Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 10/135/02. The contractual start date was in December 2015. The draft manuscript began editorial review in June 2022 and was accepted for publication in February 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Sackley et al. This work was produced by Sackley et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Sackley et al.
Chapter 1 Background
Introduction
Parkinson’s disease
Epidemiology
Parkinson’s disease (PD) is a complex neurodegenerative disorder affecting around 6.1 million people worldwide in 2016. 1 PD is more common in men (1.4 : 1.0) and mostly affects people aged over 50, with peak incidence between 85 and 89 years old. The reported prevalence of PD has increased substantially over the 20 years up to 2015, making it the fastest-growing neurological disorder worldwide. 2
Risk factors/causes
Most cases of PD are sporadic and likely to be caused by a mixture of genetic and environmental risk factors. Rarely, single gene mutations can cause PD predominantly in younger onset patients. 3
Over 60 environmental risk factors have been studied for their proposed association with PD including smoking, biomarkers, physical activity, drugs, exposure to environmental toxins and head-injury. Some risk factors such as smoking and physical activity have shown significant protective effects in larger studies. 4
Symptoms/disease progression and prognosis
Symptoms of PD are classified as motor or non-motor. The initial diagnostic motor symptoms are unilateral tremor, slowness, stiffness and mild imbalance. However, as the condition progresses, more severe motor decline occurs with imbalance leading to falls and unpredictable freezing episodes, all of which are unresponsive to medication.
There is a high prevalence of non-motor symptoms in PD, the most severe of which concern mental health. 5,6 Dementia occurs in around 40%, leading to a fluctuating state of confusion with visual hallucinations. There is a similar frequency of depression and anxiety. Sleep disturbances which include rapid eye movement sleep disorder (RBD), where people act out their dreams, are also common. Autonomic problems include orthostatic hypotension (low blood pressure on standing) which can lead to falls, and constipation requiring regular laxatives.
Parkinson’s disease is a progressive condition with severe motor decline and dementia leading to death over 15–20 years. Symptoms of PD and their progression can have an impact on the person with PD and their family and friends. However, there may be different types of PD with varying prognoses:7 A predominantly tremulous form of the condition may have a more benign outcome, compared with those with little tremor and early balance problems who develop dementia early and have a shorter life expectancy.
Treatments
At present, there are no disease-modifying therapies for PD;8 all therapies are symptomatic. First-line symptomatic treatment for the motor symptoms is pharmacological therapy with levodopa, the precursor of the neurotransmitter dopamine. 9 With disease progression, the dose of levodopa tends to be capped to avoid severe involuntary movements (dyskinesia), so in younger non-dementing patients adjuvant drugs are added to levodopa. These include dopamine agonists, monoamine oxidase B (MAOB) inhibitors and catechol-O-methyltransferase (COMT) inhibitors. The N-methyl-d-aspartate (NMDA) receptor antagonist amantadine is used to treat more severe dyskinesias if they occur.
A minority of younger mentally healthy PD patients may need more interventional therapies for severe disease once maximal oral therapies have been tried. 10 Continuous infusion of the dopamine agonist apomorphine can help smooth out gaps in ‘on’ time due to wearing off of oral medication. Surgical implantation of electrodes into the subthalamic nucleus can also provide a smoother response, but at the expense of potential postoperative complications of depression, speech disturbance, stroke and death.
Non-motor symptoms in PD are treated symptomatically as suggested in the National Institute for Health and Care Excellence (NICE) guidelines. 10
Speech and voice problems experienced by people with Parkinson's disease and their families
Impact of speech problems
Speech impairments are common among people with PD, with a reported prevalence of 68% for patient-perceived problems and 71% for listener-rated speech impairment. 11 In a study of 125 people with PD,12 38% placed speech among their top four concerns and, in another study, 29% of participants reported speech problems to be among their greatest present difficulties. 13 Changes in communication led to increased physical and mental demands during conversation, an increased reliance on family members and/or carers and an increased likelihood of social withdrawal. 14
Speech or voice problems experienced by people with PD are typically related to motor disorders of the muscles required to produce speech, known as dysarthria. Dysarthric speech is often perceived as imprecise, creating a social barrier to communication. 15 Difficulty in successfully conveying emotions is also experienced by people with PD and their listeners. 15,16 A qualitative study involving 24 people living with PD identified problems with speaking as an activity. The interviewees reported having to think more about speaking; weighing up the value versus the effort of speaking; having negative feelings about speaking; finding that speaking is influenced by different people and places; and having to adjust to the effect of speaking with the disease progression and their medication. 17 Overall, impairments of speech have been recognised to reduce the quality of life of people with PD. 15,18,19
Speech and language interventions
Speech and language therapy (SLT) in the UK aims to deliver interventions that improve communication for people with PD-related dysarthria and their families. Some interventions prescribe exercises to improve motor skills, others support the communication interaction and partnership between the person with PD and their communication partner, while others aim to augment, or provide alternative means of communication. SLT varies not only by intervention approach, content, materials and procedures but also by the component(s) of speech and voice targeted by the intervention(s) in addition to the therapy regimen [intensity, total hours of therapy (dosage), frequency and overall duration of the intervention] and tailoring to the individual (by functional needs and by level of difficulty).
The Lee Silverman Voice Treatment (LSVT) is an approach to SLT, which consists of protocol that can be personalised. 20 The commercial developers have licenced the intervention as LSVT LOUD® for use by certified clinicians or certified and supervised assistants and students who undertake initial training and updates every 2 years. The most detailed description of the intervention is in the LSVT LOUD training manual. In contrasting LSVT LOUD with other interventions, the following components are highlighted as distinct to LSVT LOUD:
-
standardised intensity (of dosage or regimen);
-
number of task repetitions and perceived effort);
-
a simple focus on LOUD;
-
three daily tasks (maximum sustained movements; directional movements;
-
functional movements);
-
a hierarchy of tasks to use from day one to move learning from daily tasks into context specific and variable speaking activities;
-
use of modelling with minimal cognitive load to shape healthy vocal loudness;
-
and sensory calibration through focusing attention on what ‘LOUD’ feels and sounds like (and training this through carryover activities and homework practice).
Surveys can help build a picture of reported practice. A 2016 survey of SLT practice for PD in Australia21 found that education and support components were commonly used and for direct intervention, motor speech was the main therapy target. The components of SLT offered included two LSVT packages (LSVT LOUD and LSVT LOUD-X) as well as developing individualised self-monitoring and prompting cues; aided augmentative and alternative communication (AAC); traditional dysarthria exercises; a pacing or alphabet board to slow speech rate; and expiratory muscle strength training which are common in NHS SLT. Loud therapy, increasing client’s insight to changes and speech tasks with increased cognitive load were also offered components common in both LSVT and NHS SLT.
Based on 2011 data, SLT provision for PD in the UK22 frequently targeted breathing control, voice quality and intelligibility. Methods of addressing these targets overlap considerably and include LSVT, breathing exercises, pacing, relaxation, articulation, and loudness and voice exercises. Use of AAC, language and psychosocial components were reported less frequently. Due to service constraints, not all therapists report delivering LSVT to the specified intensity or in the form prescribed by its developers.
Parkinson’s disease was the most common patient group reported in the 2012 UK survey of SLT treatment practices among people with progressive dysarthria. 23 The three most commonly reported SLT components for PD were general rate, volume and prosody work, functional communication and speech subsystems work.
For people with dysarthria as a consequence of PD in Australia in 201621 most sessions were individual and, for both individuals and groups, once-weekly therapy was usual. Similarly, therapy duration was most frequently reported to last 4 weeks or 6 weeks. Session length for individuals was varied from 30 to 60 minutes. Of the LSVT LOUD certified therapists, 90% reported delivery issues due to service factors including allocated time, caseload sizes and the number of therapists working part-time. 21
In 2011 in the UK, more than 50% of therapists saw PD patients as outpatients, with a third of patients needing hospital transport to participate. 22 A median of six sessions was offered over a median period of 42 days with each lasting a median of 45 minutes. Therapists reported expecting to spend 60% of their time face to face with the patient. An advice and review pattern of service delivery was dominant, and a lack of understanding of the evidence24 for motor learning principles such as regular intensive practice was also reported.
In the USA25 the most commonly recommended dosage for home education programmes by occupational therapists in the community for those with neurological injuries was 16–30 minutes a day. The content was focused on preparatory activities rather than being used directly to achieve a client’s goal. Most home practice was communicated through handouts and demonstration, with video rarely used.
Capability of and access to technology is growing rapidly, offering new opportunities for self-management and service delivery. The LSVT training manual26 describes using devices to measure time, volume, pitch and voice quality. These can be low tech (e.g. a stopwatch/Sound Level Meter/pitch pipe/tape recorder) or high tech (e.g. smartphone apps, Companion Software).
Clinical guideline recommendations
Many countries have national guidelines for PD and speech problems. 27,28
The NICE clinical guidelines10 for PD state that clinicians should consider referring people who are in the early stages of PD for assessment, education and advice from a speech and language therapist with experience of treating people with PD.
The guidelines state that people with PD who are experiencing problems with communication should be offered SLT, which should include strategies to improve speech and communication, such as attention-to-effort therapies.
Evidence base for speech and language therapy for speech problems in Parkinson‘s disease
Two previous Cochrane reviews have been published in this field (Herd et al. 29, 30). Herd et al. 29 evaluated the effectiveness of SLT interventions with placebo or no intervention. They concluded that there while there was some clinical improvement in vocal loudness (for reading and speaking), but overall, there was insufficient evidence based on three small trials (63 participants). In a linked synthesis, Herd et al. 30 compared the benefits of different types of SLT interventions. They identified six trials (159 participants) that used a variety of different restorative and compensatory approaches, but again concluded that while there were improvements in speech, there was insufficient evidence to support the selection of one SLT intervention over another.
An updated Cochrane systematic review (date of last searches 3 October 2022) builds on the two previously published Cochrane reviews. 29,30 Quasi-RCT studies were excluded and a further 20 randomised controlled trials (RCTs) were identified and published since the previous reviews were conducted. For the RCTs excluding this (the PD COMM) trial, despite no high-to-certainty evidence supporting the use of SLT interventions being identified, the review provided further confidence in the evidence of benefit for SLT interventions for the communication outcome, and vocal loudness while reading or speaking a monologue. This was a consistent finding across all three comparisons: no intervention, placebo control and when compared with another SLT intervention (where data were available). All three Cochrane reviews were in agreement regarding the need for standardisation of outcome measures (P Campbell, Glasgow Caledonian University, 2022, personal communication). Other relevant systematic reviews and evidence syntheses were identified. 31–35 Two recently published meta-analyses evaluated the effectiveness of LSVT with no intervention or another speech intervention in people with PD. 32,35 Yuan 2020 identified nine studies for inclusion (date of last search March 2020); Pu 2021 identified ten (quasi-RCTs and RCTs) studies for inclusion (date of last search December 2021). Both reviews reported that LSVT was effective in increasing vocal loudness and functional communication. Reviews have also demonstrated that there is a lack of evidence on the cost-effectiveness of different SLT techniques compared with no SLT.
Rationale and the need for the PD COMM trial
Rationale for trial
From 2009, several National Institute for Health and Care Research Health Technology Assessment (NIHR HTA) commissioned calls were issued to assess methods of SLT for improving speech intelligibility and effectiveness of communication in people with Parkinson’s disease.
The call from which the PD COMM trial was successfully funded, called for a pragmatic RCT in people with PD to assess the clinical and cost-effectiveness of NHS SLT compared with no treatment. The specifications included a follow-up of at least 1 year and the trial should follow a pilot phase. This recommendation was based on several factors:
-
dysarthria is common in PD and increases in severity and prevalence as the disease progresses;
-
quality of life is negatively affected by speech problems;
-
speech and language therapy being offered by the NHS was variable;
-
the lack of definitive studies investigating SLT for PD-related dysarthria.
Trial design
Driven by the HTA commissioned calls, the feasibility and acceptability of a large-scale SLT trial in people with PD were assessed and found to be acceptable in the PD COMM pilot trial36 funded by The Dunhill Medical Trust (grant R192/0511). The data from this pilot trial informed the design of the definitive RCT (PD COMM) to assess the clinical and cost-effectiveness of these SLT interventions. The pilot trial assessed eligibility, recruitment and retention, participant acceptability and treatment compliance. It also provided data to help inform the sample size calculations and to refine the choice of outcome measures including those used for the economic evaluation.
The 2017 NICE guideline10 for PD states that SLT should be offered to people with PD who have communication problems. The optimum treatment regimen including delivery method and theoretical basis were not and continue to remain unclear. 35 To progress the understanding of how SLT interventions are delivered and the mechanism of action, the PD COMM trial included a process evaluation.
Rationale for choice of interventions
Before the trial, guidance on SLT best practice for people with PD-related dysarthria as a result of PD could be found in two historical resources from the Royal College of Speech and language therapists. 37,38 Two Cochrane systematic reviews29,30 of the evidence published in 2012 were also available but could not provide clear, evidence-based recommendations for practice. These sources of evidence were not robust enough to be clear about the optimum treatment approach for PD-related dysarthria. The choice of treatments for the PD COMM trial was taken based on testing two commonly chosen treatment options in the UK in direct response to the HTA commissioned call.
Stakeholder involvement
This trial was supported by the Parkinson’s UK, The Dunhill Medical Trust and the Local Clinical Research Network, Division 4: Neurology.
Chapter 2 Methods
Material in this chapter has been adapted from the PD COMM trial protocol by Sackley et al. www.ncbi.nlm.nih.gov/pmc/articles/PMC7251680/). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
The trial was reported using the following reporting guidelines: CONSORT and extension for non-pharma trials, extension for three-arm trials and abstracts, GRIPP2, CHEERS and Template for Intervention Description and Replication (TIDieR) guidelines.
Objectives
The primary objective of the PD COMM trial was to evaluate the clinical and cost-effectiveness of two approaches to SLT (LSVT LOUD and NHS SLT) compared with no SLT treatment in people with PD.
An additional objective was to evaluate and compare the clinical and cost-effectiveness of two types of SLT (LSVT LOUD vs. NHS SLT) in people with PD.
The primary comparisons were:
-
Lee Silverman Voice Treatment LOUD versus no SLT (control)
-
National Health Service SLT versus no SLT (control).
An additional comparison was:
-
Lee Silverman Voice Treatment LOUD versus NHS SLT.
Research questions
-
Do people with PD-related speech or voice problems who are treated with NHS SLT report less voice handicap than those who have no SLT treatment at 3 months after randomisation?
-
Do people with PD-related speech or voice problems who are treated with LSVT LOUD treatment report less voice handicap than those who have no SLT treatment at 3 months after randomisation?
-
Do people with PD-related speech problems who are treated with LSVT LOUD report less voice handicap than those who are treated with NHS SLT treatment at 3 months after randomisation?
Trial design
The PD COMM trial was a pragmatic UK, multicentre, three-arm parallel group, unblinded, superiority, RCT with a 12-month follow-up. Participants were randomised at the level of the individual to receive either: LSVT LOUD, NHS SLT or no SLT (control) in a 1 : 1 : 1 ratio. There were two nested studies within this trial: a process evaluation and an economic evaluation. Participants randomised to the no SLT (control) group could be referred for SLT at the end of trial or, if it became medically necessary, during the trial. The type and dose of SLT for those in the control group for whom treatment became necessary were determined by the local therapists responsible for the care plan of the participant. Non-compliance with trial treatment did not constitute the participant’s withdrawal from the trial.
The trial received ethical approval on 7 December 2015 by the West Midlands NHS Research Ethics Committee (REC) (15/WM/0443). Version 4.0 (14 November 2018) of the protocol is currently in effect. Sponsored by the University of Birmingham (Research Governance Team, University of Birmingham, Birmingham), participating centres each obtained local research and development approval.
Participants
People were eligible to be included in the trial if the following criteria were met:
-
diagnosis of idiopathic PD as defined by the 1988 UK PDS Brain Bank Criteria39
-
the person with PD or their carer report problems with the person with PD’s speech or voice.
People were excluded from the trial if they met any of the following criteria:
-
dementia, usually defined clinically by the person with PD’s clinician
-
evidence of laryngeal pathology including vocal nodules or a history of vocal strain or previous laryngeal surgery as LSVT may not be appropriate in such contexts40
-
received SLT for PD speech or voice-related problems in the previous 2 years as there is some evidence that benefits of LSVT may persist for 24 months. 41
Setting
Recruitment took place at 41 sites throughout the UK, except Northern Ireland. Distribution of sites across the UK was not uniform as attempts to recruit trial sites in the whole of Northern Ireland were not successful and were only minimally successful in the southeast of England. The main reason for not joining given by potential sites contacted was a lack of capacity in their SLT service.
Participants were recruited from their routine outpatient appointments. These appointments took place in geriatric/elderly care, neurology or SLT secondary care settings.
The interventions were provided through secondary care outpatient community-based SLT departments. For some participants who had specific needs, or as a service delivery choice, the intervention was provided at home.
Interventions
Participants were encouraged to be fully compliant with their randomised treatment allocation. We anticipated that it was possible that some may have received SLT as arranged by other health or social care providers not associated with the trial. As any access to SLT for their dysarthria could dilute the intervention effect, at each assessment participants in the no SLT (control) group were asked whether they had received any SLT.
Participants randomised to either of the SLT treatment arms completed brief home-based therapy diaries to determine the level of home-based practice prescribed and undertaken by participants outside of the therapy sessions.
In order to monitor intervention delivery, therapists providing the SLT interventions completed a SLT Initial Interview Log including the abbreviated mental test42 and after each SLT session delivered, the therapists completed a treatment record form (TRF). These forms were used to monitor participant adherence (including missed or cancelled appointments), and therapist adherence to these programme protocols. In addition, for the NHS SLT intervention, the forms were used to further explore what SLT delivered within the NHS entailed.
We maintained a supportive environment to encourage therapists to make and maintain a distinction between the two SLT intervention approaches throughout the trial, including continuing professional development opportunities such as therapists’ days and an online network, and a proactive approach to encouraging queries.
Lee Silverman Voice Treatment (LSVT LOUD)
The focus of LSVT LOUD is to ‘think loud’, improving phonation and vocal loudness through better vocal fold adduction. 40 The intervention aimed to replicate the dose and content recommended by the originators and delivered in clinical practice and previous ‘standard’ LSVT trials. The LSVT LOUD intervention consisted of four face-to-face or remote 50-minute sessions per week delivered over 4 weeks. 40 Remote sessions were provided through the LSVT Companion software and could support remote intervention delivery for up to 8 of 16 sessions. Each session follows a similar structure which can be personalised for each patient: 25 minutes of repeated and intensive maximum effort drills, and 25 minutes of high-effort speech production tasks. 40 Participants were also prescribed 5 to 10 minutes of home-based practice tasks on treatment days, and up to 30 minutes of home-based practice tasks on non-treatment days. 43 The content of the intervention consisted of repeated repetitions of sustained ‘ah’ phonation, maximum fundamental frequency range high and low pitch glides, and functional sentence repetition for the first half of each session, and exercises using speech production hierarchy that progresses throughout the duration of the treatment programme (single word, phrases, sentences, paragraph reading, conversation) during the second half of the sessions. 43 Throughout all of the sessions, the focus of the intervention was to ‘think loud’, maintaining the vocal loudness produced during vowel phonation throughout all other tasks during the treatment. 40 Delivery of LSVT LOUD in the trial was as typical within an NHS setting.
National Health Service speech and language therapy
The content, intensity and dose of NHS SLT for people with PD-related voice or speech problems are poorly defined within the published literature. For this reason, the NHS SLT group took a pragmatic approach to the intervention and encompassed all local standard NHS SLT practices and techniques with the exception of LSVT (as per the LSVT LOUD Protocol). Therapists were free to tailor therapy to individual participant’s needs. NHS SLT could potentially include interventions targeted at rehabilitation of the underlying movement disorder functions that resulted in dysarthria, behavioural or compensatory strategies and AAC strategies to improve communicative function and participation. 44 The therapist involved participant’s family members or carer(s) as appropriate.
Treatments targeted at impairments of the underlying speech production movements may include exercises focused on improving capacity, control and co-ordination of respiration, techniques for improving phonation intensity and co-ordination with respiration (but not LSVT), and exercises to improve the range, strength and speed of the articulatory muscles. 45,46 Behavioural therapy includes interventions that target the reduction of prosodic abnormality47,48 such as exercises targeting pitch, intonation, stress patterns and volume variation,45–49 and techniques to address the overall rate of speech45,46 including the use of therapeutic devices such as pacing boards. 50,51 AAC strategies such as topic and alphabet supplementation through communication books and boards may be employed,44 along with AAC devices such as voice amplifiers, delayed auditory feedback systems and masking devices. 52–54
Pitch limiting voice treatment55 may also be utilised within the NHS SLT intervention. The above NHS SLT approaches may include techniques that are also used in LSVT LOUD (e.g. vocal intensity exercises) but will be distinct from that intervention as it was anticipated, based on the available literature, that they would be delivered in combination with other SLT strategies and at a different intensity and dose. Though dose and frequency were to be determined by the local therapist in response to participants’ individual needs, it was anticipated that it would reflect the median dose as reported in a survey of current UK SLT practice for PD by Miller et al. ,22 of six sessions delivered over 42 days. The PD COMM Pilot trial36 also found the median NHS SLT intervention dose to be six sessions (range 1–14) over an average of 9.6 weeks (standard deviation 6.1 weeks).
Control no speech and language therapy group
Participants were randomly allocated to the no SLT (control) arm for PD-related speech or voice problems for 12 months participation in the PD COMM trial. Participants may still receive SLT for swallowing problems (dysphagia). Since there is insufficient evidence to prove or disprove the benefit of SLT in PD, equipoise still exists. Therefore, it is ethical to randomise between SLT and no SLT. Investigators, however, remained vigilant throughout the 12-month trial follow-up period for participants randomised to the no SLT (control) group, who deteriorated to the point of needing therapy urgently for their speech or voice problems received SLT without delay via the usual local NHS services. At the end of the trial after their 12 months of patient and clinical assessments, participants in the control arm could be referred for SLT for their dysarthria by their usual care specialist through local NHS referral pathways.
Adherence to the interventions
As this was a pragmatic trial of two existing interventions in the NHS, our approach to fidelity was multidimensional. 56 The PD COMM pilot established that dosage was a key differentiating factor. For the practical purposes of statistical analysis, LSVT LOUD treatment adherence was defined as participants randomised to the LSVT LOUD arm receiving at least 14 out of the 16 prescribed LSVT LOUD sessions and having completed these sessions within 3 months of randomisation. A session was considered an LSVT LOUD session if at least 30 minutes of time was attributed to LSVT LOUD on the SLT treatment log (supervised or unsupervised). As a secondary assessment of LSVT adherence, we only included those who received all 16 sessions of LSVT within 3 months of randomisation. The first measure of adherence used a pragmatic approach, for example, a participant may have missed one session due to taking a holiday, whereas the second measure of adherence is strictly looking at those participants who received the intervention as prescribed.
As NHS SLT is not a prescriptive intervention, participants were considered adherent if they completed their SLT sessions within 3 months of randomisation that is they had their last session within 3 months of randomisation. These sessions should also not have any time attributed to LSVT LOUD. The NHS SLT was deemed to have been completed for a participant when a returned SLT log has been answered as ‘Yes’ to this being the last treatment session of the therapy course.
Adherence to the no SLT (control) arm was monitored through the resource usage form where participants could record whether they had received any SLT. If a participant in the no SLT (control) arm reported receiving SLT over the course of the 12-month follow-up, then they were considered non-adherent. The only exception to this was if SLT had been prescribed for the treatment of dysphagia only.
Training requirements
Prior to commencing recruitment, all site staff involved with the trial had to complete good clinical practice training. Key members of the site research team were required to attend either a meeting or a teleconference covering aspects of the trial design, protocol procedures, adverse event reporting, collection and reporting of data and record keeping. All therapists who took part were registered with UK regulatory body, the Health and Care Professions Council (HCPC), which sets standards for education, training and practice.
Only speech and language therapists or therapist assistants trained in LSVT LOUD, which includes a detailed manual and 2-year updates, could deliver the LSVT LOUD intervention. Therapists were provided with LSVT LOUD training through the trial by LSVT Global20 for free if they had not done this training or needed it to reregister as an LSVT therapist.
Ideally every therapist delivering LSVT therapy should have conducted at least three treatment sessions before delivering the intervention to trial participants; however, this was at the discretion of the trial sites.
Three workshops were held early into the trial, which brought together site speech and language therapists, research nurses and the research team to explore the PD COMM interventions. The workshop participants explored what was considered ‘core’ and ‘peripheral’ for each intervention and the barriers and challenges of delivering the interventions. The workshops helped to ensure that the trial ran smoothly.
Outcomes
In the pilot trial, extensive vocal assessments were carried out alongside the participant-reported outcomes. We decided not to undertake vocal assessments within the PD COMM trial as:
-
the additional time involved was prohibitive;
-
there was concern that since one of the trial interventions (LSVT LOUD) specifically focussed on vocal loudness that the results might be skewed in favour of this intervention;
-
the focus of the trial was on the participants’ self-perception of functional communication rather than vocal loudness.
Primary outcome
The primary outcome measure was the patient-reported Voice Handicap Index (VHI)57 total score at 3 months. The VHI comprises of 30 questions divided into emotional, functional and physical subscales. 57 It aims to assess the psychosocial consequences of voice disorders, and can be used to gain an overall perception of effectiveness of voice-related communication. The VHI total score ranges from 0 to 120 (with 0 being the best score and 120 the worst score) and the subscales range from 0 to 40.
Many previous trials have used vocal loudness as their primary outcome. It has been used as an outcome measure in an extended LSVT trial for PD16 and was also collected in the PD COMM Pilot trial. 36 The VHI was chosen as it was a patient-reported outcome that took little time to complete, was well-completed in our pilot trial36 and better reflected the focus of the PD COMM trial objectives.
Secondary outcomes
Patient-reported measures were used to assess the participant’s perception of how their voice impacted on daily activities and their quality of life, and to complement the primary outcome. The secondary outcome measures were:
Quality of life was measured using the PDQ-39. 59 This is a validated, health-related quality-of-life measure specific to PD,59 and was the most widely used disease-specific quality-of-life rating scale for PD completed by the participant. It comprises of 39 questions divided into the following dimensions: mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication and bodily discomfort. The PDQ-39 summary index and each of the individual dimensions provide a score that can be converted into a 0–100 metric where 0 = no problem at all and 100 = worst or maximum level of problem.
-
Questionnaire on Acquired Speech Disorders (QASD)60
Participation restriction related to speech and communication were assessed using the self-reported QASD. 60 The QASD questionnaire comprises of 30 questions which are scored 0–3 giving a total score that ranges from 0 to 90, where lower scores were better.
-
EuroQol5D (5-level version). 61
The EQ-5D-5L61,62 is a well-established standardised instrument for use as a measure of health outcome. It provides a simple descriptive profile and a single index value for health status. It is completed by the participant, and comprises of the following five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each dimension can take one of five responses: no problems, slight problems, moderate problems, severe problems or extreme problems. There is also a 100-point visual analogue scale. It is often used together with resource utilisation questionnaires (see below) to provide data to inform the cost-effectiveness analysis.
-
ICEpop Capabilities Measure for Older Adults (ICECAP-O).
The ICECAP-O63 is a measure of capability in older people for use in economic evaluations. Unlike most profile measures used in economic evaluations, the ICECAP-O focuses on well-being defined in a broader sense, rather than health. The measure covers attributes of well-being that were found to be important to older people in the UK and is completed by the participant. It comprises of five attributes: attachment (love and friendship); security (thinking about the future without concern); role (doing things that make you feel valued); enjoyment (enjoyment and pleasure) and control (independence).
-
Adverse events (AEs) (see Chapter 2, Safety reporting).
-
Hoehn and Yahr stage.
The Hoehn and Yahr stage64 is a clinician-rated measure of disease severity in PD. It is a standard staging scale for PD that is required to document the severity of PD in the participant population.
-
Carer quality of life (Parkinson’s Disease Questionnaire – Carers).
Carer quality of life will be measured using the Parkinson’s Disease Questionnaire – Carer. 65 This is the first disease-specific measure of quality of life for carers of people with PD and is a validated and reliable tool. It is completed by the carer and comprises of 29 questions with 5 responses (Never/Occasionally/Sometimes/Often/Always). It is made up of four discrete scales: social and personal activities (12 items), anxiety and depression (6 items), self-care (5 items) and stress (6 items). The raw score of each scale can be calculated and converted to a 0–100 metric where 0 = no problem at all and 100 = worst or maximum level of problem. The sum of the scale scores can provide a single figure used to assess the overall quality of life of the individual questioned.
-
Resource utilisation (collected for the Health Economic Evaluation).
Developed for use in the PD COMM Pilot, a disease-specific Resource Usage questionnaire was used to collect information on participant resource usage data. The questionnaire included items on primary care and secondary care healthcare utilisation, including the use of therapy services, and outpatient appointments. Further questions related to use of social services, including provision of meals and formal care. Finally, information was collected on time off work, participants’ out-of-pocket costs (e.g. travel, medication) and costs incurred by informal carers, to inform analysis from a societal perspective.
Outcome assessment time points
Assessments were made following informed consent, and prior to randomisation (baseline assessment), and then at 3 months (i.e. after treatment if in the SLT arms), 6 and 12 months after randomisation ([ee the trial record forms at (URL: www.birmingham.ac.uk/research/bctu/trials/pd/pd-comm/investigators/documentation)]. Assessments completed by the participants at 3, 6 and 12 months were returned to the Birmingham Clinical Trials Unit (BCTU) by post. The participants had clinical assessments at baseline and then again at 12 months after randomisation (Table 1).
| Measure | Completed by | Assessment time | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 12 months | |||
| Randomisation form | Clinician | ✓ | ||||
| Clinical data entry: Entry form or 12-month CRF | Education and living arrangements | Clinician | ✓ | |||
| Height | Clinician | ✓ | ||||
| Weight | Clinician | ✓ | ✓ | |||
| PD Medication | Clinician | ✓ | ✓ | |||
| Hoehn and Yahr stage | Clinician | ✓ | ✓ | |||
| VHI | Participant | ✓ | ✓ | ✓ | ✓ | |
| PDQ-39 | Participant | ✓ | ✓ | ✓ | ✓ | |
| QASD | Participant | ✓ | ✓ | ✓ | ✓ | |
| EQ-5D-5L | Participant | ✓ | ✓ | ✓ | ✓ | |
| ICECAP-O | Participant | ✓ | ✓ | ✓ | ✓ | |
| Resource usage questionnaire | Participant | ✓ | ✓ | ✓ | ||
| Transition item | Participant and carer | ✓ | ✓ | ✓ | ||
| PDQ-Carer | Carer | ✓ | ✓ | ✓ | ✓ | |
| Adverse event log | Clinician | ✓a | ||||
| Initial Interview Log (first session only) and treatment record form (all seasons). | Speech and language therapist | ✓b | ||||
| Home-based therapy diary | Participant | ✓c | ||||
Sample size
The primary outcome was the mean difference in the VHI total score at 3 months across the three comparisons: LSVT LOUD versus no SLT (control); NHS SLT versus no SLT (control); and LSVT LOUD versus standard NHS SLT. Data from the PD COMM Pilot trial were used to inform the sample size calculations for this trial as the minimal clinically important differences (MCID) for the VHI had not been established in PD patients. In the PD COMM Pilot trial,36 a difference of around 10 points in VHI total score was observed at 3 months between SLT and no SLT (control) for both types of SLT (NHS SLT and LSVT LOUD). To detect a 10-point difference in VHI total score between arms at 3 months (using a two-sided t-test and the upper standard deviation of 26.27 obtained from the VHI baseline data from the pilot trial; effect size 0.38), with 80% power and α = 0.01, we needed to recruit 163 participants included per arm. Allowing for 10% dropout this increased to 182 participants per arm, meaning the trial had a planned sample size of 546 participants.
Global rating scale (transition item)
The transition item is a single question asked at the 3-month time point to the participant and carer: ‘Compared to 3 months ago (when you joined the trial), has your ability to communicate using speech changed?’ with seven levels of response ranging from ‘much worse’ to ‘much better’ or ‘Compared to 3 months ago (when your partner/family member/friend joined the trial), has his/her ability to communicate using speech changed’ is used for the participant and carer respectively. It measures whether the participant or carer has noticed any change in communication by voice or speech since the participant (with PD) entered the trial. The transition item was used to calculate the MCID for the VHI.
Randomisation
Sequence generation and allocation concealment
Following informed consent and completion of all baseline data collection, the participant could be randomised into the trial. Participants were randomised at the level of the individual via a central, secure, web-based computer-generated randomisation system developed and controlled by the BCTU, thus ensuring concealment of next treatment allocation. To randomise a patient into the trial, staff delegated the task of randomising patients into the trial either logged onto the trial database or rang the BCTU randomisation telephone line. The randomisation process used a minimisation procedure. The following minimisation variables were used:
-
age (≤ 59, 60–70, > 70 years);
-
disease severity measured using the Hoehn and Yahr staging64 (1.0–2.5, 3.0–5.0); and
-
severity of speech measured using the VHI57 total score (≤ 33, mild 34–44, moderate 45–61, severe > 61).
To avoid any possibility of the treatment allocation becoming predictable, a random element was included within the randomisation process. Once the participant was randomised into the trial, they were given a unique trial identifier and the treatment allocation was confirmed by e-mail to the site.
Recruitment and selection
The trial was designed to reflect routine care, thus minimising the burden for people with PD and difficulties with movement and fatigue. Participants were usually identified during routine clinic appointments with their specialist clinician or PD specialist nurse. The healthcare professional then informed potentially eligible patients of the trial and provided a copy of the participant information sheet (PIS). Patients were given time to review the PIS and/or go through it with a member of the team, typically the research nurse, and given the opportunity to ask questions. Given the low-risk nature of the trial, and the mobility limitations of the population, potential participants could join the trial on the same day that they had discussed the trial and received the PIS or they could come back at a later date if they preferred.
Prior to randomisation, the availability of speech and language therapists at the site was checked. Availability of SLT interventions locally was investigated before randomisation in order to reduce delays between randomisation and the start of treatment. Once randomised into the trial, the aim was to start providing the NHS SLT intervention within 4 weeks and the LSVT LOUD intervention within 7 weeks of randomisation, thereby allowing for potential Ear, Nose and Throat (ENT) referrals prior to starting the intervention, and enabling the intervention to be completed prior to the primary analysis time point (at 3 months post randomisation). Randomisation could be deferred if the SLT intervention could not be initiated within the set time frames (e.g. if the therapist was on leave, or there was no service capacity to engage in LSVT LOUD at that time). However, the participant’s baseline questionnaire had to be completed within 2 weeks prior to randomisation, so this was factored into any planned delay of a patient’s randomisation. Provision of treatment at a location different to the randomisation location was permitted within the trial, depending on local practice. Participant’s General Practitioner was informed of their patient’s randomisation into the trial.
Typically, the research nurse obtained informed consent and enrolled the participant into the trial including randomising the participant via the BCTU web-based system or by telephoning BCTU telephone randomisation service. They also liaised with the local speech and language therapists to ensure that SLT, should they be randomised to therapy, started within the required time frame (Figure 1).
FIGURE 1.
Intended trial participant flow.

Blinding
Given the nature of the intervention, the trial was not blinded; participants, trial assessors or treatment providers were all aware of the intervention group to which the participant had been randomised.
Statistical methods
The primary comparisons within the PD COMM trial compared (1) those randomised to receive LSVT LOUD compared to no SLT (control) and (2) those randomised to receive NHS SLT compared to those randomised to no SLT (control). A third comparison compared those randomised to LSVT LOUD to those randomised to NHS SLT. All primary analyses were based on the intention-to-treat (ITT) principle, with participants analysed in the treatment groups to which they were randomised.
For all tests, summary statistics (e.g. mean differences) are reported along with 99% confidence intervals (CI) and p-values from two-sided tests. A p-value of < 0.01 was considered statistically significant, as per the sample size calculations to take into account the multiple treatment comparisons being undertaken. For the primary comparisons, the no SLT (control) group was the reference group. In the secondary comparison, the NHS SLT group was the reference group. All analyses were undertaken using SAS®, version 9.4 [SAS Institute Inc., Cary, NC, USA (SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc. in the USA and other countries. ® indicates USA registration)], or Stata®, version 17 (StataCorp LP, College Station, TX, USA).
The primary outcome measure was the VHI total score at 3 months. A linear regression model was used to estimate differences in the VHI total score at 3 months between the two arms of interest, with the VHI baseline score and the minimisation variables: age and severity of PD (Hoehn and Yahr) included in the model as covariates. Statistical significance of the treatment estimate was evaluated using the corresponding p-value in the model. Three per-protocol analyses were performed on the primary outcome only. These were:
-
analysing those who were adherent (see Adherence to interventions for adherence definition) to randomised intervention and those who completed the 3-month assessment form inside the assessment window (± 1 month)
-
analysing only those who were adherent
-
analysing only those who completed the 3-month assessment form within the assessment window.
Sensitivity analyses to investigate the impact of missing data were also restricted to the primary outcome and included assuming worst score and best score in place of missing responses, with different combinations by intervention group; assuming mean score of domain in place of missing response; and multiple imputation using important prognostic variables (e.g. treatment allocation; baseline value) to predict the missing response.
The majority of the secondary outcome measures (e.g. PDQ-39) are continuous measures and were analysed in the same way as for the primary outcome: a linear regression analysis adjusting for relevant baseline score and all of the minimisation variables (baseline VHI, age and severity of PD). Mean differences were reported alongside 99% CIs. The primary analysis of the secondary outcomes was at 3 months as per the primary outcome.
Participant-completed questionnaires were also completed at 6 and 12 months post randomisation. Secondary analyses assessed these data at both 6 and 12 months using linear regression analysis adjusting for relevant baseline score and minimisation variables as per the primary analysis. Continuous outcomes were also analysed using repeated measures models using all available data. Baseline value of the measure and time were included in the model along with the minimisation variables as fixed effects. Time was assumed to be a categorical (fixed) variable. To allow for a varying treatment effect over time, a time-by-treatment interaction parameter was included in the model. If the p-value of this interaction was significant, then mean differences between the groups were reported from this model, else the interaction term was removed before reporting mean differences. A general ‘unstructured’ covariance structure was assumed.
Adverse events and safety data were summarised descriptively by treatment arm and the number of events and percentage of participants experiencing any AE reported.
Subgroup analyses were performed for the primary outcome only to assess whether there were differences in the treatment effect by the minimisation variables: VHI total score (≤ 33; mild 34–44; moderate 45–61; severe > 61); age (≤ 59; 60–70; > 70 years); and PD severity as measured by Hoehn and Yahr staging (1–2.5; 3–5). The trial was not powered to detect for differences in treatment effect in these subgroups and, therefore, these analyses were to be treated as purely hypothesis generating.
Oversight and monitoring
Interim data analyses of the primary outcome and AE were supplied in confidence to the Data Monitoring Committee (DMC), which gave advice on whether the accumulated data from the trial, together with the results from other relevant research, justified the continuing recruitment of further participants. The DMC could recommend discontinuation of the trial if the recruitment rate or data quality were unacceptable, or if any issues were identified which may compromise participant safety. The trial would have stopped early if interim analyses or new evidence emerged showing differences between treatments that were deemed to be convincing to the clinical community.
The DMC was able to advise the chair of the trial steering committee (TSC) if, in their view, any of the randomised comparisons in the trial had provided both (1) ‘proof beyond reasonable doubt’66 that for all, or for some, types of patient one particular treatment is definitely indicated or definitely contraindicated in terms of a net difference in the major end points, and (2) evidence that might reasonably be expected to influence the patient management of many clinicians who are already aware of the other main trial results. The TSC would then have had to decide whether to close or modify any part of the trial. Unless this happened, however, the Trial Management Group (TMG), TSC, the investigators and all the central administrative staff (except the statisticians who supply the confidential analyses) remained unaware of the interim results.
Safety reporting
A risk assessment of the PD COMM trial was performed with the SLT interventions considered to be of low risk. From the literature, the only reported AE associated with the interventions was a small increased risk of vocal strain or abuse; however, none were reported in the PD COMM pilot trial. This risk was minimised as speech and language therapists are trained to identify and rehabilitate vocal strain. No other risks were expected to arise from taking part in the trial and therefore it was reasonable to collect only targeted AEs.
For participants in either treatment arm, any vocal strain or abuse believed to be associated with treatment was identified by the therapists at the participants’ SLT session and reported in the AE log. In all trial arms, the participant-reported resource usage form was checked to ensure that no vocal strain or abuse occurred following participants reporting outpatient appointments with ENT specialists. At the 12-month clinical visit, the medical professional also checked whether any AEs occurred since entering the trial.
Serious adverse events (SAEs) are events that cause death, are life-threatening, require or extend an existing hospitalisation, result in persistent or significant disability or incapacity; birth defect or congenital abnormality; or are otherwise considered medically significant by the investigator. SAEs that are not related to vocal strain or abuse were excluded from expedited notification during the course of the trial and were collected in the resource usage and 12-month clinical case report form (CRF).
Treatment-related AEs associated with vocal strain or abuse will be documented and reported from the date of commencement of protocol-defined SLT treatment until 30 days after the administration of the last treatment. AEs associated with vocal strain or abuse that are not considered treatment related [i.e. AEs experienced on the no SLT (control) arm] were reported from randomisation until 12 months post randomisation via the resource usage questionnaire.
Summary of any changes to the trial protocol
The following amendments and/or administrative changes were made to this study since the implementation of the first approved protocol. 67
Original application was submitted with version 1.0 of the protocol. Substantial changes to the protocol and participant CRFs were made to satisfy requirements from the REC.
Resubmitted to REC as version 1.1 and the protocol was approved subject to minor changes. This version was released to trial sites at the start of recruitment of the trial.
Version 2 of the protocol added an exclusion criterion that the investigator had to be certain that the participant would not require SLT in the 12-month trial period. A note was also added which stated that should it become medically necessary, participants in the ‘no treatment’ control group could be given SLT treatment. The severity measure H&Y was removed from 3- and 6-month data collection and height and weight were removed from the 6-month data collection. The CRFs were removed from the clinician-reported outcome measures.
Version 3 was not approved and so was not an active version of the trial protocol.
Version 4 added that participants could be contacted by letter or phone by a member of the clinical team to inform them of the trial prior to them attending clinic or afterwards should this be more practical or appropriate. The need for participants to explicitly consent to members of the research team and or members and representatives of the sponsors to be given direct access to their medical records was added. The protocol was also amended to confirm that a participant’s GP was only informed of the participant’s involvement in the trial, if they had given their consent for this to happen.
The ability to defer randomisation due to SLT service availability was added. The baseline questionnaire had to be completed within 2 weeks prior to randomisation and consent was reconfirmed if the delay was longer than 3 weeks.
For the qualitative interviews, the timeframe of interview was changed to between 3 and 6 months assessments and the length of the interviews was amended to 30 minutes from 30 minutes to 1 hour. Providing a participant information sheet for the process evaluation was amended from optional to mandatory.
Chapter 3 Results
Recruitment
The PD COMM trial opened to recruitment on 26 September 2016 and was suspended on 16 March 2020, before the recruitment target of 546 participants was achieved, due to the impact of the coronavirus disease discovered in 2019 (COVID-19) pandemic (see Appendix 1, Figure 31). Following discussions among the coinvestigator group and then with the TSC and the funder, it was decided that the COVID-19 pandemic meant that recruitment to the trial and delivering the trial interventions in the same way as pre-pandemic would have been difficult. The decision was made to not reopen the trial and recruitment closed in November 2020 without reaching this target. As of 16 March 2020, 388 participants were recruited to the trial; 130 participants were all allocated to LSVT LOUD, 129 participants were allocated to NHS SLT and 129 participants were allocated to no SLT (control).
There were 42 centres across England, Scotland and Wales opened to recruitment, of which 41 recruited at least one participant. The centres were a diverse range of secondary care facilities and SLT services based in hospitals in large urban centres to small centres serving rural communities. The numbers of participants recruited at each centre were skewed (see Appendix 1, Figure 32) with a few centres recruiting a large number of participants and the majority of centres recruiting low numbers. The highest recruiting centres were in large urban centres in Scotland. The median number of participants recruited at each centre was 8 (range 2–32).
Participant flow
The participants were randomised to treatment and progressed through the trial (Figure 2) to complete a 12-month follow-up appointment; the two active SLT intervention arms had to complete the therapy within the 3 months from randomisation. Of the 388 participants randomised, a total of 31 did not start an active trial intervention (see Appendix 1, Table 20) for a range of different reasons. None of the reasons were recorded for more than two participants. Of those that started active treatment or no SLT (control), 7 participants (2%) died during the trial, 30 participants (8%) withdrew from the trial, 2 participants were lost to follow-up and 10 participants (3%) partially withdrew from the trial: 7 (2%) of these provided clinical data only and 3 (1%) provided participant completed data only.
FIGURE 2.
Flow of participants.

