Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number 16/116/82. The contractual start date was in June 2018. The final report began editorial review in February 2020 and was accepted for publication in September 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Morris et al. This work was produced by Morris et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Morris et al.
Chapter 1 Context
Overview
Rare diseases affect many people, more than 3.5 million in the UK alone. Poor co-ordination of care is a problem faced by many people affected by rare diseases. In this introductory chapter we consider what care co-ordination means in the context of rare diseases, the problem addressed by this research and the aims and objectives of the research. In this report we use the terms ‘rare conditions’ and ‘rare diseases’ interchangeably to refer to rare, ultra-rare and undiagnosed diseases and conditions.
What does care co-ordination mean?
The focus of this study is the co-ordination of care for people affected by rare diseases. A systematic review conducted in 2007 reported no single agreed definition of co-ordinated care and proposed the following working definition:
Care co-ordination is the deliberate organisation of patient care activities between two or more participants (including the patient) involved in a patient’s care to facilitate the appropriate delivery of health care services. Organising care involves the marshalling of personnel and other resources needed to carry out all required patient care activities and is often managed by the exchange of information among participants responsible for different aspects of care. 1
As part of our research, we sought to improve this definition in the context of rare diseases.
What is the problem being addressed by this research?
There are an estimated 6172 unique rare conditions. 2 Each rare disease affects fewer than 1 in 2000 of the population,3 but combined they affect a large number of people, approximately 3.5 million in the UK4 and 30 million in the European Union. 3 The problem being addressed by this research is the variation in how care is co-ordinated for people affected by rare diseases in the UK (and in many cases the complete lack of care co-ordination), depending on where they live and the disease they are affected by.
Rare diseases are often serious, chronic and complex in nature, affecting multiple systems of the body. As a result, several health-care professionals (HCPs) are often involved in patients’ care. Many people need access to a number of different NHS services to receive the care they need, including care by specialists and care nearer to home. Care by specialists may require travelling long distances and staying away from home, which can be inconvenient, costly and stressful. Care nearer to home may involve care by the local hospital or general practitioner (GP). Receiving care from a range of people, including specialists and local providers, can cause problems because co-ordination between the different professionals and services is often poor, care plans may not be in place or followed and some patients may have gaps in their care because they do not see the right professionals and, when they do, the information to facilitate appropriate care may not be to hand. Parents/carers of children with rare conditions often face a significant care burden, needing time off work to look after their children and take them to appointments. There can also be challenges in ensuring continuity of care when children transition from paediatric to adult services.
Why is this research needed?
There is evidence to suggest that care is poorly co-ordinated for people affected by rare diseases. In addition, improving care co-ordination for people affected by rare diseases has been raised as a major concern by policy-makers.
In January 2016, Rare Disease UK (London, UK) published results from a survey of more than 1200 people (patients and carers) affected by rare diseases, which found that information on test and procedure results and treatment was not shared effectively between services, meaning that patients may have received suboptimal treatment. 5 The survey also found that patients and families frequently had to attend multiple clinics and travel significant distances to reach them. For example, one in three respondents had to attend three or more clinics and 12% of respondents attended more than five different clinics. Respondents attended clinics monthly (23%), every 6–8 weeks (32%), quarterly (55%) or at least once a year (92%). 5
In addition, not only did patients have to frequently visit multiple clinics, but nearly half the survey respondents reported that they travelled for more than 1 hour to get to their furthest clinic, with 11% of respondents reporting that they had to travel for more than 3 hours. 5 The survey found that 81% of patients did not have a care co-ordinator or advisor, and a further 8% of patients were unsure whether or not they did. The survey also found that 40% of respondents did not know if there was a specialist centre for their condition. Of the patients who were aware of a specialist centre for their condition, only 66% used it. 5 These data illustrate the heavy burden that poor care co-ordination places on patients and families dealing with rare diseases, which could be improved by better co-ordination.
In September 2016, Genetic Alliance UK (London, UK) undertook a study to identify the hidden costs of rare diseases in the UK. 6 The aims of the study were to examine how services are co-ordinated for patients with rare diseases, what is known about the impact of the lack of co-ordinated care, what costs and outcomes are important to patients and families, and how these data might best be collected. The study involved interviews with patients, families and patient organisations. The main conclusions were that receiving co-ordinated care is important for patients with rare diseases, yet remains a challenge; the full costs and benefits associated with different models of care for patients with rare disease are unknown; patients and families face significant hidden costs, both financial and psychosocial, associated with the way their care is managed; and there are limitations associated with existing research and data sets for rare diseases.
The problem of poor co-ordination of care for patients with rare diseases has also been highlighted by the UK governments, although the evidence base is largely anecdotal. In 2013, the Department of Health and Social Care, Northern Ireland Executive, Scottish Government and the National Assembly for Wales published The UK Strategy for Rare Diseases,7 which said that it was essential to co-ordinate care for people with rare diseases. The strategy also stated that more needed to be done to improve co-ordination and that research was needed on how care for people with rare diseases should be co-ordinated. In the progress report from the All Party Parliamentary Group on rare, genetic and undiagnosed conditions it was noted that care continues to be badly co-ordinated. 8
More recently, the UK government, and patients and families, further highlighted the problem of co-ordinated care for people affected by rare diseases in The UK Rare Diseases Framework. 9 The UK Rare Diseases Framework9 restated that co-ordination of care was one of the top challenges facing people affected by rare diseases and better co-ordination was listed as one of the four top priorities. In addition, better co-ordination was also listed as one of the four major challenges facing the rare diseases community. In a ‘national conversation’ survey of 6293 members of the UK rare diseases community, conducted in 2019, co-ordination of care was identified as the top challenge by 16% of patients, 19% of families and carers, 11% of rare disease patient organisations and 18% of HCPs (Table 1). 9 We note that, although co-ordination of care was noted as a major challenge in its own right, any improvement in co-ordination is also likely to have a positive impact on the other challenges (e.g. by improving diagnosis, awareness and access).
| Challenge | Stakeholder group (%) | |||
|---|---|---|---|---|
| People living with a rare disease | Family members and carers | Rare disease patient organisations | HCPs | |
| Getting the right diagnosis | 30 | 17 | 29 | 18 |
| Awareness of the rare disease among HCPs | 19 | 17 | 14 | 14 |
| Access to specialist medical care and treatment | 17 | 14 | 16 | 11 |
| Co-ordination of care | 16 | 19 | 11 | 18 |
Unfortunately, although there are indications that care needs to be better co-ordinated for people affected by rare diseases, there is not good evidence as to how this should be achieved. A 2013 report by Rare Disease UK10 provided anecdotal evidence of the benefits of having a named care co-ordinator and concluded that there was a strong case for investment in care co-ordinator posts, although quantitative evidence was lacking. Van Groenendael et al. 11 analysed the national service for an ultra-rare disease (Alström syndrome) and compared outcome and cost of the service with standard care. 11 Van Groenendael et al. 11 found that organised multidisciplinary ‘one-stop’ clinics achieved better outcomes than standard care, at similar costs. Indicative of the lack of evidence about how to improve care co-ordination for people affected by rare diseases, The UK Strategy for Rare Diseases7 called for further research in this area, in particular around how care for people with rare diseases is co-ordinated and how best it ought to be co-ordinated. Our study aimed to address these gaps.
Aims and objectives
Aims
The aims of this study were to use quantitative and qualitative research methods to investigate (1) if, and how, care of people with rare diseases is co-ordinated in the UK and (2) if, and how, patients and families affected by rare diseases, and HCPs who treat rare diseases, would like care to be co-ordinated.
Objectives
-
To undertake a scoping review to identify what ‘co-ordinated care’ means, what the components of co-ordinated care are and to identify in what ways, and why, co-ordinated care for people with rare diseases might be similar to or different from co-ordinated care for people with other conditions.
-
To understand if and how care of people with rare diseases is co-ordinated in the UK.
-
To analyse preferences for different models of co-ordinated care by patients, families and HCPs.
-
To develop a taxonomy describing how care for people with rare diseases could be co-ordinated.
-
To calculate the costs of the models of co-ordinated care identified in the taxonomy.
-
To work closely with patients and families throughout the project and disseminate findings widely.
Research questions and overview of the research project
The research questions (RQs) we addressed to meet the aims and objectives were as follows.
Research question 1
What does ‘co-ordinated care’ mean, what are the components of co-ordinated care and in what ways, and why, may co-ordinated care for people with rare diseases be similar to or different from co-ordinated care for people with other conditions?
Research question 2
Is care for people with rare diseases in the UK co-ordinated and, if so, how?
Research question 3
What are the preferences of patients, families and HCPs in relation to how care for rare diseases is co-ordinated?
Research question 4
What are the different ways in which care for people with rare diseases might be co-ordinated?
Research question 5
How much do these options cost?
Our study was interested in exploring all spectrums of co-ordination (from a lack of co-ordination through to good co-ordination).
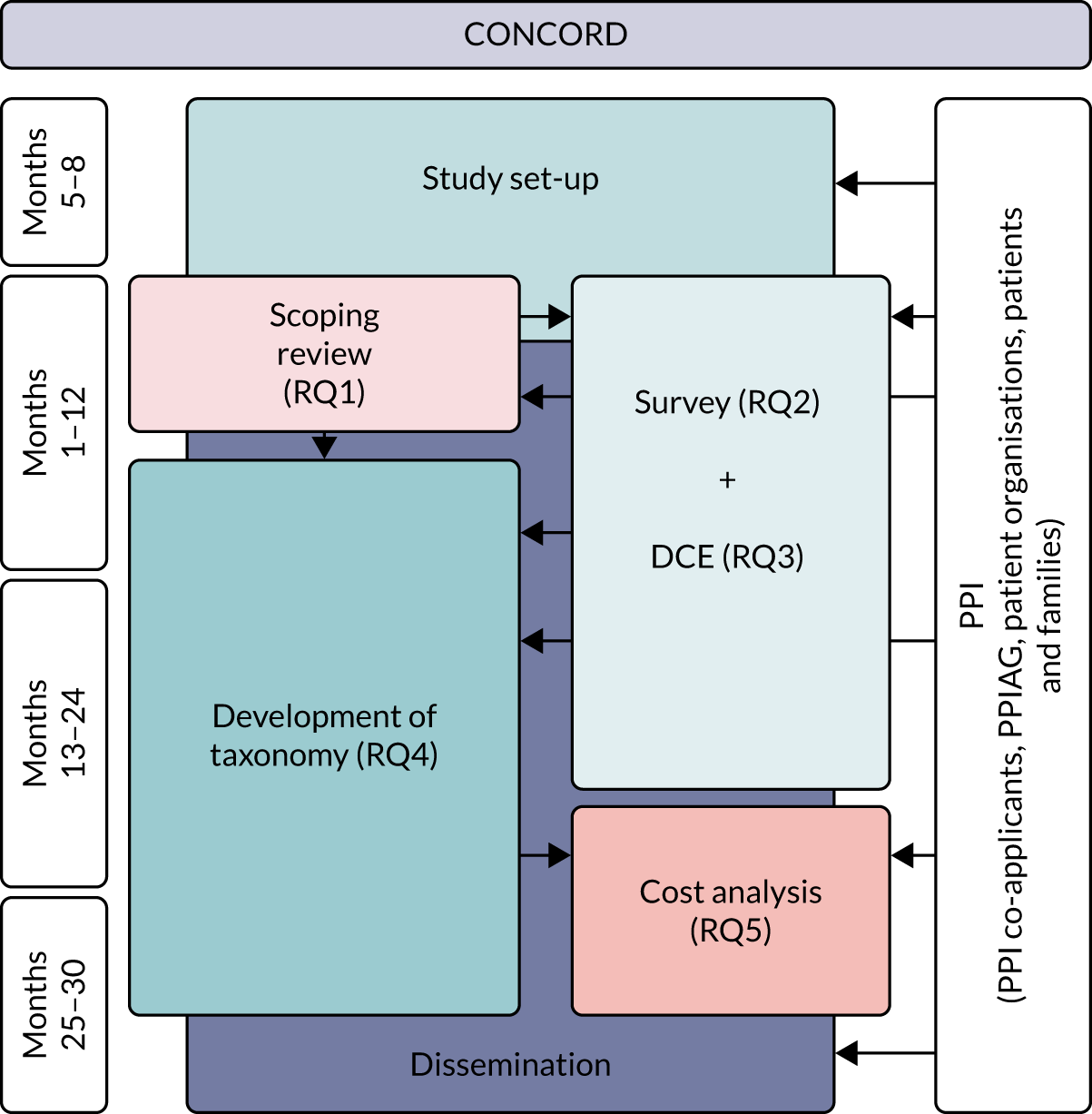
The study was funded by the National Institute for Health Research (NIHR) Health and Social Care Delivery Research programme from June 2018 to February 2021. The CONCORD (Co-ordinated Care Of Rare Diseases) study timeline is summarised in Figure 1. For RQ1, we undertook a scoping review (not only in rare diseases) that focused on care co-ordination across organisational boundaries and interventions employed to support and improve this. For RQs 2 and 3, we created a questionnaire-based survey of current experiences and costs, incorporating a discrete choice experiment (DCE) of preferences for co-ordination. In addition, we undertook an exploratory qualitative interview study to understand the impact of a lack of co-ordinated care on patients and carers, and preferences for co-ordination. For RQ4, we drew on the findings of the scoping review and also carried out interviews, focus groups and workshops with a range of stakeholders to develop a taxonomy of co-ordinated care for rare diseases. For RQ5, we reviewed the costs of different components of co-ordinated care.
FIGURE 1.
The CONCORD study flow chart. PPI, patient and public involvement; PPIAG, Patient and Public Involvement Advisory Group.
Structure of the report
This report is structured as follows.
Chapter 2 presents the overarching design of the study and provides an overview of the methods employed. (Detailed information on methods is presented within each of the findings chapters.)
Chapter 3 presents the methods and results of a scoping review to define co-ordinated care for people living with rare conditions. Chapter 3 extends previous research by providing an updated definition of co-ordinated care for rare conditions, and by identifying and categorising components of co-ordination according to their role within complex care processes.
Chapter 4 presents an exploratory qualitative interview study of patients affected by rare conditions and their carers, exploring how these groups are affected by whether or not care is co-ordinated, and the factors that might influence effective care co-ordination.
Chapter 5 presents the findings from a national cross-sectional survey of patients, parents/carers and HCPs about different aspects of care co-ordination for rare diseases, including the use of specialist centres, care co-ordinators and care plans.
Chapter 6 presents the findings of a DCE to evaluate preferences of patients, parents/carers and HCPs for characteristics of co-ordinated care.
Chapter 7 outlines the development and refinement of a taxonomy of care co-ordination for people living with rare conditions. The taxonomy outlines the six domains involved in co-ordinating care for rare conditions.
Chapter 8 presents selected models of co-ordinated care from the taxonomy and illustrative costs of different components of co-ordinated care.
Chapter 9 presents a discussion of our findings linked to our RQs and the implications for health services and research.
Chapter 2 Research methods
Overview
In this chapter we provide an overview of the design and methods employed in the CONCORD study. We outline the qualitative and quantitative methods used. Further information on methods is presented within each of the chapters that follow.
Methods
Setting
This study is concerned with how people with rare conditions are cared for across organisational settings in the UK, including the NHS, the social care sector and the third sector. The primary focus for this study was NHS care, but we were also interested in providers that are gatekeepers to social care provision and third-sector care, as significant elements of co-ordination specifically relate to the integration between health care and these other sectors. To identify as many different models of co-ordination as possible, no limits were set on the rare conditions included or where people lived in the UK.
Overview of approaches
We used the following methods in our research:
-
a scoping review (not only in rare diseases) that focused on care co-ordination across organisational boundaries, and interventions employed to support and improve this
-
an exploratory qualitative interview study to understand the impact of a lack of co-ordinated care on patients and carers
-
a questionnaire-based survey of current experiences, incorporating a DCE of preferences for co-ordination
-
interviews, focus groups and workshops with a range of stakeholders to develop a taxonomy of co-ordinated care for rare diseases
-
a review of the costs of providing co-ordinated care.
There were numerous interdependencies between the different components of the study (see Figure 1). The scoping review provided the theoretical underpinnings for the taxonomy of co-ordinated care and informed the content of the survey, the DCE and what is known about the costs of co-ordinated care. The exploratory qualitative interviews informed the scoping review, the survey and the DCE. The survey and DCE helped to identify different models of care co-ordination, which were used to create the taxonomy. In addition, the survey and the DCE were also intended to provide data for the cost analysis of the different co-ordination models, which were, in turn, based on the taxonomy (which delineated the options to be costed).
Study participants
Study participants comprised patients (aged ≥ 18 years) affected by a rare condition, parents/carers (aged ≥ 18 years) of children or adults with rare conditions, HCPs (e.g. doctors, nurses and allied health professionals) involved in the care of people with rare conditions, national leads on specialist health-care commissioning, national patient groups and charities, and local providers and commissioners of co-ordinated care. More specifically, participants were involved in various elements of the research as follows:
-
To find out if the scoping review findings applied to rare conditions and to support the design of the survey and DCE, we undertook three focus groups. The focus groups were as follows:
-
one virtual focus group with seven patients and carers affected by rare diseases
-
one face-to-face focus group with six patients and carers affected by rare diseases
-
one face-to-face focus group with four HCPs.
-
-
To explore the impact of unco-ordinated care, and to support the design of the survey and DCE, we conducted interviews with 15 patients and carers affected by rare diseases [14 interviews were via telephone and one interview was via Skype™ (Microsoft Corporation, Redmond, WA, USA)].
-
To ensure that the survey was worded appropriately and contained appropriate questions, we undertook a pilot study of the survey and DCE questionnaire with 24 patients, carers and HCPs. The study comprised four think-aloud interviews and 20 interviews that provided written or verbal feedback.
-
For the survey and DCE, we obtained 1457 responses from 760 patients affected by rare diseases, 446 parents/carers and 251 HCPs.
-
To develop the taxonomy, we planned to undertake up to 30 national and local stakeholder interviews. These interviews included national leads on specialist health-care commissioning, national patient groups and charities, local providers of co-ordinated care (including health care, social care and the voluntary sector) and local commissioners of co-ordinated care.
-
To develop the taxonomy, we undertook 30 interviews with HCPs, charity representatives and commissioners, and four focus groups involving a total of 22 patients and carers affected by rare diseases.
-
To refine the taxonomy, we conducted two workshops with 15 attendees each. Workshop participants included adult patients (aged ≥ 18 years) and carers of adult patients, carers of younger patients (aged < 18 years), care providers (including health care, social care and the voluntary sector) for adults with rare conditions, care providers (including health services, social services bridging health and social care and the voluntary sector) for children with rare conditions, and commissioners of co-ordinated care provision, including NHS England and local authorities.
Participants were accessed via patient and provider networks and organisations.
Patient and public involvement
To meet our aims, the study required substantial input from patients and families. The research team included representatives from a national charity that is an alliance of more than 180 patient organisations (Genetic Alliance UK) and from national patient organisations with direct experience of living with rare conditions. These representatives ensured that patients’ and families’ priorities and needs remained the focus of the study, and contributed to the design and management of the study, patient recruitment, data collection, interpretation of findings and dissemination. In addition, these representatives ran the study’s Patient and Public Involvement Advisory Group (PPIAG), which involved managing and working with a group of six to eight patients and carers and meeting twice a year for the duration of the project. The PPIAG supported the development of resources and participant information, patient recruitment and dissemination of findings.
Ethics approval
This study received ethics approval from University College London Research Ethics Committee (reference 8423/002) and the London–Surrey Borders Research Ethics Committee of the Health Research Authority (reference 19/LO/0250).
Overview of research methods
Scoping review
The scoping review was designed to help us understand what aspects of co-ordinated care could or should be provided for people with rare conditions, and help us build on what was already known about co-ordinated care in other contexts that might be used to enhance co-ordinated care for rare conditions. The scoping review had six stages. 12
Stage 1: defining the research questions
In stage 1, we developed three RQs.
Stage 2: identifying relevant studies
In stage 2, we conducted a review of reviews about care co-ordination for chronic conditions in general, not just rare conditions, to identify factors important to co-ordinated care. We searched for evidence from a range of different sources, including electronic databases, hand-searching of key journals and reference lists of retrieved studies. We limited the search to studies published after 2006 (as a comprehensive 2007 review1 included papers published up to 2006). Reviews published in peer-reviewed journals, as well as grey literature, were included.
Stage 3: study selection
In stage 3, selection criteria were developed iteratively and reviews were included if they focused on care co-ordination in some form, provided a definition of co-ordinated care, identified components of co-ordinated care and focused on patients with rare conditions, chronic conditions or long-term conditions. Identified studies were screened in three phases (i.e. title, abstract and full text) and a percentage were screened by a second researcher.
Stage 4: charting the data
In stage 4, we extracted data, including the characteristics of co-ordination, from the identified reviews.
Stage 5: collating, summarising and reporting results
In stage 5, we presented an overview of materials reviewed and a thematic analysis of their results.
Stage 6: stakeholder consultation
In stage 6, draft findings were shared with three focus groups and these were used to develop our analysis and interpretation of findings, including whether co-ordinated care for people with rare conditions is similar to or different from those in other contexts.
For further details about the methods employed, see Chapter 3, Methods.
Survey
We conducted a national survey to understand how care of people with rare conditions was co-ordinated in the UK. The questionnaire incorporated a DCE to quantify what aspects of care co-ordination participants preferred.
The content of the questionnaire was informed by 15 semistructured qualitative interviews with patients and carers to identify costs associated with living with rare conditions. These interviews were also used for the exploratory qualitative study to investigate the impact on patients and carers of having care that was not co-ordinated.
Survey participants were adult patients (aged ≥ 18 years) affected by a rare condition, parents/carers (aged ≥ 18 years) of children or adults with rare conditions and HCPs (e.g. doctors, nurses and allied health professionals) involved in the care of people with rare conditions. The target number of responses for each of these three groups was at least 300, with an overall target sample size of 1500. Participants were required to live in the UK, but there were no restrictions in terms of rare condition, demographic factors or geographical location within the UK.
Participants were accessed through patient networks and organisations, and through care providers and regional genetics services.
We produced a draft of the questionnaire based on the outputs of the in-depth interviews and scoping review (including the focus groups). This was reviewed by the PPIAG and amended accordingly. We then piloted the survey and made amendments according to feedback received. The survey was then finalised in discussion with the PPIAG.
The questionnaire covered a variety of topics, including experience of diagnosis, rare condition, availability/role of care co-ordinators, content and use of care plans, availability/role of specialist centres, use of health services and perceived impact of care co-ordination on quality of care.
A survey company generated online, electronic and hard-copy versions of the questionnaire ready for circulation. Most respondents completed the questionnaire via a weblink to the online questionnaire, which was made available on a dedicated website. Participants were also given options to complete the survey by mailed hard copy, electronically by e-mail or by telephone.
Analyses of the data were descriptive. The results of the categorical, ordinal and interval questions were reported as frequencies and percentages, or means and medians, with corresponding measures of spread [e.g. confidence intervals (CIs) or interquartile ranges].
For further details about the methods employed, see Chapter 5, Methods.
Discrete choice experiment
We undertook a DCE to investigate preferences for care co-ordination. 13 A DCE is a quantitative method used to elicit preferences from participants without directly asking them to state their preferred options. 14 The DCE formed one part of the survey questionnaire, eliciting preferences for the way in which care is co-ordinated for the three participant groups. A longlist of attributes was drawn from the scoping review and was shortened to six attributes based on feedback from the interviews, focus groups and the PPIAG. The levels of each of the attributes were based on feasible ranges derived from reviews of documentary evidence from the scoping review and feedback from the PPIAG and interviews. The DCE used a pairwise choice framework, describing combinations of levels and attributes of different models of co-ordinated care, including main effects only. We reduced the total number of feasible pairwise choice questions to 18, which were split into three blocks of six (i.e. each participant completed six choice questions). We also asked respondents to provide a simple ranking of the attributes according to importance.
The DCE data were analysed using conditional logit analyses. We selected this type of regression model given our focus on identifying which attributes significantly affect preferences, and which attributes are most and least important to respondents, conditional on the other attributes in the analysis. We tested for differences in preferences between responder groups. We calculated marginal rates of substitution (MRSs) with respect to costs, dividing the coefficient for each attribute by the coefficient for the cost attribute to calculate the ‘willingness to pay’ for each attribute. We also calculated the predicted probability that different combinations of the attribute levels would be selected, allowing us to rank different models of co-ordinated care in terms of their order of preference by the participants.
For further details about the methods employed, see Chapter 6, Methods.
Taxonomy of models of co-ordinated care
We developed and refined a taxonomy (classification) of different models describing how care for people with rare conditions could be co-ordinated. To do this, we conducted interviews and focus groups with stakeholders to derive a draft taxonomy. The sampling framework was designed to capture experience with different models of co-ordinated care. We aimed to conduct up to 30 interviews with national leads on specialist health-care commissioning, national patient groups and charities, and local providers and commissioners of co-ordinated care. We also conducted four focus groups with patients and carers. We then ran a series of workshops to discuss the draft taxonomy. We aimed to run up to five workshops, each with up to 20 attendees. Owing to COVID-19, we ended up amending the study to include two virtual workshops (with up to 15 attendees each), instead of five face-to-face workshops. To recruit for the interviews, focus groups and workshops, we used a range of methods, including e-mail invitation, social media, voluntary sector recruitment and recruitment via our partnerships with four NHS sites.
The interviews and focus groups used topic guides that focused on key aspects of care co-ordination, including use of specialist clinics, information-sharing between specialist and local services, transition from child to adult services, implications of co-ordination on clinic attendance and travel distances, and influential factors affecting the ability to provide co-ordinated care. The sessions were digitally recorded and professionally transcribed. Iterative and thematic analysis of all data were undertaken concurrently, accounting for outputs from the scoping review, the survey and the DCE. To develop the taxonomy, a combination of inductive and deductive thematic analysis was used (see Chapter 7 for more information).
The resulting draft taxonomy was tested in consensus-building workshops, which aimed to produce recommendations about the taxonomy (see Chapter 7 for more information). A final taxonomy was developed based on workshop feedback.
For further details about the methods employed, see Chapter 7, Methods.
Cost of co-ordinated care
We used the findings from the taxonomy to develop hypothetical models of co-ordinated care (see Chapter 8, Methods, for further details of the development process). These hypothetical models give an example of what co-ordinated care may need to look like in different situations. We aimed to calculate the costs of these models of co-ordinated care using data from both the national survey (see Chapter 5) and the workshops used to refine the taxonomy (see Chapter 7). Unfortunately, it was not possible to use either of these sources. In the case of the survey, most people did not experience co-ordinated care. In addition, it was not possible to attribute the hypothetical models that were developed to survey respondents. In the case of the workshops, the health service utilisation associated with each hypothetical model was unknown by workshop participants, primarily because this was likely to vary according to situation-specific factors. The result was that it was not possible to generate costs associated with each hypothetical model from the survey data or workshop data. Instead, we undertook a review of the costs of different components of co-ordinated care to illustrate indicative costs. For further details, see Chapter 8, Methods.
Chapter 3 Methods and results of a scoping review to define co-ordinated care for people living with rare conditions
Overview
This chapter draws on a paper by Walton et al. 15 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
What was already known?
-
Co-ordinating care may be beneficial to patients and carers, as it may ease treatment burden.
-
Many terms and definitions have previously been used to refer to co-ordination of care (mostly for common chronic conditions).
What this chapter adds
-
This chapter extends previous research by providing an updated definition of co-ordinated care for rare conditions.
-
This chapter extends previous research by identifying and categorising components of co-ordination according to their role within complex care processes.
-
This chapter highlights similarities and differences between co-ordination for common and rare conditions (i.e. many of the components apply to both common and rare conditions, but that there are additional components and context-specific issues that are relevant for rare conditions).
Background
To co-ordinate care more effectively for people living with rare conditions, we need to be able to define what co-ordination means. A clear definition could help researchers and stakeholders to understand care co-ordination for rare conditions and identify situations where services are not co-ordinated and may require improvement. Identifying key components of co-ordination could help researchers to (1) develop care co-ordination programmes and evaluate whether or not components are delivered in practice, (2) identify potential costs, (3) standardise delivery of care co-ordination programmes (where appropriate)16 and (4) identify components that are applicable to both common and rare conditions or that are most relevant to rare conditions. To the best of our knowledge, there have been no previous reviews that have focused on care co-ordination for rare conditions only6 and, therefore, it would not have been possible to focus this review on rare conditions alone. Many, if not most, rare conditions are chronic lifelong conditions. ‘Chronic disease’ is an umbrella term used to refer to a range of long-term conditions, including both common and rare conditions. Therefore, it seemed appropriate to focus this review of reviews on co-ordination for chronic conditions (including both common and rare chronic conditions). Some reviews have been conducted into care co-ordination for chronic conditions;1,17 however, there was a need to update these reviews to include new evidence, given that organisation and technological context for care is likely to have changed significantly since the previous reviews. Therefore, this review of reviews updates our understanding of care co-ordination for common and rare chronic conditions. This review will also extend previous research by supplementing review findings with stakeholder consultations with patients and HCPs who have experience of rare conditions. This will help us to understand if definitions and components of care co-ordination are shared across common and rare chronic conditions or if some are specific to rare conditions.
This review of reviews aimed to extend previous knowledge by providing, to the best of our knowledge, one of the first reviews of care co-ordination for rare conditions. We aimed to:
-
provide an updated definition of co-ordination of care for chronic conditions (both rare and common)
-
identify key components of care co-ordination for chronic conditions (both rare and common)
-
explore whether or not findings apply to rare conditions.
Methods
We followed a recommended systematic approach to our scoping review. We carried out the following six steps:12 (1) defined the RQs, (2) identified relevant studies, (3) selected reviews, (4) charted the data, (5) collated, summarised and reported the results and (6) consulted with stakeholders (Table 2). We followed reporting standards for scoping reviews. 27
| Scoping review stage | Description of our method |
|---|---|
| Defined RQ | All co-authors developed three RQs:
|
| Identified relevant studies | Information sources:
|
| Selected reviews |
|
| Charted data |
|
| Collated, summarised and reported results |
|
| Consultation with stakeholders | Sample:
|
A scoping review methodology was appropriate for this review, as defining co-ordinated care and identifying components of co-ordinated care for common and rare conditions is a broad topic that requires accumulation of evidence from a range of study designs. 12
Stages of the scoping review
We followed these six stages to complete the scoping review.
Stage 1: defined research questions
We developed three RQs (see Table 2).
Stage 2: identified relevant studies
We searched electronic databases, hand-searched key journals and reference lists of included reviews and asked experts to identify missing papers. Details of our search and inclusion and exclusion criteria are provided in Table 2 (details of the search terms are provided in Appendix 1).
Stage 3: selected reviews
One reviewer conducted the search and reviewed titles, abstracts and then full texts against the exclusion criteria. A percentage of titles (40%), abstracts (30%) and full texts (5%) were independently screened by a second researcher. Researchers met to discuss discrepancies.
Stage 4: charted data
A data charting form was developed and used to chart data for all included reviews. Data included aims of the review, type of review, outcome measures and results in relation to definitions of co-ordinated care and components of co-ordinated care. We defined components as individual aspects of care that may be important for co-ordination. We extracted information on definitions and components of co-ordination from the whole review paper. We extracted all components reported in review papers (including those reported from individual studies within the review). Although we have research demonstrating the potential benefits of co-ordinated care (see Chapter 4), we do not yet know what effective co-ordination looks like. Therefore, we did not judge effectiveness of components. Instead, we aimed to identify components across the spectrum (e.g. from lack of co-ordination through to potentially good co-ordination). A second researcher extracted data from 10% of reviews. Discrepancies were discussed and resolved.
Stage 5: collated, summarised and reported results
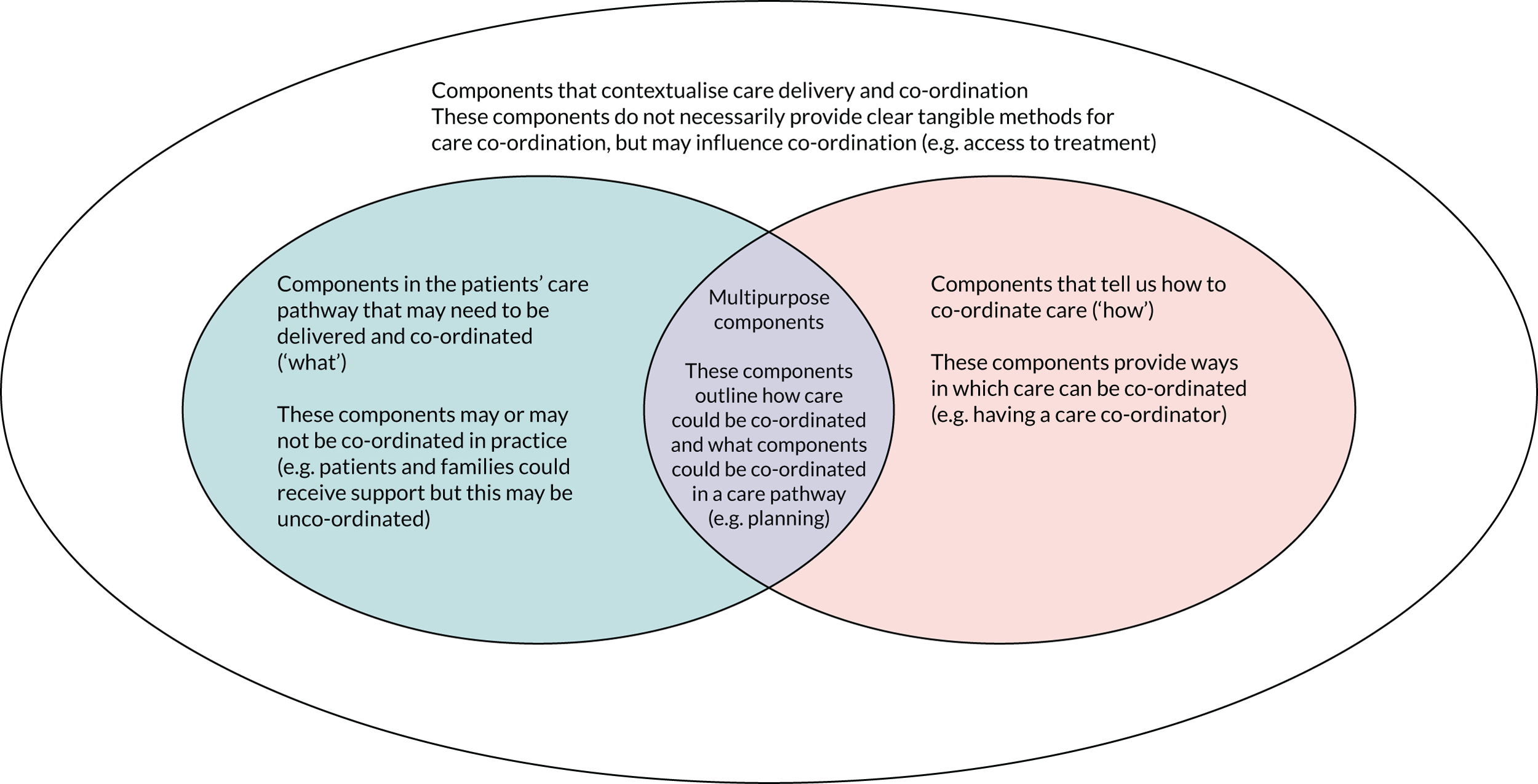
Narrative synthesis was used28 to develop definitions and identify and group components. A second researcher grouped 10% of components independently. To develop a definition of co-ordinated care, we coded individual definitions inductively. Codes were grouped by one researcher and used to develop a preliminary definition, which was reviewed and amended by the wider research team. Components were coded and grouped by one researcher. Examples of groups included ‘planning’, ‘methods of co-ordination’ and ‘approaches of co-ordination’. A second researcher double-coded 10% of components into groups. Disagreements were discussed and resolved. Groups of components were developed into themes and subthemes, and the number of reviews that reported each theme, subtheme and component was recorded. The themes were (1) care pathway (i.e. components that related to the care pathway), (2) approaches (i.e. components relating to care/co-ordination approaches), (3) support (i.e. components relating to support), (4) features (i.e. components relating to features of care) and (5) wider environment. Each theme had a number of subthemes that each contained multiple components. Once themes and subthemes of components had been developed, individual components were then reviewed and categorised into four types of components (Figure 2). The wider research team also reviewed and agreed on the categorisation of components.
FIGURE 2.
Categorisation of components of co-ordinated care. This figure is adapted from Walton et al. 15 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Stage 6: consultation with stakeholders
Three focus groups were conducted with adults aged ≥ 18 years (two focus groups with patients and carers and one focus group with HCPs). Participants provided informed consent for participation. A structured topic guide was used to facilitate conversations (see Report Supplementary Material 1). The guide included participants’ background, thoughts on definitions of co-ordinated care, views on scoping review findings, relevance of findings to rare conditions, missing components and components that worked well or were difficult. Participants were asked to reflect on a summary of early findings, including definitions from review papers and examples of components, from the review at varying stages of the review process. The short summary included information on the purpose of the review, a summary of some of the definitions that had been found so far and a table with examples of components of co-ordination (i.e. individual aspects of care that may be important for co-ordination) that we had identified from the review. Participants were asked to read this summary before the focus group.
Focus groups were audio-recorded, transcribed (by a professional transcription company), checked for accuracy and fully anonymised. Thematic analysis was used to analyse the data. Two researchers inductively coded the focus group transcripts. Thematic analysis was used to analyse the data in relation to the three RQs. Findings were discussed with the research team and refined. Stakeholder consultation findings were used to identify the relevance of the definition and components identified from the scoping review in the context of rare diseases. The Results section in this chapter integrates both the scoping review findings and the stakeholder consultation findings to highlight aspects of stakeholder consultation findings that supported, refuted or extended scoping review findings.
Results
Review characteristics
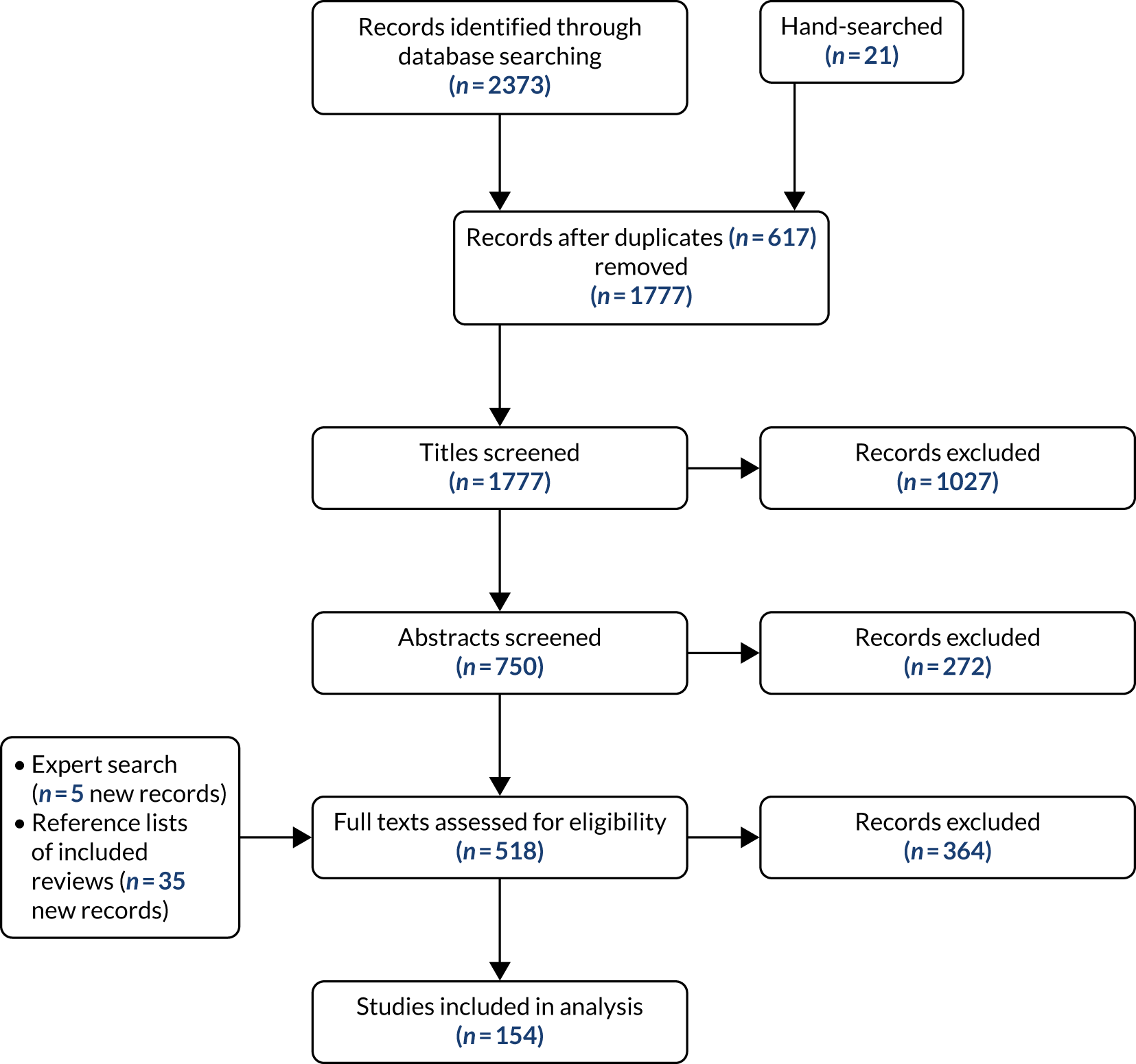
We included 154 review papers22,29–181 (see Report Supplementary Material 2 for characteristics). Figure 3 outlines the review selection process. Common chronic conditions were reviewed in 139 reviews. Only three reviews focused on a single rare condition and 12 reviews focused on both rare and common chronic conditions.
FIGURE 3.
The study selection process (based on Moher et al. 182). This figure is adapted from Walton et al. 15 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
Stakeholder consultation characteristics
Stakeholder consultation participant characteristics are shown in Table 3.
| Characteristic | Focus group (n) | Total (n) | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Mode of delivery | Virtual | Face to face | Face to face | |
| Number of participants | 7 | 4 | 6 | 17 |
| Type of participant | ||||
| Patients | 4 | N/A | 2 | 6 |
| Parents/carersa | 3 | N/A | 4 | 7 |
| HCPsb | N/A | 4 | N/A | 4 |
| Gender | ||||
| Male | 1 | 0 | 3 | 4 |
| Female | 6 | 4 | 3 | 13 |
| Age (years) | ||||
| 29–59 | 6 | N/A | 3 | 9 |
| ≥ 60 | 1 | N/A | 2 | 3 |
| Not specified | 0 | N/A | 1 | 1 |
| Diagnosis | ||||
| One specific rare condition | 4 | N/A | 3 | 7 |
| Multiple chronic conditions (including at least one rare condition) | 2 | N/A | 3 | 5 |
| Undiagnosed | 1 | N/A | 0 | 1 |
| Number of regionsc represented | 4 | 3 | 4 | 7 |
What does co-ordinated care mean for rare conditions?
Many terms and definitions were used to describe co-ordinated care (see Appendix 2 for the terms and definitions used). Stakeholder consultation findings indicated that terms and definitions were relevant for rare conditions, with some aspects emphasised [e.g. communication, expertise and multidisciplinary teams (MDTs)] or new aspects highlighted (e.g. the importance of care co-ordination being delivered equitably across geographical areas, individualisation, importance of the whole family and need to co-ordinate across a person’s whole lifetime).
From these findings, we developed a definition of co-ordinated care for rare conditions:
Co-ordination of care involves working together across multiple components and processes of care to enable everyone involved in a patient’s care (including a team of health care professionals, the patient and/or carer and their family) to avoid duplication and achieve shared outcomes, throughout a person’s whole life, across all parts of the health and care system, including: care from different health care services . . . care from different health care settings . . . care across multiple conditions or single conditions that affect multiple parts of the body, the movement from one service, or setting to another. Co-ordination of care should be family-centred, holistic (including a patient’s medical, psychosocial, educational and vocational needs), evidence-based, with equal access to co-ordinated care irrespective of diagnosis, patient circumstances and geographical location.
What are the components of co-ordinated care for rare conditions?
We identified many components of care co-ordination. Figure 4 provides a summary of components identified through the review and stakeholder consultation findings. Throughout Results, we give examples of components and the percentage of reviews (n = 154) that reported each component (see Appendix 3). We also present findings from the stakeholder consultation (see Appendix 4). In this chapter, we briefly describe the components in relation to the review findings and stakeholder consultation findings. For a more in-depth analysis of these components, please refer to the published manuscript. 15
FIGURE 4.
Summary of components of care co-ordination (from review and stakeholder consultation findings). This figure is adapted from Walton et al. 15 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
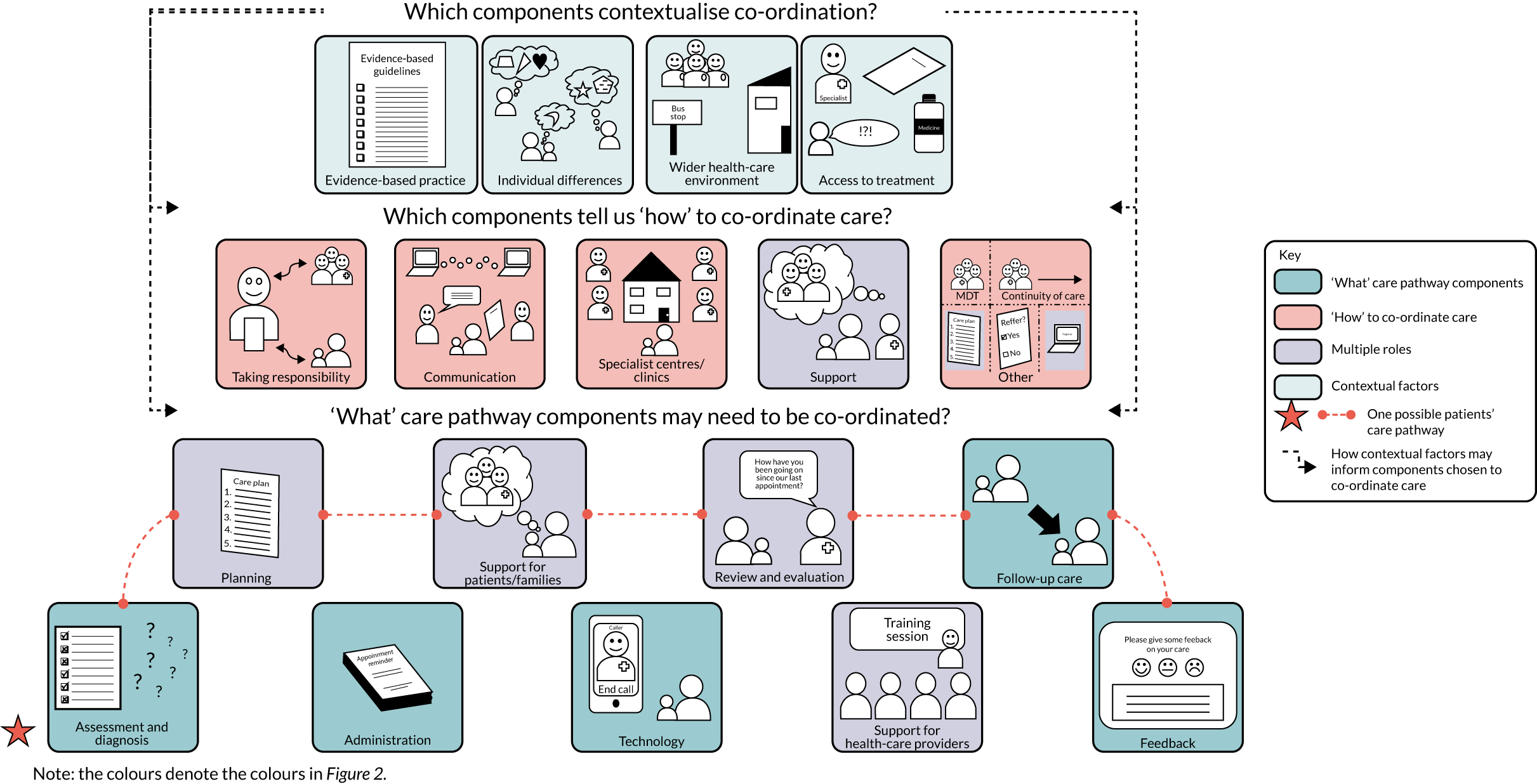
Components indicating ‘what’ care pathway components need to be co-ordinated
Scoping review findings indicated that components relating to administration, assessment and diagnosis, planning, review and evaluation, feedback, follow-up care and technology were frequently reported (development of care plans, 61.7%; follow-up care, 52.6%; monitoring, 51.3%). Stakeholder consultation findings highlighted that although these components are important, patients and carers do not always receive these components in practice (e.g. a lack of care plans and regular reviews were identified):
I’m thinking of review and evaluation with the therapist and on in-community. So, for people with rare conditions, there’ll be a period of intervention, then all will go quiet, everything’s being managed well, and then some other problem will come up, but the way therapists work is that there’ll be a period of intervention, measured outcome, closed case, no contact kept until a crisis down the road and they come back, and when I was in that position, I always wanted to be able to keep that person under review [. . .] because you were then managing a situation before it became a crisis.
FG-HCP
Scoping review findings also highlighted many support components that need to be co-ordinated, including support for patients, carers and families (education/skills training for patients, 73.4%; self-management support, 54.6%) and support for HCPs (education, 32.5%; training, 31.2%). Stakeholder consultation findings highlighted that support for patients and families across a range of needs (including medical, psychological, practical, emotional and social) and from various people (including health-care providers, peers, schools and patient support groups) is important. Findings also highlighted that support for HCPs (to access specialist knowledge and to address fears and anxieties) is necessary when co-ordinating care for rare conditions. Despite the importance of support, patients reported a lack of support and information provision for rare and undiagnosed conditions.
Components indicating ‘how’ care can be co-ordinated
Five main groups of components that outline ‘how’ care can be co-ordinated were identified. There is potential overlap between some of these components, and they are not mutually exclusive.
Someone taking responsibility
Scoping review findings highlighted frequently reported components relating to HCPs, patients and/or carers taking responsibility (co-ordination, 70.8%; responsibility for co-ordination by one health-care provider, 70.8%; patients co-ordinating own treatment, 16.9%). Stakeholder consultation findings highlighted that responsibility is key for co-ordination for rare conditions. However, it was not clear who should take responsibility (e.g. HCPs vs. patient/carers). Some participants thought that patients and carers may be best placed to co-ordinate, whereas others did not want patients to co-ordinate care:
I think having a consultant who takes the lead has been the best thing for me, that’s been the most helpful because I ring his secretary for everything and he knows that it’s his responsibility and he took responsibility but just for himself, he didn’t do it because he’s being paid to do it he just recognises that we were really sinking.
FG-PC1
Some participants wanted to be seen as partners, with control over some aspects of co-ordination, but not everything. Administrative co-ordinators were also perceived to be valuable. Findings indicated that a model of co-ordination that suits the whole family’s needs and situation should be negotiated:
Well, very similar, I mean, when it comes to co-ordination I think it’s about, for me anyway, I’d like to be in partnership with somebody rather than me doing it all but also because these sorts of illnesses, disorders, whatever, there is very little I can control about them and this might be the only thing I can have some control over but, yeah.
FG-PC2
Specialist centres/clinics
For specialist centres and clinics, components included single visit approaches (40.3%), joint clinics or consultations (14.9%) and specialist or condition-specific clinics (22.7%). Stakeholder consultation findings indicated that these components are useful for co-ordination, although some potential barriers were identified (e.g. needing funding and clinics not being delivered to standard).
Communication
Scoping review findings outlined that many components related to verbal and written communication (communication between providers and patients, 57.1%; using and sharing documentation, 46.8%; team meetings to discuss co-ordination, 76.6%). Stakeholder consultation findings highlighted a lack of communication in practice for rare conditions (e.g. lack of shared documentation, resulting in patients sharing documents between providers, and a lack of communication between professionals, resulting in patients repeating information):
. . . you know, you should have access and all those people should speak to each other because it’s an interconnected condition and they don’t, in fact it’s quite hard to find a single person who knows.
FG-PC1
Forms of identification for rare conditions may facilitate co-ordination (e.g. pendants and health passports). Many components identified in the scoping review related to technology (electronic medical records, 22.1%; teleconferencing, 22.7%; reminders for professionals, 20.1%; reminders for patients, 7.1%). Stakeholder consultation findings highlighted that technology may improve communication and, therefore, co-ordination. The need for joined-up systems was highlighted as key for co-ordination; however, this is currently not happening in practice.
Support
Scoping review findings highlighted that care could be co-ordinated through different types of support for patients and families (education and skills training, 73.4%; general support for patients, 68.2%; opportunities to familiarise with services, 9.7%; support for carers, 26%) and for HCPs (training, 31.2%; education, 32.5%; supervision, 30.5%). Stakeholder consultations highlighted the importance of patient organisations and charities that support patients and carers to develop expertise to take control over their condition and co-ordinate care. Providing patients, carers and HCPs with the opportunity to familiarise themselves with services, and development of clear expectations around co-ordination and self-management support, may help develop patients’ and carers’ expertise to co-ordinate and self-manage their care. Stakeholder consultation findings also highlight the important role that schools play in co-ordination for patients with rare conditions.
Other methods
Other components included MDT approaches (76.6%), continuity of providers (14.9%) and development of care plans (61.7%). Stakeholder consultation findings highlighted a lack of care plans for rare conditions, despite their importance for co-ordinating care in both everyday situations and emergency situations.
Components that contextualise co-ordination
Scoping review findings show that evidence-based practice (guideline-based treatment, 37%; evidence-based treatment protocols, 35.1%), individual differences and the wider health-care environment (access to care, 31.2%) may influence co-ordination. Stakeholder findings concurred with scoping review findings, but highlighted a lack of care pathways and defined standards for rare conditions and emphasised that treatments are not delivered consistently (where standards are available):
. . . it’s, having those clear pathways . . . and having something that people can, you know, work towards which is really clear . . . Even though it’s very rare, it’s, like, ‘OK, this is the process now’, and that’s really important . . . And I think that makes the systems work a lot better if there is something like that in place. I think when it’s wishy-washy or it’s not clear, or there is no clear, kind of, guidance or pathways, and because some of the situations are quite, you know, specialised, I think it’s difficult, it’s very difficult to manage.
FG-HCP
Participants reported having to travel to access care and that they were happy to do so if it meant that they received expert care. One of the key issues preventing patients with rare conditions from accessing care was a perceived limited availability of HCPs with expertise in their condition. To take limited expertise in each condition into account, findings also indicated the need to succession plan by training more experts.
Can the definitions and components of care co-ordination from common chronic conditions be applied to rare conditions?
Stakeholder consultation findings indicated that components identified through the scoping review were comprehensive and relevant for rare conditions. Although our findings highlighted that the components identified in the scoping review are relevant when co-ordinating care for people with rare conditions, we found that patients experienced a lack of co-ordination (e.g. having to attend multiple appointments on different days, gaps and delays in information-sharing and disagreements between professionals). There were particular concerns around emergencies and the necessity for patients to take control themselves to mitigate worries:
But why are we living in a world where we have to pick one or two or three of these? Why can’t it be all of them?
FG-PC1, patient
Certain factors may make it more difficult to co-ordinate care for rare conditions (e.g. difficulties in diagnosing rare conditions due to a lack of knowledge and ability to recognise symptoms and limited condition-specific expertise due to small numbers of patients).
Participants expressed views that some components were missing or may need to be emphasised for rare conditions. These included having someone to take responsibility for co-ordination, genome-based medicine/genetic screening, social support needs, counselling, and antenatal and bereavement care. In addition, participants expressed views that more focus should be given to undiagnosed patients and families for many of the components.
Discussion
Key findings
Co-ordinated care for people affected by rare conditions requires working together across multiple components and processes of care to ensure that everyone involved achieves shared outcomes throughout a person’s whole life and different parts of the health and care system. Our definition encompasses the idea that for rare conditions co-ordinated care should be family centred, evidence based and equitable for all.
These findings suggest that many of the key components and issues for co-ordinated care apply to both rare and common chronic conditions. Stakeholder consultation findings revealed additional components and context-specific issues that are relevant in the context of rare conditions.
How findings relate to previous research
Our findings extend previous research1,117 by developing a definition of care co-ordination for rare conditions.
Previous research proposed that it is difficult to distinguish between aspects of care and co-ordination components. 17,183 Our findings extend previous research, as we have grouped components according to their roles, situating components within the wider health-care pathway and environment. Our four categories overlap with those outlined in previous research,1 but clarify how co-ordination components may be involved in complex care processes.
Our review highlights that little is currently known about co-ordination for rare conditions (as most of the reviews focused on common chronic conditions). Despite similarities in need, stakeholder findings suggest that co-ordination for rare conditions may be less consistent in practice,5,8 as many of the components identified were not delivered effectively or consistently for people with rare conditions, let alone co-ordinated. These differences may be attributed to complexities associated with rare conditions (e.g. that rare conditions affect multiple body parts, children, may be lifelong, need to be co-ordinated across multiple sectors and bring complexities of diagnosis due to limited expertise). These complexities suggest that more care co-ordination is needed in cases of greater system fragmentation, clinical complexity and decreased patient capacity. 1
Limitations
We used inclusive definitions for co-ordination and chronic conditions, and reviews covered a range of countries. We are unlikely to have captured every relevant review. To identify as many studies as possible, we conducted a comprehensive search, which included contacting experts and searching the reference lists of included reviews.
It is possible that the reviews that we included in this research may not have captured all evaluations of care co-ordination in practice. Therefore, publication bias may be present. However, we used many approaches to minimise this risk, including expert consultation and stakeholder consultations.
Components reported in published descriptions of interventions do not necessarily equate to the delivery of all components and, therefore, this review is limited to the components reported in the reviews. In addition, individual studies may be included in more than one of our included reviews. We based our analysis on the wording reported in the review papers and not individual studies.
This review focused on the identification of co-ordination components, rather than testing the effectiveness of co-ordination. The effectiveness of individual components, or combinations thereof, on relevant outcomes (e.g. reduced waiting times, better health-care outcomes and better experience) is not known.
Although the scoping review provides insight into the components that are involved in co-ordination, given the small number of stakeholders included in the scoping review, it was not always possible to provide further specifics regarding whether or not different components of co-ordination may be suitable for different conditions and situations (e.g. regarding the findings on who should take responsibility for co-ordination). However, this is something that is explored in our survey, DCE and taxonomy development work (see Chapters 5–7).
Implications
Our findings provide support for various international policy initiatives. 7,19,24 We identified many components within our review that are reported as necessary for co-ordination. 7 Our findings also show that delivering co-ordinated care is complex because there are many different options for co-ordinating care. Our findings emphasised the need for someone to take responsibility for co-ordination, as outlined in the NHS Implementation Plan,19 but highlight that there are many ways in which responsibility could be managed.
Researchers and clinicians could use the components to begin to develop and evaluate existing and new models of co-ordination for common and rare chronic conditions.
Future research
This review has highlighted different components of care co-ordination. Future research could test and evaluate the clinical effectiveness, implementation and cost-effectiveness of these components in practice. This could be achieved, for example, by identifying current use of these components and evaluating them empirically, either retrospectively or prospectively.
Summary
We have defined co-ordination as working together across multiple components and processes of care to ensure that everyone involved achieves shared outcomes across a person’s whole life and throughout different parts of the health and care system. There are lots of different components that may be delivered and co-ordinated as part of a care pathway, many different ways to co-ordinate care and many factors that contextualise co-ordination. Most of the key components for co-ordinated care apply to rare and common chronic conditions, with some additional components and context-specific issues that are relevant for rare conditions.
Chapter 4 Impact of the way in which care is co-ordinated on patients with rare diseases and their carers
Overview
This chapter draws on Simpson et al. 184 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
What was already known?
-
Care co-ordination is considered important for patients with rare conditions.
-
Research addressing the impact of care co-ordination on patients and carers with experience of rare diseases is limited.
What this chapter adds
-
This chapter explores how care co-ordination (or lack of) has an impact on patients and carers.
-
Unco-ordinated care results in delays and barriers to accessing care and places a burden on patients and carers, which, in turn, has negative effects on patients and carers in terms of physical health, financial and psychosocial impacts.
-
Approaches to co-ordination that improve access to care and lessen the time and burden placed on patients and carers may be particularly beneficial.
Background
Limited research suggests that there may be both financial and non-financial ‘hidden’ impacts for patients and their families affected by rare diseases, associated with how care is co-ordinated,6,185 including:
-
psychological and emotional challenges resulting from high turnover of HCPs and a lack of information and knowledge among professionals186
-
stress and financial concerns for parents due to the burden associated with planning and co-ordinating care to meet the unique needs of their children187
-
a substantial time burden for patients and carers, in part, because of co-ordinating their care. 18
However, the evidence base is weak. Given the paucity of data in this area, the aims of this study were to explore:
-
how rare disease patients and their carers are affected by how their care is, or is not, co-ordinated
-
the factors that might influence effective care co-ordination from the patients’ and carers’ perspective.
Methods
This was an exploratory qualitative interview study of patients affected by rare conditions (including undiagnosed conditions) and their carers. We recruited patients and carers (including parents of patients and spouses/partners of adult patients) affected by rare conditions in the UK. Participants were recruited from charity networks [Genetic Alliance UK, Rare Disease UK and Syndromes Without A Name (SWAN) UK (London, UK)] using a purposive sampling method. An advert inviting interested individuals to contact the research team was disseminated via e-mail (including newsletters and members’ updates), social media and charity websites. In October 2018, 15 participants were selected from 60 interested individuals. The sample was chosen to include patients and carers, those with and without a diagnosis and those with a range of co-ordination experiences (including those who had a professional co-ordinating their care and those who co-ordinated care themselves, and those who attended a specialist centre and those who did not). Participants were also selected to represent a range of ages and locations across the UK.
Interviews were semistructured and conducted by telephone or Skype. All participants received a participant information sheet and consent form via e-mail and were given the opportunity to discuss the study and ask the researcher questions before they agreed to take part. Verbal informed consent was taken and recorded at the start of the interviews. Fifteen interviews were conducted between October 2018 and January 2019 (telephone, n = 14; Skype, n = 1). Interviews were recorded using an encrypted dictaphone and transcribed verbatim by a professional transcribing company.
