Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 11/36/41. The contractual start date was in September 2013. The draft report began editorial review in Febuary 2023 and was accepted for publication in September 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Mackenzie et al. This work was produced by Mackenzie et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Mackenzie et al.
Chapter 1 Introduction
Background
Ischaemic heart disease (IHD) remains one of the biggest health issues affecting the UK population and is responsible for significant morbidity and mortality. In 2019, 55,064 (10.4%) deaths registered in England and Wales were due to IHD. 2
Allopurinol is a xanthine oxidase inhibitor that lowers serum uric acid (SUA) levels. It is currently licensed for the treatment of symptomatic hyperuricaemia (gout) and is widely used long term in patients with gout to prevent acute gout flares. Allopurinol is not currently licensed for the treatment of asymptomatic hyperuricaemia, or for the treatment of patients with IHD, unless they also have gout. Xanthine oxidase is an enzyme that promotes inflammation and atherosclerosis via the production of reactive oxygen species and xanthine oxidase activity is raised in several conditions including coronary artery disease. 3
The role of SUA in cardiovascular (CV) disease is controversial, with some observational studies associating higher SUA levels with worse CV outcomes,4,5 but more recent Mendelian randomisation approaches suggest no major role of uric acid levels in determining CV outcomes. 6 Some observational studies have suggested that allopurinol therapy may improve CV outcomes,7,8 while others have not found any beneficial effect. 9 There is significant risk of confounding and bias within observational studies, and a need for evidence generated via prospective randomised trials to guide practice in this area was identified. 10
Several small interventional studies (with up to 100 participants) have investigated the effects of allopurinol on various CV parameters. Studies have reported improvements in some parameters with allopurinol therapy, including endothelial function,11–15 flow-mediated dilatation,16,17 blood pressure,18–20 left ventricular mass,16,21,22 carotid intimal media thickness and arterial stiffness. 20 However, other studies have reported no benefit of allopurinol therapy on blood pressure,17 left ventricular mass,23,24 myocardial perfusion25 and flow-mediated dilatation. 26
In a randomised crossover study in 65 patients with chronic stable angina with angiographically documented coronary artery disease, allopurinol therapy given at 600 mg daily improved time to onset of chest pain and exercise time compared to placebo therapy. 27 In a randomised study of 100 patients presenting with acute coronary syndrome (ACS), allopurinol therapy was found to reduce CV events over a 2-year follow-up period, improve markers of oxidative stress and inflammatory response and reduce angina compared to usual care. 28 Allopurinol given at the time of coronary bypass surgery improved outcomes in one study29 but not in another. 30
With regard to possible mechanisms of action, it is not clear whether the potential beneficial CV effects of allopurinol in some of these studies may be a result of urate lowering or due to a reduction in xanthine oxidase-mediated oxidative stress. 11,31
Given the uncertainties in the results of the observational and small interventional studies to date, there was a clear need to definitively answer the question of whether allopurinol therapy would improve outcomes for patients with IHD in a properly powered prospective randomised trial. If allopurinol were found to be beneficial for patients with IHD in terms of improving CV outcomes or quality of life, it would be a relatively inexpensive and easy to introduce therapy within the UK healthcare system.
The ALL-HEART study was designed to answer this question and was the first large, prospective, randomised trial of the effects of allopurinol therapy on major CV outcomes in patients with IHD. 32
Chapter 2 Methods
Study design
The ALL-HEART study was a multicentre, prospective, randomised, open-label, blinded end point (PROBE) trial. The protocol is available at https://njl-admin.nihr.ac.uk/document/download/2031938.
Objectives
The objectives of the ALL-HEART study were to answer the following questions.
Primary objective
Does allopurinol therapy added to usual care improve major CV outcomes in patients aged over 60 years with IHD but no gout?
Secondary objectives
Does allopurinol therapy added to usual care improve all-cause mortality or other CV outcomes in patients with IHD?
What is the cost-effectiveness of adding allopurinol up to 600 mg daily to usual care in patients with IHD?
Does allopurinol therapy added to usual care improve quality of life assessed by general health survey [EuroQol-5 Dimensions, five-level version (EQ-5D-5L)] or coronary heart disease-specific questionnaire (Seattle angina questionnaire)?
Participants
Participants were recruited from 424 primary care practices via 18 regional centres in the UK. Primary care records were searched for potentially eligible patients who were then invited by letter from their general practitioner (GP) to participate in the study. In addition, a small number of participants were referred to the study from secondary care centres. Eighty-four Clinical Commissioning Groups (CCGs) in England and 10 Health Boards in Scotland were involved in the study.
The inclusion and exclusion criteria were as follows.
Inclusion criteria
-
Male or female patients aged 60 years and over.
-
Ischaemic heart disease defined as a diagnosis of angina or myocardial infarction (MI) at any time or other evidence of IHD (investigator opinion).
Exclusion criteria
-
History of gout.
-
Known severe renal impairment [estimated glomerular filtration rate (eGFR) < 30 ml/minute/1.73 m2]. [This was previously ‘Known renal impairment eGFR < 60 ml/minute/1.73 m2’ for patients recruited from the start of the trial (7 February 2014) until 4 April 2016 when protocol v4 was implemented at all study sites. Fifty-two per cent of the target number of patients had been randomised by this date.]
-
Moderate to severe heart failure (HF) [New York Heart Association (NYHA) III–IV].
-
Significant hepatic disease [e.g. alanine transaminase (ALT) > 3 × upper limit of normal, cirrhosis, ascites] (investigator opinion).
-
Patients currently taking part in another interventional clinical trial of an investigational medicinal product or medical device (or taken part in one within the last 3 months).
-
Previous allergy to allopurinol.
-
Previous serious adverse cutaneous (skin) reaction to any drug (e.g. Stevens–Johnson syndrome, toxic epidermal necrolysis, hospitalisation due to skin reaction to drug) (investigator opinion).
-
Patients already taking urate-lowering therapy (including allopurinol, febuxostat, sulfinpyrazone, benzbromarone, probenecid, rasburicase).
-
Patients taking azathioprine, mercaptopurine, ciclosporin or theophylline.
-
Malignancy (except non-metastatic, non-melanoma skin cancers, cervical in situ carcinoma, breast ductal carcinoma in situ, or stage 1 prostate carcinoma) within the last 5 years (investigator opinion).
Screening and randomisation visit
At a single screening and randomisation visit held at the patient’s primary care practice by a research nurse (or the practice nurse or GP), written informed consent was obtained, then participants were screened and randomised to receive allopurinol therapy or to continue their usual care.
Baseline demographics, medical history, CV risk factors and concomitant medications were recorded. Blood pressure, height and weight were measured. Baseline blood tests were taken for urea, creatinine and electrolytes, full blood count and SUA. Blood tests were sent to local NHS laboratories and results were later entered into the electronic case report form (eCRF) by the nurse. Participants were randomised before screening blood results were available. When the screening blood results were available and had been checked for eligibility, a nurse telephoned those patients randomised to the allopurinol arm of the study to advise them to collect their prescription and start taking randomised therapy. If the screening visit eGFR result was below the exclusion limit, the participant did not receive any allopurinol and was excluded from the modified intention-to-treat (mITT) analysis population, whichever arm of the study they had been randomised to.
Randomisation
Randomisation was carried out using a web-based randomisation facility located at the Robertson Centre for Biostatistics, University of Glasgow, accessed using a web-based application or an interactive voice response system. Randomisation was based on randomised permuted blocks of variable size, stratified by history of MI, history of stroke and primary care practice. Participants were randomised 1 : 1 to receive either allopurinol therapy plus usual care or to continue usual care.
Interventions
Allopurinol 100 or 300 mg tablets (various manufacturers and suppliers) were prescribed to participants by their primary care physicians via the usual NHS prescription system and supplied via the patient’s local community pharmacy. Participants with a screening eGFR result ≥60 ml/minute/1.73 m2 were prescribed allopurinol at 100 mg daily for 2 weeks, then 300 mg daily for 2 weeks then 600 mg daily (given as 300 mg twice daily) thereafter if tolerated for the duration of the study (maximum 600 mg daily dose). After the amendment to include patients with moderate renal impairment was implemented across study sites on 4 April 2016, participants with a screening eGFR result 30–59 ml/minute/1.73 m2 were prescribed allopurinol at 100 mg daily for 2 weeks, then 300 mg daily thereafter if tolerated for the duration of the study (maximum 300 mg daily dose). If the screening visit eGFR result was below the exclusion criterion limit, the participant did not receive any randomised medication.
If there were tolerability issues, the dose could be decreased at any time at the discretion of a physician, or the allopurinol could be stopped. Participants experiencing any rash, however mild, that could be due to allopurinol were withdrawn from allopurinol therapy as a precaution against the development of a more serious rash such as Stevens–Johnson syndrome or toxic epidermal necrolysis. Participants who stopped randomised therapy were encouraged to continue within the rest of study follow-up. The comparison arm of the study was ‘usual care’; no placebo was given as this was a pragmatic open-label study. Randomised therapy was blinded to the endpoint adjudication committee, but not to participants, study staff or treating healthcare professionals. If allopurinol was started in participants randomised to the usual care group at any point in the study (e.g. for clinical reasons such as developing gout), this was recorded along with the date and the reason.
Other study procedures
At the screening visit, participants completed two quality-of-life questionnaires. The EQ-5D-5L33 was completed to assess general health outcomes and the Seattle angina questionnaire34 was completed to assess coronary artery disease-specific quality of life.
Participants who had taken any allopurinol therapy attended a 6-week study visit with a research nurse. Blood samples were taken for urea, creatinine and electrolytes, full blood count and SUA. Participants were asked at this visit whether they were still taking their allopurinol therapy.
The participants were then followed up remotely for the rest of the trial, with no further in-person study visits. Follow-up occurred by electronic record-linkage to centralised databases of hospitalisations, cancers and deaths held by Public Health Scotland (PHS) (https://publichealthscotland.scot) and NHS Digital (https://digital.nhs.uk) to capture details of serious adverse events (SAEs) and potential study endpoints. Participants were also asked to complete annual questionnaires (online, by post or by telephone). Data on adverse events, skin rashes, gout flares and self-reported adherence to randomised therapy were collected at the 6-week visit then annually (but could also be reported at any time by participants or health professionals). Participants also self-reported their health resource usage (number of visits to GP, practice nurse, outpatient clinic and physiotherapist in the previous 12-month period) at 1 year, at the end of the study (and annually for a randomly selected 25% of participants) for the purposes of the health economic analysis. Participant-reported data could be clarified or verified where necessary by contacting the participant’s primary care practice team or consulting medical records. Participants completed the EQ-5D-5L and Seattle angina questionnaires after 1 year and at the end of the trial. The follow-up period of the trial ended on 30 September 2021. After this date, participants stopped randomised therapy and continued to receive their usual care.
Amendments to protocol during study
The trial recruitment period was extended from 2 years to just over 3.5 years to allow the recruitment target to be reached. Final recruitment exceeded the target of 5215 randomised participants with a final total of 5937 randomised participants reached, due to an increase in recruitment rate in the final weeks of recruitment.
The trial follow-up period was extended twice due to the extended recruitment and lower than predicted CV event rates in the study population. Participants could choose to end their active involvement in the study after 5 years’ participation (the original study duration) or after 31 March 2021, when these extensions were implemented.
During a temporary shortage of allopurinol 100 mg tablets supply experienced during the year 2014, the protocol was amended temporarily to allow participants to start on 100–150 mg allopurinol for the first 2 weeks so that a half tablet of 300 mg could be administered daily if it was not possible to obtain 100 mg tablets. The dose was then up-titrated to 300 mg, then 600 mg daily as described above. The starting dose of allopurinol for the majority of participants in the study was 100 mg daily.
Originally, only patients with screening eGFR ≥ 60 ml/minute/1.73 m2 were included in the study. However, the protocol was amended during the second year of recruitment (change implemented at all study sites on 4 April 2016) to allow the inclusion of patients with moderate renal impairment (screening eGFR 30–59 ml/minute/1.73 m2) in the study with the goal of making the study results more generalisable. Fifty-two per cent of the target number of patients had been randomised by this date.
Outcomes
The primary outcome was the composite of non-fatal MI, non-fatal stroke or CV death.
The secondary outcomes were non-fatal MI; non-fatal stroke; CV death; all-cause mortality; hospitalisation for ACS; coronary revascularisation; hospitalisation for ACS or coronary revascularisation; hospitalisation for HF; all CV hospitalisations; quality of life; cost effectiveness.
Patient-reported healthcare service usage (specifically visits to GP, practice or community nurse, physiotherapist, doctor at a hospital outpatient clinic) were also reported as additional outcomes.
Serious adverse events occurring during the study (until 30 September 2021) were recorded, and any ongoing events were followed up until 30 days after the end of the study follow-up period (until 30 October 2021), unless participants had withdrawn consent. Gout flares, rashes and any treatment-related adverse events were also recorded.
All SAEs were reviewed by a study physician and any potential study endpoints were identified at the time of reporting. For any potential study endpoints, information was collected from medical records and death certificates. An anonymised endpoint package (with details of randomised therapy redacted) was prepared for each potential endpoint. This anonymised endpoint package was adjudicated by an independent clinical events adjudication committee unaware of randomised arm of the study that is blinded to study allocated treatment. The committee adjudicated all the components of the primary composite outcome, all deaths and all secondary CV outcomes (except coronary revascularisations which were confirmed by the Chair of the committee, and any ‘cardiovascular hospitalisations’ falling outwith one of the other adjudicated categories of CV events, which were confirmed by the study physicians in Dundee); the events are defined in the clinical endpoint adjudication committee charter (see Report Supplementary Material 1).
Record-linkage to centralised databases for records of hospitalisations, deaths and cancers was carried out at regular intervals throughout the trial with a final linkage performed after the end of the study follow-up period [PHS (https://publichealthscotland.scot) and NHS Digital (https://digital.nhs.uk)] to capture adverse events and study endpoints.
Approvals, oversight and study committees
The study was approved by the East of Scotland Research Ethics Committee (REC Ref. 13/ES/0104, IRAS ID 135900) and the Medicines and Healthcare Products Regulatory Agency (MHRA) (CTA number 21726/0284/001-0001). NHS R+D and HRA approvals were obtained, as were the relevant permissions to obtain record-linkage data from PHS and NHS Digital.
The study was jointly sponsored by the University of Dundee and NHS Tayside. The chief investigator was Professor Isla Mackenzie, University of Dundee. Principal investigators were appointed at each of the regional study sites. The Clinical Co-ordination Centre was at MEMO Research, University of Dundee. The Data and Biostatistics centre was at the Robertson Centre for Biostatistics, University of Glasgow. Trial monitoring was co-ordinated by the University of Dundee and NHS Tayside, Dundee.
Overall supervision of the study was provided by a Trial Steering Committee (TSC), chaired by Professor Sir Lewis Ritchie, University of Aberdeen. The committee included independent members and two lay/patient members. Trial safety was overseen by an Independent Data Monitoring Committee (IDMC) chaired by Professor Sir Mark Caulfield, Queen Mary University London. An IDMC charter outlined its roles and responsibilities (see Report Supplementary Material 2). A study management group led the day-to-day management of the study. All committees and the management group met regularly throughout the trial.
Statistical analysis
The power calculation suggested that 5215 participants would need to be randomised 1 : 1 to give 80% power to detect a 20% reduction in the primary outcome for the intervention (allowing for a 4% dropout for withdrawal of consent and non-CV deaths). A primary event rate of 14% over 4 years average follow-up was estimated from other trials in similar patient groups. The study would end when 631 adjudicated first primary events had occurred.
Baseline characteristics are presented by treatment group as means [standard deviation (SD)] and medians [interquartile range (IQR)] for continuous variables and as numbers (%) for categorical variables.
Clinical outcomes were analysed on a time to first event basis using Cox proportional hazards models. Treatment effects (allopurinol vs. usual care) were estimated as hazard ratios (HRs) and 95% confidence intervals (CIs) for the Cox models. Analyses were adjusted for the stratification variables history of MI and history of stroke and p-values were calculated from Wald statistics. Although primary care practice was a stratification variable to avoid potential bias in this open-label trial, because of the large number of practices involved, as is common practice, study site was not adjusted for in the analysis.
The primary analysis was a mITT analysis.
Any participants who had been randomised but were later found to have not met the inclusion/exclusion criteria (e.g. did not meet screening eGFR criteria on screening blood results, or had a history of recent malignancy not fully realised at time of inclusion in the study) were excluded from the mITT analysis. The mITT analysis censored follow-up after death from any cause not included in the endpoint being considered, date of withdrawal of consent to participate any longer in the study, or the end of the study, whichever occurred first. An on-treatment (OT) analysis censored follow-up after permanent stopping of randomised therapy, death from any cause not included in the endpoint being considered, date of withdrawal of consent to participate any longer in the study, or the end of the study, whichever occurred first. Similar analyses were performed for other time-to-event secondary clinical endpoints. Pre-specified subgroup analyses were carried out for the primary endpoint. p-values for the test of interaction between the variable defining the subgroup and randomised treatment allocation were calculated in each case.
Quality-of-life outcomes at each time point (1-year post randomisation and final visit) were analysed using linear regression models for the change in each quality-of-life measure. Analyses were adjusted for the stratification variables as per the clinical outcomes and the baseline quality-of-life value. Participants with a baseline value available but missing follow-up had a value imputed, with a zero if the participant had died, or using multiple imputation. Ten imputations were generated using SAS PROC MI, with the monotone regression option, using the following variables as predictors: baseline measure, treatment, stratification variables (history of MI and history of stroke) and age. Multiple imputations were combined using SAS PROC MIANALYZE procedure. Selected baseline characteristics were compared between patients with and those without EQ-5D-5L score being available at the end of the study using Wilcoxon rank sum tests for continuous variables and Fisher’s exact tests for categorical variables, for each arm of the study.
Healthcare service usage outcomes for all participants with data at each time point (1 year post randomisation and end of study) were analysed using stratified Wilcoxon tests which account for the stratification variables. The subset of participants with data additionally collected at each interim annual time point were analysed in the same way and the results were similar to those in all participants (with no statistically significant and clinically meaningful differences).
Time-to-event curves are presented as Kaplan–Meier curves for all-cause mortality, and as cumulative incidence functions adjusting for the competing risk of deaths not included in the endpoint being plotted for the other endpoints and are obtained using SAS PROC lifetest. Time to withdrawal of consent from the study and time to discontinuation of allopurinol treatment were described.
The type I error rate was set at 5% for two-sided superiority analyses. Pre-planned interim analyses were carried out for the IDMC (requiring p < 0.001 to make a recommendation for early stopping of the trial), but not shared with the study team, after approximately 50% and 75% of the target number of 631 adjudicated study outcomes had been observed. No adjustments were made for multiple statistical comparisons. Analyses other than for the primary endpoint should be considered exploratory.
All participants who were validly randomised and whose baseline eGFR did not exclude them based on their screening visit blood results were included in the mITT and OT analysis populations. The safety analysis population included all participants in the usual care arm, and all participants in the allopurinol arm who took at least one dose of randomised medication (allopurinol). The number of participants with SAEs and crude rates per 100 patient-years are reported by MedDRA system organ class for each treatment arm with rates compared between treatment arms. Rates, rather than percentages with event (as was originally intended), are compared because of the different withdrawal rates and hence length of follow-up in the two treatment arms. Crude rates are calculated as the number of participants with events, divided by the time-to-first event or censoring (years), multiplied by 100.
