | 1,844,310 | 10,882 | 50,854,402 |
| 2017 | 1,489,495 | 9,587,055 | 319,661 | 1,967,474 | 18,493 | 52,339,081 |
| Year | Population (n)a | Type of secondary care service use | ||||||
|---|---|---|---|---|---|---|---|---|
| Outpatient appointments | Emergency department attendances | Hospital admissions | Elective | Unplanned | Total days of hospital stay | Critical care admissions | ||
| 2006 | 1,104,135 | 1,808,701 | No data | 429,727 | 276,275 | 144,611 | 1.966,447 | No data |
| 2007 | 1,137,583 | 1,935,668 | 151,906 | 460,831 | 304,478 | 148,319 | 1,926,362 | No data |
| 2008 | 1,168,955 | 2,179,469 | 234,548 | 498,460 | 335,510 | 158,554 | 1,985,763 | 2854 |
| 2009 | 1,198,789 | 2,453,640 | 277,158 | 527,911 | 350,164 | 168,974 | 2,042,322 | 4710 |
| 2010 | 1,231,606 | 2,628,881 | 311,610 | 557,455 | 371,954 | 176,631 | 2,004,159 | 6139 |
| 2011 | 1,266,747 | 2,752,260 | 335,213 | 576,709 | 387,984 | 180,114 | 1,974,950 | 7049 |
| 2012 | 1,302,815 | 2,942,468 | 364,643 | 606,217 | 405,736 | 191,759 | 2,080,816 | 7565 |
| 2013 | 1,345,927 | 3,205,647 | 383,236 | 630,058 | 421,101 | 200,073 | 2,127,694 | 8216 |
| 2014 | 1,382,020 | 3,541,389 | 407,779 | 674,531 | 453,749 | 211,920 | 2,177,885 | 8626 |
| 2015 | 1,425,275 | 3,848,590 | 426,926 | 697,853 | 472,240 | 217,116 | 2,183,777 | 8924 |
| 2016 | 1,461,387 | 4,091,534 | 470,057 | 726,173 | 488,833 | 229,530 | 2,251,645 | 9147 |
| 2017 | 1,489,495 | 4,218,322 | 479,498 | 740,674 | 497,626 | 235,861 | 2,150,553 | 9412 |
| Primary care, mean cost (£) (95% confidence interval) | Secondary care, mean cost (£) (95% confidence interval) | Total care, mean cost (£) (95% confidence interval) | |
|---|---|---|---|
| Frailty category | |||
| Fit | 346.51 (346.25 to 346.76) | 612.46 (610.12 to 614.80] | 957.27 (955.16 to 959.39) |
| Mild | 810.94 (810.05 to 811.83) | 1354.79 (1347.67 to 1361.92) | 2158.95 (2152.16 to 2165.73) |
| Moderate | 1136.34 (1134.14 to 1138.53) | 2092.67 (2073.69 to 2111.64) | 3219.83 (3202.19 to 3237.47) |
| Severe | 1517.69 (1512.56 to 1522.82) | 2943.90 (2897.01 to 2990.79) | 4464.23 (4421.32 to 4507.15) |
Appendix 2 Patient and public engagement: stakeholder events
FIGURE 18.
Draft model structure discussed at SEG 1.
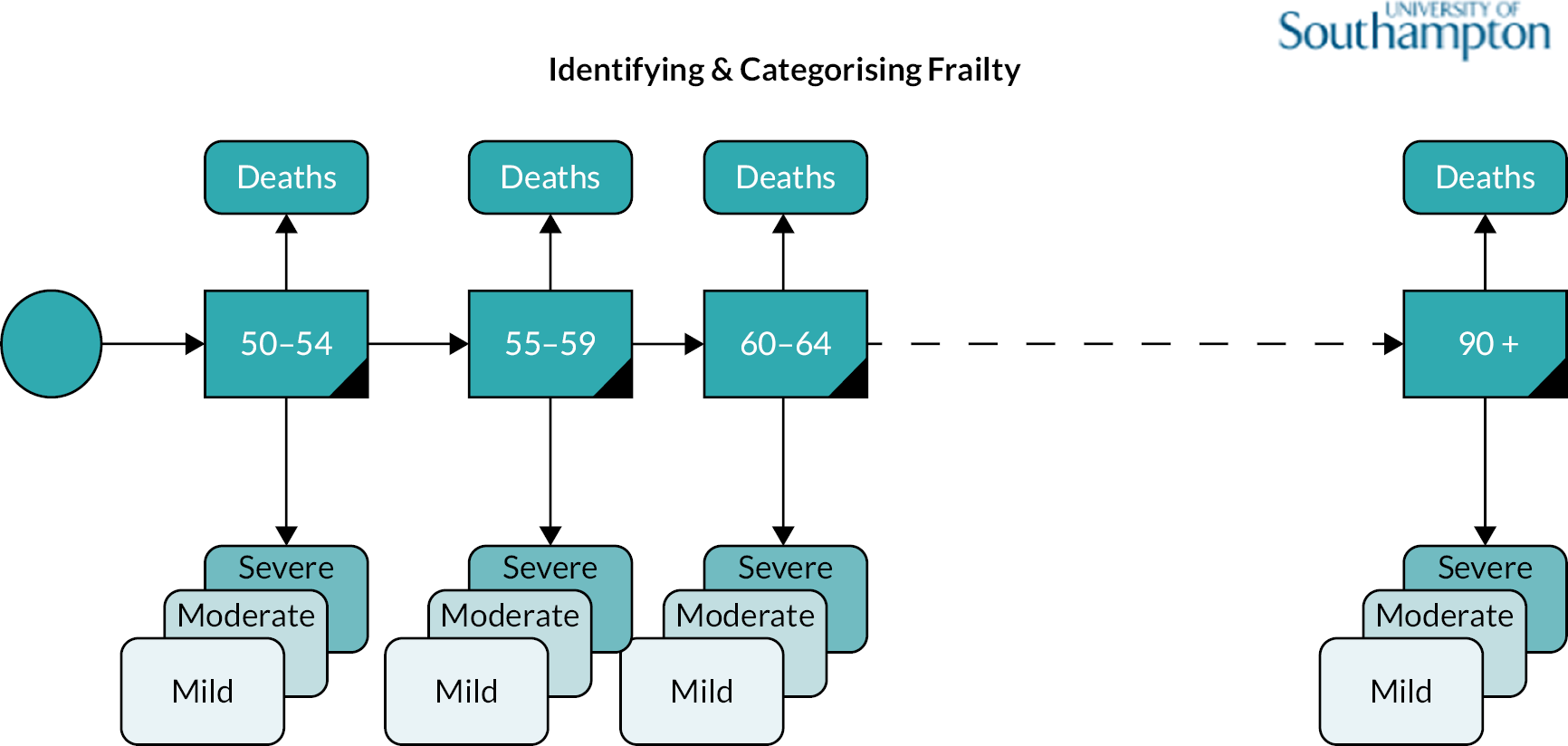
FIGURE 19.
Categorisation of frailty service sectors from SEG 1.
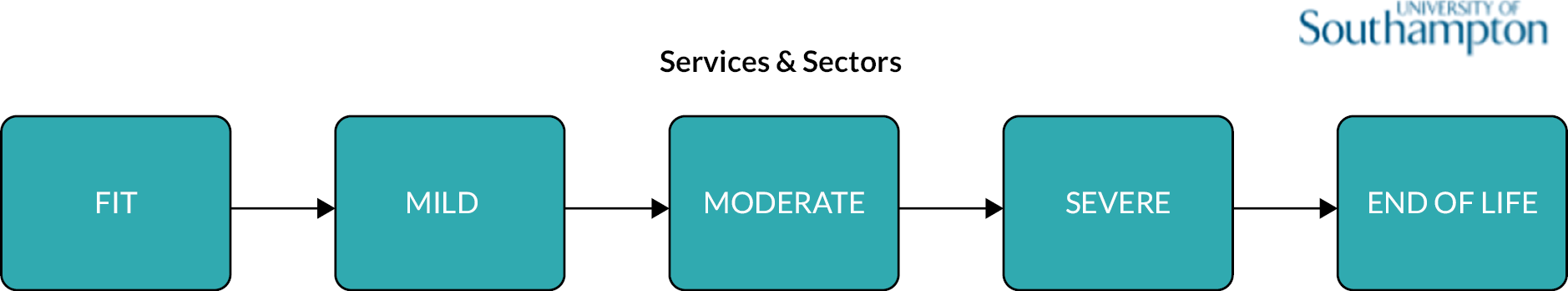
FIGURE 20.
Word cloud from Session 1/PPIE SEG 2.

FIGURE 21.
Word cloud from Session 2/PPIE SEG 2.

FIGURE 22.
SEG Event 2 – Session 1 – patients/carer sesssion summary leaflet.
FIGURE 23.
SEG Event 2 – Session 2 – patients and carer session summary leaflet.
FIGURE 24.
SEG Event 2 – Session 3 – Health and social care professional session summary leaflet.

FIGURE 25.
SEG Event 3 – Session 1 – Health and social care professionals – summary leaflet.
