Notes
Article history
The contractual start date for this research was in October 2019. This article began editorial review in October 2021 and was accepted for publication in July 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health and Social Care Delivery Research editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Thompson et al. This work was produced by Thompson et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Thompson et al.
Introduction
Until recently, healthcare professionals relied on peer-to-peer, paper-based, or standalone guidelines, and limited computer technology to support their clinical judgements and decisions. 2,3 Since the late 1980s, claims that computerised support at the point of care has potential to improve treatment or management have increased – notably in medicine. 4 The degree to which the potential of computerised support for decisions is actually realised is unclear.
In this synopsis we bring together the findings of an evidence synthesis of comparative research into the effects of CDSS on the clinical performance, behaviours and outcomes associated with the work and decisions of nurses, midwives and allied health professionals (NMAHPs, for example, physiotherapists, occupational therapists and paramedics – see www.england.nhs.uk/ahp/role/ for definitions and scope). Our aim is an accessible overview of the synthesis and associated stakeholder engagement. To improve accessibility, we have abridged some of the reporting of our results and methods.
Background
The target for CDSS: decision-making by nurses, midwives and allied health professionals
Historically, decisions about the delivery and organisation of healthcare were assumed to be the province of doctors. Whilst medical dominance has proven remarkably resistant to challenge, ‘decision-rich’ areas such as the prescribing of medications,5 the initiation of critical care outreach in acute care, nutritional management and rehabilitation planning offer the chance for professions other than medicine to formally use their decisions to shape the delivery of healthcare, how care processes are experienced and the clinical outcomes that result.
Alongside formal decisions in healthcare such as assigning a diagnosis, prescribing a treatment, or offering a prognosis, the realpolitik of healthcare delivery relies on a range of informal judgements, decisions, and negotiated positions between a range of professionals – often with fluid and overlapping roles. 6 Technology has encroached into healthcare decision-making, purporting to offer support, information and recommendations to help shape professional decisions. 7
In this synopsis we focus on nurses, midwives and allied health professionals (NMAHPs). Why? Because their work, demographic composition, educational levels, and socio-economic positions often differ from medicine and doctors, but they contribute to a complex, fluid, and – crucially – negotiated division of labour in healthcare. 8 Authority within this division of labour stems in part from the power to exercise clinical judgement, clinically reason and make or shape decisions. If we assume that work in healthcare is based on – and reflects – reasoned judgements and decisions, then it follows that different professionals in multidisciplinary teams will face different uncertainties, judgements and decisions. The support needed for tackling differing uncertainties may also be different.
Research into the decisions and decision-making of NMAHPs is relatively scarce compared to studies focusing on doctors and medical reasoning, although eminent decision scientists have studied nursing decisions since the 1960s. 9 Researchers have also described and typologised ‘nursing’ decisions. 3 Some scholars point out that there is no de facto reason why nurses – and by implication, other health professionals – should be treated as possessing their own, unique, decision-making cognition, even if decisions when viewed in context appear different. 10 Others have extended well-established descriptive and prescriptive theories of generic professional decision-making to incorporate forms of knowledge and knowing (such as ‘reflection-in-action’) associated with particular groups – notably nurses. 11
At the heart of attempts to describe, model and theorise clinical decision-making are two core constructs:
-
judgements – the weighing-up or evaluation of clinical, research or other information
-
decisions – choosing between discrete options.
The most parsimonious models of decision-making bring together judgement, choice and evaluation in a ‘feedback loop’. 12 Consequently, decision-making rarely feels like a discrete event made up separate ‘stages’ to the decision-maker. Despite the difficulties of ‘holistic’ decision-making as experienced by decision-makers, there is value in separating it into component elements; in part because the characteristics of decisions (1) determine the style of clinical reasoning best suited to a decision (intuition vs. rational information-processing), but also (2) shape the likelihood of using different forms of decision support. 3,11 The perceived time available to make choices, the perceived structure of a choice, and the need to show how you got to a judgement or decision (i.e. a choice’s visibility) can increase or decrease the chances of using technology-delivered support. 3,11
Support for decision-making often comes in the form of technologies – ranging from paper-based aids such as printed guidelines or research summaries, to web, app and computer-based decision support systems. More commonly, support can also come from informal resources such as a colleague’s advice, or, at the extreme, a professional’s own internalised resources in the form of experience, knowledge recalled from training, or just gut instinct or ‘intuition’. It is the application of computer technology to judgements and decisions that is our focus in this synopsis.
What are computerised decision support systems?
Computerised decision support systems (CDSS) are software- or computer-based technologies that offer patient-specific recommendations based on either research, expert opinion, machine learning/artificial intelligence or combinations of these, and designed to influence the clinical decision-making of health professionals. 13–15 CDSS access patient information from practitioners, healthcare staff, patients’ manual data entry or queries of electronic medical records before research or expert knowledge is assessed to provide computer-generated recommendations delivered to the clinician via a computer/tablet, mobile-phone screen or electronic medical record. Clinicians can then choose whether to use these recommendations. Examples of decision support used by NMAHPs include: assessing fall risk and preventative behaviours;16 pressure-ulcer management;17 selecting interventions for managing musculoskeletal disorders;18 screening for childhood language disorders;19 depression screening20 and, on a whole-system scale, choices faced in clinical pathways for primary care triage and prioritisation (https://digital.nhs.uk/services/nhs-pathways).
CDSS come in two main forms: (1) knowledge-based and (2) non-knowledge-based. 21 Knowledge-based CDSS use logical ‘IF-THEN-ELSE’ rules to evaluate information provided directly by a clinician or drawn from an electronic health record. These are then matched to a computerised knowledge base (in many cases expert opinion or national/international clinical practice guidelines) to provide assessments/management options/probabilities or actionable recommendations or outputs. 21,22 These forms of CDSS automate information-gathering and provide advice in line with guidelines. Examples of this type of CDSS are drug prescription/alert tools and emergency and out-of-hours telephone calls used for triaging patients. Non-knowledge-based CDSS use machine learning and artificial intelligence rather than flowchart-style rules or logic to support clinicians’ decision-making. 21 Typical examples of this type of CDSS are predictive risk models for assessing the prognosis of a disease outcome. 23 CDSS based around artificial intelligence and/or machine learning are less common than rule-based systems in NMAHP work.
CDSS systems can stand alone, integrated into, or at least capable of interacting with, wider digital infrastructure in health systems such as electronic health records (EHRs) or computerised physician order entry (CPOE – computer-based systems that automate instructions, with standardised, legible, and complete orders). They can be hosted via a computer, tablet or smartphone, and have web-based/local or ‘app’ interfaces. CDSS can present information on host devices or via the integrated EHR/CPOE system.
Why look at CDSS for NMAHPs?
NMAHPs make decisions that could benefit from digital support. Unwarranted variations in practice and outcome, for patients with seemingly similar issues and facing similar decisions and uncertainties, exist. These uncertainties make a synthesis of empirical research timely and useful. 24–26 New ways of working and support for these new roles for NMAHPs feature in many health systems. Opportunity costs associated with digital technology for learners and educators exist: professional preparation for and continuing professional development of digitally competent clinicians able to use new technologies effectively require time. 27
We had three main research-based motivations for the synthesis. First, clinical decision support systems will only be useful if they improve clinicians’ decision performance (for example, more accurate diagnoses and prognoses), improve patient health outcomes (e.g. morbidity, mortality, fewer adverse events), and offer perceived value for money for health services. 28,29 We do not know if any of these are true for NMAHPs.
Second, a previous review of studies on CDSS use by nurses found only limited impact on performance and health outcomes. 30 The review is more than 13 years old and digital technology and the research evidence base has developed significantly. The effect of CDSS on allied health professionals (AHPs) has not been reviewed systematically. Systematic reviews of studies on the impact of CDSS on healthcare delivery generally suggest they can improve practitioner performance in specific areas of decision-making such as diagnosis (4/10 systems), disease management (23/37 systems) and drug-dosing or prescribing systems (19/29 systems). 4 The impact on patient outcomes is more equivocal, with only 13% of systems (7/32) reporting improvements. 4 Reviews focusing on specific areas of clinical practice such as prescribing and drug dosing 31 or clinical subdomains such as neonatal care32 offer very limited conclusions, because the underpinning evidence is either absent,32 low quality and\or narrow in scope. 31
Third, existing reviews often neglect the fact that whilst multi-disciplinary team members may all be involved in delivering healthcare, their decisions reflect their role in the division of labour and so are likely to differ. Extant reviews often contain an implicit rationale that doctors’ decisions alone are the main mechanism for improving healthcare processes and outcomes. 4
How are clinical decision support systems supposed to improve decision-making?
Clinical decision support systems work by providing high-quality relevant useful information delivered when it is required to decision-makers. 13 The main generative mechanism by which CDSS aid NMAHPs decision-making is the combination of CDSS-generated information/suggestions with existing nurse or AHP knowledge. Thus, CDSS augment or supplement clinician decision-making rather than replacing it. CDSS are a key means of encouraging concordance with guideline-based care to reduce unwarranted variations in practice. 33
Examples of decisions supported by CDSS include:34
-
Recognising patient deterioration – CDSS can increase situational awareness or incorporation of relevant clinical and research-based information in reasoning, and tailoring of local or national guidance.
-
Determining patients with conditions that merit the application of clinical guidelines – CDSS improves the consistency of judgements and adherence to guideline recommendations and reduces (unwarranted) variation.
-
Triaging patients, often in the emergency department or primary care, to determine priority cases – the CDSS improves the reliability of judgements and simplifies choices by reducing the ‘noise’ in the situation and amplifying the appropriate ‘signals’ to encourage more appropriate decisions.
As with any health technology, CDSS will only improve care and health if actually used by nurses and AHPs in their decision-making. Whatever the quality of the underlying knowledge base, decision rules, analyses or algorithms, if unimplemented, or implemented badly, will not improve decision quality and patient benefit is less likely. Unfortunately, CDSS implementation and use by NMAHPs is rarely straightforward, and can be suboptimal. 35–37
CDSS can create the potential for harms as well as benefits. 38 These include fragmentation or disruption of work and workflow; alert fatigue; deskilling and the consequences on decisions of poor-quality or incorrect knowledge in the data used for inference or analysis. Additionally, CDSS may rely on a user’s computer literacy – something that is highly variable in nurses and AHPs. Systems can incur opportunity costs for clinicians as well as those charged with maintaining and supporting technology in health systems. 15 CDSS can also widen existing inequalities in access to high-quality care; for example, where effective CDSS are located only in prestigious teaching hospitals and associated with improved access to services, then patients who do not have access to teaching hospitals will be disadvantaged. 39,40
Thus, there are three main mechanisms by which CDSS ‘work’ in the context of decision-making by NMAHPs: successfully combining high-quality or novel CDSS information and clinician knowledge; improving quality of care processes and – by implication – outcomes, by improving the appropriateness of recommendations, management/treatment choices, accuracy of predictions or diagnoses; and successful implementation and use by clinicians.
Theoretical framework
We used theory in three ways. First, we drew on existing reviews and meta syntheses of characteristics of CDSS associated with improved outcomes and performance41,42 to test the hypothesis that possessing these characteristics would positively influence CDSS aimed at NMHAPs. Second, we used Normalisation Process Theory (NPT) as a lens through which we viewed the results of the included evidence – and their background, design, discussion and/or process evaluation/descriptions – to explore and explain the ways in which CDSS manage (or not) to become embedded and routine as a part of normal, taken-for-granted, practice. 43 NPT provided our focus for the implementation of CDSS: the ways that CDSS are used in their social context as a form of collective action by practitioners. 43 Third, NPT informed our approach to coding the qualitative responses of intended CDSS users/recipients in our stakeholder engagement/sense-check exercise (see ‘‘Calibration’ interviews’ and ‘PPI’ sections).
We considered other theoretical approaches. The NASSS framework44 (non-adoption, abandonment, scale-up, spread, sustainability) had similar a priori abilities to highlight the ways in which technologies are taken up or abandoned, but fewer people have used it in a decision support context. Actor Network Theory45 recognises that interactions between humans and technology can shift over time and are often ‘negotiated’. Applications of the theory, beyond using it as a general explanatory framework, would have entailed knowledge of the actor-networks, technologies and contexts that were often missing from study reports and beyond the scope and resources of our planned calibration exercise. NPT offered a practical, pragmatic, validated means of examining ‘what people do’ and ‘how they work’ to adopt and sustain CDSS in NMAHP work. In using this framework, this part of the study will add to the ≈130+ evaluations of varied interventions that have made explicit use of the theory. 46 It constitutes a middle-range theory of socio-technical change47 and a theoretical framework for understanding CDSS as complex interventions. 43
NPT can give a perspective on CDSS, both as a technology and as a set of practices related to that technology. 48 Whilst policy and government push the case for new technologies to deliver healthcare improvement (c.f. www.gov.uk/government/news/matt-hancock-launches-tech-vision-to-build-the-most-advanced-health-and-care-system-in-the-world) the empirical literature continues to highlight an implementation gap. 48 In using NPT we sought to address aspects of adoption (alongside our ‘core’ systematic review) sometimes downplayed in similar CDSS reviews. 4,41,49
NPT centres on four core constructs:50 ‘coherence’ – the extent to which an intervention is understood as meaningful, achievable and desirable; ‘cognitive participation’ – the enrolment of those actors necessary to deliver the intervention; collective action – the work that brings the intervention into use; and ‘reflexive monitoring’ – the ongoing process of adjusting the intervention to keep it in place. These four core constructs were used to frame our sense-check interviews with CDSS leaders, implementers and developers.
Aims and objectives
We sought to examine the impact on performance and patient outcomes associated with CDSS purporting to support the decisions and judgements of NMAHPs. To achieve this aim we had two objectives, to:
-
evaluate the clinical effectiveness and cost-effectiveness of CDSS on NMAHPs performance and patient outcomes
-
critically examine our findings in the light of interviews with people who design, implement and use CDSS systems, and to ‘calibrate’ our findings with reference to unpublished accounts.
Methods
To address our first objective, we undertook a systematic review 1 of studies comparing professionals using CDSS to those not using CDSS. Our second objective was addressed using qualitative interviews with individuals and groups seeking to encourage use of CDSS or who use or encounter them in services.
Literature searching
With an information specialist, we developed a search strategy designed to find studies focusing on CDSS and the healthcare professionals we were interested in: nurses, midwives and allied health professionals.
We ran the search strategy on multiple electronic databases and resources twice: October 2019 and February 2021. Specific databases searched included: MEDLINE (Ovid), Embase Classic+Embase (Ovid), PsycINFO (Ovid), HMIC (Ovid) Health Management Information Consortium, AMED (Allied and Complementary Medicine) (Ovid), CINAHL, Cochrane Central Register of Controlled Trials (Cochrane Database of Systematic Reviews, Wiley), Social Sciences Citation Index Expanded (Clarivate), ProQuest Dissertations & Theses Abstracts & Index, ProQuest ASSIA (Applied Social Science Index and Abstract), ClinicalTrials.gov, World Health Organisation International Clinical Trials Registry (ICTRP), Health Services Research Projects in Progress (HSRProj), OpenClinical (www.OpenClinical.org), OpenGrey (www.opengrey.eu), Health.IT.gov, Agency for Healthcare Research and Quality (www.ahrq.gov).
No date of publication and language restrictions were applied to the search. See Appendix 1 for full strategy and terms.
Deciding which studies to include or exclude
Between them, six of the research team screened all the titles and abstracts retrieved. Two of the team (CT and TM) used Cochrane Collaboration Effective Practice Organisation of Care Review Group criteria51 and the study aims and objectives to decide if studies were relevant. We restricted our review to studies which compared CDSS-use to non-use, evaluated using designs less likely to lead to biased conclusions:
-
randomised controlled trials (RCTs)
-
non-randomised trials (NRCT)
-
controlled before-and-after (CBA) studies
-
interrupted time series (ITS) and repeated measures studies.
Participants
We included studies evaluating the effects of CDSS use by NMAHPs, qualified or in training, and working in primary or secondary care. We had a long list of allied health professional categories, but in the end only paramedics, dieticians and physiotherapists were the focus of the comparative evaluations included.
Interventions
The intervention in the review was the use of any form of CDSS to aid clinical decision-making.
Comparator
The comparator was usual care: clinical practice where clinical decision-making is unsupported by CDSS. Studies must have compared care, treatment, diagnosis or management using CDSS with care, treatment or management without CDSS. We excluded CDSS aimed at diagnostic judgements where the evaluation was only against a defined reference standard. We included studies of CDSS aimed at diagnostic judgements where clinical performance with and without the CDSS featured.
Outcomes
Our primary outcome was the adherence of nurses and AHPs to evidence-based recommendations. Secondary outcomes included diagnostic accuracy, time to judgement, adverse events, health professional satisfaction, patients’ health-related quality of life and costs.
Data extraction
Data on study characteristics and outcomes were independently extracted by two reviewers (TM, CT) using the Cochrane Collaboration’s EPOC standard data-collection form. 52 A third reviewer (RR) was available to resolve disagreements if needed; none occurred.
We extracted data on:
-
methods: study design, location, study setting, and date of study
-
participants: number, mean age (age range), gender, inclusion criteria, exclusion criteria of patients and providers
-
interventions: intervention components, comparison, presence of characteristics known to increase effectiveness in CDSS generally
-
outcomes: main and other outcomes specified and collected, time points reported
-
study funder.
Quality assessment
Study quality was assessed using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions Section 8.553 and EPOC guide. 54 TM and CT assessed studies for risk of bias. Each potential source was judged as high, low, or unclear. An overall ‘Risk of bias’53 assessment was set: high – a serious bias likely to decrease certainty in the results; moderate – a risk that could plausibly raise doubts about conclusions; low – risks were unlikely to alter the results.
Data synthesis
We explored heterogeneity between CDSS systems and outcomes to determine whether meta-analysis was feasible. Heterogeneity between studies in the nature of the interventions, target groups, and outcomes measures in our initial pre-searches meant a narrative approach to synthesising findings was most appropriate. Studies were grouped and summarised by clinical similarity, for example, topics studied, type of CDSS, types of health professionals involved, patient group, outcomes reported and study design.
Intervention effects were estimated using risk difference for dichotomous data and mean differences for continuous data. We calculated 95% confidence intervals where possible. 53 Where absolute risks were not reported, these were generated from study information. Risk difference values and 95% confidence intervals were then calculated using the absolute risk values of the comparative groups.
Missing data
We contacted investigators of primary studies to verify study characteristics and obtain missing outcome data where only study abstract or results were presented in published manuscripts. Missing summary data were computed from other reported statistics wherever possible.
Investigating the effects of CDSS characteristics on outcomes
For each included study, we abstracted information on 16 system characteristics associated with effectiveness in CDSS for each study. 41 We classified each as present or absent (the predictor variable). A categorical dependent variable of either ‘success’ (CDSS better than usual care in at least one of the outcomes reported in each study) or ‘failure’ (usual care better than CDSS in one of the outcomes reported) was created for each of the 35 included studies. To evaluate whether CDSS-generated outcomes were associated with these characteristics, logistic regression models were constructed using the approach advocated by Firth for generating robust standard errors. 55 We set a 5% significance level and 95% confidence intervals for each CDSS characteristic.
‘Calibration’ interviews
We sought to access the reported experience and perceptions of key staff involved in the implementation and use of CDSS in services to sense-check our synthesis results and aid presentation. We (prior to the COVID-19 pandemic) planned a national online survey of UK NHS Chief (Nursing/AHP) Informatics Officers, but attempts at recruitment using NHSE email-based lists and forums and social media were disappointing. The COVID-19 pandemic and redeployment of key staff meant our original approach was not feasible. We eventually identified six key CDSS leaders in a range of organisations and with links to policy as well as delivery: two acute NHS Trusts (one of which was a large teaching hospital); one mixed acute and community semi-rural NHS Trust; an academic health science network lead with links to a large district general hospital-style Trust; a senior policy-level NHS lead for CDSS; a clinical academic with strategic and operational leadership role in a large urban hospital. Their implementation of CDSS varied from 20 years ago (the large teaching hospital) with most in the last three years.
Whilst two of the leaders highlighted specific system-user professional roles (such as ‘nurses’) or by clinical area (such as ‘renal’) the rest indicated that a wide multidisciplinary staff base were the intended users – including nurses and AHPs. The systems involved were intended to support a wide range of decisions; for example, disease management, detection, diagnosis, generating treatment options, forecasting/prognosis and triage.
We conducted individual virtual interviews, lasting around 40 minutes to one hour, via Zoom or telephone with our six CDSS leaders using an interview schedule developed to address the four main concepts of NPT (coherence, cognitive participation, collective action, reflexive monitoring) and contextualised for CDSS – as the ‘innovation’ or new way of working in NPT – and drawing on our main findings as prompts for discussion/sense-checking. Analysis of transcribed data and notes was abductive56 and thematic57 following the process outlined by Braun and Clarke. 58 An initial codebook was generated based on NPT constructs and sub-constructs and text read and coded. We used matrices59 with NPT constructs as columns, text from each participant as rows before comparison between participants and across columns in a version of metacoding. 60 Two of the team developed sub-themes from the initial codes of the four NPT key concepts. This was a small scale ‘pragmatic’ qualitative analysis aimed at helping understand uncertain review findings; we did not carry out inter-coder reliability checks and other qualitative-analytic techniques.