The interview included questions about how care was currently organised and how individuals would like it to be organised, what was important to them in relation to care co-ordination (and how this might change over time), and the costs and benefits associated with how care is co-ordinated.
A draft coding framework, including both anticipated and emergent codes, was developed based on previous studies6 and open-coding of two transcripts by two members of the research team. Three transcripts were then independently coded by two researchers, who met to share their coding. Any disagreements were discussed until a consensus was met. The revised coding frame was then applied to all remaining transcripts. To develop themes, a process of iterative categorisation188 was followed to systematically reduce, review and summarise the data.
Results
Participant characteristics were collected prior to interview for the 15 interviewees. Participants included patients affected by rare diseases (n = 7) and carers (n = 8). Carers were all informal carers [they were either the parent of a child with a rare disease (n = 6) or the spouse/partner of an adult with a rare disease (n = 2)]. Participants were a range of ages, from a range of geographical areas, some were affected by a diagnosed condition and some by an undiagnosed condition, and had a mix of experience in terms of access to a specialist centre.
Findings are grouped under the following three headings: (1) Experiences of unco-ordinated care for patients with rare conditions, (2) How unco-ordinated care impacts on patients and carers and (3) Examples of co-ordinated care and approaches to reduce the negative impacts of unco-ordinated care on patients and carers.
Experiences of unco-ordinated care for patients with rare conditions
Participants described a range of experiences of unco-ordinated care (Figure 5, section A), which included the following:
-
unco-ordinated appointments [i.e. unnecessary frequent appointments to see different professionals/services across different NHS settings, some of which were located far from home (i.e. at specialist centres), lack of choice about when or where their appointments took place and appointments with one professional at a time (with little evidence of medical and non-medical services being offered in the same clinic)]
-
ineffective communication between professionals and between professionals and patients/carers [i.e. lack of communication/team approach across various care professionals (particularly between those in specialist centres and local teams), no point of contact to approach with queries, problems relating to information-sharing (particularly the timeliness of information-sharing) and limited use of care plans]
-
patients and carers co-ordinating their own care (i.e. patients and carers undertaking a number of tasks, including chasing services, holding information and facilitating information-sharing, with many reporting being the main co-ordinator of care).
Experiences of unco-ordinated care varied across individuals and stages of the patient journey. For example, there were challenges associated with emergencies or acute episodes (with a lack of awareness locally and difficulties accessing timely treatment), establishing care and support pre and post diagnosis, following discharge from hospital (and receiving the appropriate care within the community) and transitioning from paediatric to adult services.
FIGURE 5.
Patients’ and carers’ experiences of unco-ordinated care and its impact. This figure is reproduced from Simpson et al. 184 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The figure includes minor additions and formatting changes to the original figure.
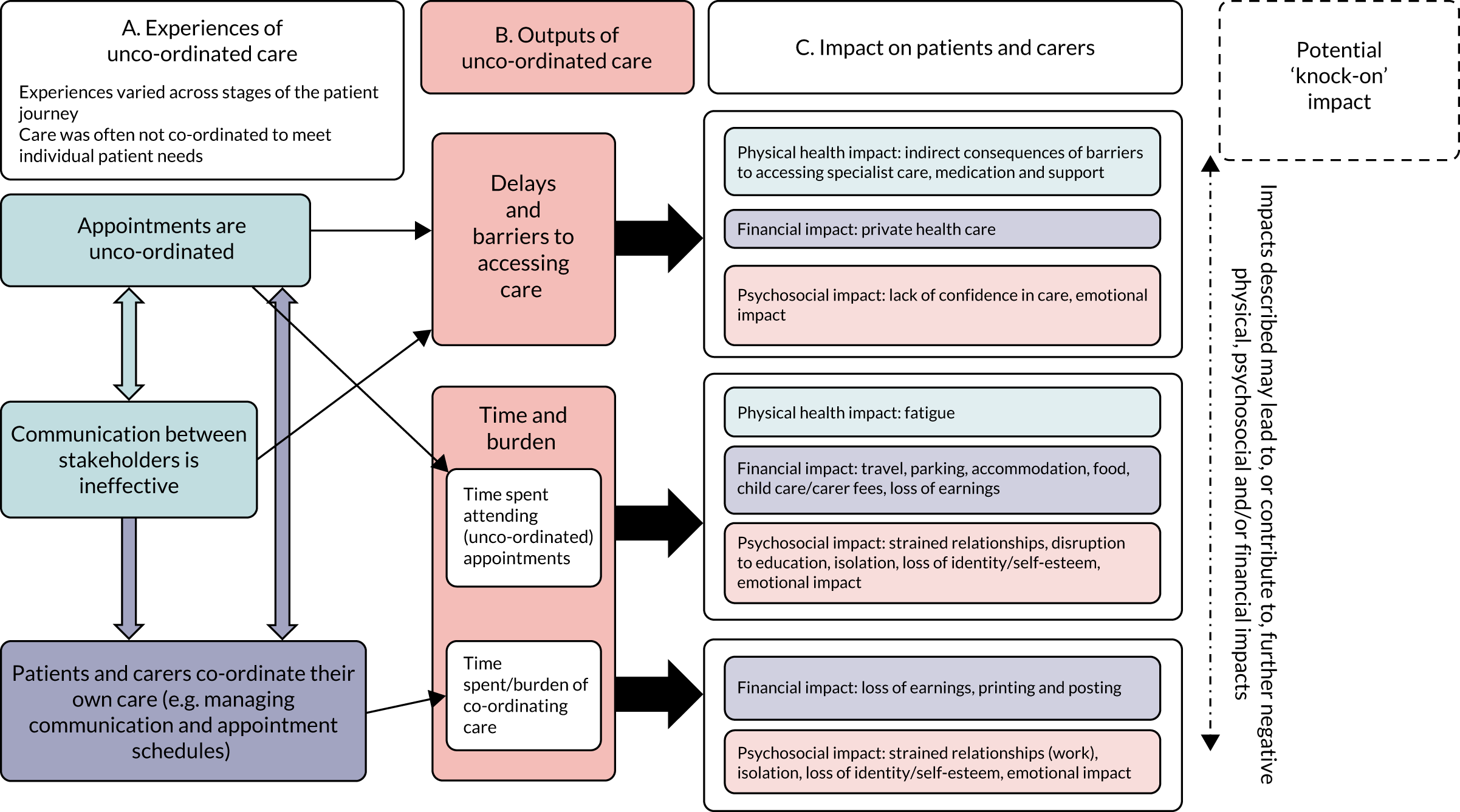
How unco-ordinated care impacts on patients and carers
Unco-ordinated care resulted in delays and barriers to accessing care and placed an additional burden on patients and carers (see Figure 5, section B). These delays and barriers, in turn, had several negative impacts for patients and carers (see Figure 5, section C).
How did unco-ordinated care influence patients’ access to care?
Interviewees reported that unco-ordinated appointments and ineffective communication between stakeholders had an impact on their ability to access care and access that care in a timely way. Seeing numerous professionals over several different appointments resulted in delays in decision-making about their care. Delays were also evident as a result of ineffective communication between professionals and information-sharing across different trusts/services. Interviewees reported wasting time during appointments due to updating professionals and/or waiting for professionals to chase results.
Initiatives that could facilitate communication between professionals (e.g. care plans or multidisciplinary meetings) were limited. Therefore, even for those patients who accessed specialist care (i.e. via a specialist centre), the location and accessibility of specialist care/advice combined with the lack of effective communication between local and specialist teams resulted in challenges, particularly in accessing local care during acute scenarios:
I would imagine a lot of people with rare diseases find this: that when they turn up at their local hospital whoever is on-shift generally has no idea what you’re talking about so you always have to go back to your consultant [and] there could be delays.
Parent of a child, diagnosed
More than one interviewee reported facing delays in accessing their medication, again as a result of ineffective communication between specialists and local services. Patients and carers reported that health and other sectors, such as social care, did not communicate with each other, sometimes preventing vital access to non-medical support:
. . . it’s terrible the co-ordination between the social work side of things and the health side of things. It took us 18 months actually to get a social worker, which seems crazy given that my son has a really profound disability.
Parent of a child, diagnosed
How did the challenges associated with access to care have a negative impact on patients and carers?
Barriers and delays were likely to have consequences for patients and carers. First, barriers and delays had a negative impact on a patient’s physical health, particularly if diagnosis or treatment was delayed. Second, barriers and delays had a financial impact on families, with participants reporting paying for private health care as a last resort as a result of delays or fighting for access to care. Third, barriers and delays had psychosocial impacts, with patients and carers reporting a significant emotional impact of having to fight for their care after experiencing a loss of confidence in the care they received:
Our main problem was obviously getting access to a doctor . . . who could do the appropriate tests . . . when [respondent’s son 1] developed that squint . . . which was kind of about a year before, he should have been referred to a neurologist at that point.
Parent of a child, diagnosed
It was also noted that impact on physical health was likely to have further ‘knock-on’ impacts for patients and carers. For example, poorer physical health could result in the need for more medical intervention, which, if unco-ordinated, could magnify many of the issues already faced, including increased challenges associated with daily activities, such as going to work (carrying further financial and psychosocial costs).
How did unco-ordinated care create additional burden on patients and carers?
Patients and carers described the time and burden placed on them to attend frequent and unco-ordinated appointments, with patients and carers spending significant time travelling to and attending various appointments. In addition, some patients and carers reported having to co-ordinate their own care, including supporting communication/information exchange and organising their vast appointment schedule. In the absence of care co-ordination (and tools, such as co-ordinators or care plans) families described having to adopt a proactive approach themselves to ensure that they received the right care. This involved spending significant amounts of time chasing services for results, appointments and advice:
I’m the one that chases appointments and makes sure that we’re where we’re supposed to be . . . a huge amount of work but how can that really be improved?
Parent of a child, diagnosed
A major task for families related to the management of information relating to the condition and the patient’s care. Participants suggested that the records kept by professionals were sometimes incomplete or inaccurate. As a result, patients and carers were often required to update or correct professionals at each appointment. Some kept detailed paper records at home, rather than relying on the records kept by professionals:
. . . If I ever got hit by a bus, we’d be screwed. Well, I wouldn’t be obviously, I’d be completely blissfully unaware, but he would be stuffed because all of this stuff is in my head.
Parent of a child, undiagnosed
The time and burden associated with attending appointments and managing a care schedule affected all patients to some extent (including those with positive experiences of co-ordinated care). However, interviewees reported that the costs were increased by (1) the unco-ordinated nature of appointments, that is they were required to travel far and frequently for services that could, in theory, be offered locally and/or in one visit rather than several, and (2) a lack of effective communication between the various professionals involved across specialties and locations.
How did the additional burden have a negative impact on patients and carers?
Participants reported that appointment schedules had a negative impact on patients’ physical health. This was a particular issue for those whose condition caused fatigue. In addition, participants reported financial costs associated with attending appointments, such as those for travel and parking, accommodation (if the distance was too great to complete a return journey on the same day), food and fees relating to child care (e.g. for patients’ siblings while parents were attending appointments) and carers (e.g. professional carers required to support parents when travelling and attending appointments). Psychosocial costs of attending appointments were also reported. For example, young patients missed time at school as a result of attending appointments:
Obviously, it’s got a financial cost, but there’s a physical cost there, you know, having to go to extra appointments when I needn’t have to.
Patient, diagnosed
Patients and carers frequently referred to the impact of attending unco-ordinated appointments and co-ordinating their care on work and employment. In part, this imposed a further financial cost to families (i.e. a loss of earnings from reducing their hours, changing the nature of their role at work or leaving paid employment to cope with the demands). Disruption to work also carried a psychosocial cost. Participants talked of strained relationships with colleagues and managers when negotiating time off and reducing hours, not having a break because they were using annual leave exclusively for appointments, and a loss of identity and self-esteem as a result of giving up their job and ‘independence’. Again, this demonstrated the multidirectional nature of some of the impacts (i.e. there may be several ‘knock-on’ impacts for patients and carers). In this instance, the financial impact of losing earnings had a psychosocial impact on participants:
. . . it has sort of an impact on your self-esteem, doesn’t it, because prior to having children I was a high-flyer and I was very independent and I earned a lot of money . . . whereas all of that has gone now; we’re living off the savings that my husband earned, I’m totally dependent on that and totally dependent on him.
Parent of a child, diagnosed
The emotional impact of managing a rare condition, in particular taking on the role of care co-ordinator, was also discussed by patients and carers. Words used to describe how the workload and burden made them feel included ‘exhausted’, ‘strained’, ‘frustrated’, ‘worried’, ‘suicidal’ and ‘terrified’. Parents, in particular, discussed feeling anxious about being the ‘expert’ and being responsible for looking out for symptom changes and receiving little support. Isolation was also a common theme among parents, which was exacerbated by their workload (e.g. they did not have the time to socialise):
The way that it’s been co-ordinated has probably added to the stress . . . having to be that person that is chasing everything, that’s definitely added to the stress.
Parent of a child, diagnosed
. . . it’s absolutely relentless and exhausting and heart-breaking. I’m on my seventh ring binder upstairs with all of the letters from the diagnoses and the medicine sheets . . . I’m just worried if I forget something.
Parent of a child, undiagnosed
There were also additional administration costs for families of printing and posting paperwork (e.g. the costs of record-keeping and sharing paperwork with relevant individuals and bodies).
Participants reported having to rely on others within their family for things, such as supporting the co-ordination of care and providing child care for children while attending appointments. Therefore, the non-financial and psychosocial impacts were also felt by wider family members who may have to support families practically and emotionally.
Examples of co-ordinated care and approaches to reduce the negative impacts of imperfect care co-ordination on patients and carers
From transcripts, we extracted data on examples of good co-ordination (currently experienced by participants) and/or participants’ suggestions for how co-ordination could be improved (Table 4).
| How care is/could be co-ordinated | What that might entail | How the approach might affect access to care and/or burden on patients and carers | Illustrative quotations from examples of co-ordinated care |
|---|---|---|---|
| Patients and carers have the support of a professional co-ordinator | Facilitating the communication and information exchange between key stakeholders (between HCPs, and between HCPs and patients and carers) |
Improves access to care (e.g. helping exchange of information between specialist and local providers) Reduces time/burden associated with co-ordinating care and attending unco-ordinated appointments for patients/carers |
I think without me having my [condition specific] specialist nurse co-ordinating the care . . . really taking some of the weight off, and doing a lot of the bread and butter . . . making sure I’m where I’m meant to be at the right time, and that the right doctor has got the right information . . . mum doesn’t have to deal with [that] . . . and I’m thankfulPatient, diagnosed |
| Scheduling appointments in a convenient way to meet patient needs | Potentially reduces time/burden associated with co-ordinating care and attending unco-ordinated appointments for patients/carers | ||
| Acting as a point of contact, including between appointments | Improves access to care (e.g. supporting patients to access specialist advice in-between appointments) | ||
| Facilitating and providing additional support, when required, in the patient journey | Improves access to care (e.g. signposting to relevant charities) | ||
| The organisation of appointments meets the needs of patients and carers | Locally and remotely where possible | Reduces time/burden associated with attending unco-ordinated appointments for patients/carers | . . . there are instances where it has been co-ordinated well by being able to condense all my appointments into 1 day, which is obviously much kinder on the bank balance . . . Obviously, it’s got a financial cost, but there’s a physical cost there, you know, having to go to extra appointments when I needn’t have toPatient, diagnosed |
| Scheduled at convenient times (supported by a care co-ordinator or use of online booking system) | Reduces time/burden associated with attending unco-ordinated appointments and co-ordinating care for patients/carers | ||
| A range of services and professionals can be accessed in one visit |
Improves access to care (e.g. care is more timely) Reduces time/burden associated with attending unco-ordinated appointments for patients/carers |
||
| Stakeholders communicate effectively | MDTs |
Improves access to care (e.g. medical and non-medical aspects of care are considered) Reduces time/burden associated with attending unco-ordinated appointments and co-ordinating care for patients/carers |
They normally ring me about a month before the appointment, ‘Here’s the list of everybody we think is currently involved with [son’s name], is this right?’ which is really useful . . . We have a meeting for about an hour or so . . . The paediatrician is normally the person that leads the meeting . . . then she’ll make some recommendations in terms of what she wants to see happen next . . . They are actually quite handy, those meetings, just to make sure that everybody knows what’s going onParent of a child, undiagnosed[Respondent’s daughter]’s got her metabolic disorder guidelines, so when we go to A&E, we’ve kind of got a triage pass to get into triage straight away so that we don’t have to be hanging around waitingParent of a child, diagnosed |
| Patients have written care plans (including plans for acute episodes) |
Improves transparency and accountability about co-ordination Improves access to care (e.g. facilitating proactive approach to care) Reduces time/burden associated with co-ordinating care for patients/carers |
||
| Patients, carers and professionals have a point of contact for specialist advice/liaison | Improves access to care (e.g. patients could access specialist information to guide self-care decisions when necessary) | ||
| Technology is used to improve communication (including between specialists and local providers) |
Improves access to care (e.g. local providers, such as GPs and staff in emergency departments, could access specialist information to guide care decisions when necessary) Reduces time/burden associated with co-ordinating care for patients/carers |
Having the support of a professional co-ordinator
One participant (patient, diagnosed) reported having a dedicated care co-ordinator within their specialist centre. The co-ordinator was a specialist nurse who acted as a point of contact for the patient, managed appointment scheduling and facilitated information-sharing between relevant professionals. The benefits reported by the participant included having a bank of expert knowledge that they could always refer to and reducing the burden on their carer. Participants suggested that a professional co-ordinator could support patients and carers in a range of other ways, including co-ordinating care across different providers (e.g. local providers), facilitating proactive care and helping families access wider support services [e.g. funding opportunities (including for specialist equipment within homes), social care and/or signposting to local charities that were seen as an important source of knowledge and support]. Such support is likely to both improve access to care (especially locally, and in both medical and non-medical care) and reduce the time/burden associated with co-ordinating and fighting to access care for patients and carers.
There were diverse views about which professionals should fulfil the role of care co-ordinator, what type of training or background would be required and when the support should be available. For example, some argued that a professional co-ordinator should be someone with a medical background or even someone who specialises in the condition, whereas some others argued that it should be someone with good organisation skills (not necessarily with a medical background). Some interviewees felt that care co-ordinators should take on specific co-ordinating tasks only as required, rather than having continuous and regular involvement. The support of a care co-ordinator may have greater benefits at key points in the patient journey, for example post diagnosis, post hospital discharge and at other transition periods (i.e. when care needs are being identified and treatment/support is being established) or during acute periods (i.e. to assist communication between local and specialist services).
It was also noted that some patients and carers may prefer to retain more control over co-ordinating their care and preferences may change as personal circumstances do. For example, as parents return to work after maternity leave their capacity to be involved in co-ordinating tasks/attending appointments may decrease. Therefore, parents may require the support of a co-ordinator more during this time:
At the moment I’m on maternity leave so I have more time to do these things, but when I’m back at work, trying to organise everything and keep track of everything, time to book appointments and stuff is tricky.
Parent of a child, undiagnosed
Changing the organisation of appointments and clinics
As demonstrated by the experience of one patient who was able to attend several appointments at the same location on the same day, the way that clinics are scheduled can reduce the time/burden on patients and carers, particularly the time/burden associated with travelling to and attending unco-ordinated appointments. Other approaches were suggested. A family-centred approach to clinics (i.e. where more than one family member with the same condition could be seen on the same day) could improve communication and decision-making between paediatric and adult services while also reducing the costs to families associated with attending two sets of appointments.
However, some approaches may not be feasible locally and despite many participants recognised the value of receiving care at a specialist centre and felt that it outweighed costs of travelling, the advantages of accessing specialist care locally or remotely were addressed. For example, the provision of services locally (such as via special schools or child development centres) were argued to have the benefit of saving time on travel (and the associated travel costs) and being a familiar environment for the family. Many participants also realised the benefits of having some consultations virtually, particularly with specialists who were based far away and where face-to-face contact was not essential for every appointment. Interviewees commented on the usefulness of virtual appointment options, especially for those who experience fatigue.
Preferences regarding the timing and scheduling of appointments were also noted to change depending on the needs of individual patients. For example, older children may be more able to cope with a full day of appointments than a younger child:
As he gets older I think sometimes it would be nice if we could have multiple appointments on the same day. That would really help, because you’re not then having three appointments . . . When they’re very small it’s difficult and one appointment a day is better because attention spans are limited and everything else.
Parent of a child, undiagnosed
Improving communication between stakeholders
One parent described an initiative (called ‘Team Around the Child’ meetings) that brought together all professionals involved in their child’s care (including HCPs, non-HCPs, local professionals and specialist professionals). This parent argued that the intervention helped promote proactive care and information-sharing. Interviewees reported other ways that professionals can or could work as a MDT, including holding MDT clinics.
The need for a care plan as a communication and co-ordination tool was evident in participants’ calls for everyone to work together to agree an approach and to provide planned, rather than reactive, care. Similarly, guidelines or a care plan for acute scenarios were viewed as useful so that patients receive timely access to care, particularly locally.
Interviewees felt that having a point of contact for specialist advice would help with communication, either for the family when making a decision about whether or not they need to go to hospital and/or a professional who can liaise between local emergency staff and specialists who know about the condition.
Interviewees highlighted ways in which they thought technology could improve communication and information-sharing. This might be, for example, a computer system that allows relevant information to be accessed by all professionals involved in a patients’ care, as well as allowing access for the patient/carer:
I would like there to be one central website where I could log in . . . I could see when he’s due to see them next, I could message consultants . . . and I could book appointments . . . maybe I could see his test results and stuff too . . . to actually access these digitally and online would be fantastic.
Parent of a child, undiagnosed
Discussion
Key findings
The study identified two key consequences of unco-ordinated care on the patient and carer experience, that is delays and barriers in accessing care and additional time/burden. A range of impacts on patients and parents/carers were identified and categorised into three overarching themes: physical health, psychosocial impacts and financial impacts. The findings also suggest ways in which service users felt negative impacts of unco-ordinated care might be reduced, including with the support of a professional co-ordinator, using MDTs, care plans, technology and/or a point of contact to improve communication, and organising appointments to meet the needs of patients and families (by providing appointments locally or virtually, where possible, offering a range of services in one visit and scheduling appointments at a convenient time for the family).
The findings demonstrated that different levels of care co-ordination (e.g. the involvement of a care co-ordinator and how clinics/appointments are delivered) may be needed at different stages of the patient’s journey and/or to meet individual patient preferences. This highlights the need for co-ordination to take into account the characteristics and preferences of the patient when new services or support are required (e.g. post diagnosis or when transitioning to adult services) and when care is needed locally in non-specialist settings. There may also be key individual differences that affect the extent to which one might experience the consequences and impacts described here. Some impacts may be magnified for some. For example, co-ordinating care may be more burdensome for a single parent without a wider support network. Such differences also need to be taken into account when considering how care should be co-ordinated.
How do the findings relate to previous research?
Our data support previous findings from others on the burden of living with a rare and/or undiagnosed condition,186,187 including struggling to access appropriate expertise, support and information, financial strain (e.g. due to changes in employment) and psychosocial impacts (e.g. disruption to everyday life, living with uncertainty and stress). However, this study offers a novel contribution to the existing knowledge base. It has identified the specific costs (financial and otherwise) and potential benefits associated with how care is co-ordinated, rather than the more general consequences and needs associated with living with or caring for someone with a rare condition.
Previous research shows that patients come up against a number of challenges within the health-care system due to the rarity and complexity of their condition, including misdiagnoses and delays in diagnosis, a lack of information and support, and low availability of treatments. 189 This study suggests that unco-ordinated care also contributes to the challenges faced by patients with rare diseases, particularly around accessing care and the burden of managing the condition.
Although this chapter presents the impacts across three overarching themes (physical health, psychosocial and financial), it is important to note the inter-relations between them. This is supported by previous research,187 which found that parents faced financial challenges as a result of their caring role, which, in turn, was a major source of stress. Another recent study highlighted a number of factors affecting the mental health of rare disease patients and their carers, including trying to access services and support (e.g. financial and non-medical support). 190 Although it is helpful to categorise different impacts (e.g. for identifying measures for evaluation and further research), the complex interdependencies found in reality should be recognised.
The findings support recent research,186,191 which has found that patients and carers take on significant tasks in relation to care co-ordination and management. Having professional support to co-ordinate care could reduce the negative impact on patients and carers (and others). However, this support could take many different forms and further research and consultation is required to establish the extent to which responsibility should be transferred from patient to professional.
Limitations
The main limitation is that only 15 participants took part in what was an exploratory qualitative study. Although efforts were made to include a variety of individuals and experiences, the sample is not representative of all those affected by rare diseases. As an exploratory and qualitative study, the intention was not to recruit a representative sample of the rare disease population, but rather to focus on in-depth individual accounts that could support the development of data collection tools and interpretation of quantitative data in the CONCORD study. The data can be used to provide an insight into an under-researched area and to inform future research (e.g. on strategies to reduce the burdens described in this chapter). In addition, although positive examples of care co-ordination were shared, it was challenges and gaps in care co-ordination that were more commonly felt among participants.
In addition, there may be other factors (not included here) that have an impact on patient and carers experience of care co-ordination. There may be institutional barriers (e.g. the availability of resources), structural barriers (e.g. the limitations of information systems within NHS organisations) and/or cultural obstacles to change.
Future research
This study identified how care could be co-ordinated in a way that benefits patients with rare conditions, and their carers. Further work is required to develop models of care co-ordination (e.g. as considered in Chapter 7), assess their feasibility and then evaluate them in practice to determine if the potential benefits we have identified can be realised.
Our findings demonstrate the need for future research to collect the views of larger numbers of patients (and carers) and consider what might influence differences in preferences. This research should also be extended to gather the views of professionals (i.e. those delivering care and supporting patients). We consider this further in the following chapter.
Summary
These findings provide evidence of both the challenges and the importance of co-ordinating care in the context of rare conditions. There are a range of negative consequences associated with poorly co-ordinated care, including delays and barriers to accessing care, and additional time and burden on patients and carers, resulting in physical, psychosocial and financial impacts. Study participants outlined a number of ways that negative impacts might be reduced for patients and carers, including having the support of a care co-ordinator, having clinics and appointments organised in a way that better meet patient needs, and effective communication between professionals and services. The findings stress the importance of approaches to care co-ordination that are flexible to individual needs and fit for purpose throughout the patient journey.
Chapter 5 National cross-sectional survey to explore experiences of co-ordinated care for people affected by rare diseases
Overview
What was already known?
-
Few studies have examined if care for people living with rare diseases is co-ordinated.
-
There are several ways in which care could be co-ordinated for people affected by rare diseases, including through the use of care co-ordinators, specialist centres and care plans.
What this chapter adds
-
We undertook a national survey involving 760 adult patients affected by rare diseases, 446 parents/carers of people affected by rare diseases and 251 HCPs who care for people affected by rare diseases to understand the extent to which, and how, care of people with rare conditions is co-ordinated in the UK.
-
There is limited access to co-ordinated care for people affected by rare diseases. For example, only 12% of adult patients affected by a rare disease reported that they had a formal care co-ordinator, 32% reported that they attended a specialist centre and 10% reported that they had a care plan.
Background
Few studies have examined whether or not care is co-ordinated for people living with rare diseases. Limited available evidence suggests that co-ordination of care in this group is poor,5,6 with experiences of delayed diagnoses, misdiagnoses, a lack of information provided about the rare condition, limited access to care co-ordinators and specialist centres, and a heavy burden placed on patients and families dealing with rare diseases due to a lack of co-ordination in care. The aim of the present study was to explore the experiences of people affected by rare conditions in the UK in greater detail, in terms of if, and how, their care is co-ordinated. Better understanding of these issues will inform how care co-ordination might be improved and centred around the needs and preferences of patients and families affected by rare conditions.
Methods
Survey instrument
A survey questionnaire was developed using data from three sources to identify elements of care co-ordination that should be explored. First, we identified themes from the scoping review of 154 reviews of co-ordinated care for rare and chronic conditions (see Chapter 3) to identify important components of co-ordinated care. Second, we ran three focus groups involving (1) patients aged ≥ 18 years affected by a rare condition, (2) parents/carers of children and adults affected by a rare condition and (3) HCPs involved in the treatment of rare conditions. One focus group was conducted virtually with four patients and three carers and two focus groups were conducted face to face (one with four HCPs and the other with two patients and four parents/carers). Third, we ran 15 one-to-one telephone or Skype interviews with seven patients and eight parents/carers. Using the findings from these activities, we identified three key areas of care co-ordination that mattered to patients and families: (1) access to care co-ordinators, (2) specialist centres and (3) care plans. We developed a first draft of the questionnaire to understand how care of people with rare conditions is co-ordinated in the UK, with specific reference to these items. The study PPIAG reviewed the draft questionnaire and modified the language and content, as necessary. The questionnaire was then piloted. Twenty respondents completed the questionnaire on their own using a draft information sheet to instruct them in the process. In addition, four ‘think-aloud’ interviews were undertaken with one patient, two parents and a HCP, who completed the draft questionnaire in the presence of a researcher. The questionnaire was modified according to the pilot data and the PPIAG performed a further review to the ensure that the language used was comprehensible and relevant to the intended focus of each question.
The survey instrument mainly contained close-ended questions with defined response categories. A smaller number of questions asked participants to provide qualitative or text information (e.g. the rare condition they were affected by) and an open-ended text box was provided for these questions. At the conclusion of each survey section, participants were also given the opportunity to provide any other comments they had on the section topic.
The first section of the survey was used to obtain consent and to determine participant eligibility. Participants were provided with a participant information sheet about the survey, which included the purpose of the survey, the organisations involved in conducting the study and assurances around anonymity and aggregation of data for reporting purposes. Participants were asked to click ‘next’, taking them to another webpage to access the survey, and were advised that by doing so they were consenting to participate in the survey. Participants were also told that they did not have to take part if they did not want to. As the survey was completed anonymously, after data were submitted by clicking the ‘submit’ button at the end of the survey it was not possible to withdraw individual respondents’ data.
The next section of the survey asked about experience of diagnosis/rare conditions. The main body of the survey followed, with three sections measuring the three key aspects of co-ordination of care identified from the preparatory research, namely experiences of care co-ordinators, specialist centres and care plans. Additional questions were then asked around use of health services. The next section included a DCE (see Chapter 6). The final section asked participants for information on socio-demographic factors. See Report Supplementary Material 1 for the patient version of the survey.
Survey sampling
Three groups of participants were eligible to complete the survey: (1) patients (aged ≥ 18 years) affected by a rare condition, (2) parents/carers (aged ≥ 18 years) of children or adults with rare conditions and (3) HCPs (e.g. doctors, nurses and allied health professionals) involved in the care of people with rare conditions. We aimed to recruit 300 participants for each group and to have an overall target sample size of 1500 participants. This was justified using two pieces of information. First, sample size calculations for surveys are possible based on population size, desired confidence level and maximum acceptable margin of error. Assuming a population size of upwards of 20,000 (predicted sample size remains close to constant for populations > 20,000), a margin of error of 3% and a confidence level of 95%, the required sample size is 1014 (calculated using SurveyMonkey®; URL: www.surveymonkey.com/mp/sample-size-calculator; Momentive Inc., San Mateo, CA, USA). Second, our target figure of 1500 participants partly stemmed from another survey5 using a similar research design in the UK. A 2016 survey by Rare Disease UK5 achieved a sample size of 1213 participants.
There were no restrictions on participants in terms of the rare condition, demographic factors (other than age ≥ 18 years) or geographical location within the UK. We deliberately did not sample from specific rare diseases, nor limit the range of rare diseases we included, to identify as many different models of co-ordination as possible, and to include as broad a range of experiences and preferences with regard to care co-ordination as possible. A complete sample frame of all adults living with a rare condition in the UK does not exist. The total number of people living with a rare condition, their contact details and their sociodemographic characteristics, such as age, gender, highest education level and location of residence, are unknown. For these reasons, purposive snowball sampling was used for this study. We discussed routes to accessing patients and parents/carers with the PPIAG. Participants were accessed via patient and provider networks and organisations, including Rare Disease UK (which has more than 2000 registered supporters, including academics, clinicians, industry, individual members and patient organisations192), Genetic Alliance UK (a national alliance of organisations with a membership of more than 180 charities that support patients and families affected by genetic disorders193) and SWAN UK (a support network for families of children and young adults with undiagnosed genetic conditions in the UK run by Genetic Alliance UK194).
An independent survey company created an electronic version of the survey using a bespoke online platform. The survey was ‘live’ from August to December 2019. Potential participants were sent a weblink to the survey either by e-mail or via social media. The message containing the weblink also included an offer to send hard copies of the questionnaire by post or e-mail or to complete it verbally over the telephone with a researcher. We also recruited patients and parents/carers via six major care providers, where research co-ordinators at each site identified potential participants and asked participants if they were willing to participate in the study. If participants were willing to participate, they were provided with further details on how to do this, as described above. HCPs were recruited using the same routes described above for patients and parents/carers. In addition, we contacted the British Society of Genetic Medicine (London, UK) and its constituent organisations and special interest groups,195 and the NIHR Clinical Research Network: Genetics. 196 These organisations circulated details of the survey to their members via their electronic mailing lists. Participants had a 48-hour window where they were able to suspend completion of the questionnaire, if they needed to do so, and then to resume where they left off at a time that was convenient to them.
Analysis of data
All data handling was conducted in compliance with General Data Protection Regulation197 requirements and all responders agreed to their data being processed for research purposes. Responses where less than 20% of all data fields were completed were removed. All the quantitative results, except for those relating to the use of health services, were reported as frequencies and percentages using Microsoft Excel™ (Microsoft Corporation, Redmond, WA, USA) and Stata® (StataCorp LP, College Station, TX, USA). For the use of services, we reported the mean values per patient, stratified by whether or not the patient had access to a care co-ordinator, a specialist centre and a care plan. We did not impute missing data. Responses were checked for any identifiable information entered in open-text boxes and redacted if necessary. All open-text data were exported by the survey company and formatted for analysis. Open-text responses specific to the topic of care co-ordinators, specialist centres and care plans were coded using inductive thematic analysis in Microsoft Excel. A list of all codes identified (per question) were summarised and were used to identify and develop cross-cutting themes and subthemes within the data. Below, we present the findings from our thematic analysis alongside the quantitative findings for the three main topics (i.e. care co-ordinators, specialist centres and care plans).
See Appendix 5 for a STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) statement pertaining to the survey.
Results
Responses and sample
In total, 1604 responses to the survey were received from 856 adult patients affected by rare diseases, 497 parents/carers of people affected by rare diseases and 251 HCPs who care for people affected by rare diseases. We excluded respondents who completed less than 20% of the data fields, which amounted to the exclusion of 96 (11%) patients and 51 (10%) carers. The final number of responses included for analysis was 1457 (adult patients affected by rare diseases, n = 760; parents/carers of people affected by rare diseases, n = 446; HCPs who care for people affected by rare diseases, n = 251). It was not possible to estimate a response rate for each group, as the survey was sent by multiple overlapping distribution routes using convenience sampling and snowball sampling techniques.
Among 760 (adult) patients with a rare condition, the modal age band was 45–54 years and more than 80% were female (Table 5). More than 95% of patients had been diagnosed with a rare disease (as opposed to being undiagnosed) and diagnoses had been confirmed by a genetic test in 30% of patients. Multiple body systems were affected, the most common being muscle, ligaments and joints (affecting 58% of the sample) and vision (affecting 57% of the sample). A total of 221 rare conditions were represented. The greatest number of responses for a single disease group was 101 (13%) for sarcoidosis. Most (57%) patients reported that they lived with a spouse or partner.
| Characteristic | Patients (N = 760), n (%) | Parents/carers (N = 446), n (%) | HCPs (N = 251), n (%) |
|---|---|---|---|
| Age of patient (years) | |||
| 0–5 | 66 (25) | ||
| 6–12 | 81 (30) | ||
| 13–17 | 34 (13) | ||
| 18–24 | 21 (4) | 33 (12) | |
| 25–34 | 75 (15) | 18 (7) | |
| 35–44 | 94 (18) | 8 (3) | |
| 45–54 | 124 (24) | 11 (4) | |
| 55–64 | 115 (23) | 12 (4) | |
| 65–74 | 66 (13) | 4 (1) | |
| ≥ 75 | 14 (3) | 1 (0) | |
| Total | 509 (100) | 268 (60) | |
| Prefer not to say | 3 | 3 (1) | |
| Missing | 248 | 175 (39) | |
| Age of parent/carer (years) | |||
| 18–24 | 5 (2) | ||
| 25–34 | 36 (13) | ||
| 35–44 | 94 (35) | ||
| 45–54 | 86 (32) | ||
| 55–64 | 36 (13) | ||
| 65–74 | 11 (4) | ||
| ≥ 75 | 1 (0) | ||
| Total | 269 (100) | ||
| Prefer not to say | 2 | ||
| Missing | 175 | ||
| Sex | |||
| Male | 73 (14) | 32 (12) | |
| Female | 434 (85) | 235 (88) | |
| Other | 2 (0) | 1 (0) | |
| Total | 509 (100) | 268 (100) | |
| Prefer not to say | 3 | 3 | |
| Missing | 248 | 175 | |
| Diagnosed with rare disease | |||
| Yes | 736 (98) | 400 (91) | |
| No | 17 (2) | 38 (8) | |
| Unsure | 7 (1) | 8 (2) | |
| Total | 760 (100) | 446 (100) | |
| Diagnosis confirmed with genetic test | |||
| Yes | 223 (30) | 255 (64) | |
| No | 402 (55) | 110 (28) | |
| Unsure | 111 (15) | 35 (9) | |
| Total | 736 (100) | 400 (100) | |
| N/A (undiagnosed) | 24 | 46 | |
| Body system affected | |||
| Muscle, ligaments and joints (rheumatology) | 438 (58) | 232 (52) | |
| Vision | 432 (57) | 114 (26) | |
| Brain, nerves and spinal cord (neurology) | 345 (45) | 229 (51) | |
| Digestion (gastroenterology) | 337 (44) | 222 (50) | |
| Hearing | 327 (43) | 201 (45) | |
| Bones and joints (orthopaedics) | 318 (42) | 205 (46) | |
| Skin (dermatology) | 314 (41) | 129 (29) | |
| Breathing and lungs (respiratory) | 302 (40) | 175 (39) | |
| Chronic pain | 296 (39) | 166 (37) | |
| Heart and circulatory (cardiology) | 230 (30) | 159 (36) | |
| Diabetes and hormones (endocrinology) | 188 (25) | 95 (21) | |
| Kidneys (nephrology) | 178 (23) | 115 (26) | |
| Behavioural difficulties | 153 (20) | 128 (29) | |
| Learning difficulties | 65 (9) | 175 (39) | |
| Mental health (psychiatry) | 35 (5) | 229 (51) | |
| Total | 760 | 446 | |
| Number of rare diseases in sample | 221 | 259 | |
| Top 10 most common rare diseases | |||
| 1 | Sarcoidosis, 101 (13) | Behçet’s syndrome, 18 (4) | |
| 2 | Behçet’s syndrome, 85 (11) | Tracheo-oesophageal fistula, 14 (3) | |
| 3 | Idiopathic intercranial hypertension, 49 (6) | Aplastic anaemia 9 (2) | |
| 4 | Lynch syndrome, 26 (3) | Ataxia, 8 (2) | |
| 5 | Ehlers–Danlos syndrome, 24 (3) | Rett syndrome, 6 (1) | |
| 6 | IgA nephropathy, 24 (3) | Tuberous sclerosis, 6 (1) | |
| 7 | Ocular melanoma, 17 (2) | Common variable immune deficiency, 5 (1) | |
| 8 | Common variable immunodeficiency, 14 (2) | Dravet syndrome, 5 (1) | |
| 9 | Scleroderma, 12 (2) | Huntington’s disease, 5 (1) | |
| 10 | Allergic bronchopulmonary aspergillosis, 11 (1) | Multiple system atrophy, 5 (1) | |
| Patient’s living arrangements | |||
| Lives alone | 115 (23) | ||
| Lives with a spouse or partner | 289 (57) | ||
| Lives with family members or friends | 99 (19) | ||
| Lives with a carer | 2 (1) | ||
| Total | 505 | ||
| Prefer not to say | 7 | ||
| Missing | 248 | ||
| Parent’s/carer’s relationship to patient | |||
| Spouse or partner | 23 (9) | ||
| Parent | 192 (71) | ||
| Guardian | 3 (1) | ||
| Grandparent | 2 (1) | ||
| Sibling | 2 (1) | ||
| Son or daughter | 41 (15) | ||
| Other relation | 1 (0) | ||
| Friend | 1 (0) | ||
| Other | 5 (2) | ||
| Total | 270 (100) | ||
| Prefer not to say | 1 | ||
| Missing | 175 | ||
| Parent’s/carer’s living arrangements | |||
| Lives with patient | 244 (91) | ||
| Does not live with patient | 24 (9) | ||
| Total | 268 (100) | ||
| Prefer not to say | 3 | ||
| Missing | 175 | ||
| Geographical region | |||
| South-east of England | 65 (13) | 35 (13) | 9 (7) |
| South-west of England | 61 (12) | 26 (10) | 12 (5) |
| Scotland | 60 (12) | 21 (8) | 6 (2) |
| London | 52 (10) | 26 (10) | 34 (14) |
| North-west of England | 51 (10) | 34 (13) | 66 (26) |
| East of England | 42 (8) | 17 (6) | 6 (2) |
| Wales | 39 (8) | 9 (3) | 1 (1) |
| Yorkshire | 35 (7) | 16 (6) | 4 (2) |
| West Midlands | 31 (6) | 48 (18) | 25 (10) |
| East Midlands | 24 (5) | 17 (6) | 11 (4) |
| North East and Cumbria | 23 (5) | 14 (5) | 7 (3) |
| Northern Ireland | 15 (3) | 1 (0) | 1 (1) |
| Other | 8 (2) | 7 (3) | 4 (2) |
| Total | 506 (100) | 271 (100) | 186 (100) |
| Prefer not to say | 4 | 0 | 0 |
| Missing | 250 | 175 | 65 |
| Ethnic group | |||
| White | 473 (94) | 245 (92) | |
| Non-white | 20 (4) | 15 (6) | |
| Other | 8 (2) | 5 (2) | |
| Total | 501 (100) | 265 (100) | |
| Prefer not to say | 9 | 6 | |
| Missing | 250 | 175 | |
| Educational attainment | |||
| No formal qualifications | 18 (4) | 6 (2) | |
| O Level or GCSE | 68 (14) | 41 (16) | |
| ONC or BTEC | 21 (4) | 14 (5) | |
| A Level (‘Higher’ in Scotland) | 35 (7) | 26 (10) | |
| Higher education qualification | 102 (21) | 40 (16) | |
| Degree or higher degree | 252 (51) | 130 (51) | |
| Total | 496 (100) | 257 (100) | |
| Prefer not to say | 14 | 14 | |
| Missing | 250 | 175 | |
| Clinical expertise in rare diseases | |||
| Yes | 136 (56) | ||
| No | 107 (44) | ||
| Total | 243 (100) | ||
| Missing | 8 | ||
| Areas of work with patients with rare conditions | |||
| Diagnosing condition | 148 (59) | ||
| Providing information/signposting or counselling | 189 (75) | ||
| Long-term care following diagnosis | 166 (66) | ||
| Long-term care in the absence of a diagnosis | 139 (55) | ||
| HCP role | |||
| Hospital doctor | 78 (31) | ||
| Nurse/midwife | 39 (16) | ||
| Allied health professional | 28 (11) | ||
| Clinical academic | 24 (10) | ||
| GP/community doctor | 12 (5) | ||
| Manager | 7 (3) | ||
| Public health professional | 5 (2) | ||
| Health informaticist | 4 (2) | ||
| Psychological therapist | 3 (1) | ||
| Patient representative | 3 (1) | ||
| Pharmacist | 1 (1) | ||
| Commissioner | 1 (1) | ||
| Other | 26 (10) | ||
The modal age category of the 446 carers who responded was 35–44 years (35%), and in around two-thirds of cases the patient being cared for was aged < 18 years. More than 80% of carers who responded were female. Most of those cared for had been diagnosed with a rare disease (8% were undiagnosed) and in more than 60% of cases the diagnosis had been confirmed with a genetic test. A total of 259 rare conditions were represented in parents/carers, and the largest number of responses for a single disease group was 18 (4%) for Behçet’s syndrome. In more than 70% of cases the carer was the parent of the person affected by the rare condition, and in more than 90% of cases the carer lived with the they person cared for.
Around 90% of both patients and parents/carers were from the white ethnic group and both groups came from a range of educational backgrounds, with having a degree or higher degree being the modal education category. Respondents in both groups were from across the UK.
More than half of the 251 HCPs reported having specific clinical expertise in rare diseases, and they worked across a range of areas with patients with rare conditions. Around 30% of respondents were hospital doctors, 16% were nurses or midwives and 11% were allied health professionals. Respondents in this group worked across the UK.
Access to formal care co-ordinators
In the questionnaire, a formal care co-ordinator was defined as:
A professional with a recognised role in helping patients and carers manage a range of needs between different professionals or across care settings. They may be a full-time co-ordinator or may co-ordinate care as part of their main job, such as a GP.
Twelve per cent of patients reported that they had a formal care co-ordinator, and this figure was comparable to the proportion of parents/carers (14%) who reported that the person they cared for had a formal care co-ordinator (Table 6 and Figure 6). However, these figures were lower than for HCPs (35% of whom reported that the majority of patients had a formal care co-ordinator). Of the patients and parents/carers who reported having access to a formal care co-ordinator, 30–40% said that this was a person employed specifically for the care co-ordinator role. In addition, the care co-ordinator had another main role, which was usually a hospital doctor, a GP or a specialist nurse. Patients and parents/carers who reported having access to a formal care co-ordinator noted that a variety of roles were undertaken, the most common for both groups being liaising between professionals (75% of patients and 73% of parents/carers reported that their formal care co-ordinator carried out this role). Other common roles were scheduling appointments, liaising between HCPs and non-HCPs, updating the patient’s care plan and being the contact for emergency or acute episodes. When asked about the main factors that determine if someone with a rare condition has access to a formal care co-ordinator, the most common responses from HCPs were complexity of disease (49%) and the extent of the patient’s need for support (45%).
| Survey question and answers | Patients (N = 760), n (%) | Parents/carers (N = 446), n (%) | HCPs (N = 251), n (%) |
|---|---|---|---|
| Do you a have a formal care co-ordinator?/Does the person you care for have a formal care co-ordinator?/Do the majority of your patients have a formal care co-ordinator? | |||
| Yes | 92 (12) | 62 (14) | 82 (35) |
| No | 570 (77) | 325 (76) | 118 (51) |
| Unsure | 76 (10) | 43 (10) | 33 (14) |
| Total | 738 (100) | 430 (100) | 233 (100) |
| Missing | 22 | 16 | 18 |
| If ‘yes’ to the previous question, is the formal care co-ordinator employed specifically for the role (or do they co-ordinate care as part of another role, e.g. as a GP or a specialist nurse?) | |||
| Yes | 33 (36) | 19 (31) | 15 (19) |
| No | 51 (56) | 38 (61) | 61 (75) |
| Unsure | 7 (8) | 5 (8) | 5 (6) |
| Total | 91 (100) | 62 (100) | 81 (100) |
| Not applicable | 646 | 368 | 151 |
| Missing | 23 | 16 | 19 |
| If ‘no’ to the previous question, what is the formal care co-ordinator’s main role? | |||
| Hospital doctor | 25 (49) | 9 (24) | 16 (26) |
| GP | 14 (27) | 5 (13) | 6 (10) |
| Specialist nurse | 7 (14) | 9 (24) | 19 (31) |
| Other | 3 (6) | 5 (13) | 6 (10) |
| Practice or community nurse | 2 (4) | 3 (8) | |
| Community paediatrician | 3 (8) | 13 (21) | |
| Palliative care specialist | 2 (5) | ||
| Charity or patient support group representative | 1 (3) | ||
| Physiotherapist | 1 (3) | ||
| Genetic counsellor | 1 (2) | ||
| Total | 51 (100) | 38 (100) | 61 (100) |
| Not applicable | 686 | 392 | 171 |
| Missing | 23 | 16 | 19 |
| Which items are managed by the formal care co-ordinator? | |||
| Liaising between HCPs | 69 (74) | 45 (73) | 75 (75) |
| Scheduling appointments | 56 (64) | 23 (37) | 41 (41) |
| Contact for emergency or acute episodes | 35 (38) | 21 (34) | 42 (42) |
| Updating the care plan | 32 (35) | 23 (37) | 55 (55) |
| Ensuring availability of health records at appointments | 25 (27) | 8 (13) | 34 (34) |
| Liaising with patient to co-ordinate multidisciplinary clinics | 21 (23) | 18 (29) | 48 (48) |
| Advocating on a patient’s behalf | 16 (17) | 19 (31) | 63 (63) |
| Out-of-hours contact | 16 (17) | 3 (5) | 21 (21) |
| Co-ordinating transitions of care | 13 (14) | 17 (27) | 60 (60) |
| Liaising between HCPs and non-HCPs (e.g. social worker, homecare) | 11 (12) | 26 (42) | 69 (69) |
| Arranging respite care | 1 (1) | 7 (11) | 36 (36) |
| Total | 92 | 62 | 100 |
| Not applicable | 668 | 384 | 151 |
| What are the main factors that determine if someone with a rare condition will have access to a formal care co-ordinator? | |||
| Complexity of disease | 124 (49) | ||
| Availability of care co-ordinators | 124 (49) | ||
| Extent of patient’s need for support | 113 (45) | ||
| Budgetary constraints | 87 (35) | ||
| Request of patient/carer/family | 80 (32) | ||
| Caseload of HCPs involved | 76 (30) | ||
| Patient’s existing support system (number and role of carers) | 67 (27) | ||
| Distance from specialist centre | 57 (23) | ||
| Unsure | 29 (12) | ||
| Total | 251 (100) | ||
FIGURE 6.
Percentage of survey respondents with access to a formal care co-ordinator. Note that the figure reports the percentage of survey respondents replying ‘yes’, ‘no’ or ‘unsure’ to the question ‘Do you a have a formal care co-ordinator?’ for patients, ‘Does the person you care for have a formal care co-ordinator?’ for parents/carers and ‘Do the majority of your patients have a formal care co-ordinator?’ for HCPs.
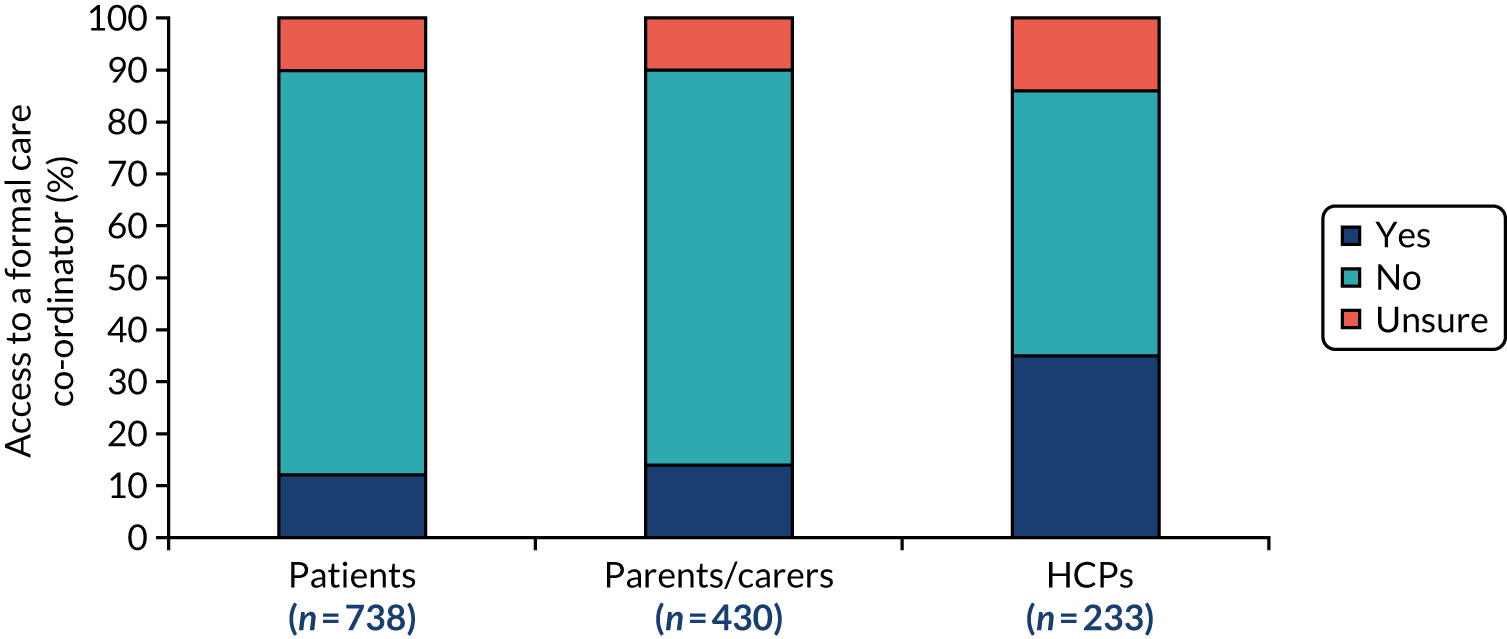
All survey respondents were given a list of roles and asked if they would prefer each of these to be undertaken by the patient, carer or the formal care co-ordinator (Table 7). Although there was some variation in the magnitudes between groups, over half of patients, parents/carers and HCPs preferred appointments to be scheduled by the patient/carer. For all other roles (i.e. liaising between professionals, updating the care plan, ensuring availability of health records, co-ordinating transitions, arranging multidisciplinary clinics and arranging respite care), the majority of respondents in all three groups preferred these to be undertaken by the formal care co-ordinator.
| Which items would you prefer to be managed by the patient/carer or formal care co-ordinator? | Patients (N = 654),a n (%) | Parents/carers (N = 391),b n (%) | HCPs (N = 208),c n (%) | |||
|---|---|---|---|---|---|---|
| Patient/carer | Formal care co-ordinator | Patient/carer | Formal care co-ordinator | Patient/carer | Formal care co-ordinator | |
| Scheduling appointments | 413 (63) | 241 (37) | 284 (75) | 107 (25) | 120 (58) | 88 (42) |
| Liaising between HCPs | 133 (20) | 521 (80) | 76 (19) | 315 (81) | 9 (4) | 199 (96) |
| Liaising between HCPs and non-HCPs (e.g. social worker, homecare) | 178 (27) | 476 (73) | 129 (33) | 262 (67) | 21 (10) | 187 (90) |
| Updating the care plan | 166 (25) | 488 (75) | 137 (35) | 254 (65) | 27 (13) | 181 (87) |
| Ensuring availability of health records at appointments | 121 (19) | 533 (81) | 59 (15) | 332 (85) | 24 (12) | 184 (88) |
| Co-ordinating transitions of care | 107 (16) | 547 (84) | 66 (17) | 325 (83) | 7 (3) | 201 (97) |
| Liaising with patient to co-ordinate multidisciplinary clinics | 121 (19) | 533 (81) | 93 (24) | 298 (76) | 20 (10) | 188 (90) |
| Arranging respite care | 158 (24) | 496 (76) | 146 (37) | 245 (63) | 61 (29) | 147 (71) |
Throughout the care co-ordinator section of the survey, respondents were asked to provide further information about their responses. In addition, at the end of the survey, respondents were asked if there was any other information they would like to provide that, specifically, related to care co-ordinators and, if so, to include this in a free-text box. The open-text qualitative information was summarised according to six overarching themes, which are described below. Issues relating to each of these themes were raised by all three respondent groups (i.e. patients, parents/carers and HCPs).
What are the potential benefits of care co-ordinators and how can they improve quality of care?
The survey asked respondents to describe how a formal care co-ordinator might improve quality of care. As noted, the majority of respondents stated that they had no experience of a formal care co-ordinator. Bearing this in mind, the following (potential) benefits were identified:
-
improving access to care, which, in turn, is likely to improve the management of conditions and impact positively on quality of life
-
acting as an advocate for patients/carers, particularly during appointments, liaising with professionals, chasing up services/appointments, dealing with issues and complaints if they arise, and navigating the care system
-
reducing the burden on patients/carers, which could, in turn, improve their mental and physical well-being
-
providing additional support for patients and carers by understanding the health and wider support needs of patients and carers and becoming a trusted professional for families to work with
-
improving communication and information-sharing between professionals, including between MDTs, specialists, local teams and different health-care providers, across boundaries (such as devolved nations) and during times of transition
-
saving costs by helping to maximise the use of resources (e.g. scheduling appointments in a rational way and avoiding duplication of efforts), by taking the pressure off and saving time for other professionals (e.g. consultant time spent co-ordinating care) and by improving the management of conditions
-
having someone whose responsibility it is to oversee care, improving both consistency and continuity of care
-
holding professionals to account and reducing errors or medical negligence
-
improving awareness of rare conditions.
It was also suggested that the benefits of the care co-ordinator role may be more significant for particular groups of patients, including those with complex and multisystem conditions (involving multiple specialists), those with brain disorders (where stress may be detrimental to the condition), those with cognitive impairment, non-English speaking families, young people in adult services (where there is no paediatrician), those not coping with the condition and those with communication difficulties.
What tasks should a co-ordinator undertake?
Many respondents provided information on the range of tasks they think co-ordinators should undertake. These were as follows:
-
liaising with families (e.g. acting as an advocate for the patient, keeping them updated and attending appointments with patients)
-
providing education and awareness about the rare condition
-
undertaking administrative tasks (e.g. improving scheduling of appointments, assembling the information required to facilitate treatment and acting as a point of contact for professionals)
-
developing and reviewing the patient’s care plan
-
undertaking clinical tasks (e.g. monitoring the patient’s condition and their treatment, and providing medical advice)
-
facilitating team working (e.g. liaising across boundaries and working with multiple services) and facilitating communication between professionals (e.g. between different specialists, and between specialists and local providers)
-
liaising with education services on behalf of young people affected by rare conditions
-
signposting patients and families to charities providing support.
What training or skills are required for the role of care co-ordinator?
Many respondents provided information on the training and skills that they felt were required for the care co-ordinator role. Some respondents argued that care co-ordinators require medical skills/training. These skills/training ranged from having an understanding of rare conditions to having specialist knowledge of a condition. Some respondents felt they should be trained medical professionals with clinical skills so that they could monitor patients clinically. However, other respondents proposed that care co-ordinators did not need a medical background and they could learn the skills required.
Many of the key skills for care co-ordinators related to the ability to work with others. This included working with the patient and their family and a range of professionals (not just from the health sector). It was suggested that co-ordinators should be good communicators and listeners.
Having a good understanding of health, social care and education systems was also identified. It was suggested that care co-ordinators should understand the system and be able to navigate it, produce care plans and be able to deal with transition issues.
Other skills suggested included organisation skills, the ability to take a proactive approach, and administration and data-handling skills. Some respondents suggested that the role had a very specific skill set and that there should be a formal training programme for co-ordinators.
Who should undertake the co-ordinator role and where should they be based?
Respondents provided details on who co-ordinated their care (including during times of transition) and discussed who should be the formal care co-ordinator. HCPs were specifically asked whether or not the role should be undertaken by a single individual. Different views emerged. Although some HCPs proposed that the role should be a standalone role, other respondents felt that it should be a team approach (rather than the role of one individual). This would encourage multidisciplinary input, improve continuity of care (i.e. there would be no issues if staff were on leave) and drive greater diversity of experience and expertise (allowing more evidence-based care). Capacity may also be a consideration when deciding how many co-ordinators are involved. It was suggested that caseloads may be too large for one person. However, respondents felt that the co-ordinator role should sit with a particular professional or be a joint role between two professionals (including a co-ordinator who worked closely with a clinical member of staff). Roles that were proposed as appropriate for the tasks that were suggested by respondents included social workers, hospital paediatricians, genetic counsellors, specialist/rare disease nurses (including condition-specific nurses), GPs and clinical leads.
Co-ordination during periods of transition was supported by a wide range of professionals in different sectors. However, these professionals were not necessarily formalised care co-ordinators, but they were professionals undertaking specific co-ordinating tasks, such as a GP making a referral or a transplant nurse co-ordinating care during a specific treatment/hospital stay.
Other suggestions for who should undertake the care co-ordinator role included someone who knows the patient, a charity-funded role, a member of the patient’s MDT and someone based at a specialist centre. It was also suggested to utilise different co-ordinators for different aspects of care (e.g. separate co-ordinators to deal with medical issues and administration).
What role does the patient/carer play in care co-ordination?
Patients and parents/carers gave many examples of unco-ordinated or poorly co-ordinated care. As a consequence of this, many patients and parents/carers carried out co-ordination tasks themselves (e.g. transferring information between professionals, chasing appointments and identifying relevant doctors and services themselves) and were the main co-ordinators of care. Respondents reported the often substantial burden this had on them. Some patients and carers stated that they preferred to retain control of co-ordination themselves. These patients and carers recognised that they had become experts in the condition and that there were limitations associated with professionals working as care co-ordinators.
What are the limitations of care co-ordinators?
Respondents flagged a number of potential limitations associated with care co-ordinator roles. First, there was some concern that a formal care co-ordinator role might exclude patients and their families. The importance of patient knowledge and partnership working (with patients and families) was highlighted. Second, the success and benefits associated with the role was dependent on many factors, including the individual co-ordinator, the condition, the patient and parent/carer, funding and capacity. The importance of wider systems and resources for co-ordination were also described, including information flow, communication between all parties, patient confidence, care plans, access to records and awareness among professionals. Third, there were some negative views about the (potential) impact of a care co-ordinator, based on the idea that they might have little impact on quality of care. It was suggested that resources for care co-ordinators might be better spent on other pressing issues for rare disease communities, such as access to treatments. Fourth, although some respondents felt that the role should be formally recognised, it was also argued that more research was needed in exploring the impact of care co-ordinator roles.
Access to specialist centres
In the survey, a specialist centre was defined as:
A centralised facility that enables patients to see a number of health-care professionals in one visit. Usually, the professionals at specialist centres will be experts in rare and undiagnosed conditions. Non-health-care professionals may also see patients at the same centre.
