Notes
Article history
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number 17/05/96. The contractual start date was in September 2018. The final report began editorial review in June 2022 and was accepted for publication in October 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Conroy et al. This work was produced by Conroy et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Conroy et al.
Chapter 1 Background
Sections of the scientific summary have been reproduced from Conroy 20191 under licence CC-BY-NC-ND 3.0.
Urgent and emergency care (UEC) is a major international issue. Older people with UEC needs and living with frailty are especially vulnerable to harms and delays that can arise in this system and the first hours have a powerful influence over the remaining UEC episode. 2–5 For example, the failure to identify delirium (new-onset confusion, often with a significant underlying medical cause) can lead to reduced attention, poor oral intake and reduced mobility, resulting in harms from the very first hours of the patient journey (e.g. dehydration, pressure sores). 6,7
It is not only the admitted population of older people who are at risk; those discharged from emergency departments (EDs) are also at risk of significant functional decline in the months following their attendance. 8,9 There is a need to improve the management of both patient groups to optimise their time in the UEC system, reduce complications and avoid unnecessary investigations and admission to hospital. This might involve a move away from ‘one problem, one solution’ approaches, which historically typifies emergency care, towards a more nuanced approach that takes account of multiple comorbidities, initial evaluations of which appear to show some promise in emergency care settings.
Demand for UEC is rising annually, especially in older people, who form one-fifth of attendees at EDs. 10 Analysis of 1.3 million attendances at 18 EDs in Yorkshire and Humber (YH) has shown that two-thirds of patients over the age of 75 years arrive by ambulance, patients over 75 years spend significantly longer in the ED and are referred for admission over half of the time. 11 We also found significant variation in admission rates by hospital studied (18–73%) in older people, which could not be fully explained by case mix. Understanding the range of variation in practice that leads to different admission rates and how this can be reduced would be timely and beneficial to health services.
The implementation of novel approaches to caring for older people living with frailty needs to be seen within the context of the complex array of different services models offered for UEC. It is therefore important that any new approaches are based on robust research evidence and their implementation is evaluated in terms of clinical and cost-effectiveness as well as understanding the impact for organisations and patients themselves. Such assessment needs to make the most of available data flows and modern approaches to understanding how patients move through care systems and the impact on effectiveness and efficiency. This proposal made use of existing data, modelling, qualitative enquiry and patient participation to describe the essential elements of a complex intervention, in keeping with Medical Research Council guidance. 12
Aims, objectives and research questions
We aimed to identify promising care models and produce guidance on implementation that addresses the needs of older people accessing UEC services. We conducted an evidence synthesis, stakeholder interviews, analysed patient pathways and outcomes in health-care data reflecting a range of models of care and used the information to conduct system dynamics modelling of the implications of changing pathways.
Work package 1: identifying best practice
-
WP1.1 – review of reviews of UEC interventions for older people, their outcomes and costs and any implementation factors identified
-
Research question (RQ) 1.1.1 – what is the evidence base for UEC interventions for older people, the outcomes of these interventions and the costs associated with these interventions?
-
RQ 1.1.2 – what factors have been described in the evidence base to date that influence implementation of UEC interventions for older people?
-
Output 1.1 – a taxonomy of UEC interventions with outcome effect sizes (where available) and descriptors of costs and implementation factors.
-
-
WP1.2 – patient and carer preferences.
-
RQ 1.2.1 – what elements of care are most important to older people and their carers with UEC needs?
-
RQ 1.2.2 – how could UEC interventions be configured to best meet the needs of older people?
-
Output 1.2 – description of patient and carer priorities for UEC.
-
-
WP1.3 – staff perspectives.
-
RQ 1.3.1 – what other interventions, not yet reported in the literature, offer promising models for improving outcomes for older people in the UEC pathway?
-
Output 1.3 – staff perspectives on the ‘state of the art’ and factors that will facilitate implementation.
-
Work package 2: qualitative study of delivery of exemplar urgent and emergency care pathways
-
RQ 2.1 – what aspects of interventions, context and approaches to implementation facilitate and hinder delivery of UEC interventions for older people?
-
Output 2.1 – context-, implementation- and intervention-related influences on delivery of interventions for older people in UEC settings.
Work package 3: routine patient-level data analysis
-
RQ 3.1 – are some UEC pathways associated with better patient outcomes than others?
-
RQ 3.2 – have UEC pathways improved or got worse over time?
-
RQ 3.3 – over and above the UEC pathway, what patient characteristics, demand and supply factors explain differences in outcomes from place to place and over time?
-
RQ 3.4 – what is the relationship between outcomes and the costs of the UEC pathway and are some pathways more cost-effective than others?
-
Output 3.1 – estimates of what drives better outcomes and lower costs for older people with UEC needs.
Work package 4: improving emergency care pathways
-
WP4.1 – baseline simulation model development.
-
RQ 4.1 – what is the best way to build an ‘archetypal’ or baseline model of patient flow into and out of one specific UEC pathway, ensuring that the model is valid and captures all the relevant factors?
-
Output 4.1 – a validated quantitative (stock-flow) system dynamics model of patient flow, describing the status quo situation in one specific setting.
-
-
WP4.2 – ‘what if’ analyses.
-
RQ 4.2 – what changes can be made to existing UEC pathways that will lead to greatest improvements, and what might the consequences of such changes be for the wider health-care system?
-
Output 4.2 – a family of system dynamics models based on output 4.1 describing the whole-system impact of evidence based, patient centred interventions applied to UEC pathways.
-
Chapter 2 Review of reviews
Sections of this chapter have been reproduced from Preston et al.,13 with permission.
Introduction
There is a large, but methodologically limited evidence base for interventions for older people in the ED. 14 Accordingly, we chose to undertake a review of reviews to answer two key RQs:
-
What is the evidence base for ED interventions for older people, the outcomes of these interventions and the costs associated with these interventions?
-
What factors have been described in the evidence base to date that influence implementation of ED interventions for older people?
In addition, the review was to inform the development of a taxonomy of models of care and any associated intervention effects to populate the system dynamics model (Chapter 7).
Methods
Reviews of reviews offer benefits in that they ‘enable broader evidence synthesis questions to be addressed … and in a faster timeframe’. 15 A review of reviews captures ‘the effects of different interventions for the same condition or population’ in this case, older people attending EDs. The protocol was published on PROSPERO in October 2018 (CRD42018111461).
Search methods for identification of reviews
Stage 1: database search
The line-by-line search strategy combined terms for UEC with terms for older people, limited by publication type (reviews), language (English language studies only) and date (2000 to current date) and was based on a previous strategy. 14 The search strategy was developed for Medline via Ovid SP (see Appendix 1) and was adapted for Embase via OVID SP, the Cumulative Index to Nursing and Allied Health Literature via EBSCO Information Services, the Cochrane Library (Database of Abstracts of Reviews of Effects) via Wiley Interscience, Web of Science Core Collection via Clarivate, Scopus via Elsevier and AgeINFO (via the Centre for Policy on Ageing: http://www.cpa.org.uk). The database search was undertaken in Autumn 2018 and an update search (Medline via Ovid SP only). References were downloaded in Endnote version 9 and then version 20 with duplicates removed.
Stage 2: search of review sources
We searched key review sources: JBI Evidence Synthesis from the Joanna Briggs Institute (https://journals.lww.com/jbisrir/Pages/default.aspx), the Campbell Collaboration (https://campbellcollaboration.org), Epistemonikos (www.epistemonikos.org) and PROSPERO (https://www.crd.york.ac.uk/PROSPERO/), using an adapted database search strategy.
Stage 3: other strategies
We undertook a cited reference search using Google Scholar and screened the results for inclusion. We also scrutinised the reference lists of included reviews and consulted with topic experts via the study executive management team (EMT). References from all sources across the three stages were downloaded in Endnote version 9 and duplicates were removed prior to screening.
Criteria for selecting reviews for inclusion
Screening of references at title and abstract level was done by one reviewer (LP or JvO), with 50% also screened by SA. All reviews that met the title and abstract screening criteria (Table 1) were screened at full text by LP and SC. Any reviews that were excluded at title stage were listed and reasons given for their exclusion. Agreement between reviewers was assessed using kappa scores.
| Criteria | Details |
|---|---|
| Publication details | Reviews published from 2000 onwards (ensuring reviews had at least 50% of their primary studies published after 2000). Peer-reviewed journal articles. Published in English. |
| Population | We included reviews with a population of whom at least 50% were people aged over 65 years or older people with living with frailty as defined by a recognised (published) frailty scale or clinical judgement. |
| Setting | Type 1 hospital-based EDs defined as ‘consultant-led, multispecialty 24-hour services with full resuscitation facilities and designated accommodation for the reception of ED patients.’ |
| Interventions | Any intervention or model of care delivered to an included population in an included setting, either initiated or wholly delivered within the ED. |
| Outcomes | Any patient, health service or staff outcome. |
| Study type | Evidence reviews, systematic reviews and meta-analyses, qualitative reviews and mixed-method reviews. |
| Model of care | Any care or model of care delivered to the population in the ED. We did not include reviews focusing solely on methods for identification of frail or high-risk older people, although where studies focusing on identification were included as part of a larger review, the review was included but data relating to these studies was not included. |
| Comparator | Usual care, no intervention, other interventions. |
| Follow up | We did not include/exclude studies based on presence or length of follow-up. |
Application of reporting standard criteria
As part of the screening process, reviews were identified which were relevant (by inclusion criteria; Table 2) but did not meet the methodological standards for a systematic review. In the protocol, we stated that we would assess reviews according to A MeaSurement Tool to Assess systematic Reviews version 2 (AMSTAR2) to assess systematic reviews. However, we adapted this approach and added an additional stage where we screened reviews to assess their methods and reporting prior to the use of AMSTAR2. This prior assessment allowed the exclusion of evidence described as a review but where data extraction and quality assessment were not possible. The reporting standards criteria developed were based on the Cochrane Handbook definition of a systematic review and criteria developed by Brunton. 16 Reviews were included in the review of reviews if they met three or more of the criteria. Conference abstracts were not assessed according to these criteria.
| Criteria | Definition |
|---|---|
| Inclusion | Review needed to report inclusion and exclusion criteria developed a priori and included primary studies needed to be screened against these criteria. |
| Search | The review needed to include a systematic search, which was described in sufficient detail to identify studies that would have met the inclusion criteria. |
| Quality assessment | An assessment of bias or reporting standards using a named tool. |
| Included studies | A list of included studies in the review, linked to findings of the review and summary statements. |
Data extraction and synthesis
Data were single extracted by one reviewer (LP, SA or JvO). The full data extraction tables are included in Appendix 2. These tables were all checked by LP and a random sample of 10% were also checked by SA. Data were extracted on review description, review methods, description of included studies, all reported outcomes (including whether they had been synthesised or reported as individual studies) and a headline message/conclusion.
Assessment of the evidence base
For the review of reviews, we used two different tools: AMSTAR2 (for reviews reporting interventions) and the Joanna Briggs Institute Critical Appraisal Checklist for Systematic Reviews and Research Syntheses (for non-intervention papers). AMSTAR2 was chosen because it allows the appraisal of reviews that include non-randomised studies of interventions, not just randomised controlled trials (RCTs). 17 We undertook a narrative assessment of the applicability of the results to the UK setting.
Size and scope of the evidence base
To examine the overall evidence base, a citation matrix18 was drawn up using EndNote 9 and Microsoft Word, mapping primary studies against the reviews in which they were cited.
Taxonomy development and validation
One of the review objectives was to develop a taxonomy of models of care delivery to older people in the ED; however, the evidence was not organised according to models of care. Three alternative frameworks (McCusker et al. 2018,53 American College of Emergency Physicians and the original framework described in the project protocol), were tested on three selected reviews by SC, JvO and LP. A process of discussion and consensus led to the choice of the Elder-Friendly Emergency Department Quality Assessment Tool. 19 This tool was used to identify whether the reviews included in our review of reviews included evidence of interventions related to any of the 13 subscales, which are as follows:
-
two‐step screening
-
standardised assessment tools
-
clinical care protocols
-
geriatric team
-
multidisciplinary staff
-
discharge planning
-
family‐centred discharge
-
linkages between ed and homecare services
-
physical environment and design
-
furniture and equipment
-
educational sessions
-
quality improvement
-
administrative data monitoring.
Again, three reviewers classified the reviews and then we discussed and agreed via consensus which of the subscales were represented in the reviews.
Results
The searching and screening in 2018 resulted in the inclusion of 18 reviews and 3 conference abstracts (Figure 1). A list of reviews excluded (and reasons) at full text are available but not included in this report. The 2021 searching and screening identified an additional 151 reviews, of which 6 were relevant, and are described below.
FIGURE 1.
Modified preferred reporting items for systematic reviews and meta-analyses (PRISMA).
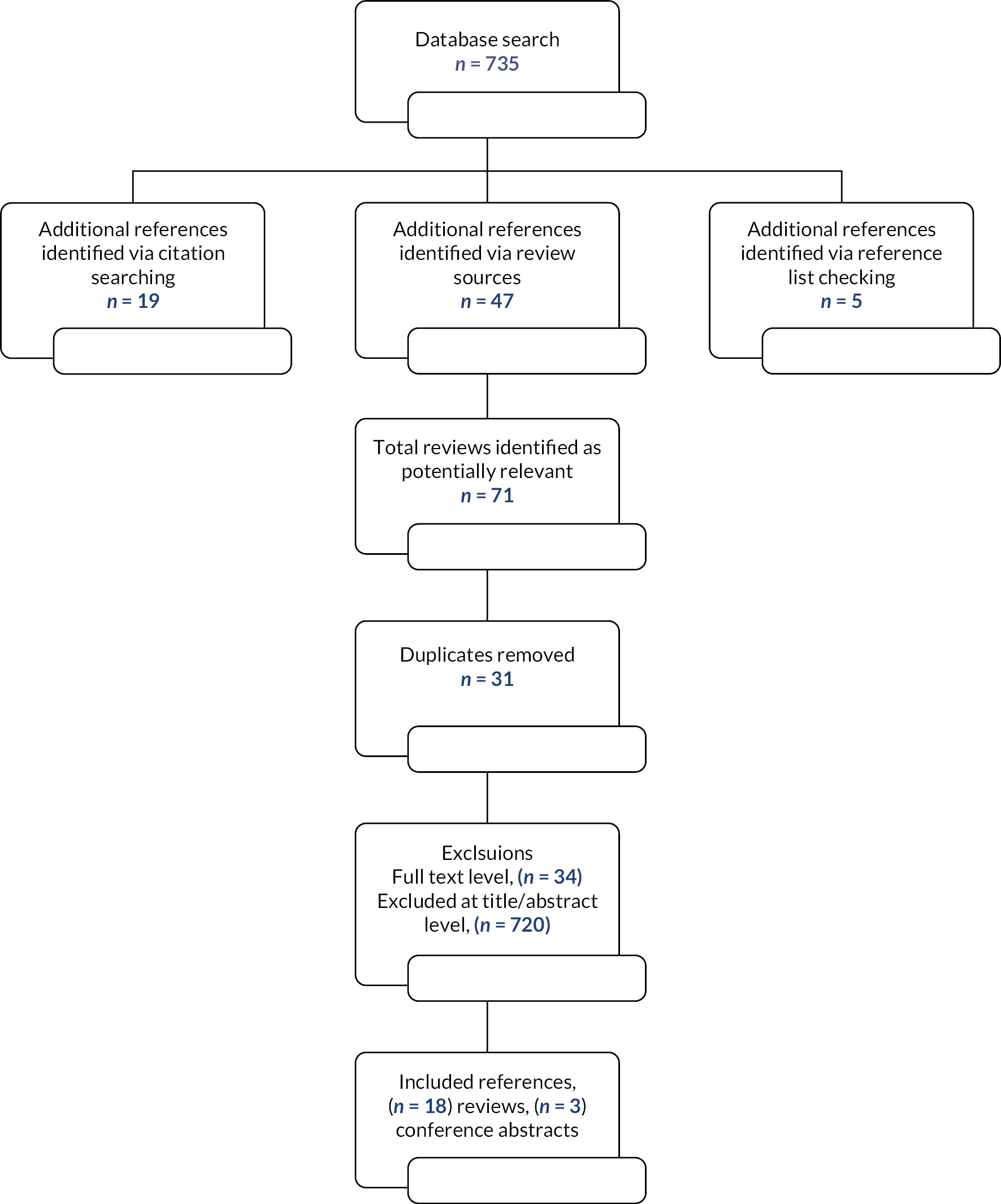
Of the 735 references originally identified in the database searches, LP screened 368 and JvO screened 367. To check the screening consistency of the two single reviewers, a third reviewer (SA) screened 50% of each of these two sets. There was moderate agreement between SA and JvO (κ 0.6, 95% confidence interval, CI, 0.48 to 0.71) and LP and SA (κ 0.6, 95% CI 0.37 to 0.76). An additional reviewer (SC) checked all references where there was disagreement or uncertainty. For the 2021 searches, LP screened titles, and LP, JvO and SC all screened any articles that had passed title screening.
Characteristics of included reviews
A total of 18 reviews and three conference abstracts were included in the review of reviews. The date range was 2004 to 2019, with primary papers included in the reviews dating from 1984 to 2018. None of the reviews limited the papers by geographical setting. Not all the papers in all the reviews were included; where evidence related to screening for frailty or frailty related syndromes, these papers were excluded. The range of (relevant) papers included in reviews was 2–28 and there were 128 unique papers included in the 18 reviews. None of the conference abstracts reported bibliographic details of included studies.
Six supplementary reviews were identified in the 2021 searches.
Methods
The methods adopted by the review authors are shown in Table 3. Shaded reviews used meta-analysis. Three reviews undertook qualitative meta-synthesis. 20–22
| Author | Description of method | Method of synthesis of primary studies | Study types included in the review |
|---|---|---|---|
| (Burkett et al.)23 | Systematic review | Narrative/tabular synthesis | All study types |
| (Conroy et al.)24 | Systematic review | Quantitative synthesis including meta-analysis | RCTs |
| (Fan et al.)25 | Literature review | Narrative synthesis of quantitative data | Any experimental or observational |
| Fealy et al.)26 | Systematic review | Narrative synthesis of quantitative data | Clinical trials, before-and-after designs and descriptive-evaluative studies |
| (Graf et al.)27 | Systematic review | Narrative synthesis of quantitative data | Re CGA: RCTs or matched controlled trials |
| (Hastings and Heflin)28 | Systematic review | Narrative synthesis of quantitative data | RCTs, non-RCTs and observational studies |
| (Hoon et al.)20 | Systematic review | Narrative synthesis of quantitative results. | All study types |
| (Hughes et al.)29 | Systematic review | Narrative synthesis and meta-analysis | Randomised or quasi-experimental study types. |
| (Jay et al.)30 | Systematic review | Narrative synthesis of quantitative data | RCTs, non-RCTs, and observational studies |
| (Karam et al.)31 | Systematic review | Narrative synthesis of quantitative data | Only included studies which used a comparison group |
| (Lowthian et al.)32 | Systematic review and meta-analysis | Quantitative synthesis of quantitative data including meta-analysis | RCTs, quantitative studies |
| (Malik et al.)33 | Systematic review | Quantitative synthesis of quantitative data including meta-analysis | RCTs, multicentre and observational studies |
| (McCusker and Verdon)34 | Systematic review | Narrative synthesis of quantitative data | RCTs, non-RCTs, before and after studies and cross-sectional studies. |
| (Parke et al.)35 | Scoping review | Narrative synthesis of quantitative data | Systematic review, meta-analysis, clinical trial, cohort study, evaluation study |
| (Pearce et al.)21 | Systematic review | Narrative synthesis of quantitative data and meta synthesis of qualitative data | Quantitative research study designs as well as narrative opinion and text |
| (Schnitker et al.)36 | Systematic literature review | Narrative synthesis of quantitative data | ‘Research based literature’ |
| (Shankar et al.)22 | Systematic review | Meta ethnography | Survey, questionnaire, focus groups or individual interviews |
| (Sinha et al.)37 | Systematic review | Narrative synthesis of quantitative data | RCT, non-RCTs, observational studies. |
Overview of included reviews
Population
The age threshold for older people adopted by the reviews tended to be aged 65 years and older, although Fealy et al. 26 and McCusker and Verdon34 included papers with age 60 years and older. Some reviews did not report a specific age but rather reported interventions for participants who were ‘older’ or ‘elderly’. Most reviews reported ED care for a general population of older people, not stratified by condition or severity. However, Schnitker et al. 36) and Parke et al. 35 both reported evidence on interventions for older people with cognitive impairment. Lowthian et al. 32 described the population of their review as ‘high risk’. This may indicate that there was some prior screening of patients before they were included in the intervention. Graf et al. 27 compared screening with intervention against intervention alone; however, this former group of papers were not included as the screening process meant that the intervention was targeted.
Interventions
Ten reviews focused on specific interventions, including discharge focused interventions19,24,28,31,32 and interventions led by specific staff. 21,26,27,30,33 Four reviews looked at measuring and classifying ED care for older people by identifying and assessing indicators for quality care23 and by describing core components of successful interventions. 25,29,37 Two reviews reported the views of older people about what constituted quality emergency care22 and the experiences of older people of the care they received. 20 Finally, two reviews looked at a variety of ED and non-ED interventions and outcomes for a specific population group (older people with cognitive impairment). 35,36
Most reviews reported interventions delivered by ED consultants, consultant geriatricians working within the ED and nurses (with or without an advanced role). There was also evidence of wider multidisciplinary team-led interventions, which included occupational/physical therapists, discharge co-ordinators, social workers, physiotherapists, and health visitors.
The review of reviews inclusion criteria stated that for interventions to be included in the review, they needed to be either initiated or wholly delivered within the ED. The discharge interventions reported in this review tended to include follow-up of patients after discharge, by ED or community/primary care professionals, although these were often reported incompletely. Only 5 of the 18 reviews reported interventions delivered exclusively within the ED.
Overview of included conference abstracts
Three conference abstracts were included in this review. Cherian et al. 39 reported interventions to enhance EDs to be more suitable for older patients. Gupta et al. 40 described a review of geriatric trauma protocols and interventions. Tran et al. 41 examined interventions for patients at high risk following discharge from the ED.
Synthesis
There was a high degree of heterogeneity in the review methods and reporting across the 18 reviews included in the review of reviews, so meta-analysis was not possible. Instead, data were reported as:
-
summary tables, reporting intervention descriptions and outcomes reported (where a statistically significant positive effect was measured)
-
narratively within evidence clusters designed to gather evidence by intervention type.
Summary tables
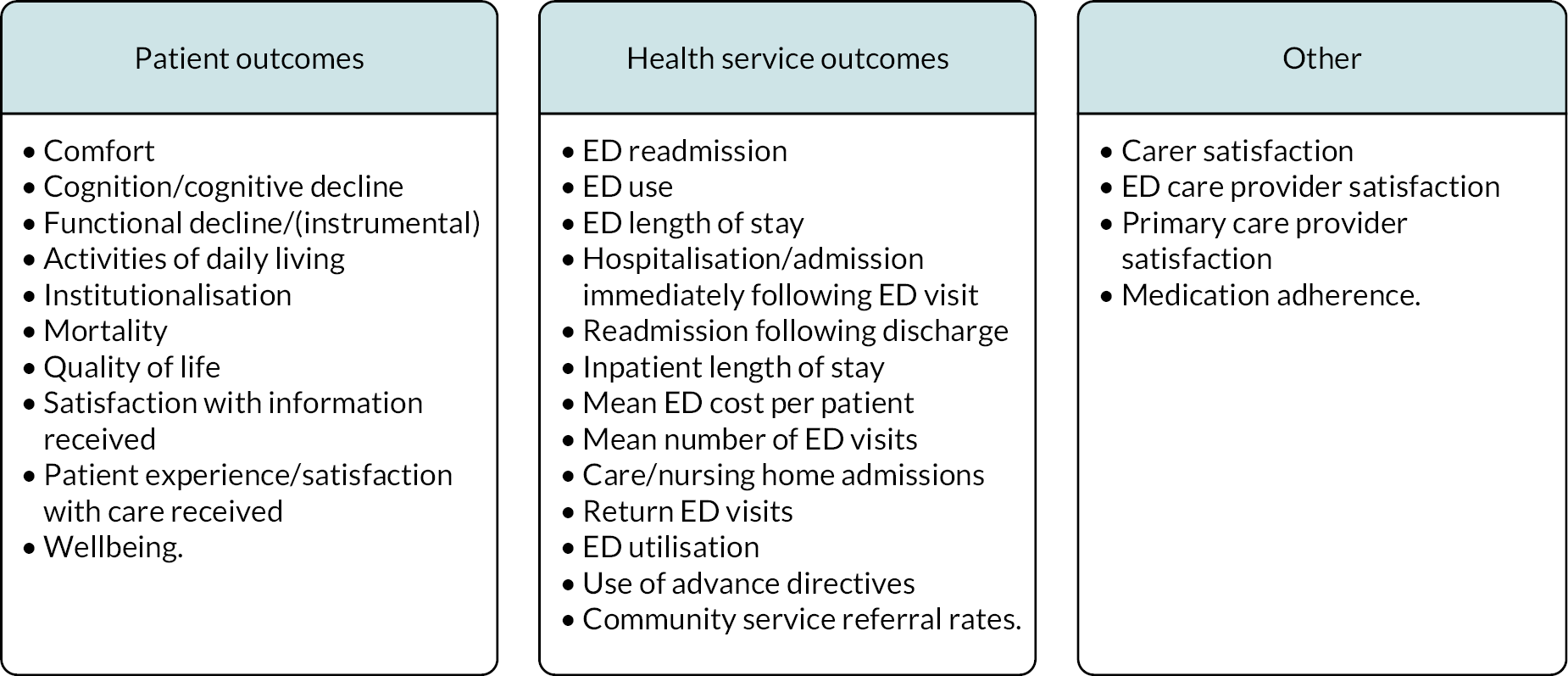
These tables report intervention descriptions (by review) and any statistically significant outcomes reported in the review, with the number of studies in which the outcome occurs. Table 4 contains data from reviews where results were reported narratively for patient outcomes. Table 5 contains from reviews where results were reported narratively for health service outcomes and Table 6 contains data from reviews where meta-analysis was undertaken. It is important to note that this table does not account for overlap across the reviews in terms of the primary studies included.
| Intervention description | Review | Outcome (positive +, negative –. mixed, no effect) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduced functional decline | Reduced dependence in IADLs | Increased satisfaction with information received | Reduced cognitive decline | Patient satisfaction | Increased medication adherence | Increased wellbeing | Mortality | Advanced directives | Caregiver satisfaction | ||
| CGA (nurse-led) | Graf et al.27 | + (4 studies) | |||||||||
| Discharge interventions | Hastings and Heflin28 | + (2 studies) | + (1 study) | + (1 study) | + (1 study) | + (1 study) | + (1 study) | + (1 study) | |||
| Sinha et al.37 | + (1 study) | + (1 study) | + (1 study) | + (1 study) | + (1 study) | + (1 study) | + (1 study) | + (1 study) | |||
| General interventions | Hughes et al.29 | + (4 studies) | |||||||||
| Intervention description | Review | Outcome (positive +, negative –. Mixed, no effect or unreported N/A) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduced admission rates | Reduced LoS | Reduced readmission rates | Reduced ED revisits | ED use | Reduced nursing home admissions | Service use | Community service referral rates | Costs | ED care provider satisfaction | Primary care provider satisfaction | ||
| CGA (consultant led) | Jay et al.30 | + (5 studies) | ||||||||||
| CGA (nurse-led) | Graf et al.27 | + (3 studies) | + (1 study) | |||||||||
| Discharge intervention | Karam et al.2 | + (5 studies) | + (2 studies) | |||||||||
| Hastings and Heflin28 | + (1 study) | + (1 study) | ||||||||||
| Sinha et al.37 | + (1 study) | + (1 stud)y | +(5 studies) | + (1 study) | + (4 studies) | + (1 study) | + (1 study) | + (1 study) | + (1 study) | |||
| Various (to reduce ED revisits) | McCusker and Verdon34 | |||||||||||
| Intervention description | Review | Outcome (positive +, negative –. Mixed, no effect or unreported N/A) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduced admission rates/hospitalisation | Reduced LoS | Reduced readmission rates | Reduced ED revisits | Reduced functional decline | Reduced Nursing home admissions | Reduced Institutionalisation | Reduced Mortality | Improved quality of life | Improved cognition | ||
| Discharge interventions | Conroy et al.24 | No effect (5 studies) | No effect (5 studies) | No effect (5 studies) | No effect (5 studies) | No effect (5 studies) | No effect (5 studies) | ||||
| Lowthian32 | No effect (5 studies) | No effect (5 studies) | No effect (5 studies) | ||||||||
| CGA (nurse-led) | Malik et al.33 | No effect (5 studies) | No effect (2 studies) | No effect (5 studies) | No effect (5 studies) | ||||||
| CGA (nurse-led) with primary care links | Malik et al.33 | No effect (2 studies) | + (2 studies) | ||||||||
Table 4 shows that reduced functional decline was the most frequently reported outcome, although in Table 5 it appears that most of the positive intervention effects were for health service outcomes.
Reviews which undertook meta-analysis24,32,33 reported on a variety of patient and health service outcomes but did not identify any statistically significant effects on outcomes as a result of interventions with the exception of Malik et al.,33 who reported reduced readmission rates.
Narrative synthesis
Table 7 describes the five evidence clusters that were developed from the review findings. These were developed iteratively and grouped thematically;42 they are presented narratively, and are summarised according to their reported effectiveness. This does not reflect the primary studies but on the strength of the findings as reported at review level.
| Cluster | Topic | Papers included |
|---|---|---|
| 1 | Staff-led interventions | Pearce et al.,21 Fealy et al.,26 Graf et al.,27 Jay et al.,30 Malik et al.,33 |
| 2 | Discharge-focused interventions | Conroy et al.,24 Hastings et al.,28 Karam et al.,31 Lothian et al.,32 McCusker et al.34 |
| 3 | Population-focused interventions | Parker et al.,35 Schnitker et al.36 |
| 4 | Patient views on ED care | Hoon et al.,20 Shankar et al.22 |
| 5 | Reviews reporting components/characteristics of successful interventions and indicators of quality care for older people in the ED | Burkett et al.,23 Fan et al.,25 Hughes et al.,29 Sinha et al.37 |
Reviews reporting interventions delivered by specific staff
Four reviews examined interventions delivered by nurses for older people within the ED21,23,26,27 and one review examined interventions delivered by consultant geriatricians. 30 For the nursing interventions, the range of included studies in reviews was 2–11 and there was a total of 15 studies from which findings were generated (7 studies were reported in more than 1 review). Graf et al. 27 also included studies reporting ED screening tools that were not part of an intervention, and these were excluded from this review. Fealy et al., Graf et al. and Pearce et al. 21,26,27 narratively summarised quantitative data, whereas Malik et al. 33 undertook narrative synthesis and meta-analysis of nine included studies across four outcomes (hospitalisation, readmissions, length of hospital stay and ED revisits).
Jay et al. 30 undertook narrative synthesis, as a meta-analysis was considered inappropriate due to heterogeneity of study design, population and interventions.
For the nurse-led interventions, Fealy et al. 26 described 11 interventions with nurse assessment, post discharge referral, patient education and follow-up. Graf et al. 27 described eight nurse-led CGA interventions, including follow up. Malik et al. 33 reported three different types of nurse intervention assessment using risk screening, CGA and nurse-led case/discharge management. The interventions reported by Pearce et al. 21 were limited to nurse-led interventions to enhance patient comfort in the ED, mainly relating to equipment.
The narrative summary of primary studies in the systematic review by Fealy et al. 26 reported reduction in service use across five studies and functional improvements across three studies; three studies found no effect. There was evidence of reduced service use in the ED being associated with increases in primary care use. The suggested characteristics of effective interventions included preintervention screening and better links with home care (as opposed to stimulating health service demand via primary care).
Graf et al. 27 reported that nurse-led CGA was effective in functional improvements. There was varying evidence on ED readmissions (both reduced and increased admissions) and potentially also nursing home admissions. Three studies found no effect. These negative findings were attributed partly to study design limitations.
The meta-analysis by Malik et al. 33 of nine studies across four outcomes found that nursing interventions did not have a significant statistical impact on any of the four domains (hospitalisation, readmissions, length of hospital stay and ED revisits). This study did not examine functional decline. Malik et al. 33 contrasted these findings with previous reviews, which are also reported in this review of reviews26,28,31 and which demonstrated both reduced service use as a result of these interventions, and also that ED risk screening led to reduced hospitalisation and nursing home admissions. These inconsistencies are attributed to methodological weaknesses in study designs, supporting an agenda for additional research on interventions which extend from the ED to the community.
Pearce et al. 21 identified only two studies which looked at patient focused outcomes. These indicated that both warming blankets and seating position had a positive impact on patient comfort and wellbeing. They noted the paucity of research around patient-centred outcomes such as nutrition, hydration and communication from a nursing perspective.
Jay et al. 30 reported statistically significant findings in terms of reduced admissions rates (which ranged between 2.6% and 9.7%). The evidence for length of stay and readmission rates was mixed. Several their included studies also reported changes in admissions rates for the control groups, indicating that CGA may have altered culture and practices around the risks of admission versus discharge.
In summary, there is contradictory evidence around the benefits of nurse-led interventions for older people in the ED. Some reviews report improved outcomes in terms of reduced service use and reduced functional decline (although there is also evidence of increased service). The strongest evidence, in the form of meta-analysis, found no effect from nurse-led interventions. In terms of consultant led CGA interventions, there was evidence of lowered admission rates. There is a common theme of methodological limitations reported across studies which limit the conclusions drawn.
Reviews reporting interventions delivered at specific points in the patient emergency department episode (and beyond)
This cluster contains five reviews. 19,24,28,31,32 These contained a range of 5–14 primary studies with 25 primary papers included, 9 of which appeared in more than 1 review and 1 of which appeared in all 5 reviews. 43 Publication dates ranged from 2004 to 2015, including primary studies from 1996 to 2013. The reviews by Conroy et al.,24 Hastings and Heflin28 and Lowthian et al. 32 were focused on the ED, whereas a subset of papers from the reviews by Karam et al. 31 and McCusker et al. 19 are reported here (as they also reported interventions initiated or delivered outside the ED).
Conroy et al. 24 focused on interventions delivered within 72 hours of ED attendance. These were CGA interventions delivered either by nurses or geriatricians and were targeted at older people with living with frailty. The review by Hastings and Heflin28 looked at evidence for interventions to improve outcomes for older people discharged from the ED; 14 of the studies reported by Hastings and Heflin28 were of interventions either initiated or concluded in the ED. A wide variety of interventions were reported, from CGA to single screening and assessment interventions, delivered by single practitioners or multidisciplinary teams. Karam et al. 31 limited inclusion criteria to interventions delivered within the ED and including CGA and other intervention types. Lowthian et al. 32 reported on discharge interventions in the form of community transition strategies (CTS) from the ED. All the CTS included geriatric assessment but this was undertaken by a variety of different staff (nurses, allied health professionals or health visitors). Follow-up interventions consisted of referral to community services or direct linkages, including telephone/general practitioner (GP) follow-up. In the review of interventions to reduce ED visits by McCusker and Verdon,34 9 of the 18 primary studies included were delivered in the ED. All these interventions had an ED and post-discharge component.
Outcomes were reported using meta-analysis24,32 and narrative synthesis. 28,31,34 Conroy et al. 24 found no clear evidence of benefit for CGA discharge interventions across all outcomes included in the review. Hastings and Heflin28 reported findings at the level of individual studies only across a wide variety of outcomes. Karam et al. 31 developed themes for intervention types (referral, follow-up, integrated model of care) and identification of study participants (risk screening or no risk screening). They found that the most effective interventions extended beyond referral and used a clinical risk prediction tool to identify those who would most benefit from the intervention. In the review by Lowthian et al.,32 four of the nine studies were included in a meta-analysis, which found no benefit of interventions in terms of ED reattendance, mortality and emergency hospitalisation. Individual studies were effective in reducing ED reattendance and nursing home admissions; Lowthian et al. 32 attributed this potentially to the methods of telephone follow up of discharged patients. The review by McCusker and Verdon34 found that there was limited evidence of benefit of discharge interventions (two studies of borderline statistical significance) on ED visits, with evidence of short-term increases in ED visits.
In summary, discharge interventions vary in their components but tend to employ improved linkages between the ED and the community, either through direct linkage or referral interventions. CGA is frequently used and is delivered by a variety of staff. There is limited evidence for the effectiveness of these interventions; two meta-analyses found no benefit and narrative synthesis demonstrated an increase in ED readmissions in patients who had received these interventions in the short term.
Reviews reporting interventions for patients with specific conditions
This cluster contained two reviews,35,36 both of which looked at interventions and best practice for older people with cognitive impairment in the ED. Findings were generated from a total of 27 primary studies. Four papers were included in both reviews. Schnitker et al. 36 also included evidence on the management of older people with cognitive impairment in acute care settings but these have been excluded from this summary. Both reviews undertook a narrative synthesis of qualitative and quantitative evidence. Schnitker et al. 36 generated themes from the evidence base. Neither review presented results in a format that could be extracted for the summary table of interventions and outcomes.
The interventions reported were around identification and management programmes dealing with the specific needs of older people with cognitive impairment. Parke et al. 35 also reported staffing interventions (team and individual changes to service delivery and staff training). Neither review reported patient or health service outcomes. Both reviews describe intervention characteristics that report positive outcomes but do not report the outcomes themselves. Both reviews summarised that interventions are not well represented or described within the ED literature but that there is more evidence from acute care, although transferability of these interventions to the ED is not well understood.
In summary, there was limited evidence which could not be synthesised. No conclusion was possible around ED interventions for older people with cognitive impairment.
Reviews reporting patient views and experiences of their care in the emergency department
This cluster contained two papers findings were generated from 28 papers. All the five papers in the review by Hoon et al. 20 were also included in the Shankar et al. 22 review. This review had a wider scope, including preferences and views of emergency care as well as patient experiences, to which the review by Hoon et al. 20 was limited. Hoon et al. undertook their review according to the methods of the Joanna Briggs Institute. Neither of the reviews presented results in a format that could be extracted for the summary table of interventions and outcomes.
Both reviews undertook qualitative synthesis to generate themes/findings. There was some commonality between these findings. Both reviews reported on the negative experience of long waiting times for older people and on the physical care needs of this population, especially in relation to pain management. The content of communication and information received by older people was also important for patient experiences and quality patient care. The review by Hoon et al. 20 commented that health-care professionals should be more aware of the positive attitude of older people to the care that they are receiving and made recommendations on changes to the physical environment that might help. Shankar et al. 22 also reported that simple changes to physical infrastructure can make a big difference to pain and physical comfort. Another important quality indicator for older people was the leadership role that health-care professionals play in ensuring quality care. 22
Summary – the total overlap between the papers in these two reviews has generated a consistent evidence base regarding patient views of their care and what represents quality care for older patients. Repeating messages are around waiting times, patient-provider-family communication, and physical adaptations to better meet the needs of older patients.
Reviews reporting components/characteristics of successful interventions and indicators of quality care for older people in the ED
The reviews by Burkett et al.; Fan et al.; Hughes et al.; Sinha et al. 23,25,29,37 all took a broader view of the care of older people within the ED. Although in some reviews25,29,37 the effectiveness of interventions was reported, this evidence was enhanced by a consideration of the key components or elements of effective interventions as follows:
-
core operational components of interventions and the role of these components in the success of interventions37
-
key elements of effective interventions25
-
the methodological quality of quality indicators of ED care23
-
intervention components and intervention strategies adopted. 29
Fan et al.,25 Hughes et al. 29 and Sinha et al. 37 included a range of 15–20 papers in their reviews. The primary studies in the review by Fan et al. 25 also related to community interventions and wider hospital interventions, so a subset of papers was reported. There were 38 papers included in these 3 reviews, with 13 of these 38 included in 2 or all the 3 reviews.
In terms of intervention effectiveness, the case management interventions reported by Sinha et al. 37 were reported as having positive effects (not statistically significant) on satisfaction levels, reductions in ED reattendances, reductions in admissions (immediate and longer term), decreases in inpatient and nursing home admissions. Fan et al. 25 and Sinha et al. 37 found negative results in terms of a small but significant negative effect on ED reattendances37 and higher ED use. 25 There was a statistically significant outcome of lowering ED use or length of stay in 5 of 20 studies examined by Fan et al. 25 Hughes et al. 29 found a small positive effect of ED interventions on functional status.
Burkett et al. 23 included 61 sources of evidence for quality indicators, of which 20 were included in peer reviewed journal articles. The 50 indicators of quality care identified related to ED processes rather than outcomes and were cross cutting. Quality, as measured using a bespoke assessment tool ranged from 39% to 67% according to specific tools.
Table 8 reports the key intervention components/elements and strategies derived from the three reviews, associated with interventions that the reviews had found to be effective (shading indicating overlap across the three reviews).
| Fan et al., 201525 | Sinha et al., 201137 | Hughes et al., 201929 |
|---|---|---|
| Multidisciplinary team and gerontological expertise | Interprofessional and capacity building work practices | |
| Integrated social and medical care | Multi strategy interventions | |
| Risk screening and geriatric assessment | High risk screening Focussed geriatric assessment |
Assessment |
| Care planning and management | Case management | |
| Discharge planning and referral coordination | Initiation of care and disposition planning in the ED | ‘Bridge’ interventions (contact before and after discharge) |
| Follow up and regular group visits | Post ED discharge follow-up with patients | Referral plus follow-up |
| Evidence based practice model | Establishment of evaluation and monitoring processes | |
| Nursing clinical delivery involvement or leadership |
In summary, there was considerable agreement across three reviews of the components of successful interventions, which should (1) integrate strategies for social and medical care involvement (2) Include screening and assessment (3) initiate care in the ED and bridge this with follow up (4) monitor and evidence successful interventions. Indicators for quality care tend to focus on care processes rather than structures or outcomes and are lacking in evidence and limited in testing.
Conference abstracts
Cherian et al. 39 looked at evidence for the implementation of a geriatric ED model and found eight different components that were related to outcomes, namely structural enhancements, operational enhancements, provider education, quality improvement, coordination of hospital resources, coordination of community resources, staffing and patient satisfaction. The review by Tran et al. 41 reported interventions to prevent ED return visits following discharge. Six interventions were identified which reported ED returns as outcomes. Limited reporting described these interventions as bundles of care (screening within the ED, home visits and referrals); one study found improved outcomes because of the intervention, while five of the six studies did not report any reduction in ED return visits. The final abstract40 reported a review of trauma protocols for geriatric patients in the ED and found that geriatric assessment within the first 24 hours of presentation was critical for patient outcomes. They reported improved outcomes (length of stay, readmission, and functional status) associated with appropriate pain management, assessment of cognition, assessment of function and assessment of psychosocial issues.
Additional reviews identified in September 2021
Gettel et al. reviewed 17 ED care transition intervention studies. 44 They found that common care transition interventions included coordination efforts, CGAs, discharge planning, and telephone or in-person follow-up.
Elliott et al. 45 identified 25 trials (13 RCTs and 12 quasi-experimental) assessing interventions supporting discharge of older people to their home from the ED. They reported a trend to reducing ED reattendances associated with ‘focussed elder discharge health interventions’.
Leaker et al. 46 reviewed the impact of geriatric emergency management nurses on the care of older people living with frailty in the ED. The main finding was a reduction in ED reattendances.
Berning et al. 47 reviewed interventions to improve older people’s experience in the ED. Department-wide interventions, including geriatric EDs and frailty units, focused care coordination with discharge planning and referral for community services, were associated with improved patient experience. Providing an assistive listening device to those with hearing loss and having a pharmacist reviewing the medication list showed an improved patient perception of quality of care provided.
van Oppen et al. 48 reported upon what older people want from emergency care, concluding that capturing individuals’ preferred outcomes could improve person-centred care.
Hughes et al. 29 reviewed ED interventions for older people, concluding that studies assessing two or more intervention strategies may be associated with the greatest effects on clinical and service outcomes.
Overall, these more recent reviews did not materially impact upon our initial findings; there were no additional empirical data that could be used in the system dynamics model.
Study assessment
A Measurement Tool to Assess Systematic Reviews, Version 2
The methodological quality of the reviews reporting either randomised or non-randomised studies of interventions was assessed for 15 of the 18 included reviews using AMSTAR2 (see Appendix 3).
Joanna Briggs Institute tool for systematic reviews
The methodological quality of the reviews reporting non-interventional papers was assessed for 320,22,23 of the 18 included reviews using the Joanna Briggs Institute tool for systematic reviews. Of these three reviews, two had generally good quality assessment, which the third was lacking. There was limited evidence that any of the reviews had considered publication bias in their assessments.
Applicability of evidence to the UK setting
Although much of the primary research summarised in the reviews emanated from North America, it is likely that the review findings will apply to UK settings. Older patients with urgent care needs will present similarly in North America compared with the UK (e.g. high prevalence of non-specific presentations). The interventions were broadly holistic in nature, consistent with the international literature supporting CGA as an intervention to improve outcomes in older people with acute care needs. Finally, the outcomes described as important are consistent with the outcomes valued by older people in the UK, as well as the service outcomes being valued by providers and commissioners of care and consistent with the International Consortium for Health Outcomes Measurement report. 38 It is therefore likely that the review findings will be relevant to the delivery of urgent care for older people in the UK.
Taxonomy
To address the research objective of developing a taxonomy of models of care, as reported in the introduction, the McCusker et al. 19 Elder Friendly Emergency Department Quality Assessment Tool was used to identify whether the reviews included in our review of reviews included evidence of interventions related to any of the 13 subscales of the tool. Use of this tool to classify the evidence allows us to describe which areas are more likely to have interventions relating to them (Table 9). The most frequently occurring subscales were interventions relating to multidisciplinary staff, with standardised assessment tools, two‐step screening, geriatric team, discharge planning and linkages between ED and homecare services also occurring in either 11 or 12 reviews. The remaining subscales were mentioned in few of the reviews, although there was often mention of issues relating to these in the background or discussion sections of reviews as being important, particularly to patients.
| McCusker subscales, in order of frequency in reviews | Total number of reviews in which subscale reported |
|---|---|
| Administrative data monitoring | 0 |
| Furniture and equipment | 1 |
| Quality improvement | 1 |
| Family‐centred discharge | 2 |
| Physical environment and design | 2 |
| Clinical care protocols | 3 |
| Educational sessions | 3 |
| Standardized assessment tools | 11 |
| Two‐step screening | 12 |
| Geriatric team | 12 |
| Discharge planning | 12 |
| Linkages between ED and homecare services | 12 |
| Multidisciplinary staff | 14 |
Discussion
The 18 reviews and 3 conference abstracts that met our inclusion criteria have demonstrated a diverse range of interventions, with varied outcomes, measured across different time periods and focussing on both individual patient and wider health service outcomes.
The evidence base is broad, with 128 primary studies being included across the 18 reviews, with the vast majority being included in only one review, but with a substantial number being included across multiple reviews, despite the review topics and titles not indicating substantial overlap. This overlap demonstrates the diverse ways in which interventions can be reported: by the health-care professional who is delivering the intervention, the type of intervention, when in the patient journey the intervention is being delivered and whether the intervention links multiple services, professionals or settings together.