Analyses and graphical displays were conducted using SAS for Windows version 9.4 and R version 3.6.1. The statistical analysis plan is in Report Supplementary Material 3.
Chapter 3 Results
Between 7 February 2014 and 29 September 2017, 6134 patients consented to take part in the trial and were assessed for eligibility. One hundred and sixty-seven were found to be ineligible and 30 did not proceed with randomisation. The final randomisation was completed on 2 October 2017. Of the 5937 randomised participants, 216 were excluded post randomisation due to their screening eGFR blood result being below the limit for the trial or were later found to have not fully met other inclusion or exclusion criteria. Five thousand seven hundred and twenty-one participants (2853 in the allopurinol arm and 2868 in the usual care arm) remained in the mITT population which formed the population for the efficacy analyses. Forty-eight participants in the allopurinol arm of the study never took any of their randomised allopurinol treatment, leaving 2805 participants in the allopurinol arm and 2868 participants in the usual care arm in the safety analysis population. The participant flow is shown in Figure 1.
FIGURE 1.
Participant flow (CONSORT diagram). a, Screening visit was not completed or participant withdrew consent before randomisation. This figure is reproduced from The Lancet, Volume 400, Issue 10359; Mackenzie IS, Hawkey CJ, Ford I Greenlaw N, Pigazzani F, Rogers A, et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, PROBE trial. P1195–205, Copyright Elsevier (2022). 1
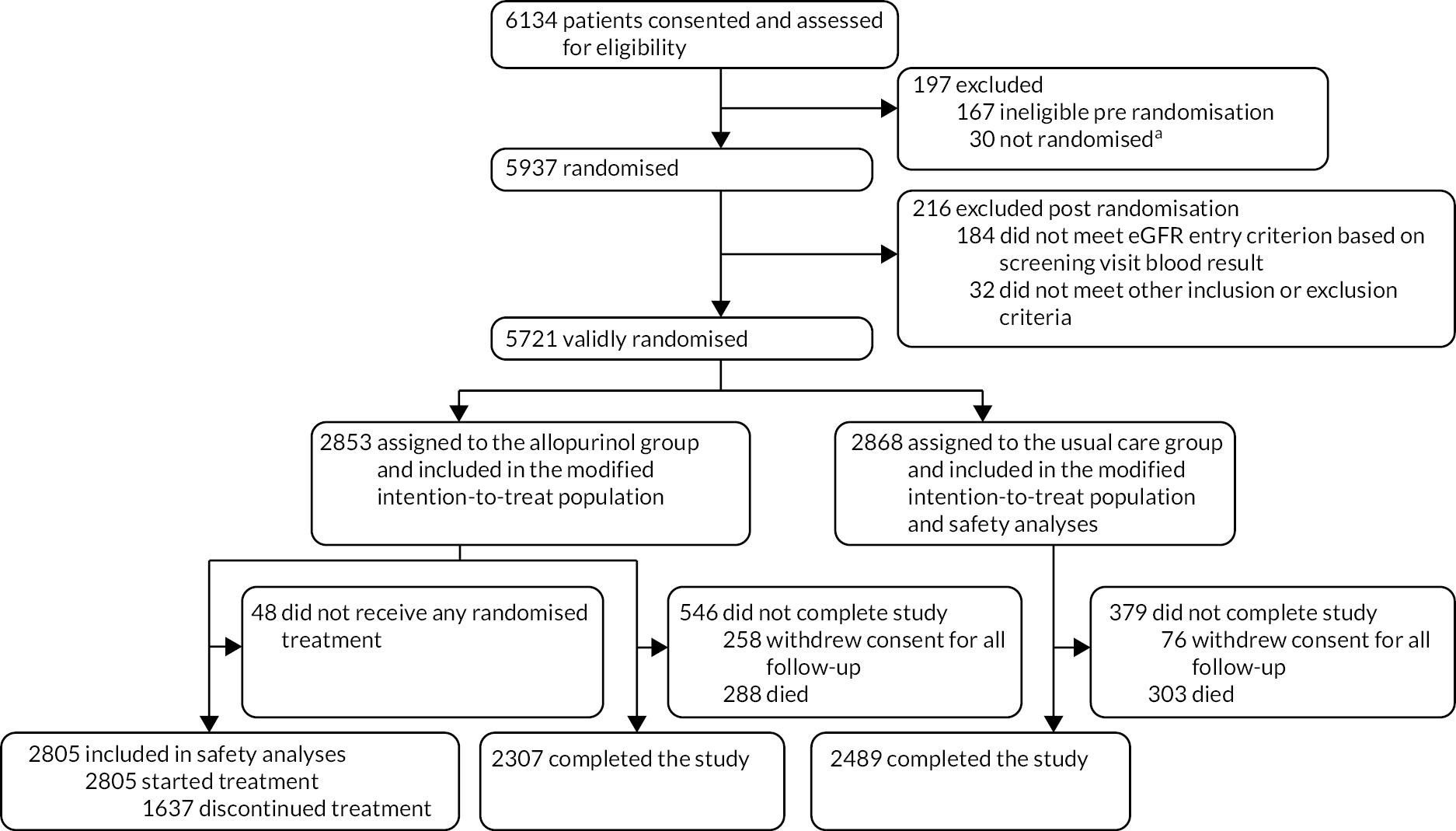
Recruitment occurred in a near-linear fashion throughout the recruitment period of the study, until the last few weeks when the recruitment rate increased (Figure 2).
FIGURE 2.
Number of participants randomised (n = 5937) by month (2014–7).
Participants were recruited from primary care practices via 18 regional sites. The breakdown of participant recruitment (consented and randomised) by regional site is given in Table 1. Three thousand four hundred and sixty-four (60.5%) participants in the analysis group (n = 5721) were recruited in England and 2257 (39.5%) in Scotland.
| Regional site | Consented (n) | Randomised (n) |
|---|---|---|
| Dundee | 1499 | 1471 |
| Edinburgh | 251 | 245 |
| Glasgow | 119 | 115 |
| Aberdeen | 144 | 136 |
| Lanarkshire (Hairmyres and Monklands sites) | 195 | 195 |
| Highland | 112 | 111 |
| Ayrshire and Arran | 47 | 46 |
| Dumfries and Galloway | 18 | 18 |
| Nottingham | 1069 | 1038 |
| West Midlands | 576 | 531 |
| Eastern | 805 | 774 |
| Kent, Surrey and Sussex | 110 | 107 |
| South West Peninsula | 325 | 316 |
| North Thames | 73 | 69 |
| North West Coast | 436 | 424 |
| North East and North Cumbria | 198 | 190 |
| Yorkshire and Humber | 157 | 151 |
| Total | 6134 | 5937 |
Participant follow-up within the study ended on 30 September 2021. Participants were asked to stop taking randomised therapy on that date. Any ongoing SAEs were followed up for outcomes for a further 30 days. Final record-linkage data were obtained from NHS Digital and PHS in December 2021. Supporting information on the remaining potential endpoints was collected over the next few weeks. The trial formally ended on 31 March 2022.
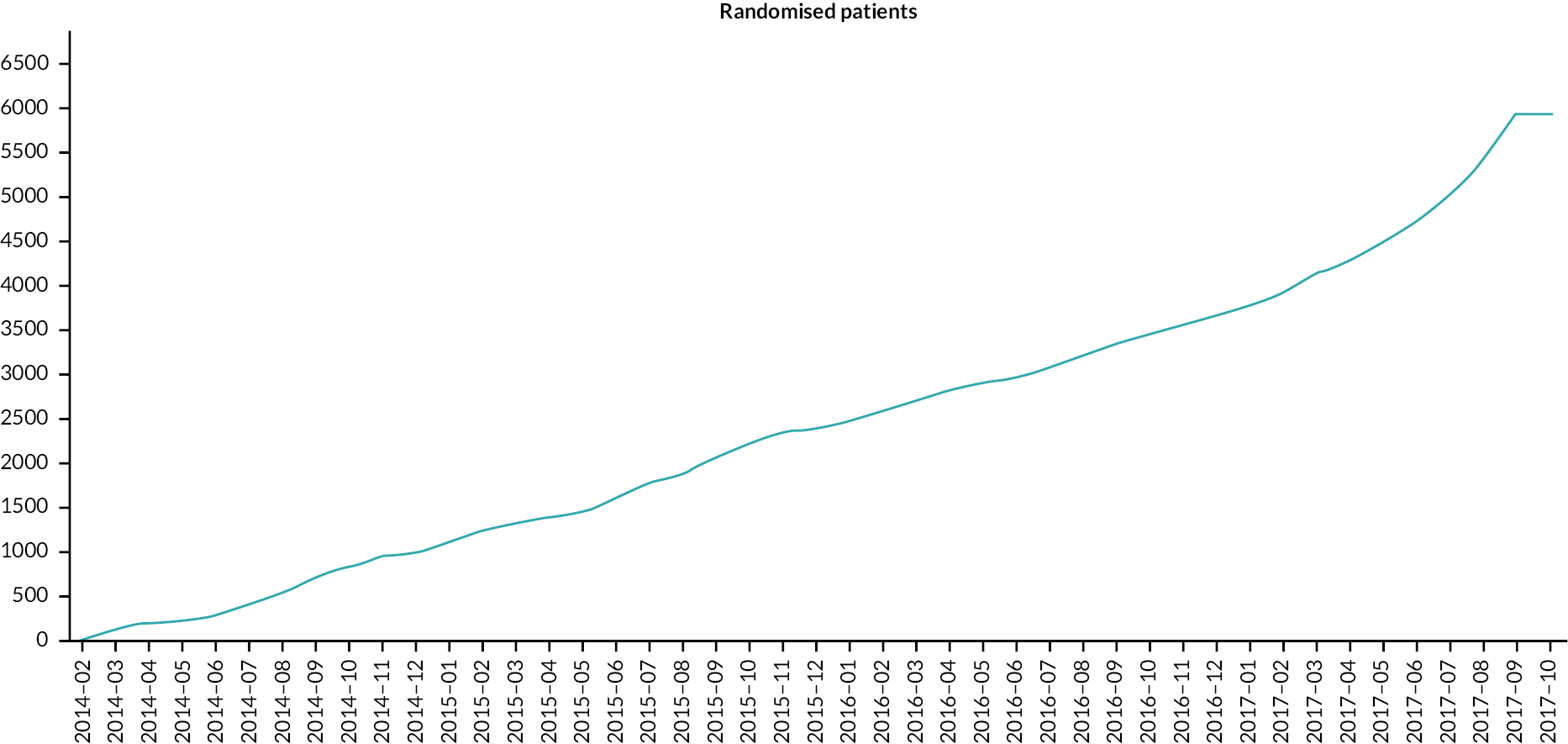
The mean duration of follow-up within the study was 4.8 years (SD 1.5). There were more withdrawals from all follow-up (withdrawals of consent) in the allopurinol group [258 (9.0%) of 2583 participants] than in the usual care group [76 (2.6%) of 2868 participants] (Figure 3).
FIGURE 3.
Cumulative incidence function – withdrawal of all follow-up (consent) from study. Randomised mITT analysis set (n = 5721).

The reasons for withdrawal from all follow-up are given in Table 2. The most common reason for withdrawal from all follow-up in both arms of the study was patient preference.
| Allopurinol (n = 2979) | Usual care (n = 2958) | ||
|---|---|---|---|
| Withdrew from study | 286 (9.6%) | 89 (3.0%) | |
| Reason | Adverse event | 23 (0.8%) | 3 (0.1%) |
| Serious adverse event | 13 (0.4%) | 2 (0.1%) | |
| Patient preference | 194 (6.5%) | 61 (2.1%) | |
| GP recommendation | 12 (0.4%) | 1 (0.0%) | |
| Non-acceptance of randomisation decision | 0 (0.0%) | 3 (0.1%) | |
| Blood sample unobtainable | 1 (0.03%) | 0 (0.0%) | |
| Limited resource for research | 7 (0.2%) | 4 (0.1%) | |
| Relocated and cannot retain | 11 (0.4%) | 5 (0.2%) | |
| Protocol violator | 20 (0.7%) | 8 (0.3%) | |
| Other | 4 (0.1%) | 0 (0.0%) | |
| Screening eGFR is <30 ml/minute/1.73 m2 | 1 (0.03%) | 2 (0.1%) |
One thousand six hundred and thirty-seven (57.4%) participants in the allopurinol group withdrew from randomised treatment (Figure 4).
FIGURE 4.
Cumulative incidence function – withdrawal from randomised treatment. Randomised mITT analysis set: allopurinol arm (n = 2853).
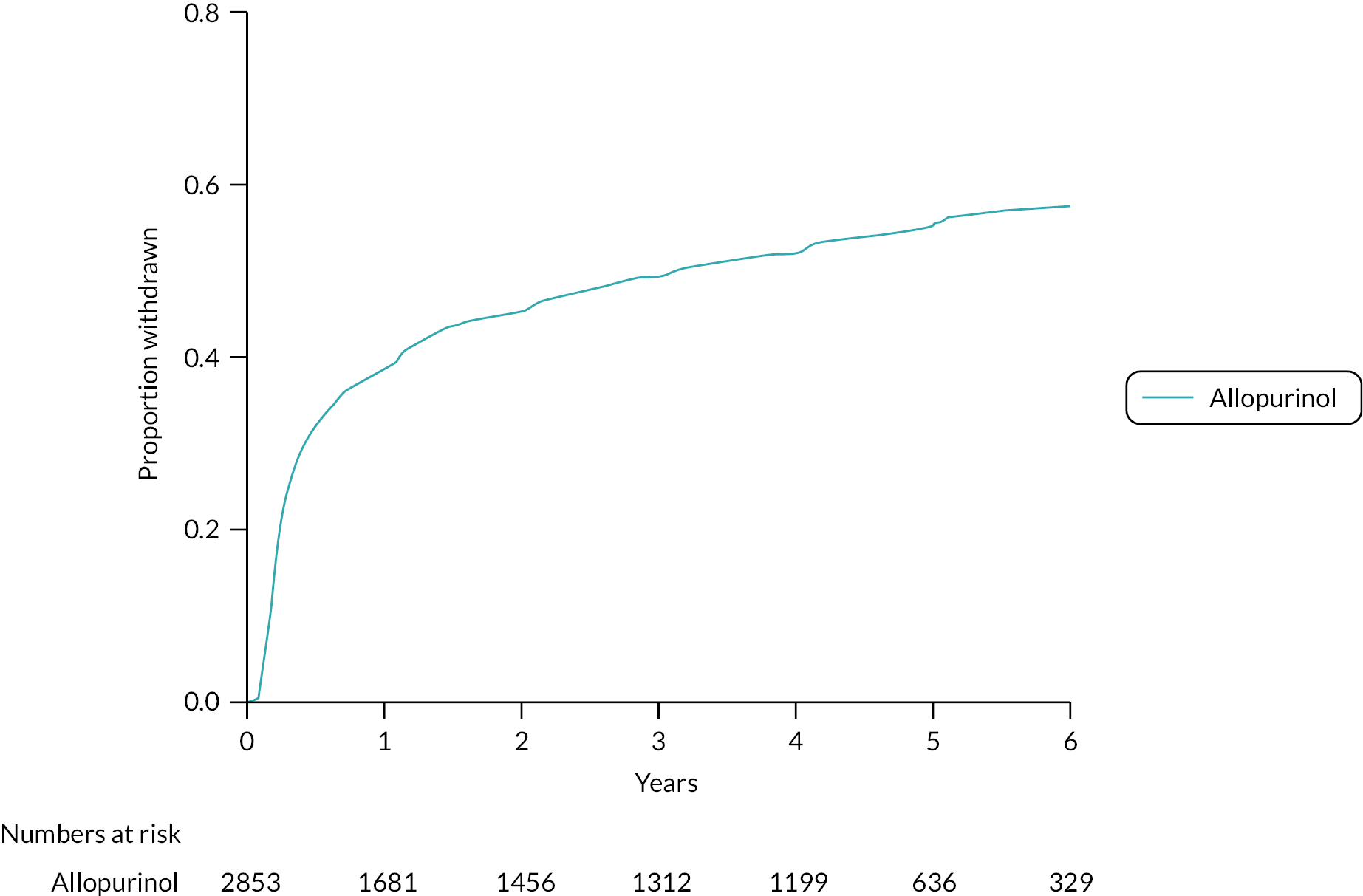
The reasons for withdrawal from randomised treatment (allopurinol) are given in Table 3.
| Allopurinol (n = 2979) | ||
|---|---|---|
| Permanent withdrawal from allopurinol | 1653 (55.5%) | |
| Reason | Adverse event | 853 (28.6%) |
| Serious adverse event | 61 (2.0%) | |
| Patient preference | 448 (15.0%) | |
| GP recommendation | 106 (3.6%) | |
| Non-acceptance of randomisation decision | 1 (0.03%) | |
| Other | 62 (2.1%) | |
| Screening eGFR is <30 ml/minute 1.73 m2 | 0 (0.0%) | |
| Medical advice | 25 (0.8%) | |
| Not started | 75 (2.5%) | |
| Prescription problem | 7 (0.2%) | |
| Protocol violator | 4 (0.1%) | |
| Unknown | 11 (0.4%) |
The two most common reasons for withdrawal from randomised treatment (allopurinol) were adverse event followed by patient preference.