Thirty-nine per cent of patients reported that a specialist centre was available for their rare condition and 83% of these patients said that they attended the specialist centre. Therefore, 32% of patients attended a specialist centre for their condition (Table 8 and Figure 7). Among carers, the respective values were 37%, 88% and 33%. Sixty per cent of HCPs reported that a specialist centre was available for the majority of their patients with a rare condition. Specialist centres provided access to HCPs with specialist knowledge. More than half of the patients, parents/carers and HCPs reported that patients at the specialist centre saw doctors who were experts in rare or undiagnosed conditions, doctors who were experts in the aspects of health affected and specialist nurses. Other professionals were seen less frequently at the specialist centre. A wide range of services were provided by the specialist centre, with the most common being appointments with an expert in rare conditions and appointments to see different types of HCPs at the centre. When HCPs were asked the main reasons why patients with rare conditions might choose not to use specialist centres, the most common responses, each reported by more than 60% of respondents, related to the extent of travelling involved, including the distance, cost and physical difficulty of travelling.
| Survey question and answers | Patients (N = 760), n (%) | Parents/carers (N = 446), n (%) | HCPs (N = 251), n (%) |
|---|---|---|---|
| Is there a specialist centre available for you?/Is a specialist centre available for the person you care for?/Is there a specialist centre available for the majority of your patients with rare conditions? | |||
| Yes | 235 (39) | 130 (37) | 122 (60) |
| No | 250 (41) | 168 (48) | 61 (30) |
| Unsure | 119 (20) | 50 (14) | 22 (11) |
| Total | 604 (100) | 348 (100) | 205 (100) |
| Missing | 156 | 98 | 46 |
| If ‘yes’, do you attend a specialist centre?/If ‘yes’, do they attend a specialist centre? | |||
| Yes | 196 (83) | 114 (88) | |
| No | 35 (15) | 14 (11) | |
| Unsure | 4 (2) | 2 (2) | |
| Total | 235 (100) | 130 (100) | |
| N/A | 369 | 218 | |
| Missing | 156 | 98 | |
| If ‘yes’, which HCPs are seen at the specialist centre? | |||
| Doctors who are experts in rare or undiagnosed conditions | 166 (85) | 86 (75) | 94 (64) |
| Specialist nurse | 123 (63) | 74 (65) | 98 (67) |
| Doctors who are experts in aspects of health affected (e.g. neurologist) | 111 (57) | 72 (63) | 94 (64) |
| Physiotherapist | 32 (16) | 35 (31) | 65 (44) |
| Psychologist | 30 (15) | 29 (25) | 67 (46) |
| Dietitian | 22 (11) | 36 (32) | 66 (45) |
| Genetic counsellor | 9 (5) | 17 (15) | 76 (52) |
| Occupational therapist | 8 (4) | 17 (15) | 55 (37) |
| Care co-ordinator | 7 (4) | 10 (9) | 29 (20) |
| Behavioural therapist | 1 (1) | 3 (3) | 13 (9) |
| Community paediatrician | 8 (7) | 22 (15) | |
| Speech and language therapist | 19 (17) | 55 (37) | |
| Other | 30 (15) | 13 (11) | 30 (20) |
| Total | 196 | 114 | 147 |
| Which services are provided by the specialist centre? | |||
| Appointments with an expert in rare conditions | 170 (87) | 83 (73) | 92 (63) |
| Appointments to see different types of HCPs at the centre | 118 (60) | 80 (70) | 92 (63) |
| Multiple appointments during a single visit | 90 (46) | 62 (54) | 75 (51) |
| Diagnostic and screening procedures | 86 (44) | 53 (46) | 89 (61) |
| Access to patient support groups or charities | 79 (40) | 35 (31) | 86 (59) |
| Access to research opportunities | 69 (35) | 41 (36) | 92 (63) |
| Contact for acute or emergency episodes | 52 (27) | 46 (40) | 63 (43) |
| Non-urgent out-of-hours contact | 50 (26) | 35 (31) | 35 (24) |
| Appointments that are not in person (e.g. virtual or telephone appointments) | 44 (22) | 23 (20) | 61 (42) |
| Support during emergency admissions | 32 (16) | 31 (27) | 62 (42) |
| Support with routine admissions | 31 (16) | 31 (27) | 53 (36) |
| Appointments to see non-HCPs (e.g. social worker) | 26 (13) | 19 (17) | 34 (23) |
| Extended hours for appointments | 12 (6) | 7 (6) | 14 (10) |
| Other | 12 (6) | 4 (4) | 5 (3) |
| Total | 196 | 114 | 147 |
| What are the main reasons why patients with rare conditions might choose not to use specialist centres? | |||
| Distance to travel to specialist centre | 179 (71) | ||
| Cost of travel to specialist centre | 166 (66) | ||
| Physical difficulty in travelling to specialist centre | 159 (63) | ||
| Patient is satisfied with quality of care provided locally | 87 (35) | ||
| Length of time between appointments at specialist centre | 81 (32) | ||
| Perceived lack of benefit from the specialist centre | 60 (24) | ||
| Length of appointment times at specialist centre | 41 (16) | ||
| Other | 39 (16) | ||
| Total | 251 | ||
FIGURE 7.
Percentage of survey respondents with access to a specialist centre. Note that the figure reports the percentage of survey respondents replying ‘yes’, ‘no’ or ‘unsure’ to the question ‘Is there a specialist centre available for you?’ for patients, ‘Is a specialist centre available for the person you care for?’ for parents/carers and ‘Is there a specialist centre available for the majority of your patients with rare conditions?’ for HCPs.
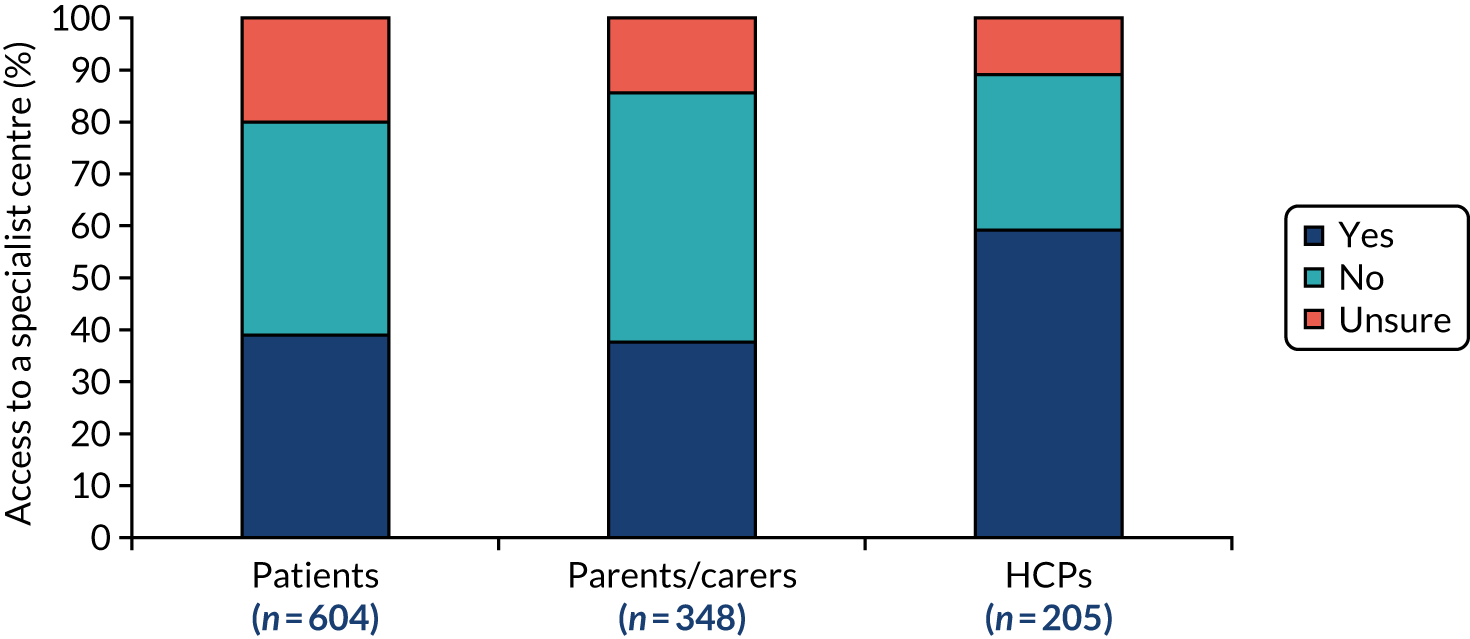
The free-text information about access to a specialist centre was summarised thematically into the six categories below. Issues relating to each of these themes were raised by all three respondent groups.
Desirable qualities of specialist centres
Participants outlined several desirable qualities of specialist centres. These qualities included being patient focused and compassionate, organised, well led by experts, accessible, up to date with research and flexible. Providing a point of contact, signposting patients to charity services and having suitable technology (e.g. shared computer systems/databases/registries that can provide access to providers in other areas and facilitate appointment booking, and having suitable technology for telemedicine appointments) were also deemed as desirable qualities. Respondents also reported that specialist centres should ideally facilitate communication with other centres and services, provide services that cannot be accessed closer to home, bring together all aspects of care and provide a holistic approach to care.
Benefits of specialist centres
Participants highlighted several benefits of specialist centres. These benefits included providing holistic treatment (e.g. managing all physical, social and mental health aspects of condition, such as diagnosis and treatment), offering shared-care approaches (e.g. with care provided by a single line-managed MDT, aligning care, results and appointments), reducing patient stress/fatigue (through multiple appointments on the same day), providing care that cannot be accessed locally and providing quicker and more effective services than non-specialist services. In addition, specialist centres were perceived as being well-run (i.e. keeping patients informed), having motivated and inspirational providers, having specialist expertise (both in terms of rare conditions and available research/clinical trials), providing an opportunity for peer support and shared experiences for patients, providing patients and families with confidence in the specialist team, having more time to deal with condition-specific queries and improving the quality of care (e.g. by utilising specialist expertise).
Limitations of specialist centres
On the other hand, several limitations were highlighted with the use of specialist centres. These limitations included inequity of access and the extent of support provided by the specialist centre (e.g. a lack of holistic support outside the specific condition, limited extent of support once the patient’s health improves and a lack of support for non-health issues, e.g. applying for benefits). Respondents emphasised that access to specialist centres was not standard practice for all rare conditions. Many participants expressed how they do not have access to a specialist centre and that it was not possible to have a specialist centre for every rare condition. A further limitation was limited accessibility of specialist centres, whereby, even if a specialist centre was available, some patients expressed difficulties in receiving a referral to a specialist centre, difficulties applying for funding to attend a specialist centre and long waiting lists for appointments. The high time and money costs associated with attending specialist centres was also commented on. Other limitations included a lack of continuity (e.g. patients not seeing the same professionals each time and having to continually repeat themselves), specialist centres raising expectations of care, but similar care not being available locally, and a lack of co-ordination between specialists and local teams.
Aspects of care needed at specialist centres but often not provided
Participants highlighted aspects of care that they felt were missing from specialist centres that could be provided. Some aspects related to lack of support, including lack of support with applying for benefits, lack of holistic support for non-medical issues, lack of peer support and a lack of interaction from professionals in a range of disciplines (e.g. psychological/mental health support, counselling, hydrotherapy, genetic counselling/genetic screening, physiotherapy, occupational therapy, podiatry, ophthalmology and support from a social worker). In addition, respondents highlighted aspects of care relating to service delivery, for example limited links with local providers and limited access to certain types of services, including specific condition clinics, specialist adult services, transition services and emergency care. Several participants commented that specialist centres were missing governance and administrative structures, including having a standardised care pathway, emergency care plans, mental health support care plans and long-term care plans. Participants highlighted that contact numbers, access to medication advice, prompt replies to queries, and information about research and clinical trials were frequently missing.
Roles within specialist centres
Several comments were made about roles within specialist centres. Some participants highlighted that specialist centres needed to have a point of contact for patients and local providers. Several participants highlighted that these points of contact do not always exist. In addition, participants highlighted that care co-ordinator and specialist nurse roles were needed. Several participants felt that services provided by MDTs at specialist centres were helpful, but others said that, in some cases, multidisciplinary clinics were not available. Some participants thought that certain individuals and roles were particularly helpful, including inspirational consultants, world experts, HCPs with specific interests in a particular rare condition and genetic counsellors. Some participants felt that specialist centres did not have access to the correct specialists or that doctors specialising in rare conditions were rare. Some participants highlighted the role of charities in the provision of care by specialist centres. This role could come in various guises, including providing support and advice alongside the specialist centre, helping with practical arrangements and travel costs to access the specialist centre, and being the first point of call for families that needed support (especially in terms of non-medical queries and emotional support). Many participants stressed the importance of specialist centres in assisting with providing access to support groups.
Relationship between specialist centres and local providers
Participants discussed the need for specialist and local care, and the relationship between specialist centres and local providers. Some participants discussed how they would like specialist centres and local providers to work together (e.g. with local providers seeking advice from the specialist centre). Some participants reported disparities between what was recommended by the specialist centre and what was delivered locally (e.g. some local hospitals/GPs were unable to provide treatment recommended by the specialist centre). Some patients highlighted that they would prefer to attend specialist clinics, whereas other patients preferred to receive treatment closer to home, thereby reducing travel distances and costs. Information on local providers that can provide support was felt to be needed. Some patients who attended specialist centres reported losing local links, meaning that they were unable to access care locally when needed. Some patients highlighted difficulties with accessing the correct care locally. There were also concerns over emergency care from local teams (e.g. by not seeking support from specialists in emergency situations). Some participants reported a lack of co-ordination and correspondence between specialist and local services (e.g. local services not using advice and information specialist centres, and vice versa).
Access to care plans
In the questionnaire, a care plan was defined as:
A paper or electronic document which describes the health services and support that are needed and should be agreed between patients, carers and professionals. The care plan may be a single document or it may be part of another record which includes non-health services such as an Education, Health and Care Plan (EHCP).
Ten per cent of adult patients reported having a care plan relating to their rare condition, compared with 44% of parents/carers (Table 9 and Figure 8). Forty per cent of HCPs said that they use care plans to document care for patients with rare conditions. In terms of the responsibility for keeping the care plan up to date, patients reported that, most commonly, it was either themselves (27%) or a hospital doctor (27%) who was responsible for keeping the care plan up to date. Parents/carers, however, reported that, most commonly, it was their responsibility to keep the care plan up to date. HCPs advised that, most commonly, this responsibility was shared between professionals (25%). Sixty-four per cent of patients and 85% of parents/carers who had a care plan reported that they were involved in developing it. The most common items included in the care plan were general information about the patient, a medical summary and an assessment of current health needs. These items were also reported to be the most useful items to be included in the care plan by all three groups of respondents, along with a plan of care for emergency or acute episodes.
| Survey question and answers | Patients (N = 760), n (%) | Parents/carers (N = 446), n (%) | HCPs (N = 251), n (%) |
|---|---|---|---|
| Do you have a care plan relating to your rare condition?/Does the person you care for have a care plan relating to their rare condition?/Do you use care plans as a means to document care for patients with rare conditions? | |||
| Yes | 59 (10) | 159 (44) | 82 (40) |
| No | 478 (78) | 165 (46) | 105 (51) |
| Unsure | 76 (12) | 37 (10) | 20 (10) |
| Total | 613 | 361 | 207 |
| Not stated | 147 | 85 | 44 |
| Who is primarily responsible for keeping the care plan up to date? | |||
| Patient | 15 (27) | 1 (1) | 4 (5) |
| Hospital doctor | 15 (27) | 5 (3) | 7 (9) |
| Shared responsibility between professionals | 8 (14) | 19 (12) | 20 (25) |
| No one holds responsibility | 5 (9) | 9 (6) | 5 (6) |
| Specialist nurse | 4 (7) | 8 (5) | 17 (21) |
| Formal care co-ordinator | 2 (4) | 2 (1) | 6 (7) |
| GP | 2 (4) | 0 (0) | 2 (2) |
| Genetic counsellor | 1 (2) | 0 (0) | 0 (0) |
| Carer | 0 (0) | 59 (37) | 0 (0) |
| Practice or community nurse | 0 (0) | 5 (3) | 1 (1) |
| Community paediatrician | 0 (0) | 2 (1) | 5 (6) |
| Other | 4 (7) | 49 (31) | 14 (17) |
| Total | 56 (100) | 159 (100) | 81 (100) |
| N/A | 554 | 202 | 125 |
| Missing | 150 | 85 | 45 |
| Were you involved in developing the care plan for your needs?/Were you, or was the person you care for, involved in developing the care plan? | |||
| Yes | 36 (64) | 135 (85) | |
| No | 14 (25) | 19 (12) | |
| Unsure | 6 (11) | 5 (3) | |
| Total | 56 (100) | 159 (100) | |
| N/A | 554 | 202 | |
| Missing | 150 | 85 | |
| What is addressed in the care plan? | |||
| General information and a medical summary | 51 (91) | 142 (89) | |
| An assessment of current health needs | 39 (70) | 117 (74) | |
| Scheduled reviews of the care plan | 20 (36) | 65 (41) | |
| Plan of care for emergency or acute episodes | 19 (34) | 77 (48) | |
| Out-of-office hours (non-urgent) contacts | 14 (25) | 33 (21) | |
| An assessment of current non-health needs (e.g. social care) | 11 (20) | 80 (50) | |
| Documented health goals | 11 (20) | 45 (28) | |
| Transition planning for changes in care | 8 (14) | 19 (12) | |
| Other | 2 (4) | 21 (13) | |
| Total | 56 | 159 | |
| What are the three most useful items that should be included in a care plan? | |||
| An assessment of current health needs | 485 (64) | 273 (61) | 149 (59) |
| General information and a medical summary | 459 (60) | 259 (58) | 155 (62) |
| Plan of care for emergency or acute episodes | 459 (47) | 196 (44) | 161 (64) |
| Scheduled reviews of the care plan | 173 (23) | 79 (18) | 19 (8) |
| Out-of-office hours (non-urgent) contacts | 108 (14) | 51 (11) | 32 (13) |
| An assessment of current non-health needs (e.g. social care) | 97 (13) | 108 (24) | 51 (20) |
| Documented health goals | 94 (12) | 51 (11) | 24 (10) |
| Transition planning for changes in care | 46 (6) | 45 (10) | 24 (10) |
| Total | 760 | 446 | 251 |
FIGURE 8.
Percentage of survey respondents with access to a care plan. Note that the figure reports the percentage of survey respondents replying ‘yes’, ‘no’ or ‘unsure’ to the question ‘Do you have a care plan relating to your rare condition?’ for patients, ‘Does the person you care for have a care plan relating to their rare condition?’ for parents/carers and ‘Do you use care plans as a means to document care for patients with rare conditions?’ for HCPs.

The open-text information responses about care plans were summarised thematically into the categories below. Issues relating to each of these themes were raised by all three respondent groups.
Why care plans are needed
Participants indicated that care plans were important for several reasons. Reasons included supporting acute admission/emergency care (e.g. informing local hospital providers on what to do to provide care); supporting local teams on how to manage the condition; providing information on who to contact and when, and what will happen when joining up care; reducing the need for patients to repeat information; helping to overcome a lack of communication; overcoming inaccuracies in letters and documents; integrating care from different services and providers; improving quality (e.g. outcomes, such as quality of life, care progress and supporting quality checks); and saving time and money. A limited number of participants indicated that care plans may not be helpful (i.e. planning may be wasting money on non-clinical staff, which could be spent on treatments, and care plans may prolong treatment and increase frustrations).
What needs to be included in a care plan
Generally, it was felt that many elements are needed in a care plan, and that all the options listed in the survey (see Table 9) should be included. In addition, it was felt that a one-page summary and a notes page for HCPs would be useful. It was also felt that research opportunities and a non-technical information section for employers may be helpful. Potential benefits of including more detail relating to responsibilities and contact, treatment and care, and personalisation were identified.
In terms of responsibilities and contact, the following elements were thought to be important for inclusion within a care plan: emergency contact numbers, a list of up-to-date professionals involved in care and their contact details, details of local hospitals that the patient can access, and responsibilities of specialist and local teams (e.g. hub and spokes staff).
In terms of treatment and care, participants felt that the care plan needed to cover health, social and educational aspects of treatment and care. For health aspects, this included medication (e.g. drugs, doses, list of medication, side effects and a drug chart), current treatments and treatment options, care history, advance care planning and end-of-life discussions, medical notes and records from all hospitals involved, key instructions and letters from each specialist, condition-specific information, a summary of medical needs, details of linked conditions, a diagnosis statement and details of check-ups and assessments. For social aspects, this included details of social care, summary of social/behavioural needs, summary of emotional needs and support, details of mental health and an assessment of impact on non-health needs. For educational aspects for children and young people, this included school information and links to the education, health and care plan (EHCP).
In terms of personalisation, participants felt that care plans should be personalised, tailored and include the following information: patient information (including their likes and dislikes, goals, allergies, cognitive skills, what’s important, preferences for support and help, days they cannot make appointments and contact details), reasonable adjustments, family support, communication style and consent for sharing information.
Respondents emphasised the need for care plans to be reviewed and updated regularly, and shared between patients, carers and all relevant settings involved in care.
Who is involved in care plans?
To develop care plans, participants indicated that they wanted a range of people to be involved. Findings highlighted that care plans require involvement from people who understood the condition and that care plans should acknowledge carer expertise. In addition, findings emphasised that care plans should be co-produced between clinicians and the patient. Findings also highlighted that involving care co-ordinators in writing care plans would be beneficial. Although care plans should be led by medical teams, it was felt that educational providers and social care providers should be involved in the development too. Although parents/carers were seen as important in planning and implementing the care plans, findings identified that, in some cases, patients were developing their own care plans.
In terms of holding responsibility for the care plan, participants felt that this required a knowledgeable individual who was familiar with medical terms and medical environments. Some participants felt that a care co-ordinator overseeing the care plan would be helpful, which was seldom seen. In some cases, patients holding their own care plans was felt to be useful.
When are care plans needed?
Care plans were felt to be necessary in different situations, including during acute admissions and emergency care, to keep track of records from different hospitals, to provide plans on elements of conditions (e.g. feeding, physiotherapy and epilepsy), for different phases of condition (i.e. through a stepped care plan), to provide a plan for the short and medium term of a patient’s condition, to update local hospitals on care, and for schools and workplaces.
Limitations of care plans
Participants reported three limitations of care plans, grouped around (1) resources, (2) comprehensiveness and accuracy and (3) lack of standardisation. In terms of resources, care plans were thought to be time-consuming to develop and update, and to create more paperwork for already overworked providers. As a consequence, participants highlighted that plans were often not updated as regularly as needed. In some cases, updating the care plan was felt to be a meaningless exercise. Findings highlighted that additional (currently non-existent) resources and training for staff were needed to deliver care plans. In terms of comprehensiveness and accuracy, most participants felt that care plans were not sufficiently comprehensive, were not always accurate, were sometimes out of date (which was felt to be potentially dangerous) and were sometimes too generic and copied from other patients. As a result, some participants felt that care plans had limited use in practice. Some participants highlighted that their care plans were up to date and comprehensive. Several participants indicated that they did not have a care plan, but would like one. In terms of the lack of standardisation, participants felt that care plans were not standardised across the patients and providers. For example, different local authorities had different plans. Some participants highlighted that care plans were not necessarily adhered to in unexpected situations. Other participants highlighted that some providers adhere to the care plan too strictly, which was thought to be problematic in situations requiring flexibility.
Accessibility of care plans
Participants indicated that care plans needed to be accessible and shared between patients and HCPs. Some patients with a care plan reported that they did not have access to it and, therefore, were not able to share it with health-care providers. It was suggested that centrally held electronic care plans might facilitate ease of access, as well as making care plans simpler to follow and more user friendly.
Access to combinations of care co-ordination
We showed that only 12% of adult patients affected by a rare disease reported that they had a formal care co-ordinator, 32% reported that they attended a specialist centre and 10% reported that they had a care plan. For parents/carers of patients affected by rare diseases, these figures were 14%, 33% and 44%, respectively. Table 10 shows access to different combinations of the three elements of care co-ordination covered by the survey. It shows that 54% of patients and 33% of parents/carers had access to neither a formal care co-ordinator, nor a care plan, nor a specialist centre (this was the modal combination). By contrast, only 2% of patients and 5% of parents/carers reported having access to all three elements.
| Combination no. | Type of care co-ordination | Patients, n (%) | Parents/carers, n (%) | ||
|---|---|---|---|---|---|
| Care co-ordinatora | Care planb | Specialist centrec | |||
| 1 | No | No | No | 326 (54) | 115 (33) |
| 2 | No | No | Yes | 169 (28) | 66 (19) |
| 5 | Yes | No | Yes | 30 (5) | 5 (1) |
| 6 | Yes | No | No | 24 (4) | 9 (3) |
| 4 | No | Yes | Yes | 22 (4) | 42 (12) |
| 3 | No | Yes | No | 17 (3) | 80 (23) |
| 7 | Yes | Yes | Yes | 14 (2) | 17 (5) |
| 8 | Yes | Yes | No | 2 (0) | 14 (4) |
| Total | 604 (100) | 348 (100) | |||
Use of services
The mean use of health services per patient over a 12-month period is shown in Table 11. The findings are stratified by whether or not the patient had access to a care co-ordinator, a specialist centre and a care plan, and by whether the respondent was a patient or parent/carer. The ranking varied between groups, but the most common types of health service use contact across all groups were visits to the general practice, appointments for diagnostic and screening procedures (including blood tests and routine scans), outpatient appointments with doctors who are experts in the aspects of health affected (e.g. neurologist), outpatient appointments with other health professionals, outpatient appointments with experts in the patient’s condition or undiagnosed conditions and visits to the practice nurse. Contacts with patient support groups/charities were the most common contact. It was difficult to detect differences between groups, except that (adult) patients had more contacts with patient support groups/charities and more GP and practice nurse visits than parents/carers. Summing across all types of contact, the mean total number of contacts per patient ranged from 48 to 74 contacts per year, or from 1 to 1.5 contacts per week.
| Health service use | Patients’ responses (n) | Parents’/carers’ responses (n) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Care co-ordinatora | Specialist centreb | Care planc | Care co-ordinatora | Specialist centreb | Care planc | |||||||
| Yes (N = 92) | No (N = 570) | Yes (N = 196) | No (N = 285) | Yes (N = 59) | No (N = 478) | Yes (N = 62) | No (N = 325) | Yes (N = 114) | No (N = 182) | Yes (N = 159) | No (N = 165) | |
| Contact with patient support groups/charities | 22.8 | 11.3 | 26.6 | 17.1 | 9.3 | 22.0 | 3.8 | 7.1 | 3.8 | 12.1 | 13.9 | 3.3 |
| GP visits | 6.1 | 6.9 | 7.4 | 10.9 | 7.0 | 9.1 | 2.8 | 4.8 | 4.5 | 4.3 | 4.2 | 4.5 |
| Appointments for diagnostic and screening procedures (including blood tests and routine scans) | 3.6 | 6.4 | 7.4 | 5.6 | 8.5 | 6.0 | 4.3 | 5.1 | 8.0 | 3.8 | 3.4 | 6.4 |
| Outpatient appointments with doctors who are experts in the aspects of health affected (e.g. neurologist) | 2.5 | 3.9 | 4.0 | 3.6 | 6.3 | 3.4 | 3.1 | 5.5 | 7.8 | 4.4 | 5.1 | 5.0 |
| Outpatient appointments with other health professionals | 1.9 | 3.9 | 6.3 | 2.6 | 5.7 | 3.5 | 3.9 | 4.5 | 5.2 | 4.3 | 4.5 | 4.0 |
| Outpatient appointments with expert in condition or undiagnosed conditions | 2.8 | 3.6 | 4.2 | 3.2 | 4.4 | 3.4 | 2.4 | 2.7 | 6.7 | 1.1 | 3.6 | 3.2 |
| Visits to practice nurse | 2.4 | 3.5 | 3.8 | 3.6 | 2.6 | 3.3 | 1.3 | 1.5 | 2.0 | 1.4 | 1.2 | 1.5 |
| Outpatient appointments with physiotherapist | 1.0 | 1.4 | 1.8 | 1.4 | 2.4 | 1.2 | 4.3 | 2.5 | 4.3 | 2.5 | 3.3 | 2.3 |
| Outpatient appointments with specialist nurse | 1.2 | 1.3 | 1.7 | 1.1 | 2.4 | 1.2 | 2.7 | 2.3 | 5.2 | 1.4 | 2.0 | 2.8 |
| Other NHS contacts | 0.6 | 1.3 | 1.3 | 1.2 | 2.5 | 1.1 | 1.8 | 4.4 | 2.7 | 5.1 | 5.7 | 1.7 |
| Outpatient appointments with psychologist | 0.3 | 1.2 | 1.0 | 0.6 | 1.8 | 0.9 | 0.8 | 1.0 | 1.3 | 0.7 | 1.1 | 0.8 |
| Emergency department attendances | 0.6 | 1.2 | 1.4 | 1.1 | 1.6 | 1.1 | 2.0 | 1.7 | 2.0 | 1.8 | 1.9 | 1.5 |
| NHS 111 calls | 0.5 | 1.1 | 0.9 | 1.1 | 1.1 | 1.0 | 1.5 | 1.7 | 2.4 | 1.3 | 1.4 | 1.3 |
| Home visits from other HCPs | 0.2 | 1.0 | 0.6 | 0.9 | 0.2 | 0.8 | 5.5 | 4.2 | 5.8 | 5.0 | 5.6 | 2.3 |
| Visits to an urgent care centre (including walk-in centre or minor injuries unit) | 0.3 | 0.8 | 0.9 | 0.6 | 1.1 | 0.6 | 0.7 | 0.8 | 0.6 | 0.9 | 0.7 | 0.9 |
| Planned admissions to hospital | 0.3 | 0.7 | 0.8 | 0.6 | 0.7 | 0.6 | 0.9 | 0.7 | 1.5 | 0.8 | 0.8 | 1.0 |
| Emergency admissions to hospital | 0.2 | 0.7 | 1.0 | 0.7 | 1.5 | 0.6 | 1.2 | 1.3 | 1.8 | 1.3 | 1.4 | 1.1 |
| Attendances at acute admissions ward | 0.2 | 0.6 | 0.7 | 0.6 | 1.2 | 0.5 | 1.8 | 0.6 | 1.4 | 0.9 | 0.9 | 0.9 |
| Outpatient appointments with occupational therapist | 0.1 | 0.4 | 0.3 | 0.4 | 0.4 | 0.3 | 4.2 | 1.6 | 3.0 | 1.9 | 2.8 | 1.3 |
| 999 calls | 0.2 | 0.3 | 0.4 | 0.3 | 0.6 | 0.2 | 1.1 | 0.6 | 0.9 | 0.7 | 0.8 | 0.6 |
| GP home visits | 0.0 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 1.0 | 0.4 | 0.5 | 0.5 | 0.5 | 0.5 |
| Outpatient appointments with behavioural therapist | 0.1 | 0.1 | 0.3 | 0.1 | 0.3 | 0.1 | 0.3 | 1.5 | 0.3 | 1.8 | 2.0 | 0.3 |
| Outpatient appointments with genetic counsellor | 0.3 | 0.1 | 0.6 | 0.2 | 1.4 | 0.1 | 0.4 | 0.3 | 0.4 | 0.3 | 0.4 | 0.3 |
| Outpatient appointments with care co-ordinator | 0.5 | 0.0 | 0.3 | 0.1 | 0.6 | 0.1 | 1.6 | 0.0 | 1.0 | 0.1 | 0.6 | 0.0 |
Perceived impact of care co-ordination on quality of care
Participants were asked to indicate how much they agreed or disagreed with the statements that having a care co-ordinator, attending a specialist centre or having a care plan improved the quality of care for people affected by rare conditions, regardless of their prior knowledge or experience of these factors. More than 70% of patients, parents/carers and HCPs agreed or strongly agreed that having a care co-ordinator would improve quality of care (Table 12), which is in sharp contrast to the numbers reporting access to this service (i.e. 12% of patients and 14% of parents/carers). More than 80% of patients, parents/carers and HCPs agreed or strongly agreed that having access to a specialist centre would improve quality of care, compared with 32% of patients and 33% of parents/carers actually having access to one. Similarly, more than 80% of participants in each group agreed or strongly agreed that having a care plan would improve quality of care, compared with 10% of patients and 44% of parents/carers actually having a care plan.
| Survey statements and answers | Patients (N = 760), n (%) | Carers (N = 446), n (%) | HCPs (N = 251), n (%) |
|---|---|---|---|
| Having a care co-ordinator improves the quality of care for people with rare conditions | |||
| Strongly agree | 257 (46) | 132 (45) | 95 (48) |
| Agree | 170 (31) | 91 (31) | 78 (39) |
| Neither agree nor disagree | 119 (21) | 64 (22) | 25 (13) |
| Disagree | 5 (1) | 3 (1) | 1 (1) |
| Strongly disagree | 3 (1) | 2 (1) | 0 (0) |
| Total | 554 (100) | 292 (100) | 199 (100) |
| Missing | 206 | 154 | 52 |
| Attending a specialist centre improves the quality of care for people with rare conditions | |||
| Strongly agree | 343 (62) | 173 (59) | 110 (55) |
| Agree | 149 (27) | 73 (25) | 65 (33) |
| Neither agree nor disagree | 55 (10) | 45 (15) | 24 (12) |
| Disagree | 3 (1) | 0 (0) | 0 (0) |
| Strongly disagree | 4 (1) | 1 (0) | 0 (0) |
| Total | 554 (100) | 292 (100) | 199 (100) |
| Missing | 206 | 154 | 52 |
| Having a care plan improves the quality of care for people with rare conditions | |||
| Strongly agree | 275 (50) | 153 (52) | 89 (45) |
| Agree | 179 (32) | 93 (32) | 93 (47) |
| Neither agree nor disagree | 96 (17) | 39 (13) | 16 (8) |
| Disagree | 3 (1) | 4 (1) | 1 (1) |
| Strongly disagree | 1 (0) | 3 (1) | 0 (0) |
| Total | 554 (100) | 292 (100) | 199 (100) |
| Missing | 206 | 154 | 52 |
Discussion
Key findings
We undertook a national survey, involving 760 adult patients affected by rare diseases, 446 parents/carers of people affected by rare diseases and 251 HCPs who care for people affected by rare diseases, to understand how care of people with rare conditions is co-ordinated in the UK. Only 12% of adult patients affected by a rare disease reported that they had a formal care co-ordinator, 32% reported that they attended a specialist centre and 10% reported that they had a care plan. For parents/carers of patients affected by a rare disease, these figures were 14%, 33% and 44%, respectively. Fifty-four per cent of patients and 33% of parents/carers had access to neither a formal care co-ordinator, nor a care plan, nor a specialist centre. By contrast, only 2% of patients and 5% of parents/carers reported having access to all three elements.
How the findings relate to previous research
There are few UK data to compare the current findings with. Nonetheless, the findings do support the findings of a limited number of previous studies,5,10 that is that levels of care co-ordination experienced by people affected by rare diseases in the UK are poor. For example, the 2016 survey by Rare Disease UK of more than 1200 people affected by rare conditions (including some carers) found that 12% of respondents had a care co-ordinator and 30% of respondents were aware of a specialist centre for their condition, with 66% of these respondents accessing it. 5
Limitations
It is not possible to determine the generalisability of the results from this study. There is a paucity of evidence regarding the total number of people living with a rare condition in the UK and their characteristics, such as gender, age distribution, ethnicity, level of education and socioeconomic status, are unknown. Respondents to this study were self-selected after receiving an e-mail or social media message about the survey, usually from a patient organisation that was on the distribution list of one or more of the study partners. We also recruited patients via NHS providers, but some patient groups may be under-represented in the survey respondents. For example, people who do not have links to one of the patient organisations and who do not use the NHS hospital services included in our study will be under-represented in the survey respondents. We offered several routes to accessing the survey (and not just via the online platform); however, there still remains a chance that people affected by rare diseases without a computer/e-mail address and those with lower electronic health literacy will be under-represented. It is assumed that the high proportion of females with a rare condition and female parents/carers in this study is over-representative. This may be because women are more likely to respond to surveys or because people were recruited to the study through patient organisations that women may be more likely to use. Sample bias may also exist in relation to the type of diseases that were represented among the study respondents. Some conditions may be over-represented in relation to the total sample size. Similarly, it is possible that more severe disorders, such as those that lead to major incapacity and/or early death, are under-represented. Despite this, our findings represent more than 200 rare diseases. In addition, as noted, it was not possible to estimate a response rate for each survey group. This was because the survey was sent via multiple overlapping distribution routes using convenience sampling and snowball sampling techniques, with multiple reminders and prompts to complete it. Therefore, although efforts were made to maximise the number of responses, it was not possible to know how many people received a request to participate in the survey. A survey undertaken by Rare Disease UK in 20165 used similar sampling processes and achieved a sample size of 1213, which suggests that our response rate was not especially low for undertaking research into people affected by are conditions.
Further research
This study provides new evidence on the extent of care co-ordination for people affected by rare conditions in the UK. We have provided evidence that only a small proportion of respondents have access to key aspects of care co-ordination. Further research would be beneficial to build on these findings and to identify new models of care co-ordination that could then be the subject of formal evaluation. Given the variation in preferences that was evident from the open-text feedback (e.g. around the benefits and limitations of different aspects of care co-ordination), further research would be valuable to gain more in-depth understanding of the diversity of preferences and the circumstances under which certain views dominate.
Summary
The findings of this study highlight that care for people affected by rare diseases is generally not well co-ordinated in the UK, with limited access to care co-ordinators, specialist centres and care plans. Better understanding of these issues can inform how care co-ordination might be improved and centred around the needs and preferences of patients and families affected by rare conditions.
Chapter 6 Discrete choice experiment to analyse preferences for co-ordinated care for rare diseases
Overview
What was already known?
-
Care for people affected by rare diseases is not well co-ordinated.
-
There are several ways in which care could be co-ordinated for people affected by rare diseases, including using care co-ordinators, specialist centres, better communication and different types of support for patients, families and HCPs.
What this chapter adds
-
We undertook a DCE to evaluate preferences for co-ordinated care by patients, parents/carers and HCPs.
-
Participants preferred services where the cost of attending appointments was lower, electronic health records were immediately accessible to staff, the lead consultant was a medical expert in the patient’s specific medical condition, care was provided with the support of a care co-ordinator, a specialist centre was available and there was a documented emergency plan in place. There were some differences between the preferences of patients and parents/carers, and HCPs.
Background
The scoping review in Chapter 3 identified several ways in which care could be co-ordinated for people affected by rare diseases, including through the use of care co-ordinators, specialist centres, better communication and different types of support for patients, families and HCPs. 15 The aim of this study was to examine patient, parent/carer and HCP preferences for different attributes of care co-ordination for people affected by rare diseases, and how these preferences varied between groups. To the best of our knowledge, there are no previous studies that have examined this topic in the context of rare diseases, although similar work has been conducted in the care of older people. 198
Methods
Overview of approach
Preferences were explored using a DCE. 13 In DCEs, respondents are typically presented with a series of questions that ask them to choose between two or more alternatives that describe a service in terms of a set of characteristics (attributes). This allows the attributes of a service that respondents prefer, as well as the trade-off they are willing to make between attributes, to be evaluated. These methods have been used to examine practitioner preferences for care co-ordination among older people. 198 DCE guidelines were followed for study design and analysis. 199 The DCE was one section of the survey described in Chapter 5.
Survey sampling
For details about the DCE sampling see Chapter 5, Survey sampling.
Attributes and levels
The attributes and levels used in the DCE, describing elements of co-ordinated care, were identified from the same sources used to develop the survey instrument (see Chapter 5, Survey instrument). First, a scoping review15 of 154 reviews of co-ordinated care for rare conditions identified components of co-ordinated care within the context of rare diseases. Second, we ran three focus groups that involved patients aged ≥ 18 years affected by a rare condition, parents/carers of children and adults affected by a rare condition and HCPs involved in the treatment of rare conditions. Third, we ran 15 one-to-one interviews that involved seven patients and eight parents/carers. In the focus groups and interviews, we asked respondents to identify the characteristics of co-ordinated care that mattered most to them. Analyses of these data identified six attributes reflecting the extent of care co-ordination for rare conditions: (1) cost to patients and carers of attending all appointments during 1 year, (2) access to health records, (3) clinical expertise, (4) role of the care co-ordinator, (5) access to a specialist centre and (6) having a documented emergency care plan (Table 13). Other potential attributes not included in the final study were the extent of patient choice, time spent by patients and carers and other aspects of co-ordinated care provision, such as mode of communication and ways of organising care. Credible levels for each attribute were chosen based on either known characteristics (e.g. the presence of that aspect of care co-ordination) or feedback from the interview and focus groups (e.g. preferred interaction with care co-ordinators and costs for attending appointments). Descriptions were developed for each of the attributes to help participants understand the nature of each attribute that they were being asked to consider (see Report Supplementary Material 1). All material was scrutinised by the PPIAG. The PPIAG agreed that the attributes and levels were appropriate and reasonable, and made changes to the questionnaire descriptions.
| Attribute | Description | Levels | |||||
|---|---|---|---|---|---|---|---|
| Cost to patients and carers of attending all appointments during 1 year | Describes the cost to patients and carers of attending all health-care appointments during 1 year (e.g. travel costs, time off work, child-care costs and subsistence) | £200 | £400 | £1000 | £2000 | ||
| Access to health records | Describes the way in which health records are shared by different HCPs in the same centre or across different health settings | Health records are not shared, test results and clinic letters are sent through the post | Electronic health records are immediately accessible to staff | ||||
| Clinical expertise | The type of medical professional who is the lead consultant and makes the majority of decisions regarding medical care | The lead consultant is a medical expert in your specific condition | The lead consultant is a medical expert in the area of the body primarily affected by your condition (e.g. neurologist) | ||||
| Role of care co-ordinator | Describes the amount of involvement of a formal care co-ordinator who is a HCP | Care is provided without the support of a care co-ordinator | Care is entirely co-ordinated on your behalf by a care co-ordinator | You have a named care co-ordinator and you decide how they support you | |||
| Access to a specialist centre | A specialist centre enables patients to see a number of HCPs in one visit. Generally, they will be experts in rare and undiagnosed conditions. Non-HCPs may also see patients at the same centre | You do not have access to a specialist centre | A specialist centre is available | ||||
| Documented emergency plan | A formal emergency plan describes the correct treatment that should be provided in urgent situations and contact details for a HCP who has knowledge of the specific condition | There is a documented emergency plan in place | No documented emergency plan exists | ||||
Discrete choice experiment questionnaire design
Respondents were asked to choose their preferred option of care from a series of pairwise choices. Each service was described by a combination of different levels of the attributes (see Table 14 for an example of a DCE ‘choice set’). An opt-out or ‘neither’ option was not included, as people are unlikely to choose none of the available options, given current levels of service provision. The number of potential combinations of attributes with four two-level attributes, one three-level attribute and one four-level attribute was 192 (24 × 31 × 41). With two options to choose from in each choice question, this gave a possible 36,672 choices (192 × 191). To reduce the number of choices to a manageable number, a fractional design was applied, where the final design was selected using relative D-efficiency to maximise the balance and orthogonality of the design (this was undertaken using the –dcreate– command in Stata). Initially, the coefficient parameters were assumed to be zero. The choice set was reduced to 18 scenarios, which were split into three blocks of six, and a third of the respondents in each group were assigned to each block. It was felt, based on the feedback received, that six choice questions was a reasonable number to ask study participants. Nine versions of the DCE questionnaire were used (i.e. three for patients, three for parents/carers and three for HCPs). The questionnaire also included a question asking respondents to rank the six attributes according to their overall importance, from 1 (most important) to 6 (least important). Information on demographic, socioeconomic and rare disease-related experience was also collected. The questionnaire was piloted in 11 respondents (three patients, four carers and four HCPs) via three think-aloud interviews (with two carers and one HCP), with the other eight respondents providing written feedback. This resulted in minor improvements being made to the wording of the questionnaire.
| Attribute | Service | |
|---|---|---|
| A | B | |
| Cost of attending all appointments over 1 year | £200 | £1000 |
| Access to health records | Health records are not shared, test results and clinic letters are sent through the post | Electronic health records are immediately accessible to staff |
| Clinical expertise | The lead consultant is a medical expert in your specific condition | The lead consultant is a medical expert in the area of the body primarily affected by your condition (e.g. neurologist) |
| Role of co-ordinator | Care is provided without the support of a care co-ordinator | Care is entirely co-ordinated on your behalf by a care co-ordinator |
| Access to specialist centre | A specialist centre is available | You do not have access to a specialist centre |
| Documented emergency plan | There is a documented emergency plan in place | No documented emergency plan exists |
Data analysis
Descriptive statistics for the characteristics of respondents were computed. Responses to the ranking questions were analysed graphically. The DCE data were analysed using alternative-specific conditional logit regression models, in which the outcome was service preference (i.e. service A or service B) and the variables in the equation were the individual attributes. A constant term was not included. Models were run for each group separately and differences in preferences between the groups were tested by comparing the coefficients for each group using chi-squared tests. Where the coefficients were not jointly different between groups, those groups were combined in subsequent analyses. The relative importance of each attribute was calculated as the difference in the coefficients between the best or most preferred level of each attribute and the worst or least preferred level of the same attribute. 200 We calculated MRSs with respect to the cost attribute (e.g. cost to patients and carers of attending all appointments during 1 year). This allows direct assessment of how much of one attribute participants are willing to trade for one unit of another attribute and, therefore, enables a comparison of different attributes on a common scale. Using the cost attribute as the denominator means that participants’ preferences and the trade-offs can be evaluated in terms of willingness to pay. The standard error of the MRS was calculated using the delta method. Findings from the regression analysis were used to calculate the predicted probabilities of choosing co-ordinated services compared with no co-ordination. No co-ordination was defined as cost to patients and carers of attending all appointments during 1 year were £1000; health records were not shared; the lead consultant was a medical expert in the area of the body primarily affected by the patient’s condition (e.g. neurologist); care was provided without the support of a care co-ordinator; a specialist centre was not available; and there was a documented emergency plan in place. In each co-ordination scenario, costs remained fixed at £1000 (i.e. co-ordination has no impact on costs) and the following potential characteristics of a co-ordinated service were amended individually and then jointly: electronic health records were immediately accessible to staff; the lead consultant was a medical expert in the patient’s specific condition; the patient/carer decided how they wished to be supported by the care co-ordinator; a specialist centre was available; and there was a documented emergency plan in place. We recalculated the predicted probabilities (1) assuming that no co-ordination was associated with high costs (£2000) and co-ordination was associated with low cost (£200) and (2) assuming that no co-ordination was associated with low costs and co-ordination was associated with high cost. All analyses were undertaken using Stata.
Results
Responses and sample
In total, 996 responses to the DCE section of the survey were received, 528 responses from patients, 280 responses from carers and 188 responses from HCPs. Descriptive statistics of respondents who completed the DCE are in Table 15. These descriptive statistics are similar to the descriptive statistics for the survey respondents overall (see Chapter 5, Responses and sample).
| Descriptive characteristic | Patients (N = 528), n (%) | Parents/carers (N = 280), n (%) | HCPs (N = 188), n (%) |
|---|---|---|---|
| Age of patient (years) | |||
| 0–5 | 66 (24 | ||
| 6–12 | 81 (29) | ||
| 13–17 | 34 (12) | ||
| 18–24 | 21 (4) | 33 (12) | |
| 25–34 | 75 (14) | 18 (6) | |
| 35–44 | 94 (18) | 8 (3) | |
| 45–54 | 124 (24) | 11 (4) | |
| 55–64 | 115 (22) | 12 (4) | |
| 65–74 | 66 (13) | 4 (1) | |
| ≥ 75 | 14 (3) | 1 (0) | |
| Missing | 19 (4) | 12 (4) | |
| Age of parent/carer (years) | |||
| 18–24 | 5 (2) | ||
| 25–34 | 36 (13) | ||
| 35–44 | 94 (34) | ||
| 45–54 | 86 (30) | ||
| 55–64 | 36 (13) | ||
| 65–74 | 11 (4) | ||
| ≥ 75 | 1 (0) | ||
| Missing | 11 (4) | ||
| Sex | |||
| Female | 434 (82) | 235 (84) | |
| Male | 73 (14) | 32 (11) | |
| Other | 2 (0) | 1 (0) | |
| Missing | 19 (4) | 12 (4) | |
| Diagnosed with rare disease | |||
| Yes | 513 (97) | 257 (92) | |
| No (undiagnosed) | 15 (3) | 23 (8) | |
| Diagnosis confirmed with genetic test | |||
| Yes | 155 (29) | 167 (60) | |
| No | 258 (54) | 68 (24) | |
| Unsure | 73 (14) | 22 (8) | |
| N/A (undiagnosed) | 15 (3) | 23 (8) | |
| Top 10 most common rare diseases | |||
| 1 | Sarcoidosis, 67 (13) | Tracheo-oesophageal fistula, 10 (4) | |
| 2 | Behçet’s syndrome, 52 (10) | Behçet’s syndrome, 6 (2) | |
| 3 | Idiopathic intracranial hypertension, 36 (7) | Rett syndrome, 5 (2) | |
| 4 | Lynch syndrome, 17 (3) | Aplastic anaemia, 4 (1) | |
| 5 | Ehlers–Danlos syndrome, 12 (2) | Tuberous sclerosis, 4 (1) | |
| 6 | IgA nephropathy, 12 (2) | Sarcoidosis, 3 (1) | |
| 7 | Familial partial lipodystrophy, 10 (2) | Growth hormone deficiency, 3 (1) | |
| 8 | Ocular melanoma, 8 (2) | Alpha thalassemia X-linked intellectual disability syndrome, 3 (1) | |
| 9 | Tarlov cyst disease, (1) | Idiopathic intracranial hypertension, 3 (1) | |
| 10 | Common variable immune deficiency, 6 (1) | Williams syndrome, 3 (1) | |
| Parent’s/carer’s relationship to patient | |||
| Spouse or partner | 23 (8) | ||
| Parent | 192 (69) | ||
| Son or daughter | 41 (15) | ||
| Other | 24 (9) | ||
| Parent’s/carer’s living arrangements | |||
| Lives with patient | 244 (87) | ||
| Does not live with patient | 24 (9) | ||
| Missing | 12 (4) | ||
| Patient’s living arrangements | |||
| Lives alone | 115 (22) | ||
| Lives with a spouse or partner | 289 (55) | ||
| Lives with family members or friends | 99 (19) | ||
| Lives with a carer | 2 (0) | ||
| Missing | 23 (4) | ||
| Geographical region | |||
| East of England | 42 (8) | 17 (6) | 6 (3) |
| East Midlands | 24 (5) | 17 (6) | 11 (6) |
| London | 52 (10) | 26 (9) | 34 (18) |
| North East and Cumbria | 23 (4) | 14 (5) | 7 (4) |
| Northern Ireland | 15 (3) | 1 (0) | 1 (1) |
| North-west of England | 51 (10) | 34 (12) | 66 (35) |
| Scotland | 60 (11) | 21 (8) | 6 (3) |
| South-east of England | 65 (12) | 35 (13) | 9 (5) |
| South-west of England | 61 (11) | 26 (9) | 12 (6) |
| Wales | 39 (7) | 9 (3) | 1 (1) |
| West Midlands | 31 (6) | 48 (17) | 25 (13) |
| Yorkshire | 35 (7) | 16 (6) | 4 (2) |
| Other | 8 (2) | 7 (3) | 4 (2) |
| Missing | 22 (4) | 9 (3) | 2 (1) |
| Ethnic group | |||
| White | 473 (90) | 245 (88) | |
| Non-white | 20 (4) | 20 (7) | |
| Missing | 35 (6) | 15 (5) | |
| Educational attainment | |||
| No formal qualifications | 18 (3) | 6 (2) | |
| O Level or GCSE, or equivalent | 68 (13) | 41 (15) | |
| ONC or BTEC, or equivalent | 21 (4) | 14 (5) | |
| A Level (‘Higher’ in Scotland) or equivalent | 35 (7) | 26 (9) | |
| Higher education qualification below degree level or equivalent | 102 (19) | 40 (14) | |
| Degree or higher degree or equivalent | 252 (48) | 130 (46) | |
| Prefer not to say | 32 (6) | 23 (8) | |
| Clinical expertise in rare diseases | |||
| Yes | 107 (57) | ||
| No | 81 (43) | ||
| Areas of work with patients with rare conditions | |||
| Diagnosing condition | 117 (62) | ||
| Providing information/signposting or counselling | 148 (79) | ||
| Long-term care following diagnosis | 127 (67) | ||
| Long-term care in the absence of a diagnosis | 109 (58) | ||
| HCP role | |||
| Allied health professional | 28 (15) | ||
| Hospital doctor | 78 (42) | ||
| GP/community doctor | 12 (6) | ||
| Nurse/midwife | 39 (21) | ||
| Clinical academic | 24 (13) | ||
| Other | 7 (4) | ||
Simple attribute ranking
The responses to the ranking question posed after the DCE questions were examined (Figure 9). Ninety-seven per cent of patients and carers and 99% of HCPs provided full responses to this question. Attributes were ranked by likelihood of being selected as the most important factor. Clinical expertise and access to a specialist centre were ranked highly by each group, and the role of the care co-ordinator was consistently ranked to be the least important factor. Attributes were ranked in the same order for each group.
FIGURE 9.
Ranking of attributes by group. (a) Patients (n = 512 respondents); (b) parents/carers (n = 271 respondents); and (c) HCPs (n = 186 respondents).
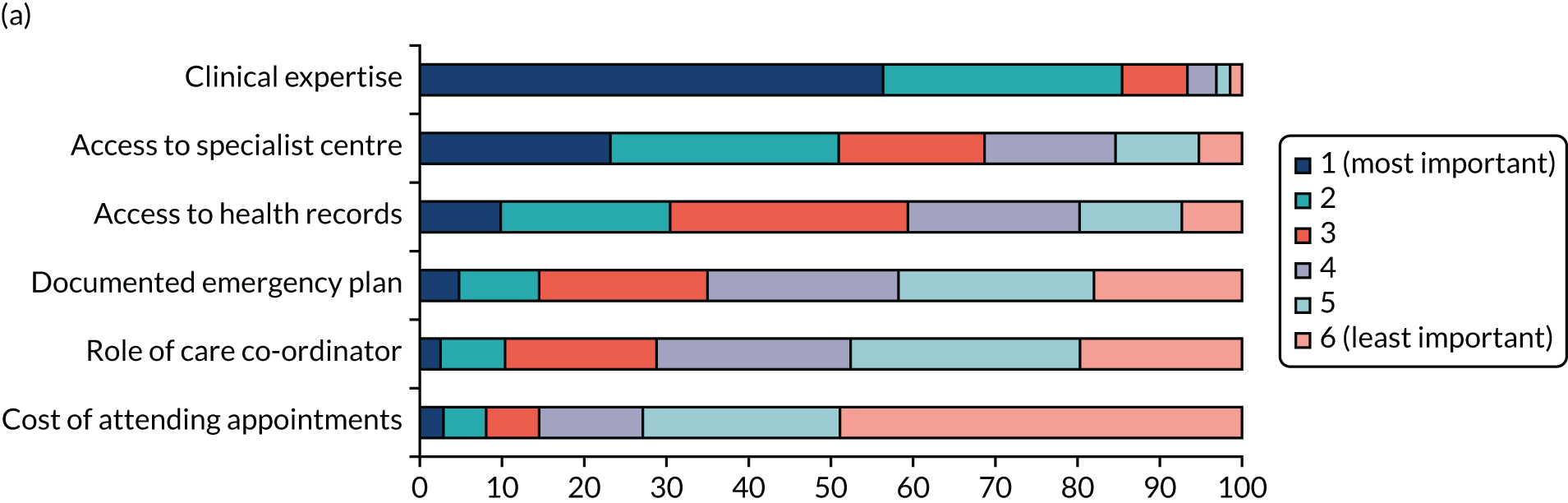
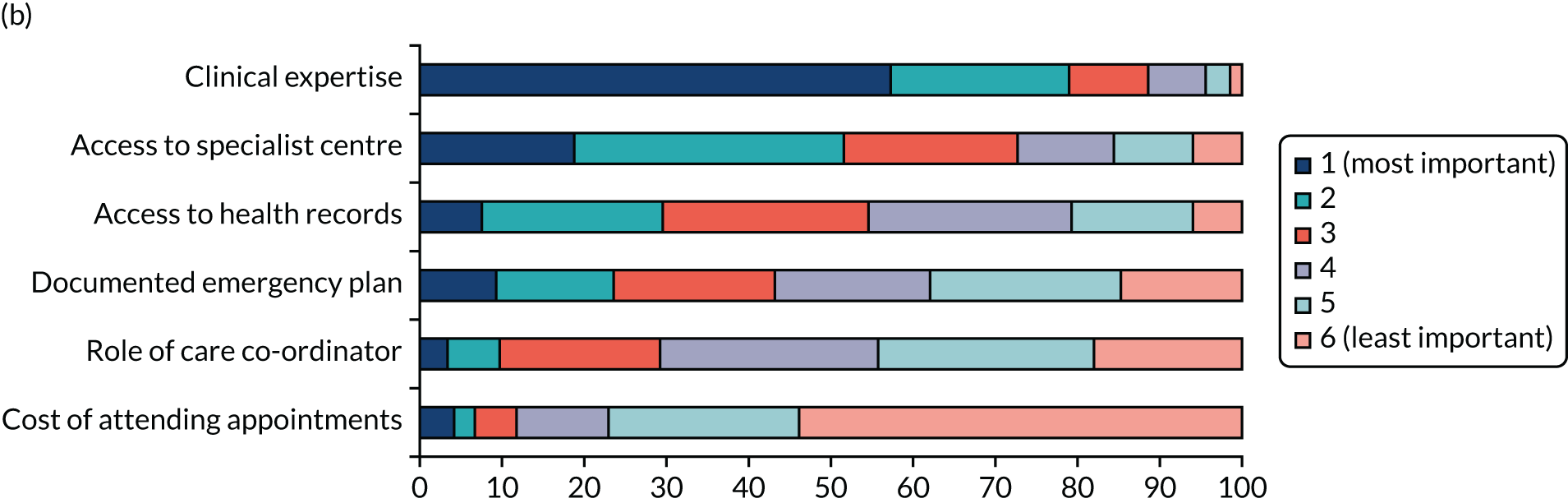

Regression analysis
There was no substantive difference in the preferences for the attributes between patients and parents/carers. The findings were quantitatively very similar (i.e. there was no statistically significant difference between the coefficients) and qualitatively identical (in terms of the sign and statistical significance of the coefficients) between the two groups, and so we reran the analyses for both groups combined (Table 16). Individuals in all groups preferred services with better co-ordination where the cost of attending appointments was lower; electronic health records were immediately accessible to staff; the lead consultant was a medical expert in the patient’s specific medical condition; care was provided with the support of a care co-ordinator; a specialist centre was available; and there was a documented emergency plan in place. There were some differences between the preferences of patients and parents/carers and the preferences of HCPs. In terms of care co-ordinators, HCPs preferred that care was entirely co-ordinated on behalf of the patient by a care co-ordinator, whereas patients and parents/carers preferred that they decided how they wished to be supported by the care co-ordinator. In terms of emergency plans, all three groups preferred there to be a documented emergency plan in place, but the preferences of HCPs for this was stronger than for patients and parents/carers.
| Analysis | Patients (n = 528) | Parents/carers (n = 280) | HCPs (n = 188) | p-valuea | p-valueb | Patients and parents/carers (n = 808) |
|---|---|---|---|---|---|---|
| Number of observations | 6336 | 3360 | 2256 | 9696 | ||
| Cost of attending appointments, coefficient (95% CI) | –0.0003 (–0.0004 to –0.0002) | –0.0002 (–0.0003 to –0.00004) | –0.0004 (–0.0006 to –0.0003) | 0.08 | 0.11 | –0.0003 (–0.0003 to –0.0002) |
| Access to health records, coefficient (95% CI) [MRS] {SE}c | ||||||
| Health records are not shared | d | d | d | d | ||
| Electronic health records are immediately accessible to staff | 0.630 (0.547 to 0.713) | 0.728 (0.611 to 0.844) | 0.761 (0.606 to 0.916) [1864] {5634} | 0.21 | 0.17 | 0.659 (0.592 to 0.723) [2442] {7828} |
| Clinical expertise, coefficient (95% CI) [MRS] {SE}c | ||||||
| The lead consultant is a medical expert in the area of the body primarily affected by the patient’s condition (e.g. neurologist) | d | d | d | d | ||
| The lead consultant is a medical expert in the patient’s specific condition | 0.685 (0.571 to 0.800) | 0.609 (0.437 to 0.780) | 0.511 (0.309 to 0.713) [1252] {4814} | 0.33 | 0.46 | 0.667 (0.592 to 0.727) [2470] {8929} |
| Role of care co-ordinator, coefficient (95% CI) [MRS] {SE}c | ||||||
| Care is provided without the support of a care co-ordinator | d | d | d | d | ||
| Care is entirely co-ordinated on behalf of the patient by a care co-ordinator | 0.236 (0.080 to 0.393) | 0.261 (0.043 to 0.480) | 0.461 (0.196 to 0.726) [1131] {5453} | < 0.01 | 0.15 | 0.249 (0.122 to 0.385) [920] {6576} |
| The patient/carer decides how they wish to be supported by the care co-ordinator | 0.312 (0.194 to 0.430) | 0.458 (0.283 to 0.634) | 0.425 (0.219 to 0.632) [1042] {4501} | 0.36 | 0.85 | 0.353 (0.255 to 0.450) [1306] {5739} |
| Access to specialist centre, coefficient (95% CI) [MRS] {SE}c | ||||||
| A specialist centre is not available | d | d | d | d | ||
| A specialist centre is available | 0.676 (0.585 to 0.766) | 0.699 (0.569 to 0.829) | 0.735 (0.561 to 0.910) [1802] {5660} | 0.83 | 0.77 | 0.677 (0.604 to 0.751) [2509] {8422} |
| Documented emergency plan, coefficient (95% CI) [MRS] {SE}c | ||||||
| No documented emergency plan exists | d | d | d | d | ||
| There is a documented emergency plan in place | 0.359 (0.270 to 0.448) | 0.393 (0.275 to 0.512) | 0.747 (0.585 to 0.909) [1832] {5617} | < 0.01 | 0.64 | 0.369 (0.298 to 0.440) [1367] {5321} |
Relative importance of the attributes
For patients and carers, access to a specialist centre was the attribute valued most highly, followed by clinical expertise, access to health records, the cost of attending all appointments during 1 year and having a documented emergency plan. The role of care co-ordinator was the attribute least valued. For HCPs, access to health records was valued most highly, followed by having a documented emergency plan, access to a specialist centre, the cost of attending all appointments during 1 year and clinical expertise. The role of care co-ordinator was valued least highly. These findings are preferred to the simple attribute ranking, as they account for the levels of the attributes.
