Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 16/162/01. The contractual start date was in September 2018. The draft manuscript began editorial review in July 2023 and was accepted for publication in January 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Wright-Hughes et al. This work was produced by Wright-Hughes et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Wright-Hughes et al.
Chapter 1 Introduction
Background and rationale
Irritable bowel syndrome (IBS) is a common, chronic, disorder of gut–brain interaction, characterised by abdominal pain in association with a change in stool form or frequency. 1 Prevalence is 5% in the community,2 and IBS accounts for > 3% of all consultations in primary care. 3 The total cost to the health service in the UK has been estimated to be > £1 billion/year. 4 Quality of life of people with IBS is impaired substantially, to a level comparable with that seen in some organic bowel disorders, such as Crohn’s disease. 5 Current first-line treatment of IBS in primary care includes dietary and lifestyle advice, fibre supplements, laxatives, antispasmodic drugs, or loperamide, but if these are ineffective, general practitioners (GPs) are often left with few treatment options, meaning people are frequently referred to see a specialist in secondary care. 6
Medical management of IBS is unsatisfactory, with no therapy proven to alter the long-term natural history and, at best, modest symptom reduction. Previous meta-analyses have suggested tricyclic antidepressants (TCAs) may be an efficacious treatment. 7–9 The most recent of these identified 12 trials, which included 787 patients. 9 Beneficial effects on IBS symptoms may arise from their well-known pain-modifying properties,10–13 as well as their influence on gut motility,14 rather than any antidepressive effects, as the doses used in randomised controlled trials (RCTs) in IBS are considerably less than the dose required to have any effect on mood. However, duration of follow-up was limited to 12 weeks, all trials were conducted in secondary or tertiary care, where patients have more severe symptoms, and most studies were small. These limitations are important. The clinical relevance of demonstrating the effectiveness of a drug over a 12-week RCT in a condition that is chronic, and often lifelong,15 is debatable. In addition, although there is evidence from pooling data from secondary and tertiary care-based trials in a meta-analysis, it is not clear whether this effect would translate into a benefit in primary care, and whether this will reduce resource use and referrals to secondary care or improve quality of life and social functioning.
The National Institute for Health and Care Excellence (NICE) guideline for the management of IBS in primary care states only that GPs should ‘consider’ TCAs as second-line treatment for IBS for their analgesic effect,16 for example ‘amitriptyline at a dose of 10 mg to 30 mg’, if dietary changes, fibre supplements, laxatives, antispasmodics or loperamide have not helped. However, this guideline also acknowledges that there is limited evidence to support this statement and proposes that a large RCT be conducted comparing a TCA with placebo in adults with IBS in primary care, with outcomes assessed at 3, 6 and 12 months, and including global improvement in IBS symptoms, effect on health-related quality of life, and adverse effects.
At present, therefore, there is uncertainty as to whether TCAs are effective for the treatment of IBS in primary care, and this may mean GPs are reluctant to consider using them. In a prior survey < 10% of GPs used them often, and only 50% believed they were effective. 17 Given that 95% of GPs use these drugs for the treatment of insomnia in primary care,18 it is presumably uncertainty over their efficacy in IBS, rather than concerns about side effects, which explains this reluctance. If a drug that is potentially efficacious for IBS is being under-utilised, this will have a negative effect on both the health service and society, in terms of worse control of IBS symptoms, which will lead to lower quality of life for people with IBS, increased sickness absences from work, and higher costs of managing IBS in secondary care, due to greater numbers of referrals and increased rates of investigation.
Given the recommendations of the NICE guideline,16 together with the fact that two of the trials in the meta-analyses used amitriptyline,19,20 both of which were small, but positive, and its proven pain-modifying properties,11,12 as well as its effects on gut motility14 and visceral hypersensitivity,21 we chose to assess amitriptyline. This study was funded successfully as part of a commissioned call by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme (NIHR award ref: 16/162/01), which identified the need to address the short- and long-term benefits of low-dose antidepressants for IBS in primary care, to help guide treatment decisions. Our work with patients and the public prior to obtaining funding confirmed a perceived need for the study but identified potential concerns about the use of a drug identified as an antidepressant for a condition like IBS. This provided a rationale for both the trial and a nested qualitative study to explore potential barriers to implementation, should low-dose amitriptyline prove to be effective.
Objectives
The objective of the AmitripTyline at Low-dose ANd Titrated for IBS as Second-line treatment (ATLANTIS) trial was to determine the clinical and cost-effectiveness of low-dose amitriptyline for IBS in primary care compared with placebo. A nested, qualitative study explored participant and GP experiences of treatments and trial participation, and implications for wider use of amitriptyline for IBS in primary care. We aimed to deliver a definitive assessment of the benefits, harms, and cost-effectiveness of low-dose amitriptyline as second-line treatment for IBS in primary care, within the NHS, to guide future adoption and implementation.
Chapter 2 Methods
Trial design
ATLANTIS was a pragmatic, randomised, multicentre, parallel-group, double-blind, placebo-controlled, superiority trial of low-dose amitriptyline as a second-line treatment for adults with IBS in primary care. The majority of participants recruited consented to 12-month study participation, consisting of an initial 6 months of trial medication with the option to continue this voluntarily for a further 6 months. Treatment duration and follow-up was curtailed to 6 months for later recruits, due to protocol changes made during the coronavirus disease discovered in 2019 (COVID-19) pandemic. Additionally, although the data to inform a cost-effectiveness analysis were collected, the analysis was unable to be completed and is planned as future work, if further funding becomes available. A nested, qualitative study explored participants’ and GPs’ experiences of treatments and participating in the trial, including acceptability, adherence, unanticipated effects, and implications for the wider use of amitriptyline for IBS.
Patient and public involvement (PPI) representatives were involved at all stages and provided valuable contributions to trial design, documentation and outputs. The final protocol and subsequent amendments were approved by Yorkshire and the Humber (Sheffield) Research Ethics Committee (19/YH/0150) and published in full. 22 The trial was conducted in accordance with the principles of Good Clinical Practice and the Declaration of Helsinki and registered with the ISRCTN (ISRCTN48075063).
Trial objectives and outcome measures
Primary
The primary objective was to determine the effect of amitriptyline on global symptoms of IBS, as measured by the IBS Severity Scoring System (IBS-SSS), 6 months after randomisation. The IBS-SSS is a validated, participant-reported, five-item questionnaire used widely in IBS trials. 23 It measures presence, severity and frequency of abdominal pain, presence and severity of abdominal distension or tightness, satisfaction with bowel habit and degree to which IBS symptoms are affecting, or interfering with, the person’s life in general. The maximum score is 500 points: a score of < 75 points indicates symptoms that are felt to be in remission, with normal bowel function; 75–174 points indicates mild IBS symptoms, 175–299 points moderate IBS and 300–500 points severe IBS.
Key secondary
The key secondary objective was to determine the effect of amitriptyline on global symptoms of IBS, according to the proportion of participants with subjective global assessment (SGA) of relief of IBS symptoms. 24 Participants rate their relief from IBS symptoms on a scale of 1 to 5 ranging from ‘completely relieved’ to ‘worse’. Scores are dichotomised so that those scoring from 1 to 3 are considered responders and those 4 or 5 non-responders. At 6 months after randomisation, response was, therefore, defined as reporting somewhat, considerable, or complete relief of IBS symptoms.
Secondary
Secondary objectives were to assess the effect of amitriptyline on:
-
Global symptoms of IBS, as measured by the IBS-SSS at 3 and 12 months.
-
Global symptoms of IBS, as measured by the proportion of participants with SGA of relief of IBS symptoms,24 at 3 and 12 months, with response defined as above.
-
Adequate relief of IBS symptoms via a weekly response to the question ‘Have you had adequate relief of your IBS symptoms?’
-
IBS-associated somatic symptoms, as measured by the Patient Health Questionnaire-12 (PHQ-12),25,26 at 6 months.
-
Anxiety and depression scores, as measured by the Hospital Anxiety and Depression Scale (HADS),27 at 3, 6 and 12 months.
-
Ability to work and participate in other activities, as measured by the Work and Social Adjustment Scale (WSAS),28–30 at 3, 6 and 12 months.
-
Acceptability of treatment, as measured by a response of ‘Yes’ to the participant-reported question at 6 months: ‘On balance do you find this medication acceptable to take and would you want to keep taking it?’. Non-acceptability defined for any participants reporting ‘No’, having discontinued treatment before 6 months, not starting trial treatment, or lost to follow-up.
-
Self-reported adherence to treatment, based on participant-report, via the question: ‘Since you were last asked, which of the options best describes how often you have taken at least one tablet of the trial medication daily?’: ‘Every day or nearly every day’, ‘Half of the days or more than half the days’, ‘Less than half of the days’, ‘None or nearly none of the days’, with an additional response for participants having discontinued or not started trial treatment or lost to follow-up, at 3, 6, 9 and 12 months.
-
Tolerability of treatment, as measured by the Antidepressant Side-Effect Checklist (ASEC),31 and mean ASEC total score [providing an approximate index of all reported adverse events (AEs) between arms], at 3, 6 and 12 months.
Cost-effectiveness objectives
Cost-effectiveness objectives were to assess the effect of amitriptyline on:
-
self-reported healthcare use, as measured by a health resource use questionnaire, at 3, 6 and 12 months
-
health-related quality of life, as measured by the EQ-5D-3L,32,33 at 3, 6 and 12 months
-
cost-effectiveness, as measured via the incremental cost-effectiveness ratio, and expressed in terms of incremental cost per quality-adjusted life-year (QALY) at 6 and 12 months.
Data were collected but analysis is on hold, awaiting further funding.
Nested qualitative study objectives
These are provided in Aims.
Participants
Adult patients with IBS in primary care, who were still symptomatic despite first-line therapies, as defined by NICE, were potentially eligible to take part in the trial. Eligible patients met all the listed inclusion criteria, and none of the exclusion criteria. Eligibility waivers to inclusion and exclusion criteria were not permitted.
Inclusion criteria
-
A diagnosis of IBS [of any subtype [IBS with constipation (IBS-C), diarrhoea (IBS-D), mixed bowel habits (IBS-M), or unclassified (IBS-U)]} in the patient’s primary care record, and fulfilling the Rome IV criteria34 (see Report Supplementary Material 1).
-
Age ≥ 18 years.
-
Ongoing symptoms, defined as an IBS-SSS score of ≥ 75 at screening,23 despite having tried dietary changes and first-line therapies as defined by NICE [fibre supplements (e.g. ispaghula husk), laxatives (e.g. bisacodyl), antispasmodics (e.g. mebeverine) or anti-diarrhoeals (e.g. loperamide)],16 which was assessed at screening via patient self-report.
-
A normal haemoglobin, total white cell count (WCC), and platelets within the last 6 months prior to screening.
-
A normal C-reactive protein (CRP) within the last 6 months prior to screening.
-
Exclusion of coeliac disease, via anti-tissue transglutaminase (tTG) antibodies, as per NICE guidance. 16
-
No evidence of active suicidal ideation, as determined by three clinical screening questions below, and no recent history of self-harm (an episode of self-harm within the last 12 months prior to screening). Any positive response on any of the three questions triggered urgent review by the patient’s GP. These clinical questions were used in preference to a formal suicidal risk rating scale, as such scales perform poorly in clinical practice:
-
whether the patient had experienced any thoughts of harming themselves, or ending their life, in the last 7–10 days
-
whether the patient currently had any thoughts of harming themselves or ending their life
-
whether the patient had any active plans or ideas about harming themselves, or taking their life, in the near future.
-
-
If female:
-
post menopausal (no menses for 12 months without an alternative medical cause), or
-
surgically sterile (hysterectomy, bilateral salpingectomy or bilateral oophorectomy), or
-
using highly effective contraception (and had to agree to continue for 7 days after the last dose of the investigational medicinal product).
-
-
Able to complete questionnaires and trial assessments.
-
Able to provide written informed consent.
Exclusion criteria
-
Age > 60 years with no GP review in the 12 months prior to screening (to assess for organic gastrointestinal disease as a cause of gastrointestinal symptoms, as this becomes more likely with increasing age).
-
Meeting locally adapted NICE 2-week referral criteria for suspected lower gastrointestinal cancer. 35
-
A known documented diagnosis of inflammatory bowel disease or coeliac disease.
-
A previous diagnosis of colorectal cancer.
-
Currently participating in, or within the 3 months prior to screening having been involved in, another clinical trial of an investigational medicinal product.
-
Pregnant or breastfeeding.
-
Planning to become pregnant within the next 18 months.
-
Currently using a TCA or using a TCA for another indication within the last 2 weeks prior to randomisation.
-
Allergy to TCAs.
-
Other known contraindications to the use of TCAs, including patients with any of the following:
-
taking monoamine oxidase inhibitors, or receiving them within the last 2 weeks
-
already prescribed a TCA for the treatment of depression
-
previous myocardial infarction
-
recorded arrhythmias, particularly heart block of any degree, prolonged Q-T interval on ECG
-
mania
-
severe liver disease
-
porphyria
-
congestive heart failure
-
coronary artery insufficiency
-
receiving concomitant drugs that prolong the QT interval (e.g. amiodarone, terfenadine or sotalol)
-
Study settings
Participants were recruited from 55 general practices [including two participant identification centres (PICs)] within urban and rural settings, with a range of sociodemographic and diversity characteristics. A further three general practices (two in West Yorkshire and one in Wessex) were opened to recruitment but did not mail-out before the recruitment period had ended. Each practice was classed as a research site with a GP as the principal investigator (PI). Practices were required to have obtained management approval and to have undertaken a site initiation meeting prior to the start of recruitment into the trial.
The 55 involved general practices were in three geographical regions, 22 in West of England (including one PIC), 13 in West Yorkshire (including one PIC) and 20 in Wessex. These three geographical regions were referred to as ‘hubs’. Each hub research team included a hub lead clinician and research nurse(s) or clinical study officer and was responsible for co-ordinating patient activity.
Screening, recruitment and registration
General practices willing to participate in the study were recruited with the assistance of the regional clinical research networks (CRNs). They searched their patient registers for potentially eligible patients aged ≥ 18 years with a diagnosis of IBS, using a SnoMed clinical terms search, which was developed previously in the NIHR-funded ACTIB trial,36,37 and updated with the support of the Wessex CRN. A GP at the practice checked the list of patients to be contacted prior to the invitation letters being sent out, to ensure that it was appropriate to contact them. Potentially eligible individuals were then contacted by letter, sent by the general practice via DocMail, informing them about the trial and inviting them to take part.
The postal invitation included a participant information sheet and informed consent form (see Report Supplementary Material 1). Potential participants interested in taking part returned a reply slip in a pre-paid envelope or contacted the study team directly via e-mail or telephone. The reply slip included a section for the potential participant to agree to be contacted about the study and, following this, for information to be requested from their GP to confirm their suitability to take part in the trial. This agreement was obtained by e-mail or telephone if the initial contact was not via returning a reply slip. The reply slip also included a ‘reason to decline’ section so that we could gather information on why people chose not to participate in the trial. Recruiting general practices were asked to provide an anonymised list of the age and sex of those invited so that the characteristics of those invited could be compared with those entering the trial.
General practitioners could also provide information about the trial to potential eligible patients opportunistically during their surgeries. Posters and leaflets were displayed in waiting rooms and the trial was advertised on general practice websites, where possible. Thus, if a patient with IBS attended a consultation, they were able to ask the GP about the study and to be given contact details for the study team, if appropriate.
Patients with IBS could also be identified by general practices working as PICs. PICs were responsible for the identification of potential patients for the trial and mailing out the invitation letter, participant information sheet, and informed consent form. Patients were then directed to respond to the invitation to the main hub research team. Patients identified from PICs were seen at the main general practice.
In an attempt to recruit an ethnically diverse sample, we reached out to minority ethnic organisations for advice as to how to make the trial more attractive to people from minority ethnic backgrounds and to publicise and raise the profile of the trial among these particular groups of potential participants.
The hub research nurse or clinical study officer contacted potential participants who replied to the study invitation to arrange a screening call. At this call, they provided further information about the trial and obtained verbal consent to telephone-screen the potential participants, using a screening form consisting of the Rome IV criteria,34 the IBS-SSS,23 and questions about the inclusion and exclusion criteria. All potential participants were assigned a unique screening identification number.
To allow generalisation of the trial results, and in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines, each recruitment hub, on behalf of each general practice, maintained and provided to the Leeds Clinical Trials Research Unit (CTRU) an anonymised screening log of the age and sex of all patients who were screened for entry into the study, including all those who confirmed interest. Documented reasons for ineligibility or declining participation were recorded and were closely monitored by the CTRU as part of a regular review of recruitment progress.
Patients who were potentially eligible, after telephone screening, were asked to attend a face-to-face appointment at their general practice to complete full eligibility screening, provide written informed consent, and obtain blood tests, if required. Patients were allowed sufficient time, and at least 24 hours, unless they wished to participate sooner, to consider participation and were given the opportunity to discuss the study with their family and healthcare professionals before they were asked whether they would be willing to take part. The research nurses and clinical study officer were trained in both the informed consent process and the ATLANTIS study and provided the patient with full and adequate oral and written information about the study, including the background, purpose, and risks and benefits of participation, as well as ensuring that the opportunity to ask questions concerning study participation was given. A GP was available to answer any questions or concerns, if required. The research nurses or clinical study officer also confirmed that the participant was free to withdraw from the study at any time without it affecting their future care. The original copy of the signed, dated, informed consent form was stored in the investigator site file. One copy was also filed in the medical records, one given to the participant, and one returned to the CTRU.
Informed consent from participants also included a request to take part in qualitative interviews at 6 and 12 months (for participants who had consented to 12-month follow-up), or 6 months only (for later participants who had only consented to 6-month follow-up), with information concerning the likely duration of these interviews provided. Finally, permission was also sought to collect longer-term routine data from electronic health records concerning amitriptyline and other IBS medication prescriptions, GP consultations for IBS, and secondary care referrals, outside the time frame of the trial itself, should further funding become available.
Following confirmation of written informed consent, participants were registered into the trial as soon as possible by an authorised member of the hub research staff. Informed consent for entry into the trial had to be obtained prior to registration. Registration was performed centrally using the CTRU automated web-based registration and randomisation system. All participants were allocated a unique trial identification number after they had been registered.
Blood test results were made available to the hub lead clinician (or delegate), the research nurse or clinical study officer, and the participant’s GP. In accordance with the trial inclusion criteria, if the blood tests showed an abnormal result (i.e. anaemia, raised or lowered total WCC, raised or lowered platelet count, a CRP over the normal laboratory range, or a positive anti-tTG antibody), the individual was not randomised into the trial, but was referred back to their GP for further assessment. However, if there were marginal, and potentially clinically insignificant, abnormalities of haemoglobin, WCC, or platelets, these were reviewed by the responsible GP and the hub lead clinician for consideration for inclusion into the study. In the case of an abnormal blood result for haemoglobin, WCC, platelets, or CRP that may have been a temporary abnormality (e.g. secondary to a recent infection), or a marginal or minor abnormality, the blood test could be repeated 2 to 4 weeks later, if the participant wished to undertake further screening for the study. If on repeat testing total haemoglobin, WCC, platelet count, and CRP were acceptable clinically, the participant could continue with screening.
If blood results were within acceptable limits, the GP from the research site was asked to confirm eligibility and sign the study-specific trial medication prescription form. Participants were then provided with web-based or postal questionnaires, depending on preference, to complete at baseline. These had to be completed no more than 7 days prior to randomisation if online, and within 14 days prior if postal. Participants were not randomised until the baseline questionnaires were completed. If randomisation had not taken place within 4 weeks of the baseline questionnaires being completed, these were repeated.
Prior to randomisation, women of childbearing potential were asked to confirm verbally that they were not pregnant and were on reliable contraception due to the potential risks of amitriptyline in pregnancy. If they were unable to do so, they were asked to perform a urine pregnancy test within the 7 days prior to randomisation. They were provided with a pregnancy test to use at home, to facilitate a result as close as possible to randomisation, and hence treatment commencing. Because this formed part of the eligibility criteria, the lead clinician at each hub reviewed and signed the final eligibility for these participants. If the test was positive or unclear the individual was not randomised into the trial but was directed to their GP.
If the participant was ineligible, or no longer wished to take part in the trial, the local research team withdrew the participant. They did not undergo any other study assessments and they were referred to their GP. Reasons for non-randomisation were documented, where available.
Randomisation
Participants were randomised 1 : 1 to receive amitriptyline or placebo. Allocation, via a web randomisation system at the University of Leeds CTRU, was performed using minimisation with a random element, to ensure balance with respect to the presence of raised HADS-depression scores (score ≥ 8), IBS subtype (IBS-C, IBS-D, IBS-M or IBS-U) and recruitment hub between treatment arms.
Blinding
As the trial was double-blind, neither the participant nor those responsible for their care and evaluation (treating team and research team) knew the treatment allocation or coding of the treatment allocation. Central pharmacy was also blinded to treatment allocation. This was achieved by identical tablets, packaging, and labelling of both the amitriptyline and placebo. The process for dose titration was the same for amitriptyline and placebo. Each bottle of amitriptyline or placebo was identified by a unique kit code, generated randomly. Lists of the kit codes and their corresponding treatments were generated by the CTRU and sent to Modepharma Limited, who supplied the kits and the code break envelopes. Management of kit codes on the kit logistics application, which was linked to the 24-hour randomisation system, were conducted by the unblinded CTRU safety statistician, in addition to maintaining the back-up kit code list.
Access to the code break envelopes at CTRU was restricted to the safety statistician and designated safety team. Interim emergency unblinding before 6- or 12-month assessments was strongly discouraged and the blind was only to be broken if information about the participant’s trial treatment was clearly necessary and would alter the appropriate medical management of the participant. Interim unblinding could be requested on the grounds of safety by the chief investigator, local PI, an authorised delegate, or a treating physician. It was anticipated that these requests would most likely originate at the time of an AE or planned change in non-trial related drug therapy. In the event of a serious adverse event (SAE), all participants were to be treated as though they were receiving the active medication (i.e. amitriptyline). Any unblinded interim reports supplied to the Data Monitoring and Ethics Committee (DMEC) were provided by the CTRU safety statistician and were password-protected securely.
At study entry, all participants were asked to provide their consent to be contacted by the CTRU to send them their treatment allocation after they had reached the 12-month assessment point in the trial, for participants who had consented to 12-month follow-up, or after they had reached the 6-month assessment point in the trial, for later participants who had consented to only 6-month follow-up. If they agreed, they and their GP were informed of their treatment allocation shortly after their final follow-up at 6 or 12 months. This was done to facilitate post-trial treatment decisions. The information was provided via e-mail, supported by an evidence-based unblinding leaflet (see Report Supplementary Material 1) to deal with any potential questions that may arise, which was developed with input from PPI representatives. Information concerning treatment allocation was provided only when CTRU had confirmation that all study assessments and contacts were complete, and all required data had been received. This information was only provided to the participant and their GP to protect and maintain the overall blind for the research team. Where participants needed further support following provision of treatment allocation and the evidence-based leaflet, they were directed to the ATLANTIS qualitative researcher, who was independent of the day-to-day running of the trial, recruitment, data collection, and treatment decisions.
Intervention
Participants were randomised 1 : 1 to receive titrated low-dose amitriptyline (Teva, the Netherlands) or identical-appearing placebo for 6 months. All participants also received the British Dietetic Association NICE-approved dietary advice sheet for IBS,38 and were provided with usual care for IBS from their GP during the trial, with the exception that amitriptyline or other TCAs could not be commenced during the trial. In addition, drugs contraindicated with TCAs, such as monoamine oxidase inhibitors or drugs prolonging the QT interval, were prohibited during the trial. Following randomisation, participants were offered an optional GP appointment at 1 month, in case of any questions, in addition to research nurse and clinical study officer support.
All participants were provided with standardised written information about dose adjustment (see Report Supplementary Material 1), developed with input from PPI representatives, to guide participant-led dose titration. This advised participants to commence at a dose of amitriptyline or placebo of 10 mg (one tablet) once-daily at night, with dose titration occurring over 3 weeks, up to a maximum of 30 mg (three tablets) once-daily at night or down to a minimum of 10 mg on alternate days, depending on side effects and response to treatment. Participants were supported throughout the titration phase, with telephone calls from the research nurse or clinical study officer at weeks 1 and 3 to assess tolerability. After an initial 3-week titration, it was expected the majority of participants would remain on a steady dose, but they could modify their dose throughout the study in response to their symptoms and any side effects, reflecting how amitriptyline would be used in usual care.
For safety purposes, due to the potential risks from amitriptyline in overdose, participants were provided with an initial 1-month supply of trial medication following randomisation. Further trial medication was dispensed at months 1 (2-month re-supply), 3 (3-month re-supply), 6 (3-month re-supply) and 9 (3-month re-supply), as appropriate, with the hub research nurse or clinical study officer contacting participants by telephone prior to re-supply at weeks 1 and 3 and months 3, 6, 9 and 12 to assess both for new evidence of suicidal ideation, adherence to trial medication, tolerability of trial medication via the ASEC, and reasons for discontinuation of trial medication (if appropriate), as well as recording concomitant medications and providing support as needed.
Participants were planned to be followed up for up to 12 months. Our PPI input prior to the grant submission revealed that 6 months of blinded treatment was felt to be the maximum reasonable initial commitment. Therefore, participants randomised in the first stage of recruitment (before 7 October 2021) received 6 months of trial medication initially and then were able to either continue blinded trial medication for a further 6 months or stop trial medication; participants randomised in the later stages of recruitment (on or after 7 October 2021) received 6 months of trial medication only. Following trial and outcome measure completion participants had the option to be unblinded and discuss amitriptyline prescription for IBS under the care of their GP if they wished.
Trial medication was supplied by Modepharma Limited and dispensed by post to the participant’s home by a central pharmacy at Leeds Teaching Hospitals NHS Trust. A copy of the study-specific trial medication prescription form was sent to the central pharmacy to facilitate prompt dispensing when the participant was randomised, although a wet ink copy was required before the study trial medication was dispensed and shipped to the participant. The participant confirmed medication receipt with the hub research team.
Assessments and data collection
Trial assessments are summarised in Table 1 with further details of assessment instruments provided below. Participants were contacted by the researcher via telephone at 1 and 3 weeks and 3, 6, 9 and 12 months (as appropriate) to support titration and ongoing treatment, including assessments of suicidal ideation, toxicity, adherence to, and acceptability of trial medication. Participants completed electronic or postal questionnaires at baseline, and 3 and 6 months, and answered a weekly question ‘Have you had adequate relief of your IBS symptoms?’ for the initial 6-month study duration. Participants recruited to 12-month follow-up, before 7 October 2021, also completed electronic or postal questionnaires at 12 months. Text message and e-mail reminders were sent at 1 week to prompt completion of questionnaires. Non-responders were telephoned with a final reminder.
| Time point | Study period | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Screening, recruitment, registration | Randomisation | Follow-up | |||||||
| −4 weeks − 0 | 0 | Week 1 | Week 3 | Week 4 | Month 3 | Month 6 | Month 9a | Month 12a | |
| Enrolment | |||||||||
| Verbal consent and eligibility screen | X | ||||||||
| Eligibility confirmation (including Rome IV criteria, blood and pregnancy tests) | X | ||||||||
| Informed consent | X | ||||||||
| Sociodemographic details: medical history; duration of IBS symptoms; previous depression or anxiety; Bristol Stool Form Scale | X | ||||||||
| Allocation | X | ||||||||
| Interventions | |||||||||
| Amitriptyline |

|
||||||||
| Placebo | |||||||||
| Study medication provision | X | X | X | X | X | ||||
| Optional GP review | X | ||||||||
| Assessments (research nurse/clinical study officer collected, while on treatment) | |||||||||
| Suicidal ideation | X | X | X | X | X | ||||
| Current dose | X | X | X | X | X | X | |||
| Concomitant Medications Review | X | X | X | X | X | X | |||
| Treatment adherence | X | X | X | X | X | ||||
| Treatment acceptability | X | X | X | X | |||||
| ASEC | X | X | X | ||||||
| Exit survey | X | ||||||||
| Assessments (self-completed questionnaire) | |||||||||
| ASEC | X | X | X | ||||||
| IBS-SSS | X | X | X | X | |||||
| HADS | X | X | X | X | |||||
| EQ-5D-3L | X | X | X | X | |||||
| WSAS | X | X | X | X | |||||
| PHQ-12 | X | X | |||||||
| SGA of relief of IBS symptoms | X | X | X | ||||||
| Health resource use | X | X | X | X | |||||
| Relief of IBS symptoms | Weekly diary with SMS text reminder | ||||||||
| Optional participant interview (nested qualitative study) | X | X | |||||||
Suicidal ideation was assessed by the researcher during all planned telephone calls via three brief questions. If yes to any, the participant was not issued with any further trial medication and their GP was contacted immediately.
-
Has the participant experienced any thoughts of harming themselves, or ending their life, in the last 7–10 days?
-
Does the participant currently have thoughts of harming themselves or ending their life?
-
Does the participant have any active plans or ideas about harming themselves, or taking their life, in the near future?
Adherence to treatment was measured by the researcher during the planned telephone calls. Participants were asked ‘Since you were last asked, which of the options best describes how often you have taken at least one tablet of the trial medication daily?’ with response options: ‘Every day or nearly every day’, ‘More than half of the days’, ‘Less than half of the days’ or ‘None or nearly none of the days’.
Acceptability of treatment was measured by participant self-report during the researcher telephone calls, as well as the decision to continue trial medication beyond 6 months. Participants were asked ‘On balance do you find this medication acceptable to take and would you want to keep taking it?’.
Adverse events were collected via a validated self-completed questionnaire, the ASEC,31 which consists of 21 potential AEs rated on a scale of 0 (absent) to 3 (severe), and also asks the individual whether they deem the AE to be treatment-related. This has been shown to demonstrate good agreement with a psychiatrist’s rating of the occurrence of treatment-related AEs with antidepressants. The ASEC was completed as part of toxicity and tolerability assessments conducted by the researcher at weeks 1 and 3, and month 9 telephone calls, and via participant-completed questionnaires at months 3, 6 and 12.
An exit survey was completed with the participant by the researcher during the 6-month telephone call to record any changes participants had made to diet, exercise levels, or IBS treatments, their experience of the ATLANTIS trial medication, and which treatment they thought they were allocated to and why.