The baseline characteristics of the mITT analysis population (n = 5721) are given in Tables 4–7. Overall, the mean age at entry to the study was 72.0 years (SD 6.8), 4321 (75.5%) participants were male and 5676 (99.2%) were white.
| Allopurinol (n = 2853) | Usual care (n = 2868) | |
|---|---|---|
| Age | ||
| Mean (SD), years | 71.9 (6.7) | 72.0 (6.8) |
| Sex | ||
| Male | 2168 (76.0%) | 2153 (75.1%) |
| Female | 685 (24.0%) | 713 (24.9%) |
| Transgender | 0 (0.0%) | 2 (0.1%) |
| Ethnicity | ||
| White | 2831 (99.2%) | 2845 (99.2%) |
| Asian/Asian British | 13 (0.5%) | 14 (0.5%) |
| Black/African/Caribbean/black British | 4 (0.1%) | 0 (0.0%) |
| Mixed/multiple ethnic groups | 2 (0.1%) | 4 (0.1%) |
| Other | 3 (0.1%) | 5 (0.2%) |
| Smoking history | ||
| Current | 253 (8.9%) | 291 (10.1%) |
| Former | 1644 (57.6%) | 1587 (55.3%) |
| Never | 956 (33.5%) | 990 (34.5%) |
| Systolic blood pressure a | ||
| Mean (SD), mmHg | 132.0 (17.8) | 133.2 (17.9) |
| Diastolic blood pressure a | ||
| Mean (SD), mmHg | 72.0 (10.5) | 72.5 (10.7) |
| Body mass index b | ||
| Mean (SD), kg/m2 | 28.9 (4.9) | 28.8 (4.9) |
| Baseline eGFR group | ||
| 30–44 ml/minute/1.73 m2 | 52 (1.8%) | 56 (2.0%) |
| 45–59 ml/minute/1.73 m2 | 201 (7.0%) | 231 (8.1%) |
| ≥60 ml/minute/1.73 m2 | 2600 (91.1%) | 2581 (90.0%) |
| Baseline SUAc | ||
| Mean (SD), mmol/l | 0.35 (0.08) | 0.34 (0.08) |
| Allopurinol (n = 2853) | Usual care (n = 2868) | |
|---|---|---|
| CV history | ||
| MI | 1348 (47.2%) | 1356 (47.3%) |
| Angina | 1845 (64.7%) | 1824 (63.6%) |
| CCS Angina grade | ||
| Grade 0 | 618 (21.7%) | 646 (22.5%) |
| Grade I | 755 (26.5%) | 702 (24.5%) |
| Grade II | 403 (14.1%) | 411 (14.3%) |
| Grade III | 61 (2.1%) | 54 (1.9%) |
| Grade IV | 8 (0.3%) | 11 (0.4%) |
| No angina | 1008 (35.3%) | 1044 (36.4%) |
| Other evidence of IHD | 1972 (69.1%) | 2000 (69.7%) |
| Duration of IHD | 10.2 (5.0, 16.3) | 10.0 (5.1, 16.0) |
| Median (IQR), years | ||
| Coronary revascularisation | 1612 (56.5%) | 1626 (56.7%) |
| Peripheral arterial revascularisation | 144 (5.0%) | 159 (5.5%) |
| Peripheral arterial disease | 199 (7.0%) | 228 (7.9%) |
| Cerebrovascular accident/stroke | 122 (4.3%) | 108 (3.8%) |
| Transient ischaemic attack | 188 (6.6%) | 166 (5.8%) |
| Hypertension | 1624 (56.9%) | 1663 (58.0%) |
| HF | 146 (5.1%) | 149 (5.2%) |
| NYHA Class I | 77 (2.7%) | 83 (2.9%) |
| NYHA Class II | 69 (2.4%) | 66 (2.3%) |
| No HF | 2707 (94.9%) | 2719 (94.8%) |
| Dyslipidaemia | 1817 (63.7%) | 1806 (63.0%) |
| Other medical history | ||
| Chronic obstructive pulmonary disease | 244 (8.6%) | 251 (8.8%) |
| Diabetes mellitus | 618 (21.7%) | 623 (21.7%) |
| Type I | 24 (0.8%) | 18 (0.6%) |
| Type II | 594 (20.8%) | 605 (21.1%) |
| No diabetes | 2235 (78.3%) | 2245 (78.3%) |
| Allopurinol (n = 2853) | Usual care (n = 2868) | |
|---|---|---|
| Statins | 2583 (90.5%) | 2558 (89.2%) |
| Ezetimibe | 93 (3.3%) | 87 (3.0%) |
| Antiplatelet agents | 2491 (87.3%) | 2504 (87.3%) |
| Anticoagulants | 253 (8.9%) | 260 (9.1%) |
| Beta blockers | 1877 (65.8%) | 1835 (64.0%) |
| ACE inhibitors | 1356 (47.5%) | 1365 (47.6%) |
| Angiotensin receptor antagonists | 530 (18.6%) | 543 (18.9%) |
| Calcium channel blockers | 848 (29.7%) | 806 (28.1%) |
| Other anti-anginal medications | 1602 (56.2%) | 1495 (52.1%) |
| Loop diuretics | 273 (9.6%) | 238 (8.3%) |
| Thiazide/thiazide-like diuretics | 214 (7.5%) | 201 (7.0%) |
| Diabetes medication: insulin | 110 (3.9%) | 80 (2.8%) |
| Diabetes medication: non-insulin | 448 (15.7%) | 435 (15.2%) |
| Non-steroidal anti-inflammatory drugs | 118 (4.1%) | 141 (4.9%) |
| Allopurinol (n = 2853) | Usual care (n = 2868) | |
|---|---|---|
| EQ-5D-5La | ||
| Health state score | 0.814 (0.197) | 0.821 (0.186) |
| Visual analogue score | 78.9 (16.7) | 78.8 (16.2) |
| Seattle angina questionnaire | ||
| Physical limitation domainb | 87.8 (19.0) | 88.0 (18.5) |
| Angina stability domain | 50.5 (9.7) | 50.7 (9.8) |
| Angina frequency domain | 90.8 (17.7) | 91.7 (16.7) |
| Treatment satisfaction domain | 95.1 (9.9) | 95.0 (10.0) |
| Disease perception domain | 84.8 (18.0) | 84.4 (18.2) |
Allopurinol dose taken and adherence to randomised therapy
The most commonly taken daily dose of allopurinol in the study was 600 mg. The median daily dose of 600 mg was observed at all time points following randomisation when considering all participants, or only those with eGFR ≥ 60 ml/minute/1.7 m2 at screening visit; a median daily dose of 300 mg was observed at all time points following randomisation in those with eGFR < 60 ml/minute/1.7 m2 at screening visit. Of those participants still taking allopurinol at each time point after randomisation, 1851 (84.8%) of 2184 participants were still taking the 600 mg daily dose at 6 weeks, 1199 (82.0%) of 1462 participants at 1 year, 1037 (80.4%) of 1290 participants at 2 years, 925 (78.7%) of 1175 participants at 3 years and 719 (82.5%) of 871 participants at 4 years. The mean daily allopurinol dose for all participants taking allopurinol was 543.8 mg at 1 year and 532 mg at the end of the study.
The total number of participants who withdrew from allopurinol therapy was 1653 (55.5%), with 853 of these being associated with adverse events, although not all of these were necessarily treatment-related events. The most common adverse event associated with withdrawal from allopurinol therapy was rash. For safety reasons, participants were withdrawn from allopurinol therapy if they experienced a rash that could have been associated with allopurinol treatment.
Two thousand four hundred and forty-seven participants in the allopurinol group had SUA measured at baseline and at 6 weeks after randomisation, and in this group, SUA concentrations decreased from 0.34 mmol/l (SD 0.08) to 0.18 mmol/l (SD 0.09). Forty-five participants assigned to the usual care group started taking allopurinol during follow-up for clinical reasons (e.g. gout).
Primary endpoint
There was no evidence of a difference in the rates of the primary endpoint between the allopurinol and usual care groups (Figure 5). Three hundred and fourteen (11.0%) of 2853 participants in the allopurinol group (2.47 events per 100 patient-years) and 325 (11.3%) of 2868 participants in the usual care group (2.37 events per 100 patient-years) had a primary endpoint [HR 1.04 (95% CI 0.89 to 1.21); p = 0.65].
FIGURE 5.
Cumulative incidence functions for the primary composite endpoint of non-fatal MI, non-fatal stroke or CV death analysed in the mITT population (n = 5721). Note: The figure was adjusted for the competing risk of deaths not included in the endpoint.
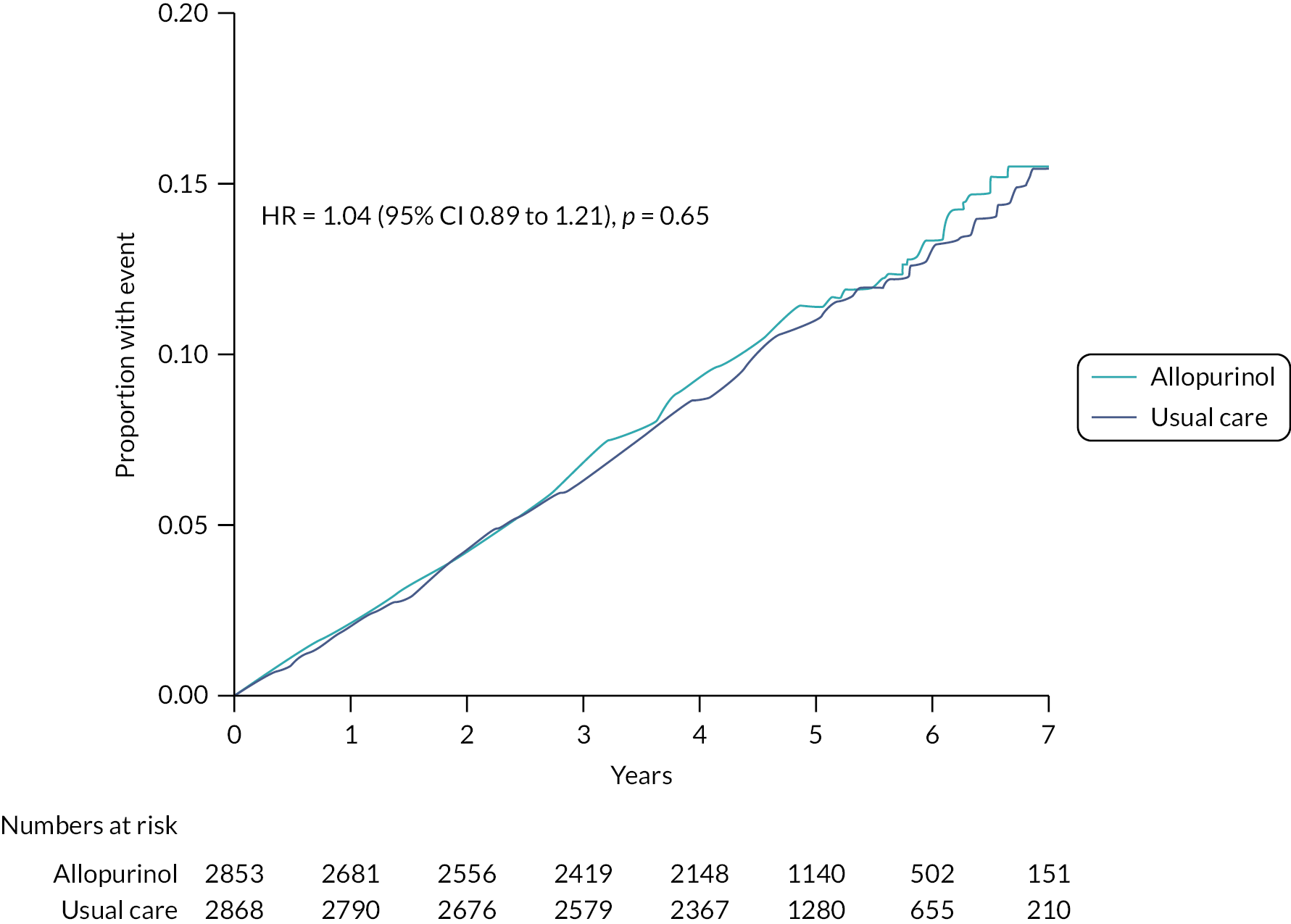
Similarly, there was no evidence of a difference between the randomised treatment groups in rates of the secondary time-to-event outcomes of non-fatal MI (Figure 6), non-fatal stroke (Figure 7), CV death (Figure 8) or all-cause mortality (Figure 9).
FIGURE 6.
Cumulative incidence functions for non-fatal MI analysed in the mITT population (n = 5721). Note: The figure was adjusted for the competing risk of deaths not included in the endpoint.

FIGURE 7.
Cumulative incidence functions for non-fatal stroke analysed in the mITT population (n = 5721). Note: The figure was adjusted for the competing risk of deaths not included in the endpoint.
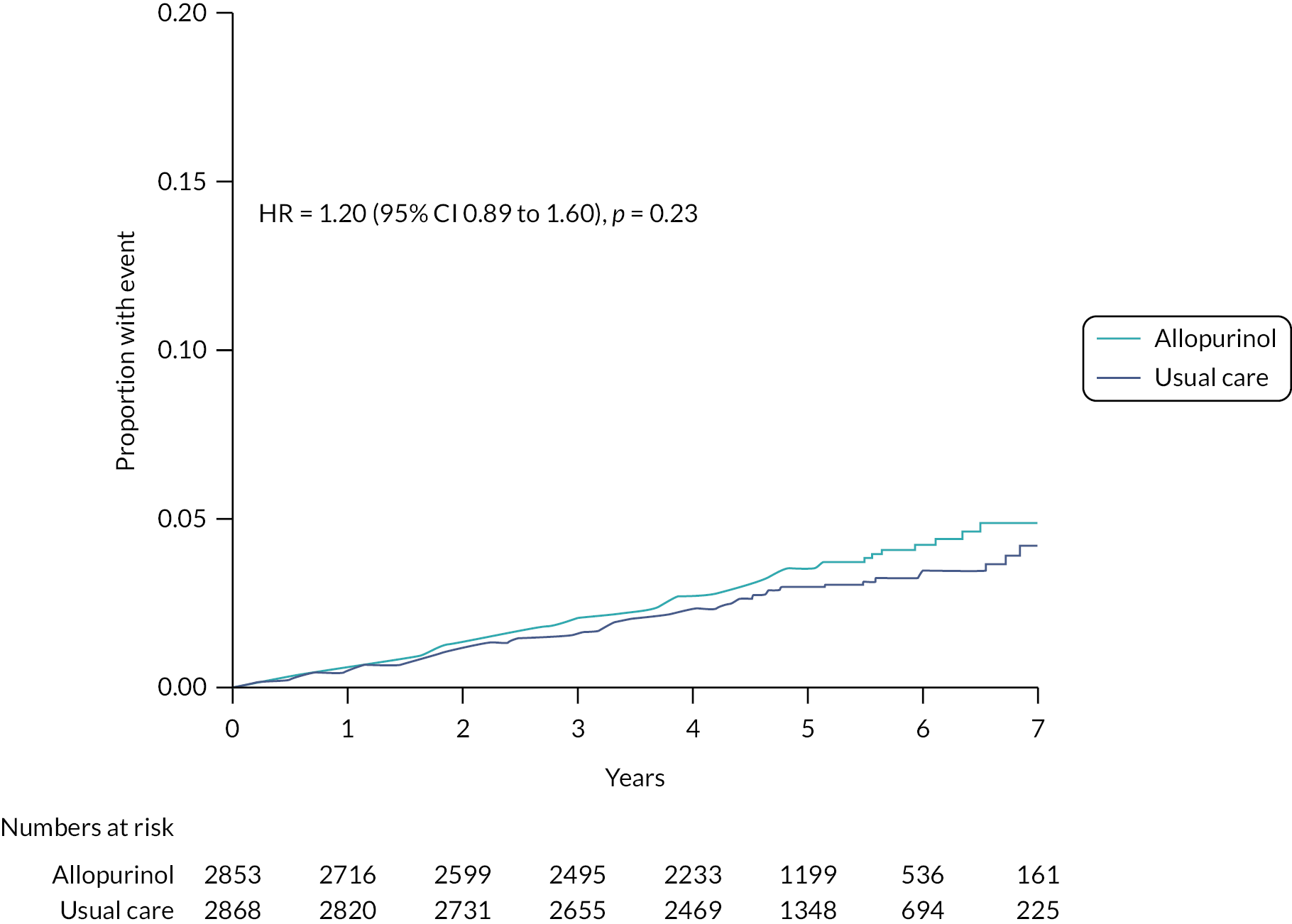
FIGURE 8.
Cumulative incidence functions for CV death analysed in the mITT population (n = 5721). Note: The figure was adjusted for the competing risk of deaths not included in the endpoint.

FIGURE 9.
Kaplan–Meier curve for all-cause mortality analysed in the mITT population (n = 5721).
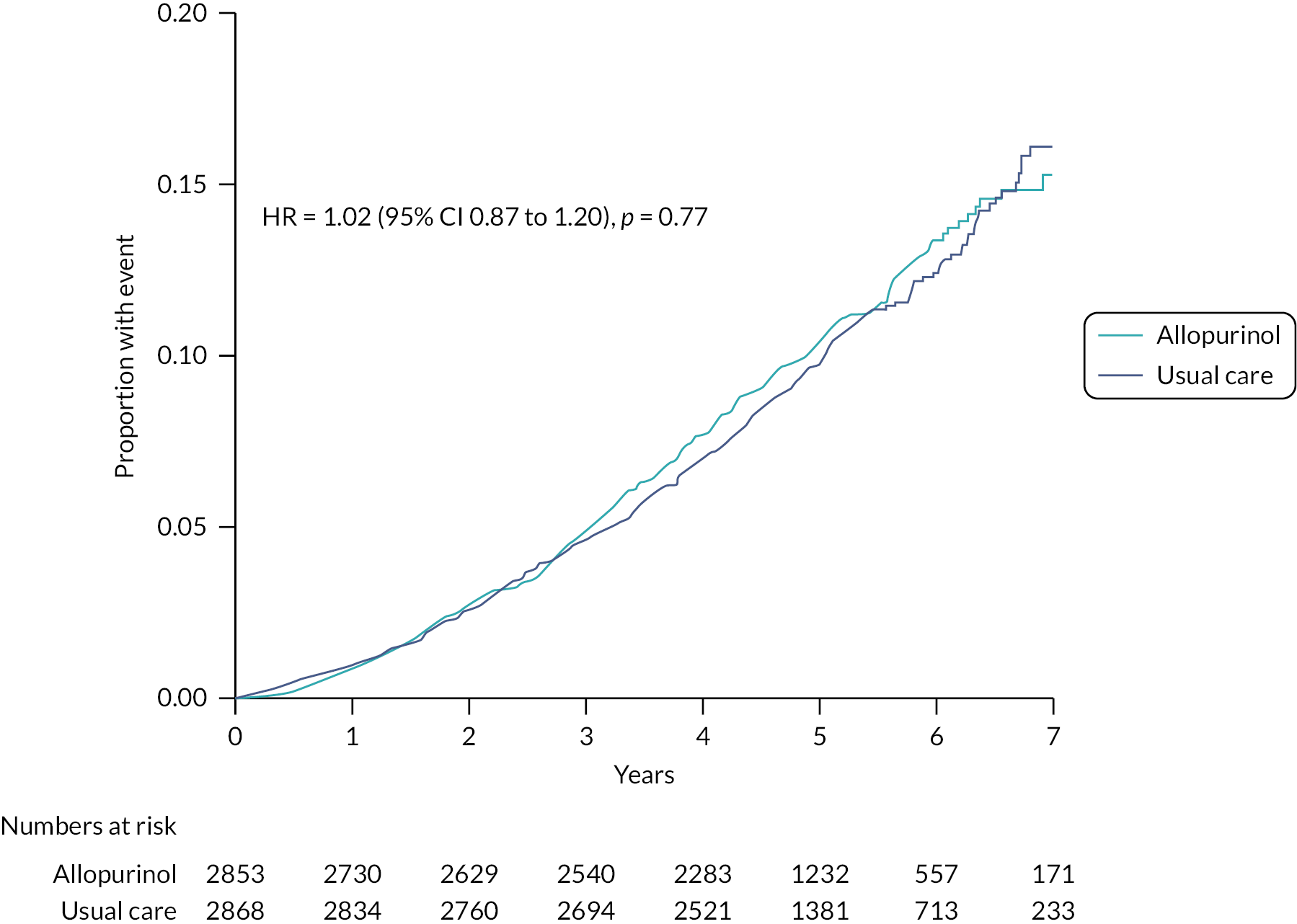
Two hundred and eighty-eight (10.1%) participants in the allopurinol group and 303 (10.6%) participants in the usual care group died from any cause [HR 1.02 (95% CI 0.87 to 1.20); p = 0.77].