Baseline data
The mean age of participants in PD COMM was approximately 70 years old, and 74% were male (286/388) (Table 2). The mean duration of Parkinson’s was between 5 and 6 years, with a broad range from newly diagnosed to over 30 years, and the majority (61%) were in Hoehn and Yahr stage of 2 or less (< 5% were in Hoehn and Yahr stage 4 or more). The participants mostly lived with a significant other (83%) or lived alone (14%). The levodopa equivalency (see Table 6) of the medication the participants were taking at baseline was similar in the two SLT intervention groups (551.4 and 557.2 mg/day) but was slightly higher (597.6 mg/day) in the no SLT (control) group.
| LSVT | NHS SLT | Control | ||
|---|---|---|---|---|
| Number of patients randomised | 130 | 129 | 129 | |
| Number of randomisation notepads available | 130 | 129 | 129 | |
| Number of entry forms available | 130 | 129 | 127 | |
| Demographics | ||||
| Age (years) | N | 130/130 | 129/129 | 129/129 |
| Mean (SD) | 69.9 (8.4) | 69.7 (9.4) | 70.2 (8.1) | |
| Range | 40.6–93.1 | 37.8–90.8 | 47.6–86.6 | |
| Age group (years) | N | 130/130 | 129/129 | 129/129 |
| < 60 (%) | 16 (12) | 17 (13) | 14 (11) | |
| 60–69 (%) | 42 (32) | 42 (33) | 43 (33) | |
| 70–79 (%) | 59 (46) | 52 (40) | 61 (47) | |
| ≥ 80 (%) | 13 (10) | 18 (14) | 11 (9) | |
| Gender | N | 130/130 | 129/129 | 129/129 |
| Male (N, %) | 91 (70) | 100 (78) | 95 (74) | |
| Weight (kg) | N | 129/130 | 128/129 | 126/127 |
| Mean (SD) | 75.4 (15.5) | 77.5 (14.3) | 76.6 (14.1) | |
| Range | 39.9–118.8 | 25.3–120.7 | 41.8–114.3 | |
| Height (m) | N | 128/130 | 129/129 | 123/127 |
| Mean (SD) | 1.71 (0.10) | 1.72 (0.09) | 1.70 (0.11) | |
| Range | 1.45–1.93 | 1.47–1.90 | 1.31–1.96 | |
| Body mass index (kg/m2) | N | 128/130 | 128/129 | 123/127 |
| Mean (SD) | 25.6 (4.4) | 26.2 (4.5) | 26.6 (4.7) | |
| Range | 15.6–41.0 | 11.5–38.4 | 18.4–44.0 | |
| Stage of Parkinson’s disease | ||||
| Duration of PD (years) | N | 130/130 | 129/129 | 127/127 |
| Mean (SD) | 5.8 (5.8) | 5.1 (4.6) | 6.1 (6.1) | |
| Median (IQR) | 3.9 (1.5–8.9) | 3.7 (1.4–7.5) | 4.1 (1.7–8.7) | |
| Range | 0.008–32.7 | 0.1–21.3 | 0.04–29.8 | |
| Hoehn and Yahr stage | N | 130/130 | 129/129 | 129/129 |
| ≤ 2.0 (%) | 78 (60) | 83 (64) | 73 (57) | |
| 2.5 (%) | 17 (13) | 10 (8) | 22 (17) | |
| 3.0 (%) | 29 (22) | 31 (24) | 33 (25) | |
| ≥ 4.0 (%) | 6 (5) | 5 (4) | 1 (1) | |
Data completeness
Data returns and data completeness were good throughout the trial (see Appendix 1, Tables 21 and 22). The participant completed VHI, PDQ-39 and QASD had response rates of over 85% at each time point. As did the quality-of-life questionnaire completed by the carer (PDQ-Carer). The clinician-completed forms had a response rate of over 90% (99% at baseline; 94% at 12 months).
Adherence to treatment
Of the 130 participants randomised to LSVT LOUD, 109 (84%) started LSVT LOUD and of these 105 completed LSVT LOUD. Twenty-one participants did not start LSVT LOUD for a variety of reasons (see Appendix 1, Table 20). Of the 129 participants randomised to NHS SLT, 119 (92%) started NHS SLT, with 118 participants considered to have completed their NHS SLT. Ten participants did not start NHS SLT, again for a variety of different reasons. In the no SLT (control) group, 120 (93%) participants received no SLT, with 9 participants receiving SLT (1 LSVT LOUD, 8 NHS SLT) (Table 3 and Figure 2).
| LSVT LOUD | NHS SLT | Control | |
|---|---|---|---|
| Number randomised to SLT | 130 | 129 | 129 |
| Number completed intervention | 105 | 118 | – |
| Adherent (%)a | 77 (73) | 70 (59) | 120 (93) |
| Non-adherent (%) | 28 (27) | 48 (41) | 9 (7) |
| Number not completed interventionb | 25 | 11 | – |
| Crossover to control | 8 (32%) | 0 (–) | – |
| Died | 1 (4%) | 0 (–) | – |
| No confirmation of treatment completion | 2 (8%) | 0 (–) | – |
| No intervention | 10 (40%) | 8 (73%) | – |
| No treatment data available | 1 (4%) | 2 (18%) | – |
| Withdrew | 3 (12%) | 1 (9%) | – |
In the LSVT LOUD group, 28 of the 105 participants who completed the treatment were considered non-adherent (27%) as they did not complete 14 or more sessions of LSVT LOUD within 3 months of randomisation. Of the participants in the LSVT LOUD group who did not complete the intervention, most had no intervention (18/25, 72%); the rest either withdrew from treatment (3/25, 12%), did not have confirmation of treatment completion (2/25, 2%) or had no treatment data in their trial records (1/25, 4%) or died (1/25, 4%). For the NHS SLT group, 48 of 118 participants (41%) were considered non-adherent as they did not have a final session confirmed by their treating therapist within 3 months of randomisation. For those who did not finish the NHS SLT intervention, the most common reason was that they did not receive the intervention (8/11, 73%). There was no treatment information available for two participants and one withdrew from treatment (see Table 3) (see Appendix 1, Table 20).
Interventions as given
The provision of the two active speech and language therapies used for this trial was monitored and recorded to provide information about the interventions as delivered (Table 4). For greater detail on the content of the interventions as delivered, see Chapter 6.
| LSVT | NHS SLT | ||
|---|---|---|---|
| No. of participants randomised | 130 | 129 | |
| No. of participants with log data | 107 | 118 | |
| No. of participants had last session | 105 | 118 | |
| N | 107 | 118 | |
| Number of sessions | Median (IQR) | 16 (16–17) | 5 (4–7) |
| Range | 1–22 | 1–15 | |
| N | 107 | – | |
| Number of sessions of LSVT LOUD | Median (IQR) | 16 (14–16) | – |
| Range | 0–18 | – | |
| N | 107 | 118 | |
| Total time (mins) | Mean (SD) | 1216.3 (453.6) | 404.1 (233.5) |
| Range | 70–2080 | 50–1331 | |
| N | 107 | 118 | |
| SLT contenta time | Mean (SD) | 962.9 (330.4) | 298.2 (171.2) |
| Range | 45–1670 | 40–1065 | |
| SLT activities by type | N = 107 | N = 118 | |
| Assessment and review time (mins)b | Mean (SD) | 132.9 (86.1) | 84.3 (51.1) |
| Range | 0–415 | 0–295 | |
| Goal setting (mins)b | Mean (SD) | 25.8 (33.2) | 25.3 (26.0) |
| Range | 0–125 | 0–130 | |
| Information provision and advice (mins)b | Mean (SD) | 36.1 (44.3) | 38.8 (32.1) |
| Range | 0–315 | 0–175 | |
| Therapy~ (mins)b | Mean (SD) | 15.0 (45.0) | 148.9 (112.7) |
| Range | 0–300 | 0–550 | |
| LSVT (mins)b | Mean (SD) | 751.8 (287.3) | – |
| Range | 0–1105 | – | |
| Indirect contact (mins)c | Mean (SD) | 5.4 (26.3) | 1.3 (6.0) |
| Range | 0–195 | 0–40 | |
| Liaison/onward referral (mins)c | Mean (SD) | 4.6 (14.0) | 5.7 (17.9) |
| Range | 0–105 | 0–130 | |
| Other for example, writing notes, phone calls (mins)c | Mean (SD) | 243.4 (184.8) | 98.8 (76.9) |
| Range | 0–710 | 0 - 380 | |
| Time per session (minutes) | N | 107 | 118 |
| Mean (SD) | 79.7 (15.9) | 74.2 (21.1) | |
| Range | 46.3–127.5 | 40.0–165.0 | |
| SLT contentd time per session | N | 107 | 118 |
| Mean (SD) | 62.7 (9.5) | 55.0 (15.6) | |
| Range | 30.9–91.7 | 23.7–100.0 | |
| LSVT LOUD time per session (minutes) | N | 98e | – |
| Mean (SD) | 54.3 (7.1) | – | |
| Range | 34.1–69.3 | – | |
| Treatment duration (weeks)f | N | 107 | 118 |
| Mean (SD) | 6.6 (6.5) | 11.4 (11.4) | |
| Range | 0–54 | 0–69 |
LSVT LOUD
Lee Silverman Voice Treatment LOUD was delivered over a median of 16 sessions and a mean of 6.6 (SD 6.5) weeks. The dose of the treatment was 1216 (SD 454) minutes, which consisted of 963 (SD 330) minutes of SLT content [mean SLT content per session: 63 (SD 10) minutes], with 752 (SD 287) minutes dedicated to LSVT LOUD [mean LSVT LOUD content per session: 54 (SD 7) minutes]. Participants were encouraged to continue with home-based practice and completed homework diaries. Ninety-six participants completed at least 1 week of the home-practice diary. Diary completion was high with a median completion of 4 weeks.
National Health Service speech and language therapy
National Health Service SLT was delivered over a median of five sessions over a mean of 11.4 (SD 11.4) weeks. The duration of NHS SLT was shorter than LSVT LOUD, totalling a mean of 404 (SD 234) minutes, with 298 (SD 171) minutes dedicated to SLT content. Mean active therapy time for NHS SLT was 149 (SD 113) minutes. The mean individual session length was similar to that of LSVT LOUD at a mean of 55 (SD 16) minutes. Therapy assistants provided a small number of treatment sessions across interventions and a few participants received the course of therapy in a group setting.
Effectiveness of speech and language therapies for Parkinson’s disease-related speech and voice problems (dysarthria) after 3 months (primary outcome)
Primary analysis
The impact of the speech or voice problems at baseline across the three randomised groups was similar: a mean of 44.6 (SD 21.9, n = 130) in the LSVT LOUD group, 46.2 (SD 24.8, n = 129) in the NHS SLT group and 44.3 (SD 22.3, n = 129) in the no SLT (control) group. At 3 months all three group’s voice impact scores were lower compared with baseline, indicating less impact from speech or voice problems: the LSVT LOUD group had a mean score of 35.0 points (SD 20.1, n = 106), the NHS SLT group had a mean score of 44.4 points (SD 24.8, n = 102) and the no SLT (control) group had a mean score of 40.5 points (SD 21.5, n = 98) (Table 5).
| Mean (SD, n) | Mean difference (99% CI) | |||||
|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. Control | LSVT vs. NHS SLT | |
| N = 130 | N = 129 | N = 129 | ||||
| Baselinea | 44.6 (21.9, 130) | 46.2 (24.8, 129) | 44.3 (22.3, 129) | – | – | – |
| Baselineb | 45.4 (22.4, 106) | 45.3 (24.2, 102) | 42.3 (21.5, 98) | – | – | – |
| 3 monthsc | 35.0 (20.1, 106) | 44.4 (24.8, 102) | 40.5 (21.5, 98) | −8.0 (−13.3 to −2.6); p = 0.0001 | 1.7 (−3.8 to 7.1); p = 0.4 | −9.6 (−14.9, −4.4); p < 0.0001 |
The interventions were compared against each other for relative impact of speech or voice problems at 3 months by calculating the mean difference in VHI total score between groups. At 3 months, the VHI total score for the LSVT LOUD group was 8 points lower than for the no SLT (control) group [−8.0, 99% CI (−13.3 to −2.6); p = 0.0001]. For NHS SLT, at 3 months, the VHI total score was 1.7 points higher than the no SLT (control) group [1.7, 99% CI (−3.8 to 7.1); p = 0.4]. In the third comparison, the LSVT LOUD group was 9.6 points lower than the NHS SLT group [−9.6, 99% CI (−14.9 to −4.4); p < 0.0001] (see Table 5).
Per-protocol analyses
The main per-protocol analysis population included only those participants who both adhered to treatment and completed the 3-month VHI outcome assessment within the 1-month time window. This analysis gave similar results to the main ITT analysis: LSVT LOUD versus no SLT: −9.7, 99% CI (−16.0 to −3.4); NHS SLT versus no SLT: 1.1, 99% CI (−5.6 to 7.8); and LSVT LOUD versus NHS SLT: −10.8, 99% CI (−17.8 to −3.8). Similar results were also seen for the two other per-protocol analyses (see Appendix 1, Table 23).
Sensitivity analyses to assess impact of missing data
Various sensitivity analyses were undertaken to assess the impact of missing data. Different assumptions were made about the reasons for missing data to investigate the impact, if any, to our analysis of the primary outcome. All these analyses gave results that were in agreement with the primary ITT analysis (see Appendix 1, Table 24). For example, the sensitivity analysis using multiple imputation gave the following results: LSVT LOUD versus no SLT: −6.9, 99% CI (−10.9 to −2.9); NHS SLT versus no SLT: 1.8, 99% CI (−2.3 to 5.9); and LSVT LOUD versus NHS SLT: −8.7, 99% CI (−12.7 to −4.7).
Exploration of the impact of potential confounders on the impact of voice problems at 3 months
Subgroup analyses were pre-specified and performed on the minimisation variables: VHI category (≤ 33 negligible; mild 34–44; moderate 45–61; severe > 61); Age category (≤ 59; 60–70; > 70 years) and Hoehn and Yahr (1–2.5; 3–5) to assess whether one particular subgroup may benefit more (or less) from the SLT interventions. There was no evidence that the effect of the interventions differed according to age (test for interaction, p = 0.7) or Hoehn and Yahr stage (test for interaction, p = 0.7) (see Appendix 1, Table 25). There was, however, evidence that the intervention effect may differ depending on the baseline VHI score (test for interaction, p = 0.007). Across all three comparisons, the treatment effect generally increased as the baseline VHI increased; for example, for LSVT LOUD greater benefits were observed in those with more severe VHI scores at baseline. A post hoc analysis excluding participants who recorded the 3-month VHI score after the start of COVID restrictions was also performed (see Appendix 1, Table 26).
Effectiveness of speech and language therapies on the impact of voice problems over 12 months
The effectiveness of the trial interventions was monitored over 12 months. The VHI total score was assessed at 6 and 12 months separately as well as over the whole 12 months using a repeated measures analysis. These were secondary analyses for the trial. LSVT LOUD scores were lower (i.e. better) than no SLT (control) and there was no evidence of a difference between NHS SLT and no SLT (control) across these time points. LSVT LOUD also had lower VHI scores (i.e. better) than NHS SLT (see Figure 3 and Appendix 1, Table 27).
FIGURE 3.
Mean VHI total score profile plot over 12 months by intervention.

Effectiveness of speech and language therapies on the emotional, functional and physical impacts of voice problems (secondary outcome)
The VHI outcome measure comprises of three different subscales: emotional, functional and physical, which were assessed separately as secondary outcomes with the aim building a detailed picture of which particular voice-related impacts were improved.
Emotional subscale
At 3 months, the LSVT LOUD group had a lower score (i.e. better) than the no SLT (control) group [−3.0 (−5.1, −0.9); p = 0.0003], while there was no difference for standard NHS SLT compared to no SLT (control) [0.2 (−1.9, 2.4); p = 0.8]. LSVT LOUD resulted in a lower (i.e. better) emotional subscale score compared to standard NHS SLT [−3.2 (−5.3, −1.1); p < 0.0001] at 3 months. Similar results were seen for the comparisons at 6 and 12 months, and over the whole 12-month trial follow-up period (see Figure 4 and Appendix 1, Table 28).
FIGURE 4.
Mean VHI emotional subscale score profile plot with error bars over 12 months by intervention.

Functional subscale
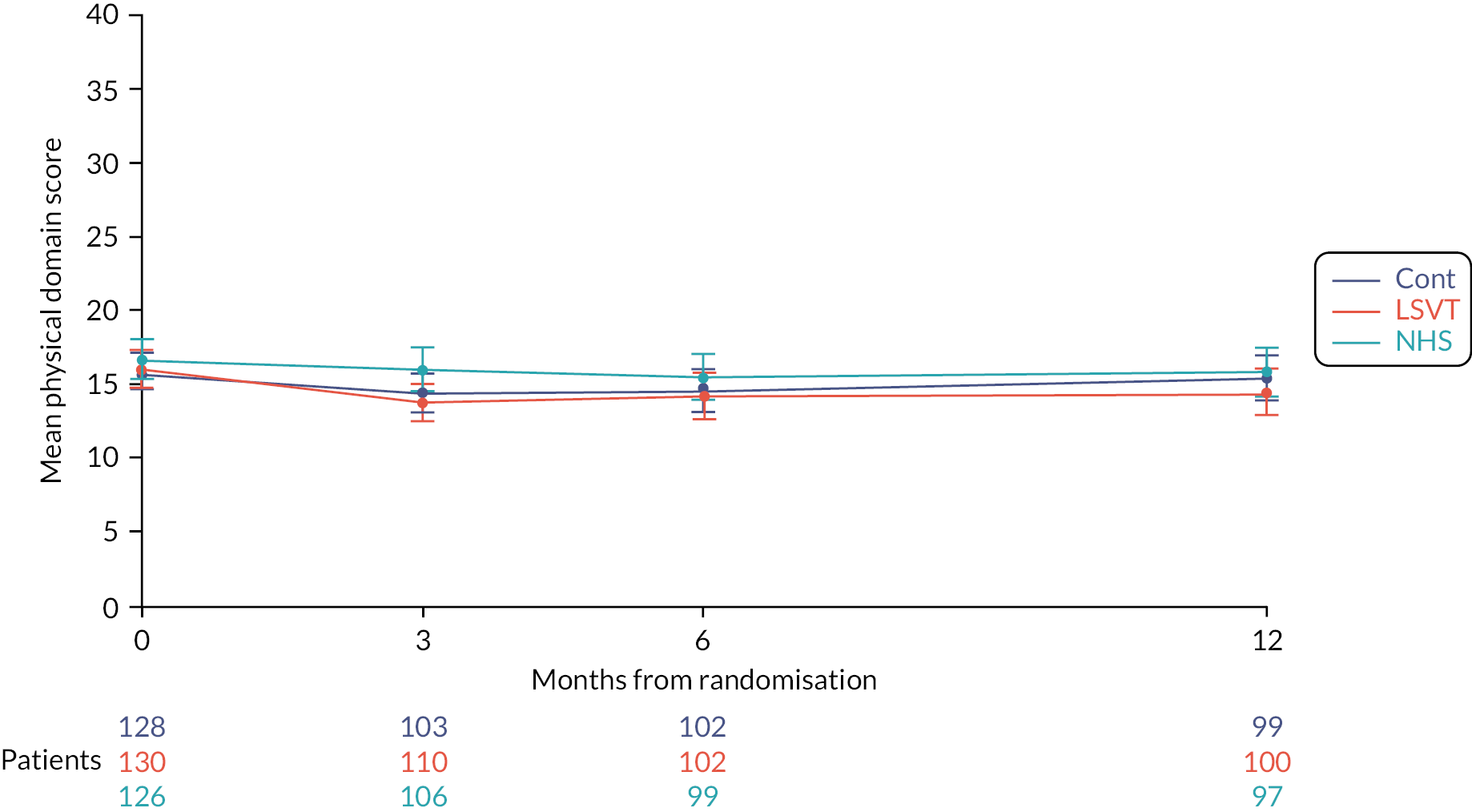
At 3 months, the LSVT LOUD group had a lower score (i.e. better) than the no SLT (control) group [−2.9 (−4.8, −1.1); p < 0.0001], while there was no difference for NHS SLT compared to no SLT (control) [−0.0 (−1.9, 1.9); p = 1.0]. LSVT LOUD resulted in a lower (i.e. better) functional subscale score compared to NHS SLT [−2.9 (−4.7, −1.1); p < 0.0001] at 3 months. Similar results were seen for the comparisons at 6 and 12 months, and over the whole 12-month trial follow-up period (see Figure 5 and Appendix 1, Table 28).
FIGURE 5.
Mean VHI functional subscale score profile plot with error bars over 12 months by intervention.
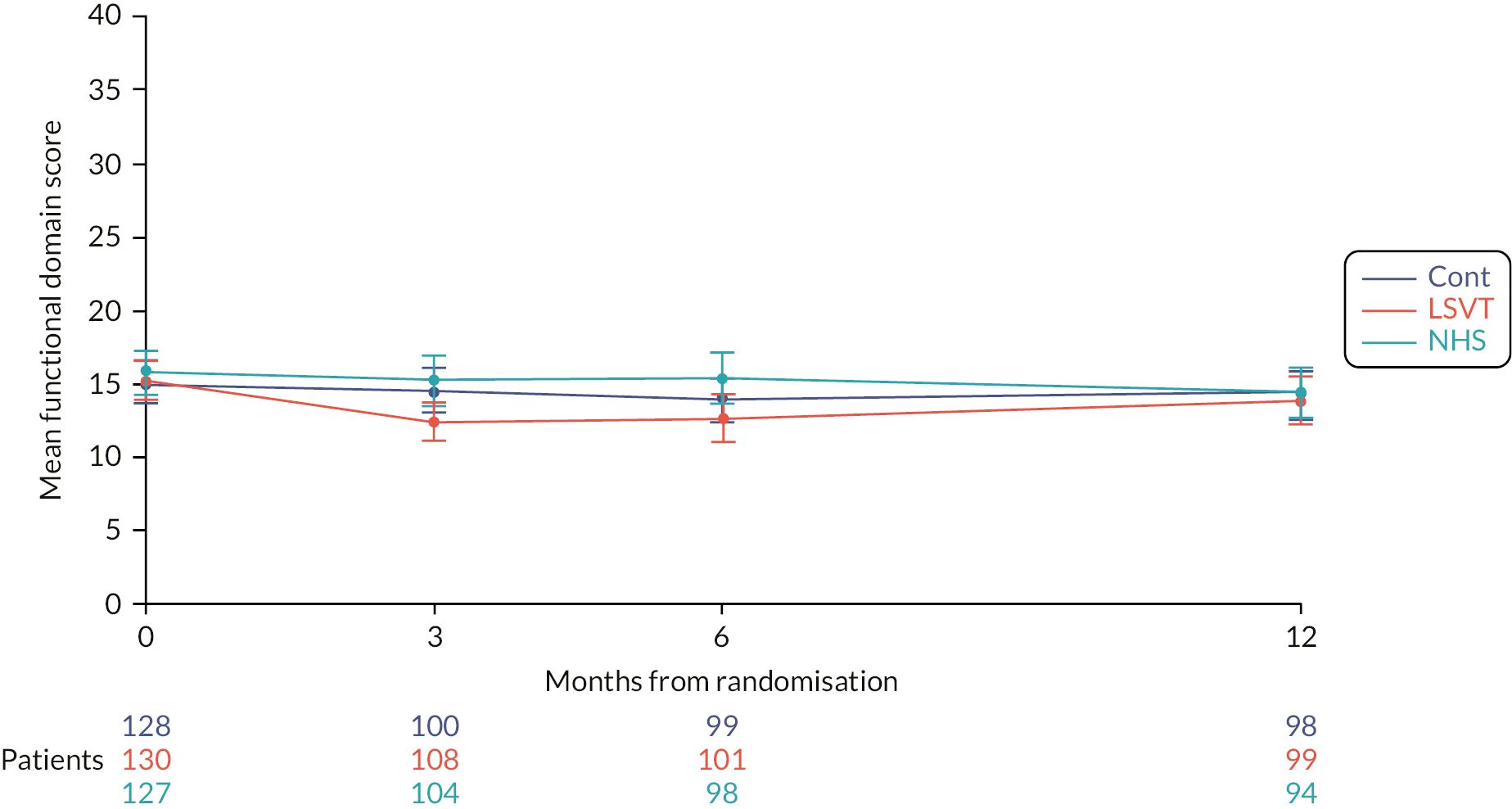
Physical subscale
Only LSVT LOUD resulted in a lower (i.e. better) physical subscale score compared to NHS SLT [−2.2 (−4.1, −0.3); p = 0.003] at 3 months. Similar results were seen for the comparisons at 6 and 12 months, and over the whole 12-month trial follow-up period (see Figure 6 and Appendix 1, Table 28).
FIGURE 6.
Mean VHI physical subscale score profile plot with error bars over 12 months by intervention.
Effect of the interventions on participant’s dysarthria-specific quality of life (secondary outcome)
To assess what impact the interventions may have on quality of life particularly associated with dysarthria (voice or speech problems), the QASD was used. At 3 months, the QASD scores were lower (i.e. better) in the LSVT LOUD group when compared to both the no SLT (control) [−5.4 (−9.8, −1.0); p = 0.002] and the NHS SLT [−4.3 (−8.7, 0.1); p = 0.01] groups. There was no evidence of a difference in the scores for NHS SLT compared to no SLT (control) at 3 months. Similar results were seen for the comparisons at 6 and 12 months, and over the whole 12-month trial follow-up period (see Figure 7 and Appendix 1, Table 29).
FIGURE 7.
Mean QASD profile plot over 12 months with error bars by intervention.

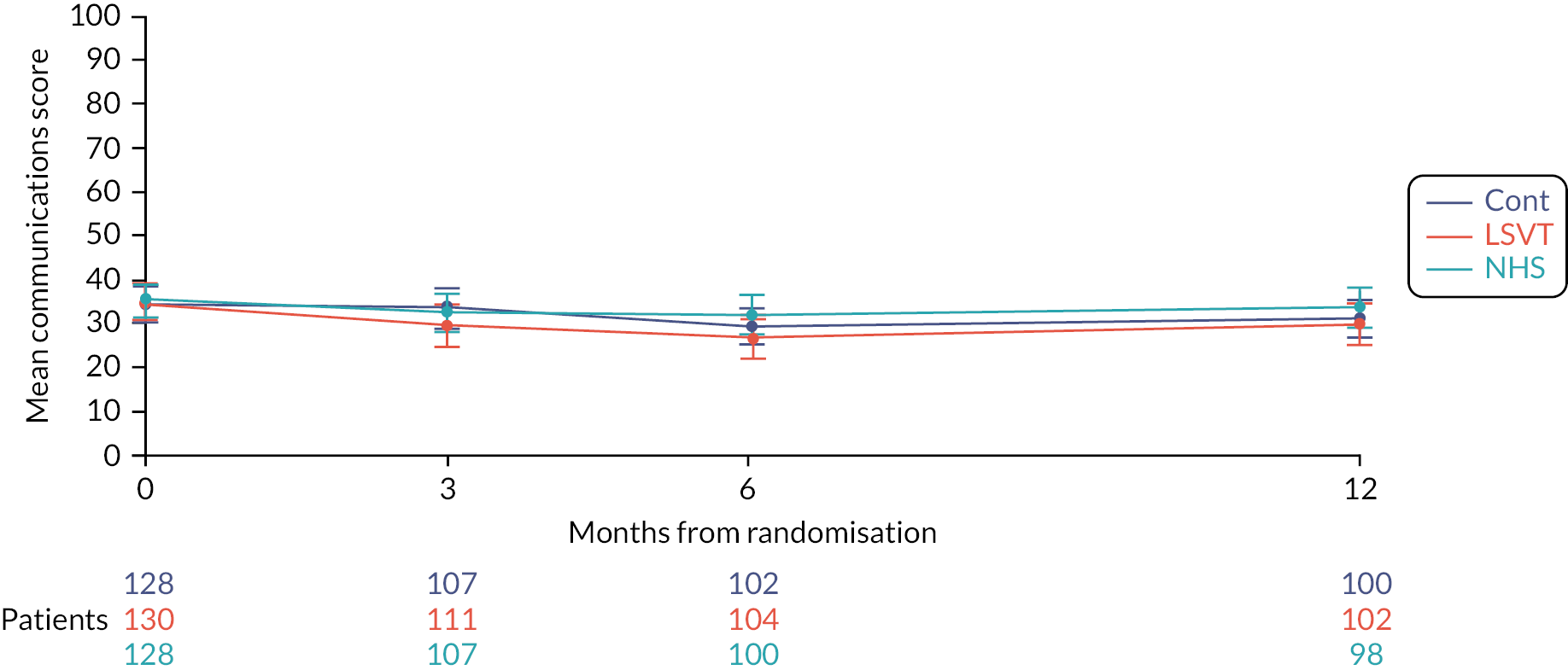
Effectiveness of speech and language therapies on the participant’s Parkinson’s disease-specific quality of life (secondary outcome)
The participant assessed the quality-of-life measure PDQ-39 assesses the impact on mobility, activities of daily living, emotional well-being, stigma, social support, cognition, communication and bodily discomfort. The plots and results of the analyses for the different domains are provided in the Appendix (see Appendix 1, Table 30, Figures 33–39). Not surprisingly, the largest differences were observed in the communication domain for the comparison of LSVT LOUD and no SLT (control). At 3 months, the mean difference between groups was −6.2 points (99% CI −11.9 to −0.6; p = 0.004) (Figure 8).
FIGURE 8.
Mean PDQ-39 communication subscale profile plot with error bars over 12 months by intervention.
Effectiveness of speech and language therapies on the participant’s general health status (secondary outcome)
The general health-related quality of life for participants was measured using the EQ-5D-5L and there was no evidence of differences between interventions (see Figures 9 and 10 and Appendix 1, Table 31).
FIGURE 9.
Mean EQ5D index score profile plot with error bars over 12 months by intervention.

FIGURE 10.
Mean EQ5D visual analogue score profile plot with error bars over 12 months by intervention.
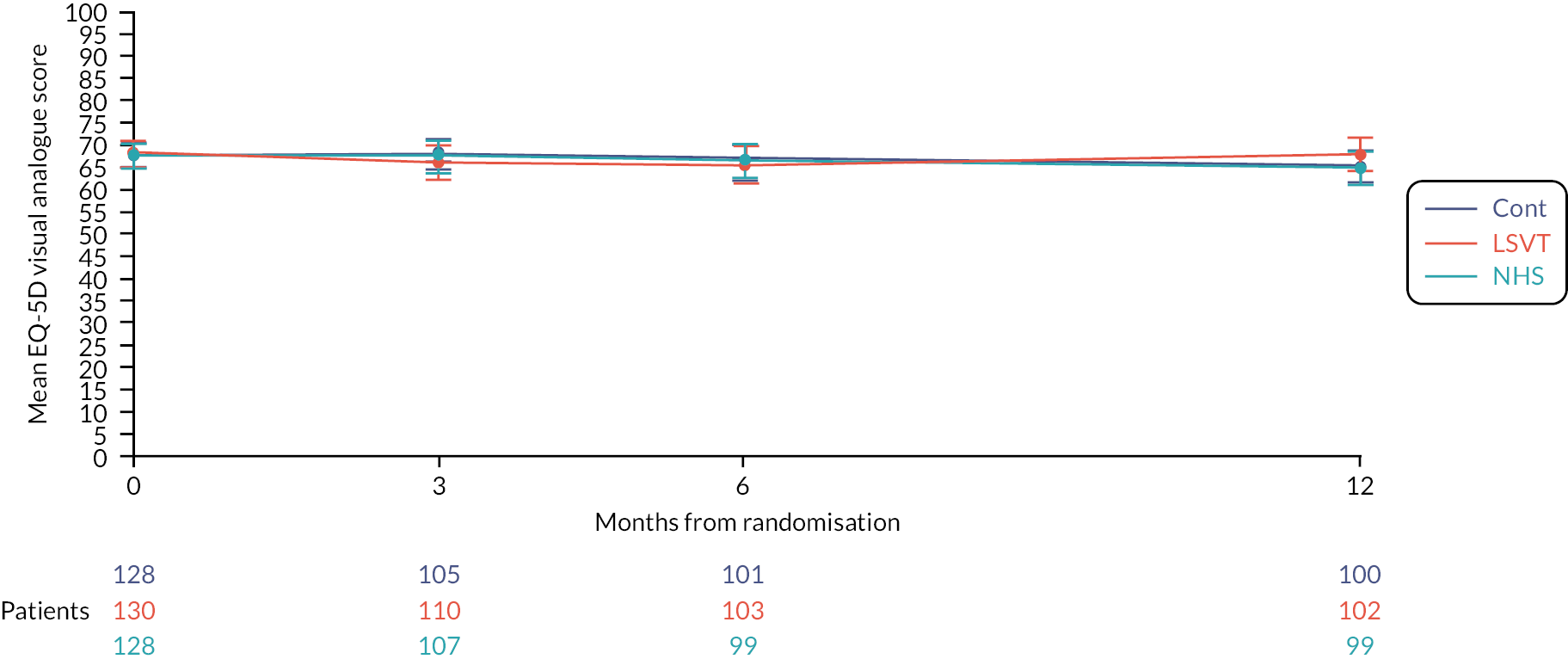
For measuring participants’ well-being, ICECAP-O was used in this trial. This is helpful for economic evaluation and consists of the total score made up of attachment, security, role, enjoyment and control domains. There was no evidence of a difference between interventions for the ICECAP-O total score at any time point (see Figure 11; and Appendix 1, Table 32).
FIGURE 11.
Mean ICECAP-O score profile plot with error bars over 12 months by intervention.
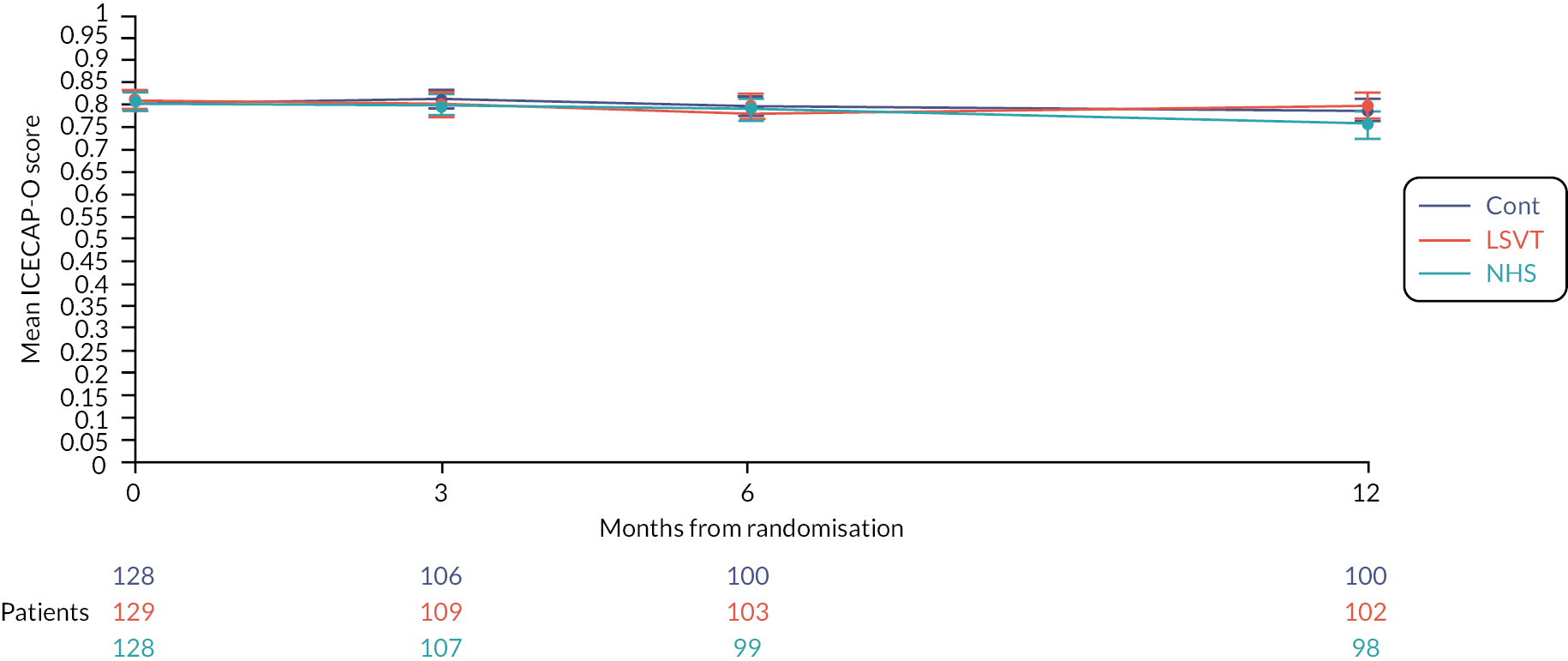
Effectiveness of speech and language therapies on carer’s quality of life (secondary outcome)
Carers were also recruited and these were mainly the spouse of participant, with a small number being a son, daughter or friend of the participant and a small number of carers with another relationship to the participant (see Appendix 1, Table 33).
The effect of the interventions on the quality of life of carers of people with Parkinson’s disease was assessed. There was a mixed pattern; the carer quality of life [Parkinson’s Disease Questionnaire–Carer) was higher (i.e. worse) after NHS SLT compared to no SLT (control) at 3 months [6.2 (0.1, 12.3); p = 0.009], but the strength of the evidence reduced when assessing the data over the whole follow-up period [5.4 (−0.4, 11.1); p = 0.02]. Conversely, the LSVT LOUD score was lower than the NHS SLT score overall [−6.3 (−11.8, −0.7); p = 0.004] with a borderline difference at 3 months [−5.6 (−11.6, 0.4); p = 0.02].
For the anxiety and depression subscale, NHS SLT had higher scores (i.e. worse) at 3 months [7.5 (0.8, 14.3); p = 0.004] compared to no SLT (control) and overall there was a borderline difference [5.9 (−0.3, 12.1); p = 0.01]. The LSVT LOUD score was lower (i.e. better) than the NHS SLT score [−7.2 (−13.8, −0.7); p = 0.005] at 3 months and overall [−6.9 (−12.9, −0.9); p = 0.003].
For the stress subscale, there was a borderline difference in favour of no SLT (control) compared to NHS SLT at 3 months and across the trial period overall. There were lower scores for LSVT LOUD versus standard NHS SLT overall, but little evidence of a difference at 3 months.
For LSVT LOUD compared to no SLT (control), there was no evidence of a difference at any time point for the overall score or the subscales (see Figures 12 and 13; Appendix 1, Table 34 and Figures 40–42).
FIGURE 12.
Mean PQD-carer summary index profile plot with error bars over 12 months by intervention.