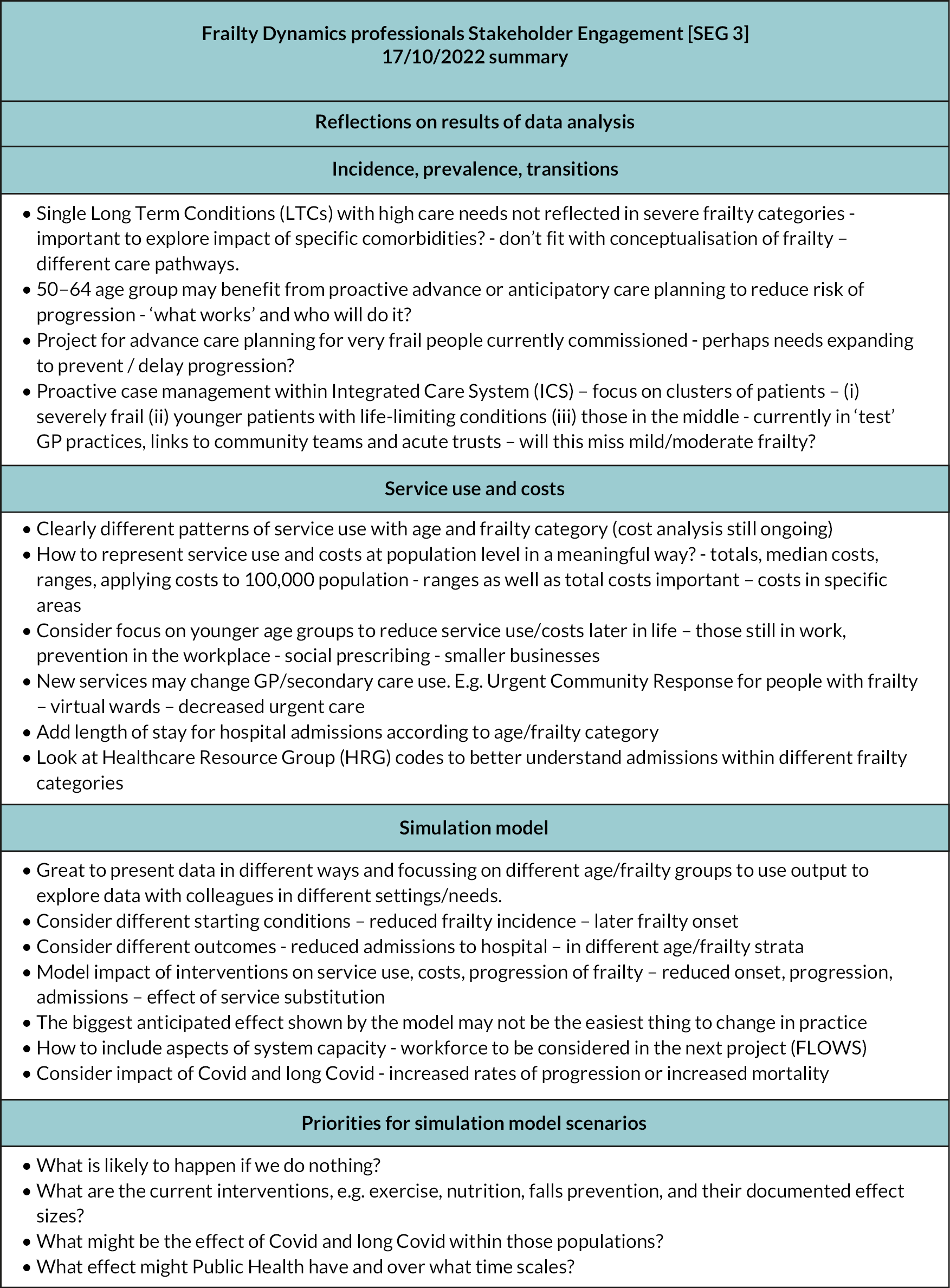
FIGURE 26.
SEG Event 3 – Session 2 – patients and carers – summary leaflet.
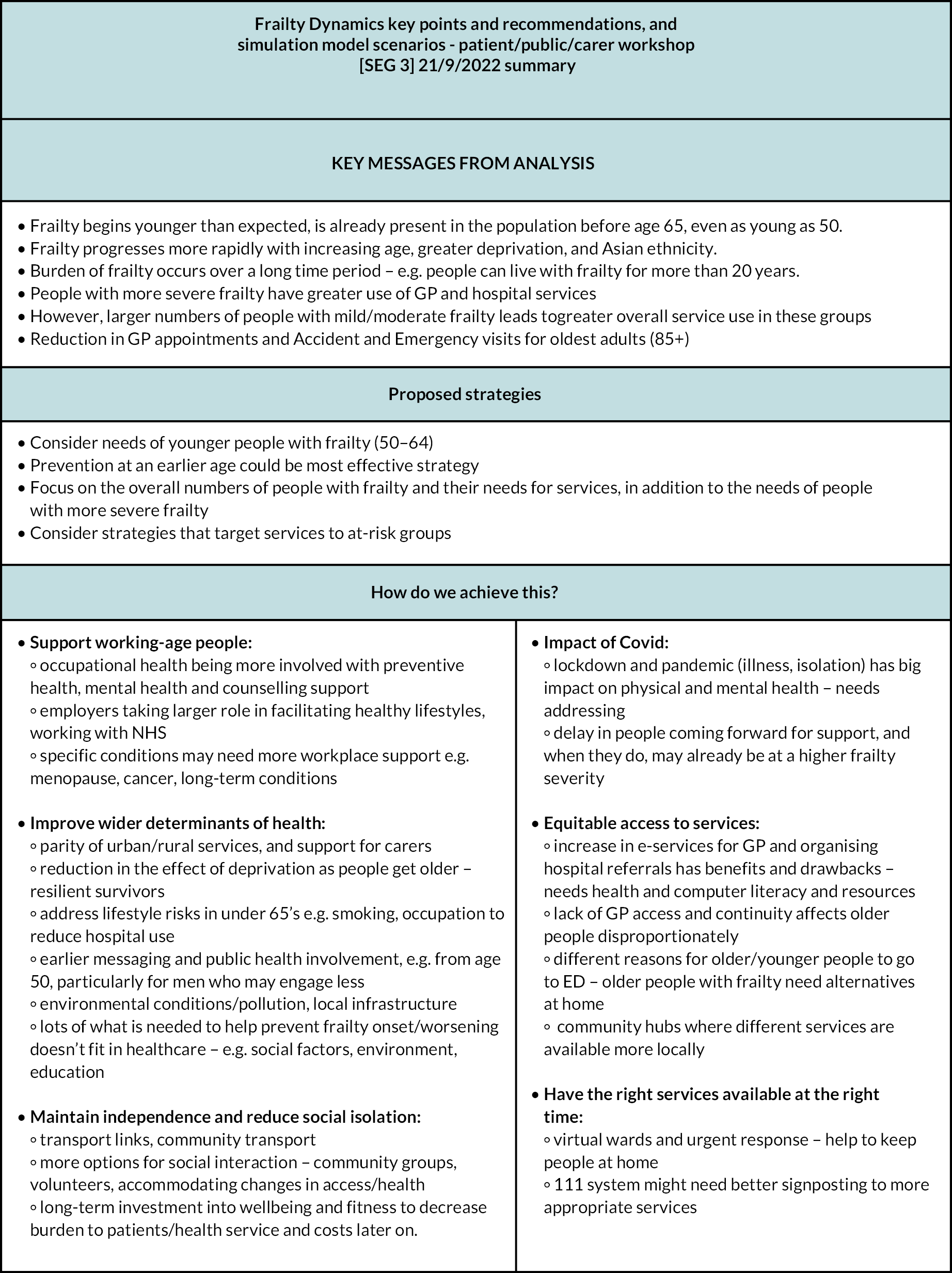
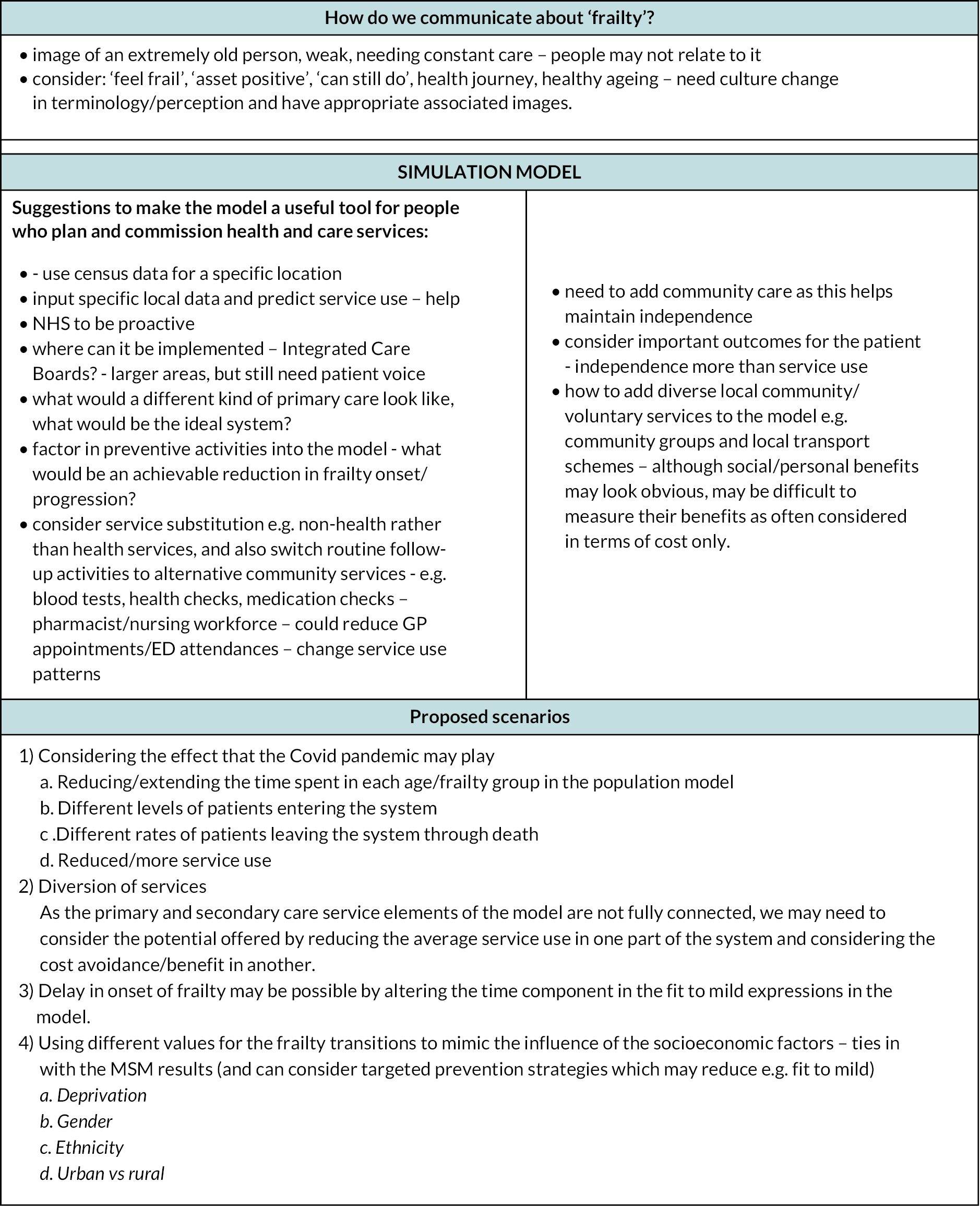
Appendix 3 Simulation model development and findings
FIGURE 27.