The IBS-SSS is used widely in trials of medical therapies in IBS. It is a five-item self-administered questionnaire, as described above. 23
The HADS is a well-validated, commonly used, self-report instrument for detecting symptoms of anxiety and depression in people with medical illnesses. 27 It consists of a total of 7 items measuring anxiety, and 7 measuring depression, scored from 0 to 3, with a total score of 21 for each. Higher scores indicate more severe symptoms of anxiety or depression.
The EQ-5D-3L is the most frequently used measure for generating QALYs. 32,33 It has been demonstrated to be appropriate in patients with IBS.
The WSAS measures the effect of chronic diseases on peoples’ ability to work and manage at home and participate in social or private leisure activities and relationships. 28–30 The WSAS has been shown to be sensitive to change in IBS trials. It has five aspects scored from 0 (not affected) to 8 (severely affected), with a total possible score of 40.
The PHQ-12 comprises 12 somatic symptoms from the full Patient Health Questionnaire-15. Each symptom is scored from 0 (‘not bothered at all’) to 2 (‘bothered a lot’). Higher scores indicate the presence of somatoform-type behaviour, which is a measure of psychological health.
The SGA of relief of IBS symptoms is frequently used in treatment trials in IBS to identify responders to therapy as described above. 24
Health resource use, including healthcare use, use of other medications for IBS, and need for referral to secondary care, was self-reported by the participant via a resource use questionnaire, using a 3-month recall period. If the participant consented to 12-month follow-up, then the recall period was extended to 6 months. This collected data concerning all resource use and medications in the community, in primary and secondary care, social care, hospitalisation, outpatient specialist visits, and diagnostic investigations. Because of the societal perspective, the questionnaire also included questions on out-of-pocket expenses, employment status, and days lost due to illness.
Relief of IBS symptoms was measured by the yes/no response to ‘Have you had adequate relief of your IBS symptoms?’ asked electronically, or via a paper-based diary. Participants were sent a weekly text reminder from CTRU to complete the assessment.
Summary of changes to the protocol
The COVID-19 pandemic resulted in the trial pausing recruitment in March 2020 for 4 months, with a series of national lockdowns, and led to subsequent reduced rates of new practice and participant recruitment. Internal pilot objectives were, therefore, difficult to evaluate in the original time frame and a costed trial extension was required to complete recruitment and follow-up of the trial. Several substantial amendments were made to the trial protocol, including an approved amendment due to the impact of COVID-19, to reduce the duration of trial medication and follow-up from 12 months to 6 months and to remove the cost-effectiveness analysis, which is now dependent on further additional funding. This was done to minimise additional funding required to complete the trial and to prioritise funds for participant recruitment. Site and hub PIs, hub researchers, and participants were informed of all protocol amendments following ethical and regulatory approvals. A summary of all protocol changes can be found in Report Supplementary Material 1.
Sample size
We estimated that an evaluable sample size of 414 participants would provide 90% power to detect the minimum clinically important difference of 35 points between amitriptyline and placebo at 6 months on the IBS-SSS,23 assuming a maximum standard deviation (SD) of 110 points on the IBS-SSS,39,40 and 5% two-sided significance level, equating to a small to moderate effect size of 0.32. The 35-point between-group difference on the IBS-SSS was agreed as a minimum clinically important difference in the ACTIB trial, which was another treatment trial in IBS in UK primary care. 36,37 The evaluable sample size gave at least 85% power to detect a 15% absolute difference in SGA of relief of IBS symptoms,24 our key secondary outcome. We planned to recruit 518 participants to allow for 20% loss to follow-up. 22
Statistical methods
A detailed statistical analysis plan was written and signed off by the Trial Management Group (TMG) and Trial Steering Committee (TSC) before analysis was undertaken.
Analyses of data up to 6 months post randomisation were conducted on the intention-to-treat (ITT) population, defined as all participants randomised, regardless of adherence to the intervention, unless otherwise indicated. Analyses of treatment-related data beyond 6 months, and secondary outcomes at 12 months, were conducted on the 12-month ITT population, defined as all participants who were randomised and who consented to 12-month follow-up, regardless of adherence to the intervention. Analysis of data beyond 6 months is presented separately in the report as results are applicable only to a subset of participants and are no longer a fully randomised comparison as participants could choose to continue treatment or not at 6 months.
An overall two-sided 5% significance level was used for all outcome comparisons. Outcome data were analysed once only after data lock, at final analysis, and no interim analyses were planned. Analyses were completed in SAS® (SAS Institute Inc., Cary, NC, USA) version 9.4. Statistical monitoring of safety data was conducted throughout the trial and reported at agreed 6-monthly intervals to the DMEC.
Descriptive analysis
Summary statistics, by treatment group (where applicable) and overall, are used to provide a descriptive analysis of the study conduct, including screening, accrual, protocol violations, withdrawals, treatment receipt, participant follow-up, and analysis populations informing the study CONSORT diagram.
A flow diagram further summarises the course of participants through the screening and recruitment process, including total number of patients approached by GP mail-out, as well as the number who expressed interest, and were screened, eligible, consented, registered, and randomised, along with reasons for drop-out at each stage. Age and sex of all patients were also summarised at each stage of the screening and recruitment process and compared with those randomised.
Baseline characteristics and questionnaire scores of recruited participants were summarised overall, by treatment group, and by availability of 6-month primary outcome data (to inform our missing data approach).
Treatment delivery and receipt were summarised overall and by treatment group, including details of treatment received and discontinuation; dosage, titration, and modifications; adherence; kit replenishment and replacement; ongoing monitoring of suicidal ideation and concomitant medications; uptake of optional GP review; and participant-reported changes in diet, exercise, other treatments, IBS symptoms, and any potential contributing factors.
The success and process of blinding are summarised overall and by arm, including details of an exit survey of participants, that is which treatment the participant thought they received and why, and end-of-trial participation unblinding.
Primary outcome analysis
A linear regression model, adjusted for true values of minimisation variables (recruitment hub, stool type, baseline HADS-depression score) and IBS-SSS score at baseline, was used to test for differences between the treatment groups on the IBS-SSS at 6 months. Missing data were imputed by treatment arm via multiple imputation by chained equations with 25 imputations, including recruitment hub, IBS subtype, sex, age, baseline questionnaire scores (IBS-SSS, PHQ-12, HADS and WSAS), 3-month IBS-SSS score and 6-month treatment status in the model. The 3-month IBS-SSS score was imputed within the same model in a preliminary step, incorporating 3-month (rather than 6-month) treatment status. Sensitivity analyses on a per-protocol population (using multiple imputation), and on participants with complete data (ITT to data availability), were performed to test robustness of results. Results were expressed as point estimates of the mean difference, together with 95% confidence intervals (CIs) and p-values.
Secondary outcome analysis
Continuous outcomes (where applicable at 3, 6 and 12 months), including IBS-SSS, HADS, WSAS and PHQ-12 scores, were analysed in the same manner as the primary outcome adjusted for the respective baseline score. Analysis of the PHQ-12 was adjusted additionally for sex due to differences in the maximum available total score for male and females. Secondary binary outcomes, including SGA of relief of IBS symptoms at 3, 6 and 12 months, and acceptability of treatment at 6 months were analysed similarly in logistic regression models with results expressed as odds ratios (ORs) with 95% CIs. Missing IBS-SSS, HADS, WSAS, PHQ-12, SGA and acceptability data were imputed in the same manner as the primary outcome, as appropriate to outcome type and incorporating both 3- and 6-month outcomes where applicable.
We planned to analyse adherence (at week 3, months 3, 6, 9 and 12) using ordinal regression. However, due to violation of the proportional odds assumption, only descriptive analyses are presented.
Adequate relief of IBS symptoms, measured weekly to 6 months, was analysed using a repeated-measures model based on available data (all participants in the ITT population with at least one weekly observation included). A likelihood-based generalised linear mixed model with population-averaged (marginal) inference and unstructured covariance matrix was used to compare response between treatment groups at each week and overall, across weeks. The treatment group, true values of minimisation variables, time (in weeks) and the treatment time interactions were fitted as fixed effects. Results were expressed as ORs, together with 95% CIs and p-values. Descriptive analysis of aggregated weekly data included responder status based on the number and proportion of participants reporting adequate relief in ≥ 50% of weeks (i.e. ≥ 13 of 25 weeks).
Tolerability of trial treatment, based on the participant’s self-reported symptoms on the ASEC at 3, 6 and 12 months, was analysed according to the safety population (see Safety analysis) and using available data for participants on trial treatment at each time point. A linear regression model, adjusted for true values of minimisation variables, was used to test for differences between the treatment groups on the total ASEC score at each time point.
Sensitivity analyses of secondary outcomes were performed to test robustness of results. These included:
-
analysis of complete data (ITT to data availability) where the primary analysis was based on multiple imputation (for IBS-SSS, HADS, WSAS, PHQ-12, SGA of relief of IBS symptoms, and acceptability)
-
analysis of SGA of relief of IBS symptoms:
-
using an alternative definition of response, with response defined as reporting only considerable or complete relief of IBS symptoms
-
as an ordinal outcome using ordinal logistic regression
-
-
analysis of acceptability, with additional multiple imputation for participants who did not start trial treatment or were lost to follow-up on or before the 6-month call (derived as not acceptable in primary analysis)
Safety analysis
All participants receiving at least one dose of trial medication were included in the safety population and analysis; participants who received at least one dose of amitriptyline were included in the amitriptyline group, regardless of the arm they were allocated to. The number of participants reporting a SAE, including serious adverse reactions (SARs) and suspected unexpected serious adverse reactions (SUSARs), and details of all SAEs were reported for each treatment group. The number and details of emergency unblinding events, pregnancies and deaths were also reported for each treatment group.
Exploratory analysis
Exploratory moderator analyses were conducted to investigate if the 6-month treatment effect on the IBS-SSS varied by baseline IBS-SSS score, recruitment hub, IBS subtype or mood (baseline HADS-depression or HADS-anxiety scores), by including an interaction between the treatment arm and each potential moderator in the primary analysis model. Moderator analyses were also conducted to investigate if the 6-month treatment effect on the key secondary outcome, SGA response, varied by IBS subtype.
Further exploratory analyses of the IBS-SSS at 3 and 6 months were conducted using logistic regression, adjusted for true values of minimisation variables, to test for differences between the treatment groups on response rates according to:
-
a ≥ 50-point decrease in the total IBS-SSS score
-
a ≥ 30% decrease in abdominal pain severity on the IBS-SSS item response score at 3 and 6 months
-
a ≥ 30% decrease in abdominal distension severity on the IBS-SSS item response score at 3 and 6 months.
Missing response data, according to the definitions above, were imputed in the same manner as the primary and secondary outcomes.
Health economics methods
We planned to perform a within-study cost-effectiveness analysis, which adopted the perspective of the NHS and Personal Social Services and a societal perspective. We planned to use a time horizon of 6 months; hence costs and outcomes were not to be discounted. Our primary outcome was intended to be cost per QALY, with uncertainty assessed using a within-trial probabilistic sensitivity analysis. This would be performed using Monte Carlo simulation, with the results presented as incremental cost-effectiveness ratios and cost-effectiveness acceptability curves. We planned to assume a willingness to pay (lambda) of £20,000 per QALY. Sensitivity analyses were planned, which would include a 12-month time horizon, as well as a scenario similar to real NHS practice, with treatment prescribed by a GP with repeated prescriptions, tests, and necessary appointments. As stated previously, health economic analyses were removed after a discussion and meeting with the HTA to minimise additional funding required to complete the trial and to prioritise funds for participant recruitment and are now on hold subject to further funding.
Qualitative study
The nested qualitative study is reported in Nested qualitative study.
Chapter 3 Clinical trial results
Study summary
The numbers of patients identified via GP mail-out, responding and screened for entry into ATLANTIS, eligible, randomised, followed up at 3, 6 and 12 months, withdrawn and analysed are presented in the CONSORT diagram in Figure 1.
FIGURE 1.
Consolidated standards of reporting trial flow diagram.
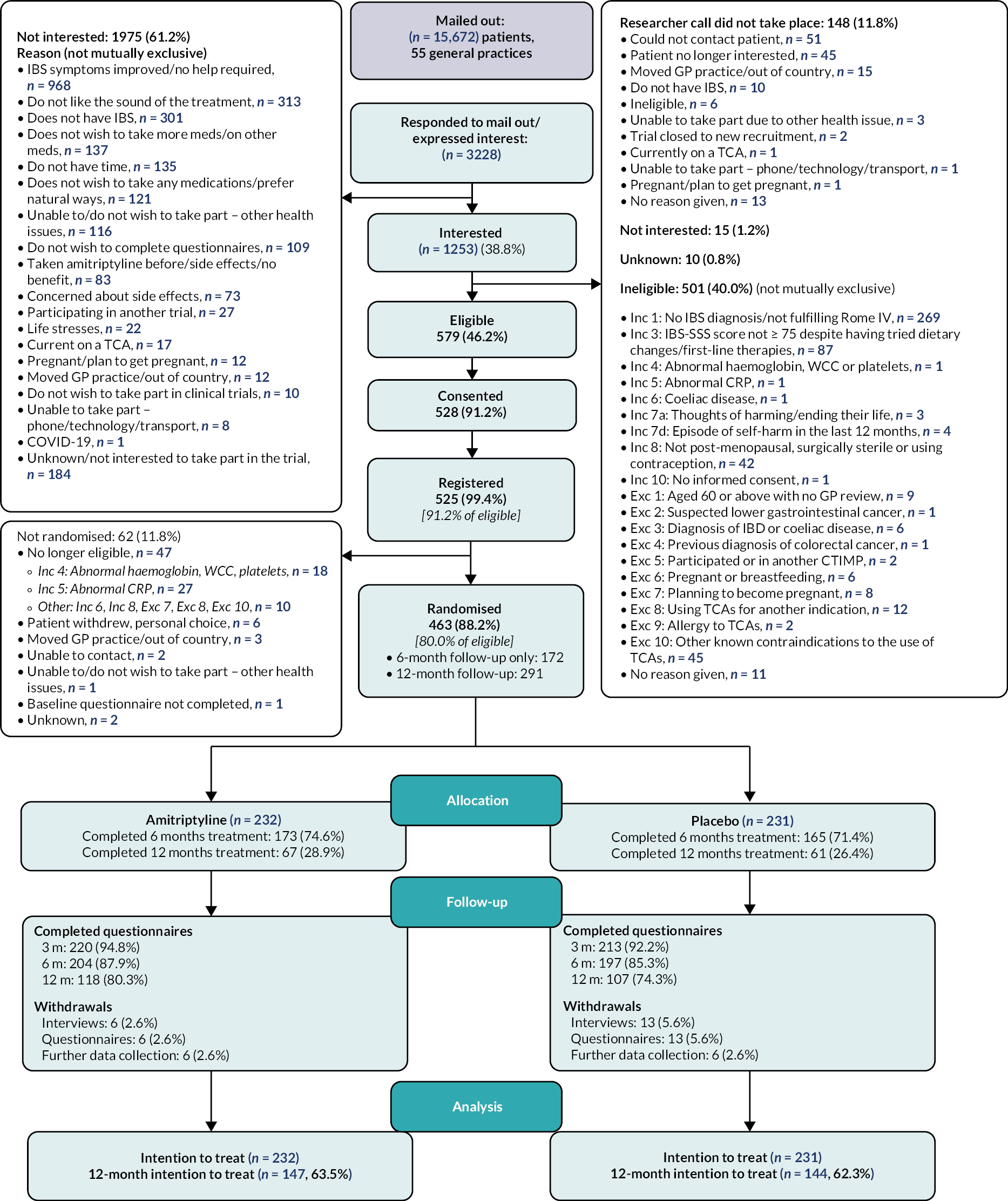
Screening and recruitment
Screening
A total of 15,672 potentially eligible patients were identified via SnoMed clinical terms searches by 55 general practices and contacted by letter to provide information and invite them to take part in the trial. Screening subsequently took place for 3228 patients who either responded via reply slip to the general practice mail-out (n = 3144, 97.4%) or were identified opportunistically following a GP visit, publicity, or other means and contacted a research nurse directly.
Of the 3228 who responded, 1253 (38.8%) expressed an interest in joining the trial, of whom 1105 (88.2%) had a screening call with a research nurse. Of these, 579 (52.4%) were eligible, of whom 528 (91.2%) consented, 525 (90.7%) were registered and 463 (80.0%) were randomised. Figure 2 and Appendix 1, Table 42 present screening and recruitment by hub.
FIGURE 2.
Screening and recruitment by hub.
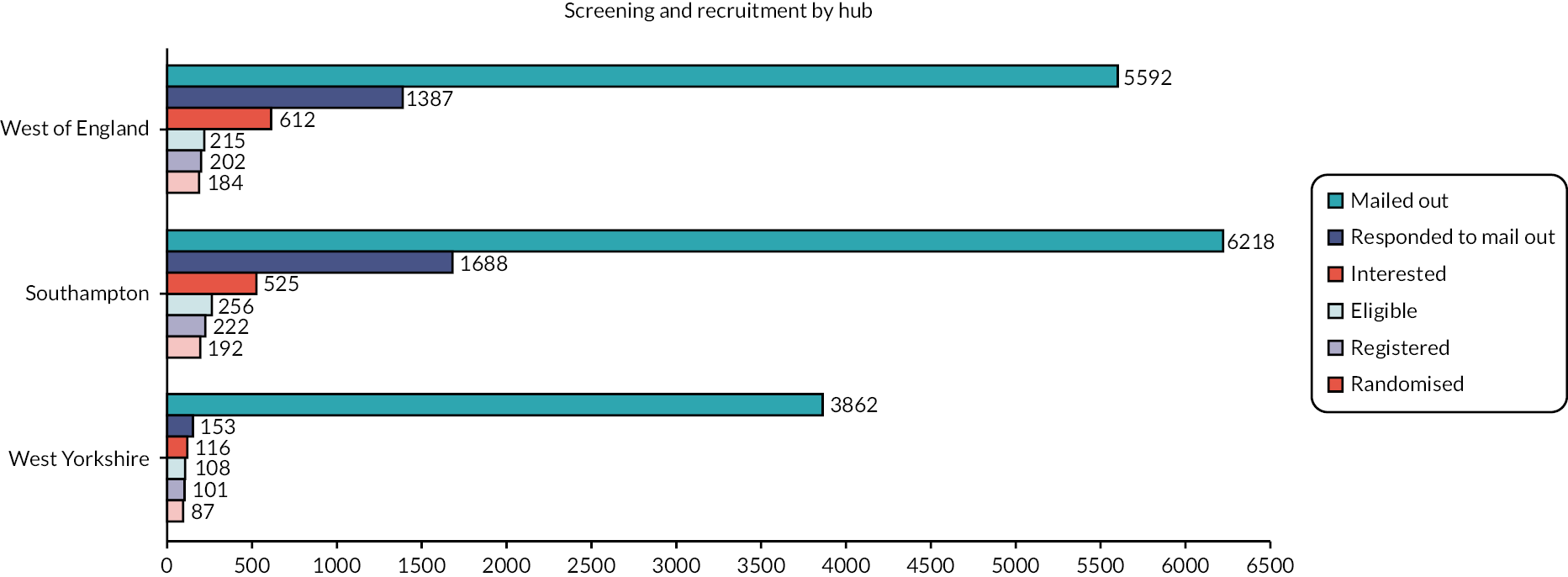
The most common reasons why the other 1975 (61.2%) patients were not interested in taking part were that their IBS symptoms had improved and no further help was required [n = 968 (49.0%) of those not interested], they did not like the sound of the treatment [n = 313 (15.8%)], they did not have IBS [n = 301 (15.2%)], they did not wish to take more medications [n = 137 (6.9%)], they did not have time [n = 135 (6.8%)], they did not wish to take any medications [n = 121 (6.1%)], their other health issues meant they felt unable to or did not wish to take part [n = 116 (5.9%)], they did not wish to complete questionnaires [n = 109 (5.5%)], they had taken amitriptyline before and experienced side effects or no benefit [n = 83 (4.2%)], or they were concerned about side effects [n = 73 (3.7%)].
The most common reasons for ineligibility of 501 (45.3% of those with a screening researcher call) patients were not having a diagnosis of IBS in the primary care record and fulfilling Rome IV criteria [n = 269 (53.7%) of those ineligible], not having ongoing symptoms, as defined by an IBS-SSS score ≥ 75 despite having tried dietary changes and first-line therapies [n = 87 (17.4%)], known contraindication to the use of TCAs [n = 45 (9.0%)], and potential for pregnancy [not post menopausal, surgically sterile or using effective contraception, n = 42 (8.4%)].
Reasons why 62 (11.8%) registered patients did not go on to be randomised included subsequently not meeting eligibility criteria [n = 47 (75.8%)], in the majority of cases due to abnormal blood test results, patient choice [n = 6 (9.7%)] or other reasons [n = 9 (14.5%)].
Recruitment
The first mail-out took place on 18 October 2019 and the first participant was randomised on 5 December 2019 and the last on 11 April 2022, with 232 participants randomised to receive low-dose amitriptyline and 231 participants randomised to receive placebo, across 55 general practices. Figure 3 shows overall, monthly and cumulative recruitment of participants into the trial. Appendix 1, Figure 8 depicts the timing between practice mail-out and subsequent randomisations.
FIGURE 3.
Recruitment graph.
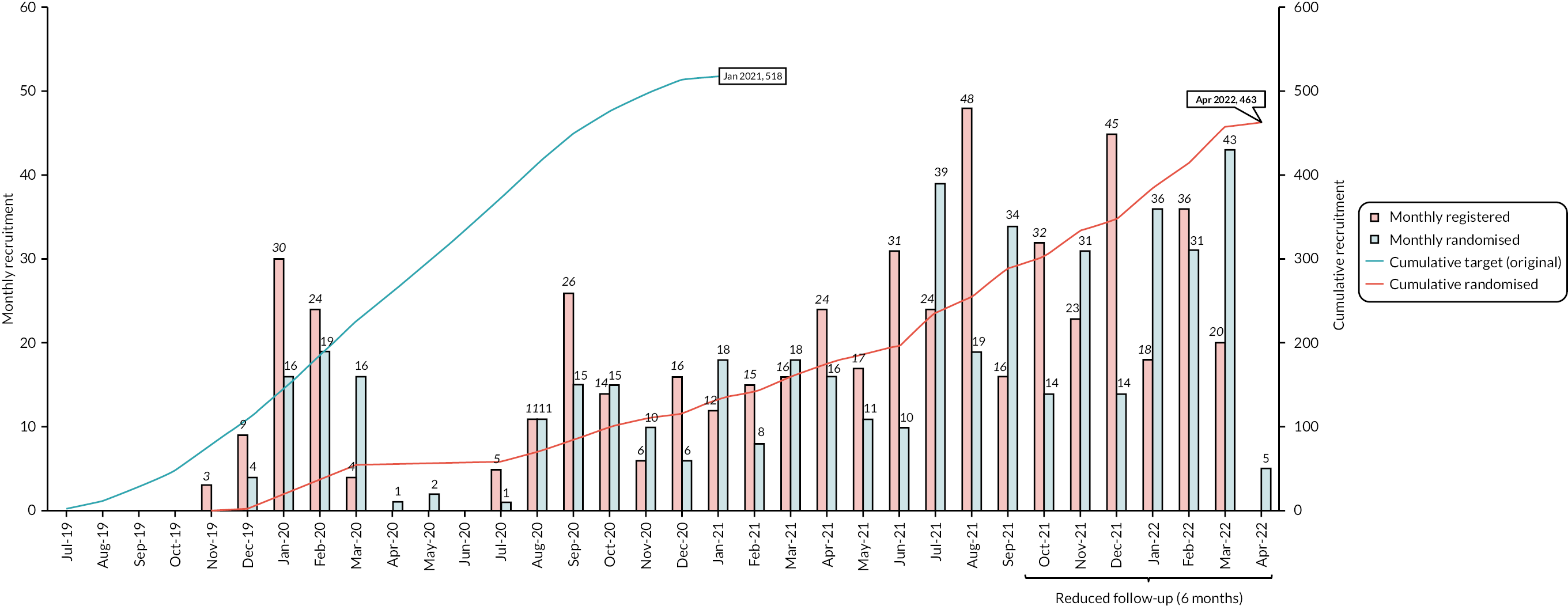
Recruitment was originally expected to be completed within 18 months. Owing to a delay in trial opening, a pause in recruitment between March and July 2020, in line with national guidance related to the COVID-19 pandemic, and subsequent slower than anticipated recruitment rates, again due to the COVID-19 pandemic, recruitment was extended and took place over 29 months. A number of changes were made to the trial protocol and study processes to enable recruitment to continue, including a reduction in follow-up from 12 to 6 months for the final cohort of recruited participants (see Report Supplementary Material 1). The first 291 (62.9%) recruited participants were therefore consented to 12-month follow-up, whereas the final 172 (37.1%) could only provide consent to 6 months of follow-up.
Characteristics of the screened, eligible and randomised participants
The age and sex of the mailed out, screened, interested, eligible and randomised patient populations were broadly similar (Table 2). Patients who responded to the mail-out or expressed interest were slightly older (mean age 57.0 vs. 48.4 years) and more likely to be female (75.5% vs. 71.3%). However, the mean age of those subsequently interested, eligible and randomised was similar to the overall mailed-out population, and the proportion of males increased through later stages of the screening process.
| Mailed-out (n = 15,672) | Responded (n = 3228) | Interested (n = 1253) | Eligible (n = 579) | Registered (n = 525) | Randomised (n = 463) | |
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean (SD) | 48.4 (16.8) | 57.0 (16.9) | 51.0 (17.0) | 48.4 (16.5) | 48.4 (16.3) | 48.4 (16.1) |
| Median (range) | 48.0 (18–100) | 59.0 (19–98) | 51.0 (19–92) | 49.0 (19–92) | 49.0 (18–92) | 49.0 (18–87) |
| Missing | 333 | 74 | 11 | 4 | 0 | 0 |
| Sex | ||||||
| Male | 4419 (28.8%) | 797 (25.5%) | 357 (29.3%) | 162 (28.7%) | 158 (30.1%) | 148 (32.0%) |
| Female | 10,918 (71.2%) | 2332 (75.5%) | 862 (70.7%) | 402 (71.3%) | 367 (69.9%) | 315 (68.0%) |
| Missing | 335 | 99 | 34 | 15 | 0 | 0 |
Protocol violations, withdrawals and follow-up
Protocol violations
Protocol violations were identified for six (1.3%) participants, three in each arm, four of which were classed as major protocol violations (see Appendix 1, Table 43). One participant was found to be ineligible 6 days after randomisation following receipt of an abnormal blood test result (positive anti-tTG antibody; major violation). Four participants experienced unplanned treatment errors, including: two participants (one in each arm) who reported having taken four tablets daily (40 mg), more than the maximum 30 mg daily allowance, at 3-month follow-up (minor violations). One participant allocated to placebo was sent the incorrect bottle of medication at randomisation due to a kit number identification error at pharmacy and received a bottle of amitriptyline; this was identified 14 days post randomisation after which the participant was asked to return the incorrect bottle and they subsequently discontinued trial medication (major violation). A further participant allocated to placebo disclosed at 6-month follow-up that they had taken 30 mg of their friend’s amitriptyline on a single day (major violation). A further protocol violation was reported for one participant allocated to amitriptyline who was asked to stop and return trial medication after reporting suicidal ideation at 3 months follow-up. However, they continued to take trial medication for a further week (major violation).
Research withdrawals
Participants could withdraw from optional interviews, weekly or monthly (3-, 6- or 12-month) questionnaires, or further data collection. A total of 23 (5.0%) participants withdrew from at least one study process: 6 (2.6%) in the amitriptyline arm and 17 (7.4%) in the placebo arm (Table 3). Participants most frequently withdrew from optional interviews and monthly questionnaires: 6 (2.6%) in the amitriptyline arm and 13 (5.6%) in the placebo arm, all but one of whom also withdrew from the weekly questionnaire, and 6 participants in each arm withdrew from further data collection. The mean time of withdrawal was 3.5 months post randomisation. The main reasons for withdrawal included treatment side effects or lack of benefit, with study withdrawal accompanying treatment discontinuation, personal choice, difficult personal circumstances, or lack of time.