There was no evidence of a difference in rates of any of the other pre-specified secondary time-to-event outcomes (hospitalisation for ACS, coronary revascularisation, hospitalisation for ACS or coronary revascularisation, hospitalisation for HF, and all CV hospitalisations) between randomised treatment groups. All primary and secondary time-to-event outcomes in each randomised treatment group, along with rates of events per 100 patient-years, HRs and p-values (calculated with the Wald test) are summarised in the mITT analysis population in Table 8.
| Outcome | Allopurinol group (n = 2853) | Usual care group (n = 2868) | HR (95% CI); p-value | ||
|---|---|---|---|---|---|
| n (%) | Rate per 100 patient-years | n (%) | Rate per 100 patient-years | ||
| Primary outcome | |||||
| Non-fatal MI, non-fatal stroke or CV death | 314 (11.0%) | 2.47 | 325 (11.3%) | 2.37 | 1.04 (0.89 to 1.21); p = 0.65 |
| Secondary time-to-event outcomes | |||||
| Non-fatal MI | 156 (5.5%) | 1.21 | 172 (6.0%) | 1.24 | 0.97 (0.78 to 1.21); p = 0.81 |
| Non-fatal stroke | 96 (3.4%) | 0.74 | 86 (3.0%) | 0.61 | 1.20 (0.89 to 1.60); p = 0.23 |
| CV death | 112 (3.9%) | 0.85 | 109 (3.8%) | 0.76 | 1.10 (0.85 to 1.43); p = 0.48 |
| All-cause mortality | 288 (10.1%) | 2.18 | 303 (10.6%) | 2.13 | 1.02 (0.87 to 1.20); p = 0.77 |
| Hospitalisation for ACS | 205 (7.2%) | 1.61 | 217 (7.6%) | 1.59 | 1.01 (0.84 to 1.23); p = 0.90 |
| Coronary revascularisation | 176 (6.2%) | 1.38 | 207 (7.2%) | 1.51 | 0.91 (0.74 to 1.11); p = 0.35 |
| Hospitalisation for ACS or coronary revascularisation | 267 (9.4%) | 2.13 | 299 (10.4%) | 2.22 | 0.95 (0.81 to 1.13); p = 0.57 |
| Hospitalisation for HF | 74 (2.6%) | 0.57 | 97 (3.4%) | 0.69 | 0.81 (0.60 to 1.10); p = 0.18 |
| All CV hospitalisations | 518 (18.2%) | 4.32 | 556 (19.4%) | 4.31 | 1.00 (0.88 to 1.12); p = 0.94 |
Subgroup analysis
A pre-specified subgroup analysis showed that results for the primary endpoint were consistent across all subgroups (Figures 10 and 11).
FIGURE 10.
Forest plot (1) for primary endpoint for intention-to-treat subgroup analysis – randomised mITT analysis set (n = 5721).
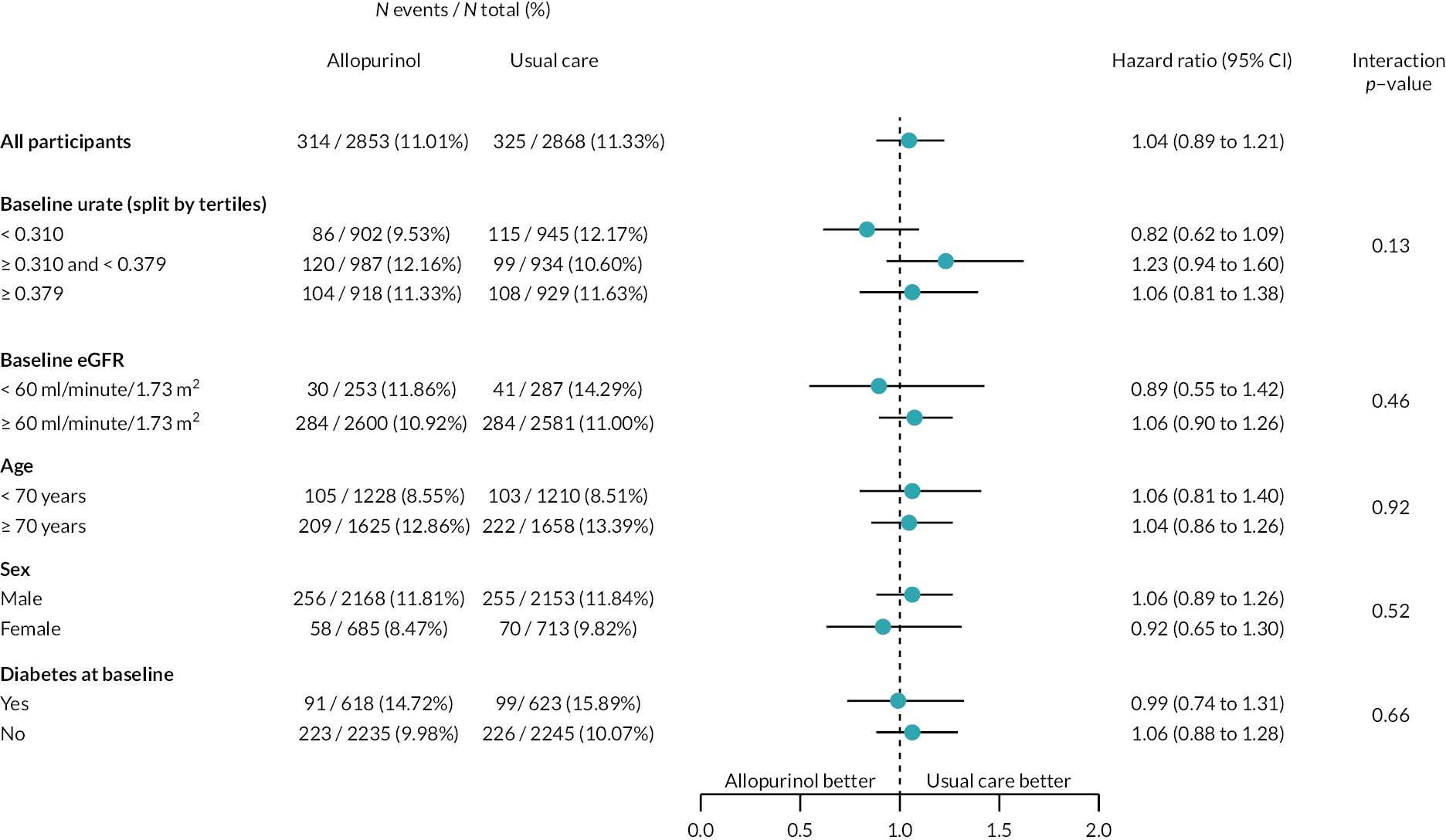
FIGURE 11.
Forest plot (2) for primary endpoint for intention-to-treat subgroup analysis – randomised mITT analysis set (n = 5721). TIA, transient ischaemic attack.

On-treatment analysis
A supporting OT analysis showed results for the time-to-event analyses that were broadly similar to those by the mITT analysis (Table 9).
| Outcome | Allopurinol group (n = 2853) | Usual care group (n = 2868) | |||
|---|---|---|---|---|---|
| n (%) | Rate per 100 patient-years | n (%) | Rate per 100 patient-years | HR (95% CI); p-value | |
| Primary outcome | |||||
| Primary outcome – non-fatal MI, non-fatal stroke or CV death | 172 (6.0%) | 2.30 | 325 (11.3%) | 2.37 | 0.98 (0.81 to 1.18); p = 0.81 |
| Secondary time-to-event outcomes | |||||
| Non-fatal MI | 75 (2.6%) | 0.99 | 172 (6.0%) | 1.24 | 0.80 (0.61 to 1.05); p = 0.10 |
| Non-fatal stroke | 59 (2.1%) | 0.78 | 86 (3.0%) | 0.61 | 1.27 (0.91 to 1.78); p = 0.16 |
| CV death | 62 (2.2%) | 0.81 | 109 (3.8%) | 0.76 | 1.10 (0.80 to 1.50); p = 0.55 |
| All-cause mortality | 153 (5.4%) | 1.99 | 303 (10.6%) | 2.13 | 0.99 (0.82 to 1.20); p = 0.93 |
| Hospitalisation for ACS | 107 (3.8%) | 1.43 | 217 (7.6%) | 1.59 | 0.88 (0.70 to 1.11); p = 0.28 |
| Coronary revascularisation | 93 (3.3%) | 1.24 | 207 (7.2%) | 1.51 | 0.81 (0.63 to 1.03); p = 0.085 |
| Hospitalisation for ACS or coronary revascularisation | 142 (5.0%) | 1.91 | 299 (10.4%) | 2.22 | 0.84 (0.69 to 1.02); p = 0.085 |
| Hospitalisation for HF | 34 (1.2%) | 0.44 | 97 (3.4%) | 0.69 | 0.66 (0.44 to 0.97); p = 0.036 |
| All CV hospitalisations | 282 (9.9%) | 3.93 | 556 (19.4%) | 4.31 | 0.90 (0.78 to 1.04); p = 0.17 |
Quality-of-life outcomes
No evidence for a difference in quality-of-life outcomes in the treatment groups was found, with no differences in EQ-5D-5L outcomes or Seattle angina questionnaire outcomes at the end of the first year (Table 10), except for a nominally significant but only slightly greater fall in the usual care group in the physical domain score of the Seattle angina questionnaire at the end of the first year [treatment difference 1.219 (95% CI 0.027 to 2.410); p = 0.045], or at the final visit (Table 11).
| Outcome | Allopurinol group (n = 2853) | Usual care group (n = 2868) | Estimated difference (95% CI); p-value |
|---|---|---|---|
| Change from baseline at 1 year from randomisation [mean (SD)] | Change from baseline at 1 year from randomisation [mean (SD)] | ||
| Secondary EQ-5D-5L outcomes | |||
| Health state scorea | −0.081 (0.205) | −0.094 (0.202) | 0.009 (−0.002 to 0.020); p = 0.11 |
| Visual analogue scoreb | −4.67 (17.11) | −4.98 (16.96) | 0.114 (−0.839 to 1.066); p = 0.81 |
| Secondary Seattle angina questionnaire scores | |||
| Physical limitation domainc | −12.50 (18.85) | −13.90 (18.84) | 1.219 (0.027 to 2.410); p = 0.045 |
| Angina stability domaind | 0.81 (15.90) | 0.35 (15.67) | 0.194 (−0.669 to 1.057); p = 0.66 |
| Angina frequency domaine | −0.45 (15.92) | −1.48 (15.42) | 0.167 (−0.814 to 1.148); p = 0.74 |
| Treatment satisfaction domainf | −1.85 (12.27) | −2.39 (13.02) | 0.064 (−0.760 to 0.888): p = 0.88 |
| Disease perception domaing | −4.16 (18.17) | −5.16 (18.35) | 0.622 (−0.371 to 1.615); p = 0.22 |
| Outcome | Allopurinol | Usual care | Estimated difference (95% CI); p-value |
|---|---|---|---|
| Change from baseline at final visit [mean (SD)] | Change from baseline at final visit [mean (SD)] | ||
| Secondary EQ-5D-5L outcomes at final visit | |||
| Health state scorea | −0.212 (0.338) | −0.219 (0.324) | 0.0046 (−0.0110 to 0.0203); p = 0.56 |
| Visual analogue scoreb | −6.01 (17.97) | −7.95 (18.69) | −0.356 (−1.823 to 1.110); p = 0.63 |
| Secondary Seattle angina questionnaire scores at final visit | |||
| Physical limitation domainc | −15.5 (20.77) | −18.2 (22.00) | 0.400 (−1.314 to 2.114); p = 0.65 |
| Angina stability domaind | 0.77 (14.00) | −0.03 (15.44) | −0.586 (−1.763 to 0.590); p = 0.33 |
| Angina frequency domaine | 0.23 (16.07) | −1.33 (16.41) | −1.059 (−2.873 to 0.755); p = 0.25 |
| Treatment satisfaction domainf | −3.84 (14.26) | −4.06 (14.44) | −1.496 (−3.099 to 0.106); p = 0.067 |
| Disease perception domaing | −4.80 (19.57) | −5.71 (20.33) | −0.668 (−2.442 to 1.106); p = 0.46 |
Health resource usage
Table 12 shows the results of the self-reported health resource usage in the preceding 12 months (visits to GP, community or practice nurse, physiotherapist, doctor in hospital outpatient clinic) collected at 1 year and at the end of the study in the allopurinol group and the usual care group. There was a lower number of reported visits to the GP at the end of the study in the allopurinol arm and a lower number of reported visits to the physiotherapist at 1 year in the allopurinol arm. However, the mean number of reported visits was generally low, and it is not clear whether these slight differences are meaningful, clinically or economically. Otherwise, self-reported health resource usage was similar in both arms of the study.
| Allopurinol (n = 2853) | Usual care (n = 2868) | p-value | ||||
|---|---|---|---|---|---|---|
| Number of GP visits | 1 year | N = 2223 | 3.09 (3.30) | N = 2427 | 3.27 (3.89) | |
| 2 (1, 4) | 2 (1, 4) | 0.064 | ||||
| End of study | N = 1241 | 1.46 (2.52) | N = 1593 | 1.66 (2.59) | ||
| 1 (0, 2) | 1 (0, 2) | 0.016 | ||||
| Number of practice or community nurse visits | 1 year | N = 2224 | 2.45 (4.35) | N = 2427 | 2.42 (4.09) | |
| 1 (1, 3) | 2 (1, 3) | 0.15 | ||||
| End of study | N = 1238 | 1.98 (4.71) | N = 1593 | 1.92 (9.14) | ||
| 1 (0, 2) | 1 (0, 2) | 0.38 | ||||
| Number of physiotherapist visits | 1 year | N = 2228 | 0.48 (1.64) | N = 2434 | 0.57 (1.99) | |
| 0 (0, 0) | 0 (0, 0) | 0.027 | ||||
| End of study | N = 1240 | 0.46 (2.06) | N = 1592 | 0.39 (1.82) | ||
| 0 (0, 0) | 0 (0, 0) | 0.99 | ||||
| Number of visits to doctor at a hospital outpatient clinic | 1 year | N = 2227 | 1.24 (2.34) | N = 2431 | 1.26 (2.19) | |
| 0 (0, 2) | 0 (0, 2) | 0.44 | ||||
| End of study | N = 1239 | 1.05 (2.47) | N = 1592 | 0.97 (3.16) | ||
| 0 (0, 1) | 0 (0, 1) | 0.39 | ||||
Serious adverse events
There was no evidence of a difference in the rates of SAEs between the allopurinol and usual care groups, except for endocrine disorders, where there were no participants with events in the allopurinol group and 14 in the usual care group (Table 13). The endocrine events were of several different types and no pattern of an excess of any one type of endocrine event was seen.
| System organ class | Allopurinol (N = 2805) | Usual care (N = 2868) | Difference in rates (95% CI) |
|---|---|---|---|
| Any SAE | 2036 (29.70) | 2194 (30.32) | −0.62 (−2.43 to 1.19) |
| Blood and lymphatic system | 99 (0.77) | 96 (0.69) | 0.09 (−0.12 to 0.29) |
| Cardiac | 577 (4.92) | 671 (5.34) | −0.42 (−0.99 to 0.15) |
| Congenital familial and genetic | 24 (0.18) | 18 (0.13) | 0.06 (−0.04 to 0.15) |
| Ear and labyrinth | 10 (0.08) | 21 (0.15) | −0.07 (−0.15 to 0.01) |
| Endocrine | 0 (0.00) | 14 (0.10) | − |
| Eye | 357 (2.94) | 383 (2.90) | 0.05 (−0.38 to 0.47) |
| Gastrointestinal | 538 (4.61) | 572 (4.51) | 0.10 (−0.44 to 0.64) |
| General and administration site | 288 (2.34) | 281 (2.08) | 0.26 (−0.10 to 0.63) |
| Hepatobiliary | 79 (0.61) | 65 (0.46) | 0.15 (−0.02 to 0.33) |
| Immune system | 6 (0.05) | 8 (0.06) | −0.01 (−0.06 to 0.04) |
| Infections and infestations | 414 (3.39) | 491 (3.72) | −0.33 (−0.79 to 0.13) |
| Injury poisoning and procedural | 251 (2.01) | 269 (1.97) | 0.05 (−0.30 to 0.39) |
| Investigations | 125 (0.98) | 162 (1.17) | −0.19 (−0.43 to 0.06) |
| Metabolism and nutrition | 110 (0.86) | 104 (0.74) | 0.12 (−0.10 to 0.33) |
| Musculoskeletal and connective tissue | 324 (2.66) | 347 (2.62) | 0.04 (−0.36 to 0.44) |
| Neoplasms benign malignant and unspecified | 485 (4.04) | 561 (4.34) | −0.30 (−0.81 to 0.20) |
| Nervous system | 295 (2.39) | 321 (2.37) | 0.02 (−0.36 to 0.39) |
| Product | 1 (0.01) | 1 (0.01) | 0.00 (−0.02 to 0.02) |
| Psychiatric | 41 (0.32) | 36 (0.25) | 0.06 (−0.07 to 0.19) |
| Renal and urinary | 143 (1.12) | 161 (1.16) | −0.04 (−0.29 to 0.22) |
| Reproductive system and breast | 51 (0.39) | 64 (0.45) | −0.06 (−0.22 to 0.09) |
| Respiratory thoracic and mediastinal | 236 (1.88) | 277 (2.04) | −0.16 (−0.49 to 0.18) |
| Skin and subcutaneous tissue | 40 (0.31) | 51 (0.36) | −0.05 (−0.19 to 0.09) |
| Social circumstances | 2 (0.02) | 8 (0.06) | −0.04 (−0.09 to 0.00) |
| Surgical and medical procedures | 328 (2.69) | 403 (3.07) | −0.38 (−0.79 to 0.04) |
| Vascular | 279 (2.25) | 299 (2.21) | 0.03 (−0.33 to 0.40) |
Fifteen participants in the allopurinol group had SAEs that were considered to be potentially treatment-related. There were 278 fatal SAEs in the allopurinol group, none of which were considered to be treatment-related. The rates of incident cancers were not different between treatment groups (Table 14).
| Allopurinol (N = 2805) | Usual care (N = 2868) | Difference in rates (95% CI) | |
|---|---|---|---|
| Any | 400 (3.27) | 457 (3.46) | −0.19 (−0.64 to 0.26) |
| Breast neoplasms (including nipple) | 12 (0.09) | 14 (0.10) | −0.01 (−0.08 to 0.07) |
| Endocrine neoplasms | 2 (0.02) | 2 (0.01) | 0.00 (−0.03 to 0.03) |
| Gastrointestinal neoplasms | 53 (0.41) | 62 (0.44) | −0.03 (−0.19 to 0.12) |
| Haematological/blood cancers | 32 (0.25) | 39 (0.28) | −0.03 (−0.15 to 0.09) |
| Hepatobiliary neoplasms | 9 (0.07) | 7 (0.05) | 0.02 (−0.04 to 0.08) |
| Mesotheliomas | 3 (0.02) | 3 (0.02) | 0.00 (−0.03 to 0.04) |
| Metastases | 1 (0.01) | 4 (0.03) | −0.02 (−0.05 to 0.01) |
| Miscellaneous and site unspecified neoplasms | 22 (0.17) | 33 (0.23) | −0.06 (−0.17 to 0.04) |
| Nervous system neoplasms | 3 (0.02) | 4 (0.03) | −0.01 (−0.04 to 0.03) |
| Ocular neoplasms | 9 (0.07) | 11 (0.08) | −0.01 (−0.07 to 0.06) |
| Renal and urinary tract neoplasms | 36 (0.28) | 33 (0.23) | 0.04 (−0.08 to 0.17) |
| Reproductive cancers | 95 (0.74) | 82 (0.58) | 0.16 (−0.04 to 0.35) |
| Respiratory and mediastinal neoplasms | 40 (0.31) | 57 (0.40) | −0.09 (−0.24 to 0.05) |
| Skeletal neoplasms | 0 (0.00) | 1 (0.01) | − |
| Skin neoplasms | 126 (0.99) | 153 (1.11) | −0.12 (−0.37 to 0.13) |
| Soft-tissue neoplasms | 1 (0.01) | 1 (0.01) | 0.00 (−0.02 to 0.02) |
Adjudicated causes of death were well balanced between the treatment groups (Table 15).
| Allopurinol (N = 2853) | Usual care (N = 2868) | ||
|---|---|---|---|
| Cause of death | CV | 108 (3.8%) | 107 (3.7%) |
| Non-CV | 176 (6.2%) | 194 (6.8%) | |
| Undetermined cause | 4 (0.1%) | 2 (0.1%) | |
| CV sub-classification | Acute MI | 16 (0.6%) | 12 (0.4%) |
| Stroke | 15 (0.5%) | 4 (0.1%) | |
| Sudden cardiac death | 47 (1.6%) | 44 (1.5%) | |
| HF | 17 (0.6%) | 30 (1.0%) | |
| CV procedure/operation | 2 (0.1%) | 3 (0.1%) | |
| Other CV cause | 11 (0.4%) | 14 (0.5%) | |
| Non-CV sub-classification | Gastrointestinal | 5 (0.2%) | 6 (0.2%) |
| General and administration site | 7 (0.2%) | 6 (0.2%) | |
| Hepatobiliary | 3 (0.1%) | 1 (0.03%) | |
| Immune system | 0 (0.0%) | 2 (0.07%) | |
| Infections and infestations | 64 (2.2%) | 67 (2.3%) | |
| Injury, poisoning and procedural | 2 (0.07%) | 3 (0.1%) | |
| Metabolism and nutrition | 1 (0.04%) | 1 (0.03%) | |
| Neoplasms benign, malignant and unspecified (including cysts and polyps) | 77 (2.7%) | 88 (3.1%) | |
| Nervous system | 4 (0.1%) | 6 (0.2%) | |
| Renal and urinary | 1 (0.04%) | 2 (0.07%) | |
| Respiratory, thoracic and mediastinal | 12 (0.4%) | 12 (0.4%) |
COVID-19 and COVID-19 pneumonia deaths and serious adverse events
Nine deaths (0.3%) in the allopurinol group and 11 deaths (0.4%) in the usual care group were adjudicated as being due to COVID-19. Seven deaths (0.2%) in the allopurinol group and seven deaths (0.2%) in the usual care group were adjudicated as being due to COVID-19 pneumonia. There were no differences between treatment groups in the numbers of participants with SAEs related to COVID-19 [30 (1.1%) in the allopurinol group vs. 31 (1.1%) in the usual care group], or COVID-19 pneumonia [9 (0.3%) in the allopurinol group vs. 8 (0.3%) in the usual care group].
