Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR132803. The contractual start date was in September 2021. The draft manuscript began editorial review in May 2023 and was accepted for publication in November 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Mistry et al. This work was produced by Mistry et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Mistry et al.
Chapter 1 Introduction
Description of health problem
Migraine is the second most common disabling disorder in the world1 and is the leading cause of years lived with disability in those aged 15–49. 2 In the UK, migraine affects 15% of adults. Women are three times more likely as men to have migraine. 3 It is also more common in young adults (late teens to 50s) with work and family commitments. 4 As such, migraine has a huge economic and social impact, costing the UK over £1.5 billion per year due to absence from work or school,4 and has substantial impacts in professional and social settings. 5 Our patient-partners describe it as a condition that ‘redefines, and can destroy, work and family life’.
Migraine is categorised into episodic and chronic migraine. Episodic migraine is diagnosed in people with migraine who have less than 15 headache days a month. 6 The definition of chronic migraine has changed over time. The Third International Classification of Headache Disorders (ICHD-3) defines chronic migraine as headaches on 15 days or more a month, for more than 3 months with features of migraine on at least 8 of those days. 6 The main focus of this report is on chronic migraine.
In 2011 the World Health Organization (WHO) called for action to address the ‘worldwide neglect’ of headache disorders. 7 Yet migraine remains a leading cause of global disease burden. 2,8,9 Around 2–4% of the world’s population meet an epidemiological definition of chronic headache. 10,11 In a 2022 trial of supportive self-management for those living with chronic headache, 99% (727/736) of those assessed for inclusion had migraine. 12 This group has the potential to benefit from effective prophylactic drugs to prevent migraine attacks. A 2017 meta-ethnography of the lived experience of people with chronic headache (four studies) identified that chronic migraine had a profound effect on people’s lives, similar to other pain conditions. Key themes identified in the findings of the meta-ethnography included the loss of control over one’s life, strained relationships and social exclusion due to chronic headache. 13 The burden on family, and the care burden for those living with a person with migraine, increases with headache frequency. 14
An evidence synthesis and an economic model on prophylactic treatments for chronic migraine is, therefore, needed to address this evidence gap and to generate recommendations.
Current treatments and existing evidence
The current state of the evidence for migraine prevention is poor, making it difficult for patients and clinicians to make decisions about which medications to consider. Various pharmacological treatments are available for the prevention of migraine. Oral medications are taken regularly (usually daily), regardless of whether a patient has a migraine at that point in time, with the aim of trying to reduce the frequency and severity of migraine attacks. For oral medications, the current evidence base for chronic migraine comes almost exclusively from data extrapolated from trials on episodic migraine. Evidence regarding the cost-effectiveness of different pharmacological treatments is also lacking.
Prophylactic medications used to treat chronic migraine include topiramate, propranolol, tricyclic antidepressants, candesartan and valproate. Topiramate and propranolol are recommended by NICE and SIGN. The evidence contained in these guidelines is of mixed quality. 15,16 Weaker evidence supports the use of amitriptyline [recommended by National Institute for Health and Care Excellence (NICE) and Scottish Intercollegiate Guidelines Network (SIGN)], and for candesartan and valproate (recommended by SIGN, but not by NICE). The NICE recommendation for amitriptyline is based on evidence comparing amitriptyline with topiramate, but not with placebo. There remains uncertainty about the effectiveness of amitriptyline as a prophylactic treatment. 17 Other prophylactic medications to treat chronic migraine in the UK include botulinum toxin type A (BTA), serotonin noradrenaline reuptake inhibitors (SNRIs) antidepressants, angiotensin-converting enzyme (ACE) inhibitors, other angiotensin receptor blockers, other beta-blockers, calcium channel blockers and pizotifen.
The most recent evidence on this topic was produced by Jackson et al. in 2015. 18 They pooled evidence from numerous randomised controlled trials (RCTs) on oral prophylactic medications for both chronic migraine and episodic migraine to explore potential differences for continuous and dichotomous outcomes. Their systematic review identified 13 trials of oral medications (n = 903, range 7–306, mean 69) which included people with chronic migraine. 19–31 Jackson et al. 18 concluded that ‘these comparisons have been somewhat haphazard, and many important potential comparisons have not been made’. The authors of a 2023 overview of systematic reviews on the use of antidepressants for pain excluded this review because of concerns about trial selection and data analysis. 32 This 2015 review needs to be updated using methods which are able to synthesise the overall evidence for a broad range of prophylactic medications for use in people with chronic migraine, for example, using a network meta-analysis (NMA). 18 NMA extends beyond the traditional pairwise meta-analysis comparison to multiple interventions and provides a more precise estimate of a treatment effect size by combining both direct (RCT of A vs. B, or B vs. C) and indirect (A vs. C compared indirectly via the common comparator B) evidence. A NMA also allows estimation of treatment rankings which can assist policymakers, clinicians and patients to select the best treatment options. 33
There has been an increase in the availability of the calcitonin gene-related peptide (CGRP) monoclonal antibodies (MAbs), usually given as monthly injections, such as erenumab, fremanezumab and galcanezumab. 34–37 These treatment options are more expensive than oral prophylactic medications. NICE recommend BTA or a CGRP MAb after patients have failed three oral medications. 38–42
A 2020 study by Forbes et al. 43 compared CGRP MAbs with placebo in the seven chronic migraine RCTs (n = 5292) and found that the additional pooled reduction in monthly migraine days (MMDs) from CGRP treatment was 2.24 days [95% confidence interval (CI) 1.82 to 2.65]. They further estimated that 68% of the apparent reduction in headache days in the intervention groups was due to contextual effects. In other words, participants in the control group would expect an average reduction in monthly headache days (MHDs) of four and half days, with the intervention group gaining an additional reduction of two and a quarter days, which is six and three-quarter days in total. Based on these data it is difficult to judge clinically if the treatment has been effective for this trial population as a whole and it is unclear how the effect sizes for CGRP MAbs compare with the effect size of more established oral medications or BTA injections.
Overall, the evidence of oral pharmacological treatments for adults with chronic migraine is of poor quality and extrapolated almost exclusively from trials on episodic migraine. As mentioned above, in the 2020 review on CGRP MAbs only 7 of the 21 included trials were on chronic migraine. 43 We cannot assume that medications shown to reduce the number of headache days in people with episodic migraine will have a positive effect on the long-term disability caused by chronic migraine. Therefore, this report aims to provide an up-to-date overview of the relative benefits, harms and costs of prophylactic medications to treat chronic migraine. Without this review, the only evidence available to decision-makers and guideline producers will be for expensive CGRP MAbs, which have a modest additional effect size compared to placebo. 44,45 For example, the use of erenumab 70 and 140 mg only reduced the number of MMDs by 2.46 and 2.45, respectively, compared with placebo. 45
Economic implications and current costs
As migraine is a leading cause of global disease burden,1 the costs associated with migraine for healthcare services and to patients and their families are significant. A 2019 review on the costs of migraine found that the direct and indirect healthcare costs of chronic migraine are 3–4 times as high as episodic migraine. 46 For example, in the USA, the total cost for episodic migraine was $2649/year and the total cost for chronic migraine was $8243/year;47 and, in Europe the direct costs for episodic migraine was €746/year and for chronic migraine this was €2427/year. 48 This cost may be partly due to the nature of the disease itself, as people with chronic and episodic migraine combined miss, on average, 10.2 work-equivalent days per year (absent on 4.4 days and reduced productivity on 11.4 days) due to headache-related disability. 46 Higher work-related difficulties are associated with chronic migraine versus episodic migraine (lost work days: 3–4 days vs. 1 day, respectively). 49 The burden on family, and the care burden for those living with a person with migraine, increases with headache frequency. 14
There are increasing pressures on the NHS to provide the newer, and more expensive, treatments when oral prophylactic medications have failed. 34–37,50 The British National Formulary (BNF) price per patient (excluding administration costs) as of December 2022 for a typical 3-month course of the CGRP MAbs – erenumab, fremanezumab and galcanezumab – are £1160, £1350 and £1800 respectively,51 whereas a BTA injection vial for a 12-week cycle costs £276.40 and the oral medications amitriptyline, candesartan, propranolol and topiramate cost on average per patient, £2.44–3.72, £4.28–6.28, £11.74–11.76 and £3.42–11.64 for 3-month treatment, respectively. 51 It is important for both patients and healthcare professionals to know the comparative effectiveness and cost-effectiveness of these older oral medications and the newer injectable treatments.
Decision problem
The commissioning brief provided the topic context:
SIGN guidance states that the global prevalence of migraine is approximately one in seven. The Global Burden of Disease study found migraine to be third in terms of the most common cause of worldwide disability in the under 50s. They estimate that migraines cost the UK around £3 billion per year in terms of healthcare, loss of productivity and disability. 9 Chronic migraine is defined (by NICE/SIGN) as headaches that occur 15 or more days per month, of which 8 or more are migraines (with or without aura) for more than 3 months.
This report presents the first evidence to compare the clinical and cost-effectiveness of prophylactic medications to treat chronic migraine for adult patients. The findings of this report will help to inform decisions made by policy-makers, clinicians and patients on the most appropriate course of drug treatment(s) for adult patients who suffer from chronic migraine.
Our aim was:
-
To review and compare the clinical and cost-effectiveness of drug treatments for adults with chronic migraine.
To fulfil the study aim, five research questions were identified which align to each of the report chapters:
-
What is the clinical effectiveness of prophylactic drugs for chronic migraine? (see Chapter 2)
-
What are the comparative incidences of adverse events (AEs) of prophylactic drugs used for migraine? (see Chapter 3)
-
What is known about the cost-effectiveness of prophylactic drugs for chronic migraine? (see Chapter 4)
-
Which prophylactic drugs for the management of chronic migraine are the most cost-effective? (see Chapter 5)
-
Based on our findings, what should the research recommendations be? (see Chapter 6)
Study population, intervention, comparators, outcomes (PICOs) and inclusion and exclusion criteria for the sub-questions are presented in each subsequent chapter.
Chapter 2 Clinical effectiveness review and network meta-analysis
Research question 1: What is the clinical effectiveness of prophylactic drugs for chronic migraine?
Introduction
This chapter presents a systematic review of published RCTs of pharmacological drug treatments for adult patients with chronic migraine. Findings from this systematic review will inform an overall synthesis of the effect of prophylactic medications for chronic migraine using a NMA.
Methods
The clinical effectiveness review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting systematic reviews52 and the Cochrane Handbook for Systematic Reviews of Interventions. 53 The protocol for the clinical effectiveness review has been registered in the PROSPERO (international prospective register of systematic reviews) database a priori. The registration number is CRD42021265990.
Search strategy
The search strategy for the clinical effectiveness review (see Chapter 2) and the AEs review (see Chapter 3) was constructed by an information specialist (AB), in consultation with the project team. The search strategy was initially constructed in MEDLINE, using both free text keywords and thesaurus (MeSH) terms for migraine/headache and the prophylactic drug interventions of interest, with the addition of a search filter for RCTs. No date or language limits were applied. The MEDLINE strategy was checked by another information specialist (not involved in the project) for any omissions or errors in spelling, search syntax, structure and use of MeSH, before being translated for the other bibliographic databases. Full search strategies can be found in Appendix 1, Table 22.
The following databases and clinical trials registers were searched between 8 and 15 September 2021:
-
MEDLINE All, 1946 to 7 September 2021 (via Ovid);
-
EMBASE Classic + EMBASE, 1947 to 7 September 2021 (via Ovid);
-
Cochrane Central Register of Controlled Trials (CENTRAL), Issue 9 of 12, September 2021 (via Cochrane Library);
-
Science Citation Index Expanded, 1970 to present (via Web of Science);
-
Global Index Medicus (all regional indexes, via WHO website);
-
ClinicalTrials.gov;
-
International Clinical Trials Registry Platform (ICTRP) (via WHO).
Records retrieved by the database and the trials register searches were exported into EndNote X9, to enable identification and systematic removal of duplicates. 54
An additional pragmatic search in MEDLINE, EMBASE and the Cochrane Database of Systematic Reviews was performed to identify recent systematic reviews of prophylactic migraine treatments. The reference lists of the outputs of this search, and those also of the NICE, SIGN and American Headache Society guidelines, were checked for relevant literature. Authors of key studies were contacted and asked for details of any articles (e.g. reports, papers published or unpublished) that may not have been captured in our search. We performed forward and backward citation tracking from all included papers using Web of Science Core Collection (and Google Scholar where papers were not available in Web of Science).
Following consultation with the study team clinical experts, we conducted a further round of searches for three medicines not previously included in the search strategies: riboflavin, magnesium and CoQ-10. Our clinical experts suggested that these medicines are currently used within the UK. This supplemental search followed the same process as initial searches and was conducted in February 2022. Searches for all the prophylactic drug interventions of interest were updated in November 2022 to identify any additional publications that had become available. Searches to check for any retractions, errata or similar, relating to included studies, were also undertaken at this time. Full details of all searches are provided in Appendix 1.
Assessing relevance and inclusion of studies
The first round of screening was based on title and abstract and was conducted by two reviewers (AB, SN). The second phase of screening was performed according to PICO criteria (see Box 1 for inclusion criteria and Box 2 for exclusion criteria). At this stage, the abstracts of the retrieved studies were reviewed independently by two out of four reviewers (MU, SN, AA, ND). The full texts of the remaining studies were retrieved, and the same combination of the reviewers conducted an additional round of full-text screening according to the pre-specified inclusion/exclusion criteria. For both the clinical effectiveness review (see Chapter 2) and AEs review (see Chapter 3), the screening process was the same.
-
RCTs in any setting.
-
RCTs with more than 100 participants per arm. (We excluded studies with fewer than 100 participants per arm, in each pairwise comparison, to avoid risk of low-quality studies contributing disproportionally to our overall conclusions.)
-
Adults (≥ 18 years old) with chronic migraine.
-
Available or anticipated to be available pharmacological medications in the UK: CGRP MAbs, BTA, antidepressants, ACE inhibitors and angiotensin receptor blockers, beta-blockers, calcium channel blockers, pizotifen, flunarizine and anti-convulsants (topiramate, valproate/divalproex, gabapentin).
-
Placebo, or
-
Usual care, or
-
Other prophylactic drugs.
-
Headache days.
-
Migraine days.
-
Headache-related quality of life.
-
Migraine-specified quality of life.
-
Headache intensity and duration.
-
Health service activity.
-
Days lost from usual activities.
-
Any other reported outcomes.
-
Non-randomised trials, quasi-randomised trials, observational studies (e.g. case reports and case series), subgroup analysis and other designs.
-
RCTs with fewer than 100 per arm.
-
Children and young people aged < 18 years.
-
Participants with menstrual migraine, acute migraine, abdominal migraine, vestibular migraine or any other conditions-related migraine.
-
Trials that examined participants with other primary headaches including tension-type headaches, cluster headaches and secondary headaches.
-
Studies comparing cognitive–behavioural therapy, psychological interventions, exercise, dietary and relaxation.
-
Studies which were dose–response trials.
-
Studies comparing different preparations of the same drug in the absence of placebo.
-
Laboratory studies without clinical outcomes.
-
Chinese traditional medicines, that is, herbal medicine/drugs and other herbal remedies which are not prescribed in the UK.
-
Drugs which are not prescribed by NHS or recommended by NICE or Scottish Medicines Consortium (SMC).
-
Non-human outcomes.
-
Outcomes with insufficient information.
We excluded studies with fewer than 100 participants per arm to ensure that we included better-quality studies and to avoid loss of precision on our NMA by including heterogenous studies. 55,56 Studies with fewer than 200 participants will not have been adequately powered to show a standardised mean difference of less than 0.5. Smaller studies are also typically older and do not use an adequate definition of chronic migraine and are of poor quality.
Data extraction for systematic review and network meta-analysis
Data for included studies were extracted by one reviewer (SN) and 20% were randomly checked for accuracy by another reviewer (SK). Data extraction forms were developed in Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) to capture the following information: ClinicalTrials.gov identifier (National Clinical Trial number), study name, study characteristics, patient demographics including baseline characteristics, intervention and comparator details, outcome(s) of interest with relevant data and measure of variability, time point of outcome measurements, duration of treatments, AEs and serious adverse events (SAEs).
Means and standard deviations (SDs) for continuous outcomes were extracted. If SDs were not provided, we calculated them from standard errors, CIs or other measures. 57 We also contacted authors by e-mail to ask for the original data in the event of any missing data.
Assessment of risk of bias for included trials
The Cochrane risk-of-bias (RoB 2) tool for randomised trials58 was applied for assessing the risk of bias of all trials independently by two members (SN, SK). The tool was used to determine whether there was high, some, or low risk of bias in the following domains: (1) arising from the randomisation process, (2) due to deviations from the intended interventions (effect of assignment to intervention), (3) missing outcome data, (4) measurement of the outcome and (5) selection of the reported result. In this approach, the rating low risk of bias ‘is judged to be at “low risk of bias” for all domains’, and the trial ‘is judged to raise “some concerns” in at least one domain for this result, but not to be at “high risk of bias” for other domains, whereas the trial ‘is judged to be at “high risk of bias” in at least one domain’ or the trial ‘is judged to have “some concerns” for multiple domains in a way that substantially lowers the confidence in the result’. 58
Assessment of certainty in evidence for included trials
We assessed the degree of certainty of evidence, all comparisons for each outcome, by using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework independently by two members (SN, SK). 59 Any discrepancies in any of the screening steps were referred to the third reviewer (MU). In GRADE, RCTs were considered as a high quality of evidence (where authors have a lot of confidence that the true effect is similar to the estimated effect) to very low-quality evidence (where authors believe that the true effect is probably markedly different from the estimated effect). There are five domains for rating down GRADE including: study limitations (risk of bias), imprecision, inconsistency, indirectness and publication bias. There are three domains for rating up GRADE including: large magnitude of effect, dose–response gradient, and all plausible confounding.
Outcomes of interest
-
Monthly headache days: As reported in the original papers.
-
Monthly migraine days: As reported in the original papers.
-
Migraine-specific quality of life (MSQ): The MSQ version 2.1 is a 14-item questionnaire that measures a patient’s quality of life over the last 4 weeks across three domains: migraine-specific quality of life-restrictive role function (MSQ-RR), seven items that assess the functional impact of migraine through limitations on a patient’s daily work and social activities; migraine-specific quality of life-preventive role function (MSQ-PR), four items that measure the impact of migraine through prevention of daily work and social activities; and migraine-specific quality of life-emotional function (MSQ-EF), three items that evaluate the emotional impact on migraine. The score ranges from 0 to 100, with a higher score indicating better quality of life. 60
-
The headache impact test-6 (HIT-6): The HIT-6 consists of six items: pain, social functioning, role functioning, vitality, cognitive functioning and psychological distress. There are five responses to each of the six items: ‘never’, ‘rarely’, ‘sometimes’, ‘very often’ or ‘always’. These responses are summed together to produce a total score for the HIT-6. A lower score (49 or less) is categorised as having little or no impact and a higher score (60–78) is categorised as having a severe impact. 61
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L): The EQ-5D-5L descriptive system is a preference-based health-related quality of life (HRQoL) measure with five dimensions that include mobility, self-care, usual activities, pain/discomfort and anxiety/depression, with each dimension assessed at five levels: from no to extreme problems. 62
-
Migraine Disability Assessment (MIDAS): The instrument was developed to assess headache-related disability for migraine patients over a 3-month recall period. 63 The questionnaire contains five questions regarding the number of days of missed work/school, reduced productivity at work/school, missed household work, reduced productivity in household work, and missed family and/or social activities. The MIDAS score is calculated by summing the five items. Higher scores depict increased disability due to headache. The total MIDAS score can be categorised according to disability: 0–5, minimal or infrequent disability; 6–10, mild or infrequent disability; 11–20, moderate disability; and 21+, severe disability. 63
-
Work Productivity and Activity Impairment – Specific Health Problem (WPAI:SHP). The WPAI:SHP questionnaire measures the effect of different health conditions on work productivity, generating scores for absenteeism, presenteeism, absenteeism plus presenteeism, and activity impairment outside work. 64 WPAI:SHP is a version of Work Productivity and Activity Impairment that can be modified for use with a specific disease, such as migraine. 64
-
Patient Health Questionnaire 9-item (PHQ-9): The PHQ-9 is a self-administered instrument for screening, diagnosing, monitoring and measuring the severity of depression over the last 2 weeks. Each question is scored from ‘0’ (not at all) to ‘3’ (nearly every day). The total score for PHQ-9 is obtained by summing the score for each question. 65
-
Functional Impact of Migraine Questionnaire (FIMQ): The FIMQ is a 20-item questionnaire that measures patient-relevant impacts of migraine in the past 7 days across three domains: activity impairment (14 items), emotional functioning (3 items) and cognitive functioning (3 items). Individual items are then transformed to a 0–100 scale, with higher scores indicating a greater level of migraine impact. 66
Selection of data and synthesising data for the network meta-analysis
Network meta-analysis is a methodology that simultaneously combines three or more interventions in a single analysis using data from several studies. Results for each pairwise comparison combine both the direct evidence in primary studies and the indirect evidence, which has not been made directly within the studies to form a network. 67 In other words, a NMA provides an estimation of treatment effect size for each pair of interventions, regardless of whether they have been compared directly in a RCT or not. 67 In addition to facilitating comparisons between interventions, NMA provides a ranking of the interventions based on their effectiveness. This can help decision-makers choose the most suitable and effective treatments. 68
The stepwise feasibility framework was used to ensure that the underlying assumptions are systematically explored, and also to ensure that pooling and comparing the treatment effects for a particular research question are transparent. 69 It comprises of four steps to illustrate the heterogeneity and differences within or between direct treatment comparisons in terms of treatment and outcome characteristics, the study and patient characteristics, baseline risk and observed treatment effects. 69
We conducted a NMA for those outcomes which were provided data for more than three interventions to obtain the treatment effect size for the clinical effectiveness review.
Two different NMA models were conducted:
-
Fixed-effects model – this model assumes that all studies included in a NMA are estimating a single true underlying effect. However, if there is significant variation in the effect sizes, known as statistical heterogeneity, then the fixed-effects model may not be appropriate.
-
Random-effects model – this model assumes that the estimated treatment effects observed across studies can differ due to both actual differences in the treatment effect in each study, as well as differences in sampling. 70
We chose between the different NMA models by using the posterior mean deviance as an indicator of model fit and the deviance information criterion (DIC). DIC is a metric used to assess the goodness of fit of a statistical model while also taking into account its complexity. It penalises models for their complexity and therefore favours simpler models over more complex ones. 71 DIC differences of three or more are considered meaningful between models. 72 When both model results were similar, we chose the results from the most parsimonious model.
Network plots were created for each analysed outcome. The node sizes of the network plots are proportional to the number of participants randomised to each of the interventions, whereas the thickness of the edges (lines) is proportional to the number of participants contributing to that comparison. 73 Stata SE17 was used to generate the forest plots for each intervention’s comparison with placebo as the reference treatment. 74 The comparisons of all interventions were interpreted using leagues tables showing all pairwise comparisons with associated 95% credible intervals (CrIs).
In our review, all outcomes are presented in continuous format (change from baseline). The calculated point estimates were mean differences (MDs) with their associated 95% CrIs. We considered follow-up periods of 12 and 16 weeks as a measurement time point for all outcomes, because most of the interventions in the included trials had reported outcomes at week 12 or 16. The only exception to this was the data for BTA as most of their outcomes were reported at week 24. Hence, data for BTA are evaluated in a longer time frame of 6 months. Where outcome data were presented for multiple time points, we took the data closest to 3 months follow-up as the main time point.
We excluded studies that had insufficient information about the mean change from baseline (SD) for each outcome per arm [e.g. in the absence of the mean change from baseline, we calculated it by subtracting the post-treatment value from the baseline. However, we were not able to produce the related SDs for those mean change because calculating the SDs requires some more data (e.g. 95% CI, or at least p-value according to the Cochrane Library guidance)]. We used a fixed-effects approach to the meta-analyses. 75 Statistical heterogeneity was quantified using the between-study SD and Tau2 or I2-statistic. The between-study SD gives a direct measure of variance in the treatment effect across studies,76,77 while the I2-statistic is used to quantify the percentage of variation in effect estimates across studies that is due to heterogeneity rather than chance. In other words, it measures the proportion of variance across studies that can be attributed to differences in population characteristics. 78,79
The statistical analyses were conducted within a Bayesian framework using multinma package80 in R software version 4.1.3. 81 We estimated the posterior densities using Markov Chain Monte Carlo (MCMC) simulations; there were four Markov chains with 4000 iterations for each chain. All baseline and intervention effect parameters were given flat (uninformative) normal (0, 1000) priors and the between-study SD flat uniform distributions with an appropriately large range given the scale of measurement. The generalised linear model settings for continuous was a normal link. 71 We assessed the convergence of the Markov chains by using the potential scale reduction factor and examining the history and autocorrelation plots for each estimated parameter. 82
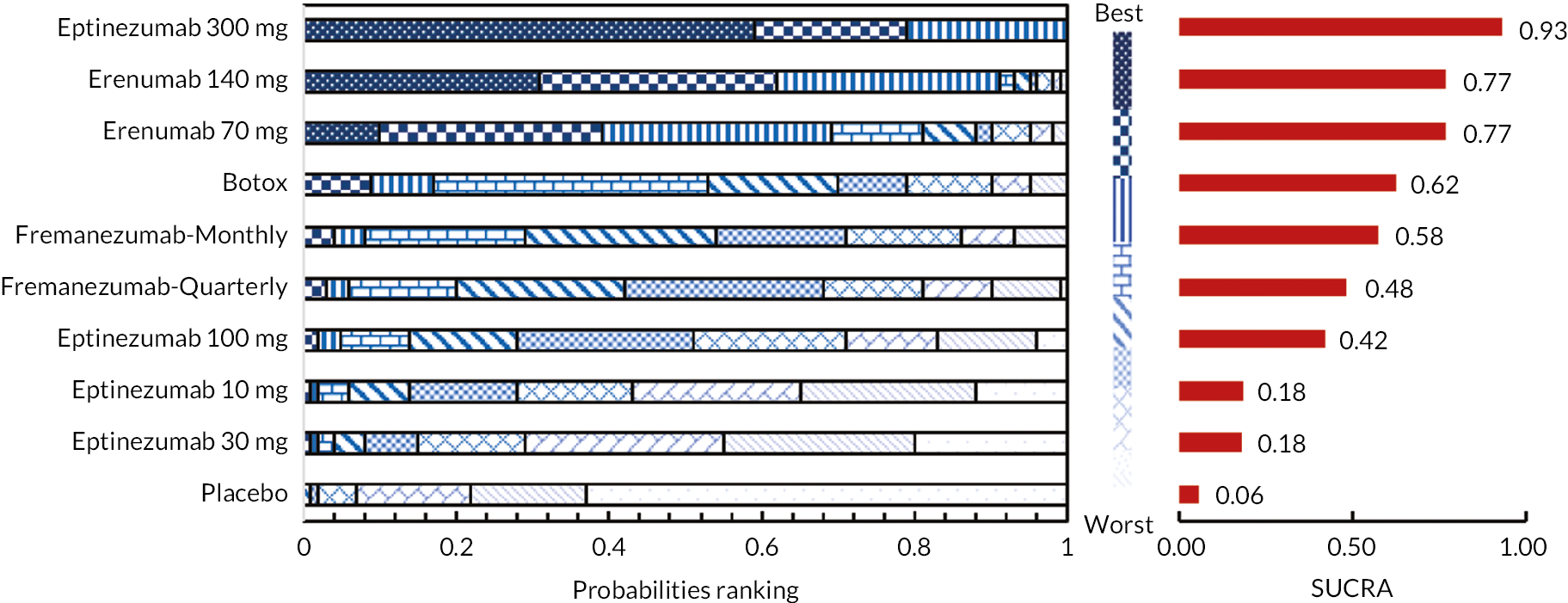
Intervention ranking
To rank the interventions, we calculated the probability of each intervention being the best, second best, and so on. In addition, we used the Surface Under the Cumulative Ranking Area (SUCRA) values (ranging from 0 to 1) to summarise the probabilities of treatment ranking. A higher SUCRA value indicates a greater likelihood of a therapy being ranked at the top. 83 The validity of the NMA depends on the main assumption that there is no effect modification of the pairwise intervention effects or similarity of the prevalence of effect modifiers in the different studies. This key assumption has been considered for exchangeability, transitivity, similarity and consistency. 84,85
To determine the overall consistency of each network, we compared the posterior mean residual deviance, the DIC, and the between-study SD for both the NMA model (consistency model) and the unrelated mean effects (UMEs) model (inconsistency model). 82 Local consistency can be obtained through the node splitting approach for agreement between the direct and indirect evidence86 within specific comparisons, which it was not possible to assess. Nevertheless, this is not necessarily a limitation because multi-arm trials are designed to allow multiple comparisons within a single trial controlling for confounding and so inconsistency is not always possible within those trials. All analyses were performed by SN and checked for accuracy by JM.
Sensitivity analysis
Based on discussions with our clinical experts, we conducted sensitivity analyses for the mean change in MHDs, and the mean change in MMDs, by excluding the lower doses of eptinezumab (10 and 30 mg), since these doses are currently not available in the UK.
Results
Included studies
Study selection
The PRISMA flow diagram in Figure 1 summarises the results of our searches for the clinical effectiveness review. The electronic searches yielded 18,528 records after the removal of duplicates. Of these, 18,184 citations were excluded at the title and abstract sifting phase. Three hundred and forty-four records were obtained for screening. We found that many articles provided a poor definition of migraine in the abstract. Of these, 293 studies were excluded based on full-text screening. A list of excluded papers and their reasons for excluding them are presented in Report Supplementary Material 1. Seven full-text articles were not available to be sifted, despite an extensive search by the University of Warwick Library Document Supply service. Thus, these seven papers were excluded. We identified 51 articles which described data from 11 trials for the clinical effectiveness review and/or NMA. Although these linked articles were cited, we used the main trial paper for the main citation, as the other linked papers only reported some subgroup analyses, were either repetitive or combined the data.
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram summarising the flow of studies for the clinical effectiveness review.

Study characteristics
The study-level baseline participant characteristics of the included RCTs are summarised in Table 1 and Appendix 2, Table 23. The participants randomised in all trials satisfied the diagnostic criteria of chronic migraine in accordance with the ICHD. 87 Trials were conducted across the world with six multi-country trials including the UK, USA, Canada, Australia, New Zealand, Japan, Korea and other European countries. The number of participants with chronic migraine randomised across the 11 trials evaluating the prophylactic effects of pharmaceutical treatment ranged from 28288 to 113037 (total of 7352). The mean age of trial participants ranged from 35.789 to 46.890 years; the mean body mass index (BMI) ranged from 22.4(25) to 29.1(24); and the percentage of female participants ranged from 79%45 to 91%. 89
| Author, year (primary study) (trial name) | Author, year (secondary publications) | Country | Definition criteria | Treatment duration (week) | Treatment | Number of participants (ITT) | Female (%) | Mean Age | Mean BMI | Mean MMD | Mean MHD | Mean MSQ-RR | Mean MSQ-PR | Mean MSQ-EF | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Dose | Route of administration | Frequency | ||||||||||||||
| Aurora, 201092 (PREEMPT1) |
Dodick, 2019;96 2010;97 Silberstein, 2020;98 Aurora, 2014;99 Lipton, 2016100 |
56 sites in North America | ICHD-3 | 24DB | Placebo | – | – | – | 338 | 85.8 | 42.1 | 27.3 | 19.1 | 19.8 | 38.8a | 56.1a | 43.3a |
| OnabotulinumtoxinA | 155U + 40U |
IM at 39 sites | Every 12 week | 341 | 89.1 | 41.2 | 26.7 | 19.1 | 20 | 39a | 56.7a | 43.3a | |||||
| Detke, 201895 (REGAIN) |
Ruff, 2019;101 Ford, 2021;102 Förderreuther, 2018;103 Ailani, 2020;104 Ament, 2021105 |
116 centres in Argentina, Canada, Czech Republic, Germany, Israel, Italy, Mexico, Netherlands, Spain, Taiwan, UK and USA | ICHD-3 | 12DB | Placebo | – | – | – | 558 | 87 | 41.6 | 26.5 | 19.6 | 21.5 | 38.4 | 55 | 44.2 |
| Galcanezumab | 120 mg | SC | Monthly | 278 | 85 | 39.7 | 26.4 | 19.4 | 21.2 | 39.3 | 55.5 | 45.3 | |||||
| 240 mg | SC | Monthly | 277 | 82 | 41.1 | 26.7 | 19.2 | 21.4 | 38.9 | 57.1 | 45.7 | ||||||
| Diener, 2010 93 (PREEMPT2) |
Dodick, 2019,96 2010;97 Silberstein, 2020;98 Aurora, 2014;99 Lipton, 2016100 |
56 sites in North America | ICHD-3 | 24DB | Placebo | – | – | – | 358 | 84.6 | 40.9 | 27.1 | 18.7 | 19.7 | 38.8a | 56.1a | 43.3a |
| OnabotulinumtoxinA | 155U + 40U | IM at 39 sites | Every 12 week | 347 | 86.2 | 41 | 26.7 | 19.2 | 19.9 | 39a | 56.7a | 43.3a | |||||
| Dodick, 201989 | – | 92 clinics/sites in USA, Australia, New Zealand and Republic of Georgia | ICHD-3 | 12DB | Placebo | – | – | – | 121 | 90 | 37.2 | 27.6 | 16.4 | 21.1 | – | – | – |
| Eptinezumab | 300 mg | IV | Single dose | 121 | 81 | 37.2 | 27.3 | 16.5 | 21.1 | – | – | – | |||||
| 100 mg | IV | Single dose | 122 | 85 | 36.7 | 27.9 | 16.9 | 21.7 | – | – | – | ||||||
| 30 mg | IV | Single dose | 122 | 91 | 35.7 | 27.1 | 16.2 | 21 | – | – | – | ||||||
| 10 mg | IV | Single dose | 130 | 87 | 36.4 | 27.4 | 16.4 | 21 | – | – | – | ||||||
| Ferrari, 201990 (FOCUS) |
Spierings, 2021106 | 104 sites in Europe and the USA | ICHD-3 | 12DB | Placebo | – | – | – | 279 | 84 | 46.8 | 25.3 | – | – | – | – | – |
| Fremanezumab | 675 mg | SC | Single dose | 276 | 83 | 45.8 | 25.1 | – | – | – | – | – | |||||
| 675 + 225 + 225 mg | SC | Monthly | 283 | 84 | 45.9 | 25.3 | – | – | – | – | – | ||||||
| Lipton, 202094 (PROMISE2) |
Diener, 2021;107 Silberstein, 2020108 |
128 sites in 13 countries across the USA and Europe |
ICHD-3 | 24DB | Placebo | – | – | – | 366 | 88.8 | 39.6 | 27 | 16.2 | 20.6 | – | – | – |
| Eptinezumab | 300 mg | IV | Single dose | 350 | 89.7 | 41 | 26.2 | 16.1 | 20.6 | – | – | – | |||||
| 100 mg | IV | Single dose | 356 | 86.2 | 41 | 26.4 | 16.1 | 20.4 | – | – | – | ||||||
| Rothrock, 201988 (FORWARD) |
Blumenfeld, 2020109 | USA | ICHD-3 | 24OL | OnabotulinumtoxinA | 155U | IM at 31 sites | Every 12 week | 140 | 84 | 40.2 | 28.9 | – | 22.1 | – | – | – |
| Topiramate | 100 mg | Oral | Twice daily | 142 | 86 | 39.4 | 28.8 | – | 21.9 | – | – | – | |||||
| Sakai, 202191 | – | 67 institutions in Japan and Korea | ICHD-3 | 12DB | Placebo | – | – | – | 191 | 85.3 | 42.1 | 22.8 | 15.4 | 21.2 | – | – | – |
| Fremanezumab | 675 mg | SC | Single dose | 191 | 86.4 | 43.5 | 22.4 | 15.2 | 21.1 | – | – | – | |||||
| 675 + 225 + 225 mg | SC | Monthly | 189 | 86.2 | 42.7 | 23.4 | 16.4 | 21.6 | – | – | – | ||||||
| Silberstein, 200728 | Silberstein, 2009;110 Dodick, 2007111 |
46 clinics/sites in USA | ICHD-2 | 16DB | Placebo | – | – | – | 153 | 86.9 | 38.6 | 28 | 15.1 | 20.8 | 42.4 | 62.4 | 40.6 |
| Topiramate | 100 mg | Oral | Twice daily | 153 | 83.7 | 37.8 | 29.1 | 15.2 | 20.4 | 43.7 | 63.5 | 43.7 | |||||
| Silberstein, 201737 (HALO) |
Winner, 2019;112 Lipton, 2020;113 Silberstein, 2020;114 Blumenfeld, 2021115 |
132 sites in 9 countries across the USA and Europe | ICHD-3 | 12DB | Placebo | – | – | – | 375 | 88 | 41.4 | 26.5 | 20.3 | 16.4 | – | – | – |
| Fremanezumab | 675 mg | SC | Single dose | 376 | 88 | 42 | 26.6 | 20.4 | 16.2 | – | – | – | |||||
| 675 + 225 + 225 mg | SC | Monthly | 379 | 87 | 40.6 | 26.5 | 20.3 | 16 | – | – | – | ||||||
| Tepper, 201745 | Brandes, 2020;116 Ashina, 2018;117 Tapper, 2019;118 Lipton, 2019119 |
69 headache and clinical research centres in Canada, USA and Europe | ICHD-3 | 12DB | Placebo | – | – | – | 286 | 79 | 42.1 | 26.3 | 18.2 | 21.1 | 42.8 | 60.3 | 53 |
| Erenumab | 70 mg | SC | Monthly | 191 | 87 | 41.4 | 26 | 17.9 | 20.5 | 44.7 | 61.9 | 53.6 | |||||
| 140 mg | SC | Monthly | 190 | 84 | 42.9 | 26 | 17.8 | 20.7 | 45.6 | 62.9 | 56.7 | ||||||
Delivery setting for all included trials were in headache and clinical research centres; the number of sites ranged from 32 to 132. Ten trials were double-blinded trials,28,37,45,88,90–95 while one trial was open label. 88 The duration of drug treatment ranged from 12 to 36 weeks for the double-blind trials and was 48 weeks for the open label trial. The included RCTs evaluated 10 different dosing regimens of CGRP MAbs (including eptinezumab 10, 30, 100 and 300 mg, erenumab 70 and 140 mg, fremanezumab 225 and 675 mg, and Galcanezumab 120 and 240 mg), BTA 155 Units (U) and topiramate 100 mg. Seven trials measured their primary outcome at week 12 (25, 26, 29–32) and the measurement time point for one trial was week 16. 28
Narrative synthesis of results by primary outcome(s) of interest
We present the summary of evidence from 11 included RCTs for each outcome of interest narratively.
-
Monthly headache days: Eight trials reported the change in MHDs from baseline. 37,88,89,92–95,110 Two double-blind RCTs evaluating BTA versus placebo in 1384 chronic migraine participants for 24 weeks, followed by a 32-week open label phase in the USA. 92,93 Reduction in headache days from baseline (mean change; 95% CI) in BTA groups in both trials were [9 (−9.69 to −8.31) and 7.8 (−8.5 to −7.1)] while for placebo groups were [6.7 (−7.39 to −6) and 6.4 (−7.11 to −5.69)]. 92,93 The efficacy and safety of BTA 155U every 12 weeks for 3 cycles was assessed in comparison with topiramate ‘immediate release’ 50–100 mg/day in 282 chronic migraine participants for 36 weeks in the open label trial. 88 After week 12, participants initially randomised to topiramate could cross over to BTA group. BTA was significantly superior to topiramate in reduction of headache days at week 32 [8.3 (−9.77 to −6.83) and 2.1 (−3.02 to −1.18), respectively]. 88
A double-blind trial evaluated the efficacy and safety of topiramate 100 mg (twice daily) with 306 chronic migraine participants in 46 clinics in the USA for 16 weeks. 28,110 Topiramate produced a statistically significant reduction in headache days compared with placebo treatment [least square mean change from baseline (95% CI); 5.8 (−6.69 to −4.91) and 4.7 (−5.59 to −3.81), respectively]. 28,110
Two double-blind trials comparing the efficacy and safety of different doses of eptinezumab against placebo in the chronic migraine population. 89,94,108 One of the trials was conducted in 128 sites across the USA and Europe with 1072 participants and outcomes were measured at weeks 12 and 24. The reduction in headache days (mean change from baseline and 95% CI) for eptinezumab 100 and 300 mg were 8.2 (−8.8 to −7.6) and 8.8 (−9.44 to −8.16), respectively versus placebo 6.4 (−7.01 to −5.79) at week 12. The reduction in headache days for 100 and 300 mg of eptinezumab at week 24 was 9.6 (−10.27 to −8.91) and 10.6 (−11.3 to −9.88), respectively compared with placebo 8.1 (−8.07 to −7.4). 94,108 Another trial was performed at 92 sites across the USA, Australia, New Zealand and the Republic of Georgia with 558 participants. Treatment duration was measured at 12 weeks. 89 The results for reduction in headache days [mean change from baseline (95% CI)] for 100, 300, 30 and 10 mg of eptinezumab were 8.9 (−10.12 to −7.67), 9.6 (−10.87 to −8.33), 9.2 (−10.35 to −8.05) and 7.5 (−8.72 to −6.28), respectively in comparison with placebo 6.9 (−8.06 to −5.74). 89
The efficacy and safety of fremanezumab was assessed in 1121 chronic migraine subjects for 12 weeks in 132 sites in 9 countries across the USA and Europe. 37 In this double-blind RCT, participants received fremanezumab quarterly (a single dose of 675 mg at baseline and placebo at weeks 4 and 8), fremanezumab monthly (675 mg at baseline and 225 mg at weeks 4 and 8) or matching placebo. 37 Headache days’ reduction from baseline was measured at 4 weeks after first dose and at 12 weeks. Fremanezumab resulted in a lower frequency of headaches than placebo in this trial. Fremanezumab quarterly reduced mean headache days per month by 4.3 (95% CI −4.89 to −3.71) and fremanezumab monthly decreased mean headache days per month by 4.6 (95% CI −5.18 to −4.01), while the reduction in MHDs for the placebo group was 2.5 (95% CI −3.09 to −1.91). 37
A double-blind RCT compared the efficacy and safety of two doses of galcanezumab in a sample of 1085 chronic migraine for a period of 12 weeks (3 injections) in 116 headache and clinical research centres across 13 countries. 95 Both doses of galcanezumab were superior to placebo in reducing the number of MHDs. The mean change in headache days from baseline for 120 and 240 mg of Galcanezumab were −4.8 (95% CI −5.58 to −4.01) and −4.6 (95% CI −5.38 to −3.8) compared with placebo −3 (95% CI −4.1 to −1.9). 95
In summary, eight trials showed that all included medications – the CGRP MAbs (fremanezumab, eptinezumab and galcanezumab), BTA and topiramate were superior to reduction in headache days in comparison with placebo. The headache days’ reduction ranged from 2.5 days for placebo37 to 10.6 days for eptinezumab 300 mg. 108
-
Monthly migraine days: Eleven studies from ten trials investigated MMDs. 28,37,45,89–95,108 Two double-blind RCTs evaluating BTA versus placebo in 1384 chronic migraine participants for 24 weeks followed by a 32-week open label phase in the USA. 92,93 Reduction in migraine days from baseline mean change (95% CI) in BTA groups in both trials were 8.7 (−9.4 to −8) and 7.6 (−8.29 to −6.91), while for placebo groups were 6.3 (−7 to −5.6) and 6.1 (−6.82 to −5.38). 92,93
-
Three trials evaluated the efficacy of fremanezumab. One of them was performed in 1121 chronic migraine subjects for 12 weeks in 132 sites in 9 countries across the USA and Europe. 37 In this double-blind RCT, participants received fremanezumab quarterly (a single dose of 675 mg at baseline and placebo at weeks 4 and 8), fremanezumab monthly (675 mg at baseline and 225 mg at weeks 4 and 8) or matching placebo. 37 MMDs reduction from baseline was measured at 12 weeks. Fremanezumab resulted in a lower frequency of migraine days than placebo in this 12-week trial. Fremanezumab quarterly reduced mean migraine days per month by 4.9 (95% CI −5.68 to −4.12) and fremanezumab monthly decreased mean migraine days per month by 5 (95% CI −5.78 to −4.22), while the reduction in MMDs for the placebo group was 3.2 (95% CI −3.98 to −2.4). 37 The other double-blind RCT which compared the efficacy of fremanezumab was conducted in 104 sites (including hospitals, medical centres, research institutes and group practice clinics) across European countries and the USA. 90 The trial population included both episodic and chronic migraine patients who had documented failure to 2 to 4 classes of migraine preventive medications in the past 10 years, although the results for reduction in MMDs was provided separately for the 837 chronic migraine participants. Fremanezumab quarterly (month 1: 675 mg; months 2 and 3: placebo), fremanezumab monthly (month 1: 675 mg; months 2 and 3: 225 mg) and matched monthly placebo for 12 weeks were administered. 90 Reductions from baseline in mean MMDs over 12 weeks were greater versus placebo; 3.9 (95% CI −4.56 to −3.23) for quarterly, −4.5 (95% CI −5.16 to −3.83) for monthly and 0.7 (−1.35 to −0.04) for placebo. 90 The double-blind trial for evaluating the efficacy and safety of fremanezumab monthly (675 mg at baseline and 225 mg at weeks 4 and 8), fremanezumab quarterly (675 mg at baseline and placebo at weeks 4 and 8) or matching placebo in Japan and Korea was conducted in 571 chronic migraine participants. 91 The change in migraine days from baseline (95% CI) for monthly and quarterly administration were −4.9 (−5.56 to −4.23), −4.1 (−5.07 to −3.12) respectively compared with placebo −2.8 (−3.78 to −1.82). 91
-
A double-blind RCT comparing the efficacy and safety of two doses (120 and 240 mg) of galcanezumab in a sample of 1085 chronic migraine participants for a period of 12 weeks (3 injections) in 116 headache and clinical research centres across 13 countries. 95 The mean change in migraine days from baseline for 120 and 240 mg of galcanezumab were superior [−4.8 (95% CI −5.58 to −4.01) and −4.6 (95% CI −5.38 to −3.8)] compared with placebo [−2.7 (95% CI −3.48 to −1.91)]. 95
-
A double-blind trial compared different doses of erenumab efficacy and safety in 69 headache and clinical research centres in North America and Europe. 667 chronic migraine patients were randomly assigned to be administered monthly 70 mg, 140 mg of erenumab or matched placebo for 12 weeks. 45 The results demonstrated that erenumab 70 and 140 mg reduced the number of MMDs compared with placebo: the mean change from baseline (95% CI) −6.64 (−7.05 to −6.23), −6.63 (−7.04 to −6.22) and −4.18 (−4.51 to −3.85), respectively. 45
-
A double-blind trial evaluated the efficacy and safety of topiramate 100 mg (twice daily) for 306 chronic migraine participants in 46 clinics in the USA for 16 weeks. 28 Topiramate treatment resulted in a mean (95% CI) reduction from baseline of 5.6 (−6.56 to −4.63) migraine days per month compared with 4.1 (−5.07 to −3.13) for the placebo group. 28
-
Two double-blind trials comparing the efficacy and safety of different doses of eptinezumab against placebo in the chronic migraine population. 89,94 One trial was conducted in 128 sites across the USA and Europe with 1072 participants and outcomes were measured at week 12. Treatment with eptinezumab 100 mg [7.7, 95% CI (−8.41 to –6.99)] and 300 mg [8.2, 95% CI (−9.13 to −7.26)] was associated with significant reductions in MMDs across weeks 1 to 12 compared with placebo [5.6, 95% CI (−6.42 to −4.78)]. The MD (95% CI) from placebo for 100 and 300 mg during 24 weeks were −1.98 (−2.94 to −1.01) and −2.65 (−3.62 to −1.68), respectively. 94 The other trial was performed across 92 sites in the USA, Australia, New Zealand and the Republic of Georgia with 558 participants. Treatment duration and time point measurement was 12 weeks. 89 Participants were assigned in eptinezumab 100, 300, 30, 10 mg or placebo, administered as a single IV infusion. The results for reduction in migraine days [mean change from baseline (95% CI)] for 100, 300, 30 and 10 mg of eptinezumab were 7.7 (−8.94 to −6.46), 8.2 (−9.48 to −6.91), 7.9 (−9.06 to −6.74) and 6.7 (−7.9 to −5.5) respectively in comparison with placebo 5.6 (−6.78 to −4.41). 89
-
In summary, 10 trials investigated different doses of CGRP MAbs drugs (including fremanezumab, erenumab, eptinezumab and galcanezumab), BTA and topiramate. These trials illustrated data from different time points in comparison with placebo. The results demonstrated superiority of pharmacological medications versus placebo in the reduction of migraine days from baseline. Migraine days were reduced ranging from 0.7 days for placebo90 to 8.7 days for BTA. 93
-
Narrative synthesis of results by secondary outcomes of interest
-
Migraine-specific quality of life: Ten studies from five trials used the MSQ questionnaire at multiple time points. 45,92,93,95,97,102,110,111,113,119 A double-blind trial compared the efficacy and safety of two doses of galcanezumab in a sample of 1085 chronic migraine patients for a period of 12 weeks (3 injections) in 116 headache and clinical research centres across 13 countries. 102 At week 12, the least-squares mean change (95% CI) in total MSQ for galcanezumab-treated patients were 20.51 (20.33 to 20.69) (120 mg) and 20.49 (20.31 to 20.67) (240 mg), both statistically significantly greater than the placebo-treated patients 14.55 (14.44 to 14.66). 102 Improvement in all domains of MSQ for both doses were significantly greater than placebo; restrictive role function [120 mg: 21.8 (19.48 to 24.12), 240 mg: 23.1 (20.62 to 25.58) than placebo 16.8 (14.65 to 18.95)], preventative role function [120 mg: 18 (15.69 to 20.32), 240 mg: 16.1 (13.77 to 18.43) than placebo 11 (8.56 to 13.14)], and emotional function [120 mg: 21 (18.3 to 23.7), 240 mg: 20.7 (17.99 to 23.41) than placebo 14.1 (11.62 to 16.58)]. 102
-
A double-blind trial evaluated efficacy and safety of topiramate 100 mg (twice daily) with 306 chronic migraine participants in 46 clinics in the USA for 16 weeks. 111 The MSQ analysis demonstrated significant improvements at week 4 in all three domains, and at weeks 8 and 16 in both restrictive role function and emotional function domains. The preventative role function closely approached, but did not reach statistical significance at week 8. 111 The mean improvement from baseline (95% CI) for topiramate-treated subjects was 23.7 (20.04 to 27.36), 16.1 (12.69 to 19.51) and 26.3 (21.9 to 30.71) for MSQ-RR, MSQ-PR and MSQ-EF, respectively. The mean improvement from baseline (95% CI) for placebo-treated subjects was 18.8 (15.22 to 22.38), 12.6 (9.27 to 15.93) and 21.0 (16.22 to 25.78) for MSQ-RR, MSQ-PR and MSQ-EF, respectively. The differences between treatment groups were statistically significant for MSQ-RR and MSQ-EF but were not statistically significant for MSQ-PR at week 16. 110
Fremanezumab efficacy and safety was assessed in 1121 chronic migraine subjects for 12 weeks in 132 sites in 9 countries across the USA and Europe. In this double-blind trial, participants received fremanezumab quarterly (a single dose of 675 mg at baseline and placebo at weeks 4 and 8), fremanezumab monthly (675 mg at baseline and 225 mg at weeks 4 and 8) or matching placebo. 113 Fremanezumab quarterly and monthly was associated with significant improvements over placebo in mean change from baseline in MSQ in all 3 domains to week 12. 113 Least square mean change in MSQ-RR (95% CI) from baseline for quarterly and monthly group was 20.3 (18.33 to 22.27) and 21 (18.77 to 23.23), respectively versus 14.7 (12.55 to 16.85) for placebo. Improvement in MSQ-PR for quarterly, monthly and placebo group was 15.9 (14.16 to 17.64), 15.5 (13.79 to 17.21) and 11.6 (9.86 to 13.34), respectively; and improvement in MSQ-EF was 20.9 (18.75 to 23.05), 20.3 (18.53 to 22.07) and 17 (15.08 to 18.92) for quarterly and monthly administration of fremanezumab and placebo. 113
A double-blind trial evaluating BTA versus placebo in 1384 chronic migraine participants for 24 weeks followed by a 32-week open label phase in the USA92,93,97 found that the improvement in MSQ-RR [mean change from baseline (95% CI)] at week 12 for BTA was 16.2 (13.55 to 18.85) against placebo 9.9 (7.26 to 12.54). For MSQ-PR, the mean change from baseline (95% CI) favoured BTA [13 (10.89 to 15.11)] rather than placebo [13 (12.41 to 13.59)]. MSQ-EF improvement was superior in the BTA group, 18.3 (15.23 to 21.37) rather than placebo 11 (7.95 to 14.05). 97
A double-blind trial conducted in 69 headache and clinical research centres in North America and Europe randomly assigned 677 chronic migraine patients to be administered monthly 70 or 140 mg of erenumab or matched placebo for 12 weeks. 45,119 Participants in the lower dose (70 mg) of erenumab experienced less improvement in MSQ-RR function [mean change from baseline (95% CI)] than the higher dose (140 mg) participants, 17.7 (14.77 to 20.63) versus 19.1 (16.15 to 22.53), while the mean change from baseline for the placebo group was 11.8 (9.25 to 14.35). The results showed participants in the 70 mg, 140 mg and placebo group had improvement in MSQ-PR function, 13 (10.51 to 15.49), 13.8 (11.31 to 16.29) and 8.9 (6.87 to 10.93), respectively. Improvement in the MSQ-EF for the 70 mg, 140 mg and placebo group was 18.2 (13.15 to 23.24), 18.8 (14.73 to 22.87) and 9.9 (5.98 to 13.82), respectively. 119
In summary, five trials reported MSQ data for three dimensions separately, including MSQ-RR, MSQ-PR and MSQ-EF. Galcanezumab, erenumab, fremanezumab, topiramate and BTA were investigated in this diverse time window in the included trials. All these drugs were associated with a better improvement in quality of life compared with placebo.
-
The HIT-6: Eleven studies from six trials evaluated headache disability through HIT-6. 37,88,89,91–94,97,100,109,119 Two trials were associated with efficacy and safety of fremanezumab. The first trial which was double blind was conducted in 1121 chronic migraine subjects for 12 weeks in 132 sites in 9 countries across the USA and Europe. The participants received fremanezumab quarterly (a single dose of 675 mg at baseline and placebo at weeks 4 and 8), fremanezumab monthly (675 mg at baseline and 225 mg at weeks 4 and 8) or matching placebo. 37 The degree of headache-related disability decreased between baseline and the 4-week period after the last dose, with significantly greater reductions [mean change from baseline (95% CI)] in HIT-6 scores with quarterly [6.4 (−7.38 to −5.42)] and monthly [6.8 (−7.58 to −6.02)] rather than with placebo [4.5 (−5.48 to −3.52)]. 37 The second trial found a greater reduction with quarterly or monthly administration of Fremanezumab compared with placebo at 4 weeks after the final (third) trial medication administration [4.1 (−4.89 to −3.31), 4.1 (−4.90 to −3.3) and 2.4 (−3.21 to −1.59), respectively]. 91 This double-blind trial assessed 571 participants with chronic migraine who received subcutaneous fremanezumab monthly (675 mg at baseline and 225 mg at weeks 4 and 8), fremanezumab quarterly (675 mg at baseline and placebo at weeks 4 and 8) or matching placebo in Japan and Korea. 91
-
Two double-blind trials evaluated BTA versus placebo in 1384 chronic migraine participants for 24 weeks followed by a 32-week open label phase in the USA. 92,93,97 The pooled results showed a statistically significant and clinically meaningful difference for BTA versus placebo at all time points starting at the first post-treatment study visit (week 4) and including week 24 for the mean change from baseline in total HIT-6 score. 97 Mean change from baseline (95% CI) at week 12 for BTA was −4.7 (−5.58 to −3.82) compared with placebo −2.6 (−3.48 to −1.72). 97 Efficacy and safety of BTA 155U every 12 weeks for 3 cycles was assessed in comparison with topiramate ‘immediate release’ 50–100 mg/day in 282 chronic migraine participants for 36 weeks in the open label trial. 88,109 After week 12, participants initially randomised to topiramate could cross over to BTA group. At week 30, BTA resulted in a mean (95% CI) reduction in HIT-6 scores from baseline of 5.6 (−6.95 to −4.25) compared with 1.3 (−2.01 to −0.59) for topiramate, with a significant between-group difference favouring BTA. 88,109
-
Two double-blind trials compared the efficacy and safety of different doses of eptinezumab against placebo in the chronic migraine population. 89,94 The first trial was conducted in 128 sites across the USA and Europe with 1072 participants, and outcomes were measured at weeks 12 and 24. 94 Patients in the eptinezumab 300 mg group demonstrated a statistically significant improvement on the HIT-6 at week 12, with an estimated MD from placebo. 94 Reduction [mean change from baseline (95% CI)] in HIT-6 for 100 and 300 mg was 6.2 (−6.92 to −5.48) and 7.3 (−8.34 to −6.26) versus placebo 4.5 (−5.27 to −3.73). 94 The second trial was performed at 92 sites across the USA, Australia, New Zealand and the Republic of Georgia with 558 participants. Participants were assigned to eptinezumab 100, 300, 30, 10 mg or placebo, administered as a single IV infusion. 89 The greatest effect of eptinezumab, as measured by the HIT-6, was observed at week 12, with changes in baseline scores of −10.0 (−11.54 to −8.46), −6.9 (−8.24 to −5.56), −6.5 (−7.83 to −5.16) and −6.5 (−7.91 to −5.09) for the 300, 100, 30 and 10 mg groups, respectively, compared with −5.8 for the placebo group. 89
-
A double-blind trial comparing different doses of erenumab in 69 headache and clinical research centres in North America and Europe had 677 chronic migraine patients who were randomly assigned monthly 70 or 140 mg of erenumab or matched placebo for 12 weeks. 45,119 The change from baseline (95% CI) in HIT-6 score was greater in the erenumab groups than in placebo as early as month 1 and this improvement was sustained throughout the trial [70 and 140 mg 5.6 (−6.80 to −4.40) and placebo 3.1 (−4.04 to −2.17)]. 119
-
In brief, six trials aimed to explore the change of disability measured by HIT-6. All pharmacological medications (BTA, fremanezumab, erenumab and eptinezumab) were more effective in the reducing the disabilities score compared with placebo. Reduction in HIT-6 score ranged from 1.3 for placebo88 to 17.4 for BTA. 109
-
-
EuroQol-5 Dimensions, five-level version (EQ-5D-5L): A double-blind, placebo RCT assessed the effect of treatment with fremanezumab on HRQoL in 1130 participants with chronic migraine. 113 Fremanezumab quarterly (675 mg at baseline, placebo at weeks 4 and 8) or monthly (225 mg at baseline, weeks 4 and 8) led to statistically significant improvements in the EQ-5D-5L visual analogue scale score, compared with placebo. Differences were reported as least-mean squares changes which were 4.6 and 4.8 for fremanezumab quarterly and monthly respectively, compared with 2.2 for placebo.
-
Migraine disability assessment: Three trials reported the MIDAS at different time points. 102,110,119 The first trial reported the MIDAS total score in a study which aimed to assess topiramate for 306 participants. 110 The MIDAS score [mean (95% CI)] decreased from baseline, indicating that improvement was greater in the topiramate group [31.4 (22.87 to 39.92)] compared with the placebo group [21.0 (12.73 to 29.27)]. The second trial evaluated the effect of erenumab in 667 participants with chronic migraine. 119 Reductions from baseline to month 3 in MIDAS total score was greater in the erenumab group compared to the placebo group, indicating better improvement. Respective differences from baseline [least-squares mean (CI)] were −11.9 (−19.3 to −4.4) and −12.2 (−19.7 to −4.8) for erenumab 70 and 140 mg. The final trial assessed galcanezumab in 1117 chronic migraine participants. 102 At week 12, the difference in the least-squares mean (CI) from baseline in the MIDAS total score for galcanezumab indicated a decrease in disability that was significantly greater for the 120 mg dose only [8.74 (−16.4 to −1.1)] and similar for the 240 mg dose [5.49 (−13.1 to 2.1)] compared with placebo.
In summary, the three trials found that there was improvement in MIDAS score for erenumab, galcanezumab and topiramate in comparison with placebo.
-
Work Productivity and Activity Impairment Questionnaire – Specific Health Problem (WPAI:SHP): An open label trial compared BTA with topiramate 100 mg for ≤ 36 weeks in people with chronic migraine. 109 Overall, 85.7% participants in the BTA group completed the study, while only 19.7% of the participants randomised to topiramate completed their initial treatment. 56.3% of those participants who discontinued topiramate, from week 12 switched to BTA. Work productivity assessed by the WPAI:SHP scores reported at week 36 revealed significant improvements with BTA versus topiramate in work productivity loss [MD: 0.67 (–1.25 to –0.09)] and activity impairment [MD: 1.53 (–2.07 to –1.0)] domains. In summary, this trial found that there was an improvement in work productivity measured by WPAI:SHP which favoured BTA compared to topiramate.
-
Patient Health Questionnaire 9-item (PHQ-9): The same trial that used the WPAI:SHP questionnaire109 also compared BTA with topiramate and reported outcomes for depression at week 36. Improvements in depression were observed via larger changes in PHQ-9 scores with BTA than topiramate [MD: 1.86 (–2.63 to –1.10)]. In summary, BTA led to a better reduction on depression in comparison with topiramate.
-
Functional Impact of Migraine Questionnaire (FIMQ): The open-label trial comparing BTA with topiramate reported FIMQ at week 30. 109 The FIMQ total score showed a greater reduction from baseline with BTA versus topiramate [MD: 11.38 (−16.01 to −6.75)] and also a greater reduction in the following domains: activity impairment [MD: 0.75 (–15.38 to –6.13)]; emotional functioning [MD: 10.81 (–15.76 to –5.86)]; and cognitive functioning [MD: 14.49 (–19.90 to –9.07)]. In brief, BTA had a favourable profile in reduction of activity and functional impairment.
Feasibility of a network meta-analysis
From the eight studies which reported MHDs, seven trials were eligible for inclusion in the NMA. Following guidance from our clinical experts, they recommended that 12 weeks can be used as the measurement time point for the NMA. They also agreed that the 16 weeks measurement time point for topiramate was comparable and can be pooled with the 12 weeks time point. The project team also decided to pool the BTA data which was measured at the 24 weeks time point. However, we excluded the open label trial evaluating BTA efficacy and safety versus topiramate88 for the NMA as the data were reported at 32 weeks. We planned to perform a sensitivity analysis to reflect the effect of the study design (open-label vs. double-blind), but it was not possible because there was insufficient information for MHDs at week 12. The other studies included in the NMA were comparable in terms of participants characteristics, treatment dosing and schedules, baseline risk and observed treatment effects.
For MMDs, 10 studies were eligible for the NMA. Five studies evaluated the change in MSQ score from baseline and were eligible in another separate NMA. Only the 12 weeks time point was included for this NMA and any other time points were excluded. From the seven trials which reported HIT-6 score, six studies were eligible to be included in NMA. We used the same reasoning for excluding the open label trial as we did for MHDs. In summary, we conducted an NMA for those outcomes which were reported in at least three trials.
Network meta-analysis results
We performed a NMA on two primary outcomes: mean change in MHD from baseline, and the mean change in MMDs from baseline.
We also performed NMA on two QoL outcomes: the mean change in MSQ score from baseline for three dimensions – (1) MSQ-RR function; (2) MSQ-PR function; and (3) MSQ-EF and the mean change in HIT-6 score from baseline.
We fitted both fixed and random-effects NMA models based on the model fit indices; we selected the fixed-effects NMA model for all outcomes (see Appendix 3, Tables 24, 28, 32, 36, 40 and 44). We found no indirect evidence in the results, as all trials included in the analysis were placebo-controlled, where no two active treatments were directly compared (Figures 2–19); thus the direct evidence and NMA estimates are the same for each outcome.
FIGURE 2.
Summary of the change in MHD from baseline – network plot. Nodes and edges are weighted according to the number of participants and studies evaluating each treatment and direct comparison, respectively. EPT10 mg, eptinezumab 10 mg IV infusion; EPT30 mg, eptinezumab 30 mg IV infusion; EPT100 mg, eptinezumab 100 mg IV infusion; EPT300 mg, eptinezumab 300 mg IV infusion; FRE-Quarterly, fremanezumab 675 mg (quarterly) SC single dose; FRE-Monthly, fremanezumab 675 + 225 + 225 mg SC; GAL120 mg, galcanezumab 120 mg SC; GAL240 mg, galcanezumab 240 mg SC; TOP100 mg, topiramate 100 mg oral; BTA, onabotulinumtoxinA 155 + 40U SC; IV, intravenous; PBO, placebo; SC, subcutaneous.

FIGURE 3.
Summary of the change in MHD from baseline – forest plot. The forest plot shows the MDs and 95% CrI compared with placebo as reference treatment. MDs lower than 0 indicate favoured results for the intervention.

FIGURE 4.
Summary of the change in MHD from baseline – rankings and SUCRA. Ranking probabilities graph (hatching blue bars) of each treatment and red bars are the SUCRA values for each treatment. The probabilities ranking ranged from 0 to 1. Due to rounding in R software these do not equate to one. SUCRA, surface under the cumulative ranking curve.

FIGURE 5.
Summary of the change in MMD from baseline – network plot. Nodes and edges are weighted according to the number of participants and studies evaluating each treatment and direct comparison, respectively. EPT10 mg, eptinezumab 10 mg IV infusion; EPT30 mg, eptinezumab 30 mg IV infusion; EPT100 mg, eptinezumab 100 mg IV infusion; EPT300 mg, eptinezumab 300 mg IV infusion; ERE70 mg, erenumab 70 mg SC; ERE140 mg, erenumab 140 mg SC; FRE-Quarterly, fremanezumab 675 mg (quarterly) SC single dose; FRE-Monthly, fremanezumab 675 + 225 + 225 mg SC; GAL120 mg, galcanezumab 120 mg SC; GAL240 mg, galcanezumab 240 mg SC; TOP100 mg, topiramate 100 mg oral; BTA, onabotulinumtoxinA 155 + 40U SC; IV, intravenous; PBO, placebo; SC, subcutaneous.
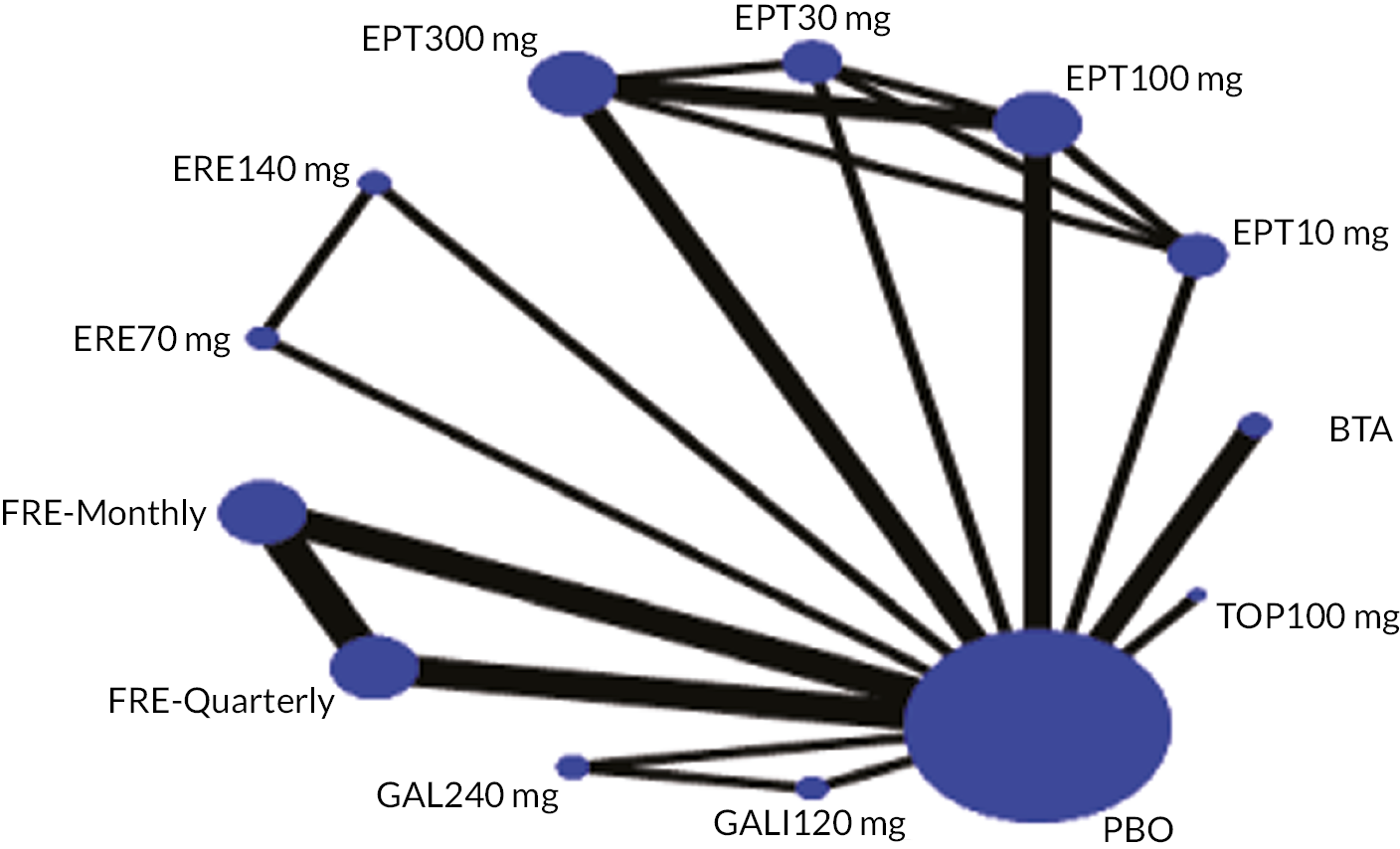
FIGURE 6.
Summary of the change in MMD from baseline – forest plot. The forest plot shows the MDs and 95% CrI compared with placebo as reference treatment. MDs lower than 0 indicate favoured results for the intervention.
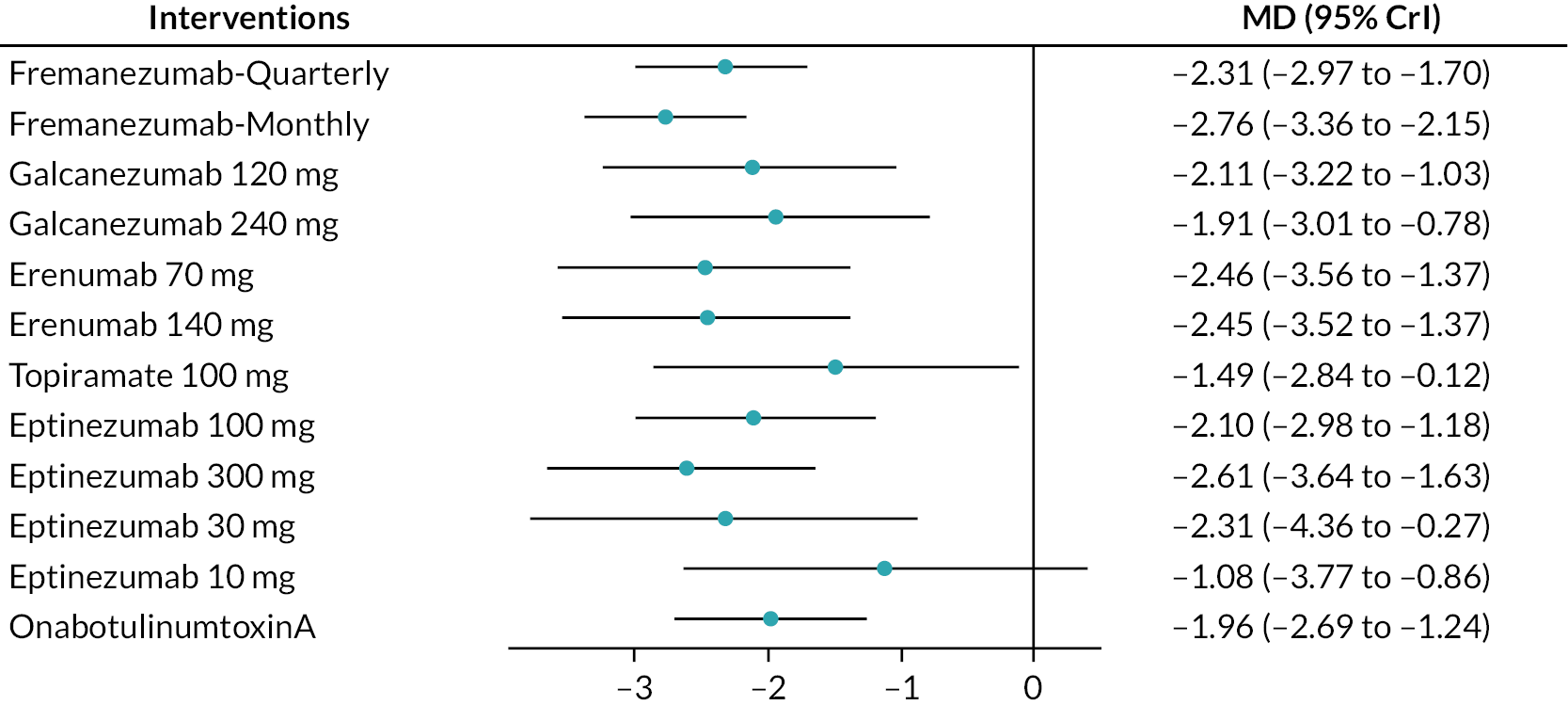
FIGURE 7.
Summary of the change in MMD from baseline – rankings and SUCRA. Ranking probabilities graph (hatching blue bars) of each treatment and red bars are the SUCRA values for each treatment. The probabilities ranking ranged from 0 to 1. Due to rounding in R software these do not equate to one. SUCRA, surface under the cumulative ranking curve.
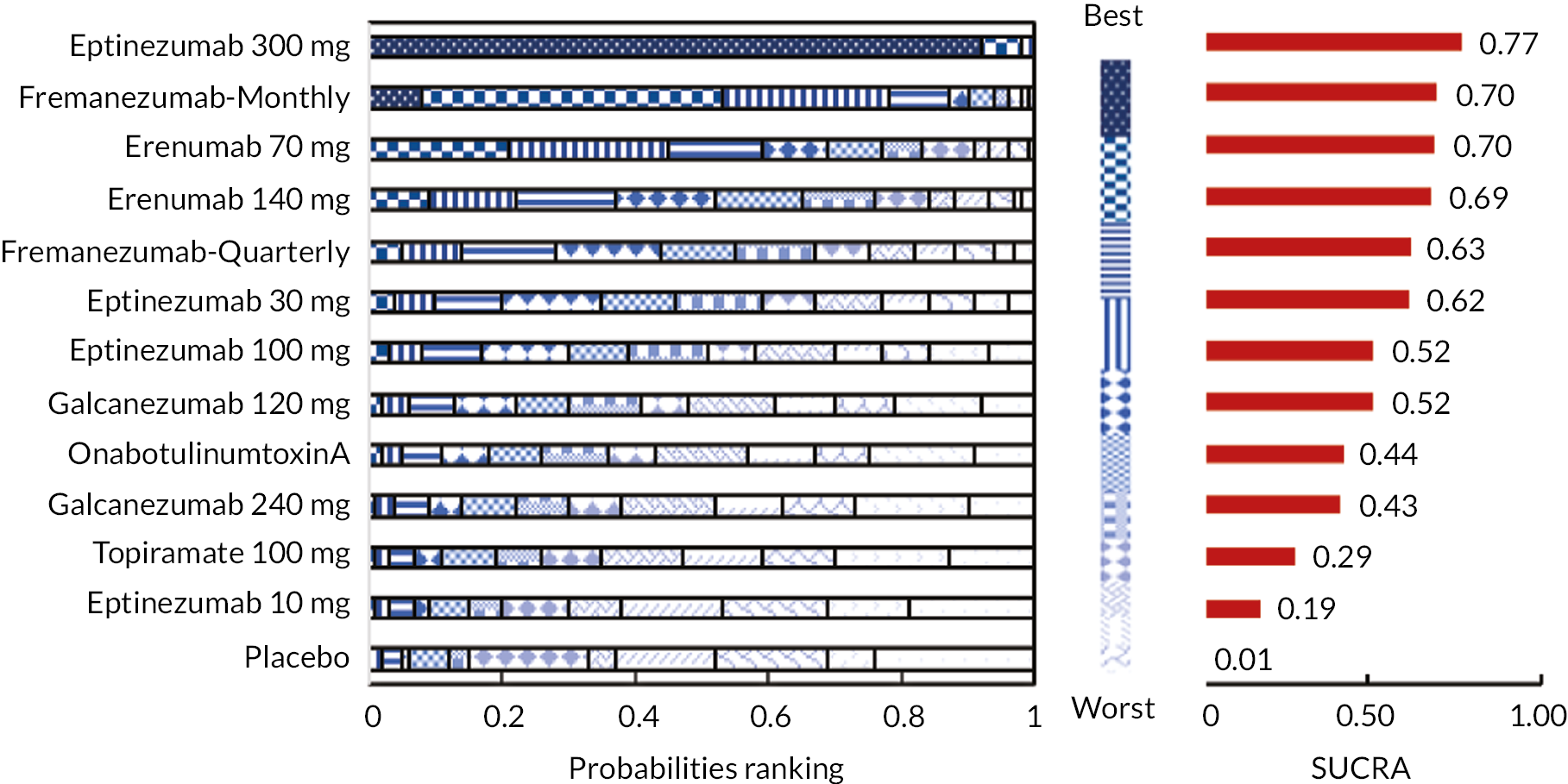
FIGURE 8.
Summary of the change in MSQ-RR from baseline – network plot. Nodes and edges are weighted according to the number of participants and studies evaluating each treatment and direct comparison, respectively.
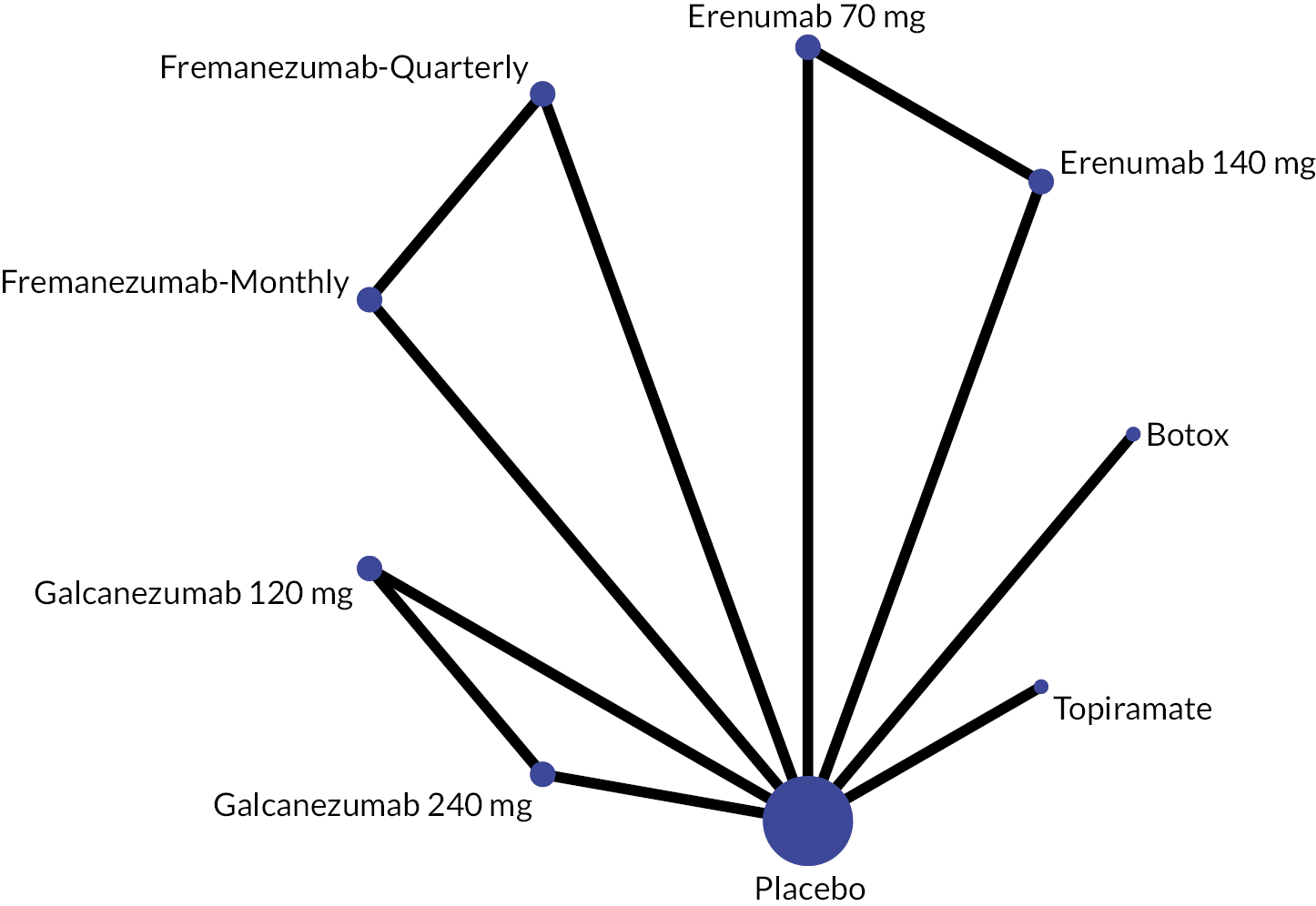
FIGURE 9.
Summary of the change in MSQ-RR from baseline – forest plot. The forest plot shows the MDs and 95% CrI compared with placebo as reference treatment. MDs lower than 0 indicate favoured results for the intervention.
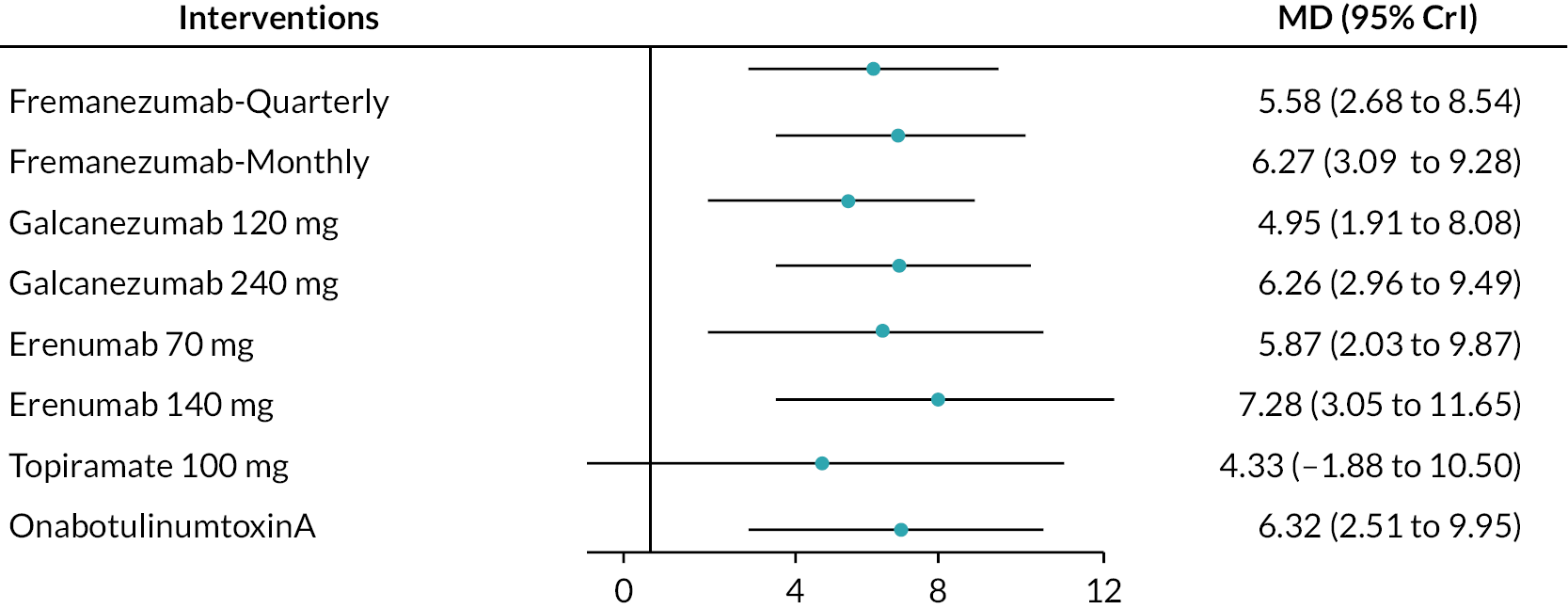
FIGURE 10.
Summary of the change in MSQ-RR from baseline – rankings and SUCRA. Ranking probabilities graph (hatching blue bars) of each treatment and red bars are the SUCRA values for each treatment. The probabilities ranking ranged from 0 to 1. Due to rounding in R software these do not equate to one. MSQ-RR, migraine-specific quality of life – restrictive role; SUCRA, surface under the cumulative ranking curve.

FIGURE 11.
Summary of the change in MSQ-PR from baseline – network plot. Network plot shows that the nodes and edges are weighted according to the number of participants and studies evaluating each treatment and direct comparison, respectively.

FIGURE 12.
Summary of the change in MSQ-PR from baseline – forest plot. The forest plot shows the MDs and 95% CrI compared with placebo as reference treatment. MDs lower than 0 indicate favoured results for the intervention.
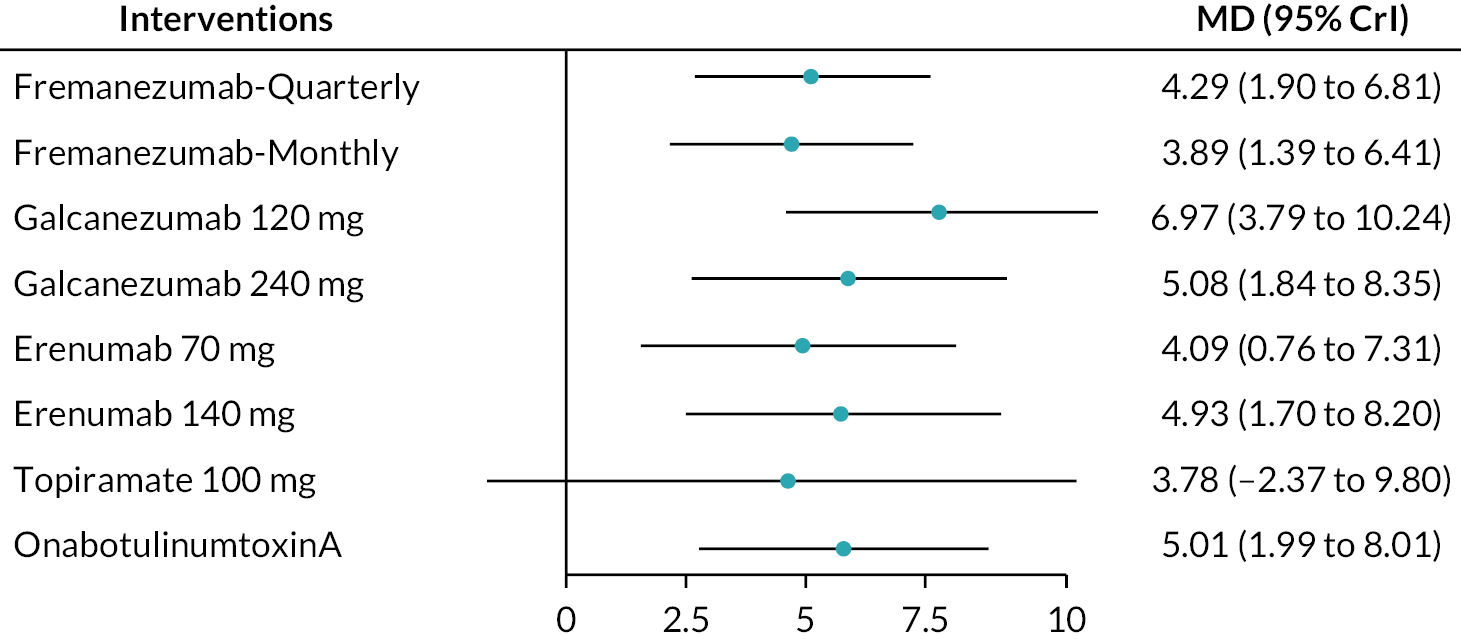
FIGURE 13.
Summary of the change in MSQ-PR from baseline – rankings and SUCRA. Ranking probabilities graph (hatching blue bars) of each treatment and red bars are the SUCRA values for each treatment. The probabilities ranking ranged from 0 to 1. Due to rounding in R software these do not equate to one. MSQ-PR, migraine-specific quality of life – preventative role; SUCRA, surface under the cumulative ranking curve.
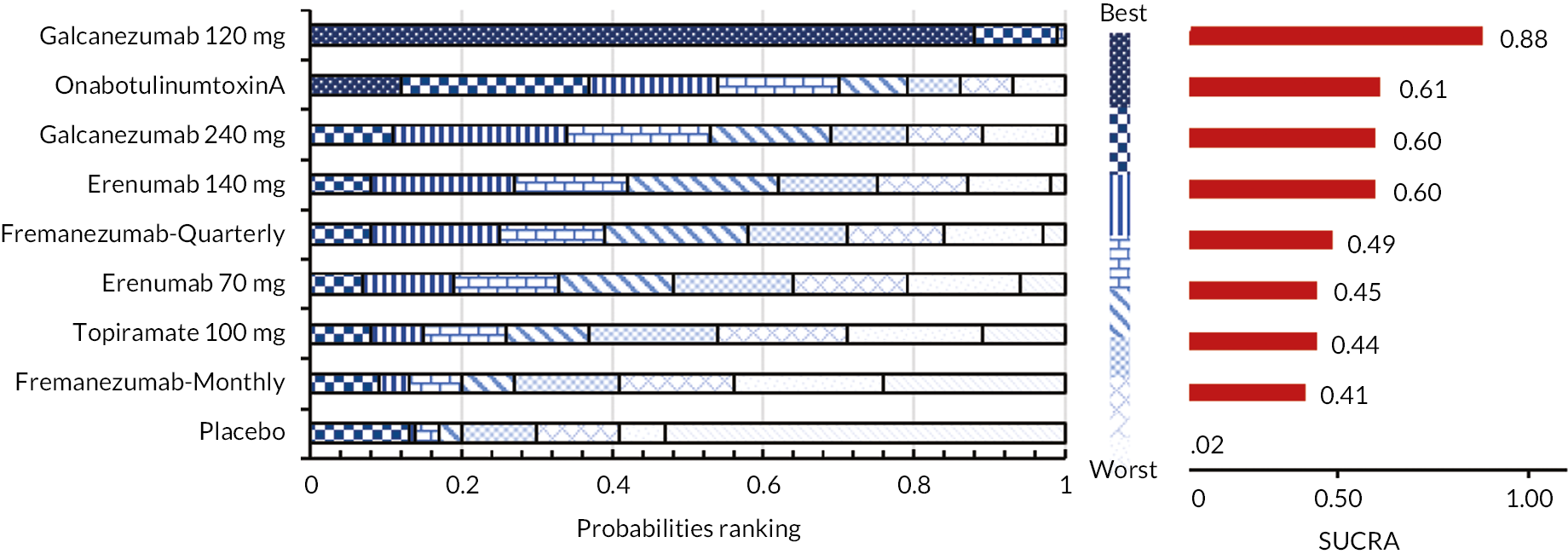
FIGURE 14.
Summary of the change in MSQ-EF from baseline – network plot. Nodes and edges are weighted according to the number of participants and studies evaluating each treatment and direct comparison, respectively.

FIGURE 15.
Summary of the change in MSQ-EF from baseline – forest plot. The forest plot shows the MDs and 95% CrI compared with placebo as reference treatment. MDs lower than 0 indicate favoured results for the intervention.

FIGURE 16.
Summary of the change in MSQ-EF from baseline – rankings and SUCRA. Ranking probabilities graph (hatching blue bars) of each treatment and red bars are the SUCRA values for each treatment. The probabilities ranking ranged from 0 to 1. Due to rounding in R software these do not equate to one. MSQ-EF, migraine-specific quality of life – emotional function; SUCRA, surface under the cumulative ranking curve.