Conceptual model of ageing and frailty transitions.
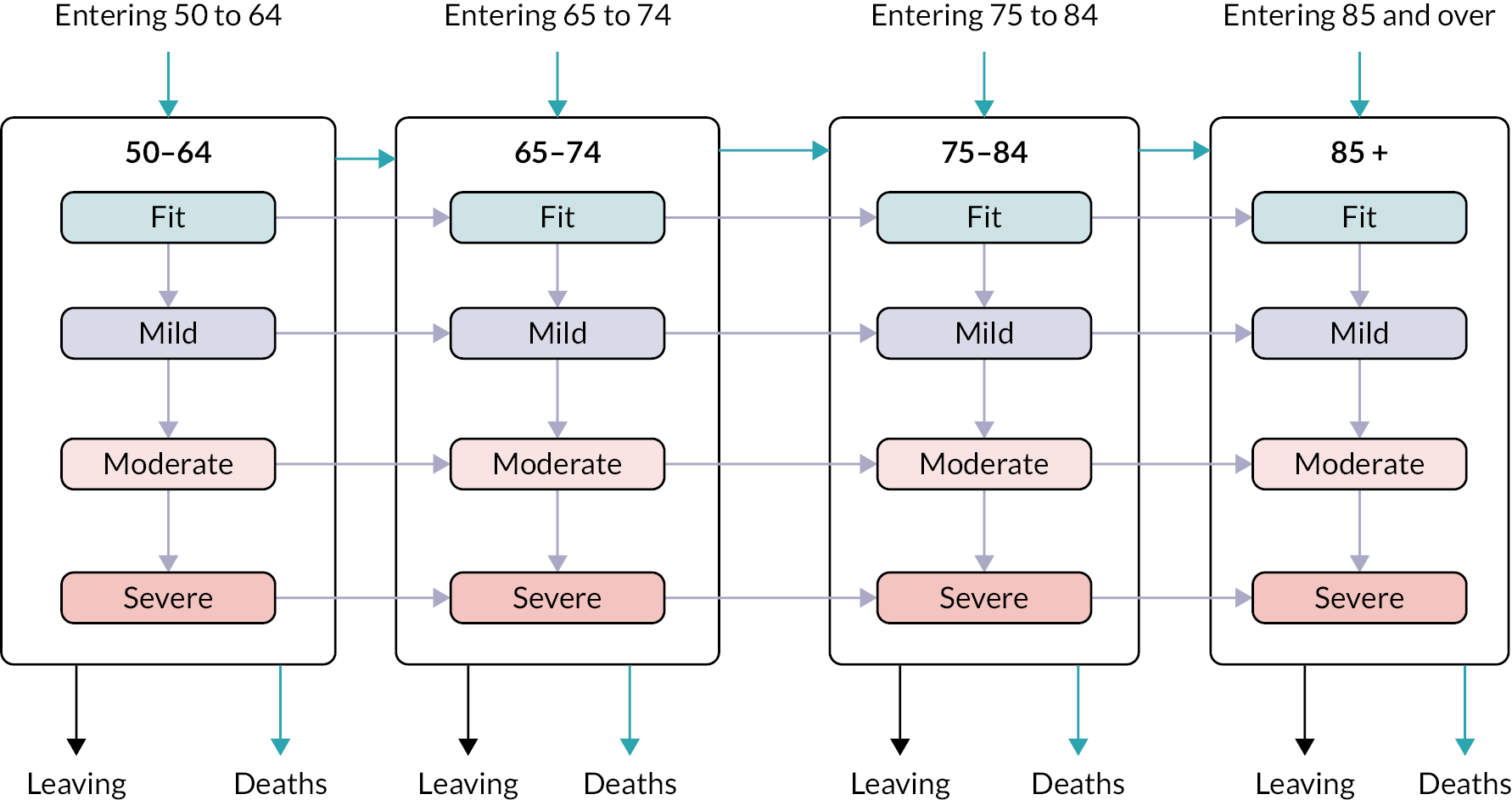
| Data set | Data items | Study phase | Access |
|---|---|---|---|
| SAIL Databank 30,000 patients Wales Primary, secondary, urgent care data, residential care |
Age Gender IMD LTC diagnoses eFI score Mortality ED attendances Ambulance calls Admissions Residential care dwelling eFI |
Workstream 2 and Workstream 4 | Host: SAIL Databank Analysis: Remote secure access Approvals: SAIL IG process and UoS Ethics Committee |
| Data | Descriptions |
|---|---|
| Patients present in the cohort in 2006 | according to (1) 4 age groups, (2) 16 age/frailty groups |
| Patients present in each calendar year | for the 16 age/frailty categories |
| Patients entering the next age group in January 2007 | for the 16 age/frailty categories |
| Patient entering the cohort in each year (2007–17) | according to (1) 4 age groups, (2) 16 age/frailty groups |
| Deaths in each calendar year (2006–17) | (1) overall, (2) by age/frailty groups |
| Death rates (deaths per person-years at risk) across the whole cohort period (2006–17) | (1) 4 age groups, (2) 4 frailty categories, (3) 16 age/frailty groups |
| Deregistrations in each calendar year | (1) overall, (2) by 16 age/frailty groups |
| Patients transitioning between age groups according to their frailty category | (1) in the first year of the new age group, (2) in the last year of the previous age group |
| Patients moving between each frailty category (fit to mild, mild to moderate, moderate to severe) | for each of the cohort calendar years (2007–17) by age group |
| Patients changing frailty category and age group at the same time | for each of the cohort calendar years (2007–17) |
| Patients changing age group and frailty category | for each of the cohort calendar years (2007–17) |
| Patients changing age group and dying in the same calendar year | for each of the cohort calendar years (2007–17) |
FIGURE 28.
Data used to inform the model development.
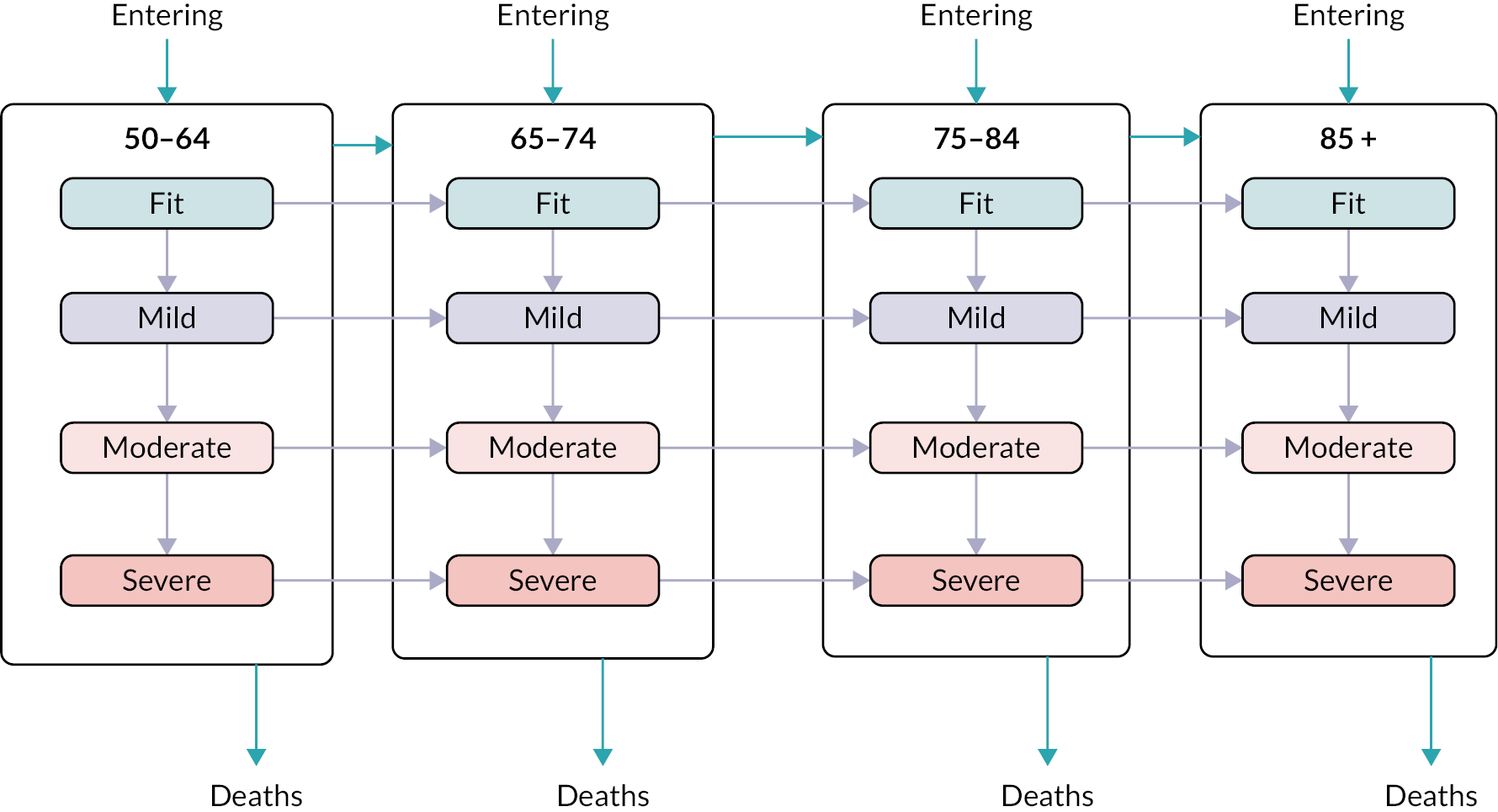
FIGURE 29.