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
|---|---|---|---|
| Number of participants with a withdrawal | 6 (2.6%) | 17 (7.4%) | 23 (5.0%) |
| Withdrawn from | |||
| Optional interviews (of participants consented to interview) | 6 (2.9%) | 13 (6.3%) | 19 (4.6%) |
| Consented to optional interview (of randomised) | 204 (87.9%) | 206 (89.2%) | 410 (88.6%) |
| Completing questionnaires | 6 (2.6%) | 13 (5.6%) | 19 (4.1%) |
| Withdrawn from monthly questionnaires only | 0 (0.0%) | 1 (0.4%) | 1 (0.2%) |
| Withdrawn from weekly and monthly questionnaires | 6 (2.6%) | 12 (5.2 %) | 18 (3.9%) |
| Further data collection | 6 (2.6%) | 6 (2.6%) | 12 (2.6%) |
| Time from randomisation to withdrawal (months) | |||
| Mean (SD) | 3.6 (4.6) | 3.5 (3.8) | 3.5 (3.9) |
| Median (range) | 2.1 (0.6, 12.7) | 1.6 (0.4, 12.3) | 1.8 (0.4, 12.7) |
| IQR | 1.0–3.0 | 0.8–5.8 | 0.8–5.8 |
| n | 6 | 17 | 23 |
| Reason for withdrawal | |||
| Side effects | 1 (16.7%) | 3 (17.6%) | 4 (17.4%) |
| Difficult personal circumstances | 1 (16.7%) | 3 (17.6%) | 4 (17.4%) |
| Lack of benefit | 0 (0.0%) | 3 (17.6%) | 3 (13.0%) |
| Do not have time | 0 (0.0%) | 3 (17.6%) | 3 (13.0%) |
| Personal choice | 0 (0.0%) | 3 (17.6%) | 3 (13.0%) |
| Due to COVID-19 | 2 (33.3%) | 0 (0.0%) | 2 (8.7%) |
| SAE/SUSAR | 0 (0.0%) | 2 (11.8%) | 2 (8.7%) |
| Unknown | 2 (33.3%) | 0 (0.0%) | 2 (8.7%) |
| Total | 6 (100%) | 17 (100%) | 23 (100%) |
Questionnaire follow-up
Monthly follow-up questionnaires were completed and returned by 433 (93.5%) of 463 participants at 3 months, 401 (86.6%) of 463 at 6 months and 225 (77.3%) of 291 participants at 12 months (Table 4). Response rates were slightly higher in the amitriptyline arm compared with the placebo arm, particularly at 12 months (80.3% vs. 74.3%). Individual questionnaire completion rates (arranged in the order they appear within each questionnaire pack in Table 4) showed a slight reduction in completion rates for questionnaires appearing later within the questionnaire packs. All baseline questionnaires were completed within 1 month prior to randomisation and follow-up questionnaires were largely completed within a 7-day window either side of the point of follow-up (see Table 4 and Appendix 1, Figure 9). The majority of the participants completed questionnaires online via REDCap, with online completion for 93.3% at baseline, and 95.2%, 96.0% and 96.9% of responders at 3, 6 and 12 months, respectively and the remainder completing paper-based questionnaires by post. Participants provided a median of 23 responses to the weekly question ‘Have you had adequate relief of your IBS symptoms?’ up to 6 months (25 weeks in total) (see Appendix 1, Figure 10). At least one response was provided by all except 10 participants in each arm, and responses were provided for at least 75% of weeks (≥ 19 weeks) for 337 (72.8%) participants, with similar rates between treatment arms.
| Month 3 | Month 6 | Month 12a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 147) | Placebo (n = 144) | Total (n = 291) | |
| Questionnaire completed? | |||||||||
| Completedb | 220 (94.8%) | 213 (92.2%) | 433 (93.5%) | 204 (87.9%) | 197 (85.3%) | 401 (86.6%) | 118 (80.3%) | 107 (74.3%) | 225 (77.3%) |
| Did not complete | 12 (5.2%) | 18 (7.8%) | 30 (6.5%) | 28 (12.1%) | 34 (14.7%) | 62 (13.4%) | 29 (19.7%) | 37 (25.7%) | 66 (22.7%) |
| Reason not completed | |||||||||
| Withdrawn questionnaires | 5 (41.7%) | 7 (38.9%) | 12 (40.0%) | 5 (17.9%) | 10 (29.4%) | 15 (24.2%) | 6 (20.7%) | 10 (27.0%) | 16 (24.2%) |
| No response | 7 (58.3%) | 11 (61.1%) | 18 (60.0%) | 23 (82.1%) | 24 (70.6%) | 47 (75.8%) | 23 (79.3%) | 27 (73.0%) | 50 (75.8%) |
| Total | 12 (100%) | 18 (100%) | 30 (100%) | 28 (100%) | 34 (100%) | 62 (100%) | 29 (100%) | 37 (100%) | 66 (100%) |
| Individual questionnairesc | |||||||||
| IBS-SSS | 219 (94.4%) | 213 (92.2%) | 432 (93.3%) | 204 (87.9%) | 197 (85.3%) | 401 (86.6%) | 118 (80.3%) | 107 (74.3%) | 225 (77.3%) |
| SGA | 220 (94.8%) | 213 (92.2%) | 433 (93.5%) | 204 (87.9%) | 195 (84.4%) | 399 (86.2%) | 118 (80.3%) | 107 (74.3%) | 225 (77.3%) |
| HADS | 220 (94.8%) | 212 (91.8%) | 432 (93.3%) | 203 (87.5%) | 193 (83.5%) | 396 (85.5%) | 117 (79.6%) | 107 (74.3%) | 224 (77.0%) |
| PHQ-12 | N/A | N/A | N/A | 202 (87.1%) | 192 (83.1%) | 394 (85.1%) | N/A | N/A | N/A |
| WSAS | 219 (94.4%) | 212 (91.8%) | 431 (93.1%) | 202 (87.1%) | 193 (83.5%) | 395 (85.3%) | 117 (79.6%) | 107 (74.3%) | 224 (77.0%) |
| ASEC | 219 (94.4%) | 212 (91.8%) | 431 (93.1%) | 201 (86.6%) | 192 (83.1%) | 393 (84.9%) | 117 (79.6%) | 107 (74.3%) | 224 (77.0%) |
| EQ-5D | 218 (94.0%) | 210 (90.9%) | 428 (92.4%) | 200 (86.2%) | 192 (83.1%) | 392 (84.7%) | 117 (79.6%) | 107 (74.3%) | 224 (77.0%) |
| Health resource use | 217 (93.5%) | 210 (90.9%) | 427 (92.2%) | 200 (86.2%) | 192 (83.1%) | 392 (84.7%) | 117 (79.6%) | 107 (74.3%) | 224 (77.0%) |
| Time to completion (days) | |||||||||
| Mean (SD) | 3.1 (0.3) | 3.1 (0.3) | 3.1 (0.3) | 6.1 (0.2) | 6.1 (0.3) | 6.1 (0.2) | 12.1 (0.2) | 12.2 (0.3) | 12.1 (0.2) |
| Median (range) | 3.0 (2.7, 5.3) | 3.0 (2.7, 4.7) | 3.0 (2.7, 5.3) | 6.0 (5.7, 7.6) | 6.0 (5.9, 7.7) | 6.0 (5.7, 7.7) | 12.0 (12.0, 13.1) | 12.1 (12.0, 13.2) | 12.0 (12.0, 13.2) |
| n | 220 | 213 | 433 | 203d | 197 | 400 | 118 | 107 | 225 |
| Timing of completion | |||||||||
| ≤ ± 7 days | 185 (84.1%) | 181 (85.0%) | 366 (84.5%) | 182 (89.2%) | 167 (84.8%) | 349 (87.0%) | 103 (87.3%) | 85 (79.4%) | 188 (83.6%) |
| ≤ ± 30 days | 31 (14.1%) | 29 (13.6%) | 60 (13.9%) | 19 (9.3%) | 27 (13.7%) | 46 (11.5%) | 14 (11.9%) | 21 (19.6%) | 35 (15.6%) |
| > ± 30 days | 4 (1.8%) | 3 (1.4%) | 7 (1.6%) | 2 (1.0%) | 3 (1.5%) | 5 (1.2%) | 1 (0.8%) | 1 (0.9%) | 2 (0.9%) |
| Total completed | 220 (100%) | 213 (100%) | 433 (100%) | 203 (100%) | 197 (100%) | 401 (100%) | 118 (100%) | 107 (100%) | 225 (100%) |
Comparison of baseline characteristics between participants with and without monthly follow-up questionnaires (Table 5) indicated that those not completing the primary 6-month follow-up questionnaire were more likely to have discontinued trial medication before 6 months, to be younger, to be in the West Yorkshire hub, and to have had more severe scores on the baseline IBS-SSS and WSAS.
| Completed (n = 401) | Not completed (n = 62) | Total (n = 463) | p-valuea | |
|---|---|---|---|---|
| Treatment allocation and receipt | ||||
| Treatment allocation | ||||
| Amitriptyline | 204 (50.9%) | 28 (45.2%) | 232 (50.1%) | |
| Placebo | 197 (49.1%) | 34 (54.8%) | 231 (49.9%) | 0.1883 |
| Did not start or discontinued trial medication before 6 months | ||||
| Yes | 80 (20.0%) | 45 (72.6%) | 125 (27.0%) | |
| No | 321 (80.0%) | 17 (27.4%) | 338 (73.0%) | < 0.0001 |
| Demographic characteristics | ||||
| Recruitment hub | ||||
| West Yorkshire | 67 (16.7%) | 20 (32.3%) | 87 (18.8%) | |
| Wessex | 171 (42.6%) | 21 (33.9%) | 192 (41.5%) | |
| West of England | 163 (40.6%) | 21 (33.9%) | 184 (39.7%) | 0.0068 |
| IBS subtype | ||||
| IBS-C | 67 (16.7%) | 10 (16.1%) | 77 (16.6%) | |
| IBS-D | 158 (39.4%) | 23 (37.1%) | 181 (39.1%) | |
| IBS-M | 163 (40.6%) | 28 (45.2%) | 191 (41.3%) | |
| IBS-U | 13 (3.2%) | 1 (1.6%) | 14 (3.0%) | 0.8896 |
| Age at randomisation | ||||
| Mean (SD) | 48.9 (15.8) | 45.7 (17.8) | 48.5 (16.1) | |
| Median (range) | 50.0 (19.0, 86.0) | 44.5 (20.0, 87.0) | 49.0 (19.0, 87.0) | 0.0489 |
| Participant sex | ||||
| Male | 132 (32.9%) | 16 (25.8%) | 148 (32.0%) | |
| Female | 269 (67.1%) | 46 (74.2%) | 315 (68.0%) | 0.4370 |
| Baseline questionnaires | ||||
| Baseline IBS-SSS score | ||||
| Mean (SD) | 269.3 (88.2) | 295.3 (100.7) | 272.8 (90.3) | |
| Median (range) | 270.0 (10.0, 480.0) | 310.0 (60.0, 480.0) | 280.0 (10.0, 480.0) | 0.0140 |
| IBS-SSS severity | ||||
| Normal (< 75) | 6 (1.5%) | 2 (3.2%) | 8 (1.7%) | |
| Mild (75–174) | 55 (13.7%) | 8 (12.9%) | 63 (13.6%) | |
| Moderate (175–299) | 184 (45.9%) | 17 (27.4%) | 201 (43.4%) | |
| Severe (300–500) | 156 (38.9%) | 35 (56.5%) | 191 (41.3%) | |
| Baseline HADS-anxiety score | ||||
| Mean (SD) | 7.5 (4.2) | 7.7 (4.6) | 7.5 (4.3) | |
| Median (range) | 7.0 (0.0, 21.0) | 7.0 (0.0, 21.0) | 7.0 (0.0, 21.0) | 0.8471 |
| Baseline HADS-depression score | ||||
| Mean (SD) | 4.2 (3.4) | 4.4 (3.4) | 4.3 (3.4) | |
| Median (range) | 4.0 (0.0, 18.0) | 4.0 (0.0, 14.0) | 4.0 (0.0, 18.0) | 0.8202 |
| Baseline PHQ-12 score | ||||
| Mean (SD) | 6.2 (3.4) | 6.8 (4.4) | 6.3 (3.5) | |
| Median (range) | 6.0 (0.0, 16.4) | 5.5 (0.0, 18.0) | 6.0 (0.0, 18.0) | 0.2396 |
| Missing | 3 | 3 | 6 | |
| Baseline WSAS score | ||||
| Mean (SD) | 10.9 (7.5) | 14.3 (9.6) | 11.4 (7.9) | |
| Median (range) | 10.0 (0.0, 36.0) | 13.5 (0.0, 40.0) | 10.0 (0.0, 40.0) | 0.0024 |
| Missing | 18 | 2 | 20 | |
Analysis populations
Intention to treat
All 463 randomised participants were included in the ITT population, including 232 allocated to amitriptyline and 232 to placebo. Prior to the protocol amendment reducing follow-up to 6 months, the first 291 randomised participants [147 (63.4%) in the amitriptyline arm; 144 (62.3%) in the placebo arm] were consented to 12-month follow-up and are included in the 12-month ITT population (Figure 1).
Per protocol
The per-protocol population included 376 (81.2%) participants at 3 months and 323 (69.8%) participants at 6 months, with slightly greater numbers of participants in the amitriptyline arm (Table 6). The majority of participants excluded from the per-protocol population were excluded because they discontinued trial medication before either 3 or 6 months. Of those excluded at 3 and 6 months, 69.0% and 72.9% had discontinued trial medication before 3 and 6 months, respectively, 12.6% and 2.1% had not responded to the treatment adherence question at the 3- and 6-month researcher follow-up calls, respectively, and 8.0% and 12.1% were lost to follow-up by 3 and 6 months, respectively. A smaller proportion of participants had not started treatment, reported inadequate levels of adherence to treatment, breached eligibility criteria, or had a major protocol violation.
| Month 3 | Month 6 | Month 12a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 147) | Placebo (n = 144) | Total (n = 291) | |
| In per-protocol population | |||||||||
| Yes | 193 (83.2%) | 183 (79.2%) | 376 (81.2%) | 172 (74.1%) | 151 (65.4%) | 323 (69.8%) | |||
| No | 39 (16.8%) | 48 (20.8%) | 87 (18.8%) | 60 (25.9%) | 80 (34.6%) | 140 (30.2%) | |||
| Total | 232 (100%) | 231 (100%) | 463 (100%) | 232 (100%) | 231 (100%) | 463 (100%) | |||
| Reasons for exclusionb | |||||||||
| Discontinued trial medication | 30 (76.9%) | 30 (62.5%) | 60 (69.0%) | 44 (73.3%) | 58 (72.5%) | 102 (72.9%) | |||
| Breach eligibility and discontinued trial medication | 1 (2.6%) | 0 (0.0%) | 1 (1.1%) | 1 (1.7%) | 0 (0.0%) | 1 (0.7%) | |||
| Major violation and discontinued trial medication | 1 (2.6%) | 1 (2.1%) | 2 (2.3%) | 1 (1.7%) | 1 (1.3%) | 2 (1.4%) | |||
| Not started treatment | 2 (5.1%) | 1 (2.1%) | 3 (3.4%) | 2 (3.3%) | 1 (1.3%) | 3 (2.1%) | |||
| Lost to follow-up | 4 (10.3%) | 3 (6.3%) | 7 (8.0%) | 11 (18.3%) | 6 (7.5%) | 17 (12.1%) | |||
| Major violation | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) | 1 (0.7%) | |||
| Not adhered to trial medication | 1 (2.6%) | 2 (4.2%) | 3 (3.4%) | 1 (1.7%) | 6 (7.5%) | 7 (5.0%) | |||
| No response to treatment adherence question | 0 (0.0%) | 11 (22.9%) | 11 (12.6%) | 0 (0.0%) | 3 (3.8%) | 3 (2.1%) | |||
| Other non-adherence | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 4 (5.0%) | 4 (2.9%) | |||
| Total excluded | 39 (100%) | 48 (100%) | 87 (100%) | 60 (100%) | 80 (100%) | 140 (100%) | |||
| Safety population | |||||||||
| Amitriptyline | 230 (100.0%) | 1 (0.4%) | 231 (50.2%) | 230 (100.0%) | 2 (0.9%) | 232 (50.4%) | 145 (100.0%) | 1 (0.7%) | 146 (50.7%) |
| Placebo | 0 (0.0%) | 229 (99.6%) | 229 (49.8%) | 0 (0.0%) | 228 (99.1%) | 228 (49.6%) | 0 (0.0%) | 142 (99.3%) | 142 (49.3%) |
| Excluded | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 1 | 3 |
Safety population
The safety population mirrored the ITT and 12-month ITT populations with the exception of three participants who were excluded as they did not start trial medication, and two participants for whom treatment cross-over was observed. One participant allocated to placebo received a bottle of amitriptyline by error within the first 2 weeks of randomisation and is included in the amitriptyline arm in the 3-, 6- and 12-month safety populations. A further participant allocated to placebo (and consenting to 6-month follow-up) reported having taken their friend’s amitriptyline on a single day at their 6-month follow-up call and is included in the amitriptyline arm for the 6-month safety population (see Table 6).
Baseline characteristics
Demographics and clinical characteristics
Participants allocated to amitriptyline and placebo were well balanced with respect to randomisation stratification factors (Table 7), demographic characteristics (Table 8), and clinical characteristics (Table 9). Wessex randomised 41.5% of participants, West of England 39.7%, and West Yorkshire 18.8% (Table 7). A small proportion of participants had IBS-U (3.0%), 41.3% had IBS-M, 39.1% IBS-D and 16.6% IBS-C. The majority of participants (84.2%) had a HADS-D score ˂ 8, indicating the absence of symptoms of depression.
| Amitriptyline (n = 232) (%) | Placebo (n = 231) (%) | Total (n = 463) (%) | |
|---|---|---|---|
| Recruitment hub | |||
| West Yorkshire | 43 (18.5) | 44 (19.0) | 87 (18.8) |
| Wessex | 97 (41.8) | 95 (41.1) | 192 (41.5) |
| West of England | 92 (39.7) | 92 (39.8) | 184 (39.7) |
| IBS subtype | |||
| IBS-C | 40 (17.2) | 37 (16.0) | 77 (16.6) |
| IBS-D | 92 (39.7) | 89 (38.5) | 181 (39.1) |
| IBS-M | 93 (40.1) | 98 (42.4) | 191 (41.3) |
| IBS-U | 7 (3.0) | 7 (3.0) | 14 (3.0) |
| Baseline HADS-D score | |||
| 0–7 (normal range) | 195 (84.1) | 195 (84.4) | 390 (84.2) |
| 8–21 (mild, moderate, severe depression) | 37 (15.9) | 36 (15.6) | 73 (15.8) |
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 49.2 (16.2) | 47.8 (15.9) | 48.5 (16.1) |
| Median (range) | 50 (19, 86) | 49 (19, 87) | 49 (19, 87) |
| Participant sex | |||
| Female | 156 (67.2%) | 159 (68.8%) | 315 (68.0%) |
| Male | 76 (32.8%) | 72 (31.2%) | 148 (32.0%) |
| Ethnic origin | |||
| White | 226 (97.4%) | 225 (97.8%) | 451 (97.6%) |
| Black | 0 (0.0%) | 1 (0.4%) | 1 (0.2%) |
| Asian | 2 (0.9%) | 2 (0.9%) | 4 (0.9%) |
| Other ethnic group | 1 (0.4%) | 1 (0.4%) | 2 (0.4%) |
| Mixed | 3 (1.3%) | 0 (0.0%) | 3 (0.6%) |
| Prefer not to say | 0 (0.0%) | 1 (0.4%) | 1 (0.2%) |
| Missing | 0 | 1 | 1 |
| Marital status | |||
| Single | 37 (15.9%) | 55 (23.8%) | 92 (19.9%) |
| Married | 123 (53.0%) | 110 (47.6%) | 233 (50.3%) |
| Living with partner | 43 (18.5%) | 36 (15.6%) | 79 (17.1%) |
| Separated | 2 (0.9%) | 4 (1.7%) | 6 (1.3%) |
| Divorced | 20 (8.6%) | 19 (8.2%) | 39 (8.4%) |
| Widowed | 7 (3.0%) | 7 (3.0%) | 14 (3.0%) |
| Highest education level achieved | |||
| No formal | 13 (5.6%) | 18 (7.8%) | 31 (6.7%) |
| GCSE/O level or equivalent | 61 (26.4%) | 61 (26.5%) | 122 (26.5%) |
| A level or equivalent | 54 (23.4%) | 54 (23.5%) | 108 (23.4%) |
| Degree | 52 (22.5%) | 58 (25.2%) | 110 (23.9%) |
| Postgraduate | 47 (20.3%) | 31 (13.5%) | 78 (16.9%) |
| Diploma | 3 (1.3%) | 3 (1.3%) | 6 (1.3%) |
| Otherb | 1 (0.4%) | 5 (2.2%) | 6 (1.3%) |
| Missing | 1 | 1 | 2 |
| IMD quintilec | |||
| 1 | 13 (5.7%) | 13 (5.7%) | 26 (5.7%) |
| 2 | 34 (14.8%) | 27 (11.7%) | 61 (13.3%) |
| 3 | 38 (16.6%) | 33 (14.3%) | 71 (15.5%) |
| 4 | 75 (32.8%) | 74 (32.2%) | 149 (32.5%) |
| 5 | 69 (30.1%) | 83 (36.1%) | 152 (33.1%) |
| Missing | 3 | 1 | 4 |
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
|---|---|---|---|
| First-line therapiesa | |||
| Antispasmodics (e.g. mebeverine) | 176 (76.5%) | 183 (79.2%) | 359 (77.9%) |
| Anti-diarrhoeals (e.g. loperamide) | 70 (30.4%) | 75 (32.5%) | 145 (31.5%) |
| Fibre supplements (e.g. ispaghula husk) | 52 (22.6%) | 52 (22.5%) | 104 (22.6%) |
| Laxatives (e.g. bisacodyl) | 51 (22.2%) | 34 (14.7%) | 85 (18.4%) |
| Peppermint oil (e.g. Mintec, Colpermin) | 18 (7.8%) | 27 (11.7%) | 45 (9.8%) |
| Otherb | 4 (1.7%) | 9 (3.9%) | 13 (2.8%) |
| Missing | 2 | 1 | 3 |
| Years from IBS diagnosis to randomisation | |||
| Mean (SD) | 13.3 (11.8) | 11.8 (10.2) | 12.5 (11.0) |
| Median (range) | 10.0 (0.0, 67.0) | 9.0 (0.0, 44.0) | 10.0 (0.0, 67.0) |
| IQR | 4.0, 21.0 | 4.0, 18.0 | 4.0, 20.0 |
| Missing | 0 | 1 | 1 |
| Years from IBS diagnosis to randomisation | |||
| ≤ 2 years | 31 (13.4%) | 36 (15.7%) | 67 (14.5%) |
| ≤ 10 years | 93 (40.1%) | 93 (40.4%) | 186 (40.3%) |
| ≤ 20 years | 47 (20.3%) | 53 (23.0%) | 100 (21.6%) |
| ≤ 30 years | 42 (18.1%) | 31 (13.5%) | 73 (15.8%) |
| > 30 years | 19 (8.2%) | 17 (7.4%) | 36 (7.8%) |
| Missing | 0 | 1 | 1 |
| Rome IV criteria | |||
| Abdominal pain | 232 (100.0%) | 231 (100.0%) | 463 (100.0%) |
| Pain relieved or aggravated by defaecation | 215 (92.7%) | 211 (91.3%) | 426 (92.0%) |
| Pain associated with change in stool frequency | 193 (83.2%) | 189 (81.8%) | 382 (82.5%) |
| Pain associated with change in stool appearance | 212 (91.4%) | 204 (88.3%) | 416 (89.8%) |
| Symptom onset 6 months prior to diagnosis | 232 (100.0%) | 231 (100.0%) | 463 (100.0%) |
The mean age at randomisation was 48.5 (SD 16.1) years and over two-thirds (68.0%) of participants were female (Table 8). Most participants were white (97.6%) with < 1% of each black, Asian, mixed or other ethnic groups. Over two-thirds of participants were married or living with a partner, and the majority (> 90%) had received formal education. Approximately a third of participants lived in each of the least 20% and 20–40% least deprived neighbourhoods in England (IMD quintile 5 and 4), while 5.7% and 13.3% lived in the 20% and 20–40% most deprived neighbourhoods (IMD quintile 1 and 2) respectively. Further details of IMD by decile and hub are in Appendix 1, Table 44.
All participants had previously tried dietary changes and other first-line therapies and met Rome IV criteria (Table 9). Over three-quarters had tried antispasmodics (77.5%), 31.3% an anti-diarrhoeal, 22.5% fibre supplements, 18.4% laxatives and 9.7% reported having tried peppermint oil. Participants were randomised a mean of 12.5 (SD 11.0) years after their IBS diagnosis (median 10 years, range < 1 week to 67 years) (see Appendix 1, Figure 11).
FIGURE 4.
Estimated treatment effect on weekly relief: logistic regression: 443 participants with at least one weekly response. The band in panel (a) represents the estimated OR and 95% CI overall across all weeks.

Baseline questionnaires
Baseline questionnaire scores were largely balanced across trial arms (Table 10). The mean IBS-SSS score was 272.8 (SD 90.33), and 41.3% of participants reported severe, 43.4% moderate and 13.6% mild IBS symptoms. The mean HADS-A and HADS-D scores were 7.5 (SD 4.3) and 4.3 (SD 3.4), respectively, with 47.1% and 15.8% reporting at least mild symptoms of anxiety or depression. Over a third of participants reported having ever received treatment for depression (38.4%) or anxiety (34.3%), with a slightly higher proportion having received treatment for depression in the placebo arm (42.9%). The mean PHQ-12 score was 6.3 (SD 3.5) and the mean WSAS score was 11.4 (SD 7.4).
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
|---|---|---|---|
| Total IBS-SSS scorec | |||
| Mean (SD) | 273.4 (90.53) | 272.1 (90.33) | 272.8 (90.33) |
| Median (range) | 280.0 (60, 480) | 270.0 (10, 470) | 280.0 (10, 480) |
| IBS-SSS level | |||
| < 75 (remission) | 3 (1.3%) | 5 (2.2%) | 8 (1.7%) |
| 75–174 (mild) | 37 (15.9%) | 26 (11.3%) | 63 (13.6%) |
| 175–299 (moderate) | 98 (42.2%) | 103 (44.6%) | 201 (43.4%) |
| 300–500 (severe) | 94 (40.5%) | 97 (42.0%) | 191 (41.3%) |
| HADS-A scores | |||
| Mean (SD) | 7.3 (4.3) | 7.7 (4.3) | 7.5 (4.3) |
| Median (range) | 7.0 (0.0, 21.0) | 7.0 (0.0, 20.0) | 7.0 (0.0, 21.0) |
| HADS-A level | |||
| 0–7 (normal range) | 126 (54.3%) | 119 (51.5%) | 245 (52.9%) |
| 8–10 (mild anxiety) | 55 (23.7%) | 58 (25.1%) | 113 (24.4%) |
| 11–14 (moderate anxiety) | 39 (16.8%) | 36 (15.6%) | 75 (16.2%) |
| 15–21 (severe anxiety) | 12 (5.2%) | 18 (7.8%) | 30 (6.5%) |
| HADS-D scores | |||
| Mean (SD) | 4.4 (3.6) | 4.1 (3.2) | 4.3 (3.4) |
| Median (range) | 4.0 (0.0, 18.0) | 4.0 (0.0, 15.0) | 4.0 (0.0, 18.0) |
| HADS-D level | |||
| 0–7 (normal range) | 195 (84.1%) | 195 (84.4%) | 390 (84.2%) |
| 8–10 (mild depression) | 23 (9.9%) | 24 (10.4%) | 47 (10.2%) |
| 11–14 (moderate depression) | 11 (4.7%) | 11 (4.8%) | 22 (4.8%) |
| 15–21 (severe depression) | 3 (1.3%) | 1 (0.4%) | 4 (0.9%) |
| Have you ever been treated for depression? | |||
| Yes | 79 (34.2%) | 99 (42.9%) | 178 (38.5%) |
| No | 152 (65.8%) | 132 (57.1%) | 284 (61.5%) |
| Missing | 1 | 0 | 1 |
| Have you ever been treated for anxiety? | |||
| Yes | 80 (34.6%) | 79 (34.2%) | 159 (34.4%) |
| No | 151 (65.4%) | 152 (65.8%) | 303 (65.6%) |
| Missing | 1 | 0 | 1 |
| Total PHQ-12 score | |||
| Mean (SD) | 6.3 (3.5) | 6.3 (3.6) | 6.3 (3.5) |
| Median (range) | 6.0 (0.0, 17.3) | 6.0 (0.0, 18.0) | 6.0 (0.0, 18.0) |
| Missing | 4 | 2 | 6 |
| WSAS total score | |||
| Mean (SD) | 11.2 (8.2) | 11.5 (7.6) | 11.4 (7.9) |
| Median (range) | 9.0 (0.0, 40.0) | 11.0 (0.0, 40.0) | 10.0 (0.0, 40.0) |
| Missing | 8 | 12 | 20 |
Six-month treatment delivery and receipt
Treatment receipt and completion
A total of 338 (73.0%) participants completed 6 months treatment, 173 (74.6%) allocated to amitriptyline, and 165 (71.4%) allocated to placebo (Table 11), and 105 (22.7%) discontinued trial medication before 6 months, 46 (19.8%) allocated to amitriptyline and 59 (25.5%) allocated to placebo. Another 17 (3.7%) participants were lost to follow-up and three participants (< 1%) did not start treatment.
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
|---|---|---|---|
| Completed 6 months treatment | |||
| Yes | 173 (74.6%) | 165 (71.4%) | 338 (73.0%) |
| Discontinued treatment before 6 months | 46 (19.8%) | 59 (25.5%) | 105 (22.7%) |
| Lost to follow-up before 6 months | 11 (4.7%) | 6 (2.6%) | 17 (3.7%) |
| Did not start treatmenta | 2 (0.9%) | 1 (0.4%) | 3 (0.6%) |
| Time from randomisation to treatment start (days) | |||
| Mean (SD) | 7.5 (5.66) | 7.4 (6.05) | 7.5 (5.86) |
| Median (range) | 6.0 (0.0, 46.0) | 6.0 (1.0, 43.0) | 6.0 (0.0, 46.0) |
| IQR | 4.0–9.0 | 4.0–9.5 | 4.0–9.0 |
| Missing | 7 | 3 | 10 |
| n | 225 | 228 | 453 |
| Time from randomisation to treatment start | |||
| ≤ 7 days | 145 (64.4%) | 152 (66.7%) | 297 (65.6%) |
| ≤ 14 days | 60 (26.7%) | 53 (23.2%) | 113 (24.9%) |
| ≤ 21 days | 13 (5.8%) | 16 (7.0%) | 29 (6.4%) |
| > 21 days | 7 (3.1%) | 7 (3.1%) | 14 (3.1%) |
| Missing | 5 | 2 | 7 |
| Optional GP review taken place? | |||
| Yes | 4 (1.7%) | 10 (4.3%) | 14 (3.0%) |
| In person | 2 | 3 | 5 |
| Over the telephone | 2 | 7 | 9 |
| Time to treatment discontinuation (months) | |||
| Mean (SD) | 2.5 (1.62) | 2.7 (1.76) | 2.6 (1.70) |
| Median (range) | 2.3 (0.2, 5.7) | 2.8 (0.2, 5.9) | 2.5 (0.2, 5.9) |
| IQR | 1.1–4.2 | 0.8–4.4 | 1.1–4.2 |
| n | 46 | 59 | 105 |
| Reason for discontinuation | |||
| Side effect | 30 (12.9%) | 20 (8.7%) | 50 (10.8%) |
| Lack of benefit | 7 (3.0%) | 18 (7.8%) | 25 (5.4%) |
| Non-specific or personal choice | 5 (2.2%) | 15 (6.5%) | 20 (4.3%) |
| SARb | 1 (0.4%) | 3 (1.3%) | 3 (0.6%) |
| Safety (including allergic reactions to IMP) | 1 (0.4%) | 2 (0.9%) | 3 (0.6%) |
| Stopped in error on advice of GP | 1 (0.4%) | 0 (0.0%) | 1 (0.2%) |
| Randomised in error | 1 (0.4%) | 0 (0.0%) | 1 (0.2%) |
| Administrative error | 0 (0.0%) | 1 (0.4%) | 1 (0.2%) |
| Total | 232 (100%) | 231 (100%) | 463 (100%) |
An optional GP review at 1-month post randomisation was requested by only 18 (3.9%) participants and was reported to have taken place either in person or over the telephone for 14 (3.0%) participants, 4 (1.7%) allocated to amitriptyline and 10 (4.3%) allocated to placebo.