FIGURE 17.
Summary of the change in HIT-6 from baseline – network plot. Nodes and edges are weighted according to the number of participants and studies evaluating each treatment and direct comparison, respectively.
FIGURE 18.
Summary of the change in HIT-6 from baseline – forest plot. The forest plot shows the MDs and 95% CrI compared with placebo as reference treatment. MDs lower than 0 indicate favoured results for the intervention.
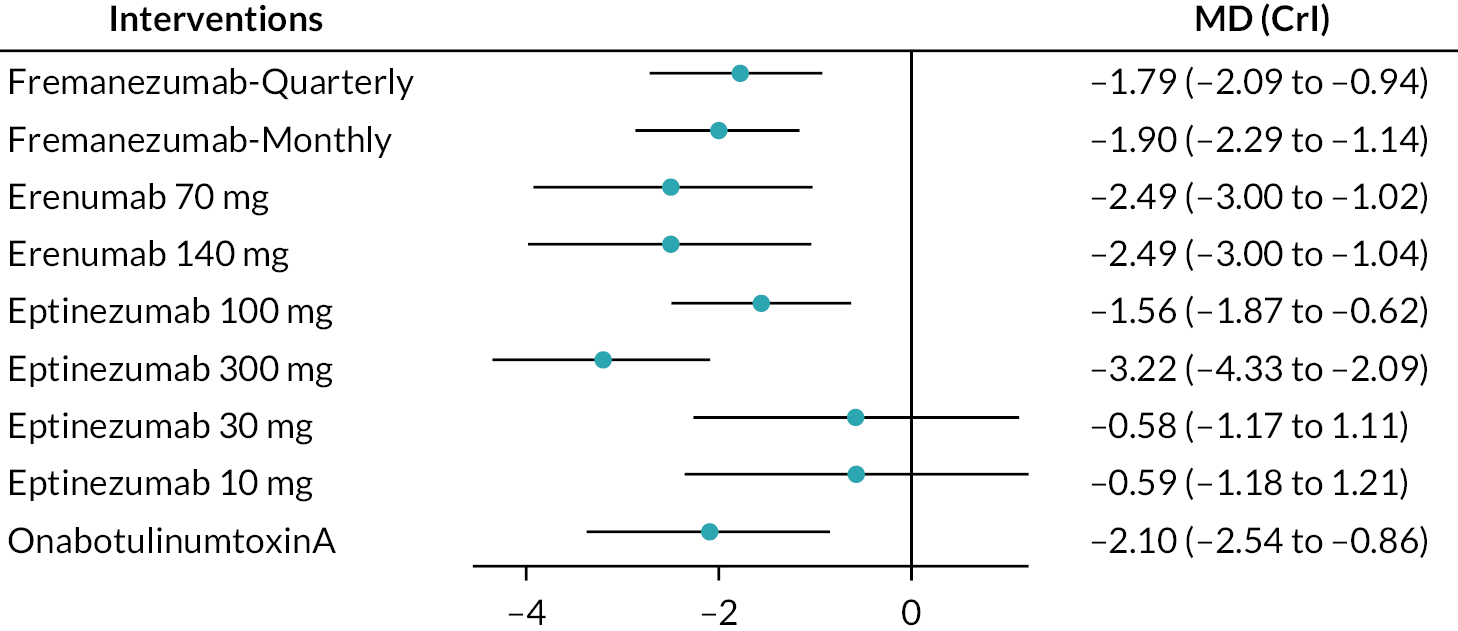
FIGURE 19.
Summary of the change in HIT-6 from baseline – rankings and SUCRA. Ranking probabilities graph (hatching blue bars) of each treatment and red bars are the SUCRA values for each treatment. The probabilities ranking ranged from 0 to 1. Due to rounding in R software these do not equate to one. HIT-6, Headache Impact Test; SUCRA, surface under the cumulative ranking curve.
Mean change in monthly headache days
For the primary outcome, mean change in MHD from baseline, this was reported in 8 RCTs with a total of 5838 participants. The NMA included two trials comparing BTA with Topiramate (27, 28) at week 24, two trials evaluating eptinezumab versus placebo (29, 41, 58) at weeks 12 and 24, a trial evaluating topiramate versus placebo (34) at week 16, a trial comparing fremanezumab with placebo37 at weeks 4 and 12, a trial evaluating galcanezumab versus placebo (30) at week 12, and a trial comparing BTA with Topiramate (33) at week 32 (Table 2).
| Eptinezumab 300 mg | ||||||||||
| −0.36 (−1.52 to 0.81) | Fremanezumab-M | |||||||||
| 0.28 (−1.18 to 0.79) | −0.09 (−1.74 to 1.54) | Eptinezumab 30 mg | ||||||||
| 0.60 (−0.47 to 1.67) | 0.23 (−0.84 to 1.34) | 0.32 (−1.25 to 1.95) | BTA | |||||||
| −0.64 (−2.02 to 0.74) | 0.28 (−1.16 to 1.67) | −0.37 (−2.22 to 1.48) | −0.05 (−1.33 to 1.23) | Galcanezumab 120 mg | ||||||
| −0.62 (−1.42 to 0.17) | 0.26 (−0.92 to 1.39) | −0.35 (−1.81 1.05) | −0.02 (−1.05 to 1.02) | −0.02 (−1.35 to 1.31) | Eptinezumab 100 mg | |||||
| −0.67 (−1.85 to 0.49) | −0.30 (−1.16 to 0.55) | −0.39 (−2.04 to 1.23) | −0.07 (−1.16 to 0.98) | −0.02 (−1.44 to 1.33) | −0.05 (−1.18 to 1.10) | Fremanezumab-Q | ||||
| −0.86 (−2.25 to 0.49) | 0.49 (−0.86 to 1.89) | −0.58 (−2.37 to 1.20) | −0.26 (−1.54 to 1.04) | 0.21 (0.85 to 1.29) | −0.23 (−1.56 to 1.08) | 0.19 (−1.20 to 1.56) | Galcanezumab 240 mg | |||
| −1.36 (−2.89 to 0.14) | 0.99 (−0.52 to 2.50) | −1.08 (−3.08 to 0.79) | −0.76 (−2.21 to 0.70) | 0.71 (−0.99 to 2.37) | −0.74 (−2.21 to 0.75) | −0.69 (−0.78 to 2.19) | 0.50 (−1.18 to 2.15) | Topiramate 100 mg | ||
| 1.98 (0.52 to 3.52) | 1.62 (−0.13 to 3.34) | 1.70 (0.85 to 3.32) | −1.38 (0.85 to 0.29) | 1.34 (−0.50. 3.27) | 1.36 (−0.13 to 2.87) | 1.31 (−0.47 to 3.03) | 1.13 (−0.70 to 3.00) | 0.62 (−1.36 to 2.69) | Eptinezumab 10 mg | |
| −2.46 (−3.24 to −1.67) | −2.10 (−2.95 to −1.23) | −2.19 (−3.63 to −0.74) | −1.87 (−2.55 to −1.18) | −1.82 (−2.91 to −0.73) | −1.84 (−2.60 to −1.08) | −1.79 (−2.62 to −0.98) | −1.61 (−2.68 to −0.54) | −1.10 (−2.38 to 0.16) | −0.48 (−2.02 to 1.01) | Placebo |
We considered follow-up periods of 12 and 16 weeks as a measurement point for the NMA. We pooled the BTA data at week 24, as the primary time point for evaluating BTA is usually 6 months. Hence, we have included 10 different doses of drugs from 7 trials for the NMA and compared this with placebo as a reference treatment.
The network plot is presented in Figure 2, where thicker edges represent comparisons with a larger number of randomised trials. Similarly, interventions with a larger number of randomised participants have larger circles. All interventions were compared with placebo. Figure 3 displays the result for the fixed-effects NMA model in comparison with placebo. According to the forest plot, all treatments significantly reduced the mean MHDs compared to placebo. The most effective intervention is eptinezumab 300 mg (MD: −2.46, 95% CrI: −3.24 to −1.67) as this reduced MHD by 2.46, followed by eptinezumab 30 mg (MD: −2.19, 95% CrI: −3.63 to −0.74), fremanezumab monthly (MD: −2.10, 95% CrI: −2.95 to −1.23), onabotulinumtoxinA (MD −1.87, 95% CrI −2.55 to −1.18), eptinezumab 100 mg (MD: −1.84, 95% CrI: −2.60 to −1.08), galcanezumab 120 mg (MD: −1.82, 95% CrI: −2.91 to −0.73), fremanezumab-quarterly (MD: −1.79, 95% CrI: −2.62 to −0.98), galcanezumab 240 mg (MD: −1.61, 95% CrI: −2.68 to −0.54) and topiramate 100 mg (MD: −1.10, 95% CrI: −2.38 to 1.01). The least effective treatment was eptinezumab 10 mg (MD: −0.48, 95% CrI: −2.02 to 1.01). We presented the league tables for all comparisons in Table 2.
The 11 node analysis in Figure 4 showed that eptinezumab 300 mg (SUCRA 0.88) had the highest probability ranking to reduce MHD, followed by fremanezumab monthly and eptinezumab 30 mg (SUCRA 0.71), onabotulinumtoxinA (SUCRA 0.59), eptinezumab 100 mg and galcanezumab 120 mg (SUCRA 0.57), fremanezumab-quarterly (SUCRA 0.55), galcanezumab 240 mg (SUCRA 0.47), topiramate 100 mg (SUCRA 0.30), eptinezumab 10 mg (SUCRA 0.14), and the lowest probability ranking is placebo (SUCRA 0.03). Treatment probabilities ranking and cumulative ranking curves were obtained and tabulated in Appendix 3, Figures 28 and 29 and Tables 25 and 26.
The global approach to test for overall consistency shows no evidence of inconsistency in the data points. It is presented in Appendix 3, Figure 30. Also, the result of comparing the fit of NMA and Unrelated Mean Effects (UME) inconsistency models illustrated a good fit which is tabulated in Appendix 3, Table 27.
Mean change in monthly migraine days
For the second primary outcome, the mean change in MMD from baseline, this was reported in 10 RCTs with a total of 7821 participants trials comparing BTA with topiramate (27, 28) at week 24, two trials evaluating eptinezumab versus placebo (29, 41, 58) at weeks 12 and 24, a trial evaluating topiramate versus placebo (34) at week 16, three trials comparing fremanezumab with placebo at weeks 4 and 12,37,90,91 a trial investigating erenumab against placebo, and a trial evaluating galcanezumab versus placebo (30) at week 12 (Table 3).
| Eptinezumab 300 mg | ||||||||||||
| 0.15 (−1.04 to 1.31) | Fremanezumab-M | |||||||||||
| −0.15 (−1.62 to 1.33) | 0.30 (−0.96 to 1.57) | Erenumab 70 mg | ||||||||||
| −0.16 (−1.65 to 1.28) | 0.31 (−0.94 to 1.57) | 0.01 (−1.10 to 1.11) | Erenumab 140 mg | |||||||||
| −0.30 (−1.48 to 0.88) | −0.45 (−1.06 to 0.17) | −0.15 (−1.42 to 1.16) | −0.13 (−1.41 to 1.13) | Fremanezumab-Q | ||||||||
| 0.30 (−1.20 to 1.78) | 0.45 (−1.13 to 2.05) | 0.15 (−1.70 to 1.94) | 0.13 (−1.65 to 1.94) | 0.00 (−1.60 to 1.66) | Eptinezumab 30 mg | |||||||
| −0.51 (−1.51 to 0.48) | 0.66 (−0.43 to 1.77) | 0.36 (−1.09 to 1.78) | 0.35 (−1.06 to 1.75) | 0.21 (−0.89 to 1.37) | −0.21 (−1.64 to 1.24) | Eptinezumab 100 mg | ||||||
| −0.50 (−1.93 to 0.97) | 0.65 (−0.61 to 1.88) | −0.35 (−1.89 to 1.21) | −0.34 (−1.87 to 1.24) | 0.20 (−1.09 to 1.46) | −0.21 (−2.01 to 1.57) | 0.01 (−1.43 to 1.43) | Galcanezumab 120 mg | |||||
| 0.65 (−0.53 to 1.89) | 0.80 (−0.15,1.75) | 0.50 (−0.79 to 1.81) | 0.49 (−0.80 to 1.76) | 0.35 (−0.62 to 1.32) | 0.36 (−1.24 to 1.99) | 0.14 (−1.01 to 1.33) | 0.15 (−1.16 to 1.47) | BTA | ||||
| −0.70 (−2.20 to 0.76) | 0.85 (−0.40 to 2.12) | −0.55 (−2.15 to 0.96) | −0.54 (−2.10 to 0.98) | 0.40 (−0.85 to 1.70) | −0.40 (−2.24 to 1.42) | −0.19 (−1.62 to 1.20) | 0.20 (−0.89 to 1.29) | −0.05 (−1.36 to 1.26) | Galcanezumab 240 mg | |||
| −1.12 (−2.85 to 0.56) | 1.27 (−0.20 to 2.78) | 0.97 (−0.78 to 2.71) | 0.96 (−0.81 to 2.71) | 0.82 (−0.68 to 2.37) | −0.83 (−2.83 to 1.23) | −0.61 (−2.27 to 1.04) | 0.62 (−1.13 to 2.38) | −0.47 (−2.01 to 1.07) | 0.42 (−1.32 to 2.19) | Topiramate 100 mg | ||
| 1.49 (−0.04 to 3.01) | 1.64 (0.08 to 3.30) | 1.34 (−0.52 to 3.23) | 1.33 (−0.53 to 3.16) | 1.19 (−0.37 to 2.90) | 1.19 (−0.47 to 2.88) | 0.98 (−0.49 to 2.46) | 0.99 (−0.85 to 2.85) | −0.84 (−2.44 to 0.83) | 0.79 (−1.03 to 2.73) | −0.37 (−1.65 to 2.39) | Eptinezumab 10 mg | |
| −2.61 (−3.64 to −1.63) | −2.76 (−3.36 to −2.15) | −2.46 (−3.56 to −1.37) | −2.45 (−3.52 to −1.37) | −2.31 (−2.97 to −1.7) | −2.31 (−4.36 to −0.27) | −2.10 (−2.98 to −1.18) | −2.11 (−3.22 to −1.03) | −1.96 (−2.69 to −1.24) | −1.91 (−3.01 to −0.78) | −1.49 (−2.84 to −0.12) | −1.08 (−3.77 to −0.86) | Placebo |
We considered follow-up periods of 12 and 16 weeks as a measurement point for NMA. We pooled the BTA data at week 24, as the primary time point for evaluating the BTA is usually 6 months. Hence, we included 12 different doses of drugs from 10 trials for the NMA and compared this with placebo as a reference treatment.
The network plot is presented in Figure 5. Figure 6 depicts the result for the fixed-effects NMA model in comparison with placebo. According to the forest plot, all treatments significantly reduced the mean MMDs compared to placebo.
The most effective drug is fremanezumab monthly (MD: −2.76, 95% CrI: −3.36 to −2.15) followed by eptinezumab 300 mg (MD: −2.61, 95% CrI: −3.64 to −1.63), erenumab 70 mg (MD: −2.46, 95% CrI: −3.56 to −1.37), erenumab 140 mg (MD: −2.45, 95% CrI: −3.52 to −1.37), fremanezumab-quarterly (MD: −2.31, CrI: −2.97 to −1.7), eptinezumab 30 mg (MD: −2.31, CrI: −4.36 to −0.27), galcanezumab 120 mg (MD: −2.11, 95% CrI: −3.22 to −1.3), eptinzumab 100 mg (MD: −2.10, 95% CrI: −2.98 to −1.18), BTA (MD: −1.96, 95% CrI: −2.69 to −1.24), galcanezumab 240 mg (MD: −1.91, 95% CrI: −3.01 to −0.78), topiramate 100 mg (MD: −1.49, 95% CrI: −2.84 to 0.72). The evidence shows the least effective treatment was eptinezumab 10 mg (MD: −1.08, 95% CrI: −3.77 to −0.86). We presented the league tables for all comparisons in Table 3.
The 13 node analysis in Figure 7 showed that eptinezumab 300 mg (SUCRA 0.77) had the highest probability ranking to reduce MMD, followed by fremanezumab monthly and erenumab 70 mg (SUCRA 0.70), erenumab 140 mg (SUCRA 0.69), fremanezumab-quarterly (SUCRA 0.63), eptinezumab 30 mg (SUCRA 0.62), eptinezumab 100 mg and galcanezumab 120 mg (SUCRA 0.52), onabotuliumtoxin A (SUCRA 0.44), galcanezumab 240 mg (SUCRA 0.43), topiramate 100 mg (0.29), eptinezumab 10 mg (SUCRA 0.19) and lowest probability ranking is placebo (SUCRA 0.01). Treatment probabilities ranking and cumulative ranking curves were estimated and tabulated in Appendix 3, Figures 31 and 32 and Tables 29 and 30.
According to the data points presented in Appendix 3, Figure 33, there is no indication of inconsistency, as determined by the global method for testing overall consistency. Also, the result of comparing the fit of NMA and UME inconsistency models illustrated a good fit which is tabulated in Appendix 3, Table 31.
Mean change in migraine-specific quality of life
Five trials provided improvement in MSQ scores at week 12 with a total 4626 participants, including a trial comparing galcanezumab against placebo,95 a trial evaluating topiramate versus placebo,111 a trial investigating fremanezumab versus placebo,113 a trial comparing erenumab against placebo,119 and a trial evaluating BTA versus placebo at week 12. 100 The network plots obtained for three dimensions are shown in Figures 8, 11 and 14 (which is the same). However, the other results are presented for each dimension separately.
Mean change in migraine-specific quality of life – restrictive role
Forest plots in Figures 9 and 10 show that all treatments were more effective than placebo. Our analysis demonstrated that erenumab 140 mg (MD: 7.28, 95% CrI: 3.05 to 11.65, SUCRA 0.75) was superior to other drugs in improvement of MSQ-RR and had the highest probability of being ranked best. This was followed by galcanezumab 240 mg (MD: 6.26, 95% CrI: 2.96. to 9.49, SUCRA 0.64) which was the next best ranked treatment, fremanezumab monthly (MD: 6.27, 95% CrI: 3.09 to 9.28, SUCRA 0.63), BTA (MD: 6.32, 95% CrI: 2.51 to 9.95, SUCRA 0.62), erenumab 70 mg (MD: 5.87, 95% CrI: 2.03 to 9.87, SUCRA 0.56), fremanezumab-quarterly (MD: 5.58, 95% CrI: 2.68 to 8.54, SUCRA 0.51), galcanezumab 120 mg (MD: 4.95, 95% CrI: 1.91 to 8.08, SUCRA 0.42), and then topiramate 100 mg (MD: 4.33, 95% CrI: −1.88 to 10.5, SUCRA 0.40). All head-to-head comparisons are shown in Table 4.
| Erenumab 140 mg | ||||||||
| 1.02 (−4.32 to 6.49) | Galcanezumab 240 mg | |||||||
| 1.02 (−4.15 to 6.29) | 0.01 (−4.58 to 4.60) | Fremanezumab-M | ||||||
| −0.96 (−6.59 to 4.94) | 0.06 (−4.79 to 4.89) | 0.06(−4.78 to 4.75) | BTA | |||||
| 1.41 (−3.01 to 5.86) | −0.39 (−5.47 to 4.59) | −0.39 (−5.42 to 4.53) | 0.45 (−5.09 to 5.81) | Erenumab 70 mg | ||||
| 1.71 (−3.49 to 6.85) | 0.68 (−3.68 to 5.07) | 0.69 (−2.38 to 3.66) | 0.75 (−4.04 to 5.37) | 0.30 (−4.68 to 5.26) | Fremanezumab-Q | |||
| 2.33 (−2.82 to 7.76) | 1.31 (−2.02 to 4.76) | −1.31 (−5.72 to 3.21) | 1.37 (−3.53 to 6.16) | 0.92 (−4.06 to 5.88) | −0.62 (−4.95 to 3.81) | Galcanezumab 120 mg | ||
| −2.95 (−10.46 to 4.43) | −1.93 (−8.71 to 5.06) | −1.93 (−8.75 to 4.92) | 1.99 (−5.23 to 9.11) | −1.54 (−9.02 to 5.87) | −1.24 (−7.91 to 5.52) | −0.62 (−7.51 to 6.17) | Topiramate 100 mg | |
| 7.28 (3.05 to 11.65) | 6.26 (2.96 to 9.49) | 6.27 (3.09 to 9.28) | 6.32 (2.51 to 9.95) | 5.87 (2.03 to 9.87) | 5.58 (2.68 to 8.54) | 4.95 (1.91 to 8.08) | 4.33 (−1.88 to 10.50) | Placebo |
The ranking probabilities and cumulative ranking probabilities for each treatment are tabulated in Appendix 3, Tables 33 and 34. Also, plots can be found in Appendix 3, Figures 34 and 35. The result of comparing the fit of NMA (consistency) model and UMEs inconsistency model is presented in Appendix 3, Table 35. As presented in Appendix 3, Figure 36 there was no evidence of inconsistency among the data points.
Mean change in migraine-specific quality of life – preventative role
Figures 12 and 13 illustrate that all treatments were more effective than placebo. The NMA results indicate that galcanezumab 120 mg (MD: 6.97, 95% CrI: 3.79 to 10.24, SUCRA 0.88) is more effective in comparison with placebo and had a larger SUCRA, followed by BTA (MD: 5.01, 95% CrI: 1.99 to 8.01, SUCRA 0.61), galcanezumab 240 mg (MD: 5.08, 95% CrI: 1.84 to 8.35, SUCRA 0.60), erenumab 140 mg (MD: 4.93, 95% CrI: 1.70 to 8.20, SUCRA 0.60), fremanezumab-quarterly (MD: 4.29, 95% CrI: 1.90 to 6.81, SUCRA 0.49), erenumab 70 mg (MD: 4.09, 95% CrI: 0.76 to 7.31, SUCRA 0.45), topiramate 100 mg (MD: 3.78, 95% CrI −2.37 to 9.80, SUCRA 0.44), and finally fremanezumab monthly (MD: 3.89 95% CrI: 1.39 to 6.41, SUCRA 0.41). Table 5 presents all head-to-head comparisons of treatment. The ranking probabilities and cumulative ranking probabilities for each treatment are tabulated in Appendix 3, Tables 37 and 38. The corresponding plots can be found in Appendix 3, Figures 37 and 38. The result of comparing the fit of NMA model and UME model are presented in Appendix 3, Table 39. Based on the available data points, there was no indication of inconsistency (see Appendix 3, Figure 39).
| Galcanezumab 120 mg | ||||||||
| −1.95 (−6.41 to 2.37) | BTA | |||||||
| −1.89 (−5.15 to 1.44) | −0.07 (−4.56 to 4.41) | Galcanezumab 240 mg | ||||||
| −2.04 (−6.59 to 2.51) | 0.09 (−4.32 to 4.60) | −0.15 (−4.26 to 4.30) | Erenumab 140 mg | |||||
| 2.68 (−1.42 to 6.73) | 0.73 (−3.30 to 4.55) | 0.79 (−3.28to 4.87) | 0.64 (−3.44 to 4.63) | Fremanezumab-Q | ||||
| −2.88 (−7.34 to 1.49) | 0.93 (−3.51 to 5.37) | −0.99 (−5.57 to 3.64) | 0.84 (−2.69 to 4.29) | −0.20 (−4.47 to 3.81) | Erenumab 70 mg | |||
| −3.19 (−10.04 to 3.51) | 1.24 (−5.50 to 8.32) | −1.30 (−8.06 to 5.64) | −1.15 (−8.18 to 5.69) | −0.51 (−7.05 to 6.02) | −0.31 (−7.11 to 6.38) | Topiramate 100 mg | ||
| 3.08 (−1.06 to 7.14) | 1.12 (−2.71 to 5.08) | 1.19 (−2.87 to 5.17) | 1.03 (−3.08 to 5.21) | −0.39 (−2.81 to 1.96) | 0.20 (−3.88 to 4.30) | −0.11 (−6.76 to 6.27) | Fremanezumab-M | |
| 6.97 (3.79 to 10.24) | 5.01 (1.99 to 8.01) | 5.08 (1.84 to 8.35) | 4.93 (1.70 to 8.20) | 4.29 (1.90 to 6.81) | 4.09 (0.76 to 7.31) | 3.78 (−2.37 to 9.80) | 3.89 (1.39 to 6.41) | Placebo |
Mean change in migraine-specific quality of life – emotional function
The study results confirmed that all treatments are more effective than placebo. The results of mean change in MSQ-EF for each drug in comparison with placebo have been presented as forest plot and ranked by SUCRA in Figures 15 and 16. Erenumab 140 mg (MD: 8.89, 95% CrI: 3.20 to 14.55, SUCRA 0.79) was the most effective in improving of MSQ-EF and was superior to others in terms of ranking, followed by erenumab 70 mg (MD: 8.30, 95% CrI: 2.10 to 14.63, SUCRA 0.73), BTA (MD: 7.34 95% CrI: 2.90 to 11.72, SUCRA 0.67), galcanezumab 120 mg (MD: 6.90, 95% CrI: 3.42 to 10.57, SUCRA 0.63), galcanezumab 240 mg (MD: 6.59, 95% CrI: 2.87 to 10.23, SUCRA 0.60), topiramate 100 mg (MD: 6.17, 95% CrI: 0.02 to 12.52, SUCRA 0.55), and fremanezumab-quarterly (MD: 3.88, 95% CrI: 1.06 to 6.75, SUCRA 0.30), while the least effective treatment was fremanezumab monthly (MD: 3.31, 95% CrI: 0.69 to 5.95, SUCRA 0.23). Table 6 presents all head-to-head comparisons of treatment. The ranking probabilities and cumulative ranking probabilities for each treatment are tabulated in Appendix 3, Tables 41 and 42. Also, the ranking graphs are available in Appendix 3, Figures 40 and 41. The result of comparing the fit of NMA model and UME model is presented in Appendix 3, Table 43. There was no evidence of inconsistency in data points (see Appendix 3, Figure 42).
| Erenumab 140 mg | ||||||||
| 0.59 (−5.82 to 7.14) | Erenumab 70 mg | |||||||
| −1.55 (−8.60 to 5.55) | −0.97 (−8.49 to 6.75) | BTA | ||||||
| 1.99 (−4.84 to 8.74) | 1.41 (−5.89 to 8.58) | 0.44 (−5.48 to 6.13) | Galcanezumab 120 mg | |||||
| 2.30 (−4.41 to 9.12) | 1.71 (−5.70 to 9.30) | 0.74 (−4.96 to 6.49) | −0.31 (−4.08 to 3.49) | Galcanezumab 240 mg | ||||
| −2.72 (−10.92 to 5.49) | −2.13 (−11.04 to 6.98) | 1.17 (−6.56 to 8.78) | −0.73 (−7.79 to 6.61) | −0.42 (−7.52 to 6.94) | Topiramate 100 mg | |||
| 5.01 (−1.35 to 11.35) | 4.42 (−2.31 to 11.35) | 3.46 (−1.88 to 8.79) | 3.02 (−1.73 to 7.69) | 2.71 (−2.00 to 7.39) | 2.29 (−4.60 to 9.13 | Fremanezumab-Q | ||
| 5.58 (−0.77 to 11.65) | 4.99 (−3.48 to 11.74) | 4.03 (−1.10 to 9.18) | 3.59 (−1.09 to 8.25) | 3.28 (−1.31 to 7.72) | 2.86 (−3.78 to 9.31) | −0.57 (−3.31 to 2.21) | Fremanezumab-M | |
| 8.89 (3.20 to 14.55) | 8.30 (2.10 to 14.63) | 7.34 (2.90 to 11.72) | 6.90 (3.42 to 10.57) | 6.59 (2.87 to 10.23) | 6.17 (0.02 to 12.52) | 3.88 (1.06 to 6.75) | 3.31 (0.69 to 5.95) | Placebo |
Mean change in headache impact test-6
Mean change in HIT-6 from baseline was reported in seven RCTs with a total of 5763 participants including a trial comparing BTA with placebo,100 two trials evaluating eptinezumab versus placebo,89,94 two trials comparing fremanezumab with placebo,37,96 a trial investigating erenumab against placebo,119 all of which have been measured at week 12, and a trial evaluating BTA versus topiramate88,109 at week 30. As mentioned earlier, we considered the follow-up period of 12 weeks as a measurement point for NMA.
We analysed the first six trials with nine different doses of drugs compared with placebo as a reference treatment. The network plot is presented in Figure 17.
The most effective treatment in the reduction of HIT-6 estimated with the highest rank was eptinezumab 300 mg (MD: −3.22, 95% CrI: −3.59 to 2.09, SUCRA 0.93), followed by erenumab 140 mg (MD: −2.49, 95% CrI: −3.00 to −1.04, SUCRA 0.77), erenumab 70 mg (MD: −2.49, 95% CrI: −3.00 to −1.02, SUCRA 0.77), BTA (MD: −2.10, 95% CrI: −2.54 to −0.86, SUCRA 0.62), fremanezumab monthly (MD: −1.99, 95% CrI: −2.29 to −1.14, SUCRA 0.58), fremanezumab-quarterly (MD: −1.79, 95% CrI: −2.09 to −0.94, SUCRA 0.48), eptinezumab 100 mg (MD: −1.56, 95% CrI: −1.87 to −0.62, SUCRA 0.42), eptinezumab 10 mg (MD: −0.59, 95% CrI: −1.18 to 1.21, SUCRA 0.18), and the least efficacious drug was eptinezumab 30 mg (MD: −0.58, 95% CrI: −1.17 to 1.11, SUCRA 0.18) as shown in Figures 18 and 19.
Table 7 presents all head-to-head comparisons of treatment. The ranking probabilities and cumulative ranking probabilities for each treatment are tabulated in Appendix 3, Tables 45 and 46. Also plots can be found in Appendix 3, Figures 43 and 44. The result of comparing the fit of NMA model and UME model is presented in Appendix 3, Table 47. There was no evidence of inconsistency in data points as shown in Appendix 3, Figure 45.
| Eptinezumab 300 mg | |||||||||
| −0.72 (−1.34 to 1.10) | Erenumab 140 mg | ||||||||
| −0.73 (−1.36 to 1.15) | 0.00 (−0.57 to 1.68) | Erenumab 70 mg | |||||||
| 1.11 (0.52 to 2.79) | 0.39 (−0.30 to 2.32) | 0.39 (−0.29 to 2.34) | BTA | ||||||
| −1.23 (−1.72 to 0.17) | −0.50 (−1.09 to 1.15) | −0.50 (−1.09 to 1.16) | −0.11 (−0.64 to 1.43) | Fremanezumab-M | |||||
| −1.42 (−1.93 to 0.00) | −0.70 (−1.28 to 1.01) | −0.70 (−1.29 to 1.01) | −0.31 (−0.86 to 1.23) | −0.20 (−0.49 to 0.65) | Fremanezumab-Q | ||||
| −1.66 (−2.03 to −0.62) | 0.93 (0.32 to 2.66) | 0.93 (0.33 to 2.67) | −0.54 (−1.07 to 1.01) | 0.43 (0.00 to 1.69) | 0.23 (−0.23 to 1.51) | Eptinezumab 100 mg | |||
| 2.63 (2.01 to 4.44) | 1.91 (1.12 to 4.19) | 1.90 (1.14 to 4.20) | −1.52 (−2.24 to 0.54) | 1.40 (0.73 to 3.36) | 1.20 (0.52 to 3.19) | 0.97 (0.37 to 2.70) | Eptinezumab 10 mg | ||
| 2.64 (2.04 to 4.39) | 1.92 (1.13 to 4.11) | 1.91 (1.17 to 4.11) | −1.52 (−2.24 to 0.54) | 1.41 (0.76 to 3.27) | 1.21 (0.55 to 3.07) | 0.98 (0.41 to 2.67) | −0.01 (−0.67 to 1.95) | Eptinezumab 30 mg | |
| −3.22 (−3.59 to −2.09) | −2.49 (−3.00 to −1.04) | −2.49 (−3.00 to −1.02) | −2.10 (−2.54 to −0.86) | −1.99 (−2.29 to −1.14) | −1.79 (−2.09 to −0.94) | −1.56 (−1.87 to −0.62) | −0.59 (−1.18 to 1.21) | −0.58 (−1.17 to 1.11) | Placebo |
Sensitivity analysis results
The results for two sensitivity analyses for the mean change in MHDs and MMDs from baseline, excluding eptinezumab 10 and 30 mg as they are not used in routine practice, are presented below. Further results are presented at Appendix 3, Tables 48 and 49 and Figures 46–49.
Mean change in monthly headache days
The results of the MHD sensitivity analysis suggest that excluding eptinezumab 10 and 30 mg from the base-case analysis changed the observed effect size from −0.02 to +0.02. This has meant some of the ranking of treatments in the SUCRA have changed. However, the top two treatments (eptinezumab 300 mg and fremanezumab monthly) have remained the same. The reordering of the new treatments is provided in Figure 20.
FIGURE 20.
Illustrative sensitivity analysis results for mean change in MHDs from baseline.
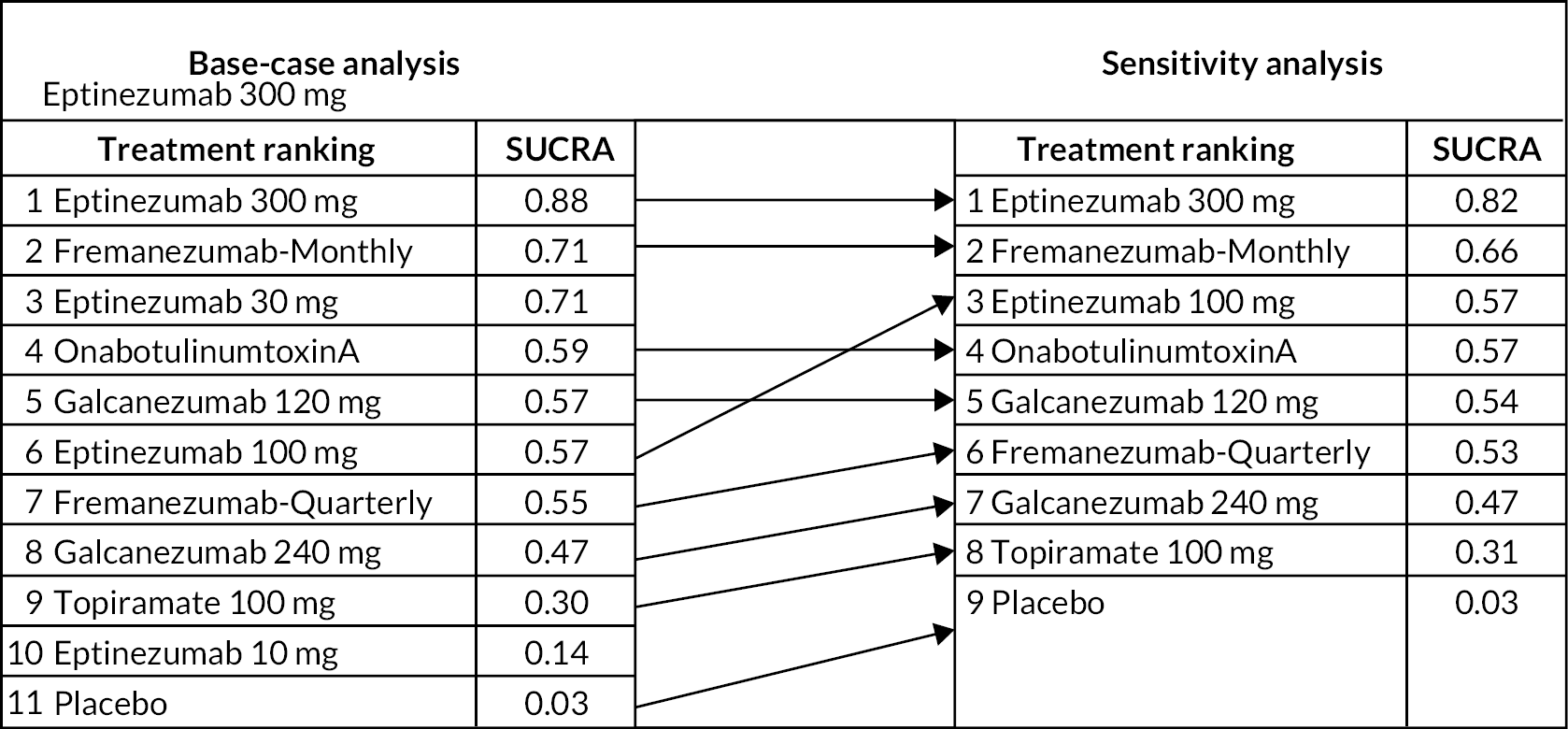
Mean change in monthly migraine days
The results of the MMD sensitivity analysis suggest that the observed effect after excluding eptinezumab 10 and 30 mg changed from −0.02 to +0.02. This has meant some of the ranking of treatments in the SUCRA have changed, including for the top two treatments: the SUCRA for eptinezumab 300 mg and fremanezumab monthly switched from 0.77 to 0.73 and 0.70 to 0.73, respectively. The reordering of the new treatments is provided in Figure 21.
FIGURE 21.
Illustrative sensitivity analysis results for mean change in MMDs from baseline.

Risk of bias in included studies
RoB assessments were undertaken using the Cochrane RoB 2 tool for randomised trials. The results of the RoB ratings by trial are summarised across the studies below and are presented in Figure 22 by RoB category. Overall, there were no major concerns that the studies were not applicable to the research question for this assessment.
FIGURE 22.
Risk of bias assessment result.

Randomisation process
Only the open label trial88 was rated as having some concerns for this domain, and other trials (10 studies) were assessed at being at low risk of bias (91%). 28,37,45,89–95
Deviations from the intended interventions
One trial (9%) was assessed as high risk of bias,88 three trials (27%) rated as having some concerns of risk of bias,45,89,95 and seven trials (64%) rated as being at low risk of bias. 28,37,90–94
Missing outcome data
The result for assessing risk of bias due to the missing outcome data was considered the same as the previous domain: one trial (9%) (high risk of bias),28 three trials (27%) (some risk of bias)37,89,95 and seven trials (64%) (low risk of bias). 45,88,90–94
Measurement of the outcome
As the measurement or ascertainment of the outcome does not have to differ between intervention groups in all trials and outcome assessors were not aware of the intervention received by study participants in most of the trials, 10 (91%) trials were rated as being at low risk of bias. 28,37,45,89–95 Only the open-label study trial was rated as having some concerns of bias due to no masking of the outcome assessor. 88
Selection of the reported result
Ten trials (91%) were in line with their pre-specified analysis plan and registered protocol, thus they were rated as being at low risk of bias,37,45,88–95 and only one trial (9.1%) was considered to have some concerns and was unclear. 28
Overall bias
Finally, the ratings for the overall risk of bias domain indicated that two trials (18%), four trials (36%) and five trials (46%) were rated as being at high,28,88 some concerns,37,45,89,95 and low risk of bias,90–94 respectively.
In summary, the studies included in our systematic review and NMA had a relative good quality in terms of risk of bias.
Certainty of evidence assessment by GRADE approach
Using the GRADE approach, we found that the certainty of evidence for each estimate was judged to be low to high (see Report Supplementary Material 2). Some estimate points were rated down by one level when the studies with high risk of bias in at least one domain contributed to the comparisons. Imprecision of some results were downgraded when the null value (zero for continuous outcomes) lies within the 95% CrI. The summary of GRADE results for outcomes of interest separately is presented in Table 8. In brief, the GRADE assessments indicated a relative certainty of evidence. The robust certainty degree was particularly highlighted for those estimations when drugs were compared with placebo.
| Outcomes | GRADE level | |||
|---|---|---|---|---|
| High | Moderate | Low | Very low | |
| MHDs | * | * | * | |
| MMDs | * | * | ||
| MSQ-RR | * | * | ||
| MSQ-PR | * | * | ||
| MSQ-EF | * | * | ||
| HIT-6 | * | * | ||
Discussion
In our analysis of 11 RCTs37,45,88–95,111 involving 7352 adult participants with chronic migraine, the results show that all pharmacological treatments for all outcomes of interest were beneficial in preventing migraine when compared to placebo. Evidence synthesis and treatments ranking was performed through a NMA, using pre-specified inclusion and exclusion criteria. The statistical analyses were conducted within a Bayesian framework using multinma package80 in R software version 4.1.381 to synthesise relevant data for each outcome of interest.
We performed six NMA for MHDs, MMDs, the three dimensions of MSQ, and the six-item HIT-6. We considered different dosing regimens for fremanezumab, galcanezumab, eptinezumab, erenumab, topiramate and BTA as separate treatments.
The results from the NMA showed that eptinezumab 300 mg and fremanezumab monthly ranked in first place in both MHD and MMD analyses (SUCRA for MHD: 0.88 and 0.71; SUCRA for MMD: 0.77 and 0.70, respectively). Eptinezumab 300 mg was the most effective drug in reduction of MHDs and also ranked as the best treatment. It should also be noted that there were no considerable differences in MHDs between eptinezumab 300 mg and eptinezumab 30 mg (MD: −2.46, 95% CrI: −3.24 to −1.67 and MD: −2.19, 95% CrI: −3.63 to −0.74, respectively). However, for eptinezumab 30 mg, which resulted in higher effect size (MD: −2.19, 95% CrI: −3.63 to −0.74) than 100 mg (MD −1.84, 95% CrI −2.60 to −1.08), this may be partly explained by the smaller sample size and the wider CIs. For MMDs, the results showed were similar to MHDs, and the best ranked treatment was for eptinezumab 300 mg; however, the most effective treatment was fremanezumab monthly. The NMA results concluded that a lower dose of erenumab (70 mg) showed a similar reduction in the MMDs as with its higher dose (140 mg). However, our clinical colleagues have noted in clinical practice that erenumab 140 mg appears to come out better; however, this is anecdotal and not evidence-based.
Furthermore, the NMA results showed that BTA ranked better in the mean change in MHD (fourth place, SUCRA: 0.59) compared with the mean change in MMD (nineth place, SUCRA: 0.44). Topiramate is ranked bottom (by a reasonable margin) in both (SUCRA: 0.3 for MHDs and 0.29 for MMDs).
The results for the three dimensions of the MSQ, and HIT-6, were provided in a NMA and the other QoL outcomes, including MIDAS, EQ-5D, PHQ-9, WPAI:SHP and FIMQ, were reported narratively. Galcanezumab 120 mg provided the best improvement in QoL for the preventative role dimension of MSQ (MSQ-PR) (MD: 6.97, 95% CrI: 3.79 to 10.24, SUCRA 0.88), but for two other dimensions including restrictive role (MSQ-RR) and emotional function (MSQ-EF), erenumab 140 mg was superior to other interventions in terms of QoL (for MSQ-RR; MD: 7.28, 95% CrI: 3.05 to 11.65, SUCRA 0.75, and for MSQ-EF; MD: 8.89, 95% CrI: 3.20 to 14.55, SUCRA 0.79). However, it was noted that the galcanezumab 120 mg showed superiority over galcanezumab 240 mg in the improvement of MSQ-PR and MSQ-EF dimensions. For the HIT-6, the results showed that eptinezumab 300 mg has the most effective treatment in reduction of the HIT-6 (MD: −3.22, 95% CrI: −3.59 to 2.09, SUCRA 0.93). It was noted that erenumab 140 mg had similar effect size with erenumab 70 mg (MD: −2.49, 95% CrI: −3.00 to −1.04, SUCRA 0.77 and MD: −2.49, 95% CrI: −3.00 to −1.02, SUCRA 0.77, respectively).
The results provided in this chapter are subject to the quality of the included studies. In this study, the results from the quality assessment found that approximately 46% of the included RCTs in this review had low RoB and 36% of the RCTs had some concerns of bias. The open label design data for BTA and topiramate carried a considerable risk of bias, but they were not incorporated into the NMA analysis due to a lack of information regarding week 12. However, the topiramate data that were included in the NMA were evaluated with a high risk of bias because it was unclear how to address the missing data. We found that the certainty of evidence for each estimate of GRADE was judged to be low to high, which highlighted the relative robustness of our findings for applying in the clinical decision-making. The main limitation of this study was the trial design for topiramate which led to grading down of certainty in MHDs, MMDs and the three dimensions of MSQ. Imprecision of estimations for eptinezumab 10 mg versus placebo resulted in downgrading in MHDs. In addition, the effect size of eptinezumab 10 and 30 mg compared with placebo gave some concerns to the imprecision.
Comparison to existing literature
To the best of our knowledge, this study is the first comprehensive NMA for pharmaceutical treatments currently available in the UK for adults with chronic migraine. Our findings for MHDs and MMDs are largely in line with a previous NMA. 72 The authors in this previous review aimed to investigate the effects of CGRP MAbs on 5164 chronic migraineurs in seven randomised trials. 72 Their focus was solely on CGRP MAbs drugs, whereas we took into account all pharmacological medications available in the UK. Our eligibility criteria allowed for the inclusion of not only CGRP MAbs, but also other drugs, such as BTA and topiramate.
In another paper, erenumab was more effective than BTA in the reduction of MMDs,120 which is in line with our results. The effectiveness of different CGRP MAbs for 3052 adult migraine patients with prior treatment failure was investigated in another review. 121 Galcanezumab 240 mg was ranked first in reducing MMDs, followed by fremanezumab monthly and then eptinezumab 300 mg. However, these findings were not in line with ours and it seems that the population with the previous treatment failures may have resulted in this discrepancy. Moreover, erenumab in our findings was ranked as the second best treatment (jointly with fremanezumab monthly) in reduction of MMDs, while in their analyses erenumab was ranked as the least effective treatment for those participants with previous treatment failures. 121 Another review and NMA aimed to assess the effect of CGRP MAbs on disability related to migraine in 7095 adult patients from nine randomised trials. 122 Fremanezumab depicted slightly better improvement in disability compared with other CGRP MAbs at 12 weeks. 122 However, our finding in improvement of MSQ score in all dimensions had the same result. Although we also compared other medications including different doses of fremanezumab, eptinezumab and BTA in our analyses, this provides a comprehensive picture of the effectiveness of different classes of drugs on participants’ QoL. Based on our results, erenumab 140 mg was the most effective treatment in the improvement of MSQ-RR and MSQ-EF but was ranked in fourth place in the effectiveness of the MSQ-PR. Our results illustrate that there are no significant differences between the two doses of erenumab in decreasing disability scores (measured by HIT-6); however, they were the second most effective treatment for MMDs.
Strengths and limitations
The main strength of this analysis is the range of migraine treatment classes including the latest treatments, such as CGRP MAbs, namely fremanezumab, eptinezumab, galcanezumab and erenumab, which are commonly used after other concurrent preventive treatments, such as BTA and topiramate have failed in the UK. This diversity can provide a comprehensive picture of the effectiveness profile of medications for decision-makers to compare migraine treatment alternatives. Therefore, this may better reflect current clinical practice. Another strength of this review is the comprehensiveness of the search strategy which was used. The search was run and updated across a broad range of electronic databases to ensure all relevant trials were included. Furthermore, we did not allow for any date or language restrictions.
However, the results of this analysis should be interpreted with caution due to its limitations. All trials included in the NMA were placebo-controlled; thus, we were not able to estimate any indirect comparisons and, hence, assess the local inconsistency. This means that there were no direct drug-to-drug comparisons in our included trials. We also included a trial which included participants who failed up to four migraine preventive drug classes90 which might result in bias in our results. Finally, we excluded studies with fewer than 100 participants per arm to include better-quality studies and to avoid loss of precision on our NMA by including heterogenous studies;55,56 hence, this excluded all other trials on oral migraine preventatives and restricted the analysis to topiramate, BTA and CGRP MAbs. This may have limited the NMA to more recently investigated treatments where the trial methodology is more precise, and which were undertaken after chronic migraine was introduced as a classification in 2007. Older trials did not separate out chronic migraine from episodic migraine or even define a difference – and including them would have resulted in a large degree of heterogeneity (e.g. between participant baseline characteristics) and results would have been at a high risk of bias and, thus, a NMA would not have been possible. Nevertheless, we might have also missed some important data by excluding these smaller trials. The quality of these older trials may be limited by a variety of factors, such as inadequate sample size, inadequate control groups and outdated methodologies. Conducting newer trials with adequate power, larger sample size and more rigorous designs can help improve our understanding of a treatment’s effectiveness and can help address these limitations of the older trials and provide more reliable and accurate results. Due to the above-mentioned restrictions to the older trials or even newer trials with no efforts to distinguish between migraine subtypes, we believe our results may have less heterogeneity and, subsequently, more precise results.
After completion of the study, we reviewed papers that we had excluded on the size criterion and we identified just one that might have been included. This study randomised 72 people to BTA or amitriptyline. It did not report on MMD, MHD or headache-related QoL. No between-group difference was found in the measures reported. 123 Another trial, that randomised 191 participants from an original target of 250, tested the addition of propranolol to topiramate in people after failure of topiramate monotherapy. This trial was stopped early on the advice of the data safety and monitoring board for futility and provides conclusive evidence that it is not worth using propranolol in this situation. 124 In our protocol design we did not consider the inclusion of trials where additional drugs were added and by default we might have included this, if the trial had reached its recruitment target. Nevertheless, it would not have fitted in our NMA, and its effect estimate does not tell us what the effect of propranolol might be when used as monotherapy. This post hoc review of excluded studies does not indicate that any relevant data have been excluded from our NMA by setting a size criterion for inclusion. The total number of trials for which we eventually extracted data was substantially fewer than we anticipated at the scoping stage because of reporting different aspects of the trials across multiple, sometimes overlapping papers, with 51 individual papers reporting just 11 trials.
In summary, the NMA findings from the included 11 RCTs indicated that pharmacological treatments are more effective than placebo in managing chronic migraine across all outcomes of interest. This review provides supportive evidence for using prophylactic medications to improve both effectiveness and QoL in chronic migraine management. According to our results, some (but not all) MAbs are better than BTA and the remainder roughly equivalent to BTA. Topiramate is worst overall. However, it is important to consider some limitations in the analyses that may affect the certainty of the results, including the lower quality of some of the included trials and the focus on larger-scale trials.
Chapter 3 Adverse events review
Research question 2: What are the comparative incidences of AEs of prophylactic drugs used for migraine?
Introduction
This chapter will explore and systematically review all published evidence on the incidence of AEs and SAEs in people with both chronic and episodic migraine. Apart from the recent trials of CGRP MAbs, AEs are poorly reported. For this reason, we extended our inclusion criteria for this review (that met the inclusion criteria for the clinical effectiveness review) to include trials with mixed populations which included episodic migraine. Thus, this allows us to give a robust estimate of the incidence of AEs in the whole population with migraine. Therefore, the list of drugs in this chapter is different from the clinical effectiveness chapter.
In this review, we applied the Common Terminology Criteria for Adverse Events (CTCAE) v5.0125 and considered the following standard definitions for AEs and SAEs.
Adverse event: An adverse event that is not a serious adverse event, meaning that it does not result in death, is not life-threatening, does not require inpatient hospitalisation or extend a current hospital stay, does not result in an ongoing or significant incapacity or interfere substantially with normal life functions, and does not cause a congenital anomaly or birth defect; it also does not put the participant in danger and does not require medical or surgical intervention to prevent one of the results listed above. 125
Serious adverse event: An adverse event that results in death, is life-threatening, requires inpatient hospitalisation or extends a current hospital stay, results in an ongoing or significant incapacity or interferes substantially with normal life functions, or causes a congenital anomaly or birth defect. Medical events that do not result in death, are not life-threatening, or do not require hospitalisation may be considered serious adverse events if they put the participant in danger or require medical or surgical intervention to prevent one of the results listed above. 125
Methods
The AEs review followed the PRISMA guidelines for reporting systematic reviews52 and the Cochrane Handbook for Systematic Reviews of Interventions. 53 The protocol for the AEs review was registered in the PROSPERO database. The registration number is CRD42021265993.
Search strategy
The search strategies for the AEs review were conducted jointly with those for the clinical effectiveness review and have already been reported in the previous chapter (see Chapter 2).
Assessing the relevance and inclusion of studies
Title and abstract screening was conducted by two reviewers (AB, SN). Screening was performed according to PICO criteria (see Box 3 for inclusion criteria and Box 4 for exclusion criteria). At this stage, the abstracts of the retrieved studies were reviewed independently by two out of four reviewers (MU, SN, AA, ND). The full texts of the remaining studies were retrieved, and the same combination of the reviewers conducted an additional round of full-text screening according to the pre-specified inclusion/exclusion criteria.
-
RCTs in any setting.
-
RCTs with more than 100 participants per arm.
-
Adults (≥ 18 years old) with chronic or episodic migraine.
-
Available or anticipated to be available pharmacological medications in the UK: CGRP MAbs, BTA, antidepressants, ACE inhibitors and angiotensin receptor blockers, beta-blockers, calcium channel blockers, pizotifen, flunarizine and anti-convulsants (topiramate, valproate/divalproex, gabapentin).
-
Placebo, or
-
Usual care, or
-
Other prophylactic drugs.
-
Adverse events and treatment-related adverse events (TAEs).
-
Serious adverse events and treatment-related serious adverse events (TSAEs).
-
Non-randomised trials, quasi-randomised trials, observational studies (e.g. case reports and case series), subgroup analysis and other designs.
-
RCTs with fewer than 100 per arm.
-
Children and young people aged < 18 years.
-
Participants with menstrual migraine, acute migraine, abdominal migraine, vestibular migraine, or any other conditions-related migraine.
-
Trials that examined participants with other primary headaches including tension-type headaches, cluster headaches, and all sorts of secondary headaches.
-
Studies comparing cognitive–behavioural therapy, psychological interventions, exercise, dietary and relaxation.
-
Studies which were dose–response trials.
-
Studies comparing different preparations of the same drug in the absence of placebo.
-
Laboratory studies without clinical outcomes.
-
Chinese traditional medicines, that is, herbal medicine/drugs and other herbal remedies which are not prescribed in the UK.
-
Drugs which are not prescribed by NHS or recommended by NICE or SMC.
-
Events data reported as discontinuation and withdrawal from trials.
Data extraction
Data for included studies were extracted by one reviewer (SN) and 20% randomly checked for accuracy by another reviewer (SK). Data extraction forms were developed in Microsoft Excel to capture the following information: ClinicalTrials.gov identifier (NCT number), study name, study characteristics including first author, year, purpose, design, date, setting and country, treatments details, participant demographics, key inclusion and exclusion criteria, AEs and SAEs definition, and information on AEs, TAEs, SAEs and TSAEs.
Assessment of risk of bias for included trials
The Cochrane RoB 2 tool for RCTs58 was applied for assessing the risk of bias of all trials independently by two members (SN, SK). The details of the tool in classifying the risk of bias in the various domains have been provided in Chapter 2.
Data synthesis
Information extracted from the included studies was summarised and tabulated. We applied the CTCAEs v5.0125 to classify the events. In addition, AEs and SAEs were pooled and the proportion of AEs and SAEs for each system organ class (SOC) for each drug was calculated where the original paper used the standard definition for AEs and SAEs.
We reported the adverse and serious adverse events from the rest of the studies (AEs from 11 studies and SAEs from 6 trials) separately, as these studies did not report events according to standard definitions for AEs and SAEs.
Results
Included studies
Study selection
The PRISMA flow diagram in Figure 23 summarises the results of our searches for the AE review. Of the 344 records which were assessed for eligibility, 277 records were excluded at full text. We identified 67 articles which described data from 40 trials22,28,35–37,45,88–95,97,107,108,117,118,126–152 for the AEs review. Although these linked articles were cited, we used the main trial paper for the main citation, as the other linked papers only reported some subgroup analyses, were either repetitive or combined the data.
FIGURE 23.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram summarising the flow of studies for the AEs review.

Study characteristics
Sixty-seven articles from 40 RCTs met the eligibility criteria to assess the AE and SAE incidences in adult with migraine (chronic or episodic). These trials evaluated 35 different dosing regimens of 12 drugs including:
-
CGRP MAbs (eptinezumab 10, 30, 100 and 300 mg, erenumab 70 and 140 mg, fremanezumab 225 and 675 mg, and galcanezumab 120, 150 and 240 mg).
-
BTA 7, 25, 40, 50, 75, 155 and 260U.
-
Topiramate 100 and 200 mg.
-
Flunarizine 5 and 10 mg.
-
Propranolol 40 and 160 mg.
-
Atogepant 10, 30 and 60 mg.
-
Amitriptyline 50 and 100 mg.
-
Divalproate 200 and 1000 mg.
-
Rimegepant 75 mg.
The study-level characteristics of the included trials are summarised in Table 9 and Appendix 4, Table 50. The participants randomised in all trials satisfied the diagnostic criteria of chronic or episodic migraine in accordance with the ICHD. 6
| Author, year | Purpose | Country and setting | Chronic/episodic | Treatment duration (week) and study design | Treatment | Number of participants (ITT) | Female (%) | Mean age | % any AEs (% TAEs) | % any SAEs (% TSAEs) | Conclusion | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Dose | Route of administration | Frequency | ||||||||||||
| Couch, 201122 | To compare amitriptyline in doses of 25–100 mg/day, depending on the tolerance of the patient, with a matched placebo | 10 American centres | Episodic | 20 DB | Placebo | – | – | – | 197 | 83 | 35.7 | 26.9 | 0 | AEs were seen roughly twice as often in the amitriptyline group | |
| Amitriptyline | 25–100 mg | Oral | One to four pills per day | 194 | 79 | 34.1 | 57.2 | 15.4 | |||||||
| Kalita, 2013137 | To compare efficacy and safety of divalproex extended release (DVA-ER) and amitriptyline (AMT) in migraine | At a tertiary care teaching hospital, and patients enrolled from the neurology outpatient service, India | Episodic | 12 OL | Divalproate | 250–1000 mg | Oral | Daily | 143 | 82 | 31.03 | 47.6 | – | The composite side effects were also not different between the two groups | |
| Amitriptyline | 12.5–50 mg | Oral | Daily | 144 | 78.7 | 32.8 | 56.3 | – | |||||||
| Dodick, 2009135 | To compare the efficacy and tolerability of topiramate and amitriptyline in the prophylaxis of EM | 32 sites in the USA | Episodic | 22 DB | Topiramate | 100 mg | Oral | Twice daily | 177 | 86.6 | 39.7 | 85.9 (68.4) | 2.3 (0) | Both appeared to be well tolerated in this population with EM | |
| Amitriptyline | 100 mg | Oral | Twice daily | 169 | 83 | 37.9 | 88.8 (75.7) | 4.7 (0.5) | |||||||
| Diener, 2002136 | To assess the efficacy and tolerability of two doses of flunarizine in the prophylaxis of migraine, in comparison with slow-release propranolol | 8 European countries | Episodic | 16 DB | Flunarizine | 5 mg | Oral | Once daily | 263 | 79 | – | 33.5 | 0.4 | No significant differences between the three treatments were found about safety: all three treatments were generally well tolerated and safe | |
| 10 mg | Oral | Once daily | 275 | 82 | – | 32 | 1.8 | ||||||||
| Propranolol | 160 mg | Oral | Once daily | 270 | 83 | – | 32.6 | 0.7 | |||||||
| Lucking, 1988128 | To compare efficacy and tolerance of flunarizine and propranolol in the prophylaxis of migraine | 99 medical practices in Germany | Episodic | 16 DB | Flunarizine | 10 mg | Oral | Once daily | 166 | 83.7 | 42 | 24.6 | 0 | Tolerance of flunarizine was similar to propranolol | |
| Propranolol | 40 mg | Oral | Three times a day | 170 | 80 | 42 | 29.6 | 0 | |||||||
| Diener,a 2007152 | To assess the effects of discontinuation of topiramate after a treatment period of 6 months | 88 neurology clinics in 21 European countries and the Middle East | Episodic | 26 DB | Placebo | – | – | – | 258 | 89 | 40.1 | 59 | 4 | Satisfaction with tolerability was similar in both treatment groups | |
| Topiramate | 200 mg | Oral | Twice per day | 254 | 85 | 40.1 | 68 | 3 | |||||||
| Lipton, 2011139 | To evaluate whether topiramate would prevent the transformation of EM to chronic daily headache (CDH) in patients with a HFEM | At 81 sites in the USA | HFEM | 26 DB | Placebo | – | – | – | 185 | 91.2 | 40.9 | 73.5 | 2.7 (0.5) | Topiramate was generally well tolerated | |
| Topiramate | 100 mg | Oral | Twice daily [two 25-mg tablets (50 mg)] | 176 | 86.8 | 39.6 | 82.4 | 1.7 (1.1) | |||||||
| Silberstein, 200728 | To evaluate the efficacy and safety of topiramate compared with placebo for the treatment of CM | 46 clinics/sites in the USA | Chronic | 16 DB | Placebo | – | – | – | 153 | 86.9 | 38.6 | 70.2 | 0 | Topiramate is safe and generally well tolerated | |
| Topiramate | 100 mg | Oral | Twice daily | 153 | 83.7 | 37.8 | 82.5 | 0 | |||||||
| Fazlalizadeh, 2008147 | To determine the comparative efficacy of topiramate and sodium valproate in the management of migraine headache | One hospital in Iran | Episodic | 4 DB | Topiramate | 100 mg | Oral | Daily | 284 | – | – | 14 | – | No statistically significant differences between therapeutic safety of sodium valproate and topiramate | |
| Sodium valproate | 200 mg | Oral | Daily | 285 | – | – | 14.4 | – | |||||||
| Aurora, 2007132 | To evaluate the safety and efficacy of multiple treatments of BTA for EM | 20 North American study centres | Episodic | 36 DB | Placebo | – | – | – | 182 | - | 43.9 | 59.9 (21.4) | (0. 2) | Multiple treatments with BTA were shown to be safe and well tolerated over an active treatment period lasting 9 months | |
| BTA | 105–260U | IM | Every 12 weeks | 187 | - | 46 | 81.3 (60.4) | 0 | |||||||
| Dodick, 201097 (pooled Aurora, 2010,92 Diener, 2010 93) |
To assess efficacy, safety and tolerability of BTA as headache prophylaxis in adults with CM. | 56 sites in North America | Chronic | 24 DB | Placebo | – | – | – | 687 | 85.2 | 41.5 | 51.7 (13.7) | 2.3 (0) | BTA treatments were safe and well tolerated | |
| BTA | 155U +40U |
IM at 39 sites | Every 12 weeks | 692 | 87.6 | 41.1 | 62.4 (33.4) | 4.8 (0.3) | |||||||
| Elkind,b 2006145 | To examine the effects of multiple treatments with low doses of BTA versus placebo for prophylaxis of EM | – | Episodic | 12 DB | Study I | Placebo | – | – | – | 106 | 84.9 | 43.8 | 47.2 (6.6) | (0) | AEs were similar among the groups within each study. BTA was safe and well tolerated |
| BTA | 7U | IM | Every 4 months | 105 | 84.3 | 44.3 | 49.5 (6.7) | (0) | |||||||
| 25U | IM | Every 4 months | 101 | 82.2 | 43.6 | 46.5 (21.8) | (0) | ||||||||
| 50U | IM | Every 4 months | 106 | 86.8 | 44.6 | 56.6 (30.2) | (0) | ||||||||
| Study II | BTA | 25U | IM | Every 4 months | 173 | – | – | 78 (24.9) | (0) | ||||||
| 50U | IM | Every 4 months | 180 | – | – | 77.2 (29.4) | (0) | ||||||||
| Study III | Placebo | – | – | – | 100 | – | – | 60 | (0) | ||||||
| BTA | 25U | IM | Every 4 months | 50 | – | – | 70 | (0) | |||||||
| 50U | IM | Every 4 months | 51 | – | – | 68.6 | (0) | ||||||||
| Relja, 2007138 | To evaluate the safety and efficacy of onabotulinumtoxinA for prophylaxis of EM | At 37 study centres in 9 countries | Episodic | 36 DB | Placebo | – | – | – | 118 | – | 42.4 | 54.2 (31.4) | 1.7 (0) | BTA was safe and well tolerated but did not result in significantly greater improvement than placebo | |
| BTA | 225U | IM | Every 12 weeks | 129 | – | 42..8 | 76.7 (67.4) | 1.5 (0) | |||||||
| 150U | IM | Every 12 weeks | 125 | – | 44.9 | 77.6 (63.2) | 1.62 (0) | ||||||||
| 75U | IM | Every 12 weeks | 123 | – | 42.8 | 77.2 (62.6) | 0.81 (0) | ||||||||
| Rothrock, 201988 | To compare the effectiveness of BTA and topiramate for CM prevention | USA (number of sites is not reported) | Chronic | 24 OL | BTA | 155U | IM | Every 12 weeks | 140 | 84 | 40.2 | 48 (17) | 2 (0) | BTA is safe; (51% of patients discontinued topiramate due to AEs) | |
| Topiramate | 100 mg | Oral | Twice daily | 142 | 86 | 39.4 | 79 (70) | 4 (1) | |||||||
| Ashina, 2020131 | To evaluate the efficacy and safety of eptinezumab in the preventive treatment of EM | 84 sites in the USA and the Republic of Georgia | Episodic | 24 DB | Placebo | – | – | – | 222 | 83.8 | 39.9 | 59.5 | 0. 4 | Eptinezumab was well tolerated, and had an acceptable safety profile | |
| Eptinezumab | 30 mg | IV | Every 12 weeks | 219 | 84.5 | 39.1 | 58.4 | 1.83 (0) | |||||||
| 100 mg | IV | Every 12 weeks | 223 | 80.3 | 40 | 63.2 | 1.79 (0) | ||||||||
| 300 mg | IV | Every 12 weeks | 224 | 88.8 | 40.2 | 57.6 | 1.34 (0) | ||||||||
| Ashina, 2022151 | To investigate the safety and efficacy of eptinezumab for migraine prevention in adults with migraine and two to four previous failures | 96 study locations across Europe (n = 93) and the USA (n = 3) | Episodic and chronic | 24 DB | Placebo | – | – | – | 298 | 88 | 43.8 | 40 | 1.3 (0) | The safety and tolerability of eptinezumab were similar to placebo | |
| Eptinezumab | 100 mg | IV | Every 12 weeks | 299 | 93 | 44.6 | 42 | 1.7 (0) | |||||||
| 300 mg | IV | Every 12 weeks | 294 | 89 | 43.1 | 41 | 2.4 (0.7) | ||||||||
| Dodick, 201989 | To determine the safety, tolerability and effectiveness of four dose levels of eptinezumab and to inform the phase 3 development programme | 92 clinics/sites in the USA, Australia, New Zealand and the Republic of Georgia | Chronic | 12 DB | Placebo | – | – | – | 121 | 90 | 37.2 | 56.2 (14) | 0.8 (0) | Eptinezumab appeared effective and well tolerated | |
| Eptinezumab | 300 mg | IV | Single dose | 121 | 81 | 37.2 | 63.6 (17.4) | 5.8 (0) | |||||||
| 100 mg | IV | Single dose | 122 | 85 | 36.7 | 57.5 (19.8) | 3.3 (0) | ||||||||
| 30 mg | IV | Single dose | 122 | 91 | 35.7 | 45.9 (14.8) | 0 | ||||||||
| 10 mg | IV | Single dose | 130 | 87 | 36.4 | 56.9 (16.2) | 0.8 (0) | ||||||||
| Lipton, 202094 | To evaluate the efficacy and safety of eptinezumab, in the preventive treatment of CM | 128 sites in 13 countries across the USA and Europe |
Chronic | 24 DB | Placebo | – | – | – | 366 | 88.8 | 39.6 | 46.7 | 0.81 | The day after IV administration through week 12, was well tolerated, and demonstrated an acceptable safety profile | |
| Eptinezumab | 300 mg | IV | Single dose | 350 | 89.7 | 41 | 52 | 1.1 | |||||||
| 100 mg | IV | Single dose | 356 | 86.2 | 41 | 43.5 | 0.84 | ||||||||
| Winner, 2021149 | To evaluate the efficacy and safety of the preventive migraine treatment, eptinezumab, initiated during a migraine attack | 47 sites in the USA and the Republic of Georgia | Episodic | 4 DB | Placebo | – | – | – | 242 | 83.1 | 44.1 | 10.3 | 0 | No notable safety findings were identified | |
| Eptinezumab | 100 mg | IV | Single dose on day 0 | 238 | 84.9 | 44.9 | 10.9 | 0 | |||||||
| Dodick, 2018134 | To evaluate the efficacy and safety of AMG 334 in migraine prevention | 69 sites (neurology clinics, and clinical research sites) across North America and Europe (including Russia) | Episodic | 12 DB | Placebo | – | – | – | 289 | 84.9 | 42 | 54.7 | 1.7 | AEs were similar in both, and did not suggest any particular safety risk with erenumab administration | |
| AMG 334 (erenumab) | 70 mg | SC | Once a month | 283 | 85.7 | 42 | 48.1 | 1.1 | |||||||
| Goadsby, 201736 | To compare the efficacy and safety of erenumab for the preventive treatment of EM | 121 sites across North America, Europe and Turkey | Episodic | 24 DB | Placebo | – | – | – | 319 | 85.9 | 41.3 | 63 | 2.2 | The overall safety profile of erenumab was similar to that of placebo | |
| Erenumab | 70 mg | SC | Monthly | 314 | 84.5 | 41.1 | 57.3 | 2.5 | |||||||
| 140 mg | SC | Monthly | 319 | 85.3 | 40.4 | 55.5 | 2.5 | ||||||||
| Reuter, 2018143 | To compare the efficacy and tolerability of erenumab with placebo in a well-defined group of patients with EM | 59 sites in 16 countries | Episodic | 12 DB | Placebo | – | – | – | 124 | 82 | 44.2 | 54 | 1 | The tolerability and safety profiles of erenumab and placebo were similar | |
| Erenumab | 140 mg | SC | Monthly | 119 | 80 | 44.6 | 55 | 2 | |||||||
| Reuter, 2022142 | To compare the tolerability and efficacy of erenumab to topiramate for migraine in adults | 82 sites in Germany | Episodic and chronic | 24 DB | Erenumab | 140 mg | SC | Monthly | 388 | 85.3 | 40.8 | 65.21 (55.4) | 2.58 (0.3) | Erenumab demonstrated a favourable tolerability and efficacy profile compared to topiramate | |
| Topiramate | 100 mg | Oral | Daily | 388 | 86.3 | 40.7 | 85.31 (81.2) | 4.9 (0.5) | |||||||
| Sun, 2016130 | To assess the safety and efficacy of erenumab (AMG 334) for the prevention of migraine | 59 headache and clinical research centres in North America and Europe | Episodic | 12 DB | Placebo | – | – | – | 153 | 83 | 41.4 | 54 | 0 | No apparent association was recorded between patients with positive anti-AMG 334 antibodies and AEs | |
| AMG 334 (Erenumab) |
7 mg | SC | Monthly | 108 | 81 | 40.3 | 50 | 1 (0) | |||||||
| 21 mg | SC | Monthly | 105 | 81 | 39.9 | 51 | 0 | ||||||||
| 70 mg | SC | Monthly | 106 | 77 | 42.6 | 54 | 1 (0) | ||||||||
| Tepper, 201745 | To assess the safety and efficacy of erenumab 70 mg and 140 mg in CM patients | 69 headache and clinical research centres in Canada, the USA and Europe | Chronic | 12 DB | Placebo | – | – | – | 286 | 79 | 42.1 | 39 | 2 | Erenumab 70 and 140 mg have a safety profile similar to placebo | |
| Erenumab | 70 mg | SC | Monthly | 191 | 87 | 41.4 | 44 | 3 | |||||||
| 140 mg | SC | Monthly | 190 | 84 | 42.9 | 47 | 1 | ||||||||
| Wang, 2021 144 |
To evaluate the efficacy and safety of erenumab in adults with EM | 83 sites in Asia, the Middle East and Latin America | Episodic | 12 DB | Placebo | – | – | – | 335 | 83.1 | 38 | 36.7 (9.6) | 1.5 (0) | The safety profile of erenumab was comparable with placebo; no new safety signals were observed | |
| Erenumab | 70 mg | SC | Monthly | 335 | 80.5 | 37.3 | 34.9 (11.3) | 2.9 (0.3) | |||||||
| 140 mg | SC | Monthly | 224 | 82.1 | 37.1 | 34.4 (10.7) | 0 | ||||||||
| Dodick, 201835 | To compare the efficacy and safety of fremanezumab for the preventive treatment of EM | 123 investigative sites in 9 countries | Episodic | 12 DB | Placebo | – | – | – | 293 | 84 | 41.3 | 58.4 (37.2) | 2.4 | The most common AE reported was injection site pain, greater incidence with fremanezumab than with placebo | |
| Fremanezumab | 675 mg | SC | Single dose | 291 | 86.3 | 41.1 | 66.3 (47.1) | 1 | |||||||
| 225/225/225 mg | SC | Monthly | 289 | 84.1 | 42.9 | 66.2 (47.6) | 1 | ||||||||
| Ferrari, 201990 | To investigate the efficacy and tolerability of fremanezumab in patients with difficult-to-treat episodic or chronic migraine | 104 sites in Europe and the USA | Chronic and episodic | 12 DB | Placebo | – | – | – | 279 | 84 | 46.8 | 48 (20) | 1 (0) | Fremanezumab was well tolerated in patients with difficult-to-treat migraine who had previously not responded to up to four classes of migraine preventive medications | |
| Fremanezumab | 675 mg | SC | Single dose | 276 | 83 | 45.8 | 55 (21) | 0.7 (0) | |||||||
| 225 + 225 + 225 mg | SC | Monthly | 283 | 84 | 45.9 | 45 (19) | 1 (0) | ||||||||
| Sakai, 202191 | To determine the efficacy and safety of fremanezumab administration in Japanese and Korean patients with CM | 67 institutions in Japan and Korea | Chronic | 12 DB | Placebo | – | – | – | 191 | 85.3 | 42.1 | 61.8 (28.3) | 0.5 (0) | Fremanezumab was well tolerated. No safety signal was detected | |
| Fremanezumab | 675 mg | SC | Single dose | 191 | 86.4 | 43.5 | 61.1 (32.1) | 0.5 (0) | |||||||
| 225 + 225 + 225 mg | SC | Monthly | 189 | 86.2 | 42.7 | 61.7 (29.3) | 1.6 (0) | ||||||||
| Sakai, 2021126 | To evaluate the efficacy and safety of fremanezumab in Japanese and Korean patients with EM | 57 institutions in Japan and 10 institutions in Korea | Episodic | 12 DB | Placebo | – | – | – | 117 | 85.5 | 44.2 | 65.8 (23.9) | 0 | No new safety concerns for fremanezumab in Japanese and Korean patients with EM | |
| Fremanezumab | 675 mg | SC | Single dose | 118 | 84.9 | 41.9 | 62.7 (28.9) | 0 | |||||||
| 225 + 225 + 225 mg | SC | Monthly | 121 | 83.5 | 44.4 | 57 (26.4) | 0 | ||||||||
| Silberstein, 201737 |
To compare two fremanezumab dosing regimens with placebo for the prevention of CM | 132 sites in 9 countries across the USA and Europe | Chronic | 12 DB | Placebo | – | – | – | 375 | 88 | 41.4 | 64 | 1.7 (0) | Injection-site reactions to fremanezumab were common. The long-term durability and safety of fremanezumab requires further study | |
| Fremanezumab | 675 mg | SC | Single dose | 376 | 88 | 42 | 70 | 0.8 (0) | |||||||
| 225 + 225 + 225 mg | SC | Monthly | 379 | 87 | 40.6 | 71 | 1.3 (0) | ||||||||
| Bo Hu, 2022150 | To assess the efficacy and safety of galcanezumab in patients with EM from China, India and Russia | 40 centres in China (n = 26), India (n = 10), and Russia (n = 4) | Episodic | 12 DB | Placebo | – | – | – | 259 | 75.7 | 36.8 | 43.2 | 1.54 | Galcanezumab 120 mg once monthly was well tolerated in patients with episodic migraine | |
| Galcanezumab | 120 mg (240 mg in the first month followed by 120 mg) | SC | Monthly | 261 | 72 | 37.2 | 49.8 | 0.76 | |||||||
| Detke, 201895 | To evaluate the efficacy and safety of Galcanezumab in the preventive treatment of CM | 116 headache and clinical research centres in Argentina, Canada, Czech Republic, Germany, Israel, Italy, Mexico, Netherlands, Spain, Taiwan, UK and USA | Chronic | 12 DB | Placebo | – | – | – | 558 | 87 | 41.6 | 50 | 0.71 | Galcanezumab appears safe, and well tolerated for the preventive treatment of CM | |
| Galcanezumab | 120 mg | SC | Monthly | 278 | 85 | 39.7 | 58 | 0.18 | |||||||
| 240 mg | SC | Monthly | 277 | 82 | 41.1 | 57 | 1.8 | ||||||||
| Dodick, 2014133 | To assess the safety and efficacy of galcanezumab (LY2951742) for migraine prevention | 35 centres in the USA | Episodic | 12 DB | Placebo | – | – | – | 110 | 87 | 41.9 | 67 | 3.6 | AEs were reported to a similar extent in both groups | |
| Galcanezumab | 150 mg | SC | Every 2 weeks | 107 | 82 | 40.9 | 72 | 1.9 | |||||||
| Mulleners, 2020146 | To assess the safety and efficacy of galcanezumab in patients with migraine who had not benefited from preventive medications from two to four categories. | 64 sites (hospitals, clinics or research centres) in 12 countries | Episodic and chronic | 12 DB | Placebo | – | – | – | 230 | 88 | 45.7 | 53 (15) | 1 | Galcanezumab was safe and well tolerated in patients for whom multiple previous standard-of-care preventive treatments had failed | |
| Galcanezumab | 120 mg | SC | Monthly | 232 | 84 | 45.9 | 51 (16) | 1 | |||||||
| Sakai, 2020127 | To assess the efficacy and safety of galcanezumab in comparison with placebo for the prevention of migraine in Japanese patients with EM | 40 sites in Japan | Episodic | 24 DB | Placebo | – | – | – | 230 | 85.2 | 44.2 | 64.8 | 0 | Galcanezumab was safe and well tolerated in Japanese patients with episodic migraine | |
| Galcanezumab | 120 mg | SC | Monthly | 115 | 82.6 | 43.2 | 85.2 | 2.6 | |||||||
| 240 mg | SC | Monthly | 114 | 84.2 | 44.8 | 81.6 | 0.9 | ||||||||
| Skljarevski, 2018141 | To evaluate the efficacy and safety of two dosing regimens of galcanezumab in patients with EM | 109 study sites in 12 countries | Episodic | 24 DB | Placebo | – | – | – | 461 | 85.3 | 42.3 | 62.3 | 1.1 | Galcanezumab 120 or 240 mg given once monthly was safe and well tolerated | |
| Galcanezumab | 120 mg | SC | Monthly | 226 | 85.3 | 40.9 | 65.0 | 2.2 | |||||||
| 240 mg | SC | Monthly | 228 | 85.7 | 41.9 | 71.5 | 3.1 | ||||||||
| Stauffer, 2018140 | To demonstrate that Galcanezumab is superior to placebo in the prevention of EM with or without aura | 90 sites in North America | Episodic | 24 DB | Placebo | – | – | – | 432 | 83.6 | 41.3 | 60.4 | 1.16 (0) | The incidence rate of AEs was low, showing the favourable tolerability profile of galcanezumab | |
| Galcanezumab | 120 mg | SC | Monthly | 206 | 85 | 40.9 | 65.5 | 2.91 (0) | |||||||
| 240 mg | SC | Monthly | 220 | 82.6 | 39.1 | 67.7 | 0 (0) | ||||||||
| Ailani, 2021129 | To examine the efficacy and safety of atogepant compared with placebo for the prevention of migraine in participants with EM | 128 sites in the USA | Episodic | 12 DB | Placebo | – | – | – | 222 | 89.2 | 40.3 | 56.8 (9) | 0.9 (0) | Most common AEs were constipation and nausea across atogepant | |
| Atogepant | 10 mg | Oral | Once daily | 221 | 90.5 | 41.4 | 52.9 (23.1) | 0.9 (0.5) | |||||||
| 30 mg | Oral | Once daily | 228 | 89.5 | 42.1 | 52.2 (14.9) | 0 | ||||||||
| 60 mg | Oral | Once daily | 231 | 86.1 | 42.5 | 53.7 (19.5) | 0 | ||||||||
| Croop, 2021148 | To compare the efficacy of rimegepant with placebo for preventive treatment of migraine. | 92 sites in the USA | Episodic | 12 DB | Placebo | – | – | – | 371 | 84 | 41.1 | 36 (9) | 1 (0.26) | Tolerability was similar to that of placebo, and no unexpected or serious safety issues were noted | |
| Rimegepant | 75 mg | Oral | Daily | 370 | 81 | 41.3 | 36 (11) | 1 (0) | |||||||
Only two trials were conducted in a single site (Iran and India);137,147 the remainder were multicentre studies from a list of countries. Twenty-seven trials included only participants with episodic migraine and nine trial studies included only participants with chronic migraine. Four trials had a mixed population of chronic and episodic migraine. The number of participants randomised across the 40 trials evaluating the safety of pharmacological treatment ranged from 217133 to 137997 with a total of 25,891 participants. The mean age of trial participants ranged from 32137 to 4690 years; and the percentage of female participants ranged from 74%150 to 91%. 151
Only two trials were designed as open-label treatment trials. 88,137 Most trials were double-blind trials. Treatment duration varied across the trials, from 2 trials which had a 4-week treatment duration,147,149 3 trials reported 16 weeks,28,128,136 19 trials reported 12 weeks,35,37,45,89–91,95,127,129,130,133,134,137,143–146,148,150 1 trial reported 20 weeks,22 1 trial reported 22 weeks,110 10 trials reported 24 weeks,36,88,94,97,127,131,140–142,151 2 trials reported 26 weeks139,152 and 2 final trials reported 36 weeks. 132,138
In summary, the majority of included trials were conducted across multi sites in a list of countries for the episodic migraine population and the trials were double blind with a 12-week duration.
Risk of bias in included studies
Risk-of-bias ratings by trial are summarised across the studies below and are presented in Figure 24. For this purpose, the Cochrane RoB 2 tool for RCTs58 was applied.
FIGURE 24.
Risk of bias assessment result.

