Notes
Article history
The research reported here is the product of an HSDR Evidence Synthesis Centre, contracted to provide rapid evidence syntheses on issues of relevance to the health service, and to inform future HSDR calls for new research around identified gaps in evidence. Other reviews by the Evidence Synthesis Centres are also available in the HSDR journal.
The research reported in this issue of the journal was funded by the HSDR programme or one of its preceding programmes as project number NIHR133541. The contractual start date was in November 2020. The final report began editorial review in July 2021 and was accepted for publication in October 2021. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HSDR editors and production house have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the final report document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2022 Chambers et al. This work was produced by Chambers et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2022 Chambers et al.
Chapter 1 Background
Cognitive impairment is an overarching term that refers to deficits in one or more of the areas of memory, problems with communication, attention, thinking and judgement. Impairment can range from mild to severe. Mild cognitive impairment (MCI) is defined as objective cognitive symptoms (e.g. memory problems) in the absence of dementia. 1 MCI is common in older people, affecting 20% of those aged > 65 years. 1 Subjective cognitive decline (SCD), in which people report problems but perform normally on cognitive tests, affects half of those aged > 65 years.
Although most people with MCI do not go on to develop dementia, the condition is associated with increased dementia risk, and this may lead people with MCI (or SCD) to seek help from health services. People with MCI may also be identified as a result of treatment for other conditions in a range of settings.
In the UK, the 2009 National Dementia Strategy2 and associated Prime Minister’s challenge3 emphasised the importance of prevention and prompt diagnosis, both of which involve a focus on people with MCI and other memory problems. The responsibility for prevention of dementia and support for people with the condition is divided between public health, the NHS and social care, although recent policy increasingly favours the integration of health and social care. Health and social care are devolved matters, with some differences between the nations of the UK.
Access to services for people with MCI is a complex issue. Lifestyle changes can reduce modifiable risk factors for dementia, including cardiometabolic dysfunction (i.e. diabetes and cardiovascular risks), physical inactivity, social isolation, hearing loss, mental illness, alcohol and smoking. 1 Although there are numerous interventions aimed at modifying lifestyle, there appear to be no evidence-based interventions aimed specifically at preventing dementia and suitable for delivery on a large scale. Responsibility for preventing dementia also falls into a grey area between public health (i.e. the responsibility of local authorities) and the NHS. A review of policies and strategies for dementia prevention in England found limited evidence for their implementation at the clinical level. 4 NHS memory services are limited to people with a diagnosis of dementia and are unable to help those with MCI beyond ‘signposting’ to other services. 1 Although early identification of people with MCI may facilitate monitoring, those who do not meet criteria for referral to memory services may remain on their general practitioner (GP)’s books without access to specialist services.
The current configuration of services leads some health professionals to question the value of identifying people with MCI. These health professionals argue that a ‘label’ of MCI may worsen anxiety or other mental health problems without offering access to effective treatments that are not otherwise available. On the other hand, prevention of dementia is a high priority for those directly affected and society as a whole.
In 2017, the National Institute for Health and Care Research (NIHR) Health and Social Care Delivery Research (HSDR) programme issued a call for research into cognitive impairment. The response to this call was limited. The HSDR programme team requested that the Sheffield HSDR Evidence Synthesis Centre review the current evidence base, taking different perspectives into account, to identify key implications for research and service delivery.
An initial scoping search of the MEDLINE database (November 2020) identified some potentially relevant papers. In particular, a consensus meeting held in Manchester in 2019 led to the publication of a clinical guideline on MCI in November 2020. 5 The authors stated that the guideline covers ‘the use of neuroimaging, fluid biomarkers, cognitive testing, follow-up and diagnostic terminology’ in MCI. 5 Although clearly important for UK practice, this guideline does not cover the full range of topics of interest to the HSDR programme. Indeed, one of the authors’ key recommendations is that the National Institute for Health and Care Excellence (NICE) should produce guidance on MCI. In the absence of such guidance, a targeted evidence review may be of value to both research commissioners and decision-makers in health and social care.
Chapter 2 Overview of methods
Patient and public involvement
The Sheffield HSDR Evidence Synthesis Centre’s strategic Public Advisory Group were involved from the outset of the review. The group commented on the review scope, including whether or not the research questions were the right ones, what they thought were the most important outcomes, if there was anything else they thought should be covered and what might be the priority areas for the public, before the protocol was finalised. A topic-specific patient and public involvement group was set up with the assistance of members of the Strategic Advisory Group. This group provided further input on their experience of services for people with MCI and the advantages and disadvantages of MCI as a diagnostic label. Near the end of the review, there was a second meeting where the patient and public involvement group commented on the review findings and were involved in writing the Plain English summary. A further meeting was held to discuss dissemination of the review findings to patients and the public. Approximately 15 members of the public were involved, including people with MCI, carers and those without direct experience of the condition.
Review questions
This report addresses the following questions:
-
What is the evidence base around the assessment and management pathway of older adults with MCI in acute hospital wards, community/primary care and residential settings? In particular –
-
How are older adults presenting with memory problems investigated to understand the underlying cause of impairment?
-
What are the advantages and disadvantages of a ‘diagnosis’ of MCI? (We will aim to address both patient and health/social care provider perspectives.)
-
What is known about the experience of health and care services from the perspective of people with memory problems and their support networks (e.g. family, friends and other carers)?
-
The report comprises two separate evidence reviews: (1) a descriptive review with narrative synthesis, focusing on diagnosis, service provision and patient experience; and (2) a critical interpretive synthesis (CIS) of evidence on the advantages and disadvantages of MCI as a diagnostic label. The first subquestion is addressed in Chapters 7 and 8, the second subquestion is introduced in Chapter 6 and is the main focus of review 2 (see Chapters 11–13) and the third subquestion is covered in Chapter 9 and review 2.
Identification of evidence
A broad literature search was developed to identify research on the assessment and management pathways of older adults with MCI. The search strategy developed by the information specialist combined thesaurus and free-text terms and relevant synonyms for the population (e.g. older adults with memory problems with or without a diagnosis of MCI), intervention (e.g. screening and assessment tools, management pathways and service modules) and uses. The search terms were then combined using Boolean operators appropriately.
The search strategy was developed on MEDLINE and then translated to the other major medical and health-related bibliographic databases. The MEDLINE search strategy is presented in Appendix 1.
The search was limited to research published in English between 2010 and 2020. The date range was chosen to reflect the introduction of the UK National Dementia Strategy in 2009. 2 Earlier publications were incorporated by including relevant literature and systematic reviews. A search filter for UK studies was applied to the search to ensure that retrieved studies were relevant to the UK context. In addition, editorials, comments and letters were excluded where database functionality allowed.
The search was conducted in January 2021 on the following databases: MEDLINE, EMBASE, PsycInfo®, Scopus, Cumulative Index to Nursing and Allied Health Literature, The Cochrane Library (i.e. Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials), Science Citation Index and Social Science Citation Index.
In addition, grey literature searches were performed to retrieve clinical guidelines, policy documents and reports related to MCI from relevant websites. A full list of the websites searched is provided in Appendix 2. Reference and citation searching of included studies and relevant existing reviews were conducted for areas where more evidence was needed.
Inclusion and exclusion criteria
Inclusion criteria
Participants
Participants were older adults (likely to be aged ≥ 60 years or ≥ 65 years) with memory problems, with or without a diagnosis of MCI, and relevant health and social care professionals, family caregivers and volunteers.
Interventions
Interventions included screening and assessment tools (including staff training), management pathways and service models for people with MCI.
Comparator
The most relevant comparator was no treatment/standard care. Quantitative studies with and without a control/comparator group were included when they met other criteria.
Outcomes
Outcomes of interest included quality of life, mental health and other patient/carer outcomes, as well as health system outcomes (e.g. measures of costs/resource use).
Study designs
Study designs that were included were quantitative research studies of any design; qualitative research involving, for example, interviews and focus groups; mixed-methods studies; service evaluations (from the UK only); UK-relevant guidelines; policy documents and grey literature; and systematic and narrative literature reviews.
Context/setting
Studies with a health and social care context/setting, including acute hospital wards, community/primary care and residential settings, were included. Although the main focus was the UK, studies from other OECD (Organisation for Economic Co-operation and Development) countries were included to address gaps in the UK evidence base.
Other criteria
Other criteria included studies published after 2010 and grey literature from the UK.
Exclusion criteria
-
Studies in which people had a formal diagnosis of dementia.
-
Lifestyle interventions intended to reduce the risk of developing dementia.
-
Editorials, commentaries, news and discussion articles, unless they provided full details of a service or pathway.
-
Books and book chapters, theses, articles in professional magazines and conference abstracts.
Study selection
Search results were downloaded to a reference management system (EndNote X9.2, Clarivate Analytics, Philadelphia, PA, USA) and duplicates removed. Unique references were imported into EPPI-Reviewer 4 (Evidence for Policy and Practice Information and Co-ordinating Centre, University of London, London, UK) systematic review software for screening and analysis. Titles/abstracts of imported references were screened against the inclusion criteria by four members of the review team, with any queries resolved by discussion among the review team. A 10% sample of excluded references were checked by one of the reviewers to ensure consistency and guard against premature exclusion. References that appeared potentially relevant were screened as full-text documents for a final decision on inclusion or exclusion, with any uncertainties resolved by discussion among the review team.
Data extraction and quality assessment
Key data were extracted and tabulated from the included studies, including study type, area of study, population, setting, study methods, findings, conclusions and key limitations. For the CIS, data extraction included positioning in argument, cited affiliations, study methods and CIS themes. Data extraction was undertaken using the coding and reporting functions of EPPI-Reviewer 4. Data extraction was performed by the four reviewers (DC, AB, AC and KS) and a 20% sample of each other’s work was checked.
Quality (risk-of-bias) assessment was undertaken for all studies that used a recognised design for which an appropriate quality assessment tool is available. Quality assessment tools used in this review included the Joanna Briggs Institute checklist for quasi-experimental studies, the CASP (Critical Appraisal Skills Programme) tool for qualitative studies, AMSTAR (A MeaSurement Tool to Assess systematic Reviews) for systematic reviews, the Swedish Agency for Health Technology Assessment tool to assess methodological limitations of qualitative evidence synthesis, and risk of bias for cohort/cross-sectional studies and diagnostic studies from the National Heart, Lung and Blood Institute (Bethesda, MD, USA) and Cochrane Collaboration, respectively. Quality assessment was performed by the four reviewers (DC, AB, KS and AC), who also checked a 20% sample of each other’s work. Assessment of the overall strength (quality and relevance) of evidence for each research question formed part of the narrative synthesis.
Synthesis of evidence
A narrative synthesis of the evidence was undertaken based on the predefined research questions and includes textual and tabular summary and critique of the included studies. Quantitative and qualitative evidence will be synthesised using methods based on the principles of CIS. 6 Briefly, CIS is a synthesis approach designed to analyse a broad range of relevant sources and use analytical outputs to develop a conceptual framework. We planned to use a variant that mobilises the literature to construct two alternative conceptual frameworks (i.e. one that assumes a pivotal role for the establishment of a definitive diagnosis of MCI and one that progresses a management pathway in the absence of a definitive diagnosis).
We have chosen a CIS methodology given its acknowledged strengths as a form of systematic review that draws on both traditions of qualitative research inquiry and systematic review methodology. A CIS is best suited to study a phenomenon that emerges over time and that constitutes a challenge to define, as is the case for MCI. In contrast to conventional systematic reviews, in which a precise question is tightly focused, CIS methodology offers the flexibility to draw from diverse relevant sources. Furthermore, CIS is not constrained to include only prespecified designs or quality of documents. Documents are selected according to relevance and their capacity to address the research question. Starting from an initial compass question relating to the assessment and management of older adults with MCI, two alternative management pathways were created and iteratively modified and defined as the synthesis progressed. In particular, we explored the extent to which assignment of a defining diagnosis or label determined the management pathway and eventual outcome. Quantitative and qualitative empirical studies was classified by the extent to which they supported each management pathway or to which they shared a common ground between the alternative pathways. The effect of contextual factors and their influence on the likelihood that individuals will progress down one or the other pathway was also explored.
Chapter 3 Review 1: definition, diagnosis and patient experience – introduction and results of literature search
Review 1 was a descriptive systematic review, incorporating evidence from primary studies and supplemented by systematic reviews. Included studies were allocated to one or more of the following groups for a narrative synthesis: conceptual studies, screening and diagnosis, services and pathways, and patient/carer experience. Of the 29 studies included in review 2 (see Chapters 11–13), 24 were included in review 1.
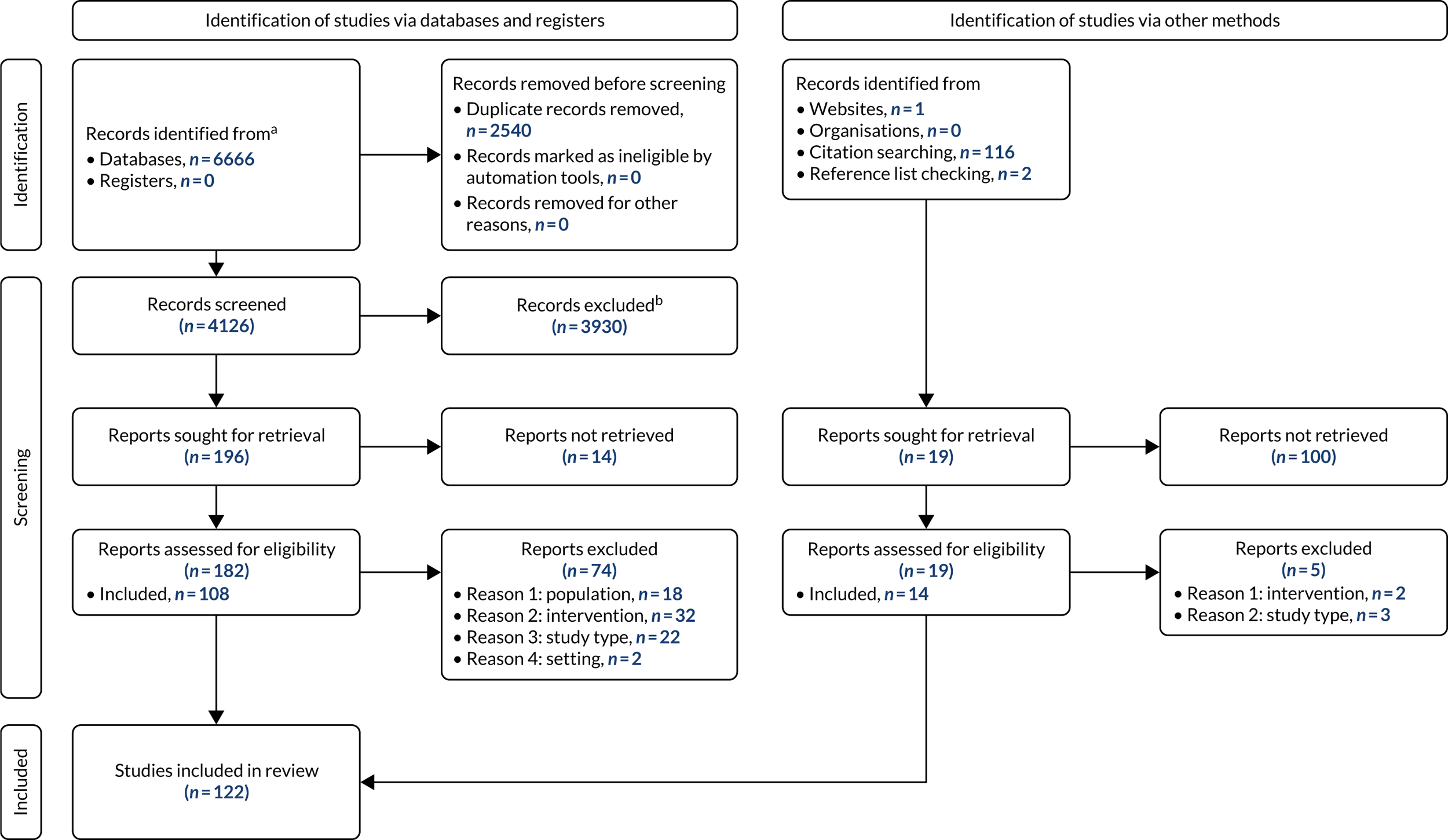
From a database of 4126 citations, we included 108 studies, with a further 14 studies identified by other methods, primarily citation searching. Figure 1 is a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) diagram illustrating the process of study selection and inclusion.
FIGURE 1.
A PRISMA flow diagram. 7 a, Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers); b, if automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
Chapter 4 Summary of included study characteristics
Table 1 summarises the study designs of papers included in review 1. The most common study types were quantitative, systematic review and narrative review, with a predominance of qualitative studies for patient/carer experience. Most quantitative studies had a cohort or cross-sectional design, but a small number of cluster-randomised trials were also included. Study participants were most commonly recruited from populations of community-living older adults or those who had sought medical help for memory problems.
| Study design | Screening/diagnosis (n) | Services/pathways (n) | Patient/carer experience (n) | Conceptual (n) |
|---|---|---|---|---|
| Quantitative | 34 | 12 | 3 | 3 |
| Qualitative | 9 | 7 | 17 | 2 |
| Mixed methods | 2 | 6 | 2 | 1 |
| Conceptual/historical | 3 | 1 | 3 | 5 |
| Systematic review | 17 | 3 | 5 | 0 |
| Narrative review | 15 | 8 | 2 | 5 |
| Commentary | 2 | 2 | 1 | 2 |
| Other | 3 | 5 | 2 | 0 |
Summary tables of study characteristics are presented as part of the narrative synthesis in Chapters 6–9.
Chapter 5 Quality (risk-of-bias) assessment
For a summary of the risk-of-bias assessment, see Appendix 3, Tables 19–23. The wide range of study designs included in the review required us to use numerous different checklists. In general, systematic reviews and qualitative studies were rated as being of reasonable quality, with limitations being most commonly related to reporting of conflicts of interest (systematic reviews) and consideration of relationships between participants and researchers (qualitative studies). The quality of the cross-sectional and cohort studies varied widely, with common issues being small samples, lack of blinded outcome assessment and adjustment for confounders. Each of the other study designs (e.g. diagnostic, qualitative evidence synthesis, cluster-randomised trials, quasi-experimental and economic evaluations) were used in just a few studies. The review also included studies, such as service evaluations and audits, that were relevant to the review question, not designed as research studies and difficult to assess for risk of bias.
Chapter 6 Conceptual studies
This review included a category of publications to offer a conceptual overview of MCI. This group of publications aimed to identify key conceptual features of MCI over time in the context of UK services. The category of publications included conceptual (non-empirical) papers or reviews and empirical papers containing conceptual elements. Publications explored diagnostic definitions and causes of MCI, the application of diagnostic concepts, the concept of MCI as it relates to services and the patient experiential conceptualisation of MCI.
Features of included papers
There were various types of included publications from the 13 publications8–20 identified. Conceptual overview papers were identified as a result of searches. Ball et al. 8 explored underlying disorders in relation to functional cognitive disorder. Forlenza et al. 9 critically reviewed existing knowledge on the conceptual limits and clinical usefulness of the diagnosis of MCI and the neuropsychological assessment (including short- and long-term prognosis). Stewart10 presented a narrative review of literature relevant to the presentation and detection of MCI in primary care. Stephan et al. 11 conducted a systematic review of the neuropathological profile of MCI, synthesising 162 clinical studies. Blackburn et al. 12 compared MCI, SCD and functional memory disorder (FMD). Brayne and Kelly13 explored the desirability of early diagnosis. The discussion paper by Swallow et al. 20 explored the process of negotiating risk in constructing classification boundaries in the memory clinic.
Other sources contained conceptual explanations for MCI as part of their objectives. One type was an evidence briefing by the British Psychological Society (Leicester, UK). 14 Such publications distil current narratives around MCI, which influence practice. The authors note that MCI is not a simple entity and the foremost narrative is the complexity of the conceptual understanding that is required. 14 There is a continued need to provide an overview of the current evidence for MCI. Evidence is framed as the support that may be needed by those for whom problems are detected before a dementia may be diagnosed.
The remainder of the studies were empirical. Swallow et al. ’s qualitative study20 examined practitioners’ accounts of the complexity associated with constructing the boundaries around MCI, Alzheimer’s disease (AD) and age in the clinic. Pierce et al. 15 presented a qualitative discourse analysis study of seven people with MCI. A service evaluation conducted by Jenkins et al. 16 consisted of an e-mail-based questionnaire, containing a vignette of an individual presenting with subjective cognitive impairment (SCI), distributed to 112 memory clinics. Stephan et al. 17 conducted a quantitative study of the suitability of forms of diagnostic tests based on criteria for MCI to predict dementia risk in the clinic. Data were from the Medical Research Council Cognitive Function and Ageing Study. 21 The study by Guzman et al. 18 consisted of a cross-sectional study of 34 participants with MCI (47% female and 53% male, with a mean age of 76.4 years) and evaluated the relationships between cognitive impairment, illness perceptions and cognitive fusion and their effects on levels of distress and quality of life. Rodda et al. 19 conducted a survey of clinicians on the Royal College of Psychiatrist’s (London, UK) Old Age Psychiatry Register about their perspectives on MCI.
The concept of mild cognitive impairment over time
Forlenza et al. 9 described the emergence of the MCI concept in the USA from the first use of the term in New York University (New York, NY, USA) (Reisburg et al. 1988 cited in Forlenza et al. 9) to the work carried out at the Mayo Clinic (Rochester, MN, USA) in the 1990s to suggest additional criteria (Petersen 1999, Petersen 2001 and Winblad 2004 cited in Forlenza et al. 9). Following this work, the US National Institute on Aging (Bethesda, MD, USA) and the Alzheimer’s Association (Chicago, IL, USA) placed emphasis on biomarkers for early diagnosis.
The study by Stephan et al. 11 was conducted prior to standardisation of the definition of MCI and reporting of pathology and, therefore, barriers existed to the creation of an integrated picture of the clinical and neuropathological profile of MCI.
Blackburn et al. 12 explored memory loss and dementia. The authors12 identified considerable confusion about the most appropriate diagnostic labels for people with memory problems at this point in time.
Issues remain in defining MCI, most prominently the conceptualisation of MCI as a stage on a pathway to dementia lacking diagnostic specificity. 8,14 MCI is described within the context of dementia, whereby an asymptomatic phase is followed by an early symptomatic phase with subjective memory complaints or MCI. 13
Definitions of mild cognitive impairment and causes of impairment
Ball et al. 8 explored underlying disorders in relation to functional cognitive disorder. Current research, such as this, has identified that some MCI cases are due to non-neurodegenerative processes and Ball et al. 8 point out the weaknesses in conceptualising MCI as a stage on a pathway to dementia lacking diagnostic specificity. In addition, Ball et al. 8 emphasise that MCI is an aetiology-neutral description and includes patients with a wide range of underlying causes. Cognitive disorder was a way of defining MCI for some time. Forlenza et al. 9 defined the criteria for the MCI concept as a ‘[s]ubjective complaint of cognitive impairment with objective cognitive impairment adjusted for age. Normal general intellectual function. Intact basic and instrumental activities of daily living’. 9
Recommendations from the British Psychological Society evidence briefing14 also identified shifting definitions of MCI. One of the central diagnostic definitions surrounds the existence of a prodromal stage of dementia. The authors14 stressed that this does not necessarily imply that everything between normal ageing and dementia (currently labelled as MCI) should be considered prodromal dementia. A key principle that emerged is the distinct pathway of MCI because it is recognised that many people labelled with MCI do not go on to develop dementia.
The same briefing paper14 provided another example of the way that a more sophisticated understanding of MCI has developed through recognition of the flaws in accepted criteria for MCI (specifically the measurement of cognitive impairment tests and the inclusion of memory impairment, which many do not experience). The authors argued that MCI should not be defined by performance below a cut-off point, but by evidence of decline from previous performance. It was suggested that subjective memory complaints are not necessarily a precursor of MCI and may reflect other conditions, such as low mood. The final areas of concern in the criteria are the possible impact of MCI symptoms on daily living and potential difficulties in defining significant impact. 14
Mild cognitive impairment has been underpinned conceptually with reference to memory loss and dementia, leading to challenges in understanding MCI at a conceptual and diagnostic level. 12 Blackburn et al. 12 pointed out that there is considerable confusion about the most appropriate diagnostic labels for people with memory problems. Despite a defined MCI label, it is widely misunderstood. Blackburn et al. 12 focused on concepts of MCI, SCD and FMD and, therefore, locating MCI within the suite of memory complaints conditions. The findings of Blackburn et al. 12 suggest that more work is required to help clinicians differentiate between non-progressive subjective memory dysfunction and the early stages of progressive memory disorders, such as AD.
The construct of SCI has been linked to MCI, although also emerging in its own right. Jenkins et al. 16 argued that SCI may be a risk factor for both MCI and dementia. Variable responses to a questionnaire containing a vignette to describe the presentation of a patient with SCI suggested a lack of a clear concept of SCI and how to manage the condition. Jenkins et al. 16 provided an insight into the relative increased clarity for MCI:
What is also clear is that several years ago the concept of MCI was the topic of similar debate to that surrounding SCI today and that MCI is now a widely recognized clinical diagnosis.
Jenkins et al. 16
The neuropathological profile of MCI was presented by Stephan et al. 11 Findings from included studies indicated that MCI is neuropathologically complex and cannot be understood within a single framework. The pathological changes identified included plaque and tangle formation, vascular pathologies, neurochemical deficits, cellular injury, inflammation, oxidative stress, mitochondrial changes, changes in genomic activity, synaptic dysfunction, disturbed protein metabolism and disrupted metabolic homeostasis. Therefore, determining causation is problematic (i.e. which factors primarily drive neurodegeneration and dementia and which are secondary features of disease progression).
The application of mild cognitive impairment diagnostic concepts
Stewart10 wrote a review paper on the challenge of real-world detection and diagnosis. The paper’s central focus is the construct of MCI and controversy surrounding the way it was defined (i.e. Stewart10 echoed the point made by the British Psychological Association14 about diagnostic cut-off points for measuring cognitive decline). Stewart10 stated that MCI is both a relatively simple and an important construct. Its importance depends on the assumption that early diagnosis and intervention are likely to be beneficial. The MCI construct is controversial because it ‘involves the imposition of categorical entities on what is essentially a continuous and mostly gradual process of decline in people who do develop dementia, not to mention incorporating the heterogeneity of cognitive changes in people who do not have underlying neurodegeneration’. 10 Stewart10 discussed the diagnostic process as likely to be based on subjective memory complaints under current definitions, rather than screening at a population level (which is unlikely to be feasible or acceptable). Yet, subjective memory complaints are heterogeneous in their aetiology, poorly predict medium-term dementia risk and are unlikely to be reported to GPs. In the process of diagnosis, the review raised the issue of distinguishing MCI from dementia and the challenges of identifying declines in functional activities of patients. The paper10 highlighted the subjectivity of the judgement in aspects such as cultural expectations for functional activity levels, individuals with increased support who may have higher function as a result and individuals with existing physical conditions that can have an impact on function. Findings10 relating to identifying MCI in clinical practice stress the importance of understanding health-seeking behaviours.
Previous concepts have focused on areas such as clinical characteristics and predictors of dementia9 and identification of dementia risk through identification of MCI. 17 Forlenza et al. ’s9 critical review of the limits of the diagnostic concept for MCI argued that the diagnostic criteria are complex, requiring sophisticated assessments:
Thus, there is a need for development of assessment strategies that are cost-effective, easy to administer and that generate results that are easy to interpret, while maintaining good sensitivity and specificity to identify MCI cases.
Forlenza et al. 9
The study by Stephan et al. 17 showed that definitions are not suitable for identifying people with MCI and at high risk of dementia in the general population. However, the definitions were able to identify people at elevated risk (possibly suitable for ‘watchful waiting’), as well as those considered to be not at risk. According to Stephan et al. ,17 identification could be achieved by using a simple test, such as the Mini Mental State Examination (MMSE), and more complex MCI criteria were not required.
Narratives questioning the desirability of the diagnosis have gained increasing traction. The desirability of early diagnosis was addressed by Brayne and Kelly. 13 Brayne and Kelly13 set the concept of early diagnosis in a UK policy context and the Prime Minister’s Challenge on Dementia. 3 Although the paper by Brayne and Kelly13 focused on dementia, it has relevancy for the exploration of the concept of early diagnosis and framed MCI in the context of dementia. Brayne and Kelly13 described a process of an asymptomatic phase followed by an early symptomatic phase with subjective memory complaints or MCI. Crucially, the authors point out that this challenge called for improved dementia diagnosis rates and is based on assumptions of benefit to individuals and those who care for them. In addition, the paper13 questions what is meant by ‘early’. Dictionary definitions include ‘in good time’, ‘before the usual time’ and ‘prematurely’. ‘In good time’ suggests a time that is appropriate for an individual, ‘before the usual time’ suggests a process that may be beneficial or harmful and ‘prematurely’ suggests the possibility of harm. 13 This concept of timely diagnosis means disclosure of the diagnosis at the ‘right time for the individual with consideration of their preferences and unique circumstances’ (Watson et al. 2019 cited in Brayne and Kelly13). This interpretation surrounds help-seeking at the point of noticing symptoms and referral to specialist secondary care assessment, such as memory clinics. Therefore, a timely diagnosis is delivered at the point people are ready for and will benefit from it. However, this has to coincide with adequate services to account for benefits costs and harms.
Mild cognitive impairment concept relating to services
In a study by Rodda et al. ,19 almost all respondents (99%) were moderately or extremely familiar with MCI. One hundred and ten (24%) respondents thought that the concept was more useful for doctors, 47 (10%) felt that it was more useful for patients and 292 (65%) rated it as the same for both. Findings from the Rodda et al. study19 indicated that psychiatrists thought that it would be helpful for patients to have a name for their symptoms. However, the study demonstrated the contentiousness of the diagnosis construction and related practices at the time among this group of professionals. For example, some respondents did not consider MCI a helpful concept and a few wrote in the free-response section that they did not consider it to be a diagnosis.
The discussion paper by Swallow et al. 20 explored the process of negotiating risk in constructing classification boundaries in the memory clinic. Swallow et al. 20 framed the process of constituting the diagnostic boundaries of disease in the clinic as interpretive and relational. In addition to diagnosis as a process to sort the ‘real from the imagined’ (Jutel 2009 cited in Swallow et al. 20), it was also a space for contestation (Bowker and Star 2000, Jutel 2009 and 2015, Jutel and Nettleton 2011, and Rosenberg 2002, 2003 and 2006 cited in Swallow et al. 20). Swallow et al. 20 pointed to the complications of the diagnostic conceptualisation that categorises pathology at earlier stages and in which there is a blurring of boundaries. According to a sociology of diagnosis perspective, the discussion highlighted the way that the grey area of the diagnosis is a liminal and ‘ “uncomfortable” space for patients and practitioners, favouring more conventional categories’. 20 The ‘functionality’ of diagnosis (Jutel 2015 and Timmermans and Buchbinder 2010 cited in Swallow et al. 20) is, therefore, called into question if underpinned by uncertainty [including potential positive aspects, such as uncertainty serving to return the act of decision-making to the clinic (Latimer 2013 and Reed et al. 2016 cited in Swallow et al. 20)]. Swallow et al. 20 also incorporated wider themes, such as ageing and differing positive or negative sociocultural constructions of ageing, and loss of self and related conditions, such as AD or MCI (Beard and Neary 2013, Latimer 2018, and Swallow and Hillman 2018 cited in Swallow et al. 20). Swallow et al. 20 argued that these constructions may exist at some level within the practices of the memory clinic.
Stewart et al. 10 pointed out that most research in this area has focused on the applicability of cognitive assessments in primary care. However, in general, the detection of MCI in primary care is low and, therefore, detection may need to be expanded. The authors10 suggested the use of routine clinical data and nurse practitioner involvement.
Experience-related aspects of the mild cognitive impairment concept
Psychosocial adjustment is a conceptual term emphasised by Guzman et al. 18 and their cross-sectional study of standardised measures for cognitive assessment, illness perceptions, cognitive fusion, depression, anxiety and quality of life. Guzman et al. 18 outlined the features of a MCI diagnosis. Receiving a MCI diagnosis can evoke a broad range of emotional responses in people diagnosed with MCI, including worry, ambivalence or relief. 22,23 MCI is a vague term and makes the person confused as to whether or not they will go on to develop dementia. Some researchers argued that a MCI diagnosis merely causes undue distress for individuals and their caregivers about what may be part of a ‘normal’ ageing process. 24,25 Among their findings,24,25 the researchers showed that illness perceptions were a stronger predictor of depression and quality of life than cognitive impairment. However, limited research has focused on individual experiences of receiving this diagnosis. Recommendations24,25 include multiple treatment targets to help people adjust and accept a diagnosis, which is a factor in secondary conditions, such as depression, and in improving quality of life.
Discourses of patients with a MCI diagnosis were explored by Pierce et al. 15 A central narrative was ‘[k]nowingly not wanting to know’15 about the possibility MCI would develop into dementia. Another MCI discourse was characterised as not knowing about MCI. In addition, Pierce et al. 15 explained a third discourse: ‘[i]n the absence of a coherent discourse [of MCI], around a diagnosis given to them by experts in a memory clinic, participants turned to a more familiar discourse to help them ascertain their positioning – that of ambivalent ageing and certainty of death’. 15 Therefore, the conclusions of the study suggest that the conceptualisation of MCI by patients should be considered by clinicians in terms of how information is presented to people about MCI and, in particular, how MCI is positioned in respect to normal ageing and dementia.
Summary
The conceptual landscape of MCI has changed significantly over time, gaining its own diagnostic label. However, conceptual features of MCI remain challenging for diagnosis and patient acceptance. A conceptual understanding of MCI as purely a pre-dementia condition persists, with a reliance on categorical cut-off points to distinguish between normal ageing and MCI. In addition, conceptualisations include both those who go on to develop dementia as well as those with a wide range of cognitive problems but no underlying neurodegenerative decline. This produces a level of conceptual complexity in a single framework that locates MCI definitions in the context of dementia, leading to understandings that incorporate an absence of diagnostic boundaries with the neuropathological complexity of the condition. In practice, this means that diagnosis is subjective and there is a reliance on assessment of function and memory. Clinicians call for a more sophisticated assessment of function relative to the individual.
Chapter 7 Screening/diagnosis
We included 82 studies in the screening/diagnosis group. This group includes strategies to identify people with ‘memory problems’ in broader population samples to distinguish those with MCI from those with related conditions [e.g. SCD and functional cognitive decline (FCD)] and to identify the underlying problem in people with the MCI label. The group overlaps with, but does not fully cover, the larger topic of diagnosis of dementia, given the specific focus of the review on MCI. Primary research studies were supplemented by evidence from reviews and clinical guidelines (Table 2). The group also includes help-seeking, which is an essential requirement for memory problems to be identified in routine practice.
| Study | Type of study | Study aims/objectives | Study sample/population | Setting | Findings: screening/diagnosis | Study limitations |
|---|---|---|---|---|---|---|
| Dunne et al.5 |
Narrative review Expert consensus guideline with narrative literature review |
To describe the scope of use of MCI as a diagnostic category, determine its utility and explore the implications of its continued use in research and clinical practice To create a clear problem statement as a framework for future national guidance on minimum standards in diagnosis and management of MCI |
Not applicable (literature review/guideline) Clinical/health service perspective |
Guideline focuses on UK clinical and research settings | Clinical benefits of accurate diagnosis may include resolution of uncertainty for patient and clinician, discharge from regular clinic visits, referral to more appropriate specialties, advance care planning, access to clinical trials, advice on current and future treatments, and counselling, support and education. Decisions about investigations should be made on an individual basis | Expert guideline with no apparent patient or carer involvement |
| Langa and Levine26 | Narrative review | To present evidence on the diagnosis, treatment and prognosis of MCI and to provide physicians with an evidence-based framework for caring for older patients with MCI and their caregivers | Review focuses on people with MCI | General review with no specific setting | The prevalence of MCI in adults aged ≥ 65 years is 10–20%. Risk increases with age and appears to be higher in men. Substantial variation in (dementia) risk estimates (from < 5% to 20% annual conversion rates). A suggested approach to diagnosis based on history, physical/neurological examination, laboratory testing and cognitive testing is presented | Narrative review with an apparent US focus |
In the context of MCI, screening refers to administration of cognitive tests, often in primary care settings, to identify people who may need specialist investigation. A secondary use of the term relates to cognitive testing of older adults as part of research studies. Screening of older adults without symptoms of cognitive decline is not recommended by the Canadian Task Force on Preventive Health Care27 or the US Preventive Services Task Force because of lack of evidence of benefit. 28,29
Help-seeking
We identified three studies30–32 of barriers to and facilitators of help-seeking for memory problems and two UK studies33,34 of interventions aimed at encouraging people with concerns to consult their GP (Table 3). A 2018 systematic review30 found that there is often a long delay between noticing symptoms and seeking help and that help-seeking is often precipitated by a ‘pivotal event’. In the absence of such an event, people may discount their symptoms as normal, reserve judgement about them or misattribute them. In a small study31 of Irish adults, facilitators of help-seeking were family, friends and peers, alongside well-informed health professionals. Barriers to seeking help were a lack of knowledge, fear, loss, stigma and inaccessible services. 31 Ethnic minority groups may encounter specific barriers to help-seeking. A qualitative study32 of South Asian people identified barriers, including not knowing what help is available, a perception of lack of time in GP consultations and a belief that ‘good families’ care for people with dementia themselves. Barriers to accessing early/timely diagnosis at the level of the health system are discussed below (see Recognition and recording in primary care).
| Study | Type of study | Study aims/objectives | Study sample/population | Setting | Findings: screening/diagnosis | Study limitations |
|---|---|---|---|---|---|---|
| Chan et al.33 | Quantitative pilot study | To determine if a leaflet campaign by the Alzheimer’s Society to raise awareness of memory problems increases the number of people presenting to their GP with memory problems |
Intervention practices combined population: 88,924 Control practices combined population: 53,863 |
Fourteen UK general practices. Seven general practices in neighbouring locality acted as a control. The intervention and control locality referred to the same specialist service | Just under 40% of people presented with memory problems had a blood test in control and intervention localities. Referral to secondary care occurred in approximately 80% of people presenting with memory problems and was more likely in the intervention group. Patients were more likely to be referred than to have investigations or be prescribed antidepressants | Authors noted that the study demonstrated the strengths and weaknesses of routinely collected data |
| Devoy and Simpson31 | Mixed methods | To identify factors that may increase intentions to seek help for an early dementia diagnosis among people experiencing memory problems |
People aged 50–69 years living in Dublin or Kildare, Ireland (n = 22 for focus groups and n = 95 for survey) Patient/public perspective |
Community groups serving older people | Content analysis revealed that participants had knowledge of the symptoms of dementia but not about available interventions. Facilitators of help-seeking were family, friends and peers, alongside well-informed health professionals. Barriers to seeking help were a lack of knowledge, fear, loss, stigma and inaccessible services. The main predictors of help-seeking were knowledge of dementia and subjective norm, accounting for 6% and 8% of the variance, respectively | Limitations to TPB; some participants considered survey too long/repetitive. Irish population and so may not generalise to UK |
| Livingston et al.34 | Quantitative | To assess whether a GP’s personal letter with an evidence-based leaflet about overcoming barriers to accessing help for memory problems increases timely dementia diagnosis and patient presentation to general practice | Patients aged ≥ 70 years without a diagnosis of dementia and living in their own homes (n = 6387 in 11 intervention practices and n = 8171 in control practices). Health service/GP perspective | Twenty-two general practices and 13 corresponding secondary care memory services in London, Hertfordshire and Essex, UK | There was no between-group difference in cognitive severity (MMSE score) at diagnosis. GP consultations with patients with suspected memory disorders increased in the intervention group compared with the control group (odds ratio 1.41, 95% confidence interval 1.28 to 1.54). There was no between-group difference in the proportion of patients referred to memory clinics | It is not known if the additional patients presenting to GPs had objective as well as subjective memory problems and, therefore, should have been referred. In addition, the intervention aimed to empower patients but did not do anything to change GP practice |
| Mukadam et al.32 | Qualitative | To determine barriers to timely help-seeking for dementia among people from South Asian backgrounds and what the features of an intervention to overcome them would be | Purposively recruited 53 English- or Bengali-speaking South Asian adults without a known diagnosis of dementia through community centres and snowballing. (Number of adults with dementia or MCI unknown) | Community settings in and around Greater London | Health-care system. Not knowing what help is available. Perception that GPs do not have enough time in consultations. Perception of good families look after people with dementia themselves | Study is not necessarily representative of that whole community. Reliability of a hypothetical case (i.e. opinions could change in reality) |
| Perry-Young et al.30 | Systematic review | The aim of this review was to systematically search, critically appraise and present a synthesis of the literature on the social dynamics of help-seeking for dementia | A total of 249 participants were represented, including 32 people with dementia and 217 carers (171 spouses, 39 children, sons- or daughters-in-law and one sibling). (Searches include MCI, but this number of participants is not given) | Three of the studies were conducted in Canada, two in the USA, two in the UK, one in the Netherlands and one in Sweden | Delays due to services (e.g. immigrant caregivers faced particular difficulties in accessing resources). Participants experienced active reflection and seeking of further evidence about memory problems (leads to the redefinition of the situation). Person with dementia’s denial and refusal to seek help causes a moral dilemma for the significant other, who may feel that seeking medical help or consulting lay networks would be a betrayal | Limitations of representativeness of third-order interpretations. Findings may not account for all complexities involved in the interpersonal aspects of decision-making. Recollection and hindsight may have affected data accuracy. Carer reflection may have been self-censored to avoid hurting the feelings of the person with dementia/MCI |
Chan et al. 33 and Livingston et al. 34 reported on interventions in the English NHS to encourage people with memory concerns to consult their GP. Both studies33,34 involved distribution of information to patients at the level of the general practice. Chan et al. 33 found that recording and management of memory problems improved during the period of the study, but there was a greater improvement in a control area where no additional information was distributed. In the study by Livingston et al. ,34 consultations about memory problems increased in intervention practices compared with control practices. However, there was no increase in diagnoses or referrals to memory clinics. This limited evidence suggests that provision of information to patients may not be sufficient to overcome barriers to help-seeking and obtaining help for memory problems.