Participants started treatment a median of 6 days post randomisation, and the majority (88.6%) started treatment within 2 weeks. In participants who discontinued treatment before 6 months, the median time to discontinuation was 2.5 months, with a slightly earlier time to discontinuation in the amitriptyline arm (median 2.3 vs. 2.8 months). The most common reason for treatment discontinuation was side effects in 30 (12.9%) and 20 (8.7%) participants allocated to amitriptyline and placebo respectively, followed by lack of benefit in 7 (3.0%) amitriptyline and 18 (7.8%) placebo participants, and non-specific or personal choice in 5 (2.2%) amitriptyline and 15 (6.5%) placebo participants. The most common side effects leading to treatment discontinuation were drowsiness (in 13 participants allocated to amitriptyline and 6 to placebo), deterioration of mood (9 amitriptyline, 5 placebo), constipation (4 amitriptyline, 3 placebo) and headache (2 amitriptyline, 4 placebo) (see Appendix 1, Figure 12). Note that side effects in all participants according to the ASEC are reported in detail in Tolerability at 3 and 6 months.
FIGURE 5.
Participant-reported tolerability on the ASEC at 3 months (safety population for participants on treatment).

Across all randomised participants (ITT population), the median time from randomisation to the end-of-trial treatment was 5.8 months, with similar duration observed across trial arms (Table 12). Appendix 1, Figure 13 further presents the distribution of time from randomisation to treatment end date for all participants and by follow-up duration, and Further 12-month treatment delivery and secondary end points provides further details of treatment beyond 6 months for participants in the 12-month ITT population.
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
|---|---|---|---|
| Months to last dose (from randomisation) | |||
| Mean (SD) | 7.3 (3.74) | 6.8 (3.72) | 7.0 (3.73) |
| Median (range) | 5.9 (0.2, 14.0) | 5.8 (0.2, 12.2) | 5.8 (0.2, 14.0) |
| IQR | 5.7–11.9 | 4.9–11.9 | 5.5–11.9 |
| Missing | 21 | 16 | 37 |
| n | 211 | 215 | 426 |
| Months to last dose (from randomisation) | |||
| ≤ 1 month | 10 (4.7%) | 16 (7.4%) | 26 (6.1%) |
| ≤ 2 months | 11 (5.2%) | 9 (4.2%) | 20 (4.7%) |
| ≤ 3 months | 11 (5.2%) | 6 (2.8%) | 17 (4.0%) |
| ≤ 4 months | 2 (0.9%) | 12 (5.6%) | 14 (3.3%) |
| ≤ 5 months | 9 (4.3%) | 11 (5.1%) | 20 (4.7%) |
| ≤ 6 months | 79 (37.4%) | 85 (39.5%) | 164 (38.5%) |
| ≤ 7 months | 9 (4.3%) | 8 (3.7%) | 17 (4.0%) |
| ≤ 8 months | 2 (0.9%) | 2 (0.9%) | 4 (0.9%) |
| ≤ 9 months | 6 (2.8%) | 2 (0.9%) | 8 (1.9%) |
| ≤ 10 months | 2 (0.9%) | 1 (0.5%) | 3 (0.7%) |
| ≤ 11 months | 2 (0.9%) | 1 (0.5%) | 3 (0.7%) |
| ≤ 12 months | 37 (17.5%) | 43 (20.0%) | 80 (18.8%) |
| > 12 months | 31 (14.7%) | 19 (8.8%) | 50 (11.7%) |
| Total | 211 (100%) | 215 (100%) | 426 (100%) |
FIGURE 6.
Participant-reported tolerability on the ASEC at 6 months (safety population for participants on treatment).
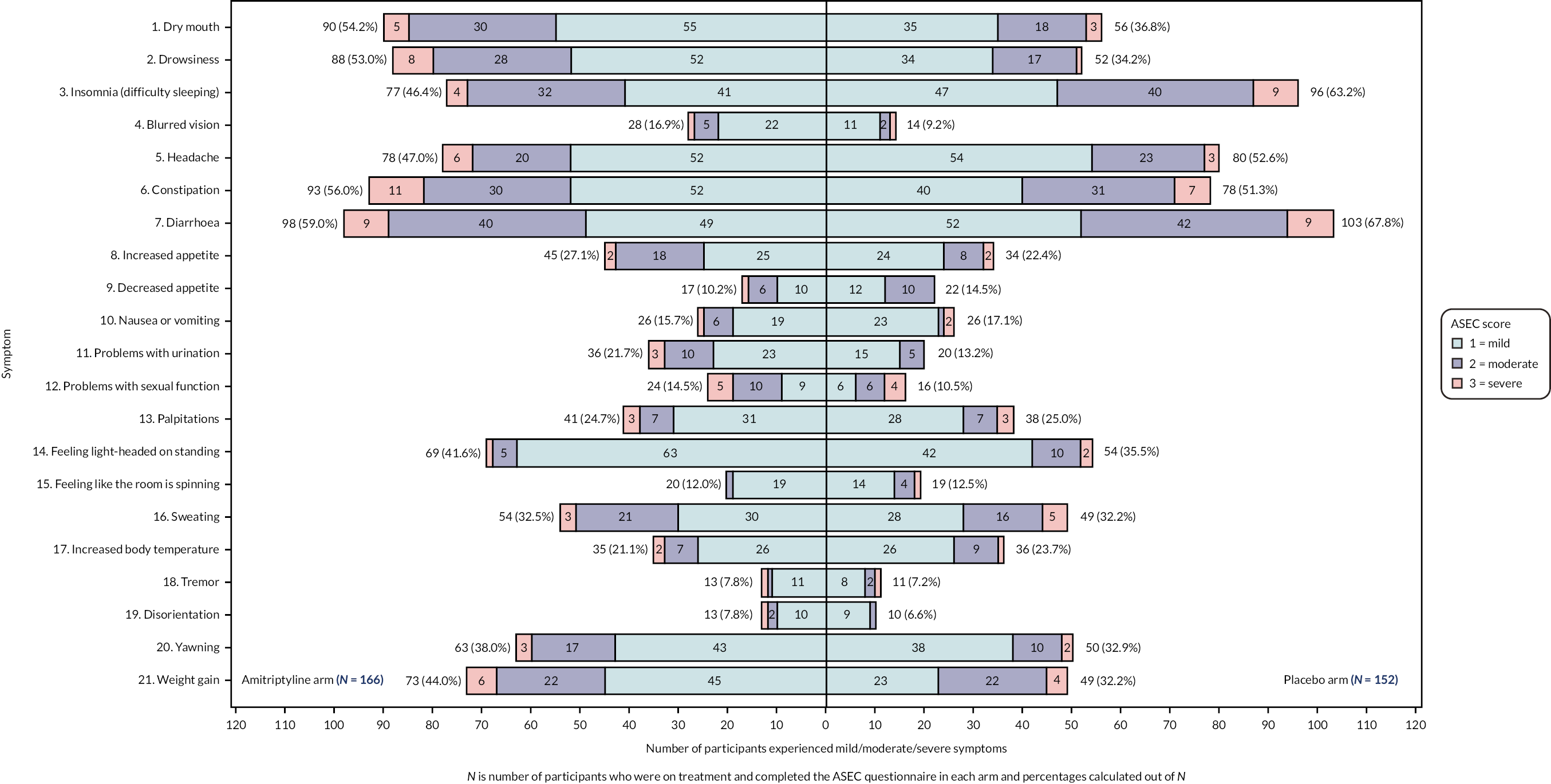
Treatment adherence, dosage titration and modification
Over 90% of participants on trial medication at each of week 3, month 3 and month 6, reported the highest level of treatment adherence, taking at least one tablet ‘every day or nearly every day’, with similar rates across trial arms at each time point (see Table 13 and Appendix 1, Figure 14). At 3 weeks post randomisation, most participants were taking a dose of 10 mg (44.9% amitriptyline vs. 35.4% placebo) or 20 mg (37.0% amitriptyline vs. 39.2% placebo) once daily, with a slightly higher proportion of participants in the placebo arm taking 30 mg once-daily (see Table 13 and Appendix 1, Figure 15). By 3 months, similar proportions of participants randomised to amitriptyline were taking 20 mg (35.2%) or 30 mg (37.8%) once daily, although by 6 months this increased to 42.8% taking 30 mg once daily. However, in the placebo arm, 57.0% of participants titrated their dose to 30 mg once daily by 3 months and this remained similar at 6 months.
| Week 3 | Month 3 | Month 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 222) | Placebo (n = 223) | Total (n = 445) | Amitriptyline (n = 194) | Placebo (n = 196) | Total (n = 390) | Amitriptyline (n = 173) | Placebo (n = 165) | Total (n = 338) | |
| Participant taken at least one tablet daily | |||||||||
| Every/nearly every day | 212 (98.1%) | 204 (95.8%) | 416 (97.0%) | 185 (95.4%) | 179 (96.8%) | 364 (96.0%) | 163 (94.2%) | 146 (90.1%) | 309 (92.2%) |
| ≥ half the days | 4 (1.9%) | 6 (2.8%) | 10 (2.3%) | 8 (4.1%) | 4 (2.2%) | 12 (3.2%) | 9 (5.2%) | 9 (5.6%) | 18 (5.4%) |
| < half of the days | 0 (0.0%) | 2 (0.9%) | 2 (0.5%) | 0 (0.0%) | 2 (1.1%) | 2 (0.5%) | 1 (0.6%) | 4 (2.5%) | 5 (1.5%) |
| None/nearly no days | 0 (0.0%) | 1 (0.5%) | 1 (0.2%) | 1 (0.5%) | 0 (0.0%) | 1 (0.3%) | 0 (0.0%) | 3 (1.9%) | 3 (0.9%) |
| Missing | 6 | 10 | 16 | 0 | 11 | 11 | 0 | 3 | 3 |
| Current dose of trial medication | |||||||||
| 1 × 10 mg every other day | 1 (0.5%) | 4 (1.9%) | 5 (1.2%) | 3 (1.6%) | 4 (2.2%) | 7 (1.8%) | 6 (3.5%) | 4 (2.5%) | 10 (3.0%) |
| 1 × 10 mg daily | 97 (44.9%) | 75 (35.4%) | 172 (40.2%) | 49 (25.4%) | 23 (12.4%) | 72 (19.0%) | 40 (23.1%) | 22 (13.9%) | 62 (18.7%) |
| 2 × 10 mg daily | 80 (37.0%) | 83 (39.2%) | 163 (38.1%) | 68 (35.2%) | 53 (28.5%) | 121 (31.9%) | 53 (30.6%) | 43 (27.2%) | 96 (29.0%) |
| 3 × 10 mg daily | 38 (17.6%) | 50 (23.6%) | 88 (20.6%) | 73 (37.8%) | 106 (57.0%) | 179 (47.2%) | 74 (42.8%) | 89 (56.3%) | 163 (49.2%) |
| Missing | 6 | 11 | 17 | 1 | 10 | 11 | 0 | 7 | 7 |
| Dose modification since last follow-up call | |||||||||
| Yes | 96 (50.5%) | 100 (55.2%) | 196 (52.8%) | 33 (19.2%) | 17 (11.2%) | 50 (15.4%) | |||
| Higher dose | 79 (82.3%) | 88 (88.0%) | 167 (85.2%) | 17 (51.5%) | 8 (47.1%) | 25 (50.0%) | |||
| Lower dose | 17 (17.7%) | 12 (12.0%) | 29 (14.8%) | 16 (48.5%) | 9 (52.9%) | 25 (50.0%) | |||
| No | 94 (49.5%) | 81 (44.8%) | 175 (47.2%) | 139 (80.8%) | 135 (88.8%) | 274 (84.6%) | |||
| Missing | 4 | 15 | 19 | 1 | 13 | 14 | |||
FIGURE 7.
Participant-reported tolerability on the ASEC at 12 months (safety population for participants on treatment).
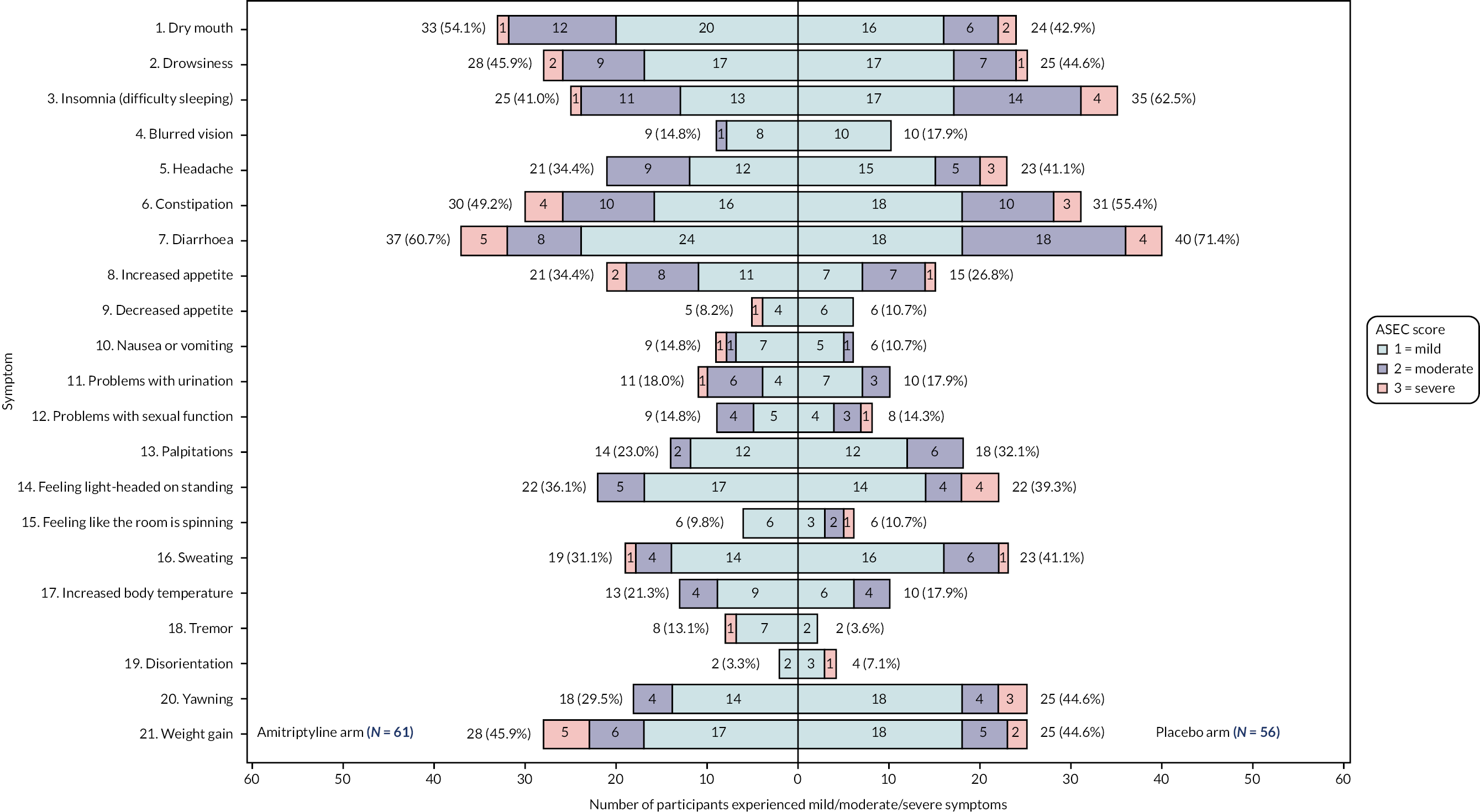
Between 3 weeks and 3 months post randomisation, just over half of participants still on treatment had modified their dose (50.5% amitriptyline, 55.2% placebo) of whom the majority had an increase in dose (82.3% amitriptyline, 88.0% placebo). Dose modifications were less frequent between 3 and 6 months, reported in 33 (19.2%) and 17 (11.2%) participants still receiving amitriptyline and placebo respectively, with half the modifications to a higher and half to a lower dose, and with similar proportions across trial arms.
Dosage and adherence details reported in participants who had discontinued treatment are presented in Appendix 1, Tables 45 and 46.
Further treatment summaries
Off-trial amitriptyline
One participant (0.2% of all participants, 1.0% of participants who discontinued trial medication before 6 months) reported taking amitriptyline off-trial after treatment discontinuation within 6 months of randomisation.
Suicidal ideation and drugs leading to discontinuation
Two participants (one in the placebo arm and one in the amitriptyline arm) reported experiencing thoughts of self-harm, one at the 3-week and one at the 3-month follow-up call. Both events were reported as a SAR (see Safety) and participants discontinued trial medication subsequently. No participants reported taking, or discontinued trial medication due to taking, monoamine oxidase inhibitors or drugs prolonging the QT interval during the study during researcher concomitant medication reviews at the 1-week, 3-week, 3-month or 6-month follow-up call.
Treatment replenishment and replacement
Treatment replenishment at 3 weeks and 3 months is presented in Appendix 1, Table 47. A total of 20 treatment kit replacements were conducted for 18 participants (10 amitriptyline, 8 placebo) throughout the trial. The reasons for the replacement requests were damaged or lost bottles, participants running out of medication before their next scheduled follow-up call, or administrative errors, where a replacement was performed instead of replenishment, or a pharmacy error occurred.
Changes in diet, exercise, other IBS treatments, and IBS symptoms at 6 months
Of 338 participants completing 6 months treatment and researcher follow-up, 67 (19.9%) reported having tried at least one new diet for IBS during the study, 66 (19.6%) reported increasing the amount of exercise they did to help their IBS symptoms, whereas 11 (3.3%) reported reducing their exercise, and 34 (10.1%) tried one or more other treatments for their IBS symptoms, with similar rates across trial arms (Table 14). Over two-thirds of participants on amitriptyline [118 (68.2%)] reported having experienced improved IBS symptoms, compared with just over half of participants on placebo [89 (54.6%)]. The majority of participants reporting improvement attributed this to the ATLANTIS trial medication [103 (87.3%) on amitriptyline; 75 (84.3%) on placebo]. Among participants who had discontinued treatment before 6 months, a lower proportion reported experiencing improved IBS symptoms [13 (28.3%) on amitriptyline; 10 (29.1%) on placebo]. Appendix 1, Table 48 further details the types of new diets, other treatments and attributed reasons for improvement in IBS symptoms.
| Month 6 | Discontinued treatment before month 6 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 173) | Placebo (n = 165) | Total (n = 338) | Amitriptyline (n = 46) | Placebo (n = 59) | Total (n = 105) | |
| Tried a new diet for IBS | 33 (19.1%) | 34 (20.9%) | 67 (19.9%) | 0 (0.0%) | 6 (12.8%) | 6 (7.6%) |
| Changed amount of exercise | ||||||
| Increased the amount of exercise | 38 (22.0%) | 28 (17.2%) | 66 (19.6%) | 3 (9.3%) | 5 (10.6%) | 8 (10.1%) |
| Reduced the amount of exercise | 4 (2.3%) | 7 (4.3%) | 11 (3.3%) | 1 (3.1%) | 0 (0.0%) | 1 (1.3%) |
| Tried other treatments for IBS symptoms | 18 (10.4%) | 16 (9.8%) | 34 (10.1%) | 5 (15.6%) | 2 (4.3%) | 7 (8.9%) |
| Experienced improved IBS symptoms | 118 (68.2%) | 89 (54.6%) | 207 (61.6%) | 13 (28.3%) | 10 (21.3%) | 23 (29.1%) |
| Missing | 0 | 2 | 2 | 14 | 12 | 26 |
Primary end point: 6-month global symptoms of irritable bowel syndrome (irritable bowel syndrome Severity Scoring System)
Summary statistics of available data for the IBS-SSS up to and including the 6-month follow-up are presented in Table 15 and Appendix 1, Figure 16. The IBS-SSS item level scores can be found in Appendix 1, Table 49 and Appendix 1, Figure 17. The total IBS-SSS score was available at 6 months for 401 (86.6%) participants [204 (87.9%) on amitriptyline; 197 (85.3%) on placebo]. Although IBS-SSS scores in each arm were similar at baseline, the mean 6-month scores were 170.4 (SD 107.7) and 200.1 (SD 114.4) in participants allocated to amitriptyline and placebo, respectively. A higher proportion of participants had remission of IBS symptoms according to the IBS-SSS (score < 75 points) at 6 months in the amitriptyline arm compared with placebo (23.5% vs. 15.7%), and a lower proportion of participants had severe IBS symptoms on the IBS-SSS (score ≥ 300 points) in the amitriptyline arm compared with placebo (15.2% vs. 23.4%).
| Baseline | Month 3 | Month 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
| Total IBS-SSS score | |||||||||
| Mean (SD) | 273.4 (90.53) | 272.1 (90.33) | 272.8 (90.33) | 173.0 (106.63) | 194.6 (107.53) | 183.7 (107.50) | 170.4 (107.73) | 200.1 (114.46) | 185.0 (111.94) |
| Median (range) | 280.0 (60, 480) | 270.0 (10, 470) | 280.0 (10, 480) | 180.0 (0, 460) | 190.0 (0, 430) | 180.0 (0, 460) | 170.0 (0, 500) | 190.0 (0, 450) | 180.0 (0, 500) |
| IQR | 210.0–330.0 | 200.0–330.0 | 210.0–330.0 | 80.0–250.0 | 110.0–270.0 | 100.0–260.0 | 80.0–250.0 | 110.0–290.0 | 90.0–270.0 |
| Missing | 0 | 0 | 0 | 13 | 18 | 31 | 28 | 34 | 62 |
| Mean difference (95% CI), p-value | −23.30 (−41.96 to −4.64), 0.014 | −27.01 (−46.91 to −7.10), 0.008 | |||||||
| IBS-SSS severity | |||||||||
| < 75 (remission) | 3 (1.3%) | 5 (2.2%) | 8 (1.7%) | 49 (22.4%) | 34 (16.0%) | 83 (19.2%) | 48 (23.5%) | 31 (15.7%) | 79 (19.7%) |
| 75–174 (mild) | 37 (15.9%) | 26 (11.3%) | 63 (13.6%) | 59 (26.9%) | 64 (30.0%) | 123 (28.5%) | 58 (28.4%) | 58 (29.4%) | 116 (28.9%) |
| 175–299 (moderate) | 98 (42.2%) | 103 (44.6%) | 201 (43.4%) | 84 (38.4%) | 70 (32.9%) | 154 (35.6%) | 67 (32.8%) | 62 (31.5%) | 129 (32.2%) |
| 300–500 (severe) | 94 (40.5%) | 97 (42.0%) | 191 (41.3%) | 27 (12.3%) | 45 (21.1%) | 72 (16.7%) | 31 (15.2%) | 46 (23.4%) | 77 (19.2%) |
| Missing | 13 | 18 | 31 | 28 | 34 | 62 | |||
| Difference in IBS-SSS score from baseline | |||||||||
| Mean (SD) | −99.8 (107.67) | −76.1 (107.09) | −88.1 (107.92) | −99.2 (112.88) | −68.9 (109.26) | −84.3 (112.01) | |||
| Median (range) | −80.0 (−370.0 to 170.0) | −70.0 (−430.0 to 250.0) | −80.0 (−430.0 to 250.0) | −90.0 (−360.0 to 160.0) | −50.0 (−380.0 to 200.0) | −80.0 (−380.0 to 200.0) | |||
| IQR | −170.0 to −20.0 | −140.0 to 0.0 | −160.0 to −20.0 | −170.0 to −20.0 | −130.0 to 0.0 | −160.0 to −10.0 | |||
| Missing | 13 | 18 | 31 | 28 | 34 | 62 | |||
| ≥ 50-point reduction in total IBS-SSS score from baseline | |||||||||
| Yes | 149 (68.0%) | 126 (59.2%) | 275 (63.7%) | 131 (64.2%) | 106 (53.8%) | 237 (59.1%) | |||
| No | 70 (32.0%) | 87 (40.8%) | 157 (36.3%) | 73 (35.8%) | 91 (46.2%) | 164 (40.9%) | |||
| Missing | 13 | 18 | 31 | 28 | 34 | 62 | |||
| ≥ 30% reduction in abdominal pain severity from baseline (Item 1b) | |||||||||
| Yes | 114 (52.3%) | 100 (46.9%) | 214 (49.7%) | 113 (55.7%) | 84 (42.6%) | 197 (49.3%) | |||
| No | 104 (47.7%) | 113 (53.1%) | 217 (50.3%) | 90 (44.3%) | 113 (57.4%) | 203 (50.8%) | |||
| Missing | 14 | 18 | 32 | 29 | 34 | 63 | |||
| ≥ 30% reduction in abdominal distention severity from baseline (Item 3b) | |||||||||
| Yes | 100 (45.7%) | 86 (40.4%) | 186 (43.1%) | 95 (46.6%) | 74 (37.6%) | 169 (42.1%) | |||
| No | 119 (54.3%) | 127 (59.6%) | 246 (56.9%) | 109 (53.4%) | 123 (62.4%) | 232 (57.9%) | |||
| Missing | 13 | 18 | 31 | 28 | 34 | 62 | |||
Amitriptyline was superior to placebo at 6 months in ITT analysis, using linear regression adjusted for covariates with multiple imputation, with strong evidence (p < 0.05) of a reduced total IBS-SSS score at 6 months with amitriptyline compared with placebo and an estimated adjusted mean difference of −27.01 (95% CI −46.91 to −7.10; p = 0.008). Results of sensitivity analyses were consistent, albeit with larger reductions in the 6-month IBS-SSS score, with amitriptyline compared with placebo (Table 16).
| Primary analysis (n = 463, multiple imputation) | Complete case (n = 401) | Per-protocol (n = 323, multiple imputationsb) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimate (95% CI) | SE | p-value | Parameter estimate (95% CI) | SE | p-value | Parameter estimate (95% CI) | SE | p-value | |
| Intercept | 57.71 (21.03 to 94.40) | 18.71 | 0.002** | 58.90 (21.05 to 96.74) | 19.25 | 0.002** | 48.38 (7.28 to 89.49) | 20.97 | 0.021* |
| Treatment: amitriptyline (vs. placebo) | −27.01 (−46.91 to −7.10) | 10.15 | 0.008** | −30.87 (−50.88 to −10.86) | 10.18 | 0.003** | −31.76 (−54.18 to −9.33) | 11.44 | 0.006** |
| Baseline IBS-SSS score | 0.51 (0.40 to 0.62) | 0.06 | < 0.001** | 0.50 (0.39 to 0.62) | 0.06 | < 0.001** | 0.52 (0.39 to 0.65) | 0.07 | < 0.001** |
| IBS subtype (vs. IBS-M or IBS-U) | 0.240 | ||||||||
| IBS-C | 24.68 (−3.50 to 52.87) | 14.37 | 0.086 | 23.81 (−4.95 to 52.57) | 14.63 | 0.104 | 31.33 (−0.92 to 63.58) | 16.46 | 0.057 |
| IBS-D | 0.36 (−21.41 to 22.12) | 11.10 | 0.974 | 1.75 (−20.23 to 23.73) | 11.18 | 0.876 | 15.40 (−9.02 to 39.83) | 12.46 | 0.216 |
| Baseline HADS-D score | 0.66 (−2.36 to 3.68) | 1.54 | 0.669 | 0.71 (−2.28 to 3.71) | 1.52 | 0.640 | −0.44 (−3.91 to 3.04) | 1.77 | 0.805 |
| Recruitment hub (vs. Wessex) | 0.081 | ||||||||
| West of England | 7.87 (−13.52 to 29.25) | 10.91 | 0.471 | 6.49 (−15.44 to 28.43) | 11.16 | 0.561 | 2.52 (−21.46 to 26.50) | 12.23 | 0.837 |
| West Yorkshire | −17.93 (−46.39 to 10.52) | 14.50 | 0.217 | −26.58 (−55.60 to 2.44) | 14.76 | 0.072 | −23.37 (−57.13 to 10.38) | 17.22 | 0.175 |
Baseline IBS-SSS score was strongly associated with outcome such that higher baseline scores were associated with higher 6-month scores, with a 0.51 (95% CI 0.40 to 0.62; p < 0.001) increase in 6-month IBS-SSS score for every unit increase in baseline score in the primary analysis (Table 16). Similar effects were observed in sensitivity analysis. There were no other statistically significant covariate effects.
There was weak evidence (p < 0.1) that participants with IBS-C had higher 6-month IBS-SSS scores compared with participants with IBS-M or IBS-U, with a mean adjusted difference of 24.7 (95% CI −3.5 to 52.9; p = 0.086) in primary analysis, and an extenuated effect in per-protocol analysis and a reduced effect in complete case analysis. Participants recruited in West Yorkshire tended to have lower scores than participants recruited from Wessex or West of England. However, only weak evidence of a statistically significant effect was observed in complete case analysis and not in the primary or per-protocol analysis.
Missing data
Overall, across trial arms, univariable analysis identified recruitment hub as predictive (p < 0.05) of missing data status, baseline HADS-D, baseline HADS-A and baseline PHQ-12 scores as predictive of outcome, and age, baseline IBS-SSS, and baseline WSAS scores, and 6-month treatment status as key characteristics predictive of both missing data status and outcome (see Table 5 and Appendix 1, Table 50).
Primary analysis, therefore, imputed missing 6-month IBS-SSS scores using multiple imputation (stratified by treatment group) and incorporated IBS-SSS score at baseline and 3 months, the planned covariates of recruitment hub, IBS subtype, and HADS-D score, as well as additional variables found to be predictive of missingness and/or outcomes including 6-month treatment status, age, baseline WSAS, HADS-A and PHQ-12 score. Sex was also included in the imputation model to allow for consistency across imputation models for all outcomes (as a covariate in PHQ-12 analysis).
