Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/57/85. The contractual start date was in May 2017. The draft report began editorial review in October 2021 and was accepted for publication in September 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Przulj et al. This work was produced by Przulj et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – Journals Library, and the DOI of the publication must be cited.
2023 Przulj et al.
Chapter 1 Introduction
Smoking in pregnancy is associated with increased risks of miscarriage, stillbirth, prematurity, low birthweight, perinatal morbidity and mortality, neonatal death and sudden infant death. 1 In the UK, the annual smoking-attributable maternal and infant health-care costs are estimated at £20–87.5 million. 2 As smoking in pregnancy remains highest among socially disadvantaged women,3 eliminating it could reduce socioeconomic inequalities in stillbirths and infant deaths. 4 There is systematic review-level evidence that when pregnant smokers successfully quit, infants’ birth outcomes improve5 and it is likely that other morbidities in both mothers and children can be reduced if mothers stop smoking. 1
Finding effective interventions that help pregnant smokers quit has proven difficult. Although around 50% of pregnant smokers try quitting,3 spontaneous quit rates in pregnant smokers in the UK are low. 6 Advice by health professionals combined with behavioural support and pharmacotherapy can provide some help, but the effect is limited. 7–9 The National Institute for Health and Care Research (NIHR) and Chief Scientist Office funded several large randomised controlled trials (RCTs) to address this problem. An exercise intervention with multi-session behavioural support improved exercise levels but did not help with smoking cessation10 [7% of pregnant smokers achieved abstinence by the end of pregnancy (EOP)]. Financial incentives have shown promising results, but there are practical issues in applying this approach in routine care. 8,11 Trials of nicotine patches (NPs)12,13 provided reassuring data on the safety of nicotine replacement in pregnancy, but patches lacked efficacy (9% maintained abstinence to delivery). The two other smoking cessation medications, varenicline and bupropion, include labelling that cautions against use in pregnancy. E-cigarettes (ECs) appear to be the most promising of the remaining options, with a realistic chance of providing practical help to pregnant smokers and their children, but no study has tested them in this context so far.
E-cigarettes are used with increasing frequency by smokers wishing to limit or stop smoking. 14 ECs allow self-titration of nicotine intake15 and apart from nicotine, they also provide a degree of sensorimotor replacement and enjoyment. 16 This is the most likely explanation of the fact that ECs are more popular among smokers than nicotine replacement therapy (NRT)14 and generate higher user ratings and better adherence. 17
E-cigarettes do not contain most of the chemicals responsible for health risks of smoking and those that are present are there at levels much lower than in cigarette smoke. 18 A recent Cochrane review reported no safety concerns over EC use for up to 2 years. 19 Some dangers may yet emerge over long-term use, but taking such uncertainty into account, the overall risks are estimated to be some 95% lower than risks of smoking. 18 A controversy continues over EC use among young people, in particular, whether this is deflecting them away from smoking or attracting them to it,20 but expanding evidence suggests that ECs have a potential to act as treatment for smokers who cannot stop smoking unaided. In cohort studies, promising proportions of hard-to-reach smokers given ECs reduced or stopped smoking, including smokers unwilling to quit and smokers with schizophrenia. 21–23 Some UK Stop Smoking Services (SSSs) are now using ECs and report encouraging results. 24,25 SSS monitoring data show that smokers using ECs have higher cessation rates than smokers using other approaches. 25 Several randomised trials have evaluated ECs for smoking cessation and the Cochrane review concluded that ECs are an effective treatment. The conclusion is marked as of moderate certainty due to the limited number of trials, but the uncertainty concerns the size of the effect rather than its direction because ECs deliver nicotine and there is ample evidence that NRT is effective. 26
Pregnant smokers typically use NRT reluctantly, only when asked to do so by their health-care providers, but some use ECs spontaneously despite the fact that NRT is provided free on the NHS while ECs have to be purchased. 27,28 A survey of 252 US obstetrician-gynaecologists found that the majority want to know more about the safety and efficacy of ECs in pregnancy29 and commentators have called for trials of efficacy and safety of ECs in pregnancy including commentators with an anti-EC stance. 27,28 The results from these independent sources of information are consistent in suggesting that pregnant smokers are likely to be generally open to EC use, and that there is an urgent need for information on EC safety and efficacy in this group.
Some of the barriers to NRT use may not apply to EC use. Pregnant smokers seem to dislike oral NRT products and adherence to NP use is poor,12,30 for example, only 7% of women used patches for >1 month in a large UK trial. 12 Pregnant smokers may be finding patch use by itself unrewarding as the patches provide nicotine slowly without any immediate feedback or sensorimotor reinforcement. Pregnant women also metabolise nicotine faster than other smokers and may need higher nicotine doses delivered at a faster rate than standard NRT formulations provide. 31
In contrast to NRT products, ECs are more attractive to smokers, allow extensive individualisation (of flavour, ease of use and other product characteristics) and deliver nicotine faster than NPs and oral NRT products. 32 Unlike NPs, ECs allow for titration of nicotine intake to smokers’ needs, which can be expected to translate to better withdrawal relief. It can thus be expected that pregnant smokers will adhere to EC treatment better than to NRT treatment.
In summary, there is a good rationale for testing the effectiveness of ECs as a stop-smoking treatment for pregnant smokers. This is an important group for whom few practicable and effective interventions exist. ECs have a theoretical potential to be of help and are already used by some pregnant smokers. Finding out whether ECs do help is an important priority.
Apart from questions of efficacy, there are also important questions about whether ECs are safe to use by pregnant smokers. Concerns about safety of nicotine in ECs are the same as concerns about safety of nicotine in NRT. Pregnant smokers already consume nicotine from cigarettes at doses that are higher than those provided by NRT and ECs. 18 There is a consensus that NRT is much safer than smoking in pregnancy and NRT is routinely used by UK pregnancy SSSs. 33 In addition to this, the large UK trial which monitored infant outcomes after NP use in pregnancy has shown better infant development at 2 years among children born to women randomised to NRT than those randomised to placebo. 13 The smoking-attributable fetal harm is likely caused by products of tobacco combustion such as carbon monoxide (CO) and chemicals such as polycyclic aromatic hydrocarbons which are strongly associated with reduced fetal growth in utero34 and which are virtually absent in both NRT and ECs.
Regarding adverse effects of other chemicals in EC aerosol, the main components of EC liquid are propylene glycol, which is approved for use in pregnancy (e.g. in asthma nebulisers), and glycerine, which has no known adverse effects (e.g. glycerine syrup is not considered to pose risks in pregnancy). ECs are used by millions of smokers and pose little risk over short-term use (up to 2 years). 19 To our knowledge, no chemicals other than nicotine have been identified that would be inhaled by vapers (EC users) in quantities likely to affect the health of the fetus.
In summary, there are some safety concerns, particularly regarding nicotine, but they are tempered by the fact that ECs are used as a replacement for cigarettes and that cigarette smoke contains numerous other chemicals responsible for fetal harm.
Chapter 2 Methods
Overview of trial design
This was a pragmatic multi-centre RCT comparing ECs with NPs, with both interventions accompanied by the same brief behavioural support.
Objectives and hypothesis
The objective of the study was to compare ECs and NPs in effectiveness in helping pregnant smokers quit and in their safety. We hypothesised that ECs would be more effective than NPs. We did not formulate any hypotheses regarding comparative safety of the two products.
Changes to trial design
Table 1 provides a summary of the changes made to the study protocol.
| Approved versiona | Date | Summary |
|---|---|---|
| 3.4 | 30 June 2017 | Ethics committee recommended that age eligibility criteria be included in protocol (missed in error) |
| 4.0 | 3 January 2018 | Change in sponsor representative; change to storage of paper forms |
| 5.0 | 28 February 2019 | Amendment to named statistician and change of name and address for CTU, data collection to include CO measurement to verify abstinence in those using a nicotine product, replacement of participants randomised but later found ineligible; addition of new questions on respiratory health; addition of online survey method for follow-up data collection; addition of collection of smoking status from hospital records at delivery |
| 6.0 | 14 August 2019 | Professor Christopher Griffiths added as new chief investigator; update to saliva payments |
| 7.0 | 3 February 2020 | Updates to protocol to reflect finalised statistical analysis plan |
Participants
Inclusion/exclusion criteria
Participants were eligible if they were pregnant (between 12 and 24 weeks’ gestation); aged ≥18 years or over; smoked on a daily basis and wanted help in stopping smoking; were willing to be randomised to use either NPs or ECs and to receive weekly support and follow-up calls; and could understand English to a level that permitted data collection.
Participants were ineligible if they had a known allergic reaction to nicotine skin patches; were currently using any NRT or EC on a daily basis; were taking part in another stop-smoking study; or had a serious medical problem or high-risk pregnancy at the time of initial consent. (Note: we did not withdraw participants if their pregnancy became high risk during the trial as this could have biased the evaluation of safety outcomes.)
Recruitment
Participants were recruited in several ways, depending on the resources of the site: identified by research midwives from patient records and sent the patient information sheet and invitation letters (alongside 12- or 20-week scan appointment letters if appropriate); invited via telephone, email or text; or approached in person and asked to complete a screening questionnaire when they attended antenatal hospital appointments. Community midwives who saw pregnant smokers at booking appointments or at other routine care appointments could refer pregnant smokers interested in the study to the research team. Specialist pregnancy stop-smoking advisors could also refer smokers interested in the study, and posters advertising the study were placed within the sites’ clinics (see Appendix 1).
Settings and locations
Participants were recruited from 23 hospital sites across England, and one NHS SSS in Scotland. Once randomised into the study, stop-smoking advisors and researchers at the Health and Lifestyle Research Unit (HAL), Queen Mary University of London (QMUL), delivered the interventions and conducted follow-up calls. Data were collected in person at the baseline visit, and over the phone or via online/postal questionnaires at follow-ups.
Trial procedures
Smokers who were interested in the study were provided with the patient information sheet. If they decided to take part, they were invited to the baseline visit, arranged where possible to coincide with their hospital appointments.
Baseline visit and informed consent procedures
At the baseline visit, a research midwife further explained the study and any queries were discussed. Eligibility to take part in the study was then checked, and participants signed the informed consent form and were given a copy for their records. Midwives taking consent undertook Good Clinical Practice training. After providing consent, participants were asked to complete a baseline questionnaire and to provide a saliva sample (which was then posted to HAL). They were then randomised to use either NPs or ECs. The midwife then demonstrated the relevant product to the participant and answered any questions. A convenient date and time was then arranged for the first support call with the stop-smoking advisor. This was usually in a week’s time.
Participants were informed that their product would be posted to them in time for their first call (for details see Interventions).