Marginal rates of substitution
As an indication of their strength of preference, and the value they put on each attribute, patients and parents/carers were willing to pay £2509 for access to a specialist centre, £2470 for a consultant who was a medical expert in the patient’s condition, £2442 for electronic health records that were immediately accessible to staff, £1367 for a documented emergency plan and £1306 for the support of a care co-ordinator where the patient/carer decided how they wished to be supported (see Table 16). HCPs were willing to pay £1864 for electronic health records that were immediately accessible to staff, £1832 for a documented emergency plan, £1802 for patient access to a specialist centre, £1252 for a consultant who was a medical expert in the patient’s condition and £1131 for a care co-ordinator who entirely co-ordinated care on behalf of the patient. These MRS values reflect the relative importance of the attributes.
Predicted probabilities
The probability that respondents would choose a service with different types of care co-ordination compared with no co-ordination is shown in Figure 10. We defined ‘no co-ordination’ as a service where health records are not shared, the lead consultant is a medical expert in the area of the body primarily affected by the patient’s condition (e.g. neurologist), care is provided without the support of a care co-ordinator, a specialist centre is not available and there is not a documented emergency plan in place. Compared with this option, we found that respondents had a higher probability of choosing a service that had any of the individual attributes of co-ordination. For patients and parents/carers, the probabilities ranged from 0.60 to 0.67, depending on which individual attribute was selected, with the attributes ranked in terms of their predicted probability in the same order as the relative importance (see Figure 10a). If a service achieved all of the attributes of co-ordination, then the probability that patients and carers would prefer to use that service was 0.94. For HCPs, the probabilities for each individual attribute ranged from 0.59 to 0.66. For a service that achieved all of the attributes of co-ordination the probability was 0.96 (see Figure 10b). When co-ordination reduced (increased) costs compared with no co-ordination the probability that respondents would choose a service with the different types of care co-ordination increased (see Appendix 6).
FIGURE 10.
Predicted probabilities of choosing co-ordinated services. (a) Patients and parents/carers combined; and (b) HCPs. Note that for ‘no co-ordination’, health records are not shared, the lead consultant is a medical expert in the area of the body primarily affected by the patient’s condition (e.g. neurologist), care is provided without the support of a care co-ordinator, a specialist centre is not available and there is not a documented emergency plan in place. For ‘full co-ordination’, electronic health records are immediately accessible to staff, the lead consultant is a medical expert in the patient’s specific condition, the patient/carer decides how they wish to be supported by the care co-ordinator (patients/carers) or care is entirely co-ordinated by a care co-ordinator (HCPs), a specialist centre is available and there is a documented emergency plan in place. All other co-ordination scenarios are as for ‘no co-ordination’ except for the attribute indicated. In all scenarios the cost to patients and carers of attending all health-care appointments during 1 year is held constant at £1000. Scenarios are ordered from left to right in ascending order of magnitude of the predicted probability of choosing the co-ordination service (note that the ordering is different for patients and carers combined and for HCPs).
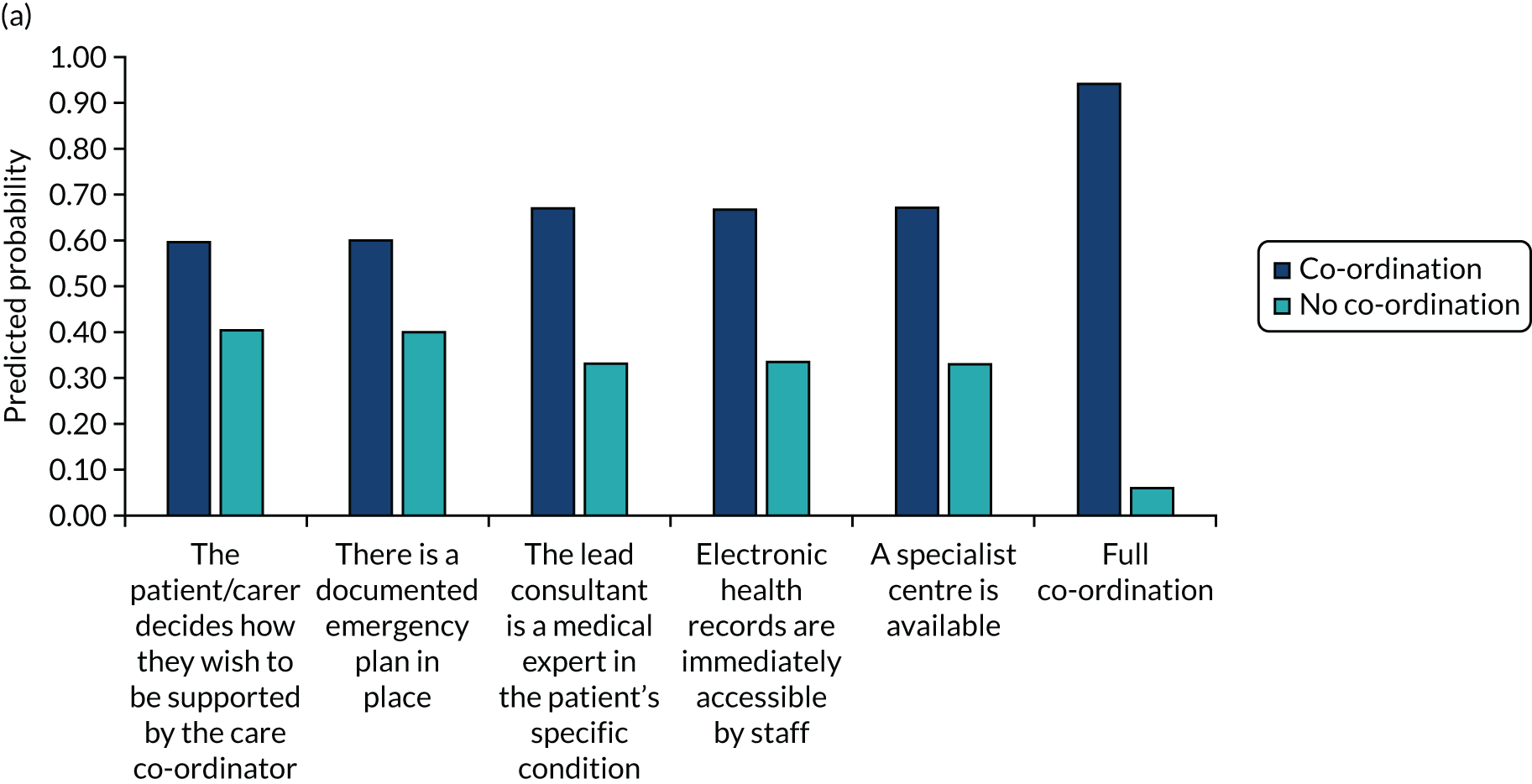
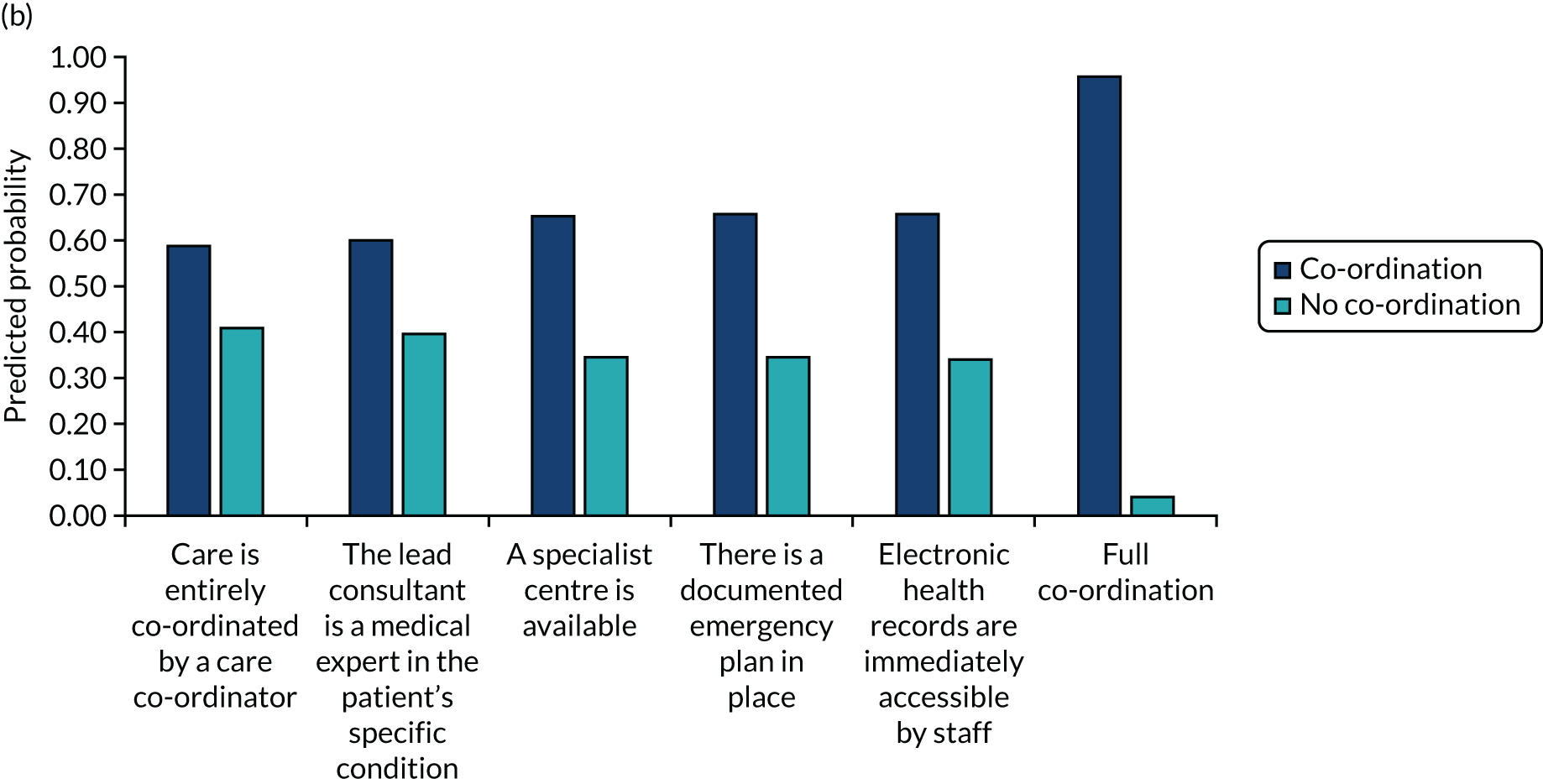
Discussion
Key findings
Patients, parents/carers and HCPs all preferred services where the cost of attending appointments was lower, electronic health records were immediately accessible to staff, the lead consultant was a medical expert in the patient’s specific medical condition, care was provided with the support of a care co-ordinator, a specialist centre was available and there was a documented emergency plan in place. Preferences were found to be consistent with better co-ordination of care, although there were some differences between the preferences of patients and parents/carers, and HCPs. The probability that participants would choose a service with all the elements of co-ordination studied in place was high.
How the findings relate to previous research
There are several studies that have explored how people affected by rare diseases would like their care to be co-ordinated, although these studies tend to focus on single options, such as care co-ordinators10 or specialist centres. 5 We are not aware of any studies that have compared between multiple aspects and, to the best of our knowledge, none have used a DCE-based approach.
Limitations
Several limitations are acknowledged. DCEs elicit hypothetical choices and, therefore, might lack external validity if individuals do not make the same choices in real-life situations. Some aspects of the DCE might be difficult for respondents to understand, such as the forced choices between services, probabilities and clinical concepts. The representativeness of the samples used might be limited by the recruitment strategies, yielding potential sampling bias (e.g. there was a high proportion of female patients and parents/carers). The modal education category was those who were educated to degree level or higher, and it is unclear if costs would have been the least important attribute if, for example, the sample was, on average, less well educated. Although the overall sample size was large, we obtained fewer responses from parents/carers and HCPs than targeted. There might be other components of co-ordinated care that are important, but were not included in the present analysis. Unfortunately, the number of attributes that can be included in a DCE is limited by the number of data that participants can process. The nature of our piloting work meant that we were unable to produce initial estimates of the model coefficients, which could have been used to inform the final study design. Initially, the coefficient parameters were assumed to be zero. Preferences might vary by subgroups within our study groups (e.g. parents of children affected by rare diseases vs. carers of adults with a rare disease), but sample size considerations make subgroup analyses problematic.
Further research
This study provides new evidence on the elements of care co-ordination that matter to people affected by rare diseases. Further research would be beneficial to develop different models based on people’s preferences, as described in this study, describing how care for people with rare conditions could be co-ordinated. These models could then be the focus of further formal evaluation. Further research would also be helpful to understand the reasons for the differences in preferences between patients and parents/carers on the one hand and for HCPs on the other.
Summary
The findings of this study highlight that people value better co-ordinated care, which is in line with policy documents that emphasise commitments to co-ordinated care for people affected by rare diseases. 7 These findings are relevant to policy-makers, service planners and providers who are designing services for people affected by rare conditions. These findings show the factors that could be included in service provision as ways of improving the co-ordination of care.
Chapter 7 Developing a taxonomy of care co-ordination for people living with rare conditions
Overview
This chapter draws on Walton et al. 201 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for non-commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc/4.0/. The text below includes minor additions and formatting changes to the original text. This chapter also draws on Walton et al. 202 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
What was already known?
-
Improving care co-ordination for people with rare conditions may help to reduce the burden associated with co-ordinating their own care.
-
Care co-ordination has many components and, therefore, there are many different ways of co-ordinating care for rare conditions.
-
Taxonomies can be used to help us to organise complex phenomena, such as care co-ordination.
What this chapter adds
-
We have developed a taxonomy of care co-ordination for rare conditions, which outlines the six domains involved in co-ordinating care for rare conditions. The six domains that we identified were (1) ways of organising care, (2) ways of organising teams, (3) responsibilities, (4) how often care appointments and co-ordination take place, (5) access to records and (6) mode.
-
We have outlined qualifying factors related to each of the taxonomy domains. The qualifying factors provide insight into different participants’ preferences, benefits and challenges of different models of care co-ordination, factors that influence co-ordination, and barriers to and facilitators of co-ordination. These findings provide information on which models of co-ordination may be suitable in different situations.
Background
To understand and evaluate care co-ordination for rare conditions, it is necessary to develop a method for organising different ways of co-ordinating care. One way to organise and understand care co-ordination is to develop a taxonomy. Taxonomies are systems used to organise complex phenomena into common conceptual domains and dimensions based on similarities. 203,204 Taxonomies are, therefore, relevant to organising a concept such as care co-ordination, which has been shown to be multifaceted with multiple components (see Chapter 3). For example, previous research has developed a taxonomy of burden of treatment for patients with chronic conditions. 205 A taxonomy can be used to outline existing and potentially new ways of co-ordinating care. A taxonomy of care co-ordination for rare diseases has the potential to facilitate the measurement of the clinical effectiveness and cost-effectiveness of pre-existing and new options for co-ordinating care, and may also help researchers to develop new models. If resulting care co-ordination strategies are piloted, evaluated and eventually implemented more widely within the NHS, then this will hopefully lead to better care and reduced burden for people living with rare diseases. 18,20
To our knowledge, no previous studies have attempted to develop a taxonomy of care co-ordination for rare conditions.
This study aimed to develop and refine a proposed taxonomy of care co-ordination for people living with rare conditions.
We explored the following RQs:
-
What ways of co-ordinating care exist currently and are possible?
-
What are stakeholders’ preferences in relation to different ways of co-ordinating care?
-
What are stakeholders’ recommendations to improve the taxonomy?
Methods
Design
Our study used qualitative methods (i.e. interviews, focus groups and workshops).
Using a qualitative approach allows for a more in-depth understanding of complex phenomena. 206,207 In addition, the use of qualitative methods allows for the direct involvement of those with most experience in the phenomena being studied and classified, such as patients, HCPs and carers. This is particularly important in health-care service research in which patients, carers and HCPs are the key stakeholders. 208 By understanding patients’, carers’ and HCPs’ views on the organisation of care co-ordination for rare diseases, we could improve health-care services and optimise the patient experience, therefore, reducing burden. 209 It has also been proposed that qualitative studies are well suited to explore new concepts. 207 As co-ordination of care is a relatively new field, using qualitative methods will offer a rich perspective on care and stakeholders’ preferences.
This research was conducted in a two-stage process. First, interviews and focus groups were conducted to develop an initial taxonomy. Interviews and focus groups were felt to be appropriate methods for exploring and gathering in-depth perspectives on stakeholders’ experiences of co-ordination and the different models of care co-ordination that currently exist, together with preferences for potential new models of co-ordination. Second, workshops were conducted to refine the proposed taxonomy. Workshops were felt to be appropriate for helping to gather consensus around whether or not the taxonomy was appropriate and to develop recommendations to improve the taxonomy. Figure 11 outlines the two stages.
FIGURE 11.
Overview of the two stages involved in developing the taxonomy.
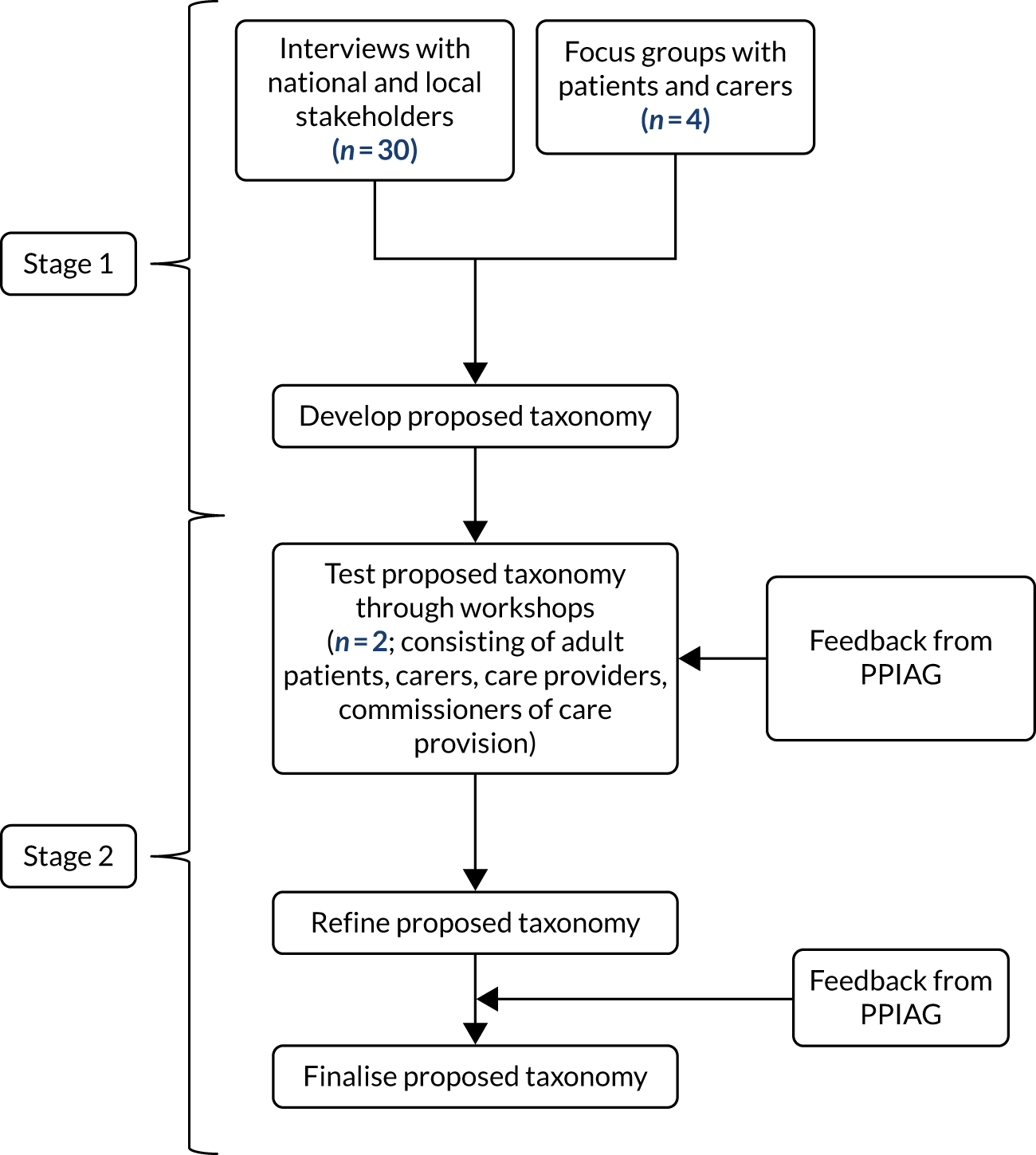
Sample
We recruited a range of participants, including patients with rare, ultra-rare or undiagnosed conditions, carers/parents, HCPs, charity representatives and commissioners, to take part in the interviews (n = 30), focus groups (four groups of between six and eight participants210) and workshops (two workshops, one for patients/carers and one for professionals, of approximately 15 participants each). We originally planned for five face-to-face workshops; however, we had to reduce this to two remote workshops because of the COVID-19 pandemic. Interview and focus group participants informed the development of the taxonomy. Workshop participants informed the refinement of the taxonomy.
To take part in the study, participants needed to be aged ≥ 18 years. Children were not included because of ethics issues relating to recruiting participants aged < 18 years. One focus group participant withdrew from the study after the focus group, resulting in 22 patients and carers taking part in the focus groups.
Participants were recruited using a range of methods, including e-mail invitation, social media, via the voluntary sector and through our partnerships with four NHS sites.
As there are currently between 6000 and 8000 rare diseases,20 it was not possible to include participants affected by every rare disease. To ensure that different models of co-ordinated care (including different types of care co-ordination and no co-ordination) and a wide range of experience and expertise were captured, we used purposive sampling. We sampled professionals based on their area of the UK, job role and experience with different types of care co-ordination. We sampled patients and carers based on their area of the UK, condition, role, age and experience with different types of care co-ordination.
Measures
To gather data to inform the development of the taxonomy, two topic guides (one for interviews and one for focus groups) were developed and used to collect data (see Report Supplementary Material 1). Questions focused on stakeholders’ experiences of co-ordinated care, implications of co-ordinated care, preferences for key aspects of care co-ordination (including preferred way of co-ordinating care, format, access, frequency, location, information-sharing and transition), benefits and challenges, and factors that help and get in the way of co-ordination. Feedback on the topic guide was sought from the PPIAG prior to data collection.
To gather data to refine the taxonomy, one topic guide for both workshops was developed and used to collect data (see Report Supplementary Material 1). The topic guide was based around the six categories identified in the taxonomy and prompted participants about whether or not they had feedback on the category (e.g. if we had missed anything and whether or not findings seemed appropriate based on participant experiences), the appropriateness of options in the light of the COVID-19 pandemic and asked for recommendations to improve the category.
Procedure
Participants were recruited using a range of methods, including e-mail invitation, social media, via the charity sector and through our partnerships with four NHS sites. Potential participants were asked to contact the study researcher via e-mail or telephone.
To ensure that a range of participants with different experiences were recruited, potential participants were asked to provide responses to eligibility questions when registering their interest. Participants were sent these eligibility questions by e-mail. For HCPs, these eligibility questions included questions regarding their occupation, specialty and geographical region. For patients and carers, these eligibility questions included questions regarding age, ethnicity, geographical region, role, whether or not they receive co-ordinated care (i.e. a specialist service and who co-ordinates) and whether or not they have a diagnosis. The researcher checked that participants met the eligibility criteria for the study.
Selected individuals were asked to complete consent forms (one for the researcher and one for the participant) prior to taking part in the interviews, focus groups or workshops. Participants who took part virtually or via telephone were asked to return written consent forms in advance. Participants were informed that their data would be kept confidential, fully anonymised and that they could withdraw at any time without providing a reason. Focus group participants were informed that any data collected up until the point of withdrawal would be kept because of difficulties removing individual participants from focus group data. We took steps to ensure that quotations from the participant who withdrew from the study were not included in publications. These steps included removing withdrawn quotations from the analysis spreadsheet.
To gather data to inform the development of a taxonomy, interviews with HCPs, charity representatives and commissioners, and focus groups with patients and carers, were conducted.
One researcher conducted interviews either by telephone or face to face, depending on participants’ preferences. The interviews lasted approximately 1 hour (range 44–74 minutes). Two researchers (HW and AS) conducted the four focus groups (one researcher facilitated and one researcher took notes). 210 A third researcher (EH) observed one of the focus groups. Two focus groups were conducted face to face (one in London and one in Birmingham) and two focus groups were conducted virtually using Skype. Focus groups were up to 3 hours in length, including a break (range 149–154 minutes). Interviews and focus groups were digitally recorded using an encrypted dictaphone (with consent from participants) and professionally transcribed. Transcripts were checked for accuracy and fully anonymised (including names and places). Data were stored in the University College London Data Safe Haven (a secure electronic environment, certified to ISO27001 information security standard, conforming to the NHS Information Governance Toolkit) and coded using NVivo 12 (QSR International, Warrington, UK).
To refine the taxonomy, workshops were conducted virtually. Workshop participants were sent a brief 15-minute video prior to the workshop, which outlined the findings of the taxonomy (including each domain and the options within each domain). The presentation also covered qualifier findings, including preferences, barriers/facilitators, factors influencing co-ordination and benefits/challenges of different options. During the workshops, participants were given an introduction to the workshop before being split into three breakout groups. Each breakout group had one facilitator (HW, EH or AIGR) and one note taker (JJ, SM or AH). During the breakout groups, facilitators encouraged the small groups to discuss each of the six domains in the taxonomy. Facilitators prompted participants about whether or not they had feedback on the category (i.e. whether or not anything had been missed and whether or not findings seemed appropriate based on participant experiences), the appropriateness of options in the light of the COVID-19 pandemic and asked for recommendations to improve the category. After the breakout groups, participants reconvened in the main group and each group provided feedback on their discussions. Workshops were recorded using an encrypted dictaphone. Notes were checked for thoroughness and summarised prior to being sent to a graphic facilitator (New Possibilities, Birmingham, UK) to create a graphical representation of the findings.
Analysis
Thematic analysis was used to analyse interview and focus group data. It is recommended that inductive and deductive data analysis approaches are used to develop taxonomies. 204 Therefore, to generate codes, a combination of inductive and deductive coding was used.
To develop an initial coding frame, six interview transcripts were coded inductively by two researchers (HW and AS). From this, a coding framework was developed and agreed. The coding framework included codes on aspects of care co-ordination (e.g. types, who is involved, mode, information-sharing, where, frequency, transition and methods of access) and qualifier codes (e.g. preferences, benefits/challenges, barriers/facilitators and factors influencing co-ordination). The coding framework was used to code all interview and focus group transcripts (HW). A second researcher (AS) also coded 20% of the data (for six interviews and one focus group transcript). Coding was discussed and discrepancies were resolved and agreed.
Next, findings were grouped into themes and subthemes using Braun and Clarke’s211 thematic analysis methodology, supplemented by Neale’s188 iterative categorisation process, which is designed to support thematic analysis. Given the large number of data, this was carried out in two stages: (1) development of themes and subthemes for the data on aspects of co-ordination (to develop initial taxonomy options) and (2) development of themes and subthemes for the data on qualifying codes (to develop models). The following five themes were developed: (1) ways of organising care, (2) ways of organising teams, (3) responsibilities for co-ordination, (4) access to co-ordination and (5) mode of co-ordination. Themes and subthemes were discussed by co-authors and used to develop a taxonomy. Our taxonomy included different options, ranging from lack of co-ordination through to strategies to improve co-ordination.
To develop the proposed taxonomy, Nickerson et al. ’s212 six stages of taxonomy development were followed:
-
Identify the meta-characteristic that will inform the choice of characteristics in the taxonomy.
-
Identify ending conditions (i.e. requirements that the taxonomy needs to meet to be finalised).
-
Choose approach.
-
Identify a subset of objects to classify, using findings from the interviews and focus groups.
-
Identify common characteristics (i.e. similarities and differences will be identified to identify common characteristics and discriminatory characteristics for co-ordinated care).
-
Group the characteristics using a manual or graphical process. 203
Table 17 outlines how we applied these six steps.
| Step | Our outcome |
|---|---|
| 1. Identify meta-characteristic | Meta-characteristic = different ways in which care can be co-ordinated for rare conditions |
| 2. Identify ending conditions | Our ending conditions:
|
| 3. Decide on approach | We used an empirical–conceptual approach. We based the taxonomy on our findings from interviews and focus groups and earlier CONCORD findings |
| 4. Use a subset of objects to classify | We used themes and subthemes from the interviews and focus groups as objects to classify. The subthemes outline types of co-ordination that can be used as objects (e.g. nationally commissioned services and condition-specific clinics). A list of ‘objects’ (i.e. example ways of co-ordinating care) was identified from themes and subthemes |
| 5. Identify common characteristics | Similarities and differences were identified to identify common characteristics and discriminatory characteristics. These were identified through the summaries of themes and subthemes |
| 6. Group characteristics using a manual or graphical process | We used a manual process to group characteristics into domains to form the first draft of the taxonomy |
To refine the taxonomy, the workshop notes were analysed by one researcher using inductive thematic analysis. Workshop notes were coded and grouped into themes relating to stakeholders’ experiences of the model of co-ordination, benefits and challenges of the model of co-ordination, factors influencing co-ordination, missing aspects and the impact of COVID-19. Feedback on aspects that were missing in the taxonomy were used to refine and finalise the taxonomy. Findings were discussed with the wider research team.
Results
Participant characteristics
Seventy-nine participants took part in this study (including patients, carers, HCPs, commissioners and charity representatives). Data from 52 participants informed the development of the taxonomy (interviews, n = 30; focus group participants, n = 22) and data from 27 workshop participants informed the refinement of the taxonomy (patient/carer workshop, n = 12; professional workshop, n = 15). Two of the interview participants also took part in a workshop. Demographic characteristics are shown in Table 18.
| Characteristic | Development of taxonomy (n) (N = 52) | Refinement of taxonomy (n) (N = 27) | Total (n) | ||
|---|---|---|---|---|---|
| Interviews | Focus groups | Patient/carer workshop | Professional workshop | ||
| Number of participants | 30 | 22a | 12 | 15 | 79 (77 different peopleb) |
| Type of participant | |||||
| Patients | N/A | 16 | 5 | N/A | 21 |
| Parents/carers of children aged < 18 years | N/A | 5 | 4 | N/A | 9 |
| Parents/carers of adults aged ≥ 18 years | N/A | 1 | 3 | N/A | 4 |
| HCPs | 15 | N/A | N/A | 2 | 17 |
| HCPs employed by a charity | 2 | N/A | N/A | 2 | 4 |
| Charity representatives | 5 | N/A | N/A | 8 | 13 |
| Commissioners | 3 | N/A | N/A | 3 | 6 |
| Multiple rolesc | 5 | N/A | N/A | N/A | 5 |
| Age (years) | |||||
| 18–25 | N/A | 2 | 0 | N/A | 2 |
| 29–59 | N/A | 16 | 10 | N/A | 26 |
| ≥ 60 | N/A | 4 | 2 | N/A | 6 |
| Locations represented | |||||
| National role (UK) | 2 | 0 | 0 | 8 | 10 |
| National role (England and Wales) | 1 | 0 | 0 | 1 | 2 |
| National role (England) | 5 | 0 | 0 | 3 | 8 |
| Scotland | 1 | 0 | 1 | 0 | 2 |
| Wales | 1 | 1 | 0 | 0 | 2 |
| East of England | 1 | 2 | 1 | 1 | 5 |
| London | 4 | 7 | 0 | 0 | 11 |
| Yorkshire and the Humber | 1 | 2 | 0 | 0 | 3 |
| North-east of England | 1 | 2 | 0 | 0 | 3 |
| North of England | 1 | 0 | 0 | 0 | 1 |
| North-west of England | 2 | 3 | 1 | 0 | 6 |
| South-east of England | 1 | 2 | 3 | 0 | 6 |
| South-west of England | 4 | 0 | 4 | 1 | 9 |
| West Midlands | 5 | 2 | 1 | 1 | 9 |
| East Midlands | 0 | 1 | 1 | 1d | 3 |
Overview of care co-ordination taxonomy
Our taxonomy of care co-ordination consists of six domains: (1) ways of organising care, (2) ways of organising the team, (3) responsibility for co-ordination, (4) how often appointments and co-ordination take place, (5) access and (6) mode (Table 19). Each domain has different options for co-ordinating care (labelled ‘characteristics’). ‘Objects’, which outline the different ways of co-ordinating care for each option are also highlighted, for example, quotations illustrating each option within the taxonomy (Table 20). A summary of the domains and options are highlighted in Figure 12. The examples given in this taxonomy refer to those identified through interviews and focus groups (and validated within the workshops). Many of the examples highlight examples of real-world practice; however, some of the examples are hypothesised and relate to potential new ways of co-ordinating care.
| Domain | Characteristic | Object | Example |
|---|---|---|---|
| 1. Ways of organising care | Local | Local care delivery | All care delivered locally – in one place, or multiple places – including hospital and home visits, emergency care |
| Local care co-ordination | All co-ordination delivered locally – e.g. co-ordination appointments local to the patient | ||
| Hybrid (combination of specialist and local) (e.g. hub and spokes models) | Co-ordination nationally centralised but delivered locally | Specialist service co-ordinating care but care delivery is done locally (e.g. at local hospital or GP) | |
| Care nationally centralised but delivered locally | Care nationally centralised with outreach, specialist providers with routine care from local providers | ||
| Types of outreach models | Outreach support for professionals, outreach clinics, outreach care co-ordination, outreach education | ||
| Regionally centralised care | Regional network models, regionally delivered services | ||
| Nationally centralised | Care delivered and co-ordinated centrally | Specialist centre, rare disease centre or service | |
| Care delivered centrally (in one nationally commissioned service or centre) | Nationally commissioned service or rare disease centres, adult and paediatric centres or condition-specific centres | ||
| Care delivered centrally in multiple services/centres or as part of a network | National network models to delivery care and co-ordination and share expertise, nationally commissioned services | ||
| 2. Ways of organising the team | Little collaboration between professionals | Professionals not working together [health care, social care, third sector (if appropriate), etc.] | Lack of MDT, lack of collaborative working |
| Some collaboration between professionals | Some professionals working together to provide care [health care, social care, third sector (if appropriate), etc.] | Joint clinics with specialist and local providers or adult and paediatric providers | |
| Continuity of professionals | Same professionals throughout care, professionals attending appointments with patients | ||
| High levels of collaboration between professionals | All professionals working together to provide care [health care, social care, third sector (if appropriate), etc.] | Condition-specific clinics – run by health-care professionals, within specialist service, one-stop shop, carousel clinic | |
| All professionals meeting together to discuss care (health care, social care, etc.) | MDT meeting, or health-care professionals attending education, health and care plan meetings | ||
| Little collaboration between professionals and patients | Professionals not working with patients | Lack of collaboration with patients (e.g. lack of involvement in MDT meetings) | |
| Some collaboration between professionals and patients | Professionals working with patients to prepare them | Orientation visits/transition events/advice and support | |
| Patients meeting to discuss care | |||
| High levels of collaboration between professionals and patients | Professionals meeting together with patient/carer [health care, social care, third sector (if appropriate), etc.] | Patient involvement in MDT meeting where appropriate | |
| 3. Responsibilities | Administrative support | Administrator | Administrator, charity worker, administrator + patient/carer |
| Point of contact for patients | Administrator/charity worker/nurse or allied health professional/doctor | ||
| Point of contact for professionals (health care, social care, etc.) | Co-ordinator, specialist | ||
| Formal roles/responsibilities | Administrative co-ordinator | Clinic co-ordinator – could be range of roles, including patient/carer, non-medical professional, charity-employed support worker, nurse or allied health professional equivalent | |
| Care co-ordinator | Someone with system and condition knowledge, such as a nurse or allied health professional equivalent or hospice/community nurse/social care professional/non-medical professional/charity-employed support worker/transition co-ordinator/doctor equivalent role | ||
| Clinical co-ordinator | Someone with sufficient clinical expertise to co-ordinate complexity – doctor equivalent role, GP | ||
| Clinical lead | Someone with oversight over care, such as a nurse, doctor-equivalent role, GP | ||
| GP | Co-ordination, and implementing care plans from specialist | ||
| Charities/patient support networks (in some situations) | Direct roles in co-ordination (e.g. clinic co-ordinators/co-ordinating care), supporting co-ordination and advocating on patients’ behalf | ||
| Supportive roles | Charities/patient support networks | Direct roles in co-ordination (e.g. clinic co-ordinators/co-ordinating care), supporting co-ordination and advocating on patients’ behalf | |
| Patients and carers | Direct role as co-ordinators, providing education to professionals, part of the MDT and information provision | ||
| Peers | Providing support for co-ordination | ||
| No responsibility | No point of contact/co-ordinator/clinical lead/GP/hospital ownership | ||
| 4. How often care appointments and co-ordination appointments take place | Regular | Care appointments | Ranging from multiple weekly – weekly – every 3 months – every 6 months – annually |
| Co-ordination appointments | Ranging from more than once a month – monthly – every 2 months – every 6 months – annually | ||
| Meetings | Ranging from before every clinic – weekly – twice a month – monthly – every 3 or 4 months – every 6 months – annually | ||
| On demand when needed | Care appointments | On-demand care appointments, co-ordination or specialist centre appointments when needed | |
| Hybrid (combination of regular and on demand) | Regular appointments (as above) with on demand in between as and when needed | Regular appointments but with on-demand appointments (care appointments, co-ordination appointments or specialist centre appointments) as and when needed | |
| 5. Access to records | Full access | HCPs | Health-care professionals having full access to records |
| Patients and/or carers | Patients and/or carers having full access to records | ||
| Filtered access (i.e. information filtered to necessary information that is needed by the relevant individuals) | HCPs | Health-care professionals having access to the relevant necessary information that is needed | |
| Patients | Patients and/or carers having access to the relevant necessary information that is needed | ||
| Third sector (where deemed necessary) | Charity organisations having access to relevant necessary information if needed (e.g. when involved in care delivery/co-ordination) | ||
| 6. Modea of communication | Digital | Information-sharing | Digital records, digital letters, digital databases and registries, digital portals, mobile applications for patients and digital patient information |
| Co-ordinated care delivery | Video appointments with professionals, virtual MDT clinics, digital ways of tracking symptoms (e.g. electronic wearable devices), virtual tours of wards, applications to record test results, diagnostic technology, virtual centres | ||
| Co-ordination | Video appointments with co-ordinator, co-ordination in the cloud, virtual review (as lowest level of co-ordination) | ||
| Communication (between professionals) | Virtual panels to discuss cases with experts, e-mail hotlines, virtual MDT meetings and clinics, e-mail contact | ||
| Communication (between professionals, patients and carers) | E-mail contact | ||
| Face to face | Co-ordinated care delivery | Initial meetings, key treatment phases, such as diagnosis and stabilisation, physical examinations, clinic appointments, home appointments | |
| Co-ordination | Face-to-face meetings between patients and co-ordinator | ||
| Communication (between professionals) | Face-to-face team meetings | ||
| Information-sharing | Via co-ordinator and meetings | ||
| Telephone | Co-ordinated care delivery | Telephone clinics and consultations, conference calls, appointments (such as GP appointments), telephone calls when needed, discharge calls and follow-up appointments | |
| Co-ordination | Telephone calls with co-ordinators, initial introductions, co-ordination of care via phone, NHS 111-style phone service to co-ordinate care for rare conditions, WhatsApp [Facebook, Inc., Menlo Park, CA, USA] contact with co-ordinator | ||
| Communication (between professionals) | Phone calls with other professionals, contacting specialists, professional conference calls, discussing treatment plans, asking local teams to implement care plans | ||
| Communication (between professionals, patients and carers) | Telephone advice services or direct line to team, regular check-ups, phoning departments, WhatsApp contact, phone calls between patient and professionals, messaging peers | ||
| Written | Information-sharing: care documentation |
Written records, such as condition-specific passports and alert cards Written letters, such as clinic letters, discharge letters and summary letters Care plans for patients, such as agreed care plans, shared care protocols, education health and care plans, transition plans Reports, such as written reports and handover packs and transition reports and booklets and summary of records |
|
| Information-sharing: service planning |
Plans to specify hospital and health-care professional roles and responsibilities Standard operating procedures to record MDT working |
||
| Information-sharing: guidelines and care pathways |
Service specifications Quality assurance standards Governance frameworks National guidelines, such as NICE, charity produced or specialist service produced International best practice Lack of evidence-based pathways For co-ordinators |
||
| Information-sharing: training policies and frameworks | For co-ordinators, supervisors | ||
| Lack of (communication mode) | Information-sharing | Lack of letters, care plans | |
| Communication | Between professionals or professionals and patients |
| Domain | Subdomain | Example quotation |
|---|---|---|
| 1. Ways of organising care | a. National | Yeah, we’ve been running our multi-specialty clinics for about 18 months now in our new rare disease centreInterviewee, HCP |
| b. Hybrid | So, [place 3] is our lead paediatric centre, so they see all the local [place 3] patients, and they are our hub, we are a spoke, so we look after the patients locally in [place 2]. But [place 3] very much do like the guidelines that we follow and everything like that, and they are available to contact . . . and like I said once a year they will see every patient in our clinicInterviewee, charity representative and HCP | |
| c. Local | I live in deepest darkest, it’s rural [region 1], the most southerly tip, nearly as far away from the central hospitals of [place 3] and [place 2] as you can get. So I want all my care in the community and that of my son, I want everything down here, because you know, there’s no public transport, there’s no, I mean, literally there are no buses where we live, anywhere. To get anywhere, yeah, there’s just nothing. And so we need something that is definitely in the community, and also communities can be very differentInterviewee, charity representative | |
| 2. Ways of organising professionals involved in a patient’s care | a. High collaboration | The [rare condition x] clinic does try to address some of those deficiencies by providing a platform for co-ordinated care. . . they can come to the clinic here and see six different specialties simultaneously, and those different specialties can then try and formulate a care plan which incorporates aspects of each specialty’s contributionInterviewee, HCP |
| b. Some collaboration | But what we try to do is to ensure that there is a joint transition clinic between the paediatrician and the receiving adult clinician and a visit to the hospital, which is usually supported . . . by one of the workers from the children’s unitInterviewee, commissioner | |
| c. Low collaboration | My experience currently of co-ordinated care is that there is none. It sounds like a complete and utter fantasy to meFocus group participant, parent/carer | |
| 3. Responsibilities | a. Administrative support | . . . we’ve got an admin person and she’s quite instrumental at helping us set those up as well . . . so that’s a useful, really useful resource that we haveInterviewee, HCP |
| Yeah, we have a – when a patient is new to the service they’ll get given quite a lot of contacts, including our health e-mailInterviewee, HCP | ||
| b. Formal responsibilities | . . . there could be a stratified level of lead with a, sort of, triangle, an upturned triangle with a base at the bottom, the pinnacle at the top, and then, actually, the other way around, that the digital is at the bottom along with the smallest amount of care, and then, you know, you might have a patient requiring, you know, a quarterly or even a monthly telephone call with the co-ordinator or the community nurse, or whatever . . . Certainly, you start with digital and then you would have a monthly phone call or a quarterly phone call depending on what the anticipated need of that patient is, and then it could be escalated up as requiredInterviewee, commissioner | |
| I guess it’s fairly, sort of, just everyone, sort of, chipping in, but I guess, obviously, the consultant’s there and, ultimately, they will try and . . . You know, if we’re struggling with it, then they might, sort of, take more control of that conversation and be, like – or suggest, ‘Why don’t you do it like this?’ but, generally, it’s, kind of, us just, sort of, negotiating between ourselvesInterviewee, HCP | ||
| I think that a GP is the closest thing I have to a care co-ordinator . . . feel like they might be best equipped to sort of co-ordinate care if they had more time and training to do it or even budget to do itFocus group participant, patient | ||
| c. Supportive roles | . . . but they [patient support groups] are very good at picking up the pieces, supporting patients and providing information that the health-care professionals don’t provide, so they’re key I thinkInterviewee, HCP | |
| I’m pretty much [name]’s care co-ordinator. She sees about 15 to 16 different specialistsFocus group participant, parent/carer | ||
| 4. How often care appointments and co-ordination take place | a. Regular | . . . so there could be kind of like different levels of how often you need to see people, but I think definitely for us it would be that it would be ongoing at the minuteFocus group participant, parent/carer |
| b. On demand | I find sometimes if you have yearly or 6-monthly appointments time and time again, they can be a bit fruitlessFocus group participant, patient | |
| 5. Access to records | a. Full access | Well, that gets us back to the electronic patient record, doesn’t it? You know, ideally, I think there should be an electronic patient record that is accessible to everyone involved in someone’s care. Unless that is available, communication always ends up as a weak link, doesn’t it?Interviewee, HCPI just want it to be shared with me, and it can’t, and they never let you see everythingFocus group participant, patient |
| b. Restricted access | Yeah, so in essence, the way . . . what I’ve just really said, I think the information needs to be available to all who need to have it, obviously with appropriate restrictionsInterviewee, HCPI would like something like that on my health records of who wants to look at it, with a little bit of why, then yes, I’ll just tick yes, but also, I’d like a list of who has accessed it . . . Because I want to know who’s reading my, you know, someone did say at one time, ‘Oh, the psychiatric team are looking at your notes’, I haven’t given them permission to do that . . . You know, why are they looking at my notes and for what reason?Focus group participant, patient | |
| 6. Mode of contact | a. Information-sharing | Well it is having it, so basically so there is communication from one place to the next . . . if everything’s joined up beautifully electronically, that’ll be there anyway almostInterviewee, HCP |
| . . . it’s really helpful that there’s a sort of overarching operating policy or operating manual for any serviceInterviewee, commissioner | ||
| b. Care and co-ordination appointments | . . . there needs at least to be a connection with a multidisciplinary physical structure . . . And otherwise the co-ordination of care could also be digital, as we said beforehand. You know, it could be on the cloudInterviewee, HCP | |
| . . . a new diagnostic result. I think this requires face-to-face contact with, you know, an expert or a co-ordinating clinician. This is, you know, it’s like giving someone a new name. So, I think it is very important that there’s a face-to-face contact with a medical professional when this happens. Then I think there is a need for face-to-face contact when there’s a new kind of clinical or medical complication, but that face-to-face contact need not necessarily be with the co-ordinating clinician; that could be with the relevant clinicianInterviewee, HCP |
FIGURE 12.
A summary of the taxonomy domains (numbered) and care co-ordination options (bullets).

The taxonomy outlined in this chapter is the final version that has been refined from workshop feedback. Findings from the workshops highlighted key aspects that needed to be clarified within the taxonomy, including the need to emphasise that care is not just medical (i.e. it also includes social and educational aspects of care) and that care is lifelong. Workshop findings highlighted the need to separate out collaborations that include patients/carers from collaborations between professionals, the need for third-sector involvement in collaboration (where appropriate), the need to emphasise the role that charities and patients/carers play in care co-ordination, a hybrid model of frequency and the need to clarify aspects of the mode domain. We amended the taxonomy in line with this feedback. See Report Supplementary Material 3 for visual representations of the workshop findings.
‘Qualifiers’ refers to information relating to people’s preferences, the benefits and challenges of different ways of co-ordinating care, factors influencing co-ordination, and barriers to and facilitators of underpinning co-ordination. A summary of qualifying information, relating to preferences (see Appendix 7), benefits and challenges (see Appendix 8) and factors influencing co-ordination (see Appendix 9), identified through the interviews and focus groups is shown in Table 21.
| Domain | Participants’ preference for options within domain | Example benefits for options within domain | Example challenges for options within domain | Factors influencing choice of option within domain |
|---|---|---|---|---|
| 1. Ways of organising care | Nationally commissioned services |
✓ Improved co-ordination ✓ Motivated staff ✓ Holistic ✓ Reduce travel ✓ Expertise |
✗ Not accessible to all ✗ Not suitable for some conditions ✗ Not able to cover all aspects of care |
Patient factors (e.g. condition complexity, severity, clarity over who patient needs to see, age, diagnosis, location) Health-care environment [e.g. resources (funding and availability) and environment (access and suitability)] Societal factors (e.g. funding and availability of guidelines) |
| Hub and spokes, networks and outreach (e.g. specialist co-ordinating care, local delivering, outreach clinics, support for local providers) |
✓ Education for local providers ✓ Reduce travel ✓ Set standards |
✗ Resources | ||
| 2. Ways of organising the team | Condition-specific clinics or joint clinics (some to high collaboration) |
✓ Allow teams to figure out who patient needs to see ✓ Reduce travel ✓ Message consistency ✓ Holistic care |
✗ Difficulty organising ✗ Lack of involvement from some disciplines ✗ Tiring clinics |
Patient factors (e.g. age, condition and how many disciplines patient needs to see) Provider factors (e.g. knowledge, understanding and expertise) Health-care environment (e.g. resources and availability of clinics) |
| Meetings (some to high collaboration) |
✓ Shared conclusion ✓ Message consistency |
✗ Difficulty organising ✗ Time ✗ Lack of sharing or reading information ✗ Meetings without patient: disliked by patients |
||
| Transition methods (e.g. half appointment with adult, half appointment with child) |
✓ Helping patient take responsibility ✓ Smoother transition ✓ Build confidence |
✗ Differences in adult and child services ✗ Reluctance to transition ✗ Takes time |
||
| 3. Responsibilities | Point of contact (administrative support) |
✓ Answer queries ✓ Build rapport |
✗ Time ✗ Not available |
Patient factors (e.g. diagnosis, age, condition, individual needs and preferences) Provider factors (e.g. skills and capability, attitudes and opportunity) Health-care environment (e.g. availability of roles) Societal (e.g. resources and attitudes) |
Co-ordinator (formal role):
|
✓ Organise appointments ✓ Relationships between patient and team ✓ Support patient ✓ Point of contact |
✗ Need time and dedicated role ✗ Lack of co-ordinators ✗ Need cover |
||
|
✓ Expertise ✓ Holistic care ✓ Facilitates collaboration |
|||
GP (formal role):
|
✓ Speed of referral |
✗ Time ✗ Motivation ✗ Referral pathways |
||
| Support from charities (supporting co-ordination, HCPs, clinics and providing materials) |
✓ Administrative support ✓ Push for standards |
✗ Not available for all conditions ✗ Reliant on donations |
||
| 4. How often | Regular | ✓ Ability to check in and update on care |
Patient factors [e.g. diagnosis, age, ability to travel, condition (including stability and severity)] Provider factors (e.g. time, knowledge and understanding) Health-care environment (e.g. availability of roles, time and funding) |
|
| On demand |
✓ Helping to access care when needed ✓ Not wasting providers’ time |
|||
| Predetermined schedules |
✓ Evidence based ✓ Suitable for condition ✓ Accounts for genetic breakthroughs |
|||
| 5. Access | Access to records for providers: limited/restricted by relevance | ✓ Providers only see the information they need |
Patient factors (e.g. diagnosis, consent and the condition) Health-care environment (e.g. resources, environmental factors and attitudes) Societal (e.g. funding) |
|
| Access to records for patients | ✓ Beneficial for patients | |||
| Access to out-of-hours support, holistic care and individualised care |
✓ Able to access care when needed ✓ Saving time ✓ Rapport |
✗ Information not always available in emergencies | ||
| 6. Mode: information-sharing | Digital (e.g. online portals, online records, apps, e-mail and databases) |
✓ Easy access to information (portals, records, apps, e-mails) ✓ Quicker (portals, e-mails) ✓ Secure (portals, records) ✓ Patient control over access (apps) |
✗ IT failures (portals, records) ✗ Difficulties keeping up to date (portals, databases) ✗ Too much information (records) ✗ Security (apps, e-mails) |
Patient factors (e.g. age and condition) Health-care environment (e.g. access to technology) |
| Written (e.g. care plans, letters, written agreements, patient-held records and condition-specific passports) |
✓ Keeping everyone updated (letters, care plans) ✓ Quicker (letters) ✓ On hand when needed (condition-specific passports) ✓ Patient ownership (patient-held records) ✓ Ensuring accountability (written agreements) |
✗ Lost or delayed (letters) ✗ Not always accepted or used by providers (condition-specific passports, care plans) |
||
| 6. Mode: care delivery and co-ordination | Digital (e.g. Skype or virtual appointments) |
✓ Reducing travel ✓ Suitable for updating, reviewing and answering questions ✓ Consistent messaging |
✗ Cannot fully replace specialist appointments ✗ Not appropriate for all conditions ✗ Not appropriate for first meeting ✗ Information security |
Patient factors (e.g. age, individual needs and condition) |
| Face to face |
✓ Physical examination of patients ✓ Problem-solving ✓ Relationship building ✓ Support |
✗ Not appropriate for all conditions because of travel ✗ Difficulties organising ✗ Tiring ✗ Time ✗ Funding |
||
| Telephone |
✓ Reduces travel ✓ Joint decision-making |
✗ Not suitable for all conditions ✗ Not preferred by patients/carers ✗ Cannot see body language |
||
| Combination |
✓ Keeping everyone in the loop ✓ Reducing travel ✓ Saving time and money ✓ Sharing information/consistent messaging |
|||
| 6. Mode: communication | Face to face |
✓ Easier to address issues and reduce misunderstandings ✓ Agree plans moving forward |
✗ Lack of capacity to attend | |
| Digital |
✓ Convenient if face to face not possible ✓ Agreeing solutions ✓ Reducing time |
|||
| Telephone |
✓ Suitable for answering queries ✓ Reduces chance of patients getting lost in system |
✗ Not guaranteed a response ✗ Not suitable for all conditions |
Our interview and focus group findings also identified many barriers and facilitators underpinning these domains of care co-ordination. Barriers and facilitators fit within the following five themes: (1) ability, (2) attitudes, (3) opportunity, (4) resources and (5) environment. We grouped barriers and facilitators into patient factors (i.e. ability, attitude and opportunity), provider factors (i.e. ability, attitudes and opportunity), health-care environment factors (i.e. resources, environment and attitudes) and societal factors (i.e. resources and attitudes) (see Appendix 10).
Taxonomy domains and qualifiers
Ways of organising care
Our findings highlighted different ways of organising care (see Table 19). These ways ranged from local care provision where all care is delivered locally through to care being delivered in national centres that serve all patients in the country with a particular rare condition. There are also some ‘hybrid’ options, which combine both specialist and local care (e.g. outreach clinics that are delivered by clinicians from specialist centres but in a patient’s local area). See Table 19 for further examples and Table 20 for sample quotations.
Workshop findings indicated that participants experienced a change in services due to COVID-19 (e.g. reduced access to specialists and limited capacity for local services).
Figure 13 provides a summary of the different ways of organising care.
FIGURE 13.
Ways of organising care (visual representation of taxonomy domain 1).
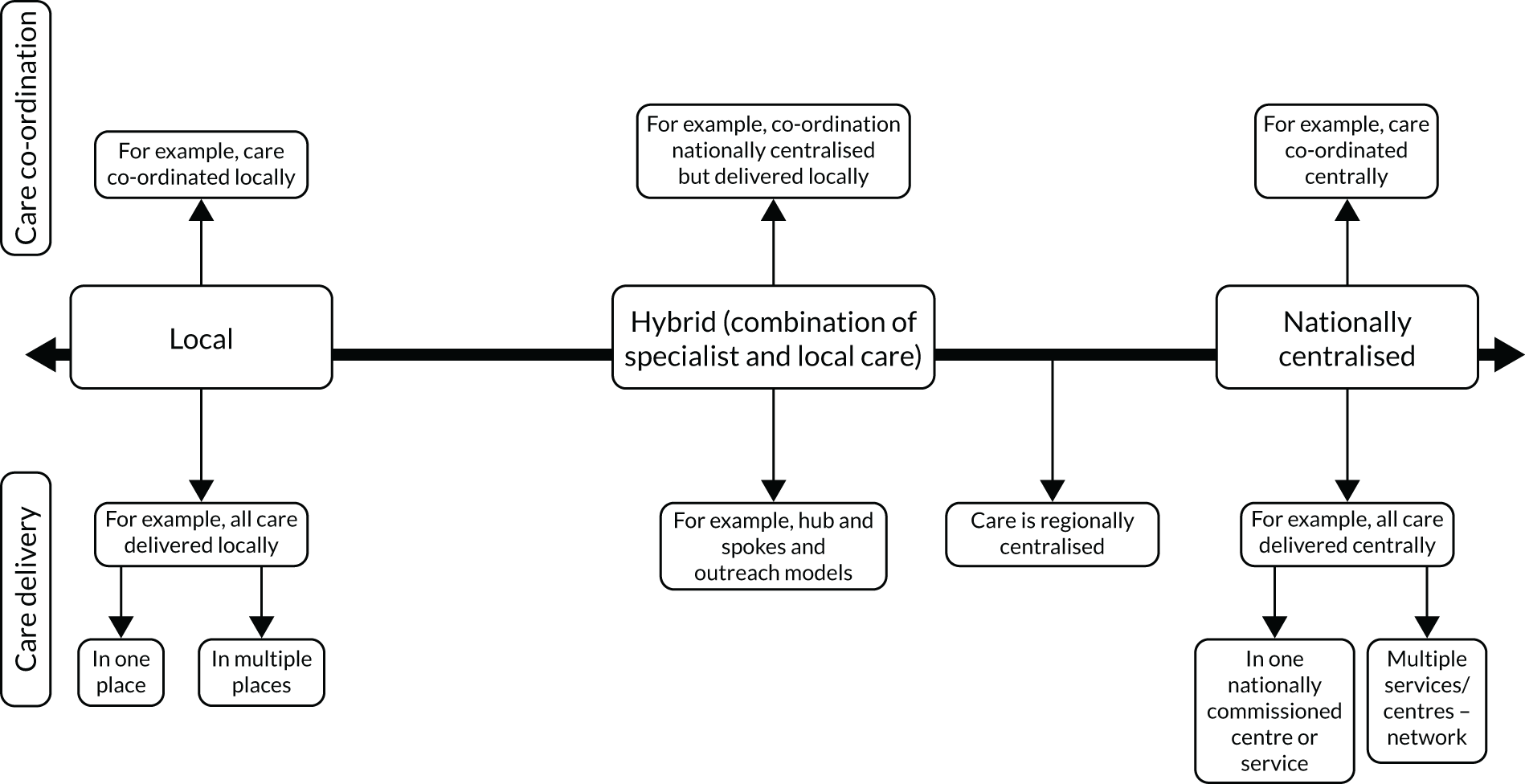
In terms of qualifying factors (see Table 21), findings indicated that participants from all stakeholder groups may prefer nationally commissioned services and hybrid models. Hybrid models include specialist centres co-ordinating care and local services delivering care, outreach clinics and provision of support for local providers:
Hmm . . . well obviously ideally close to home but I think the majority of our patients, if they feel they’re going to be getting a good service and a specialist service, they are willing to travel to a specialist centre.
Interviewee, HCP
Both of these options have benefits and challenges. For example, nationally centralised locations, such as specialist centres, might improve co-ordination and increase access to expertise. However, these services are not available for all conditions and may not cover all aspects of care that the patient needs. For hybrid options, people pointed out benefits, such as reducing travel and the ability to provide education to local health-care providers.
Many factors were perceived to influence the type of centralisation, including patient factors (e.g. age, ability to travel and rare condition), health-care environment factors (e.g. availability of resources, such as funding issues and availability of experts and models of co-ordination), environmental factors (e.g. ease of access and suitability of the environment, and relationships between care teams, such as specialist and local teams) and societal factors (e.g. funding and availability of service specifications and policies). For example, the patient’s condition was perceived to influence how care is organised in a number of ways, including the nature of the condition (e.g. the complexity of the condition, whether or not the condition affects multiple body systems, the number of disciplines involved in a patient’s care and need for co-ordination across a whole spectrum of care and not just acute medical situations). Participants also felt that specialist services work only if the condition has a discrete phenotype and if services know exactly who a patient will need to see. Conditions that do not fit into a clinical group or are difficult to define may not be well placed to be cared for within a specialist service. In addition, conditions that are more stable may require less co-ordination (e.g. may just require a point of contact within a specialist centre). Where the patient lives also determines how care should be co-ordinated. Findings indicated that patients and families may fit into three groups: (1) those who live far away from the specialist centre but can travel, (2) those who live far away from the specialist centre but cannot travel and (3) those who live close to the specialist centre. Different models of co-ordination may be needed for these different types of families. For example, those who live far away from the specialist centre may require visits to specialist centres to be minimised and care to be delivered locally or online.
Ways of organising teams
Our findings highlighted different ways of organising teams (see Table 19). Options ranged from little collaboration (e.g. not having a MDT) to high levels of collaboration (e.g. all HCPs working together to provide or discuss care in a condition-specific clinic or MDT meeting). Other options included some HCPs working together (e.g. in joint clinics). See Table 20 for example quotations.
Workshop findings highlighted that COVID-19 may have offered new opportunities for collaboration, such as the ability for local team members to dial into MDT meetings. Figure 14 provides a summary of the different ways of organising teams.
FIGURE 14.
Ways of organising teams (visual representation of taxonomy domain 2).