PPI
In conjunction with our PPI co-applicant (AL) we invited eight members of a single GP practice’s Patient Participation Group to a virtual meeting in early 2021. Our PPI co-applicant (AL) hosted the meeting, supported by one of the research team. Participants were sent a description of the purpose and use of CDSS several days before the meeting and asked to consider issues related to patient care and experience of consultations. The practice had a CDSS system embedded into its EHR system. Advanced practitioners, practice nurses and the practice physiotherapist accessed the EHR and CDSS system both during and outside consultations or treatment. In the meeting participants were presented with some of the uncertainties that the team felt were unaddressed by the included studies and offered the chance to ask new questions. The meeting resulted in 12 frequently asked questions (FAQs) that a patient faced with a nurse or AHP using a CDSS might ask. After the meeting, participants were asked to vote via email on the five FAQs they identified as ‘most important’.
The project team and the primary research had dedicated PPI expertise from our co-applicant and team member Alison Ledward, an experienced partner in health research and with a background in education and social work. The virtual stakeholders were from a semi-rural area of southern England with only small pockets of socio-economic deprivation. Of note is the almost complete absence of PPI and information related to diversity, inclusion and equality in the research study reports synthesised (Figure 1).
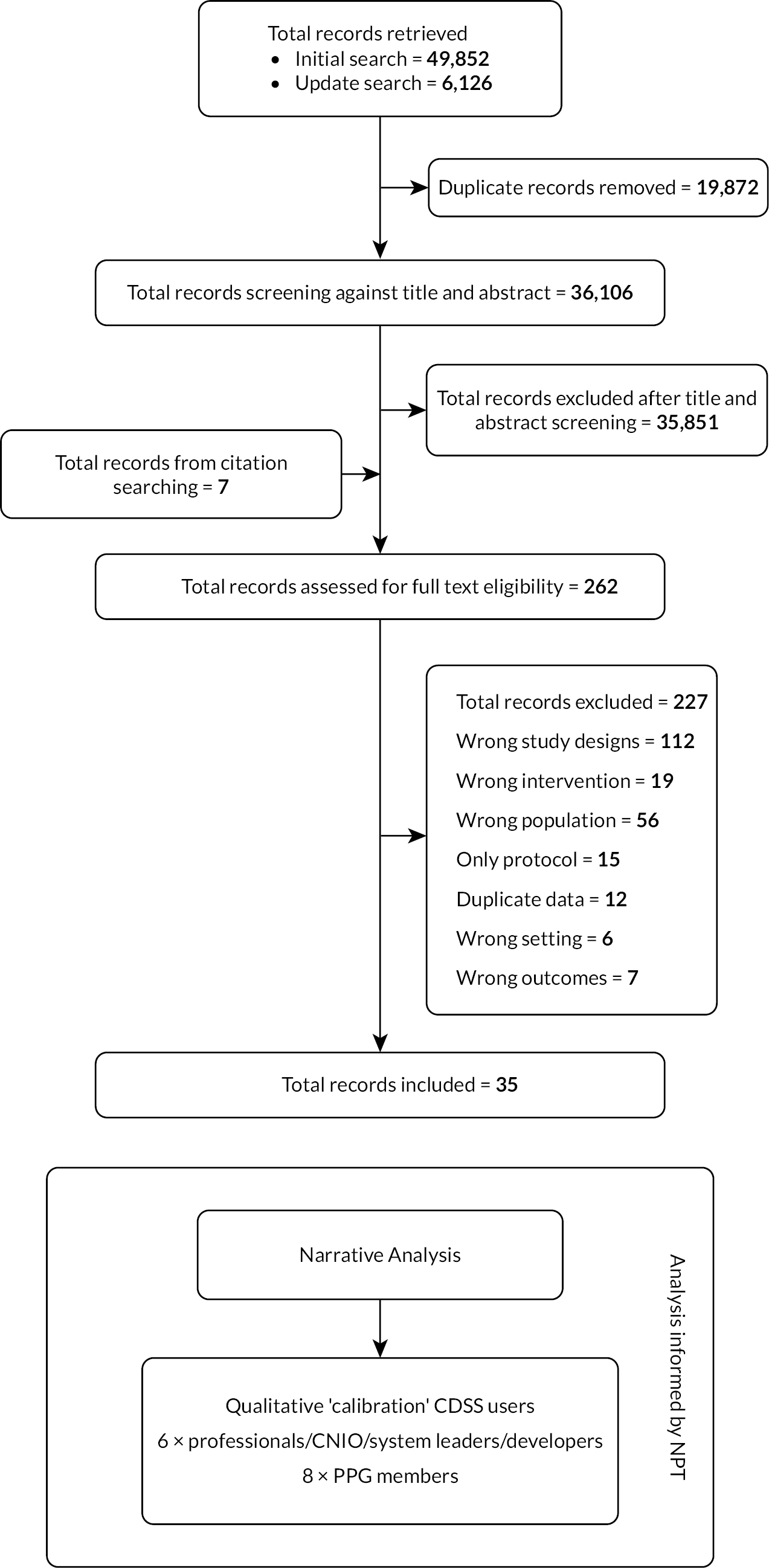
Figure 1.
Research Pathway Diagram including PRISMA flow chart of study selection. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71.
Findings from the systematic review
From 36,106 initially identified publications, we screened the full text of 262 papers to arrive at our final synthesis of 35 studies. The included studies (see Table 1) were mainly randomised controlled trials (RCTs) (n = 28, 80%) with the other 20% a mix of controlled before-and-after (CBA) studies, interrupted time series (ITS) and a single non-randomised trial (NRCT). Eighty-three per cent (n = 29) of the included studies were published after our previous systematic review of decision support in 2007;30 most examined the effects on hospital staff (57%) and Western healthcare systems (USA, UK, Netherlands, Czech Republic and Norway provided the backdrop for 75% of the studies). A single study reported theory to inform the design of the intervention and/or implementation. Just less than a third of the studies (28%) had a published protocol to compare the reported study against.
| Author and year | Country | Design | Setting | Number of sites | Study duration | HPs involved | Outcomes | Interventions |
|---|---|---|---|---|---|---|---|---|
| Beeckman et al. 201389 | Belgium | RT | Nursing homes | 4 | 5 months | Nurses and physios | Risk of pressure ulcers; HP knowledge and attitude | Pre-vPlan (a six-step clinical practice to reduce pressure ulcers using CDSS) A standard protocol (a hard copy with no implementation strategy) of reducing pressure ulcers |
| Bennet et al. 201661 | UK | ITS | Emergency department, district general hospital | 1 | 1 year | Nurses | Triage prioritization; pain assessment and management; management of neutropenic sepsis | Triage CDSS [intervention period] Triage CDSS [pre-intervention period] |
| Blaha et al. 200975 | Czech Republic | RT | ICU post elective cardiac surgery, university hospital | 1 | 48 hours | Nurses | Intensive care glycaemic control/diabetes | Intervention (CDSS-model predictive control algorithm) Control-1 (paper based-Matias protocol) Control-2 (paper based-Bath protocol) |
| Byrne 200583 | USA | CBA | Nursing homes | 90 | 33 months | Nurses | Falls and pressure ulcer reduction (assessment and prevention) | CDSS use CDSS non-use |
| Canbolat et al. 201976 | Turkey | Non-RT | ICU university general hospital | 1 | 22 months | Nurses (and physicians) | ICU glycaemic control | CDSS use Usual care |
| Cavalcanti et al. 200977 | Brazil | Clustered RT | ICU general hospital | 5 | 19 months | Nurses | ICU glycaemic control | Intervention (CDSS use computer-assisted insulin protocol) Control-1 (Leuven protocol) Control-2 (conventional treatment) |
| Cleveringa et al. 200871 | Netherlands | Clustered RT | Primary care practices | 26 | 1 year | Nurses (and physicians) | Management and prevention of diabetes (and CV risk factors) | CDSS use Usual care |
| Cleveringa et al. 201072 | Netherlands | Clustered RT | Primary care practices | 26 | 1 year | Nurses | Management and prevention of diabetes (and CV risk factors) | Same as Cleveringa et al. 2008 but a cost effectiveness study. |
| Cortez 201466 | USA | Clustered RT | Academic medical centre oncology clinics | 4 | 11 weeks | Nurses | Management of cancer symptoms | Intervention (drop down boxes) Control (no drop-down boxes) |
| Dalaba 201586 | Ghana | CBA | Primary care health centres (midwifery) | 12 | 2 years | Nurses | Maternal care | CDSS use Usual care (CDSS non-assisted) |
| Dowding et al. 201290 | USA | ITS | General hospitals | 29 | 6 years | Nurses | Risk assessment, falls and pressure ulcer prevention | CDSS use Usual care (CDSS non-assisted) |
| Duclos et al. 201584 | France | Clustered RT |
Paediatric wards in a university hospital | 6 | 2 years | Dieticians | Nutritional care in malnourished children | CDSS use Usual care (CDSS non-assisted) |
| Dumont et al. 201278 | USA | RT | ICU wards in a regional referral hospital | 1 | 4 months | Nurses | Glycaemic control | CDSS use paper protocol (modified Portland protocol) |
| Dykes et al. 201091,92 | USA | Clustered RT | Urban hospitals | 4 | 6 months | Nurses | Fall prevention | CDSS use |
| Usual care | ||||||||
| Dykes et al. 202092 | USA | ITS | Academic medical centres | 3 | 42 months | Nurses | Fall prevention | Pre-intervention period |
| Post-intervention period | ||||||||
| Fitzmaurice et al. 200067 | UK | RT | Primary care/general practice | 12 | 1 year | Nurses | Oral anticoagulation care | CDSS use (nurses) CDSS non-assisted physicians |
| Forberg et al. 201685 | Sweden | Clustered RT | Paediatric university hospital | 12 | 3 months | Nurses | Management of peripheral venous catheters in paediatrics | CDSS use Usual care (CDSS non-assisted) |
| Fossum et al. 201193 | Norway | CBA | Nursing homes | 15 | 2 years | Nurses | Preventative behaviours and management of nutrition | CDSS use Usual care (CDSS non-assisted) |
| Geurts et al. 201773 | Netherlands | RT | University paediatric hospital | 1 | 2 years | Nurses | Management of (re)hydration in children | Nurse-led CDSS Usual care |
| Hovorka et al. 200779 | Czech Republic | RT | Cardiac surgery, university hospital | 1 | 48 hours | Nurses | Glycaemic control | CDSS use Usual care (CDSS non-assisted) |
| Kroth et al. 200668 | USA | RT | University hospital | 1 | 9 months | Nurses | Body temperature assessment | CDSS use Usual care (CDSS non-assisted) |
| Lattimer et al. 199864 | UK | RT | Primary care practices | 1 | 1 year | Nurses & physicians | Emergency call assessment | Nurses with CDSS) Control (doctors with no CDSS) |
| Lattimer et al. 200065 | UK | RT | Primary care practices | 1 | 1 year | Nurses & physicians | Cost analysis of emergency call assessments | Nurses with CDSS) Control (doctors with no CDSS) |
| Lee et al. 200974 | USA | RT | University trainee –school of nursing | 1 | 8 months | Nurses | Obesity management | CDSS use Usual care (CDSS non-assisted) |
| Lv et al. 201994 | China | RT | Community healthcare centres | 4 | 1 year | Nurses | Chronic asthma management | CDSS use |
| Usual care | ||||||||
| Mann et al. 201180 | USA | RT | Surgical military hospital ICU | 1 | 6 days | Nurses | Glycaemic control in burn intensive care patients | CDSS use Usual care (paper-based protocol) |
| McDonald et al. 201788 | USA | RT | Nursing care homes | 1 | 2 months | Nurses | Management of chronic medical condition | CDSS use Usual care |
| Paulson et al. 202095 | Norway | RT | University hospital | 1 | 10 months | Nurses | Management of malnutrition | CDSS use |
| Usual care | ||||||||
| Plank et al. 200681 | Mixed (Austria, Czech Republic, UK) | RT | University hospitals | 3 | 48 hours | Nurses | Glycaemic control | Intervention (CDSS-model predictive control (MPC)) Control (Routine Treatment Protocol (RTP)) |
| Rood et al. 200569 | Netherlands | RT | Surgical ICU in a teaching hospital | 1 | 10 weeks | Nurses | Glycaemic control | Intervention (CDSS based guideline) Control (paper-based guideline) |
| Roukema et al. 200887 | Netherlands | RT | Children’s hospital | 1 | 27months | Nurses | Management of children with fever without apparent source | Nurses (CDSS use) Physicians (CDSS non-assisted) |
| Sassen et al. 201470 | Netherlands | RT | University research centre | recruited Online | 17 months | Nurses and physios | professionals’ behaviour | Intervention (CDSS use) Control (no CDSS use) |
| Snooks et al. 201462 | UK | RT | Emergency ambulance services | 13 | 1 year | Paramedics | Assessment and management of falls | CDSS (used hand-held tablet computers for decisions) Usual care (no CDSS use) |
| Vadher et al. 199782 | UK | RT | Cardiovascular medicine, general hospital | 1 | A nurse and trainee doctors | oral anticoagulant control | Intervention (nurse with CDSS) Control (trainee doctor without CDSS) |
|
| Wells 201363 | UK | RT | Emergency ambulance services | 13 | 1 year | Paramedics | Emergency fall assessment and management | CDSS (used hand-held tablet computers for decisions) Usual care (no CDSS use) |
Who are the users of evaluated CDSS?
Overwhelmingly, evaluations focused on single disciplines using CDSS compared to similar professionals making unaided decisions: nurses (n = 25, 71%) and paramedics (6%). Fewer evaluations compared CDSS supported nurses to CDSS unsupported doctors, or a multidisciplinary mix of nurses and physiotherapists in intervention and control groups.
What about the CDSS systems?
Most CDSS come as standalone computer-based systems (89%, n = 31) with less than 10% accessed via mobile technology or the web. All the CDSS were ‘knowledge-based’ (see earlier typology) and whilst single-function systems (such as disease management) were the norm, there were examples of multi-function CDSS (for example, diagnosis and management).
-
Triage – five studies in emergency care61–63 and primary care. 64,65
-
Disease management – five studies: managing cancer symptoms,66 oral anticoagulation,67 temperature monitoring,68 blood glucose monitoring,69 and optimising shared decision-making for self-management. 70
-
Diagnosing and managing disease – four studies; diagnosing and treating diabetes;71,72 recognising and acting on clinical dehydration in acute gastroenteritis;73 and screening, automated diagnosis, and care-planning for people with obesity. 74
-
Drug dosing – eight studies: mainly in blood glucose control in intensive and emergency environments75–81 and oral anticoagulant regimens in hospital cardiovascular patients. 82
-
Reminder systems – three studies used CDSS for reminders on disease prevention,83 disease diagnosis,84 and disease management. 85 Three others used reminders for multiple functions: disease prevention and management,86 disease diagnosis and management,87 and disease diagnosis reminder/alert along with disease diagnosis and management. 88
Are evaluations of CDSS for nurses, midwives and AHPs biased?
With the exception of three RCTs (classed as ‘unclear’), all the studies’ risks of generating biased conclusions were ‘high’ (Table 2). The threat of bias did not diminish over time. In RCTs, NRCTs, and CBA studies, sources of bias encountered included no randomisation (13%) or unclear randomisation (27%); unclear (38%) or not done (17%) allocation; only a third of studies (n = 10) reported similar baseline measures of outcome and a single study only adjusted their analysis for any differences. Seventeen studies did not specify baseline outcome measurements in their report (57%) and whilst baseline characteristics of providers and patients were similar in around a third of the studies, they differed in another third, and in a further third only patient characteristics were reported – despite the fact that the decisions supported were made primarily by professionals. Half of the 32 randomised studies did not specify missing data and 15 of 32 (47%) studies failed to specify whether CDSS users had knowledge of how they had been allocated to intervention and control groups. In 60% of evaluations (18 studies), ‘contamination’ was likely or could not be ruled out. More positively, there was no evidence of selective reporting of outcomes. For the three evaluations based around an interrupted time series (Table 3), whilst confounding was an issue, there were no issues with selective outcome reporting, missing data or lack of clarity about when the ‘interruption’ happened.
| Author and year | Risk of bias domains and scores | Overall bias score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Baseline outcome measurements similar | Baseline characteristics similar | Incomplete outcome data | Knowledge of the allocated interventions adequately prevented during the study | Protection against contamination | Selective outcome reporting | Other bias | ||
| Beeckman et al. 201389 | Low | High | Low | Low | Unclear | High | Low | Low | Low | High |
| Blaha et al. 200975 | Unclear | Unclear | Low | Unclear | Low | Low | Unclear | Low | Low | Unclear |
| Byrne 200583 | High | High | Low | High | Unclear | Unclear | Low | Low | High | High |
| Canbolat et al. 201976 | High | High | Unclear | High | Unclear | Unclear | High | Low | Unclear | High |
| Cavalcanti et al. 200977 | Low | Low | Unclear | High | Low | Unclear | Unclear | Low | Low | High |
| Cleveringa et al. 200871 | Low | Low | Low | High | Unclear | Unclear | Low | Low | Low | High |
| Cleveringa et al. 201072 | Unclear | Low | Low | High | Low | Unclear | Low | Low | Low | High |
| Cortez 201466 | Unclear | Low | High | Low | Low | Low | Low | Low | Low | High |
| Dalaba et al. 201586 | High | High | High | High | Unclear | Unclear | Low | Low | Low | High |
| Duclos et al. 201584 | Low | Low | High | High | Low | Unclear | Unclear | Low | Low | High |
| Dumont et al. 201278 | Unclear | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Low | High | High |
| Dykes et al. 201091 | Unclear | Low | Low | Unclear | Low | High | High | Low | Low | high |
| Fitzmaurice et al. 200067 | Low | Unclear | Low | High | Low | Low | Unclear | Low | Low | High |
| Forberg et al. 201685 | Low | Unclear | Low | Low | Low | Unclear | High | Low | Low | High |
| Fossum et al. 201193 | High | High | Low | Unclear | Low | Unclear | Low | Low | Low | High |
| Geurts et al. 201673 | Low | Low | Unclear | Low | Low | Low | High | Low | High | High |
| Hovorka et al. 200779 | Low | Low | Unclear | Unclear | Unclear | Low | High | Low | Low | High |
| Kroth et al. 200668 | Low | Unclear | Unclear | High | Low | Low | Low | Low | Low | High |
| Lattimer et al. 199864 | Low | Low | Unclear | Unclear | Unclear | Low | Low | Low | Low | Unclear |
| Lattimer et al. 200065 | Unclear | Unclear | Unclear | High | Unclear | Low | Low | Low | Low | Unclear |
| Lee et al. 200974 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High | Low | Low | High |
| Lv et al. 201994 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | High | Low | Low | High |
| Mann et al. 201180 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | High | Low | Low | High |
| McDonald et al. 201788 | Low | Low | Unclear | Low | Low | High | Unclear | Low | High | High |
| Paulson et al. 202095 | Low | Low | Unclear | Low | High | Low | Unclear | Low | Low | High |
| Plank et al. 200681 | Unclear | Unclear | Unclear | High | Low | Low | High | Low | Low | High |
| Rood et al. 200569 | Low | Unclear | Unclear | Unclear | Unclear | Unclear | High | Low | Low | High |
| Roukema et al. 200887 | Low | Low | Unclear | Unclear | Unclear | Unclear | High | Low | Low | High |
| Sassen et al. 201470 | Unclear | Low | Low | Low | High | High | Low | Low | Low | High |
| Snooks et al. 201462 | Low | Low | Unclear | Unclear | Unclear | Low | Low | Low | Low | Unclear |
| Vadher et al. 199782 | Low | Unclear | Unclear | Low | Unclear | Low | High | Low | High | High |
| Wells 201363 | Low | Unclear | Unclear | Low | Unclear | Low | High | Low | Low | High |
| Author and year | Risk of bias domains and scores | Overall bias | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention independent of other changes | Shape of the intervention effect pre-specified | Intervention unlikely to affect data collection | Knowledge of the allocated interventions adequately prevented during the study | Incomplete outcome data adequately | Selective outcome reporting | Other bias | ||
| Bennet 201661 | High | Low | Low | Low | Low | Low | Low | High |
| Dykes et al. 202096 | High | Low | Low | Low | Low | Low | Low | High |
| Dowding et al. 201290 | High | Low | Low | Low | Low | Low | Low | High |
The impact of CDSS on performance and outcomes
A broad range of outcomes are used in evaluations of CDSS: 119 outcomes, with 111 different measures. They can be grouped into five areas: (1) care processes, (2) care outcomes, (3) professional knowledge, beliefs and behaviour, (4) safety and (5) economic costs and consequences.