FIGURE 13.
Mean PDQ-39 anxiety and depression subscale profile plot with error bars over 12 months by intervention.
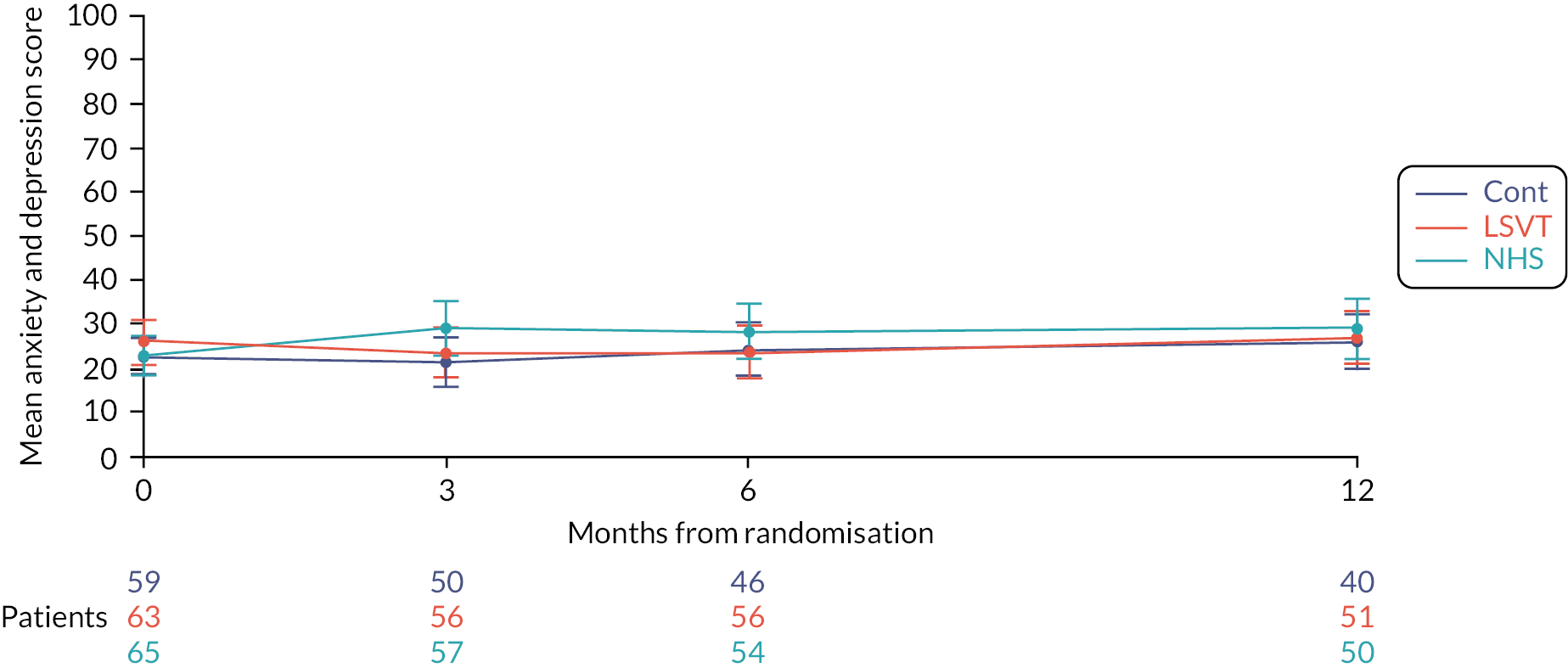
Clinical data
Clinical outcomes included assessing Hoehn and Yahr (excluding any reference to communication, speech or voice abilities) and PD-related medication (assessed using levodopa equivalency) at 12 months. There was a median Hoehn and Yahr value of 2.0 across all groups at baseline, and apart from a slightly higher median in the no SLT (control) group at 12 months of 2.5 (IQR 2.0–3.0), Parkinson’s disease severity remained the same over 12 months.
The amount of Parkinson’s treatment given was similar across the two active treatment groups at both baseline and 12 months and was slightly higher in the no SLT (control) group at both time points. In all groups, the amount of treatment given at 12 months had increased since baseline (Table 6).
| LSVT | NHS SLT | Control | ||
|---|---|---|---|---|
| N = 130 | N = 129 | N = 129 | ||
| Hoehn and Yahr stage | ||||
| N = 130 | N = 129 | N = 129 | ||
| Baseline | Median (IQR) | 2.0 (2.0–3.0) | 2.0 (1.5–3.0) | 2.0 (2.0–3.0) |
| N = 87 | N = 91 | N = 82 | ||
| 12 months | Median (IQR) | 2.0 (2.0–3.0) | 2.0 (2.0–3.0) | 2.5 (2.0–3.0) |
| Levodopa equivalency (mg/day) | ||||
| N = 130 | N = 129 | N = 127 | ||
| Baseline | Mean (SD) | 551.4 (342.8) | 557.2 (365.1) | 597.6 (416.9) |
| N = 110 | N = 109 | N = 108 | ||
| 12 months | Mean (SD) | 573.0 (352.7) | 569.7 (327.3) | 633.7 (371.8) |
Adverse events
There were no SAEs reported. A total of 139 AEs were recorded among a trial population of 388 participants. There were no AEs reported in the no SLT (control) group. The LSVT LOUD group reported 93 AEs which occurred in 36 (28%) participants, of which the majority (80 events) were vocal strain with a small number of throat ache (6 events), cold virus (5 events) and perceived hard glottal attack (2 events). Almost all the 46 events experienced by 16 participants receiving NHS SLT were vocal strain (45 events) and there was one throat infection (see Appendix 2, Table 35).
Chapter 4 Meta-analysis
Background and pilot trial design
Prior to submission of the application for NIHR funding for the PD COMM study, a pilot study funded by The Dunhill Trust was conducted to assess the feasibility of undertaking a large-scale trial comparing LSVT LOUD, NHS SLT and a no SLT intervention control in PD, as well as determine the procedures, outcome measures and sample size for a full-scale trial. The PD COMM pilot trial results have been published. 36
In summary, the PD COMM pilot was a multicentre, assessor-blinded, three-armed parallel group RCT. People with idiopathic PD, with self-reported or carer-reported problems with speech, were randomised in a 1 : 1 : 1 ratio to receive either LSVT LOUD; NHS SLT; or no treatment in first 6 months unless deemed medically necessary. Potential participants diagnosed with dementia, judged as likely to need SLT in the near term or given SLT in the previous 2 years were excluded. Outcomes were assessed at baseline (prior to randomisation), and then at 3, 6 and 12 months post randomisation.
PD COMM pilot results
The trial opened to recruitment in May 2012. Over a period of 23 months, a total of 89 patients were recruited from 12 sites and randomised to the three arms of the trial (30 LSVT LOUD, 30 NHS SLT, 29 control). The trial was completed by 27/30 (90%) participants in both therapy arms and 29/29 (100%) participants in the control arm. Data return rates were excellent, with the participant-completed questionnaires returned for > 90% of expected forms across all time points. Participants entering the trial were on average 67 years old, male (78%), and had a BMI of 27 kg/m2. On average participants had PD for 5.5 years and were in Hoehn and Yahr stage ≤ 2.0 (66%) (Table 7). Participants were taking on average the equivalent of 580 mg a day of levodopa in PD medication at entry.
| LSVT | NHS SLT | Control | ||
|---|---|---|---|---|
| Number of patients randomised | 30 | 30 | 29 | |
| Demographics | ||||
| Age (years) | Mean (SD) | 67 (8.4) | 68 (10.3) | 65 (7.5) |
| Range | 45–80 | 42–86 | 49–79 | |
| Age group (years) | < 60 (%) | 5 (17) | 5 (17) | 8 (28) |
| 60–69 (%) | 11 (37) | 10 (33) | 11 (38) | |
| 70–79 (%) | 13 (43) | 12 (40) | 10 (34) | |
| ≥ 80 (%) | 1 (3) | 3 (10) | 0 (-) | |
| Gender | Male (N, %) | 23 (77) | 23 (77) | 23 (79) |
| Stage of PD | ||||
| Duration of PD (years) | N | 30 | 30 | 29 |
| Mean (SD) | 6.1 (3.7) | 5.6 (4.2) | 4.9 (3.4) | |
| Median (IQR) | 5.4 (4.0–8.3) | 4.8 (2.5–8.3) | 4.9 (2.5–7.2) | |
| Range | 0.3–16.8 | 0.2–14.7 | 0.06–11.7 | |
| N | 30 | 29 | 26 | |
| Hoehn and Yahr stage | ≤ 2.0 (%) | 20 (67) | 16 (55) | 20 (77) |
| 2.5 (%) | 5 (17) | 2 (7) | 5 (19) | |
| 3.0 (%) | 4 (13) | 9 (31) | 1 (4) | |
| ≥ 4.0 (%) | 1 (3) | 2 (7) | 0 (–) | |
There were a total of six withdrawals: three in the LSVT LOUD and three in the NHS SLT group. In the LSVT LOUD group, participants had a median of 16 sessions lasting on average 61 minutes over 4.7 weeks. In the NHS SLT group, participants had a median of six sessions lasting on average 54 minutes over 9.6 weeks. In total, seven participants randomised to LSVT LOUD either did not start LSVT LOUD (n = 3) or stopped the LSVT LOUD early (i.e. did not complete 16 sessions; n = 4). The four participants that did not complete all 16 sessions of LSVT LOUD received 1, 2 or 3 sessions. Three of these seven participants withdrew from the trial all citing the intensity and time commitment of LSVT LOUD as the reason for withdrawal. One participant randomised to NHS SLT did not start therapy. The reason for not starting therapy was that the participant could not receive therapy within the designated time period as their daughter was taken ill, and then the participant had a planned operation. This participant withdrew from the trial. One participant in the control group was referred to SLT within 6 months of randomisation.
The PD COMM Pilot study was not powered to detect differences between the groups in any of the outcome measures included. However, the direction of the outcome measures was in favour of the SLT groups. The PD COMM trial demonstrated that a full-scale trial would be feasible as trial procedures worked well and the interventions were well accepted by patients with no AEs. Furthermore, the design of the full-scale trial was refined, determining the sample size and thus the number of sites and recruitment time required, reducing the burden on both participants and speech and language therapists (Voice Related Quality of Life Questionnaires and vocal recording measurements were removed) and the VHI was chosen as the primary outcome.
Meta-analysis of PD COMM and PD COMM Pilot
As the populations from both PD COMM Pilot and PD COMM are similar, with trial design comparable, a meta-analysis has been performed to combine results from both trials. Analysis was rerun on the pilot trial data (Table 8) to match with the analysis of the full trial (see Table 2).
| Mean (SD, n) | Mean difference (99% CI) | |||||
|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | |
| Baseline | 42 (20.2, 26) | 42 (25.5, 28) | 42 (21.0, 26) | – | – | – |
| 3 monthsa | 33 (22.4,22) | 36 (21.2, 22) | 46 (25.1, 28) | −7.25 (−19.0 to 4.5) | −4.7 (−16.9 to 7.6) | −2.6 (−14.9 to 9.7) |
The data from the 388 participants in the PD COMM trial and the 89 participants in the PD COMM Pilot trial were combined using random-effects meta-analysis methods. These meta-analyses included 372 participants [126 in LSVT LOUD group; 123 in NHS SLT group and 123 in the no SLT (control) group]. A difference of 8.0 points in the VHI total score at 3 months in favour of LSVT LOUD was found when compared with no SLT (control) [−7.9, 99% CI (−12.7 to −3.0); p < 0.01] (Figure 14). There was no evidence of a difference between NHS SLT and no SLT (control) in the meta-analysis [−0.08, 99% CI (−7.3 to 7.2); p = 0.2] (Figure 15), and borderline evidence of a difference when LSVT LOUD was compared with NHS SLT [−7.4, 99% CI (−15.9 to 1.0); p = 0.02] (Figure 16).
FIGURE 14.
Forest plot of LSVT vs. control from PD COMM and PD COMM pilot.
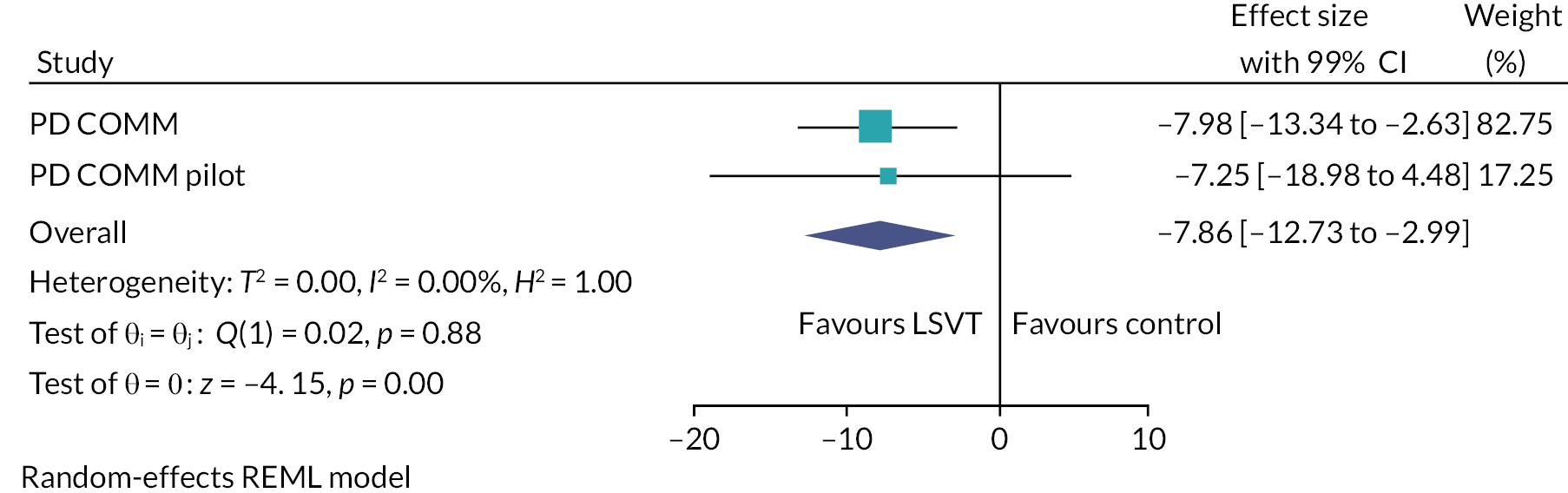
FIGURE 15.
Forest plot of NHS SLT vs. control from PD COMM and PD COMM pilot.
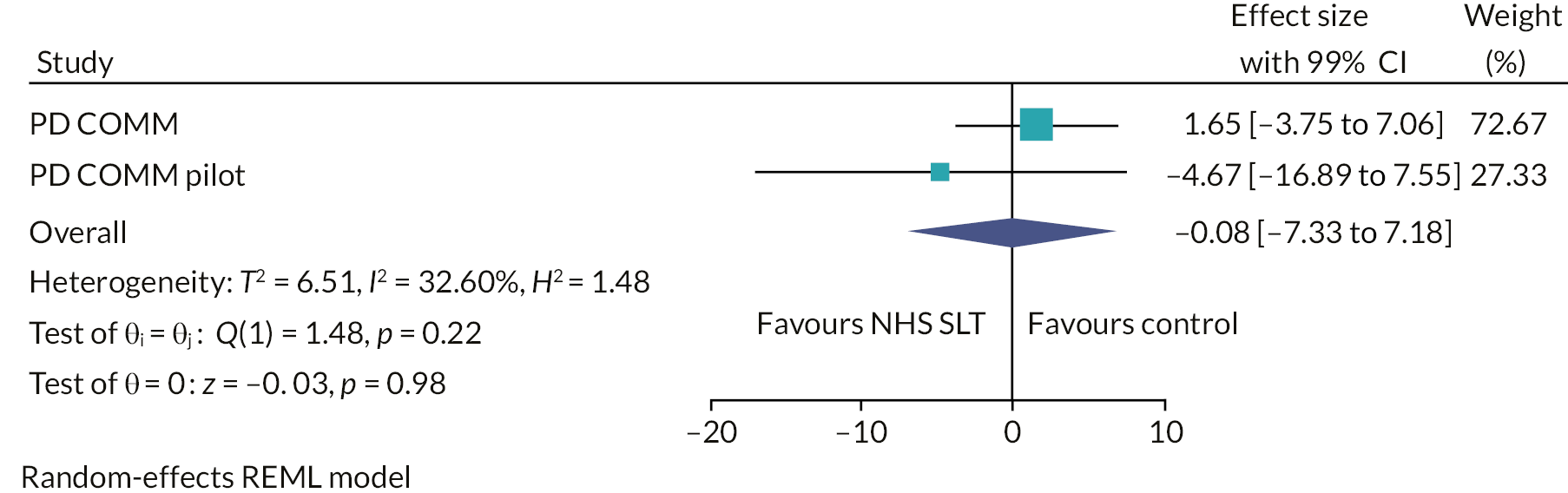
FIGURE 16.
Forest plot of NHS SLT vs. LSVT from PD COMM and PD COMM pilot.

Chapter 5 Implementation of PD COMM interventions: process evaluation
Material in this chapter has been adapted from the PD COMM trial process evaluation protocol by Masterson-Algar et al. (https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-017-2130-1). This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
This chapter describes the work carried out as part of the process evaluation alongside the PD COMM trial. As the Medical Research Council guidance68 suggests, process evaluations serve a very important role, not only when checking whether the trial intervention was delivered as planned (fidelity) but also in providing detailed insight into the experiences of those who engage with the complex intervention. 69,70 By carrying out a process evaluation it will be possible to identify if observed impacts are solely due to the trial intervention, or if these impacts are a result of a number of external and internal variables that are closely linked to the environment and the context in which the interventions take place. Pragmatic trials of complex interventions, such as PD COMM, which include a process evaluation will produce higher quality results that can help clarify the potential generalisability and optimisation of PD COMM interventions in routine practice. 68,71
In order to identify the causal processes around intervention implementation and fidelity taking place during the PD COMM trial, this process evaluation has used the normalisation process theory (NPT) which is highly attuned to the challenges of complex interventions as it encourages looking at systems as a whole. 72 This sociological theory has been widely used to understand how healthcare interventions are implemented, embedded and finally integrated into a healthcare setting. 73 The major focus of analysis within NPT is the individual and collective work that is undertaken to implement and enact, in this case, the PDCOMM trial and the interventions. This work is considered within four domains, in this case, as follows:
-
Coherence: the work undertaken to understand and make sense of what is required.
-
Cognitive participation: the degree to which people become and remain engaged and committed.
-
Collective action: the work undertaken to implement what is required.
-
Reflexive monitoring: what is done to reflect on and evaluate the trial and interventions.
We considered that its conceptual framework74 would assist the interpretation and synthesis of data and analysis to explain what implementation processes took place and the interactions and gaps between the two PD COMM SLT interventions, the changing context, speech and language therapists and their practice.
Process evaluation aims and objectives
The overall aim of this process evaluation was to evaluate and understand PD COMM intervention trial implementation and fidelity in order to provide insights that will assist in the interpretation of the PD COMM trial results.
Specific objectives were to:
-
investigate therapists’ experience of implementation and familiarity with PD COMM interventions, identifying the actual practices received by participants, likely tailoring to contexts, and other influences on implementation within the healthcare setting
-
investigate the influence of therapists’ factors pre-trial, including their:
-
relevant skills and training
-
previous experience of LSVT and NHS SLT and familiarity with its methods and procedures
-
level of acceptance of the interventions both at a personal level and in their implementation context
-
expectations in terms of intervention provision within the context of a pragmatic trial and intervention impact.
-
-
identify therapists’ learning over time and its potential impact on implementation outcomes
-
investigate study participants’ experiences, views and level of engagement in the PD COMM study, and their influence on participants’ participation, motivation and (where relevant) adherence to therapy for those in treatment and control groups
-
synthesise all process evaluation data collected and link it to the explanation of trial findings.
Methods
Design
The process evaluation was conducted as an integral part of the PD COMM trial (Figure 17) and it applied NPT to examine normalisation from the perspectives of both staff delivering the intervention and patient participants who needed to embed the trial interventions into their daily routine. All details of the process evaluation design and methods were published as a protocol for the study. 75
FIGURE 17.
PD COMM process evaluation flow chart.

This process evaluation used a variety of data collection methods. 75
Therapist questionnaire (TQ): All speech and language therapists and SLT assistants involved in the trial were asked to complete an online questionnaire at two time points: prior to the start of intervention delivery and after they had treated their last PD COMM participant. The TQ consisted of three sections A, B and C. Section A collected data on therapists’ roles and years of experience; section B consisted of an adapted version of the NPT NoMAD tool76 to evaluate attitudes towards the LSVT and NHS SLT interventions across the four NPT domains and the therapists’ self-efficacy as measured by the general self efficacy (GSE) questionnaire. 77 Section C comprised the LSVT skill set questionnaire78 to evaluate therapists’ confidence with completing LSVT in practice settings.
Semistructured in-depth interviews with study participants. Purposeful sampling ensured engagement of trial participants with different ages, PD severity (Hoehn and Yahr stage) and the presence of a family carer.
Semistructured in-depth interviews with study therapists responsible for delivering the two active treatment arms. Interviews were carried out at two time points: estimated midway through therapists’ involvement in the trial, and at least 1 year after they joined the trial.
All interviews were digitally recorded and fully transcribed and then checked by the interviewer, keeping all names of participants anonymous. All participants were asked to give consent to the recording prior to the start of the interview.
Quantitative and qualitative sets of data were analysed independently before being combined.
Quantitative data
Descriptive statistics were carried out on the data from the TQ to explore the frequency of responses to items and investigate where therapists have shown a more positive or negative response.
Qualitative data
All interviews were recorded and transcribed verbatim after anonymisation. The software package Atlas.ti (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany) was used to facilitate analysis.
All interview data were coded around a theoretical coding framework informed by the NPT constructs72 (More details are available at https://www.birmingham.ac.uk/research/bctu/trials/pd/pd-comm/investigators/process-evaluation). The analysis followed the phases of thematic analysis described by Braun and Clarke. 79 First, two of the authors (PMA and MC) read and re-read all transcripts and notes to gain familiarity with the data. The researchers then coded all data informed by the coding framework and assigned relevant data extracts to each code. New codes were created for data falling outside of the coding framework to avoid missing important concepts.
Themes were identified as meaningful patterns across coded data and were then reviewed and agreed by all authors. The qualitative data analysis was conducted within source prior to synthesis across data sources. Following the process described in Figure 17, all identified themes generated from the analysis of both, participant and therapists’ interviews were mapped onto and synthesised with the rest of the data from TQs. The quantitative data (TQ) on therapists’ level of experience and familiarity with the interventions together with qualitative themes on participants’ engagement in the trial and therapist’s experiences were synthesised during two, one-day workshops with all researchers in the team. Workshops were informed by:
-
Previous research carried out by two of the report authors identified the ‘balancing’ act and the ‘learning overtime’ that takes place when therapists have a research role and
-
The NPT framework. As a result of this workshop an explanatory framework emerged from the data.
Results
Quantitative data: therapist questionnaires
The online TQ was completed by 71 therapists prior to start of intervention delivery (time point 1). Of those therapists, 26 (36.6%) went on to complete the questionnaire approximately 1 year into the trial (time point 2). Therapists who completed the questionnaire were spread across all trial sites. Descriptive statistics were carried out to indicate the frequency of responses. Due to the limited sample size for time point 2 no within-group and between-group analyses were possible.
Of those therapists who completed the TQ 8% were Agenda for Change Band80 5, 39% were band 6 and 53% were band 7 or above. Therapists had different levels of experience of working as SLTs (Figure 18).
FIGURE 18.
Distribution of PD COMM speech and language therapists according to years of experience.
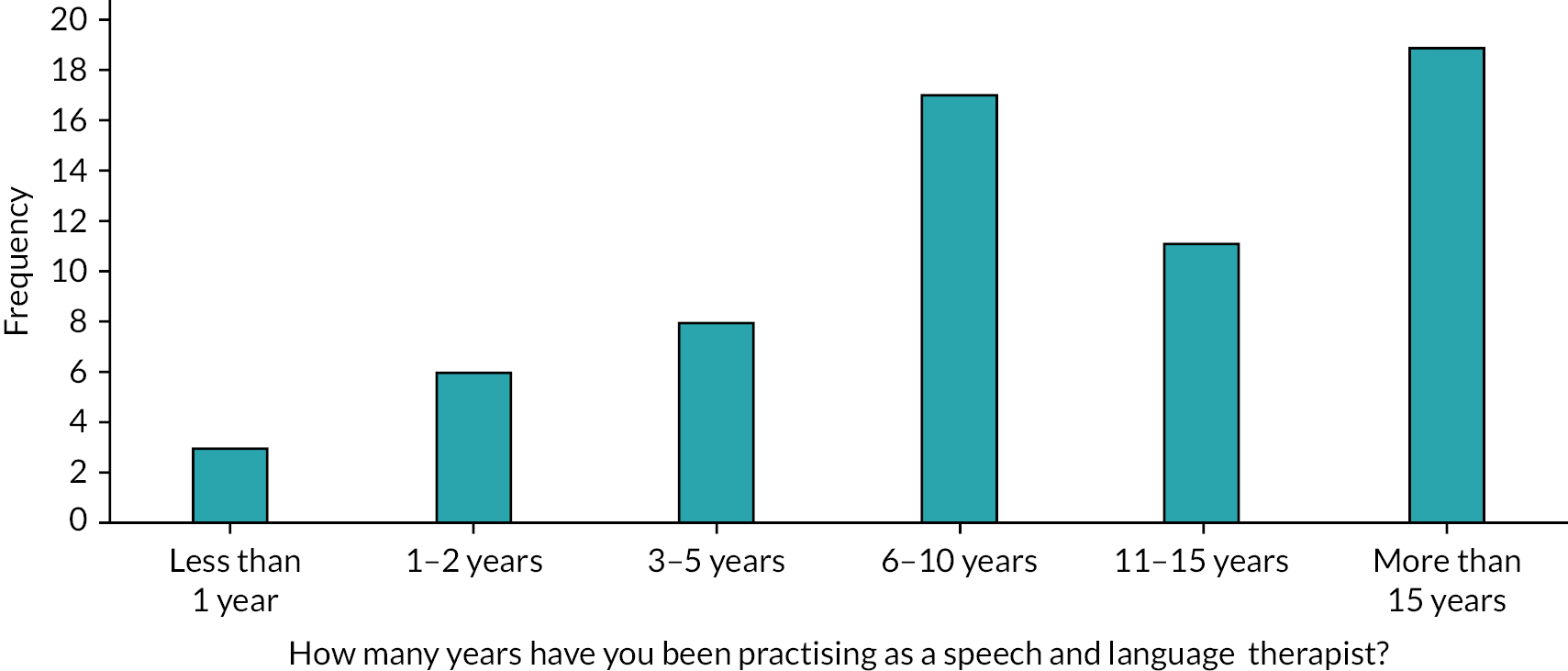
Sixty-nine per cent of therapists delivered LSVT LOUD as part of their current role in their Trust. Among different years of experience there was a similar spread across those who normally delivered LSVT as part of their role and those who did not (Figure 19).
FIGURE 19.
Distribution of SLTs according to years of experience and delivering LSVT as part of their current role.
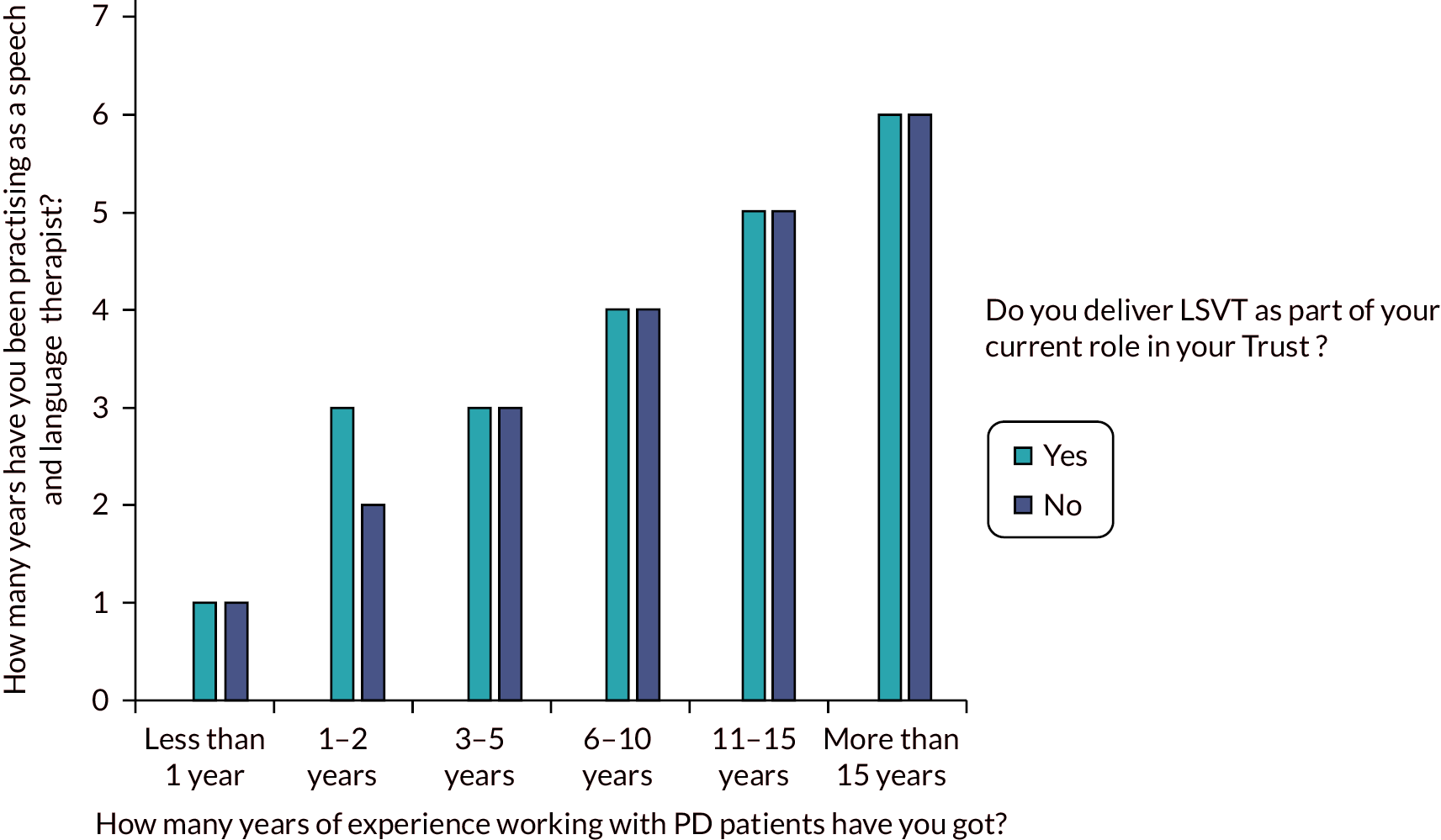
Eighty-four per cent of the therapists had a GSE Total Score of 30 or higher. All therapists had high scores regardless of their years of experiences practising as a speech and language therapist (Figures 20 and 21).
FIGURE 20.
Distribution of GSE scores across all PD COMM therapists with different levels of experience grouped depending on whether SLTs deliver LSVT as part of their current role.

FIGURE 21.
Distribution of degree of familiarity of SLTs with LSVT and NHS SLT interventions across different levels of professional experience.

The answers to questions included in the adapted version of the NoMAD tool (Part B of the TQ) that addressed issues around familiarity with PD COMM interventions, the results show that therapists were overall familiar with both NHS and LSVT interventions and saw them as a normal part of their role. Therapists showed equipoise when it came to both interventions regardless of whether or not they delivered LSVT as part of their normal everyday job. Therapists were asked to rank from 1 to 10 how familiar both interventions felt to them. Survey data show a similar spread of responses across both interventions (see Figure 21).
All questions/items in section B of the TQ were grouped according to four of the NPT constructs that they represented (Coherence, Cognitive participation, Collective action and Reflexive monitoring). Data were analysed to describe the spread of responses (1 – Strongly disagree up to 5 – Strongly agree) and the mean value for each of the constructs for each of the interventions. Results show further evidence that confirms balance across both interventions (Figure 22).
FIGURE 22.
Mean score across four NPT constructs for each of the PD COMM interventions.
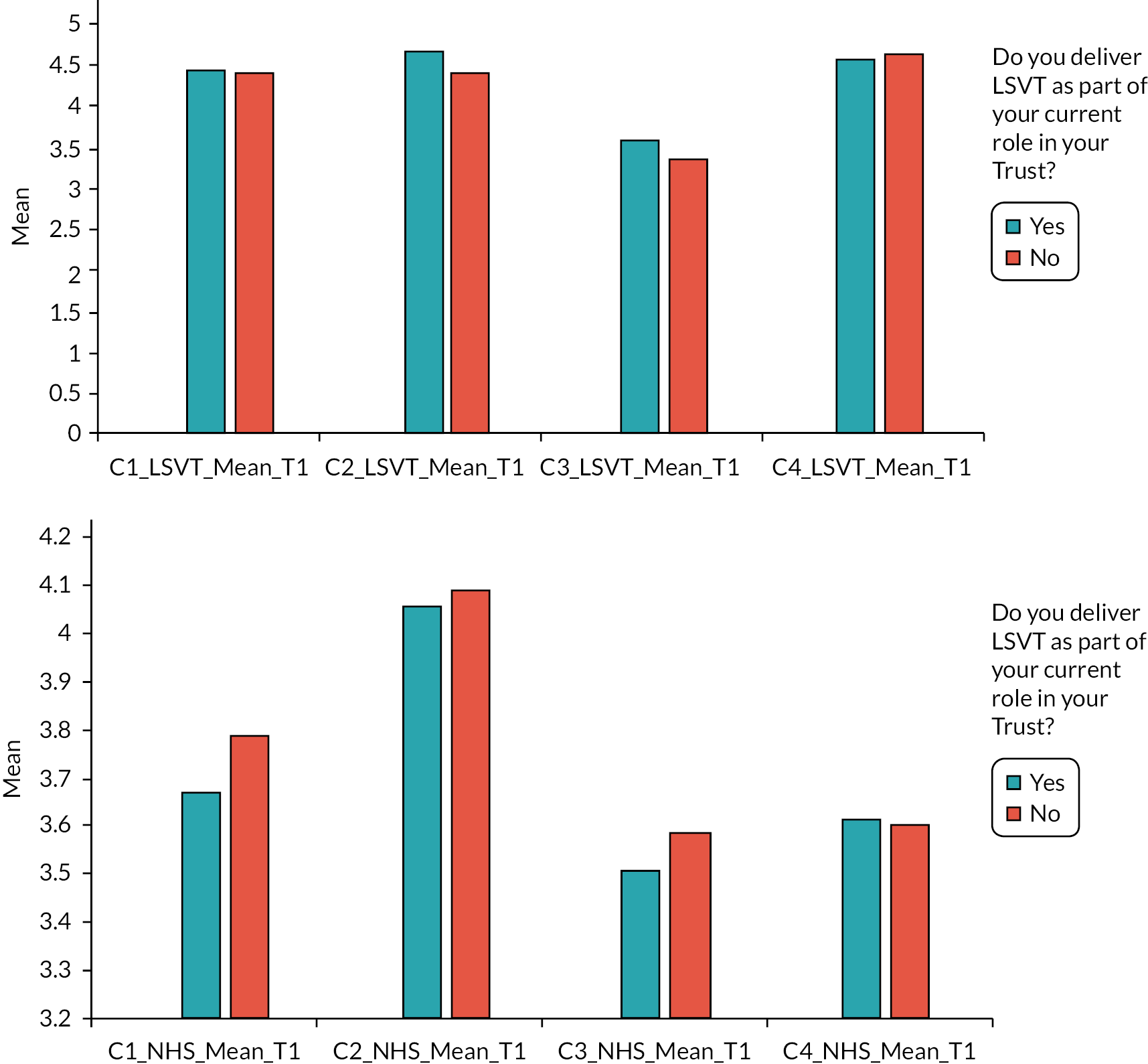
The last section of the questionnaire, section C (LSVT Skills level questionnaire), which explored therapists’ level of confidence in their ability to deliver all components of LSVT, showed that overall, all therapists who completed the questionnaire felt they were able to implement all aspects of the LSVT intervention (3 – ‘Got it’). This was true even for those who had recently completed their pre-registration training (Figure 23).
FIGURE 23.
Mean scores (from 1 – ‘I don’t get it’ to 3 – ‘Got it’) for each of the eight sections of the LSVT skills questionnaire, across all therapists with different levels of experience.

In summary, data collected in this questionnaire provided clear evidence of equivalence across PD COMM therapists with regard to their familiarity and preparedness to deliver both PD COMM SLT interventions.
Qualitative semistructured interviews
Therapist interviews
A total of 20 PD COMM SLTs consented were interviewed over the phone at time point 1, approximately midway through therapists’ involvement in the trial. Among these, nine completed an interview at time point 2, approximately 1 year into the trial. Therapists who were interviewed worked across 17 study sites and had different levels of experience. Speech and language therapists were highly engaged and interested in discussing the work they had carried out during their involvement in the trial. Overall, they were aware of the need for research evidence to support their professional practices.
Trial participant interviews
A total of 24 trial participants consented to complete a phone interview. Interviews were carried out between July 2017 and April 2019. Out of the 24, 19 had a carer and 5 lived on their own. Ten were randomised to the control group, eight to LSVT and six to NHS SLT. For those from whom a score could be accessed, 13 participants had a Hoehn and Yahr score of between 1 and 2.5 and 5 participants had a score between 3 and 5. Apart from six participants who were younger, all the rest were older than 65 years of age.
Interviews produced in-depth descriptions of experiences and engagement of participants throughout their involvement in the PD COMM trial. A number of themes and subthemes were identified for both sets of interviews (Table 9).
| Themes | Subthemes |
|---|---|
| PD COMM therapists | |
| Before the trial |
|
| Research – a ‘new’ part of my role |
|
| Am I (and my service) ready for this? |
|
| Do I feel supported? |
|
| The impact of what I am doing |
|
| PD COMM participants | |
| Before the trial |
|
| Being a research participant |
|
| Experiencing change/impact |
|
PD COMM therapists – identified themes
Before the trial
Data show that PD COMM therapists had different personal motivations to join the trial. In some cases, therapists’ interest in research drove them to ask their team members and line managers about joining the trial when they became aware of it. For other SLTs this was not the case, and it was their trust/service that decided to join the trial with little involvement or decision-making on the part of the therapist.
Overall, SLTs reported a strong awareness of the need for research to show the effectiveness of the interventions they regularly deliver as part of their professional role. Hence, their wish to be evidence-based practitioners was reported as a strong motivation to want to join the trial. This was particularly true for those therapists who had been involved in the PD COMM pilot study and had found the experience highly rewarding.
Furthermore, for some of those therapists who had never been involved in research, taking part in PD COMM was perceived as a ‘great opportunity’ for professional development.
Although some therapists had a pre-conceived idea of what being involved in research would be like (this is particularly true for those who had been involved in the pilot or in other research trials), data show that for some of them research was an ‘unknown’. Therapists overwhelmingly reported that the time-consuming nature of it (often linked to paperwork and administrative tasks) was by far the biggest surprise for them.
Research – a ‘new’ part of my role
Interview data show that prior to the trial, each service, to a certain extent had a clinical pathway that drove them to use one intervention in preference of the other. This was directly linked to the value that each service team as a whole attributed to each of the therapies. Similarly, each therapist (and very closely linked, their practice/Trust) had a particular approach to practice that was driven by:
-
LSVT principles focusing on a different degree of treatment intensity or
-
National Health Service approaches (often very varied).
Therapists’ approaches were mirrored by the whole service itself. Those practices with little LSVT ethos were often the ones where no therapists, prior to PD COMM, were LSVT trained. On the other hand, in services where the higher-grade speech and language therapists were LSVT trained, the ‘LSVT philosophy’ often cascaded down to all junior members of the team.
However, data from interviews also indicate that therapists showed an ability to develop ‘equipoise’ with respect to the trial interventions, and therapists were able to have a balanced perspective on both of the interventions. This is clear in the data that show how throughout their involvement in PD COMM therapists succeeded at balancing any natural tendency to apply their normal clinical ways of working (focusing on addressing patients’ needs) with the need to follow the trial protocol. This was because they did not perceive trial interventions as a ‘threat’ to their normal way of working, and that they were aware of the importance for both trialled interventions to be ‘different’ in terms of content and delivery.
Sticking to protocol was described as more or less challenging depending on which intervention therapists were talking about. Following the LSVT LOUD protocol was often described as a straightforward task due to its strong prescriptive nature that was widely considered ‘fit for purpose’, reducing the demands on the therapist to tailor it to patients’ needs. Tailoring did take place but, as therapists explained, not in the content of the intervention, which remained highly standard. However, necessary practical adaptations such as the use of the ‘LSVT companion software’ or the sharing of therapy sessions between two therapists were made.
Following the NHS SLT intervention protocol was unanimously described by therapists as more challenging. One of the reasons for this being that in services driven by LSVT, therapists were in charge of ‘putting together’ the protocol (content) for the NHS intervention, which was often new to them and often described as a ‘watered down LSVT’. The way in which therapists and services as a whole followed a pragmatic approach to make decisions on the content of the NHS intervention could have resulted in a potential degree of overlap between both trialled interventions. However, most therapists did not see this necessarily as a weakness of the NHS intervention and reported feeling confident about it being clearly different from the LSVT intervention.
Am I (and my service) ready for this?
Most therapists reported being aware of the fact that taking part in research would potentially have an impact on service capacity. This impact was more acutely linked to the delivery of LSVT LOUD, as it was seen as resource intense, involving 4 hours a week for 4 weeks. Data show that therapists and their service were proactive at taking the necessary steps to minimise the impact on service capacity. Two strategies were most popular among those therapists who were interviewed. Firstly, speech and language therapists reported having a system in place by which they would regularly communicate with their service research nurse to limit the number of patients’ being randomised and match it to the service capacity at that point in time.
Secondly, as already mentioned above, most services decided that LSVT LOUD would be delivered by a team of two therapists in order to be able to deliver the 4 hours per week for 4 weeks.
All therapists discussed their opinions and experiences of the randomisation process. Those with little or no research experience explained that it was a new concept that needed adjusting. A number of therapists showed some frustration at the fact that although, as previously explained, they had planned ahead of starting the trial in order to be able to deliver LSVT LOUD due to randomisation, they ended up with only participants in the no SLT (control) group.
Some therapists reported feeling that randomisation had challenged their clinical decision-making as it had impacted on their ability to decide which patients to refer for LSVT. As they explained, this led them to deliver LSVT LOUD to patients who, in normal (outside of research) circumstances would have been deemed as ‘not appropriate for LSVT’ due to its intensity or high level of commitment.
Therapists considered that randomisation also had, at times, a negative impact on recruitment as the risk of ‘ending up in the control group’ had often been mentioned by participants as a reason to decline the invitation to take part in the trial. As therapists explained, this was particularly true in those services where there was no waiting list to receive LSVT (at that point in time) or the waiting time, on average, was short. In contrast, in those services with long waiting lists for LSVT or where LSVT was not normally offered, patients who had heard about LSVT and its potential positive impact were happy to take the risk just to have some chances of being randomised to the LSVT LOUD group.
Therapists explained that randomisation was not the only factor impacting on recruitment; issues around patient/therapy fit were equally important. Therapists were aware that, in some cases patients had declined to take part because they felt they could not commit to the intensity and prescriptive nature of LSVT LOUD.
Do I feel supported in my role as a researcher?
Most therapists reported having been engaged in regular team meetings within their service in order to discuss and plan issues related to their involvement in PD COMM. For a number of therapists, this ‘team working spirit’ was seen as a potential positive outcome of being involved in research at both, a personal level and at service level.
Unanimously, therapists felt supported by the trial team throughout their involvement. This support was reflected in a number of ways. Firstly, therapists valued the prompt replies from the trial’s administration team whenever they contacted them with a query, very often linked to paperwork completion. Secondly, therapists valued their participation in the ‘collaborators/therapists one day events’ organised throughout their involvement in the study. Overall, therapists saw these events as ‘great networking opportunities’ that gave them the chance to ‘bounce ideas’ with colleagues and also address some of the challenges and voice their concerns in a safe and understanding environment. Finally, therapists valued the monetary incentives that the trial had provided such as, budget to train more LSVT therapists or ‘up the hours’ of some of the therapists in order to be able to do the research work (this was only included in the budget for sites in Scotland).
An online discussion platform (JISCMAIL list) set up as an alternative method for therapists to be able to be in touch with each other was well received and a number of therapists used it in order to distribute or disseminate delivery plans or ‘site specific’ plans that could help other sites struggling with similar challenges (e.g. time allocation planning prior to randomisation – in preparation in case it was LSVT LOUD).
The impact of what I am doing
Therapists reported their research experience as a ‘learning curve’. For example, they reported that they had needed time to adapt and learn how to deal, for example, with paperwork and ‘admin tasks’ linked to the research process. As time passed, these tasks, which at the beginning were perceived as tedious and time-consuming, became a natural part of their role often thanks to the support of trial team members or support from a colleague with more research experience. For therapists this learning curve meant that with time they were able to focus their energy in, as they reported the ‘important bit’, delivering the interventions.
With time, therapists also became better at delivering the trial interventions. This was particularly true for those therapists who had recently completed their LSVT training. They reported that as time went on, they had become more relaxed and more familiar with LSVT components and this had in turn allowed them to ‘enjoy’ the sessions with patients more. Therapists for whom the NHS SLT intervention was ‘new’ reporting similar feelings.
Data show that taking part in PD COMM had an impact on therapists’ normal way of working and future clinical decision-making. Therapists acknowledged how their preconceptions or assumptions in regard to PD COMM interventions had been ‘put to the test’. For example, a number of therapists explained that they had found themselves (for reasons out of their control, such as randomisation) delivering LSVT LOUD successfully to patients who, in normal circumstances (outside of their research role) they would have never identified as ‘appropriate’ for LSVT.
For a number of LSVT driven therapists delivering the NHS SLT intervention had also been, in some cases, an ‘eye opener’ that had forced them to come out of their comfort zone. This sort of impact was also seen at a service level. Therapists explained witnessing positive outcomes for patients receiving the NHS SLT intervention that they had ‘put together’ as part of their involvement in the trial. Witnessing this positive impact had led to the therapists (and the service) deciding to continue to deliver this type of intervention even after the trial was completed.
However, therapists also explained that although, as previously mentioned, taking part in the trial had had an impact on both their individual and their team’s practice. Further implementation after the trial or sustainable changes/adaptations to reflect the outcomes of research would heavily rely on whether managers were interested and invested in driving evidence-based practice linked to research or had access to the necessary funding.
Data show that for most therapists, being able to take part in the study and witnessing the improvement, or not, of PD patients has helped them feel reassured about what works for whom. Therapists reported feeling excited/expectant about the prospect of having confirmation of the impact, or not, of the interventions with trial results. In some cases, therapists were able to explain, during the interview, how they had seen a very clear positive impact of LSVT LOUD. They had witnessed how the intervention had improved the person’s well-being significantly and how patients had been very grateful for it. This being particularly true for those patients who had been extremely motivated and engaged throughout the process.
Overall, therapists did not have strong feelings regarding the appropriateness of the chosen outcome measures and record forms. A number of therapists did voice a concern about the complex structure of home-based diaries, which made them difficult for some patients to complete. SLTs worked around this problem in different ways such as spending the first minutes of each session with a participant going through the diaries and making sure they were correctly completed.
Finally, therapists were aware that regardless of what they had experienced and the impact they had witnessed during their time delivering the PD COMM interventions to participants, it would be the final results of the trial that would determine its impact on future services.
PD COMM participants – identified themes
Before the trial
Participants had been impacted by their health in different ways. Although most participants reported that their health condition made them feel low in mood at times, participants also explained how they were trying to make every effort to continue to ‘live as normal’, not letting the illness ‘bring them down’. One participant explained ‘generally speaking, I feel different 10% of the time and 90% I am my usual self’.
During the interviews, participants explained how getting a diagnosis was in some way perceived as a relief. One participant explained that it was ‘good to have a name and something to work on’. For some participants, being diagnosed provided an explanation as to why they were not coping at work or at home with everyday tasks or why they were quieter than normal.
Overwhelmingly, participants explained that after the diagnosis they had decided to ‘take control’ of their health. Participants also described this as ‘helping oneself’. As one participant explained:
‘I decided that it wasn’t going to take me, as such. And I started doing the Wii Fit, the yoga and balance and all that sort of stuff. And I used to run a lot as well. I was running a lot before I was diagnosed so I just carried that on’.
Participants’ attitude towards the illness played a major role in their ability to deal with its impacts. However, one participant explained that it is hard to remain positive:
‘It’s difficult on a day-to-day to keep positive and keep going. [ … ] Three years after I was diagnosed, I was walking with a walking stick and I changed my car to an automatic because I thought my left leg wouldn’t last much longer. So, I made myself into an invalid. About a year after that, I thought, "Bugger this!" Excuse the language. I’ve not used a walking stick since. A lot of it is state of mind. I could quite easily, tomorrow, make myself disabled and very needy. But I feel I’ve got to keep positive and keep pushing’.
Understanding the illness by learning and reading about it, its trajectory and medication options was described by participants as an effective coping strategy. Most participants explained how they were aware of the progressive nature of PD and how the uncertainty of the condition worsening with time was a worry to them:
‘I realise that there’s no cure for this disease, which is not very good. It’s a negative thought, isn’t it? And I know these drugs I’m taking; they’re supposed to delay everything. I don’t quite know whether they are delaying it’.
Another participant explained: ‘It’s quite a strange feeling. I’m guessing that I’ll eventually become … take over or try and take over. [ … ] It just creeps up behind you and off you go. So, I’m watching and monitoring it’.
For many participants, learning about medication was vital but understanding how it worked best for them was often a slow complex process that often needed ‘tweaking’ and regular reviewing. One participant explained:
‘It’s been a real problem, but I think we’ve got the solution now. I’ve had about six months, really, of trying out various combinations of things to find a new treatment that’s better than what I was on before. I think we’ve finally got there’.
The unpredictable nature of the condition and the daily variability in the intensity of symptoms such as tiredness, slowness and mobility difficulties were seen as difficult to adapt to. Participants explained that it was hard to never know whether it will be a good or a bad day:
‘On the days I’m having a hard time, I feel quite miserable about it. And days like today, I just don’t think about it’.
Another participant explained:
‘I do have days when my voice breaks up and it may be all day, may be half a day, may be for an hour, and may be for ten minutes’.
Participants explained how important it was for them to live an independent life and ‘to be in control’, not becoming a burden to others (mainly spouses). However, they also admitted that it was equally important to learn how to manage expectations and be realistic about their own limitations. Taking the right precautions and making the necessary adaptations was seen as vital in order to increase their chances of success at doing what they want to do. Not driving long distances or using shopping areas with sitting areas for resting were some of the examples mentioned by participants. One participant explained:
‘I’ve had to re-learn the way I play (the guitar). I did stop playing at one point. I stopped playing for about six months because I was so frustrated that I couldn’t play as much as I used to, but then I missed playing so much, I decided to give it a go again. I found different ways of playing, different ways of managing, lowering my expectations about what I can play and keeping things simple’.
Another participant reported:
‘I do the things that I always did, I just have to do them in moderation now. Still go out and socialise but it’s not like I can go out until 11/12 o’clock. I go out a bit earlier and come home a bit earlier. Just got to tailor it. I still do the things I enjoy, which for me is a big thing, really. Like I say, that’s a message. Just try and keep doing the things that you like and enjoying it. You have to moderate and tailor them. Try not to let it beat you and stop you doing the things that you like. It’s tough’.
However, a number of participants explained how these adaptations/changes were often imposed on them and driven by the intensity of symptoms, not by their own choice. In regard to work, several participants explained that they had had to ‘scale down’ (e.g. work part-time) very much against their will but rather forced to by the condition. As one participant explained:
‘I was a teacher and then I was doing teacher training and at that point, training courses and teaching demonstration lessons, things like that, were becoming increasingly difficult, partly because of the fatigue, partly because of my voice, and also there was the concern because my balance wasn’t very good at times, I might actually fall on a child and hurt them. So, I think that was the point that they said, “Would you like to take early retirement?” So, I did that last year, which was a bit of a pain. I didn’t want to, but it seemed like the sensible thing to do’.
The condition has stopped many participants from doing what they want to do because as one participant explained,
‘I just can’t seem to make my body do things that I want it to do’. This was described by participants as ‘being very hard’.
Participants described a number of sources of support such as the PD nurses involved in their care and overall had felt supported by NHS. As one participant explained:
‘Just a no-nonsense approach. I don’t want people to pity me. I mean, I like the Parkinson’s nurse I’ve got. She’s attentive and curious and knowledgeable and well clued-up, has her own opinions and is not afraid to tell me, to make it clear. That’s the kind of support I need’.
Participants described the different sources of support available to them (e.g. support groups, exercise classes) and were clear as to which ones were ‘their thing’ and which ‘just not for me’.
However, unanimously, it was family members, wives/husbands that were described as the main source of support. As one participant put it: ‘I rely on her 100%, she is with me 100% of the way’. Another participant explained: ‘It’s enormous. It’s a great help. They nag me all the time to make sure I’m doing things. [Laughs] They are concerned about me and they do try to help all the time’.
Being a research participant
All participants were happy with the role they had played as trial participants, and that there were a range of reasons that drove them to take part in the trial. Firstly, taking part in research gave them a purpose and made them feel proactive in terms of dealing with their illness. Secondly, they explained that they felt like they wanted to contribute to society where they could and give something back.
Other reasons for taking part in PD COMM were rarely mentioned, such as ‘skipping waiting lists’ or getting better or faster treatment. One participant explained:
‘Our local hospital is small and there tends to be a little bit of a wait, particularly for non-urgent. I, personally, feel that I got speech therapy faster than I would have. It’s circumstantial, I know, that this study happened to be running but I personally felt that I got the treatment that I needed, that was good for me, faster through this route than I would have by saying I needed speech therapy and an appointment and a referral from my GP’.
As previously discussed, overall participants were aware of the randomisation process and had a pragmatic approach to it. As put by one participant: ‘I thought I’d take my chances and got lucky’. Those that were randomised to the LSVT LOUD group reported feeling ‘pleased’: ‘I was quite pleased about it because the nurse recommended it strongly, yeah. Pleased to get into the right section’. However, not all participants felt the same, particularly those randomised to the control group: ‘I wish it was a different group because I’ve not been very happy in the control group’.
Another participant explained: ‘I knew nothing about Lee Silverman. I knew I didn’t want to be in the placebo group. But as an aside, I was hoping that I might derive benefit from being part of the study. I was keen to get treatment. The NHS stuff was, for my purposes, more than adequate, because it was about mitigation rather than cure’.
Not all participants joined the trial hoping to be randomised to the LSVT LOUD group; data show that some had been worried about being allocated to it mainly due to two reasons. Firstly, because they were worried about the high level of commitment that LSVT LOUD required and secondly because they did not consider the severity of their symptoms to be serious enough (or affecting them enough): ‘In fact, we were discussing it with my therapist and I was saying that the Lee Silverman would’ve been pretty intensive for me. It would’ve been more difficult for me to fully participate’. Or: ‘I didn’t feel in urgent need of speech therapy, so I wasn’t disappointed on that score. And like I say, I was just a little bit concerned about how much time and effort I need to put into the lessons. Because if I do something, I like to do it properly. Put my best effort in’.
Overall, participants were happy with the PD COMM paperwork they were asked to complete. However, data show that some participants found the home practice diaries hard to follow and time-consuming. Participants reported: ‘I found it quite frustrating, which she explained it would be. I mean, even doing it myself, I tried to do it myself to the best of my ability but it’s never quite the same as if you’ve got a professional with you, with anything, I suppose. But she would remind me if I was dropping my voice or various things. It was quite different’.
Participants, in some cases, reported for example, not fully understanding the scales in the outcome measures, or not feeling like the items in the questionnaires reflect accurately what they wanted to convey: ‘All these questionnaires, the tick-boxes, the answers aren’t always exactly what you would have said’.
Overall, participants reported feeling supported by the trial team throughout their involvement in the trial and valuing the way the trial was run:
‘I thought the trial was incredibly well-run, really. I didn’t have any criticisms with the way it was run, the way it works. I know it was an intensive one because I had to go to [Hospital] four times a week but I can see why that was essential. There’s no other real way of doing it, I suppose. I suppose the therapist could have come to me but that wouldn’t have helped her with her time-management. She’s got loads of other things to do, I guess. I’ve got no fault, really, with the way the trial was run. It was excellent, really. It all worked really well’.
The role of spousal partners was instrumental in the running of the trial as they played a vital role in supporting participants to, for example, attend sessions or complete the paperwork. As one participant explained: ‘My writing is pretty poor at the moment so when we filled it in, it was rather a long questionnaire and my wife filled it in, did the writing, and I told her what to put. Then she had to fill one in as the carer, except she doesn’t like to be called a carer for some reason’.
During the interviews participants explained how they were highly committed to the study and ‘up for a challenge’. One participant explained: ‘It was a lot more concentrated than I thought it would be, because I was going like five times a week for four weeks. But even that wasn’t a problem’. Participants were keen to keep their commitment particularly when they noticed a positive impact: ‘I thought it was my own advantage that I attend the appointment, so I didn’t find it inconvenient’. This commitment was maintained, in the case of LSVT LOUD, regardless of its intensity and frequency of appointments. Participants and in many cases their close family members organised themselves and found support around them in order to travel the distance to appointments four times a week. The commitment of participants was clear in the data, as this example shows: ‘The last meeting for that, we ended up parking in the wrong place and got a £40 fine for it because we couldn’t find anywhere to park. Even though the carpark would let us in, there was no spaces. So my wife was driving around while I went to the appointment’.
However, a number of participants admitted that their involvement had only been possible/feasible because they lived in close proximity to the hospital and, as they explained, if this hadn’t been the case they wouldn’t have been able to attend appointments. For example: ‘It worked because it’s only half a mile up the road from me. On my doorstep’.
Participants’ commitment was also reflected in their efforts at completing the paperwork as best as they could:
‘Oh no, got to do it again. But no, since I was going to see her every day, I didn’t want to let her down because I was on the course and everything. Although it felt like proper homework like at school, it’s still something that I wanted to do’. However, as this participant explains, it wasn’t always possible to complete it: ‘It took a lot of time to keep the – a lot of discipline – to keep the home side of it up. It’s tempting to leave it but there were occasions where I didn’t do my homework. I did notice a difference. It was really worth doing home-based therapies, but I had to be really strict about it’.
Experiencing change/impact
Trial interventions had an impact on study participants’ well-being. Participants who received a trial intervention reported noticing a difference in the way they were able to communicate with others. As one participant put it:
‘I’m speaking to neighbours now that I’ve been living beside for 14 years and that was from before and I was saying hello to them and they’re saying hello back. So I’ve been reaching out, taking advice from the therapist, and if I can manage then I must be doing something right’.
For those participants that, prior to the trial, had experienced a negative impact of PD on their speaking volume, the intervention had shown a greater, more noticeable impact. One participant explained: ‘In a nutshell, it’s made me normal again. So therapy has helped me with that for a different reason, but it has opened up a bit. But it’s hard to describe. It’s more than restoring self-confidence, it’s sort as if you’ve had something you haven’t had before, to communicate’.
Another participant explained: ‘Huge, huge change, actually. Made a very big difference. It was about two weeks that I started to notice that my voice felt a lot more strength and people could hear me a lot better than before’.
Participants reported how, as a result of the intervention they had been able to resume normal everyday activities such as speaking on the phone:
‘I speak better. If you’d have asked me 10 weeks ago, you wouldn’t have been able to understand me on the phone. The main therapy is speaking loud. I think I’m shouting most of the time but the girls said, “No, don’t worry. That’s correct. You’re not shouting. You’re speaking like I would speak normally.” So I found it very helpful, yeah, indeed’.
For others, the impact had been more subtle: ‘I can read something out, I produce quite good sound and pronunciation of the words, but then when I’m speaking off-the-cuff to people and in conversations, I can do it ok but I’m not as good at it as I used to be’.
This was also particularly true for those participants who didn’t consider that the PD had significantly impacted on the volume of their voice prior to the trial. As one participant explained:
‘Well, not terribly. I think I realised it did strengthen my voice a bit. I tried to keep up, as it were, in certain ways, with the exercises, because as she explained, it’s repetition building up, like doing any exercise. So I did notice perhaps an increase in volume when I was singing, maybe. But I don’t think I was having huge problems with it before. [Laughs] So it was slightly difficult to say, “Oh yes, that’s made a massive difference to me,” or not’.
Mapping of identified themes on to the normalisation process theory constructs
Once all themes from participants’ and therapists’ interviews were described in detail the research team mapped them on to the NPT framework74 and its five constructs (Table 10). This exercise revealed that for both, therapists and participants:
| NPT construct | PD COMM | |
|---|---|---|
| Therapists | Participants | |
|
Coherence
Does it make sense? How is it different from something else? Does everyone involved understand their roles and the changes they will need to make? |
The trial made sense to speech and language therapists: therapists being the ones ‘Wanting’ to take part in the trial and pushing for it within their service. Interventions not posing a ‘threat’ to speech and language therapists’ normal way of working – overall the trial did not ask therapists for significant changes to their normal routine. Speech and language therapists aware of the need to reach ‘equipoise’ – a balanced perspective on both interventions but balanced with the reality of their own working environments: therapists and the practice ‘driving one’ of the interventions depending on the value attributed to each of them – chance for ‘unconscious bias’. |
Taking part in research makes sense because it is in line with participants:
|
Speech and language therapists confident that both interventions were ‘different enough’:
|
||
|
Cognitive participation
Relational work Who is willing/or not to drive this? Who thinks it is the right thing to do/or not? |
Speech and language therapists being involved in the trial ‘just because’ their service is, ‘by default’ vs. therapists actively seeking support from line managers and service to be able to join the trial. It makes sense to be involved in research: therapists wanting to gain ‘research experience’ (they see this as a part of their role). Identifying the need for the trial: speech and language therapists keen to help produce robust evidence to back up the interventions they are already delivering. |
Participants reported a number of reasons for wanting to take part in the trial. Health benefits were not the only reason. Taking part in research was also seen as:
|
| Participants and their close family members (mainly spouses) motivated to take part in the trial regardless of the chance of being assigned to the control group. | ||
|
Collective action
How did (someone) make it happen? Who is doing/not doing what needs to be done to make it happen? What resources have been used to help get it done? |
Speech and language therapists with prior research experience (e.g. involvement in the pilot) ready to start vs. speech and language therapists with no research experience taking more time to ‘get going’. Therapists learning over time to deal with research tasks (e.g. trial admin). Speech and language therapists are able and confident to follow a prescriptive LSVT LOUD protocol vs. a more pragmatic and tailored NHS SLT intervention protocol. A whole team approach – each service team (e.g. therapists, research nurses, site PI) ‘planned ahead’ and discussed potential ‘capacity issues’ and set up clear communication pathways: speech and language therapists sharing LSVT LOUD delivery with a member of the team. Therapists and research nurses communicating in order to plan ahead of randomisation. |
Those participants randomised to an intervention arm have planned ahead of intervention delivery:
|
Versus challenges such as:
|
Participants felt supported by SLTs (e.g. help with home-based diaries and trial paperwork, community visits) and this increased their level of motivation/engagement in the trial. Participants supported by family members (mainly spouses) – great impact on adhering to the intensity of LSVT and completing outcome measures. |
|
Speech and language therapists felt well supported by trial team and this has helped the process:
|
||
|
Reflexive monitoring
Appraisal work, was it worth doing? How and who is noticing that it is or is not working? What has been the impact on therapists/patients and how do they feel about it? What would you have done differently? |
Speech and language therapists’ reflections on the trial process:
|
Overall participants were positive about having taken part in the trial and they felt it was a worthwhile experience. Participants understanding the randomisation process and accepting the trial arm they were assigned to vs. a number of participants disappointed with being assigned to the control arm. Participants who had more severe symptoms prior to taking part in the trial reporting an impact of the intervention (particularly LSVT) vs. participants with mild impact on their voice reporting less/no impact. |
Speech and language therapists' ‘pre-assumptions’ being challenged (e.g. what does the ‘ideal’ LSVT LOUD patient look like?):
|
Participants reflected on the fact that they were not sure to what level they had adhered to completing ‘home practice’ exercise in both of the intervention arms. Participants also reflected that the level of support (from SLTs and family members) to enhance home practice was, in some cases, low. |
|
| Speech and language therapists ‘seeing’ that what they are doing as part of their research role (delivering PD COMM interventions) works – witnessing impact on participants. | ||
-
the trial and its aims ‘made sense’ in its own right
-
that they were ready to ‘drive it’ and ‘make it happen’
-
that they considered it the ‘right thing to do’ in order to bring about a positive outcome for those people affected by PD, their therapists and their SLT services
-
that therapists and participants were able to identify their own and the trial’s limitations and how these could play a role in the final outcome of the study.
An explanatory framework and model of ‘equipoise’ for the PD COMM trial interventions
The analysis and final synthesis of all data from all sources show that, via carrying out this process evaluation, the research team has been able to understand the process by which therapists have been able to deliver and implement and PD COMM intervention and participants have received such interventions. Based on the data, we are confident that equipoise between both PD COMM interventions was reached; in other words, there are no data to suggest that such equipoise has been compromised (Figure 24).
FIGURE 24.
Model of equipoise between PD COMM interventions.