Gout and skin rashes
At the end of the first year of participation in the study, a similar number of participants in the allopurinol group and the usual care group reported a new attack of gout occurring in the previous 12 months [112 (5.0%) of 2805 vs. 114 (4.6%) of 2868, p = 0.55]. At the end of the first year, more participants in the allopurinol group than in the usual care group reported a history of a new skin rash within the previous 12 months [291 (13.1%) vs. 223 (9.1%), p < 0.0001]. At the end of the second year of the study, occurrences of gout and skin rash were reported similarly in both groups. At the end of the third and fourth years of the study, both gout and skin rash in the previous 12 months were reported more commonly by participants in the usual care group than in the allopurinol group of the study.
Chapter 4 Health economic analysis
Aim
The aim of the ALL-HEART health economic analysis was to assess the cost effectiveness of allopurinol versus usual care as a treatment to reduce the risk of the primary endpoint of CV death, non-fatal MI or non-fatal stroke for patients with IHD.
Methods
General approach
The health economic analysis plan (HEAP) is available in Report Supplementary Material 4. The methods to be used depended on the results of the trial. If the trial failed to demonstrate a statistically significant reduction in its primary endpoint and in the outcome of all-cause mortality, then a within-trial cost-effectiveness analysis was to be carried out. Otherwise, the cost-effectiveness analysis was to be based on a lifetime approach with future costs and benefits based on a Markov model. In fact, the trial did not show evidence of benefit in reducing the risk of the primary endpoint or all-cause mortality. Hence, the analysis presented herein is based on a within-trial assessment of the incremental cost-effectiveness ratio (ICER). Similarly, as there were no meaningful clinical differences between treatment arms, we have not reported subgroup or sensitivity analyses in the health economic analyses. The ICER is defined to be the ratio of the mean ‘between treatment group’ difference in costs divided by the mean difference in quality-adjusted life-years (QALYs), derived from EQ-5D-5L index. To take account of potential biases due to treatment group-dependent censoring, costs and QALYs were estimated using inverse probability of censoring weighting (IPCW). The primary analysis was to be based on costs associated with the recurrent components of the primary endpoint (hospitalisation for MI or stroke) and death due to CV causes. All of these events were adjudicated by a Clinical Endpoint Committee, blinded to the treatment group assignation. Costs associated with resource use other than hospitalised events (such as GP visits) were patient-reported and were recorded in the eCRF at the end of year 1 and at the end of the study for all patients and at intermediate annual visits for a random sample of 25% of the patients. EuroQol-5 Dimensions, five-level version questionnaires were completed at the end of year 1 and at the end of the study.
Costs and QALYs were calculated for each period of 6 months from the beginning of the trial, up to a maximum of 5 years. Costs for hospitalisations were assigned to the time period associated with the admission to hospital. Results are displayed graphically in the incremental cost-effectiveness plane and in a plot of the probability of cost-effectiveness as a function of ceilings for willingness to pay. Because of the relatively short timeframe of the analysis, we have not applied time preference discounting to costs or benefits. Missing data were handled using multiple imputation with SAS PROC MI and intermediate time-point EQ-5D-5L index values calculated using linear interpolation. The accuracy of mean costs and QALYs was determined using the bootstrap (1000 iterations) to derive 95% CIs. To maximise the efficiency of the programming a single imputation of missing values was generated within each bootstrap iteration.
As hospitalisation costs are based on a cost per event (such as stroke), it was originally intended to present a sensitivity analysis that focused on the costs of length of stay. However, problems arose in ascertaining discharge dates in a significant number of cases. In addition, where data were available there were a number of outliers suggesting that these data might be unreliable. Hence, this sensitivity analysis was not carried out.
Costs
-
Drug costs: The costs of allopurinol treatment are based on the doses in the study until withdrawal, using the costs in Table 16. Costs for a given dose were created by combining the costs of 100 and 300 mg doses; for example, a 600 mg dose was costed as twice the cost of a 300 mg tablet. Drug costs in the usual care arm were set to zero.
-
Clinical event costs: Costs of hospitalisations for MI and stroke (including all recurrent events) and CV deaths not involving a hospitalisation for stroke or MI were calculated from Table 16.
-
Non-hospital costs: Costs associated with healthcare resource use outside of the hospital were costed from Table 16. Costs recorded at the final study visit were assigned to year 5 if recorded after year 5 or to the nearest year if recorded before year 5. Within each bootstrap replication, SAS PROC MI was used to create a complete data set of annual costs up to 5 years. Half of the annual costs for each year were assigned to the two corresponding 6-month periods. Costs after death or complete withdrawal from the study were set to zero.
-
Total costs: Total costs were defined to be the sum of drug costs + clinical event (MI, stroke, CV death) costs + other resource use (GP, practice of community nurse, physiotherapist, doctor at a hospital outpatient clinic) costs (Table 17).
-
QALYs: Within each bootstrap sample we used one run of SAS PROC MI to generate a complete list of EQ5D scores for baseline, year 1 and end of study (data only captured at baseline, Y1 and end of study). EuroQol-5 Dimensions scores recorded at the final study visit were assigned to year 5 if recorded after year 5 or to the nearest year if recorded before year 5. Six-month scores were calculated by linear interpolation. If the subject was censored before 5 years, scores after the censoring date were set to zero. Quality-adjusted life-years for each 6-month period were calculated as 0.5 × (average of scores at beginning and end of the period). This score was assigned to the beginning of the period. All QALYs for periods after the date of death or censoring were set as equal to zero.
-
IPCW: Probability of censoring was calculated using the Kaplan–Meier method treating censoring times as event times and death times as censoring times. Inverse probability of censoring weighting estimates of costs and QALYs were calculated by weighting the costs (or QALYs) associated with each 6-month time period with respect to the inverse of the estimated probability of censoring at the beginning of each time period. Kaplan–Meier estimates of the probability of censoring were recalculated within each bootstrap sample.
-
Data analysis: For each bootstrap sample, data for IPCW costs and QALYs were outputted. Bootstrap estimates with 95% CIs were calculated for each quantity of interest. Incremental costs were plotted against incremental QALYs in the cost-effectiveness plane and a cost-effectiveness acceptability curve created (probability of being cost-effective vs. ceiling of willingness to pay).
-
Software: All calculations were carried out in SAS version 9.3 for Windows. Graphics were created in Minitab version 20.3.
| Item | Cost (£) | Source |
|---|---|---|
| Allopurinol 100 mg | 0.86 per 28 tablets | British National Formulary35 |
| Allopurinol 300 mg | 1.20 per 28 tablets | British National Formulary35 |
| GP consultation | 39 | PSSRU36 page 111 |
| Practice nurse consultation | 14 | PSSRU36 page 109 (20 minutes) |
| Community nurse contact | 22 | PSSRU36 page 108 (30 minutes) |
| Outpatient clinic | 140 | NICE HTA papers, TA77337 |
| Physiotherapy visit | 56 | https://beta.isdscotland.org/topics/finance/file-listings-fy-2019-to-2020/ and Excel file R04638 |
| Acute stroke hospitalisation | 8767 | Luengo-Fernandez et al.39 |
| Acute MI hospitalisation | 5415 | Palmer et al.40 |
| CV death not occurring during a hospitalisation for stroke or MI | 3126 | McEwan et al.41 |
| Cost per day for a CV hospitalisation | 919 | https://beta.isdscotland.org/topics/finance/file-listings-fy-2019-to-2020/ and Excel file R04042 |
| Allopurinol (n = 2853) | Usual care (n = 2868) | Incremental values | |
|---|---|---|---|
| Drug costs (£) | 64.5 (64.1, 65.0) | 0 | 64.5 (64.1, 65.0) |
| Primary endpoint costs (£) | 532 (482, 582) | 499 (451, 548) | 35.6 (−39.8, 99.7) |
| Resource use costsa (£) | 1188 (1089, 1267) | 1174 (1082, 1240) | 16.2 (−45.2, 79.4) |
| Total costs (£) | 1782 (1674, 1888) | 1669 (1566, 1761) | 115.4 (17.0, 210.2) |
| QALYs | 2.541 (2.496, 2.586) | 2.543 (2.501, 2.580) | −0.000 (−0.061, 0.060) |
Results
Inverse probability of censoring weighting costs and QALYs are given for each treatment arm and as incremental values (difference: allopurinol − usual care). The plot of incremental costs versus incremental QALYs is given in Figure 12.
FIGURE 12.
Plot of incremental costs vs. incremental QALYs in the cost-effectiveness plane.
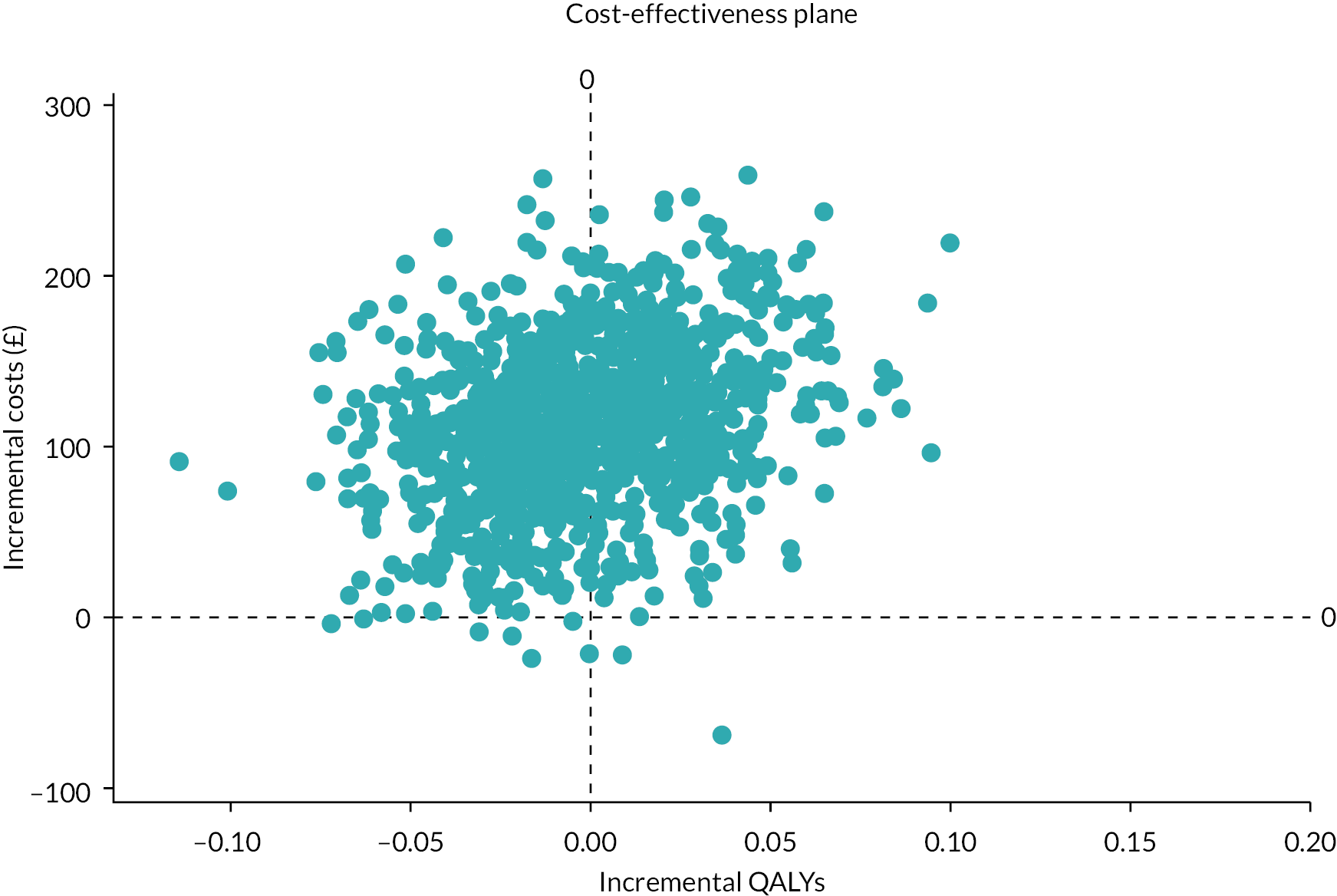
It is evident from the plot and summary results that there is strong evidence that allopurinol treatment is associated with incremental costs relative to usual care [incremental cost per patient £115.4, 95% CI (£17.0 to £210.2)], with little evidence of improvement in relation to incremental QALYs [incremental QALYs −0.000, 95% CI (−0.061 to 0.060)]. An alternative presentation of these results is given in the cost-effectiveness acceptability curve (Figure 13).
FIGURE 13.
Cost-effectiveness acceptability curve.
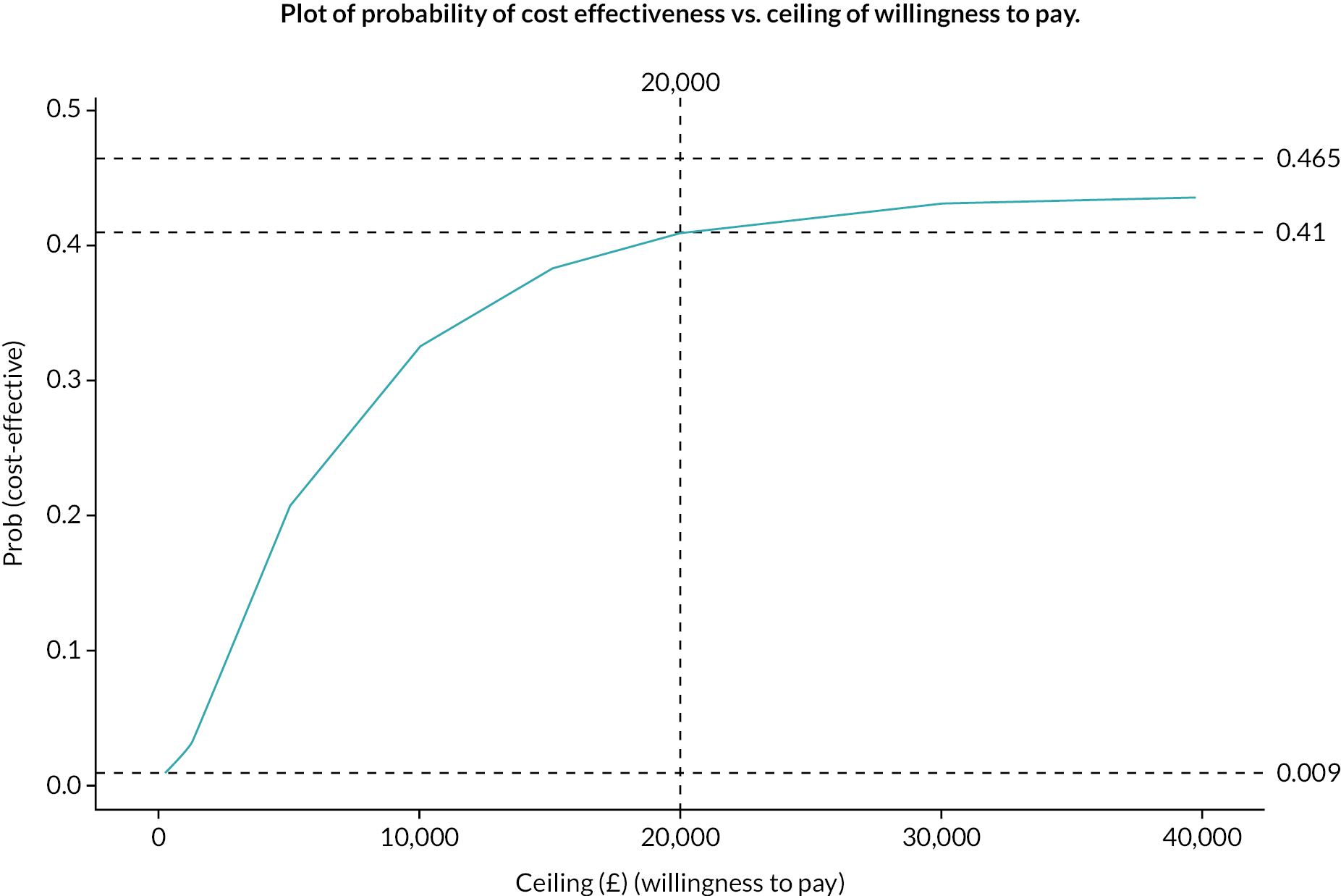
This curve asymptotes at a probability of 0.465, meaning that even with a willingness to pay of an infinite amount the probability of cost effectiveness is only 0.465. At a willingness to pay of £20,000 the probability of cost effectiveness is 0.41.
Conclusions of health economic analysis
In keeping with the clinical results of the ALL-HEART trial, the cost-effectiveness analysis provides no evidence to support the use of allopurinol in the treatment of patients with IHD with the objective of reducing the risk of major CV events (MI, stroke and CV death).