Care processes
There were 34 process outcomes reported. CDSS improved just less than half of these (16/34, 47%) in four evaluations 69,78,82,85 (Table 4). Conversely, outcomes were worse or no different for 53% of process outcomes (18/34) (Table 5).
| Author and year | Interventions | Health professionals | Patient participants | Outcome measured | Outcome values reported | Change of value within a groupa | Risk difference (95% CI)a |
|---|---|---|---|---|---|---|---|
| Adherence to guidelines | |||||||
| Dumont et al. 201278 | CDSS use | Nurses (OA = 44) | 141 adults | Deviations from the protocol, out of 10 (mean (SD)) | 4 months = 0.39(1.0) | – | Mean difference: –2.61 (–4.5 to –0.71) |
| Paper protocol | Nurses | 159 adults | 4 months = 3.0(4.3) | ||||
| Forberg et al. 201685 | CDSS use | 108 nurses | Not applicable | Nurses adherence to guidelines on disinfection of hands | Baseline = 97/108 3 months = 93/105 |
–1.2% | 6.7% (4.9 to 8.5) |
| CDSS non-use | 103 nurses | Not applicable | Baseline = 96/103 3 months = 87/102 |
–7.9% | |||
| CDSS use | Nurses adherence to guidelines on usage of disposable gloves (n/N) | Baseline = 80/108 3 months = 76/105 |
–1.7% | –1.4% (–2.2 to –0.5) | |||
| CDSS non-use | Baseline = 71/103 3 months = 70/102 |
–0.3% | |||||
| CDSS use | Nurses adherence to guidelines on daily inspection of peripheral venous catheters (PVC) site (n/N) | Baseline = 58/108 3 months = 58/103 |
2.6% | –5.2% (–7.1 to –3.3) | |||
| CDSS non-use | Baseline = 47/102 3 months = 55/102 |
7.8% | |||||
| Rood et al. 200569 | CDSS-based GL | ICU nurses | 66 adults | Adherence to insulin dose advice (n/N) | 10 weeks = 1818/2352 | – | 22% (19 to 25) |
| Paper-based GL | ICU nurses | 54 adults | 10 weeks = 1667/2597 | – | |||
| CDSS-based GL | ICU nurses | 66 adults | Adherence to the guideline for taking blood samples on time (n/N) | 10 weeks = 945/2352 | – | 4.7% (2.0 to 7.4) | |
| Paper-based GL | ICU nurses | 54 adults | 10 weeks = 922/2597 | – | |||
| Vadher et al. 199782 | CDSS | 1 nurse | 87 adults | Dose advice ‘acceptance’ in patients with therapeutic range 2–3 | Post-test = 188/214 | – | 28% (20.4 to 35.5) |
| Control | 3 trainee doctors | 90 adults | Post-test = 145/242 | – | |||
| CDSS | 1 nurse | Dose advice ‘acceptance’ in patients with therapeutic range 3–4.5 (n/N) | Post-test = 160/239 | – | –6.2% (–14.7 to 2.2) | ||
| Control | 3 trainee doctors | Post-test = 150/205 | – | ||||
| CDSS | 1 nurse | Interval advice ‘acceptance’ (%) in patients with therapeutic range 2–3 | Post-test = 170/230 | – | 23.9% (15.6 to 32.2) | ||
| Control | 3 trainee doctors | Post-test = 133/266 | – | ||||
| CDSS | 1 nurse | Interval advice ‘acceptance’ (%) in patients with therapeutic range 3–4.5 | Post-test = 129/239 | – | 3.9% (–5.4 to 13.3) | ||
| Control | 3 trainee doctors | Post-test = 101/202 | |||||
| Patient assessment, diagnosis, and treatment practices | |||||||
| Bennett et al. 201661 | CDSS use period | Pain assessment | Post-test = 97.7% | – | 62.7% (59.6 to 65.8) | ||
| CDSS non use | Pre-test = 35% | ||||||
| CDSS use | IV antibiotics in 1hour for sepsis | Post-test = 5.6% | – | –5.9% (–8.3 to –3.5) | |||
| CDSS non use | Pre-test = 11.5% | ||||||
| Duclos et al. 201584 | CDSS | Dieticians | 667 children | Investigation of malnutrition aetiology | Post-test = 284/667 | 21.2% (15.9 to 26.5) | |
| Usual care | Dieticians | 477 children | Post-test = 102/477 | ||||
| CDSS | Dieticians | 667 children | Managed by a dietitian | Post-test = 305/667 | 12% (6.3 to 17.7) | ||
| Usual care | Dieticians | 477 children | Post-test = 161/477 | ||||
| CDSS | Dieticians | 667 children | prescribed refeeding protocol | Post-test = 230/667 | –4.5% (–10.2 to 1.2) | ||
| Usual care | Dieticians | 477 children | Post-test = 186/477 | ||||
| Geurts et al. 201773 | CDSS | Nurses | 113 children | Patient consultation time(min)-median (IQR) | Post-test = 136(108) | – | 3 minutes |
| Usual care | Nurses | 109 children | Post-test = 133(92) | ||||
| CDSS | Nurses | 113 children | Electrolyte level test | Post-test = 15/113 | – | –7.8% (–17.7 to 2.1) | |
| Usual care | Nurses | 109 children | Post-test = 23/109 | ||||
| CDSS | Nurses | 113 children | Acid-base balance test | Post-test = 13/113 | – | –3.2% (–12.1 to 5.7) | |
| Usual care | 109 children | Post-test = 16/109 | |||||
| CDSS | Nurses | 113 children | Oral rehydration solution (nasogastric tube) | Post-test = 17/113 | – | 6.7% (–1.6 to 15.2) | |
| Usual care | Nurses | 109 children | Post-test = 9/109 | ||||
| CDSS | Nurses | 113 children | IV rehydration given | Post-test = 0/113 | – | –1.8% (–4.4 to 0.7) | |
| Usual care | Nurses | 109 children | Post-test = 2/109 | ||||
| CDSS | Nurses | 113 children | Other liquid given | Post-test = 18/113 | – | –11.6% (–22.4 to –0.8) | |
| Usual care | Nurses | 109 children | Post-test = 30/109 | ||||
| Roukema et al. 200887 | CDSS use | Nurses | 74 children | Time spent in ED (minutes), median (IQR) | 27 months = 138 (77) | – | 15 minutes |
| Control | Nurses | 90 children | 27 months = 123 (96) | ||||
| CDSS use | Nurses | 74 children | Time spent in ED for lab test (minutes), median (IQR) | 27 months = 140 (68) | – | –20 minutes | |
| Control | Nurses | 90 children | 27 months = 160 (98) | ||||
| Snooks et al. 201462 | CDSS | 17 paramedics | 436 adults | Mean length of episode of care (minutes) | CDSS vs. control | – | –5.7 min (–38.5 to 27.2)b |
| Control | 19 paramedics | 343 adults | |||||
| Wells 201363 | CDSS | 22 paramedics | 436 adults | Respiratory rate recorded, % | 1 year = 405/436 | – | –1.2% (–4.7 to 2.2) |
| Control | 20 paramedics | 341 adults | 1 year = 321/341 | ||||
| CDSS | 22 paramedics | 436 adults | Pulse rate recorded | 1 year = 414/436 | – | 0.9% (–3.9 to 2.0) | |
| Control | 20 paramedics | 341 adults | 1 year = 327/341 | ||||
| CDSS | 22 paramedics | 436 adults | Consciousness recorded | 1 year = 405/436 | – | –5.1% (–7.9 to –2.2) | |
| Control | 20 paramedics | 341 adults | 1 year = 334/341 | ||||
| Kroth et al. 200668 | CDSS use | 164 nurses | Not applicable | Proportion of erroneously recorded temperatures | 9 months = 248/45823 | – | –0.8% (–0.9 to –0.6) |
| Control | 173 nurses | Not applicable | 9 months = 575/44339 | ||||
| Documenting of events | |||||||
| Dowding et al. 201290 | CDSS use | Nurses | Fall documentation ratio | Post-CDSS use vs. pre-CDSS use period | – | 1.4 (0.03 to 73.7) b | |
| CDSS non-use | Nurses | ||||||
| CDSS use | Hospital acquired pressure ulcer (HAPU) risk documentation ratio | Post-CDSS use vs. pre-CDSS use period | 9.1 (1.95 to 42.5) b | ||||
| CDSS non-use | |||||||
| Paulson et al. 202095 | CDSS use | Nurses | 44 adults | Documentation of nutritional intake compared to requirements | 10 months = 37/44 | 80% (67 to 92) | |
| Usual care | Nurses | 50 adults | 10 months = 2/50 | ||||
| CDSS use | Nurses | 44 adults | Documentation of a nutritional care plan | 10 months = 31/44 | 54.4% (37.6 to 71.3) | ||
| Usual care | Nurses | 50 adults | 10 months = 8/50 | ||||
| CDSS use | Nurses | 44 adults | Documentation of nutritional treatment | 10 months = 36/44 | 23.8% (6 to 41.6) | ||
| Usual care | Nurses | 50 adults | 10 months = 29/50 | ||||
| Patient referrals | |||||||
| Snooks et al. 201462 | CDSS | 17 paramedics | 436 adults | Patients referred to falls service | 1 year = 42/436 | 4.7% (1.1 to 8.3) | |
| Control | 19 paramedics | 343 adults | 1 year = 17/343 | ||||
| Author and year | Interventions | Health professionals | Patient participants | Outcome measured | Outcome values reported | Change of value within a groupa | Risk difference (95% CI)a |
|---|---|---|---|---|---|---|---|
| Glycaemic control | |||||||
| Blaha et al. 200975 | CDSS (eMPC) | ICU nurses | 40 adults | Entire study time in target range (blood glucose) (mmol/l) | After 48 hrs = 46% | – | Versus Mathias: 7.8% (–13.7 to 29.4) Versus Bath 6.3% (–3.9 to 16.5) |
| Mathias protocol | 40 adults | After 48 hrs = 38.2% | – | ||||
| Bath-protocol | 40 adults | After 48 hrs = 39.7% | |||||
| CDSS (eMPC) | ICU nurses | 40 adults | Entire study mean blood glucose (SE) (mmol/l) | Baseline = 8.1(0.6) 48 hrs = 5.9(0.2) |
–2.2 mmol/l | Versus Mathias: –1 mmol/l Versus Bath: –0.7 mmol/l |
|
| Mathias protocol | 40 adults | Baseline = 7.9(0.4) 48 hrs = 6.7(0.1) |
–1.2 mmol/l | ||||
| Bath-protocol | 40 adults | Baseline = 8.0(0.2) 48 hrs = 6.5(0.2) |
–1.5 mmol/l | ||||
| Canbolat et al. 201976 | CDSS (automated BG control) | Nurses | 33 adults | Occasions for BG out of target (120 to 180 mg/dl) range | 22 months = 2101/5789 | – | –21.8% (–23.7 to –20.0) |
| Standard protocol | Physicians | 33 adults | 22 months = 2977/5122 | ||||
| CDSS (automated BG control) | Occasions for BG out of target range due to insulin treatment | 22 months = 745/5789 | – | –28.1% (–29.7 to –26.5) | |||
| Standard protocol | 22 months = 2099/5122 | ||||||
| Cavalcanti et al. 200977 | CDSS (computer-assisted insulin protocol) | ICU nurses | 56 adults | Mean blood glucose (mmol/dl) | 19 months = 125 | – | Versus Leuven –2.1 mmol/dl Versus conventional –33.5 mmol/dl |
| Control (Leuven protocol) | ICU nurses | 58 adults | 19 months = 127.1 | – | |||
| Control (conventional treatment) | ICU nurses | 53 adults | 19 months = 158.5 | ||||
| CDSS (computer-assisted insulin protocol) | ICU nurses | 56 adults | Patients with hypoglycaemia | 19 months = 12/56 | – | Versus Leuven –20% (–36.6 to –3.4) Versus conventional 17.6% (5.7 to 29.5) |
|
| Control (Leuven protocol) | ICU nurses | 58 adults | 19 months = 24/58 | ||||
| Control (conventional treatment) | ICU nurses | 53 adults | 19 months = 2/53 | – | |||
| Cleveringa et al. 200871 | CDSS use in diabetic patients | Nurses | 1699 adults | A1C<7% | Baseline = 60.8% 1 year = 68% |
7.2% | 4.6% (2.7 to 6.5) |
| Usual care | Nurses | 1692 adults | Baseline = 61.6% 1 Year = 64.2% |
2.6% | |||
| CDSS use in diabetic patients | 1699 adults | Systolic BP < 140 | Baseline = 41% 1 year = 53.9% |
12.9% | 10.2% (7.9 to 12.5) | ||
| Usual care | 1692 adults | Baseline = 39.5% 1 year = 42.2% |
2.7% | ||||
| CDSS use in diabetic patients | 1699 adults | Total cholesterol < 4.5 mmol/l | Baseline = 36.2% 1 year = 49.0% |
10.5% | 3.7% (1.2 to 6.2) | ||
| Usual care | 1692 adults | Baseline = 38.5% 1 year = 45.3% |
6.8% | ||||
| Hovorka et al. 200779 | CDSS (eMPC) | ICU nurses | 30 adults | Proportion in target range (4–6.1 mmol/l) | 48 hrs = 60.4% | – | 32.9% (20.0 to 46.0) |
| Usual care | ICU nurses | 30 adults | 48 hrs = 27.5% | ||||
| CDSS (eMPC) | Entire study mean blood glucose (mmol/l) (SD) | 48 hrs = 6.2 (1.1) | – | –1 mmol/l | |||
| Usual care | 48 hrs = 7.2 (1.1 | ||||||
| CDSS (eMPC) | Time in target range (hours) | 48 hrs = 14.5 | 7.9 hrs | ||||
| Usual care | 48 hrs = 6.6 | ||||||
| Mann et al. 201180 | CDSS use | ICU nurses | 18 adults | Occasions glucose range on target (80 to 110 mg/dl) | 72 hrs = 47% | – | 6% (–7.7 to 19.7) |
| Paper protocol | ICU nurses | 18 adults | 72 hrs = 41% | ||||
| CDSS use | ICU nurses | Occasions over target range (over 110 mg/dl) | 72 hrs = 49% | – | –5% (–18.8 to 8.8) | ||
| Paper protocol | ICU nurses | 72 hrs = 54% | |||||
| CDSS use | Occasions under target (under 80 mg/dl) range | 72 hrs = 4.5% | – | –0.3% (–2.1 to 1.5) | |||
| Paper protocol | 72 hrs = 4.8% | ||||||
| Plank et al. 200681 | CDSS (MPC) use | ICU nurses | Not reported | Occasions within the target glycaemic range (80–110 mg/dl) | 48 hrs = 52% | – | 33% (20.5 to 45.4) |
| Usual care | ICU nurses | Not reported | 48 hrs = 19% | ||||
| CDSS (MPC) use | ICU nurses | Not reported | Improvement glycaemic control for 48 hours | 48 hrs = 65% | – | 40% (27.4 to 52.6) | |
| Usual care | ICU nurses | Not reported | 48 hrs = 25% | ||||
| CDSS (MPC) use | Not reported | Occasions over the target glycaemic range (>110 mg/dl) | 48 hrs = 46% | – | –31% (–43.7 to –18.2) | ||
| Usual care | Not reported | 48 hrs = 77% | |||||
| CDSS (MPC) use | Not reported | Average glucose (mg/dl) | 48 hrs = 117 mg/dL | – | –14 mg/dl | ||
| Usual care | Not reported | 48 hrs = 131 mg/dL | |||||
| Blood coagulation management | |||||||
| Fitzmaurice et al. 200067 | CDSS use | Nurses | 122 adults | Proportion of tests in range | Baseline = 223/366 1 year = 732/1181 |
1.1% | –1.9% (–3.1 to –0.7) |
| CDSS non-use | Physicians | 245 adults | Baseline = 264/480 1 year = 986/1700 |
3% | |||
| CDSS use | Nurses | International Normalised Ratio (INR) results within range point prevalence | Baseline = 74/118 1 year = 86/121 |
8.4% | –2.6% (–5.3 to –0.1) | ||
| CDSS non-use | Physicians | Baseline = 129/244 1 year = 157/245 |
11% | ||||
| CDSS use | Nurses | Time spent within INR target range | Baseline = 64/113 1 year = 76/110 |
12% | 7% (–0.7 to 14.7) | ||
| CDSS non-use | Physicians | Baseline = 99/174 1 year = 143/230 |
5% | ||||
| Antenatal and peripartum care | |||||||
| Dalaba et al. 201586 | CDSS use | Nurses | Not reported | Antenatal complications per 1000 attendance | Before = 9 After = 12 |
0.3% | 0.3% (–0.03 to 0.6) |
| CDSS non-use | Nurses | Not reported | Before = 16 After = 16 |
0% | |||
| CDSS use | Delivery complications per 1000 attendances | Before = 107 After = 96 |
–0.9% | 2.4% (1.1 to 3.7) | |||
| CDSS non-use | Before = 133 After = 100 |
–3.3% | |||||
| Managing patients with chronic co-morbid diseases | |||||||
| McDonald et al. 201788 | CDSS use | 165 nurses | 2550 adults | Medication regimen complexity index <24.5 | Post-test = 158/2550 | – | 0% (–1.1 to 1.1) |
| Usual care | 335 nurses | 5369 adults | Post-test = 333/5369 | ||||
| CDSS use | 165 nurses | 2550 adults | Emergency-room use | Post-test = 421/2550 | – | –0.2 (–1.9 to 1.6) | |
| Usual care | 335 nurses | 5369 adults | Post-test = 897/5369 | ||||
| CDSS use | 165 nurses | 2550 adults | Hospitalisation | Post-test = 502/2550 | – | –1.4% (–3.3 to 0.5) | |
| Usual care | 335 nurses | 5369 adults | Post-test = 1133/5369 | ||||
| Lv et al. 201994 | CDSS use | Nurses | 70 children | Asthma exacerbations (median and inter-quartile range) | Baseline = 9(3) 1 year = 3(2) |
– | |
| Usual care | Nurses | 73 children | Baseline = 9 (4) 1 year = 4(2) |
– | |||
| Outpatient obesity screening | |||||||
| Lee et al. 200974 | CDSS use | 13 nurses | 807 adults | Encounters with obesity related diagnosis | 8 months = 91/807 | – | 10.3% (8.0 to 12.5) |
| Usual care | 16 nurses | 997 adults | 8 months = 10/997 | ||||
| CDSS use | 13 nurses | 807 adults | Encounters with missed obesity-related diagnosis | 8 months = 51/208 | – | –41.9% (–48.8 to –35.1) | |
| Usual care | 16 nurses | 997 adults | 8 months = 440/662 | ||||
| Fall and pressure ulcer management | |||||||
| Beeckman et al. 201389 | CDSS (Pre-vPlan) | 65 nurses and physios | 225 adults | Pressure-ulcer prevention | Day 1 = 15/58 Day 120 = 41/65 |
37.2% | 2.3% (–11.0 to 15.6) |
| Standard protocol | 53 nurses and physios | 239 adults | Day 1 = 16/63 Day 120 = 41/68 |
34.9% | |||
| CDSS (Pre-vPlan) | 65 nurses and physios | 225 adults | Prevalence of pressure ulcer | Day 1 = 34/225 Day 120 = 16/225 |
–8% | –6.3% (–10.2 to –2.4) | |
| Standard protocol | 53 nurses and physios | 239 adults | Day 1 = 39/239 Day 120 = 35/239 |
–1.7% | |||
| Byrne 200583 | CDSS use | 89 nurses | Not reported | Fall rate | Before = 0.312 After = 0.318 |
0.6% | 3.1% |
| CDSS non-use | Not reported | Before = 0.315 After = 0.29 |
–2.5% | ||||
| CDSS use | Not reported | Pressure-ulcer rate | Before = 0.085 After = 0.088 |
–0.3% | –0.6% | ||
| CDSS non-use | Not reported | Before = 0.091 After = 0.094 |
0.3% | ||||
| Dowding et al. 201290 | CDSS use | Fall rate | Post-CDSS use vs. pre-CDSS use period | – | 0.91 (0.75 to 1.12)b | ||
| CDSS non-use | |||||||
| CDSS use CDSS non-use |
HAPU ratio | Post-CDSS use vs. pre-CDSS use period | – | 0.47 (0.25 to 0.85) b | |||
| Dykes et al. 201091 | CDSS use | Nurses | 5160 adults | Fall rate difference (per 1000 patient days) | CDSS use vs. usual care | –1.16 (–2.16 to –0.17) b | |
| Usual care | Nurses | 5104 adults | |||||
| Dykes et al. 202092 | UDSS use | Nurses | 19,283 adults | Fall rate difference (per 1000 patient days) | Post-CDSS use vs. pre-CDSS use period | –0.15 (–0.04 to –0.25) b | |
| CDSS non-use | Nurses | 17,948 adults | |||||
| Fossum et al. 201193 | CDSS use | Nurses | 367 adults | Prevalence of pressure ulcers | Before = 16/167 After = 23/200 |
1.9% | 4.2% (0.2 to 8.2) |
| CDSS non-use | Nurses | 274 adults | Before = 17/150 After = 11/122 |
–2.3% | |||
| Triaging | |||||||
| Bennett et al. 201661 | CDSS use period | Nurses | 400 adults | Correct triage prioritisation | Post-test = 85.2% | – | 24.7% (18.8 to 30.6) |
| CDSS non-use | Nurses | 400 adults | Pre-test = 60.5% | ||||
| Lattimer et al. 199864 | CDSS | Nurses | Not applicable | Calls managed with telephone advice from GP | Post-test = 1109/7184 | – | –34.2% (–35.6 to –32.8) |
| Usual care | Physicians | Not applicable | Post-test = 3629/7308 | ||||
| CDSS | Nurses | Patient attended primary care centre | Post-test = 1177/7184 | – | –10% (–11.4 to –8.8) | ||
| Usual care | Physicians | Post-test = 1934/7308 | |||||
| CDSS | Nurses | Patient visited at home by duty GP | Post-test = 1317/7184 | – | –5.5% (–6.9 to –4.2) | ||
| Usual care | Physicians | Post-test = 1745/7308 | |||||
| Lattimer et al. 200097 | CDSS | Nurses | Total admissions within 3 days | 1 year = 428/7184 | – | –0.98% (–1.8 to –0.2) | |
| Usual care | Physicians | 1 year = 507/7308 | |||||
| Snooks et al. 2014 | CDSS | Paramedics | 436 adults | Patients left at scene without conveyance to emergency department | 1 year = 183/436 | – | 5.2% (–1.7 to 12.1) |
| Control | Paramedics | 343 adults | 1 year = 126/343 | ||||
| CDSS | 436 adults | Patients with further emergency admission to hospital or death | 1 year = 69/436 | – | 1.5% (–3.5 to 6.6) | ||
| Control | 343 adults | 1 year = 49/343 | |||||
| CDSS | Patients with ED attendance/emergency admission to hospital/death | 1 year = 92/436 | – | 3.3% (–2.3 to 8.9) | |||
| Control | 1 year = 61/343 | ||||||
| CDSS | Patients who reported >1 further fall | 1 year = 135/236 | – | –6.8% (–16.3 to 2.7) | |||
| Control | 1 year = 112/175 | ||||||
| Quality of life and patients’ satisfaction | |||||||
| Cleveringa et al. 201072 | CDSS use | Life-years gained | CDSS vs. usual care | 0.14 (–0.12 to 0.40)b | |||
| Usual care | |||||||
| CDSS use | Healthy years (QALYs, discounted) | CDSS vs. usual care | 0.037 (–0.066 to 0.14)b | ||||
| Usual care | |||||||
| Snooks et al. 201462 | CDSS | Paramedics | 239 adults | Quality of life (SF12 MCS), mean (SD) | 1 year = 41.9(10.3) | –1 (–3.1 to 1.1) | |
| Control | Paramedics | 177 adults | 1 year = 42.9(10.9) | ||||
| CDSS | Paramedics | 239 adults | Quality of life (SF12 PCS), mean (SD) | 1 year = 29(8) | –1 (–2.6 to 0.6) | ||
| Control | Paramedics | 177 adults | 1 year = 30(8.5) | ||||
| CDSS | Paramedics | 228 adults | Patient satisfaction (QC Technical), mean (SD) | 1 year = 97.8(10.7) | –0.4 (–2.4 to 1.6) | ||
| Control | Paramedics | 165 adults | 1 year = 98.2(9.4) | ||||
CDSS had mixed effects on guideline adherence – sometimes within the same study. In the only trial which took into account baseline and follow-up data,85 nurses in both arms showed lower adherence to hand disinfection (CDSS = −1.2%, Control = −7.9%) and disposable-glove guidance (CDSS = −1.7%, Control = −0.3%), but improved daily inspection of peripheral venous catheters (CDSS = 2.6%, Control = 7.8%). Compared to their non-CDSS-using colleagues, CDSS-using nurses were slightly better at adhering to hand-disinfection guidelines (risk difference = 6.7%; 95% CI: 4.9 to 8.5%) but worse at adhering to policies on disposable gloves (risk difference = −1.4%; 95% CI: −2.2 to −0.5%) and inspection of peripheral venous catheter sites (risk difference = −5.2%; 95% CI: −7.2 to −3.3%).