Model checking
Graphical and statistical tests of the adequacy of the linear regression model for treatment and covariates were generally satisfactory and are presented in Appendix 1, Figure 18.
Key secondary end point: subjective global assessment of relief of irritable bowel syndrome symptoms at 6 months
A total of 399 (86.2%) participants [204 (87.9%) on amitriptyline; 195(84.4%) on placebo] provided a response to the SGA of relief of IBS symptoms during the past week at 6-month follow-up. Table 17 and Appendix 1, Figure 19 show a higher proportion of participants reported IBS symptoms as being completely, considerably, or somewhat relieved in the amitriptyline arm compared with placebo (61.3% vs. 45.1%, primary responder definition), with response rates of 35.8% versus 22.6% for complete or considerable relief (sensitivity responder definition).
| Month 3 | Month 6 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
| Reliefa of IBS symptoms | ||||||
| 1-Completely relieved | 7 (3.2%) | 5 (2.3%) | 12 (2.8%) | 8 (3.9%) | 4 (2.1%) | 12 (3.0%) |
| 2-Considerably relieved | 67 (30.5%) | 42 (19.7%) | 109 (25.2%) | 65 (31.9%) | 40 (20.5%) | 105 (26.3%) |
| 3-Somewhat relieved | 65 (29.5%) | 58 (27.2%) | 123 (28.4%) | 52 (25.5%) | 44 (22.6%) | 96 (24.1%) |
| 4-Unchanged | 78 (35.5%) | 97 (45.5%) | 175 (40.4%) | 73 (35.8%) | 99 (50.8%) | 172 (43.1%) |
| 5-Worse | 3 (1.4%) | 11 (5.2%) | 14 (3.2%) | 6 (2.9%) | 8 (4.1%) | 14 (3.5%) |
| Missing | 12 | 18 | 30 | 28 | 36 | 64 |
| Responder (response 1–3) | ||||||
| Yes | 139 (63.2%) | 105 (49.3%) | 244 (56.4%) | 125 (61.3%) | 88 (45.1%) | 213 (53.4%) |
| Odds ratio (95% CI), p-valueb | 1.70 (1.15 to 2.53), 0.008 | 1.78 (1.19 to 2.66), 0.005 | ||||
| Responder (response 1–2) | ||||||
| Yes | 74 (33.6%) | 47 (22.1%) | 121 (27.9%) | 73 (35.8%) | 44 (22.6%) | 117 (29.3%) |
Amitriptyline was superior to placebo at 6 months in ITT analysis (Table 18), using logistic regression adjusted for covariates with multiple imputation, with strong evidence of an increased odds of response (IBS symptoms completely, considerably or somewhat relieved) on the SGA at 6 months with amitriptyline compared with placebo and an OR of 1.78 (95% CI 1.19 to 2.66; p = 0.005). Results of sensitivity analyses were consistent, albeit with larger estimated treatment effects, in both complete case and sensitivity analysis using the alternative response (complete or considerable relief) definition (Table 18).
| Primary analysis (Responder 1–3 vs. 4–5), (n = 463) | Sensitivity analysis | Secondary analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete case (Responder 1–3 vs. 4–5), (n = 399) | Alternative responder definition (Responder 1–2 vs. 3–5), (n = 463) | Ordinal regressiona, (n = 463) | ||||||||||||||
| P. est. | SE | p-value | Odds ratio (95% CI) | P. est. | SE | p-value | Odds ratio (95% CI) | P. est. | SE | p-value | Odds ratio (95% CI) | P. est. | SE | p-value | Odds ratio (95% CI) | |
| Intercept | −0.08 | 0.25 | 0.751 | −0.02 | 0.25 | 0.945 | −1.10 | 0.28 | < 0.0001 | |||||||
| 1-Completely relieved | −3.74 | 0.36 | <0.001 | |||||||||||||
| 2-Considerably relieved | −1.10 | 0.24 | <0.001 | |||||||||||||
| 3-Somewhat relieved | −0.07 | 0.23 | 0.780 | |||||||||||||
| 4-Unchanged | 3.10 | 0.34 | <0.001 | |||||||||||||
| Treatment: amitriptyline (vs. placebo) | 0.58 | 0.20 | 0.005 ** | 1.78 (1.19 to 2.66) | 0.69 | 0.21 | 0.001 ** | 2.00 (1.33 to 3.00) | 0.63 | 0.23 | 0.006 ** | 1.88 (1.20 to 2.95) | 0.54 | 0.18 | 0.003 ** | 1.72 (1.20 to 2.46) b |
| IBS subtype (vs. IBS-M or IBS-U) | 0.188 | |||||||||||||||
| IBS-C | −0.54 | 0.30 | 0.066 | 0.58 (0.32 to 1.04) | −0.54 | 0.30 | 0.068 | 0.58 (0.33 to 1.04) | −0.26 | 0.33 | 0.421 | 0.77 (0.40 to 1.46) | −0.40 | 0.26 | 0.124 | 0.67 (0.40 to 1.12) |
| IBS-D | −0.11 | 0.22 | 0.625 | 0.90 (0.58 to 1.38) | −0.13 | 0.23 | 0.569 | 0.88 (0.56 to 1.37) | −0.13 | 0.24 | 0.588 | 0.88 (0.54 to 1.41) | −0.13 | 0.20 | 0.501 | 0.88 (0.60 to 1.29) |
| Baseline HADS-D score | −0.02 | 0.03 | 0.518 | 0.98 (0.92 to 1.04) | −0.03 | 0.03 | 0.373 | 0.97 (0.92 to 1.03) | −0.06 | 0.03 | 0.102 | 0.95 (0.89 to 1.01) | −0.03 | 0.03 | 0.321 | 0.97 (0.92 to 1.03) |
| Recruitment hub (vs. Wessex) | 0.140 | |||||||||||||||
| West of England | −0.11 | 0.22 | 0.631 | 0.90 (0.59 to 1.38) | −0.07 | 0.22 | 0.767 | 0.94 (0.60 to 1.45) | −0.11 | 0.25 | 0.671 | 0.90 (0.55 to 1.47) | −0.09 | 0.20 | 0.665 | 0.92 (0.62 to 1.36) |
| West Yorkshire | 0.44 | 0.28 | 0.115 | 1.55 (0.90 to 2.69) | 0.52 | 0.30 | 0.084 | 1.69 (0.93 to 3.07) | 0.70 | 0.30 | 0.017* | 2.02 (1.13 to 3.61) | 0.49 | 0.26 | 0.057 | 1.63 (0.98 to 2.69) |
There were no statistically significant covariate effects in primary analysis. As seen in the primary outcome, there was weak evidence (p < 0.1) that participants with IBS-C had reduced odds of responding compared with participants with IBS-M or IBS-U (OR 0.58, 95% CI 0.32 to 1.04; p = 0.066). A similar effect was observed in complete case analysis but not in sensitivity analysis using the alternative response (complete or considerable relief) definition. Although there was no evidence of a difference across hub in primary analysis, there was good evidence of an increased odds of response in participants in West Yorkshire compared with Wessex in sensitivity analysis using the alternative response definition (OR 2.02, 95% CI 1.13 to 3.61; p = 0.017) and weak evidence in complete case analysis.
Secondary analysis
The treatment effect estimated from secondary analysis using ordinal logistic regression, rather than dichotomising responses, was consistent with the primary analysis (Table 18). The estimated odds of a better response (ordinal regression models the odds of a better response, assuming proportional odds between the cumulative odds of: complete relief vs. less than complete relief; at least considerable relief vs. less than considerable relief; at least some relief vs. less than some relief; and at least unchanged vs. worse symptoms) in the amitriptyline arm compared with the placebo arm was 1.72 (95% CI 1.20 to 2.46; p = 0.003).
Model checking
Graphical and statistical tests of the adequacy of the logistic regression and ordinal logistic regression models were satisfactory (see Appendix 1, Figure 20 and Appendix 1, Key secondary end point).
Secondary end points
Global symptoms of irritable bowel syndrome (irritable bowel syndrome Severity Scoring System) at 3 months
The total IBS-SSS score was available at 3 months for 432 (93.3%) participants [219 (94.4%) on amitriptyline; 213 (92.2%) on placebo]. The mean 3-month total IBS-SSS scores were 173.0 (SD 106.6) and 194.6 (SD 107.5) in participants allocated to amitriptyline and placebo, respectively (Table 15). A higher proportion of participants had remission of IBS symptoms on the IBS-SSS (IBS-SSS score < 75 points) at 3 months in the amitriptyline arm compared with placebo (22.4% vs. 16.0%), and a lower proportion of participants had severe IBS on the IBS-SSS (IBS-SSS score ≥ 300 points) in the amitriptyline arm compared with placebo (12.3% vs. 21.1%).
As per the 6-month outcome, amitriptyline was superior to placebo, with strong evidence (p < 0.05) of a reduced total IBS-SSS score at 3 months with amitriptyline compared with placebo and an estimated adjusted mean difference of −23.30 (95% CI −41.96 to −4.64; p = 0.014) (Tables 15 and 19). There were no statistically significant covariate effects (see Appendix 1, Table 51). Similar results were obtained in the complete case and per-protocol analysis, with increased treatment effects.
| IBS-SSS | Mean differencea (amitriptyline – placebo) | p -value | N |
| Primary analysis | −23.30 (−41.96, −4.64) | 0.014 | 463 |
| Complete case | −23.95 (−42.35, −5.56) | 0.011 | 433 |
| Per protocol | −27.70 (−47.23, −8.17) | 0.005 | 373 |
| SGA of relief | Odds ratiob (amitriptyline vs. placebo) | p -value | N |
| Primary analysis (Responder 1–3 vs. 4–5) | 1.70 (1.15, 2.53) | 0.008 | 463 |
| Sensitivity analysis | |||
| Complete case (Responder 1–3 vs. 4–5) | 1.81 (1.23, 2.67) | 0.003 | 433 |
| Alternative responder definition (Responder 1–2 vs. 3–5) | 1.81 (1.17, 2.79) | 0.008 | 463 |
| Secondary analysis | |||
| Ordinal regression | 1.80 (1.26, 2.58) | 0.001 | 463 |
Subjective global assessment of relief of irritable bowel syndrome symptoms at 3 months
A total of 433 (93.5%) participants [220 (94.8%) on amitriptyline; 213 (92.2%) on placebo] provided a response to SGA of relief of IBS symptoms during the past week at 3-month follow-up. A higher proportion of participants reported IBS symptoms as being completely, considerably, or somewhat relieved in the amitriptyline arm compared with placebo (63.2% vs. 49.3%), with response rates of 33.6% vs. 22.1% for complete or considerable relief (see Table 17 and Appendix 1, Figure 19).
As per the 6-month outcome, amitriptyline was superior to placebo, with strong evidence (p < 0.05) of an increased odds of response for SGA of relief of IBS symptoms at 3 months in the amitriptyline arm compared with the placebo arm and an OR of 1.70 (95% CI 1.15 to 2.53; p = 0.008) (Tables 17 and 19). Similar results were obtained in sensitivity and secondary analysis, with increased odds of response with amitriptyline (Table 19).
There were no statistically significant covariate effects (see Appendix 1, Table 52). There was weak evidence (p < 0.1) that participants with IBS-C had reduced odds of responding compared with participants with IBS-M or IBS-U (OR 0.60, 95% CI 0.34 to 1.05; p = 0.071) in primary analysis and complete case analysis but not in secondary analysis using ordinal regression or in sensitivity analysis using the alternative responder definition.
Hospital Anxiety and Depression Scale-A scores at 3 and 6 months
An improvement in participants’ HADS-A scores was seen in both groups over time and compared with baseline, with a mean score of 7.5 (SD 4.3) at baseline, 6.6 (SD 4.2) at 3 months, and 6.8 (SD 4.2) at 6 months (Table 20). There was no evidence of a treatment effect on HADS-A scores at 3 or 6 months in primary or sensitivity analysis (see Tables 20 and 21, Appendix 1, Table 53 and Figure 21). Adjusted mean differences indicated higher scores, but not statistically significantly different, in the amitriptyline arm by 0.05 (95% CI −0.53 to 0.63; p = 0.861) at 3 months and 0.08 (95% CI −0.49 to 0.63; p = 0.775) at 6 months in primary analysis. However, the direction of effect was reversed in 6-month complete case analysis, which indicated lower scores, again not statistically significantly different, in the amitriptyline arm by −0.07 (95% CI −0.66 to 0.51; p = 0.808).
| Baseline | Month 3 | Month 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
| HADS-A score | |||||||||
| Mean (SD) | 7.3 (4.3) | 7.7 (4.3) | 7.5 (4.3) | 6.5 (4.4) | 6.6 (4.0) | 6.6 (4.2) | 6.7 (4.4) | 6.9 (4.0) | 6.8 (4.2) |
| Median (range) | 7.0 (0.0, 21.0) | 7.0 (0.0, 20.0) | 7.0 (0.0, 21.0) | 6.0 (0.0, 21.0) | 7.0 (0.0, 17.0) | 6.0 (0.0, 21.0) | 6.0 (0.0, 20.0) | 7.0 (0.0, 16.0) | 7.0 (0.0, 20.0) |
| Missing | 0 | 0 | 0 | 12 | 19 | 31 | 29 | 38 | 67 |
| Mean difference (95% CI), p-value | 0.05 (−0.53 to 0.63), 0.861 | 0.08 (−0.49 to 0.65), 0.775 | |||||||
| Anxiety level | |||||||||
| 0–7 (Normal) | 126 (54.3%) | 119 (51.5%) | 245 (52.9%) | 138 (62.7%) | 131 (61.8%) | 269 (62.3%) | 127 (62.6%) | 112 (58.0%) | 239 (60.4%) |
| 8–10 (Mild anxiety) | 55 (23.7%) | 58 (25.1%) | 113 (24.4%) | 46 (20.9%) | 46 (21.7%) | 92 (21.3%) | 41 (20.2%) | 44 (22.8%) | 85 (21.5%) |
| 11–14 (moderate anxiety) | 39 (16.8%) | 36 (15.6%) | 75 (16.2%) | 24 (10.9%) | 26 (12.3%) | 50 (11.6%) | 22 (10.8%) | 31 (16.1%) | 53 (13.4%) |
| 15–21 (severe anxiety) | 12 (5.2%) | 18 (7.8%) | 30 (6.5%) | 12 (5.5%) | 9 (4.2%) | 21 (4.9%) | 13 (6.4%) | 6 (3.1%) | 19 (4.8%) |
| HADS-D score | |||||||||
| Mean (SD) | 4.4 (3.6) | 4.1 (3.2) | 4.3 (3.4) | 3.5 (3.3) | 3.6 (3.2) | 3.5 (3.3) | 3.9 (3.6) | 4.0 (3.5) | 4.0 (3.6) |
| Median (range) | 4.0 (0.0, 18.0) | 4.0 (0.0, 15.0) | 4.0 (0.0, 18.0) | 3.0 (0.0, 15.0) | 3.0 (0.0, 14.0) | 3.0 (0.0, 15.0) | 3.0 (0.0, 18.0) | 3.0 (0.0, 16.0) | 3.0 (0.0, 18.0) |
| Missing | 0 | 0 | 0 | 12 | 19 | 31 | 30 | 38 | 68 |
| Mean difference (95% CI), p-value | −0.22 (−0.71 to 0.26), 0.369 | −0.20 (−0.75 to 0.34), 0.462 | |||||||
| Depression level | |||||||||
| 0–7 (normal) | 195 (84.1%) | 195 (84.4%) | 390 (84.2%) | 196 (89.1%) | 187 (88.2%) | 383 (88.7%) | 175 (86.6%) | 158 (81.9%) | 333 (84.3%) |
| 8–10 (mild depression) | 23 (9.9%) | 24 (10.4%) | 47 (10.2%) | 12 (5.5%) | 15 (7.1%) | 27 (6.3%) | 14 (6.9%) | 29 (15.0%) | 43 (10.9%) |
| 11–14 (moderate depression) | 11 (4.7%) | 11 (4.8%) | 22 (4.8%) | 8 (3.6%) | 10 (4.7%) | 18 (4.2%) | 10 (5.0%) | 4 (2.1%) | 14 (3.5%) |
| 15–21 (severe depression) | 3 (1.3%) | 1 (0.4%) | 4 (0.9%) | 4 (1.8%) | 0 (0.0%) | 4 (0.9%) | 3 (1.5%) | 2 (1.0%) | 5 (1.3%) |
| WSAS scoreb | |||||||||
| Mean (SD) | 11.2 (8.2) | 11.5 (7.6) | 11.4 (7.9) | 9.3 (7.6) | 9.5 (6.3) | 9.4 (7.0) | 8.1 (7.6) | 9.4 (7.8) | 8.7 (7.7) |
| Median (range) | 9.0 (0.0, 40.0) | 11.0 (0.0, 40.0) | 10.0 (0.0, 40.0) | 8.0 (0.0, 39.0) | 9.0 (0.0, 28.0) | 8.0 (0.0, 39.0) | 6.0 (0.0, 40.0) | 8.0 (0.0, 39.0) | 7.0 (0.0, 40.0) |
| Missing | 8 | 12 | 20 | 22 | 33 | 55 | 37 | 47 | 84 |
| Mean difference (95% CI), p-value | −0.27 (−1.36 to 0.83), 0.633 | −1.04 (−2.30 to 0.23), 0.108 | |||||||
| PHQ-12 scorec | |||||||||
| Mean (SD) | 6.3 (3.5) | 6.3 (3.6) | 6.3 (3.5) | 5.7 (3.4) | 5.9 (3.2) | 5.8 (3.3) | |||
| Median (range) | 6.0 (0.0, 17.3) | 6.0 (0.0, 18.0) | 6.0 (0.0, 18.0) | 5.2 (0.0, 19.0) | 6.0 (0.0, 16.0) | 5.5 (0.0, 19.0) | |||
| Missing | 4 | 2 | 6 | 30 | 39 | 69 | |||
| Mean difference (95% CI), p-value | −0.04 (−0.58 to 0.49), 0.877 | ||||||||
| 3 months | 6 months | |||||
|---|---|---|---|---|---|---|
| Mean difference (amitriptyline – placebo) | p-value | N | Mean difference (amitriptyline – placebo) | p-value | N | |
| HADS-A | ||||||
| Primary analysis | 0.05 (−0.53, 0.63) | 0.861 | 463 | 0.08 (−0.49, 0.65) | 0.775 | 463 |
| Complete case | 0.07 (−0.50, 0.64) | 0.815 | 432 | −0.07 (−0.66, 0.51) | 0.808 | 396 |
| HADS-D | ||||||
| Primary analysis | −0.22 (−0.71, 0.26) | 0.369 | 463 | −0.20 (−0.75, 0.34) | 0.462 | 463 |
| Complete case | −0.27 (−0.75, 0.21) | 0.264 | 432 | −0.37 (−0.89, 0.15) | 0.161 | 395 |
| WSAS | ||||||
| Primary analysis | −0.27 (−1.36, 0.83) | 0.633 | 463 | −1.04 (−2.30, 0.23) | 0.108 | 463 |
| Complete case | −0.38 (−1.48, 0.72) | 0.499 | 398 | −1.29 (−2.61, 0.02) | 0.054 | 367 |
| PHQ-12 | ||||||
| Primary analysis | −0.04 (−0.58, 0.49) | 0.877 | 463 | |||
| Complete case | −0.22 (−0.73, 0.29) | 0.400 | 392 | |||
Hospital Anxiety and Depression Scale-D scores at 3 and 6 months
An improvement in participants’ HADS-D scores was seen in both groups over time and compared with baseline, with a mean score of 4.3 (SD 3.4) at baseline, 3.5 (SD 3.3) at 3 months, and 4.0 (SD 3.6) at 6 months (Table 20). There was no evidence of a treatment effect on HADS-D scores at 3 or 6 months in primary or sensitivity analysis (see Tables 20 and 21, Appendix 1, Table 54 and Figure 21). Scores were lower, but not statistically significantly different, in the amitriptyline arm by −0.22 (95% CI −0.71 to 0.26; p = 0.369) at 3 months and −0.20 (95% CI −0.75 to 0.34; p = 0.462) at 6 months in primary analysis, with similar but extenuated non-significant effects in complete case analysis.
Ability to work and participate in other activities (work and social adjustment scale scores) at 3 and 6 months
An improvement in participants’ WSAS scores was seen in both groups compared with baseline, with a mean score of 11.4 (SD 7.9) at baseline, 9.4 (SD 7.0) at 3 months, and 8.7 (SD 7.7) at 6 months (Table 20). There was no evidence of a treatment effect on WSAS scores at either 3 or 6 months in primary or sensitivity analysis (see Tables 20 and 21, Appendix 1, Table 55 and Figure 22). Scores were lower, but not statistically significantly different, in the amitriptyline arm by −0.27 (95% CI −1.36 to 0.83; p = 0.369) at 3 months and −1.04 (95% CI −2.30 to 0.23; p = 0.108) at 6 months in primary analysis, with extenuated non-significant effects in complete case analysis.
Irritable bowel syndrome-associated somatic symptom-reporting (patient health questionnaire-12 scores) at 6 months
Again, an improvement in participants’ PHQ-12 scores was observed in both groups compared with baseline, with a mean score of 6.3 (SD 3.5) at baseline and 5.8 (SD 3.3) at 6 months (Table 20). There was no evidence of a treatment effect on PHQ-12 scores at 6 months in primary or sensitivity analysis (see Tables 20 and 21, Appendix 1, Table 56). Scores were lower, but not statistically significantly different, in the amitriptyline arm by −0.04 (95% CI −0.58 to 0.49; p = 0.877) at 6 months in primary analysis, with an extenuated non-significant effect in complete case analysis.
Participant-reported weekly adequate relief of irritable bowel syndrome symptoms
Summary statistics of available data for weekly adequate relief of IBS symptoms up to 26 weeks post randomisation are presented in Table 22 and Appendix 1, Figure 23. In 443 (96%) participants reporting data for at least 1 week, the mean number and proportion of weeks with adequate relief was 10.2 (SD 7.77) weeks and 48.3% (SD 33.6) of weeks in the amitriptyline arm, and 8.0 (7.76) weeks and 38.0% (SD 34.2%) of weeks in the placebo arm.
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
|---|---|---|---|
| Number of weekly questions completed | |||
| Mean (SD) | 19.9 (6.59) | 19.5 (6.91) | 19.7 (6.74) |
| Median (range) | 23.0 (0.0, 25.0) | 23.0 (0.0, 25.0) | 23.0 (0.0, 25.0) |
| IQR | 18.0–24.0 | 17.0–24.0 | 17.0–24.0 |
| Number of weekly questions completed N (%) | |||
| 0 week | 10 (4.3%) | 10 (4.3%) | 20 (4.3%) |
| 1–12 weeks | 24 (10.3%) | 32 (13.9%) | 56 (12.1%) |
| 13–18 weeks | 28 (12.1%) | 22 (9.5%) | 50 (10.8%) |
| 19–24 weeks | 134 (57.8%) | 135 (58.4%) | 269 (58.1%) |
| 25 weeks | 36 (15.5%) | 32 (13.9%) | 68 (14.7%) |
| Number of weeks with adequate relief | |||
| Mean (SD) | 10.2 (7.77) | 8.0 (7.76) | 9.1 (7.84) |
| Median (range) | 11.0 (0.0, 25.0) | 5.0 (0.0, 25.0) | 8.0 (0.0, 25.0) |
| IQR | 3.0–17.0 | 0.0–14.0 | 1.0–16.0 |
| Missinga | 10 | 10 | 20 |
| % of weeks with adequate relief (of 25 weeks)b | |||
| Mean (SD) | 41.0 (31.1) | 32.0 (31.1) | 36.5 (31.4) |
| Median (range) | 44.0 (0.0, 100) | 20.0 (0.0, 100) | 32.0 (0.0, 100) |
| IQR | 12.0–68.0 | 0.0–56.0 | 4.0–64.0 |
| Relief for at least ≥ 13/25 weeksa | |||
| Yes | 90 (40.5%) | 67 (30.3%) | 157 (35.4%) |
| No | 132 (59.5%) | 154 (69.7%) | 286 (64.6%) |
Amitriptyline was superior to placebo, with strong evidence of an increased likelihood of adequate relief with amitriptyline compared with placebo and an overall OR of 1.56 (95% CI 1.20 to 2.03; p < 0.001) across all weeks (see Figure 4, Appendix 1 and Table 57) in repeated-measures analysis. The odds of relief were increased (OR > 1) with amitriptyline compared with placebo at all weeks, with good evidence (p < 0.05) for 12 of 25 weeks (weeks 3, 6, 8–9, 11–16, 23, 24), weak evidence (p < 0.1) for 5 weeks (weeks 10, 17–18, 22, 24), and the effect was not statistically significant for 8 weeks (weeks 1–2, 4–5, 7, 19–21).
Acceptability of trial medication at 6 months
Acceptability of trial medication, based on participants’ 6-month response and prior treatment discontinuation, was available for a total of 424 (91.6%) participants (with similar rates by arm). A higher proportion of participants reported acceptability in the amitriptyline arm compared with placebo (57.8% vs. 46.9%) (Table 23). Conversely a lower proportion of participants reported they did not find the medication acceptable (14.2% vs. 21.6%) and had discontinued treatment before 6 months (21.8% vs. 27.7%) in the amitriptyline arm compared with placebo. Three participants did not start treatment and 18 participants were lost to follow-up before 6 months; these participants were classed as not finding the medication acceptable in the primary analysis.
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
|---|---|---|---|
| Does the participant find the medication acceptable at 6 months? | |||
| Yes | 122 (57.8%) | 100 (46.9%) | 222 (52.4%) |
| Answered ‘Yes’ to the acceptability at 6 months | 122 (57.8%) | 99 (46.5%) | 221 (52.1%) |
| Acceptability missing but remained on treatment > 6 months | 0 (0.0%) | 1 (0.5%) | 1 (0.2%) |
| No | 89 (42.2%) | 113 (53.1%) | 202 (47.6%) |
| Answered ‘No’ to the acceptability at 6 months | 30 (14.2%) | 46 (21.6%) | 76 (17.9%) |
| Discontinued treatment < 6 months | 46 (21.8%) | 59 (27.7%) | 105 (24.8%) |
| Did not start trial medication | 2 (0.9%) | 1 (0.5%) | 3 (0.7%) |
| Lost to follow-up ≤ 6 months | 11 (5.2%) | 6 (2.7%) | 17 (4.0%) |
| Othera | 0 (0.0%) | 1(0.5%) | 1 (0.2%) |
| Missing | 21 | 18 | 39 |
Data were missing at random for 39 participants due to an administrative error in one site where acceptability was not asked of participants recruited to the reduced 6-month follow-up. As all participants’ missing data were from the same hub and still on treatment at 6 months, multiple imputation was performed by allocation within participants on treatment from the missing hub only.
Amitriptyline was superior to placebo in the ITT analysis, with good evidence (p < 0.05) of an increased odds of acceptability with amitriptyline compared with placebo and an adjusted OR of 1.60 (95% CI 1.08 to 2.35; p = 0.018) (Table 24). Similar results were obtained in sensitivity analysis.
| Adjusted OR (amitriptyline vs. placebo) | p-value | N | |
|---|---|---|---|
| Primary analysis | 1.60 (1.08, 2.35) | 0.018 | 463 |
| Sensitivity analysis | |||
| Complete case | 1.59 (1.08, 2.35) | 0.020 | 424 |
| Complete case (excluding n = 21 lost to follow-up or did not start treatment) | 1.74 (1.16, 2.60) | 0.007 | 403 |
Adherence
The number and proportion of participants with the highest level of adherence to therapy (taking one tablet every day or nearly every day) decreased over time and was reported by 416/447 (93.1%) participants at week 3, 364/452 (80.5%) at 3 months and 309/460 (67.2%) at 6 months (Table 25, excluding participants on treatment with missing data). The majority of the remaining participants were classified according to the lowest level of adherence, having discontinued or not started trial medication, or having been lost to follow-up. Rates were similar across trial arms at 3 weeks and 3 months. However, by 6 months a higher proportion of participants in the amitriptyline arm reported the highest level of adherence compared with placebo, 163 (70.3%) versus 146 (64.0%) participants respectively; and a slightly lower proportion of participants had the lowest level of adherence (25.4% vs. 28.9%, respectively).
| Has the participant taken at least one tablet daily | Week 3 | Month 3 | Month 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | |
| Every or nearly every day | 212 (93.8%) | 204 (92.3%) | 416 (93.1%) | 185 (79.7%) | 179 (81.4%) | 364 (80.5%) | 163 (70.3%) | 146 (64.0%) | 309 (67.2%) |
| ≥ half the days | 4 (1.8%) | 6 (2.7%) | 10 (2.2%) | 8 (3.4%) | 4 (1.8%) | 12 (2.7%) | 9 (3.9%) | 9 (3.9%) | 18 (3.9%) |
| < half of the days | 0 (0.0%) | 2 (0.9%) | 2 (0.4%) | 0 (0.0%) | 2 (0.9%) | 2 (0.4%) | 1 (0.4%) | 4 (1.8%) | 5 (1.1%) |
| None or nearly none of the days | 0 (0.0%) | 1 (0.5%) | 1 (0.2%) | 1 (0.4%) | 0 (0.0%) | 1 (0.2%) | 0 (0.0%) | 3 (1.3%) | 3 (0.7%) |
| Discontinued, did not start treatment, or lost to follow-up | 10 (4.4%) | 8 (3.6%) | 18 (4.0%) | 38 (16.4%) | 35 (15.9%) | 73 (16.2%) | 59 (25.4%) | 66 (28.9%) | 125 (27.2%) |
| Missing | 6 | 10 | 16 | 0 | 11 | 11 | 0 | 3 | 3 |
Due to the large proportion of participants reporting the highest and lowest levels of adherence (every day or nearly every day through to discontinued, did not start treatment, or lost to follow-up), and small numbers of participants reporting adherence within these extremes, the proportional hazards assumption was not met, and only descriptive analyses are presented.