Randomisation process
Two trials88,128 were rated as having some concerns (3%) and high level of risk (3%) for this domain, and the other trials (n = 38) were assessed at being at low risk of bias (94%). 22,28,35–37,45,89–91,94,95,97,126,127,129–152
Deviations from the intended interventions
Two trials (5%)88,137 were assessed as high risk of bias, and 38 trials (95%) were rated as being at low risk of bias. 22,28,35–37,45,89–91,94,95,97,126–136,138–152
Missing outcome data
Assessment for missing outcome data showed four trials (10%)37,95,131,147 had some concerns, and 36 trials (90%) trials were assessed as low risk of bias. 22,28,35,36,45,88–91,94,97,126–130,132–146,148–152
Measurement of the outcome
Outcome assessors were aware of the intervention received by study participants in the 21 trials, hence, 53% of trials were rated as having some concerns22,88,89,128,132,133,135,137–143,145–148,150–152 and 19 trials (47%) were rated as being at low risk of bias. 28,35–37,45,90,91,94,95,97,126,127,129–131,134,136,144,149
Selection of the reported result
Twenty-nine trials (73%) adhered to their pre-specified analysis plan and registered protocol. Thus, they were rated as being at low risk of bias,35–37,45,88–91,94,95,97,126,127,129–134,140–144,146,148–151 and 11 trials (27%) were considered to have some concerns and were assessed as ‘unclear’ as the trial protocol was unavailable. 22,28,128,135–139,145,147,152
Overall risk of bias assessment
The ratings for the overall risk of bias domain indicated that 3 trials (7%), 23 trials (58%) and 14 trials (35%) were rated as being at high,88,128,137 some concerns,22,28,37,89,95,131–133,135,136,138–143,145–148,150–152 and low risk35,36,45,90,91,94,97,126,127,129,130,134,144,149 of bias, respectively (see Figure 24).
In brief, most of the included RCTs were assessed with some concerns regarding the risk of bias.
Adverse events results
Of the 40 included trials, 29 trials with 20,694 participants applied a standard definition for AEs which we use in our review. We used SOC to classify and illustrate the proportion of attributed AEs to each drug. A list of classified AEs are presented in Appendix 5, Table 51. Appendix 5, Table 68 shows the percentage of total incidence of any AEs reported for 20 different dosing regimens of 9 drugs. Among them, the most reported AEs belonged to amitriptyline 25–100 mg and galcanezumab 150 mg with 89%133,135 and 72.0%,133 respectively. The lowest number of any AEs is for erenumab 140 mg (33%). 36,45,142–144 Arm level AEs incidence are presented in Appendix 5, Tables 52–67.
Table 10 provides a detailed summary of classified AEs reported in 29 trials. The table illustrates the percentage of attributed AEs of each SOC.
| Treatments | Doses | Participants (N) | Investigations (%) | Skin and subcutaneous (%) | Gastrointestinal disorders (%) | Ear and labyrinth disorders (%) | Eye disorders (%) | Psychiatric disorders (%) | Metabolism and nutrition disorders (%) | Vascular disorders (%) | Renal and urinary disorders (%) | Musculoskeletal and connective tissue disorders (%) | Nervous system disorders (%) | Infection and infestation (%) | General disorders and administration site conditions (%) | Respiratory, thoracic and mediastinal disorders (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline135 | 25–100 mg | 169 | 23 (13.6) | 0 | 100 (59.2) | 0 | 0 | 0 | 8 (4.7) | 0 | 0 | 0 | 73 (43.4) | 40 (23.7) | 41 (24.3) | 7 (4.1) |
| Atogepant129 | 10 mg | 221 | 8 (3.7) | 0 | 28 (12.7) | 0 | 0 | 2 (0.9) | 0 | 0 | 0 | 0 | 7 (3.2) | 25 (11.4) | 3 (1.4) | 1 (0.5) |
| 30 mg | 228 | 4 (1.8) | 0 | 26 (11.4) | 0 | 0 | 1 (0.4) | 0 | 0 | 0 | 0 | 4 (1.8) | 40 (17.5) | 7 (3.1) | 2 (0.9) | |
| 60 mg | 231 | 9 (3.9) | 0 | 30 (13) | 0 | 0 | 5 (2.2) | 0 | 0 | 0 | 0 | 4 (1.7) | 39 (17) | 9 (3.9) | 4 (1.7) | |
| BTA88,97 | 150U | 907 | 0 | 0 | 1 (0.1) | 0 | 29 (3.2) | 5 (0.5) | 0 | 0 | 0 | 141 (15.6) | 5 (5.0) | 14 (1.5) | 23 (2.5) | 0 |
| Eptinezumab89,94,131,149,151 | 100 mg | 1238 | 5 (0.4) | 0 | 32 (2.6) | 0 | 0 | 0 | 0 | 0 | 0 | 19 (1.5) | 38 (3.1) | 148 (12) | 26 (2.1) | 19 (1.5) |
| Eptinezumab89,94,131,151 | 300 mg | 989 | 0 | 0 | 47 (4.8) | 0 | 0 | 0 | 0 | 0 | 0 | 9 (0.9) | 18 (1.8) | 191 (19.3) | 20 (2) | 17 (1.7) |
| Eptinezumab89 | 10 mg | 130 | 0 | 0 | 6 (4.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 (10) | 23 (17.7) | 0 | 4 (3.1) |
| Eptinezumab89,131 | 30 mg | 341 | 0 | 0 | 17 (5) | 0 | 0 | 0 | 0 | 0 | 0 | 4 (1.2) | 14 (4.2) | 65 (19.1) | 5 (1.5) | 10 (3) |
| Erenumab130 | 21 mg | 105 | 0 | 0 | 2 (2) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (4) | 12 (11) | 2 (2) | 1 (1) |
| Erenumab130 | 7 mg | 108 | 0 | 0 | 3 (3) | 0 | 0 | 0 | 0 | 0 | 0 | 4 (4) | 5 (5) | 12 (11) | 5 (5) | 2 (2) |
| Erenumab36,45,130,134,144 | 70 mg | 1228 | 0 | 0 | 50 (4.1) | 0 | 0 | 0 | 0 | 5 (0.4) | 0 | 15 (1.2) | 23 (1.9) | 161 (13.1) | 59 (4.8) | 0 |
| Erenumab36,45,142–144 | 140 mg | 1238 | 4 (0.3) | 0 | 144 (11.6) | 17 (1.4) | 0 | 42 (3.4) | 9 (0.7) | 0 | 0 | 28 (2.3) | 87 (7) | 110 (8.9) | 68 (5.5) | 0 |
| Fremanezumab35,37,90,91,126 | Monthly | 1263 | 4 (0.3) | 8 (0.6) | 24 (1.9) | 0 | 0 | 8 (0.6) | 0 | 1 (0.1) | 0 | 11 (0.9) | 19 (1.5) | 155 (12.3) | 794 (62.9) | 63 (5) |
| Quarterly | 1251 | 8 (0.6) | 4 (0.3) | 48 (3.8) | 0 | 0 | 9 (0.7) | 0 | 3 (0.2) | 0 | 13 (1) | 18 (1.4) | 170 (13.6) | 762 (60.9) | 8 (0.6) | |
| Galcanezumab95,127,140,141,146,150 | 120 mg | 1313 | 13 (1) | 8 (0.6) | 56 (4.3) | 7 (0.5) | 0 | 5 (0.4) | 0 | 0 | 7 (0.5) | 32 (2.4) | 34 (2.6) | 197 (15) | 284 (21.6) | 11 (0.8) |
| Galcanezumab95,127,140,141 | 240 mg | 844 | 2 (0.2) | 13 (1.5) | 37 (4.4) | 4 (0.5) | 0 | 0 | 0 | 0 | 0 | 19 (2.3) | 20 (2.4) | 101 (12) | 272 (32.2) | 18 (2.1) |
| Galcanezumab133 | 150 mg | 107 | 0 | 5 (5) | 15 (14) | 0 | 3 (3) | 0 | 0 | 5 (5) | 0 | 18 (17) | 5 (5) | 28 (26) | 28 (26) | 0 |
| Placebo35–37,45,89–91,94,95,97,126,127,129–131,133,134,140,141,143,144,146,148–151 | - | 7569 | 16 (0.2) | 8 (0.1) | 228 (3) | 0.0 | 8 (0.1) | 6 (0.1) | 0.0 | 7 (0.1) | 0.0 | 132 (1.7) | 150 (2) | 874 (11.5) | 996 (13) | 55 (0.7) |
| Rimegepant148 | 75 mg | 370 | 0 | 0 | 11 (3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 (8) | 0 | 0 |
| Topiramate88,135,142 | 100 mg | 707 | 22 (3.1) | 0 | 194 (27.4) | 23 (3.2) | 21 (2.9) | 88 (12.5) | 63 (8.9) | 0 | 0 | 3 (0.4) | 426 (60.2) | 52 (7.3) | 115 (16.3) | 9 (1.3) |
-
Investigations: amitriptyline 25–100 mg (14%), atogepant 60 mg (4%), atogepant 30 mg and galcanezumab 240 mg (2%), fremanezumab quarterly and galcanezumab 120 mg (1%).
-
Skin and subcutaneous: galcanezumab 150 mg (5%), galcanezumab 240 mg (2%), galcanezumab 120 mg and Fremanezumab monthly (1%).
-
Gastrointestinal disorders: amitriptyline 25–100 mg (59%), topiramate 100 mg (27%), galcanezumab 150 mg (14%), atogepant 60 and 10 mg (13%), erenumab 140 mg (12%), atogepant 30 mg (11%), eptinezumab 300, 30 and 10 mg (5%), erenumab 70 mg, galcanezumab 120 and 240 mg and fremanezumab quarterly (4%), eptinezumab 100 mg, erenumab 7 mg, rimegepant 75 mg and placebo (3%), erenumab 21 mg and fremanezumab monthly (2%).
-
Ear and labyrinth disorders: topiramate 100 mg (3%), erenumab 140 mg, galcanezumab 120 and 240 mg (1%).
-
Eye disorders: onabotulinumtoxinA 150U and galcanezumab 150 mg and Topiramate 100 mg (3%).
-
Psychiatric disorders: topiramate 100 mg (13%), erenumab 140 mg (3%), atogepant 60 mg, fremanezumab monthly and quarterly, atogepant 10 mg and OnabotulinumtoxinA 150U (1%).
-
Metabolism and nutrition disorders: topiramate 100 mg (9%), amitriptyline 25–100 mg (5%), erenumab 140 mg (1%).
-
Vascular disorders: galcanezumab 150 mg (5%).
-
Renal and urinary disorders: galcanezumab 120 mg (1%).
-
Musculoskeletal and connective tissue disorders: galcanezumab 150 mg (17%), onabotulinumtoxinA 150U (16%), erenumab 7 mg (4%), erenumab 140 mg, galcanezumab 120 and 240 mg, eptinezumab 100 mg and placebo (2%), eptinezumab 300 and 30 mg, erenumab 70 mg, fremanezumab monthly and quarterly (1%).
-
Nervous system disorders: topiramate 100 mg (60%), amitriptyline 25–100 mg (44%), eptinezumab 10 mg (10%), erenumab 140 mg (7%), onabotulinumtoxinA 150U, galcanezumab 150 mg and erenumab 7 mg (5%), eptinezumab 30 mg and erenumab 21 mg (4%), atogepant 10 mg, galcanezumab 120 mg and eptinezumab 100 mg (3%), atogepant 30 and 60 mg, eptinezumab 300 mg, fremanezumab monthly, galcanezumab 240 mg, erenumab 70 mg and placebo (2%), fremanezumab quarterly (1%).
-
Infection and infestation: galcanezumab 150 mg (26%), amitriptyline 25–100 mg (24%), eptinezumab 300 and 30 mg (19%), atogepant 30 mg and eptinezumab 10 mg (18%), atogepant 60 mg (17%), galcanezumab 120 mg (15%), fremanezumab quarterly (14%), erenumab 70 mg (13%), eptinezumab 100 mg, fremanezumab monthly, galcanezumab 240 mg and placebo (12%), atogepant 10 mg, erenumab 7 and 21 mg (11%), erenumab 140 mg (9%), rimegepant 75 mg (8%), topiramate 100 mg (7%), onabotulinumtoxina 150U (2%).
-
General disorders and administration site conditions: fremanezumab monthly (63%), fremanezumab quarterly (61%), galcanezumab 240 mg (32%), galcanezumab 150 mg (26%), amitriptyline 25–100 mg (24%), galcanezumab 120 mg (22%), topiramate 100 mg (16%), placebo (13%), erenumab 140 mg (6%), erenumab 7 and 70 mg (5%), atogepant 60 mg (4%), atogepant 30 mg and onabotulinumtoxina 150U (3%), eptinezumab 30, 100 and 300 mg and erenumab 21 mg (2%), atogepant 10 mg (1%).
-
Respiratory, thoracic and mediastinal disorders: topiramate 100 mg (9%), fremanezumab monthly (5%), amitriptyline 25–100 mg (4%), eptinezumab 10 and 30 mg (3%), atogepant 60 mg, eptinezumab 100 and 300 mg, erenumab 7 mg and galcanezumab 240 mg (2%), atogepant 10 and 30 mg, erenumab 21 mg, galcanezumab 120 mg, fremanezumab quarterly and placebo (1%).
Eleven of the forty included trials did not mention what criteria they considered as AEs and just reported the incidence of AEs accrued. Appendix 5, Tables 69 and 75 present the AEs reported in these trials as per publication.
Serious adverse events results
From the 40 RCTs, 30 trials reporting data from 21,529 participants have applied a standard definition for SAEs. These trials evaluated 20 different dosing regimens of 9 drugs. Among them, one study (a series of three sequential, randomised, controlled studies of repeated treatments with BTA for migraine prophylaxis)145 did not explicitly report the number of people with SAEs, however, the results showed that there were no treatment-related SAEs. Thus, SAEs from 29 trials with 20,557 participants were combined. Appendix 6, Table 98 shows the percentage of any SAEs reported for each dosing regimen of these drugs. Table 11 provides more details of classified SAEs and illustrates the percentage of attributed SAEs of each SOC.
| Treatments | Doses | Total participants (n) | Neoplasms benign malignant and unspecified (%) | Nervous system disorders (%) | Injury, poisoning and procedural complications (%) | Respiratory, thoracic and mediastinal disorders (%) | Gastrointestinal disorders (%) | Renal and urinary disorders (%) | Infections and infestations (%) | Cardiac disorders (%) | Congenital, familial and genetic disorders (%) | Hepatobiliary disorders (%) | Psychiatric disorders (%) | Musculoskeletal and connective tissue disorders (%) | Investigations (%) | Metabolism and nutrition disorders (%) | Reproductive system and breast disorders (%) | Skin and subcutaneous tissue disorders (%) | Vascular disorders (%) | General disorders and administration site conditions (%) | Eye disorders (%) | Ear and labyrinth disorders (%) | Immune system disorders (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline135 | 25–100 mg | 169 | 2 (1.18) | 1 (0.59) | 0 | 0 | 1 (0.59) | 1 (0.59) | 1 (0.59) | 0 | 0 | 1 (0.59) | 0 | 0 | 0 | 0 | 1 (0.59) | 0 | 0 | 0 | 0 | 0 | 0 |
| Atogepant129 | 10 mg | 221 | 0 | 0 | 0 | 1 (0.45) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.45) | 0 | 0 |
| 30 mg | 228 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 60 mg | 231 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| BTA88,92 | 150U | 907 | 11 (1.21) | 5 (0.55) | 2 (0.22) | 7 (0.77) | 3 (0.33) | 1 (0.11) | 1 (0.11) | 5 (0.55) | 0 | 0 | 4 (0.44) | 1 (0.11) | 0 | 1 (0.11) | 1 (0.11) | 0 | 1 (0.11) | 1 (0.11) | 0 | 0 | 0 |
| Eptinezumab89 | 10 mg | 130 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.77) | 1 (0.77) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eptinezumab89,131 | 30 mg | 341 | 0 | 0 | 1 (0.29) | 0 | 0 | 2 (0.59) | 0 | 0 | 0 | 0 | 0 | 1 (0.29) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eptinezumab89,94,131,149,151 | 100 mg | 1238 | 1 (0.08) | 3 (0.24) | 6 (0.48) | 0 | 2 (0.16) | 0 | 1 (0.08) | 1 (0.08) | 0 | 3 (0.24) | 5 (0.40) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.08) | 0 | 0 |
| Eptinezumab89,94,131,151 | 300 mg | 989 | 1 (0.98) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.98) |
| Erenumab36,45,142–144 | 140 mg | 1238 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.96) | 0 | 0 | 0 | 0 | 0 |
| Erenumab36,45,130,134,144 | 70 mg | 1228 | 2 (1.18) | 1 (0.59) | 0 | 0 | 1 (0.59) | 1 (0.59) | 1 (0.59) | 0 | 0 | 1 (0.59) | 0 | 0 | 0 | 0 | 1 (0.59) | 0 | 0 | 0 | 0 | 0 | 0 |
| Erenumab130 | 7 mg | 108 | 0 | 0 | 0 | 1 (0.45) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.45) | 0 | 0 |
| Erenumab130 | 21 mg | 105 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fremanezumab35,37,90,91,126 | Monthly | 1262 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quarterly | 1251 | 11 (1.21) | 5 (0.55) | 2 (0.22) | 7 (0.77) | 3 (0.33) | 1 (0.11) | 1 (0.11) | 5 (0.55) | 0 | 0 | 4 (0.44) | 1 (0.11) | 0 | 1 (0.11) | 1 (0.11) | 0 | 1 (0.11) | 1 (0.11) | 0 | 0 | 0 | |
| Galcanezumab95,127,140,141,146,150 | 120 mg | 1313 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.77) | 1 (0.77) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galcanezumab95,127,140,141 | 240 mg | 844 | 0 | 0 | 1 (0.29) | 0 | 0 | 2 (0.59) | 0 | 0 | 0 | 0 | 0 | 1 (0.29) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Galcanezumab (LY2951742)133 | 150 mg | 107 | 1 (0.08) | 3 (0.24) | 6 (0.48) | 0 | 2 (0.16) | 0 | 1 (0.08) | 1 (0.08) | 0 | 3 (0.24) | 5 (0.40) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.08) | 0 | 0 |
| Placebo35–37,45,89–91,94,95,97,126,127,129–131,133,134,140,141,143,144,146,148–151 | - | 7570 | 11 (0.14) | 12 (0.15) | 18 (0.23) | 11 (0.14) | 8 (0.1) | 2 (0.03) | 17 (0.22) | 4 (0.05) | 1 (0.01) | 5 (0.06) | 3 (0.04) | 9 (0.12) | 1 (0.01) | 2 (0.03) | 9 (0.12) | 0 | 1 (0.01) | 3 (0.04) | 1 (0.01) | 0 | 4 (0.05) |
| Rimegepant148 | 75 mg | 370 | 1 (0.27) | 0 | 0 | 0 | 0 | 0 | 1 (0.27) | 0 | 0 | 0 | 1 (0.27) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Topiramate 88,135,142 |
100 mg | 707 | 1 (0.14) | 1 (0.14) | 1 (0.14) | 2 (0.28) | 2 (0.28) | 1 (0.14) | 8 (1.13) | 1 (0.14) | 0 | 1 (0.14) | 1 (0.14) | 1 (0.14) | 1 (0.14) | 2 (0.28) | 4 (0.57) | 0 | 2 (0.28) | 0 | 4 (0.42) | 0 | 1 (0.14) |
From the 40 trials included to assess the safety data, 4 trials have not reported any SAEs data. These four trials evaluated efficacy and safety of topiramate for the treatment of chronic migraine,28 flunarizine versus propranolol in the prophylaxis of migraine,128 amitriptyline versus divalproate in migraine,137 and a comparative study of topiramate versus sodium valproate in the prevention of migraine headaches. 147
Six trials did not provide any definitions used to identify SAEs. We have reported them separately from those trials with a standard definition:
-
A trial evaluated BTA (105–260U) prophylactic treatment of episodic migraine for 369 participants. 132 Only four participants (three in the BTA group and one in the placebo group) experienced four SAEs, of which none were reported by the investigator to be related to study medication.
-
A study of multiple treatments of BTA (75, 150 and 225U) for the prophylaxis of episodic migraine headaches in 495 participants138 reported that seven participants experienced SAEs. No further details were provided.
-
Amitriptyline 25–100 mg in the prophylactic treatment of migraine in 393 participants found that no serious events occurred. 22
-
Efficacy and tolerability in migraine prophylaxis of flunarizine (5 and 10 mg) in comparison with propranolol 160 mg daily were evaluated in 808 participants with episodic migraine. 136 The results depicted one participant in the flunarizine 5 mg group experienced malaise and vertigo. In the flunarizine 10 mg group, five participants reported a SAE: urinary incontinence (n = 1), injury (n = 1), cholelithiasis (n = 1), breast neoplasm (n = 1) and depression (n = 1). In the propranolol group, two participants reported a SAE: one injury and one menstrual disorder.
-
A trial evaluated whether topiramate (100 mg) would prevent the transformation of episodic migraine to chronic daily headache (CDH) in 361 participants with a high-frequency episodic migraine (HFEM). 139 Eight participants (three in the topiramate group and five in the placebo group) reported a total of nine SAEs including spontaneous abortion (x2), bradycardia, bipolar disorder, suicidal thoughts, neuropathy, fractured pelvis secondary to a motor vehicle accident, chest pain and worsening of migraine.
-
A trial assessed the effects of discontinuation of topiramate (200 mg) after a treatment period of 6 months in 512 participants with episodic migraine. 152 Six of the 25 reported SAEs were judged by investigators to be possibly (urinary calculus, dyspnoea, pyrexia and urticaria), probably (depressed mood), or very likely to be (nephrolithiasis) related to the use of topiramate.
List of classified SAEs are presented in Appendix 6, Table 76. Arm level SAE incidence of the 36 trials can be found in Appendix 6, Tables 77–97.
Discussion
We systematically reviewed and narratively synthesised the incidences of AEs and SAEs from 40 RCTs22,28,35–37,45,88–95,97,107,108,117,118,126–151 which investigated pharmacological interventions to manage chronic or episodic migraine. These trials included 25,891 participants. Results suggest that all the pharmacological interventions included in this review were found to be tolerable, although it was apparent that the rate of AEs on a particular organ is different for each drug. For example, nervous system disorders occurred more frequently with amitriptyline and topiramate drugs, whereas rimegepant was not responsible for these disorders in any of the included trials. Psychiatric disorders were more frequent in participants taking topiramate. Infection and infestation were reported for all included pharmacological interventions, among them, BTA had the least infection rate; however, musculoskeletal, and connective tissue disorders were highly reported for BTA than any of the other medications. Amitriptyline and topiramate had a major role in contributing to gastrointestinal disorders in participants, while participants who were taking fremanezumab suffered more from general disorders and administration site conditions than any of the other medications.
The number of included trials and subsequently the number of participants for those AEs and SAEs are different. Among them, the safety profiles for erenumab at different doses,36,45,130,134,142–144 topiramate 100 mg,28,88,135,139,142,147,152 and galcanezumab at three different doses95,127,133,140,141,146,150 have been investigated more than the other medications, with seven trials for each of these medications. Then there are five RCTs for each of the following medications: eptinezumab at different ranges of doses,89,94,131,149,151 BTA at different ranges of doses88,97,132,138,145 and fremanezumab monthly and quarterly. 35,37,90,91,126 Then we have three RCTs for amitriptyline at two doses,22,135,137 two RCTs for divalproate extended release (250–1000 mg) and sodium valproate (200 mg);137,147 flunarizine at two doses,128,136 and propranolol at two doses. 128,136 The lowest amount of evidence about the AEs and SAEs are for rimegepant and atogepant at different ranges of doses.
Thus, it is important to note that the AEs and SAEs for erenumab at different doses, topiramate 100 mg, and galcanezumab may be better established and have a long history of records and documentation rather than the other medications. It is also important to consider that most of the included trials have some concerns in terms of the risk of bias (see below for further details).
For the drugs, with different doses of administration the results vary based on the drug of interest. For example, the safety profiles for erenumab, atogepant, BTA, galcanezumab, amitriptyline, propranolol, eptinezumab and flunarizine were all analysed at different doses. The included evidence showed that the higher doses for erenumab (140 mg) and atogepant (30 mg) had a lower incidence of the AEs and SAEs. However, for BTA, galcanezumab, amitriptyline and propranolol the lower doses seem to be associated with lower incidences of AEs and SAEs. Eptinezumab at a mid-dose (100 mg) also benefits from a lower incidence of AEs. Finally, for flunarizine (5 and 10 mg), there is a marginal difference in the incidence of the AEs and its safety profile does not seem to vary among the different doses.
Another interesting finding from our review is that placebo-related AEs in the included RCTs are more than erenumab at different doses, rimegepant, topiramate, and eptinezumab at doses 100 and 300 mg. The percentage of reported AEs for placebo is similar to that of atogepant, while it is lower for all other medications.
It is also important to note that in some trials the safety profiles for the medications have been investigated solely for episodic migraine, while in other trials, it is solely for chronic migraine or a combination of both episodic and chronic migraine. For example, for rimegepant, atogepant, amitriptyline, divalproate extended release (250–1000 mg), sodium valproate (200 mg), flunarizine and propranolol, the AEs and SAEs profiles have been among patients with episodic migraine only; while for eptinezumab, the participants have chronic migraine only; and for the rest of the medications the participants included in the trials are a combination of episodic and chronic migraineurs. However, regardless of the type of migraine, it seems that the medications generally have a satisfactory incidence of AEs and SAEs, and the type of the migraine does not seem to be a crucial determinant for the safety profiles of these medications.
Comparison with previous literature
When comparing with other studies, we have found some review studies that support our findings and a few reviews which may not be aligned with the conclusions we have reached about the AEs and SAEs in this review. Comparisons by each of the drugs are:
-
Topiramate 100 mg: One open-label trial was assessed as having a high risk of bias,88 and the rest were considered as having some concerns. 28,135,139,142,147,152 Overall, topiramate was reported as well tolerated with the most common AEs related to the nervous system and gastrointestinal disorders, although erenumab demonstrated a favourable tolerability profile compared to topiramate in a trial. 142 In another crossover trial, 51% of patients discontinued topiramate due to AEs and swapped to the BTA group. 88 Although these trials have been conducted with relatively similar inclusion and exclusion criteria and design, AEs incidences reported for topiramate 100 mg in a trial are meaningfully lower than others. 147 This gap might be justified by the short treatment duration, 4 weeks compared with at least 12 weeks. In another meta-analysis, the safety profile was in favour of the CGRP MAbs, with a higher likelihood to help than to harm compared with topiramate. 153
-
BTA at different ranges of doses: The safety profile of BTA at doses between 7.5 and 260 mg was investigated in five RCTs with 2237 subjects with chronic or episodic migraine. Two studies were rated as having a high88 or a low risk of bias,97 and three were rated as having some concerns. 132,138,145 The results showed that lower doses of BTA have a safer AEs profile than higher doses. It is worth mentioning that the lower doses are only prescribed for episodic migraine. Musculoskeletal and connective tissue disorders were the most common AEs for BTA. A pairwise meta-analysis shows that total AEs for BTA was higher than placebo with a relative risk ratio of 1.22 (95% CI 1.07 to 1.14). 154 This is in line with our results which show that BTA has a higher rate of AEs compared with placebo.
-
Eptinezumab at different ranges of doses: The safety profile of eptinezumab at doses 10, 30, 100 and 300 mg was investigated in five RCTs with 2696 subjects with chronic or episodic migraine. Two studies were rated as being at low risk of bias,89,94 and three were rated as having some concerns. 131,149,151 However, all doses of eptinezumab were generally reported to be tolerable and acceptable. Eptinezumab 100 mg showed a more desirable AE profile (a smaller proportion of AEs), which may be due the short treatment duration (4 weeks). 149 Hou et al. synthesised results from five trials and found that total AEs in migraine patients with CGRP MAbs therapy were not significantly different from those observed in placebo groups (OR 1.17, 95% CI 0.91 to 1.51). 155 The most common AEs for all doses were depicted for infection and infestation in the SOC. These results align with the other review results, which presented upper respiratory tract infection and urinary tract infection as the frequent AEs. 155 For SAEs, it appears from the data reviewed that a lower dose has a more favourable profile rather than higher doses.
-
Erenumab at different ranges of doses: The results of two meta-analyses, one by Lattanzi et al. and another by Zhu et al. , align with our review and concluded that there were no differences in the occurrence of AEs and SAEs between the erenumab and placebo groups. 156,157 In our results for erenumab 21 mg, no SAEs were reported. 130 Our results showed that the least AEs incidence belonged to erenumab 140 mg by SOC, although AEs for gastrointestinal disorders were high. While participants who underwent erenumab 70 mg reported a higher number of infections and infestations, the results were in line with another review. 155 Two erenumab RCTs had some concerns regarding the risk of bias;142,143 the rest were rated as having a low risk of bias. 36,45,130,134,144
-
Fremanezumab monthly and quarterly: The safety profile of fremanezumab was investigated in five RCTs with 2514 subjects with chronic or episodic migraine. Four studies were assessed as being at low risk of bias,35,90,91,126 and one had some concerns. 37 There were differences in terms of participants who were included in the trials, that is, subjects with medication overuse, history of failed treatment, and those using preventive migraine medications. AEs incidence in monthly groups was reported to be lower than in quarterly groups.
-
Galcanezumab at three doses: The results for evaluating the safety of galcanezumab 120, 150 and 240 mg were reported in seven trials with 2264 participants with chronic or episodic migraine. Of these studies, one trial was rated as being low risk of bias,127 and six trials as having some concerns. 95,133,140,141,146,150 There were differences in eligibility criteria, for example, including participants with a history of documented treatment failure of two to four migraine preventive medications,146 while another trial excluded participants having failed treatment with three or more migraine prevention medications. 141 Overall, galcanezumab for all doses was tolerable and accepted, although it appears from the data reviewed that the AEs incidence in the studies with 12 weeks of treatment occurred in a lower proportion than at 24 weeks. General disorders and administration site conditions, followed by infection and infestations, were the most frequent AEs for all doses. However, for Hou et al. they presented upper respiratory infections and viral infections (infections and infestations) as their most common AEs. 155 This discrepancy may be because they only included one trial.
-
Rimegepant 75 mg: The results for rimegepant 75 mg were reported in one trial with 375 participants with episodic migraine which had some concerns in terms of risk of bias, although it showed similar tolerability to placebo, and there were no unexpected or serious safety issue noted. 148,158 Gao et al. included four RCTs (3827 subjects) and their results showed that rimegepant 75 mg had good safety for episodic migraine. Similar to our finding, there was no statistically significant increase in AEs compared with the placebo. 159
-
Atogepant at different ranges of doses: The safety profile of atogepant at doses 10, 30 and 60 mg was investigated in a low risk of bias trial with 680 episodic migraine subjects. 129 The AEs for all doses were approximately the same and well tolerated, although atogepant 30 mg had fewer treatment-related AEs incidences. No SAEs were reported for atogepant 30 and 60 mg. A systematic review found that atogepant was well tolerated and had a low frequency of AEs. 160
-
Amitriptyline at two doses: The results for evaluating the safety of amitriptyline 50 and 100 mg were reported in three trials with 504 subjects with episodic migraine. An open label trial was assessed as being at high risk of bias,137 and the other two trials as having some concerns. 22,135 AEs experienced the most by the participants were for gastrointestinal disorders followed by nervous system disorders. The results showed that a lower dose of amitriptyline had more AEs. We could not find any evidence for the safety profile of amitriptyline that had been synthesised through systematic review or meta-analysis.
-
Divalproate extended release (250–1000 mg) and sodium valproate (200 mg): Two RCTs with 428 episodic migraine subjects. One trial was rated as having a high risk of bias,137 and the other trial was rated as having some concerns. 147 Both were found to be tolerable, which is supported by two reviews. 161,162
-
Flunarizine at two doses: Two RCTs with 698 episodic migraine subjects investigated the safety profile of flunarizine at two doses (5 and 10 mg). One trial was rated as having a high risk of bias,128 and the other was rated as having some concerns. 136 Both doses were well tolerated and acceptable. There were no considerable safety differences between the doses. Anker et al. ’s systematic review on flunarizine efficacy and safety for episodic migraine supports our findings. 163
-
Propranolol at two doses (40 and 160 mg): The results from two trials with 440 subjects suffering from episodic migraine showed that the lower dose had a more desirable safety profile than the higher dose. One of these trials was rated as having a high risk of bias,128 and the other was rated as having some concerns. 136 Gastrointestinal disorders, general disorders and administration site conditions were reported as the most frequent AEs.
Wang et al. 144 compared the different MAbs against CGRP or its receptor for adult patients with migraine; however, this NMA was limited to direct comparisons between placebo and eptinezumab, erenumab, fremanezumab and galcanezumab, for both AEs and SAEs. 164 The results of this NMA showed that galcanezumab ranked the highest for causing at least one SAE, followed by eptinezumab, erenumab and fremanezumab. However, this result is constrained by massive variations in reported SAEs among the included RCTs. Also, there was no available indirect comparisons between the trials. 164
Strengths and limitations
Almost all the available evidence from the systematic reviews focused on one drug or fewer drugs than what we have considered in this review. Therefore, one of the key strengths of this analysis is the inclusion of a range of migraine treatments, including the latest therapies, such as CGRPs, specifically fremanezumab, eptinezumab, galcanezumab and erenumab, which are commonly used after other concurrent preventive treatments, such as BTA and topiramate have failed. As the inclusion criteria for this chapter comprised both episodic and/or chronic migraine, we have also included some oral medications which were not included in the clinical effectiveness chapter. This diversity provides a comprehensive overview of the medication effectiveness profile, allowing decision-makers to compare alternative treatments and obtain a better reflection of clinical practice. Another main strength of this review is the comprehensiveness of the search strategy employed. The search was conducted and updated across a wide range of electronic databases, without any restrictions on dates or language, to ensure that all relevant trials were included in the analysis.
It is important to note that all the systematic reviews that we have compared to our review have mentioned that there are shortcomings in their included RCTs and that further head-to-head RCTs are required for more robust results for AEs. We recommend further head-to-head RCTs to assess the safety profile of oral medications in the chronic migraine population because our review only found data evaluating the AEs incidence in chronic migraine participants which is limited to newer CGRP treatments, BTA and topiramate. In addition, for some of the considered drugs in our review including amitriptyline, divalproate, sodium valproate and Propranolol, our searches couldn’t identify any relevant systematic reviews to enable us to compare our results. Hence, further studies are needed to have a clear understanding of the safety profile of these drugs.
We should also note that there are further limitations of some of the trials included in this review. For example, the trials which included the drugs atogepant and rimegepant – even though they have product licences, they have not yet been approved by NICE or SIGN; the trial for BTA for episodic migraine patients used non-standard doses, whereas the current standard dose for chronic migraine patients is 155U; and galcanezumab 150 mg dose is not used in standard practice and had a significantly higher AEs profile.
Finally, the results of this analysis should be interpreted with caution due to its limitations. We used CTCAE Version 5.0 for classifying the AEs and SAEs. However, there are some reported AEs and SAEs in the included studies which are not in the CTCAE, thus as a solution for this issue our clinical experts discussed what would be the best respective category for those events. For instance, panic attack was categorised as a psychiatric disorder. The included studies were not consistent in terms of the reporting of AE and SAE definitions, and due to this limitation, our clinical advisers reached a consensus on pooling the results for those studies with the same definitions and reporting the results for the other studies by each study narratively.
In summary, all medications were tolerable, but they had different side effects. Rimegepant had a favourable AEs profile. Amitriptyline and topiramate were associated with a higher occurrence of nervous system disorders and gastrointestinal disorders. Topiramate also was linked to a higher prevalence of psychiatric disorders. All medications had infections and infestations as a side effect. However, the medications did not follow a similar incidence pattern; BTA had the least infection rate, while it had a higher incidence of musculoskeletal and connective tissue disorders than other medications. Participants taking fremanezumab and galcanezumab experienced more general disorders and administration site conditions, while erenumab and eptinezumab had a higher rate of infection and infestation, similar to atogepant.
Chapter 4 Cost-effectiveness review
Research question 3: What is known about the cost-effectiveness of prophylactic drugs for chronic migraine?
Introduction
This chapter will explore and review all published cost-effectiveness studies including economic models of the use of different pharmacological treatments for adult patients with chronic migraine. Studies providing information on resource use, costs, utilities and probabilities, useful to inform the economic model in Chapter 5, were also identified.
Methods
The protocol for the cost-effectiveness review has been registered in the PROSPERO database. The registration number is CRD42021265995.
Search strategy
The search strategy was constructed by an information specialist (AB), in consultation with the project team. MEDLINE and EMBASE searches were based on those used in the searches for Chapters 2 and 3, with the addition of filters for economic and costs studies (instead of a RCTs filter) and search terms for prophylactic drug treatments of migraine in general (as well as specific named interventions). Search strategies in economics/Health Technology Assessment (HTA) specific sources included only terms for migraine/headache, with the addition of general terms for drug treatment or prevention in some cases. No language or date limits were applied. Full search strategies including bibliographic database names and dates searched can be found in Appendix 7, Table 99, along with the targeted internet searches using Google and Google Scholar. Furthermore, Appendix 7 also contains information on the websites of the government agencies which were also searched for publications relating to migraine or headache.
Records retrieved by the database searches were exported into EndNote X9, to enable systematic removal of duplicates. 54 In addition, we did forward and backward citation tracking from included journal articles, using Web of Science Core Collection (and the Citation Finder tool or Google Scholar where articles were not available in Web of Science). Searches were re-run in November 2022 to identify any new studies or publications since the original searches, and we also ran searches to check for any retractions, errata or similar relating to included journal articles. Additional searches for utility data to inform the economic model were also undertaken at this time.
Assessment of eligibility
The citations including title and abstracts were first assessed against the eligibility criteria by two reviewers (HM, SK). Full-text articles meeting the eligibility criteria were then obtained and reviewed. Any disagreements between the reviewers were resolved by discussion or by a third reviewer if necessary (MU). No language restrictions were applied.
Inclusion criteria
Only studies meeting the following inclusion criteria were included:
-
Study type: Full economic evaluations in which both the costs and the outcomes of interventions and comparators are examined, including both trial-based and model-based evaluations.
-
Population: Adults with chronic migraine, where the headache occurred for 15 or more days/month for more than 3 months.
-
Interventions: Prophylactic drugs to treat chronic migraine as listed in Box 1 (see Chapter 2).
-
Comparators: Placebo, usual care, or other prophylactic drugs as in Box 1 (see Chapter 2).
-
Outcomes: Measures included headache/migraine days, headache-related QoL, MSQ and incremental cost-effectiveness ratios (ICERs) amongst others.
Exclusion criteria
Studies meeting the following exclusion criteria were excluded:
-
Partial economic evaluations
-
Systematic reviews and/or meta-analyses
-
Qualitative studies
-
Study protocols
-
Conference abstracts
-
Editorials and short commentaries
-
Articles comparing pharmacotherapy with non-pharmacological interventions.
Data extraction
Using a pre-specified data extraction form, data extraction for full-text studies was carried out by one reviewer (SK) and then checked by a second reviewer (SN). Data extracted included:
-
Study context – authors, publication year, country, setting, study population, intervention and comparators.
-
Economic evaluation methods – economic evaluation type, model type, study perspective, time horizon, currency and price year, discount rate, resource use/costs, outcome measures and analytical methods.
-
Economic evaluation results – study results, sensitivity analyses, generalisability and conclusion.
-
Other – funding sources and conflicts of interest.
Data synthesis
Information extracted from the included studies was summarised and tabulated. Findings from individual studies were compared narratively. We summarised the published journal articles separately to the reports, as the latter will not have had a formal peer-reviewed process.
Quality appraisal of economic evaluations
The quality of both the trial-based and model-based economic evaluations was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 checklist. 165 The Philips checklist was also used to assess the quality of each model based economic evaluation. 166 Quality assessment was undertaken by one reviewer (SK) and was checked for completeness and accuracy by a second reviewer (SN).
Results
Search results
A total of 7309 citations were retrieved from the database searches. After deduplication and title and abstract review, 88 articles were reviewed at the full-text stage. Nine articles met the inclusion criteria for published peer-reviewed journal articles. 167–175 The excluded studies with their reasons for exclusions are provided in Report Supplementary Material 3. Two articles were translated into English language. 173,175 The targeted internet and website searches identified an additional 72 reports, and after the screening we included 7 of these reports. 176–182 The PRISMA diagram is shown in Figure 25. We have narratively synthesised the reporting of the nine published journal articles separately from the seven reports.
FIGURE 25.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for cost-effectiveness studies. CADTH, Canadian Agency for Drugs andTechnology in Health.
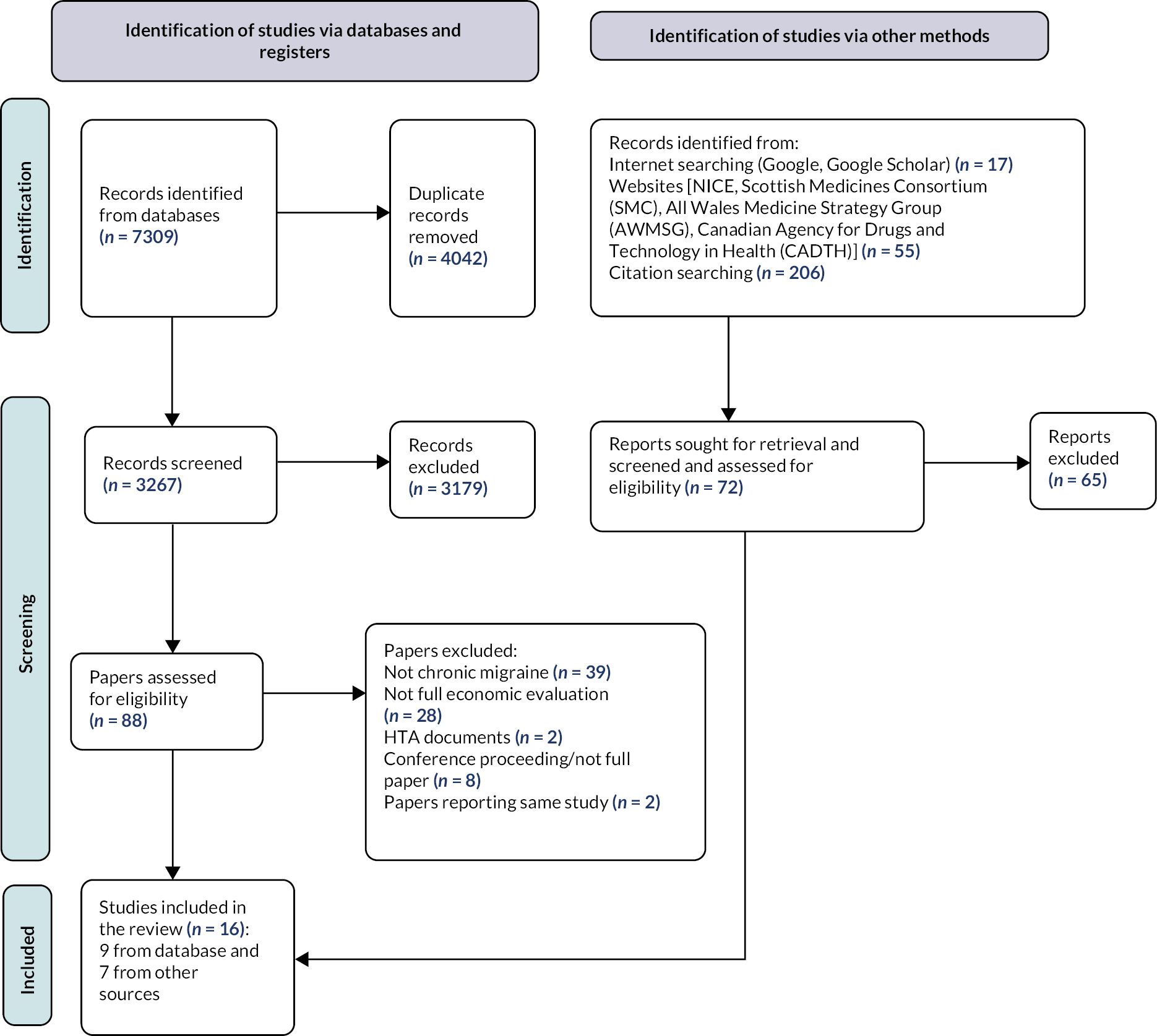
Journal articles (n = 9)
All journal based studies were model based cost-utility analyses and were from high-income countries including two from the UK. 167,170 Four studies167,169,170,173 evaluated BTA and the remaining five studies evaluated erenumab. 168,171,172,174,175 All studies evaluating BTA compared the treatment with placebo or best supportive care; and studies evaluating erenumab compared their outcomes with placebo or BTA (see Appendix 8, Table 100).
Five studies167,169–171,173 utilised a Markov state-transition model structure (see Appendix 8, Table 101). The Markov models included health states which were stratified by the number of migraine or headache days. Of the other four studies, one used a decision tree,168 and the other three developed hybrid models. 172,174,175 The majority of studies used a 2-year time horizon in the economic models. An NHS perspective was adopted for the UK studies, the European studies were based on the societal perspective and the American studies were based on societal and payers’ perspectives.
The majority of studies included the direct costs of the prophylactic and comparator drugs plus administration costs, and any associated costs for general practitioner (GP) visits, consultant, neurology or specialist nurse appointments and/or accident and emergency department visits (see Appendix 8, Table 101). The study by Vekov and Izmaylov175 only included the costs of the drugs as they assumed all other healthcare costs for both arms were equal. For studies adopting a societal perspective, indirect costs were reported, such as wages lost as a result of not working. Some studies explicitly presented the productivity costs associated with presenteeism and absenteeism. 171,172 Resource use data for three studies167,170,173 were obtained from the International Burden of Migraine Studies (IBMS), and for other studies, resource use was obtained from published studies including trial data or other local databases. For the UK studies,167,170 the NHS reference costs were the main source of cost inputs, and for the non-UK studies the main source of cost inputs were previously published studies, publicly available local databases or published price lists. All nine studies reported the currency and price year in which the unit costs were calculated and reported (see Appendix 8, Table 101).
All studies longer than the 1-year time frame used discounting. The two UK studies in line with the NICE guidelines183 used a 3.5% discount rate for both costs and outcomes. 167,170 All other studies discounted costs and outcomes at a rate of 3%, except one study which used a 5% discount rate. 175 The two UK studies167,170 used a £20,000–30,000 per quality-adjusted life- year (QALY) as the willingness-to-pay (WTP) threshold, whereas the other studies had different WTP thresholds (see Appendix 8, Table 102 for more information).
All studies used the EQ-5D as the main outcome measure to estimate QALYs (see Appendix 8, Table 102). The EQ-5D scores were mapped from the MSQ to produce utility values in six studies. 167–169,171–173 Hollier-Hann et al. 170 administered the EQ-5D-5L as part of the REPOSE trial, and utility values were estimated using a UK tariff. Sussman et al. 174 used EQ-5D-5L scores from the erenumab and BTA trials to estimate utility values; however, they did not state whose values or what tariff was used to estimate them. 174 Other outcomes measures included the number of headache days or migraine days avoided.
All studies167,169,170,173 which used BTA as the treatment found BTA to be more cost-effective than placebo, with ICERs ranging from £15,028 (€17,720) to £16,598 (€19,572). Erenumab was found to be cost-effective in two studies in participants for whom previous preventive treatments had not worked,168,171 and erenumab dominated placebo in two studies. 172,174 When erenumab was compared to BTA from a societal perspective, the ICER was above the most common WTP thresholds £182,128 (€218,870). 168 One study found erenumab not cost-effective compared to placebo. 175 All of the studies conducted deterministic and/or probabilistic sensitivity analysis (PSA). In most cases, the results were sensitive to changes in MMDs, health utilities and treatment costs, but were cost-effective overall – see Appendix 8, Table 101 for more information.
Other reports (n = 7)
Four of the seven reports were from the UK,179–182 two from Canada176,177 and one from the USA178 (see Appendix 8, Table 100). All reports were cost-utility model based analyses. The key interventions assessed for cost-effectiveness were: BTA,176,182 erenumab,177,178,180 fremanezumab178,179 and galcanezumab. 181 All reports compared the intervention to placebo (best supportive care) and four reports also compared the main intervention to BTA. 177,179–181
Two main models were employed (see Appendix 8, Table 101): a Markov model with health states stratified by number of headache days per 28 days or hybrid model with decision tree and Markov model using 12-week cycle lengths. The time horizon ranged from 2 years to a lifetime. The UK-based studies adopted an NHS perspective, and a healthcare payer perspective was adopted for the three North American studies.
All studies reported similar resource usage data. All studies reported the currency and price year for unit costs and also the discount rate (see Appendix 8, Table 101). All the studies used the MSQ scores which were mapped on to the EQ-5D to estimate utility values (see Appendix 8, Table 102). 176–182
All CGRP inhibitors like erenumab, galcanezumab and fremanezumab were found to be cost-effective in the chronic migraine population for whom the previous treatments did not work under the widely accepted WTP thresholds177,179–181 and have been recommended for such group of participants (see Appendix 8, Table 102 for more information). The sensitivity analyses reported in each of the reports was more comprehensive than what was reported in the journal articles.
Generalisability
To assess the level of generalisability, all studies were classified as: (1) generalisable; (2) transferable; and (3) context-specific. Three journal articles167,170,173 were transferable, and the remaining six studies were considered to be context-specific. Two journal articles did not report the source of funding. 168,175 All of the reports were considered to be context-specific and none of them declared any conflicts of interest, although they were all funded by the pharmaceutical industries except one report182 (see Appendix 8, Table 103).
Quality appraisal of economic evaluations
None of the included studies fulfilled all of the quality criteria for the CHEERS 2022 checklist165 (see Appendix 8, Table 104); however, the majority of studies fulfilled a large number of quality criteria. The criteria that were the least well addressed were the items on heterogeneity and generalisability. Most of the studies fulfilled a large number of the quality criteria according to the Phillips checklist. 166 The criteria that were least well addressed were whether the data has been assessed appropriately, the principles of uncertainty, heterogeneity, and assumption about the continuity of treatment and its effect, including sensitivity analysis around the assumption of different alternatives of treatment effect.
Discussion
We undertook a systematic search for economic evaluation studies for the cost-effectiveness of chronic migraine medications in the adult population and identified nine peer-reviewed journal articles and seven published reports. All articles were model based and were generally classed as high quality when appraised by the CHEERS reporting tool. None of the studies were trial-based economic evaluations.
The main strength of this review is that it included the latest CGRPs which have been approved for the treatment of chronic migraine. Although these newer drugs are more costly than the oral preventatives, they were cost-effective. Another strength is the comprehensiveness of the search strategy used and that the search was performed using a broad range of electronic databases of published studies. Furthermore, there were no country and language restrictions. The main limitation of our review is that we only included full economic evaluations and therefore important data contained within partial economic evaluations might have been missed. Another limitation of the included studies is that they did not define the comparators (i.e. best supportive care, placebo and preventative treatment) clearly.
Our review is more comprehensive and provides more worldwide evidence than the review published by Mahon and colleagues in 2020. 184 Their review only included eight studies which compared BTA or topiramate as the main intervention and is limited to studies published in the UK. 184
In summary, based on the findings from the review, BTA and CGRPs were cost-effective compared to placebo, although the CGRPs had more incremental economic benefits compared to BTA. CGRPs might provide an acceptable cost-effective prophylactic medication for chronic migraine including for participants for whom the other treatments including BTA have been unsuccessful.
Chapter 5 Economic model
Research question 4: Which prophylactic drugs for the management of chronic migraine are the most cost-effective?
Introduction
We built a Markov model to assess the cost-effectiveness of different pharmacological medications compared to usual care (placebo) to treat or prevent chronic migraine in the adult population. The economic model has only compared the drugs for which the trials were included in the NMA (see Chapter 2). The following seven treatments were compared in the base-case analysis: (1) onabotulinumtoxinA (BTA), (2) eptinezumab 100 mg, (3) eptinezumab 300 mg, (4) fremanezumab (monthly dose), (5) fremanezumab (quarterly dose), (6) galcanezumab and (7) topiramate with placebo. We also compared erenumab (70 and 140 mg) with placebo in a sensitivity analysis. This chapter describes the structure of the model, the model inputs, the assumptions made, the various scenarios which have been evaluated, the results and key sensitivity analyses.
Model structure and assumptions
To assess the cost-effectiveness of the different pharmacological medications for chronic migraine, we developed a Markov (state-transition) model in Microsoft Excel. The model structure was informed by the cost-effectiveness review (see Chapter 4)167,182 and inputs from clinical and non-clinical team members. A Markov model was considered to be the most appropriate choice because progression of chronic migraine can evolve over time, and during this time patients can move between various states of headache severity based on the number of headache days (health states) or can die (due to all-cause mortality).
The model comprised 13 health states (as shown by the ovals), including death which is an absorbing state, once you have entered you cannot leave (Figure 26). The remaining 12 states were split into 2 parallel levels: on treatment and off treatment. Each health state was subdivided into categories based on the number of headache days per 28 days: 0–3, 4–9, 10–14 (episodic migraine) or 15–19, 20–23, 24–28 (chronic migraine) headache days per 28 days (see Figure 26). The arrows represent the transitions that patients can make in the model, and any recurring arrows show that the patients can stay in that health state for more than one cycle.
FIGURE 26.
Economic model structure.