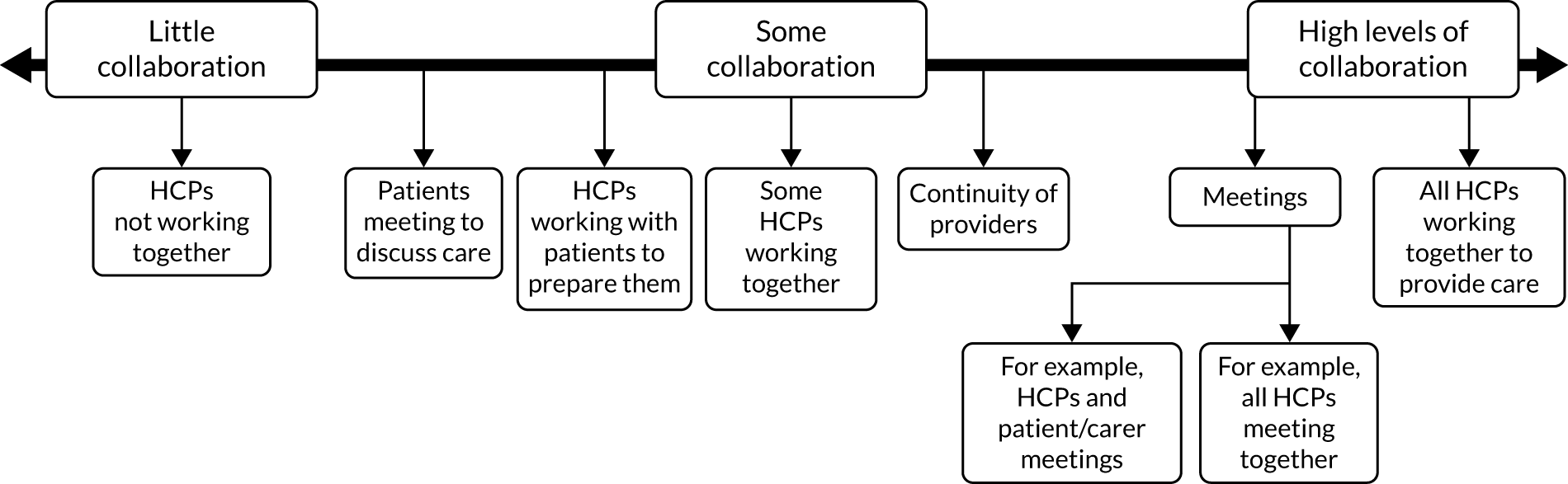
In terms of qualifying factors (see Table 21), findings outlined preferences for condition-specific clinics or joint clinics, meetings and some transition methods to support patients. Each of these options have benefits and challenges. For example, condition-specific clinics and MDTs allow teams to figure out who patients need to see, ensure that all those involved in a person’s care receive the same messages and may reduce travel. However, these options are difficult to organise and may be tiring for patients:
. . . we do support this idea of multidisciplinary team clinics, and then that those MDTs develop good lines of communication with GPs and other providers. That seems to work the best, and we think that there’s some evidence that patients do better when they’re under the care of those sorts of clinics.
Interviewee, charity representative and HCP
Many factors were perceived to influence the type of collaboration, including patient factors (e.g. age and condition), provider factors (e.g. knowledge, understanding and whether or not the team has multidisciplinary expertise) and health-care environment factors (e.g. resources and availability of collaboration models, such as joint clinics, MDT clinics and orientation visits, and availability of experts). Patient factors included age (e.g. clinics varying for adults and children) and the patient’s condition. For example, the nature of the condition influences collaboration, as the type of clinic used depends on how multisystemic the condition is and how many disciplines it involves. Carousel clinics (where the patient sees different HCPs one after another) or MDT clinics may be suitable for only those conditions that affect multiple body systems, and MDTs may only work if there is a discrete phenotype with clarity over which professionals need to be seen.
Responsibilities
Our findings highlighted the different types of responsibility involved in co-ordinating care for rare conditions (see Table 19), including administrative, formal and supportive roles. Administrative support included help with organising appointments and having a point of contact. Many different professionals were identified as doing, or having potential to do, these roles (e.g. administrators, co-ordinators, rare disease charities, and a combination of patients and administrators). Formal co-ordination responsibilities were identified across three roles: (1) those conducted by a co-ordinator (i.e. an administrative, general or clinical co-ordinator), (2) those conducted by a clinical lead (e.g. overseeing care) and (3) those conducted by a GP (e.g. being a point of contact, co-ordinating care, referrals and signposting). Findings outlined different options for who the co-ordinator could be, including those in doctor-equivalent roles, nurse or allied health professional roles, non-medical roles and social care sector roles. Supportive roles were also identified, including those conducted by patients and carers (e.g. in a direct role as co-ordinators, or involvement in education, MDTs and information provision) and those conducted by charities (e.g. direct roles in co-ordination, providing support for co-ordination and advocating on the patient’s behalf). Figure 15 provides a summary of the different types of responsibility. See Table 20 for sample quotations.
FIGURE 15.
Types of responsibilities for co-ordination (visual representation of taxonomy domain 3).
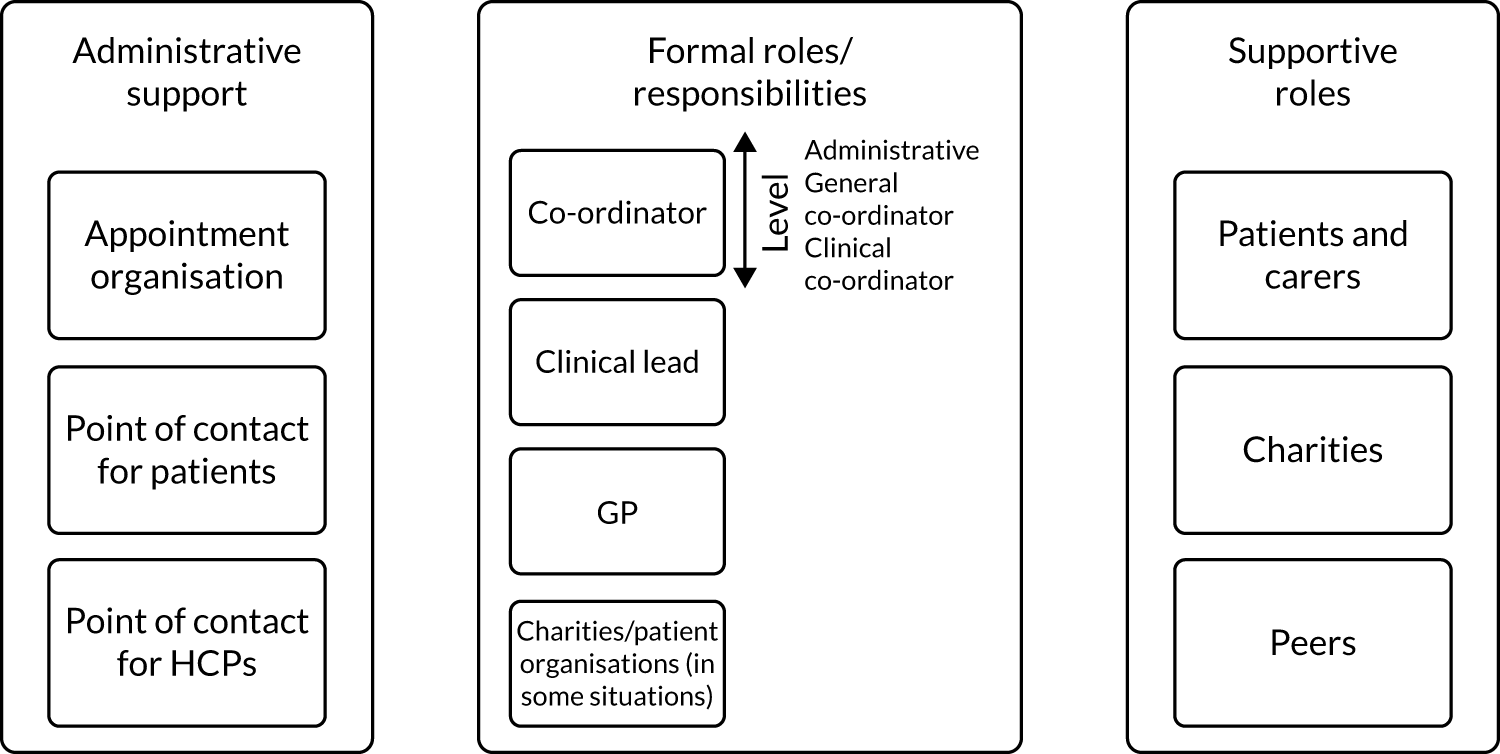
In terms of qualifying factors (see Table 21), findings indicated that participants would prefer a point of contact to answer queries, a co-ordinator (e.g. a nurse or allied health professional), a clinical lead, support from their GP and support from charities. Workshop findings highlighted the importance of charities in care co-ordination and the importance of patients and carers who are often co-ordinating their own care:
I agree. I don’t think it’s difficult. I think you can have a . . . I think you need a named consultant as the overall co-ordinator . . . But then maybe the person you have face to face with, the person who is a co-ordinator or . . . Often I do think the senior nurse is really good.
Focus group participant, patient
Each of these options have benefits and challenges. For example, benefits of co-ordinators include helping build relationships between patients and the team, and supporting patients. However, co-ordinators need time and a dedicated role. These roles may not always exist currently. People felt that clinical leads provide expertise, holistic care and facilitate collaboration between professionals. People felt that GPs were lacking time, sometimes motivation and clear methods to refer patients to services.
Many factors were perceived to influence who takes responsibility, including patient factors (e.g. diagnosis, age of patient, condition and the individual’s needs and preferences), provider factors (e.g. knowledge, support and education, understanding of the health-care system, interest and motivation, and time and availability of a team to work with), health-care environment factors (e.g. resources, such as availability of co-ordinator roles) and societal factors (e.g. availability of patient organisations, stigma and willingness to change). The patient factor that was discussed most frequently was the patient’s individual needs and preferences. For example, patient choice on who sees their records, which HCPs they see, who co-ordinates their care and the extent to which the patient/carer are involved in co-ordination and meetings. In addition, individual patient needs influence who is involved in co-ordination (e.g. the need for co-ordinated care and who is involved to be tailored and take individual family needs and ambitions into account). For example, a national care co-ordinator model that takes the person’s individual needs into account to determine how much contact they have with their co-ordinator or the level of co-ordination. An additional factor relating to individual needs was the patient’s ability to self-manage and co-ordinate their own care. Some patients may be able to co-ordinate their own care, but others would struggle and, therefore, need a co-ordinator.
How often care appointments and co-ordination take place
Our findings highlighted different time periods for care appointments and co-ordination activities. Options included regular appointments and on-demand appointments (see Table 19). Workshop findings highlighted the need for a hybrid category that combines both regular care (at a minimum) with on-demand support. See Table 20 for example quotations.
Workshop findings highlighted that COVID-19 may have provided opportunities for on-demand appointments for those with stable conditions (as long as safety nets are in place).
See Figure 16 for a summary of this domain.
FIGURE 16.
Different options for how often care appointments and co-ordination appointments take place (visual representation of taxonomy domain 4).

In terms of qualifying factors (see Table 21), there was less agreement regarding preferences for different time periods, with some participants preferring on-demand appointments for care and/or co-ordination (as this enables them to access care when needed and not waste providers’ time) and other participants preferring regular appointments (e.g. to receive check-ups and updates on care).
Many factors were perceived to influence frequency, including patient factors (e.g. diagnosis, age, ability to travel, condition, stability or progression associated with the condition, phase, severity, individual needs and time since treatment), provider factors (e.g. time and knowledge) and health-care environment factors (e.g. availability of job roles, guidelines, time within job roles and funding):
I guess it depends on the condition and how much things are changing, and whether it is a life-limiting condition, because if it is a life-limiting condition there is probably more things that are changing more rapidly. So, I think it has to be condition specific, so I guess you would be guided by what the experts think is appropriate.
Interviewee, charity representative and HCP
Access
Our findings highlighted different types of access to records. Options ranged from full to restricted access to records for patients and providers (see Table 19). Workshop participants highlighted that full access to records with a summary of important details may be helpful. See Figure 17 for a summary of this domain. See Table 20 for sample quotations.
FIGURE 17.
Options for access to records (visual representation of taxonomy domain 5).
In terms of qualifying factors (see Table 21), patients and HCPs having access to records was seen as important throughout the interviews and focus groups, but it was less clear what people would prefer in terms of full or filtered access. For example, for HCPs, our findings show that it is important for HCPs to have access to information and records. However, the extent to which HCPs can access information and records was not as clear. Some participants felt that any HCP should be able to access the records. Other participants felt that access to records should be limited (e.g. to only necessary information, such as current and relevant information/information necessary for each discipline). Reasons for this tended to differ across patients, carers and HCPs. For example, some patients and carers felt that they would not want all of their HCPs to have access to all aspects of their records (e.g. those parts that are irrelevant to the current condition or situation/aspects of childhood records that are no longer relevant) and that they would want control over who has access. Some HCPs spoke about how access to complete records can also be overwhelming and that it may be necessary to filter information by relevance:
I mean, I personally wouldn’t mind it shared with anyone. I’d rather the more people.
Yeah I’m the same.
I’m the same. I mean, with my daughter, I was, like, ‘Yay. If you want to look at this and you want to use this to help her . . . if this can make you more informed, if this can connect you to my daughter, please do ahead and do it’, you know.
Factors that were perceived to influence access included patient factors (e.g. diagnosis and consent), health-care environment factors (e.g. resources, environmental factors and attitudes) and societal factors (e.g. funding).
Mode of communication
Our findings highlighted different modes, including modes for information-sharing, care delivery and/or co-ordination and communication (see Table 19). Figure 18 provides a summary of this domain. See Table 20 for sample quotations.
FIGURE 18.
Different options for mode of co-ordination (visual representation of taxonomy domain 6).
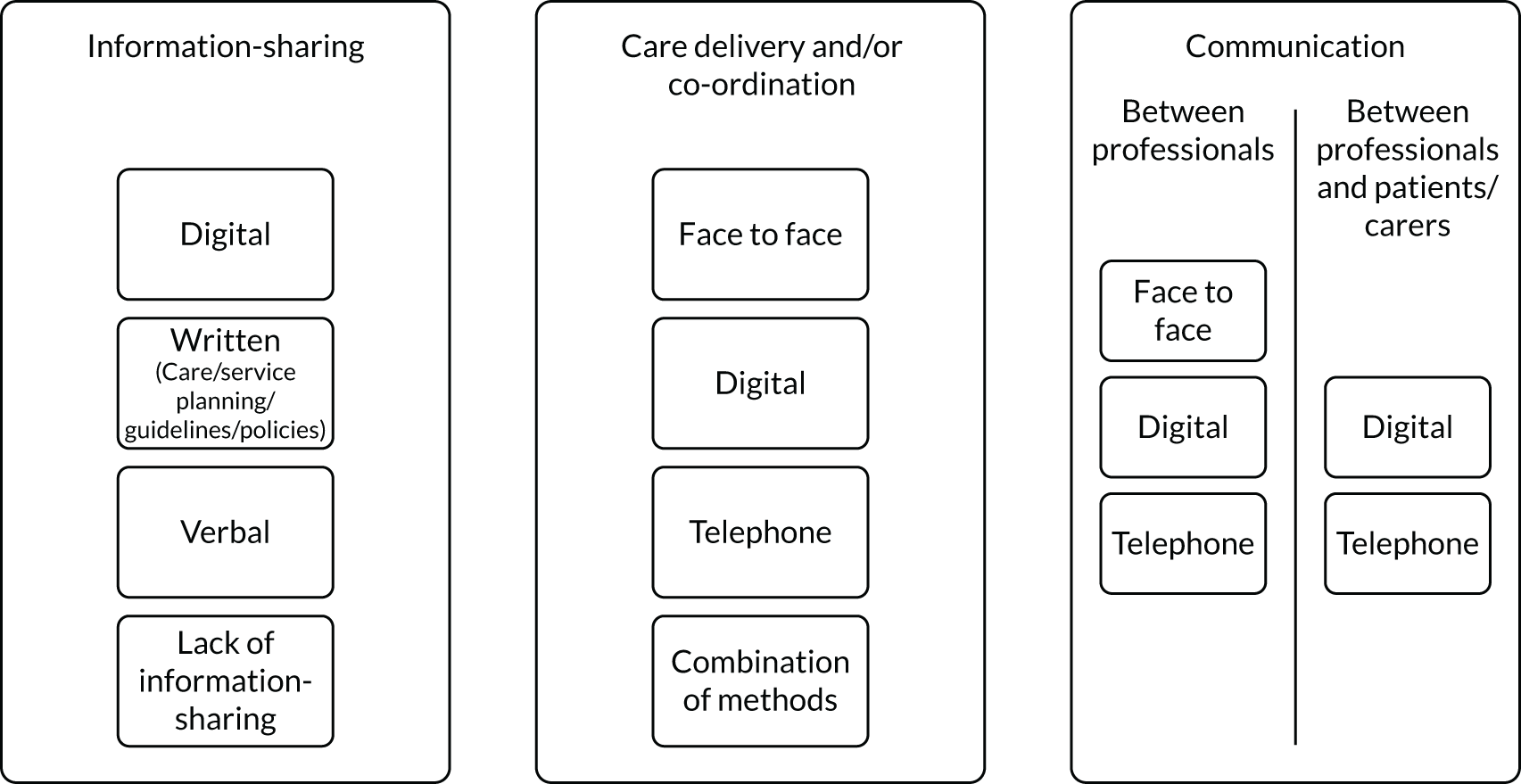
For information-sharing, our participants described many different modes, including digital methods, written methods, verbal methods or a lack of information-sharing. In terms of qualifying factors (see Table 21), our participants preferred digital methods (e.g. online portals, records, mobile applications, e-mails and databases) and written methods (e.g. care plans, letters, written agreements of responsibility, patient-held records and condition-specific passports). Digital methods were seen to provide easier and quicker access to information, but were limited by information technology failures and were difficult to keep up to date. Written methods were seen to keep everyone up to date and ensure accountability, but were considered to have the potential to get lost or delayed:
I think that in a totally ideal world – see, it’s pie in the sky, but in a totally ideal world, if all of the NHS had electronic patient records that were all on the same system and could be shared automatically between units then, you know, we’d be able to see things more nationally.
Interviewee, charity representative and HCP
In terms of care and co-ordination appointments, our participants described many different modes, including face to face, digital, telephone and a combination of methods. Workshop findings highlighted that COVID-19 has accelerated the shift from traditional methods of delivery of care (i.e. face to face) to digital and telephone delivery of care for people living with rare conditions. In terms of qualifying factors (see Table 21), it was less clear what people preferred and each mode had benefits and challenges. We found that digital appointments may reduce travel and may be suitable for reviews and updates, but these appointments cannot fully replace face-to-face appointments. Using a combination of methods was felt to keep everyone updated, reduce travel, save time and money, and ensure that everybody involved is informed/in agreement and has the same information.
For communication, participants described many different modes, including face to face, digital and telephone methods. In terms of qualifying factors (see Table 21), participants’ preferred different modes and each mode had many different benefits and challenges. Face-to-face methods were perceived to reduce misunderstandings and help to agree plans, but were limited by availability. Digital methods were seen as good for reducing time and agreeing solutions. Telephone methods were considered suitable for answering patient queries.
Perceived factors influencing mode of information-sharing and care delivery included patient factors (e.g. age, condition and individual needs) and health-care environment factors (e.g. access to technology).
Discussion
Key findings
We have developed a taxonomy of care co-ordination for rare conditions. We identified the following six domains of care co-ordination: (1) ways of organising care (i.e. local, national or a hybrid), (2) ways of organising the team (i.e. high collaboration, some collaboration or low collaboration), (3) responsibility for co-ordination (i.e. administrative support, formal roles and responsibilities, supportive roles and no responsibility), (4) how often appointments and co-ordination take place (i.e. regular appointments, on-demand appointments or a hybrid), (5) access (i.e. full or filtered access to records) and (6) mode of information-sharing, care co-ordination/delivery and communication.
Our findings highlighted various stakeholder preferences, benefits/challenges and factors influencing co-ordination for different options within each of the six domains. These findings indicate that different models of care co-ordination for rare conditions may be appropriate in different situations.
How findings relate to previous research
These findings extend knowledge on care co-ordination for rare conditions. National policy documents and previous research has highlighted the importance of care co-ordination. 8,15,19 However, findings indicate that little is known about co-ordination for rare conditions15 (see Chapter 3). Previous research has shown that co-ordination for rare and common chronic conditions has many components,1,15,117,184 but care co-ordination had not been formally categorised. In addition, although previous taxonomies have been developed for other complex health concepts, such as integrated care213 and the burden of treatment for patients with chronic conditions,209 to the best of our knowledge, there have been no taxonomies that have focused specifically on care co-ordination for chronic or rare conditions. The taxonomy presented in this chapter extends previous research by formalising care co-ordination for rare conditions into six domains (each with different options).
In addition, this research offers insight into participants’ preferences, the benefits and challenges of different models of co-ordination, factors influencing co-ordination, and the barriers to and facilitators of co-ordination in general. These findings extend previous knowledge by identifying possible situations in which different models of co-ordination may be appropriate. For example, previous research has highlighted that some aspects of care co-ordination may be necessary for rare conditions (e.g. care co-ordinators and specialist centres). 5,10,11 However, to the best of our knowledge, there has been little research on the benefits and challenges of each model and how they work in practice. This research extends this knowledge by outlining the benefits and challenges associated with each model.
Given that there are many different rare conditions, it was not known whether or not one taxonomy would be applicable across different rare conditions. These findings highlight that although different conditions have different characteristics and challenges, it is possible to develop a taxonomy that covers a range of rare conditions. Previous research1 has indicated that more care co-ordination is needed in complex situations (e.g. limited patient capacity and clinical complexity). Our findings concur with this and highlight a range of factors that need to be considered when choosing how to co-ordinate care, including patient factors, provider factors, environmental factors and societal factors. Examples of patient factors included severity and complexity of condition, where patients live, the patient’s ability to travel and the patient’s ability to co-ordinate care. Therefore, findings indicate that a ‘one size fits all’ approach to care co-ordination is not appropriate and that we should develop models of care co-ordination that take into account a range of individual, organisational and societal factors, rather than developing different models of co-ordination for each individual condition. Models can then be tailored to individual situations.
Our findings highlighted three main options for organising care. Findings extend previous research11,214–216 by demonstrating that participants from all stakeholder groups indicated a strong preference for nationally commissioned services and hybrid models (including hub and spokes models, network models and outreach models) because of the associated benefits (e.g. increased co-ordination, access to expertise and reducing travel). This supports previous research, which highlights the potential benefits of specialist services,11 hub and spokes models214,215 and outreach models216 for different health conditions. However, for rare conditions, findings indicated that these models may not be appropriate in all situations and, in some situations, patients may prefer local care (e.g. if they are unable to travel or do not live near to a specialist centre). In addition, specialist services may not be appropriate for every condition (e.g. for conditions that do not have discrete phenotypes). These findings highlight that different models of co-ordination are needed for different types of families (e.g. those who live near to specialist centres, those who live far away but can travel and those who live far away but cannot travel).
Our findings on the organisation of teams for rare conditions supports previous research that indicates the importance of collaboration and MDTs for rare conditions and other conditions. For example, previous research has highlighted negative implications for patients associated with co-ordinating their own care, including repeating information to different HCPs. 184,186 Findings also support previous research that has indicated a need to join up care appointments from different disciplines and hospitals into one appointment (e.g. condition-specific clinics) to facilitate co-ordination. 15,184 However, findings indicate that collaboration does not always happen in practice and that improvements in collaboration/joined-up working are needed.
Our findings extend previous research by highlighting the different types of responsibility needed to co-ordinate care for rare conditions. Previous research has indicated the importance of care co-ordinators. 5,10,15,184 However, our findings extend previous research by highlighting the importance of the many different roles needed to co-ordinate care. These roles include administrative support, co-ordinators, clinical leads, GPs and charities. Patients and carers currently play large roles in care co-ordination; however, we found that patient involvement in co-ordination was not always appropriate if patients were unable to, or did not want to, co-ordinate their own care. This finding is consistent with previous research that has indicated the negative impact co-ordinating care can have on patients and families184,186 and treatment burden more generally. 205,209 These findings indicate that different models of co-ordination are needed to take into account those who are able and want to co-ordinate their care and those who cannot. For example, the level/type of co-ordinator offered should vary depending on complexity and the patients’ ability to co-ordinate their own care. However, findings indicate that these roles do not always exist in practice and that further resources are needed (e.g. specific co-ordinator roles and training pathways).
Clinical guidelines for rare conditions (where available) outline how often patients with certain conditions should be seen in practice for their care appointments. Our findings offer support for including timings of appointments within such guidelines, as the findings demonstrated the importance of regular care and co-ordination appointments, particularly at key stages of a patient’s journey or condition. However, in some situations, on-demand appointments or a combination of regular and on-demand appointments were felt to be appropriate. Therefore, these findings suggest that the frequency of co-ordination and care appointments/meetings need to take into account factors such as patient and provider preferences.
Findings support previous research that indicates that a combination of methods can be used to deliver health care, including methods to share information, methods to facilitate co-ordination and methods to communicate with professionals and patients. In particular, findings highlighted the potential for remote methods of co-ordination, including digital information-sharing (e.g. through electronic records), virtual clinics and care co-ordination appointments. This shift to digital methods has been accelerated during the COVID-19 pandemic and supports previous research217,218 that indicates that digital methods may show some potential for use in health-care delivery. Our findings suggest that this may also apply to care co-ordination. However, each mode of communication has benefits and challenges, and findings indicate that the mode of co-ordination should take into account many factors, including individual preferences and resources. In addition, findings indicate that digital appointments must not replace face-to-face appointments completely in terms of care delivery and co-ordination. Face-to-face appointments were felt to be integral, particularly at key points of the patients’ journey (e.g. initial meetings and diagnosis), for certain conditions or for patients requiring more in-depth clinical care co-ordination because of additional difficulties. This extends previous research219 by highlighting the limits of digital methods of care delivery and co-ordination, and emphasises the need to offer multiple modes when co-ordinating care for patients with rare conditions.
Limitations
Although our sample of participants included a variety of rare conditions, locations and sectors, we were unable to include every rare condition. Some groups of participants, including individuals from minority ethnic groups and certain roles (e.g. GPs), were under-represented in our sample. Therefore, we are unlikely to have captured every possible option of care co-ordination for rare conditions. However, we included as many different views as possible throughout the study.
Our topic guides for both the interviews and focus groups were comprehensive and included an extensive number of questions. Therefore, it is possible that this may have compromised depth. However, we obtained a large number of in-depth data from these interviews and focus groups and, therefore, it is unlikely that depth was compromised.
Care co-ordination is a complex concept. Therefore, it is possible that we may have missed relevant constructs. However, we have minimised this risk through the extensive data collected in this study, which, together with the survey and scoping review findings, provide a comprehensive overview of the different ways in which care can be co-ordinated for rare conditions.
In addition, owing to COVID-19, we were unable to conduct five face-to-face workshops as planned. Instead, we held two remote workshops with patients/carers and HCPs. This resulted in fewer participants overall. However, in-depth discussions and feedback indicated that findings were appropriate and, therefore, this did not have an overall impact on the study.
Our taxonomy included examples of care co-ordination that currently exist in practice but also included potential new options for co-ordinating care. Therefore, it is not fully clear from the findings presented in this chapter which options of care co-ordination are currently available in practice and which areas may need improvement. However, our findings demonstrate that there are examples of real-world practice for each of the six domains and subdomains presented in this chapter, and existing services may not include elements from each of the six domains at present. Example quotations (see Table 20) provide context regarding the availability of different options in practice. Our study aimed to identify existing and new models of care co-ordination and including both existing examples and new options enabled us to achieve this aim to develop a taxonomy that captures as many options as possible, offering a broader view of findings in relation to potential hypothesised future models of care co-ordination.
Implications
The taxonomy developed in this study can be used as a menu for service planners, researchers and commissioners to consider when developing new and/or existing models of co-ordination. For example, we have used the taxonomy, together with the qualifier findings presented in this chapter, to develop some hypothetical models of care co-ordination that may be applicable in different situations (see Chapter 8). We have also developed a flow chart that may inform how the findings are used to develop such models (see Chapter 8). These models can be costed and evaluated by researchers and services.
The qualifier findings can also be used to inform decisions about which models of care co-ordination may be suitable for use in different situations. This is particularly helpful given the complexity of care pathways and funding for rare conditions.
Future research
Future research is needed to explore the implementation, clinical effectiveness and cost-effectiveness of different models of care co-ordination for rare conditions in practice. This is important, given that it is not yet clear whether or not co-ordinated care leads to better outcomes (e.g. patient outcomes, professional outcomes and organisational outcomes). Further research is also needed to operationalise these models of care co-ordination so that delivery of care co-ordination can be measured.
Summary
Six domains of care co-ordination were identified within this taxonomy. Findings indicate that there are different options for co-ordinating care. Although different stakeholders have different preferences for options of care co-ordination, each type of care co-ordination has associated benefits and challenges. For each domain, there are many factors that influence co-ordination, including patient factors, provider factors, environmental factors and societal factors. In addition, there are underlying barriers and facilitators that influence care co-ordination for rare conditions that must be taken into account when deciding how to co-ordinate care.
Chapter 8 Illustrative models of co-ordination care
Overview
This chapter draws on Walton et al. 202 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.
What was already known?
-
There are numerous ways to co-ordinate care for people living with rare conditions.
-
There are also several factors to take into account when deciding how best to co-ordinate care, including stakeholder preferences, the benefits and challenges of different models of co-ordination, factors influencing co-ordination, and barriers to and facilitators of co-ordination.
What this chapter adds
-
Very little is known about the costs of different ways of co-ordinating care for people affected by rare diseases. This chapter outlines 10 hypothetical models of care co-ordination. These models take into account different factors that influence co-ordination and provide an insight into which models of care co-ordination may suit different circumstances.
Background
There are many ways to co-ordinate care. Our findings have highlighted the following six domains of care co-ordination for rare conditions, each of which have different options within them: (1) ways of organising care (e.g. centralised, local or a hybrid), (2) ways of organising teams (e.g. low collaboration or high collaboration), (3) responsibilities (e.g. administrative roles, formal roles and informal roles), (4) frequency (e.g. regular, on demand or a hybrid), (5) access to records (e.g. full access or restricted access) and (6) mode of communication (e.g. digital, telephone or face to face) (see Chapter 7, Overview of care co-ordination taxonomy). We also highlighted the many qualifying factors that may influence how care should be co-ordinated. For example, patient and HCP preferences, benefits and challenges of different models of co-ordination, factors influencing co-ordination (including patient, provider, health-care environment and societal factors) and barriers to and facilitators of co-ordination more generally (see Chapter 7, Taxonomy domains and qualifiers). The taxonomy that we have developed as part of this project, together with the in-depth findings on qualifiers of care co-ordination model, can help us to develop new models of co-ordination.
In this chapter, we develop and refine hypothetical and illustrative models of care co-ordination for rare conditions using our taxonomy. We also review what evidence exists on the costs of different components of co-ordinated care.
Methods
Development of illustrative models
Development of a CONCORD flow chart
To develop illustrative models of care co-ordination, we first developed a flow chart of co-ordinated care (see Appendix 11). The flow chart was developed using the taxonomy and qualitative findings on qualifiers of care co-ordination (i.e. preferences, benefits/challenges, factors influencing co-ordination and barriers/facilitators) outlined in Chapter 7, Taxonomy domains and qualifiers. The flow chart is based on 30 interviews with health-care providers, commissioners and charity representatives and four focus groups with patients and carers with experience of rare, ultra-rare and undiagnosed conditions. The CONCORD flow chart is a visual representation of the CONCORD taxonomy findings (see Chapter 7). One researcher (HW) developed the flow chart to visualise how different ways of co-ordinating care can be used in certain situations.
The CONCORD flow chart includes the six domains from the CONCORD taxonomy. We included all six domains as they were all found to be important when co-ordinating care. Within the flow chart, a series of questions are asked to help users to think about which option of co-ordination may best suit patient, family and service circumstances. The flow chart has decision boxes (i.e. boxes that are fully shaded). Within each decision box, there are multiple options that may be suitable (e.g. the type of technology, mode of communication or who co-ordinates care). The flow chart is not designed to account for all possible situations, but, instead, aims to support discussion and thinking around which models may suit different situations. In addition to the flow chart, we have also designed a cover note to help users to understand how the flow chart can be used (see Appendix 11).
To provide insight into how the flow chart was developed from the findings, we provide an example of how the qualifier findings were used to consider how different options with the domains of the taxonomy could be adapted in different situations. For example, for domain 1 (ways care is organised), the findings on preferences, benefits/challenges and factors influencing co-ordination indicate that specialist centres may not be available for all conditions and, therefore, it is necessary to first ask whether or not specialist centres are available. Findings indicated that specialist centres work better for some conditions than for others (e.g. specialist centres may work better for patients with discrete phenotypes as services can identify who a patient will need to see, whereas patients with conditions that do not fit into a clinical group or are difficult to define may not currently be as well placed for care within specialist centres). Therefore, it is necessary to find out whether or not the patient can benefit from attending a specialist service. Findings indicated the need to consider where the patient lives and if the patient can, and wants to, travel to attend a specialist centre. This would help us to determine whether care is most appropriate at a national or regional specialist centre, or, alternatively, whether a hybrid model (i.e. care split between specialist centre and local care) or outreach care (i.e. in the local area) may be more appropriate for their situation.
Development of illustrative models
Using the CONCORD flow chart and the taxonomy presented in Chapter 7, we next developed some hypothetical illustrative models of care co-ordination. These models were designed to illustrate the use of the taxonomy and the CONCORD flow chart. We developed hypothetical models instead of actual care co-ordination models, as the findings from Chapter 7 indicated that there were many different ways care could be co-ordinated and we may not be able to fully represent all situations, domains and options of care co-ordination if using real-life examples. However, some real-life examples of different aspects of co-ordination are shown in Chapter 7.
To develop the illustrative models, we considered different scenarios in terms of:
-
where the patient and parent/carer live in relation to a specialist centre
-
whether or not the patient and parent/carer can, or want to, travel to a specialist centre
-
whether or not the patient and parent/carer have the ability (and want) to co-ordinate their own care
-
whether or not the patient and parent/carer have access to a specialist centre
-
whether or not it is clear whom the patient needs to see for the management of the condition.
Initially, we developed eight models, including models of care co-ordination for patients who have access to specialist centres (e.g. models relating to those who live nearby centres, those who live far from centres away but who are able to travel and those who live far away from centres but cannot travel) and models of care co-ordination for patients who do not have access to specialist centres (e.g. models relating to patients without a specialist centre and where it is not clear who they need to see and to patients without a specialist centre where it is clear who they need to see). Each of these scenarios had two models: (1) a model for patients or parents/carers who had the ability to co-ordinate their own care and (2) a model for patients or parents/carers who were unable co-ordinate their own care.
When developing the models, we also highlighted how additional situation-specific decisions would need to be considered within each model. For example, when considering how best to co-ordinate someone’s care there would need to be a decision-making process regarding the provider and patient. These situation-specific factors may include one or more of the following:
-
the level of co-ordinator support available and needed
-
who the co-ordinator is and who the clinical lead is
-
who should be involved in MDT meetings
-
the extent to which different modes are used for information-sharing, communication, care delivery and co-ordination
-
the extent to which information is shared
-
the extent to which providers have access to records
-
how often care co-ordination and care appointments are needed
-
transition needs.
These decisions are likely to be based on considerations such as the availability of resources, the health-care economy and environment, patient- and parent/carer-level circumstances and factors, and provider-level factors.
Refinement of hypothetical illustrative models
To refine the models, we sent a handout by e-mail or post depending on the preference of the respondent that summarised the hypothetical models to CONCORD workshop participants (i.e. patients, carers, HCPs, commissioners and charity representatives) who consented to provide feedback after the workshops (see Chapter 7). We asked participants for their views on whether or not the models seemed appropriate based on their experiences, and why, and whether or not we had missed any obvious models of co-ordination.
We received written feedback from eight workshop participants and members of the CONCORD research team. To address the feedback and refine the models, we grouped the feedback into two categories: (1) ‘feedback on the models’ and (2) ‘suggested improvements’.
Generally, findings indicated positive feedback on the hypothetical models. However, participants highlighted that, in practice, these models may have some overlap, may not currently be seen and/or may not be feasible in the current climate (e.g. because of funding and local commissioning). Yet, participants affirmed that these models should be aimed for and that these models may be possible to achieve in future.
A number of improvements were suggested by workshop participants, including the need to add transition into all models; the need to include broader use of digital and remote technologies; the need to mention formal shared care models; the need to clarify that who is involved in outreach clinics may vary (e.g. it may not always be a whole MDT); the need to add information about emergency health-care planning; the need to signpost patients with undiagnosed/ultra-rare conditions to relevant patient support groups; arranging appointment frequency based on need, and explaining the role of care co-ordinators. In addition, feedback from the research team highlighted that there were more models for those with access to a specialist centre than for those without. It was proposed that further models of co-ordination for those without access to a specialist centre should be included. We identified amendments to the models for each of the suggested improvements. For example, one of the suggested improvements was to include transition in more of the models and so we ensured that transition was mentioned in models 1–8.
The models were amended to take into account the feedback received, resulting in 10 hypothetical illustrative models of care co-ordination (see Report Supplementary Material 4).
Measuring costs
In accordance with our study protocol, to address RQ5 (i.e. ‘how much do different models of co-ordinated care cost?’) we originally planned to undertake preliminary cost analyses of the models of co-ordinated care that we developed. These analyses were to include the cost to set up and implement each model and the cost to run each model. We envisaged that these analyses would not be a formal analysis of the incremental costs of care co-ordination (as such an analysis would not be possible without detailed evidence of the long-term impacts of co-ordination on health outcomes and health-care use), with the focus, instead, on the ‘intervention’ costs associated with setting up and running the different models of co-ordinated care. We envisaged that data for these analyses would be based on data from the survey (see Chapter 5) and data from the workshops used to refine the taxonomy (see Chapter 7). Unfortunately, it was not possible to use either of these sources. In the case of the survey, as shown in Chapter 5, most people did not experience co-ordinated care. In addition, the survey did not ask questions about the ‘intervention costs’ associated with different types of care co-ordination. Furthermore, it was not possible to attribute the 10 hypothetical models that were developed to survey respondents’ experiences. In the case of the workshops, the health service utilisation associated with each hypothetical model was unknown by workshop participants. This was primarily because the use of services associated with each model was likely to vary according to the situation-specific factors described above (see Chapter 8, Development of hypothetical models). The result was that it was not possible to generate costs associated with each hypothetical model from the survey data or from the workshop data.
In an attempt to find indicative costs of the models, we instead undertook a review of the costs of different characteristics of co-ordinated care. We identified from the 10 hypothetical models that costs would likely be incurred for the following characteristics:
-
whether or not the patient attended a specialist centre
-
whether or not the patient had a care co-ordinator
-
whether or not the patient had a care plan (i.e. a formalised care agreement)
-
whether or not the patient’s care was discussed in MDT meetings.
We then attempted to identify the costs of each of these characteristics from previously conducted research and administrative data.
We know that evidence on the costs of interventions to co-ordinate care is extremely limited. 219 Nonetheless, to identify what costs were available, we did the following:
-
We adapted the search strategy we used for the scoping review in Chapter 3 (see Appendix 1). We reran the PubMed search, replacing the search terms relating to the type of study (search number #17; the scoping review was a review of reviews) with ‘cost*’ in any field. This meant that the search focused on studies of care co-ordination of rare and chronic diseases where costs were mentioned. We reviewed the titles and abstracts of the identified papers to identify cost analyses of the characteristics of co-ordinated care included in the models. Studies focusing on any condition were included, not just rare conditions. As we are interested in the costs of co-ordination from a UK perspective, we included UK studies only.
-
We searched the NHS Economic Evaluation Database (NHS EED)220 using search terms ‘coord* OR care plan OR specialist centre OR care co-ordinator’. Note that NHS EED includes economic evaluations published up until 31 March 2015 only. We reviewed the titles and abstracts of the identified studies. As above, studies focusing on any condition were included, not just rare conditions, and we included UK studies only.
-
We reviewed commonly used sources of unit costs for undertaking economic evaluations in the UK (NHS reference costs for 2018/19221 and Unit Costs of Health and Social Care 2019222) to identify unit costs for each characteristic. We included unit costs relating to any condition, not only rare conditions.
We tried to identify UK-based ‘intervention’ costs for each of the four characteristics (i.e. attending a specialist centre, access to a care co-ordinator, having a care plan and having care discussed at MDT meeting) and converted these into 2019/20 GBP where necessary.
Results
Illustrative models
We developed 10 hypothetical models of care co-ordination. These models are summarised in Table 22 (see Report Supplementary Material 4 for further details). Each type of model is a function of where the patient and parent/carer lives in relation to a specialist centre, if the patient and parent/carer can, or wants to, travel to a specialist centre, if the patient and parent/carer has the ability (and want) to co-ordinate their own care, if the patient and parent/carer has access to a specialist centre and whether or not it is clear who the patient needs to see for the management of the condition. The characteristics of the models are centred around attending a specialist centre or outreach clinic, having a formalised care agreement (i.e. a care plan), having a care co-ordinator to organise appointments (or providing a point of contact), whether or not there are meetings between HCPs to discuss care and the type of a HCP who oversees care. The 10 models described in Table 22 are essentially combinations of these characteristics. As noted above, the specificity of these characteristics will be determined by situation-specific factors.
| Model number | Type of model | Characteristics of model |
|---|---|---|
| 1 | Patient (adult or child) lives near to a specialist centre/service (or condition-specific clinic/joint clinic) plus patient or parent/carer has ability and want to co-ordinate own care |
|
| 2 | Patient (adult or child) lives near to specialist service/centre (or condition-specific clinic/joint clinic) plus patient or parent/carer cannot co-ordinate own care | |
| 3 | Patient (adult or child) lives far away from specialist service/centre (or condition-specific clinic/joint clinic) and can travel only if necessary plus patient or parent/carer has ability and want to co-ordinate own care |
|
| 4 | Patient (adult or child) lives far away from the specialist service/centre (or condition-specific clinic/joint clinic) and can travel only if necessary plus patient or parent/carer cannot co-ordinate own care | |
| 5 | Patient (adult or child) lives far away from specialist service/centre (or condition-specific clinic/joint clinic) and is unable to travel to access specialist centre plus patient or parent/carer has ability and want to co-ordinate own care |
|
| 6 | Patient (adult or child) lives far away from specialist service/centre (or condition-specific clinic/joint clinic) and is unable to travel to access specialist centre plus patient or parent/carer cannot co-ordinate own care | |
| 7 | Patient (adult or child) with an ultra-rare/undiagnosed condition does not have access to a specialist centre and it is not clear who they need to see plus patient or parent/carer has ability and wants to co-ordinate own care |
|
| 8 | Patient (adult or child) with an ultra-rare/undiagnosed condition does not have access to a specialist centre and it is not clear who they need to see plus patient or parent/carer cannot co-ordinate own care | |
| 9 | Patient (adult or child) with a rare/ultra-rare or undiagnosed condition does not have access to a specialist centre but it is clear who they need to see plus patient or parent/carer has ability and wants to co-ordinate own care |
|
| 10 | Patient (adult or child) with a rare/ultra-rare or undiagnosed condition does not have access to a specialist centre but it is clear who they need to see plus patient or parent/carer cannot co-ordinate own care |
Costs of model characteristics
Our reviews of PubMed and NHS EED identified 7254 and 190 hits, respectively. However, after reviewing the titles and abstracts of these reports, we found that evidence on the UK costs of characteristics of co-ordinated care was extremely limited. We found one study11 that calculated the costs of treatment at a specialist centre for a rare condition (Alström syndrome). This study estimated the mean cost per patient per annum, including clinic attendances and contacts with HCPs, plus consumables and capital. The estimated cost was £748 per patient per annum (i.e. £690 per patient per annum in 2015/16 prices). We found one study223 that evaluated the costs of a care co-ordinator (a key worker for disabled children), which included telephone calls and face-to-face contacts with the patient and their family and non-contact time (e.g. writing case notes, travelling, liaising with staff from their own and other organisations, and attending meetings and reviews). The estimated cost was £834 per patient per annum (i.e. £151 for 3 months in 2002/3 prices) for time spent in contact with families, which rose to £1251 to £1668 per patient per annum when including non-contact time. 223 We found estimates for the costs of maintaining and reviewing a care plan – for looked after children – with a total cost of £568 per patient per annum (i.e. £556 per annum in 2018/19 prices). 222 We were unable to find estimates of the UK costs per patient of MDT meetings, but found national average costs of £94–140 per patient per meeting for cancer MDT meetings. 221 With the exception of the cost of attending the specialist centre, it is not clear if these costs are applicable to co-ordinated care for people affected by rare conditions. In addition, we were unable to use these estimates to represent the impact of the situation-specific factors identified in the illustrative models.
Discussion
Key findings
In this chapter, we developed and refined 10 illustrative models of care co-ordination for rare conditions using our taxonomy. The type of model was a function of where the patient and parent/carer lives in relation to a specialist centre, if the patient and parent/carer can, and wants to, travel to a specialist centre, if the patient and parent/carer have the ability (and want) to co-ordinate their own care, if the patient and parent/carer have access to a specialist centre and whether or not it is clear who the patient needs to see for the management of the condition.
We attempted to calculate the costs of each model using data from the study, but this was not possible. Instead, we reviewed what evidence exists on the UK costs of different components of co-ordinated care, focusing on the costs of attending a specialist centre, the costs of having a care co-ordinator, the costs of having a care plan and the costs of discussing a patient’s management at MDT meetings. Although some UK cost data were found, these data were limited and it was not possible to apply these data to the 10 illustrative models.
Limitations
The main limitation of the research presented in this chapter was the lack of cost data, which prevented us from estimating the costs of the illustrative models. We were unable to use data from the national survey or the taxonomy and so, instead, searched for previously estimated costs from UK-based studies for the key characteristics of the models. In terms of the survey, it was not possible for survey respondents to report the costs of different aspects of care co-ordination, partly because many of these aspects are not experienced by participants and also because the NHS costs incurred or resources used to provide these aspects of care were unknown. For the work on the taxonomy, we had originally envisaged that for each of the models that were developed it would have been possible to provide specific information around the resources needed to provide that model (e.g. the number, type and hours of staff required, and the non-staff resources required). With these data, it would have been possible to undertake a detailed bottom-up costing. However, this information was not known by study participants and so this level of specificity was not possible. There were few UK studies that calculated the costs of care co-ordination and it was unclear if the available data are applicable to the co-ordination of care for rare diseases. In addition, we were unable to use these estimates to represent the impact of the situation-specific factors identified in the illustrative models.
Further research
Further research would be beneficial to produce accurate estimates of the costs of the different elements of co-ordinated care for people affected by rare conditions in the UK. One possible approach would be to find empirical examples of each element of co-ordinated care currently in existence and undertake a detailed bottom-up costing. In addition, further research into the feasibility of adapting existing rare disease services or implementing these hypothetical models into rare disease services in future is needed.
Summary
The findings of this chapter highlight that it is possible to create models of care co-ordination from the taxonomy. These findings provide an insight into which models of care co-ordination may suit different circumstances, and can support discussion and thinking around which models may suit different situations. UK data on the costs of providing co-ordinated care are sparse and further research is needed to evaluate these costs.
Chapter 9 Discussion
Overview
This research used qualitative and quantitative research methods to report and analyse the co-ordination of care for people affected by rare diseases in the UK to investigate (1) if and how care of people with rare diseases is co-ordinated in the UK and (2) if and how patients and families affected by rare diseases, and HCPs who treat rare diseases, would like them to be co-ordinated.
Our study posed five RQs (see Chapter 1, Research questions and overview of the research project). To address RQ1, we undertook a scoping review that focused on care co-ordination across organisational boundaries and the interventions employed to support and improve this. For RQs 2 and 3, we created a questionnaire-based survey of current experiences and costs, incorporating a DCE of preferences for co-ordination. In addition, we undertook an exploratory qualitative interview study to understand the impact of a lack of co-ordinated care on patients and carers. For RQ4, we undertook interviews, focus groups and workshops with a range of stakeholders to develop a taxonomy of co-ordinated care for rare diseases. For RQ5, we aimed to calculate the costs of models developed from this taxonomy.
To address these RQs required substantial input from patients and families, in terms of both helping to design and participating in the research. The research team included representatives from Genetic Alliance UK (a national charity that is an alliance of more than 180 patient organisations) and from national patient organisations with direct experience of living with rare conditions. These representatives ensured that patients’ and families’ priorities and needs were the focus of the study and contributed to the design and management of the study, patient recruitment, data collection, interpretation of findings and dissemination. These representatives also ran the study’s PPIAG, which involved managing and working with a group of six to eight patients and carers and meeting twice a year for the duration of the project. This group supported the development of resources and participant information, patient recruitment and dissemination of findings.
In this chapter, we present a summary of our main findings linked to our RQs. We then discuss the implications of these findings, the strengths and limitations of our study and propose a future research agenda.
Main findings
In terms of our investigation into RQ1 (i.e. what ‘co-ordinated care’ means, what the components of co-ordinated care are and in what ways and why co-ordinated care for people with rare diseases may be similar or different from co-ordinated care for people with other conditions), our main findings were as follows:
-
Our definition of co-ordinated care for rare conditions is as follows:Reproduced from Walton et al. 15 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/2020Walton et al. https://creativecommons.org/licenses/by/4.0/
Co-ordination of care involves working together across multiple components and processes of care to enable everyone involved in a patient’s care (including a team of health care professionals, the patient and/or carer and their family) to avoid duplication and achieve shared outcomes, throughout a person’s whole life, across all parts of the health and care system, including: care from different health care services . . . care from different health care settings . . . care across multiple conditions or single conditions that affect multiple parts of the body, the movement from one service, or setting to another. Co-ordination of care should be family-centred, holistic (including a patient’s medical, psychosocial, educational and vocational needs), evidence-based, with equal access to co-ordinated care irrespective of diagnosis, patient circumstances and geographical location.
-
Our review highlighted that little was known about co-ordination for rare conditions because most reviews focused on common chronic conditions. Our definition reflects important differences between rare conditions and common chronic conditions, specifically the complexities associated with rare conditions. For example, rare conditions may affect multiple body parts, can affect children and may be lifelong. Rare conditions need to be co-ordinated across multiple sectors. In addition, there are difficulties with regard to diagnosis because of limited expertise. These complexities suggest that more care co-ordination is needed in cases of greater system fragmentation, clinical complexity and decreased patient capacity.
-
Components of care for rare diseases that require co-ordination relate to administration, assessment and diagnosis, planning, review and evaluation, feedback, follow-up care, use of technology, support for patients carers and families, and support for HCPs.
-
Components that outline how care can be co-ordinated relate to someone taking responsibility, use of specialist centres/clinics, communication, support for patients, families and HCPs, MDT approaches, continuity of providers and development of care plans.
-
Components that may influence or contextualise co-ordination are evidence-based practice (e.g. guideline-based treatment), individual differences in needs, wants and preferences, the wider health-care environment and access to treatment.
-
Many of the key components and issues for co-ordinated care apply to both rare and common chronic conditions. Important factors that may make it more difficult to co-ordinate care for rare conditions are difficulties in diagnosing rare conditions due to limited knowledge and ability to recognise symptoms and a lack of condition-specific expertise due to small numbers of patients.
In terms of our second RQ, which asked if care for people with rare diseases in the UK was co-ordinated, our main findings were as follows:
-
Care for people affected by rare diseases is generally not well co-ordinated in the UK, with limited access to care co-ordinators, specialist centres and care plans.
-
Only 12% of patients affected by a rare disease, and 14% of parents and carers, reported having a formal care co-ordinator.
-
Only 39% of patients reported that a specialist centre for their rare condition was available and 32% of patients attended a specialist centre for their condition. Among parents/carers, these values were 37% and 33%, respectively.
-
Ten per cent of patients reported having a care plan relating to their rare condition, compared with 44% of parents/carers.
-
Fifty-four per cent of patients and 33% of parents/carers had no access to a formal care co-ordinator, a care plan or a specialist centre. By contrast, only 2% of patients and 5% of parents/carers reported having access to all three elements.
-
Lack of co-ordination resulted in delays/barriers to accessing care, and placed a significant burden on patients and carers, and these effects had a negative impact on patients’ and carers’ physical and mental health, and their financial well-being.
In relation to the preferences of patients, families and HCPs in relation to how care for rare diseases is co-ordinated (i.e. RQ3), we found the following:
-
Preferences of patients, parents/carers and HCPs were all found to be consistent with better co-ordination of care.
-
More than 70% of patients, parents/carers and HCPs agreed or strongly agreed that having a care co-ordinator would improve quality of care.
-
More than 80% of patients, parents/carers and HCPs agreed or strongly agreed that having access to a specialist centre would improve quality of care.
-
More than 80% in each group agreed or strongly agreed that having a care plan would improve quality of care.
-
All three groups preferred services where the cost of attending appointments was lower, electronic health records were immediately accessible to staff, the lead consultant was a medical expert in the patient’s specific medical condition, care is provided with the support of a care co-ordinator, a specialist centre was available and there was a documented emergency plan in place.
-
All participant groups were prepared to make trade-offs for better care co-ordination. For example, patients and parents/carers were willing to pay £2509 for access to a specialist centre, £2470 for a consultant who was a medical expert in the patient’s condition, £2442 for electronic health records that were immediately accessible to staff, £1367 for a documented emergency plan and £1306 for the support of a care co-ordinator.
-
There were some differences between the preferences of patients and parents/carers compared with HCPs. HCPs preferred that care was entirely co-ordinated on behalf of the patient by a care co-ordinator, whereas patients and carers preferred that they decided how they wish to be supported by the care co-ordinator. In terms of emergency plans, all three groups preferred there to be a documented emergency plan in place, but HCPs felt more strongly about this than patients and carers.
Research question 4 asked about the different ways in which care for people with rare diseases might be co-ordinated. Our main findings were as follows:
-
We developed a taxonomy, which classified the co-ordination of care for rare conditions into the following six domains: (1) ways of organising care, (2) ways of organising teams, (3) responsibilities, (4) how often care appointments and co-ordination take place, (5) access to records and (6) modes of communication.
-
Ways of organising care ranged from all care being delivered locally to care being delivered in a national centre that serves all patients in the country with a particular rare condition. There were also ‘hybrid’ options, combining both specialist and local care.
-
Ways of organising teams ranged from little collaboration (e.g. not having a MDT) to high levels of collaboration (e.g. all professionals working together to provide or discuss care in a condition-specific clinic or a MDT meeting). Intermediate options included some HCPs working together (e.g. in joint clinics).
-
We identified different types of responsibility involved in co-ordinating care for rare conditions, including administrative, formal and supportive roles. Administrative support included help in organising appointments for patients and having a point of contact. Formal co-ordination responsibilities were those conducted by a co-ordinator, a clinical lead or a GP. Supportive roles were also identified, including those played by patients and carers and those conducted by charities.
-
Care appointments and co-ordination activities can be arranged at regular intervals or on demand, or a hybrid approach combining regular care (at a minimum) with on-demand support could be adopted.
-
Patients’ and providers’ access to records ranged from full access to restricted access.
-
A range of different modes of information-sharing, care delivery and/or co-ordination and communication were identified. Perceived factors influencing mode of information-sharing and care delivery included patient factors (e.g. age, condition and individual needs) and health-care environment factors (e.g. access to technology).
-
A combination of methods can be used to deliver health care, highlighting the potential for remote methods of co-ordination, including digital information-sharing (e.g. through electronic records), virtual clinics and care co-ordination appointments. This shift to digital methods has been accelerated during the COVID-19 pandemic. Our findings also highlight that each mode of communication has benefits and challenges, and findings indicate that the mode of co-ordination should take into account many factors, including individual preferences and resources. In addition, our findings indicate that, despite the COVID-19 pandemic, digital appointments should not replace face-to-face appointments completely in terms of care delivery and co-ordination. Face-to-face appointments were felt to be essential, particularly at key points of the patients’ journey (e.g. initial meetings and diagnosis), for certain conditions or for patients requiring more in-depth clinical care co-ordination because of additional difficulties. This extends previous research219 by highlighting the limits of digital methods of care delivery and co-ordination, and emphasises the need to offer multiple modes when co-ordinating care for patients with rare conditions.
In terms of RQ5 (i.e. how much do these options cost) our main findings were as follows:
-
Using the taxonomy it was possible to develop some hypothetical models of care co-ordination that may be applicable in different situations. These models are a function of a range of scenarios that commonly apply to people affected by rare conditions, namely where the patient and parent/carer lives in relation to a specialist centre, if the patient and parent/carer can (and wants to) travel to a specialist centre, if the patient and parent/carer have the ability (and want) to co-ordinate their own care, if the patient and parent/carer have access to a specialist centre and whether or not it is clear who the patient needs to see for the management of the condition.
-
We developed a flow chart that may inform how the findings are used to develop such models.
-
We undertook a review of the costs of different components of co-ordinated care to illustrate indicative costs, and found a lack of cost data.
Implications of these findings
There are two main implications of the findings of this study. The first relates to whether or not care for rare diseases is co-ordinated, the second relates to the ways in which care for people with rare diseases might be co-ordinated.
Implications relating to whether or not care for rare diseases is co-ordinated
The UK government recently highlighted the problem of co-ordinated care for people affected by rare diseases in The UK Rare Diseases Framework,9 although the evidence base was largely anecdotal. 9 The UK Rare Diseases Framework9 states that co-ordination of care is one of the top challenges facing people affected by rare diseases and better co-ordination was listed as one of the four top priorities. In a ‘national conversation’ survey of 6293 members of the rare diseases community, conducted in 2019, co-ordination of care was identified as one of the top challenges facing the rare diseases community. Our study findings are consistent with The UK Rare Diseases Framework,9 but add further detail of the extent of these problems. We identified gaps in provision of services that might better facilitate co-ordination, identified aspects of care that are not co-ordinated, provided evidence of the impact of these problems and provided further evidence that people affected by rare diseases have a strong preference for better co-ordination.
Our definition of care co-ordination for rare diseases highlights the complexity of achieving co-ordinated care, indicating that there are several components of care that ought to be addressed to improve co-ordination. This definition serves as a useful guide for researchers, policy-makers and other stakeholders seeking to improve care co-ordination.
Evidence of the lack of co-ordinated care for people affected by rare diseases is supported by our national survey, which found that for the majority of people affected by rare diseases care is not well co-ordinated, with limited access to care co-ordinators, specialist centres and care plans.
The importance of the finding from our national survey that people affected by rare diseases have limited access to co-ordinated care was made clear by our exploratory qualitative interview study, which highlighted how patients and carers are negatively affected by poorly co-ordinated care in terms of their physical and mental health and their financial well-being.
The importance of co-ordinated care was further strengthened by the findings of our taxonomy and our analysis of preferences, which showed that patients, parents/carers and HCPs all have a clear preference for better co-ordinated care. This preference related to all the aspects of co-ordinated care that were considered.
Implications relating to the ways in which care for people with rare diseases might be co-ordinated
As well as providing further evidence of lack of care co-ordination, our findings also have implications for the ways in which care for people with rare diseases might be co-ordinated and propose new models of care co-ordination. Following the publication of The UK Rare Diseases Framework,9 the four devolved nations of the UK will develop action plans that set out how the four priorities identified in the framework will be addressed (note that the third priority is ‘better co-ordinated care’). Our findings around the hypothetical models of co-ordination have potentially useful implications for this work, for example by being included in national and local action plans, and for others seeking to improve co-ordination of care.
Our definition of care co-ordination and description of the components of care co-ordination can be taken into account when considering how to improve co-ordinated care.
The taxonomy developed in this study can be used as a menu for service planners, researchers and commissioners to consider when developing new and/or existing models of co-ordination. In addition, the qualifier findings from the taxonomy can also be used to inform decisions about which models of care co-ordination may be suitable for use in different situations, accounting for the preferences of stakeholders. This is particularly helpful given the complexity of care pathways and service funding for rare conditions.
We have developed a flow chart that may inform how the findings from the taxonomy may be used to develop such models, and their potential costs.
The trade-offs from the DCE could be used to value the potential benefits of different models of care co-ordination. For example, the willingness to pay for each aspect of care co-ordination is a measure of the value of the benefit of each aspect on average per patient. The willingness to pay could be summed across all patients receiving that aspect of care and balanced against the total costs of providing that aspect of care in a future cost–benefit analysis.
Main strengths and limitations
Strengths
There are several strengths of our study. The study used a combination of qualitative and quantitative research methods to better understand (1) what co-ordinated care for people affected by rare diseases means and (2) how to address the problem of poorly co-ordinated care. Input into addressing the RQs was obtained from a range of stakeholders, including patients, parents, carers, HCPs from a range of occupations and clinical backgrounds, commissioners and patient organisations. We undertook a large and comprehensive national survey, covering a wide range of people affected by rare diseases. The study had a strong patient and public involvement (PPI) component throughout all aspects of the study. Members of Genetic Alliance UK, PPI co-applicants on the study team and the study PPIAG, inter alia, advised on the content and wording of the survey, gave feedback on the findings of the scoping review and taxonomy, and advised on dissemination opportunities.
Limitations
There were several limitations to the study. These limitations are described in detail in each chapter, but the main limitations were as follows.
First, it was not possible to capture the experiences of people affected by every rare condition. However, the principles of care co-ordination, and what these ought to entail, are likely to be common across many rare conditions, which means that it was still feasible to make recommendations about what co-ordinated care for rare conditions should involve. We ensured that we included a wide range of models of care co-ordination by involving many different stakeholders during the study and eliciting their views throughout. These stakeholders included patient organisations/charities that work on behalf of people affected by rare conditions, patients and carers affected by rare conditions, HCPs caring for people with rare conditions, commissioners and providers at local and national levels who commission/provide health services for people affected by rare conditions, academics interested in the organisation of care for people with rare conditions and policy-makers with an interest in rare conditions.
Second, our sampling for both the qualitative and quantitative aspects of the study may have been biased if study participants were systematically different from the population affected by rare conditions. For our national survey and DCE in Chapters 5 and 6, it was not possible to determine the representativeness of the sample and, therefore, the generalisability of the results. We had high proportions of patients and parents/carers who were female and from more highly educated groups, which is likely to indicate that these groups were over-represented, but the true extent of this is difficult to quantify. There is a paucity of evidence regarding the total number of people living with rare conditions in the UK, and their characteristics, such as gender, age distribution, ethnicity, level of education and socioeconomic status, are unknown. Moreover, the methods used to recruit meant that people without links to a patient organisation that was known to the study partners, and who do not use NHS hospital services, were likely to be under-represented in the survey respondents. For the taxonomy and models in Chapters 7 and 8, although we sampled from a variety of rare conditions, locations, sectors and populations, some groups are likely to have been under-represented in our research and, therefore, we are unlikely to have captured every possible option of care co-ordination for rare conditions. Although we used the wealth of experience at Genetic Alliance UK in undertaking research among people affected by rare diseases throughout the study, and worked with the PPIAG to explore the right methods for including hard-to-reach patients and families affected by rare conditions, our sampling strategy may have introduced bias into our results and conclusions.
Third, our review of reviews in Chapter 3 was a scoping review and, therefore, was unlikely to have captured every relevant study. To identify as many studies as possible, we conducted a comprehensive search that included contacting experts and searching the reference lists of included reviews.