Screenshot of the SD model in AnyLogic.
| Entering | Becoming older | Transitioning to a higher frailty category | |
|---|---|---|---|
| Entering an age/frailty subgroup | |||
| Fit | |||
| 50–64 | 5780.58 | ||
| 65–74 | 641.51 | ||
| 75–84 | 253.8 | ||
| 85 + | 117.61 | ||
| Mild | |||
| 50–64 | 548.9 | ||
| 65–74 | 187.5 | ||
| 75–84 | 199.87 | ||
| 85 + | 151.24 | ||
| Moderate | |||
| 50–64 | 51.14 | ||
| 65–74 | 32.43 | ||
| 75–84 | 68.19 | ||
| 85 + | 80.74 | ||
| Severe | |||
| 50–64 | 6.16 | ||
| 65–74 | 5.2 | ||
| 75–84 | 18.28 | ||
| 85 + | 30.27 | ||
| Becoming older while remaining fit | |||
| Ageing to 65 | 1455.9 | ||
| Ageing to 75 | 646.76 | ||
| Ageing to 85 | 190.04 | ||
| Becoming older while mildly frail | |||
| Ageing to 65 | 495.75 | ||
| Ageing to 75 | 655.83 | ||
| Ageing to 85 | 315 | ||
| Becoming older while moderately frail | |||
| Ageing to 65 | 116.08 | ||
| Ageing to 75 | 226.32 | ||
| Ageing to 85 | 276.18 | ||
| Becoming older while severely frail | |||
| Ageing to 65 | 16.64 | ||
| Ageing to 75 | 51.81 | ||
| Ageing to 85 | 100 | ||
| Becoming mildly frail (in each age group) | |||
| 50–64 | 1202.5 | ||
| 65–74 | 1437.5 | ||
| 75–84 | 750.7 | ||
| 85 + | 324.3 | ||
| Becoming moderately frail (in each age group) | |||
| 50–64 | 368.79 | ||
| 65–74 | 527.3 | ||
| 75–84 | 928.7 | ||
| 85 + | 542.1 | ||
| Becoming severely frail (in each age group) | |||
| 50–64 | 64.7 | ||
| 65–74 | 172.6 | ||
| 75–84 | 450.7 | ||
| 85 + | 458.3 | ||
| Die | Leave participating practice | |
|---|---|---|
| Fit | ||
| 50–64 | 0.000266374 | 0.002503695 |
| 65–74 | 0.000694083 | 0.001925978 |
| 75–84 | 0.001860235 | 0.001852182 |
| 85 + | 0.006824661 | 0.003708615 |
| Mild | ||
| 50–64 | 0.000884341 | 0.002050559 |
| 65–74 | 0.001603123 | 0.001536819 |
| 75–84 | 0.003374953 | 0.001607223 |
| 85 + | 0.009814731 | 0.002702485 |
| Moderate | ||
| 50–64 | 0.001954172 | 0.002007755 |
| 65–74 | 0.003262494 | 0.001626885 |
| 75–84 | 0.005780752 | 0.001915927 |
| 85 + | 0.013033851 | 0.00302172 |
| Severe | ||
| 50–64 | 0.00405949 | 0.001924223 |
| 65–74 | 0.006395763 | 0.002003816 |
| 75–84 | 0.009734493 | 0.002369443 |
| 85 + | 0.017655853 | 0.003521731 |
| Living | Entering the cohort | Dying | Deregistration | Frailty transitions | Ageing | |
|---|---|---|---|---|---|---|
| 50–64 | ||||||
| Fit | 0.45 | 1.96 | 5.35 | 5.77 | 10.35 | |
| Mild | 1.42 | 2.37 | 6.55 | 6.93 | 11.53 | |
| Moderate | 6.93 | 5.57 | 7.58 | 7.94 | 14.91 | |
| Severe | 7.77 | 15.84 | 4.00 | 10.22 | 19.45 | |
| Fit to Mild | 24.04 | |||||
| Mild to Moderate | 3.43 | |||||
| Moderate to Severe | 4.83 | |||||
| 65–74 | ||||||
| Fit | 3.89 | 5.43 | 5.64 | 4.93 | 8.03 | |
| Mild | 2.91 | 6.18 | 7.39 | 7.24 | 6.99 | |
| Moderate | 3.44 | 7.15 | 5.75 | 9.90 | 11.82 | |
| Severe | 5.39 | 22.48 | 7.40 | 9.45 | 9.55 | |
| Fit to Mild | 9.30 | |||||
| Mild to Moderate | 7.83 | |||||
| Moderate to Severe | 10.49 | |||||
| 75–84 | ||||||
| Fit | 3.75 | 4.39 | 8.16 | 1.83 | 10.94 | |
| Mild | 1.53 | 5.42 | 6.30 | 5.58 | 9.07 | |
| Moderate | 3.50 | 4.43 | 5.91 | 6.52 | 4.35 | |
| Severe | 3.99 | 11.02 | 4.47 | 7.62 | 8.54 | |
| Fit to Mild | 14.46 | |||||
| Mild to Moderate | 5.50 | |||||
| Moderate to Severe | 6.54 | |||||
| 85 + | ||||||
| Fit | 4.70 | 4.67 | 5.49 | 6.12 | n/a | |
| Mild | 2.43 | 4.01 | 3.22 | 4.71 | n/a | |
| Moderate | 2.23 | 5.52 | 2.51 | 8.38 | n/a | |
| Severe | 10.44 | 16.08 | 5.26 | 11.87 | n/a | |
| Fit to Mild | 6.23 | |||||
| Mild to Moderate | 6.79 | |||||
| Moderate to Severe | 5.29 | |||||
| Frailty category and age group | Entry into cohort | Death | Deregistrations | Ageing into next age group | Frailty transition |
|---|---|---|---|---|---|
| Fit | |||||
| 50–64 | 0.504 | 1.208 | 0.411 | No change | 1.8 |
| 65–74 | 0.279 | 1.159 | 0.503 | 0.90 | Adjusted parametric form for Fit to Mild transition in 65–74 age group: 0.1134 – 0.0003t + 0.0000008t2 |
| 75–84 | 0.210 | 1.130 | 0.494 | 0.895 | No change |
| 85 + | 0.155 | 1.069 | 0.64 | Adjusted parametric form for Fit to Mild transition in the 85 + age group: 0.2907–0.0011t + 0.000003t2 | |
| Mild | |||||
| 50–64 | 1.100 | 0.835 | 0.288 | 1.16 | 1.1 |
| 65–74 | 0.399 | 0.921 | 0.353 | No change | No change |
| 75–84 | 0.277 | 0.953 | 0.412 | No change | No change |
| 85 + | 0.200 | 0.94 | 0.407 | Adjusted parametric form for Mild to Moderate transition in the 85 + age group: 0.1936 + 0.0003t – 0.000003t2 | |
| Moderate | |||||
| 50–64 | 1.368 | 0.947 | 0.362 | 1.389 | 1.17 |
| 65–74 | 0.570 | 0.944 | 0.469 | 1.15 | No change |
| 75–84 | 0.356 | 0.879 | 0.396 | 0.853 | No change |
| 85 + | 0.273 | 0.987 | 0.389 | No change | |
| Severe | |||||
| 50–64 | 1.370 | 1.062 | 0.370 | 1.701 | |
| 65–74 | 0.95 | 1.000 | 0.468 | 1.23 | |
| 75–84 | 0.592 | 0.994 | 0.381 | No change | |
| 85 + | 0.491 | 0.993 | 0.434 | ||
| Living | Entering the cohort | Dying | Deregistration | Frailty transitions | Ageing | |
|---|---|---|---|---|---|---|
| 50–64 | ||||||
| Fit | 2.09 | 5.05 | 9.48 | 4.77 | 8.17 | |
| Mild | 1.41 | 5.64 | 8.36 | 7.38 | 12.3 | |
| Moderate | 6.45 | 8.35 | 5.37 | 11.73 | 10.26 | |
| Severe | 6.73 | 18.86 | 5.12 | 10.92 | 21.38 | |
| Fit to Mild | 3.05 | |||||
| Mild to Moderate | 4.94 | |||||
| Moderate to Severe | 3.85 | |||||
| 65–74 | ||||||
| Fit | 2.4 | 16.99 | 9.69 | 10.95 | 6.08 | |
| Mild | 7.10 | 25.24 | 9.84 | 7.26 | 9.21 | |
| Moderate | 5.58 | 28.12 | 4.01 | 18.2 | 2.78 | |
| Severe | 11.05 | 48.84 | 1.13 | 15.36 | 14.05 | |
| Fit to Mild | 6.68 | |||||
| Mild to Moderate | 8.03 | |||||
| Moderate to Severe | 3.33 | |||||
| 75–84 | ||||||
| Fit | 3.31 | 19.97 | 11.2 | 4.13 | 10.12 | |
| Mild | 4.32 | 21.98 | 10.7 | 6.6 | 3.24 | |
| Moderate | 1.38 | 23.23 | 6.62 | 12.34 | 7.39 | |
| Severe | 2.33 | 27.49 | 1.99 | 4.32 | 3.19 | |
| Fit to Mild | 10.61 | |||||
| Mild to Moderate | 4.39 | |||||
| Moderate to Severe | 5.37 | |||||
| 85 + | ||||||
| Fit | 9.12 | 12.02 | 8.01 | 7.38 | n/a | |
| Mild | 4.15 | 15.76 | 6.70 | 8.23 | n/a | |
| Moderate | 2.93 | 19.19 | 3.09 | 11.13 | n/a | |
| Severe | 8.92 | 34.64 | 4.43 | 4.3 | n/a | |
| Fit to Mild | 4.45 | |||||
| Mild to Moderate | 5.97 | |||||
| Moderate to Severe | 7.38 | |||||
FIGURE 30.