To limit contamination, participants were asked, should they wish to use the non-allocated product (ECs in the NP arm and NRT in the EC arm – use of other NRT products than patches in the NP arm was allowed), to refrain from doing so for at least the first 4 weeks of the intervention period.
Eligibility confirmation
Medically qualified site Principal Investigators (PIs) (usually a senior obstetrician) were asked to review the study documents and confirm the participants’ eligibility. As the site PIs were not present at baseline visits, this was normally done following randomisation, but PIs were blinded to treatment allocation during this process. In eight cases (four in each study arm), randomised participants were withdrawn before starting the intervention. In four cases the PIs considered the participants ineligible, in one case only verbal consent was taken, one pregnancy was terminated before the study product was received and one participant was using patches daily. One participant was randomised twice.
Interventions
E-cigarette arm
Participants were provided with a Tobacco Product Directive approved refillable EC starter kit (One Kit by the UK ECIG STORE®, Wembley, UK), together with two 10 ml bottles of tobacco-flavoured e-liquid (18 mg/ml) and a pack of five replacement coils. An instructional leaflet was also provided (see Appendix 2). The EC starter pack was posted to participants a few days ahead of their first support call. Further supplies of e-liquid were posted out to participants for up to 8 weeks. A lower strength e-liquid (11 mg) and fruit flavours were available if participants did not like the original e-liquid posted out, and participants were encouraged to shop around if these were also unsuitable or they preferred a different EC device to the one provided.
Nicotine patch arm
Participants were posted an initial 2-week supply of Nicorette® Invisi (McNeil Products Ltd., High Wycombe, UK) 15 mg/16-hour NPs a few days ahead of their first support call. They were instructed to use them every day, placing them on a suitable area of the skin in the morning upon waking and removing before bedtime. Further supplies were posted out to participants for up to 8 weeks. A lower strength patch (10 mg/16 hours) was available as an alternative. Participants were encouraged to seek out other NRT products (such as the gum, lozenges, spray, etc.) via their general practitioner (GP) or local SSS if they felt they were struggling with the patch alone. Standard stop-smoking advice was also provided (see Weekly support calls).
Weekly support calls
Participants in both arms were scheduled to receive six support calls on a weekly basis. The support provided followed that of usual advice given in the UK SSS35 and was conducted by trained stop-smoking advisors. Calls were scheduled at times convenient to the participants. If calls were not answered, a further two attempts to contact the participant were made via phone, followed by a text asking for when would be a good time to call.
A text reminder was sent the day before the first call. At this first call, the receipt of the product was checked and participants were guided on how to use it. They discussed their past experience with quit attempts and set up their target quit date (TQD). Participants could choose to start using their product immediately or wait until the TQD.
A second call was conducted on or near the TQD. The advisor checked on any product issues and discussed quitting strategies (e.g. preparing for the TQD, avoiding triggers to smoking and distracting themselves with other tasks). Participants were encouraged to avoid taking even a single puff of a cigarette from the TQD onwards and to use their products on a regular basis. The first call took up to 20 minutes, the other calls took on average 10 minutes.
If participants wanted to have their TQD straight after the first call, the contents of the first and second calls were combined.
During the further four weekly calls, the advisors checked on clients’ progress, supplies of e-liquid/patches and any product-related issues, and offered guidance on maintaining abstinence/stopping smoking. At the final call, participants were given information on how to get further product supplies if they wished to continue using the products (e.g. purchase own e-liquid or obtain NRT prescriptions from GP or from local SSS) and were informed that a final supply could be sent out to them in the next 2 weeks upon request. Participants were also reminded that they would be contacted for a follow-up around their due date.
Follow-up calls
Two follow-ups were conducted, one at the EOP to collect effectiveness and safety data and another at 3 months post partum, requested by the study funder, to collect additional health outcomes of the mother and infant. Text reminders were sent prior to these calls. If participants were not contactable via phone, then emails, texts, postal and online questionnaires were sent to obtain the data. The follow-up efforts at EOP used the following protocol: two calls and text in week 1; two calls and text in week 2; one call in week 3 followed by posting a questionnaire, emailing it and a text; no contact during weeks 4–5 to allow return of questionnaires; two calls in week 6; one text in week 7; two calls in week 8; one text in week 9; one call in week 10; one text in week 11 followed by an email; one call in week 12; one text in week 13; one call in week 14; and a final text in week 15. The follow-up efforts at 3 months post partum were as follows: two calls and text in week 1; one call in week 2 followed by posting and emailing of questionnaire and a text; no contact during weeks 3–4 to allow return of questionnaires; one call and one text in week 5; and a final call in week 6.
To prevent distress to participants, the co-ordinating office sent a list of participants due for follow-up to the local research teams to identify participants who had experienced an event which could make follow-up distressing, and to advise whether follow-up should be attempted. A text reminder was also sent to all participants the day before their follow-up call was due to take place. Participants were asked to text back if they did not wish to be called. Participants were also able to text back their smoking status if a call was not convenient.
As the majority of pregnant smokers who abstain during pregnancy return to smoking after delivery,6,36 the first follow-up call was made at 35 weeks’ gestation. Following an example from a recent trial,37 to increase the chance of reaching participants, the period for data collection was from 35 weeks’ gestation to 10 weeks post estimated delivery date.
Participants reporting abstinence from smoking, those who were using both cigarettes and NRT/ECs (‘dual users’) and those who reported a reduction of cigarette consumption of 50% or more were asked to provide a saliva sample. Saliva sample kits and instruction on use were posted with a self-addressed envelope for the return of the sample. Once received by the study team, the participants were sent a text to thank them and £20 was sent in the post for their time and effort.
During the last 14 months of the study, we also asked self-reported abstainers using nicotine-containing products to attend local study sites to provide a CO reading. Once the site sent the CO reading to the study team, the participants were sent a text to thank them and £20 was sent in the post for their time and effort.
A further amendment was approved in January 2020 to see if any improvement in sample returns could be achieved by including £10 with the sampling kit (an upfront payment) and sending a further £10 when the saliva sample was returned (total remained £20). This was implemented in February 2020.
Measures
The following were collected at baseline (for categories and units, e.g. age in years, ng/ml, see Table 3):
| Trial arm | ||
|---|---|---|
| Baseline characteristic | ECs (N = 571) | NPs (N = 569) |
| Age, years, median (IQR) | 26.6 (22.5–30.9) | 27.3 (23.6–31.1) |
| Education, n (%) | ||
| Primary and secondary school | 229 (40.1) | 234 (41.1) |
| Further education | 288 (50.4) | 273 (48.0) |
| Higher education | 54 (9.5) | 62 (10.9) |
| Employed, n (%) | 274 (48.0) | 257 (45.2) |
| Ethnicity, n (%) | ||
| White British | 513 (89.8) | 495 (87.0) |
| Other | 58 (10.2) | 74 (13.0) |
| Cigarettes per day, median (IQR) | 10 (7–15) | 10 (7–15) |
| FTCD, mean (SD) | 4.0 (2.1) | 4.3 (2.1) |
| Salivary cotinine levels, ng/ml, median (IQR) (N = 529 and 531, respectively)a | 111 (75.8–165) | 118 (73.9–176) |
| Lives with smoker, n (%) | 342 (59.9) | 328 (57.6) |
| Past treatment,b n (%) | ||
| Varenicline | 69 (12.1) | 79 (13.9) |
| NRT | 268 (46.9) | 273 (48.0) |
| Bupropion | 7 (1.2) | 5 (0.9) |
| None | 272 (47.6) | 267 (46.9) |
| Tried ECs in the past, n (%) | 288 (50.4) | 267 (46.9) |
-
demographics: age, ethnicity, employment, whether living with a partner and level of education
-
smoking history: Fagerström Test for Cigarette Dependence (FTCD),38 cigarettes per day, previous stop-smoking product use, longest previous period of abstinence and whether they live with other smokers
-
saliva sample to measure nicotine intake via cotinine levels.
The following were collected weekly (from 1 to 4 weeks post TQD), and at EOP:
-
smoking status
-
allocated product use during the trial: on how many days per week; product type; reasons for stopping/switching (where applicable); for ECs only: e-liquid flavour and strength
-
non-allocated product use (same as above)
-
contact with local SSS
-
adverse events (AEs) and adverse reactions (ARs), and infant AEs at 3 months post partum
-
at EOP only: weeks of using products regularly (on ≥5 days/week) and weeks of using occasionally (at least once a week)
-
respiratory health questionnaire asking about shortness of breath, phlegm, wheezing and cough39
-
in participants reporting abstinence from smoking: salivary cotinine and anabasine or CO reading (in abstainers using nicotine products)
-
in participants reporting reduction of smoking by at least 50% and in those reporting both smoking and using nicotine products: salivary cotinine.
Birth outcomes were also collected by research staff at the participating site via hospital records.
Chapter 3 Outcomes
Primary outcome
Prolonged abstinence from smoking from 2 weeks after the TQD until the EOP defined as per Russell Standard (up to five lapses allowed with no smoking at all during the previous week at the time of final follow-up)40 and validated by salivary cotinine (<10 ng/ml41) for those not reporting using any nicotine product or a CO of <8 parts per million or anabasine (<1 ng/ml) for those reporting other forms of nicotine use at the EOP follow-up.
We supplemented anabasine validation with CO validation when we noticed during an unrelated study that we were conducting at the time that salivary anabasine may be a less reliable indicator of smoking than thought. We used anabasine validation where CO readings were not available.
Participants with missing validation or missing information on smoking status were included as non-abstainers.
Sensitivity analyses for primary outcome
Three sensitivity analyses were conducted:
-
A per-protocol analysis where participants who did not set up a TQD, did not start product use or never established contact with the study team (unless EOP follow-up finds that they did start product use) were excluded from the analysis.
-
An analysis where missing smoking status was estimated using multiple imputation by chained equations and data were imputed 50 times separately for each arm. 42,43 The imputation model included variables associated with the primary outcome and/or its missingness: FTCD, living with a smoker, number of cigarettes smoked, education, occupation, daily use of allocated products on all 4 intervention weeks and point abstinence at week 4.
-
An analysis where abstainers who used non-allocated products (ECs in NP arm and NRT in EC arm) were excluded from the analysis. Such use was defined as use for at least 5 consecutive days during the 4 weeks post TQD or current use at EOP or reporting use during pregnancy for at least 1 week or occasional use for at least 3 weeks.
Secondary smoking status outcomes
Self-reported prolonged abstinence at EOP, 7-day and 2-month validated and self-reported abstinence at EOP and self-reported abstinence at 4 weeks. (Note: if participants were reached only post delivery and reported smoking now, but were abstinent at the time of delivery, they were included as self-reported abstainers.)
Proportion of participants who reduced their cigarette consumption by at least 50% at EOP compared to baseline, self-reported and also validated by a reduction of at least 50% in salivary cotinine levels (including abstainers; and only including non-abstainers).