Fourth, our qualitative study in Chapter 4 was based on only 15 participants.
Fifth, in terms of the DCE, this method elicits hypothetical choices and, therefore, might lack external validity if individuals do not make the same choices in real-life situations. We also acknowledge that preferences from the DCE might vary by subgroups within our study groups, but sample size considerations make subgroup analyses problematic.
Sixth, our cost analysis in Chapter 8 was limited in scope because of the paucity of available data, and there is considerable uncertainty in the costs associated with different co-ordination models.
Future research
Our findings demonstrate the need for future research to improve the co-ordination of care for people affected by rare diseases in the UK. In particular, future work should consider the following:
-
Further work is required to develop specific models of care co-ordination, as may be derived from our taxonomy, accounting for the views and preferences of patients, parents/carers and HCPs. This work could involve qualitative research, comprising interviews and focus groups with stakeholders, including those who already have experience of different aspects of care co-ordination.
-
In terms of preferences for different models of care co-ordination, further research would be valuable to gain a more in-depth understanding of the diversity of preferences by different subgroups of people affected by rare diseases and the circumstances under which certain views dominate. This could be achieved via qualitative and quantitative research methods, focusing on specific subgroups of stakeholders.
-
We noted in our national survey that a high proportion of our respondents were female. Further research to elicit the preferences among males would be beneficial, for example by repeating the survey and focusing, in particular, on sampling from males.
-
Further research to evaluate the resource use and costs of different models of care co-ordination would be valuable given the dearth of economic evidence. This could be achieved, for example, by adopting a bottom-up costing of models of care that currently exist. These models should include the use of care co-ordinators, specialist centres and/or care plans, and could consist of the illustrative models from Chapter 8. The feasibility of these models should also be addressed and should then be evaluated in practice to determine whether or not the potential benefits we have identified can be realised.
-
Such evaluations should test the clinical effectiveness and cost-effectiveness of different co-ordination models using a mixture of qualitative and quantitative approaches, as used in this study. The trade-offs from the DCE in Chapter 6 could be used to value the potential benefits of different models of care co-ordination, and be balanced against the costs.
-
Future research should also consider how best to implement these models into practice, for example using improvement science research methods.
-
We identified, from the regression analysis in our DCE, that there were some differences between the preferences of patients and parents/carers, and HCPs. In terms of care co-ordinators, HCPs preferred that care was entirely co-ordinated on behalf of the patient by a care co-ordinator, whereas patients and carers preferred that they decided how they wished to be supported by the care co-ordinator. In terms of emergency plans, all three groups preferred there to be a documented emergency plan in place; however, HCPs felt more strongly about this than patients and carers. Further research would be useful to understand these differences in preferences to facilitate making recommendations about preferred models of care co-ordination.
Acknowledgements
We are grateful to all of the research participants who took part in this study and to our contacts at each of the study sites who helped to co-ordinate the study and recruit participants. We thank the members of the PPIAG who contributed an insightful viewpoint to inform protocol development, study design, data collection tools development and dissemination. We also thank the members of the Study Steering Committee for their support and critical thinking.
Contributions of authors
Stephen Morris (https://orcid.org/0000-0002-5828-3563) (RAND Professor of Health Services Research) was the principal investigator and led the study. He provided oversight for the survey and DCE. He contributed to design and analysis of all other aspects of the research, wrote the first drafts of Chapters 1, 2, 8 and 9, and is lead author of the final report.
Emma Hudson (https://orcid.org/0000-0002-0505-5049) (Health Economist) led the design and analysis of the survey and DCE, and contributed to the scoping review. She contributed to the design and analysis of all other aspects of the research and wrote the first drafts of Chapters 5 and 6.
Lara Bloom (https://orcid.org/0000-0002-6168-8094) (Chief Executive Officer and President of The Ehlers–Danlos Society) provided PPI input, co-led the PPIAG and contributed to the design and analysis of all aspects of the research.
Lyn S Chitty (https://orcid.org/0000-0002-4857-7138) (Professor of Genetics and Fetal Medicine) provided clinical input to the study from a clinical genetics perspective and contributed to the design and analysis of all aspects of the research.
Naomi J Fulop (https://orcid.org/0000-0001-5306-6140) (Professor of Health Care Organisation and Management) provided oversight for the qualitative aspects of the study, and contributed to the design and analysis of all other aspects of the research.
Amy Hunter (https://orcid.org/0000-0001-5076-8761) (Director of Research) provided oversight of PPI activities and of the qualitative exploratory study, and contributed to the design and analysis of all other aspects of the research.
Jennifer Jones (https://orcid.org/0000-0001-6936-6092) (Researcher) contributed to PPI activities and to the design and analysis of all aspects of the research.
Joe Kai (https://orcid.org/0000-0001-9040-9384) (Professor of Primary Care) provided clinical input to the study from a primary health-care perspective, and contributed to the study design and analysis of all aspects of the research.
Larissa Kerecuk (https://orcid.org/0000-0001-5003-3183) (Rare Disease Lead and Consultant Paediatric Nephrologist) provided clinical input to the study from the perspective of an expert in the treatment of rare diseases.
Maria Kokocinska (https://orcid.org/0000-0001-7262-1314) (Nephrology and Rare Diseases Research Co-ordinator) contributed to the design and conduct of the survey.
Kerry Leeson-Beevers (https://orcid.org/0000-0001-8826-5086) (National Development Manager and Project Lead) provided PPI input, co-led the PPIAG and contributed to the design and analysis of all aspects of the research.
Pei Li Ng (https://orcid.org/0000-0001-8411-220X) (Research Project Manager) provided project management input and co-ordinated research activities.
Sharon Parkes (https://orcid.org/0000-0002-9817-1160) (Nephrology and Rare Diseases Research Co-ordinator) contributed to the design and conduct of the survey.
Angus IG Ramsay (https://orcid.org/0000-0002-4446-6916) (Senior Research Fellow) provided oversight for the qualitative aspects of the study and contributed to the design and analysis of all other aspects of the research.
Amy Simpson (https://orcid.org/0000-0001-7880-4218) (Senior Researcher) led the design and analysis of the exploratory qualitative study and the open-text data from the survey, and contributed to the scoping review. She contributed to the design and analysis of all other aspects of the research and wrote the first draft of Chapter 4.
Alastair Sutcliffe (https://orcid.org/0000-0001-8542-6155) (Professor of General Paediatrics) provided clinical input to the study from a general paediatrics perspective, and contributed to the study design and all aspects of the research.
Christine Taylor (https://orcid.org/0000-0003-0120-0365) (Research Project Manager) provided project management input and co-ordinated research activities.
Holly Walton (https://orcid.org/0000-0002-8746-059X) (Research Associate) led the design and analysis of the scoping review, the open-text data from the survey, the taxonomy and the resulting models. She contributed to the design and analysis of all other aspects of the research and wrote the first drafts of Chapters 3 and 7.
All authors contributed to integrating the findings of the study. All authors made critical revisions to the report for important intellectual content and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved.
Publications
Walton H, Hudson E, Simpson A, Ramsay AIG, Kai J, Morris S, et al. Defining co-ordinated care for people with rare conditions: a scoping review. Int J Integr Care 2020;20:14:1–16.
Simpson A, Bloom L, Fulop NJ, Hudson E, Leeson-Beevers K, Morris S, et al. How are patients with rare diseases and their carers in the UK impacted by the way care is co-ordinated? An exploratory qualitative interview study. Orphanet J Rare Dis 2021;16:76.
Walton H, Simpson A, Ramsay A, Hudson E, Hunter A, Jones J, et al. Developing a taxonomy of care coordination for people living with rare conditions: a qualitative study. medRxiv 2021;2021.11.16.21266387.
Walton H, Simpson A, Ramsay AI, Hunter A, Jones J, Ng PL, et al. Development of models of care coordination for rare conditions: a qualitative study. Orphanet J Rare Dis 2022;17:1–24.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HSDR programme or the Department of Health and Social Care.
References
- Shojania KG, McDonald KM, Wachter RM, Owens DK. Technical Review 9. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies. Volume 7 – Care Coordination 2007. www.ahrq.gov/downloads/pub/evidence/pdf/caregap/caregap.pdf (accessed 22 October 2021).
- Nguengang Wakap S, Lambert DM, Olry A, Rodwell C, Gueydan C, Lanneau V, et al. Estimating cumulative point prevalence of rare diseases: analysis of the Orphanet database. Eur J Hum Genet 2020;28:165-73. https://doi.org/10.1038/s41431-019-0508-0.
- European Commission . EU Research on Rare Diseases n.d. https://ec.europa.eu/info/research-and-innovation/research-area/health-research-and-innovation/rare-diseases_en (accessed 17 January 2021).
- Rare Disease UK. What Is a Rare Disease? n.d. www.raredisease.org.uk/what-is-a-rare-disease/ (accessed 17 January 2021).
- Rare Disease UK. The Rare Reality – An Insight into the Patient and Family Experience of Rare Disease 2016. www.raredisease.org.uk/media/2361/patient-experiences-2015.pdf (accessed 22 October 2021).
- Genetic Alliance UK. The Hidden Costs of Rare Diseases: A Feasibility Study 2016. www.geneticalliance.org.uk/media/2502/hidden-costs-full-report_21916-v2-1.pdf (accessed 22 October 2021).
- Department of Health and Social Care . The UK Strategy for Rare Diseases n.d. www.gov.uk/government/uploads/system/uploads/attachment_data/file/260562/UK_Strategy_for_Rare_Diseases.pdf (accessed 22 October 2021).
- The All Party Parliamentary Group on Rare, Genetic and Undiagnosed Conditions . Leaving No One Behind: Why England Needs an Implementation Plan for the UK Strategy for Rare Diseases 2017. www.raredisease.org.uk/media/2757/final-for-website.pdf (accessed 22 October 2021).
- Department of Health and Social Care . The UK Rare Disease Framework n.d. www.gov.uk/government/publications/uk-rare-diseases-framework/the-uk-rare-diseases-framework (accessed 17 January 2021).
- Rare Disease UK. Rare Disease Care Coordination: Delivering Value, Improving Services 2013. www.raredisease.org.uk/media/1639/rduk-care-coordinator-report.pdf (accessed 17 January 2021).
- Van Groenendael S, Giacovazzi L, Davison F, Holtkemper O, Huang Z, Wang Q, et al. High quality, patient centred and coordinated care for Alstrom syndrome: a model of care for an ultra-rare disease. Orphanet J Rare Dis 2015;10. https://doi.org/10.1186/s13023-015-0366-y.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19-32. https://doi.org/10.1080/1364557032000119616.
- Ryan M, Watson W, Gerard K, Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Healthcare. Dordrecht: Springer; 2008.
- York Health Economics Consortium . Discrete Choice Experiment (DCE) n.d. https://yhec.co.uk/glossary/discrete-choice-experiment-dce (accessed 10 February 2021).
- Walton H, Hudson E, Simpson A, Ramsay AIG, Kai J, Morris S, et al. Defining coordinated care for people with rare conditions: a scoping review. Int J Integr Care 2020;20. https://doi.org/10.5334/ijic.5464.
- Gerolamo AM, Schore J, Brown RS, Schraeder C, Schraeder C, Shelton PS. Comprehensive Care Coordination for Chronically Ill Adults. Chichester: Wiley-Blackwell; 2011.
- Powell Davies G, Harris M, Perkins D, Roland M, Williams A, Larsen K, et al. Coordination of Care Within Primary Health Care and With Other Sectors: A Systematic Review 2006. https://rsph.anu.edu.au/files/final_25_powell_davies_pdf_17464.pdf (accessed 25 October 2021).
- EURORDIS . Juggling Care and Daily Life: The Balancing Act of the Rare Disease Community 2017. www.eurordis.org/publication/juggling-care-and-daily-life-balancing-act-rare-disease-community (accessed 25 October 2021).
- NHS England . Implementation Plan for the UK Strategy for Rare Diseases 2018. www.england.nhs.uk/wp-content/uploads/2018/01/implementation-plan-uk-strategy-for-rare-diseases.pdf (accessed 25 October 2021).
- Rare Disease UK. Understanding Children and Young People’s Experiences 2018. www.raredisease.org.uk/media/3348/understanding-children-and-young-peoples-experiences-website.pdf (accessed 25 October 2021).
- Ferrara L, Morando V, Tozzi V. The transition of patients with rare diseases between providers: the patient journey from the patient perspective. Int J Integr Care 2017;17. https://doi.org/10.5334/ijic.3465.
- Yeung CH, Santesso N, Zeraatkar D, Wang A, Pai M, Sholzberg M, et al. Integrated multidisciplinary care for the management of chronic conditions in adults: an overview of reviews and an example of using indirect evidence to inform clinical practice recommendations in the field of rare diseases. Haemophilia 2016;22:41-50. https://doi.org/10.1111/hae.13010.
- Shojania KG, Bero LA. Taking advantage of the explosion of systematic reviews: an efficient MEDLINE search strategy. Eff Clin Pract 2001;4:157-62.
- Dharssi S, Wong-Rieger D, Harold M, Terry S. Review of 11 national policies for rare diseases in the context of key patient needs. Orphanet J Rare Dis 2017;12. https://doi.org/10.1186/s13023-017-0618-0.
- Higgins JP, Deeks JJ, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Hoboken, NJ: Wiley; 2008.
- Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010;5. https://doi.org/10.1186/1748-5908-5-69.
- Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467-73. https://doi.org/10.7326/M18-0850.
- Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. A Product from the ESRC Methods Programme 2006. www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf (accessed 25 October 2021).
- McDonald KM, Sundaram V, Bravata DM, Lewis R, Lin N, Kraft SA, et al. Closing the Quality Gap: A Critical Analysis of Quality Improvement Strategies (Vol. 7: Care Coordination). Rockville, MD: Agency for Healthcare Research and Quality; 2007.
- Adli M, Bauer M, Rush AJ. Algorithms and collaborative-care systems for depression: are they effective and why? A systematic review. Biol Psychiatry 2006;59:1029-38. https://doi.org/10.1016/j.biopsych.2006.05.010.
- Allen J, Hutchinson AM, Brown R, Livingston PM. Quality care outcomes following transitional care interventions for older people from hospital to home: a systematic review. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-346.
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD006525.pub2.
- Barr N, Vania D, Randall G, Mulvale G. Impact of information and communication technology on interprofessional collaboration for chronic disease management: a systematic review. J Health Serv Res Policy 2017;22:250-7. https://doi.org/10.1177/1355819617714292.
- Bearne LM, Byrne AM, Segrave H, White CM. Multidisciplinary team care for people with rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol Int 2016;36:311-24. https://doi.org/10.1007/s00296-015-3380-4.
- Bettger JP, Alexander KP, Dolor RJ, Olson DM, Kendrick AS, Wing L, et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med 2012;157:407-16. https://doi.org/10.7326/0003-4819-157-6-201209180-00004.
- Betz CL, O’Kane LS, Nehring WM, Lobo ML. Systematic review: health care transition practice service models. Nurs Outlook 2016;64:229-43. https://doi.org/10.1016/j.outlook.2015.12.011.
- Bhawra J, Toulany A, Cohen E, Moore Hepburn C, Guttmann A. Primary care interventions to improve transition of youth with chronic health conditions from paediatric to adult healthcare: a systematic review. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011871.
- Binks JA, Barden WS, Burke TA, Young NL. What do we really know about the transition to adult-centered health care? A focus on cerebral palsy and spina bifida. Arch Phys Med Rehabil 2007;88:1064-73. https://doi.org/10.1016/j.apmr.2007.04.018.
- Boland MR, Tsiachristas A, Kruis AL, Chavannes NH, Rutten-van Mölken MP. The health economic impact of disease management programs for COPD: a systematic literature review and meta-analysis. BMC Pulm Med 2013;13. https://doi.org/10.1186/1471-2466-13-40.
- Bongaerts BW, Müssig K, Wens J, Lang C, Schwarz P, Roden M, et al. Effectiveness of chronic care models for the management of type 2 diabetes mellitus in Europe: a systematic review and meta-analysis. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-013076.
- Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care. Making sense of a complex intervention: systematic review and meta-regression. Br J Psychiatry 2006;189:484-93. https://doi.org/10.1192/bjp.bp.106.023655.
- Breland JY, Mignogna J, Kiefer L, Marsh L. Models for treating depression in specialty medical settings: a narrative review. Gen Hosp Psychiatry 2015;37:315-22. https://doi.org/10.1016/j.genhosppsych.2015.04.010.
- Brooks AJ, Smith PJ, Cohen R, Collins P, Douds A, Forbes V, et al. UK guideline on transition of adolescent and young persons with chronic digestive diseases from paediatric to adult care. Gut 2017;66:988-1000. https://doi.org/10.1136/gutjnl-2016-313000.
- Burke L, Kirkham J, Arnott J, Gray V, Peak M, Beresford MW. The transition of adolescents with juvenile idiopathic arthritis or epilepsy from paediatric health-care services to adult health-care services: a scoping review of the literature and a synthesis of the evidence. J Child Health Care 2018;22:332-58. https://doi.org/10.1177/1367493517753330.
- Busetto L, Luijkx KG, Elissen AM, Vrijhoef HJ. Intervention types and outcomes of integrated care for diabetes mellitus type 2: a systematic review. J Eval Clin Pract 2016;22:299-310. https://doi.org/10.1111/jep.12478.
- Butler M, Kane RL, McAlpine D, Kathol R, Fu SS, Hagedorn H, et al. Does integrated care improve treatment for depression? A systematic review. J Ambul Care Manage 2011;34:113-25. https://doi.org/10.1097/JAC.0b013e31820ef605.
- Cairo SB, Gasior A, Rollins MD, Rothstein DH. Delivery of Surgical Care Committee of the American Academy of Pediatrics Section on Surgery . Challenges in transition of care for patients with anorectal malformations: a systematic review and recommendations for comprehensive care. Dis Colon Rectum 2018;61:390-9. https://doi.org/10.1097/DCR.0000000000001033.
- Campbell F, Biggs K, Aldiss SK, O’Neill PM, Clowes M, McDonagh J, et al. Transition of care for adolescents from paediatric services to adult health services. Cochrane Database Syst Rev 2016;4. https://doi.org/10.1002/14651858.CD009794.pub2.
- Cape J, Whittington C, Bower P. What is the role of consultation-liaison psychiatry in the management of depression in primary care? A systematic review and meta-analysis. Gen Hosp Psychiatry 2010;32:246-54. https://doi.org/10.1016/j.genhosppsych.2010.02.003.
- Chhabra PT, Rattinger GB, Dutcher SK, Hare ME, Parsons KL, Zuckerman IH. Medication reconciliation during the transition to and from long-term care settings: a systematic review. Res Social Adm Pharm 2012;8:60-75. https://doi.org/10.1016/j.sapharm.2010.12.002.
- Chu PY, Maslow GR, von Isenburg M, Chung RJ. Systematic review of the impact of transition interventions for adolescents with chronic illness on transfer from pediatric to adult healthcare. J Pediatr Nurs 2015;30:e19-27. https://doi.org/10.1016/j.pedn.2015.05.022.
- Chuah FLH, Haldane VE, Cervero-Liceras F, Ong SE, Sigfrid LA, Murphy G, et al. Interventions and approaches to integrating HIV and mental health services: a systematic review. Health Policy Plan 2017;32:iv27-iv47. https://doi.org/10.1093/heapol/czw169.
- Coelho A, Leone C, Ribeiro V, Sá Moreira P, Dussault G. Integrated disease management: a critical review of foreign and Portuguese experience. Acta Med Port 2014;27:116-25. https://doi.org/10.20344/amp.4758.
- Coffey A, Mulcahy H, Savage E, Fitzgerald S, Bradley C, Benefield L, et al. Transitional care interventions: relevance for nursing in the community. Public Health Nurs 2017;34:454-60. https://doi.org/10.1111/phn.12324.
- Collet J, de Vugt ME, Verhey FR, Schols JM. Efficacy of integrated interventions combining psychiatric care and nursing home care for nursing home residents: a review of the literature. Int J Geriatr Psychiatry 2010;25:3-13. https://doi.org/10.1002/gps.2307.
- Coventry PA, Hudson JL, Kontopantelis E, Archer J, Richards DA, Gilbody S, et al. Characteristics of effective collaborative care for treatment of depression: a systematic review and meta-regression of 74 randomised controlled trials. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0108114.
- Coyne B, Hallowell SC, Thompson M. Measurable outcomes after transfer from pediatric to adult providers in youth with chronic illness. J Adolesc Health 2017;60:3-16. https://doi.org/10.1016/j.jadohealth.2016.07.006.
- Craven MA, Bland R. Better practices in collaborative mental health care: an analysis of the evidence base. Can J Psychiatry 2006;51:7-72.
- Cronin J, Murphy A, Savage E. Can chronic disease be managed through integrated care cost-effectively? Evidence from a systematic review. Ir J Med Sci 2017;186:827-34. https://doi.org/10.1007/s11845-017-1600-5.
- Crowley R, Wolfe I, Lock K, McKee M. Improving the transition between paediatric and adult healthcare: a systematic review. Arch Dis Child 2011;96:548-53. https://doi.org/10.1136/adc.2010.202473.
- Cucciare MA, Coleman EA, Timko C. A conceptual model to facilitate transitions from primary care to specialty substance use disorder care: a review of the literature. Prim Health Care Res Dev 2015;16:492-505. https://doi.org/10.1017/S1463423614000164.
- Dallimore DJ, Neukirchinger B, Noyes J. Why is transition between child and adult services a dangerous time for young people with chronic kidney disease? A mixed-method systematic review. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0201098.
- Damery S, Flanagan S, Combes G. Does integrated care reduce hospital activity for patients with chronic diseases? An umbrella review of systematic reviews. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011952.
- Powell Davies G, Williams AM, Larsen K, Perkins D, Roland M, Harris MF. Coordinating primary health care: an analysis of the outcomes of a systematic review. Med J Aust 2008;188:S65-8. https://doi.org/10.5694/j.1326-5377.2008.tb01748.x.
- de Bruin SR, Baan CA, Struijs JN. Pay-for-performance in disease management: a systematic review of the literature. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-272.
- de Bruin SR, Heijink R, Lemmens LC, Struijs JN, Baan CA. Impact of disease management programs on healthcare expenditures for patients with diabetes, depression, heart failure or chronic obstructive pulmonary disease: a systematic review of the literature. Health Policy 2011;101:105-21. https://doi.org/10.1016/j.healthpol.2011.03.006.
- de Bruin SR, Versnel N, Lemmens LC, Molema CC, Schellevis FG, Nijpels G, et al. Comprehensive care programs for patients with multiple chronic conditions: a systematic literature review. Health Policy 2012;107:108-45. https://doi.org/10.1016/j.healthpol.2012.06.006.
- Desmedt M, Vertriest S, Hellings J, Bergs J, Dessers E, Vankrunkelsven P, et al. Economic impact of integrated care models for patients with chronic diseases: a systematic review. Value Health 2016;19:892-90. https://doi.org/10.1016/j.jval.2016.05.001.
- Doug M, Adi Y, Williams J, Paul M, Kelly D, Petchey R, et al. Transition to adult services for children and young people with palliative care needs: a systematic review. Arch Dis Child 2011;96:78-84. https://doi.org/10.1136/adc.2009.163931.
- Drewes HW, Steuten LM, Lemmens LC, Baan CA, Boshuizen HC, Elissen AM, et al. The effectiveness of chronic care management for heart failure: meta-regression analyses to explain the heterogeneity in outcomes. Health Serv Res 2012;47:1926-59. https://doi.org/10.1111/j.1475-6773.2012.01396.x.
- Ehrlich C, Kendall E, Muenchberger H, Armstrong K. Coordinated care: what does that really mean?. Health Soc Care Community 2009;17:619-27. https://doi.org/10.1111/j.1365-2524.2009.00863.x.
- Ekers D, Murphy R, Archer J, Ebenezer C, Kemp D, Gilbody S. Nurse-delivered collaborative care for depression and long-term physical conditions: a systematic review and meta-analysis. J Affect Disord 2013;149:14-22. https://doi.org/10.1016/j.jad.2013.02.032.
- Elissen AM, Steuten LM, Lemmens LC, Drewes HW, Lemmens KM, Meeuwissen JA, et al. Meta-analysis of the effectiveness of chronic care management for diabetes: investigating heterogeneity in outcomes. J Eval Clin Pract 2013;19:753-62. https://doi.org/10.1111/j.1365-2753.2012.01817.x.
- Farooq S. Collaborative care for depression: a literature review and a model for implementation in developing countries. Int Health 2013;5:24-8. https://doi.org/10.1093/inthealth/ihs015.
- Feltner C, Jones CD, Cené CW, Zheng ZJ, Sueta CA, Coker-Schwimmer EJ, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med 2014;160:774-84. https://doi.org/10.7326/M14-0083.
- Flanagan S, Damery S, Combes G. The effectiveness of integrated care interventions in improving patient quality of life (QoL) for patients with chronic conditions. An overview of the systematic review evidence. Health Qual Life Outcomes 2017;15. https://doi.org/10.1186/s12955-017-0765-y.
- Franx G, Dixon L, Wensing M, Pincus H. Implementation strategies for collaborative primary care-mental health models. Curr Opin Psychiatry 2013;26:502-10. https://doi.org/10.1097/YCO.0b013e328363a69f.
- Fraser MW, Lombardi BM, Wu SY, Zerden LD, Richman EL, Fraher EP. Integrated primary care and social work: a systematic review. J Soc Social Work and Res 2018;9:175-21. https://doi.org/10.1086/697567.
- Fuller JD, Perkins D, Parker S, Holdsworth L, Kelly B, Roberts R, et al. Effectiveness of service linkages in primary mental health care: a narrative review part 1. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-72.
- Gallagher C, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan R, et al. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart 2017;103:1947-53. https://doi.org/10.1136/heartjnl-2016-310952.
- Garralda E, Hasselaar J, Carrasco JM, Van Beek K, Siouta N, Csikos A, et al. Integrated palliative care in the Spanish context: a systematic review of the literature. BMC Palliat Care 2016;15. https://doi.org/10.1186/s12904-016-0120-9.
- Gensichen J, Beyer M, Muth C, Gerlach FM, Von Korff M, Ormel J. Case management to improve major depression in primary health care: a systematic review. Psychol Med 2006;36:7-14. https://doi.org/10.1017/S0033291705005568.
- Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006;166:2314-21. https://doi.org/10.1001/archinte.166.21.2314.
- Goeman D, Renehan E, Koch S. What is the effectiveness of the support worker role for people with dementia and their carers? A systematic review. BMC Health Serv Res 2016;16. https://doi.org/10.1186/s12913-016-1531-2.
- Göhler A, Januzzi JL, Worrell SS, Osterziel KJ, Gazelle GS, Dietz R, et al. A systematic meta-analysis of the efficacy and heterogeneity of disease management programs in congestive heart failure. J Card Fail 2006;12:554-67. https://doi.org/10.1016/j.cardfail.2006.03.003.
- Haldane V, Legido-Quigley H, Chuah FLH, Sigfrid L, Murphy G, Ong SE, et al. Integrating cardiovascular diseases, hypertension, and diabetes with HIV services: a systematic review. AIDS Care 2018;30:103-15. https://doi.org/10.1080/09540121.2017.1344350.
- Hayes SL, Mann MK, Morgan FM, Kelly MJ, Weightman AL. Collaboration between local health and local government agencies for health improvement. Cochrane Database Syst Rev 2012;10. https://doi.org/10.1002/14651858.CD007825.pub6.
- Health Quality Ontario . Electronic tools for health information exchange: an evidence-based analysis. Ont Health Technol Assess Ser 2013;13:1-76.
- Health Quality Ontario . Continuity of care to optimize chronic disease management in the community setting: an evidence-based analysis. Ont Health Technol Assess Ser 2013;13:1-41.
- Heath G, Farre A, Shaw K. Parenting a child with chronic illness as they transition into adulthood: a systematic review and thematic synthesis of parents’ experiences. Patient Educ Couns 2017;100:76-92. https://doi.org/10.1016/j.pec.2016.08.011.
- Hoeft TJ, Fortney JC, Patel V, Unutzer J. Task-sharing approaches to improve mental health care in rural and other low-resource settings: a systematic review. J Rural Health 2018;34:48-62. https://doi.org/10.1111/jrh.12229.
- Homer CJ, Klatka K, Romm D, Kuhlthau K, Bloom S, Newacheck P, et al. A review of the evidence for the medical home for children with special health care needs. Pediatrics 2008;122:e922-37. https://doi.org/10.1542/peds.2007-3762.
- Hopman P, de Bruin SR, Forjaz MJ, Rodriguez-Blazquez C, Tonnara G, Lemmens LC, et al. Effectiveness of comprehensive care programs for patients with multiple chronic conditions or frailty: a systematic literature review. Health Policy 2016;120:818-32. https://doi.org/10.1016/j.healthpol.2016.04.002.
- Huang Y, Wei X, Wu T, Chen R, Guo A. Collaborative care for patients with depression and diabetes mellitus: a systematic review and meta-analysis. BMC Psychiatry 2013;13. https://doi.org/10.1186/1471-244X-13-260.
- Huffman JC, Adams CN, Celano CM. Collaborative care and related interventions in patients with heart disease: an update and new directions. Psychosomatics 2018;59:1-18. https://doi.org/10.1016/j.psym.2017.09.003.
- Hussain M, Seitz D. Integrated models of care for medical inpatients with psychiatric disorders: a systematic review. Psychosomatics 2014;55:315-25. https://doi.org/10.1016/j.psym.2013.08.003.
- Jackson GL, Powers BJ, Chatterjee R, Bettger JP, Kemper AR, Hasselblad V, et al. The patient centered medical home. A systematic review. Ann Intern Med 2013;158:169-78. https://doi.org/10.7326/0003-4819-158-3-201302050-00579.
- Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RW, Guzman J, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database Syst Rev 2014;9. https://doi.org/10.1002/14651858.CD000963.pub3.
- Ke Y, Ng T, Chan A. Survivorship care models for breast cancer, colorectal cancer, and adolescent and young adult (AYA) cancer survivors: a systematic review. Support Care Cancer 2018;26:2125-41. https://doi.org/10.1007/s00520-018-4197-y.
- Kerr H, Price J, Nicholl H, O’Halloran P. Transition from children’s to adult services for young adults with life-limiting conditions: a realist review of the literature. Int J Nurs Stud 2017;76:1-27. https://doi.org/10.1016/j.ijnurstu.2017.06.013.
- Khan F, Ng L, Amatya B, Brand C, Turner-Stokes L. Multidisciplinary care for Guillain–Barré syndrome. Cochrane Database Syst Rev 2010;10. https://doi.org/10.1002/14651858.CD008505.pub2.
- Kooij L, Groen WG, van Harten WH. The effectiveness of information technology-supported shared care for patients with chronic disease: a systematic review. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.7405.
- Kruis AL, Smidt N, Assendelft WJ, Gussekloo J, Boland MR, Rutten-van Mölken M, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2013;10. https://doi.org/10.1002/14651858.CD009437.pub2.
- Krumholz HM, Currie PM, Riegel B, Phillips CO, Peterson ED, Smith R, et al. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation 2006;114:1432-45. https://doi.org/10.1161/CIRCULATIONAHA.106.177322.
- Le Berre M, Maimon G, Sourial N, Guériton M, Vedel I. Impact of transitional care services for chronically ill older patients: a systematic evidence review. J Am Geriatr Soc 2017;65:1597-608. https://doi.org/10.1111/jgs.14828.
- Le Roux E, Mellerio H, Guilmin-Crépon S, Gottot S, Jacquin P, Boulkedid R, et al. Methodology used in comparative studies assessing programmes of transition from paediatrics to adult care programmes: a systematic review. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-012338.
- Lemmens KM, Nieboer AP, Huijsman R. A systematic review of integrated use of disease-management interventions in asthma and COPD. Respir Med 2009;103:670-91. https://doi.org/10.1016/j.rmed.2008.11.017.
- Lemmens KM, Lemmens LC, Boom JH, Drewes HW, Meeuwissen JA, Steuten LM, et al. Chronic care management for patients with COPD: a critical review of available evidence. J Eval Clin Pract 2013;19:734-52. https://doi.org/10.1111/j.1365-2753.2011.01805.x.
- Lemmens LC, Molema CC, Versnel N, Baan CA, de Bruin SR. Integrated care programs for patients with psychological comorbidity: a systematic review and meta-analysis. J Psychosom Res 2015;79:580-94. https://doi.org/10.1016/j.jpsychores.2015.07.013.
- Lewis ME, Myhra LL. Integrated care with indigenous populations: a systematic review of the literature. Am Indian Alsk Native Ment Health Res 2017;24:88-110. https://doi.org/10.5820/aian.2403.2017.88.
- Lim LL, Lau ESH, Kong APS, Davies MJ, Levitt NS, Eliasson B, et al. Aspects of multicomponent integrated care promote sustained improvement in surrogate clinical outcomes: a systematic review and meta-analysis. Diabetes Care 2018;41:1312-20. https://doi.org/10.2337/dc17-2010.
- Lion KC, Mangione-Smith R, Britto MT. Individualized plans of care to improve outcomes among children and adults with chronic illness: a systematic review. Care Manag J 2014;15:11-25. https://doi.org/10.1891/1521-0987.15.1.11.
- Lupari M, Coates V, Adamson G, Crealey GE. ‘We’re just not getting it right’ – how should we provide care to the older person with multi-morbid chronic conditions?. J Clin Nurs 2011;20:1225-35. https://doi.org/10.1111/j.1365-2702.2010.03620.x.
- MacInnes J, Williams L. A review of integrated heart failure care. Prim Health Care Res Dev 2018;20. https://doi.org/10.1017/S1463423618000312.
- Mackie S, Darvill A. Factors enabling implementation of integrated health and social care: a systematic review. Br J Community Nurs 2016;21:82-7. https://doi.org/10.12968/bjcn.2016.21.2.82.
- Manderson B, McMurray J, Piraino E, Stolee P. Navigation roles support chronically ill older adults through healthcare transitions: a systematic review of the literature. Health Soc Care Community 2012;20:113-27. https://doi.org/10.1111/j.1365-2524.2011.01032.x.
- Martínez-González NA, Berchtold P, Ullman K, Busato A, Egger M. Integrated care programmes for adults with chronic conditions: a meta-review. Int J Qual Health Care 2014;26:561-70. https://doi.org/10.1093/intqhc/mzu071.
- McBrien KA, Ivers N, Barnieh L, Bailey JJ, Lorenzetti DL, Nicholas D, et al. Patient navigators for people with chronic disease: a systematic review. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0191980.
- McCallum S, Mikocka-Walus A, Turnbull D, Andrews JM. Continuity of care in dual diagnosis treatment: definitions, applications, and implications. J Dual Diagn 2015;11:217-32. https://doi.org/10.1080/15504263.2015.1104930.
- McColl MA, Shortt S, Godwin M, Smith K, Rowe K, O’Brien P, et al. Models for integrating rehabilitation and primary care: a scoping study. Arch Phys Med Rehabil 2009;90:1523-31. https://doi.org/10.1016/j.apmr.2009.03.017.
- McIntosh D, Startsman LF, Perraud S. Mini review of integrated care and implications for advanced practice nurse role. Open Nurs J 2016;10:78-89. https://doi.org/10.2174/187443460160101078.
- Medical Advisory Secretariat . Community-based care for the specialized management of heart failure: an evidence-based analysis. Ont Health Technol Assess Ser 2009;9:1-42. www.ncbi.nlm.nih.gov/pmc/articles/PMC3377506/pdf/ohtas-09-42a.pdf.
- Miller CJ, Grogan-Kaylor A, Perron BE, Kilbourne AM, Woltmann E, Bauer MS. Collaborative chronic care models for mental health conditions: cumulative meta-analysis and metaregression to guide future research and implementation. Med Care 2013;51:922-30. https://doi.org/10.1097/MLR.0b013e3182a3e4c4.
- Mitchell GK, Brown RM, Erikssen L, Tieman JJ. Multidisciplinary care planning in the primary care management of completed stroke: a systematic review. BMC Fam Pract 2008;9. https://doi.org/10.1186/1471-2296-9-44.
- Mitchell GK, Burridge L, Zhang J, Donald M, Scott IA, Dart J, et al. Systematic review of integrated models of health care delivered at the primary-secondary interface: how effective is it and what determines effectiveness?. Aust J Prim Health 2015;21:391-408. https://doi.org/10.1071/PY14172.
- Muntingh AD, van der Feltz-Cornelis CM, van Marwijk HW, Spinhoven P, van Balkom AJ. Collaborative care for anxiety disorders in primary care: a systematic review and meta-analysis. BMC Fam Pract 2016;17. https://doi.org/10.1186/s12875-016-0466-3.
- Ngune I, Jiwa M, McManus A, Hughes J. Do patients with long-term side effects of cancer treatment benefit from general practitioner support? A literature review. Int J Integr Care 2015;15. https://doi.org/10.5334/ijic.1987.
- Nicoll R, Robertson L, Gemmell E, Sharma P, Black C, Marks A. Models of care for chronic kidney disease: a systematic review. Nephrology 2018;23:389-96. https://doi.org/10.1111/nep.13198.
- Niesink A, Trappenburg JC, de Weert-van Oene GH, Lammers JW, Verheij TJ, Schrijvers AJ. Systematic review of the effects of chronic disease management on quality-of-life in people with chronic obstructive pulmonary disease. Respir Med 2007;101:2233-9. https://doi.org/10.1016/j.rmed.2007.07.017.
- Ouwens M, Hulscher M, Hermens R, Faber M, Marres H, Wollersheim H, et al. Implementation of integrated care for patients with cancer: a systematic review of interventions and effects. Int J Qual Health Care 2009;21:137-44. https://doi.org/10.1093/intqhc/mzn061.
- Parker S, Fuller J. Are nurses well placed as care coordinators in primary care and what is needed to develop their role: a rapid review?. Health Soc Care Community 2016;24:113-22. https://doi.org/10.1111/hsc.12194.
- Peterson K, Helfand M, Humphrey L, Christensen V, Carson S. VA Evidence-Based Synthesis Program Reports. Evidence Brief: Effectiveness of Intensive Primary Care Programs 2011.
- Peytremann-Bridevaux I, Staeger P, Bridevaux PO, Ghali WA, Burnand B. Effectiveness of chronic obstructive pulmonary disease-management programs: systematic review and meta-analysis. Am J Med 2008;121:433-43.e4. https://doi.org/10.1016/j.amjmed.2008.02.009.
- Peytremann-Bridevaux I, Arditi C, Gex G, Bridevaux PO, Burnand B. Chronic disease management programmes for adults with asthma. Cochrane Database Syst Rev 2015;5. https://doi.org/10.1002/14651858.CD007988.pub2.
- Pilotto A, Cella A, Pilotto A, Daragjati J, Veronese N, Musacchio C, et al. Three decades of comprehensive geriatric assessment: evidence coming from different healthcare settings and specific clinical conditions. J Am Med Dir Assoc 2017;18:192.e1-192.e11. https://doi.org/10.1016/j.jamda.2016.11.004.
- Pimouguet C, Le Goff M, Thiébaut R, Dartigues JF, Helmer C. Effectiveness of disease-management programs for improving diabetes care: a meta-analysis. CMAJ 2011;183:e115-27. https://doi.org/10.1503/cmaj.091786.
- Powell Davies G, Harris M, Perkins D, Roland M, Williams A, Larsen K, et al. Coordination of Care Within Primary Health Care and with Other Sectors: A Systematic Review. Sydney, NSW: University of New South Wales; 2017.
- Prior M, McManus M, White P, Davidson L. Measuring the ‘triple aim’ in transition care: a systematic review. Pediatrics 2014;134:e1648-61. https://doi.org/10.1542/peds.2014-1704.
- Pugh JD, McCoy K, Williams AM, Bentley B, Monterosso L. Rapid evidence assessment of approaches to community neurological nursing care for people with neurological conditions post-discharge from acute care hospital. Health Soc Care Community 2018;16. https://doi.org/10.1111/hsc.12576.
- Ranaghan C, Boyle K, Meehan M, Moustapha S, Fraser P, Concert C. Effectiveness of a patient navigator on patient satisfaction in adult patients in an ambulatory care setting: a systematic review. JBI Database System Rev Implement Rep 2016;14:172-218. https://doi.org/10.11124/JBISRIR-2016-003049.
- Rochester-Eyeguokan CD, Pincus KJ, Patel RS, Reitz SJ. The current landscape of transitions of care practice models: a scoping review. Pharmacotherapy 2016;36:117-33. https://doi.org/10.1002/phar.1685.
- Rodrigues CR, Harrington AR, Murdock N, Holmes JT, Borzadek EZ, Calabro K, et al. Effect of pharmacy-supported transition-of-care interventions on 30-day readmissions: a systematic review and meta-analysis. Ann Pharmacother 2017;51:866-89. https://doi.org/10.1177/1060028017712725.
- Santomassino M, Costantini GD, McDermott M, Primiano D, Slyer JT, Singleton JK. A systematic review on the effectiveness of continuity of care and its role in patient satisfaction and decreased hospital readmissions in the adult patient receiving home care services. JBI Libr Syst Rev 2012;10:1214-59. https://doi.org/10.11124/jbisrir-2012-56.
- Savic M, Best D, Manning V, Lubman DI. Strategies to facilitate integrated care for people with alcohol and other drug problems: a systematic review. Subst Abuse Treat Prev Policy 2017;12. https://doi.org/10.1186/s13011-017-0104-7.
- Schultz AT, Smaldone A. Components of interventions that improve transitions to adult care for adolescents with type 1 diabetes. J Adolesc Health 2017;60:133-46. https://doi.org/10.1016/j.jadohealth.2016.10.002.
- Sendall M, McCosker L, Crossley K, Bonner A. A structured review of chronic care model components supporting transition between healthcare service delivery types for older people with multiple chronic diseases. Health Inf Manag 2017;46:58-6. https://doi.org/10.1177/1833358316681687.
- Shah B, Forsythe L, Murray C. Effectiveness of interprofessional care teams on reducing hospital readmissions in patients with heart failure: a systematic review. Medsurg Nurs 2018;27:177-85.
- Sigfrid L, Murphy G, Haldane V, Chuah FLH, Ong SE, Cervero-Liceras F, et al. Integrating cervical cancer with HIV healthcare services: a systematic review. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0181156.
- Siouta N, Van Beek K, van der Eerden ME, Preston N, Hasselaar JG, Hughes S, et al. Integrated palliative care in Europe: a qualitative systematic literature review of empirically-tested models in cancer and chronic disease. BMC Palliat Care 2016;15. https://doi.org/10.1186/s12904-016-0130-7.
- Siouta N, van Beek K, Preston N, Hasselaar J, Hughes S, Payne S, et al. Towards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways. BMC Palliat Care 2016;15. https://doi.org/10.1186/s12904-016-0089-4.
- Smith SM, Allwright S, O’Dowd T. Effectiveness of shared care across the interface between primary and specialty care in chronic disease management. Cochrane Database Syst Rev 2007;3. https://doi.org/10.1002/14651858.CD004910.pub2.
- Smith SM, Allwright S, O’Dowd T. Does sharing care across the primary-specialty interface improve outcomes in chronic disease? A systematic review. Am J Manag Care 2008;14:213-24.
- Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Managing patients with multimorbidity: systematic review of interventions in primary care and community settings. BMJ 2012;345. https://doi.org/10.1136/bmj.e5205.
- Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev 2012;4. https://doi.org/10.1002/14651858.CD006560.pub2.
- Smith SM, Cousins G, Clyne B, Allwright S, O’Dowd T. Shared care across the interface between primary and specialty care in management of long term conditions. Cochrane Database Syst Rev 2017;2. https://doi.org/10.1002/14651858.CD004910.pub3.
- Somme D, Trouve H, Dramé M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimers Dement 2012;8:426-36. https://doi.org/10.1016/j.jalz.2011.06.004.
- Strand H, Parker D. Effects of multidisciplinary models of care for adult pre-dialysis patients with chronic kidney disease: a systematic review. Int J Evid Based Healthc 2012;10:53-9. https://doi.org/10.1111/j.1744-1609.2012.00253.x.
- Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systematic review and meta-analysis. Int J Geriatr Psychiatry 2013;28:889-902. https://doi.org/10.1002/gps.3906.
- Thomas R, Huntley A, Mann M, Huws D, Paranjothy S, Elwyn G, et al. Specialist clinics for reducing emergency admissions in patients with heart failure: a systematic review and meta-analysis of randomised controlled trials. Heart 2013;99:233-9. https://doi.org/10.1136/heartjnl-2012-302313.
- Thota AB, Sipe TA, Byard GJ, Zometa CS, Hahn RA, McKnight-Eily LR, et al. Collaborative care to improve the management of depressive disorders: a community guide systematic review and meta-analysis. Am J Prev Med 2012;42:525-38. https://doi.org/10.1016/j.amepre.2012.01.019.
- Tricco AC, Antony J, Ivers NM, Ashoor HM, Khan PA, Blondal E, et al. Effectiveness of quality improvement strategies for coordination of care to reduce use of health care services: a systematic review and meta-analysis. CMAJ 2014;186:E568-78. https://doi.org/10.1503/cmaj.140289.
- Tummers JF, Schrijvers AJ, Visser-Meily JM. Economic evidence on integrated care for stroke patients; a systematic review. Int J Integr Care 2012;12. https://doi.org/10.5334/ijic.847.
- Turk E, Zaletel J, Ormstad SS, Micetic-Turk D, Isola A. Is a multi-disciplinary approach in the delivery of care for patients with diabetes mellitus type 2 cost effective? A systematic review. Healthmed 2012;6:711-19.
- Valentijn PP, Pereira FA, Ruospo M, Palmer SC, Hegbrant J, Sterner CW, et al. Person-centered integrated care for chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2018;13:375-86. https://doi.org/10.2215/CJN.09960917.
- van der Klauw D, Molema H, Grooten L, Vrijhoef H. Identification of mechanisms enabling integrated care for patients with chronic diseases: a literature review. Int J Integr Care 2014;14. https://doi.org/10.5334/ijic.1127.
- van Dongen JJ, van Bokhoven MA, Daniëls R, van der Weijden T, Emonts WW, Beurskens A. Developing interprofessional care plans in chronic care: a scoping review. BMC Fam Pract 2016;17. https://doi.org/10.1186/s12875-016-0535-7.
- van Servellen G, Fongwa M, Mockus D’Errico E. Continuity of care and quality care outcomes for people experiencing chronic conditions: a literature review. Nurs Health Sci 2006;8:185-95. https://doi.org/10.1111/j.1442-2018.2006.00278.x.
- Vanasse A, Courteau M, Ethier JF. The ‘6W’ multidimensional model of care trajectories for patients with chronic ambulatory care sensitive conditions and hospital readmissions. Public Health 2018;157:53-61. https://doi.org/10.1016/j.puhe.2018.01.007.
- Vedel I, Khanassov V. Transitional care for patients with congestive heart failure: a systematic review and meta-analysis. Ann Fam Med 2015;13:562-71. https://doi.org/10.1370/afm.1844.
- Viggiano T, Pincus HA, Crystal S. Care transition interventions in mental health. Curr Opin Psychiatry 2012;25:551-8. https://doi.org/10.1097/YCO.0b013e328358df75.
- Wagner A, Brucker SY, Ueding E, Gröber-Grätz D, Simoes E, Rall K, et al. Treatment management during the adolescent transition period of girls and young women with Mayer–Rokitansky–Küster–Hauser syndrome (MRKHS): a systematic literature review. Orphanet J Rare Dis 2016;11. https://doi.org/10.1186/s13023-016-0536-6.
- Watson R, Parr JR, Joyce C, May C, Le Couteur AS. Models of transitional care for young people with complex health needs: a scoping review. Child Care Health Dev 2011;37:780-91. https://doi.org/10.1111/j.1365-2214.2011.01293.x.
- Watson LC, Amick HR, Gaynes BN, Brownley KA, Thaker S, Viswanathan M, et al. Practice-based interventions addressing concomitant depression and chronic medical conditions in the primary care setting: a systematic review and meta-analysis. J Prim Care Community Health 2013;4:294-306. https://doi.org/10.1177/2150131913484040.
- Watt N, Sigfrid L, Legido-Quigley H, Hogarth S, Maimaris W, Otero-García L, et al. Health systems facilitators and barriers to the integration of HIV and chronic disease services: a systematic review. Health Policy Plan 2017;32:iv13-iv26. https://doi.org/10.1093/heapol/czw149.
- Wood E, Ohlsen S, Ricketts T. What are the barriers and facilitators to implementing Collaborative Care for depression? A systematic review. J Affect Disord 2017;214:26-43. https://doi.org/10.1016/j.jad.2017.02.028.
- Xyrichis A, Lowton K. What fosters or prevents interprofessional teamworking in primary and community care? A literature review. Int J Nurs Stud 2008;45:140-53. https://doi.org/10.1016/j.ijnurstu.2007.01.015.
- Yang F, Xiong ZF, Yang C, Li L, Qiao G, Wang Y, et al. Continuity of care to prevent readmissions for patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. COPD 2017;14:251-61. https://doi.org/10.1080/15412555.2016.1256384.
- Yiu KC, Rohwer A, Young T. Integration of care for hypertension and diabetes: a scoping review assessing the evidence from systematic reviews and evaluating reporting. BMC Health Serv Res 2018;18. https://doi.org/10.1186/s12913-018-3290-8.
- Zhu QM, Liu J, Hu HY, Wang S. Effectiveness of nurse-led early discharge planning programmes for hospital inpatients with chronic disease or rehabilitation needs: a systematic review and meta-analysis. J Clin Nurs 2015;24:2993-3005. https://doi.org/10.1111/jocn.12895.
- Zlateva I, Anderson D, Coman E, Khatri K, Tian T, Fifield J. Development and validation of the medical home care coordination survey for assessing care coordination in the primary care setting from the patient and provider perspectives. BMC Health Serv Res 2015;15. https://doi.org/10.1186/s12913-015-0893-1.
- Zwar N, Harris M, Griffiths R, Roland M, Dennis S, Powell Davies G, et al. A Systematic Review of Chronic Disease Management 2006. https://rsph.anu.edu.au/files/final_25_zwar_pdf_85791.pdf (accessed 28 January 2022).
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
- McDonald KM, Schultz E, Albin L, Pineda N, Lonhart J, Sundaram V, et al. Care Co-Ordination Measures Atlas Update 2014. www.ahrq.gov/ncepcr/care/co-ordination/atlas.html (accessed 25 October 2021).
- Simpson A, Bloom L, Fulop NJ, Hudson E, Leeson-Beevers K, Morris S, et al. How are patients with rare diseases and their carers in the UK impacted by the way care is coordinated? An exploratory qualitative interview study. Orphanet J Rare Dis 2021;16. https://doi.org/10.1186/s13023-020-01664-6.
- Schlander M, Holm S, Nord E, Richardson J, Garattini S, Kolominsky-Rabas P, et al. 8th European Conference on Rare Diseases & Orphan Products (ECRD 2016). Orphanet J Rare Dis 2016;11:1-9. https://doi.org/10.1186/s13023-016-0515-y.
- von der Lippe C, Diesen PS, Feragen KB. Living with a rare disorder: a systematic review of the qualitative literature. Mol Genet Genomic Med 2017;5:758-73. https://doi.org/10.1002/mgg3.315.
- Pelentsov LJ, Laws TA, Esterman AJ. The supportive care needs of parents caring for a child with a rare disease: a scoping review. Disabil Health J 2015;8:475-91. https://doi.org/10.1016/j.dhjo.2015.03.009.
- Neale J. Iterative categorization (IC): a systematic technique for analysing qualitative data. Addiction 2016;111:1096-106. https://doi.org/10.1111/add.13314.
- Kole A, Faurisson F. Rare diseases social epidemiology: analysis of inequalities. Adv Exp Med Biol 2010;686:223-50. https://doi.org/10.1007/978-90-481-9485-8_14.
- Rare Disease UK. Living With a Rare Disease: The Effect on Mental Health 2018. www.raredisease.org.uk/wp-content/uploads/sites/7/2018/07/living-with-a-rare-condition-the-effect-on-mental-health-pdf.pdf (accessed 25 October 2021).
- Currie G, Szabo J. ‘It is like a jungle gym, and everything is under construction’: the parent’s perspective of caring for a child with a rare disease. Child Care Health Dev 2019;45:96-103. https://doi.org/10.1111/cch.12628.
- Rare Disease UK . Rare Disease UK n.d. www.raredisease.org.uk/ (accessed 9 October 2020).
- Genetic Alliance UK . Genetic Alliance n.d. www.geneticalliance.org.uk/ (accessed 9 October 2020).
- Syndromes Without A Name . SWAN UK n.d. www.undiagnosed.org.uk/ (accessed 9 October 2020).
- British Society of Genetic Medicine . British Society of Genetic Medicine n.d. www.bsgm.org.uk/ (accessed 9 October 2020).
- NIHR Clinical Research Network . Genomics and Rare Diseases n.d. www.nihr.ac.uk/explore-nihr/specialties/genomics-and-rare-diseases.htm (accessed 28 January 2022).
- GDPR.EU . Complete Guide to GDPR Compliance n.d. https://gdpr.eu (accessed 7 October 2021).
- Jasper R, Chester H, Hughes J, Abendstern M, Loynes N, Sutcliffe C, et al. Practitioners preferences of care coordination for older people: a discrete choice experiment. J Gerontol Soc Work 2018;61:151-70. https://doi.org/10.1080/01634372.2017.1417342.
- Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health – a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health 2011;14:403-13. https://doi.org/10.1016/j.jval.2010.11.013.
- Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices taskforce. Value Health 2016;19:300-15. https://doi.org/10.1016/j.jval.2016.04.004.
- Walton H, Simpson A, Ramsay A, Hudson E, Hunter A, Jones J, et al. Developing a taxonomy of care coordination for people living with rare conditions: a qualitative study. MedRxiv 2021. https://doi.org/10.1101/2021.11.16.21266387.
- Walton H, Simpson A, Ramsay AI, Hunter A, Jones J, Ng PL, et al. Development of models of care coordination for rare conditions: a qualitative study. Orphanet J Rare Dis 2022;17:1-24. https://doi.org/10.1186/s13023-022-02190-3.
- Bailey KD. Typologies and Taxonomies. Thousand Oaks, CA: SAGE Publications Ltd; 2011.
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res 2007;42:1758-72. https://doi.org/10.1111/j.1475-6773.2006.00684.x.
- Tran VT, Barnes C, Montori VM, Falissard B, Ravaud P. Taxonomy of the burden of treatment: a multi-country web-based qualitative study of patients with chronic conditions. BMC Med 2015;13. https://doi.org/10.1186/s12916-015-0356-x.
- Bogardus ST, Bradley EH, Tinetti ME. A taxonomy for goal setting in the care of persons with dementia. J Gen Intern Med 1998;13:675-80. https://doi.org/10.1046/j.1525-1497.1998.00203.x.
- Bradley EH, Holmboe ES, Mattera JA, Roumanis SA, Radford MJ, Krumholz HM. A qualitative study of increasing β-blocker use after myocardial infarction: why do some hospitals succeed?. JAMA 2001;285:2604-11. https://doi.org/10.1001/jama.285.20.2604.
- Ferris R, Blaum C, Kiwak E, Austin J, Esterson J, Harkless G, et al. Perspectives of patients, clinicians, and health system leaders on changes needed to improve the health care and outcomes of older adults with multiple chronic conditions. J Aging Health 2018;30:778-99. https://doi.org/10.1177/0898264317691166.
- May CR, Eton DT, Boehmer K, Gallacher K, Hunt K, MacDonald S, et al. Rethinking the patient: using burden of treatment theory to understand the changing dynamics of illness. BMC Health Serv Res 2014;14. https://doi.org/10.1186/1472-6963-14-281.
- Kreuger RA, Casey MA. Designing and Conducting Focus Group Interviews 2002. www.eiu.edu/ihec/Krueger-FocusGroupInterviews.pdf (accessed 25 October 2021).
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Nickerson RC, Varshney U, Muntermann J. A method for taxonomy development and its application in information systems. Eur J Inf Syst 2013;22:336-59. https://doi.org/10.1057/ejis.2012.26.
- Valentijn PP, Boesveld IC, van der Klauw DM, Ruwaard D, Struijs JN, Molema JJ, et al. Towards a taxonomy for integrated care: a mixed-methods study. Int J Integr Care 2015;15. https://doi.org/10.5334/ijic.1513.
- Elrod JK, Fortenberry JL. The hub-and-spoke organization design: an avenue for serving patients well. BMC Health Serv Res 2017;17:25-33. https://doi.org/10.1186/s12913-017-2341-x.
- Morris S, Ramsay AIG, Boaden RJ, Hunter RM, McKevitt C, Paley L, et al. Impact and sustainability of centralising acute stroke services in English metropolitan areas: retrospective analysis of hospital episode statistics and stroke national audit data. BMJ 2019;364. https://doi.org/10.1136/bmj.l1.
- Bond M, Bowling A, Abery A, McClay M, Dickinson E. Evaluation of outreach clinics held by specialists in general practice in England. J Epidemiol Community Health 2000;54:149-56. https://doi.org/10.1136/jech.54.2.149.
- Badawy SM, Radovic A. Digital approaches to remote pediatric health care delivery during the COVID-19 pandemic: existing evidence and a call for further research. JMIR Pediatr Parent 2020;3. https://doi.org/10.2196/20049.
- Shaw J, Agarwal P, Desveaux L, Palma DC, Stamenova V, Jamieson T, et al. Beyond ‘implementation’: digital health innovation and service design. NPJ Digit Med 2018;1. https://doi.org/10.1038/s41746-018-0059-8.
- Ovreteit J. Does Clinical Coordination Improve Quality and Save Money? 2011. www.health.org.uk/publications/does-clinical-coordination-improve-quality-and-save-money (accessed 25 October 2021).
- University of York Centre for Reviews and Dissemination . CRD Database n.d. www.crd.york.ac.uk/CRDWeb/HomePage.asp (accessed 14 February 2021).
- NHS . National Cost Collection for the NHS n.d. www.england.nhs.uk/national-cost-collection/ (accessed 14 February 2021).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2019. Canterbury: PSSRU, University of Kent; 2019.
- Beecham J, Sloper P, Greco V, Webb R. The costs of key worker support for disabled children and their families. Child Care Health Dev 2007;33:611-18. https://doi.org/10.1111/j.1365-2214.2007.00740.x.