Throughout their involvement in PD COMM, speech and language therapists have successfully balanced the demands placed on them as both, therapists and researchers. They have also been able to reach agreement balancing their clinical decision-making versus their need to remain true to the PD COMM intervention protocol. This balancing act has been a direct result of the learning that has taken place over time.
Chapter 6 Content of Parkinson's disease COMM interventions: process evaluation
The PD COMM used active interventions: NHS SLT and LSVT LOUD as well as a no SLT control group. To replicate current clinical practice as closely as possible, the exact content of the SLT was not prescribed in detail for the trial and instead with the specific exclusion of LSVT LOUD approaches, local SLTs developed an intervention program that drew from a range of possible SLT approaches and techniques in response to each participant’s needs. In contrast, the LSVT LOUD intervention was more formally defined but also reflected the way in which this intervention was delivered in the NHS setting in the UK. In this chapter, we consider the nature and content of the two SLT approaches delivered by therapists within the PD COMM trial based on the treatment records provided by participants and therapists. We compared the reporting of the rationale for the therapy approaches, the materials and procedures used, the mode and location of delivery, tailoring and modifications.
At the point of therapy data analysis, we had access to 187 individual participants’ data (IPD) of which only 105 met our inclusion criteria; LSVT LOUD (n = 51) and NHS SLT (n = 54). We excluded participants’ data where SLT intervention was ongoing (n = 17), we lacked three or more TRFs (n = 19) or therapy notes (TNs) (n = 39). Thus, 105 (LSVT n = 51; standard NHS n = 54) therapy records were included in our therapy data extraction.
Methods of capturing PD COMM intervention information
Individual participant data (IPD) recorded by participants and therapists during the PD COMM trial was extracted from TRFs and TNs.
To enable comparison between NHS and LSVT LOUD interventions, individual participant therapy data were entered using the same headings for the TRFs and the TNs.
We developed extraction category headings iteratively and then applied them systematically. These headings were shaped by:
-
the TIDieR81 reporting checklist for complex interventions
-
the TRF therapy coding categories
-
inductive analysis of eight sets of Standard NHS therapy notes
-
LSVT training manual and proformas
-
background literature review
-
ongoing refinement of the categories continued as data extraction progressed.
Speech and language therapy treatment record forms
Speech and language therapists or assistants completed one TRF for each therapy session. They documented minutes spent undertaking broad categories of therapy activities.
Broad categories for intervention components (Table 11) were agreed and piloted36 prior to the development of the TRF. Categories reflected clinical dysarthria management guidelines from the Royal College of Speech and Language Therapists which recommend a combination of educational, physiological, compensatory and augmentative approaches within a framework of body function and structures (impairment), activity, participation and contextual factors.
| Assessment and review |
|---|
| Goal setting |
| Information provision (to the person or the caregiver) |
| Impairment exercises |
| Compensatory strategies |
| Alternative and augmentative communication strategies |
| Generalisation activities |
| Training of caregivers |
| LSVT |
| Indirect contact/liaison |
| Other |
Therapy notes
Speech and language therapists (or assistants) routinely completed TNs as part of their usual practice after each therapy session. Data extraction from TNs was conducted under the following categories:
-
why the therapy was being done (rationale, goals and delivery plans)
-
what materials and equipment were reported as being used (technologies, biofeedback, numerical measurement, named approaches)
-
what therapy processes were reported (targets, cues, vocal activities, practice structure)
-
tailoring (personal and interpersonal).
Meanwhile, TRFs recorded the number of sessions (with/without direct contact), nature of sessions delivered (assessment/intervention), the provider (SLT/assistant/supervised student), duration of therapy (first to last session), therapy location, mode of delivery, time allocation assessment, goal setting, information provision and therapy (impairment, compensatory, AAC, generalisation, training, LSVT), use of LSVT companion, other therapist activities relating to patient treatment (liaison, other).
Speech and language therapy intervention rationale and goals
Based on the subset of data reviewed, we found that almost all NHS SLT interventions had a rationale recorded from therapist’s and participant’s perspective. Documentation of a rationale in LSVT LOUD TNs was less likely from either perspective.
Therapy goals were commonly recorded in NHS treatment notes unlike LSVT LOUD treatment notes. Participant’s goals were recorded across both interventions [NHS SLT n = 22 (41%); LSVT LOUD n = 14; (27%)] and where these were unreported, therapist-led goals were recorded (outcomes, task or session based) more frequently in NHS LOUD® (n = 30; 56%) than in LSVT LOUD treatment notes (n = 7; 14%).
Materials used
Technologies
Use of exercise materials was reported for almost all participant intervention sessions for both the NHS SLT and the LSVT LOUD; sheets, lists, pictures for description, reading passages and participant’s own materials such as magazines or books. LSVT-specific materials such as LOUD card prompts and sheets from the manual are featured. 26 Reference to using telephones or video calls as part of intervention (whether in the session or as a home-based practice task) was made in 69% of LSVT LOUD but only 13% of NHS LOUD® notes.
The reported use of apps differed between the two therapy interventions (LSVT LOUD 37%; NHS SLT 17%), but the range of apps reported was more varied in NHS LOUD® (which uniquely featured Voice Meter Pro,82 DAF pro,83 Bla Bla Bla84 and a trial of ClaroCom85 as AAC). In contrast, 10/19 reported apps used in the LSVT LOUD interventions specified Voice Analyst,86 and one used an app to encourage vocal effort through creating background noise. Parkinson’s UK apps87 and Decibel 1088 were reported in both interventions.
Biofeedback
Where biofeedback was mentioned in therapy activities, it was difficult to extract where visual or auditory biofeedback was being used. Visual biofeedback was described in 86% (44/51) LSVT LOUD and 57% (31/54) NHS LOUD® interventions. Auditory biofeedback was reported in 41% (21/51) LSVT LOUD and 37% (20/51) NHS SLT interventions. Use of both visual and auditory biofeedback was reported in 37% (19/51) LSVT LOUD and 27% (14/54) NHS SLT interventions.
Numerical measurement
Numerical measurement was common in LSVT LOUD, whether from an object (such as a sound meter; 78%, n = 40/51) or Companion Software (16%; n = 8/51). This is compared with 52% (n = 28/54) of participants receiving NHS SLT using numerical measurement and two participants (4%) that used Companion Software. Use of self-rating scales was reported in 53% (n = 27/51) of LSVT LOUD and 39% (n = 21/54) of NHS SLT notes. Self-rating of effort is an expected component of LSVT interventions.
Intervention procedures
Intervention targets (speech subsystems)
A high proportion of LSVT LOUD interventions had one speech impairment target. Few had two. In contrast, half of NHS SLT interventions described a focus on three or more speech subsystems followed by two subsystems and the remaining interventions focused on one speech subsystem impairment target. The most common speech subsystems focused on within NHS SLT were phonation and breath support. Effectively all LSVT LOUD targeted one impairment target – phonation; one was ‘unreported’ due to limited participant therapy (Table 12).
| Intervention procedures | NHS SLT (n = 54) (%) | LSVT LOUD (n = 51) (%) |
|---|---|---|
| Targets | ||
| One speech subsystem | 19 | 86 |
| Two speech subsystems | 28 | 8 |
| Over two speech subsystems | 52 | |
| Phonation | 78 | 98 |
| Breath support | 67 | |
| Insight (calibration) | 76 | 92 |
| Strategies to modify the environment | 22 | 16 |
| Voice cues | ||
| ‘Loud’ or ‘Think Loud’ | 70 | 96 |
| ‘clear’ | 26 | – |
| ‘slow’ | 17 | – |
| referencing exaggerated movements | 28 | – |
| referencing volume | 22 | – |
| referencing chunking of words | 9 | – |
Other intervention targets
Therapists described activities to increase participants’ insight into what was different in their communication as a consequence of PD, why this would make it harder for other people to understand them, and what they need to do to address it. In LSVT interventions this re-training of sensory perceptions is referred to as calibration. Both NHS SLT and LSVT LOUD interventions had high numbers of therapy reports targeting improved insight, with LSVT LOUD higher than the NHS SLT intervention. Both interventions reported a similarly low-level use of strategies to modify the environment (e.g. by reducing background noise) or deliberate use of background noise to encourage participants to use their loud voice.
Voice and speech cues
‘Loud’ or ‘Think Loud’ was reported by almost all LSVT LOUD records and in most cases, was the only recorded cue. In comparison, although a majority of NHS SLT cues reported were also ‘Loud’ or ‘Think Loud’ there was more variation with another cue referencing volume such as ‘strong’ voice described. Other cues documented in NHS SLT included ‘clear’ referencing articulation; those that referenced exaggerated movements; and those that cued ‘slow’ or ‘chunking’ of speech into smaller groups of words with pauses.
Vocal activities
Vocal activities were reported and there were differences in the use of most of the activities mentioned between the SLT treatment groups (Table 13).
| Vocal activities | NHS SLT (n = 54) (%) | LSVT LOUD (n = 51) (%) |
|---|---|---|
| Non-speech oral motor exercise (NSOME) | 19a | < 1b |
| Automatic speech | 28 | 0 |
| Vocal play | 30 | 12 |
| C/V/CV | 69 | 98 |
| Functional phrases and hierarchical speech | – | 96 |
| Interaction | 22 | 67 |
| Functional-everyday phrases | 50 | – |
| Hierarchical activities | 83 | – |
Tailoring
Tailoring materials to a participant’s interests (such as scuba diving, cars, DIY, model railway, yoga) was described in 75% (n = 38/51) of LSVT LOUD interventions compared to 35% (n = 19/54) of NHS SLT. Tailoring to a client’s interactions was reported in 67% (n = 34/51) LSVT LOUD and 37% (n = 20/54) of NHS SLT. Descriptions of tailoring to participants’ communication goals, however (e.g. practising a planned lecture), were reported in 24% (n = 12/51) of LSVT LOUD interventions and 39% (n = 21/54) of NHS SLT. Although intensive therapeutic interventions can raise tolerance concerns, tailoring of NHS SLT interventions to the PD COMM participants’ health was documented in both NHS SLT (39%, n = 21/54) and LSVT LOUD records (33%, n = 17/51).
In LSVT LOUD, therapists are taught to model communication behaviours (‘do as I do’) and to avoid explanation. Use of modelling was reported in 73% (n = 37/51) of LSVT LOUD and 54% (n = 29/54) of NHS SLT records. Task titration or facilitating an augmentative response to a task was reported in both interventions (LSVT LOUD 96%, n = 49/51; NHS SLT 89%, n = 48/54). Advice and educational elements to therapy were more commonly reported in NHS SLT (89%, n = 48/54) than in LSVT LOUD (65%, n = 33/51). Therapists in both interventions used encouragement (LSVT LOUD 45%, n = 23/51; NHS SLT 50%, n = 27/54) and described involving others to provide feedback to the participant (LSVT LOUD 31%, n = 16/51; NHS SLT 31%, n = 17/54).
Home-based practice
Prescription of home-based practice was reported in almost all LSVT LOUD notes in our sample (98%, n = 50/51). Prescribed home-based practice was also reported in 98% (n = 53/54) of NHS SLT notes. Some NHS SLT home-based practice content was similar to LSVT LOUD: practise of ‘ahs’ (44%, n = 24/54), everyday (functional) phrases (52%, n = 28/54), hierarchical tasks (76%, n = 41/54) and applied (carryover) tasks (41%, n = 22/54). A LOUD or volume focus was reported in 67% (n = 36/54). The structure of the prescription also had similarities, with 87% (n = 47/54) advising daily practice and 91% (n = 49/54) prescribing exercises.
Chapter 7 Economic evaluation
Overview
The chapter reports the economic evaluation conducted alongside the PD-COMM clinical trial using data collected within the trial. The economic evaluation aimed to estimate the cost-effectiveness of LSVT LOUD with NHS SLT and no SLT (control group) in patients with PD who have dysarthria.
Methods
The NICE recommendations89 were used to guide the methods of the PD COMM trial economic evaluation and the analysis was reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines. 90
A within-trial economic evaluation in the form of cost–utility analysis was conducted from the perspective of the UK NHS. The primary results were expressed in terms of cost per quality-adjusted life-year (QALY) gained at 12 months for the three comparisons. Additional secondary analyses were performed from the NHS and personal social services (PSS) perspective, and broader societal perspective and using the capability approach utilising broader measures of capability well-being.
Outcome measures
The PD-COMM pilot study suggested that EQ-5D scores were related to voice impairment and would capture improvement in voicing/speech between trial arms. Therefore, this measurement was used for the economic evaluation. 91 Health-related quality of life (HRQoL) was measured using the EuroQol5D (5-level version) (EQ-5D-5L) completed by participants or their carer92 at baseline, 3, 6 and 12 months. The EQ-5D-5L questionnaire comprises five domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. Each domain consists of five levels ranging from no problems to extreme problems or unable to perform. 61 In line with NICE recommendations,92 the crosswalk value set was applied to participant responses for mapping the 5L responses to 3L preference-based summary scores. 93 Health utility values over 12-month follow-up were summarised by trial arm and assessment time point and QALY was generated for individual participant.
The ICECAP-O63 was used to capture changes in participant’s capability at 12 months, allowing a broader assessment of benefit in participants with PD that may not be captured with EQ-5D-5L. ICECAP-O is a preference-based measure of capability and well-being for older people over the age of 65, and consists of five attributes (attachment, security, role, enjoyment and control). 94 It is recommended by NICE in the economic evaluation of social care, has been used in several studies in the UK, and is a validated instrument to measure capability well-being. 95,96 Although the pilot study found that ICECAP-O was weakly correlated with voice measures, some PD-related QoL dimensions could be captured using this outcome measure and we felt it was important to capture wider aspects of well-being.
A set of tariff values has been elicited for ICECAP-O from a sample of older people in the UK using best-worst scaling. The scale ranges from one (a measure of 44444 reflecting a full capability) to zero (a measure of 11111 reflecting no capability). 63 These values are used to generate a capability score that is combined with time to generate a secondary outcome expressed as year of full capability (YFC). 97 To explore an alternative decision rule, the sufficient capability approach was used where a measure of ‘33333’ represents the threshold for maximum capability instead of ‘44444’. 98 Years of sufficient capability (YSCs) were generated from rescaled ICECAP-O utility scores to represent gains in sufficient capability over time. 98 Utility scores from EQ-5D-5L and capability scores from ICECAP-O were separately combined with time and analysed using the trapezium rule (the area under the curve approach), to generate QALYs, YFCs and YSCs at 12 months, respectively. 99
Resource use and costs
Trial interventions
All resource use involved in the delivery of the trial interventions was obtained from the SLT TRFs, where the number and duration of NHS SLT and LSVT LOUD sessions attended by each participant were recorded. Details on who conducted the session, location and type of the session (one to one/group) were also collected. Additional sessions that were recorded in the SLT Initial Interview Log and not in the SLT treatment log were also added.
Sessions were costed individually based on the duration and location assuming that all sessions were conducted one to one. Both PD COMM SLT interventions were mainly delivered by a therapist, therefore, a cost of a therapist time in minutes (average of band 6 and 7) was used for all sessions. The cost of time of a therapist was multiplied by duration of contact (in minutes) to determine the total cost of the intervention per patient for LSVT LOUD and NHS SLT. The duration of contact included the time spent for data management, preparation and delivering the intervention and these were considered in the cost of the intervention. However, the duration of PD COMM SLT content was also estimated to calculate the cost of SLT content and presented as a subcategory of cost. Where a session was conducted in the participant’s home, 60 minutes of travel time was added to the total session time. For LSVT LOUD remote sessions, the costs of a computer loan from the NHS and companion software were added, and NHS SLT remote sessions were assumed to have delivered over the phone. The cost of LSVT training for therapists per patient was estimated using national data from SLT service providers with assumptions (see Appendix 2, Table 36) and was also included in the cost of the intervention. Experts were consulted to confirm that the assumptions were appropriate.
Additional SLT sessions for speech and/or dysphagia (outside the trial interventions) reported by participants were recorded and costed as 60 minutes standard sessions by a therapist. Reported SLT sessions in the treatment log by participants randomised to the control arm who had NHS SLT were considered as additional sessions outside the trial intervention. To avoid double counting, therapist reported SLT sessions and SLT services from the follow-up questionnaire completed by participants were compared and trial sessions were deducted from total additional SLT services (primary or secondary).
NHS resource use/costs
Data on visits to primary care, therapists and other healthcare professionals, secondary care and social care services related to PD were collected using a patient-completed resource usage questionnaire at 3, 6 and 12 months, with 3- and 6-month questionnaires referring to the previous 3 months and the 12-month questionnaire, the previous 6 months. Data were collected on the number of primary care contacts such as the number of visits to GP practices, PD specialist nurses and health visitors in addition to secondary care (outpatient appointments) attendance. In addition, data on the use of other health services including visits to psychotherapists, occupational therapists, additional SLT for speech and/or dysphagia (other than the trial interventions) were also captured.
Details on PD medication were obtained at baseline and an average dose of medication was calculated and multiplied by the unit cost over a 12-month period. Given that SLT is not affected by medication, it was assumed that medication was not changed within the trial period. All health and social services were considered to occur within the NHS unless otherwise stated.
Unit costs were obtained from national sources such as Unit Costs of Health and Social Care, The British National Formulary and NHS reference costs. 100–102 Where different categories associated with a resource use were identified, weighted averages were used. Where participants recorded that a healthcare service was used without providing the number of contacts, a minimum number of contacts was assumed. For example, if a GP visit was reported without further data, it was assumed that there had been one GP visit. A micro-costing approach was used and assigned costs to each component of resource use to capture participant-level variation in costs. 103 Total costs were calculated for each patient and total mean per patient costs estimated by trial arm.
Social care costs
Data on resources related to use of social services, including home help, provision of meals and formal care were also reported. The average number of visits, days or meals per week was provided at each time point and the total number of services utilised over 12 months follow-up was calculated.
Productivity losses
The questionnaire also captured the loss of productivity due to reduced working hours as a result of PD. Questions asked if participants were in paid employment, and those who responded positively were requested to report the reduced number of working hours per week due to PD. These estimates were converted to total working hours over the 12-month period to calculate any loss of earnings associated with reduced productivity. Where a loss of working time was reported but actual hours were not given, the minimum reported number of working hours lost was imputed. Participants who were not currently employed were asked to report if they had completely stopped working due to PD during the recall period of the questionnaire (3 months or 6 months). These responses were used to generate the work loss at each follow-up point assuming that they worked 26 hours per week (average of full- and part-time job) as participants were not asked for data on their average working hours. Using the human capital approach104–106 the average wage for employees over the age of 60, obtained from annual earnings data106 was used to assign costs to the lost value of productivity.
Patient-incurred costs
Data on the use of private healthcare services and out-of-pocket expenditure on travel, medications and any other PD-related costs incurred by participants were also obtained from the questionnaire. In the absence of national unit cost estimates for private healthcare use, NHS equivalent costs were assumed to represent the costs of private health services. Where out-of-pocket costs were stated but not specified, the minimum amount of reported cost was imputed.
In the initial PD COMM SLT interview, participants were asked to provide information on the method of transportation and the number of miles travelled to attend PD COMM SLT sessions. The recorded miles were doubled to estimate the total distance travelled to attend a single session. This value was then combined with the number of attended outpatient sessions for each participant to calculate the total cost of travel in the trial. Unit costs for different methods of transportation were obtained from national sources (see Appendix 2, Table 37).
Costs were reported in UK currency [Great British pounds (£GBP)] for 2019–20. Cost estimates from earlier years were inflated, using the Hospital and Community Health Services Pay and Prices Index to 2019–20 prices. 100 Relevant items of health and social care resource use, employment and travel and out-of-pocket costs with their associated unit costs, description and the source from which these costs were obtained can be found in Appendix 2, Table 37.
Missing data
Participants who returned the follow-up questionnaire (resource use and effectiveness) at any follow-up time point were included in the base-case analysis. A multiple imputation approach was applied to replace missing values for costs and health and capability outcomes, under the assumption that the variables with missing values were Missing At Random (MAR). This approach replaces missing observations with a set of plausible predicted values, derived from a posterior predictive distribution of the missing data given the available data. 107 Twenty-five data sets were imputed (reflecting the percentage of missing data), which were then combined using Rubin’s rules. 108 Age, sex, severity of PD (Hoehn and Yahr) and severity of speech impairment (VHI) were included in the imputation models. Total costs over 12 months were imputed for different cost components, whereas missing values for the EQ-5D-5L and ICECAP-O were imputed at each time point.
For participants who were known to have died after the first follow-up visit, a value of zero at the next follow-up point was assigned to EQ-5D-5L and ICECAP-O scores based on the date of death. For participants who died, resource use and costs were given a value of zero for all subsequent follow-up time points. EQ-5D-5L and ICECAP-O overall scores were considered as missing if the participant failed to respond to all questionnaire items. For all participants, if they returned the resource usage questionnaire and a response to an individual item was not completed, it was considered that the service was not utilised.
To explore the impact of missing data on cost-effectiveness, a complete case analysis was carried out where only patients with complete data on outcome measures and costs were included.
Statistical analysis
Outcome measures, resource use and costs were presented as means and standard deviations (SDs) and mean bootstrapped differences between interventions with 95% CIs, where applicable. Due to cost data being positively skewed, 95% CIs around the mean difference in costs were calculated using the bias-corrected and accelerated (BCa) bootstrap method using the analyses of 1000 resamples. 109 If the CIs of the difference in mean costs or outcomes between treatment arms did not cross zero, this indicated a significant difference between treatment arms. 109
Differences between treatments for all outcomes and cost components were adjusted for age at randomisation, outcome and sex as well as severity of PD (Hoehn and Yahr) and severity of dysarthria (VHI). The baseline score (EQ-5D-5L and ICECAP-O) was also included in the adjustment for outcome measures and calculation of total QALYs, YFC and YSC. 110
All analyses were performed using Stata® version 16 (StataCorp LP, College Stations, TX, USA).
Economic evaluation
The first stage of the analysis involved reporting resource use, costs and health outcomes in a disaggregated manner by treatment arm. 103
The base-case analysis took the form of a cost–utility analysis (CUA) from the perspective of the UK NHS using total costs and QALYs for each trial arm of the trial. In line with the statistical analysis, economic analyses were conducted on an ITT basis. Differences between treatment arms in total costs were divided by differences in the outcome measure (QALYs) to calculate the incremental cost-effectiveness ratio (ICER), expressed as the additional cost per QALY gained. ICERs were generated for three comparisons: LSVT LOUD versus no SLT (control) NHS SLT versus no SLT (control); and LSVT LOUD versus NHS SLT. The NICE upper and lower thresholds of £30,000 and £20,000 per QALY were used to determine if an intervention was cost-effective. 89 Secondary analyses presenting the incremental cost per YFC and YSC calculated from ICECAP-O were also conducted. Given that trial involvement was for only 12 months, discounting was not applied to either the costs or outcomes. Further analyses were undertaken from the NHS/PSS perspective to include social care costs and a wider societal perspective to include the costs incurred by the participants and productivity losses.
Analysis of uncertainty
Bootstrapping was used to account for the overall uncertainty that occurs because of variations in sampling, by jointly bootstrapping mean cost and QALY differences. 111 The technique generated 5000 paired values of incremental costs and QALYs for the three treatment comparisons and were presented on a cost-effectiveness plane as a scatterplot to aid interpretation. 111,112 A cost-effectiveness acceptability curve (CEAC) was then constructed to reflect the probability that an intervention is cost-effective at different willingness-to-pay values per QALY gained. 113
Deterministic sensitivity analyses were conducted to explore the uncertainty in assumptions related to the delivery of the intervention and resource use. A series of different scenarios for LSVT LOUD delivery were explored by varying the location of session delivery (online or face to face), the staff grade of the session supervisor and supervised or unsupervised sessions. These scenarios were discussed with experts to agree on a typical scenario and sensible alternative options to reflect potential modes of delivery. Session delivery settings (outpatient or home delivery), level of session supervisors (therapist or therapy assistant), the method of delivery (face to face, online) and supervised or unsupervised sessions were varied using a combination of scenarios.
The following scenarios were explored:
-
All sessions are delivered by a therapist.
-
Scenario 1: 100% of session in an outpatient setting.
-
Scenario 2: 50% of the sessions are face to face in an outpatient setting and 50% as online sessions.
-
Scenario 3: 50% of the sessions are in an outpatient setting and 50% at participant’s home.
-
Scenario 4: 50% of the sessions are face to face at participant’s home and 50% as online sessions.
-
Scenario 5: 100% of sessions at participant’s home.
-
-
50% of the sessions are delivered by a therapist and 50% by a trained therapy assistant.
-
Scenario 6: 100% in an outpatient setting.
-
Scenario 7: 50% of the sessions are face to face in an outpatient setting and 50% as online sessions.
-
Scenario 8: 50% of the sessions are in an outpatient setting and 50% at participant’s home.
-
Scenario 9: All sessions delivered by a therapist where 50% of the sessions are face to face at participant’s home and 50% as online sessions.
-
Scenario 10: 100% at participant’s home.
-
-
50% of sessions are supervised and 50% are unsupervised.
-
Scenario 11: 50% of the sessions are in an outpatient setting and the remaining 50% unsupervised at participant’s home.
-
Scenario 12: 50% of the sessions are supervised at participant’s home and the remaining 50% unsupervised at participant’s home.
-
A single scenario for NHS SLT was used assuming that sessions were conducted in an outpatient setting. Online LSVT LOUD sessions and a mix of face-to-face and unsupervised LSVT LOUD sessions were assumed to be as effective as face-to-face sessions in terms of quality of life and speech outcomes. 114 Therefore, the QALY values for intervention groups from the trial data were held constant and used to calculate cost per QALY for each scenario. Further analyses were carried out. The first was related to adherence where only participants who were adherent to SLT interventions were included. The second assuming a vocal folds examination by an ENT consultant was undertaken for participants in the LVST LOUD arm before their first SLT initial session.
Results
Data completion
Complete EQ-5D-5L data at baseline, 3, 6 and 12 months were available for 273 participants (92 LSVT LOUD, 89 NHS SLT and 92 no SLT (control)) out of 388 participants recruited, and 270 participants [91 LSVT LOUD, 89 NHS SLT and 90 no SLT (control)] had completed ICECAP-O at all time points.
The SLT TRF was completed by therapists or therapy assistants for 107 (82%) participants in the LSVT LOUD group and 117 (91%) in the NHS SLT group. Resource use data at all time points were available for 93 participants in the LSVT LOUD arm, 92 in the NHS SLT arm and 92 in the no SLT (control) arm. A total of 116 (88% of recruited) participants in the LSVT LOUD arm returned follow-up questionnaires (on effectiveness and resource use) at any time point, 113 (88%) in the NHS SLT and 112 (87%) in the no SLT (control) groups and were included in the base-case analysis where missing values were imputed.
Of the 388 participants randomised in the trial, 253 (63%) had complete data on resource use, treatment, EQ-5D-5L and ICECAP-O. Details on data completion and proportion of returned forms/questionnaires are presented in Appendix 2, Table 38.
Outcome measures
Mean EQ-5D-5L and ICECAP-O scores per participant with complete outcome data for each trial arm at baseline, 3, 6 and 12 months are presented in Appendix 2, Table 39). At baseline, participants in the LSVT LOUD arm had higher EQ-5D-5L and ICECAP-O scores compared to those in the NHS SLT and no SLT (control) groups. On average, there was a reduction in EQ-5D-5L and ICECAP-O scores in the three trial arms over the 12-month follow-up period.
Total imputed and adjusted QALYs, YFC and YSC over 12 months were calculated and are presented in Table 14. In the base-case analysis LSVT LOUD was slightly more effective with an average 0.008 (95% CI, −0.020 to 0.040) QALYs gained compared with no SLT (control) and 0.012 (95% CI, −0.017 to 0.042) additional QALYs compared with NHS SLT. In terms of capability measures, LSVT LOUD was also associated with improved YFC and YSC compared with both no SLT (control) and NHS SLT (see Table 14). NHS SLT was less effective than no SLT (control) with 0.005 (95% CI, −0.039 to 0.026) fewer QALYs, and gave lower capability scores for YFC and YSC. Mean differences for all three comparisons were not statistically significant for all outcome measures except for YFC and YSC when comparing LSVT LOUD versus NHS SLT.
| Mean (SD) | Mean adjusteda bootstrapped difference (95% CI) | |||||
|---|---|---|---|---|---|---|
| LSVT LOUD | NHS SLT | No SLT (control) | LSVT LOUD vs. no SLT (control) | NHS SLT vs. no SLT (control) | LSVT LOUD vs. NHS SLT | |
| Base-case analysis – Imputedb | ||||||
| n | 116 | 113 | 112 | |||
| QALYs | 0.606 (0.205) | 0.583 (0.215) | 0.598 (0.193) | 0.008 (−0.020 to 0.040) | −0.005 (−0.039 to 0.026) | 0.012 (−0.017 to 0.042) |
| YFC | 0.800 (0.136) | 0.780 (0.126) | 0.797 (0.123) | 0.018 (−0.002 to 0.039) | −0.003 (−0.023 to 0.018) | 0.020 (0.003 to 0.036) |
| YSC | 0.889 (0.139) | 0.871 (0.131) | 0.891 (0.127) | 0.013 (−0.008, 0.035) | −0.006 (−0.020 to 0.018) | 0.018 (0.002 to 0.038) |
| Complete case analysisc | ||||||
| n | 82 | 81 | 90 | |||
| QALYs | 0.630 (0.197) | 0.607 (0.204) | 0.597 (0.195) | 0.019 (−0.015 to 0.051) | 0.004 (−0.031 to 0.040) | 0.014 (−0.020 to 0.048) |
| YFC | 0.821 (0.120) | 0.794 (0.123) | 0.795 (0.124) | 0.030 (0.007 to 0.054) | 0.006 (−0.018 to 0.028) | 0.023 (0.000 to 0.042) |
| YSC | 0.912 (0.120) | 0.887 (0.127) | 0.889 (0.128) | 0.026 (0.001 to 0.053) | 0.004 (−0.022 to 0.029) | 0.021 (−0.002 to 0.043) |
In the complete case analysis, the mean difference in QALY, YFC and YSC between LSVT LOUD and no SLT (control) was much greater, and NHS SLT was associated with improved QALYs, YFC and YSC compared with no SLT (control).
Resource use and costs
Trial interventions
Table 15 presents the mean resource use and costs for trial interventions per participant including therapist time and travel time with additional costs including phone calls, LSVT training, companion software and laptop loan. The average cost of LSVT LOUD was £1315.55 (SD, 570.47) and £412.06 (SD, 275.01) for NHS SLT. Total session time was higher in LSVT LOUD group (1232.70 minutes compared with 412.31 minutes in the NHS SLT arm) and contributed to almost 82%–88% of the total cost of the SLT interventions. Very few participants used the LSVT companion software. The average number of SLT sessions was 16 for LSVT LOUD and 6 for NHS SLT.
| LSVT LOUD (n = 107) | NHS SLT (n = 118) | |||||
|---|---|---|---|---|---|---|
| Resource unit, mean (SD) | Cost (£), mean (SD) | Resource unit, mean (SD) | Cost (£), mean (SD) | No SLT (control) | ||
| Trial session and intervention set up | ||||||
| SLT therapist time (minutes) | ||||||
| Total session time | 1232.70 (449.61) | 1084.87 (395.65) | 412.31 (239.75) | 362.83 (210.98) | – | – |
| SLT contenta | 963.76 (330.85) | 848.11 (291.15) | 306.50 (178.68) | 269.72 (157.24) | ||
| Travel time | 235.51 (367.07) | 207.25 (323.02) | 54.41 (128.90) | 47.88 (113.43) | – | – |
| Phone call (minutes) | 0 | 0 | 19.41 (76.64) | 1.36 (5.36) | – | – |
| LSVT training | – | 21 | 0 | – | – | |
| Companion software | – | 0.45 (2.65) | 0 | – | – | |
| Computer loan | – | 2.07 (12.27) | 0 | – | – | |
| Total cost of trial intervention | 1315.55 (570.47) | 412.06 (275.01) | ||||
| Non-trial additional SLT sessions – (60 minutes per session) | ||||||
| N | 93 | 92 | 92 | |||
| Mean (SD)b | 2.53 (6.45) | 133.92 (341.66) | 2.01 (3.07) | 106.83 (162.83) | 0.90 (2.72) | 47.81 (144.25) |
The data from the treatment log showed that the majority of therapy sessions took place in an outpatient setting (73% in LSVT LOUD group vs. 77% in NHS SLT group). Almost 94% of LSVT LOUD sessions and 92% of NHS SLT sessions were supervised by a therapist. Only 26% of LSVT LOUD sessions took place in participants’ homes (6% of these sessions were unsupervised) compared with 16% of NHS SLT sessions. Remote sessions contributed to 1% of LSVT LOUD sessions and 6% of NHS SLT sessions.
Participants in both LSVT LOUD and NHS SLT arms required non-trial additional SLT sessions (an average of 2.53 per participant for LSVT and 2.01 for NHS SLT) with a small number of sessions reported by those in the no SLT (control) arm (average 0.90). These additional sessions were in part due to outliers, where 7 LSVT LOUD participants had attended at least 10 additional sessions. The clinical reason for these additional sessions (e.g. speech or voice problems, dysphagia) was not recorded.
National Health Service and social services resource use
Disaggregated mean resource use and costs per participant for those with complete resource use data over 12 months are presented by treatment group in Table 16. The level of resource utilisation was similar across treatment arms. The main cost drivers of primary care and therapy services were GP practice visits and PD nurse and physiotherapy appointments. PD-related outpatient appointments and consultations were comparatively more frequent in the NHS SLT arm compared to LSVT LOUD and no SLT (control) arms.
| Resource item | LSVT LOUD n = 93 |
NHS SLT n = 92 |
No SLT (control) n = 92 |
|||
|---|---|---|---|---|---|---|
| Mean visits (SD) | Mean cost £ (SD) | Mean visits (SD) | Mean cost £ (SD) | Mean visits (SD) | Mean cost £ (SD) | |
| NHS | ||||||
| Primary and community services | ||||||
| GP home | 0.09 (0.38) | 8.65 (38.27) | 0.16 (0.76) | 16.40 (76.45) | 0.07 (0.44) | 6.56 (44.25) |
| GP practice | 2.96 (3.93) | 115.32 (153.21) | 2.82 (3.01) | 109.79 (117.35) | 2.73 (2.64) | 106.40 (102.80) |
| Nurse Home | 0.28 (1.80) | 5.87 (37.71) | 0.41 (1.42) | 8.67 (29.89) | 0.14 (0.51) | 2.97 (10.59) |
| Nurse practice | 1.37 (2.48) | 14.82 (26.85) | 1.51 (2.38) | 16.39 (25.86) | 1.16 (1.57) | 12.62 (17.04) |
| PD nurse | 2.48 (2.56) | 69.55 (71.82) | 2.16 (2.10) | 60.57 (58.74) | 2.05 (2.10) | 57.52 (58.46) |
| Health visitor | 0.15 (0.74) | 2.86 (13.99) | 0.18 (0.82) | 3.51 (15.67) | 0.065 (0.32) | 1.24 (6.17) |
| Therapists and other professional staff | ||||||
| Physiotherapist | 2.31 (4.41) | 122.53 (233.52) | 2.14 (3.38) | 113.49 (178.93) | 2.33 (5.44) | 123.28 (288.49) |
| Occupational therapist | 0.71 (2.18) | 37.61 (115.53) | 0.64 (1.61) | 33.99 (85.56) | 0.50 (1.35) | 26.50 (71.37) |
| Other servicesa | 0.27 (0.82) | 13.73 (36.38) | 0.50 (1.75) | 22.22 (78.83) | 0.22 (0.97) | 8.49 (29.76) |
| Secondary care | ||||||
| Outpatient appointment | 2.04 (2.20) | 337.21 (321.49) | 2.30 (2.78) | 391.43 (442.75) | 1.87 (1.80) | 339.69 (331.81) |
| Social services | ||||||
| Home care/home help (an hour visit)b | 20.41 (97.04) | 816.34 (3881.79) | 9.18 (29.89) | 367.39 (1195.61) | 7.21 (30.00) | 288.26 (1200.14) |
| Day centre (days) | 2.10 (16.63) | 127.90 (1014.53) | 2.26 (14.24) | 137.91 (868.76) | 3.25 (16.42) | 198.25 (1001.87) |
| Luncheon Club (meal) | 1.26 (7.19) | 8.81 (50.32) | 2.83 (13.19) | 19.78 (92.32) | 0.85 (5.72) | 5.93 (40.03) |
| Sitting service (days) | – | – | 0.42 (4.07) | 50.87 (487.92) | – | – |
| Institutional care (days)c | 1.94 (18.67) | 342.58 (3303.73) | 0.98 (9.38) | 749.11 (2240.57) | – | – |
Social services resource use was reported by 20 (17%) LSVT LOUD, 17 (15%) NHS SLT and 16 (14%) no SLT (control) participants. Use of social services resources was much greater per person in the LSVT LOUD arm compared to NHS SLT and no SLT (control) arms and home help was the main cost component due to the presence of a small number of outliers. An observation was considered to be an outlier where the patient had over 120 visits over the 12-month period. Given that SLT interventions are highly unlikely to have any impact on physical functioning, normal daily activities or the requirement for institutional care, a decision was made to deviate from the original protocol and to exclude social services costs for the base-case analysis. Therefore, the base case was presented for the NHS perspective.
The results indicated that all arms had large standard deviations for the costs, particularly in the LSVT LOUD arm, driven by the skewed cost data caused by outliers.
Private health services
Privately funded health care and social services were required by a small number of participants in all treatment arms (see Table 40). Participants in the LSVT LOUD arm required more frequent physiotherapy sessions and institutional care compared with those in NHS SLT and no SLT (control) arms.
Patient-incurred costs and productivity losses
Details on the mean PD-related out-of-pocket expenses and loss of earnings as a result of reduced productivity are presented in Appendix 2, Table 40. Other PD-related expenses were greatest in the in the no SLT (control) arm. Travel costs obtained from the initial SLT interview form were higher in the LSVT LOUD arm, directly related to the greater number of sessions required for delivery of LSVT LOUD compared with NHS SLT. These direct SLT travel costs were included in the total societal cost. General travel costs were also reported in patient questionnaires which included both travel to SLT appointments and visits not specifically related to the intervention. As there was a high likelihood of double counting of SLT travel costs, these are presented as part of the disaggregated costs (see Appendix 2, Table 40) but not included in final total societal costs.
Very few participants were in paid employment across the treatment arms, reflecting the age of participants and the presence of PD. Overall, participants in the LSVT LOUD arm had reduced their working hours more than those in the other treatment arms and more participants receiving the NHS SLT intervention had to stop work completely (see Appendix 2, Table 40).
Total costs
The mean total costs per participant for different cost categories across treatment arms are presented in Table 17. The greatest costs were PD medications, the cost of the SLT interventions and visits to primary care services and therapists. The cost of medication was highest for participants in the LSVT LOUD arm, who also had a higher mean baseline severity of PD. The aggregated cost of primary care, therapist and secondary care services were similar across treatment arms.
| LSVT LOUD | NHS SLT | No SLT (control) | LSVT LOUD vs. no SLT (control) | NHS SLT vs. no SLT (control) | LSVT LOUD vs. NHS SLT | |
|---|---|---|---|---|---|---|
| Cost (£), mean (SD) | Mean adjusteda bootstrapped difference (£) (95% CI) | |||||
| Base-case analysis – Imputeda | ||||||
| n | 116 | 113 | 112 | |||
| Healthcare costs | ||||||
| Trial intervention | 1341.47 (521.90) | 414.71 (264.61) | - | 1342.99 (1253.08 to 1433.07) | 414.95 (367.90 to 467.42) | 924.21 (824.39 to 1032.84) |
| Additional SLT sessions | 133.64 (306.99) | 105.34 (148.67) | 52.38 (132.53) | 83.08 (31.33 to 146.06) | 53.86 (16.25 to 86.20) | 28.77 (−26.41 to 96.23) |
| Primary and community care and therapists | 389.62 (307.96) | 383.90 (341.78) | 348.26 (335.70) | 44.18 (−39.66 to 131.36) | 44.56 (−52.35 to 119.73) | 2.43 (−80.33 to 80.19) |
| Secondary care | 340.19 (290.19) | 391.54 (402.64) | 338.79 (302.61) | 5.45 (−76.45 to 87.62) | 64.31 (−24.76 to 174.27) | −55.60 (−148.10 to 31.27) |
| Medications | 1402.71 (874.93) | 1383.11 (855.03) | 1290.21 (809.13) | 106.48 (−114.84 to 319.94) | 75.07 (−133.76 to 291.63) | 24.38 (−188.58 to 232.85) |
| Total NHS costs | 3607.63 (1165.01) | 2677.60 (1157.76) | 2029.65 (1026.04) | 1582.183 (1325.21 to 1892.23) | 652.76 (381.69 to 934.26) | 924.20 (641.08 to 1219.78) |
| Social servicesb | 1183.57 (4660.48) | 697.57 (2027.56) | 486.52 (2013.38) | 795.92 (−20.57 to 1874.39) | 247.18 (−138.84 to 712.22) | 412.45 (−331.91 to 1394.22) |
| Total NHS/PSS costs | 4791.36 (5067.15) | 3375.17 (2409.37) | 2516.17 (2013.38) | 2378.10 (1554.54 to 3586.44) | 899.93 (386.37 to 1435.69) | 1336.65 (426.94 to 2411.46) |
| Wider costs | ||||||
| Productivity cost | 794.88 (2655.16) | 1932.68 (4347.68) | 1318.25 (3662.85) | −731.16 (−1559.11 to -55.95) | 262.85 (−689.36 to 115.25) | −1038.44 (−1909.89 to -280.69) |
| Private health services | 125.41 (407.07) | 101.84 (305.51) | 71.03 (210.71) | 56.54 (−18.73 to 160.03) | 102.05 (−834.08 to 1036.32) | 22.62 (−61.35 to 124.12) |
| Out-of-pocket expenditurec | 430.79 (1315.01) | 246.07 (564.69) | 468.90 (1572.24) | −41.16 (−436.77 to 366.21) | −218.03 (−618.77 to 47.45) | 175.06 (−41.96 to 480.49) |
| Travel costd | 57.94 (140.69) | 24.31 (63.73) | 0 | 55.96 (39.51 to 86.45) | 24.31 (15.57 to 39.44) | 33.98 (14.49 to 73.74) |
| Total societal costs | 5016.09 (3144.11) | 4980.73 (4675.37) | 3887.04 (4093.56) | 922.56 (−26.65 to 1788.62) | 754.03 (−251.52 to 1757.57) | 118.67 (−834.29 to 1022.71) |
| Total societal/PPS costs | 6199.67 (6072.71) | 5678.30 (4990.53) | 4373.56 (4564.23) | 1718.48 (490.00 to 3403.83) | 1001.20 (−183.49 to 2120.68) | 531.12 (−555.82 to 1927.70) |
| Complete case analysis | ||||||
| n | 82 | 81 | 90 | |||
| Trial intervention | 1393.61 (548.82) | 423.91 (274.67) | - | 1390.00 (1272.63 to 1514.59) | 424.07 (363.50 to 488.71) | 962.11 (842.74 to 1095.28) |
| Additional SLT sessions | 124.10 (290.80) | 104.04 (162.37) | 48.88 (145.68) | 74.19 (14.68 to 154.62) | 52.94 (5.13 to 95.76) | 21.06 (−48.01 to 91.85) |
| Primary and community care and therapists | 378.20 (348.09) | 361.70 (354.09) | 352.08 (369.83) | 21.48 (−87.28 to 124.54) | 25.23 (−79.19 to 140.10) | 6.14 (−105.20 to 109.39) |
| Secondary care | 341.70 (333.17) | 392.34 (454.89) | 343.08 (334.72) | −6.04 (−103.08 to 84.39) | 63.68 (−63.47 to 178.22) | −61.76 (−190.94 to 63.11) |
| Medications | 1297.93 (783.07) | 1317.15 (808.65) | 1296.98 (817.48) | 7.72 (−227.37 to 257.08) | 26.70 (−196.21 to 270.90) | −13.49 (−248.35 to 244.19) |
| Total NHS costs | 3535.55 (1024.37) | 2599.13 (1137.22) | 2026.61 (1093.30) | 1487.34 (1175.47 to 1823.01) | 592.62 (300.05 to 919.79) | 914.06 (591.50 to 1208.67) |
In terms of mean cost differences by category of resource use (see Table 17), the greatest differences were for the LSVT LOUD and NHS SLT interventions, both compared with each other and the no SLT (control) arm, and differences were statistically significant. Differences in the cost of additional SLT sessions were also statistically significant for both SLT interventions versus no SLT (control) but not when LSVT LOUD and NHS SLT were compared.
The mean total NHS cost differences indicated that LSVT was associated with additional costs of £1582.18 (95% CI, £1325.21 to £1892.23) and £652.76 (95% CI, £381.69 to £934.26) compared with no SLT (control) and NHS SLT, respectively. NHS SLT was £924.20 (95% CI, £641.08 to £1219.78) more expensive than no SLT (control). Differences in total costs were statistically significant. There were only small differences between trial arms for primary care services, therapists, secondary care services and medication. The NHS/PSS cost difference between LSVT LOUD and the other trial arms was much greater due to high social services costs reported by a small number of participants in the LSVT LOUD arm.
When considering the wider societal perspective with PSS costs, the difference in costs between LSVT LOUD and no SLT (control) was lower, and LSVT LOUD was associated with an additional cost of £922.56 (95% CI, £−26.65 to £1788.62). Compared with NHS SLT, LSVT LOUD had slightly higher costs of £118.67 (95% CI, £−834.29 to £1022.71). The difference in costs between NHS SLT and no SLT (control) was £754.03 (95% CI, £−251.52 to £1757.57). All differences were not statistically significant except for the difference between LSVT LOUD and no SLT (control). The reduced cost difference when the societal perspective was adopted was mainly related to the inclusion of productivity costs. These cost differences increased when social services costs were also included.
Economic evaluation
Base-case analysis
Results of the base-case analyses from the perspective of the UK NHS are presented in Table 18. The results suggest that LSVT LOUD was associated with an ICER of £197,772 per QALY gained and £77,017 per QALY gained compared to no SLT (control) and NHS SLT, respectively. Using the capability outcome measure, the cost of achieving an additional YFC for the LSVT LOUD arm was £87,899 compared with no SLT (control) and £46,210 compared with NHS SLT. The ICERs using the YSC as the measure of outcome were £121,706 and £51,344 per YSC gained for LSVT LOUD versus no SLT (control) and NHS SLT, respectively. For all three outcomes, standard NHS SLT was less effective and more costly than no SLT (control) and is therefore by definition ‘dominated’ by no SLT (control) and was not cost-effective.
| Incremental cost (£) Mean (95% CI) |
Incremental effectiveness Mean (95% CI) |
ICER Cost (£) per outcome |
|
|---|---|---|---|
| Primary outcome – QALY gained | |||
| LSVT vs. no SLT (control) | 1582.18 (1325.21 to 1892.23) | 0.008 (−0.020 to 0.040) | 197,772 |
| NHS SLT vs. no SLT (control) | 652.76 (381.69 to 934.26) | −0.005 (−0.039 to 0.026) | Dominated |
| LSVT vs. NHS SLT | 924.20 (641.08 to 1219.78) | 0.012 (−0.017 to 0.042) | 77,017 |
| Secondary outcome – YFC gained | |||
| LSVT vs. no SLT (control) | 1582.18 (1325.21 to 1892.23) | 0.018 (−0.002 to 0.039) | 87,899 |
| NHS SLT vs. no SLT (control) | 652.76 (381.69, 934.26) | −0.003 (−0.023 to 0.018) | Dominated |
| LSVT vs. NHS SLT | 924.20 (641.08 to 1219.78) | 0.020 (0.003 to 0.036) | 46,210 |
| Secondary outcome – YSC gained | |||
| LSVT vs. no SLT (control) | 1582.18 (1325.21 to 1892.23) | 0.013 (−0.008 to 0.035) | 121,706 |
| NHS SLT vs. no SLT (control) | 652.76 (381.69 to 934.26) | −0.006 (−0.020 to 0.018) | Dominated |
| LSVT vs. NHS SLT | 924.20 (641.08 to 1219.78) | 0.018 (0.002 to 0.038) | 51,344 |
The distribution of bootstrapped incremental costs and incremental effects (QALYs) presented on the cost-effectiveness planes supported the base-case results. The replications for LSVT LOUD versus no SLT (control) were in the north-west and north-east quadrants (Figure 25) showing that LSVT LOUD was more costly, with great uncertainty around the effectiveness (either more effective or marginally less effective). Similarly, compared with NHS SLT, LSVT LOUD was also more expensive and associated with both marginally more and fewer QALYs as illustrated in Figure 26. Estimates of incremental costs and QALYs between NHS SLT and no SLT (control) were also located in the northeast and northwest quadrants as demonstrated in Figure 27, with more points showing fewer QALYs. Overall, the wide spread of the replications with outliers reveals the degree of uncertainty around the effectiveness results.
FIGURE 25.
Scatterplot on the cost-effectiveness plane representing 5000 estimates of incremental costs and QALYs for the base-case analysis (NHS perspective) comparing LSVT vs. no SLT (control).