As a result of the inconsistent reporting across reviews, the clusters that we have developed have significant overlap, for example an RCT of CGA with a multidisciplinary team follow-up post ED visit was reported in ten reviews across four of the seven clusters, indicating overlap between the reviews themselves and the evidence-based clusters that we developed.
The evidence base in terms of primary studies and reviews is extensive. The interventions developed and evaluated for this population in the ED setting are numerous. However, the evidence for their effectiveness is unconvincing and the messages around further research using experimental study designs is consistent.
In terms of effectiveness, the four reviews that undertook numerical synthesis of primary studies24,29,32,33 all found no or little evidence for the effectiveness of interventions across a broad range of patient and health service outcomes. The review by Malik et al. 33 was limited to nursing interventions; the other three reviews were more general, although Conroy et al. 24 and Lowthian et al. 32 focused on discharge interventions. Across these reviews, there was overlap in the 14 included studies – 2 primary studies were included in all 4 reviews and a further 3 primary studies were included in 2 or more reviews.
The remaining 11 reviews of interventions were similarly equivocal in their findings with reviews reporting mixed findings on the effects of interventions, demonstrating seemingly negative effects such as increased service use, alongside evidence of no effect and evidence of effectiveness, with either clinical or statistical significance. The systematic and narrative reviews did report evidence of the effectiveness of interventions on some outcomes. Fealy et al. 26 found evidence in terms of reduced service use and reduced functional decline from nursing interventions and Jay found consultant led CGA (which was variously implemented) did reduce admission rates. 30 Looking at a wider range of ED interventions, Graf et al. 27 found evidence for CGA in reducing functional decline and ED readmissions. Hastings and Heflin28 found mixed results with some evidence for reduced functional decline and health service use.
There was no synthesis of cost data, although reviews did include evidence from three studies that looked at the costs and cost effectiveness of interventions, so analysis would have been possible.
There was limited evidence for factors that influence the implementation of these interventions. The nature of the type and urgency of care delivered within the ED is a theme that runs throughout the included reviews as a challenge to developing and measuring interventions, but this is common to all the interventions that are either delivered or initiated within the ED. Evidence on implementation may more readily be extracted from the primary studies included in these reviews.
Additional evidence was reported from the four reviews23,25,29,37 which sought to develop overarching descriptions of effective interventions. Across these reviews, there were several areas of commonality that demonstrate where interventions may best be focused. However, as this list of descriptions of interventions demonstrates, most of them encompass delivery of care both within an outside the ED and care delivery in the acute setting is not limited to ED staff only:
-
interprofessional work practices/teams
-
integrating social and medical care
-
geriatric assessment including screening
-
case management
-
discharge planning with contact after discharge
-
post discharge referrals or follow ups
-
evaluation and monitoring/evidence-based practice.
The list of 26 outcomes reported across the 18 papers included in our review of reviews was broadly in line with the core outcome measures as reported in the review by Hastings and Heflin. 37 This agreement indicates that our included reviews measured a wide variety of outcomes, as reported in the primary studies, rather than restricting reporting to specific outcome measures.
The McCusker et al. 19 tool has allowed us to look at the interventions and consider their relative importance in terms of which types of interventions have been tested. This process highlights the findings within our reviews on CGA (screening and assessment interventions), discharge planning and follow-up and the importance of the staff delivering care.
Adopting a review of reviews methodology necessitates that the unit of analysis is each review, rather than the primary studies that are contained within that review. Therefore, the confidence that we can take from review findings depends upon authors’ interpretations of the evidence. Within some of the included systematic reviews, primary studies were organised by intervention type or by outcome, whereas other reviews reported studies sequentially, which makes analysis and reporting of the overall review unit challenging.
There is little consistency in how individual studies have been classified; for example, the same study appears in reviews both reporting discharge interventions and staff interventions. This is a limitation of the reviews and a limitation of the way in which we have chosen to organise the data.
The summary tables of outcomes and interventions are limited. It is not possible to map the primary studies to the outcomes reported. We are also reliant on the review authors to have determined whether an intervention is statistically significant. There was little reporting of clinical significance, so this has not been included in these tables.
The literature reported here is predominantly US focused. This is not unusual but does present challenges in terms of our interpretation of the evidence; for example, the promising McCusker et al. 19 tool, which reports elements of interventions, includes reference to linking together services that are not delivered by a single provider, such as the NHS.
Conclusion
This review of reviews found a diverse but overlapping evidence base supporting a range of interventions. We have sought to develop typologies of effective interventions to guide implementation of interventions to improve the care of older people in the ED and after discharge from the ED. However, there was no consistent typology found in the literature that served this purpose. There was evidence that interventions initiated within the ED and continued into the community or primary care setting had better outcomes than those delivered in the ED alone. However, this does not represent a model of care or care pathway as described in our review protocol.
A wide range of tested interventions were identified across the 18 reviewed reviews. There was inconsistency in how these were reported whether by the clinician delivering the intervention or the point in the patient journey where it was being delivered. The evidence on outcomes of these interventions was inconclusive.
Using the review as a unit of analysis limits the extent to which conclusions can be drawn on the effectiveness and outcomes of specific interventions. Perhaps the most useful outcome from this work is the classification of existing interventions using the McCusker et al. 19 framework, which has allowed a summary of the strengths and weakness of the evidence base. This has also highlighted the importance of multifaceted and holistic assessments, but also the important absence of evidence for patient-reported outcome measures (PROM) being fed-back into the system.
Chapter 3 Patient and carer preferences
Sections of this chapter have been reproduced from Phelps et al. 49 with permission.
The aim of this interview study was to create an understanding of what ‘good looks like’, adding a strong patient perspective to the literature described in Chapter 2, which contained relatively little on patient priorities and outcomes. The patient perspectives were also used to inform the implementation toolkit (Chapter 5) and the modelling exercise detailed in Chapters 6 and 7.
We report patient experience and patient outcomes or preferences separately, although the data were ascertained using the same methods and participants. We understand that there is a significant overlap between experience and outcomes, particularly in older people living with severe frailty. 38 These individuals are typically in the last year or two of life, during which period, the experience of health care – being treated with dignity, comfort, and respect – can be as important if not more so that outcomes such as mortality or improving function. Indeed, previous authors have combined outcomes and experience in PROMs designed for older people. 50 However, for ease of reading, we have presented patients’ experiences in this section and outcomes in Patient reported outcomes below.
Patient experience
Introduction
Little is known about the experiences and preferences of older people for emergency care, even less for older people living with frailty. Reviews of existing evidence highlight waiting times, physical and psychological support alongside good communication and information provision in shaping the experiences of older patients in the ED. 20,22,48 Although existing studies included people with stereotypical markers of frailty, none used a validated frailty assessment. As such there is currently limited evidence about whether or how the presence of frailty impacts upon older people’s experiences and preferences for emergency care. 48 These needs must be understood and addressed to provide person-centred care.
Methods
Sampling and recruitment
Older people (≥ 75 years) and/or their carers with experience of emergency care were recruited from three EDs in England between January and June 2019. Recruitment was undertaken by experienced research nurses (sites 1 and 2) and clinical staff (site 3) using a purposive recruitment strategy which tried to reflect the population of interest according to: frailty, age, sex, ethnicity, cognitive impairment, place of residence, mode of arrival (ambulance or independent), whether seen in ‘majors’ or ‘minors’, different days of the week and different times of day as summarised below. Participants were included if they were at least mildly frail [≥ 5 on the clinical frailty scale (CFS)]. 51 Informed consent was taken by staff who recruited participants within 72 hours of patients’ entry to ED, with the majority being recruited on the day of their ED attendance.
Data collection
Data were collected via semistructured in-depth interviews using a topic guide informed by Chapter 213 and designed in collaboration with lay co-researchers. Interviews explored patients’ views on their recent experience of emergency care, their priorities and preferred outcomes. Where desired and possible, carers/relatives were also interviewed, alongside or separate from patients according to individual preferences.
In most cases, interviews (conducted by ER or KP) took place within 30 days of the patient’s ED attendance. Most took place in the patient/carer’s usual place of residence, typically their own home, but sometimes the family home, sheltered accommodation or care home. Four interviews, all with carers, took place via telephone at their request. Interviews were audio recorded and lasted approximately 60 minutes each.
Data analysis
All interviews were transcribed verbatim by a professional transcription service and analysed in NVivo (Lumivero, Denver CO) using the framework approach. 52 After reading the first transcripts, initially identified themes were used to generate a coding framework; transcripts were coded by two qualitative researchers (ER or KP). Themes generated during the analysis process were discussed and validated by wider members of the research team (SC and/or JvO) and our lay collaborators on a regular basis. Data collection and analysis were concurrent, with early findings directing further enquiries in interviews.
Patient and public involvement
The study was supported by the involvement of two lay collaborators (PR and JL) who provided advice on recruitment and helped in the design of topic guides and in the analysis. In addition, study updates were shared with the wider Leicester, Leicestershire and Rutland Ageing Patient and Public Involvement Research Forum on a quarterly basis, where useful feedback was provided.
Ethical approvals
The study was submitted to the London South East Research Ethics Committee for review which classified it as service evaluation. Accordingly ethical approval was granted by the University of Leicester’s Sub Committee for Medicine and Biological Sciences.
Results
Participants
In total, 40 participants were interviewed: 24 patients and 16 carers who, between them, described ED attendances for 28 patients across the 3 sites. Over two-thirds of patients (68%) were female; 43% were aged 75–84 years and 57% were aged over 85 years, including three aged over 95 years. The majority were white British; 12 had CFS scores of 5, 12 had CFS 6 and 4 had CFS 7. Table 10 shows demographic and ED attendance details for the patients whose ED experiences are described in the study.
| Site 1 | Site 2 | Site 3 | Total | |
|---|---|---|---|---|
| Sex: | ||||
| Male | 1 | 3 | 5 | 9 |
| Female | 7 | 9 | 3 | 19 |
| Age (years): | ||||
| 75–84 | 2 | 6 | 4 | 12 |
| ≥ 85 | 6 | 6 | 4 | 16 |
| CFS frailty score: | ||||
| 5–6 | 7 | 9 | 8 | 24 |
| 7 | 1 | 3 | 0 | 4 |
| Ethnicity: | ||||
| White British | 7 | 11 | 8 | 26 |
| White other | 1 | 0 | 0 | 1 |
| Asian | 0 | 1 | 0 | 1 |
| Black | 0 | 0 | 0 | 0 |
| ED attendance: | ||||
| Majors | 8 | 11 | 7 | 26 |
| Minors | 0 | 1 | 1 | 2 |
| Accommodation: | ||||
| Own home | 6 | 9 | 7 | 22 |
| With family | 2 | 1 | 1 | 4 |
| Care home | 0 | 2 | 0 | 2 |
| Travel to hospital: | ||||
| Ambulance | 8 | 11 | 7 | 26 |
| Independently/with family | 0 | 1 | 1 | 2 |
| Cognitive impairment: | ||||
| Yes | 1 | 1 | 3 | 5 |
| No | 7 | 11 | 5 | 23 |
| Attendance at ED: | ||||
| Weekday | 4 | 12 | 4 | 20 |
| Weekend | 4 | 0 | 4 | 8 |
| In hours (9 a.m. to 5 p.m. Mon-Fri) | 2 | 10 | 3 | 15 |
| Out of hours | 6 | 2 | 5 | 13 |
Over one-third of patients had fallen; other common conditions included breathing difficulties, heart problems, confusion and stomach or back pain.
Reluctance to attend the emergency department
Most participants were reluctant to be taken to ED. Reasons for this reluctance included previous poor experiences in hospital, only recently having come out of hospital and therefore not wanting to go back in, fear of ‘never coming home again’, fear of a specific hospital, fear of ‘picking up a bug’ and the belief that it would be a ‘waste of time’:
M I resisted it. Told them [the ambulance crew] that I’d gone through the same thing before. Anyway, I went in and my treatment there exactly duplicated the previous time.
I … So you said you resisted that.
M Not physically.
I But you just made it –
M Yeah, I made it clear that I didn’t want to go …
I … And why is it that you didn’t want to go in …?
M Because the last time I went in, they wasted so much time.
In the face of pain, distress and anxiety, initial feelings of reluctance and resistance about being taken to ED gave way to feelings of resignation in most cases. In this context, the ‘decision’ to take participants to ED cannot really be seen as a decision at all, certainly not one in which participants could engage in any meaningful way. Ultimately, most accepted the decision recommended by paramedics who were seen as ‘knowing best’. Family members had, in several cases, played a role in persuading patients that they needed to be taken to ED:
F So we had to convince my Mom that taking to the hospital.
I Why did she not want to go?
F At first she didn’t want to but then when she, when we mentioned that was as soon as the treatment is finished we can bring you back home.
I Right.
F Everything will be fine and she trusts us of course, so I went into hospital with her in the ambulance. [S2 08C]
Staff care and attitudes
Participants emphasised attitudes, manner and responsiveness, rather than technical competence, when reflecting on the staff they had encountered in ED. Overall, participants were very positive about ED staff who were seen as being very caring, reassuring and pleasant in their manner:
F1 I didn’t doubt for one minute they cared.
I Why did you not doubt it?
F1 I don’t know. They kept saying ‘You’ll be all right [name] in a little while’ and they were gently caring. They weren’t sort of saying ‘Well, right…’ and talking amongst themselves as much to say, ‘Well right’ you know ‘here comes another one through the machine sort of thing.’ But they weren’t. They were genuinely caring. [S1 07PC]
The importance of being treated with respect, particularly in the context of older age, was highlighted. Comments made by some indicate that they had not expected a positive experience (fearing that they would be treated negatively because of their age) but this had not been the case:
F1 I refer to older people, to my age group, normally I’d be the first to say ‘Her age. They haven’t got the patience.’ But having been in the last time, well four times, I can’t fault it. [S1 07PC]
A small number of participants, however, did feel that they had been treated negatively because of their age, describing what they saw as a lack of input or intervention during their time in ED/hospital:
I Right. Okay. It doesn’t sound like much happened whilst you were there?
F No. It didn’t. To be quite frank with you. They don’t want to know us old ones.
I Is that how you feel?
F That’s how I feel…
F Did you feel the same in every single part of the hospital that you went in?
F Yeah. They didn’t. If you were over 80 they didn’t want to know. [S2 06P]
A key concern related to staff being unresponsive when patients called for their attention. This was a particular issue for patients who needed assistance with toileting. While highlighted by a small number of patients, this issue had a significantly negative impact upon their ED experience, relating as it does to personal comfort and dignity. One patient described how upon requesting assistance to go to the toilet she was effectively told it was acceptable for her to soil herself as she was wearing incontinence protection:
M You get the impression because you were wearing counter measures
F Oh –
M Yeah, proper pants –
I Incontinence –
M It’s basically – it’s all right, we’ll sort it out later. Which isn’t very good for dignity.
I No, it’s not –
F And I was told 2 or 3 times, when I say I don’t like getting wet underneath – I was told 2 or 3 times oh it doesn’t matter, that’s what the nurses are for.
I Really?
F I was more or less told it’s quite alright if you do it in your pants.
Information and communication
The provision of information and effective communication between staff and patients were highlighted as crucial by patients and relatives during their time in ED. Being kept informed of what was happening (tests and treatment), what was likely to happen (i.e. admission, transfer or discharge) and some indication of what was wrong was absolutely key. Experiences, however, varied significantly. Some patients reflected positively: staff had kept them informed, explaining pending tests and procedures using a clear and friendly communication style. On the other hand, some participants described negative experiences concerning the provision of information and communication which had caused frustration and bewilderment, as they were left with little sense of what was going on:
I Did you feel that they were communicating with you what tests had been done and did you get any results back or -?
M Erm … no, I think it’s just that waiting thing that kills it. So my wife and daughter went off and then at eleven o’clock, two ambulance guys turn up ‘can you get your kit, we’re off to [name of another hospital].’
I At what point did you know you were going to be admitted, taken to the [name of other hospital], was that decided quite quickly or did that take quite a while?
M I think the system knew but I didn’t really…the information really doesn’t go to the patient until it’s happening I think, you know, […] the system seems to know what’s happening, but sometimes the patient doesn’t. [S2 01P]
Linked to the provision of information, some patients commented on the extent to which they had been involved in discussions and decision making about their care. Again, experiences were mixed. Some patients reported that they felt listened to and had participated in decision-making; others, however, had not felt involved in important decisions about their care:
I When did you know that you were going to be moved on to a ward?
F Not till the stretcher come. I said, ‘Where am I going?’ They said, ‘You’re going on a ward.’ I said, ‘What for?’ ‘Oh, we don’t know whether you broke your leg or not.’ Well if they didn’t know if I broke my leg or not who did know?
I So you had had an x ray?
F Yeah. But nobody ever says, ‘Oh your x ray is fine’ or this that and the other. No. Very badly done.
I So nobody asked you whether or not you wanted to go onto a ward?
F No.
I It was just kind of done without asking you?
F Without asking me. As though I’m an idiot. I know I’m 82 years but I’ve still got it all up here. [S2 06P]
Together with the provision of information, the way ED staff communicated and the language they used were important to patients and relatives while in ED. Some patients had been impressed by the way in which staff had introduced themselves and explained what they did. Others, however, were not always clear on these details and this was seen as an impediment to communication; patients were often unclear as to whom they had seen and should speak to with queries. A few patients commented that the various coloured uniforms on display in ED (with no explanation as to what these represented) added to the confusion:
I Yeah. So you’re in the A&E bit. How many different doctors did you see, do you know?
F I think I saw two different ones. And some nurses, you don’t know who’s who, to be honest, even when I was in hospital, I said to my husband ‘do you know, there’s so many different colours, you know –
I Uniforms you mean?
M Yeah.
F - one’s got green, one’s got blue and one’s got light blue, one’s got white, one’s got pink’ and so in the end I never knew who really was the Sister and who wasn’t, you know. [S3 05PC]
A few participants commented on the language and communication style used by ED staff; again, painting a mixed picture. Some had appreciated the clear, candid and honest language employed by staff when discussing their cases. Others, in contrast, stated that they could not always hear or understand what they were being told or that things were not always explained properly. Staff, they found, did not always take time to speak slowly and clearly to ensure that information was received and understood.
Environment and personal comfort
The ED environment created a significant impression upon participants’ experiences of emergency care. Several patients at one site drew attention to what they described as an overcrowded, noisy, and sometimes rather ‘chaotic’ ED environment:
F Yeah. And it was, you know, chaotic I thought.
I Just because of the number of people there or -?
F Yes, it was a huge amount of people there, seemed to be, you know, and there was somebody calling out ‘I want some biscuits, can you get me some biscuits I want some biscuits!’ and then she turned round and started to sing! And I thought I’ve landed in a mental home! [Laughs]
F You know, it sort of went on like that for quite a while. And there was no music, nothing to listen to, except these chaotic things that were happening around me and I thought for crying out loud, you know, it would be nice if somebody turned on some music or if you could watch a screen and, you know –
I But there was nothing at all.
F No. [S1 08P]
In contrast, patients at another site, which had recently been refurbished and used glass, rather than curtained cubicles, generally talked about the ED environment in overwhelmingly positive terms. The resulting modern, calm, and quieter atmosphere had in turn made them feel calmer as they experienced less rushed and ‘smoother’ care:
F Well that’s something else, I don’t know – well you know the old A&E – they’re always, in the old A&E was this rustling and bustling about, now there’s a quiet sort of everything’s done equally as quickly but there’s no noise, it’s so quiet and you don’t get this trolleys rushing about and that and handovers and that, they seem to be so easy now, you know, from your ambulance stretcher onto your sort of bed sort of thing, yeah, no, as I say, the handover as I say is good. Everything I found was, it’s done, I don’t know, much smoother – everybody is hurrying about but they don’t seem to be rushing about, you know.
I So it feels, is it calmer?
F Oh calmer, definitely. [S2 12P]
Comments regarding privacy while in ED were, overall, quite positive. Several patients who had experienced the new glass cubicles in one of the sites felt that these had resulted in improved privacy. Interestingly, even those patients who described care in busy and overcrowded conditions in other EDs did not raise privacy as a concern even though the systems employed by staff to maintain privacy appeared to be far from ideal:
F No, no. But it was funny because, as I said before, if you needed to be examined privately, if a doctor wanted to, they’d yank somebody out of this cubicle, push them in, shut the curtain, the doctor would do what he’d got to do, the curtain would come out again and then somebody had gone in! And it was funny I suppose but -
I How did you feel in terms of kind of privacy and dignity and things like that in this ridiculous situation?!
F Well, er, you have a blanket over you so…and they don’t do anything, they only take your blood pressure in view of everybody else, you know, everybody, they take you into one of these little cubicles – [S1 06P]
Being comfortable and having basic physical needs met were highlighted as having an important impact upon participants’ ED experience. There were two main aspects to this: the availability of food and drink and the comfort of hospital trolleys/beds. Having access to food and drink while waiting in ED (often for considerable periods of time) was an important element of patients’ and relatives’ well-being and personal comfort. Some patients reported positive experiences in this regard, with ED staff proactively offering drinks and/or light refreshments which were very much appreciated:
I Right. And you hadn’t … had anybody got you up and moved you or asked you if you needed the toilet or anything like that?
F Oh yes they came and did … they gave me a buzzer, so said if you need the toilet or anything, just buzz. They did make me a drink and brought me a sandwich, because I hadn’t had anything since breakfast. [S2 04P]
Other participants, however, including several with diabetes, reported that staff had not enquired as to whether they would like something to eat or drink and did not offer anything. Some participants said that they had been given refreshments when they asked for them, but one relative had had to make their own arrangements:
F Not this time, but the time before, I actually went up to one of the nurses and said, ‘Is there any possibility of organising something for my husband to eat, he’s dia-.’ ‘Oh we don’t usually.’ I said, ‘Well he’s diabetic and he’s not ate for,’ whatever it was, six hours or whatever. She said ‘well there’s a cafe,’ is it Costa, whatever it is.
I Oh right, OK. So they, as a matter of course, no matter how long someone’s in the emergency department they won’t give them anything to eat?
F Well I don’t –
I That was the message that you received?
F Yeah. [S3 06PC]
Several participants described uncomfortable and even painful hours lying on hospital trolleys or beds unable to sleep or rest because of the discomfort. The problem was exacerbated by the fact that patients often had to wait in ED for many hours. Trolleys and beds were too hard, too small, and very uncomfortable:
M See this is the concern I’ve got about it, is that there’s this target of you know, once you get into A&E you’ll be in a bed in four hours. Well, I suppose technically they meet that when they put you into a cubicle. But that’s certainly not a bed, and I wouldn’t recommend it. There’s no way I slept in that. I mean I’m only five feet ten, I’m not huge. There’s no way I can lie in that bed comfortably, no matter what they do. I would imagine that if I’m five feet ten, there’s only about five feet six of that bed.
I So it’s uncomfortable.
M It’s uncomfortable. [S1 10PC]
The importance of having family members present during an ED attendance was highlighted by interviewees. Just over half of patients had someone with them in ED, typically spouses or children, while approximately one-third reported that they were alone during this time (information was missing in the remaining cases). Family members provided a source of practical and emotional support during what could otherwise be a quite daunting experience. In contrast, some of those on their own in ED described the experience as frightening, lonely or simply boring:
I Did you feel safe?
F Yes, I felt safe, yes. I wasn’t upset or anything.
I No, you weren’t frightened or worried or anxious?
F No, I don’t think I was because [name of grand-daughter] was with me, if she hadn’t have been I probably would have been.
I Right, OK, yeah. So it was important to have somebody with you.
F That’s the way I felt, yes. [S1 05P]
Time waiting in the emergency department
The issue of waiting times in ED emerged as a prominent theme during interviews. Just over a quarter of patients had waited four hours or less in ED before being admitted, transferred or discharged. These participants expressed satisfaction and sometimes surprise at such ‘swift’ treatment. In contrast, another quarter reported they had waited in ED approximately 12 hours or longer before being admitted to a ward. Such long waits were difficult to endure, especially when combined with having to lie on uncomfortable trolleys/beds and going without food and drink for extended periods of time:
F Oh yes. The first time I ever went in there, oh boy, I’ve never got over it, my bum is still suffering from that first time.
I Really.
F Yeah.
I Were you on like one of those narrow trolley things?
F Yeah.
I Oh dear. And were you on there for a long time?
F Oh I was, fifteen hours before they put me into a bed.
F The first time I went and I were there for fifteen hours before they found me a bed but, apart from that, I have no complaints. [S1 04P]
Comments made by some relatives during interviews provide insight into the impact long waits in ED can have upon them as well as their loved ones. They were often older people themselves and had also experienced discomfort, difficulties obtaining refreshments and lack of sleep:
F Well I’ve been very well but I think visiting [name of husband] for five weeks and the first two days I spent 60 hours without going to bed –
I Oh my goodness.
F - simply because he went in as an emergency –
F - and I sat all night with him while they were waiting for the consultant to have a look at him and then we went to the [name of another hospital] for the next night and I sat all night there as well. [S2 09C]
Discussion
People wished to be treated by caring, responsive staff and with respect, which was understood in terms of dignity and appropriate care. The provision of timely information communicated clearly and honestly to both patients and relatives was also highlighted, not least in enabling patients to be involved in decision-making about their own care. Participants wanted to experience emergency care in a calm, quiet environment where basic needs for privacy, comfort and food/drink are met, and where they can be supported by relatives and friends. The desire for shorter waiting times was also prominent. Study participants described very mixed and sometimes negative experiences in terms of whether these preferences were met. This explains the reluctance expressed by many about attending hospital in the first place.
The findings reported from this study are consistent with previous research on the experiences of emergency care for older people, which emphasise the importance of information and communication, staff attitudes, waiting times and physical/environmental needs in shaping those experiences. 20,22,48,53,54 These aspects of care have also been identified as instrumental in determining the experiences of adult patients in the ED. 55–57 In many respects, the needs and priorities of older people living with frailty in the ED mirror those of patients of all ages, not least in the import attached to relational aspects of care (communication, compassion and empathy) over technical competence. 55–57 Our findings suggest, however, that these needs and preferences are particularly pressing for older people living with frailty as they are perhaps more vulnerable in the ED environment both physically and emotionally when these needs are not met. 48
For older people living with frailty, interactions with staff are inextricably linked to their own sense of self and dignity. Being treated with respect and kindness provides validation and legitimacy, whereas being ignored or dismissed (e.g. when seeking help with toileting) can have a long lasting negative impact upon self-esteem. 20 Similarly with regard to information and communication, older people living with frailty may need particular support to be involved in decision-making,58 such as timely information communicated clearly, and for staff to check that patients have understood. 59 Communication barriers relating to hearing/visual problems, language or cognitive impairment need to be identified and overcome. 22 The presence, involvement and advocacy of relatives is imperative for both older people living with frailty and their carers. 59,60
Our findings show that the noisy, busy ED environment poses a particular challenge to older people living with frailty who prefer to receive care in a calm and quiet setting. They are particularly sensitive to and distressed by the physical privations they often experience in ED in the form of uncomfortable trolleys and a lack of food and drink. While previous studies remark on the ‘resilience’ or ‘tolerance’ demonstrated by older people encountering long waits in ED,61,62 this may be more challenging for older people with frailty. Participants reflected very negatively on this element of their experience; they had no choice but to ‘tolerate’ long and/or uncomfortable waits which took a considerable toll upon them at the time and appeared to make them more reluctant to attend the ED when faced with future emergency episodes.
Participants included those living with mild to severe frailty and, as such, we report the views of a group of patients who have previously been excluded from research in this area yet represent a sizeable and growing number of those accessing emergency care. Although views on desirable outcomes from ED attendance are not explored here, these data were collected during the study and are presented separately.
Despite the efforts of recruiting staff, the lack of ethnic diversity within our sample is a limitation. We did not recruit any patients from the very severe frailty categories (CFS 8 and 9). Further, we were unable to explore whether there were any relationships or associations between distinct levels of frailty (i.e. mild/moderate/severe) to experiences and preferences for emergency care. This would require a larger mixed methods study including more patients within each frailty classification. Almost one in five participants had cognitive impairment; the inability to gain consent (or consultee consent) or the inability to participate in an interview may have influenced greater representation of people with cognitive impartment. Although most interviews were conducted within 30 days of the ED attendance there is always the possibility of inaccurate recall. Moreover, the focus on relational aspects of care may reflect the purview of both patients and interviewers who, having little knowledge of medical processes and procedures, focused upon interpersonal elements of the ED experience. 55
This study provides important evidence about the extent to which frailty impacts upon older people’s experiences of emergency care. Our research suggests that frailty can result in a particular vulnerability in ED if physical (ED environment, personal comfort, waiting) and emotional (sense of dignity, communication, involvement, family support) needs are not met.
At the level of practice, we make several recommendations which might potentially be delivered in clinical practice (Figure 3). Recommendations relating to staff care and attitudes and information and communication could make a significant positive difference to the experiences of older people living with frailty in ED. While the ED environment and waiting times may be harder to change, health care professionals can help older people living with frailty by being mindful of their comfort, physical needs, the flow of information and the importance of patient/carer involvement.
FIGURE 3.
Meeting the needs of frail older people in ED: recommendations for practice.
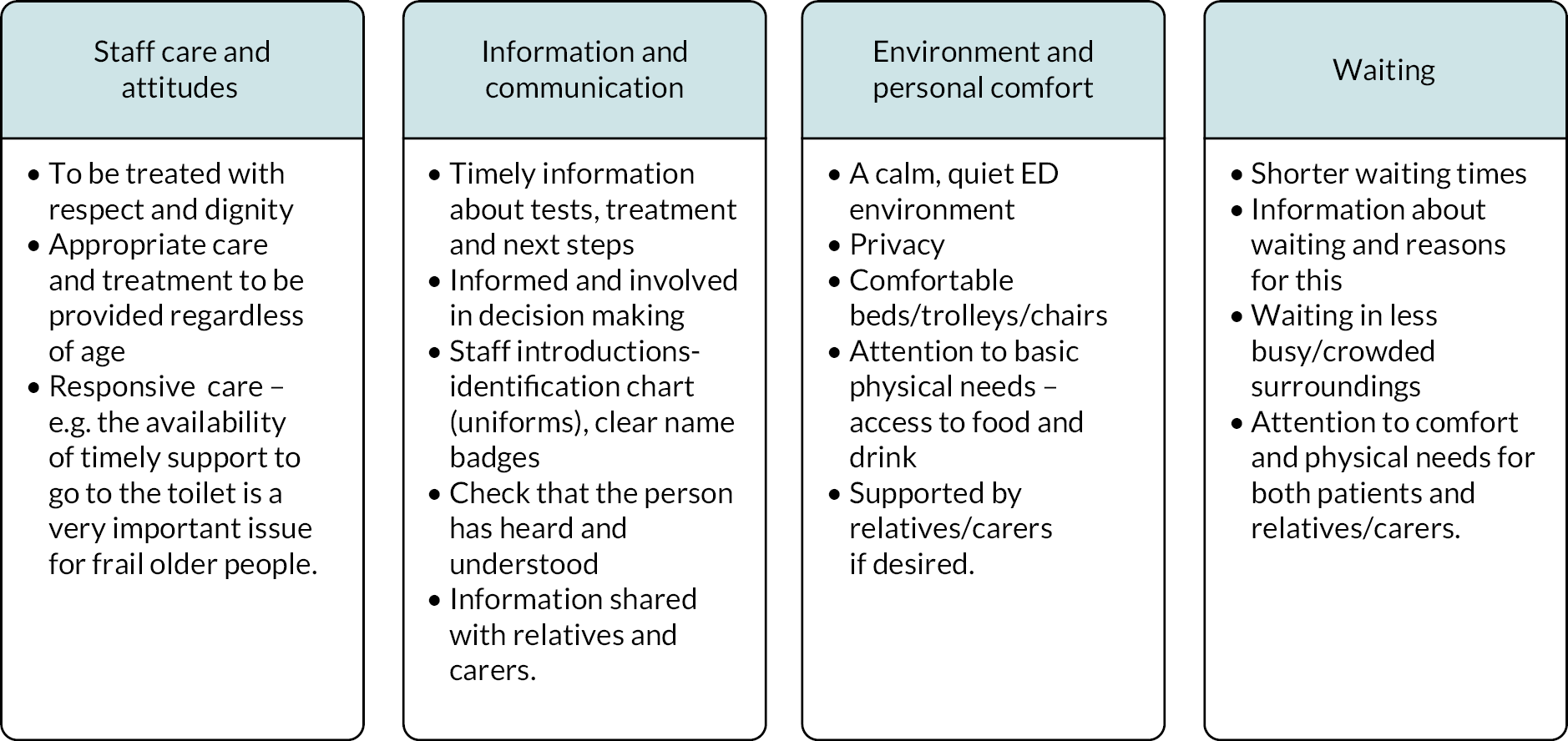
More broadly and given the challenges of more fundamental changes to the fabric of the ED and the pressures on this part of the health-care system, policy-makers and practitioners need to consider service development changes when responding to the needs of older people living with frailty requiring UEC. These may include the adoption of new approaches closer to the hospital ‘front door’, interventions which minimise or even bypass the time these patients need to spend in ED, as well as frailty-friendly design in ED. 63,64 Any new models of care must be based on robust research evidence, which should relate to clinical and cost effectiveness but also to patient and carer perspectives. 65
Conclusion
Frailty reflects a vulnerability and a need for support in basic activities of daily living, which EDs in this study, and perhaps more widely, are not set up to provide. Changes at the levels of clinical practice and service design are required to deliver even the most basic care for older people living with frailty in the ED environment.
Patient reported outcomes
Methods
Using the same study design, participants and data but by analysing with a focus on outcomes rather than preferences, we report here on patients’ preferred outcomes from emergency care. The methods and recruited participants data are not repeated here, but the reader is referred to the Methods section above.
Results
Findings are presented as goals of care and attainment of those goals (expressed verbatim or illustrated through experiences).
Physical and medical goals of care
The views of participants on their physical and medical goals of care are illustrated in Box 1. Some participants attended ED to be ‘checked out’ or simply to have their medical problem diagnosed and treated. Those with chronic conditions wanted the current exacerbation of their condition to be controlled and a plan made for ongoing symptom control. Although this may seem like an obvious goal, what was striking was that it was rarely attained. Some (though not all) participants felt that they had not been diagnosed and their condition had not been treated. Some felt they were sent home as their symptoms dissipated, with no explanation of the cause. Some respondents felt that they had not had diagnostics and treatment due to their age.
Participants attended ED hoping, but not necessarily expecting, that they would leave feeling better. Many did not feel better when they were sent home, and about one-third felt that they had deteriorated while in hospital (admitted participants). Reasons for deterioration in hospital included falls, changes in medication, hospital-acquired conditions, deconditioning and loss of function caused by reduced mobility.
Several participants who were spouses of hospitalised participants felt that they themselves deteriorated whilst their partner was in hospital due to hours spent travelling and visiting or lack of help at home.
I So what’s important to you is that the clinical side –
P Is that they get the diagnosis and the treatment right and send me off home. [S1 07PC]
P Well I just wanted to make sure everything was checked out. You know, I didn’t know whether I’d have to have a scan or an x-ray, and it was just the X-ray. [S2 03P]
P The pain in my legs I want medication pretty quickly. Because it can get really painful. And then I don’t know what to do with myself. [S2 15P]
P Getting better.
I OK.
P That is your only priority. [S1 09PC]
P I was hoping they’d find a reason for my distended stomach [S2 05P]
Participant views on attainment of care goals
C … they said, ‘well we’ll put him on antibiotics’, I said ‘yes and he’ll be in for a couple of days and you’ll send him home, nobody seems to ask the question why’. [S3 06PC]
I … did you ever get a diagnosis or did they say what they thought it was?
P No, I didn’t know what it was … because they didn’t know what it was, I felt I’d take up everybody’s time. [S1 02P]
C Three weeks later we were still chasing this scan because she’d been moved and they didn’t know on the new ward that she was after a scan [S1 07PC]
P I feel it was a waste of time … [S1 10PC]
P Even when you go up the doctor’s they say, you know, ‘he’s 85 years old, you know, what do you want me to do?’ … That’s their attitude, you know.
I Did you feel that that was the attitude in hospital as well?
P Well yeah I do, yeah.
C He was just left like a piece of meat. [S3 02PC]
C He was actually worse when he come out than when he went in. [S3 02PC]
Goals of care concerning information and involvement in decision-making
The views of participants on the information provided and their involvement in decision-making are illustrated in Box 2. When admitted, participants and their carers wanted information about their tests, diagnosis, treatment and care. They wanted to be informed about what was wrong with them, what was happening to them and why, and for that information to be accurate and consistent. Six respondents stated that they felt that they had been well informed about their condition and their treatment. However, participants, relatives and carers often felt uninformed about their tests, diagnoses, treatment, care, prognosis and discharge. Participants left with unanswered questions, with some feeling unable or not knowing what to ask. There were examples of participants being moved, being told they couldn’t eat, or undergoing tests without being told when or why.
Admitted participants stated that it was important for them to be involved in decisions about their treatment and care, though not everyone felt this way. Some wanted the clinicians to make decisions for them. Being involved in decision-making required being given enough information to make an informed choice, and is therefore linked to information about diagnosis, treatment and care. Some participants did feel involved in decisions about their care and that their wishes were listened to. Others, however, felt that decisions were made about their treatment and care without consultation.
When admitted, participants felt it important to share information with relatives and carers to assist with understanding and recall of information provided, advocacy and arranging family support. On many occasions poor communication or missing information caused frustration, especially when participants had hearing difficulties or cognitive impairment. Part of the issue for relatives was that there was rarely anyone around to explain things during visiting hours. Relatives and carers perceived staff as too busy to take time out to talk or even as unwilling to give information.
Medication changes were not explicitly mentioned as desired by our participants; that is, they did not go to hospital with this in mind yet changes in medication whilst in hospital were common. What participants did want was information about why medications were indicated or changed. Those who had medication changes did not always know why and could experience problems such new side effects and deterioration in conditions. Some participants were unclear about what their medication should be at discharge and whether changes implemented in hospital were to be continued. GPs were not always informed of medication changes, and in some cases, GPs revoked these changes and reverted to the prehospital medication regimen.
Participants and carers were unsure about what they should do after discharge in terms of self-care; they felt this information should be given prior to discharge.
P I’d also like the thorough checks to be done as quickly as possible and to be kept informed as to what the checks are for [S110PC]
I And do you think is it important to you that they involve you in any decisions?
P Oh lord, yeah. It is for me. Because I like to know what’s happening to my body, I mean, OK if it’s bad news, you’ve got to deal with it but, yeah, I think it’s much better to, from my point of view, to know what’s facing you as opposed to sort of pushing it under the carpet sort of thing. And then eventually, I mean, I think you know when there’s something really wrong with your body but, as I say, that’s me, not everybody wants that [S2 12P]
C What we call, it’s reverse triage. The doctors will come and do their spiel and then the patients will turn round and say ‘Excuse me what did he say? What did he mean?’ so we’re then doing another triage but after the doctor’s done it, just explaining as to what … [S1 07PC]
P I always want to know what it is and if they introduce me to a new tablet I want to know why. [S2 15P]
Participant views on attainment of care goals
P … he didn’t tell me anything and I thought well why doesn’t somebody tell me, maybe I should have asked, but you’re in their territory, not your own, you know. [S1 08P]
P No. You’re just an idiot. They treat you if you’re over 80 they treat the older generation as an idiot. And I don’t like that. You might as well know what I think of them. [S2 06P]
C … my general kind of issue is that nobody talks to me and trying to find out information is so hard and trying to find somebody to talk to. So you will see the health-care assistants, so they come round and then it’s all very much ‘well I need to go and find a nurse to speak to you’, but you can’t find them, they’re so busy, there’s never anybody around … there’s nobody around to talk to you, to update you on what’s going on. And it’s so frustrating. [S2 02C]
C when dad got discharged they said ‘we’ve stopped the propanol tablet’…. So we took dad a week later and spoke to the GP, [name], and he said ‘ah, you’re here because they’ve stopped your losartan tablet’, ‘no, they’ve stopped the propanol tablet’, ‘no, not on this record’ … [S3 02PC]
C … She was sent home with a morphine patch, there was no others supplied. They finally came when there were new Dosette boxes that had come from the chemist, but nobody had told me that it was supposed to be me that was changing them and giving me any advice on what I was supposed to do. [S2 02C]
Discharge and discharge planning
The views of participants on discharge and discharge planning are illustrated in Box 3. A key goal identified by participants was to get home as quickly as possible; most did not want to be admitted. But participants wanted to feel ‘fit enough’, and ‘able to manage’ before being sent home. For some this related to previous experiences of readmissions after going home ‘too soon’. This is not to say that participants expected to feel completely well and, indeed, some felt they would recover better at home. Some participants were sent home before they felt well enough, suggesting to them that the hospital needed the bed. Some felt guilty about ‘taking up a bed’, and one was told they were fit to go home despite their carer also being in hospital. Relatives, who themselves were often living with frailty, were often anxious about coping post discharge.
Participants wanted to be involved in their discharge planning and often needed their relatives’ and carers’ support. Decisions about discharge could feel very last minute and information confused and conflicting. Some participants felt that they were discharged suddenly without warning. One participant, whose family had not been told she was being discharged, was woken early in the morning and taken to the discharge lounge in night clothes and told to put on someone else’s clothes.
Participants and relatives wanted clear information about when a discharge would happen, and what home or community support was being arranged. Going home during daytime, having a carer or relative present and being helped into the house were seen as important. Discharges were not always well planned. Participants and family were not always asked about whether help was needed at home on discharge. Very long, uncomfortable waits were common and distressing and were usually caused by waiting for medication, discharge letters, porters and transport. One patient was discharged without medication. Participants were discharged alone at night, sometimes with minimal assistance; one patient was left on her doorstep at 2 a.m.
Participants did not want repeat admission and wanted their health issues to be addressed to prevent this from happening. Of the 14 participants who had experienced a previous admission, 10 perceived that the subsequent admission was for the same problem. They felt that if their issues had been investigated and resolved the first time, then a reattendance would not have been necessary. One respondent felt that if an adequate discharge package of care had been provided then a subsequent admission may not have happened.
P That was number one. To go into hospital to be treated, and to get out as fast as you can. … You know, as soon as you get in, you want to get out.[S1 09PC]
P on the Tuesday night [day of discharge from hospital] I thought how am I going to get upstairs? So I went up on my bottom. My husband was at the back of me. And when I got to the top – oh no, I can’t push myself up to stand.… Came all the way downstairs on my bottom again and I slept in this chair. [S2 03P]
P I did say. I said ‘I want to get home’ but I said ‘I’m not going home without I’m right this time’ because obviously something wasn’t right last time … After the last few times I thought I’m going to be cautious this time … [S1 07PC]
P I’ve said to the doctors, the consultants … As long as I’m, I’m fine you know, and I can get back home and I feel well. But I don’t want to come back again. [S1 07PC]
Participant views on attainment of care goals
P … they were going to send me home then, I wasn’t really fit enough, I didn’t feel fit enough, but they were going to send me home [S1 05P]
C (hospital) rang me and … she said ‘you need carers’, I thought well nothing’s been discussed about him coming home yet, let alone think about carers, you know [S3 08C]
P … the arrangement was that they’d let my wife know and my granddaughter would come down and pick me up. Now unbeknownst to me they rang my wife and told her that they were releasing me but they were providing transport for me. Now about two or three hours after they told her, I was wondering why she hadn’t rung me or anything. They told me the same thing and I was – I was devastated. I was so mad about it. [S1 10PC]
I ‘Cause I mean did they ask you anything about that in hospital, whether you needed any equipment or anything like that?
P No they didn’t. [S2 03P]
P Then the second time was ten days after I’d been discharged the first time. [S1 06P]
Continuing treatment and care
The views of participants on their continuing treatment and care are illustrated in Box 4. Participants and their relatives hoped that they would be referred on for inpatient or outpatient specialist care, if required. Five participants in our study were transferred to a different hospital for specialist care. Others were referred for outpatient appointments, although they did not always know for what.
Participants expected that their GP would be given information about their emergency care episode, informed of diagnoses, changes in medication and continuing care. There was an expectation that if continuing support from community health services was required then this would be communicated and coordinated for them. Respondents hoped to be visited at home by community nursing or therapy services following their emergency. In some cases, this appeared to have happened smoothly, often due to proactive communication by the patient or their relatives. However, communication with primary health care and community services on discharge was generally felt to be poor, and referrals or appointments were not always completed.
Participants wanted to receive social care support on discharge if needed. Some stated that they were not asked if they needed support. Eight participants received temporary or permanent additional support at home after discharge. There were problems coordinating discharges with (re)starting new and existing care packages. One patient with serious mental health issues had her medication changed by the hospital, but no information on this had been passed to her family or care staff who prompted medication.
C So in theory, in that case, you could have – I know it’s very difficult but you could have something like a checklist within the hospital, if a person’s come in with heart failure, you know refer heart nurse, you know age query, continence query, the things, and use it as a tick list before a patient’s discharged. [S1 07PC]
C I think as a result as well, this time, rather than the first-time mum went in, they’re getting the heart nurse to do a home visit, sensory team, continence team. Now this in theory could have all gone on her first discharge. I mean it’s people obviously like [name], carers that come, that say ‘Oh are so and so involved?’ We don’t know about things like that and we weren’t pointed in that direction. So with help from everybody else we’re more aware of what mum – and I’m not talking about Benefits, I’m talking about health, medical you know. [S1 07PC]
Participant views on attainment of care goals
C She’s seen the letter, she opened the letter, and she was sitting here in the dark when I come over and I said, ‘what’s the matter with you?’, she said ‘thank God you’ve come, I’m panicking’.
I It doesn’t say what it’s for. How bizarre. It doesn’t say what it’s for, does it?
C Well no. She read the letter and she was on one because she was panicking because she thought she had to go back in. [S3 03PC]
P Now here, I’m afraid here we come to a hitch, because on 4 March they informed my doctor’s surgery when I came out of hospital what my new regime was and I’m afraid it must have got lost in the post or something because it didn’t get put properly onto my notes until 23rd April [S1 06P]
C Fortunately this week I’ve been with social services and I think maybe the district nurse has been out to do it. The care home didn’t get the new care plan so she was assessed by occupational therapy I think maybe on the Monday, sent home on the Wednesday. She was assessed because before she went in she only had carers twice a day. It was assessed that she needed carers at a minimum three times a day but her medication had been set up as four times a day … [S2 02C]
Function and mobility
The views of participants on their function and mobility are illustrated in Box 5. Participants want to retain or regain their mobility by being supported and encouraged to be mobile in hospital and at home after discharge. They wanted physiotherapy, adaptations such as handrails at home, and equipment such as walking frames. Many participants in our study felt that they lost mobility because of their urgent care episode. Some felt prevented from being mobile in hospital, being told they could not go anywhere alone but then staff not being available to go with them. Participants also felt unsupported to regain mobility at home, with only four receiving physiotherapy after discharge and six receiving equipment. Many purchased their own equipment.
Participants want to return to their normal activities such as personal care, cooking, housework, enjoying their gardens and going out. Participants wanted to maintain or regain their independence, living in their own homes with minimal support. Most of our respondents maintained their independence after their emergency care, either because their health and function did not deteriorate to the extent that they lost their functionality, or because they received help at home. Care needs often increased during hospitalisation, with two participants being discharged to care homes, two having increased care packages, and a further six receiving temporary support.