Adherence outcomes
Number setting TQD, weekly support contacts completed, number receiving support from local SSS; number of weeks products were used regularly (≥5 days/week) and occasionally (<5 days/week), amount used per day, products used, product changes; and use of non-allocated products (same details as above).
Safety measures and health outcomes
Proportion of participants reporting AEs, serious adverse events (SAEs), ARs [using Medical Dictionary for Regulatory Activities (MedDRA) terminology] and new respiratory symptoms (phlegm, shortness of breath, cough and wheezing).
Birth outcomes
Terminations, miscarriage (non-live birth prior to 24 weeks’ gestation), stillbirth (non-live birth at 24 weeks’ gestation or later), neonatal death (from live birth to 28 days), post-neonatal death (29 days to 2 years), preterm birth (<37 weeks’ gestation), low birthweight (<2500 g), neonatal unit admissions, congenital abnormalities, caesarean section delivery, birthweight and gestational age.
Data collection and entry
A web-based application, using an Oracle 11g database, was used to collect data. Oracle Database 11g Enterprise Edition Release 11.2.0.1.0 (Oracle Corporation, Austin, TX, USA) was used for building the database and Java programming language for the building of the study application. This was set up and hosted by the Centre for Cancer Prevention, QMUL. Data were kept in accordance with good clinical practice and data protection requirements.
Sample size
We estimated the quit rate at delivery in the NP arm at 8%12 and in the EC arm at 14%39 [odds ratio 1.87, risk ratio (RR) = 1.75]. Using the power command in Stata® version 15 (StataCorp LP, College Station, TX, USA) indicated that 1140 participants would be needed for 90% power (570 in each condition; alpha = 0.05, two-tailed test) to detect this difference. This sample size would allow detecting smaller treatment effects with lower power, for example, 80% power to detect a difference between 8% and 13.1% (odds ratio 1.74, RR = 1.64).
Randomisation
Participants were randomised (1:1) via a pre-programmed list generated by an independent statistician using R Statistical Software (Foundation for Statistical Computing, Vienna, Austria). 44 The randomisation scheme consisted of a sequence of blocks of random size. The randomisation list was programmed into the study database application by the study programmer who was not involved in the recruitment of participants. Research midwives at participating sites enrolled participants and conducted the randomisation through the study database application. Study staff were unaware of the randomisation sequence.
Treatment blinding
Researchers and participants were blind to treatment allocation until the point of randomisation which was generated via the study database. Once randomised, participants were informed by the research midwife which treatment they would receive. Researchers conducting follow-up calls were blind to treatment allocation until condition-specific questions were reached. The trial statistician was blind to participant allocation until the analysis was complete.
Statistical methods
General principles
To describe participants’ demographics and smoking characteristics at baseline by arms, descriptive statistics are used: mean and standard deviation (SD) for continuous measures that are approximately symmetric; median and quartiles if the distribution was skewed. Discrete outcomes are described using both the number and proportion (percentage). Similarly, summary measures of the primary and secondary outcomes are presented.
Analyses follow intention-to-treat principles, meaning that all randomised participants are included in the analysis in the treatment group to which they were randomised. For each outcome measure we report:
-
the number of participants included in the analysis, by treatment group
-
a treatment effect (e.g. RR for ECs relative to NPs), with a 95% confidence interval (CI)
-
a two-sided p-value.
Binary outcomes are analysed using a binomial regression with a logarithmic link, which allows estimating RRs, calculated with the NP arm as the reference. If the model were not to converge, we would use a Poisson regression model with robust standard errors. For continuous outcomes, we opted for linear regression if the data were symmetric or non-parametric methods, if the data were not approximately symmetric.
Participants who withdrew from the study or were lost to follow-up were included as non-abstainers.
Further information on sensitivity analyses
The three sensitivity analyses of the primary outcome are described in Outcomes. The inclusion of multiple imputation analysis is problematic as it relies on the assumption that data are missing at random, while in smoking cessation trials, participants who fail in stopping smoking sometimes feel embarrassed and may be less likely to respond to follow-up contact. 45 It is, however, included for completeness. Below we present more details on the rationale for the sensitivity analysis that exclude abstainers using non-allocated products.
In our previous trial, participants assigned to NRT were more likely to use ECs than the other way round. 39 Our plan to control for this occurrence was to conduct a sensitivity analysis that excluded abstainers who could be assumed to have achieved abstinence via the non-allocated product (ECs in the NP arm and NRT in the EC arm). A statistical adjustment could not be used because non-allocated products were different in the two study arms. An alternative to our approach would be to exclude all users of non-allocated products, rather than just those who achieved abstinence. This, however, is not useful if contamination rates in the two study arms are different and the two contaminators have different efficacy. In this case, this approach overestimates the cessation rate in the arm allocated to the less effective treatment and underestimates the difference between the study arms. To illustrate this effect, let us assume that the quit rate is 10% with treatment A and 20% with treatment B. If the intervention is tested in a sample of 100 participants in each study arm, there will be 10 successful quitters in A and 20 in B. If all who fail with A (n = 90) try B and 20% succeed (n = 18) while half of those who fail with B try A (n = 40) and 10% succeed (n = 4), quit rates will be 28% [(10 + 18)/100)]and 24% [(20 + 4)/100] in the A and B arms, respectively, masking the real 10% versus 20% treatment difference. To try to control for the bias by excluding all users of non-allocated products (‘switchers’) changes this to an even less accurate success rates of 100% [10/(100 − 90)] versus 33% [20/(100 − 40)]. Excluding only abstinent switchers results in quit rates of 12% [10/(100 − 18)] versus 21% [20/(100 − 4)], the closest value to the true treatment effect. The true treatment effect (10% vs. 20%) would be shown if only abstinence that was achieved without regular use of non-allocated product is included, with ‘switchers’ re-classified as non-abstainers.
COVID-19 study impact
During the COVID-19 lockdown period (March 2020 onwards), it was noticed that the proportion of saliva samples returned to the research team was lower than observed pre-lockdown. We conducted an additional sensitivity analysis for the primary outcome (i.e. validated abstinence at EOP) to take into account the impact of lockdown on missing data (see Appendix 3).
Birth and maternal outcomes
The primary analysis was of singleton births. A sensitivity analysis included multiple births. Due to the correlated structure of this sensitivity analysis, we estimated standard errors allowing for intragroup correlation and estimated 95% CIs that account for clustering at the mother level. When no adverse birth outcome was recorded, we assumed that none had occurred. Elective terminations were excluded.
We also explored whether nicotine product use affects gestation age and birthweight in abstinent smokers (unadjusted) and while adjusting for time since last cigarettes using regression analysis or a non-parametric alternative if data are not symmetric.
For the safety analyses, the denominator excluded participants who withdrew from the study prior to delivery (n = 6).
Adverse events
The number and percentage of women reporting AEs, ARs and SAEs affecting them or their babies is reported. Participants/babies with multiple entries related to the same underlying cause are included in the most severe events category.
Summary statistics are presented to compare respiratory symptoms in the two study arms.
The statistical analysis plan was pre-registered on Open Science Framework (https://osf.io/dvh4a).
Bayes factor
For the key comparisons, we calculated Bayes factor (BF), which is the ratio of the likelihood of an alternative hypothesis to a null hypothesis. 46 A BF of 1–3 suggests no or low evidence of an effect, ≥3 to <10 moderate evidence and 10 and above strong evidence for the alternative hypothesis (H1). We specified a half-normal distribution (i.e. top half of a normal distribution with mode = 0) with the SD set to the expected effect size (i.e. log RR). The expected effect size was based on our previous EC versus NRT study for smoking cessation39 and on a study comparing nicotine and placebo NPs for effects of nicotine on birthweight. 12
Statistical software
The analyses were carried out using Stata version 16.1. 47
Participant withdrawal
Participants were able to withdraw from the study at any time. Participants who withdrew their consent for further data collection were not replaced. Those who withdrew prior to the EOP follow-up were included as non-abstainers. Data collected up to the point of the consent withdrawal were used.
Participants who were randomised in error and found ineligible by the site PIs, or during the first contact with the study team, or who were randomised twice by mistake, were withdrawn and replaced.
Patient and public involvement
Study design and protocol were discussed with smokers and stop-smoking advisers. A member of the public served on the Trial Steering Committee (TSC) and advised on participant engagement.
At our annual ‘Update and Supervision Days’ for SSS staff, we asked for input from advisers working with pregnant smokers. The advisors noted that EC use by pregnant smokers is becoming more common. There was a consensus that information on safety and efficacy of such use is urgently needed and that the trial results will have an important practical impact.
Study approvals and trial conduct
The study was sponsored by the QMUL Joint Management Research Office. Ethical approval was obtained from the National Research Ethics Service Committee London –South East (ref: 17/LO/0962) on 29 June 2017 and approvals were obtained from the Health Research Authority on 24 July 2017.
The study was approved by the Medicines and Healthcare Products Regulatory Agency on 5 June 2017 via the Clinical Trial of Investigational Medicinal Product (CTIMP) Notification Scheme. The study was pre-registered on ISRCTN, ref: ISRCTN62025374.
A Data Monitoring and Ethics Committee and a TSC were convened every 6–12 months during the recruitment and follow-up phase of the trial. All members were independent. A Trial Monitoring Group consisting of members of the study team also met regularly. Table 2 shows the members of the committees.
| Data Monitoring and Ethics Committee | Paul Aveyard (Chair) |
| Dominic Stringer (statistician) | |
| Anne Greenough (neonatologist) | |
| TSC | Jamie Brown (Chair) |
| Eleni Vangeli (expert in smoking cessation) | |
| Leoni Brose (expert in smoking cessation) | |
| Maryjane Winston (lay member) |
Quality control
A risk assessment was carried out in conjunction with the study sponsor and Barts CTU (the CTU at time of study commencement), which was used as a basis for the study monitoring plan. During the recruitment phase, a monitor from the co-ordinating site carried out a monitoring visit at each of the sites within 3 months of the first participant being enrolled. Monitoring visits were then carried out every 6 months. The monitoring was initially at the study site but later moved to remote working during the COVID-19 pandemic. The sponsor was responsible for oversight of the monitoring process and monitoring of the co-ordinating centre. Trial oversight was provided by the Barts CTU (between May 2017 and March 2019) and the King’s CTU (from April 2019).
Saliva analysis
ABS Laboratories was used for cotinine and anabasine analysis. ABS Laboratories is a good laboratory practice (GLP) accredited contract research organisation that specialises in the quantification of drugs, metabolites and biomarkers in biological and non-biological samples. Cotinine and anabasine in saliva samples were determined using high-performance liquid chromatography coupled to tandem mass spectrometry with multiple reaction monitoring.