In trials that did not take into account baseline values,69,78,82 CDSS-supported nurses adhered more to guidelines on insulin dosing (risk difference = 22%; 95% CI: 19 to 25%), blood sampling on time (risk difference = 4.7%; 95% CI: 2.0 to 7.4%), and deviated less from protocols (mean score difference out of 10 = −2.6; 95% CI: −4.5 to −0.71)69,78 and were more accepting of recommended medication doses than trainee doctors. 82
Assessing and treating patients
Six studies63,68,73,84,87 61 examined 18 indicators of the quality of patient assessment and treatment. In single studies, CDSS-using nurses assessed pain more readily in emergency department patients (62.7% higher than non CDSS-users [95% CI: 59.6 to 65.8%]) and investigated more paediatric malnutrition aetiology by 21.2% (95% CI: 15.9 to 26.5%), but were slower to provide IV antibiotics within an hour of sepsis onset (5.9% slower, 95% CI: −8.3 to −3.5%). They were no more likely to order laboratory tests (electrolyte levels, acid–base balance test) or nutrition supplements (oral rehydration solution and IV rehydration) in children seen in a paediatric university hospital.
CDSS-enabled nurses recorded fewer incorrect temperatures (risk difference = −0.8%, 95% CI: −0.9 to −0.6%) on wards. CDSS-supported paramedics were no more complete in their assessment of vital signs (respiratory rate, pulse rate and consciousness).
Documenting care
In two single studies, documentation of fall risk (risk ratio = 1.4, 95% CI: 0.03 to 73.7),90 pressure-ulcer risk (risk ratio = 9.1, 95% CI: 1.95 to 42.5),90 nutritional care planning, nutritional intake and treatment were all better when nurses were using CDSS. 95
Referring to expertise
Paramedics using CDSS in one study avoided unnecessary use of the ER by referring more patients to a community falls service rather than hospital (risk difference = 4.7%, 95% CI: 1.1. to 8.3%). 62
Care outcomes
CDSS were associated with better nurse or AHP influenced outcomes in less than half of the indicators reported (22/54, 40.7%) in six RCTs. In one indicator (delivery complications per 1000 births) CDSS reduced fewer harms than not using CDSS (whose harms also diminished over time). 86
Blood glucose control
Seven trials 71,75–77,79–81 reporting on 19 separate indicators of glycaemic control suggest CDSS can improve:
-
glucose levels in ICU nurses compared to non-tailored protocols75
-
proportion of patients with glycated haemoglobin (A1C) <7% (as well as systolic blood pressure and cholesterol levels)
-
proportion of patients in target range
-
number of occasions in target range and reduce the numbers over the target range
-
control over 48 hours.
Blood coagulation management
Whilst CDSS-using nurses generated more ‘tests in range’ than unsupported doctors there were no differences in prevalence of INR ‘in range’ or time spent ‘in range’ between CDSS-enabled nurses and non-using doctors.
Antenatal and peripartum care
A single controlled before-and-after study showed that using a CDSS resulted in less of a reduction in delivery complications than not using one (risk difference = 2.4%, 95% CI: 1.1 to 3.7%) – although users and non-users both improved over time.
Managing those with chronic co-morbid conditions
For those patients with complex, co-morbid, conditions, CDSS use by nurses did not reduce ER use or hospitalisations or lead to rationalised, simpler, medication regimens.
Screening for obesity
A single trial revealed that trainee nurses using a CDSS saw more patients with obesity-related diagnoses and lower numbers of patients with missed obesity-related diagnoses.
Assessing for fall and pressure-ulcer risk factors
These complex and uncertain areas of nursing practice yielded mixed results and effects altered across differing study designs. A trial89 saw CDSS users associated with fewer pressure ulcers (although non-users’ also saw pressure ulcers reduced by a smaller amount). CBA studies saw more patients with pressure ulcers amongst CDSS users 93 but lower levels of malnutrition in patients cared for by CDSS-supported nurses93 or no difference (in pressure ulcers or falls) in CDSS users and non-users. 83 In the single time series study that exists, where CDSS implementation constituted the interruption, there were fewer pressure ulcers or falls when nurses were using the decision support.
Triage
CDSS-using nurses and paramedics using CDSS made fewer calls needing advice from general practitioners (GP) (risk difference = −34.2%, 95% CI: −36 to −33%), lower numbers of patients visited at home by a GP (risk difference = −5.5%, 95% CI: −6.9 to −4.2%), and fewer admissions to hospital within 3 days of nurse input (risk difference = −0.98%, 95% CI: −1.8 to −0.2%). They were also no more likely to ‘leave a patient at the scene without conveying to an emergency department’ (risk difference = 5.2%, 95% CI: −1.7 to 12.1%). The proportion of ‘correct’ triage prioritisation judgments was higher when professionals used CDSS (risk difference = 24.7%; 95% CI: 18.8 to 30.6%).
Health-related quality of life and satisfaction with care
Only two studies attempted to measure HRQOL and one also reported patient satisfaction with care; neither study demonstrated differences in HRQOL or patient satisfaction attributable to CDSS use.
Do CDSS increase knowledge and shape positive behaviours?
Professions exist in part due to their own claims of specialist – superior to non-professional – knowledge and modes of behaviour. 98 It follows then those professional decisions are in part shaped by their knowledge and associated with behaviours. CDSS as a technology to support professional decision impacts on only some of the component parts of professional knowledge and behaviour (Table 6).
| Author and year | Interventions | Health professionals | Patient participants | Outcome measured | Outcome values reported | Change of value within a groupa | Mean or risk difference (95% CI)a |
|---|---|---|---|---|---|---|---|
| Beeckman et al. 201389 | CDSS (Pre-vPlan) | 65 nurses and physios | 225 adults | Positive knowledge change | Baseline = 28/65 5 months = 26/50 |
8.9% | 6.5% (0.8 to 13.2) |
| Standard protocol | 53 nurses and physios | 239 adults | Baseline = 21/53 5 months = 16/38 |
2.4% | |||
| CDSS (Pre-vPlan) | 65 nurses and physios | 225 adults | Positive attitude change | Baseline = 48/65 5 months = 42/50 |
10.2% | 12.7% (5.9 to 19.5) | |
| Standard protocol | 53 nurses and physios | 239 adults | Baseline = 39/53 5 months = 27/38 |
–2.5% | |||
| Cortez 201466 | CDSS (drop-down boxes) | 26 nurses | NA | Research utilisation | Baseline = 35% 11 weeks = 38% |
3% | 9% (3.3 to 14.7) |
| Control | 24 nurses | NA | Baseline = 19% 11 weeks = 13% |
–6% | |||
| Dumont et al. 201278 | CDSS use | Nurses (OA = 44) | 141 adults | Nurses’ satisfaction, out of 10 (mean [SD]) | 4 months = 8.4(1.4) | – | 3.6 (2.4 to 4.8) |
| Paper protocol | Nurses | 159 adults | 4 months = 4.8(2.4) | ||||
| CDSS use | Perception of how often needed to deviate from the protocol, out of 10 (mean [SD]) | 4 months = 2.7(2.2) | – | –4.7 (–6.1 to –3.3) | |||
| Paper protocol | 4 months = 7.4(2.4) | ||||||
| Sassen et al. 201470 | CDSS use | 42 nurses and physios | Not reported | Behaviour, mean (SD) | Baseline = 4.5 (1.02) 17 months = 4.6 (0.85) |
0.1 (0.93) | 0.1 (–0.32 to 0.53) |
| Control | 27 nurses and physios | Not reported | Baseline = 4.8 (0.69) 17 months = 4.8 (0.82) |
0 (0.75) | |||
| CDSS use | 42 nurses and physios | Intention, mean (SD) | Baseline = 6.3 (1.0) 17 months = 6.1 (1.1) |
0.2 (1.05) | 0.3 (–0.22 to 0.82) | ||
| Control | 27 nurses and physios | Baseline = 5.9 (1.15) 17 months = 6.0 (0.91) |
–0.1(1.05) | ||||
| CDSS use | 42 nurses and physios | Attitude, mean (SD) | Baseline = 6.3 (0.44) 17 months = 6.3 (0.56) |
0.0(0.05) | –0.1 (–0.13 to –0.07) | ||
| Control | 27 nurses and physios | Baseline = 6.2 (0.69) 17 months = 6.3 (0.68) |
0.1 (0.09) | ||||
| CDSS use | 42 nurses and physios | Perceived behavioural control, mean (SD) | Baseline = 4.7 (0.79) 17 months = 5.0 (0.73) |
0.3 (0.77) | –0.1 (–0.49 to 0.29) | ||
| Control | 27 nurses and physios | Baseline = 4.9 (0.87) 17 months = 5.3 (0.8) |
0.4 (0.85) | ||||
| CDSS use | 42 nurses and physios | Subjective norms, mean (SD) | Baseline = 5.5 (0.55) 17 months = 5.6 (0.63) |
0.1 (0.59) | 0 (0.34 to 0.34) | ||
| Control | 27 nurses and physios | Baseline = 5.6 (0.93) 17 months = 5.7 (0.76) |
0.1 (0.84) | ||||
| CDSS use | 42 nurses and physios | Moral norms, mean (SD) | Baseline = 6.0 (0.63) 17 months = 6.2 (0.7) |
0.2 (0.67) | 0.1 (–0.21 to 0.41) | ||
| Control | 27 nurses and physios | Baseline = 6.2 (0.59) 17 months = 6.3 (0.55) |
0.1 (0.57) | ||||
| CDSS use | 42 nurses and physios | Barriers, mean (SD) | Baseline = 3.1 (1.17) 17 months = 3.2 (1.12) |
0.1 (1.14) | 0.3 (–0.23 to 0.83) | ||
| Control | 27 nurses and physios | Baseline = 2.8 (1.01) 17 months = 2.6 (0.96) |
–0.2 (0.98) |
CDSS, on the basis of four RCTs, positively influenced perceptions of frequency of needing to deviate from protocols, positive knowledge and attitude changes. Conversely, measured knowledge itself and key elements of ‘planned behaviour’99 (intention, attitude, self-efficacy, and subjective and moral norms) were no different in CDSS-using professionals and non-users. Using more research knowledge in decisions, as a consequence of CDSS exposure, was equivocal: one RCT suggests it can be improved, whilst studies with nurses and physiotherapists suggests not.
Do CDSS improve safety in nurse, midwife and AHP performance?
CDSS do not by default make services safer (Table 7). The complex socio-technical systemic location of CDSS and the work required to embed and sustain systems mean unintended consequences are not uncommon. Aside from simply providing ‘ineligible’ or suboptimal advice, unintended consequences can also include (1) errors in information entry and retrieval associated with a poor human-computer interface, and (2) inflexible digital systems leading to errors in coordination and communication. 100 Of course, errors also occur in unsupported practice. CDSS, however, have the potential to hardwire such errors into the socio-technical system of clinical practice, making mistakes systematic and systemic. Iatrogenic harm can also arise from ignoring the complex socio-technical system that the technology must operate in: e-iatrogenesis. 38,101 No studies focused specifically on the effects of CDSS use on patient safety in hospital care. In primary and first-response care, CDSS use by nurses is associated with lower probability of cardiovascular events in people having their diabetes managed by nurses (as opposed to CDSS-unsupported physicians) – an 11% risk difference (95% CI −18 to −4). However, serious adverse incidents and deaths in people who had fallen and were attended by CDSS-informed paramedics were unaffected.
| Author and year | Interventions | Health professionals | Patient participants | Outcome measured | Outcome values reported | Change of value within a groupa | Risk difference (95% CI)a |
|---|---|---|---|---|---|---|---|
| Cleveringa et al. 201072 | CDSS use in diabetic patients | Nurses | 1699 adults | Cardiovascular events occurring | CDSS vs. usual care | – | –11% (–18 to –4)b |
| Usual care | Nurses | 1692 adults | |||||
| Fitzmaurice et al. 200067 | CDSS nurse | Nurses | 224 adults | Serious adverse reaction events | 1 year = 3 (1.3%) | –5.7% (–10.1 to –1.2) | |
| CDSS non-use | Physicians | 143 adults | 1 year = 10 (7%) | ||||
| CDSS nurse | Nurses | 224 adults | Deaths | 1 year = 3 (1.3%) | –5% (–9.2 to –0.7) | ||
| CDSS non-use | Physicians | 143 adults | 1 year = 9 (6.3%) | ||||
| Snooks et al. 201462 | CDSS | 17 Paramedics | 436 adults | Patients dying | 1 year = 19/436 (4.4%) | – | 1.2% (–1.5 to 3.8) |
| Control | 19 Paramedics | 343 adults | 1 year=11/343 (3.2%) |
The effects of ‘good’ design features in CDSS
We examined the effects of incorporating 16 design or system features known to positively influence performance and outcomes in 32 of the studies reported41 (Tables 8 and 9). None were associated with greater effectiveness of the CDSS and all were highly uncertain (as seen in their wide 95% CIs in Table 8).
| Factor | CDSS better than usual carea | Usual care better than CDSSa |
|---|---|---|
| Some of study’s authors are also systems’ developers | 0.60 (0.1 to 3.5) | 0.23 (0.01 to 4.6) |
| System provides advice automatically within practitioner’s workflow | 0.71 (0.16 to 3.2) | 0.39(0.08 to 2.1) |
| System provides advice at time of care | 0.85 (0.18 to 3.9) | 0.10 (0.01 to 1.95) |
| Advice presented in electronic charting or order entry systems | – | – |
| Provides advice for patients | 1.12 (0.04 to 29.9) | 1.22 (0.04 to 33.1) |
| Requires reason for over-ride | 1.12 (0.04 to 29.9) | 1.22 (0.04 to 33.1) |
| System facilitates or automates recommended actions | 0.30 (0.04 to 2.1) | 0.36 (0.02 to 7.5) |
| Advice is evidence based | 2.8 (0.13 to 60.2) | 0.49 (0.02 to 10.5) |
| Critiquing function | – | – |
| Practitioner does not enter data into system | – | – |
| Modern system (study published after 2000) | 0.26 (0.01 to 5.4) | 0.59 (0.07 to 4.8) |
| Advice or reminders provided directly to patients | 1.12 (0.04 to 29.9) | 1.22 (0.04 to 33.1) |
| Trained users | 3.08 (0.46 to 20.7) | 1.1 (0.2 to 6.0) |
| Local users were consulted during creation of recommendations | 1.94 (0.08 to 44.2) | 0.71 (0.03 to 16.4) |
| System presents its reasoning | – | – |
| System cites research evidence | – | – |
| Author and year | Factors associated with effectiveness of a CDSS | CDSS better than the usual care in at least one reported outcomea | CDSS worse than the usual care in at least one outcomea | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Some of study’s authors are also system’s developers | System provides advice automatically within practitioner’s workflow | System provides advice at time of care | Advice presented in electronic charting or order entry systems | Provides advice for patients | Requires reason for over-ride | System facilitates or automates recommended actions | Advice is evidence based | Critiquing function | Practitioner does not enter data into system | Modern system (study published after 2000) | Advice or reminders provided directly to patients | Trained users | Local users were consulted during creation of recommen-dations | System presents its reasoning | System cites research evidence | |||
| Beeckman 201389 | √ | √ | √ | √ | ||||||||||||||
| Bennet 201661 | √ | √ | √ | |||||||||||||||
| Blaha 200975 | √ | √ | √ | |||||||||||||||
| Byrne 200583 | √ | √ | ||||||||||||||||
| Canbolat 201976 | √ | √ | √ | √ | √ | |||||||||||||
| Cavalcanti 200977 | √ | √ | √ | √ | ||||||||||||||
| Cleveringa 200871 | √ | |||||||||||||||||
| Cleveringa 201072 | √ | √ | ||||||||||||||||
| Cortez 201466 | √ | |||||||||||||||||
| Dalaba 201586 | √ | √ | √ | |||||||||||||||
| Dowding 201290 | √ | √ | ||||||||||||||||
| Duclos 201584 | √ | √ | √ | √ | √ | √ | √ | |||||||||||
| Dumont 201278 | √ | √ | ||||||||||||||||
| Dykes 200991 | √ | √ | √ | √ | √ | √ | √ | |||||||||||
| Dykes 202092 | √ | √ | √ | √ | ||||||||||||||
| Fitzmaurice 200067 | √ | √ | ||||||||||||||||
| Forberg 201685 | √ | √ | √ | √ | ||||||||||||||
| Fossum 201193 | √ | √ | √ | √ | ||||||||||||||
| Geurts 201773 | √ | √ | ||||||||||||||||
| Hovorka 200779 | √ | √ | √ | |||||||||||||||
| Kroth 200668 | √ | √ | √ | √ | √ | |||||||||||||
| Lattimer 199864 | √ | √ | ||||||||||||||||
| Lattimer 200065 | √ | √ | ||||||||||||||||
| Lee 200974 | √ | √ | √ | √ | √ | |||||||||||||
| Lv 201994 | √ | √ | √ | |||||||||||||||
| Mann 201180 | √ | √ | ||||||||||||||||
| McDonald 201788 | √ | √ | √ | √ | ||||||||||||||
| Paulsen 202095 | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||
| Plank 200681 | √ | √ | √ | √ | ||||||||||||||
| Rood 200569 | √ | √ | √ | √ | ||||||||||||||
| Roukema 200887 | √ | √ | √ | |||||||||||||||
| Sassen et al. 201470 | √ | √ | √ | √ | ||||||||||||||
| Snooks 201462 | √ | √ | ||||||||||||||||
| Vadher 199782 | √ | |||||||||||||||||
| Wells 201363 | √ | √ | √ | |||||||||||||||
Economic costs and benefits
Four randomised trials62,65,72,73 included 20 economic indicators of costs and consequences (Table 9). The costs of managing cardiovascular disease were lower in CDSS-using groups (cost difference = −€587, 95% CI −880 to −294) but cost more when supporting diabetes care protocol implementation (cost difference = €326, 95% CI 315 to 318). Clinical work took longer: ‘mean length of job cycle time’ was significantly higher (difference in minutes = 8.9, 95% CI 2.3 to 15.3) in CDSS users (see Table 9). Cost per quality adjusted life-year (QALY) was €38,243 (£32,333) – more than NICE’s normal cost-effectiveness threshold of £20,000 to 30,000 per QALY gained for implementation in the UK NHS (Table 10).