Example screenshot showing the instability of the model.
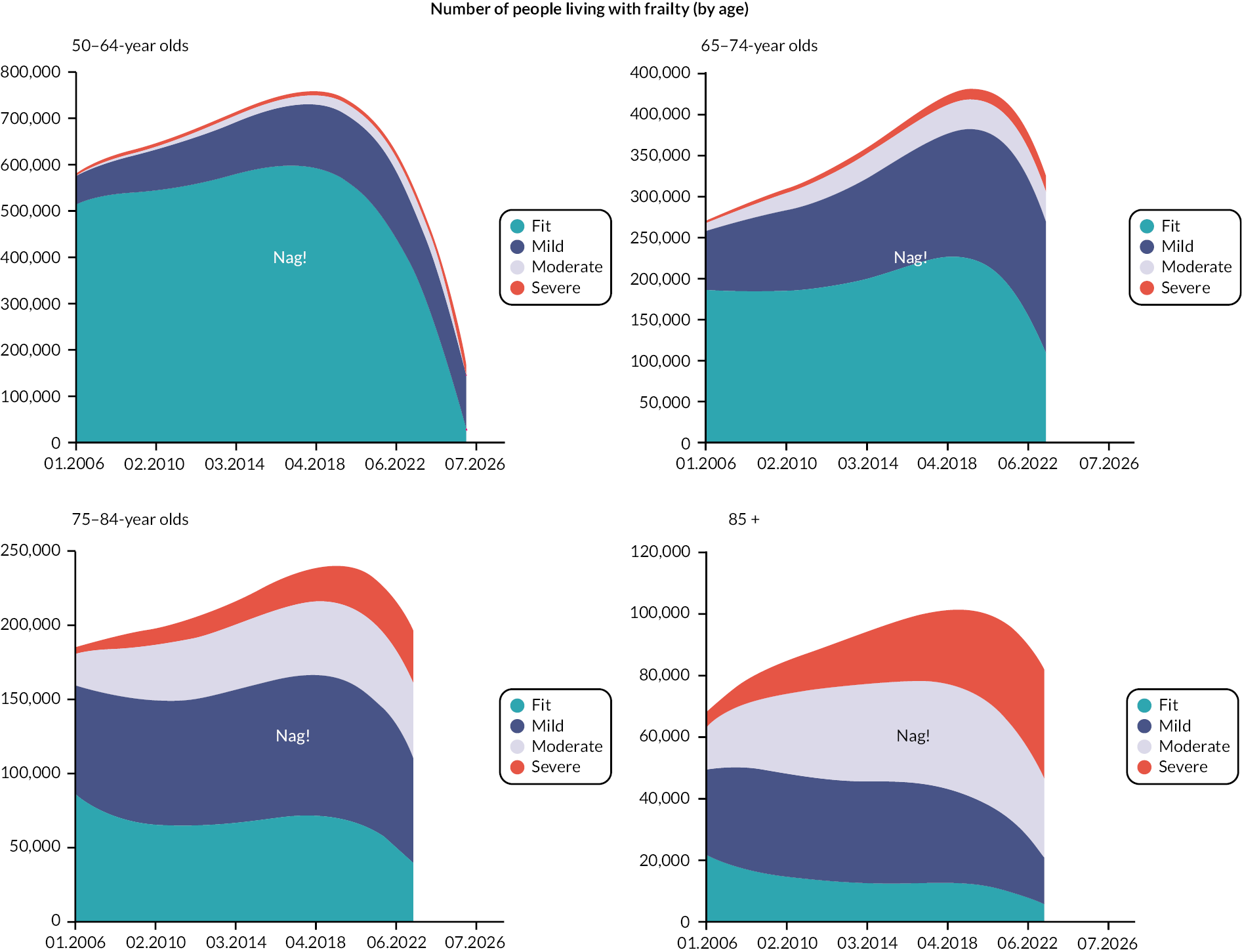
FIGURE 31.
Example screenshot showing improved stability of the model.
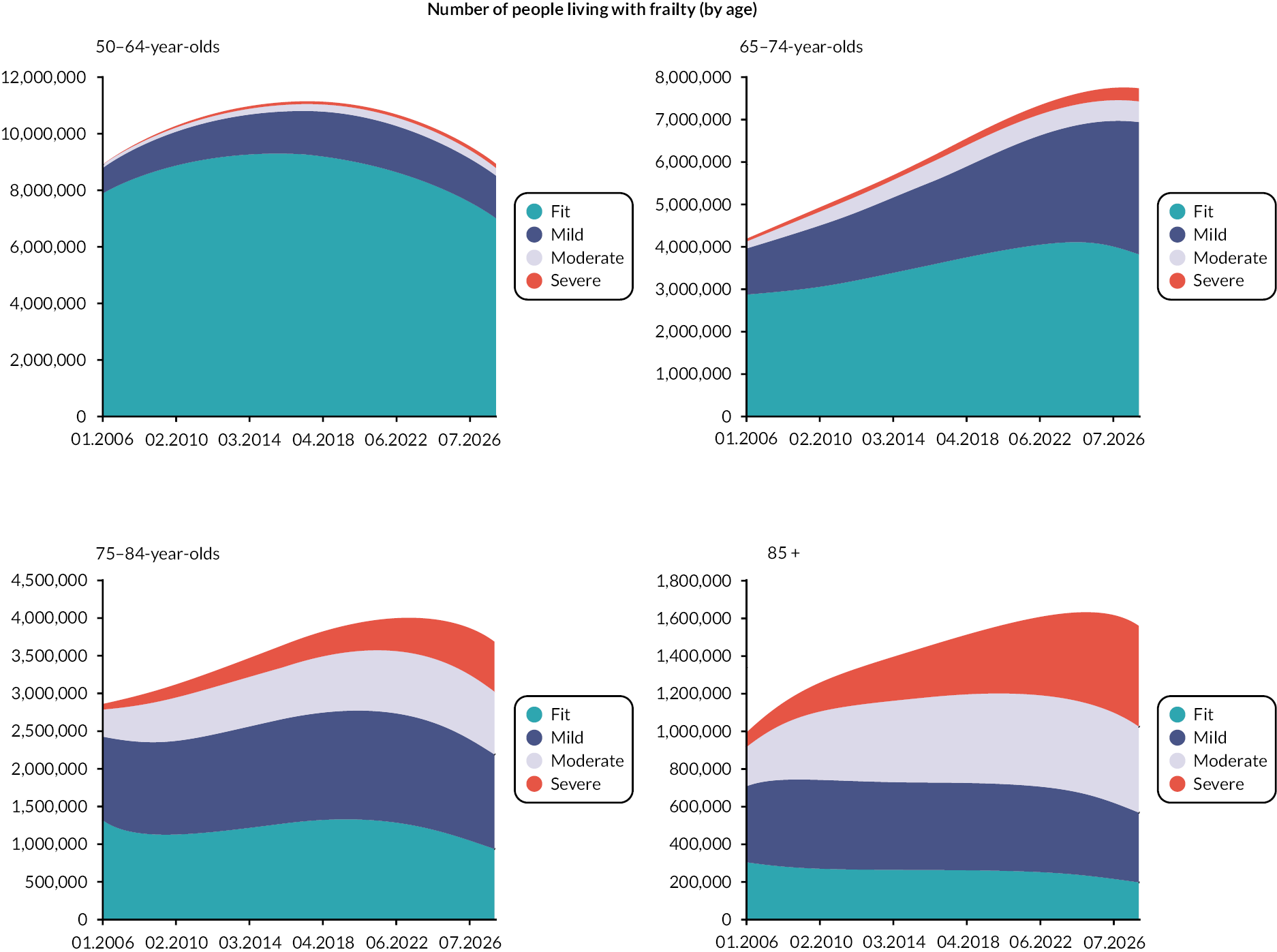
FIGURE 32.