The model starts by assigning a hypothetical cohort of 1000 people presenting with chronic migraine into one of the three chronic migraine health states. The proportion of people starting the model in the three health states was based on the PREEMPT trial as it is one of the largest chronic migraine trials: 15–19 MHDs – 530 patients; 20–23 MHDs – 280 patients and 24–28 MHDs – 190 patients. 92,93 In the first cycle, the patient can stay in that health state, or move to a lesser headache severity health state, or move to a more headache severity health state, or move to the corresponding ‘off treatment’ health state or move to the death health state. For example, if a patient started in the 15–19 MHD health state, they can stay in this health state, or move to any of these ‘on treatment’ health states (0–3, 4–9, 10–14, 20–23 or 24–28 MHDs), or move to the 15–19 MHD ‘off treatment’ health state or die. In the second cycle and onwards, this pattern continues. If someone transitions to an ‘off treatment’ health state, we have assumed that they cannot move back to an ‘on treatment’ health state. The cycle length for the model is 3 months (12 weeks) and transitions between each health state occur at the end of each cycle.
Model inputs
Transition probabilities
For the base-case analysis, the transition probabilities were calculated in the following way: for the placebo group, the transition probabilities were calculated by digitising the transition probability image (see Figure 2 in the Batty et al. paper) which showed a visual representation of transition probabilities. 167 These transition probabilities were based on the PREEMPT trial. 92,93
For the different prophylactic medications, transition probabilities were estimated using treatment effect estimates from the NMA (see Figure 2). Since treatment effects were parameterised as MD in MHDs, we derived transition probabilities from these as follows: we assumed that the number of headache days was uniformly distributed across the range covered by each state, and that the mean and variance of the treatment effect was independent of baseline disease severity. This allowed us to derive the post-treatment distribution of MHDs for each state, and therefore the proportion arriving in each state post-treatment. Based on advice from clinical members of the team and following NICE guidance, we also applied a discontinuation rate for each medication. For those who were on BTA we applied a 10% discontinuation rate and for all other medications we applied a 20% discontinuation rate. 39–41
These probability matrices for each prophylactic medication were then multiplied with the placebo’s transition probability matrix mentioned earlier (a two-step transition) to obtain transition probabilities for each individual prophylactic drug. The transition probabilities used for each drug are shown in Appendix 9, Table 105.
Utilities
The utility values for each of the health states were based on the EQ-5D-5L data from a RCT for educational and supportive self-management intervention for people with chronic headaches (CHESS). 12 We categorised participants in the CHESS trial regardless of their treatment group, based on the ‘number of days over the last 4 weeks that they had a migraine/headache’ to obtain MHD health states. The EQ-5D-5L data from the CHESS trial were obtained from the participants at 4 time points (baseline, 4, 8 and 12 months). These EQ-5D-5L responses were converted into health state utilities based on values mapped on to the EQ-5D-3L descriptive system185 using the Hernandez-Alava crosswalk algorithm186 for the baseline time point. In consultation with our clinical colleagues, we assumed that the utility is the same for all prophylactic drugs, but they differ by the MHD health states that the patient is in (Table 12).
| Health states | Mean | SE |
|---|---|---|
| 0–3 MHD | 0.7573 | 0.1662 |
| 4–9 MHD | 0.6449 | 0.2817 |
| 10–14 MHD | 0.6764 | 0.2458 |
| 15–19 MHD | 0.6420 | 0.2543 |
| 20–23 MHD | 0.5916 | 0.2549 |
| 24–28 MHD | 0.5040 | 0.2835 |
| 0–3 Off TX | 0.7573 | 0.1662 |
| 4–9 Off TX | 0.6449 | 0.2817 |
| 10–14 Off TX | 0.6764 | 0.2458 |
| 15–19 Off TX | 0.6420 | 0.2543 |
| 20–23 Off TX | 0.5916 | 0.2549 |
| 24–28 Off TX | 0.5040 | 0.2835 |
Resource use and costs
The costs of the drugs for a 3-monthly cycle were obtained from the BNF. 51 For each of the drugs (except for BTA and eptinezumab as these are administered in hospitals/clinics and topiramate as this is an oral drug) we assumed that the first injection/infusion would be administered by a nurse (30 minutes) and in this appointment they would show the patient how to administer the drug by themself. In the NICE appraisals for these drugs, the manufacturers for these prophylactic drugs assumed that patients would then be able to self-administer these drugs; however, based on the information and guidance provided by NICE we have assumed that 10% of patients would not be able to self-administer and we have included a cost for this in each subsequent cycle. 179,181 For the drugs which are administered in a hospital/clinic setting we have assumed that this would be a 15-minute appointment with a nurse. The hourly cost of the nurse’s time was obtained from the Unit Costs of Health and Social Care 2021. 187 These unit costs are shown in Table 13 and further information is provided in Appendix 9, Table 106. The price year for costs is 2021/22 and any costs not in this financial year were bought in line using the NHS cost inflation index. 187
| Resource use item | Unit cost (£) | Source |
|---|---|---|
| Prophylactic drugs (3-monthly cycle) – 2022 prices | ||
| BTA | 276.40 | https://bnf.nice.org.uk/ 51 |
| Eptinezumab 100 mg | 1350.00 | |
| Eptinezumab 300 mg | 4050.00 | |
| Fremanezumab – monthly | 1350.00 | |
| Fremanezumab – quarterly | 1350.00 | |
| Galcanezumab | 1350.00a | |
| Topiramate | 5.10 | |
| Staff time in 2021–2 prices | ||
| Nurse (hourly cost) | 42.00 | Unit Costs of Health and Social Care, 2021187 |
| Specialist consultant – neurologist (hourly cost) | 122.00b | Latest tariff did not include costs for neurology outpatient therefore assumed to be a follow-up attendance – single professional (WF01A) for a neurology outpatient visits (code 400).188 |
| Other resource items in 2021–2 prices | ||
| GP visit | 39.23 | Unit Costs of Health and Social Care, 2021187 |
| A&E visit | 165.00 | A&E worksheet. ‘VB08Z’, Emergency Medicine, Category 2 Investigation with Category 1 Treatment.189 |
| Hospital admission | 618.00 | Non-elective tariff for code AA31E (Headache, Migraine or Cerebrospinal Fluid Leak, with CC Score 0–6) in worksheet ‘1 APC & OPROC’ HRG code: AA31E.189 |
| Triptan usage | 3.99 | The cost of triptans per attack was based on the weighted average of triptan costs in the UK, taken from NHS Prescriptions Cost Analysis.167,182 |
For each 12-week cycle (regardless of the prophylactic medication), we assumed that there was a cost of care associated for each health state. This included GP visits, accident and emergency (A&E) visits, hospital admissions and triptan use. The frequency of usage for these resource items was obtained from the IBMS for UK patients. 190 In addition, we checked the NICE guidance for the various prophylactic medications and we also included any additional neurology consultant and nursing visits (see Table 13 and Appendix 9, Table 107).
All-cause mortality
Age-specific mortality rates used in the model were based on the UK general population lifetime tables from the Office for National Statistics (ONS). 191 Using the ONS data, the average probability of death for males and females were combined. As the cohort ages, mortality rates generally increase throughout the time horizon in the model.
Base-case and sensitivity analysis
We developed a Markov model from a UK NHS and personal social service (PSS) perspective to estimate the costs and QALY gains associated with the different prophylactic drugs for chronic migraine compared with placebo. For the base-case analysis, we have adopted a 2-year time horizon and the starting age for the patient cohort is 30 years. Costs are in 2021–2 prices and health outcomes are expressed in terms of QALY gains. Cost-effectiveness was measured in terms of an incremental cost per QALY gained (ICER). Discount rates of 3.5% were applied to both costs and outcomes.
We present both deterministic and probabilistic results. To represent the uncertainty in the parameters used in the model and to illustrate sampling uncertainty, a PSA was implemented via Monte Carlo simulations involving 1000 draws for all model inputs except the drug costs which were entered as deterministic values. We used a gamma distribution for costs and the beta distribution was used for utility values. These bootstrapped simulations enabled us to simulate 1000 replicates of the base-case ICER (displayed on cost-effectiveness planes) and to calculate the probability of cost-effectiveness at threshold values ranging from £0 to £50,000 per QALY gained [cost-effectiveness acceptability curves (CEACs)]. When comparing all prophylactic medications, we used a cost-effectiveness acceptability frontier (CEAF) which summarises the uncertainty around the cost-effectiveness of the various medications by indicating which strategy is preferred at different threshold values for cost-effectiveness.
Scenario and sensitivity analyses
We conducted scenario and sensitivity analyses by altering base-case inputs into the model. We did the following analyses:
-
Changing time horizon – in the base-case analysis we chose a 2-year time horizon, in the sensitivity analyses we have used a 5-year and a lifetime horizon.
-
Utility inputs – in the base-case analysis we chose to use utility values as recommended by NICE, currently using the Hernandez-Alava crosswalk algorithm,186 in the sensitivity analyses we have used the van Hout crosswalk algorithm. 192
-
Drug administration – in the base-case analysis we assumed that 10% of patients would not be able to self-administer (in line with NICE guidance); however, in practice our clinical colleagues said that all patients should be able to self-administer, and we have changed this assumption to only 1% of patients not being able to self-administer their medications.
-
MMDs – in the base-case analysis our main outcome was using MHDs, however, in this sensitivity analysis we have used MMDs as the outcome measure. This has enabled the addition of another medication for the analysis (erenumab – 70 and 140 mg). Furthermore, we have also used utility values from the Lipton et al. study which estimated utility values using MMDs. 171
-
Reducing drug costs for MAbs – we know that there are confidential discounts agreed via the Patient Access Scheme between the NHS and manufacturers, however, we do not know what these discounts are. In this sensitivity analysis, we have reduced the drugs costs by 25% and 50% for eptinezumab 100 and 300 mg, fremanezumab monthly and quarterly and galcanezumab.
-
Eptinezumab – using MHDs as the primary outcome we compared eptinezumab 100 versus 300 mg.
-
BTA versus topiramate – using MHDs as the primary outcome we compared BTA versus topiramate.
Expected value of perfect information
Value-of-information analyses explore the likelihood that additional evidence might alter the recommendation by reducing decision uncertainty, and determine parameters of study design (e.g. choice of comparator(s), length of follow-up, choice of outcome measures) that maximise the value of any future RCTs. The expected value of perfect information (EVPI) is the maximum expected gain in net benefit per patient that can be obtained from reducing uncertainty in model parameters. 193 To estimate the maximum expected gain in net benefit across the whole population, we can multiply the individual EVPI by the expected future population to benefit from the interventions.
To estimate the total population that would benefit from these prophylactic drugs, we need to know the incidence of chronic migraine per year in the UK. To the best of our knowledge, we could not find any reasonable estimate for the UK. We know that 2–4% of the world population meets the definition for chronic migraine;10,11 and 15% of the UK population have a migraine,3 but this is not split into migraine type. The annual global incidence of migraine is 1142.5 per 10,000 population. 194 Assuming that 2% of the UK population195 are at risk of chronic migraine, the incidence of migraine per year is 153,095.
If the population EVPI is not significantly greater than the cost of doing a specific piece of research, then there is no value in doing that research.
Results
Base-case analysis: cost-effectiveness results
In the base-case analysis we compared the cost-effectiveness of the different medications for chronic migraine using data from the NMA based on MHDs. The time horizon was 2 years, with a starting age of 30 years for the patient cohort. Costs are in 2021–2 prices and utility was estimated using the Hernandez-Alava crosswalk algorithm. Table 14 shows the deterministic (undiscounted and discounted) and probabilistic (discounted) results. The results are presented in terms of increasing costs.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER: cost per QALY gained (£) | |
|---|---|---|---|---|---|
| Deterministic results – undiscounted | |||||
| Placebo | 1784 | 1.4006 | – | – | – |
| Topiramate | 1675 | 1.4491 | −109 | 0.0485 | Dominated |
| Placebo | 1784 | 1.4006 | – | – | – |
| BTA | 3766 | 1.4802 | 1982 | 0.0796 | 24,900 |
| Placebo | 1784 | 1.4006 | – | – | – |
| Fremanezumab (monthly) | 10,458 | 1.4815 | 8674 | 0.0809 | 107,187 |
| Placebo | 1784 | 1.4006 | – | – | – |
| Fremanezumab (quarterly) | 10,498 | 1.4719 | 8714 | 0.0723 | 120,526 |
| Placebo | 1784 | 1.4006 | – | – | – |
| Eptinezumab 100 | 10,521 | 1.4745 | 8737 | 0.0739 | 118,235 |
| Placebo | 1784 | 1.4006 | – | – | – |
| Galcanezumab | 10,945 | 1.4734 | 9160 | 0.0728 | 125,795 |
| Placebo | 1784 | 1.4006 | – | – | – |
| Eptinezumab 300 | 28,219 | 1.4916 | 26,435 | 0.0910 | 290,453 |
| Deterministic results – discounted | |||||
| Placebo | 1729 | 1.3531 | – | – | – |
| Topiramate | 1624 | 1.3995 | –104 | 0.0464 | Dominated |
| Placebo | 1729 | 1.3531 | – | – | – |
| BTA | 3654 | 1.4294 | 1925 | 0.0763 | 25,238 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Fremanezumab (monthly) | 10,155 | 1.4307 | 8427 | 0.0776 | 108,604 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Fremanezumab (quarterly) | 10,193 | 1.4224 | 8465 | 0.0693 | 122,126 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Eptinezumab 100 | 10,216 | 1.4239 | 8487 | 0.0708 | 119,796 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Galcanezumab | 10,640 | 1.4229 | 8912 | 0.0698 | 127,649 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Eptinezumab 300 | 27,401 | 1.4403 | 25,672 | 0.0873 | 294,151 |
| Probabilistic results – discounted | |||||
| Placebo | 1728 | 1.3460 | – | – | – |
| Topiramate | 1624 | 1.4045 | –104 | 0.0584 | Dominated |
| Placebo | 1728 | 1.3460 | – | – | – |
| BTA | 3654 | 1.4270 | 1926 | 0.0810 | 23,775 |
| Placebo | 1728 | 1.3460 | – | – | – |
| Fremanezumab (monthly) | 10,161 | 1.4350 | 8433 | 0.0890 | 94,748 |
| Placebo | 1728 | 1.3460 | – | – | – |
| Fremanezumab (quarterly) | 10,196 | 1.4273 | 8467 | 0.0812 | 104,251 |
| Placebo | 1728 | 1.3460 | – | – | – |
| Eptinezumab 100 | 10,221 | 1.4199 | 8492 | 0.0739 | 114,894 |
| Placebo | 1728 | 1.3460 | – | – | – |
| Galcanezumab | 10,646 | 1.4161 | 8917 | 0.0701 | 127,279 |
| Placebo | 1728 | 1.3460 | – | – | – |
| Eptinezumab 300 | 27,411 | 1.4365 | 25,683 | 0.0904 | 284,030 |
When comparing each of the medications separately against placebo, the deterministic discounted results showed that topiramate was cheaper (£104 less expensive) and more effective (0.0464 more QALYs) than placebo, therefore topiramate dominated placebo. Each of the other medications, when compared separately, were more expensive than placebo, however, they generated more QALY gains than placebo. In terms of the cost per QALY gained, BTA was more cost-effective than placebo with an ICER of £25,238 per QALY gained. The other five medications (fremanzumab monthly, fremanzumab quarterly, eptinezumab 100 mg, eptinezumab 300 mg and galcanuzmab) when compared with placebo had ICERs which would not be considered cost-effective if using a £20,000–30,000 per QALY threshold. Probabilistic results were in line with deterministic results (see Table 14).
The cost-effectiveness planes for each of the medications versus placebo are shown in Appendix 10, Figures 50–56. For topiramate versus placebo, the ICER points are scattered across the four quadrants, with the majority of points in the bottom two quadrants (indicating that toprimate is cheaper but the effectiveness is varied in terms of topiramate either being less or more effective than placebo). For the other medications versus placebo, the ICER points were in the top two quadrants, indicating that each medication was more expensive than placebo and the effectiveness also varied (being less or more effective).
Figures 57–63 show the CEACs for each medication against placebo. For any amount (up to £50,000 maximum as shown in the graph) that a decision-maker is willing to pay for an additional QALY, topiramate was always 60% more cost-effective than placebo. When comparing BTA with placebo, if a decision-maker is willing to pay anything above £23,700 per QALY, BTA was the more cost-effective option. For the other medications, when comparing with placebo, placebo always remained the most cost-effective option (up to £50,000 maximum a decision-maker is willing to pay as shown in the graph).
Table 15 shows the base-case results when comparing all medications for the 2-year time horizon, ranked by the least costly option. For the discounted deterministic results, topiramate was the least costly option and had slightly more QALY gains than placebo, whereas eptinezumab 300 mg was the more costly option but generated the most QALY gains. Options placebo (dominated by topiramate), fremanezumab quarterly, eptinezumab 100 mg and galcanezumab (all dominated by fremanezumab monthly) were all eliminated as they were dominated by other medications. Then we compared topiramate, BTA, fremanezumab monthly and eptinezumab 300 mg. Fremanezumab monthly was extendedly dominated by a linear combination of BTA and eptinezumab 300 mg and therefore was eliminated. The ICER between BTA and topiramate and the ICER between eptinezumab 300 mg and BTA were not within plausible cost-effectiveness thresholds.
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER: cost per QALY gained (£) | Comparison for | |
|---|---|---|---|---|---|---|
| Deterministic results – discounted | ||||||
| Topiramate | 1625 | 1.3995 | – | – | – | |
| Placebo | 1729 | 1.3531 | 104 | −0.0464 | Dominated | Placebo vs. topiramate |
| BTA | 3654 | 1.4294 | 2029 | 0.0298 | 68,002 | BTA vs. topiramate |
| Fremanezumab (monthly) | 10,155 | 1.4403 | 6501 | 0.0013 | Extendedly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 10,193 | 1.4224 | 38 | −0.0083 | Dominated | Fremanezumab (quarterly vs. monthly) |
| Eptinezumab 100 | 10,216 | 1.4239 | 60 | −0.0067 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Galcanezumab | 10,640 | 1.4229 | 485 | −0.0078 | Dominated | Galcanezumab vs. fremanezumab (monthly) |
| Eptinezumab 300 | 27,401 | 1.4403 | 23,747 | 0.0110 | 2,160,037 | Eptinezumab 300 vs. BTA |
| Probabilistic results – discounted | ||||||
| Topiramate | 1624 | 1.4045 | – | – | – | |
| Placebo | 1728 | 1.3460 | 104 | −0.0584 | Dominated | Placebo vs. topiramate |
| BTA | 3654 | 1.4270 | 2030 | 0.0226 | 89,939 | BTA vs. topiramate |
| Fremanezumab (monthly) | 10,161 | 1.4350 | 6507 | 0.0080 | Extendedly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 10,196 | 1.4273 | 34 | −0.0078 | Dominated | Fremanezumab (quarterly vs. monthly) |
| Eptinezumab 100 | 10,221 | 1.4199 | 59 | −0.0151 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Galcanezumab | 10,646 | 1.4161 | 485 | −0.0189 | Dominated | Galcanezumab vs. fremanezumab (monthly) |
| Eptinezumab 300 | 27,411 | 1.4365 | 23,757 | 0.0094 | 2,524,429 | Eptinezumab 300 vs. BTA |
For the discounted probabilistic results, again topiramate was the least costly option and had slightly more QALY gains than placebo, whereas eptinezumab 300 mg was the more costly option but generated the most QALY gains, in line with the deterministic results. Options placebo (dominated by topiramate), fremanezumab quarterly, eptinezumab 100 mg and galcanezumab (all dominated by fremanezumab monthly) were all eliminated as they were dominated by other medications. Then we compared topiramate, BTA, fremanezumab monthly and eptinezumab 300 mg. Fremanezumab monthly was extendedly dominated by a linear combination of BTA and eptinezumab 300 mg and therefore was eliminated. The ICER between BTA and topiramate and the ICER between eptinezumab 300 mg and BTA were not within plausible cost-effectiveness thresholds. This is represented graphically using a CEAF, where topiramate is the most cost-effective medication if the decision-maker is willing to pay up to £50,000 per QALY (Figure 27).
FIGURE 27.
Base-case CEAF.
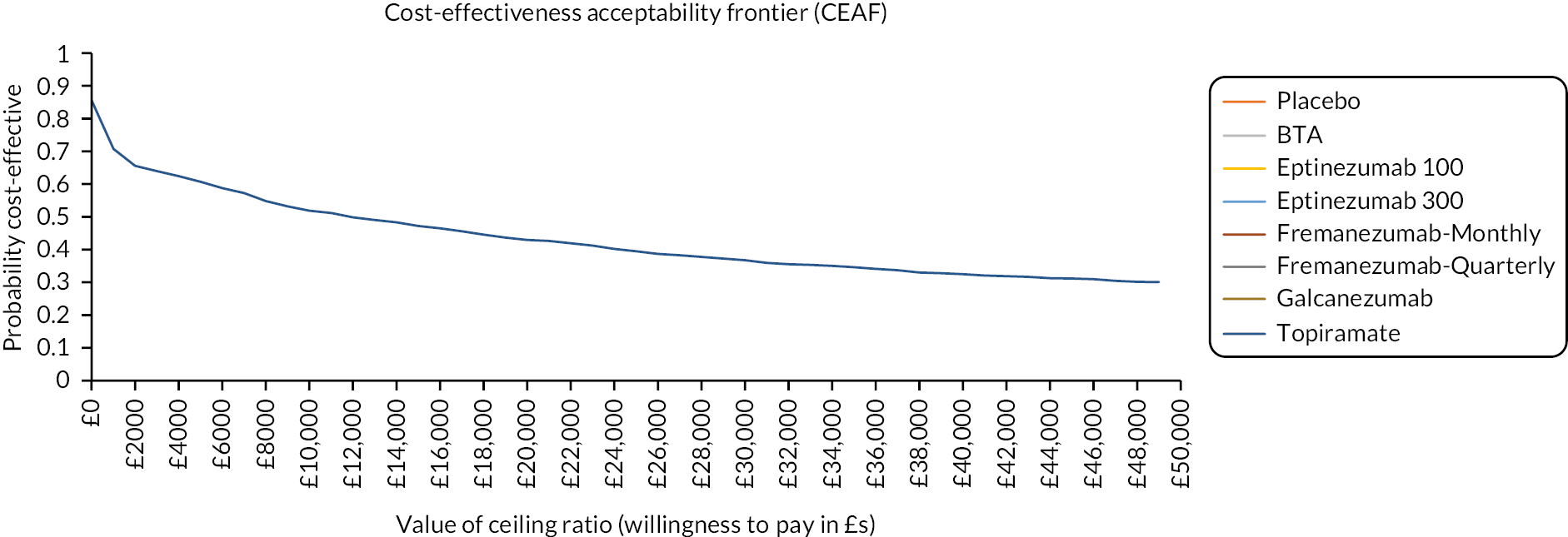
Sensitivity analysis: cost-effectiveness results
The results for the discounted deterministic and discounted PSA when comparing each medication separately against placebo are shown in Appendix 10, Table 108. When changing the time horizon from 2 to 5 years, using van Hout crosswalk algorithm instead of the Hernandez-Alava crosswalk algorithm for utility values, and assuming that 1% of patients could not self-administer their medication, and reducing the drug costs for the MAbs to 25% and 50%, these results were are all in line with the base-case analyses. When a lifetime horizon was adopted, topiramate still dominated placebo; however, when the other medications (apart from eptinezumab 300 mg) were compared with placebo separately, they were all deemed cost-effective with the ICERs less than £20,000 per QALY gained. Using MMDs as an outcome measure instead of MHDs, only BTA was below the £20k cost per QALY gained threshold, all other medications did not fall below the recommended £20,000–30,000 threshold by NICE. When comparing eptinezumab 100 mg with 300 mg, and BTA with topiramate, the ICERs did not fall within a cost-effectiveness range (see Appendix 10, Table 108).
The results for the discounted deterministic and discounted PSA when comparing all medications together are shown in Appendix 10, Table 109. For all the different scenarios, and in line with the base-case results, topiramate was the least costly option and had slightly more QALY gains than placebo, whereas eptinezumab 300 mg was the more costly option but generated the most QALY gains (except for when using MMDs as an outcome measure, fremanezumab monthly generated slightly more QALY gains then eptinezumab 300 mg). Only for the lifetime horizon, when removing the dominated options, BTA was more cost-effective than topiramate and the cost per QALY gained was always within the £20,000 WTP threshold. When left with the non-dominated options – comparing either fremanezumab monthly or eptinezumab 300 mg with BTA, the ICERs were not within plausible cost-effectiveness threshold ranges.
Expected value of perfect information results
The EVPI per person per year is £2374 at a cost-effectiveness threshold of £20,000 per QALY and £4047 at a cost-effectiveness threshold of £30,000 per QALY. To calculate the full EVPI for this decision, these figures need to be multiplied by the number of people whose treatment depends on the decision being made, and then aggregated (with discounting) over the time period until the decision is revisited. Assuming an annual decision population of 153,095, this gives an annual EVPI of £363 million at a cost-effectiveness threshold of £20,000 per QALY and £620 million at a cost-effectiveness threshold of £30,000 per QALY.
Assuming conservatively that the decision might be updated in 2 years, the total EVPI would therefore be £720 million at a cost-effectiveness threshold of £20,000 per QALY and at £1228 million at a cost-effectiveness threshold of £30,000 per QALY. This is an upper bound on the value of research, and the expected value of sample information of a specific trial would be less. The cost-effectiveness of further research will also depend on what treatment is offered in the interim. Nevertheless, the EVPI is substantial, suggesting there would be considerable value to further research in the form of a clinical trial to reduce decision uncertainty.
Discussion
We developed a Markov (state-transition) model to assess the cost-effectiveness of different pharmacological medications compared to usual care (placebo) to treat or prevent chronic migraine in the adult population based on evidence from the cost-effectiveness review in Chapter 4 and in consultation with our clinical colleagues.
The model used the effectiveness data – the reduction in the MD in MHDs – for the different medications from the NMA in Chapter 2. In the base-case analysis, costs were in 2021–2 prices and calculated from an NHS and PSS perspective over a 2-year time horizon. EQ-5D-5L scores from the CHESS trial were converted into health state utility values using the Hernandez-Alava crosswalk algorithm. The health state utilities were expressed in terms of QALYs.
Our base-case deterministic results showed that when comparing each of the medications seperately against placebo, topiramate dominated placebo. Each of the other medications, when compared separately, were more expensive than placebo, however, they generated more QALY gains. In terms of the cost per QALY gained, BTA was more cost-effective than placebo with an ICER of £25,328 per QALY gained. When comparing all medications together, topiramate was the least costly option and had the fewest QALY gains, whereas eptinezumab 300 mg was the more costly option but generated the most QALY gains. The ICER between BTA and topiramate, fremanezumab monthly and BTA, and eptinezumab 300 mg and fremanezumab monthly were not within plausible cost-effectiveness thresholds. Base-case probabilistic sensitivity analyses based on 1000 Monte Carlo simulations were in line with the base-case deterministic results; the CEAF showed that when comparing all medications, topiramate was the most cost-effective medication if the decision-maker is willing to pay up to £50,000 per QALY. Extensive sensitivity/scenario analyses were conducted, and when using MHDs as an outcome measure, the results were generally in line with the base-case results. The main exception was when using MMDs as an outcome measure instead of MHDs, fremanezumab monthly generated more QALY gains than eptinezumab 300 mg; and furthermore, when a lifetime horizon was adopted, all medications when compared separately against placebo were regarded as cost-effective as the cost per QALY gained fell below the £20,000 willingness-to-pay threshold, although eptinezumab 300 mg did not fall into this category.
A number of limitations apply to the model. Firstly, as there were not enough data in the literature, we assumed that once a participant enters the ‘off treatment’ health state, they cannot return to an ‘on treatment’ health state. However, in practice we know that a participant can come off a prophylactic medication if their migraines are better, or if they cannot tolerate a medication; however, their migraine may return sometime later, and they may be prescribed another medication for their migraine.
Secondly, there were no data on MHDs for erenumab, hence, we only compared erenumab when using MMDs as an outcome measure. However, erenumab may have the potential to be a cost-effective treatment as it generated more QALY gains than BTA, even though it was slightly more expensive. Furthermore, there was no effectiveness data on the cheaper oral drugs, such as amitriptyline and propranolol, and including these medications in the economic model may have changed the overall cost-effectiveness results.
Thirdly, the small differences in QALY gains between some of the medications, namely fremanezumab and BTA, meant that they produced huge ICERs and therefore these drugs may not appear to be cost-effective.
Fourthly, the length of follow-up used in the base-case model was 2 years as there is not enough long-term data on the success or failure of these medications. However, in a sensitivity analysis we used a lifetime horizon and nearly all of the medications, including the costly CGRP MAbs, were considered to be cost-effective against placebo and within plausible WTP thresholds.
Fifthly, we know that a lot of these medications are heavily discounted. We conducted a sensitivity analysis reducing the costs of these expensive CGRP MAbs by 25% and 50% and although the cost of these medications fell, the ICERs were still huge. This was mainly due to the small QALY gain differences between the prophylactic medications which were compared.
Sixthly, we used utility data based on MHDs based on the CHESS trial. There was very limited data in the literature on utility values which were based on MMDs. Also, there were no studies that mapped EQ-5D or SF-6D data to generate utility values for specific headache day health states which we have used in our model.
Seventhly, as the model is from an NHS and PSS perspective, we have not taken into account any broader societal costs, such as the costs to the patient for time off work and loss of pay (productivity). Finally, the model did not take into account any adverse effects associated with migraines as described in Chapter 3.
In summary, we found that topiramate was the least costly option and had the fewest QALY gains, whereas eptinezumab 300 mg was more costly but generated the most QALY gains. The CEAF showed that topiramate was the most cost-effective medication if the decision-maker is willing to pay up to £50,000 per QALY.
Chapter 6 Consensus workshop and recommendations for research priorities
Research question 5: Based on our findings, what should the research recommendations be?
Introduction
In our final work package, we used consensus methodology to develop a set of research recommendations based on the results from the systematic reviews and the economic modelling.
Methods
Ethical approval
We obtained ethical approval for the consensus workshop from the University of Warwick’s Biomedical and Scientific Research Ethics Committee (BSREC 49/22-23).
Design
Nominal Group Technique (NGT) is a method used in health research to enable a large diverse group to generate ideas and make decisions quickly. 196–199 The method facilitates everyone to participate and contribute to the decision-making. The workshop was designed together with our patient and public involvement (PPI) representative who suggested that we add a breakaway session, wherein people with migraine and headache experts meet separately, to share thoughts and reflect on any challenges in the mixed groups. This was an additional way of ensuring that all voices were heard at the workshop. Small group work facilitated discussion between patients and healthcare professionals, moderated by experienced facilitators. Facilitators were not members of the study team, minimising the possibility of influencing the conversation in a particular direction. At the end of the workshop, participants voted anonymously using an online polling software (Vevox). 200
Sample and recruitment
We aimed to recruit around 15 people with chronic migraine and 10 healthcare professionals. People with migraine were approached through the National Migraine Centre (NMC) mailing list. An invitation was sent out by administrators of the NMC containing information about the workshop and a link to an online expression of interest (EoI) form and contact details for the study team. Healthcare professionals were approached directly by the study team using personal networks. We aimed for a mix of specialties and backgrounds, such as neurologists, GPs with a special interest and headache nurses. Clinicians were invited to express an interest by contacting the study team.
Results
Participants
We received 147 EoIs in response to the invitation shared by NMC. Nineteen people were sampled for maximum variation in terms of age, years living with chronic migraine and ethnicity, and were invited to the workshop. Eight people with chronic migraine attended on the day. Fourteen clinicians expressed an interest in response to information circulated by the team to their networks, and all were invited to the workshop. Eleven attended on the day; all of the eight neurologists worked in a secondary care setting and four of these worked solely as headache specialists (tertiary referral headache neurologists). Although we invited more people with migraine than health professionals, on the day the balance of attendees was tipped in favour of health professionals. Demographics of our sample are shown in Table 16.
| Characteristic | Number | ||
|---|---|---|---|
| People with migraine | Age | 18–39 | 3 |
| 40–59 | 3 | ||
| 60+ | 2 | ||
| Ethnicity | White British/other | 4 | |
| Mixed heritage | 3 | ||
| Asian British/other | 1 | ||
| Number of years with CM | 0–9 | 4 | |
| 10–20 | 2 | ||
| 20+ | 2 | ||
| Health professionals | Role | Neurologists | 8 |
| Specialist nurse | 1 | ||
| GP with special interest | 2 | ||
The workshop
Prior to the workshop, we sent out a summary of the study findings (see Report Supplementary Material 4). The workshop took place online using Microsoft Teams. The workshop began with a presentation to summarise the research and the findings of work packages 1–4. We explained the aim of the workshop, and the scope of research recommendations. Our PPI representative spoke briefly about the importance of equal voice within the small groups. Following this, we split into three breakout groups with around seven participants (approximately four healthcare professionals and three people with migraine).
In the small groups, participants were asked to agree on their top five drug-placebo comparisons, and their top five drug-drug comparisons. They were asked to consider:
-
how much evidence we have on the drug
-
safety (side effects)
-
efficacy (how effective the drugs were found to be in this study)
-
feasibility (cost, availability, ease of administration).
We provided a crib sheet reporting the study findings to support the discussion and decision-making (see Report Supplementary Material 4). Participants could suggest comparisons of drugs not included in this study, and they were reminded that they held valid knowledge and perspectives to bring to the discussion.
We then split into two groups: one group with people with migraine and the other group with healthcare professionals, to reflect on the success of/any issues with equal voice in the small group sessions. During a break, the scribes sent their notes from the breakout group sessions to the team. We then held a plenary session to discuss the outcomes of the group discussions, took a break and prepared for the voting (to take place using Vevox, an anonymous polling website). 200 Voting took place in the last part of the workshop, followed by a brief discussion of the results and explanation of the next steps for the team. All attendees were provided with a certificate of attendance (health professionals) or thank you letter and payment (patients) by e-mail after the meeting.
Results
Each group provided ten top comparisons [five drug vs. placebo (Table 17), and five drug vs. drug (Table 18)]. We removed duplicate questions to create the following two lists of top comparisons:
| Beta-blocker | Placebo |
| Candesartan | Placebo |
| Doxycycline | Placebo |
| Flunarizine | Placebo |
| Melatonin | Placebo |
| Rimegepant | Placebo |
| SNRIs (duloxetine, venlafaxine) | Placebo |
| Tricyclic antidepressant | Placebo |
| All CGRP MAbs rotation | All CGRP MAbs rotationa |
|---|---|
| BTA + topiramate | CGRP MAbs |
| CGRP MAbs | BTA |
| CGRP MAbs | CGRP MAbs + gepant |
| CGRP MAbs + BTA | BTA |
| CGRP MAbs + BTA | CGRP MAbs |
| CGRP MAb receptor | MAb ligand |
| Flunarizine | BTA |
| Melatonin | Amitriptyline |
| Propranolol | BTA |
| Topiramate | Flunarizine |
Participants then anonymously voted for their top five choices of the drug versus placebo (Table 19) and drug versus drug (Table 20) comparisons. The results were:
| 1 | Candesartan | Placebo |
| 2 | Flunarizine | Placebo |
| 3 | Melatonin | Placebo |
| 4 | Beta-blocker | Placebo |
| 5 | Tricyclic antidepressant | Placebo |
| 1 | CGRP MAbs + BTA | CGRP MAbs |
| 2 | CGRP MAbs | BTA |
| 3 | CGRP MAb receptor | Mab ligand |
| 4 | CGRP MAbs + BTA | BTA |
| 5 | CGRP MAbs | CGRP MAbs + gepant |
We then combined these 10 and asked participants to rank them in order of priority (Table 21). The results were:
| 1 | CGRP MAbs + BTA | CGRP MAbs |
| 2 | Candesartan | Placebo |
| 3 | Flunarizine | Placebo |
| 4 | CGRP MAbs | BTA |
| 5 | CGRP MAbs + BTA | BTA |
| 6 | CGRP MAb receptor | MAb ligand |
| 7 | Tricyclic antidepressant | Placebo |
| 8 | CGRP MAbs | CGRP MAbs + gepant |
| 9 | Melatonin | Placebo |
| 10 | Beta-blocker | Placebo |
Discussion
The results indicate that comparisons of CGRP MAbs and BTA were a top priority for our group. They also raised the question of whether there might be additive effects of combining these medications, which was not something we anticipated. The effect sizes, in terms of MHDs/MMDs days for each of the drugs we included in our reviews, are at best modest; the largest being 2.76 days for fremanezumab monthly dose. As these drugs work through different pathways, it might be that more substantial effects are possible. Adding together the effects of BTA and a CGRP MAb, assuming no negative interaction, might have a mean effect size of 4–5 days that would be transformative for many people with chronic migraine. Candesartan and flunarizine were the top drugs the group wanted compared against placebo. There was no evidence for these drugs in our clinical and cost-effectiveness study, and the group felt strongly that these were important drugs to study.
Chapter 7 Discussion and conclusions
Statement of principal findings
In Chapter 2, we identified 11 RCTs with more than 100 participants per arm from 51 publications which comprised 7352 adult participants with chronic migraine. We found that all pharmacological medications for all outcomes of interest were beneficial in preventing chronic migraine compared with placebo; however, there were no trials of sufficient quality of the commonly used drugs, such as propranolol or amitriptyline. Overall, the CGRP MAbs reduced headache/migraine days by 2.0–2.5 days per month. Eptinezumab 300 mg reduced MHDs by 2.46 days and fremanezumab monthly reduced MMDs by 2.76 days. BTA reduced headache/migraine days by fewer than 2 days per month. The NMA results showed that eptinezumab 300 mg had the highest probability ranking to reduce MHDs and MMDs; and BTA ranked better in the NMA in terms of the mean change in MHD compared with the mean change in MMD. Topiramate reduced headache/migraine days by less than 1.5 fewer days per month. The CGRP MAbs provided a worthwhile improvement on the HIT-6 measure – eptinezumab 300 mg reducing the HIT-6 by a score of 3.22 points and BTA had a worthwhile effect on the HIT-6 measure, reducing the HIT-6 score by 2.10 points. There was no convincing benefit of topiramate on the MSQ measure. Galcanezumab 120 mg provided the best improvement in QoL for the MSQ-PR dimension, but for two other dimensions of the MSQ-RR and MSQ-EF, erenumab 140 mg was superior to other treatments. The quality assessment results found that approximately 46% of the included RCTs in this review had a low RoB and 36% of the RCTs had some concerns of bias.
In Chapter 3, the incidence of AEs review found evidence from 40 RCTs reported across 67 articles, which investigated pharmacological interventions to manage chronic or episodic migraine. These trials included 25,891 participants and three additional drugs were included – amitriptyline, atogepant and rimegepant. There were very few SAEs – none of which were linked to the use of these drugs. Non-SAEs were common, and results suggested that all the pharmacological medications included in this review were found to be tolerable. There were differences in the incidence of AEs between the CGRP MAbs with most people using fremanezumab and one in four people using galcanezumab reporting injection site issues. These issues were much less common in people using eptinezumab or erenumab. Most people using topiramate or amitriptyline had nervous system or gastrointestinal side effects; topiramate was also linked to a higher prevalence of psychiatric disorders; and AEs related to BTA were uncommon.
In Chapter 4, the cost-effectiveness review identified nine peer-reviewed journal articles and seven published reports of economic evaluation studies of chronic migraine prophylactic medications in the adult population. All articles were model based evaluations, and none were trial-based economic evaluations. We found that although these newer drugs (BTA and CGRP MAbs) were more costly than the oral preventatives, they were however deemed cost-effective. Generally, the articles were classed as high quality when appraised by the CHEERS reporting tool.
In Chapter 5, our economic model to assess the cost-effectiveness of different pharmacological medications to treat chronic migraine found that when comparing each of the medications separately against placebo, topiramate dominated placebo (cheaper and more effective); and the best value medication was BTA, with the cost per QALY around £25,000. When comparing all medications together, the results showed that topiramate was the least costly option and had slightly more QALY gains than placebo, whereas eptinezumab 300 mg was the more costly option but generated the most QALY gains. Most medications were eliminated due to dominance. The ICER between BTA and topiramate, and the ICER between eptinezumab 300 mg and BTA weres not within plausible cost-effectiveness thresholds. Probabilistic results were similar to deterministic results. The CEAF showed that when comparing all medications topiramate was the most cost-effective medication if the decision- maker is willing to pay up to £50,000 per QALY. None of the CGRP MAbs represented good value for money in this comparative analysis. However, it is likely that CGRP MAbs will be cost-effective in people who have failed treatment with BTA.
In Chapter 6, our consensus workshop brought together 8 participants with chronic migraine and 11 health professionals with expertise in chronic migraine. The small groups found that the cheaper, oral medications, tricyclic antidepressants, SNRIs were ranked highly when compared with placebo; and when comparing the medications with each other, the CGRP MAbs and BTA separately or in combination with each other were ranked highly. The final (anonymised) rankings showed that the top three medications were: (1) candesartan, (2) flunarizine and (3) melatonin when compared with placebo; and (1) CGRP MAbs and BTA versus CGRP MAbs, (2) CGRP MAbs versus BTA and (3) CGRPs MAbs receptor (erenumab) versus CGRP MAbs ligand (eptinezumab, fremanezumab and galcanezumab), when all medications were compared together. In terms of priority, general consensus was reached on the top three choices of medications for chronic migraine: (1) CGRP MAbs and BTA versus CGRP MAbs, (2) candesartan versus placebo and (3) flunarizine versus placebo.
Strengths and limitations
To the best of our knowledge, this study is the first, most comprehensive NMA and economic model for pharmacological medications for chronic migraine in adult participants in the UK. A key strength of this project is the range of chronic migraine treatments we have included. These include the latest CGRP MAbs, namely fremanezumab, eptinezumab, galcanezumab and erenumab, which are commonly used after other concurrent preventive treatments, such as BTA and topiramate have failed in the UK.
A further strength of this project is that we applied a methodological approach following internationally recognised systematic review guidance for the systematic reviews and network meta-analyses. For example, we used a robust, comprehensive systematic search strategy, where the search was run on a broad range of electronic databases to identify all relevant trials, which did not allow for any date or language restrictions; and studies were selected by at least two reviewers independently and data extraction was performed by one reviewer and checked by a second to ensure accuracy and completion. We also conducted risk of bias and quality assessments of the included articles. For the clinical effectiveness review, we not only included the key outcomes in terms of headache days and migraine days, but we also looked at headache-related quality of life. For the AEs review, we widened our inclusion criteria to include episodic migraine participants as well as chronic migraine participants, this enabled us to include three more additional migraine medications. The economic model was based on a previous peer-reviewed published economic model which we adapted, and we conducted a fully probabilistic economic analysis, which meant that it avoids assuming that uncertain parameters are fixed. Finally, we held a consensus workshop and consensus was reached on the top three choices of medication for chronic migraine.
A potential limitation of this study is that we could not include all medications used to treat chronic migraine. This was due to excluding any RCTs with less than 100 participants per arm to ensure that we included better-quality studies and to avoid loss of precision on our NMA by including heterogeneous studies. We made this decision based on our initial scoping review where we found many studies that appeared to be of chronic migraine.
On further examination during the research process, it became clear that many studies apparently of chronic migraine were in fact of episodic migraine, of mixed populations. Additionally, the 11 trials we did include were reported across 51 papers meaning the pool of trials was very much smaller than it appeared at the start of the study.
Disappointingly, we did not identify any eligible studies for other commonly used drugs, recommended by NICE and/or SIGN, such as amitriptyline, candesartan, flunarizine or propranolol. This emphasises the need for high-quality trials on these older oral medications to ensure that we are appropriately using them.
Our post hoc re-examination of the characteristics of studies excluded on the basis of size identified just one small trial (n = 72) comparing amitriptyline and BTA that might have met our inclusion criteria. 123 This trial did not report on our outcomes of interest. One other trial, testing the addition of propranolol to topiramate (n = 191), would have met our size criterion except it was closed early for futility. 124 Furthermore, the trial would not have contributed to our NMA. Even though the trial did produce a very clear result on the futility of adding propranolol to topiramate, it does not tell us anything about how effective propranolol might be as monotherapy.
Furthermore, some of these older trials did not define whether the migraine was either chronic or episodic, or even define a difference, and including them would have resulted in a large degree of heterogeneity; this has limited our NMA to more recently investigated treatments when chronic migraine was introduced as a classification in 2007. Overall, this means that we only included the more recently investigated treatments where the trial methodology is more precise and excluded some of the pertinent data from smaller, usually older, trials such as the oral preventatives.
All of our included trials were industry funded, therefore caution is needed when interpreting these results. For the additional three drugs included in the AEs review, two of these drugs, atogepant and rimegepant, have product licences, but these have not yet been approved by NICE or SMC for the preventative treatment of CM. The main limitation of our cost-effectiveness review is that we only included full economic evaluations (i.e. studies which compared both costs and outcomes of the intervention and comparator), so we may have missed potential important information relating to the costs and outcomes of these medications. In our economic model, we did not have data on MHDs for one of the most commonly used MAbs, erenumab; hence, this medication was excluded from the main base-case analysis and including this medication may have resulted in slightly different findings as shown by the sensitivity analysis when erenumab was included when using MMDs as the outcome measure. Furthermore, we included eptinezumab 300 mg as a dose, but only the 100 mg dose for eptinezumab was approved by NICE and SMC, hence further caution is needed when interpreting these results.
Patient and public involvement
We would like to thank Andrew Cooklin for providing a patient and public perspective. He contributed to the design of the protocol, the study methods and findings, and the writing of the Plain Language Summary, and helped with the consensus workshop. Furthermore, we would like to thank the participants with chronic migraine and headache experts who took part in the consensus workshop. As a result of PPI involvement, we identified the need for trials where all medications currently used for chronic migraine can be compared concurrently, and this has contributed to our recommendations for future research.
Equality, diversity and inclusion
The report contains data from published peer-reviewed articles and reports. We cannot take responsibility for any information that does not abide by equality, diversity and inclusion in the inclusion of studies in this report.
Implications for practice
Our clinical effectiveness results suggested that the CGRP MAbs overall were consistently the best choices for headache days, migraine days and headache-related quality of life. However, our economic model suggested that topiramate was the best value drug if you are prepared to pay up to £50,000 per QALY. However, there is uncertainty in these results as not all medications were included in the base-case economic analysis as we did not have information on MHDs for some of the medications; and thus, if all current chronic migraine medications were included, the cost-effectiveness results may have been different.
Topiramate is the only established oral drug we can make any observations for and compare with CGRP MAbs. The CGRP MAbs appear to be clinically superior, but even so, topiramate, despite its high incidence of AEs, represents the best value for money. Within the current care pathway, it is unlikely that BTA or CGRP MAbs will be recommended ahead of topiramate without a very substantial reduction in price. What is perhaps a more critical decision point is whether BTA or CGRP MAbs might be preferred as the first choice in patients where oral medications are not effective. Our findings support continuing with the current care pathway since our CEAF found that only topiramate met an acceptable threshold. However, as noted in our sensitivity analysis, if the price of the CGRPs MAbs was reduced then these medications are more likely to be cost-effective. Data from our health economics review, however, does support the use of CGRP MAbs for chronic migraine in patients where BTA is not effective.
It is disappointing that we did not find an evidence base to support the use of medications such as amitriptyline, candesartan, flunarizine and propranolol that are recommended by NICE and/or SIGN. Our consensus meeting identified the need for trials comparing candesartan and flunarizine with placebo as the top priorities for placebo-controlled trials. Furthermore, our consensus group identified the direct comparison of BTA and CGRP MAbs as a key research question. They also identified the question of whether the clinical effectiveness of these drugs might be additive. The effect sizes, in terms of mean monthly migraine/headache days for each of these drugs, are at best modest, the largest being 2.76 days for Fremanezumab monthly dose. As these drugs work through different pathways, it might be that more substantial effects are possible. Adding together the effects of BTA and a CGRP MAb, assuming no negative interaction, might have a mean effect size of 4–5 days that would be transformative for many people with chronic migraine. Our consensus group identified the comparative, and additive, effects of BTA and CGRP MAbs as high-priority research questions, although it should be noted that a previous study of multiple drugs for chronic migraine was terminated for futility. 124
Recommendations for future research
Further research is needed where all medications currently used for chronic migraine can be compared concurrently, using common outcome measures, such as MHDs or MMDs. Head-to-head RCTs of these common medications for chronic migraine are very much needed to assess both the clinical and cost-effectiveness evidence for adults with chronic migraine in the UK.
Conclusions
The CGRP MAbs overall were consistently best choices for headache days, migraine days and headache-related quality of life. BTA was less likely to be the best choice than some (but not all) CGRP MAbs for headache days, migraine days and headache-related quality of life. Topiramate was very unlikely to be the best choice for headache days, migraine days and headache-related quality of life when compared to CGRP MAbs or BTA. The economic model found that topiramate was the best value drug if you are prepared to pay up to £50,000 per QALY. It is likely that CGRP MAbs are likely to be cost-effective in people who have failed treatment with BTA. We reached general consensus on the top three choices of medication for preventing chronic migraine.
In conclusion, we have summarised the existing clinical and cost-effectiveness data on preventive drugs for chronic migraine and identified which directions future research on these drugs might take. We did not find convincing evidence that the CGRP MAbs are more clinically effective and cost-effective compared to topiramate or BTA.
Additional information
Acknowledgements
The authors would like to thank Dr Rachel Potter who helped with obtaining ethical approval for the consensus workshop, Dr Felix Achana who provided some economic advice and Dr Nicky Welton who provided some guidance for the network meta-analysis. We are also grateful to Dr Susanne Arnold, Professor David Ellard and Dr Vivien Nichols who helped with facilitating the groups for the consensus workshop. Finally, we would also like to say thank you to Felicity Langar and Kimberley Stewart who helped out with administrative queries and the consensus workshop.
CRediT contribution statement
Hema Mistry (https://orcid.org/0000-0002-5023-1160): Conceptualisation (equal), Data curation (equal), Formal analysis (equal), Funding acquisition (lead), Methodology (equal), Project Administration (lead), Supervision (lead), Validation (lead), Writing – original draft (equal), Writing – editing and reviewing (lead).
Seyran Naghdi (https://orcid.org/0000-0001-5504-7189): Data curation (equal), Formal analysis (equal), Methodology (equal), Validation (supporting), Writing – original draft (equal), Writing – editing and reviewing (supporting).
Anna Brown (https://orcid.org/0000-0002-4541-6232): Data curation (equal), Funding acquisition (supporting), Writing – original draft (supporting).
Sophie Rees (https://orcid.org/0000-0003-4399-2049): Data curation (supporting), Funding acquisition (supporting), Writing – original draft (equal).
Jason Madan (https://orcid.org/0000-0003-4316-1480): Formal analysis (supporting), Funding acquisition (supporting), Methodology (supporting), Validation (supporting), Writing – original draft (supporting).
Amy Grove (https://orcid.org/0000-0002-8027-7274): Funding acquisition (supporting), Writing – original draft (supporting).
Saval Khanal (https://orcid.org/0000-0001-5201-0612): Data curation (supporting), Formal analysis (supporting), Validation (supporting).
Callum Duncan (https://orcid.org/0000-0002-4516-9024): Conceptualisation (equal), Funding acquisition (supporting), Writing – original draft (supporting).
Manjit Matharu (https://orcid.org/0000-0002-4960-2294): Conceptualisation (equal), Funding acquisition (lead), Writing – original draft (supporting).
Andrew Cooklin: Funding acquisition (supporting), Writing – original draft (supporting).
Aiva Aksentyte (https://orcid.org/0000-0002-3242-855X): Data curation (supporting).
Natasha Davies (https://orcid.org/0000-0001-6635-7035): Data curation (supporting).
Martin Underwood (https://orcid.org/0000-0002-0309-1708): Conceptualisation (equal), Data curation (supporting), Formal analysis (supporting), Funding acquisition (supporting), Methodology (supporting), Supervision (supporting), Validation (supporting), Writing – original draft (equal), Writing – editing and reviewing (supporting).
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/AYWA5297.
Primary conflicts of interest: Hema Mistry is a member of the NIHR HTA General Funding Commissioning Committee. She is co-investigator on NIHR grants.
Jason Madan is co-investigator on NIHR grants (NIHR award IDs: 14/199/14; 17/149/12; 17/84/07; 17/86/06; NIHR132871; NIHR200846; 14/25/05; RP-PG-1209-10071).
Amy Grove is a member of the NIHR HTA Commissioning Committee and a member of the NIHR DSE Fellowship Funding Committee. She is supported by NIHR Advanced Fellowship NIHR300060 and also by the NIHR Applied Research Collaboration (ARC) West Midlands NIHR200165. She is co-investigator on NIHR grants.
Callum Duncan is chair of Scottish Intercollegiate Guideline Network (SIGN) 155 and has provided advice on the use of BTA, CGRP monoclonal antibodies and CGRP antagonists to the Scottish Medicines Consortium and on eptinezumab to NICE. He was the Secretary for the British Association for the Study of Headache 2015–22 and he is a board member of Anglo Dutch Migraine Association. He is co- investigator on NIHR grants.
Manjit Matharu is the president of the medical advisory board of the CSF Leak Association. He has received consulting fees from AbbVie, TEVA, Lundbeck, Eli Lilly, Salvia and Pfizer. He has received payment for the development of educational presentations from AbbVie, Pfizer and Eli Lilly and support for attending a meeting from Pfizer. He is on the advisory board for AbbVie, TEVA, Lunbeck, Eli Lilly, Salvia and Pfizer. He has the following patent issued WO2018051103A1: System and method for diagnosing and treating headaches. He has stock options with Tesla, Adobe, Nvidia, META and Microsoft. He has received grants from Abbott, Medtronic and The Ehlers Danlos society. He is co-investigator on NIHR grants.
Martin Underwood is chief investigator or co-investigator on multiple previous and current research grants from the UK National Institute for Health and Care Research (NIHR award IDs: 13/146/02; 14/224/04; 15/15/09; 16/61/18; 16/77/02; 17/129/02; NIHR128768; NIHR131316; NIHR131407; NIHR131629; NIHR132046; NIHR132871; 14/25/05; 17/60/22; NIHR134398; 16/167/56), and is a co-investigator on grants funded by the Australian NHMRC and Norwegian MRC. He was part of the NIHR Journals Library Editors Group 1 March 2016 to 31 March 2019. He was an NIHR senior investigator until March 2021. He is a director and shareholder of Clinvivo Ltd that provides electronic data collection for health services research. He is part of an academic partnership with Serco Ltd, funded by the European Social Fund, related to return to work initiatives. He receives some salary support from University Hospitals Coventry and Warwickshire NHS Trust. He is a co-investigator on two current and one completed NIHR-funded studies that have, or have had, additional support from Stryker Ltd. He has published multiple papers on headache disorders, some of which are cited in this monograph.
Data-sharing statement
All data requests should be submitted to the Warwick Clinical Trials Unit data accessing committee. Access to anonymised data may be granted following review.
Ethics statement
Ethical approval for the consensus workshop was obtained from the University of Warwick’s Biomedical and Scientific Research Ethics Committee (BSREC 49/22-23) on 13 February 2023.
Information governance statement
University of Warwick is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, University of Warwick is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://warwick.ac.uk/services/legalandcomplianceservices/dataprotection/privacynotices/research/.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211-59.
- Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: will health politicians now take notice?. J Headache Pain 2018;19. https://doi.org/10.1186/s10194-018-0846-2.
- Russell MB, Rasmussen BK, Thorvaldsen P, Olesen J. Prevalence and sex-ratio of the subtypes of migraine. Int J Epidemiol 1995;24:612-8.
- British Association for the Study of Headache (BASH) . Guidelines for All Healthcare Professionals in the Diagnosis and Management of Migraine, Tension-Type, Cluster and Medication Overuse Headache 2010:1-52.
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF;. AMPP Advisory Group . Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343-9.
- Olesen J. International classification of headache disorders. Lancet Neurol 2018;17:396-7.
- World Health Organization . Atlas of Headache Disorders and Resources in the World 2011 2011.
- Institute for Health Metrics and Evaluation . Global Burden of Disability Compare 2020. https://vizhub.healthdata.org/gbd-compare/ (accessed 9 September 2020).
- Steiner TJ, Stovner LJ, Vos T. GBD 2015: migraine is the third cause of disability in under 50s. J Headache Pain 2016;17. https://doi.org/10.1186/s10194-016-0699-5.
- Hagen K, Zwart J, Vatten L, Stovner L, Bovim G. Prevalence of migraine and non-migrainous headache – head-HUNT, a large population-based study. Cephalalgia 2000;20:900-6.
- Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27:193-210.
- Underwood M, Achana F, Carnes D, Eldridge S, Ellard DR, Griffiths F, et al. upportive self-management program for people with chronic headaches and migraine: a randomized controlled trial and economic evaluation. Neurology 2023;100:e1339-52. https://doi.org/10.1212/wnl.0000000000201518.
- Nichols VP, Ellard DR, Griffiths FE, Kamal A, Underwood M, Taylor SJC. CHESS Team . The lived experience of chronic headache: a systematic review and synthesis of the qualitative literature. BMJ Open 2017;7.
- Steiner TJ, Stovner LJ, Katsarava Z, Lainez JM, Lampl C, Lantéri-Minet M, et al. The impact of headache in Europe: principal results of the Eurolight project. J Headache Pain 2014;15.
- National Institute for Health and Care Excellence . Headache in over 12s: Diagnosis and Management 2021. www.nice.org.uk/guidance/cg150 (accessed 23 July 2024).
- Scottish Intercollegiate Guidelines Network (SIGN) . Pharmacological Management of Migraine: A National Clinical Guideline 2018.
- National Institute for Health and Care Excellence . Amitriptyline to Prevent Recurrent Migraine: Is Amitriptyline a Clinically and Cost Effective Prophylactic Treatment for Recurrent Migraine? 2021. www.nice.org.uk/researchrecommendation/amitriptyline-to-prevent-recurrent-migraine-is-amitriptyline-a-clinically-and-cost-effective-prophylactic-treatment-for-recurrent-migraine (accessed 9 February 2023).
- Jackson JL, Cogbill E, Santana-Davila R, Eldredge C, Collier W, Gradall A, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLOS ONE 2015;10.
- Bartolini M, Silvestrini M, Taffi R, Lanciotti C, Luconi R, Capecci M, et al. Efficacy of topiramate and valproate in chronic migraine. Clin Neuropharmacol 2005;28:277-9.
- Behan PO, Connelly K. Prophylaxis of migraine: a comparison between naproxen sodium and pizotifen. Headache 1986;26:237-9.
- Beran RG, Spira PJ. Levetiracetam in chronic daily headache: a double-blind, randomised placebo-controlled study. (The Australian KEPPRA Headache Trial [AUS-KHT]). Cephalalgia 2011;31:530-6.
- Couch JR. Amitriptyline Versus Placebo Study Group . Amitriptyline in the prophylactic treatment of migraine and chronic daily headache. Headache 2011;51:33-51.
- Diener HC, Bussone G, Van Oene JC, Lahaye M, Schwalen S, Goadsby PJ. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia 2007;27:814-23.
- Domingues RB, Silva AL, Domingues SA, AUuino CC, Kuster GW. A double-blind randomized controlled trial of low doses of propranolol, nortriptyline, and the combination of propranolol and nortriptyline for the preventive treatment of migraine. Arq Neuropsiquiatr 2009;67:973-7.
- Mei D, Ferraro D, Zelano G, Capuano A, Vollono C, Gabriele C, et al. Topiramate and triptans revert chronic migraine with medication overuse to episodic migraine. Clin Neuropharmacol 2006;29:269-75.
- Saper JR, Lake AE, Cantrell DT, Winner PK, White JR. Chronic daily headache prophylaxis with tizanidine: a double‐blind, placebo‐controlled, multicenter outcome study. Headache 2002;42:470-82.
- Saper JR, Silberstein SD, Lake AE, Winters ME. Double‐blind trial of fluoxetine: chronic daily headache and migraine. Headache 1994;34:497-502.
- Silberstein SD, Lipton RB, Dodick DW, Freitag FG, Ramadan N, Mathew N, et al. Topiramate Chronic Migraine Study Group . Efficacy and safety of topiramate for the treatment of chronic migraine: a randomized, double‐blind, placebo‐controlled trial. Headache 2007;47:170-80.
- Silvestrini M, Bartolini M, Coccia M, Baruffaldi R, Taffi R, Provinciali L. Topiramate in the treatment of chronic migraine. Cephalalgia 2003;23:820-4.
- Stensrud P, Sjaastad O. Comparative trial of Tenormin (atenolol) and Inderal (propranolol) in migraine. Headache 1980;20:204-7.
- Yurekli VA, Akhan G, Kutluhan S, Uzar E, Koyuncuoglu HR, Gultekin F. The effect of sodium valproate on chronic daily headache and its subgroups. J Headache Pain 2008;9:37-41.
- Ferreira GE, Abdel-Shaheed C, Underwood M, Finnerup NB, Day RO, McLachlan A, et al. Efficacy, safety, and tolerability of antidepressants for pain in adults: overview of systematic reviews. BMJ 2023;380.
- Zheng H, Huang SL, Chen YY, Tang TC, Qin D, Chen M. Topiramate, acupuncture, and BoNT‐A for chronic migraine: a network meta‐analysis. Acta Neurol Scand 2021;143:558-68.
- Diener HC, Charles A, Goadsby PJ, Holle D. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol 2015;14:1010-22.
- Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA 2018;319:1999-2008.
- Goadsby PJ, Reuter U, Hallström Y, Broessner G, Bonner JH, Zhang F, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med 2017;377:2123-32.
- Silberstein SD, Dodick DW, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med 2017;377:2113-22.
- National Institute for Health and Care Excellence . Botulinum Toxin Type A for the Prevention of Headaches in Adults With Chronic Migraine 2012. https://www.nice.org.uk/guidance/ta260 (accessed 23 July 2024).
- National Institute for Health and Care Excellence . Galcanezumab for Preventing Migraine 2020. https://www.nice.org.uk/guidance/ta260 (accessed 23 July 2024).
- National Institute for Health and Care Excellence . Erenumab for Preventing Migraine 2021. https://www.nice.org.uk/guidance/ta260 (accessed 23 July 2024).
- National Institute for Health and Care Excellence . Fremanezumab for Preventing Migraine 2022. https://www.nice.org.uk/guidance/ta260 (accessed 23 July 2024).
- National Institute for Health and Care Excellence . Eptinezumab for Preventing Migraine 2023. https://www.nice.org.uk/guidance/ta260 (accessed 23 July 2024).
- Forbes RB, McCarron M, Cardwell CR. Efficacy and contextual (placebo) effects of CGRP antibodies for migraine: systematic review and meta‐analysis. Headache 2020;60:1542-57.
- Bigal ME, Dodick DW, Rapoport AM, Silberstein SD, Ma Y, Yang R, et al. Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 2014;14:1081-90.
- Tepper S, Ashina M, Reuter U, Brandes JL, Dolezil D, Silberstein S, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017;16:425-34.
- Leonardi M, Raggi A. A narrative review on the burden of migraine: when the burden is the impact on people’s life. J Headache Pain 2019;20.
- D’Amico D, Sansone E, Grazzi L, Giovannetti AM, Leonardi M, Schiavolin S, et al. Multimorbidity in patients with chronic migraine and medication overuse headache. Acta Neurol Scand 2018;138:515-22.
- Bloudek L, Stokes M, Buse D, Wilcox TK, Lipton RB, Goadsby PJ, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). J Headache Pain 2012;13:361-78.
- Raggi A, Covelli V, Guastafierro E, Leonardi M, Scaratti C, Grazzi L, et al. Validation of a self-reported instrument to assess work-related difficulties in patients with migraine: the HEADWORK questionnaire. J Headache Pain 2018;19.
- Roberts M. Migraine: New Drug Works When Others Fail, Researchers Say 2018. www.bbc.co.uk/news/health-43781227 (accessed 10 September 2020).
- National Institute for Health and Care Excellence . British National Formulary (BNF) 2022. https://bnf.nice.org.uk/ (accessed 9 February 2023).
- Page MJ, McKenzie JE, Bossuyt PM, Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021;10:1-11.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons; 2019.
- The EndNote Team . Endnote 2013.
- Beets MW, Weaver RG, Ioannidis JPA, Pfledderer CD, Jones A, von Klinggraeff L, et al. Influence of pilot and small trials in meta-analyses of behavioral interventions: a meta-epidemiological study. Syst Rev 2023;12. https://doi.org/10.1186/s13643-023-02184-7.
- Patel S, Hee SW, Mistry D, Jordan J, Brown S, Dritsaki M, et al. Identifying back pain subgroups: developing and applying approaches using individual patient data collected within clinical trials. Programme Grants Appl Res 2016;4. https://doi.org/10.3310/pgfar04100.
- Page MJ, Higgins JP, Sterne JA. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons; 2019.
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutran I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366. https://doi.org/10.1136/bmj.l4898.
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1 Introduction – GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383-94.
- Rendas-Baum R, Bloudek LM, Maglinte GA, Varon SF. The psychometric properties of the Migraine-Specific Quality of Life Questionnaire version 21 (MSQ) in chronic migraine patients. Qual Life Res 2013;22:1123-33.
- Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia 2011;31:357-67.
- EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001;56:S20-8.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. PharmacoEconomics 1993;4:353-65.
- Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13.
- Kawata AK, Hareendran A, Shaffer S, Mannix S, Thach A, Desai P, et al. Evaluating the psychometric properties of the migraine functional impact questionnaire (MFIQ). Headache 2019;59:1253-69.
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013;159:130-7.
- Chaimani A, Caldwell DM, Li T, Higgins Julian PT, Salanti G. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons; 2019.
- Cope S, Zhang J, Saletan S, Smiechowski B, Jansen JP, Schmid P. A process for assessing the feasibility of a network meta-analysis: a case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancer. BMC Med 2014;12:1-17.
- Jones B, Roger J, Lane PW, Lawton A, Fletcher C, Cappelleri JC, et al. PSI Health Technology Special Interest Group, Evidence Synthesis sub-team . Statistical approaches for conducting network meta‐analysis in drug development. Pharm Stat 2011;10:523-31.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17.
- Soni P, Chawla E. Efficacy and safety of anti-calcitonin gene-related peptide monoclonal antibodies for treatment of chronic migraine: a systematic review and network meta-analysis. Clin Neurol Neurosurg 2021;209.
- van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Res Synth Methods 2012;3:285-99.
- StataCorp . Stata Statistical Software: Release 17 2021.
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105-24.
- Caldwell DM, Dias S, Welton NJ. Extending treatment networks in health technology assessment: how far should we go?. Value Health 2015;18:673-81.
- Dias S, Welton NJ, Sutton AJ, Ades AE. London: National Institute for Health and Care Excellence (NICE); 2011.
- Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 2005;25:646-54.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002;21:1539-58.
- Phillippo DM. multinma: an R Package for Bayesian Network Meta-Analysis of Individual and Aggregate Data n.d.
- R Core Team. R: A Language and Environment for Statistical Computing 2020.
- Thomas KH, Dalili MN, López-López JA, Keeney E, Phillippo D, Munafò MR, et al. Smoking cessation medicines and e-cigarettes: a systematic review, network meta-analysis and cost-effectiveness analysis. Health Technol Assess 2021;25:1-224.
- Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev 2017;6:1-5.
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279-301.
- Smith PH, Weinberger AH, Zhang J, Emme E, Mazure CM, McKee SA. Sex differences in smoking cessation pharmacotherapy comparative efficacy: a network meta-analysis. Nicotine Tob Res 2017;19:273-81.
- van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node‐splitting models for assessment of inconsistency in network meta‐analysis. Res Synth Methods 2016;7:80-93.
- Arnold M. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders. Cephalalgia 2018;38:1-211.
- Rothrock JF, Adams AM, Lipton RB, Silberstein SD, Jo E, Zhao X, et al. FORWARD Study Investigative Group . FORWARD study: evaluating the comparative effectiveness of onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. Headache 2019;59:1700-13.
- Dodick DW, Lipton RB, Silberstein S, Goadsby PJ, Biondi D, Hirman J, et al. Eptinezumab for prevention of chronic migraine: a randomized phase 2b clinical trial. Cephalalgia 2019;39:1075-85.
- Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 2019;394:1030-40.
- Sakai F, Suzuki N, Kim BK, Igarashi H, Hirata K, Takeshima T, et al. Efficacy and safety of fremanezumab for chronic migraine prevention: multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial in Japanese and Korean patients. Headache 2021;61:1092-101.
- Aurora SK, Dodick DW, Turkel CC, DeGryse RE, Silberstein SD, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010;30:793-80.
- Diener HC, Dodick DW, Aurora SK, Turkel CC, DeGryse RE, Lipton RB, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010;30:804-14.
- Lipton RB, Goadsby PJ, Smith J, Schaeffler BA, Biondi DM, Hirman J, et al. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology 2020;94:e1365-77.
- Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology 2018;91:e2211-21.
- Dodick DW, Silberstein SD, Lipton RB, DeGryse RE, Adams AM, Diener HC. Early onset of effect of onabotulinumtoxinA for chronic migraine treatment: analysis of PREEMPT data. Cephalalgia 2019;39:945-56.
- Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, et al. PREEMPT Chronic Migraine Study Group . OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache 2010;50:921-36.
- Silberstein SD, Diener HC, Dodick DW, Manack Adams A, DeGryse RE, Lipton RB. The impact of onabotulinumtoxinA vs. placebo on efficacy outcomes in headache day responder and nonresponder patients with chronic migraine. Pain Ther 2020;9:695-707.
- Aurora SK, Dodick DW, Diener HC, DeGryse RE, Turkel CC, Lipton RB, et al. OnabotulinumtoxinA for chronic migraine: efficacy, safety, and tolerability in patients who received all five treatment cycles in the PREEMPT clinical program. Acta Neurol Scand 2014;129:61-70.
- Lipton RB, Rosen NL, Ailani J, DeGryse RE, Gillard PJ, Varon SF. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine over one year of treatment: pooled results from the PREEMPT randomized clinical trial program. Cephalalgia 2016;36:899-908.
- Ruff DD, Ford JH, Tockhorn-Heidenreich A, Sexson M, Govindan S, Pearlman EM, et al. Efficacy of galcanezumab in patients with chronic migraine and a history of preventive treatment failure. Cephalalgia 2019;39:931-44.
- Ford J, Tassorelli C, Leroux E, Wang S, Ayer D, Nichols R, et al. Changes in patient functioning and disability: results from a phase 3, double-blind, randomized, placebo-controlled clinical trial evaluating galcanezumab for chronic migraine prevention (REGAIN). Qual Life Res 2021;30:105-15.
- Förderreuther S, Zhang Q, Stauffer VL, Aurora SK, Láinez MJA. Preventive effects of galcanezumab in adult patients with episodic or chronic migraine are persistent: data from the phase 3, randomized, double-blind, placebo-controlled EVOLVE-1, EVOLVE-2, and REGAIN studies. J Headache Pain 2018;19:1-9.
- Ailani J, Andrews JS, Rettiganti M, Nicholson RA. Impact of galcanezumab on total pain burden: findings from phase 3 randomized, double-blind, placebo-controlled studies in patients with episodic or chronic migraine (EVOLVE-1, EVOLVE-2, and REGAIN trials). J Headache Pain 2020;21:1-9.
- Ament M, Day K, Stauffer VL, Skljarevski V, Rettiganti M, Pearlman E, et al. Effect of galcanezumab on severity and symptoms of migraine in phase 3 trials in patients with episodic or chronic migraine. J Headache Pain 2021;22:1-10.
- Spierings ELH, Ning X, Ramirez Campos V, Cohen JM, Barash S, Buse DC. Improvements in quality of life and work productivity with up to 6 months of fremanezumab treatment in patients with episodic and chronic migraine and documented inadequate response to 2 to 4 classes of migraine‐preventive medications in the phase 3b FOCUS study. Headache 2021;61:1376-86.
- Diener HC, Marmura MJ, Tepper SJ, Cowan R, Starling AJ, Diamond ML, et al. Efficacy, tolerability, and safety of eptinezumab in patients with a dual diagnosis of chronic migraine and medication‐overuse headache: subgroup analysis of PROMISE‐2. Headache 2021;61:125-36.
- Silberstein S, Diamond M, Hindiyeh NA, Biondi DM, Cady R, Hirman J, et al. Eptinezumab for the prevention of chronic migraine: efficacy and safety through 24 weeks of treatment in the phase 3 PROMISE-2 (Prevention of migraine via intravenous ALD403 safety and efficacy–2) study. J Headache Pain 2020;21:1-12.
- Blumenfeld AM, Patel AT, Turner IM, Mullin KB, Manack Adams A, Rothrock JF. Patient-reported outcomes from a 1-year, real-world, head-to-head comparison of onabotulinumtoxinA and topiramate for headache prevention in adults with chronic migraine. J Prim Care Community Health 2020;11.
- Silberstein S, Lipton R, Dodick D, Freitag F, Mathew N, Brandes J, et al. Topiramate treatment of chronic migraine: a randomized, placebo‐controlled trial of quality of life and other efficacy measures. Headache 2009;49:1153-62.
- Dodick DW, Silberstein S, Saper J, Freitag FG, Cady RK, Rapoport AM, et al. The impact of topiramate on health‐related quality of life indicators in chronic migraine. Headache 2007;47:1398-408.
- Winner PK, Spierings ELH, Yeung PP, Aycardi E, Blankenbiller T, Grozinski-Wolff M, et al. Early onset of efficacy with fremanezumab for the preventive treatment of chronic migraine. Headache 2019;59:1743-52.
- Lipton RB, Cohen JM, Gandhi SK, Yang R, Yeung PP, Buse DC. Effect of fremanezumab on quality of life and productivity in patients with chronic migraine. Neurology 2020;95:e878-88.
- Silberstein SD, Cohen JM, Seminerio MJ, Yang R, Ashina S, Katsarava Z. The impact of fremanezumab on medication overuse in patients with chronic migraine: subgroup analysis of the HALO CM study. J Headache Pain 2020;21:1-10.
- Blumenfeld AM, Stevanovic DM, Ortega M, Cohen JM, Seminerio MJ, Yang R, et al. No ‘wearing‐off effect’ seen in quarterly or monthly dosing of fremanezumab: subanalysis of a randomized long‐term study. Headache 2020;60:2431-43.
- Brandes JL, Diener HC, Dolezil D, Freeman MC, McAllister PJ, Winner P, et al. The spectrum of response to erenumab in patients with chronic migraine and subgroup analysis of patients achieving ≥50%, ≥75%, and 100% response. Cephalalgia 2020;40:28-3.
- Ashina M, Tepper S, Brandes JL, Reuter U, Boudreau G, Dolezil D, et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia 2018;38:1611-21.
- Tepper SJ, Diener HC, Ashina M, Brandes JL, Friedman DI, Reuter U, et al. Erenumab in chronic migraine with medication overuse: subgroup analysis of a randomized trial. Neurology 2019;92:e2309-20.
- Lipton RB, Tepper SJ, Reuter U, Silberstein S, Stewart WF, Nilsen J, et al. Erenumab in chronic migraine: patient-reported outcomes in a randomized double-blind study. Neurology 2019;92:e2250-60.
- Mahon R, Vo P, Pannagl K, Tiwari S, Heemstra H, Ferraris M, et al. Assessment of the relative effectiveness of erenumab compared with onabotulinumtoxinA for the prevention of chronic migraine. Curr Med Res Opin 2022;39:105-12.
- Wang X, Wen D, He Q, You C, Ma L. Efficacy and safety of monoclonal antibody against calcitonin gene-related peptide or its receptor for migraine patients with prior preventive treatment failure: a network meta-analysis. J Headache Pain 2022;23:1-10.
- Soni P, Chawla E. Quality of life related to functional disability in migraine patients: a systematic review and network meta-analysis. Clin J Pain 2021;37:845-51.
- Magalhães E, Menezes C, Cardeal M, Melo A. Botulinum toxin type A versus amitriptyline for the treatment of chronic daily migraine. Clin Neurol Neurosurg 2010;112:463-6.
- Silberstein SD, Dodick DW, Lindblad AS, Holroyd K, Harrington M, Mathew NT, et al. Randomized, placebo-controlled trial of propranolol added to topiramate in chronic migraine. Neurology 2012;78:976-84.
- U.S. Department of Health and Human Services . Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 n.d.
- Sakai F, Suzuki N, Kim BK, Tatsuoka Y, Imai N, Ning X, et al. Efficacy and safety of fremanezumab for episodic migraine prevention: multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group trial in Japanese and Korean patients. Headache 2021;61:1102-11.
- Sakai F, Ozeki A, Skljarevski V. Efficacy and safety of galcanezumab for prevention of migraine headache in Japanese patients with episodic migraine: a phase 2 randomized controlled clinical trial. Cephalalgia Rep 2020;3.
- Lucking CH, Oestreich W, Schmidt R, Soyka D. Flunarizine vs. propranolol in the prophylaxis of migraine: two double-blind comparative studies in more than 400 patients. Cephalalgia 1988;8:21-6.
- Ailani J, Lipton RB, Goadsby PJ, Guo H, Miceli R, Severt L, et al. ADVANCE Study Group . Atogepant for the preventive treatment of migraine. N Engl J Med 2021;385:695-706.
- Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M, et al. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 2016;15:382-90.
- Ashina M, Saper J, Cady R, Schaeffler BA, Biondi DM, Hirman J, et al. Eptinezumab in episodic migraine: a randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia 2020;40:241-54.
- Aurora SK, Gawel M, Brandes JL, Pokta S, Vandenburgh AM. BOTOX North American Episodic Migraine Study Group . Botulinum toxin type a prophylactic treatment of episodic migraine: a randomized, double‐blind, placebo‐controlled exploratory study. Headache 2007;47:486-99.
- Dodick DW, Goadsby PJ, Spierings EL, Scherer JC, Sweeney SP, Grayzel DS. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 2014;13:885-92.
- Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, et al. ARISE: a phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 2018;38:1026-37.
- Dodick DW, Freitag F, Banks J, Saper J, Xiang J, Rupnow M, et al. CAPSS-277 Investigator Group . Topiramate versus amitriptyline in migraine prevention: a 26-week, multicenter, randomized, double-blind, double-dummy, parallel-group noninferiority trial in adult migraineurs. Clin Ther 2009;31:542-59.
- Diener HC, Matias-Guiu J, Hartung E, Pfaffenrath V, Ludin HP, Nappi G, et al. Efficacy and tolerability in migraine prophylaxis of flunarizine in reduced doses: a comparison with propranolol 160 mg daily. Cephalalgia 2002;22:209-21.
- Kalita J, Bhoi SK, Misra UK. Amitriptyline vs. divalproate in migraine prophylaxis: a randomized controlled trial. Acta Neurol Scand 2013;128:65-72.
- Relja M, Poole AC, Schoenen J, Pascual J, Lei X, Thompson C. A multicentre, double-blind, randomized, placebo-controlled, parallel group study of multiple treatments of botulinum toxin type A (BoNTA) for the prophylaxis of episodic migraine headaches. Cephalalgia 2007;27:492-503.
- Lipton RB, Silberstein S, Dodick D, Cady R, Freitag F, Mathew N, et al. Topiramate intervention to prevent transformation of episodic migraine: the topiramate INTREPID study. Cephalalgia 2011;31:18-30.
- Stauffer VL, Dodick DW, Zhang Q, Carter JN, Ailani J, Conley RR. Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol 2018;75:1080-8.
- Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia 2018;38:1442-54.
- Reuter U, Ehrlich M, Gendolla A, Heinze A, Klatt J, Wen S, et al. Erenumab versus topiramate for the prevention of migraine – a randomised, double-blind, active-controlled phase 4 trial. Cephalalgia 2022;42:108-18.
- Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet 2018;392:2280-7.
- Wang S-J, Roxas AA Jr, Saravia B, Kim BK, Chowdhury D, Riachi N, et al. Randomised, controlled trial of erenumab for the prevention of episodic migraine in patients from Asia, the Middle East, and Latin America: the EMPOwER study. Cephalalgia 2021;41:1285-97.
- Elkind AH, O’Carroll P, Blumenfeld A, DeGryse R, Dimitrova R. BoNTA-024-026-036 Study Group . A series of three sequential, randomized, controlled studies of repeated treatments with botulinum toxin type A for migraine prophylaxis. J Pain 2006;7:688-96.
- Mulleners WM, Kim BK, Láinez MJA, Lanteri-Minet M, Pozo-Rosich P, Wang S, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 2020;19:814-25.
- Fazlalizadeh H, Khamseh F, Soleimani B, Tajik A. Comparative study of topiramate versus sodium valproate in the prevention of migraine headaches. Med Sci J Islamic Azad Univ 2009;19:105-9.
- Croop R, Lipton RB, Kudrow D, Stock DA, Kamen L, Conway CM, et al. Oral rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet 2021;397:51-60.
- Winner PK, McAllister P, Chakhava G, Ailani J, Ettrup A, Krog Josiassen M, et al. Effects of intravenous eptinezumab vs. placebo on headache pain and most bothersome symptom when initiated during a migraine attack: a randomized clinical trial. JAMA 2021;325:2348-56.
- Hu B, Li G, Li X, Wu S, Yu T, Li X, et al. Galcanezumab in episodic migraine: the phase 3, randomized, double-blind, placebo-controlled PERSIST study. J Headache Pain 2022;23:1-11.
- Ashina M, Lanteri-Minet M, Pozo-Rosich P, Ettrup A, Christoffersen CL, Josiassen MK, et al. Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 2022;21:597-60. https://doi.org/10.1016/S1474-4422(22)00185-5.
- Diener HC, Agosti R, Allais G, Bergmans P, Bussone G, Davies B, et al. TOPMAT-MIG-303 Investigators Group . Cessation versus continuation of 6-month migraine preventive therapy with topiramate (PROMPT): a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2007;6:1054-62.
- Overeem LH, Raffaelli B, Mecklenburg J, Kelderman T, Neeb L, Reuter U. Indirect comparison of topiramate and monoclonal antibodies against CGRP or its receptor for the prophylaxis of episodic migraine: a systematic review with meta-analysis. CNS Drugs 2021;35:805-20.
- Herd CP, Tomlinson CL, Rick C, Scotton WJ, Edwards J, Ives N, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev 2018;6.
- Hou M, Xing H, Cai Y, Li B, Wang X, Li P, et al. The effect and safety of monoclonal antibodies to calcitonin gene-related peptide and its receptor on migraine: a systematic review and meta-analysis. J Headache Pain 2017;18:1-12.
- Lattanzi S, Brigo F, Trinka E, Vernieri F, Corradetti T, Dobran M, et al. Erenumab for preventive treatment of migraine: a systematic review and meta-analysis of efficacy and safety. Drugs 2019;79:417-31.
- Zhu C, Guan J, Xiao H, Luo W, Tong R. Erenumab safety and efficacy in migraine: a systematic review and meta-analysis of randomized clinical trials. Medicine (Baltimore) 2019;98.
- Lipton RB, Croop R, Stock EG, Stock DA, Morris BA, Frost M, et al. Rimegepant, an oral calcitonin gene-related peptide receptor antagonist, for migraine. N Engl J Med 2019;381:142-9.
- Gao B, Yang Y, Wang Z, Sun Y, Chen Z, Zhu Y, et al. Efficacy and safety of rimegepant for the acute treatment of migraine: evidence from randomized controlled trials. Front Pharmacol 2020;10.
- Singh A, Balasundaram MK. Atogepant for migraine prevention: a systematic review of efficacy and safety. Clin Drug Investig 2022;42:301-8.
- Cui X, Sun S, Liu J, Wu QY, Zhang JF, Li X. The efficacy and safety of valproate medications for migraine in adults: a meta-analysis. Eur Rev Med Pharmacol Sci 2020;24:5734-41.
- Linde M, Mulleners WM, Chronicle EP, McCrory DC. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev 2013;6. https://doi.org/10.1002/14651858.CD010611.
- Stubberud A, Flaaen NM, McCrory DC, Pedersen SA, Linde M. Flunarizine as prophylaxis for episodic migraine: a systematic review with meta-analysis. Pain 2019;160:762-72.
- Wang X, Chen Y, Song J, You C. Efficacy and safety of monoclonal antibody against calcitonin gene-related peptide or its receptor for migraine: a systematic review and network meta-analysis. Front Pharmacol 2021;12.
- Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Int J Technol Assess Health Care 2022;38. https://doi.org/10.1017/S0266462321001732.
- Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al. Review of guidelines for good practice in decision-analytic modelling in health technology assessment. Health Technol Assess 2004;8:iii-v, ix.
- Batty AJ, Hansen RN, Bloudek LM, Varon SF, Hayward EJ, Pennington BW, et al. The cost-effectiveness of onabotulinumtoxinA for the prophylaxis of headache in adults with chronic migraine in the UK. J Med Econ 2013;16:877-87. https://doi.org/10.3111/13696998.2013.802694.
- Giannouchos TV, Mitsikostas DD, Ohsfeldt RL, Vozikis A, Koufopoulou P. Cost-effectiveness analysis of erenumab versus onabotulinumtoxinA for patients with chronic migraine attacks in Greece. Clin Drug Investig 2019;39:979-90. https://doi.org/10.1007/s40261-019-00827-z.
- Hansson-Hedblom A, Axelsson I, Jacobson L, Tedroff J, Borgström F. Economic consequences of migraine in Sweden and implications for the cost-effectiveness of onabotulinumtoxinA (Botox) for chronic migraine in Sweden and Norway. J Headache Pain 2020;21. https://doi.org/10.1186/s10194-020-01162-x.
- Hollier-Hann G, Curry A, Onishchenko K, Akehurst R, Ahmed F, Davies B, et al. Updated cost-effectiveness analysis of onabotulinumtoxinA for the prevention of headache in adults with chronic migraine who have previously received three or more preventive treatments in the UK. J Med Econ 2020;23:113-23. https://doi.org/10.1080/13696998.2019.1675417.
- Lipton RB, Brennan A, Palmer S, Hatswell AJ, Porter JK, Sapra S, et al. Estimating the clinical effectiveness and value-based price range of erenumab for the prevention of migraine in patients with prior treatment failures: a US societal perspective. J Med Econ 2018;21:666-75. https://doi.org/10.1080/13696998.2018.1457533.
- Mahon R, Lang A, Vo P, Huels J, Cooney P, Danyliv A, et al. Cost-effectiveness of erenumab for the preventive treatment of migraine in patients with prior treatment failures in Sweden. PharmacoEconomics 2021;39:357-72. https://doi.org/10.1007/s40273-020-00996-2.
- Ruggeri M, Carletto A, Marchetti M. Cost-effectiveness of onabotulinumtoxinA for the prophylaxis of chronic migraine [Italian, English]. PharmacoEcon: Italian Res Art 2013;15:19-33. https://doi.org/10.1007/s40276-013-0003-5.
- Sussman M, Benner J, Neumann P, Menzin J. Cost-effectiveness analysis of erenumab for the preventive treatment of episodic and chronic migraine: results from the US societal and payer perspectives. Cephalalgia 2018;38:1644-57. https://doi.org/10.1177/0333102418796842.
- Vekov T, Izmaylov A. Cost-effectiveness analysis of CGRP inhibitors for treatment of patients with chronic or episodic migraine [Bulgarian]. Gen Med 2019;21:33-8.
- Canadian Agency for Drugs and Technologies in Health . CADTH Common Drug Review: Pharmacoeconomic Review Report for OnabotulinumtoxinA (Botox) 2019.
- Canadian Agency for Drugs and Technologies in Health . CADTH Common Drug Review: Pharmacoeconomic Review Report for Erenumab (Aimovig) 2019.
- Institute for Clinical and Economic Review . Calcitonin Gene-Related Peptide (CGRP) Inhibitors As Preventive Treatments for Patients With Episodic or Chronic Migraine: Effectiveness and Value - Final Evidence Report 2018.
- National Institute for Health and Care Excellence . Single Technology Appraisal: Fremanezumab for Preventing Migraine [ID1368] - Committee Papers 2019.
- National Institute for Health and Care Excellence . Single Technology Appraisal: Erenumab for Preventing Migraine [ID1188] - Committee Papers 2019.
- National Institute for Health and Care Excellence . Single Technology Appraisal: Galcanezumab for Preventing Migraine [ID1372] - Committee Papers 2020.
- Warwick Evidence . Botulinum Toxin Type A for the Prophylaxis of Headaches in Adults With Chronic Migraine: A Single Technology Assessment 2011.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013.
- Mahon R, Huels J, Hacking V, Cooney P, Danyliv A, Vudumula U, et al. Economic evaluations in migraine: systematic literature review and a novel approach. J Med Econ 2020;23:864-76.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41.
- Hernandez-Alava M, Pudney S. EQ5Dmap: a command for mapping between EQ-5D-3L and EQ-5D-5L. Stata J 2018;18:395-41.
- Jones KC, Burns A. Unit Costs of Health and Social Care 2021. Canterbury: University of Kent; 2021.
- NHS England . NHS Tariff 2018 2019 2020. www.england.nhs.uk/publication/past-national-tariffs-documents-and-policies/ (accessed 7 March 2023).
- NHS England . NHS Tariff 2021 22 2022. www.england.nhs.uk/publication/past-national-tariffs-documents-and-policies/ (accessed 7 March 2023).
- Blumenfeld AM, Varon SF, Wilcox TK, Buse DC, Kawata AK, Manack A, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia 2011;31:301-15.
- Office for National Statistics 2021. https://www.gov.uk/government/statistics/national-life-tables-life-expectancy-in-the-uk-2018-to-2020 (accessed 23 July 2024).
- van Hout B, Janssen M, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15.
- Briggs AH, Weinstein MC, Fenwick EA, Karnon J, Sculpher MJ, Paltiel AD;. ISPOR-SMDM Modeling Good Research Practices Task Force . Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group–6. Med Decis Making 2012;32:722-32.
- Safiri S, Pourfathi H, Eagan A, Mansournia MA, Khodayari MT, Sullman MJM, et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain 2022;163:e293-309.
- Office for National Statistics . Population Estimates for the UK, England, Wales, Scotland and Northern Ireland: Mid-2021 2022. www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2021 (accessed 22 September 2023).
- Mars T, Ost B, Ellard DR, Roberts N, McGregor A, Smith A, et al. Intraarticular facet injections for low back pain: design considerations, consensus methodology to develop the protocol for a randomized controlled trial. Pain Physician 2015;18.
- Nair R, Aggarwal R, Khanna D. Seminars in Arthritis and Rheumatism. Philadelphia, PA: Elsevier; 2011.
- Potter R, Hee SW, Griffiths F, Dodd K, Hoverd E, Underwood M, et al. Development and validation of a telephone classification interview for common chronic headache disorders. J Headache Pain 2019;20:1-10.
- Van de Ven AH, Delbecq AL. The nominal group as a research instrument for exploratory health studies. Am J Public Health 1972;62:337-42.
- Vevox . Vevox: The #1 Rated Polling and Q&Amp;A Platform 2023. www.vevox.com/ (accessed 30 May 2023).
- Khanal S, Underwood M, Naghdi S, Brown A, Duncan C, Matharu M, et al. A systematic review of economic evaluations of pharmacological treatments for adults with chronic migraine. J Headache Pain 2022;23.
Appendix 1 Literature searches for clinical effectiveness review and adverse events review
| Bibliographic databases and clinical trials registers | ||
| Database | Date searched | Number of records |
| MEDLINE All (via Ovid) | 8 September 2021 | 4029 |
| EMBASE (via Ovid) | 8 September 2021 | 8404 |
| Cochrane CENTRAL (via Cochrane Library) | 8 September 2021 | 6754 |
| Science Citation Index (via Web of Science) | 8 September 2021 | 4737 |
| Global Index Medicus (via World Health Organization) | 14 September 2021 | 200 |
| ClinicalTrials.gov | 15 September 2021 | 338 |
| ICTRP (World Health Organization) | 15 September 2021 | 512 |
|
Total number of records retrieved: 24,974
Duplicates removed (EndNote): 8368 Final number for screening: 16,606 |
||
| Bibliographic databases and clinical trials registers; additional search for riboflavin, magnesium and coenzyme Q10 | ||
| Source | Date searched | Number of records |
| MEDLINE All (via Ovid) | 8 February 2022 | 163 |
| EMBASE (via Ovid) | 8 February 2022 | 587 |
| Cochrane CENTRAL (via Cochrane Library) | 8 February 2022 | 331 |
| Science Citation Index (via Web of Science) | 8 February 2022 | 359 |
| Global Index Medicus (via World Health Organization) | 8 February 2022 | 24 |
| ClinicalTrials.gov | 8 February 2022 | 15 |
| ICTRP (World Health Organization) | 8 February 2022 | 38 |
|
Total number of records retrieved: 1517
Duplicates removed within this set (EndNote): 481 Duplicates removed against original search (EndNote): 448 Final number for screening: 588 |
||
| Pragmatic search for recent systematic reviews, to check reference lists/included studies | ||
| Database | Date searched | Number of records |
| MEDLINE All (via Ovid) | 14 February 2022 | 114 |
| EMBASE (via Ovid) | 14 February 2022 | 164 |
| Cochrane Database of Systematic Reviews (via Cochrane Library) | 14 February 2022 | 4 |
|
Total number of records retrieved: 282
Duplicates removed within this set (EndNote): 103 Final number for screening: 179 |
||
| Bibliographic databases and clinical trials registers; search update November 2022 (including all relevant drug terms) | ||
| Database | Date searched | Number of records |
| MEDLINE All (via Ovid) | 7 November 2022 | 390 |
| EMBASE (via Ovid) | 7 November 2022 | 710 |
| Cochrane CENTRAL (via Cochrane Library) | 7 November 2022 | 713 |
| Science Citation Index (via Web of Science) | 7 November 2022 | 440 |
| Global Index Medicus (via World Health Organization) | 7 November 2022 | 222 |
| ClinicalTrials.gov | 8 November 2022 | 390 |
| ICTRP (World Health Organization) | 8 November 2022 | 631 |
|
Total number of records retrieved: 3496
Duplicates removed within this set (EndNote): 1096 Duplicates removed against previous searches (EndNote): 1066 Final number for screening: 1334 |
||
| Other sources; citation tracking | ||
| Source | Date searched | Number of records |
| Reference lists – included studies (Web of Science) | 23 November 2022 | 875 |
| Forwards citation tracking: Science Citation Index (Web of Science) |
22–23 November 2022 | 2710 |
| Forwards citation tracking: Google Scholar (for studies not found in Web of Science only) | 23 November 2022 | 23 |
|
Total number of records retrieved: 3608
Duplicates removed (both within this set and against previous searches) (Endnote): 2122 Final number for screening: 1486 |
||
| Checking for retraction notices, errata and comments relating to included studies | ||
| Source | Date searched | Number of records |
| MEDLINE All (via Ovid) | 22 November 2022 | 23 |
| EMBASE (via Ovid) | 22 November 2022 | 0 |
| Retraction Watch website | 22 November 2022 | 0 |
| Total number of records retrieved: 23 | ||
Search strategies: original searches, September 2021
MEDLINE (via Ovid)
Date searched: 8 September 2021
Database: Ovid MEDLINE(R) ALL <1946 to 7 September 2021>
Search Strategy:
––––––– –––––––– ––––––––– –––––––– ––––––––
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kf,ti. (112921)
-
Headache/ or exp Headache Disorders/ (61239)
-
1 or 2 [population: migraine/headache] (124144)
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kf,ti. (216437)
-
Calcitonin Gene-Related Peptide/ai (436)
-
Antibodies, Monoclonal/ or Antibodies, Monoclonal, Humanized/ (217039)
-
Calcitonin Gene-Related Peptide Receptor Antagonists/ (701)
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kf,ti,nm. (507)
-
(rimegepant or ubrogepant or atogepant or gepant?).ab,kf,ti,nm. (214)
-
exp Botulinum Toxins/ (17105)
-
(botulin* adj toxin*).ab,kf,ti,nm. (21943)
-
(botulinum* or botox* or onabotulinum*).ab,kf,ti,nm. (25159)
-
(antidepress* or anti depress*).ab,kf,ti. (73890)
-
exp Antidepressive Agents/ (153122)
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kf,ti,nm. (17955)
-
exp ‘Serotonin and Noradrenaline Reuptake Inhibitors’/ (5005)
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kf,ti. (2908)
-
exp Angiotensin Converting Enzyme Inhibitors/ (45324)
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kf,ti. (37937)
-
acei.ab,kf,ti. (4344)
-
lisinopril.ab,kf,ti,nm. (3086)
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kf,ti. (14474)
-
(ARB or ARBs).ab,kf,ti. (7873)
-
exp Angiotensin Receptor Antagonists/ (25403)
-
candesartan.ab,kf,ti,nm. (3374)
-
((beta adj3 block*) or betablock*).ab,kf,ti. (55697)
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagon* or block*)).ab,kf,ti. (34997)
-
exp Adrenergic beta-Antagonists/ (85444)
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kf,ti,nm. (67114)
-
(calcium adj2 (block* or antagon* or inhibit*)).ab,kf,ti. (41676)
-
(CCB or CCBs).ab,kf,ti. (2619)
-
exp Calcium Channel Blockers/ (88532)
-
(flunarizine or verapamil).ab,kf,ti,nm. (27700)
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kf,ti. (53599)
-
exp Anticonvulsants/ (147158)
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kf,ti,nm. (31200)
-
Pizotyline/ (250)
-
(pizotifen or pizotyline).ab,kf,ti,nm. (418)
-
(alpha adj4 agonist*).ab,kf,ti. (15369)
-
exp Adrenergic alpha-Agonists/ (164069)
-
(clonidine or guanfacine).ab,kf,ti,nm. (19180)
-
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 [Interventions: named drugs/drug classes or types] (1098623)
-
randomized controlled trial.pt. (542809)
-
controlled clinical trial.pt. (94373)
-
randomized.ab. (533045)
-
placebo.ab. (221237)
-
clinical trials as topic.sh. (197235)
-
randomly.ab. (365421)
-
trial.ti. (247114)
-
43 or 44 or 45 or 46 or 47 or 48 or 49 (1392358)
-
exp animals/ not humans.sh. (4882975)
-
50 not 51 [RCTs filter] (1281368)
-
3 and 42 and 52 [population and interventions and RCTs filter] (3949)
-
(‘in data review’ or in process or publisher or ‘pubmed not medline’).st. (4677722)
-
(random* or controlled trial* or clinical trial* or rct).ab,kf,ti. (1547833)
-
54 and 55 [pragmatic filter to pick up RCTs that have not been fully indexed for MEDLINE yet] (236445)
-
3 and 42 and 56 [population and interventions and non-MEDLINE RCT filter] (365)
-
53 or 57 (4029)
The migraine/headache search terms (lines 1–3) and botox search terms (lines 10–12) are based on those used in:
Herd CP, Tomlinson CL, Rick C, Scotton WJ, Edwards J, Ives N, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev 2018;6:CD011616. https://doi.org/10.1002/14651858.CD011616.pub2
The search filter for RCTs (lines 43–52) is the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity- and precision-maximizing version (2008 revision); Ovid format:
Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Technical Supplement to Chapter 4: Searching for and Selecting Studies. In Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane; 2021. URL: www.training.cochrane.org/handbook.
EMBASE (via Ovid)
Date searched: 8 September 2021
Database: EMBASE Classic+EMBASE <1947 to 7 September 2021>
Search strategy:
–––––– ––––––– –––––––– –––––––– –––––– –––––
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kw,ti. (186,741)
-
‘headache and facial pain’/ or exp chronic daily headache/ or headache/ or exp migraine/ or vascular headache/ (294,109)
-
headache/si not exp ‘headache and facial pain’/dm, dt, pc, th (78,809)
-
(1 or 2) not 3 [population: headache/migraine, not indexed with headache only as a side effect] (253,432)
-
antimigraine agent/ (2568)
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kw,ti. (274,731)
-
exp calcitonin gene-related peptide receptor antagonist/ (3874)
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kw,ti,tn. (1446)
-
(rimegepant or ubrogepant or atogepant or gepant?).ab,kw,ti,tn. (465)
-
botulinum toxin/ or botulinum toxin A/ (39,617)
-
(botulin* adj toxin*).ab,kw,ti,tn. (23,049)
-
(botulinum* or botox* or onabotulinum*).ab,kw,ti,tn. (34,514)
-
(antidepress* or anti depress*).ab,kw,ti. (108,574)
-
exp antidepressant agent/ (515,170)
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kw,ti,tn. (22,239)
-
exp serotonin noradrenalin reuptake inhibitor/ (200,894)
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kw,ti. (4807)
-
exp dipeptidyl carboxypeptidase inhibitor/ (184,029)
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kw,ti. (55,292)
-
acei.ab,kw,ti. (9043)
-
lisinopril.ab,kw,ti,tn. (4456)
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kw,ti. (22,208)
-
(ARB or ARBs).ab,kw,ti. (15,639)
-
exp angiotensin receptor antagonist/ (100,628)
-
candesartan.ab,kw,ti,tn. (4072)
-
((beta adj3 block*) or betablock*).ab,kw,ti. (83,019)
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagon* or block*)).ab,kw,ti. (44,785)
-
exp beta adrenergic receptor blocking agent/ (316,412)
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kw,ti,tn. (69,432)
-
(calcium adj2 (block* or antagonis* or inhibit*)).ab,kw,ti. (55,273)
-
(CCB or CCBs).ab,kw,ti. (4501)
-
exp calcium antagonist/ (289,500)
-
(flunarizine or verapamil).ab,kw,ti,tn. (29,550)
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kw,ti. (84,444)
-
exp anticonvulsive agent/ (451,887)
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kw,ti,tn. (43,826)
-
pizotifen/ (1970)
-
(pizotifen or pizotyline).ab,kw,ti,tn. (443)
-
(alpha adj4 agonist*).ab,kw,ti. (12,528)
-
exp alpha 2 adrenergic receptor stimulating agent/ (114,998)
-
(clonidine or guanfacine).ab,kw,ti,tn. (19,865)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 [Interventions: named drugs/drug classes or types] (1,819,814)
-
Clinical trial/ (1,033,371)
-
Randomized controlled trial/ (676,452)
-
Randomization/ (91,991)
-
Single blind procedure/ (43,673)
-
Double blind procedure/ (189,813)
-
Crossover procedure/ (68,369)
-
Placebo/ (381,079)
-
Randomi?ed controlled trial$.tw. (265,730)
-
Rct.tw. (43,428)
-
Random allocation.tw. (2293)
-
Randomly allocated.tw. (39,355)
-
Allocated randomly.tw. (2722)
-
(allocated adj2 random).tw. (993)
-
Single blind$.tw. (27,624)
-
Double blind$.tw. (228,282)
-
((treble or triple) adj blind$).tw. (1438)
-
Placebo$.tw. (336,008)
-
Prospective study/ (711,345)
-
or/43-60 (2,456,634)
-
Case study/ (89,939)
-
Case report.tw. (486,887)
-
Abstract report/ or letter/ (1,209,603)
-
or/62-64 (1,774,111)
-
61 not 65 [Ovid EMBASE RCTs filter, available from: https://tools.ovid.com/ovidtools/expertsearches.html] (2,397,874)
-
4 and 42 and 66 [population and interventions and RCTs filter] (11,374)
-
conference abstract.pt. (4,171,170)
-
67 not 68 (8404)
The search filter for RCTs (lines 43–66) is the Ovid search filter: RCT – EMBASE, available from: https://tools.ovid.com/ovidtools/expertsearches.html.
Cochrane CENTRAL (via www.cochranelibrary.com)
Date searched: 8 September 2021
Database: Cochrane Central Register of Controlled Trials. Issue 9 of 12, September 2021
ID Search Hits
-
(headache* OR (head NEXT ache*) OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*):ti,ab,kw 36,987
-
[mh Headache] OR [mh ‘Headache Disorders’] 5399
-
#1 or #2 36,987
-
(((‘calcitonin gene related peptide’ OR CGRP) NEAR/5 (antibod* OR antagon* OR inhibit* OR block*)) OR ‘anti CGRP’ OR ‘anti calcitonin gene-related peptide’ OR (monoclonal NEXT antibod*) OR mAb OR mAbs OR moAb OR moAbs):ti,ab,kw 11,615
-
[mh ‘Calcitonin Gene-Related Peptide’/AI] 24
-
[mh ^‘Antibodies, Monoclonal’] OR [mh ^”Antibodies, Monoclonal, Humanized”] 8639
-
[mh ^‘Calcitonin Gene-Related Peptide Receptor Antagonists’] 55
-
(erenumab OR galcanezumab OR fremanezumab OR eptinezumab):ti,ab,kw 879
-
(rimegepant OR ubrogepant OR atogepant OR gepant*):ti,ab,kw 199
-
[mh ‘Botulinum Toxins’] 1981
-
(botulin* NEXT toxin*):ti,ab,kw 4218
-
(botulinum* OR botox* OR onabotulinum*):ti,ab,kw 4788
-
(antidepress* OR (anti NEXT depress*)):ti,ab,kw 16,855
-
[mh ‘Antidepressive Agents’] 5919
-
(amitriptyline OR venlafaxine OR mirtazapine OR duloxetine):ti,ab,kw 6344
-
[mh ‘Serotonin and Noradrenaline Reuptake Inhibitors’] 59
-
(SNRI OR SNRIs OR ((serotonin NEAR/2 (noradrenaline OR norepinephrine)) NEXT (‘reuptake inhibitor’ OR ‘reuptake inhibitors’ OR ‘reuptake inhibition’))):ti,ab,kw 832
-
[mh ‘Angiotensin Converting Enzyme Inhibitors’] 4056
-
((‘Angiotensin Converting Enzyme’ NEXT Inhibit*) OR (ACE NEXT inhibit*)):ti,ab,kw 9206
-
acei:ti,ab,kw 1658
-
lisinopril:ti,ab,kw 1304
-
((‘angiotensin receptor’ OR ‘angiotensin II receptor’) NEXT (block* OR antagon*)):ti,ab,kw 4639
-
(ARB OR ARBs):ti,ab,kw 2490
-
[mh ‘Angiotensin Receptor Antagonists’] 2218
-
candesartan:ti,ab,kw 1247
-
((beta NEAR/3 block*) OR betablock*):ti,ab,kw 11,414
-
((adrenergic OR adrenoreceptor* OR adrenoceptor*) NEAR/3 (antagon* OR block*)):ti,ab,kw 10,435
-
[mh ‘Adrenergic beta-Antagonists’] 4597
-
(propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol):ti,ab,kw 13,764
-
(calcium NEAR/2 (block* OR antagon* OR inhibit*)):ti,ab,kw 7875
-
(CCB OR CCBs):ti,ab,kw 727
-
[mh ‘Calcium Channel Blockers’] 2877
-
(flunarizine OR verapamil):ti,ab,kw 2752
-
(anticonvuls* OR antiepilep* OR (anti NEXT convuls*) OR (anti NEXT epilep*)):ti,ab,kw 5822
-
[mh Anticonvulsants] 2470
-
(topiramate OR valproate OR divalproex OR ‘valproic acid’ OR gabapentin):ti,ab,kw 6356
-
[mh ^Pizotyline] 36
-
(pizotifen OR pizotyline):ti,ab,kw 85
-
(alpha NEAR/4 agonist*):ti,ab,kw 2130
-
[mh ‘Adrenergic alpha-Agonists’] 1145
-
(clonidine OR guanfacine):ti,ab,kw 4432
-
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 98,163
-
#3 and #42 in Trials 6754
The Ovid MEDLINE search strategy was translated for use in the Cochrane Library with the aid of the Polyglot Search Translator:
Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc 2020;108:195–207. https://doi.org/10.5195/jmla.2020.834
Science Citation Index Expanded (via Web of Science)
Date searched: 8 September 2021
SCI-EXPANDED – 1970–present
Search history
-
29 #27 AND #28 4737
-
28 TS = (random* OR ‘controlled trial*’ OR ‘clinical trial*’ OR rct OR placebo* OR ((single* OR doubl* OR trebl* OR tripl*) NEAR/0 (blind* OR mask* OR dummy))) 2,167,277
-
27 (#1) AND #26 10,871
-
26 #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 713,606
-
25 TS = (clonidine OR guanfacine) 15,906
-
24 TS = (alpha NEAR/4 agonist*) 19,843
-
23 TS = (pizotifen OR pizotyline) 224
-
22 TS = (topiramate OR valproate OR divalproex OR ‘valproic acid’ OR gabapentin) 37,381
-
21 TS = (anticonvuls* OR antiepilep* OR ‘anti convuls*’ OR ‘anti epilep*’) 55,882
-
20 TS = (flunarizine OR verapamil) 24,117
-
19 TS = (CCB OR CCBs) 2897
-
18 TS = (calcium NEAR/2 (block* OR antagon* OR inhibit*)) 46,563
-
17 TS = (propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol) 49,544
-
16 TS=((adrenergic OR adrenoreceptor* OR adrenoceptor*) NEAR/3 (antagon* OR block*)) 29,120
-
15 TS=((beta NEAR/3 block*) OR betablock*) 56,780
-
14 TS = (candesartan) 4054
-
13 TS = (ARB OR ARBs) 8763
-
12 TS=((‘angiotensin receptor’ OR ‘angiotensin II receptor’) NEAR/0 (block* OR antagon*)) 14,815
-
11 TS = (lisinopril) 3148
-
10 TS=(‘Angiotensin Converting Enzyme Inhibit*’ OR ‘ACE inhibit*’ OR acei) 39,677
-
9 TS = (SNRI OR SNRIs OR (serotonin NEAR/2 (noradrenaline OR norepinephrine) NEAR/0 ‘reuptake inhib*’)) 2774
-
8 TS = (amitriptyline OR venlafaxine OR mirtazapine OR duloxetine) 18,231
-
7 TS = (antidepress* OR ‘anti depress*’) 80,971
-
6 TS = (botulinum* OR botox* OR onabotulinum*) 29,222
-
5 TS = (botulin* NEAR/0 toxin*) 19,563
-
4 TS = (rimegepant OR ubrogepant OR atogepant OR gepant$) 402
-
3 TS = (erenumab OR galcanezumab OR fremanezumab OR eptinezumab) 1260
-
2 TS=(((‘calcitonin gene-related peptide’ OR CGRP) NEAR/5 (antibod* OR antagon* OR inhibit* OR block*)) OR ‘anti-CGRP’ OR ‘anti-calcitonin gene-related peptide’ OR ‘monoclonal antibod*’ OR mAb OR mAbs OR moAb OR moAbs) 284,268
-
1 TS = (headache* OR ‘head ache*’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*) 106,395
The Ovid MEDLINE search strategy was translated for use in the Cochrane Library with the aid of the Polyglot Search Translator:
Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the Polyglot Search Translator: a randomized controlled trial. J Med Libr Assoc 2020;108:195–207. https://doi.org/10.5195/jmla.2020.834
The search filter for RCTs (line 28) incorporates some terms used in literature searches for the development of NICE clinical guideline CG155: Psychosis and schizophrenia in children and young people: recognition and management. NICE, 2013. Appendix 8: search strategies for the identification of clinical studies. Available from: www.nice.org.uk/guidance/cg155/evidence.
Global Index Medicus www.globalindexmedicus.net/
Date searched: 14 September 2021
Databases:
All the Regional Indexes Medici: African Index Medicus (AIM), Index Medicus for the Eastern Mediterranean Region (IMEMR), Index Medicus for the South-East Asia Region (IMSEAR), Latin America and the Caribbean Literature on Health Sciences (LILACS), Western Pacific Region Index Medicus (WPRO).
Search screen: Advanced, available at: https://search.bvsalud.org/gim/advanced/?lang=en.
Search strategy: (note tw fields are Title, Abstract, Subject)
1.
tw:((tw:(headache* OR ‘head ache’ OR ‘head aches’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*))
AND
(tw:(((‘calcitonin gene related peptide’ OR cgrp) AND (antibod* OR antagon* OR inhibit* OR block*)) OR ‘anti-CGRP’ OR ‘anti-calcitonin gene-related peptide’ OR ‘monoclonal antibody’ OR ‘monoclonal antibodies’ OR mab OR mabs OR moab OR moabs OR erenumab OR galcanezumab OR fremanezumab OR eptinezumab OR rimegepant OR ubrogepant OR atogepant OR gepant* OR (botulin* AND toxin*) OR botulinum* OR botox OR onabotulinum* OR antidepress* OR ‘anti depressant’ OR ‘anti depressants’ OR ‘anti depressive’ OR amitriptyline OR venlafaxine OR mirtazapine OR duloxetine OR (serotonin AND (noradrenaline OR norepinephrine) AND reuptake AND inhibit*) OR snri OR snris OR (‘angiotensin converting enzyme’ AND inhibit*) OR ‘ACE inhibitor’ OR ‘ACE inhibitors’ OR acei OR lisinopril OR ((‘angiotensin receptor’ OR ‘angiotensin II receptor’) AND (block* OR antagon*)) OR arb OR arbs OR candesartan OR ‘beta blocker’ OR ‘beta blockers’ OR ‘beta blocking’ OR ‘beta blockade’ OR betablock* OR ((adrenergic OR adrenoreceptor* OR adrenoceptor*) AND beta AND (antagon* OR block*)) OR propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol OR (calcium AND (block* OR antagon* OR inhibit*)) OR flunarizine OR verapamil OR anticonvuls* OR antiepilep* OR ‘anti convulsive’ OR ‘anti convulsant’ OR ‘anti convulsives’ OR ‘anti convulsants’ OR ‘anti epileptic’ OR ‘anti epileptics’ OR topiramate OR valproate OR divalproex OR ‘valproic acid’ OR gabapentin OR pizotifen OR pizotyline OR (alpha AND agonist*) OR clonidine OR guanfacine))
AND
(tw:(random* OR placebo* OR sham OR trial* OR ‘double blind’ OR ‘single blind’ OR ‘triple blind’ OR ‘treble blind’ OR ‘control group’ OR ‘control groups’ OR allocat* OR ‘phase 3’ OR ‘phase III’ OR ‘open label’ OR quasirandom* OR rct)))
[uses free text sensitive filter for RCTs developed by the Information Specialist (Anna Brown)]
199 results
2.
tw:((tw:(headache* OR ‘head ache’ OR ‘head aches’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*))
AND
(tw:(((‘calcitonin gene related peptide’ OR cgrp) AND (antibod* OR antagon* OR inhibit* OR block*)) OR ‘anti-CGRP’ OR ‘anti-calcitonin gene-related peptide’ OR ‘monoclonal antibody’ OR ‘monoclonal antibodies’ OR mab OR mabs OR moab OR moabs OR erenumab OR galcanezumab OR fremanezumab OR eptinezumab OR rimegepant OR ubrogepant OR atogepant OR gepant* OR (botulin* AND toxin*) OR botulinum* OR botox OR onabotulinum* OR antidepress* OR ‘anti depressant’ OR ‘anti depressants’ OR ‘anti depressive’ OR amitriptyline OR venlafaxine OR mirtazapine OR duloxetine OR (serotonin AND (noradrenaline OR norepinephrine) AND reuptake AND inhibit*) OR snri OR snris OR (‘angiotensin converting enzyme’ AND inhibit*) OR ‘ACE inhibitor’ OR ‘ACE inhibitors’ OR acei OR lisinopril OR ((‘angiotensin receptor’ OR ‘angiotensin II receptor’) AND (block* OR antagon*)) OR arb OR arbs OR candesartan OR ‘beta blocker’ OR ‘beta blockers’ OR ‘beta blocking’ OR ‘beta blockade’ OR betablock* OR ((adrenergic OR adrenoreceptor* OR adrenoceptor*) AND beta AND (antagon* OR block*)) OR propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol OR (calcium AND (block* OR antagon* OR inhibit*)) OR flunarizine OR verapamil OR anticonvuls* OR antiepilep* OR ‘anti convulsive’ OR ‘anti convulsant’ OR ‘anti convulsives’ OR ‘anti convulsants’ OR ‘anti epileptic’ OR ‘anti epileptics’ OR topiramate OR valproate OR divalproex OR ‘valproic acid’ OR gabapentin OR pizotifen OR pizotyline OR (alpha AND agonist*) OR clonidine OR guanfacine)))
AND
(type_of_study:(‘clinical_trials’))
[uses the ‘Type of study’ filter available from the results page for ‘Controlled clinical trial’]
71 results, of which 1 unique (i.e. not found by search 1 – deduplicated on import into EndNote)
Final number of unique results from Global Index Medicus: 200
ClinicalTrials.gov https://clinicaltrials.gov/
Date searched: 15 September 2021
Search screen: basic/home page
Search strategy:
| Condition or disease | Other terms | Filter applied | Hits |
|---|---|---|---|
| headache OR migraine | ‘calcitonin gene related peptide’ OR CGRP OR ‘monoclonal antibody’ OR ‘monoclonal antibodies’ | Study Type: Interventional | 100 |
| headache OR migraine | erenumab OR galcanezumab OR fremanezumab OR eptinezumab OR rimegepant OR ubrogepant OR atogepant OR gepant OR gepants | Study Type: Interventional | 115 |
| headache OR migraine | botox OR ‘botulinum toxin’ OR onabotulinumtoxin | Study Type: Interventional | 50 |
| headache OR migraine | antidepressant OR amitriptyline OR venlafaxine OR mirtazapine OR duloxetine | Study Type: Interventional | 38 |
| headache OR migraine | ‘serotonin noradrenaline reuptake inhibitor’ OR SNRI | Study Type: Interventional | 8 |
| headache OR migraine | ‘angiotensin converting enzyme inhibitor’ OR lisinopril | Study Type: Interventional | 2 |
| headache OR migraine | ‘angiotensin receptor blocker’ OR candesartan | Study Type: Interventional | 5 |
| headache OR migraine | ‘beta blocker’ OR propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol | Study Type: Interventional | 28 |
| headache OR migraine | calcium AND (blocker OR antagonist) | Study Type: Interventional | 32 |
| headache OR migraine | flunarizine OR verapamil | Study Type: Interventional | 18 |
| headache OR migraine | anticonvulsant OR anticonvulsive OR topiramate OR valproate OR divalproex OR valproic acid OR gabapentin | Study Type: Interventional | 99 |
| headache OR migraine | alpha agonist OR clonidine OR guanfacine | Study Type: Interventional | 5 |
| headache OR migraine | pizotifen OR pizotyline | Study Type: Interventional | 0 |
| Total number of records retrieved: 500 Total number of unique records (after deduplication using EndNote): 338 |
|||
International Clinical Trials Registry Platform (WHO ICTRP) https://trialsearch.who.int/
Date searched: 15 September 2021
Search screen: basic/home page
| Search | Number of trials found |
|---|---|
| (migrain* OR headache*) AND (calcitonin gene related peptide OR CGRP OR monocolonal antibod*) | 55 |
| (migrain* OR headache*) AND (erenumab OR amg334 OR amg-334 OR galcanezumab OR LY2951742 OR fremanezumab OR TEV-48125 OR eptinezumab OR ALD403) | 166 |
| (migrain* OR headache*) AND (rimegepant OR BHV-3000 OR BHV3000 OR BMS-927711 OR ubrogepant OR MK-1602 OR atogepant OR AGN-241689 OR MK-8031 OR gepant*) | 40 |
| (migrain* OR headache*) AND (botulin* OR botox OR onabotulinum* OR AGN 191622 OR NT 201) | 70 |
| (migrain* OR headache*) AND (antidepress* OR anti depress* OR anti-depress* OR serotonin norepinephrine reuptake inhibitor OR serotonin noradrenaline reuptake inhibitor OR SNRI OR SNRIs) | 2 |
| (migrain* OR headache*) AND (amitriptyline OR venlafaxine OR mirtazapine OR duloxetine OR LY248686) | 49 |
| (migrain* OR headache*) AND (angiotensin converting enzyme inhibit* OR ACE inhibit* OR lisinopril) | 1 |
| (migrain* OR headache*) AND (angiotensin OR ARB OR ARBs OR candesartan) | 7 |
| (migrain* OR headache*) AND (beta block* OR beta-block* OR betablock*) | 2 |
| (migrain* OR headache*) AND (propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol) | 64 |
| (migrain* OR headache*) AND calcium AND (block* OR antagon* OR inhibit*) | 0 |
| (migrain* OR headache*) AND (flunarizine OR verapamil) | 37 |
| (migrain* OR headache*) AND (anticonvuls* OR antiepilep* OR anti convuls* OR anti epilep* OR anti-convuls* OR anti-epilep*) | 6 |
| (migrain* OR headache*) AND (topiramate OR RWJ-17021 OR USL255 OR valproate OR divalproex OR valproic acid OR gabapentin) | 136 |
| (migrain* OR headache*) AND (clonidine OR guanfacine OR SPD503 OR pizotifen OR pizotyline) | 6 |
| Total number of records retrieved: 641 Total number of unique records (after deduplication using EndNote): 512 |
|
Search strategies: additional searches for riboflavin, magnesium and coenzyme-Q10, February 2022
MEDLINE (via Ovid)
Date searched: 8 February 2022
Ovid MEDLINE(R) ALL <1946 to 7 February 2022>
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kf,ti. 115,846
-
Headache/ or exp Headache Disorders/ 62,888
-
1 or 2 [population: migraine/headache] 127,140
-
Riboflavin/ 9019
-
(riboflavin or vitamin b2 or vitamin b 2).ab,kf,ti,nm. 14,667
-
Ubiquinone/ 9986
-
(coenzyme q* or co enzyme q* or ubidecarenone or ubiquino* or coq10 or co q10).ab,kf,ti,nm. 17,133
-
Magnesium/ or exp Magnesium Compounds/ 83,822
-
magnesium.ab,kf,ti,nm. 113,129
-
4 or 5 or 6 or 7 or 8 or 9 [interventions: 3 drugs added February 2022] 147,736
-
randomized controlled trial.pt. 558,117
-
controlled clinical trial.pt. 94,685
-
randomized.ab. 550,007
-
placebo.ab. 225,467
-
clinical trials as topic.sh. 199,113
-
randomly.ab. 375,668
-
trial.ti. 256,318
-
11 or 12 or 13 or 14 or 15 or 16 or 17 1,425,517
-
exp animals/ not humans.sh. 4,955,382
-
18 not 19 [Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity- and precision-maximizing version (2008 revision)] 1,311,348
-
3 and 10 and 20 [population + interventions + RCT filter] 161
-
(‘in data review’ or in process or publisher or ‘pubmed not medline’).st. 4,673,502
-
(random* or controlled trial* or clinical trial* or rct).ab,kf,ti. 1,597,122
-
22 and 23 [filter to pick up RCTs that have not been fully indexed for MEDLINE yet] 231,267
-
3 and 10 and 24 [population + interventions + RCT filter for non indexed studies] 18
-
21 or 25 163
EMBASE (via Ovid)
Date searched: 8 February 2022
EMBASE Classic+EMBASE <1947 to 7 February 2022>
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kw,ti. 192,971
-
‘headache and facial pain’/ or exp chronic daily headache/ or headache/ or exp migraine/ or vascular headache/ 304,535
-
headache/si not exp ‘headache and facial pain’/dm, dt, pc, th 80,538
-
(1 or 2) not 3 263,170
-
exp riboflavin/ 22,643
-
(riboflavin or vitamin b2 or vitamin b 2).ab,kw,ti,tn. 15,440
-
ubidecarenone/ 9897
-
(coenzyme q* or co enzyme q* or ubidecarenone or ubiquino* or coq10 or co q10).ab,kw,ti,tn. 18,062
-
magnesium/ or magnesium citrate/ or magnesium sulfate/ or magnesium oxide/ or magnesium derivative/ 125,171
-
magnesium.ab,kw,ti,tn. 83,078
-
5 or 6 or 7 or 8 or 9 or 10 198,610
-
Clinical trial/ 1047586
-
Randomized controlled trial/ 697,078
-
Randomization/ 93,174
-
Single blind procedure/ 45,106
-
Double blind procedure/ 194,609
-
Crossover procedure/ 69,726
-
Placebo/ 387,209
-
Randomi?ed controlled trial$.tw. 277,050
-
Rct.tw. 45,402
-
Random allocation.tw. 2364
-
Randomly allocated.tw. 40,516
-
Allocated randomly.tw. 2775
-
(allocated adj2 random).tw. 996
-
Single blind$.tw. 28,417
-
Double blind$.tw. 232,753
-
((treble or triple) adj blind$).tw. 1530
-
Placebo$.tw. 343,527
-
Prospective study/ 746,134
-
or/12-29 2,526,798
-
Case study/ 92,743
-
Case report.tw. 502,347
-
Abstract report/ or letter/ 1,226,797
-
or/31-33 1,809,075
-
30 not 34 [Ovid EMBASE RCTs filter, available from: https://tools.ovid.com/ovidtools/expertsearches.html] 2,466,633
-
4 and 11 and 35 690
-
conference abstract.pt. 4,311,641
-
36 not 37 587
Cochrane CENTRAL (via www.cochranelibrary.com)
Date searched: 8 September 2021
Database: Cochrane Central Register of Controlled Trials. Issue 2 of 12 February 2022
ID Search Hits
-
(headache* OR (head NEXT ache*) OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*):ti,ab,kw 38,109
-
[mh Headache] OR [mh ‘Headache Disorders’] 5556
-
#1 or #2 38,109
-
[mh ^Riboflavin] 370
-
(riboflavin OR ‘vitamin b2’ OR ‘vitamin b 2’):ti,ab,kw 1059
-
[mh ^Ubiquinone] 585
-
((coenzyme NEXT q*) OR (‘co enzyme’ NEXT q*) OR ubidecarenone OR ubiquino* OR coq10 OR ‘co q10’):ti,ab,kw 1446
-
[mh ^Magnesium] OR [mh ‘Magnesium Compounds’] 2639
-
(magnesium):ti,ab,kw 8245
-
#4 or #5 or #6 or #7 or #8 or #9 10,644
-
#3 and #10 in Trials 331
Science Citation Index Expanded (via Web of Science)
Date searched: 8 February 2022
SCI-EXPANDED – 1970–present
Search history
-
7 #6 AND #5 AND #1 359
-
6 TS = (random* OR ‘controlled trial*’ OR ‘clinical trial*’ OR rct OR placebo* OR ((single* OR doubl* OR trebl* OR tripl*) NEAR/0 (blind* OR mask* OR dummy))) 2,233,208
-
5 #2 OR #3 OR #4 203,563
-
4 TS = (magnesium) 171,475
-
3 TS=(‘coenzyme q*’ OR ‘co enzyme q*’ OR ubidecarenone OR ubiquino* OR coq10 OR ‘co q10’) 18,925
-
2 TS = (riboflavin OR ‘vitamin b2’ OR ‘vitamin b 2’) 13,950
-
1 TS = (headache* OR ‘head ache*’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*) 109,965
Global Index Medicus www.globalindexmedicus.net/
Date searched: 8 February 2022
Databases:
All the Regional Indexes Medici: African Index Medicus (AIM), Index Medicus for the Eastern Mediterranean Region (IMEMR), Index Medicus for the South-East Asia Region (IMSEAR), Latin America and the Caribbean Literature on Health Sciences (LILACS), Western Pacific Region Index Medicus (WPRO)
Search screen: Advanced, available at: https://search.bvsalud.org/gim/advanced/?lang=en
Search strategy: (note tw fields are Title, Abstract, Subject)
1.
tw:((tw:(headache* OR ‘head ache’ OR ‘head aches’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*)) AND (tw:(riboflavin OR ‘vitamin b2’ OR ‘vitamin b 2’ OR ‘coenzyme q’ OR ‘coenzyme q10’ OR ‘co enzyme q’ OR ‘co enzyme q10’ OR ubidecarenone OR ubiquino* OR coq10 OR ‘co q10’ OR magnesium)) AND (tw:(random* OR placebo* OR sham OR trial* OR ‘double blind’ OR ‘single blind’ OR ‘triple blind’ OR ‘treble blind’ OR ‘control group’ OR ‘control groups’ OR allocat* OR ‘phase 3’ OR ‘phase III’ OR ‘open label’ OR quasirandom* OR rct)))
[uses free text sensitive filter for RCTs developed by the Information Specialist (Anna Brown)]
24 results
2.
tw:((tw:(headache* OR ‘head ache’ OR ‘head aches’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*)) AND (tw:(riboflavin OR ‘vitamin b2’ OR ‘vitamin b 2’ OR ‘coenzyme q’ OR ‘coenzyme q10’ OR ‘co enzyme q’ OR ‘co enzyme q10’ OR ubidecarenone OR ubiquino* OR coq10 OR ‘co q10’ OR magnesium))) AND (type_of_study:(‘clinical_trials’))
[uses the ‘Type of study’ filter available from the results page for ‘Controlled clinical trial’]
6 results, of which 0 unique (i.e. not found by search 1 – deduplicated on import into EndNote)
Final number of unique results from Global Index Medicus: 24
ClinicalTrials.gov https://clinicaltrials.gov/
Date searched: 8 February 2022
Search screen: basic/home page
Search strategy:
| Condition or disease | Other terms | Filter applied | Hits |
|---|---|---|---|
| headache OR migraine | riboflavin | Study Type: Interventional | 3 |
| headache OR migraine | ‘coenzyme Q10’ | Study Type: Interventional | 5 |
| headache OR migraine | magnesium | Study Type: Interventional | 14 |
| Total number of records retrieved: 22 Total number of unique records (after deduplication using EndNote): 15 |
|||
International Clinical Trials Registry Platform (WHO ICTRP) https://trialsearch.who.int/
Date searched: 8 February 2022
Search screen: basic/home page
| Search | Number of trials found |
|---|---|
| (migrain* OR headache*) AND (riboflavin OR vitamin b2 OR vitamin b 2) | 11 |
| (migrain* OR headache*) AND (coenzyme q OR coenzyme q10 OR co enzyme q OR co enzyme q10 OR ubidecarenone OR ubiquino* OR coq10 OR co q10) | 11 |
| (migrain* OR headache*) AND magnesium | 23 |
| Total number of records retrieved: 45 Total number of unique records (after deduplication using EndNote): 38 |
|
Search strategies: pragmatic search for recent systematic reviews, to check reference lists/included studies, February 2022
MEDLINE (via Ovid)
Date searched: 14 February 2022
Ovid MEDLINE(R) ALL <1946 to 11 February 2022>
-
exp Migraine Disorders/pc 2569
-
‘migrain*’.ab,hw,kf,ti. 43,508
-
((prevent* or prophyla*) adj2 (treatment? or therap* or medication? or drug?)).ab,hw,kf,ti. 179,039
-
2 and 3 3218
-
(migrain* adj4 (prevent* or prophyla*)).ab,hw,kf,ti. 3883
-
1 or 4 or 5 5846
-
(metaanalys* or ‘meta analys*’).tw. 222,321
-
(systematic* adj3 review*).mp. 276,043
-
meta analysis.pt. 152,804
-
7 or 8 or 9 [pragmatic systematic review filter] 392,108
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kf,ti. 219,332
-
Calcitonin Gene-Related Peptide/ai 452
-
Antibodies, Monoclonal/ or Antibodies, Monoclonal, Humanized/ 221,635
-
Calcitonin Gene-Related Peptide Receptor Antagonists/ 781
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kf,ti,nm. 588
-
(rimegepant or ubrogepant or atogepant or gepant?).ab,kf,ti,nm. 247
-
exp Botulinum Toxins/ 17,563
-
(botulin* adj toxin*).ab,kf,ti,nm. 22,444
-
(botulinum* or botox* or onabotulinum*).ab,kf,ti,nm. 25,677
-
(antidepress* or anti depress*).ab,kf,ti. 75,518
-
exp Antidepressive Agents/ 155,320
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kf,ti,nm. 18,204
-
exp ‘Serotonin and Noradrenaline Reuptake Inhibitors’/ 5141
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kf,ti. 2996
-
exp Angiotensin-Converting Enzyme Inhibitors/ 45,974
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kf,ti. 38,458
-
acei.ab,kf,ti. 4519
-
lisinopril.ab,kf,ti,nm. 3114
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kf,ti. 14,830
-
(ARB or ARBs).ab,kf,ti. 8220
-
exp Angiotensin Receptor Antagonists/ 26,157
-
candesartan.ab,kf,ti,nm. 3407
-
((beta adj3 block*) or betablock*).ab,kf,ti. 56,350
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagon* or block*)).ab,kf,ti. 35,141
-
exp Adrenergic beta-Antagonists/ 85,957
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kf,ti,nm. 67,483
-
(calcium adj2 (block* or antagon* or inhibit*)).ab,kf,ti. 41,979
-
(CCB or CCBs).ab,kf,ti. 2692
-
exp Calcium Channel Blockers/ 89,276
-
(flunarizine or verapamil).ab,kf,ti,nm. 27,822
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kf,ti. 54,399
-
exp Anticonvulsants/ 149,062
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kf,ti,nm. 31,789
-
Pizotyline/ 250
-
(pizotifen or pizotyline).ab,kf,ti,nm. 420
-
(alpha adj4 agonist*).ab,kf,ti. 15,482
-
exp Adrenergic alpha-Agonists/ 165,206
-
(clonidine or guanfacine).ab,kf,ti,nm. 19,260
-
Riboflavin/ 9020
-
(riboflavin or vitamin b2 or vitamin b 2).ab,kf,ti,nm. 14,670
-
Ubiquinone/ 9995
-
(coenzyme q* or co enzyme q* or ubidecarenone or ubiquino* or coq10 or co q10).ab,kf,ti,nm. 17,147
-
Magnesium/ or exp Magnesium Compounds/ 83,845
-
magnesium.ab,kf,ti,nm. 113,174
-
or/11-54 1,249,348
-
6 and 10 and 55 182
-
limit 56 to yr=‘2017 - 2022’ 114
EMBASE (via Ovid)
Date searched: 14 February 2022
EMBASE Classic+EMBASE <1947 to 11 February 2022>
-
exp migraine/pc [Prevention] 4944
-
‘migrain*’.ab,hw,kf,ti. 82,866
-
((prevent* or prophyla*) adj2 (treatment? or therap* or medication? or drug?)).ab,hw,kf,ti. 254,616
-
2 and 3 6551
-
(migrain* adj4 (prevent* or prophyla*)).ab,hw,kf,ti. 7162
-
1 or 4 or 5 11,916
-
(metaanalys* or ‘meta analys*’).tw. 285,785
-
(systematic* adj3 review*).mp. 446,604
-
‘systematic review’/ 331,996
-
exp meta analysis/ 238,368
-
7 or 8 or 9 or 10 587,214
-
antimigraine agent/ 2621
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kw,ti. 278,976
-
exp calcitonin gene-related peptide receptor antagonist/ 4505
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kw,ti,tn. 1846
-
(rimegepant or ubrogepant or atogepant or gepant?).ab,kw,ti,tn. 634
-
botulinum toxin/ or botulinum toxin A/ 40,825
-
(botulin* adj toxin*).ab,kw,ti,tn. 22677
-
(botulinum* or botox* or onabotulinum*).ab,kw,ti,tn. 35,359
-
(antidepress* or anti depress*).ab,kw,ti. 110,521
-
exp antidepressant agent/ 544,182
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kw,ti,tn. 22,630
-
exp serotonin noradrenalin reuptake inhibitor/ 205,435
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kw,ti. 4833
-
exp dipeptidyl carboxypeptidase inhibitor/ 188,015
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kw,ti. 56,131
-
acei.ab,kw,ti. 9324
-
lisinopril.ab,kw,ti,tn. 4542
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kw,ti. 21,397
-
(ARB or ARBs).ab,kw,ti. 16,145
-
exp angiotensin receptor antagonist/ 104,492
-
candesartan.ab,kw,ti,tn. 4099
-
((beta adj3 block*) or betablock*).ab,kw,ti. 82,776
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagon* or block*)).ab,kw,ti. 43,748
-
exp beta adrenergic receptor blocking agent/ 322,377
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kw,ti,tn. 70,087
-
(calcium adj2 (block* or antagonis* or inhibit*)).ab,kw,ti. 53,952
-
(CCB or CCBs).ab,kw,ti. 4594
-
exp calcium antagonist/ 294,836
-
(flunarizine or verapamil).ab,kw,ti,tn. 29,749
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kw,ti. 85,741
-
exp anticonvulsive agent/ 473,685
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kw,ti,tn. 44,825
-
pizotifen/ 1985
-
(pizotifen or pizotyline).ab,kw,ti,tn. 447
-
(alpha adj4 agonist*).ab,kw,ti. 12,307
-
exp alpha 2 adrenergic receptor stimulating agent/ 119,247
-
(clonidine or guanfacine).ab,kw,ti,tn. 20,022
-
exp riboflavin/ 22,670
-
(riboflavin or vitamin b2 or vitamin b 2).ab,kw,ti,tn. 15,448
-
ubidecarenone/ 9906
-
(coenzyme q* or co enzyme q* or ubidecarenone or ubiquino* or coq10 or co q10).ab,kw,ti,tn. 18,078
-
magnesium/ or magnesium citrate/ or magnesium sulfate/ or magnesium oxide/ or magnesium derivative/ 125,280
-
magnesium.ab,kw,ti,tn. 83,136
-
or/12-54 2,046,652
-
6 and 11 and 55 513
-
limit 56 to yr=‘2017 - 2022’ 267
-
conference abstract.pt. 4,317,835
-
57 not 58 164
Cochrane Database of Systematic Reviews (via www.cochranelibrary.com)
Date searched: 14 February 2022
ID Search Hits
-
MeSH descriptor: [Migraine Disorders] explode all trees and with qualifier(s): [prevention&control - PC] 514
-
(migrain*):ti,ab,kw 8739
-
((prevent* or prophyla*) NEAR/2 (treatment? or therap* or medication? or drug?)):ti,ab,kw 39,315
-
#2 AND #3 1778
-
(migrain* NEAR/4 (prevent* or prophyla*)):ti,ab,kw 2616
-
#1 OR #4 OR #5 with Cochrane Library publication date Between Jan 2017 and Feb 2022, in Cochrane Reviews 4
Search strategies: update searches, November 2022
MEDLINE (via Ovid)
Date searched: 7 November 2022
Ovid MEDLINE(R) ALL <1946 to 4 November 2022>
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kf,ti. 121,076
-
Headache/ or exp Headache Disorders/ 64,821
-
1 or 2 [population: migraine/headache, based on Cochrane botox review] 132,425
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kf,ti. 224,346
-
Calcitonin Gene-Related Peptide/ai 463
-
Antibodies, Monoclonal/ or Antibodies, Monoclonal, Humanized/ 227,720
-
Calcitonin Gene-Related Peptide Receptor Antagonists/ 887
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kf,ti,nm. 730
-
(rimegepant or ubrogepant or atogepant or gepant?).ab,kf,ti,nm. 300
-
exp Botulinum Toxins/ 18,153
-
(botulin* adj toxin*).ab,kf,ti,nm. 23,232
-
(botulinum* or botox* or onabotulinum*).ab,kf,ti,nm. 26,565
-
(antidepress* or anti depress*).ab,kf,ti. 78,168
-
exp Antidepressive Agents/ 158,352
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kf,ti,nm. 18,641
-
exp ‘Serotonin and Noradrenaline Reuptake Inhibitors’/ 5336
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kf,ti. 3138
-
exp Angiotensin Converting Enzyme Inhibitors/ 46,764
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kf,ti. 39,244
-
acei.ab,kf,ti. 4749
-
lisinopril.ab,kf,ti,nm. 3155
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kf,ti. 15,370
-
(ARB or ARBs).ab,kf,ti. 8687
-
exp Angiotensin Receptor Antagonists/ 27,181
-
candesartan.ab,kf,ti,nm. 3449
-
((beta adj3 block*) or betablock*).ab,kf,ti. 57,470
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagon* or block*)).ab,kf,ti. 35,378
-
exp Adrenergic beta-Antagonists/ 86,663
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kf,ti,nm. 68,123
-
(calcium adj2 (block* or antagon* or inhibit*)).ab,kf,ti. 42,541
-
(CCB or CCBs).ab,kf,ti. 2828
-
exp Calcium Channel Blockers/ 90,326
-
(flunarizine or verapamil).ab,kf,ti,nm. 28,045
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kf,ti. 55,690
-
exp Anticonvulsants/ 152,010
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kf,ti,nm. 32842
-
Pizotyline/ 252
-
(pizotifen or pizotyline).ab,kf,ti,nm. 425
-
(alpha adj4 agonist*).ab,kf,ti. 15,644
-
exp Adrenergic alpha-Agonists/ 16,6795
-
(clonidine or guanfacine).ab,kf,ti,nm. 19,418
-
Riboflavin/ 9260
-
(riboflavin or vitamin b2 or vitamin b 2).ab,kf,ti,nm. 15,160
-
Ubiquinone/ 10,256
-
(coenzyme q* or co enzyme q* or ubidecarenone or ubiquino* or coq10 or co q10).ab,kf,ti,nm. 17,694
-
Magnesium/ or exp Magnesium Compounds/ 85,028
-
magnesium.ab,kf,ti,nm. 115,926
-
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 [Interventions: named drugs/drug classes or types] 1,275,840
-
randomized controlled trial.pt. 579,949
-
controlled clinical trial.pt. 95,083
-
randomized.ab. 580,977
-
placebo.ab. 232,922
-
clinical trials as topic.sh. 200,534
-
randomly.ab. 394,586
-
trial.ti. 273,031
-
49 or 50 or 51 or 52 or 53 or 54 or 55 148,2588
-
exp animals/ not humans.sh. 5,060,853
-
56 not 57 [Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity- and precision-maximizing version (2008 revision)] 1,364,006
-
3 and 48 and 58 [population and interventions and RCT filter] 4313
-
(‘in data review’ or in process or publisher or ‘pubmed not medline’).st. 4,897,386
-
(random* or controlled trial* or clinical trial* or rct).ab,kf,ti. 1,688,331
-
60 and 61 [filter to pick up RCTs that have not been fully indexed for MEDLINE yet] 242,577
-
3 and 48 and 62 [population and interventions and non-MEDLINE RCT filter] 328
-
59 or 63 4390
-
limit 64 to ed = 20210908-20221107 303
-
limit 64 to ep = 20210908-20221107 211
-
limit 64 to dt = 20210908-20221107 259
-
limit 64 to ez = 20210908-20221107 259
-
limit 64 to da = 20210908-20221107 366
-
65 or 66 or 67 or 68 or 69 390
EMBASE (via Ovid)
Date searched: 7 November 2022
EMBASE <1974 to 4 November 2022>
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kw,ti. 191,138
-
‘headache and facial pain’/ or exp chronic daily headache/ or headache/ or exp migraine/ or vascular headache/ 308,443
-
headache/si not exp ‘headache and facial pain’/dm, dt, pc, th 84,165
-
(1 or 2) not 3 264,484
-
antimigraine agent/ 2699
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kw,ti. 287,081
-
exp calcitonin gene related peptide receptor antagonist/ 5131
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kw,ti,tn. 2201
-
(rimegepant or ubrogepant or atogepant or gepant?).ab,kw,ti,tn. 796
-
botulinum toxin/ or botulinum toxin A/ 42,134
-
(botulin* adj toxin*).ab,kw,ti,tn. 23,263
-
(botulinum* or botox* or onabotulinum*).ab,kw,ti,tn. 35,729
-
(antidepress* or anti depress*).ab,kw,ti. 112,171
-
exp antidepressant agent/ 544,332
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kw,ti,tn. 22,582
-
exp serotonin noradrenalin reuptake inhibitor/ 202,776
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kw,ti. 5032
-
exp dipeptidyl carboxypeptidase inhibitor/ 195,131
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kw,ti. 57,476
-
acei.ab,kw,ti. 9813
-
lisinopril.ab,kw,ti,tn. 4703
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kw,ti. 22,228
-
(ARB or ARBs).ab,kw,ti. 17,008
-
exp angiotensin receptor antagonist/ 111,549
-
candesartan.ab,kw,ti,tn. 4178
-
((beta adj3 block*) or betablock*).ab,kw,ti. 81,083
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagon* or block*)).ab,kw,ti. 39,737
-
exp beta adrenergic receptor blocking agent/ 321,800
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kw,ti,tn. 67,224
-
(calcium adj2 (block* or antagonis* or inhibit*)).ab,kw,ti. 54,558
-
(CCB or CCBs).ab,kw,ti. 4814
-
exp calcium antagonist/ 338,915
-
(flunarizine or verapamil).ab,kw,ti,tn. 29,819
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kw,ti. 83,876
-
exp anticonvulsive agent/ 465,496
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kw,ti,tn. 46,634
-
pizotifen/ 1959
-
(pizotifen or pizotyline).ab,kw,ti,tn. 430
-
(alpha adj4 agonist*).ab,kw,ti. 12,529
-
exp alpha 2 adrenergic receptor stimulating agent/ 122,600
-
(clonidine or guanfacine).ab,kw,ti,tn. 20,035
-
exp riboflavin/ 18,899
-
(riboflavin or vitamin b2 or vitamin b 2).ab,kw,ti,tn. 12,921
-
ubidecarenone/ 10,384
-
(coenzyme q* or co enzyme q* or ubidecarenone or ubiquino* or coq10 or co q10).ab,kw,ti,tn. 17,792
-
magnesium/ or magnesium citrate/ or magnesium sulfate/ or magnesium oxide/ or magnesium derivative/ 111,389
-
magnesium.ab,kw,ti,tn. 76,887
-
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 2,044,855
-
Clinical trial/ 1,047,984
-
Randomized controlled trial/ 735,096
-
Randomization/ 95,413
-
Single blind procedure/ 48,155
-
Double blind procedure/ 200,384
-
Crossover procedure/ 71,963
-
Placebo/ 387,396
-
Randomi?ed controlled trial$.tw. 299,187
-
Rct.tw. 49,280
-
Random allocation.tw. 2441
-
Randomly allocated.tw. 42,860
-
Allocated randomly.tw. 2848
-
(allocated adj2 random).tw. 933
-
Single blind$.tw. 29,805
-
Double blind$.tw. 235,200
-
((treble or triple) adj blind$).tw. 1684
-
Placebo$.tw. 350,987
-
Prospective study/ 806,517
-
or/49-66 2,623,005
-
Case study/ 89,478
-
Case report.tw. 50,970
-
Abstract report/ or letter/ 1,257,280
-
or/68-70 1,836,926
-
67 not 71 [Ovid EMBASE RCTs filter, available from: https://tools.ovid.com/ovidtools/expertsearches.html] 2,560,333
-
4 and 48 and 72 12,827
-
conference abstract.pt. 4,583,125
-
73 not 74 9141
-
limit 75 to dc = 20210908-20221107 710
Cochrane CENTRAL (via www.cochranelibrary.com)
Date searched: 7 November 2022
Database: Cochrane Central Register of Controlled Trials. Issue 10 of 12, October 2022
ID Search Hits
-
(headache* OR (head NEXT ache*) OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*):ti,ab,kw 39,419
-
[mh Headache] OR [mh ‘Headache Disorders’] 5788
-
#1 or #2 39,419
-
(((‘calcitonin gene related peptide’ OR CGRP) NEAR/5 (antibod* OR antagon* OR inhibit* OR block*)) OR ‘anti CGRP’ OR ‘anti calcitonin gene-related peptide’ OR (monoclonal NEXT antibod*) OR mAb OR mAbs OR moAb OR moAbs):ti,ab,kw 13,047
-
[mh ‘Calcitonin Gene-Related Peptide’/AI] 26
-
[mh ^‘Antibodies, Monoclonal’] OR [mh ^”Antibodies, Monoclonal, Humanized”] 9475
-
[mh ^‘Calcitonin Gene-Related Peptide Receptor Antagonists’] 80
-
(erenumab OR galcanezumab OR fremanezumab OR eptinezumab):ti,ab,kw 1138
-
(rimegepant OR ubrogepant OR atogepant OR gepant*):ti,ab,kw 339
-
[mh ‘Botulinum Toxins’] 2130
-
(botulin* NEXT toxin*):ti,ab,kw 4497
-
(botulinum* OR botox* OR onabotulinum*):ti,ab,kw 5138
-
(antidepress* OR (anti NEXT depress*)):ti,ab,kw 17,488
-
[mh ‘Antidepressive Agents’] 6090
-
(amitriptyline OR venlafaxine OR mirtazapine OR duloxetine):ti,ab,kw 6524
-
[mh ‘Serotonin and Noradrenaline Reuptake Inhibitors’] 62
-
(SNRI OR SNRIs OR ((serotonin NEAR/2 (noradrenaline OR norepinephrine)) NEXT (‘reuptake inhibitor’ OR ‘reuptake inhibitors’ OR ‘reuptake inhibition’))):ti,ab,kw 854
-
[mh ‘Angiotensin Converting Enzyme Inhibitors’] 4125
-
((‘Angiotensin Converting Enzyme’ NEXT Inhibit*) OR (ACE NEXT inhibit*)):ti,ab,kw 9390
-
acei:ti,ab,kw 1753
-
lisinopril:ti,ab,kw 1331
-
((‘angiotensin receptor’ OR ‘angiotensin II receptor’) NEXT (block* OR antagon*)):ti,ab,kw 4748
-
(ARB OR ARBs):ti,ab,kw 2624
-
[mh ‘Angiotensin Receptor Antagonists’] 2308
-
candesartan:ti,ab,kw 1256
-
((beta NEAR/3 block*) OR betablock*):ti,ab,kw 11,523
-
((adrenergic OR adrenoreceptor* OR adrenoceptor*) NEAR/3 (antagon* OR block*)):ti,ab,kw 10,392
-
[mh ‘Adrenergic beta-Antagonists’] 4648
-
(propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol):ti,ab,kw 13,983
-
(calcium NEAR/2 (block* OR antagon* OR inhibit*)):ti,ab,kw 7926
-
(CCB OR CCBs):ti,ab,kw 732
-
[mh ‘Calcium Channel Blockers’] 2910
-
(flunarizine OR verapamil):ti,ab,kw 2808
-
(anticonvuls* OR antiepilep* OR (anti NEXT convuls*) OR (anti NEXT epilep*)):ti,ab,kw 6069
-
[mh Anticonvulsants] 2555
-
(topiramate OR valproate OR divalproex OR ‘valproic acid’ OR gabapentin):ti,ab,kw 6651
-
[mh ^Pizotyline] 36
-
(pizotifen OR pizotyline):ti,ab,kw 86
-
(alpha NEAR/4 agonist*):ti,ab,kw 2206
-
[mh ‘Adrenergic alpha-Agonists’] 1159
-
(clonidine OR guanfacine):ti,ab,kw 4662
-
[mh ^Riboflavin] 377
-
(riboflavin OR ‘vitamin b2’ OR ‘vitamin b 2’):ti,ab,kw 1112
-
[mh ^Ubiquinone] 606
-
((coenzyme NEXT q*) OR (‘co enzyme’ NEXT q*) OR ubidecarenone OR ubiquino* OR coq10 OR ‘co q10’):ti,ab,kw 1507
-
[mh ^Magnesium] OR [mh ‘Magnesium Compounds’] 2714
-
(magnesium):ti,ab,kw 8693
-
#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 113,318
-
#3 and #48 with Cochrane Library publication date Between Sep 2021 and Nov 2022, in Trials 713
Science Citation Index Expanded (via Web of Science)
Date searched: 7 November 2022
| Query | Results | |
|---|---|---|
| #33 | #1 AND #31 AND #32 and Science Citation Index Expanded (SCI-EXPANDED) Timespan [Index date]: 2021-09-08 to 2022-11-07 | 440 |
| #32 | TS = (headache* OR ‘head ache*’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*) Editions: WOS.SCI | 114,776 |
| #31 | #26 OR #30 | 981,863 |
| #30 | #27 OR #28 OR #29 Editions: WOS.SCI | 212,609 |
| #29 | TS = (magnesium) Editions: WOS.SCI | 179,306 |
| #28 | TS=(‘coenzyme q*’ OR ‘co enzyme q*’ OR ubidecarenone OR ubiquino* OR coq10 OR ‘co q10’) Editions: WOS.SCI | 19,547 |
| #27 | TS = (riboflavin OR ‘vitamin b2’ OR ‘vitamin b 2’) Editions: WOS.SCI | 14,589 |
| #26 | #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 | 772,406 |
| #25 | clonidine OR guanfacine (Topic) | 17,184 |
| #24 | alpha NEAR/4 agonist* (Topic) | 21,033 |
| #23 | pizotifen OR pizotyline (Topic) | 236 |
| #22 | topiramate OR valproate OR divalproex OR ‘valproic acid’ OR gabapentin (Topic) | 41,733 |
| #21 | anticonvuls* OR antiepilep* OR ‘anti convuls*’ OR ‘anti epilep*’ (Topic) | 62,154 |
| #20 | flunarizine OR verapamil (Topic) | 25,018 |
| #19 | CCB OR CCBs (Topic) | 3729 |
| #18 | calcium NEAR/2 (block* OR antagon* OR inhibit*) (Topic) | 49,286 |
| #17 | propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol (Topic) | 52,463 |
| #16 | (adrenergic OR adrenoreceptor* OR adrenoceptor*) NEAR/3 (antagon* OR block*) (Topic) | 30,107 |
| #15 | (beta NEAR/3 block*) OR betablock* (Topic) | 61,243 |
| #14 | candesartan (Topic) | 4293 |
| #13 | ARB OR ARBs (Topic) | 10,693 |
| #12 | (‘angiotensin receptor’ OR ‘angiotensin II receptor’) NEAR/0 (block* OR antagon*) (Topic) | 16,515 |
| #11 | lisinopril (Topic) | 3398 |
| #10 | ‘Angiotensin Converting Enzyme Inhibit*’ OR ‘ACE inhibit*’ OR acei (Topic) | 42,930 |
| #9 | SNRI OR SNRIs OR (serotonin NEAR/2 (noradrenaline OR norepinephrine) NEAR/0 ‘reuptake inhib*’) (Topic) | 3230 |
| #8 | amitriptyline OR venlafaxine OR mirtazapine OR duloxetine (Topic) | 20,245 |
| #7 | antidepress* OR ‘anti depress*’ (Topic) | 92,351 |
| #6 | botulinum* OR botox* OR onabotulinum* (Topic) | 32,959 |
| #5 | botulin* NEAR/0 toxin* (Topic) | 22,325 |
| #4 | rimegepant OR ubrogepant OR atogepant OR gepant$ (Topic) | 648 |
| #3 | erenumab OR galcanezumab OR fremanezumab OR eptinezumab (Topic) | 1801 |
| #2 | TS = ((((‘calcitonin gene-related peptide’ OR CGRP) NEAR/5 (antibod* OR antagon* OR inhibit* OR block*)) OR ‘anti-CGRP’ OR ‘anti-calcitonin gene-related peptide’ OR ‘monoclonal antibod*’ OR mAb OR mAbs OR moAb OR moAbs)) | 301,595 |
| #1 | TS = (random* OR ‘controlled trial*’ OR ‘clinical trial*’ OR rct OR placebo* OR ((single* OR doubl* OR trebl* OR tripl*) NEAR/0 (blind* OR mask* OR dummy))) Editions: WOS.SCI | 2,354,770 |
Global Index Medicus www.globalindexmedicus.net/
Date searched: 7 November 2022
Databases:
All the Regional Indexes Medici: African Index Medicus (AIM), Index Medicus for the Eastern Mediterranean Region (IMEMR), Index Medicus for the South-East Asia Region (IMSEAR), Latin America and the Caribbean Literature on Health Sciences (LILACS), Western Pacific Region Index Medicus (WPRO)
Search screen: Advanced, available at: https://search.bvsalud.org/gim/advanced/?lang=en
Search strategy: (note tw fields are Title, Abstract, Subject)
1.
tw:((tw:(headache* OR ‘head ache’ OR ‘head aches’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*))
AND
(tw:(((‘calcitonin gene related peptide’ OR cgrp) AND (antibod* OR antagon* OR inhibit* OR block*)) OR ‘anti-CGRP’ OR ‘anti-calcitonin gene-related peptide’ OR ‘monoclonal antibody’ OR ‘monoclonal antibodies’ OR mab OR mabs OR moab OR moabs OR erenumab OR galcanezumab OR fremanezumab OR eptinezumab OR rimegepant OR ubrogepant OR atogepant OR gepant* OR (botulin* AND toxin*) OR botulinum* OR botox OR onabotulinum* OR antidepress* OR ‘anti depressant’ OR ‘anti depressants’ OR ‘anti depressive’ OR amitriptyline OR venlafaxine OR mirtazapine OR duloxetine OR (serotonin AND (noradrenaline OR norepinephrine) AND reuptake AND inhibit*) OR snri OR snris OR (‘angiotensin converting enzyme’ AND inhibit*) OR ‘ACE inhibitor’ OR ‘ACE inhibitors’ OR acei OR lisinopril OR ((‘angiotensin receptor’ OR ‘angiotensin II receptor’) AND (block* OR antagon*)) OR arb OR arbs OR candesartan OR ‘beta blocker’ OR ‘beta blockers’ OR ‘beta blocking’ OR ‘beta blockade’ OR betablock* OR ((adrenergic OR adrenoreceptor* OR adrenoceptor*) AND beta AND (antagon* OR block*)) OR propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol OR (calcium AND (block* OR antagon* OR inhibit*)) OR flunarizine OR verapamil OR anticonvuls* OR antiepilep* OR ‘anti convulsive’ OR ‘anti convulsant’ OR ‘anti convulsives’ OR ‘anti convulsants’ OR ‘anti epileptic’ OR ‘anti epileptics’ OR topiramate OR valproate OR divalproex OR ‘valproic acid’ OR gabapentin OR pizotifen OR pizotyline OR (alpha AND agonist*) OR clonidine OR guanfacine))
AND
(tw:(random* OR placebo* OR sham OR trial* OR ‘double blind’ OR ‘single blind’ OR ‘triple blind’ OR ‘treble blind’ OR ‘control group’ OR ‘control groups’ OR allocat* OR ‘phase 3’ OR ‘phase III’ OR ‘open label’ OR quasirandom* OR rct)))
204 results
2.
tw:((tw:(headache* OR ‘head ache’ OR ‘head aches’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*))
AND (tw:(((‘calcitonin gene related peptide’ OR cgrp) AND (antibod* OR antagon* OR inhibit* OR block*)) OR ‘anti-CGRP’ OR ‘anti-calcitonin gene-related peptide’ OR ‘monoclonal antibody’ OR ‘monoclonal antibodies’ OR mab OR mabs OR moab OR moabs OR erenumab OR galcanezumab OR fremanezumab OR eptinezumab OR rimegepant OR ubrogepant OR atogepant OR gepant* OR (botulin* AND toxin*) OR botulinum* OR botox OR onabotulinum* OR antidepress* OR ‘anti depressant’ OR ‘anti depressants’ OR ‘anti depressive’ OR amitriptyline OR venlafaxine OR mirtazapine OR duloxetine OR (serotonin AND (noradrenaline OR norepinephrine) AND reuptake AND inhibit*) OR snri OR snris OR (‘angiotensin converting enzyme’ AND inhibit*) OR ‘ACE inhibitor’ OR ‘ACE inhibitors’ OR acei OR lisinopril OR ((‘angiotensin receptor’ OR ‘angiotensin II receptor’) AND (block* OR antagon*)) OR arb OR arbs OR candesartan OR ‘beta blocker’ OR ‘beta blockers’ OR ‘beta blocking’ OR ‘beta blockade’ OR betablock* OR ((adrenergic OR adrenoreceptor* OR adrenoceptor*) AND beta AND (antagon* OR block*)) OR propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol OR (calcium AND (block* OR antagon* OR inhibit*)) OR flunarizine OR verapamil OR anticonvuls* OR antiepilep* OR ‘anti convulsive’ OR ‘anti convulsant’ OR ‘anti convulsives’ OR ‘anti convulsants’ OR ‘anti epileptic’ OR ‘anti epileptics’ OR topiramate OR valproate OR divalproex OR ‘valproic acid’ OR gabapentin OR pizotifen OR pizotyline OR (alpha AND agonist*) OR clonidine OR guanfacine)))
AND
(type_of_study:(‘clinical_trials’))
71 results, of which 1 unique (i.e. not found by search 1 – deduplicated in EndNote)
3.
tw:((tw:(headache* OR ‘head ache’ OR ‘head aches’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*))
AND
(tw:(riboflavin OR ‘vitamin b2’ OR ‘vitamin b 2’ OR ‘coenzyme q’ OR ‘coenzyme q10’ OR ‘co enzyme q’ OR ‘co enzyme q10’ OR ubidecarenone OR ubiquino* OR coq10 OR ‘co q10’ OR magnesium))
AND
(tw:(random* OR placebo* OR sham OR trial* OR ‘double blind’ OR ‘single blind’ OR ‘triple blind’ OR ‘treble blind’ OR ‘control group’ OR ‘control groups’ OR allocat* OR ‘phase 3’ OR ‘phase III’ OR ‘open label’ OR quasirandom* OR rct)))
26 results, of which 17 unique (i.e. not found by searches 1 or 2 – deduplicated on import into EndNote)
4.
tw:((tw:(headache* OR ‘head ache’ OR ‘head aches’ OR migrain* OR cephalgi* OR cephalalgi* OR hemicrani*)) AND (tw:(riboflavin OR ‘vitamin b2’ OR ‘vitamin b 2’ OR ‘coenzyme q’ OR ‘coenzyme q10’ OR ‘co enzyme q’ OR ‘co enzyme q10’ OR ubidecarenone OR ubiquino* OR coq10 OR ‘co q10’ OR magnesium))) AND (type_of_study:(‘clinical_trials’))
6 results, of which 0 unique (i.e. not found by searches 1–3 – deduplicated on import into EndNote)
Total unique results: 222
ClinicalTrials.gov https://clinicaltrials.gov/
Date searched: 8 November 2022
Search screen: basic/home page
Search strategy:
| Condition or disease | Other terms | Filter applied | Hits | |
|---|---|---|---|---|
| 1 | headache OR migraine | ‘calcitonin gene related peptide’ OR CGRP OR ‘monoclonal antibody’ OR ‘monoclonal antibodies’ | Study Type: Interventional | 112 |
| 2 | headache OR migraine | erenumab OR galcanezumab OR fremanezumab OR eptinezumab OR rimegepant OR ubrogepant OR atogepant OR gepant OR gepants | Study Type: Interventional | 139 |
| 3 | headache OR migraine | botox OR ‘botulinum toxin’ OR onabotulinumtoxin | Study Type: Interventional | 57 |
| 4 | headache OR migraine | antidepressant OR amitriptyline OR venlafaxine OR mirtazapine OR duloxetine | Study Type: Interventional | 41 |
| 5 | headache OR migraine | ‘serotonin noradrenaline reuptake inhibitor’ OR SNRI | Study Type: Interventional | 8 |
| 6 | headache OR migraine | ‘angiotensin converting enzyme inhibitor’ OR lisinopril | Study Type: Interventional | 2 |
| 7 | headache OR migraine | ‘angiotensin receptor blocker’ OR candesartan | Study Type: Interventional | 6 |
| 8 | headache OR migraine | ‘beta blocker’ OR propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol | Study Type: Interventional | 30 |
| 9 | headache OR migraine | calcium AND (blocker OR antagonist) | Study Type: Interventional | 33 |
| 10 | headache OR migraine | flunarizine OR verapamil | Study Type: Interventional | 18 |
| 11 | headache OR migraine | anticonvulsant OR anticonvulsive OR topiramate OR valproate OR divalproex OR valproic acid OR gabapentin | Study Type: Interventional | 106 |
| 12 | headache OR migraine | alpha agonist OR clonidine OR guanfacine | Study Type: Interventional | 6 |
| 13 | headache OR migraine | pizotifen OR pizotyline | Study Type: Interventional | 0 |
| 14 | headache OR migraine | riboflavin | Study Type: Interventional | 3 |
| 15 | headache OR migraine | ‘coenzyme Q10’ | Study Type: Interventional | 6 |
| 16 | headache OR migraine | magnesium | Study Type: Interventional | 15 |
| Total number of records retrieved: 582 Total number of unique records (after deduplication using EndNote): 390 |
||||
International Clinical Trials Registry Platform (WHO ICTRP) https://trialsearch.who.int/
Date searched: 8 November 2022
Search screen: basic/home page
| Search | Number of trials found | |
|---|---|---|
| 1 | (migrain* OR headache*) AND (calcitonin gene related peptide OR CGRP OR monocolonal antibod*) | 66 |
| 2 | (migrain* OR headache*) AND (erenumab OR amg334 OR amg-334 OR galcanezumab OR LY2951742 OR fremanezumab OR TEV-48125 OR eptinezumab OR ALD403) | 167 |
| 3 | (migrain* OR headache*) AND (rimegepant OR BHV-3000 OR BHV3000 OR BMS-927711 OR ubrogepant OR MK-1602 OR atogepant OR AGN-241689 OR MK-8031 OR gepant*) | 58 |
| 4 | (migrain* OR headache*) AND (botulin* OR botox OR onabotulinum* OR AGN 191622 OR NT 201) | 75 |
| 5 | (migrain* OR headache*) AND (antidepress* OR anti depress* OR anti-depress* OR serotonin norepinephrine reuptake inhibitor OR serotonin noradrenaline reuptake inhibitor OR SNRI OR SNRIs) | 2 |
| 6 | (migrain* OR headache*) AND (amitriptyline OR venlafaxine OR mirtazapine OR duloxetine OR LY248686) | 51 |
| 7 | (migrain* OR headache*) AND (angiotensin converting enzyme inhibit* OR ACE inhibit* OR lisinopril) | 1 |
| 8 | (migrain* OR headache*) AND (angiotensin OR ARB OR ARBs OR candesartan) | 7 |
| 9 | (migrain* OR headache*) AND (beta block* OR beta-block* OR betablock*) | 2 |
| 10 | (migrain* OR headache*) AND (propranolol OR metoprolol OR timolol OR atenolol OR nadolol OR nebivolol OR pindolol) | 67 |
| 11 | (migrain* OR headache*) AND calcium AND (block* OR antagon* OR inhibit*) | 0 |
| 12 | (migrain* OR headache*) AND (flunarizine OR verapamil) | 37 |
| 13 | (migrain* OR headache*) AND (anticonvuls* OR antiepilep* OR anti convuls* OR anti epilep* OR anti-convuls* OR anti-epilep*) | 7 |
| 14 | (migrain* OR headache*) AND (topiramate OR RWJ-17021 OR USL255 OR valproate OR divalproex OR valproic acid OR gabapentin) | 140 |
| 15 | (migrain* OR headache*) AND (clonidine OR guanfacine OR SPD503 OR pizotifen OR pizotyline) | 6 |
| 16 | (migrain* OR headache*) AND (riboflavin OR vitamin b2 OR vitamin b 2) | 8 |
| 17 | (migrain* OR headache*) AND (coenzyme q OR coenzyme q10 OR co enzyme q OR co enzyme q10 OR ubidecarenone OR ubiquino* OR coq10 OR co q10) | 11 |
| 18 | (migrain* OR headache*) AND magnesium | 25 |
| Total number of records retrieved: 730 Total number of unique records (after deduplication using EndNote): 631 |
||
Reference lists and forward citation searches
Web of Science Core Collection: Science Citation Index Expanded – 1970–present; Social Sciences Citation Index (SSCI) – 1900–present; Arts and Humanities Citation Index (AHCI) – 1975–present; Conference Proceedings Citation Index – Science (CPCI-S) – 1990–present; Conference Proceedings Citation Index – Social Science and Humanities (CPCI-SSH) – 1990–present; Emerging Sources Citation Index (ESCI) – 2015–present.
Dates searched: 22–24 November 2022
Searched for each included study by combinations of author and title keywords
69/72 included study papers had records in Web of Science, yielding 2710 citing paper results and 875 reference list results
Google Scholar https://scholar.google.co.uk/
Date searched: 23 November 2022
The remaining 3 study papers were found via Google Scholar; 2 had 0 citing papers in Google Scholar, 1 had 23 citing papers.
Searches to check for retraction notices, errata and comments relating to included studies
MEDLINE (Ovid) search strategy, date searched: 22 November 2022
Database: Ovid MEDLINE(R) ALL <1946 to 21 November 2022>
Search Strategy:
------------ ------------ ------------ -------------- ---------------- --------------
-
(‘33069214’ or ‘34407343’ or ‘33549036’ or ‘34246226’ or ‘33231489’ or ‘33400330’ or ‘32075406’ or ‘29984601’ or ‘24107267’ or ‘20647170’ or ‘17445098’ or ‘32985341’ or ‘31816249’ or ‘21070231’ or ‘33338437’ or ‘30446596’ or ‘17988947’ or ‘20647171’ or ‘33314079’ or ‘12047461’ or ‘15316798’ or ‘29471679’ or ‘19393844’ or ‘25127173’ or ‘31234642’ or ‘18052949’ or ‘29800211’ or ‘31112399’ or ‘20487038’ or ‘17018329’ or ‘31427046’ or ‘32930994’ or ‘30594122’ or ‘30982348’ or ‘29171821’ or ‘23406477’ or ‘32747522’ or ‘31291516’ or ‘32209650’ or ‘27288354’ or ‘20974598’ or ‘30996060’ or ‘3180198’ or ‘32949542’ or ‘31721185’ or ‘17428299’ or ‘30360965’ or ‘31559634’ or ‘31104507’ or ‘34324700’ or ‘34323290’ or ‘33023473’ or ‘19719543’ or ‘32958075’ or ‘33026630’ or ‘29171818’ or ‘17300356’ or ‘29255900’ or ‘33250209’ or ‘34374086’ or ‘29813147’ or ‘30942898’ or ‘26879279’ or ‘28460892’ or ‘30996056’ or ‘34171973’).ui. (66)
Annotation: MEDLINE accession numbers/PubMed IDs of 66 included studies identified via MEDLINE, exported from EndNote
-
(cin or comment or con or concern or cri or crf or ecf or eci or efr or ein or erratum or expression or republished or retracted or retraction or rin or rof or rpf or rpi or rrf or rri or uin or uof or update).cm. (2,146,340)
-
1 and 2 (22)
-
fazlalizadeh h.au. (4)
-
Erenumab versus topiramate for the prevention of migraine.m_titl. (1)
-
‘10.1177/2515816320932573’.do,cm. (0)
-
(Efficacy and safety of galcanezumab for prevention of migraine headache in Japanese patients with episodic migraine).m_titl. (0)
-
Time course of efficacy of atogepant for the preventive treatment of migraine: Results from the randomized, double-blind ADVANCE trial.m_titl. (1)
-
Early onset of efficacy with fremanezumab for the preventive treatment of chronic migraine.m_titl. (1)
-
5 or 8 or 9 (3)
Annotation: 3 additional included studies now available in MEDLINE
-
2 and 10 (1)
-
3 or 11 (23)
EMBASE (Ovid) search strategy, date searched: 22 November 2022
Checking for Sakai et al. , 2020 only (as not in MEDLINE):
EMBASE Classic+EMBASE <1947 to 21 November 2022>
-
‘2005611510’.rr.0
-
(Efficacy and safety and galcanezumab and ‘prevention of migraine’ and Japanese).mp.2
-
erratum/ or ‘expression of concern’/ or retraction notice/262,282
-
Retracted article/13,012
-
yes.ne.5434
-
(erratum or tombstone).pt.268,823
-
3 or 4 or 5 or 6272,030
-
(retraction or retracted).ti.16,218
-
(comment on or erratum or corrigendum or withdrawn).ti.236,362
-
7 or 8 or 9312,106
-
2 and 100
Retraction Watch Database http://retractiondatabase.org/RetractionSearch.aspx
Date searched: 22 November 2022
Searched for ‘migraine’ in Title field (as all included studies include this word in the title): 7 results, none of which are in the included studies.
Appendix 2 Baseline characteristics of the included studies for clinical effectiveness review
| First author, year/country | Purpose/study design and date | Key inclusion criteria | Key exclusion criteria | Conclusion |
|---|---|---|---|---|
| Author, year: Silberstein, 200728 Country: USA |
Purpose: To evaluate the efficacy and safety of topiramate (100 mg/day) compared with placebo for the treatment of chronic migraine Study design: randomised, double-blind, placebo-controlled, parallel-group, multicentre trial Date: September 2003–March 2005 |
|
|
Topiramate resulted in statistically significant improvements compared with placebo in mean monthly migraine and migraine headache days. Topiramate is safe and generally well tolerated |
|
|
|||
| Author, year: Rothrock, 201988 Country: USA |
Purpose: To compare effectiveness of onabotulinumtoxinA and topiramate for CM prevention Study design: multicentre, randomised, parallel-group, post-authorisation, open label prospective study. After 12 weeks, patients initially randomised to topiramate could cross over to onabotulinumtoxinA treatment Date: August 2014–September 2017 |
|
|
In those few patients who were randomised to the oral medication and completed the treatment phase, topiramate was at least as efficacious as onabotulinumtoxin A. However, the high discontinuation rate associated with topiramate [the majority (51%) of patients discontinued treatment because of AEs] appears to diminish its clinical value significantly, compared to only 4% of BTA group. Results also demonstrate that BTA is a safe and often effective alternative for patients with CM who discontinue treatment with topiramate |
|
|
|||
| Author, year: Tepper, 201745 Country: North America (Canada and the USA) and Europe (Czech Republic, Denmark, Finland, Germany, Norway, Poland, Sweden, and the UK) |
Purpose: To assess the safety and efficacy of erenumab 70 and 140 mg in patients with chronic migraine Study design: Phase 2, randomised, double-blind, placebo-controlled, multicentre Date: 3 April 2014–4 December 2015 |
|
|
In patients with chronic migraine, erenumab 70 and 140 mg reduced the number of MMDs with a safety profile similar to placebo, providing evidence that erenumab could be a potential therapy for migraine prevention. Further research is needed to understand long-term efficacy and safety of erenumab, and the applicability of this study to real- world settings |
|
|
|||
| Author, year: Dodick, 201989 Country: 82 in the USA, 4 in Australia, and 3 each in New Zealand and the Republic of Georgia |
Purpose: To determine the safety, tolerability, and effectiveness of 4 dose levels of eptinezumab and to inform the phase 3 development programme Study design: Phase 2b, parallel-group, double-blind, randomised, placebo-controlled, dose-ranging clinical trial Date: December 2014–December 2016 |
|
|
The results of this trial demonstrate that eptinezumab appears effective and well tolerated for the preventive treatment of chronic migraine and justifies the conduct of pivotal phase 3 trials for migraine prevention |
|
|
|||
| Author, year: Detke, 201895 Country: Argentina, Canada, Czech Republic, Germany, Israel, Italy, Mexico, the Netherlands, Spain, Taiwan, UK and USA |
Purpose: To evaluate the efficacy and safety of galcanezumab, a humanised monoclonal antibody that selectively binds to calcitonin gene-related peptide, in the preventive treatment of chronic migraine Study design: Phase 3, randomised, double-blind, placebo-controlled study Date: January 2016–March 2017 |
|
|
Both doses of galcanezumab were superior to placebo in reducing the number of monthly MHDs. Galcanezumab appears efficacious, safe and well tolerated for the preventive treatment of chronic migraine |
|
|
|||
| Author, year: Aurora, 201092 Country: 56 North American sites |
Purpose: To assess efficacy, safety and tolerability of BTA as headache prophylaxis in adults with chronic migraine Study design: Phase 3 study, with a 24-week, double-blind, parallel-group, placebo-controlled phase followed by a 32-week, open label phase Date: 23 January 2006–16 July 2008 |
|
|
There was no between-group difference for the primary endpoint, headache episodes. However, significant reductions from baseline were observed for BTA for headache and migraine days, cumulative hours of headache-on-headache days and frequency of moderate/severe headache days, which in turn reduced the burden of illness in adults with disabling chronic migraine |
|
|
|||
| Author, year: Diener, 2010 93 Country: At 66 global sites in North America and 16 in Europe |
Purpose: To evaluate the efficacy and safety of BTA for prophylaxis of headaches in adults with chronic migraine Study design: Phase 3 study, with a 24-week, double-blind, placebo-controlled phase, followed by a 32-week, open label phase Date: 7 February 2006–11 August 2008 |
|
|
The results of PREEMPT 2 demonstrate that BTA is effective for prophylaxis of headache in adults with chronic migraine. Repeated BTA treatments were safe and well tolerated |
|
||||
| Author, year: Ferrari, 201990 Country: Belgium, the Czech Republic, Denmark, Finland, France, Germany, Italy, the Netherlands, Poland, Spain, Sweden, Switzerland, UK and USA |
Purpose: To investigate the efficacy and tolerability of monthly and fremanezumab quarterly compared with placebo in patients with difficult-to-treat episodic or chronic migraine, who had documented failure to two to four pharmacological classes of migraine preventive medications Study design: Phase 3 FOCUS trial, randomised, double-blind, placebo-controlled, parallel-group |
|
|
Fremanezumab was effective and well tolerated in patients with difficult-to-treat migraine who had previously not responded to up to four classes of migraine preventive medications |
|
|
|||
| Author, year: Sakai, 202191 Country: Japan and Korea |
Purpose: To determine the efficacy and safety of fremanezumab administration in Japanese and Korean patients with chronic migraine Study design: Multicentre, randomised, double-blind, placebo-controlled, parallel-group Date: November 2017 and November 2019 |
|
|
Fremanezumab effectively prevents CM in Japanese and Korean patients and was well tolerated. No safety signal was detected |
|
|
|||
| Author, year: Silberstein, 201737 Country: 132 sites in 9 countries |
Purpose: To compare two fremanezumab dose regimens with placebo for the prevention of chronic migraine Study design: Randomised, double-blind, placebo-controlled, parallel-group trial Date: March 2016–January 2017 |
|
|
Fremanezumab as a preventive treatment for chronic migraine resulted in a lower frequency of headache than placebo in this 12-week trial. Injection-site reactions to the drug were common |
|
|
|||
| Author, year: Lipton, 202094 Country: 13 countries (USA, Spain, Ukraine, Russian Federation, UK, Republic of Georgia, Hungary, Italy, Slovakia, Germany, Czech Republic, Denmark and Belgium) |
Purpose: To evaluate the efficacy and safety of eptinezumab, a humanised CGRP MAb in the preventive treatment of chronic migraine Study design: Phase 3, double-blind, randomised, placebo-controlled, parallel-group Date: 30 November 2016–20 April 2018 |
|
|
In patients with CM, eptinezumab 100 and 300 mg was associated with a significant reduction in MMDs from the day after IV administration through to week 12, was well tolerated, and demonstrated an acceptable safety profile |
|
|
|||
|
Appendix 3 Further results from the network meta-analysis
Mean change in monthly headache day from baseline
| Residual deviance (20 data points) | pDa | DICb | |
|---|---|---|---|
| Fixed model | 18.7 | 17 | 35.6 |
| Random model | 18.7 | 18.2 | 36.9 |
FIGURE 28.
Treatment probabilities ranking curves for each treatment (mean change in MHD from baseline). Probabilities ranking graph shows the probability of each intervention to being 1st best, 2nd best, etc.

| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 | P. rank 10 | P. rank 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eptinezumab 300 mg | 0.38 | 0.29 | 0.16 | 0.08 | 0.04 | 0.03 | 0.01 | 0 | 0 | 0 | 0 |
| Fremanezumab monthly | 0.14 | 0.16 | 0.17 | 0.15 | 0.13 | 0.11 | 0.08 | 0.04 | 0.02 | 0 | 0 |
| Eptinezumab 30 mg | 0.27 | 0.16 | 0.11 | 0.09 | 0.08 | 0.08 | 0.07 | 0.07 | 0.06 | 0.01 | 0 |
| OnabotulinumtoxinA | 0.04 | 0.08 | 0.12 | 0.15 | 0.17 | 0.16 | 0.13 | 0.1 | 0.04 | 0.01 | 0 |
| Galcanezumab 120 mg | 0.08 | 0.09 | 0.11 | 0.11 | 0.12 | 0.12 | 0.14 | 0.13 | 0.08 | 0.02 | 0 |
| Eptinezumab 100 mg | 0.02 | 0.07 | 0.13 | 0.14 | 0.17 | 0.16 | 0.15 | 0.11 | 0.05 | 0 | 0 |
| Fremanezumab-quarterly | 0.03 | 0.07 | 0.1 | 0.14 | 0.13 | 0.16 | 0.15 | 0.14 | 0.07 | 0.01 | 0 |
| Galcanezumab 240 mg | 0.03 | 0.06 | 0.07 | 0.09 | 0.1 | 0.11 | 0.15 | 0.2 | 0.15 | 0.04 | 0 |
| Topiramate 100 mg | 0.01 | 0.02 | 0.02 | 0.04 | 0.04 | 0.06 | 0.08 | 0.14 | 0.35 | 0.2 | 0.04 |
| Eptinezumab 10 mg | 0 | 0 | 0.01 | 0.01 | 0.01 | 0.02 | 0.03 | 0.07 | 0.17 | 0.41 | 0.26 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0.29 | 0.7 |
FIGURE 29.
Treatment cumulative ranking curves for each treatment (mean change im MHD from baseline). Cumulative rank probabilities graph shows likelihood of therapy to be at the top rank and presented by the SUCRA values (ranges 0–1).
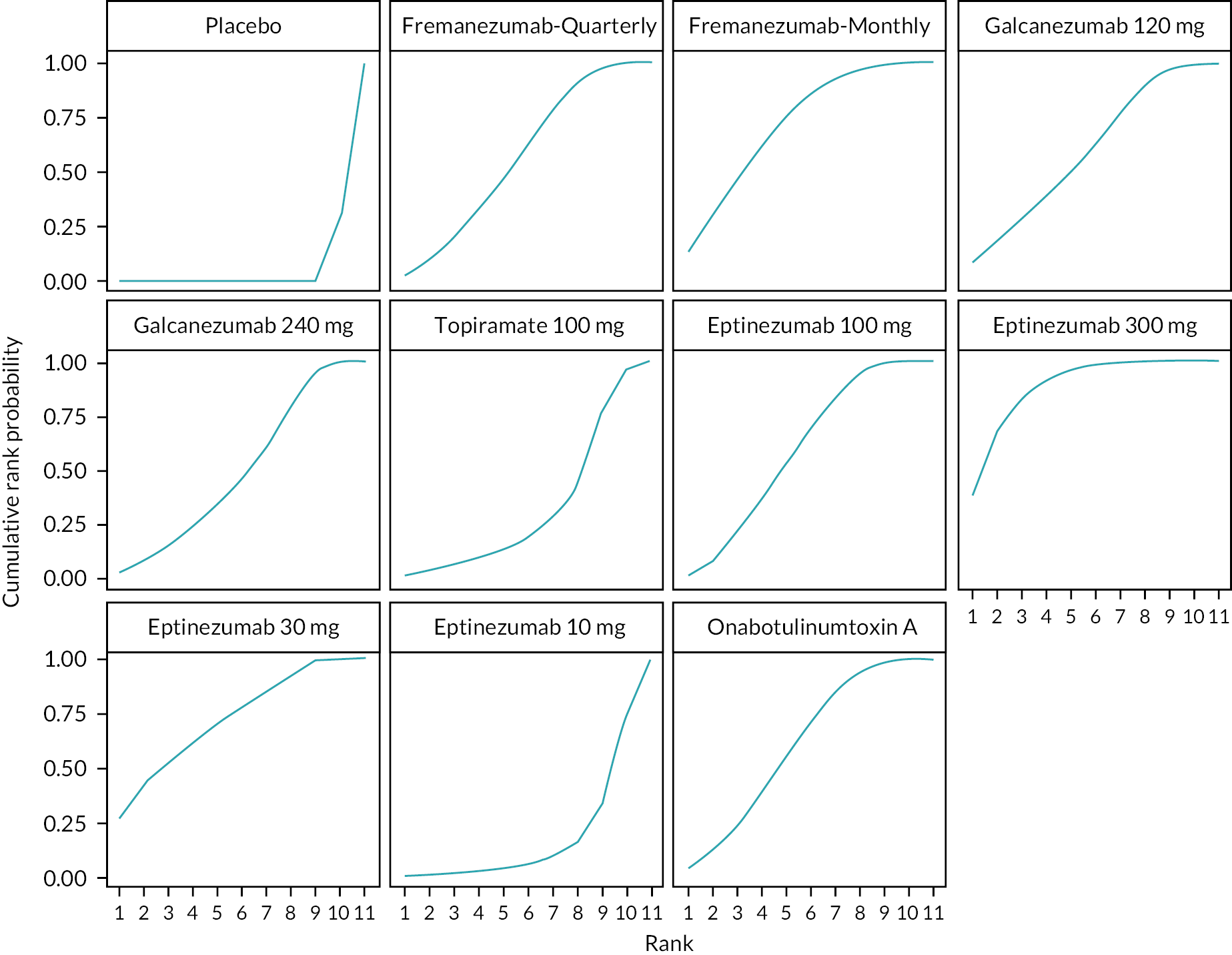
| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 | P. rank 10 | P. rank 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Eptinezumab 300 mg | 0.39 | 0.68 | 0.83 | 0.91 | 0.96 | 0.98 | 1 | 1 | 1 | 1 | 1 |
| Fremanezumab monthly | 0.14 | 0.3 | 0.47 | 0.63 | 0.76 | 0.86 | 0.94 | 0.98 | 1 | 1 | 1 |
| Eptinezumab 30 mg | 0.27 | 0.43 | 0.54 | 0.62 | 0.71 | 0.78 | 0.86 | 0.93 | 0.99 | 1 | 1 |
| OnabotulinumtoxinA | 0.04 | 0.12 | 0.24 | 0.39 | 0.56 | 0.72 | 0.85 | 0.95 | 0.99 | 1 | 1 |
| Galcanezumab 120 mg | 0.08 | 0.17 | 0.28 | 0.39 | 0.51 | 0.63 | 0.77 | 0.9 | 0.98 | 1 | 1 |
| Eptinezumab 100 mg | 0.02 | 0.08 | 0.22 | 0.37 | 0.53 | 0.69 | 0.84 | 0.95 | 1 | 1 | 1 |
| Fremanezumab-quarterly | 0.03 | 0.1 | 0.2 | 0.34 | 0.47 | 0.63 | 0.78 | 0.92 | 0.99 | 1 | 1 |
| Galcanezumab 240 mg | 0.03 | 0.09 | 0.15 | 0.24 | 0.34 | 0.46 | 0.61 | 0.81 | 0.95 | 1 | 1 |
| Topiramate 100 mg | 0.01 | 0.03 | 0.06 | 0.09 | 0.14 | 0.19 | 0.28 | 0.42 | 0.77 | 0.96 | 1 |
| Eptinezumab 10 mg | 0 | 0 | 0.01 | 0.02 | 0.03 | 0.05 | 0.09 | 0.15 | 0.33 | 0.74 | 1 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0.3 | 1 |
| Residual deviance (20 data points) | pDa | DICb | |
|---|---|---|---|
| NMA (Consistency) model | 18.7 | 17 | 35.6 |
| UMEs (Inconsistency) model | 18.9 | 17.2 | 36.1 |
FIGURE 30.
Global consistency test for mean change in MHD from baseline [UMEs (Inconsistency) Model and Fixed NMA model]. Dev-Dev plot to identify inconsistent data points, the data points are scattered in a further distance from the bivector from the origin, and these show global inconsistency.

Mean change in monthly migraine day from baseline
| Residual deviance (29 data points) | pDa | DICb | |
|---|---|---|---|
| Fixed model | 34 | 21.8 | 55.8 |
| Random model | 28.7 | 25.8 | 54.5 |
FIGURE 31.
Treatment probabilities ranking curves for each treatment (mean change in MMD from baseline). Probabilities ranking graph shows the probability of each intervention to being 1st best, 2nd best, etc.

| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 | P. rank 10 | P. rank 11 | P. rank 12 | P. rank 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eptinezumab 300 mg | 0.24 | 0.19 | 0.13 | 0.1 | 0.09 | 0.08 | 0.07 | 0.04 | 0.03 | 0.02 | 0.01 | 0 | 0 |
| Fremanezumab monthly | 0.07 | 0.12 | 0.17 | 0.17 | 0.16 | 0.13 | 0.09 | 0.05 | 0.03 | 0.01 | 0 | 0 | 0 |
| Erenumab 70 mg | 0.17 | 0.16 | 0.11 | 0.11 | 0.08 | 0.09 | 0.07 | 0.07 | 0.06 | 0.04 | 0.03 | 0.01 | 0 |
| Erenumab 140 mg | 0.15 | 0.15 | 0.12 | 0.1 | 0.1 | 0.09 | 0.07 | 0.07 | 0.06 | 0.05 | 0.03 | 0.01 | 0 |
| Fremanezumab-quarterly | 0.04 | 0.08 | 0.12 | 0.14 | 0.14 | 0.13 | 0.12 | 0.1 | 0.07 | 0.04 | 0.02 | 0 | 0 |
| Eptinezumab 30 mg | 0.18 | 0.1 | 0.09 | 0.08 | 0.07 | 0.07 | 0.07 | 0.08 | 0.08 | 0.08 | 0.08 | 0.02 | 0 |
| Eptinezumab 100 mg | 0.03 | 0.05 | 0.07 | 0.08 | 0.1 | 0.11 | 0.12 | 0.13 | 0.12 | 0.11 | 0.06 | 0.02 | 0 |
| Galcanezumab 120 mg | 0.06 | 0.06 | 0.08 | 0.08 | 0.08 | 0.08 | 0.09 | 0.11 | 0.11 | 0.13 | 0.08 | 0.04 | 0 |
| OnabotulinumtoxinA | 0.01 | 0.02 | 0.04 | 0.05 | 0.07 | 0.09 | 0.13 | 0.15 | 0.16 | 0.15 | 0.1 | 0.03 | 0 |
| Galcanezumab 240 mg | 0.03 | 0.04 | 0.04 | 0.05 | 0.06 | 0.07 | 0.09 | 0.1 | 0.14 | 0.15 | 0.14 | 0.09 | 0 |
| Topiramate 100 mg | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.04 | 0.05 | 0.06 | 0.09 | 0.13 | 0.24 | 0.25 | 0.02 |
| Eptinezumab 10 mg | 0 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.03 | 0.04 | 0.05 | 0.09 | 0.21 | 0.45 | 0.06 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.08 | 0.92 |
FIGURE 32.
Treatment cumulative ranking curves for each treatment (mean change in MMD from baseline). Cumulative rank probabilities graph shows likelihood of therapy to be at the top rank and presented by the SUCRA values (ranges 0–1).
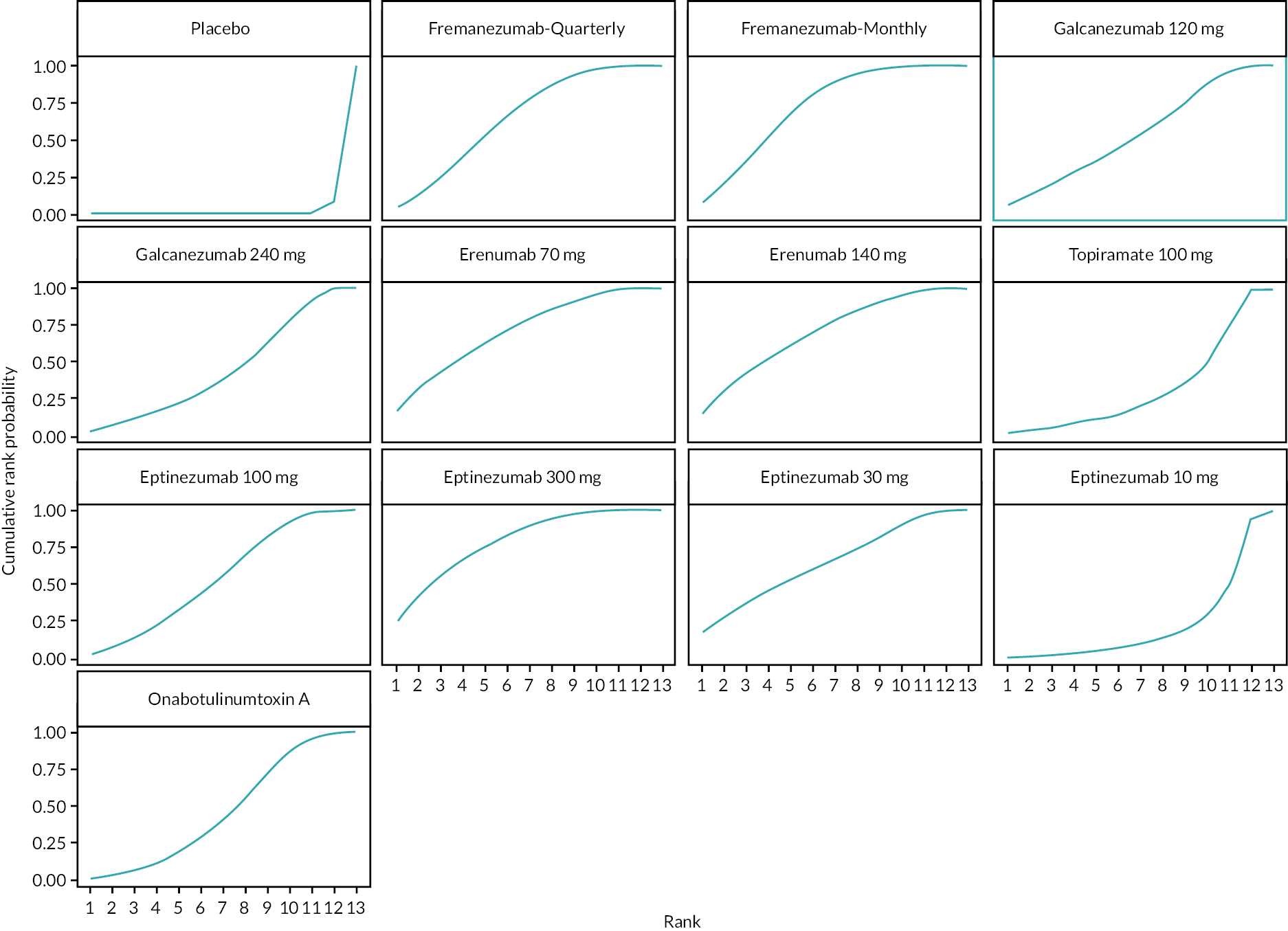
| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 | P. rank 10 | P. rank 11 | P. rank 12 | P. rank 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eptinezumab 300 mg | 0.24 | 0.43 | 0.56 | 0.66 | 0.75 | 0.83 | 0.89 | 0.94 | 0.97 | 0.99 | 1 | 1 | 1 |
| Fremanezumab monthly | 0.07 | 0.19 | 0.35 | 0.52 | 0.68 | 0.8 | 0.89 | 0.95 | 0.98 | 1 | 1 | 1 | 1 |
| Erenumab 70 mg | 0.17 | 0.33 | 0.44 | 0.55 | 0.63 | 0.72 | 0.79 | 0.86 | 0.91 | 0.96 | 0.99 | 1 | 1 |
| Erenumab 140 mg | 0.15 | 0.3 | 0.43 | 0.53 | 0.62 | 0.71 | 0.78 | 0.85 | 0.91 | 0.96 | 0.99 | 1 | 1 |
| Fremanezumab-quarterly | 0.04 | 0.12 | 0.24 | 0.39 | 0.52 | 0.65 | 0.77 | 0.87 | 0.94 | 0.98 | 1 | 1 | 1 |
| Eptinezumab 30 mg | 0.18 | 0.28 | 0.37 | 0.45 | 0.52 | 0.59 | 0.66 | 0.74 | 0.81 | 0.89 | 0.98 | 1 | 1 |
| Eptinezumab 100 mg | 0.02 | 0.07 | 0.14 | 0.23 | 0.33 | 0.44 | 0.57 | 0.7 | 0.82 | 0.93 | 0.99 | 1 | 1 |
| Galcanezumab 120 mg | 0.06 | 0.12 | 0.2 | 0.28 | 0.36 | 0.44 | 0.54 | 0.64 | 0.75 | 0.88 | 0.96 | 1 | 1 |
| OnabotulinumtoxinA | 0.01 | 0.03 | 0.07 | 0.12 | 0.19 | 0.29 | 0.42 | 0.56 | 0.72 | 0.87 | 0.97 | 1 | 1 |
| Galcanezumab 240 mg | 0.03 | 0.07 | 0.11 | 0.16 | 0.23 | 0.3 | 0.39 | 0.49 | 0.63 | 0.78 | 0.92 | 1 | 1 |
| Topiramate 100 mg | 0.02 | 0.04 | 0.06 | 0.09 | 0.12 | 0.15 | 0.21 | 0.27 | 0.36 | 0.49 | 0.73 | 0.99 | 1 |
| Eptinezumab 10 mg | 0 | 0.01 | 0.02 | 0.03 | 0.05 | 0.07 | 0.1 | 0.14 | 0.19 | 0.29 | 0.49 | 0.94 | 1 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.08 | 1 |
| Residual deviance (20 data points) | pDa | DICb | |
|---|---|---|---|
| NMA (Consistency) Model | 41.1 | 22 | 63.1 |
| UMEs (Inconsistency) Model | 41.1 | 22 | 63 |
FIGURE 33.
Global consistency test for mean change in MMD from baseline [UMEs (Inconsistency) Model and Fixed NMA model]. Dev-Dev plot to identify inconsistent data points, the data points are scattered in a further distance from the bivector from the origin, and these show global inconsistency.
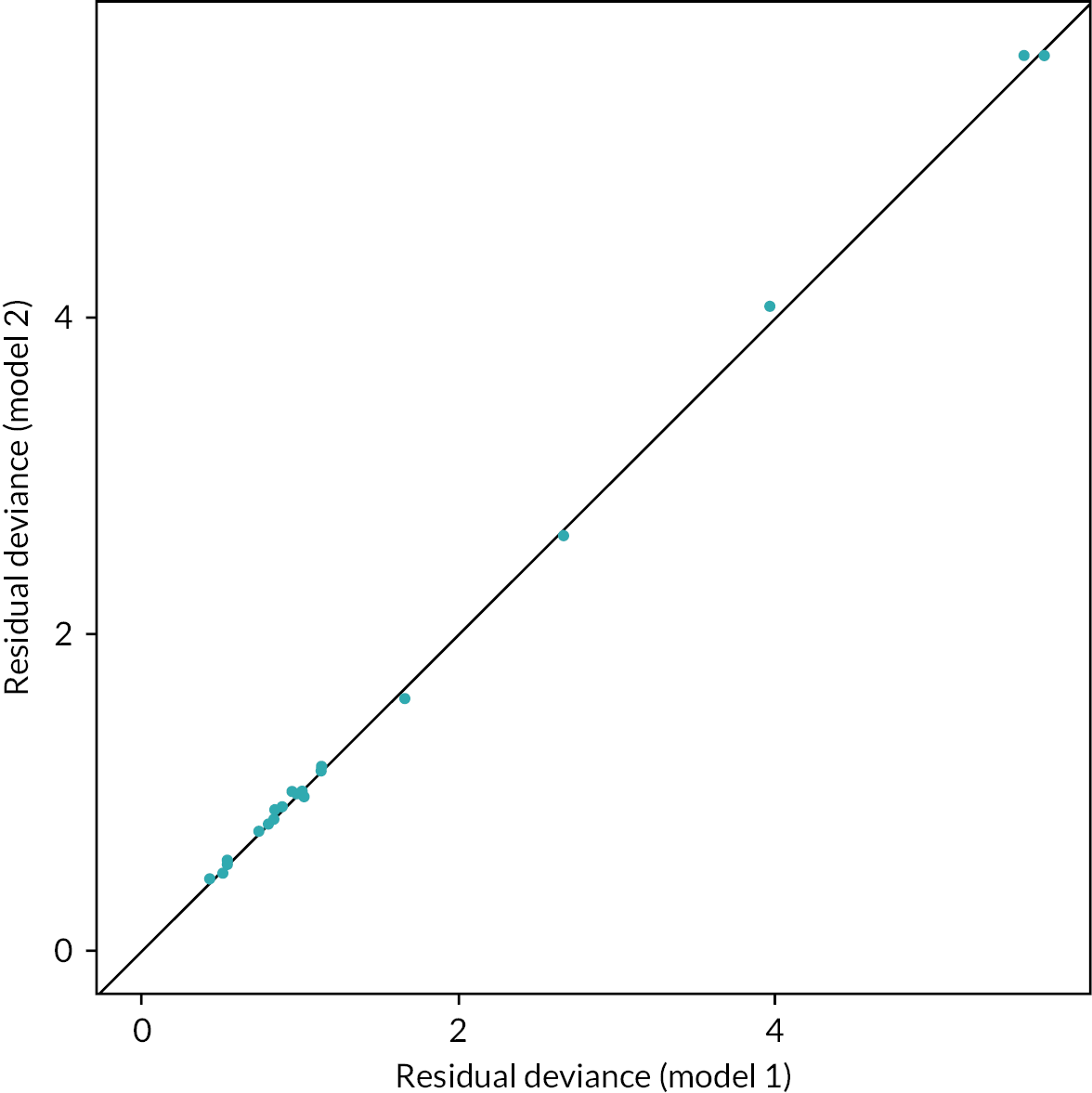
Mean change in migraine-specific quality of life – restrictive role from baseline
| Residual deviance (13 data points) | pDa | DICb | |
|---|---|---|---|
| Fixed model | 12.9 | 12.9 | 25.8 |
| Random model | 13.1 | 13.1 | 26.3 |
FIGURE 34.
Treatment probabilities ranking curves for each treatment (mean change in MSQ-RR from baseline). Probabilities ranking graph shows the probability of each intervention to being 1st best, 2nd best, etc.
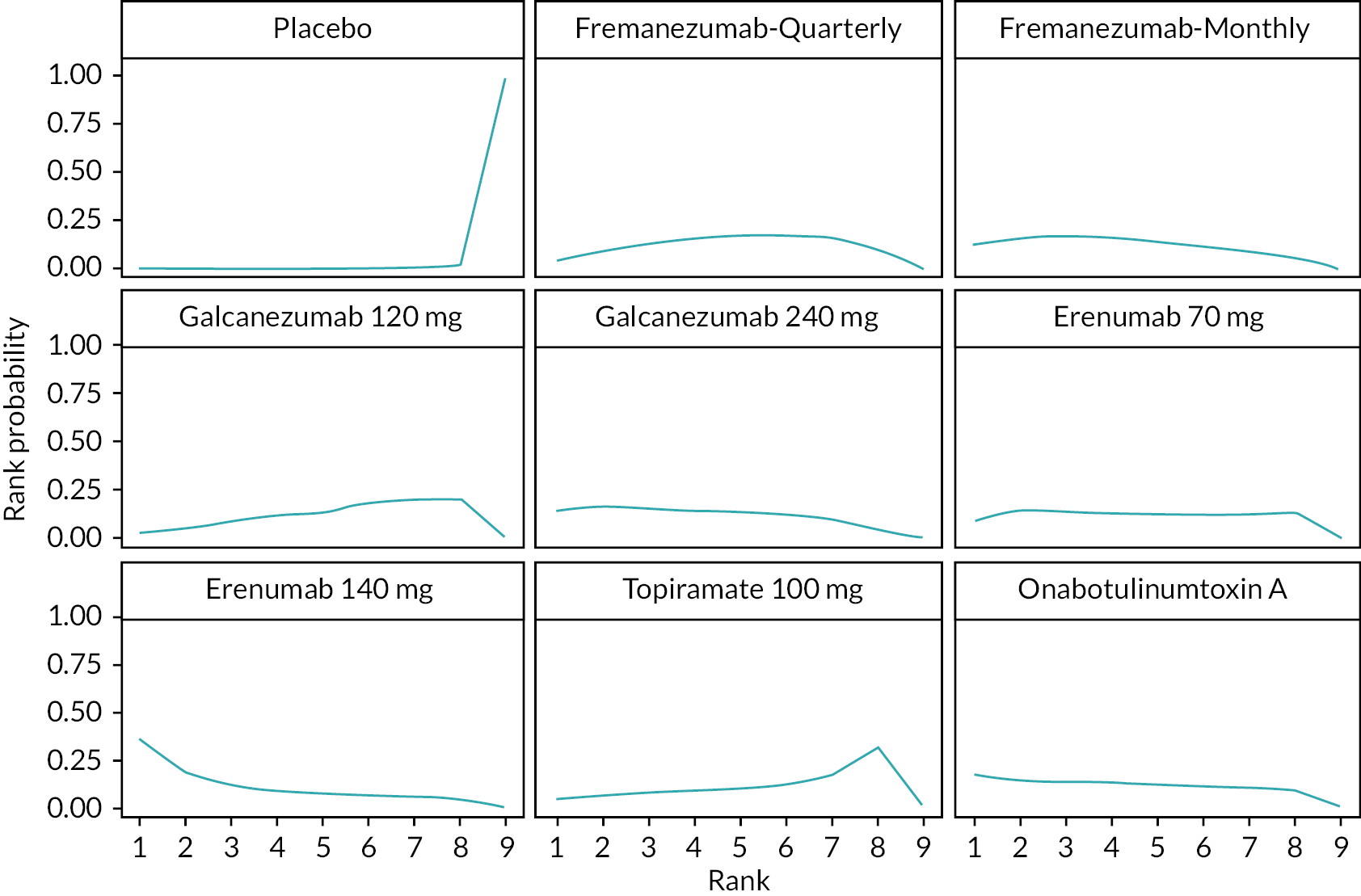
| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 |
|---|---|---|---|---|---|---|---|---|---|
| Erenumab 140 mg | 0.36 | 0.18 | 0.12 | 0.09 | 0.08 | 0.07 | 0.06 | 0.04 | 0 |
| Galcanezumab 240 mg | 0.15 | 0.16 | 0.15 | 0.14 | 0.14 | 0.12 | 0.1 | 0.04 | 0 |
| Fremanezumab monthly | 0.12 | 0.16 | 0.17 | 0.15 | 0.14 | 0.11 | 0.09 | 0.06 | 0 |
| OnabotulinumtoxinA | 0.17 | 0.15 | 0.13 | 0.12 | 0.12 | 0.11 | 0.1 | 0.1 | 0 |
| Erenumab 70 mg | 0.09 | 0.14 | 0.14 | 0.13 | 0.13 | 0.12 | 0.12 | 0.13 | 0 |
| Fremanezumab-quarterly | 0.04 | 0.09 | 0.13 | 0.16 | 0.16 | 0.16 | 0.16 | 0.1 | 0 |
| Galcanezumab 120 mg | 0.03 | 0.05 | 0.08 | 0.12 | 0.13 | 0.18 | 0.2 | 0.21 | 0 |
| Topiramate 100 mg | 0.04 | 0.07 | 0.08 | 0.09 | 0.1 | 0.13 | 0.17 | 0.31 | 0.01 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0.99 |
FIGURE 35.
Treatment cumulative ranking curves for each treatment (mean change in MSQ-RR from baseline). Cumulative rank probabilities graph shows likelihood of therapy to be at the top rank and presented by the SUCRA values (ranges 0–1).

| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 |
|---|---|---|---|---|---|---|---|---|---|
| Erenumab 140 mg | 0.36 | 0.54 | 0.66 | 0.75 | 0.83 | 0.9 | 0.96 | 1 | 1 |
| Galcanezumab 240 mg | 0.15 | 0.31 | 0.46 | 0.6 | 0.74 | 0.86 | 0.96 | 1 | 1 |
| Fremanezumab monthly | 0.12 | 0.28 | 0.45 | 0.61 | 0.75 | 0.86 | 0.95 | 1 | 1 |
| OnabotulinumtoxinA | 0.17 | 0.32 | 0.45 | 0.57 | 0.7 | 0.8 | 0.91 | 1 | 1 |
| Erenumab 70 mg | 0.09 | 0.24 | 0.38 | 0.5 | 0.63 | 0.75 | 0.87 | 1 | 1 |
| Fremanezumab-quarterly | 0.04 | 0.13 | 0.26 | 0.41 | 0.57 | 0.74 | 0.9 | 1 | 1 |
| Galcanezumab 120 mg | 0.03 | 0.08 | 0.16 | 0.28 | 0.41 | 0.59 | 0.79 | 1 | 1 |
| Topiramate 100 mg | 0.04 | 0.11 | 0.18 | 0.28 | 0.38 | 0.5 | 0.68 | 0.99 | 1 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 1 |
| Residual deviance (13 data points) | pDa | DICb | |
|---|---|---|---|
| NMA (Consistency) model | 12.9 | 12.9 | 25.8 |
| UMEs (Inconsistency) model | 13 | 13 | 26 |
FIGURE 36.
Global consistency test for mean change in MSQ-RR from baseline [UMEs (Inconsistency) model and fixed NMA model]. Dev-Dev plot to identify inconsistent data points, the data points are scattered in a further distance from the bivector from the origin, and these show global inconsistency.
Mean change in migraine-specific quality of life – preventative role from baseline
| Residual deviance (13 data points) | pDa | DICb | |
|---|---|---|---|
| Fixed model | 13.1 | 13.1 | 26.2 |
| Random model | 12.8 | 12.8 | 25.7 |
FIGURE 37.
Treatment probabilities ranking curves for each treatment (mean change in MSQ-PR from baseline). Probabilities ranking graph shows the probability of each intervention to being 1st best, 2nd best, etc.
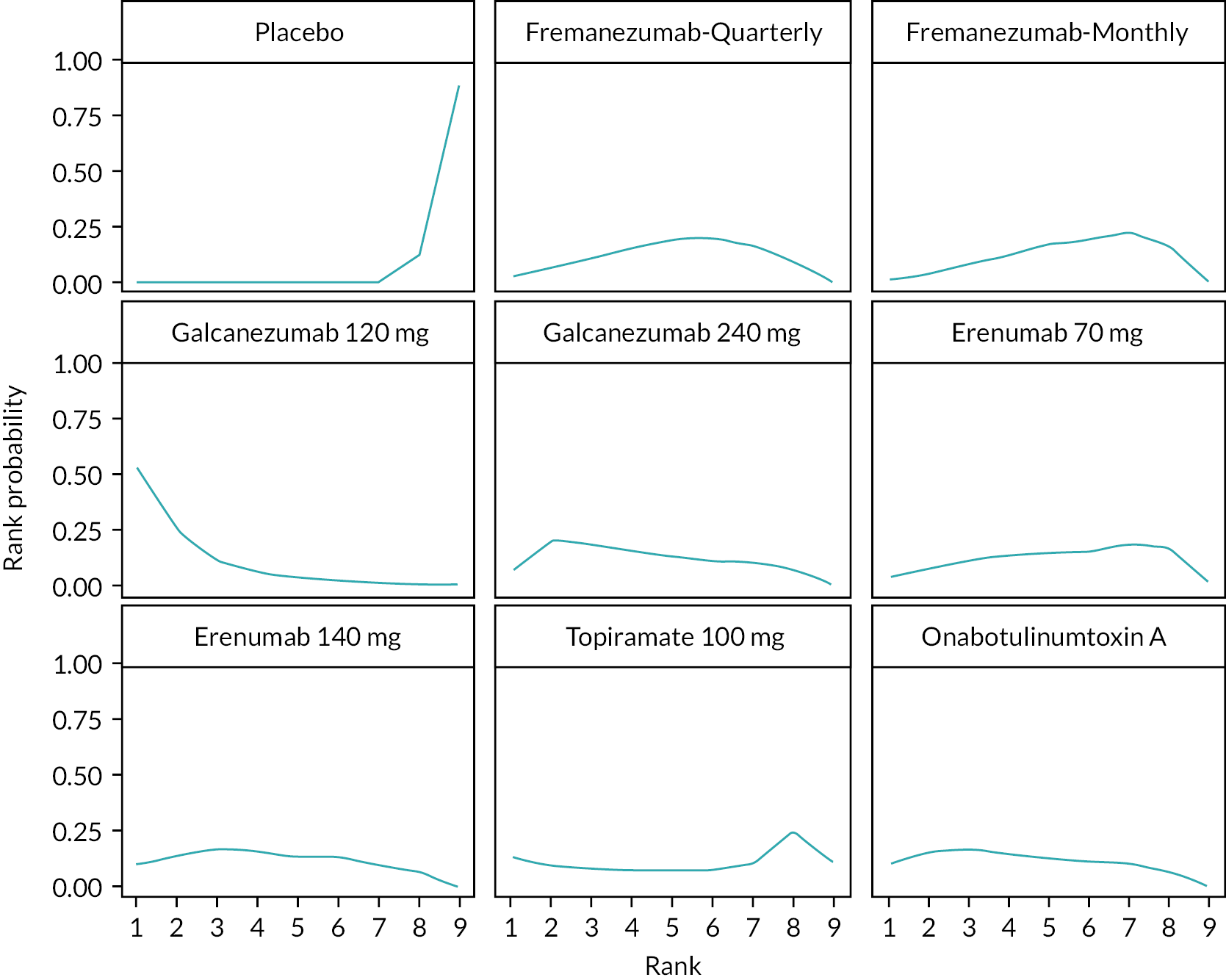
| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 |
|---|---|---|---|---|---|---|---|---|---|
| Galcanezumab 120 mg | 0.53 | 0.24 | 0.11 | 0.06 | 0.03 | 0.02 | 0.01 | 0 | 0 |
| OnabotulinumtoxinA | 0.11 | 0.15 | 0.17 | 0.15 | 0.13 | 0.12 | 0.1 | 0.07 | 0 |
| Galcanezumab 240 mg | 0.06 | 0.2 | 0.18 | 0.15 | 0.13 | 0.11 | 0.1 | 0.07 | 0 |
| Erenumab 140 mg | 0.1 | 0.14 | 0.17 | 0.16 | 0.13 | 0.13 | 0.1 | 0.07 | 0 |
| Fremanezumab-quarterly | 0.03 | 0.07 | 0.11 | 0.15 | 0.19 | 0.2 | 0.16 | 0.09 | 0 |
| Erenumab 70 mg | 0.03 | 0.07 | 0.11 | 0.14 | 0.14 | 0.15 | 0.19 | 0.16 | 0.01 |
| Topiramate 100 mg | 0.13 | 0.09 | 0.08 | 0.07 | 0.08 | 0.08 | 0.11 | 0.25 | 0.11 |
| Fremanezumab monthly | 0.01 | 0.04 | 0.07 | 0.12 | 0.17 | 0.19 | 0.23 | 0.17 | 0 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.12 | 0.88 |
FIGURE 38.
Treatment cumulative ranking curves for each treatment (mean change in MSQ-PR from baseline). Cumulative rank probabilities graph shows likelihood of therapy to be at the top rank and presented by the SUCRA values (ranges 0–1).
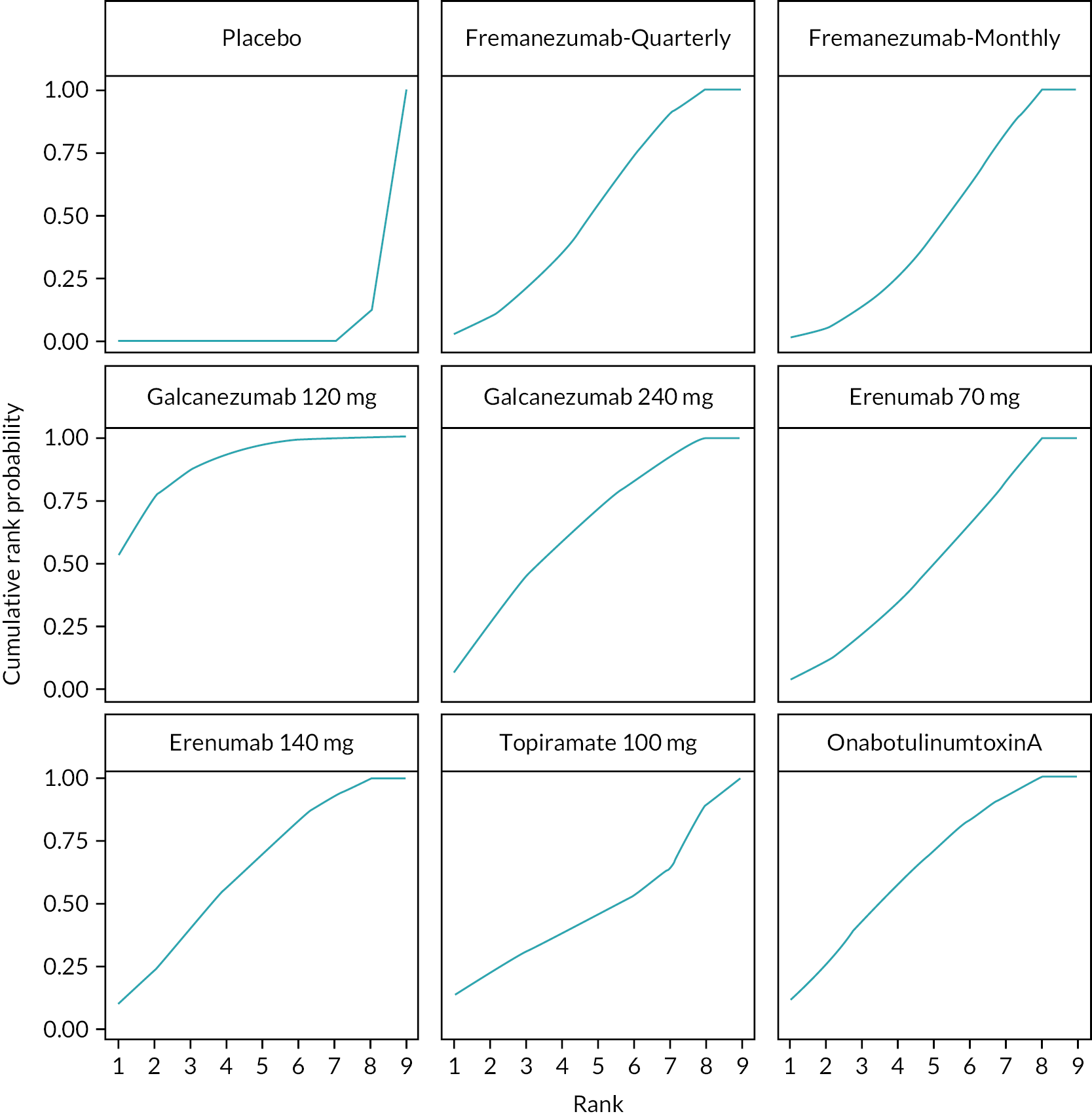
| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 |
|---|---|---|---|---|---|---|---|---|---|
| Galcanezumab 120 mg | 0.53 | 0.77 | 0.87 | 0.93 | 0.96 | 0.98 | 1 | 1 | 1 |
| OnabotulinumtoxinA | 0.11 | 0.26 | 0.43 | 0.58 | 0.71 | 0.83 | 0.93 | 1 | 1 |
| Galcanezumab 240 mg | 0.06 | 0.26 | 0.44 | 0.59 | 0.72 | 0.83 | 0.93 | 1 | 1 |
| Erenumab 140 mg | 0.1 | 0.24 | 0.41 | 0.57 | 0.7 | 0.83 | 0.93 | 1 | 1 |
| Fremanezumab-quarterly | 0.03 | 0.09 | 0.2 | 0.36 | 0.55 | 0.74 | 0.91 | 1 | 1 |
| Erenumab 70 mg | 0.03 | 0.1 | 0.21 | 0.34 | 0.49 | 0.64 | 0.83 | 0.93 | 1 |
| Topiramate 100 mg | 0.13 | 0.22 | 0.31 | 0.38 | 0.45 | 0.53 | 0.64 | 0.89 | 1 |
| Fremanezumab monthly | 0.01 | 0.05 | 0.13 | 0.25 | 0.42 | 0.61 | 0.84 | 1 | 1 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.12 | 1 |
| Residual deviance (13 data points) | pDa | DICb | |
|---|---|---|---|
| NMA (Consistency) model | 13.1 | 13.1 | 26.2 |
| UMEs (Inconsistency) model | 12.9 | 12.9 | 25.8 |
FIGURE 39.
Global consistency test for mean change in MSQ-PR from baseline [UMEs (Inconsistency) Model and fixed NMA model]. Dev-Dev plot to identify inconsistent data points, the data points are scattered in a further distance from the bivector from the origin, and these show global inconsistency.

Mean change in migraine-specific quality of life – emotional function from baseline
| Residual deviance (13 data points) | pDa | DICb | |
|---|---|---|---|
| Fixed model | 13 | 13 | 26 |
| Random model | 12.8 | 12.8 | 25.6 |
FIGURE 40.
Treatment probabilities ranking curves for each treatment (mean change in MSQ-EF from baseline). Probabilities ranking graph shows the probability of each intervention to being 1st best, 2nd best, etc.
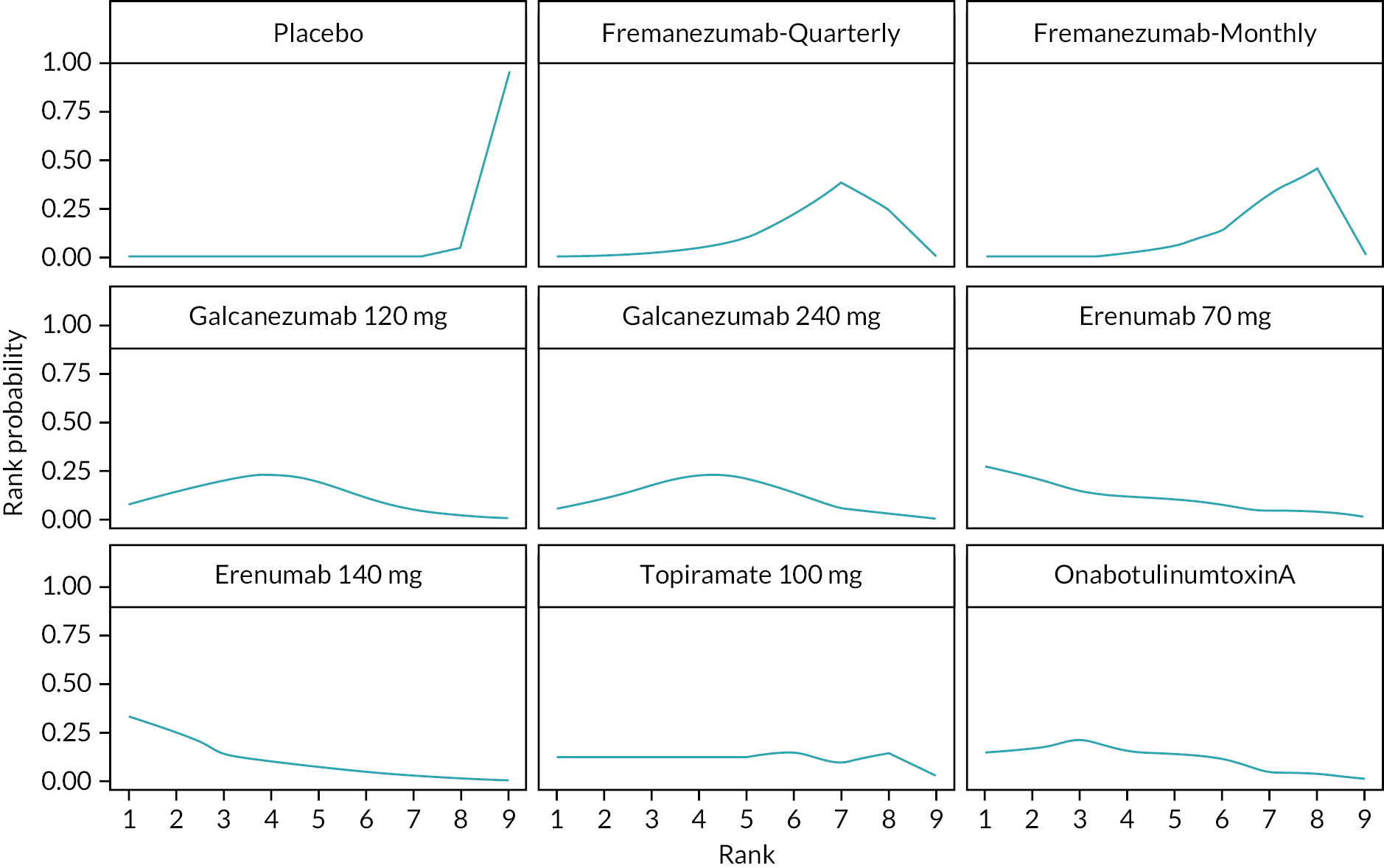
| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 |
|---|---|---|---|---|---|---|---|---|---|
| Erenumab 140 mg | 0.34 | 0.25 | 0.14 | 0.1 | 0.07 | 0.05 | 0.03 | 0.02 | 0 |
| Erenumab 70 mg | 0.27 | 0.22 | 0.14 | 0.11 | 0.1 | 0.07 | 0.04 | 0.04 | 0.01 |
| OnabotulinumtoxinA | 0.14 | 0.17 | 0.21 | 0.16 | 0.14 | 0.11 | 0.04 | 0.03 | 0 |
| Galcanezumab 120 mg | 0.07 | 0.13 | 0.2 | 0.23 | 0.2 | 0.11 | 0.04 | 0.02 | 0 |
| Galcanezumab 240 mg | 0.06 | 0.1 | 0.17 | 0.23 | 0.21 | 0.15 | 0.05 | 0.03 | 0 |
| Topiramate 100 mg | 0.12 | 0.12 | 0.12 | 0.12 | 0.13 | 0.15 | 0.09 | 0.13 | 0.02 |
| Fremanezumab-quarterly | 0 | 0.01 | 0.02 | 0.04 | 0.1 | 0.22 | 0.38 | 0.23 | 0 |
| Fremanezumab monthly | 0 | 0 | 0 | 0.01 | 0.05 | 0.14 | 0.33 | 0.46 | 0.01 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.04 | 0.96 |
FIGURE 41.
Treatment cumulative ranking curves for each treatment (mean change in MSQ-EF from baseline). Cumulative rank probabilities graph shows likelihood of therapy to be at the top rank and presented by the SUCRA values (ranges 0–1).

| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 |
|---|---|---|---|---|---|---|---|---|---|
| Erenumab 140 mg | 0.34 | 0.59 | 0.73 | 0.83 | 0.91 | 0.96 | 0.98 | 1 | 1 |
| Erenumab 70 mg | 0.27 | 0.49 | 0.63 | 0.74 | 0.84 | 0.92 | 0.95 | 0.99 | 1 |
| OnabotulinumtoxinA | 0.14 | 0.31 | 0.52 | 0.68 | 0.81 | 0.92 | 0.97 | 1 | 1 |
| Galcanezumab 120 mg | 0.07 | 0.21 | 0.4 | 0.63 | 0.83 | 0.94 | 0.98 | 1 | 1 |
| Galcanezumab 240 mg | 0.05 | 0.16 | 0.33 | 0.56 | 0.77 | 0.92 | 0.97 | 1 | 1 |
| Topiramate 100 mg | 0.12 | 0.24 | 0.36 | 0.48 | 0.6 | 0.75 | 0.84 | 0.98 | 1 |
| Fremanezumab-quarterly | 0 | 0.01 | 0.02 | 0.06 | 0.16 | 0.38 | 0.76 | 1 | 1 |
| Fremanezumab monthly | 0 | 0 | 0.01 | 0.02 | 0.07 | 0.21 | 0.54 | 0.99 | 1 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.04 | 1 |
| Residual deviance (13 data points) | pDa | DICb | |
|---|---|---|---|
| NMA (Consistency) Model | 13 | 13 | 26 |
| UMEs (Inconsistency) Model | 13 | 13 | 26 |
FIGURE 42.
Global consistency test for mean change in MSQ-EF from baseline [UMEs (Inconsistency) Model and Fixed NMA model]. Dev-Dev plot to identify inconsistent data points, the data points are scattered in a further distance from the bivector from the origin, and these show global inconsistency.
Mean change in headache impact test-6 from baseline
| Residual deviance (19 data points) | pDa | DICb | |
|---|---|---|---|
| Fixed model | 18.3 | 15.1 | 33.4 |
| Random model | 18.1 | 16.6 | 34.7 |
FIGURE 43.
Treatment probabilities ranking curves for each treatment (mean change in HIT-6 from baseline). Probabilities ranking graph shows the probability of each interventions to being 1st best, 2nd best, etc.
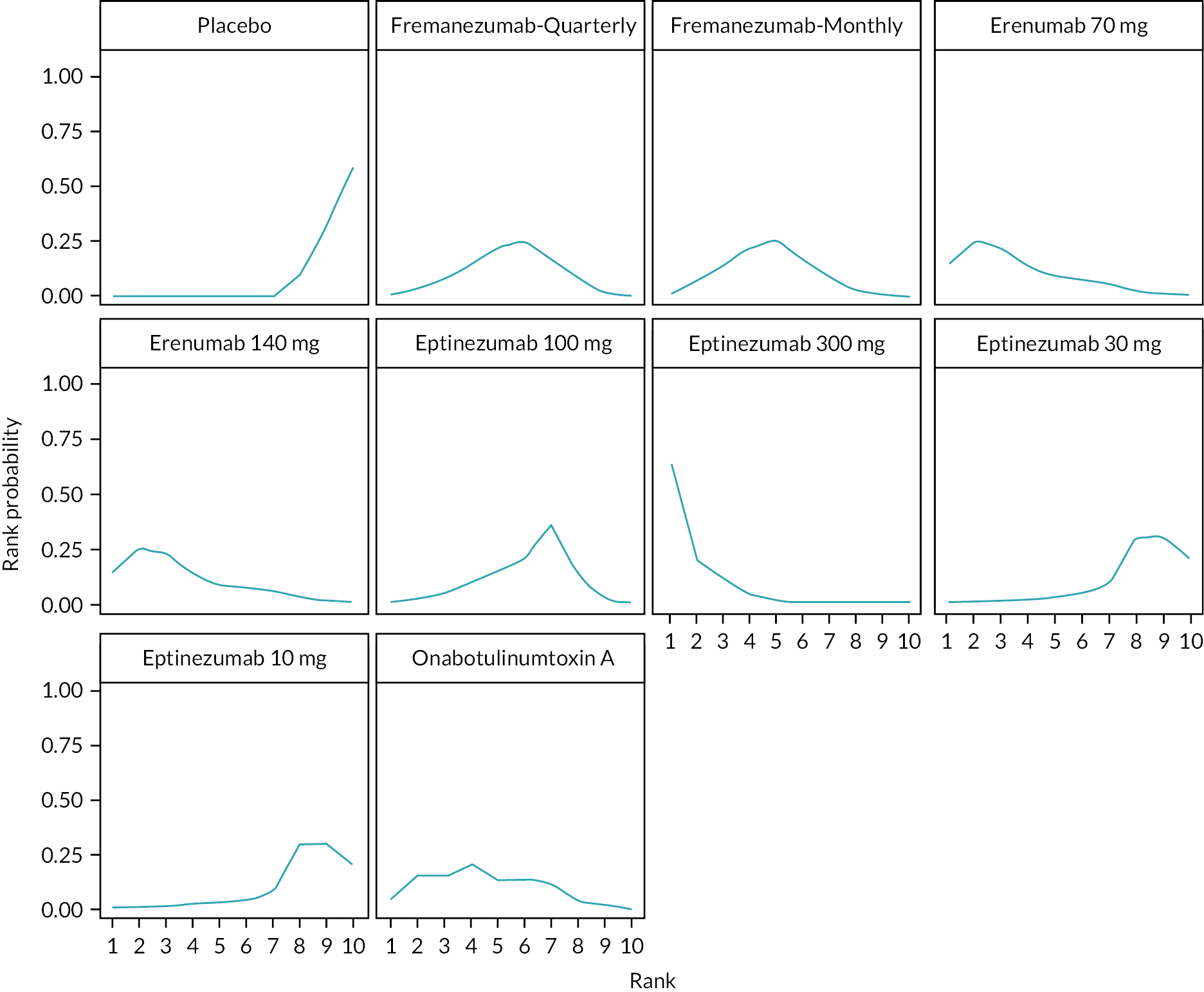
| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 | P. rank 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Eptinezumab 300 mg | 0.63 | 0.2 | 0.11 | 0.04 | 0.01 | 0 | 0 | 0 | 0 | 0 |
| Erenumab 140 mg | 0.15 | 0.25 | 0.23 | 0.13 | 0.09 | 0.07 | 0.05 | 0.02 | 0.01 | 0 |
| Erenumab 70 mg | 0.15 | 0.26 | 0.22 | 0.13 | 0.09 | 0.07 | 0.05 | 0.03 | 0.01 | 0 |
| BTA | 0.05 | 0.15 | 0.15 | 0.2 | 0.13 | 0.14 | 0.11 | 0.04 | 0.02 | 0 |
| Fremanezumab monthly | 0.01 | 0.07 | 0.14 | 0.23 | 0.26 | 0.17 | 0.09 | 0.02 | 0.01 | 0 |
| Fremanezumab-quarterly | 0.01 | 0.04 | 0.08 | 0.15 | 0.22 | 0.25 | 0.17 | 0.07 | 0.02 | 0 |
| Eptinezumab 100 mg | 0 | 0.02 | 0.04 | 0.1 | 0.15 | 0.21 | 0.36 | 0.12 | 0.02 | 0 |
| Eptinezumab 10 mg | 0 | 0.01 | 0.01 | 0.03 | 0.03 | 0.04 | 0.08 | 0.3 | 0.29 | 0.21 |
| Eptinezumab 30 mg | 0 | 0.01 | 0.01 | 0.02 | 0.03 | 0.04 | 0.09 | 0.29 | 0.31 | 0.2 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.31 | 0.59 |
FIGURE 44.
Treatment cumulative ranking curves for each treatment (mean change in HIT-6 from baseline). Cumulative rank probabilities graph shows likelihood of therapy to be at the top rank and presented by the SUCRA values (ranges 0–1).
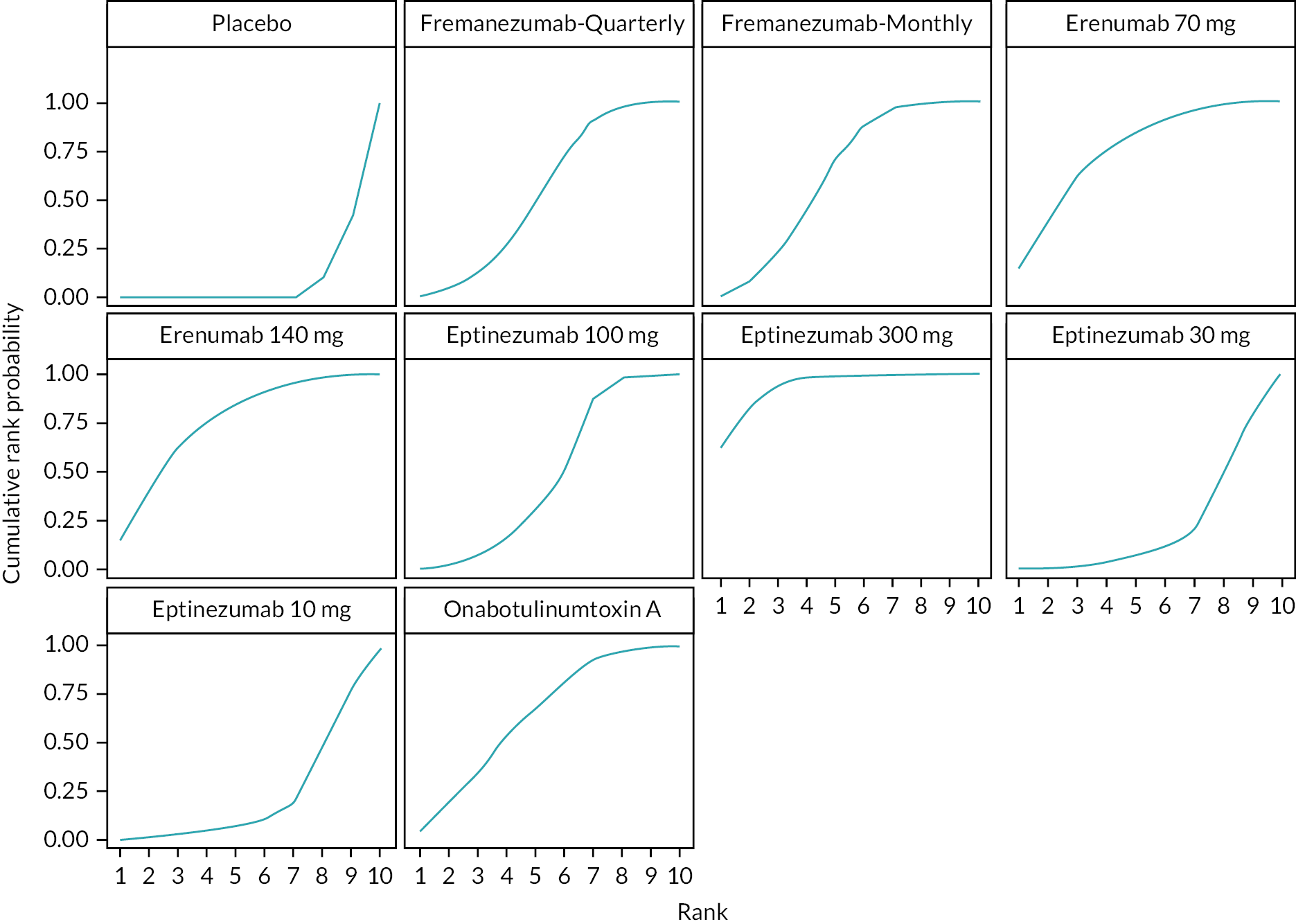
| Interventions | P. rank 1 | P. rank 2 | P. rank 3 | P. rank 4 | P. rank 5 | P. rank 6 | P. rank 7 | P. rank 8 | P. rank 9 | P. rank 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Eptinezumab 300 mg | 0.63 | 0.83 | 0.94 | 0.98 | 0.99 | 1 | 1 | 1 | 1 | 1 |
| Erenumab 140 mg | 0.16 | 0.44 | 0.69 | 0.81 | 0.89 | 0.95 | 0.99 | 1 | 1 | 1 |
| Erenumab 70 mg | 0.16 | 0.46 | 0.68 | 0.81 | 0.88 | 0.95 | 0.99 | 0.99 | 1 | 1 |
| BTA | 0.01 | 0.1 | 0.26 | 0.56 | 0.77 | 0.93 | 0.98 | 1 | 1 | 1 |
| Fremanezumab monthly | 0.02 | 0.09 | 0.21 | 0.41 | 0.65 | 0.85 | 0.96 | 0.99 | 1 | 1 |
| Fremanezumab-quarterly | 0.01 | 0.04 | 0.11 | 0.23 | 0.42 | 0.67 | 0.89 | 0.98 | 1 | 1 |
| Eptinezumab 100 mg | 0 | 0.01 | 0.04 | 0.12 | 0.28 | 0.48 | 0.88 | 0.98 | 1 | 1 |
| Eptinezumab 10 mg | 0 | 0.01 | 0.02 | 0.03 | 0.06 | 0.09 | 0.16 | 0.48 | 0.8 | 1 |
| Eptinezumab 30 mg | 0 | 0.01 | 0.01 | 0.03 | 0.05 | 0.09 | 0.15 | 0.48 | 0.8 | 1 |
| Placebo | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0.4 | 1 |
| Residual deviance (19 data points) | pDa | DICb | |
|---|---|---|---|
| NMA (Consistency) Model | 18.3 | 15.1 | 33.4 |
| UMEs (Inconsistency) Model | 18 | 16.5 | 34.5 |
FIGURE 45.
Global Consistency Test for mean change in HIT-6 from baseline [UMEs (Inconsistency) Model and Fixed NMA model]. Dev-Dev plot to identify inconsistent data points, the data points are scattered in a further distance from the bivector from the origin, and these show global inconsistency.

Sensitivity analysis results
Mean change in monthly headache day from baseline
The forest plot shows the MDs and 95% CrI compared with placebo as reference treatment. MDs were lower than zero indicating favourable results for the intervention (Figure 46).
FIGURE 46.
Forest plot for mean change in MHD from baseline (MDs, 95% CrI).

| Eptinezumab 300 mg | ||||||||
| −0.36 (−1.47 to 0.78) | Fremanezumab-M | |||||||
| −0.62 (−1.38 to 0.16) | 0.27 (−0.89 to 1.37) | Eptinezumab 100 mg | ||||||
| 0.61 (−0.48 to 1.65) | 0.25 (−0.82 to 1.35) | −0.01 (−1.06 to 1.02) | OnabotulinumtoxinA | |||||
| −0.66 (−1.97 to 0.67) | 0.30 (−1.02 to 1.72) | −0.04 (−1.31 to 1.28) | −0.05 (−1.41 to 1.26) | Galcanezumab 120 mg | ||||
| −0.65 (−1.77 to 0.49) | −0.30 (−1.11 to 0.50) | −0.03 (−1.15 to 1.10) | −0.04 (−1.11 to 1.06) | 0.01 (−1.33 to 1.42) | Fremanezumab-Q | |||
| −0.86 (−2.16 to 0.46) | 0.51 (−0.88 to 1.85) | −0.24 (−1.52 to 1.06) | −0.25 (−1.56 to 1.06) | 0.21 (−0.87 to 1.32) | 0.21 (−1.18 to 1.58) | Galcanezumab 240 mg | ||
| −1.36 (−2.84 to 0.06) | 1.01 (−0.53 to 2.52) | −0.74 (−2.20 to 0.67) | −0.75 (−2.18 to 0.68) | 0.71 (−0.93 to 2.37) | 0.71 (−0.76 to 2.22) | 0.50 (−1.12 to 2.18) | Topiramate 100 mg | |
| −2.46 (−3.23 to −1.69) | −2.11 (−2.94 to −1.29) | −1.84 (−2.59 to −1.08) | −1.85 (−2.59 to −1.13) | −1.81 (−2.87 to −0.66) | −1.81 (−2.63 to −0.98) | −1.60 (−2.69 to −0.50) | −1.10 (−2.33 to 0.17) | Placebo |
The SUCRA values range from 0 to 1; presents the likelihood of drug being at the top rank (Figure 47).
FIGURE 47.
The surface under the cumulative ranking curve (SUCRA) for mean change in MHD from baseline.
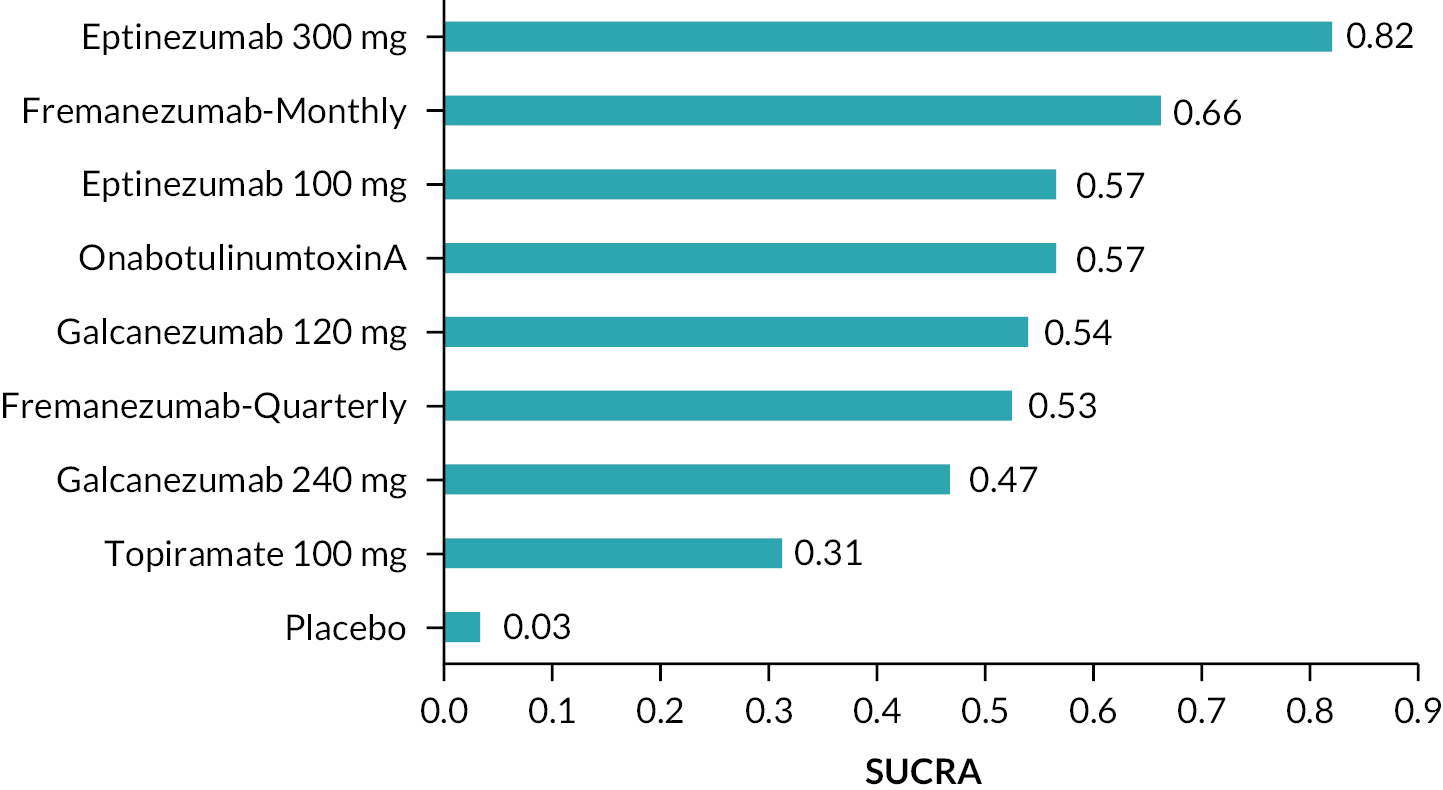
Mean change in monthly migraine day from baseline
The forest plot shows the MDs and 95% CrI compared with placebo as reference treatment. MDs lower than 0 indicate favoured results for the intervention (Figure 48).
FIGURE 48.
Forest plot for mean change in MMD from baseline (MDs, 95% CrI).