P I just wanted to be normal. I couldn’t understand why I couldn’t get them other three yards to the toilet because of that wire, it was connected up and they wouldn’t disconnect it and I asked for it to be disconnected, which they disconnected it for a couple of minutes, a few minutes, that’s all that it took. [S3 06PC]
P I want to be right … [I would like to] be able to get outside and sit outside ‘cause I like my garden. [S1 07PC]
P Yes, I want to get back I want to get back at walking about.
I Ok.
P If I can walk round here potter into the kitchen and back which I was doing until the fall came. [S2 15P]
P … So yeah, my ideal is to be, if I can be semi-independent, let’s put it – semi-independent. That’s the best thing I can describe it. There’s things that I won’t be able to do like change my bedding for a start now, and certain things. And I have a cleaner anyway. But that’s it really. [S1 07PC]
Participant views on attainment of care goals
C Well when you go into hospital, you always come, your mobility is always worse when you come out. Dad couldn’t walk from the car up to here.
P I can hardly walk now.
C We had to put him on the Strollator, the four-wheel drive chair, and drag dad up because he couldn’t walk.
I Whereas when he went in he could.
C Yeah. [S3 02PC]
P Yeah, for some reason. I tried to walk a bit in there but of course there’s not many places, you know, I kept going to the toilet, I mean, I’d wash myself and just walk up the top and come back again but perhaps it wasn’t enough, I don’t know. [S3 05PC]
PYou can’t get out, you’re stuck in here, I’m dependent on my son to take me out in the car, I can’t go anywhere, I belong to three walking groups, which I’ve had to give up. [S1 06P]
Discussion
This analysis explored the goals of care of older people living with frailty and their families/carers during an urgent care episode. The key finding was the discrepancy between what people sought and what was attained (Table 11).
| Care goals | Were these attained? |
|---|---|
| Physical and medical: | |
| Getting the medical problem sorted – diagnosis and treatment Getting checked out – diagnostic tests, X-rays etc. Pain and symptom relief Feeling better |
Many felt they were not diagnosed and treated Most people had diagnostic tests Most had symptom relief Most stated that they did not feel better when discharged Admitted participants often deteriorated Spouses of admitted participants struggled to maintain their own health |
| Information and involvement in decision-making: | |
| Information about diagnosis and treatment Involvement in decision making about care and treatment Information sharing with relatives and carers Information about medication changes Information about aftercare |
Lack of information about diagnosis and treatment Lack of involvement in decision making about care and treatment Lack of information sharing with relatives Lack of information about changes in medication Lack of information about aftercare |
| Continuing treatment and care: | |
| Referral for specialist health services Communication and coordination with primary care and community health services Communication and coordination with social care services |
Some referrals, but much confusion Lack of communication and coordination with primary care Lack of communication and coordination with social care services |
| Function and mobility: | |
| Retaining/regaining mobility Returning to normal activities Maintaining Independence |
Many experienced deterioration in mobility due to hospital stay Many felt they had not returned to usual activities at time of interview Most had retained their independence |
This research adds to existing evidence by expanding the issues identified by older people, those living with frailty, who are poorly represented in previous work. 48,53 Existing outcomes measures for older people38 do not address all of the goals of urgent care identified by our participants from their perspective; for example: addressing the medical problem and feeling better; receiving information about diagnosis and treatment, medication changes and involving relatives and carers; involvement in discharge planning and appropriate discharge; referral, communication and coordination with primary and social care; returning to usual activities; not being readmitted or needing to reattend ED. It is somewhat surprising and disappointing that some of these goals of care were really very modest yet were often not attained.
Our participants did not particularly discuss mental health as desired goals of care other than perceiving these as medical needs requiring attention. They did not expect urgent health care episodes to tackle their experiences of loneliness and isolation. Carer burden was evident from our interviews.
To our knowledge, this is the first paper discussing goals of care and their (non)attainment for older people living with frailty and emergency care needs. Participants were interviewed by expert, non-clinical researchers with subsequent clinical validation, facilitating free and open discourse about health care. In this cohort of older people living with frailty, we were able to identify multiple domains of meaningful goals of care not apparently addressed in previous reviews,48 adding to the understanding of how these might be better elicited and addressed. This study took place in three English hospitals with quite different emergency care processes, yet the goals of care identified were consistent across all three. There was a lack of ethnic diversity in our sample, despite the efforts of the recruitment staff. We also failed to recruit participants with high frailty scores, who may have a different focus in terms of goals of care.
Many older people living with frailty have poor service-centred outcomes from urgent care. 9 These interviews describe limited attainment of person-centred goals of care, with many participants reporting unattained goals or deteriorating while in hospital; this qualitative description adds depth to previous studies reporting on poor outcomes from emergency66,67 and acute care68–70 in older people living with frailty.
Emergency care research and quality improvement usually focuses upon service metrics such as length of stay and readmission rates, which do not necessarily align with patient perspectives. 23,71 Such service metrics are easy to generate but may not be meaningful to older people living with frailty. 48,72 In particular, holistic, person-centred care which can best serve people living with frailty, is poorly captured using such metrics. 48
There is a need for improved communication across the whole pathway of care, with patients being able to feel involved at every stage. Readmissions are a concern within increasingly pressurised systems. The data presented here fit with other reports that have identified that many reasons for readmission might reasonably be identified during the index acute admission. 48,73 It might be that a focus on delivering patient-centred care, driven by eliciting and addressing patient care goals, might lead not only to higher quality care but also to more efficient care. For example, if patients report that their goal is to improve function, and this is the focus of the acute admission then readmissions might be avoided.
Future research could explore if capturing goals of care at the beginning and throughout an urgent care episode might influence clinical care and pathways, and thence the achievement of said goals. Engagement in research of people of minority-ethnic backgrounds is needed, as is engagement of people with high levels of frailty.
Most participants in our study did not attain their goals of care and many deteriorated or experienced adverse effects in hospital and difficulties after discharge. Attainment of care goals from first contact to post discharge for older people living with frailty and urgent care needs should be improved. Further work is needed to develop goal-oriented care for older people living with frailty which is personalised to their specific needs.
Conclusion
Older people living with frailty have heterogeneous urgent care goals which require individual ascertainment. Identifying these goals of care early could result in improved attainment through person-centred care.
Chapter 4 Staff perspectives
Background
Alongside patients and carers, the direct experience of staff of providing emergency care to older people living with frailty represents a valuable resource on which to draw in determining what a high-quality model of care might comprise, and from which to identify promising approaches to implementing models of care put forward in the literature in real-world emergency care contexts. WP1.3 involved qualitative data collection with commissioners, practitioners and others closely involved in the development and implementation of approaches to providing UEC for older people.
The objectives of this work package (WP) were, first, to identify emergent approaches to care that were not yet fully specified in the literature, and so risked not being accounted for in the review of reviews (WP1.1) and, second, to characterise the key features of high-quality UEC for older people and the key influences on implementation.
Methods
We interviewed clinicians and/or managers working in UEC sampled through a national multidisciplinary reference group focusing on geriatric emergency medicine (n~100) and from the NHS Elect national frailty networks. These groups comprised medicine (geriatrics, emergency care, acute medicine), as well as nursing and therapy. The sampling frame was as follows:
-
managers of frailty emergency care services
-
doctors – both ED and geriatric specialists who work in ED; emergency frailty units; emergency frailty teams (acute physicians and surgeons who participate in frailty interventions)
-
nurses – specialist frailty nurses working in ED or frailty
-
physiotherapists and occupational therapists – who work specifically in ED/urgent care
-
social workers – who work in ED/urgent care
-
paramedics.
The participants were invited for interview either face to face or over the telephone, and/or to a focus group held during one of the reference group events in London. Interviews focused on the nature of the novel interventions and approaches to care being led and delivered by participants, including the nature of the clinical and organisational problems being addressed, the rationale (theory and empirical evidence) behind the approach adopted, details about the composition of the intervention and the challenges involved in implementation, and emerging evidence for impact (including intended and unintended consequences for patients, carers, staff, the ED, the wider hospital and the wider system). Interview schedules were informed by insights from WP1.1, and by theoretical frameworks for the study of the implementation and normalisation of innovative approaches to providing care. 74,75 A particular focus was the fit of plausible interventions with clinical and organisational problems and the challenges involved in implementing them in practice in real-world UEC settings, with a view to informing the focus of the further qualitative work to be undertaken in WP2. In common with WP1.2, interviews were digitally audio-recorded, transcribed and then analysed using the framework approach. 76
Results
We involved 42 participants (Box 6) from a range of clinical and non-clinical backgrounds about the challenges, opportunities and innovations in providing high-quality, tailored care to older people living with frailty in and around ED settings in Feb to April 2019.
One to one telephone interviews (n = 21) (February to April 2019):
Two focus groups (n = 23) at BGS urgent care meeting (February 2019)
42* participants in total:
-
consultant geriatricians 5 + 12 (17)
-
EM consultants 3 + 1 (4)
-
community or ED nurses 4 + 4 (8)*
-
therapists 3 + 3 (6)
-
pharmacists 2 + 1 (3)
-
commissioner 1 (1)
-
paramedic 1 (1)
-
service improvement managers 2 (2)
-
specialty not known 2 (2).
* Two nurses took part in both one to one interviews and focus groups
We present our findings in this chapter under four headings. First, we outline and classify the main types of intervention identified by participants. By and large, these reflected the types identified in the review of reviews, covering interventions in the community, interventions in the ED, and interventions elsewhere in the hospital. Data from WP1.3 indicate some of the key points of difference in how these three approaches are put into practice. Second, we identify the most important components of high-quality care as seen by participants as common across these approaches, including organisational arrangements, operational features and skills requirements. We then consider implications for staffing UEC for older people in further detail, comparing the typical staffing profiles of the three broad approaches and identifying some of their common implications for multidisciplinary team working. Finally, we consider in more detail the principal influences on implementation as experienced by participants, particularly those that appeared peculiar to novel approaches to emergency care for older people, including, for example, the precarity of funding arrangements, the need for a risk-management approach that not all staff or systems found comfortable, and the balance between developing expertise and ensuring that other parts of the system were appropriately skilled to identify and cater for the needs of older people.
Approaches to urgent and emergency care for older people
Participants in interviews and focus groups described a wide range of models of care for older people seeking UEC, with various labels and some variation in terms of staffing profile, referral criteria, objectives and care provided. However, they tended to fall under three broad headings, with alternative models of provision under each: community-based teams focused on reducing attendances and admissions; interventions delivered within the ED providing specialised care and support; and interventions offered elsewhere in the acute hospital, either as an alternative to care in the main ED or an alternative to admission to a ward from the ED.
Community-based teams tended to offer a mixture of reactive and proactive interventions. Proactive interventions sought to pre-empt crises and associated emergency attendances and admissions, involving for example community frailty teams, multidisciplinary teams in general practices and community matrons. They tended to include a wide range of multidisciplinary staff, collaborating closely with GPs to identify patients at risk of crisis, assess needs through CGA and plan care accordingly. Reactive interventions responded to immediate needs for emergency care among older people with a view to avoiding an unnecessary attendance, by providing an assessment service equivalent to that offered in the ED in a patient’s own home or in a separate medical assessment unit, together with referral routes to appropriate diagnostic, treatment and care services. Services of this kind typically took referrals from GPs, call centres (emergency hubs and NHS 111) and ambulance crews and had rapid response times (e.g. under two hours), although they tended not to be available 24/7. Some included ‘in-reach’ functions, to ‘pull’ patients out from an ED following attendance and provide assessment and care in a community setting. Typically, they were nurse or therapist led with access to geriatrician input as necessary; some also included input from pharmacists and other specialist practitioners.
Services within the ED included dedicated teams and/or dedicated areas of the department. Dedicated teams included those led by practitioners who were usually community-based, such as community matrons, and those led by staff based in the ED or wider hospital. Their remits overlapped, although the focus of community-led teams tended to be on identifying and assessing patients who could potentially return home swiftly and safely once appropriate support was put in place. These teams would look to identify such patients early after arrival and take advantage of strong connections with community-based providers of health and social care to set up support packages rapidly, ideally within a few hours and usually the same day. Teams led by ED and wider hospital staff would also look to discharge patients rapidly where appropriate, but also had links to provision within the hospital, such as frailty units (see below), which could offer the right multidisciplinary input to support safe and prompt discharge. Typically, these teams were geriatrician led, with input from nurses, therapists and other relevant clinicians. Whether formed of community or hospital based practitioners, dedicated teams did not generally offer 24/7 input. Dedicated areas of the ED comprised beds or chairs specifically set aside for older people living with frailty, with a view to providing a calmer environment, and were served by dedicated teams. Some could be accessed directly by GPs or ambulance crews, while others relied on triage at the ED’s ‘front door’. Again, a key aim was to rapidly assess the potential for discharge to usual place of residence, with support as required, and to secure admission to an appropriate unit within the hospital if necessary.
The third main form of intervention identified by participants was services elsewhere in the hospital. These fell into two main categories: services offered as an alternative to the ED, such as frailty assessment units, and those offering an alternative to admission to a regular ward, with the expectation of a short (< 72-hour) stay, such as frailty units. Alternatives to the ED were located in physically distinct areas of the hospital and generally led by geriatricians, with multidisciplinary staff, and tended to operate during ‘office hours’, typically between 8 and 12 hours per day on weekdays only. Access arrangements varied but, in some cases, they could be accessed directly by ambulance crews, rather than requiring triage in the ED. Short-stay services were provided for individuals for whom admission was necessary but expected to be brief, though in practice, pressures on bed availability meant that patients perhaps more suitable for other wards were often accommodated, sometimes for longer periods. Some units included fairly substantial numbers of beds (20–30); others were much smaller. In some cases, short-stay frailty units and frailty assessment units were integrated, providing a ‘one-stop’ service for frail older patients that avoided both the ED and the main hospital wards.
Key components of urgent and emergency care interventions
Participants identified a range of components that they saw as important in the delivery of UEC interventions, which were necessary regardless of the specific approach taken. To a large extent, these mapped well to the McCusker framework used to organise the components of care described in the literature reviewed by WP1.11 There were some omissions in what participants described; for example, there was relatively little reference to the use of routine data for audit and evaluation purposes or to the use of quality improvement methods. Participants’ focus was primarily on components of care delivery rather than on continuous monitoring or improvement.
Common to many participants was the view that the physical environment of the ED was ill suited to the needs of older people living with frailty, and that the noise and bustle could even lead to deterioration. These physical features were partly what drove efforts to develop dedicated areas within or outside the ED, such as assessment units. Where dedicated areas had been set up, much care had often gone into making them ‘frailty friendly’. However, other than short-stay frailty units that provided an alternative to admission to a ward, few services in and around the ED were available at all hours and, indeed, many were accessible only during ‘office hours’ on weekdays. The general view among participants was that the ebbs and flows of patients meant that 24/7 community or ED-based services might be excessive, but that opening 12 hours a day, 7 days a week would help to ensure availability to patients who could most benefit from such provision.
While the physical limitations of the ED environment were one key reason for setting up separate dedicated areas for older people, a more pressing driver was their distinctive needs in terms of care, assessment and care planning and the implications of these needs for skill mix, organisation of care pathway and timescales. Prompt and thorough assessment of older people, ideally covering all of the domains of CGA, was seen as a vital component of good care, but the ED itself offered a poor setting in which to deliver it. Assessments led by non-specialised ED staff were often patchy and rushed and tended not to be prioritised given the need to make decisions within the window of the four-hour standard. Delivering a high-quality, multidisciplinary assessment required either a highly coordinated and focused process or a more decompressed timescale, offered respectively by dedicated teams and spaces outside the ED. Comprehensive assessment offered by community teams, for example, could offer a key part of durable plans to avoid unnecessary attendance, while assessments by dedicated teams or in in dedicated areas ensured rapid and accurate identification of needs for the duration of any admission, and to secure a safe and timely discharge.
[Describing the approach taken in a frailty unit] The way the patients were being managed in the pitstop, everybody had [CGA] so by the time they leave the pitstop, they had a medical plan, they had an OT plan, a physio plan, pharmacy review, everything done. So there was not a lot that needed to be done once they actually went into the ward except just follow the plan that was made by them, they didn’t have to chase physios, chase OT because it was all done.
(P02a AMD, service improvement)
Though some EDs did seek to offer CGA to appropriate patients routinely, without the input of specialist teams, there was scepticism among participants about the adequacy and accuracy of such approaches. Where assessments were begun in the ED, they were often incomplete at the point of discharge or admission and left to teams in the hospital or community to complete—an inherently fallible process.
With the proper CGA it takes a lot of time and resources. And unfortunately you know they don’t have that in ED with the volume of patients. So what happens is that this is just an eyeballing sort of tool to categorise patients not necessarily with a proper CGA (P19 Pharmacist, Lead care of older people and stroke)
There’s a real time pressure in A&E to get it all done. […] Because obviously you don’t necessarily complete the whole of the comprehensive geriatric assessment in the time, if there are outstanding bits, so we need to make sure that we can then hand that back onto the community to follow up.
(P05 consultant physician and geriatrician)
Closely related to timely and accurate assessment was the need for discharge planning right from the start of contact. Again, the coordination of multidisciplinary input across sectors was central to this process, and a fundamental part of many of the models of care put forward as alternatives to the standard pathway through the ED. There was a sense among some participants that proactive efforts put into discharge planning by frailty units and other alternative models meant that admissions were more likely to be avoided in such settings (even accounting for case mix). Participants argued that this was due to better assessment and better links with community service providers, as well as to staff who were more confident and less risk-averse than decision-makers in EDs, and able to make decisions based on a wide range of disciplinary inputs.
So it might be that when the consultants are seeing the patients, some of them will spend a lot more time than an acute medical consultant would spend with them. But that is the point of having us there because we’re trying to deliver the patient a more in-depth care, if you like, because if we’re going to discharge them we want to make sure they don’t come bouncing back or that we tie them into the right service.
(P20, advanced nurse practitioner)
Correspondingly, strong links across sectors and services were seen as vital, particularly with primary care and other community-based teams, whose input would be vital to ensuring that care plans were delivered and arrangements for avoiding admissions were more than short-term patch-ups. As well as intervening to prevent unnecessarily attendances, some community-based teams also had close links with ED and other hospital-based staff, providing additional expert input and broadening the range of options available for assessment and care. Similarly, links from community-based teams to hospitals also offered streamlined access to diagnostic facilities for patients in the community, reducing the need for admission. Establishing consistent links across a hospital’s catchment area, though, could be challenging when it spanned multiple primary care networks, community health-care providers and local authorities, and this could create uncertainty and inequality in discharge decisions.
I would say the biggest negative is around readmission. So, it really highlighted how poor we are as an integrated care system. So as we were balancing risk and taking more risk around discharge it almost feels often that your discharging is a bit of a black hole. And the next real step change I think that needs to happen is around that integrated pathway. And proper discharge from hospital and trying to create a fluid team really that works across into the community.
(P09 consultant geriatrician)
Good-quality communication was at the heart of creating such links, and participants reported that information could sometimes go missing in discussions across teams and sectors–for example in relation to patients’ preferences regarding admission, treatment, and resuscitation. Participants also noted inconsistencies in the quality of communication with care homes and with patients’ families, both important to ensuring durable returns to living in the community.
Other critical dependencies with other services included palliative care and ambulance services. Patients nearing the end of their lives were seen as frequent attenders at EDs, sometimes due to poor symptom control, and at risk of inappropriate admission and intervention if coordination with palliative care teams was lacking. Participants saw the decisions of ambulance crews as representing a potentially crucial point in selecting the right emergency care pathway for older people but reported mixed experiences of the extent to which ambulance staff were skilled and empowered to diverge from the default of conveyance to the ED. In some cases, protocols had been established for ambulance crews to refer patients to community teams or hospital-based frailty teams, based on initial screening and assessment undertaken by crews. In others, ambulance crews could contact frailty teams or geriatricians by telephone for advice about how to manage patients on a case-by-case basis. Elsewhere, however, ambulance staff were seen as lacking in knowledge about frailty and unwilling to take risks, even if patients themselves expressed a preference to avoid hospital.
Yeah, but because of the area I’m in, the older people—because as well one of the things that we’ve tried to do is say actually older people going to hospital is not the safest option, because there’s always been this kind of mentality in the ambulance service, ‘Well you can’t harm them if you take them to hospital, you can’t get in trouble if you take them in’, and they’ve always had this issue about leaving people at home.
(P07)
Similarly, interfaces with other groups who might channel patients towards frail-friendlier services were also critical, but inconsistent in quality. One issue was responsibility for frailty assessment and ensuring that it identified the patients most likely to benefit from available services in a timely way. Staff in the ED, and in other ‘gatekeeping’ roles such as ambulance crews, were seen as well placed to assess for frailty, but ensuring reliable and accurate screening could be challenging. What frailty teams saw as a vital and relatively straightforward process was not always perceived that way by other teams expected to implement it. Despite the apparent simplicity of frailty scoring systems, some participants reported systematic differences between their own scores and those derived by colleagues involved in triaging in the ED. Some efforts had been undertaken to provide training and to routinise frailty screening in the workflows of colleagues, but with mixed success, in part due to disagreements about where responsibility lay.
We were told – this was in discussion recently – that [ambulance service] can’t implement anything new to the paramedics that isn’t in their core training, even though it’s just a pictorial.
(Participant, focus group 2)
Participants also identified other opportunities to undertake frailty assessment to better anticipate, manage and avert demand on emergency care, for example in primary care and in care homes, with a view to early detection of deterioration.
Staffing urgent and emergency care interventions
Across service models, participants perceived multidisciplinary team working as vital. Multidisciplinary input into assessment was seen to help to expedite decision-making and improve the quality of care and strengthened the breadth and quality of connections with other services available to teams. A breadth of expertise and experiences could also help to ensure that all pathways available to patients were given due consideration and avoid defaulting to seemingly lower-risk options such as admission.
We are sharing to resolve the problem, so there is a cross boundary in terms of practices, in terms of opinion, in terms of clinical decision. I don’t know, I may be right, I may be wrong, but I feel like in frailty it has to be like interdisciplinary so we kind of help each other rather than a strong opinion like that is not a safe discharge.’
(P03 physiotherapist, frailty lead)
‘I think there’s always that risk of over-treating, but we talk about that a lot. We have a weekly MDT where we talk about patients and we talk about that a lot and reflect a lot on scenarios where perhaps there may have been some—not conflicts, but slight differences of opinions in the team about whether we should escalate to IVs or not for example.
(P13, advanced nurse practitioner)
The three overarching types of intervention varied in terms of the staff available but typically either comprised or had agreed arrangements for access to key forms of specialist input, including from geriatricians, pharmacists, and therapists. Leads included physicians (including geriatricians, less commonly ED doctors, and occasionally GPs for community-based services), nurses and therapists.
Community-based teams mainly consisted of nurses, therapists and geriatricians, with others included in or readily accessible to team members as necessary. They tended to have close relationships with community nursing teams, integrated care teams, care homes, general practices and mental health teams. Medical cover was provided variously by community geriatricians and GPs, with geriatricians usually on hand to provide advice to GPs when needed. Senior nurses, including community matrons and advanced nurse practitioners, were often central to the activity of community-based teams, leading assessments, decisions about prescriptions, and day-to-day patient care.
Teams in the ED varied in scale and disciplinary involvement. In some cases, large, multidisciplinary teams of frailty specialists were available, including geriatricians, advanced nurse practitioners, therapists, pharmacists and social workers. These teams were largely geriatrician led, although in some cases emergency care clinicians had been involved in developing, staffing and leading them. While some teams had dedicated time from geriatricians, including in some cases geriatricians who devoted most of their time to working with the ED, others relied on ‘on call’ geriatrician input, and some participants reported that geriatrician colleagues could be reticent to attract geriatricians with an appropriate interest.
They couldn’t appoint a geriatrician; it was like an hour or two hours for one of our geriatricians. So it happened that our medical director during the time is actually a geriatrician, so she dedicated two hours of her time every afternoon, so from one to three in the afternoon, that she will see patients who have been identified as needing frailty assessment.
(P03 physiotherapist, frailty lead)
Teams and units outside the ED but within the hospital also tended to be led by geriatricians, with dedicated specialised nurses leading on day-to-day care, and in some cases input from therapists, social workers and pharmacists.
The services described by participants faced various recruitment and retention challenges. Particularly notable were the issues some faced in securing consistent geriatrician input. For services where medical oversight was provided by physicians from a different specialty, such as general practice or emergency medicine, obtaining consultative input from geriatricians was not always straightforward and could make informed and patient-centred decision-making more difficult. Particularly for services that operated across sectors, clear agreements about service level and commitment were crucial to underpin high-quality care and decision-making.
So most units – and my previous unit from a different trust had the GOD phone, whatever you want to call it, geriatrician for the day, silver phone after the Silver Book, there’s lots of different name for it, where the GPs can call any geriatrician. So attached to that is advice, very quick assessments.
(Focus group 1)
We’ve got […] a phone-based service that GPs can use to get advice. But the commitment to respond to those phone calls is quite low. Part of the thing in [trust] is the consultants, there isn’t paid time to answer those calls. They don’t necessarily see it culturally as something that they absolutely should be doing. […] I think if you went back 20 years there was much more of a sense that that was absolutely a core part of a consultant’s job. So we’ve lost a little bit of that. And I think pressures on consultant time doesn’t help.
(P18 commissioner)
Also seen as vital was the input of pharmacists, but here too securing consistent access could be challenging. Pharmacists had a distinctive knowledge base that could feed into both immediate decision-making and assessment for longer-term care planning, but while some participants reported that pharmacists were a core part of the multidisciplinary team, others had patchier access to their expertise. Notably, pharmacists’ contributions at the point of ED attendance were often not consistently built into care pathways, due to capacity constraints and the time needed for a full review of medications management, with many services only able to obtain pharmacist input post admission to hospital wards or to short-term frailty units.
We don’t have pharmacists in our ED unfortunately. Within our trust two of the hospitals have got emergency departments but only one of them has got a specialist pharmacist in that ED. But unfortunately that pharmacist sees only the highly complex patients and they’re absolutely swamped because the resources are not there. […] So once they’re admitted then the service then has a greater impact because then they will be seen by OTs and pharmacists within the hospital, the ward admission structure. [… But] they may get discharged so quickly that they can possibly something put in place. It’s only the patients who are then admitted that may require a greater input of service.’
(P19 pharmacist, lead care of older people and stroke)
Community-based services and dedicated teams within the hospital but outside the ED reported more consistent involvement of pharmacists in assessment and decision-making, and continuing connections across sectors following a return to the community were again seen as important, not least to ensure that any changes to medications regimens made by pharmacists to reduce polypharmacy and risk of admission were not reversed by GPs following discharge.
We’re also now starting to have care home pharmacists, so pharmacists that will review patients in hospital and then follow them up in the community to make sure that the GPs are following the medication plans. We’re finding a lot of patients are getting readmitted because GPs don’t follow the plan.
(Focus group 1)
Overall, key features of approaches to staffing included the importance of dedicated teams, comprising experienced and confident staff, who were comfortable in working with each and in taking an approach to multidisciplinary decision-making that was not always common in other parts of the health-care system. Where the responsibility of teams was well demarcated and working relationships and practices well established, participants felt that they had better ownership of decisions and were able make good decisions more quickly.
I’ve got key members of the team. We’ve had very little change over in our staff which has been key. Because they all feel like they’re making an impact. (P17 consultant geriatrician)
It’s not just about their presentation or their age, it’s about their level of dependence and, you know, what’s going to happen to them if we do bring them in? Where is the best place for them to be looked after? Often more junior doctors, specifically in the emergency department, they don’t have the confidence or the expertise to make that decision. It’s far easier [to say], ‘This is really complicated, let’s just get them in. There’s too much going on and I can’t tell which is the most important thing to deal with today’. Whereas a senior clinician can often get to the bottom of it quite quickly.
(Focus group 1)
Being comfortable with a degree of managed risk-taking was seen as crucial to effective service provision, and again was underwritten by experience, multidisciplinary input, and trust in the competence and judgement of colleagues. This was true for those with overall accountability for decision-making.
There is an element of what is an acceptable risk. And I think that’s different for a lot of people, you know, and certainly even with our therapists and nursing, they will absolutely stop clinicians where somebody is saying, ‘Well they can’t possibly go home’, and actually our therapists and nurses are saying, ‘This is what they are like all the time, they manage at home like this, we can let this person go home’. And actually I think that’s having knowledge, you know, because we often see a lot of patients again and again, we know them relatively well, but there is something about that sort of level of risk. And also knowing the backup that you have to support that level of risk going out into community.
(P10 consultant geriatrician)
One concomitant risk of building teams with strong experience and clearly bounded remits, however, was a depletion of skills and confidence among colleagues in the ED and elsewhere. As noted above, effective service delivery depended in part on accurate triage to identify the patients who could benefit most from the input of dedicate teams, but the willingness and ability of ED and ambulance staff to assess and route patients was mixed. Equally, there was a sense among some participants that enhancing the skills and profile of frailty-focused teams could result in a deskilling elsewhere or promote a sense that care of older people was the responsibility of those teams alone, rather than a core part of emergency health-care delivery. Conversely, from the perspective of those outside specialised teams, there was sometimes a sense that the mainstream service was being deprived of capacity that it badly needed.
Using the therapists, the OT and physio in a frailty practitioner role, I think some of the other therapists were a little bit, sort of, territorial and didn’t really know what the frailty OTs were doing and then I think the OT has now found it a little bit difficult because she was a bit like, ‘Well should I be getting the equipment out of the cupboard or should I be filling out the CGA?’, you know, there was a little bit of, you know, because obviously these are kind of new roles so there was a little bit of finding feet.
(P05 consultant geriatrician, frailty lead)
The emergency department side of it, it can sometimes because they have that early intervention team, that maybe the medics can be a little bit hands off you know, in sort of identifying people. ‘Oh yeah, they’re for early intervention team’, and then they’re not getting the other aspects of what the patients require so that’s taken a bit of time to get that.
(P12)
In places where hospitals were setting these units up, they would then say, ‘Well we have to provide this service, so we’re now not going to participate in the acute medicine rota.’ And then the acute medicine rota falls over because all of a sudden if you’re a small hospital and you’ve only got, I don’t know, 12 consultants and all of a sudden three of them are not playing, it’s a quarter of your consultants gone. […] The next thing you know you’ve got a massive artificial workforce shortage. […] So actually it heavily dichotomises aspects of the patient’s care depending on how you set your unit up, you know, if you set up a unit, you can only be there for two days and everything’s brilliant and then you go to another ward that can’t deliver anything because someone’s hogged all the resource, actually it makes the team resentful and it’s not good for the patient.
(P11 senior clinical fellow and acute physician)
Given demographic changes, the limited capacity and opening hours of frailty-oriented services, and the need for risk stratification and involvement in the care of patients with less complex needs, broad-based knowledge of the needs of older people and how best to care for them was seen as vital by participants, as was continued input from geriatricians and other specialists in the management of frailty into mainstream care provided in the ED and elsewhere in the hospital.
Influences on implementation and consolidation of services
More broadly, the dispositions of staff at various levels towards the value that frailty-oriented services could add to a health economy’s urgent and emergency system services appeared crucial to the extent to which such services were initiated and sustained. In most cases, it was needs and opportunities identified by frontline clinicians that had led to services being set up, often on a pilot basis and with ‘soft’, non-recurrent, funding. While some had become increasingly mainstreamed, others remained precarious; often, participants felt, they were the first services to be curtailed when there were staffing shortages or surges in demand in the short term, while in the long-term arrangements for their funding were uncertain.
It has been challenging. I think we were able to fund the service initially for the early supported discharge aspect. The funding for our nursing staff, which was part of the move to another ward and because of the budget that was already there for that ward, we were actually able to argue for two frailty nurses to develop the service and say that, you know, ‘If you want us to do more, this is what we need.’ So we were able to negotiate that, but that was quite difficult and that took a while to get through. I think we have been struggling to expand the service to seven days and that has been a challenge in trying to get funding for that despite knowing that that would be a helpful move, so that’s just within the financial environment that our trust is in.
(P10, consultant geriatrician).
In practice, frailty-oriented services were often held together by just a single individual or a small team, leaving them vulnerable to changes in personnel or changes in priorities. The need for experienced staff, comfortable with risk and confident in multidisciplinary team working, compounded the fragility of such teams, especially when such highly skilled staff were in strong demand in other parts of the system.
I’ve had comments from a lot of my colleagues who are I suppose a bit surprised that I’m just doing it all myself and it seems that I don’t have a huge team to sort of help me get this off the ground or help this be sustainable, so there is a sort of…I do have a worry that it’s not going to be a sustainable thing forever, but I hope so.
(P21, lead pharmacist, elderly care).
The problem is that there’s still just one person in the hospital that is running the whole show basically. So, the resources are still quite thin. The aim is that every pharmacist should be trained to be able to offer the service. But it comes down to resources, it comes down to time. Those are your biggest challenges. And also retaining staff. That’s a big thing. You train staff and then they move unfortunately.
(P19, pharmacist, lead care of older people and stroke)
In contrast, participants reported that some organisations displayed more progressive, facilitative approaches to provision for older people. Personal buy-in and engagement from senior figures within a trust could help to ensure that the importance of UEC for older people was recognised, and that interdependencies with other aspects of provision were understood; recognition of the issue as a whole-system challenge could also help to ensure co-ordinated approaches to addressing it across integrated care systems (ICS), overcoming sometimes divergent priorities across sectors.
So one of the key things is having people like [colleague], so [they’ve] been very proactive in terms of supporting and promoting it in the right channels, which if we don’t have that support then it’s very difficult to really—you can make progress, but you’re never going to make the progress that you need.
(P02b, consultant geriatrician)
We’re an integrated care system, so we’re very much taking what we would describe as a system approach to frailty, so we are looking at the kind of proactive, preventative stuff that we can do in the community using population screening tools basically to identify people at high risk and then obviously once we’ve looked, making sure that we’re then supporting the community to try and keep them at home and obviously, if it is appropriate, then to come back to hospital, that’s fine, but if it’s not appropriate for them to come back to hospital then we can obviously give a bit of support to the community team about what we can do.
(P05, consultant physician and geriatrician)
In contrast, a lack of joined-up thinking in local health economies could frustrate the efforts of individual organisations to try to improve emergency and urgent care pathways.
Another issue which is kind of generic, but it does impact on our frail elderly when we’re trying to get them home, is our availability for transport. That’s a huge problem sometimes because you can’t get the ambulance because they’re too busy bringing people into hospital.
(P13, advanced nurse practitioner, rapid response and treatment service).
At the level of the organisation of care on a day-to-day basis, too, there was a need for well-developed relationships and protocols with adjacent teams in the ED and elsewhere to ensure that frailty-oriented services could work effectively. As noted above, a careful line needed to be followed between upskilling dedicated teams and ensuring that skill and responsibility remained distributed across the UEC pathway, particularly given the volume of patients and variation in the complexity of their needs. Some colleagues were sceptical about the value of frailty-specific teams, and time and care were needed in agreeing the role of frailty-oriented services and the distribution of responsibilities across teams.
I think ED would just prefer the patient to be taken away and it took us quite a long time to establish that actually no, the frailty team work with you, we’re not going to take the patient off your hands, you know, we’re working as part of your team, not as a separate team that’s going to do everything else, we’re going to be part of the team, we’re going to work together, so that provided some of the more difficult conversations at the beginning.
(P10, consultant geriatrician)
So they became ours, well we don’t have a team for that, so even things like, ‘Well your patient wants to go to the toilet,’ ‘Well I can’t provide all the nursing care for all the older people down here on my own and do my job’. People were really awful, I mean, it was really difficult.
(P06, nurse).
Lack of clarity regarding the boundaries of responsibility or how frailty-oriented services were intended to complement ‘regular’ emergency and urgent care pathways could result not just in poor working relationships but in reluctance to take responsibility for the high-quality frailty screening necessary for the identification of patients who could most benefit from such services.
Conversely, overlapping jurisdictions between professionals and teams could also pose problems. Participants highlighted a need for clear governance arrangements for frailty-oriented teams, including how multidisciplinary team arrangements for decision-making interfaced with intraprofessional norms of accountability.
My colleague, who’s a physio, she struggled in the early days because she was trying to be really, really proactive which was part of our role and some of the therapists were very junior and very risk averse, and also the processes were quite constrained. So for example people have to be signed off by a therapist before certain things can happen, but it has to be a therapist who does it. […] They weren’t really sure whether [colleague] was able to say that to them because she wasn’t in their hierarchy, so she was a separate physio.
(P16, Advanced clinical practitioner in frailty, nurse)
Training and awareness-raising regarding the needs of frail patients, the process of frailty screening and the roles of different teams were identified as helping to address these challenges, but again were time consuming and vulnerable to staff turnover.
Several participants identified the importance of extra-organisational networks in supporting their work to develop and sustain frailty services, including the Acute Frailty Network (AFN), Health Improvement Scotland and various professional associations. Such networks were seen as offering structured approaches to develop services using improvement methodologies, as well as frameworks for measuring, benchmarking, and demonstrating impact.
Being a member of the Frailty Network […] gave me a lot of resources in terms of different hospitals, I can quote them or I can use their data to show them that look this is what I’ve done, this is what happened and kind of putting it more personalised according to their staff group. So it gives me a better confidence actually, the confidence to talk about it’s not just me, I’m not creating it, it happens everywhere.
(P03, physiotherapist, frailty lead)
Staff described a range of approaches to seeking to demonstrate the impact of their services, with mixed outcomes. Evidencing impact on measures such as admission rates or length of stay could help to secure longer-term funding for services; some also sought to collect data on patient-oriented outcomes. In cases where participants felt that their services were comparatively well established, some form of data-based case had been crucial, even if only derived from audits or before-and-after comparisons.
That just sold it to the chief executive and everybody when we presented this, that it just was sold and they didn’t even need a proper business case, they said ‘just go and recruit as many people as you need to get this eight til eight working.’ So even that recruitment has been an issue, but definitely the support was there and they could see that you would not need the beds that they predicted that they would need if we did this.
(P02a AMD, service improvement)
I think now we’ve proven our worth. We’re established because we’re able to demonstrate reduced length of stay that has then been sited at unscheduled care meetings. This is what [name of service] are doing. So we’re kind of proven now.
(P17, consultant in medicine for the elderly).
Complementing generalisable evidence about the likely value of frailty-oriented services with data demonstrating local impact could, in some cases, provide a sufficient foundation for a sustained service, though ongoing challenges of staffing and competing priorities meant that relatively few participants felt confident that their services were truly embedded and normalised.
Discussion
Our interviews and focus groups with those at the sharp end of UEC interventions for older people living with frailty suggest that the models presented in the literature (Chapter 2) reflect current practice. While there are variations in the scope and focus of what different organisations offer, the approaches we found could all be covered under the headings of community-based interventions, interventions provided by distinct teams or in distinct areas of the ED and interventions offered elsewhere in the hospital, either as an alternative to ED care or after it, as a short-term alternative to admission to a regular ward. Participants identified key components of good-quality care across these approaches; for example, a focus on prompt and thorough assessment and meticulous discharge planning from the start, and the professional contributions and skill sets that are important to making them work.
Our analysis also highlights some of the key influences on implementation of these interventions. Many of these reflect generic challenges involved in organisational change in health care and well documented elsewhere, such as alignment with organisational priorities and the need for leadership buy-in. Some, however, played out in distinctive ways in relation to emergency care for older people and merit careful consideration from other groups looking to enhance their services. For example, a common theme across participants was the need for personnel who were comfortable with risk management, and in team-based decision-making that sought to foreground the needs and preferences of older people and be creative and enterprising in identifying approaches to supporting patients that prevented medically unnecessary admissions wherever possible. This meant that services needed staff who were both relatively experienced and senior and were happy with new ways of working that prioritised crossdisciplinary collaboration over intradisciplinary hierarchy. Such ways of working, moreover, did not always fit comfortably with established jurisdictional boundaries, such that there were sometimes tensions between responsibilities and accountabilities of professional groups (particularly those in non-medical roles) and rapid decisions and implementation of care plans by teams.
Boundaries between frailty teams and colleagues in the ED and broader system could also pose some wider challenges, with efforts to set up services for older people needing to tread a narrow line between upskilling dedicated teams and neglecting (or being perceived to neglect) the role of others in high-quality care. High-quality care in dedicated teams depended in part on effective triage and risk stratification by others, but both the skill and the enthusiasm for tasks such as frailty screening were reported as patchy. In some places, services focused on older people were seen as replacing input from other colleagues even when they were not resourced to do so, while elsewhere there was some evidence of resentment among those providing care to patients on ‘regular’ emergency care pathways that resources had been requisitioned for a particular group. Framing and addressing emergency care pathways as a system problem and ensuring that interventions accounted for indirect consequences elsewhere in the system, appears important both to ensuring that solutions are effective and lasting, and sustaining a shared sense of responsibility.
Many of the participants we spoke to in these interviews and focus groups, however, expressed concerns about the longevity of the services in which they were involved, with funding through ‘soft money’ common, and vulnerability to loss of staff and temporary withdrawals due to staff shortages apparent. All data collection for WP1.3 took place prior to the onset of the COVID-19 pandemic, which has exacerbated staffing pressures, created immediate pressures on emergency care, and may have suppressed other demands that now appear to be resurging with a vengeance. We explored the impact of the pandemic in a smaller sample of case studies in WP2 (Chapter 4), but the precarity of these services may have been exacerbated by the various pressures created by the pandemic and by current efforts to recover elective health-care provision. Current efforts to enhance coordination of care across health economies through the development of ICS may have the potential to support emergency care of older people as a system priority and system responsibility, but it was clear from the testimony of our participants that they did not always find it easy to bring the issue to the fore of senior decision-makers attention and sell the benefits – for systems and patients – of more tailored approaches.
This substudy has some limitations. While participants covered a good range of the professional groups involved in developing and delivering care models for older people seeking urgent or emergency care, we cannot be certain that the approaches identified are comprehensive. Our data also do not provide any basis for assessing the relative prevalence of the approaches described. Most participants were also directly involved in developing, delivering, managing or commissioning dedicated services; while they described the views of colleagues in ‘mainstream’ emergency care, we do not have direct access to the views of this group on such services. Additionally, while they identified a range of approaches, more of our participants worked in acute hospitals than in community settings, and so they may have been more familiar with approaches to care that take place upon or after attendance at the ED, rather than those seeking to prevent it.
Conclusion
Although UEC interventions for older people are broadly aligned with the literature (Chapter 2), there is much variation in the scope and focus between organisations, and many respondents highlighted tensions relating to staff skill mix and service stability.
Chapter 5 Qualitative study of delivery of exemplar urgent and emergency care pathways
Background
The objective of WP2 was to build on the insights offered by the three substudies of WP1 by examining the organisation and delivery of exemplar pathways for UEC of older people in practice. We intended to sample between four and six case study organisations that reflected different approaches to delivering care, with a view to understanding how these pathways were put into practice and the influences of context and implementation approach on activity. We planned to use a combination of in-depth interviews, ethnographic observation and analysis of relevant documents to examine these issues in each site.
Methods
Based on the care models identified in WP1.1 and drawing on preliminary analyses of routine data from YH conducted for WP3 (Chapter 6) and informal conversations with UEC leads across EDs in the region to provide background information on their approach, we developed a sampling frame for WP2 that categorised EDs according to the following features:
-
Size (average attendances per week) – ascertained from routine data.
-
Adoption of ‘frail friendly’ practices – ascertained from informal conversations.
-
Performance against four-hour standard, admissions ratio, emergency reattendances rate – ascertained from routine data.
We selected four EDs that exemplified difference on these features, including:
-
one larger site, adopting frail friendly practices, with consistently better performance (site 2.1)
-
one smaller site, adopting frail friendly practices, with consistently worse performance (site 2.2)
-
one larger site, not adopting frail friendly practices, with consistently worse performance (site 2.3)
-
one smaller site, not adopting frail friendly practices, with consistently better performance (initially identified site subsequently replaced by site 2.4).
With a view to enhancing the quality of data collection, the team responsible for leading WP2 was initially blinded as to which study site exemplified which characteristics.
We received an initial favourable ethical opinion to undertake this study in September 2019 (reference 19/YH/0258), subject to review and recommendation by the Health Research Authority Confidentiality Advisory Group for access to patient information without consent under precedent set pathway 10 (since the observational work could involve incidental exposure to patient details, although these would not be recorded or otherwise processed by the research team). Provisional approval was provided in November 2019 (reference 19/CAG/0194) but data collection was not permitted to start in any site until all four had received assurance of the adequacy of their data protection arrangements through review of their data security and protection toolkits by NHS Digital. Confirmation for the final site was not received until June 2020.
Significant further disruption to the plans for WP2 then followed with the onset of the COVID-19 pandemic from March 2020. With research and development departments in NHS organisations prioritising urgent public health research and data collection infeasible given the need to prioritise clinical care, WP2 was paused, initially with a view to resuming in autumn 2020. We reviewed the situation continually and sought to recommence WP2 following the NIHR’s ‘Restart’ framework from summer 2020, initially with a view to undertaking (remote) interview data collection and collation of documents only (given the ethical and practical challenges associated with ethnographic observation). Sponsor approval was granted in July 2020 and three of the sites granted permission to begin data collection in August, September and December 2020. The fourth did not provide approval, owing to constraints on capability and capacity; we continued to seek approval until early 2022 but it was not forthcoming. We therefore recruited a replacement site (site 2.4) with similar characteristics; approval was provided by the organisation in February 2021.
Initial data collection in sites 2.1, 2.2 and 2.3 coincided with the second and third waves of the COVID-19 pandemic in autumn and winter 2020; recruitment to interviews was consequently slow. With the introduction of lockdown in January 2021, in response to the rise of the alpha variant of the coronavirus, we again suspended data collection. In view of this, and in anticipation of an improved public health outlook ahead, in February 2021, following consultation with NIHR, we applied for and were granted a six-month costed extension to the project, with a view to allowing more time for interview data to be collected and, subject to feasibility, risk assessment and organisational permissions, ethnographic data collection. We resumed data collection in the initial three sites and in the newly added replacement site 2.4 in spring 2021. We also explored the possibility of ethnographic data collection, as originally planned, with each site. Three were willing to permit site access and in autumn 2021 we completed periods of observation in two sites (site 2.3 and site 2.4). We curtailed data collection early in site 2.3 and took the decision not to go ahead with observational work in site 2.2, scheduled for December 2021, because of the rise of the omicron variant, and the associated transmission risks and pressures on EDs.
Data collection was thus heavily disrupted. Ultimately, it was possible to produce a reasonable dataset, although it fell a little short of original plans in terms of both quantity (fewer interviews than intended: 10–12 per site rather than 15–20) and complexion (fewer observational data: 0–20 hours per site, rather than around 20 hours in each). The final dataset is summarised in Table 12.
| Site | Size | ‘Frail friendly?’ | Performance | Interviews | Hours of observation |
|---|---|---|---|---|---|
| 2.1 | Larger | Yes | Better | 10 | 0 |
| 2.2 | Smaller | Yes | Worse | 11 | 0 |
| 2.3 | Larger | No | Worse | 12 | 10 |
| 2.4 | Smaller | No | Better | 12 | 20 |
Additionally, various documentary sources were collected in each site; a more detailed description of the sites is included in Report supplementary material 1.