Recognition and recording in primary care
We included six reviews10,35–39 and 12 primary studies40–51 in this section.
As noted above, identification and investigation of memory problems generally begins when patients or family members are concerned about a person’s symptoms, leading to a consultation with the patient’s GP. The objective of the investigations is often presented as ‘early diagnosis’ of the underlying cause of the memory problems (i.e. dementia or other). 10 Brooker et al. 35 have developed the concept of a ‘timely diagnosis’ (of dementia), stating that ‘citizens should have access to accurate diagnosis at a time in the disease process when it can be of most benefit to them’. 35 The concept of timely diagnosis is discussed further in review 2 (see Chapter 11).
Two included studies40,41 identified barriers to early diagnosis in general or for disadvantaged groups. A survey of primary and secondary care physicians (n = 1365) found that barriers included patients seeing cognitive decline as a normal part of ageing and not disclosing symptoms, long waiting lists, and a lack of treatment options and definitive biomarker tests. 40 Although a relatively large sample, this survey reflected the perceptions of a self-selected group of physicians only. A mixed-methods study of homeless older people living in hostels reported that the memory assessment service (MAS) was difficult to access and not patient centred. 41
People who report memory concerns to their GP are likely to be assessed using ‘pencil and paper’ cognitive tests in the first instance (see Cognitive tests). Six included studies36,42–46 assessed recognition and recording of memory problems by GPs in the UK and the Netherlands. A meta-analysis from 201136 found that GPs have considerable difficulty identifying people with MCI or mild dementia using clinical judgement, and diagnoses are poorly recorded in medical records. 36 For MCI, GPs recognised 44.7% of cases and the diagnostic label was recorded for only 10.9%. This review was published in 2011 and so may not reflect current practice. 36
Given the limitations of clinical judgement, efforts have been made to promote the use of cognitive screening tests in primary care. In the UK, these include guidance from NICE and the Social Care Institute for Excellence and the National Dementia Strategy, published in 2006 and 2009, respectively. 2,42 A comparison of GP referrals to memory clinics before and after the launch of this guidance found that the number of referrals increased, but the rate of dementia diagnoses decreased and the use of cognitive screening tools did not change. 42 This suggests an absence of evidence for the effects of the guidance in promoting screening, at least in the short term. As an alternative or complement to screening, Olazarán et al. 43 investigated the role of data routinely collected by GPs in identifying MCI and predicting its course (i.e. progression to dementia or reversion to normal cognition). For example, old age, source of information about symptoms (informant or primary care physician), short duration and low education were associated with MCI or dementia at baseline.
The implications of failing to diagnose or record cases of MCI or dementia in primary care have been investigated in more recent research (three included studies44–46). A recent UK study,44 which used linked records, found that 34% of participants with known dementia had a primary care diagnosis in 2008–11 and 44% had a primary care diagnosis in 2011–13. In both periods, a further 21% of participants had a record of a concern or a referral, but no diagnosis. There was a lack of relationship between severity of non-memory symptoms and diagnosis, suggesting possible low awareness of such symptoms. In a study in the Netherlands,45 GPs showed variable awareness of the presence of cognitive impairment in their older patients (i.e. those aged ≥ 65 years) without a firm diagnosis of dementia. Lack of awareness of a problem was highest for the older age groups. Training GPs to diagnose MCI and dementia, followed by case finding, resulted in a non-significant increase in new MCI diagnoses when compared with control practices. 46
Although the common model in the UK is for people with memory problems to be identified in primary care and referred to a memory clinic for more detailed assessment, primary care-led services are available in some areas. Three included studies47–49 focused on screening and diagnosis, and the topic of different service models is discussed in more detail below (see Chapter 8). An evaluation of primary care-led services in Bristol found that, in practice, GPs rarely made independent diagnoses of dementia. 48 A need for specialist support with diagnosis was also identified in a study aimed at developing a multidisciplinary memory clinic in south-western Sydney, NSW, Australia. 49 Eye clinics have been suggested as an alternative setting for screening,47 but this approach does not appear to have been tested.
Finally, two scoping reviews37,38 have examined the potential use of telehealth technologies to support diagnosis of memory problems, especially in remote areas, as well as highlighting limitations of the available evidence. Two overlapping articles50,51 by the same author reviewed the role of mobile applications, but the review focused on features of the applications rather than evidence of their accuracy or value for diagnosis. A review39 of the international literature, included for completeness, provided few new data, but emphasised the lack of evidence from low- and middle-income countries, especially the former.
Screening in hospital settings
We identified only one study52 of screening for memory problems in a hospital setting. A Commissioning for Quality and Innovation payment led to improvements in practice, as demonstrated by audit of case notes in three wards at a large university teaching hospital in England. This study53 involved hospital inpatients aged > 75 years who were admitted as emergencies. The study was subsequently withdrawn.
Diagnostic terminology
Conceptual studies of MCI are summarised in Chapter 6 and the advantages and disadvantages of MCI as a ‘diagnostic label’ are the focus of review 2 (i.e. the CIS). We found evidence from two included studies (surveys)19,54 that indicated that clinicians regard MCI as a valuable diagnostic label, suggesting that any change to the labelling of patients may be difficult to achieve. A survey of UK psychiatrists (n = 453) found that only 4.4% of clinicians did not view MCI as a useful concept, although they showed less uniformity in their diagnostic practices. 19 The authors reported that psychiatrists saw MCI as a transitional stage between normal ageing and dementia. A more recent survey of members of the European Academy of Neurology (Vienna, Austria) and the European Alzheimer’s Disease Consortium (EADC) (Mannheim, Germany) (n = 102) reported that 92% of respondents used MCI as a diagnostic label in clinical practice. 54 A majority (68%) of respondents also used the labels prodromal AD or MCI due to AD. Over 70% of respondents thought that these labels had additional value compared with a label of MCI and influenced decisions about treatment, as well as communication with patients.
Patient/carer experience of diagnosis
This section deals specifically with experience of the process of diagnosis and labelling of memory problems. The broader topic of patient and carer experience of services is covered below (see Chapter 9), although some overlap is inevitable. This section is based on evidence from four reviews22,55–57 and six primary (mainly qualitative) studies. 1,15,24,58–60
An early scoping review55 of consent for the investigation and diagnosis of memory problems noted that early discovery of AD biomarkers in research or clinical settings is problematic because it requires patients to consent to disclosure of findings that indicate an uncertain risk of developing AD in the future. The authors of the review55 argue that expectation of future decline may lead to stigma, isolation and discrimination, but offer no solutions beyond the need for more nuanced analysis of the ethics issues involved. Closely linked to consent is the question of how patients and carers understand and describe the concept of MCI. Two studies15,58 specifically addressed this question. Roberts and Clare58 used a qualitative methodology (interpretive phenomenological analysis) to analyse transcripts of interviews with 25 people with a clinical diagnosis of MCI who had been informed of their diagnosis. Four higher-order themes were identified, but participants did not use the term MCI, suggesting that the term was not meaningful for them, although they did want a clear explanation for their memory problems. Pierce et al. 15 applied ‘discourse analysis’ to interviews with seven people diagnosed with MCI. One of the discourses identified was ‘not knowing’ about MCI. This was accompanied by ‘knowing about aging and death’ and ‘not wanting to know about dementia’. These two complementary studies set the scene for difficulties in patient experience of diagnosis and labelling.
Three included systematic reviews22,56,57 cover experience of the diagnostic pathway from diagnosis of MCI22 to investigation in memory clinics56 and diagnosis of dementia. 57 Dean and Wilcock22 noted a lack of studies on the effect of disclosing a diagnosis of MCI on patients and carers. Patients and carers receiving a diagnosis of MCI produced a range of negative and positive emotional responses, but there were no studies of experience of the diagnostic process. The review by Robinson et al. 56 included experiences of the diagnosis of both MCI and dementia, with similar findings to those of Dean and Wilcock. 22 Specifically, for MCI, a diagnosis could lead to feelings of exclusion, but could also reinforce a shared identity with others. Participants wished to be proactive in their response to a MCI diagnosis. 56 Bunn et al. 57 identified more concrete information on experience of the diagnostic process. Bunn et al. 57 reported that patients and carers perceived that, in some cases, doctors were slow to recognise symptoms or reluctant to give a diagnosis, and that even when people were referred to memory services the process could be slow, with long periods of waiting. 57
The four primary qualitative studies1,24,59,60 (Table 4) provided more detailed and richer data than those that were available from the reviews. The topic is discussed in more detail below (see Chapter 9) because the studies also include patient experience of services. Overall, the studies identified substantial difficulties with the diagnostic/labelling process from the viewpoint of patients and carers. These are well summarised by the titles of the studies, which refer to ‘making sense of nonsense’,24 ‘falling through the cracks’1 and ‘negotiating a labyrinth’. 60
| Study | Type of study | Study aims/objectives | Study sample/population | Setting | Findings: screening/diagnosis | Study limitations |
|---|---|---|---|---|---|---|
| Beard and Neary24 | Qualitative | The purpose of the study (a subset analysis) was to examine the specific experiences of memory loss for individuals diagnosed with MCI | All participants had sought cognitive evaluation following memory problems and had been given a diagnosis of amnestic MCI within the previous 3 years. All were community dwelling | Study participants were recruited from a research registry at an AD centre in a large Midwestern US city | MCI is undergoing a medicalisation process and narrative accounts of the condition do not support this (despite advances in diagnostic criteria). Authors suggest that the MCI label is used inconsistently by clinicians and researchers. Understanding of MCI following diagnosis: location on a continuum of age-associated cognitive challenges causes confusion | None provided |
| Manthorpe et al.59 | Qualitative | To increase understanding of the experiences of people developing dementia, and of their carers, to inform practice and decision-making |
Participants had early dementia or MCI (and carers) Fifty-three participants in total (27 individuals with memory problems and 26 carers; 20 were matched pairs) |
Memory clinics situated in London (n = 1), the north-west of England (n = 1) and the north-east of England (n = 2) | Few participants experienced the process of memory assessment as patient centred. Where assessment processes were lengthy and drawn out, participants experienced considerable uncertainty. Many participants experienced tests and assessments as distressing, sometimes in settings that were perceived as alarming or potentially stigmatising by association. Information provision and communication were variable and practitioners were not always thought to help people to make sense of their experiences | Interpretative nature of qualitative research |
| Poppe et al.1 | Qualitative | To investigate how services respond to people with memory concerns and how a future effective and inclusive dementia prevention intervention might be structured | Eighteen people aged ≥ 60 years with subjective or objective memory problems, six family members, 10 health and social care professionals and 11 third-sector workers. Mixed perspective | NHS and third-sector organisations supporting older people | Diagnosis of MCI seen as entering a transitional state between health and dementia. Patients perceived that MCI was a medical problem, but responsibility for preventing dementia and seeking help if symptoms worsened was placed on them | Diverse sample of health professionals but authors note that the patient sample was primarily people who had recently sought help for memory problems and so may not be representative |
| Samsi et al.60 | Qualitative | This study explores the experience of the assessment and diagnostic pathway for people with cognitive impairment and their family carers |
Twenty-seven people with cognitive impairment and 26 carers (20 dyads). Most participants with cognitive impairment were recruited and interviewed once when first referred to the memory service. Purposeful sampling and a variable sample matrix applied Inclusion criteria were people who: |
London (one site), north-west England (one site) and north-east England (two sites). In site 2, assessments were conducted at home and diagnosis communicated in the memory clinic. However, by the end of the study, staff in site 2 were conducting all assessments in the memory clinic |
(1) Initial service encounters: primary care seen as gateway (2) Assessment processes (2a) Confusing referral process. Lack of clarity on when and who referral was about (2b) Entering the labyrinth: patient confusion, tests included ‘X-rays of the head’, general scans, MRI, electrocardiography and blood and cholesterol tests (2c) Waiting times. Time from the first consultation in primary care to the point of diagnosis ranged from 3 to 9 months. Diagnosis communicated in a way patients thought it enhanced shock (3b) Lack of information (3c) Relationship with practitioners. Everyone who had been assessed and diagnosed in their own homes reported a positive experience (4a) Memory retraining. One memory service held ‘classes’ on practical strategies for managing memory problems. Independence was a key priority (4b) Planning. Advice welcomed (4c) Dashed expectations. Long waiting times also had the added disadvantage of raising expectations, which were then dashed at diagnosis |
Generalisability of context: community-dwelling older people excluded those living in care homes. The study excluded people with severe communication difficulties and those unable to speak English. Finally, there was an absence of professional perspectives |
Cognitive tests
Cognitive tests are the main tools available to GPs and other primary care clinicians to supplement clinical judgement in assessing people with memory problems. There is an extensive literature on the diagnostic accuracy of such tests, but this review focused on the role of cognitive tests in aiding decisions about further investigations and referral or treatment pathways.
Evidence from systematic reviews indicate that a number of comprehensive screening tests, such as the Addenbrooke’s Cognitive Examination – Revised, the Cambridge Cognitive Examination, the Montreal Cognitive Assessment (MoCA) and the Consortium to Establish a Registry for Alzheimer’s Disease, have > 80% sensitivity for distinguishing people with MCI from healthy volunteers. 61 However, the time and expertise required to administer some tests may limit access to them in primary care settings. 5 An international survey of physicians (n = 1365, 63% specialists) found that most reported using cognitive tests for early detection of MCI or dementia, with the MMSE being the most commonly used tool. 62
We included two systematic reviews63,64 and three primary studies65–67 of specific tests or types of test (Table 5). A 2018 systematic review63 of automated tests included 16 studies of 11 tools, but none was suitable for monitoring disease progression or response to treatment. A systematic review64 of the Ascertain Dementia 8 questionnaire concluded that that this was a suitable tool for use in busy primary care settings because, on average, it took < 3 minutes to administer.
| Study | Type of study | Study aims/objectives | Study sample/population | Setting | Findings: screening/diagnosis | Study limitations |
|---|---|---|---|---|---|---|
| Aslam et al.63 | Systematic review | To determine whether or not automated computerised tests accurately identify patients with progressive cognitive impairment and, if so, to investigate their role in monitoring disease progression and/or response to treatment | People with MCI or early dementia | Various countries and settings (e.g. primary care, memory clinic) | Sixteen studies assessing 11 diagnostic tools were included. No studies were eligible for inclusion in the review of tools for monitoring progressive disease and response to treatment | The wide range of tests assessed and non-standardised reporting of diagnostic accuracy outcomes meant that statistical analysis was not possible |
| Chen et al.64 | Systematic review | To assess the diagnostic accuracy of the AD8 questionnaire for cognitive impairment | Population appears to be people with cognitive impairment (i.e. MCI or dementia). Seven studies with 3728 participants were included | Primary care (i.e. community, clinic or hospital) | Studies were classified into two subgroups according to the severity of cognitive impairment (MCI/dementia vs. normal cognition, and dementia vs. non-dementia). The overall sensitivity across the subgroups MCI/dementia vs. normal cognition, and dementia vs. non-dementia, respectively (0.72, 0.91), was superior to specificity (0.67, 0.78). The pooled negative likelihood ratio (0.17, 0.13) was better than the positive likelihood ratio (2.52, 3.94). The areas under the summary receiver operating characteristic curve were 0.83 and 0.92, respectively. Meta-regression indicated that location (community vs. non-community) may be a source of heterogeneity. The average administration time was < 3 minutes | Only seven studies included and three did not include MCI. There were diverse populations and settings |
| Ehrensperger et al.65 | Quantitative | To describe the development and test the feasibility and validity of a newly developed case-finding tool (BrainCheck) for cognitive decline |
Feasibility study: 52 GPs rated the feasibility and acceptance of the patient-directed tool Validation study: an independent group of 288 memory clinic patients with diagnoses of MCI (n = 80), probable AD (n = 185) or major depression (n = 23) and 126 demographically matched cognitively healthy controls Medical/health service perspective |
Memory clinics in Switzerland |
Feasibility study: GPs rated the patient-directed tool as highly feasible and acceptable Validation study: a classification and regression tree analysis generated an algorithm to categorise patient-directed data, which resulted in a correct classification rate of 81.2% (sensitivity = 83.0%, specificity = 79.4%). The correct classification rate of the combined patient- and informant-directed instruments (BrainCheck) was 89.4% (sensitivity = 97.4%, specificity = 81.6%) |
Memory clinic patients may not be representative of those seen in primary care |
| Hancock and Larner66 | Quantitative | To investigate the diagnostic utility of the TYM as an independent test to differentiate patients with and without dementia in memory clinics |
Consecutive patients (n = 224; 58% male, 35% meeting clinical diagnostic criteria for dementia) attending two memory clinics in the UK Clinical/health service perspective |
A memory clinic based in a psychiatric hospital and a cognitive function clinic based in a regional neuroscience centre | TYM was easy to use and acceptable to patients. Downwards adjustment of the TYM test cut-off point to ≤ 30/50 (vs. ≤ 42/50, which was used in the index study) was necessary to maximise test accuracy and specificity. Using this revised cut-off point, TYM showed comparable diagnostic utility (sensitivity = 0.73, specificity = 0.88, positive predictive value = 0.77, negative predictive value = 0.86, area under receiver operating characteristic curve = 0.89) to the MMSE and the ACE-R for the differentiation of dementia from non-dementia | Further work is needed to assess test performance in other settings and effect of factors like age and education |
| O’Malley et al.67 | Quantitative | To evaluate a fully automated system (CognoSpeak), which enables risk stratification at the primary–secondary care interface and ongoing monitoring of patients with memory concerns | Fifteen participants in each of four groups: AD, MCI, FCD and healthy controls. Groups were 40–67% male, with a mean age ranging from 55 to 68 years. Clinician/health service perspective | Memory clinic and community (i.e. controls) in Sheffield, UK | CognoSpeak distinguished between participants in the AD or MCI groups and those in the FCD or healthy control groups with a sensitivity of 86.7%. Patients with MCI were identified with a sensitivity of 80% | Initial evaluation with a relatively small sample |
Tests evaluated in single studies were ‘BrainCheck’, TYM (Test your Memory) and ‘CognoSpeak’ (a fully automated screening tool). Ehrensperger et al. 65 developed and evaluated ‘BrainCheck’, which was a brief screening instrument for use in primary care and combined different sources of data. ‘BrainCheck’ was rated highly feasible and acceptable by GPs and correctly classified 89.4% of cases (including MCI, probable AD, depression and healthy controls). The TYM test, which was designed to be self-administered under medical supervision, was evaluated in a memory clinic population. 66 TYM was considered easy to use and acceptable to patients (n = 224, 35% meeting criteria for dementia). Using a revised cut-off point, the TYM showed comparable diagnostic utility to the MMSE and the Addenbrooke’s Cognitive Examination – Revised for the differentiation of people with and without dementia. The authors66 suggested that self-administered tests, such as TYM, may be particularly useful where clinicians’ time is limited. In a pilot study involving 15 people with MCI, people with other conditions and healthy controls, the automated system achieved levels of accuracy comparable to those of current manually administered screening tools. 67 This suggests potential to improve the efficiency of screening processes in the future.
An important point about cognitive tests is that the prevalence of the condition of interest (e.g. MCI or dementia) depends on the test used and the selected cut-off point. In an early study involving people aged 70–90 years without dementia (n = 981), Kochan et al. 68 found that the prevalence of MCI ranged from 4% to 70%, depending on the criteria used. Klekociuk et al. 69 noted that existing MCI diagnostic criteria resulted in an unacceptably high rate of false-positive diagnoses of MCI. The authors69 reported that a combination of measures of complex sustained attention, semantic memory, working memory, episodic memory and selective attention correctly classified the outcome in > 80% of cases. The rate of false-positive diagnoses (5.93%) was lower than that reported in previously published MCI studies. The issue of ‘false-positive’ labelling of MCI (i.e. reversion to normal cognition) is discussed below. In practice, sophisticated combinations of tests, such as those used by Klekociuk et al. ,69 are unlikely to be available in normal clinical practice.
NHS London Clinical Networks guidance recommends the use of a validated brief screening test, such as MoCA, for the detection of MCI,70 but this appears to be in the context of a memory service assessment rather than in primary care.
Two papers by Sabbagh et al. 71,72 focus on early detection of MCI in different settings. The review,71 which was focused on primary care, identifies barriers to effective screening in primary care related to time constraints and test validation. Tests delivered in ≤ 10 minutes are unlikely to fully evaluate all dimensions of cognition. In addition, tests developed and tested on highly homogeneous and well-educated populations may perform less well in routine practice with more diverse populations. Factors such as level of education and proficiency in English may affect test performance, potentially reducing test scores and resulting in people being wrongly labelled with MCI.
A further paper72 by the same group of authors deals with early detection of MCI at home. The time constraints of primary care are less of an issue in this setting and a range of digital technologies are available as supplements or alternatives to conventional ‘pencil and paper’ tests. These technologies are less well validated than tests in clinical settings and other barriers include costs and concerns about reliability of the technology. However, the authors conclude that ‘passive’ technologies that work by monitoring user behaviour could ultimately provide a low-effort, easy-to-use solution for widespread cognitive performance monitoring. 72
We identified one economic evaluation73 of cognitive testing for MCI and dementia in primary care. The authors73 examined three cognitive tests and concluded that any of them could be considered cost-effective compared with unassisted clinical judgement by GPs. The most cost-effective option in the base case was the GP assessment of cognition. It should be noted that benefits from testing were due to early access to medication for dementia.
In summary, the time and expertise needed to administer cognitive tests is variable. Although much research has focused on diagnostic accuracy, quick tests suitable for use in primary care may effectively identify people who need further investigation for cognitive impairment.
Imaging
We included two narrative reviews and a systematic review, giving a broad overview of imaging (scanning) in the investigation of MCI and dementia, together with a study of service variation and a cost study (which also included biomarkers). Two included studies (one a systematic review) reported on the effects of amyloid imaging on diagnosis and treatment, whereas a further systematic review focused on the impact of disclosing amyloid imaging results to people without dementia. The Manchester consensus guidance5 also covers imaging and is more recent than the general reviews.
In their 2013 review, Burhan et al. 74 focused mainly on functional magnetic resonance imaging (MRI), magnetic resonance spectroscopy and diffusion tensor imaging. The authors74 concluded that, because of the limitations of current evidence, these technologies were not recommended for clinical investigation. In their 2012 review, Sullivan et al. 75 reached similar conclusions, noting that neuroimaging provided little additional benefit over cognitive testing in predicting progression from MCI to dementia. Sullivan et al. 75 did not recommend routine scanning in all patients with MCI or suspected dementia, stating that health professionals should use their clinical judgement. A 2018 systematic review76 evaluated the potential of machine learning combined with neuroimaging for the diagnosis of AD or MCI. The review76 included 111 studies and concluded that machine learning was most accurate for distinguishing people with AD from healthy controls, but poor for distinguishing MCI from normal cognition. Overall, machine learning was not considered sufficiently developed for use in routine practice.
Evidence from UK services suggests considerable variation in the use of imaging in the investigation of cognitive impairment. The 2019 London memory service audit77 reported that the percentage of patients deemed not to require a scan for dementia diagnosis varied from 6% to 46%. Of those patients who did have a scan, the percentage who received computerised tomography (CT) (rather than MRI) varied from 2% to 58%. The Manchester consensus guidance5 noted that clinicians who request neuroimaging may not have access to the original images and, instead, have to rely on written reports. CT may be used as an alternative to MRI because the latter is more expensive or not available (e.g. because of lack of access to equipment or the service is not commissioned locally).
Availability of positron emission tomography (PET) in the UK is also patchy. The Manchester consensus guidance5 noted that there were only 62 PET scanners in the country at the time of writing, with most being in university teaching hospitals or research centres. There is increasing evidence for the value of PET in the diagnosis and management of MCI and suspected dementia. In a cohort study78 of memory clinic patients [n = 507 patients, 114 (23%) with MCI], amyloid PET results were positive for 242 (48%) patients. The suspected aetiology changed for 125 (25%) patients after undergoing amyloid PET. The mean diagnostic confidence increased from 80% (standard deviation 13) to 89% (standard deviation 13). In 123 (24%) patients, there was a change in patient treatment after PET, mostly related to additional investigations and therapy. Fantoni et al. 79 performed a systematic review and combined data from seven studies. The authors79 concluded that amyloid PET contributed to diagnostic revision in almost one-third of cases and demonstrated value in increasing diagnostic confidence and refining management plans.
Disclosure of amyloid PET scanning results is controversial in view of the uncertain predictive value of the result for individuals and the lack of disease-modifying treatments for early dementia. A systematic review80 of empirical evidence and theoretical arguments for and against disclosure identified 51 different arguments. The authors80 concluded that widespread disclosure of results to people without dementia requires further research on the impact of disclosure, as well as the predictive value of the test. Communication materials and strategies to support disclosure should be developed and evaluated.
The importance of PET and biomarkers (see below) is expected to increase with the availability of disease-modifying therapies for dementia. Intensive investigation will be required to identify those patients who could benefit from such treatments (e.g. those with MCI) and evidence of underlying damage suggestive of early (or prodromal) dementia. Wittenberg et al. 81 attempted to estimate the potential future costs of expanding the number of PET and cerebrospinal fluid (CSF) tests in the UK. Based on a focused literature review and consultation with experts, annual costs of 100,000 extra amyloid PET scans and 100,000 extra CSF tests were estimated at £113M and £48M, respectively. These costs are likely to be higher in the first year because of the need for additional staff training. However, as noted by the authors,81 these costs are likely to be small compared with the acquisition costs of disease-modifying drugs and the impacts of inaccurate diagnoses. Ongoing economic evaluation will be needed to clarify the costs and benefits.
Biomarkers
Blood and CSF biomarkers are increasingly important as alternatives to imaging in investigating the underlying cause of cognitive impairment. Biomarkers are particularly important in investigating MCI when the underlying cause of the impairment is thought to be AD. 5
The review included four publications82–85 focused on the use of biomarkers (with the extensive clinical and diagnostic accuracy literature falling outside our scope). In line with the Manchester consensus guidance,5 a 2017 expert review82 recommends biomarker testing in people with MCI or dementia when there is diagnostic uncertainty and the result will affect diagnosis or treatment. A 2015 survey83 of EADC members revealed that the participating memory clinics were using biomarker testing for clinical as well as research purposes. Survey participants were specialist centres regarded as early adopters of new technologies and so the results may not be representative of routine practice. In addition, a further limitation is that only four of eight eligible centres in the UK participated in the study. 83
In 2020, the EADC published a survey on biomarker counselling, disclosure of diagnosis and follow-up in patients with MCI,84 and guidance on the same topic (jointly with the European Academy of Neurology). 85 The survey involved 34 centres across 20 countries. The majority of respondents had access to biomarker tests and found them useful. Arrangements for pre-test counselling and advice and referral after testing varied between centres. The study authors identified a need to improve counselling and training for clinicians to improve their communication skills. As with the previous EADC survey, this research involved specialist tertiary centres (only one from the UK) and so the findings may not be applicable to routine practice. The guidance85 emphasises the need to involve dementia specialists in counselling before biomarker testing and in disclosure of the findings. Follow-up is recommended for patients with MCI and should include advice on reducing dementia risk and, if applicable, treatment of specific underlying causes. The authors85 note that advice on advance directives may also be relevant.
Other tests
We included three studies86–88 of other tests that have been used to support investigation of the underlying cause in people with MCI. Alongside the cognitive impact of MCI, effects on gait and balance have been extensively evaluated. A 2017 systematic review86 (with 14 included studies) reported that MCI affects specific aspects of gait, particularly when patients are challenged to complete a cognitive task at the same time (i.e. dual-task conditions). The authors86 concluded that gait assessment with an additional cognitive task is useful for diagnosis and outcome analysis in the population of people being investigated for possible MCI. Interventions targeting specific components of gait could potentially slow progression to dementia.
The other two studies87,88 looked at tests intended for use in memory clinic populations. A clinical decision support tool (PredictND) was evaluated in 747 patients with MCI, FCD or dementia. 87 Compared with clinical assessment alone, the aetiological diagnosis changed in 13% of patients when the decision support tool was also used. Diagnostic accuracy (using follow-up diagnosis as the reference standard) did not change, but confidence in the diagnosis, measured on a visual analogue scale, increased by three percentage points. The authors87 concluded that clinical decision support tools could help clinicians in the differential diagnosis of dementia. Elsey et al. 88 explored the use of conversation analysis to distinguish people with FMDs from those with memory problems caused by dementia. In a small study (n = 30, including four patients with amnestic MCI), patients with functional disorders responded differently than those with dementia or MCI to questions about memory problems. The authors88 concluded that conversational profiles have the potential to assist with screening and referral in primary care and diagnosis in secondary care.
In summary, tests such as gait and conversation analysis may have the potential to aid investigation of memory problems, but the relative lack of studies suggests that they may have been supplanted by the rapid development of imaging and biomarker technologies.
Differential diagnosis
This section covers investigations to distinguish between MCI and two related conditions (i.e. SCD and FCD). SCD does not have a universally accepted definition, but can be distinguished from MCI on the basis that perceived memory problems or cognitive decline are not supported by the results of cognitive tests. 1 FCD is defined as persistent subjective cognitive difficulties that are not consistent with a recognised disease process and not supported by objective measures of cognitive functioning. 89 An additional feature is described as ‘internal inconsistency’ (i.e. an ability to perform a task well at some times but not others). 8
Two included studies8,89 focused on differential diagnosis of FCD and MCI. A recent expert review8 on FCD noted that early diagnosis of FCD can provide reassurance to patients compared with a diagnosis of MCI. A comparative study89 of 21 people diagnosed with FCD, 17 people diagnosed with MCI and 25 healthy controls found that both groups were impaired relative to controls, with the main difference being that the FCD group were younger. The authors89 concluded that clinicians need to be aware that FCD and MCI symptoms overlap and that the diagnosis can change over time. The potential for conversation analysis to identify people with FCD was noted above (see Other tests).
We included four studies90–93 on differential diagnosis of MCI and SCD. A cross-sectional survey90 of people aged ≥ 65 years in south London (n = 126) reported SCD in two-thirds of participants, and the SCD was described as significant in 31%. However, only one participant had sought help from their GP. This is consistent with studies of help-seeking reported above (see Help-seeking). A 2015 narrative review91 characterised SCD as a form of early cognitive decline that cannot be detected by current objective tests and, hence, a risk factor for progression to MCI and dementia. A small observational case series92 recruited 62 patients with SCD who were followed for a mean of 44 months. At the time of follow-up, 24% of patients had developed dementia or amnestic MCI, with the main factors predicting progression being older age at onset of symptoms and first assessment.
Some definitions of MCI include subjective memory complaints as a requirement. Yates et al. 93 investigated the presence of SCD and its relationship with mood in people with MCI. In a 2-year follow-up study, a clear association was found between SCD and mood both at a single time point and over time. Mood problems (e.g. depression or anxiety) were more closely related to the presence of SCD than that of objective cognitive impairment. The authors93 concluded that SCD may be a function of anxiety and depression rather than objectively detectable cognitive decline.
Evidence,8,89–93 varying in quantity and quality, suggests that distinguishing MCI from FCD and SCD is highly complex. FCD may be a separate entity and early diagnosis may be reassuring for the patient that they do not have dementia. SCD may, in at least some cases, be a precursor to progression to objective MCI and dementia.
Alternative causes of mild cognitive impairment
Expert reviews and clinical guidelines emphasise the importance of considering alternative (non-neurodegenerative) causes of MCI, but we found a lack of studies evaluating such investigations. An expert review26 of diagnosis and management recommended that clinicians should consider depression, polypharmacy and uncontrolled cardiovascular risk factors. Similarly, the Manchester consensus guidance5 identifies physical and psychiatric illness as alternative causes of cognitive symptoms.
Reversion to ‘normal’ status
Four studies43,69,94,95 included in the review investigated factors associated with reversion from MCI to normal cognition (Table 6). Awareness of this possibility is important for the investigation, counselling and management of people with MCI. Interpretation of the studies is complicated by different definitions of MCI and normal cognition. Overall, reversion to normal cognition was associated with younger age and a higher level of education. One study94 reported that people who reverted to normal cognition had more comorbidities (e.g. respiratory, urological and psychiatric conditions) than those who went on to develop dementia, suggesting that comorbidities may have been a factor in developing MCI and treatment of these conditions may have improved cognition.
| Study | Type of study | Study aims/objectives | Study sample/population | Setting | Findings: screening/diagnosis | Study limitations |
|---|---|---|---|---|---|---|
| Grande et al.94 | Quantitative | To evaluate the proportion of MCI subjects who revert to normal cognition in a memory clinic context, focusing on the role of comorbidities | A total of 503 people with MCI, of whom 374 (mean age 75.1 years, 60% female) completed follow-up and were included in the analysis | A memory clinic in Milan, Italy | During a mean time of 32 (SD± 25.5) months, 21 subjects (5.6%) reverted to normal cognition. Subjects who reverted to normal cognition were younger (p = 0.0001), more educated (p = 0.0001), had better global cognition (p = 0.0001) and suffered from more comorbidities (p = 0.002) than those who developed dementia | The study was described as a preliminary analysis that does not take account of the possibility of developing new comorbidities during follow-up. Authors also note possible selection bias related to the specific clinical setting |
| Hadjichrysanthou et al.95 | Quantitative | To investigate factors associated with observed cognitive improvements in people diagnosed with MCI or dementia |
Longitudinal data sets provided by (1) ADNI (1737 people) and (2) NACC (9927 people) Clinical/health service perspective |
Clinical trial sites (ADNI) and AD centres (NACC) in the USA and Canada | In both data sets, transitions from MCI to normal cognition were significantly associated with younger age, better cognitive function and the absence of ApoE e4 alleles. Better cognitive function and, in some cases, the absence of ApoE e4 alleles were also significantly associated with transitions from types of dementia to less severe clinical states. The effect of gender and education was not clear-cut | Limitations not discussed, but diagnostic processes in North America may not reflect UK practice |
| Klekociuk et al.69 | Quantitative | To identify a set of neuropsychological measures able to differentiate true-positive cases of MCI from those who were unimpaired at 11 months’ follow-up |
Sample of participants from a longitudinal study tracking the neuropsychological profile of MCI (N = 118; female, n = 72) Clinical/health service perspective |
Community research study in Australia | A combination of measures of complex sustained attention, semantic memory, working memory, episodic memory and selective attention correctly classified outcome in > 80% of cases. The rate of false-positive diagnoses (5.93%) was lower than reported in previously published MCI studies | Study focused on identifying people with MCI for research purposes |
| Olazarán et al.43 | Quantitative | To explore the diagnostic and prognostic value of variables that are routinely part of the medical history of patients with suspected cognitive impairment |
Patients (n = 176) aged > 50 years with suspected cognitive impairment (mean age 72.1 years, 70.5% female) Clinician/health service perspective |
Primary care (public health centre in Madrid, Spain) | Of 176 patients analysed, 81 (46.0%) had MCI and 18 (10.2%) had dementia at baseline. After 1 year, eight (9.9%) MCI patients had progressed to dementia, but 48 (59.3%) had reverted to normal cognition. Old age, source of information about symptoms (informant or primary care physician), short duration and low education were associated with MCI or dementia at baseline. Low education predicted progression to dementia in MCI patients, and fewer chronic medical conditions and younger age predicted reversion from MCI to normal cognition | Small sample size and short follow-up |
Interestingly, for the investigation of MCI, Olazarán et al. 43 reported that data routinely collected as part of the patient history in primary care had value both for detecting MCI and predicting the outcome. Klekociuk et al. 69 took a contrasting approach, using a battery of tests to identify the best combination for distinguishing people with true MCI and reducing the rate of false-positive diagnoses.