Tolerability at 3 and 6 months
An overall summary of 21 possible treatment-emergent AEs self-reported by participants at 3 and 6 months, as captured by the ASEC for participants in the safety population and still on treatment, are reported in Table 26.
| Month 3 | Month 6 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 231) | Placebo (n = 229) | Total (n = 460) | Amitriptyline (n = 232) | Placebo (n = 228) | Total (n = 460) | |
| N on treatment/unknownb | 194 (84.0%) | 196 (85.6%) | 390 (84.8%) | 174 (75.0%) | 164 (71.9%) | 338 (73.5%) |
| N on treatment and ASEC completed | 193 (83.5%) | 192 (83.8%) | 385 (83.7%) | 166 (71.6%) | 152 (66.7%) | 318 (69.1%) |
| Total score | ||||||
| Mean (SD) | 9.9 (6.00) | 8.4 (5.67) | 9.2 (5.88) | 9.3 (6.07) | 8.7 (6.19) | 9.0 (6.13) |
| Median (range) | 9.0 (0.0, 32.0) | 8.0 (0.0, 33.0) | 8.0 (0.0, 33.0) | 8.0 (0.0, 30.0) | 7.0 (0.0, 28.0) | 7.0 (0.0, 30.0) |
| IQR | 6.0–13.0 | 4.0–12.0 | 5.0–12.0 | 5.0–12.0 | 4.0–12.0 | 4.0–12.0 |
| N | 193 | 192 | 385 | 166 | 152 | 318 |
| Mean differencec (95% CI) | 1.39 (0.29 to 2.50) | 0.26 (−0.98 to 1.51) | ||||
| p-value | 0.013 | 0.681 | ||||
| ASEC symptoms | ||||||
| No symptoms | 5 (2.6%) | 7 (3.6%) | 12 (3.1%) | 2 (1.2%) | 3 (2.0%) | 5 (1.6%) |
| ≥ 1 mild-severe symptom | 188 (97.4%) | 185 (96.4%) | 373 (96.9%) | 164 (98.8%) | 149 (98.0%) | 313 (98.4%) |
| ≥ 1 moderate – severe symptom | 156 (80.8%) | 154 (80.2%) | 310 (80.5%) | 127 (76.5%) | 113 (74.3%) | 240 (75.5%) |
| ≥ 1 severe symptom | 58 (30.1%) | 46 (24.0%) | 104 (27.0%) | 45 (27.1%) | 37 (24.3%) | 82 (25.8%) |
| N mild-severe symptoms | ||||||
| Mean (SD) | 6.8 (3.74) | 5.8 (3.56) | 6.3 (3.68) | 6.5 (3.57) | 6.0 (3.65) | 6.3 (3.61) |
| Median (range) | 6.0 (0.0, 19.0) | 5.0 (0.0, 19.0) | 6.0 (0.0, 19.0) | 6.0 (0.0, 17.0) | 5.0 (0.0, 17.0) | 6.0 (0.0, 17.0) |
| IQR | 4.0–8.0 | 3.0–8.0 | 4.0–8.0 | 4.0–9.0 | 3.0–8.0 | 3.0–9.0 |
| N | 193 | 192 | 385 | 166 | 152 | 318 |
Of 385 (83.7%) and 318 (69.1%) participants on treatment (or treatment status unknown) and completing the ASEC at 3 and 6 months respectively, most participants reported at least one mild to severe side effect (> 95%), and 310 (80.5%) participants at 3 months and 440 (75.5%) participants at 6 months reported at least one moderate to severe side effect, with similar rates across trial arms. A slightly greater proportion of participants reported at least one severe side effect with amitriptyline compared with placebo at both 3 [58 (30.1%) amitriptyline; 46 (24.0%) placebo] and 6 months [45 (27.1%) amitriptyline; 37 (24.3%) placebo]. The total ASEC score (ranging from 0 to 63), which quantifies both the number and severity of symptoms reported, was slightly higher in the amitriptyline arm compared with placebo, with an overall mean of 9.2 (SD 5.88) at 3 months and 9.0 (SD 6.13) at 6 months. In adjusted complete case analysis, there was a statistically significant increase in the total ASEC score with amitriptyline compared with placebo at 3 months (1.39, 95% CI 0.29 to 2.50; p = 0.013) but not at 6 months (0.26, 95% CI −0.98 to 1.51; p = 0.681). Further details of the types of AEs can be found in Figures 5 and 6, Appendix 1, Table 58. Adverse events occurred more frequently in the amitriptyline arm compared with placebo, related mainly to its known anticholinergic effects, including dry mouth, drowsiness, blurred vision, and problems with urination. However, rates fell generally between 3 and 6 months and few (< 5%) were severe, except for constipation and diarrhoea (< 10%).
Further 12-month treatment delivery and secondary end points
Treatment receipt, adherence, dosage and titration
Of the 291 participants in the 12-month ITT population, 128 (44%) completed 12 months treatment; 67 (45.6%) of 147 allocated to amitriptyline, and 61 (42.4%) of 144 allocated to placebo (Table 27). The median time from randomisation to the end-of-trial treatment was 10.4 months, with a longer duration observed in the amitriptyline arm compared with placebo (median 11.5 vs. 8.3 months). Similar to all randomised participants, just under a quarter (24.4%) discontinued trial medication before 6 months; 33 (22.4%) of 147 allocated to amitriptyline, and 38 (26.4%) of 144 allocated to placebo. At the 6-month follow-up time point, participants were given the choice to continue treatment or not; of 208 participants who were still on treatment at 6 months, 91 (85.8%) of 106 and 81 (79.4%) of 102 participants chose to continue treatment in the amitriptyline and placebo arms, respectively, with 15 (14.2%) participants in the amitriptyline arm and 21 (20.6%) in the placebo arm choosing to stop treatment. Of the 172 participants who continued treatment beyond 6 months, a further 16 (16.5%) of 91 in the amitriptyline arm, and 11 (13.6%) of 81 in the placebo arm discontinued treatment before the full 12-month period. The most common reason for treatment discontinuation after 6 months was lack of benefit in 3 (2.0%) of 147 and 6 (4.2%) of 144 participants allocated to amitriptyline and placebo respectively, followed by non-specific or personal choice in 5 (3.4%) amitriptyline and 3 (2.1%) placebo participants, and side effects in 4 (2.7%) amitriptyline and 1 (< 1%) placebo participant.
| Amitriptyline (n = 147) | Placebo (n = 144) | Total (n = 291) | |
|---|---|---|---|
| Participant continued trial medication beyond 6 months | |||
| Yes | 91 (61.9%) | 81 (56.3%) | 172 (59.1%) |
| No | 15 (10.2%) | 21 (14.6%) | 36 (12.4%) |
| Did not start/discontinued/lost to follow-up < 6 months | 41 (27.9%) | 42 (29.2%) | 83 (28.5%) |
| Completed 12 months’ treatment | |||
| Yes | 67 (45.6%) | 61 (42.4%) | 128 (44.0%) |
| Discontinued trial medication | 64 (43.5%) | 70 (48.6%) | 134 (46.0%) |
| Before 6 months | 33 (22.4%) | 38 (26.4%) | 71 (24.4%) |
| Chose not to continue treatment beyond 6 months | 15 (10.2%) | 21 (14.6%) | 36 (12.4%) |
| After 6 months | 16 (10.9%) | 11 (7.6%) | 27 (9.3%) |
| Lost to follow-up | 14 (9.5%) | 12 (8.3%) | 26 (8.9%) |
| Before 6 months | 6 (4.1%) | 3 (2.1%) | 9 (3.1%) |
| After 6 months | 8 (5.4%) | 9 (6.3%) | 17 (5.8%) |
| Not started treatment | 2 (1.4%) | 1 (0.7%) | 3 (1.0%) |
| Reason for discontinuation > 6 months | |||
| Lack of benefit | 3 (2.0%) | 6 (4.2%) | 9 (3.1%) |
| Non-specific or personal choice | 5 (3.4%) | 3 (2.1%) | 8 (2.7%) |
| Side effecta | 4 (2.7%) | 1 (0.7%) | 5 (1.7%) |
| Administrative error | 1 (0.7%) | 1 (0.7%) | 2 (0.7%) |
| SAE or SAR | 1 (0.7%) | 0 (0.0%) | 1 (0.3%) |
| Safety (including allergic reactions to IMP) | 1 (0.7%) | 0 (0.0%) | 1 (0.3%) |
| Other reasonb | 1 (0.7%) | 0 (0.0%) | 1 (0.3%) |
| Months to last dose (from randomisation) | |||
| Mean (SD) | 8.5 (4.16) | 8.0 (4.14) | 8.3 (4.15) |
| Median (range) | 11.5 (0.2, 14.0) | 8.3 (0.2, 12.2) | 10.4 (0.2, 14.0) |
| Missing | 16 | 13 | 29 |
| n | 131 | 131 | 262 |
| Months to last dose (from randomisation) | |||
| ≤ 1 month | 9 (6.9%) | 8 (6.1%) | 17 (6.5%) |
| ≤ 2 months | 5 (3.8%) | 6 (4.6%) | 11 (4.2%) |
| ≤ 3 months | 8 (6.1%) | 6 (4.6%) | 14 (5.3%) |
| ≤ 4 months | 1 (0.8%) | 7 (5.3%) | 8 (3.1%) |
| ≤ 5 months | 7 (5.3%) | 6 (4.6%) | 13 (5.0%) |
| ≤ 6 months | 15 (11.5%) | 25 (19.1%) | 40 (15.3%) |
| ≤ 7 months | 6 (4.6%) | 5 (3.8%) | 11 (4.2%) |
| ≤ 8 months | 2 (1.5%) | 2 (1.5%) | 4 (1.5%) |
| ≤ 9 months | 6 (4.6%) | 2 (1.5%) | 8 (3.1%) |
| ≤ 10 months | 2 (1.5%) | 1 (0.8%) | 3 (1.1%) |
| ≤ 11 months | 2 (1.5%) | 1 (0.8%) | 3 (1.1%) |
| ≤ 12 months | 37 (28.2%) | 43 (32.8%) | 80 (30.5%) |
| > 12 months | 31 (23.7%) | 19 (14.5%) | 50 (19.1%) |
| Total | 131 (100%) | 131 (100%) | 262 (100%) |
Over 90% of participants on trial medication continued to report the highest level of treatment adherence beyond 6 months at month 9 and 12, taking at least one tablet daily ‘every day or nearly every day’ with similar rates across trial arms (Table 28 and Appendix 1, Figure 14). In the amitriptyline arm, the proportion of participants on each dose remained similar at 6, 9 and 12 months, with just under half of participants on the highest dose of 30 mg (see Table 28 and Appendix 1, Figure 15). In contrast, in the placebo arm, 55 (57.9%) participants were on 30 mg at 6 months, 47 (69.1%) at 9 months and 40 (65.6%) at 12 months. Relatively few participants changed their dose after 6 months. A total of 17 (12.4%) changed dose between 6 and 9 months; rates were similar across arms. However, participants in the amitriptyline arm were more likely to lower their dose, whereas those on placebo were more likely to increase their dose. Only 12 (9.5%) participants remaining on treatment changed dose between and 9 and 12 months post randomisation, with a higher number in the amitriptyline arm.
| Month 6 | Month 9 | Month 12 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 106) | Placebo (n = 102) | Total (n = 208)a | Amitriptyline (n = 74) | Placebo (n = 69) | Total (n = 143) | Amitriptyline (n = 67) | Placebo (n = 61) | Total (n = 128) | |
| Has the participant taken at least one tablet daily? | |||||||||
| Every or nearly every day | 99 (93.4%) | 89 (89.9%) | 188 (91.7%) | 65 (92.9%) | 64 (94.1%) | 129 (93.5%) | 61 (91.0%) | 57 (93.4%) | 118 (92.2%) |
| ≥ half the days | 6 (5.7%) | 5 (5.1%) | 11 (5.4%) | 5 (7.1%) | 4 (5.9%) | 9 (6.5%) | 5 (7.5%) | 3 (4.9%) | 8 (6.3%) |
| < half of the days | 1 (0.9%) | 3 (3.0%) | 4 (2.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.6%) | 1 (0.8%) |
| None or nearly none of the days | 0 (0.0%) | 2 (2.0%) | 2 (1.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.5%) | 0 (0.0%) | 1 (0.8%) |
| Missing | 0 (0.0%) | 3 | 3 | 4 | 1 | 5 | |||
| Current dose of trial medication | |||||||||
| 1 × 10 mg every other day | 4 (3.8%) | 4 (4.2%) | 8 (4.0%) | 2 (2.8%) | 1 (1.5%) | 3 (2.2%) | 2 (3.0%) | 1 (1.6%) | 3 (2.3%) |
| 1 × 10 mg daily | 26 (24.5%) | 10 (10.5%) | 36 (17.9%) | 17 (23.9%) | 5 (7.4%) | 22 (15.8%) | 15 (22.4%) | 5 (8.2%) | 20 (15.6%) |
| 2 × 10 mg daily | 30 (28.3%) | 26 (27.4%) | 56 (27.9%) | 20 (28.2%) | 15 (22.1%) | 35 (25.2%) | 20 (29.9%) | 15 (24.6%) | 35 (27.3%) |
| 3 × 10 mg daily | 46 (43.4%) | 55 (57.9%) | 101 (50.2%) | 32 (45.1%) | 47 (69.1%) | 79 (56.8%) | 30 (44.8%) | 40 (65.6%) | 70 (54.7%) |
| Missing | 0 | 7 | 7 | 3 | 1 | 4 | |||
| Dose modification since last follow-up call | |||||||||
| Yes | 24 (22.9%) | 13 (14.1%) | 37 (18.8%) | 9 (12.7%) | 8 (12.1%) | 17 (12.4%) | 8 (12.3%) | 4 (6.6%) | 12 (9.5%) |
| Higher dose | 13 (54.2%) | 7 (53.8%) | 20 (54.1%) | 3 (33.3%) | 7 (87.5%) | 10 (58.8%) | 5 (62.5%) | 2 (50.0%) | 7 (58.3%) |
| Lower dose | 11 (45.8%) | 6 (46.2%) | 17 (45.9%) | 6 (66.7%) | 1 (12.5%) | 7 (41.2%) | 3 (37.5%) | 2 (50.0%) | 5 (41.7%) |
| No | 81 (77.1%) | 79 (85.9%) | 160 (81.2%) | 62 (87.3%) | 58 (87.9%) | 120 (87.6%) | 57 (87.7%) | 57 (93.4%) | 114 (90.5%) |
| Missing | 1 | 10 | 11 | 4 | 15 | 19 | 1 | 13 | 14 |
No participants in the 12-month ITT population who discontinued treatment after 6 months reported taking amitriptyline off-trial after treatment discontinuation.
Secondary end points
Global symptoms of irritable bowel syndrome (irritable bowel syndrome Severity Scoring System) at 12 months
The total IBS-SSS score was available at 12 months for 225 (77.3%) participants [118 (80.3%) on amitriptyline; 107 (74.3%) on placebo]. The mean 12-month total IBS-SSS scores were 160.7 (SD 113.7) and 176.7 (SD 107.2) in participants allocated to amitriptyline and placebo, respectively (Table 29). A higher proportion of participants had remission of IBS symptoms on the IBS-SSS (IBS-SSS score < 75 points) at 12 months in the amitriptyline arm compared with placebo (28.8% vs. 20.6%). There was weak evidence (p < 0.10) of a reduced total IBS-SSS score with amitriptyline compared with placebo, with an estimated adjusted mean difference of −22.59 (95% CI −49.35 to 4.16; p = 0.098) in 12-month ITT analysis. Similar results were obtained in sensitivity analysis (Table 30 and Appendix 1, Table 59).
| Baseline | Month 12 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 147) | Placebo (n = 144) | Total (n = 291) | |
| Total IBS-SSS score | ||||||
| Mean (SD) | 273.4 (90.53) | 273.4 (90.53) | 273.4 (90.53) | 160.7 (113.69) | 176.7 (107.23) | 168.3 (110.71) |
| Median (range) | 280.0 (60, 480) | 280.0 (60, 480) | 280.0 (60, 480) | 145.0 (0, 440) | 170.0 (0, 390) | 160.0 (0, 440) |
| IQR | 210.0–330.0 | 210.0–330.0 | 210.0–330.0 | 70.0–240.0 | 90.0–270.0 | 80.0–250.0 |
| Missing | 0 | 0 | 0 | 29 | 37 | 66 |
| Mean difference (95% CI), p-valuea | −22.59 (−49.35 to 4.16), 0.098 | |||||
| IBS-SSS level | ||||||
| < 75 (remission) | 3 (1.3%) | 3 (1.3%) | 3 (1.3%) | 34 (28.8%) | 22 (20.6%) | 56 (24.9%) |
| 75–174 (mild) | 37 (15.9%) | 37 (15.9%) | 37 (15.9%) | 36 (30.5%) | 34 (31.8%) | 70 (31.1%) |
| 175–299 (moderate) | 98 (42.2%) | 98 (42.2%) | 98 (42.2%) | 34 (28.8%) | 33 (30.8%) | 67 (29.8%) |
| 300–500 (severe) | 94 (40.5%) | 94 (40.5%) | 94 (40.5%) | 14 (11.9%) | 18 (16.8%) | 32 (14.2%) |
| Difference in IBS-SSS score from baseline | ||||||
| Mean (SD) | −109.2 (117.58) | −80.4 (98.86) | −95.5 (109.80) | |||
| Median (range) | −110.0 (−370.0, 280.0) | −70.0 (−410.0, 110.0) | −90.0 (−410.0, 280.0) | |||
| IQR | −200.0 to −30.0 | −140.0 to 0.0 | −170.0 to −20.0 | |||
| ≥ 50 point reduction in total IBS-SSS score from baseline | ||||||
| Yes | 84 (71.2%) | 65 (60.7%) | 149 (66.2%) | |||
| No | 34 (28.8%) | 42 (39.3%) | 76 (33.8%) | |||
| ≥ 30% reduction in abdominal pain severity from baseline (item 1b) | ||||||
| Yes | 65 (55.1%) | 49 (45.8%) | 114 (50.7%) | |||
| No | 53 (44.9%) | 58 (54.2%) | 111 (49.3%) | |||
| ≥ 30% reduction in abdominal distention severity from baseline (item 3b) | ||||||
| Yes | 58 (49.2%) | 45 (42.1%) | 103 (45.8%) | |||
| No | 60 (50.8%) | 62 (57.9%) | 122 (54.2%) | |||
| SGA of relief of IBS symptomsb | ||||||
| 1 – Completely relieved | 6 (5.1%) | 4 (3.7%) | 10 (4.4%) | |||
| 2 – Considerably relieved | 36 (30.5%) | 26 (24.3%) | 62 (27.6%) | |||
| 3 – Somewhat relieved | 28 (23.7%) | 20 (18.7%) | 48 (21.3%) | |||
| 4 – Unchanged | 47 (39.8%) | 53 (49.5%) | 100 (44.4%) | |||
| 5 – Worse | 1 (0.8%) | 4 (3.7%) | 5 (2.2%) | |||
| Missing | 29 | 37 | 66 | |||
| Responder (response 1–3) | ||||||
| Yes | 70 (59.3%) | 50 (46.7%) | 120 (53.3%) | |||
| Odds ratio (95% CI), p-valuec | 1.58 (0.94 to 2.64), 0.083 | |||||
Subjective global assessment of relief of irritable bowel syndrome symptoms at 12 months
A higher proportion of participants reported IBS symptoms as being completely, considerably, or somewhat relieved in the amitriptyline arm compared with placebo, with response rates of 59.3% versus 46.7% at 12 months (Table 29). There was weak evidence of an increased odds of response for SGA of relief of IBS symptoms with amitriptyline compared with placebo, with an OR of 1.58 (95% CI 0.94 to 2.64; p = 0.083, n = 291) (Table 30) in 12-month ITT analysis and good evidence in sensitivity analysis (OR 1.73, 95% CI 1.01 to 2.95; p = 0.046, n = 225) (Table 30 and Appendix 1, Table 60).
| Mean difference (amitriptyline – placebo) | p-value | N | |
|---|---|---|---|
| IBS-SSS | |||
| Primary analysis | −22.59 (−49.35, 4.16) | 0.098 | 291 |
| Complete case | −24.34 (−50.49, 1.81) | 0.068 | 225 |
| Anxiety (HADS-A) | |||
| Primary analysis | −0.38 (−1.22, 0.47) | 0.385 | 291 |
| Complete case | −0.18 (−1.02, 0.65) | 0.662 | 224 |
| Depression (HADS-D) | |||
| Primary analysis | −0.88 (−1.71, −0.06) | 0.036 | 291 |
| Complete case | −0.85 (−1.63, −0.07) | 0.032 | 224 |
| Ability to work and participate in activities (WSAS) | |||
| Primary analysis | −2.14 (−3.80, −0.49) | 0.011 | 291 |
| Complete case | −1.70 (−3.24, −0.15) | 0.031 | 202 |
| Odds ratio (amitriptyline – placebo) | p -value | N | |
| SGA | |||
| Primary analysis | 1.58 (0.94, 2.64) | 0.083 | 291 |
| Complete case | 1.73 (1.01, 2.95) | 0.046 | 225 |
Hospital Anxiety and Depression Scale-A scores at 12 months
As observed at 3 and 6 months, an improvement in participants’ HADS-A scores was seen in both groups at 12 months compared with baseline. However, although scores were lower in the amitriptyline compared with the placebo arm there was no evidence of an effect between treatment arms in 12-month ITT analysis (−0.38, 95% CI −1.22 to 0.47; p = 0.385) or sensitivity analysis (Tables 30 and 31).
| Baseline | Month 12 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 147) | Placebo (n = 144) | Total (n = 291) | |
| HADS-A score | ||||||
| Mean (SD) | 7.3 (4.3) | 7.7 (4.3) | 7.5 (4.3) | 6.9 (4.6) | 7.3 (4.1) | 7.1 (4.4) |
| Median (range) | 7.0 (0.0, 21.0) | 7.0 (0.0, 20.0) | 7.0 (0.0, 21.0) | 6.0 (0.0, 21.0) | 7.0 (0.0, 18.0) | 7.0 (0.0, 21.0) |
| Missing | 0 | 0 | 0 | 30 | 37 | 67 |
| Mean difference (95% CI), p-value | −0.38 (−1.22 to 0.47), 0.385 | |||||
| Anxiety level | ||||||
| 0–7 (normal) | 126 (54.3%) | 119 (51.5%) | 245 (52.9%) | 72 (61.5%) | 56 (52.3%) | 128 (57.1%) |
| 8–10 (mild anxiety) | 55 (23.7%) | 58 (25.1%) | 113 (24.4%) | 21 (17.9%) | 28 (26.2%) | 49 (21.9%) |
| 11–14 (moderate anxiety) | 39 (16.8%) | 36 (15.6%) | 75 (16.2%) | 14 (12.0%) | 19 (17.8%) | 33 (14.7%) |
| 15–21 (severe anxiety) | 12 (5.2%) | 18 (7.8%) | 30 (6.5%) | 10 (8.5%) | 4 (3.7%) | 14 (6.3%) |
| HADS-D score | ||||||
| Mean (SD) | 4.4 (3.6) | 4.1 (3.2) | 4.3 (3.4) | 3.8 (3.5) | 4.6 (3.8) | 4.2 (3.7) |
| Median (range) | 4.0 (0.0, 18.0) | 4.0 (0.0, 15.0) | 4.0 (0.0, 18.0) | 3.0 (0.0, 18.0) | 4.0 (0.0, 17.0) | 3.0 (0.0, 18.0) |
| Missing | 0 | 0 | 0 | 30 | 37 | 67 |
| Mean difference (95% CI), p-value | −0.88 (−1.71 to −0.06), 0.036 | |||||
| Depression level | ||||||
| 0–7 (Normal) | 195 (84.1%) | 195 (84.4%) | 390 (84.2%) | 103 (88.0%) | 87 (81.3%) | 190 (84.8%) |
| 8–10 (Mild depression) | 23 (9.9%) | 24 (10.4%) | 47 (10.2%) | 6 (5.1%) | 13 (12.1%) | 19 (8.5%) |
| 11–14 (Moderate depression) | 11 (4.7%) | 11 (4.8%) | 22 (4.8%) | 6 (5.1%) | 5 (4.7%) | 11 (4.9%) |
| 15–21 (Severe depression) | 3 (1.3%) | 1 (0.4%) | 4 (0.9%) | 2 (1.7%) | 2 (1.9%) | 4 (1.8%) |
| WSAS scoreb | ||||||
| Mean (SD) | 11.2 (8.2) | 11.5 (7.6) | 11.4 (7.9) | 7.9 (7.0) | 10.1 (7.2) | 8.9 (7.2) |
| Median (range) | 9.0 (0.0, 40.0) | 11.0 (0.0, 40.0) | 10.0 (0.0, 40.0) | 6.0 (0.0, 34.0) | 10.0 (0.0, 35.0) | 7.0 (0.0, 35.0) |
| Missing | 8 | 12 | 20 | 37 | 41 | 78 |
| Mean difference (95% CI), p-value | −2.14 (−3.80 to −0.49), 0.011 | |||||
Hospital Anxiety and Depression Scale-D scores at 12 months
Despite no evidence of a treatment effect on participants’ HADS-D at 3 months and 6 months, amitriptyline was found to be superior to placebo at 12 months, with a significant difference in mean HADS-D scores between arms in 12-month ITT analysis (−0.88, 95% CI −1.71 to −0.06; p = 0.036) (Table 31). Similar results were obtained in sensitivity analysis (Table 30).
Ability to work and participate in other activities (Work and Social Adjustment Scale scores) at 12 months
Amitriptyline was also superior to placebo in terms of ability to work or participate in other activities at 12 months according to the WSAS, with a significant difference in mean WSAS score between arms in 12-month ITT analysis (−2.14, 95% CI; −3.80 to −0.49; p = 0.011) (Table 31). Similar results were obtained in sensitivity analysis (Table 30).
Tolerability at 12 months
Of 128 (44.4%) participants on treatment (or treatment status unknown) and completing the ASEC at 12 months, most participants reported at least one mild to severe side effect (95.7%) at 12 months, with similar rates across trial arms (Table 32). Although the total ASEC score was higher with amitriptyline compared with placebo, there was no evidence of an effect between treatment arms in 12-month safety analysis (−1.04, 95% CI −3.32 to 1.24; p = 0.368, n = 117). Further details of the types of AEs can be found in Figure 7 and Appendix 1, Table 58.
| Amitriptyline (n = 146) | Placebo (n = 142) | Total (n = 288) | |
|---|---|---|---|
| Number on treatmentb | 67 (45.9%) | 61 (43.0%) | 128 (44.4%) |
| Number on treatment and completed the ASEC | 61 (41.8%) | 56 (39.4%) | 117 (40.6%) |
| Total score | |||
| Mean (SD) | 8.6 (5.65) | 9.6 (7.41) | 9.0 (6.54) |
| Median (range) | 9.0 (0.0, 24.0) | 8.0 (0.0, 36.0) | 8.0 (0.0, 36.0) |
| IQR | 4.0–12.0 | 4.0–13.0 | 4.0–13.0 |
| N | 61 | 56 | 117 |
| Mean difference (95% CI), p-valuec | −1.04 (−3.32 to 1.24), 0.368 | ||
| ASEC symptoms | |||
| No symptoms | 2 (3.3%) | 3 (5.4%) | 5 (4.3%) |
| ≥ 1 mild-severe symptom | 59 (96.7%) | 53 (94.6%) | 112 (95.7%) |
| ≥ 1 moderate – severe symptom | 41 (67.2%) | 40 (71.4%) | 81 (69.2%) |
| ≥ 1 severe symptom | 16 (26.2%) | 15 (26.8%) | 31 (26.5%) |
| Total number of mild-severe symptoms | |||
| Mean (SD) | 6.0 (3.56) | 6.6 (4.15) | 6.3 (3.85) |
| Median (range) | 6.0 (0.0, 15.0) | 6.5 (0.0, 19.0) | 6.0 (0.0, 19.0) |
| N | 61 | 56 | 117 |
Exploratory analysis
50-point reduction in irritable bowel syndrome Severity Scoring System at 3 and 6 months
Compared with baseline, there was a mean reduction in the total IBS-SSS score of 99.8 (SD 107.7) and 76.1 (SD 107.1) in participants allocated to amitriptyline and placebo, respectively at 3 months, and a mean reduction of 99.2 (SD 112.9) and 68.9 (SD 109.3) at 6 months. A higher proportion of participants had a reduction of ≥ 50 points in the amitriptyline arm compared with placebo at both 3 (68.0% vs. 59.2%) and 6 months (64.2% vs. 53.8%) (Table 15). Adjusted analysis found weak evidence of an association with treatment, such that participants in the amitriptyline arm were 1.49 times (95% CI 0.97 to 2.28; p = 0.068) more likely to have a ≥ 50-point reduction than those in the placebo arm at 3 months and 1.48 times (95% CI 0.97 to 2.2; p = 0.068) more likely at 6 months (see Appendix 1, Table 61).
Thirty per cent reduction in 6-month irritable bowel syndrome Severity Scoring System abdominal pain and distention
A higher proportion of participants reported a ≥ 30% reduction on individual abdominal pain and abdominal distention severity items (items 1b and 3b) in the amitriptyline arm compared with placebo at both 3 and 6 months (Table 15). With amitriptyline versus placebo, 52.3% versus 46.9% at 3 months, and 55.7% versus 42.6% at 6 months reported a ≥ 30% reduction in abdominal pain, and 45.7% versus 40.4% at 3 months and 46.6% versus 37.6% at 6 months reported a ≥ 30% reduction in abdominal distention.
Adjusted analysis found good evidence of an increased likelihood of a ≥ 30% reduction in abdominal pain severity with amitriptyline compared with placebo (OR 1.66, 95% CI 1.12 to 2.46; p = 0.012) at 6 months, but no evidence of a statistically significant effect at 3 months. There was no evidence of an effect on abdominal distention at 3 or 6 months. Full analysis model effects are presented in Appendix 1, Tables 62 and 63.