Chapter 4 Results
The main results presented below were published in Nature Medicine (https://doi.org/10.1038/s41591-022-01808-0).
Participant flow
Figure 1 shows the Consolidated Standards of Reporting Trials (CONSORT) flow diagram. 48
FIGURE 1.
CONSORT flow diagram. * Four in each arm.
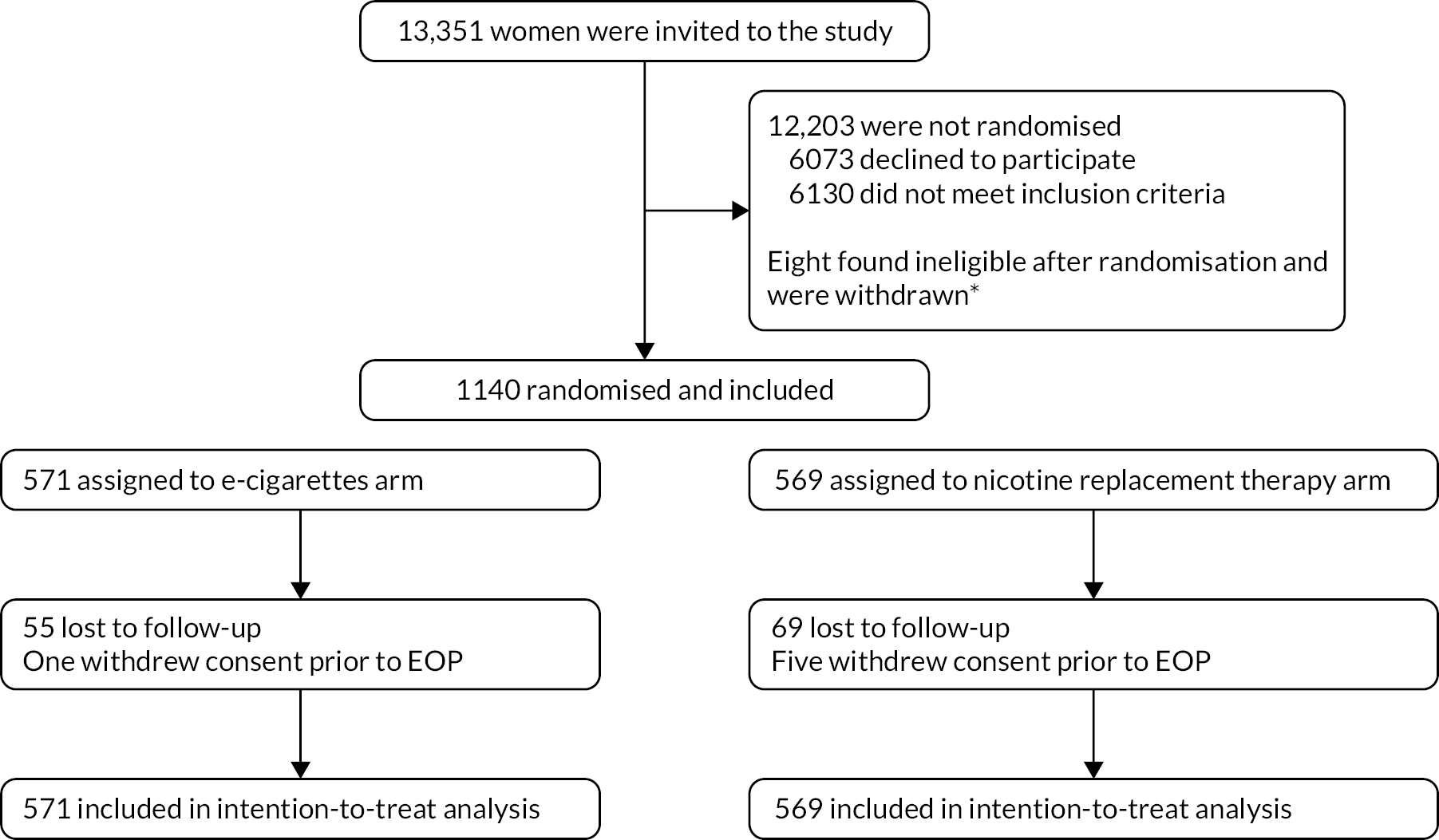
We were able to establish self-reported smoking status in 531 (93%) and 516 (91%) participants in the EC and NP arms, respectively, either via direct contact or from hospital records.
Table 3 presents the sample characteristics. The participants in the two study arms had similar profiles.
Effectiveness outcomes
For the primary outcome, saliva samples were required from participants who reported abstinence at EOP. Obtaining the samples proved difficult, with only 108 of 196 self-reported abstainers (55.1%) providing useable samples. Availability of useable samples in participants reporting reduction in smoking (46.9%) and those reporting dual use (60.5%) was broadly similar. We checked for an effect of COVID-19 lockdown on data collection, but it was only minor (see Appendix 3).
Due to problems with saliva sampling, validated prolonged abstinence rates were low (6.8% vs. 4.4% in the EC and NP arms, respectively). The difference between the two study arms was not significant (Table 4). The BF = 2.69 which suggests the data are insensitive. 49
| Trial arm | |||
|---|---|---|---|
| Primary outcome | ECs (N = 571) | NPs (N = 569) | RR (95% CI) |
| Validated prolonged abstinence at EOP, n (%) | 39 (6.8) | 25 (4.4) | 1.55 (0.95 to 2.53) |
| Sensitivity analyses | |||
| Per protocol (N = 483 and 382, respectively), n (%) | 39 (8.1) | 23 (6.0) | 1.34 (0.82 to 2.21) |
| Multiple imputation | (9.9) | (7.1) | 1.39 (0.90 to 2.14) |
| Abstainers using non-allocated products excluded (N = 571 and 564, respectively), n (%) | 39 (6.8) | 20 (3.6) | 1.93 (1.14 to 3.26) |
The results of ‘per-protocol’ and multiple-imputation analyses were similar. However, in the analysis excluding abstainers who regularly used the non-allocated product (0 ECs vs. 5 NRT), the difference between the two study arms (6.8% vs. 3.6%) was significant (Table 4). The BF = 10.0 which indicates a strong effect.
Regarding secondary outcomes, the EC arm had generally higher abstinence rates, but the difference only reached statistical significance for abstinence at 4 weeks and for self-reported abstinence at EOP. Regarding regular use of non-allocated products, six self-reported PP abstainers in the EC arm and 25 in the NP arm reported such use. With these participants excluded, all differences between the two study arms became significant (Table 5). Results for abstinence at 4 weeks and abstinence over 2-month pre-EOP are in Appendix 4.
| Trial arm | |||
|---|---|---|---|
| Secondary abstinence outcomes | ECs (N = 571) | NPs (N = 569) | RR (95% CI) |
| Self-reported prolonged abstinence at EOP, n (%) | 63 (11.0) | 44 (7.7) | 1.43 (0.99 to 2.06) |
| Validated PP abstinence at EOP, n (%) | 58 (10.2) | 40 (7.0) | 1.44 (0.98 to 2.13) |
| Self-reported PP abstinence at EOP, n (%) | 118 (20.7) | 78 (13.7) | 1.51 (1.16 to 1.96) |
| Sensitivity analyses with abstainers using the non-allocated product excluded | |||
| Self-reported prolonged abstinence at EOP (N = 569 and 556, respectively), n (%) | 61 (10.7) | 31 (5.6) | 1.92 (1.27 to 2.92) |
| Validated PP abstinence at EOP (N = 569 and 558, respectively), n (%) | 56 (9.8) | 29 (5.2) | 1.89 (1.23 to 2.92) |
| Self-reported PP abstinence at EOP (N = 565 and 544, respectively), n (%) | 112 (19.8) | 53 (9.7) | 2.03 (1.50 to 2.76) |
We also conducted an exploratory post hoc sensitivity analysis that assumed that abstainers using non-allocated products would not succeed in stopping smoking without such use and instead of excluding them, included them as non-abstainers. This approach allows the inclusion of the whole sample and maintains randomisation and statistical power. The results are shown in Table 6.
| Trial arm | |||
|---|---|---|---|
| ECs (N = 571) | NPs (N = 569) | RR (95% CI) | |
| Validated prolonged abstinence at EOP | 39 (6.8) | 20 (3.5) | 1.94 (1.15 to 3.29) |
| Self-reported prolonged abstinence at EOP | 61 (10.7) | 31 (5.5) | 1.96 (1.29 to 2.97) |
| Validated PP abstinence at EOP | 56 (9.8) | 29 (5.1) | 1.92 (1.25 to 2.97) |
| Self-reported PP abstinence at EOP | 112 (19.6) | 53 (9.3) | 2.11 (1.55 to 2.86) |
The rates of at least a 50% reduction in cigarettes smoked per day accompanied by at least a 50% reduction in salivary cotinine compared to baseline in participants who did not achieve abstinence were very low (in part due to the problem with saliva sampling) and did not differ between the study arms. Self-reported smoking reduction favoured the EC arm, but the difference only became significant when reducers using non-allocated products were excluded (Table 7).
| Trial arm | |||
|---|---|---|---|
| Smoking reduction | ECs (N = 571) | NPs (N = 569) | RR (95% CI) |
| Validateda 50% reduction at EOP in non-abstainers (N = 453 and 491, respectively), n (%) | 12 (2.7) | 12 (2.4) | 1.08 (0.49 to 2.39) |
| Sensitivity analysis: Reducers using non-allocated product excluded (N = 453 and 489, respectively), n (%) | 12 (2.7) | 10 (2.0) | 1.30 (0.57 to 2.97) |
| Self-reported 50% reduction at EOP in non-abstainers (N = 453 and 491, respectively), n (%) | 192 (42.4) | 166 (33.8) | 1.25 (1.06 to 1.48) |
| Sensitivity analysis: Reducers using non-allocated product excluded (N = 448 and 450, respectively), n (%) | 187 (41.7) | 125 (27.8) | 1.50 (1.25 to 1.81) |
| Self-reported 50% reduction at EOP in the overall sample, including abstainers | 248 (43.3) | 198 (34.8) | 1.25 (1.08 to 1.44) |
| Sensitivity analysis: Reducers using non-allocated product excluded (N = 562 and 516, respectively), n (%) | 239 (42.5) | 145 (28.1) | 1.51 (1.28 to 1.79) |
| Validated 50% reduction at EOP in the overall sample, including abstainers | 22 (3.9) | 22 (3.9) | 1.00 (0.56 to 1.78) |
| Sensitivity analysis: Reducers using non-allocated product excluded (N = 570 and 564, respectively), n (%) | 21 (3.7) | 17 (3.0) | 1.22 (0.65 to 2.29) |
Treatment adherence
Regarding treatment adherence, about 30% of participants did not set a TQD, with rates similar in the two study arms (Table 8). The uptake of support phone calls was low in both study arms. Product use was initially also low, especially in the NP arm. More participants returned to using their products later in pregnancy, with product use being significantly higher in the EC arm across different indicators. Additional product use data are in Appendix 5. A third of participants in the EC arm were using ECs at EOP, compared to 6% using NRT in the NP arm. Use of non-allocated products mirrored the difference, with significantly more participants in the NP arm becoming EC users than the other way round.