Prognosis and progression to dementia
Progression from MCI to dementia is the reverse of return to normal cognition and the studies in the previous section may also be relevant. We included four studies17,96–98 specifically on the topic of predicting progression to dementia. A 4-year follow-up study96 of patients with amnestic MCI (n = 44) reported that 41% of patients had progressed to dementia by the end of the study. A combination of cognitive tests distinguished those who progressed from those who did not with 74% accuracy. Using a different technology, Montero-Odasso et al. 97 reported that dual-task gait analysis could be used to predict progression. The authors97 noted that gait testing is easy to administer and could be used to decide on further biomarker testing, preventative strategies and follow-up planning in patients with MCI.
An early study by Stephan et al. 17 examined different definitions of MCI and concluded that none reliably predicted risk of developing dementia over 2 years when applied to a general population, although people who were not at risk were more reliably identified. In contrast to the findings of Grande et al. 94 on comorbidities (see Reversion to ‘normal’ status), a further study by Stephan et al. 98 found no association between comorbidities and progression from MCI to dementia.
Chapter 8 Services/pathways
Services or pathways were investigated in 39 of the included studies, with the included studies published from 2010 to 2020. Studies investigated pathways and services in a range of countries. The majority of the studies were UK based. Two studies investigated populations in Australia and two studies investigated populations in the Netherlands. One study investigated populations in each of Sweden, Sweden and Spain, respectively. The narrative and systematic reviews reviewed international literature, apart from the narrative review, which focused on diagnostic care pathways in England. 99 The included studies consisted of designs with known methodological weaknesses (i.e. cohort studies) and were often small and included pilot and feasibility studies, meaning than any conclusions from these studies are tentative. Overall, the services/pathways studies suggested that there is variation across services in terms of the skills mix of staff, diagnostic tests available, treatments and a need for post-diagnostic support, including annual follow-up.
The authors of the Manchester consensus,5 which is discussed in greater detail in Chapter 11, state that there is a need for NICE guidance on the diagnosis and management of MCI. Currently, many memory clinics discharge patients with a MCI diagnosis back into primary care unless they deteriorate and the authors of the report thought that annual follow-up for patients with MCI would be beneficial, although expensive, to implement. 5 A recent UK qualitative study1 found that memory clinics were generally commissioned for people with dementia and there were insufficient resources for people with MCI and SCI (Table 7).
| Study | Type of study | Study aims/objectives | Study sample/population | Study limitations |
|---|---|---|---|---|
| Dunne et al.5 | Expert consensus guideline with narrative literature review |
To describe the scope of use of MCI as a diagnostic category, determine its utility and explore the implications of its continued use in research and clinical practice To create a clear problem statement as a framework for future national guidance on minimum standards in diagnosis and management of MCI |
Not applicable (literature review/guideline) Clinical/health service perspective |
Expert guideline with no apparent patient or carer involvement |
| Poppe et al.1 | Qualitative | To investigate how services respond to people with memory concerns and how a future effective and inclusive dementia prevention intervention might be structured |
Eighteen people aged ≥ 60 years with subjective or objective memory problems, six family members, 10 health and social care professionals and 11 third-sector workers Mixed perspective |
Diverse sample of health professionals, but authors note that the patient sample were primarily people who had recently sought help for memory problems and so may not be representative |
Dementia care pathways
Three narrative reviews99–101 researched dementia care pathways; further details are provided in Table 8. One review, published in 2014,100 reviewed the evidence around dementia care pathways and discussed the service pathways and the referral routes that are used by services in England and internationally. The review100 found that the term ‘dementia care pathway’ has several potentially overlapping meanings. For people with memory problems, the first encounter with health services, which is generally via a GP consultation, seems to be particularly important. The process of further assessment is not always straightforward, with barriers such as GPs reluctance to refer patients to memory services and the fact that the initial assessment appointment can be daunting and confusing for patients. The other narrative review,99 published in 2017, reviewed studies on the involvement of primary care in diagnostic care pathways for people with memory problems in England. The review found evidence of service redesign and the implementation of innovative approaches to attempt to improve dementia assessment, diagnosis and subsequent support. The development of innovative approaches, such as (1) GPs being primarily responsible for diagnosis and treatment, and only the more complex cases are referred to MASs or (2) diagnosis led by secondary care with support from older people’s mental health teams, should be encouraged and properly evaluated. In addition, attempts to improve access and signposting to postdiagnostic support should also be encouraged.
| Study | Type of study | Study aims/objectives | Study sample/population | Findings: services/pathways | Study limitations |
|---|---|---|---|---|---|
| Draper et al.101 | Narrative review |
A review of how integrated care has been used in assessment and care of adults with dementia and other cognition disorders To assess the evidence for integrated care, including the enablers of and barriers to this approach |
Adults with dementia and other cognition disorders | Varying amount of involvement of primary and specialist care was found in models of integrated community dementia assessment and management, but all focused on improving care co-ordination, interdisciplinary teamwork and personalised care | None reported |
| Samsi and Manthorpe100 | Narrative review | To review the evidence around dementia care pathways and to discuss service pathways and referral routes used by some services in England and internationally |
Not applicable Included studies focus on people at different stages of diagnosis, assessment and living with dementia |
The term ‘dementia care pathway’ has several potentially overlapping meanings. For people with memory problems, the first encounter with health services, generally via a GP consultation, seems to be particularly important. The process of further assessment is not always straightforward, with barriers to referral to memory services, and, in addition, the process can be daunting for patients. There is limited research on patients’ experience of the assessment process | Narrative review, which focused on the pathway from MCI to dementia rather than other outcomes |
| Wells and Smith99 | Narrative review | To review the involvement of primary care in diagnostic care pathways for people with memory problems | Studies on diagnostic care pathways for people with memory problems | This rapid review found that substantial effort is being made to integrate primary care to improve the assessment and diagnosis of dementia. Service redesign and implementing innovative approaches are ways of achieving this. Primary care involvement was generally found to be beneficial. Service (re)design, however, needs to consider how it can meet the needs of all the different patient groups. Providing access to training and support for all primary care practitioners is important when developing existing or new services | None reported |
Another narrative review101 investigated how care is integrated for people with AD and other cognition disorders. This review101 found varying amounts of involvement of primary and specialist care within models of integrated community dementia assessment and management. All of these models focused on improving care co-ordination, interdisciplinary teamwork and personalised care. Although integrated care for people with dementia is highly desirable, the review101 found that efforts to improve integrated dementia care have had mixed results in terms of diagnosis rates, patient behaviour, carer burden and well-being. This limited success is partly due to the inherent complexity involved and the many different types of integration that can occur. Further steps towards a goal of integrated care would focus on improving interprofessional training, early diagnosis in primary care, technology, integrating dementia care into mainstream health and social care, and the encouragement of dementia-friendly communities.
Memory clinics/memory assessment services
Memory clinics or MASs were investigated in 16 studies16,46,48,49,70,77,102–111 (Table 9).
| Study | Type of study | Study aims/objectives | Study sample/population | Findings: services/pathways | Study limitations |
|---|---|---|---|---|---|
| Abley et al.108 | Qualitative | To explore the views of patients and carers on what constitutes high-quality communication and information provision when undergoing assessment in memory clinics | Twenty-seven people with cognitive impairment (13 with confirmed dementia) and 26 carers; 20 matched pairs |
Being kept informed, two subthemes: (1) the need for professionals to ensure understanding and manage expectations during the assessment process and (2) the need for memory clinic professionals to provide specific information What do patients and carers find helpful? People appeared to want individually tailored information. The environment/context is also important [e.g. face-to-face (oral) information supplemented by written information]. Patients wanted information provided as part of an early intervention service. Clinic visits allowed clinicians to monitor medication effects and provide emotional support and practical advice, as well as discussing the diagnosis. Memory retraining classes and memory strategy groups run by memory clinics were reported to have positive outcomes for patients and carers |
The study did not seek the views of professionals in the memory clinics or observe their practice. Further research directly observing practice and, more specifically, what information is relayed would be valuable. The study explored the information needs of only those people with dementia and, therefore, some of the participants with memory problems who did not receive a diagnosis of dementia may have subsequently had a diagnosis confirmed. Therefore, it would be valuable to follow these patients up to explore their experiences |
| Chrysanthaki et al.102 | Quantitative | To determine if a typology of MASs can be constructed, based on shared structural and process characteristics | Seventy-three MASs (about one-third of all MASs in England) from an original randomly selected group of 80 | It was not possible to group characteristics to form the basis of a typology of MASs. There was considerable variation in staff numbers (20-fold), new patients per whole-time equivalent staff (20-fold), skill mix and the nurse-to-doctor ratio (1 : 10). The operational performance also varied. All MASs provided pharmacological treatment after diagnosis, but the availability of non-pharmacological support varied | Identified limitations included incomplete data collection and reliance on self-reported data. The study was limited to distinct MASs and did not take account of assessment in primary care |
| Cook et al.77 | Quantitative service audit | To find variation between London memory services and develop service improvement projects to address them | A total of 590 patients from 10 London memory services. Median age of patients at referral ranged from 79 to 82 years. Female referrals ranged from 51% to 68%. A total of 33% of referrals were of non-white British ethnicity, varying from 10% to 54% per service (comparable to contemporaneous population projection data) | Postdiagnostic support information is provided for patients with a diagnosis of dementia only | Information on the service specification of the memory services was not collected as part of the audit. Further discussions with services has highlighted some variation in staffing numbers, and professional and grade mix, which could have contributed to the variation found in the audit |
| Cook et al.103 | Quantitative questionnaire and case note audit | To collect data on memory service performance and identify areas for improvement | Eighty-five memory services from five regions of England. Data on 3978 patients | The audit demonstrated marked variation in almost every aspect of the memory service pathway, from assessment practices, to the choice of investigations, to the final diagnosis and access to treatment and support | Not all regions were represented and there was possible selection bias, with services that were more interested in audit/quality improvement more likely to participate |
| Dean et al.109 | Qualitative | The aim of this study was to investigate the experiences of people with MCI and their advocates, particularly within health-care services |
Twenty-three people with MCI were recruited from research databases and memory clinics Inclusion criteria: diagnosis of MCI All had been referred by their GPs and 20 advocates were also interviewed |
Patients’ suggested improvements to services Information provision: likely prognosis, treatment options and what the patient advocates themselves could do for the people with MCI Changes: the process of assessment and those relating to the interaction with memory service staff |
Data were obtained at one time point and, therefore, it was not possible to comment on how experiences and needs of people with MCI within health-care services might change over time. Recall and/or insight regarding the topics discussed were impaired in the participants with MCI and the results of the interviews should be interpreted with this in mind |
| Dodd et al.48 | Qualitative | To compare patient, family member and professional experience of primary care- and secondary (usual) care-led memory services |
Health professionals (n = 18), patients recently diagnosed with dementia (n = 13) and carers/relatives (n = 15) (i.e. 23 people with experience of primary care services and 23 from secondary care) Mixed perspective |
Key themes from interviews included GPs and memory nurses working together and an absence of postdiagnostic support | There was a low response from primary care patients, a reliance on health professionals for recruitment from secondary care services and findings are not generalisable to other service models |
| Ramakers and Verhey107 | Quantitative | To understand in greater depth how memory clinics have developed in the Netherlands since 1998 | People attending memory clinics in the Netherlands | Since 1998, the number of memory clinics had increased from 12 to 43 in 2004, and to 63 in 2009. In addition, the number of new patients referred had increased by 73% from 1998 to 2008. The most common diagnosis at memory clinics was still dementia, but a significant proportion of patients were also diagnosed with milder memory problems (i.e. MCI or subjective cognitive complaints). By 2009, memory clinics were part of regional care services and the services that they provided placed less significance on research from universities. Most of the memory clinics were using brain imaging, blood assessments and diagnostic tools. Memory clinics were found to be increasing their use of extensive neuropsychological assessments and CSF diagnostics. Most (80%) memory clinics employed a neurologist, 68% had a clinical geriatrician and 52% employed a psychiatrist | The survey could have missed some services and not all memory clinics completed the surveys, which could lead to underestimation of results. Estimations were generally used for results and not official figures. The study did not collect information on subject demographics |
| Jenkins et al.16 | Mixed-methods quantitative and qualitative analysis of survey data | To evaluate how much is known about SCI and how it is managed in specialist clinical practice in the UK | Representatives of UK memory clinics (n = 23) | The response rate was 21% (23/112). Four main themes emerged from the free-text answers: (1) patient factors influencing what action is taken, (2) further investigations, (3) possible outcomes of the process and (4) barriers clinicians may encounter | The study had a low response rate and was based on vignette rather than real cases |
| Minstrell et al.104 | Quantitative | To identify the demographics, assessment scores and diagnostic profiles of those attending a nurse-led memory clinic with an open referral policy and to assess how it differs from other memory clinic profiles | Patients attending the nurse-led memory clinic over a 25-month period in 2011–13 (n = 106) | Key differences between the nurse-led memory clinic sample and other data sets were history of falls being more common, higher mean MMSE scores and fewer dementia diagnoses. Sixty-four (60%) patients were self-referred, of whom 19 (30%) were diagnosed with MCI or dementia. Overall, 48 (45%) patients received diagnoses of MCI or dementia |
Descriptive study with no control group and a relatively small sample Non-UK setting |
| NHS London Clinical Networks70 | Slide set summarising guidance on diagnosis and management of people with MCI and FCD in primary care | To provide commissioners and clinicians in memory services and primary care with guidance on the appropriate pathways for patients who present with memory complaints due to a range of non-dementia causes | Not applicable. Guidance applies to people with MCI, FCD and other conditions (e.g. depression/anxiety and alcohol misuse) that may cause memory problems | The 2019 London memory service audit looked at 988 case notes across 20 services. There was considerable variation between services. The audit found that 40% of patients (including 85% of patients aged < 65 years) were not given a diagnosis of dementia. The most common non-dementia diagnosis was MCI (46%) |
Describes guidance rather than actual practice Specific to London and may not be applicable to other regions |
| Park et al.105 | Quantitative | To describe change in patients’ health-related quality of life 6 months after referral to MASs and to examine associations with patient characteristics and use of post-diagnostic interventions | Patients (n = 883) referred to 69 MASs and their informal caregivers (n = 569) | Mean health-related quality of life improved, irrespective of diagnosis. Self-reported health-related quality of life increased by 3.4 points (95% CI 2.7 to 4.1 points) and proxy-reported health-related quality of life by 1.3 points (95% CI 0.5 to 2.1 points). Health-related quality of life change was not associated with any of the patient characteristics studied. Patients (54%) with dementia receiving antidementia drugs reported greater improvement in their health-related quality of life, but those using non-pharmacological therapies reported less improvement than those not receiving therapy | |
| Park et al.106 | Quantitative | To detail changes in patients’ health-related quality of life 1 year after their initial appointment at MASs and to consider any correlations with patient or MAS characteristics |
A total of 702 patients attending first appointment at a MAS and 452 lay carers Mean age: respondents, 77.3 years; non-respondents, 78.6 years Female: respondents, n = 340 (48.4%); non-respondents, n = 389 (55.7%) Ethnic minority: respondents, n = 33 (4.7%); non-respondents, n = 38 (5.5%) |
Self-reported health-related quality of life improved in all study participants over the year. People with a diagnosis of dementia saw greater improvement than people with MCI or no diagnosis. The presence of advisory and support staff at MASs was the only characteristic that was associated with large increases in health-related quality of life |
There was no control arm and so it was not possible to know if health-related quality of life improvement was due to attending a MAS The study had a recruitment rate of 42% at the start of the study and only half of participants replied after 1 year. In addition, there were some differences between patient characteristics at the start of the study Other patient factors could have had an impact on outcomes MAS characteristics were hard to standardise across services |
| Pennington et al.110 | Quantitative | To estimate the costs of diagnosis and support for patients with suspected dementia over 6 months at different MASs in England | Eighty MASs were recruited at random, with each MAS to recruit 25 patients and their carers if patients were accompanied. Final sample of 69 MASs. Recruited patients who spoke English who were attending MASs for their first assessment | MASs’ mean monthly staff costs were £73,000, with an extra £3500 per month for imaging and assessment costs. Each new patient assessed had a monthly clinic cost that varied from £320 to £5400 across clinics. Carers reported primary health and social care costs of £130–220 a month between baseline and 6 months, and these costs were additional to MAS. Carers reported that costs of pharmacological and non-pharmacological treatments were small. Informal care costs dwarfed health and social care costs when valued at a modest unit cost. Costs for assessment and support of patients presenting with memory problems over the first 6 months were £1582–2497 and half of these costs were from services directly provided by MASs | Authors noted the following:
|
| Rubinsztein et al.111 | Quantitative | To compare a MCS with a traditional CMHT service on cost and quality of service |
People referred for assessment for cognitive impairment at a MCS or a CMHT from August to November 2011 Mean age: MCS, 80 years; CMHT, 84 years Female: MCS, n = 19 (58%); CMHT, n = 22 (67%) Patients with early-onset dementia were excluded from the study. All patients referred had not been previously diagnosed with dementia. For each service, coincidentally, the medical notes of 33 participants were reviewed. |
Mean cost for MCS was £742, which was cheaper than the mean cost for CMHT service, which was £807; however, the difference was not statistically significant. Key difference was that in the MCS arm 97% of patients were seen by a doctor and also a non-medical clinician, whereas in the CMHT arm 45% of patients saw a doctor only and then were discharged. The MCS provided more comprehensive and multidisciplinary care | The study had a small number of participants, meaning that the findings are only preliminary. Retrospective service evaluation meant that data from case notes had not been collected for research purposes originally |
| Steiner et al.49 | To co-create a model of care for a new multidisciplinary memory clinic |
The study involved 20 GPs, 53 older people and community/local government representatives, and 25 community health-care workers Mixed health service/patient perspective |
Community forum participants felt that they had a good knowledge of available dementia resources and services. However, participants felt that these services were highly fragmented and needed to be easier to navigate for the patient/carer. Recommendations included a ‘one-stop shop’ and the provision of a dementia key worker | The non-UK setting limits generalisability (although many underlying issues will be similar). The study presents data to support design of a care model rather than evaluating an actual model | |
| van den Dungen et al.46 | Quantitative cluster-randomised controlled trial | To assess the effect of a two-component intervention of case finding and subsequent care on diagnostic yield and mental health of patients and carers |
Patients aged ≥ 65 years at 15 primary care practices (n = 647) Mixed clinical and patient/carer perspective |
Among patients and relatives who consented to stage 2 of the trial (n = 145; 25%), there were no differences in mental health between the intervention and control groups | The study had a low consent rate for stage 2, lower prevalence of MCI and dementia than expected and a possible ‘Hawthorne effect’ in the control group, with some GPs very interested in dementia and offering nurse-led care |
Four studies70,77,102,103 considered the organisation and characteristics of memory clinics throughout the UK and found considerable variability.
Chrysanthaki et al. 102 attempted to categorise MASs in the UK. The study randomly surveyed 73 MASs in 2015, finding considerable variation in staff numbers, new patients per whole-time equivalent, skills mix and nurse-to-doctor ratio, length of first appointment, follow-up time and frequency of follow-up within the first year. Following diagnosis, all MASs surveyed provided pharmacological treatment, but the availability of non-pharmacological support varied. These variations made it impossible to group characteristics within a typology of MAS, and the impact of individual structural and process characteristics should be considered in future evaluations. Another audit77 of 10 London memory services was undertaken in 2016 to compare memory services and develop a quality improvement programme. This audit, again, found considerable variation between services. An important finding was that postdiagnostic support information was provided for patients receiving a diagnosis of dementia only, meaning that patients diagnosed with MCI would generally receive no information or support. In addition, waiting times are potentially increased by people without dementia attending memory services. Services could consider developing specific pathways for these patients. In 2019, Cook et al. 103 undertook a larger audit of 85 MASs from five regions of England. This audit,103 again, revealed considerable differences across all stages of the memory service pathway, from assessment practices to choice of investigations, final diagnosis and access to support and treatment.
In 2020, the London Dementia Clinical Networks produced guidance70 for commissioners and clinicians working in memory services. The guidance70 discusses appropriate pathways for people presenting with memory problems that are not due to dementia. The London audit of memory services,77 discussed above, had found that 40% of patients were not given a dementia diagnosis, which increased to 85% for patients aged < 65 years. MCI was the most common non-dementia diagnosis. The guidance advised that people with MCI should be reviewed at least annually until a non-dementia cause is established, the condition has resolved or the person has been diagnosed with dementia. Follow-up may take place in primary or secondary care, depending on local commissioning arrangements. Similarly, the audit of memory services in London highlights the importance of follow-up for these patients, together with the likelihood that non-dementia patients can increase waiting lists and thereby delay dementia diagnosis. 103
Outside the UK, the development of memory clinics in Netherlands from 1998 to 2009 was described by Ramakers and Verhey. 107 The number of memory clinics and people accessing the services have increased significantly. In the Netherlands, memory clinics are now part of the regular care for people with memory problems and early stages of dementia.
Different ways of organising memory clinics were investigated in five studies. 16,48,49,104,108 The UK studies are discussed, followed by the studies from Australia.
A qualitative evaluation,48 published in 2014, of primary care-led dementia diagnostic services in Bristol found important themes of GPs and memory nurses working together and a lack of support following diagnosis. Although patients and carers were generally satisfied with primary care- or secondary care-led dementia diagnosis, they, together with health professionals, expressed concern about the lack of postdiagnostic support, which was a problem that was also highlighted in the 2016 audit of memory services. 77
One study108 explored the views of patients and carers on communication and information provision during memory services assessment. People with cognitive impairment and their carers were recruited from four memory clinics (two in north-east England, one in London and one in north-west England). The patients interviewed discussed the lack of information about the clinic that they were attending, the need for professionals to ensure that patients understand the process, and long waits for test results. Conversely, patients and carers found tailored information helpful, as well as signposting to local voluntary sector services that could provide information or support. The study108 concluded that the communication and information provided needs to be improved for patients being assessed for possible dementia, particularly for patients with MCI and for patients less likely to improve with medication.
A preliminary service evaluation16 in the UK used vignettes to consider knowledge of SCI and how it is managed in specialist clinical practice. The evaluation included 23 memory clinics located throughout the UK and found that most clinicians would discharge patients with SCI and only a small number of clinicians would arrange follow-up or diagnose SCI. The study16 concluded that there is a need for a coherent and consistent framework for the management of people with SCI.
Two further studies14,104 described service initiatives in Australia that could be introduced in the UK. A descriptive study104 aimed to identify differences between a nurse-led memory clinic with an open referral policy and other memory clinics in Australia. The characteristics of profiles to be identified were demographics, assessment scores and diagnostic profiles of people attending. The open referral policy led to a large proportion of patients being self-referred and nearly one-third of these patients were diagnosed with MCI or dementia. Open referral policies and nurse-led services may overcome current barriers to early diagnosis. An evidence briefing from the British Psychological Society14 noted that increasing access to memory clinics could lead to identification of more people with MCI.
A qualitative study49 elicited the views of GPs, older people, community/government local representation and community health-care workers to co-create a multidisciplinary memory clinic in Australia. Community forum participants felt that they had a good knowledge of available dementia resources and services, but that services were highly fragmented and needed to be easier to navigate for the patient/carer. A ‘one-stop shop’ and the provision of a dementia key worker were potential recommendations. Participants also recommended that the memory clinic should offer diagnostic services, rapid referrals, case management, education, legal services, culturally sensitive and appropriate services, allied health, research participation opportunities and clear communication with GPs. The study also highlighted that it is important to work with stakeholders to co-design models of care for people with dementia that take into account the needs of the community.
Patient outcomes were investigated in four studies. 46,105,106,109
A cohort study105 investigated changes in patients’ health-related quality of life 6 months after referral to a MAS in England. Patients’ mean health-related quality of life improved in the first 6 months irrespective of diagnosis. Another cohort study106 found that self-reported health-related quality of life improved in all study participants over the first year after their initial appointment at a MAS. People with a diagnosis of dementia saw greater improvement in health-related quality of life than people with MCI or no diagnosis. The presence of advisory and support staff at MASs was the only characteristic that was associated with large increases in health-related quality of life.
A qualitative study investigated the health care experiences of people with MCI and their caregivers,109 and this is discussed in greater detail in Chapter 9. Patients were recruited from six mental health trusts in the south of England. The study found that the specific needs of patients with MCI should be considered in the design of memory clinics and other related services.
A cluster-randomised controlled trial46 assessed the effect of a two-stage intervention of GP education case finding on diagnostic yield and the mental health of patients and carers in the Netherlands (i.e. a potential service innovation for the UK). Among patients and relatives who consented to stage 2 of the trial (n = 145, 25%), there were no differences in mental health between the intervention and control groups. There was a non-significant increase in the number of new MCI diagnoses in the intervention arm. 46
The costs of memory clinics were investigated in two studies110,111 and audits of memory clinics have demonstrated significant differences in the provision of services. A cohort study,111 published in 2015, compared the costs and quality of memory clinics with a traditional Community Mental Health Teams (both services were based in eastern areas of England). The mean cost for the memory clinic-based service was £742, which was cheaper than the mean cost for Community Mental Health Team service, which was £807. However, the difference was not statistically significant. The memory clinic-based service provided more comprehensive and multidisciplinary care, including counselling for patients before and after diagnosis, more screening of blood tests for comorbidities or reversible causes of dementia, increased use of structured assessment tools for cognitive screening of patients and carers, more regular copying of letters to patients/carers and signposting to third-sector services. Another cohort study110 assessed the cost of diagnosis and early support for patients with cognitive decline of patients attending randomly selected MASs in England. The study found considerable variation in costs across the MASs. Differences appeared to be due to differences in the workload of different staff. Further research to investigate the impact of this variation on patient outcomes would be helpful.
Role of other services in early or increasing dementia diagnosis
Four studies33,47,112,113 researched the role of other services in early or increasing diagnosis of people with MCI. These studies included primary care services, such as cancer clinics and an ophthalmology clinic. Further details of these studies are provided in Table 10.
| Study | Type of study/service focus | Study aims/objectives | Study sample/population | Findings: services/pathways | Study limitations |
|---|---|---|---|---|---|
| Chan et al.33 |
Quantitative pilot study Focus: general practice |
To determine if a leaflet campaign by the Alzheimer’s Society to raise awareness of memory problems increases the number of people presenting to their GP with memory problems |
Intervention practices combined population: 88,924 Control practices combined population: 53,863 For the intervention practices, the proportion of patients aged > 55 years was above the national average, whereas for the control locality the proportion of patients aged > 65 years was below the national average Recording of patient ethnicity was poor in control and intervention practices and so is not reported Baseline recordings of the prevalence of memory problems and dementia were nearly double in the intervention locality |
Referral to secondary care occurred in approximately 80% of people presenting with memory problems and was more likely in the intervention group. Antidepressant use was greater in control locality. The proportion of people with memory problems prescribed cholinesterase inhibitors was not significantly greater in the intervention practices | The study demonstrated the strengths and weaknesses of routinely collected data. Demographic details were strong, with the exception of ethnicity recording. With computerised laboratory links, pathology data were largely complete, although other investigations, such as brain scans, may be reported in hospital letters only and not coded in the general practice computer record. Computerised referral, and more recent changes in the general practice contract to encourage the use of screening questionnaires, may improve these data. The study did not look at any link between health economic status and prescribing. Comparing a single locality and its neighbour has limitations. The authors were informed that contamination was unlikely, but it is likely that some ‘cross-border’ communication between community, staff and patients occurred |
| Dickens and Ramaesh47 |
Narrative review Focus: ophthalmology |
To review evidence on the role of ophthalmology clinics in screening for early AD | The population of interest was people receiving surgical treatment for cataracts, although most studies in the review did not focus on this group | Following testing by eye clinic staff, abnormal findings were communicated to primary care physicians for further follow-up and assessment | Review appears to cite no studies of the proposed approach and so the intervention is best seen as a suggestion for research. Current policy is against population screening for MCI/early dementia |
| Giebel et al.113 |
Qualitative Focus: general practice |
Exploration of the perceptions of dementia (e.g. symptoms, causes, consequences and treatments) held by South Asians and to discern how these understandings vary by age and by the self-recognition of memory problems, as these influence help-seeking behaviour | Younger and middle-aged adults aged 30–59 years (group A, n = 72), older adults aged ≥ 60 years without memory problems (group B, n = 88) and older adults ≥ 60 years with subjective memory problems (group C, n = 33) | For people with memory problems, treatments included talking to their GP/nurse; taking medication; talking to family or friends; socialising; self-administered psychological; accepting and dealing with fate; self-administered behavioural; waiting for things to come back to memory; using formal support services/groups (e.g. day care centres, care homes) or talking to people with similar problems | The study included people with memory problems (aged ≥ 60 years), but not specifically MCI. The study did not adjust for level of education, ethnicity and religion. The authors did not know how many people had direct experience of someone with dementia and how many used the vignette provided as a reference for undertaking the assessment. Group C consisted of people with both organic and functional causes of poor memory, such as higher levels of depression |
| Hopkinson et al.112 |
Mixed-methods case study Focus: oncology |
To consider how cancer clinics could improve the experience of cancer treatment and treatment outcomes for people with self-reported memory problems | Patients aged ≥ 18 years with a self-reported memory problem attending cancer clinic sessions. Observation of 33 encounters between people with a memory problem and a staff member, and 10 consultations were recorded. People were attending for treatment for breast cancer or urologic cancer, or people were undergoing radiotherapy for mixed cancers (five staff members, six people receiving cancer treatment and five carers) | Within the cancer clinic, there were five actions that could improve the experience for people with dementia or MCI: (1) communicating common problems that people with memory problems could experience to increase awareness, (2) providing an environment that would help people to feel that they could discuss their memory problems, (3) training for staff on memory issues in patients, (4) providing tools that could help people to self-manage their memory problems and (5) tackling the support needs of carers | The study was conducted at just one cancer centre. Patients observed in the clinic had self-reported memory issues, whereas patients who were interviewed were assessed and it was confirmed that they had cognitive impairment. A range of clinicians, health professionals and members of the public assessed the model of the experience of cancer treatment for credibility, but they were living and working in south-east Wales and, therefore, other parts of the UK and other countries could have different cancer care pathways |
Two studies47,112 investigated how services (ophthalmology clinics47 and cancer clinics112) could help to identify people with memory problems or cognition disorders when providing services. A narrative review47 explored the role of ophthalmology clinics in screening for early AD. Ophthalmology services and eye clinics could have a role in the early diagnosis of AD by communicating abnormal eye test findings to primary care physicians to follow-up and assess as required. However, the review47 did not include any studies evaluating this approach and was more a suggestion for further research. In addition, a outpatient cancer clinic in Wales investigated112 how it could identify people with memory problems to improve treatment experience and outcomes. The mixed-methods case study112 found five actions that could potentially improve the experience for people with dementia or MCI: (1) communicating common problems that people with memory problems could experience to increase awareness, (2) providing an environment that would help people to feel that they could discuss their memory problems, (3) training for staff on memory issues in patients, (4) providing tools that could help people to self-manage their memory problems and (5) tackling the support needs of carers. For people with dementia or MCI, embedding the clinical treatment of cancer within an environment that is dementia friendly could improve the cancer treatment experience for people with dementia or MCI.
Chan et al. 33 undertook a locality-based controlled study to evaluate whether or not a leaflet campaign to raise awareness of memory problems led to more people visiting their general practice to discuss concerns about their memory. The leaflet campaign increased the recording and management of memory problems, but the improvement was greater in control than intervention practice. This study,33 which was set in the UK, from 2010, may have questionable relevance to the current situation because of increased ease of accessing information using the internet, etc.
Certain ethnic groups can be reticent about reporting memory problems to their GP. A qualitative study113 investigated perceptions of associated symptoms, causes, consequences and preferred treatments of dementia for South Asian adults with memory problems in Manchester, UK. Perceptions varied among South Asians in different circumstances and in different age cohorts, and understanding these variations in perceptions of dementia can enable targeted information and interventions and lead to identification of those older adults at greater risk of distress.
Treatment/follow-up
Treatment or follow-up for MCI and dementia were investigated in six studies29,114–118 and further details of the studies are provided in Table 11.
| Study | Type of study | Study aims/objectives | Study sample/population | Findings: services/pathways | Study limitations |
|---|---|---|---|---|---|
| Patnode et al.29 | Systematic review | To systematically review the test accuracy of cognitive screening instruments and benefits and harms of interventions to treat cognitive impairment in older adults (aged ≥ 65 years) | Studies of people aged ≥ 65 years living in the community were eligible for inclusion. Treatment studies included people with MCI or mild to moderate dementia | Two hundred and twenty-four RCTs and three observational studies, including more than 240,000 patients or caregivers, addressed the treatment of MCI or mild to moderate dementia. None of the treatment trials was linked with a screening programme. Medications approved to treat AD improved scores on cognitive tests. Psychoeducation interventions for caregivers resulted in a small benefit for caregiver burden over 3–12 months. Intervention benefits were small and of uncertain clinical importance | The authors noted limitations of the evidence base. The review included international literature, but has a US focus |
| Peach et al.114 | Qualitative | To explore the perceptions of older people with mild dementia and MCI, and their family carers, about falling, falls risk and the acceptability of falls prevention interventions | Twenty patient–relative dyads recruited from MASs and falls prevention services in the UK | Participants would consider taking exercise specifically to prevent falls. In addition, patients reported that they would join a group if invited. Relatives wanted to provide practical support, but not to the point of undermining the patient’s independence | None provided |
| Quinn et al.115 | Systematic review | To review the evidence on group-based psychosocial interventions that include an important component of self-management for people with MCI or dementia | Studies published in English up to November 2013 that report a group-based intervention for ≥ 6 months and include a significant component of self-management for people with MCI or dementia | The review included 15 interventions (12 aimed at people with dementia and three aimed at people with MCI). The most common self-management elements were information, communication, social support and skills training. There is preliminary evidence that interventions with self-management elements are feasible and acceptable and can potentially help these populations | The review included studies with different methodologies. The review included interventions for people with MCI and dementia, and differences between these conditions. Difficult to categorise the self-managements elements in the different interventions. Review included interventions that had five or more self-management elements |
| Regan and Varanelli116 | Systematic review | To review the evidence on a community-based intervention to improve depression, anxiety or adjustment in people with MCI or early dementia sufferers | Patients with MCI, early dementia or depression or anxiety disorders | Sixteen studies were included in the review (seven randomised controlled trials and eight pre–post studies). It was difficult to make comparisons between the 16 studies because of differences in outcome measures, inclusion criteria, psychotherapeutic approaches, how interventions were delivered (e.g. group or individual) and settings. Studies to treat depression and anxiety used problem-solving therapy (n = 3) and CBT (n = 2), and findings were promising but would need to be investigated further. Studies to improve quality of life or adjustment in people with MCI or early dementia used a variety of approaches, predominantly psychological, which included CBT and psychotherapy, multimodal and intergenerational. Of these approaches, modified CBT showed promising improvements for patients with MCI and early dementia | Included studies varied substantially in their quality, with many being pilot or feasibility studies. Differences in findings between similar approaches may have been due to study quality. Many of the included studies used a waitlist control group instead of an active control group, which is weaker methodologically. The interventions varied in their approaches, outcomes measures and baseline characteristics, making it difficult to compare them. The review included studies in English only and could have missed important research from Asia and parts of Europe |
| Royal College of Psychiatrists118 | Mixed methods | To develop consensus guidelines for clinicians on driving with dementia or MCI. The guidelines aimed to give clinicians information about their legal and clinical responsibilities and provide a framework for managing patients who have a diagnosis of dementia or MCI and drive | Working group of a diverse range of clinicians and carers | Clinicians with immediate concerns about the driving safety of an individual with a diagnosis of MCI need to advise them to stop driving while awaiting a decision by the DVLA. Functional impairment is not significant for most people with a MCI diagnosis, meaning that most will not need to notify the DVLA. Clinicians should also consider any comorbidities that could have an impact on safety to drive. It is the responsibility of the person with MCI to contact the DVLA, but anticipated that the clinician will advise if notifying the DVLA is necessary | None reported |
| The British Psychological Society117 | Narrative review | To summarise the research evidence on psychological therapies for people who have a confirmed diagnosis of dementia and their families | People with a diagnosis of dementia and their families |
Psychological therapies have an important role in dementia care and can take a formal or informal format. These interventions need to be delivered by trained professionals who are registered with and supervised on a regular basis by an appropriate regulatory body. The evidence for psychological therapies for people with dementia is increasing and, although the studies are of variable quality, there is enough evidence of effectiveness to start to draw initial conclusions. Conclusions are provided for therapies for people with mild dementia, which could be comparable to MCI Pre-assessment counselling: people will have a variety of feelings about diagnosis and this can include arguments with their family about the benefit of assessment. Talking through the potential implications of being assessed can help people to decide whether or not they want to be assessed for dementia Counselling and support following a diagnosis of dementia: generally a group format to help people and their families with adapting to the diagnosis of dementia. Therapies that include self-management have found preliminary promising evidence that these groups can potentially enhance self-efficacy. There is promising evidence for the effectiveness of psychological therapies to treat people with dementia at a mild or moderate level and anxiety or depression, although the evidence is insufficient to enable recommendation of a specific type of therapy. Psychological therapies that focus on carers emotional and psychological needs were found to lower depression levels in family carers Examples of good practice are provided in the briefing |
None reported |
A systematic review115 reviewed self-management interventions for people with dementia and MCI. The review115 found that group-based interventions for people with dementia or MCI included components of self-management, information, communication, social support and skills training. Preliminary evidence reported that self-management interventions can potentially help people with MCI or dementia and are feasible in this population. However, included studies had important methodological issues, with many not discussing the intervention’s theoretical basis and many not reporting measurable outcomes. To find the effectiveness of these interventions, more methodologically strong research is required.
A systematic review,116 published in 2013, reviewed the evidence on the impact of psychological intervention studies on adjustment depression and anxiety in MCI and early dementia. Several studies treating depression in people with early dementia using problem-solving and modified cognitive–behavioural therapy approaches had promising findings. Modified cognitive–behavioural therapy showed promise for improving adjustment and quality of life in patients with MCI and early dementia. Strong methodological studies are needed to properly test these interventions and enable the development of clinical recommendations. The British Psychological Society published an evidence briefing117 in 2016 on a similar topic, reviewing the evidence on psychological therapies for people with dementia. The briefing117 found that the evidence base is developing and, although methodological quality remains variable, conclusions can start to be drawn. Psychological therapies should be performed by trained professionals. For people with a dementia, diagnosis can either be formal or informal. These different formats consider the context of the therapy and the patient’s specific needs. The briefing identified the following three actions for moving forward. First, psychological therapies should be generally available in dementia care throughout the journey, from pre-assessment counselling to support following diagnosis, and at times when people with dementia become anxious or distressed. Second, psychological therapies, where necessary, should focus on carers’ and family needs and help a patient’s wider family to understand the diagnosis of dementia and enable planning for the future. Third, routine care should integrate psychotherapy skills to help improve interactions between people with dementia and their carers.