Adapting the conceptual model for national level – final FD Simulation Model. Reproduced with permission from England et al.,91 Health Systems – under review. This is an Open Access article distributed under the terms of the Creative Commons Attribution Licence, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
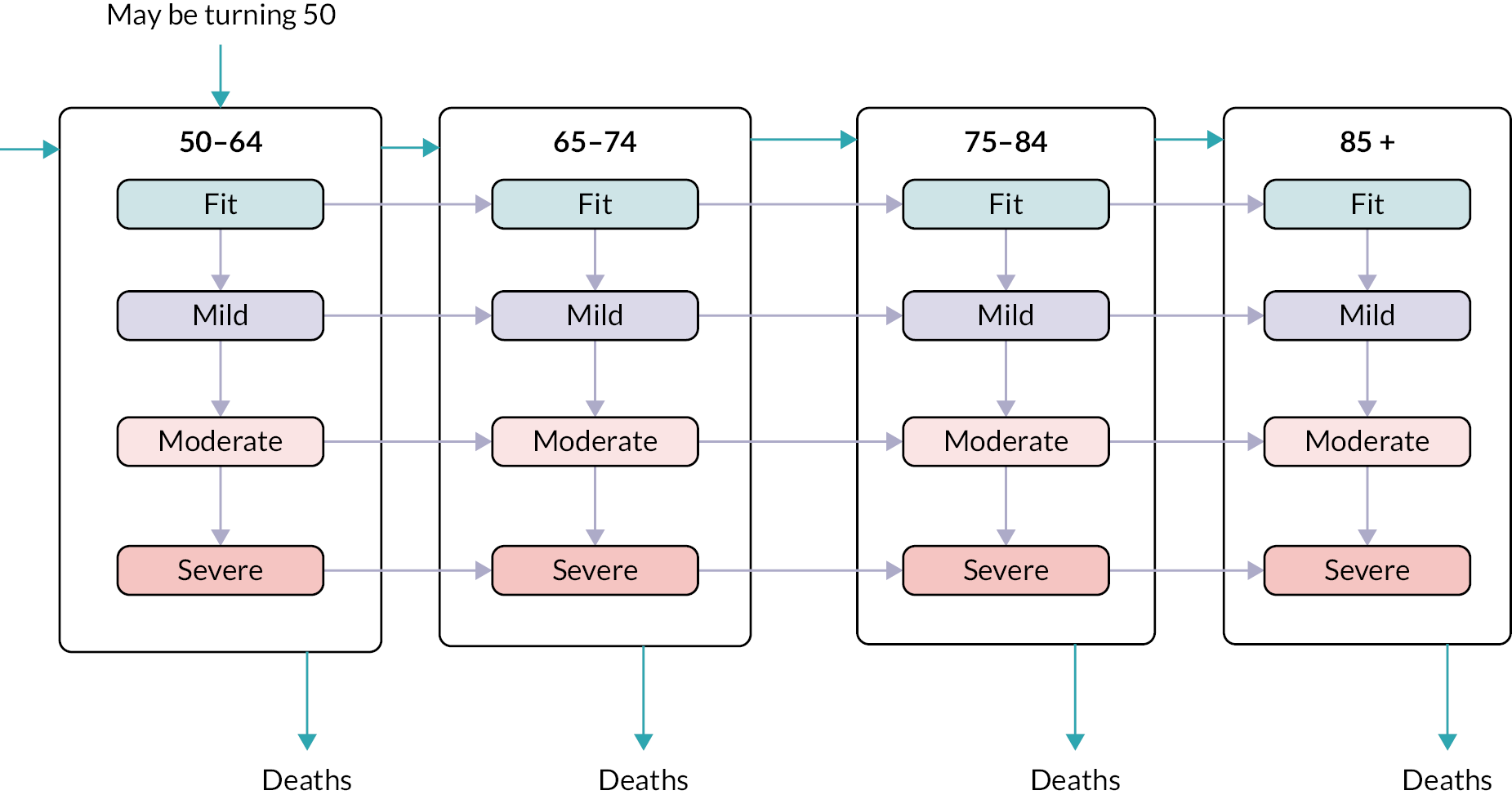
| Mid-year | 50–64 | 65–74 | 75–84 | 85–90 | Total (50 +) |
|---|---|---|---|---|---|
| 2005 | 8,913,695 | 4,186,147 | 2,855,158 | 986,704 | 16,941,704 |
| 2006 | 9,041,649 | 4,164,253 | 2,850,283 | 1,039,944 | 17,096,129 |
| 2007 | 9,153,186 | 4,186,667 | 2,855,667 | 1,082,834 | 17,278,354 |
| 2008 | 9,258,322 | 4,273,848 | 2,864,519 | 1,111,614 | 17,508,303 |
| 2009 | 9,353,488 | 4,387,775 | 2,876,775 | 1,135,737 | 17,753,775 |
| 2010 | 9,460,262 | 4,490,464 | 2,908,101 | 1,165,050 | 18,023,877 |
| 2011 | 9,588,371 | 4,592,171 | 2,944,178 | 1,193,318 | 18,318,038 |
| 2012 | 9,590,084 | 4,844,490 | 2,991,512 | 1,220,506 | 18,646,592 |
| 2013 | 9,671,508 | 5,023,573 | 3,043,739 | 1,237,867 | 18,976,687 |
| 2014 | 9,817,800 | 5,162,873 | 3,099,319 | 1,275,516 | 19,355,508 |
| 2015 | 9,994,043 | 5,285,755 | 3,130,528 | 1,295,289 | 19,705,615 |
| 2016 | 10,181,728 | 5,413,344 | 3,141,405 | 1,328,092 | 20,064,569 |
| 2017 | 10,369,150 | 5,495,181 | 3,183,274 | 1,352,056 | 20,399,661 |
| 2018 | 10,533,154 | 5,547,393 | 3,266,882 | 1,364,978 | 20,712,407 |
| 2019 | 10,689,947 | 5,576,066 | 3,380,599 | 1,397,051 | 21,043,663 |
| 2020 | 10,833,946 | 5,598,428 | 3,459,181 | 1,406,410 | 21,297,965 |
| 2021 | 10,977,156 | 5,631,714 | 3,549,656 | 1,430,287 | 21,588,813 |
| 2022 | 11,075,420 | 5,550,409 | 3,765,160 | 1,456,722 | 21,847,711 |
| 2023 | 11,124,001 | 5,549,290 | 3,921,170 | 1,491,088 | 22,085,549 |
| 2024 | 11,132,224 | 5,594,279 | 4,037,206 | 1,524,157 | 22,287,866 |
| 2025 | 11,115,673 | 5,669,799 | 4,135,406 | 1,551,184 | 22,472,062 |
| 2026 | 11,059,725 | 5,785,082 | 4,229,151 | 1,562,550 | 22,636,508 |
| 2027 | 10,968,901 | 5,928,869 | 4,289,495 | 1,594,604 | 22,781,869 |
| Mid-year | Number becoming 50 | Number becoming 65 | % of 50– 64-year olds that become 65 | Number becoming 75 | % of 65– 74-year olds that become 75 | Number becoming 85 | % of 75– 84-year olds that become 85 |
|---|---|---|---|---|---|---|---|
| 2004 | 616,718 | 469,943 | 371,782 | 220,242 | |||
| 2005 | 633,354 | 443,865 | 4.98 | 374,394 | 8.88 | 223,963 | 7.71 |
| 2006 | 653,741 | 473,187 | 5.23 | 369,093 | 8.99 | 214,767 | 7.86 |
| 2007 | 676,990 | 519,732 | 5.68 | 361,340 | 8.82 | 207,387 | 7.52 |
| 2008 | 688,599 | 547,018 | 5.91 | 361,230 | 8.45 | 206,451 | 7.24 |
| 2009 | 702,356 | 545,922 | 5.84 | 374,108 | 8.23 | 206,894 | 7.18 |
| 2010 | 729,121 | 555,040 | 5.87 | 380,763 | 8.33 | 210,353 | 7.11 |
| 2011 | 753,474 | 709,646 | 7.40 | 388,454 | 8.29 | 211,093 | 7.14 |
| 2012 | 768,644 | 646,777 | 6.74 | 398,269 | 8.02 | 212,846 | 7.06 |
| 2013 | 783,842 | 606,763 | 6.27 | 400,106 | 7.93 | 219,220 | 6.99 |
| 2014 | 791,217 | 587,146 | 5.98 | 394,967 | 7.75 | 228,883 | 7.07 |
| 2015 | 787,915 | 571,273 | 5.72 | 372,072 | 7.47 | 231,569 | 7.31 |
| 2016 | 787,964 | 563,731 | 5.54 | 406,717 | 6.87 | 232,310 | 7.37 |
| 2017 | 776,394 | 576,498 | 5.56 | 448,970 | 7.40 | 228,736 | 7.30 |
| 2018 | 777,217 | 580,732 | 5.51 | 476,842 | 8.09 | 231,314 | 7.00 |
| 2019 | 759,708 | 576,200 | 5.39 | 473,332 | 8.55 | 242,740 | 6.84 |
| 2020 | 777,997 | 594,643 | 5.49 | 482,547 | 8.45 | 246,429 | 7.02 |
| 2021 | 753,929 | 614,414 | 5.60 | 619,426 | 8.57 | 252,668 | 6.94 |
| 2022 | 723,734 | 635,908 | 5.74 | 564,721 | 11.16 | 258,416 | 6.71 |
| 2023 | 695,219 | 647,286 | 5.82 | 530,203 | 10.18 | 260,541 | 6.59 |
| 2024 | 684,320 | 660,828 | 5.94 | 512,877 | 9.48 | 257,525 | 6.45 |
| 2025 | 671,949 | 687,799 | 6.19 | 499,189 | 9.05 | 243,679 | 6.23 |
| 2026 | 660,493 | 711,397 | 6.43 | 492,965 | 8.63 | 267,702 | 5.76 |
| 2027 | 671,498 | 726,964 | 6.63 | 504,590 | 8.31 | 296,385 | 6.24 |
| Year | 50–64 | 50–64 | 50–64 | 50–64 | 65–74 | 65–74 | 65–74 | 65–74 | 75–84 | 75–84 | 75–84 | 75–84 | 85 + | 85 + | 85 + | 85 + | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fit | Mild | Moderate | Severe | Fit | Mild | Moderate | Severe | Fit | Mild | Moderate | Severe | Fit | Mild | Moderate | Severe | ||
| 2017 | 369.2 | 206.7 | 61.6 | 14.1 | 183.6 | 215.3 | 83.1 | 31.6 | 75.0 | 144.7 | 109.0 | 64.7 | 16.6 | 57.2 | 66.8 | 55.1 | 1754.4 |
| 2018 | 371.9 | 215.2 | 66.4 | 15.8 | 183.1 | 223.7 | 86.1 | 34.2 | 73.2 | 142.5 | 109.4 | 69.2 | 15.9 | 56.4 | 67.3 | 60.1 | 1790.2 |
| 2019 | 374.0 | 223.2 | 71.1 | 17.5 | 182.0 | 230.6 | 88.2 | 36.5 | 72.5 | 142.0 | 110.3 | 74.3 | 15.1 | 55.2 | 67.4 | 65.4 | 1825.5 |
| 2020 | 375.6 | 231.0 | 75.8 | 19.2 | 179.9 | 235.8 | 89.3 | 38.4 | 72.9 | 143.6 | 112.3 | 80.4 | 14.0 | 53.4 | 66.9 | 71.1 | 1859.7 |
| 2021 | 377.2 | 238.7 | 80.6 | 21.0 | 177.0 | 239.7 | 89.6 | 40.0 | 73.4 | 146.1 | 115.0 | 87.6 | 12.9 | 51.3 | 66.1 | 77.1 | 1893.2 |
| 2022 | 377.5 | 245.7 | 85.1 | 22.8 | 175.2 | 244.0 | 89.6 | 41.4 | 72.8 | 147.6 | 117.6 | 95.4 | 12.0 | 49.3 | 65.2 | 83.8 | 1925.2 |
| 2023 | 377.9 | 252.3 | 89.6 | 24.6 | 173.9 | 248.2 | 89.3 | 42.7 | 72.0 | 149.3 | 120.6 | 104.3 | 11.0 | 47.1 | 64.0 | 90.9 | 1957.7 |
| 2024 | 376.6 | 258.0 | 93.8 | 26.4 | 169.7 | 247.0 | 86.8 | 42.7 | 74.7 | 156.6 | 126.2 | 115.6 | 9.98 | 44.6 | 62.4 | 98.2 | 1989.3 |
| 2025 | 373.7 | 262.7 | 97.7 | 28.1 | 167.9 | 248.9 | 85.1 | 43.1 | 74.0 | 159.9 | 131.2 | 128.1 | 9.06 | 42.1 | 60.6 | 106.0 | 2018.2 |
| 2026 | 369.4 | 266.4 | 101.2 | 29.8 | 167.9 | 253.0 | 83.9 | 43.7 | 71.3 | 160.4 | 135.4 | 141.9 | 8.07 | 39.4 | 58.5 | 113.9 | 2044.3 |
| 2027 | 364.0 | 269.1 | 104.4 | 31.3 | 169.3 | 259.1 | 83.1 | 44.4 | 67.7 | 159.2 | 139.0 | 157.2 | 7.00 | 36.2 | 55.8 | 121.9 | 2068.7 |
| Year | 50–64 | 50–64 | 50–64 | 50–64 | 65–74 | 65–74 | 65–74 | 65–74 | 75–84 | 75–84 | 75–84 | 75–84 | 85 + | 85 + | 85 + | 85 + | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fit | Mild | Moderate | Severe | Fit | Mild | Moderate | Severe | Fit | Mild | Moderate | Severe | Fit | Mild | Moderate | Severe | ||