Results
Analysis of interview and observational data identified several influences on the development and delivery of UEC interventions. We present them under the following headings:
-
Intervention-related implementation influences:
-
staff: frailty mindset and behaviours
-
resources: workforce, space, and physical environment
-
operational influences: referral criteria, frailty assessment, operating hours, transport.
-
-
Context-related implementation influences:
-
links with community, social and primary care
-
organisation and management support
-
COVID-19 pandemic.
-
-
Approaches to implementation:
-
service/quality improvement networks
-
engaging staff and building relationships
-
education about frailty
-
evidence.
-
Apart from COVID-19, these influences on implementation have much in common with the analysis of data collected for WP1.3. Data from the four study sites serve to underline the significance of these influence; our analysis in this chapter offers a more detailed account of how these factors help and hinder the delivery of UEC interventions for older people.
Intervention-related implementation factors
Staff: frailty mindset and behaviours
The behaviour and disposition of staff appeared to have an important influence on the delivery of UEC for frail older people across the four sites. In the context of an older person attending ED, being ‘frailty minded’ (in other words, actively considering the impact of frailty on an attending older person and its implications for the course of action given their presenting condition) seemed to make a notable difference to the pathway followed and the ultimate outcome for the patient. Frailty-minded staff proactively considered alternatives to hospital admission with the aim of getting patients ‘home’ as quickly as possible if safe to do so. Typically, this involved employing interventions which could facilitate rapid discharge with appropriate support put in place. Staff described this mindset as being informed by views and experiences that hospital was not always the best or safest option for older people requiring UEC and that they may be better served by alternative interventions:
People go ‘oh well, what if such a thing has happened?’, but by putting people in bed to stop them from falling and decompensating is more likely to make them fall. So it’s getting that message across that actually if the patient is capacious, when you’re frail you live with a degree of risk. Keeping you in hospital does not reduce that risk it actually increases it. [S2.1 08]
A frailty mindset was generally well embedded in services developed specifically for older people requiring UEC via the recruitment of appropriately skilled staff with an interest in frailty and education about frailty. In common with the traits identified by participants in WP1.3 as important to high-quality care for frail older people, certain behaviours were identified by staff in all sites as key features of a frailty mindset, and as crucial to implementing effective services for older people in UEC settings. These included challenging the ‘status quo’ (including defaulting to admitting patients), positive risk-taking, viewing patients holistically and knowing when not to intervene. Staff in site 2.2 described how, in the past, there had been a tendency to admit patients among both ED doctors and, perhaps more surprisingly, geriatricians. The establishment of UEC services for older people had necessitated challenging existing practices and embedding new ways of thinking, something helped by the employment of therapists in senior roles:
Even the doctors it used to be you come in, you get admitted, you know, so to say to them actually you come in, we treat your symptoms, we ensure you’ve got a safe discharge and wraparound services and off you go – it was almost like a shock to the system. So we have had to do a lot of work with all of the staff groups really to really get them into that mindset and that ethos and I’ve actually had to put in some quite senior roles within the therapy team [ … ] to get it established and to get the leadership to support some of the junior staff into that way of thinking. [S2.2 08]
Positive risk-taking was described by interviewees in terms of balancing the risks associated with hospital admission for many older people (e.g. deconditioning, an unfamiliar environment, lack of support networks) with those associated with supporting older people in their usual place of residence if stable enough for discharge. Sites 2.3 and 2.4 had also sought to instil this kind of ethos in recent years, through new interventions and additions to staff. With the establishment of frailty-related interventions for UEC, site 2.3 had seen a shift towards positive risk-taking in recent years:
There was also a very cautious approach of if we’re not 100% sure they’re safe we’re not going to let them go home. And that’s definitely changed, which I think is probably good. So there’s much more of a sort of we can support this person at home and yes they may fall again but if we put things in place then it’s unlikely. [S2.3 01]
Alongside therapists, obtaining greater input from geriatricians was also seen as an important means of leading, supporting and validating a less risk-averse approach:
And I think geriatricians are really good at risk-taking … I was just thinking of one in particular who really just sort of looked at the person, doesn’t look at the blood results, this, that and the other, the story – looks at the person and just manages to – because if the patient is post-taken by a geriatrician, I think they’ve got more chance of getting home that day. [S2.4 10]
Resources
Reflecting the findings of WP1.3, insufficient resources were identified as an important obstacle in developing and delivering UEC interventions for older people in the study sites. This included both workforce and the physical environment.
Workforce
Although mentioned by staff in all sites, problems relating to workforce were especially highlighted in sites 2.1 and 2.4, particularly insufficient geriatricians and therapists. In site 2.1, ED and frailty team staff commented that clinical cover on the frailty unit had not been adequate. This could mean that specialised frailty services were only available for part of the week:
Geriatrics is propping up the general medical rota and we won’t be able to progress it unless we have a 7-day service. So again it’s the buy-in about geriatrics as a specialty and treating a frail patient as a skill in itself. [S2.1 08]
Even within those limited operating hours, the reliability of the frailty service could be patchy:
The biggest issue […] for eternity in [site 2.1] is the lack of consistency, so how well that model operated was slightly dependent on who was working, not who was working that day, but how many people were working that day. […] So if they rang, if ED rang us, if they’d identified somebody that they thought might be suitable for us and they rang the frailty phone, you know, would we be able to take them or not, we were not, in my opinion, consistently able to say yes we’ll take them…And therefore I think that eroded a bit of trust almost between ED and us as a frailty team. [S2.1 03]
Similarly, in site 2.4, there was support for developing frailty-oriented pathways in ED, but workforce pressures, exacerbated by the COVID pandemic, had impeded such plans:
We are, as a department, we have recently identified a number of people who are interested, particularly in frailty, and in trying to improve the services that we offer within the ED such as just better identification of frail older patients but that work is really at a very early stage and we have significant capacity issues to be able to undertake such kind of additional developmental work, shall we say, particularly in light of COVID and what’s happening now in terms of recovery, so that’s had a big impact on our ability to do anything new and different. [S2.4 04]
ED doctors in site 2.4 generally felt that they lacked the capacity to deliver such interventions due to their own staffing pressures. The small consultant cohort in ED was attributed to the small size of the hospital, a lack of funding for posts and difficulties in recruiting staff to work in the area. From the perspective of geriatrics, finite resources and pressures relating to the stability of the acute medical workforce (which impacted on their own workload), meant that an increased focus on ED would mean withdrawing from other areas:
It is just about which bits we try and do because there’s too many bits to do but there is general willingness to do quite a lot of things, quite a lot of us who’d quite like to be doing a bit more in community and there’s also people who’d be quite happy to go and do some ED assessments. It’s just what don’t you do in order to do that. [S2.4 01]
Space and physical environment
Interviews and observations revealed several examples of how space constraints and the physical environment had impacted negatively upon the delivery of UEC for older people. In site 2.1, a lack of space in ED meant that the frailty unit was located in a separate part of the hospital. Several staff felt this was far from ideal in terms of being able to discharge patients quickly from ED:
Both my team and the acute frailty team should be working right at the front door, so we should really be based more in the A&E sort of Department, rather than – so in my view the frailty bed area should be a part of the A&E Department… It shouldn’t be a separate unit, so that we can all work together in that one area and discharge patients directly from there. [S2.1 09]
Similarly, the ED in site 2.4 was described as too small and not really fit for purpose. Observations and interviews indicated that the layout made it difficult to keep a check on patients and this was felt to impact upon older people. The same-day emergency care unit in site 2.4 was also seen as too small to meet demand and accommodate the needs of older patients.
We don’t have any space for a commode, but we do often, we are very limited in what we have. We don’t have one piece of equipment to be able to enable anybody to walk, you know a Zimmer frame or anything like that … So I think just better facilities for the elderly really, more commodes. We don’t even have enough storage space for the equipment that elderly people might need. [S2.4 09]
While lack of space did not emerge as an issue during interviews with staff working in site 2.3, some challenges were identified during observations in that site. The initial assessment or triage area appeared very cramped, especially during busy periods when additional ‘makeshift’ bays were added, raising questions about patient privacy. The site had recently started a new intervention, ‘the silver shift’, which aimed to identify and assess frail older people waiting in the ED quickly and proactively. While this initiative was in its infancy, it was reported that a lack of space or cubicles in which to conduct assessments was already posing a significant challenge. Staff in site 2.2 were looking forward to the rebuilding of their ED, which it was hoped would provide a purpose-built environment for assessment:
So I think the physical space could be a lot better and we could do a lot better and going forward I think they want to push the whole frailty service and have a separate area if they can going forward and actually they’re looking in the new build to have the RATS [rapid assessment and treatment services] team are going to have their own area, physio assessment area, its own kitchen, its own garden so they can properly assess patients to see how they’re going to get on at home and I think that’ll be really beneficial. [S2.2 10]
Operational influences
Various issues around to the way UEC interventions for older people operated were identified as having both positive and negative influences upon delivery in the study sites: referral criteria and ‘protection’ of services; frailty assessment in ED; operating hours; and the availability of hospital transport.
Referral criteria and ‘protection’ of services
Clear eligibility criteria for referral to UEC interventions for older people from ED were identified as crucial in ensuring that services saw the ‘right’ patients and could therefore be most effective and impactful. Sites 2.2 and 2.3 had both worked to develop clear referral criteria for their acute frailty services but also underlined the need for continual ‘enforcement’ of these in the face of pressure from ED to accept patients. Enforcement was associated with experience, confidence, and seniority in terms of staff refusing inappropriate referrals:
You have to be very kind of confident in terms of pushing back to other colleagues that patients, seeing patients that are acceptable, because obviously you’ve got a set directive which is kind of try and do the most for the most … you’ve got to make sure patients are turnaround-able, if you like, generally. And that takes quite a lot of confidence and pushback from the senior doctors to actually make sure we get the right. [S2.2 09]
Frailty staff in site 2.3 held weekly meetings with ED to review referrals and were engaged in development work to proactively identify patients suitable for the frailty assessment unit by increasing their presence in the initial triage area. This had been prompted by a desire to improve the UEC experience for older people (by shortening waits in ED) and to maximise the impact the frailty unit could have by intervening as early as possible in the patient’s UEC journey.
Experiences in site 2.1 illustrate what can happen when frailty services are not protected by clear eligibility criteria or are not enforced. Interviewees reported that the frailty unit and chaired area had been vulnerable to wider hospital pressures since its inception. For example, during winter pressures, the ambulatory chaired area would commonly be turned over to accommodate beds. For extended periods during the COVID-19 pandemic, the frailty unit ceased to operate altogether; the chaired area was closed and ‘frailty’ beds were used for general medical patients:
Whilst we’ve had COVID, they’ve not had any ambulatory, any chaired area at all, it’s all been beds and, again, because of bed pressures and because of having to move across the negative people around the hospital and ensure that infection control’s adhered to then it’s ended up being more like a medical ward, we’ve had young people on what should be the frailty ward. [S2.1 09]
Staff expressed their frustration that the service was almost regraded as a luxury which could be dispensed with during times of pressure. They took the opposite view: that the frailty unit could have made a positive contribution during times of stress for the hospital:
It’s when the times are tough that’s when the service can offer its most benefit … And it’s when we’re overwhelmed with pressure that (a) you lose your ambulatory area to beds and (b) the criteria goes out the window, so instead of having the type of patients that you think you can make a difference for, you have all sorts of different types of clinical problems which you may or may not be able to help with and when you go down that route, length of stay increases and the hospital grinds to a halt. [S2.1 04]
As noted in WP1.3, executive buy-in to the benefits of dedicated UEC pathways for frail older people could vary. Protecting them at times of challenge could be especially difficult.
Frailty assessment in ED
Linked to the above, and raised in WP1.3, interviewees in all four sites felt that routine frailty assessment in ED would improve access to UEC interventions for older people by facilitating the early identification of potential patients. Unfortunately, the routine use of frailty identification tools did not appear to be fully embedded in any of the EDs studied. While interviewees described various initiatives and attempts to make frailty assessment mandatory at the front door in all sites, compliance among ED staff was not high, largely due to competing pressures and perhaps more significantly (and again reflecting concerns raised in WP1.3), a lack of understanding of the value of such assessment. Site 2.4 sought to address this challenge through greater education of relevant ED staff. Participants in site 2.2 were also trying to improve frailty assessment in ED. They highlighted the importance of this work in underpinning the other interventions they were seeking to introduce:
We’ve had to re-tweak the comprehensive geriatric assessment now numerous times to meet the needs of our patients and we’ve also struggled quite a lot to get staff to do electronic frailty scoring using the Rockwood frailty score, that then feeds into our criteria. So when you talk of establishing a model and a service, they’re the things that take time trying to embed all of the changes for it to work. If I was giving advice to anyone else I’d be saying get those embedded from day one because we did it further down the line because we were learning and on reflection we should have had that good to go from day one. [S2.2 08]
Operating hours
The ‘opening hours’ of UEC interventions influenced their perceived usefulness and, consequently, the extent to which they were embedded within the system of care. While frail-friendly practices had been adopted in site 2.1 for some time, the ‘frailty’ of its frailty service was evident in relation to workforce issues and lack of ring-fencing, described above. The fact that the service was only available Monday to Friday during the daytime was identified as another weakness both by ED and frailty team staff. Not only did the service operate for limited hours; several interviewees commented that these hours did not actually reflect peaks in ED attendances by older people (usually in the early evening). As a result, older people often had to be admitted to acute medical unit overnight before they could be seen by the frailty team the following morning:
Yeah. So a frail, elderly person arrives at 4 o’clock so they couldn’t then go to the frailty unit and if they’re not fit straight away for discharge, which quite often they’re not, they essentially are stuck till the morning and that’s quite a long time for a frail, elderly person … You then have quite often found a reason that they need an admission by the morning. [S2.1 02]
In contrast, the frailty unit in site 2.3 operated on a 24/7 basis. Moreover, clinical staff on the unit were supported by ward clerks who were also available 24/7. This initiative had been implemented to facilitate same day discharges and swift transfers from the unit to the base wards when required. The role of the ward clerks was expanded to include chasing medication and booking transport so that clinical staff could focus on clinical work rather than administration. Meanwhile, in site 2.4, remarks about operating hours centred upon the availability of the discharge service which was generally described as a highly valued UEC intervention, particularly regarding older people. The service had been expanded from 5 to 7 days, operating from 8.00 a.m. to 8.00 p.m. Despite this expansion, though, several participants reported that older people sometimes had to be admitted unnecessarily outside these hours. Again, the hours during which the service was available didn’t necessarily reflect peak demand:
I would imagine if you were wanting to start afresh in A&E you would probably want it more like the twilight, the people who come in later on in the evening would be – being able to support the assessment later on in the day would be good. [S2.4 05]
Transport
Finally in terms of operational influences, the lack of hospital transport outside daytime hours was identified as a barrier to efficient discharge in two sites (2.1 and 2.4). The issue appeared to be particularly pressing in site 2.4 where hospital transport was unavailable in the evening:
We often obviously get lots of ambulance attendances for elderly population, found on floor, off legs, we get a huge amount of care home referrals as well like unwitnessed falls, yeah, it’s our bread and butter really is this, yeah. And we unfortunately do end up admitting quite a lot of them and depending sort of what time of day they arrive, if they arrive after five o’clock it’s a no-go to try and get anyone home … so we don’t do the best service for them, I don’t think. Ultimately, I think people are admitted sometimes that don’t need to be in hospital. [S2.4 02]
The contrast between what they were able to offer during daytime and out-of-hours provision was a source of frustration for staff in site 2.4:
It’s a real frustration to me really in that we can have a set up where during office hours there’s a real push to try and get people home but then just the simple issue of not being able to transport somebody home out of hours is meaning that people are getting admitted solely for that, so that doesn’t happen uncommonly. I’m sure in my nights last week there was at least one that that happened with. [S2.4 07]
Context-related implementation influences
Links with community, social and primary care
Access to responsive and reliable services in the community was identified as the most important contextual factor in the delivery of UEC interventions for older people by staff in the case study sites, receiving greater emphasis than during interviews conducted for WP1.3. Some interviewees made the point that to be effective, UEC interventions required a ‘whole systems’ approach, underpinned by trust and good relationships between organisations:
The activity that we have as a secondary care organisation is generated by the community, you know, what’s going on out there dictates what comes in and when and equally how we get it out and when! [Laughs] So the strength of your community services and the seamless linkage between community, primary care, and secondary care if it’s needed, that’s the bit that needs to be quite, I think, quite seamless and good communication, good relationship, trust in the system. [S2.1 03]
Sites 2.3 and 2.4 reported more positive experiences in this regard. In both, acute and community services were vertically integrated, provided by a single organisation, and this supported the development of UEC interventions for older people. In site 2.3, integration had been further enhanced by having community services and geriatric medicine located within one directorate, operationally managed as one within a single financial envelope. This was felt to have enabled effective use of resources and service innovation:
So we have one operational director who within her financial envelope is both geriatric beds and the community, so when looking after somebody in their own home costs a quarter of a teaching hospital geriatric medicine bed and they’re both on your spreadsheet, it makes it really helpful I think to encourage redesign. [S2.3 09]
One such innovation was the establishment of a specialist community team focused on enabling older people to be rapidly discharged from hospital and assessed in their own home with immediate support in place. This also encompassed support for GPs with concerns about older patients, who could access the same team and benefit from immediate home care packages and therapy to support patients, with the GP continuing to provide medical oversight. GPs in this area were also able to refer to the frailty unit and rapid assessment clinic. Such interventions were facilitated by the establishment of good relationships with primary and social care, partly achieved by engaging these organisations and individuals in service redesign from the start and aided by vertical integration.
Acute and community services were also integrated in site 2.4. While specific UEC interventions for older people here were less developed than in site 2.3, the arrangement permitted integration of acute and community rehabilitation and therapy services across urgent care, ward-based and community settings (including the discharge service). Therapists worked across all three setting, gaining additional skills, knowledge and confidence, and the integrated service offered continuity of care for patients:
Where maybe it was a bit more tricky four years ago, but definitely now it’s a big part of the development of this [name of initiative] is to integrate and link us all together as closely as we possibly can in one service, so that we follow the patient rather than patient moves through silo working teams. [S2.4 05]
Therapy staff spoke positively about these developments both during interviews and observations and highlighted the opportunities for increased focus upon admission avoidance work in the community by providing support to GPs.
In contrast, access to and links with community provision in site 2.2 appeared to be rather mixed; one interviewee commented that while the site’s rapid assessment and treatment team did their best, they could be hampered by lack of support in the community with the result that older patients sometimes had to be admitted unnecessarily. Experiences reported in site 2.1, meanwhile, illustrated the barriers presented by limited access to and links with community services when trying to deliver UEC interventions for older people. Therapy staff in the urgent care service expressed frustration at having to admit patients who could have been discharged due to community services that it was felt were not sufficiently responsive:
One of the things I’ve alluded to is the frustration when you’ve assessed somebody in A&E and you know exactly what they need to go home and those services aren’t available, […] so that’s frustrating when people have to be admitted because they’re waiting for services and when everything, we’ve done everything else and they have to come into the hospital because the services aren’t responsive enough. […] There might be struggles to get them the appropriate package of care, all the rehab beds might be full even though we get priority for rehab beds in A&E, if the beds are all full then there’s nothing we can do about that. [S2.1 09]
Operational difficulties regarding links between acute and community services in site 2.1 reflected an absence of strong relationships at the strategic level. One interviewee noted that relationships, particularly with primary care, had been more positive some years earlier when the site was working with the AFN. Since then, a change in key personnel was one factor that had contributed to a weakening in this relationship and the site was operating in a more isolated fashion:
We’re a long, long way away from being able to develop that model of care in [Site 2.1]. We are very much – I would argue quite an isolated secondary care service. So I don’t feel that we have particularly strong links to the CCG – Whereas I think post that intensive year with the frailty network, that sort of link has dissolved and disappeared a bit. Also the person in that role has changed and I think we never quite built the same relationship with the person who come into the role. [S2.1 03]
Organisational and management support
The role played by organisational and management support in the delivery of UEC interventions for older people was not highlighted evenly across the four sites. In sites 2.2 and 2.3, the issue was not mentioned frequently but when it was, it was identified as a positive influence upon service developments. Greater attention was paid to the role of organisational and management support as a contextual factor in site 2.4. Interviewees were mixed as to whether this was positive or negative, perhaps reflecting the changing nature of organisational priorities and uncertainty about future plans for service innovation in this area. There was a sense that historically the trust had failed to recognise that frailty was a priority for the whole organisation and was not the sole concern of geriatric medicine. This was felt to be changing though, with a shift in the language from ‘older people’ to ‘frailty’ identified as instrumental in driving the issue from geriatric medicine into the consciousness of the ED and, more importantly, the organisation as a whole:
It very much seemed like it was a problem about geriatricians for geriatricians rather than being an organisational priority. I think it took a long time for them to acknowledge that actually 90% of people in the building at any one time were older people with frailty ... but the language is definitely changing so you know everyone uses frailty as a term and that is very much I think, much more acceptable as an organisational issue rather than older people perhaps. I think they could say well older people belong to geriatric medicine and medicine, whereas frailty has got a bit more that feels like it can reach into other directorates. [S2.4 01]
Site 2.4 did not have any specific interventions for older people in ED and although some staff were working with the AFN to examine potential new models, nothing specific had been proposed or piloted by the time of fieldwork. Moreover, some staff involved in progressing AFN work remained uncertain about the organisation’s commitment to such developments.
The role of management received most attention in site 2.1, where, despite its previous adoption of frail-friendly practices, a perceived lack of support was identified as a significant barrier in the establishment of the frailty unit. As noted above, during times of pressure (winter, the COVID-19 pandemic) the frailty unit/chaired area in site 2.1 was closed and replaced with beds, reportedly without the knowledge or involvement of clinical staff:
The consultants knew nothing of it, the nursing team knew nothing of it, they literally came in on Monday to find that the ward had been closed and the nurses were then disbanded to various other locations across the hospital. And the rest of the junior doctor population were distributed across the other care of the elderly wards and it was the same for the consultants and we had no ambulatory area, no frailty ward and no team essentially. [S2.1 04]
While weak referral criteria and staffing issues were recognised as contributing factors to the vulnerability of the frailty unit in site 2.1, those working on the unit identified a lack of management commitment to the service, exemplified in such decisions, as fundamental. Some emphasised a lack of support at the most senior (executive) levels within the organisation, noting that they had failed to protect or value the service even in the face of evidence demonstrating its effectiveness:
We’ve not only presented a vision but we’ve backed it up with data, we’ve backed it up with local numbers in the way that we were able to operate out of ward [number] when we were up there and you know when you’re kind of, like, just like ‘well …’ – and that’s the thing that I find most disheartening is, do you know what, if you can’t be convinced with what the evidence base says, so we know that ambulatory care, keep them in their own homes, don’t bring them into hospital because they decondition, make sure we’re doing Rockwood scoring for identification and CGAs as your gold standard. [S2.1 03]
Other decision-makers were also identified as important in the fate of the frailty unit, for example the bed management and senior nursing teams. Some participants reflected that earlier engagement with these groups might have been beneficial:
So we did have therapists and we had the exec sponsors and things like this, but we didn’t really have a lot of buy in from the matron and kind of senior nursing side of things … and I think that kind of filters down to the bed management team who are largely a lot who are kind of quite closely linked to the senior nursing team and so there wasn’t really – it was quite hard to try and sell the idea, sell the benefit to them and if they couldn’t necessarily see the benefit, there isn’t necessarily as much motivation to try and protect the pathway. [S2.1 04]
Staff in site 2.1 hoped that the frailty unit would be ‘relaunched’ following its closure during COVID-19, this time with the involvement of a matron and perhaps as a ‘nurse-led’ rather than ‘doctor-led’ initiative.
The experiences reported in site 2.1 contrast sharply with those of site 2.3, where management support for the frailty unit was such that the unit had been designed specifically with older people in mind and situated close to the ED:
The Trust values it [the frailty unit] so much they completely refitted the wards directly by A&E so it’s completely designed for older people’s care, so we are closer to A&E than the Medical Assessment Unit and that’s lovely to work in an organisation that has that sort of signal of appreciation of the importance of older people’s care. [S2.3 09].
Again, in contrast to site 2.1, those involved in driving the development of UEC interventions for older people in site 2.3 adopted an approach to service improvement which systematically engaged all relevant staff and stakeholders both in diagnosing problems and designing solutions.
COVID-19 pandemic
Interviews and observations for WP2 took place against the backdrop of the COVID-19 pandemic; the pandemic was identified as an important recent influence the delivery and development of UEC interventions for older people. The pandemic was described as both a barrier and facilitator, and one that had interacted with other influences for better or worse. The data presented in this section relates to the impact of the pandemic upon UEC interventions for older people in the study sites. Its impact upon staff and patients, while profound, is not described here.
Positives: frailty assessment, advanced care planning, service relocation, ‘frailty mindset’
Staff in three of the four sites reported that COVID-19 had resulted in an increased emphasis upon frailty assessment (use of CFS) in ED and having earlier conversations with patients and their relatives about advanced care decisions (including escalation plans and the use of ‘do not attempt resuscitation’ notices) for people with suspected COVID-19. Interviews suggest that these tools and approaches, recognised as being helpful in ensuring appropriate care for older people with UEC needs, were becoming more widely used in EDs during the pandemic:
I think we’re probably better at trying to find out people’s clinical frailty scores which we do in our potential COVID area, which I don’t think most people even knew they existed before that. So, yeah, we are better at doing that, it’s usually done by a registrar or consultant. We have like a drop-down box on the computer for anybody who has potential COVID which says clinical frailty scores, escalation decisions, discussions with family. Because it can be really helpful in those cohorts because it can help decide placements and, you know, it’s just useful for prioritising assessment perhaps on the wards. [S2.3 01]
In site 2.4, a new COVID-19 clerking booklet had been developed in ED, and participants noted its role in ensuring that conversations were undertaken and documented about treatment escalation plans/DNARs.
Interventions of this kind, participants noted, would usually take many months to develop, but during the pandemic could be put into practice within a matter of weeks. Participants commented favourably on the role of the pandemic in removing ‘red tape’ and enabling important changes to be made more quickly:
The clerking document is a great example. So previously that would have taken months to roll out, meetings upon meetings and sending it for feedback to everywhere and medical records. It would have taken months, but we got that out within a couple of weeks or so and said ‘We’re going to use this and we’ll trial it, and if there’s a problem we’ll change it’ so it became much more of a dynamic, iterative process rather than the previous bureaucracy, so we’ve removed a lot of that red tape which has been helpful. [S2.4 11]
What was less clear, however, was whether interventions of this kind, specifically focused on patients with COVID, might lay the foundations for longer-term, embedded changes within the ED more broadly. Regarding frailty assessment, for example, interviews suggest that this was still far from routine in all four sites.
In site 2.2, reconfiguration of services in response to the pandemic had facilitated the relocation of the emergency assessment unit to a much larger ward right next to ED. Those involved in the delivery of the service had previously argued for a move from their former very cramped surroundings to a larger, more flexible space, but to no avail. Given the success of the unit in preventing admissions during COVID-19, participants anticipated that the new arrangement would remain in place until the planned rebuild of the ED.
Here, too, there were examples of new developments that had been hastened by the pandemic. Staff gave the example of a ‘trusted assessor’ form for discharging patients. Rather than having to fill in separate forms for each service referral needed, staff completed a standardised ‘trusted assessor’ form for all referrals which was sent to a discharge command hub. The new process saved time and seemed to result in a swifter return home for patients. Again, something which may have taken months to put in place was established rapidly due to the pandemic:
That’s one of the biggest positives that have come out of COVID I think is having this method, a standardised method of being able to – because it was always on our agenda, it was always one of our goals to get trusted assessor forms and it was almost – it was a real uphill battle – and actually COVID ended up fast forwarding that for us. [S2.2 08]
Finally, in terms of the positive impact of COVID-19 upon UEC interventions for older people, several interviewees commented that the pandemic had served to reinforce the frailty mindset that many saw as crucial by underlining the idea that hospital may not always be the best place of care for older people living with frailty. This applied both to colleagues and managers within the hospital, and to stakeholders elsewhere in the system, such as nursing homes:
But I think they’ve got a bit better in COVID because I think – not that they never had the best interest for the patient – but I think they have a greater understanding that hospital isn’t the right place. I think they don’t want the patient to get COVID and bring it back to the home. I think they have that understanding that the nursing home is the best place for them, you know, so that’s really helped us. [S2.2 06]
Negatives: delays and suspension of urgent and emergency care interventions for older people
The main negative impact of the pandemic upon UEC interventions for older people reported by the sites was in delaying planned service developments and the suspension of services in a couple of cases. While some new initiatives may have been expedited by the pandemic, others were moved onto the backburner. In site 2.4, some staff felt that their work with the AFN had been hampered by the pandemic as staff were exhausted and lacked capacity to engage in and deliver change:
As a department, we have recently identified a number of people who are interested, particularly in frailty, and in trying to improve the services that we offer within the ED such as just better identification of frail older patients but that work is really at a very early stage and we have significant capacity issues to be able to undertake such kind of additional developmental work, shall we say, particularly in light of COVID and what’s happening now in terms of recovery, so that’s had a big impact on our ability to do anything new and different. [S2.4 04]
In site 2.3, planned work with the older persons psychiatric team to improve the ED environment and pathways for patients with dementia had also been put on hold during the COVID-19 pandemic, although it was hoped that this would be revisited as the pandemic eased:
We were having such a hard time with COVID patients coming in, that any sort of improvement work, any service improvement, any quality improvement priorities took a back seat, and I think we’re now at a point where we’re reinvigorating everything only over the last couple of months. [S2.3 12]
The greatest negative impact of COVID-19 was reported in site 2.1 where, as described above, the frailty unit/chaired area was closed to make room for beds following reconfiguration of hospital services, with the unit operating as a general medical and ‘hot’ COVID-19 ward at times during the pandemic. Here, the fact that other influences (such as staffing, operating hours, lack of ring-fencing/management support) had stopped the unit from becoming fully embedded within the hospital left it particularly vulnerable to additional pressures such as the pandemic. Those working within the frailty team were at a loss to understand why the frailty unit/chaired area, which had a record of discharging older people quickly, had been closed to make way for beds which ultimately would not improve patient flow:
COVID has taken over and it took a lot of time to re-establish the need for a frailty unit and convince people that actually having more beds doesn’t mean, it actually stops turnaround, so that ambulatory unit will help bed flow. People can’t always get their heads round it if they’re not in geriatrics. [S2.1 08]
Approaches to implementation
Service/quality improvement networks
Reflecting the analysis of WP1.3, all four sites had engaged with improvement networks when setting up UEC interventions for older people. Sites 2.1 and 2.4 had worked with the AFN which supports the development of UEC initiatives for older people living with frailty. Site 2.2 had worked with the emergency care improvement support team to establish acute frailty provision and same-day emergency care in response to concerns about emergency care standards and admission levels within the Trust. Via the team, colleagues involved in developments had attended regional network meetings and visited exemplar models of care to share experiences and learning to inform their thinking:
So we could all get together, we were all on tables and all the organisations could work in groups to sort of share ideas of what have you done that works, what have you found doesn’t work and each organisation was at a very different stage in the journey, some were very well established, some were just setting up, we were sort of in the middle and that was really helpful, but yes in answer to your other questions, some of our – I think it was our deputy care group managers at the time did, with some of the other staff, did go off and visit various …yeah they went to a few different places and spoke to different people about models to try and get a sense of what was happening in the region whilst we were in the development stage. [S2.2 08].
Site 2.3 had adopted the Flow Coaching Academy approach in the design and implementation of UEC interventions for older people. The key feature of the Flow Coaching Academy model was the use of weekly ‘big room’ meetings in which relevant stakeholders, including patients/patient representatives, met to discuss how services could be redesigned to better meet patient needs. Small scale changes were then made as part of plan-do-study-act (PDSA) cycles to test, collect evidence and evaluate impact. The frailty unit had been designed in this way, with patients’ views driving developments. Striking from interviews in this site was that this approach was still in use to continually improve the care of frail older people with urgent care needs. Site 2.3 still held weekly frailty ‘big room’ meetings, as well as weekly internal meetings to ensure continuing service development and improvement. There was a strong sense from those who had led developments in this site that implementation was a continuous process that would never be complete. This was distinctive when compared with the other sites:
It’s not a project. We’re never going to be perfect so the work never finishes. We have a rhythm and so if you come onto our [frailty unit] for example there is an internal improvement meeting every Tuesday trying to further improve the internal processes of that, then on a Thursday there’s the frailty big room meeting where we’re working on the interfaces with frailty care and with A&E and with community colleagues and such like. And it’ll never finish, you know, so yes we’ve gone from one of the worst performing to now knowing that we’re the best, but we’re not stopping there. [S2.3 09]
Engaging staff and building relationships
Engaging staff in change was identified as important in implementing new UEC interventions for older people, particularly in site 2.3 where, as above, the ‘big room’ methodology had enabled the involvement of both internal (ED and frailty staff) and external stakeholders (GPs, social care) in diagnosing problems and designing and testing solutions. This had been vital in fostering a sense of ownership and resulting in less ‘pushback’ when the frailty unit was set up. It was also important in ensuring that plans made at the strategic level had buy-in from, and were informed by the understanding of, those who would be involved in delivering change were not involved or invested in them:
Like yes, it’s quite a high-level meeting with quite a few of the directors and consultants, but I’ve asked my Band 7 and my matron to attend, because I think they’re the ones who know what needs to happen on the shop floor. I can make a strategic plan but actually if the staff don’t have their hearts and minds in it, it’s not going to work. So if they work to develop some system with the frailty team which is what we’re piloting at the minute, then it’s more likely to be effective. [S2.3 12]
Although at an earlier stage in the development of UEC interventions for older people, staff in site 2.4 were similarly mindful of the need to engage and involve staff at an early stage for interventions to be implemented successfully. Communication and education were highlighted as key mechanisms:
So yes I think getting buy in from people, so if this new service, hopefully, is advertised as what it is and what it can deliver, I think then people will be on-board with that and education around what we’re wanting to do and what’s the best outcome for our patients is key, rather than it just arriving and then saying ‘this is how we’re working now’ – that never works really does it – you need to educate people before it happens and then – get their opinions and their advice and what they think needs to be seen and stuff so that people have some ownership of it. I think that’s what needs to happen… Yeah, I think making it feel like it’s part of us and making sure that everyone sort of knows about what the plan is, what we’re wanting to achieve and educating them about it. [S2.4 02]
Interviewees also highlighted the importance of good relationships more broadly, notably between ED and intervention/frailty staff, in delivering UEC for older people. In site 2.4, building relationships between ED and other staff focused on UEC for frail older people (the discharge service and geriatrics short stay team) was another focus of their early work. Interviewees spoke positively of having good foundations on which to build and of a shared understanding of what was possible given resource constraints; some offered concrete examples of the benefits that followed:
I don’t think there’s particularly going to be an issue with hearts and minds and ED. I think that’s actually, that feels like that’s going to be a lot easier than I thought because I think I was worried about them saying yes that’s fine, you’d be very welcome, what can you do? To which the answer was well I can’t do an awful lot at the moment, whereas actually what they are asking is what can they do, which is awesome. [S2.4 01]
I had one arrangement that I was very impressed with and I knew that probably that wouldn’t have happened maybe two months ago. I was trying to get an elderly patient home and I called the [discharge service] as you do and […] a registered nurse specialist who came down looked at the patient and it was 7 o’clock, so it was on the cusp of not being able to go home, and she managed to arrange a wheelchair taxi and her staff to follow the patient home. I’d never saw them do that before! So they’re obviously trying their best with the limited resources and the fact that there’s no ambulance. [S2.4 08]
Some interviewees in site 2.4 referred to the small size of the hospital as helpful in fostering good relationships, as people tended to meet one another and knew each other personally:
It’s the smallest hospital I’ve ever worked in, but in many ways it’s the easiest place to build those relationships just because there’s fewer of us so you just bump into people more readily, you are talking to the same specialists on the phone more regularly, so you just develop those kind of close relationships more easily. [S2.4 07]
Education about frailty
Educating staff about frailty was identified as contributing to the successful implementation of UEC interventions for older people in several ways. Education served to raise awareness and engage staff in new developments; it helped to establish a ‘frailty mindset’ among staff, and it was seen as leading to improved patient care, for example by promoting the use of frailty assessment in ED. The importance of frailty education was a particular focus in sites 2.2 and 2.4.
Managers in site 2.2 had held training for therapy staff using patient stories to illustrate the benefits of the ‘home first approach’ and identifying ‘missed opportunities’ to understand how care might have been delivered differently. At a more informal level, participants also described provided education for junior staff to underline the needs of older patients living with frailty:
Some of it I look at it as it’s partly that education of our junior doctors, particularly around the frailty patients of what we need to do and that hospital’s not necessarily the best place for them to be… So I do quite a lot of education at the board round. So when I do my board round I tend to do a five-minute education piece at the end of it. I quite often talk about frailty patients. [S2.2 04]
There was, though, a sense even from those engaged in such informal training that a more systematic approach was needed and was lacking compared with some specialties:
We’re very good at paediatric training for our trainees, we’re not so good for our, on the frailty side of things, and that’s an area I think we just need to get a lot better and say actually this is, these are the significant parts of our work you know, for the rest of our lives effectively in emergency medicine. [S2.2 04]
There were signs of work towards systematising education and training more fully and articulating it with service development priorities. Work with the AFN in site 2.4, for example, had led to education about frailty being identified as a key focus for their initial work in developing new services for older people with UEC needs. Accordingly, a frailty education fellow had been appointed here with a specific remit for promoting education about frailty within the organisation, including community teams. At the time of fieldwork, the focus was on establishing a baseline of what training staff had received about frailty and what they would like. Staff were reported as being enthusiastic about this, and this was reflected in observations.
Evidence
Also mentioned during WP1.3 interviews, staff across all four sites highlighted the importance of gathering locally relevant evidence as part of the development and implementation of UEC interventions for older people. The benefits of collecting evidence or data were described in two main ways: demonstrating the impact of new interventions (sites 2.1, 2.2 and 2.4); and informing new service initiatives (site 2.3). Being able to demonstrate impact and effectiveness was regarded as part of the implementation process in terms of securing organisational support, future funding and embedding new services. Staff in sites 2.1, 2.2 and 2.4 reported that data on outcome measures such as saved bed days, admissions and length of stay were routinely collected and had demonstrated positive impacts. In sites 2.2 and 2.4, data relating to recently introduced services had led to additional funding and expansion:
We’ve been able to boil down quite extensive data to create, so we’re able to say at the end of the month how many acute beds of activity we’ve completed in the community rather than the patients being in acute bed base, so we’re able to demonstrate that as our overall currency and that’s taken quite a long time to develop, but that is something that’s described in the organisation, so we’re able to say what we achieve using bed days. [S2.4 05]
As well collecting outcomes data, some interviewees drew attention to the importance of gathering experiential data from patients and staff regarding UEC interventions for older people. Both were seen as a significant source of feedback which could help to shape and improve services:
The experience of the person receiving the service, you know, perhaps their friends and relatives as well, because we always like the friends and family test, but also the staff, like, real time feedback from your staff members, they’ve got such an untapped resource of knowledge. […] Things you don’t know are going to be an issue until you’re suddenly there and doing it, where is the mechanism for those to be raised and dealt with before they become ingrained, entrenched issues! [S2.1 03]
Interviewees in site 2.3 also highlighted the central role played by evidence and data in implementing new services for older people, but here the emphasis was on collecting data to inform developments as part of the ‘big room’ approach via testing and PDSA cycles described above. Gathering evidence to shape and inform new interventions (rather than collecting data following the implementation of services) had been part of the learning process for this improvement approach. Changes made to the role of ward clerks on the frailty unit in this site, expanded to support clinical staff and made available 24/7, were also only trialled following a time-and-motion study which had estimated that significant clinical time could be saved by adopting this change. Data, including staff feedback, were then collected during the trial which confirmed the benefits of this arrangement which was subsequently made permanent.
Conclusion
Though disrupted by delays to approvals and by the COVID-19 pandemic, our case studies of the influences on implementation of care of older people in emergency care identified some important factors. Many of these converged with influences identified by the wider population of clinicians involved in emergency care of older people covered by WP1.3, although there were some differences of emphasis, reflecting local issues and priorities. Three points brought out by our analysis in particular merit further discussion.
First, while our theoretically informed sampling approach sought to obtain variation in our sample reflecting the breadth of practices and conditions in EDs regionally and nationally, in practice the influence of these contextual features appeared marginal and inconsistent. This may in part be the product of a strength of our approach to data collection and analysis: the lead researchers for this WP were blinded to these features during fieldwork and the initial stages of analysis, reducing the risk of confirmation bias. Thus, it may be that the impact of these contextual influences on practice was indeed comparatively limited. However, this inconsistency may also reflect the fact that the information that formed the basis for sampling decisions was some years old by the time of data collection; while major changes in the size of the EDs seems unlikely, shifts in the outcomes and the approaches to delivery of care in the ED are more plausible. Indeed, the two sites sampled for their non-adoption of frail-friendly practices (sites 2.3 and 2.4) were both active in seeking to improve and innovate, particularly site 2.3 with its uptake of Flow Coaching Academy techniques and focus on continuous improvement. Participants in site 2.1, in contrast, felt that their approach to UEC for older people had slipped back in recent years, after an earlier concerted focus. Likewise, and reflecting the findings of WP1.3, practices and interventions introduced earlier were often vulnerable to attenuation or disinvestment through time, particularly during the pandemic, as site 2.1 found in relation to its frailty unit.
Second, in contrast to the frailty of implementation of organisational innovations in and around the ED, the lasting impact of more structurally embedded change was more apparent. The vertical integration of community and acute care services in sites 2.3 and 2.4 seemed to compensate substantially for any absence, current or historic, of frail-friendly practices in the ED, and participants saw it as offering an excellent platform for cross-working that could help to ensure that informed decisions made in the ED about the most appropriate course of action for older attendees could be put into action promptly and effectively. Integration was no panacea: support services that were available only during working hours left gaps in provision, and several participants noted a mismatch between availability of supply and peak demand for such support. However, it did appear to offer the opportunity for both better coordination across sectors, and the development of skills and understanding across professional groups. The formalisation of ICS in the Health and Care Act 2022 may offer an opportunity to develop further such collaborative models in other systems, although the organisational detail of these will no doubt be important.
Finally, and relatedly, the heavy interdependence of the ED with other parts of the hospital and wider care system was very clear from our analysis. This is not a new finding, but the insights offered by interviewees and observations showed very clearly how good-quality care in the ED was crucially dependent on well-formed community links, on flow through the hospital, and on a focus spanning sectors on the common agreed objective regarding approaches to caring for frail older people. Participants also pointed to how relatively minor omissions in related services around the system could make a tangible difference to the quality of care that could be provided in the ED; for example, the absence of out-of-hours support teams in several cases, and the issues with non-emergency ambulance provision in site 2.4. Again, these influences appeared from our analysis to be more important on quality of care, at least as perceived by staff, then what went on inside the ED; certainly, the impact of frail-friendly practices seemed vitally dependent on a wider systems-based approach to older people’s care.
Implementation guidance will be located on the FutureNHS website.
Chapter 6 Analysis of routine patient level data to describe urgent and emergency care pathways, outcomes and costs
Sections of this chapter have been reproduced from Street et al. 77 under licence CC-BY-4.0.
Introduction
People have different UEC pathways and, consequently, they are likely to experience different outcomes and have different health-care costs. The analytical aim of this WP was to assess the extent to which patient and pathway characteristics influence these outcomes and costs. To perform this assessment, we had to meet the following objectives:
-
determine each patient’s UEC pathway
-
measure their outcomes
-
assess the health service costs of each patient’s UEC journey
-
identify the patient and pathway characteristics that explain why people experience different outcomes.
To achieve these objectives, we analysed the longitudinal Centre for Urgent and Emergency Care Research (CUREd) database (University of Sheffield School of Health and Related Reseach, Sheffield, UK). This database allowed us to track patient journeys from the initial emergency call, through conveyancing by ambulance to the ED, to hospital admission and to discharge. It was also possible to calculate the health service costs of the UEC pathway for each patient.
For most patients, their UEC pathway takes them to the ED, and we analysed three outcomes for these patients: whether they were admitted, transferred or discharged within 4 hours; whether they were transferred to hospital; and whether they reattended the ED within 30 days of discharge. We also analysed these three outcomes separately for those who had an ambulance journey to the ED and for those who did not.
For many patients, their UEC pathway involved hospital care, whether they were transferred from the ED or admitted directly to hospital. For these patients we analysed three sets of outcomes: their length of stay, whether they died in hospital and whether they were subsequently readmitted to hospital.
We hypothesised that the outcomes that patients experienced, and the costs of their care would be related to their individual characteristics as well as their UEC pathway. The CUREd database contains a rich set of sociodemographic and clinical information that made it possible to identify the patient and pathway characteristics associated with better outcomes and lower costs. The database also included information about the nature of their contact with emergency services as their journeyed along the UEC pathway. The analysis of these data involved estimating regression models that specified the relationship between these patient and pathway characteristics, outcome and costs.
Data and methods
Data
We conducted a data analysis of linked routine health-care data for the entire YH region of the UK (population 5.5 million). Emergency care in the region over the period was provided by one ambulance service, the Yorkshire Ambulance Service (YAS), with a single emergency phone number (999), an NHS telephone triage service (NHS 111), 18 type 1 EDs (24-hour consultant-led services with resuscitation facilities) and 13 acute hospital trusts. We extracted data from the CUREd database, which collates routine NHS data from NHS 111, the YAS computer-aided dispatch data and the ED and inpatient administration systems. The database makes it possible to track each patient from their initial contact, through transit to and from the ED, and during and after their hospital stay. The full dataset runs from 1 April 2011 until 31 March 2017, but we ran the analyses from 1 April 2013 so that the hospital frailty risk score (HFRS) was always constructed consistently using a full two years’ worth of historic data for all patients admitted to hospital. 77
The full CUREd dataset contains data on over 5.5 million individuals who accessed urgent care in the region. Of these, we identified 359,945 people aged 75 years or older who had an ED attendance or emergency hospital admission between 1 April 2013 and 31 March 2017. Many patients attended the ED or hospital on multiple occasions (e.g. 1% of patients attended more than 12 times over the full period), generating 1,035,045 separate observations.
Outcome variables
We analysed three outcomes for patients attending ED:
-
Whether they were seen and discharged from the ED within four hours of arrival. This was calculated by comparing time of arrival in ED and departure time from ED.
-
Whether they were transferred from the ED and admitted to hospital.
-
Whether they reattended the ED within 30 days of discharge either from the ED or hospital, this being readily ascertained from the linked data. This analysis took account of whether the patient died.
For patients admitted to hospital, we analysed three sets of outcomes:
-
Whether they had a short, long or excessive length of stay. These were defined according to Getting it Right First Time (GIRFT: https://gettingitrightfirsttime.co.uk) standards for geriatric medicine which define short length of stay as 2 or fewer days, long length of stay as more than 7 days and excessive length of stay as more than 21 days.