A qualitative study114 interviewed patients and their relatives from MASs and falls prevention services in the UK. The study114 reported that participants would consider taking exercise specifically to prevent falls and reported that participants would join a group if invited. Relatives wanted to provide practical support, but without undermining the patient’s independence.
The Royal College of Psychiatrists developed a consensus guideline118 that considers the role of clinicians in advising patients with MCI about driving. Clinicians managing patients with a MCI diagnosis should consider if patients are able to drive safely. Concerned clinicians should advise patients to contact the Driver and Vehicle Licensing Agency. Clinical reassessment of an individual’s ability to drive should be part of the ongoing management of a patient with a MCI diagnosis. The Manchester consensus5 felt that annual follow-up would be beneficial, although expensive to implement, and this could include assessment of driving ability.
A systematic evidence review,29 undertaken to inform the US Preventive Services Task Force, assessed the evidence on screening and treatment for cognitive impairment in older adults. The review29 included a large number of studies, but found that it remains unclear whether or not interventions for patients or caregivers provide clinically important benefits for older adults with earlier detected cognitive impairment or their caregivers.
Specific populations
Two studies41,119 investigated services and pathways for specific populations of people with dementia (i.e. prisoners119 and older homeless people41). A mixed-methods study119 investigated the prevalence of dementia and cognitive impairment in prisoners aged ≥ 50 years in England and Wales. In terms of services, the study found that local initiatives to improve the experience of prisoners had been developed but were difficult to sustain, given that they were not commissioned services. Multidisciplinary working in prisons is hindered by agencies continuing to work separately and the limited or inadequate communication between different professionals. Another mixed-methods study41 investigated service provision for homeless people experiencing memory problems. The study41 found that the links between different hostels and local primary care and mental health services varied significantly and some staff had substantially more sector experience than others, but high levels of staff turnover were reported. Training levels and availability varied, and differences existed in the extent to which hostel staff were permitted to access local NHS and local authority courses. The study41 concluded that, given the declining number of hostels in England, the limits of NHS engagement with this sector and growing homelessness, homeless people with memory problems are under-recognised and excluded from other sources of support. Services and support for prisoners and homeless people with MCI are insufficient and need to be developed.
The role of technology
Four studies38,120–122 investigated the use of technology in diagnosis and interventions for people with MCI and dementia (Table 12). For details of the study by Sabbagh et al. ,122 see Table 16.
| Study | Type of study | Study aims/objectives | Study sample/population | Findings: services/pathways | Study limitations |
|---|---|---|---|---|---|
| Costanzo et al.38 |
Narrative review Descriptive review with search but no quality assessment |
To provide a narrative synthesis of the literature about the implementation of telemedicine for diagnosis, treatment and follow-up of patients with AD and MCI and their caregivers | Studies of people with a diagnosis of MCI or AD were included | Forty-one included studies focused on supporting patients during the stages of the disease (28 articles), patients’ caregivers (nine articles) or both (four articles), with generally positive results |
A narrative review with search and inclusion criteria but no quality assessment Limited descriptive synthesis |
| Lindqvist et al.120 | Qualitative | The aim of this study was to investigate the areas of concern in which persons with cognitive deficits meet challenges in everyday life, in what environments these challenges appear and how technology might be involved as part of the challenge and/or the solution to the challenge |
Participants that live with cognitive deficits (n = 5) or cohabit with a person with cognitive deficit (n = 5) Members of volunteer health organisations, health professionals at memory investigation clinics and researchers in the area of MCI/early-stage dementia and public environment/technology (n = 5) |
The involvement of technology in everyday activities could be hindering and evoke stress, or it could bring about feelings of control. The involvement of technology was especially obvious in challenges linked to managing personal finances | The subjectivity of the areas of reaction to the use of technology from qualitative approach within this particular context. Most participants in the two focus groups representing voluntary health organisations were female. It is possible that new technologies have become part of new problems, just as much as they might be part of new solutions |
| Quintana et al.121 | Quantitative feasibility study | To test the feasibility and usability of SMART4MD. SMART4MD is a tablet app that is modified to meet the needs of people with mild dementia | Nineteen people with MCI and their carers | Users testing the SMART4MD app found it to be a useable interface and users were generally satisfied with the interface | The feasibility study could have included more people and those from countries other than Spain and Sweden in SMART4MD |
| Sabbagh et al.122 |
Other Expert panel |
A US expert panel considering the detection of MCI at home72 found that, despite barriers yet to be addressed, electronic point-of-contact testing holds great promise. It offers a critical method to support large-scale cognitive screening for the early detection of MCI. Supplementing in-clinic evaluation with at-home assessment may help to identify individuals with MCI, allowing physicians to intervene and ultimately to monitor progression, potentially without requiring the individual to present to the physician’s office.
A literature review38 of telemedicine in AD and MCI considered the diagnostic and interventional implications. Included studies reporting the use of telemedicine to support patients, their carers or both during the disease stages generally had positive results, although the review38 did not perform quality appraisal of the included studies.
A small focus group study120 researched how technology could help or hinder the daily lives of people with mild cognitive deficits. Participants in the study were from Sweden, and had cognitive deficits, lived with someone with cognitive deficits or were members of volunteer health organisations or health professionals at memory investigation clinics. The study found that technology could help, cause stress or bring about feelings of control. In addition, the study found that technology was used for orientation and managing finances. A small feasibility-usability study121 investigated a tablet application (app) that was adapted for people with cognitive impairment. The app was tested in home and clinical settings in Sweden and Spain. The app was found to be feasible and had an acceptable interface for people with MCI. The testing of the app resulted in further development and improved procedures.
COVID-19
Two studies123,124 covered the challenges arising from the COVID-19 pandemic for diagnosis and memory services and how services could develop (Table 13).
| Study | Type of study | Study aims/objectives | Study sample/population | Findings: services/pathways | Study limitations |
|---|---|---|---|---|---|
| NHS England and NHS Improvement123 | Other service description/specification | To describe changes to the operation of memory services in England resulting from the COVID-19 pandemic | Not applicable. Paper describes and discusses general principles for configuring and managing memory services | MASs will need to offer a blended model, offering telephone, video and face-to-face appointments. The report offers advice on components of the diagnostic pathway, peri-diagnostic and post-diagnostic report, advance care planning and other issues | Policy/guidance document with limited data |
| Owens et al.124 | Narrative review | To review the evidence and current practice and guidelines for remote cognitive assessment in memory clinics | Older adults with possible cognitive impairment needing to attend a memory clinic for assessment |
Level 1: ad hoc adaptations of traditional clinic assessments. Most current instruments were considered straightforward to use over the telephone or by video, but instruments might not be as accurate when administered by telephone or video Level 2: specific adaptations with psychometric data available. Instruments that have electronic versions or adaptations, for example for people with visual impairment, could be harder for people with dementia, but these instruments have been tested for validity and reliability Level 3: cognitive remote measurement technologies for remote memory clinics. Findings from new technologies were organised into online platforms, device-based tests, wearable remote measurement technologies and virtual and augmented reality and game consoles. Authors thought that these platforms presented good opportunities for the future and the COVID-19 pandemic gave them a chance to start using them |
None reported |
One policy/guidance,123 produced by NHS England and NHS Improvement, described changes to memory services in England due to COVID-19 and potential new ways of working, discussing general principles for configuring and managing memory services. A blended model, incorporating telephone, video and face to face, is needed. Personalised care remains key to MAS delivery and needs to evolve to adapt safely to COVID-19 restrictions while maintaining patient choice wherever possible. COVID-19 may become endemic and adaptation to a new way of working is required to maintain high-quality care. In addition, a narrative review124 considered the international evidence on remote clinics during and after the COVID-19 pandemic and potential solutions for after the pandemic. Memory services have experienced considerable challenges due to COVID-19; however, this has provided the opportunity for services to consider new technologies and approaches for cognition assessment. Solutions are available that could enable clinical assessment in people who are old, frail and limited by social distance measures. Moving forward, this approach, both now and in the future, could enable people who are living remotely or who would find attending a memory clinic stressful to be assessed in their own homes. This is linked to the study in technology section on home assessment (see The role of technology). 122
Chapter 9 Patient/carer experience
Patient/carer experiences were investigated in 30 of the included studies (Table 14). Included studies were published from 2010 to 2020. The year 2020 had the most included studies. Other years were relatively equally represented, with one study published in 2011, three in 2012, four in 2013, three in 2014, four in 2015, two in 2016, three in 2017 and two in 2018. None of the included studies was published in either 2010 or 2019. Patient/carer experiences from diverse countries were presented, primarily from the UK (17 studies), with one study investigating each of Australia, Singapore, Sweden and the USA. There were two multicentre studies, with one combining Europe and the USA and the other representing the UK and North America. Two further US papers and one of the European studies were consensus or position statements. The four literature reviews included studies from Canada, Denmark, France, the Netherlands, the USA, the UK, Sweden and Taiwan (Province of China). One study included in the literature reviews was a European multicentre study. One literature review did not identify any of the countries of origin for the source studies. Owing to the nature of the question, the majority of studies recruited patients from memory clinics (see Table 14).
| Study | Country | Setting | Title | Year |
|---|---|---|---|---|
| Abley et al.108 | UK | Four memory clinics: one in London, one in north-west England and two in north-east England | Patients’ and carers’ views on communication and information provision when undergoing assessments in memory services | 2013 |
| Beard and Neary24 | USA | An AD centre in a large Midwestern US city | Making sense of nonsense: experiences of mild cognitive impairment | 2013 |
| Begum et al.90 | UK | Two primary care services in south London | Subjective memory impairment in older adults: aetiology, salience and help seeking | 2012 |
| Birt et al.125 | UK | NHS and community in four areas of England | Relational experiences of people seeking help and assessment for subjective cognitive concern and memory loss | 2020 |
| Boyd et al.126 | UK | Community-dwelling participants in Northern Ireland, UK | Community-based trials of mobile solutions for the detection and management of cognitive decline | 2017 |
| Buckley et al.127 | Multiple (literature review) | Diverse settings, including community, hospitals and nursing homes | Subjective cognitive decline from a phenomenological perspective: a review of the qualitative literature | 2015 |
| Bunn et al.57 | UK and North America | Health systems of various countries, primarily the UK and North America | Psychosocial factors that shape patient and carer experiences of dementia diagnosis and treatment: a systematic review of qualitative studies | 2012 |
| Cheong et al.128 | Singapore | Patients undergoing a counselling service for persons with early cognitive impairment | Advance care planning in people with early cognitive impairment | 2015 |
| Dean and Wilcock22 | Multicentre: Denmark, France, the Netherlands, the USA, Canada and the UK | Literature review | Living with mild cognitive impairment: the patient’s and carer’s experience | 2012 |
| Dean et al.109 | UK | Six mental health trusts in the south of England | Exploring the experiences of people with mild cognitive impairment and their caregivers with particular reference to healthcare – a qualitative study | 2014 |
| Dodd et al.48 | UK | Primary and secondary care dementia services in Bristol, UK | An evaluation of primary care led dementia diagnostic services in Bristol | 2014 |
| Dooley et al.129 | UK | Nine UK-based secondary care memory clinics across rural and urban settings | Communication and understanding of mild cognitive impairment diagnoses | 2020 |
| Frederiksen et al.84 | Europe | Position statement | Biomarker counseling, disclosure of diagnosis and follow-up in patients with mild cognitive impairment: a European Alzheimer’s disease consortium survey | 2020 |
| Giebel et al.130 | UK | South Asian community in Greater Manchester, UK | Perceptions of self-defined memory problems vary in South Asian minority older people who consult a GP and those who do not: a mixed-method pilot study | 2016 |
| Gomersall et al.23 | Literature review: USA, UK, Sweden, the Netherlands, Canada and Taiwan (Province of China) | The USA (10 studies), the UK (three studies), Sweden (two studies) and the Netherlands, Canada, and Taiwan (Province of China) (one study each) | Living with ambiguity: a metasynthesis of qualitative research on mild cognitive impairment | 2015 |
| Gomersall et al.131 | UK | A memory clinic in South Yorkshire, UK | ‘It’s definitely not Alzheimer’s’: perceived benefits and drawbacks of a mild cognitive impairment diagnosis | 2017 |
| Guzman et al.18 | UK | Six NHS memory clinics and a specialist old age psychology service between March 2017 and February 2018 | Psychosocial adjustment to mild cognitive impairment: the role of illness perceptions, cognitive fusion and cognitive impairment | 2020 |
| Lindqvist et al.120 | Sweden | Sweden | The contrasting role of technology as both supportive and hindering in the everyday lives of people with mild cognitive deficits: a focus group study | 2018 |
| Manthorpe et al.59 | UK | Memory clinics situated in London (n = 1), north-west England (n = 1) and north-east England (n = 2) | From forgetfulness to dementia: clinical and commissioning implications of diagnostic experiences | 2013 |
| Mukadam et al.32 | UK | Community settings in and around Greater London, UK | What would encourage help-seeking for memory problems among UK-based South Asians? A qualitative study | 2015 |
| Peach et al.114 | UK | MASs and falls prevention services in the UK | Attitudes of older people with mild dementia and mild cognitive impairment and their relatives about falls risk and prevention: a qualitative study | 2017 |
| Perry-Young et al.30 | Literature review: Canada, the USA, the UK, the Netherlands and Sweden | Three studies in Canada, two studies in the USA, two studies in the UK, one study in the Netherlands and one study in Sweden | How people come to recognise a problem and seek medical help for a person showing early signs of dementia: a systematic review and meta-ethnography | 2018 |
| Pierce et al.15 | UK | Memory clinics across north Wales | Knowingly not wanting to know: discourses of people diagnosed with mild cognitive impairment | 2016 |
| Poppe et al.1 | UK | NHS and third-sector organisations supporting older people | ‘Falling through the cracks’; stakeholders’ views around the concept and diagnosis of mild cognitive impairment and their understanding of dementia prevention | 2020 |
| Roberts and Clare58 | UK | North Wales | Meta-representational awareness in mild cognitive impairment: an interpretative phenomenological analysis | 2013 |
| Robinson et al.56 | Europe and the USA | Europe (18 studies) and the USA (17 studies) | The transition to dementia – individual and family experiences of receiving a diagnosis: a review | 2011 |
| Sabbagh et al.71 | USA | US statement | Early detection of mild cognitive impairment (MCI) in primary care | 2020 |
| Sabbagh et al.72 | USA | US statement | Early detection of mild cognitive impairment (MCI) in an at-home setting | 2020 |
| Samsi et al.60 | UK | London (one site), north-west England (one site) and north-east England (two sites) | Negotiating a labyrinth: experiences of assessment and diagnostic journey in cognitive impairment and dementia | 2014 |
| Steiner et al.49 | Australia | Memory clinic in south-western Sydney, NSW, Australia | ‘We need a one-stop-shop’: co-creating the model of care for a multidisciplinary memory clinic with community members, GPs, aged care workers, service providers, and policy-makers | 2020 |
Overall pathway summary
In the UK, access to specialist medical care is through referral from primary care. When a person or their family or carer starts to identify memory problems and/or suspects dementia, they often first go to a primary care service. 60 Specific triggers for help-seeking for subjective cognitive concern include being prompted by family and knowing a relative with dementia. 125 Patients may have concerns about impaired memory, mood and personality changes or psychosis that could be caused by dementia. The GP will conduct an initial assessment to rule out any underlying physical or mental health causes. If further investigation is required, an onward referral is made to a MAS. The stages of the pathway are shown in Box 1 (based on NHS London Clinical Networks70) and are then explored narratively in the following sections. Table 15 summarises coverage of the pathway in the studies included in this section.
1. Patient/carer suspects dementia.
2. Presentation to primary care.
3. Initial GP assessment.
4. Treat or review.
5. Referral to MAS.
6. Referral accepted.
7. Person offered dementia assessment.
8. Dementia assessment.
9a. MCI diagnosis.
9b. Diagnosis of dementia.
10a. Care plan.
10b. Exit pathway.
Contains public sector information licensed under the Open Government Licence v3.0. 70
| Study | MCI patient pathway | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient/carer suspects dementia | Presentation to primary care | Initial GP assessment | Treat or review | Referral to MAS | Referral accepted | Person offered dementia assessment | Dementia assessment | MCI diagnosis | Care plan | Exit pathway | Diagnosis of dementia | |
| Abley et al.108 | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Beard and Neary24 | ✓ | |||||||||||
| Begum et al.90 | ✓ | |||||||||||
| Birt et al.125 | ✓ | ✓ | ||||||||||
| Boyd et al.126 | ✓ | ✓ | ||||||||||
| Buckley et al.127 | ✓ | |||||||||||
| Bunn et al.57 | ✓ | ✓ | ||||||||||
| Cheong et al.128 | ✓ | |||||||||||
| Dean and Wilcock22 | ✓ | ✓ | ✓ | |||||||||
| Dean et al.109 | ✓ | ✓ | ✓ | |||||||||
| Dodd et al.48 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Dooley et al.129 | ✓ | ✓ | ✓ | |||||||||
| Frederiksen et al.84 | ✓ | ✓ | ✓ | |||||||||
| Giebel et al.130 | ✓ | ✓ | ||||||||||
| Gomersall et al.23 | ✓ | |||||||||||
| Gomersall et al.131 | ✓ | ✓ | ||||||||||
| Guzman et al.18 | ✓ | |||||||||||
| Lindqvist et al.120 | ✓ | ✓ | ||||||||||
| Manthorpe et al.59 | ✓ | ✓ | ||||||||||
| Mukadam et al.32 | ✓ | ✓ | ✓ | ✓ | ||||||||
| Peach et al.114 | ✓ | ✓ | ✓ | |||||||||
| Perry-Young et al.30 | ✓ | ✓ | ✓ | |||||||||
| Pierce et al.15 | ✓ | ✓ | ✓ | |||||||||
| Poppe et al.1 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Roberts and Clare58 | ✓ | ✓ | ✓ | ✓ | ||||||||
| Robinson et al.56 | ✓ | ✓ | ✓ | |||||||||
| Sabbagh et al.71 | ✓ | ✓ | ||||||||||
| Sabbagh et al.72 | ✓ | ✓ | ||||||||||
| Samsi et al.60 | ✓ | |||||||||||
| Steiner et al.49 | ✓ | |||||||||||
Patient/carer suspects dementia
Carers commonly precipitate the search for help from a doctor. 57 Patients may differ in their willingness and readiness to receive a diagnosis. 57 Persistent barriers to early diagnosis include stigma, the normalisation of symptoms and a lack of awareness about the signs and symptoms of dementia. 132 It often takes a trigger event or tipping point, such as a hospitalisation or bereavement, before people seek help. 57,100 Family members often recognise something is wrong before the person with dementia does and are frequently instrumental in obtaining a diagnosis. 56,57 Carers commonly report changes in behaviour, effects on the patient’s ability to socialise and exacerbation of pre-existing problems, such as marital difficulties and medical conditions. 22,56,133 Carers attribute changes to multiple causes, including the effect of ‘normal’ ageing, incipient dementia, longstanding personality traits and physical health problems. 22 The conceptual studies, reviewed earlier (see Chapter 6), reveal an emerging emphasis that the diagnosis pathway should make more consideration of the relative function of each individual.
Subsequently, family members may continue to find decision-making being deferred to them, particularly in relation to advance care planning. 128 Patients and relatives associate dementia with ‘loss of the self’. 56 Whether or not patients and relatives will want to be informed of the diagnosis depends on how they perceive the consequences of dementia or how they react to previous family experiences. 56 Studies describe how patients may display the phenomenon of ‘knowingly not wanting to know’. 15 Patients fear a loss of control (particularly with regard to issues such as continence) and express a loss of well-being. Patients are concerned that their family will be upset and they fear having to move to a care home. At this particular point in the pathway, patients do not hold specific expectations of support services, but they express a desire for advice. 56
Minority ethnic populations often perceive greater stigma for dementia symptoms. 32,57 These populations are less likely to recognise symptoms of dementia as an illness than white individuals, and more likely to ascribe these symptoms to the ageing process. 32,57 In addition, symptoms of dementia are sometimes given cultural or religious explanations. 32,57 One study reported that some people who have not had a consultation consider memory problems to be given by God32 and, consequently, see acceptance of fate as an alternative response. 32 Another study found that some people do not identify medical support as appropriate. 130
The evidence is inconclusive regarding whether or not level of education or socioeconomic status has an impact on awareness of dementia and help-seeking behaviour. 57
Presentation to primary care
Some people with memory problems approach the GP themselves, whereas others make a joint approach with family members, although carers often act proactively. In a few instances, the carer approaches the GP without informing the person who is experiencing difficulties. Those with memory problems or other symptoms may seek a diagnosis in the belief that catching the disorder early is crucial to receiving treatment for it. The media may be influential in this decision:
We’re still waiting for something to happen. Because they reckon early diagnosis don’t they and then they can do something and try and hold it if that is the case. Give you something to slow it up a bit. But we’re still waiting.
Carer59
A thematic analysis identified the point of deciding to seek help as one of three ‘transition points’ at which trust can either be developed or undermined. 125 Patients who perceive health-care practitioners’ behaviour as dismissive are less likely to trust the outcome of the health-care encounter.
Initial general practitioner assessment
Most patients report either positive or neutral experiences of consulting their GP about their memory problems. 109 In contrast, most patient advocates report at least some negative aspects to their encounter with the GP. 109 Patients who are referred to the memory service tend to recall little, if any, diagnostic input from their GP. However, these patients consistently acknowledge the GP’s importance as the person who starts the ball rolling at the beginning of the diagnostic process. 48 Many patients recognise the important gatekeeping role of the GP. Carers spoke of GPs not always taking action when concerns are first raised, but instead telling them to ‘keep an eye on it and in a couple months if it is still the same we’ll refer her for tests’. 48 Carers may feel that they are not being listened to by the GP or that they are being ‘fobbed off’ and that memory problems are not being taken seriously by GPs. 48 Patient advocates also report issues relating to ‘reporting’ on the person with MCI in their presence. 109
Studies suggest that sometimes doctors are slow to recognise symptoms or reluctant to give a diagnosis. 48,57 In terms of communication with professionals, people with memory problems and their carers identify two areas as most important to them: (1) being kept informed during the assessment process and (2) being told the outcomes of the assessment, including the results of tests and the diagnosis. 108 The health-care practitioners’ response to help-seeking is the second ‘transition point’ at which trust can be developed or undermined. 125 Although rates of detection of MCI in primary care have increased from the low rates of the early 2010s,10 a need to expand coverage by nurse practitioners and routine clinical data remains.
Treat or review
Experiences of referral are mixed, with some patients reporting a delay in referral to memory services and others reporting confidentiality obstacles, with doctors reluctant to talk to carers about their family member with dementia. 57 South Asians who have had a consultation are more likely to identify forgetfulness and loss of social meaning as symptoms of dementia. 130
Referral to a memory assessment service
Even when people have been referred to memory services, the process may be slow, with long periods of waiting. 57,59,60 Some experience significant delay between appointments. While waiting, patients report having little expectation of what they were waiting for. Consequently, intervals between appointments for results and explanations may be seen as burdensome. 59
Delays in the assessment process sometimes lead patients to believe that a diagnosis could not be confirmed. This can lead to disappointment with the health system. 59 The passage of time does not always resolve uncertainty about the diagnosis and how to respond to it and, in fact, it can exacerbate patient concerns. 59
Referral accepted
Seeking medical advice may offer practical benefits. Changing a set of signs and symptoms into a ‘known’ condition may offer the prospect of clinical management and the relief of suffering. However, in the specific case of MCI (i.e. with no recommended treatments being available) this otherwise promising pathway may become a dead end, leading to potential frustration. The discussion of timely diagnosis in the conceptual papers and the CIS reveal the importance of disclosure of the diagnosis at the ‘right time for the individual with consideration of their preferences and unique circumstances’. 13 This requires that help-seeking can be met by adequate services to optimise the benefits and minimise the costs and harms, as itemised below. An appropriate service response would include multiple treatment targets to help people to accept and adjust to their diagnosis. This would then realise benefits in terms of reduction of secondary conditions, such as depression, and improved quality of life. 18
Common sources of support for carers are friends, social groups and family members, including, in some cases, the person with MCI themselves. 109
Person offered dementia assessment
The process and outcome of assessment is a third and final ‘transition point’ at which trust can be developed or undermined. 125 Misunderstandings and absence of trust in assessment processes may lead to patients failing to fully agree with the outcomes of the assessment. 125
Dementia assessment
Patients express variable experience of a memory clinic. 109 Many patients experience the assessment process as confusing, with one study describing it as ‘labyrinth like’. 60 Patients are able to recall in detail what happened, the impact it had on their lives and the meanings they attributed to the process as they began to accept their condition. 60 Many patients experience rising anxiety as they approach the point of diagnosis. 60 Patients receive few explanations about the various medical tests, examinations and scans they underwent, including ‘X-rays of the head’60 and ‘general scans, magnetic resonance imaging scans, electrocardiography and blood and cholesterol tests’. 60 Even when these procedures are discussed, patients may remain confused about details and what the tests would reveal. 60
Neuropsychological testing can be particularly daunting when patients try to interpret their scores. 60 Patients respond to testing with words such as ‘worry’, ‘concern’ and ‘anxious’, starting from being visited by a specialist doctor to being concerned about what the tests are for, what scores mean and whether or not they have underperformed. 60 Without a clear explanation of the meaning of these scores, test results appear misunderstood or meaningless, making patients even more anxious. 60 Interpreting scan results can be frightening for patients and, for some, it is the first time that the possibility of AD is raised. 60 Patients express relief at the outcome of the assessment58 and relay positive comments on how the service is run. 109 However, most patients receive little or no information about their diagnosis. 58,109
Information reported by informants is important to confirm that the patient remains able to perform activities of daily living. An informant might report, for example, that the patient needs more time to complete tasks, but is still able to do them independently. 70
Clinicians are recommended to use a brief screening tool validated for the detection of people with MCI, such as the MoCA. Neuroimaging to identify underlying brain pathology may be useful, but is not currently considered mandatory. Neuropsychological assessment is potentially helpful in cases where screening test results are inconsistent with the results of clinical assessment.
Patients with early-stage dementia may struggle to complete the early cognitive decline assessment without some help. 126 Completion of the assessment using a computer may provoke frustration in patients. 126 Many may experience ‘dexterity limitations associated with ageing despite having no formal diagnosis of conditions affecting motor control e.g. lack of familiarity with touch screen technology, manifested in frequent accidental screen presses’. 126
Patients generally want professionals to provide understandable information and manage expectations during the assessment process. In addition, patients want memory clinic staff to provide specific information about local services. 108 Information needs to be provided in an appropriate form for people with memory problems and their carers. Abley et al. 108 found that few patients could recall positive examples of direct communication between memory services and themselves, or their carers, such as letters that could be easily understood. Overall, the memory services appeared to have few mechanisms to enable patients to remain informed. In some cases, families were informed of the outcomes of the investigations before the patient. 108
Being told outcomes of the assessment
Patients and carers desire explanations of both positive results and uncertain or negative findings. The type and range of investigations people experience, including scans, blood tests and memory tests, form an important part of the assessment process for people with memory problems. Patients may feel that clinicians explain the results of scans well. 108 Other patients report non-receipt of test results, long delays in receiving them or having to contact different services to obtain results that appeared lost. Communication may be inconsistent, with mixed messages (i.e. being told different things by different people), which may increase anger and distress. 108 Patients particularly want to know what is wrong with them when tests do not reveal abnormal findings. 108 Some patients find it reassuring to be clearly told that they do not have AD, whereas others merely see this as delaying the inevitable. 131 One patient found it unsettling to be told several possibilities for her problems, namely AD, brain damage as a result of falls or ‘just wear and tear and getting old’. 108
Communication about the diagnosis is not always co-ordinated within multidisciplinary team members before being relayed to patients and families. 131 One carer had found it particularly traumatic to indirectly receive a diagnosis and, to some extent, this had also had an impact on her husband with memory problems. The couple had received a copy of a letter to a consultant that included a diagnosis of dementia without having previously been told the probable diagnosis. This led to considerable distress. 108
Mild cognitive impairment diagnosis
Mild cognitive impairment is characterised by the subjective experience of a decline from a previous level of cognitive functioning. Diagnosis of MCI requires objective evidence of impairment in performance on one or more cognitive domains relative to that expected given the individual’s age and general level of intellectual functioning. The cognitive impairment should not be sufficiently severe to significantly interfere with independence in the person’s performance of activities of daily living,9 but is not entirely attributable to normal ageing. Patients with MCI occupy a difficult middle ground where they often require assistance to maintain optimum function, but do not require enough help for it to be clearly classified as ‘caregiving’. 22 As discussed in Chapter 6, the use of cut-off points to distinguish between MCI and normal ageing may be problematic. For this reason, some authors argue that MCI should not be defined by performance below a cut-off point, but by evidence of decline from previous performance.
Studies of the communication of the diagnosis129,134 reveal that doctors commonly present a diagnosis of MCI as ‘not dementia’. Over three-quarters of doctors specifically name MCI, with almost a half of these doctors explaining MCI as a stage between ‘normal’ ageing and dementia. Where diagnosis is not named, doctors’ explanations focus on patient symptoms, often attributing these to vascular disease. 129
Emotional responses to a diagnosis of MCI range from the positive (e.g. relief that the diagnosis was not dementia) to negative (e.g. fear about the future). 22 Patients in all diagnostic groups face challenges in dealing with cognitive changes and with their changing self-identity. 127 Patients specifically with a MCI diagnosis face the challenge of causal attribution of cognitive decline, as well as anxiety and concern related to perceived decline. 127 Even where their cognitive state does not decline, patients still face anxiety, fearing that decline will occur.
People respond very emotionally, and often differently, to diagnosis, with responses that include sadness, anger, loss, rationalisation and humour. 131 The two most common responses to the diagnosis are worry and relief. 131 Many people who expect to receive a diagnosis of dementia or AD describe relief when this is not the case. One participant who had previously been told she had AD based on the result of her CT describes being ‘devastated, absolutely wiped out’ on hearing this and when she was later given the MCI diagnosis she was ‘over the moon’. 131
Following disclosure of a diagnosis, people with dementia develop diverse coping mechanisms from positive responses, such as feeling empowered, to difficulty in accepting the diagnosis and active denial. Some people respond to their diagnosis by seeing it as an opportunity to ‘reconsider their priorities and make positive changes, such as spending more time with family, focusing on positive tasks which bring enjoyment, undertaking future care planning and sharing the diagnosis with the outside world’. 56 People with MCI display varied coping strategies, being initially aware of their difficulties, but then, if progressing to dementia, losing this insight. The ability to cope and how to do so relate to how people perceive normal ageing and how they have previously experienced people with dementia. People find the label of AD more difficult to cope with than dementia or memory loss.
This sense of relief contrasts with previous widespread fear about the possibility of developing dementia or, specifically, AD. Other concerns may relate to brain cancer or other illnesses affecting cognition, after which being diagnosed with ‘a touch of Alzheimer’s’ may still be met with relief. 131 Nevertheless, many patients and carers continue to worry that MCI could develop into dementia. The MCI diagnostic label may fail to assuage anxiety given the similarities between MCI and dementia. However, other patients are reassured at the diagnosis. The relationship of MCI to dementia and AD may be interpreted in diverse ways. Some patients may see a diagnosis of MCI as implying no further danger from AD. Other patients may miss the mention of the term MCI all together, perhaps latching on to familiar lay terms, such as memory loss. 131 People with MCI and their families report struggling to deal with ongoing changes in relationships, with both parties fearing that the person with MCI could become dependent. 131
Patients struggle to make sense of the MCI label. 131 The term ‘cognitive’ is particularly problematic. Patients do not usually encounter the term outside their contact with medical and research professionals. However, it is not only the ‘cognitive’ part of the label that patients have a problem with, but describing their impairments as ‘mild’ fails to grasp the depth of their difficulties. 131 This phenomenon has been described as ‘not knowing’ about MCI. 15
Many patients question the MCI diagnosis, rating their own experience over ‘the abstract discourse of medicine’. 131 The ability to trust the doctor, or to rely on the ability of the doctor, is critical, with many patients believing that more is going on than the doctors uncover. Ruling out an AD diagnosis may be seen as a clinician’s strategy to mask something more serious or sinister, or to stop the patient from contemplating serious actions. The converse is that some patients believe that MCI is a form of overmedicalisation whereby a diagnostic label is inappropriately attached to the normal ageing process. However, not all patients react against the MCI label, as others trust the diagnosis because of their respect for clinical judgement. Such patients may also express a willingness to leave clinical issues in the hands of the clinicians so that they can get on with living their own lives. 58
A tension regarding medication sees some people expressing a desire for medication, whereas others are cautious of overmedicating. Medical advice may be solicited, not as a way to medically ‘treat’ memory loss, but to secure a sense that support is available to be called on as and when needed. People with MCI come to view clinical services not only as a source of appropriate treatments, but also as a way of receiving reassuring support as and when they need it. It is not unusual, in the absence of a definitive medicine to slow down or prevent cognitive decline, for people with MCI to draw on ‘self-management strategies to maintain cognition – crosswords, puzzles, “brain training” games, and diary keeping’. 23 Clinical services are, therefore, viewed very much as a back-up service, as persons with MCI take the lead on managing the changes they experienced. Patients may be provided with information, such as the NHS Health Check Dementia Leaflet,135 which is available in different languages and as a video.
Those with MCI are frequently not told or given written information about the clinic or service they are attending. Professionals may ask people with memory problems if they would like copies of the clinic letter sent to their GP, but this is not always actioned. 108 Some patients are told that they have MCI. 108 Although seemingly accurate from a professional perspective, on its own, without additional information or practical help, this may seem meaningless and not particularly helpful. 108 Patients may react by questioning whether or not their condition is a disease. 24 On diagnosis, patients struggle with the social implications of a potential future diagnosis of AD. 24 The resulting ambiguity may precipitate both social and psychological tensions. 24 Patients particularly resist attribution of their symptoms to AD, explained by the considerable stigma associated with that condition24 and this illustrates that a significant psychosocial impact is associated with how MCI is framed and stigmatised. 24 Subjective memory impairment can be more concerning than angina, asthma, hypertension or a previous heart attack.
Care plan
In MCI, the difficulties patients experience with their mental abilities, such as memory or thinking, are worse than would normally be expected for a healthy person of their age. However, the symptoms are not severe enough to interfere significantly with daily life. Many people with MCI remain stable or improve in cognition, but this condition does involve an increased risk of dementia.
Care plans should include strategies to help those with MCI to cope with practical problems, for example no longer being able to deal with finances and withdrawing their involvement in hobbies and other activities, as well as with difficulties in planning and spatial orientation. Those with MCI need assistance with activities of daily living. They not only need to be able to recognise their neuropsychiatric symptoms, but also need to develop coping strategies that help them to come to terms with their memory problems.
Memory retraining classes and memory strategy groups run by memory clinics are reported to have positive outcomes for patients and carers. 108 Where patients attend weekly sessions at memory sessions, they find information integrated within these sessions beneficial. 108 Memory clinics also offer opportunities to meet others ‘in the same boat’ in addition to providing practical strategies for dealing with memory problems. Written handouts provided at the clinics may be considered accessible and useful. 108 The source of the information can be important. Carers may contact voluntary organisations, particularly when services do not provide timely or sufficient information. Such information is found to be helpful, including ‘buddying schemes, carers’ leaflets and regular newsletters’. 108
It is considered good clinical practice to assess people with MCI on a yearly basis, provided that they consent to this. This should take place until:
-
a non-dementia cause is established
-
it has resolved, such as treated sleep apnoea (using screening tools such as the Epworth Sleepiness Scale or STOP-Bang)
-
the patient has been diagnosed with dementia.
Follow-up can occur in primary or secondary care, according to local commissioning. Some general practices keep their own MCI registers so that they can recall patients for their annual review and, if there are concerns, then a referral is made to the memory service. Efforts to embed review within each general practice may be facilitated by commissioners adding this to their QIPP (quality, innovation, productivity and prevention) workstream.
Exit pathway
Patients will continue to experience changing levels of insight and also to maintain differing perceptions of well-being in ageing. 127 Diagnostic labels can take multiple sources of complex information and reduce them to a label that explains the whole. 131 As Verhaeghe136 puts it:
. . . a name always carries with it [ . . . ] the illusion of control and mastery. Nothing is worse than not being able to name something; once a name has been found, it seems manageable.
Verhaeghe136
Relief at receiving a serious diagnosis has even been noted in studies of dementia. 56
Diagnosis of dementia
Making the diagnosis of dementia and conveying it to patients and carers is challenging. As discussed earlier (see Chapter 6), controversy surrounds whether or not everything between normal ageing and dementia (currently labelled as MCI) should be considered as prodromal dementia. 137 Negative connotations associated with dementia, inconsistent symptoms and not knowing enough about the signs and symptoms are commonly reported barriers to early dementia diagnosis. Being told one has dementia has a large impact on a patient’s identity and often causes feelings of loss, anger, fear and frustration. 22,57,108 Spouses have to adjust to increasingly unequal relationships and the transition to a role as carer. 22,108 Carers may be vulnerable to the strain associated with these relationships, often leading to their own health problems. 57 On the other hand, studies examining the experience of couples often report how they find ways to continue working together as a team.
Adjusting to a dementia diagnosis is a complex process. 57 Initially, patients and carers experience conflicts, such as a conflict between autonomy and safety, between recognising the need for help and reluctance to accept it, or between living in the present and dealing with anxiety about and preparing for the future. 57 Clinicians need to be able to identify the levels of emotional distress and difficulty in coping with the illness in people with dementia and their family carers in a timely manner, offering early referral for specialist psychological support. 56
Regardless of culture and context, individuals often share common experiences of becoming a person with dementia. Dementia has an impact on identity,57 leading to feelings of loss, anger, uncertainty and frustration. 22,57 People with dementia struggle to preserve aspects of their former self. Family carers may support them by focusing on the person’s abilities, rather than drawing attention to mistakes. 57 A desire to preserve a pre-dementia identity sometimes leads to people being reluctant to disclose their diagnosis,57 which could lead to social isolation. 57 Despite this, studies suggest that eventually individuals with dementia and their carers come to accept their situation. 57
Dementia has a significant impact on both the individuals with dementia and their families. Spouses have to recalibrate to increasingly unequal relationships57 and communication between the couple is often affected. However, studies looking at the experiences of couples often found an emphasis on working together as a team, with a high degree of mutuality. 57 Significant strain on carers may, subsequently, have an adverse impact on their own health. 57
Some individuals respond to receiving a diagnosis as the beginning of adjustment, whereas other individuals who have been experiencing symptoms for some time have already made considerable adjustment. Although diagnosis is commonly traumatic,57 the validation of suspicions can come as a relief. 57 Some individuals with dementia and their carers may continue to consider memory loss insignificant even after diagnosis. 57
Tensions and conflicts tend to resolve over time as the disease progresses. Patients and carers become more balanced and accepting of the dementia. Many patients and their families adopt strategies to cope with the impact of dementia on their lives to manage the disease and maintain a ‘normal life’. Practical strategies include use of reminders22 and social strategies may require relying on family support. Carers seek to maintain patients’ previous level of function and activity through encouragement and by planning activities together. 22
Some patients utilise emotional strategies, such as using humour. 57 At some point, patients and carers report that they are able to adopt positive mindsets and incorporate dementia in their lives.