Appendix 1 Search terms used in scoping review
| Database | Search number | Search |
|---|---|---|
| MEDLINE | 1 | (Care OR Service).ab,ti. |
| 2 | (Co-ordination OR Coordinat* OR Co-ordinat* OR Coordination OR Collaborat* OR Collaborative OR Integrat* OR Integrated OR Shared OR Synchronised OR Synchronis* or Synchroniz* OR Synchronized OR Interdisciplinary OR Interdisciplin* OR Transitional OR Transition* OR cooperat* OR co-operat*).ab,ti. | |
| 3 | 1 AND 2 | |
| 4 | (Co-ordination of care OR Coordination of care OR Co-ordinat* of care OR Coordinat* of care OR Care co-ordination OR Care coordination OR Care co-ordinator OR Care coordinator OR Care coordinat* OR Care co-ordinat* OR Coordinated care OR Co-ordinated care OR Co-ordinated treatment OR Coordinated treatment OR Coordinating care OR Co-ordinating care OR Coordinat* care OR Co-ordinat* care OR Co-ordinat* treatment OR Coordinat* treatment OR Named coordinator OR Named coordinat* OR Named co-ordinator OR Named co-ordinat* OR Care advisor OR Patient navigator OR Care navigator OR Care organisation OR Care organisat* OR Care organization OR Care organizat* OR Care management OR Care manage* OR Case management OR Case manage* OR Disease management OR Disease manage* OR Condition management OR Condition manage* OR Organisation of patient care activities OR Organization of patient care activities OR Interprofessional network OR Interdisciplinary partnerships OR Integrated care OR Integrated care systems OR Co-management OR Co management OR Patient care planning OR Progressive patient care OR Multidisciplinary teams OR Multidisciplin* teams OR Multidisciplinary treatment OR Multidisciplin* treatment OR Multidisciplinary care OR Multidisciplin* care OR Collaboration OR Teamwork OR Model of care OR Continuity of care OR Continuity of patient care OR Care transitions OR Transition between care providers OR Participatory care OR Cross border cooperation OR Coordination across boundaries OR Co-ordination across boundaries OR Care pathway OR Care pathways OR Models of Care OR Care models OR Centres of excellence OR Specialist services OR Specialised services OR Speciali* services OR Specialist care OR Specialised care OR Speciali* care OR shared care OR transition* care OR transition of care OR transition* services OR transition* OR transfer of care OR patient care team OR patient transfer OR transition to adult care).ab,ti. | |
| 5 | 3 OR 4 | |
| 6 | (Co-ordination OR Coordination).ab,ti. | |
| 7 | (Component* OR Element* OR Activit* OR Feature* OR characteristic*).ab,ti. | |
| 8 | 6 AND 7 | |
| 9 | 5 OR 8 | |
| 10 | Condition* OR Disease* OR Disorder* OR illness* OR syndrome* | |
| 11 | Chronic OR Complex Chronic OR Long-term OR Long term OR Co-morbid* OR Co morbid OR comorbid OR Multi-morbid* OR multimorbid or multi morbid* OR Rare OR Very rare OR Ultra rare OR Ultra-rare OR Genetic OR undiagnosed OR undiagnosed genetic OR unknown OR unknown genetic OR without a name OR orphan | |
| 12 | 10 AND 11 | |
| 13 | 9 AND 12 | |
| 14 | Health OR Healthcare OR Health care OR Health-care | |
| 15 | Delivery OR Delivery, Integrated OR Integrated OR Delivery of OR Service* | |
| 16 | Delivery of healthcare, Integrated | |
| 17/18 | (14 AND 15) OR 16 | |
| 19 | Intervention OR Evaluation | |
| 20 | 19 AND 18 | |
| 21 | 13 AND 20 | |
| 22 | ((meta-analysis.pt or meta-analysis.tw or metanalysis.tw or ((review.pt or guideline.pt or consensus.ti or guideline*.ti or literature.ti or overview.ti or review.ti) and ((Cochrane.tw or Medline.tw or CINAHL.tw or (National.tw and Library.tw) or (handsearch*.tw or search*.tw or searching.tw)) and (hand.tw or manual.tw or electronic.tw or bibliographi*.tw or database* or (Cochrane.tw or Medline.tw or CINAHL.tw or (National.tw and Library.tw))))) or ((synthesis.ti or overview.ti or review.ti or survey.ti) and (systematic.ti or critical.ti or methodologic.ti or quantitative.ti or qualitative.ti or literature.ti or evidence.ti or evidence-based.ti))) not (case*.ti or report.ti or editorial.pt or comment.pt or letter.pt)) | |
| 23 | 21 and 22 | |
| 24 | 23+ filters > 2006 | |
| Scopus | (((((TITLE-ABS (care OR service) AND TITLE-ABS (co-ordination OR coordinat* OR co-ordinat* OR coordination OR collaborat* OR collaborative OR integrat* OR integrated OR shared OR synchronised OR synchronis* OR synchroniz* OR synchronized OR interdisciplinary OR interdisciplin* OR transitional OR transition* OR cooperat* OR co-operat*)) OR TITLE-ABS (“co-ordination of care” OR “coordination of care”OR “co-ordinat* of care”OR “coordinat* of care” OR “care co-ordination” OR “care coordination” OR “care co-ordinator”OR “care coordinator” OR “care coordinat*”OR “care co-ordinat*” OR “coordinated care”OR “co-ordinated care” OR “co-ordinated treatment”OR “coordinated treatment” OR “coordinating care” OR “co-ordinating care” OR “coordinat* care” OR “co-ordinat* care” OR “co-ordinat* treatment” OR “coordinat* treatment” OR “named coordinator” OR “named coordinat*”OR “named co-ordinator” OR “named coordinat*”OR “care advisor”OR “patient navigator”OR “care navigator” OR “care organisation” OR “care organisat*” OR “care organization” OR “care organizat*” OR “care management”OR “care manage*”OR “case management” OR “case manage*” OR “disease management” OR “disease manage*” OR “condition management” OR “condition manage*” OR “organisation of patient care activities” OR “organization of patient care activities” OR “interprofessional network” OR “interdisciplinary partnerships” OR “integrated care” OR “integrated care systems” OR co-management OR “co management” OR “patient care planning” OR “progressive patient care” OR “multidisciplinary teams” OR “multidisciplin* teams” OR “multidisciplinary treatment” OR “multidisciplin* treatment” OR “multidisciplinary care” OR “multidisciplin* care” OR collaboration OR teamwork OR “model of care” OR “continuity of care” OR “continuity of patient care” OR “care transitions” OR “transition between care providers” OR “participatory care” OR “cross border cooperation” OR “coordination across boundaries” OR “co-ordination across boundaries” OR “care pathway”OR “care pathways” OR “models of care” OR “care models” OR “centres of excellence” OR “specialist services” OR “specialised services” OR “speciali* services” OR “specialist care” OR “specialised care” OR “speciali* care” OR “shared care” OR “transition* care” OR “transition of care” OR “transition* services” OR transition* OR “transfer of care” OR “patient care team”OR “patient transfer” OR “transition to adult care”)) OR (TITLE-ABS (co-ordination OR coordination) AND TITLE-ABS (component* OR element* OR activit* OR feature* OR characteristic*))) AND ((condition* OR disease* OR disorder* OR illness* OR syndrome*) AND (chronic OR complex AND chronic OR long-term OR long AND term OR co-morbid* OR “co morbid” OR comorbid OR multi-morbid* OR multimorbid OR “multi morbid*” OR rare OR “very rare”OR “ultra rare”OR ultra-rare OR genetic OR undiagnosed OR “undiagnosed genetic”OR unknown OR “unknown genetic”OR “without a name”OR orphan))) AND ((((health OR healthcare OR “health care” OR health-care) AND (delivery OR “delivery, integrated” OR integrated OR “delivery of service*”)) OR (“delivery of healthcare, integrated”)) AND (intervention OR evaluation))) AND ((meta-analysis OR meta-analysis OR meta analysis OR ((review OR guideline OR consensus OR guideline* OR literature OR overview OR review) AND ((cochrane OR medline OR cinahl OR (national AND library) OR (handsearch* OR search* OR searching)) AND (hand OR manual OR electronic OR bibliographi* OR database* OR (cochrane OR medline OR cinahl OR (national AND library))))) OR ((synthesis OR overview OR review OR survey) AND (systematic OR critical OR methodologic OR quantitative OR qualitative OR literature OR evidence OR evidence-based))) AND NOT (case* OR report OR editorial OR comment OR letter)) | |
| Limits: English, human, 2006-2018 and review | ||
| CINAHL Plus | 1 | TI ( (Care OR Service) ) OR AB ( (Care OR Service) ) |
| 2 | TI ( Co-ordination OR Coordinat* OR Co-ordinat* OR Coordination OR Collaborat* OR Collaborative OR Integrat* OR Integrated OR Shared OR Synchronised OR Synchronis* or Synchroniz* OR Synchronized OR Interdisciplinary OR Interdisciplin* OR Transitional OR Transition* OR cooperat* OR co-operat* ) OR AB ( Co-ordination OR Coordinat* OR Co-ordinat* OR Coordination OR Collaborat* OR Collaborative OR Integrat* OR Integrated OR Shared OR Synchronised OR Synchronis* or Synchroniz* OR Synchronized OR Interdisciplinary OR Interdisciplin* OR Transitional OR Transition* OR cooperat* OR co-operat* ) | |
| 3 | S1 AND S2 | |
| 4 | TI ( “Co-ordination of care” OR “Coordination of care” OR “Co-ordinat* of care” OR “Coordinat* of care” OR “Care co-ordination” OR “Care coordination” OR “Care co-ordinator” OR “Care coordinator” OR “Care coordinat*” OR “Care co-ordinat*” OR “Coordinated care” OR “Co-ordinated care” OR “Co-ordinated treatment” OR “Coordinated treatment” OR “Coordinating care” OR “Co-ordinating care” OR “Coordinat* care” OR “Co-ordinat* care” OR “Co-ordinat* treatment” OR “Coordinat* treatment” OR “Named coordinator” OR “Named coordinat*” OR “Named co-ordinator” OR “Named co-ordinat*” OR “Care advisor” OR “Patient navigator” OR “Care navigator” OR “Care organisation” OR “Care organisat*” OR “Care organization” OR “Care organizat*” OR “Care management” OR “Care manage*” OR “Case management” OR “Case manage*” OR “Disease management” OR “Disease manage*” OR “Condition management” OR “Condition manage*” OR “Organisation of patient care activities” OR “Organization of patient care activities” OR “Interprofessional network” OR “Interdisciplinary partnerships” OR “Integrated care” OR “Integrated care systems” OR Co-management OR “Co management” OR “Patient care planning” OR “Progressive patient care” OR “Multidisciplinary teams” OR “Multidisciplin* teams” OR “Multidisciplinary treatment” OR “Multidisciplin* treatment” OR “Multidisciplinary care” OR “Multidisciplin* care” OR Collaboration OR Teamwork OR “Model of care” OR “Continuity of care” OR “Continuity of patient care” OR “Care transitions” OR “Transition between care providers” OR “Participatory care” OR “Cross border cooperation” OR “Coordination across boundaries” OR “Co-ordination across boundaries” OR “Care pathway” OR “Care pathways” OR “Models of Care” OR “Care models” OR “Centres of excellence” OR “Specialist services” OR “Specialised services” OR “Speciali* services” OR “Specialist care” OR “Specialised care” OR “Speciali* care” OR “shared care” OR “transition* care” OR “transition of care” OR “transition* services” OR transition* OR “transfer of care” OR “patient care team” OR “patient transfer” OR “transition to adult care”) OR AB (“Co-ordination of care” OR “Coordination of care” OR “Co-ordinat* of care” OR “Coordinat* of care” OR “Care co-ordination” OR “Care coordination” OR “Care co-ordinator” OR “Care coordinator” OR “Care coordinat*” OR “Care co-ordinat*” OR “Coordinated care” OR “Co-ordinated care” OR “Co-ordinated treatment” OR “Coordinated treatment” OR “Coordinating care” OR “Co-ordinating care” OR “Coordinat* care” OR “Co-ordinat* care” OR “Co-ordinat* treatment” OR “Coordinat* treatment” OR “Named coordinator” OR “Named coordinat*” OR “Named co-ordinator” OR “Named co-ordinat*” OR “Care advisor” OR “Patient navigator” OR “Care navigator” OR “Care organisation” OR “Care organisat*” OR “Care organization” OR “Care organizat*” OR “Care management” OR “Care manage*” OR “Case management” OR “Case manage*” OR “Disease management” OR “Disease manage*” OR “Condition management” OR “Condition manage*” OR “Organisation of patient care activities” OR “Organization of patient care activities” OR “Interprofessional network” OR “Interdisciplinary partnerships” OR “Integrated care” OR “Integrated care systems” OR Co-management OR “Co management” OR “Patient care planning” OR “Progressive patient care” OR “Multidisciplinary teams” OR “Multidisciplin* teams” OR “Multidisciplinary treatment” OR “Multidisciplin* treatment” OR “Multidisciplinary care” OR “Multidisciplin* care” OR Collaboration OR Teamwork OR “Model of care” OR “Continuity of care” OR “Continuity of patient care” OR “Care transitions” OR “Transition between care providers” OR “Participatory care” OR “Cross border cooperation” OR “Coordination across boundaries” OR “Co-ordination across boundaries” OR “Care pathway” OR “Care pathways” OR “Models of Care” OR “Care models” OR “Centres of excellence” OR “Specialist services” OR “Specialised services” OR “Speciali* services” OR “Specialist care” OR “Specialised care” OR “Speciali* care” OR “shared care” OR “transition* care” OR “transition of care” OR “transition* services” OR transition* OR “transfer of care” OR “patient care team” OR “patient transfer” OR “transition to adult care” ) | |
| 5 | S3 or S4 | |
| 6 | TI (Co-ordination OR Coordination) OR AB (Co-ordination OR Coordination) | |
| 7 | TI(Component* OR Element* OR Activit* OR Feature* OR characteristic*) OR AB (Component* OR Element* OR Activit* OR Feature* OR characteristic*) | |
| 8 | S6 AND S7 | |
| 9 | S5 OR S8 | |
| 10 | Condition* OR Disease* OR Disorder* OR illness* OR syndrome* | |
| 11 | Chronic OR Complex Chronic OR Long-term OR Long term OR Co-morbid* OR “Co morbid” OR comorbid OR Multi-morbid* OR multimorbid or “multi morbid*” OR Rare OR “Very rare” OR “Ultra rare” OR Ultra-rare OR Genetic OR undiagnosed OR “undiagnosed genetic” OR unknown OR “unknown genetic” OR “without a name” OR orphan | |
| 12 | S10 AND S11 | |
| 13 | S9 AND S12 | |
| 14 | Health OR Healthcare OR “Health care” OR Health-care | |
| 15 | Delivery OR “Delivery, Integrated” OR Integrated OR Delivery of OR Service* | |
| 16 | “delivery of health care, integrated” | |
| 17 | S14 AND S15 | |
| 18 | S16 OR S17 | |
| 19 | Intervention OR evaluation | |
| 20 | S18 AND S19 | |
| 21 | S13 AND S20 | |
| 22 | PT meta-analysis OR TX meta-analysis OR TX metanalysis | |
| 23 | ( TX Cochrane OR TX Medline OR TX CINAHL ) OR ( TX National AND TX library ) | |
| 24 | TX handsearch* OR TX search* OR TX searching | |
| 25 | TX hand OR TX manual OR TX electronic OR TX bibliographi* OR database | |
| 26 | S23 OR S25 | |
| 27 | S24 AND S26 | |
| 28 | PT review OR PT guideline OR TI consensus OR TI guideline* OR TI literature OR TI overview OR TI review | |
| 29 | S23 OR S27 | |
| 30 | S28 AND S29 | |
| 31 | ( TI synthesis OR TI overview OR TI review OR TI survey ) AND ( TI systematic OR TI critical OR TI methodologic OR TI quantitative OR TI qualitative OR TI literature OR TI evidence OR TI evidence-based ) | |
| 32 | S22 OR S30 OR S31 | |
| 33 | ( S22 OR S30 OR S31 ) NOT ( TI case* OR TI report OR PT editorial OR PT comment OR PT letter ) | |
| 34 | S21 AND S33 | |
| 35 | **add filters to #34 here*** | |
| Web of Science | 1 | TS=(care "OR" service) |
| 2 | TS=(Co-ordination OR Coordinat* OR Co-ordinat* OR Coordination OR Collaborat* OR Collaborative OR Integrat* OR Integrated OR Shared OR Synchronised OR Synchronis* or Synchroniz* OR Synchronized OR Interdisciplinary OR Interdisciplin* OR Transitional OR Transition* OR cooperat* OR co-operat*) | |
| 3 | #2 AND #1 | |
| 4 | TS = (Co-ordination of care OR Coordination of care OR Co-ordinat* of care OR Coordinat* of care OR Care co-ordination OR Care coordination OR Care co-ordinator OR Care coordinator OR Care coordinat* OR Care co-ordinat* OR Coordinated care OR Co-ordinated care OR Co-ordinated treatment OR Coordinated treatment OR Coordinating care OR Co-ordinating care OR Coordinat* care OR Co-ordinat* care OR Co-ordinat* treatment OR Coordinat* treatment OR Named coordinator OR Named coordinat* OR Named co-ordinator OR Named co-ordinat* OR Care advisor OR Patient navigator OR Care navigator OR Care organisation OR Care organisat* OR Care organization OR Care organizat* OR Care management OR Care manage* OR Case management OR Case manage* OR Disease management OR Disease manage* OR Condition management OR Condition manage* OR Organisation of patient care activities OR Organization of patient care activities OR Interprofessional network OR Interdisciplinary partnerships OR Integrated care OR Integrated care systems OR Co-management OR Co management OR Patient care planning OR Progressive patient care OR Multidisciplinary teams OR Multidisciplin* teams OR Multidisciplinary treatment OR Multidisciplin* treatment OR Multidisciplinary care OR Multidisciplin* care OR Collaboration OR Teamwork OR Model of care OR Continuity of care OR Continuity of patient care OR Care transitions OR Transition between care providers OR Participatory care OR Cross border cooperation OR Coordination across boundaries OR Co-ordination across boundaries OR Care pathway OR Care pathways OR Models of Care OR Care models OR Centres of excellence OR Specialist services OR Specialised services OR Speciali* services OR Specialist care OR Specialised care OR Speciali* care OR shared care OR transition* care OR transition of care OR transition* services OR transition* OR transfer of care OR patient care team OR patient transfer OR transition to adult care) | |
| 5 | #4 OR #3 | |
| 6 | TS=(Co-ordination OR Coordination) | |
| 7 | TS=(Component* OR Element* OR Activit* OR Feature* OR characteristic*) | |
| 8 | #7 AND #6 | |
| 9 | #8 OR #5 | |
| 10 | TS=(Condition* OR Disease* OR Disorder* OR illness* OR syndrome*) | |
| 11 | TS=(Chronic OR Complex Chronic OR Long-term OR Long term OR Co-morbid* OR Co morbid OR comorbid OR Multi-morbid* OR multimorbid or multi morbid* OR Rare OR Very rare OR Ultra rare OR Ultra-rare OR Genetic OR undiagnosed OR undiagnosed genetic OR unknown OR unknown genetic OR without a name OR orphan) | |
| 12 | #11 AND #10 | |
| 13 | #12 AND #9 | |
| 14 | TS=(Health OR Healthcare OR Health care OR Health-care) | |
| 15 | TS=(Delivery OR Delivery, Integrated OR Integrated OR Delivery of OR Service*) | |
| 16 | TS=(Delivery of healthcare, Integrated) | |
| 17 | #14 AND #15 | |
| 18 | #16 OR #17 | |
| 19 | TS=(Intervention OR Evaluation) | |
| 20 | #18 AND #19 | |
| 21 | #20 AND #13 | |
| 22 | TS=(meta-analysis OR meta-analysis OR metanalysis) | |
| 23 | TS=((Cochrane OR Medline OR CINAHL) OR (National AND Library)) | |
| 24 | TS=(handsearch* OR search* OR searching) | |
| 25 | TS=(hand OR manual OR electronic OR bibliographi* OR database*) OR #23 | |
| 26 | #25 AND #24 | |
| 27 | TS=(review OR guideline) | |
| 28 | TI=(consensus OR guideline* OR literature OR overview OR review) | |
| 29 | #28 OR #27 | |
| 30 | #26 OR #23 | |
| 31 | #29 AND #30 | |
| 32 | TI=((synthesis OR overview OR review OR survey) AND (systematic OR critical OR methodologic OR quantitative OR qualitative OR literature OR evidence OR evidence-based)) | |
| 33 | #32 OR #31 OR #22 | |
| 34 | TI=(case* OR report) | |
| 35 | TS=(editorial OR comment OR letter) | |
| 36 | #34 OR #35 | |
| 37 | #33 NOT #36 | |
| 38 | #21 AND #37 | |
| 39 | (#38) ***english and date** | |
| PubMed | 1 (title/abstract) | (Care OR Service) AND (Co-ordination OR Coordinat* OR Co-ordinat* OR Coordination OR Collaborat* OR Collaborative OR Integrat* OR Integrated OR Shared OR Synchronised OR Synchronis* or Synchroniz* OR Synchronized OR Interdisciplinary OR Interdisciplin* OR Transitional OR Transition* OR cooperat* OR co-operat*) |
| 2 (title/abstract) | “Co-ordination of care” OR “Coordination of care” OR “Co-ordinat* of care” OR “Coordinat* of care” OR “Care co-ordination” OR “Care coordination” OR “Care co-ordinator” OR “Care coordinator” OR “Care coordinat*” OR “Care co-ordinat*” OR “Coordinated care” OR “Co-ordinated care” OR “Co-ordinated treatment” OR “Coordinated treatment” OR “Coordinating care” OR “Co-ordinating care” OR “Coordinat* care” OR “Co-ordinat* care” OR “Co-ordinat* treatment” OR “Coordinat* treatment” OR “Named coordinator” OR “Named coordinat*” OR “Named co-ordinator” OR “Named co-ordinat*” OR “Care advisor” OR “Patient navigator” OR “Care navigator” OR “Care organisation” OR “Care organisat*” OR “Care organization” OR “Care organizat*” OR “Care management” OR “Care manage*” OR “Case management” OR “Case manage*” OR “Disease management” OR “Disease manage*” OR “Condition management” OR “Condition manage*” OR “Organisation of patient care activities” OR “Organization of patient care activities” OR “Interprofessional network” OR “Interdisciplinary partnerships” OR “Integrated care” OR “Integrated care systems” OR Co-management OR “Co management” OR “Patient care planning” OR “Progressive patient care” OR “Multidisciplinary teams” OR “Multidisciplin* teams” OR “Multidisciplinary treatment” OR “Multidisciplin* treatment” OR “Multidisciplinary care” OR “Multidisciplin* care” OR Collaboration OR Teamwork OR “Model of care” OR “Continuity of care” OR “Continuity of patient care” OR “Care transitions” OR “Transition between care providers” OR “Participatory care” OR “Cross border cooperation” OR “Coordination across boundaries” OR “Co-ordination across boundaries” OR “Care pathway” OR “Care pathways” OR “Models of Care” OR “Care models” OR “Centres of excellence” OR “Specialist services” OR “Specialised services” OR “Speciali* services” OR “Specialist care” OR “Specialised care” OR “Speciali* care” OR “shared care” OR “transition* care” OR “transition of care” OR “transition* services” OR transition* OR “transfer of care” OR “patient care team” OR “patient transfer” OR “transition to adult care” | |
| 3 (title/abstract) | (Co-ordination OR Coordination) AND (Component* OR Element* OR Activit* OR Feature* OR characteristic*) | |
| 4 | 1 OR 2 OR 3 | |
| 5 | Condition* OR Disease* OR Disorder* OR illness* OR syndrome* | |
| 6 | Chronic OR Complex Chronic OR Long-term OR Long term OR Co-morbid* OR “Co morbid” OR comorbid OR Multi-morbid* OR multimorbid or “multi morbid*” OR Rare OR “Very rare” OR “Ultra rare” OR Ultra-rare OR Genetic OR undiagnosed OR “undiagnosed genetic” OR unknown OR “unknown genetic” OR “without a name” OR orphan | |
| 7 | 5 AND 6 | |
| 8 | 4 AND 7 | |
| 9 | Health OR Healthcare OR “Health care” OR Health-care | |
| 10 | Delivery OR “Delivery, Integrated” OR Integrated OR Delivery of OR Service* | |
| 11 | “Delivery of healthcare, Integrated” | |
| 12/13 | (9 AND 10) OR 11 | |
| 14 | Intervention OR Evaluation | |
| 15 | 13 AND 14 | |
| 16 | 8 AND 15 | |
| 17 | ((meta-analysis [pt] OR meta-analysis [tw] OR metanalysis [tw]) OR ((review [pt] OR guideline [pt] OR consensus [ti] OR guideline* [ti] OR literature [ti] OR overview [ti] OR review [ti]) AND ((Cochrane [tw] OR Medline [tw] OR CINAHL [tw] OR (National [tw] AND Library [tw])) OR (handsearch* [tw] OR search* [tw] OR searching [tw]) AND (hand [tw] OR manual [tw] OR electronic [tw] OR bibliographi* [tw] OR database* OR (Cochrane [tw] OR Medline [tw] OR CINAHL [tw] OR (National [tw] AND Library [tw]))))) OR ((synthesis [ti] OR overview [ti] OR review [ti] OR survey [ti]) AND (systematic [ti] OR critical [ti] OR methodologic [ti] OR quantitative [ti] OR qualitative [ti] OR literature [ti] OR evidence [ti] OR evidence-based [ti]))) BUTNOT (case* [ti] OR report [ti] OR editorial [pt] OR comment [pt] OR letter [pt]) | |
| 18 | 16 AND 17 | |
| 19 | 18 + filters > 2006, peer reviewed, English | |
| Cochrane Database of systematic reviews | 1 | (Care OR Service):ti,ab |
| 2 | (Co-ordination OR Coordinat* OR Co-ordinat* OR Coordination OR Collaborat* OR Collaborative OR Integrat* OR Integrated OR Shared OR Synchronised OR Synchronis* or Synchroniz* OR Synchronized OR Interdisciplinary OR Interdisciplin* OR Transitional OR Transition* OR cooperat* OR co-operat*):ti,ab | |
| 3 | #1 AND #2 | |
| 4 | (“Co-ordination of care” OR “Coordination of care” OR “Co-ordinat* of care” OR “Coordinat* of care” OR “Care co-ordination” OR “Care coordination” OR “Care co-ordinator” OR “Care coordinator” OR “Care coordinat*” OR “Care co-ordinat*” OR “Coordinated care” OR “Co-ordinated care” OR “Co-ordinated treatment” OR “Coordinated treatment” OR “Coordinating care” OR “Co-ordinating care” OR “Coordinat* care” OR “Co-ordinat* care” OR “Co-ordinat* treatment” OR “Coordinat* treatment” OR “Named coordinator” OR “Named coordinat*” OR “Named co-ordinator” OR “Named co-ordinat*” OR “Care advisor” OR “Patient navigator” OR “Care navigator” OR “Care organisation” OR “Care organisat*” OR “Care organization” OR “Care organizat*” OR “Care management” OR “Care manage*” OR “Case management” OR “Case manage*” OR “Disease management” OR “Disease manage*” OR “Condition management” OR “Condition manage*” OR “Organisation of patient care activities” OR “Organization of patient care activities” OR “Interprofessional network” OR “Interdisciplinary partnerships” OR “Integrated care” OR “Integrated care systems” OR Co-management OR “Co management” OR “Patient care planning” OR “Progressive patient care” OR “Multidisciplinary teams” OR “Multidisciplin* teams” OR “Multidisciplinary treatment” OR “Multidisciplin* treatment” OR “Multidisciplinary care” OR “Multidisciplin* care” OR Collaboration OR Teamwork OR “Model of care” OR “Continuity of care” OR “Continuity of patient care” OR “Care transitions” OR “Transition between care providers” OR “Participatory care” OR “Cross border cooperation” OR “Coordination across boundaries” OR “Co-ordination across boundaries” OR “Care pathway” OR “Care pathways” OR “Models of Care” OR “Care models” OR “Centres of excellence” OR “Specialist services” OR “Specialised services” OR “Speciali* services” OR “Specialist care” OR “Specialised care” OR “Speciali* care” OR “shared care” OR “transition* care” OR “transition of care” OR “transition* services” OR transition* OR “transfer of care” OR “patient care team” OR “patient transfer” OR “transition to adult care”):ti,ab | |
| 5 | #3 OR #4 | |
| 6 | (Co-ordination OR Coordination):ti,ab | |
| 7 | (Component* OR Element* OR Activit* OR Feature* OR characteristic*):ti,ab | |
| 8 | #6 AND #7 | |
| 9 | #5 OR #8 | |
| 10 | Condition* OR Disease* OR Disorder* OR illness* OR syndrome* | |
| 11 | Chronic OR Complex Chronic OR Long-term OR Long term OR Co-morbid* OR “Co morbid” OR comorbid OR Multi-morbid* OR multimorbid or “multi morbid*” OR Rare OR “Very rare” OR “Ultra rare” OR Ultra-rare OR Genetic OR undiagnosed OR “undiagnosed genetic” OR unknown OR “unknown genetic” OR “without a name” OR orphan | |
| 12 | #10 AND #11 | |
| 13 | #9 AND #12 | |
| 14 | Health OR Healthcare OR “Health care” OR Health-care | |
| 15 | Delivery OR “Delivery, Integrated” OR Integrated OR Delivery of OR Service* | |
| 16 | “Delivery of healthcare, Integrated” | |
| 17 | #14 AND #15 | |
| 18 | #17 OR #16 | |
| 19 | Intervention or evaluation | |
| 20 | #18 AND #19 | |
| 21 | #13 AND #20 | |
| 22 | (meta-analysis):pt OR (meta-analysis OR metanalysis):ti | |
| 23 | (Cochrane OR Medline OR CINAHL) OR (National AND Library) | |
| 24 | (search* OR searching OR handsearch*) | |
| 25 | (hand OR manual OR electronic OR bibliographi* OR database*) | |
| 26 | #24 AND (#25 OR #23) | |
| 27 | (review OR guideline):pt AND (Consensus OR guideline* OR literature OR overview OR review):ti | |
| 28 | #27 AND (#23 OR #26) | |
| 29 | (synthesis OR overview OR review OR survey):ti AND (Systematic OR critical OR methodological OR quantitative OR qualitative OR literature OR evidence OR evidence-based):ti | |
| 30 | #22 OR #28 OR #29 | |
| 31 | (case* OR report):ti OR (editorial OR comment OR letter):pt | |
| 32 | #30 NOT #31 | |
| 33 | #21 AND #32 | |
| Nursing and Allied Health and Social Sciences (ProQuest) (two databases) | 1 | ti((Care OR Service) AND (Co-ordination OR Coordinat* OR Co-ordinat* OR Coordination OR Collaborat* OR Collaborative OR Integrat* OR Integrated OR Shared OR Synchronised OR Synchronis* or Synchroniz* OR Synchronized OR Interdisciplinary OR Interdisciplin* OR Transitional OR Transition* OR cooperat* OR co-operat*)) OR ab((Care OR Service) AND (Co-ordination OR Coordinat* OR Co-ordinat* OR Coordination OR Collaborat* OR Collaborative OR Integrat* OR Integrated OR Shared OR Synchronised OR Synchronis* or Synchroniz* OR Synchronized OR Interdisciplinary OR Interdisciplin* OR Transitional OR Transition* OR cooperat* OR co-operat*)) |
| 2 | ti(“Co-ordination of care” OR “Coordination of care” OR “Co-ordinat* of care” OR “Coordinat* of care” OR “Care co-ordination” OR “Care coordination” OR “Care co-ordinator” OR “Care coordinator” OR “Care coordinat*” OR “Care co-ordinat*” OR “Coordinated care” OR “Co-ordinated care” OR “Co-ordinated treatment” OR “Coordinated treatment” OR “Coordinating care” OR “Co-ordinating care” OR “Coordinat* care” OR “Co-ordinat* care” OR “Co-ordinat* treatment” OR “Coordinat* treatment” OR “Named coordinator” OR “Named coordinat*” OR “Named co-ordinator” OR “Named co-ordinat*” OR “Care advisor” OR “Patient navigator” OR “Care navigator” OR “Care organisation” OR “Care organisat*” OR “Care organisation” OR “Care organizat*” OR “Care management” OR “Care manage*” OR “Case management” OR “Case manage*” OR “Disease management” OR “Disease manage*” OR “Condition management” OR “Condition manage*” OR “Organisation of patient care activities” OR “Organization of patient care activities” OR “Interprofessional network” OR “Interdisciplinary partnerships” OR “Integrated care” OR “Integrated care systems” OR “Co-management” OR “Co management” OR “Patient care planning” OR “Progressive patient care” OR “Multidisciplinary teams” OR “Multidisciplin* teams” OR “Multidisciplinary treatment” OR “Multidisciplin* treatment” OR “Multidisciplinary care” OR “Multidisciplin* care” OR Collaboration OR Teamwork OR “Model of care” OR “Continuity of care” OR “Continuity of patient care” OR “Care transitions” OR “Transition between care providers” OR “Participatory care” OR “Cross border cooperation” OR “Coordination across boundaries” OR “Co-ordination across boundaries” OR “Care pathway” OR “Care pathways” OR “Models of Care” OR “Care models” OR “Centres of excellence” OR “Specialist services” OR “Specialised services” OR “Speciali* services” OR “Specialist care” OR “Specialised care” OR “Speciali* care” OR “shared care” OR “transition* care” OR “transition of care” OR “transition* services” OR transition* OR “transfer of care” OR “patient care team” OR “patient transfer” OR “transition to adult care”) OR ab(“Co-ordination of care” OR “Coordination of care” OR “Co-ordinat* of care” OR “Coordinat* of care” OR “Care co-ordination” OR “Care coordination” OR “Care co-ordinator” OR “Care coordinator” OR “Care coordinat*” OR “Care co-ordinat*” OR “Coordinated care” OR “Co-ordinated care” OR “Co-ordinated treatment” OR “Coordinated treatment” OR “Coordinating care” OR “Co-ordinating care” OR “Coordinat* care” OR “Co-ordinat* care” OR “Co-ordinat* treatment” OR “Coordinat* treatment” OR “Named coordinator” OR “Named coordinat*” OR “Named co-ordinator” OR “Named co-ordinat*” OR “Care advisor” OR “Patient navigator” OR “Care navigator” OR “Care organisation” OR “Care organisat*” OR “Care organisation” OR “Care organizat*” OR “Care management” OR “Care manage*” OR “Case management” OR “Case manage*” OR “Disease management” OR “Disease manage*” OR “Condition management” OR “Condition manage*” OR “Organisation of patient care activities” OR “Organization of patient care activities” OR “Interprofessional network” OR “Interdisciplinary partnerships” OR “Integrated care” OR “Integrated care systems” OR “Co-management” OR “Co management” OR “Patient care planning” OR “Progressive patient care” OR “Multidisciplinary teams” OR “Multidisciplin* teams” OR “Multidisciplinary treatment” OR “Multidisciplin* treatment” OR “Multidisciplinary care” OR “Multidisciplin* care” OR Collaboration OR Teamwork OR “Model of care” OR “Continuity of care” OR “Continuity of patient care” OR “Care transitions” OR “Transition between care providers” OR “Participatory care” OR “Cross border cooperation” OR “Coordination across boundaries” OR “Co-ordination across boundaries” OR “Care pathway” OR “Care pathways” OR “Models of Care” OR “Care models” OR “Centres of excellence” OR “Specialist services” OR “Specialised services” OR “Speciali* services” OR “Specialist care” OR “Specialised care” OR “Speciali* care” OR “shared care” OR “transition* care” OR “transition of care” OR “transition* services” OR transition* OR “transfer of care” OR “patient care team” OR “patient transfer” OR “transition to adult care”) | |
| 3 | 1 OR 2 | |
| 4 | ti((Co-ordination OR Coordination) AND (Component* OR Element* OR Activit* OR Feature* OR characteristic*)) OR ab((Co-ordination OR Coordination) AND (Component* OR Element* OR Activit* OR Feature* OR characteristic*)) | |
| 5 | 3 OR 4 | |
| 6 | Condition* OR Disease* OR Disorder* OR illness* OR syndrome* | |
| 7 | Chronic OR Complex Chronic OR Long-term OR Long term OR Co-morbid* OR “Co morbid” OR comorbid OR Multi-morbid* OR multimorbid or “multi morbid*” OR Rare OR “Very rare” OR “Ultra rare” OR Ultra-rare OR Genetic OR undiagnosed OR “undiagnosed genetic” OR unknown OR “unknown genetic” OR “without a name” OR orphan | |
| 8 | 5 AND 6 | |
| 9 | 4 AND 9 | |
| 10 | ((Health OR Healthcare OR “Health care” OR Health-care) and (Delivery OR “Delivery, Integrated” OR Integrated OR Delivery of OR Service*)) OR (“Delivery of healthcare, Integrated”) | |
| 11 | Intervention OR Evaluation | |
| 12 | 10 AND 11 | |
| 13 | 9 AND 11 | |
| 14 | ((meta-analysis OR ft(meta-analysis OR metanalysis)) OR (((review OR guideline) OR ti(consensus OR guideline* OR literature OR overview OR review)) AND ((ft(Cochrane OR Medline OR CINAHL) OR ft(National AND Library)) OR (ft(handsearch* OR search* OR searching) AND ((ft(Cochrane OR Medline OR CINAHL) OR ft(National AND Library)) OR ft(hand OR manual OR electronic OR bibliographi* OR database*))))) OR (ti(synthesis OR overview OR review OR survey) AND ti(systematic OR critical OR methodologic OR quantitative OR qualitative OR literature OR evidence OR evidence-based))) NOT (ti(case* OR report) OR (editorial OR comment OR letter)) | |
| 15 | 13 AND 14 | |
| Limits: English, human, 2006-2018 and possibly review |
Appendix 2 Terms and definitions used in scoping review
| Type of co-ordination | Number of reviews (%) | Review references | Example definition |
|---|---|---|---|
| Integrated care models/integrated care | 34 (22.1) | Breland et al.;42 Busetto et al.;45 Butler et al.;46 Chuah et al.;52 Coelho et al.;53 Collet et al.;55 Cronin et al.;59 Damery et al.;63 Desmedt et al.;68 Flanagan et al.;76 Gallagher et al.;80 Haldane et al.;86 Hussain and Seitz;96 Lemmens et al.;109 Lewis and Myhra;110 Lim et al.;111 MacInnes and Williams;114 Mackie and Darvill;115 Martínez-González et al.;117 McColl et al.;120 McIntosh et al.;121 Mitchell et al.;125 Ouwens et al.;130 Savic et al.;144 Sigfrid et al.;148 Siouta et al.;149 Smith et al.;153,154 Siouta et al.;150 Tummers et al.;162 Valentijn et al.;164 van der Klauw et al.;165 Watt et al.;176 Yiu et al.178 | A coherent set of methods and models on the funding, administrative, organisational, service delivery and clinical levels designed to create connectivity, alignment and collaboration within and between the cure and care sectors [Kodner & Spreeuwenberg, 2002]Cronin et al.59 |
| Transition/care transition | 18 (11.7) | Allen et al.;31 Bhawra et al.;37 Bettger et al.;35 Chu et al.;51 Coffey et al.;54 Coyne et al.;57 Cucciare et al.;61 Chhabra et al.;50 Doug et al.;69 Feltner et al.;75 La Berre et al.;105 Manderson et al.;116 Rochester-Eyeguokan et al.;141 Rodrigues et al.;142 Sendall et al.;146 Vanasse et al.;168 Viggiano et al.;170 Vedel and Khanassov169 | The transition period encompasses multiple steps in a process including thoughtful planning, the actual transfer from paediatric to adult care, and adjustment to the new system afterwardsBhawra et al.37 |
| Transition: child to adult | 15 (9.7) | Betz et al.;36 Binks et al.;38 Brooks et al.;43 Burke et al.;44 Cairo et al.;47 Campbell et al.;48 Crowley et al.;60 Dallimore et al.;62 Heath et al.;90 Kerr et al.;100 Le Roux et al.;106 Schultz and Smaldone;145 Prior et al.;138 Wagner et al.;171 Watson et al.172 | Health care transition has been defined as ‘purposeful, planned movement of adolescents and young adults with chronic physical and medical conditions from child-centered to adult-oriented health care systems that is uninterrupted, coordinated, developmentally appropriate, psychosocially sound, and comprehensive’ (Blum et al., 1993, p. 570)Betz et al.36 |
| Collaborative care | 15 (9.7) | Adli et al.;30 Archer et al.;32 Bower et al.;41 Coventry et al.;56 Craven and Bland;58 Farooq;74 Franx et al.;77 Gilbody et al.;83 Huang et al.;94 Hayes et al.;87 Huffman et al.;95 Miller et al.;123 Muntingh et al.;126 Thota et al.;160 Wood et al.175 | Collaborative care involves providers from different specialities, disciplines, or sectors working together to offer complementary services and mutual support, to ensure that individuals receive the most appropriate service from the most appropriate provider in the most suitable location, as quickly as necessary, and with minimal obstaclesCraven and Bland58 |
| Disease management programmes | 14 (9.1) | Boland et al.;39 de Bruin et al.;65,66 Elissen et al.;73 Göhler et al.;85 Kruis et al.;103 Krumholz et al.;104 Lemmens et al.;107 Medical Advisory Secretariat;122 Niesink et al.;129 Peytremann-Bridevaux et al.;133,134 Pimouguet et al.;136 Zwar et al.181 | Disease management programmes are multidisciplinary approaches to care for chronic diseases that co-ordinate comprehensive care strategies along the disease continuum and across healthcare delivery systems |
| Care co-ordination | 9 (5.8) | Powell Davies et al.;64 Ehrlich et al.;71 Ekers et al.;72 McDonald et al.;1 Mitchell et al.;124 Tricco et al.;161 Parker and Fuller;131 Powell Davies et al.;137 Zlateva et al.180 | Care coordination is the deliberate organization of patient care activities between two or more participants (including the patient) involved in a patient’s care to facilitate the appropriate delivery of health care services. Organizing care involves the marshalling of personnel and other resources needed to carry out all required patient care activities, and is often managed by the exchange of information among participants responsible for different aspects of careMcDonald et al.1 |
| Case management/patient navigator | 8 (5.2) | Gensichen et al.;82 Goeman et al.;84 Lupari et al.;113 McBrien et al.;118 Pugh et al.;139 Ranaghan et al.;140 Somme et al.;156 Tam-Tham et al.158 | Case management was defined as a nurse providing targeted care to individual patients which included support/clinical and social support, assessment, planning, implementation and monitoring or organising care provision to prevent and/or minimise exacerbations in the individual’s chronic condition(s) (DH, 2005)Lupari et al.113 |
| Shared care | 7 (4.6) | Kooij et al.;102 Ngune et al.;127 Smith et al.;151,152,155 van Dongen et al.;166 Yiu et al.178 | Shared care is a means to improve integration and is defined as ‘the joint participation of GPs and hospital consultants in the planned delivery of care for patients with a chronic condition, informed by an enhanced information exchange over and above routine discharge and referral letters’ [Hickman, Drummond & Grimshaw, 1994]Kooij et al.102 |
| Continuity of care | 6 (3.9) | Health Quality Ontario;88,89 McCallum et al.;119 Santomassino et al.;143 Van Servellen et al.;167 Yang et al.177 | CoC [continuity of care] as a one-dimensional outcome measure referring to the successful linkage of a patient from one level of care to another and CoC as an overarching construct referring to a multidimensional series of care practices during treatmentMcCallum et al.119 |
| Interprofessional collaboration/interprofessional teams | 5 (3.3) | Barr et al.;33 Fraser et al.;78 Garralda et al.;81 Shah et al.;147 Xyrichis and Lowton176 | These benefits accrue from greater system integration and continuity of patient care by replacing professional silos with a cooperative team approachBarr et al.33 |
| MDT | 4 (2.6) | Bearne et al.;34 Pilotto et al.;135 Strand and Parker;157 Yeung et al.22 | An MDT intervention was defined as a team involving two or more health and social care professionals working in a coordinated wayBearne et al.34 |
| Multidisciplinary care | 3 (2.0) | Bongaerts et al.;40 Khan et al.;101 Turk et al.163 | Multidisciplinary care in GBS [Guillain–Barré syndrome] refers to delivery of co-ordinated care with clearly identified goals within a specified time period, utilising at least two disciplines (medicine, physiotherapy, occupational therapy, dietetics and other allied health professions)Khan et al.101 |
| Comprehensive care programmes | 2 (1.3) | de Bruin et al.;67 Hopman et al.93 | Comprehensive care programs can be defined as those initiatives that proactively seek to structure and coordinate care and improve health outcomes while constraining healthcare expendituresHopman et al.93 |
| Chronic care model | 2 (1.3) | Drewes et al.;70 Lemmens et al.108 | Chronic care model – ‘interventions consisting of > 2 components of the Chronic care model’Lemmens et al.108 |
| Plans of care | 1 (0.7) | Lion et al.112 | Given the lack of a standard definition or terminology around IPCs, we used the AAP [American Academic of Pediatrics] concept of a written ‘plan of care [that] is developed by the physician, child or youth, and family and is shared with other providers, agencies and organizations involved with the care of the patient’ (AAP, 2002)Lion et al.112 |
| Models of care | 1 (0.7) | Nicoll et al.128 | . . . a model of care must have been capable of delivering more than one type of intervention targeted at more than one aspect of disease managementNicoll et al.128 |
| Discharge planning | 1 (0.7) | Zhu et al.179 | Nurse-led early DP [discharge planning] programmes, which consisted of initial nurse visit within 48 hours of hospital admission, predischarge assessment, structured home visits and telephone follow-ups after discharge, are led by a nurse, supported by a multidisciplinary teamZhu et al.179 |
| Interdisciplinary care | 1 (0.7) | Peterson et al.132 | Multi-component, interdisciplinary intensive primary care programsPeterson et al.132 |
| Specialist clinics | 1 (0.7) | Thomas et al.159 | Specialist clinics were defined as units providing access to multidisciplinary teams including specialist heart failure nurses, physicians or cardiologists delivering advanced diagnostic or treatment servicesThomas et al.159 |
| Practice-based interventions | 1 (0.7) | Watson et al.173 | We defined ‘practice-based’ as any intervention that (a) targets the care process within a system of care and (b) aims to improve depression or both depression and chronic medical conditions. Examples of practice based interventions include: coordinated care, integrated care, collaborative careWatson et al.173 |
| Linkage | 1 (0.7) | Fuller et al.79 | A primary mental health care linkage was defined as follows: 1. The linkage is the process used to connect two or more services in the provision of clinical primary mental health care. 2. One part of the linkage must involve a primary health care practitioner such as a GP, community nurse or practice nurse. The other part of the linkage can be any health or human service entity including hospital or community based mental health specialists, private practitioners, or non-health agencies such as housing, education or welfare etc. Linkages must be two-way which excludes a single referral without feedback or continuing relationshipFuller et al.79 |
| Medical home | 1 (0.7) | Homer et al.92 | These data and the experience of families led to the formulation of a model of family-centred, community-based care for CSHCN [children with special health care needs] termed ‘the medical home’ (MH) (American Academy of Pediatrics, 1992; Sia & Jacob, 1992; Sia, Tonnings, Osterhus, & Taba, 2004)Homer et al.92 |
| Consultation liaison | 1 (0.7) | Cape et al.49 | Consultation-liaison was defined as an intervention where patients were seen once or twice by a mental health professional for assessment (consultation) and advice to the GP about management (liaison), but where no treatment was provided by the mental health professionalCape et al.49 |
| Task-sharing | 1 (0.7) | Hoeft et al.91 | We conceptualize task sharing not as a referral to other providers (eg, to an urban mental health specialist without involving a local provider) but instead a sharing of care among rural providers or between rural and urban providersHoeft et al.91 |
| Patient-centred medical home | 1 (0.7) | Jackson et al.97 | 1. Team-based care . . . 2. The intervention includes ≥ 2 of . . . Enhanced access to care . . . Coordinate care . . . Comprehensiveness . . . A systems-based approach to improving quality and safety . . . A sustained partnership and personal relationship over time . . . structural changes to the traditional practiceJackson et al.97 |
| Multidisciplinary biopsychosocial rehabilitation | 1 (0.7) | Kamper et al.98 | MBR [multidisciplinary biopsychosocial rehabilitation] was defined as an intervention that involves a physical component (for example an exercise program) and at least one other element from the biopsychosocial model, that is psychological or social and occupational. The intervention program had to have been delivered by clinicians from different disciplinesKamper et al.98 |
Appendix 3 Themes, subthemes, components and type of components identified from reviews
| Theme | Subtheme (number of reviews, %) | Components (number of reviews, %) | Type of component | ||
|---|---|---|---|---|---|
| What? | How? | Contextual factors | |||
| 1. Care pathway (including analysis and decision-making) | Assessment of patient and carer (100, 64.9) | Physical and mental health status (58, 37.7) | ✗ | ||
| Assessment of personal factors (e.g. challenges, knowledge, patient understanding, preferences, goals, support, barriers, risk, activities of daily living, motivation and self-management) (35, 22.7) | ✗ | a | |||
| Screening (physical and mental health) (31, 20.1) | ✗ | ||||
| Needs (e.g. physical and mental health, neighbourhood) (27, 17.5) | ✗ | ✗ | |||
| Environment (e.g. work, home and social situation) (22, 14.3) | ✗ | ||||
| Medication and medication adherence (22, 14.3) | ✗ | ||||
| Resources (6, 3.9) | ✗ | ||||
| Process and outcome measurements (6, 3.9) | ✗ | ||||
| Discharge assessment (5, 3.3) | ✗ | ||||
| Readiness to engage (4, 2.6) | ✗ | ||||
| Readiness to transfer (4, 2.6) | ✗ | ||||
| Self-assessment (3, 2.0) | ✗ | ||||
| Caregiver well-being, capabilities and support (3, 2.0) | ✗ | ||||
| Diagnostic testing (3, 2.0) | ✗ | ||||
| Behavioural evaluation (1, 0.7) | ✗ | ||||
| Eligibility (1, 0.7) | ✗ | ||||
| Planning (111, 72.1) | Development of care plan/treatment plan, medication plan, maintenance plan, relapse prevention plan, follow-up plan, discharge plan, action plan, self-management plan (95, 61.7) | ✗ | ✗ | ||
| Goal-setting, joint goal-setting and shared decision-making (50, 32.5) | ✗ | ✗ | |||
| Agreeing care with patient/carer (20, 13.0) | ✗ | ✗ | |||
| Planning who is responsible for which aspects of care (18, 11.7) | ✗ | ||||
| Preparation (10, 6.5) | ✗ | ||||
| Condition-specific passport (5, 3.3) | ✗ | ✗ | |||
| Care contract between provider and patient (1, 0.7) | ✗ | ✗ | |||
| Financial planning (1, 0.7) | ✗ | ✗ | |||
| Review and evaluation (95, 61.7) | Monitoring (reviewing progress with care plan, symptoms, progress, performance, treatment, adherence, watchful waiting and outcomes, and telemonitoring) (79, 51.3) | ✗ | ✗ | ||
| Identifying and/or addressing problems/problem-solving/relapse prevention (38, 24.7) | ✗ | ✗ | |||
| Medication review (28, 18.2) | ✗ | ||||
| Self-monitoring of behaviours/outcomes (24, 15.6) | ✗ | ||||
| Amending plan, care or goal to overcome difficulties (21, 13.6) | ✗ | ✗ | |||
| Evaluation (18, 11.7) | ✗ | ✗ | |||
| Clinical review/monitoring (11, 7.1) | ✗ | ||||
| Feedback (48, 31.2) | Feedback for health-care provider (28, 18.2) | ✗ | ✗ | ||
| Feedback (non-specific) (includes benchmarking) (18, 11.7) | ✗ | ||||
| Feedback to patients (9, 5.8) | ✗ | ||||
| Feedback from patients/carers about care (9, 5.8) | ✗ | ✗ | |||
| Feedback from health-care providers (4, 2.6) | ✗ | ✗ | |||
| Management of patient complaints (1, 0.7) | ✗ | ||||
| Biofeedback (1, 0.7) | ✗ | ||||
| Follow-up (91, 59.1) | Follow-up (e.g. conducted over telephone/face to face/web/mailing) (81, 52.6) | ✗ | |||
| Post-discharge follow-up (31, 20.1) | ✗ | ||||
| Follow-up arranged with patient (15, 9.7) | ✗ | ||||
| Structured/systematic follow-up/used register to follow-up (13, 8.4) | ✗ | ||||
| Pharmacotherapy/medication follow-up (2, 1.3) | ✗ | ||||
| No formal follow-up (1, 0.7) | ✗ | ||||
| Follow-up of test results (1, 0.7) | ✗ | ||||
| Administration (62, 40.3) | Reminders (41, 26.6) | ✗ | |||
| Documentation/record-keeping/reports (29, 18.8) | ✗ | ✗ | |||
| Appointment scheduling (24, 15.6) | ✗ | ✗ | |||
| Administrative support (7, 4.6) | ✗ | ✗ | |||
| Client consent (1, 0.7) | ✗ | ||||
| Technological difficulties/safeguarding support (1, 0.7) | ✗ | ||||
| 2. Approaches | Methods of co-ordination (149, 96.8) | Team approach (e.g. multi/interdisciplinary) (118, 76.6) | ✗ | ||
| Co-ordination/collaboration/case management/disease management/integrated approach (109, 70.8) | ✗ | ||||
| Responsibility of co-ordination by one person (e.g. co-ordinator/care managers/care led by one provider or set of providers) (109, 70.8) | ✗ | ||||
| Communication between providers, other providers and/or patients (88, 57.1) | ✗ | ||||
| Using and sharing documentation/information (e.g. patient records/notes/medical summary) (72, 46.8) | ✗ | ||||
| Single visit approach (including colocation, specific clinics and medical homes) (62, 40.3) | ✗ | ||||
| Referral systems (57, 37.0) | ✗ | ||||
| Meetings to bring team together to discuss co-ordination (48, 31.2) | ✗ | ||||
| Transition (41, 26.6) | X | ||||
| Task-sharing/delegation/shared care (29, 18.8) | ✗ | ||||
| Patient/carer co-ordinating own treatment/patient carer involvement/patients as partners in care/patient-held records (26, 16.9) | ✗ | ||||
| Continuity of care (23, 14.9) | ✗ | ||||
| Joint clinics/consultation (23, 14.9) | ✗ | ||||
| Alternating appointments/visits (5, 3.3) | ✗ | ||||
| Professional communities or practices (4, 2.6) | ✗ | ||||
| None/lack of co-ordination (3, 2.0) | ✗ | ||||
| Expert care (97, 63.0) | Specialist care/specialist teams/expert knowledge/condition-specific expertise/specialist referrals/expert review of guidelines (89, 57.8) | ✗ | |||
| Specialist clinics/condition-specific clinics (35, 22.7) | ✗ | ||||
| Patient and family expertise (2, 1.3) | ✗ | ||||
| Lack of expertise (1, 0.7) | ✗ | ||||
| Technology (93, 60.4) | Telecare (including telehealth, home-based monitoring system, use of monitoring equipment or technological aids, telemedicine, electronic prescriptions, telepsychiatry) (patients/carers) (37, 24.0) | ✗ | ✗ | ||
| Algorithms/decision support aids for HCPs (35, 22.7) | ✗ | ||||
| Communication systems/teleconferencing (HCPs) (35, 22.7) | ✗ | ||||
| Electronic medical records/personal health records/continuity of care records (34, 22.1) | ✗ | ||||
| Clinical information systems (HCPs) (e.g. patient tracking) (32, 20.8) | ✗ | ||||
| Reminders/recall and alert system (HCPs) (31, 20.1) | ✗ | ✗ | |||
| Centralised database of patients/registry (HCPs) (28, 18.2) | ✗ | ||||
| Automated performance monitoring (HCPs)/feedback systems (24, 15.6) | ✗ | ||||
| Support/education using technology [e.g. via online platforms (patients/carers)/e-consultations] (24, 15.6) | ✗ | ||||
| Non-specific IT (19, 12.3) | ✗ | ||||
| Use of online platform (HCPs) (e.g. online learning, general IT, websites, IT platforms, shared IT platforms and computerised lab records) (18, 11.7) | ✗ | ✗ | |||
| Electronic appointment reminders (patients/carers) (11, 7.1) | ✗ | ✗ | |||
| Automated summaries and reports and other materials (HCPs) (10, 6.5) | ✗ | ||||
| Delivery of questionnaires (HCPs, patients and carers) (6, 3.9) | ✗ | ||||
| Technological support (4, 2.6) | ✗ | ||||
| Use of other digital tools (e.g. digital camera) (1, 0.7) | ✗ | ||||
| 3. Support | Support provided to patients and carers (144, 93.5) | Education and information for patients/skills training (e.g. understanding condition and management; transition; honest, open and age appropriate; available services; support transition; and communication skills) (113, 73.4) | ✗ | ✗ | |
| General support for patients (including social support, practical support, non-specific support, general support for transition and support from different modalities, e.g. face to face, letter, telephone, e-mail and newsletters) (105, 68.2) | ✗ | ✗ | |||
| Self-management support (84, 54.6) | ✗ | ✗ | |||
| Psychological support (e.g. counselling, CBT, MI, emotional support, behavioural therapy) (77, 50) | ✗ | ||||
| Medical treatment (including support for medication, medical treatment and surgery) (73, 47.4) | ✗ | ||||
| Home visits (62, 40.3) | ✗ | ✗ | |||
| Physical health support (e.g. diet, exercise, smoking cessation and health promotion) (54, 35.1) | ✗ | ||||
| Involvement of social workers/other community personnel (including volunteers) (54, 35.1) | ✗ | ✗ | |||
| Signposting, linking to community resources and community-based referrals and care (51, 33.1) | ✗ | ✗ | |||
| Support for carers, including education and information (e.g. how they can support their child, information about co-ordination and sectors) (40, 26.0) | ✗ | ✗ | |||
| Information about/support for accessing services/resources or using health-care aspects (e.g. records) (25, 16.2) | ✗ | ✗ | |||
| Support from pharmacist (25, 16.2) | ✗ | ✗ | |||
| Peer mentoring/involvement of peers/mentor programmes/peer support/peer educators (24, 15.6) | ✗ | ||||
| Opportunities to become familiar with co-ordinated approach (e.g. visits, tours, lunch and joint visits) (15, 9.7) | ✗ | ||||
| Family support/involvement (11, 7.1) | ✗ | ✗ | |||
| Palliative care support (7, 4.6) | ✗ | ||||
| Support with other aspects of care (e.g. social welfare benefits, legal services, finances and housing) (6, 3.9) | ✗ | ||||
| Consultations out of clinic (3, 2.0) | ✗ | ✗ | |||
| Social networking (2, 1.3) | ✗ | ||||
| Genetic counselling (1, 0.7) | ✗ | ||||
| Support provided to HCPs (85, 55.2) | Education (including summer camps) (50, 32.5) | ✗ | ✗ | ||
| Training (48, 31.2) | ✗ | ✗ | |||
| Supervision/support (47, 30.5) | ✗ | ||||
| Support tools (10, 6.5) | ✗ | ✗ | |||
| Non-specific interventions (6, 3.9) | ✗ | ✗ | |||
| Administrative/technical support (5, 3.3) | ✗ | ✗ | |||
| Behaviour plans (1, 0.7) | ✗ | ||||
| Capacity building (1, 0.7) | ✗ | ✗ | |||
| 4. Features | Individual differences (69, 44.8) | Individualised care/care plans/patient-centred care (51, 33.1) | ✗ | ||
| Taking into account culture, health, demographic factors (e.g. age or gender), readiness to transition, goals and expectations and independence (35, 22.7) | ✗ | ||||
| Flexible (health care/timing) (12, 7.8) | ✗ | ||||
| Evidence-based practice (100, 64.9) | Guideline-based treatment (57, 37.0) | ✗ | |||
| Evidence-based standardised treatment protocols (54, 35.1) | ✗ | ||||
| Standardised/structured care (including use of manual, checklists, competency frameworks and criteria) (30, 19.5) | ✗ | ||||
| Evidence-based pathways/evidence-based care/evidence-based screening (28, 18.2) | ✗ | ||||
| Treatment algorithms (22, 14.3) | ✗ | ||||
| Evidence-based tools (8, 5.2) | ✗ | ||||
| Best practice/previous treatments/recommendations (8, 5.2) | ✗ | ||||
| Policy/policy template (7, 4.6) | ✗ | ||||
| Unstructured/non- guideline-based care (1, 0.7) | ✗ | ||||
| Treatment targets (1, 0.7) | ✗ | ||||
| Other (31, 20.1) | Qualified staff (11, 7.1) | ✗ | |||
| Length of intervention (9, 5.8) | ✗ | ||||
| Amount of contact (3, 2.0) | ✗ | ||||
| Interested/willing providers (3, 2.0) | ✗ | ||||
| Type of co-ordination (2, 1.3) | ✗ | ||||
| Characteristics of staff (2, 1.3) | ✗ | ||||
| 5. Wider environment | Health-care environment (87, 56.5) | Supportive environment for co-ordination (e.g. distance from treatment facilities and access to care) (48, 31.2) | ✗ | ||
| Resources (e.g. expertise, staffing and resources) (33, 21.4) | ✗ | ||||
| Quality improvement/evaluation of services (31, 20.1) | ✗ | ||||
| Financial incentives (including providers, institution and patients) (27, 17.5) | ✗ | ||||
| Organisation of health-care system (including service planning) (26, 16.9) | ✗ | ||||
| Structural changes (25, 16.2) | ✗ | ||||
| Organisational support (including support from organisation and agreements/discussions between organisations and professional networks) (25, 16.2) | ✗ | ||||
| Outreach (17, 11.0) | ✗ | ||||
| Governance (9, 5.8) | ✗ | ✗ | |||
| Identification of barriers to care/assessment of need (8, 5.2) | ✗ | ||||
| Use of existing services (2, 1.3) | ✗ | ||||
| Lack of supportive environment (1, 0.7) | ✗ | ||||
| Wider environment (15, 9.7) | National policy changes (9, 5.8) | ✗ | |||
| Campaigns (5, 3.3) | ✗ | ||||
| Funding collaborations/changes to funding (2, 1.3) | ✗ | ||||
| Geographical coverages and rostering (2, 1.3) | ✗ | ||||
Appendix 4 Example quotations from the stakeholder consultations for ‘what’, ‘how’ and ‘facilitating components’
| Type of component | Subtheme | Example quotation(s) |
|---|---|---|
| Components that need to be co-ordinated during a patients’ care pathway (‘what’) | Support for patients, carers and HCPs | Well, the first thing that [name 2] was diagnosed when he was seven and the day that I got in touch with the [organisation 4] which was a group of parents doing, you know, that was the most important thing, that was the most important day of my life and when I say my life I mean that, not just my son’s lifeFG-PC2Not only for us but for the professionals as well because if they are uneducated or unaware of certain things, they need the support just as much as we do to sort of be able to treat us and allow us to get the care that we needFG-PC1 |
| Elements of care that need to be co-ordinated | I’m thinking of review and evaluation with the therapist and on in-community. So, for people with rare conditions, there’ll be a period of intervention, then all will go quiet, everything’s being managed well, and then some other problem will come up, but the way therapists work is that there’ll be a period of intervention, measured outcome, closed case, no contact kept until a crisis down the road and they come back, and when I was in that position, I always wanted to be able to keep that person under review . . . because you were then managing a situation before it became a crisisFG-HCPYeah definitely for me, definitely for my son diagnosis has changed everything, I feel completely empowered to look after him because all I need to say are the words [condition 1] and then people go away they’ve got Google [URL: www.google.com; Google Inc., Mountain View, CA, USA] they can find . . . Prior to that I felt completely alone with it and like I said before, I felt like a fraud even my own family didn’t really until he got diagnosed really believed that there could possibly be something so randomly wrong. And so I think diagnosis is key and we only [cuts off] the genome 100,000 scheme that’s why he got diagnosed and, you know, we’re eternally grateful for that reallyFG-PC1 | |
| Components that tell us how to co-ordinate care (‘how’) | Co-ordination through taking responsibility | We do need leadership. If this was in a theatre, you know, if you were having an operation the surgeon’s in charge, it’s the same with this, it’s no different really, somebody has got to be in charge, I’ve actually had to tell doctors you are the main man, until you make a decision the other three aren’t going to do anything so I think this thing about co-ordination is very, very importantFG-PC2I think having a consultant who takes the lead has been the best thing for me, that’s been the most helpful because I ring his secretary for everything and he knows that it’s his responsibility and he took responsibility but just for himself, he didn’t do it because he’s being paid to do it he just recognises that we were really sinkingFG-PC1Well, very similar I mean when it comes to co-ordination I think it’s about, for me anyway, I’d like to be in partnership with somebody rather than me doing it all but also because these sorts of illnesses, disorders, whatever, there is very little I can control about them and this might be the only thing I can have some control over but, yeahFG-PC2And I do wonder with rare conditions, whether there ought to be an advocate all the way through. So, somebody in maternity, somebody in special care, somebody on the paediatric ward, somebody in the community nurse team, the health visitor, that there’s just one person, so that that person can link to that person that can link to that person, almostFG-HCP |
| Co-ordination through specialist centres | That’s absolutely got to be gold standard for any of those kinds of conditions. To really have, if you’re talking about your evaluation, your reassessment, your planning and implementation, and getting that real, you know, assurance about that follow-up, you know, to do that annually, do an MDT, I mean . . .FG-HCP. . . he actually does attend a rare disease clinic and there is some attempt at co-ord[ination] – so he’s actually seen three doctors on the same dayFG-PC2 | |
| Co-ordination through technology | P4:And, again, if there is one system, it would be good if everybody does it, you know . . .Interviewer:All of the different NHS trustsP4Yeah. Everyone’s doing their own things at the momentP3:I think the key is that systems talk to each other because it’s so time-consuming and arduous to get the information to the right people in an efficient way. It takes hours on just getting it right, and things are going to get missed, and it isn’t good enough, and I think technology could be something that could be much easier to join up, you would imagineFG-HCP | |
| Co-ordination through communication | [Name 2] has a health passport that it takes into hospital with him but it’s very basic, it’s about his condition and it’s about certain things, he doesn’t like this, he doesn’t like that because he’s no communication but I think everybody really, everybody should have one of those even if you’ve only got a basic condition that when you’re going into hospital you’ve got something that you can say, yes, that’s meFG-PC2. . . you know, you should have access and all those people should speak to each other because it’s an interconnected condition and they don’t, in fact it’s quite hard to find a single person who knowsFG-PC1 | |
| Co-ordination through support | . . . my son has a rare condition that no-one knows anything about and so therefore there’s no support group, there’s no charity group, there’s nothing for my daughter, you know, who watches my son in hospital week after week after week, I’m away for stretches at a time, there’s nothing in place and I think that is part of co-ordinated care because that’s care for my familyFG-PC1. . . so the defining moment really and truly is having somebody that knew about the condition, you know, OK we’ve got a diagnosis but the help that we got and the ability to say we need or we want come from that, and this is why I would suggest to anybody join a support group, go to the association if there is one and if there’s not an association or a support group make one which, you know? That’s my contributionFG-PC2Yeah, and if the school’s been set up, I always find, then the parents truly get some respite whilst the child’s there because they don’t have to worry about them the whole time if they know the care is being, continuing to be co-ordinated there on a safe basis, so I think that’s a really important(?) supportFG-HCPI know, from a family point of view, we do, kind of, family integrated care on our neonatal unit where we get the parents, you know, presenting their babies on ward rounds and being involved with patients’ decisions so that it becomes . . . if they’ve got that experience while they’re in hospital, you know, they get quite confidentFG-HCPI always felt that transition skills to develop were more important because that person was going on to advocate for themselves and manage their own health condition, and would need to be alert and know when to go to an orthopaedic consultant, when to go to a community therapistFG-HCP | |
| Other: team approach | I think it’s quite accurate on the fourth point as a team approach because I think that is it’s not a team approach it’s very much in depending as long as the doctor that’s seeing you maybe that day gets his stuff done he’s not concerned if it impacts or anything else from any of the other doctors, I think there’s not a team approach in that respect whereas I think if they had more of a mentality around a team approach things might work better. And again that just goes back to anything in life, if you have one goal and you all have a common goal then you’re going to have a better chance of hitting it if you’re all going in the same direction than if you were going right, left, north, south, east, west, so that kind of stood out to meFG-PC2 | |
| Other: continuity of care | I think what could be useful is if you have a family history of a rare or chronic condition if you had one specialist who deals with all members of the same family because if you are clones then you are very likely to respond to the same treatment, that has happened in my family and that’s very useful because you have one specialist who knows the lot of you and he then knows that if a treatment suits one of you it should suit all of you and in my case for that particular illness it has been very useful and we’ve all responded well to the treatmentFG-PC1 | |
| Other: planning | Now we’ve always asked for a proper care plan and I’m sure every . . . I mean you were saying earlier on you’ve been there done that . . . If you look at the rare disease strategy a care plan is probably the most common thing that patients ask for and there’s a lot of reasons for that, one you can monitor health over the year, you know what you’re doing and if you’ve got more than one issue like you’ve got central nervous issues or you’ve got liver issues or you’ve got movement issues it’s all co-ordinated and the place you can remember and it restores compliance but the important thing it actually monitors the evolution of the rare disease and you can monitor performance. Now why there is such a resistance to a proper care plan I do not know because I’ve never ever been able to get oneFG-PC2Yes, the only thing I’d say hasn’t changed is a care plan in place [cuts off], I’ve still not received a care plan for him and they’re still . . . we won’t take him to [place 3] hospital anymore, we just will not go it’s too dangerous . . . because there’s nothing on their system and that has to change, it’s ridiculous, it has to change thatFG-PC1 | |
| Components that facilitate co-ordination (‘facilitating’) | Evidence-based practice | . . . it’s, having those clear pathways . . . and having something that people can, you know, work towards which is really clear. . . . Even though it’s very rare, it’s, like, ‘OK, this is the process now’, and that’s really important. . . . And I think that makes the systems work a lot better if there is something like that in place. I think when it’s wishy-washy or it’s not clear, or there is no clear, kind of, guidance or pathways, and because some of the situations are quite, you know, specialised, I think it’s difficult, it’s very difficult to manageFG-HCP |
| Individualised treatment | Everybody is put into the same category, I mean me and [patient 2] both here have [condition 3] but I bet our stories are also very different and I find that I’m put in a box because I’ve got [condition 3] I’m just seen to some doctors as being a little bit bendy and actually there’s a hell of a lot more going on than just that and I find everybody is either put in that tick box scenario and we’re not getting the treatment and care that we deserve and needFG-PC1Every single person in this phone call every single person with a rare condition their experience is different, so they may want to take these identified points, routes, identified points and they may want to either put them in a chain of command, how they want it to happen, they may want to put it as if someone’s running a project on it in a project management style thing or in a hierarchy of importance with the very minimum that we need and the pinnacle being the optimum that we want or in another form of pyramid where it says the top of the hierarchy is the absolute base that we need and going down to what we want, there are various ways of actually using what are really good groups and identified elements but in a different manner | |
| Access to care | And like you said before, when you realise that you have to push for things you just do it and then you start doing it naturally with everything and at the beginning you got emotional about it but after a bit you don’t get emotional and you just go through the motions and you go I know how it works now, you just keep going, I think remove the emotion from it because I found at the start I was picking all different things, you realise it’s a game really of semantics and stuff like thatFG-PC2 | |
| Access to health-care environment | The other thing that I was wondering from a parent point of view is how few specialists there are and they’re all old, my age, so we don’t seem to say, right, we’ve got this . . . and how medical professions develop how do you attract new talent in to actually take over from the older clinicians who are leaving? And that is a concern because we’ve only got four or five in the UK who even you would use to diagnosis and many rare diseases as I sayFG-PC2 |
Appendix 5 STROBE statement for Chapter 5 (checklist of items that should be included in reports of cross-sectional studies)
| Item | Item number | Recommendation |
|---|---|---|
| Title and abstract | 1 |
(a) Indicate the study’s design with a commonly used term in the title or the abstract The title of Chapter 5 is ‘National cross-sectional survey to explore experiences of co-ordinated care for people affected by rare diseases’ |
|
(b) Provide in the abstract an informative and balanced summary of what was done and what was found The Scientific Summary of the report provides a detailed summary of the chapter |
||
| Introduction | ||
| Background/rationale | 2 |
Explain the scientific background and rationale for the investigation being reported See Chapter 5, Background |
| Objectives | 3 |
State specific objectives, including any prespecified hypotheses The aim of this study is presented in Chapter 5, Background |
| Methods | ||
| Study design | 4 |
Present key elements of study design early in the paper |
| Setting | 5 |
Describe the setting, locations and relevant dates, including periods of recruitment, exposure, follow-up and data collection See Chapter 5, Methods and Survey sampling |
| Participants | 6 |
(a) Give the eligibility criteria, and the sources and methods of selection of participants See Chapter 5, Methods and Survey sampling |
| Variables | 7 |
Clearly define all outcomes, exposures, predictors, potential confounders and effect modifiers. Give diagnostic criteria, if applicable See Chapter 5, Methods and Survey instrument |
| Data sources/measurement | 8 |
For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group See Chapter 5, Methods and Survey instrument |
| Bias | 9 |
Describe any efforts to address potential sources of bias See Chapter 5, Methods and Analysis of data |
| Study size | 10 |
Explain how the study size was arrived at See Chapter 5, Methods and Survey sampling |
| Quantitative variables | 11 |
Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why See Chapter 5, Methods and Analysis of data |
| Statistical methods | 12 |
(a) Describe all statistical methods, including those used to control for confounding See Chapter 5, Methods and Analysis of data |
|
(b) Describe any methods used to examine subgroups and interactions See Chapter 5, Methods and Analysis of data |
||
|
(c) Explain how missing data were addressed See Chapter 5, Methods and Analysis of data |
||
|
(d) If applicable, describe analytical methods taking account of sampling strategy See Chapter 5, Methods and Analysis of data |
||
|
(e) Describe any sensitivity analyses See Chapter 5, Methods and Analysis of data |
||
| Results | ||
| Participants | 13 |
(a) Report numbers of individuals at each stage of study, e.g. numbers potentially eligible, examined for eligibility, confirmed eligible, included in the study, completing follow-up and analysed Sample numbers are reported in Chapter 5, Results and Responses and sample |
|
(b) Give reasons for non-participation at each stage In Chapter 5, Results and Responses and sample, we explain why it was not possible to estimate a response rate |
||
|
(c) Consider use of a flow diagram A flow diagram is not needed. The sample numbers are easily explained in the text in Chapter 5, Results and Responses and sample |
||
| Descriptive data | 14 |
(a) Give characteristics of study participants (e.g. demographic, clinical and social) and information on exposures and potential confounders Sample characteristics are reported in Chapter 5, Results and Responses and sample, and in Table 5 |
|
(b) Indicate number of participants with missing data for each variable of interest Missing descriptive data are reported in Table 5 |
||
| Outcome data | 15 |
Report numbers of outcome events or summary measures Outcomes (access to and use of co-ordinated care) are reported in Tables 6–12 |
| Main results | 16 |
(a) Give unadjusted estimates and, if applicable, confounder-adjusted estimates and their precision (e.g. 95% CI). Make clear which confounders were adjusted for and why they were included |
|
(b) Report category boundaries when continuous variables were categorised Not applicable |
||
|
(c) If relevant, consider translating estimates of relative risk into absolute risk for a meaningful time period Not applicable |
||
| Other analyses | 17 |
Report other analyses done (e.g. analyses of subgroups and interactions, and sensitivity analyses) |
| Discussion | ||
| Key results | 18 |
Summarise key results with reference to study objectives See Chapter 5, Discussion and Key findings |
| Limitations | 19 |
Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias See Chapter 5, Discussion and Limitations |
| Interpretation | 20 |
Give a cautious overall interpretation of results, considering objectives, limitations, multiplicity of analyses, results from similar studies and other relevant evidence See Chapter 9, Discussion, for a summary of the main findings of the study, including the survey and a discussion of the implications of these findings |
| Generalisability | 21 |
Discuss the generalisability (external validity) of the study results See Chapter 5, Discussion and Limitations |
| Other information | ||
| Funding | 22 |
Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based |
Appendix 6 Figures showing the predicted probabilities of choosing co-ordinated services
FIGURE 19.