| Author and year | Interventions | Health professionals | Patient participants | Outcome measured | Outcome values reported | Difference (95% CI)‡ |
|---|---|---|---|---|---|---|
| Cleveringa et al. 201072 | CDSS use | Nurses | Diabetes-related costs (excluding CHD)-€ discounted | CDSS vs. usual care | 1698.00 (187 to 3209) b | |
| Usual care | Nurses | |||||
| CDSS use | Cardiovascular disease cost-€ discounted | CDSS vs. usual care | −587.00 (−880 to −294) b | |||
| Usual care | ||||||
| CDSS use | Diabetic care protocol cost-€ discounted | CDSS vs. usual care | 316.00 (315 to 318) b | |||
| Usual care | ||||||
| CDSS use | Total cost-€ discounted | CDSS vs. usual care | 1,415.00 (−130 to 2961) b | |||
| Usual care | ||||||
| CDSS use | Total costs per QALY gained (Euro) | CDSS vs. usual care | 38,243.00 b | |||
| Usual care | ||||||
| Geurts et al. 201773 | CDSS use | Nurses | 113 children | Average emergency department visit cost (Euro) | 156.4 | 0.00 |
| Usual care | Nurses | 109 children | 156.4 | |||
| CDSS use | Average diagnostics cost (Euro) | 1.09 | −0.46 | |||
| Usual care | 1.55 | |||||
| CDSS use | Average treatment cost (Euro) | 4.48 | 1.90 | |||
| Usual care | 2.58 | |||||
| CDSS use | Average follow-up/hospitalisation (Euro) | 134. | 26.60 | |||
| Usual care | 107.4 | |||||
| CDSS use | Average costs of missed diagnoses/adverse events (Euro) | 49.70 | −32.10 | |||
| Usual care | 81.8 | |||||
| CDSS use | Average cost of CDSS implementation (Euro) | 61.95 | 61.95 | |||
| Usual care | 0.0 | |||||
| CDSS use | Overall average cost | 408 | 58.00 | |||
| Usual care | 350 | |||||
| Lattimer et al. 200065 | CDSS | Nurses | Not applicable | Net savings [of CDSS use] in a year (£) | CDSS vs. usual care | 13,185 (−77,509 to 123,824) b |
| Usual care | Physicians | Not applicable | ||||
| CDSS | Cost saved from inpatient stay | CDSS vs. usual care | 51,059 b | |||
| Usual care | ||||||
| Snooks et al. 201462 | CDSS | Paramedics | Implementing cost of CCDS in one month (in 100s £) | 74 | 74 | |
| Control | Paramedics | |||||
| CDSS | Total cost of implementation in one month (in 100s £) | 2,773 2,526 |
247 (−247 to 741) b | |||
| Control | ||||||
| CDSS | Net resources saved by CCDS per patient year (£) |
39 b | ||||
| Control | ||||||
| CDSS | Net cost resources saved by CCDS per patient year (£) | 208–308 b | ||||
| Control | ||||||
| CDSS | Mean length of Job cycle time (minutes) | CDSS vs. control | 8.9 min (2.3 to 15.3) b | |||
| Control | ||||||
| CDSS | Mean length of episode of care (minutes) | CDSS vs. control | −5.7 min (−38.5 to 27.2)b | |||
| Control |
Insights from developers, intended users and implementers of CDSS
Coherence
Coherence, or the extent to which an intervention is understood as meaningful, achievable and desirable, in an important factor in adoption. Participants emphasised a number of key differences from CDSS-unsupported care and used these to increase understanding and help encourage professional adoption:
-
Reducing documentation whilst simultaneously utilising best evidence. One example was encouraging appropriate pain assessment whilst assessing pressure-ulcer risks.
-
CDSS as ‘teacher’ – forcing users to engage with new information – such as guidelines – in practice.
-
Making decisions ‘visible’ and facilitating appropriate action – an example being greater propensity for escalation in response to NEWS score above the Trust’s threshold for action.
-
Technology making information more ‘palatable’ to younger clinicians who were perceived as having preferences for technology-delivered information.
-
CDSS facilitating QI and audit and accreditation to a desired standard: something that was much harder prior to the technology being introduced. An example provided was the measurement of ‘care not done’ such as a Waterlow pressure risk assessment within four hours of admission.
-
Standardisation – of care planning, collecting and organising data. Examples would be the assessment, recording and suggested actions associated with the North American Nursing Diagnostic assessment system.
The extent to which these perceived advantages were shared by everyday users of the system was unknown. On probing, it was clear that leaders recognised variability in take-up and adoption, implying not all clinicians may feel the same way. Variation was often attributed to user characteristics such as age (‘older users being reluctant’) and clinical role (‘pharmacist reviewing medications invokes less CDSS-use than initiating medications’) rather than the systems themselves or the fit between decision-work and system suitability.
Interviewees highlighted the importance of fostering shared understanding of CDSS functions, stated benefits, limitations and links to concepts such as safety, accountability and (unwarranted) variability as levers for adoption and sustainability. One system developer and evaluator emphasised the understanding required to know when not to use the CDSS:
it’s critical that the users of any of these tools understand the limitations. So, they understand when to use it when not to use it (participant’s emphasis). So, in that patient in front of them, how do they assess how safe and appropriate it is to use that tool?
Participant 4. National Policy Team on Digital Health
Collective action
In terms of the work required to bring the CDSS into use (collective action), it was clear that for all the interviewees that adjustment and pragmatic relaxing of some prerequisites (such as the overriding of alerts) – arising from reflexive monitoring – was required to sustain and encourage use. Implicit in many accounts was a recognition that systems sometimes possessed, and generated, unwanted characteristics that needed active countering. Examples included:
-
fine tuning or muting alerts to reduce alert fatigue
-
ensuring that over-riding a CDSS recommendation was handled ‘sensitively’ in any post-decision scrutiny – healthcare is uncertain and sometimes over-riding may be necessary or appropriate given the uniqueness of the individual to whom the CDSS is applied
-
guarding against ‘garbage in and garbage out’ in both knowledge-based (poor-quality protocols) and machine learning systems (poor-quality ‘training’ data).
The extent to which CDSS developers and proponents expected work to adapt to the CDSS (as opposed to the CDSS having the flexibility to fit into work-as-done) was a strong thread in all the interviews. All the participants reported an awareness of ‘workflow’ (variously referred to as ‘context’, ‘work’, ‘environment’ or ‘practice’) as an important component in the adoption and enactment of CDSS advice.
For example, we had some clinical decision support in a discharge planning assessment … Depending on the boxes [ticked] and the information provided, we gave it some guidance and then if it was greater than a score of 10 it would create a referral to the discharge team, to help with complex discharge planning. Now staff didn’t even use the discharge planning assessment, never mind the associated decision support associated with it.
Participant 1. CNIO large metropolitan teaching hospital
A pharmacist system-developer relayed the importance of understanding the decision tasks and ‘work’ to which the CDSS was to be applied.
Or they may split the work amongst the team so that instead of having one nurse doing a drug round for all 28 patients, they might have four nurses, only giving drugs to six or seven patients. So I think when you’re looking for from my perspective I look at medication safety and I always try to understand, well, if this is happening what are the things that are contributing to that? It’s not just the individual, you know? Yes, there clearly is an individual aspect into this. But is it to do also with them finding and retrieving medicines? Is it to do with interruptions within the environment? Is it to do with the team dynamics the way they work? Is it to do with the trolley, so you know some of the equipment that they use actually isn’t conducive to finding medicines, is not arranged in the right way, so … trying to understand how they interact with the environment around them to do the task at hand.
Participant 2. CNIO large semi-urban NHS acute trust
All the accounts highlighted the need for supportive infrastructure and new ‘roles’ to enable sustained use of the systems.
What we need is more infrastructure and resources to support better implementation of these technologies, and these are tools, these are absolutely our tools, but they need people to use these tools properly. You can’t just give it to the organisations, so we need more specialists who are able to look at the data and analyse who can develop different ways of configuring the system and adapt it to what we need.
Participant 2. CNIO large semi-urban NHS acute trust
Cognitive participation
To encourage cognitive participation, interviewees emphasised formal mechanisms for engagement and increased understanding: training, education, professional committees in areas such as guidance development (rule/knowledge-based systems) and implementation. The accounts revealed a strongly multidisciplinary work environment and decisions that involved multiple professions. However, the training, the committees, and the support infrastructure were almost always mono-professional: all of the respondents’ accounts relayed work in which nurses and AHPs primarily restricted their work scope and communications to other nurses and AHPs.
All the interviewees had roles that involved responsibility for either CDSS introduction or maintaining and/or developing it in services. Aside from the aforementioned mono-professional focus, all discussed strategies that seemingly involved ‘listening’ to users, and encouraging ‘buy-in’, whilst simultaneously emphasising the advantages of ‘forcing’ CDSS use as part of work flow:
Before [CDSS], we were relying on people’s memory, how to manage, literally more than one patient every five minutes arriving at triage for example. So, it did change the way people work because it forced them to assess people in a set way and always ask the same questions.
Participant 4. National Policy Team on Digital Health
Reflexive monitoring
Various accounts relayed this as a valuable mechanism and ‘reality check’ on assumed implementation and the use of formal methods to encourage cognitive participation in clinicians. All the accounts highlighted training and training materials as ‘intended’ mechanisms for supporting initiation and use. One pharmacist’s account, though, revealed both the limitations of self-reported behaviour and the benefits of evaluating efforts:
The thing I would say though, while there’s a lot of these resources available, I don’t know if everybody is accessing it. Certainly, our junior doctors, we did a survey a little while back to try and understand how supported they were in terms of prescribing safely on the electronic system and a lot of them thought they were quite happy. They felt quite confident about using the system to prescribe safely and accurately …. but when we asked them about training types and resources available, a lot of people did not know about them or never used it or didn’t attend the classroom training.
Participant 2. CNIO large semi-urban NHS acute trust
Exogenous factors outside formal strategy were highlighted by a participant CNIO in a large metropolitan teaching hospital with almost 100% adoption of an EHR with decision support capabilities. Strategically, the historic roll-out of a trust-wide EHR system was seen as only partly successful; but the COVID pandemic had catalysed pragmatic adoption and positive feedback. One participant suggested that the CDSS and EHR adoption process was sped up by ‘5 years’. The technology was also being used by the trust to prevent – or at least question – returning to ‘business as usual’ even as the pandemic context changed and more face-to-face care and treatment were possible.
Despite informal reflexive monitoring (primarily communal and individual appraisal) figuring strongly in accounts, the relative absence of formal systematisation-evaluation in accounts was striking. Only one of the interviewees referred to formal evaluation of CDSS effects on work and outcomes: a time series evaluation of a system – they had developed – focused on a relatively rare disease in the emergency department; perhaps tellingly, they were a system developer and a clinical academic. Two CNIOs mentioned ‘informal evaluation’, mainly the collection of anecdotal evidence and committee discussion of first-hand experience of use and adoption. Despite the lack of formal evaluation, participants recognised the need for rapid and relevant evaluation – often in the form of real-time monitoring:
If 80% of nurses are clicking past and just ignoring it, what’s the clinical risk in that? Do we need to go and change practice and process and education to train and encourage people to actually read these things? And I don’t think we do enough of that yet.
Participant 1. CNIO large metropolitan teaching hospital
Where it’s really important like NEWS scoring, they also need to be monitored for compliance. It’s no point having CDSS if you’re not going to monitor if they’re effective.
Participant 3. CAHPIO mixed acute and community, semi-urban NHS trust
Other insights
As with studies in the synthesis, users’ accounts of system development and implementation did not contain references to formal or explicit theory. Phrases such as ‘listening to users’, ‘understanding the work’ and ‘alert fatigue being counter-productive’ implied some awareness of theoretically important concepts, but none of the stakeholders located their points within relevant theories of systems or implementation.
The dangers of ignoring interactional workability and unwanted artefacts of CDSS, such as generating more work for other parts of the healthcare system, were highlighted by one leader. To the extent that adoption itself can be compromised:
You do a nutritional screening tool and then the dietician says ‘stop doing it because actually we’re getting too many referrals’, that’s not the right reason to give it up; and that for me is about how you use the data to inform your future and your resourcing and your requirements and escalating risk where appropriate.
Participant 3. CAHPIO mixed acute and community, semi-urban NHS trust
Standardisation, safety and applying research evidence were the reported primary drivers for initiation, although the extent to which variability, safety and lack of evidence use were historical problems in their organisations – or indeed improved with the advent of CDSS – was not clear.
CDSS as a vehicle for improving knowledge application to practice was highlighted. Whilst two participants referred explicitly to research-based knowledge in the guidance underpinning the CDSS recommendations, for others it was codified, collective (committee) knowledge that was the basis for advice.
Where participants highlighted economic aspects of CDSS use, they generally focused on cost-effectiveness – but in the lay (costs-consequence) rather than formal economic sense (£ per QALY). One participant highlighted how economic context can hinder implementation:
You get paid a set amount for each out-patient appointment that’s face-to-face … let’s say it’s £100 for each patient, and a follow-up is £60 per patient. But there was a lot of, ‘so what is this, it’s not face-to-face physically but it’s face-to-face virtually, is that still classed as a face-to-face appointment, is there a different cost associated. A lesser cost associated with a virtual consultation than a physical one?’ So, it’s not all just about tech it’s about cost as well.
Participant 1. CNIO large metropolitan teaching hospital
Potential recipients of CDSS-mediated advice
As part of the process of calibrating the results with users (a form of stakeholder engagement) we used a voting process to derive the ‘top 5’ (see Box 1) frequently asked questions (FAQs) that might act as prompts for conversations between CDSS professional users and/or those in receipt of, or participating in, CDSS-enabled healthcare decision-making.
-
Does the CDSS make the decisions for my nurse/AHP and is my care now decided by a computer?
-
Will CDSS mean that I will have less time talking to someone?
-
Are nurses/AHPs appropriately trained to use CDSS?
-
Can I check that the CDSS is using correct information about me (e.g. the medications I take/up-to-date information on my medical history)?
-
Will the CDSS share my information with anyone else (e.g. Big Pharma)?
None of the studies included in the synthesis reported addressing these uncertainties from the perspective of people likely to receive the CDSS-enabled care as part of the implementation of the systems.
These five FAQs, whilst useful as a potential start point for further (primary) research, must be interpreted cautiously and are not indicative of representative uncertainties. The aim of engaging with the users was to sense-check our results; the questions did not arise from adequately scaled, sampled, or theoretically scrutinised research efforts.
Discussion
Reasons often cited for CDSS – and present in our stakeholder accounts – include safety, reducing unwarranted variation in practice and outcomes, and greater efficiency and effectiveness associated with the application of evidence to patient care. Our results suggest CDSS cannot yet be relied upon to make healthcare safer or increase standardisation or the application of high-quality research (or indeed, other kinds of knowledge) in nurse and AHP-led decision-informed healthcare. Whilst services may be safer, vary less and evidence feature in choices in CDSS-enabled care some of the time, we still do not necessarily know why. Evidence on efficiency is extremely sparse and inconclusive.
Two specific issues highlighted in our previous review remain relevant. First, knowledge-based CDSS need high-quality knowledge if they are to improve processes and outcomes. Until AI and machine learning becomes more widespread in NMAHP decision-making, knowledge-based systems will rely on protocols, algorithms and ‘if-then-else’ rules. The evidential basis for many of these rules and protocols is often unclear. CDSS need populating with well-evaluated rules in which the clinical significance of any pre-CDSS evaluation of rules, logic and generative mechanisms for the knowledge underpinning systems is established. CDSS are a form of ‘complex intervention’ and whilst guidance on developing complex interventions was less developed in 2009, the latest iteration of guidance makes the necessity of testing logic and mechanisms before adding more complexity by translating evidence into technologies explicit. 104
Second, the need to unpack the heterogeneity and mixed results by examining the effects of components of systems remains. We looked at features known to be associated with effectiveness in CDSS aimed primarily at doctors (see Table 8). There was little impact on NMAHP-focused CDSS. Other quality improvement and implementation behaviour-change methods – notably audit and feedback – have recognised that using theory explicitly in design and evaluation105,106 and conducting iterative programmes of head-head evaluation of key modifiable characteristics can lead to stronger and more sustained implementation of technologies. 107 We think this iterative development in NMAHP-focused CDSS would arguably lead to compounded learning and more efficient implementation of more effective systems.
The variable adoption of systems evaluated in our included studies suggests that CDSS are not uniformly acceptable to all clinicians. Many of the systems, and some of our stakeholder interviews, imply that CDSS often appear as a non-negotiable aspect of works experienced after the decision has already been made to adopt a system – usually by managers who will not have to work with the system for real-time decision-making. We found no instances of outright resistance to the imposition of CDSS in the primary studies. This was not surprising. Dune in his study of evaluations of CDSS systems in the NHS also found that whilst outright resistance was rare, other forms of sub-optimal engagement were more common. Clinicians doing the ‘bare minimum’, simply ‘ticking boxes’, deferring assessments (and avoiding computerised input), or providing poor excuses for missing specified time limits for activities were all features of professional-culture-influenced, subtle, resistance. 108
What’s changed since the last review of CDSS and NMAHPs?
The volume of studies focusing on CDSS has tripled since the last systematic review undertaken with a similar focus in 2009. The body of comparative research now contains a number of randomised comparisons between CDSS and unsupported practice, which is welcome. However, just less than half of the 35 included studies occur in a single site and all remain at high risk of biased conclusions. The increased number and comparative nature of studies has not led to more information to inform decision-making – whether as a clinical decision-maker or someone with responsibility for commissioning, implementing or purchasing systems in healthcare environments.
The studies included in the review suggest that CDSS have potential to improve both processes and outcomes – especially in nursing, and a more limited range of professions allied to medicine – for specific decision-focused work. However, the limited empirical evidence means that the reproducibility and systematic realisation of this potential are hard to predict. Aside from the limited and contradictory empirical research, systems and evaluations lack an explicit theoretical basis. The net effect is that the evidence base still feels essentially scattergun as a corpus of work on which to base local improvement efforts. When systems work (which, in our review, is approximately half of the time) the reasons why can only be speculated upon. But as importantly, when these systems fail (which, in our review, was approximately half of the time) the often considerable financial and opportunity costs are difficult to justify as – to use an oft quoted idiom in healthcare – lessons cannot be learned. Lists of features known to increase effectiveness of CDSS systems41,42 are only partially useful in a complex socio-technical context in which professional knowledge, work as done (rather than work as imagined by CDSS designers or implementers), interdisciplinary norms and dynamics influence technology use and decision-making itself. Varghese and colleagues in a review of CDSS suggest uncertain and dynamic clinical environments shape the significant differences in impact of CDSS. 109 Many studies – 73% in Varghese et al.’s review – are single-site studies from a single unit, practice or hospital. 109 We might exhort designers, implementers and evaluators to consider the system-level factors that might generate differences in organisational dynamics, but it is only middle-range theories (such as NPT or NASS) that allow technologists, clinicians and researchers to think systematically about the diagnostic, remedial, proactive and evaluative tweaks needed to repair, improve and optimise systems.
For complex interventions such as CDSS, the context surrounding the system is as important as the mechanisms by which they generate outcomes. Whilst not a feature of the shifting research evidence base, the context for technology use in healthcare has changed dramatically since spring 2020 and the COVID-19 pandemic. Technologies that were an exceptional component of service delivery, such as video consultation, have become part of mainstream service provision – rapidly accepted and seemingly sustained. 110 The evidence in our synthesis has not captured this shift, but it is possible that many of the barriers to implementation or positive effects may be less important in the current delivery context.
Study strengths and weaknesses
A strength of our review is our inclusive approach to searching for a broad range of studies of differing research designs to reflect the potential breadth of nursing, midwifery and AHP decision-informed care and treatment. We have also undertaken a tighter – methodologically speaking – systematic review focusing only on randomised controlled comparisons:102 the results are not substantially different. This suggests that risk of bias associated with study design is unlikely to be driving the overall research picture.
A related strength of the synthesis overall was the, limited, use of calibration interviews. These helped us unpack some of the apparent effects and both reinforced the importance of good design and implementation and served to highlight the relative absence of both in the included studies.
Including nurses, midwives and AHPs meant that differences in decision context could be examined. Specifically, we thought that certain uncertainty-related decision types (such as triage) or decision components (recording assessments) might be able to be controlled for and we would be able to look for differences between professions faced with similar uncertainties or tasks and using similar CDSS. To some extent this was realised – for example, in relation to triage. But the dearth of inter-professional studies for similar work using CDSS prevented any meaningful comparison. To the best of our knowledge this attempt to control for similar decisions in different contexts with the same system has only been undertaken once by Pope and colleagues in a qualitative case study of CDSS aimed at nurses in emergency and urgent care. 48 They found that consistency in the use of CDSS (i.e. as the designers intended) was as dependent on the implementation and resourcing to support the work required to enact the system as the technology itself. A theme that was apparent in our stakeholder accounts and also a viable hypothesis arising from the studies included in our synthesis is the lack of explicit theory used to design or evaluate reported implementation.
This was the first large-scale review of CDSS technology for both nurses and AHPs since 2009. We envisaged a larger number of studies, reflecting advances in computing power and CDSS types (e.g. machine learning). In contrast to the rapid development of machine learning and changes to tech-enabled choices in the commercial world (such as predictive analytics from Amazon, Google etc.) the research evidence for CDSS in nursing, midwifery and AHPs seemed somewhat dated and lacking behind wider societal technology adoption. Nonetheless, our findings are based on three-times the number of studies in our earlier review, suggesting further and faster growth in the evidence base may be possible.