FIGURE 26.
Scatterplot on the cost-effectiveness plane representing 5000 estimates of incremental costs and QALYs for the base-case analysis (NHS perspective) comparing LSVT vs. NHS SLT.

FIGURE 27.
Scatterplot on the cost-effectiveness plane representing 5000 estimates of incremental costs and QALYs for the base-case analysis (NHS perspective) comparing NHS SLT vs. no SLT (control).
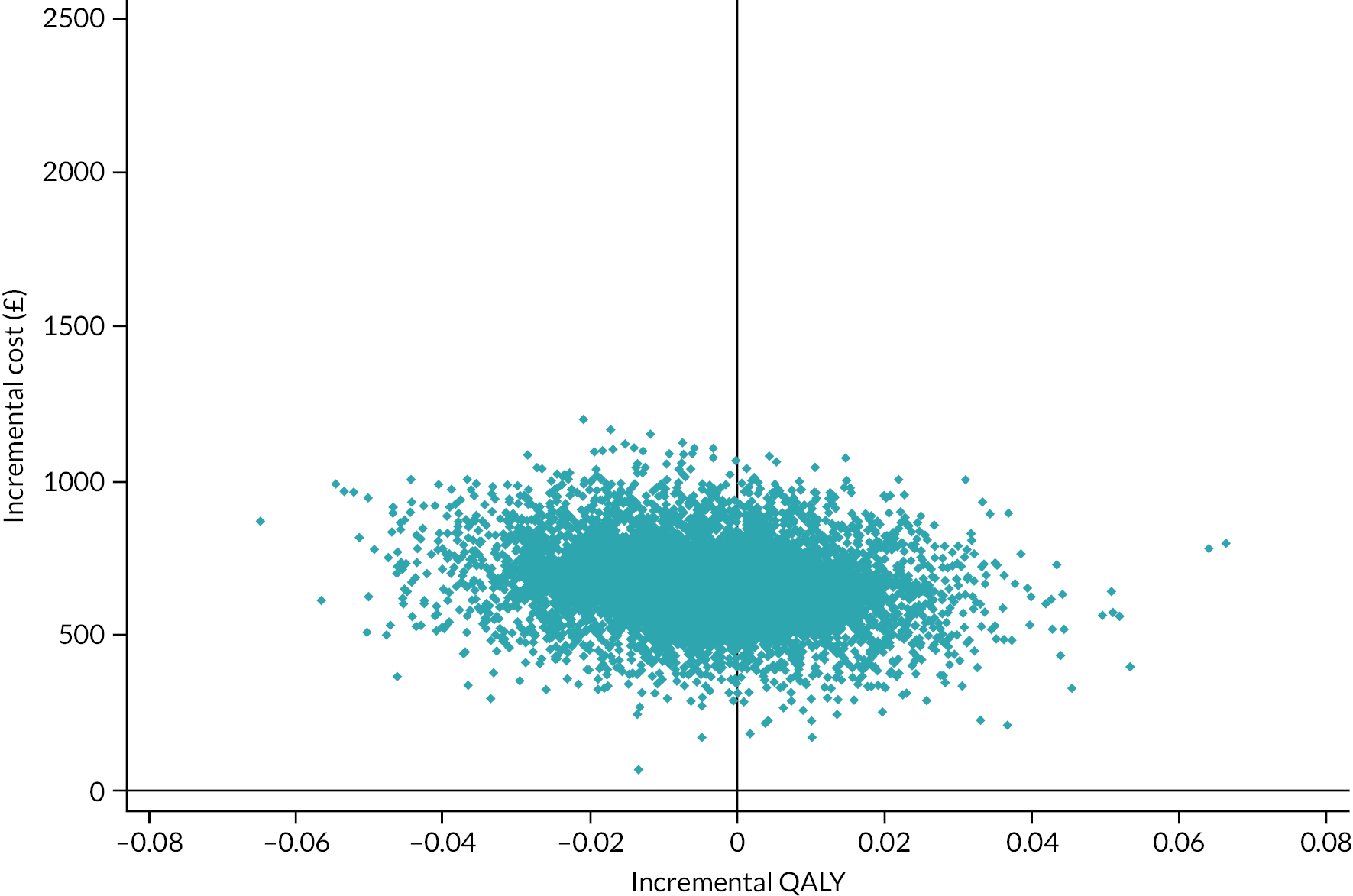
At NICE’s threshold of £20,000 per QALY, the CEACs show that the probability of LSVT LOUD being cost-effective was 0% and 3% compared to no SLT (control) NHS SLT, respectively (Figure 28). The probability of NHS SLT being cost-effective versus no SLT (control) was 2% (Figure 29). Considering the higher willingness-to-pay threshold of £30,000 per QALY, LSVT still had a low probability being cost-effective compared with NHS SLT and no SLT (control), with a probability of being cost-effective of 14% compared when compared with NHS SLT (Figure 30).
FIGURE 28.
Cost-effectiveness acceptability curve (CEAC) showing the probability of LSVT LOUD being cost-effective compared to no SLT (control) for the base-case analysis (NHS perspective).