| 2017 | 83.8 | 91.9 | 37.0 | 10.3 | 52.3 | 97.9 | 46.2 | 21.2 | 22.2 | 63.4 | 58.5 | 42.6 | 5.13 | 26.7 | 38.7 | 39.2 | 737.0 |
| 2018 | 84.4 | 95.6 | 39.9 | 11.5 | 52.2 | 101.7 | 47.8 | 22.9 | 21.7 | 62.4 | 58.7 | 45.5 | 4.94 | 26.4 | 39.0 | 42.8 | 757.4 |
| 2019 | 84.9 | 99.2 | 42.7 | 12.7 | 51.9 | 104.8 | 49.0 | 24.5 | 21.4 | 62.2 | 59.2 | 48.9 | 4.68 | 25.8 | 39.1 | 46.6 | 777.6 |
| 2020 | 85.3 | 102.6 | 45.6 | 14.0 | 51.3 | 107.2 | 49.6 | 25.7 | 21.6 | 62.9 | 60.2 | 53.0 | 4.35 | 25.0 | 38.8 | 50.6 | 797.6 |
| 2021 | 85.6 | 106.1 | 48.4 | 15.3 | 50.4 | 108.9 | 49.8 | 26.8 | 21.7 | 64.0 | 61.7 | 57.7 | 4.01 | 24.0 | 38.3 | 54.9 | 817.6 |
| 2022 | 85.7 | 109.2 | 51.2 | 16.6 | 49.9 | 110.9 | 49.8 | 27.8 | 21.5 | 64.7 | 63.1 | 62.8 | 3.72 | 23.0 | 37.8 | 59.6 | 837.4 |
| 2023 | 85.8 | 112.1 | 53.8 | 17.9 | 49.6 | 112.8 | 49.6 | 28.6 | 21.3 | 65.4 | 64.7 | 68.7 | 3.40 | 22.0 | 37.1 | 64.7 | 857.5 |
| 2024 | 85.5 | 114.6 | 56.3 | 19.2 | 48.4 | 112.3 | 48.2 | 28.6 | 22.1 | 68.6 | 67.7 | 76.1 | 3.09 | 20.9 | 36.2 | 69.9 | 877.7 |
| 2025 | 84.8 | 116.8 | 58.7 | 20.4 | 47.9 | 113.1 | 47.3 | 28.9 | 21.9 | 70.1 | 70.4 | 84.3 | 2.81 | 19.7 | 35.1 | 75.4 | 897.6 |
| 2026 | 83.9 | 118.4 | 60.8 | 21.6 | 47.8 | 115.0 | 46.6 | 29.3 | 21.1 | 70.3 | 72.7 | 93.4 | 2.50 | 18.4 | 33.9 | 81.1 | 916.8 |
| Es2027 | 82.6 | 119.6 | 62.7 | 22.7 | 48.2 | 117.8 | 46.2 | 29.8 | 20.0 | 69.8 | 74.6 | 103.5 | 2.17 | 16.9 | 32.4 | 86.8 | 935.7 |
| Year | Fit | Mild | Moderate | Severe | Total |
|---|---|---|---|---|---|
| 2017 | 17.89 | 17.39 | 11.67 | 7.87 | 54.81 |
| 2018 | 17.89 | 17.76 | 11.98 | 8.52 | 56.15 |
| 2019 | 17.89 | 18.11 | 12.27 | 9.22 | 57.49 |
| 2020 | 17.86 | 18.45 | 12.54 | 9.96 | 58.80 |
| 2021 | 17.82 | 18.76 | 12.78 | 10.76 | 60.12 |
| 2022 | 17.74 | 19.04 | 13.01 | 11.62 | 61.41 |
| 2023 | 17.67 | 19.30 | 13.23 | 12.54 | 62.73 |
| 2024 | 17.56 | 19.54 | 13.43 | 13.53 | 64.06 |
| 2025 | 17.39 | 19.73 | 13.62 | 14.60 | 65.33 |
| 2026 | 17.15 | 19.85 | 13.77 | 15.75 | 66.52 |
| 2027 | 16.89 | 19.93 | 13.87 | 16.97 | 67.65 |
| Year | Fit | Mild | Moderate | Severe | Totals |
|---|---|---|---|---|---|
| 2017 | 30,880 | 32,885 | 24,321 | 12,001 | 100,089 |
| 2018 | 30,827 | 33,891 | 25,099 | 13,003 | 102,820 |
| 2019 | 30,783 | 34,738 | 25,822 | 14,042 | 105,384 |
| 2020 | 30,712 | 35,381 | 26,504 | 15,130 | 107,726 |
| 2021 | 30,608 | 35,902 | 27,151 | 16,277 | 109,938 |
| 2022 | 30,452 | 36,427 | 27,755 | 17,483 | 112,117 |
| 2023 | 30,310 | 36,898 | 28,324 | 18,763 | 114,295 |
| 2024 | 30,141 | 36,920 | 28,845 | 20,102 | 116,008 |
| 2025 | 29,846 | 37,090 | 29,363 | 21,568 | 117,868 |
| 2026 | 29,466 | 37,332 | 29,831 | 23,157 | 119,787 |
| 2027 | 29,068 | 37,596 | 30,222 | 24,863 | 121,749 |
| Year | Fit | Mild | Moderate | Severe | Total |
|---|---|---|---|---|---|
| 2017 | 17,151,226 | 18,452,057 | 10,134,799 | 5,176,542 | 50,914,623 |
| 2018 | 17,145,843 | 18,898,797 | 10,446,927 | 5,607,819 | 52,099,387 |
| 2019 | 17,138,080 | 19,328,069 | 10,736,203 | 6,059,828 | 53,262,179 |
| 2020 | 17,110,857 | 19,735,244 | 11,007,266 | 6,537,405 | 54,390,772 |
| 2021 | 17,069,080 | 20,120,510 | 11,264,039 | 7,044,591 | 55,498,220 |
| 2022 | 16,994,759 | 20,473,899 | 11,503,088 | 7,583,229 | 56,554,975 |
| 2023 | 16,927,208 | 20,811,112 | 11,726,928 | 8,157,799 | 57,623,048 |
| 2024 | 16,830,442 | 21,103,703 | 11,934,481 | 8,768,199 | 58,636,825 |
| 2025 | 16,667,304 | 21,348,499 | 12,135,953 | 9,433,307 | 59,585,064 |
| 2026 | 16,455,093 | 21,546,445 | 12,312,637 | 10,151,213 | 60,465,387 |
| 2027 | 16,226,238 | 21,709,923 | 12,451,901 | 10,917,653 | 61,305,715 |
| Year | Fit | Mild | Moderate | Severe | Total |
|---|---|---|---|---|---|
| 2017 | 2,319,998 | 2,456,302 | 1,385,929 | 661,607 | 6,823,836 |
| 2018 | 2,319,191 | 2,517,493 | 1,432,154 | 717,570 | 6,986,408 |
| 2019 | 2,318,108 | 2,576,539 | 1,475,233 | 775,669 | 7,145,549 |
| 2020 | 2,314,284 | 2,632,864 | 1,515,888 | 836,515 | 7,299,551 |
| 2021 | 2,308,254 | 2,686,260 | 1,554,604 | 900,591 | 7,449,710 |
| 2022 | 2,298,249 | 2,735,390 | 1,590,764 | 967,964 | 7,592,368 |
| 2023 | 2,289,355 | 2,782,561 | 1,624,898 | 1,039,268 | 7,736,082 |
| 2024 | 2,275,758 | 2,823,233 | 1,655,992 | 1,113,781 | 7,868,765 |
| 2025 | 2,254,111 | 2,857,993 | 1,686,684 | 1,194,990 | 7,993,779 |
| 2026 | 2,226,572 | 2,887,066 | 1,714,363 | 1,282,612 | 8,110,614 |
| 2027 | 2,197,438 | 2,912,254 | 1,737,403 | 1,376,197 | 8,223,292 |
| Year | Fit | Mild | Moderate | Severe | Total |
|---|---|---|---|---|---|
| 2017 | 756,047 | 1,083,928 | 859,233 | 633,736 | 3,332,944 |
| 2018 | 753,263 | 1,101,993 | 879,410 | 686,411 | 3,421,076 |
| 2019 | 750,416 | 1,119,272 | 897,686 | 742,396 | 3,509,769 |
| 2020 | 746,846 | 1,135,493 | 914,306 | 802,200 | 3,598,845 |
| 2021 | 742,684 | 1,150,645 | 929,686 | 866,390 | 3,689,406 |
| 2022 | 737,049 | 1,164,035 | 943,798 | 935,419 | 3,780,300 |
| 2023 | 731,417 | 1,176,215 | 956,531 | 1,009,612 | 3,873,775 |
| 2024 | 726,519 | 1,189,242 | 969,316 | 1,089,813 | 3,974,890 |
| 2025 | 717,452 | 1,197,603 | 980,848 | 1,176,672 | 4,072,575 |
| 2026 | 705,346 | 1,201,546 | 989,633 | 1,269,936 | 4,166,460 |
| 2027 | 691,994 | 1,201,911 | 994,425 | 1,368,778 | 4,257,107 |
| Year | Fit | Mild | Moderate | Severe | Total |
|---|---|---|---|---|---|
| 2017 | 340,376 | 520,157 | 492,218 | 416,805 | 1,769,556 |
| 2018 | 338,858 | 526,544 | 502,030 | 451,457 | 1,818,889 |
| 2019 | 337,340 | 532,567 | 510,758 | 488,467 | 1,869,133 |
| 2020 | 335,540 | 538,079 | 518,495 | 528,143 | 1,920,257 |
| 2021 | 333,489 | 543,099 | 525,509 | 570,877 | 1,972,974 |
| 2022 | 330,735 | 547,338 | 531,861 | 617,025 | 2,026,959 |
| 2023 | 327,944 | 550,913 | 537,393 | 666,741 | 2,082,991 |
| 2024 | 325,769 | 555,722 | 543,342 | 720,762 | 2,145,596 |
| 2025 | 321,506 | 557,875 | 548,361 | 779,137 | 2,206,878 |
| 2026 | 315,732 | 557,513 | 551,621 | 841,687 | 2,266,552 |
| 2027 | 309,311 | 555,040 | 552,356 | 907,790 | 2,324,497 |
| Year | Fit | Mild | Moderate | Severe | Total |
|---|---|---|---|---|---|
| 2017 | 25,252 | 68,738 | 85,626 | 89,722 | 269,338 |
| 2018 | 24,821 | 68,532 | 86,524 | 97,353 | 277,230 |
| 2019 | 24,354 | 68,180 | 87,155 | 105,611 | 285,299 |