Chapter 5 Discussion
Previous smaller interventional and observational studies exploring the impact of urate-lowering therapy on CV outcomes in patients with gout and various CV conditions have given conflicting results, hence the importance of a prospective, randomised trial to determine whether allopurinol improves outcomes in patients with IHD. The ALL-HEART study is the first, large, prospective, randomised outcome trial of allopurinol versus usual care in patients with IHD. It has definitively shown that allopurinol therapy has no benefit on major CV outcomes in patients with IHD, but no gout. The primary intention-to-treat analysis found no difference in rates of the primary or secondary time-to-event clinical outcomes. A similar result was found in the supporting OT analysis, albeit this is likely to be significantly biased due to treatment discontinuation effects. Hospitalisations for HF were significantly lower in the supporting OT analysis in the allopurinol arm, but the numbers were small, in addition to the risk of bias as above, so this finding should be interpreted with caution. The pre-specified subgroup analysis for the primary outcome found no evidence of benefit of allopurinol therapy for any subgroups.
Participants self-reported occurrences of gout at the end of each year of participation in the study. A lower incidence of gout in the previous 12 months was reported by participants in the allopurinol arm than in the usual care arm at the end of the third and fourth years of participation in the study, which might be expected with chronic urate-lowering therapy in a population at risk of gout. However, this may be subject to some reporting bias and would likely not be an indication to start allopurinol prophylactically as it could be started after onset of gout if required. No other clinical, quality-of-life or health economic benefits of allopurinol therapy were demonstrated in the ALL-HEART study.
It is known that allopurinol may cause skin rash in a proportion of patients, and this is most likely to occur during the first few weeks of therapy, in certain ethnic groups with higher prevalence of certain human leucocyte antigen (HLA)-types, and in patients with pre-existing liver or kidney disease. Occasionally, allopurinol therapy may cause serious rashes such as Stevens–Johnson syndrome or toxic epidermal necrolysis, and the risk of these conditions developing or progressing is reduced by stopping allopurinol therapy immediately if any skin rash occurs. To reduce this risk for participants in the ALL-HEART study, it was advised that allopurinol therapy should be stopped immediately if participants reported any rash that might be related to allopurinol. This conservative approach likely resulted in a higher rate of withdrawal from allopurinol therapy than ideal, but it also reflects how allopurinol would be stopped within real-life clinical care in the NHS. Similar rates of withdrawal from therapy have been seen in other trials of urate-lowering therapy. For example, there was a 56.6% withdrawal from randomised therapy in the North American Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout trial (CARES),43 despite CARES trial participants having a clear clinical indication for urate-lowering therapy (gout). The majority of participants who stopped allopurinol therapy in ALL-HEART continued within study follow-up, so any events occurring following their withdrawal from allopurinol therapy would have been detected. More withdrawals of consent from the study occurred in the allopurinol arm than in the usual care arm, with the difference appearing early in the study. Withdrawals of consent may have been partly driven by the extra study visit for participants in the allopurinol arm at 6 weeks and by withdrawal of consent accompanying early withdrawals from allopurinol therapy. The fact that a placebo or blinding to therapy was not used in the study, instead having an open-label study with a pragmatic usual care arm, may have led to more unbalanced withdrawal from randomised therapy and from the study, as well as some bias in reporting of more subjective adverse events, both participant reported outcomes and those reported by health professionals. However, the components of the primary outcome are objective and were detected from record-linkage hospitalisation data, supported by information from medical records and adjudicated by an endpoint committee blinded to treatment allocation so hopefully free from such biases. Serious adverse events were detected using record-linkage information in addition to participant or health professional reports so should be relatively unbiased. The number of missing data for the self-reported quality of life and health resource usage outcomes is acknowledged as a limitation.
No significant safety issues arose with the short- or long-term use of allopurinol in the ALL-HEART study. SAEs, deaths, incident cancers and COVID-19-related deaths and SAEs were found similar in both allopurinol and usual care groups. This is reassuring for those patients with IHD who may require allopurinol therapy for clinical reasons such as gout in the future.
The final approximately 1.5 years of the study follow-up period coincided with the COVID-19 pandemic, which particularly impacted the UK and clinical trials being conducted within the UK, from March 2020 onwards. ALL-HEART was designed from the start as a partly decentralised trial, with remote follow-up processes in place including questionnaires and record-linkage to national databases for outcomes and pharmacovigilance purposes and with no ‘in-person’ participant visits after the first 6 weeks of participation. This gave the trial added resilience and allowed it to continue without interruption, unlike many other clinical trials, with its processes relatively unaffected during the pandemic. However, despite the use of record-linkage to detect potential endpoints, there may have been some under-reporting of CV endpoints within the trial at the height of the pandemic as fewer patients attended hospitals with acute CV conditions during lockdown periods in early 2020. 44 The decentralised nature of the ALL-HEART trial also resulted in significant cost savings and allowed participants to take part in a major clinical trial lasting several years without the inconvenience of having to travel repeatedly to a central site for trial follow-up visits. The initial screening and randomisation visit and the 6-week visit were carried out locally to participants, usually at their local primary care practice. In recent years, decentralised clinical trial processes have been gaining popularity, having accelerated in their use during the COVID-19 pandemic, and knowledge about the best approaches to optimising participant experiences, inclusivity and study processes is expanding significantly. 45–48
Allopurinol is generally the first-line urate-lowering therapy for patients with gout. However, a newer and more potent xanthine oxidase inhibitor, febuxostat, also became available a few years ago. Two large, randomised trials of CV outcomes with allopurinol versus febuxostat in patients with gout reported their results during the conduct of the ALL-HEART study. These were the North American Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout trial (CARES)43 and the Febuxostat versus Allopurinol Streamlined Trial (FAST). 49 The CARES trial, in patients with gout and established CV disease, reported that febuxostat was non-inferior to allopurinol for the primary composite endpoint of death from CV causes, MI, stroke, or unstable angina with urgent revascularisation. However, in the CARES trial, rates of the secondary outcomes of adverse CV outcomes, all-cause death and CV death were significantly higher with febuxostat than with allopurinol. Warnings were issued by regulators against the use of febuxostat in patients with pre-existing CV disease. However, in contrast, the FAST trial, which had better retention in study follow-up and adherence to randomised treatment, found no increased CV risk with febuxostat, and reported a lower rate of all-cause deaths and CV deaths in the febuxostat group than in the allopurinol group. Unlike the ALL-HEART study, the CARES and FAST trials did not include placebo or usual care arms. Although the CARES and FAST trials primarily included patients with gout, both trials included a significant number of patients with co-existing IHD. Patients with pre-existing gout were excluded from participation in the ALL-HEART study, but no selection of patients based on baseline SUA occurred; so, while the mean baseline SUA level in ALL-HEART participants was not particularly high, participants with a wide range of SUA levels were included in the ALL-HEART study. It is possible that some of those patients with IHD with the highest SUA levels would already have expressed some clinical gout symptoms and would therefore not have been included in the ALL-HEART study. It is possible that allopurinol therapy may have had more anti-oxidative activity in patients with higher baseline SUA levels and that in the absence of hyperuricaemia, allopurinol may have had pro-oxidant effects. However, the pre-specified subgroup analysis for the primary outcome in ALL-HEART found no difference in outcomes in patients in the different tertiles of baseline SUA levels. Further analysis of the changes in urate levels with allopurinol therapy and the CV outcomes within the ALL-HEART study may be of interest.
Trials of urate-lowering therapy in patients with asymptomatic hyperuricaemia have not shown positive effects on reducing major CV outcomes. For example, a randomised trial of febuxostat versus control in 1070 elderly patients in Japan with asymptomatic hyperuricaemia at risk for cerebral, CV or renal events, the Febuxostat for Cerebral and CardioRenovascular Events PrEvEntion StuDy (FREED) showed a reduction in the primary composite cardiorenal endpoint in the febuxostat arm, but this was driven by reduced progression of renal dysfunction, rather than an effect on other CV endpoints. 50 Another Japanese trial of febuxostat therapy versus lifestyle modification in 483 patients with asymptomatic hyperuricaemia found no effect on progression of carotid atherosclerosis (carotid-intima medial thickness) over a 2-year treatment period. 51 At present, there remains no clear place for urate-lowering therapy in CV care, outwith certain situations such as clinical gout.
The daily dose of allopurinol given in the ALL-HEART study (600 mg for the majority of participants) was higher than that generally given to patients with gout in the UK (most commonly 100–300 mg daily40), but within the licensed dose range of 100–900 mg daily. The 600 mg dose was selected for the ALL-HEART study based on earlier studies that suggested that high doses of allopurinol were needed to achieve beneficial CV effects. 11,27,52 In the ALL-HEART study, SUA levels were approximately halved, to levels well below normal ranges or treatment targets in patients with gout, after 6 weeks of allopurinol therapy. This suggested good adherence to allopurinol therapy in the study, at least in the first few weeks. Adherence to randomised therapy was self-reported at the 6-week visit and annually thereafter, which is a limitation; however, it was possible to check with practices to confirm whether patients were still receiving prescriptions for allopurinol if there was any uncertainty, and generally patient reports and practice records correlated well.
The generalisability of the ALL-HEART study results to the population of patients with IHD is likely to be high. The study was conducted in patients’ usual primary care setting, with long-term remote follow-up after the first 6 weeks, so that there would have been minimal impact on participants’ usual care during the study. ALL-HEART was a pragmatic study that was a successful example of how a major CV trial could be delivered within the NHS primary care setting in the UK, involving collaborations with several hundred primary care practices, academic institutions, secondary care facilities and research networks. This allowed widespread geographical inclusion of patients, including from some remote and rural practices, and from areas with different levels of social deprivation. Patients with recent-onset coronary disease, or recent ACSs, were not excluded from participation in the ALL-HEART study; however, the median duration of IHD at study entry was 10.1 years, suggesting that most of the participants had chronic, and therefore potentially more stable, IHD. It is possible that different results may have been obtained if a more acute patient population had been recruited to the study. Most participants were taking secondary CV prevention medications including statins and antiplatelet agents at the time of entry to the study, reflecting the high quality of care for patients with IHD in the UK at the time.
Equality, diversity and inclusion
Population characteristics
The mean age of the population included in the study was 72.0 years. A lower age limit of 60 years was selected to ensure the study would have an adequate event rate and that prescriptions for this age group would be free of charge (in England) to avoid issues associated with prescription charges affecting participation. There was no upper age limit for inclusion. Similarly to recruitment into many other CV trials, fewer participants were female (24.5%) than male. All ethnicities were included in the study. However, despite hopes to ensure inclusion of a broad range of ethnicities, 99.2% of participants within the ALL-HEART study reported white ethnicity. The study team recruited from 424 primary care practices across Scotland and England that included city and rural practices, some of which had much higher ethnic diversity than others. We had hoped that the geographical spread of primary care sites would ensure ethnic diversity in the patient populations we were able to recruit from. We had staff from a wide range of ethnic backgrounds involved in the study in different roles. Images used on the public study website portrayed people of different genders, ethnicities and with visible disabilities to encourage a culture of inclusivity and diversity. In any future trial, further efforts to recruit from underserved populations, particularly regarding ethnicity with reference to the INCLUDE Ethnicity guidance,53 should be made to ensure that results are as generalisable as possible. The ALL-HEART study also completed recruitment prior to publication of the INCLUDE framework and guidance,54 the principles of which should also lead to better diversity in studies in the future. One observation was that no participants suffered severe Stevens–Johnson syndrome within the ALL-HEART study. If the study had been performed in a more diverse ethnic group, the potential risk of Stevens–Johnson syndrome would likely have been higher. The fact that initial visits were generally held in primary care practices and much of the study follow-up was remote could have facilitated the participation of patients with mobility disorders, who may have struggled logistically or physically to attend study visits at central sites some distance from their homes. Options for preferred methods of contact were provided to all participants – e-mails, letters or telephone calls, and options for completion of follow-up questionnaires were also offered – online, paper or by telephone. Participants were able to change their preferences throughout the study follow-up period to adapt to changing circumstances and to aid continuing participation in the study.
Research team and wider involvement
The research team included those from groups who are generally under-represented in CV clinical trials. For example, the chief investigator was female, which is still relatively uncommon in the leadership of major CV trials. Regional principal investigators included both genders and wide ethnic diversity. The research team included senior and junior members, with a wide range of background experience, clinically, academically and in public engagement. The team encouraged and embraced inclusivity regarding neurodiversity, disabilities, caring responsibilities, less than fulltime employees, genders and different ethnicities. Flexibility in working patterns was provided to accommodate individual needs and ensure effective working. National Institute for Health and Care Research allowed flexibility during the two-stage grant application process to accommodate maternity leave of the chief investigator and allowed remote attendance at a monitoring meeting (before the days this was commonplace) to facilitate attendance during maternity leave.
Continuing professional development opportunities were provided to all members of the team, appropriate to their needs at the time. The diversity, flexibility, skill mix and enthusiasm within the study team led to a good working environment and collaborative approach which benefitted the successful conduct of the study and resulted in appropriate problem solving throughout the trial.
Patient and public involvement
Input was obtained at the planning stage of the study from lay people with experience of cardiac disease on the design of the ALL-HEART study and on the acceptability of randomisation to a usual care arm.
The TSC included two patient or lay members throughout the project. Three different patient or lay members held this role during the study, with one member serving on the steering committee from start to end of the study. These members had knowledge of, or personal experience of, cardiac disease, including IHD, and some had been members of local cardiac support groups and other committees previously. The members contributed fully to meetings of the steering committee, also commenting on the extension applications, communications to participants during the study, patient information sheets, end of study letters and dissemination of the results, including communication of the results to participants. Very positive feedback was obtained at the final steering committee meeting, with one lay member reporting that it had been the best experience of patient participation in a committee experienced to date, because their views were always sought and listened to. The commitment and dedication of the patient and lay members throughout the trial were very much appreciated, as it offered a patient perspective on all aspects of the trial and guided the trial team when making important decisions about the conduct and future of the trial.
Patient and public involvement input was obtained into the wording of the plain language summary of this report. Suggested improvements to the wording to improve understanding and readability were incorporated.
The initial study results were presented as a hotline session at the European Society of Cardiology conference in Barcelona in August 2022, then the full paper published in The Lancet in October 2022. A lay summary of the study results and a link to the full Lancet paper were disseminated to ALL-HEART study participants by e-mail or letter, according to participant contact preference, within days of the Lancet publication and lifting of the embargo. A lay summary was also published on the ALL-HEART public study website just after the embargo was lifted. Results were disseminated quickly to all participating GP practices and staff involved in the study. Several positive messages of thanks were received back from participants and staff after being sent the study results. Some of these messages were reviewed at the final TSC meeting. Participant feedback included thanking the study team for the concise and clear summary of the results, thanks for sharing the results and feedback that they had been happy to participate in the trial.
Chapter 6 Conclusions
The ALL-HEART study showed that treatment with allopurinol 600 mg daily did not improve CV outcomes compared to usual care in patients aged over 60 years with IHD. There were no long-term safety issues with allopurinol therapy if it was initially tolerated and continued. There was no evidence that allopurinol used in line with the study protocol is cost-effective within the NHS system. The results of the ALL-HEART study suggest that allopurinol should not be recommended for use for the secondary prevention of CV events in patients with IHD.
Recommendations for research
-
Future research should explore other therapeutic options for the improvement of CV outcomes and quality of life in patients with IHD.
-
Further exploration of the effects of allopurinol on CV outcomes in patients with co-existing IHD and clinical gout or hyperuricaemia could be considered (patients with gout were excluded from this study).
Acknowledgements
We wish to thank all participants for giving up their time and taking part in the ALL-HEART study. We also thank the principal investigators, committee chairs and members and the numerous clinical and non-clinical staff who worked with us on this study in primary care practices, university and secondary care centres and research networks. We thank the patients and members of the public who contributed PPI input to the study.
The study was supported by the Scottish Primary Care Research Network, Support for Science Scotland (Grampian, Highlands, Tayside, Fife, Forth Valley, Greater Glasgow and Clyde, Lothian, Ayrshire and Arran, Dumfries and Galloway, Lanarkshire) and the NIHR Local Clinical Research Networks (East Midlands, West Midlands, Eastern, North Thames, Yorkshire and Humber, North East and North Cumbria, North West Coast, Kent, Surrey and Sussex and South West Peninsula).
We thank Public Health Scotland and NHS Digital for providing data linkage.
Contributions of authors
Isla S Mackenzie (https://orcid.org/0000-0002-3680-7127) (Professor of Cardiovascular Medicine) was the chief investigator, with overall responsibility for the design, co-ordination, conduct and delivery of the study. With co-investigators, she led on the conception, design and the original grant application. She drafted the report with input from co-authors.
Christopher J Hawkey (https://orcid.org/0000-0002-6031-1017) (Professor of Gastroenterology) was a regional principal investigator and a co-investigator on the grant application. He contributed to study conduct and reviewed and commented on the final report.
Ian Ford (https://orcid.org/0000-0001-5927-1823) (Senior Research Fellow) was the lead trial statistician and a co-investigator on the grant application. He performed the statistical analysis and contributed to study design, drafting of the statistical analysis plan and drafting of the report. He reviewed and commented on the final report.
Nicola Greenlaw (https://orcid.org/0000-0003-3847-1126) (Consultant Biostatistician) was a trial statistician. She performed the statistical analysis, and contributed to drafting of the statistical analysis plan and drafting of the final report. She performed the health economic analysis and drafted the health economic analysis chapter with support from Andrew Walker and Ian Ford. She reviewed and commented on the final report.
Filippo Pigazzani (https://orcid.org/0000-0002-8726-306X) (Clinical Research Fellow) contributed to study conduct and data processing. He reviewed and commented on the final report.
Amy Rogers (https://orcid.org/0000-0001-5207-7032) (Clinical Research Fellow) contributed to study conduct and data processing. She reviewed and commented on the final report.
Allan D Struthers (https://orcid.org/0000-0002-2926-2528) (Emeritus Professor of Cardiovascular Medicine) was a co-investigator on the grant application. He reviewed and commented on the final report.
Alan G Begg (https://orcid.org/0000-0002-9712-9227) (General Practitioner and Honorary Senior Lecturer) was a co-investigator on the grant application. He contributed to patient and public involvement activities and reviewed and commented on the final report.
Li Wei (https://orcid.org/0000-0001-8840-7267) (Professor of Pharmacoepidemiology and Drug Safety Research) was a co-investigator on the grant application. She reviewed and commented on the final report.
Anthony J Avery (https://orcid.org/0000-0001-7591-4438) (Professor of Primary Health Care) was a co-investigator on the grant application and contributed to study design. He contributed to study conduct and reviewed and commented on the final report.
Jaspal S Taggar (https://orcid.org/0000-0002-5031-0977) (Clinical Associate Professor in Primary Care) was a co-investigator on the grant application. He contributed to study conduct and reviewed and commented on the final report.
Andrew Walker (https://orcid.org/0000-0002-5372-856X) (Health Economist) was a co-investigator on the grant application and designed the health economic analysis. He supported the health economic analysis and reviewed and commented on the final report.
Suzanne L Duce (https://orcid.org/0000-0001-6539-1311) (Project Manager) contributed to study conduct and project management. She contributed to drafting the final report, and reviewed and commented on the final report.
Rebecca J Barr (https://orcid.org/0000-0003-4820-4240) (Senior Research Manager) contributed to study conduct and project management. She contributed to drafting the final report, and reviewed and commented on the final report.
Jennifer S Dumbleton (https://orcid.org/0000-0001-9099-5555) (Senior Clinical Trials Manager) contributed to study conduct and project management. She reviewed and commented on the final report.
Evelien D Rooke (https://orcid.org/0000-0002-9174-777X) (Clinical Research Fellow) contributed to study conduct and data processing. She reviewed and commented on the final report.