-
Whether they died while in hospital. In-hospital death is indicated by the discharge status of the patient.
-
Whether, conditional on the patient surviving the hospital admission, they were readmitted to hospital as an emergency within 30 days of discharge, and whether the patient had more than three emergency admissions within 90 and 365 days, these also being GIRFT standards.
For all patients, we analysed the full pathway costs of their use of emergency care service, calculated by applying national tariffs to each element of care delivery from each patient’s first contract with emergency services through to their discharge either from the ED or hospital (but not including subsequent reattendances or admissions). We also analysed hospital costs for those admitted to hospital. The methods by which costs were calculated are detailed in Appendix 4.
Patient characteristics
All analyses controlled for the patients’ age, categorised into five-year age bands, sex and the socioeconomic conditions of where they lived using the deciles of index of multiple deprivation (IMD) with IMD = 1 indicating the worst-off communities,78 number of emergency admissions in the past year, whether patients were care home residents, as recorded in the CUREd dataset, and for the estimated travel time by road between the patient´s residence and the hospital. 79
For those admitted to hospital, we controlled for whether the patient was admitted to hospital through the ED, and for frailty risk using the HFRS. 77 The Charlson comorbidity index80 uses age and International Classification of Diseases 10th revision indicators of comorbidity to estimate mortality risk and takes values from 0 to 17 but was categorised for the analysis into four groups (0, 1, 2 and 3+). 81 The analyses also accounted for the number of emergency admissions in the past year, counts of the number of operation codes, and whether or not they had ambulatory care sensitive conditions. 82
In the analyses of hospital length of stay, we accounted for whether the patient died in hospital. In the analyses of in-hospital death and 30-day readmission, we accounted for the patient’s length of stay during the index admission. The analysis of ED reattendance accounted for whether the patient died in ED or in hospital while the analysis of 30-day readmission is conditioned on the patient surviving the ED or hospital admission.
Pathway characteristics
All analyses included variables capturing the patient’s UEC journey prior to ED attendance or hospital admission; namely, the number and length of the individual’s emergency (NHS 111 and 999) calls, time of the ambulance on scene (arrival to departure), time taken between calling the ambulance and arrival at the ED, and the urgency with which the ambulance was dispatched, assigned by the NHS Pathways triage system based on answers from the caller to scripted questions asked by the call-handler. 83
Site and time variables
In the analyses of ED outcomes, ED size was measured using the number of attendances (in 1000s) during the year that the patient attended. Differences in staffing ratios across EDs were captured by the number of attendances divided by the number of senior doctors. We accounted for out-of-hours ED attendances occurring on weekends, public holidays and weekdays from 6.30 p.m. to 8 a.m. 84
Although suppressed in the tables, we also included variables accounting for the day of ED attendance or hospital admission and whether this was on a public holiday, and month and year variables to capture seasonal effects and annual trends.
Estimation model
We estimated multivariate models to analyse the outcomes, recognising that patients are clustered in Eds and in hospital sites. The models took the general form:
where Y indicates one of the outcomes. Patient and pathway characteristics are indicated by vectors X and P, respectively. Vector S includes the size and staffing of the ED, vector T comprises the set of time variables, while vector Z captures ED or hospital site effects.
We employed a logit model for the three ED outcomes: whether the patient was admitted, transferred or discharged from the ED within four hours of arrival; whether they were admitted from ED to hospital; whether they reattended the ED within 30 days of discharge either from the ED or hospital.
For those patients admitted to hospital, we also used logit models to assess short, long and excessive length of stay and to evaluate the probability of having more than three emergency admissions within 90 or 365 days of the original admission. We employed a Cox proportional hazard model for in-hospital death and a sample selection bivariate probit model for 30-day readmission. As detailed in Appendix 5, the latter model recognises that in-hospital mortality is a competing risk for 30-day readmission and conditions the probability of readmission on whether the patient survived the previous hospitalisation. 85 Applying the sample selection bivariate probit model involved first estimating a selection equation to explain the probability of survival before estimating the probability of readmission. The survival model accounts for the day of the admission, the argument being that this influences in-hospital mortality but has no bearing on the probability of readmission. 85 Appendix 5 provides support for this assumption.
Total pathway costs and hospital costs were analysed using a generalised linear model. 86 For the logit models, the effect of each variable was captured by ORs. Effects were captured by HRs for in-hospital death and by average marginal effects for emergency readmission and costs.
We included a fixed effect for each of the 18 type 1 EDs or the 49 hospital sites, denoted as vector Z. Standard errors are clustered at patient level to capture correlation across multiple admissions, to different EDs or hospital sites, by the same patient.
Analyses were conducted according the specific UEC journey that patients experienced; namely, for those who attended a type 1 ED, for those conveyed to the ED by ambulance and for those who arrived at the ED by other means, and for those whose UEC pathway involved admission to hospital.
Statistical analysis was conducted using Stata 15 (College Station, TX).
Results
Descriptive statistics
The dataset comprise a total of 1,035,045 observations, of which 867,902 were ED attendances and 167,143 were emergency admissions direct to hospital. Table 10 reports summary statistics across all those observations with non-missing values for each of the outcome and explanatory variables.
Attendances lasting more than four hours totalled 256,215 (29.5%) of the 867,902 ED attendances. Following receipt of care, 363,526 (41.9%) were discharged from the ED (of whom 3901 (0.5%) died) while the remaining 504,376 (58.1%) were admitted to hospital for further treatment; 178,553 (20.6%) reattended the ED within 30 days of discharge.
Of the 671,519 patients admitted to hospital, 504,376 (75.1%) had been transferred from the ED while 167,143 (24.9%) were admitted directly; 199,506 (29.7%) had a short stay, 233,600 (34.8%) stayed more than 7 days and 73,665 (11%) stayed more than 21 days; 51,323 (7.6%) of patients died in hospital, and 129,971 (19.4%) had an emergency readmission within 30 days of being discharged, with 21,579 (3.2%) having three subsequent emergency admissions within 90 days and 90,639 (13.5%) within 365 days.
The average cost of hospital care for those admitted was £2760 while the average cost of the entire UEC pathway amounted to £2007.
In terms of patient characteristics, the mean age was 83.5 years, 42% were male, 17% lived in the most deprived areas (IMD 1) with 7% in the least deprived areas (IMD 10); 16.5% of attendances were by care home residents and the average travel time was 14 minutes from the patient’s place of residence to the ED or hospital.
For those admitted to hospital, 39% were classified to the low frailty risk category, 39.1% to the intermediate risk category and 21.8% to the high-risk category. 20.9% were classified to the 0 Charlson comorbidity category and 31.4% to the 3+ category. On average, patients had 1.28 operations and 19.4% were diagnosed with an ambulatory care sensitive condition.
Figure 4 summarises the possible routes to the ED and hospital, with summary statistics about the pathway reported in the bottom part of Table 13. Of the total, 94,178 (9.1%) first phoned NHS 111, 6.7% of whom more than one call that same day and the average call lasting 15 minutes, and they were either advised to go directly to the ED (10,024, 1%) or were taken by ambulance (84,154, 8.1%). A further 451,030 (43.6%) made a 999 call to the ambulance service, 4.4% calling more than once that same day, and were transferred to the ED by ambulance. Of all the 535,184 conveyances by ambulance, 50.1% were designated by the call handlers as less urgent (category 4), 18.8% as urgent (category 3), 30.2% as an emergency (category 2) and 0.9% as in a life-threatening condition (category 1). 83 The average time between the call for an ambulance and arrival at the ED was 85 minutes, with the average time on scene being 40 minutes. The remaining 489,837 (47.3%) went directly to the ED or hospital, without prior NHS 111 or 999 phone contact and no ambulance journey.
FIGURE 4.
The emergency care pathway.
| Variables | N | Mean | SD |
|---|---|---|---|
| ED outcomes | |||
| ED duration (> 4 hours) | 867,411 | 0.295 | 0.456 |
| Hospital admission from ED | 867,902 | 0.581 | 0.493 |
| ED death | 867,902 | 0.004 | 0.067 |
| ED reattendance within 30 days | 867,467 | 0.206 | 0.404 |
| Inpatient outcomes | |||
| Length of stay: | |||
| < 2 days | 671,028 | 0.297 | 0.457 |
| > 7 days | 671,028 | 0.348 | 0.476 |
| > 21 days | 671,028 | 0.110 | 0.313 |
| In-hospital death | 671,519 | 0.076 | 0.266 |
| Readmission 30 days | 671,519 | 0.194 | 0.395 |
| > 3 admissions in 365 days | 671,519 | 0.135 | 0.342 |
| > 3 admissions in 90 days | 671,519 | 0.032 | 0.176 |
| HRG tariff inpatient (in £1000s) | 671,028 | 2.760 | 3.130 |
| Total pathway cost (in £1000s) | 1,034,956 | 2.007 | 2.854 |
| Patient characteristics | |||
| Age: | |||
| Total | 1,034,430 | 83.544 | 5.896 |
| 75–80 | 1,034,430 | 0.297 | 0.457 |
| 80–85 | 1,034,430 | 0.294 | 0.456 |
| 85–90 | 1,034,430 | 0.239 | 0.426 |
| 90–95 | 1,034,430 | 0.131 | 0.337 |
| > 95 | 1,034,430 | 0.039 | 0.193 |
| Sex (= 1 male) | 1,035,018 | 0.420 | 0.493 |
| IMD decile: | |||
| 1 | 1,029,932 | 0.170 | 0.376 |
| 2 | 1,029,932 | 0.099 | 0.299 |
| 3 | 1,029,932 | 0.107 | 0.309 |
| 4 | 1,029,932 | 0.084 | 0.278 |
| 5 | 1,029,932 | 0.090 | 0.286 |
| 6 | 1,029,932 | 0.101 | 0.301 |
| 7 | 1,029,932 | 0.102 | 0.303 |
| 8 | 1,029,932 | 0.089 | 0.285 |
| 9 | 1,029,932 | 0.088 | 0.283 |
| 10 | 1,029,932 | 0.070 | 0.255 |
| Care home (= 1 yes) | 1,030,682 | 0.165 | 0.371 |
| Road travel distance (minutes) (LSOA to ED/hospital) | 1,030,066 | 13.576 | 9.514 |
| Frailty risk: | |||
| Low | 671,028 | 0.390 | 0.488 |
| Intermediate | 671,028 | 0.391 | 0.488 |
| High | 671,028 | 0.218 | 0.413 |
| Charlson Comorbidity Index: | |||
| 0 | 671,519 | 0.209 | 0.407 |
| 1 | 671,519 | 0.275 | 0.446 |
| 2 | 671,519 | 0.203 | 0.402 |
| 3+ | 671,519 | 0.314 | 0.464 |
| Unique operations (OPCS) | 671,028 | 1.284 | 2.398 |
| ACSC (= 1 yes) | 671,519 | 0.194 | 0.396 |
| Pathway variables | |||
| Length of NHS 111 call (minutes)a | 94,178 | 14.927 | 13.997 |
| Calls to NHS 111 per day (> 1)a | 94,178 | 0.067 | 0.250 |
| Ambulance calls per day (> 1)a | 535,184 | 0.044 | 0.206 |
| Call handler designation: | |||
| Less urgent | 535,184 | 0.501 | 0.500 |
| Urgent | 535,184 | 0.188 | 0.391 |
| Emergency | 535,184 | 0.302 | 0.459 |
| Life threatening | 535,184 | 0.009 | 0.096 |
| Length ambulance on scene (minutes)a | 519,322 | 39.605 | 18.916 |
| Length ambulance service (minutes)a,b | 535,183 | 85.178 | 44.078 |
| Admission through ED (= 1 yes) | 671,519 | 0.751 | 0.432 |
| Out of hours in ED (= 1 yes) | 867,902 | 0.542 | 0.498 |
| ED attendances in past year: | |||
| 0 | 827,467 | 0.445 | 0.497 |
| 1 | 827,467 | 0.241 | 0.428 |
| 2 | 827,467 | 0.130 | 0.337 |
| 3+ | 827,467 | 0.183 | 0.387 |
| Hospital admissions in past year: | |||
| 0 | 671,028 | 0.475 | 0.499 |
| 1 | 671,028 | 0.247 | 0.431 |
| 2 | 671,028 | 0.127 | 0.333 |
| 3+ | 671,028 | 0.151 | 0.358 |
| ED site variables | |||
| Site size in 1000 | 867,902 | 13.393 | 4.440 |
| Attendances per consultant | 867,902 | 1618.25 | 626.812 |
Some 54.2% attended the ED out of hours, 44.5% had not attended an ED in the preceding 12 months, but 18.73% had attended three times or more. Of those admitted to hospital, 47.5% had not been admitted previously in the preceding year while 15.1% had been admitted three or more times.
ED pathway outcomes
Figures 5–7 and Table 14 present the results from the analyses of the three ED outcomes. Patients in older age categories were more likely to experience poor outcomes. For example, compared with those aged 75–79 years, those aged ≥ 95 years were more likely to stay more than 4 hours in the ED (OR 1.146), to be admitted to hospital (OR 1.345) and to reattend the ED within 30 days (OR 1.091). Men were more likely to be admitted to hospital (OR 1.139) and had a higher likelihood of ED reattendance (OR 1.123). Compared with those living in the most deprived areas (IMD 1), those in better-off areas (IMD 10) were less likely to spend more than four hours in the ED (OR 0.921), to be admitted to hospital (OR 0.882) or to reattend (OR 0.862).
| Dependent variable | (1) | (2) | (3) |
|---|---|---|---|
| ED duration (> 4 hours) | Hospital admission from ED | 30-day reattendance | |
| Logit (OR) | Logit (OR) | Logit (R) | |
| Age (years): | |||
| 80–85 | 1.076* (0.008) | 1.148* (0.008) | 1.027* (0.010) |
| 85–90 | 1.124* (0.009) | 1.301* (0.010) | 1.081* (0.011) |
| 90–95 | 1.157* (0.011) | 1.391* (0.014) | 1.102* (0.013) |
| 95+ | 1.146* (0.017) | 1.345* (0.021) | 1.091* (0.019) |
| Sex (= 1 male) | 1.008 (0.006) | 1.139* (0.007) | 1.123* (0.008) |
| IMD decile: | |||
| 2 | 0.984 (0.011) | 0.992 (0.012) | 0.969** (0.016) (0.016) |
| 3 | 0.968* (0.011) | 1.001 (0.012) | 0.965*** (0.014) |
| 4 | 0.979** (0.012) | 1.008 (0.013) | 0.942* (0.014) |
| 5 | 0.971*** (0.011) | 0.983 (0.012) | 0.914* (0.015) |
| 6 | 0.960* (0.011) | 0.957* (0.011) | 0.902* (0.013) |
| 7 | 0.948* (0.011) | 0.964* (0.011) | 0.886* (0.013) |
| 8 | 0.933* (0.011) | 0.929* (0.011) | 0.878* (0.013) |
| 9 | 0.941* (0.012) | 0.922* (0.011) | 0.869* (0.013) |
| 10 | 0.921* (0.012) | 0.882* (0.012) | 0.862* (0.014) |
| Care home (= 1 yes) | 1.095* (0.008) | 0.795* (0.007) | 1.014 (0.009) |
| Road travel distance (minutes) | 1.001* (0.0003) | 1.006* (0.0003) | 0.993* (0.0004) |
| Length NHS 111 call (minutes) | 0.998* (0.0004) | 0.989* (0.0004) | 0.998* (0.001) |
| NHS 111 calls per day (> 1) | 1.076*** (0.035) | 0.987 (0.032) | 1.076*** (0.038) |
| Ambulance calls per day (> 1) | 1.144* (0.020) | 1.342* (0.024) | 1.107* (0.021) |
| Call handler designation: | |||
| Less urgent | 1.238* (0.015) | 2.155* (0.025) | 1.049* (0.014) |
| Urgent | 1.359* (0.019) | 2.590* (0.035) | 1.086* (0.017) |
| Emergency | 1.350* (0.015) | 3.172* (0.036) | 1.053* (0.014) |
| Life-threatening | 1.448* (0.052) | 3.603* (0.144) | 1.026 (0.045) |
| Length ambulance on scene (minutes) | 1.003* (0.0002) | 1.009* (0.0002) | 1.001*** (0.0002) |
| Length ambulance service (minutes) | 1.002* (0.0001) | 0.999* (0.0001) | 1.000* (0.0001) |
| Out of hours (= 1 yes) | 1.419* (0.009) | 1.261* (0.008) | 1.071* (0.008) |
| ED attendances in past year: | |||
| 1 | 1.046* (0.007) | 1.122* (0.007) | 1.432* (0.011) |
| 2 | 1.073* (0.009) | 1.180* (0.009) | 1.845* (0.016) |
| 3+ | 1.087* (0.009) | 1.146* (0.010) | 3.133* (0.036) |
| Site size (attendances in 1000s) | 1.083* (0.004) | 0.729* (0.003) | 1.018* (0.004) |
| Attendances per consultant | 0.999* (1.67e-05) | 0.999* (1.52e-05) | 0.999 (1.89e-05) |
| N | 823,006 | 823,471 | 823,062 |
| Sites | 18 | 18 | 18 |
| Time fixed-effects (day, month, year) | yes | yes | yes |
| Site fixed-effects | yes | yes | yes |
| SE clustered | patient | patient | patient |
| Pseudo R2 | 0.144 | 0.089 | 0.060 |
| Years | 2013–2017 | 2013–2017 | 2013–2017 |
FIGURE 5.
ED pathway outcomes: ED duration (more than four hours).
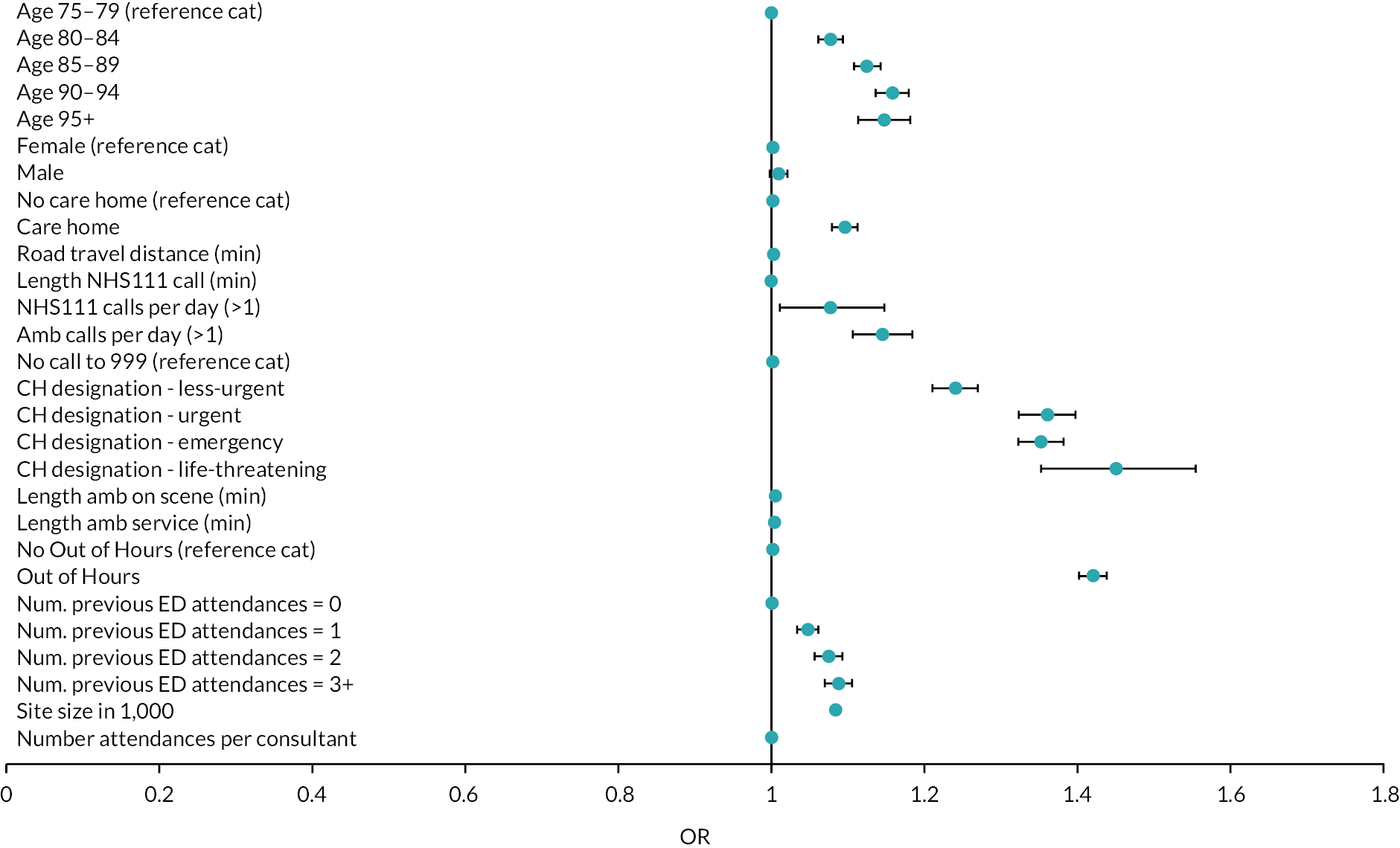
FIGURE 6.
ED pathway outcomes: 30-day reattendance.
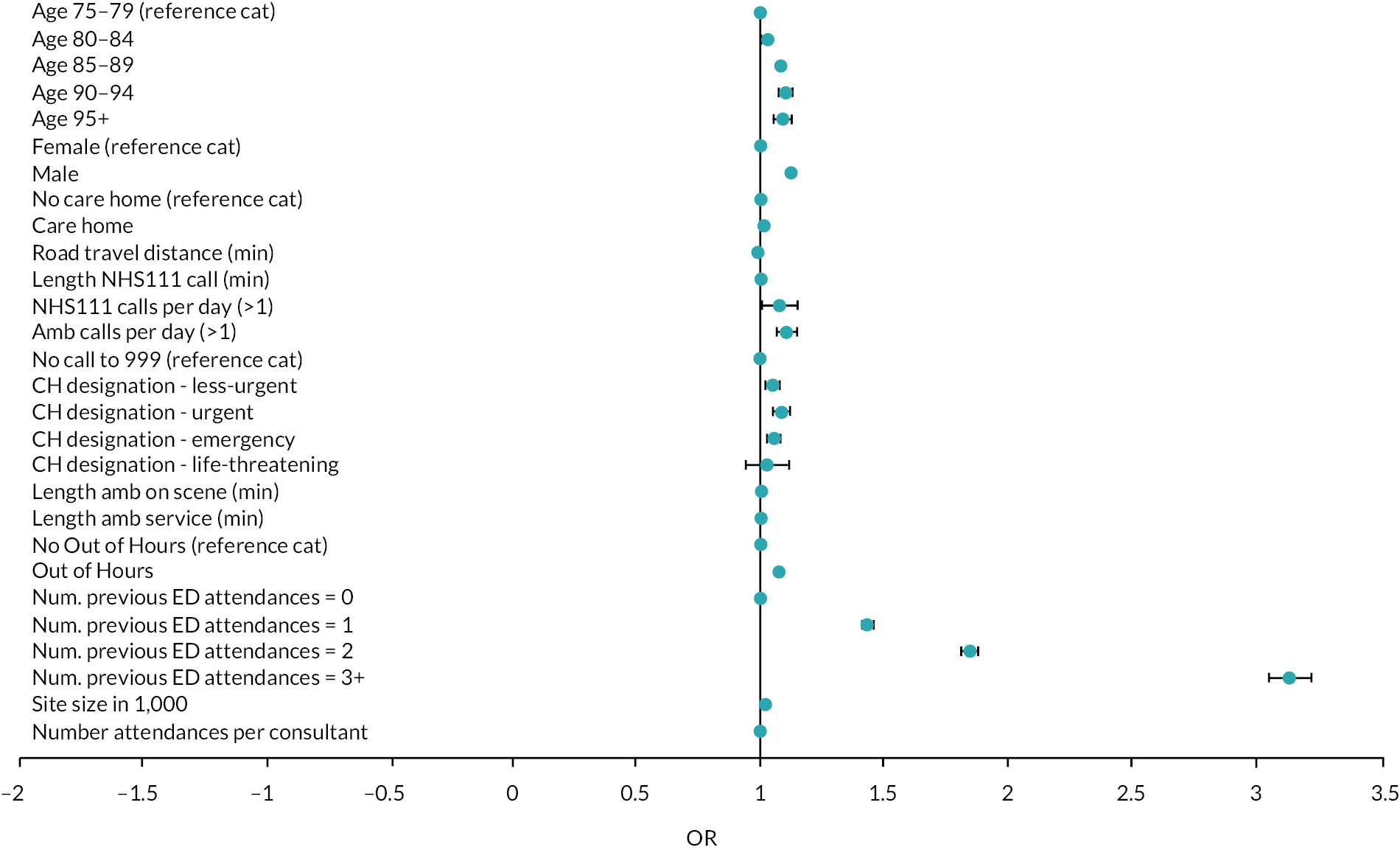
FIGURE 7.
ED pathway outcomes: hospital admission from ED.

Care home residents were more likely to spend more than four hours in the ED (OR 1.095) and less likely to be admitted to hospital (OR 0.795). People living closer to hospital were more likely to be admitted to hospital (OR. 1.006) and were less likely to reattend (OR 0.993).
Patients were more likely to spend more than four hours in the ED or to reattend if they made more than one NHS 111 or 999 call on the day of ED attendance. The call handler designation of urgency for those conveyed by ambulance was associated with all three outcomes. Compared with those who made their own way to the ED, the probability of waiting more than four hours was higher for those designated as less urgent (OR 1.238), urgent (OR 1.359), emergency (OR 1.35) and with a life-threatening condition (OR 1.448). There is also clear gradient across urgency categories in the likelihood of hospital admission, increasing from OR 2.155 for those designated less urgent to OR 3.603 for those with life-threatening conditions. This pattern is not evident and the effects are smaller or insignificant when considering reattendance. The longer the ambulance was on the scene, the higher the likelihood that the patient stayed more than four hours in the ED (OR 1.003) or was admitted to hospital (OR 1.009).
Patients who attended the ED out of hours spent longer in the ED (OR 1.419) were more likely to be admitted to hospital (OR 1.261) and were more likely to reattend (OR 1.071). Previous attendances were significantly associated with all outcomes, particularly for frequent attenders. Compared with those who had not previously attended the ED in the past year, those who had attended three or more times were more likely to spend more than four hours in ED (OR 1.087), to be admitted to hospital (OR 1.146) and to reattend the ED within 30 days (OR 3.133). The larger the ED (expressed as the number of attendances), the higher the likelihood of waiting more than four hours and of reattendance but the lower the likelihood of hospital admission. The effect sizes associated with number of attendances per senior doctor are very close to OR 1 for all three outcomes.
Ambulance pathway outcomes
Table 15 and Figures 8–10 report the analyses of the ED outcomes for those who were or were not conveyed to the ED by ambulance, revealing clear differences between these two groups in what factors were associated with their outcomes.
| Dependent variable | ED duration (> 4 hours) | Hospital admission from ED | 30-day reattendance | |||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Sample | Ambulance | Non-ambulance | Ambulance | Non-ambulance | Ambulance | Non-ambulance |
| Logit (OR) | Logit (OR) | Logit (OR) | ||||
| Age (years): | ||||||
| 80–85 | 1.015 (0.010) | 1.133* (0.012) | 1.074* (0.012) | 1.189* (0.011) | 1.017 (0.013) | 1.033* (0.013) |
| 85–90 | 1.017 (0.011) | 1.253* (0.015) | 1.143* (0.013) | 1.422* (0.015) | 1.052* (0.014) | 1.114* (0.015) |
| 90–95 | 1.030** (0.012) | 1.333* (0.020) | 1.181* (0.015) | 1.590* (0.022) | 1.072* (0.016) | 1.138* (0.019) |
| > 95 | 0.999 (0.019) | 1.374* (0.035) | 1.104* (0.021) | 1.618* (0.038) | 1.067* (0.023) | 1.117* (0.030) |
| Sex (= 1 male) | 0.997 (0.008) | 1.021** (0.009) | 1.115* (0.009) | 1.161* (0.009) | 1.117* (0.011) | 1.131* (0.012) |
| IMD decile: | ||||||
| 2 | 0.984 (0.014) | 0.991 (0.017) | 0.995 (0.016) | 0.999 (0.016) | 0.975 (0.019) | 0.964 (0.022) |
| 3 | 0.973*** (0.014) | 0.968*** (0.016) | 1.008 (0.016) | 1.000 (0.016) | 0.982 (0.018) | 0.945* (0.019) |
| 4 | 1.002 (0.015) | 0.959** (0.018) | 1.035** (0.017) | 0.991 (0.017) | 0.959** (0.018) | 0.921* (0.020) |
| 5 | 0.972*** (0.015) | 0.974 (0.017) | 1.001 (0.017) | 0.961** (0.016) | 0.909* (0.017) | 0.919* (0.021) |
| 6 | 0.983 (0.015) | 0.944* (0.017) | 1.038** (0.016) | 0.891* (0.014) | 0.891* (0.016) | 0.916* (0.018) |
| 7 | 0.964** (0.014) | 0.948* (0.016) | 1.026*** (0.016) | 0.928* (0.015) | 0.890* (0.017) | 0.884* (0.018) |
| 8 | 0.962** (0.015) | 0.912* (0.017) | 0.991 (0.017) | 0.888* (0.015) | 0.868* (0.017) | 0.893* (0.019) |
| 9 | 0.976 (0.016) | 0.921* (0.017) | 0.992 (0.017) | 0.879* (0.014) | 0.868* (0.017) | 0.873* (0.018) |
| 10 | 0.950* (0.016) | 0.900* (0.019) | 0.959** (0.018) | 0.832* (0.016) | 0.840* (0.018) | 0.886* (0.021) |
| Care home (=1 yes) | 1.044* (0.010) | 1.184* (0.016) | 0.658* (0.006) | 1.071* (0.013) | 0.992 (0.011) | 1.054* (0.015) |
| Road travel distance (minutes) | 0.998* (0.001) | 1.004* (0.0004) | 1.002* (0.001) | 1.009* (0.0003) | 0.994* (0.001) | 0.992* (0.001) |
| Length of NHS 111 call (minutes) | 0.999 (0.001) | 0.990* (0.001) | 0.991* (0.0004) | 0.976* (0.001) | 0.999 (0.001) | 0.992* (0.001) |
| NHS 111 calls per day (> 1) | 1.095* (0.037) | 0.660** (0.108) | 0.965 (0.032) | 0.882 (0.115) | 1.080** (0.039) | 0.919 (0.143) |
| Ambulance calls per day (> 1) | 1.124* (0.020) | 1.360* (0.024) | 1.100* (0.0204) | |||
| Call handler designation: | ||||||
| Urgent | 0.930* (0.013) | 1.183* (0.017) | 1.020 (0.017) | |||
| Emergency | 1.086* (0.010) | 1.467* (0.013) | 1.000 (0.010) | |||
| Life-threatening | 1.020 (0.038) | 1.732* (0.070) | 0.968 (0.043) | |||
| Length of time ambulance on scene (minutes) | 1.004* (0.0002) | 1.009* (0.0002) | 1.001* (0.0002) | |||
| Length ambulance service (minutes)a | 1.002* (0.0001) | 1.000 (0.0001) | 1.000* (0.0001) | |||
| Out of hours (= 1 yes) | 1.295* (0.011) | 1.622* (0.017) | 1.051* (0.009) | 1.531* (0.014) | 1.041* (0.010) | 1.119* (0.013) |
| ED attendances in past year: | ||||||
| 1 | 1.045* (0.009) | 1.046* (0.011) | 1.077* (0.009) | 1.148* (0.009) | 1.388* (0.014) | 1.473* (0.016) |
| 2 | 1.063* (0.012) | 1.081* (0.014) | 1.082* (0.012) | 1.263* (0.014) | 1.772* (0.021) | 1.919* (0.025) |
| 3+ | 1.037* (0.011) | 1.172* (0.016) | 0.975** (0.011) | 1.371* (0.017) | 3.008* (0.040) | 3.268* (0.053) |
| Site size in 1000 | 1.104* (0.005) | 1.038* (0.007) | 0.727* (0.004) | 0.739* (0.004) | 1.018* (0.005) | 1.016** (0.007) |
| Attendances per consultant | 1.000* (2.48e-05) | 1.000* (2.29e-05) | 1.000* (2.54e-05) | 1.000 (1.93e-05) | 1.000 (2.83e-05) | 1.000 (2.47e-05) |
| N | 436,771 | 386,235 | 437,027 | 386,444 | 436,801 | 386,261 |
| Time fixed-effects (day, month, year) | Yes | Yes | Yes | Yes | Yes | Yes |
| Site fixed-effects | Yes | Yes | Yes | Yes | Yes | Yes |
| SE clustered | patient | patient | patient | patient | patient | patient |
| Pseudo R2 | 0.150 | 0.116 | 0.037 | 0.043 | 0.064 | 0.054 |
| Years | 2013–2017 | 2013–2017 | 2013–2017 | 2013–2017 | 2013–2017 | 2013–2017 |
FIGURE 8.
Ambulance pathway outcomes: 30-day reattendance.
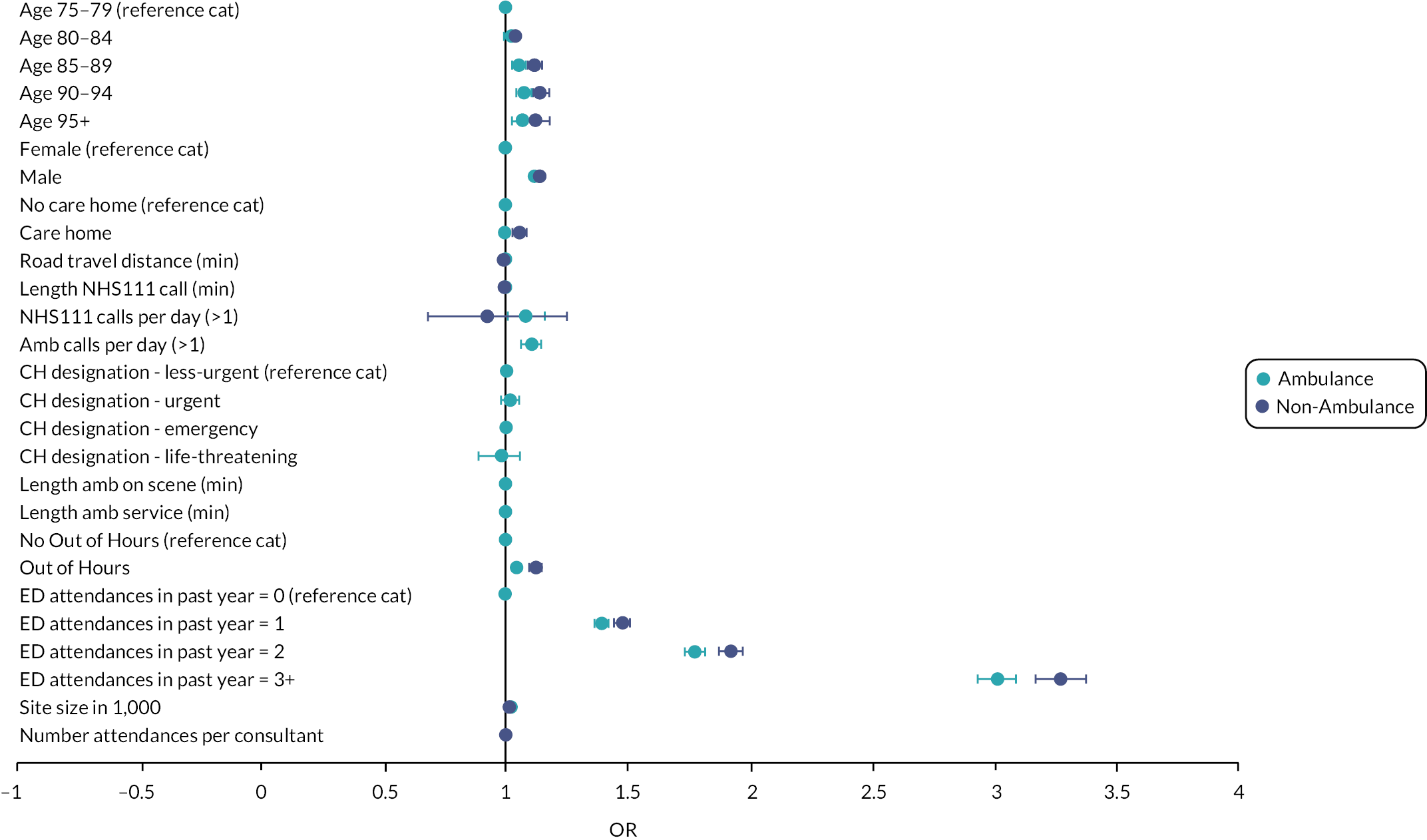
FIGURE 9.
Ambulance pathway outcomes: hospital admission from ED.
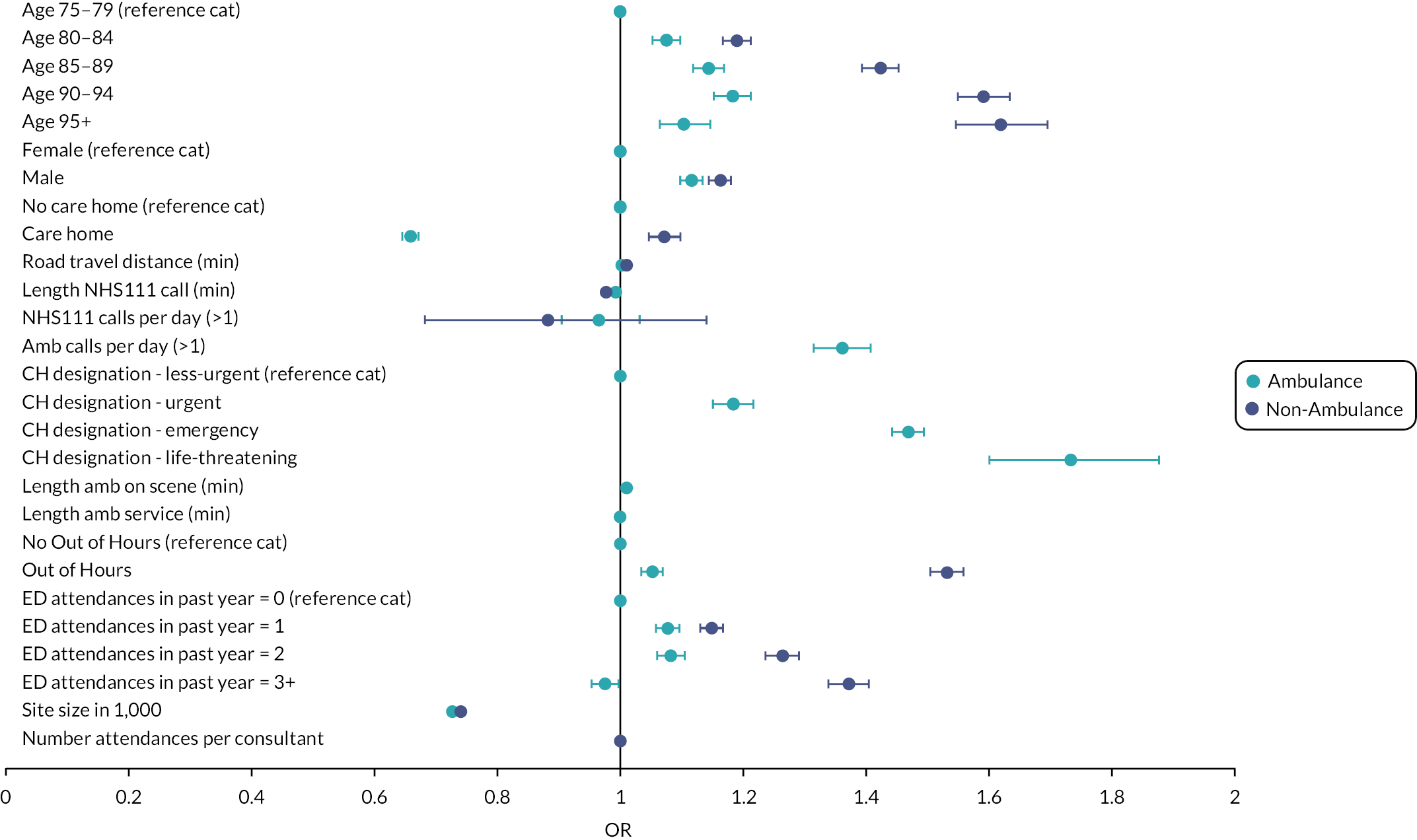
FIGURE 10.
Ambulance pathway outcomes: ED duration (more than four hours).

In terms of the likelihood of waiting more than four hours (Table 15 columns 1 and 2 and Figure 5), for those conveyed by ambulance, few of the patient characteristics are particularly influential, suggesting that people who arrived by ambulance were afforded priority based primarily upon their clinical urgency rather than any other characteristic. For this group, the most influential factor was whether they attended out of hours (OR 1.295).
Out-of-hours attendance was also the most influential factor (OR 1.622) in explaining the probability of waiting more than four hours for those who did not arrive at the ED by ambulance, but there is also an age gradient, older people facing longer ED waits (e.g. OR 1.374 for those ≥ 95 years) as did those from care homes (OR 1.184).
In terms of admission to hospital (Table 15 columns 3 and 4 and Figure 7), for those arriving by ambulance the most important factors were the call-handlers designation of urgency, those considered to have urgent (OR 1.183), emergency (OR 1.467) or life-threatening conditions (OR 1.732) being more likely to be admitted than those designated less-urgent. Older people were also more likely to be admitted (e.g. OR 1.104 for those ≥ 95 years).
Age was also an important predictor of admission for those who did not arrive by ambulance (e.g. OR 1.59 for those ≥ 95 years) and the likelihood of admission increased the more times they had previously attended the ED in the past year (e.g. OR 1.371 if three times) and if they attended out of hours (OR 1.531).
The factors associated with 30-day reattendance were little different for those who did and did not arrive at the ED by ambulance, previous attendances being the dominant factor.
Hospital pathway outcomes
Figures 11–13 and Table 16 report the analyses of the GIRFT measures of hospital length of stay. This reveals that frailty risk is more important at explaining length of stay than any of the other patient or pathway characteristics considered. Those with intermediate and high frailty risk were significantly less likely to have a short stay (OR 0.476 and OR 0.293, respectively) and significantly more likely to have a long stay (OR 2.909 and OR 5.872, respectively) and excessive stay (OR 4.25 and OR 11.78, respectively). When considering length of stay in excess of either 7 or 21 days, none of the other variables has an OR of more than 2. Of these other variables, the Charlson index (e.g. OR 1.891 for Charlson of 3+) and presence of an ambulatory care sensitive conditions (OR 1.384) were most important in explaining short length of stay and long stay, with similar effects for excessive length of stay.
FIGURE 11.
Hospital outcomes – length of stay (> 21 days).
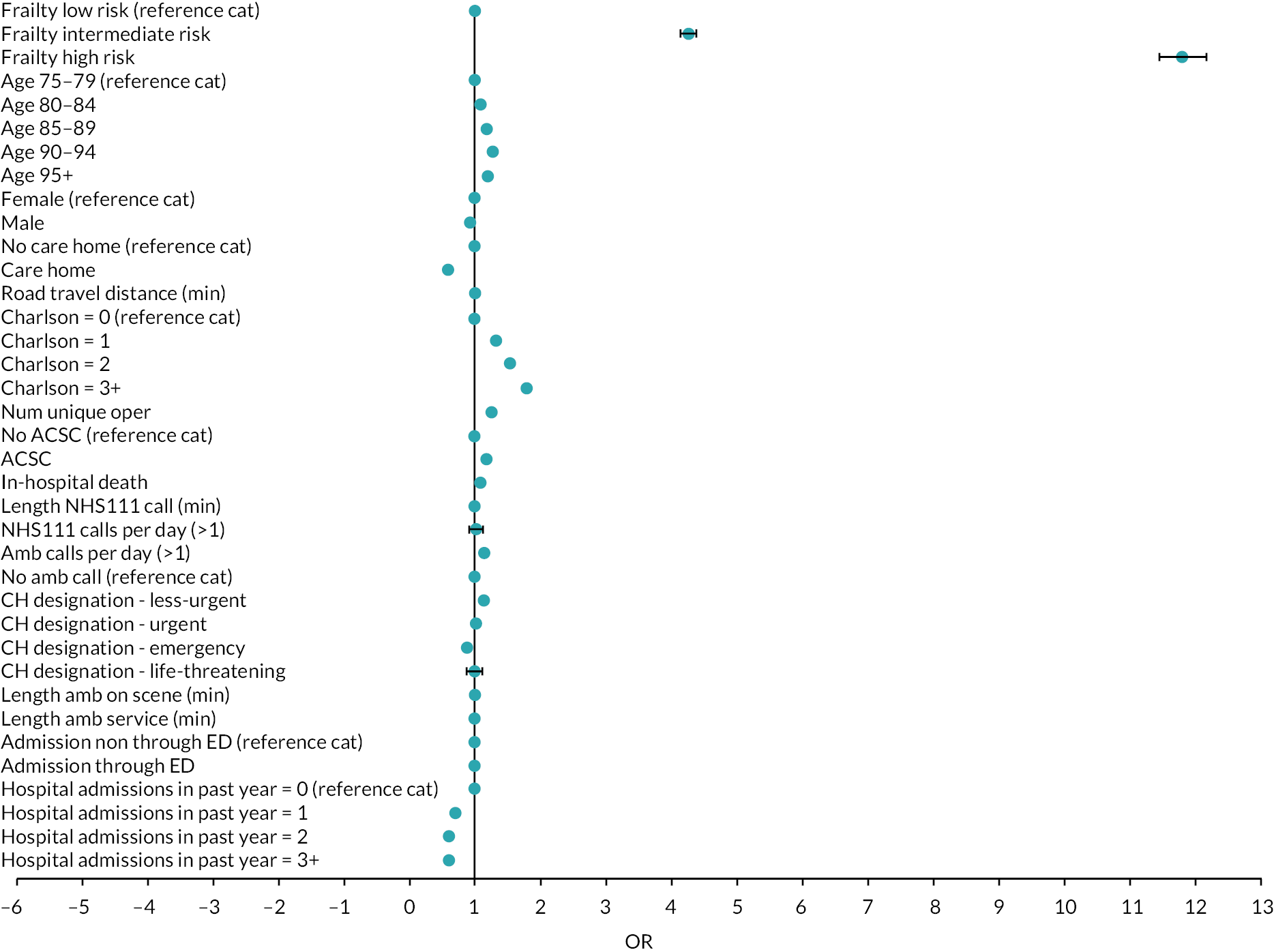
FIGURE 12.
Hospital outcomes – length of stay (> 7 days).

FIGURE 13.
Hospital outcomes – length of stay (< 2 days).