Viewing our synthesis through the lens of NPT proved feasible and highlighted issues that may have been missed if we focused only on our results in a pragmatic way. Thinking of implementation using higher-order concepts as part of systems development and implementation planning may be more useful than trying to simply ‘build in’ system characteristics. Whilst these are associated with positive outcomes in the wider CDSS literature they were not indicative of improvement in studies of nursing, midwifery and AHPs – suggesting, it is work/roles/professional lines of demarcation that matter more than the architecture or ingredients of decisions. Interviews with system developers, adopters and leaders suggest that – whilst the language may not map onto NPT terminology exactly – concepts of coherence (understanding/sense-making), cognitive participation (building and sustaining), collective action (enacting) and reflexive monitoring (evaluation) are present in accounts.
As with any review of research evidence, our synthesis is a function of the evidence. Research into CDSS aimed at nurses, midwives and AHPs is overwhelmingly low quality, with an absence of theoretical perspectives on design or implementation that could help explain apparent effects/failure or which others could systematically build on. Empirically, studies overwhelmingly focused on nurses and ‘nursing’ decisions in a professionally demarcated way. This is unfortunate as healthcare work is essentially multi-disciplinary and team-based – especially in acute environments. The evidence on the economics of CDSS aimed at nurses and AHPs is patchy and scarce. Our synthesis is unable to adequately answer policy questions of value-for-money associated with systems.
A key working assumption behind the review was that the comparative research evidence base would have matured sufficiently in quantity and quality to produce a substantive series of ‘effects’ and ‘differences’ between systems with less uncertainty that our last review. We felt describing these effects (and associated uncertainty) in a rigorous and systematic way, and having sufficient volume of similar research – amenable to statistical meta-analysis – would offer useful insights for decision-makers faced with commissioning and choosing CDSS. This assumption did not hold up.
We searched only for comparative research studies and failed to include study designs that – whilst less suited to statistical synthesis – might have yielded a richer insight into the implementation processes and mediating effects, mechanisms and contexts that were clearly influential in explaining the mixed picture of uncertain results. As the evidence base matures and the use of theory and well-conducted process evaluations increases, alternatives to traditional systematic review approaches – for example, realist synthesis – may provide more insight and actionable recommendations for implementation, particularly as a basis for nested evaluation alongside CDSS roll-outs.
Beyond reductionist ideas of ‘satisfaction’ with systems, a sophisticated and nuanced view of user perspective was largely absent in studies. Studies since the 1980s have highlighted the complex way in which people interact and socially create meaning, satisfaction, justifications and reinforcement for their use of technology generally and CDSS specifically. 48,103 This is not a trivial omission; how people feel about systems influences their later behaviours and the consequent effects of systems. Artificially simplifying user perspectives on CDSS use to abstract concepts such as ‘satisfaction’ has limited utility for those seeking targets for optimising and evaluating systems.
The lack of common reporting standards for CDSS made comparison between studies to assess similarities difficult and we cannot rule out the possibility that there was more homogeneity between systems and therefore greater potential for statistical synthesis. In the absence of detailed, structured, reporting we were conservative in our estimates. Out of pragmatic necessity, we abstracted descriptions and system features and effects to higher-order concepts such as ‘knowledge-based’ (features) or ‘care processes’ (outcomes). This meant discarding potentially ‘richer’ information. Similarly, studies rarely addressed issues of context and exogenous factors shaping use, implementation and effects. This meant issues of ‘context’ were hard to unpack and incorporate into our analysis. Other kinds of reviews – for example, realist syntheses – may have produced more information on what works for whom, when and in what circumstances.
A final weakness relates to our limited range of interviews with what can be termed ‘stakeholders’ in CDSS. Our original plans had to be changed as a result of lack of engagement arising from the redeployment of staff (and changed priorities) arising from the COVID-19 pandemic. Consequently, the numbers, extent and richness of the data were less than we would have liked. Nonetheless, the limited work yielded some valuable insights and the approach was promising and we will seek to maximise this technique in future syntheses generally and seek approvals and funds to try again with the communities of practice that surround CDSS design, adoption and implementation.
What could we have done differently?
We started from an optimistic estimate of fit-for-purpose research evidence and sufficient quality for inclusion. Our initial pre-synthesis exploratory work suggested substantially more evaluations than when we undertook our last similar review in the 1990s. This sanguine position was justified on the grounds that we expected the numbers of evaluations of CDSS to mirror wider technological and digital expansion in healthcare; for example, EHRs. The growth – at least rhetorically – of technology in the delivery of nursing, midwifery and AHP decision-informed care has not led to similar growth in evaluations.
Our planned analysis was SECONDARY|primary; a synthesis of a rich and detailed extensive pool of well-conducted comparative evaluations that might even merit meta-analysis of common systems, followed by smaller-scale qualitative interviews with those with a stake in decision support. On reflection, a secondary|PRIMARY balance may have been more productive. Whilst the pool of experimental/quasi-experimental research has more than tripled since the last comparable review in 2009 it is still very limited and heterogeneous and still unsuited to statistical synthesis and aggregation. Our primary data collection was hampered by the effects of the COVID-19 pandemic on accessing services and users of CDSS in healthcare. We struggled to recruit, arrange and conduct interviews remotely with the numbers of CDSS implementers, service supporters and service-users. But our interviews and virtual meetings suggest that there is still much to be gained from understanding the experiences and perceptions of those who develop, implement, use and receive/take part in CDSS-mediated professional advice/choices. The uncertainty in the quantitative evaluations synthesised, the depth of perceptions and experience in the limited calibration undertaken, and the role that theory – and theory revised in the light of empirical and perceptual description – could play in improving systems and their implementation make the need for well-conducted, theory-informed, primary qualitative research more urgent.
Study/trial registration details
The main study protocol was registered with PROSPERO1 [number: CRD42019147773] and a protocol for our (sub)review of randomised controlled trials was published in the Cochrane Library. 102
Implications for decision-makers
At the system level, the passion of advocates for decision support systems as part of ‘digital’ healthcare is evident in social media and dedicated professional forums. Amongst policy-makers, the investment and intent to use more technology are regularly expressed by ministers and government. In the UK for example, it is a governmental ambition that:
care professionals should be able to use decision support tools to provide the best care, medicine or device for a patient based on accurate and available data. 111
Whilst developers, service leaders and advocates may push for greater tech-based decision support, our synthesis suggests that the decision-maker experience and real-world results may not warrant such enthusiasm. Governments and health systems investing in CDSS-enabled care should adequately fund well-designed evaluations with sufficient data and methodological support to illuminate the return on investment from CDSS and enable truly informed choices about purchasing or development. There is an urgent need for well-designed health economic evaluations of CDSS to inform system and organisational-level decisions.
Organisations considering implementing or investing in CDSS aimed at nursing, midwifery and AHP work should be aware that there is no evidence on the ‘best’ systems to purchase or invest in. Claimed effects are likely to be accompanied by considerable uncertainty and an equal amount of effort needs to be put into the implementation and resourcing of the ‘work’ needed to initiate, embed and sustain CDSS in a varied workforce whose decision-informed work is heavily contextualised and shapes technology use and its interaction with practice and clinical experience. Whilst some of the technical skills in ICT and data science required to instigate or adopt a CDSS might be planned for, it is our experience that skills required to embed CDSS in work effectively are less evident in many services: theory-informed quality improvement and behaviour-change expertise; human factors and other engineering-focused skills; evaluation of complex interventions. Organisations should pay as much attention to building teams with these skills to support CDSS embedding as they might ICT or clinical champions/advocates. Such teams should include an active patient focus and voice to aid adoption. The five FAQs from our patients – if addressed by providers – might also usefully help encourage ‘coherence’ (shared understanding between potential users/adopter in NPT). Marketing and information aimed at users (both professional and patient) should aim to provide answers tailored to a local service context.
Individual clinicians are often told that the recommendations of CDSS are just that: recommendations. It is tempting to downplay the need for critical appraisal of CDSS recommendations; indeed, prescriptive models of searching for research evidence to apply to patient care decisions have been taken by some to imply that (computerised decision support) ‘systems’ do not require appraisal – their major advantage. 112 The architects of these recommendations are more cautious and are clear that some responsibilities lie on both the system developers and implementers:
The only more compiled source would be a [computerised decision support] system, such as an electronic medical [sic.] record, in which the individual patient’s characteristics were automatically linked to the current best evidence that matched their specific circumstances, with caregivers being reminded or notified of key aspects of management. Such computerised decision support systems are currently few and far between, and those in existence often fall short of ensuring that the evidence supporting the system is the best available and is kept up to date. 113
And the CDSS user:
Users of evidence reports at any level of the 5S pyramid need to be aware of the underlying methods of assembly and assure themselves that these methods are sound. At each level, the standards for evidence generation, retrieval, selection, and analysis should be explicit and at the highest evidence standard possible. For example, systems based on guidelines for patient care should be explicit about the source of the guidelines, and the guidelines should be based on systematic reviews of the pertinent evidence to date. 113
Whilst recognising that, for ad hoc individual clinical decisions and clinicians, critical appraisal of a CDSS system’s evidence base is unrealistic, we recommend that where a CDSS system is being commissioned or implemented, then those with responsibility for others’ use of CDSS-generated advice assure themselves of the quality of the evidence underpinning the system. Ideally guidance should come from the most reliable, trustworthy and clinically useful research evidence. It was clear from our interviews with system implementers that some systems in use in settings are adapted and based on the collective knowledge of ‘experts’, often from within the hospital, or standardised terminological systems (for example, NANDA) where the effects on clinical outcomes of decisions informed by them are largely unknown.
Research recommendations
Evaluators (as well as system designers) should consider appropriate middle-range implementation theory as a means of negotiating the complex socio-technical relationship between technology and associated work. As with quality improvement106 and implementation-related behaviours,105 testing theory-based predictions systematically and building on extant theory in design and evaluation of CDSS will do much to reduce the probability of a similarly ‘broad, but shallow’ pool of empirical studies facing researchers updating this synthesis in another 10 years.
Second, future syntheses should be made easier and more informative by journals and authors adhering to guidelines for conducting and reporting CDSS when publishing findings. These guidelines for CDSS, in general, already exist; there is no reason to believe that they cannot be applied to nursing, midwifery and AHP-focused CDSS. 114,115
Whilst the growth in the number of comparative evaluations in the past decade is welcome, simple head-to-head comparisons rarely help us understand and learn from failure and success of systems. CDSS are a complex healthcare intervention and the development and evaluation of the systems needs to reflect this complexity. Well-established guidance exists for developing and evaluating such interventions and should form an explicit part of studies. 104 This is particularly the case where the comparative evidence is – relatively – well developed (for example, triage decisions or drug dosing), where stronger use of explicit theory could lead to better-designed studies and more insight – especially from modest CDSS effects or ‘failure’.
The absence of PPI involvement in primary studies of CDSS should be remedied. In an era in which shared decision-making is an expected norm in many areas of healthcare practice, it is hard to conceive of good reasons why patients should not have a stronger role in informing the design, implementation and evaluation of CDSS. Missing opportunities for better PPI means researchers may unwittingly perpetuate existing inequalities in areas such as technological access or literacy between demographic and professional groups.
Given the well-established potential for CDSS to introduce harm into clinical work, studies should incorporate the possibility of both e-iatrogenesis and harm into their designs. As with any other health technology evaluation, they should measure safety events and unintended consequences in their research. This is particularly important in any economic analysis of the costs and benefits of systems.
Like issues of safety, future research should seek to build the economic evidence surrounding CDSS. As well as the sorts of cost-effectiveness analysis that would allow potential adopters to choose between different systems – studies we acknowledge may be complex to design and conduct for in-house evaluation teams – even the simplest cost-consequence analysis could and should consider the costs of implementing the systems. These are often missing from studies and – as with quality improvement in healthcare generally – have the effect of making systems seem more attractive than they might be if the true costs were known. 116
Conclusion
Our review of available evidence comparing CDSS support to unsupported (by CDSS) decision-related performance and outcomes in nurses and – to a far lesser extent – midwives and AHPs synthesises 35 studies and reveals a contradictory picture. Studies reported that around half the process measures improved and half were worse or no different. CDSS-attributable outcomes fared little better, with only ≈40% of outcomes reported actually improving with CDSS use and, in one study, producing fewer delivery complications less efficiently than not using the system. Characteristics previously believed to improve general CDSS effectiveness were not associated with improved processes or outcomes. The within-study variation in take-up and wide confidence intervals around effects suggest two things. First, 13 years since the last substantive non-medical review, uncertain effects are still evident. Second, simply adding more studies into the heterogeneous bundle of extant evaluations, without systematically testing and refining relevant mid-range theories, will not lead to the step change in replicable implementation that policy-makers and advocates of digital technology in health desire so urgently.
This is the first systematic review that set out to control for the same systems used in different professional and organisational contexts. The limited empirical and theoretical treatment of the complex socio-technical context in which CDSS operate means we are unable to offer definitive conclusions about which systems best improve processes and outcomes in NMAHP work. The synthesis does allow us to add to the growing realisation that CDSS planners, implementers and evaluators should use well-developed and rigorously tested mid-range theory that both already exists and is designed to produce technologies better able to work within complex socio-technical systems. Doing this before systems are designed and evaluated, rather than having to use theory to scrutinise processes and effects, would be more efficient. Justifying greater investment in implementing CDSS, unaccompanied by well-designed, theoretically informed evaluation, is made more challenging by the absence of economic comparisons of the costs and benefits of different systems aimed at NMAHPs. Studies that focus on real-world implementation of CDSS should – by default – incorporate some form of health economic evaluation.
CDSS are often sold on the promise that they can support and release the potential of NMAHPs’ decisions in multidisciplinary healthcare to increase quality efficiently. Our synthesis suggests there is still some way to go before this is a routine reality for both clinicians and patients.
Acknowledgements
We would like to thank the CDSS experts and patients that gave their time to help us contextualise our results at a point in our history when providing time for others is a generous gift.
Contributions of authors
Carl Thompson (https://orcid.org/0000-0002-9369-1204) drafted this synthesis and was responsible for the initial research idea and bid.
Teumzghi Mebrahtu (https://orcid.org/0000-0003-4821-2304) and Carl Thompson (https://orcid.org/0000-0002-9369-1204) were responsible for the review design, extraction and analysis; all team members at some point helped screen titles and abstracts, informed our analysis, and helped draft work and articles.
Sarah Skyrme (https://orcid.org/0000-0002-6173-0117) and Alison Ledward carried out the virtual meeting of PPG members and production of patient FAQs on CDSS.
Sarah Skyrme (https://orcid.org/0000-0002-6173-0117) also interviewed our system experts.
Ethical approval for the qualitative components of the project was sought and granted by the University of Leeds Faculty of Medicine and Health Ethics Committee HREC 20-002 - Effects of computerised clinical decision support systems (CDSS) on nursing and Allied Health Professional performance and patient outcomes: A systematic review and user contextualisation.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to available anonymised data may be granted following review.
Ethics statement
Ethical approval for the study was granted by the School of Healthcare Research Ethics Committee, University of Leeds, on 20 October 2020: approval reference HREC 20-002.
This article
The contractual start date for this research was in October 2019. This article began editorial review in October 2021 and was accepted for publication in July 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The Health and Social Care Delivery Research editors and publisher have tried to ensure the accuracy of the authors’ article and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Disclaimer
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
This manuscript reports on one component of the research award Effects of computerised clinical decision support systems (CDSS) on nursing and Allied Health Professional performance and patient outcomes: A systematic review and user contextualisation. For more information about this research please view the award page [https://www.fundingawards.nihr.ac.uk/award/NIHR127926]
References
- Thompson C, Randell R, Keenan AM, . Effects of computerised clinical decision support systems (CDSS) on nursing and allied health professional performance and patient outcomes: a systematic review and user contextualisation n.d. www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019147773.
- Covell DG, Uman GC, Manning PR. Information needs in office practice: are they being met? [published Online First: 1985/10/01]. Ann Intern Med 1985;103:596-9. https://doi.org/10.7326/0003-4819-103-4-596.
- Thompson C, Cullum N, McCaughan D, . Nurses, information use, and clinical decision making – the real world potential for evidence-based decisions in nursing [published Online First: 2004/07/16]. Evid Based Nurs 2004;7:68-72. https://doi.org/10.1136/ebn.7.3.68.
- Garg AX, Adhikari NKJ, McDonald H, . Effects of computerized clinical decision support systems on practitioner performance and patient outcomes a systematic review. JAMA 2005;293:1223-38. https://doi.org/10.1001/jama.293.10.1223.
- Cooper RJ, Bissell P, Ward P, . Further challenges to medical dominance? The case of nurse and pharmacist supplementary prescribing. Health 2012;16:115-33. https://doi.org/10.1177/1363459310364159.
- Noordegraaf M. Hybrid professionalism and beyond: (New) Forms of public professionalism in changing organizational and societal contexts. J Prof Organ 2015;2:187-206. https://doi.org/10.1093/jpo/jov002.
- Hallgren KA, Bauer AM, Atkins DC. Digital technology and clinical decision making in depression treatment: current findings and future opportunities [published Online First: 2017/04/28]. Depress Anxiety 2017;34:494-501. https://doi.org/10.1002/da.22640.
- Hughes DJ, Allen D. Nursing and the Division of Labour in Healthcare. Basingstoke: Palgrave; 2002.
- Hammond KR, Kelly KJ, Schneider RJ, . Clinical inference in nursing: revising judgments. Nurs Res 1967;16:38-45.
- Buckingham CD, Adams A. Classifying clinical decision making: a unifying approach [published Online First: 2000/11/30]. J Adv Nurs 2000;32:981-9.
- Standing M. Clinical judgement and decision-making in nursing - nine modes of practice in a revised cognitive continuum [published Online First: 2008/03/21]. J Adv Nurs 2008;62:124-34. https://doi.org/10.1111/j.1365-2648.2007.04583.x.
- Elstein AS, Schwartz A. Clinical problem solving and diagnostic decision making: selective review of the cognitive literature [published Online First: 2002/03/23]. BMJ 2002;324:729-32. https://doi.org/10.1136/bmj.324.7339.729.
- Osheroff JA, Teich JM, Middleton B, . A roadmap for national action on clinical decision support [published Online First: 2007/01/11]. J Am Med Inform Assoc 2007;14:141-5. https://doi.org/10.1197/jamia.M2334.
- Sim I, Gorman P, Greenes RA, . Clinical decision support systems for the practice of evidence-based medicine. J Am Med Inform Assoc 2001;8:527-34. https://doi.org/10.1136/jamia.2001.0080527.
- Sutton RT, Pincock D, Baumgart DC, . An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med 2020;3. https://doi.org/10.1038/s41746-020-0221-y.
- Lytle KS, Short NM, Richesson RL, . Clinical decision support for nurses: a fall risk and prevention example [published Online First: 2015/11/17]. Comput Inform Nurs 2015;33:530-7. https://doi.org/10.1097/CIN.0000000000000192.
- Khong PC, Hoi SY, Holroyd E, . Nurses’ clinical decision making on adopting a wound clinical decision support system [published Online First: 2015/06/13]. Comput Inform Nurs 2015;33:295-30. https://doi.org/10.1097/CIN.0000000000000164.
- Gross DP, Armijo-Olivo S, Shaw WS, . Clinical decision support tools for selecting interventions for patients with disabling musculoskeletal disorders: a scoping review [published Online First: 2015/12/17]. J Occup Rehabil 2016;26:286-318. https://doi.org/10.1007/s10926-015-9614-1.
- Martin Ruiz ML, Valero Duboy MA, Torcal Loriente C, . Evaluating a web-based clinical decision support system for language disorders screening in a nursery school [published Online First: 2014/05/30]. J Med Internet Res 2014;16. https://doi.org/10.2196/jmir.3263.
- Mahabir C, Kuperman G, Lantigua R, . Improving depression screening using CDS and patient access to information using PHRs. Comput Inform Nurs 2014;32:364-65. https://doi.org/10.1097/01.NCN.0000453183.49477.ee.
- Berner ES. Clinical Decision Support Systems: Theory and Practice. New York, NY: Springer; 2007.
- Cresswell K, Majeed A, Bates DW, . Computerised decision support systems for healthcare professionals: an interpretative review [published Online First: 2012/01/01]. Inform Prim Care 2012;20:115-28. https://doi.org/10.14236/jhi.v20i2.32.
- Rahbari NN, Reissfelder C, Koch M, . The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients [published Online First: 2011/06/16]. Ann Surg Oncol 2011;18:3640-9. https://doi.org/10.1245/s10434-011-1829-6.
- Cook DA, Pencille LJ, Dupras DM, . Practice variation and practice guidelines: attitudes of generalist and specialist physicians, nurse practitioners, and physician assistants. PLOS ONE 2018;13. https://doi.org/10.1371/journal.pone.0191943.
- Stride SL, Hundley VA, Way S, . Identifying the factors that influence midwives’ perineal practice at the time of birth in the United Kingdom. Midwifery 2021;102. https://doi.org/10.1016/j.midw.2021.103077.