Moderator analysis of 6-month irritable bowel syndrome Severity Scoring System score
Moderator analysis found no evidence of a moderating effect of recruitment hub, IBS subtype, baseline IBS-SSS, HADS-A scores or HADS-D scores on the primary 6-month treatment effect on the primary outcome (Table 33 and Appendix 1, Table 64). Although no statistically significant interaction effects were observed, larger treatment effects, in favour of amitriptyline, were observed in participants:
| Moderator | Primary analysis | Complete case | ||||
|---|---|---|---|---|---|---|
| Mean difference (amitriptyline vs. placebo) | Interaction estimate (95% CI) | Interaction, p-value | Mean difference (amitriptyline vs. placebo) | Interaction estimate (95% CI) | Interaction, p-value | |
| Recruitment hub | 0.457 | |||||
| Wessex | −21.95 (−52.77, 8.86) | −26.89 (−57.69, 3.90) | ||||
| West Yorkshire | −48.13 (−96.39, 0.13) | −26.17 (−84.71 to 32.36) | 0.380 | −59.13 (−108.14, −10.11) | −42.4 (−83.3 to −1.62) | |
| West of England | −22.28 (−53.52, 8.97) | −0.32 (−44.29 to 43.64) | 0.989 | −23.40 (−54.88, 8.08) | 8.08 (−22.8 to 39.0) | |
| IBS subtype | 0.246 | |||||
| IBS-M or IBS-U | −7.98 (−38.13, 22.16) | −12.07 (−42.31, 18.17) | ||||
| IBS-C | −50.24 (−99.14, −1.34) | −42.26 (−99.56 to 15.04) | 0.148 | −54.20 (−103.53, −4.88) | −42.13 (−100.14 to 15.88) | 0.154 |
| IBS-D | −38.66 (−69.70, −7.61) | −30.67 (−74.15 to 12.81) | 0.167 | −41.89 (−73.73, −10.04) | 29.82 (−73.72 to 14.09) | 0.183 |
| Baseline IBS-SSS score | −0.14 (−0.37 to 0.09) | 0.236 | −0.14 (−0.369 to 0.091) | 0.235 | ||
| Lower quartile = 210 | −18.23 (−41.77, 5.31) | |||||
| Median = 280 | −28.01 (−48.11, −7.91) | |||||
| Upper quartile = 330 | −35.00 (−59.87, −10.13) | |||||
| Baseline HADS-A score | 1.86 (−2.91 to 6.64) | 0.444 | 2.43 (−2.30 to 7.16) | 0.313 | ||
| Lower quartile = 4.0 | −31.64 (−57.06, −6.22) | |||||
| Median = 7.0 | −26.05 (−45.89, −6.21) | |||||
| Upper quartile = 10.0 | −20.45 (−43.95, 3.04) | |||||
| Baseline HADS-D score | −1.69 (−7.68 to 4.31) | 0.581 | −0.86 (−6.84 to 5.11) | 0.776 | ||
| Lower quartile = 1.0 | −21.51 (−48.74, 5.71) | |||||
| Median = 4.0 | −26.57 (−46.47, −6.68) | |||||
| Upper quartile = 7.0 | −31.63 (−58.06, −5.21) | |||||
-
from West Yorkshire compared with Wessex and West of England (see Appendix 1, Figures 24 and 25)
-
with IBS-C or IBS-D compared with those with IBS-M or IBS-U (see Appendix 1, Figures 26 and 27)
-
with higher baseline IBS-SSS scores (see Appendix 1, Figure 28)
-
with lower HADS-A scores (see Appendix 1, Figure 29)
-
with higher baseline HADS-D scores (see Appendix 1, Figure 30).
Moderator analysis of 6-month subjective global assessment of relief of irritable bowel syndrome symptoms
Moderator analysis found no evidence of a moderating effect of IBS subtype on the 6-month treatment effect on the key secondary outcome SGA of relief of IBS symptoms (Tables 34 and 35). Although no statistically significant interaction was observed, larger treatment effects, in favour of amitriptyline, were observed in participants with IBS-D compared with those with IBS-C and IBS-M or IBS-U (see Appendix 1, Figures 31 and 32).
| Primary analysis | Complete case analysis | ||||
|---|---|---|---|---|---|
| Subgroup effect, OR (95% CI) (amitriptyline vs. placebo) | Interaction, OR (95% CI) | Interaction, p-value | Subgroup effect, OR (95% CI) (amitriptyline vs. placebo) | Interaction, p-value | |
| IBS subtype (amitriptyline vs. placebo) | |||||
| IBS-M or IBS-U | 1.50 (0.83 to 2.73) | 1.71 (0.93 to 3.14) | 0.604 | ||
| IBS-C | 1.56 (0.58 to 4.20) | 1.04 (0.33 to 3.28) | 0.947 | 1.65 (0.62 to 4.43) | 0.959 |
| IBS-D | 2.27 (1.21 to 4.26) | 1.51 (0.64 to 3.60) | 0.347 | 2.59 (1.35 to 4.95) | 0.360 |
| IBS-C | IBS-D | IBS-M or IBS-U | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 40) | Placebo (n = 37) | Total (n = 77) | Amitriptyline (n = 92) | Placebo (n = 89) | Total (n = 181) | Amitriptyline (n = 100) | Placebo (n = 105) | Total (n = 205) | |
| SGA of relief of IBS symptoms | |||||||||
| 1 – Completely relieved | 1 (2.8%) | 3 (9.7%) | 4 (6.0%) | 3 (3.7%) | 0 (0.0%) | 3 (1.9%) | 4 (4.6%) | 1 (1.1%) | 5 (2.9%) |
| 2 – Considerably relieved | 11 (30.6%) | 3 (9.7%) | 14 (20.9%) | 27 (33.3%) | 14 (18.4%) | 41 (26.1%) | 27 (31.0%) | 23 (26.1%) | 50 (28.6%) |
| 3 – Somewhat relieved | 6 (16.7%) | 6 (19.4%) | 12 (17.9%) | 22 (27.2%) | 18 (23.7%) | 40 (25.5%) | 24 (27.6%) | 20 (22.7%) | 44 (25.1%) |
| 4 – Unchanged | 17 (47.2%) | 17 (54.8%) | 34 (50.7%) | 28 (34.6%) | 40 (52.6%) | 68 (43.3%) | 28 (32.2%) | 42 (47.7%) | 70 (40.0%) |
| 5 – Worse | 1 (2.8%) | 2 (6.5%) | 3 (4.5%) | 1 (1.2%) | 4 (5.3%) | 5 (3.2%) | 4 (4.6%) | 2 (2.3%) | 6 (3.4%) |
| Missing | 4 | 6 | 10 | 11 | 13 | 24 | 13 | 17 | 30 |
| Responder (score 1–3) | |||||||||
| Yes | 18 (50.0%) | 12 (38.7%) | 30 (44.8%) | 52 (64.2%) | 32 (42.1%) | 84 (53.5%) | 55 (63.2%) | 44 (50.0%) | 99 (56.6%) |
| No | 18 (50.0%) | 19 (61.3%) | 37 (55.2%) | 29 (35.8%) | 44 (57.9%) | 73 (46.5%) | 32 (36.8%) | 44 (50.0%) | 76 (43.4%) |
| Missing | 4 | 6 | 10 | 11 | 13 | 24 | 13 | 17 | 30 |
Safety
Serious adverse events and reactions
A total of 10 SAEs and SARs were reported for 10 (2.2%) of 460 participants in the safety population; 6 (2.6%) and 4 (1.7%) participants in the amitriptyline and placebo safety populations, respectively (Table 36). SAEs were reported for five participants, four in the amitriptyline arm and one in the placebo arm. Five SARs (expected and suspected to be related to the trial medication) were reported for a further five participants, two in the amitriptyline arm and three in the placebo arm.
| Amitriptyline (n = 231) | Placebo (n = 229) | Total (n = 460) | |
|---|---|---|---|
| Total participants with a SAE or SAR | 6 (2.6%) | 4 (1.7%) | 10 (2.2%) |
| Total number of SAE/SARS | 6 | 4 | 10 |
| Total participants with a SAE | 4 (1.7%) | 1 (0.4 %) | 5 (1.1 %) |
| Total number of SAEs | 4 | 1 | 5 |
| Total participants with a SAR | 2 (0.9%) | 3 (1.3%) | 5 (1.1 %) |
| Total number of SARs | 2 | 3 | 5 |
| Out of all SAEs and SARs | 6 (100%) | 4 (100%) | 10 (100%) |
| MedDRA/body system codea | |||
| Cardiac disorders | 0 (0.0%) | 2 (50.0%) | 2 (20.0%) |
| Gastrointestinal disorders | 1 (16.7%) | 0 (0.0%) | 1 (10.0%) |
| Injury, poisoning and procedural complications | 1 (16.7%) | 0 (0.0%) | 1 (10.0%) |
| Psychiatric disorders | 2 (33.3%) | 1 (25.0%) | 3 (30.0%) |
| Renal and urinary disorders | 2 (33.3%) | 1 (25.0%) | 3 (30.0%) |
| Seriousness criteria (not mutually exclusive)b | |||
| Life-threatening | 1 (16.7%) | 1 (20.0%) | 2 (18.2%) |
| Required or prolonged hospitalisation | 3 (50.0%) | 3 (60.0%) | 6 (54.5%) |
| Other important medical event | 2 (33.3%) | 1 (20.0%) | 3 (27.3%) |
| Outcome | |||
| Recovered | 6 (100.0%) | 3 (75.0%) | 9 (90.0%) |
| Recovered with sequelae | 0 (0.0%) | 1 (25.0%) | 1 (10.0%) |
| Days from randomisation to onset | |||
| Mean (SD) | 142.3 (63.31) | 94.3 (89.96) | 123.1 (74.44) |
| Median (range) | 124.5 (83.0, 231.0) | 76.0 (20.0, 205.0) | 118.0 (20.0, 231.0) |
| IQR | 85.0–206.0 | 21.0–167.5 | 83.0–205.0 |
| Days from onset to recovery | |||
| Mean (SD) | 52.2 (75.72) | 12.5 (17.75) | 36.3 (60.91) |
| Median (range) | 15.0 (3.0, 194.0) | 4.5 (2.0, 39.0) | 10.0 (2.0, 194.0) |
| IQR | 3.0–83.0 | 2.5–22.5 | 3.0–39.0 |
| Action taken | |||
| None | 3 (50.0%) | 1 (25.0%) | 4 (40.0%) |
| Treatment delayed | 2 (33.3%) | 0 (0.0%) | 2 (20.0%) |
| Treatment stopped | 1 (16.7%) | 3 (75.0%) | 4 (40.0%) |
| Most recent dose | |||
| 10 mg | 1 (16.7%) | 2 (50.0%) | 3 (30.0%) |
| 20 mg | 2 (33.3%) | 0 (0.0%) | 2 (20.0%) |
| 30 mg | 3 (50.0%) | 2 (50.0%) | 5 (50.0%) |
| Other causes | |||
| Yesc | 3 (50.0%) | 2 (50.0%) | 5 (50.0%) |
| No | 3 (50.0%) | 2 (50.0%) | 5 (50.0%) |
Other safety events
No SUSARs, deaths, or pregnancies occurred during the trial.
Blinding
Emergency unblinding
No emergency unblinding requests were made throughout the trial.
Treatment allocation exit survey
A treatment allocation exit survey was undertaken by the research nurse or clinical study officer, asking participants which treatment they thought they had received, at the participants’ 6-month treatment call or at the point of treatment discontinuation for participants who discontinued treatment before 6 months. Of the participants who completed 6 months treatment, 129 (74.6%) in the amitriptyline arm and 91 (56.5%) in the placebo arm guessed their allocation correctly (Table 37). A slightly higher proportion of participants who discontinued treatment before 6 months guessed their allocation correctly [27 (78.1%) amitriptyline; 30 (63.8%) placebo]. On a scale of 0 (not at all certain) to 10 (completely sure), participant certainty about their choice was a mean of 7.3 (SD 1.79) at 6 months and 7.5 (SD 2.22) at treatment discontinuation.
| Month 6 | Discontinued treatment before month 6 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 173) | Placebo (n = 165) | Total (n = 338) | Amitriptyline (n = 46) | Placebo (n = 59) | Total (n = 105) | |
| Treatment participant thought they received | ||||||
| Active drug | 129 (74.6%) | 70 (43.5%) | 199 (59.6%) | 25 (78.1%) | 17 (36.2%) | 42 (53.2%) |
| Placebo | 44 (25.4%) | 91 (56.5%) | 135 (40.4%) | 7 (21.9%) | 30 (63.8%) | 37 (46.8%) |
| Missing | 0 | 4 | 4 | 14 | 12 | 26 |
| How certain was the participant (0 = Not at all, to 10 = Completely sure) | ||||||
| Mean (SD) | 7.4 (1.81) | 7.2 (1.77) | 7.3 (1.79) | 7.6 (2.39) | 7.4 (2.11) | 7.5 (2.22) |
| Median (range) | 8.0 (1.0, 10.0) | 7.0 (0.0, 10.0) | 7.0 (0.0, 10.0) | 8.0 (0.0, 10.0) | 8.0 (2.0, 10.0) | 8.0 (0.0, 10.0) |
| Missing | 0 | 3 | 3 | 14 | 13 | 27 |
| Reason for treatment allocation predictiona | ||||||
| Treatment worked | 80 (46.2%) | 58 (36.0%) | 138 (41.3%) | 7 (21.9%) | 4 (8.5%) | 11 (13.9%) |
| Treatment didn’t work | 33 (19.1%) | 70 (43.5%) | 103 (30.8%) | 7 (21.9%) | 25 (53.2%) | 32 (40.5%) |
| Participant had a side effect | 61 (35.3%) | 22 (13.7%) | 83 (24.9%) | 20 (62.5%) | 10 (21.3%) | 30 (38.0%) |
| Just a guess | 17 (9.8%) | 19 (11.8%) | 36 (10.8%) | 2 (6.3%) | 7 (14.9%) | 9 (11.4%) |
| Participant had no side effects | 9 (5.2%) | 16 (9.9%) | 25 (7.5%) | 0 (0.0%) | 3 (6.4%) | 3 (3.8%) |
| Appearance or taste of the tablet | 5 (2.9%) | 1 (0.6%) | 6 (1.8%) | 1 (3.1%) | 1 (2.1%) | 2 (2.5%) |
| Taken amitriptyline previously | 2 (1.2%) | 4 (2.5%) | 6 (1.8%) | 2 (6.3%) | 5 (10.6%) | 7 (8.9%) |
| Otherb | 1 (0.6%) | 0 (0.0%) | 1 (0.3%) | 0 | 0 | 0 |
Eighty participants (46.2%) in the amitriptyline arm made their report of which treatment they thought they had received at 6 months because the treatment worked, whereas 33 participants (19.1%) made their choice because the treatment did not work and 61 (35.3%) because they had a side effect. Of the participants in the placebo arm at 6 months, 58 (36.0%) made their report of which treatment they thought they had received because it worked, whereas 70 (43.5%) made their report because it did not work and 22 (13.7%) because they had a side effect. Of participants who discontinued treatment before 6 months, a higher proportion of participants made their report because they had a side effect [20 (62.5%) amitriptyline; 10 (21.3%) placebo] and a lower proportion of participants in both arms made their report because the treatment worked [7 (21.9%) amitriptyline; 4 (8.5%) placebo].
End-of-trial participation unblinding
A total of 346 (74.7%) participants were provided with their treatment allocation following their trial participation, 176 (75.9%) participants in the amitriptyline arm and 170 (73.6%) in the placebo arm (Table 38). Most [312 (90.2%)] participants requested that their GP be provided with their treatment allocation directly via the research team, and the remainder [34 (9.8%)] requested their GP not be provided with their allocation. The primary method by which participants could request their treatment allocation was via completion of, and response within, the final follow-up questionnaire, the method used by 314 (90.8%) of participants, while the remainder [32 (9.2%)] requested their allocation via correspondence with the trial researcher at their final 12-month researcher treatment follow-up call prior to implementation of the allocation request within participants’ final questionnaire pack. Only 10 (2.9%) participants were provided with their allocation via post, where participants’ preference was post or where a valid e-mail address was not provided, with the remainder via e-mail.
| Amitriptyline (n = 232) (%) | Placebo (n = 231) (%) | Total (n = 463) (%) | |
|---|---|---|---|
| Participant unblinded following trial participation | |||
| Yes | 176 (75.9) | 170 (73.6) | 346 (74.7) |
| No | 56 (24.1) | 61 (26.4) | 117 (25.3) |
| Of unblinded participants | |||
| Did the participant want to unblind their GP? | |||
| Yes | 158 (89.8) | 154 (90.6) | 312 (90.2) |
| No | 18 (10.2) | 16 (9.4) | 34 (9.8) |
| Unblinding requested via | |||
| Final questionnaire | 163 (92.6) | 151 (88.8) | 314 (90.8) |
| Researcher correspondence/e-mail | 13 (7.4) | 19 (11.2) | 32 (9.2) |
| Participant contacted the qualitative team to discuss their allocation? | |||
| Yesa | 1 (0.6) | 3 (1.8) | 4 (1.2) |
| No | 175 (99.4) | 167 (98.2) | 342 (98.8) |
| Total unblinded | 176 (100) | 170 (100) | 346 (100) |
| Reason participants not unblinded | |||
| Did not complete final questionnaire | 34 (60.7) | 50 (82.0) | 84 (71.8) |
| Did not respond to unblinding question in final questionnaire | 8 (14.3) | 1 (1.6) | 9 (7.7) |
| Ended trial before final questionnaire included unblinding request/lost to follow-upb | 12 (21.4) | 4 (6.6) | 16 (13.7) |
| Requested NOT to be unblinded in final questionnaire | 2 (3.6) | 6 (9.8) | 8 (6.8) |
| Total not unblinded | 56 (100) | 61 (100) | 117 (100) |
Of the 117 (25.3%) participants who were not provided their treatment allocation following trial participation, in most cases this was because participants had not completed their final questionnaire or had not responded to the allocation request within their completed final questionnaire. Only 8 (6.8%) participants requested not to be provided with their allocation, and the remaining 16 (13.7%) participants had completed their final questionnaire prior to implementation of the allocation request within the final questionnaire pack and had also discontinued trial medication before the final 12-month researcher treatment follow-up call.
Research nurse/clinical study officer unblinding
The research nurse or clinical study officer was inadvertently unblinded for four participants after their final follow-up and treatment allocation had been revealed: in three cases by the participant, and in one case by a GP research nurse to clarify participant details.
Chapter 4 Nested qualitative study
Aims
The overarching aim was to explore participants’ and GPs’ experiences of treatments and participating in the ATLANTIS trial. The objectives were to identify factors that facilitate or impede prescribing and uptake of low-dose amitriptyline in IBS, to identify participants’ and GPs’ perspectives on the broader impact of the trial, and to explore psychosocial and contextual factors that might shape wider use of amitriptyline for IBS. The purpose was to use these in-depth findings to support the interpretation of trial outcomes and to inform future efforts to promote wider use of amitriptyline for IBS where appropriate.
Methods
Design
The qualitative study was nested within the main ATLANTIS trial. The qualitative team comprised a female research fellow with experience of conducting qualitative research about IBS, a female health psychologist, two female GPs, a male psychology student and a female psychology student. Semistructured telephone interviews were conducted with a sub-sample of trial participants and GPs involved in the trial. Reflexive thematic analysis,41,42 incorporating techniques from grounded theory,43 was used to analyse the qualitative data. Data collection and initial analyses proceeded iteratively: that is, coding started after the first few interviews were conducted and informed subsequent interviews. Although analysis was primarily inductive, that is, driven by the data, the common-sense model of illness perception44 and normalisation process theory45 informed the development of the interview topic guides and were consulted during the analysis to aid our interpretation of data around experiences of IBS and treatments and wider implementation of amitriptyline for IBS in primary care. Qualitative findings were related to the findings of the main trial by comparing themes across participant groups and triangulating the themes against key quantitative findings.
Ethical considerations
As part of the main trial consent procedures, all participants could consent to be contacted about taking part in optional semi-structured telephone interviews at two time points: 6 months and 12 months post randomisation. All trial participants who had consented to be contacted about the qualitative study and had reached their 6-month post-randomisation time point were sent a qualitative study invitation pack by the ATLANTIS trial team (CTRU). The qualitative study invitation pack comprised a covering letter, participant information sheet and qualitative interview consent form (see Report Supplementary Material 1). Trial participants were invited to express their interest in taking part in an interview by e-mailing a completed written consent form to the qualitative researcher (EJT). All GPs from participating practices (excluding those GPs also on the trial management team) were invited directly by the qualitative researcher to take part in one telephone interview. A list of general practices and PI contact details (name and e-mail addresses) was sent to the qualitative researcher by the ATLANTIS trial team (CTRU) via a secure messaging system. GPs were contacted about the qualitative study following their participant recruitment period (approximately 5 months after they completed their mail-out). Each PI was e-mailed a copy of the GP participant information and consent form and asked to forward this information to all GPs involved in participant recruitment for ATLANTIS at their practice (see Report Supplementary Material 1). GPs were asked to complete and return the consent form if they were interested in taking part in an interview. Written informed consent was obtained by the qualitative team prior to carrying out all interviews.
In addition to obtaining written consent, interviewers reiterated the purpose of the interviews and obtained verbal consent prior to starting each interview. At the end of each interview, participants were given another opportunity to ask any questions and were thanked for their contribution. They were also offered a copy of their transcript and a copy of study findings when available.
All interviews were pseudonymised on transcription and participant ID numbers were assigned to ensure confidentiality and minimise the risk of participant identification in sharing/reporting findings.
The qualitative study was included in the main ethics application approved by Yorkshire and the Humber (Sheffield) Research Ethics Committee (19/YH/0150).
Sampling and recruitment
The aim was to interview a diverse sample of trial participants to include approximately 20 interviewees from each arm of the trial (amitriptyline/placebo). The lead qualitative researcher (EJT) was blinded to participant allocation for most but not all qualitative interviews: she was blinded for all 6-month interviews; however, for participants who had been told their treatment allocation beforehand, this was discussed during their 12-month interview (this happened for seven participants); EJT also knew the treatment allocation of four trial participants through her role supporting unblinding to treatment allocation (one of whom was interviewed as part of the qualitative study). The rest of the qualitative team remained blinded to participant allocation throughout recruitment, data collection, and data analysis until preliminary analysis of the main trial data was complete (1 March 2023). The final sample size was dependent on saturation, and when we determined we had achieved a rigorous, credible analysis in relation to our aims. Interviewing participants from the amitriptyline arm allowed us to identify factors related to acceptability, uptake, and psychosocial context. Interviewing participants from the placebo arm enabled us to explore between-group qualitative comparisons to provide insight into the quantitative results. Interviewing the same participants at 6 and 12 months allowed us greater depth to explore changes over time and the potential to better understand any differences in the quantitative results between 6 and 12 months.
Our aim was to purposively sample trial participants to incorporate variety in sex, age, recruitment hub (West of England, West Yorkshire, Wessex), baseline symptom severity scores (IBS-SSS), and those who decided to continue or stop treatment at 6 months. Sampling for variety on key characteristics helps ensure that the qualitative findings encapsulate the breadth of participants’ experiences and are not overshadowed by any one subgroup. However, such sampling was not possible due to the impact of the COVID-19 pandemic delaying qualitative study recruitment. Instead, we employed convenience sampling and interviewed all trial participants who expressed an interest in the qualitative study and responded to the qualitative researcher’s request to arrange an interview. We were still able to recruit and explore experiences from a variety of trial participants, generating a comprehensive account of participants’ perspectives based on interviews with 20 participants from each trial arm.
Between October 2019 and April 2022, the ATLANTIS trial recruited a total of 463 participants. Between April 2020 and March 2022, 140 qualitative study invitations were sent to all trial participants who had consented to be contacted about the qualitative study. Forty-two of these participants were interviewed, a further three participants contacted the qualitative researcher to decline to take part due to ill health or going away and the rest did not respond. Sixteen interviews were conducted in the pilot phase of the trial (between April and June 2020) with participants who had reached their 2-month time point. The timing of this first interview for participants in the pilot phase was brought forward (to 2 instead of 6 months) to fit within the time frame for the internal pilot. Twenty-six interviews were conducted with participants who had reached their 6-month time point between April 2021 and March 2022. Participants were invited in batches to permit iterative interviewing and data analysis. Each month the ATLANTIS trial team sent out batches of qualitative study invitations to participants who had consented to be contacted about the qualitative study and had reached the required time point. Recruitment to interviews ended when no new themes emerged, and existing themes were well developed within a diverse sample.
Of the 42 participants interviewed at 2 or 6 months, only 29 were eligible for follow-up interviews at 12 months. This was because following national guidance trial recruitment paused from March to July 2020 due to COVID-19, which resulted in 13 participants reaching their 12-month time point beyond the end of the qualitative study interviewing period. Of the 29 trial participants invited to take part in a follow-up interview, 19 individuals were interviewed, 2 declined due to lack of availability and 8 did not respond.
In total, 55 general practices were involved in the ATLANTIS trial, 42 of which were available to be contacted during the qualitative study time frame. Forty-two qualitative study invitations were sent to general practices inviting any GP involved in the ATLANTIS trial to take part in an interview about their experiences. Ten GPs declined to take part due to lack of capacity and 16 did not respond. Sixteen GP interviews with a diverse sample (full and part-time, gender and years as a GP) were conducted between October 2020 and March 2022.
Qualitative interviews
Semistructured telephone interviews were used to elicit trial participants’ and GPs’ experiences of the trial treatments and trial participation. Three separate topic guides were developed: (1) 6-month participant interview, (2) 12-month participant interview, (3) GP interview (see Report Supplementary Material 1). Each topic guide was developed collaboratively by the qualitative research team by drawing on existing literature, relevant theories (as detailed below), and input from patient collaborators. The topic guides consisted of open-ended questions and prompts used by the interviewer to elicit participants’ views and experiences of the trial and their thoughts and feelings about the trial treatments and managing IBS. Topic guides were used flexibly to ensure that interviewers explored all required topics while remaining open to exploring participants’ individual experiences and perspectives in-depth, to allow novel and unanticipated insights to emerge. All interviews (GP and trial participant) were audio-recorded using a digital audio-recorder (except one interview which was audio-recorded via MS Teams). Field notes were taken to capture the interviewer’s impressions and reflections, and any aspects not captured by the audio-recorder. These notes were used to aid initial coding of each transcript and were reflected upon during the analysis. At the end of the interview, interviewees were thanked and debriefed.
Participant interviews
Participant topic guides were informed by the common-sense model of illness perception,44 which provides a conceptual framework for understanding how participants experience treatments and make treatment decisions within the context of chronic illness; this has proved relevant in previous qualitative work on IBS. 46
The extended common-sense model (CSM) including the necessity-concerns framework is relevant to theorising how the beliefs of patients around symptoms and treatment may relate to adherence to medication. It suggests that patients construct cognitive and emotional representations around the identity, cause, duration, consequences and controllability of a health condition, and that treatment adherence is related to their understanding of the condition and its treatments, as well as the perceived need for treatment and concerns about any negative effects. 47
The 6-month telephone interviews explored trial participants’ experiences of taking part in the trial, their trial treatment (while still blinded), their experiences of other treatments for IBS, their thoughts about the COVID-19 pandemic, their thoughts about the future and the process of unblinding to treatment allocation. Most of the interviews were conducted by a female qualitative research fellow (n = 36) and the rest (n = 6) were conducted by a male doctoral student. The 6-month interviews lasted from 17 to 65 minutes (mean 40 minutes). They took place over a 23-month period (between April 2020 and March 2022).
The 12-month telephone interviews explored trial participants’ reflections on taking part in the trial and the treatment they were allocated to (where possible), their thoughts about the unblinding process, any other treatments they had tried since the 6-month interviews, their thoughts about the pandemic and about the future. Most of the 12-month interviews were conducted by a female qualitative research assistant (n = 15) and the rest (n = 4) were conducted by a male doctoral student. These interviews lasted from 15 to 60 minutes (mean 29 minutes). They took place over a 16-month period (from October 2020 to February 2022).
General practitioner interviews
The GP interview topic guide was informed by key domains from normalisation process theory (NPT),45 which provides a conceptual framework that specifies the factors and processes likely to hinder or enable widespread implementation of new practices. The four constructs of NPT are coherence (people making sense of the processes), cognitive participation (engaging with the process), collective action (the work that is required to operationalise the process), and reflexive monitoring (reflecting on and appraising new working practices). Drawing on this theory helps to explain the factors and processes encountered during implementation and how these can facilitate the embedding of an intervention (such as amitriptyline for IBS in primary care) into everyday practice.
General practitioner telephone interviews explored GPs’ experiences of prescribing amitriptyline within the trial (and within the broader contexts of primary care and IBS management), and potential barriers and facilitators to widespread post-trial implementation in primary care. Most of the GP interviews were conducted by a female qualitative research fellow (n = 15) and one was conducted by a female master’s student. GP interviews lasted from 18 to 45 minutes (mean 27 minutes). They took place over a 17-month period (between October 2020 and March 2022).
Qualitative data analysis
All interviews were transcribed verbatim by a professional transcription company and any identifiable data (e.g. person names, place names) removed. Analysis started after the first few interviews and proceeded iteratively. This enabled early insights to be explored more fully in later interviews and the topic guides to be revised accordingly, for example, adding questions about unblinding to treatment allocation. The six phases of reflexive thematic analysis42 were implemented alongside coding techniques from grounded theory43 (e.g. open coding, line-by-line coding, constant comparison) to explore the interview data. In phase 1 (data familiarisation), one author (EJT) repeatedly read through all the transcripts and listened back to the audio-recordings. In phase 2 (generating initial codes), EJT conducted line-by-line coding. Trial participant 6-month and 12-month interviews and GP interviews were all coded separately. Codes were derived inductively from the data and grouped together to produce separate initial coding frames for the three interview data sets. After coding all the transcripts, in phase 3 (searching for themes) the codes were then sorted into potential themes by recognising meaningful repeated patterns and identifying key concepts in the data. To enhance the quality and credibility of the analysis, detailed coding manuals were produced containing names and descriptions of potential themes and subthemes along with examples quotes. At this stage participants’ 6- and 12-month data were compared and combined into a participant coding manual. A separate coding manual was produced for the GP interview data.