Jonathan N Townend (https://orcid.org/0000-0002-5881-5806) (Professor of Cardiology) chaired the clinical endpoint adjudication committee. He reviewed and commented on the final report.
Lewis D Ritchie (https://orcid.org/0000-0002-9380-7641) (Honorary Professor of General Practice) chaired the trial steering committee. He reviewed and commented on the final report.
Thomas M MacDonald (https://orcid.org/0000-0001-5189-6669) (Professor of Clinical Pharmacology and Pharmacoepidemiology) was a co-investigator on the grant application, deputised as chief investigator and was involved in the conception, design and conduct of the study. He reviewed and commented on the final report.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used – #datasaveslives. You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
The data sets generated or analysed are not expected to be made available owing to the limitations of participant consent and approvals in place regarding data sharing between organisations involved in the study. The data will be held in the University of Dundee. Any applications for potential data sharing or collaboration should be made to the corresponding author.
Ethics statement
The study was approved by the East of Scotland Research Ethics Committee (REC Ref. 13/ES/0104, IRAS ID 135900) on 12 September 2013. All participants gave written informed consent.
Information governance statement
The University of Dundee is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, The University of Dundee is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: www.dundee.ac.uk/information-governance/data-protection.
The Robertson Centre for Biostatistics, University of Glasgow was a Data Processor.
Disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre the HTA programme or the Department of Health and Social Care.
Publications
Mackenzie IS, Hawkey CJ, Ford I, Greenlaw N, Pigazzani F, Rogers A, et al.; ALL-HEART study group. Allopurinol and cardiovascular outcomes in patients with ischaemic heart disease: a multicentre, prospective, randomised, open-label, blinded endpoint clinical trial the ALL-HEART study. Lancet 2022;400:1195–205.
Disclaimers
This manuscript presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Mackenzie IS, Hawkey CJ, Ford I, Greenlaw N, Pigazzani F, Rogers A, et al. ALL-HEART study group . Allopurinol and cardiovascular outcomes in patients with ischaemic heart disease: a multicentre, prospective, randomised, open-label, blinded endpoint clinical trial the ALL-HEART study. Lancet 2022;400:1195-205.
- Office of National Statistics n.d. www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/ischaemicheartdiseasesdeathsincludingcomorbiditiesenglandandwales/2019registrations (accessed 19 September 2022).
- Spiekermann S, Landmesser U, Dikalov S, Bredt M, Gamez G, Tatge H, et al. Electron spin resonance characterization of vascular xanthine and NAD(P)H oxidase activity in patients with coronary artery disease: relation to endothelium-dependent vasodilation. Circulation 2003;107:1383-9. https://doi.org/10.1161/01.cir.0000056762.69302.46.
- Muiesan ML, Agabiti-Rosei C, Paini A, Salvetti M. Uric acid and cardiovascular disease: an update. Eur Cardiol 2016;11:54-9. https://doi.org/10.15420/ecr.2016:4:2.
- Chang CC, Wu CH, Liu LK, Chou RH, Kuo CS, Huang PH, et al. Association between serum uric acid and cardiovascular risk in nonhypertensive and nondiabetic individuals: the Taiwan I-Lan longitudinal aging study. Sci Rep 2018;8. https://doi.org/10.1038/s41598-018-22997-0.
- Efstathiadou A, Gill D, McGrane F, Quinn T, Dawson J. Genetically determined uric acid and the risk of cardiovascular and neurovascular diseases: a mendelian randomization study of outcomes investigated in randomized trials. J Am Heart Assoc 2019;8. https://doi.org/10.1161/JAHA.119.012738.
- Singh J, Weisman A, Tomlinson GA, Lipscombe LL, Perkins BA, Hawker GA. Association between allopurinol and cardiovascular outcomes and all-cause mortality in diabetes: a retrospective, population-based cohort study. Diabetes Obes Metab 2019;21:1322-9.
- Lai S-W, Lin C-L, Liao K-F. Case–control study examining the association between allopurinol use and ischemic cerebrovascular disease. J Investig Med 2019;67:48-51.
- Ju C, Lai RWC, Li KHC, Hung J, Lai J, Ho J, et al. Comparative cardiovascular risk in users versus non-users of xanthine oxidase inhibitors and Febuxostat versus Allopurinol users. Rheumatology 2019;59:2340-49. https://doi.org/10.1093/rheumatology/kez576.
- Suissa S, Suissa K, Hudson M. Effectiveness of allopurinol in reducing mortality: time-related biases in observational studies. Arthritis Rheumatol 2021;73:1749-57. https://doi.org/10.1002/art.41710.
- George J, Carr E, Davies J, Belch J, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 2006;114:2508-16.
- Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt D, Reaveley D, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation 2002;105:2619-24.
- Farquharson CA, Butler R, Hill A, Belch J, Struthers A. Allopurinol improves endothelial dysfunction in chronic heart failure. Circulation 2002;106:221-6.
- Butler R, Morris AD, Belch J, Struthers A. Allopurinol normalises endothelial dysfunction in type 2 diabetics with mild hypertension. Hypertension 2000;35:746-51.
- Guthikonda S, Sinkey C, Barenz T, Haynes W. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation 2003;107:416-21.
- Rekhraj S, Gandy S, Szwejkowski BR, Nadir MA, Noman A, Houston JG, et al. High dose allopurinol reduces left ventricular mass in patients with ischaemic heart disease. J Am Coll Cardiol 2013;61:926-32.
- Gaffo AL, Calhoun DA, Rahn EJ, Oparil S, Li P, Dudenbostel T, et al. Effect of serum urate lowering with allopurinol on blood pressure in young adults: a randomized, controlled, crossover trial. Arthritis Rheumatol 2021;73:1514-22. https://doi.org/10.1002/art.41749.
- Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 2008;300:924-32.
- Morgan BJ, Teodorescu M, Pegelow DF, Jackson E, Schneider D, Plante D, et al. Effects of losartan and allopurinol on cardiorespiratory regulation in obstructive sleep apnoea. Exp Physiol 2018;103:941-55. https://doi.org/10.1113/EP087006.
- Higgins P, Walters MR, Murray HM, McArthur K, McConnachie A, Lees K, et al. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: a randomised controlled trial. Heart 2014;100:1085-92.
- Szwejkowski BR, Gandy SJ, Rekhraj S, Houston JG, Lang C, Morris A, et al. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol 2013;62:2284-93.
- Kao MP, Ang DS, Gandy SJ, Nadir MA, Houston JG, Lang C, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol 2011;22:1382-9.
- Gingles CR, Symon R, Gandy SJ, Struthers A, Houston G, MacDonald T, et al. Allopurinol treatment adversely impacts left ventricular mass regression in patients with well-controlled hypertension. J Hypertens 2019;37:2481-9. https://doi.org/10.1097/HJH.0000000000002189.
- Rutherford E, Ireland S, Mangion K, Stewart G, MacGregor M, Roditi G, et al. A randomized, controlled trial of the effect of allopurinol on left ventricular mass index in hemodialysis patients. Kidney Int Rep 2020;6:146-55.
- Pop-Busui R, Stevens MJ, Raffel DM, White EA, Mehta M, Plunkett CD, et al. Effects of triple antioxidant therapy on measures of cardiovascular autonomic neuropathy and on myocardial blood flow in type 1 diabetes: a randomised controlled trial. Diabetologia 2013;56:1835-44. https://doi.org/10.1007/s00125-013-2942-9.
- Lim TK, Noman A, Choy AMJ, Khan F, Struthers AD, Lang CC. The APEX trial: effects of allopurinol on exercise capacity, coronary and peripheral endothelial function, and natriuretic peptides in patients with cardiac syndrome X. Cardiovasc Ther 2018;36. https://doi.org/10.1111/1755-5922.12311.
- Noman A, Ang DS, Ogston S, Lang C, Struthers A. High dose allopurinol prolongs exercise in chronic stable angina: a randomised placebo controlled trial. Lancet 2010;375:2161-7.
- Huang Y, Zhang C, Xu Z, Shen Z, Zhang X, Du H, et al. Clinical study on efficacy of allopurinol in patients with acute coronary syndrome and its functional mechanism. Hellenic J Cardiol 2017;58:360-5. https://doi.org/10.1016/j.hjc.2017.01.004.
- Johnson WD, Kayser KL, Brenowitz JB, Saedi SF. A randomized controlled trial of allopurinol in coronary bypass surgery. Am Heart J 1991;121:20-4. https://doi.org/10.1016/0002-8703(91)90950-m.
- Taggart DP, Young V, Hooper J, Kemp M, Walesby R, Magee P, et al. Lack of cardioprotective efficacy of allopurinol in coronary artery surgery. Br Heart J 1994;71:177-81. https://doi.org/10.1136/hrt.71.2.177.
- Rajendra NS, Ireland S, George J, Belch JJ, Lang CC, Struthers AD. Mechanistic insights into the therapeutic use of high-dose allopurinol in angina pectoris. J Am Coll Cardiol 2011;58:820-8. https://doi.org/10.1016/j.jacc.2010.12.052.
- Mackenzie IS, Ford I, Walker A, Hawkey C, Begg A, Avery A, et al. Multicentre, prospective, randomised, open-label, blinded endpoint trial of the efficacy of allopurinol therapy in improving cardiovascular outcomes in patients with ischaemic heart disease: protocol of the ALL-HEART study. BMJ Open 2016;6.
- EQ-5D-5L n.d. www.euroqol.org (accessed 4 August 2022).
- Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol 1995;25:333-41.
- Joint Formulary Committee . British National Formulary n.d. www.bnf.nice.org.uk (accessed 16 May 2022).
- Personal Social Services Research Unit (PSSRU) . Unit Costs of Health and Social Care 2021. www.pssru.ac.uk/project-pages/unit-costs/ (accessed 16 May 2022).
- National Institute for Health and Care Excellence Single Technology Appraisal . Empagliflozin for Treating Chronic Heart Failure With Reduced Ejection Fraction [ID3826] n.d. www.nice.org.uk/guidance/ta773/evidence/committee-papers-pdf-11004994333 (accessed 16 May 2022).
- Public Health Scotland . Data and Intelligence. Finance n.d. https://beta.isdscotland.org/topics/finance/file-listings-fy-2019-to-2020/ (accessed 16 May 2022).
- Luengo-Fernandez R, Gray AM, Rothwell PM. on behalf of the Oxford Vascular Study . A population-based study of hospital care costs during 5 years after transient ischemic attack and stroke. Stroke 2012;43:3343-51.
- Palmer S, Sculpher M, Philips Z, Robinson M, Ginnelly L, Bakhai A, et al. A Cost-Effectiveness Model Comparing Alternative Management Strategies for the Use of Glycoprotein IIb IIIa Antagonists in Non-ST Elevation Acute Coronary Syndrome 2002. www.nice.org.uk/guidance/ta47/documents/glycoprotein-iibiiia-modelling-report2 (accessed 16 May 2022).
- McEwan P, Darlington O, McMurray JJV, Jhund P, Docherty K, Bohm M, et al. Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health-economic analysis of DAPA-HF. Eur J Heart Fail 2020;22:2147-56.
- Public Health Scotland . Data and Intelligence. Finance n.d. https://beta.isdscotland.org/topics/finance/file-listings-fy-2019-to-2020/ (accessed 16 May 2022).
- White WB, Saag KG, Becker MA, Borer J, Philip B, Gorelick MD, et al. Cardiovascular safety of Febuxostat or Allopurinol in patients with Gout. N Engl J Med 2018;378:1200-10.
- Mafham MM, Spata E, Goldacre R, Gair D, Curnow P, Bray M, et al. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet 2020;396:381-9. https://doi.org/10.1016/S0140-6736(20)31356-8.
- Coyle J, Rogers A, Copland R, De Paoli G, MacDonald TM, Mackenzie IS. on behalf of the Trials@Home Consortium . Learning from Remote Decentralised Clinical Trial (RDCT) experiences: a qualitative analysis of interviews with trial personnel, patient representatives and other stakeholders. Br J Clin Pharmacol 2021;88:1031-42. https://doi.org/10.1111/bcp.15003.
- Rogers A, De Paoli G, Subbarayan S, Copland R, Harwood K, Coyle J, et al. on behalf of the Trials@Home consortium . A systematic review of methods used to conduct decentralised clinical trials. Br J Clin Pharmacol 2021;88:2843-62. https://doi.org/10.1111/bcp.15205.
- Coyle J, Rogers A, Copland R, De Paoli G, MacDonald TM, Mackenzie IS. Trials@Home Consortium . A secondary qualitative analysis of stakeholder views about participant recruitment, retention, and adherence in decentralised clinical trials (DCTs). Trials 2022;23. https://doi.org/10.1186/s13063-022-06521-4.
- NIHR Remote Trial Delivery n.d. https://sites.google.com/nihr.ac.uk/remotetrialdelivery/home (accessed 19 December 2022).
- Mackenzie IS, Ford I, Nuki G, Hallas J, Hawkey C, Webster J, et al. FAST Study Group. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet 2020;396:1745-57. https://doi.org/10.1016/S0140-6736(20)32234-0.
- Kojima S, Matsui K, Hiramitsu S, Hisatome I, Waki M, Uchiyama K, et al. Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy. Eur Heart J 2019;40:1778-86.
- Tanaka A, Taguchi I, Teragawa H, Ishizaka N, Kanzaki Y, Tomiyama H, et al. PRIZE Study Investigators . Febuxostat does not delay progression of carotid atherosclerosis in patients with asymptomatic hyperuricemia: a randomized, controlled trial. PLOS Med 2020;17.
- MacIsaac RL, Salatzki J, Higgins P, Walters MR, Padmanabhan S, Dominiczak AF, et al. Allopurinol and cardiovascular outcomes in adults with hypertension. Hypertension 2016;67:535-40.
- NIHR INCLUDE ethnicity guidance n.d. www.trialforge.org/trial-forge-centre/include/ (accessed 19 December 2022).
- NIHR INCLUDE guidance n.d. https://sites.google.com/nihr.ac.uk/include/ (accessed 19 December 2022).