Patients with a dementia diagnosis have an urgent need for support from outside the family, both immediately after diagnosis and subsequently. 57 GPs play an important role in helping patients and carers to gain access to information, social and psychological support, and community care. This ongoing need for information requires information from diverse sources and in varied formats. 108 Several studies57,108 attest to the need for patients and carers to access information on financial aids and entitlements early on. The studies also highlight a need for continued access to supportive professionals and specialists.
Families with a member who has experience of dealing with dementia as a health professional or with a relative with dementia are more likely to completely acknowledge a diagnosis than those with no previous exposure to dementia. 57 Adjusting to a diagnosis is complex. Several conflicts and tensions need to be resolved to accommodate a diagnosis. For example, studies identify the conflict that may arise as people strive to preserve identity and autonomy in the face of increasing symptoms. This sometimes leads to apparent unawareness of or resistance to acknowledging a diagnosis. 57 Although this might be interpreted as denial, it may be seen ‘as a self-maintaining strategy or a deliberate choice to be seen as an agent rather than an object’. 57 Although some people with dementia and their carers actively seek information,57 others reject new knowledge. However, understanding of and attitudes towards dementia are not fixed and can evolve throughout the disease trajectory.
The GP is generally the first point of contact for people with dementia and their carers. 57 GPs have an important role to play in facilitating service access. 57 Attending memory clinics could be shocking or frightening57 and receiving a diagnosis could lead to increased tension as someone negotiates a new identity as a person with dementia. 57 Barriers to memory clinic use could include transport access, funding, awareness and costs. 49
Patients express a clear need for greater support after diagnosis, including advice, social and psychological support, access to community care and respite. 57 Valuable support is provided by voluntary organisations, such as the Alzheimer’s Society. 57,108 Signposting to voluntary organisations needs to be improved. Information provision is seen as key in many studies, but better knowledge-sharing at the point of diagnosis is not always the solution. 57 The information needs of patients vary over time and information provision needs to be ongoing, offering flexibility in timing and format. 57,108
Many people find peer support valuable. 57 However, for others, there could be negative consequences, as the inclusion of people at different stages in the dementia trajectory could make people aware of what the future held for them. 57 The timing of referral to community-based support groups may be key. 57 Appropriate referral to such groups is more likely to occur within a continuous therapeutic relationship between the person with dementia and practitioners involved in their care.
Patients consistently express negative perceptions attached to a diagnosis of dementia. 127
In terms of receiving a positive diagnosis of dementia, some patients and carers appreciated honest and clear communication and the opportunity for follow-up discussion. Sometimes, however, the amount and timing of follow-up was thought to be inadequate, leading to distress. 108 When opportunities are provided, patients do not always know what questions to ask, especially immediately following a diagnosis. 108
Information along the pathway
People appeared to want information provision to be tailored to their needs and provided at an appropriate time. Most people wanted higher levels of provision (e.g. ‘knowing everything was better than not knowing’),108 as this helps to ‘plan and be aware of things that may happen. 108 For a minority, too much written information can be a problem, with a few being happy to receive none. ‘Staggered approaches’ to disclosure and information provision are relatively uncommon. 108 Where patients receive a diagnosis of AD and an offer of ‘antidementia’ medication, visits to their homes from nurses, although primarily to monitor medication effects, afford supplementary opportunities for communication, including emotional support or practical advice, as well as for discussing the diagnosis. 108
Written information about the different types of dementia is welcomed. Commonly, patients do not receive written information about their diagnosis, unless in the form of a booklet from a voluntary organisation. People wanting further written information are often carers of people with less common dementias. 108
The principal benefit to patients and carers of knowing what is wrong is in being able to choose whether or not to plan ahead. Practical advice about, for example, managing personal finances is welcomed. 60,108 Although some patients appreciate immediate practical advice, for others the benefits of such discussions are more emotional and help to develop confidence. 60 Those patients not receiving practical advice reported frustration, helplessness, loss of independence and autonomy, and an inability to assert control over their future. 60
Implications for pathways
Patients who have been diagnosed are less likely to be anxious and upset if they have had a previous opportunity to discuss the possibility of dementia as a diagnosis with health professionals. 108 In England, patients can access memory clinic services via a GP referral only. Although the pathway presents referral from GP as an interim stage on the way to assessment, it is clear that formal discussion between GPs and their patients with memory problems prior to referral should be seen as key in communication. 108 Information leaflets that outline what a patient and their family can expect from their local memory clinic are also helpful. Changes in the commissioning of services in England may enhance GPs’ roles in this area. 108 Commissioning guidance for diagnostic and early assessment dementia services has already emerged. 59 Although national guidance is useful in terms of setting evidence-based standards, local services will need to construct flexible diagnostic disclosure pathways. 108
Chapter 10 Review 1 discussion and conclusions
This section briefly considers the main findings of the descriptive review. Methodological issues and implications for service delivery and research are considered in Chapter 14. The review has synthesised evidence from over 100 studies, following a pathway from the fundamental concept of MCI through the process of screening and diagnosis to a description and evaluation of relevant services and pathways to patient and carer perspectives on the services available to them.
The concept of MCI as a state between normal ageing and dementia has been in use since before the period covered by the descriptive review. The conceptual studies section of the review indicates that the concept is understood by many clinicians as a pre-dementia (prodromal) condition related to AD or other forms of dementia. Although the amnestic form of MCI is strongly associated with progression to dementia, the diagnostic label of MCI is applied to people with a variety of underlying conditions whose cognitive status may remain stable or improve over time. Investigations and services offered to people with memory problems need to be considered against the background of the unclear and contested conceptualisation of MCI. The pros and cons of the diagnostic label from the patient and clinician viewpoints are considered in review 2, but it is clear that the label is valued and used by clinicians, whereas it is more problematic for patients and their carers.
The review included a large number of studies that covered the process of screening and diagnosis of MCI. Screening of older people without symptoms for memory problems is not recommended and so screening in practice (outside research studies) normally involves people seeking help from their GP. Screening in other settings, such as hospitals, may be justified, but we found only one study52 addressing this topic and it appears that the study was subsequently retracted. 53 Barriers to help-seeking are widespread and may affect different groups differently, resulting in disparities in access to services.
Investigation of memory problems in primary care is normally supported by cognitive testing. Evidence suggests that GPs may have difficulty recognising and recording memory problems using clinical judgement alone. A large number of cognitive tests have been evaluated, although diagnostic accuracy was outside the scope of this review. These cognitive tests include rapid tests suitable for use in primary care to identify people needing a more detailed assessment. Screening by GPs using cognitive tests may be a good use of resources73 and is likely to be of increasing importance with the development of disease-modifying treatments that may benefit people in the early stages of dementia.
Further investigation involves tests that are generally available at specialist centres only (e.g. MRI or PET and analysis of biomarkers in blood and CSF). The limited availability of such tests in the UK health system has been highlighted by recent expert guidance. 5 Other types of test have been evaluated in research studies. The investigation of MCI is complicated by potential overlap with at least two other recognised conditions (i.e. SCD and FCD).
Diagnosis or labelling with MCI is likely to need regular review, as patients may progress to dementia, remain stable or improve over time. This may involve follow-up in primary care or referral to a memory clinic/memory service. The services/pathways section of this review (see Chapter 8) highlights the wide variation in organisation and access to memory clinics in the UK and internationally. A key finding is that memory clinics are primarily commissioned to identify and support people with dementia, suggesting that different service models may be needed for people with MCI. The lack of an evidence-based population-level dementia prevention programme may be a barrier to distinctive services for people with MCI. Limited evidence supports the possibility of screening for memory problems alongside other services, such as ophthalmology or cancer clinics.
The available evidence allowed us to describe patient and carers’ experience of the investigation of memory problems in considerable detail, based on 30 included studies (see Table 14), covering both UK and international evidence. People with MCI interviewed for qualitative studies frequently portrayed their experiences prior to diagnosis in negative terms, for example ‘falling through the cracks’1 and ‘negotiating a labyrinth’. 60 Disclosure of a diagnosis, whether of MCI or dementia, may come as a shock to the patient, although a diagnosis of MCI may be tinged with relief that it is not dementia. Carrying a label of MCI has been described as ‘living with ambiguity’23 and ‘making sense of nonsense’. 24 These findings suggest a need for research and practice to make the investigation and management of MCI more patient centred. They also raise questions about the balance of advantages and disadvantages associated with the MCI label. This topic is investigated in depth in Chapter 11 using the techniques of CIS.
Chapter 11 Review 2: advantages and disadvantages of mild cognitive impairment as a diagnostic ‘label’ – critical interpretive synthesis methods
This synthesis builds on initial mapping work (see Chapter 2) that followed systematic review principles, namely in undertaking a robust and transparent searching strategy and explicit data extraction. Our decision to use CIS6 recognises the characteristics of a complex body of literature that combines qualitative and quantitative studies and spans multiple disciplines. MCI is associated with two principal narratives. The first narrative is driven by the quest towards medicalisation and the need for early diagnosis, lifestyle modification and, increasingly, disease-modifying therapies. 20,138 The second narrative asserts that unnecessary diagnosis is clinically unhelpful and ethically questionable and may, on occasion, result in premature, and even unwarranted, anxiety in the patient and their carers. 139,140
As well as starting from an open research question, five further activities are factored into the CIS process:6
-
The literature search uses a broad searching strategy (e.g. website search, reference chaining and contacting experts) to complement a structured bibliographic database approach.
-
Literature is selected based on likely relevance, including purposive selection with flexible inclusion criteria (i.e. not necessarily aiming to identify and include all relevant literature). Ongoing literature selection is directed by an emerging conceptual framework informed by theoretical saturation.
-
Quality appraisal is based on the content of the paper, its likely relevance and theoretical contribution to the CIS. Papers that are considered to be ‘fatally flawed’ may be excluded from the synthesis.
-
Data extraction for CIS demands constant reflexivity, with an ongoing critical orientation to the material by placing the literature within context. The construction of a theoretical framework utilises similar analytical techniques to meta-ethnography.
-
The concepts are constantly compared with the data to identify the relationships among them. The aim is to develop a synthesising line of argument that links constructs and the relationships between them within a coherent theoretical framework.
Protocol
The review was registered on the PROSPERO database (reference CRD42021232535). PRISMA guidelines have been followed in the reporting of this synthesis. In addition, recent observations on reporting from a subset of CISs have been factored into the methods and the documentation of the report. 141
Open research question
Critical interpretive synthesis typically starts with an open research question. For this study, we used ‘how are people with a cognitive impairment investigated to understand the underlying cause of impairment?’. The question was then refined into meaningful subquestions during progress towards a finalised question, which became apparent within the line of argument constructed by the end of the review.
Literature search
See Chapter 2.
Inclusion criteria
In addition to the inclusion criteria for review 1, studies included in the CIS had to meet the following criteria.
Phenomenon of interest
-
Early or timely diagnosis and referral to memory clinics for people assigned the label MCI.
Timing
-
‘Early’ versus ‘late’ diagnosis and its sequelae (i.e. early differentiation of MCI from normal cognitive ageing vs. later differentiation).
Study selection and data extraction
See Chapter 2.
Quality appraisal
For CIS, quality appraisal explores the likely relevance and contribution to the theory that is being developed. A form of triage took place to ensure that the included studies were specifically robust as an example of the type of study under consideration (e.g. a systematic review or a qualitative study). However, the subsequent synthesis attached emphasis to the likely relevance of items, but not to a consideration of relative rigour. This decision is compatible with interpretive forms of synthesis in which the contribution of an article to a line of argument is considered important, in comparison with the risk of gaps in the overall argumentation.
Formulation of a synthesising argument
A CIS was performed to identify unifying or explanatory themes emerging from the literature. Essentially, the CIS requires analysis and familiarisation with the studies, iterative exploration of the extracted data and consideration of the identified themes. Critical examination of the ways that the literature had constructed two competing narratives helped in constructing the research questions. The first narrative relates to the nature and adverse consequences of diagnostic uncertainty and the desirability of attaching a label for the patient, clinician and health service. The second narrative relates to the ethics challenges of offering a potential diagnosis when diagnosis is unclear, prognosis uncertain and disease-modifying therapies unavailable. Communication between clinician and patient and their carer was isolated as a central issue within the care pathway. Several studies of patient–clinician communication were prioritised in the analysis. 129,142–144 CIS offers a versatile tool for handling qualitative, quantitative and mixed research without excluding methodologically weaker studies of clear relevance. Within the framework of the two competing overall narratives, we utilised an inductive approach to identify recurring themes and develop a synthesising line of argument.
Chapter 12 Critical interpretive synthesis results
A summary of the study screening and selection process is seen in Figure 2. Characteristics of the included literature can be seen below. From 4126 unique citations retrieved by the initial search, we identified 29 papers for inclusion in the CIS. A further 11 papers were identified from follow-up of citations, including use of the scite web tool (scite, Brooklyn, NY, USA) for analysing citations in context. Collectively, this meant that 40 publications were included, comprising both empirical papers (i.e. quantitative, qualitative and mixed methods) and opinion-based papers, as well as consensus statements and guidelines informed by evidence.
FIGURE 2.
A PRISMA flow diagram for CIS (searches of databases and citation searching). 7 a, Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers); b, if automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
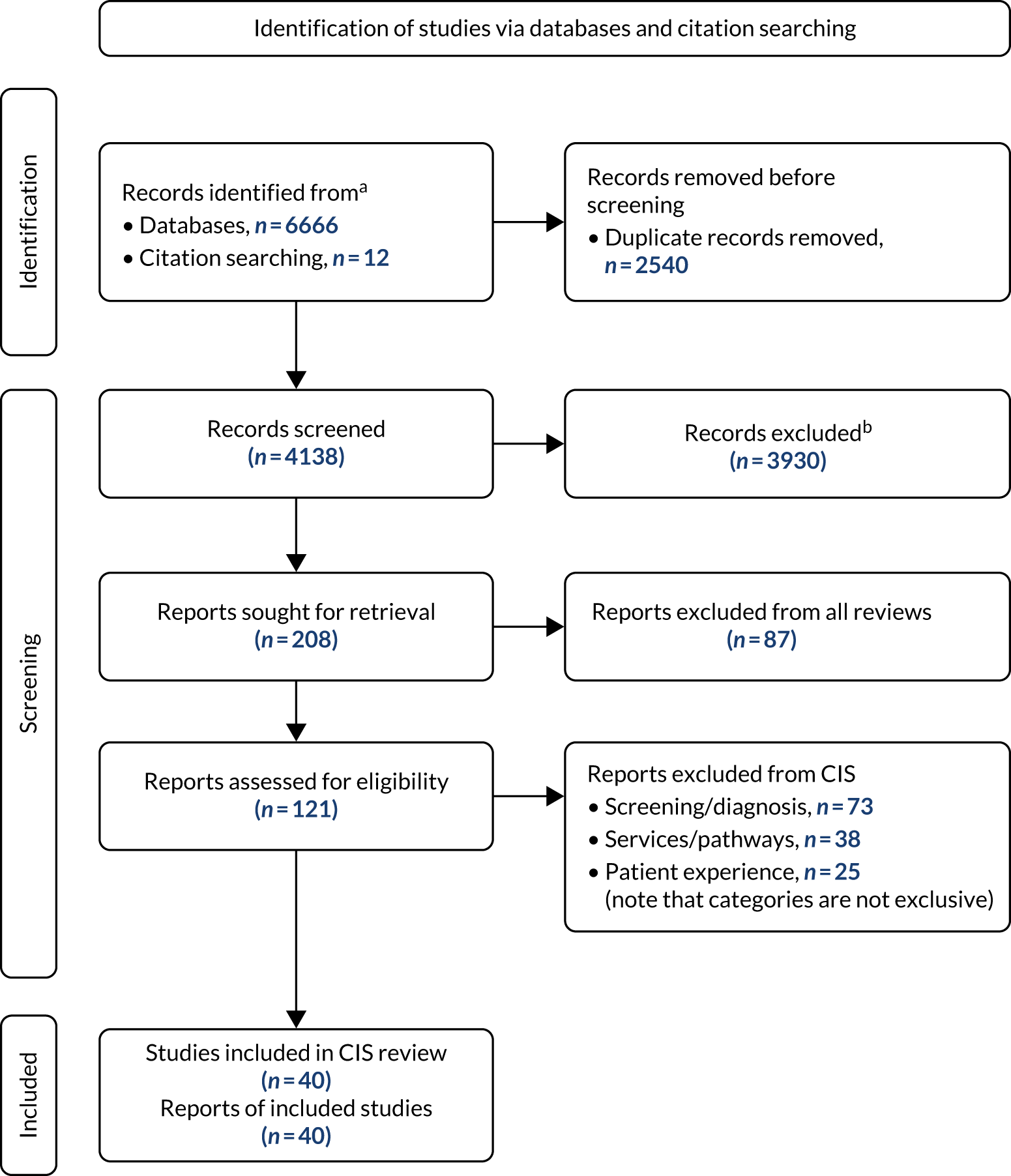
Characteristics of included literature
Table 16 shows details of articles included in the synthesis together with their methodologies. Forty publications were identified, including 10 qualitative studies, with a further three qualitative studies that used either conversation analysis or discourse analysis, two mixed methods studies, four surveys and one audit study. Only two quantitative studies were identified to inform the debate (a cross sectional study and a cluster-randomised controlled trial). We examined six expert consensus statements and two guidelines. In addition, we selected seven literature reviews, comprising three systematic reviews, one qualitative meta-synthesis, two scoping reviews and one unspecified literature review. The remaining two items represented editorial opinion contributions. All items exclusively related to high-income countries. The majority of papers (n = 19) originated from the UK, including three papers from Wales and one each from Northern Ireland and Scotland. Other studies represented the Netherlands (n = 4), the USA (n = 3), Canada (n = 2) and Germany (n = 1). Four studies were from Europe more generally and two were ‘international’. One of the European studies also included survey respondents from the USA and Canada and so was coded multiple times. The remaining seven items were literature reviews for which their coverage, not their geographic origin, is salient to this discourse (see Table 16).
| Study | Aims/objectives | Country of study | Article type, research methods, data collection and analysis | Key results |
|---|---|---|---|---|
| Ball et al.8 | Not stated | UK | Expert consensus | It may be easier to identify those who meet criteria for FCD than those with underlying AD pathology because of limited access and imperfect precision of current AD biomarkers |
| Bertens et al.54 | To investigate attitudes of clinicians in Europe on the clinical utility of MCI and prodromal AD/MCI, and to investigate whether prodromal AD/MCI has an impact on the management of MCI patients | Europe | Survey | Diagnostic criteria of MCI and prodromal AD/MCI due to AD are commonly used among EAN/EADC members. The prodromal AD/MCI due to AD were considered clinically useful and had an impact on patient management and communication |
| Brayne and Kelly13 | Not stated | UK | Opinion | Current evidence base and treatment options do not support screening for dementia, with little empirical evidence that intensive case identification and early diagnosis for dementia is justified |
| Brooker et al.35 | To produce a set of evidence-based recommendations on dementia for policy-makers | Experts from 24 European Union countries | Iterative process with patients, carers and professionals | ‘Timely diagnosis’ can help people with MCI and their families make sense of what is happening, make lifestyle changes and plan for the future. Principles to maximise benefit/reduce harm include reducing stigma about dementia |
| Canadian Task Force on Preventive Health Care27 | To provide evidence-based recommendations on screening for cognitive impairment in adults. Focuses on screening asymptomatic adults | Canada | Guideline | Recommends not screening asymptomatic adults aged ≥ 65 years for cognitive impairment. (Strong recommendation, low-quality evidence) |
| Cook et al.77 | To determine variation in London memory services and address this through service improvement projects | UK | Audit | Memory services should streamline pathways to reduce waiting times and implement pathways for people with MCI. Memory services should also work with commissioners and primary care to ensure that access to interventions is consistent with updated NICE dementia guideline |
| de Wilde et al.80 | To systematically review the literature on disclosure of amyloid PET in cognitively normal individuals and people with MCI in both research and clinical settings | Literature review | Systematic review | Before PET result disclosure for individuals without dementia in a research or clinical setting is ready for widespread application, research is needed about its psychological impact and its predictive value at an individual level |
| Dooley et al.129 | To identify how MCI is communicated and to explore the relationship with patient and companion understanding | UK | Conversation analysis | Increased risk of dementia was not discussed in half the diagnostic feedback meetings. Clear consistent communication, particularly about increased risk of dementia, may increase patient understanding and enable lifestyle changes to prevent some progressing to dementia |
| Dubois et al.145 | To comprehensively review existing scientific evidence on the benefits and potential challenges of making a timely diagnosis of AD | Literature review | Literature review (2000–June 2014) | Timely diagnosis potentially offers opportunities of early intervention, implementation of co-ordinated care plans, better management of symptoms, patient safety, cost savings and postponement of institutionalisation |
| Dunne et al.5 | To outline the need for national guidance on the use of neuroimaging, fluid biomarkers, cognitive testing, follow-up and diagnostic terminology in MCI | UK | Consensus statement | Cognitive testing, neuroimaging and fluid biomarkers can improve the sensitivity and specificity of diagnosis and may also help guide prognosis. Improved availability of disease-modifying therapies would require definitive diagnosis, but would present major challenges to the NHS |
| Erdmann and Langanke146 | To argue that the benefits of early AD detection in research outweigh the risk of potential adverse effects when studies are conducted with symptomatic people actively seeking support only | Germany | Opinion | Disclosing results can, at least initially, cause severe distress and harm. Study suggests a research ethics ‘principle of caution’ that supports a restrictive disclosure policy |
| Fang et al.25 | To explore the conceptual development of MCI and identify the resulting ethics, political and technological implications for the care of older adults with MCI | Literature review | Scoping review (1999–2013) | Reflects on conceptual, ethics and policy responses together with needs of older adults diagnosed with MCI to highlight opportunities for technological interventions to reposition MCI in the ageing care discourse |
| Frederiksen et al.84 | To survey the clinical practices of physicians in terms of biomarker counselling, management and follow-up in European expert centres diagnosing people with MCI | Europe | Survey | Clinical variation calls for better biomarker counselling and better training in communication skills. Future initiatives should address importance of communicating preventative strategies and advance planning |
| Giebel et al.130 | To investigate how South Asian people with self-defined memory problems, with and without GP consultation, construe the symptoms, causes, consequences and treatment of the condition | UK | Mixed-method pilot study | Perceptions of dementia varied by GP consultation for memory problems. A greater proportion of older adults without a consultation considered memory problems to be given by God, saw acceptance of fate as an alternative treatment and did not identify medical support as appropriate. Consultation for memory problems appears linked to physical health problems and mental health consultation (depression) |
| Gomersall et al.23 | To examine the published qualitative literature on experiences of being diagnosed and living with MCI using meta-synthesis as the methodological framework | Literature review | Qualitative meta-synthesis | Two overarching conceptual themes:
|
| Gomersall et al.131 | To understand the perceived benefits and drawbacks of a MCI diagnosis from the perspective of those living with the label | UK | Semistructured interviews | There is a need to clarify how clinicians and patients communicate about MCI, and how people can be helped to live well with the label. The emotional impact of a MCI diagnosis is complex and raises conflicting and fluctuating emotions, most notably worry and relief |
| Guzman et al.18 | To explore the influence of illness perceptions and cognitive fusion on coping and emotional responses in a sample of people diagnosed with MCI | UK | Cross-sectional study of 34 participants with MCI | Data suggest multiple potential treatment targets in helping people with MCI to successfully adapt and adjust. Targeting appraisals (illness perceptions) using cognitive therapy is one potential treatment target |
| Hagan147 | To examine how social support is promoted in the diagnostic process | Northern Ireland, UK | Qualitative study | Respondents reported both positive and negative experiences of diagnosis. Explicit links to navigators or other services at the point of diagnosis need to prioritise information regarding opportunities for social engagement for those being diagnosed |
| Hughes et al.55 | To explore issues that arise in connection with a ‘diagnosis’ of pre-clinical dementia | Literature review | Scoping review | The study discusses stigma, ethics issues, psychological burden, language and issues of meaning, medicalisation, virtues and values. The study suggests a need for biopsychosocial and ethics research to understand these issues |
| Jenkins et al.16 | To determine how much is known about SCI and how it is currently managed in specialist clinical practice in the UK | UK | Qualitative service evaluation using e-mail questionnaire | Analysis revealed (1) factors that influence what action is taken when an individual presents and what further investigations are performed, (2) multiplicity of potential outcomes and (3) barriers clinicians face. The study suggests a need for a coherent, consistent framework for managing SCI |
| Judge et al.40 | (1) To understand clinical practices/barriers related to the diagnostic process for patients with suspected MCI or AD and (2) to evaluate how primary care physician perspectives differ from specialists | Europe, Canada and USA | Online survey | Major themes included patients seeing cognitive decline as a normal part of ageing and not disclosing symptoms, long waiting lists, and a lack of disease-modifying therapies and definitive biomarker tests |
| Livingston et al.148 | To call for nations and individuals to be ambitious about preventing dementia, and to lay out a set of policies and lifestyle changes to help | International | Statement of expert: Lancet Commission on Dementia | Update to the 2017 Lancet Commission. Expands modifiable risk factors from 9 to 12 to include head injuries, excessive alcohol consumption and exposure to air pollution. Forty per cent of dementia cases could be prevented or delayed by targeting these 12 modifiable risk factors |
| NHS London Clinical Networks70 | To provide commissioners and clinicians in memory services and primary care with guidance on appropriate pathways for patients who present with memory complaints due to non-dementia causes | UK | Regional guidance | The London Dementia Clinical Network reviewed pathways for non-dementia diagnosis and produced guidance aimed at commissioners and clinicians within memory services and primary care |
| Peel142 | To look at how dementia-related diagnostic information is actually verbally communicated and whether or not the absence of explicit terminology is problematic | UK | Conversation analysis | Diagnostic communication, which is sensitive and responsive to the patient and their carers, is not predicated on presence/absence of particular lexical choices. How diagnostic information is communicated is sensitive to problems associated with ‘insight’ in terms of delivery and receipt or non-receipt of diagnosis |
| Pierce et al.15 | To identify through discourse analysis how people with a diagnosis of MCI used language to reveal societal views and shared meanings of the diagnosis, and the positions taken by people | Wales, UK | Discourse analysis | In addition to ‘not knowing’ about MCI, participants went on to position themselves between ‘knowing’ about ageing and dying and ‘not wanting to know’ about dementia. Clinicians must consider how information is presented to people about MCI, including where MCI is positioned in respect to normal ageing and dementia |
| Poppe et al.1 | To explore how services respond to people with memory concerns currently, and how a future, effective and inclusive dementia prevention intervention might be structured for people with memory concerns | UK | Qualitative interviews | The study identified three main themes:
|
| Roberts and Clare58 | To focus on (1) what the psychological impact of living with the symptoms of MCI is and how do people with MCI cope with this; (2) how awareness of symptoms influence the experience of MCI and (3) how people with an MCI label describe their difficulties | Wales, UK | Qualitative interviews | The study proposes an exploratory model with a dominant theme of ‘fear and uncertainty’. Themes indicate that MCI symptoms are perceived as a threat to psychological well-being, which results in context-specific appraisal of the symptoms of MCI |
| Rodda et al.19 | To investigate current practice for, as well as familiarity with and attitudes towards the concept of, MCI among UK old age psychiatrists | UK | Anonymised postal survey to clinicians on Royal College of Psychiatrists Old Age Psychiatry register | Only 4.4% of old age psychiatrist respondents thought that the concept of MCI was not useful. Eighty-two per cent of respondents required no or minimal impairment in activities of daily living to assign a diagnosis of MCI. Clinicians find MCI a useful term to conceptualise the transitional stage between normal ageing and dementia |
| Sabbagh et al.71 | To summarise a conceptual framework and provide guidance to researchers, test developers and suppliers to inform ongoing refinement of cognitive evaluation | USA | Expert statement | The study acknowledges that cognitive screening by default is not recommended and proposes large-scale evaluation of individuals with a concern or interest in their cognitive performance. Such a strategy can increase likelihood of timely and effective identification and management of MCI |
| Sabbagh et al.72 | To explore the potential of direct-to-consumer tools, as they relate to cognitive evaluation at home | International | Expert statement | Direct-to-consumer tools offer potential to sidestep barriers associated with cognitive evaluation in primary care, therefore, improving access to cognitive assessments |
| Sabbagh et al.122 | To provide guidance to developers of cognitive tests and tools to facilitate transition towards globally accessible cognitive screening aimed at early detection, diagnosis and management of MCI due to AD | International | Expert statement | The working group developed consensus perspectives on new algorithms for large-scale screening, detection and diagnosis of individuals with MCI within primary medical care delivery. The expert panel also addressed operational aspects of the implementation of unsupervised at-home testing of cognitive performance |
| Samsi et al.60 | To explore the experience of the assessment and diagnostic pathway for people with cognitive impairment and their family carers | UK | Qualitative interviews with people with MCI and carers using four memory services | Service providers should review assessment and diagnosis disclosure processes for people with MCI and their carers and develop a process that is person centred and accommodates individual preferences |
| Smedinga et al.149 | To critically review the arguments in favour of or against AD biomarker testing in people with no cognitive impairmentor MCIand to explicate their underlying moral values | Literature review | Systematic review using qualitative data-analysis within an ethics framework | The right to know, which derives from the moral value of respect for autonomy, is a central argument in favour of biomarker testing. Although AD biomarkers may have value for research, advantage for clinical practice appears limited |
| Swallow150 | To examine the ways in which the hopeful promissory claims of early diagnosis, as it maintains the dominant biomedical model for managing AD, are negotiated by health-care practitioners | UK | Qualitative research | Early diagnosis has the potential to ‘close off’ hopeful promissory visions of the future in two ways:
|
| Swallow20 | To examine practitioner accounts of the complexity associated with constructing the boundaries around MCI, AD and age in the clinic | UK | Qualitative data gathered in outpatient memory service | Practitioners utilise uncertainty by classifying people with MCI to keep them on for review to account for the possibility that patients may go on to develop AD, but they also recognise the difficulty in predicting future progression to AD |
| Tromp et al.151 | To explore the ethics considerations that shape current clinical practice regarding early AD diagnostics and the use of biomarkers | The Netherlands | Qualitative study | Identifies six clusters of considerations that influence physicians’ diagnostic decision-making: (1) preferences and characteristics of people, (2) test characteristics, (3) impact on care, (4) type of setting, (5) disease concepts and (6) issues on a societal level |
| van den Dungen et al.46 | To assess the effect of a two-component intervention of case-finding and subsequent care on these outcomes | The Netherlands | Cluster-randomised controlled trial | The study found a non-significant increase in the number of new MCI diagnoses. A larger, more highly powered study is warranted |
| Vanderschaeghe et al.152 | To consider ethics issues related to the process of biomarker testing and the impact on the diagnostic disclosure to people with MCI due to prodromal AD | Literature review | Systematic review of the theoretical bioethics literature (2003–16) | Challenges include the uncertainty and predictive value of the biomarker-based diagnosis where patients can be amyloid positive without full certainty of if and, if so, when they will develop symptomatic decline due to AD. Another challenge was tension between right to know and wishing not to know |
| Visser et al.143 | To examine, using both quantitative and qualitative methods, uncertainty communicated by memory clinic clinicians in postdiagnostic testing consultations with patients and their caregivers | The Netherlands | Observational study with quantitative and qualitative data | Most clinicians openly discussed the limits of scientific knowledge and diagnostic testing with patients and caregivers in the dementia context. Knowledge can support clinicians to optimally convey uncertainty and facilitate patient uncertainty management |
| Visser et al.144 | To explore clinician communication, including discussion of diagnosis, cause, prognosis and care planning, in routine postdiagnostic testing consultations with people with MCI | The Netherlands | Thematic content analysis of audio-recorded consultations | Clinicians (1) differed in how they informed patients about the MCI label, (2) tentatively addressed cause of symptoms, (3) (implicitly) steered against further biomarker testing, (4) rarely informed patients about their risk of developing dementia, (5) often emphasised potential symptom stabilisation/improvement and (6) did not engage in conversation on long-term (care) planning |
What is known about the communication of diagnostic uncertainty in mild cognitive impairment?
The clinician perspective
Thirteen studies1,16,19,20,40,54,84,129,142–144,150,151 described the clinician perspective solely,16,19,20,40,54,84,150,151 within mixed groups of stakeholders1 or in focusing on patient–clinician communication. 129,142–144 The detection of cognitive impairment at the MCI stage is considered by some to be clinically useful in that it allows for better communication between doctors, patients and caregivers as a starting point for a care and treatment plan. 54 Non-pharmacological interventions at the MCI stage have been shown to stabilise or even improve patients’ cognitive functioning. 153 However, little is known about how physicians who manage patients with MCI carry out biomarker counselling or how the results and consequences of biomarker sampling are communicated to patients. 143
Clear and consistent findings were identified across the set of studies. In the UK, the label of MCI has become a feature of everyday clinical practice. Clinicians find MCI a useful term to conceptualise the transitional stage between normal ageing and dementia. 19 In practice, the label of MCI offers little explanatory power. 28 Clinicians find it challenging to communicate the meaning of MCI to patients and their carers. Commentators identify a need to clarify how clinicians and patients communicate about MCI and how people can be helped to live well with the label. 131
Some clinicians choose not to discuss the relationship of MCI with dementia and AD with their patients, whereas others use analogies from other diseases or situations. 151 Collectively, doctors draw on heterogenous definitions that variously clarify or confuse the patient’s understanding. Technically, some clinicians suggest the utility of other labels, such as FCD,8 but these do not seem to address the fundamental communication issue that underlies the condition. Particularly problematic are (1) MCI may or may not progress to dementia,131 (2) reliable and definitive biomarker tests are not available in routine clinical practice40 and (3) aside from lifestyle-modifying changes that rely on early intervention,148 disease-modifying therapies are not available. 62
Even the most widely accepted guidelines leave the clinician with considerable uncertainty in their subjective assessment of MCI and AD. This uncertainty is not limited to differentiating MCI from dementia. Even with a fairly certain clinical diagnosis of MCI, predicting the future prognosis of the underlying abnormality remains uncertain. 131 In clinical practice, such prognostic uncertainty154 creates additional worries and anxieties in the patient and their families.
An increasingly recognised group of people are those who have been diagnosed with MCI or early dementia, but actually have functional cognitive disorder. This is characterised by complaints of cognitive impairment that are internally inconsistent, last for prolonged periods of time and fail to respond to reassurance. 155 This diagnosis represents the most likely explanation for many individuals who have received a dementia diagnosis, but show no discernible deterioration in cognitive functioning or disability over several years. 155 A substantial proportion of individuals with MCI will later return to normal cognitive function or maintain stable cognition, rather than showing progressive deterioration. 8
In practice, there is variability in diagnostic practice. Cognitive screening by default is not recommended. Some observers suggest large-scale evaluation of individuals with a concern or interest in their cognitive performance. Disclosure of PET results requires further exploration of its psychological impact and its predictive value at an individual level. 80 In addition, this practice also requires the further development and evaluation of communication resources and strategies to support disclosure of PET results. 80 Clinicians report that increased risk of dementia was not discussed in half the diagnostic feedback meetings. 129 Service providers should review the assessment and diagnosis disclosure process for people with MCI and their carers. 60
Clinicians do, however, consistently find it useful to have diagnostic criteria for MCI and prodromal AD/MCI. Only 4.4% of respondents thought that the concept of MCI was not useful. 19 However, the belief that the current diagnostic criteria for MCI are useful may fail to recognise the role of clinical expertise and experience in enhancing the value of these criteria. Seventy-nine per cent of clinicians required a memory complaint from the patient/informant for a diagnosis. 19 Most clinicians do not operationalise a specific cut-off point on cognitive testing. Eighty-two per cent of clinicians required no or minimal impairment in activities of daily living for a diagnosis of MCI. 19 Generally, clinicians consider these diagnostic criteria as clinically useful, with the potential to impact on patient management and communication. 54 Clinically, diagnosing MCI is helpful as a way of facilitating communication between health professionals. 131 However, clinicians display considerable heterogeneity in the definition, cause and likely prognosis of MCI presentations. 129
Patient perspective of the label
When the clinician is uncertain, patients and their families remain uncertain and worried. 131 ‘Making sense of nonsense’ generated from confusing or partial information from medical providers was identified as a major theme in qualitative studies of persons with MCI. 23,131 Ambiguity and uncertainty surrounding the diagnosis provoke many patients to desire more information about their condition, such as how it differs from ‘normal’ ageing and how it differs from dementia. 23 Patients specifically express a threat of the unknown when they, or a care partner, notice memory problems for which they (1) have been offered no clear explanation and (2) harbour uncertainty about addressing. 156 Wide-ranging benefits to the patient, the family and caregivers, and to resources and services, in diagnosing dementia earlier are suggested to far outweigh the concerns. 157 It has also been suggested that ‘catastrophic’ reactions to the diagnosis of dementia from individuals are relatively uncommon. 157 Receiving information regarding disease prognosis and treatment planning can induce a sense of relief in patients by changing a group of unknown symptoms into a known condition. 131 Therefore, delivering educative content on the condition is believed to have a stress-lowering effect and improve neuropsychiatric symptoms. 158
For patients, a diagnosis of MCI can profoundly affect a person’s understanding of their place in the world. 23 Patients construct various explanations for memory problems. Single studies130 found that some older adults from South Asian ethnic backgrounds may consider memory problems to be given by God, see acceptance of fate as an alternative treatment and/or they may not identify medical support as appropriate. How people respond depends on the availability of their social support networks, which daily activities are affected and what people understand MCI to mean. 131
Patients particularly face a challenge from experiencing the emotions of fear, anxiety and uncertainty. 20 The emotional impact of a MCI diagnosis is complex and the patient may fluctuate between worry and relief. 131 ‘Fear and uncertainty’ may trigger such coping responses as ‘interdependence’, ‘life goes on as normal’ and ‘disavowal of difficulty’. 58 Some patients see cognitive decline as a normal part of ageing and do not disclose their symptoms. Some patients choose ‘not knowing’ about MCI. Other patients position themselves between the familiar discourses of ‘knowing’ about ageing and dying and ‘not wanting to know’ about dementia. 15 Clinicians face having to consider how they present information to people about MCI, including how they position MCI in relation to normal ageing and dementia. Patients tend not to use the term MCI, suggesting that the label holds little meaning for them. 159 Nevertheless, patients express a need for a definitive explanation of the difficulties that they experience. 58 MCI symptoms are perceived as a threat to psychological well-being and so patients respond with a context-specific appraisal of the symptoms of MCI. 58
Uncertainty is seen in the need for ‘living with ambiguity’, which describes the difficulty that people experience in making sense of their diagnosis. 23 Not only does this uncertainty relate to a lack of clear and consistent information received by people with MCI,23 but, in some cases, whether or not patients are even told that MCI is the diagnosis. 23 Patients often felt that they were without support to manage their uncertainties and emotions and expressed difficulty at not knowing where to turn. 60 More evidence is needed on the beneficial and/or harmful effects on patients of discussing uncertainty. 143 Knowledge can support clinicians to optimally convey uncertainty and facilitate the management of patient uncertainty. 143
Patients and family members commonly report that they believe that symptoms of cognitive decline are not an illness, but an inevitable part of normal ageing. 13,40 For this reason, dementia diagnosis usually occurs after the disease has progressed and the patient is at least partially dependent on a caregiver, causing multiple unmet needs for patients with dementia and their families. 160 If patients do not realise that it is a disease, then they are unable to determine what cognitive tools are needed to delay the consequences of dementia and if this is the eventual prognosis. 23 Timely diagnosis could support patients and caregivers in optimal management of the disease. Patients diagnosed with MCI were relieved at not being diagnosed with AD or brain cancer. 23
Patients were highly critical of the process of assessment and diagnosis disclosure, but generally positive about the practice of individual professionals. 60 All stakeholders, including people with MCI and their carers, should be involved in development of services to provide continuous relevant information and clarity to service users. Service providers need to develop a process that is person centred and accommodates individual preferences. 60
Ethics issues associated with communicating (or not communicating) diagnostic uncertainty
A MCI diagnosis leads to an ethics tension. 23 Earlier support and services afforded by a diagnosis may come at the expense of anxiety about the future, with continued uncertainty about how concerns and needs can be addressed. 23 Despite emerging prognostic studies, people with MCI continue to live with significant uncertainty. 131 Fear of the disease and lack of a disease-modifying treatment may result in a negative balance of good over inflicted harms, which argues against its use. 149 There remains a need to differentiate between what we hope or expect from research and where we currently stand. 149 Although acknowledging that many more ethics issues exist, Fang et al. 25 identifies the following seven specific ethics issues:
-
Social implications arise from a MCI diagnosis, with little known about the ‘subjective and psychological impact of receiving an MCI diagnosis’, making it ‘difficult to establish whether the benefit of having a diagnosis outweighs the potential harm’. 25
-
There is a lack of a clear and adequate understanding of the consequences of a diagnosis of MCI when seeking to maintain personal freedom to make one’s own decisions, work, drive and maintain personal and family relationships.