| Trial arm | |||
|---|---|---|---|
| Treatment adherence | ECs (N = 571) | NPs (N = 569) | RR (95% CI) |
| TQD set, n (%) | 418 (73.2) | 394 (69.2) | 1.06 (0.98 to 1.14) |
| Support sessions completed, median (IQR) | 1 (0–3) | 1 (0–2) | 0 (−0.31 to 0.31)a |
| Accessed local SSS support, n (%) | 29 (5.1) | 34 (6.0) | 0.85 (0.53 to 1.38) |
| Allocated product use, n (%) | |||
| Did not use allocated product at all | 88 (15.4) | 184 (32.3) | 0.48 (0.38 to 0.60) |
| Requested more after initial 2-week supply | 315 (55.2) | 207 (36.4) | 1.52 (1.33 to 1.73) |
| Current use at 4 weeks | 228 (39.9) | 128 (22.5) | 1.78 (1.48 to 2.13) |
| Regular use during studyb | 438 (76.7) | 292 (51.3) | 1.49 (1.36 to 1.64) |
| Current use at EOP | 193 (33.8) | 32 (5.6) | 6.01 (4.21 to 8.58) |
| Non-allocated product use, n (%) | |||
| Current use at 4 weeks | 11 (1.9) | 56 (9.8) | 0.20 (0.10 to 0.37) |
| Regular use during studyb | 16 (2.8) | 101 (17.8) | 0.16 (0.09 to 0.26) |
| Current use at EOP | 4 (0.7) | 49 (8.6) | 0.08 (0.03 to 0.22) |
Table 8 shows the product use among the full sample. We also looked at product use in participants reporting abstinence from smoking at EOP. Among this sample, 58 (49.2%; 57 allocated and one non-allocated) participants in the EC arm were using a nicotine product, either ECs or NRT. In the NP arm, 15 (19.2%; five allocated, eight non-allocated and two both) reported such use [χ2 (df = 1) = 18.0; p < 0.001].
Among various NRT products, NPs, which were the starting product, remained in use by far the most frequently. Of the 238 participants in the NP arm who reported at EOP that they had used NRT during pregnancy, 236 (99.2%) used NPs, including 16 who used a combination of NPs with other NRT products; one used only a mouth spray and one used only an inhaler. Regarding patch strength, of 351 patch products dispensed by the study team, only 29 (8.3%) were 10 mg NPs, while the rest were 15 mg NPs.
Among available EC products, refillable ECs, which were the starting product, remained by far the most frequently used. Among 344 EC users in the EC arm, 94.2% used refillable ECs. E-liquids with a higher nicotine content (11–20 mg/ml) and tobacco and fruit flavours were popular (Table 9).
| Products used during the initial 4 weeks ( N = 344) a | ||
| Product, n (%) | Refillable EC | 324 (94.2) |
| Cig-a-like | 1 (0.3) | |
| Cartridge/pod | 1 (0.3) | |
| Information missing | 18 (5.2) | |
| Nicotine strength, n (%) | 0 mg/ml | 7 (2.0) |
| 1–10 mg/ml | 47 (13.7) | |
| 11–20 mg/ml | 199 (57.9) | |
| Information missing | 91 (26.5) | |
| Flavour, n (%) | Fruit | 180 (52.3) |
| Tobacco | 24 (7.0) | |
| Mint/menthol | 22 (6.4) | |
| Chocolate, dessert, candy | 11 (3.2) | |
| Other | 21 (6.1) | |
| Information missing | 86 (25.0) | |
| Products used since last contact at EOP (N = 371)a | ||
| Product, n (%) | Refillable EC | 330 (89.0) |
| Cig-a-like | 0 (0) | |
| Cartridge/pod | 2 (0.5) | |
| Information missing | 39 (10.5) | |
| Nicotine strength, n (%) | 0 mg/ml | 8 (2.2) |
| 1–10 mg/ml | 77 (20.8) | |
| 11–20 mg/ml | 61 (16.4) | |
| Information missing | 225 (60.7) | |
| Flavour, n (%) | Fruit | 97 (26.2) |
| Mint/menthol | 38 (10.2) | |
| Chocolate, dessert, candy | 19 (5.1) | |
| Tobacco | 17 (4.6) | |
| Other | 24 (6.5) | |
| Information missing | 176 (47.4) | |
A total of 244 participants provided information on their products at 4 weeks and at EOP. Nicotine concentrations in their e-liquids decreased significantly over time [Bhapkar χ2 (df = 2) = 32.0; p < 0.001].
Safety outcomes
There were 1095 singleton births and 13 pairs of twins (nine in the EC and four in the NP arm). Safety data were available from 1110 women (97.4% of the sample; 97.4% in each arm). A total of 39 participants (20 in the EC and 19 in the NP arm) delivered infants in non-study sites and no data were available on birthweight for 10 of them and on gestational age and birthweight for 29. Two women (one in each arm) had an elective termination and were excluded from the analyses.
Participants in the NP arm had more infants with low birthweight (<2.5 kg; see Table 10), BF = 10.3. In a post hoc exploratory analysis, we also compared the proportions of babies with birthweight below the 10th centile (2.38 kg). This too was more frequent in the NP (10.9%) than in the EC arm (7.1%); RR = 0.65, 95% CI 0.44 to 0.96.
| Birth outcomes | Trial arm | ||
|---|---|---|---|
| ECs (N = 546)a,b | NPs (N = 549)a,b | RR (95% CI) | |
| Miscarriage, n (%) | 2 (0.4) | 3 (0.6) | 0.67 (0.11 to 4.00) |
| Stillbirth, n (%) | 2 (0.4) | 0 (0) | N/C |
| Neonatal death, n (%) | 2 (0.4) | 3 (0.6) | 0.67 (0.11 to 4.00) |
| Post-neonatal death, n (%) | 0 | 3 (0.6) | N/C |
| Maternal death, n (%) | 0 | 0 | N/C |
| Preterm birth, n (%) | 46 (8.4) | 63 (11.5) | 0.73 (0.51 to 1.05) |
| Low birthweight, n (%) (N = 541 and 541, respectively) | 52 (9.6) | 80 (14.8) | 0.65 (0.47 to 0.90) |
| Neonatal unit admission, n (%) | 51 (9.3) | 46 (8.4) | 1.11 (0.76 to 1.63) |
| Congenital abnormalities, n (%)c | 25 (4.6) | 15 (2.7) | 1.68 (0.89 to 3.14) |
| Terminations, n (%) | |||
| Due to congenital abnormalities | 1 (0.2) | 2 (0.4) | 1.51 (0.25 to 9.00) |
| Due to premature rupture of membranes | 2 (0.4) | 0 | N/C |
| Number of women with adverse birth outcomes, n (%) | 112 (20.5) | 119 (21.7) | 0.95 (0.75 to 1.19) |
| Delivery by caesarean section, n (%) | 131 (24.0) | 148 (27.0) | 0.89 (0.73 to 1.09) |
| Gestational age, weeks (N = 545 and 547, respectively), mean (SD) | 38.4 (3.0) | 38.2 (3.1) | 0.23 (−0.14 to 0.59)d |
| Birthweight, kg (N = 541 and 541, respectively), mean (SD) | 3.1 (0.60) | 3.1 (0.62) | 0.03 (−0.04 to 0.10)d |
The rates of other adverse birth outcomes and mean birthweight were similar in the two study arms. The analysis including twins provided similar results (see Appendix 6).
The two study arms were also similar in other AEs (Table 10). SAEs and AEs that occurred fewer than three times and that are marked as ‘Other’ in Table 11 are listed in Appendix 7. ARs deemed possibly related to study products consisted primarily of skin irritation and nausea in the NP arm, and cough and throat irritation in the EC arm.
| Event | Trial arm | |
|---|---|---|
| ECs | NPs | |
| Other severe AEs mother | ||
| Premature rupture of the membranes | 5 | 5 |
| Pre-eclampsia | 3 | 3 |
| Threatened labour | 3 | 3 |
| Vaginal haemorrhage | 2 | 4 |
| Genitourinary tract infection | 0 | 4 |
| Haemorrhage in pregnancy | 2 | 2 |
| Abdominal pain | 2 | 1 |
| Migraine | 1 | 2 |
| Premature labour | 1 | 2 |
| Other (see list) | 18 | 21 |
| Other severe AEs baby | ||
| Newborn respiratory disorders | 9 | 7 |
| Jaundice | 3 | 2 |
| Vomiting | 2 | 2 |
| Meconium aspiration syndrome | 3 | 1 |
| Drug withdrawal syndrome | 3 | 1 |
| Sepsis neonatal | 4 | 0 |
| Hypoglycaemia neonatal | 3 | 0 |
| Tonsillitis | 2 | 1 |
| Fetal growth restriction | 1 | 2 |
| Other (see list) | 20 | 20 |
| AEs mother | ||
| Nasopharyngitis | 25 | 17 |
| Lower respiratory tract infection | 15 | 9 |
| Nausea | 12 | 11 |
| Headache | 11 | 9 |
| Cough | 8 | 8 |
| Gestational diabetes | 6 | 11 |
| Influenza-like illness | 7 | 6 |
| Migraine | 2 | 7 |
| Urinary tract infection | 3 | 5 |
| Abortion induced | 4 | 2 |
| Perinatal depression | 4 | 2 |
| Vaginal haemorrhage | 4 | 2 |
| Asthma | 2 | 3 |
| Oropharyngeal pain | 1 | 4 |
| Vomiting | 3 | 2 |
| Hypertension | 0 | 4 |
| Viral infection | 2 | 2 |
| Abdominal pain upper | 3 | 1 |
| Depression | 1 | 2 |
| Dyspepsia | 1 | 2 |
| Hypotension | 1 | 2 |
| Other | 48 | 35 |
| Total AEs mother | 163 | 146 |
| Total severe AEs mother | 19 | 35 |
| AEs baby | ||
| Fetal growth restriction | 1 | 2 |
| Other | 4 | 8 |
| Total AEs baby | 5 | 10 |
| Total severe AEs baby | 1 | 2 |
| Total number of other mother/infant SAEs and AEs | 255 | 239 |
| Number of participants with other SAEs and AEs, n (%) | 181 (33.2) | 162 (29.5) |
| ARs potentially related to treatment | ||
| Application site irritation, hypoaesthesia, rash, pain or pruritus | 0 | 81 |
| Nausea | 17 | 36 |
| Cough | 42 | 0 |
| Oropharyngeal pain or irritation | 39 | 0 |
| Rash | 0 | 14 |
| Headache | 4 | 9 |
| Dizziness | 1 | 8 |
| Chest pain or discomfort | 11 | 0 |
| Vomiting | 1 | 3 |
| Dyspnoea | 3 | 0 |
| Migraine | 1 | 2 |
| Myalgia | 0 | 3 |
| Other (see list) | 7 | 6 |
| Total number of ARs | 126 | 162 |
| Total number of severe ARs | 1 | 1 |
| Number of participants with ARsa | 108 | 148 |
| Action following ARs b | ||
| Study drug discontinuation/interruption following AR | 36 | 111 |
| Study drug dose change following AR | 41 | 12 |
There were no differences between the two study arms in overall number of SAEs and AEs (476 vs. 479 in the EC and NP arm, respectively) or in the number of participants experiencing any SAEs or AEs (285 vs. 292 in the EC and NP arm, respectively; RR = 0.97, 95% CI 0.87 to 1.09).