Predicted probabilities of choosing co-ordinated services: low cost for co-ordination and high cost for no co-ordination. (a) Patients and parents/carers combined; and (b) HCPs. No co-ordination: cost to patients and carers of attending all health-care appointments during 1 year is £2000; health records are not shared; the lead consultant is a medical expert in the area of the body primarily affected by the patient’s condition (e.g. neurologist); care is provided without the support of a care co-ordinator; a specialist centre is not available and there is not a documented emergency plan in place. Full co-ordination: cost to patients and carers of attending all health-care appointments during 1 year is £200; electronic health records are immediately accessible to staff; the lead consultant is a medical expert in the patient’s specific condition; the patient/carer decides how they wish to be supported by the care co-ordinator (patients/carers) or care is entirely co-ordinated by a care co-ordinator (HCPs); a specialist centre is available and there is a documented emergency plan in place. All other co-ordination scenarios are as for no co-ordination except for the attribute indicated. Scenarios are ordered from left to right in ascending order of magnitude of the predicted probability of choosing the co-ordination service (note that the ordering is different for patients and carers combined and HCPs).
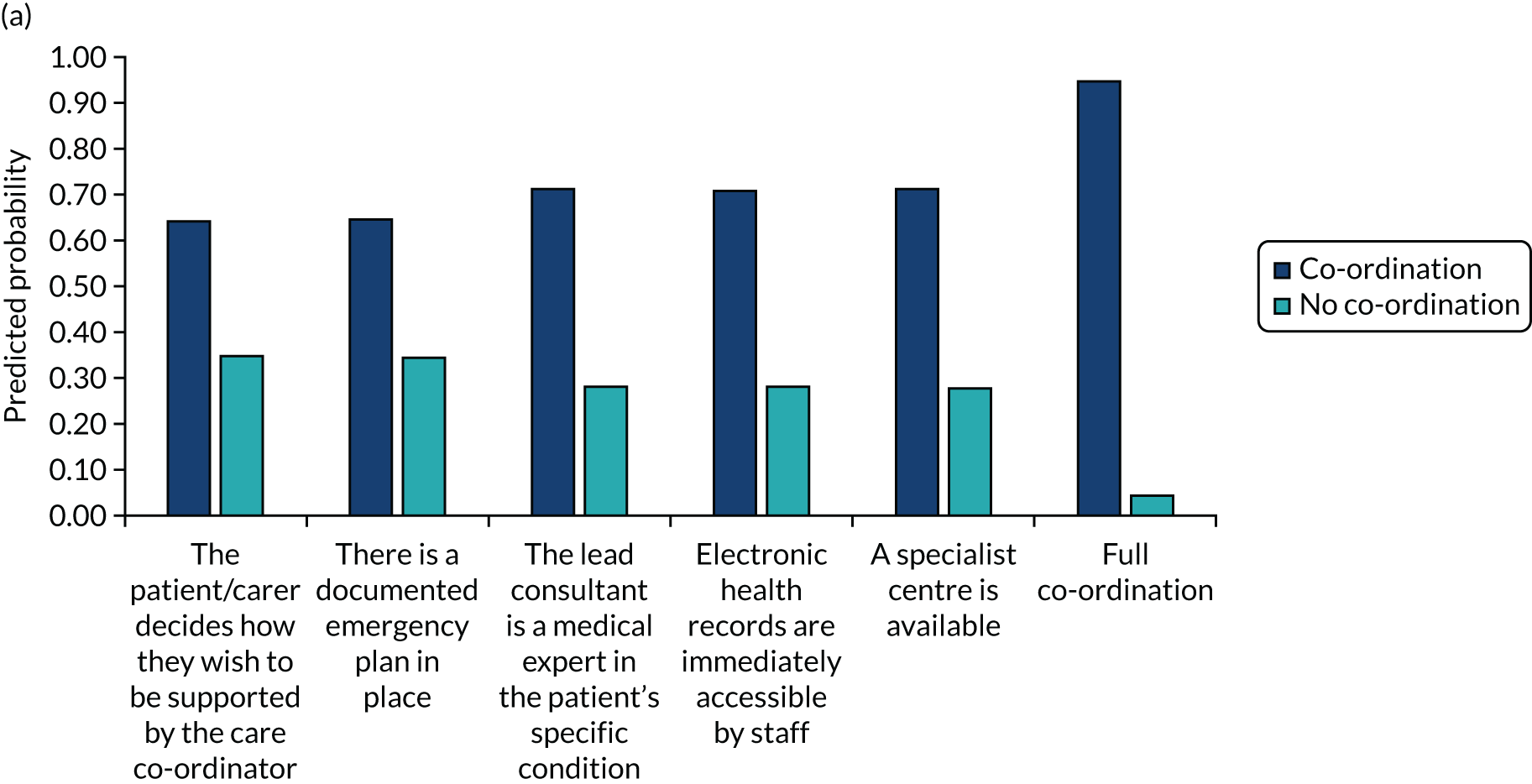

FIGURE 20.
Predicted probabilities for choosing co-ordinated services: high cost for co-ordination and low cost for no co-ordination. (a) Patients and parents/carers combined; and (b) HCPs. No co-ordination: cost to patients and carers of attending all health-care appointments during 1 year is £200; health records are not shared; the lead consultant is a medical expert in the area of the body primarily affected by the patient’s condition (e.g. neurologist); care is provided without the support of a care co-ordinator; a specialist centre is not available and there is not a documented emergency plan in place. Full co-ordination: cost to patients and carers of attending all health-care appointments during 1 year is £2000; electronic health records are immediately accessible to staff; the lead consultant is a medical expert in the patient’s specific condition; the patient/carer decides how they wish to be supported by the care co-ordinator (patients/carers) or care is entirely co-ordinated by a care co-ordinator (HCPs); a specialist centre is available and there is a documented emergency plan in place. All other co-ordination scenarios are as for no co-ordination except for the attribute indicated. Scenarios are ordered from left to right in ascending order of magnitude of the predicted probability of choosing the co-ordination service (note that the ordering is different for patients and carers combined and HCPs).
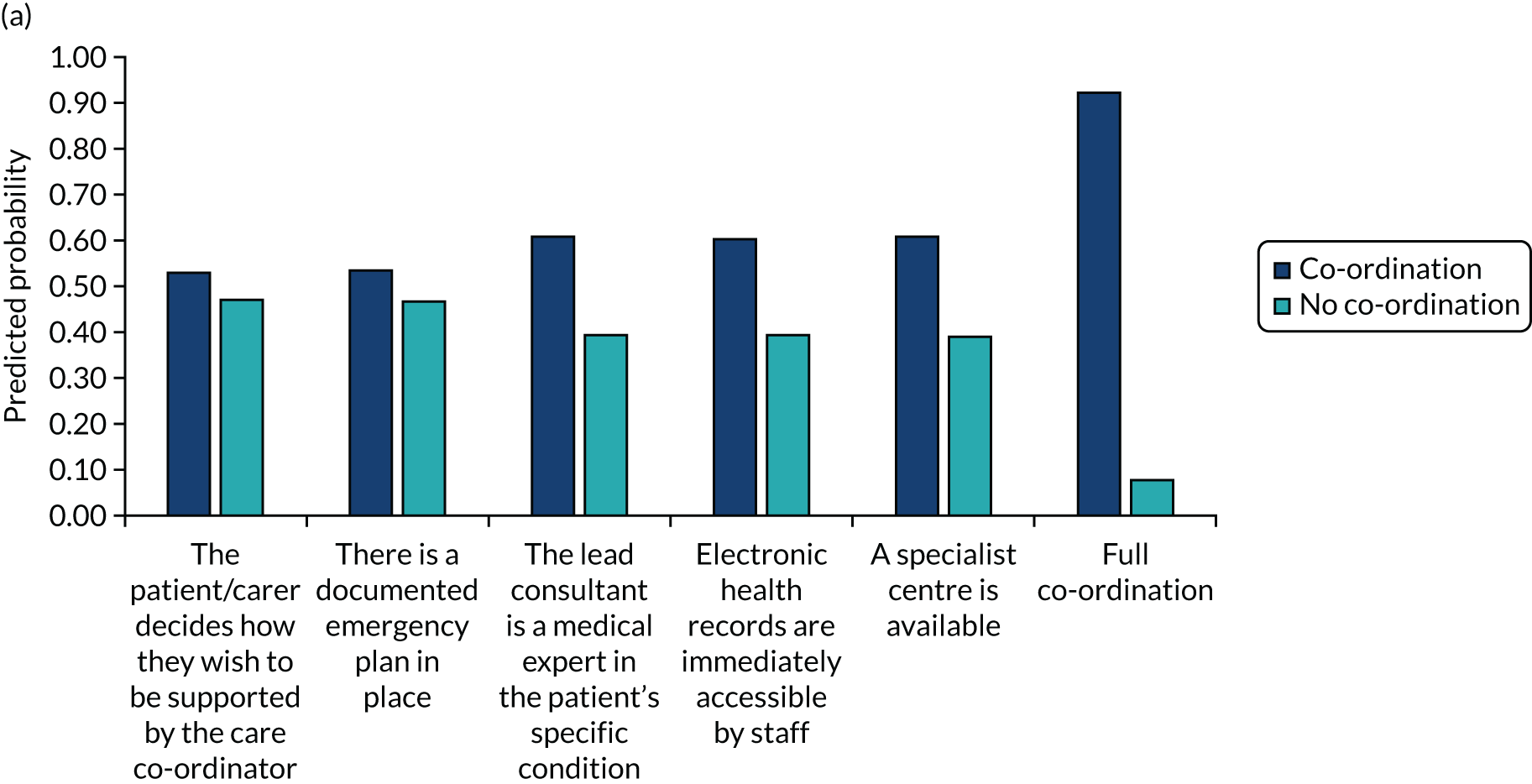
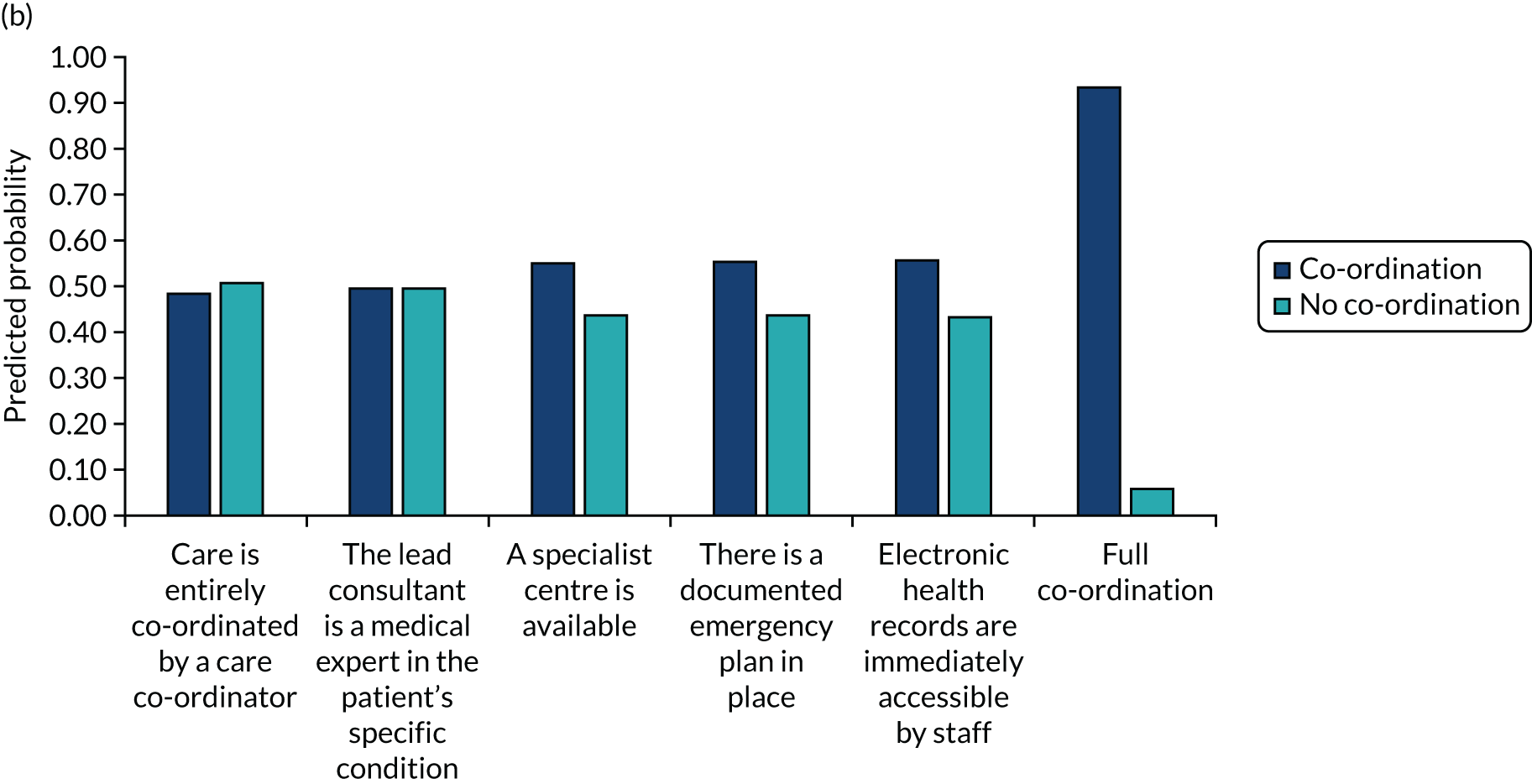
Appendix 7 Preferences for co-ordination across the themes highlighted in the taxonomy
| Theme | Model | Subtype of model |
|---|---|---|
| Theme 1: centralisation (domain 1) | ||
| Way of organising care | Commissioned services | National |
| Local/regional | ||
| Physical or virtual | ||
| Hub and spokes/outreach/networks | Hub and spokes: specialist co-ordinating, local implementing | |
| Outreach clinics | ||
| Networks of expertise | ||
| Colocation | Of appointments and co-ordinator | |
| Of sectors | ||
| Of paediatric and adult providers | ||
| Theme 2: collaboration (domain 2) | ||
| Type of collaboration | Condition-specific clinics | Joint clinics |
| MDT clinics | ||
| Transition clinics/joint appointments | ||
| Joint working | Negotiation and agreements of timings | |
| MDTs | MDT | |
| Meetings | MDT meetings | |
| Joint meetings | ||
| Patient forums | ||
| Transition as part of EHC meetings | ||
| Primary–secondary care interface | Primary–secondary care interface | |
| Other methods that facilitate transition | Continuity of child services into adulthood | |
| Preparation support | ||
| Referrals | ||
| Summaries of care | ||
| Individualised transition | ||
| Orientation visit | ||
| Continued protection | ||
| Part of the appointment on own and part with parent | ||
| Transition assessment | ||
| Theme 3: responsibilities (domain 3) | ||
| Administration | Administration | Administrative support |
| Point of contact | ||
| Formal responsibilities | Co-ordinator | General |
| Administrator | ||
| Charity role | ||
| Clinic co-ordinator role | ||
| Non-medical | ||
| Nurse or allied health professional | ||
| GP/primary care | ||
| Doctor-equivalent role | ||
| More than one | ||
| Play therapist | ||
| Social worker | ||
| Specialist | ||
| Technology | ||
| Transition co-ordinator role | ||
| Patient | ||
| Co-ordinator and patient/carer | ||
| Co-ordinator (including administrative and clinical) | ||
| Clinical lead | Doctor-equivalent role | |
| Continuity of provider | Same consultant/GP throughout | |
| Disciplines involved in care | Psychological support | |
| Flexibility | ||
| GP | First port of call | |
| Specialist interest located in genetics | ||
| Information provision | ||
| Referrers | ||
| Provider training | Provider training | |
| Raising awareness of services | ||
| Informal responsibilities | Charities | Supporting HCPs |
| Help with co-ordination/clinics | ||
| Provision of co-ordination materials | ||
| Role of patients | Records | |
| On-demand co-ordination | ||
| Expertise | ||
| Meetings | ||
| Partnership with patients | ||
| Patient choice | ||
| Patient support | Access to support | |
| Peer support | Patient forums | |
| Peer support | ||
| Buddying: patients/parents | ||
| Specialist and local providers | Specialist and local providers | Communication/information-sharing between specialist and local providers |
| Specialists supporting local staff | ||
| Local providers attending specialist meetings | ||
| Local care providers | ||
| Maximising expertise in limited centres | ||
| Specialist care provider | ||
| Theme 4: access (domains 1, 4 and 5) | ||
| Where (domain 1) | Care delivery | Specialist centre |
| Local area | ||
| Online | ||
| Multiple hospitals | ||
| Not A&E | ||
| Care co-ordination | Specialist centres | |
| Locally | ||
| Online | ||
| Primary care | ||
| Primary–secondary care interface | ||
| Non-medical department | ||
| Care delivery and co-ordination | Co-ordinated in specialist centres, implemented locally | |
| Specialist centres: both | ||
| Frequency (domain 4) | Care delivery | Regular appointments |
| On demand | ||
| Ongoing | ||
| Less frequent appointments | ||
| Care co-ordination | Regular appointments | |
| On demand | ||
| Ongoing | ||
| Meetings | Regular | |
| Access to information (domain 5) | Access to records | General |
| Providers | ||
| Patients | ||
| Third parties | ||
| Access to specialists | Access to specialists/expertise | |
| Provider access to specialists | ||
| Access to care | Out-of-hours support | |
| Registered pharmacies | ||
| Personalised budgets | ||
| Equal access to care | ||
| Appropriate length of appointments | ||
| Reduced need for rereferrals | ||
| Easier access to care | ||
| Financial support | ||
| Holistic care | ||
| Individualised care | ||
| Theme 5: mode of co-ordination (domain 6) | ||
| Information-sharing | Digital methods | Database |
| Non-specific | ||
| Instant messaging | ||
| Shared IT systems | ||
| Mobile applications | ||
| Online portals | ||
| Registries | ||
| Shared electronic records | ||
| Spreadsheets | ||
| Wearable technology | ||
| Written methods | Alert card | |
| Care pathway | ||
| Care plan | ||
| Condition-specific passport | ||
| Development of expert guides | ||
| Emergency protocols | ||
| Guidelines | ||
| Handover information-sharing | ||
| Information-sharing guidelines | ||
| Letters | ||
| Patient-held records | ||
| Summaries of care | ||
| Information handover (transition) | ||
| Transition assessment | ||
| Transition documentation | ||
| Written-down responsibility agreements | ||
| Verbal methods | Communication | |
| Information-sharing via co-ordinator | ||
| Information-sharing via meetings | ||
| Telephone | ||
| Lectures | ||
| Signposting | ||
| Care delivery | Mode of care appointments | Face to face |
| Digital | ||
| Telephone | ||
| Combination of digital and face to face | ||
| Combination of telephone and face to face | ||
| Combination of all three (i.e. telephone, digital and face to face) | ||
| Combination of telephone and digital | ||
| Mode of monitoring | Written | |
| Technology: application | ||
| Mode of co-ordination | Digital | |
| Face to face | ||
| Telephone | ||
| Digital or telephone | ||
| Mode of communication between HCPs | Face to face | |
| E-mail, telephone or letters | ||
| Digital | ||
| Telephone | ||
| Mode of communication between HCPs and patients | Digital | |
| Telephone | ||
| Written | ||
| Mode of meetings | Digital | |
| Mode of peer support | Face to face | |
| Telephone | ||
| Mode of transition | Telephone | |
Appendix 8 Benefits and challenges associated with ways of co-ordinating care across the themes highlighted in the taxonomy
| Theme | Model | Subtype of model | Benefits, benefits and challenges, or challenges | Type of benefit/challenge |
|---|---|---|---|---|
| Theme 1: centralisation (domain 1) | ||||
| Way of organising care | Commissioned services | Specialist centre | Benefits | Improved co-ordination |
| Reducing travel | ||||
| Holistic view | ||||
| Reducing appointments and faster decisions | ||||
| Agreement of plans: everyone knows what is going on | ||||
| Improved outcomes | ||||
| Setting standards for other countries | ||||
| Motivated staff | ||||
| Benefits and challenges | Access to expertise | |||
| Need for flexibility | ||||
| Not available for all conditions | ||||
| Resource availability | ||||
| Support for local providers | ||||
| Involvement of charities | ||||
| Challenges | Not accessible for all | |||
| Not covering all aspects of condition | ||||
| Not suitable for some types of condition | ||||
| Not always useful: needs to be useful to be worth travelling | ||||
| Reliance on clinic | ||||
| Difficulties organising all appointments in 1 day | ||||
| Lack of continuity | ||||
| Hub and spoke/outreach/networks | Hub and spoke | Benefits | Education and support/communication for local providers | |
| Reduces travel while providing specialist care | ||||
| Setting standards | ||||
| Reducing worries about who is responsible for which bits | ||||
| Family benefits | ||||
| Challenges | Resources | |||
| Reliance on both specialists and local teams | ||||
| Not always lifelong | ||||
| Outreach clinics | Benefits | Education and support for local providers | ||
| Less missed appointments | ||||
| Reduced travel | ||||
| Enables joint working/consistency | ||||
| Quicker | ||||
| Easy access to specialist | ||||
| Challenges | Missing some members of the team | |||
| Not always available | ||||
| More frequent appointments | ||||
| Not always having a suitable environment | ||||
| Not available across all ages | ||||
| Not always working well | ||||
| Daunting | ||||
| Not suitable for all conditions | ||||
| Network model | Benefits | Education and support of local providers | ||
| Setting standards | ||||
| Balance of partnership between local and specialist teams | ||||
| Suitable for some conditions | ||||
| Able to provide more care options | ||||
| Reducing patient travel | ||||
| Overcoming limited expertise | ||||
| Increasing patient engagement | ||||
| Improve co-ordination | ||||
| Benefits and challenges | Resources | |||
| European reference networks | Benefits | Networking | ||
| Challenges | Reliant on political decisions | |||
| Funding/time | ||||
| Theme 2: collaboration (domain 2) | ||||
| Type of collaboration | Condition-specific clinics | Condition-specific clinics | Benefits | Facilitates organisation of clinic |
| MDT | MDT | Benefits | Reduces travel | |
| Inclusion of named professional | ||||
| Consistency of messages and decisions | ||||
| Variety of skills | ||||
| Improves co-ordination | ||||
| Works well for conditions with clear guidelines on who is involved | ||||
| Benefits and challenges | Holistic care | |||
| Challenges | Difficulties with organisation and transparency | |||
| Lack of involvement from some disciplines | ||||
| Lack of agreement when seen separately | ||||
| Involvement not always long term | ||||
| Tiring: busy day | ||||
| MDT not always available | ||||
| Not having MDTs is perceived to be ineffective | ||||
| Inequity | ||||
| Need to build expertise | ||||
| Requires time and funding | ||||
| Meetings | Meetings | Benefits | Shared conclusions and agreement of progression | |
| Able to discuss freely (meetings without family) | ||||
| Consistency of messaging | ||||
| Benefits to family | ||||
| Benefits and challenges | Time | |||
| Challenges | Difficulties organising | |||
| Lack of sharing | ||||
| Lack of reading | ||||
| Unpopular with families (meetings without family) | ||||
| Primary–secondary care interface | Primary–secondary care interface | Benefits | Greater expertise | |
| Better links between primary and secondary care | ||||
| Other transition methods | Other transition methods | Benefits | Enhanced care journey/smoother transition | |
| Helping to take responsibility | ||||
| Continuity | ||||
| Support | ||||
| Agree on care plan | ||||
| Benefits and challenges | Appropriate information-sharing | |||
| Differences in child to adult care | ||||
| Confidence | ||||
| Relationship-building | ||||
| Challenges | Time | |||
| Knowledge | ||||
| Excessive support | ||||
| Wider life factors | ||||
| Motivation of parents to stay involved | ||||
| Dependent on condition | ||||
| Theme 3: responsibilities (domain 3) | ||||
| Administrative support | Administrator | General | Benefits | Improve co-ordination |
| Reduce stress of patients | ||||
| Administrator | Benefits | Improve co-ordination | ||
| Benefits and challenges | Support patients | |||
| Challenges | Time restrictions | |||
| Administrator and patient | Benefits | Easier for patients | ||
| Charities | Benefits | Providing support for specialist clinics | ||
| Challenges | Regulatory problems | |||
| Point of contact | General | Benefits | Someone who can answer questions | |
| Building rapport | ||||
| Doctor equivalent | Challenges | Not well placed to answer questions | ||
| Not available | ||||
| Nurse equivalent | Benefits | Facilitating access to the hospital | ||
| Time | ||||
| Passing information on | ||||
| Benefits and challenges | Answer questions | |||
| Finances | ||||
| Challenges | Not in place across the whole of UK | |||
| Formal responsibilities | Co-ordinator | General | Benefits | Support in local area |
| Helping families make decisions | ||||
| Passing on information | ||||
| Facilitating organisation of appointments and care | ||||
| Supporting patients | ||||
| Point of contact | ||||
| Liaising across sectors and aspects of care | ||||
| Holistic view | ||||
| Motivation | ||||
| Facilitating patient choice | ||||
| Well evidenced | ||||
| Benefits and challenges | Need dedicated time and capacity | |||
| Need dedicated role/profession | ||||
| Facilitating relationships between patients and care team | ||||
| Challenges | Lack of co-ordinators | |||
| Time spent chasing results | ||||
| Unable to have one for every condition | ||||
| Need more than one | ||||
| Need flexibility | ||||
| Charities | Benefits | Support with administration | ||
| Relationships with patients | ||||
| Point of contact | ||||
| Capacity to work with families | ||||
| Linking health and social care sectors | ||||
| Challenges | Lack of consistency across charities | |||
| Lack of knowledge about treatment | ||||
| Not suitable for all conditions | ||||
| Time | ||||
| Not having infrastructure within NHS | ||||
| HCP negative attitudes | ||||
| Doctor and nurse | Benefits | Clinical leadership and co-ordination | ||
| Doctor-equivalent role | Benefits | Patient preferences | ||
| Reducing burden on family | ||||
| Benefits and challenges | Taking responsibility for patient and continuity | |||
| Interest and motivation | ||||
| Need to be specialist | ||||
| Challenge | Needing to plan cover | |||
| Negative attitudes | ||||
| GPs | Benefits | Access to information and well equipped to co-ordinate | ||
| Knowledge and ability | ||||
| Holistic | ||||
| Suitable for certain conditions | ||||
| Benefits and challenges | Continuity | |||
| Challenges | Time | |||
| Funding | ||||
| Not always getting a response | ||||
| Not always kept in the loop | ||||
| HCP plus patient | Benefits | Support when needed | ||
| Non-medical personnel | Benefits | Planning of clinics | ||
| Advocate | ||||
| Smooth clinic running | ||||
| Understanding of condition | ||||
| Challenges | Not holistic | |||
| Not invested | ||||
| Resources | ||||
| Time | ||||
| Need cover | ||||
| Supported living | Benefits | No budget gap | ||
| Nurse- or allied health professional-equivalent roles | Benefits | Knowing the patients | ||
| Helping navigate multidisciplinary world | ||||
| Able to explain to patients | ||||
| Passing on information to team | ||||
| More empathetic and compassionate | ||||
| Understanding of condition | ||||
| Taking responsibility | ||||
| Benefits and challenges | Time | |||
| Availability of dedicated positions | ||||
| Other | ||||
| Liaise and chase on patients’ behalf | ||||
| Challenges | Finances | |||
| Lack of detailed job description | ||||
| Patients/carers | Benefits | Motivation to co-ordinate/ownership | ||
| Knowledge | ||||
| Benefits and challenges | Ability to self manage | |||
| Challenges | Viewed negatively | |||
| Stressful/tiring | ||||
| Impact on family life | ||||
| Child protection | ||||
| Ignored | ||||
| Transition changes | ||||
| Social care | Benefits | More frequent care | ||
| Signposting ability | ||||
| Holistic care | ||||
| Improving co-ordination | ||||
| Challenges | Time | |||
| Lack of condition knowledge | ||||
| Lack of dedicated position | ||||
| Geneticists | Benefits and challenges | Ability to facilitate more than just diagnosis | ||
| Clinical lead | General | Benefits | Facilitate collaboration | |
| Holistic | ||||
| Consultant | Benefits | Expertise | ||
| Relationship | ||||
| Holistic | ||||
| Taking responsibility | ||||
| Challenges | Difficult to reach | |||
| Discipline specific | Benefits | Flexibility | ||
| GP role | Gatekeepers | Benefits | Speed of referral | |
| Challenges | Time | |||
| Motivation | ||||
| Referral pathways | ||||
| Implementing care plans from specialists | Benefits | Appropriate person | ||
| Facilitate co-ordination with patient | ||||
| Benefits and challenges | Ability to provide care | |||
| Challenges | Time | |||
| Unable to help | ||||
| Not discussing frequently | ||||
| Lack of knowledge about who to refer to | ||||
| Informal responsibilities | Charities | Other involvement | Benefits | Administrative support |
| Support role | ||||
| Pushing for standards | ||||
| Challenges | Not available for all conditions | |||
| Reliant on donations | ||||
| Patients | Other involvement | Benefits | Education of doctors and peers | |
| Choice over who sees records | ||||
| Challenges | Psychological challenges | |||
| Time | ||||
| Involvement of peer support | Other involvement | Benefits | Help to co-ordinate own care | |
| Reduce isolation | ||||
| Benefits and challenges | Education | |||
| Challenges | HCP attitudes | |||
| GDPR | ||||
| Lack of peer support | ||||
| Specialist vs. local teams | Specialist and local involvement | Specialist and local involvement | Benefits | Educating providers |
| Challenges | Confusion over who to talk to | |||
| Lack of co-ordination | ||||
| Local teams not listening to specialist teams | ||||
| Specialist vs. local providers | Specialist vs. local providers | Benefits | Specialists seen as having expertise | |
| Agreement of care between them | ||||
| Ability to co-ordinate from specialist centre | ||||
| Co-ordinator as a go-between for specialist and local | ||||
| Convenience of local and remote specialist | ||||
| Challenges | Local providers not believing specialists | |||
| Lack of resources locally | ||||
| Challenges of information-sharing | ||||
| Excess travelling to specialist centres | ||||
| Separate specialists as not holistic | ||||
| Time and funding | ||||
| Theme 4: access (domains 1, 4 and 5) | ||||
| Where (domain 1) | Care delivery | Care delivered locally with specialist centre input | Benefits | Overcoming limited expertise |
| Some conditions requiring local care | ||||
| Challenges | Requires local buy-in | |||
| Not always kept in the loop | ||||
| Not able to provide care | ||||
| Care delivered locally | Challenges | Differences in care delivery | ||
| Specialist centre | Benefits | Child-friendly care | ||
| Care co-ordination | Local | Benefits | Convenience | |
| Overcomes time limitations (CCG based) | ||||
| Benefits and challenges | Reduces travel | |||
| Frequency (domain 4) | Care delivery | Regular | Benefits | Able to check in, update on care and check nothing has changed |
| Relationship building (seeing same person more than once) | ||||
| On demand | Benefits | Helping people access care when needed | ||
| Benefits and challenges | Not wasting time | |||
| Less frequent appointments, but all on same day | Benefits | Reduces travel | ||
| Scheduled times | Benefits | Evidence based | ||
| Suitable for condition | ||||
| Takes into account genetic breakthrough | ||||
| Co-ordination | Regular | Benefits | Able to check in, update on care and check nothing has changed | |
| On demand | Benefits | Not wasting time | ||
| Benefits and challenges | Helping people access care when needed | |||
| Access to information (domain 5) | Equity of access | Equity of access | Benefits | Facilitating co-production (formal conflict resolution process) |
| Flagging patients up when in need of care (pathways) | ||||
| Individualised care (personalised budgets) | ||||
| Kept up to date (technology pathway) | ||||
| Facilitating access despite communication difficulties (webchat or e-mail) | ||||
| Providing clarity on who to contact (documentation showing who to contact) | ||||
| Quality assurance (written care agreements) | ||||
| Reducing burden (access to care co-ordination department) | ||||
| Reducing exclusion from system (on-demand access) | ||||
| Reducing time wasting (on-demand access) | ||||
| Smoothing care (guidelines, access to specialist, care plan) | ||||
| Benefits and challenges | Access to records | |||
| Accessing care | ||||
| Awareness | ||||
| Knowledge | ||||
| Challenges | Commissioning | |||
| Depending on severity of symptoms | ||||
| Models of co-ordination not available for all conditions | ||||
| Delays | ||||
| Different care in different areas | ||||
| Different guidelines | ||||
| Doctors ignoring recommendations | ||||
| Ethics issues | ||||
| HCP worries (A&E) | ||||
| Lack of clarity over diagnosis | ||||
| Lack of ownership from GPs | ||||
| Lack of registered pharmacies | ||||
| Lack of services (mental health) | ||||
| Need for support for third-sector providers (e.g. education) | ||||
| Delays in emergency care (patient worries) | ||||
| Physical ability of patients | ||||
| Unresponsive services (111) | ||||
| Information-sharing with employer | Information-sharing with employer | Challenges | Stigma | |
| Theme 5: mode of co-ordination (domain 6) | ||||
| Information-sharing | Digital methods | Online portal | Benefits | Everything in one place |
| Access to information | ||||
| Quicker appointment organisation | ||||
| Easy access to information | ||||
| Track appointments | ||||
| Quicker | ||||
| Cheaper | ||||
| Benefits and challenges | Secure | |||
| Challenges | Failures of IT | |||
| Too much information | ||||
| Keeping information up to date | ||||
| IT issues | ||||
| Online records | Benefits | Improving co-ordination | ||
| Reducing problems associated with written communication | ||||
| Everyone knows what is going on | ||||
| Patient control over who has access | ||||
| Up-to-date records | ||||
| Saving time | ||||
| Benefits and challenges | Easy access from anywhere | |||
| Shared system, helping to co-ordinate | ||||
| Data protection | ||||
| Accessibility | ||||
| Security and restriction | ||||
| Unwieldy | ||||
| Challenges | IT failures | |||
| Not accessible to everyone | ||||
| Cost | ||||
| Errors | ||||
| Acceptability | ||||
| Expense | ||||
| Mobile applications for records | Benefits | Patient control over who information is shared with | ||
| Easy access | ||||
| Monitoring platform | ||||
| Suitability for some groups of patients | ||||
| Benefits and challenges | Data protection | |||
| Instant communication | ||||
| Security and restriction | ||||
| Challenges | Negative HCP attitudes | |||
| Not talking to each other | ||||
| Expense | ||||
| Need technological ability | ||||
| Difficulties for some conditions | ||||
| Not all hospitals use applications | ||||
| Not talking to each other | ||||
| E-mail correspondence | Benefits | Quick and easy | ||
| Written record of communication | ||||
| Useful for scene-setting | ||||
| Useful for updates and reviews | ||||
| Good for advice | ||||
| Ability to flag urgency | ||||
| Time to reply | ||||
| More continuity | ||||
| Environmentally friendly/cheap | ||||
| Transparent | ||||
| Benefits and challenges | Confidentiality | |||
| Not guaranteed a response | ||||
| Challenges | Not suitable for all conditions | |||
| Unwieldy if lots to go through | ||||
| Misunderstandings | ||||
| Data protection | ||||
| Lack of IT infrastructure | ||||
| Database | Benefits | Able to pull out information when needed | ||
| Signposting | ||||
| Improves co-ordination | ||||
| Reduces A&E attendance | ||||
| Benefits and challenges | Not kept up to date | |||
| Challenges | Difficult to keep track of | |||
| Not interacting with other hospitals | ||||
| ERN forums online | Benefits | Networking | ||
| Challenges | IT systems | |||
| Voluntary | ||||
| Wearable devices | Benefits | Convenience | ||
| Online appointment reminders | Benefits | Easier for patients | ||
| Registry | Benefits | Data comparisons | ||
| Written methods | Letters | Benefits | Keeping everyone in the loop | |
| Quicker | ||||
| Written record | ||||
| Patients are kept up to date | ||||
| Nicer when addressed to patients | ||||
| More tangible | ||||
| Challenges | Lost or delayed | |||
| Errors | ||||
| Intense | ||||
| Written records | Benefits | Ownership | ||
| Back-up | ||||
| Good for full reports | ||||
| Condition-specific passport | Benefits and challenges | Information on hand when needed | ||
| Challenges | Creating worries for providers | |||
| Needs to be accepted by providers | ||||
| Patient-held records | Benefits | Accurate recording | ||
| Ownership and easily shared | ||||
| Holistic | ||||
| Challenges | Funding | |||
| Written agreements of responsibility | Benefits | Accountability | ||
| Benefits and challenges | Accessibility | |||
| Care plan | Benefits | Reduces repetition | ||
| Information provision | ||||
| Helps patients, carers and HCPs to remember | ||||
| Challenges | Not used by doctors | |||
| Impact on care outcomes | ||||
| Not shared or used | ||||
| Summary sheet | Benefits | Information provision | ||
| Confidence it will be acted on | ||||
| Paper records | Challenges | Not available when needed | ||
| Documentation: co-ordination | Benefits | Monitoring symptoms | ||
| Highlight key information in emergencies | ||||
| Alert cards | Benefits | Information provision | ||
| Challenges | Not consistently liked | |||
| Ignored by HCPs | ||||
| Transition booklet | Benefits | Increase responsibility | ||
| Verbal | Evening lectures | Benefits | Sharing information | |
| Care delivery | Digital | Skype/virtual teleconferencing | Benefits | Reduces travel |
| Good for updates and reviews | ||||
| Able to see the patient | ||||
| Consistent messages | ||||
| Reduces stress | ||||
| Reduces time-wasting | ||||
| Easy | ||||
| Appropriateness for age | ||||
| Benefits and challenges | Good for answering questions | |||
| Not able to replace specialist appointments | ||||
| Time | ||||
| Appropriateness for conditions | ||||
| Challenges | Not working for first meetings | |||
| Data protection | ||||
| Not able to pick up all nuances | ||||
| Funding | ||||
| Requires confidence | ||||
| Virtual meetings | Benefits | Good technology | ||
| Agreeing solutions | ||||
| Good if face-to-face meetings are not possible | ||||
| Reduces time | ||||
| Face to face | Face-to-face appointments | Benefits | Helps to work things out and build relationships | |
| Able to physically examine patients | ||||
| Able to pick up problems | ||||
| Able to offer physical support | ||||
| Easier discussions | ||||
| Preventing information getting lost | ||||
| Overcoming communication challenges | ||||
| Benefits and challenges | Not appropriate for all conditions | |||
| Need to be useful appointments | ||||
| Requires travelling | ||||
| Challenges | Difficult to organise | |||
| Tiring and impactful | ||||
| Time | ||||
| Funding | ||||
| Peer support | Benefits | Reducing isolation | ||
| Meetings | Benefits | Easier to address issues | ||
| Reduces misunderstandings | ||||
| Demonstrations | ||||
| Easier to agree plans and move forward | ||||
| More effective than written reports | ||||
| Challenges | Lack of capacity to go to meetings | |||
| Telephone | Telephone point of contact | Benefits | Enables queries or messages | |
| Reduces chance of getting lost in the system | ||||
| Easier than e-mail | ||||
| Options for all ages | ||||
| Building relationships | ||||
| Good for discussing with GP | ||||
| Empowering | ||||
| Safer than e-mail | ||||
| Able to do verbal demonstrations | ||||
| Challenges | Not guaranteed a response | |||
| Not suitable for all conditions | ||||
| Misunderstandings | ||||
| Resources needed | ||||
| Telephone appointments | Benefits | Reducing travel where appointments can be done remotely | ||
| Consistent messaging | ||||
| Facilitates joint decision-making | ||||
| Agreeing plans moving forwards | ||||
| Can send non-confidential information (WhatsApp) | ||||
| Benefits and challenges | Appropriateness for conditions | |||
| Challenges | Not a preference | |||
| Not able to pick up body language | ||||
| Connection difficulties | ||||
| Miscommunication | ||||
| Combination | Combination of digital and face to face | Benefits | Keeping everyone in the loop | |
| Reducing travel | ||||
| Saving time and money | ||||
| Sharing information | ||||
| Message consistency | ||||
Appendix 9 Factors influencing care co-ordination across the themes highlighted in the taxonomy
| Theme | Type of factor | Theme | Subtheme |
|---|---|---|---|
| Theme 1: centralisation (domain 1) | |||
| The way that care is organised | Patient factors | Diagnosis | Diagnosis: rarity |
| Type of condition | |||
| Grouping conditions | |||
| Age | Adults vs. children | ||
| Developmental age | |||
| Stage of life | |||
| Age that condition affects | |||
| Condition | Condition: general | ||
| Condition: discreteness | |||
| Condition: severity | |||
| Condition: nature | |||
| Condition: stability | |||
| Condition: availability of treatment/funding | |||
| Health-care environment | Resources | Funding | |
| Differences in child and adult services | |||
| Availability of experts | |||
| Availability of different types of co-ordination | |||
| Availability of local resources | |||
| Quality standards | |||
| Environment | Access to hospital | ||
| Suitability of environment | |||
| Referral boundaries | |||
| Care delivered across multiple hospitals | |||
| Size of hospital | |||
| Colocation | |||
| Attitudes | Relationships between care teams | ||
| Societal | Resources | Funding | |
| Geographical differences in funding | |||
| Lack of centralised budgets | |||
| Guidelines | |||
| Theme 2: collaboration (domain 2) | |||
| Collaboration | Patient factors | Age | Clinics varying for adults and children |
| Care assessment needs in childhood | |||
| Need for adults to have co-ordinated appointments on similar days | |||
| Diagnosis | Type of diagnosis affecting collaboration | ||
| Condition | Type of condition (whole spectrum of care not just acute) | ||
| Availability of MDT clinics for conditions | |||
| How discrete condition is | |||
| Nature of condition | |||
| Patient’s needs | |||
| Severity of condition | |||
| Symptoms of condition | |||
| Provider factors | Knowledge and understanding | Team needing multidisciplinary expertise (in line with guidelines) | |
| International expertise | |||
| Health-care environment | Resources | Funding | |
| Availability of experts | |||
| Availability of different types of co-ordination | |||
| Number of professionals involved affecting greater number of appointments needed | |||
| Theme 3: responsibilities (domain 3) | |||
| Responsibilities | Patient factors | Diagnosis | Availability of co-ordinators/who co-ordinates care seen as differing, depending on type of condition |
| Rarity of condition | |||
| Lack of diagnosis: difficult to know who is involved | |||
| Lack of awareness for some rare conditions | |||
| Age of patient | Parent vs. patient role, as differing in childhood and adult | ||
| Who is involved, as differing with age | |||
| Ownership in childhood vs. adulthood | |||
| Care gap between childhood and geriatric care | |||
| HCP lack of trust in adults | |||
| Someone to help co-ordinate schools and employment | |||
| Condition | Condition complexity | ||
| Stability | |||
| Type of condition | |||
| Symptoms of condition | |||
| Consistency across conditions | |||
| Phase of condition | |||
| Individual patient needs and preferences | Choice | ||
| Individual patient needs | |||
| Ability to self-manage | |||
| Family needs | |||
| Family demographics | |||
| Medical knowledge | |||
| Intelligence or ability to push | |||
| Communication needs | |||
| Access needs | |||
| Individual presentation of condition | |||
| Holistic care | |||
| Family preferences | |||
| Parent involvement | |||
| Employment | |||
| Feedback | |||
| School | |||
| Consent | Consent and level of co-ordinators | ||
| Consent for third parties’ involvement | |||
| Consent for peer support | |||
| Provider factors | Skills and capability | Knowledge and understanding | |
| Support and education | |||
| Understanding of how the health-care system works | |||
| Level of clinical skill | |||
| Training pathways and regulation | |||
| Understanding about family and resources | |||
| People skills | |||
| Attitudes | Interest | ||
| Motivation | |||
| Preferences | |||
| Anxiety about treating | |||
| Attitudes | |||
| Opportunity | Type of provider | ||
| Time | |||
| Teamwork | |||
| Health-care environment | Resources | Funding | |
| Availability of providers | |||
| Availability of job roles: co-ordinator | |||
| Access to HCPs | |||
| Societal | Resources | Availability of patient organisations | |
| Funding: staff | |||
| Attitudes | Willingness to change | ||
| Stigma | |||
| Theme 4: access (domains 1, 4 and 5) | |||
| Where (domain 1) | Patient factors | Diagnosis | Diagnosis determines where care should be co-ordinated |
| Age | Location of care as dependent on age-related needs | ||
| Where care is co-ordinated as differing with age | |||
| Some locations of care are better for children | |||
| Condition | Rarity | ||
| Progression/stability | |||
| Stage | |||
| Type | |||
| Symptoms | |||
| Ability to travel | Depending on ability to travel | ||
| Where patients live influences co-ordination | |||
| Patients who live far away from specialist centre, but can travel | |||
| Patients who live far away from specialist centre, but cannot travel | |||
| Patients who live close to specialist centre | |||
| National guidelines | |||
| Where care is delivered seen as depending on where HCPs are based | |||
| Where care delivered seen as depending on balancing community life | |||
| For those far away, access to specialists relying on referrals | |||
| Lack of access to records means travel is wasted | |||
| Capacity of patients who can be treated seen as a factor influencing co-ordination models | |||
| Team who work closely seen as having better co-ordination | |||
| Availability of access to advice from specialists for local provider | |||
| Health-care environment | Resources | Geographical differences in care provision | |
| Availability of local resources | |||
| Environment | Suitability of environment | ||
| Size of hospital | |||
| Access to HCPs | |||
| Number of professionals involved in condition | |||
| Colocation | |||
| Societal factors | Resources | Commissioning | |
| Geographical differences in funding | |||
| Staff funding | |||
| Lack of centralised budgets | |||
| Frequency (domain 4) | Patient factors | Diagnosis | Diagnosis seen as influencing frequency |
| Age of patient | Care co-ordination as needing to be lifelong | ||
| Frequency dependent on age-related needs | |||
| Condition-specific pathways determining frequency | |||
| Condition | Progression | ||
| Phase of condition | |||
| Stability | |||
| Symptoms | |||
| Type of condition | |||
| Severity | |||
| Time since treatment | |||
| Individual needs | |||
| Ability to travel/location | Patient’s location | ||
| Provider factors | Knowledge and understanding | Knowledge and understanding | |
| Time | Time | ||
| Health-care environment | Resources | Guidelines | |
| Availability of job roles: co-ordinator | |||
| Not enough time to treat patients | |||
| Funding | |||
| Access (domain 5) | Patient factors | Diagnosis | Diagnosis: easier to access care and co-ordination |
| Lack of diagnosis, making it harder to access care and co-ordination | |||
| Lack of care due to type of diagnosis received | |||
| Condition | Severity | ||
| Availability of guidelines | |||
| Awareness | |||
| Access to records | |||
| Consent | Consent over records | ||
| Consent over anonymisation | |||
| Consent for registry | |||
| Consent for online portal | |||
| Consent for national IT | |||
| Health-care environment | Attitudes | Relationships | |
| Hospital attitudes | |||
| Willingness of local area | |||
| Resources | Funding | ||
| Availability of job roles: co-ordinator | |||
| Access to technology | |||
| Availability of experts | |||
| Access to hospital | |||
| Availability of different types of co-ordination | |||
| Availability of local resources | |||
| Quality standards | |||
| Training programmes | |||
| Not enough time to treat patients | |||
| Referral boundaries | |||
| Care delivered across multiple hospitals | |||
| Access to HCPs | |||
| Number of professionals involved in condition | |||
| Lack of registered care providers | |||
| Environment | Differences in child and adult services | ||
| Suitability of environment | |||
| Geographical differences in care provision | |||
| Size of hospital | |||
| Location of hospital | |||
| Societal factors | Funding | Commissioning | |
| Geographical differences in funding | |||
| Funding staff | |||
| Funding: lack of centralised budgets | |||
| Guidelines | |||
| Theme 5: mode of co-ordination (domain 6) | |||
| Information-sharing | Patient factors | Age | Lack of information-sharing in adults |
| Condition | Severity | ||
| Condition type | |||
| Health-care environment | Resources | Access to technology | |
| Care delivery | Patient factors | Age | Age as influencing mode |
| Individual needs | Individual needs as influencing mode | ||
| Condition | Stability | ||
| Symptoms | |||
| Type of condition | |||
| Phase of treatment | |||
| Type of appointment | |||
| Time since treatment | |||
| Ability to travel | |||
| Purpose of mode | |||
Appendix 10 Barriers to and facilitators of care co-ordination
| Type of factor | Theme | Subtheme | Barrier/facilitator |
|---|---|---|---|
| Patient factors | Ability | Ability to self-manage | Barrier |
| Facilitator | |||
| Knowledge | Barrier | ||
| Facilitator | |||
| Medical knowledge | Barrier/facilitator | ||
| Barrier | |||
| Facilitator | |||
| Health | Barrier | ||
| Facilitator | |||
| Attitudes | Anxieties | Barrier | |
| Facilitator | |||
| Confidence | Facilitator | ||
| Relationship with providers | Barrier/facilitator | ||
| Barrier | |||
| Facilitator | |||
| HCP perception of patients | Barrier | ||
| Opportunity | Ability to access hospital | Barrier | |
| Finances | Barrier | ||
| Facilitator | |||
| Geographical location | Barrier | ||
| Facilitator | |||
| Time | Barrier | ||
| Work/school | Barrier | ||
| Provider factors | Ability | Knowledge/awareness | Barrier |
| Facilitator | |||
| Education | Barrier | ||
| Facilitator | |||
| Need for training | Barrier | ||
| Facilitator | |||
| Qualifications | Facilitator | ||
| Attitudes | Confidence/anxiety | Barrier | |
| Facilitator | |||
| Interest | Barrier | ||
| Facilitator | |||
| Motivation | Barrier | ||
| Facilitator | |||
| Opinion | Barrier | ||
| Personality: people skills | Barrier | ||
| Facilitator | |||
| Relationships | Barrier | ||
| Facilitator | |||
| Reluctance | Barrier | ||
| Opportunity | Time | Barrier | |
| Facilitator | |||
| Administrative support | Facilitator | ||
| Availability of providers | Barrier | ||
| Facilitator | |||
| Gatekeeping structures | Barrier | ||
| Lack of access to information | Barrier | ||
| Lack of involvement | Barrier | ||
| Health-care environment | Resources | Availability of providers | Barrier |
| Facilitator | |||
| Availability of technology | Barrier | ||
| Facilitator | |||
| Capacity | Barrier | ||
| Facilitator | |||
| Capacity building | Barrier | ||
| Facilitator | |||
| Funding | Barrier | ||
| Facilitator | |||
| Guidelines and specification of working procedures | Barrier | ||
| Facilitator | |||
| Environment | Different provision of services across different sectors | Barrier | |
| Co-location | Barrier | ||
| Facilitator | |||
| Cross organisational relationships | Barrier | ||
| Facilitator | |||
| Facilities | Barrier | ||
| Facilitator | |||
| Organisational time restraints/flexibility | Barrier | ||
| Facilitator | |||
| Attitudes | Organisational politics | Barrier | |
| Facilitator | |||
| Societal factors | Resources | Funding | Barrier |
| Facilitator | |||
| National politics | Barrier | ||
| Availability of patient group | Barrier | ||
| Facilitator | |||
| Societal professions | Barrier | ||
| Facilitator | |||
| Separation of sectors | Barrier | ||
| Guidelines | Barrier | ||
| Facilitator | |||
| Technology interoperability | Barrier | ||
| Lack of certain services | Barrier | ||
| Attitudes | Supportive trust and CCG | Barrier | |
| Facilitator | |||
| Stigma | Barrier | ||
| Employment support | Barrier | ||
| Facilitator | |||
| Awareness | Facilitator | ||
| Law and regulation | Barrier | ||
| Facilitator | |||
| Support from other organisations | Facilitator | ||
| School support | Facilitator |
Appendix 11 CONCORD flow chart of co-ordinated care and cover note to help users understand how the flow chart can be used
List of abbreviations
- CI
- confidence interval
- CONCORD
- Co-ordinated Care Of Rare Diseases
- DCE
- discrete choice experiment
- EHCP
- education, health and care plan
- GP
- general practitioner
- HCP
- health-care professional
- MDT
- multidisciplinary team
- MRS
- marginal rate of substitution
- NHS EED
- NHS Economic Evaluation Database
- NIHR
- National Institute for Health Research
- PPI
- patient and public involvement
- PPIAG
- Patient and Public Involvement Advisory Group
- RQ
- research question
- STROBE
- STrengthening the Reporting of OBservational studies in Epidemiology
- SWAN
- Syndromes Without A Name
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/LNZZ5321).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