FIGURE 29.
Cost-effectiveness acceptability curve (CEAC) showing the probability of NHS SLT being cost-effective compared to no SLT (control) for the base-case analysis (NHS perspective).
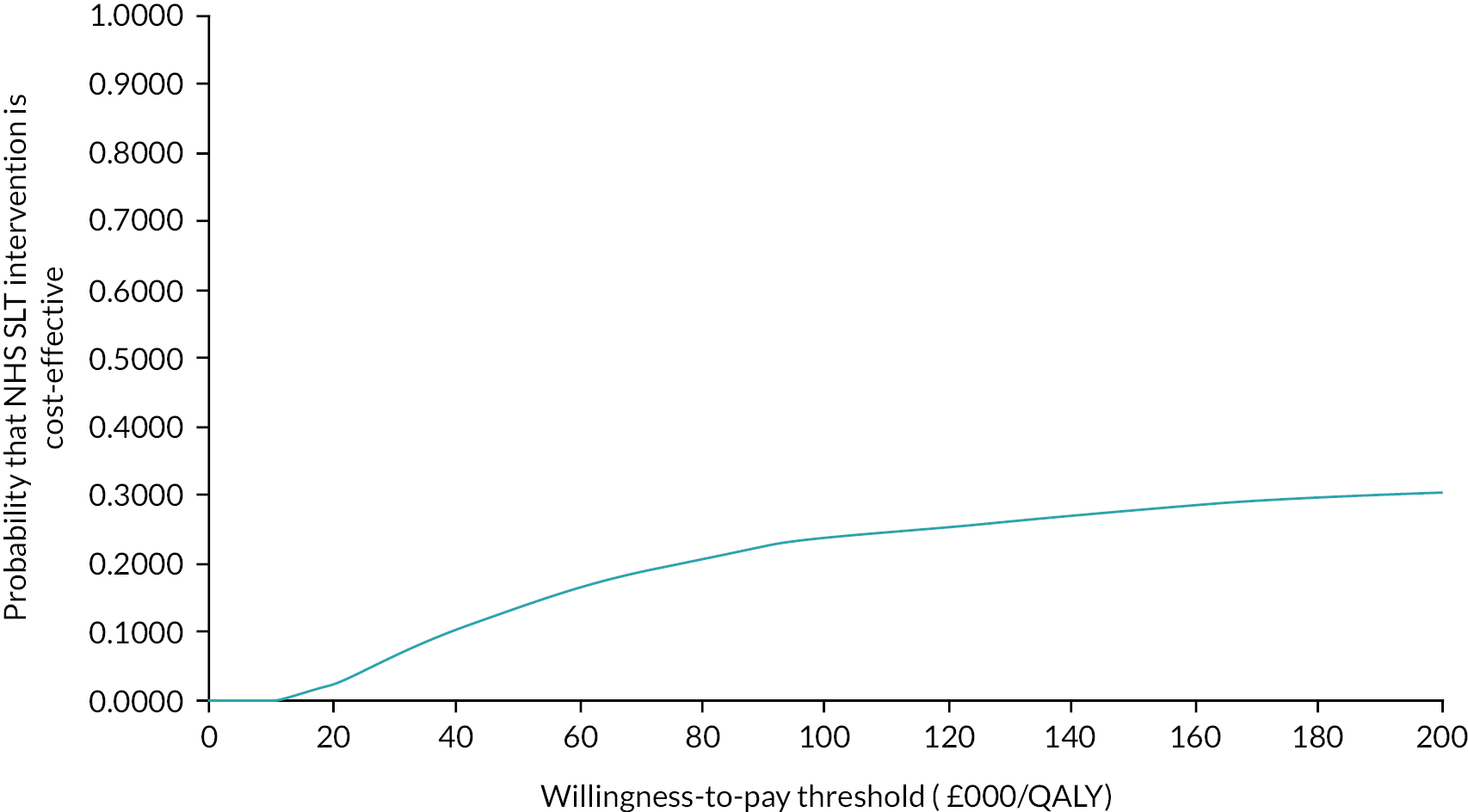
FIGURE 30.
Cost-effectiveness acceptability curve (CEAC) showing the probability of LSVT LOUD being cost-effective compared to NHS SLT for the base-case analysis (NHS perspective).
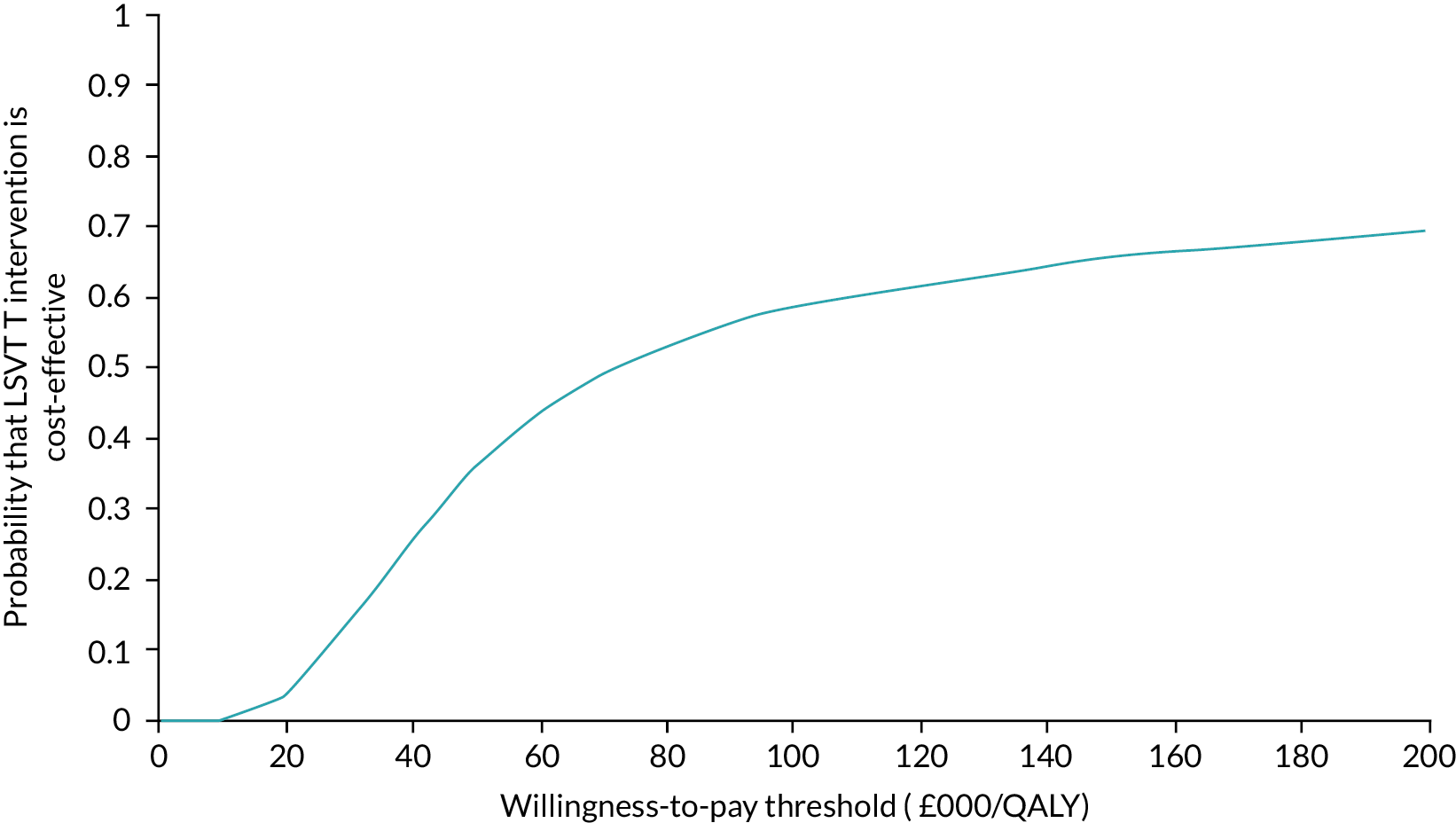
Sensitivity analysis
A number of sensitivity analyses were conducted to explore the impact of including social care costs, considering the wider societal perspective (with and without social care costs), missing data, adding the cost of ENT examination at the beginning of the treatment and participant adherence with the SLT protocol. Almost all the findings supported the results from the results of base-case analysis (see Appendix 2, Table 41). For the societal perspective (with and without social care costs) LSVT LOUD was also not cost-effective when compared to no SLT (control) if the society’s willingness to pay was £20,000 per QALY. However, when excluding social care costs, for LSVT LOUD versus NHS SLT the ICER was £9899 per QALY, suggesting that LSVT LOUD may be cost-effective. This result was due to the lower productivity costs in the LSVT LOUD arm. For both productivity costs and social services costs, the results were influenced by outliers from a very small number of participants. These costs are unlikely to be related to the intervention and therefore these estimates should be interpreted with caution.
The inclusion of only patients with complete data on all NHS costs and outcomes reduced the ICER to £78,281 per QALY for LSVT LOUD versus no SLT (control) £65,290 per QALY versus NHS SLT. Participant adherence resulted in greater QALY gains for the LSVT LOUD arm and a reduced ICER for LSVT LOUD versus NHS SLT of £32,291 per QALY.
Scenario analyses
Alternative scenarios were explored for the delivery of LSVT LOUD sessions. The cost of LSVT LOUD from each scenario was used to replace the trial LSVT LOUD cost and added to the other healthcare costs to generate the total cost per participant from the perspective of the NHS. The results of the scenario analysis are presented in Table 19 and show the new cost of delivering LSVT LOUD, the mean differences in costs and QALYs and the ICERs for each scenario. The analysis demonstrated that the least expensive scenario for LSVT LOUD delivery was a combination of supervised and unsupervised sessions. This scenario reduced the ICER to £27,808 per QALY compared with NHS SLT. If all sessions were supervised, conducting all sessions in an outpatient setting was the least costly method to deliver LSVT LOUD where sessions delivered by a mix of therapists and therapy assistants less costly than all sessions conducted by a therapist.
| Scenario | Cost of LSVT LOUDa intervention (£) | Comparisons | Incremental total cost (£) Mean (95% CI) |
Incremental QALY Mean (95% CI) |
ICER Cost (£) per QALY |
| Session’s supervisor: therapist | |||||
| 16 outpatient sessions | 866 | LSVT LOUD vs. no SLT (control) | 1101.49 (837.89 to 1359.77) | 0.008 (−0.020 to 0.040) | 137,686 |
| NHS SLT vs. no SLT (control) | 433.72 (163.45 to 732.84) | −0.005 (−0.039 to 0.026) | Dominated | ||
| LSVT LOUD vs. NHS SLT | 666.10 (389.26 to 957.29) | 0.012 (−0.017 to 0.042) | 55,508 | ||
| 8 outpatient sessions + 8 online sessions | 956 | LSVT LOUD vs. no SLT (control) | 1191.49 (932.58 to 1460.59) | 0.008 (−0.020 to 0.040) | 148,936 |
| LSVT LOUD vs. NHS SLT | 756.10 (461.96 to 1077.71) | 0.012 (−0.017 to 0.042) | 63,008 | ||
| 8 outpatient sessions + 8 participant’s home sessions | 1288 | LSVT LOUD vs. no SLT (control) | 1523.89 (1251.72 to 1783.23) | 0.008 (−0.020 to 0.040) | 190,486 |
| LSVT LOUD vs. NHS SLT | 1088.50 (798.83 to 1381.26) | 0.012 (−0.017 to 0.042) | 90,708 | ||
| 8 participant’s home + 8 online sessions | 1378 | LSVT LOUD vs. no SLT (control) | 1613.89 (1346.62 to 1918.38) | 0.008 (−0.020 to 0.040) | 201,736 |
| LSVT LOUD vs. NHS SLT | 1178.50 (881.47 to 1449.14) | 0.012 (−0.017 to 0.042) | 98,208 | ||
| 16 participant’s home sessions | 1711 | LSVT LOUD vs. no SLT (control) | 1946.29 (1666.39 to 2215.93) | 0.008 (−0.020 to 0.040) | 243,286 |
| LSVT LOUD vs. NHS SLT | 1510.90 (1249.65 to 1803.25) | 0.012 (−0.017 to 0.042) | 125,908 | ||
| Session’s supervisor: therapist + therapy assistant | |||||
| 16 outpatient sessions | 693 | LSVT LOUD vs. no SLT (control) | 928.69 (659.66 to 1185.23) | 0.008 (−0.020 to 0.040) | 116,086 |
| LSVT LOUD vs. NHS SLT | 493.30 (213.68 to 778.66) | 0.012 (−0.017 to 0.042) | 41,108 | ||
| 8 outpatient sessions + 8 online sessions | 783 | LSVT LOUD vs. no SLT (control) | 1018.69 (768.19 to 1283.56) | 0.008 (−0.020 to 0.040) | 127,250 |
| LSVT LOUD vs. NHS SLT | 583.30 (307.12 to 863.88) | 0.012 (−0.017 to 0.042) | 48,608 | ||
| 8 outpatient sessions + 8 participant’s home sessions | 1029 | LSVT LOUD vs. no SLT (control) | 1264.69 (999.01 to 1543.61) | 0.008 (−0.020 to 0.040) | 158,086 |
| LSVT LOUD vs. NHS SLT | 829.30 (551.60 to 1140.67) | 0.012 (−0.017 to 0.042) | 69,108 | ||
| 8 participant’s home + 8 online sessions | 1119 | LSVT LOUD vs. no SLT (control) | 1354.69 (1088.50 to 1623.43) | 0.008 (−0.020 to 0.040) | 169,336 |
| LSVT LOUD vs. NHS SLT | 919.30 (665.85 to 1217.97) | 0.012 (−0.017 to 0.042) | 76,608 | ||
| 16 participant’s home sessions | 1365 | LSVT LOUD vs. no SLT (control) | 1600.69 (1332.83 to 1867.38) | 0.008 (−0.020 to 0.040) | 200,086 |
| LSVT LOUD vs. NHS SLT | 1165.30 (873.64 to 1428.48) | 0.012 (−0.017 to 0.042) | 97,108 | ||
| Supervised and unsupervised sessions | |||||
| 8 outpatient sessions + 8 online sessions | 533 | LSVT LOUD vs. no SLT (control) | 769.09 (486.29 to 1026.58) | 0.008 (−0.020 to 0.040) | 96,136 |
| LSVT LOUD vs. NHS SLT | 333.70 (33.19 to 620) | 0.012 (−0.017 to 0.042) | 27,808 | ||
| 8 participant’s home sessions + 8 online sessions | 869 | LSVT LOUD vs. no SLT (control) | 1105.09 (830.97 to 1377.58) | 0.008 (−0.020 to 0.040) | 138,136 |
| LSVT LOUD vs. NHS SLT | 669.70 (384.39 to 975.77) | 0.012 (−0.017 to 0.042) | 55,808 | ||
Chapter 8 Discussion and conclusions
Summary of findings and interpretation
Clinical outcomes
LSVT LOUD was more effective at reducing participant-assessed impact of voice and speech problems than NHS SLT and no SLT (control) after 3 months. These results remain robust when the potential effects of non-adherence to treatment were investigated. The continued benefit at 12 months of LSVT LOUD on the impact of voice problems compared to NHS SLT and no SLT (control) is encouraging, but it remains possible that re-intervention may be required should the treatment effect wear off beyond 12 months. There was a benefit in communication-related quality of life for participants shortly after LSVT LOUD which exceeded the MCIC. In terms of AEs, the higher rate of vocal strain with LSVT LOUD treatment is generally a minor issue and is at an acceptable rate in relation to the level of benefit.
National Health Service SLT, which reflected current non-LSVT practice, was not effective when compared to both no SLT (control) or LSVT LOUD after 3 months. Moderately wide confidence limits perhaps reflect the variability in the pragmatically provided treatment and therefore do not rule out a beneficial effect for NHS SLT or alternative protocolised SLT approaches, delivered to a heterogeneous PD population with dysarthria.
Although we found benefits for LSVT LOUD in the voice-related outcomes, there were no differences in the scores for all three treatment comparisons in any of the general quality-of-life secondary outcomes: EQ-5D-5L, ICECAP-O and PDQ-39.
Severity of speech disorder measured by VHI measure at baseline showed a significant interaction with treatment and, although hypothesis generating only, provides a signal which may be worth exploring further. There was no evidence that the intervention effect differed according to age, Parkinson’s disease severity and receiving treatment during the COVID-19 pandemic.
Process evaluation findings
Implementation of the interventions
Investigating implementation fidelity through high-quality process evaluations can increase understanding of why a complex intervention works or fails. To date, there are a limited number of published process evaluation protocols, the number being even lower when linked to trials investigating rehabilitation therapies. Regardless of this, there has been a significant increase in the funding allocated to carry out process evaluations. In line with what other researchers115 have already argued, there is no single way to design and carry out a process evaluation; this is particularly true when dealing with complex interventions such as the ones investigated in the PD COMM trial. The explanatory burden being placed on process evaluations is often unrealistic116 and can lead the researcher into a mis-design ending up with ‘too much’ data for a high cost. The complex multicomponent nature of the PD COMM interventions means that the research team was faced with choices about what aspects of the intervention and its delivery to focus on, and what methods to select in order to address these while avoiding the collection of unnecessary data. By having a strong theoretical frame underpinning our design choices and by tailoring the evaluation to meet the intrinsic characteristics of this trial this process evaluation can provide an understanding of the implementation of PD COMM interventions and influencing factors. It aimed to bring clarity as to how therapists’ level of ‘preparedness’ and therapist learning and tailoring throughout the implementation of a complex intervention can impact on implementation outcomes.
Rehabilitation interventions such as PD COMM interventions are likely to be tailored, as therapists become more experienced and ‘learn’ how to best target participants’ needs while staying true to the protocol. In today’s healthcare research, where client-centredness plays a major role, there is an increased awareness of the need to tailor interventions to patients’ needs and cultural background. 117 This process evaluation will have a role in monitoring how this is done while exploring individual contexts; this may help to explain variations in the effectiveness of the intervention.
Content of the interventions
We identified similarities and differences in the reported provision of the two SLT interventions. Encouraging participants’ use of insight or ‘calibration’ was often mentioned, as was targeting phonation and using the word ‘loud’ or ‘think loud’ as a cue. Descriptions of exercise materials (sheets, list and pictures) were common in both types of SLT. The extent to which auditory feedback, daily practice and home-based practice review were reported was also very similar between approaches as was the amount of time spent on information provision. Tailoring of the SLT interventions according to participant’s health or titration of tasks or facilitating an augmentative response to a task was mentioned in TNs a similar number of times across interventions. Therapists recorded the use of encouragement and involving others to provide feedback a similar number of times in both types of SLT.
In addition to the differences in the therapy regimen (average number of weekly therapy hours and sessions and total therapy hours), the two SLT interventions had documented differences in content and procedures. LSVT LOUD TNs commonly described the use of numerical measurements (tools or software), therapeutic use of telephones or video calls and a focus on a single speech system impairment. In contrast, NHS SLT rarely mentioned these tools, and more commonly targeted three or more speech subsystems and the use of breath support. The use of everyday/functional phrases and hierarchical activities were commonly described LSVT LOUD TNs but less so in NHS SLT. In contrast, the use of automatic speech, ‘clear’ and ‘slow’ voice cues, encouraging exaggerated movements, volume and chunking of words was reported for NHS SLT but not for LSVT LOUD where applied tasks and tailoring to the participant’s interest and interactions were more commonly documented.
Health economics findings
The base-case economic evaluation demonstrated that LSVT LOUD was not a cost-effective intervention when compared with either NHS SLT or no SLT (control). Over a 12-month follow-up period, primary analysis from the NHS perspective estimated the ICER for LSVT versus no SLT (control) to be £197,772 per QALY gained and £77,017 per QALY gained versus NHS SLT. NHS SLT was more expensive but lacked evidence that it was any more effective (in terms of QALYs) than no SLT (control) and was therefore by definition ‘dominated’. The LSVT LOUD arm had a high cost per participant and this cost was primarily driven by the predominantly therapist-led delivery of the intensive intervention, and while QALY gains were demonstrated with LSVT for both comparisons, the differences were small. Therefore, at the NICE willingness-to-pay threshold of £20,000–30,000 per QALY gained, both LSVT LOUD and NHS SLT were not cost-effective.
The high additional cost of delivering LSVT LOUD was not surprising given the intensity of the intervention which comprised of four sessions weekly for 4 weeks and a total of 16 sessions (if delivered as per protocol). The cost of therapist time to deliver the sessions contributed to over 80% of the total cost, consistent with results from a recent Australian LSVT study. 118 Analysis of alternative LSVT LOUD delivery scenarios revealed that the involvement of therapy assistants in LSVT LOUD sessions reduced the overall cost. Assuming the first eight sessions were supervised by a therapist with an assistant then conducting the remaining eight sessions, the ICER versus NHS SLT was reduced to £41,108 per QALY gained. Given that remote interventions have evolved in recent years, we also explored scenarios containing online sessions; however, these incurred extra one-off costs of companion software and laptop loan. This approach was also advocated by Saiyed et al. , who suggested that moving to online (supervised) delivery would be optimal to patients living in rural/remote areas where there is limited access to SLT services and significant travel costs incurred in traveling to urban centres. 118 The most cost-effective approach to delivering LSVT LOUD was a combination of supervised and unsupervised sessions where the LSVT LOUD intervention was delivered using the LSVT companion software, and resulted in an ICER of £27,808 per QALY. However, the scenario analysis assumed the same effectiveness as LSVT LOUD delivery in the trial and it is unknown whether unsupervised sessions are as effective as supervised sessions and have the same impact on the quality of life. Further analysis explored including only those participants who were fully compliant with the protocol, and this resulted in greater QALY gains and a lower ICER of £32,291 per QALY compared with NHS SLT.
When considering capability as the measure of effect, LSVT LOUD resulted in greater levels of capability compared with NHS SLT and no SLT (control), with an ICER of £46,210 per YFC versus NHS SLT. There is no agreed willingness-to-pay threshold per YFC, although preliminary work has suggested the threshold may be in the region of £33,500 per YFC,119 suggesting LSVT LOUD may not be cost-effective when using a broader measure of well-being.
The use of other healthcare services was similar for all participants in all treatment groups, and this was expected as the SLT interventions are unlikely to affect the physical health of patients with PD. The severity of PD is an important predictor of resource use and this study showed that patients randomised to different treatment groups had similar severity. 120 However, social services utilisation was higher in LSVT LOUD arm, and this was driven by the presence of a number of outliers, amplified by the relatively modest sample size of approximately 115 per trial arm. Social care utilisation is most likely to be related to physical symptoms and abilities unrelated to difficulty in communication; therefore, while the initial analysed proposed the NHS/PSS perspective, a decision was made to present the NHS perspective as the primary analysis. The SLT interventions remained not cost-effective when compared with no SLT (control) from the broader societal perspective. However, the cost per QALY gained for LSVT LOUD compared to NHS SLT was significantly reduced to £9889 due to less work absence, but this finding should be treated with extreme caution due to the small number of participants contributing to this and the presence of outliers.
Results in context
Impact of voice problems
This trial does not provide any evidence about the relative benefits of NHS SLT and LSVT LOUD at any dosage apart from that delivered in the trial. For LSVT LOUD treatment, there is a protocol which must be followed and sets the dosage; however, NHS SLT reflects a multitude of possible approaches as chosen by the local therapist in response to the individual patient needs and thus did not have a defined protocol. Similarly, the dose was set by therapists though we did expect that the treatment would end within 3 months which may have curtailed long-term follow-up and review plans in this therapy arm. There is evidence that the dose of a therapy can alter the effectiveness of therapy interventions. The dose of NHS SLT which participants were receiving was not constrained, in order to replicate the clinical reality of using this intervention and no difference in the impact on voice problems was found compared to not receiving SLT. The extent to which any SLT approach is protocolised and the dosage regulated is an area which needs attention when considering whether to give SLT other than LSVT LOUD.
Suitability and acceptability
LSVT LOUD can be effectively delivered in the NHS context and appeared to be acceptable to most participants with few crossing-over from their allocated group, and a very small number citing the intensity of the intervention as a reason for cross-over or withdrawal. We are however also aware that LSVT LOUD has been recognised as ‘a selective and elective therapy’121 and the required intensity may have influenced some patients’ decisions about participation in the trial.
Thus, taking the limitations of the trial in account, on average, people with PD-related dysarthria will benefit more from LSVT LOUD than usual care and therapists should use the findings to challenge their perception about who is and is not suitable for LSVT LOUD. The choice about participation in LSVT LOUD should be given to people with Parkinson’s disease and UK SLT speech and language therapists should therefore offer LSVT to people with Parkinson’s who report problems with their speech or voice and avoid making that decision for the patient.
Health economics
The analysis of clinical effectiveness, concentrating on a speech-specific outcome, showed statistically significant results in favour of LSVT LOUD. In contrast, the EQ-5D-5L utility scores used as the health economic outcome measure and the total QALYs calculated from EQ-5D-5L showed positive but non-significant differences. Given that EQ-5D-5L dimensions focus on pain and discomfort, self-care and mobility and exclude a focus on speech and communication may thus be inadequately captured using this generic quality-of-life measure, and in turn the effectiveness of LSVT LOUD (and other speech and communication services) may be underestimated. 122 Previous studies have also questioned the validity of the EQ-5D-5L questionnaire in other patients with communication difficulties such as aphasia. 123,124 Therefore, there is a tension between using a frequently used questionnaire to calculate QALYs, a common outcome measure used by decision-makers for comparing interventions across diseases, and using an alternative outcome measure focusing solely on patient communication, but prohibiting across disease comparison of cost-effectiveness.
Strengths
Trial design
Establishing a minimum clinically significant change for the primary outcome measure, the VHI, was one of the aims of this trial. We used a transitional outcome measure but did not conclusively establish an MCIC for VHI using anchor-based methods and ROC analysis curves as the sample size of the trial was not large enough. There was a signal that the MCIC for VHI may be a 3- to 4-point difference so the 8- and 9.6-point differences established for LSVT LOUD compared to NHS SLT and no SLT may be clinically relevant.
This is the first full economic evaluation evaluating the cost-effectiveness of SLT interventions within the UK NHS in patients with PD. The analysis has provided a comprehensive summary of different costs and outcomes from several perspectives and detailed resource use of health and social services follow-up costs and those concerned with delivery of the intervention. Different evaluative spaces have also been explored to assess the effect of SLT intervention on maximising not only health but also capability/well-being. The collection of patient-reported resource use (as primary and secondary care services) and SLT treatment logs completed by supervisors ensured all SLT sessions were captured and were not double counted. The analysis also explored a variety of LSVT LOUD delivery scenarios to provide information for commissioners of SLT services and the costs of each scenario were estimated to determine typical scenarios for LSVT LOUD delivery by local SLT service providers. These scenarios were validated by experienced therapists and clinicians working within different service providers within the NHS.
Pragmatic delivery of interventions
The applicability of these trial results is high as the pragmatic nature of the trial allowed for close replication of the interventions as they are, or can be, currently provided through the NHS. Large pragmatic trials in SLT for PD-related dysarthria are a relatively recent development, and PD COMM is the first large-scale trial to test SLT interventions which as closely as possible replicate those provided to people with PD-related dysarthria in a clinical setting.
However, LSVT LOUD is unusual within UK SLT interventions in a number of ways. Firstly, it is highly protocolised in content, dosage and delivery, which involves considerable reorganisation from standard practice. Secondly, therapists can only deliver it if they participate in mandatory training and 2-yearly updates (managed centrally by LSVT Global) and they receive ongoing support with access to trainers and an online community. Thirdly, patients are expected to meet certain criteria and demonstrate a high level of commitment to LSVT from the outset. Thus LSVT LOUD is a relatively ‘homogenous’ intervention which supported the intervention delivery and evaluation of its effectiveness.
Primary outcome choice
Most previous trials of PD-related dysarthria have used clinician-assessed vocal loudness as a primary outcome measure. This is the first large definitive RCT to use the participant-rated outcome measure of VHI which measures how the participant perceives the impact their voice problems are having on their daily activities and their quality of life.
Limitations
The PD COMM trial closed early at the start of the COVID-19 pandemic due to the impact on the impact of participants and potential participants in clinic, the availability of SLT services with redeployment of therapists to other roles (or training to support redeployment). Consequently, the trial did not recruit the planned 388 participants. The original sample size was based on having sufficient (80%) power to detect a 10-point difference in the VHI total score at 3 months. To help to understand the impact of not reaching the intended power for the trial, a conditional power calculation was performed which showed that when comparing LSVT LOUD with NHS SLT, had we recruited the intended 163 in each arm, we would have had at least 90% power even if the remaining data had been following the null hypothesis that is that there was no difference between the three trial comparisons.
The nature of the interventions made blinding unfeasible for this trial. Participant’s, carers’ and therapists’ knowledge and expectations about the different trial interventions, particularly receiving no treatment, could have contributed to performance or detection bias in the results.
There were unavoidable differences in delivery of the interventions across different sites. These differences arose from normal variation in clinical practice across the NHS: the different resources available to sites, variation in what was ‘standard’ practice locally, and site size and geographical location. The highest recruiting sites for this trial were in large urban settings. Particularly, the proportion of home and remote visits compared to outpatient sessions may have limited the external validity of the results.
A limitation with general quality-of-life outcomes is that they are less likely to pick up benefits due to a specific improvement in communication alone as this is only one facet out of multiple subscales, including anxiety and depression. This does not mean that improvement in communication cannot confer a general improvement in quality of life. While general quality-of-life measures were used for this trial, as they were necessary to assess cost-effectiveness, they may not have been able to adequately detect an improvement due to reduction of communication difficulties.
As this was a trial to assess the effectiveness of SLT interventions, it could be argued that only collection of resource use data related to communication difficulties was necessary in the economic evaluation. However, for completeness all PD-related resource use was considered and for the majority of resource use categories, this was broadly similar across arms and did not influence the results. The two areas where outliers had an impact on the results were social care use and productivity losses. A decision was made to deviate from the protocol and exclude the costs of social care services from the base-case analysis due to outliers, although this may have underestimated the total cost across all treatment arms. An alternative approach could be implemented in the analysis by excluding the outliers instead of social care costs. However, LSVT LOUD was not cost-effective irrespective of the inclusion of social care costs. Productivity losses were reported by only a small number of participants and but changed the results from the societal perspective to favour LSVT LOUD versus standard NHS SLT. However, data on participants’ baseline employment status were not collected; therefore, it was not possible to determine if the intervention had a direct impact on time off work or work continuity. Furthermore, data were not collected on presenteeism in those who worked, although speech problems may have an impact on a person’s ability to do their job. Therefore, the results of this analysis of productivity costs may be limited in their usefulness.
It was difficult to determine from the SLT log the time spent on PD COMM trial activity, so the overall cost of sessions may also include protocol-driven costs. Within-trial patient compliance and severity of PD may have underestimated or overestimated the actual time spent by a therapist to deliver SLT interventions and the effect on health-related and capability outcomes. Therefore, we also conducted a per-protocol analysis to see if the recommendation would change if the participants adhered to the recommended method of delivery. Our estimates for the cost of medication were higher than previous studies that reported the average annual cost of PD medication to be within the range of £300–600 per patient;125,126 however, there were only small differences between trial arms and these costs did not influence the results.
Inevitably, the limitations of this process evaluation relate principally to the challenges of engaging with participants due to practical issues, such as other demands on therapists’ time, and participants’ willingness to engage in telephone interviews. Changes within the workforce such as staff turnover also made it challenging to follow the same participants up over time, and so we did not focus on this aspect of our planned analysis. People with dysarthria have described a reluctance to engage in telephone conversations127 and thus some trial participants may have been reluctant or less able to engage in telephone interviews. The components of the process evaluation reported in this chapter were framed principally around NPT, and so there is some potential for data collection and analysis to have missed some issues. However, we paid attention to data that sat outside of our analytical framework and explored the credibility of alternative interpretations throughout the analysis process. Our analysis of therapy content depended on therapy records, and thus if a particular approach, tool or method was not documented in the TNs, it did not necessarily reflect that it had not occurred.
Conclusions
This is the first large-scale pragmatic RCT comparing two SLT approaches and no treatment. LSVT LOUD is beneficial compared to no SLT (control) for reduction of PD-related speech or voice impacts, which persists to at least 12 months from starting treatment. There is lack of evidence of effectiveness for ‘standard’ NHS SLT, as currently provided, for PD-related speech impacts. Both LSVT LOUD and NHS SLT were not cost-effective compared with no SLT (control) and while LSVT LOUD is more effective in terms of QALYs it is also associated with high cost due to the intensive delivery of the intervention. Alternative delivery models may reduce LSVT LOUD intervention costs sufficiently to approach cost-effectiveness.
Equality, diversity and inclusion
Participant representation
People with communication impairment such as dysarthria related to PD are an under-researched population often excluded from research activities because of the challenges associated with their involvement. The trial population not only had a communication impairment, but additionally had a progressive disease which involves mobility and impacts on participation, raising questions about involvement and gatekeeping (participant health, cognitive concerns, support from others for attendance at therapy, etc., in addition to ability to participate). These concerns were taken into account when deciding the inclusion criteria including levels of PD severity and the details of trial design.
Steps taken included:
-
we conducted a successful pilot to demonstrate the feasibility of recruitment in this population;
-
an inclusive approach to recruitment where all levels of PD severity and dysarthria severity were eligible to enrol on the trial;
-
this was a UK-wide study, with the aim of recruiting regardless of geographical location;
-
the trial team worked with SLTs and gerontologists to highlight the need for high-level RCT evidence from trials such as PD COMM in order to inform clinical use of common interventions.
Speech-related problems associated with PD do not typically impact on language abilities and so accessible information sheets and consent forms were not necessary. Previous Cochrane reviews highlighted the paucity of data. Rapid searching identified few additional RCTs on treating PD-related speech problems and all of them were small and underpowered. People with dementia were excluded and are not routinely included in these clinical interventions and so further work is required to understand how to include people with substantial cognitive impairments.
Geographical areas with high proportions of different ethnic groups were included (Birmingham, Glasgow, London) and our previous work found that recruiting via the NHS was the most effective approach. 128 However, we did not record ethnicity in the screening logs and so cannot comment.
Future PD rehabilitation trials should refer to the recent Include Ethnicity Framework in the development of the trial, which aims to support the relevance and facilitate the inclusion of under-represented ethnic groups.
Reflections on your research team and wider involvement
There is a range of experience and expertise across the research team. The team was balanced in age and gender and included people from a range of ethnic and cultural backgrounds.
Patient and public involvement
In total the pilot trial and the main trial have taken over 10 years. In this time, we have accessed patient and public involvement (PPI) in different ways, via Parkinson’s UK, both local and national involvement, through a group of individuals (and their carers) and via individuals.
People with PD and their carers have been involved by the NIHR in the identification of the call question, in the design of the study, the conduct, the oversight, the reporting and dissemination. However, we have not completed the dissemination at the time of writing this report and will be working with individuals and Parkinson’s UK on this aspect.
A questionnaire was shared with the PPI group prior to the grant application and 25 responses were received which helped clarify the application. There was a patient representative on TSC. PPI representatives reviewed HBT diaries, consent forms and the participant information sheets. Participants were invited to a PD COMM workshop to discuss the process evaluation of the PD COMM trial in 2017. The Birmingham Clinical Trial Unit Parkinson’s PPI Group meeting was held in 2019.
A patient representative attended the final results meeting in February 2022 and the health economics final results meeting in March 2022 as well as the collaborator’s final results meeting in April 2022. The same patient representative also reviewed the final statistical results report. We informed all patients and public representatives who were involved with the trial of the results of the trial as soon as this was feasible.
A number of recommendations have been made regarding PPI input in health economics since the economic evaluation was designed for PD COMM. PPI input within economic evaluation analysis is now required in the UK to advise and comment on methods to collect cost and outcome data. However, this requires careful selection of suitable tasks that are relevant to the research, prior training and maintaining involvement over the research period.
Implications for decision-makers
This is the first large-scale, pragmatic trial comparing two commonly used speech and language therapies against each other and against no treatment. There is a robust, strong signal that 3 months of LSVT LOUD is beneficial compared to no SLT (control) for reduction of PD-related speech problems, which persists to at least 12 months from starting treatment. There is lack of evidence of effectiveness for ‘standard’ NHS SLT, as currently provided, for PD-related speech problems.
The main finding from the economic evaluation was that both LSVT LOUD and NHS SLT were not cost-effective compared with no SLT (control) and while LSVT LOUD is more effective in terms of QALYs it was also associated with high cost due to the intensive delivery of the intervention in this trial. Alternative modes of delivery may sufficiently reduce the cost of LSVT LOUD for the intervention to approach cost-effectiveness at a threshold of £30,000/QALY. Patient preferences should also be considered when exploring different options for LSVT LOUD delivery to evaluate what factors could affect patient uptake and adherence. With the advancement in technology, the move to virtual therapy may affect SLT service uptake and patient compliance. LSVT LOUD can be delivered virtually as remote sessions instead of in an outpatient setting at a slightly higher cost, especially for PD patients who have problems in their functional mobility. Costs associated with the delivery of remote LSVT LOUD must consider the cost of training, companion software and computer loan where applicable.
An informed discussion about the shape and nature of SLT provision in this population should be progressed, with the evidence of benefit for LSVT LOUD as a foundation. The gap between the NHS cost-effectiveness threshold and the cost-effectiveness of LSVT LOUD is an important consideration for decisions related to the provision of SLT for PD-related speech problems, particularly with respect to the delivery format.
Attention should also be focused on factors beyond the treatment content when determining the make-up and delivery of future SLT services: the current availability of speech and language therapists in the UK and their support staff such as assistants and administrators; access to outpatients, home and remote visits, virtual therapy, costs and frequency of treatment required. As services seek to rebuild following the COVID-19 pandemic, there are further questions to address around LSVT implementation and cost-effectiveness; how do we ensure UK services have the capacity and resources to offer LSVT, whatever their size or geography? Do the costs of LSVT reduce over time as it becomes embedded in practice? Health service and delivery research would be useful to address some of the unknowns. Can the modifications in delivery models we proposed lower the cost of LSVT LOUD without reducing effectiveness?
The PD COMM findings should also generate discussions about alternative approaches to LSVT. Where LSVT is unacceptable to a patient, what effective therapy intervention should be offered in its place? Would the effectiveness of standard NHS SLT improve if a more intensive regimen was adopted?
In including NHS SLT as a second version of a control arm (i.e. usual care in all its guises other than LSVT), PD COMM has provided vital evidence about the current content, delivery and implementation of ‘standard’ care which future RCTs can build upon including the evaluation of new interventions with different treatment targets such as conversation partner training. This is particularly important when our findings have drawn attention to carers and the potential for therapy to impact negatively on them.
In PDCOMM the NHS SLT intervention was characterised by heterogeneous content, approaches, speech system targets and delivery models delivered at a low dose. It is possible that alternative SLT approaches exist that should be formally defined, protocolised and empirically tested in the context of an RCT.
Recommendations for future research
This is the largest and most impactful study of SLT in PD. The findings are really important and have the potential to improve patient outcomes. One obvious next step would be to look at the implementation of LSVT LOUD in the NHS. Acknowledging the limitations of the inclusion and exclusion criteria and the cost-effectiveness, it would still be valuable to investigate implementation models that incorporated different delivery patterns and therefore have different cost-effectiveness.
Future research on the effects of SLT dose and delivery effectiveness could provide valuable information when investigating alternatives to LSVT LOUD. Some patients could not tolerate LSVT LOUD and so understanding how NHS could be better tailored for those patients has the potential to be useful. Both SLT interventions were found to have questionable cost-effectiveness based on current UK NICE rates of payment; however, modelling a change in delivery mode for LSVT LOUD to more online delivery did reduce the high cost burden of this treatment. The effects of adjusting both types of SLT to a more online delivery format should be investigated. SLT has a wide scope to be provided at wide range of doses and delivery methods. The signal that the intervention effect differed according to baseline severity of VHI score may also be worth investigating.
The impact of speech and language therapies on carers was mixed, with some evidence of improvement in quality of life for LSVT LOUD. As carers are vital for those with PD-related speech problems accessing care and supporting home practice, investigating further the impact of SLT on carers is important. We would suggest more detailed qualitative work would be required to better understand carer burden and how that can be addressed.
Building on the earlier PD COMM pilot, this RCT adopted the VHI at 3 months as the primary outcome measure which is a self-reported measure of the impact of speech and voice problems on the individual. Historically, much emphasis was placed on changes in outcome measurement instruments that captured aspects such as vocal loudness. Few included PROMS in trials of SLT for PD-related dysarthria. Our PD COMM trial demonstrated the effectiveness of one SLT intervention over a ‘standard’ care and a no therapy control group based on changes experienced by people with PD-related dysarthria in their VHI rated-functional physical and emotional impacts. We would encourage future trials to also use PROMS such as the VHI to capture important impacts of SLT interventions from the perception of people with PD. Similarly, following participants up for 12 months is vital to capture changes over time and therapy maintenance.
There are several areas where further research could be conducted in health economics. The lack of sensitivity of the EQ-5D-5L measure in the economic evaluation to changes in speech and voice suggests that more responsive preference-based outcome measures are required for the calculation of QALYs. Future research could include the use of alternative measures such as the Health Utilities Index 3 (HUI3) which has a version with a speech attribute, or the development of a speech ‘bolt-on’ dimension for the more commonly used EQ-5D. Speech and communication changes may have positive or negative spillover effects on carers, therefore future economic evaluations need to consider carer’ quality of life using HRQoL outcome measures or carer-specific measures, such as the Carer Experience Scale or CarerQol. Future economic evaluations are also encouraged to conduct subgroup analyses to explore differential responses to SLT interventions in specific groups of patients. Finally, a more robust evaluation of the effectiveness and cost-effectiveness of different delivery methods and doses of the intervention is required to test the simplifying assumptions made in the analysis presented here.
The PD COMM trial successfully built both research and clinical capacity in SLT for PD and the wider neurological specialism. People with PD experience other problems which are managed by SLT. For example, the network established for PD COMM could be used to investigate swallowing problems in PD.
Additional information
Contributions of authors
Catherine M Sackley (https://orcid.org/0000-0002-8580-6622) (Chief Investigator) obtained the funding for the trial, designed the trial and oversaw the running of the trial, interpreted the data and contributed to writing the report.
Caroline Rick (https://orcid.org/0000-0001-7713-9834) (Associate Professor of Clinical Trials) obtained the funding for the trial, designed the trial, oversaw the running of the trial, interpreted the data and contributed to writing the report.
Marian C Brady (https://orcid.org/0000-0002-4589-7021) (Professor of Rehabilitation; Speech and Language Therapist) contributed to the conception and design of the trial, obtained funding for the trial, Scottish recruitment site set-up and data collection, data analysis and interpretation for the main trial data, co-led the therapy data process evaluation (Chapter 6) and contributed to drafting and critical revision of the final report.
Christopher Burton (https://orcid.org/0000-0003-1159-1494) (Professor of Health Services Research) obtained the funding for the trial, designed the trial, developed the data collection and analysis plan, oversaw the implementation process evaluation analysis and the drafting of the implementation process evaluation chapter (Chapter 5), interpreted the data and contributed to writing the report.
Sue Jowett (https://orcid.org/0000-0001-8936-3745) (Professor of Health Economics) obtained the funding for the trial, designed the trial, developed the economic data collection and analysis plan, oversaw the health economic analysis and the drafting of the health economics chapter (Chapter 7), interpreted the data and contributed to writing the report.
Smitaa Patel (https://orcid.org/0000-0002-4997-3829) (Senior Statistician) obtained the funding for the trial, designed the trial, oversaw the running of the trial, developed and led on the statistical aspects of the study, supervised and performed the interim and final data analyses, interpreted the data and contributed to writing the report.
Rebecca Woolley (https://orcid.org/0000-0001-5119-1431) (Trial Statistician) inputted into the design of the trial, oversaw the running of the trial, performed the interim and final data analyses, interpreted the data and contributed to writing the report.
Patricia Masterson-Algar (https://orcid.org/0000-0001-6344-1346) (Researcher, Health Sciences) developed, designed and conducted the implementation process evaluation, interpreted the data, wrote the implementation process evaluation chapter (Chapter 5) and contributed to writing the report.
Avril Nicoll (https://orcid.org/0000-0001-9677-4817) (Researcher, Health Services Research) supported Scottish site start up and recruitment and data acquisition, co-led the therapy data process evaluation (Chapter 6), interpretation and drafting of findings, and contributed to writing, revision and approval of the final report.
Christina H Smith (https://orcid.org/0000-0001-7803-9616) (Associate Professor, Speech and Language Therapy) contributed to developing the project, provided specialist input, interpreted the data and contributed to writing the report.
Zainab Abdali (https://orcid.org/0000-0002-2736-5427) (Research Fellow in Health Economics) worked on the health economic analyses and data interpretation and drafted the health economics chapter (Chapter 7), interpreted the data and contributed to writing the report.
Natalie Ives (https://orcid.org/0000-0002-1664-7541) (Reader in Clinical Trials) obtained the funding for the trial, designed the trial, provided statistical oversight of the study, oversaw the final data analyses, interpreted the data and contributed to writing the report.
Gillian Beaton (https://orcid.org/0000-0002-8539-9318) (Clinical Specialist Lead Speech and Language Therapist) reviewed and contributed to the delivery of the trial in clinical settings, data collection methods, clinical interpretation of the data and writing the report.
Sylvia Dickson (https://orcid.org/0000-0002-4137-5752) (Researcher, Health Services Research) supported Scottish site start up and recruitment, data acquisition, therapy data analysis, interpretation of data and contributed to writing the report.
Ryan Ottridge (https://orcid.org/0000-0002-8685-2145) (Trial Management Team Leader) oversaw the running of the trial, oversaw data collection, data quality, day-to-day management, administrative support of the study, contributed to interpreting the data and writing the report.
Helen Nankervis (https://orcid.org/0000-0003-4352-1626) (Researcher, Health Services Research) contributed to interpretation of the data and writing the report.
Carl E Clarke (https://orcid.org/0000-0003-2478-3436) (Professor of Clinical Neurology and Honorary Consultant Neurologist) obtained the funding for the trial, designed the trial, oversaw the running of the trial, interpreted the data and contributed to writing the report.
Acknowledgements
The authors would like to acknowledge the support of Dr Pauline Campbell.
The study design, collection, management, analysis and interpretation of the data, publishing the data is the responsibility of the chief investigator and the Collaborative Group. The funder does not have any role in the above.
Trial management
The study design, collection, management, analysis and interpretation of the data and publishing the data were the responsibility of the chief investigator and the Collaborative Group. The trial was managed by the BCTU, with additional support for Scottish sites being provided by Glasgow Caledonian University. The process evaluation was managed by the University of Bangor and the intervention description analysis was managed by Glasgow Caledonian University. The funder did not have a role in the above activities. Oversight of the trial was performed by the Trial Management Group, an independent Data Monitoring Committee (DMC) and Trial Steering Committee (TSC). The DMC and TSC membership was agreed with the funder; these committees met at least annually to review the data and progress of the trial. Annual reports were submitted to the NHS REC and monthly updates against pre-determined milestones were sent to the funder.
Patient and public involvement
T Jefferys, L Sharp, T Farnsworth, C Jeffery.
Members of the PD COMM Collaborative Group
Members of the Trial Steering Committee
Lisa Shaw (Chair), Senior Research Associate, University of Newcastle upon Tyne
Mr Chris Jeffery, Patient and Public Involvement Representative
Michelle Collinson, Senior Medical Statistician, University of Leeds
Linsay Pennington, Senior Lecturer and Speech and Language Therapist, University of Newcastle upon Tyne
Catherine Sackley, Professor of Rehabilitation, King’s College London
Paul Worth, Consultant in Neurology, Cambridge University Hospitals NHS Foundation Trust
Members of the Data Monitoring Committee
Carl Counsell (Chair), Consultant Neurologist, University of Aberdeen
Katherine Deane, Senior Lecturer, University of East Anglia
Louise Hiller, Statistician, University of Warwick
Members of the Trial Management Group
Professor Catherine Sackley (Chief Investigator)
Professor Carl E Clarke (Deputy Chief Investigator)
Ms Pui Au
Mr Naveed Saeed
Ms Jane Futterer
Ms Gillian Beaton
Professor Marian Brady
Professor Sue Jowett
Ms Smitaa Patel
Ms Natalie Ives
Members of the Birmingham Clinical Trials Unit
F Dowling
S Tearne
L Sturdy
R Dhaliwal
D Hingley
P Au
Participating centres and PD COMM Collaborative Group members
*Indicates current principal investigator
#Indicates former principal investigator
Aneurin Bevan University Health Board: A Church*, A Davey, C Gallagher, A Conroy, S Bailey, B Done, D Davies
Basildon and Thurrock University Hospitals NHS Foundation Trust: S Sveinbjornsdottir*, M Kasti#, K Allen, J Colnet, J Riches, L Kittridge, L Morris, C Waszkiewicz
Bath and North East Somerset Community Health and Care Services; V Lyell*, V Page, N Bassford, H Rayner, E Henderson
Betsi Cadwaladr University Health Board: S Abraham*, JV Hindle#, S Jones, P Martin-Forbes, C Watkins, A Roberts, E Newcombe, L Bibby, L Matthews, J Roberts, S Thomas, H Hawthorne, K Clewley, S Lord, J Roberts, G Bretag, S Noble
Blackpool Teaching Hospitals NHS Foundation Trust: D McGhee*, H Mcclure, A Ling Zhi Teo, R Wheeldon, C Wilkinson, M Oprea, G Van Duyvenvoorde, N Wilson, L Evans, R Belshaw, A Clarke, M Turner, C Thompson
Brighton and Sussex University Hospitals NHS Trust: R Saha*, J Aram, D Mullan, J Newman, K Micabel, H Robinson, K Chick, J Gaylard, J Cochrane
Cardiff and Vale University Health Board: E Christopher Thomas*, A Kissick, P Wright, B Mohamed, S Mahon, T Williams, S Appleton, N Elliott, L Evans, J Ridley
Central London Community Healthcare NHS Trust: A Funaki*, P Daly, J Hackworth, K Timms, L Evison, A Bajracharya
East Cheshire NHS Trust: M Silverdale*, K Andrews, A Davies, R Bedford, A Jones, D Mournfield, L Howieson, V Price, J Hunter, A Baggs, L Evans, R Norton, M Holland, K Pointon
Gloucestershire Hospitals NHS Foundation Trust: S Kulkarni*, S Bobeldijk, S Beames, J Cavanagh, S Sudlow
Great Western Hospitals NHS Foundation Trust: G Lennox*, R Whittaker, D Nelson, S Pegler, C Drayson, P Turner, J Stockwell Guy’s and St Thomas’ NHS Foundation Trust: T Andrews*, E White, E Turner, T Burnay, C Hickey, S Warner
Harrogate and District NHS Foundation Trust: R Buccoliero*, C Isles, C Stemp, J Guy, C Bennett, S Smith, P Randall, L Ware, J Cann
Homerton University Hospital NHS Foundation Trust: L Sautin*, S Grobler#, S Adjei#, A Hankin, D Ovayolu, M Dobbs, O Joyce, R Humphreys, A Jha, C Holbrook, K Rowsell, F Johnson, H Thornley, S Conway
Hywel Dda University Health Board: C James*, S Murrow, M Hughes, K Pope, C MacPhee, E Williams, R Hughes, A Evans, A Richmond, K Pilborough, K Campbell, S Elizabeth Davies, A Taylor, S Thomas, D Asandei
Lancashire Teaching Hospitals NHS Trust: T Majeed*, J Dawber, S Furey, A Oppetit, J Birt, M Hare, V Fleming, A Timoroksa, H Al-Nufoury, S Sharp, L Freimann
Leeds Teaching Hospitals NHS Trust: I Mavroudis*, J Alty#, E Richfield, J Harrison, V Smith, T Joyce, J Bamford, S Jamieson, J Cosgrove, S Butterworth, E Sacre, L Makawa, P Duggan-Carter, C Arnold, K Brown, P Mpofu, C Joyce, S Henderson, C Wiseman, L Hyne, B Stevens, A Wood, D Holland, V Smith
London North West Healthcare NHS Trust: J Juada*, S Molloy#, C Pavel, M Dhanarante, T Adedoyin, C Rowbottom, L Little, R Choudhury, L Prados, A Kelly, R Eggers, T Saifee, P Poku, L Niepage
Manchester University NHS Foundation Trust: D Ahearn*, A Fountain, A Curran NHS Ayrshire and Arran: A Watt*, M Wilson, A Anderson, M Graham, J Taylor, P Hewat
NHS Dumfries and Galloway: S Donaldson*, H Moores-Poole, C Angus, S Coull, R Davy, A Gilmour-Graham, T Mcilroy, A Kendall
NHS Forth Valley: S Pal*, E Messeder, D Thomson, V Johnston, P Raby, S Kinnear, P Faunce Smith
NHS Greater Glasgow and Clyde: S Wishart*, D Grosset, J Burns, A Louise Cunnington, E Newman, C Vennard, C Dalton, T Murphy, G Ralph, A Ritchie, C Nelson, A McEntee, P Fowley, H Hare, G Beaton
NHS Highland: M Wilson*, D McDonald, J Finlayson, A Donaldson, S Sutherland, S Bramley, C Dunn, M Wallis, S Hewitt
NHS Lanarkshire: H Morgan*, A Falconer#, L Peacock, A McAlpine, C McBrearty, A Lowe, N Findlay, A Adam, C Tearney, J Picken, K MacKenzie, L McCallum, L Smith, M Sidney, P Downie, L Donnelly, R McAllister, S Campbell, S Maclachlan, L Shearer, K Campbell
NHS Lothian: G Duncan*, S Marrinan, M Dewar, J Kerr, L Killin, A Peters, A Stewart, T Daniels, A Darbyshire, I McCoy, U Duff, F Young, S Orr, C Telford, D Fraser, S Borthwick, H Bailey, L Karbownicki, E McLeod
NHS Tayside: D Sutherland*, E Sammler, L Whyte, C Young, L Gillies, L Gall, J Dallas, L Cassidy, E Letham, V Salisbury, L Anderson, C Hutton, S Waggett, D Anderson, A Mcgee
Norfolk and Norwich University Hospitals NHS Trust: S Cooper*, G Mamutse, A Niruban, A Bath, A Wiltshire, M Harmer, C Wright, J Graham, K Richardson, J Tyler
Norfolk Community Health and Care NHS Trust: L Isaacs*, N Crow, S Pinnell, W Neale, L Maloney, R Weller, K Young, C Squire North Bristol NHS Trust: A Whone*, Y Hernandez, H Findlay, K King, L Gethin, S Ticehurst, A Swift, J Short, J Dean, C Westcott, K Thomas, S Cottrell, D Kruszynska
Northern Lincolnshire and Goole NHS Foundation Trust: S Kamath*, Q Ma, J Hall, R Wilson, H Goodhand, K Mellows, L Jayne Cottam, T Behan, J Gibson, E Lomas, J Kirk, L Smith, J Benson
Pennine Acute Hospitals NHS Trust: J Raw*, P Mulligan, A Ansari, R Irving, A Javed, S Hussain, L Johnson, R Joseph, J Brooke, J Melville, M McCormack, J Stockley, D Ganderton, A Cherriman
Powys Teaching Health Board: J Price*, C Douglas, C Cooter, J Bushell
Royal Devon and Exeter NHS Foundation Trust: R Sheridan*, C Browning, K Polverino, T Malone, S Jackson, A Foden, R James, S Hayes, L Roberts, E Davis
Sandwell and West Birmingham Hospitals NHS Trust: C Clarke*, D Nicholl, A Majeed, MT Oo, K Blachford, A Boughey, J Kaur, S Kaur, M Awan, S Rahman, J Round
St Helens and Knowsley Hospitals NHS Trust: D Gandecha*, S Williams, S Dealing, H Moss, L Talbot Taunton and Somerset NHS Foundation Trust: S Cooper*, R Sophia, J Allen, S Cox, C Moreira, D Woolven, D Sharratt, E Foster, H Hurren, J Watson, S Northover, D Green, A Treloggen, C Pawley, K Beesley, K Milne, L Howard, S Craw, A Lewis, A Whitcher, C Vickers, T Russell, A Sykes, H Meikle, N Loraine
The Walton Centre NHS Foundation Trust: M Steiger*, H Treloar, L Roebuck, M Taylor
Torbay and South Devon NHS Foundation Trust: R Nashed*, J Garfield-Smith, S Mills, H Griffin, C Marshall, G De Selincourt, V Queen, M Stone, M FarrowJones, E Sturdy, K Almedilla, F Fitzsimmons, M Alison, F Rogers, B Reed
University Hospitals Birmingham NHS Foundation Trust: M Pinkney*, S Jones#, S Muzerengi, M Johnson, S Stafford, E Parmar, J Albutt, S Kaur, M Awan, S Rahman, K Leahy
University Hospitals Bristol NHS Foundation Trust: T Allain*, M Sritharan#, A Daniell, K Kunsteinaite, S Slade, F Pimbblet, C Killourhey
Wye Valley NHS Trust: E Wales*, C Hughes, G Horsfield, L Mercer, Z Roberts, K Stock, M Evans, S Boyd, L King, J Birch, S Anderson, C Evans, N Stapleton, U Magennis, R Vernall
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Data-sharing statement
Following the publication of the main trial results anonymous data sets will be available on an individual case-by-case basis in accordance with the University of Birmingham, Birmingham Clinical Trials Unit’s Standard Operating Procedures with agreement from corresponding author Professor Catherine Sackley and King’s College, London.
Ethics statement
Ethical approval was granted on 17/12/2015 by West Midlands – Coventry & Warwickshire Research Ethics Committee. Reference number: 15/WM/0443.
Information governance statement
University of Birmingham is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, University of Birmingham is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://www.birmingham.ac.uk/privacy.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/ADWP8001.
Primary conflicts of interest: Catherine M Sackley declares she is the holder of NIHR grant GH 17/63/66 and a Dunhill Medical Trust award. She was supported to attend the Movement Disorder Society meeting for dissemination of results in Madrid 2022. Marian C Brady declares she is an SLT representative in the WHO Rehabilitation Package for Parkinson’s disease, publication pending 2022. She has a family member with Parkinson’s disease and speech problems. Avril Nicholl declares she is a Member and Fellow of the Royal College of Speech and Language Therapists and registered as a speech and language therapist with the Health and Care Professions Council. Gillian Beaton declares consulting fees for NMAHP Glasgow paid to NHSGGC. Sue Jowett declares she was a member of the NIHR HAT Clinical Evaluation and Trials Funding Committee 2016–20. Caroline Rick, Patricia Masterton-Algar, Christopher Burton, Christina H Smith, Sylvia Dickson, Ryan Ottridge, Zainab Abdali, Rebecca Woolley, Smitaa Patel, Natalie Ives, Helen Nankervis and Carl E Clarke declare no competing interests. The PD COMM trial design was informed by our PD COMM Pilot trial, which was funded by The Dunhill Medical Trust. Further support was provided by Professor Lori Ramig in the form of free LSVT LOUD training.
The PD COMM trial design was informed by our PD COMM Pilot trial, which was funded by The Dunhill Medical Trust. Further support was provided by Professor Lori Ramig in the form of free LSVT LOUD® training.
Publications
Sackley CM, Smith CH, Rick C, Brady MC, Ives N, Patel R, et al. Lee Silverman voice treatment versus standard NHS speech and language therapy versus control in Parkinson’s disease (PD COMM pilot): study protocol for a randomized controlled trial. Trials 2014;15:213. https://doi.org/10.1186/1745-6215-15-213.
Masterson-Algar P, Burton CR, Brady MC, et al. The PD COMM trial: a protocol for the process evaluation of a randomised trial assessing the effectiveness of two types of SLT for people with Parkinson’s disease. Trials 2017;18:397. https://doi.org/10.1186/s13063-017-2130-1
Sackley CM, Smith CH, Rick CE, Brady MC, Ives N, Patel S, et al. ; PD COMM Pilot Collaborative Group. Lee Silverman Voice Treatment versus standard speech and language therapy versus control in Parkinson’s disease: a pilot randomised controlled trial (PD COMM pilot). Pilot Feasibility Stud 2018;4:30. https://doi.org/10.1186/s40814-017-0222-z
Sackley CM, Rick C, Au P, Brady MC, Beaton G, Burton C, et al. ; PD COMM Collaborative Group. A multicentre, randomised controlled trial to compare the clinical and cost-effectiveness of Lee Silverman Voice Treatment versus standard NHS Speech and Language Therapy versus control in Parkinson’s disease: a study protocol for a randomised controlled trial. Trials 2020;21(1):436. https://doi.org/10.1186/s13063-020-04354-7
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- GBDPsD Collaborators . Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:939-53. https://doi.org/10.1016/S1474-4422(18)30295-3.
- GBDNDC Group . Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol 2017;16:877-97. https://doi.org/10.1016/S1474-4422(17)30299-5.
- Kalinderi K, Bostantjopoulou S, Fidani L. The genetic background of Parkinson’s disease: current progress and future prospects. Acta Neurol Scand 2016;134:314-26. https://doi.org/10.1111/ane.12563.
- Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson’s disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord 2016;23:1-9. https://doi.org/10.1016/j.parkreldis.2015.12.008.
- Rodriguez-Blazquez C, Schrag A, Rizos A, Chaudhuri KR, Martinez-Martin P, Weintraub D. Prevalence of non-motor symptoms and non-motor fluctuations in Parkinson’s disease using the MDS-NMS. Mov Disord Clin Pract 2021;8:231-9. https://doi.org/10.1002/mdc3.13122.
- Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. PRIAMO Study Group . The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord 2009;24:1641-9. https://doi.org/10.1002/mdc3.22643.
- Lawton M, Ben-Shlomo Y, May MT, Baig F, Barber TR, Klein JC, et al. Developing and validating Parkinson’s disease subtypes and their motor and cognitive progression. J Neurol Neurosurg Psychiatry 2018;89:1279-87. https://doi.org/10.1136/jnnp-2018-318337.
- Vijiaratnam N, Simuni T, Bandmann O, Morris HR, Foltynie T. Progress towards therapies for disease modification in Parkinson’s disease. Lancet Neurol 2021;20:559-72. https://doi.org/10.1016/S1474-4422(21)00061-2.
- Group PDMC, Gray R, Ives N, Rick C, Patel S, Gray A, et al. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet 2014;384:1196-205. https://doi.org/10.1016/S0140-6736(14)60683-8.
- Parkinson’s Disease in Adults: Diagnosis and Management. 2017. www.ncbi.nlm.nih.gov/books/NBK447153/.
- Kalf JG, Bloem BR, Munneke M. Prevalence of speech impairments in Parkinson’s disease: a systematic review. Mov Disord 2009;24.
- Miller N, Allcock L, Jones D, Noble E, Hildreth AJ, Burn DJ. Prevalence and pattern of perceived intelligibility changes in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2007;78:1188-90. https://doi.org/10.1136/jnnp.2006.110171.
- Hartelius L, Svensson P. Speech and swallowing symptoms associated with Parkinson’s disease and multiple sclerosis: a survey. Folia Phoniatr Logop 1994;46:9-17. https://doi.org/10.1159/000266286.
- Miller N, Noble E, Jones D, Burn D. Life with communication changes in Parkinson’s disease. Age Ageing 2006;35:235-9. https://doi.org/10.1093/ageing/afj053.
- Pell MD, Cheang HS, Leonard CL. The impact of Parkinson’s disease on vocal-prosodic communication from the perspective of listeners. Brain Lang 2006;97:123-34. https://doi.org/10.1016/j.bandl.2005.08.010.
- Barnish MS, Horton SMC, Butterfint ZR, Clark AB, Atkinson RA, Deane KHO. Speech and communication in Parkinson’s disease: a cross-sectional exploratory study in the UK. BMJ Open 2017;7. https://doi.org/10.1136/bmjopen-2016-014642.
- Yorkston K, Baylor C, Britton D. Speech versus speaking: the experiences of people with Parkinson’s disease and implications for intervention. Am J Speech Lang Pathol 2017;26:561-8. https://doi.org/10.1044/2017_AJSLP-16-0087.
- Heberlein I, Vierrege P. The influence of speech disturbances on quality of life and coping strategies on Parkinson’s disease patients. Forum Logopadie 2005;19:26-31.
- Miller N. Communication changes in Parkinson’s disease. Pract Neurol 2017;17:266-74. https://doi.org/10.1136/practneurol-2017-001635.
- LR G. LSVT Loud. LSVT Global; 2021.
- Swales M, Theodoros D, Hill AJ, Russell T. Communication service provision and access for people with Parkinson’s disease in Australia: a national survey of speech-language pathologists. Int J Speech Lang Pathol 2019;21:572-83. https://doi.org/10.1080/17549507.2018.1537372.
- Miller N, Deane KH, Jones D, Noble E, Gibb C. National survey of speech and language therapy provision for people with Parkinson’s disease in the United Kingdom: therapists’ practices. Int J Lang Commun Disord 2011;46:189-201. https://doi.org/10.3109/13682822.2010.484849.
- Collis J, Bloch S. Survey of UK speech and language therapists’ assessment and treatment practices for people with progressive dysarthria. Int J Lang Commun Disord 2012;47:725-37. https://doi.org/10.1111/j.1460-6984.2012.00183.x.
- Miller N, Bloch S. A survey of speech-language therapy provision for people with post-stroke dysarthria in the UK. Int J Lang Commun Disord 2017;52:800-15. https://doi.org/10.1111/1460-6984.12316.
- Proffitt R. Home exercise programs for adults with neurological injuries: a survey. Am J Occup Ther 2016;70:7003290020p1-8. https://doi.org/10.5014/ajot.2016.019729.
- LSVT Global store . LSVT Global n.d. www.lsvtglobal.com/store/IdaStoreLSVT (accessed 27 September 2022).
- 2014.
- Guidelines for Speech-language Therapy in Parkinson’s Disease. Nijmegen: Parkinson Net/National Parkinson Foundation (NPF); 2011.
- Herd CP, Tomlinson CL, Deane KH, Brady MC, Smith CH, Sackley CM, et al. Speech and language therapy versus placebo or no intervention for speech problems in Parkinson’s disease. Cochrane Database Syst Rev 2012;2012. https://doi.org/10.1002/14651858.CD002812.pub2.
- Herd CP, Tomlinson CL, Deane KH, Brady MC, Smith CH, Sackley CM, et al. Comparison of speech and language therapy techniques for speech problems in Parkinson’s disease. Cochrane Database Syst Rev 2012;2012. https://doi.org/10.1002/14651858.CD002814.pub2.
- Linares-Del Rey M, Vela-Desojo L, Cano-de la Cuerda R. Mobile phone applications in Parkinson’s disease: a systematic review. Neurologia (Engl Ed) 2019;34:38-54. https://doi.org/10.1016/j.nrl.2017.03.006.
- Pu T, Huang M, Kong X, Wang M, Chen X, Feng X, et al. Lee Silverman voice treatment to improve speech in Parkinson’s disease: a systemic review and meta-analysis. Parkinsons Dis 2021;2021. https://doi.org/10.1155/2021/3366870.
- van de Wetering-van Dongen VA, Kalf JG, van der Wees PJ, Bloem BR, Nijkrake MJ. The effects of respiratory training in Parkinson’s disease: a systematic review. J Parkinson’s Dis 2020;10:1315-33.
- Xu H, Bao Z, Liang D, Li M, Wei M, Ge X, et al. Speech and language therapy for voice problems in Parkinson’s disease: a meta-analysis. J Neuropsychiatry Clin Neurosci 2020;32:344-51. https://doi.org/10.1176/appi.neuropsych.19020044.
- Yuan F, Guo X, Wei X, Xie F, Zheng J, Huang Y, et al. Lee Silverman voice treatment for dysarthria in patients with Parkinson’s disease: a systematic review and meta-analysis. Eur J Neurol 2020;27:1957-70. https://doi.org/10.1111/ene.14399.
- Sackley CM, Smith CH, Rick CE, Brady MC, Ives N, Patel S, et al. PD COMM Pilot Collaborative Group . Lee Silverman Voice Treatment versus standard speech and language therapy versus control in Parkinson’s disease: a pilot randomised controlled trial (PD COMM pilot). Pilot Feasibility Stud 2018;4. https://doi.org/10.1186/s40814-017-0222-z.
- Royal College of Speech and Language Therapists . Clinical Guidelines 2005.
- Royal College of Speech & Language Therapists . Communicating Quality 3: RCSLT’s Guidance on Best Practice in Service Organisation and Provision 2006.
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988;51:745-52.
- Ramig LO, Countryman S, Thompson LL, Horii Y. Comparison of two forms of intensive speech treatment for Parkinson disease. J Speech Hear Res 1995;38:1232-51.
- Ramig LO, Sapir S, Countryman S, Pawlas AA, O’Brien C, Hoehn M, et al. Intensive voice treatment (LSVT) for patients with Parkinson’s disease: a 2 year follow up. J Neurol Neurosurg Psychiatry 2001;71:493-8.
- Hodkinson HM. Evaluation of a mental test score for assessment of mental impairment in the elderly. 1972. Age Ageing 2012;41:iii35-40. https://doi.org/10.1093/ageing/afs148.
- Spielman J, Ramig LO, Mahler L, Halpern A, Gavin WJ. Effects of an extended version of the Lee Silverman voice treatment on voice and speech in Parkinson’s disease. Am J Speech Lang Pathol 2007;16:95-107. https://doi.org/10.1044/1058-0360(2007/014).
- Hustad KC. The relationship between listener comprehension and intelligibility scores for speakers with dysarthria. J Speech Lang Hear Res 2008;51:562-73. https://doi.org/10.1044/1092-4388(2008/040).
- Robertson SJ, Thomson F. Speech therapy in Parkinson’s disease: a study of the efficacy ad long term effects of intensive treatment. Br J Disord Commun 1984;19:213-24.
- Johnson JA, Pring TR. Speech therapy and Parkinson’s disease: a review and further data. Br J Disord Commun 1990;25:183-94.
- Scott S, Caird FI. Speech therapy for Parkinson’s disease. J Neurol Neurosurg Psychiatry 1983;46:140-4.
- Scott S, Caird FI. The response of the apparent receptive speech disorder of Parkinson’s disease to speech therapy. J Neurol Neurosurg Psychiatry 1984;47:302-4.
- Scott S, Caird FI. Speech therapy for patients with Parkinson’s disease. Br Med J (Clin Res Ed) 1981;283.
- Lang AE, Fishbein V. The ‘pacing board’ in selected speech disorders of Parkinson’s disease. J Neurol Neurosurg Psychiatry 1983;46.
- Healey V . A comparison of the efficacy of two methods of rate control in the speech of people with Parkinson’s disease. Parkinson’s News: A Quarterly Bulletin for Health and Social Care Professionals 2006;1:6-7.
- Adams SG, Lang AE. Can the Lombard effect be used to improve low voice intensity in Parkinson’s disease?. Eur J Disord Commun 1992;27:121-7.
- Schulz GM, Grant MK. Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson’s disease: a review of the literature. J Commun Disord 2000;33:59-88.
- Rousseau B, Watts C. Susceptibility of speakers with Parkinson disease to delayed feedback. J Med Speech-Lang Patholo 2002;10:41-9.
- de Swart BJ, Willemse SC, Maassen BA, Horstink MW. Improvement of voicing in patients with Parkinson’s disease by speech therapy. Neurology 2003;60:498-500.
- An M, Dusing SC, Harbourne RT, Sheridan SM, Consortium ST-P. What really works in intervention? Using fidelity measures to support optimal outcomes. Phys Ther 2020;100:757-65. https://doi.org/10.1093/ptj/pzaa006.
- Jacobson BH, Johnson A, Grywalski C, Silbergleit A, Jacobson G, Benninger MS, et al. The voice handicap index (VHI): development and validation. Am J Speech-Lang Pathol 1997;6:66-70.
- Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson’s disease summary index score. Age Ageing 1997;26:353-7.
- Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson’s disease quality of life questionnaire and its associated measures. J Neurol 1998;245:S10-4. https://doi.org/10.1007/pl00007730.
- Hartelius L, Elmberg M, Holm R, Lovberg AS, Nikolaidis S. Living with dysarthria: evaluation of a self-report questionnaire. Folia Phoniatr Logop 2008;60:11-9. https://doi.org/10.1159/000111799.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
- Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, et al. Valuing the ICECAP capability index for older people. Soc Sci Med 2008;67:874-82. https://doi.org/10.1016/j.socscimed.2008.05.015.
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427-42.
- Jenkinson C, Dummett S, Kelly L, Peters M, Dawson J, Morley D, et al. The development and validation of a quality of life measure for the carers of people with Parkinson’s disease (the PDQ-Carer). Parkinsonism Relat Disord 2012;18:483-7. https://doi.org/10.1016/j.parkreldis.2012.01.007.
- de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol 2006;5:525-35. https://doi.org/10.1016/S1474-4422(06)70471-9.
- PD COMM Protocol Versions n.d. www.birmingham.ac.uk/research/bctu/trials/pd/PD-COMM/investigators/documentation.aspx (accessed 29 September 2022).
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: medical research council guidance. BMJ 2015;350. https://doi.org/10.1136/bmj.h1258.
- Process Evaluation for Public Health Interventions and Research. San Francisco, CA: Jossey-Bass; 2002.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J, Team RS. Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-6. https://doi.org/10.1136/bmj.332.7538.413.
- Bonell C, Fletcher A, Morton M, Lorenc T, Moore L. Realist randomised controlled trials: a new approach to evaluating complex public health interventions. Soc Sci Med 2012;75:2299-306. https://doi.org/10.1016/j.socscimed.2012.08.032.
- May CR, Mair F, Finch T, MacFarlane A, Dowrick C, Treweek S, et al. Development of a theory of implementation and integration: normalization process theory. Implement Sci 2009;4. https://doi.org/10.1186/1748-5908-4-29.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8. https://doi.org/10.1186/1741-7015-8-63.
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7. https://doi.org/10.1186/1472-6963-7-148.
- Masterson-Algar P, Burton CR, Brady MC, Nicoll A, Clarke CE, Rick C, et al. The PD COMM trial: a protocol for the process evaluation of a randomised trial assessing the effectiveness of two types of SLT for people with Parkinson’s disease. Trials 2017;18. https://doi.org/10.1186/s13063-017-2130-1.
- Finch TL, Girling M, May CR, Mair FS, Murray E, Treweek S, et al. Improving the normalization of complex interventions: part 2 – validation of the NoMAD instrument for assessing implementation work based on normalization process theory (NPT). BMC Med Res Methodol 2018;18. https://doi.org/10.1186/s12874-018-0591-x.
- Schwarzer R, Jerusalem M. Measures in Health Psychology: A User’s Portfolio. Windsor: NFER-NELSON; 1995.
- Global L. LSVT Skills Checklist 2018. https://lcc17.lsvtglobal.com/wp-content/uploads/2018/04/LSVT-LOUD-Skills-Checklist_04-18.pdf (accessed 27 September 2022).
- Braun V, Clarke V. Using thematic analysis in psychology. Qualitat Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- National Job Profiles n.d. www.nhsemployers.org/articles/national-job-profiles (accessed 27 September 2022).
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. https://doi.org/10.1136/bmj.g1687.
- Voice Meter Pro. n.d. https://dragonflyapps.com/voice-meter-pro/ (accessed 27 September 2022).
- DAF PRO n.d. https://speechtools.co/daf-pro (accessed 27 September 2022).
- Bla Bla Bla n.d. https://speechtools.co/daf-pro (accessed 27 September 2022).
- ClaroCom n.d. www.clarosoftware.com/portfolio/clarocom/ (accessed 27 September 2022).
- Voice Analyst n.d. www.parkinsons.org.uk/information-and-support/voice-analyst (accessed 27 September 2022).
- Parkinsons UK Apps n.d. www.parkinsons.org.uk/information-and-support/apps-and-devices-parkinsons (accessed 27 September 2022).
- Decibel 10 n.d. https://skypaw.com/decibel10.html (accessed 27 September 2022).
- Guide to the Methods of Technology Appraisal 2013. London: National Institute for Health and Care Excellence; 2013.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 Explanation and Elaboration: a report of the ISPOR CHEERS II good practices task Force. Value Health 2022;25:10-31. https://doi.org/10.1016/j.jval.2021.10.008.
- Scobie S, Jowett S, Lambe T, Patel S, Woolley R, Ives N, et al. Lee Silverman Voice Treatment versus standard speech and language therapy versus control in Parkinson’s disease: preliminary cost-consequence analysis of the PD COMM pilot randomised controlled trial. Pilot Feasibility Stud 2021;7. https://doi.org/10.1186/s40814-021-00888-y.
- NICE . Position Statement on Use of the EQ-5D-5L Value Set for England 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 3 February 2020).
- van Hout B, Janssen M, Fau-Feng Y-S, Feng YS, Fau-Kohlmann T, Kohlmann T, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15. https://doi.org/10.1016/j.jval.2012.02.2008.
- Grewal I, Lewis J, Flynn T, Brown J, Bond J, Coast J. Developing attributes for a generic quality of life measure for older people: preferences or capabilities?. Soc Sci Med 2006;62:1891-901. https://doi.org/10.1016/j.socscimed.2005.08.023.
- Flynn TN, Chan P, Coast J, Peters TJ. Assessing quality of life among British older people using the ICEPOP CAPability (ICECAP-O) measure. Appl Health Econ Health Policy 2011;9:317-29. https://doi.org/10.2165/11594150-000000000-00000.
- Shah KK, Murtagh FEM, McGeechan K, Crail S, Burns A, Tran AD, et al. Health-related quality of life and well-being in people over 75 years of age with end-stage kidney disease managed with dialysis or comprehensive conservative care: a cross-sectional study in the UK and Australia. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-027776.
- Flynn TN, Huynh E, Peters TJ, Al-Janabi H, Clemens S, Moody A, et al. Scoring the Icecap – a capability instrument. Estimation of a UK general population tariff. Health Econ 2015;24:258-69. https://doi.org/10.1002/hec.3014.
- Mitchell PM, Roberts TE, Barton PM, Coast J. Assessing sufficient capability: a new approach to economic evaluation. Soc Sci Med 2015;139:71-9. https://doi.org/10.1016/j.socscimed.2015.06.037.
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford; 2015.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2020. Personal Social Services Research Unit, University of Kent. Canterbury: University of Kent; 2020.
- Joint Formulary Committee . British National Formulary n.d. www.medicinescomplete.com (accessed 14 December 2021).
- NHS National Schedule of Reference Costs 2019 20 n.d. www.england.nhs.uk/publication/2019-20-national-cost-collection-data-publication/ (accessed 7 December 2021).
- Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Krol M, Brouwer W. How to estimate productivity costs in economic evaluations. PharmacoEcon 2014;32:335-44. https://doi.org/10.1007/s40273-014-0132-3.
- Schulpher MJ. Economic Evaluation in Health Care: Merging Theory with Practice. Oxford: Oxford University Press; 2001.
- Office for National Statistics . Annual Survey of Hours and Earnings 2020. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2020 (accessed 23 February 2022).
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEcon 2014;32:1157-70. https://doi.org/10.1007/s40273-014-0193-3.
- Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med 1991;10:585-98. https://doi.org/10.1002/sim.4780100410.
- Barber JA, Thompson SG. Analysis and interpretation of cost data in randomised controlled trials: review of published studies. BMJ 1998;317:1195-200. https://doi.org/10.1136/bmj.317.7167.1195.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. https://doi.org/10.1002/hec.944.
- Glick HA, Briggs AH, Polsky D. Quantifying stochastic uncertainty and presenting results of cost-effectiveness analyses. Expert Rev Pharmacoecon Outcomes Res 2001;1:25-36. https://doi.org/10.1586/14737167.1.1.25.
- Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212-4. https://doi.org/10.1177/0272989X9001000308.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. https://doi.org/10.1002/hec.635.
- Theodoros Deborah G, Hill Anne J, Russell Trevor G. Clinical and quality of life outcomes of speech treatment for Parkinson’s disease delivered to the home via telerehabilitation: a noninferiority randomized controlled trial. Am J Speech-Lang Pathol 2016;25:214-32. https://doi.org/10.1044/2015_AJSLP-15-0005.
- Grant A, Treweek S, Dreischulte T, Foy R, Guthrie B. Process evaluations for cluster-randomised trials of complex interventions: a proposed framework for design and reporting. Trials 2013;14. https://doi.org/10.1186/1745-6215-14-15.
- Munro A, Bloor M. Process evaluation: the new miracle ingredient in public health research?. Qual Res 2010;10:699-713. https://doi.org/10.1177/1468794110380522.
- Morrison DM, Hoppe MJ, Gillmore MR, Kluver C, Higa D, Wells EA. Replicating an intervention: the tension between fidelity and adaptation. AIDS Educ Prev 2009;21:128-40. https://doi.org/10.1521/aeap.2009.21.2.128.
- Saiyed M, Hill AJ, Russell TG, Theodoros DG, Scuffham P. Cost analysis of home telerehabilitation for speech treatment in people with Parkinson’s disease. J Telemed Telecare 2020;28:524-9. https://doi.org/10.1177/1357633X20948302.
- Kinghorn P, Afentou N. Eliciting a monetary threshold for a year of sufficient capability to inform resource allocation decisions in public health and social care. Soc Sci Med 2021;279. https://doi.org/10.1016/j.socscimed.2021.113977.
- McIntosh E, Gray A, Daniels J, Gill S, Ives N, Jenkinson C, et al. PD SURG Collaborators Group . Cost–utility analysis of deep brain stimulation surgery plus best medical therapy versus best medical therapy in patients with Parkinson’s: economic evaluation alongside the PD SURG trial. Mov Disord 2016;31:1173-82. https://doi.org/10.1002/mds.26423.
- Wight S, Miller N. Lee Silverman Voice Treatment for people with Parkinson’s: audit of outcomes in a routine clinic. Int J Lang Commun Disord 2015;50:215-25. https://doi.org/10.1111/1460-6984.12132.
- Latimer NR, Bhadhuri A, Alshreef A, Palmer R, Cross E, Dimairo M, et al. Self-managed, computerised word finding therapy as an add-on to usual care for chronic aphasia post-stroke: an economic evaluation. Clin Rehabil 2021;35:703-17. https://doi.org/10.1177/0269215520975348.
- Whitehurst DGT, Latimer NR, Kagan A, Palmer R, Simmons-Mackie N, Victor JC, et al. Developing accessible, pictorial versions of health-related quality-of-life instruments suitable for economic evaluation: a report of preliminary studies conducted in Canada and the United Kingdom. PharmacoEcon Open 2018;2:225-31. https://doi.org/10.1007/s41669-018-0083-2.
- Whitehurst DGT, Latimer NR, Kagan A, Palmer R, Simmons-Mackie N, Hoch JS. Preference-based health-related quality of life in the context of aphasia: a research synthesis. Aphasiology 2015;29:763-80. https://doi.org/10.1080/02687038.2014.985581.
- McCrone P, Allcock LM, Burn DJ. Predicting the cost of Parkinson’s disease. Mov Disord 2007;22:804-12. https://doi.org/10.1002/mds.21360.
- Weir S, Samnaliev M, Kuo TC, Tierney TS, Walleser Autiero S, Taylor RS, et al. Short- and long-term cost and utilization of health care resources in Parkinson’s disease in the UK. Mov Disord 2018;33:974-81. https://doi.org/10.1002/mds.27302.
- Brady MC, Clark AM, Dickson S, Paton G, Barbour RS. The impact of stroke-related dysarthria on social participation and implications for rehabilitation. Disabil Rehabil 2011;33:178-86. https://doi.org/10.3109/09638288.2010.517897.
- Hoppitt TJ, Shah S, Bradburn P, Gill PS, Calvert MJ, Pall HS, et al. Reaching the ‘hard to reach’: strategies to recruit black and minority ethnic service users with rare long-term neurological conditions. Int J Soc Res Methodol 2012;15:485-95.
- Palmer R, Dimairo M, Latimer N, Cross E, Brady M, Enderby P, et al. Computerised speech and language therapy or attention control added to usual care for people with long-term post-stroke aphasia: the Big CACTUS three-arm RCT. Health Technol Assess 2020;24:1-176. https://doi.org/10.3310/hta24190.
- Burns Curtis L. Canterbury: Personal Social Services Research Unit, University of Kent; 2015.
- Curtis L. Canterbury: Personal Social Services Research Unit, University of Kent; 2014.
- HMRC . Travel — Mileage and Fuel Rates and Allowances n.d. www.gov.uk/government/publications/rates-and-allowances-travel-mileage-and-fuel-allowances/travel-mileage-and-fuel-rates-and-allowances (accessed 9 February 2022).
- Regtransfers . The UK Taxi Price Index n.d. www.regtransfers.co.uk/fungames/uk-taxi-price-index (accessed 9 February 2022).
- Whatprice? . Train Prices n.d. www.whatprice.co.uk/travel/train-prices.html (accessed 9 February 2022).
Appendix 1 Results
FIGURE 31.
Rate of recruitment against target recruitment.