-
There is a tendency of health-care providers to try to avoid diagnostic disclosure of dementia or AD to patients, families and caregivers, providing ‘the less threatening and more palatable disclosure of MCI’25 and, therefore, inadvertently hindering appropriate treatment for the more serious conditions.
-
There is an ongoing ‘medicalization of underperformance’25 in old age through inclusion of the symptomatic predementia phases (i.e. MCI) alongside the dementia phases.
-
There is increasing evidence from qualitative studies of MCI of significant levels of fear, suffering and uncertainty in the lived experience of MCI in a ‘hypercognitive society’25 and a paucity of treatment options currently available to treat these aspects of MCI.
-
The testing of biological agents on people who are asymptomatic but considered at risk for future MCI is ethically questionable and effectively turns ‘people into patients in the absence of demonstrated health issues’. 25
-
The value of identifying individuals at risk for dementia as opposed to furthering knowledge of MCI itself (i.e. whether or not it is ethically sound to continue to focus on MCI only as a precursor, prodromal stage of dementia) is questionable. Critics contend that the focus should be placed on MCI as ‘a multifaceted and heterogeneous condition in its own right’,25 which may or may not be related to progression to dementia. 161
Ethics issues extend beyond diagnosis and treatment to include issues regarding research. 55,146
Service-level issues
Diagnosis can be important for patients who need support and education. 131 In terms of service-level responses, commentators suggest a need to identify an alternative pathway that does not specifically lead to dementia. 90 People express frustration at long waiting lists and a lack of treatment options. People experience a discordant response between medicalising memory concerns and situating responsibilities for their management with patients and families. 1 Effective dementia programmes must be evidence based,1 remain sufficiently flexible to allow new activities to be fitted into people’s current lives and be mindful of risks of pathologising memory concerns. 1
Current memory services are neither commissioned nor financially and clinically resourced to support people with memory concerns without dementia. 1 Referral rates in the UK are already high and are expected to rise further. Many countries are seeing increased referrals to memory clinics and other specialist services and this is partially due to a raised awareness of the benefits of an early or ‘timely’ diagnosis35 for accessing health and social care interventions, enabling future care planning and increasing the efficacy of both drug and non-drug treatments. 162 Delayed diagnosis of dementia is common among older people from culturally and linguistically diverse backgrounds, as they tend to present to health services at a later stage of the disease. 163
Clinicians may react to the uncertainty by labelling patients with MCI and seeing them in memory clinics for review. This allows for the possibility that patients may go on to develop dementia, but also recognises the difficulty in predicting future prognosis. The process is not only about managing uncertainty. Decisions should also take into account the wider social and political context in which ageing and cognitive deterioration occurs. 20
Memory services should streamline pathways to reduce waiting times and implement pathways for patients who do not have dementia. 77 Memory services should also be monitoring the appropriateness of neuroimaging and be working with commissioners and primary care to ensure that access to post-diagnostic interventions is consistent with the updated NICE dementia guideline. 77 The London Dementia Clinical Network has reviewed pathways for non-dementia diagnosis and developed guidance aimed at commissioners and clinicians within memory services and primary care. 70 However, large-scale dementia prevention requires a broad societal response. 1
Emerging evidence also suggests that early detection of MCI may provide an economic benefit to health-care systems. 122 Evidence suggests that the financial burden associated with caring for MCI patients is significant, but less than the financial burden for patients who have developed AD. 164 Economic evaluations suggest that routine cognitive assessment may be cost-effective. 73 Additional research is needed to further understand the economic benefits of early detection of MCI. In the meantime, existing literature suggests that health-care systems may derive significant benefits from implementing early detection practices. 122
Many countries utilise a stepwise case-finding diagnostic strategy. 122 This strategy consists of non-specialist screening in a primary care setting and, if positive, the patient is referred to a secondary care service for a full evaluation. 165 If the evaluation is negative, then the patient is generally referred to a subsequent follow-up screening at a later date. Patients with mild to moderate dementia unidentified at the first screening are likely to request a new referral a very short time later,165,166 as a result of the significant cognitive and/or behavioural disturbances that continue to cause distress to themselves and their families. 165 On the other hand, patients going undetected on first screening might not feel the need for a new referral for a long time because of subtle or selective disturbances. 165 Therefore, an inaccurate first detection can become the worst enemy of a timely diagnosis. Missed diagnosis is, therefore, likely to incur the loss of potential benefits for patients (e.g. improving quality of life), caregivers (e.g. delay of appropriate care plans), health-care professionals (e.g. delayed information provision to patients and families) and society (e.g. reducing health-care costs). 165
Commentators have suggested an intermediate ‘1.5 stage of full detection’165,166 where a ‘frontline’ dementia specialist (i.e. a behavioural neurologist, a neuropsychologist, a geriatrician, an old age psychiatrist or an advanced practice nurse) would work alongside a primary care doctor, reviewing data from the first screening of all negative cases and supplementing these with clinical evaluations of the patient’s cognitive, affective and behavioural status. 165 This new model requires shared spaces (e.g. district memory clinics) and conversion of some specialists to full-time consultants for primary care services. 165 Potentially, this model seeks to achieve cost-effectiveness, simultaneously reducing the number of missed first detections of prodromal dementia or MCI and saving the high cost of full assessments provided in specialist settings. 165,166
Direct-to-consumer tools offer the potential to sidestep barriers associated with cognitive evaluation in primary care, therefore improving access to cognitive assessments. 72 However, the health service does not yet have risk-averse strategies in place to handle most types of consumer self-diagnosis and, for that reason, cannot be seen to encourage use of such approaches. Although direct-to-consumer cognitive assessment is associated with barriers, including test validation, user experience and technological concerns, these issues can conceivably be addressed so that a large-scale self-assessed cognitive evaluation as an initial cognitive screen may become feasible. 72 Large-scale screening, detection and diagnosis of individuals with MCI within primary medical care delivery could conceivably be supplemented by implementation of well-managed at-home testing of cognitive performance. 122
Timely diagnosis
‘Timely diagnosis’ is increasingly emerging as the preferred term in connection with the pathway to AD. 35,145,167 This concept acknowledges that patients who display MCI do not share a common point at which diagnosis is best made. Instead, ‘timely diagnosis’ requires intervention at an individual patient-defined point that allows the patient and their carer to undertake mitigating changes in lifestyle and/or to make preparations, should they choose to do so, for future activities of living and financial arrangements162,168 in anticipation or in advance of a worsening of their symptoms. 145 It is claimed that timely intervention facilitates the early initiation of treatment, including pharmacological and psychosocial interventions, such as cognitive stimulation therapy. 142 Studies169 have demonstrated that timely intervention can delay admission to nursing homes and increase the time to dependency. Timely diagnosis here reflects ‘access to diagnosis at a time when people can use this information to make sense of what is happening to them, make lifestyle changes and plan for the future’. 35 What constitutes timely diagnosis may, therefore, change as new information or treatments become available. 145,170 Timely diagnosis of dementia is recommended throughout Europe, as it maximises autonomy, including the ability to make future choices while patients still hold decisional capacity. Discussing MCI with patients and their care partners might provide relief, enabling both to label the patient’s condition. 131
‘Timely diagnosis’ is seen to help people with MCI and their families make sense of what is happening, make lifestyle changes and plan for the future. 35,145 Concerns relate to the need to reduce stigma about dementia and respect the rights of the individual. In particular, the right to know, which derives from the moral value of respect for autonomy, is rehearsed as a central argument in favour of biomarker testing. 149,152 However, the right to know is also seen to contend with the wish not to know. 152
Timely diagnosis potentially offers opportunities for early intervention, implementation of co-ordinated care plans, better management of symptoms, patient safety, cost savings and postponement of institutionalisation. Timely diagnosis further adds the possibility of patients with MCI participating in trials with potentially disease-modifying therapy. For this reason, patients may want to have the opportunity to participate in support groups, as well as an opportunity to access counseling on how to mitigate the risk of progression. 84
Patients were pleased to be able to call on clinical support, but were frustrated at the lack of available ‘treatments’ and were often anxious to slow down cognitive decline. Barriers to timely diagnosis included stigma, suicide risk, lack of training, diagnostic uncertainty, shortage of specialised diagnostic services and the reluctance of health-care providers to make a diagnosis when no effective disease-modifying options are available. 145
An early diagnosis occurs when individuals retain autonomy to self-manage their health. Following diagnosis, most individuals continue to reside in the community, leading active lives and making their own decisions. 171 An early diagnosis can offer a concrete answer for enquirer uncertainty over distressing symptomology. Pharmacological treatments are considered at their most effective during early stages. 147
An early diagnosis is not necessarily timely, as some patients feel unprepared for the diagnostic burden when memory problems first arise. 13 Timeliness relates to when the enquirer wants to seek help. 145 Diagnosing early may exert undue pressure on already stretched resources13 and so an appropriate time for diagnosis may align with perceived eligibility for services. 172 Timeliness was interpreted to mean ‘as soon as possible’ by 92% of Australian respondents. 171 Timeliness is dependent not just on practitioner expertise, but also on the duration individuals and families take to identify cognitive problems, commonly 2–3 years from the onset of symptoms, by which time significant deteriorations may have occurred. 147 Diagnosis at a younger age may take substantially longer. 173 This delay may reflect greater reluctance to prescribe dementia as the source of symptoms.
Individuals may fear diagnosis because of uncertainties about the condition. Individuals may hold perceptions that nothing can be done to help. Being diagnosed can be stigmatising, intrusive and accompanied by significant losses, such as employment. 174 Timeliness and earliness should converge to facilitate access to appropriate treatment and support. 145 Optimally, this timeliness and earliness should give individuals and their families time to comprehend what is happening and to make plans for future care and financial management. 145 As early diagnoses, by definition, occur when individuals retain control and capacity, clinicians should encourage opportunities for meaningful social engagement. 175
Another priority involves recognising that how diagnosis is communicated can have an impact on subsequent adjustment and the need for postdiagnostic support. Clear and consistent communication, particularly about increased risk of dementia, preventative strategies and advance planning, may increase patient understanding and enable lifestyle changes to prevent some patients progressing to dementia. 23,129 Specifically, clinicians need to be equipped to deliver better biomarker counseling, including referral to support groups. 84 Consideration of ethics issues are required before disclosure of a biomarker-based diagnosis to the patient. 152 Discussion concerning an early AD diagnosis based on biomarkers has not entered clinical practice structurally. 151 Clinicians have been found to implicitly steer patients away from further biomarker testing. 144 Clinicians should be encouraged to discuss difficult but important issues, such as driving and long-term care planning. 85
Prevalence of tensions
Although the main tension being considered is between early and late diagnosis of MCI, numerous other stresses were surfaced by this analysis. Patients may differ in how they react to diagnosis. Some patients view the diagnosis of MCI as an early opportunity to get their affairs in order, whereas others see this as unwelcome news and either go into denial or respond by just focusing on the here and now as a planned coping strategy. Earlier support and services afforded by a diagnosis may come at the expense of anxiety about the future, with continued uncertainty about how concerns and needs can be addressed. 23 Ethically, this may present a tension between right to know and a wish not to know. 152
Future perspectives
The relationship between these tensions continues to change as disease-modifying therapies become increasingly available, requiring a definitive diagnosis, but presenting major challenges to the NHS. Investment is required in training, infrastructure and provision of biomarkers and neuroimaging. 5 A combination of multiple biomarkers may offer greater sensitivity and specificity than any single disease marker, but their practical usefulness depends on future large-scale studies. 5 Although AD biomarkers may have value for research, the immediate advantage for clinical practice appears limited. 5 There is a need for biopsychosocial and ethics research to understand these issues. 55 Such studies require increased research participation among those with MCI. 5
Diagnostic biomarkers and future treatments are likely to challenge the status quo. Biomarkers that predict Alzheimer’s pathology, in particular, or neurodegeneration, more generally (including but not limited to MRI and PET, genetics and blood or CSF measurement), are already finding utility in clinical trials. 8,122 Increasingly, biomarkers are used to identify risk of clinical progression on an individual basis, but they are, as yet, imperfect and not always available. 8 Notwithstanding NICE advice to use biomarkers in ‘suspected early dementia’, there is no corresponding guidance specifically for use in MCI. 5 However, current diagnostic criteria do allow for a diagnosis of AD to be made at the MCI stage in the presence of AD biomarkers. In the UK, CSF examination is rarely performed as part of the diagnostic work-up, although it is safe, well tolerated and is cheaper than PET imaging. 5 The advent of disease-modifying therapies for prodromal AD would require significant investment in biomarker and neuroimaging infrastructure if NHS patients are to receive timely intervention and significant planning and engagement with commissioners and providers will consequently be required. 5 The views of medical practitioners regarding early AD diagnostics and the use of AD biomarkers in persons with no or MCI, as well as the scientific and ethics bases for this practice, remain under-reported in the scientific literature. 151,152
Timely diagnosis of AD when people first seek help for changes in cognition, behaviour or functioning not necessarily resulting in dementia has the potential to reduce the impact of no or delayed diagnosis or misdiagnosis. 145 Timely diagnosis may offer many benefits to patients and caregivers. 145 These benefits could include treatment to control symptoms, avoidance of medications that may worsen symptoms and, in the future, access to interventions that slow or lessen the disease process. 145 Patients could put into place advance care planning and make end-of-life decisions, consider changing unhealthy lifestyles and seek better medical care. 145,176 Most ideas are based on expert opinion and, perhaps, belief, given that evidence is lacking. 165 Further studies are needed to demonstrate not only that a timely diagnosis is feasible, but also that it has benefits. 145 Such evidence would support the cultural shift towards diagnosis at the pre-dementia stage of AD. 145 Table 17 summarises some of the major arguments in relation to early diagnosis of MCI and Table 18 summarises some of the major arguments in relation to the label itself.
| Argument in favour of . . . | Argument against . . . |
|---|---|
| Early diagnosis of MCI | |
| More time to plan for social, financial and medical decisions (e.g. driving, day-to-day life) | Anxiety of diagnosis |
| Timeliness equated to ‘as soon as possible’ for 92% of respondents31 | Consequences of disclosure of a diagnosis of AD in those with minimal symptoms who have full insight |
| Increasing global accessibility of digital consumer electronics | Impact on patient’s autonomy and capacity (e.g. insurance premiums, driving licence) |
| Potential use of biomarkers | Potential stigmatisation |
| Potential treatment of symptomatic conditions | Social stigma of very mild AD |
| Considerable savings in medical and long-term care costs for both patients (particularly in USA) and governments | Some feel unprepared for the diagnostic burden when memory problems first arise13 |
| Uncertainty of prognosis5 | |
| Repeated assessment of competence | |
| Cost of diagnostic tests | |
| Access to and cost of treatment | |
| Clinicians do not consider it beneficial for patients’ overall health and well-being | |
| Clinicians perceive that there are no effective treatments | |
| Clinicians consider that a diagnosis early in the disease continuum may actually be harmful to patients | |
| Early diagnostic disclosure172 | |
| Facilitate planning for the future | Risk of causing emotional distress and anxiety; avoiding maleficence |
| Psychological benefit to person with dementia and/or family members and carers | Inability of person with dementia to understand and/or retain the diagnosis |
| Maximise opportunity for patient to contribute to the management of their own dementia | No perceived benefits, or perceived costs outweigh perceived benefits |
| Person’s ‘right to know’ | Persons right (or wish) ‘not to know’ |
| Maximise treatment possibilities | Diagnosis needs to be timely in relation to when the enquirer wants to seek help13 |
| Obtain access to a second opinion | An appropriate time for diagnosis may align with perceived eligibility for services172 |
| Facilitate access to patient support services | Lack of robust evidence of improvements to well-being from strategies aimed at earlier diagnosis |
| Poor access to necessary specialists and/or support services | Potential risk of ‘overdiagnosis’ |
| Patient is already aware of problems and wishes to know | Lack of cure or effective treatments |
| Stigma associated with the diagnosis of dementia | Diversion of resources away from activities of proven value |
| Late diagnosis | |
| No clear differentiation between brain structure of healthy people and early MCI people | Cost/benefit of drugs, reimbursement issues and rules about discontinuation |
| Competency of patient to consent to participate in research studies | |
| Use of placebo in randomised clinical trials for new drugs | |
| Delays addressing discussion of patient’s best interests and planning for the future | |
| Other considerations | |
| Neutral | |
| Diagnostic uncertainty based on biomarkers that have not been fully validated; no ‘gold-standard’ biomarker or specific diagnostic threshold values currently available | |
| Need for clear communication and clinical pathways | |
| Given that elderly patients often have multiple conditions, GPs report that it is difficult to integrate multiple clinical guidelines with ‘dementia or memory problems right at the bottom of the list’ | |
| With good support and preparation, the feelings of shock, grief, anger and loss that people with dementia and families may experience can be balanced by feelings of reassurance and empowerment167 | |
| Balance of benefits and harms may be influenced strongly by the manner in which the diagnosis is made and disclosed, and the support offered after diagnosis, influencing later adjustment167 | |
| Carer considerations | |
| Some patients/families experience negative reactions to disclosure of a diagnosis of AD | |
| Other reports find no long-term effects of a dementia diagnosis and conclude that individual preferences should be taken into account | |
| Important theoretical advantage is opportunity to achieve added value from earlier treatment or intervention with disease-modifying therapy in a clinical trial before the onset of dementia. (Entirely speculative as no effective disease-modifying therapies are currently available) | |
| Potential to improve quality of life of patients and their informal family caregivers, both of whom are often relieved once patient is diagnosed | |
| Timely diagnosis may improve patient access to support services or pathways of care and enable future planning | |
| Risks or challenges associated with a timely diagnosis of AD, including ethics issues, competency questions, discrimination and stigmatisation | |
| Misdiagnosis can lead to inappropriate treatment of patients who could take unnecessary medications for AD or not receive correct therapy for potentially treatable disorders | |
| Health service considerations | |
| Increased risk of misdiagnosis and uncertainty about the rate of progression to dementia, which can vary considerably among individuals | |
| Diagnosing early may exert undue pressure on already stretched resources13 | |
| Argument in favour of label | Argument against label |
|---|---|
| Patient | |
| Eligibility for clinical trials | Stigma |
| Eligibility for disease-modifying therapies, when available | Directed down inappropriate memory clinic pathway |
| Label not meaningful for patient | |
| Professional | |
| Facilitates communication with other health professionals | Many individuals diagnosed with MCI stay cognitively stable or even improve over time177 |
| Explanatory value in conversation with patients and carers | |
| Other considerations | |
| Carer considerations | |
| Labelling provides access to support, but may carry stigma and may not be meaningful | |
| Health service considerations | |
| Monetary costs to society of establishing systems for timely diagnosis and intervention may prove burdensome | |
Chapter 13 Review 2 (critical interpretive synthesis) discussion
In aviation (and by analogy, lorries awaiting entry to the Channel Tunnel) stacking refers to a situation whereby pilots go into a holding pattern, circling over the runaway. Therefore, ‘holding stacks’ function as a ‘waiting room’ for an aircraft before landing. From this ‘waiting room’, the air traffic controller efficiently organises subsequent landing directions and runway allocation. Memory clinics appear to mimic this stacking function (i.e. the stack itself is a contingency situation of organisational convenience within which the patients’ cognitive resources are gradually being depleted). Once these resources reach a critical point, the patient is admitted to a dementia pathway. Increasingly, the prospect of ‘redirection’ to a more appropriate pathway is being offered through the provision of biomarkers for diagnosis and disease-modifying therapies.
Modification and explicit sharing of expectations – from patient, clinician and carer – may offer a feasible way of modifying the current zone of diagnostic uncertainty and an apparent reluctance on the part of both clinician and patient to accept a definitive MCI diagnosis. 178 Such an approach corresponds with guidance from the General Medical Council, which recommends that if doctors are uncertain about the diagnosis then they should explain this to the patient. 178,179
Summary
This review reveals that MCI has fulfilled its original pragmatic purpose as a mid-way ‘holding stack’ between normal cognitive functioning and dementia. However, the absence of unambiguous and operational definition has resulted in persistent uncertainty as to whether it refers to cognitive difficulties from any cause or constitutes a prodrome of AD. In the presence of such uncertainty, commentators advocate selective avoidance of the label in diagnostic practice until more is known about its core features and predictive value. Since Ritchie and Ritchie’s previous editorial,161 the major shift in the categorisation of MCI has been the introduction of biomarkers to inform the identification – if not yet the prognosis – of AD. 180 However, the introduction of biomarkers has led to a reconceptualisation of AD (seen in the popularisation of the newly proposed concepts of ‘preclinical’ and ‘prodromal’ AD for those who test positive for AD biomarkers but have no dementia). 151
Synthesising argument for diagnosis of mild cognitive impairment
Our synthesising argument (i.e. the output generated by CIS) reads as follows.
The core concept along the pathway of diagnosis and treatment for dementia is ‘timely diagnosis’. 145,167 This concept acknowledges that an attempt to pinpoint a precise point for intervention that is common to all patients who display MCI is likely to meet only limited success. Historically, ‘timely diagnosis’ can be considered intervention at an individual patient-defined point that allows the patient and their carer to undertake mitigating changes in lifestyle and/or to make preparations for future activities of living and financial arrangements162,168 in anticipation or in advance of a worsening of their symptoms. 145 Planning for information provision and social support become important in this context. 162 Alternatively, patients and carers may make an active decision to handle their situation by living in the present and taking one day at a time. Against this patient-oriented conception of timely diagnosis has been ranged a health service imperative for early intervention, driven by a prevalent assumption that earliest possible intervention is necessarily a good thing and supported by indicative evidence that medical costs decrease with an earlier, more accurate diagnosis. 162,181 Finally, the clinical perspective sees the previously limited ambition of intervention being potentially transformed by biomarkers, both in diagnosis and in treatment with disease-modifying agents. These clinical developments have served to bring the clinical horizon more sharply in view for individual patients.
When viewed against these three competing drivers, the decision on diagnosis of MCI transforms from a debate between early and late diagnosis to, instead, an attempt to optimise and synchronise the three competing time cycles of patient, service and clinician. Adoption of the preferred objective ‘timely’ suppresses the relative value of both ‘early’ and ‘late’ and seeks to synchronise timelines informed by patient values, health service policy and evidence-based clinical care.
Furthermore, the subsidiary debate about the value of attribution of the label of MCI is deflected from its questionable value as an intermediate ‘holding’ stage where the patient may deny or conceal symptoms,40 which may or may not lead to subsequent dementia162 and in which a patient is typically ‘parked’ in a memory clinic. Instead, the label can be interpreted as a timely detection point at which the patient and carer are able to prepare and the clinician is able to meaningfully intervene, using cost-effective drug (e.g. acetylcholinesterase inhibitors) or non-drug interventions (e.g. cognitive stimulation therapy) to delay cognitive deterioration and improve quality of life. 162 Although subverting the early diagnosis targets arbitrarily specified for health services planning and delivery, appropriate, timely and acceptable intervention represents an effective and, largely, cost-effective strategy for health systems. 73
Implications of ‘timely diagnosis’ for the clinical encounter
Based on the synthesis and the overall synthesising argument, we were able to identify the implications of operationalising ‘timely diagnosis’. Communication is key so that the clinician can identify what exactly the patient wants to know and when, and any information requirements that will require supplementary information. A focus on activities of daily life and how memory problems affect the activities in which an individual patient is likely to engage is to be privileged over a standard approach based on some ‘typical’ disease trajectory. The US Alzheimer’s Association (Chicago, IL, USA) has developed best practice clinical guidelines for the evaluation of neurodegenerative cognitive behavioural syndromes, AD and dementias. 179
Critical interpretive synthesis: strengths and limitations
This was a comprehensive review of the literature on issues surrounding the label of MCI. However, the studies identified were heterogeneous, with primarily the qualitative studies facilitating richer analysis. All of the studies were conducted in high-income countries. By including published papers in English only, we may have missed research more relevant to other cultural settings. In addition, we included only studies published between 2010 to 2021. However, several articles25,145,152 adopted a historical approach to the development of the MCI concept, offering a longer time perspective.
Limitations to our CIS include the fact that reliance on the label MCI may have excluded some studies that document a pre-dementia stage in less specific terms. However, we compensated for this weakness by following up systematically through citation searching and use of a citations-in-context web tool (scite).
As characteristic of interpretivist research, our values and research experiences have informed how we interpreted the data and, therefore, presented the advantages, disadvantages and considerations for each clinical pathway. Therefore, other researchers may interpret the analysis differently because of different preferred paradigms and experiences. In addition, as we sought neither to ‘vote count’ nor to balance equal numbers of papers supporting or negating the claims of early diagnosis, it is possible that we have quantitatively given more attention to one of the two counterarguments. Nevertheless, the overall resting position that we have adopted of a need for ‘timely diagnosis’ places value on both competing positions and, indeed, seeks to reconcile their imperatives. Finally, the authors of this study identify with a patient, rather than a clinician, perspective. This may privilege social and psychological concerns over clinical and technological considerations and may endorse the rights of the individual over the concerns of the health service. Nevertheless, our perspectives and experience as health service researchers do acknowledge the need to make decisions that seek to optimise the effectiveness, efficiency and appropriateness of service delivery.
Beyond the specific context of MCI, the issues explored in this study provide a valuable exemplar of how clinical uncertainty, which subsequently translates to patient and carer uncertainty, acts out in a face-to-face context. The questionable ethics and clinical value of a diagnosis that is not tied securely in with a specific prognosis, and of screening against the acknowledged principles of mass screening, namely in the absence of a treatment, are confirmed by the clinical and patient perspectives that were engaged by this synthesis. This potential sticking point has been eroded by the development of biomarkers and more so by promising treatments. However, escape from this particular impasse should not mask the fact that effective communication, which involves acknowledging and learning to deal with uncertainty, is key not only to this situation, but also to other clinical areas of uncertain diagnosis.
Chapter 14 Overall discussion and conclusions
Main findings
The two reviews presented in this report represent complementary approaches to the synthesis of a substantial body of evidence on the pathway from awareness of memory problems to a potential diagnosis of dementia. The CIS (review 2) includes additional evidence from commentary and opinion to understand the pros and cons of MCI as a diagnostic label from the clinician and patient/carer perspectives. Although our brief was to examine the investigation of cognitive impairment and not the diagnosis of dementia, some degree of overlap was inevitable.
The descriptive review highlights multiple barriers to efficient diagnosis of memory problems, starting with patient reluctance to seek help. Interventions to encourage people with concerns about their memory to see their GP have been evaluated, but without clear evidence of effectiveness. 33,34 GPs have a variety of cognitive tests available, but recent evidence suggests that substantial numbers of patients meeting criteria for dementia do not have a diagnosis recorded. 44 Options for management for people with memory problems include follow-up in the community (with individual advice about reducing dementia risk) and referral to a memory clinic/memory service for further assessment. Memory clinics are mainly intended to identify and support people with dementia and those not meeting diagnostic criteria may find themselves being discharged back to the care of their GP.
The review identified considerable variation in the way memory clinics in the UK are organised and their approach to investigating the underlying cause of memory problems. 102,103 Recent expert consensus guidance suggests that the availability of imaging and biomarker tests is limited outside specialist tertiary centres. 5 During the COVID-19 pandemic, memory clinics have changed their way of working to include virtual assessments. 123 Another recent development that is likely to have major implications for service providers and patients with MCI is the development of aducanumab (Aduhelm®, Biogen Inc., Cambridge, MA, USA) [the first disease-modifying therapy for dementia (specifically AD)], which was recently approved by regulators in the USA (see Implications for research and Implications for service delivery).
The descriptive review found clear evidence from qualitative studies that patients with MCI and their carers find the process of investigation and diagnosis difficult and frustrating to negotiate. 1,60 Receiving a diagnostic label of MCI involves living with uncertainty23 and the terminology itself may be problematic for patients.
The CIS (review 2) investigated the advantages and disadvantages of MCI as a diagnostic label from various standpoints. The key finding from the synthesis is that the need for a ‘timely’ diagnosis outweighs the ongoing debate about the value, or otherwise, of early investigation and labelling of memory problems. Determining what is a timely diagnosis involves balancing the perspectives of the patient, the health system and the clinician. For the patient, a timely diagnosis is one that enables them to make changes and provision for the future while avoiding unnecessary anxiety and stress. The health service in the UK has to balance a policy that favours early diagnosis and treatment of memory problems against the limitation of available resources and interventions. From the clinical perspective, scanning and biomarker tests offer the possibility of a diagnosis of early dementia prior to the appearance of symptoms, with associated ethics issues. 85 The concept of what is a timely diagnosis may be transformed in the relatively near future if disease-modifying treatments for dementia prove to be effective and enter routine clinical practice.
Strengths and limitations
Strengths and limitations of the included studies
The multicomponent nature of the review and the requirement to address multiple questions resulted in a heterogeneous group of included studies. Individual studies were rated as being of variable quality and differed in their ability to address the individual review questions and, as a consequence, some topics enjoy robust evidence whereas others had to include a broader range of study designs. In particular, the CIS incorporated study designs of variable quality. However, this was considered acceptable because interpretive approaches to review value insights even when they are not supported by a majority of sources or by studies of high quality.
A particular characteristic of the included studies was variability in the criteria for inclusion of participants. For example, studies that explore pathways to AD are not always clear on the diagnostic status of included patients. This means that experiences of those with confirmed AD may not be sufficiently distinct from those diagnosed with MCI. In addition, different thresholds have been used to classify MCI and so there may be geographic or chronological differences even in contexts that primarily relate to the UK. This is a potential issue given that the MCI pathway is continually subject to changes in relation to biomarkers for diagnosis and to the emerging prospect of disease-modifying treatments.
The large proportion of UK studies across the different home nations is a considerable strength of the included studies, thereby strengthening applicability. Perspectives of minority ethnic groups were considered in some studies. Similarities were observed between the roles of UK memory clinics and this offered consistency to many of the findings. Much of the evidence was cross-sectional, making it challenging to link people’s experiences to their eventual outcome. This is important, given that the prognosis for a diagnosis of MCI is not always AD but improvement or relative stability.
Strengths and limitations of the reviews
This review shares the characteristics of resource-constrained evidence syntheses conducted for the NIHR HSDR Evidence Synthesis Centres. Given that the synthesis is primarily descriptive and does not seek precise estimates of effect, the risk to the robustness and credibility of the review is minor. Nevertheless, it is important to explore the implications of the review process so that readers can have confidence in the findings.
A considerable strength is the broad coverage of the sources searched. This coverage, in terms of bibliographic databases and web sites, compares favourably with that for a conventional systematic review, increasing the confidence that relevant items have been identified. On the negative side, within retrieved items, it was challenging to identify where MCI was specifically being explored. Supplementary searches for completion of the CIS revealed several studies and discursives relating to the labelling and diagnosis of AD that had not been identified from manual study selection. Examining and including all studies of AD would prove of questionable value.
Inclusion of systematic reviews provided an effective way of summarising the vast literature on screening and diagnostic tests, with an emphasis on impact on decision-making rather than diagnostic accuracy per se. We also included three high-qualitative evidence syntheses. This pragmatic decision creates a risk of ‘double-counting’ if evidence is included from a study in its own right and as part of a systematic review. This is unlikely to be a major issue for a descriptive review in which the majority of included systematic reviews dealt with specific tests that were not covered elsewhere. We are confident that the main issues and concerns have been identified, even if it was not possible within the limited resources to include all relevant studies.
For the CIS, it was particularly important to access views both for and against early screening and we are confident that we were not only able to do this, but also to benefit from studies and reviews that presented ‘both sides of the story’. CIS benefits from combining the coverage of systematic reviews with the interpretive power of metaethnography. We believe that we were able to produce a clear and compelling ‘line of argument’ as the acknowledged output of the CIS. Conduct of a CIS is considered particularly challenging, given the unclear specification of its methods and a small number of published examples, relative to other types of qualitative evidence synthesis. Nevertheless, as well as being chosen as the most appropriate review design because of the pre-existence of two dominant narratives (in favour of and against screening), we were able to benefit from the methods of the original example of CIS and from a recent review of multiple published CIS reviews. 6
We appreciate that our review was able to include studies published in English only. However, this coincides with our intended target population of inhabitants of the UK. Research from other Organisation for Economic Co-operation and Development countries was included to supplement the UK evidence base (e.g. for studies of distinctive service models that might be adapted for use in the UK). A limitation is that coverage of this literature was not comprehensive. This reflects the fact that the principal methodology of the initial stage of the descriptive review was a mapping review. It is known that this method is vulnerable to limitations in the indexing and abstracting of retrieved and non-retrieved studies and in potential inconsistencies in coding. It was not possible to employ double observer study selection, data extraction or quality assessment, although processes of checking and verification were used throughout the review. Although single abstract screening may miss up to 13% of relevant studies,182 it is possible that a higher percentage of eligible studies may have been missed because of the added complication of imprecise diagnosis combined with indistinct definitions. It was not possible to contact study authors to clarify unclear information; however, we were able to access one author of both a primary study131 and a systematic review23 for internal peer review. Nevertheless, we are confident that none of these methodological limitations would change the overall conclusions of this review.
We also sought to access the views of patient and public involvement representatives (two meetings) and to invite input from those working with commissioners and in primary care. Although time constraints limited the extent and timing of such consultations, we believe that we have produced a review output that reflects the current state of MCI diagnosis and management in the UK at this point in time.
Implications for service delivery
We identified the following implications for service delivery:
-
Our results suggest the need for a more formalised discussion between GPs and their patients with memory problems prior to memory clinic referral, covering the implications of dementia as a possible diagnosis.
-
Services should consider the potential value of efforts to improve the recording of diagnoses of dementia in primary care (e.g. by provision of training and support for GPs to perform assessments using validated tools). 44
-
Quality improvement work at a local and national level is expected to produce benefits in terms of improving and standardising services provided in memory clinics. 103
-
Changes to the operation of memory clinics necessitated by the COVID-19 pandemic will require evaluation to ensure that services are delivered efficiently and effectively in the aftermath of the pandemic.
-
The Manchester consensus guidance5 identified a need for NICE guidance on diagnosis and management of MCI and such guidance could reduce variation in service delivery. Although national guidance is useful in terms of setting evidence-based standards, local services will need to construct flexible diagnostic disclosure pathways. 70
-
There is likely to be a need to plan and resource services to optimise the delivery of disease-modifying therapies if such therapies are approved for use in the NHS. This is a priority for both service delivery and research.
Implications for research
We identified the following priorities for research:
-
There is a need to strengthen the evidence base for primary care-led investigation (including appropriate tools and thresholds for referral) and management of memory problems compared with service delivery through hospital-based memory clinics.
-
The descriptive review identified limited research on screening for memory problems outside general practice. 47 Research to evaluate models of service for other settings, such as emergency departments, acute hospital wards and care homes, would be of value.
-
In view of the move towards remote delivery of health care forced by the COVID-19 pandemic,123,124 research is needed to evaluate remote methods of memory assessment as part of mainstream services, as well as for remote locations.
-
Further qualitative research is needed to ensure that services for people with memory problems are patient centred and provide people with a timely diagnosis that is expressed in terms that they can understand and act on. This research should include people with diverse memory problems (i.e. SCD and FCD, as well as MCI) and different underlying causes (for those diagnosed with MCI).
-
As noted above, research is needed to optimise the introduction of disease-modifying treatments for early dementia on approval. This research could build on modelling work already carried out to estimate the costs of increased use of scanning and biomarkers, including equipment and training costs. 81
-
Research should continue to develop and evaluate evidence-based programmes to reduce dementia risk for people with MCI, which can be implemented at scale, taking into the needs and preferences of people with MCI. 1 Research should consider the needs of minority groups and potential impact on health inequalities.