| Fremanezumab-M | ||||||||||
| 0.17 (−0.98 to 1.33) | Eptinezumab 300 mg | |||||||||
| 0.29 (−0.96 to 1.54) | −0.12 (−1.72 to 1.42) | Erenumab 70 mg | ||||||||
| 0.31 (−0.96 to 1.61) | −0.14 (−1.71 to 1.36) | 0.v02 (−1.11 to 1.17) | Erenumab 140 mg | |||||||
| −0.46 (−1.06 to 0.17) | −0.29 (−1.51 to 0.09) | −0.17 (−1.47 to 1.10) | −0.15 (−1.48 to 1.16) | Fremanezumab-Q | ||||||
| 0.67 (−0.0.60 to 1.94) | −0.01 (−2.03 to 0.98) | −0.39 (−1.93 to 1.23) | −0.36 (−1.93 to 1.25) | 0.22 (−1.05 to 1.46) | Galcanezumab120 mg | |||||
| 0.66 (−0.44 to 1.80) | −0.49 (−1.49 to 0.48) | 0.38 (−1.15 to 1.80) | 0.35 (−1.16 to 1.88) | 0.20 (−0.91 to 1.33) | −0.01 (−1.47 to 1.45) | Eptinezumab100 mg | ||||
| 0.81 (−0.14 to 1.74) | 0.64 (−0.59 to 1.87) | 0.52 (−0.81 to 1.87) | 0.50 (−0.86 to 1.83) | 0.35 (−0.64 to 1.28) | 0.14 (−1.18 to 1.49) | 0.15 (−1.02 to 1.31) | OnabotulinumtoxinA | |||
| 0.88 (−0.35 to 2.17) | −0.71 (−2.24 to 0.77) | −0.59 (−2.20 to 0.96) | −0.57 (−2.14 to 1.03) | 0.42 (−0.84 to 1.73) | 0.21 (−0.91 to 1.31) | −0.22 (−1.69 to 1.20) | −0.07 (−1.39 to 1.23) | Galcanezumab 240 mg | ||
| 1.27 (−0.21 to 2.76) | −1.10 (−2.83 to 0.57) | 0.99 (−0.76 to 2.73) | 0.96 (−0.82 to 2.74) | 0.81 (−0.69 to 2.34) | 0.60 (−1.12 to 2.36) | −0.61 (−2.25 to 1.07) | −0.46 (−1.98 to 1.02) | 0.39 (−1.34 to 2.19) | Topiramate 100 mg | |
| −2.77 (−3.38 to −2.16) | −2.60 (−3.63 to −1.63) | −2.48 (−3.59 to −1.35) | −2.46 (−3.60 to −1.33) | −2.31 (−2.93 to −1.67) | −2.10 (−3.20 to −0.97) | −2.11 (−3.04 to −1.18) | −1.96 (−2.69 to −1.24) | −1.89 (−3.00 to −0.77) | −1.50 (−2.83 to −0.10) | Placebo |
The SUCRA values ranges from 0 to 1; presents the likelihood of therapy to be at the top rank (Figure 49).
FIGURE 49.
The SUCRA for mean change in MMD from baseline.
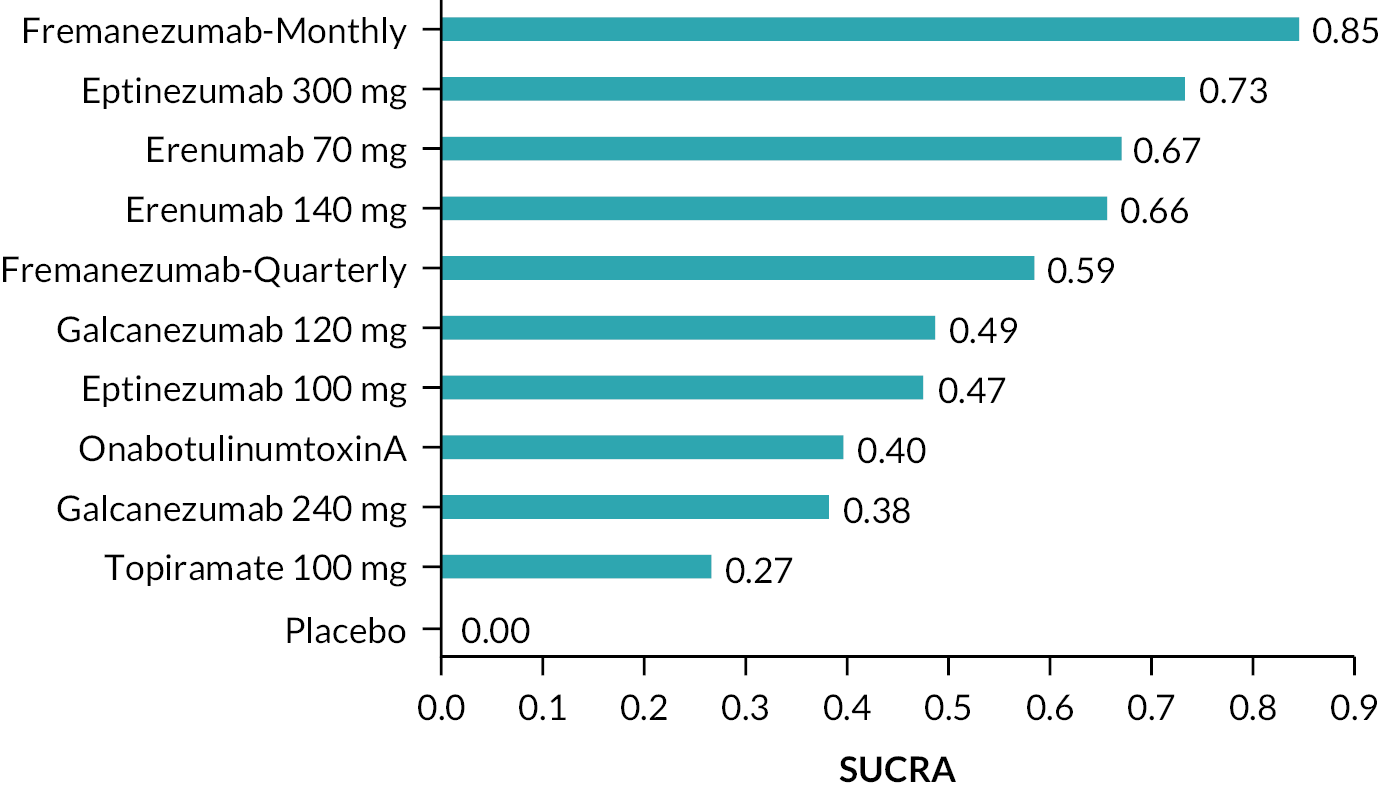
Appendix 4 Baseline characteristics of the included studies for adverse events review
| First author, year/country | Study design and date | Key inclusion criteria | Key exclusion criteria |
|---|---|---|---|
| Author, year: Silberstein SD, 200728 Country: USA |
Study design: Randomised, double-blind, placebo-controlled, parallel-group, multicentre trial Date: September 2003–March 2005 |
|
|
|
|||
| Author, year: Rothrock, 201988 Country: USA |
Study design: Multicentre, randomised, parallel-group, post-authorisation, open label prospective study. After 12 weeks, patients initially randomised to topiramate could cross over to BTA treatment Date: August 2014–September 2017 |
|
|
|
|
||
| Author, year: Tepper, 201745 Country: North America (Canada and USA) and Europe (Czech Republic, Denmark, Finland, Germany, Norway, Poland, Sweden and UK) |
Study design: Phase 2, randomised, double-blind, placebo-controlled, multicentre Date: April 2014–December 2016 |
|
|
|
|
||
| Author, year: Dodick, 201989 Country: 82 in USA, 4 in Australia, and 3 each in New Zealand and the Republic of Georgia |
Study design: Phase 2b, parallel-group, double-blind, randomised, placebo-controlled, dose-ranging clinical trial Date: December 2014–December 2016 |
|
|
|
|
||
| Author, year: Detke, 201895 Country: Argentina, Canada, Czech Republic, Germany, Israel, Italy, Mexico, the Netherlands, Spain, Taiwan, UK and USA |
Study design: Phase 3, randomised, double-blind, placebo-controlled study Date: January 2016–March 2017 |
|
|
|
|
||
|
|
||
| Author, year: Dodick 2010;97 (pooled Aurora 2010,92 Diener 2010 93) Country: 56 North American sites |
Study design: Phase 3 study, with a 24-week, double-blind, parallel-group, placebo-controlled phase followed by a 32-week, open label phase Date: 23 January 2006–16 July 2008 and 7 February 2006–11 August 2008 |
|
|
|
|||
| Author, year: Ferrari, 201990 Country: Belgium, Czech Republic, Denmark, Finland, France, Germany, Italy, Netherlands, Poland, Spain, Sweden, Switzerland, UK and USA |
Study design: Phase 3 FOCUS trial, randomised, double-blind, placebo-controlled, parallel-group Date: October 2017–May 2019 |
|
|
|
|||
| Author, year: Sakai F, 202191 Country: Japan and Korea |
Study design: Multicentre, randomised, double-blind, placebo-controlled, parallel-group Date: November 2017 and November 2019 |
|
|
|
|
||
| Author, year: Silberstein SD, 201737 Country: 132 sites in 9 countries |
Study design: Randomised, double-blind, placebo-controlled, parallel-group trial Date: March 2016 to January 2017 |
|
|
| Author, year: Lipton, 202094 Country: 13 countries (USA, Spain, Ukraine, Russian Federation, UK, Republic of Georgia, Hungary, Italy, Slovakia, Germany, Czech Republic, Denmark and Belgium) |
Study design: Phase 3, double-blind, randomised, placebo-controlled, parallel-group Date: November 2016–April 2018 |
|
|
|
|
||
| Author, year: Silberstein, 200728 Country: USA |
Study design: Randomised, double-blind, placebo-controlled, parallel-group, multicentre trial Date: September 2003–March 2005 |
|
|
|
|||
| Author, year: Lucking, 1988128 Country: Germany |
Study design: Double-blind |
|
|
| Author, year: Ailani, 2021129 Country: USA |
Study design: Multicentre, double-blind, parallel-group, randomised, placebo-controlled trial Date: December 2018–June 2020 |
|
|
|
|||
| Author, year: Sun, 2016130 Country: North America (Canada, USA) and Europe (Denmark, Finland, Germany, Norway, Sweden and Portugal) |
Study design: Multicentre, randomised, double-blind, placebo-controlled trial Date: August 2013–November 2019 |
|
|
|
Category 1: Divalproex Sodium, Sodium Valproate; Category 2: Topiramate; Category 3: Beta-blockers (e.g. Atenolol, Bisoprolol, Metoprolol, Nadolol, Nebivolol, Pindolol, Propranolol, Timolol); Category 4: Tricyclic antidepressants (e.g. Amitriptyline, Nortriptyline, Protriptyline); Category 5: Venlafaxine, Desvenlafaxine, Duloxetine, Milnacipran; Category 6: Flunarizine, Verapamil; Category 7: Lisinopril, Candesartan; Category 8: Butterbur, Feverfew, Magnesium (≥ 600 mg/day), Riboflavin (≥ 100 mg/day) |
||
|
|||
| Author, year: Ashina, 2020131 Country: USA and Republic of Georgia |
Study design: Multicentre, randomised, double-blind, placebo-controlled, parallel-group study Date: September 2015–December 2017 |
|
|
|
|
||
| Author, year: Aurora, 2007132 Country: North America |
Study design: Randomised, double-blind, placebo-controlled, parallel-group, multicentre clinical study |
|
|
|
|
||
| Author, year: Couch, 201122 Country: USA |
Study design: Double-blind, placebo-controlled study Date: 1976 and 1979 |
|
|
|
|||
| Author, year: Dodick, 2014133 Country: USA |
Study design: Randomised, double-blind, placebo-controlled, phase 2 proof-of-concept study, parallel assignment Date: July 2012–September 2013 |
|
|
|
|
||
| Author, year: Dodick, 2018134 Country: 69 sites across North America and Europe (including Russia) |
Study design: Multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 3 trial Date: July 2015–March 2017 |
|
|
|
|
||
| Author, year: Dodick, 2009135 Country: USA |
Study design: Multicentre, randomised, double-blind, double-dummy, parallel-group noninferiority study Date: February 2004–October 2005 |
|
|
|
|||
| Author, year: Diener, 2002136 Country: 8 countries: Belgium, Denmark, Spain, France, Germany, Italy, Portugal, Switzerland |
Study design: A phase-IV double-blind equivalence trial Date: April 1992–March 1996 |
|
|
|
|
||
| Author, year: Dodick, 201835 Country: Canada, Czech Republic, Finland, Israel, Japan, Poland, Russia, Spain, USA |
Study design: Randomised, double-blind, placebo-controlled, parallel group Date: March 2016–April 2017 |
|
|
|
|
||
| Author, year: Goadsby, 201736 Country: 121 sites across North America, Europe, and Turkey |
Study design: Multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 3 trial Date: July 2015–September 2016 |
|
|
|
|
||
| Author, year: Kalita, 2013137 Country: India |
Study design: Single-centre prospective study with randomised controlled open labelled design Date: - |
|
|
| Author, year: Relja, 2007138 Country: 37 study centres in 9 countries (1 centre in Belgium, 6 in Croatia, 1 in Denmark, 3 in Finland, 6 in France, 5 in Germany, 2 in Norway, 1 in Switzerland and 12 in UK) |
Study design: Randomised, double-blind, placebo-controlled, parallel-group, multicentre clinical study of multiple treatments of BTA Date: - |
|
|
|
|
||
| Author, year: Lipton, 2011139 Country: 81 sites in the USA |
Study design: Multicentre, randomised, double blind, placebo-controlled, parallel-group study Date: September 2005 and August 2007 |
|
|
|
|
||
|
|||
| Author, year: Sakai, 2020127 Country: Japan from 40 sites |
Study design: Phase 2, randomised, double-blind, placebo-controlled parallel-design study Date: December 2016–January 2019 |
|
|
|
|
||
| Author, year: Sakai, 2021126 Country: Japan and Korea |
Study design: Multicentre, randomised, double-blind, placebo-controlled, parallel-group Phase 2b/3 trial Date: November 2017 and November 2019 |
|
|
|
|
||
| Author, year: Stauffer, 2018140 Country: 90 sites in North America |
Study design: Phase 3, randomised, double-blind, placebo-controlled study, parallel design Date: November 2015–August 2018 |
|
|
|
|
||
| Author, year: Skljarevski, 2018141 Country: 109 study sites in USA, UK, Netherlands, Spain, Czech Republic, Germany, Argentina, Israel, Korea, Taiwan and Mexico |
Study design: Phase 3, multicentre, placebo-controlled, double-blind, randomised Date: January 2016 and March 2017 |
|
|
|
|
||
| Author, year: Reuter, 2018143 Country: 59 sites in 16 countries across Europe and Australia |
Study design: Randomised, double-blind, placebo-controlled, phase 3b study Date: March 2017–January 2021 |
|
|
| Author, year: Reuter, 2022142 Country: 82 study sites in Germany |
Study design: Randomised, double-blind, double dummy, active-controlled, parallel-group phase 4 Date: February 2019–July 2020 |
|
|
|
|
||
| Author, year: Wang, 2021144 Country: 83 sites across 11 countries in Asia, the Middle East and Latin America |
Study design: Multicentre, randomised, double-blind, placebo-controlled, parallel-group, phase 3 study Date: February 2018–January 2020 |
|
|
|
|
||
| Author, year: Diene, 2007152 Country: 88 neurology clinics in 21 countries in Europe and the Middle East |
Study design: Randomised, double-blind, placebo-controlled trial Date: December 2003–February 2005 |
|
|
|
|||
| Author, year: Elkind, 2006145 Country: USA |
Study design: A series of 3 sequential, randomised, controlled studies Date: - |
|
|
|
|
||
| Author, year: Mulleners, 2020146 Country: 64 sites (hospitals, clinics or research centres) in 12 countries (Belgium, Canada, Czech Republic, France, Germany, Hungary, Japan, the Netherlands, South Korea, Spain, UK and USA) |
Study design: Multicentre, randomised, double-blind, placebo-controlled, phase 3b trial Date: September 2018–21 March 2019 |
|
|
|
|
||
| Author, year: Fazlalizadeh, 2008147 Country: Iran |
Study design: Double-blind randomised clinical trial Date: 2006–7 |
|
|
| Author, year: Croop, 2021148 Country: 92 sites in USA |
Study design: Multicentre, randomised, double-blind, placebo-controlled trial Date: November 2018–August 2019 |
|
|
|
|
||
|
|
||
| Author, year: Winner, 2021149 Country: 47 sites in USA and Republic of Georgia |
Study design: Phase 3, multicentre, parallel-group, double-blind, randomised, placebo-controlled trial Date: November 2019–July 2020 |
|
|
|
|||
|
|||
|
|||
| Author, year: Hu, 2022150 Country: 40 centres in China (n = 26), India (n = 10) and Russia (n = 4) |
Study design: Phase 3, randomised, double-blind, placebo-controlled study Date: July 2019–March 2022 |
|
|
|
|
||
| Author, year: Ashina, 2022151 Country: 96 study locations across Europe (n = 93) and USA (n = 3) |
Study design: Multicentre, multi-arm, double-blind, placebo-controlled Date: June 2020–October 2021 |
|
|
|
|
||
|
|
Appendix 5 Further results for adverse events
| SOC | AEs |
|---|---|
| Cardiac disorders | Acute myocardial infarction, atrial fibrillation, syncope |
| Ear and labyrinth disorders | Labyrinthitis, sudden hearing loss, vertigo, vestibular neuronitis |
| Eye disorders | Angle closure glaucoma, diplopia, optic neuritis, retinal detachment, rhegmatogenous retinal detachment |
| Gastrointestinal disorders | Abdominal pain, alcoholic pancreatitis, appendicitis, diverticulitis, oesophagitis, gastric ulcer haemorrhage, gastritis, haemorrhoids, intestinal haemorrhage, irritable bowel syndrome, mechanical ileus, obstructive defaecation, pancreatitis, pancreatitis acute, parotitis, small intestinal obstruction, vomiting |
| General disorders and administration site conditions | Abdominal adhesions, asthenia, chest pain, oedema peripheral, malaise, nasal septum deviation, non-cardiac chest pain, tooth impacted, vocal cord thickening |
| Hepatobiliary disorders | Cholecystitis, cholecystitis acute, cholelithiasis, common bile duct stone |
| Immune system disorders | Anaphylactic reaction, anaphylactic shock, hypersensitivity |
| Infections and infestations | Acute pyelonephritis, bacterial pharyngitis, bacteriuria, clostridium difficile colitis, COVID-19 pneumonia, gastroenteritis, gastrointestinal infection, infected dermal cyst, influenza, kidney infection, nasopharyngitis, papilloma viral infection, parasitic gastroenteritis, pyelonephritis, pyrexia, sepsis, tonsillitis, urinary tract infection, viral gastroenteritis, viral infection |
| Injury | Accident, ankle fracture, brain contusion, cartilage injury, clavicle fracture, concussion, contusion, fall, foot fracture, hand fracture, humerus fracture, injury, ligament rupture, limb injury, lower limb fracture, meniscus injury, radius fracture, respiratory fume inhalation, rib fracture, road traffic accident, skin laceration, sternal fracture, tendon injury, thoracic vertebral fracture, traumatic orbital fracture, ulna fracture, wrist fracture |
| Investigations | Alanine aminotransferase increased, aspartate aminotransferase increased, hepatic enzyme increased, weight decreased |
| Metabolism and nutrition disorders | Decreased appetite, hypokalaemia, hyponatraemia |
| Musculoskeletal and connective tissue disorders | Arthralgia, back pain, Behçet syndrome, costochondritis, flank pain, intervertebral disc protrusion, osteoarthritis, periarthritis, post-traumatic neck syndrome |
| Neoplasms: benign, malignant and unspecified (incl cysts and polyps) | Adenocarcinoma of the cervix, brain neoplasm, breast cancer, colon cancer, fibroma, gall bladder polyp, ovarian cyst, polycystic ovaries, rectal polyp, ruptured ovarian cyst, uterine leiomyoma, breast neoplasm, fibroadenoma of breast, malignant melanoma, neoplasm malignant, vulval cancer |
| Nervous system disorders | Cerebellar syndrome, cerebral venous thrombosis, cervical radiculopathy, hypoaesthesia, lumbar spinal stenosis, migraine, migraine aggravated, migraine with aura, nervous system disorders, neuropathy, seizure, speech disorder, transient ischaemic attack |
| Neurological | Spinal pain |
| Poisoning and procedural complications | Overdose, intentional overdose |
| Pregnancy, puerperium and perinatal conditions | Pregnancy |
| Psychiatric disorders | Confusional state, depression, disorientation, major depression, psychogenic seizure, suicidal ideation, suicide attempt |
| Psychiatry | Panic attack |
| Renal and urinary disorders | Bladder dysfunction, calculus urinary, nephrolithiasis, renal calculus, renal colic, urinary incontinence |
| Reproductive system and breast disorders | Cervical dysplasia, dysmenorrhoea, endometriosis, menorrhagia, menstrual disorder and vaginal haemorrhage, metrorrhagia, ovarian disorder, spontaneous abortion, threatened abortion |
| Respiratory, thoracic and mediastinal | Asthma, chronic obstructive pulmonary disease, COPD and apnoea related to COPD, dyspnoea, epistaxis, pneumonia, post-surgical laryngospasm with hypoxic brain injury |
| Skin and subcutaneous tissue disorders | Erythema nodosum |
| Vascular disorders | Hypertensive crisis, orthostatic hypotension, peripheral vascular disease, pulmonary embolism |
| Study ID | Author | Year | Intervention | Participants | Any AEs | Treatment-related AEs | AEs definition |
|---|---|---|---|---|---|---|---|
| 666 | Hu | 2022 | Galcanezumab 120 mg | 261 | 49.8 | Standard | |
| 666 | Hu | 2022 | Placebo | 259 | 43.2 | Standard | |
| 555 | Ashina | 2022 | Eptinezumab 100 mg | 299 | 42 | 3 | Standard |
| 555 | Ashina | 2022 | Eptinezumab 300 mg | 294 | 41 | 1 | Standard |
| 555 | Ashina | 2022 | Placebo | 298 | 40 | 3 | Standard |
| 158 | Sakai | 2021 | Fremanezumab-M | 188 | 61.7 | 29.3 | Standard |
| 158 | Sakai | 2021 | Fremanezumab-Q | 190 | 61.1 | 32.1 | Standard |
| 158 | Sakai | 2021 | Placebo | 191 | 61.8 | 28.3 | Standard |
| 8 | Ailani | 2021 | Atogepant 10 mg | 221 | 52.9 | 23.1 | Standard |
| 8 | Ailani | 2021 | Atogepant 30 mg | 228 | 52.2 | 14.9 | Standard |
| 8 | Ailani | 2021 | Atogepant 60 mg | 231 | 53.7 | 19.5 | Standard |
| 8 | Ailani | 2021 | Placebo | 222 | 56.8 | 9 | Standard |
| 157 | Sakai | 2021 | Fremanezumab-M | 121 | 57 | 26.4 | Standard |
| 157 | Sakai | 2021 | Fremanezumab-Q | 118 | 62.7 | 31.4 | Standard |
| 157 | Sakai | 2021 | Placebo | 117 | 65.8 | 23.9 | Standard |
| 197 | Reuter | 2021 | Erenumab 140 mg | 388 | 55.4 | Standard | |
| 197 | Reuter | 2021 | Topiramate 100 mg | 388 | 81.2 | Standard | |
| 203 | Wang | 2021 | Erenumab 70 mg | 335 | 34.9 | 11.3 | Standard |
| 203 | Wang | 2021 | Erenumab 140 mg | 224 | 34.4 | 10.7 | Standard |
| 203 | Wang | 2021 | Placebo | 335 | 36.7 | 9.6 | Standard |
| 777 | Winner | 2021 | Eptinezumab 100 mg | 238 | 10.9 | Standard | |
| 777 | Winner | 2021 | Placebo | 242 | 10.3 | Standard | |
| 105 | Lipton | 2020 | Eptinezumab 100 mg | 356 | 43.5 | Standard | |
| 105 | Lipton | 2020 | Eptinezumab 300 mg | 350 | 52 | Standard | |
| 105 | Lipton | 2020 | Placebo | 366 | 46.7 | Standard | |
| 19 | Ashina | 2020 | Eptinezumab 30 mg | 219 | 58.4 | Standard | |
| 19 | Ashina | 2020 | Eptinezumab 100 mg | 223 | 63.2 | Standard | |
| 19 | Ashina | 2020 | Eptinezumab 300 mg | 224 | 57.6 | Standard | |
| 19 | Ashina | 2020 | Placebo | 222 | 59.5 | Standard | |
| 156 | Sakai | 2020 | Galcanezumab 120 mg | 115 | 85.2 | Standard | |
| 156 | Sakai | 2020 | Galcanezumab 240 mg | 114 | 81.6 | Standard | |
| 156 | Sakai | 2020 | Placebo | 230 | 64.8 | Standard | |
| 221 | Mulleners | 2020 | Galcanezumab 120 mg | 232 | 51 | 15 | Standard |
| 221 | Mulleners | 2020 | Placebo | 230 | 53 | 16 | Standard |
| 888 | Croop | 2020 | Rimegepant 75 mg | 370 | 36 | 11 | Standard |
| 888 | Croop | 2020 | Placebo | 371 | 36 | 9 | Standard |
| 61 | Dodick | 2019 | Eptinezumab 100 mg | 122 | 57.5 | 19.8 | Standard |
| 61 | Dodick | 2019 | Eptinezumab 300 mg | 121 | 63.6 | 17.4 | Standard |
| 61 | Dodick | 2019 | Eptinezumab 30 mg | 122 | 45.9 | 14.8 | Standard |
| 61 | Dodick | 2019 | Eptinezumab 10 mg | 130 | 56.9 | 16.2 | Standard |
| 61 | Dodick | 2019 | Placebo | 121 | 56.2 | 14 | Standard |
| 217 | Ferrari | 2019 | Fremanezumab-Q | 276 | 55 | 21 | Standard |
| 217 | Ferrari | 2019 | Fremanezumab-M | 285 | 45 | 19 | Standard |
| 217 | Ferrari | 2019 | Placebo | 277 | 48 | 20 | Standard |
| 148 | Rothrock | 2019 | BTA 150U | 220 | 48 | 17 | Standard |
| 148 | Rothrock | 2019 | Topiramate 100 mg | 142 | 79 | 70 | Standard |
| 49 | Detke | 2018 | Galcanezumab 120 mg | 273 | 58 | Standard | |
| 49 | Detke | 2018 | Galcanezumab 240 mg | 282 | 57 | Standard | |
| 49 | Detke | 2018 | Placebo | 558 | 50 | Standard | |
| 45 | Dodick | 2018 | Erenumab 70 mg | 283 | 48.1 | Standard | |
| 45 | Dodick | 2018 | Placebo | 289 | 54.7 | Standard | |
| 60 | Dodick | 2018 | Fremanezumab-M | 290 | 66.2 | 47.6 | Standard |
| 60 | Dodick | 2018 | Fremanezumab-Q | 291 | 66.3 | 47.1 | Standard |
| 60 | Dodick | 2018 | Placebo | 293 | 58.4 | 37.2 | Standard |
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 65.5 | Standard | |
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 67.7 | Standard | |
| 181 | Stauffer | 2018 | Placebo | 432 | 60.4 | Standard | |
| 201 | Vladimir | 2018 | Galcanezumab 120 mg | 226 | 65 | Standard | |
| 201 | Vladimir | 2018 | Galcanezumab 240 mg | 228 | 71.5 | Standard | |
| 201 | Vladimir | 2018 | Placebo | 461 | 62.3 | Standard | |
| 196 | Reuter | 2018 | Erenumab 140 mg | 119 | 55 | Standard | |
| 196 | Reuter | 2018 | Placebo | 124 | 54 | Standard | |
| 173 | Silberstein | 2017 | Fremanezumab-Q | 376 | 70 | 49 | Standard |
| 173 | Silberstein | 2017 | Fremanezumab-M | 379 | 71 | 51 | Standard |
| 173 | Silberstein | 2017 | Placebo | 375 | 64 | 42 | Standard |
| 185 | Tepper | 2017 | Erenumab 70 mg | 190 | 44 | Standard | |
| 185 | Tepper | 2017 | Erenumab 140 mg | 188 | 47 | Standard | |
| 185 | Tepper | 2017 | Placebo | 282 | 39 | Standard | |
| 77 | Goadsby | 2017 | Erenumab 70 mg | 314 | 57.3 | Standard | |
| 77 | Goadsby | 2017 | Erenumab 140 mg | 319 | 55.5 | Standard | |
| 77 | Goadsby | 2017 | Placebo | 319 | 63 | Standard | |
| 88 | Hong Sun | 2016 | Erenumab 7 mg | 108 | 50 | Standard | |
| 88 | Hong Sun | 2016 | Erenumab 21 mg | 105 | 51 | Standard | |
| 88 | Hong Sun | 2016 | Erenumab 70 mg | 106 | 54 | Standard | |
| 88 | Hong Sun | 2016 | Placebo | 153 | 54 | Standard | |
| 44 | Dodick | 2014 | Galcanezumab 150 mg | 107 | 72 | Standard | |
| 44 | Dodick | 2014 | Placebo | 110 | 67 | Standard | |
| 94 | Kalita | 2013 | Divalproate 250–1000 mg | 143 | 47.6 | No definition | |
| 94 | Kalita | 2013 | Amitriptyline 50 mg | 144 | 56.3 | No definition | |
| 41 | Couch | 2011 | Amitriptyline 100 mg | 194 | 57.2 | No definition | |
| 41 | Couch | 2011 | Placebo | 197 | 26.9 | No definition | |
| 143 | Lipton | 2011 | Topiramate 100 mg | 176 | 82.4 | No definition | |
| 143 | Lipton | 2011 | Placebo | 185 | 73.5 | No definition | |
| 59 | Dodick | 2010 | BTA 150U | 687 | 62.4 | 29.4 | Standard |
| 59 | Dodick | 2010 | Placebo | 692 | 51.7 | 12.7 | Standard |
| 47 | Dodick | 2009 | Topiramate 100 mg | 177 | 85.9 | 68.4 | Standard |
| 47 | Dodick | 2009 | Amitriptyline 100 mg | 169 | 88.8 | 75.7 | Standard |
| 999 | Fazlalizadeh | 2008 | Topiramate 100 mg | 284 | 14.4 | No definition | |
| 999 | Fazlalizadeh | 2008 | Sodium valproate 200 mg | 285 | 14 | No definition | |
| 170 | Silberstein | 2007 | Topiramate 100 mg | 160 | 82.5 | Standard | |
| 170 | Silberstein | 2007 | Placebo | 161 | 70.2 | Standard | |
| 111 | M Relja | 2007 | BTA 225U | 129 | 76.7 | 67.4 | No definition |
| 111 | M Relja | 2007 | BTA 150U | 125 | 77.6 | 63.2 | No definition |
| 111 | M Relja | 2007 | BTA 75U | 123 | 77.2 | 62.6 | No definition |
| 111 | M Relja | 2007 | Placebo | 118 | 54.2 | 31.4 | No definition |
| 215 | Diener | 2007 | Topiramate 200 mg | 254 | 68 | No definition | |
| 215 | Diener | 2007 | Placebo | 258 | 59 | No definition | |
| 21 | Aurora | 2006 | BTA 105 to 260U | 187 | 81.3 | 60.4 | No definition |
| 21 | Aurora | 2006 | Placebo | 182 | 59.9 | 21.4 | No definition |
| 216 | Elkind (study1) | 2006 | BTA 7U | 105 | 49.5 | 6.7 | No definition |
| 216 | Elkind (study1) | 2006 | BTA 25U | 101 | 46.5 | 21.8 | No definition |
| 216 | Elkind (study1) | 2006 | BTA 50U | 106 | 56.6 | 30.2 | No definition |
| 216 | Elkind (study1) | 2006 | Placebo | 106 | 47.2 | 6.6 | No definition |
| 216 | Elkind (study2) | 2006 | BTA 25U | 173 | 77.2 | 29.4 | No definition |
| 216 | Elkind (study2) | 2006 | BTA 50U | 180 | 78 | 24.9 | No definition |
| 216 | Elkind (study3) | 2006 | BTA 25U | 50 | 70 | No definition | |
| 216 | Elkind (study3) | 2006 | BTA 50U | 51 | 68.8 | No definition | |
| 216 | Elkind (study3) | 2006 | Placebo | 100 | 60 | No definition | |
| 53 | Diener | 2002 | Flunarizine 5 mg | 263 | 33.5 | No definition | |
| 53 | Diener | 2002 | Flunarizine 10 mg | 275 | 32 | No definition | |
| 53 | Diener | 2002 | Propranolol 160 mg | 270 | 32.6 | No definition | |
| 109 | Lucking | 1988 | Flunarizine 10 mg | 160 | 24.6 | No definition | |
| 109 | Lucking | 1988 | Propranolol 40 mg | 170 | 29.6 | No definition |
| Study ID | Author(s) | Year of publication | Intervention | Participants | Weight increase | Weight decrease | Increased blood creatine kinase level | Blood creatinine phosphokinase increased | INR increased | Alanine aminotransferase > 3 × ULN | Aspartate aminotransferase ≥ 3× ULN | Total bilirubin ≥ 2× ULN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 666 | Hu | 2022 | Galcanezumab 120 mg | 261 | 1.5 | 1.9 | ||||||
| 666 | Hu | 2022 | Placebo | 259 | 0 | 0 | ||||||
| 555 | Ashina | 2022 | Eptinezumab 100 mg | 299 | 1.5 | |||||||
| 555 | Ashina | 2022 | Eptinezumab 300 mg | 294 | 0 | |||||||
| 555 | Ashina | 2022 | Placebo | 298 | ||||||||
| 8 | Ailani | 2021 | Atogepant 10 mg | 221 | 2.3 | 1.4 | ||||||
| 8 | Ailani | 2021 | Atogepant 30 mg | 228 | 0.9 | 0.9 | ||||||
| 8 | Ailani | 2021 | Atogepant 60 mg | 231 | 3 | 0.9 | ||||||
| 8 | Ailani | 2021 | Placebo | 222 | 0.9 | 2.7 | ||||||
| 197 | Reuter | 2021 | Erenumab 140 mg | 388 | 0.8 | |||||||
| 197 | Reuter | 2021 | Topiramate 100 mg | 388 | 5.7 | |||||||
| 217 | Ferrari | 2019 | Fremanezumab-Q | 276 | 1 | |||||||
| 217 | Ferrari | 2019 | Fremanezumab-M | 285 | 0.5 | |||||||
| 217 | Ferrari | 2019 | Placebo | 277 | 0.5 | |||||||
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 1.9 | |||||||
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 0.9 | |||||||
| 181 | Stauffer | 2018 | Placebo | 432 | 1.4 | |||||||
| 173 | Silberstein | 2017 | Fremanezumab-Q | 376 | 0.26 | 0.26 | 0.6 | |||||
| 173 | Silberstein | 2017 | Fremanezumab-M | 379 | 0.26 | 0.26 | 0 | |||||
| 173 | Silberstein | 2017 | Placebo | 375 | 0 | 0 | 0 | |||||
| 94 | Kalita | 2013 | Divalproate 250–1000 mg | 143 | 61.7 | |||||||
| 94 | Kalita | 2013 | Amitriptyline 50 mg | 144 | 58.7 | |||||||
| 41 | Couch | 2011 | Amitriptyline 100 mg | 194 | 1.5 | |||||||
| 41 | Couch | 2011 | Placebo | 197 | 1.01 | |||||||
| 47 | Dodick | 2009 | Topiramate 100 mg | 177 | 0 | |||||||
| 47 | Dodick | 2009 | Amitriptyline 100 mg | 169 | 13.6 | |||||||
| 215 | Diener | 2007 | Topiramate 200 mg | 254 | 9 | |||||||
| 215 | Diener | 2007 | Placebo | 258 | 7 | |||||||
| 53 | Diener | 2002 | Flunarizine 5 mg | 263 | 9.9 | |||||||
| 53 | Diener | 2002 | Flunarizine 10 mg | 275 | 5.6 | |||||||
| 53 | Diener | 2002 | Propranolol 160 mg | 270 | 2.6 |
| Study ID | First author | Year of publication | Intervention | Participants | Ecchymosis | Injury | Contusion |
|---|---|---|---|---|---|---|---|
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 2.4 | ||
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 0 | ||
| 181 | Stauffer | 2018 | Placebo | 432 | 1.2 | ||
| 143 | Lipton | 2011 | Topiramate 100 mg | 176 | 1.7 | ||
| 143 | Lipton | 2011 | Placebo | 185 | 9.2 | ||
| 170 | Silberstein | 2007 | Topiramate 100 mg | 160 | 5 | ||
| 170 | Silberstein | 2007 | Placebo | 161 | 1.2 | ||
| 21 | Aurora | 2006 | BTA 105–260U | 187 | 1.1 | ||
| 21 | Aurora | 2006 | Placebo | 182 | 1.6 | ||
| 53 | Diener | 2002 | Flunarizine 5 mg | 263 | 1.9 | ||
| 53 | Diener | 2002 | Flunarizine 10 mg | 275 | 1.5 | ||
| 53 | Diener | 2002 | Propranolol 160 mg | 270 | 2.6 |
| Study ID | Author | Year of publication | Intervention | Participants | Anorexia | Decreased appetite |
|---|---|---|---|---|---|---|
| 197 | Reuter | 2021 | Erenumab 140 mg | 388 | 2.1 | |
| 197 | Reuter | 2021 | Topiramate 100 mg | 388 | 9 | |
| 148 | Rothrock | 2019 | BTA 150U | 220 | 0 | |
| 148 | Rothrock | 2019 | Topiramate 100 mg | 142 | 11 | |
| 143 | Lipton | 2011 | Topiramate 100 mg | 176 | 8.5 | |
| 143 | Lipton | 2011 | Placebo | 185 | 2.7 | |
| 47 | Dodick | 2009 | Topiramate 100 mg | 177 | 6.8 | |
| 47 | Dodick | 2009 | Amitriptyline 100 mg | 169 | 4.7 | |
| 170 | Silberstein | 2007 | Topiramate 100 mg | 160 | 5 | |
| 170 | Silberstein | 2007 | Placebo | 161 | 5.6 | |
| 215 | Diener | 2007 | Topiramate 200 mg | 254 | 5 | |
| 215 | Diener | 2007 | Placebo | 258 | 3 |
| Study ID | Author(s) | Year of publication | Intervention | Participants | Menstrual irregularity | Dysmenorrhoea |
|---|---|---|---|---|---|---|
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 0.6 | |
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 2.2 | |
| 181 | Stauffer | 2018 | Placebo | 432 | 0.6 | |
| 94 | Kalita | 2013 | Divalproate 250–1000 mg | 143 | 4.8 | |
| 94 | Kalita | 2013 | Amitriptyline 50 mg | 144 | 0 |
| Study ID | Author(s) | Year of publication | Intervention | Participants | Eczema | Urticaria | Pruritus | Hair fall | Skin tightness | Rash | Alopecia | Sweat discoloration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 666 | Hu | 2022 | Galcanezumab 120 mg | 261 | 1.5 | |||||||
| 666 | Hu | 2022 | Placebo | 259 | 0.8 | |||||||
| 157 | Sakai | 2021 | Fremanezumab-M | 121 | 2.5 | |||||||
| 157 | Sakai | 2021 | Fremanezumab-Q | 118 | 0.8 | |||||||
| 157 | Sakai | 2021 | Placebo | 117 | 0 | |||||||
| 156 | Sakai | 2020 | Galcanezumab 120 mg | 115 | 1.7 | |||||||
| 156 | Sakai | 2020 | Galcanezumab 240 mg | 114 | 6.1 | |||||||
| 156 | Sakai | 2020 | Placebo | 230 | 0 | |||||||
| 217 | Ferrari | 2019 | Fremanezumab-Q | 276 | 0.5 | 0.5 | ||||||
| 217 | Ferrari | 2019 | Fremanezumab-M | 285 | 1 | 0.5 | ||||||
| 217 | Ferrari | 2019 | Placebo | 277 | 0.5 | 0.5 | ||||||
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 1 | |||||||
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 2.7 | |||||||
| 181 | Stauffer | 2018 | Placebo | 432 | 0.2 | |||||||
| 44 | Dodick | 2014 | Galcanezumab 150 mg | 107 | 5 | |||||||
| 44 | Dodick | 2014 | Placebo | 110 | 0 | |||||||
| 94 | Kalita | 2013 | Divalproate 250–1000 mg | 143 | 38.5 | |||||||
| 94 | Kalita | 2013 | Amitriptyline 50 mg | 144 | 1.4 | |||||||
| 41 | Couch | 2011 | Amitriptyline 100 mg | 194 | 0.5 | 3.1 | ||||||
| 41 | Couch | 2011 | Placebo | 197 | 1.5 | 2.5 | ||||||
| 21 | Aurora | 2006 | BTA 105–260U | 187 | 7.5 | |||||||
| 21 | Aurora | 2006 | Placebo | 182 | 0.5 |
| Study ID | Author | Year of publication | Intervention | Participants | Belpharotosis | Abnormal vision | Visual disturbance | Vision blurred | Eyelid oedema |
|---|---|---|---|---|---|---|---|---|---|
| 148 | Rothrock | 2019 | BTA 150U | 220 | 3 | ||||
| 148 | Rothrock | 2019 | Topiramate 100 mg | 142 | 8 | ||||
| 44 | Dodick | 2014 | Galcanezumab 150 mg | 107 | 3 | ||||
| 44 | Dodick | 2014 | Placebo | 110 | 2 | ||||
| 41 | Couch | 2011 | Amitriptyline 100 mg | 194 | 2.1 | ||||
| 41 | Couch | 2011 | Placebo | 197 | 2.5 | ||||
| 59 | Dodick | 2010 | BTA 150U | 687 | 3.3 | ||||
| 59 | Dodick | 2010 | Placebo | 692 | 0.3 | ||||
| 47 | Dodick | 2009 | Topiramate 100 mg | 177 | 5.1 | ||||
| 47 | Dodick | 2009 | Amitriptyline 100 mg | 169 | 5.3 | ||||
| 21 | Aurora | 2006 | BTA 105–260U | 187 | 15.5 | 6.4 | |||
| 21 | Aurora | 2006 | Placebo | 182 | 1.6 | 0 | |||
| 216 | Elkind (study1) | 2006 | BTA 7U | 105 | 1.9 | 1 | |||
| 216 | Elkind (study1) | 2006 | BTA 25U | 101 | 5 | 0 | |||
| 216 | Elkind (study1) | 2006 | BTA 50U | 106 | 7.6 | 6.6 | |||
| 216 | Elkind (study1) | 2006 | Placebo | 106 | 0 | 0 | |||
| 216 | Elkind (study2) | 2006 | BTA 25U | 173 | 4 | ||||
| 216 | Elkind (study2) | 2006 | BTA 50U | 180 | 8.9 | ||||
| 216 | Elkind (study3) | 2006 | BTA 25U | 50 | 0 | ||||
| 216 | Elkind (study3) | 2006 | BTA 50U | 51 | 5.9 | ||||
| 216 | Elkind (study3) | 2006 | Placebo | 100 | 0 |
| Study ID | Author | Year of publication | Intervention | Participants | Urinary retention | Protein urine present |
|---|---|---|---|---|---|---|
| 666 | Hu | 2022 | Galcanezumab 120 mg | 261 | 2.3 | |
| 666 | Hu | 2022 | Placebo | 259 | 1.5 | |
| 41 | Couch | 2011 | Amitriptyline 100 mg | 194 | 3.1 | |
| 41 | Couch | 2011 | Placebo | 197 | 0 |
| ID | Author | Year | Intervention | Vascular disorders | Cardiac disorders | ||
|---|---|---|---|---|---|---|---|
| Participants | Hypotension | Hypertension | Tachycardia | ||||
| 217 | Ferrari | 2019 | Fremanezumab quarterly | 276 | 1 | ||
| 217 | Ferrari | 2019 | Fremanezumab monthly | 285 | 0.5 | ||
| 217 | Ferrari | 2019 | Placebo | 277 | 0.5 | ||
| 77 | Goadsby | 2017 | Erenumab 70 mg | 314 | 1.6 | ||
| 77 | Goadsby | 2017 | Erenumab 140 mg | 319 | 0 | ||
| 77 | Goadsby | 2017 | Placebo | 319 | 2.5 | ||
| 44 | Dodick | 2014 | Galcanezumab 150 mg | 107 | 5 | ||
| 44 | Dodick | 2014 | Placebo | 110 | 0 | ||
| 216 | Elkind (study 3) | 2006 | BTA 25U | 50 | 2 | ||
| 216 | Elkind (study 3) | 2006 | BTA 50U | 51 | 2 | ||
| 216 | Elkind (study 3) | 2006 | Placebo | 100 | 5 | ||
| 53 | Diener | 2002 | Flunarizine 5 mg | 263 | 1.1 | ||
| 53 | Diener | 2002 | Flunarizine 10 mg | 275 | 1.1 | ||
| 53 | Diener | 2002 | Propranolol 160 mg | 270 | 1.5 | ||
| 41 | Couch | 2011 | Amitriptyline 100 mg | 194 | 3.6 | ||
| 41 | Couch | 2011 | Placebo | 197 | 3 | ||
| Study ID | Author | Year of publication | Intervention | Participants | Nasal congestion | Bronchitis | Rhinitis | Sinus congestion | Cough | Asthma |
|---|---|---|---|---|---|---|---|---|---|---|
| 158 | Sakai | 2021 | Fremanezumab-M | 188 | 1.1 | |||||
| 158 | Sakai | 2021 | Fremanezumab-Q | 190 | 2.1 | |||||
| 158 | Sakai | 2021 | Placebo | 191 | 0 | |||||
| 8 | Ailani | 2021 | Atogepant 10 mg | 221 | 0.5 | |||||
| 8 | Ailani | 2021 | Atogepant 30 mg | 228 | 0.9 | |||||
| 8 | Ailani | 2021 | Atogepant 60 mg | 231 | 1.7 | |||||
| 8 | Ailani | 2021 | Placebo | 222 | 2.3 | |||||
| 19 | Ashina | 2020 | Eptinezumab 30 mg | 219 | 2.3 | <1 | ||||
| 19 | Ashina | 2020 | Eptinezumab 100 mg | 223 | 2.7 | 3.6 | ||||
| 19 | Ashina | 2020 | Eptinezumab 300 mg | 224 | 3.1 | 2.7 | ||||
| 19 | Ashina | 2020 | Placebo | 222 | 3.6 | 3.2 | ||||
| 221 | Mulleners | 2020 | Galcanezumab 120 mg | 232 | 1 | |||||
| 221 | Mulleners | 2020 | Placebo | 230 | 2 | |||||
| 61 | Dodick | 2019 | Eptinezumab 100 mg | 122 | 3.3 | |||||
| 61 | Dodick | 2019 | Eptinezumab 300 mg | 121 | 3.3 | |||||
| 61 | Dodick | 2019 | Eptinezumab 30 mg | 122 | 3.3 | |||||
| 61 | Dodick | 2019 | Eptinezumab 10 mg | 130 | 3.1 | |||||
| 61 | Dodick | 2019 | Placebo | 121 | 7.4 | |||||
| 60 | Dodick | 2018 | Fremanezumab-M | 290 | 21 | |||||
| 60 | Dodick | 2018 | Fremanezumab-Q | 291 | 1.4 | |||||
| 60 | Dodick | 2018 | Placebo | 293 | 1 | |||||
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 0.5 | 1.5 | 1.9 | |||
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 2.3 | 3.2 | 2.7 | |||
| 181 | Stauffer | 2018 | Placebo | 432 | 0.9 | 1.4 | 1.6 | |||
| 88 | Hong Sun | 2016 | Erenumab 7 mg | 108 | 2 | |||||
| 88 | Hong Sun | 2016 | Erenumab 21 mg | 105 | 1 | |||||
| 88 | Hong Sun | 2016 | Erenumab 70 mg | 106 | 0 | |||||
| 88 | Hong Sun | 2016 | Placebo | 153 | 2 | |||||
| 47 | Dodick | 2009 | Topiramate 100 mg | 177 | 5.1 | |||||
| 47 | Dodick | 2009 | Amitriptyline 100 mg | 169 | 4.1 | |||||
| 216 | Elkind (study2) | 2006 | BTA 25U | 173 | 3.5 | |||||
| 216 | Elkind (study2) | 2006 | BTA 50U | 180 | 5.6 | |||||
| 216 | Elkind (study3) | 2006 | BTA 25U | 50 | 2 | |||||
| 216 | Elkind (study3) | 2006 | BTA 50U | 51 | 3.9 | |||||
| 216 | Elkind (study3) | 2006 | Placebo | 100 | 7 | |||||
| 53 | Diener | 2002 | Flunarizine 5 mg | 263 | 1.5 | |||||
| 53 | Diener | 2002 | Flunarizine 10 mg | 275 | 2.2 | |||||
| 53 | Diener | 2002 | Propranolol 160 mg | 270 | 2.2 |
| Study ID | Author | Year of publication | Intervention | Participants | Abdominal pain | Oropharyngeal pain | Abdominal discomfort | Diarrhoea | Flatulence | Dry mouth | Oropharyngeal pain | Toothache | Upper abdominal pain | Dyspepsia | Nausea | Dry mucous membrane | Constipation | Vomiting | Gastrointestinal symptoms | Vertigo | Giddiness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 666 | Hu | 2022 | Galcanezumab 120 mg | 261 | 1.9 | 1.5 | |||||||||||||||
| 666 | Hu | 2022 | Placebo | 259 | 0.8 | 2.3 | |||||||||||||||
| 555 | Ashina | 2022 | Eptinezumab 100 mg | 299 | 0 | 2 | 1 | ||||||||||||||
| 555 | Ashina | 2022 | Eptinezumab 300 mg | 294 | 2 | 1 | 2 | ||||||||||||||
| 555 | Ashina | 2022 | Placebo | 298 | 2 | 1 | 1 | ||||||||||||||
| 158 | Sakai | 2021 | Fremanezumab-M | 188 | 1.6 | 1.1 | |||||||||||||||
| 158 | Sakai | 2021 | Fremanezumab-Q | 190 | 2.1 | 2.6 | |||||||||||||||
| 158 | Sakai | 2021 | Placebo | 191 | 0 | 1 | |||||||||||||||
| 8 | Ailani | 2021 | Atogepant 10 mg | 221 | 5 | 7.7 | |||||||||||||||
| 8 | Ailani | 2021 | Atogepant 30 mg | 228 | 4.4 | 7 | |||||||||||||||
| 8 | Ailani | 2021 | Atogepant 60 mg | 231 | 6.1 | 6.9 | |||||||||||||||
| 8 | Ailani | 2021 | Placebo | 222 | 1.8 | 0.5 | |||||||||||||||
| 157 | Sakai | 2021 | Fremanezumab-M | 121 | 0 | 0.8 | 0.8 | ||||||||||||||
| 157 | Sakai | 2021 | Fremanezumab-Q | 118 | 2.5 | 2.5 | 0 | ||||||||||||||
| 157 | Sakai | 2021 | Placebo | 117 | 0 | 0 | 2.6 | ||||||||||||||
| 197 | Reuter | 2021 | Erenumab 140 mg | 388 | 1.8 | 2.1 | 2.8 | 1.5 | 6.7 | 11.3 | 4.4 | ||||||||||
| 197 | Reuter | 2021 | Topiramate 100 mg | 388 | 4.1 | 4.6 | 2.6 | 2.3 | 6.7 | 3.1 | 5.9 | ||||||||||
| 203 | Wang | 2021 | Erenumab 70 mg | 335 | 5.7 | ||||||||||||||||
| 203 | Wang | 2021 | Erenumab 140 mg | 224 | 5.4 | ||||||||||||||||
| 203 | Wang | 2021 | Placebo | 335 | 1.5 | ||||||||||||||||
| 777 | Winner | 2021 | Eptinezumab 100 mg | 238 | 0 | ||||||||||||||||
| 777 | Winner | 2021 | Placebo | 242 | 0.8 | ||||||||||||||||
| 105 | Lipton | 2020 | Eptinezumab 100 mg | 356 | 1.7 | ||||||||||||||||
| 105 | Lipton | 2020 | Eptinezumab 300 mg | 350 | 3.4 | ||||||||||||||||
| 105 | Lipton | 2020 | Placebo | 366 | 1.9 | ||||||||||||||||
| 19 | Ashina | 2020 | Eptinezumab 30 mg | 219 | 1.8 | 4.1 | |||||||||||||||
| 19 | Ashina | 2020 | Eptinezumab 100 mg | 223 | 1.3 | 2.2 | |||||||||||||||
| 19 | Ashina | 2020 | Eptinezumab 300 mg | 224 | 3.6 | 2.2 | |||||||||||||||
| 19 | Ashina | 2020 | Placebo | 222 | 1.4 | 3.6 | |||||||||||||||
| 221 | Mulleners | 2020 | Galcanezumab 120 mg | 232 | 1 | 1 | 2 | 2 | 2 | ||||||||||||
| 221 | Mulleners | 2020 | Placebo | 230 | 2 | 2 | 2 | 2 | 0.004 | ||||||||||||
| 888 | Croop | 2020 | Rimegepant 75 mg | 370 | 3 | ||||||||||||||||
| 888 | Croop | 2020 | Placebo | 371 | 1 | ||||||||||||||||
| 61 | Dodick | 2019 | Eptinezumab 100 mg | 122 | 7.4 | ||||||||||||||||
| 61 | Dodick | 2019 | Eptinezumab 300 mg | 121 | 6.6 | ||||||||||||||||
| 61 | Dodick | 2019 | Eptinezumab 30 mg | 122 | 3.3 | ||||||||||||||||
| 61 | Dodick | 2019 | Eptinezumab 10 mg | 130 | 4.6 | ||||||||||||||||
| 61 | Dodick | 2019 | Placebo | 121 | 7.4 | ||||||||||||||||
| 217 | Ferrari | 2019 | Fremanezumab-Q | 276 | 3 | 1 | 1 | 3 | |||||||||||||
| 217 | Ferrari | 2019 | Fremanezumab-M | 285 | 0.5 | 0.5 | 0.5 | 0.5 | |||||||||||||
| 217 | Ferrari | 2019 | Placebo | 277 | 1 | 0 | 2 | 0.5 | |||||||||||||
| 148 | Rothrock | 2019 | BTA 150U | 220 | 0.5 | ||||||||||||||||
| 148 | Rothrock | 2019 | Topiramate 100 mg | 142 | 13 | ||||||||||||||||
| 49 | Detke | 2018 | Galcanezumab 120 mg | 273 | 2 | 1 | 1 | 1 | |||||||||||||
| 49 | Detke | 2018 | Galcanezumab 240 mg | 282 | 1 | 2 | 2 | 2 | |||||||||||||
| 49 | Detke | 2018 | Placebo | 558 | 2 | 1 | 1 | 1 | |||||||||||||
| 45 | Dodick | 2018 | Erenumab 70 mg | 283 | 2.5 | 1.4 | |||||||||||||||
| 45 | Dodick | 2018 | Placebo | 289 | 4.5 | 2.1 | |||||||||||||||
| 60 | Dodick | 2018 | Fremanezumab-M | 290 | 1.4 | ||||||||||||||||
| 60 | Dodick | 2018 | Fremanezumab-Q | 291 | 2.4 | ||||||||||||||||
| 60 | Dodick | 2018 | Placebo | 293 | 1.7 | ||||||||||||||||
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 1.9 | 1.9 | 2.4 | 1 | |||||||||||||
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 1.4 | 1.4 | 3.6 | 1.8 | |||||||||||||
| 181 | Stauffer | 2018 | Placebo | 432 | 0.7 | 0.7 | 3.5 | 0.5 | |||||||||||||
| 201 | Vladimir | 2018 | Galcanezumab 120 mg | 226 | 3.1 | ||||||||||||||||
| 201 | Vladimir | 2018 | Galcanezumab 240 mg | 228 | 1.3 | ||||||||||||||||
| 201 | Vladimir | 2018 | Placebo | 461 | 2.4 | ||||||||||||||||
| 173 | Silberstein | 2017 | Fremanezumab-Q | 376 | 1 | ||||||||||||||||
| 173 | Silberstein | 2017 | Fremanezumab-M | 379 | 2 | ||||||||||||||||
| 173 | Silberstein | 2017 | Placebo | 375 | 3 | ||||||||||||||||
| 185 | Tepper | 2017 | Erenumab 70 mg | 190 | 2 | 0 | |||||||||||||||
| 185 | Tepper | 2017 | Erenumab 140 mg | 188 | 3 | 4 | |||||||||||||||
| 185 | Tepper | 2017 | Placebo | 282 | 2 | 0.5 | |||||||||||||||
| 77 | Goadsby | 2017 | Erenumab 70 mg | 314 | 2.2 | 1.6 | |||||||||||||||
| 77 | Goadsby | 2017 | Erenumab 140 mg | 319 | 1.9 | 3.4 | |||||||||||||||
| 77 | Goadsby | 2017 | Placebo | 319 | 1.9 | 1.3 | |||||||||||||||
| 88 | Hong Sun | 2016 | Erenumab 7 mg | 108 | 0 | 3 | |||||||||||||||
| 88 | Hong Sun | 2016 | Erenumab 21 mg | 105 | 1 | 1 | |||||||||||||||
| 88 | Hong Sun | 2016 | Erenumab 70 mg | 106 | 1 | 3 | |||||||||||||||
| 88 | Hong Sun | 2016 | Placebo | 153 | 3 | 1 | |||||||||||||||
| 44 | Dodick | 2014 | Galcanezumab 150 mg | 107 | 6 | 4 | 4 | ||||||||||||||
| 44 | Dodick | 2014 | Placebo | 110 | 3 | 1 | 9 | ||||||||||||||
| 94 | Kalita | 2013 | Divalproate 250–1000 mg | 143 | 9.1 | 1.8 | 0 | 12.6 | 2.1 | ||||||||||||
| 94 | Kalita | 2013 | Amitriptyline 50 mg | 144 | 56.5 | 1.1 | 0.7 | 8.3 | 3.6 | ||||||||||||
| 41 | Couch | 2011 | Amitriptyline 100 mg | 194 | 2.1 | 35 | 11.8 | ||||||||||||||
| 41 | Couch | 2011 | Placebo | 197 | 1.5 | 7.2 | 4.1 | ||||||||||||||
| 143 | Lipton | 2011 | Topiramate 100 mg | 176 | 6.3 | 6.8 | 10.8 | ||||||||||||||
| 143 | Lipton | 2011 | Placebo | 185 | 3.2 | 2.7 | 9.2 | ||||||||||||||
| 47 | Dodick | 2009 | Topiramate 100 mg | 177 | 6.8 | 5.1 | 10.2 | 3.4 | |||||||||||||
| 47 | Dodick | 2009 | Amitriptyline 100 mg | 169 | 35.5 | 8.3 | 7.1 | 8.3 | |||||||||||||
| 170 | Silberstein | 2007 | Topiramate 100 mg | 160 | 9.4 | 8.8 | |||||||||||||||
| 170 | Silberstein | 2007 | Placebo | 161 | 3.1 | 8.1 | |||||||||||||||
| 215 | Diener | 2007 | Topiramate 200 mg | 254 | 2 | 4 | |||||||||||||||
| 215 | Diener | 2007 | Placebo | 258 | 2 | 4 | |||||||||||||||
| 53 | Diener | 2002 | Flunarizine 5 mg | 263 | 1.1 | 13 | |||||||||||||||
| 53 | Diener | 2002 | Flunarizine 10 mg | 275 | 1.5 | 17 | |||||||||||||||
| 53 | Diener | 2002 | Propranolol 160 mg | 270 | 1.9 | 8 | |||||||||||||||
| 109 | Lucking | 1988 | Flunarizine 10 mg | 160 | 7.1 | 5.2 | |||||||||||||||
| 109 | Lucking | 1988 | Propranolol 40 mg | 170 | 9.8 | 7.2 |
| Study ID | Author | Year of publication | Intervention | Participants | Anxiety | Agitation | Sleep disorder | Nervousness | Insomnia | Mood swings | Irritability | Confusion | Depressed mood | Depression |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 197 | Reuter | 2021 | Erenumab 140 mg | 388 | 4.1 | 1.5 | 2.1 | 1.3 | 0.3 | 1.5 | ||||
| 197 | Reuter | 2021 | Topiramate 100 mg | 388 | 1.5 | 2.6 | 4.1 | 4.6 | 3.6 | 4.1 | ||||
| 221 | Mulleners | 2020 | Galcanezumab 120 mg | 232 | 2 | |||||||||
| 221 | Mulleners | 2020 | Placebo | 230 | 0 | |||||||||
| 217 | Ferrari | 2019 | Fremanezumab-Q | 276 | 1 | 2 | ||||||||
| 217 | Ferrari | 2019 | Fremanezumab-M | 285 | 0.5 | 2 | ||||||||
| 217 | Ferrari | 2019 | Placebo | 277 | 0 | 0.5 | ||||||||
| 148 | Rothrock | 2019 | BTA 150U | 220 | 2 | |||||||||
| 148 | Rothrock | 2019 | Topiramate 100 mg | 142 | 6 | |||||||||
| 41 | Couch | 2011 | Amitriptyline 100 mg | 194 | 7.2 | 5.2 | 3.6 | 2.1 | ||||||
| 41 | Couch | 2011 | Placebo | 197 | 4.1 | 8.12 | 7.1 | 1 | ||||||
| 143 | Lipton | 2011 | Topiramate 100 mg | 176 | 5.7 | |||||||||
| 143 | Lipton | 2011 | Placebo | 185 | 1.6 | |||||||||
| 215 | Diener | 2007 | Topiramate 200 mg | 254 | 5 | |||||||||
| 215 | Diener | 2007 | Placebo | 258 | 5 | |||||||||
| 53 | Diener | 2002 | Flunarizine 5 mg | 263 | 2.7 | |||||||||
| 53 | Diener | 2002 | Flunarizine 10 mg | 275 | 0.7 | |||||||||
| 53 | Diener | 2002 | Propranolol 160 mg | 270 | 1.9 |
| Study ID | Author | Year of publication | Intervention | Participants | Muscular weakness | Muscle spasms | Muscle tightness | Myalgia | Musculoskeletal stiffness | Back pain | Musculoskeletal pain | Arthralgia | Neck pain | Arm pain |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 555 | Ashina | 2022 | Eptinezumab 100 mg | 299 | 2 | 2 | ||||||||
| 555 | Ashina | 2022 | Eptinezumab 300 mg | 294 | 1 | 1 | ||||||||
| 555 | Ashina | 2022 | Placebo | 298 | 1 | 0 | ||||||||
| 158 | Sakai | 2021 | Fremanezumab-M | 188 | 2.7 | |||||||||
| 158 | Sakai | 2021 | Fremanezumab-Q | 190 | 0.5 | |||||||||
| 158 | Sakai | 2021 | Placebo | 191 | 0.5 | |||||||||
| 157 | Sakai | 2021 | Fremanezumab-M | 121 | 0 | |||||||||
| 157 | Sakai | 2021 | Fremanezumab-Q | 118 | 2.5 | |||||||||
| 157 | Sakai | 2021 | Placebo | 117 | 0 | |||||||||
| 777 | Winner | 2021 | Eptinezumab 100 mg | 238 | 0 | |||||||||
| 777 | Winner | 2021 | Placebo | 242 | 0.8 | |||||||||
| 19 | Ashina | 2020 | Eptinezumab 30 mg | 219 | 1.8 | |||||||||
| 19 | Ashina | 2020 | Eptinezumab 100 mg | 223 | 3.1 | |||||||||
| 19 | Ashina | 2020 | Eptinezumab 300 mg | 224 | 1.3 | |||||||||
| 19 | Ashina | 2020 | Placebo | 222 | 3.2 | |||||||||
| 221 | Mulleners | 2020 | Galcanezumab 120 mg | 232 | 3 | |||||||||
| 221 | Mulleners | 2020 | Placebo | 230 | 2 | |||||||||
| 217 | Ferrari | 2019 | Fremanezumab-Q | 276 | 2 | 0.5 | 0.5 | |||||||
| 217 | Ferrari | 2019 | Fremanezumab-M | 285 | 0.5 | 0.5 | 1 | |||||||
| 217 | Ferrari | 2019 | Placebo | 277 | 2 | 1 | 0 | |||||||
| 148 | Rothrock | 2019 | BTA 150U | 220 | 4 | |||||||||
| 148 | Rothrock | 2019 | Topiramate 100 mg | 142 | 2 | |||||||||
| 49 | Detke | 2018 | Galcanezumab 120 mg | 273 | 3 | 0 | 3 | |||||||
| 49 | Detke | 2018 | Galcanezumab 240 mg | 282 | 1 | 2 | 0 | |||||||
| 49 | Detke | 2018 | Placebo | 558 | 3 | 1 | 1 | |||||||
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 2.4 | 1.5 | ||||||||
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 3.2 | 1.8 | ||||||||
| 181 | Stauffer | 2018 | Placebo | 432 | 1.4 | 0.9 | ||||||||
| 196 | Reuter | 2018 | Erenumab 140 mg | 119 | 4 | 3 | ||||||||
| 196 | Reuter | 2018 | Placebo | 124 | 2 | 0 | ||||||||
| 185 | Tepper | 2017 | Erenumab 70 mg | 190 | < 1 | |||||||||
| 185 | Tepper | 2017 | Erenumab 140 mg | 188 | 4 | |||||||||
| 185 | Tepper | 2017 | Placebo | 282 | 1 | |||||||||
| 77 | Goadsby | 2017 | Erenumab 70 mg | 314 | 1.9 | 2.2 | ||||||||
| 77 | Goadsby | 2017 | Erenumab 140 mg | 319 | 1.9 | 2.2 | ||||||||
| 77 | Goadsby | 2017 | Placebo | 319 | 2.2 | 1.9 | ||||||||
| 88 | Hong Sun | 2016 | Erenumab 7 mg | 108 | 0 | 3 | 1 | |||||||
| 88 | Hong Sun | 2016 | Erenumab 21 mg | 105 | 0 | 0 | ||||||||
| 88 | Hong Sun | 2016 | Erenumab 70 mg | 106 | 0 | 1 | ||||||||
| 88 | Hong Sun | 2016 | Placebo | 153 | 2 | 3 | ||||||||
| 44 | Dodick | 2014 | Galcanezumab 150 mg | 107 | 7 | 6 | 4 | |||||||
| 44 | Dodick | 2014 | Placebo | 110 | 7 | 6 | 2 | |||||||
| 143 | Lipton | 2011 | Topiramate 100 mg | 176 | 5.7 | |||||||||
| 143 | Lipton | 2011 | Placebo | 185 | 5.4 | |||||||||
| 59 | Dodick | 2010 | BTA 150U | 687 | 5.5 | 2.6 | 2.3 | 2.2 | 6.7 | |||||
| 59 | Dodick | 2010 | Placebo | 692 | 0.3 | 0.3 | 0.7 | 0.7 | 2.2 | |||||
| 21 | Aurora | 2006 | BTA 105–260U | 187 | 26.2 | 1.6 | 17.1 | 7.5 | ||||||
| 21 | Aurora | 2006 | Placebo | 182 | 1.1 | 0.5 | 4.4 | 1.1 |
| ID | Author | Year | Intervention | Participants | Neck rigidity | Dysesthesia | Paraesthesia | Hypertonia | Hypoesthesia | Difficulty with memory | Difficulty with concentration |
Taste perversion | Migraine | Dizziness | Aphasia | Dysgeusia | Cognitive disorder | Headache | Somnolence | Drowsiness | Facial paralysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 666 | Hu | 2022 | Galcanezumab 120 mg | 261 | 3.4 | ||||||||||||||||
| 666 | Hu | 2022 | Placebo | 259 | 2.3 | ||||||||||||||||
| 555 | Ashina | 2022 | Eptinezumab 100 mg | 299 | 1 | ||||||||||||||||
| 555 | Ashina | 2022 | Eptinezumab 300 mg | 294 | 1 | ||||||||||||||||
| 555 | Ashina | 2022 | Placebo | 298 | 2 | ||||||||||||||||
| 8 | Ailani | 2021 | Atogepant 10 mg | 221 | 3.2 | ||||||||||||||||
| 8 | Ailani | 2021 | Atogepant 30 mg | 228 | 1.8 | ||||||||||||||||
| 8 | Ailani | 2021 | Atogepant 60 mg | 231 | 1.7 | ||||||||||||||||
| 8 | Ailani | 2021 | Placebo | 222 | 0.9 | ||||||||||||||||
| 157 | Sakai | 2021 | Fremanezumab-M | 121 | 0 | 0 | 1.7 | ||||||||||||||
| 157 | Sakai | 2021 | Fremanezumab-Q | 118 | 0 | 0.8 | 1.7 | ||||||||||||||
| 157 | Sakai | 2021 | Placebo | 117 | 2.6 | 2.6 | 3.4 | ||||||||||||||
| 197 | Reuter | 2021 | Erenumab 140 mg | 388 | 0.5 | 4.4 | 0.5 | 0.3 | 4.6 | 0 | 5.2 | 0.5 | 0.8 | 0.5 | |||||||
| 197 | Reuter | 2021 | Topiramate 100 mg | 388 | 2.1 | 39.9 | 3.4 | 2.6 | 16.2 | 6.2 | 13.1 | 2.8 | 5.7 | 2.1 | |||||||
| 203 | Wang | 2021 | Erenumab 70 mg | 335 | 0.9 | ||||||||||||||||
| 203 | Wang | 2021 | Erenumab 140 mg | 224 | 3.1 | ||||||||||||||||
| 203 | Wang | 2021 | Placebo | 335 | 1.8 | ||||||||||||||||
| 105 | Lipton | 2020 | Eptinezumab 100 mg | 356 | 1.7 | ||||||||||||||||
| 105 | Lipton | 2020 | Eptinezumab 300 mg | 350 | 2.3 | ||||||||||||||||
| 105 | Lipton | 2020 | Placebo | 366 | 4.4 | ||||||||||||||||
| 19 | Ashina | 2020 | Eptinezumab 30 mg | 219 | 3.7 | ||||||||||||||||
| 19 | Ashina | 2020 | Eptinezumab 100 mg | 223 | 4.5 | ||||||||||||||||
| 19 | Ashina | 2020 | Eptinezumab 300 mg | 224 | 1.8 | ||||||||||||||||
| 19 | Ashina | 2020 | Placebo | 222 | 3.6 | ||||||||||||||||
| 221 | Mulleners | 2020 | Galcanezumab 120 mg | 232 | 2 | ||||||||||||||||
| 221 | Mulleners | 2020 | Placebo | 230 | 0 | ||||||||||||||||
| 61 | Dodick | 2019 | Eptinezumab 100 mg | 122 | 5.7 | 9.8 | |||||||||||||||
| 61 | Dodick | 2019 | Eptinezumab 300 mg | 121 | 0.8 | 1.7 | |||||||||||||||
| 61 | Dodick | 2019 | Eptinezumab 30 mg | 122 | 2.5 | 2.5 | |||||||||||||||
| 61 | Dodick | 2019 | Eptinezumab 10 mg | 130 | 1.5 | 8.5 | |||||||||||||||
| 61 | Dodick | 2019 | Placebo | 121 | 1.7 | 7.4 | |||||||||||||||
| 217 | Ferrari | 2019 | Fremanezumab-Q | 276 | 0.5 | 2 | |||||||||||||||
| 217 | Ferrari | 2019 | Fremanezumab-M | 285 | 1 | 1 | |||||||||||||||
| 217 | Ferrari | 2019 | Placebo | 277 | 3 | 1 | |||||||||||||||
| 148 | Rothrock | 2019 | BTA 150U | 220 | 0.5 | 0 | 3 | 3 | 5 | ||||||||||||
| 148 | Rothrock | 2019 | Topiramate 100 mg | 142 | 31 | 8 | 2 | 13 | 13 | ||||||||||||
| 49 | Detke | 2018 | Galcanezumab 120 mg | 273 | 2 | ||||||||||||||||
| 49 | Detke | 2018 | Galcanezumab 240 mg | 282 | 1 | ||||||||||||||||
| 49 | Detke | 2018 | Placebo | 558 | 1 | ||||||||||||||||
| 45 | Dodick | 2018 | Erenumab 70 mg | 283 | 2.1 | ||||||||||||||||
| 45 | Dodick | 2018 | Placebo | 289 | 2.8 | ||||||||||||||||
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 1 | 2.6 | |||||||||||||||
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 2.3 | 2.3 | |||||||||||||||
| 181 | Stauffer | 2018 | Placebo | 432 | 0.9 | 2.6 | |||||||||||||||
| 201 | Vladimir | 2018 | Galcanezumab 120 mg | 226 | 3.5 | ||||||||||||||||
| 201 | Vladimir | 2018 | Galcanezumab 240 mg | 228 | 3.1 | ||||||||||||||||
| 201 | Vladimir | 2018 | Placebo | 461 | 2.2 | ||||||||||||||||
| 196 | Reuter | 2018 | Erenumab 140 mg | 119 | 3 | ||||||||||||||||
| 196 | Reuter | 2018 | Placebo | 124 | 2 | ||||||||||||||||
| 173 | Silberstein | 2017 | Fremanezumab-Q | 376 | 2 | ||||||||||||||||
| 173 | Silberstein | 2017 | Fremanezumab-M | 379 | 3 | ||||||||||||||||
| 173 | Silberstein | 2017 | Placebo | 375 | 1 | ||||||||||||||||
| 185 | Tepper | 2017 | Erenumab 70 mg | 190 | 2 | ||||||||||||||||
| 185 | Tepper | 2017 | Erenumab 140 mg | 188 | 3 | ||||||||||||||||
| 185 | Tepper | 2017 | Placebo | 282 | 1 | ||||||||||||||||
| 77 | Goadsby | 2017 | Erenumab 70 mg | 314 | 1.3 | ||||||||||||||||
| 77 | Goadsby | 2017 | Erenumab 140 mg | 319 | 0.9 | ||||||||||||||||
| 77 | Goadsby | 2017 | Placebo | 319 | 3.1 | ||||||||||||||||
| 88 | Hong Sun | 2016 | Erenumab 7 mg | 108 | 1 | 4 | |||||||||||||||
| 88 | Hong Sun | 2016 | Erenumab 21 mg | 105 | 3 | 1 | |||||||||||||||
| 88 | Hong Sun | 2016 | Erenumab 70 mg | 106 | 3 | 3 | |||||||||||||||
| 88 | Hong Sun | 2016 | Placebo | 153 | 1 | 1 | |||||||||||||||
| 44 | Dodick | 2014 | Galcanezumab 150 mg | 107 | 5 | ||||||||||||||||
| 44 | Dodick | 2014 | Placebo | 110 | 3 | ||||||||||||||||
| 94 | Kalita | 2013 | Divalproate 250–1000 mg | 143 | 4.9 | ||||||||||||||||
| 94 | Kalita | 2013 | Amitriptyline 50 mg | 144 | 47.3 | ||||||||||||||||
| 41 | Couch | 2011 | Amitriptyline 100 mg | 194 | 1.5 | 10.1 | 27.3 | ||||||||||||||
| 41 | Couch | 2011 | Placebo | 197 | 1 | 5.6 | 8.6 | ||||||||||||||
| 143 | Lipton | 2011 | Topiramate 100 mg | 176 | 32.4 | 6.8 | 9.7 | 11.4 | 5.1 | ||||||||||||
| 143 | Lipton | 2011 | Placebo | 185 | 0.7 | 2.7 | 1.6 | 7.6 | 1.6 | ||||||||||||
| 59 | Dodick | 2010 | BTA 150U | 687 | 2.9 | ||||||||||||||||
| 59 | Dodick | 2010 | Placebo | 692 | 1.6 | ||||||||||||||||
| 47 | Dodick | 2009 | Topiramate 100 mg | 177 | 29.9 | 10.7 | 6.8 | 5.6 | 8.5 | 5.1 | 11.9 | ||||||||||
| 47 | Dodick | 2009 | Amitriptyline 100 mg | 169 | 4.7 | 3.6 | 3 | 3.6 | 10.7 | 0 | 17.8 | ||||||||||
| 170 | Silberstein | 2007 | Topiramate 100 mg | 160 | 28.8 | 9.4 | 6.9 | 9.4 | 9.4 | 3.8 | 5.6 | ||||||||||
| 170 | Silberstein | 2007 | Placebo | 161 | 7.5 | 0 | 6.2 | 2.5 | 2.5 | 7.5 | 4.3 | ||||||||||
| 215 | Diener | 2007 | Topiramate 200 mg | 254 | 30 | 4 | 0.7 | 3 | |||||||||||||
| 215 | Diener | 2007 | Placebo | 258 | 21 | 5 | 0.7 | 4 | |||||||||||||
| 21 | Aurora | 2006 | BTA 105 to 260U | 187 | 10.2 | 2.1 | 7 | 3.7 | 3.2 | 2.1 | 5.9 | 1.6 | |||||||||
| 21 | Aurora | 2006 | Placebo | 182 | 3.3 | 0 | 1.1 | 1.6 | 0.5 | 0 | 4.9 | 0 | |||||||||
| 216 | Elkind (study 1) | 2006 | BTA 7U | 105 | 1 | ||||||||||||||||
| 216 | Elkind (study 1) | 2006 | BTA 25U | 101 | 2 | ||||||||||||||||
| 216 | Elkind (study 1) | 2006 | BTA 50U | 106 | 7.6 | ||||||||||||||||
| 216 | Elkind (study 1) | 2006 | Placebo | 106 | 1.9 | ||||||||||||||||
| 216 | Elkind (study 2) | 2006 | BTA 25U | 173 | 1.2 | 4.6 | |||||||||||||||
| 216 | Elkind (study 2) | 2006 | BTA 50U | 180 | 5 | 5.6 | |||||||||||||||
| 216 | Elkind (study 3) | 2006 | BTA 25U | 50 | 0 | ||||||||||||||||
| 216 | Elkind (study 3) | 2006 | BTA 50U | 51 | 5.9 | ||||||||||||||||
| 216 | Elkind (study 3) | 2006 | Placebo | 100 | 0 | ||||||||||||||||
| 53 | Diener | 2002 | Flunarizine 5 mg | 263 | 1.5 | 1.9 | |||||||||||||||
| 53 | Diener | 2002 | Flunarizine 10 mg | 275 | 1.1 | 2.5 | |||||||||||||||
| 53 | Diener | 2002 | Propranolol 160 mg | 270 | 3.3 | 0.7 | |||||||||||||||
| 109 | Lucking | 1988 | Flunarizine 10 mg | 160 | 2.4 | ||||||||||||||||
| 109 | Lucking | 1988 | Propranolol 40 mg | 170 | 2.2 |
| Study ID | Author | Year of publication | Intervention | Participants | Infection | Nasopharyngitis | Sinus infection | Sinusitis | Upper respiratory tract infection | Urinary tract infection | Cystitis | Influenza | Pyrexia | COVID-19 | Viral infection | Viral gastroenteritis | Flu syndrome | Gastroenteritis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 666 | Hu | 2022 | Galcanezumab 120 mg | 261 | 2.7 | 5.4 | 2.3 | |||||||||||
| 666 | Hu | 2022 | Placebo | 259 | 3.5 | 5 | 1.2 | |||||||||||
| 555 | Ashina | 2022 | Eptinezumab 100 mg | 299 | 2 | 0.33 | 7 | |||||||||||
| 555 | Ashina | 2022 | Eptinezumab 300 mg | 294 | 3 | 2 | 6 | |||||||||||
| 555 | Ashina | 2022 | Placebo | 298 | 1 | 1 | 5 | |||||||||||
| 158 | Sakai | 2021 | Fremanezumab-M | 188 | 16.6 | 0 | 2.1 | |||||||||||
| 158 | Sakai | 2021 | Fremanezumab-Q | 190 | 21.1 | 2.5 | 1.1 | |||||||||||
| 158 | Sakai | 2021 | Placebo | 191 | 18.8 | 1 | 1.6 | |||||||||||
| 8 | Ailani | 2021 | Atogepant 10 mg | 221 | 1.8 | 1.8 | 4.1 | 1.4 | 1.4 | 0.9 | ||||||||
| 8 | Ailani | 2021 | Atogepant 30 mg | 228 | 3.5 | 1.3 | 5.7 | 3.9 | 0.9 | 2.2 | ||||||||
| 8 | Ailani | 2021 | Atogepant 60 mg | 231 | 3.5 | 2.2 | 3.9 | 3.9 | 2.2 | 1.3 | ||||||||
| 8 | Ailani | 2021 | Placebo | 222 | 3.6 | 1.4 | 4.5 | 3.6 | 0.9 | 1.8 | ||||||||
| 157 | Sakai | 2021 | Fremanezumab-M | 121 | 14 | 5 | ||||||||||||
| 157 | Sakai | 2021 | Fremanezumab-Q | 118 | 12.7 | 1.7 | ||||||||||||
| 157 | Sakai | 2021 | Placebo | 117 | 13.7 | 0.9 | ||||||||||||
| 203 | Wang | 2021 | Erenumab 70 mg | 335 | 0.6 | 2.7 | 3 | |||||||||||
| 203 | Wang | 2021 | Erenumab 140 mg | 224 | 3.6 | 1.8 | 2.2 | |||||||||||
| 203 | Wang | 2021 | Placebo | 335 | 2.4 | 2.1 | 4.5 | |||||||||||
| 777 | Winner | 2021 | Eptinezumab 100 mg | 238 | 0.8 | 0.8 | ||||||||||||
| 777 | Winner | 2021 | Placebo | 242 | 0.8 | 0.8 | ||||||||||||
| 105 | Lipton | 2020 | Eptinezumab 100 mg | 356 | 5.3 | 2 | 4.2 | 2.2 | ||||||||||
| 105 | Lipton | 2020 | Eptinezumab 300 mg | 350 | 9.4 | 2.6 | 5.4 | 3.4 | ||||||||||
| 105 | Lipton | 2020 | Placebo | 366 | 6 | 4.1 | 5.5 | 1.6 | ||||||||||
| 19 | Ashina | 2020 | Eptinezumab 30 mg | 219 | 6.4 | 3.2 | 11.4 | 1.4 | ||||||||||
| 19 | Ashina | 2020 | Eptinezumab 100 mg | 223 | 7.6 | 2.7 | 9.9 | 1.8 | ||||||||||
| 19 | Ashina | 2020 | Eptinezumab 300 mg | 224 | 6.3 | 4.9 | 10.3 | 3.6 | ||||||||||
| 19 | Ashina | 2020 | Placebo | 222 | 5.4 | 6.3 | 7.2 | 2.3 | ||||||||||
| 156 | Sakai | 2020 | Galcanezumab 120 mg | 115 | 7.8 | |||||||||||||
| 156 | Sakai | 2020 | Galcanezumab 240 mg | 114 | 0.9 | |||||||||||||
| 156 | Sakai | 2020 | Placebo | 230 | 1.3 | |||||||||||||
| 221 | Mulleners | 2020 | Galcanezumab 120 mg | 232 | 9 | 2 | 2 | 2 | 3 | 1 | ||||||||
| 221 | Mulleners | 2020 | Placebo | 230 | 7 | 2 | 2 | 1 | 5 | 2 | ||||||||
| 888 | Croop | 2020 | Rimegepant 75 mg | 370 | 4 | 2 | 2 | |||||||||||
| 888 | Croop | 2020 | Placebo | 371 | 2 | 3 | 2 | |||||||||||
| 61 | Dodick | 2019 | Eptinezumab 100 mg | 122 | 6.6 | 2.5 | 6.6 | |||||||||||
| 61 | Dodick | 2019 | Eptinezumab 300 mg | 121 | 7.4 | 6.6 | 10.7 | |||||||||||
| 61 | Dodick | 2019 | Eptinezumab 30 mg | 122 | 2.5 | 4.9 | 5.7 | |||||||||||
| 61 | Dodick | 2019 | Eptinezumab 10 mg | 130 | 4.6 | 6.2 | 6.9 | |||||||||||
| 61 | Dodick | 2019 | Placebo | 121 | 5 | 5 | 5 | |||||||||||
| 217 | Ferrari | 2019 | Fremanezumab-Q | 276 | 5 | 1 | 1 | 0.5 | 1 | |||||||||
| 217 | Ferrari | 2019 | Fremanezumab-M | 285 | 2 | 3 | 1 | 2 | 1 | |||||||||
| 217 | Ferrari | 2019 | Placebo | 277 | 4 | 1 | 2 | 0.5 | 3 | |||||||||
| 148 | Rothrock | 2019 | BTA 150U | 220 | 6 | |||||||||||||
| 148 | Rothrock | 2019 | Topiramate 100 mg | 142 | 7 | |||||||||||||
| 49 | Detke | 2018 | Galcanezumab 120 mg | 273 | 6 | 1 | 3 | 2 | 2 | 2 | ||||||||
| 49 | Detke | 2018 | Galcanezumab 240 mg | 282 | 3 | 3 | 3 | 1 | 1 | 0 | ||||||||
| 49 | Detke | 2018 | Placebo | 558 | 5 | 1 | 2 | 1 | 1 | 2 | ||||||||
| 45 | Dodick | 2018 | Erenumab 70 mg | 283 | 5.3 | 2.1 | 6.4 | 3.9 | ||||||||||
| 45 | Dodick | 2018 | Placebo | 289 | 5.9 | 2.1 | 4.8 | 3.5 | ||||||||||
| 60 | Dodick | 2018 | Fremanezumab-M | 290 | 3.8 | 1.4 | 5.5 | 2.4 | ||||||||||
| 60 | Dodick | 2018 | Fremanezumab-Q | 291 | 3.8 | 0.7 | 3.8 | 3.4 | ||||||||||
| 60 | Dodick | 2018 | Placebo | 293 | 3.1 | 2.7 | 5.1 | 1.4 | ||||||||||
| 181 | Stauffer | 2018 | Galcanezumab 120 mg | 206 | 7.8 | 4.6 | 3.9 | 2.4 | ||||||||||
| 181 | Stauffer | 2018 | Galcanezumab 240 mg | 220 | 2.7 | 3.6 | 5.9 | 1.8 | ||||||||||
| 181 | Stauffer | 2018 | Placebo | 432 | 6.3 | 3 | 3.5 | 1.2 | ||||||||||
| 201 | Vladimir | 2018 | Galcanezumab 120 mg | 226 | 8.4 | 5.8 | 1.3 | |||||||||||
| 201 | Vladimir | 2018 | Galcanezumab 240 mg | 228 | 7 | 5.3 | 4.4 | |||||||||||
| 201 | Vladimir | 2018 | Placebo | 461 | 8.9 | 3.5 | 3 | |||||||||||
| 196 | Reuter | 2018 | Erenumab 140 mg | 119 | 4 | 3 | ||||||||||||
| 196 | Reuter | 2018 | Placebo | 124 | 10 | 0 | ||||||||||||
| 173 | Silberstein | 2017 | Fremanezumab-Q | 376 | 5 | 3 | 5 | |||||||||||
| 173 | Silberstein | 2017 | Fremanezumab-M | 379 | 4 | 1 | 4 | |||||||||||
| 173 | Silberstein | 2017 | Placebo | 375 | 5 | 3 | 4 | |||||||||||
| 185 | Tepper | 2017 | Erenumab 70 mg | 190 | 3 | 3 | ||||||||||||
| 185 | Tepper | 2017 | Erenumab 140 mg | 188 | 2 | 3 | ||||||||||||
| 185 | Tepper | 2017 | Placebo | 282 | 6 | 1 | ||||||||||||
| 77 | Goadsby | 2017 | Erenumab 70 mg | 314 | 9.9 | 2.2 | 6.7 | 1.6 | 1.3 | |||||||||
| 77 | Goadsby | 2017 | Erenumab 140 mg | 319 | 11 | 3.4 | 4.7 | 2.2 | 2.5 | |||||||||
| 77 | Goadsby | 2017 | Placebo | 319 | 10 | 2.2 | 5.6 | 2.2 | 1.9 | |||||||||
| 88 | Hong Sun | 2016 | Erenumab 7 mg | 108 | 9 | 1 | 1 | |||||||||||
| 88 | Hong Sun | 2016 | Erenumab 21 mg | 105 | 5 | 2 | 4 | |||||||||||
| 88 | Hong Sun | 2016 | Erenumab 70 mg | 106 | 6 | 3 | 1 | |||||||||||
| 88 | Hong Sun | 2016 | Placebo | 153 | 8 | 2 | 3 | |||||||||||
| 44 | Dodick | 2014 | Galcanezumab 150 mg | 107 | 4 | 3 | 17 | 2 | ||||||||||
| 44 | Dodick | 2014 | Placebo | 110 | 7 | 5 | 9 | 4 | ||||||||||
| 94 | Kalita | 2013 | Divalproate 250–1000 mg | 143 | 1.5 | |||||||||||||
| 94 | Kalita | 2013 | Amitriptyline 50 mg | 144 | 1.1 | |||||||||||||
| 143 | Lipton | 2011 | Topiramate 100 mg | 176 | 9.1 | 9.1 | 9.7 | |||||||||||
| 143 | Lipton | 2011 | Placebo | 185 | 8.1 | 6.5 | 9.2 | |||||||||||
| 47 | Dodick | 2009 | Topiramate 100 mg | 177 | 7.9 | 7.9 | 7.9 | |||||||||||
| 47 | Dodick | 2009 | Amitriptyline 100 mg | 169 | 10.7 | 6.5 | 6.5 | |||||||||||
| 170 | Silberstein | 2007 | Topiramate 100 mg | 160 | 4.4 | 13.8 | ||||||||||||
| 170 | Silberstein | 2007 | Placebo | 161 | 5 | 12.4 | ||||||||||||
| 216 | Elkind (study 1) | 2006 | BTA 7U | 105 | 3.8 | 11.4 | 10.5 | |||||||||||
| 216 | Elkind (study 1) | 2006 | BTA 25U | 101 | 6.9 | 9.9 | 4 | |||||||||||
| 216 | Elkind (study 1) | 2006 | BTA 50U | 106 | 3.8 | 10.4 | 6.6 | |||||||||||
| 216 | Elkind (study 1) | 2006 | Placebo | 106 | 2.8 | 11.3 | 8.5 | |||||||||||
| 216 | Elkind (study 2) | 2006 | BTA 25U | 173 | 11.6 | 9.2 | 8.1 | 6.9 | ||||||||||
| 216 | Elkind (study 2) | 2006 | BTA 50U | 180 | 8.3 | 8.3 | 6.7 | 7.8 | ||||||||||
| 216 | Elkind (study 3) | 2006 | BTA 25U | 50 | 6 | 4 | 12 | 4 | ||||||||||
| 216 | Elkind (study 3) | 2006 | BTA 50U | 51 | 5.9 | 7.8 | 15.7 | 5.9 | ||||||||||
| 216 | Elkind (study 3) | 2006 | Placebo | 100 | 3 | 4 | 9 | 7 | ||||||||||
| 53 | Diener | 2002 | Flunarizine 5 mg | 263 | 4.5 | |||||||||||||
| 53 | Diener | 2002 | Flunarizine 10 mg | 275 | 6.5 | |||||||||||||
| 53 | Diener | 2002 | Propranolol 160 mg | 270 |
| Study ID | Author | Year | Intervention | Participants | Influenza-like illness | I-S pain | I-S reaction | I-S haemorrhage | Pain | Pain in extremity | I-S rash | I-S paraesthesia | I-S bruising | Infusion-S extravasation | I-S discoloration | I-S discomfort | I-S induration | I-S warmth | I-S pruritus | I-S oedema | I-S erythema | I-S swelling | Asthenia | Fatigue | Non-cardiac chest pain | I-S hypersensitivity | I-S haematoma |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 666 | Hu | 2022 | GAL 120 | 261 | 7.3 | 3.8 | 2.3 | 5 | 1.9 | ||||||||||||||||||
| 666 | Hu | 2022 | PBO | 259 | 6.2 | 0.4 | 0 | 0 | 0 | ||||||||||||||||||
| 555 | Ashina | 2022 | EPT 100 | 299 | 1 | ||||||||||||||||||||||
| 555 | Ashina | 2022 | EPT 300 | 294 | 2 | ||||||||||||||||||||||
| 555 | Ashina | 2022 | PBO | 298 | 1 | ||||||||||||||||||||||
| 158 | Sakai | 2021 | FRE-M | 188 | 7.4 | 29.3 | 17.6 | 5.3 | 15.4 | ||||||||||||||||||
| 158 | Sakai | 2021 | FRE-Q | 190 | 12.6 | 26.8 | 12.1 | 1.6 | 12.1 | ||||||||||||||||||
| 158 | Sakai | 2021 | PBO | 191 | 8.9 | 25.1 | 12.6 | 2.6 | 11 | ||||||||||||||||||
| 8 | Ailani | 2021 | ATO 10 | 221 | 1.4 | ||||||||||||||||||||||
| 8 | Ailani | 2021 | ATO 30 | 228 | 3.1 | ||||||||||||||||||||||
| 8 | Ailani | 2021 | ATO 60 | 231 | 3.9 | ||||||||||||||||||||||
| 8 | Ailani | 2021 | PBO | 222 | 1.8 | ||||||||||||||||||||||
| 157 | Sakai | 2021 | FRE-M | 121 | 9.1 | 25.6 | 0.8 | 14.9 | 5.8 | 15.7 | 3.3 | ||||||||||||||||
| 157 | Sakai | 2021 | FRE-Q | 118 | 13.6 | 29.7 | 3.4 | 11.9 | 1.7 | 11.9 | 1.7 | ||||||||||||||||
| 157 | Sakai | 2021 | PBO | 117 | 6 | 21.4 | 0.9 | 10.3 | 0 | 12.8 | 0 | ||||||||||||||||
| 197 | Reuter | 2021 | ERE 140 | 388 | 9.8 | ||||||||||||||||||||||
| 197 | Reuter | 2021 | TOP 100 | 388 | 17.3 | ||||||||||||||||||||||
| 203 | Wang | 2021 | ERE 70 | 335 | 1.2 | ||||||||||||||||||||||
| 203 | Wang | 2021 | ERE 140 | 224 | 0.4 | ||||||||||||||||||||||
| 203 | Wang | 2021 | PBO | 335 | 2.4 | ||||||||||||||||||||||
| 777 | Winner | 2021 | EPT 100 | 238 | 0.8 | 2.1 | |||||||||||||||||||||
| 777 | Winner | 2021 | PBO | 242 | 0.8 | 0 | |||||||||||||||||||||
| 105 | Lipton | 2020 | EPT 100 | 356 | 2.2 | ||||||||||||||||||||||
| 105 | Lipton | 2020 | EPT 300 | 350 | 1.7 | ||||||||||||||||||||||
| 105 | Lipton | 2020 | PBO | 366 | 1.9 | ||||||||||||||||||||||
| 19 | Ashina | 2020 | EPT 30 | 219 | 2.3 | ||||||||||||||||||||||
| 19 | Ashina | 2020 | EPT 100 | 223 | 3.6 | ||||||||||||||||||||||
| 19 | Ashina | 2020 | EPT 300 | 224 | 3.6 | ||||||||||||||||||||||
| 19 | Ashina | 2020 | PBO | 222 | < 1 | ||||||||||||||||||||||
| 156 | Sakai | 2020 | GAL 120 | 115 | 6.1 | 8.7 | 14.8 | 10.4 | |||||||||||||||||||
| 156 | Sakai | 2020 | GAL 240 | 114 | 7 | 20.2 | 27.2 | 10.5 | |||||||||||||||||||
| 156 | Sakai | 2020 | PBO | 230 | 1.3 | 0 | 2.2 | 1.3 | |||||||||||||||||||
| 221 | Mulleners | 2020 | GAL 120 | 232 | 6 | 3 | 1 | 2 | 2 | 0 | 0 | 3 | 0 | ||||||||||||||
| 221 | Mulleners | 2020 | PBO | 230 | 2 | 0 | 0 | 1 | 1 | 3 | 2 | 0 | |||||||||||||||
| 217 | Ferrari | 2019 | FRE-Q | 276 | 4 | 0.5 | 1 | 1 | 0.5 | 4 | 0.5 | 1 | 7 | 0.5 | 3 | ||||||||||||
| 217 | Ferrari | 2019 | FRE-M | 285 | 3 | 1 | 1 | 1 | 2 | 5 | 1 | 2 | 6 | 1 | 3 | ||||||||||||
| 217 | Ferrari | 2019 | PBO | 277 | 3 | 1 | 0.5 | 1 | 0.5 | 4 | 0 | 1 | 5 | 1 | 1 | ||||||||||||
| 148 | Rothrock | 2019 | BTA 150 | 220 | 0.5 | ||||||||||||||||||||||
| 148 | Rothrock | 2019 | TOP 100 | 142 | 13 | ||||||||||||||||||||||
| 49 | Detke | 2018 | GAL 120 | 273 | 6 | 3 | 0 | 1 | 2 | ||||||||||||||||||
| 49 | Detke | 2018 | GAL 240 | 282 | 7 | 5 | 2 | 5 | 2 | ||||||||||||||||||
| 49 | Detke | 2018 | PBO | 558 | 4 | 2 | 0 | 1 | 2 | ||||||||||||||||||
| 45 | Dodick | 2018 | ERE 70 | 283 | 6 | 3.5 | |||||||||||||||||||||
| 45 | Dodick | 2018 | PBO | 289 | 4.2 | 2.1 | |||||||||||||||||||||
| 60 | Dodick | 2018 | FRE-M | 290 | 30 | 1 | 24 | 17.9 | 0.7 | ||||||||||||||||||
| 60 | Dodick | 2018 | FRE-Q | 291 | 29.6 | 3.1 | 19 | 18.9 | 2.1 | ||||||||||||||||||
| 60 | Dodick | 2018 | PBO | 293 | 25.9 | 2 | 15 | 14 | 1.4 | ||||||||||||||||||
| 181 | Stauffer | 2018 | GAL 120 | 206 | 16 | 3.4 | 1 | 4.4 | 4.9 | ||||||||||||||||||
| 181 | Stauffer | 2018 | GAL 240 | 220 | 20.5 | 5.5 | 1.8 | 4.6 | 4.1 | ||||||||||||||||||
| 181 | Stauffer | 2018 | PBO | 432 | 17.4 | 0.9 | 1.4 | 0.2 | 2.6 | ||||||||||||||||||
| 201 | Vladimir | 2018 | GAL 120 | 226 | 9.3 | 3.1 | 2.7 | 2.7 | 2.2 | 2.7 | |||||||||||||||||
| 201 | Vladimir | 2018 | GAL 240 | 228 | 8.8 | 7.9 | 3.1 | 3.1 | 0.4 | 2.2 | |||||||||||||||||
| 201 | Vladimir | 2018 | PBO | 461 | 8.5 | 0 | 0 | 0.9 | 0 | 2.6 | |||||||||||||||||
| 196 | Reuter | 2018 | ERE 140 | 119 | 6 | 3 | 3 | ||||||||||||||||||||
| 196 | Reuter | 2018 | PBO | 124 | 6 | 3 | 2 | ||||||||||||||||||||
| 173 | Silberstein | 2017 | FRE-Q | 376 | 30 | 2 | 20 | 21 | |||||||||||||||||||
| 173 | Silberstein | 2017 | FRE-M | 379 | 26 | 2 | 24 | 20 | |||||||||||||||||||
| 173 | Silberstein | 2017 | PBO | 375 | 28 | 3 | 18 | 16 | |||||||||||||||||||
| 185 | Tepper | 2017 | ERE 70 | 190 | 4 | ||||||||||||||||||||||
| 185 | Tepper | 2017 | ERE 140 | 188 | 4 | ||||||||||||||||||||||
| 185 | Tepper | 2017 | PBO | 282 | 1 | ||||||||||||||||||||||
| 77 | Goadsby | 2017 | ERE 70 | 314 | 3.2 | 1.9 | |||||||||||||||||||||
| 77 | Goadsby | 2017 | ERE 140 | 319 | 0.3 | 2.2 | |||||||||||||||||||||
| 77 | Goadsby | 2017 | PBO | 319 | 0.3 | 2.5 | |||||||||||||||||||||
| 88 | Hong Sun | 2016 | ERE 7 | 108 | 5 | ||||||||||||||||||||||
| 88 | Hong Sun | 2016 | ERE 21 | 105 | 2 | ||||||||||||||||||||||
| 88 | Hong Sun | 2016 | ERE 70 | 106 | 4 | ||||||||||||||||||||||
| 88 | Hong Sun | 2016 | PBO | 153 | 2 | ||||||||||||||||||||||
| 44 | Dodick | 2014 | GAL 150 | 107 | 17 | 4 | 5 | ||||||||||||||||||||
| 44 | Dodick | 2014 | PBO | 110 | 6 | 5 | 0 | ||||||||||||||||||||
| 41 | Couch | 2011 | AMI 100 | 194 | 8.2 | 7.7 | 1.5 | ||||||||||||||||||||
| 41 | Couch | 2011 | PBO | 197 | 4.5 | 4.1 | 0.5 | ||||||||||||||||||||
| 143 | Lipton | 2011 | TOP 100 | 176 | 14.8 | ||||||||||||||||||||||
| 143 | Lipton | 2011 | PBO | 185 | 8.6 | ||||||||||||||||||||||
| 47 | Dodick | 2009 | TOP 100 | 177 | 16.9 | ||||||||||||||||||||||
| 47 | Dodick | 2009 | AMI 100 | 169 | 24.3 | ||||||||||||||||||||||
| 170 | Silberstein | 2007 | TOP 100 | 160 | 11.9 | ||||||||||||||||||||||
| 170 | Silberstein | 2007 | PBO | 161 | 9.9 | ||||||||||||||||||||||
| 215 | Diener | 2007 | TOP 200 | 254 | 7 | ||||||||||||||||||||||
| 215 | Diener | 2007 | PBO | 258 | 4 | ||||||||||||||||||||||
| 21 | Aurora | 2006 | BTA 260 | 187 | 2.1 | 0 | 1.6 | ||||||||||||||||||||
| 21 | Aurora | 2006 | PBO | 182 | 0.5 | 2.2 | 0 | ||||||||||||||||||||
| 216 | Elkind (study 2) | 2006 | BTA 25 | 173 | 5.2 | 7.5 | |||||||||||||||||||||
| 216 | Elkind (study 2) | 2006 | BTA 50 | 180 | 2.2 | 7.8 | |||||||||||||||||||||
| 216 | Elkind (study 3) | 2006 | BTA 25 | 50 | 4 | ||||||||||||||||||||||
| 216 | Elkind (study 3) | 2006 | BTA 50 | 51 | 2 | ||||||||||||||||||||||
| 216 | Elkind (study 3) | 2006 | PBO | 100 | 5 | ||||||||||||||||||||||
| 53 | Diener | 2002 | FLU 5 | 263 | 3.8 | ||||||||||||||||||||||
| 53 | Diener | 2002 | FLU 10 | 275 | 1.5 | ||||||||||||||||||||||
| 53 | Diener | 2002 | PRO 160 | 270 | 3.7 | ||||||||||||||||||||||
| 109 | Lucking | 1988 | FLU 10 | 160 | 8.1 | ||||||||||||||||||||||
| 109 | Lucking | 1988 | PRO 40 | 170 | 8.1 |
| Intervention | Dose | Frequency | Total participants | Participants with AEs (%)a |
|---|---|---|---|---|
| Erenumab36,45,142–144 | 140 mg | Monthly | 1238 | 408 (33) |
| Rimegepant148 | 75 mg | Once daily | 370 | 133 (36) |
| Topiramate88,135,142 | 100 mg | Twice daily | 707 | 264 (37) |
| Eptinezumab89,94,131,149,151 | 100 mg | Single dose on day 0 | 1238 | 517 (42) |
| Erenumab36,45,130,134,144 | 70 mg | Monthly | 1228 | 574 (47) |
| Erenumab130 | 7 mg | Monthly | 108 | 54 (50) |
| Erenumab130 | 21 mg | Monthly | 105 | 54 (51) |
| Eptinezumab89,94,131,149,151 | 300 mg | Single dose on day 0 | 989 | 509 (51) |
| Placebo35–37,45,89–91,94,95,97,126,127,129–131,133,134,140,141,143,144,146,148–151 | – | Matched with active treatments | 7569 | 3831 (51) |
| Atogepant129 | 30 mg | Once daily | 228 | 119 (52) |
| Atogepant129 | 10 mg | Once daily | 221 | 117 (53) |
| Atogepant129 | 60 mg | Once daily | 231 | 124 (54) |
| Eptinezumab89,131 | 30 mg | Single dose on day 0 | 341 | 184 (54) |
| Eptinezumab89 | 10 mg | Single dose on day 0 | 130 | 74 (57) |
| OnabotulinumtoxinA (BTA)88,97 | 150U | Every 12 weeks | 907 | 534 (59) |
| Galcanezumab95,127,140,141,146,150 | 120 mg | Monthly | 1313 | 786 (60) |
| Fremanezumab35,37,90,91,126 | Monthly (225 mg) |
Monthly | 1263 | 774 (61) |
| Fremanezumab35,37,90,91,126 | Quarterly (675 mg) |
Single dose on day 0 | 1251 | 798 (64) |
| Galcanezumab95,127,140,141,146 | 240 mg | Monthly | 844 | 566 (67) |
| Galcanezumab133 | 150 mg | Every 2 weeks | 107 | 77 (72) |
| Amitriptyline135 | 25–100 mg | Twice daily | 169 | 150 (89) |
| Author | Year of publication | Intervention | Dose (units) | Participants | Eye disorders (%) | Vascular disorders (%) | Nervous system disorders (%) | Infection and infestation (%) | General disorders and administration site conditions (%) | Respiratory disorders (%) | Any AEs (%) | Treatment-related AEs (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ble pharoptosis | Eyelid oedema | Hypertension | Dizziness | Headache | Infection | Flu syndrome | Sinus infection | Upper respiratory tract infection | Injection site pain | Pain | Bronchitis | |||||||
| Elkind (study 1)145 | 2006 | BTA | 7.5U | 105 | 2 (1.9) | 1 (1) | 0 | 0 | 1 (1) | 0 | 11 (10.5) | 4 (3.8) | 12 (11.4) | 0 | 0 | 0 | 52 (49.5) | 7 (6.6) |
| Elkind (study 1)145 | 2006 | BTA | 25U | 101 | 5 (5) | 0 | 0 | 0 | 2 (2) | 0 | 4 (4) | 7 (6.9) | 10 (9.9) | 0 | 0 | 0 | 47 (46.5) | 22 (21.78) |
| Elkind (study 2)145 | 2006 | BTA | 25U | 173 | 7 (4) | 0 | 0 | 2 | 8 | 20 | 12 | 16 | 14 | 9 | 13 | 6 | 134 | 43 |
| Elkind (study 3)145 | 2006 | BTA | 25U | 50 | 0 | 0 | 1 (2) | 0 | 0 | 3 (6) | 2 (4) | 2 (4) | 6 (12) | 0 | 2 (4) | 1 (2) | 35 (70) | - |
| Elkind (study2)145 | 2006 | BTA | 50U | 180 | 16 (8.9) | 0 | 0 | 9 (5) | 10 (5.6) | 15 (8.3) | 14 (7.8) | 15 (8.3) | 12 (6.7) | 4 (2.2) | 14 (7.8) | 10 (5.6) | 139 (77.2) | 53 (29.44) |
| Elkind (study 3)145 | 2006 | BTA | 50U | 51 | 3 (5.9) | 0 | 1 (2) | 3 (5.9) | 0 | 3 (5.9) | 3 (5.9) | 4 (7.8) | 8 (15.7) | 0 | 1 (2) | 2 (3.9) | 35 (68.6) | - |
| Elkind (study 1)145 | 2006 | BTA | 50U | 106 | 8 (7.6) | 7 (6.6) | 0 | 0 | 8 (7.6) | 0 | 7 (6.6) | 4 (3.8) | 11 (10.4) | 0 | 0 | 0 | 60 (56.6) | 32 (30.18) |
| Elkind (study 1)145 | 2006 | Placebo | - | 106 | 0 | 0 | 0 | 0 | 2 (1.9) | 0 | 9 (8.5) | 3 (2.8) | 12 (11.3) | 0 | 0 | 0 | 50 (47.2) | 7 (6.6) |
| Elkind (study 3)145 | 2006 | Placebo | - | 100 | 0 | 0 | 5 (5) | 0 | 0 | 3 (3) | 7 (7) | 4 (4) | 9 (9) | 0 | 5 (5) | 7 (7) | 60 (60) | - |
| Author | Intervention | Participants | Injury (%) | Skin and subcutaneous (%) | Eye disorders (%) | Musculoskeletal and connective tissue disorders (%) | Nervous system disorders (%) | General disorders and injection site condition (%) | Gastrointestinal disorders (%) | Any AEs (%) | Treatment-related AEs (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ecchymosis | Skin tightness | Blepharoptosis | Eyelid oedema | Muscular weakness | Back pain | Neck pain | Arm pain | Neck rigidity | Hypertonia | Hypesthesia | Migraine | Dizziness | Headache | Asthenia | Injection-site pain | Injection site haemorrhage | Pain | Dysphagia | Nausea | |||||
| Aurora, 2006132 | BTA 105 to 260U | 187 | 2 (1) | 14 (8) | 29 (16) | 12 (6) | 49 (26) | 3 (2) | 32 (17) | 14 (7) | 19 (10) | 13 (7) | 7 (4) | 6 (3) | 4 (2) | 11 (6) | 0 | 4 (2) | 0 | 3 (2) | 0 | 0 | 152 (81) | 113 (60) |
| Relja, 2007138 | BTA 225U | 129 | 0 | 6 (5) | 18 (14) | 3 (2) | 35 (27) | 0 | 30 (23) | 6 (5) | 22 (17) | 4 (3) | 0 | 1 (1) | 2 (2) | 2 (2) | 5 (4) | 3 (2) | 0 | 5 (4) | 4 (3) | 4 (3) | 99 (77) | 87 (67) |
| Relja, 2007138 | BTA 150U | 125 | 0 | 9 (7) | 12 (10) | 0 | 35 (28) | 0 | 24 (19) | 6 (5) | 20 (16) | 3 (2) | 0 | 3 (2) | 3 (2) | 5 (4) | 3 (2) | 9 (7) | 2 (2) | 3 (2) | 3 (2 | 2 (2) | 97 (78) | 79 (63) |
| Relja, 2007138 | BTA 75U | 123 | 0 | 7 (6) | 3 (2) | 2 (2) | 30 (2) | 0 | 22 (18) | 7 (6) | 13 (11) | 3 (2) | 0 | 2 (2) | 3 (2) | 4 (3) | 4 (3) | 4 (3) | 3 (2) | 3 (2) | 1 (1 | 1 (1) | 95 (77) | 77 (63) |
| Relja, 2007138 | Placebo | 118 | 0 | 2 (2) | 0 | 0 | 2 (2) | 0 | 6 (5) | 0 | 5 (4) |
0 | 0 | 1 (1) | 3 (3) | 3 (3) | 3 (3) | 2 (2) | 0 | 2 (2) | 0 | 1 (1) | 64 (54) | 37 (31) |
| Aurora, 2006132 | Placebo | 182 | 3 (2) | 1 | 3 (2) | 0 | 2 (1) | 1 | 8 (4) | 2 (1) | 6 (3) | 2 (1) | 3 (2) | 1 | 0 | 9 (5) | 0 | 1 | 4 (2) |
0 | 0 | 0 | 109 (60) | 39 (21) |
| Author | Year | Intervention | Dose | Participants | General AEs (%) | Liver AEs (%) | Immunologic AEs (%) | Skin AEs (%) | Any AEs (%) |
|---|---|---|---|---|---|---|---|---|---|
| Fazlalizadeh147 | 2008 | Sodium valproate | 200 mg | 285 | 19 (6.66) | 14 (4.91) | 0 | 7 (2.46) | 40 (14%) |
| Fazlalizadeh147 | 2008 | Topiramate | 100 mg | 284 | 29 (10.21) | 6 (2.11) | 6 (2.11) | 0 | 41 (14.4) |
| Author | Year | Intervention | Participants | Investigations (%) | Reproductive system (%) | Skin and subcutaneous (%) | Gastrointestinal disorders (%) | Ear disorders (%) | Nervous system disorders (%) | Any AEs (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight increase | Menstrual irregularity | Hair loss | Dry mouth | Vomiting | Gastrointestinal symptoms | Giddiness | Drowsiness | |||||
| Kalita137 | 2013 | Amitriptyline (12.5– 50 mg) | 144 | 71 (58.7) | 0 | 2 (1.4) | 78 (56.5) | 1 (0.7) | 12 (8.3) | 4 (3.6) | 69 (47.3) | 81 (56.3) |
| Kalita137 | 2013 | Divalproate (250–1000 mg) | 143 | 79 (61.7) | 6 (4.8) | 55 (38.5) | 13 (9.1) | 0 | 18 (12.6) | 3 (2.1) | 7 (4.9) | 68 (47.6) |
| Author | Intervention | Participants | Investigations (%) | Skin and subcutaneous (%) | Gastrointestinal disorders (%) | Eye disorders (%) | Psychiatric disorders (%) | Renal and urinary disorders (%) | Nervous system disorders (%) | Cardiac disorders (%) | General disorders and administration site conditions (%) | Any AEs (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight increase | Rash | Sweat discoloration | Nausea | Dry mucous membrane | Constipation | Visual disturbance | Agitation | Nervousness | Insomnia | Depression | Urinary retention | Paraesthesia | Dizziness | Somnolence | Tachycardia | Asthenia | Fatigue | Non-cardiac chest pain | ||||
| Couch, 201122 | Amitriptyline 100 mg | 194 | 3 (1.5) | 1(0.5) | 6 (3) | 4 (2) | 68 (35) | 23 (11) | 4 (2) | 14 (7) | 10 (5) | 7 (3.6) | 4 (2) | 6 (3) | 3 (1.5) | 20 (10) | 53 (27) | 7(3.6) | 16 (8) | 15 (7.7) | 3 (1.5) | 111 (57) |
| Couch, 201122 | Placebo | 197 | 2 (1) | 3 (1.5) | 5 (2.5) | 3 (1.5) | 14 (7) | 8 (4) | 5 (2.5) | 8 (4) | 16 (8) | 14 (7) | 2 (1) | 0 | 2 (1) | 11 (5.5) | 17 (8.6) | 6 (3) | 9 (4.5) | 8 (4) | 1 (0.5) | 53 (27) |
| Author | Intervention | Participants | Investigations (%) | Injury (%) | Gastrointestinal disorders (%) | Psychiatric disorders (%) | Nervous system disorders (%) | General disorders (%) | Vascular disorders (%) | Respiratory disorder (%) | Ear and labyrinth disorders (%) | Any AEs (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight increase | Injury | Abdominal pain | Nausea | Gastrointestinal disorders | Depression | Dizziness | Hypoesthesia | Somnolence | Fatigue | Hypotension | Rhinitis | Vertigo | ||||
| Lucking, 1998128 | Propranolol 40 mg | 170 | 8 (3.6) | 0 | 0 | 0 | 22 (9.8) | 0 | 0 | 5 (2.2) | 0 | 18 (8.1) | 0 | 0 | 16 (7.2) | 16 (7.2) |
| Diener, 2002136 | Propranolol 160 mg | 270 | 7 (2.6) | 7 (2.6) | 5 (1.9) | 22 (8) | 0 | 5 (1.9) | 9 (3.3) | 0 | 2 (0.7) | 10 | 4 (1.5) | 6 (2.2) | 0 | 88 (27) |
| Diener, 2002136 | Flunarizine 5 mg |
263 | 26 (9.9) | 5 (1.9) | 3 (1.1) | 34 (13) | 0 | 7 (2.7) | 4 (1.5) | 0 | 5 (1.9) | 10 (3.8) | 3 (1.1) | 4 (1.5) | 0 | 88 (33.5) |
| Diener, 2002136 | Flunarizine 10 mg | 275 | 18 (5.6) | 4 (1.5) | 4 (1.5) | 47 (17) | 0 | 2(0.7) | 3 (1.1) | 0 | 7 (2.5) | 14 (5.9) | 3 (1.1) | 6 (2.2) | 0 | 88 (32) |
| Lucking, 1998128 | Flunarizine 10 mg | 160 | 6 (2.8) | 0 | 0 | 0 | 15 (7.1) | 0 | 0 | 5 (2.4) | 0 | 17 (8.1) | 0 | 0 | 11 (5.2) | 11 (5.2) |
| Author | Intervention | Participants | Investigations (%) | Injury (%) | Gastrointestinal disorders (%) | Psychiatric disorders (%) | Metabolism and nutrition disorders (%) | Musculoskeletal and connective tissue (%) disorders | Nervous system disorders (%) | Infection (%) | General disorders (%) | Any AEs (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight decrease | Injury | Diarrhoea | Dry mouth | Nausea | Abdominal pain | Confusion | Depression | Anorexia | Back pain | Paraesthesia | Hypoesthesia | Difficulty with attention | Taste perversion | Dizziness | Somnolence | Sinusitis | Upper respiratory tract infection | Fatigue | ||||
| Lipton, 2011139 | Topiramate 100 mg | 176 | 0 | 3 (2) | 11 (6) | 12 (7) | 19 (11) | 0 | 10 (6) | 0 | 15 (9) | 10 (6) | 57 (32) | 12 (7) | 0 | 17 (10) | 20 (11) | 9 (5) | 16 (9) | 16 (9) | 26 (15) | 145 (82) |
| Silberstein, 200728 | Topiramate 100 mg | 160 | 0 | 8 (5) | 0 | 15 (9) | 14 (8) | 0 | 0 | 0 | 8 (5) | 0 | 46 (29) | 15 (9) | 15 (9 | 15 (9) | 6 (4) | 9 (6) | 7 (4) | 22 (14) | 19 (12) | 132 (83) |
| Diener, 2007152 | Topiramate 200 mg | 254 | 23 (9) | 0 | 0 | 0 | 11 (4) | 6 (2) | 0 | 13 (5) | 13 (5) | 0 | 77 (30) | 0 | 11 (4) | 8 (3) | 1 | 0 | 0 | 0 | 18 (7) | 173 (68) |
| Lipton, 2011139 | Placebo | 185 | 0 | 17 (9) | 6 (3) | 5 (3) | 17 (9) | 0 | 3 (2) | 0 | 5 (3) | 10 (5) | 13 (7) | 5 (3) | 0 | 3 (2) | 14 (8) | 3 (2) | 15 (8) | 12 (7) | 16 (9) | 136 (74) |
| Silberstein, 200728 | Placebo | 161 | 0 | 2 (1) | 0 | 5 (3) | 13 (8) | 0 | 0 | 9 (6) | 0 | 12 (8) | 0 | 4 (2.5) | 4 (3) | 12 (8) | 7 (4) | 8 (5) | 20 (13) | 16 (10) | 113 (70) | |
| Diener, 2007152 | Placebo | 258 | 18 (7) | 0 | 0 | 0 | 10 (4) | 5 (2) | 0 | 13 (5) | 8 (3) | 0 | 55 (21) | 0 | 12 (5) | 9 (3) | 1 | 0 | 0 | 0 | 10 (4) | 151 (59) |
Appendix 6 Further results for serious adverse events
| SOC | SAEs |
|---|---|
| Cardiac disorders | Acute myocardial infarction, atrial fibrillation, syncope |
| Ear and labyrinth disorders | Labyrinthitis, sudden hearing loss, vertigo, vestibular neuronitis |
| Eye disorders | Angle closure glaucoma, diplopia, optic neuritis, retinal detachment, rhegmatogenous retinal detachment |
| Gastrointestinal disorders | Abdominal pain, alcoholic pancreatitis, appendicitis, diverticulitis, oesophagitis, gastric ulcer haemorrhage, gastritis, haemorrhoids, intestinal haemorrhage, irritable bowel syndrome, mechanical ileus, obstructive defaecation, pancreatitis, pancreatitis acute, parotitis, small intestinal obstruction, vomiting |
| General disorders and administration site conditions | Abdominal adhesions, asthenia, chest pain, oedema peripheral, malaise, nasal septum deviation, non-cardiac chest pain, tooth impacted, vocal cord thickening |
| Hepatobiliary disorders | Cholecystitis, cholecystitis acute, cholelithiasis, common bile duct stone |
| Immune system disorders | Anaphylactic reaction, anaphylactic shock, hypersensitivity |
| Infections and infestations | Acute pyelonephritis, bacterial pharyngitis, bacteriuria, clostridium difficile colitis, COVID-19 pneumonia, gastroenteritis, gastrointestinal infection, infected dermal cyst, influenza, kidney infection, nasopharyngitis, papilloma viral infection, parasitic gastroenteritis, pyelonephritis, pyrexia, sepsis, tonsillitis, urinary tract infection, viral gastroenteritis, viral infection |
| Injury | Accident, ankle fracture, brain contusion, cartilage injury, clavicle fracture, concussion, contusion, fall, foot fracture, hand fracture, humerus fracture, injury, ligament rupture, limb injury, lower limb fracture, meniscus injury, radius fracture, respiratory fume inhalation, rib fracture, road traffic accident, skin laceration, sternal fracture, tendon injury, thoracic vertebral fracture, traumatic orbital fracture, ulna fracture, wrist fracture |
| Investigations | Alanine aminotransferase increased, aspartate aminotransferase increased, hepatic enzyme increased, weight decreased |
| Metabolism and nutrition disorders | Decreased appetite, hypokalaemia, hyponatraemia |
| Musculoskeletal and connective tissue disorders | Arthralgia, back pain, Behçet syndrome, costochondritis, flank pain, intervertebral disc protrusion, osteoarthritis, periarthritis, post-traumatic neck syndrome |
| Neoplasms: benign, malignant and unspecified (including cysts and polyps) | Adenocarcinoma of the cervix, brain neoplasm, breast cancer, colon cancer, fibroma, gall bladder polyp, ovarian cyst, polycystic ovaries, rectal polyp, ruptured ovarian cyst, uterine leiomyoma, breast neoplasm, fibroadenoma of breast, malignant melanoma, neoplasm malignant, vulval cancer |
| Nervous system disorders | Cerebellar syndrome, cerebral venous thrombosis, cervical radiculopathy, hypoaesthesia, lumbar spinal stenosis, migraine, migraine aggravated, migraine with aura, nervous system disorders, neuropathy, seizure, speech disorder, transient ischaemic attack |
| Neurological | Spinal pain |
| Poisoning and procedural complications | Overdose, intentional overdose |
| Pregnancy, puerperium and perinatal conditions | Pregnancy |
| Psychiatric disorders | Confusional state, depression, disorientation, major depression, psychogenic seizure, suicidal ideation, suicide attempt |
| Psychiatry | Panic attack |
| Renal and urinary disorders | Bladder dysfunction, calculus urinary, nephrolithiasis, renal calculus, renal colic, urinary incontinence |
| Reproductive system and breast disorders | Cervical dysplasia, dysmenorrhoea, endometriosis, menorrhagia, menstrual disorder and vaginal haemorrhage, metrorrhagia, ovarian disorder, spontaneous abortion, threatened abortion |
| Respiratory, thoracic and mediastinal | Asthma, chronic obstructive pulmonary disease, COPD and apnoea related to COPD, dyspnoea, epistaxis, pneumonia, post-surgical laryngospasm with hypoxic brain injury |
| Skin and subcutaneous tissue disorders | Erythema nodosum |
| Vascular disorders | Hypertensive crisis, orthostatic hypotension, peripheral vascular disease, pulmonary embolism |
| Study ID | Author, year | Interventions | Participants | Any SAEs | Treatment-related SAEs |
Death | SAEs definitions |
|---|---|---|---|---|---|---|---|
| 8 | Ailani, 2021 | Atogepant 10 mg | 221 | 0.9 | 0.5 | 0 | Standard |
| 8 | Ailani, 2021 | Atogepant 30 mg | 228 | 0 | 0 | 0 | Standard |
| 8 | Ailani, 2021 | Atogepant 60 mg | 231 | 0 | 0 | 0 | Standard |
| 8 | Ailani, 2021 | Placebo | 222 | 0.9 | 0 | 0 | Standard |
| 19 | Ashina, 2020 | Eptinezumab 100 mg | 223 | 1.79 | 0 | 0 | Standard |
| 19 | Ashina, 2020 | Eptinezumab 30 mg | 219 | 1.83 | 0 | 0 | Standard |
| 19 | Ashina, 2020 | Eptinezumab 300 mg | 224 | 1.34 | 0 | 0 | Standard |
| 19 | Ashina, 2020 | Placebo | 222 | 2.8 | 0 | 0 | Standard |
| 44 | Dodick, 2014 | Galcanezumab 150 mg | 107 | 0 | – | 0 | Standard |
| 44 | Dodick, 2014 | Placebo | 110 | 0.91 | 0 | Standard | |
| 45 | Dodick, 2018 | Erenumab 70 mg | 283 | 1.1 | – | 0 | Standard |
| 45 | Dodick, 2018 | Placebo | 289 | 1.7 | – | 0 | Standard |
| 47 | Dodick, 2009 | Amitriptyline 100 mg | 169 | 4.7 | 0.5 | 0 | Standard |
| 47 | Dodick, 2009 | Topiramate 100 mg | 177 | 2.3 | 0 | 0 | Standard |
| 49 | Detke, 2018 | Galcanezumab 120 mg | 273 | 0.18 | – | 0 | Standard |
| 49 | Detke, 2018 | Galcanezumab 240 mg | 282 | 1.8 | – | 0 | Standard |
| 49 | Detke, 2018 | Placebo | 558 | 0.7 | – | 0 | Standard |
| 53 | Diener, 2002 | Flunarizine 10 mg | 275 | 1.8 | – | 0 | No definition |
| 53 | Diener, 2002 | Flunarizine 5 mg | 263 | 0.4 | – | 0 | No definition |
| 53 | Diener, 2002 | Propranolol 160 mg | 270 | 0.7 | – | 0 | No definition |
| 59 | Dodick, 2010 | BTA 150U | 687 | 4.8 | 0.1 | 0 | Standard |
| 59 | Dodick, 2010 | Placebo | 692 | 2.3 | 0 | 0 | Standard |
| 60 | Dodick, 2018 | Fremanezumab-M | 289 | 1 | 0 | 0 | Standard |
| 60 | Dodick, 2018 | Fremanezumab-Q | 291 | 1 | 0 | 0.3 | Standard |
| 60 | Dodick, 2018 | Placebo | 293 | 2.4 | 0 | 0 | Standard |
| 61 | Dodick, 2019 | Eptinezumab 10 mg | 130 | 0.8 | 0 | 0 | Standard |
| 61 | Dodick, 2019 | Eptinezumab 100 mg | 122 | 3.3 | 0 | 0 | Standard |
| 61 | Dodick, 2019 | Eptinezumab 30 mg | 122 | 0 | 0 | 0 | Standard |
| 61 | Dodick, 2019 | Eptinezumab 300 mg | 121 | 5.8 | 0 | 0 | Standard |
| 61 | Dodick, 2019 | Placebo | 121 | 0.8 | 0 | 0 | Standard |
| 77 | Goadsby, 2017 | Erenumab 140 mg | 319 | 2.51 | – | 0 | Standard |
| 77 | Goadsby, 2017 | Erenumab 70 mg | 314 | 2.5 | – | 0 | Standard |
| 77 | Goadsby, 2017 | Placebo | 319 | 2.2 | – | 0 | Standard |
| 88 | Hong Sun, 2016 | Erenumab 21 mg | 105 | 1 | 0 | 0 | Standard |
| 88 | Hong Sun, 2016 | Erenumab 7 mg | 108 | 0 | 0 | 0 | Standard |
| 88 | Hong Sun, 2016 | Erenumab 70 mg | 106 | 0 | 0 | 0 | Standard |
| 88 | Hong Sun, 2016 | Placebo | 153 | 1 | 0 | Standard | |
| 105 | Lipton, 2020 | Eptinezumab 100 mg | 356 | 0.84 | – | 0 | Standard |
| 105 | Lipton, 2020 | Eptinezumab 300 mg | 350 | 1.1 | – | 0 | Standard |
| 105 | Lipton, 2020 | Placebo | 366 | 0.81 | – | 0 | Standard |
| 109 | Lucking, 1998 | Flunarizine 10 mg | 160 | 0 | 0 | 0 | No definition |
| 109 | Lucking, 1998 | Propranolol 40 mg | 170 | 0 | 0 | 0 | No definition |
| 111 | Relja, 2007 | BTA 150U | 125 | 1.62 | – | 0 | No definition |
| 111 | Relja, 2007 | BTA 225U | 129 | 1.5 | – | 0 | No definition |
| 111 | Relja, 2007 | BTA 75U | 123 | 0.81 | – | 0 | No definition |
| 111 | Relja, 2007 | Placebo | 118 | 1.7 | 0 | 0 | No definition |
| 143 | Lipton, 2011 | Placebo | 185 | 2.7 | 0.5 | 0 | No definition |
| 143 | Lipton, 2011 | Topiramate 100 mg | 176 | 1.7 | 1.1 | 0 | No definition |
| 148 | Rothrock, 2019 | BTA 150U | 220 | 2 | 0 | 0 | Standard |
| 148 | Rothrock, 2019 | Topiramate 100 mg | 142 | 4 | 1 | 0 | Standard |
| 156 | Sakai, 2020 | Galcanezumab 120 mg | 115 | 2.6 | – | 0 | Standard |
| 156 | Sakai, 2020 | Galcanezumab 240 mg | 114 | 0.9 | – | 0 | Standard |
| 156 | Sakai, 2020 | Placebo | 230 | 0 | 0 | 0 | Standard |
| 157 | Sakai, 2021 | Fremanezumab-M | 121 | 0 | 0 | 0 | Standard |
| 157 | Sakai, 2021 | Fremanezumab-Q | 118 | 0 | 0 | 0 | Standard |
| 157 | Sakai, 2021 | Placebo | 117 | 0 | 0 | 0 | Standard |
| 158 | Sakai, 2021 | Fremanezumab-M | 188 | 1.6 | 0 | 0 | Standard |
| 158 | Sakai, 2021 | Fremanezumab-Q | 190 | 0.5 | 0 | 0 | Standard |
| 158 | Sakai, 2021 | Placebo | 191 | 0.5 | 0 | 0 | Standard |
| 170 | Silberstein, 2007 | Placebo | 161 | 0 | 0 | 0 | No definition |
| 170 | Silberstein, 2007 | Topiramate 100 mg | 160 | 0 | 0 | 0 | No definition |
| 173 | Silberstein, 2017 | Fremanezumab-M | 379 | 1.32 | 0 | 0 | Standard |
| 173 | Silberstein, 2017 | Fremanezumab-Q | 376 | 0.8 | 0.26 | Standard | |
| 173 | Silberstein, 2017 | Placebo | 375 | 1.6 | – | 0 | Standard |
| 181 | Stauffer, 2018 | Galcanezumab 120 mg | 206 | 2.91 | 0 | 0 | Standard |
| 181 | Stauffer, 2018 | Galcanezumab 240 mg | 220 | 0 | 0 | 0 | Standard |
| 181 | Stauffer, 2018 | Placebo | 432 | 1.16 | 0 | 0 | Standard |
| 185 | Tepper, 2017 | Erenumab 140 mg | 188 | 1 | – | 0 | Standard |
| 185 | Tepper, 2017 | Erenumab 70 mg | 190 | 3 | – | 0 | Standard |
| 185 | Tepper, 2017 | Placebo | 282 | 2 | – | – | Standard |
| 196 | Reuter, 2018 | Erenumab 140 mg | 119 | 1.68 | 0 | 0 | Standard |
| 196 | Reuter, 2018 | Placebo | 124 | 0.8 | 0 | 0 | Standard |
| 197 | Reuter, 2021 | Erenumab 140 mg | 388 | 2.58 | 0.3 | 0 | Standard |
| 197 | Reuter, 2021 | Topiramate 100 mg | 388 | 4.9 | 0.5 | 0 | Standard |
| 201 | Vladimir, 2018 | Galcanezumab 120 mg | 226 | 2.2 | – | 0 | Standard |
| 201 | Vladimir, 2018 | Galcanezumab 240 mg | 228 | 3.1 | – | 0 | Standard |
| 201 | Vladimir, 2018 | Placebo | 461 | 1.1 | – | 0 | Standard |
| 203 | Wang, 2021 | Erenumab 140 mg | 224 | 0 | 0 | 0 | Standard |
| 203 | Wang, 2021 | Erenumab 70 mg | 335 | 2.99 | 0.3 | 0 | Standard |
| 203 | Wang, 2021 | Placebo | 335 | 1.94 | 0 | 0 | Standard |
| 215 | Diener, 2007 | Placebo | 258 | 4 | 0 | 0 | No definition |
| 215 | Diener, 2007 | Topiramate 200 mg | 254 | 3 | 0.39 | 0 | No definition |
| 216 | Elkind, 2006 (study 1) | BTA 25U | 101 | – | 0 | 0 | No definition |
| 216 | Elkind, 2006 (study 2) | BTA 25U | 173 | – | 0 | 0 | No definition |
| 216 | Elkind, 2006 (study 3) | BTA 25U | 50 | – | 0 | 0 | No definition |
| 216 | Elkind, 2006 (study 1) | BTA 50U | 106 | – | 0 | 0 | No definition |
| 216 | Elkind, 2006 (study 2) | BTA 50U | 180 | – | 0 | 0 | No definition |
| 216 | Elkind, 2006 (study 3) | BTA 50U | 51 | – | 0 | 0 | No definition |
| 216 | Elkind, 2006 (study 1) | BTA 7U | 105 | – | 0 | 0 | No definition |
| 216 | Elkind, 2006 (study 1) | Placebo | 106 | – | 0 | 0 | No definition |
| 216 | Elkind, 2006 (study 3) | Placebo | 100 | – | 0 | 0 | No definition |
| 217 | Ferrari, 2019 | Fremanezumab-M | 285 | 3.86 | 0 | 0 | Standard |
| 217 | Ferrari, 2019 | Fremanezumab-Q | 276 | 3.62 | 0 | 0 | Standard |
| 217 | Ferrari, 2019 | Placebo | 277 | 1 | 0 | 0 | Standard |
| 221 | Mulleners, 2020 | Galcanezumab 120 mg | 232 | 1 | – | 0 | Standard |
| 221 | Mulleners, 2020 | Placebo | 230 | 1 | – | 0 | Standard |
| 555 | Ashina, 2022 | Eptinezumab 100 mg | 299 | 1.67 | 0 | 0 | Standard |
| 555 | Ashina, 2020 | Eptinezumab 300 mg | 294 | 2.38 | 0.68 | Standard | |
| 555 | Ashina, 2022 | Placebo | 298 | 1.34 | 0 | 0 | Standard |
| 666 | Hu, 2022 | Galcanezumab 120 mg | 261 | 0.76 | – | 0 | Standard |
| 666 | Hu, 2022 | Placebo | 259 | 1.54 | – | 0 | Standard |
| 777 | Winner, 2021 | Eptinezumab 100 mg | 238 | 0 | 0 | 0 | Standard |
| 777 | Winner, 2021 | Placebo | 242 | 0 | 0 | 0 | Standard |
| 888 | Croop, 2020 | Placebo | 371 | 1 | 0.26 | 0 | Standard |
| 888 | Croop, 2020 | Rimegepant 75 mg | 370 | 0.81 | 0 | 0 | Standard |
| 999 | Fazlalizadeh, 2008 | Sodium valproate 200 mg | 285 | – | – | 0 | No definition |
| 999 | Fazlalizadeh, 2008 | Topiramate 100 mg | 284 | – | – | 0 | No definition |
| Author, year | Interventions | Participants | Breast cancer | Fibroadenoma of breast | Breast neoplasm | Polycystic ovaries | Thyroid adenoma | Vulval cancer | Benign colonic neoplasm | Anal polyp | Uterine leiomyoma | Gall bladder polyp | Lentigo maligna | Malignant melanoma in situ | Malignant melanoma | Pelvic pain | Squamous cell carcinoma | Papillary thyroid cancer | Ruptured ovarian cyst | Adenocarcinoma of the cervix | Ovarian cyst | Colon cancer | Rectal polyp | Brain neoplasm | Fibroma |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hong Sun, 2016 | Erenumab 70 mg | 106 | 0 | ||||||||||||||||||||||
| Hong Sun, 2016 | Erenumab 7 mg | 108 | 0.1 | ||||||||||||||||||||||
| Hong Sun, 2016 | Erenumab 21 mg | 105 | 0 | ||||||||||||||||||||||
| Dodick, 2009 | Amitriptyline 100 mg | 169 | 0.6 | 0.6 | |||||||||||||||||||||
| Dodick, 2010 | BTA 150U | 687 | 0.44 | 0.15 | 0.3 | 0.15 | 0.15 | 0.15 | 0.15 | ||||||||||||||||
| Rothrock, 2019 | BTA 150U | 220 | 0.45 | ||||||||||||||||||||||
| Dodick, 2019 | Eptinezumab 100 mg | 122 | 0.82 | ||||||||||||||||||||||
| Ashina, 2020 | Eptinezumab 300 mg | 224 | 0.45 | 0.45 | |||||||||||||||||||||
| Dodick, 2019 | Eptinezumab 300 mg | 121 | 0.83 | 0.83 | |||||||||||||||||||||
| Tepper, 2017 | Erenumab 70 mg | 190 | 0.53 | ||||||||||||||||||||||
| Goadsby, 2017 | Erenumab 70 mg | 314 | 0.31 | ||||||||||||||||||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0 | 0.36 | 0 | ||||||||||||||||||||
| Detke, 2018 | Galcanezumab 120 mg | 273 | 0.36 | ||||||||||||||||||||||
| Vladimir, 2018 | Galcanezumab 120 mg | 226 | 0 | 0.44 | 0.44 | ||||||||||||||||||||
| Croop, 2020 | Rimegepant 75 mg | 370 | 0.27 | ||||||||||||||||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | ||||||||||||||||||||||
| Rothrock, 2019 | Topiramate 100 mg | 142 | 0.7 | ||||||||||||||||||||||
| Sakai, 2021 | Placebo | 191 | 0.5 | ||||||||||||||||||||||
| Silberstein, 2017 | Placebo | 375 | 0.26 | ||||||||||||||||||||||
| Dodick, 2010 | Placebo | 692 | 0.28 | ||||||||||||||||||||||
| Ferrari, 2019 | Placebo | 277 | 0.36 | 0.36 | 0.36 | 0.36 | |||||||||||||||||||
| Ashina, 2020 | Placebo | 222 | 0.45 | ||||||||||||||||||||||
| Dodick, 2018 | Placebo | 289 | 0.3 | ||||||||||||||||||||||
| Dodick, 2018 | Placebo | 293 | 0.34 | ||||||||||||||||||||||
| Vladimir, 2018 | Placebo | 461 | 0.2 | 0 | 0 |
| Author, year | Interventions | Participants | Migraine with aura | Dizziness | Migraine aggravated | Neuropathy | Hypoesthesia | Intracranial aneurysm | Multiple sclerosis | Optic neuritis | Transient ischaemic attack | Tonic-clonic seizure | Spinal pain | Serotonin syndrome | Migraine | Headache | Convulsion | Seizure | Cervical radiculopathy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hong Sun, 2016 | Erenumab 70 mg | 106 | 0.1 | ||||||||||||||||
| Dodick, 2009 | Amitriptyline 100 mg | 169 | 0.6 | ||||||||||||||||
| Dodick, 2010 | BTA 150U | 687 | 0.59 | 0.15 | |||||||||||||||
| Dodick, 2019 | Eptinezumab 100 mg | 122 | 0.82 | ||||||||||||||||
| Ashina, 2022 | Eptinezumab 100 mg | 299 | 0 | 0 | 0.33 | ||||||||||||||
| Ashina, 2020 | Eptinezumab 300 mg | 294 | 0 | 0.34 | 0 | ||||||||||||||
| Dodick, 2019 | Eptinezumab 300 mg | 121 | 0.83 | 0.83 | |||||||||||||||
| Goadsby, 2017 | Erenumab 140 mg | 319 | 0.26 | 0 | |||||||||||||||
| Reuter, 2018 | Erenumab 140 mg | 119 | 0.84 | ||||||||||||||||
| Dodick, 2018 | Erenumab 70 mg | 283 | 0.4 | ||||||||||||||||
| Goadsby, 2017 | Erenumab 70 mg | 314 | 0 | 0.31 | |||||||||||||||
| Dodick, 2018 | Fremanezumab-M | 289 | 0.35 | ||||||||||||||||
| Ferrari, 2019 | Fremanezumab-M | 285 | 0 | 0.35 | 0.35 | ||||||||||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0 | 0.35 | |||||||||||||||
| Vladimir, 2018 | Galcanezumab 240 mg | 228 | 0.44 | 0 | |||||||||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0 | 0.26 | |||||||||||||||
| Silberstein, 2017 | Placebo | 375 | 0.26 | ||||||||||||||||
| Dodick, 2010 | Placebo | 692 | 0.28 | ||||||||||||||||
| Tepper, 2017 | Placebo | 282 | 0.35 | ||||||||||||||||
| Ferrari, 2019 | Placebo | 277 | 0.36 | 0.36 | |||||||||||||||
| Ashina, 2020 | Placebo | 222 | 0.45 | ||||||||||||||||
| Dodick, 2018 | Placebo | 289 | 0.3 | ||||||||||||||||
| Dodick, 2018 | Placebo | 293 | 0.34 | 0.34 | |||||||||||||||
| Vladimir, 2018 | Placebo | 461 | 0 | 0.2 | |||||||||||||||
| Wang, 2021 | Placebo | 335 | 0.3 | ||||||||||||||||
| Ashina, 2022 | Placebo | 298 | 0.34 | 0 | 0 | ||||||||||||||
| Lipton, 2011 | Placebo | 0.5 | 0.5 |
| Author, year | Interventions | Participants | Respiratory fume inhalation | Seroma | Incarcerated incisional hernia | Foot fracture | Clavicle fracture | Accident | Cartilage injury | Wrist fracture | Ulna fracture | Thoracic vertebral fracture | Lower limb fracture | Injury | Hand fracture | Humerus fracture | Ankle fracture | Traumatic orbital fracture | Meniscus injury | Radius fracture | Fall | Tendon injury | Ankle fracture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rothrock, 2019 | BTA 150U | 220 | 0.45 | ||||||||||||||||||||
| Ashina, 2022 | Eptinezumab 100 mg | 299 | 0.33 | ||||||||||||||||||||
| Tepper, 2017 | Erenumab 140 mg | 188 | 0.53 | ||||||||||||||||||||
| Goadsby, 2017 | Erenumab 140 mg | 319 | 0.26 | ||||||||||||||||||||
| Reuter, 2018 | Erenumab 140 mg | 119 | 0.84 | ||||||||||||||||||||
| Reuter, 2021 | Erenumab 140 mg | 388 | 0.26 | 0.26 | |||||||||||||||||||
| Silberstein, 2017 | Fremanezumab-M | 379 | 0.26 | 0.26 | 0.26 | ||||||||||||||||||
| Ferrari, 2019 | Fremanezumab-M | 285 | 0.35 | ||||||||||||||||||||
| Silberstein, 2017 | Fremanezumab-Q | 376 | 0.26 | ||||||||||||||||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.36 | 0.36 | |||||||||||||||||||
| Dodick, 2018 | Fremanezumab-Q | 291 | 0.34 | ||||||||||||||||||||
| Sakai, 2020 | Galcanezumab 120 mg | 115 | 0.9 | ||||||||||||||||||||
| Stauffer, 2018 | Galcanezumab 120 mg | 206 | 0.49 | 0.49 | |||||||||||||||||||
| Vladimir, 2018 | Galcanezumab 240 mg | 228 | 0.44 | ||||||||||||||||||||
| Dodick, 2009 | Topiramate 100 mg | 177 | 0.5 | ||||||||||||||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | ||||||||||||||||||||
| Rothrock, 2019 | Topiramate 100 mg | 142 | 0.7 | ||||||||||||||||||||
| Silberstein, 2017 | Placebo | 375 | 0.26 | 0.26 | |||||||||||||||||||
| Ferrari, 2019 | Placebo | 277 | 0.36 | 0.35 | |||||||||||||||||||
| Dodick, 2018 | Placebo | 293 | 0.34 | 0.34 | |||||||||||||||||||
| Goadsby, 2017 | Placebo | 319 | 0.26 | ||||||||||||||||||||
| Vladimir, 2018 | Placebo | 461 | 0.2 | ||||||||||||||||||||
| Mulleners, 2020 | Placebo | 230 | 0.43 | ||||||||||||||||||||
| Ashina, 2022 | Placebo | 298 | 0.34 | 0 | |||||||||||||||||||
| Diener, 2002 | Propranolol 160 mg | 0.36 | |||||||||||||||||||||
| Diener, 2002 | Flunarizine 10 mg | 0.37 |
| Author, year | Interventions | Participants | Ligament rupture | Sternal fracture | Skin laceration | Limb injury | Stomal hernia | Procedural pain | Postprocedural constipation | Postprocedural complication | Abdominal wound dehiscence | Road traffic accident | Head injury | Concussion | Brain contusion | Contusion | Rib fracture | Radius fracture | Overdose | Intentional overdose |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rothrock, 2019 | BTA 150U | 220 | 0.45 | |||||||||||||||||
| Ashina, 2020 | Eptinezumab 30 mg | 219 | 0.46 | |||||||||||||||||
| Ashina, 2020 | Eptinezumab 100 mg | 223 | 0.45 | 0.45 | ||||||||||||||||
| Ashina, 2020 | Eptinezumab 300 mg | 224 | 0.45 | 0.45 | ||||||||||||||||
| Dodick, 2019 | Eptinezumab 300 mg | 121 | 0.83 | 0.83 | ||||||||||||||||
| Reuter, 2021 | Erenumab 140 mg | 388 | 0.26 | 0.26 | 0.26 | 0.26 | 0 | 0.26 | ||||||||||||
| Tepper, 2017 | Erenumab 70 mg | 190 | 0.53 | |||||||||||||||||
| Sakai, 2021 | Fremanezumab-M | 188 | 0.53 | |||||||||||||||||
| Ferrari, 2019 | Fremanezumab-M | 285 | 0.36 | |||||||||||||||||
| Silberstein, 2017 | Fremanezumab-Q | 376 | 0.26 | |||||||||||||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.36 | 0.35 | ||||||||||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | |||||||||||||||||
| Rothrock, 2019 | Topiramate 100 mg | 142 | 0.7 | |||||||||||||||||
| Dodick, 2014 | Placebo | 110 | 0.91 | |||||||||||||||||
| Dodick, 2018 | Placebo | 293 | 0.34 | |||||||||||||||||
| Goadsby, 2017 | Placebo | 319 | 0.26 | |||||||||||||||||
| Vladimir, 2018 | Placebo | 461 | 0.2 | 0.2 | 0.2 | |||||||||||||||
| Croop, 2020 | Placebo | 371 | 0.27 | |||||||||||||||||
| Ashina, 2022 | Placebo | 298 | 0.34 | 0.34 |
| Author, year | Interventions | Participants | Pneumonia | Postsurgical laryngospasm with hypoxic brain injury | COPD and apnoea related to COPD | COPD | Asthma | Respiratory distress | Dyspnoea | Vocal cord thickening | Pulmonary embolism | Pulmonary sarcoidosis | Sleep apnoea syndrome | Hypoxia | Epistaxis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ailani, 2021 | Atogepant 10 mg | 221 | 0.45 | ||||||||||||
| Dodick, 2010 | BTA 150U | 687 | 0.44 | 0.15 | 0.15 | ||||||||||
| Rothrock, 2019 | BTA 150U | 220 | 0.45 | 0.45 | |||||||||||
| Dodick, 2019 | Eptinezumab 300 mg | 121 | 0.83 | ||||||||||||
| Sakai, 2021 | Fremanezumab-M | 188 | 0.53 | ||||||||||||
| Ferrari, 2019 | Fremanezumab-M | 285 | 0.35 | ||||||||||||
| Silberstein, 2017 | Fremanezumab-Q | 376 | 0.26 | 0.26 | 0 | 0 | |||||||||
| Rothrock, 2019 | Topiramate 100 mg | 142 | 0.7 | 0.7 | |||||||||||
| Silberstein, 2017 | Placebo | 375 | 0 | 0 | 0.26 | 0.26 | |||||||||
| Dodick, 2010 | Placebo | 692 | 0.28 | 0.28 | |||||||||||
| Detke, 2018 | Placebo | 558 | 0.18 | ||||||||||||
| Ailani, 2021 | Placebo | 222 | 0.45 | 0 | |||||||||||
| Ashina, 2020 | Placebo | 222 | 0.45 | 0.45 | |||||||||||
| Stauffer, 2018 | Placebo | 432 | 0.23 | ||||||||||||
| Croop, 2020 | Placebo | 371 | 0.27 |
| Author, year | Interventions | Participants | Mechanical ileus | intestinal haemorrhage | Haemorrhoids | Irritable bowel syndrome | Esophagitis | Pancreatitis acute | Pancreatitis acute | Colitis ischaemic | Colitis | Pancreatitis | Gastroesophageal reflux | Inguinal hernia | Parotitis | Gastric ulcer haemorrhage | Vomiting | Diverticulitis | Abdominal pain | Gastritis | Small intestinal obstruction | Obstructive defaecation | Alcoholic pancreatitis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dodick, 2009 | Amitriptyline 100 mg | 169 | 0.6 | ||||||||||||||||||||
| Dodick, 2010 | BTA 150U | 687 | 0.15 | 0.15 | 0.15 | ||||||||||||||||||
| Tepper, 2017 | Erenumab 140 mg | 188 | 0.53 | ||||||||||||||||||||
| Reuter, 2021 | Erenumab 140 mg | 388 | 0.26 | 0.26 | |||||||||||||||||||
| Sakai, 2021 | Fremanezumab-M | 188 | 0.53 | ||||||||||||||||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.36 | 0.36 | |||||||||||||||||||
| Dodick, 2018 | Fremanezumab-Q | 291 | 0.34 | ||||||||||||||||||||
| Mulleners, 2020 | Galcanezumab 120 mg | 232 | 0.43 | ||||||||||||||||||||
| Stauffer, 2018 | Galcanezumab 120 mg | 206 | 0.5 | 0.5 | |||||||||||||||||||
| Vladimir, 2018 | Galcanezumab 120 mg | 226 | 0.44 | ||||||||||||||||||||
| Detke, 2018 | Galcanezumab 240 mg | 282 | 0.35 | ||||||||||||||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | 0.26 | |||||||||||||||||||
| Detke, 2018 | Placebo | 558 | 0.18 | 0.18 | |||||||||||||||||||
| Tepper, 2017 | Placebo | 282 | 0.35 | 0.35 | 0.35 | 0 | |||||||||||||||||
| Ailani, 2021 | Placebo | 222 | 0.45 |
| Author, year | Interventions | Participants | Nephrolithiasis | Urinary incontinence | Kidney injury | Calculus urinary | Renal calculus | Renal colic | Bladder dysfunction |
|---|---|---|---|---|---|---|---|---|---|
| Dodick, 2009 | Amitriptyline 100 mg | 169 | 0.6 | ||||||
| Dodick, 2010 | BTA 150U | 687 | 0.15 | ||||||
| Ashina, 2020 | Eptinezumab 30 mg | 219 | 0.46 | 0.46 | |||||
| Silberstein, 2017 | Fremanezumab-M | 379 | 0.26 | ||||||
| Ferrari, 2019 | Fremanezumab-M | 285 | 0.7 | ||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.35 | ||||||
| Vladimir, 2018 | Galcanezumab 120 mg | 226 | 0.44 | ||||||
| Vladimir, 2018 | Galcanezumab 240 mg | 228 | |||||||
| Detke, 2018 | Galcanezumab 240 mg | 282 | 0.35 | 0.35 | |||||
| Rothrock, 2019 | Topiramate 100 mg | 142 | 0.7 | ||||||
| Silberstein, 2017 | Placebo | 375 | 0.26 | ||||||
| Diener, 2002 | Flunarizine 10 mg | 0.37 |
| Author, year | Interventions | Participants | Gastrointestinal infection | Viral infection | Nasopharyngitis | Tonsillitis | Upper respiratory tract infection bacterial | Sepsis | Pyelonephritis | Kidney infection | Vaginal abscess | Viral gastroenteritis | Gastroenteritis | Pharyngitis streptococcal | Infected dermal cyst | Sinusitis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dodick, 2009 | Amitriptyline 100 mg | 169 | 0.6 | |||||||||||||
| Dodick, 2010 | BTA 150U | 687 | 0.5 | |||||||||||||
| Dodick, 2019 | Eptinezumab 300 mg | 121 | 0.83 | 0.83 | ||||||||||||
| Goadsby, 2017 | Erenumab 140 mg | 319 | 0.26 | 0.26 | 0.26 | 0.26 | ||||||||||
| Wang, 2021 | Erenumab 70 mg | 335 | 0.3 | |||||||||||||
| Mulleners, 2020 | Galcanezumab 120 mg | 232 | 0.43 | |||||||||||||
| Hu, 2022 | Galcanezumab 120 mg | 261 | 0.38 | 0.38 | ||||||||||||
| Croop, 2020 | Rimegepant 75 mg | 370 | 0.27 | |||||||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | 0.26 | 0.26 | 0.26 | ||||||||||
| Dodick, 2010 | Placebo | 692 | 0.28 | 0.28 | 0.28 | 0.28 | ||||||||||
| Ferrari, 2019 | Placebo | 277 | 0.35 | |||||||||||||
| Reuter, 2018 | Placebo | 124 | 0.8 | |||||||||||||
| Wang, 2021 | Placebo | 335 | 0.3 | 0.3 | ||||||||||||
| Croop, 2020 | Placebo | 371 | 0.27 |
| Author, year | Interventions | Participants | Peri tonsillitis | Diverticulitis | Dengue fever | Cellulitis | Labyrinthitis | Clostridium difficile colitis | Influenza | Papilloma viral infection | Appendicitis | Parasitic gastroenteritis | Bacteriuria | Pyrexia | Acute pyelonephritis | COVID-19 pneumonia | Urinary tract infection | Bacterial pharyngitis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ashina, 2022 | Eptinezumab 100 mg | 299 | 0.33 | |||||||||||||||
| Ashina, 2020 | Eptinezumab 300 mg | 294 | 0.68 | |||||||||||||||
| Goadsby, 2017 | Erenumab 140 mg | 319 | 0.26 | |||||||||||||||
| Reuter, 2021 | Erenumab 140 mg | 388 | 0.26 | |||||||||||||||
| Tepper, 2017 | Erenumab 70 mg | 190 | 0.53 | |||||||||||||||
| Dodick, 2018 | Erenumab 70 mg | 283 | 0.4 | |||||||||||||||
| Goadsby, 2017 | Erenumab 70 mg | 314 | 0.31 | |||||||||||||||
| Wang, 2021 | Erenumab 70 mg | 335 | 0.3 | |||||||||||||||
| Dodick, 2018 | Fremanezumab-M | 289 | 0.35 | |||||||||||||||
| Sakai, 2021 | Fremanezumab-Q | 190 | 0.5 | |||||||||||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.35 | |||||||||||||||
| Vladimir, 2018 | Galcanezumab 120 mg | 226 | 0.44 | |||||||||||||||
| Vladimir, 2018 | Galcanezumab 240 mg | 228 | 0.44 | 0.44 | ||||||||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | 0.26 | 0.26 | 0.26 | ||||||||||||
| Tepper, 2017 | Placebo | 282 | 0.35 | |||||||||||||||
| Ferrari, 2019 | Placebo | 277 | 0.35 | 0.35 | ||||||||||||||
| Ashina, 2020 | Placebo | 222 | 0.45 | |||||||||||||||
| Wang, 2021 | Placebo | 335 | 0.3 | |||||||||||||||
| Croop, 2020 | Placebo | 371 | 0.27 | |||||||||||||||
| Hu, 2022 | Placebo | 259 | 0.38 |
| Author, year | Interventions | Participants | Atrial fibrillation | Acute coronary syndrome | Tachycardia | Atrial fibrillation | Palpitations | Pericarditis | Syncope | Acute myocardial infarction |
|---|---|---|---|---|---|---|---|---|---|---|
| Dodick, 2010 | BTA 150U | 687 | 0.15 | 0.15 | 0.15 | 0.15 | ||||
| Rothrock, 2019 | BTA 150U | 220 | 0.45 | 0.45 | ||||||
| Ferrari, 2019 | Fremanezumab-M | 285 | 0.35 | |||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.36 | |||||||
| Vladimir, 2018 | Galcanezumab 240 mg | 228 | 0.44 | |||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | |||||||
| Detke, 2018 | Placebo | 558 | 0.18 | |||||||
| Ferrari, 2019 | Placebo | 277 | 0.36 | |||||||
| Ashina, 2020 | Placebo | 222 | 0.45 |
| Author, year | Interventions | Participants | Congenital diaphragmatic hernia | Metrorrhagia | Menometrorrhagia | Ovarian disorder | Abortion threatened | Spontaneous abortion | Uterine Prolapse | Endometriosis | Menstrual disorder and vaginal haemorrhage | Dysmenorrhoea | Menorrhagia | Cervical dysplasia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dodick, 2009 | Amitriptyline 100 mg | 169 | 0.6 | |||||||||||
| Dodick, 2010 | BTA 150U | 687 | 0.15 | |||||||||||
| Lipton, 2020 | Eptinezumab 300 mg | 350 | 0.38 | |||||||||||
| Reuter, 2021 | Erenumab 140 mg | 388 | 0.26 | 0.26 | ||||||||||
| Dodick, 2018 | Fremanezumab-M | 289 | 0.35 | |||||||||||
| Ferrari, 2019 | Fremanezumab-M | 285 | 0.35 | 0.35 | ||||||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.35 | 0.35 | ||||||||||
| Dodick, 2009 | Topiramate 100 mg | 177 | 0.5 | 0.5 | 0.5 | |||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | |||||||||||
| Dodick, 2010 | Placebo | 692 | 0.28 | |||||||||||
| Lipton, 2020 | Placebo | 366 | 0.27 | |||||||||||
| Ferrari, 2019 | Placebo | 277 | 0.36 | 0.36 | ||||||||||
| Ashina, 2020 | Placebo | 222 | 0.45 | |||||||||||
| Dodick, 2018 | Placebo | 293 | 0.34 | |||||||||||
| Goadsby, 2017 | Placebo | 319 | 0.26 | |||||||||||
| Wang, 2021 | Placebo | 335 | 0.5 | |||||||||||
| Lipton, 2011 | Placebo | 0.5 | ||||||||||||
| Diener, 2002 | Propranolol 160 mg | 0.3 |
| Author, year | Interventions | Participants | Cholelithiasis | Hepatic cholestatic | Cerebral venous thrombosis | Cholecystitis acute |
|---|---|---|---|---|---|---|
| Dodick, 2009 | Amitriptyline 100 mg | 169 | 0.6 | |||
| Ashina, 2020 | Eptinezumab 30 mg | 219 | 0.46 | |||
| Dodick, 2019 | Eptinezumab 100 mg | 122 | 0.5 | |||
| Ashina, 2020 | Eptinezumab 100 mg | 223 | 0.45 | |||
| Ashina, 2022 | Eptinezumab 100 mg | 299 | 0.33 | |||
| Goadsby, 2017 | Erenumab 140 mg | 319 | 0.63 | 0.26 | ||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.36 | 0.36 | ||
| Vladimir, 2018 | Galcanezumab 240 mg | 228 | 0.44 | |||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | |||
| Dodick, 2010 | Placebo | 692 | 0.28 | |||
| Tepper, 2017 | Placebo | 282 | 0.35 | |||
| Dodick, 2018 | Placebo | 289 | 0.3 | |||
| Stauffer, 2018 | Placebo | 432 | 0.5 | |||
| Diener, 2002 | Flunarizine 10 mg | 0.37 |
| Author, year | Interventions | Participants | Major depression | Depression | Stress | Conversion disorder | Suicidal ideation | Disorientation | Substance-induced mood disorders | Panic attack | Menorrhagia | Suicide attempt | Psychogenic seizure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dodick, 2010 | BTA 150U | 687 | 0.3 | 0.15 | 0.15 | ||||||||
| Dodick, 2019 | Eptinezumab 10 mg | 130 | 0.77 | ||||||||||
| Dodick, 2019 | Eptinezumab 100 mg | 122 | 0.82 | 0.82 | |||||||||
| Ashina, 2020 | Eptinezumab 100 mg | 223 | 0.45 | 0.45 | 0.45 | ||||||||
| Ashina, 2020 | Eptinezumab 300 mg | 294 | 0.34 | ||||||||||
| Reuter, 2021 | Erenumab 140 mg | 388 | 0.26 | ||||||||||
| Silberstein, 2017 | Fremanezumab-M | 379 | 0.26 | ||||||||||
| Vladimir, 2018 | Galcanezumab 240 mg | 228 | 0.44 | ||||||||||
| Croop, 2020 | Rimegepant 75 mg | 370 | 0.27 | ||||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | ||||||||||
| Vladimir, 2018 | Placebo | 461 | 0.2 | ||||||||||
| Ashina, 2022 | Placebo | 298 | 0.34 | ||||||||||
| Diener, 2002 | Flunarizine 10 mg | 0.37 |
| Author, year | Interventions | Participants | Costochondritis | Tendonitis | Vertebral osteophyte | Rhabdomyolysis | Periarthritis | Post-traumatic neck syndrome | Back pain | Behcet syndrome | Intervertebral disc protrusion | Osteoarthritis | Lumbar spinal stenosis | Arthralgia | Flank pain |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dodick, 2010 | BTA 150U | 687 | 0.15 | ||||||||||||
| Ashina, 2020 | Eptinezumab 30 mg | 219 | 0.46 | ||||||||||||
| Ashina, 2020 | Eptinezumab 300 mg | 294 | 0.34 | ||||||||||||
| Tepper, 2017 | Erenumab 140 mg | 188 | 0.52 | ||||||||||||
| Reuter, 2021 | Erenumab 140 mg | 388 | 0.26 | ||||||||||||
| Tepper, 2017 | Erenumab 70 mg | 190 | 0.53 | 0 | |||||||||||
| Dodick, 2018 | Erenumab 70 mg | 283 | 0.4 | ||||||||||||
| Goadsby, 2017 | Erenumab 70 mg | 314 | 0.31 | 0.31 | |||||||||||
| Silberstein, 2017 | Fremanezumab-M | 379 | 0.26 | ||||||||||||
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.35 | ||||||||||||
| Stauffer, 2018 | Galcanezumab 120 mg | 206 | 0.46 | ||||||||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | ||||||||||||
| Dodick, 2010 | Placebo | 692 | 0.28 | ||||||||||||
| Tepper, 2017 | Placebo | 282 | 0.35 | ||||||||||||
| Ashina, 2020 | Placebo | 222 | 0.45 | ||||||||||||
| Dodick, 2018 | Placebo | 289 | 0.3 | ||||||||||||
| Goadsby, 2017 | Placebo | 319 | 0.26 | 0.26 | |||||||||||
| Stauffer, 2018 | Placebo | 432 | 0.23 | ||||||||||||
| Mulleners, 2020 | Placebo | 230 | 0.43 | ||||||||||||
| Ashina, 2022 | Placebo | 298 | 0.34 |
| Author, year | Interventions | Participants | Weight decreased | International normalised ratio abnormal |
|---|---|---|---|---|
| Ferrari, 2019 | Fremanezumab-Q | 276 | 0.35 | |
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 |
| Author, year | Interventions | Participants | Hypokalaemia | Hypoglycaemia | Dehydration | Hyponatraemia | Decreased appetite |
|---|---|---|---|---|---|---|---|
| Dodick, 2010 | BTA 150U | 687 | 0.15 | ||||
| Detke, 2018 | Galcanezumab 240 mg | 282 | 0.35 | ||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | ||||
| Rothrock, 2019 | Topiramate 100 mg | 142 | 0.7 | ||||
| Dodick, 2018 | Placebo | 289 | 0.3 | ||||
| Dodick, 2018 | Placebo | 293 | 0.34 |
| Author, year | Interventions | Participants | Hypertensive crisis | Peripheral arterial occlusive disease | Deep vein thrombosis | Pulmonary embolism |
|---|---|---|---|---|---|---|
| Dodick, 2010 | BTA 150U | 687 | 0.15 | |||
| Silberstein, 2017 | Fremanezumab-M | 379 | 0.26 | |||
| Detke, 2018 | Galcanezumab 240 mg | 282 | 0.35 | |||
| Rothrock, 2019 | Topiramate 100 mg | 142 | 0.7 | 0.7 | ||
| Stauffer, 2018 | Placebo | 432 | 0.23 |
| Author, year | Interventions | Participants | Non-cardiac chest pain | Malaise | Nasal septum deviation | Tooth impacted | Chest pain | Abdominal adhesions | Asthenia | Oedema peripheral |
|---|---|---|---|---|---|---|---|---|---|---|
| Dodick, 2010 | BTA 150U | 687 | 0.15 | |||||||
| Tepper, 2017 | Erenumab 140 mg | 188 | 0 | 0.53 | ||||||
| Goadsby, 2017 | Erenumab 140 mg | 319 | 0.31 | |||||||
| Tepper, 2017 | Erenumab 70 mg | 190 | 0.53 | |||||||
| Goadsby, 2017 | Erenumab 70 mg | 314 | 0.26 | |||||||
| Wang, 2021 | Erenumab 70 mg | 335 | 0.3 | |||||||
| Sakai, 2020 | Galcanezumab 120 mg | 115 | 0.9 | |||||||
| Sakai, 2020 | Galcanezumab 240 mg | 114 | 0.9 | |||||||
| Silberstein, 2017 | Placebo | 375 | 0.26 | |||||||
| Goadsby, 2017 | Placebo | 319 | 0.26 | |||||||
| Hu, 2022 | Placebo | 259 | 0.38 | |||||||
| Lipton, 2011 | Placebo | 0.5 | ||||||||
| Diener, 2002 | Flunarizine 5 mg | 0.38 |
| Author, year | Interventions | Participants | Diplopia | Retinal tear | Rhegmatogenous retinal detachment | Angle closure glaucoma | Retinal detachment | Optic neuritis |
|---|---|---|---|---|---|---|---|---|
| Ailani, 2021 | Atogepant 10 mg | 221 | 0.45 | |||||
| Ashina, 2022 | Eptinezumab 100 mg | 299 | 0.33 | |||||
| Ferrari, 2019 | Fremanezumab-M | 285 | 0.35 | |||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | 0.26 | 0.26 | |||
| Silberstein, 2017 | Placebo | 375 | 0.26 |
| Author, year | Interventions | Participants | Ear and labyrinth disorders | Immune system disorders | Blood and lymphatic system disorders | ||||
|---|---|---|---|---|---|---|---|---|---|
| Vestibular neuronitis | Sudden hearing loss | Vertigo | Hypersensitivity | Anaphylactic reaction | Anaphylactic shock | Thrombocytopenia | |||
| Hong Sun, 2016 | Erenumab 70 mg | 106 | 0.1 | ||||||
| Ashina, 2020 | Eptinezumab 300 mg | 224 | 0.45 | ||||||
| Ashina, 2020 | Eptinezumab 300 mg | 294 | 0.68 | ||||||
| Goadsby, 2017 | Erenumab 140 mg | 319 | 0.26 | ||||||
| Ferrari, 2019 | Fremanezumab-M | 285 | 0.35 | ||||||
| Sakai, 2020 | Galcanezumab 120 mg | 115 | 0.9 | ||||||
| Reuter, 2021 | Topiramate 100 mg | 388 | 0.26 | ||||||
| Silberstein, 2017 | Placebo | 375 | 0.26 | ||||||
| Dodick, 2010 | Placebo | 692 | 0.28 | ||||||
| Dodick, 2018 | Placebo | 289 | 0.3 | ||||||
| Dodick, 2018 | Placebo | 293 | 0.3 | ||||||
| Goadsby, 2017 | Placebo | 319 | 0.26 | ||||||
| Treatments | Doses | Frequency | Total participants (n) | Participants with any SAEsa (%) |
|---|---|---|---|---|
| Atogepant129 | 30 mg | Once daily | 228 | 0 |
| Atogepant129 | 60 mg | Once daily | 231 | 0 |
| Erenumab130 | 21 mg | Monthly | 105 | 0 |
| Galcanezumab133 | 150 mg | Every 2 weeks | 107 | 0 |
| Eptinezumab89 | 10 mg | Single dose on day 0 | 130 | 1 (0.77) |
| Rimegepant148 | 75 mg | Once daily | 370 | 3 (0.81) |
| Atogepant129 | 10 mg | Once daily | 221 | 2 (0.9) |
| Erenumab130 | 7 mg | Monthly | 108 | 1 (0.93) |
| Eptinezumab89,131 | 30 mg | Single dose on day 0 | 341 | 4 (1.17) |
| Fremanezumab35,37,90,91,126 | Quarterly, 625 mg | Single dose on day 0 | 1251 | 15 (1.2) |
| Eptinezumab89,94,131,149,151 | 100 mg | Single dose on day 0 | 1238 | 16 (1.29) |
| Galcanezumab95,127,140,141 | 240 mg | Monthly | 844 | 12 (1.42) |
| Placebo35–37,45,89–91,94,95,97,126, 127,129,131,133,134,140,141,143,144,146,148–151,155 | - | Matched with active treatments | 7570 | 109 (1.42) |
| Galcanezumab95,127,140,141,146,150 | 120 mg | Monthly | 1313 | 20 (1.52) |
| Fremanezumab35,37,90,91,126 | Monthly, 225 mg | Monthly | 1262 | 22 (1.74) |
| Erenumab36,45,142–144 | 140 mg | Monthly | 1238 | 22 (1.78) |
| Eptinezumab89,94,131,151 | 300 mg | Single dose on day 0 | 989 | 21 (2.12) |
| Erenumab36,45,134,144 | 70 mg | Monthly | 1228 | 28 (2.28) |
| BTA88,97 | 150U | Every 12 weeks | 907 | 37 (4.08) |
| Topiramate88,135,142 | 100 mg | Twice daily | 707 | 29 (4.1) |
| Amitriptyline135 | 25–100 mg | Twice daily | 169 | 8 (4.73) |
Appendix 7 Literature searches for cost-effectiveness studies
Overview
| Bibliographic databases | ||
| Database | Date searched | Number of records |
| MEDLINE All, 1946–3 September 2021 (via Ovid) | 6 September 2021 | 568 |
| EMBASE Classic + EMBASE, 1947–3 September 2021 (via Ovid) | 6 September 2021 | 2531 |
| EconLit (via EBSCOhost) | 6 September 2021 | 66 |
| NHS EED (via CRD website) | 6 September 2021 | 116 |
| HTA database (via CRD website) | 6 September 2021 | 123 |
| International HTA database (via INAHTA website) | 6 September 2021 | 138 |
| Cost-effectiveness Analysis Registry (via Tufts Medical Center website) | 6 September 2021 | 32 |
| EconPapers [via Research Papers in Economics (RePEc)] | 6 September 2021 | 30 |
|
Total number of records retrieved: 3604
Duplicates removed (EndNote): 677 Final number for screening: 2927 |
||
| Other sources | ||
| Source | Date searched | Documents retrieved |
| NICE website | 7 September 2021 | 25 |
| SMC website | 7 September 2021 | 5 |
| AWMSG website | 7 September 2021 | 0 |
| CADTH website | 7 September 2021 | 14; plus 1 record of ongoing review |
| 13 September 2021 | 5 | |
| Google Scholar | 13 September 2021 | 1 |
|
Total number sought for retrieval: 50
Reports not retrieved/available: 0 Final number for screening: 50 (+1 ongoing review) |
||
| Bibliographic databases – update search (with three additional drug terms where applicable), November 2022 | ||
| Database | Date searched | Number of records |
| MEDLINE All (via Ovid) | 10 November 2022 | 644 |
| EMBASE (via Ovid) | 10 November 2022 | 2767 |
| EconLit (via EBSCOhost) | 10 November 2022 | 76 |
| NHS EED (via CRD) | As these databases are no longer updated, searches were not re-run; however the original search results were rescreened for any studies relating to riboflavin, coenzyme Q10 or magnesium (0 found) | |
| HTA database (via CRD) | ||
| International HTA database (via INAHTA website) | 10 November 2022 | 157 |
| Cost-effectiveness Analysis Registry (via Tufts Medical Center website) | 10 November 2022 | 34 |
| EconPapers [via Research Papers in Economics (RePEc)] | 14 November 2022 | 27 |
|
Total number of records retrieved: 3705
Duplicates removed within this set (EndNote): 546 Duplicates removed against previous searches (EndNote): 2819 Final number for screening: 340 |
||
| Other sources – update search (with three additional drug terms), November 2022 | ||
| Source | Date searched | Documents retrieved |
| NICE website | 15 November 2022 | 6 |
| SMC website | 16 November 2022 | 0 |
| AWMSG website | 16 November 2022 | 0; 2 records of ongoing NICE TAs |
| CADTH website | 16 November 2022 | 4; plus 2 records of ongoing/suspended reviews |
| 16 November 2022 | 3 | |
| Google Scholar | 16 November 2022 | 8 |
|
Total number sought for retrieval: 21
Reports not retrieved/available: 0 Final number for screening: 21 (+3 ongoing reviews and 1 suspended review) |
||
| Citation tracking | ||
| Source | Date searched | Number of records |
| Reference lists – included studies (Web of Science and Citation Finder) | 16 May 2022 and 25 May 2022 | 255 |
| Forwards citation tracking: Web of Science | 30 November 2022 | 62 |
| Forwards citation tracking: Google Scholar (for studies not found in Web of Science only) | 30 November 2022 | 49 |
|
Total number of records retrieved: 366
Duplicates removed (both within this set and against previous searches) (EndNote): 160 Final number for screening: 206 |
||
| Checking for retraction notices, errata and comments relating to included articles | ||
| Source | Date searched | Number of records |
| MEDLINE All (via Ovid) | 22 November 2022 | 1, not related to included studies |
| EMBASE (via Ovid) | 22 November 2022 | 0 |
| Retraction Watch website | 22 November 2022 | 0 |
| Total number of records retrieved: 0 | ||
| Additional search for utility data | ||
| Database | Date searched | Number of records |
| MEDLINE All (via Ovid) | 21 November 2022 | 860 |
| Cost-effectiveness Analysis Registry (via Tufts Medical Center website) | 21 November 2022 | 118 |
| ISPOR Presentations Database (via ISPOR website) | 21 November 2022 | 32 |
| ScHARRHUD | 21 November 2022 | 2 |
| EQ-5D website | 22 November 2022 | 0 |
| Total number of records retrieved: 1012 | ||
Search strategies: original searches, September 2021
MEDLINE (via Ovid)
Date searched: 6 September 2021
Database: Ovid MEDLINE(R) ALL <1946 to 3 September 2021>
Search strategy:
----------- ----------- ------------- -------------- --------------- ----------------
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kf,ti. (112,847)
-
Headache/ or exp Headache Disorders/ (61,218)
-
1 or 2 [population: migraine/headache] (124,069)
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kf,ti. (216,382)
-
Calcitonin Gene-Related Peptide/ai (436)
-
Antibodies, Monoclonal/ or Antibodies, Monoclonal, Humanized/ (216,971)
-
Calcitonin Gene-Related Peptide Receptor Antagonists/ (700)
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kf,ti,nm. (506)
-
(brogepant or brogepant or atogepant or gepant?).ab,kf,ti,nm. (213)
-
exp Botulinum Toxins/ (17,099)
-
(botulin* adj toxin*).ab,kf,ti,nm. (21,932)
-
(botulinum* or botox* or onabotulinum*).ab,kf,ti,nm. (25,143)
-
(antidepress* or anti depress*).ab,kf,ti. (73,848)
-
exp Antidepressive Agents/ (153,091)
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kf,ti,nm. (17,952)
-
exp ‘Serotonin and Noradrenaline Reuptake Inhibitors’/ (5001)
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kf,ti. (2907)
-
exp Angiotensin Converting Enzyme Inhibitors/ (45,311)
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kf,ti. (37,925)
-
acei.ab,kf,ti. (4337)
-
lisinopril.ab,kf,ti,nm. (3085)
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kf,ti. (14,463)
-
(ARB or ARBs).ab,kf,ti. (7863)
-
exp Angiotensin Receptor Antagonists/ (25,388)
-
candesartan.ab,kf,ti,nm. (3374)
-
((beta adj3 block*) or betablock*).ab,kf,ti. (55,677)
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagonist* or block*)).ab,kf,ti. (34,501)
-
exp Adrenergic beta-Antagonists/ (85,429)
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kf,ti,nm. (67,109)
-
(calcium adj2 (block* or antagonis* or inhibit*)).ab,kf,ti. (41,544)
-
(CCB or CCBs).ab,kf,ti. (2617)
-
exp Calcium Channel Blockers/ (88,521)
-
(flunarizine or verapamil).ab,kf,ti,nm. (27,699)
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kf,ti. (53,578)
-
exp Anticonvulsants/ (147,133)
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kf,ti,nm. (31,187)
-
Pizotyline/ (250)
-
(pizotifen or pizotyline).ab,kf,ti,nm. (418)
-
(alpha adj4 agonist*).ab,kf,ti. (15,366)
-
exp Adrenergic alpha-Agonists/ (164,048)
-
(clonidine or guanfacine).ab,kf,ti,nm. (19,179)
-
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 [Interventions: named drugs/drug classes or types] (1,098,078)
-
Economics/ (27,362)
-
exp ‘Costs and Cost Analysis’/ (248,833)
-
Economics, Nursing/ (4006)
-
Economics, Medical/ (9151)
-
Economics, Pharmaceutical/ (3015)
-
exp Economics, Hospital/ (25,285)
-
Economics, Dental/ (1919)
-
exp ‘Fees and Charges’/ (30,859)
-
exp Budgets/ (13,884)
-
budget*.ti,ab,kf. (32,036)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kf. (248,167)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2 (323,416)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf. (179,847)
-
(value adj2 (money or monetary)).ti,ab,kf. (2646)
-
exp models, economic/ (15,779)
-
economic model*.ab,kf. (3649)
-
markov chains/ (15,222)
-
markov.ti,ab,kf. (24,937)
-
monte carlo method/ (30,091)
-
monte carlo.ti,ab,kf. (53,356)
-
exp Decision Theory/ (12,574)
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kf. (28,316)
-
43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 [economic evaluations/cost/economic models filter] (791,472)
-
3 and 42 and 65 [population + named drug interventions + economic filter] (209)
-
exp Migraine Disorders/dt, pc (9891)
-
‘migrain*’.ab,hw,kf,ti. (42,481)
-
((prevent* or prophyla*) adj2 (treatment? or therap* or medication? or drug?)).ab,hw,kf,ti. (173,556)
-
((pharmacolog* or pharmaceutical or drug? or medical) adj1 (treatment? or therap* or management)).ab,hw,kf,ti. (455,613)
-
68 and (69 or 70) (4510)
-
67 or 71 (12,167)
-
65 and 72 [economics filter + general terms for migraine prevention/drug treatment] (477)
-
66 or 73 (568)
The migraine/headache search terms (lines 1–3) and Botox search terms (lines 10–12) are based on those used in:
Herd CP, Tomlinson CL, Rick C, Scotton WJ, Edwards J, Ives N, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev 2018;6:CD011616. https://doi.org/10.1002/14651858.CD011616.pub2
The search filter for economic and cost studies (lines 43–65) is the CADTH filter for Economic Evaluations/Cost/Economic Models – Ovid MEDLINE:
Strings attached: CADTH database search filters (Internet). Ottawa: CADTH; 2016. Available from: www.cadth.ca/resources/finding-evidence/.
EMBASE (via Ovid)
Date searched: 6 September 2021
Database: EMBASE Classic+EMBASE <1947 to 3 September 2021>
Search Strategy:
----------- ------------ ----------- ------------ -------------- --------------------
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kw,ti. (186,676)
-
‘headache and facial pain’/ or exp chronic daily headache/ or headache/ or exp migraine/ or vascular headache/ (294,055)
-
headache/si not exp ‘headache and facial pain’/dm, dt, pc, th (78,809)
-
(1 or 2) not 3 [population: migraine/headache; not as side effect only] (253,367)
-
antimigraine agent/ (2568)
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kw,ti. (274,669)
-
exp calcitonin gene related peptide receptor antagonist/ (3872)
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kw,ti,tn. (1445)
-
(rimegepant or ubrogepant or atogepant or gepant?).ab,kw,ti,tn. (465)
-
botulinum toxin/ or botulinum toxin A/ (39,609)
-
(botulin* adj toxin*).ab,kw,ti,tn. (23,,041)
-
(botulinum* or botox* or onabotulinum*).ab,kw,ti,tn. (34504)
-
(antidepress* or anti depress*).ab,kw,ti. (108,538)
-
exp antidepressant agent/ (515,062)
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kw,ti,tn. (22,238)
-
exp serotonin noradrenalin reuptake inhibitor/ (200,859)
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kw,ti. (4807)
-
exp dipeptidyl carboxypeptidase inhibitor/ (184,019)
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kw,ti. (55,283)
-
acei.ab,kw,ti. (9041)
-
lisinopril.ab,kw,ti,tn. (4455)
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kw,ti. (22,206)
-
(ARB or ARBs).ab,kw,ti. (15,636)
-
exp angiotensin receptor antagonist/ (100,617)
-
candesartan.ab,kw,ti,tn. (4072)
-
((beta adj3 block*) or betablock*).ab,kw,ti. (83,000)
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagonist* or block*)).ab,kw,ti. (44,164)
-
exp beta adrenergic receptor blocking agent/ (316,392)
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kw,ti,tn. (69,423)
-
(calcium adj2 (block* or antagonis* or inhibit*)).ab,kw,ti. (55,268)
-
(CCB or CCBs).ab,kw,ti. (4499)
-
exp calcium antagonist/ (289,477)
-
(flunarizine or verapamil).ab,kw,ti,tn. (29,545)
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kw,ti. (84,420)
-
exp anticonvulsive agent/ (451,825)
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kw,ti,tn. (43,812)
-
pizotifen/ (1970)
-
(pizotifen or pizotyline).ab,kw,ti,tn. (443)
-
(alpha adj4 agonist*).ab,kw,ti. (12,525)
-
exp alpha 2 adrenergic receptor stimulating agent/ (114,971)
-
(clonidine or guanfacine).ab,kw,ti,tn. (19,862)
-
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 [named drug or drug class interventions] (1,819,269)
-
Economics/ (244,858)
-
Cost/ (63,187)
-
exp Health Economics/ (917,483)
-
Budget/ (31,270)
-
budget*.ti,ab,kw. (43,208)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kw. (313,082)
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2 (458,270)
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kw. (252,987)
-
(value adj2 (money or monetary)).ti,ab,kw. (3646)
-
Statistical Model/ (167,169)
-
economic model*.ab,kw. (5435)
-
Probability/ (123,900)
-
markov.ti,ab,kw. (32,912)
-
monte carlo method/ (44,164)
-
monte carlo.ti,ab,kw. (55,487)
-
Decision Theory/ (1821)
-
Decision Tree/ (15,564)
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kw. (39,985)
-
or/43-60 [Filter for economic studies] (1,788,926)
-
4 and 42 and 61 (2163)
-
exp migraine/dt, pc [Drug Therapy, Prevention] (18,205)
-
‘migrain*’.ab,hw,kw,ti. (79,663)
-
((prevent* or prophyla*) adj2 (treatment? or therap* or medication? or drug?)).ab,hw,kw,ti. (247,454)
-
((pharmacolog* or pharmaceutical or drug? or medical) adj1 (treatment? or therap* or management)).ab,hw,kw,ti. (1,207,792)
-
64 and (65 or 66) (12,279)
-
63 or 67 (27,128)
-
61 and 68 [economics filter + general terms for migraine prevention/drug treatment] (2170)
-
62 or 69 (3083)
-
conference abstract.pt. (4,170,650)
-
70 not 71 (2531)
The search filter for economic and cost studies (lines 43–61) is the CADTH filter for Economic Evaluations/Cost/Economic Models – OVID EMBASE:
Strings attached: CADTH database search filters (Internet). Ottawa: CADTH; 2016. Available from: www.cadth.ca/resources/finding-evidence/
EconLit (via EBSCOhost)
Date searched: 6 September 2021
Database: EconLit with Full Text
Search modes – Boolean/Phrase
Search Screen – Advanced Search
| # | Query | Results |
|---|---|---|
| S5 | S1 AND S4 | 66 |
| S4 | S2 OR S3 | 255,123 |
| S3 | AB (therap* or treat* or prevent* or prophyla* or management) OR TI (therap* or treat* or prevent* or prophyla* or management) | 134,407 |
| S2 | TX pharmac* OR health* or medic* or drug or drugs | 138,404 |
| S1 | AB (headache* or ‘head ache*’ or migrain* or cephalgi* or cephalalgi* or hemicrani*) OR SU (headache* or ‘head ache*’ or migrain* or cephalgi* or cephalalgi* or hemicrani*) OR TI (headache* or ‘head ache*’ or migrain* or cephalgi* or cephalalgi* or hemicrani*) | 88 |
NHS Economic Evaluation Database (NHS EED) and HTA database (via CRD) www.crd.york.ac.uk/CRDWeb/
Date searched: 6 September 2021
| Search | Hits |
|---|---|
| (headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*) [All fields] IN NHSEED | 116 |
| (headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*) [All fields] IN HTA | 123 |
International HTA database (via INAHTA website) https://database.inahta.org/
Date searched: 6 September 2021
| Line | Query | Hits |
|---|---|---|
| 4 | #1 OR #2 OR #3 | 138 |
| 3 | ‘Headache Disorders’[mhe] | 57 |
| 2 | ‘Headache’[mh] | 30 |
| 1 | headache* or ‘head ache*’ or migrain* or cephalgi* or cephalalgi* or hemicrani* | 135 |
Cost-effectiveness Analysis Registry (via Tufts Medical Center website) https://cevr.tuftsmedicalcenter.org/databases/cea-registry
Date searched: 6 September 2021
Basic search screen: Methods selected
Results of each search were copied and pasted into Excel, to easily identify unique results, which were then found in PubMed for easy export/import into EndNote.
| Search term/s | Results |
|---|---|
| headache | 24 |
| head ache | 0 |
| migraine | 21, of which 8 unique/13 already found with ‘headache’ search |
| Total unique results: | 32 |
EconPapers [via Research Papers in Economics (RePEc)] https://econpapers.repec.org/
Date searched: 6 September 2021
Advanced search screen: https://econpapers.repec.org/scripts/search.pf
30 documents matched the search for (headache* OR ‘head ache*’ OR migrain*) AND (pharmac* OR medic* OR drug OR drugs OR therap* OR treat* OR prevent* OR prophyla*) in titles and keywords in working papers, articles, books and chapters.
National Institute for Health and Care Excellence website www.nice.org.uk/
Date searched: 7 September 2021
Browsed Guidance section: Conditions and diseases > Neurological conditions > Headaches
23 published products on this topic:
25 documents relating to 6 published guidelines or other evidence reviews were judged to contain potentially useful information for the cost-effectiveness review.
Scottish Medicines Consortium website www.scottishmedicines.org.uk/
Date searched: 7 September 2021
Search box on homepage:
Migraine 12 results
headache 6 results, none unique
5 documents relating to 5 drugs were judged to contain potentially useful information for the cost-effectiveness review
All Wales Medicines Strategy Group (AWMSG) website https://awmsg.nhs.wales/
Date searched: 7 September 2021
Search box on homepage:
migraine 3 results, all refer to NICE technology appraisals identified above
headache 0 results
0 documents for retrieval
Canadian Agency for Drugs and Technologies in Health (CADTH) website https://cadth.ca/
Date searched: 7 September 2021
Search box on homepage, results limited to ‘Reports’ tab.
migraine 30 results, of which 8 potentially relevant
headache 40 results, of which 1 potentially relevant and not already identified
14 documents relating to 9 projects/reviews were judged to contain potentially useful information for the cost-effectiveness or clinical reviews; 1 ongoing reimbursement review also identified.
Google www.google.co.uk
Date searched: 13 September 2021
Results (10 per page) were browsed until yielding very few results containing all search terms.
Documents were retrieved if judged to be potentially useful for the cost-effectiveness review, and if they had not already been identified via the bibliographic databases or HTA websites searches.
| Search string | Number of results browsed | Documents retrieved |
|---|---|---|
| migraine prevention OR prophylaxis technology assessment | 30 | 0 |
| migraine prevention OR prophylaxis economic | 60 | 3 (2 × ICER reports; Clarke et al., 1996) |
| migraine prevention OR prophylaxis cost-effectiveness | 60 | 2 [NCPE Ireland report on fremanezumab, after which checked www.ncpe.ie for further reports on migraine (using ‘migraine’ in website search box) and identified a further report on erenumab] |
| Total documents retrieved: | 5 |
Google Scholar https://scholar.google.co.uk
Date searched: 13 September 2021
Results (10 per page) were browsed until yielding very few results containing all search terms.
Documents were retrieved if judged to be potentially useful for the cost-effectiveness review, and if they had not already been identified via the bibliographic databases, HTA websites or Google searches.
| Search string | Number of results browsed | Documents retrieved |
|---|---|---|
| migraine prevention OR prophylaxis technology assessment | 20 | 0 |
| migraine prevention OR prophylaxis economic | 60 | 1 (Serrano et al., 2013) |
| migraine prevention OR prophylaxis cost-effectiveness | 70 | 0 |
| migraine prevention OR prophylaxis costs | 20 | 0 |
| Total documents retrieved: | 1 |
Search strategies: update searches, November 2022
MEDLINE (via Ovid)
Date searched: 10 November 2022
Ovid MEDLINE(R) ALL <1946 to 9 November 2022>
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kf,ti. 12,1183
-
Headache/ or exp Headache Disorders/ 64,883
-
1 or 2 [population: migraine/headache, based on Cochrane botox review] 132,533
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kf,ti. 224,447
-
Calcitonin Gene-Related Peptide/ai 463
-
Antibodies, Monoclonal/ or Antibodies, Monoclonal, Humanized/ 227,782
-
Calcitonin Gene-Related Peptide Receptor Antagonists/ 890
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kf,ti,nm. 728
-
(rimegepant or ubrogepant or atogepant or gepant?).ab,kf,ti,nm. 302
-
exp Botulinum Toxins/ 18,168
-
(botulin* adj toxin*).ab,kf,ti,nm. 23,254
-
(botulinum* or botox* or onabotulinum*).ab,kf,ti,nm. 26,590
-
(antidepress* or anti depress*).ab,kf,ti. 78,227
-
exp Antidepressive Agents/ 158,386
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kf,ti,nm. 18,644
-
exp ‘Serotonin and Noradrenaline Reuptake Inhibitors’/ 5339
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kf,ti. 3142
-
exp Angiotensin Converting Enzyme Inhibitors/ 46,784
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kf,ti. 39,275
-
acei.ab,kf,ti. 4756
-
lisinopril.ab,kf,ti,nm. 3161
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kf,ti. 15,381
-
(ARB or ARBs).ab,kf,ti. 8696
-
exp Angiotensin Receptor Antagonists/ 27,199
-
candesartan.ab,kf,ti,nm. 3451
-
((beta adj3 block*) or betablock*).ab,kf,ti. 57,496
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagonist* or block*)).ab,kf,ti. 34,884
-
exp Adrenergic beta-Antagonists/ 86,681
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kf,ti,nm. 68,133
-
(calcium adj2 (block* or antagonis* or inhibit*)).ab,kf,ti. 42,422
-
(CCB or CCBs).ab,kf,ti. 2832
-
exp Calcium Channel Blockers/ 90,332
-
(flunarizine or verapamil).ab,kf,ti,nm. 28,047
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kf,ti. 55,700
-
exp Anticonvulsants/ 152,024
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kf,ti,nm. 32,859
-
Pizotyline/ 252
-
(pizotifen or pizotyline).ab,kf,ti,nm. 425
-
(alpha adj4 agonist*).ab,kf,ti. 15,650
-
exp Adrenergic alpha-Agonists/ 166,822
-
(clonidine or guanfacine).ab,kf,ti,nm. 19,417
-
Riboflavin/ or Ubiquinone/ or Magnesium/ or exp Magnesium Compounds/ [additional drugs identified 2022] 104,342
-
(riboflavin or vitamin b2 or vitamin b 2 or coenzyme q* or co enzyme q* or ubidecarenone or ubiquino* or coq10 or co q10 or magnesium).ab,kf,ti,nm. [additional drugs identified 2022] 147,923
-
4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 [Interventions: named drugs/drug classes or types] 1,275,996
-
Economics/ 27,469
-
exp ‘Costs and Cost Analysis’/ 261,027
-
Economics, Nursing/ 4013
-
Economics, Medical/ 9230
-
Economics, Pharmaceutical/ 3084
-
exp Economics, Hospital/ 25,645
-
Economics, Dental/ 1920
-
exp ‘Fees and Charges’/ 31,239
-
exp Budgets/ 14,053
-
budget*.ti,ab,kf. 34,526
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kf. 269,051
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2 359,511
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kf. 198,450
-
(value adj2 (money or monetary)).ti,ab,kf. 2893
-
exp models, economic/ 16,156
-
economic model*.ab,kf. 4009
-
markov chains/ 15,834
-
markov.ti,ab,kf. 27,574
-
monte carlo method/ 31,696
-
monte carlo.ti,ab,kf. 57,654
-
exp Decision Theory/ 12,981
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kf. 34,084
-
45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 [economic evaluations/cost/economic models filter from CADTH www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters#eco] 858,537
-
3 and 44 and 67 [population + named drug interventions + economic filter] 268
-
exp Migraine Disorders/dt, pc 10,729
-
‘migrain*’.ab,hw,kf,ti. 45,173
-
((prevent* or prophyla*) adj2 (treatment? or therap* or medication? or drug?)).ab,hw,kf,ti. 189,003
-
((pharmacolog* or pharmaceutical or drug? or medical) adj1 (treatment? or therap* or management)).ab,hw,kf,ti. 477,844
-
70 and (71 or 72) 4964
-
69 or 73 13,104
-
67 and 74 [economics filter + general terms for migraine prevention/drug treatment] 532
-
68 or 75 644
EMBASE (via Ovid)
Date searched: 10 November 2022
EMBASE Classic+EMBASE <1947 to 9 November 2022>
-
(headache* or head ache* or migrain* or cephalgi* or cephalalgi* or hemicrani*).ab,kw,ti. 202,354
-
‘headache and facial pain’/ or exp chronic daily headache/ or headache/ or exp migraine/ or vascular headache/ 322,004
-
headache/si not exp ‘headache and facial pain’/dm, dt, pc, th 84,245
-
(1 or 2) not 3 278,483
-
antimigraine agent/ 2699
-
(((calcitonin gene-related peptide or CGRP) adj5 (antibod* or antagon* or inhibit* or block*)) or anti-CGRP or anti-calcitonin gene-related peptide or monoclonal antibod* or mAb or mAbs or moAb or moAbs).ab,kw,ti. 287,217
-
exp calcitonin gene related peptide receptor antagonist/ 5139
-
(erenumab or galcanezumab or fremanezumab or eptinezumab).ab,kw,ti,tn. 2207
-
(rimegepant or ubrogepant or atogepant or gepant?).ab,kw,ti,tn. 797
-
botulinum toxin/ or botulinum toxin A/ 42,654
-
(botulin* adj toxin*).ab,kw,ti,tn. 23,636
-
(botulinum* or botox* or onabotulinum*).ab,kw,ti,tn. 36,798
-
(antidepress* or anti depress*).ab,kw,ti. 114,256
-
exp antidepressant agent/ 568,155
-
(amitriptyline or venlafaxine or mirtazapine or duloxetine).ab,kw,ti,tn. 23,369
-
exp serotonin noradrenalin reuptake inhibitor/ 211,940
-
(SNRI or SNRIs or (serotonin adj2 (noradrenaline or norepinephrine) adj reuptake inhib*)).ab,kw,ti. 5037
-
exp dipeptidyl carboxypeptidase inhibitor/ 195,496
-
(Angiotensin Converting Enzyme Inhibit* or ACE inhibit*).ab,kw,ti. 57,500
-
acei.ab,kw,ti. 9819
-
lisinopril.ab,kw,ti,tn. 4705
-
((angiotensin receptor or angiotensin II receptor) adj (block* or antagon*)).ab,kw,ti. 22,240
-
(ARB or ARBs).ab,kw,ti. 17,026
-
exp angiotensin receptor antagonist/ 111,581
-
candesartan.ab,kw,ti,tn. 4182
-
((beta adj3 block*) or betablock*).ab,kw,ti. 84,862
-
((adrenergic or adrenoreceptor* or adrenoceptor*) adj3 (antagonist* or block*)).ab,kw,ti. 43,408
-
exp beta adrenergic receptor blocking agent/ 333,504
-
(propranolol or metoprolol or timolol or atenolol or nadolol or nebivolol or pindolol).ab,kw,ti,tn. 71,299
-
(calcium adj2 (block* or antagonis* or inhibit*)).ab,kw,ti. 54,855
-
(CCB or CCBs).ab,kw,ti. 4827
-
exp calcium antagonist/ 340,528
-
(flunarizine or verapamil).ab,kw,ti,tn. 30,070
-
(anticonvuls* or antiepilep* or anti convuls* or anti epilep*).ab,kw,ti. 88,096
-
exp anticonvulsive agent/ 491290
-
(topiramate or valproate or divalproex or valproic acid or gabapentin).ab,kw,ti,tn. 46,687
-
pizotifen/ 2006
-
(pizotifen or pizotyline).ab,kw,ti,tn. 450
-
(alpha adj4 agonist*).ab,kw,ti. 12,572
-
exp alpha 2 adrenergic receptor stimulating agent/ 126,725
-
(clonidine or guanfacine).ab,kw,ti,tn. 20,327
-
exp riboflavin/ or ubidecarenone/ or magnesium/ or magnesium citrate/ or magnesium sulfate/ or magnesium oxide/ or magnesium derivative/ [additional drugs identified 2022] 160,786
-
(riboflavin or vitamin b2 or vitamin b 2 or coenzyme q* or co enzyme q* or ubidecarenone or ubiquino* or coq10 or co q10 or magnesium).ab,kw,ti,tn. [additional drugs identified 2022] 119,146
-
5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 2,136,027
-
Economics/ 246,769
-
Cost/ 64,938
-
exp Health Economics/ 1,002,939
-
Budget/ 32,775
-
budget*.ti,ab,kw. 45,859
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ti,kw. 305,827
-
(economic* or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic* or pharmaco-economic* or expenditure or expenditures or expense or expenses or financial or finance or finances or financed).ab./freq = 2 505,669
-
(cost* adj2 (effective* or utilit* or benefit* or minimi* or analy* or outcome or outcomes)).ab,kw. 269,096
-
(value adj2 (money or monetary)).ti,ab,kw. 3909
-
Statistical Model/ 172,230
-
economic model*.ab,kw. 5843
-
Probability/ 137,823
-
markov.ti,ab,kw. 34,476
-
monte carlo method/ 47,954
-
monte carlo.ti,ab,kw. 58,507
-
Decision Theory/ 1848
-
Decision Tree/ 18,902
-
(decision* adj2 (tree* or analy* or model*)).ti,ab,kw. 45,947
-
or/45-62 [Filter for economic studies from CADTH: www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters#health] 1,934,921
-
4 and 44 and 63 2509
-
exp migraine/dt, pc [Drug Therapy, Prevention] 19,315
-
‘migrain*’.ab,hw,kw,ti. 86,560
-
((prevent* or prophyla*) adj2 (treatment? or therap* or medication? or drug?)).ab,hw,kw,ti. 269,120
-
((pharmacolog* or pharmaceutical or drug? or medical) adj1 (treatment? or therap* or management)).ab,hw,kw,ti. 1,299,091
-
66 and (67 or 68) 14,004
-
65 or 69 29,664
-
63 and 70 2398
-
64 or 71 3457
-
conference abstract.pt. 4,588,873
-
72 not 73 2767
EconLit (via EBSCOhost)
Date searched: 10 November 2022
Database: EconLit with Full Text
Search modes – Boolean/Phrase
Search Screen – Advanced Search
| # | Query | Results |
|---|---|---|
| S5 | S1 AND S4 | 76 |
| S4 | S2 OR S3 | 275, 263 |
| S3 | AB (therap* or treat* or prevent* or prophyla* or management) OR TI (therap* or treat* or prevent* or prophyla* or management) | 143, 783 |
| S2 | TX pharmac* OR health* or medic* or drug or drugs | 151, 216 |
| S1 | AB (headache* or ‘head ache*’ or migrain* or cephalgi* or cephalalgi* or hemicrani*) OR SU (headache* or ‘head ache*’ or migrain* or cephalgi* or cephalalgi* or hemicrani*) OR TI (headache* or ‘head ache*’ or migrain* or cephalgi* or cephalalgi* or hemicrani*) | 99 |
International HTA database (via INAHTA website) https://database.inahta.org/
Date searched: 10 November 2022
| Line | Query | Hits |
|---|---|---|
| 4 | #1 OR #2 OR #3 | 157 |
| 3 | ‘Headache Disorders’[mhe] | 69 |
| 2 | ‘Headache’[mh] | 32 |
| 1 | headache* or ‘head ache*’ or migrain* or cephalgi* or cephalalgi* or hemicrani* | 154 |
Cost-effectiveness Analysis Registry (via Tufts Medical Center website) https://cevr.tuftsmedicalcenter.org/databases/cea-registry
Date searched: 10 November 2022
Basic search screen: Methods selected
Results of each search were copied and pasted into Excel, to easily identify unique results, which were then found in PubMed for easy export/import into EndNote.
| Search term/s | Results |
|---|---|
| headache | 22 |
| head ache | 0 |
| migraine | 23, of which 12 unique/11 already found with ‘headache’ search |
| Total unique results: | 34 |
EconPapers [via Research Papers in Economics (RePEc)] https://econpapers.repec.org/
Date searched: 14 November 2022
Advanced search screen: https://econpapers.repec.org/scripts/search.pf
27 documents matched the search for (headache* OR ‘head ache*’ OR migrain*) AND (pharmac* OR medic* OR drug OR drugs OR therap* OR treat* OR prevent* OR prophyla*) in titles and keywords in working papers, articles, books and chapters that were added to EconPapers in the last 2 years.
National Institute for Health and Care Excellence website www.nice.org.uk/
Date searched: 15 November 2022
Browsed Guidance section: Conditions and diseases > Neurological conditions > Headaches
20 published products on this topic:
6 documents relating to 1 technology appraisal guidance and 1 clinical guideline new/updated since the previous search and judged to contain potentially useful information
Scottish Medicines Consortium website www.scottishmedicines.org.uk/
Date searched: 16 November 2022
Search box on homepage:
migraine 13 results
headache 6 results, 0 unique/new since previous search
0 documents were new/updated since the previous search and judged to contain potentially useful information
All Wales Medicines Strategy Group (AWMSG), via All Wales Therapeutics and Toxicology Centre website https://awttc.nhs.wales/
Date searched: 16 November 2022
Search box on homepage:
migraine 6 results, of which 3 refer to NICE technology appraisals identified above, 2 refer to ongoing NICE technology appraisals and 1 is a review article (not AWMSG)
headache 9 results, of which 0 are relevant/AWMSG documents
0 documents for retrieval, 2 ongoing NICE appraisals identified
Canadian Agency for Drugs and Technologies in Health (CADTH) website https://cadth.ca/
Date searched:
Search box on homepage, results limited to ‘Reports’ tab.
migraine 87 results, of which 4 potentially relevant
headache 556 results, browsed first 60 results (sorted by relevance), by which point no relevant results were appearing (non-headache conditions). 0 potentially relevant and not already identified
4 documents relating to 2 projects/reviews were judged to contain potentially useful information for the cost-effectiveness or clinical reviews; 1 ongoing reimbursement review and 1 suspended review were also identified.
Google www.google.co.uk
Date searched: 16 November 2022
Results (10 per page) were browsed until yielding very few results containing all search terms.
Documents were retrieved if judged to be potentially useful for the cost-effectiveness review, and if they had not already been identified via the bibliographic databases or HTA websites searches.
| Search string | Number of results browsed | Documents retrieved |
|---|---|---|
| migraine prevention OR prophylaxis technology assessment date range: 1 Sept 2021 – 16 Nov 2022 |
30 | 3 |
| migraine prevention OR prophylaxis economic date range: 1 Sept 2021 – 16 Nov 2022 |
40 | 0 |
| migraine prevention OR prophylaxis cost-effectiveness date range: 1 Sept 2021 – 16 Nov 2022 |
50 | 0 |
| Total documents retrieved: | 3 |
Google Scholar https://scholar.google.co.uk
Date searched: 16 November 2022
Results (10 per page) were browsed until yielding very few results containing all search terms.
Documents were retrieved if judged to be potentially useful for the cost-effectiveness review, and if they had not already been identified via the bibliographic databases, HTA websites or Google searches.
| Search string | Number of results browsed | Documents retrieved |
|---|---|---|
| migraine prevention OR prophylaxis technology assessment Since 2021 |
30 | 1 |
| migraine prevention OR prophylaxis economic Since 2021 |
50 | 2 |
| migraine prevention OR prophylaxis cost-effectiveness Since 2021 |
50 | 5 |
| migraine prevention OR prophylaxis costs Since 2021 |
30 | 0 |
| Total documents retrieved | 8 |
Reference lists search (included studies; journal articles only)
Web of Science Core Collection: Science Citation Index Expanded–1970–present; ***Social Sciences Citation Index (SSCI)–1900–present; Arts and Humanities Citation Index (AHCI)–1975–present; Conference Proceedings Citation Index – Science (CPCI-S)–1990–present; Conference Proceedings Citation Index – Social Science and Humanities (CPCI-SSH)–1990–present; Emerging Sources Citation Index (ESCI)–2015–present.
Date searched: 30 November 2022
Searched for each included study by combinations of author and title keywords
6/9 included study papers had records in Web of Science, yielding 219 reference list results.
Citation Finder https://citation-finder.vercel.app/
Date searched: 30 November 2022
Reference lists from Batty et al. , 2013 (36 references) and Ruggeri et al. , 2013 (30 references) were copied into Citation Finder, where 36 results were available/downloadable and exported to EndNote
The reference list from Vekov et al., 2019 could not be checked, due to being in non-Roman alphabet.
Forward citations search (included studies; journal articles only)
Web of Science Core Collection: Science Citation Index Expanded-1970–present; SSCI–1900–present; AHCI–1975–present; CPCI-S–1990–present; CPCI-SSH–1990–present; ESCI–2015–present.
Date searched: 30 November 2022
Searched for each included study by combinations of author and title keywords
6/9 included study papers had records in Web of Science, yielding 62 citing paper results
Google Scholar https://scholar.google.co.uk/
Date searched: 30 November 2022
2/3 study papers not found in Web of Science were found via Google Scholar; 1 had 0 citing papers in Google Scholar, 1 had 49 citing papers. Vekov et al. , 2019 was not found.
Searches to check for retraction notices, errata and comments relating to included journal articles
MEDLINE (Ovid) search strategy, date searched: 30 November 2022
Database: Ovid MEDLINE(R) ALL <1946 to 29 November 2022>
Search strategy:
---------- ------------ ---------- ----------- ------------ ------------- ------------
-
(‘23647483’ or ‘31302899’ or ‘32787820’ or ‘31578100’ or ‘29571276’ or ‘33491167’ or ‘30142988’).ui. (7) [these are the 7 included journal articles available in MEDLINE]
-
(cin or comment or con or concern or cri or crf or ecf or eci or efr or ein or erratum or expression or republished or retracted or retraction or rin or rof or rpf or rpi or rrf or rri or uin or uof or update).cm. (2,147,964)
-
1 and 2 (0)
-
((cost-effectiveness or economic or price range) and (onabotulinum* or erenumab)).mp. and (migraine or headache).ti. [mp = title, book title, abstract, original title, name of substance word, subject heading word, floating subheading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] (34)
-
(retracted publication or retraction of publication).pt. (25,344)
-
expression of concern.pt. or expression of concern.af. (2922)
-
(ecf or eci or rin or rof).cm. (27,157)
-
(retraction or retracted).ti. (16,522)
-
5 or 6 or 7 or 8 (33,054)
-
(comment or ‘corrected and republished article’ or published erratum).pt. (1,113,471)
-
(cin or con).cm. [‘comment in’ or ‘comment on’] (1,748,037)
-
(cri or crf or ecf or eci or efr or ein or rin or rof or rpf or rpi or rrf or rri or uin or uof).cm. (430,242)
-
(comment on or erratum or corrigendum or withdrawn).ti. (94,317)
-
9 or 10 or 11 or 12 or 13 (2,171,292)
-
4 and 14 (1)
0 retractions or comments.
1 erratum found – not related to any included studies.
EMBASE (Ovid) search strategy, date searched: 30 November 2022
Checking for Ruggeri et al., 2013 and Vekov et al., 2019 only, as these are not available in MEDLINE.
EMBASE Classic+EMBASE <1947 to 29 November 2022>
-
(‘369487386’ or ‘630983838’).rr.0[accession nos. of the 2 included journal articles not available in MEDLINE]
-
(cost-effectiveness or economic or price range).rt.745
-
(onabotulinum* or erenumab or CGRP).rt.61
-
(migraine or headache).rt.386
-
2 and 3 and 40
-
(cost-effectiveness or economic or price range).ti.96,194
-
(onabotulinum* or erenumab or CGRP).ti.5625
-
(migraine or headache).ti.63,709
-
6 and 7 and 828
-
erratum/ or ‘expression of concern’/ or retraction notice/262,872
-
Retracted article/13,016
-
yes.ne.5454
-
(erratum or tombstone).pt.269,407
-
10 or 11 or 12 or 13272,633
-
(retraction or retracted).ti.16,297
-
(comment on or erratum or corrigendum or withdrawn).ti.236,791
-
14 or 15 or 16312,777
-
9 and 170
No errata, retractions or comments found.
Retraction Watch Database http://retractiondatabase.org/RetractionSearch.aspx
Date searched: 22 November 2022
Searched for ‘migraine’ in Title field (as all included studies include this word in the title): 7 results, none of which are in the included studies.
Additional search for utility data to inform the economic model
MEDLINE (Ovid)
Date searched: 21 November 2022
Ovid MEDLINE(R) ALL <1946 to 18 November 2022>
-
exp Migraine Disorders/ 31,004
-
‘migrain*’.ab,kf,ti. 41,133
-
1 or 2 [migraine PRECISE] 45,319
-
Quality-Adjusted Life Years/ 15,238
-
(quality-adjusted or adjusted life year$).ti,ab,kf. 21,931
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab,kf. 13,787
-
(illness state$1 or health state$1).ti,ab,kf. 7951
-
(hui or hui1 or hui2 or hui3).ti,ab,kf. 1874
-
(multiattribute$ or multi attribute$).ti,ab,kf. 1221
-
(utility adj3 (score$1 or valu$ or health$ or cost$ or measur$ or disease$ or mean or gain or gains or index$)).ti,ab,kf. 19,025
-
utilities.ti,ab,kf. 8926
-
(eq-5d or eq5d or eq-5 or eq5 or euro qual or euroqual or euro qual5d or euroqual5d or euro qol or euroqol or euro qol5d or euroqol5d or euro quol or euroquol or euro quol5d or euroquol5d or eur qol or eurqol or eur qol5d or eur qol5d or eur?qul or eur?qul5d or euro$ quality of life or european qol).ti,ab,kf. 16,014
-
(euro$ adj3 (5 d or 5d or 5 dimension$ or 5dimension$ or 5 domain$ or 5domain$)).ti,ab,kf. 5587
-
(sf36$ or sf 36$ or sf thirtysix or sf thirty six).ti,ab,kf. 25,680
-
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).ti,ab,kf. 2259
-
quality of life/ and ((quality of life or qol) adj (score$1 or measure$1)).ti,ab,kf. 14,819
-
quality of life/ and ec.fs. 10,872
-
quality of life/ and (health adj3 status).ti,ab,kf. 11,245
-
(quality of life or qol).ti,ab,kf. and Cost-Benefit Analysis/ 7420
-
((qol or hrqol or quality of life).ti,kf. or *quality of life/) and ((qol or hrqol$ or quality of life) adj2 (increas$ or decrease$ or improv$ or declin$ or reduc$ or high$ or low$ or effect or effects or worse or score or scores or change$1 or impact$1 or impacted or deteriorat$)).ab. 50,021
-
Cost-Benefit Analysis/ and (cost-effectiveness ratio$ and (perspective$ or life expectanc$)).ti,ab,kf. 4925
-
*quality of life/ and (quality of life or qol).ti. 62,829
-
quality of life/ and ((quality of life or qol) adj3 (improv$ or chang$)).ti,ab,kf. 38,413
-
quality of life/ and health-related quality of life.ti,ab,kf. 42,320
-
models, economic/ 11,038
-
or/4-25 [Filter FSF – sensitivity maximizing filter to identify HSU studies, from Arber et al. , 2017 https://doi.org/10.1017/S0266462317000897] 209,664
-
3 and 26 860
Lines 4–26 are ‘filter FSF1 – sensitivity maximizing’ from Arber M, Garcia S, Veale T, Edwards M, Shaw A, Glanville JM. Performance of Ovid MEDLINE search filters to identify health state utility studies. Int J Technol Assess Health Care 2017;33:472–80. https://doi.org/10.1017/S0266462317000897
Cost-effectiveness Analysis Registry (via Tufts Medical Center website) https://cevr.tuftsmedicalcenter.org/databases/cea-registry
Date searched: 21 November 2022
Basic search screen: Utilities selected
| Search term/s | Results |
|---|---|
| migraine | 118 |
ISPOR Presentations Database
www.ispor.org/heor-resources/presentations-database/search
Date searched: 21 November 2022
| Search term/s (keyword field) | Results |
|---|---|
| migrain* AND (utilit* OR HSUV*) | 32, of which 8 duplicates = 24 unique results |
| migrain* AND disutilit* | 3 duplicates/already found above = 0 unique |
| migrain* AND (EQ-5D OR euroqol) | 21, of which 10 duplicates, 3 already found above = 8 unique |
| Total: 32 posters/records downloaded (posters downloaded where available) | |
ScHARRHUD
Date searched: 21 November 2022
Search: migrain* in Any field 3 results, of which 1 already found by MEDLINE search above
Total: 2 records downloaded
EQ-5D website
Search for EQ-5D documents: https://euroqol.org/publications/search-for-eq-5d-documents/
Date searched: 22 November 2022
migraine 0 results
headache 0 results
Appendix 8 Cost-effectiveness review – further information
Reproduced with permission from Khanal et al. (2022). 201 This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license, which permits others to copy, redistribute, remix, transform and build upon this work for any purpose, provided the original work is properly cited, a link to the licence is given, and indication of whether changes were made. See: https://creativecommons.org/licenses/by/4.0/. The table includes minor additions and formatting changes to the original table.
| Author, year, country | Objective(s) | Study design | Study population | Subgroups | Sample size (n) | Intervention | Comparators | Type of economic evaluation |
|---|---|---|---|---|---|---|---|---|
| Journal articles | ||||||||
| Batty, 2013167 UK | To evaluate the cost-effectiveness of BTA compared with placebo for the prophylaxis of headaches in adults with CM | Model- based economic evaluation | Participants in the Phase III PREEMPT Trial were considered for the model | The groups were: (1) Licensed population, of all CM participants (n = 401), (2) Participants who previously received 1 or more oral drugs (only topiramate was a licensed treatment for migraine) (n = 983), and (3) Participants who previously received 3 or more oral drugs (n = 439) | 1384 | BTA | Placebo | CUA |
| Giannouchos, 2019168 Greece | To evaluate the differences in costs and outcomes of erenumab versus BTA in CM participants | Model- based economic evaluation | Participants with CM who failed initial preventive treatment with BTA or erenumab. Adults with a mean age 41 years; and 86% were females | None | Not reported | Erenumab | BTA | CUA |
| Hansson-Hedblom, 2020169 Norway and Sweden | To describe the economic consequences of migraine using cost of illness survey data and the cost-effectiveness of BTA for the treatment of CM | Model- based economic evaluation | Participants in Phase III PREEMPT trial | As in other study using PREEMPT trial participants | Not reported | BTA | Placebo | CUA |
| Hollier-Hann, 2020170 UK | To evaluate the cost-effectiveness of BTA compared with placebo for the prophylaxis of headaches in adults with CM | Model- based economic evaluation | Participants with CM who have previously received three or more oral preventive therapies in PREEMPT trial | None | 439 | BTA | Placebo | CUA |
| Lipton, 2018171 USA | To estimate value-based pricing ranges for erenumab 140 mg, administered subcutaneously every 4 weeks, in patients who have failed at least 1 prior preventive treatment compared to BSC | Model- based economic evaluation | Participants that were either naıve to preventive treatment or previously treated with preventive medication but failed due to lack of efficacy or intolerability. The populations considered in the model are subgroups of participants who have previously failed 1 prior preventive therapy | CM and EM group | Not reported | Erenumab | Placebo (vs. BTA as a scenario analysis) | CUA |
| Mahon, 2021172 Sweden | To determine the cost-effectiveness of erenumab for the preventive treatment of migraine | Model- based economic evaluation | Participants with CM and EM. The base-case analysis for ‘total migraine’ assumed that 66.7% of the participants had CM and 33.3% had EM, which aligns with the reported percentage of participants with CM for whom prophylactic treatment fails | None | Not reported | Erenumab | Placebo (vs. BTA as a scenario analysis) | CUA |
| Ruggeri, 2013173 Italy | To evaluate the cost-effectiveness of BTA versus placebo in participants with CM | Model- based economic evaluation | Participants with CM from PREEMPT trial | None | 1384 patients. (n = 686 – BTA; n = 698 – placebo) | BTA | Placebo | CUA |
| Sussman, (2018174 USA | To assess the cost-effectiveness of erenumab for the prophylactic treatment of EM and CM | Model- based economic evaluation | Participant with EM and CM. The analyses were done separately | CM and EM groups | Not stated | Erenumab | Placebo (vs. BTA as a scenario analysis) | CUA |
| Vekov, 2019175 Bulgaria | To develop a model based on costs and health benefits of CGRP inhibitors | Model- based economic evaluation | Participants with EM and CM | CM and EM groups. For the CM group only participants who have not improved with standard preventive therapy were included | 667 | Erenumab | Preventative treatment | CUA |
| Other reports | ||||||||
| CADTH (BTA), 2019176 Canada | To compare cost-effectiveness of BTA with existing treatments | Canada | Model- based economic evaluation | Participants with CM from PREEMPT trial. Adult participants with CM, defined as headache 15 or more days per month and headache lasting 4 hours a day or longer | 1384 | BTA | BSC | CUA |
| CADTH (erenumab), 2019177 Canada |
To compare cost-effectiveness of erenumab with existing treatments | Canada | Model- based economic evaluation | Adult participants with CM, defined as headache 15 or more days per month and headache lasting 4 hours a day or longer. (Study 295, STRIVE trial and LIBERTY trial) | Not stated | Erenumab | BSC (vs. BTA in scenario analysis) | CUA |
| ICER^ (CGRP), 2018178 USA | To compare cost-effectiveness of CGRP inhibitors as the preventative treatments for participants with EM or CM | USA | Model- based economic evaluation | Patients with CM who fail initial preventive treatment with BTA or other treatment for the prevention of migraine attack | Not stated | Erenumab, fremanezumab | BSC (no preventative care) | CUA |
| NICE: erenumab, 2019180 UK | To compare cost-effectiveness of erenumab with existing treatments | UK | Model- based economic evaluation | Patients with CM who fail initial preventive treatment with BTA or other treatment for the prevention of migraine attack | 439 | Erenumab | BSC and BTA | CUA |
| NICE: fremanezumab, 2019179 UK | To compare cost-effectiveness of fremanezumab with existing treatments | UK | Model- based economic evaluation | Patients with CM who fail initial preventive treatment with BTA or other treatment for the prevention of migraine attack | 439 | Fremanezumab | BSC and BTA | CUA |
| NICE: galcanezumab, 2020181 UK | To compare cost-effectiveness of galcanezumab with existing treatments | UK | Model- based economic evaluation | Patients with CM who fail initial preventive treatment with BTA or other treatment for the prevention of migraine attack | 439 | Galcanezumab | BSC and BTA | CUA |
| Warwick Evidence, 2011182 UK | To compare cost-effectiveness of BTA with existing treatments | UK | Model- based economic evaluation | Patients in the Phase III PREEMPT trial were considered for the model | 1384 | BTA | Placebo | CUA |
| Authors, year | Model type | Perspective | Time horizon | Cost included in the model | Source of cost and resource inputs | Currency, price year |
|---|---|---|---|---|---|---|
| Journal articles | ||||||
| Batty, 2013167 | A Markov model with 13 health states, including death. The 12 states were split into 2 parallel stages: on treatment and off treatment. A 12-week cycle length was employed. The model also considered negative and positive stopping rule for the treatment | NHS | 2 years | Cost of BTA; consultant time to take participant history, tailor prophylactic and acute treatment; consultant time to administer the injections; cost of care including GP visits, ED visits, hospitalisation and triptan costs | Resource used was informed by IBMS, with unit costs taken from NHS reference cost, cost of triptans per attack was based on the weighted average costs in the UK in 2010 | UK £ 2010 |
| Giannouchos, 2019168 | Decision tree model | Payer and societal perspective | 1 year | Direct costs included the cost of the 2 drugs and administration, the use of acute drugs under usual care, and hospitalisation costs, physician, and ED visits. Indirect costs for the societal perspective analysis included wages lost on workdays | Resource utilisation data were obtained from 4 previously published studies and the cost inputs were obtained from publicly available data for the Greek healthcare sector and on the governmental pricing system derived from a public Greek hospital | Euro € 2019 |
| Hansson-Hedblom, 2020169 | A Markov model with 13 health states, including death as mentioned in Batty AJ, et al. | Payer and societal perspective | 10 years | Direct cost included cost of BTA, neurology consultant appointment, specialist nurse appointment, cost of care including GP visits, ED visits, hospitalisation and triptan costs.Indirect cost involved productivity cost | SEK, 2018 for Sweden; NOK, 2018 for Norway |
|
| Hollier-Hann, 2020170 | CUA using Markov model with 13 health states, including death as mentioned in Batty AJ, et al. | NHS | 2 years | Cost of BTA; consultant time to take participant history, tailor prophylactic and acute treatment; consultant time to administer the injections; cost of care including GP visits, ED visits, hospitalisation and triptan costs | Resource used was informed by IBMS, with unit costs taken from NHS reference cost, cost of triptans per attack was based on the weighted average costs in the UK in 2010 | UK £ 2010 |
| Lipton, 2018171 | A Markov model was implemented based on the clinical data from the Episodic Migraine (EM) and Chronic Migraine (CM) studies for the subgroups of participants with prior treatment failures. The cycle length was 28 days |
US societal perspective | 10 years | Direct medical costs included cost of medicine and administration, GP visits, ED visits, hospitalisations, and specialist neurologist consultations based on published unit costs. Cost of medicines to treat acute attacks. Indirect costs included productivity cost associated with presenteeism and absenteeism | Average annual medical resource use is taken from a published 2009 analysis of survey data from 7437 migraine participants in the USA |
USD $ 2017 |
| Mahon, 2021172 | A hybrid decision tree plus Markov model was developed | Swedish societal perspective | 10 years | Direct cost included cost of medicine and administration, ED visit, hospitalisation, GP visit, consultant visit, nurse/physician visit, triptan medication and other medications. Indirect cost related to absenteeism and presenteeism were included | Resource utilisation and efficacy data were sourced from four trials (CM295, STRIVE, ARISE and LIBERTY). Study 178, which had an open label phase of 256 weeks, was used to inform long-term assumptions regarding those who continued on treatment. Resource usage costs were obtained from the price list of Sweden. Productivity costs were included from the published literature | SEK, 2018 |
| Ruggeri, 2013173 | A Markov model with 13 health states, including death as mentioned in Batty AJ, et al. | Italian National Health Service and a societal perspective |
2 years | Direct cost included cost of medicine and administration, GP visit or outpatient cost, ED visit, hospitalisation and cost of triptans. Indirect costs included productivity cost | Resource utilisation data were derived from IBMS study. Costs were obtained from the local government data | Euro € 2013 |
| Sussman, 2018174 | A hybrid Monte Carlo participant simulation and Markov cohort model was constructed. Both EM and CM participants must have failed at least 1 previous therapy prior to model entry since CGRP pathway antagonists are expected to be used as second-line therapies. Participants in the EM cohort must have had between 4 and 14 MMDs and participants in the CM cohort must have had at least 15 MMDs at baseline | US societal and payers perspective | 2 years | Direct cost included – acute medication cost, physician visit, ED visit, AEs and hospitalisation cost. Indirect costs included productivity cost | Data inputs for the model were derived from the erenumab pivotal and open labelled extended trials, and BTA pivotal trial, published literature, and publicly available sources | USD $ 2017 |
| Vekov, 2019175 | A hybrid model including a Monte Carlo simulation and a Markov cohort model. The input data to the model are the primary and secondary clinical end-points in the randomised trials NCT02066415 and NCT02483585. They measure the change in the number of days with migraine per month at weeks 12 and 24, the number of days per month with symptomatic migraine therapy | Payers’ perspective | 2 years | Only the cost of medicines was included; other healthcare costs were assumed to be equal for both therapies and hence excluded | Resource utilisation (medicine usage) data were obtained from the randomised trial NCT02066415 | Bulgarian Lev (BGN) 2019 |
| Other reports | ||||||
| CADTH (BTA), 2019176 | Hybrid model with decision tree for 12-week assessment period, classifying patients as responders and non-responders, and Markov model for post-assessment with 12-week cycle lengths | Canadian public healthcare payer perspective | 3 years | Direct costs included cost of medicine and administration, GP visits or outpatient cost, ED visits, hospitalisation and cost of triptans. Indirect costs included productivity cost | Resource used was informed by IBMS, with unit costs taken from NHS reference costs, cost of triptans per attack was based on the weighted average costs in the UK in 2010 | CAD $ 2019 |
| CADTH (erenumab), (2019)177 | Hybrid model with decision tree for 12-week assessment period, classifying participants as responders and non-responders, and Markov model for post-assessment with 12-week cycle lengths | Canadian public healthcare payer perspective | 3 years | Direct costs included cost of medicine and administration, GP visits or outpatient cost, ED visits, hospitalisation and cost of triptans. Indirect costs included productivity cost | Resource used was informed by the trial, and cost data were obtained from manufacturer and other local data resources | CAD $ 2019 |
| ICER^ (CGRP), (2018)178 | Markov model comprising CGRP inhibitor versus no preventive treatment arms. The intervention arm of the model includes 3 health states: (1) CGRP inhibitor treatment, (2) no preventive treatment, and (3) death. The comparator arm includes two health states: (1) no preventive treatment and (2) death | Health system payer perspective | 2 years | Direct medical care cost including cost of medicine, GP visit, outpatient visit, ED visit and hospitalisation | Resource used was informed by IBMS, with unit costs taken from the local data resources | USD $ 2018 |
| NICE: erenumab, (2019)180 | A decision tree plus Markov model included 2 health states – on treatment and discontinuation of treatment once patients were classified as responders or non-responders | NHS perspective | Lifetime | Migraine-specific cost related to hospitalisation and ED visits, healthcare professional visits and use of acute medication | Resource used was informed by National Health and Wellness survey conducted in migraine population, with unit costs taken from the local data resources | UK £ 2018 |
| NICE: fremanezumab, (2019)179 | A decision tree plus Markov model included 2 health states – on treatment and discontinuation of treatment once patients were classified as responders or non-responders | NHS perspective | 10 years | Migraine-specific cost related to hospitalisation and ED visits, healthcare professional visits and use of acute medication | Resource used was informed by National Health and Wellness survey conducted in migraine population, with unit costs taken from the local data resources | UK £ 2019 |
| NICE: galcanezumab, (2020)181 | A decision tree plus Markov model included 2 health states – on treatment and discontinuation of treatment once patients were classified as responders or non-responders | NHS perspective | Lifetime | Migraine-specific cost related to hospitalisation and ED visits, healthcare professional visits and use of acute medication | Trial-specific (CONQUER) data and the resource utilisation data from Lipton et al. (2018) | UK £ 2020 |
| Warwick Evidence, (2011)182 | A Markov model with 13 health states, including death. The 12 states were split into 2 parallel stages: on treatment and off treatment. Each treatment state was subdivided into categories based on the number of headache days per 28 days. 3 health states for EM (0–3, 4–9 and 10–14 headache days per 28 days), and 3 health states for CM (15–19, 20–23 and 24 + headache days per 28 days). A 12-week cycle length was employed. The model also considered negative and positive stopping rule for the treatment | NHS perspective | 2 years | Migraine-specific cost related to hospitalisation and ED visits, healthcare professional visit and use of acute medication | Resource used was informed by IBMS, with unit costs taken from NHS reference costs, cost of triptans per attack was based on the weighted average costs in the UK in 2010 | UK £ 2011 |
| Authors, year | Discount rate (%) | Utilities (QALYs) and outcomes | Results/ICER | WTP threshold | Sensitivity analyses | ||
|---|---|---|---|---|---|---|---|
| Preference-based measure used to estimate utilities | Whose utility values? | Other outcomes | |||||
| Journal articles | |||||||
| Batty, 2013167 | 3.5 | MSQ v2.1 was used to collect HRQoL information at baseline and 24 weeks after the intervention. The MSQ scores were mapped to EuroQol EQ-5D to produce utility values | Utility values from the participants of the PREEMPT trial | Headache per day/year, cost per headache day avoided | At 2 years, BTA treatment was associated with an increase in costs of £1367 and an increase in QALYs of 0.1 compared to placebo, resulting in an ICER of £15,028. Treatment with BTA reduced headache days by 38 days per year at a cost of £18 per headache day avoided | £20,000–30,000/QALY | Both deterministic and PSA were performed |
| Giannouchos, 2019168 | None | QALYs were calculated by using the health utility data (MSQ to EQ-5D) for participants with CM from 10 countries obtained from the IBMS | General public | Number of migraines avoided | CM treatment with erenumab compared to BTA resulted in ICERs of €218,870 and €231,554 per QALY gained and €620 and €656 per migraine avoided, from the societal and the payer’s perspective, respectively. Using a cost-effectiveness threshold equal to three times the local GDP per capita (€49,000), for erenumab the ICERs fall below this threshold | EURO 49,000/QALY | Both PSA and deterministic sensitivity analyses were performed |
| Hansson-Hedblom, 2020169 | 3 | The IBMS study was used to map EQ-5D scores from MSQ score | Utility values from the participants of PREEMPT trial | In Sweden, BTA was associated with 0.223 additional QALYs at an additional cost of EUR 4126 compared to placebo, resulting in an ICER of EUR 18,506. In Norway, BTA was associated with 0.216 additional QALYs at an additional cost of EUR 4301 compared to placebo, resulting in an ICER of EUR 19,954 | SEK 280,000 (Sweden) and NOK 495,000 (Norway) | Both PSA and deterministic sensitivity analyses were performed | |
| Hollier-Hann, 2020170 | 3.5 | Utility values were directly obtained from the EQ-5D data collected in the REPOSE study. EQ-5D was administered at baseline and each follow-up visit (at intervals of approx. 12 weeks) | UK tariff | Headache per day/year, cost per headache day avoided | BTA treatment resulted in incremental costs of £1204 and an incremental QALY gain of 0.07 compared with placebo in CM participants who have previously failed 3 or more preventive treatments, corresponding to an ICER of £16,306 per QALY gained | £20,000–30,000/QALY | Both PSA and deterministic sensitivity analyses were performed |
| Lipton, 2018171 | 3 | MSQ responses from the erenumab EM and CM pivotal studies were mapped to the EQ-5D-3L, then pooled to generate one complete migraine data set | General public | Erenumab resulted in incremental QALYs of 0.185 vs. BSC and estimated cost offsets due to reduced MMDs of $8482 over 10 years, with an average duration of treatment of 2 years | $100,000–200,000/ QALY |
Both PSA and deterministic sensitivity analyses were performed | |
| Mahon, 2021172 | 3 | Two trials included in this study used the MSQ score which was mapped onto EQ-5D | Not stated | Cost per migraine day avoided | Erenumab treatment resulted in ICERs of SEK 34,696 and SEK 301,565 per QALY gained in the total migraine and EM populations, respectively. Erenumab was dominant in the CM population | SEK 300,000/QALY | Both PSA and deterministic sensitivity analyses were performed |
| Ruggeri, 2013173 | 3 | The IBMS study was used to map EQ-5D scores from MSQ score | UK tariff | Headache per day/year, cost per headache day avoided | BTA compared with placebo gained an incremental 0.04 more QALYs per participant; the incremental cost per participant was €208; the ICER was €4899 per QALY gained | €20,000–30, 000/QALY | Both PSA and deterministic sensitivity analyses were performed |
| Sussman, 2018174 | 3 | EQ-5D scores were used | Not stated | Headache-related disability, lost work productivity, anxiety and depression | From a societal perspective treatment with erenumab compared with no preventive treatment ranges from a dominant strategy among CM participants to an ICER of $122,167 for EM participants. When excluding indirect costs (i.e. payer perspective), the ICERs are cost-effective among CM participants ($23,079 and $65,720 vs. no preventive treatment and BTA, respectively), but not among EM participants | USD 50,000/QALY | Both PSA and deterministic sensitivity analyses were performed |
| Vekov, 2019175 | 5 | EQ-5D scores were used | Not stated | HIT-6, MIDAS | Erenumab was not cost-effective compared to placebo (standard prevention therapy) with ICER of 637,000 BGN per QALY | Three times the national annual GDP per capita | PSA |
| Other reports | |||||||
| CADTH (BTA), 2019176 | 3 | MSQ was used to collect HRQoL information at baseline and 24 weeks after the intervention. The MSQ scores then were mapped into EQ-5D to produce utility values | Utility values from the participants of PREEMPT trial were used | Headache per day/year, cost per headache day avoided | ICER was CAD 134,601/QALY gained for BTA vs. BSC. At a WTP of CAD 50,000 per QALY, BTA was associated with a 9% probability of being the optimal intervention. A price reduction of more than 75% is required to achieve an ICER of less than CAD 50,000/QALY | CAD 50,000 | Sensitivity analysis showed that utility values had the greatest influence on model results |
| CADTH (erenumab), 2019177 | 3 | MSQ was used to collect HRQoL information at baseline and 24 weeks after the intervention. The MSQ scores then were mapped into EQ-5D to produce utility values | Utility values from the participants of PREEMPT trial were used | Headache per day/year, cost per headache day avoided | Erenumab dominated BTA in the population for whom the previous treatment including BTA had failed | CAD 50,000 | Sensitivity analyses involved analysing different time horizons were performed |
| ICER^ (CGRP), 2018178 | 3 | MSQ was used to collect HRQoL information at baseline and 24 weeks after the intervention. The MSQ scores then were mapped into EQ-5D to produce utility values | Utility values from the participants of PREEMPT trial were used | Headache per day/year, cost per headache day avoided | The ICER for erenumab vs. no preventative treatment was USD 86,000/QALY and fremanezumab vs. no preventative treatment was USD 115,000/QALY, both way above the baseline WTP of USD 50,000/QALY | USD 50,000 | Sensitivity analyses were performed using topiramate as the alternative treatment to BTA and this resulted in an estimated ICER of USD 28,960/QALY |
| NICE: erenumab, 2019180 | 3.5 | MSQ was used to collect HRQoL information at baseline and 24 weeks after the intervention. The MSQ scores then were mapped into EQ-5D to produce utility values | Utility values obtained from erenumab trials (Study 295, STRIVE and ARISE) data | Headache per day/year, cost per headache day avoided | The blended dose of erenumab was cost-effective in treating CM population vs. BTA and vs. BSC with an ICER of £18,893 and £17,212 per QALY gained, respectively. Erenumab 140 mg is cost-effective treatment vs. both BTA and BSC, with an ICER of £17,832 and £13,340 per QALY gained, respectively | £20,000–30,000/QALY | Both PSA and deterministic sensitivity analyses were performed including using the whole migraine population and a societal perspective |
| NICE: fremanezumab, 2019179 | 3.5 | MSQ was used to collect HRQoL information. The MSQ scores then were mapped into EQ-5D to produce utility values | Utility values obtained from patient level MSQ data from FOCUS trial | Headache per day/year, cost per headache day avoided | Fremanezumab had higher costs, but also gained more QALYs than both BSC and BTA. The ICERs showed that fremanezumab was a cost-effective treatment compared to BSC (£11,825/QALY gained) and BTA (£16,227/QALY gained) | £20,000–30,000/QALY | Both PSA and deterministic sensitivity analyses were performed |
| NICE: galcanezumab, 2020181 | 3.5 | MSQ was used to collect HRQoL information. The MSQ scores then were mapped into EQ-5D to produce utility values | Utility values obtained from patient level MSQ data from CONQUER trial | Headache per day/year, cost per headache day avoided | The actual ICERS were confidential and masked. However, the report indicated that ICER for galcanezumab fell below the lower threshold (£20,000/QALY gained) as defined by standard WTP for UK | £20,000–30,000/QALY | Both PSA and deterministic sensitivity analyses were performed |
| Warwick Evidence, 2011182 | 3.5 | MSQ was used to collect HRQoL information. The MSQ scores then were mapped into EQ-5D to produce utility values | Utility values obtained from patient level MSQ data from PREEMPT Trial | Headache per day/year, cost per headache day avoided | The reported ICER was £5828/QALY gained | £20,000–30,000/QALY | Both PSA and deterministic sensitivity analyses were performed |
| Author, year | Limitations | Generalisability |
|---|---|---|
| Journal articles | ||
| Batty, 2013167 | Placebo was not representative of standard care | Transferable |
| Giannouchos, 2019168 | Limitations were mostly presented for the assumptions made in the model | Context-specific |
| Hansson-Hedblom, 2020169 | The clinical trial may not be representative of everyday practice and physicians and participants may adjust treatment practices. The model was limited by only using MMDs, and other dimensions of migraine, such as duration and severity, were not considered | Context-specific |
| Hollier-Hann, 2020170 | Limitations included the assumptions made for the model including that treatment response, HRQoL, and resource utilisation were based on MMD frequency alone | Transferable |
| Lipton, 2018171 | The model was created based on primary efficacy data from a mixed population of participants (EM and CM). There were also limited data beyond week 12 for CM participants. Also, treatment response, HRQoL and resource utilisation were based on MMD frequency alone | Context-specific |
| Mahon, 2021172 | Limitations included the assumptions made for the model including that treatment response, HRQoL and resource utilisation were based on MMD frequency alone | Context-specific |
| Ruggeri, 2013173 | Same limitations as Lipton et al. (see above) and also the study used the UK tariff for the utility scores in the base model | Transferable |
| Sussman, 2018174 | Same limitations as Lipton et al. (see above). | Context-specific |
| Vekov, 2019175 | Limitations were not stated | Context-specific |
| Reports | ||
| CADTH (BTA), 2019176 | The severity of CM was not captured in the model and there was no good quality of comparative evidence | Context-specific |
| CADTH (erenumab), 2019177 | There was no good quality of comparative evidence | Context-specific |
| ICER^ (CGRP), 2018178 | Since the data were obtained from the trial, there was uncertainty about the long-term effectiveness of the drugs | Context-specific |
| NICE: erenumab, 2019180 | Uncertainty due to not having long-term effectiveness data | Context-specific |
| NICE: fremenzumab, 2019179 | Uncertainty due to not having long-term effectiveness data. There was also a lack of granularity within the published data for BTA, which led to limitations within the NMA conducted to compare the efficacy of fremanezumab and BTA | Context-specific |
| NICE: galcanezumab, 2020181 | The limitations included the model’s inability to capture the natural progression of diseases, the use of short-term estimates of mean change in MHDs, and response rates for extrapolating to different time horizons | Context-specific |
| Warwick Evidence, 2011182 | Limitations included the trials limitation to deal with correlated data, predicted ED-5D scores and the integrity around utility scores | Context-specific |
| Journal articles | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Batty et al.167 | Giannouchos et al.168 | Hansson-Hedblom et al.169 | Hollier-Hann et al.170 | Lipton et al.171 | Mahon et al.172 | Ruggeri et al.173 | Sussman et al.174 | Vekov et al.175 | |
| Other reports | |||||||||
| CADTH (BTA)176 | CADTH (Erenumab)177 | ICER^ (CGRP)178 | NICE (Erenumab)180 | NICE (Fremenzumab)179 | NICE (Galcanezumab)181 | Warwick Evidence (BTA)182 | |||
| CHEERS 2022 (n = 27) | |||||||||
| Yes | 25 | 23 | 23 | 23 | 22 | 25 | 23 | 25 | 12 |
| No | 2 | 2 | 2 | 3 | 2 | 2 | 2 | 2 | 10 |
| Partial | 1 | 3 | 3 | 2 | 4 | 1 | 3 | 1 | 6 |
| Unclear | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Not applicable | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Philips criteria (n = 57) | |||||||||
| Yes | 51 | 50 | 50 | 49 | 51 | 51 | 50 | 51 | 20 |
| No | 3 | 4 | 4 | 6 | 4 | 3 | 3 | 3 | 16 |
| Partial | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 3 | 9 |
| Unclear | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 10 |
| Not applicable | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 2 |
| CHEERS 2022 (n = 27) | |||||||||
| Yes | 26 | 26 | 25 | 26 | 26 | 26 | 24 | ||
| No | 1 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| Partial | 0 | 1 | 1 | 0 | 0 | 0 | 3 | ||
| Unclear | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Not applicable | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Philips criteria (n = 57) | |||||||||
| Yes | 50 | 49 | 52 | 53 | 54 | 55 | 55 | ||
| No | 3 | 2 | 1 | 2 | 1 | 0 | 0 | ||
| Partial | 4 | 6 | 4 | 1 | 1 | 1 | 2 | ||
| Unclear | 0 | 0 | 0 | 1 | 1 | 1 | 0 | ||
| Not applicable | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
Appendix 9 Model inputs for the economic model
| Transitions | Placebo | BTA | Eptinezumab 100 | Eptinezumab 300 | Fremanezumab (monthly) | Fremanezumab (quarterly) | Galcanezumab | Topiramate |
|---|---|---|---|---|---|---|---|---|
| 0–3 MHD–0–3 MHD | 0.56200 | 0.68563 | 0.67243 | 0.69976 | 0.68342 | 0.66996 | 0.67054 | 0.62965 |
| 0–3 MHD–4–9 MHD | 0.28100 | 0.16337 | 0.16182 | 0.14079 | 0.15336 | 0.16373 | 0.16336 | 0.19681 |
| 0–3 MHD–10–14 MHD | 0.05400 | 0.03862 | 0.03823 | 0.03368 | 0.03640 | 0.03863 | 0.03847 | 0.04386 |
| 0–3 MHD–15–19 MHD | 0.01500 | 0.01660 | 0.01614 | 0.01934 | 0.01743 | 0.01586 | 0.01604 | 0.01363 |
| 0–3 MHD–20–23 MHD | 0.03400 | 0.02601 | 0.02585 | 0.02090 | 0.02386 | 0.02629 | 0.02605 | 0.03053 |
| 0–3 MHD–24–28 MHD | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 0–3 MHD–0–3 Off TX | 0.05400 | 0.06977 | 0.08553 | 0.08553 | 0.08553 | 0.08553 | 0.08553 | 0.08553 |
| 4–9 MHD–0–3 MHD | 0.17200 | 0.40152 | 0.39196 | 0.43971 | 0.41116 | 0.38764 | 0.38866 | 0.31857 |
| 4–9 MHD–4–9 MHD | 0.49100 | 0.33880 | 0.33422 | 0.31423 | 0.32618 | 0.33606 | 0.33601 | 0.37326 |
| 4–9 MHD–10–14 MHD | 0.23800 | 0.15606 | 0.15482 | 0.13033 | 0.14497 | 0.15701 | 0.15615 | 0.18513 |
| 4–9 MHD–15–19 MHD | 0.02800 | 0.02325 | 0.02281 | 0.02362 | 0.02314 | 0.02274 | 0.02283 | 0.02300 |
| 4–9 MHD–20–23 MHD | 0.02800 | 0.02483 | 0.02460 | 0.02122 | 0.02324 | 0.02490 | 0.02471 | 0.02738 |
| 4–9 MHD–24–28 MHD | 0.00600 | 0.00249 | 0.00249 | 0.00179 | 0.00221 | 0.00255 | 0.00254 | 0.00355 |
| 4–9 MHD–4–9 Off TX | 0.03700 | 0.05305 | 0.06910 | 0.06910 | 0.06910 | 0.06910 | 0.06910 | 0.06911 |
| 10–14 MHD–0–3 MHD | 0.08400 | 0.21276 | 0.20761 | 0.23435 | 0.21836 | 0.20519 | 0.20576 | 0.16652 |
| 10–14 MHD–4–9 MHD | 0.27500 | 0.26762 | 0.26233 | 0.27559 | 0.26766 | 0.26118 | 0.26200 | 0.25391 |
| 10–14 MHD–10–14 MHD | 0.34300 | 0.27936 | 0.27567 | 0.25747 | 0.26835 | 0.27729 | 0.27666 | 0.29818 |
| 10–14 MHD–15–19 MHD | 0.18700 | 0.12471 | 0.12346 | 0.10850 | 0.11744 | 0.12480 | 0.12437 | 0.14399 |
| 10–14 MHD–20–23 MHD | 0.04700 | 0.04675 | 0.04622 | 0.04159 | 0.04436 | 0.04662 | 0.04634 | 0.04928 |
| 10–14 MHD–24–28 MHD | 0.01900 | 0.00789 | 0.00789 | 0.00567 | 0.00699 | 0.00809 | 0.00804 | 0.01126 |
| 10–14 MHD–10–14 Off TX | 0.04500 | 0.06092 | 0.07683 | 0.07683 | 0.07683 | 0.07683 | 0.07683 | 0.07686 |
| 15–19 MHD–0–3 MHD | 0.02100 | 0.08076 | 0.07868 | 0.09103 | 0.08364 | 0.07756 | 0.07782 | 0.05973 |
| 15–19 MHD–4–9 MHD | 0.12700 | 0.16688 | 0.16293 | 0.18265 | 0.17086 | 0.16118 | 0.16204 | 0.14218 |
| 15–19 MHD–10–14 MHD | 0.27500 | 0.28304 | 0.27802 | 0.28198 | 0.27961 | 0.27766 | 0.27780 | 0.27311 |
| 15–19 MHD–15–19 MHD | 0.30900 | 0.21684 | 0.21418 | 0.19665 | 0.20713 | 0.21577 | 0.21541 | 0.24132 |
| 15–19 MHD–20–23 MHD | 0.12700 | 0.13237 | 0.13077 | 0.11949 | 0.12624 | 0.13174 | 0.13100 | 0.13715 |
| 15–19 MHD–24–28 MHD | 0.06200 | 0.02575 | 0.02573 | 0.01850 | 0.02283 | 0.02639 | 0.02623 | 0.03673 |
| 15–19 MHD–15–19 Off TX | 0.07900 | 0.09435 | 0.10970 | 0.10970 | 0.10970 | 0.10970 | 0.10970 | 0.10978 |
| 20–23 MHD–0–3 MHD | 0.00000 | 0.03029 | 0.02942 | 0.03564 | 0.03192 | 0.02885 | 0.02899 | 0.01989 |
| 20–23 MHD–4–9 MHD | 0.06400 | 0.06680 | 0.06541 | 0.06992 | 0.06723 | 0.06502 | 0.06526 | 0.06167 |
| 20–23 MHD–10–14 MHD | 0.09200 | 0.17809 | 0.17350 | 0.20102 | 0.18456 | 0.17104 | 0.17200 | 0.13944 |
| 20–23 MHD–15–19 MHD | 0.32800 | 0.26864 | 0.26370 | 0.27076 | 0.26654 | 0.26312 | 0.26398 | 0.26828 |
| 20–23 MHD–20–23 MHD | 0.31100 | 0.34413 | 0.33962 | 0.31612 | 0.33018 | 0.34164 | 0.33991 | 0.34896 |
| 20–23 MHD–24–28 MHD | 0.18700 | 0.07768 | 0.07762 | 0.05580 | 0.06884 | 0.07959 | 0.07912 | 0.11078 |
| 20–23 MHD–20–23 Off TX | 0.01800 | 0.03437 | 0.05073 | 0.05073 | 0.05073 | 0.05073 | 0.05073 | 0.05098 |
| 24–28 MHD–0–3 MHD | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 | 0.00000 |
| 24–28 MHD–4–9 MHD | 0.00000 | 0.00891 | 0.00860 | 0.01140 | 0.00972 | 0.00835 | 0.00845 | 0.00514 |
| 24–28 MHD–10–14 MHD | 0.02400 | 0.04885 | 0.04757 | 0.05550 | 0.05075 | 0.04686 | 0.04713 | 0.03775 |
| 24–28 MHD–15–19 MHD | 0.09200 | 0.08665 | 0.08464 | 0.09417 | 0.08847 | 0.08382 | 0.08443 | 0.07881 |
| 24–28 MHD–20–23 MHD | 0.13900 | 0.50391 | 0.49180 | 0.55323 | 0.51651 | 0.48619 | 0.48697 | 0.38584 |
| 24–28 MHD–24–28 MHD | 0.70000 | 0.29077 | 0.29055 | 0.20887 | 0.25771 | 0.29795 | 0.29619 | 0.41468 |
| 24–28 MHD–24–28 Off TX | 0.04500 | 0.06092 | 0.07683 | 0.07683 | 0.07683 | 0.07683 | 0.07684 | 0.07777 |
| 0–3 Off TX–0–3 Off TX | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 |
| 4–9 Off TX–4–9 Off TX | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 |
| 10–14 Off TX–10–14 Off TX | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 |
| 15–19 Off TX–15–19 Off TX | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 |
| 20–23 Off TX–20–23 Off TX | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 |
| 24–28 Off TX–24–28 Off TX | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 | 1.00000 |
| Interventions | Available preparations | Strength | Route of administration | Recommended dose | Administration |
|---|---|---|---|---|---|
| BTA 150U | 200-unit powder for solution for injection vials | 200 U | Subcutaneous injection | The recommended total dose is 155 units administered intramuscularly (into the muscle) | Administered by a nurse in a hospital/clinic every 12 weeks. 15-minute appointment |
| Eptinezumab 100 mg |
Eptinezumab 100 mg/ml injection single use vial | 100 mg | Intravenous infusion | Recommended dose is 100 mg every 12 weeks. Review treatment 6 months after initiation | Administered by a nurse in a hospital/clinic every 12 weeks. 15-minute appointment |
| Eptinezumab 300 mg |
Eptinezumab 100 mg/ml injection single use vial | 100 mg | Intravenous infusion | Recommended dose is 300 mg every 12 weeks. Review treatment 6 months after initiation | Administered by a nurse in a hospital/clinic every 12 weeks. 15-minute appointment |
| Fremanezumab – monthly | 225 mg/1.5 ml solution for injection pre-filled pens/injection | 225 mg | Subcutaneous injection | Recommended dose is 225 mg once a month, review treatment within first 3 months and regularly thereafter | First dose administered by a nurse in a hospital/clinic, a 30-minute appointment. Subsequent doses assumed 10% would not be able to self-administer |
| Fremanezumab – quarterly | 225 mg/1.5 ml solution for injection pre-filled pens/injection | 675 mg | Subcutaneous injection | Recommended dose is 675 mg every 3 months, review treatment within first 3 months and regularly thereafter | First dose administered by a nurse in a hospital/clinic, a 30-minute appointment. Subsequent doses assumed 10% would not be able to self-administer |
| Galcanezumab 120 mg | 120 mg/1 ml solution for injection pre-filled pens | 120 mg | Subcutaneous injection | Loading dose 240 mg for 1 dose, then maintenance 120 mg once a month. Maintenance dosing to start 1 month after loading dose | First dose administered by a nurse in a hospital/clinic, a 30-minute appointment. Subsequent doses assumed 10% would not be able to self-administer |
| Topiramate | Topiramate 25 mg tablets. 60 tablets in one pack | 25 mg | Tablet | Initially 25 mg once daily for 1 week, then increased in steps of 25 mg every week; usual dose 50–100 mg daily in 2 divided doses; maximum 200 mg per day | No administration required |
| For all prophylactic drugs including placebo | ||||
|---|---|---|---|---|
| Health states | GP visits | A&E visits | Hospital admissions | Triptan usage |
| 0–3 MHD | 0.69 | 0.10 | 0.03 | 1.88 |
| 4–9 MHD | 0.69 | 0.10 | 0.03 | 5.07 |
| 10–14 MHD | 0.69 | 0.10 | 0.03 | 5.07 |
| 15–19 MHD | 2.07 | 0.41 | 0.09 | 7.29 |
| 20–23 MHD | 2.07 | 0.41 | 0.09 | 7.29 |
| 24–28 MHD | 2.07 | 0.41 | 0.09 | 7.29 |
| 0–3 Off TX | 0.69 | 0.10 | 0.03 | 1.88 |
| 4–9 Off TX | 0.69 | 0.10 | 0.03 | 5.07 |
| 10–14 Off TX | 0.69 | 0.10 | 0.03 | 5.07 |
| 15–19 Off TX | 2.07 | 0.41 | 0.09 | 7.29 |
| 20–23 Off TX | 2.07 | 0.41 | 0.09 | 7.29 |
| 24–28 Off TX | 2.07 | 0.41 | 0.09 | 7.29 |
| Source | IBMS190 | IBMS190 | IBMS190 | IBMS190 |
| Placebo and topriamate | BTA | Eptinezumab, fremanezumab, galcanezumab | ||
| Health statesa | Consultant visit (15 minutes) |
Consultant visit (30 minutes) |
Nurse visits | Consultant visits |
| 0–3 MHD | 1.00 | 1.00 | 0.277 | 0.036 |
| 4–9 MHD | 1.00 | 1.00 | 0.398 | 0.064 |
| 10–14 MHD | 1.00 | 1.00 | 0.144 | 0.114 |
| 15–19 MHD | 1.00 | 1.00 | 0.381 | 0.219 |
| 20–23 MHD | 1.00 | 1.00 | 0.381 | 0.219 |
| 24–28 MHD | 1.00 | 1.00 | 0.381 | 0.219 |
| Source | 182 | 182 | 179,181 | 179,181 |
Appendix 10 Economic model results
Base-case cost-effectiveness planes
FIGURE 50.
Cost-effectiveness plane – topiramate vs. placebo.