In the sample of participants who answered the questions regarding respiratory symptoms (n = 64 in the EC arm and n = 79 in the NP arm), 84.4% in the EC arm and 79.8% in the NP arm reported new symptoms since the start of treatment (RR = 1.06, 95% CI 0.91 to 1.23). Among those who rated the severity of their symptoms (n = 53 in the EC and n = 63 in the NP arm), 5.7% in the EC arm and 19.1% in the NP arm reported experiencing severe symptoms (symptoms stopped them from carrying out everyday activities ‘a lot’ vs. ‘a little’ or ‘had no effect’).
Chapter 5 Discussion
The trial results suggest that ECs are more effective than NPs in helping pregnant smokers quit, but lead to more continued use. Regarding EC safety, birth and safety outcomes were similar in the two study arms, but there were fewer cases of low birthweight in the EC arm. Here we discuss separately the study findings concerning effectiveness and those that concern EC safety.
Effect of e-cigarettes on smoking cessation
The measure that comprised the primary outcome required a biochemical validation of abstinence from smoking via salivary sampling. This required participants to read the instructions on the sampling kit posted to their address, provide a sample in sufficient quantity and mail the sample back to the study team. The compliance with this request was limited, with only about half of the eligible participants providing useable samples. It is possible that the timing of the sampling was particularly inconvenient for women in late pregnancy or with newborn babies. Future studies should consider other approaches. Due to this issue, the validated sustained abstinence rates were low. The difference favoured the EC arm, but it was not statistically significant.
Six times as many participants in the NP arm used ECs regularly during pregnancy than participants in the EC arm who used any form of NRT (18% vs. 3%). A pre-specified sensitivity analysis of the primary outcome that excluded abstainers who regularly used the non-allocated product showed a significantly higher abstinence rate in the EC arm (RR = 1.93, 95% CI = 1.14 to 3.26). When using the same adjustment for other abstinence outcomes, ECs were almost twice as effective as NPs across different outcome measures (RRs ranging from 1.89 to 2.03).
The two treatments thus did not differ significantly in the primary outcome, but the effect of ECs appears to have been masked by EC use in the NP study arm. When this was controlled for, ECs were superior to NPs. The difference between the two treatments in biochemically validated abstinence (7% vs. 4%) is low because of the saliva sampling problem, but the difference in self-reported 7-day PP abstinence at EOP (21% vs. 14%, or 20% vs. 10% with adjustment for contamination) suggests a clinically important effect.
Behaviour change trials that compare an old and a new treatment are at a risk that some participants who did not benefit from the old treatment join the trial to access the new treatment and if allocated to the old one, are disappointed and drop out. In this trial, similar proportions of participants had previous experience with NRT and ECs (just under 50%). They all stopped using the products and continued to smoke. Familiarity with the two products and the rate of previous failure with them was thus similar. Reassuringly, the proportions of participants in the two study arms who never set up a TQD were also similar in the two study arms. However, more participants in the NP arm never tried their allocated product (32% vs. 15%). This, however, seems to have been due to participants being keen to try ECs, rather than not wanting NPs, because NP use was higher than in the previous large UK Smoking, Nicotine and Pregnancy (SNAP) trial of nicotine versus placebo patches that recruited pregnant smokers in the same way and from largely the same locations. 10,12,50–53
For any treatment to make an impact, clients must be willing to use it. In previous studies of pregnant smokers, treatment adherence was limited. 12 For instance, in the SNAP trial behavioural support was rarely used and only 14% (72 out of 521) of participants in the NP arm requested further patches after the first 4-week supply. 12 Here, the adherence to behavioural advice was also low. Almost a third of participants did not set up a TQD, and the response to support calls was low. In the NP arm, 23% of participants used their allocated products for at least 4 weeks. ECs had a higher initial appeal, with 40% of participants in the EC arm using their allocated product at 4 weeks. The two products further diverged during the follow-up period. At EOP, 6% and 34% were using their products in the NP and EC study arms, respectively. The contrast was even stronger among self-reported EOP abstainers, with 9% in the NP arm versus 57% in the EC arm using their allocated product at EOP. Interviews with trial participants suggested that positive beliefs about the necessity of vaping to achieve smoking cessation outweighed concerns about vaping. 54 The difference at EOP could have been in part at least due to participants expecting that NPs are supposed to be used for only 3 months, although in this study, participants in both study arms were encouraged to use their products for as long as needed. They also needed to ask for a prescription for NRT, while this was not needed for ECs. On the other hand, participants were able to obtain NPs and any other NRT they asked for free of charge, but had to source EC supplies and pay for them themselves. The number of participants who switched to regular use of the non-allocated product can be considered another indication of treatment attractiveness. This was much more frequent in the NP arm. As in previous studies with general cohorts of smokers,39,55 ECs seem to be a more attractive option than NPs in this client group as well.
The higher rate of ongoing use of ECs compared to ongoing use of NRT raises the question of what effects this may have over time on participants who stopped smoking, as well as on those who became dual users. Smokers who switch to dual use can be expected to reduce their smoking and toxicant intake and so gain a degree of harm reduction. 56–58 The effect on ex-smokers is less clear. It could be negative because EC use is likely to carry some health risks if used over an extended time,59 and for some vapers at least, the cost and/or compulsive nature of ongoing nicotine use is likely to be undesirable. Ongoing EC use, however, could also have some positive effects if it helps with reducing irritability39 and weight gain60 that can accompany cessation of nicotine use and possibly help with maintaining enjoyment that was previously derived from smoking. 61 Perhaps the key issue is whether extended EC use facilitates or prevents relapse back to smoking. Future studies should examine quality of life, relapse risk and health outcomes in comparable ex-smokers who did and did not switch to EC use.
Regarding the type of EC products used by participants, refillable ECs were used almost exclusively. In the USA, pod-based ECs, such as JUUL™ (Juul Labs Inc., San Francisco, CA, USA), are now the most popular EC product. 62 JUUL has a high nicotine content (59%) and provides nicotine in a way similar to cigarettes. 63 The UK, however, is currently subject to European Union (EU) regulations that ban ECs with nicotine content above 20%. This means that pod products on sale in the EU provide only low nicotine levels that are unlikely to be helpful for smokers trying to stop smoking and that indeed did not become popular in the UK. 63
As in previous studies,39,55 fruit flavour was the most popular EC flavour choice. The study also replicated an unexpected previous finding39 of EC users reducing nicotine content of their e-liquid over time. This could be the result of a conscious effort at weaning oneself off nicotine, but it could also be due to an improved vaping technique64 or using more effective EC devices.
Regarding treatment costs, it is worth noting that while ECs were more costly for participants, NRT was much more costly to treatment providers.
Safety of e-cigarettes compared to safety of nicotine patches
Existing data on safety of ECs in pregnancy come from observational studies. In two reports, infants of exclusive vapers had a higher risk of smallness for gestational age65 and a higher incidence of low birthweight and pre-term birth66 compared to non-smokers. The findings are difficult to interpret as most or all vapers had been smoking during early pregnancy, and no comparison is provided with ex-smokers who quit without using ECs. One study found birthweight of infants born to exclusive vapers matching that of infants of never-smokers, and significantly higher than in infants of smokers. 67 A study of Neonatal Behavioural Assessment Scale scores reported a greater number of abnormal reflexes in infants of both smokers and EC users compared to non-smokers. 68 This could be related to EC use, but as in the two studies above, the finding could reflect differences between smoking and non-smoking mothers, or tobacco exposure in early pregnancy.
In our study, AEs and pregnancy outcomes were similar in the two study arms, but there were more infants born with low birthweight (<2500 g) in the NP arm. The BF of 10.3 indicates strong evidence for the effect, though a possibility needs to be considered that as the two study arms were compared on a number of health outcomes, the finding could be an artefact of multiple testing. The most likely explanation of the finding is that participants in the EC arm reduced their smoking more than those randomised to NPs. The finding echoes the result from the SNAP trial mentioned earlier, where infants born to women randomised to NPs had better outcomes than those randomised to placebo patches. 69 The secondary analyses could not explain this finding in terms of smoking cessation70 but smoking reduction was not measured and it is plausible that women on NPs reduced their smoking more than those on placebo patches. The potentially important implication of both of these findings is that the risks of negative birth outcomes in smokers is due to other chemicals in cigarette smoke rather than nicotine.
It is important to note that these results only concern effects of nicotine in later pregnancy. All study participants were smoking for at least the first 3 months of pregnancy and so were exposed to both nicotine and to other tobacco chemicals early on. The results do not rule out possible detrimental effects of nicotine during these early stages. However, they provide reassuring evidence that providing pregnant smokers with alternative nicotine delivery devices such as NRT or ECs does not generate any additional risks and is likely in fact to reduce the risk of low birthweight. In addition, a recent study looking at women who stopped smoking at various stages of their pregnancy showed that smoking during the second trimester or through the entire pregnancy is associated with a higher incidence of low birthweight. 71 Our results suggest that nicotine is not implicated in these effects. If the effects of smoking in the first trimester are due to the same processes, nicotine on its own may not affect intrauterine growth.
Trial strengths
This was a large ‘real-world’ trial. The participants were representative of the population of pregnant smokers in the UK and the two interventions were delivered in a way that is economic and practicable and can be applied in routine care. The trial included detailed examinations of smoking behaviours as well as monitoring of product safety and of birth outcomes for both women and infants.