- Cheyne H, Dowding DW, Hundley V. Making the diagnosis of labour: midwives’ diagnostic judgement and management decisions. J Adv Nurs 2006;53:625-35. https://doi.org/10.1111/j.1365-2648.2006.03769.x.
- Royal College of Nursing n.d. www.rcn.org.uk/professional-development/publications/pub-006129.
- Bright TJ, Wong A, Dhurjati R, . Effect of clinical decision-support systems: a systematic review. Annals of Internal Medicine 2012;157:29-43. https://doi.org/10.7326/0003-4819-157-1-201207030-00450 %.
- Moja L, Kwag KH, Lytras T, . Effectiveness of computerized decision support systems linked to electronic health records: a systematic review and meta-analysis [published Online First: 2014/10/17]. Am J Public Health 2014;104:e12-22. https://doi.org/10.2105/AJPH.2014.302164.
- Randell R, Mitchell N, Dowding D, . Effects of computerized decision support systems on nursing performance and patient outcomes: a systematic review. J Health Serv Res Policy 2007;12:242-51. https://doi.org/10.1258/135581907782101543.
- Gillaizeau F, Chan E, Trinquart L, . Computerized advice on drug dosage to improve prescribing practice. Cochrane Database Syst Rev n.d.;2013.
- Tan K, Dear PR, Newell SJ. Clinical decision support systems for neonatal care. Cochrane Database Syst Rev n.d.;2005.
- Coiera E, Westbrook J, Wyatt J. The safety and quality of decision support systems [published Online First: 2006/10/20]. Yearb Med Inform 2006:20-5.
- Dunn Lopez K, Gephart SM, Raszewski R, . Integrative review of clinical decision support for registered nurses in acute care settings. J Am Med Inform Assoc 2016;24:441-50. https://doi.org/10.1093/jamia/ocw084.
- Dowding D, Mitchell N, Randell R, . Nurses’ use of computerised clinical decision support systems: a case site analysis. J Clin Nurs 2009;18:1159-67. https://doi.org/10.1111/j.1365-2702.2008.02607.x.
- Randell R, Mitchell N, Dowding D, . Effects of computerized decision support systems on nursing performance and patient outcomes: a systematic review [published Online First: 2007/10/11]. J Health Serv Res Policy 2007;12:242-9. https://doi.org/10.1258/135581907782101543.
- Porter A, Dale J, Foster T, . Implementation and use of computerised clinical decision support (CCDS) in emergency pre-hospital care: a qualitative study of paramedic views and experience using Strong Structuration Theory. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0786-x.
- Weiner JP, Kfuri T, Chan K, . ‘e-Iatrogenesis’: the most critical unintended consequence of CPOE and other HIT [published Online First: 2007/03/03]. J Am Med Inform Assoc 2007;14:387-8. https://doi.org/10.1197/jamia.M2338.
- Langhorne P, Ramachandra S. Organised inpatient (stroke unit) care for stroke: network meta-analysis. Cochrane Database Syst Rev 2020. https://doi.org/10.1002/14651858.CD000197.pub4.
- Sintchenko V, Coiera E. Decision complexity affects the extent and type of decision support use. AMIA Annu Symp 2006:724-8.
- Roshanov PS, Fernandes N, Wilczynski JM, . Features of effective computerised clinical decision support systems: meta-regression of 162 randomised trials. BMJ 2013;346.
- Van de Velde S, Heselmans A, Delvaux N, . A systematic review of trials evaluating success factors of interventions with computerised clinical decision support. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0790-1.
- May C, Finch T, Mair F, . Understanding the implementation of complex interventions in health care: the normalization process model [published Online First: 2007/09/21]. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-148.
- Greenhalgh T, Wherton J, Papoutsi C, . Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies [published Online First: 2017/11/03]. J Med Internet Res 2017;19. https://doi.org/10.2196/jmir.8775.
- Cresswell KM, Worth A, Sheikh A. Actor-Network Theory and its role in understanding the implementation of information technology developments in healthcare. BMC Medical Inform Decis Mak 2010;10. https://doi.org/10.1186/1472-6947-10-67.
- May CR, Cummings A, Girling M, . Using Normalization Process Theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review [published Online First: 2018/06/09]. Implement Sci 2018;13. https://doi.org/10.1186/s13012-018-0758-1.
- Finch TL, Rapley T, Girling M, . Improving the normalization of complex interventions: measure development based on normalization process theory (NoMAD): study protocol [published Online First: 2013/04/13]. Implement Sci 2013;8. https://doi.org/10.1186/1748-5908-8-43.
- Pope C, Halford S, Turnbull J, . Using computer decision support systems in NHS emergency and urgent care: ethnographic study using normalisation process theory [published Online First: 2013/03/26]. BMC Health Serv Res 2013;13. https://doi.org/10.1186/1472-6963-13-111.
- Kawamoto K, Houlihan CA, Balas EA, . Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330. https://doi.org/10.1136/bmj.38398.500764.8F.
- May CR, Finch T, Ballini L, . Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit [published Online First: 2011/10/04]. BMC Health Serv Res 2011;11. https://doi.org/10.1186/1472-6963-11-245.
- Cochrane Effective Practice Organisation of Care (EPOC) n.d. http://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 15 April 2020).
- Cochrane Effective Practice and Organisation of Care (EPOC) n.d. https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 30 March 2020).
- Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane 2019.
- Cochrane Effective Practice and Organisation of Care (EPOC) n.d. https://epoc.cochrane.org/resources/epoc-resources-review-authors.
- Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002;21:2409-19. https://doi.org/10.1002/sim.1047.
- Lipscomb M. Abductive reasoning and qualitative research [published Online First: 2012/09/07]. Nurs Philos 2012;13:244-56. https://doi.org/10.1111/j.1466-769X.2011.00532.x.
- Roberts K, Dowell A, Nie JB. Attempting rigour and replicability in thematic analysis of qualitative research data; a case study of codebook development [published Online First: 2019/03/30]. BMC Med Res Methodol 2019;19. https://doi.org/10.1186/s12874-019-0707-y.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. Thousand Oaks, CA, London: Sage; 1994.
- Ryan GW, Bernard HR. Techniques to identify themes. Field Methods 2003;15:85-109. https://doi.org/10.1177/1525822X02239569.
- Bennett P, Hardiker N. A Quantitative study investigating the effects of computerised clinical decision support in the emergency department. Stud Health Technol Inform 2016;225:53-7.
- Snooks HA, Carter B, Dale J, . Support and Assessment for Fall Emergency Referrals (SAFER 1): cluster randomised trial of computerised clinical decision support for paramedics. PLOS ONE 2014;9. https://doi.org/10.1371/journal.pone.0106436.
- Wells B. Implementation of Computerised Clinical Decision Support (CCDS) in a Prehospital Setting: Processes of Adoption and Impact on Paramedic Role and Practice. Swansea University; 2013.
- Lattimer V, George S, Thompson F, . Safety and effectiveness of nurse telephone consultation in out of hours primary care: randomised controlled trial. BMJ 1998;317:1054-9.
- Lattimer V, Sassi F, George S, . Cost analysis of nurse telephone consultation in out of hours primary care: evidence from a randomised controlled trial. BMJ 2000;320:1053-7.
- Cortez S. Measuring active Clinical Decision Support Influence on Nursing Research Utilization. University of Phoenix; 2014.
- Fitzmaurice DA, Hobbs FD, Murray ET, . Oral anticoagulation management in primary care with the use of computerized decision support and near-patient testing: a randomized, controlled trial. Arch Intern Med 2000;160:2343-8.
- Kroth PJ, Dexter PR, Overhage JM, . A computerized decision support system improves the accuracy of temperature capture from nursing personnel at the bedside. AMIA Annu Symp 2006:444-8.
- Rood E, Bosman RJ, van der Spoel JI, . Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation. J Am Med Inform Assoc 2005;12:172-80. https://doi.org/10.1197/jamia.M1598.
- Sassen B, Kok G, Schepers J, . Supporting health care professionals to improve the processes of shared decision making and self-management in a web-based intervention: randomized controlled trial. J Med Internet Res 2014;16.
- Cleveringa FGW, Gorter KJ, Van Donk MD, . Combined task delegation, computerized decision support, and feedback improve cardiovascular risk for type 2 diabetic patients. Diabetes Care 2008;31:2273-5.
- Cleveringa FGW, Welsing PMJ, Van Den Donk M, . Cost-effectiveness of the diabetes care protocol, a multifaceted computerized decision support diabetes management intervention that reduces cardiovascular risk. Diabetes Care 2010;33:258-63.
- Geurts D, de Vos-Kerkhof E, Polinder S, . Implementation of clinical decision support in young children with acute gastroenteritis: a randomized controlled trial at the emergency department. Eur J Pediatr 2017;176:173-81.
- Lee NJ, Chen ES, Currie LM, . The effect of a mobile clinical decision support system on the diagnosis of obesity and overweight in acute and primary care encounters. ANS Adv Nurs Sci 2009;32:211-21. https://doi.org/10.1097/ANS.0b013e3181b0d6bf.
- Blaha J, Kopecky P, Matias M, . Comparison of three protocols for tight glycemic control in cardiac surgery patients. Diabetes Care 2009;32. https://doi.org/10.2337/dc08-1851.
- Canbolat O, Kapucu S, Kilickaya O. Comparison of routine and computer-guided glucose management for glycemic control in critically ill patients. Crit Care Nurse 2019;39:20-7. https://doi.org/10.4037/ccn2019431.
- Cavalcanti AB, Silva E, Pereira AJ, . A randomized controlled trial comparing a computer-assisted insulin infusion protocol with a strict and a conventional protocol for glucose control in critically ill patients. J Crit Care 2009;24:371-8. https://doi.org/10.1016/j.jcrc.2009.05.005.
- Dumont C, Bourguignon C. Effect of a computerized insulin dose calculator on the process of glycemic control. Am J Crit Care 2012;21:106-15. https://doi.org/10.4037/ajcc2012956.
- Hovorka R, Kremen J, Blaha J, . Blood glucose control by a model predictive control algorithm with variable sampling rate versus a routine glucose management protocol in cardiac surgery patients: a randomized controlled trial. J Clin Endocrinol Metab 2007;92:2960-4. https://doi.org/10.1210/jc.2007-0434.
- Mann EA, Jones JA, Wolf SE, . Computer decision support software safely improves glycemic control in the burn intensive care unit: a randomized controlled clinical study. J Burn Care Res 2011;32:246-55.
- Plank J, Blaha J, Cordingley J, . Multicentric, randomized, controlled trial to evaluate blood glucose control by the model predictive control algorithm versus routine glucose management protocols in intensive care unit patients. Diabetes Care 2006;29. https://doi.org/10.2337/diacare.29.02.06.dc05-1689.
- Vadher BD, Patterson DL, Leaning M. Comparison of oral anticoagulant control by a nurse-practitioner using a computer decision-support system with that by clinicians. Clin Lab Haematol 1997;19:203-7.
- Byrne CM. Impact of Prospective Computerized Clinical Decision Support Information and Targeted Assistance on Nursing Home Resident Outcomes. State University of New York at Albany; 2005.
- Duclos A, Touzet S, Restier L, . Implementation of a computerized system in pediatric wards to improve nutritional care: a cluster randomized trial. Eur J Clin Nutr 2015;69:769-75. https://doi.org/10.1038/ejcn.2014.288.
- Forberg U, Unbeck M, Wallin L, . Effects of computer reminders on complications of peripheral venous catheters and nurses’ adherence to a guideline in paediatric care-a cluster randomised study. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0375-9.
- Dalaba MA, Akweongo P, Aborigo RA, . Cost-effectiveness of clinical decision support system in improving maternal health care in Ghana. PLOS ONE 2015;10. https://doi.org/10.1371/journal.pone.0125920.
- Roukema J, Steyerberg EW, van der Lei J, . Randomized trial of a clinical decision support system: impact on the management of children with fever without apparent source. J Am Med Inform Assoc 2008;15:107-13. https://doi.org/10.1197/jamia.M2164.
- McDonald MV, Feldman PH, Barron-Vaya Y, . Outcomes of clinical decision support (CDS) and correlates of CDS use for home care patients with high medication regimen complexity: a randomized trial. J Eval Clin Pract 2017;22:10-9.
- Beeckman D, Clays E, Van Hecke A, . A multi-faceted tailored strategy to implement an electronic clinical decision support system for pressure ulcer prevention in nursing homes: a two-armed randomized controlled trial. Int J Nurs Stud 2013;50:475-86. https://doi.org/10.1016/j.ijnurstu.2012.09.007.
- Dowding DW, Turley M, Garrido T. The impact of an electronic health record on nurse sensitive patient outcomes: an interrupted time series analysis [published Online First: 2011/12/17]. J Am Med Inform Assoc 2012;19:615-20. https://doi.org/10.1136/amiajnl-2011-000504.
- Dykes PC, Carroll DL, Hurley A, . Fall prevention in acute care hospitals: a randomized trial. Jama 2010;304:1912-8.
- Dykes PC, Burns Z, Adelman J, . Evaluation of a patient-centered fall-prevention tool kit to reduce falls and injuries: a nonrandomized controlled trial. JAMA Netw Open 2020;3:e2025889-e89.
- Fossum M, Alexander GL, Ehnfors M, . Effects of a computerized decision support system on pressure ulcers and malnutrition in nursing homes for the elderly. Int J Med Inf 2011;80:607-17. https://doi.org/10.1016/j.ijmedinf.2011.06.009.
- Lv S, Ye X, Wang Z, . A randomized controlled trial of a mobile application-assisted nurse-led model used to improve treatment outcomes in children with asthma. J Adv Nurs 2019;75:3058-67.
- Paulsen MM, Paur I, Gjestland J, . Effects of using the MyFood decision support system on hospitalized patients’ nutritional status and treatment: a randomized controlled trial. Clin Nutr 2020;39:3607-17.
- Dykes PC, Burns Z, Adelman J, . Evaluation of a patient-centered fall-prevention tool kit to reduce falls and injuries: a nonrandomized controlled trial. JAMA Netw Open 2020;3:e2025889-e89. https://doi.org/10.1001/jamanetworkopen.2020.25889.
- Lattimer V, Sassi F, George S, . Cost analysis of nurse telephone in out of hours primary care: evidence from a randomised controlled trial. BMJ 2000;320:1053-7.
- Abbott AD. The System of Professions: An Essay on the Division of Expert Labor. Chicago, IL: University of Chicago Press; 1988.
- Fishbein M, Ajzen I. Predicting and Changing Behavior: The Reasoned Action Approach. New York, NY; Hove: Psychology Press; 2009.
- Coiera E, Ash J, Berg M. The unintended consequences of health information technology revisited [published Online First: 2016/11/11]. Yearb Med Inform n.d.;2016:163-9. https://doi.org/10.15265/IY-2016-014.
- Sittig DF. A socio-technical model of health information technology-related e-iatrogenesis [published Online First: 2008/11/13]. AMIA Annu Symp Proc 2008:1209-10.
- Mebrahtu TF, Bloor K, Ledward A, . Effects of computerised clinical decision support systems (CDSS) on nursing and allied health professional performance and patient outcomes. Cochrane Database Syst Rev n.d.;2021. https://doi.org/10.1002/14651858.CD014699.
- Greenhalgh T, Hinder S, Stramer K, . Adoption, non-adoption, and abandonment of a personal electronic health record: case study of HealthSpace [published Online First: 2010/11/18]. BMJ 2010;341. https://doi.org/10.1136/bmj.c5814.
- O’Cathain A, Croot L, Duncan E, . Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2019–029954.
- Atkins L, Francis J, Islam R, . A guide to using the Theoretical Domains Framework of behaviour change to investigate implementation problems [published Online First: 2017/06/24]. Implement Sci 2017;12. https://doi.org/10.1186/s13012-017-0605-9.
- Grol R, Baker R, Moss F. Quality improvement research: understanding the science of change in health care [published Online First: 2002/11/27]. Qual Saf Health Care 2002;11:110-1. https://doi.org/10.1136/qhc.11.2.110.
- Ivers NM, Sales A, Colquhoun H, . No more ‘business as usual’ with audit and feedback interventions: towards an agenda for a reinvigorated intervention [published Online First: 2014/01/21]. Implement Sci 2014;9. https://doi.org/10.1186/1748-5908-9-14.
- Dune R. Evaluating Evaluations of Clinical Decision Support Systems: Case Studies From NHS Clinical Settings. Open University; 2016.
- Varghese J, Kleine M, Gessner SI, . Effects of computerized decision support system implementations on patient outcomes in inpatient care: a systematic review [published Online First: 2017/10/17]. J Am Med Inform Assoc 2018;25:593-602. https://doi.org/10.1093/jamia/ocx100.
- Hutchings R. The Impact of Covid-19 on The Use of Digital Technology in the NHS. London: Nuffield Trust; 2020.
- Department of Health and Social Care n.d. www.gov.uk/government/publications/the-future-of-healthcare-our-vision-for-digital-data-and-technology-in-health-and-care/the-future-of-healthcare-our-vision-for-digital-data-and-technology-in-health-and-care (accessed 1 July 2021).
- Dicenso A, Bayley L, Haynes RB. Accessing pre-appraised evidence: fine-tuning the 5S model into a 6S model [published Online First: 2009/09/26]. Evid Based Nurs 2009;12:99-101. https://doi.org/10.1136/ebn.12.4.99-b.
- Haynes RB. Of studies, syntheses, synopses, summaries, and systems: the ‘5S’ evolution of information services for evidence-based healthcare decisions [published Online First: 2007/01/11]. Evid Based Med 2006;11:162-4. https://doi.org/10.1136/ebm.11.6.162-a.
- Kawamoto K, McDonald CJ. Designing, conducting, and reporting clinical decision support studies: recommendations and call to action. Ann Intern Med 2020;172:S101-9. https://doi.org/10.7326/M19-0875.
- National Health Services (NHS) n.d. www.digitalhealth.net/2020/10/nhsx-launches-assessment-criteria-for-digital-health-tools/ (accessed 29 October 2020).
- Thompson C, Pulleyblank R, Parrott S, . The cost-effectiveness of quality improvement projects: a conceptual framework, checklist and online tool for considering the costs and consequences of implementation-based quality improvement [published Online First: 2015/07/24]. J Eval Clin Pract 2016;22:26-30. https://doi.org/10.1111/jep.12421.
Appendix 1 Example Ovid MEDLINE(R) 1946 to 12 February 2021 search strategy
-
exp Decision Making/ (207895)
-
decision support techniques/ (20911)
-
(decision* adj2 making).ti,ab,kf. (159754)
-
(decision* adj2 support*).ti,ab,kf. (24230)
-
(decision* adj2 aid*).ti,ab,kf. (6501)
-
or/1–5 (354546)
-
exp Computers/ (79322)
-
exp information systems/ (238259)
-
exp Informatics/ (537355)
-
Internet/ (74916)
-
Software/ (112580)
-
Cell Phone/ (8821)
-
Mobile Applications/ (6962)
-
exp Telemedicine/ (32559)
-
Medical Records Systems, Computerized/ (19076)
-
exp Electronic Health Records/ (21793)
-
computer*.ti,ab,kf. (313610)
-
electronic*.ti,ab,kf. (291368)
-
(internet or web or online or on-line).ti,ab,kf. (310071)
-
(software or computer program*).ti,ab,kf. (193359)
-
(automate* or automation).ti,ab,kf. (136436)
-
(pda or pdas).ti,ab,kf. (13229)
-
personal digital assistant*.ti,ab,kf. (1012)
-
(app or apps).ti,ab,kf. (31717)
-
(application* adj2 mobile*).ti,ab,kf. (4834)
-
(iPad* or iPhone* or smartphone* or smart phone* or smart device* or mobile phone or android phone* or cellphone* or cell phone*).ti,ab,kf. (26450)
-
(tablet adj2 (pc or device* or comput*)).ti,ab,kf. (1603)
-
((hand held or handheld) adj2 (pc or device* or comput*)).ti,ab,kf. (2669)
-
(telehealth or telecare or telemedicine or ehealth or mhealth).ti,ab,kf. (29130)
-
or/7–29 (1674343)
-
6 and 30 (66042)
-
exp Decision Making, Computer-Assisted/ (149528)
-
Decision Support Systems, Clinical/ (8302)
-
(computer assisted adj2 (decision* or diagnos* or therap* or support or treatment? or management)).ti,ab,kf. (1545)
-
(computer aided adj2 (decision* or diagnos* or therap* or support or treatment? or management)).ti,ab,kf. (3921)
-
(decision adj2 support adj2 (system* or tool*)).ti,ab,kf. (9917)
-
(decision making adj2 (system* or tool*)).ti,ab,kf. (2560)
-
Expert Systems/ (3420)
-
(expert adj2 system*).ti,ab,kf. (3613)
-
Reminder Systems/ (3568)
-
((computer* or electronic* or CDSS) adj2 (reminder* or alert*)).ti,ab,kf. (1210)
-
((medication or medicine or treatment or therapy) adj2 (reminder* or alert*)).ti,ab,kf. (857)
-
reminder system*.ti,ab,kf. (875)
-
Medical Order Entry Systems/ (2303)
-
((computer* or electronic*) adj2 order entry).ti,ab,kf. (1874)
-
(computer adj2 decision support*).ti,ab. (412)
-
CPOE.ti,ab,kf. (1139)
-
or/32–47 (177952)
-
31 or 48 [all computerised clinical decision support systems terms] (228840)
-
Allied Health Personnel/ (11925)
-
Allied Health Occupations/ (587)
-
Physical Therapist Assistants/ (16)
-
Physical Therapy Specialty/ (2889)
-
Speech-Language Pathology/ (3172)
-
Occupational Therapy/ (13482)
-
Nutritionists/ (1290)
-
dietetics/ (7837)
-
Anesthesiologists/ (1163)
-
podiatry/ (2273)
-
exp Osteopaths/ (321)
-
osteopathic physicians/ (321)
-
anesthesiologist*.ti,ab,kf. (22810)
-
podiatrist*.ti,ab,kf. (910)
-
prosthetist*.ti,ab,kf. (397)
-
chiropodist*.ti,ab,kf. (132)
-
orthoptist*.ti,ab,kf. (319)
-
orthotist*.ti,ab,kf. (220)
-
osteopath*.ti,ab,kf. (5983)
-
radiographer*.ti,ab,kf. (1803)
-
art therapist*.ti,ab,kf. (89)
-
drama therapist*.ti,ab,kf. (3)
-
music therapist*.ti,ab,kf. (368)
-
(allied adj2 health adj2 (profession* or worker* or personnel or occupation* or staff)).ti,ab,kf. (3421)
-
((physical or occupational or language or speech or physio*) adj2 therap*).ti,ab,kf. (50227)
-
physiotherapist*.ti,ab,kf. (8544)
-
dietetic*.ti,ab,kf. (9828)
-
dietitian*.ti,ab,kf. (6580)
-
nutritionist*.ti,ab,kf. (3020)
-
Patient care team/ (66483)
-
((multidisciplinary or multi-disciplinary or multiprofessional or multi-professional or interdisciplinary or interprofessional) adj2 team*).ti,ab,kf. (32126)
-
Emergency Medical Technicians/ (5756)
-
Emergency Medical Services/ (43736)
-
Ambulances/ (6210)
-
Air Ambulances/ (2874)
-
paramedic*.ti,ab,kf. (8537)
-
HEMS.ti,ab,kf. (767)
-
ems.ti,ab,kf. (13017)
-
emt.ti,ab,kf. (25232)
-
prehospital.ti,ab,kf. (13136)
-
pre-hospital.ti,ab,kf. (4836)
-
first responder*.ti,ab,kf. (2449)
-
emergency medical technician*.ti,ab,kf. (1168)
-
emergency services.ti,ab,kf. (4115)
-
ambulance*.ti,ab,kf. (11269)
-
field triage.ti,ab,kf. (275)
-
out-of-hospital.ti,ab,kf. (11317)
-
(nurse or nurses or nursing).ti,ab,kf. (462330)
-
exp nurses/ (89638)
-
exp nursing staff/ (67063)
-
Midwifery/ (19460)
-
(midwif* or midwiv*).ti,ab,kf. (25895)
-
or/50–101 [allied health professionals or nurses or midwives] (836031)
-
49 and 102 [all CDSS and allied health professionals or nurses or midwives] (9549) (see Appendix 2, Table 11).