Conclusions
The concept of MCI as a state between normal ageing and dementia has been in use for many years. Conceptual studies indicate that MCI is understood by many clinicians as a pre-dementia (prodromal) condition related to AD or other forms of dementia. In practice, the diagnostic label of MCI is applied to people with a variety of underlying conditions whose cognitive status may decline, remain stable or improve over time. The pros and cons of the diagnostic label from the patient and clinician viewpoints are considered in review 2 and the findings suggest that the label is valued by clinicians, but is more problematic for patients and their carers.
Investigation of memory problems normally starts when people seek help from their GP. Delays in seeking help after noticing symptoms are common and members of ethnic minority groups may face specific barriers to help-seeking.
Evidence suggests that GPs may have difficulty recognising and recording memory problems using clinical judgement alone. Screening with cognitive tests may be a good use of resources73 and is likely to be of increasing importance with the development of disease-modifying treatments that may benefit people in the early stages of dementia.
Further investigation involves tests (i.e. MRI or PET and analysis of biomarkers in blood and CSF) that are generally available at specialist centres only. The process is complicated by potential overlap with at least two other recognised conditions (i.e. SCD and FCD).
Pathways for people with memory problems may involve follow-up in primary care or referral to a memory clinic/service. We found wide variation in organisation and access to memory clinics in the UK and internationally. A key finding is that memory clinics are primarily commissioned to identify and support people with dementia, suggesting that different service models may be needed for people with MCI. The lack of an evidence-based population-level dementia prevention programme may be a barrier to developing such services.
People with MCI interviewed for qualitative studies frequently portrayed their experiences prior to diagnosis in negative terms. The findings suggest a need for research and practice to make the investigation and management of MCI more patient centred.
The key finding from the CIS (review 2) is that the need for a ‘timely’ diagnosis outweighs the ongoing debate about the value, or otherwise, of early investigation and labelling of memory problems. Determining what is a timely diagnosis involves balancing the perspectives of the patient, the health system and the clinician.
The two reviews reported here have applied different ‘lenses’ to the same body of evidence (supplemented by some additional studies for the CIS). Taken together, the reviews identify the concept of a timely diagnosis for memory problems and identify barriers to obtaining such a diagnosis, from reluctance to seek help through to patchy availability of advanced diagnostic tests. We have also identified priorities for service delivery and research. The report is particularly timely in the light of the recent US approval of a disease-modifying treatment for AD. 183
Acknowledgements
We thank the members of the Sheffield HSDR Evidence Synthesis Centre Public Advisory Group and the topic-specific advisory group for this review. We are grateful to our internal peer reviewers, Dr Tim Gomersall and Dr Debasish Kar.
Contributions of authors
Duncan Chambers (https://orcid.org/0000-0002-0154-0469) (Research Fellow in Public Health) commented on drafts of the protocol and drafts of the report, and was responsible for project co-ordination, study selection, data extraction, quality assessment and report writing.
Anna Cantrell (https://orcid.org/0000-0003-0040-9853) (Research Fellow in Health Economics and Decision Science) commented on drafts of the protocol and drafts of the report, and was responsible for information retrieval, study selection, data extraction, quality assessment and report writing.
Katie Sworn (Research Associate in Health Economics and Decision Science) commented on drafts of the report, and was responsible for study selection, data extraction, quality assessment and report writing.
Andrew Booth (https://orcid.org/0000-0003-4808-3880) (Reader in Evidence-Based Information Practice) commented on drafts of the protocol and drafts of the report, and was responsible for information retrieval, study selection, data extraction, quality assessment and report writing.
Data-sharing statement
Any additional data not included in this report and its appendices are available on request. All queries should be submitted to the corresponding author.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HSDR programme or the Department of Health and Social Care.
References
- Poppe M, Mansour H, Rapaport P, Palomo M, Burton A, Morgan-Trimmer S, et al. ‘Falling through the cracks’; stakeholders’ views around the concept and diagnosis of mild cognitive impairment and their understanding of dementia prevention. Int J Geriatr Psychiatry 2020;35:1349-57. https://doi.org/10.1002/gps.5373.
- Department of Health and Social Care . Living Well With Dementia: A National Dementia Strategy 2009. www.gov.uk/government/publications/living-well-with-dementia-a-national-dementia-strategy (accessed 16 December 2021).
- Department of Health and Social Care . Prime Minister'S Challenge on Dementia 2012. www.gov.uk/government/publications/prime-ministers-challenge-on-dementia (accessed 16 December 2021).
- Collins R, Silarova B, Clare L. Dementia primary prevention policies and strategies and their local implementation: a scoping review using England as a case study. J Alzheimers Dis 2019;70:S303-18. https://doi.org/10.3233/JAD-180608.
- Dunne RA, Aarsland D, O’Brien JT, Ballard C, Banerjee S, Fox NC, et al. Mild cognitive impairment: the Manchester consensus. Age Ageing 2020;50:72-80. https://doi.org/10.1093/ageing/afaa228.
- Dixon-Woods M, Cavers D, Agarwal S, Annandale E, Arthur A, Harvey J, et al. Conducting a critical interpretive synthesis of the literature on access to healthcare by vulnerable groups. BMC Med Res Methodol 2006;6. https://doi.org/10.1186/1471-2288-6-35.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372. https://doi.org/10.1136/bmj.n71.
- Ball HA, McWhirter L, Ballard C, Bhome R, Blackburn DJ, Edwards MJ, et al. Functional cognitive disorder: dementia’s blind spot. Brain 2020;143:2895-903. https://doi.org/10.1093/brain/awaa224.
- Forlenza OV, Diniz BS, Stella F, Teixeira AL, Gattaz WF. Mild cognitive impairment. Part 1: clinical characteristics and predictors of dementia. Braz J Psychiatry 2013;35:178-85. https://doi.org/10.1590/1516-4446-2012-3503.
- Stewart R. Mild cognitive impairment – the continuing challenge of its ‘real-world’ detection and diagnosis. Arch Med Res 2012;43:609-14. https://doi.org/10.1016/j.arcmed.2012.10.011.
- Stephan BC, Hunter S, Harris D, Llewellyn DJ, Siervo M, Matthews FE, et al. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry 2012;17:1056-76. https://doi.org/10.1038/mp.2011.147.
- Blackburn DJ, Wakefield S, Shanks MF, Harkness K, Reuber M, Venneri A. Memory difficulties are not always a sign of incipient dementia: a review of the possible causes of loss of memory efficiency. Br Med Bull 2014;112:71-8. https://doi.org/10.1093/bmb/ldu029.
- Brayne C, Kelly S. Against the stream: early diagnosis of dementia, is it so desirable?. BJPsych Bull 2019;43:123-5. https://doi.org/10.1192/bjb.2018.107.
- British Psychological Society . Evidence Briefing: Mild Cognitive Impairment n.d. www.bps.org.uk/sites/www.bps.org.uk/files/Policy/Policy%20-%20Files/%E2%80%8B%E2%80%8B%E2%80%8B%E2%80%8B%E2%80%8B%E2%80%8B%E2%80%8BEvidence%20Briefing%20-%20Mild%20Cognitive%20Impairment_0.pdf (accessed 20 December 2021).
- Pierce S, Lamers C, Salisbury K. Knowingly not wanting to know: discourses of people diagnosed with mild cognitive impairment. Dementia (London) 2016;15:1246-59. https://doi.org/10.1177/1471301215600895.
- Jenkins A, Tales A, Tree J, Bayer A. Are we ready? The construct of subjective cognitive impairment and its utilization in clinical practice: a preliminary UK-based service evaluation. J Alzheimers Dis 2015;48:25-31. https://doi.org/10.3233/JAD-150541.
- Stephan BCM, Savva GM, Brayne C, Bond J, McKeith IG, Matthews FE, et al. Optimizing mild cognitive impairment for discriminating dementia risk in the general older population. Am J Geriatr Psychiatry 2010;18:662-73. https://doi.org/10.1097/JGP.0b013e3181e0450d.
- Guzman A, Gillanders D, Stevenson A, Ross K. Psychosocial adjustment to mild cognitive impairment: the role of illness perceptions, cognitive fusion and cognitive impairment. Dementia (London) 2021;20:464-84. https://doi.org/10.1177/1471301219893862.
- Rodda J, Gandhi SD, Mukadam N, Walker Z. Attitudes of UK psychiatrists to the diagnosis of MCI in clinical practice. Int Psychogeriatr 2013;25:286-91. https://doi.org/10.1017/S1041610212001500.
- Swallow J. Constructing classification boundaries in the memory clinic: negotiating risk and uncertainty in constituting mild cognitive impairment. Sociol Health Illn 2020;42:99-113. https://doi.org/10.1111/1467-9566.13016.
- Brayne C, McCracken C, Matthews FE. Cohort profile: the Medical Research Council Cognitive Function and Ageing Study (CFAS). Int J Epidemiol 2006;35:1140-5. https://doi.org/10.1093/ije/dyl199.
- Dean K, Wilcock G. Living with mild cognitive impairment: the patient’s and carer’s experience. Int Psychogeriatr 2012;24:871-81. https://doi.org/10.1017/S104161021100264X.
- Gomersall T, Astell A, Nygård L, Sixsmith A, Mihailidis A, Hwang A. Living with ambiguity: a metasynthesis of qualitative research on mild cognitive impairment. Gerontologist 2015;55:892-91. https://doi.org/10.1093/geront/gnv067.
- Beard RL, Neary TM. Making sense of nonsense: experiences of mild cognitive impairment. Sociol Health Illn 2013;35:130-46. https://doi.org/10.1111/j.1467-9566.2012.01481.x.
- Fang ML, Coatta K, Badger M, Wu S, Easton M, Nygård L, et al. Informing understandings of mild cognitive impairment for older adults: implications from a scoping review. J Appl Gerontol 2017;36:808-39. https://doi.org/10.1177/0733464815589987.
- Langa KM, Levine DA. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA 2014;312:2551-61. https://doi.org/10.1001/jama.2014.13806.
- Canadian Task Force on Preventive Health Care . Recommendations on screening for cognitive impairment in older adults. CMAJ 2016;188:37-46. https://doi.org/10.1503/cmaj.141165.
- Lin JS, O’Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;159:601-12. https://doi.org/10.7326/0003-4819-159-9-201311050-00730.
- Patnode CD, Perdue LA, Rossom RC, Rushkin MC, Redmond N, Thomas RG, et al. Screening for cognitive impairment in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2020;323:764-85. https://doi.org/10.1001/jama.2019.22258.
- Perry-Young L, Owen G, Kelly S, Owens C. How people come to recognise a problem and seek medical help for a person showing early signs of dementia: a systematic review and meta-ethnography. Dementia 2018;17:34-60. https://doi.org/10.1177/1471301215626889.
- Devoy S, Simpson EEA. Help-seeking intentions for early dementia diagnosis in a sample of Irish adults. Aging Ment Health 2017;21:870-8. https://doi.org/10.1080/13607863.2016.1179262.
- Mukadam N, Waugh A, Cooper C, Livingston G. What would encourage help-seeking for memory problems among UK-based South Asians? A qualitative study. BMJ Open 2015;5. https://doi.org/10.1136/bmjopen-2015-007990.
- Chan T, van Vlymen J, Dhoul N, de Lusignan S. Using routinely collected data to evaluate a leaflet campaign to increase the presentation of people with memory problems to general practice: a locality based controlled study. Inform Prim Care 2010;18:189-96. https://doi.org/10.14236/jhi.v18i3.771.
- Livingston G, Baio G, Sommerlad A, de Lusignan S, Poulimenos S, Morris S, et al. Effectiveness of an intervention to facilitate prompt referral to memory clinics in the United Kingdom: cluster randomised controlled trial. PLOS Med 2017;14. https://doi.org/10.1371/journal.pmed.1002252.
- Brooker D, Fontaine JL, Evans S, Bray J, Saad K. Public health guidance to facilitate timely diagnosis of dementia: ALzheimer’s COoperative Valuation in Europe recommendations. Int J Geriatr Psychiatry 2014;29:682-93. https://doi.org/10.1002/gps.4066.
- Mitchell AJ, Meader N, Pentzek M. Clinical recognition of dementia and cognitive impairment in primary care: a meta-analysis of physician accuracy. Acta Psychiatr Scand 2011;124:165-83. https://doi.org/10.1111/j.1600-0447.2011.01730.x.
- Barth J, Nickel F, Kolominsky-Rabas PL. Diagnosis of cognitive decline and dementia in rural areas – a scoping review. Int J Geriatr Psychiatry 2018;33:459-74. https://doi.org/10.1002/gps.4841.
- Costanzo MC, Arcidiacono C, Rodolico A, Panebianco M, Aguglia E, Signorelli MS. Diagnostic and interventional implications of telemedicine in Alzheimer’s disease and mild cognitive impairment: a literature review. Int J Geriatr Psychiatry 2020;35:12-28. https://doi.org/10.1002/gps.5219.
- Pelegrini LNC, Mota GMP, Ramos CF, Jesus E, Vale FAC. Diagnosing dementia and cognitive dysfunction in the elderly in primary health care: a systematic review. Dement Neuropsychol 2019;13:144-53. https://doi.org/10.1590/1980-57642018dn13-020002.
- Judge D, Roberts J, Khandker R, Ambegaonkar B, Black CM. Physician perceptions about the barriers to prompt diagnosis of mild cognitive impairment and Alzheimer’s disease. Int J Alzheimers Dis 2019;2019. https://doi.org/10.1155/2019/3637954.
- Manthorpe J, Samsi K, Joly L, Crane M, Gage H, Bowling A, et al. Service provision for older homeless people with memory problems: a mixed-methods study. Health Serv Deliv Res 2019;7. https://doi.org/10.3310/hsdr07090.
- Menon R, Larner AJ. Use of cognitive screening instruments in primary care: the impact of national dementia directives (NICE/SCIE, National Dementia Strategy). Fam Pract 2011;28:272-6. https://doi.org/10.1093/fampra/cmq100.
- Olazarán J, Torrero P, Cruz I, Aparicio E, Sanz A, Mula N, et al. Mild cognitive impairment and dementia in primary care: the value of medical history. Fam Pract 2011;28:385-92. https://doi.org/10.1093/fampra/cmr005.
- Aldus CF, Arthur A, Dennington-Price A, Millac P, Richmond P, Dening T, et al. Undiagnosed dementia in primary care: a record linkage study. Health Serv Deliv Res 2020;8. https://doi.org/10.3310/hsdr08200.
- van den Dungen P, Moll van Charante EP, van de Ven PM, Foppes G, van Campen JP, van Marwijk HW, et al. Dutch family physicians’ awareness of cognitive impairment among the elderly. BMC Geriatr 2015;15. https://doi.org/10.1186/s12877-015-0105-1.
- van den Dungen P, Moll van Charante EP, van de Ven PM, van Marwijk HW, van der Horst HE, van Hout HP. Case finding of mild cognitive impairment and dementia and subsequent care; results of a cluster RCT in primary care. PLOS ONE 2016;11. https://doi.org/10.1371/journal.pone.0156958.
- Dickens P, Ramaesh K. The evolving role of ophthalmology clinics in screening for early Alzheimer’s disease: a review. Vision 2020;4. https://doi.org/10.3390/vision4040046.
- Dodd E, Cheston R, Fear T, Brown E, Fox C, Morley C, et al. An evaluation of primary care led dementia diagnostic services in Bristol. BMC Health Serv Res 2014;14. https://doi.org/10.1186/s12913-014-0592-3.
- Steiner GZ, Ee C, Dubois S, MacMillan F, George ES, McBride KA, et al. ‘We need a one-stop-shop’: co-creating the model of care for a multidisciplinary memory clinic with community members, GPs, aged care workers, service providers, and policy-makers. BMC Geriatr 2020;20. https://doi.org/10.1186/s12877-019-1410-x.
- Thabtah F, Peebles D, Retzler J, Hathurusingha C. Dementia medical screening using mobile applications: a systematic review with a new mapping model. J Biomed Inform 2020;111. https://doi.org/10.1016/j.jbi.2020.103573.
- Thabtah F, Peebles D, Retzler J, Hathurusingha C. A review of dementia screening tools based on mobile application. Health Technol 2020;10:1011-22. https://doi.org/10.1007/s12553-020-00426-5.
- Mills JK, Minhas JS, Robotham SL. An assessment of the dementia CQUIN – an audit of improving compliance. Dementia 2014;13:697-703. https://doi.org/10.1177/1471301213519894.
- Mills JKA, Minhas JS, Robotham SL. ‘An assessment of the dementia CQUIN – an audit of improving compliance’: withdrawn. Dementia 2017;16. https://doi.org/10.1177/1471301213515575.
- Bertens D, Vos S, Kehoe P, Wolf H, Nobili F, Mendonça A, et al. Use of mild cognitive impairment and prodromal AD/MCI due to AD in clinical care: a European survey. Alzheimers Res Ther 2019;11. https://doi.org/10.1186/s13195-019-0525-9.
- Hughes JC, Ingram TA, Jarvis A, Denton E, Lampshire Z, Wernham C. Consent for the diagnosis of preclinical dementia states: a review. Maturitas 2017;98:30-4. https://doi.org/10.1016/j.maturitas.2017.01.008.
- Robinson L, Gemski A, Abley C, Bond J, Keady J, Campbell S, et al. The transition to dementia – individual and family experiences of receiving a diagnosis: a review. Int Psychogeriatr 2011;23:1026-43. https://doi.org/10.1017/S1041610210002437.
- Bunn F, Goodman C, Sworn K, Rait G, Brayne C, Robinson L, et al. Psychosocial factors that shape patient and carer experiences of dementia diagnosis and treatment: a systematic review of qualitative studies. PLOS Med 2012;9. https://doi.org/10.1371/journal.pmed.1001331.
- Roberts JL, Clare L. Meta-representational awareness in mild cognitive impairment: an interpretative phenomenological analysis. Aging Ment Health 2013;17:300-9. https://doi.org/10.1080/13607863.2012.732033.
- Manthorpe J, Samsi K, Campbell S, Abley C, Keady J, Bond J, et al. From forgetfulness to dementia: clinical and commissioning implications of diagnostic experiences. Br J Gen Pract 2013;63:e69-75. https://doi.org/10.3399/bjgp13X660805.
- Samsi K, Abley C, Campbell S, Keady J, Manthorpe J, Robinson L, et al. Negotiating a labyrinth: experiences of assessment and diagnostic journey in cognitive impairment and dementia. Int J Geriatr Psychiatry 2014;29:58-67. https://doi.org/10.1002/gps.3969.
- Allan CL, Behrman S, Ebmeier KP, Valkanova V. Diagnosing early cognitive decline –when, how and for whom?. Maturitas 2017;96:103-8. https://doi.org/10.1016/j.maturitas.2016.11.018.
- Judge D, Roberts J, Khandker RK, Ambegaonkar B, Black CM. Physician practice patterns associated with diagnostic evaluation of patients with suspected mild cognitive impairment and Alzheimer’s disease. Int J Alzheimers Dis 2019;2019. https://doi.org/10.1155/2019/4942562.
- Aslam RW, Bates V, Dundar Y, Hounsome J, Richardson M, Krishan A, et al. A systematic review of the diagnostic accuracy of automated tests for cognitive impairment. Int J Geriatr Psychiatry 2018;33:561-75. https://doi.org/10.1002/gps.4852.
- Chen HH, Sun FJ, Yeh TL, Liu HE, Huang HL, Kuo BI, et al. The diagnostic accuracy of the Ascertain Dementia 8 questionnaire for detecting cognitive impairment in primary care in the community, clinics and hospitals: a systematic review and meta-analysis. Fam Pract 2018;35:239-46. https://doi.org/10.1093/fampra/cmx098.
- Ehrensperger MM, Taylor KI, Berres M, Foldi NS, Dellenbach M, Bopp I, et al. BrainCheck – a very brief tool to detect incipient cognitive decline: optimized case-finding combining patient- and informant-based data. Alzheimers Res Ther 2014;6. https://doi.org/10.1186/s13195-014-0069-y.
- Hancock P, Larner AJ. Test Your Memory test: diagnostic utility in a memory clinic population. Int J Geriatr Psychiatry 2011;26:976-80. https://doi.org/10.1002/gps.2639.
- O’Malley RPD, Mirheidari B, Harkness K, Reuber M, Venneri A, Walker T, et al. Fully automated cognitive screening tool based on assessment of speech and language [published online ahead of print November 20 2020]. J Neurol Neurosurg Psychiatry 2020. https://doi.org/10.1136/jnnp-2019-322517.
- Kochan NA, Slavin MJ, Brodaty H, Crawford JD, Trollor JN, Draper B, et al. Effect of different impairment criteria on prevalence of ‘objective’ mild cognitive impairment in a community sample. Am J Geriatr Psychiatry 2010;18:711-22. https://doi.org/10.1097/JGP.0b013e3181d6b6a9.
- Klekociuk SZ, Summers JJ, Vickers JC, Summers MJ. Reducing false positive diagnoses in mild cognitive impairment: the importance of comprehensive neuropsychological assessment. Eur J Neurol 2014;21. https://doi.org/10.1111/ene.12488.
- NHS London Clinical Networks . Non-Dementia Pathways: Guidance from the London Dementia Clinical Networks 2020. www.england.nhs.uk/london/wp-content/uploads/sites/8/2019/07/Final-non-dementia-pathways-V2.pdf (accessed 20 December 2021).
- Sabbagh MN, Boada M, Borson S, Chilukuri M, Dubois B, Ingram J, et al. Early detection of mild cognitive impairment (MCI) in primary care. J Prev Alzheimers Dis 2020;7:165-70. https://doi.org/10.14283/jpad.2020.21.
- Sabbagh MN, Boada M, Borson S, Doraiswamy PM, Dubois B, Ingram J, et al. Early detection of mild cognitive impairment (MCI) in an at-home setting. J Prev Alzheimers Dis 2020;7:171-8. https://doi.org/10.14283/jpad.2020.22.
- Tong T, Thokala P, McMillan B, Ghosh R, Brazier J. Cost effectiveness of using cognitive screening tests for detecting dementia and mild cognitive impairment in primary care. Int J Geriatr Psychiatry 2017;32:1392-400. https://doi.org/10.1002/gps.4626.
- Burhan AM, Bartha R, Bocti C, Borrie M, Laforce R, Rosa-Neto P, et al. Role of emerging neuroimaging modalities in patients with cognitive impairment: a review from the Canadian Consensus Conference on the Diagnosis and Treatment of Dementia 2012. Alzheimers Res Ther 2013;5. https://doi.org/10.1186/alz192.
- Sullivan V, Majumdar B, Richman A, Vinjamuri S. To scan or not to scan: neuroimaging in mild cognitive impairment and dementia. Adv Psychiatr Treat 2012;18:457-66. https://doi.org/10.1192/apt.bp.110.008813.
- Pellegrini E, Ballerini L, Hernandez MDCV, Chappell FM, González-Castro V, Anblagan D, et al. Machine learning of neuroimaging for assisted diagnosis of cognitive impairment and dementia: a systematic review. Alzheimers Dement 2018;10:519-35. https://doi.org/10.1016/j.dadm.2018.07.004.
- Cook LD, Nichol KE, Isaacs JD. The London memory service audit and quality improvement programme [published online ahead of print March 22 2019]. BJPsych Bull 2019. https://doi.org/10.1192/bjb.2019.18.
- de Wilde A, van der Flier WM, Pelkmans W, Bouwman F, Verwer J, Groot C, et al. Association of amyloid positron emission tomography with changes in diagnosis and patient treatment in an unselected memory clinic cohort: the ABIDE project. JAMA Neurol 2018;75:1062-70. https://doi.org/10.1001/jamaneurol.2018.1346.
- Fantoni ER, Chalkidou A, O’Brien JT, Farrar G, Hammers A. A systematic review and aggregated analysis on the impact of amyloid pet brain imaging on the diagnosis, diagnostic confidence, and management of patients being evaluated for Alzheimer’s disease. J Alzheimers Dis 2018;63:783-96. https://doi.org/10.3233/JAD-171093.
- de Wilde A, van Buchem MM, Otten RHJ, Bouwman F, Stephens A, Barkhof F, et al. Disclosure of amyloid positron emission tomography results to individuals without dementia: a systematic review. Alzheimers Res Ther 2018;10. https://doi.org/10.1186/s13195-018-0398-3.
- Wittenberg R, Knapp M, Karagiannidou M, Dickson J, Schott J. Economic impacts of introducing diagnostics for mild cognitive impairment Alzheimer’s disease patients. Alzheimers Dement 2019;5:382-7. https://doi.org/10.1016/j.trci.2019.06.001.
- Sheikh-Bahaei N, Sajjadi SA, Pierce AL. Current role for biomarkers in clinical diagnosis of Alzheimer disease and frontotemporal dementia. Curr Treat Options Neurol 2017;19. https://doi.org/10.1007/s11940-017-0484-z.
- Bocchetta M, Galluzzi S, Kehoe PG, Aguera E, Bernabei R, Bullock R, et al. The use of biomarkers for the etiologic diagnosis of MCI in Europe: an EADC survey. Alzheimers Dement 2015;11:195-206.e1. https://doi.org/10.1016/j.jalz.2014.06.006.
- Frederiksen KS, Nielsen TR, Appollonio I, Andersen BB, Riverol M, Boada M, et al. Biomarker counseling, disclosure of diagnosis and follow-up in patients with mild cognitive impairment: a European Alzheimer’s disease consortium survey. Int J Geriatr Psychiatry 2021;36:324-33. https://doi.org/10.1002/gps.5427.
- Frederiksen KS, Nielsen TR, Winblad B, Schmidt R, Kramberger MG, Jones RW, et al. European Academy of Neurology/European Alzheimer’s Disease Consortium position statement on diagnostic disclosure, biomarker counseling, and management of patients with mild cognitive impairment. Eur J Neurol 2021;28:2147-55. https://doi.org/10.1111/ene.14668.
- Bahureksa L, Najafi B, Saleh A, Sabbagh M, Coon D, Mohler MJ, et al. The impact of mild cognitive impairment on gait and balance: a systematic review and meta-analysis of studies using instrumented assessment. Gerontology 2017;63:67-83. https://doi.org/10.1159/000445831.
- Bruun M, Frederiksen KS, Rhodius-Meester HFM, Baroni M, Gjerum L, Koikkalainen J, et al. Impact of a clinical decision support tool on dementia diagnostics in memory clinics: the PredictND validation study. Curr Alzheimer Res 2019;16:91-101. https://doi.org/10.2174/1567205016666190103152425.
- Elsey C, Drew P, Jones D, Blackburn D, Wakefield S, Harkness K, et al. Towards diagnostic conversational profiles of patients presenting with dementia or functional memory disorders to memory clinics. Patient Educ Couns 2015;98:1071-7. https://doi.org/10.1016/j.pec.2015.05.021.
- Pennington C, Ball H, Swirski M. Functional cognitive disorder: diagnostic challenges and future directions. Diagnostics 2019;9. https://doi.org/10.3390/diagnostics9040131.
- Begum A, Morgan C, Chiu CC, Tylee A, Stewart R. Subjective memory impairment in older adults: aetiology, salience and help seeking. Int J Geriatr Psychiatry 2012;27:612-20. https://doi.org/10.1002/gps.2760.
- Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, Teipel SJ, et al. Evolving evidence for the value of neuroimaging methods and biological markers in subjects categorized with subjective cognitive decline. J Alzheimers Dis 2015;48:171-91. https://doi.org/10.3233/JAD-150202.
- Fonseca JA, Ducksbury R, Rodda J, Whitfield T, Nagaraj C, Suresh K, et al. Factors that predict cognitive decline in patients with subjective cognitive impairment. Int Psychogeriatr 2015;27:1671-7. https://doi.org/10.1017/S1041610215000356.
- Yates JA, Clare L, Woods RT, MRC CFAS. Subjective memory complaints, mood and MCI: a follow-up study. Aging Ment Health 2017;21:313-21. https://doi.org/10.1080/13607863.2015.1081150.
- Grande G, Cucumo V, Cova I, Ghiretti R, Maggiore L, Lacorte E, et al. Reversible mild cognitive impairment: the role of comorbidities at baseline evaluation. J Alzheimers Dis 2016;51:57-6. https://doi.org/10.3233/JAD-150786.
- Hadjichrysanthou C, McRae-McKee K, Evans S, de Wolf F, Anderson RM. Alzheimer’s Disease Neuroimaging Initiative . Potential factors associated with cognitive improvement of individuals diagnosed with mild cognitive impairment or dementia in longitudinal studies. J Alzheimers Dis 2018;66:587-600. https://doi.org/10.3233/JAD-180101.
- Lonie JA, Parra-Rodriguez MA, Tierney KM, Herrmann LL, Donaghey C, O’Carroll RE, et al. Predicting outcome in mild cognitive impairment: 4-year follow-up study. Br J Psychiatry 2010;197:135-40. https://doi.org/10.1192/bjp.bp.110.077958.
- Montero-Odasso MM, Sarquis-Adamson Y, Speechley M, Borrie MJ, Hachinski VC, Wells J, et al. Association of dual-task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol 2017;74:857-65. https://doi.org/10.1001/jamaneurol.2017.0643.
- Stephan BC, Brayne C, Savva GM, Matthews FE. Medical Research Council Cognitive Function and Ageing Study . Occurrence of medical co-morbidity in mild cognitive impairment: implications for generalisation of MCI research. Age Ageing 2011;40:501-7. https://doi.org/10.1093/ageing/afr057.
- Wells CE, Smith SJ. Diagnostic care pathways in dementia: a review of the involvement of primary care in practice and innovation. J Prim Care Community Health 2017;8:103-11. https://doi.org/10.1177/2150131916678715.
- Samsi K, Manthorpe J. Care pathways for dementia: current perspectives. Clin Interv Aging 2014;9:2055-63. https://doi.org/10.2147/CIA.S70628.
- Draper B, Low LF, Brodaty H. Integrated care for adults with dementia and other cognitive disorders. Int Rev Psychiatry 2018;30:272-91. https://doi.org/10.1080/09540261.2018.1564021.
- Chrysanthaki T, Fernandes B, Smith S, Black N. Can memory assessment services (MAS) in England be categorized? A national survey. J Public Health 2017;39:828-40. https://doi.org/10.1093/pubmed/fdx018.
- Cook L, Souris H, Isaacs J. The 2019 National Memory Service Audit. London: Dementia Clinical Network, NHS England and Improvement; 2020.
- Minstrell M, Bentley M, Bucher H, Morrissey M, Higgs C, Robinson A, et al. Open referral policy within a nurse-led memory clinic: patient demographics, assessment scores, and diagnostic profiles. Int Psychogeriatr 2015;27:967-79. https://doi.org/10.1017/S1041610214002361.
- Park MH, Smith SC, Chrysanthaki T, Neuburger J, Ritchie CW, Hendriks AAJ, et al. Change in health-related quality of life after referral to memory assessment services. Alzheimer Dis Assoc Disord 2017;31:192-9. https://doi.org/10.1097/WAD.0000000000000190.
- Park MH, Smith SC, Ritchie CW, Hendriks AAJ, Black N. Memory assessment services and health-related quality of life: 1-year follow-up [published online ahead of print May 30 2018]. Int J Geriatr Psychiatry 2018. https://doi.org/10.1002/gps.4913.
- Ramakers IH, Verhey FR. Development of memory clinics in the Netherlands: 1998 to 2009. Aging Ment Health 2011;15:34-9. https://doi.org/10.1080/13607863.2010.519321.
- Abley C, Manthorpe J, Bond J, Keady J, Samsi K, Campbell S, et al. Patients’ and carers’ views on communication and information provision when undergoing assessments in memory services. J Health Serv Res Policy 2013;18:167-73. https://doi.org/10.1177/1355819613479945.
- Dean K, Jenkinson C, Wilcock G, Walker Z. Exploring the experiences of people with mild cognitive impairment and their caregivers with particular reference to healthcare – a qualitative study. Int Psychogeriatr 2014;26:475-85. https://doi.org/10.1017/S104161021300207X.
- Pennington M, Gomes M, Chrysanthaki T, Hendriks J, Wittenberg R, Knapp M, et al. The cost of diagnosis and early support in patients with cognitive decline. Int J Geriatr Psychiatry 2018;33:5-13. https://doi.org/10.1002/gps.4641.
- Rubinsztein JS, van Rensburg MJ, Al-Salihy Z, Girling D, Lafortune L, Radhakrishnan M, et al. A memory clinic v. traditional community mental health team service: comparison of costs and quality. BJPsych Bull 2015;39:6-11. https://doi.org/10.1192/pb.bp.113.044263.
- Hopkinson J, King A, Courtier N, Milton R, Elias J. Potential for identification of memory problems in the cancer clinic to enable improved treatment experience and outcomes: mixed methods case study research. Eur J Oncol Nurs 2020;48. https://doi.org/10.1016/j.ejon.2020.101777.
- Giebel CM, Worden A, Challis D, Jolley D, Bhui KS, Lambat A, et al. Age, memory loss and perceptions of dementia in South Asian ethnic minorities. Aging Ment Health 2019;23:173-82. https://doi.org/10.1080/13607863.2017.1408772.
- Peach T, Pollock K, van der Wardt V, das Nair R, Logan P, Harwood RH. Attitudes of older people with mild dementia and mild cognitive impairment and their relatives about falls risk and prevention: a qualitative study. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0177530.
- Quinn C, Toms G, Anderson D, Clare L. A review of self-management interventions for people with dementia and mild cognitive impairment. J Appl Gerontol 2016;35:1154-88. https://doi.org/10.1177/0733464814566852.
- Regan B, Varanelli L. Adjustment, depression, and anxiety in mild cognitive impairment and early dementia: a systematic review of psychological intervention studies. Int Psychogeriatr 2013;25:1963-84. https://doi.org/10.1017/S104161021300152X.
- British Psychological Society . Evidence Briefing: Psychological Therapies for People With Dementia 2016.
- Royal College of Psychiatrists . Driving With Dementia or Mild Cognitive Impairment: Consensus Guidelines for Clinicians 2019.
- Forsyth K, Heathcote L, Senior J, Malik B, Meacock R, Perryman K, et al. Dementia and mild cognitive impairment in prisoners aged over 50 years in England and Wales: a mixed-methods study. Health Serv Deliv Res 2020;8. https://doi.org/10.3310/hsdr08270.
- Lindqvist E, PerssonVasiliou A, Hwang AS, Mihailidis A, Astelle A, Sixsmith A, et al. The contrasting role of technology as both supportive and hindering in the everyday lives of people with mild cognitive deficits: a focus group study. BMC Geriatr 2018;18. https://doi.org/10.1186/s12877-018-0879-z.
- Quintana M, Anderberg P, Sanmartin Berglund J, Frögren J, Cano N, Cellek S, et al. Feasibility-usability study of a tablet app adapted specifically for persons with cognitive impairment-SMART4MD (Support Monitoring and Reminder Technology for Mild Dementia). Int J Environ Res Public Health 2020;17. https://doi.org/10.3390/ijerph17186816.
- Sabbagh MN, Boada M, Borson S, Chilukuri M, Doraiswamy PM, Dubois B, et al. Rationale for early diagnosis of mild cognitive impairment (MCI) supported by emerging digital technologies. J Prev Alzheimers Dis 2020;7:158-64. https://doi.org/10.14283/jpad.2020.19.
- NHS England and NHS Improvement . Memory Service Assessments: A New Way of Working 2020.
- Owens AP, Ballard C, Beigi M, Kalafatis C, Brooker H, Lavelle G, et al. Implementing remote memory clinics to enhance clinical care during and after COVID-19. Front Psychiatry 2020;11. https://doi.org/10.3389/fpsyt.2020.579934.
- Birt L, Poland F, Charlesworth G, Leung P, Higgs P. Relational experiences of people seeking help and assessment for subjective cognitive concern and memory loss. Aging Ment Health 2020;24:1356-64. https://doi.org/10.1080/13607863.2019.1592111.
- Boyd A, Synnott J, Nugent C, Elliott D, Kelly J. Community-based trials of mobile solutions for the detection and management of cognitive decline. Healthc Technol Lett 2017;4:93-6. https://doi.org/10.1049/htl.2016.0102.
- Buckley RF, Saling MM, Frommann I, Wolfsgruber S, Wagner M. Subjective cognitive decline from a phenomenological perspective: a review of the qualitative literature. J Alzheimers Dis 2015;48:125-40. https://doi.org/10.3233/JAD-150095.
- Cheong K, Fisher P, Goh J, Ng L, Koh HM, Yap P. Advance care planning in people with early cognitive impairment. BMJ Support Palliat Care 2015;5:63-9. https://doi.org/10.1136/bmjspcare-2014-000648.
- Dooley J, Bailey C, Xanthopoulou P, Bass N, McCabe R. Communication and understanding of mild cognitive impairment diagnoses. Int J Geriatr Psychiatry 2020;35:662-70. https://doi.org/10.1002/gps.5284.
- Giebel C, Challis D, Worden A, Jolley D, Bhui KS, Lambat A, et al. Perceptions of self-defined memory problems vary in south Asian minority older people who consult a GP and those who do not: a mixed-method pilot study. Int J Geriatr Psychiatry 2016;31:375-83. https://doi.org/10.1002/gps.4337.
- Gomersall T, Smith SK, Blewett C, Astell A. ‘It’s definitely not Alzheimer’s’: perceived benefits and drawbacks of a mild cognitive impairment diagnosis. Br J Health Psychol 2017;22:786-804. https://doi.org/10.1111/bjhp.12255.
- Parker M, Barlow S, Hoe J, Aitken L. Persistent barriers and facilitators to seeking help for a dementia diagnosis: a systematic review of 30 years of the perspectives of carers and people with dementia [published online ahead of print February 6 2020]. Int Psychogeriatr 2020. https://doi.org/10.1017/S1041610219002229.
- Sharp BK. Stress as experienced by people with dementia: an interpretative phenomenological analysis. Dementia 2019;18:1427-45. https://doi.org/10.1177/1471301217713877.
- Dooley J, Bass N, McCabe R. How do doctors deliver a diagnosis of dementia in memory clinics?. Br J Psychiatry 2018;212:239-45. https://doi.org/10.1192/bjp.2017.64.
- NHS Health Check . NHS Health Check Dementia Leaflet n.d. www.healthcheck.nhs.uk/commissioners-and-providers/marketing/dementia-resources/ (accessed 22 December 2021).
- Verhaeghe P. On Being Normal and Other Disorders: A Manual for Clinical Psychodiagnostics. London: Karnac Books; 2008.
- Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med 2014;275:214-28. https://doi.org/10.1111/joim.12190.
- Leibing A. The earlier the better: Alzheimer’s prevention, early detection, and the quest for pharmacological interventions. Cult Med Psychiatry 2014;38:217-36. https://doi.org/10.1007/s11013-014-9370-2.
- Leuzy A, Gauthier S. Ethical issues in Alzheimer’s disease: an overview. Expert Rev Neurother 2012;12:557-67. https://doi.org/10.1586/ern.12.38.