Trial limitations
Validation of smoking status via postal saliva sampling proved problematic. Almost half of eligible participants did not provide useable samples, which led to low primary outcome abstinence rates and reduced the study power. The trial results concern primarily NPs rather than NRT combinations. Although participants were encouraged to use additional NRT products, this was used only rarely. In non-pregnant smokers, combinations of patches with other NRT products were shown more effective than single NRT. 72 Participants could only access ECs with a maximum of 20 mg/ml nicotine because EU regulations do not allow higher nicotine concentrations. The results may not generalise to modern ‘pod’ EC products with higher nicotine delivery.
Implications for health care
Although nicotine in late pregnancy may not have any detrimental effects on pregnancy outcomes, given the question marks regarding possible effects of continuing nicotine use on quality of life, health outcomes and risk of relapse, stopping smoking without nicotine-containing aids is preferable to switching to such products. However, where the choice is between using nicotine products such as NRT or EC, or continuing to smoke, nicotine product use would be the recommended option. Specialist SSSs should include EC starter packs among the treatment options offered to pregnant smokers. Such an offer is likely to reach more smokers and generate better smoking reduction at lower cost than the offer of NPs.
Recommendations for research
As noted earlier, future studies should avoid postal saliva sampling. Regarding use of non-allocated products, this is likely to continue to occur in future studies. Researchers should pre-specify how this will be controlled for. We opted for excluding abstainers who used the non-allocated products, but re-classifying them as non-abstainers could be a better approach, as discussed above. Future studies may also consider partially randomised patient preference design. The inclusion of long-term follow-up of the offspring would address concerns about effects of nicotine on later offspring development. Regarding more general research recommendations, in this field, long-term health effects of EC use are the main current research priority. Studies are needed that compare biomarkers or risks and eventually also long-term health outcomes, quality of life and relapse rates in comparable ex-smokers who either stopped smoking without switching to EC use, or stopped smoking and use ECs.
Conclusion
In the unadjusted primary analysis, there was insufficient evidence to confidently demonstrate that ECs are more effective in helping pregnant smokers quit than NPs. EC effects appear to have been masked by EC use in the NP arm. When this was controlled for, ECs were more effective than patches. Regarding product safety, ECs do not seem to pose more risks to birth outcomes that were assessed in this study than NPs and may reduce the incidence of low birth weight.
Acknowledgements
We thank the trial participants; the staff at participating sites; the members of the TSC and Data Monitoring and Ethics Committee; NIHR research project managers; and the staff of the Barts CTU and the King’s CTU. We are especially grateful to the NIHR Health Technology Assessment programme who provided the main study funding and to Public Health England for providing funding for the study NP supplies.
Contributions of authors
Dunja Przulj (https://orcid.org/0000-0003-1133-8835) (Research Fellow) was the study manager. She co-wrote the original grant application, co-designed the trial, trained staff, delivered the interventions, contributed to data collection and interpretation of the trial findings and assisted with the drafting of the report.
Francesca Pesola (https://orcid.org/0000-0002-2054-7930) (Study Statistician) led the statistical analysis plan, analysed the trial data and assisted with drafting of the report.
Katie Myers Smith (https://orcid.org/0000-0003-1837-3924) (Senior Research Fellow) was the study manager and co-wrote the original grant application, co-designed the trial, co-wrote the statistical analysis plan, trained staff, delivered the interventions, contributed to data collection and assisted with interpretation of the trial findings and drafting of the report.
Hayden McRobbie (https://orcid.org/0000-0002-7777-1845) (Professor in Public Health Interventions) was a co-investigator. He co-wrote the original grant application, co-designed the trial and assisted with interpretation of the trial findings and drafting of the report.
Tim Coleman (https://orcid.org/0000-0002-7303-4805) (Professor of Primary Care) was a co-investigator. He co-wrote the original grant application, co-designed the trial and assisted with interpretation of the trial findings and drafting of the report. Professor Coleman is an NIHR Senior Investigator.
Sarah Lewis (https://orcid.org/0000-0001-5308-6619) (Professor of Medical Statistics) was a co-investigator. She co-wrote the original grant application, co-designed the trial, co-wrote the statistical analysis plan and provided statistical oversight and assisted with interpretation of the trial findings and drafting of the report.
Christopher Griffiths (https://orcid.org/0000-0001-7935-8694) (Professor of Primary Care) was the chief investigator (from June 2019 onwards). He oversaw the management of the trial and assisted with interpretation of the trial findings and drafting of the report.
Robert Walton (https://orcid.org/0000-0001-7700-1907) (Clinical Professor of Primary Health Care) was the chief investigator (from the start of the study to June 2019). He oversaw the management of the trial and assisted with interpretation of the trial findings and drafting of the report.
Rachel Whitemore (https://orcid.org/0000-0002-7599-8640) (Senior Trial Manager) was a site co-ordinator for the English sites. She identified recruiting sites, conducted site staff training and monitoring and assisted with the drafting of the report.
Miranda Clark (https://orcid.org/0000-0002-6179-046X) (Senior Trial Manager) was a site co-ordinator for the English sites. She identified recruiting sites, conducted site staff training and monitoring and assisted with the drafting of the report.
Michael Ussher (https://orcid.org/0000-0002-0995-7955) (Professor of Behavioural Medicine) was a co-investigator. He co-wrote the original grant application, co-designed the trial and assisted with interpretation of the trial findings and drafting of the report.
Lesley Sinclair (https://orcid.org/0000-0002-2210-8181) (Research Fellow/Trial Manager) was a site co-ordinator for the Scottish sites. She identified recruiting sites, conducted site staff training and monitoring and assisted with the drafting of the report.
Emily Seager (https://orcid.org/0000-0001-6672-5555) (Research Health Psychologist) was a research assistant who delivered the interventions and contributed to data collection.
Sue Cooper (https://orcid.org/0000-0002-1994-6395) (Principal Research Fellow and NIHR Programme Manager) was a co-investigator. She co-wrote the original grant application, co-designed the trial and assisted with interpretation of the trial findings and drafting of the report.
Linda Bauld (https://orcid.org/0000-0001-7411-4260) (Bruce and John Usher Professor of Public Health) was a co-investigator. She co-wrote the original grant application, co-designed the trial and assisted with interpretation of the trial findings and drafting of the report.
Felix Naughton (https://orcid.org/0000-0001-9790-2796) (Associate Professor in Health Psychology) was a co-investigator. He co-wrote the original grant application, co-designed the trial and assisted with interpretation of the trial findings and drafting of the report.
Peter Sasieni (https://orcid.org/0000-0003-1509-8744) (Academic Director of King’s CTU and Professor of Cancer Prevention) was a co-investigator and lead of the CTU providing trial oversight. He co-wrote the original grant application, co-designed the trial and assisted with interpretation of the trial findings and drafting of the report.
Isaac Manyonda (https://orcid.org/0000-0003-3080-2357) (Consultant in Obstetrics and Gynaecology) was a co-investigator. He co-wrote the original grant application, co-designed the trial and assisted with interpretation of the trial findings and drafting of the report.
Peter Hajek (https://orcid.org/0000-0001-9160-4296) (Professor of Clinical Psychology) was the PI. He led the original grant application, co-designed the trial and led the drafting of the report.
Ethics statement
The study was approved by the National Research Ethics Service Committee London – South East (ref: 17/LO/0962) and the Medicines and Healthcare Regulatory Agency via the CTIMP (Clinical Trial of an Investigational Medicinal Product) Notification Scheme. The trial was conducted in compliance with the Medicines for Human Use (Clinical Trials) Regulations 2004 (SI 2004/1031), Research Governance Framework, Good Clinical Practice guidelines, and the World Medical Association Declaration of Helsinki (1996).
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
Appendix 1 Example of study adverts


Pregnant smokers required for cutting edge study

We are testing new ways to help pregnant smokers quit. If you can help us with this
ground breaking study, speak to your midwife today or call/email on XXXXXXXXXXXX.
Appendix 2 E-cigarette use instructions

Appendix 3 Effects of COVID-19 lockdown on study data
During the COVID-19 lockdown period (March 2020 onwards), it was noticed that the proportion of saliva samples returned to the research team was lower than observed pre-lockdown and we included in the statistical analysis plan an additional sensitivity analysis for the primary outcome to take into account the impact of the pandemic on missing data (assumed missing at random).
In total, five saliva kits were posted to self-reported abstainers between 1 March and 5 May 2020, during the period of pandemic lockdown in the UK (three in the EC arm and two in the NP arm). Of these, two were returned. The rates of sample return in the pre-COVID period were 73.6% and 66.7% in the EC and NP arms, respectively. The rates of passed validation observed pre-COVID were 92.1% and 100% in the EC and NP arms, respectively. Therefore, we assumed that 66.0% in the EC arm and 66.7% in NP arm would be returned and pass validation. We estimated validation status (pass/fail) for the three non-responders for each arm separately and found that two could be expected to pass validation (one in each arm). This did not alter the study findings.