FIGURE 32.
Trial site recruitment.

| LSVT (N = 130) | Reason |
|---|---|
| 21 patients did not start LSVT | |
| − Cross-over to control (7) | Outside catchment for SLT |
| Patient caring for family then uncontactable | |
| Patient felt no issue with voice | |
| Site told patient they were randomised to control | |
| SLT too intense | |
| Travel issues (2) | |
| − Dead (1) | Died before they could start intervention |
| − No intervention (9) | Change in diagnosis after consent |
| Declined SLT due to pandemic | |
| Hospitalised | |
| No record of intervention at site | |
| Did not get a response to parking cost refund | |
| No SLT available due to pandemic (2) | |
| Uncontactable for SLT (2) | |
| − No treatment data available (1) | No staff to complete forms |
| − Withdrew (3) | Felt SLT too troublesome |
| Not well enough for SLT | |
| SLT too intense | |
| 109 patients started LSVT | |
| − 105 patients completed LSVT | |
| − 4 patients did not complete LSVT | |
| − Cross-over to control (1) | Outside catchment area for SLT |
| − No confirmation of treatment completion (2) | Had 6 sessions but no confirmation if they had their last session |
| Had 2 sessions but no confirmation if they had their last session | |
| − No intervention (1) | Incomplete investigation of swallowing |
| NHS SLT (N = 129) | |
| 10 patients did not start NHS SLT | |
| − No intervention (7) | Did not attend SLT sessions |
| Moved away, unable to have SLT | |
| No SLT available due to pandemic (3) | |
| Patient did not need SLT | |
| Staffing issue at site | |
| − No treatment data available (2) | Forms not completed by site |
| No staff to complete forms | |
| − Withdrew (1) | Deteriorating condition |
| 119 patients started NHS SLT | |
| − 118 patients completed NHS SLT | |
| − 1 patient did not complete NHS SLT | |
| − No intervention (1) | No information on treatment at site |
| No. of forms expected |
Returned (comp) (%) |
Returned (incomp) (%) |
Not returned (%) |
Response rate (%) |
Median time to completion (months) (range) |
|
|---|---|---|---|---|---|---|
| VHI | ||||||
| Baseline | 388 | 382 (98) | 4 (1) | 2 (1) | 99 | 0.0 (−1.9–9.1) |
| 3 months | 375 | 306 (82) | 17 (4) | 52 (14) | 86 | 3.0 (1.8–12.2) |
| 6 months | 355 | 294 (83) | 12 (3) | 49 (14) | 86 | 6.0 (4.9–15.5) |
| 12 months | 342 | 285 (83) | 15 (5) | 42 (12) | 88 | 12.1 (11.6–26.0) |
| PDQ-39 | ||||||
| Baseline | 388 | 370 (95) | 16 (4) | 2 (1) | 99 | 0.0 (−1.9–9.1) |
| 3 months | 375 | 310 (83) | 15 (4) | 50 (13) | 87 | 3.0 (1.8–12.2) |
| 6 months | 355 | 290 (82) | 16 (4) | 49 (14) | 86 | 6.0 (4.9–15.5) |
| 12 months | 342 | 288 (84) | 12 (4) | 42 (12) | 88 | 12.1 (11.6–26.0) |
| QSAD (LwD) | ||||||
| Baseline | 388 | 368 (95) | 18 (4) | 2 (1) | 99 | 0.0 (−1.9–9.1) |
| 3 months | 375 | 306 (82) | 18 (5) | 51 (13) | 86 | 3.0 (1.8–12.2) |
| 6 months | 355 | 289 (82) | 15 (4) | 51 (14) | 86 | 6.0 (4.9–15.5) |
| 12 months | 342 | 286 (84) | 15 (4) | 41 (12) | 88 | 12.1 (11.6–26.0) |
| Carer QoL | ||||||
| Baseline | 194 | 184 (95) | 4 (2) | 6 (3) | 97 | 0.0 (−1.6–2.8) |
| 3 months | 189 | 160 (85) | 5 (2%) | 24 (13) | 87 | 3.0 (2.5–6.6) |
| 6 months | 175 | 154 (88) | 2 (1) | 19 (11) | 89 | 6.0 (5.5–10.1) |
| 12 months | 169 | 139 (82) | 3 (2) | 27 (16) | 84 | 12.1 (0.1–14.5) |
| Clinical data | ||||||
| Baseline | 388 | 354 (91) | 32 (8) | 2 (1) | 99 | 0.0 (−1.8–51.5) |
| 12 months | 347 | 224 (64) | 103 (30) | 20 (6) | 94 | 13.4 (8.6–57.1) |
| LSVT | NHS SLT | |
|---|---|---|
| Number randomised | 130 | 129 |
| Initial interview | ||
| Not returned | 21 | 11 |
| Returned | 109 | 118 |
| Completed 0–1 week after randomisation (%) | 10 (9) | 12 (10) |
| Completed 1–2 weeks after randomisation (%) | 14 (13) | 14 (12) |
| Completed 2–3 weeks after randomisation (%) | 26 (24) | 32 (27) |
| Completed 3–4 weeks after randomisation (%) | 21 (19) | 29 (25) |
| Completed 4–6 weeks after randomisation (%) | 20 (19) | 18 (15) |
| Completed 6–8 weeks after randomisation (%) | 9 (8) | 6 (5) |
| Completed > 8 weeks after randomisation (%) | 9 (8) | 7 (6) |
| Intervention logs | ||
| Not returned | 2 | 1a |
| Returned | 107 | 118b |
| Has had last session | 105 | 118 |
| Completed intervention within 3 months of randomisation c | 88 (84%) | 70 (59%) |
| Completed intervention within 3–4 months of randomisation | 10 (9%) | 13 (11%) |
| Completed intervention within 4–5 months of randomisation | 1 (1%) | 13 (11%) |
| Completed intervention within 5–6 months of randomisation | 1 (1%) | 5 (4%) |
| Completed > 6 months after randomisation | 5 (5%) | 17 (15%) |
| Sensitivity – adherence | ||||||
|---|---|---|---|---|---|---|
| Mean (SD, n) | Mean difference (99% CI)a | |||||
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | |
| Per-protocol analysis | ||||||
| Baseline | 49.5 (23.6, 67) | 47.0 (26.4, 55) | 43.0 (21.2, 83) | – | – | – |
| 3 months | 35.0 (20.6, 64) | 44.3 (26.4, 52) | 39.9 (20.9, 77) | −9.7 (−16.0 to −3.4); p < 0.0001 | 1.1 (−5.6 to 7.8); p = 0.7 | −10.8 (−17.8 to −3.8); p < 0.0001 |
| Analysis including just those adherent to treatment | ||||||
| Baseline | 47.9 (23.7, 75) | 46.1 (26.0, 61) | 42.0 (21.5, 98) | – | – | – |
| 3 months | 34.6 (20.3, 71) | 43.2 (25.8, 58) | 40.1 (21.5, 92) | −9.8 (−15.8 to −3.9); p < 0.0001 | −0.2 (−6.5 to 6.2); p = 0.9 | −9.7 (−16.3 to −3.0); p = 0.0002 |
| Analysis including just those who completed VHI within the assessment window (3 ± 1 months) | ||||||
| Baseline | 46.7 (22.8, 93) | 46.1 (24.8, 96) | 43.6 (21.1, 88) | – | – | – |
| 3 months | 34.7 (19.6, 89) | 45.2 (25.7, 91) | 39.9 (20.7, 81) | −7.8 (−13.6 to −2.0); p = 0.0005 | 2.7 (−3.1 to 8.5); p = 0.2 | −10.5 (−16.1 to −4.9); p < 0.0001 |
| Sensitivity - analyses for missing scores | ||||||
|---|---|---|---|---|---|---|
| Mean (SD, n) | Mean difference (99% CI)a | |||||
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | |
| Assuming worst score for missing response | ||||||
| Baseline | 45.8 (22.4, 111) | 45.1 (24.5, 107) | 43.1 (21.6, 105) | – | – | – |
| 3 months | 36.5 (21.4, 111) | 44.3 (25.2, 107) | 41.6 (21.9, 105) | −7.2 (−12.6 to −1.7); p = 0.0007 | 1.1 (−4.3 to 6.6); p = 0.6 | −8.3 (−13.7 to −2.9); p < 0.0001 |
| Assuming best score for missing response | ||||||
| Baseline | 45.8 (22.4, 111) | 45.1 (24.5, 107) | 43.1 (21.6, 105) | – | – | – |
| 3 months | 36.4 (21.1, 111) | 44.1 (25.2, 107) | 41.2 (21.7, 105) | −7.0 (−12.4 to −1.6); p = 0.0009 | 1.3 (−4.1 to 6.7); p = 0.5 | −8.3 (−13.6 to −2.9); p < 0.0001 |
| Assuming worst score in LSVT and NHS SLT arm and best score in control | ||||||
| Baseline | 45.8 (22.4, 111) | 45.1 (24.5, 107) | 43.1 (21.6, 105) | – | – | – |
| 3 months | 36.5 (21.4, 111) | 44.3 (25.2, 107) | 41.2 (21.7, 105) | −6.8 (−12.2 to −1.4); p = 0.001 | 1.5 (−4.0 to 6.9); p = 0.5 | −8.3 (−13.7 to −2.9); p < 0.0001 |
| Assuming best score in LSVT and NHS SLT arm and worst score in control | ||||||
| Baseline | 45.8 (22.4, 111) | 45.1 (24.5, 107) | 43.1 (21.6, 105) | – | – | – |
| 3 months | 36.4 (21.1, 111) | 44.1 (25.2, 107) | 41.6 (21.9, 105) | −7.3 (−12.7 to −1.9); p = 0.0005 | 1.0 (−4.5 to 6.4); p = 0.7 | −8.3 (−13.6 to −2.9); p < 0.0001 |
| Assuming best score in LSVT and Control arm and worst score in NHS SLT arm | ||||||
| Baseline | 45.8 (22.4, 111) | 45.1 (24.5, 107) | 43.1 (21.6, 105) | – | – | – |
| 3 months | 36.4 (21.1, 111) | 44.3 (25.2, 107) | 41.2 (21.7, 105) | −7.0 (−12.4 to −1.6); p = 0.0009 | 1.5 (−3.9 to 6.9); p = 0.5 | −8.5 (−13.8 to −3.1); p <0.0001 |
| Assuming best score in LSVT and worst score in NHS SLT and control arm | ||||||
| Baseline | 45.8 (22.4, 111) | 45.1 (24.5, 107) | 43.1 (21.6, 105) | – | – | – |
| 3 months | 36.4 (21.1, 111) | 44.3 (25.2, 107) | 41.6 (21.9, 105) | −7.3 (−12.7 to −1.9); p = 0.0005 | 1.1 (−4.3 to 6.6); p = 0.6 | −8.5 (−13.8 to −3.1); p < 0.0001 |
| Assuming mean score of complete responses per domain | ||||||
| Baseline | 45.8 (22.4, 111) | 45.1 (24.5, 107) | 43.1 (21.6, 105) | – | – | – |
| 3 months | 36.5 (21.3, 111) | 44.2 (25.3, 107) | 41.4 (21.9, 105) | −7.0 (−12.4 to −1.6); p = 0.0009 | 1.2 (−4.2 to 6.7); p = 0.6 | −8.2 (−13.6 to −2.9); p < 0.0001 |
| Using multiple imputation | ||||||
| 3 months | 36.0 (2.0, 20)b | 44.2 (2.4, 20)b | 40.8 (2.1, 20)b | −6.9 (−10.9 to −2.9); p = 0.0008 | 1.8 (−2.3 to 5.9); p = 0.4 | −8.7 (−12.7 to −4.7); p < 0.0001 |
| Mean (SD, n) | Interaction p-value |
Mean difference (99% CI)a | |||||
|---|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | ||
| VHI subgroup | |||||||
| VHI ≤ 33 | 21.6 (16.0, 34) | 24.3 (13.1, 31) | 18.6 (12.1, 30) | 0.007 | −0.6 (−10.1 to 8.9) | 4.0 (−5.7 to 13.6) | −4.6 (−13.9 to 4.8) |
| VHI 34−44 (mild) | 31.9 (13.4, 19) | 34.2 (18.5, 20) | 38.5 (14.7, 22) | −6.5 (−18.2 to 5.3) | −3.4 (−15.0 to 8.3) | −3.1 (−15.2 to 9.0) | |
| VHI 45−61 (moderate) | 39.4 (15.6, 27) | 49.3 (12.6, 31) | 49.6 (15.7, 27) | −10.0 (−20.2 to 0.3) | −1.3 (−11.2 to 8.6) | −8.6 (−18.5 to 1.3) | |
| VHI > 61 (severe) |
50.1 (21.6, 26) | 78.4 (19.7, 20) | 64.4 (10.3, 19) | −16.0 (−27.4 to −4.7) | 8.2 (−4.2 to 20.5) | −24.2 (−35.5 to −12.9) | |
| Age subgroup | |||||||
| Age ≤ 59 years | 40.6 (19.3, 10) | 65.5 (28.4, 13) | 56.7 (22.4, 9) | 0.7 | −11.6 (−28.9 to 5.7) | 0.9 (−15.5 to 17.3) | −12.5 (−28.5 to 3.4) |
| Age 60–70 years | 30.5 (21.7, 43) | 40.3 (25.0, 38) | 35.0 (21.0, 35) | −8.8 (−17.4 to −0.2) | 3.1 (−5.7 to 11.9) | −11.9 (−20.3 to −3.5) | |
| Age > 70 years | 37.5 (18.5, 53) | 42.1 (21.3, 51) | 41.4 (20.5, 54) | −6.0 (−13.3 to 1.3) | 0.6 (−6.7 to 8.0) | −6.6 (−14.0 to 0.8) | |
| Hoehn and Yahr subgroup | |||||||
| H&Y 1–2.5 | 33.0 (20.2, 78) | 41.4 (23.0, 77) | 38.1 (22.4, 75) | 0.7 | −8.5 (−14.7 to −2.2) | 0.6 (−5.6 to 6.8) | −9.0 (−15.2 to −2.9) |
| H&Y 3–5 | 40.5 (19.1, 28) | 53.8 (28.1, 25) | 48.4 (16.2, 23) | −6.4 (−17.2 to 4.3) | 5.0 (−6.0 to 16.0) | −11.4 (−22.0 to −0.9) | |
| Pre- vs. post-COVID-19 subgroup | |||||||
| Pre COVID | 34.6 (20.0, 98) | 45.1 (25.2, 96) | 41.2 (21.0, 90) | 0.1 | −8.9 (−14.5 to −3.4) | 1.8 (−3.8 to 7.3) | −10.7 (−16.1 to −5.2) |
| During COVID | 39.6 (22.7, 8) | 33.0 (14.5, 6) | 32.1 (25.6, 8) | 3.5 (−15.6 to 22.5) | −0.2 (−20.8 to 20.4) | 3.6 (−16.9 to 24.2) | |
| Mean (SD, n) | Mean difference (99% CI)a | |||||
|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | |
| N = 103 | N = 101 | N = 97 | ||||
| Baseline | 46.5 (22.5, 103) | 45.9 (24.8, 101) | 43.9 (21.8, 97) | – | – | – |
| 3 months | 34.6 (20.0, 98) | 45.1 (25.2, 96) | 41.2 (21.0, 90) | −8.9 (−14.5 to −3.4); p < 0.0001 | 1.7 (−3.8 to 7.3); p = 0.4 | −10.7 (−16.1 to −5.2); p < 0.0001 |
| Mean (SD, n) | Mean difference (99% CI)a | p-valueb | |||||
|---|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | ||
| Baseline | 44.6 (21.9, 130) | 46.2 (24.8, 129) | 44.3 (22.3, 129) | – | – | – | |
| 6 months | 36.7 (24.1, 100) | 43.6 (25.0, 96) | 40.6 (22.4, 98) | −7.2 (−13.3 to −1.1) | −0.01 (−6.2 to 6.2) | −7.2 (−13.3 to −1.0) | |
| 12 months | 38.2 (24.0, 97) | 42.0 (24.1, 92) | 42.5 (22.4, 96) | −6.7 (−12.6 to −0.8) | −1.1 (−7.1 to 4.9) | −5.6 (−11.5 to 0.4) | |
| Overallc | – | – | – | −6.7 (−11.4 to −2.0); p = 0.0002 | 0.6 (−4.2 to 5.3); p = 0.8 | −7.3 (−12.0 to −2.6); p < 0.0001 | 0.6 |
| Mean (SD, n) | Mean difference (99% CI)a | p-valueb | |||||
|---|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | ||
| Emotional subscale | |||||||
| Baseline | 13.3 (8.8, 130) | 14.2 (10.1, 127) | 13.6 (9.0, 126) | – | – | – | |
| 3 months | 9.7 (8.0, 110) | 13.0 (9.6, 106) | 12.2 (8.4, 104) | −3.0 (−5.1 to −0.9); p = 0.0003 | 0.2 (−1.9 to 2.4); p = 0.8 | −3.2 (−5.3 to −1.1); p < 0.0001 | |
| 6 months | 9.8 (9.0, 102) | 12.8 (9.6, 99) | 11.7 (8.8, 100) | −3.0 (−5.5 to −0.6) | −0.3 (−2.7 to 2.2) | −2.8 (−5.2 to −0.4) | |
| 12 months | 10.8 (9.0, 102) | 12.6 (9.6, 97) | 12.7 (8.2, 99) | −2.6 (−5.0 to −0.3) | −0.4 (−2.8 to 2.0) | −2.2 (−4.6 to 0.1) | |
| Overallc | – | – | – | −2.7 (−4.5 to −0.9); p = 0.0002 | 0.001 (−1.9 to 1.9); p = 1.0 | −2.7 (−4.5 to −0.9); p = 0.0001 | 0.7 |
| Functional subscale | |||||||
| Baseline | 15.3 (7.4, 130) | 15.7 (8.6, 127) | 15.1 (8.0, 128) | – | – | – | |
| 3 months | 12.5 (6.8, 108) | 15.3 (8.7, 104) | 14.6 (7.7, 100) | −2.9 (−4.8 to −1.1); p < 0.0001 | −0.0 (−1.9 to 1.9); p = 1.0 | −2.9 (−4.7 to −1.1); p < 0.0001 | |
| 6 months | 12.7 (7.8, 101) | 15.4 (8.8, 98) | 14.0 (7.7, 99) | −2.5 (−4.5 to −0.6) | −0.1 (−2.0 to 1.9) | −2.5 (−4.4 to −0.5) | |
| 12 months | 13.9 (8.0, 99) | 14.4 (8.3, 94) | 14.4 (7.9, 98) | −1.5 (−3.5 to 0.5) | −0.4 (−2.4 to 1.6) | −1.1 (−3.0 to 0.9) | |
| Overallb | − | – | – | -2.2 (−3.8 to −0.6); p = 0.0004 | −0.1 (−1.7 to 1.5); p = 0.9 | −2.1 (−3.7 to −0.5); p = 0.0006 | 0.4 |
| Physical subscale | |||||||
| Baseline | 16.0 (7.9, 130) | 16.7 (7.6, 126) | 15.8 (6.8, 128) | – | – | – | |
| 3 months | 13.7 (7.5, 110) | 16.0 (7.9, 106) | 14.4 (6.8, 103) | −1.5 (−3.4 to 0.4); p = 0.04 | 0.7 (−1.3 to 2.6); p = 0.4 | −2.2 (−4.1 to −0.3); p = 0.003 | |
| 6 months | 14.2 (8.4, 102) | 15.5 (7.0, 99) | 14.5 (7.2, 102) | −1.5 (−3.6 to 0.5) | −0.3 (−2.4 to 1.7) | −1.2 (−3.2 to 0.8) | |
| 12 months | 14.3 (8.5, 100) | 15.8 (8.2, 97) | 15.4 (7.6, 99) | −2.0 (−4.1 to 0.1) | −0.4 (−2.5 to 1.7) | −1.6 (−3.7 to 0.5) | |
| Overallc | – | – | – | −1.5 (−3.2 to 0.1); p = 0.02 | 0.1 (−1.6 to 1.8); p = 0.8 | −1.7 (−3.3 to −0.02); p = 0.009 | 0.6 |
| Mean (SD, n) | Mean difference (99% CI)a | p-valueb | |||||
|---|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | ||
| Baseline | 29.9 (19.9, 123) | 31.5 (19.9, 122) | 30.2 (19.0, 123) | – | – | – | |
| 3 months | 23.5 (19.4, 103) | 27.6 (21.0, 100) | 26.6 (19.1, 103) | −5.4 (−9.8 to −1.0); p = 0.002 | −1.1 (−5.6 to 3.3); p = 0.5 | −4.3 (−8.7 to 0.1); p = 0.01 | |
| 6 months | 24.6 (21.3, 98) | 28.0 (20.6, 92) | 26.4 (20.0, 99) | −4.0 (−8.6 to 0.7) | −1.0 (−5.8 to 3.7) | −2.9 (−7.7 to 1.8) | |
| 12 months | 25.6 (21.2, 94) | 30.4 (21.6, 95) | 28.1 (19.4, 97) | −4.6 (−9.8 to 0.6) | 0.3 (−4.9 to 5.5) | −4.8 (−10.1 to 0.4) | |
| Overallc | – | – | – | −4.9 (−8.7 to −1.1); p = 0.0009 | −0.8 (−4.7 to 3.0); p = 0.6 | −4.1 (−7.9 to −0.3); p = 0.006 | 0.7 |
| Mean (SD, n) | Mean difference (99% CI)a | p-valueb | |||||
|---|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | ||
| Summary index | |||||||
| Baseline | 27.9 (16.6, 130) | 29.5 (16.5, 128) | 28.4 (15.2, 128) | – | – | – | |
| 3 months | 27.6 (17.6, 111) | 28.8 (16.1, 107) | 29.2 (15.9, 107) | −2.2 (−5.6 to 1.1); p = 0.08 | −1.2 (−4.6 to 2.2); p = 0.4 | −1.0 (−4.4 to 2.3); p = 0.4 | |
| 6 months | 27.4 (18.4, 104) | 29.2 (17.7, 100) | 27.6 (15.7, 102) | −1.6 (−5.4 to 2.2) | −0.03 (−3.9 to 3.8) | −1.6 (−5.4 to 2.2) | |
| 12 months | 28.5 (17.6, 102) | 31.6 (17.1, 98) | 29.8 (16.8, 100) | −2.1 (−6.1 to 2.0) | 1.1 (−3.0 to 5.2) | −3.1 (−7.2 to 0.9) | |
| Overallc | – | – | – | −1.8 (−4.9 to 1.4); p = 0.1 | −0.4 (−3.5 to 2.8); p = 0.8 | −1.4 (−4.5 to 1.7); p = 0.2 | 0.3 |
| Mobility | |||||||
| Baseline | 34.9 (28.6, 130) | 32.6 (27.6, 128) | 35.2 (27.9, 128) | – | – | – | |
| 3 months | 35.1 (28.7, 111) | 33.9 (28.6, 107) | 34.3 (27.3, 107) | −0.4 (−5.7 to 4.9); p = 0.9 | 1.5 (−3.9 to 6.9); p = 0.5 | −1.9 (−7.2 to 3.4); p = 0.4 | |
| 6 months | 36.0 (29.0, 104) | 33.7 (28.4, 100) | 33.8 (26.6, 102) | 0.6 (−5.1, 6.2) | 1.1 (−4.6, 6.8) | −0.5 (−6.2 to 5.1) | |
| 12 months | 37.1 (29.9, 102) | 38.5 (29.4, 98) | 37.5 (28.1, 100) | −1.0 (−7.6 to 5.7) | 2.9 (−3.8 to 9.7) | −3.9 (−10.6 to 2.8) | |
| Overallc | – | – | – | 0.1 (−4.8 to 4.9); p = 1.0 | 1.5 (−3.4 to 6.3); p = 0.4 | −1.4 (-6.2 to 3.4); p = 0.5 | 0.4 |
| Activities of daily living | |||||||
| Baseline | 35.6 (25.5, 130) | 36.0 (23.8, 128) | 34.1 (22.0, 128) | – | – | – | |
| 3 months | 35.3 (25.3, 111) | 32.9 (23.7, 107) | 35.1 (23.4, 107) | −2.9 (−7.8 to 2.0); p = 0.1 | −4.8 (−9.7 to 0.2); p = 0.01 | 1.8 (−3.0 to 6.7); p = 0.3 | |
| 6 months | 35.3 (26.1, 104) | 34.2 (25.0, 100) | 34.0 (21.8, 102) | −1.3 (−6.6 to 4.1) | −3.0 (−8.4 to 2.4) | 1.7 (−3.7 to 7.1) | |
| 12 months | 36.8 (26.0, 102) | 38.7 (24.6, 98) | 36.7 (23.5, 100) | −2.4 (−8.8 to 3.9) | −0.4 (−6.8 to 6.0) | −2.1 (−8.4 to 4.3) | |
| Overallc | – | – | – | −2.1 (−6.6 to 2.3); p = 0.2 | −3.8 (−8.3 to 0.7); p = 0.03 | 1.7 (−2.8 to 6.1); p = 0.3 | 0.2 |
| Emotional well-being | |||||||
| Baseline | 25.7 (20.1, 130) | 28.9 (21.7, 128) | 26.6 (19.1, 128) | - | - | - | |
| 3 months | 25.7 (21.2, 111) | 28.3 (21.0, 107) | 29.1 (19.6, 107) | −4.3 (−8.9 to 0.3); p = 0.02 | −3.1 (−7.7 to 1.5); p = 0.09 | −1.2 (−5.8 to 3.4); p = 0.5 | |
| 6 months | 25.0 (22.3, 104) | 29.0 (22.6, 100) | 26.8 (19.1, 102) | −3.6 (−8.8 to 1.5) | −1.0 (−6.3 to 4.2) | −2.6 (−7.8 to 2.6) | |
| 12 months | 26.2 (21.8, 102) | 29.6 (20.9, 98) | 29.1 (19.8, 100) | −3.5 (−8.9 to 1.9) | −1.0 (−6.4 to 4.5) | −2.6 (−8.0 to 2.9) | |
| Overall~ | -– | -– | – | – | |||
| Stigma | |||||||
| Baseline | 19.0 (22.9, 130) | 21.4 (23.3, 128) | 19.6 (18.0, 128) | – | – | - | |
| 3 months | 18.4 (21.5, 111) | 18.4 (21.6, 107) | 17.7 (19.7, 107) | 0.3 (−4.8 to 5.4); p = 0.9 | −0.02 (−5.2 to 5.2); p = 1.0 | 0.3 (−4.8 to 5.4); p = 0.9 | |
| 6 months | 19.8 (22.5, 104) | 19.5 (22.5, 100) | 17.9 (20.8, 102) | 0.01 (−5.4 to 5.4) | 0.3 (−5.2 to 5.7) | −0.2 (−5.6 to 5.1) | |
| 12 months | 19.4 (22.0, 102) | 21.0 (22.9, 98) | 19.1 (19.8, 100) | −0.8 (−6.7 to 5.2) | 1.0 (−5.0 to 7.0) | −1.8 (−7.8 to 4.1) | |
| Overallc | – | – | – | 0.3 (−4.1 to 4.8); p = 0.8 | 0.2 (−4.3 to 4.6); p = 0.9 | 0.2 (−4.3 to 4.6); p = 0.9 | 0.8 |
| Social support | |||||||
| Baseline | 9.3 (15.5, 130) | 11.0 (17.7, 128) | 11.6 (16.4, 128) | – | – | – | |
| 3 months | 10.8 (16.0, 111) | 12.4 (18.3, 107) | 12.5 (16.1, 107) | −1.3 (−6.2 to 3.6); p = 0.5 | −0.4 (−5.3 to 4.6); p = 0.8 | −0.9 (−5.8 to 4.0); p = 0.6 | |
| 6 months | 11.6 (17.4, 104) | 13.4 (19.1, 100) | 11.9 (16.0, 102) | −0.001 (−5.3 to 5.3) | 1.7 (−3.6 to 7.0) | −1.7 (−6.9 to 3.6) | |
| 12 months | 10.6 (15.9, 102) | 14.5 (20.8, 98) | 13.1 (18.6, 100) | −2.1 (−7.9 to 3.7) | 1.6 (−4.3 to 7.4) | −3.7 (−9.4 to 2.1) | |
| Overallc | – | – | – | −0.3 (−4.7 to 4.0); p = 0.8 | 0.9 (−3.5 to 5.3); p = 0.6 | −1.2 (−5.5 to 3.1); p = 0.5 | 0.6 |
| Cognition | |||||||
| Baseline | 27.5 (21.2, 130) | 33.8 (22.4, 128) | 29.9 (21.0, 128) | – | – | – | |
| 3 months | 28.7 (22.0, 111) | 31.1 (19.8, 107) | 32.4 (23.3, 107) | −1.6 (−7.0 to 3.8); p = 0.4 | −2.1 (−7.5 to 3.4); p = 0.3 | 0.5 (−4.9 to 5.9); p = 0.5 | |
| 6 months | 28.1 (22.0, 104) | 32.7 (21.8, 100) | 31.1 (22.5, 102) | −2.3 (−8.0 to 3.4) | −0.6 (−6.4 to 5.2) | −1.7 (−7.5 to 4.0) | |
| 12 months | 29.1 (21.7, 102) | 34.5 (21.0, 98) | 32.3 (20.7, 100) | −1.7 (−6.9 to 3.5) | 1.5 (−3.8 to 6.8) | −3.2 (−8.5 to 2.0) | |
| Overallc | – | – | – | −1.3 (−5.7 to 3.1); p = 0.4 | 0.4 (−4.0 to 4.9); p = 0.8 | −1.7 (−6.2 to 2.7); p = 0.3 | 0.3 |
| Communication | |||||||
| Baseline | 34.7 (22.0, 130) | 35.4 (23.3, 128) | 34.4 (24.2, 128) | – | – | – | |
| 3 months | 29.5 (24.6, 111) | 32.6 (22.4, 107) | 33.4 (23.9, 107) | −6.2 (−11.9 to −0.6); p = 0.004 | −2.7 (−8.4 to 3.0); p = 0.2 | −3.5 (−9.1 to 2.1); p = 0.1 | |
| 6 months | 26.5 (22.7, 104) | 32.0 (23.1, 100) | 29.4 (20.7, 102) | −5.8 (−11.6 to −0.05) | −0.7 (−6.5 to 5.1) | −5.1 (−10.9 to 0.7) | |
| 12 months | 29.8 (24.0, 102) | 33.6 (22.7, 98) | 31.1 (21.6, 100) | −3.8 (−9.8 to 2.1) | 1.0 (−5.0 to 7.1) | −4.9 (−10.9 to 1.1) | |
| Overall~ | – | – | – | – | |||
| Bodily discomfort | |||||||
| Baseline | 36.5 (26.3, 130) | 37.3 (24.3, 128) | 35.5 (21.6, 128) | – | – | – | |
| 3 months | 37.7 (26.0, 111) | 40.5 (25.0, 107) | 39.1 (23.0, 107) | −3.0 (−9.1 to 3.1); p = 0.2 | 1.3 (−4.9 to 7.5); p = 0.6 | −4.3 (−10.4 to 1.8); p = 0.07 | |
| 6 months | 36.6 (25.1, 104) | 38.9 (24.7, 100) | 36.0 (24.9, 102) | −2.0 (−8.5 to 4.5) | 1.6 (−4.9 to 8.2) | −3.6 (−10.1 to 2.9) | |
| 12 months | 38.6 (25.4, 102) | 42.3 (24.7, 98) | 39.7 (23.8,100) | −2.2 (−8.8 to 4.4) | 1.6 (−5.1 to 8.3) | −3.7 (−10.4 to 2.9) | |
| Overallc | – | – | – | −2.2 (−7.2 to 2.7); p = 0.2 | 1.8 (−3.3 to 6.8); p = 0.4 | −4.0 (−8.9 to 1.0); p = 0.04 | 1.0 |
FIGURE 33.
Mean PQD-39 summary index profile plot with error bars over 12 months by intervention.
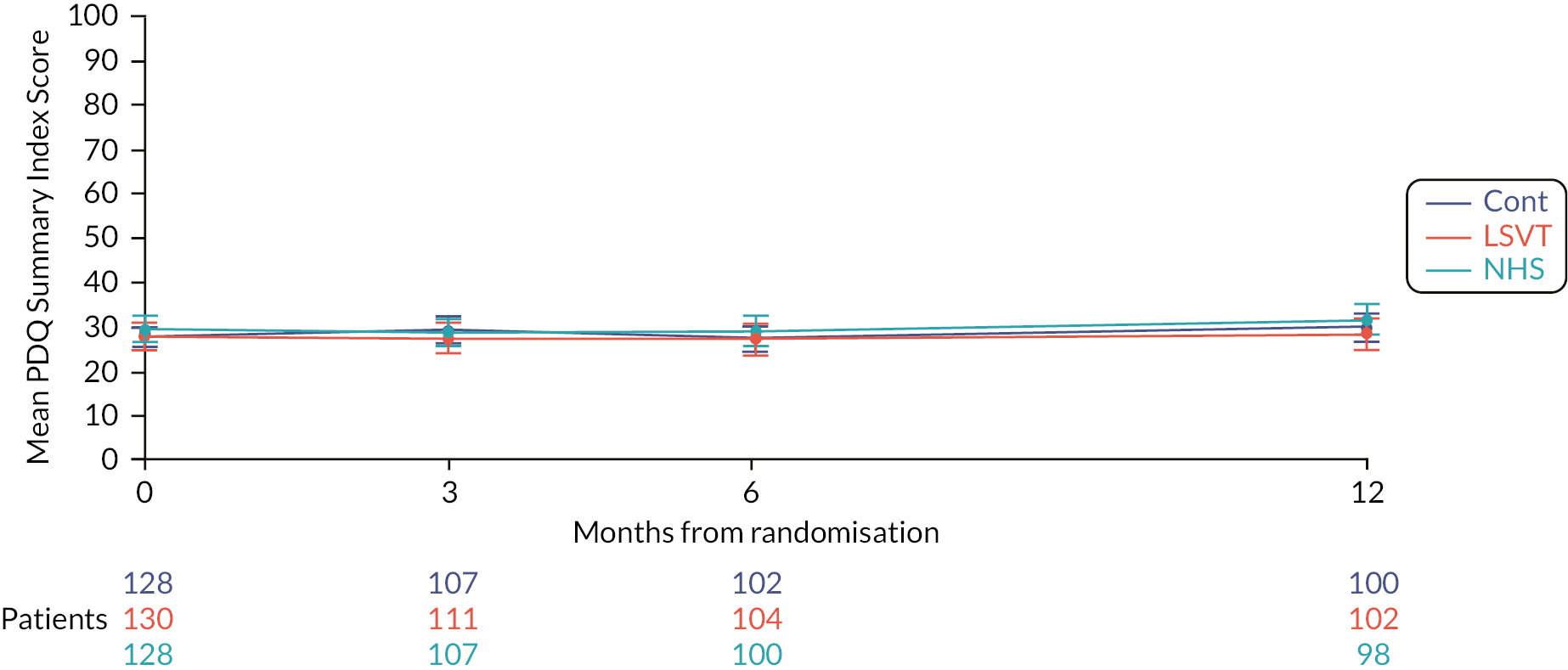
FIGURE 34.
Mean PDQ-39 mobility subscale profile plot with error bars over 12 months by intervention.