Appendix 2 Additional information on studies and interventions
| Author and year | Type of report | Funding source | Interventions and their details | CDSS function category |
|---|---|---|---|---|
| Beeckman et al. 201389 | Journal article | None | •A standalone computer-based CDSS (Pre-vPlan-a six-step clinical practice to reduce pressure ulcers using CDSS): Step-1: Analysis of current practice, target group, and context Step-2: Match of research findings and/or existing guidelines to practice Step-3: description of the specific change outcome Step-4: Selection/development of the implementation strategies Step-5: Development and execution of the implementation process Step-6: Continuous evaluation and adaptation of the implementation •A standard protocol (a hard copy with no implementation strategy) of reducing pressure ulcers |
Disease prevention |
| Bennet et al. 201661 | Journal article | Not stated | •A standalone computer-based Triage CDSS [intervention period]: the CDSS was developed in-house by engineers and ED clinician to be used in triage in emergency department •Triage without CDSS [pre-intervention period] |
Triaging |
| Blaha et al. 200975 | Journal article | Public sector | •A standalone computer-based CDSS: a predictive model algorithm that, automates blood glucose control in critically ill patients, by calculating the current insulin of each individual patient and generating advice on the new insulin infusion rate. •Control-1 (paper based-Matias protocol): continuous insulin infusion combined with iv insulin boluses (RMP) to maintain euglycemia (target range 4.4–6.1 mmol/liter) •Control-2 (paper based-Bath protocol): initial insulin infusion rate is dependent on the initial blood glucose. Subsequent changes to the infusion rate are made after comparing the latest blood glucose both to the target range and also to the previous blood glucose value |
Drug dosing |
| Byrne 200583 | PhD Thesis | Public sector | •A standalone computer-based CDSS: computerized information to alert nursing and other staff to the resident-specific risk factors •Usual care (CDSS non-use) |
Disease prevention reminders |
| Canbolat et al. 201976 | Journal article | Public sector | •A standalone computer based CDSS: automated blood glucose monitoring system using newly developed glycaemic control software. •Usual care: ICU nurses measured blood glucose levels and reported them to ICU physicians, who made dosing and treatment decisions based on their own knowledge and assessment. |
Drug dosing |
| Cavalcanti et al. 200977 | Journal article | Public sector | •A standalone computer/handheld-based CDSS: a computer program running on an ICU desktop or a handheld (electronic supplemental material) used for adjustinginsulin doses. •Control-1 (Leuven protocol): continuous intravenous insulin infusion with adjustments according to a protocol developed and used by Van den Berghe et al •Control-2 (conventional treatment): intermittent subcutaneous insulin administration according to a sliding scale. |
Drug dosing |
| Cleveringa et al. 200871 | Journal article | Private sector-commercial | •A standalone computer-based CDSS: an intervention with the following components:1) diabetes consultation hour run by a practice nurse,2) a CDSS that contained a diagnostic and treatment algorithm based on the Dutch type2 diabetes guidelines and provided patient-specific treatment advice,3) a recall system, and 4) feedback every 3 months regarding the percentage of patients meeting the treatment target. The primary care physicians (PCPs) were advised that they should prescribe new medication and refer patients if necessary. •Usual care: diabetes care provided by the Primary carephysicians (PCP)or by a practice nurse under PCP responsibility |
Disease diagnosis and management |
| Cleveringa et al. 201072 | Journal article | Private sector-commercial | •Same as Cleveringa et al. 2008 but a cost effectiveness study. | Disease diagnosis and management |
| Cortez 201466 | PhD Thesis | Not stated | •A standalone computer-based CDSS: clinical decision support in the form of evidence-based drop-down boxes in the nursing electronic documentation system •Control (no drop-down boxes) |
[chronic] Disease management |
| Dalaba 201586 | Journal article | Public sector | •A standalone computer [laptop]-based CDSS: intervention software that prompts health workers to provide pre-natal and delivery care and then gives recommendations or alerts the health worker when there are danger signs •Usual care: pre-natal and delivery care without the use of CDSS |
Disease management/prevention reminders |
| Dowding et al. 201290 | Journal article | Public sector | •A standalone computer-based CDSS [intervention period]: an organization-wide electronic health record (EHR), Kaiser Permanent Health-Connect •Usual care (CDSS non-assisted)-pre-intervention period |
Disease prevention |
| Duclos et al. 201584 | Journal article | Public sector | •A standalone computer-based CDSS: computerized system aimed to (1) detect automatically the malnourished patient by calculating Weight/Height and Height/Age ratio from the weight, height and age of children at admission and (2) automatically alert doctors and dietitians when the child was below the normal ratio. The alert was presented to doctors as a ‘red flag’ on the display alongside electronically prescribed drugs; for dietitians a monitoring dashboard was updated daily so they could intervene without waiting for a doctor. •Usual care (CDSS non-assisted) |
Disease diagnosis reminder |
| Dumont et al. 201278 | Journal article | Public sector | •A standalone computer-based CDSS: a computerised insulin-dosing calculator •paper protocol (modified Portland protocol) |
Drug dosing |
| Dykes et al. 201091 | Journal article | Public sector/not for profit | •A standalone computer-based CDSS: ◦Phase 1: qualitative inquiry to identify barriers and facilitators to fall risk communication and interventions. ◦Phase 2: developing fall prevention tool kit prototype by using the Morse Falls Scale (MFS) risk factors ◦Phase 3: identify valid icons for the fall prevention tool kit using an iterative process involving domain experts, end users, and an illustrator. ◦Phase 4: implement the CDSS/conducting the trial |
Disease prevention |
| •Usual care | ||||
| Dykes et al. 202092 | Journal article | Public sector/not for profit | •A standalone computer-based CDSS: A Five-Phase Fall Tailoring Interventions for Patient Safety (TIPS) tool kit ◦Phase 1: Problem analysis: learn about the needs and preferences of patients and providers and other social-technical factors that relate to fall prevention ◦Phase 2–3: Design and development: implement content, display, and workflow integration strategies most likely to address requirements and overcome barriers ◦Phase 4: Implementation: conduct a pilot test of fall TIPS and compare for effectiveness in engaging patients and families in the 3-step fall prevention process ◦Phase 5: Evaluation: evaluate the toolkit’s efficacy on patient activation, falls, and injurious falls |
Disease prevention |
| •Usual care | ||||
| Fitzmaurice et al. 200067 | Journal article | Public sector/MRC | •A standalone computer-based CDSS: anticoagulation management system used by Nurses •Usual care: anticoagulation management by physicians without the use of CDSS |
Disease management |
| Forberg et al. 201685 | Journal article | Public sector | •A standalone computer-based CDSS: a reminders system for peripheral venous catheter (PVC) intravenous treatment/management •Usual care (CDSS non-assisted) |
Disease management reminders |
| Fossum et al. 201193 | Journal article | Public sector | •A standalone computer-based CDSS: a system composed of CDSS integrated into the EHR based on two research-based risk assessment instruments: the Risk Assessment Pressure Scale (RAPS) for pressure ulcer risk screening and the Mini Nutritional Assessment (MNA®) tool for screening nutritional status. •Usual care (CDSS non-assisted) |
Disease prevention |
| Geurts et al. 201773 | Journal article | Public sector/ non-for-profit | •A standalone computer-based CDSS: clinical dehydration scale and guidelines on treatment of acute gastroenteritis were incorporated in an electronic, easily accessible clinical decision support system, available at each desktop at the emergency department •Usual care |
Disease diagnosis and management |
| Hovorka et al. 200779 | Journal article | Public sector/EC | •A standalone computer-based CDSS: enhanced model predictive control (eMPC) that adapts itself to the input-output relationship observed during tight glucose control. •Usual care (CDSS non-assisted) |
Drug dosing |
| Kroth et al. 200668 | Journal article | Not stated | •A standalone computer-based CDSS: consists of a DatascopeAccutor Plus patient monitor (Datascope Corp., Paramus, NJ) and a bed side PC (keyboard & LCD screen). The DataScope delivers its results (Blood pressure, pulse, body temperature and Oximetry) to the PC via an RS-232 serial interface that uses a vendor developed, data-exchange protocol. •Usual care (CDSS non-assisted) |
Disease management |
| Lattimer et al. 199864 | Journal article | Public sector/EC | •A standalone computer-based CDSS: a computer based telephone advice system (TAS) used by nurses in primary care call management •Usual care (Doctors with no CDSS) |
Triaging |
| Lattimer et al. 200065 | Journal article | Public sector/MRC | •A standalone computer-based CDSS: a computer based telephone advice system (TAS) used by nurses in primary care call management •Usual care (doctors with no CDSS) |
Triaging |
| Lee et al. 200974 | Journal article | Public sector | •A hand-held- or mobile-based CDSS: a personal digital assistant-based clinical decision support system for obesity used for the screening and management of obesity, smoking, and depression and consists of 3 parts: screening, automated generation of diagnosis, and care planning •Usual care (CDSS non-assisted): personal digital assistant-based clinical log without decision support features for obesity |
Disease diagnosis and management |
| Lv et al. 201994 | Journal article | Public sector/ non-for-profit | •A standalone computer-based CDSS: the software included the following basic modules: medication reminder, adherence management, alert of acute asthma exacerbations, assessment of exacerbation severity, treatment recommendation, keeping a health diary, instant communication with healthcare providers and health education | Disease prevention |
| •Usual care | ||||
| Mann et al. 201180 | Journal article | Public sector | •A standalone computer-based CDSS: an EndoTool® insulin dosing CDSS package for acute glycaemic control •Usual care (paper-based protocol) |
Drug dosing |
| McDonald et al. 201788 | Journal article | Public sector | •A standalone computer-based CDSS: with three components: (i) an algorithm to identify patients with complex medication regimens at increased risk of medication problems or adverse outcomes; (ii) a clinical alert comprising an email to the nurse’s mobile device flagging specific patients with complex medication regimens and signposting to a ‘medication regimen complexity care management module’; (iii) a complex medication management module as part of the Patient Care Record System (PCRS) that suggested nursing goals and interventions for patients with multiple co-morbidities and complex medications. •Usual care: nurses with pen-based tablet computers running the PCRS electronic health record who could access referral, medication, care plan, assessment information. Using clinical judgment alone, they decided issues to communicate to doctors, PCRS problems to ‘pull down’ and their priority. Before and/or seeing a patient nurses reviewed their Plan of Care, reviews and updates current medications and documents progress on the PCRS sub modules/problems. |
Disease diagnosis and management; and, diagnosis alerts |
| Paulsen et al. 202095 | Journal article | •A standalone computer-based CDSS: MyFood digital decision support system is developed for useamong hospitalized patients who are malnourished or at risk of malnutrition | Disease prevention | |
| •Usual care | ||||
| Plank et al. 200681 | Journal article | Public sector | •A standalone computer-based CDSS: a model predictive control system used for glycaemic control. It enables the prediction of the glucose excursion by a dose optimizer. The dose optimizer proposes future insulin infusion rates and tunes the rates until the predicted glucose excursion fits into a desired glucose excursion •Usual care (Routine Treatment Protocol (RTP)): blood glucose values were provided to the ICU staff as required by the routine glucose management protocol implemented in the respective ICU |
Drug dosing |
| Rood et al. 200569 | Journal article | Not stated | •A standalone computer-based CDSS: guideline-based advice provided via aclinical information system (CIS) decision support software module (Event Manager) and a custom-made Visual Basic application integrated within the CIS. The application displayed glucose and insulin data and suggested current treatment and the interval to the next glucose measurement. •Usual care(paper-based guideline) |
Disease management |
| Roukema et al. 200887 | Journal article | Public sector | •A standalone computer-based CDSS: nurses used a computer system that automatically identified children with high risk score and recommends laboratory test •Usual care: Physiciansassess patients without the help of CDSS |
Diagnosis/management reminders |
| Sassen et al. 201470 | Journal article | Not stated | •Web-based CDSS: clinical decision support system used to optimize shared decision-making and the self-management of patients •Control (no CDSS use) |
Disease management |
| Snooks et al. 201462 | Journal article | Public sector | •A hand-held tablet [computer]-basedCDSS: paramedics use CDSS to decide whether to take patients who had fallen to an Emergency Department or leave them at home with referral to a community-based falls service •Usual care: paper-based protocols comprised assessment, treatment on scene as required and default conveyance to the Emergency Department unless the patient refused to travel to hospital. |
Triaging |
| Vadher et al. 199782 | Journal article | Public sector | •A standalone computer-based CDSS: nurses used oral anticoagulant control model to monitor doses •Usual care (trainee doctor without CDSS) |
Drug dosing |
| Wells 201363 | PhD Thesis | Not stated | •A hand-held tablet [computer]-based CDSS: paramedics use CDSS to decide whether to take patients who had fallen to an emergency department or leave them at home with referral to a community-based falls service •Usual care: paper-based protocols comprised assessment, treatment on scene as required and default conveyance to the Emergency Department unless the patient refused to travel to hospital. |
Triaging |
| CDSS, computerised decision support system; EC, European Commission; ICU, intensive care unit; MRC, Medical research Council. | ||||
Appendix 3 Study information on protocols and CDSS implementation theory/models
| Author and year | Implementation model/theory for CDSS | Protocol publisheda | Science of implementation published elsewherea |
|---|---|---|---|
| Beeckman et al. 201389 | Implementation model by Grol and Wensing (2005): a stepped approach to implementation, justifying the implementation need to reduce target group possible resistance. Starting with in-depth analysis of current practice, target group, and context a series of steps employed (1) matching research findings and/ or existing guidelines to the relevant practice, (2) describing desired change outcomes, (3) selecting/ developing implementation strategies, (4) developing and executing implementation processes, and (5) evaluation and adaptation of processes. | None | None |
| Bennet et al. 201661 | Although author discusses ‘theory of decision-making’ in nursing (e.g: four stages of medical decision-making by Elstein et al. (1978), page 44 of the thesis), it was not explicitly described that if implementation was based on any model or theory. | None | None |
| Blaha et al. 200975 | None described | Yes (Trial ID: NCT00764712); protocol outcomes and manuscript outcomes match. However, the protocol does not provide any additional information. | None |
| Byrne 200583 | Author discusses Rycroft-Malone et al.’s conceptual framework for adoption (page 43 of Thesis) but not explicitly mentioned that it was used as a model of implementation. | None | None |
| Canbolat et al. 201976 | None described | None | None |
| Cavalcanti et al. 200977 | None described | None | None |
| Cleveringa et al. 200871 | None described | Yes (Trial ID: ISRCTN21523044); protocol outcomes and manuscript outcomes match. However, the protocol does not provide any additional information. | None |
| Cleveringa et al. 201072 | None described | Yes (Trial ID: ISRCTN21523044); Cost effectiveness outcomes in manuscript are not listed in protocol. The protocol does not provide any additional information. | |
| Cortez 201466 | None described | None | None |
| Dalaba 201586 | None described | None | None |
| Dowding et al. 201290 | None described | None | None |
| Duclos et al. 201584 | None described | None | None |
| Dumont et al. 201278 | None described | None | None |
| Dykes et al. 201091 | None described | Yes (Trial ID: NCT00675935); manuscript outcomes match the listed outcomes in the protocol. However, the protocol does not provide any additional information. | None |
| Dykes et al. 202092 | None described | Yes (Trial ID: NCT02969343); manuscript outcomes match the listed outcomes in the protocol. However, the protocol does not provide any additional information. | None |
| Fitzmaurice et al. 200067 | None described | None | None |
| Forberg et al. 201685 | None described | Yes (Trial ID: ISRCTN44819426); manuscript outcomes match the listed secondary outcomes in the protocol. However, the protocol does not provide any additional information. | None |
| Fossum et al. 201193 | None described | None | None |
| Geurts et al. 201773 | None described | Yes (Trial ID: NTR2304); manuscript outcomes match the listed outcomes in the protocol. However, the protocol does not provide any additional information. | None |
| Hovorka et al. 2007 79 | None described | None | None |
| Kroth et al. 200668 | None described | None | None |
| Lattimer et al. 199864 | None described | None | None |
| Lattimer et al. 200065 | None described | None | None |
| Lee et al. 200974 | None described | None | None |
| Lv et al. 201994 | None described | Authors cite protocol number (ChiCTR1800016726) but not accessible. | None |
| Mann et al. 201180 | None described | None | None |
| McDonald et al. 201788 | None described | None | The authors published about ‘Automating the medication regimen complexity index’ (https://doi.org/10.1136/amiajnl-2012-001272) a year before but hardly give any theoretical background or models for adoption other than just ‘testing’ if the technology works. |
| Paulsen et al. 202095 | None described | Yes (Trial ID: NCT03412695); manuscript outcomes match the listed outcomes in the protocol. However, the protocol does not provide any additional information. | The authors published about ‘Automating the medication regimen complexity index’ (https://mhealth.jmir.org/2018/9/e175/) but hardly give any theoretical background or models for adoption other than just ‘testing’ if the technology works. |
| Plank et al. 200681 | None described | None | None |
| Rood et al. 200569 | None described | None | None |
| Roukema et al. 200887 | None described | None | None |
| Sassen et al. 201470 | None described | Yes (Trial ID: NTR2584); manuscript outcomes match the listed outcomes in the protocol. However, the protocol does not provide any additional information. | None |
| Snooks et al. 201462 | None described | Yes (Trial ID: ISRCTN10538608); manuscript outcomes match the listed outcomes in the protocol. However, the protocol does not provide any additional information. | None |
| Vadher et al. 199782 | None described | None | None |
| Wells 201363 | None described | Yes (Trial ID: ISRCTN10538608); manuscript outcomes match the listed outcomes in the protocol. However, the protocol does not provide any additional information. | None |
List of abbreviations
- AHP
- allied health professional/profession as defined and outlined at www.england.nhs.uk/ahp/role/
- CBA
- controlled before-and-after studies
- CDSS
- computerised/computerized decision support system
- CNIO/CAHPIO
- Chief Nursing Information Officer; Chief Allied Health Profession Information Officer
- CPOE
- computerised physician order entry
- EHR
- electronic health record
- ER/D
- emergency room/department
- HRQOL
- health-related quality of life
- INR
- international normalised ratio (a measure of time for blood to clot)
- ITSs
- interrupted time series study
- NASSS
- non-adoption, abandonment, scale-up, spread, sustainability (framework)
- NMAHP
- nurses, midwives and allied health professions
- NPT
- normalisation/normalization process theory
- NRCTs
- non-randomised controlled trials
- PPI
- Patient Public Involvement
- RCTs
- randomised controlled trials