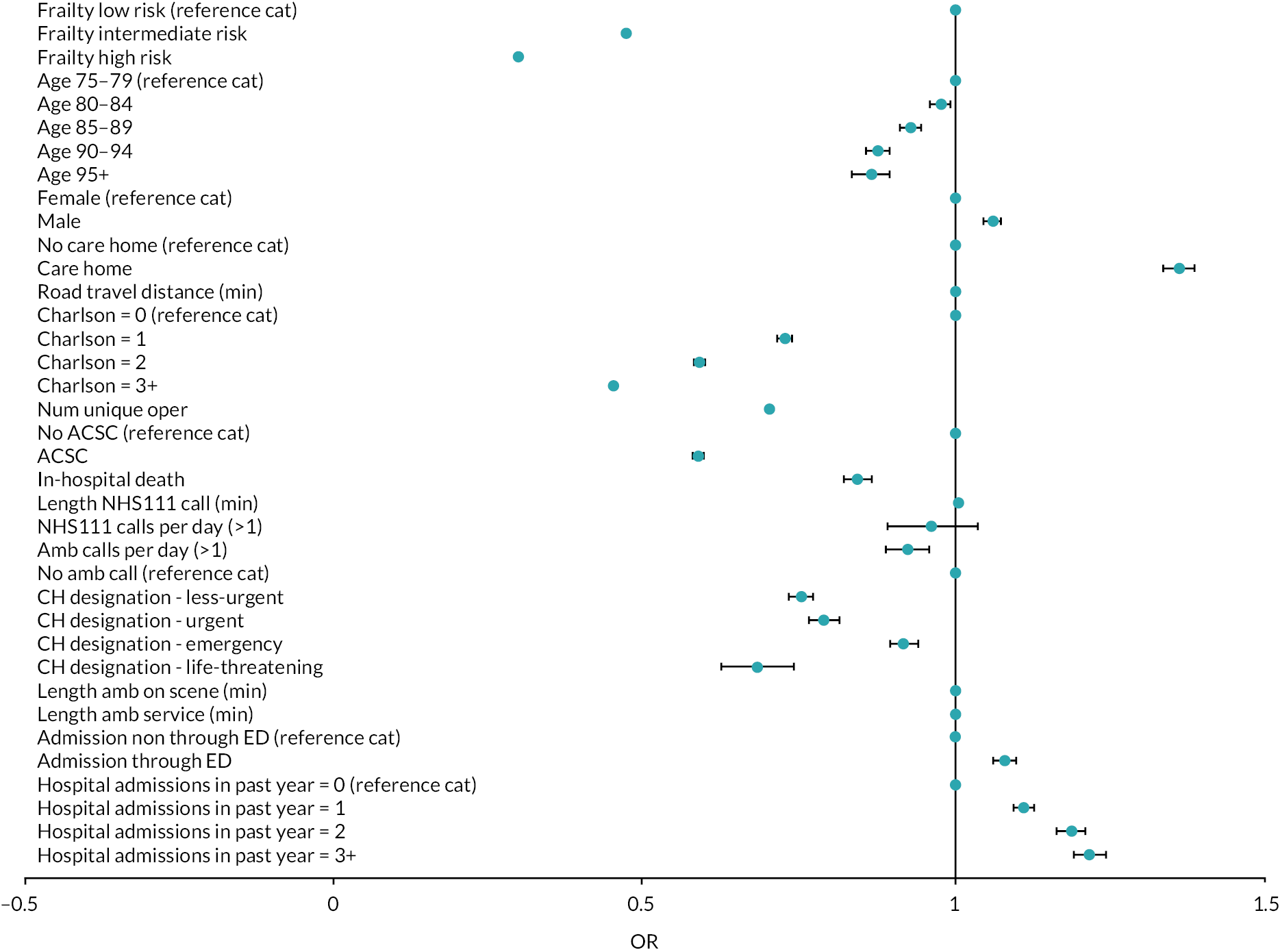
| Dependent variable | (1) | (2) | (3) |
|---|---|---|---|
| Length of stay | |||
| < 2 days | > 7 days | >21 days | |
| Logit (OR) | Logit (OR) | Logit (OR) | |
| Frailty risk: | |||
| Intermediate | 0.476* (0.003) | 2.909* (0.022) | 4.250* (0.058) |
| High | 0.293* (0.003) | 5.872* (0.057) | 11.78* (0.182) |
| Age: | |||
| 80–85 | 0.976* (0.008) | 1.082* (0.009) | 1.073* (0.014) |
| 85–90 | 0.928* (0.008) | 1.197* (0.011) | 1.165* (0.015) |
| 90–95 | 0.875* (0.010) | 1.286* (0.013) | 1.243* (0.019) |
| > 95 | 0.863* (0.015) | 1.278* (0.021) | 1.194* (0.027) |
| Sex (= 1 male) | 1.059* (0.007) | 0.882* (0.006) | 0.922* (0.009) |
| IMD decile: | |||
| 2 | 1.001 (0.013) | 1.006 (0.013) | 1.017 (0.018) |
| 3 | 0.968** (0.013) | 1.025** (0.013) | 1.043** (0.019) |
| 4 | 0.987 (0.014) | 1.015 (0.014) | 1.028 (0.020) |
| 5 | 1.008 (0.014) | 1.002 (0.013) | 1.002 (0.019) |
| 6 | 0.976*** (0.013) | 1.021 (0.013) | 1.000 (0.018) |
| 7 | 1.000 (0.013) | 1.016 (0.013) | 1.004 (0.018) |
| 8 | 1.011 (0.014) | 1.000 (0.013) | 1.002 (0.019) |
| 9 | 0.995 (0.014) | 1.003 (0.014) | 1.017 (0.020) |
| 10 | 1.031** (0.016) | 0.996 (0.015) | 1.004 (0.021) |
| Care home (= 1 yes) | 1.360* (0.013) | 0.654* (0.006) | 0.592* (0.007) |
| Road travel distance (minutes) | 0.998* (0.0004) | 0.999 (0.0004) | 0.998* (0.0005) |
| Charlson comorbidity index: | |||
| 1 | 0.725* (0.006) | 1.274* (0.012) | 1.320* (0.019) |
| 2 | 0.587* (0.006) | 1.495* (0.015) | 1.532* (0.024) |
| 3+ | 0.449* (0.004) | 1.891* (0.017) | 1.782* (0.026) |
| Number of unique operations | 0.701* (0.002) | 1.305* (0.002) | 1.249* (0.002) |
| ACSC (= 1 yes) | 0.585* (0.005) | 1.384* (0.011) | 1.188* (0.013) |
| In-hospital death | 0.842* (0.011) | 1.079* (0.012) | 1.044* (0.015) |
| Length of NHS 111 call (minutes) | 1.003* (0.001) | 0.996* (0.001) | 0.996* (0.001) |
| NHS 111 calls per day (> 1) | 0.960 (0.037) | 1.049 (0.037) | 0.997 (0.052) |
| Ambulance calls per day (> 1) | 0.923* (0.018) | 1.082* (0.019) | 1.143* (0.028) |
| Call handler designation: | |||
| Less-urgent | 0.752* (0.010) | 1.148* (0.014) | 1.134* (0.021) |
| Urgent | 0.788* (0.012) | 1.011 (0.015) | 1.019 (0.023) |
| Emergency | 0.916* (0.012) | 0.874* (0.011) | 0.867* (0.016) |
| Life-threatening | 0.679* (0.030) | 0.996 (0.040) | 0.984 (0.059) |
| Length of time ambulance on scene (minutes) | 0.998* (0.0002) | 1.004* (0.0002) | 1.003* (0.0003) |
| Length ambulance service (minutes)a | 0.999* (0.0001) | 1.001* (9.54e-05) | 1.000* (0.0001) |
| Admission through ED | 1.079* | 0.957* | 0.994 |
| Hospital admissions in past year: | (0.009) | (0.008) | (0.013) |
| 1 | 1.110* (0.008) | 0.797* (0.006) | 0.699* (0.008) |
| 2 | 1.186* (0.012) | 0.716* (0.007) | 0.603* (0.008) |
| 3+ | 1.216* (0.013) | 0.716* (0.007) | 0.607* (0.008) |
| N | 668,786 | 668,781 | 668,779 |
| Time fixed-effects (day, month, year) | Yes | Yes | Yes |
| Site fixed-effects | Yes | Yes | Yes |
| SE clustered | patient | patient | patient |
| Pseudo R2 | 0.136 | 0.151 | 0.174 |
| Years | 2013–2017 | 2013–2017 | 2013–2018 |
Column (1) of Table 17 and Figure 16 report the results pertaining to the analysis of in-hospital death. Frailty risk is associated with greater likelihood of in-hospital death (HR 1.872 for intermediate risk and 2.042 for high risk). The likelihood also increases for older people (e.g. HR 1.832 for those ≥ 95 years), in line with the Charlson measures (e.g. HR 3.239 for Charlson of 3+), for those with an ambulatory care sensitive condition (HR 1.483) and according to the call-handler’s designation of urgency (e.g. HR 1.838 for those designated as having a life-threatening condition).
| Dependent variable | (1) | (2) | (3) | (4) |
|---|---|---|---|---|
| In-hospital death | 30-day readmissions | > 3 admissions 90 days | > 3 admissions 365 days | |
| HR | Bivariate problem AME | Logit (OR) | Logit (OR) | |
| Frailty risk: | ||||
| Intermediate | 1.872* (0.025) | 0.006* (0.001) | 0.898* (0.023) | 1.115* (0.014) |
| High | 2.042* (0.034) | −0.010* (0.002) | 0.671* (0.024) | 0.964** (0.017) |
| Age: | ||||
| 80–85 | 1.134* (0.015) | −0.004* (0.001) | 0.881* (0.029) | 0.972*** (0.016) |
| 85–90 | 1.337* (0.019) | −0.001 (0.002) | 0.894* (0.033) | 0.976 (0.017) |
| 90–95 | 1.580* (0.025) | −0.004** (0.002) | 0.847* (0.036) | 0.925* (0.019) |
| 95+ | 1.832* (0.042) | −0.009* (0.003) | 0.823* (0.056) | 0.841* (0.029) |
| Sex (=1 male) | 1.211* (0.012) | 0.014* (0.001) | 1.177* (0.031) | 1.094* (0.014) |
| IMD decile: | ||||
| 2 | 0.947* (0.018) | −0.008* (0.002) | 0.899** (0.047) | 0.952** (0.023) |
| 3 | 0.930* (0.017) | −0.003 (0.002) | 0.943 (0.046) | 0.929* (0.022) |
| 4 | 0.942* (0.019) | −0.006* (0.002) | 0.869* (0.045) | 0.875* (0.023) |
| 5 | 0.927* (0.018) | −0.008* (0.002) | 0.879** (0.051) | 0.864* (0.023) |
| 6 | 0.885* (0.017) | −0.011* (0.002) | 0.807* (0.040) | 0.846* (0.020) |
| 7 | 0.917* (0.017) | −0.013* (0.002) | 0.819* (0.046) | 0.811* (0.021) |
| 8 | 0.895* (0.018) | −0.015* (0.002) | 0.732* (0.041) | 0.762* (0.020) |
| 9 | 0.887* (0.018) | −0.014* (0.002) | 0.742* (0.039) | 0.773* (0.020) |
| 10 | 0.834* (0.019) | −0.018* (0.002) | 0.676* (0.041) | 0.704* (0.021) |
| Care home (= 1 yes) | 0.789* (0.010) | −0.015* (0.001) | 0.762* (0.028) | 0.651* (0.012) |
| Road travel distance (minutes) | 0.996* (0.0005) | −0.0004* (5.91e-05) | 0.994* (0.001) | 0.993* (0.001) |
| Charlson comorbidity index: | ||||
| 1 | 1.736* (0.033) | 0.013* (0.002) | 1.209* (0.041) | 1.146* (0.017) |
| 2 | 2.346* (0.045) | 0.025* (0.002) | 1.335* (0.046) | 1.283* (0.021) |
| 3+ | 3.239* (0.059) | 0.027* (0.002) | 1.374* (0.047) | 1.298* (0.021) |
| Unique operations | 1.064* (0.002) | −0.002* (0.0002) | 0.977* (0.004) | 0.968* (0.002) |
| ACSC (= 1 yes) | 1.483* (0.016) | 0.003** (0.001) | 1.189* (0.027) | 1.173* (0.014) |
| Length of stay | 0.997* (0.0003) | −0.0001* (3.92e-05) | – | – |
| Length NHS 111 call (minutes) | 0.993* (0.001) | −4.18e-05 (8.70e-05) | 1.000 (0.001) | 1.000 (0.001) |
| NHS 111 calls per day (> 1) | 1.161** (0.071) | 0.004 (0.006) | 1.133 (0.094) | 0.994 (0.047) |
| Ambulance calls per day (> 1) | 1.036 (0.031) | 0.026* (0.003) | 1.159* (0.050) | 1.021 (0.025) |
| Call handler designation: | ||||
| Less-urgent | 1.123* (0.023) | 0.003 (0.002) | 0.949 (0.033) | 0.999 (0.017) |
| Urgent | 1.198* (0.031) | 0.001 (0.003) | 1.019 (0.045) | 0.785* (0.019) |
| Emergency | 1.423* (0.029) | −0.003 (0.002) | 1.057 (0.036) | 1.081* (0.018) |
| Life-threatening | 1.838* (0.099) | −0.011*** (0.006) | 0.821 (0.107) | 0.498* (0.049) |
| Length of time ambulance on scene (minutes) | 1.002* (0.0003) | 0.0002* (3.48e-05) | 1.001** (0.001) | 1.002* (0.0003) |
| Length ambulance service (minutes) | 1.000*** (0.0001) | 2.05e-05 (1.63e-05) | 1.000 (0.0003) | 1.000 (0.0001) |
| Admission through ED | 0.984 (0.012) | −0.004** (0.001) | 1.139* (0.029) | 1.165* (0.015) |
| Hospital admissions in past year: | ||||
| 1 | 1.001 (0.012) | 0.047* (0.001) | 1.903* (0.038) | 1.747* (0.015) |
| 2 | 0.996 (0.015) | 0.084* (0.002) | 3.031* (0.077) | 2.653* (0.034) |
| 3+ | 1.044* (0.016) | 0.150* (0.002) | 7.525* (0.255) | 5.156* (0.096) |
| N | 261,686 | 668,795 | 617,268 | 617,511 |
| Time fixed-effects (day, month, year) | Yes | Yes | Yes | Yes |
| Site fixed-effects | Yes | Yes | Yes | Yes |
| SE clustered | patient | patient | patient | patient |
| Pseudo R2 or AIC/BIC | 0.089 | 0.092 | 0.101 | |
| Years | 2013–2017 | 2013–2017 | 2013–2017 | 2013–2017 |
The remaining columns of Table 17 report the results for the three GIRFT measures of emergency readmission to hospital, which are also summarised in Figures 14 and 15. The number of past admissions in the previous year stand out as by far the most important explanatory variables. For example, those who had three or more admissions in the past year are 15% more likely to be readmitted within 30 days (average marginal effect 0.15) and to have more than three admissions within 90 days (OR 7.525) and 365 days (OR 5.156; Figure 14).
FIGURE 14.
Hospital pathway outcomes: > 3 admissions in 365 or 90 days.

FIGURE 15.
Hospital pathway outcomes: 30-day readmissions.
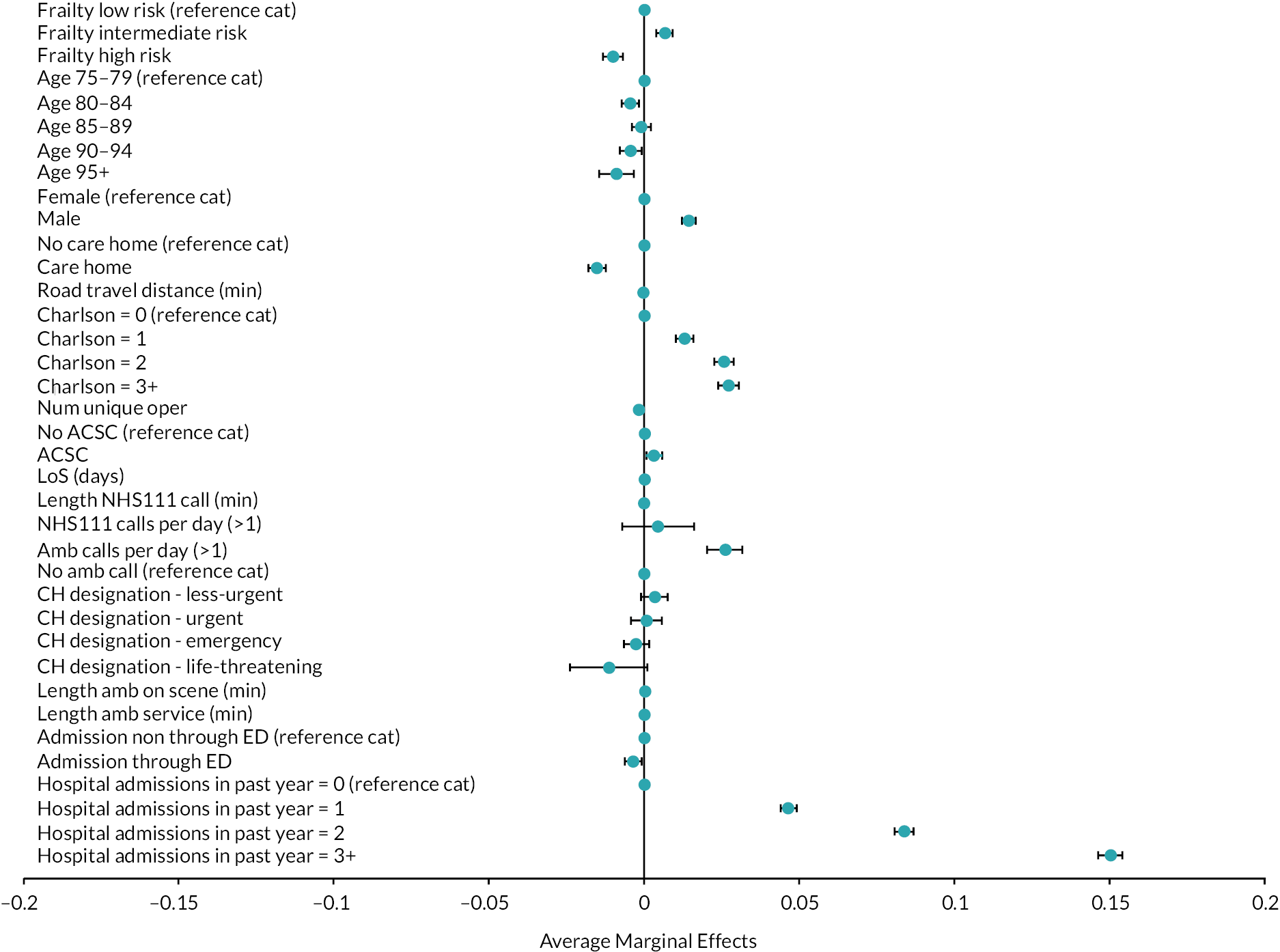
FIGURE 16.
Hospital pathway outcomes – in-hospital death.

Pathway and hospital costs
In Table 18 and Figures 17 and 18, the results of the cost analyses are presented. The most important factor influencing the costs of the overall UEC pathway is whether there is a hospital admission, costs being £4262 higher for those admitted to hospital compared with those who were not. Costs were £1328 and £2209 higher for patients designated by call-handlers to have an emergency or life-threatening condition and £1236 higher for those who died, whether in the ED or hospital.
| Dependent variable | Total pathway cost | HRG tariff inpatient |
|---|---|---|
| GLM. AME | GLM. AME | |
| Age (years): | ||
| 80–85 | 7.104 (7.819) | −1.957 (5.037) |
| 85–90 | −5.092 (8.252) | −4.502 (5.245) |
| 90–95 | −47.13* (10.20) | −2.875 (6.053) |
| 95+ | −96.41* (16.35) | −3.323 |
| Sex (= 1 male) | 15.47** (6.165) | −26.71* (4.062) |
| IMD decile: | ||
| 2 | 13.03 (11.81) | 1.952 (7.370) |
| 3 | 20.04*** (11.64) | 13.89*** (7.284) |
| 4 | 23.08*** (12.51) | 17.83** (7.838) |
| 5 | 30.76** (12.47) | 19.10** (7.802) |
| 6 | 33.53* (12.11) | 30.37* (7.801) |
| 7 | 23.50** (11.90) | 19.16** (7.506) |
| 8 | 25.77** (12.68) | 27.88* (8.198) |
| 9 | 28.68** (12.85) | 27.90* (8.190) |
| 10 | 15.41 (14.17) | 23.91* (8.954) |
| Care home (= 1 yes) | 113.2* (10.63) | 21.24* (5.344) |
| Road travel distance (minutes) | 11.55* (0.749) | 4.975* (0.360) |
| Hospital admission | 4262* (243.7) | – |
| ED duration | 54.57* (3.320) | – |
| Death | 1236* (72.06) | 333.1* (20.43) |
| Length of stay (days) | 217.5* (13.08) | 87.04* (5.315) |
| Frailty risk: | ||
| Intermediate | – | 269.3* (15.38) |
| High | – | 287.7* (16.38) |
| Charlson comorbidity index: | ||
| 1 | – | 165.5* (10.71) |
| 2 | – | 287.2* (17.39) |
| 3+ | – | 459.3* (26.97) |
| Unique operations | – | 179.3* (10.39) |
| ACSC (= 1 yes) | – | −74.07* (5.896) |
| Length of NHS 111 call (minutes) | −3.786* (0.476) | −2.622* (0.345) |
| NHS 111 calls per day (> 1) | −18.25 (33.10) | −30.42 (20.22) |
| Ambulance calls per day (> 1) | 232.8* (23.17) | 136.9* (13.17) |
| Call handler designation: | ||
| Less urgent | 417.7* (26.32) | 117.2* (10.06) |
| Urgent | 543.3* (34.12) | 40.65* (9.342) |
| Emergency | 1328* (77.97) | −92.07* (8.732) |
| Life-threatening | 2209* (137.9) | 202.8* (27.22) |
| Length of time ambulance on scene (minutes) | 2.613* (0.263) | 1.391* (0.147) |
| Length ambulance service (minutes) | −0.317* (0.108) | 0.152* (0.0584) |
| Admission through ED | – | −30.20* (5.462) |
| Hospital admissions in past year: | ||
| 1 | – | −45.64* (5.137) |
| 2 | – | −63.98* (6.702) |
| 3+ | – | −85.97* (7.769) |
| N | 1,028,856 | 668,795 |
| Time fixed-effects (day, month, year) | yes | yes |
| Site fixed-effects | yes | yes |
| SE clustered | patient | patient |
| AIC/BIC | 2.043/−1.39e+07 | 3.562/−8657482 |
| Years | 2013–2017 | 2013–2017 |
FIGURE 17.
Pathway and hospital costs: total pathway cost (in 1000 pounds).
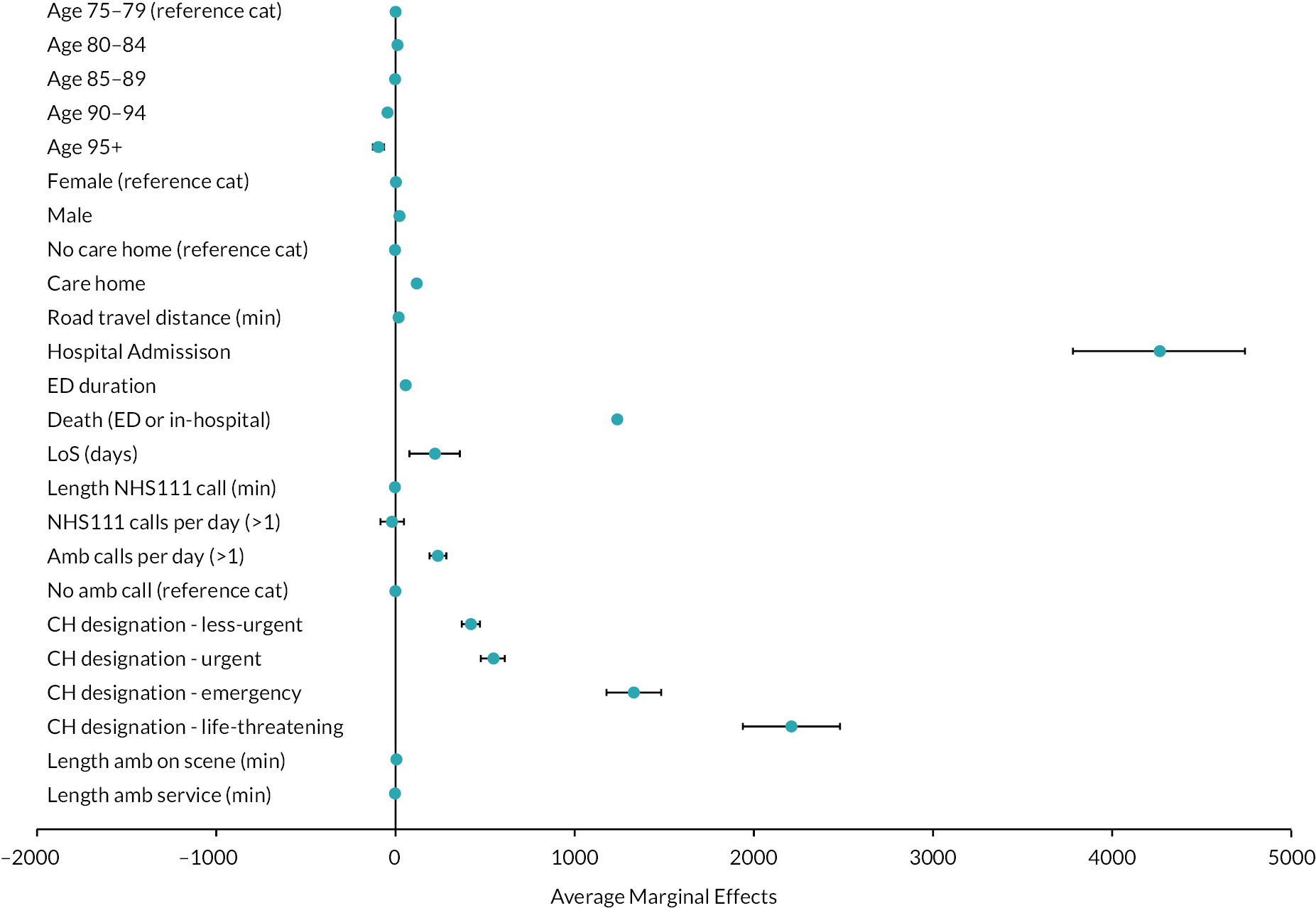
FIGURE 18.
Pathway and hospital costs: healthcare resource group tariff inpatient (in £1000s).
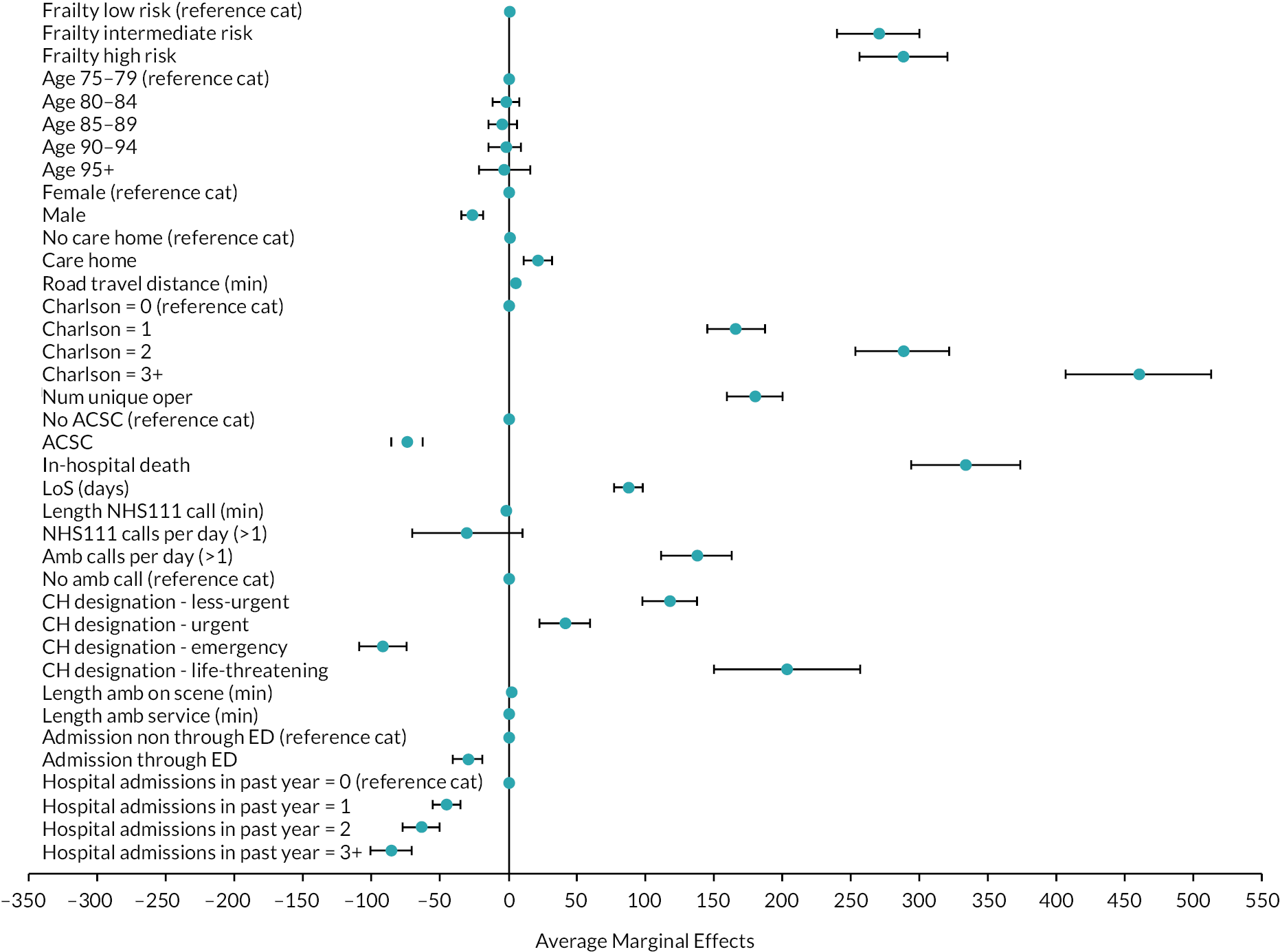
The analysis of hospital costs is reported in Table 18 column (2) and allows for the inclusion of a wider set of explanatory variables derived from the hospital data. Compared with those with low frailty risk, hospital costs were £269 higher for those of intermediate frailty risk and £288 higher for those of high frailty risk. Cost was £333 higher for those who died and increased by £87 for each day in hospital. Costs increased according to the Charlson categories, being £459 higher for those categorised as 3+ and by £179 for each operation conducted. Costs were also £137 higher for those who made more than one call for an ambulance on the day. Compared with those not conveyed to hospital by ambulance, costs were higher for those designated by the call-handlers as less urgent (£117), urgent (£41) and life-threatening (£202), but lower for those designated as an emergency (£92).
Discussion
Key results
To our knowledge, this study is the first to analyse a set of ED and hospital outcomes and costs, contextualised as a function of the UEC pathway. This allowed us to identify a number of factors associated with adverse outcomes using readily available system-level data. Our analyses of the three ED outcomes identified some factors that are already well known, notably that those of higher age, from more deprived areas and who attend out-of-hours had a greater likelihood of a longer ED wait, hospital admission and ED reattendance. But we offer novel insights, notably in recognising the importance of call-handler designation for those conveyed to the ED by ambulance.
Turing to the hospital outcomes, we find that frailty risk is a strong predictor of length of stay and in-hospital death, according with other studies that have applied the HFRS to older people. 87,88 We found that being categorised as having intermediate or high frailty risk was a more important predictor of length of stay than any of the other rich set of control variables included in our analyses. These categories also proved important explanators of in-hospital mortality, only the Charlson comorbidity index offering greater explanatory power. The fact that both the HFRS and Charlson categories were highly significant predictors when jointly considered demonstrates that they capture different risk factors associated with in-hospital death.
In contrast, frailty risk was not a strong predictor of 30-day readmission, as with McAlister87,89 but unlike Gilbert88 and Mitsutake. 90 The inability of the HFRS to predict readmission may be because of the care that frail patients receive: if post-discharge care packages are put in place to support frail individuals when they return to their usual place of residence, their risk of readmission may be reduced.
Strengths and limitations
The strengths of this study include the large longitudinal dataset which captured information from a populous region with diverse EDs. We benefited from a large sample size, thereby generating more precise estimates than for small samples. We controlled for a wide range of patient characteristics, included measures of the urgent care pathway not considered in previous studies, and were able to perform analyses of different UEC journeys.
The study has limitations. Although not a national study, the data are from large, representative region of the UK. Our analysis was limited to routinely available data, which prioritise service and system-level over patient-centred outcomes. 91 The data only reflect the period 2011–2017 and patterns of care may have changed in the interim. The explanatory variables can be improved, for instance by accounting for ethnicity, known to be poorly coded in English hospital data, and by using better measures of socioeconomic status. Even though diagnoses are likely to be important predictors of ED outcomes, unfortunately the ED data in the CUREd database do not capture diagnoses codes accurately or in a consistent fashion across EDs, with 61.5% of attendances having no diagnostic information recorded at all. While frailty identification is now commonplace in EDs,64 no measure of frailty could be constructed from our data for those attending the ED, only for those admitted to hospital. 88 For those admitted to hospital, the positive association between the HFRS and longer stay may be upwardly biased since patients with longer stays tend to have more extensive documentation of frailty-related diagnoses,92 though use of three-character rather than four-character ICD-10 codes offers some protection against this bias.
Implications for practice
Call handler designation of urgency could be relatively easily embedded in EDs that wish to focus efforts on those at the highest risk of a long ED wait, hospital admission and ED reattendance upon arrival at ED. Systems might test the use of some simple urgent care risk stratification based on call-handler designation to identify cohorts of people who might especially benefit from alternative care pathways, for instance with the use of a GP or geriatric emergency medicine-led telephone advice line for paramedics, or routine notation of the call-handler designation during ambulance handovers to ED triage practitioners. Call-handler designations are routinely captured in electronic patient report forms but often not in handwritten ones, and may not be routinely considered by professionals working in the ED. Given the characteristics of those prioritised urgent by call-handlers, and the emerging evidence base for frailty-attuned interventions in the ED,13,93 it might be that senior decision-makers delivering initial assessment could use the designation to inform early intervention discussions or involvement of professionals with frailty expertise.
Call handler designation of urgency could be relatively easily embedded in EDs that wish to focus efforts on those at the highest risk of adverse events upon arrival at ED. Systems might test the use of some simple urgent care risk stratification based on call-handler designation to identify cohorts of people who might especially benefit from alternative care pathways, such as early senior decision-making that can facilitate early intervention of transfer to a bed. Given the characteristics of those prioritised as requiring urgent attention by call handlers, and the emerging evidence base for frailty-attuned interventions in the ED,13,93 it might be that frailty expertise in combination with emergency medicine expertise could be usefully deployed to support these individuals.
Our analyses of hospital outcomes indicated the importance of frailty risk in explaining length of stay and in-hospital mortality. While no frailty tool discriminates well enough to direct clinical care at the individual patient level, tools can be used to identify cohorts at greater risk of poor outcomes. 94 If embedded into hospital electronic health records, the HFRS could be used to target frailty interventions, thereby avoiding the burden of implementing manual scores such as the CFS and improving the standardisation of frailty assessment. The combination of the electronic Frailty Index (eFI) for primary care95 and the HFRS for secondary care risk stratification would advance frailty-friendly care, as called for in various policy documents. 65,96 The eFI and HFRS identify different at-risk cohorts,97 so their combination offers opportunity to identify the whole population at risk. Further research could examine how well the HFRS performs in other health care systems, refine the HFRS through the incorporation of real-time data, and develop frailty-attuned clinical decision support systems. 98
Conclusion
Call-handler designation was the most powerful predictor of a four-hour wait and of transfer to hospital. We confirmed that frailty risk was a strong, independent predictor of length of stay and in-hospital death, but not 30-day readmission.
Chapter 7 Baseline simulation model development
The aim of WP4 was to develop a user-friendly simulation model to support future planning and service redesign by showing the impact on the wider health-care system of a range of ED based interventions for older people. The tool is intended to be used by clinicians, commissioners, and service managers. The simulation model and the user interface were developed in software widely used for simulation models (AnyLogic version 8.7.3, AnyLogic, Loughborough, UK). The model allows the user to use their own data (imported from a Microsoft Excel® file) or in-built data from WP3 and other external data to provide the baseline on which to compare the chosen intervention.
System dynamics has frequently been used to model whole systems such as those identified in this study. The scope of the model is shown in Figure 19. Patients are managed in primary care until they need help. Following a call for help, they may attend the ED where they may need to be admitted or are discharged. The model also includes those patients that are readmitted following their discharge home or to a long-term care facility.
FIGURE 19.
Scope of the model.
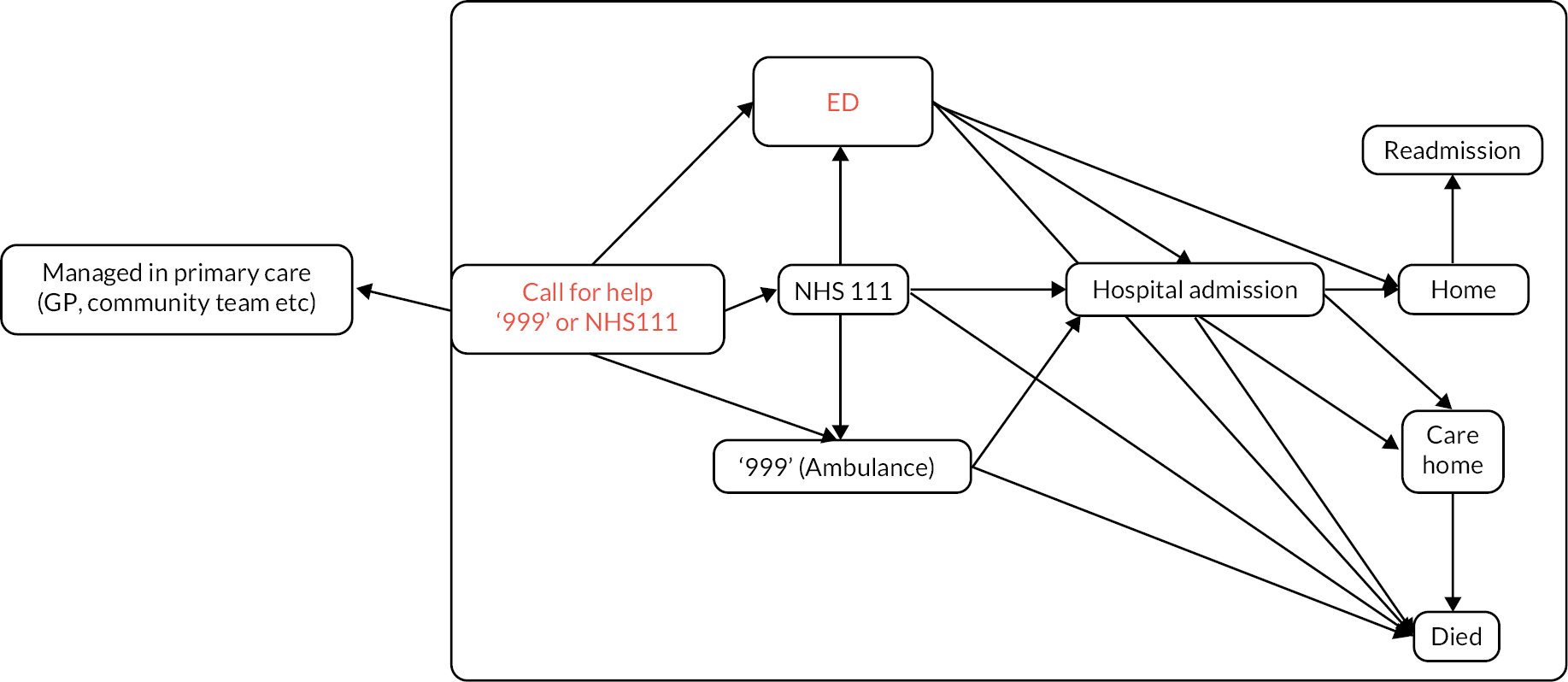
The analysis from WP3 informed the ED attendance rates, readmission rates, the number of emergency admissions as well as the proportion of patients admitted from the ED. The number of hospital related deaths was also estimated from the WP3 analysis, whereas deaths in care homes and the wider population were estimated using Office for National Statistics (ONS) mortality figures.
The earlier WPs informed the model’s development: WP1.1 identified the potential interventions and their effects on mortality, admission, readmission and length of stay. WP2 identified how the interventions are implemented in hospital settings and WP3 provided the baseline parameters for the model (Figure 20). The multidisciplinary nature of the study has been extremely beneficial in the model’s development, with clinicians and patient groups offering feedback on the model’s design and usability.
FIGURE 20.
How each component of the emergency care for older people study informs the system dynamics decision support tool.

Figures 21 and 22 show the front screen of the user interface and the summary screen where all the model parameters are captured prior to the model running. The front screen describes the purpose of the tool and provides some helpful hints on how to use the model. The parameter summary screen captures both demographic information on the population as well as hospital parameters such as the daily number of ED attendances, emergency admissions and readmissions. The screen also allows the user to select the chosen intervention and its hours of operation (24/7, weekdays, other).
FIGURE 21.
Initial screen of the decision support tool.
FIGURE 22.
Summary screen capturing all the model parameters.
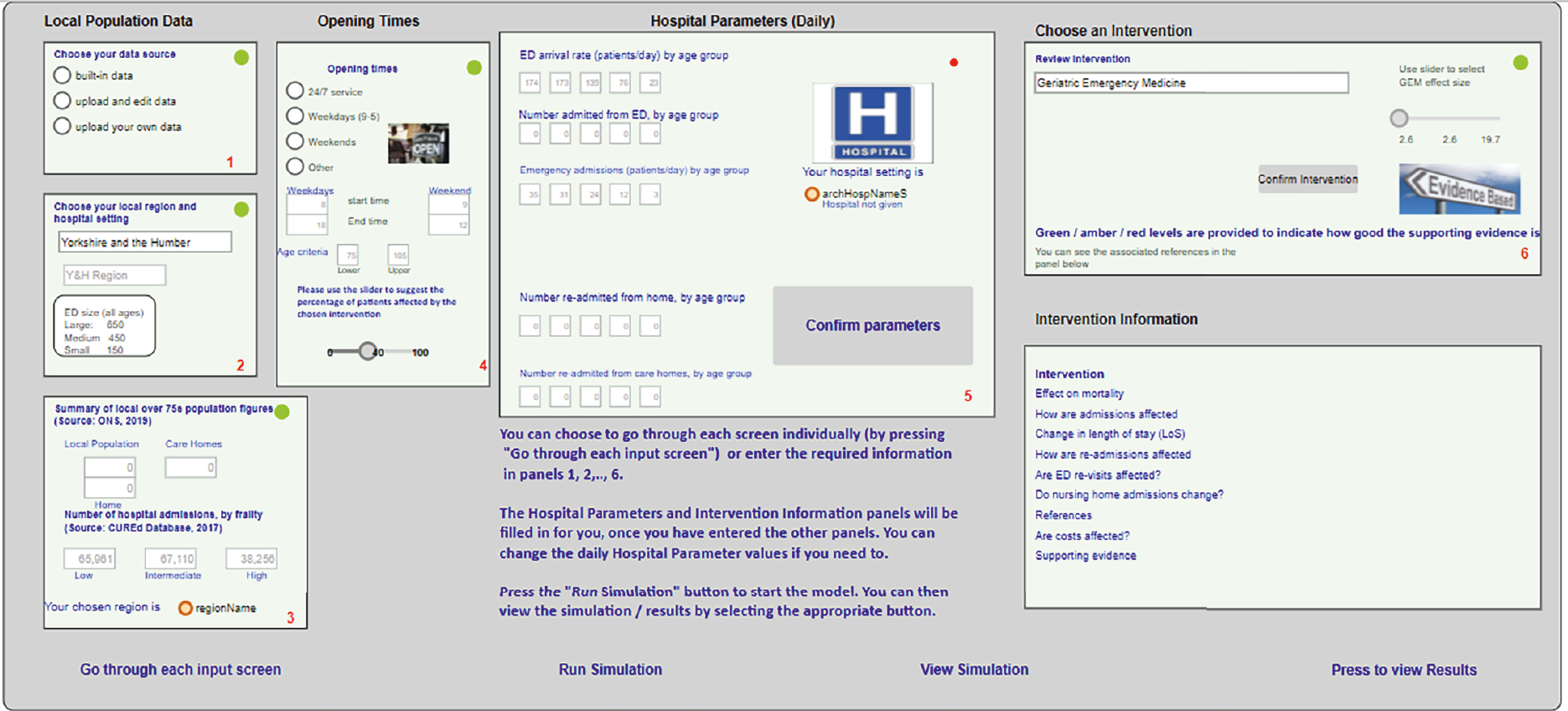
The user can select one of three hospital archetypes (large, medium, and small) which are based on the CUREd dataset (Table 19) that best reflects their setting.
| Age groups (years) | 75–79 | 80–84 | 85–89 | 90–94 | 95+ | |
|---|---|---|---|---|---|---|
| Large | Daily ED attendances | 23 | 22 | 18 | 10 | 3 |
| Medium | 11 | 11 | 9 | 5 | 2 | |
| Small | 8 | 8 | 6 | 4 | 1 | |
| Large | Daily emergency admissions | 5 | 4 | 4 | 2 | 1 |
| Medium | 3 | 2 | 2 | 1 | 1 | |
| Small | 2 | 2 | 2 | 1 | 1 |
The underlying system dynamics model is shown in Figure 23. The model uses a daily time-step and runs over one year. As the model is providing a high-level view, it does not distinguish between weekdays and weekends, but this could be considered in future iterations of the model. The model does not consider resource constraints such as staffing, but does include daily variation on ED arrivals, emergency admissions, readmissions, and discharges.
FIGURE 23.
Underlying system dynamics model.
The model has been constructed in panels which represent various parts of the system:
-
whole system population
-
ED attendances
-
ED admissions
-
other (non-ED) admissions
-
care home admissions
-
deaths.
Within each model, the number of patients is captured in entities called ‘stocks’ and the movement between them as ‘flows’. The daily changes in stocks and flows are determined by differential equations which measure the rate of change derived from the model parameters derived in WP3. The structures of the baseline and intervention models are the same. In the intervention model, the parameters for ED attendance, readmission, conversion rates (ED attendance leading to admission), length of stay and discharge have been adjusted from the baseline values depending on the effect sizes documented in the literature (WP1.1). The interventions included in the decision support tool are described in Chapter 8.
As well as the model that has been developed specifically for the YH region, there is a more generic version where the user can select from a list of 42 ICS in England. The need for a more generic model was identified in the stakeholder events as participants were keen to see the tool working in their own area and hospital setting. The generic model is essentially the same as the YH model with one main exception in the screen that captures the local population figures. In the YH model, the user can select from a list of 25 towns, cities or counties within the region, whereas in the generic version there are 42 ICS regions to choose from. Both use ONS data to inform the population statistics for the different age groups. The system dynamics model will be hosted, free to access, on the AnyLogic Cloud and links to this and a repository of all the accompanying information will be housed on the NHS Futures website.