FIGURE 51.
Cost-effectiveness plane – BTA vs. placebo.
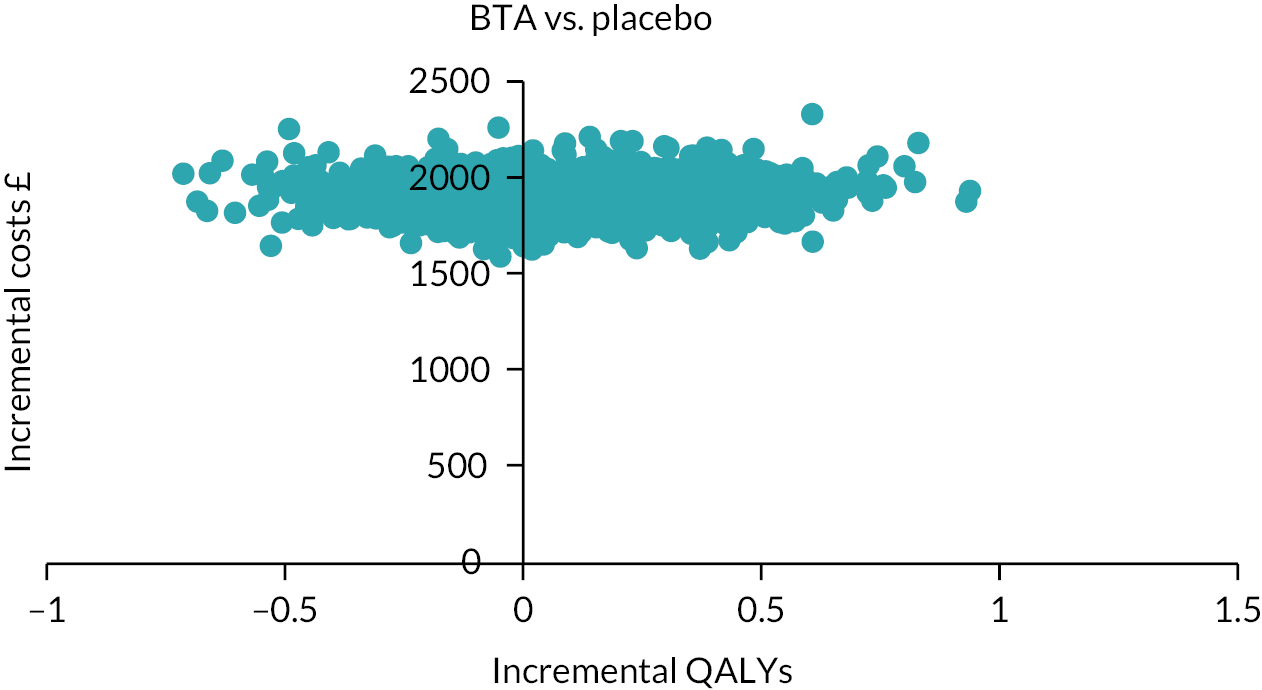
FIGURE 52.
Cost-effectiveness plane – eptinezumab 100 mg vs. placebo.