- Gauthier S, Leuzy A, Racine E, Rosa-Neto P. Diagnosis and management of Alzheimer’s disease: past, present and future ethical issues. Prog Neurobiol 2013;110:102-13. https://doi.org/10.1016/j.pneurobio.2013.01.003.
- Depraetere J, Vandeviver C, Keygnaert I, Beken TV. The critical interpretive synthesis: an assessment of reporting practices. Int J Soc Res Methodol 2020:1-21. https://doi.org/10.31235/osf.io/e7g3u.
- Peel E. Diagnostic communication in the memory clinic: a conversation analytic perspective. Aging Ment Health 2015;19:1123-30. https://doi.org/10.1080/13607863.2014.1003289.
- Visser LNC, Pelt SAR, Kunneman M, Bouwman FH, Claus JJ, Kalisvaart KJ, et al. Communicating uncertainties when disclosing diagnostic test results for (Alzheimer’s) dementia in the memory clinic: the ABIDE project. Health Expect 2020;23:52-6. https://doi.org/10.1111/hex.12964.
- Visser LNC, van Maurik IS, Bouwman FH, Staekenborg S, Vreeswijk R, Hempenius L, et al. Clinicians’ communication with patients receiving a MCI diagnosis: the ABIDE project. PLOS ONE 2020;15. https://doi.org/10.1371/journal.pone.0227282.
- Dubois B, Padovani A, Scheltens P, Rossi A, Dell’Agnello G. Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. J Alzheimers Dis 2016;49:617-31. https://doi.org/10.3233/JAD-150692.
- Erdmann P, Langanke M. The ambivalence of early diagnosis - returning results in current Alzheimer research. Curr Alzheimer Res 2018;15:28-37. https://doi.org/10.2174/1567205014666170908101237.
- Hagan RJ. What next? Experiences of social support and signposting after a diagnosis of dementia. Health Soc Care Community 2020;28:1170-9. https://doi.org/10.1111/hsc.12949.
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673-734. https://doi.org/10.1016/S0140-6736(17)31363-6.
- Smedinga M, Tromp K, Schermer MHN, Richard E. Ethical arguments concerning the use of Alzheimer’s disease biomarkers in individuals with no or mild cognitive impairment: a systematic review and framework for discussion. J Alzheimers Dis 2018;66:1309-22. https://doi.org/10.3233/JAD-180638.
- Swallow J. Expectant futures and an early diagnosis of Alzheimer’s disease: knowing and its consequences. Soc Sci Med 2017;184:57-64. https://doi.org/10.1016/j.socscimed.2017.05.017.
- Tromp K, Smedinga M, Richard E, Perry M, Schermer MHN. Views on early diagnosis of Alzheimer’s disease among Dutch physicians: a qualitative interview study. J Alzheimers Dis 2021;79:917-27. https://doi.org/10.3233/JAD-200884.
- Vanderschaeghe G, Dierickx K, Vandenberghe R. Review of the ethical issues of a biomarker-based diagnoses in the early stage of Alzheimer’s disease. J Bioeth Inq 2018;15:219-30. https://doi.org/10.1007/s11673-018-9844-y.
- York Health Economics Consortium . Overview of Systematic Reviews of Nonpharmacological Interventions for Dementia 2017. www.nice.org.uk/guidance/ng97/evidence/appendix-o-yhec-report-pdf-174697045530 (accessed 21 December 2021).
- Lawson RA, Yarnall AJ, Duncan GW, Breen DP, Khoo TK, Williams-Gray CH, et al. Stability of mild cognitive impairment in newly diagnosed Parkinson’s disease. J Neurol Neurosurg Psychiatry 2017;88:648-52. https://doi.org/10.1136/jnnp-2016-315099.
- Howard R, Schott J. When dementia is misdiagnosed. Int J Geriatr Psychiatry 2021;36:799-801. https://doi.org/10.1002/gps.5538.
- Morris JL, Hu L, Hunsaker A, Liptak A, Seaman JB, Lingler JH. Patients’ and family members’ subjective experiences of a diagnostic evaluation of mild cognitive impairment. J Patient Exp 2020;7:124-31. https://doi.org/10.1177/2374373518818204.
- Moore V, Cahill S. Diagnosis and disclosure of dementia – a comparative qualitative study of Irish and Swedish general practitioners. Aging Ment Health 2013;17:77-84. https://doi.org/10.1080/13607863.2012.692763.
- Lin RS, Yu DS, Li PW, Masika GM. The effectiveness of non-pharmacological interventions targeting neuropsychiatric symptoms among persons with preclinical and mild dementia: A systematic review and network meta-analysis. Int J Geriatr Psychiatry 2021;36:479-92. https://doi.org/10.1002/gps.5460.
- Kurtz K, Matuseski R, McKee A, Murphy V, Peterson M, Polland R, et al. Examining the Lived Experience of Mild Cognitive Impairment (MCI): An Evidence-Based Practice Project 2019. https://sophia.stkate.edu/cgi/viewcontent.cgi?article=1003&context=ot_grad (accessed 21 December 2021).
- De Cola MC, Lo Buono V, Mento A, Foti M, Marino S, Bramanti P, et al. Unmet needs for family caregivers of elderly people with dementia living in Italy: what do we know so far and what should we do next?. Inquiry 2017;54. https://doi.org/10.1177/0046958017713708.
- Ritchie K, Ritchie CW. Mild cognitive impairment (MCI) twenty years on. Int Psychogeriatr 2012;24:1-5. https://doi.org/10.1017/S1041610211002067.
- Robinson L, Tang E, Taylor JP. Dementia: timely diagnosis and early intervention. BMJ 2015;350. https://doi.org/10.1136/bmj.h3029.
- Lee SM, Lin X, Haralambous B, Dow B, Vrantsidis F, Tinney J, et al. Factors impacting on early detection of dementia in older people of Asian background in primary healthcare. Asia Pac Psychiatry 2011;3:120-7. https://doi.org/10.1111/j.1758-5872.2011.00130.x.
- Ton TGN, DeLeire T, May SG, Hou N, Tebeka MG, Chen E, et al. The financial burden and health care utilization patterns associated with amnestic mild cognitive impairment. Alzheimers Dement 2017;13:217-24. https://doi.org/10.1016/j.jalz.2016.08.009.
- Abbate C, Trimarchi PD, Maggioni C. A missed detection of prodromal dementia may be the worst enemy of a timely diagnosis. Alzheimers Dement 2016;4:37-8. https://doi.org/10.1016/j.dadm.2016.03.010.
- Abbate C, Trimarchi PD, Inglese S, Gallucci A, Tomasini E, Bagarolo R, et al. The two-step strategy could be inadequate and counteracting to diagnose prodromal dementia or mild cognitive impairment. Front Aging Neurosci 2020;12. https://doi.org/10.3389/fnagi.2020.00229.
- Woods B, Arosio F, Diaz A, Gove D, Holmerová I, Kinnaird L, et al. Timely diagnosis of dementia? Family carers’ experiences in 5 European countries. Int J Geriatr Psychiatry 2019;34:114-21. https://doi.org/10.1002/gps.4997.
- Jensen-Hart S. Practical support for caregivers in navigating financial issues associated with dementia. Fam Soc 2018;99:329-32. https://doi.org/10.1177/1044389418806701.
- Leung KK, Finlay J, Silvius JL, Koehn S, McCleary L, Cohen CA, et al. Pathways to diagnosis: exploring the experiences of problem recognition and obtaining a dementia diagnosis among Anglo-Canadians. Health Soc Care Community 2011;19:372-81. https://doi.org/10.1111/j.1365-2524.2010.00982.x.
- Donaghy PC, McKeith IG. The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther 2014;6. https://doi.org/10.1186/alzrt274.
- Watson R, Bryant J, Sanson-Fisher R, Mansfield E, Evans TJ. What is a ‘timely’ diagnosis? Exploring the preferences of Australian health service consumers regarding when a diagnosis of dementia should be disclosed. BMC Health Serv Res 2018;18. https://doi.org/10.1186/s12913-018-3409-y.
- Dhedhi SA, Swinglehurst D, Russell J. ‘Timely’ diagnosis of dementia: what does it mean? A narrative analysis of GPs’ accounts. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2013-004439.
- Draper B, Cations M, White F, Trollor J, Loy C, Brodaty H, et al. Time to diagnosis in young-onset dementia and its determinants: the INSPIRED study. Int J Geriatr Psychiatry 2016;31:1217-24. https://doi.org/10.1002/gps.4430.
- Robertson D, Kirkpatrick P, McCulloch S. Sustaining adults with dementia or mild cognitive impairment in employment: a systematic review protocol of qualitative evidence. JBI Database System Rev Implement Rep 2015;13:124-36. https://doi.org/10.11124/jbisrir-2015-2033.
- Campbell S, Manthorpe J, Samsi K, Abley C, Robinson L, Watts S, et al. Living with uncertainty: mapping the transition from pre-diagnosis to a diagnosis of dementia. J Aging Stud 2016;37:40-7. https://doi.org/10.1016/j.jaging.2016.03.001.
- Goldfarb D, Sheard S, Shaughnessy L, Atri A. Disclosure of Alzheimer’s disease and dementia: patient- and care partner-centric decision-making and communication. J Clin Psychiatry 2019;80. https://doi.org/10.4088/jcp.ms18002br1c.
- Glynn K, O’Callaghan M, Hannigan O, Bruce I, Gibb M, Coen R, et al. Clinical utility of mild cognitive impairment subtypes and number of impaired cognitive domains at predicting progression to dementia: a 20-year retrospective study. Int J Geriatr Psychiatry 2021;36:31-7. https://doi.org/10.1002/gps.5385.
- Cox CL, Miller BM, Kuhn I, Fritz Z. Diagnostic uncertainty in primary care: what is known about its communication, and what are the associated ethical issues?. Fam Pract 2021;38:654-68. https://doi.org/10.1093/fampra/cmab023.
- Atri A, Norman M, Knopman D. Alzheimer’s Association Best Clinical Practice Guidelines for the Evaluation of Neurodegenerative Cognitive Behavioral Syndromes, Alzheimer’s Disease and Dementias in the United States n.d.
- Saunders S, Ritchie K, Russ TC, Muniz-Terrera G, Ritchie CW. Evolution and future directions for the concept of mild cognitive impairment. Int Psychogeriatr 2018;30:1431-4. https://doi.org/10.1017/S1041610217002812.
- Tang EY, Brayne C, Albanese E, Stephan BC. Mild cognitive impairment definitions: more evolution than revolution. Neurodegener Dis Manag 2015;5:11-7. https://doi.org/10.2217/nmt.14.42.
- Gartlehner G, Affengruber L, Titscher V, Noel-Storr A, Dooley G, Ballarini N, et al. Single-reviewer abstract screening missed 13 percent of relevant studies: a crowd-based, randomized controlled trial. J Clin Epidemiol 2020;121:20-8. https://doi.org/10.1016/j.jclinepi.2020.01.005.
- US Food and Drug Administration . FDA Grants Accelerated Approval for Alzheimer’s Drug n.d. www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug (accessed 14 March 2022).
- Lin JS, O’Connor E, Rossom RC, Perdue LA, Burda BU, Thompson M, et al. Screening for Cognitive Impairment in Older Adults: A Systematic Review for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013.
- Great Britain . Mental Capacity Act 2005.
Appendix 1 MEDLINE search strategy
MEDLINE search strategy
Database: Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily.
Date of search: January 2021.
Dates searched: 1946–8 January 2021.
Search strategy
-
cognition disorders/or cognitive dysfunction/ (83,692)
-
mild.ab,ti. (361,771)
-
1 and 2 (15,704)
-
“mild cognitive impairment$”.ab,ti. (17,633)
-
“mild neurocognitive disorder$”.ab,ti. (174)
-
mci.ab,ti. (18,337)
-
“subjective cognitive decline”.ab,ti. (577)
-
scd.ab,ti. (12,367)
-
“functional cognitive disorder$”.ab,ti. (21)
-
fcd.ab,ti. (1433)
-
(memor$ adj (problem$ or lapse$ or impairment$)).ab,ti. (15,313)
-
Dementia/pc [Prevention & Control] (1735)
-
Dementia/ (52,474)
-
Primary Prevention/ (18,859)
-
prevent$.ab,ti. (1,466,709)
-
14 or 15 (1,473,041)
-
13 and 16 (3114)
-
3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 17 (61,523)
-
Diagnosis/or Delayed Diagnosis/or Early Diagnosis/ (50,994)
-
diagnos$.ab,ti. (2,548,327)
-
manag$.ab,ti. (1,404,024)
-
Primary Prevention/ (18,859)
-
prevent$.ab,ti. (1,466,709)
-
labelling.ab,ti. (40,317)
-
service pathway$.ab,ti. (58)
-
screening.ab,ti. (545,191)
-
“service model$”.ab,ti. (1766)
-
assessment tool$.ab,ti. (26,285)
-
or/19-28 (5,284,586)
-
18 and 29 (23,792)
-
exp United Kingdom/ (369,592)
-
(national health service$ or nhs$).ab,in,ti. (19,172)
-
(english not ((published or publication$ or translat$ or written or language$ or speak$ or literature or citation$) adj5 english)).ti,ab. (96,653)
-
(gb or “g.b.” or britain$ or (british$ not “british columbia”) or uk or “u.k.” or united kingdom$ or (england$ not “new england”) or northern ireland$ or northern irish$ or scotland$ or scottish$ or ((wales or “south wales”) not “new south wales”) or welsh$).ab,in,jw,ti. (2,131,833)
-
(bath or “bath’s” or ((birmingham not alabama*) or (“birmingham’s” not alabama*) or bradford or “bradford’s” or brighton or “brighton’s” or bristol or “bristol’s” or carlisle* or “carlisle’s” or (cambridge not (massachusetts* or boston* or harvard*)) or (“cambridge’s” not (massachusetts* or boston* or harvard*)) or (canterbury not zealand*) or (“canterbury’s” not zealand*) or chelmsford or “chelmsford’s” or chester or “chester’s” or chichester or “chichester’s” or coventry or “coventry’s” or derby or “derby’s” or (durham not (carolina* or nc)) or (“durham’s” not (carolina* or nc)) or ely or “ely’s” or exeter or “exeter’s” or gloucester or “gloucester’s” or hereford or “hereford’s” or hull or “hull’s” or lancaster or “lancaster’s” or leeds* or leicester or “leicester’s” or (lincoln not nebraska*) or (“lincoln’s” not nebraska*) or (liverpool not (new south wales* or nsw)) or (“liverpool’s” not (new south wales* or nsw)) or ((london not (ontario* or ont or toronto*)) or (“london’s” not (ontario* or ont or toronto*)) or manchester or “manchester’s” or (newcastle not (new south wales* or nsw)) or (“newcastle’s” not (new south wales* or nsw)) or norwich or “norwich’s” or nottingham or “nottingham’s” or oxford or “oxford’s” or peterborough or “peterborough’s” or plymouth or “plymouth’s” or portsmouth or “portsmouth’s” or preston or “preston’s” or ripon or “ripon’s” or salford or “salford’s” or salisbury or “salisbury’s” or sheffield or “sheffield’s” or southampton or “southampton’s” or st albans or stoke or “stoke’s” or sunderland or “sunderland’s” or truro or “truro’s” or wakefield or “wakefield’s” or wells or westminster or “westminster’s” or winchester or “winchester’s” or wolverhampton or “wolverhampton’s” or (worcester not (massachusetts* or boston* or harvard*)) or (“worcester’s” not (massachusetts* or boston* or harvard*)) or (york not (“new york*” or ny or ontario* or ont or toronto*)) or (“york’s” not (“new york*” or ny or ontario* or ont or toronto*))))).ti,ab,in. (1,466,105)
-
(bangor or “bangor’s” or cardiff or “cardiff’s” or newport or “newport’s” or st asaph or “st asaph’s” or st davids or swansea or “swansea’s”).ti,ab,in. (58,046)
-
(aberdeen or “aberdeen’s” or dundee or “dundee’s” or edinburgh or “edinburgh’s” or glasgow or “glasgow’s” or inverness or (perth not australia*) or (“perth’s” not australia*) or stirling or “stirling’s”).ti,ab,in. (217,258)
-
(armagh or “armagh’s” or belfast or “belfast’s” or lisburn or “lisburn’s” or londonderry or “londonderry’s” or derry or “derry’s” or newry or “newry’s”).ti,ab,in. (27,491)
-
or/31-38 (2,724,361)
-
(exp africa/or exp americas/or exp antarctic regions/or exp arctic regions/or exp asia/or exp oceania/) not (exp great britain/or europe/) (2,942,630)
-
39 not 40 (2,570,511)
-
30 and 41 (2589)
-
limit 42 to yr = “2010 -Current” (2037)
-
limit 43 to english language (2019)
-
(editorial or comment or letter).pt. (1,920,184)
-
44 not 45 (1975)
-
from 46 keep 1-1975 (1975)
Appendix 2 Grey literature search
Mild cognitive impairment grey search
Grey literature searches were performed in January 2021 to retrieve clinical guidelines, policy documents and reports related to MCI from relevant websites:
-
Age UK (URL: www.ageuk.org.uk)
-
American Academy of Neurology (URL: www.aan.com/)
-
Alzheimer’s Research UK (URL: www.alzheimersresearchuk.org)
-
Alzheimer’s Society (URL: www.alzheimers.org.uk)
-
British Geriatrics Society (URL: www.bgs.org.uk)
-
The British Psychological Society (URL: www.bps.org.uk)
-
GOV.UK (URL: www.gov.uk).
In addition, searches were conducted on the Google search engine™ (Google Inc., Mountain View, CA, USA; URL: www.google.com/) for relevant literature (MCI and guideline, MCI and policy, etc.).
Appendix 3 Quality assessment tables
| Study | Was an ‘a priori’ design provided? | Was there duplicate study selection and data extraction? | Was a comprehensive literature search performed? | Was the status of publication (i.e. grey literature) used as an inclusion criterion? | Was a list of studies (included and excluded) provided? | Were the characteristics of the included studies provided? | Was the scientific quality of the included studies assessed and documented? | Was the scientific quality of the included studies used appropriately in formulating conclusions? | Were the methods used to combine the findings of studies appropriate? | Was the likelihood of publication bias assessed? | Was the conflict of interest stated? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aslam et al.63 | Yes: followed Cochrane Handbook | Yes: data extraction checked rather than duplicated | Yes | No: appears not, as theses were eligible | No: only included studies | Yes | Yes | Yes | Not applicable | No: not mentioned | Yes: none declared |
| Bahureksa et al.86 | Cannot answer | Yes: yes study selection, no data extraction | Yes | No | Yes: yes included, no excluded | Yes | Yes | Cannot answer: unclear | Yes | Yes | Yes |
| Chen et al.64 | Yes: inferred from introduction | Yes: reported for selection and quality assessment | Yes | No: not mentioned | No: included only | Yes | Yes | Yes: all classed as high quality | Yes | Yes | Yes |
| de Wilde et al.80 | Cannot answer | Yes | Yes | Yes: only articles from peer-reviewed journals included | No: included only | Yes | No | Not applicable | Not applicable | No | Yes: reported for authors |
| Dean and Wilcock22 | No: no protocol; no risk of bias | No: NR | Cannot answer: unclear – three databases searched and there was some supplementary searches but no justification of publication restrictions | No | No: no excluded | Yes, although no time frame | No | Not applicable | No: methods to combine findings NR | No | Yes |
| Fang et al.25 | Yes | Cannot answer | Yes | Yes | No | No | No | No | Cannot answer | Yes | No |
| Fantoni et al.79 | Yes: based on reported methods/objectives | Yes | Yes | Yes: peer-reviewed journal or peer-selected conference abstract | Yes: included and final stage excluded | Yes | Yes | No: QUADAS ‘was independent of the analysis and not designed to weigh data’ | Cannot answer: appears to be IPD but no details reported | No | Yes: reported for authors |
| Lin et al.184 | Yes | Yes: second reviewer verified extracted data | Yes | No: grey literature searched for ongoing trials | Yes | Yes | Yes | Yes | Yes: mainly narrative synthesis, but some meta-analyses included | Yes | No: not mentioned in report |
| Lin et al.28 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes: stated for authors |
| Mitchell et al.36 | Yes: protocol mentioned | Yes: only mentioned explicitly for extraction | Yes | Cannot answer | No: included studies only | Yes | Yes | Yes: studies regarded as generally high quality | Yes | Yes | Yes: declared for authors (none) |
| Patnode et al.29 | Yes: update of previous review (2013) | Yes | Yes | Cannot answer | No: included only in journal paper | Cannot answer: link to full report not working | Yes | Yes | Yes | Yes | Yes: reported for authors |
| Pelegrini et al.39 | Cannot answer | Yes: more explicit for selection | Yes | Yes: dissertations, etc., excluded | No: included only | Yes | No | No | Not applicable | No | No |
| Pellegrini et al.76 | Yes: protocol registered (PROSPERO) | Yes: partial – duplicate study selection, unclear if duplicate data extraction | Yes: partial – searched at least two databases, provided keywords, completed search with 24 months of completing the review and included conference papers if sufficient data reported. Does not mention searching trial/study registries, reference lists of included studies, contact with experts or searches for grey literature | Cannot answer: publication status not mentioned | No: included studies in tables in appendix. For excluded studies, just reasons for exclusion with numbers | Yes: in tables in supplementary material | Yes: used QUADAS-2 criteria | Yes | Yes | No | No |
| Perry-Young et al.30 | Cannot answer: no information on protocol | Cannot answer: extraction NR | Yes: partial – more than two databases searched, key words given and reference lists scanning, but no consultation with experts or grey literature | No: not listed | No: no exclusions | Cannot answer: unknown how many people had MCI | Cannot answer: two reviewers (LP and GO) independently assessed the 16 studies using the CASP assessment tool for qualitative research (2013). Eight exclusions but full results not given | Cannot answer: exclusions made on quality but no breakdown of quality appraisal | Yes: explanation of line of argument synthesis and data sufficiently presented | No | Yes |
| Quinn et al.115 | Yes | No | Yes | Yes: included studies had to have been published in English | No | Yes | Cannot answer: there is discussion about methodological quality of included studies, but no information in the methodology about assessment of study quality | Yes | Yes | No | Yes |
| Regan and Varanelli116 | Yes | Cannot answer | Yes | Yes | No: included, but not excluded | Yes | Yes | Yes | Yes | Cannot answer | Yes |
| Study | Was there a clear statement of aims? | Is a qualitative methodology appropriate? | Was the research design appropriate? | Was the recruitment strategy appropriate? | Was the data collected in a way that addressed the research issue? | Has the relationship between researcher and participants been adequately considered? | Have ethics issues been taken into consideration? | Was the data analysis sufficiently rigorous? | Is there a clear statement of findings? |
|---|---|---|---|---|---|---|---|---|---|
| Abley et al.108 | Yes | Yes: aim to capture the experiences of people with memory problems | Yes: in-depth information required from participants | Yes: purposive sampling guided the selection of participants. The initial sampling frame was expanded to recruit more women and a wider variation in socioeconomic status. Recruited from four memory clinics: London, north-west England and two in north-east England. Patients were referred to them from GPs and the clinics had responsibility for the assessment and diagnosis of dementia. Memory clinics were chosen as a recruitment source because they see people relatively early in the development of dementia and these experiences. Consent was an element of recruitment. Prediagnosis and postdiagnosis patients recruited | Yes: one group was interviewed about their experiences before and after diagnostic assessment, whether or not a diagnosis was made. However, no topic guide. Data were transcribed | Yes: researchers wrote regular reflexive diaries that served as field notes. Interview summaries were sent to each participant and follow-up telephone calls corroborated the issues. This was an opportunity for summaries to be amended according to participant feedback | Yes: only people who were able to consent to participate in the research were included. Assessment of a participant’s capacity to make the decision to participate was determined by the completion of a short pro forma. No mention of an ethics committee | Yes: in-depth description of process, sufficient data and quotations | Yes |
| Beard and Neary24 | Yes | Yes: exploring experiences of MCI. Given the paucity of previous studies on the topic and exploratory nature of this research, grounded theory methods were deemed well-suited to the project | Yes: understanding the social aspects of MCI diagnoses was the basis for analysis. Qualitative interviews to challenge the stigma that people with AD are often deemed incapable (and perhaps unworthy) of contributing to the social discourse surrounding their illness experience | Cannot tell: unclear – this study is part of a larger study. The research was based on a non-probability sample using convenience, snowball and theoretical sampling. Place where patients recruited given | Cannot tell: setting and interview topic guide not given. Data collection methods given, but not justified | No: NR | Cannot tell: ethics issues around consent and ethics approval NR. Participants had agreed to be contacted about future research studies and, therefore, at least implicitly self-identified with the label they had been given | Yes: thick description of process. Quotations supporting themes. Contradictory data | Yes: findings located in context of other findings. Link to research question. However, credibility not discussed |
| Birt et al.125 | Yes | Yes | Yes | Yes: broad inclusion criteria, including people not referred for further assessment | Yes: repeat interviews | Cannot tell: nothing reported | Yes: ethics approval reported | Yes | Yes |
| Dean et al.109 | Yes: aims reported | Yes | Yes: exploring the experiences. A qualitative approach was used as there is relatively little background information on this topic and it was, therefore, deemed an appropriate method to explore participants’ experiences | Yes: how and why patients were recruited was explained | Yes: description and saturation discussed. Topic guide given | Yes | Yes: interest in study described and informed consent. Ethics approval | Yes: analysis process described. Sufficient data provided. Contradictory data taken into account | Yes: credibility of findings discussed |
| Dodd et al.48 | Yes | Yes | Cannot tell: unclear why themes were generated before interviews | Yes | Yes: semistructured interviews | No: section entitled ‘research team and reflexivity’ does not really cover this | Yes: ethics approval reported | Cannot tell: limited description of data analysis | Yes |
| Dooley et al.129 | Yes | Yes | Yes | Yes | Yes | No | Cannot tell | Cannot tell | Yes |
| Giebel et al.113 | Yes: aims provided | Yes: perceptions of dementia | Yes: semistructured Barts Explanatory Model Inventory for Dementia schedule used, whereas older adults also completed measures of cognition (MMSE), and depression (GDS) | Yes: although a little vague on approach to public via community organisations | Yes: general topics of inventories given | Cannot tell: some information about interviewer training, but role and relationship not discussed | Yes: ethics approval noted and consent procedure described | Yes: quantitative analysis significance levels given. Analysis consistent with the inventory approach | Yes: refers to wider evidence. Limitation’s provided |
| Gomersall et al.131 | Yes | Yes | Yes | Yes: opportunity sampling because of small pool of potential participants | Yes: semistructured interviews | Yes: section on ‘trustworthiness and reflexivity’ | Yes: authors report on ethics approval and on an issue that arose during the study | Yes: grounded theory analysis described | Yes |
| Lindqvist et al.120 | Yes: aims and relevance provided | Yes: perspectives on support explored | Yes: focus groups justified by authors | Yes: those with cognitive difficulties and spouses of those with cognitive difficulties all volunteered to participate as equal members of volunteer health organisation (organisation unclear). In addition, recruited researcher and health professionals at memory clinics (convenience sampling) | Yes: focus groups mixed with people with cognitive difficulties and spouses and both voices were given equal weight. Separate groups between three types of participant (health professionals, researchers and members of health organisations). Process of data collection provided | No: researchers were facilitators. No discussion of relationship to participants with memory problems | Cannot tell: ethics approval, but no further information | Yes: process described, sufficient data | Yes: findings discussed in relation to other literature and credibility discussed |
| Manthorpe et al.59 | Yes: aims and relevance | Yes: exploration of experience of patients | Yes: this qualitative study had both retrospective and prospective elements to capture diagnosis | Yes: memory clinics were the recruitment source because they encounter individuals at a relatively early point in the transition to dementia. Purposive: three study areas were selected on the basis that together they served populations that were diverse in terms of socioeconomic status and ethnicity | Yes: focus groups process provided | Yes: process of consent well described. Conducted interviews in participants’ homes. The researcher summarised the salient points, which were sent to each participant | Yes: in keeping with the Mental Capacity Act.185 The interviewers started with the phrase ‘the diagnosis of the cause of your memory problems’, to give an opportunity to participants to frame the context of the conversation | Yes: constant comparative analysis method well described. Sufficient data presented and contradictory data discussed | Yes: credibility and wider literature discussed |
| Mukadam et al.32 | Yes: aims and relevance provided | Yes: perspectives on barriers | Yes: interview option, groups of participants from a similar background. Focus groups of people from same background justified | Yes: maximum variation sample to capture perspectives across Asian community | Yes: procedures well described. Use of a case vignette | Cannot tell: Mental Capacity Act185 considered. Need for individual interview; however, specific relationship between researcher and participant not reported. Trained researchers mentioned | Yes: Mental Capacity Act185 consent considered. Ethics approval took place. Translation of material undertaken. Groups consisted of people from same background, as homogeneity within the group can facilitate more detailed and free-flowing consideration of topics and also allows comparison between groups | Yes: interpretative phenomenological analysis method. Sufficient detail and disconfirming cases discussed | Yes: credibility discussed, but not set in context of wider literature |
| Peach et al.114 | Yes: aims and relevancy given | Yes: aimed to capture attitudes | Yes | Cannot tell: recruited through another study about falls. Convenience sample not clear | Yes: semistructured interviews were conducted in participants’ homes using an interview guide. Interviews were audio-recorded and transcribed verbatim for thematic analysis | Yes: memory problems managed with attention to communication strategies, such as simplifying question structure and giving plenty of time for responses. How patient was referred to discussed. Implications of presence of partner not discussed. Patients contacted by a researcher (TP) who was a falls specialist occupational therapist and was not involved in the participants’ clinical care | Yes: all participants were deemed to have mental capacity to consent by the recruiting clinician following a structured assessment and all participants provided written consent to take part. The study was approved by an NHS Research Ethics Committee | Yes: sufficient data. Processes discussed. No critical examination of researcher’s own role. Contradictions discussed | Cannot tell: main findings clear. No critical examination of findings and limitations |
| Poppe et al.1 | Yes | Yes | Cannot tell: no discussion about why they chose study methods | Yes | Yes | Cannot tell: not discussed in article | Yes | Yes | Yes |
| Roberts and Clare58 | Yes | Yes | Yes | Yes | Yes | Yes | Yes: ethics approval granted by relevant NHS and university ethics committees | Yes: two researchers scrutinised data and analyses at each stage independently | Yes |
| Samsi et al.60 | Yes: aim and relevance | Yes | Yes: captured experiences, justified in text | Yes | Yes: processes described and topic guide developed from other studies | Cannot tell | Cannot tell: only a mention of ethics approval with some discussion of consent | Yes: process and consensus discussed. Sufficient data presented, contradictions discussed | Yes: credibility of small sample discussed, reflections in relation to other literature |
| Steiner et al.49 | Yes | Yes | Cannot tell: no justification for research design | Cannot tell: study gained insights of community, health workers and policy-makers, but not hospital-based staff | Yes | Cannot tell: conclusion discusses limitation that findings were hard to disentangle from research teams’ preconceived ideas about memory clinics | Yes | Yes | Yes |
| Study | Questiona | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Aldus et al.44 | Yes: multiple research questions | Yes | Yes: 94% of records obtained | Yes: CFAS-II study | No | No: exposure = dementia (vs. controls) | Yes | Yes: diagnosis of dementia | Not applicable: used existing records | Yes: covariates |
| Begum et al.90 | Unclear: aims only | Yes: sample defined | No: 126/1977 | Unclear | Unclear: NR. No discussion of power of study | Unclear: level of help available at the GPs for subjective memory problems not provided | Yes: salience definition, seeking help definition and number of symptoms described | Yes: Likert scale described briefly | No: practice staff called participants and they may have known their history of symptoms | Unclear |
| Boyd et al.126 | No: NR | Unclear | No | Unclear | No: NR | No: usability exposure NR | No | No: not for EnCare diagnostics (sic) (intervention) assessment | No: assessors not blinded | Unclear |
| Bruun et al.87 | Yes | Yes | Unclear: non-participation rate not reported | Yes: different hospitals, but strict inclusion criteria | No | No: exposure = PredictND tool | Not applicable | Yes | No: the study design was ‘a trade-off between the importance of mimicking the clinical setting and absolute blinding’ | Not applicable |
| Cook et al.77 | Yes | Yes | Not applicable | Yes | Not applicable | Unclear | Unclear | Not applicable | Not applicable | Not applicable |
| de Wilde et al.78 | Yes | Yes | Yes: 55% | Yes: older people with memory problems | No | Not applicable: exposure = PET | Not applicable | Yes | No | No |
| Forsyth et al.119 | Yes | Yes | Unclear | Yes | Yes | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Grande et al.94 | Yes | Yes | Yes | Yes | Not applicable: all patients diagnosed with MCI between 2004 and 2013 were eligible to participate | Not applicable | Yes: exposure = comorbidities? | Yes | No: no reference to blinding | Yes: Cox regression model |
| Kochan et al.68 | Yes | Yes | Yes: 987/1037 | Yes: community-dwelling older people | No | Yes: level/stringency of cognitive tests | Yes: tests and associated rules for classifying MCI vs. unimpaired | Yes | Not applicable | Yes: for comparison of (M)CI criteria using sample-based data (sample from English-speaking backgrounds) |
| Lonie et al.96 | Yes | Yes | Yes: 46/87 | Yes | No | Not applicable | Not applicable | Yes | No | Yes |
| Menon and Larner42 | Yes | Yes | Yes: consent not needed | Yes | No | No | Unclear: cannot tell whether GPs were actually exposed to guidance | Yes | No | No |
| Montero-Odasso et al.97 | Yes | Yes | Unclear: sample was 112 individuals with dementia | Yes | No: part of larger cohort study | No | Not applicable | Yes | Yes | Yes: analyses adjusted for covariates, including age, sex and education level, number of comorbidities and baseline cognition |
| Olazarán et al.43 | Unclear | Yes | Yes: 182 patients were recruited, four declined participation and two lost to follow-up and so 176 patients described and analysed | Yes | Unclear: limitation section mentions small sample size | No | Not applicable | Yes: diagnosis of cognitive impairment was performed independently by an expert | Yes | No: studies investigating if variables usually collected in a patients medical history were helpful in the detection and prognosis of MCI and dementia and so analyses were unadjusted. Chi-square test, Mann–Whitney test, t-test and unadjusted analysis of variance were used to compare the clinical variables of the different groups of interest |
| Park et al.105 | Yes | Yes | Yes: 67% for patients and 60% for carers | Yes | No: attempted to recruit all referred patients from participating MASs | No: exposure is MAS and associated interventions | Not applicable | Yes | No | Yes: adjusted for patient characteristics |
| Park et al.106 | Yes | Yes | Yes: participation rate for MAS | Yes | Unclear: again, refers to MAS | Not applicable: exposure is MAS | Not applicable | Yes | Not applicable: not appropriate as only one group | Yes |
| Ramakers and Verhey107 | Yes | Yes | Yes | Yes | Not applicable: attempted to survey all memory clinics in the Netherlands | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Rubinsztein et al.111 | Yes | Yes | Not applicable: retrospective data collection | Yes | Not applicable: sample determined by time frame of study | Not applicable | Not applicable | Yes | Not applicable: case note review | Unclear |
| Stephan et al.98 | Yes | Yes | Unclear | Yes | No | Yes: exposure = comorbidity | Yes | Yes | No | Yes: univariate/multivariate regression models |
| Yates et al.93 | Yes | Yes | Yes | Yes | No | No | Yes: exposure = subjective memory complaints? | Yes: outcome = cognitive status? | No | Yes? Logistic regression |
| Study | Representative spectrum? | Acceptable reference standard? | Acceptable delay between tests? | Partial verification avoided? | Differential verification avoided? | Was the reference standard independent of the index test? | Index test results blinded? | Reference standard results blinded? | Relevant clinical information? | Were uninterpretable results reported? | Were withdrawals from the study explained? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ehrensperger et al.65 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | No | Yes |
| Hancock and Larner66 | Yes | Yes | Yes: all administered on the same day | Yes | Yes | Yes | Yes: TYM results not used for diagnosis | Unclear | Yes | No | Yes: attributed to test being self-administered |
| Klekociuk et al.69 | Yes | Not applicable: compares new model with current diagnostic criteria, but no reference standard per se | Unclear: compares current (Winblad) criteria at screening with stable diagnosis over approximately 2 years | Yes: whole sample evaluated | Yes | Not applicable | Not applicable | Not applicable | Yes | No | No: approximately 9% withdrawal rate |
| O’Malley et al.67 | Unclear: study includes patients with AD, MCI, FCD and healthy control patients. Mentions need for future studies to include patients with non-AD dementias | Yes | Unclear: not reported | Yes | Yes | Yes: reference standard completed by specialist memory clinic and index test by CognoSpeak (a fully automated system) | Yes: index test completed by automated system | Yes: reference standard completed before CognoSpeak | Not applicable: index test completed by automated system | Not applicable | No: no withdrawals |
| van den Dungen et al.45 | Unclear: patients who agreed to participate were younger and more often male than non-respondents | Yes | No: due to delays in ethics approval the time between the index and reference test was 9 months | No: only patients assessed as possible cognitive impairment, dementia or no signs of cognitive impairment were offered reference test. Significant number of patients declined. Random sample patients assessed as no signs of cognitive impairment were offered reference test | Yes | Yes | Yes | Yes: all index tests were completed before the reference test | Yes | Yes | Yes: numbers of patients assessed for index test that refused participation or were excluded from the study for various reasons are listed |
| Study | Questiona | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Buckley et al.127 | Yes | Yes | No | Yes | Yes | No information | No | No information | Yes | No information | Yes | High concern | Yes | No |
| Bunn et al.57 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Minor concern | Yes | No information |
| Gomersall et al.23 | Yes | Yes | Yes | Yes: research team backgrounds mentioned in text | Yes | No information | No | No information | Yes | Yes | Yes | Moderate concern: lack of quality/risk-of-bias assessment and information on study selection | Yes | No: GRADE-CERQual probably not available when this review was published |
List of abbreviations
- AD
- Alzheimer’s disease
- AMSTAR
- A MeaSurement Tool to Assess systematic Reviews
- app
- application
- CASP
- Critical Appraisal Skills Programme
- CIS
- critical interpretive synthesis
- CSF
- cerebrospinal fluid
- CT
- computerised tomography
- EADC
- European Alzheimer’s Disease Consortium
- FCD
- functional cognitive decline
- FMD
- functional memory disorder
- GP
- general practitioner
- HSDR
- Health and Social Care Delivery Research
- MAS
- memory assessment service
- MCI
- mild cognitive impairment
- MMSE
- Mini Mental State Examination
- MoCA
- Montreal Cognitive Assessment
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- PET
- positron emission tomography
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SCD
- subjective cognitive decline
- SCI
- subjective cognitive impairment
- TYM
- Test Your Memory