| 2020 | 23,845 | 67,622 | 87,461 | 114,489 | 293,417 |
| 2021 | 23,321 | 66,952 | 87,547 | 124,100 | 301,920 |
| 2022 | 22,770 | 66,253 | 87,555 | 134,632 | 311,210 |
| 2023 | 22,176 | 65,393 | 87,347 | 145,999 | 320,916 |
| 2024 | 21,815 | 64,863 | 87,183 | 158,345 | 332,206 |
| 2025 | 21,211 | 63,917 | 86,765 | 171,519 | 343,412 |
| 2026 | 20,414 | 62,519 | 85,962 | 185,444 | 354,338 |
| 2027 | 19,511 | 60,659 | 84,585 | 199,862 | 364,617 |
| Year | Fit | Mild | Moderate | Severe | Total | Total frail |
|---|---|---|---|---|---|---|
| 2017 | 10.068 | 12.755 | 8.545 | 5.536 | 36.904 | 13.220 |
| 2018 | 10.042 | 13.001 | 8.758 | 5.996 | 37.797 | 15.134 |
| 2019 | 10.017 | 13.236 | 8.952 | 6.483 | 38.688 | 16.814 |
| 2020 | 9.981 | 13.459 | 9.130 | 7.004 | 39.573 | 18.298 |
| 2021 | 9.936 | 13.667 | 9.296 | 7.562 | 40.461 | 19.623 |
| 2022 | 9.873 | 13.854 | 9.448 | 8.162 | 41.338 | 20.822 |
| 2023 | 9.812 | 14.029 | 9.587 | 8.806 | 42.235 | 21.925 |
| 2024 | 9.750 | 14.198 | 9.724 | 9.502 | 43.175 | 22.972 |
| 2025 | 9.641 | 14.322 | 9.850 | 10.257 | 44.069 | 23.976 |
| 2026 | 9.497 | 14.401 | 9.951 | 11.068 | 44.917 | 24.948 |
| 2027 | 9.341 | 14.447 | 10.015 | 11.929 | 45.731 | 25.900 |
Appendix 4 Equations used within the frailty dynamics system dynamics population model
Introduction
The equations included in the population model are used to estimate the number of patients in certain categories at a certain point after the start of January 2006.
The six categories we are interested in are:
-
The number of patients in each of the age/frailty groups who are alive at time t.
-
The number of patients that change frailty status (remaining in the same age group), per month – assuming worsening of frailty, captured by the eFi score: that is, Fit to Mild, Mild to Moderate, Moderate to Severe.
-
The number of patients that age and move into the next age group, per month – assuming they stay in the same frailty category.
-
The number of new patients joining each of the age/frailty groups, per month.
-
The number of patients that die each month.
-
The number of patients that deregister or are lost to follow-up each month.
Solving differential equations related to these six categories will provide estimates of the number of patients in each age/frailty group over time (the stocks and flows within our model).
We have 16 population subgroups – our over 50s population is divided into four age bands (50–64, 65–74, 75–84 and 85 +) and each band has four measures of frailty according to the patients’ eFi scores (Fit, Mild, Moderate and Severe).
In the population model based on the RCGP RSC cohort data, we are initially considering 12 years from 1 January 2006 through to 31 December 2017 with a monthly timestep.
Solving the model equations: high-level approach
As the equations within our model cannot be typically solved analytically to get a nice easy expression to work with, we use a numerical approximation algorithm. This involves starting with an initial number of patients in each of our 16 age/frailty groups and then adding on/subtracting a given number of patients each month (Equation 1). The number of patients that are added on/removed depends on a group of equations (currently 80 for the population model due to the 16 population subgroups). Full details of the equations can be provided on request.
The typical structure of the equation capturing the monthly change in each age/frailty population subgroup is as follows with the variables described in Table 46:
| Variable | Description |
|---|---|
| entryflowj | Number of patients that join an age/frailty subgroup in the population |
| frailtytransitionflowj−1 to j | Number of patients who have moved from a lower frailty category in the previous month to their current frailty score, e.g. from Fit to Mild |
| frailtytransitionflowj to j+1 | Number of patients who during the month have moved into the next frailty category, e.g. from Mild to Moderate |
| ageing flowj | Number of patients that move from the current age band into the next age band, e.g. those that were in the 65–74 group turn 75 |
| ageing flowj−1 | Number of patients who have aged during the month, e.g. those that have recently turned 65 and were previously in the 50–64 age band |
| deathsj | Number of patients in a population subgroup that die during the month |
| deregistrationsj | Number of patients that have deregistered from a RCGP RSC GP practice/are lost to follow-up in the month |
The expressions for the entry flow are typically of the form given in Equation 2 where a, b, c and d are constants.
The expressions for the frailty transition flows are typically of the form given in Equation 3 where a, b, c and d are constants.
The expressions for the ageing flows are typically of the form given in Equation 4 where a, b, c, d and e are constants.
The expressions for the exit flows due to death are typically of the form given in Equation 5.
The expressions for the exit flows due to deregistration are typically of the form given in Equation 6.
List of abbreviations
- A&E
- Accident and Emergency
- BMI
- body mass index
- CAG
- Confidentiality Advisory Group
- CGA
- Comprehensive Geriatric Assessment
- DARS
- Data Access Request Service
- ED
- emergency department
- eFI
- electronic Frailty Index
- EHR
- electronic health record
- FI
- frailty index
- GLM
- generalised linear model
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HRA
- Health Research Authority
- ID
- identifier
- IDAOPI
- Income Deprivation Affecting Older People Index
- IMD
- Index of Multiple Deprivation
- LSOA
- lower super output area
- LTC
- long-term condition
- MDT
- multidisciplinary team
- MSM
- multistate Markov
- ONS
- Office for National Statistics
- PPIE
- public patient involvement and engagement
- PYAR
- person-years at risk
- RCGP RSC
- Royal College of General Practitioners Research and Surveillance Centre
- RDS
- Research Design Service
- REC
- Research Ethics Committee
- SAIL
- Secure Anonymised Information Linkage
- SD
- system dynamics
- SEG
- Stakeholder Engagement Group
- SO
- study outcome
- St.D
- standard deviation
- TRE
- trusted research environment