| Trial arm | |||
|---|---|---|---|
| ECs (N = 571) | NPs (N = 569) | RR (95% CI) | |
| COVID-19 effect, n (%) | 40 (7.0) | 26 (4.6) | 1.53 (0.95 to 2.48) |
Appendix 4 Additional smoking cessation outcomes
| Trial arm | |||
|---|---|---|---|
| EC (N = 571) | NP (N = 569) | RR (95% CI) | |
| Self-reported abstinence at 4 weeks, n (%) | 89 (15.6) | 61 (10.7) | 1.45 (1.07 to 1.97) |
| Validated abstinence for the past 2 months | 46 (8.1) | 35 (6.2) | 1.31 (0.86 to 2.00) |
| Self-reported abstinence for the past 2 months | 68 (11.9) | 59 (10.4) | 1.15 (0.83 to 1.60) |
Appendix 5 Additional data on product use
| Trial arm | |||
|---|---|---|---|
| ECs (N = 571) | NPs (N = 569) | Median difference (95% CI) | |
| Used product at each time point | |||
| Week 1 | 232 (40.6) | 169 (29.7) | |
| Week 2 | 213 (37.3) | 139 (24.4) | |
| Week 3 | 187 (32.8) | 103 (18.1) | |
| Week 4 | 228 (39.9) | 128 (22.5) | |
| EOP | 371 (65.0) | 236 (41.5) | |
| Median (IQR) number of weeks of regular use (participant N = 465 and 434, respectively)a | 8 (2–19) | 1 (0–5) | −7 (−5.33 to 8.67) |
| Median (IQR) number of weeks of occasional use (participant N = 465 and 434, respectively)a | 0 (0–1) | −2 (1–4) | −2 (−2.16 to 1.84) |
| Non-allocated product use, n (%) | |||
| Median (IQR) number of weeks of regular use (participant N = 21 and 102, respectively)a | 1 (0–3) | 1 (0–2) | 0 (−1.21 to 1.21) |
| Median (IQR) number of weeks of occasional use (participant N = 21 and 102, respectively)a | 1 (1–1) | 1 (0–1) | 0 (−0.61 to 0.61) |
Appendix 6 Birth outcomes including twin births (nine in the EC arm and four in the NP arm)
| Trial arm | |||
|---|---|---|---|
| ECs (N = 564)a | NPs (N = 557)a | RR (95% CI) | |
| Miscarriage, n (%) | 3 (0.5) | 3 (0.5) | 0.99 (0.20 to 4.87) |
| Stillbirth, n (%) | 2 (0.4) | 0 (0) | N/C |
| Neonatal death, n (%) | 2 (0.4) | 3 (0.5) | 0.66 (0.11 to 3.93) |
| Post-neonatal death, n (%) | 0 | 3 (0.5) | N/C |
| Maternal death, n (%) | 0 | 0 | N/C |
| Preterm birth, n (%) | 56 (9.9) | 69 (12.4) | 0.80 (0.56 to 1.14) |
| Low birthweight (N = 558 and 549, respectively), n (%) | 63 (11.3) | 86 (15.7) | 0.72 (0.53 to 0.99) |
| Neonatal unit admission, n (%) | 58 (10.3) | 46 (8.3) | 1.25 (0.85 to 1.81) |
| Congenital abnormalities, n (%)b | 26 (4.6) | 15 (2.7) | 1.71 (0.92 to 3.20) |
| Terminations, n (%) | |||
| Due to congenital abnormalities | 1 (0.2) | 2 (0.4) | 1.48 (0.25 to 8.84) |
| Due to premature rupture of membranes | 2 (0.4) | 0 | N/C |
| Total number of adverse birth outcomes | 213 | 227 | |
| Number of women with adverse birth outcomes (N = 555 and 553, respectively), n (%) | 120 (21.6) | 122 (22.1) | 0.98 (0.78 to 1.22) |
| Delivery by caesarean section, n (%) | 145 (25.7) | 152 (27.2) | 0.94 (0.77 to 1.15) |
| Gestational age, weeks (N = 562 and 555, respectively), mean (SD) | 38.3 (3.1) | 38.2 (3.1) | 0.12 (−0.25 to 0.49)c |
| Birthweight, kg (N = 558 and 549, respectively), mean (SD) | 3.1(0.63) | 3.1 (0.63) | 0.01 (−0.07 to 0.08)c |
Appendix 7 Serious adverse events and AEs that occurred only once or twice
| Event | Trial arm | |
|---|---|---|
| ECs | NPs | |
| Other SAEs mother | ||
| Abdominal pain upper | 1 | 0 |
| Acute myocardial infarction | 1 | 0 |
| Alcoholism | 1 | 0 |
| Cellulitis | 0 | 1 |
| Cerebral haemorrhage | 1 | 0 |
| Cervix inflammation | 0 | 1 |
| Dehydration | 1 | 0 |
| Diarrhoea | 0 | 1 |
| Eclampsia | 0 | 1 |
| Endometritis decidual | 0 | 1 |
| Epilepsy | 1 | 1 |
| Gastritis | 0 | 1 |
| Gestational diabetes | 0 | 1 |
| Haemoglobin decreased | 0 | 1 |
| Hyperemesis gravidarum | 0 | 1 |
| Influenza | 1 | 1 |
| Kidney infection | 1 | 0 |
| Lower respiratory tract infection | 0 | 1 |
| Mastitis | 1 | 0 |
| Nephrolithiasis | 1 | 0 |
| Pneumonia | 1 | 1 |
| Post-partum haemorrhage | 1 | 0 |
| Premature separation of placenta | 0 | 1 |
| Preterm premature rupture of membranes | 0 | 1 |
| Puerperal pyrexia | 1 | 0 |
| Pulmonary thrombosis | 0 | 1 |
| Pyelonephritis acute | 0 | 1 |
| Renal pain | 1 | 0 |
| Retained products of conception | 0 | 1 |
| Sciatica | 1 | 0 |
| Sepsis | 1 | 1 |
| Tooth infection | 0 | 1 |
| Upper respiratory tract infection | 0 | 1 |
| Ureteric injury | 1 | 0 |
| Wound infection | 1 | 0 |
| Other SAEs baby | ||
| Abdominal distension | 1 | 0 |
| Asthma | 1 | 0 |
| Benign neonatal sleep myoclonus | 0 | 1 |
| Beta haemolytic streptococcal infection | 1 | 0 |
| Bradycardia | 1 | 0 |
| Bronchiolitis | 0 | 1 |
| Bronchitis | 0 | 1 |
| Cardiac arrest neonatal | 0 | 1 |
| Cholecystectomy | 0 | 1 |
| Fetal cardiac arrest | 1 | 0 |
| Fetal hypokinesia | 1 | 1 |
| Haematoma | 0 | 1 |
| Hospitalisation for further diagnosis | 1 | 0 |
| Hypertonia neonatal | 0 | 1 |
| Hypothermia neonatal | 1 | 1 |
| Hypoxic–ischaemic encephalopathy | 1 | 0 |
| Immune thrombocytopenia | 1 | 0 |
| Infantile apnoea | 1 | 1 |
| Intraventricular haemorrhage neonatal | 0 | 1 |
| Low birthweight baby | 1 | 1 |
| Necrotising enterocolitis neonatal | 1 | 0 |
| Neonatal infection | 1 | 1 |
| Neonatal pneumothorax | 0 | 1 |
| Neonatal seizure | 2 | 0 |
| Perinatal stroke | 0 | 1 |
| Poor feeding infant | 0 | 1 |
| Poor weight gain neonatal | 0 | 1 |
| Shoulder dystocia | 0 | 1 |
| Skin discolouration | 1 | 0 |
| Skull fracture | 0 | 1 |
| Spinal cord neoplasm | 1 | 0 |
| Viral infection | 2 | 0 |
| AEs mother | ||
| Abdominal discomfort | 1 | 0 |
| Abdominal pain | 0 | 2 |
| Abdominal pain lower | 0 | 1 |
| Acne | 1 | 0 |
| Acute sinusitis | 1 | 0 |
| Anaemia of pregnancy | 2 | 0 |
| Anxiety | 1 | 1 |
| Application site irritation | 2 | 0 |
| Back pain | 1 | 1 |
| Bartholin’s abscess | 1 | 0 |
| Bile output increased | 0 | 1 |
| Bronchitis | 0 | 1 |
| Chest discomfort | 1 | 1 |
| Chest pain | 1 | 0 |
| Cholestasis | 1 | 1 |
| Constipation | 1 | 1 |
| Crohn’s disease | 0 | 1 |
| Deep vein thrombosis | 1 | 0 |
| Decreased appetite | 0 | 1 |
| Depressed mood | 0 | 1 |
| Dermatitis allergic | 1 | 0 |
| Diarrhoea | 1 | 0 |
| Dizziness | 1 | 1 |
| Dyspnoea | 1 | 1 |
| Ear infection | 1 | 0 |
| Eczema | 1 | 0 |
| Fatigue | 1 | 0 |
| Fluid retention | 1 | 0 |
| Food poisoning | 1 | 0 |
| Gastroenteritis | 0 | 1 |
| Haemorrhage urinary tract | 0 | 1 |
| Iron deficiency | 1 | 0 |
| Kidney infection | 1 | 0 |
| Ligament injury | 1 | 1 |
| Mastitis | 1 | 0 |
| Mental disorder | 0 | 1 |
| Mood swings | 0 | 1 |
| Mouth ulceration | 1 | 0 |
| Muscle spasms | 0 | 1 |
| Neck pain | 1 | 0 |
| Oedema peripheral | 1 | 0 |
| Oligohydramnios | 1 | 0 |
| Otitis media acute | 1 | 0 |
| Pain | 0 | 1 |
| Palpitations | 0 | 1 |
| Panic attack | 1 | 1 |
| Pelvic pain | 1 | 0 |
| Peripheral swelling | 0 | 1 |
| Placenta praevia | 1 | 0 |
| Post-procedural complication | 0 | 1 |
| Pre-eclampsia | 1 | 0 |
| Premature rupture of membranes | 0 | 1 |
| Pulmonary embolism | 1 | 0 |
| Pyrexia | 1 | 0 |
| Rash | 0 | 1 |
| Rhesus antibodies | 0 | 1 |
| Road traffic accident | 1 | 0 |
| Seasonal allergy | 1 | 0 |
| Sinusitis | 0 | 1 |
| Suicidal ideation | 0 | 1 |
| Subcutaneous abscess | 1 | 0 |
| Symphysiolysis | 0 | 1 |
| Thrombosis | 1 | 0 |
| Tonsillitis | 0 | 1 |
| Tooth extraction | 0 | 1 |
| Tooth fracture | 1 | 0 |
| Wheezing | 1 | 0 |
| AEs baby | ||
| Acid reflux | 1 | 0 |
| Bilateral ventriculomegaly | 0 | 1 |
| Bronchitis | 1 | 1 |
| Chesty cough | 0 | 1 |
| Cyst, NOS | 0 | 1 |
| Difficulty breathing | 0 | 1 |
| Fetal hypokinesia | 1 | 0 |
| Infection | 0 | 1 |
| Respiratory distress | 0 | 1 |
| Viral infection | 1 | 1 |
| ARs potentially related to treatment | ||
| Arthralgia | 0 | 1 |
| Asthma | 2 | 0 |
| Dyspepsia | 2 | 0 |
| Eczema | 0 | 1 |
| Functional gastrointestinal disorder | 0 | 1 |
| Hyperhidrosis | 0 | 1 |
| Lip swelling | 1 | 0 |
| Muscle swelling | 0 | 1 |
| Nightmare | 0 | 1 |
| Stomatitis | 1 | 0 |
| Wheezing | 1 | 0 |
List of abbreviations
- AE
- adverse event
- AR
- adverse reaction
- BF
- Bayes factor
- CI
- confidence interval
- CO
- carbon monoxide
- CONSORT
- Consolidated Standards of Reporting Trials
- CTU
- Clinical Trials Unit
- EC
- e-cigarette
- EOP
- end of pregnancy
- EU
- European Union
- FTCD
- Fagerström Test for Cigarette Dependence
- GP
- general practitioner
- HAL
- Health and Lifestyle Research Unit
- IQR
- interquartile range
- NIHR
- National Institute for Health and Care Research
- NP
- nicotine patch
- NRT
- nicotine replacement therapy
- PI
- Principal Investigator
- PP
- point prevalence
- QMUL
- Queen Mary University of London
- RCT
- randomised controlled trial
- RR
- risk ratio
- SAE
- serious adverse event
- SD
- standard deviation
- SNAP
- Smoking, Nicotine and Pregnancy
- SSS
- Stop Smoking Service
- TQD
- target quit date
- TSC
- Trial Steering Committee