FIGURE 53.
Cost-effectiveness plane – eptinezumab 300 mg vs. placebo.
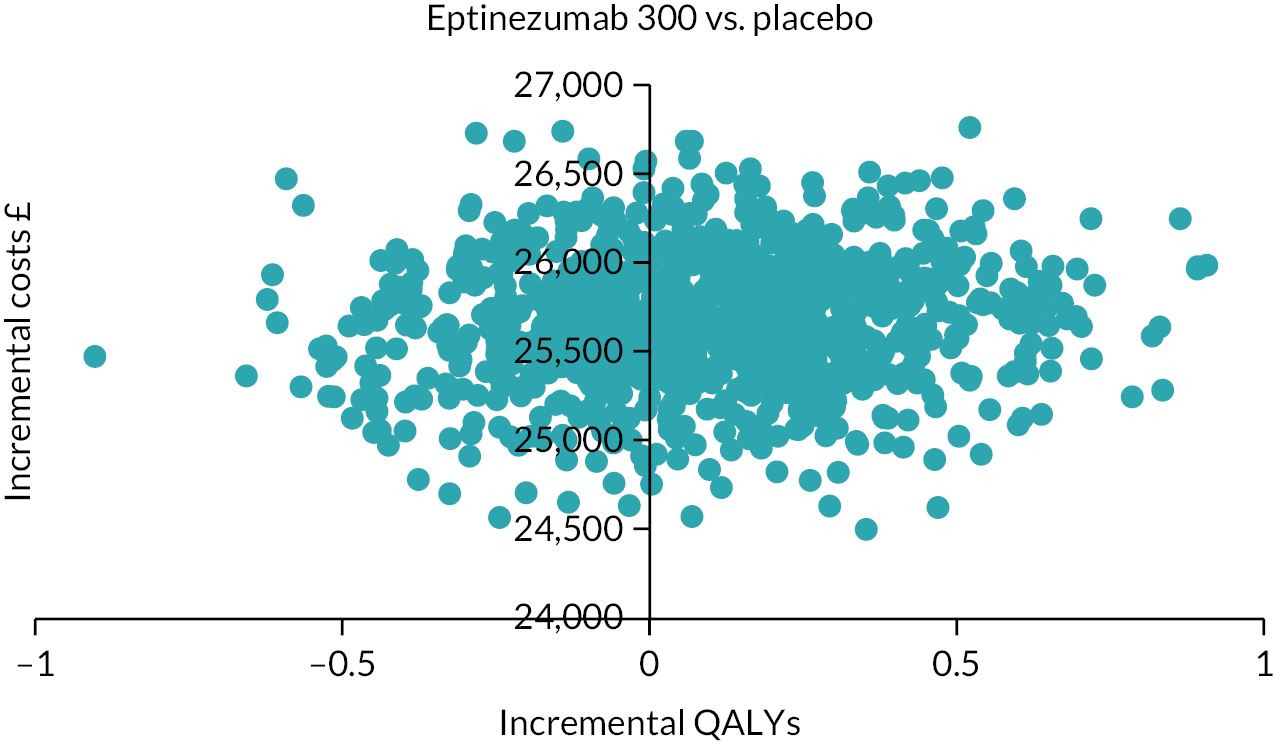
FIGURE 54.
Cost-effectiveness plane – fremanezumab monthly vs. placebo.

FIGURE 55.
Cost-effectiveness plane – fremanezumab quarterly vs. placebo.

FIGURE 56.
Cost-effectiveness plane – galcanezumab vs. placebo.

Base-case cost-effectiveness acceptability curves
FIGURE 57.
Cost-effectiveness acceptability curve – placebo vs. topiramate.

FIGURE 58.
Cost-effectiveness acceptability curve – placebo vs. BTA.

FIGURE 59.
Cost-effectiveness acceptability curve – placebo vs. eptinezumab 100 mg.
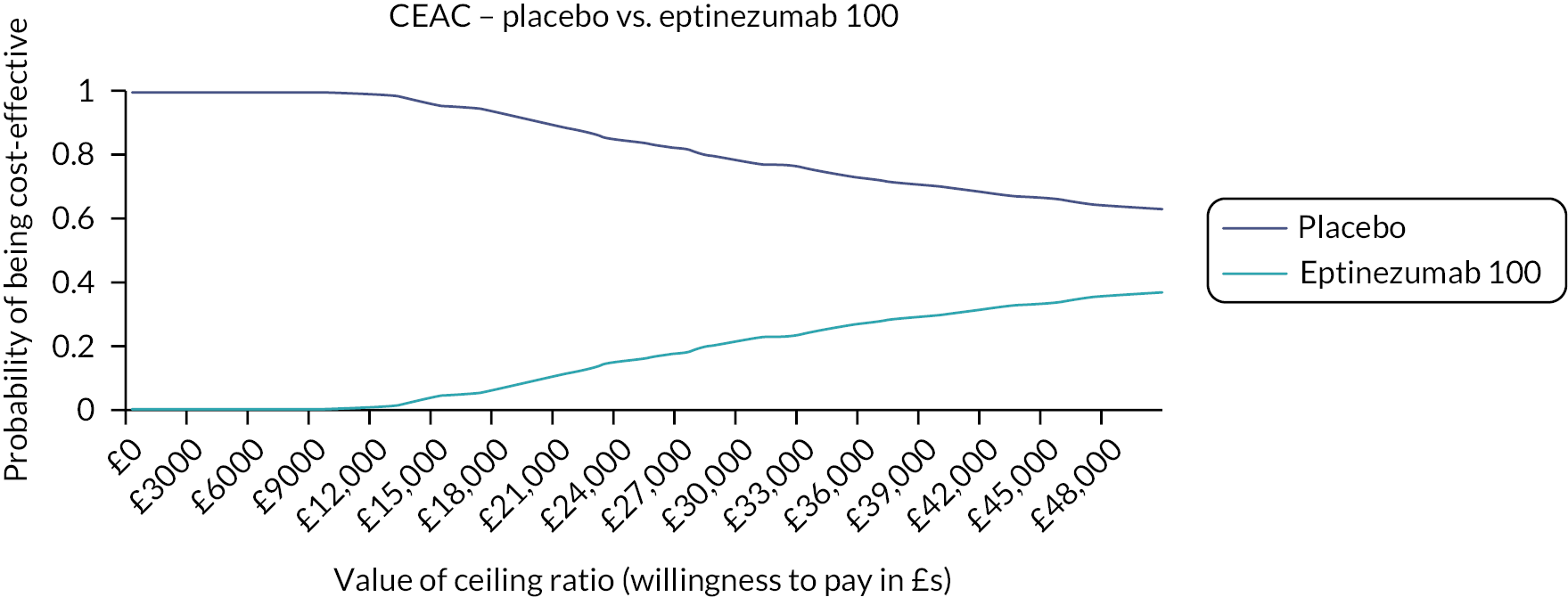
FIGURE 60.
Cost-effectiveness acceptability curve – placebo vs. eptinezumab 300 mg.

FIGURE 61.
Cost-effectiveness acceptability curve – placebo vs. fremanezumab monthly.
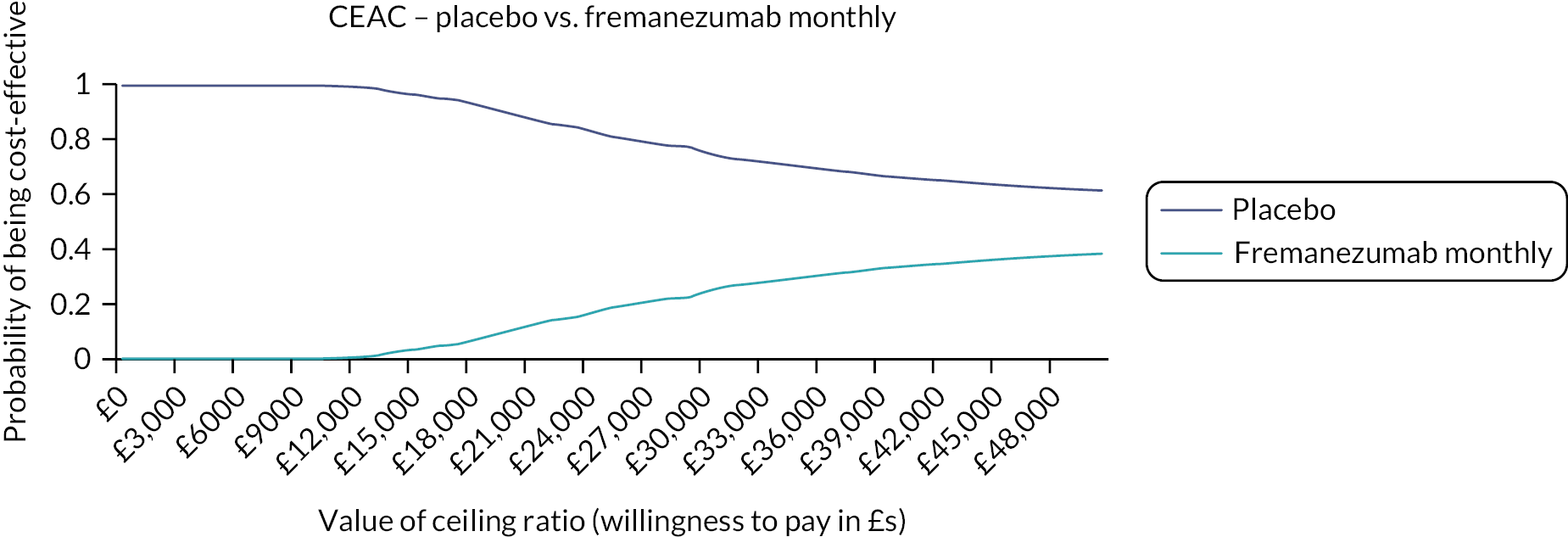
FIGURE 62.
Cost-effectiveness acceptability curve – placebo vs. fremanezumab quarterly.

FIGURE 63.
Cost-effectiveness acceptability curve – placebo vs. galcanezumab.

| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER: cost per QALY gained (£) | |
|---|---|---|---|---|---|
| (a) Changing time horizon – 5 years | |||||
| Deterministic results – discounted | |||||
| Placebo | 3488 | 3.0463 | – | – | – |
| Topiramate | 3165 | 3.1629 | −323 | 0.1166 | Dominated |
| Placebo | 3488 | 3.0463 | – | – | – |
| BTA | 6376 | 3.2414 | 2888 | 0.1951 | 14,804 |
| Placebo | 3488 | 3.0463 | – | – | – |
| Fremanezu mab (monthly) | 16,005 | 3.2398 | 12,517 | 0.1935 | 64,686 |
| Placebo | 3488 | 3.0463 | – | – | – |
| Fremanezumab (quarterly) | 16,103 | 3.2200 | 12,615 | 0.1737 | 72,640 |
| Placebo | 3488 | 3.0463 | – | – | – |
| Eptinezumab 100 | 16,138 | 3.2237 | 12,651 | 0.1774 | 71,327 |
| Placebo | 3488 | 3.0463 | – | – | – |
| Galcanezumab | 16,545 | 3.2212 | 13,057 | 0.1748 | 74,680 |
| Placebo | 348 | 3.0463 | – | – | – |
| Eptinezumab 300 | 42,120 | 3.2637 | 38,633 | 0.2164 | 178,540 |
| Probabilistic results – discounted | |||||
| Placebo | 3491 | 3.0348 | – | – | – |
| Topiramate | 3159 | 3.171 | −333 | 0.1369 | Dominated |
| Placebo | 3491 | 3.0348 | – | – | – |
| BTA | 6383 | 3.2497 | 2892 | 0.2149 | 13,458 |
| Placebo | 3491 | 3.0348 | – | – | – |
| Fremanezumab (monthly) | 16,039 | 3.2483 | 12,548 | 0.2135 | 58,778 |
| Placebo | 3491 | 3.0348 | – | – | – |
| Fremanezumab (quarterly) | 16,120 | 3.2283 | 12,629 | 0.1935 | 65,265 |
| Placebo | 3491 | 3.0348 | – | – | – |
| Eptinezumab 100 | 16,145 | 3.2163 | 12,654 | 0.1815 | 69,737 |
| Placebo | 3491 | 3.0348 | – | – | – |
| Galcanezumab | 16,577 | 3.2071 | 13,086 | 0.1723 | 75,937 |
| Placebo | 3491 | 3.0348 | – | – | – |
| Eptinezumab 300 | 42,184 | 3.2573 | 38,693 | 0.2225 | 173,923 |
| (b) Changing time horizon – lifetime | |||||
| Deterministic results – discounted | |||||
| Placebo | 15,117 | 15.1901 | – | – | – |
| Topiramate | 13,324 | 15.7707 | −1792 | 0.5805 | Dominated |
| Placebo | 15,117 | 15.1901 | – | – | – |
| BTA | 16,326 | 16.2092 | 1210 | 1.0190 | 1187 |
| Placebo | 15,117 | 15.1901 | – | – | – |
| Fremanezumab (monthly) | 27,364 | 16.1691 | 12,247 | 0.9790 | 12,510 |
| Placebo | 15,117 | 15.1901 | – | – | – |
| Fremanezumab (quarterly) | 27,741 | 16.0690 | 12,625 | 0.8789 | 14,365 |
| Placebo | 15,117 | 15.1901 | – | – | – |
| Eptinezumab 100 | 27,736 | 16.0878 | 12,620 | 0.8976 | 14,059 |
| Placebo | 15,117 | 15.1901 | – | – | – |
| Galcanezumab | 28,165 | 16.0748 | 13,048 | 0.8847 | 14,749 |
| Placebo | 15,117 | 15.1901 | – | – | – |
| Eptinezumab 300 | 57,524 | 16.2834 | 42,407 | 1.0932 | 38,790 |
| Probabilistic results – discounted | |||||
| Placebo | 15,138 | 15.1467 | – | – | – |
| Topiramate | 13,351 | 15.7628 | −1787 | 0.6161 | Dominated |
| Placebo | 15,138 | 15.1467 | – | – | – |
| BTA | 16,381 | 16.2613 | 1243 | 1.1146 | 1115 |
| Placebo | 15,138 | 15.1467 | – | – | – |
| Fremanezumab (monthly) | 27,469 | 16.1774 | 12,331 | 1.0307 | 11,964 |
| Placebo | 15,138 | 15.1467 | – | – | – |
| Fremanezumab (quarterly) | 27,840 | 16.0931 | 12,702 | 0.9464 | 13,421 |
| Placebo | 15,138 | 15.1467 | – | – | – |
| Eptinezumab 100 | 27,846 | 16.1319 | 12,708 | 0.9852 | 12,899 |
| Placebo | 15,138 | 15.1467 | – | – | – |
| Galcanezumab | 28,194 | 16.1418 | 13,056 | 0.9951 | 13,120 |
| Placebo | 15,138 | 15.1467 | – | – | – |
| Eptinezumab 300 | 57,609 | 16.3428 | 42,471 | 1.1961 | 35,507 |
| (c) Utility inputs – van Hout crosswalk algorithm | |||||
| Deterministic results – discounted | |||||
| Placebo | 1729 | 1.3733 | – | – | – |
| Topiramate | 1625 | 1.4174 | −104 | 0.0440 | Dominated |
| Placebo | 1729 | 1.3733 | – | – | – |
| BTA | 3654 | 1.4458 | 1925 | 0.0725 | 25,561 |
| Placebo | 1729 | 1.3733 | – | – | – |
| Fremanezumab (monthly) | 10,155 | 1.4470 | 8427 | 0.0737 | 114,365 |
| Placebo | 1729 | 1.3733 | – | – | – |
| Fremanezumab (quarterly) | 10,193 | 1.4391 | 8465 | 0.0658 | 128,613 |
| Placebo | 1729 | 1.3733 | – | – | – |
| Eptinezumab 100 | 10,216 | 1.4406 | 8487 | 0.0673 | 126,158 |
| Placebo | 1729 | 1.3733 | – | – | – |
| Galcanezumab | 10,640 | 1.4396 | 8912 | 0.0663 | 134,439 |
| Placebo | 1729 | 1.3733 | – | – | – |
| Eptinezumab 300 | 27,401 | 1.4562 | 25,672 | 0.0829 | 309,695 |
| Probabilistic results – discounted | |||||
| Placebo | 1723 | 1.3807 | – | – | – |
| Topiramate | 1627 | 1.4063 | –96 | 0.0256 | Dominated |
| Placebo | 1723 | 1.3807 | – | – | – |
| BTA | 3656 | 1.4475 | 1933 | 0.0668 | 28,937 |
| Placebo | 1723 | 1.3807 | – | – | – |
| Fremanezumab (monthly) | 10,161 | 1.4608 | 8438 | 0.0801 | 105,328 |
| Placebo | 1723 | 1.3807 | – | – | – |
| Fremanezumab (quarterly) | 10,193 | 1.4532 | 8470 | 0.0725 | 116,804 |
| Placebo | 1723 | 1.3807 | – | – | – |
| Eptinezumab 100 | 10,221 | 1.4346 | 8498 | 0.0539 | 157,699 |
| Placebo | 1723 | 1.3807 | – | – | – |
| Galcanezumab | 10,650 | 1.4436 | 8927 | 0.0629 | 141,848 |
| Placebo | 1723 | 1.3807 | – | – | – |
| Eptinezumab 300 | 27,411 | 1.4512 | 25,688 | 0.0705 | 364,182 |
| (d) Drug administration – 1% of patients can’t self–administer medication | |||||
| Deterministic results – discounted | |||||
| Placebo | 1729 | 1.3531 | – | – | – |
| Topiramate | 1625 | 1.3995 | −104 | 0.0464 | Dominated |
| Placebo | 1729 | 1.3531 | – | – | – |
| BTA | 3654 | 1.4294 | 1925 | 0.0763 | 25,238 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Fremanezumab (monthly) | 10,140 | 1.4307 | 8411 | 0.0776 | 108,407 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Fremanezumab (quarterly) | 10,178 | 1.4224 | 8449 | 0.0693 | 121,905 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Eptinezumab 100 | 10,216 | 1.4239 | 8487 | 0.0708 | 119,796 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Galcanezumab | 10,625 | 1.4229 | 8896 | 0.0698 | 127,430 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Eptinezumab 300 | 27,401 | 1.4403 | 25,672 | 0.0873 | 294,151 |
| Probabilistic results – discounted | |||||
| Placebo | 1730 | 1.3513 | – | – | – |
| Topiramate | 1626 | 1.3988 | −104 | 0.0475 | Dominated |
| Placebo | 1730 | 1.3513 | – | – | – |
| BTA | 3655 | 1.4336 | 1925 | 0.0823 | 23,373 |
| Placebo | 1730 | 1.3513 | – | – | – |
| Fremanezumab (monthly) | 10,148 | 1.4218 | 8418 | 0.0768 | 109,651 |
| Placebo | 1730 | 1.3513 | – | – | – |
| Fremanezumab (quarterly) | 10,184 | 1.4189 | 8454 | 0.0676 | 125,025 |
| Placebo | 1730 | 1.3513 | – | – | – |
| Eptinezumab 100 | 10,220 | 1.4220 | 8490 | 0.0707 | 120,142 |
| Placebo | 1730 | 1.3513 | – | – | – |
| Galcanezumab | 10,638 | 1.4212 | 8908 | 0.0699 | 127,362 |
| Placebo | 1730 | 1.3513 | – | – | – |
| Eptinezumab 300 | 27,416 | 1.4392 | 25,686 | 0.0879 | 292,306 |
| (e) Using MMDs instead of MHDs | |||||
| Deterministic results – discounted | |||||
| Placebo | 1729 | 1.2257 | – | – | – |
| Topiramate | 1582 | 1.3212 | −147 | 0.0955 | Dominated |
| Placebo | 1729 | 1.2257 | – | – | – |
| BTA | 3646 | 1.3606 | 1917 | 0.1348 | 14.216 |
| Placebo | 1729 | 1.2257 | – | – | – |
| Erenumab 70 | 8945 | 1.3747 | 7216 | 0.1489 | 48,450 |
| Placebo | 1729 | 1.2257 | – | – | – |
| Erenumab 140 | 8946 | 1.3742 | 7217 | 0.1484 | 48,624 |
| Placebo | 1729 | 1.2257 | – | – | – |
| Fremanezumab (monthly) | 10,070 | 1.3919 | 8341 | 0.1661 | 50,212 |
| Placebo | 1729 | 1.2257 | – | – | – |
| Fremanezumab (quarterly) | 10,133 | 1.3652 | 8404 | 0.1395 | 60,263 |
| Placebo | 1729 | 1.2257 | – | – | – |
| Eptinezumab 100 | 10,184 | 1.3558 | 8456 | 0.1300 | 65,021 |
| Placebo | 1729 | 1.2257 | – | – | – |
| Galcanezumab | 10,604 | 1.3564 | 8875 | 0.1307 | 67,905 |
| Placebo | 1729 | 1.2257 | – | – | – |
| Eptinezumab 300 | 27,377 | 1.3823 | 25,648 | 0.1565 | 163,865 |
| Probabilistic results – discounted | |||||
| Placebo | 1731 | 1.2245 | – | – | – |
| Topiramate | 1585 | 1.3220 | −146 | 0.0975 | Dominated |
| Placebo | 1731 | 1.2245 | – | – | – |
| BTA | 3645 | 1.3566 | 1914 | 0.1321 | 14,493 |
| Placebo | 1731 | 1.2245 | – | – | – |
| Erenumab 70 | 8944 | 1.3754 | 7213 | 0.1508 | 47,824 |
| Placebo | 1731 | 1.2245 | – | – | – |
| Erenumab 140 | 8949 | 1.3749 | 7218 | 0.1504 | 48,001 |
| Placebo | 1730 | 1.2245 | – | – | – |
| Fremanezumab (monthly) | 10,072 | 1.3916 | 8341 | 0.1670 | 49,934 |
| Placebo | 1731 | 1.2245 | – | – | – |
| Fremanezumab (quarterly) | 10,140 | 1.3644 | 8409 | 0.1399 | 60,111 |
| Placebo | 1731 | 1.2245 | – | – | – |
| Eptinezumab 100 | 10,188 | 1.3584 | 8457 | 0.1339 | 63,178 |
| Placebo | 1731 | 1.2245 | – | – | – |
| Galcanezumab | 10,610 | 1.3584 | 8879 | 0.1339 | 66,322 |
| Placebo | 1731 | 1.2245 | – | – | – |
| Eptinezumab 300 | 27,377 | 1.3850 | 25,646 | 0.1605 | 159,779 |
| (f) Reducing costs of MAbs by 25% | |||||
| Deterministic results – discounted | |||||
| Placebo | 1729 | 1.3531 | – | – | – |
| Topiramate | 1625 | 1.3995 | –104 | 0.0464 | Dominated |
| Placebo | 1729 | 1.3531 | – | – | – |
| BTA | 3654 | 1.4294 | 1925 | 0.0763 | 25,238 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Fremanezumab (monthly) | 7996 | 1.4307 | 6267 | 0.0776 | 80,774 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Fremanezumab (quarterly) | 8033 | 1.4224 | 6304 | 0.0693 | 90,952 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Eptinezumab 100 | 8055 | 1.4239 | 6326 | 0.0708 | 89,300 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Galcanezumab | 8367 | 1.4229 | 6639 | 0.0698 | 95,091 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Eptinezumab 300 | 20,928 | 1.4403 | 19,199 | 0.0873 | 219,985 |
| Probabilistic results – discounted | |||||
| Placebo | 1727 | 1.3513 | – | – | – |
| Topiramate | 1627 | 1.3980 | –101 | 0.0467 | Dominated |
| Placebo | 1727 | 1.3513 | – | – | – |
| BTA | 3653 | 1.4275 | 1926 | 0.0762 | 25,264 |
| Placebo | 1727 | 1.3513 | – | – | – |
| Fremanezumab (monthly) | 7997 | 1.4303 | 6269 | 0.0790 | 79,328 |
| Placebo | 1727 | 1.3513 | – | – | – |
| Fremanezumab (quarterly) | 8039 | 1.4225 | 6311 | 0.0712 | 88,656 |
| Placebo | 1727 | 1.3513 | – | – | – |
| Eptinezumab 100 | 8057 | 1.4369 | 6329 | 0.0856 | 73,978 |
| Placebo | 1727 | 1.3513 | – | – | – |
| Galcanezumab | 8366 | 1.4249 | 6638 | 0.0736 | 90,251 |
| Placebo | 1727 | 1.3513 | – | – | – |
| Eptinezumab 300 | 20,938 | 1.4533 | 19,211 | 0.1019 | 188,442 |
| (g) Reducing costs of MAbs by 50% | |||||
| Deterministic results – discounted | |||||
| Placebo | 1729 | 1.3531 | – | – | – |
| Topiramate | 1625 | 1.3995 | –104 | 0.0464 | Dominated |
| Placebo | 1729 | 1.3531 | – | – | – |
| BTA | 3654 | 1.4294 | 1925 | 0.0763 | 25,238 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Fremanezumab (monthly) | 5837 | 1.4307 | 4108 | 0.0776 | 52,944 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Fremanezumab (quarterly) | 5872 | 1.4224 | 4143 | 0.0693 | 59,778 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Eptinezumab 100 | 5895 | 1.4239 | 4166 | 0.0708 | 58,804 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Galcanezumab | 6094 | 1.4229 | 4366 | 0.0698 | 62,532 |
| Placebo | 1729 | 1.3531 | – | – | – |
| Eptinezumab 300 | 14,455 | 1.4403 | 12,727 | 0.0873 | 145,820 |
| Probabilistic results – discounted | |||||
| Placebo | 1729 | 1.3415 | – | – | – |
| Topiramate | 1625 | 1.4078 | –105 | 0.0663 | Dominated |
| Placebo | 1729 | 1.3415 | – | – | – |
| BTA | 3653 | 1.4218 | 1923 | 0.0803 | 23,965 |
| Placebo | 1729 | 1.3415 | – | – | – |
| Fremanezumab (monthly) | 5835 | 1.4395 | 4106 | 0.0980 | 41,902 |
| Placebo | 1729 | 1.3415 | – | – | – |
| Fremanezumab (quarterly) | 5869 | 1.4321 | 4140 | 0.0906 | 45,706 |
| Placebo | 1729 | 1.3415 | – | – | – |
| Eptinezumab 100 | 5896 | 1.4210 | 4166 | 0.0795 | 52,409 |
| Placebo | 1729 | 1.3415 | – | – | – |
| Galcanezumab | 6097 | 1.4272 | 4367 | 0.0856 | 50,991 |
| Placebo | 1729 | 1.3415 | – | – | – |
| Eptinezumab 300 | 14,455 | 1.4358 | 12,7276 | 0.0942 | 135,025 |
| (h) Eptinezumab 100 vs. 300 mg | |||||
| Deterministic results – discounted | |||||
| Eptinezumab 100 | 10,216 | 1.4239 | – | – | – |
| Eptinezuma b 300 | 27,401 | 1.4403 | 17,185 | 0.0164 | 1,045,846 |
| Probabilistic results – discounted | |||||
| Eptinezumab 100 | 10,219 | 1.4247 | – | – | – |
| Eptinezumab 300 | 27,415 | 1.4416 | 17,195 | 0.0169 | 1,018,261 |
| (i) Topiramate vs. BTA | |||||
| Deterministic results – discounted | |||||
| Topiramate | 1625 | 1.3995 | – | – | – |
| BTA | 3654 | 1.4294 | 2029 | 0.0298 | 68,002 |
| Probabilistic results – discounted | |||||
| Topiramate | 1626 | 1.3969 | – | – | – |
| BTA | 3655 | 1.4319 | 2030 | 0.0351 | 57,881 |
| Costs (£) | QALYs | Incremental costs (£) | Incremental QALYs | ICER: cost per QALY gained (£) | Comparison | |
|---|---|---|---|---|---|---|
| a) 5-year time horizon | ||||||
| Deterministic results – discounted | ||||||
| Topiramate | 3165 | 3.1629 | – | – | – | – |
| Placebo | 3488 | 3.0463 | 323 | −0.1166 | Dominated | Placebo vs. topiramate |
| BTA | 6376 | 3.2414 | 3211 | 0.0784 | 40,928 | BTA vs. topiramate |
| Fremanezumab (monthly) | 16,005 | 3.2398 | 9629 | −0.0016 | Dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 16,103 | 3.2200 | 9727 | −0.0214 | Dominated | Fremanezumab (quarterly) vs. BTA |
| Eptinezumab 100 | 16,138 | 3.2237 | 9763 | −0.0177 | Dominated | Eptinezumab 100 vs. BTA |
| Galcanezumab | 16,545 | 3.2212 | 10,170 | −0.0202 | Dominated | Galcanezumab vs. BTA |
| Eptinezumab 300 | 42,120 | 3.2627 | 35,745 | 0.0213 | 1,676,779 | Eptinezumab 300 vs. BTA |
| Probabilistic results – discounted | ||||||
| Topiramate | 3159 | 3.1717 | – | – | – | – |
| Placebo | 3491 | 3.0348 | 333 | −0.1369 | Dominated | Placebo vs. topiramate |
| BTA | 6383 | 3.2497 | 3224 | 0.0779 | 41,366 | BTA vs. topiramate |
| Fremanezumab (monthly) | 16,039 | 3.2483 | 9656 | −0.0014 | Dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 16,120 | 3.2283 | 9737 | −0.0214 | Dominated | Fremanezumab (quarterly) vs. BTA |
| Eptinezumab 100 | 16,145 | 3.2163 | 9762 | −0.0334 | Dominated | Eptinezumab 100 vs. BTA |
| Galcanezumab | 16,577 | 3.2071 | 10,194 | −0.0425 | Dominated | Galcanezumab vs. BTA |
| Eptinezumab 300 | 42,184 | 3.2573 | 35,801 | 0.0076 | 4,707,286 | Eptinezumab 300 vs. BTA |
| b) Lifetime horizon | ||||||
| Deterministic results – discounted | ||||||
| Topiramate | 13,324 | 15.7707 | – | – | – | – |
| Placebo | 15,117 | 15.1901 | 1792 | −0.5805 | Dominated | Placebo vs. topiramate |
| BTA | 16,326 | 16.2092 | 3002 | 0.4385 | 6846 | BTA vs. topiramate |
| Fremanezumab (monthly) | 27,364 | 16.1691 | 11,038 | −0.0400 | Dominated | Fremanezumab (monthly) vs. BTA |
| Eptinezumab 100 | 27,736 | 16.0878 | 11,410 | −0.1214 | Dominated | Eptinezumab 100 vs. BTA |
| Fremanezumab (quarterly) | 27,741 | 16.0690 | 11,415 | −0.1402 | Dominated | Fremanezumab (quarterly) vs. BTA |
| Galcanezumab | 28,165 | 16.0748 | 11,838 | −0.1344 | Dominated | Galcanezumab vs. BTA |
| Eptinezumab 300 | 57,524 | 16.2834 | 41,197 | 0.0742 | 555,210 | Eptinezumab 300 vs. BTA |
| Probabilistic results – discounted | ||||||
| Topiramate | 13,351 | 15.7628 | – | – | – | – |
| Placebo | 15,138 | 15.1467 | 1787 | −0.6161 | Dominated | Placebo vs. topiramate |
| BTA | 16,381 | 16.2613 | 3030 | 0.4985 | 6077 | BTA vs. topiramate |
| Fremanezumab (monthly) | 27,469 | 16.1774 | 11,088 | −0.0840 | Dominated | Fremanezumab (monthly) vs. BTA |
| Eptinezumab 100 | 27,846 | 16.1319 | 11,465 | −0.1294 | Dominated | Fremanezumab (quarterly) vs. BTA |
| Fremanezumab (quarterly) | 27,840 | 16.0931 | 11,459 | −0.1682 | Dominated | Eptinezumab 100 vs. BTA |
| Galcanezumab | 28,194 | 16.1418 | 11,813 | −0.1195 | Dominated | Galcanezumab vs. BTA |
| Eptinezumab 300 | 57,609 | 16.3428 | 41,228 | 0.0815 | 505,711 | Eptinezumab 300 vs. BTA |
| c) Utility inputs – van Hout crosswalk algorithm | ||||||
| Deterministic results – discounted | ||||||
| Topiramate | 1625 | 1.4174 | – | – | – | – |
| Placebo | 1729 | 1.3733 | 104 | −0.0440 | Dominated | Placebo vs. topiramate |
| BTA | 3654 | 1.4458 | 2029 | 0.0284 | 71,339 | BTA vs. topiramate |
| Fremanezumab (monthly) | 10,155 | 1.4470 | 6501 | 0.0012 | Extendedly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 10,193 | 1.4391 | 38 | −0.0079 | Dominated | Fremanezumab (quarterly) vs. fremanezumab (monthly) |
| Eptinezumab 100 | 10,216 | 1.4406 | 60 | −0.0064 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Galcanezumab | 10,640 | 1.4396 | 485 | −0.0074 | Dominated | Galcanezumab vs. fremanezumab (monthly) |
| Eptinezumab 300 | 27,401 | 1.4562 | 23,747 | 0.0104 | 2,280,271 | Eptinezumab 300 vs. BTA |
| Probabilistic results – discounted | ||||||
| Topiramate | 1627 | 1.4063 | – | – | – | |
| Placebo | 1723 | 1.3807 | 96 | −0.0256 | Dominated | Placebo vs. topiramate |
| BTA | 3656 | 1.4475 | 2029 | 0.0412 | 49,265 | BTA vs. topiramate |
| Fremanezumab (monthly) | 10,161 | 1.4608 | 6505 | 0.0133 | Extendendly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 10,193 | 1.4532 | 32 | −0.0076 | Dominated | Fremanezumab (quarterly) vs. fremanezumab (monthly) |
| Eptinezumab 100 | 10,221 | 1.4346 | 60 | −0.0262 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Galcanezumab | 10,650 | 1.4436 | 489 | −0.0172 | Dominated | Galcanezumab vs. fremanezumab (monthly) |
| Eptinezumab 300 | 27,411 | 1.4512 | 23,755 | 0.0037 | 6,353,726 | Eptinezumab 300 vs. BTA |
| d) Drug administration – 1% of patients can’t self-administer medication | ||||||
| Deterministic results – discounted | ||||||
| Topiramate | 1625 | 1.3995 | – | – | – | – |
| Placebo | 1729 | 1.3531 | 104 | −0.0464 | Dominated | Placebo vs. topiramate |
| BTA | 3654 | 1.4294 | 2029 | 0.0298 | 68,002 | BTA vs. topiramate |
| Fremanezumab (monthly) | 10,140 | 1.4307 | 6486 | 0.0013 | Extendedly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 10,178 | 1.4224 | 38 | −0.0083 | Dominated | Fremanezumab (quarterly) vs. fremanezumab (monthly) |
| Eptinezumab 100 | 10,216 | 1.4239 | 76 | −0.0067 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Galcanezumab | 10,625 | 1.4229 | 485 | −0.0078 | Dominated | Galcanezumab vs. fremanezumab (monthly) |
| Eptinezumab 300 | 27,401 | 1.4403 | 23,747 | 0.0110 | 2,160,037 | Eptinezumab 300 vs. BTA |
| Probabilistic results – discounted | ||||||
| Topiramate | 1626 | 1.3988 | – | – | – | – |
| Placebo | 1730 | 1.3513 | 104 | −0.0475 | Dominated | Placebo vs. topiramate |
| BTA | 3655 | 1.4336 | 2028 | 0.0349 | 58,183 | BTA vs. topiramate |
| Fremanezumab (monthly) | 10,148 | 1.4281 | 6493 | −0.0056 | Dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 10,184 | 1.4189 | 6529 | −0.0147 | Dominated | Fremanezumab (quarterly) vs. BTA |
| Eptinezumab 100 | 10,220 | 1.4220 | 6566 | −0.0117 | Dominated | Eptinezumab 100 vs. BTA |
| Galcanezumab | 10,638 | 1.4212 | 6984 | −0.0124 | Dominated | Galcanezumab vs. BTA |
| Eptinezumab 300 | 27,416 | 1.4392 | 23,762 | 0.0055 | 4,294,946 | Eptinezumab 300 vs. BTA |
| e) Using MMDs instead of MHDs | ||||||
| Deterministic results – discounted | ||||||
| Topiramate | 1582 | 1.3212 | – | – | – | – |
| Placebo | 1729 | 1.2257 | 147 | −0.0955 | Dominated | Placebo vs. topiramate |
| BTA | 3646 | 1.3606 | 2064 | 0.0394 | 52,428 | BTA vs. topiramate |
| Erenumab 70 | 8945 | 1.3747 | 5299 | Extendedly dominated | Erenumab 70 vs. BTA | |
| Erenumab 140 | 8946 | 1.3742 | 1 | 0.0141 | Erenumab 140 vs. erenumab 70 | |
| Fremanezumab (monthly) | 10,070 | 1.3919 | 6424 | −0.0005 | Dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 10,133 | 1.3652 | 63 | 0.0313 | 205,481 | Fremanezumab (quarterly) vs. fremanezumab |
| Eptinezumab 100 | 10,184 | 1.3558 | 115 | −0.0267 | Dominated | (monthly) |
| Galcanezumab | 10,604 | 1.3564 | 534 | −0.0361 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Eptinezumab 300 | 27,377 | 1.3828 | 17,307 | −0.0354 −0.0096 |
Dominated Dominated |
Galcanezumab vs. fremanezumab (monthly) eptinezumab 300 vs. fremanezumab (monthly) |
| Probabilistic results – discounted | ||||||
| Topiramate | 1585 | 1.3220 | – | – | – | – |
| Placebo | 1731 | 1.2245 | 146 | −0.0975 | Dominated | Placebo vs. topiramate |
| BTA | 3645 | 1.3566 | 2060 | 0.0346 | 59,596 | BTA vs. topiramate |
| Erenumab 70 | 8944 | 1.3754 | 5299 | 0.0188 | Extendendly dominated | Erenumab 70 vs. BTA |
| Erenumab 140 | 8949 | 1.3749 | 5 | −0.0005 | Dominated | Erenumab 140 vs. erenumab 70 |
| Fremanezumab (monthly) | 10,072 | 1.3916 | 6427 | 0.0350 | 183,732 | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 10,140 | 1.3644 | 68 | −0.0272 | Dominated | Fremanezumab (quarterly) vs. fremanezumab (monthly) |
| Eptinezumab 100 | 10,188 | 1.3584 | 116 | −0.0332 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Galcanezumab | 10,610 | 1.3584 | 538 | −0.0332 | Dominated | Galcanezumab vs. fremanezumab (monthly) |
| Eptinezumab 300 | 27,377 | 1.3850 | 17,305 | −0.0065 | Dominated | Eptinezumab 300 vs. fremanezumab (monthly) |
| f) Reducing costs of MAbs by 25% | ||||||
| Deterministic results – discounted | ||||||
| Topiramate | 1625 | 1.3995 | – | – | – | – |
| Placebo | 1729 | 1.3531 | 104 | −0.0464 | Dominated | Placebo vs. topiramate |
| BTA | 3654 | 1.4294 | 2029 | 0.0298 | 68,002 | BTA vs. topiramate |
| Fremanezumab (monthly) | 7996 | 1.4307 | 4342 | 0.0013 | Extendedly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 8033 | 1.4224 | 37 | −0.0083 | Dominated | Fremanezumab (quarterly) vs. fremanezumab (monthly) |
| Eptinezumab 100 | 8055 | 1.4239 | 59 | −0.0067 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Galcanezumab | 8367 | 1.4229 | 371 | −0.0078 | Dominated | Galcanezumab vs. fremanezumab (monthly) |
| Eptinezumab 300 | 20,928 | 1.4403 | 17,274 | 0.0110 | 1,571,264 | Eptinezumab 300 vs. BTA |
| Probabilistic results – discounted | ||||||
| Topiramate | 1623 | 1.4026 | – | – | – | – |
| Placebo | 1730 | 1.3398 | 107 | −0.0628 | Dominated | Placebo vs. topiramate |
| BTA | 3653 | 1.4388 | 2031 | 0.0362 | 56,100 | BTA vs. topiramate |
| Fremanezumab (monthly) | 7998 | 1.4364 | 4344 | −0.0025 | Dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 8033 | 1.4284 | 4380 | −0.0104 | Dominated | Fremanezumab (quarterly) vs. BTA |
| Eptinezumab 100 | 8060 | 1.4232 | 4406 | −0.0157 | Dominated | Eptinezumab 100 vs. BTA |
| Galcanezumab | 8372 | 1.4174 | 4718 | −0.0215 | Dominated | Galcanezumab vs. BTA |
| Eptinezumab 300 | 20,928 | 1.4385 | 17,275 | −0.0003 | Dominated | Eptinezumab 300 vs. BTA |
| g) Reducing costs of MAbs by 50% | ||||||
| Deterministic results – discounted | ||||||
| Topiramate | 1625 | 1.3995 | – | – | – | – |
| Placebo | 1729 | 1.3531 | 104 | −0.0464 | Dominated | Placebo vs. topiramate |
| BTA | 3654 | 1.4294 | 2029 | 0.0298 | 68,002 | BTA vs. topiramate |
| Fremanezumab (monthly) | 5837 | 1.4307 | 2183 | 0.0013 | Extendedly dominated | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 5872 | 1.4224 | 35 | −0.0083 | Dominated | Fremanezumab (quarterly) vs. fremanezumab (monthly) |
| Eptinezumab 100 | 5895 | 1.4239 | 58 | −0.0067 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Galcanezumab | 6094 | 1.4229 1.4403 |
258 | −0.0078 | Dominated | Galcanezumab vs. fremanezumab (monthly) |
| Eptinezumab 300 | 14,455 | 10,801 | 0.0110 | 982,491 | Eptinezumab 300 vs. BTA | |
| Probabilistic results – discounted | ||||||
| Topiramate | 1625 | 1.4078 | – | – | – | – |
| Placebo | 1729 | 1.3415 | 105 | −0.0663 | Dominated | Placebo vs. topiramate |
| BTA | 3653 | 1.4218 | 2028 | 0.0140 | 144,881 | BTA vs. topiramate |
| Fremanezumab (monthly) | 5835 | 1.4395 | 2182 | 0.0177 | 123,111 | Fremanezumab (monthly) vs. BTA |
| Fremanezumab (quarterly) | 5869 | 1.4321 | 34 | −0.0074 | Dominated | Fremanezumab (quarterly) vs. fremanezumab (monthly) |
| Eptinezumab 100 | 5896 | 1.4210 | 61 | −0.0185 | Dominated | Eptinezumab 100 vs. fremanezumab (monthly) |
| Galcanezumab | 6097 | 1.4272 | 261 | −0.0123 | Dominated | Galcanezumab vs. fremanezumab (monthly) |
| Eptinezumab 300 | 14,455 | 1.4358 | 8620 | −0.0037 | Dominated | Eptinezumab 300 vs. fremanezumab (monthly) |
List of abbreviations
- ACE
- angiotensin-converting enzyme
- A&E
- accident and emergency
- AEs
- adverse events
- AMG334
- erenumab
- AWMSG
- All Wales Medicine Strategy Group
- BMI
- body mass index
- BNF
- British National Formulary
- BTA
- onabotulinumtoxinA/botulinum toxin type A/Botox
- CADTH
- Canadian Agency for Drugs and Technology in Health
- CEAC
- cost-effectiveness acceptability curve
- CGRP
- calcitonin gene-related peptide
- CEAF
- cost-effectiveness acceptability frontier
- CHD
- chronic daily headache
- CHEERS
- Consolidated Health Economic Evaluation Reporting Standards
- CM
- chronic migraine
- CrI
- credible interval
- CTCAE
- Common Terminology Criteria for Adverse Events
- DIC
- deviance information criterion
- ED
- emergency department
- EoI
- expression of interest
- EQ-5D
- EuroQol EQ-5D
- EVPI
- expected value of perfect information
- FIMQ
- Functional Impact of Migraine Questionnaire
- Fremanezumab-M
- fremanezumab monthly
- Fremanezumab-Q
- fremanezumab quarterly
- GP
- general practitioner
- GRADE
- Grading of Recommendations, Assessment, Development and Evaluations
- HIT-6
- headache impact test-6
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- IBMS
- International Burden of Migraine Studies
- ICER
- incremental cost-effectiveness ratio
- ICHD
- International Classification of Headache Disorders
- ICHD-2
- International Classification of Headache Disorders, version 2
- ICHD-3
- International Classification of Headache Disorders, version 3
- ICTRP
- International Clinical Trials Registry Platform
- IM
- intramuscular
- ITT
- intention to treat
- IV
- intravenous
- MAb
- monoclonal antibody
- MD
- mean difference
- MHD
- monthly headache day
- MIDAS
- Migraine Disability Assessment
- MMD
- monthly migraine day
- MSQ
- migraine-specific quality of life
- MSQ-EF
- migraine-specific quality of life – emotional function
- MSQ-PR
- migraine-specific quality of life – preventative role
- MSQ-RR
- migraine-specific quality of life – restrictive role
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- NMC
- National Migraine Centre
- OL
- open label
- ONS
- Office for National Statistics
- PBO
- placebo
- PHQ-9
- Patient Health Questionnaire 9-item
- PICO
- population, intervention, comparators, outcomes
- PPI
- patient and public involvement
- PREEMPT
- Participants in The Phase III REsearch Evaluating Migraine Prophylaxis Therapy
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PSS
- personal social service
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RoB
- risk of bias
- SAE
- serious adverse event
- SC
- subcutaneous
- SD
- standard deviation
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMC
- Scottish Medicines Consortium
- SNRIs
- serotonin noradrenaline reuptake inhibitors
- SOC
- system organ class
- SUCRA
- Surface Under the Cumulative Ranking Area
- TAE
- treatment-related adverse event
- TSAE
- treatment-related serious adverse event
- U
- units
- UME
- unrelated mean effect
- WHO
- World Health Organization
- WPAI:SHP
- Work Productivity and Activity Impairment – Specific Health Problem
- WTP
- willingness to pay
Notes
-
List of excluded studies for clinical effectiveness and adverse events reviews
-
Supplementary Information: The GRADE approach for rating the quality of estimates of treatment effect size
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/AYWA5297).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