Stakeholder engagement
The tool was designed to be used by clinicians, commissioners and planners via a user-friendly interface that allows users with no prior knowledge of simulation to select the input data and run the models. Stakeholder engagement was conducted throughout all stages of development to ‘sense-check’ the emerging findings and comment on the usability of the user interface:
-
The research team (clinicians from primary care, emergency and geriatric medicine) reviewed the model monthly over three years.
-
An independent study steering committee (also including clinical and methodological experts) provided high level oversight and gave a strong steer on which intervention scenarios to include, considering the level of supporting evidence.
-
A series of four one-hour long, external stakeholder events (3 aimed at clinicians and commissioners and 1 at patients and carers, totalling around 40 individuals) considered the structure and usability of the user interface and results screens.
-
The tool was also demonstrated at two national NHS measurement classes attended by 60 attendees from 20 NHS organisations. The attendees included service managers, improvement and transformation leads, clinical directors, consultants and specialty doctors from frailty and emergency care departments within NHS trusts, commissioning groups and local councils.
The stakeholder events and NHS measurement classes gathered feedback from potential end-users of the tool. One key element of their feedback was to have a tool that could be used in any area of NHS England, leading to the development of a version of the tool for use in any of the 42 ICS of NHS England. This generic version is currently being tested through a series of workshops and webinars (August 2022).
Chapter 8 ‘What if’ analyses
This chapter discusses the ‘what-if’ scenarios (informed by WP 1.1) that have been included in the system dynamics model. The interventions are divided into three categories: Pre-ED, in the ED and Post-ED. The interventions included are:
-
Pre-ED:
-
In the ED:
-
Post ED:
-
frailty unit, older persons assessment unit. 104
-
Each of these interventions can be selected from a dropdown menu within the user interface of the tool (Figure 24). Once selected, the user is provided with a summary of the effects of the intervention from either systematic review, RCTs or National Institute for Health and Care Excellence guidance. The tool also gives a summary of the intervention along with an indication of the quality of the evidence (red, amber or green) and references. Most of the interventions are described as having the highest level of evidence quality with only the post-ED (amber) scoring lower.
FIGURE 24.
Selecting an intervention.

The decision support tool allows the user to see the following process-based graphical outputs over a simulated year in the chosen hospital setting for both the baseline (‘as is’ situation) and chosen intervention:
-
average number of patients in each location (ED, ward, care home, own home)
-
average daily discharges from ED
-
average daily admissions: from ED and direct to the wards (emergency admissions only)
-
average daily discharges to own home
-
average daily discharges to care home
-
readmissions from own home or a care home within 30 days of discharge
-
cumulative number of deaths in hospital, or within 30 days of discharge.
In each graph, the baseline is depicted in red and the intervention in blue. A selection of results is included for each of the interventions.
Importantly, and reflecting the real world, the model allows users to specify the opening hours of the service, as well as the population that it would target. For example, a scenario running between 8 a.m. and 8 p.m. for seven days a week leads to 50% of patients being eligible, thus reducing the system impact. If this service only accepted people aged 85 years or older, it would not affect outcomes of those aged less than 85 years, further diluting the impact.
Pre-emergency department
Under proactive care, which involves risk stratification and primary care-led holistic assessment and care planning, the evidence suggests that there is no statistically significant effect on the number of patients admitted to hospital, mortality or nursing home admissions. Accordingly, Figure 25 shows that there is no significant difference between the baseline and intervention results for the number of patients admitted from ED into a hospital bed in a large hospital setting.
FIGURE 25.
Proactive care – number of patients admitted from ED.
With hospital at home (‘virtual wards’), where holistic care is provided at home in the event of a crisis, there are slightly fewer patients admitted from ED (Figure 26). The evidence suggests that a larger proportion of older patients are living at home and that there is a slight reduction in mortality.
FIGURE 26.
Hospital at home – number of patients admitted from ED.
In the emergency department
Geriatric emergency medicine (run during the week, Monday to Friday, 9 a.m. to 5 p.m.) involves consultant-led CGA, and the evidence suggests fewer admissions and readmissions.
Figure 27 shows that the number of over 75-year-old patients admitted from ED is slightly reduced, as is the number readmitted from their own home (Figure 28).
FIGURE 27.
Geriatric emergency nedicine intervention – number of patients admitted from ED.
FIGURE 28.
Geriatric emergency nedicine intervention – number of patients readmitted from their own homes.

Under ‘front-door frailty’ (run during the week) and involves community matron/in reach, discharge planning, clinical navigator, etc., the evidence suggests reduced admissions and readmissions and fewer hospital related deaths. Figure 29 shows slightly fewer hospital-related deaths as expected.
FIGURE 29.
Front-door frailty – hospital related deaths.
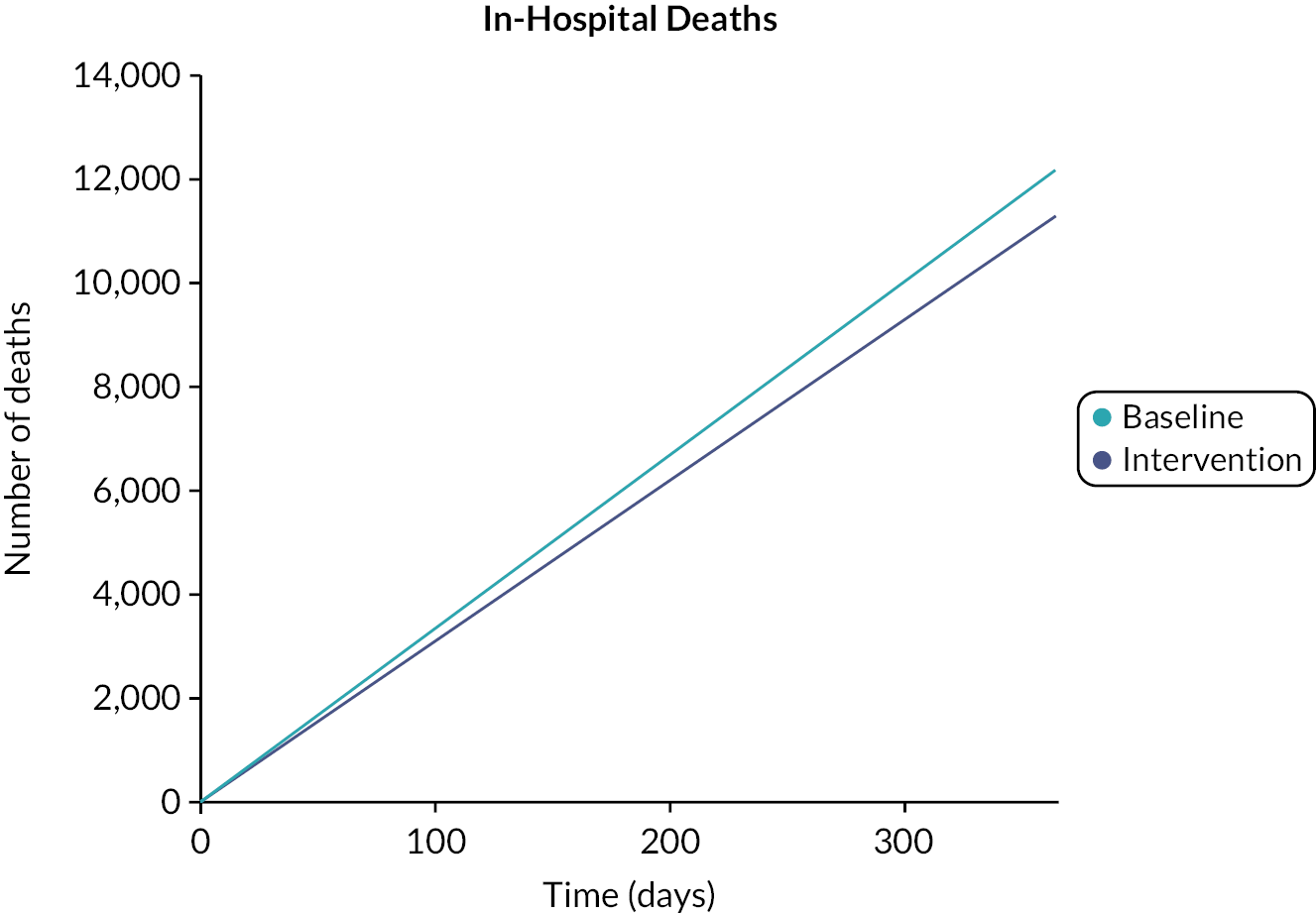
Post emergency department
Under older persons assessment or acute frailty units, the evidence suggests reduced readmissions and reduced hospital related mortality. Figure 30 shows slightly fewer patients admitted to hospital from the ED and readmitted after 30 days (Figure 31).
FIGURE 30.
Older persons assessment unit – number of patients admitted from ED.

FIGURE 31.
Older persons assessment unit – number of patients readmitted from their own homes.
Discussion
To our knowledge, this is the first whole-system model focusing on urgent care for older people. It allows users to run one of five evidence-based scenarios to answer the ‘what if’ or ‘so what’ question – what might happen if we implemented this service in our ED. It is configurable to allow users to input their own data or chose from a range of archetypes that reflect their own setting. It takes account not only of the hours of provision, but the population targeted. It should support decisions about which model of urgent care might best suit the setting in question, according to the outcomes (length of stay, readmissions, institutionalisation, mortality) that the system wants to address.
Strengths and limitations of the model
Key strengths of the system dynamics model are that it uses robust published evidence from WP 1.1 to create the scenarios, an integrated dataset reflecting the whole of the UEC pathway (WP3), and extensive stakeholder engagement to ensure that it is both user friendly and a realistic representation of the system.
Another strength of both versions of the model is in the ability of being able to visualise the impact of the intervention through the comprehensive set of graphs which compare the baseline and intervention results for several key hospital metrics over a simulated year of operation. The user has the option to view the model as it updates or run the model and view the output results at the end of the simulated year. The involvement of interdisciplinary research team, coupled with stakeholder meetings have provided very useful feedback on the model throughout its development, especially in how it looks and feels to the end user.
One final strength of the tool is that the underlying system dynamics model includes feedback loops which enable the user to see how various parts of the system connect, mirroring the real world. For example, in the acute frailty unit intervention scenario, the evidence suggests that with the intervention increasing a patient’s length of stay by 0.5 days, there should be more patients in hospital. However, the reduction in patients readmitted leads to an overall reduction in hospital numbers.
However, there are limitations to the model. First, we were not able to include frailty measures into the model, as these were not routinely embedded into the CUREd dataset. While it would have been possible to capture HFRS for the admitted cohorts, this would not be available to include in the whole system model, which also captures outcomes on people attending but discharged from EDs. We have only used interventions that have been reported in peer-reviewed papers; emerging care models, such as prehospital frailty services, which offer promise but the effect sizes are uncertain. Future iterations of the system-dynamics model could allow for these novel interventions to be incorporated. We were unable to model jointly delivered interventions, such as front-door frailty in combination with acute frailty units, as only separate effects have been reported, so we do not know the combined effect sizes. A final limitation is that the model does not include an estimate of the staffing resources needed to provide a service and would need to be considered separately by those seeking to implement a new or different service for older people accessing emergency care in their region.
Chapter 9 Overarching discussion
Summary of findings
This programme of work brought together a comprehensive body of existing evidence, combined with qualitative work to understand the context of emergency care from patient, carer and staff perspectives, and detailed econometric analysis of a large, linked dataset reflecting the emergency care pathway from a system perspective to inform the development of a clinical decision support tool that will be useful to teams considering service improvements in their emergency care pathways, including clinicians, service managers and commissioners. In brief, we have described what works, how it can be implemented and what benefits five evidence-based interventions might offer older people in the emergency care setting.
Patients and their loved ones emphasised the importance of being treated with respect and dignity, provision of timely and accurate information and involvement in decision making. Receiving care in a calm environment with attention to personal comfort, basic physical needs and being supported by family members were key, as were shorter waiting times. Goals of care included medical problems being diagnosed and resolved; being informed about tests and treatment; appropriate well-planned discharge home with support where needed and avoiding reattendance; and retaining basic activities of daily living. While many of these needs are likely shared by a range of patient groups, maintaining function and delivering care in a comfortable environment are especially important for older people, who are by definition more vulnerable because of their pre-existing frailty. Sadly, many of these really rather modest needs were not consistently met.
There is some good evidence that interventions delivered at the ED level impact on patient outcomes, which may address some of the issues that patients and families voiced. Yet our qualitative field work emphasised the fragility of frailty services in the ED, in particular highlighting deficiencies in staffing (skill mix and mindset), lack of joined up services linking to the community and sustainability (evidencing impact and spreading best practice throughout the workforce).
Our study of routine data identified pathways of care that indicate the system currently does not address the needs of patients and carers and is deficient in terms of the existing pathways. We found there were some strong predictors of outcome which included age, previous attendance, out-of-hours attendance and a new finding: call-handler designation of urgency There is potential for some of these to be used in the development and implementation of future interventions. For example, call-handler designation might be used to alert EDs to the impending arrival of older people at the highest risk of adverse outcomes. This might generate a frailty attuned senior assessment that, based upon the evidence in our review of reviews,13 could start to change the existing paradigm of high admission, rates, long lengths of stay, high rates of readmission, institutionalisation and mortality. The system dynamics model can be used by hospital teams and/or commissioners to understand the impact of different frailty attuned interventions on their own systems.
Strengths of this work include the interdisciplinary approach and the qualitative methods which added depth to the breadth of insight that the literature and econometric analysis were able to generate. Person-centredness was at the heart of this work, supported by strong patient and public involvement (PPI), as well as the patient and carer interviews in WP 1.2 that ensure that the patient voice was at the heart of our considerations. We also engaged widely with professional stakeholders who added their ‘lived experience’ about service improvements relating to frailty in the ED, as well as providing extensive input into the design of the system dynamics tool. Professional stakeholders were drawn from a range of relevant organisations reflecting frailty, acute and emergency care pathway. Notably, we were able to engage with national improvement collaboratives focusing upon whole system approaches to the care of older people, who were able to guide the design (and appearance) of the system dynamics decision support tool.
Limitations
There are limitations to the work. Our review of review method took a big picture overview of the evidence to date, rather than a more detailed examination, for example in the form of a meta-analysis. Despite our best efforts, people with severe frailty and those from ethnic minorities were under-represented in the patient and care interviews, which may limit the transferability of the findings from this part of the study. The planned ethnography was curtailed due to COVID restrictions, but we were still able to gather a wide range of professional perspectives on making improvements in the ED setting, which should prove useful. We also partnered with NHS Elect, a national improvement collaborative focusing upon frailty, to include wider improvement expertise perspectives in the implementation guide. This offsets the paucity of evidence in the formal literature relating to implementation in the urgent care setting. The measures identified in the literature included PROMs as well as service metrics, but we were unable to model the PROMs in the econometric analysis, as these were not available in the routine data. The econometric analysis reflected the whole system, but we were similarly unable to model frailty in the ED as frailty measures were not routinely available in the dataset used. Nevertheless, we were able to generate a detailed analysis of emergency care pathways taking account of the wider context. The system dynamics model was limited to five evidence-based interventions or scenarios, primarily constrained by the robustness of the underpinning literature. However, emerging evidence-based interventions could be added in the future should this change. It also focused on service metrics and mortality, rather than PROMs, but again these could be added should such data become routinely available. We have included information on which interventions might improve PROMs in the accompanying guidance for the system dynamics model.
Implications for policy and practice
We have shown that experience and outcomes remain poor for older many people accessing emergency care. We have also shown what works, and what needs to be done to sustain service developments. We have provided tools that will help systems (clinicians, service mangers, commissioners and others) identify which service model might offer the greatest impact in their specific context (https://future.nhs.uk/ECOPDecisionSupportTool). With the expansion of Virtual Wards and Same Day emergency care for older people living with frailty, as well as emerging pre-hospital support schemes, there is now a real opportunity for the NHS to create frailty-friendly emergency care services that could improve patient experience and service outcomes – allowing olde people to get home sooner and healthier. Indeed, the totality of the evidence presented here would suggest that ED-based, integrated frailty services should be routine, in keeping with NHS England policy,64,105 but oddly not with NICE guidance from 2018104 – which might be refreshed based on this work.
Our work is also timely as ICSs start to become more operational, and the system dynamics model provides a useful whole system perspective. The tool can be a vehicle upon which to generate whole system discussions, informed by evidence and data. Also in keeping with systems thinking, in which simple rules are often best for navigating complex systems, our finding that the ambulance triage codes are powerful predictors of emergency care outcomes might be a useful measure to include in system wide dashboards.
Implications for research
The patient and care interviews in WP 1.3, as well as the professional interviews from WP 1.2 and WP 2, provide some rather distressing evidence about the gap between what good looks like, and the reality of frailty care delivered on the ground. We suggest that the totality of evidence presented should promote a more concerted effort to deliver frailty attuned emergency care, as discussed above. But moreover, unless the impact of such care is measured, it may not be valued. We have frequently described the limitations of service data to describe what matters to older people living with frailty who have urgent care needs. It might be that a PROM designed for population and setting could start to drive more person-centred care. For example, a PROM applied in the first hours of an urgent care period might generate a clinical conversation that focuses upon what matters to the patient (rather than protocolised care). Such data could be collected and systematised, for example included within the emergency care data set and/or Hospital Episode Statistics, linked to routine measures of frailty – such as the HFRS. 77,88 This would the allow services and the wider system to visualise the person-centredness of care being delivered, stratified by frailty. Work is underway to develop such a PROM from members of our team, and a HFRS system wide dashboard is already available here: AFS Counting Activity – same day emergency care (SDEC) Collaboration Platform – FutureNHS Collaboration Platform (generated in our previous HSDR study, 17/05/96). A future system dynamics model including PROMs, frailty segmentation and further evidence-based scenarios could be an extremely powerful tool at the clinical, service and system level (see Box 7).
During this research, we also became aware of a growing number of service developments in which senior clinical decision makers with geriatric emergency medicine expertise provide real-time support to ambulance services, in an attempt to avoid unnecessary and often unwanted hospitalisation, instead directing patients towards community-based services. Indeed, we did include this as a scenario in the system dynamics model, but in the end the evidence was insufficiently robust, so it was removed. Future research should evaluate these schemes more robustly, as they may have potential to increase the proportion of acute care delivered at home (see Box 7). Several of the schemes report an approximate 50% reduction in conveyance rates for the frailest, and the draft system dynamics modelling suggested that such a service running 8–8pm 7 days per week might release half an acute ward in a typical ICS (population circa 1 million).
-
Develop and implement Patient Reported Outcomes for older people living with frailty who have urgent care needs
-
Explore an evaluation of emerging pre-hospital services for older people living with frailty who have urgent care needs
Equality, diversity, and inclusion
Participant representation
WP 1.2 attempted to recruit a diverse range of patient and carer participants, using frailty data as well as ethnicity from the local systems to inform the sampling frame. Despite which, people with severe frailty and those from ethnic minorities were under-represented. This is in part related to the complexities associated with recruiting people living with severe frailty, who typically have barriers to providing informed consent (e.g. sensory or cognitive impairment). Although our team have considerable experience in recruiting people without capacity, we worked with the Clinical Research Networks, who may be less comfortable using consultee consent processes. We are aware that they are wider issues beyond appearance and language – for example, trust in the NHS, which might have had an impact on the relative under-representation of people from ethnic minorities. In contrast, the econometric analysis and the stakeholder engagement broadly reflected the distribution of ethnicity found in the community and workforce, respectively.
Reflections on the research team and wider involvement
We are aware that our research team was predominantly white (although we had PPI representation from ethnic minutes on our EMT meetings), and this may have had some unconscious influence on our ability to recruit people from ethnic minorities. We did include early career researchers in our EMT meetings – James van Oppen, undertaking a NIHR doctoral research fellowship, who contributed actively, not least in developing the PROM for older people with emergency care needs. We also included Abdullah Alshibani, a University of Leicester PhD student who was working on pre-hospital frailty, although more from a trauma perspective.
Patient and public involvement
We have been fortunate to have substantial PPI for this study. First, we had two lay members attending our EMT meetings, as well as separate discussion which required us to prepare lay summaries and share these with our PPI colleagues throughout the programme. Second, we consulted regularly with the Leicester older people’s PPI forum, both in developing this proposal, but also reporting upon progress and the emerging findings. Each of the WP leads presented to the PPI forum on at least one occasion. WP 1.2 was designed explicitly to insert the patients voice into the research, and we believe did so successfully given the limitations of the data that we had to work with. Our outputs led by James van Oppen are very person centred, relating to the development of a PROM for older people living with frailty who have urgent care needs. Our plans for a system wide frailty dashboard that includes PROMS for this group of people are a good example of the practical outputs that a person-centred approach can deliver.
Conclusions
We have reaffirmed the poor outcomes experienced by many older people living with frailty who have urgent care needs. We have identified some of the barriers to better care delivery, measures available now and in the near future that could start to generate different conversations more aligned to person centred care and created tools that might support clinicians, service managers and commissioners in choosing which evidence-based intervention might work best for their context. There is an opportunity in the current policy context to transform emergency care for older people.
Future work will focus on refining the system dynamics model, specifically including PROMs and prehospital services for older people living with frailty who have urgent care needs.
This report contains transcripts of interviews conducted in the course of the research, or similar, and contains language which may offend some readers.
Acknowledgements (including associated publications)
We would like to thank all the staff and patients that supported this work, especially during the pandemic. Without their input, none of this would have been possible; we hope that in return some of the findings might improve care and outcomes!
An especial thanks goes to the Leicester older people’s PPI forum, who have supported this work from conception though to completion, providing valuable insights and commentary.
Thanks also to NHS Elect, which has played an invaluable role in informing the study but also supporting dissemination at scale via the frailty networks.
Finally, a big thank you to Dave Evenden (visiting research fellow, Clinical and Experimental Sciences, University of Southampton) who played a key role in developing the system dynamics model at the outset of the project.
Contributions of authors
Simon Conroy (https://orcid.org/0000-0002-4306-6064) (Geriatrician, Chief Investigator) took led overall on this report and on the patient interviews.
Sally Brailsford (https://orcid.org/0000-0002-6665-8320) (Lead for Systems Dynamics) led on the system dynamics model section.
Christopher Burton (https://orcid.org/0000-0003-0233-2431) (Implementation, and Primary Care Expertise) supported Maynou and Street with the CUREd analysis.
Tracey England (https://orcid.org/0000-0001-8904-3071) (Systems Dynamics Model Designer) led on the system dynamics model section.
Jagruti Lalseta (Lay Advisor) reviewed the lay summary and executive report.
Graham Martin (https://orcid.org/0000-0003-1979-7577) (Lead Qualitative Researcher) led on the staff interviews and ethnography.
Suzanne Mason (https://orcid.org/0000-0002-6053-9062) (Professor of Emergency Medicine) constructed the CUREd dataset, provided emergency care expertise, and supported Maynou and Street with the CUREd analysis.
Laia Maynou-Pujolras (https://orcid.org/0000-0002-0447-2959) (Lead Analyst on the CUREd data) led on the CUREd analysis, supported by Mason and Burton.
Kay Phelps (https://orcid.org/0000-0003-4735-4669) (Qualitative Interviewer and Ethnographer) led on the patient interviews, staff interviews, and ethnography.
Louise Preston (https://orcid.org/0000-0001-7477-4517) (Senior Research Fellow, Division of Population Health, School of Medicine and Population Health, University of Sheffield) led on the literature review and on the review of reviews.
Emma Regen (https://orcid.org/0000-0002-8786-2783) (Qualitative Interviewer and Ethnographer) led on the patient interviews, staff interviews, and ethnography.
Peter Riley (Lay Advisor) reviewed the lay summary and executive report.
Andrew Street (https://orcid.org/0000-0002-2540-0364) (Lead for the Econometric Analysis) led on the CUREd analysis, supported by Mason and Burton.
James van Oppen (https://orcid.org/0000-0002-2570-7112) (PhD Candidate) supported the qualitative elements, provided emergency care expertise, developed a PROM for older people living with frailty who have urgent care needs, and led on the review of reviews.
All the authors have reviewed and approved the final version of the report.
Conferences/meetings
‘Emergency Care for Older People: the simulation model’ presented at Operational Research Applied to Health Services (ORAHS), 5–9 July 2021.
‘Emergency Care for Older People (ECOP): a system dynamics approach’ presented at Operational Research Society 63rd annual conference, 14–16 September 2021.
Stakeholder meetings held in March (clinical staff) and May 2021 (patient and carers).
Workshops and webinars to present and discuss the ECOP decision support tool, planned for June and November 2022, facilitated by the NHS Elect frailty networks, British Geriatrics Society and Royal College of Emergency Medicine.
Finally, we have planned a series of bespoke webinars to support the dissemination of the system dynamics tool, supported by the NHS Elect frailty networks (Figure 32).
FIGURE 32.
NHS Elect supported system dynamics model dissemination events.
Publications
-
van Oppen JD, Alshibani A, Coats TJ, Graham B, Holch P, Lalseta, J., et al. A systematic review and recommendations for prom instruments for older people with frailty in emergency care. J Patient Rep Outcomes 2022;6(1):30. https://doi.org/10.1186/s41687-022-00438-x
-
Mooijaart SP, Carpenter CR, Conroy SP. Geriatric emergency medicine-a model for frailty friendly healthcare. Age Ageing 2022;51(3): afab280. https://doi.org/10.1093/ageing/afab280
-
van Oppen JD, Coats TJ, Conroy SP, Lalseta J, Phelps K, Regen E, et al. What matters most in acute care: an interview study with older people living with frailty. BMC Geriatr 2022;22(1):156. https://doi.org/10.1186/s12877-022-02798-x
-
Conroy S, Thomas M. Urgent care for older people. Age Ageing 2022;51(1):afab019. https://doi.org/10.1093/ageing/afab019
-
Gilbert T, Cordier Q, Polazzi S, Bonnefoy M, Keeble E, Street A, et al. External validation of the Hospital Frailty Risk Score in France. Age Ageing 2022;51(1):afab126. https://doi.org/10.1093/ageing/afab126
-
van Oppen J, Conroy S. Are emergency departments responding to the aging demography? Ann Emerg Med 2022;79(4):364–66. https://doi.org/10.1016/j.annemergmed.2021.11.024
-
Lucke JA, Mooijaart SP, Heeren P, Singler K, McNamara R, Gilbert T, et al. Providing care for older adults in the Emergency Department: expert clinical recommendations from the European Task Force on Geriatric Emergency Medicine. Eur Geriatr Med 2022;13(2):309–17. https://doi.org/10.1007/s41999-021-00578-1
-
Lucke JA, Mooijaart SP, Conroy S, Blomaard LC, De Groot B, Nickel CH. Mortality risk for different presenting complaints amongst older patients assessed with the Manchester triage system. Eur Geriatr Med 2022;13(2):323–8. https://doi.org/10.1007/s41999-021-00568-3
-
Alshibani A, Alharbi M, Conroy S. Under-triage of older trauma patients in prehospital care: a systematic review. Eur Geriatr Med 2021;12(5):903–19. https://doi.org/10.1007/s41999-021-00512-5
-
Elliott A, Taub N, Banerjee J, Aijaz F, Jones W, Teece L, et al. Does the CFS at Triage Predict Outcomes From Emergency Care for Older People? Annals Emerg Med 2021;77(6):620–7. https://doi.org/10.1016/j.annemergmed.2020.09.006
-
Hogervorst VM, Buurman BM, De Jonghe A, Van Oppen JD, Nickel CH, Lucke J, et al. Emergency department management of older people living with frailty: A guide for emergency practitioners. Emerg Med J 2021;38(9):724–9. https://doi.org/10.1136/emermed-2020-210014
-
Alshibani A, Banerjee J, Lecky F, Coats TJ, Alharbi M, Conroy S. New horizons in understanding appropriate prehospital identification and trauma triage for older adults. Open Access Emerg Med 2021;13:117–35. https://doi.org/10.2147/OAEM.S297850
-
Street A, Maynou L, Gilbert T, Stone T, Mason S, Conroy S. The use of linked routine data to optimise calculation of the Hospital Frailty Risk Score on the basis of previous hospital admissions: a retrospective observational cohort study. Lancet Healthy Longev 2021;2(3):e154–62. https://doi.org/10.1016/S2666-7568(21)00004-0
-
Conroy S, Maynou L. Frailty: time for a new approach to health care? Lancet Healthy Longev 2021;2(2):e60–1. https://doi.org/10.1016/S2666-7568(20)30064-7
-
Alshibani A, Singler B, Conroy S. Towards improving prehospital triage for older trauma patients. Z Gerontol Geriatr 2021;54(2):125–9. https://doi.org/10.1007/s00391-021-01844-4
-
Mooijaart SP, Nickel CH, Conroy SP, Lucke JA, van Tol LS, Olthof M, et al. A European research agenda for geriatric emergency medicine: a modified Delphi study. Eur Geriatr Med 2020;12(2):413–22. https://doi.org/10.1007/s41999-020-00426-8
-
Preston L, Van Oppen JD, Conroy SP, Ablard S, Buckley Woods H, Mason SM. Improving outcomes for older people in the emergency department: a review of reviews. Emerg Med J 2021;38(12):882–8. https://doi.org/10.1136/emermed-2020-209514
-
van Oppen JD, Valderas JM, Mackintosh NJ, Conroy SP. Patient-reported outcome and experience measures in geriatric emergency medicine. Z Gerontol Geriatr 2020;54(2):122–4. https://doi.org/10.1007/s00391-020-01777-4
-
Alshibani A, Banerjee J, Lecky F, Coats TJ, Prest R, Mitchell A, et al. A consensus building exercise to determine research priorities for silver trauma. BMC Emerg Med 2020;20(1):63. https://doi.org/10.1186/s12873-020-00357-4
-
Todd OM, Burton JK, Dodds RM, Hollinghurst J, Lyons RA, Quinn TJ, et al. New Horizons in the use of routine data for ageing research. Age Ageing 2020;49(5):716–22. https://doi.org/10.1093/ageing/afaa018
-
van Oppen JD, Keillor L, Mitchell A, Coats TJ, Conroy SP. What older people want from emergency care: a systematic review. Emerg Med J 2019;36(12):754–61. https://doi.org/10.1136/emermed-2019-208589
-
Phelps K, Regen E, van Oppen JD, Riley P, Lalseta J, Martin G, et al. What are the goals of care for older people living with frailty when they access urgent care? Are those goals attained? A qualitative view of patient and carer perspectives. Int Emerg Nurs 2022;63:101189. https://doi.org/10.1016/j.ienj.2022.101189
-
England T, Brailsford S, Evenden D, Street A, Maynou L, Mason SM, et al. Examining the effect of interventions in emergency care for older people using a system dynamics decision support tool. Age Ageing 2023;52(1):afac336. https://doi.org/10.1093/ageing/afac336
-
Regen E, Phelps K, van Oppen JD, Riley P, Lalseta J, Martin G, et al. Emergency care for older people living with frailty: patient and carer perspectives. Emerg Med J 2022;39(10):726–32. http://dx.doi.org/10.1136/emermed-2022-212420
-
Maynou L, Street A, Burton C, Mason SM, Stone T, Martin G, et al. Factors associated with longer wait times, admission and reattendances in older patients attending emergency departments: an analysis of linked healthcare data. Emerg Med J 2023;40(4):248–56.
Ethics statement
WP 1.2: University of Leicester ethics: 17525-spc3-ls:healthsciences. Approval granted 18/9/2018.
WP 2: IRAS 262143, Yorkshire and the Humber – Leeds West Research Ethics Committee; CAG Health Research Authority 19/CAG/0194. Approval granted 25 September 2019.
WP 3: IRAS 215818, REC 17/YH/0024, Yorkshire and the Humber – Leeds East Research Ethics Committee, approved 26 June 2018; CAG Health Research Authority 17/CAG/0024, approved 25 October 2018.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
Appendix 1 Review of reviews: search strategy
-
*Emergency Service, Hospital/
-
*Emergency Medical Services/
-
*Emergency Medicine/
-
(emergency adj2 service$).ti,ab.
-
emergency care. ti,ab.
-
urgent care.ti,ab.
-
emergency department*.ti,ab.
-
(accident adj2 emergency).ti,ab.
-
casualty.ti,ab.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9
-
*‘Aged, 80 and over’/or *Health Services for the Aged/
-
*Frail Elderly/
-
*Aged/or *Aging/)
-
(ageing or elderly or geriatric or frail or aged or old or older).ti.
-
11 or 12 or 13 or 14
-
10 and 15
-
meta analysis.mp,pt. or review.pt. or search:.tw.
-
16 and 17
-
limit 18 to (English language and year=‘2000 -Current’)
Appendix 2 Review of reviews
The review methods are summarised in Table 20, the study descriptions in Tables 21–23. The results are presented in Tables 24–26.
| Reference | RQ/objectives | Author description of review method/our description of data analysis | Study types included in the review | Search process, databases/dates/other sources searched | Data analysis methods | Review inclusion criteria | Review exclusion criteria | Papers included (n), date range of included papers, including all or subset of primary included papers? |
|---|---|---|---|---|---|---|---|---|
| Burkett et al.23 | 1. Identify indicators of quality ED care for older people. 2. Critically evaluate their methodological quality. 3. Map them using three frameworks to assess the balance of indicators. | SR. Narrative/tabular synthesis of critical appraisal of quality indicators | Any studies reporting QI | Databases: 3; dates: search in 2014; other sources: 22 websites listed in the paper | QI were mapped by consensus. QI were assessed independently and resolved by consensus | Inclusion criteria unit of assessment was the QI rather than the paper: (1) definition of the QI; (2) QI for older people (> 65 years); (3) QI developed for ED use or evidence of use in ED setting; | Lack of explicit definition, not used in the ED, not specifically for older people, inability of ED provider to influence QI) | 20 papers, 1997–2013. Includes all papers reported as sources of quality indicators (table 2 of article) |
| (4) QI that can be influenced by the ED team (ED provider assessment or management or ED system of care) | ||||||||
| Cherian et al.39 | SR of evidence for implementation of geriatric ED model | SR/narrative synthesis | No information (conference abstract) | Databases: 7; dates: 1980–2012; other sources: none | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) |
| Conroy et al.24 | Aim was to examine evidence for services for older patients who developed a crisis and attended hospital but who were assessed, treated and discharged, either immediately, or within a short time period (up to 72 hours) from an AMU or ED | SR/quantitative synthesis of quantitative data | RCTs | Databases: 11; dates: inception–2009 (rerun in 2010); other sources: none | Outcomes expressed as RR with 95% CI. Where studies could be combined, a fixed effects model was used (apart from where there was high heterogeneity I squared over 30%) where a random effects model was used. Where studies could not be combined, results were presented within subgroups. Funnel plots were used to identify possible publication bias. | Age: Participants aged ≥ 65 years; setting (ED): acute hospital setting (ED/MAU); intervention description: CGA; other intervention details: discharged rapidly from an acute setting (< 72 hours); other intervention details: | Condition- specific interventions; interventions to reduce hospital use; trials relating to children’ trials relating to psychiatric disorders | 5 papers, 1999–2004; includes all papers |
| Fan et al.25 | Review aimed to appraise the effectiveness of interventions targeted at ED use by the elderly in terms of reducing ED utilising and identify core characteristics shared by successful intervention models | Literature review; narrative synthesis of quantitative data | Experimental or observational studies were included | Databases: 5; dates: no information; other sources: reference lists of identified articles were manually searched for additional literature | A qualitative approach was used, owing to the heterogeneity of study designs, interventions and outcome measures that precluded meta-analysis | Studies were included if they: (1) focused on the effectiveness evaluation of strategies targeting the elderly population to reduce ED use; (2) reported a measure of ED use as study outcome | Studies were excluded if they: (1) did not provide a description of intervention; (2) were investigating interventions limited to patients with particular medical diagnoses; (3) used non-elderly population as the study sample, unless the results for the younger subjects and the older subjects were presented separately; | 20 papers, 1996–2012; includes a subset (hospital-based interventions) |
| (4) reported only on patients or health providers subjective perceptions; (5) failed to report sufficient quantitative data to measure the outcome of interest; (6) had no control or comparison group | ||||||||
| Fealy et al.26 | How effective are gerontologically informed nursing assessment and referral interventions aimed at older ED attendees? | SR/narrative synthesis of quantitative data | Clinical trials, before-and-after designs, and descriptive–evaluative studies | Databases: 4; dates: 1992–2008; other sources searched: reference lists | Narrative synthesis approach | Age: ≥ 60; setting: index ED visit; intervention: undertaken by or involving a nurse | Interventions outside the scope of nursing practice and/or not directly associated with the index ED visit were excluded, as were reports of interventions led by medical researchers, and reports of the development and testing of risk screening instruments. To avoid reporting bias, findings from review papers were excluded from the results | 11 papers, 1996–2006; includes all papers |
| Graf et al.27 | The use and value of CGA in ED for evaluations of older patients and its influence on adverse outcomes. This approach is compared with an alternative one using existing screening tools | SR/narrative synthesis of quantitative data | Re CGA: RCTs or matched controlled trials (cohort, case–control, case-matched and cross-sectional) | Databases: 1; dates: inception to 2009; other sources: none | Narrative synthesis | Age: geriatric problems; setting: ED; intervention: complete CGA, screening tools, adverse outcome assessment | No control or match. Not geriatric. Not ED based. No outcomes reported | 8 papers, 1996–2005; includes a subset (studies on CGA efficiency) |
| Gupta et al.40 | To review the most recent literature regarding management of elderly trauma patients | Literature review/narrative synthesis | No information (conference abstract) | Databases: 2; dates: 2006–2013; other sources: none | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) |
| Hastings and Heflin28 | To evaluate the evidence for interventions designed to improve outcomes for elders discharged from the ED | SR/narrative synthesis of quantitative data | RCTs, non-RCTs and observational studies | Databases: 2; dates: 1966–2005; other sources: reference lists of identified articles were manually searched for additional literature. The authors also consulted with content experts to identify unpublished work relevant to the goals of this review | Descriptive; a meta-analysis was judged infeasible due to obvious heterogeneity in trial design, interventions, and outcome measures | Patients of interest were community-dwelling elder patients discharged home from the ED. Citations that seemed to describe an intervention to improve outcomes for senior adults discharged from the ED | Excluded articles that described and/or tested an intervention limited to patients with a single presentation or diagnosis (e.g. falls, delirium) or delivered only to patients who would have otherwise been hospitalised | 14 papers, 1996–2004; includes a subset (papers from the following categories: trained staff in the ED/education interventions/discharge interventions) |
| Hughes et al.29 | To evaluate the effect of ED interventions on clinical, use and care experience outcomes for older adults | SR | Randomised or quasi-experimental (non-randomised trial, case-matched controlled prospective before-and-after cohort study, interrupted time series) | Medline via PubMed, Embase, CINAHL and PsycINFO; search to December 2017; reference list checking, ClinicalTrials.gov, Scopus for conference abstracts | Where possible, used random effects models for dichotomous outcomes using RRs and mean difference for continuous outcomes | Adults ≥ 65 years presenting to ED. Evaluate an intervention strategy. Meet study design criteria, be conducted in an OECD country, report one or more clinical or use outcome of interest | Not reported | 17 articles describing 15 studies (9 randomised and 6 non-randomised); 1996–2018; includes all papers. |
| Hoon et al.20 | 1. What are elderly patients’ experiences of overall care received in the ED? 2. What are elderly patients’ experiences of the nursing care received in the ED? 3. What are the needs identified by elderly patients in the ED? |
SR undertaken using methods of the Joanna Briggs Institute/narrative synthesis of quantitative results. JBI-QARI (JBI, University of Adelaide, Adelaide, Australia) to synthesise qualitative results | Quantitative and qualitative evidence | Databases: 5 (plus Cochrane Library and JBI to check no prior reviews; dates: not given (assume studies published prior to 2010); other sources searched: reference lists, bibliographies | Narrative summary of quantitative study, qualitative studies pooled using JBI-QARI (meta-synthesis of qualitative statements generated through synthesis of findings arranged according to quality | Age: ≥ 65 years (male and female); admitted to ED with urgent and non-urgent health related issues. Phenomena of interest: elderly patients experiences of care in the ED setting including evaluation of factors that influence experiences of care | Excluded cognitive/mental disabilities, patients using drugs/alcohol, unconscious patients, GP or drop in clinics | 5 papers, 1999–2007; includes all papers |
| Jay et al.30 | SR seeks to assess whether consultant geriatrician-led CGA within the ED can reduce admission rates, and how this subsequently impacts upon inpatient length of stay and readmission rates | SR/narrative synthesis of quantitative data | RCTs, non-RCTs and observational studies | Databases: 5; dates: from conception to week 3 of March 2016; other sources searched: unpublished literature and trial registry databases were searched using Open Grey, UKCRN portfolio Database and the UK National Research Register Archive. References of related articles and all potentially eligible studies were hand searched for additional papers and included studies were forward cited | The studies were reviewed using a narrative analysis approach | (1) studies of older persons > 65 years presenting non-electively to EDs; (2) studies in which the intervention consisted of CGA performed in the ED by a team that included at least a consultant geriatrician; (3) RCTs and observational studies; (4) studies reporting, at the least, inpatient admission rates | Studies in which CGA was performed after the final decision to admit as an inpatient or discharge had been made | 5 studies, 2012–2014; includes all papers |
| Karam et al.31 | To review the literature on ED-based interventions and examine the evidence on reductions in ED re-visits, hospitalisations, nursing home admissions and deaths among older adults | SR/narrative synthesis of quantitative data | Only included studies which used a comparison group | Databases: 4; dates: search end date of 2012; other sources: reference lists | 2 themes emerged and were used to create a framework for presenting the results | Age: ‘older adults of which age cut-offs correspond with the general understanding’. Setting: patients were discharged from the ED. Intervention: an intervention aimed to reduce adverse events after an index ED visit. 3 additional inclusion criteria were added at data extraction stage: | Literature reviews, letters to the editor, commentaries, editorials | 9 studies, 1996–2008; includes all papers |
| ‘(1) The manuscript consisted of an evaluation of the intervention; (2) a concurrent or historical comparison group was used to make comparisons against the intervention group; (3) at least one of the specified health services events was included as a study outcome’ | ||||||||
| Lowthian et al.32 | (1) profile effective care transition models; (2) provide robust estimates of effect of these care models | SR and meta-analysis/quantitative synthesis of quantitative data including meta-analysis | RCT, quantitative studies | Databases: 3; dates: 1937–2013; other sources searched: Reference lists | Meta-analyses employing random effects models | Age: > 65 years who were discharged home; setting: ED; intervention: discharge and care transition strategies | Patients admitted to hospital, single disease management models, inadequate information re post-discharge outcomes. | 11 papers, 1996–2013; includes all papers |
| Malik et al.33 | This SR aims to appraise the impact of geriatric focused nurse assessment and interventions in the ED in terms of admission rate, ED revisits and length of hospital stay | SR/quantitative synthesis of quantitative data including meta-analysis | Quantitative research consisting of RCTs, multicentre and observational studies were included | Databases: 6, dates: 1990 to 2016; other sources: reference lists | Results for dichotomous variables were presented as RRs with 95% CI. Results for continuous variables are presented as a mean difference with a 95% CI. Meta-analysis results presented in Forest plots. A narrative synthesis was conducted on data considered to be unsuitable for meta-analysis | Age: ≥ 65 years; setting: presenting to the ED; intervention: geriatric-focused interventions and assessments undertaken independently by a lone nurse or as part of a MDT (within ED, or community if within 24 hours) | Those evaluating interventions and assessments on inpatients, in the community, nursing home and where the nurse was not conducting the assessment and intervention. Studies primarily assessing functional decline, patients ≤ 65 years, non-English studies, non-RCT or descriptive, case studies, expert opinions and qualitative research were excluded | 9 papers, 1996–2015; includes all papers |
| McCusker and Verdon34 | CGA interventions for older hospital and community-based populations, to explore what characteristics of the intervention (site, type, duration) are associated with ED use | SR/narrative synthesis of quantitative data | RCTs, non-RCTs, before-and-after studies and cross-sectional studies | Databases: Medline and the Cochrane database of clinical trials, 1965–2004; other sources searched: hand of the bibliographies of relevant studies and review articles; the authors also consulted with colleagues | Descriptive | Original research (written in English or French) on interventions conducted in non-institutionalised populations ≥ 60 years, not restricted to a particular medical condition in which ED visits were a study outcome | (1) did not report data from original study; (2) study sample included patients < 60 years (unless the results for those ≥ 60 years were presented separately); (3) study sample was from a nursing home or other long-term care facility; (4) no intervention was investigated or the intervention did not meet the criteria for CGA; | 8 papers, 1996–2004; includes a subset (papers on the ED) |
| (5) the study outcomes did not include a measure of ED use; (6) the paper was written in a language other than English or French; (7) studies restricted to a particular medical diagnosis or procedure; (8) studies that did not compare those individuals who received a CGA intervention with those in comparison group | ||||||||
| Parke et al.35 | To synthesise evidence on the needs and challenges of providing health care to older adults with cognitive impairment in the ED | Scoping review/narrative synthesis of quantitative data | SR; meta-analysis; clinical trial; cohort study; evaluation study | Databases: 9; dates: 1990–2008 (initiated search alerts with an end date of June 2009); other sources searched: none | Descriptive | Qualitative or quantitative data on delirium or dementia from an aged population defined as ≥ 65 years who received care in the ED | Excluded studies if there was insufficient focus on the topic, and other exclusions including nursing home populations and non-English papers | 15 papers, 1994–2009; includes all papers |
| Pearce et al.21 | SR aimed to identify any age-friendly nursing interventions that have shown improvements in the management of the older people presenting to the ED | SR/narrative synthesis of quantitative data and meta synthesis of qualitative data | Quantitative research study designs as well as narrative opinion and text | Databases: 9; dates: 1999 to 2010; other sources searched: reference lists, unpublished databases, Google Scholar | Narrative summary | Age: ≥ 65 years; setting: presenting to the ED; intervention: any age-friendly nursing interventions | None reported | 2 papers, 2002–2005; includes a subset (only included research papers, did not include policy and expert opinion documents) |
| Schnitker et al.36 | ‘To identify practices designed to meet the specific care needs of older cognitively impaired patients in EDs’ | Systematic literature review/narrative synthesis of quantitative data | ‘Research based literature’ | CINAHL, Medline, PubMed, PsycINFO, Cochrane Library | Narrative summary of results with themes generated | English language; ≥ 65 years with cognitive impairment; ED in acute care hospitals; interventions or best practice | Non-intervention studies; interventions with a duration of over 24 hours | 12 papers, 1998–2011; includes a subset of ED papers (cognitive screening – 9, falls prevention – 1, delirium prevention – 2) |
| Shankar et al.22 | ‘To synthesize the current knowledge about the elderly patients preferences and views of their emergency care’ | SR/meta ethnography of patient views | Survey, questionnaire, focus groups or individual interviews | PubMed and CINAHL | Narrative synthesis and meta ethnography to identify themes. Meta-ethnography involved listing concepts, themes and metaphors from each study that were then organised, related and linked to each other and synthesised into broader ideas which were integrated into specific themes. Articles were classified as to whether they did or did not include the theme | (1) Hospital-based emergency care; (2) outcomes relating to one or more of the IOM 6 dimensions of patient care | Articles were only included if they addressed one of the IOM 6 dimensions of patient care | 28 papers, 1986–2011; includes all papers |