Appendix 1 Members of ALL-HEART study group* and other contributors
ALL-HEART Trial Steering Committee
*Prof. Sir Lewis Ritchie (Chair), University of Aberdeen
*Prof. Isla Mackenzie (Chief Investigator), University of Dundee
*Prof. Thomas MacDonald, University of Dundee
*Mr Gordon Snedden (Patient/lay representative)
*Ms Sharon Ham (Patient/lay representative)
*Prof. Stuart Ralston, University of Edinburgh
*Prof. David Newby, University of Edinburgh
*Prof. Morris Brown, Queen Mary University of London
*Prof. Saad Shakir, Drug Safety Research Unit, Southampton
Former member:
*Mr Tom Brighton (Patient/lay representative)
ALL-HEART Independent Data Monitoring Committee
*Prof. Sir Mark Caulfield (Chair), Queen Mary University of London
*Prof. Christopher Weir, University of Edinburgh
*Dr Martin Denvir, University of Edinburgh
ALL-HEART Clinical Endpoint Adjudication Committee
*Prof. Jon Townend (Chair), University of Birmingham
*Dr Adnan Nadir, University Hospitals Birmingham NHS Foundation Trust
*Dr Jasper Trevelyan, Worcestershire Acute Hospitals NHS Trust
*Dr Sohail Khan, University Hospitals Birmingham NHS Foundation Trust
*Dr Kailash Krishnan, Nottingham University Hospitals NHS Trust
*Dr Don Sims, University Hospitals Birmingham NHS Foundation Trust
*Dr Marc Randall, Leeds Teaching Hospitals NHS Trust
Former members:
*Dr Helen Routledge, Worcestershire Acute Hospitals NHS Trust
*Dr Sagar Doshi, University Hospitals Birmingham NHS Foundation Trust
ALL-HEART Regional Principal Investigators
*Prof. Isla Mackenzie (Chief Investigator), University of Dundee [Tayside, Fife, Forth Valley; Dumfries and Galloway (formerly *Dr Husnat Ahmed)]
*Prof. Chris Hawkey, University of Nottingham (East Midlands, West Midlands, Eastern, North Thames)
*Prof. Michael Eddleston, University of Edinburgh (Lothian)
*Dr Jim Finlayson, NHS Highland (Highlands)
*Dr Richard Johnson, Saltcoats Group Practice (Ayrshire and Arran)
*Dr Colin Petrie, Monklands Hospital (Lanarkshire – Monklands Hospital)
*Dr Robin Weir, Hairmyres Hospital (Lanarkshire – Hairmyres Hospital)
*Dr Peter Arthur, Claughton Medical Centre (North West Coast)
*Prof. Jesse Dawson, University of Glasgow [Greater Glasgow and Clyde (formerly *Prof. David Preiss)]
*Dr Mary-Joan Macleod, University of Aberdeen [Grampian (formerly *Dr Jacqueline Furnace)]
*Prof. Terry McCormack, Whitby Group Practice (Yorkshire and Humber)
*Dr Raj Sharma, East Sussex Healthcare NHS Trust (Kent, Surrey and Sussex)
*Prof. Ahmet Fuat, Carmel Medical Practice (North East and North Cumbria)
*Dr Lawrence Barnes, Rame Group Practice [South West Peninsula (formerly *Dr Paul McEleny)]
Data management and biostatistics (Robertson Centre for Biostatistics, University of Glasgow)
Local PI: *Prof. Ian Ford
Statisticians: *Nicola Greenlaw, *Kirsty Wetherall, Michele Robertson
Software Developers: *Jane Aziz, *Robbie Wilson
Data managers: *Sarah Boyle
Medical Coders: Kirstin Sweeney, David Jamieson
Project managers: *Claire Kerr, Mairi Warren
Formerly Assistant Director of Information Systems: Sharon Kean
Other key contributors
Dundee (lead site):
Project managers: *Rebecca Barr, *Suzanne Duce, *Adam Wilson, *Susan Long, Wendy Saywood, Evelyn Findlay
Study physicians: *Amy Rogers, *Filippo Pigazzani, *Evelien Rooke, *Greg Guthrie, *Claudine Jennings, *JW Kerr Grieve, *Alexander Doney, Ronald MacWalter
Research nurses: *Bridget Shepherd, *Moira Dryburgh, *Anne Mackintosh, Caroline Hall, Dawn Ross, Lesley Riley, Fiona Gowans, Wendy Urquhart, Elizabeth Cowan, Caroline Paterson, Catherine Deas, Helen Waldie, Gwyneth Stewart, Ronnie Rebecca, Linda Crighton
IT/website: *Kris Zutis
Administrators: Caitlin McKay, Sara Edwards, Joanne Elwin, Dawn Thompson, Catriona Young, Carolyn Boyle, Shirley Fraser, Alison McGinnis, Vivian Taylor
Sponsor representatives (TASC, University of Dundee and NHS Tayside):
Governance: Patricia Burns, Graeme Boyle, Jacob George, Russell Petty
Monitoring: Marney Keiller, Louise Boldy, Anna Barnett, Louise Crowe, Tiffany Stewart, Emma Hutchison, Adele Maxwell, Mairi Wilson, Michelle Armstrong, Lorna Campbell, Caron Innes, Debbie Pankhurst, Susie Waugh
Audit: Valerie Godfrey
Contracts manager: Euan Banyard
Nottingham (East Midlands, West Midlands, Eastern, North Thames):
Project manager: *Jen Dumbleton
Research nurses: *Monique Morar, *Emma Isard
Dumfries and Galloway:
Assistant investigator: Dr Hossam Elmahy
Project manager: Janie Keggans
Kent, Surrey and Sussex:
Research facilitator: Charlotte Gosden
Lothian:
Research facilitators: Avril Cairns, Frances Dougherty
Greater Glasgow and Clyde:
Research facilitators: Linda Wilson, Fiona McIntosh, Rhona Mackay, Victoria Patterson
South West Peninsula:
Research facilitator: Tania Crabb
Grampian:
Research facilitator: Joan Henderson
Lanarkshire:
Research facilitators: Bernie Welsh, Tracy Baird, Maxine Fernon
Highlands:
Research facilitator: Avril Donaldson
North West Coast:
Research facilitators: Kate Maitland, Kay Aymer
Yorkshire and Humber:
Research facilitator: Michelle Platton, Kim Williams
Ayrshire and Arran:
Research facilitator: Margo Henry
Dumfries and Galloway:
Research facilitator: Janie Candlish
North East and North Cumbria:
Research facilitator: Nicola Coverdale, Helen Riding, Emma Murray, Sally Dunn
Recruiting nurses and doctors:
Bridget Shepherd, Moira Dryburgh, Ann Mackintosh, Angela Andrew, Vic Shepherd, Gwyneth Stewart, Frances Dougherty, Joan Henderson, Monique Morar, Emma Isard, Avril Cairns, Avril Donaldson, Michelle Platton, Margo Henry, Kate Maitland, Rhona McKay, Elizabeth Sabin, Carla Christie, Sarah Leonard, Victoria Paterson, Lesley Patience, Fiona Wright, Catherine Beveridge, Michelle Chalke, Howard Fairey, Ronald Rebecca, Laura Dunk, Mishelle Cunningham, Alison Vermeer, Kim Fell, Ruth Osborne, Jenny Johnson, Kate McCloskey, Shelina Rajan, Iain Swetman, Lynne Shoebottom, Caroline Mansfield, John Cvancara, Vasilica Munteanu, Amanda Ayers, Theresa Leighton, Amy Townrow, Joanna Toohey, Mark Pinkerton, Bridgid Joughin, Allison Daniels, Jagjit Takhar, Michelle Lowry-Bartram, Angela Helmstetter, Loraine Leggett, Katrina Young, Tracey Adcock, Elaine Butcher, Claire Talbot, Sarah Joshi, Eleanor Hoverd, Jonathan Davies, Pauline Darbyshire, William Cole, Sorela Mazilu-Wood, Susan Zhao, Sian Jones, Sandy Smith, Jo Bishop, Michelle Linsell, Lisa Palmer, Victoria Warmington, Angela Langrish-Smith, Somi Spannuth, Janet Wilson, Julie Timmins, Helen Jung, Siobhan Campbell, Anita Agasou, Carol Bartlett, Lucy Pepiatt, Lisa Westwood, Jacqui Vicars, Nicky Todd, Rachel Nixon, Jill Ducker, Clare Jones, Jo Bannister, Linda Crighton, Sharleen Blundell, Lesley Miller, Alice Mackie, Heather Warwick, Christine Howell, Pam Parry, Nicola Harding, Julie Robey, Sarah Bland, Tracy Baird, Claire Beith, Margaret Clarke, Louise Burgess, Joanna Beldon, Helen Wadeson, Elizabeth Butterworth, Alison Crumbie, Louise Chivers, Rachel Osbaldeston, Kirsti Withington, Elizabeth Weight, Lynn Siddle, Robert Seeley, Gail Timcke, Paula Melrose, Tina Goult, Brenda Deboys, Susan Lees, Joann Batty, Marie Roy, Helen Clough, Jackie Smith, Stephanie Moodie, Elizabeth Harmer, Lisa Manning, Kirsty Bignell, Kate Wallace, Pam Cashin, Sara Yearsley, Lisa Van-Kuyk, Michelle Orton, Preeti Pandya, Stella Oldham, Nicola Shea, David Seamark, Emma Lambon, Lucy Sadler, Janet Graham, Jane Stewart, Rachel Minty, Helen Patel, Dawn Clayton, Nick Cartmell, Joyce Pickering, Becky George, Carrie Gelhardt, Debra Chatterton, Jacquie Spencer, Dawn Young, Angela Sunne, Keith Elliott, Helen Permain, Melanie Robinson, Lucy Saunders, Mary-Jo Anson, Sara Jetten, David Ratcliffe, Geri Carson, Joanna Young, Kathleen Polverino, Joanne Turner, Jodie Rice, Hazel Hunt, Lindsey Dougall, Pat Edwards, Mark Pugsley, Lou Johnson, Claire Brown, Ina Burokiene, Julie Blundell, Denise Linscott, Janet Drinkwater, Suzie Cimelli, Julia Rooney, Vicky Honour, Deborah Cooper, Jane Howe, Helen Cox, Pam Godwin, Sandra Mononga
Participating general practitioners:
Thomas Dymock, Marc Jacobs, Alistair Acheson, Rebecca Wheater, Lesley Prentice, Yuk Chan, Neil Macintosh, Elaine Thomson, Theo Morrocco, Gillian Thomson, Helen Kirkwood, Robert Deuchar, Iain Craig, Michelle Duncan, Duncan Brown, Ailsa McEwen, Peter Fox, Andrew Thomson, David Herron, Paul Baughan, Gerald Burnett, Julie Anderson, Richard Humble, John Kennedy, Ronnie Ip, Nico Grunenberg, Carolyn Mackay, Colin Fraser, Joseph Trewavas, Rajesh Muvva, Alistair McCracken, David McElnea, Iain Taylor, Joanna Coy, Hilary Duffy, Lynne Christie, Joanna Wrate, Kenneth Thompson, Manasi Wheal, Phil Tipping, Yvonne Wedekind, Karen Scallan, Anjali Patil, Iain Thomson, Joyce Meikle, Kenneth Bell, Haval Jelal, Ruth Morris, Laura Holden, Derek Dunbar, Neil McLeod, Teresa Cannavina, Derek Dundas, Niamh Mullins, Fiona Mauritzen, John Marston, Keith Ferguson, Julie Reid, Alexis Dunsmore, Nick Haldane, Jonathan Dickson, Alan Thomson, Scott Jamieson, Julia Hamilton, David Ratcliffe, Andrew Brett, Shophia Kuganlipava, Mark Pugsley, Pippa Lally, Andre Beattie, Paul Vinson, Hugo Wilson, Helen Horsfall, Lai Fun Russell, Claire Pedder, Guy Dixon, Tom McMillan, Paul Stimpson, Ian Burns-Brown, Rachel Morrison, Neil Chalmers, David Chan, Mary Rouse, Steven Halliday, Aldon Kennedy, Christine Kirk, Kennneth O’Neill, Susan Langridge, Louise Sheil, Elaine Cameron, Caroline Oates, Amy Kerr, Hilary Melrose, Anne Reid, Andrew Wright, Michael McKenzie, William Graham, Luis Alguero, Pervez Ghaus, Jimmy Khouly, Richard Veale, Pamela Grills, Lisa Van Kuyk, Hayley Reed, Louisa Beringer, Nick Cartmell, Alastair Corfield, Ewart Jackson Voyzey, Ben Ward, Daniel Thomas, Nigel de Sousa, Kevin Douglas, Frances da Cunha, Tim Bray, David Watson, Jim Repper, Mary Craik, James Stewart, Victoria Jack, Lorna Hamilton, Sheila Smyth, Rosemary Tierney, Mobeen Ashraf, Helen Macleod, David Barr, Grant Leckie, Katharine Stenhouse, Bruce Taylor, Simon Wetherell, Patrick Haslam, Alison MacLeod, Bruce Helme, Kathryn Morgan, David Cowling, Nour Ghazal, Paul Hanlon, Mammen Ninan, Tanveer Ahmed, Fahad Yousaf, Julie Colclough, SJ Gardner, SJ Gardner, Michael Woodbridge, Christinne Hunt, William Lumb, James Cowdery, Umesh Chauhan, Umesh Chauhan, Peter Leftwick, Amar Ali, Helen Downs, Andrew Gallagher, Rahul Keith, Preeti Pandya, Fiona Laing, Irfan Zafar, Jonathan Mark Wimborne, David Lewis, Catja Schmitgen, Richard Stokell, Susan Jespersen, Ibrahim Hassan, Claire Vicary, Davinder Singh, Karen Forshaw, Kate Rowell, Mark Boon, Diane Shepherd, Ram Vasudevan, Nazish Ahmad, Stuart Saunders, Olaleye Oginni, Philip Williams, Catherine Liley, Howard Lewis, Helen Effingham, Theyvanai Dodd, Pippa Rutter, Andrew Perkins, Mark Richardson, Penny Blackwell, Bijoy Sinha, James Gray, Mark Howard, Nick Smith, Tim Parkin, Jane Coleman, Paul Cregor, Helen Lever, Jonathan Marsden, Upendra Bhatia, Penny Shields, David Hughes, Faiz Ismail, Herbel Pabla, James Threlfall, Azhar Farooqi, Mark Loveland, Helen Little, Shafiq Shafi, Rohit Sahdev, Satish Kumar, Jasvinder Dhaliwal, Paul Danaher, Clare Swaebe, Tej Mehta, Jacqueline Barney, Steph Short, David Tull, Diane Geatch, Aboo Thamby, Elise Maclachlan, Shora Montgomerie, Julian Hick, Asifa Akhtar, Saqib Bhatti, Christopher Trzcinski, Alan Lewis, Rakesh Patel, Leah Robinson, Janet Hall, Wendy Stammers, Sophia Shah, Andrew Jordan, Matthew Capehorn, Vikas Gupta, Moses Bandrapalli, Ken Brown, Humphrey Emery, Mark Rooney, Devi Srinivasan, Richard Oliver, Jon Dickson, Leslie Jeffers, Steve Jones, Imran Azizullah, Ritabrata Ray, Jaspal Taggar, Richard Park, Peter Bridgwood, Lisa Boruch, Parveen Ghatora, Subramanian Sivan, Imran Arshad, Azhra Zafar, Anthony Sinnott, Lisa Steiner, Ross Martin, Shahid Ansari, Shiraz Makda, Dalveer Samra, Karen Fearn, Mike Eaves, Sam Adcock, Subbannan Sukumar, Steven Lloyd, Debasish Kar, Yvonne Owen, Alistair Park, Khudija Irfan, Tom Bailey, Richard Benn, Catherine Ash, Anisa Malik, Lucy Cormack, Eleanor Farrands, Feisal Docrat, Deborah Curran, Helena Cobb, Mayur Lakhani, Victoria Hodges, Vipul Masharani, Tom Holdsworth, Robert Thornton, William Francis, Fiona Buckley, Gail Sanderson, Duncan Rogers, Gary Lees, Kanan Pande, Scot Richardson, Frances Adams, Michael Brookes, Charu Tangri, Rikin Patel, Gary Solomons, Tim Rundell, Monique Aurora, Chas Thenuwara, Khalid Mirza, Babafemi Salako, Rob Howlett, Alex Carefull, Varun Potluri, Shah Chowdhury, Alison Dow, Stephen Thurston, Philip Pinney, Kevin Brinkhurst, Paulo Fargnoli, Basia Uszycka, Mohammed Subhi, Theresa Leighton, Angela Hallatt, Jehad Aldegather, Carsten Dernedde, Helena Simper, Richard West, Tamer Okasha, James Atkins, Judith Somers Heslam, Lindsey Crockett, Emily Foster, Brian Ainsworth, Vivien St Joseph, Chaminda Dooldeniya, Tara Laidler, Walter Tobias, Martin Hadley-Brown, Frances Scouller, John Tweedale, Chris Schramm, Jovenus Mosakamowo, Costas Paschalides, Jagit Takhar, Katrina Young, Jo Farnell, Laurence Kemp, Neil Modha, Sil Tan, Farah Paruk, Arul Nambi, Peter Robinson, Sam Howell, Ike Nnene, Vanaja Santosh, Gordon McAnsh, Simon Lomax, Victoria Warmington, Salah Estifanos, Angela Langrish-Smith, Satish Singh, Hywel Jones, Johanna Fitzgerald, Tajammal Mirza, Andrew Emerson, Christopher Pearce, Will Elson, Peter Spofforth, Ian Smith, Kevin Elsby, Gary Taylor, Sid Sidhu, Samir Pai, Joanna Skinner, Sharon Woods, Ayotunde Ajala, Martin Aylward, Nicola Shea, Elizabeth Christie, Corinne Bakker, Heather Griffin, Crispin Dunne, Elizabeth Albright, Faith Airey, David Hallam, Claire Jones, Richard Edwards, Naresh Aggarwal, Nasrullah Cheema, Mohammed Huda, Hergeven Dosanjh, Shahid Merali, Mark Lindsey, Suparna Behura, Andrew Godfrey, Naresh Chauhan, Ulka Choudhary, Cristina Ramos, David Buckley, Loy Yap, Dhanumjayarao Chunduri, Adriana Zilveti, Crispin Fisher, Nancy Yadava, Rajendra Yadava, Andrew Wallis, Harinder Grewal, Abbas Walji, Grania O’Mahony, Sharon Turner, Nerinda Narhlya, John Ward, Tim Marshall, Asad Sabir, F Lockhat, Mark Stone, Sri Sundaram, Susan Price, Clare Pilkinton, Trisha Wildbore, Rajul Patodi, Norman Ullah, Richard Dales, Richard Woof, David Shukla, Vattakkatt Premchand, Emma Spiller, Peter Gregory, Sonal Nicum, Imran Zaman, Touseef Safdar, Catherine Faarup, Kulyadi Pai, Kim Knowles, Anil Joshi, W Htike, Paul de Cates, Iqbal More, Kate Day, Bhaskar Appanna, Wendy Cooper, Toby Helliwell, Caron Bates, Gareth Rowland, Raj Kanwar, Gemma Moore, Helen Tyrell, Matt Wilson, Alison Shearman, Bhupinder Rai, Linda Thompson, Marie Imlach, Grace Watts, Joanna Bell, Clive Loraine, Olagoke Aiyegbayo, Sally Bastiman, V Kumar, Saul Miller, Julia Cook, Mary Philipsz, Catherine Bromham, Justine Norman, Farzan Kamali, Richard Holdsworth, Tom Schatzberger, Enda Chadwick, Ann Rajan, Edward Russell-Smith, Amanda Pal, Philip Rathbone, Kam Singh, Stephen Clay.
Other supporting staff
Susan Willis, Andrea Brown, Andrew Perugia, Rebecca Bale, Robert Hirst, Yvonne McIlvenna, Leah Chick, Mike Cox, Clive Sillitoe, Laura Renwick, Claire Graham, Lisa Atkinson, Tricia Holloway, Lorraine Underwood, Cate Atkins, Sara Eddy, Lauren Merrin, Kevin Windsor, Kate Dodd, Carol Stanton, Diane Stevenson, Helen Yoward, Wendy Godfrey, Katie Hill, Ruth James-Morse, Sarah Galbraith-Gibbons, Clare Seamark, Sue Blake, Sarah Goodall, Anne Wood, Nina Joint, Stacy Wilson, Jayne Ashton, Lucinda Ralph, Amanda Cardy, Maria Coward, Caroline Cooke, Julie Birch, Barbara Jukes, Karen Bates, Bev Browitt, Nula Smart, Nicola Vardy, Christine Smith, Jackie Frater, Mary Start, Katie Budd, Robert Whitehead, Paula Davies, Michelle Farmer, Catherine Watkins, Jennie Wilson, Louise Green, Phillipa Sheilds, Gillian Stitson, Teresa Buglass, Kirsty Owen, Jill Palmer, Jenny Day, Karen Harmer, Louise Armstrong, Claire Thompson, Melanie Piggot, Jessica Payne, Lucy King, India Mills, Marie Whittington, Catherine Engall, Jodie Button, Samantha Jones, Roberta Butter, Denise Steward, Jennet Ashton, Carole Bacon, Michelle Linsell, Eric Salisbury, Nicky Currie, Dawn Boyce, Jan Wright, Kate Bywater, Emma Edwards, Louise Cooper, Kate Sloper, David Adams, Sue Turner, Kayleigh Dorey, Lauren Hoskin, Jessica Bane, Miranda Hoad, Aimee Longfoot, Lisa Coleman, Kirsty Burton, Paula West, Liesl Crowe-Broekhuijsen, Elisabeth Gaunt, Becky Dilley, Lan Jenner, Yvonne Tul, Jessica Cox, Kay Scott, Maya Leach, Jill Barlow, David Harris, Laura Howe, Rob Forder, Susan Longfoot, Yvonne McIlvenna, Lauren Clarke, Alison Talbot, Andrea Isaew, Andy Gibson, Angela Atkinson, Angela Dunne, Ann Selby, Ann Grain, Ann Whitaker, Anne Hitchens, Ashley Dawson, Barbara Robinson, Bob Seeley, Carla Bratten, Carol Keel, Catherine Allan, Chrissie Starsmeare, Christine Wright, Claire Cox, Clare Mestanza, Crystal Chetwood, Dale Carter, David Nash, Dawn Clayton, Debbie Kelly, Debbie D’Cruz, Denise Jarvis, Denise Williams, Diane Hammond, Donna Watson, Elaine Nickson, Eleanor Miller, Elizabeth Harmer, Elizabeth Habershon, Emma Harrison, Fenglin Guo, Gemma Palfreman, Glennis Lowdon, Gurdip Heer, Hazel Mole, Helen Permain, Jackie Gardiner, Jacqueline Alland, Jacqueline Mburu, Jane Hall, Jane Macdonald, Janet Field, Janice Irvine, Jennifer Titley, Joann Barker, Joanna Morrison, Julie Mason, Julie Friend, Kamil Klosowicz, Karen Kilby, Karen Norcot, Karen Parish, Katrina Snelling, Kay Holden, Kim Burke, Linda Field, Lisa Brightmore, Lisa Gibbons, Lisa Zeidan, Lucy Pointin, Lynda Melvin, Marie Crook, Merilyn Green, Michelle Line, Nicola Walker, Nikki King, Penny Lavis, Peter Bradburn, Rachel Lister, Rachel Walker, Rajvinder Gill, Rebecca Harrison, Ruth Holden, Saif Uddin, Sally Gordon, Samantha Hunt, Sandeep Virdee, Sara McNamara, Sara Stearn, Sarah Browne, Sarah Fisher, Sarah Manby, Sarah Wytrykowski, Sharon Johnston, Shirley Boden, Sian Clifton, Sisi Oo, Susan Rawson, Susan Read, Susan Wilkinson, Vicky Taylor, Viji George, Wendy Dwornik.
Appendix 2 Comparisons of EQ-5D-5L data availability at the end of the study by selected baseline characteristics in each arm of the study
List of abbreviations
- ACS
- acute coronary syndrome
- CI
- confidence interval
- CV
- cardiovascular
- eCRF
- electronic case report form
- eGFR
- estimated glomerular filtration rate
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- GP
- general practitioner
- HF
- heart failure
- HR
- hazard ratio
- ICER
- incremental cost-effectiveness ratio
- IDMC
- independent data monitoring committee
- IHD
- ischaemic heart disease
- IPCW
- inverse probability of censoring weighting
- IQR
- interquartile range
- MI
- myocardial infarction
- mITT
- modified intention to treat
- NIHR
- National Institute for Health and Care Research
- NYHA
- New York Heart Association
- PROBE
- prospective, randomised, open-label, blinded endpoint
- QALY
- quality-adjusted life-year
- SAE
- serious adverse event
- SD
- standard deviation
- SUA
- serum uric acid
- TSC
- Trial Steering Committee
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/ATTM4092).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