FIGURE 35.
Mean PDQ-39 activities of daily living subscale profile plot with error bars over 12 months by intervention.
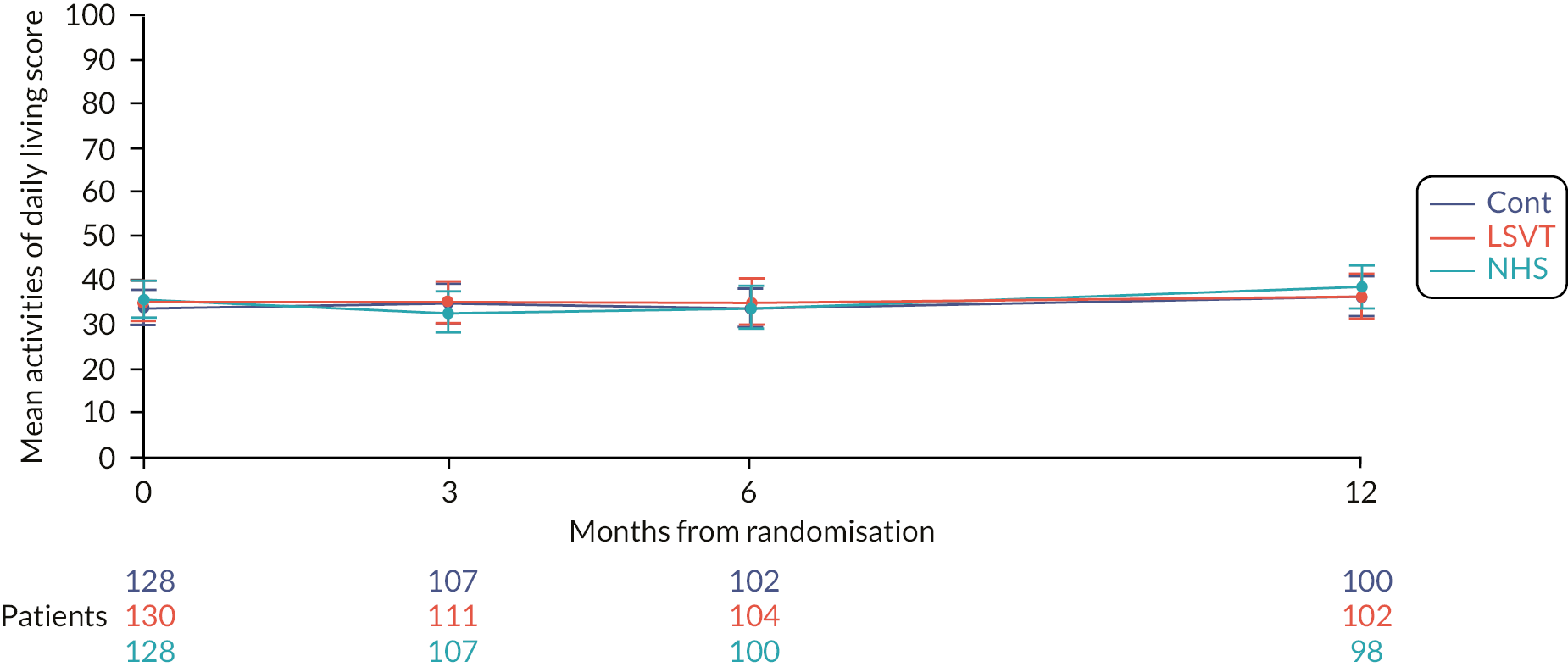
FIGURE 36.
Mean PDQ-39 emotional well-being subscale profile plot with error bars over 12 months by intervention.

FIGURE 37.
Mean PQD-39 stigma subscale profile plot with error bars over 12 months by intervention.

FIGURE 38.
Mean PDQ-39 cognition subscale profile plot with error bars over 12 months by intervention.
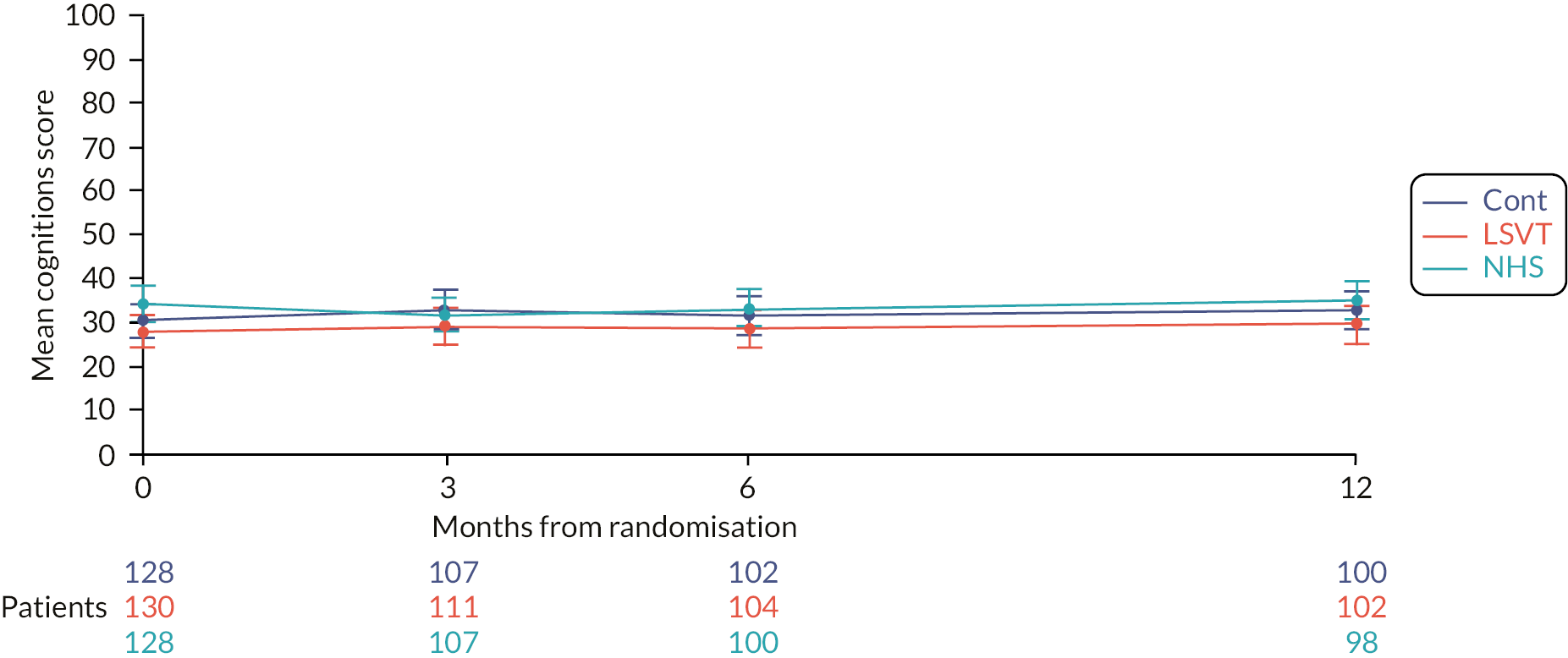
| Mean (SD, n) | Mean difference (99% CI)a | ||||||
|---|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | p-valueb | |
| EQ5D index score | |||||||
| Baseline | 0.64 (0.20, 129) | 0.61 (0.23, 128) | 0.61 (0.22, 128) | - | - | - | |
| 3 months | 0.61 (0.22, 111) | 0.61 (0.23, 106) | 0.63 (0.22, 106) | −0.02 (−0.08 to 0.03); p = 0.3 | −0.02 (−0.07 to 0.04); p = 0.5 | −0.01 (−0.07 to 0.05); p = 0.7 | |
| 6 months | 0.61 (0.23, 104) | 0.59 (0.23, 100) | 0.60 (0.22, 101) | 0.01 (−0.05 to 0.07) | −0.0009 (−0.06 to 0.06) | 0.01 (−0.05 to 0.07) | |
| 12 months | 0.59 (0.24, 102) | 0.56 (0.24, 98) | 0.56 (0.23, 100) | 0.02 (−0.04 to 0.09) | −0.01 (−0.07 to 0.05) | 0.03 (−0.03 to 0.10) | |
| Overallc | - | - | - | 0.002 (−0.5 to 0.05); p = 0.9 | −0.01 (−0.05 to 0.04); p = 0.7 | 0.01 (−0.04 to 0.06); p = 0.7 | 0.4 |
| EQ5D visual analogue score | |||||||
| Baseline | 68.2 (18.1, 130) | 67.4 (16.6, 128) | 67.5 (18.7, 128) | – | -– | – | |
| 3 months | 66.1 (20.8, 110) | 67.5 (18.9, 107) | 68.1 (17.6, 105) | −0.9 (−5.9 to 4.2); p = 0.7 | 1.3 (−3.8 to 6.4); p = 0.5 | −2.1 (−7.1 to 2.8); p = 0.3 | |
| 6 months | 65.4 (20.4, 103) | 66.4 (18.1, 99) | 66.0 (19.2, 101) | 0.9 (−4.9 to 6.7) | 2.5 (−3.4 to 8.4) | −1.6 (−7.4 to 4.3) | |
| 12 months | 68.0 (18.9, 102) | 64.7 (19.7, 99) | 65.1 (19.2, 100) | 3.2 (−2.7 to 9.1) | 1.7 (−4.3 to 7.6) | 1.6 (−4.4 to 7.5) | |
| Overallc | – | – | – | 0.7 (−3.7 to 5.1); p = 0.7 | 1.6 (−2.9 to 6.1); p = 0.4 | −0.9 (−5.3 to 3.5); p = 0.6 | 0.3 |
FIGURE 39.
Mean PDQ-39 bodily discomfort subscale profile plot with error bars over 12 months by intervention.

| Mean (SD, n) | Mean difference (99% CI)a | p-valueb | |||||
|---|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | ||
| Baseline | 0.81 (0.13, 129) | 0.81 (0.11,128) | 0.81 (0.13, 128) | – | – | – | |
| 3 months | 0.80 (0.15,109) | 0.80 (0.11, 107) | 0.82 (0.12, 106) | 0.001 (−0.03 to 0.03); p = 0.9 | −0.003 (−0.03 to 0.03); p = 0.8 | 0.004 (−0.03 to 0.04); p = 0.7 | |
| 6 months | 0.80 (0.15,103) | 0.79 (0.13, 99) | 0.80 (0.12, 100) | 0.02 (−0.01 to 0.05) | 0.005 (−0.03 to 0.04) | 0.01 (−0.02 to 0.05) | |
| 12 months | 0.80 (0.15, 102) | 0.76 (0.16, 98) | 0.79 (0.14, 100) | 0.02 (−0.01 to 0.06) | −0.02 (−0.06 to 0.02) | 0.04 (0.003 to 0.08) | |
| Overallc | – | – | – | 0.01 (−0.02 to 0.04); p = 0.4 | −0.003 (−0.03 to 0.02); p = 0.8 | 0.01 (−0.01 to 0.04); p = 0.2 | 0.2 |
| LSVT | NHS SLT | Control | |
|---|---|---|---|
| N = 130 | N = 129 | N = 129 | |
| Participants with carer (%) | 78 (60) | 86 (67) | 83 (64) |
| Number of consented carers (%) | 67/78 (86) | 67/86 (78) | 60/83 (72) |
| Relation of carer | |||
| Spouse (%) | 64 (96) | 61 (91) | 52 (86) |
| Child (%) | 2 (3) | 2 (3) | 4 (7) |
| Friend | 1 (1%) | 0 (–) | 0 (–) |
| Other | 0 (–) | 4 (6%) | 4 (7%) |
| Mean (SD, n) | Mean difference (99% CI)a | ||||||
|---|---|---|---|---|---|---|---|
| LSVT | NHS SLT | Control | LSVT vs. control | NHS SLT vs. control | LSVT vs. NHS SLT | p-valueb | |
| Summary index | |||||||
| Baseline | 28.4 (19.9, 61) | 24.8 (19.2, 65) | 24.9 (17.1, 58) | – | – | – | |
| 3 months | 27.5 (22.6, 54) | 29.9 (22.5, 56) | 22.8 (19.3, 50) | 0.6 (−5.6 to 6.9); p = 0.8 | 6.2 (0.1 to 12.3); p = 0.009 | −5.6 (−11.6 to 0.4); p = 0.02 | |
| 6 months | 26.4 (22.7, 55) | 31.2 (23.1, 54) | 25.1 (20.5, 45) | −3.3 (−10.3 to 3.6) | 3.7 (−3.2 to 10.6) | −7.0 (−13.6 to −0.4) | |
| 12 months | 30.9 (21.4, 51) | 34.1 (25.0, 48) | 28.6 (21.0, 40) | −1.3 (−9.5 to 7.0) | 6.2 (−2.1 to 14.5) | −7.5 (−15.3 to 0.3) | |
| Overallc | – | – | – | −0.9 (−6.6 to 4.9); p = 0.7 | 5.4 (−0.4 to 11.1); p = 0.02 | −6.3 (−11.8 to −0.7); p = 0.004 | 0.5 |
| Social and personal activities | |||||||
| Baseline | 26.5 (19.7, 62) | 24.9 (18.1, 66) | 24.4 (16.6, 58) | – | – | – | |
| 3 months | 27.4 (21.1, 55) | 30.0 (20.4, 57) | 23.7 (18.2, 51) | 0.6 (−5.3 to 6.5); p = 0.8 | 4.3 (−1.5 to 10.1); p = 0.06 | −3.7 (−9.3 to 1.9); p = 0.09 | |
| 6 months | 26.9 (22.1, 55) | 30.2 (21.6, 54) | 25.0 (20.7, 46) | −2.2 (−9.1 to 4.7) | 1.9 (−5.0 to 8.8) | −4.1 (−10.6 to 2.4) | |
| 12 months | 30.4 (19.5, 52) | 33.2 (23.9, 49) | 27.1 (20.0, 40) | 0.01 (−8.4 to 8.4) | 5.4 (−3.0 to 13.9) | −5.4 (−13.2 to 2.4) | |
| Overallb | – | – | – | −0.2 (−5.7 to 5.4); p = 0.9 | 4.0 (−1.6 to 9.5); p = 0.06 | −4.2 (−9.5 to 1.2); p = 0.04 | 0.7 |
| Anxiety and depression | |||||||
| Baseline | 26.1 (19.6, 63) | 22.8 (19.1, 65) | 22.7 (16.5, 59) | – | – | – | |
| 3 months | 23.5 (21.2, 56) | 29.0 (23.4, 57) | 21.3 (19.8, 50) | 0.3 (−6.5 to 7.1); p = 0.9 | 7.5 (0.8 to 14.3); p = 0.004 | −7.2 (−13.8 to −0.7); p = 0.005 | |
| 6 months | 23.6 (22.0, 56) | 28.5 (22.9, 54) | 24.1 (20.6, 46) | −3.5 (−11.4 to 4.4) | 2.9 (−5.0 to 10.8) | −6.4 (−14.0 to 1.1) | |
| 12 months | 27.0 (21.4, 51) | 28.7 (23.8, 50) | 25.9 (19.3, 40) | −1.5 (−9.5 to 6.6) | 3.6 (−4.5 to 11.8) | −5.1 (−12.8 to 2.5) | |
| Overallb | – | – | – | −1.0 (−7.1 to 5.2); p = 0.7 | 5.9 (−0.3 to 12.1); p = 0.01 | −6.9 (−12.9 to −0.9); p = 0.003 | 0.6 |
| Self-care | |||||||
| Baseline | 29.4 (22.0, 63) | 25.5 (22.2, 66) | 23.3 (19.4, 59) | – | – | – | |
| 3 months | 26.0 (24.1, 56) | 28.3 (26.0, 56) | 20.2 (20.4, 51) | 1.1 (−7.2 to 9.3); p = 0.7 | 5.8 (−2.4 to 14.0); p = 0.07 | −4.8 (−12.7 to 3.2); p = 0.1 | |
| 6 months | 26.5 (25.6, 55) | 29.9 (24.8, 54) | 24.0 (21.7, 45) | −2.8 (−11.8 to 6.2) | 2.8 (−6.0 to 11.7) | −5.7 (−14.1 to 2.8) | |
| 12 months | 29.3 (23.7, 52) | 34.2 (26.7, 50) | 27.9 (23.0, 40) | −3.7 (−13.8 to 6.3) | 6.3 (−3.7 to 16.4) | −10.1 (−19.5 to −0.6) | |
| Overallb | – | – | – | −1.3 (−8.8 to 6.1); p = 0.6 | 4.3 (−3.1 to 11.8); p = 0.1 | −5.7 (−12.8 to 1.5); p = 0.04 | 0.3 |
| Stress | |||||||
| Baseline | 33.3 (25.9, 62) | 26.8 (22.7, 66) | 29.1 (22.9, 59) | – | – | – | |
| 3 months | 30.7 (27.7, 56) | 32.9 (26.1, 57) | 25.6 (24.9, 51) | 1.8 (−6.0 to 9.6); p = 0.6 | 7.7 (−0.0 to 15.4); p = 0.01 | −5.9 (−13.4 to 1.7); p = 0.04 | |
| 6 months | 29.2 (26.2, 56) | 36.2 (27.6, 54) | 27.6 (24.2, 45) | −2.7 (−11.1 to 5.8) | 6.5 (−1.9 to 15.0) | −9.2 (−17.2 to −1.2) | |
| 12 months | 35.3 (27.0, 52) | 37.2 (30.7, 49) | 33.6 (27.4, 40) | 0.6 (−9.8 to 11.0) | 7.0 (−3.5 to 17.5) | −6.4 (−16.3 to 3.4) | |
| Overallb | – | – | – | −0.6 (−7.7 to 6.5); p = 0.8 | 6.4 (−0.6 to 13.4); p = 0.02 | −7.0 (−13.8 to −0.2); p = 0.008 | 0.4 |
FIGURE 40.
Mean PDQ-carer social and personal activities subscale profile plot with error bars over 12 months by intervention.

FIGURE 41.
Mean PDQ-carer self-care subscale profile plot with error bars over 12 months by intervention.
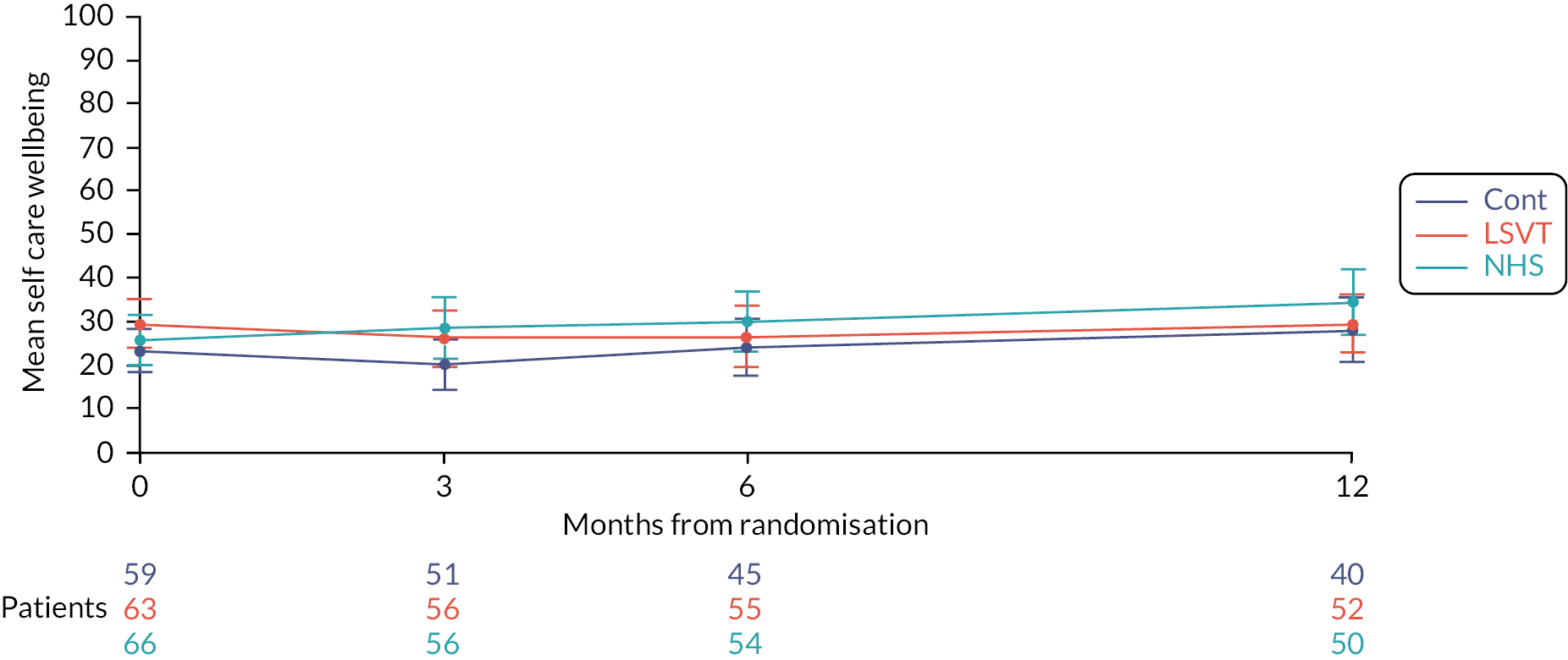
FIGURE 42.
Mean PQD-carer stress subscale profile plot with error bars over 12 months by intervention.
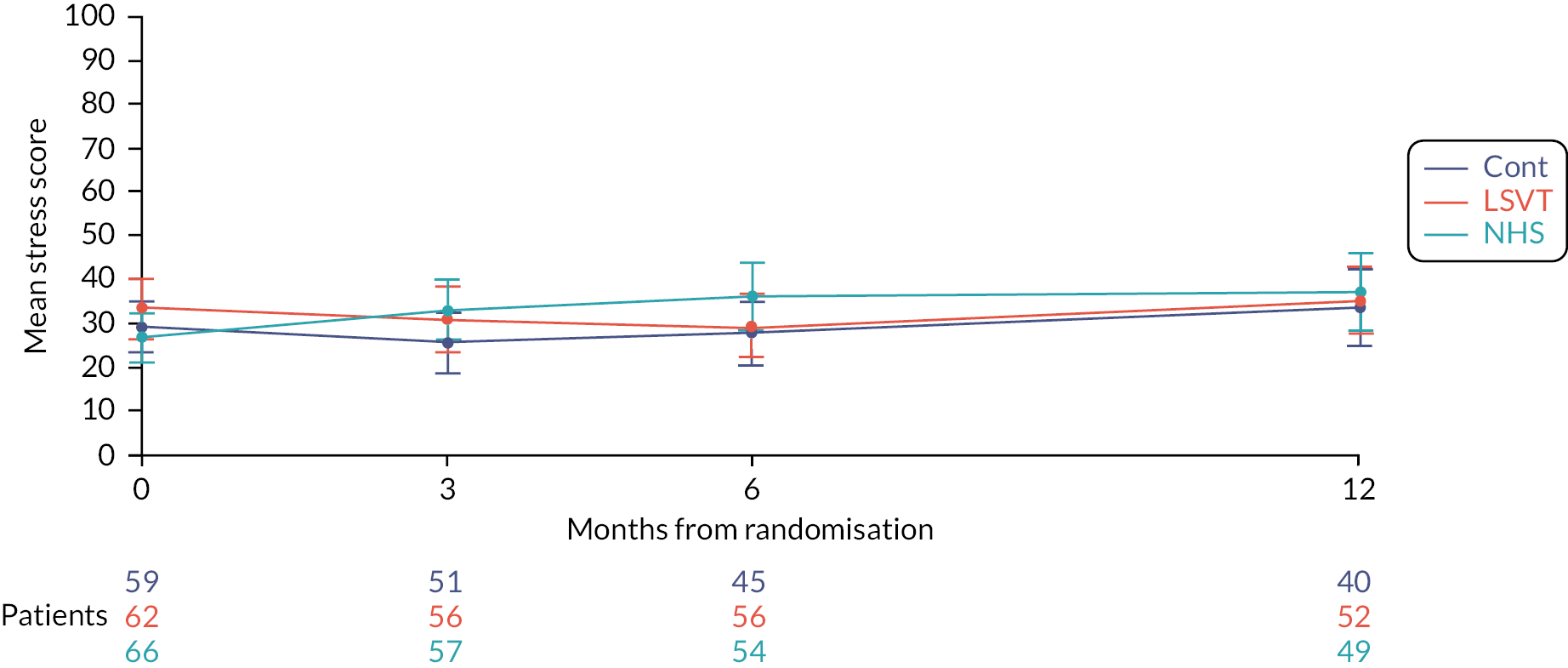
| AEs and SAEs by treatment group | ||||
|---|---|---|---|---|
| LSVT | NHS SLT | Control | ||
| N = 130 | N = 129 | N = 129 | ||
| Adverse events | ||||
| Number of AEs | 93 | 46 | 0 | |
| Description of AE | ||||
| Throat infection | 0 | 1 | – | |
| Throat ache | 6 | 0 | – | |
| Hard glottal attack | 2 | 0 | – | |
| Vocal strain | 80 | 45 | – | |
| Cold virus | 5 | 0 | – | |
| Number of participants who had an AE | N (%) | 36 (28) | 16 (12) | – |
| Serious adverse events | ||||
| Number of SAEs | 0 | 0 | 0 | |
| Number of participants who had an SAE | N (%) | – | – | – |
Appendix 2 Health economics
| Description of costs | Values | Assumptions |
|---|---|---|
| Number of trained LSVT SLTs per service | 2 | From the pilot study – most services were staffed with 1–3 full time equivalent therapists seeing PwPD. We are assuming that the therapist will work for 5 years. |
| Cost for training + update | £1374 over 5 years (£275 per year) | LSVT training cost of $580 (£440) per therapist plus estimate of £100/person travel plus $200 (£147) representing the cost of LSVT training update (every 2 years). The cost is spread over 5 years. |
| Companion software – clinician edition | £1020 over 5 years (£204 per year) | Cost of $683 (£510) per therapist over 5 years. |
| Average PwPD on SLT service caseload per year | 13 | From the pilot study – estimated from 65 services with a combined caseload of 820 PwPD. |
| Cost of training per PwPD | £21 per year | |
| Cost of companion software per PwPD – clinician edition | £16 per year | All PwPD in a service will at least use the software one time. |
| Type of resource use | NHS cost (£) | Unit of measure | Description/assumptions | Source |
|---|---|---|---|---|
| Trial intervention | ||||
| Training and set up | 21 | /participant | Details in Table 15 | Scobie et al. 202191 |
| LSVT companion software | 16 | /participant | LSVT Website20 | |
| NHS–laptop loan | 74 | /participant | Palmer et al. 2020129 | |
| Phone call | 0.07 | /minute | ||
| Speech and language therapy | ||||
| Community-based sessions by a therapist | 0.88 | /minute | Scientific and professional staff average of band 6 and 7 | PSSRU 2020100 |
| Community-based sessions by an assistant therapist | 0.52/minute | /minute | Scientific and professional staff band 4 | PSSRU 2020100 |
| Primary care and social care | ||||
| GP at practice | 39 | /visit | 9.22 minutes contact | PSSRU 2020100 |
| GP home visit | 100.62 | /visit | 11.4 minutes consultation + 12 minutes travel | PSSRU 2020100 |
| Nurse at practice | 10.85 | /visit | 15.5 minutes contact | |
| Nurse home visits | 21 | /visit | 30 minutes including travel time | PSSRU 2020100 |
| PD nurse specialist | 28 | /visit | Hospital-based nurse – band 6 assumes 30 minutes contact | PSSRU 2020100 |
| Health visitor | 19 | /visit | 20 minutes direct care (inflated to 2020 costs) | PSSRU 2015130 |
| Therapists and other healthcare professionals (community based) | ||||
| Physiotherapist | 53 | /session | Scientific and professional staff average of band 6 and 7 | PSSRU 2020100 |
| Occupational therapist | 49 | /session | Community occupational therapist (local authority) | PSSRU 2020100 |
| Additional SLT | 53 | /session | Scientific and professional staff average of band 6 and 7 | PSSRU 2020100 |
| Chiropodist/podiatrist specialist | 48 | /session | Scientific and professional staff average of band 6 | PSSRU 2020100 |
| District nurse | 42 | /session | – | NHS reference cost 19/20102 |
| Secondary care | ||||
| Outpatient | ||||
| A&E | 99.91 | /visit | Outpatient – non-consultant-led/follow-up visit | NHS reference cost 19/20102 |
| Audiology Medicine | 80.59 | /visit | Outpatient – non-consultant-led/follow-up visit | NHS reference cost 19/20102 |
| ENT | 109.23 | /visit | Outpatient – consultant-led/follow-up visit | NHS reference cost 19/20102 |
| Geriatric medicine | 250.08 | /visit | Outpatient – consultant-led/follow-up visit | NHS reference cost 19/20102 |
| Liaison psychiatrist | 258.78 | /visit | Outpatient – consultant-led/follow-up visit | NHS reference cost 19/20102 |
| Neurology | 187.17 | /visit | Outpatient – consultant-led/follow-up visit | NHS reference cost 19/20102 |
| Neurology nurse | 147.08 | /visit | Neurology outpatient – non-consultant-led/follow-up visit | NHS reference cost 19/20102 |
| Pain | 192.45 | /visit | Outpatient – consultant-led/follow-up visit | NHS reference cost 19/20102 |
| Physiotherapy | 60.80 | /visit | Outpatient – consultant-led/follow-up visit | NHS reference cost 19/20102 |
| Urology | 110.52 | /visit | Outpatient – consultant-led/follow-up visit | NHS reference cost 19/20102,131 |
| Social services | ||||
| Home care | 40 | /hour | Community care package for older person | PSSRU 2014131 |
| Day centre | 61 | /attendance | Community care package for older person | PSSRU 2014131 |
| Luncheon Club | 7 | /meal | Community care package for older person – meal on wheel | PSSRU 2014131 |
| Sitting service | 120 | /day | Private company | |
| Medication | ||||
| Levodopa Levodopa/Entacapone Dopamine MAOB inhibitor COMT inhibitor |
Participant specific | BNF101 | ||
| Institutional care | ||||
| Residential or nursing home (NHS) | 177 | /day | Local authority own-provision residential care for older people (age 65+) | PSSRU 2020100 |
| Residential or nursing home (privately funded) | 123 | /day | Private sector nursing homes for older people (age 65+) | PSSRU 2020100 |
| Productivity | ||||
| Full time | 15.64 | /hour | Hourly pay – average for male and female/full time and part time over the age of 60 years | ONS 2020106 |
| Transportation | ||||
| Bicycle | 0.20 | /mile | Travel–mileage and fuel rates and allowances (business mile) | HR revenue and customs132 |
| Bus | Free | /mile | Assuming over 60s concessionary bus pass | |
| Car | 0.25 | /mile | Travel–mileage and fuel rates and allowances (business mile) | HR revenue and customs132 |
| Taxi | 2.8 | /mile | Obtained from private company | Online source133 |
| Train | 0.11 | /mile | Price for people over 60s assuming that they pay 2/3 of the original price | Online source134 |
| Questionnaire | LSVT | NHS SLT | No SLT (control) |
|---|---|---|---|
| n (%) | 130 | 129 | 129 |
| EQ-D | |||
| Intention to treat | |||
| Completed any follow-up data (%) | 116 (89) | 113 (88) | 112 (87) |
| Available data | |||
| Completed – baseline | 129 | 128 | 128 |
| Completed – 3 months | 111 | 106 | 107 |
| Completed – 6 months | 104 | 101 | 103 |
| Completed – 12 months | 102 | 100 | 103 |
| Complete data | |||
| Completed at all time points (%) | 92 (71) | 89 (69) | 92 (71) |
| ICECAP-O | |||
| Intention to treat (any follow-up data) | |||
| Completed any follow-up data (%) | 116 (89) | 113 (88) | 112 (87) |
| Available data | |||
| Completed – baseline | 129 | 128 | 128 |
| Completed – 3 months | 109 | 107 | 106 |
| Completed – 6 months | 103 | 100 | 101 |
| Completed – 12 months | 102 | 99 | 102 |
| Complete data | |||
| Completed at all time points (%) | 91 (70) | 89 (69) | 90 (70) |
| Resource use | |||
| Intention to treat (any follow-up data) | |||
| (%) | 116 (89) | 113 (88) | 112 (87) |
| Complete data | |||
| Completed at all time points (%) | 93 (72) | 92 (71) | 92 (71) |
| SLT treatment log | |||
| Complete data | |||
| Completed forms (%) | 107 (82) | 117 (91) | 5 (4) |
| All questionnaires | |||
| Complete case (%) | 80 (62) | 81 (63) | 90 (70) |
| Outcome measure/follow-up point | LSVT | Standard NHS SLT | No SLT (control) |
|---|---|---|---|
| EQ-5D-5L scores | |||
| Baseline | |||
| N | 129 | 128 | 128 |
| Mean (SD) | 0.637 (0.196) | 0.605 (0.228) | 0.613 (0.220) |
| 3 months | |||
| n | 111 | 106 | 107 |
| Mean (SD) | 0.605 (0.219) | 0.611 (0.225) | 0.624 (0.223) |
| 6 months | |||
| n | 104 | 101 | 103 |
| Mean (SD) | 0.606 (0.232) | 0.585 (0.237) | 0.590 (0.231) |
| 12 months | |||
| n | 102 | 99 | 103 |
| Mean (SD) | 0.590 (0.242) | 0.552 (0.247) | 0.547 (0.247) |
| ICECAP-O scores (full capability) | |||
| Baseline | |||
| n | 129 | 128 | 128 |
| Mean (SD) | 0.812 (0.126) | 0.808 (0.113) | 0.811 (0.133) |
| 3 months | |||
| N | 109 | 107 | 106 |
| Mean (SD) | 0.803 (0.146) | 0.802 (0.114) | 0.816 (0.116) |
| 6 months | |||
| n | 103 | 100 | 101 |
| Mean (SD) | 0.799 (0.151) | 0.782 (0.150) | 0.790 (0.144) |
| 12 months | |||
| n | 102 | 99 | 102 |
| Mean (SD) | 0.799 (0.148) | 0.749 (0.173) | 0.773 (0.175) |
| Cost category | LSVT n = 93 |
NHS SLT n = 92 |
No SLT (control) n = 92 |
|||
|---|---|---|---|---|---|---|
| Resource unit, mean (SD) | Cost (£), mean (SD) | Resource unit, mean (SD) | Cost (£), mean (SD) | Resource unit, mean (SD) | Cost (£), mean (SD) | |
| Health and social care services (privately funded) | ||||||
| Physiotherapist | 1.77 (7.64) | 94.03 (405.16) | 0.98 (4.16) | 51.85 (220.42) | 0.62 (3.10) | 32.84 (164.22) |
| Additional SLT | 0.01 (0.10) | 0.57 (5.50) | 0.00 | 0.00 | 0.17 (1.67) | 9.22 (88.41) |
| Occupational therapist | 0.04 (0.33) | 2.11 (16.04) | 0.01 (0.10) | 0.53 (5.11) | 0.07 (0.53) | 3.20 (25.99) |
| Institutional care (privately funded) | 1.94 (18.67) | 238.06 (2295.81) | 0.98 (9.38) | 120.326 (1154.13) | – | – |
| Other servicesa | 2.03 (4.27) | 27.75 (86.29) | 3.15 (11.43) | 44.26 (229.66) | 1.86 (4.84) | 29.22 (128.27) |
| Out-of-pocket expenditure | ||||||
| Travelb | – | 99.60 (240.90) | – | 52.02 (114.11) | – | 51.69 (102.29) |
| PD medication | – | 2.63 (9.94) | – | 1.53 (12.667) | – | 2.30 (13.59) |
| Other expenses | – | 444.93 (1457.57) | – | 227.73 (618.76) | – | 463.11 (1728.41) |
| Travel to attend outpatient sessions (miles)c | 182.12 (225.06) | 60.81 (155.89) | 61.55 (74.61) | 26.14 (69.91) | – | – |
| Productivity | ||||||
| Reduced work (hours)d | 20.69(111.34) | 323.56 (1741.36) | 12.86 (68.67) | 201.11 (1073.96) | 10.88 (52.51) | 170.17 (821.22) |
| Lost time from stopping work completely (hours)d | 29.07 (128.57) | 454.74 (2010.84) | 99.20 (258.13) | 1551.42 (4037.22) | 73.48 (244.59) | 1149.2 (3825.38) |
| Incremental cost (£) Mean (95% CI) |
Incremental effectiveness | ICER Cost (£) per QALY |
|
|---|---|---|---|
| Analysis from the NHS/PSS perspective | |||
| LSVT vs. no SLT (control) | 2378.10 (1554.54 to 3586.44) | 0.008 (−0.020 to 0.040) | 297,262 |
| NHS SLT vs. no SLT (control) | 899.93 (386.37 to 1435.69) | −0.005 (−0.039 to 0.026) | Dominated |
| LSVT vs. NHS SLT | 1336.65 (426.94 to 2411.46) | 0.012 (−0.017 to 0.042) | 111,387 |
| Analysis from the broader societal perspective (excluding PSS costs) | |||
| LSVT vs. no SLT (control) | 922.56 (−26.65 to 1788.62) | 0.008 (−0.020 to 0.040) | 115,320 |
| NHS SLT vs. no SLT (control) | 754.03 (−251.52 to 1757.57) | −0.005 (−0.039 to 0.026) | Dominated |
| LSVT vs. NHS SLT | 118.67 (−834.29 to 1022.71) | 0.012 (−0.017 to 0.042) | 9889 |
| Analysis from the broader societal perspective (including PSS costs) | |||
| LSVT vs. no SLT (control) | 1718.48 (490.00 to 3403.83) | 0.008 (−0.020 to 0.040) | 214,810 |
| NHS SLT vs. no SLT (control) | 1001.20 (−183.49 to 2120.68) | −0.005 (−0.039 to 0.026) | Dominated |
| LSVT vs. NHS SLT | 531.12 (−555.82 to 1927.70) | 0.012 (−0.017 to 0.042) | 44,260 |
| Vocal folds examination by an ENT consultant for LVST | |||
| LSVT vs. no SLT (control) | 1686.91 (1403.58 to 1967.04) | 0.008 (−0.020 to 0.040) | 210,864 |
| NHS SLT vs. no SLT (control) | 650.13 (334.92 to 915.46) | −0.005 (−0.039 to 0.026) | Dominated |
| LSVT vs. NHS SLT | 1031.33 (745.82 to 1352.63) | 0.012 (−0.017 to 0.042) | 85,944 |
| Complete case analysis | |||
| LSVT vs. no SLT (control) | 1487.34 (1175.47 to 1823.01) | 0.019 (−0.015 to 0.051) | 78,281 |
| NHS SLT vs. no SLT (control) | 592.62 (300.05 to 919.79) | 0.004 (−0.031 to 0.040) | 148,155 |
| LSVT vs. NHS SLT | 914.06 (591.50 to 1208.67) | 0.014 (−0.020 to 0.048) | 65,290 |
| Per protocol – adherent | |||
| LSVT vs. no SLT (control) | 1674.30 (1382.17 to 2000.42) | 0.030 (−0.018 to 0.076) | 55,810 |
| NHS SLT vs. no SLT (control) | 607.70 (308.19 to 901.47) | −0.014 (−0.072 to 0.034) | Dominated |
| LSVT vs. NHS SLT | 1065.62 (728.12 to 1404.41) | 0.033 (−0.021 to 0.09) | 32,291 |
Glossary
Intention to treat Where an analysis is based on the initial treatment assignment regardless of subsequent treatment or not.
Behavioural compensatory strategies Modification to behaviour to compensate for a deficit, weakness, injury or inadequacy in a specific skill.
Dysarthria Difficulty with the muscle movements required to produce speech as a result of neurological changes or damage.
Equipoise Uncertainty regarding the relative value of therapeutic arms of a clinical trial.
Minimisation Method of adaptive stratified randomisation.
List of abbreviations
- AAC
- augmentative and alternative communication
- AE
- adverse event
- BCTU
- Birmingham Clinical Trials Unit
- CRF
- case report form
- DMC
- Data Monitoring Committee
- EQ-5D-5L
- EuroQol5D (5-level version)
- GSE
- general self-efficacy scale
- HTA
- Health Technology Assessment
- ICECAP-O
- ICEpop Capabilities Measure for Older Adults
- ICER
- incremental cost-effectiveness ratio
- LSVT LOUD
- Lee Silverman Voice Treatment
- MCID
- minimal clinically important difference
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NPT
- normalisation process theory
- NSOME
- non-speech oral motor exercises
- PD
- Parkinson’s disease
- PDQ-39
- Parkinson’s Disease Questionnaire-39
- PIS
- patient information sheet
- PPI
- patient and public involvement
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- QASD
- Questionnaire on Acquired Speech Disorders
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SLT
- speech and language therapy
- TRF
- treatment record form
- TSC
- Trial Steering Committee
- VHI
- Voice Handicap Index
- YSC
- year of sufficient capability