| Sinha et al.37 | ‘existing evidence for ED based case management models’ | SR according to PRISMA methods/narrative synthesis of quantitative data | RCTs, non-RCTs, observational studies/programme descriptions | Medline and CINAHL, 1966–2010, English language | Interventions were examined for operational components. These operational components were then put into categories. Individual papers were examined in terms of their components and whether they adhered to the list of components. Then the relative importance of the presence of a component in terms of intervention effectiveness was determined using a collated list of outcome measures | ‘Descriptions of clinical interventions with assessment and management components to improve outcomes for older adults’ | Articles that did not quantify outcomes; screening instruments; interventions for patients with a single diagnosis | 20 papers, 1994–2007; includes all papers |
| Tran et al.41 | Literature review attempted to identify risk factors and interventions to prevent the most common complications, ED returns | Literature review/narrative synthesis | No information (conference abstract) | Databases: 2; dates: no information; other sources searched: manual reference search | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) |
| Reference | Studies included in the review | Topic | Aim of the review | How were data collected? | Findings | Any other information? |
|---|---|---|---|---|---|---|
| Burkett et al.23 | QIs for ED care of older persons (defined as those aged ≥ 65 years) | QIs | (1) To identify existing QIs for older people. (2) To map the domains of the QIs to assess the balance of the existing indicators. (3) To critically evaluate these quality indicators | QI mapped by: (1) IOM quality of care (timeliness, effectiveness, efficiency, safety, patient centeredness, equity). (2) Donabedian (structures, processes, outcomes). (3) Alessandrini (general, disease specific or cross cutting). (4) QI quality assessed by a modified tool, using AIRE and QUALIFY. 5 different categories (purpose, relevance and organisational context, stakeholder involvement, scientific evidence, specifications, feasibility | 50 QIs identified. 36/50 process indicators, 8/50 outcome indicators and 6/50 structure indicators. 30/50 were cross cutting, 16/50 were general and 4/50 were disease specific. Quality appraisal demonstrated a range of 39.4–67% and 18/50 scored 50% or more for all 5 domains | No indicators addressed equity – disaggregating age data would allow examination of whether all older people are receiving the same care. Donabedian and IOM considered to be important by review authors in considering QIs. Limited field testing of QIs |
| Hoon et al.20 | Studies reporting patients experience of care within the ED | Patient experience and satisfaction | (1) To determine best available evidence on care provided in the ED. (2) To aggregate the findings of this evidence to generate a set of statements about older peoples care in the ED | SR according to Joanna Briggs Institute methodology | 5 papers identified from which 12 unequivocal findings were extracted. Quantitative evidence demonstrated satisfaction with care was related to length of waiting time and information and pain management received. Qualitative synthesis generated found that health-care professionals should be aware of the intolerable factors of waiting experiences and the positive attitudes of older people during these waits. in addition, there is a need for improvement for nursing staff to deliver the attention and expectations that older people require | Implications for practice are that: waiting times should be shortened; physiological and psychological needs should be considered; physical environment and facility design should be more patient friendly; complete information and clear answers to questions should be provided |
| Shankar et al.22 | Qualitative studies and surveys addressing elderly patients views of emergency care | A meta-ethnography of patient experience and satisfaction | To develop themes of quality emergency care for older people in the ED from qualitative studies and surveys addressing elderly patients views of emergency care | 28 articles described specific views and attitudes of older people about the quality of hospital based emergency care. Meta-ethnography was used to identify ideas and themes, initially linked to the IOM 6 dimensions of patient centred care | 6 themes identified: (1) role of health-care providers (emergency physicians and nurses) 11/26 studies; (2) content of communication and patient education 23/26 studies; (3) barriers to communication; (4) waiting times; (5) physical needs in the emergency care setting; (6) general elder care needs | These themes can be used to structure improvements to the care of older people |
| Reference | Population: participants, n (range); centres, n (range); conditions included: control group |
Topic | Who delivers the intervention(s)? | What do the intervention(s) consist of? | Where are the intervention(s) delivered? | When are the intervention(s) delivered? | How often are the interventions delivered? | Length of follow-up across studies (range) |
|---|---|---|---|---|---|---|---|---|
| Cherian et al.39 | Participants: no information; centres: no information; conditions included: limited information – geriatric population; studies included: no information | Geriatric ED practices | Not reported | Geriatric ED | Hospital (ED) | Not reported | Not reported | Not reported |
| Gupta et al.40 | Participants: no information; centres: no information; conditions included: geriatric trauma patients, largely defined as aged ≥ 65 years; 26 studies included | Geriatric trauma management | Not reported | Geriatric assessment with recognition of geriatric syndromes for example delirium and polypharmacy | ED | Within 24 hours of presentation | Not reported | Not reported |
| Tran et al.41 | Participants: no information; centres: no information; conditions included: geriatric population whose age is > 60 years; 23 studies included | Interventions to prevent post ED discharge complications | Majority of studies used nurses for screening | ‘Majority of interventions are bundle of care, which consisted of nurse screening for patients at risk, home visits and referrals for appropriate social/medical assistance’ | Hospital and community | Not reported | Not reported | Not reported |
| Reference | Population: participants, n (range); centres, n (range); conditions included | Topic | Who delivers the intervention(s)? | What do the intervention(s) consist of? | Where are the intervention(s) delivered? | When are the intervention(s) delivered? (range) | How often are the interventions delivered? (range) | Length of follow-up across studies (range) |
|---|---|---|---|---|---|---|---|---|
| Conroy et al.24 | Participants 313–739; centres 5; conditions included: falls | Rapid-access nurse-led/geriatrician supported assessment – and CGA for patients post-ED | Nurse (3), geriatrician (2) | CGA with additional services | Hospital (ED) with evidence of additional interventions delivered in the home and in the community setting as well as follow up MDT in the hospital setting | < 72 hours after presenting to the ED | Not reported | 0 days–12 months |
| Fan et al.25 | Participants: no information; centres: no information; conditions included: limited information – geriatric population | MDT and gerontological expertise | Not reported | Hospital-based strategies were most characterised by risk screening and geriatric assessment for the purpose of identifying potential risk factors and unresolved problems and discharge planning and referral coordination | Hospital and community | Not reported | Not reported but include community follow up | ‘Relatively long term’; only 2 were < 6 months |
| Fealy et al.26 | Participants: 224–2139; centres: 1–4; conditions included: ED attendees | Nursing assessment and referral interventions | Advanced nurse/nurse | Nurse assessment/post discharge referral/patient education/telephone follow-up | ED/home | During ED visit or post-discharge | Single/for 4 weeks/every 6 weeks | 4 weeks to 12 months |
| Graf et al.27 | Participants: 114–2679; centres: 8; conditions included: older people | CGA interventions delivered by nurses in ED | Nurse (7), research assistant (1) | CGA (8) ± referral to community resources and telephone follow-up | CGA in ED (5)/at home (2) | Some in ED; up to 16 weeks between ED visit and intervention (1) | Gagnon study gave 6-weekly repeat telephone calls | 10 days to 1 year |
| Hastings and Heflin28 | Participants: not reported; centres: could gather this info for the clinical trials – 8; conditions included: community-dwelling elder patients discharged home from the ED – mixture of > 65 and over > 75 years | Interventions delivered by all staff | Geriatric nurse practitioner (3); MDT including geriatrician (1); consultation service consisting of OT and physical therapists (1); nurse discharge plan coordinator (1); nurse-practitioner-led intervention (1) | CGA (1); geriatric consultation service (2); functional assessment (1); care co-ordination team (1); ED-based nurse discharge plan co-ordinator (1); 30-minute assessment by geriatric nurse (1); ISAR screening instrument (1); quick response programme (1); nurse practitioner-led intervention (1) | Hospital (ED) | During the ED visit | Not reported | Not reported |
| Hughes et al.29 | 4561 (randomised) and 11,580 (non-randomised) | Interventions according to strategy type, single or multi strategy, intervention components. | Not uniformly reported | Various types but classified as (1) discharge planning; (2) case management; (3) medication safety (4) geriatric EDs | Within ED, outside of ED or both (bridge strategies) | Not reported | The majority are multiple times | 1–18 months following the index ED visit |
| Jay et al.30 | Participants: 28,434; centres: 6; conditions included: >65 years presenting non-electively to EDs | Consultant-led CGA | All interventions had at least a consultant geriatrician and older peoples’ nurse; 3 studies employed a physiotherapist and occupational therapist; and 1 also had access to a dedicated social worker | Geriatrician-led CGA | Hospital (ED) | All the interventions consisted of MDT assessment of patients in the ED with the goal of overcoming medical and social barriers to safe discharge | Not reported | Not applicable for most reported outcomes; 2 papers examine interventions effect upon readmission rates and report a range of 7–90 days |
| Karam et al.31 | Participants: 86–2532; centres: not reported but assume 9 studies; conditions included: age threshold main inclusion criteria, conditions such as falls also included | Nurse/social worker/geriatrician-led integrated (discharge) assessment interventions | Nurse/social worker (8) ± geriatrician (1) | Referral to community resources (5); follow-up (CGA/care plan) (3), care facilitator (1) | ED (1 study commenced an integrated care facilitator for 90 days) | Initiated in ED | 1 study started ongoing facilitator | 8 days to 12 months |
| Lowthian et al.104 | Participants: 345–14,658; centres: 1–4; conditions included: patients at high risk of adverse outcomes | ED CTS | Nurses, allied health professionals or trained health visitors (11) | Geriatric assessment (9) | ED and until community-based services became available | ED and until community-based services became available | Multiple ED with community follow up | 14 days to 18 months |
| Malik et al.33 | Participants: 110–2139; centres: 7; no specific conditions mentioned | Nurse/ANP-led ED CGA | Nurse/ANP (9) | Nurse assessment using risk screening tool (5); CGA (2); nurse case management/discharge coordinator (2) | ED only (5); ED & home follow-up (2); home (2) | Prior to ED discharge (7); at home (2) | ED & follow-up (5) | 30–120 days (not all studies reported) |
| McCusker and Verdon34 | Participants: 12–1724; centres: 7 conditions included: no restriction on medical condition – ED-based samples | CGA | Social worker in consultation with physician (1) or nurse in consultation with physician (1) or nurse (3) | CGA with additional services | Not clear whether all of the interventions are delivered in the ED as the studies used ‘ED-based samples’ not necessarily ED based interventions | Not reported but do include home visits | for 12 months (1); initial assessment + 4 week follow-up (1); for 10 months (1); for 14 days (1); one-time, limited telephone follow-up (2); one-time (1) | 0 days–12 months |
| Parke et al.35 | Participants 1005; centres: 4; conditions included: delirium or dementia from an aged population defined as ≥ 65 years who received care in the ED | Prevalence and identification of cognitive impairment in ED (CGA, training) | Geriatric teams (2); geriatric consultant (1) | Case finding using quick-to-administer screening tools (2); geriatric teams that target all older ED patients to provide CGA (2); Specialised geriatric training for ED physicians (1); addition of a geriatric consultant on the ED roster (1) | ED | Not reported (assume during attendance) | Not reported | 3–18 months (not all studies reported) |
| Pearce et al.21 | Participants: 49–136; centres: 1; conditions included: no specific condition mentioned | Nursing interventions in the ED to enhance older peoples comfort | Generic approaches | Physical equipment (2); education re nursing care (7) | ED | Physical equipment: during ED attendance | Once | Up to ED discharge |
| Schnitker et al.36 | Participants: not reported; centres: not reported; conditions included: older people with delirium | Screening, preventing and managing cognitive impairment | Various | (1) Screening for the detection of cognitive impairment in the ED and provision of care when early cognitive screening applied in Eds; (2) delirium prevention; (3) falls prevention | ED | Within 24-hour ED window | Not reported | Up to 12 months (not all studies reported) |
| Sinha et al.37 | Participants: mean sample size of 890 (range 12–3977); centres: 18 conditions included: age thresholds only | ED-based case-management models | Various: nurse, social worker, MDT, allied health-care workers | Discharge coordination, home visits and onward referrals, care planning, in home services | ED and home | ED and post discharge (ED based geriatric assessment and discharge risk assessment) | From once up to total length of 12 months’ duration of intervention | Up to 12 months |
| Reference (any outcomes not reported in the paper) | Outcome | When measured? | Studies in which outcome measured (n) | Participants (n) | Outcome | Comment |
|---|---|---|---|---|---|---|
| Conroy et al.24 (not reported: cost, cost–benefit, cost-effectiveness, health status, length of stay, discharge, satisfaction, carer strain, carer burden) | Mortality | Final follow up (1–18 months) | 5 of 5 | 2474 | RR 0.92 (95% CI 0.55 to 1.52) | |
| Institutionalisation | Final follow up (4–18 months) | 3 of 5 | 1816 | RR: nurse-led 0.75 (95% CI 0.44 to 1.29); geriatrician-led 1.16 (95% CI 0.62 to 2.16) | ||
| Functional outcomes | Not reported – ‘of doubtful clinical importance’ | |||||
| Quality of life | Not reported – ‘not clinically meaningful’ | |||||
| Cognition | Not reported ‘unlikely to be clinically important’ | |||||
| Readmissions | Final follow-up | 5 of 5 | 2474 | RR 0.95 (95% CI 0.83 to 1.08) | No significant difference between intervention and control; however, evidence of heterogeneity using I2 (42%) | |
| Fan et al.25 | ED use and ED length of stay (reported together) | Not reported | Not reported | Not reported | Not reported | 5 studies demonstrated statistical significance in lowering ED use and 2 studies demonstrated higher use; 2 tended towards higher use/7 studies showed no statistical significance of the intervention, 3 of which documented increases in ED use and 4 reported reductions. |
| Fealy et al.26 | ED revisits | Various | 5 | Various | Various | 5 studies demonstrated reduced number of admissions and representations |
| Readmissions | Various | 5 | Various | Various | ||
| Hospital days | Various | 1 | Various | Various | ||
| Nursing home admissions | Various | 1 | Various | Various | ||
| Long term hospital admissions | Various | 1 | Various | Various | ||
| Graf et al.27 | ED revisits | 10 days to 3 months | 7 | 114–2679 | No meta-analysis | |
| Institutionalisation | 1–4 months | 3 | 326–385 | |||
| Death | 1–18 months | 5 | 178–385 | |||
| Functional decline | 1–10 months | 5 | 178–385 | |||
| Hastings and Heflin28 (not reported: institutionalisation; ED admission rates; caregiver burden; patient satisfaction; expenditures; change in depressive symptoms; change in caregiver physical and mental health) | Return ED visits | 1 study reported follow-up period: 8 days and 14 days | 2 | Not reported | 1 study reported decrease in unscheduled revisits at day 8 and day 14; 1 study reported trend toward fewer subsequent ED visits in intervention group | |
| Hospital admission | 2 years | 1 | Not reported | Not reported | high rates of hospital admission | |
| Mortality | One study reported 2-year follow-up | 2 | Not reported | Not reported | Only one study reported outcome: high rates of mortality 2 years post intervention | |
| Nursing home admissions | 1 month | 1 | Not reported | Not reported | Decreased admissions at 1 month | |
| Functional decline | 4 months | 1 | Not reported | Not reported | Reduction in functional decline | |
| Hughes et al.29 | Functional decline | 1–18 months | 6 (5 randomised) | 2233 randomised, 687 non-randomised | 3 of 5 showed benefit: beneficial interventions were multistrategy | |
| QoL | Not reporting as no effect | Not reporting as no effect | Not reporting as no effect | No effect | ||
| Patient experience | Not reporting as no effect | Not reporting as no effect | Not reporting as no effect | No effect | 2 of 4 randomised showed benefit for satisfaction, helpfulness or self-esteem, beneficial interventions were multistrategy or case management | |
| Hospitalisation (at or after initial visit) | Not reporting as no effect | Not reporting as no effect | Not reporting as no effect | No effect | RR 0.96; no consistent effects on readmission | |
| ED return visit | Not reporting as no effect | Not reporting as no effect | Not reporting as no effect | No effect | RR 1.13; 2 of 5 showed lower readmission | |
| Jay et al.30 | Inpatient admission rates (same day) | Not reported | 5 of 5 | 28,434 | Not reported | All 5 reported statistically significant reductions in admission rates of older patients following the introduction of geriatrician-led CGA to the ED. However, there was a degree of variation in the magnitude of the results (reductions between 2.6–9.7%) leading to some uncertainty to the clinical and economic benefits of the intervention |
| Inpatient length of stay | Not reported | 4 of 5 | 23,169 | Not reported | One paper reported no sig difference, two papers report a decrease in length of stay and one paper reported an increase. | |
| Readmission rates | 7, 30 and 90 days | 2 of 5 | 16,679 | Not reported | Two papers found no significant difference in readmission rates at 7 and 30 days. One study reported a fall in readmissions among > 85 years at 90 days | |
| Karam et al.31 | ED revisits | 1 day–18 months | 9 | Not reported | Reductions in ED revisits in 4/9 studies | |
| Hospitalisation | 30 days–18 months | 5 | Not reported | Reductions in readmissions in 4/5 studies | ||
| Nursing-care home admission | 30 days–3 months | 2 | Not reported | Only in 1 subgroup of one study | ||
| Death | 30 days–18 months | 3 | Not reported | No improvements in outcomes relating to mortality | ||
| Lowthian et al.104 | Unplanned ED re-presentation/ | 1 month | 4 | 1389 | OR 1.32 (95% CI 0.99 to 1.76) | No benefit |
| Emergency hospitalisation/admission | 1 month | 4 | 1389 | OR 0.9 (95% CI 0.7 to 1.16) | No benefit | |
| Mortality | 18 months | 4 | 1794 | No benefit | ||
| Malik et al.33 | Hospitalisation | 30 days and 14 days (2 meta analyses) | 6 | Not reported | OR 0.90 (95% CI 0.71 to 1.13) and OR 0.84 (95% CI 0.70 to 1.02) | Pooled data did not demonstrate a difference in hospitalisation among study groups |
| Readmissions | 30 days (data incomplete) | 5 | Not reported | OR 0.99 (95% CI 0.62 to 1.61) | Results showed no difference in readmission rates between study groups | |
| Length of hospital stay | Not reported | 2 | Not reported | HR 1.1 (95% CI 0.5 to 3.3), MD 1.10 (95% CI −2.19 to 4.39) | Results indicated no difference between groups | |
| ED revisits | Not reported | 5 | Not reported | OR 1.03 (95% CI 0.84 to 1.26) | Pooled data analysis did not reveal a difference in ED revisits between groups | |
| McCusker and Verdon34 | Mean number of ED visits | 3 months to 1 year | 4 of 7 | 1554 | Only reported at individual study level | Long-term case management significantly increased ED visits and two others showed a trend to short-term (30-day) increase in ED visits. These short-term increases had disappeared by 4 months in both studies |
| Mean number of ED hours | 1 year | 1 of 7 | 12 | Only reported at individual study level | ||
| Mean ED cost per patient | 1 year | 1 of 7 | 12 | Only reported at individual study level | ||
| ED return visit | 14–30 days | 4 of 7 | 3458 | Only reported at individual study level | Only 2 of the interventions for ED patients reduced ED visits; neither was an RCT and the effects were of borderline statistical significance | |
| Parke et al.35 | Review reports intervention characteristics but no patient or health service outcomes are reported | |||||
| Pearce et al.21 (not reported: pressure area status, hydration status, nutritional status, reported level of comfort and pain status) | Comfort | Before ED discharge | 2 | 185 | Not reported | Warmed blankets improved comfort and reclining chairs reduced pain from gurneys |
| Schnitker et al., 201336 (not reported: numerous variables) | Whether recognition of cognitive impairment can improve outcomes | Not reported | Not reported | Not reported | Not reported | Several short screening tools were identified to improve recognition of cognitive impairment. Also important to be able to identify whether the patient has delirium or cognitive impairment. |
| Sinha et al.37 (28 outcomes measures) | Outcome measures reported in each study: 28 outcome measures in total occurring across 18 studies. | Between once and 12 months across the 18 studies | 18 | Not reported | Measures were: hospital admission rate (3); hospital admissions avoided (3); length of inpatient stay (5); ED revisitation rate (13); time to first ED revisitation (1); subsequent hospital admission rate (7); acute bed days used (2); nursing home admission rates (4); home care referral rates (2); health and social care services use (3); outpatient visits (2); costs related to health/social care use (4); mortality at 3 months (3); patient satisfaction with service (12); patient satisfaction with information (1); adherence to new medication (1); adherence to follow-up appointments (1); presence of advance directives (1); ADL/IADL/functional decline (6); cognitive decline (1); change in depressive symptoms (1); health status measured by SF-36 (3); Quality of life/wellbeing (4); carer satisfaction (4); carer health status measured by SF-36 (1); carer burden (1); ED/hospital care providers satisfaction with service (5); primary care providers satisfaction with service (3) | In addition to collating the intervention components and the intervention outcomes, looked at whether the studies reported a positive effect, whether this positive effect was significant and then compared this with how many of the characteristic components the studies had. Several themes emerged: (1) effective interventions use validated risk stratification tools prior to assessment, care plan or referral with a specialised clinician; (2) effectiveness was influenced by team composition, in particular nurse leadership (as nurses and mid-level clinicians have training and experience that span both health and social care which helps with integrated interventions) |
| Reference | Key outcomes | Findings |
|---|---|---|
| Burkett et al.23 | Methodological quality of quality indicators | They were mostly process (36) followed by outcome (8) and structure (6) indicators; 16 were for all older people, 4 were disease specific and 30 were cross cutting. Quality of QIs was variable. Range 39.4–67% with 18 scoring 50% or more for all domains. 30/50 did not use a systematic review of evidence to develop the QI and only 4/50 appraised included evidence |
| Hoon et al.20 | Qualitative: experiences of overall care, experiences of nursing care, needs of elderly patients in the ED. Quantitative: factors influencing experiences of care | 12 unequivocal findings from the evidence generated two syntheses. (1) HCPs should be aware of how intolerable wait times are and appreciate the positive attitudes of staff. (2) There is a need for improvement in nursing staff to deliver appropriate attention and meet patient expectations |
| Shankar et al.22 | Relating to one of the six IOM dimensions of patient-centred care: (1) respectful to patients’ values, preferences and expressed needs; (2) coordinated and integrated; (3) provide information, communication and education; (4) ensure physical comfort; (5) provide emotional support – relieving fear and anxiety; 6) involve family and friends | All 26 included articles presented in tabular format by the 6 individual themes of quality defined by the elderly adults. These themes presented as strategies to improve emergency care: (1) physicians should care for both health and social care needs and refer these on if necessary; (2) increase provider communication to decrease anxiety and manage expectations; (3) providers should minimise barriers to communication by tailoring questions/knowledge/communication to patients; (4) where long waiting times are unavoidable, staff should communicate with patients the reasons and the expected wait; (5) simple changes to infrastructure can make a difference to pain and physical comfort; (6) patients are often anxious when in the ED and this anxiety is high on discharge – improving communication between patients, caregivers and family carers can alleviate this through family-centred care |
| Reference (a priori outcome measures) | Key outcomes | Outcome | When measured | Studies in which outcome measured (n) | Participants (n) | Risk ratio | Comment |
|---|---|---|---|---|---|---|---|
| Cherian et al.39 (no information, (conference abstract) | Structural enhancements, operational enhancements, provider education, quality improvement, coordination of hospital resources, coordination of community resources, staffing, patient satisfaction and outcome evidence | No outcomes reported (conference abstract) | Not reported | Not reported | Not reported | Not reported | All these outcomes needed to design a geriatric ED |
| Gupta et al.40 | Length of stay; readmission rates; functional status; mortality | Length of stay | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) | Decreased length of stay |
| Readmission rates | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) | Decreased readmission rates | ||
| Functional status | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) | No information (conference abstract) | Increased return to functional status | ||
| Mortality | No information (conference abstract) | 3 of 26 | No information (conference abstract) | No information (conference abstract) | Significant difference in mortality | ||
| Tran et al.41 | ED returns and risk factors for these returns | ED return visit | 14 days/28 days/and longer | One study looked at 14 day return; no information about how many other studies looked at longer lengths | No information (conference abstract) | 14-day ED return (95% CI 0.57 to 0.96) | 1 study reported successful reduction of short term 14-day ED return (16% control vs. 12.9% intervention, 95% CI 0.57 to 0.96). However, other studies reported rates of ED return after 28 days or longer post discharge were not significantly reduced by these ED interventions |
Appendix 3 Review of reviews: quality assessment
We report on the quality assessments of the selected review in Table 27.
| Conroy et al.24 | Fan et al.25 | Fealy et al.26 | 123Graf et al.27 | Hastings and Heflin28 | Hughes et al.29 | Jay et al.30 | Karam et al.31 | Lowthian et al.104 | Malik et al.33 | McCusker and Verdon34 | Parke et al.35 | Pearce et al.21 | Schnitker et al.36 | Sinha et al.37 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Did the RQs and inclusion criteria for the review include the components of PICO? For Y, all should be ticked. | Population | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Intervention | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | |
| Comparator | Y | Y | N | Y | N | Y | N | Y | N | N | Y | N | N | N | N | |
| Outcome | Y | Y | N | Y | N | Y | Y | Y | Y | N | Y | N | Y | Y | Y | |
| Y/N | Y | Y | N | Y | N | Y | N | Y | N | N | Y | N | N | N | N | |
| 2. Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol? For PY, criteria 1–4; for Y, criteria 1–8 | Review question | Y | Y | N | N | Y | Y | Y | N | Y | N | Y | Y | N | Y | Y |
| Search strategy | Y | Y | N | N | Y | Y | Y | N | Y | N | Y | Y | N | Y | Y | |
| Inclusion/exclusion criteria | Y | Y | N | N | Y | Y | Y | N | Y | N | Y | Y | N | Y | Y | |
| RoB assessment | Y | Y | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | N | Y | |
| Protocol registered | N | N | N | N | N | Y | Y | N | Y | N | N | N | N | N | N | |
| Meta-analysis plan (if appropriate) | Y | NA | N | N | NA | Y | NA | N | Y | N | NA | NA | N | NA | NA | |
| Causes of heterogeneity plan | Y | NA | N | N | NA | Y | NA | N | Y | N | NA | NA | N | NA | NA | |
| Justification for protocol deviations | N | NA | N | N | NA | N | Y | N | NA | N | NA | N | N | N | N | |
| Y/PY/N | P | P | N | N | P | Y | P | N | Y | N | N | P | N | N | N | |
| 3. Did the review authors explain their selection of the study designs for inclusion in the review? For Y, review should satisfy ONE of the following | Explanation for including only RCTs | Y | NA | NA | N | NA | NA | NA | NA | NA | NA | NA | N | NA | NA | |
| Explanation for including only NRSI | NA | Y | NA | NA | NA | NA | Y | NA | NA | NA | NA | N | NA | NA | Y | |
| Explanation for including both RCTs and NRSI | NA | NA | N | ? | Y | NA | N | Y | N | Y | N | N | NA | |||
| Y/N | Y | Y | N | N | ? | Y | Y | N | Y | N | Y | N | N | N | Y | |
| 4. Did the review authors use a comprehensive literature search strategy? For PY, criteria 1–3; for Y, criteria 1–8 | Searched at least 2 databases (relevant to RQ) | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Provided key word and/or search strategy | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Justified publication restrictions (e.g. language) | Y | Y | N | NA | Y | Y | Y | NA | NA | Y | Y | Y | N | N | N | |
| Searched the reference lists/bibliographies of included studies | N | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Searched trial/study registries | N | N | Y | N | N | Y | Y | N | Y | Y | N | Y | Y | N | N | |
| Included/consulted content experts in the field | N | N | N | N | Y | N | N | Y | Y | N | Y | Y | N | N | N | |
| Where relevant, searched for grey literature | N | N | N | N | N | N | Y | NA | N | N | N | N | Y | Y | N | |
| Conducted search within 24 months of completion of the review | Y | Y | Y | Y | Y | ? | Y | Y | Y | Y | Y | Y | Y | ? | ? | |
| Y/PY/N | P | P | N | N | P | P | P | P | P | P | P | P | N | N | N | |
| 5. Did the review authors perform study selection in duplicate? For Y, ONE of the following | At least 2 reviewers independently agreed on selection of eligible studies and achieved consensus on which studies to include | N | ? | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | N | N |
| 2 reviewers selected a sample of eligible studies and achieved good agreement (at least 80%), with the remainder selected by one reviewer | N | ? | ? | N | N | N | N | N | ||||||||
| Y/N | N | ? | Y | N | Y | Y | Y | Y | Y | Y | N | Y | Y | N | N | |
| 6. Did the review authors perform data extraction in duplicate? For Y, ONE of the following | At least 2 reviewers achieved consensus on which data to extract from included studies | N | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | N | N | |
| 2 reviewers extracted data from a sample of eligible studies and achieved good agreement (at least 80%), with the remainder extracted by one reviewer | N | N | N | N | N | |||||||||||
| Y/N | N | Y | Y | N | ? | Y | Y | Y | Y | N | Y | Y | Y | N | N | |
| 7. Did the review authors provide a list of excluded studies and justify the exclusions? For PY, criterion 1; for Y, criteria 1 and 2 | Provided a list of all potentially relevant studies that were read in full-text form but excluded from the review | Y | N | N | N | Y | N | Y | N | N | N | N | Y | N | N | N |
| Justified the exclusion from the review of each potentially relevant study | Y | Y | N | N | Y | N | Y | N | N | N | N | Y | N | N | N | |
| Y/PY/N | Y | P | N | N | Y | N | Y | N | N | N | N | Y | N | N | N | |
| 8. Did the review authors describe the included studies in adequate detail? For PY, criteria 1–5; for Y, criteria 1–10 | Described populations | Y | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y |
| Described interventions | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Described comparators | Y | Y | N | Y | Y | Y | N | Y | N | N | Y | Y | Y | N | N | |
| Described outcomes | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
| Described research designs | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | |
| Described population in detail | Y | ? | N | N | N | Y | Y | N | N | N | Y | Y | N | N | Y | |
| Described intervention in detail (including doses where relevant) | Y | ? | N | Y | N | Y | Y | Y | N | Y | Y | Y | Y | N | Y | |
| Described comparator in detail (including doses where relevant) | Y | ? | N | N | N | Y | N | N | N | N | Y | Y | Y | N | Y | |
| Described study’s setting | Y | ? | Y | N | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | |
| Timeframe for follow up | Y | ? | Y | Y | P | Y | N | Y | Y | N | Y | N | Y | N | Y | |
| Y/PY/N | Y | ? | N | N | P | Y | N | N | N | N | Y | P | Y | N | Y | |
| 9. Did the review authors use a satisfactory technique for assessing the RoB in individual studies that were included in the review? | Name | Van Tulder | EPHPP | Grimshaw checklist | None | Bespoke tool | Cochrane ROB | RoBANS | None | Cochrane Risk of Bias and Newcastle-Ottawa | Cochrane Risk of Bias and EBL | None | CASP | JBI | NHMRC Levels of evidence | Cochrane Risk of Bias and MOOSE |
| RCTs, for PY, criteria 1 and 2; for Y, criteria 1–4 | Unconcealed allocation | N | NA | Y | N | NA | Y | NA | N | Y | Y | N | ? | N | NA | NA |
| Lack of blinding of patients and assessors when assessing outcomes (unnecessary for objective outcomes such as all cause mortality) | N | NA | Y | N | NA | Y | NA | N | Y | Y | N | ? | N | NA | NA | |
| Allocation sequence that was not truly random | N | NA | Y | N | NA | Y | NA | N | Y | Y | N | ? | N | NA | NA | |
| Selection of the reported result from among multiple measurements or analyses of a specified outcome | N | NA | N | N | NA | Y | NA | N | Y | Y | N | ? | N | NA | NA | |
| Y/PY/No/includes only NRSI | N | NSRI | P | N | NA | Y | NA | N | Y | Y | N | N | N | N | N | |
| NRSI For PY, criteria 1 and 2; for Y, criteria 1-4 | From confounding | NA | N | NA | NA | ? | ? | Y | NA | NA | NA | NA | NA | NA | NA | NA |
| From selection bias | NA | N | NA | NA | ? | ? | Y | NA | NA | NA | NA | NA | NA | NA | NA | |
| methods used to ascertain exposures and outcomes | NA | N | NA | NA | NA | Y | NA | NA | NA | NA | NA | NA | NA | NA | ||
| selection of the reported result from among multiple measurements or analyses of a specified outcome | NA | N | NA | NA | NA | ? | N | NA | NA | NA | NA | NA | NA | NA | NA | |
| Y/PY/N/includes only RCT | N | N | N | N | N | N | P | N | N | N | N | N | N | N | N | |
| 10. Did the review authors report on the sources of funding for the studies included in the review? | Must have reported on the sources of funding for individual studies included in the review. Note: reporting that the reviewers looked for this information but it was not reported by study authors also qualifies | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Y/N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | |
| 11. If meta-analysis was performed did the review authors use appropriate methods for statistical combination of results? | ||||||||||||||||
| RCTs; for Y, criteria 1–3 | The authors justified combining the data in a meta-analysis | Y | NA | NA | NA | NA | Y | NA | NA | Y | Y | NA | NA | NA | NA | NA |
| AND they used an appropriate weighted technique to combine study results and adjusted for heterogeneity if present | Y | NA | NA | NA | NA | NA | NA | Y | Y | NA | NA | NA | NA | NA | ||
| AND investigated the causes of any heterogeneity | Y | NA | NA | NA | NA | Y | NA | NA | Y | N | NA | NA | NA | NA | NA | |
| Y/N/no meta-analysis conducted (–) | Y | – | – | – | – | Y | – | – | Y | N | – | – | – | – | – | |
| NRSI, for Y, criteria 1–4 | The authors justified combining the data in a meta-analysis | NA | NA | NA | NA | NA | Y | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| AND they used an appropriate weighted technique to combine study results and adjusted for heterogeneity if present | NA | NA | NA | NA | NA | Y | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| AND they statistically combined effect estimates from NRSI that were adjusted for confounding, rather than combining raw data, or justified combining raw data when adjusted effect estimates were not available | NA | NA | NA | NA | NA | Y | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| AND they reported separate summary estimates for RCTs and NRSI separately when both were included in the review | NA | NA | NA | NA | NA | Y | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Y/N/no meta-analysis conducted | N | N | N | N | N | Y | N | N | N | N | N | N | N | N | N | |
| 12. If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? For Y, criterion 1 or 2 | Included only low risk of bias RCTs | Y | NA | NA | NA | NA | N | NA | NA | Y | N | NA | NA | NA | NA | NA |
| OR, if the pooled estimate was based on RCTs and/or NRSI at variable RoB, the authors performed analyses to investigate possible impact of RoB on summary estimates of effect | NA | NA | NA | NA | NA | N | NA | NA | NA | N | NA | NA | NA | NA | NA | |
| Y | - | - | - | - | N | Y | N | - | - | - | - | - | - | - | ||
| 13. Did the review authors account for RoB in individual studies when interpreting/discussing the results of the review? For Y, criteria 1 OR 2 | Included only low RoB RCTs | Y | N | N | N | N | Y | NA | NA | Y | N | N | NA | N | NA | NA |
| OR, if RCTs with moderate or high or NRSI were included the review provided a discussion of the likely impact of RoB on the results | NA | N | N | NA | N | Y | Y | NA | NA | N | N | NA | N | NA | NA | |
| Y/N | Y | N | N | N | N | Y | Y | N | Y | N | N | N | N | |||
| 14. Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review? For Y, criterion 1 or 2 | There was no significant heterogeneity in the results | N | N | N | Y | N | Y | N | N | N | NA | |||||
| Or if heterogeneity was present the authors performed an investigation of sources of any heterogeneity in the results and discussed the impact of this on the results of the review | N | N | N | N | Y | Y | N | N | N | NA | ||||||
| Y/N | N | N | - | N | N | Y | N | Y | Y | Y | N | N | N | N | ||
| 15. If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review? | Performed graphical or statistical tests for publication bias and discussed the likelihood and magnitude of impact of publication bias | Y | N | N | N | N | N | N | N | N | N | N | N | |||
| Y/N/no meta-analysis conducted | Y | - | - | N | - | N | - | - | N | N | - | - | - | - | - | |
| 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? For Y, criterion 1 or 2 | The authors reported no competing interests or | N | Y | Y | N | N | Y | Y | Y | Y | N | Y | Y | Y | Y | |
| The authors described their funding sources and how they managed potential conflicts of interest | Y | Y | N | N | N | Y | Y | Y | Y | Y | ||||||
| Y/N | Y | Y | Y | N | ? | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | |
Appendix 4 Analysis of hospital costs for those admitted to hospital – methods
Costs are assigned to each individual patient [i] based on the 2017/18 reference cost data;106 a collation of healthcare resource group (HRG) costs reported by official mandate in a standardised format by all English hospitals to the Department of Health.
The national cost of a 999 or NHS 111 call ccall amounts to £7, as reported in column D of worksheet AMB of the NHS national tariff payment system. The ambulance cost, csamb depends on the type of service s, also reported in column D. The national costs of the ED attendance ckae are reported in the AE worksheet,6 where costs are differentiated according to AE service codes [k].
For non-elective patients admitted to hospital, costs depend on how long they spent in hospital. Patients that stayed less than two days are costed on the basis of the short-stay emergency tariff reported on worksheet NES6 for each HRG [h]. This information is used to assign the cost chss for patients with a short-stay non-elective according to the HRG to which they are allocated.
For patients that stay more the two days, the cost calculation comprises two elements. The first is the national average base cost cb for those patients with a typical length of stay for their HRG, reported on worksheet NEL. 6 The second element is an excess per diem cost cx for each extra day spent in hospital by patients with an exceptionally long stay for their HRG, with this information reported on worksheet NEL_XS. 6 Thus, the full cost cne of hospital treatment for each non-elective patient allocated to HRG [h] takes the form:
Where indicates the patient’s actual length of stay and dihx indicates the additional number of days that the patient stays in hospital above the typical length of stay for their HRG.
The total cost of each individual patient’s emergency care pathway TCi therefore, is the sum of these elements:
Appendix 5 Sample selection bivariate probit model
The sample selection bivariate probit model for 30-day readmission recognises that in-hospital mortality is a competing risk for 30-day readmission and conditions the probability of readmission on whether the patient survived the previous hospitalisation. This involved first estimating a selection equation to explain the probability of survival before estimating the probability of readmission. The survival model accounts for the day of the week of the admission, the argument being that this influences in-hospital mortality but has no bearing on the probability of readmission.
Accounting for day of the week accords with the literature showing that there is a ‘weekend effect’ associated with hospital survival, perhaps because of differences in staffing levels and mix across the week. Hence the survival equation recognises that the probability of in-hospital mortality varies by day of admission day to day.
In contrast, the day of the week that the patient was originally admitted has no bearing on the probability that they will be readmitted to hospital 30 days later. The argument by Laudicella et al. 85 is that the day of the week of the original admission ‘can be assumed to be uncorrelated with the risk of a readmission, which depends on postoperative care that can be provided more flexibly over a long period of time once survival has been assured.’ For our data, the proportionate shares across days of the week are almost identical for all patients and for those who are readmitted, as shown in Figure 33. This confirms that the day of the original admission does not influence the probability of readmission.
FIGURE 33.
Day of week of original admission.

Appendix 6 The system dynamics model technical features
This appendix describes the differential equations used in the underlying system dynamics model to explain the stocks and flows used to represent the emergency care of older people in the YH region. The appendix also describes the risk ratio (RR) calculations used to determine the effect size of a chosen intervention in the model. The differential equations and the RR calculations are the same in the generic version of the model, the only difference is the local population figures fed in from the user interface which relate to the 42 ICS in England rather than the YH region.
Stocks and flows
The model uses the stocks shown in Table 28 to monitor the number of patients in each part of the hospital system or surrounding population.
| Stock | Description |
|---|---|
| Local population | Number of people in the population and is the sum of the number in their home/care home/hospital |
| ED | Number of patients in the ED |
| APC | Number of patients that have been admitted and are in hospital |
| UPR | Number of patients in their home |
| CH | Number of people in a care home |
| A_DN | Number of in-hospital deaths (cumulative total) |
| U_DN | Number of people that die at home (cumulative total) |
| CH_D | Number of care home deaths (cumulative total) |
Each of the stocks is divided into five-year age groups: (75–79), (80–84), (85–89), (90–94) and (95 and over).
The model uses the following flows described in Table 29 to represent patient flow through the system.
| Stock | Description |
|---|---|
| ED attends | Number of people attending the ED (per day); governed by the attendance rate and read in from the Excel file |
| Sent_home | Number of patients discharged from the ED each day; depends on the number in the ED and the proportion of people not admitted |
| emAdms | Number of people admitted through an emergency admission; the parameter values for each age group are entered in an Excel file and can be altered in the tool |
| adms | Number of patients admitted from the ED; depends on the number in the ED and the proportion of people admitted |
| disch | Number of patients discharged from hospital; depends on the number in hospital and the proportion discharged |
| readm | Number of patients readmitted from their home; read in from the Excel file but can be altered using the tool’s interface. |
| CHreadm | Number of patients readmitted from care homes; read in from the Excel file but can be altered using the tool’s interface. |
| dischCH | Number of patients discharged from hospital into a care home; depends on the proportion discharged and the number in hospital |
| APC_deathsN | Number of hospital deaths; depends on the hospital mortality rate and the number of patients in hospital |
| UPR_deathsN | Number of deaths; depends on the mortality rate and the number who live at home |
| CH_deathsN | Number of deaths in care homes; depends on the mortality rate associated with care homes and the number in care home population |
The daily change in the stocks and flows are governed by the following differential equations. As each of the stocks is divided according to age group, this is reflected in the equations using the subscript, AgeGroup.
The change in the size of the local population (see Equation 6) is determined by the difference in the growth and death rates associated with the overall population minus the number of people that attend ED and are an emergency admission.
The daily number of patients in the ED is governed by the number of patients attending ED, and the number of readmissions (from a patient’s home or care home) minus the number discharged from the ED and those admitted into hospital (see Equation 6).
The number of patients in hospital is determined by the number of patients admitted from the ED and the number of emergency admissions minus the number of hospital related deaths and those that have been discharged to their home or a care home (see Equation 7).
The number of patients in their own homes is determined by the number discharged from either the ED or the hospital minus the number that have been admitted into hospital (from the ED, through readmission or emergency admission) or have died (see Equation 8).
The movement of patients in care homes is determined by the number of hospital patients discharged into a care home minus the number that die, and the number of care home patients readmitted to hospital (see Equation 9).
The number of hospital related deaths is given by APC_deathsN, which is determined by the number of patients in hospital multiplied by the hospital mortality rate (see Equation 10).
Where
The daily number of deaths of patients (in their own homes) is given by UPR_deathsN, which is determined by the number of patients in their own homes multiplied by the death rate for the area (see Equation 11).
Where
The number of care home deaths is governed by the number of patients in care homes multiplied by the death rate associated with care homes (see Equation 12).
Where
The daily number of patients attending ED is determined by the average number expected (calculated using the CUREd database) and adjusted by a random number sampled from a uniform distribution. This enables the system dynamics model to incorporate daily variation in ED attendance figures (see Equation 13).
The number of patients sent home from the ED each day is determined by the number of patients in the ED multiplied by the proportion discharged and adjusted for the by the average time spent in ED (see Equation 14).
The number of emergency admissions is governed by the average number of emergency admissions, calculated from the CUREd database, and adjusted using a random number sampled from a uniform distribution to enable daily variation to be captured (see Equation 15).
The daily number of patients admitted into hospital depends on the number in the ED multiplied by the proportion admitted and adjusted by the length of stay in the ED (see Equation 16).
The daily number of discharges (to a patient’s home) is determined by the number of patients in hospital and adjusted for by the average length of stay and a random number sampled from a uniform distribution to allow for daily variation in the discharge numbers (see Equation 17).
The number of patients readmitted from their home into hospital is determined by the average number of readmissions calculated from the CUREd database and adjusted to include variation (see Equation 18).
The number of patients readmitted from a care home into hospital is determined by the average number of readmissions from care homes calculated from the CUREd database and adjusted to include variation (see Equation 19).
The number of patients discharged from hospital to a care home depends on the number of patients in hospital and adjusted for due to their length of stay and daily variation in discharge figures (see Equation 20).
Model parameters
Most of the parameters were estimated using the YH data in the CUREd dataset. The non-hospital death rates and the care home parameters were estimated using ONS data for the region. The parameter values for the baseline model are given in Table 30. In the decision support tool, users can enter their own values of each parameter or use the default values given for the region. The decision support tool allows the user the option to select a hospital setting nearest their own and use the relevant model parameters in the baseline and scenario runs. The model parameters are divided into five-year age bands.
| Parameter | ‘75–79’ | ‘80–84’ | ‘85–89’ | ‘90–94’ | ‘95+’ |
|---|---|---|---|---|---|
| Hospital mortality (%) | 2.4 | 2.6 | 3.5 | 4.3 | 5.6 |
| ED attendance | 23 | 22 | 18 | 10 | 3 |
| Emergency admission | 5 | 4 | 4 | 2 | 1 |
| Patients admitted from ED (%) | 48.9 | 54.3 | 58.79 | 61.67 | 59.1 |
| Proportion of Readmissions (care home) | 0.0000184 | 0.0000408 | 0.0000859 | 0.0001526 | 0.0001872 |
| Proportion of Readmission (home) | 0.00002 | 0.00002 | 0.00004 | 0.00005 | 0.00004 |
| Death rate (home) | 0.0000242584 | 0.000027 | 0.0000969444 | 0.00028 | 0.0002824 |
| Death rate (care home) | 0.00031 | 0.00064 | 0.00095 | 0.0018 | 0.0025 |
Intervention effects using risk ratios
In several of the intervention strategies considered in the system dynamics model, the effect on the patient outcome is described in the literature in terms of a RR (ratio of the probability of an outcome in an exposed group to the probability of an outcome in an unexposed group).
List of abbreviations
- A&E
- accident and emergency
- AFN
- acute frailty network
- AMSTAR2
- a measurement tool to assess systematic reviews, version 2
- CFS
- clinical frailty scale
- CGA
- comprehensive geriatric assessment
- CTS
- community transition strategies
- CUREd
- Centre for Urgent and Emergency Care Research
- ECOP
- Emergency care for older people
- ED
- emergency department
- eFI
- electronic Frailty Index
- EMT
- Executive Management Team
- GIRFT
- getting it right first time
- GP
- general practitioner
- HFRS
- hospital frailty risk score
- HR
- hazard ratio
- ICS
- integrated care system
- IMD
- index of multiple deprivation
- ONS
- Office for National Statistics
- OR
- odds ratio
- PDSA
- plan-do-study-act
- PPI
- patient and public involvement
- PRISMA
- preferred reporting items for systematic reviews and meta-analyses
- PROM
- patient-reported outcome measure
- RCT
- randomised controlled trial
- RQ
- research question
- RR
- risk ratio
- UEC
- urgent and emergency care
- YH
- Yorkshire and Humber
- WP
- work package
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/NLCT5104).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.