The initial three phases were conducted by one author (EJT), a qualitative research fellow. In phase 4 (reviewing themes), potential themes/subthemes detailed in the coding manuals were discussed with, and iteratively developed by, members of the research team (EJT, FB, HE, SA) and PPI members (MC, EJW) to offer diverse inferences and interpretation of the data and to avoid idiosyncratic interpretations. Reviewing themes for fit with coded extracts and entire data set involved reviewing the original data for relevant incidents for each potential theme and expanding, merging, or generating new themes. A negative case analysis was carried out, which explicitly searched for ideas in the data that were potentially inconsistent with our interpretations. This allowed us to identify and reflect upon important, but rare, views and the limits of the analysis.
In phase 5 (defining and naming themes), matrices and diagramming were used to compare the themes’ similarities and differences between the 6-month and 12-month trial participant interviews and between the trial participant and GP interviews. Themes were reviewed and refined to form broader cross-cutting themes producing an overarching narrative that drew on the participant 6-month, participant 12-month, and GP interviews. In phase 6 (reporting), quotes were reviewed and discussed by the qualitative team to provide compelling examples for theme and subthemes and to write up our main report.
Although primarily inductive, the common-sense model of illness perception and normalisation process theory were reviewed against the coding manuals to help in interpreting initial findings related to (1) participants’ experiences of IBS and treatments and (2) wider implementation of amitriptyline for IBS in primary care. NVivo (version 12) (QSR International, Warrington, UK)48 was used to manage data, implement and record coding and to perform thematic comparisons as described above.
After drafting the main report and when the quantitative trial results became available, the qualitative team then returned to the data to undertake further analysis of the qualitative themes in relation to the quantitative trial results. Two key analyses were undertaken.
-
NVivo’s48 Matrix Queries function was used to cross-tabulate talk about key themes by participants’ treatment allocation (amitriptyline vs. placebo). This enabled an exploration of similarities and differences between how people in each trial arm talked about their experiences of IBS and the ATLANTIS trial; findings from this analysis are integrated into the presentation of themes in Barriers and facilitators to uptake of low-dose, Experiences of taking part in the ATLANTIS trial, Reflections on managing IBS during the COVID-19 pandemic and Reflections on treatment allocation.
-
Quantitative and qualitative findings were cross-tabulated, considering points of convergence, divergence, and explanation. This enabled the qualitative findings to be related to the quantitative findings in a systematic manner. This analysis is presented in Relating the qualitative and quantitative findings.
The findings are presented in five main sections, which discuss: participants’ and GPs’ barriers and facilitators to low-dose amitriptyline for IBS, and how these relate to the context of treatment-seeking; trial participants’ experiences of taking part in the ATLANTIS trial; trial participants’ experiences of being blinded to low-dose amitriptyline or placebo; trial participants’ reflections on managing IBS during the COVID-19 pandemic in the first lockdown in 2020 (during which 16 interviews were conducted) and during subsequent lockdowns and ongoing social restrictions in 2021–2.
Findings
Participants
Forty-two trial participants took part in a 6-month telephone interview. Fifty-five per cent (n = 23) were allocated to the amitriptyline treatment arm and 45% (n = 19) were allocated to the placebo treatment arm. Of these 42 participants, 19 took part in a 12-month telephone interview. Nine of these 19 participants (47%) were allocated to the amitriptyline treatment arm and 10 were allocated to receive placebo (53%). Baseline characteristics of trial participants who took part in the 6-month and 12-month interviews are shown in Table 39. Sixteen GPs took part in a telephone interview; their characteristics are shown in Table 40.
| Baseline characteristics | 6 months (n = 42) | 12 months (n = 19) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Female | 30 | 71 | 15 | 79 |
| Male | 12 | 29 | 4 | 21 |
| Age | ||||
| Median age (years) | 54 | 54 | ||
| Range (years) | 21–83 | 25–83 | ||
| Recruitment hub | ||||
| Wessex | 16 | 38 | 6 | 32 |
| West Yorkshire | 9 | 22 | 6 | 32 |
| West of England | 17 | 40 | 7 | 37 |
| Educational level | ||||
| No formal | 1 | 2 | 1 | 5 |
| GCSE | 7 | 17 | 3 | 16 |
| A level | 11 | 26 | 5 | 26 |
| Degree | 9 | 21 | 4 | 21 |
| Postgraduate | 13 | 31 | 5 | 26 |
| Professionally qualified | 1 | 2 | 1 | 5 |
| Ethnicity | ||||
| White | 41 | 98 | 18 | 95 |
| Asian | 1 | 2 | 1 | 5 |
| IBS-SSS at baseline | ||||
| Mild | 9 | 22 | 4 | 21 |
| Moderate | 19 | 45 | 9 | 47 |
| Severe | 14 | 33 | 6 | 32 |
| N | % | |
|---|---|---|
| Sex | ||
| Female | 8 | 50 |
| Male | 8 | 50 |
| Age | ||
| Median age (years) | 45 | |
| Range (years) | 34–60 | |
| Recruitment hub | ||
| Wessex | 7 | 44 |
| West Yorkshire | 0 | 0 |
| West of England | 9 | 56 |
| Ethnicity | ||
| White | 12 | 75 |
| Asian | 3 | 19 |
| Mixed | 1 | 6 |
| Employment status | ||
| Part-time | 11 | 69 |
| Full-time | 5 | 31 |
| Years worked as GP | ||
| Median (years) | 18 | |
| Range (years) | 3–30 | |
Barriers and facilitators to uptake of low-dose
Amitriptyline for irritable bowel syndrome
Thematic analysis of trial participant and GP interviews highlighted barriers and facilitators to low-dose amitriptyline for IBS, as well as an overarching theme that explained why participants and GPs in this trial were willing to try it despite some concerns. Key barriers, that is, factors likely to hinder prescribing and uptake of low-dose amitriptyline for IBS, include concerns about antidepressant use, medicalising IBS, and side effects. Key facilitators include the familiarity of amitriptyline, the low and flexible recommended dosage, its potential to offer benefits beyond IBS symptom relief and perceived ease of treatment. The barriers and facilitators to low-dose amitriptyline for IBS were expressed in the context of desire for a novel approach to IBS: GPs were keen to offer more options for IBS and patients sought a cure for their symptoms. The next three sections explore and illustrate these barriers, facilitators, and the desire for a cure.
Potential barriers to uptake of low-dose amitriptyline for irritable bowel syndrome
Concern about amitriptyline being an antidepressant was raised by both trial participants and GPs as a potential barrier to patient uptake. A dominant worry for participants was the potential psychological effects of taking an antidepressant such as amitriptyline in that it might ‘alter their mind’ or ‘zombify them’.
My only concern was that it would alter my mind in some way because it’s used to treat depression. I don’t know how it works treating depression, but that’s not anything I know anything about.
P2, female aged 45–54 years with severe IBS, 6-month interview
Patient reluctance towards taking an antidepressant for a ‘functional physical health problem’ was also highlighted among GP interviewees. Typically, GPs expressed doubt about prescribing amitriptyline for people with IBS, especially with mild symptoms, as they felt such patients would be reluctant to take an antidepressant for IBS.
It would only be for the ones who are really struggling … I think at that level of symptoms when people are feeling a bit desperate about their symptoms. There may be some reticence at the slightly lower-end spectrum of IBS for people to start taking an antidepressant … in patients potentially with mild symptoms, they may be reluctant to use a medication that’s labelled an antidepressant for their physical health problem.
GP2, male GP aged 35–44 years with 15–20 years’ experience
Perceived social stigma of taking an antidepressant was highlighted by both GPs and participants as a barrier to uptake due to concern that amitriptyline may be perceived negatively by others and not easily understood to be a treatment for other (non-mental health) conditions such as IBS.
The worst thing about it is that it can be used as an antidepressant, and it’s almost like, if somebody saw that on your prescription, they wouldn’t think, oh, she’s taking that because she has IBS; they would think, oh, she must have stress or depression, and there is still a stigma about that. I think that’s probably the biggest negative, is that kind of stigma … a lot of people are aware of the name of amitriptyline, and then it’s got that connotation, so I think that’s a negative. If you called it something completely different, and said, ‘It’s a treatment for IBS’, you probably wouldn’t worry about somebody knowing you were having it.
P22, female aged 55–64 years with mild IBS, 12-month interview
General practitioners were concerned about the wider consequences of prescribing a long-term ‘drug’ for people with IBS and suggested that this may be a barrier to prescribing amitriptyline for IBS patients. They predominantly worried that prescribing amitriptyline for IBS could ‘medicalise’ IBS and highlighted potential contraindications to its prescription and polypharmacy issues as potential barriers.
I think that we’re very cautious or wary of the fact that there are within a stage where we’re thinking about polypharmacy and about multiple medications and is the prescribing of medicines the right thing to do for everybody? Obviously, it’s not necessarily … I think that there is both caution in general about prescribing – particularly in elderly patients, older patients – and caution about polypharmacy … I think there is an issue to explore about then the potential for medicalising, introducing a medicine for instance to somebody who therefore may not require a medicine and may just require lifestyle options.
GP11, male GP aged 45–54 years with over 20 years’ experience
Furthermore, GP interviewees felt that prescribing low-dose amitriptyline for IBS could lead to increased administrative burden from the resultant repeat prescriptions and follow-up review appointments.
More drugs to sign off! I keep prescribing, my list is already too long.
GP1, female GP aged 55–64 years with over 20 years’ experience
Amitriptyline has side effects and it has to be prescription only, whereas Buscopan and Colofac are over the counter. It’s medicalising the situation and increasing the workload in terms of monitoring a drug and repeat prescription system, etc. That is an additional workload compared to somebody who the diagnosis is made and they’re sending them off to get their own meds.
GP7, female GP aged 55–64 years with over 20 years’ experience
General practitioner interviewees also expressed concerns about patient tolerability of side effects, potential of overdose and wider impacts of prescribing amitriptyline, including anticholinergic burden in older patients. Careful consideration of the suitability of low-dose amitriptyline for people with IBS was thus evident in the data.
Often these patients are quite young, but as they get older, then it’s an anticholinergic and it adds to the anticholinergic burden and we know that people fall over and get confused and having long-term anticholinergic burden is not a great idea in terms of overall well-being.
GP1, female GP aged 55–64 years with over 20 years’ experience
Reluctance to ‘medicalise’ IBS was also evident in the trial participant interview data. Concerns about drug side effects/dependency were expressed in the participant interviews but this seemed to have been tempered by the sense that if they experienced any adverse effects, they would likely be known side effects and as such manageable. This may be reflective of the fact that they are participants who have all signed up for a drug trial and were provided with detailed information about possible side effects.
I suppose I was a bit concerned that amitriptyline was a drug that I’d heard of, and I was worried that I might get addicted to it in some way, because it’s antidepressant I understood.
P6, female aged over 65 years with mild IBS, 6-month interview
I suppose with any low-dose medication that can be used for depression, anxiety, and whatnot, I did think weight gain might be part of it, but again I thought if it helps with my stomach I’m prepared to deal with any sort of mild side effects. If it were to help, I’d be up for a bit of weight gain if that’s what it took.
P42, female aged 18–25 years with severe IBS, 6-month interview
Potential facilitators of uptake of low-dose amitriptyline for irritable bowel syndrome
The familiarity of amitriptyline appeared to be an important factor helping participants and GPs feel reassured about using amitriptyline for IBS. Trial participants seemed to take comfort in amitriptyline being a well-established drug rather than a new or experimental treatment. Prior personal experience or knowing others already taking amitriptyline for non-mental health conditions appeared to help normalise a drug treatment, and specifically amitriptyline, for IBS.
From what I understand, it’s been used for years so it’s quite a well-known drug, so I didn’t feel uneasy using it. It’s been tried and tested, so I think if we can use existing drugs to treat other things, then it’s worth giving them a go.
P18, female aged 25–34 years with moderate IBS, 6-month interview
Well, I’ve got a couple of friends that are on them, but not for their tummies, they’ve got them because they’ve got a bit of stress in their lives, and have lost their husbands and stuff, and it helps them sleep. I was quite happy to go on them, because I knew that I’d got friends on it; it wasn’t something that I didn’t recognise.
P9, female aged over 65 years with moderate IBS, 6-month interview
The familiarity of amitriptyline was also highlighted in the GP interviews as a potential facilitator to prescribing. Amitriptyline was commonly viewed as a widely available and inexpensive treatment with well-established knowledge of side effects, that anecdotally it ‘seems logical’ that it could be successful is helping to resolve IBS symptoms.
It’s freely available and, as I said, very cheap, so there isn’t going to be that barrier in terms of an expensive, new medication.
GP2, male GP aged 35–45 years with 15–20 years’ experience
My initial thoughts were that it was actually a very good idea in that anecdotally there’s certainly been a suggestion about amitriptyline helping people with multiple issues regarding pain and possibly pain, certainly neuropathic-type pain.
GP11, male GP aged 45–54 years with over 20 years’ experience
General practitioners demonstrated existing knowledge of amitriptyline and seemed confident in making prescribing decisions about people with IBS. A common view was that it is more appropriate to prescribe low-dose amitriptyline to people experiencing pain and diarrhoea rather than constipation due to not wanting to ‘give them something that’s going to also constipate them’.
I think I’d more likely use it for somebody who’s got cramps and bloating and maybe loose motions, than constipation really. If they’ve got those sorts of symptoms, particularly pain I think, then I’m more likely to use it for that I’m more inclined to use it for the pain aspect of IBS.
GP8, female GP aged 45–54 years with over 20 years’ experience
Distinguishing low-dose use for IBS from traditional antidepressant use was highlighted as a potential facilitator of uptake. Trial participants felt assured that amitriptyline for IBS is prescribed at a lower dose that it would be for treating depression, feeling that it would be safer and that there was more distance/separation from being on a treatment for mental health conditions.
I think it’s an antidepressant, isn’t it, or it’s used as an antidepressant obviously in larger doses. I wasn’t really concerned because the dosages are pretty low to be fair and it is used for other things, so you’d like to think it was relatively safe to be used for IBS as well.
P7, female, aged 45–54 years with moderate IBS, 6-month interview
Similarly, GP interviewees reflected on the importance of addressing patients’ concerns about amitriptyline being an antidepressant. A common belief was that patients could be reassured by explaining that, although traditionally an antidepressant, amitriptyline for IBS is prescribed at a much lower dose and is also commonly used for a variety of other conditions. Some GP interviewees also reflected that explaining that amitriptyline is being prescribed for ‘physical symptoms’ can help ensure that people with IBS are not left feeling that the GP thinks IBS is ‘all in their head’.
Not to blow my own trumpet, but I’m always really careful with, I mean, amitriptyline can be used for a variety of conditions, can’t it? I’m always very careful that I, because the common thing is, and usually patients I haven’t spoken to who come back and tend to want to talk about the amitriptyline they’ve been prescribed and usually the case of, ‘You’ve prescribed me an antidepressant, I’m not depressed’. I’m very careful to say or remind patients that if you’re taking, amitriptyline is used for a number of reasons and it traditionally was used as an antidepressant, but we’re using it at much lower doses to what you want as an antidepressant.
GP4, male GP aged 35–44 years, with 10–15 years’ experience
I’m always quite careful when I prescribe amitriptyline for anything to explain to people that, ‘Look, this is an antidepressant, but we’re not looking at using it as an antidepressant’. I put that in as one of the first things I say because otherwise, people go away, read the leaflet, ring up and say, ‘Why have you given me this?’ I always tend to couch it like that and say, ‘Look, it’s been around for a long time. That was originally its use, but it’s been found to helpful in lots of other conditions’. Then I’ll often say to patients, ‘It’s used for migraine prophylaxis, sciatica, chronic pains, etc.’, because otherwise, they think you just think it’s all in their head and you’re giving them an antidepressant. So I definitely couch it like that. I find that when I put it like that, people seem to be reasonably receptive to it as an idea.
GP14, male GP aged 45–54 years with 15–20 years’ experience
Recognising potential benefits beyond IBS symptom relief was also highlighted as a potential facilitator to prescribing and uptake. Taking amitriptyline was viewed by trial participants as potentially having other benefits beyond IBS symptom relief, including improving emotional health (e.g. elevating mood/reducing stress), relieving symptoms in other pain-related conditions and improving sleep.
I think because it’s to do with, yes, it was a very, very small amount of, like an antidepressant, but maybe that would help control any emotional feelings that I was going through, maybe that would then help with my IBS.
P18, female aged 25–34 years with moderate IBS, 6-month interview
It was amitriptyline, and I have taken amitriptyline in the past for migraines and also, I was recommended to take them for my back. I’ve got lower back problems, so I thought, oh, that’d be quite good. I could kill all the birds with one stone! IBS, migraines, back problems!
P3, female aged over 65 years with moderate IBS, 6-month interview
Some participants described experiencing side effect of drowsiness but welcomed the opportunity of a good night’s sleep, which had been disrupted by their IBS symptoms and/or stress.
I just wait for the Channel 4 News to end, and then take it at eight o’clock because I’ve worked out clockwise, that if I take it at bedtime, then I wake up a bit groggy. Whereas if I take it at eight o’clock, it sort of kicks in about the time I go to bed. I’m only doing it for the sleep. No, I’m not, but it is helpful. So the spin-off from this is if anybody wants a sleeping tablet, then amitriptyline’s a really good one.
P40, male aged 45–54 years with moderate IBS, 6-month interview
I definitely found an improved sleep, because I was drowsy, going to bed. When I have stressful periods, that is something that suffers, is my sleep; getting off to sleep – because I have all sorts going through my head. The tablets actually really helped with that.
P25, female aged 25–34 years with moderate IBS, 12-month interview
Similarly, in GP interviews amitriptyline was viewed as an advantageous choice for some people. As well as providing reassurance about the low and flexible dose range of amitriptyline for IBS, it appeared to be common practice among GP interviewees to stress to patients that they ‘could use some of the side effects to their advantage’ and reassure patients of other potential benefits taking amitriptyline such as addressing sleep problems.
I generally sell it to them like if they struggle to sleep, then the amitriptyline can help them sleep.
GP5, male GP aged 25–34 years with 5–10 years’ experience
I think the people who I’m thinking of who would probably take this amitriptyline are the people where they’re quite debilitated by their symptoms. They’re probably affecting their sleep; it’s probably affecting their mood, so the fact that it was previously used as an antidepressant, even though that’s not what we’re using it for in this context, may actually also be a benefit. I think it’s really looking at both arguments and saying, ‘Well, it may help improve your mood even though we don’t use it in that context and the doses are much lower’. Being able to explain the benefits, the sleep – if you’re struggling with your sleep, it may help with that.
GP10, female GP aged 45–54 years with 15–20 years’ experience
Emphasising ease of treatment was viewed as another important facilitator of patient uptake of amitriptyline. Trial participants seemed to appreciate the small size of the tablets making them easier to swallow and only having to take tablets once a day making it easy to fit into their daily routine.
Well, they’re nice and small, I suppose, physically! They’re easy to swallow and all that sort of thing.
P6, female aged over 65 years with mild IBS, 6-month interview
I don’t like taking pills per se. I’m not a pill-popper, but I have been taking them, and I would be quite happy to continue to take them. Easy. Easy to swallow. Yes, not a problem.
P32, female aged 45–54 years with mild IBS, 6-month interview
I take them at seven o’clock now, on the dot. Taking the tablets wasn’t a problem. I take other tablets as well. I take a, the smallest dose of statin in the evenings as well, so it goes hand in hand with that.
P5, male aged over 65 years with moderate IBS, 6-month interview
Another important factor was having the flexibility of adjusting the dose from one 10 mg tablet (10 mg) to 2 × 10 mg (20 mg) or 3 × 10 mg tablets (30 mg). Although some participants were uneasy about adjusting the dose themselves and felt they would prefer any dose adjustment to be a medical judgement and ‘not just willy-nilly by yourself’, most trial participants reported a sense of empowerment and appreciation at having the flexibility to adjust the dose according to their needs and increasing dosage at their own pace.
I felt like I could reduce them or increase – do you know what I mean? There was a sort of sense of being able to say, ‘No, I don’t need three every day to make them work’. Do you know what I mean? It was like there was that sense of, okay, I can see that by reducing them it got worse, and by increasing them it got better. There was that sort of flexibility within the study to try that out a bit.
P23, female aged 45–54 years with severe IBS, 12-month interview
I have more control with this. Whereas the previous medical trials, it was you have to take this at certain times. This, I could take one tablet or two tablets, or three tablets, whatever I felt was suitable for me. That was quite nice to have the flexibility, definitely … having control over it and going with what my body felt like if you see what I mean. The maximum tablets I did go up to was two. I just felt three was a bit too much. I just didn’t feel comfortable with it. Whereas the two was the good in between and I was happy enough with that.
P17, female aged 25–34 years with severe IBS, 6-month interview
Participants described using the written instructions and flow chart provided by the trial team and talking to the researchers to guide their dose titration. Most found the written information helpful and straightforward. A few participants felt the amount of written information was potentially overwhelming (‘I was absolutely deluged with stuff’). A few mentioned they would have liked further guidance on how to manage the process of stopping their tablets.
It [dose titration] was fine. There was a flowchart to follow. I followed the flowchart and I took the extra medicine.
P37, male aged 35–44 years with severe IBS, 6-month interview
Interviewer: How has it been taking the tablets, then, thinking about the process of adjusting the dose, and just taking the tablets?
P23: Fine. I think, because again, there was quite a lot of information sent about that, so there was the sort of suggestion that you could start at one a day, but then, if you felt you had any side effects go to every other day, or increase to two a day, or two every other day. So there was that sort of information about, okay, you’ve got to trial and error this a bit to see what you feel is right for you. P23, female aged 45–54 years with severe IBS, 6-month interview
I actually had some quite bad, kind of, what I considered potentially withdrawal symptoms from the study, as well. […] It might be kind of attributed to a stressful time, but I feel like if there was more information on weaning off the tablets, that might have been useful to kind of prevent that potentially from happening.
P25, female aged 25–34 years with moderate IBS, 6-month interview
Similarly, GP interviewees also reflected on how prescribing a ‘dose range rather than straight dose’ of amitriptyline was a ‘good idea’ and is ‘now common practice’. They also expressed that explaining to patients that they can self-titrate the dose (increasing or reducing as required) helps GPs to promote the benefits of taking amitriptyline for IBS to patients, potentially increasing adherence and reducing appointment time.
I think you need to empower patients to be able to increase the dose themselves otherwise that’s three appointments just to get them to 30 milligrams. … I think the idea that they can titrate themselves is a good one.
GP3, male GP aged 45–54 years with 15–20 years’ experience
I think it’s a good idea to be able to wax and wane it. People know their own body and particularly with IBS things change so much even for one person … so I think it’s a good idea.
GP16, female GP aged 35–44 years with ˂ 5 years’ experience
Desire for a cure
Participants’ desire for a cure and GPs’ desire to offer more patient choice around IBS treatments seemed to explain how patients contribute to an overarching theme that the potential positives of trying amitriptyline for IBS outweighed any concerns. Participants expressed frustration at having unresolved symptoms and feeling they tried everything else (‘you name it; I think I’ve tried it; nothing seems to work’) and as such were highly motivated to try something new, especially if it offered the hope of sustained symptom relief. Hopes for a cure seemed to be a key motivator for taking part in the ATLANTIS trial.
That’s one of the reasons I was quite keen to come on this trial because it offered a ray of hope. A miracle cure! There are things you can try but none of them seem to be very effective. So really, if they could find those little blue pills that cure it, it would be brilliant.
P1, male aged over 65 years with severe IBS, 6-month interview
If you can get some sort of cure for it. That’s what the appealing thing was, if they can come up with some cure.
P28, male aged 45–64 years with moderate IBS, 6-month interview
While GP interviewees did not view amitriptyline as a potential cure for IBS, they recognised the potential benefit of providing another IBS treatment option and being able to offer increased patient choice. GP interviewees commonly appreciated having something else to offer people with IBS and described how prescribing amitriptyline for IBS would allow them to add ‘another string to your bow’ or have ‘another tool in the box’.
Well, I think it’s always useful to have another tool in your box, isn’t it? If you’ve tried other things that haven’t worked, it’s useful to have something else you can give a patient and it’s cheap.
GP3, male GP aged 45–54 years with 15–20 years’ experience
Well, it’s another string to your bow, so it’s another thing to be able to offer. Yes, I think it’s just another offer, really … and these patients have – not necessarily something else.
GP14, male GP aged 45–54 years with 15–20 years’ experience
Being able to provide reliable patient information about amitriptyline for IBS was viewed as an important component in offering more choice and encouraging future prescribing of amitriptyline for IBS. GPs also reflected on the need to update national guidelines and the patient information leaflets that accompany amitriptyline tablets if ATLANTIS demonstrated successful trial outcomes.
If it is effective, then getting that into the patient literature so that when we send them something to have a think about … patients can have a think about it and the pros and cons … having that resource to hand. I mean, if it’s integrated into our online systems round the country to describe the physician support information and integrated into the computer system would be amazing.
GP1, female GP aged 55–65 years, over 20 years’ experience
Comparison between participants allocated to placebo and low-dose amitriptyline
Talk about barriers and facilitators to low-dose amitriptyline for IBS was very similar among trial participants who had been allocated to placebo and those who had been allocated to low-dose amitriptyline.
Experiences of taking part in the ATLANTIS trial
Participants described volunteering for ATLANTIS in the hope that an effective treatment could be found for them and/or others with IBS, often in the context of support from significant others. They described experiencing fluctuating IBS symptoms and described symptom changes over time ranging from improved symptoms through no clear change to possibly worsening symptoms. The predominant view among trial participants was that ATLANTIS was a straightforward and convenient trial to be involved with. Two main aspects of the trial led to positive evaluations of trial participation: the convenience of the tablets and questionnaires; and receiving support from the trial researchers (three research nurses and one clinical study officer). A few small delays were frustrating, but glitches were experienced as well-managed by the trial team and frustrations with specific questionnaires were also noted but did not detract from overall positive evaluations of experiences in the trial.
Volunteering to find something to relieve irritating bowel syndrome symptoms
Trial participants described joining the ATLANTIS trial within the context of ongoing symptoms, to find a way to resolve or to relieve IBS symptoms or to ‘make a difference’. Some participants emphasised the desire for personal benefit while others also spoke about the potential for the trial results to benefit others.
As I’ve had quite extreme problems with IBS, I was quite happy of any chance it might improve things. […] that’s one of the reasons I was quite keen to come on this trial because it offered a ray of hope.
P1, male aged over 65 years with severe IBS, 6-month interview
Yes, so I was contact by my doctors just to ask if I would take part as I’ve been suffering with IBS for quite some time now, so it was really just, I’ve tried different things, I thought I’d give this a try and see if it worked for me. Or if it doesn’t, if it’s helpful for other people who suffer with it as well.
P18, female aged 25–34 years with moderate IBS, 6-month interview
Participants described talking with family members and/or friends about the ATLANTIS trial and being supported to take part.
Well, I’ve spoken to a couple of my good friends, and said this is what I have gone on to, and they’re saying, ‘Well, we wish you luck, that you’ll hopefully find something to sort it’, because I probably show in my face when things are really, really bad because everything’s knotting and grinding, and all the rest of it. I’m sure I’m frowning, and close friends tend to pick up on stuff like that anyway. They were pleased when I said that I’d been selected and was going to give it a go, it was like, ‘Good luck, I hope it works out for you’.
P4, male aged over 65 years with mild IBS, 6-month interview
Symptom fluctuations and changes
Trial participants described experiencing fluctuating IBS symptoms and changes over time which ranged from greatly improved symptoms through no clear change to potentially slightly worsening symptoms.
I think it’s virtually disappeared. There’s still a few things I can’t eat, but not many. Before, I couldn’t eat anything with wheat in; it would make it worse, whereas now I can. I’ve just got a few things left, like lentils I don’t seem to do well with. They’re a very tiny number, so now I can just go out to eat; there’s always something to eat. The same as friends, I don’t have to ask them to do special meals they wouldn’t otherwise do. So yes, I don’t have any problems sleeping; I’m not woken up by cramps and pains. It’s made a huge difference.
P26, female aged over 65 years with severe IBS, 6-month interview
It’s come back a bit, but not as bad as it used to be. I used to get a bout maybe every two or three weeks, whereas now it’s maybe once a month. So, it is an improvement, but it’s not as good as it was at the beginning of the trial.
P8, female aged 55–64 years with mild IBS, 12-month interview
Some weeks it’s worse than others, but yes, I’d say most weeks I experience some form of IBS. Sometimes it’s less painful. Sometimes it’s more. Sometimes it’s, yes, it’s always very up and down. I think it’s to do with my stress levels and stuff. It depends on how I’m feeling.
P18, female aged 25–34 years with moderate IBS, 12-month interview
Yes, when I started the study, I started off taking one tablet a day. Then the instructions were that if I’d had no ill effects, I should start to increase it to two after a certain period and then again if there were no ill effects, I should increase it to three. So, I’ve been taking three a day for quite a long time and it doesn’t seem to make any difference at all.
P2, female aged 45–54 years with severe IBS, 6-month interview
It’s probably worse than it was before. Yes, the last couple of months it’s not been great. Well, as it is sometimes, it goes better and sometimes it goes worse, and it just seems to be slightly worse at the moment.
P10, male aged 55–64 years with mild IBS, 12-month interview
The convenience of trial treatments and questionnaires
Having to take tablets just once a day, ‘Just taking tablets, not a strain’, completing short online questionnaires (at a time convenient to participants) and receiving links and reminders via text and e-mail all seemed to fuel a common perception that the ATLANTIS trial was straightforward and not a burden on participants’ time.
It’s been very non-intrusive. They’ve been very efficient sending the medication and I’ve filled in the weekly surveys. It’s been easy enough. It’s not been arduous. They’re quite short, generally. The survey was just one question. It hasn’t involved any pain or disruption to my life, really. It’s been quite easy to fit in.
P26, female aged over 65 years with severe IBS, 6-month interview
It was all fairly straightforward. It’s not a particular hassle to take the tablets. I think I always felt as though I didn’t really have to think about it very much, because if I needed new tablets, or if there was a questionnaire to fill in or something I’d get a text, so I was always prompted, wasn’t in any way difficult. No, it’s just all fairly straightforward.
P24, female aged 45–54 years with mild IBS, 12-month interview
Receiving support from the researchers
Trial participants reported highly valuing the researcher support throughout the trial. Researchers were seen as friendly, helpful, supportive, and informative, ‘always available to answer any participant queries or concerns’ without being bothersome.
In terms of communication and everything, that’s been really, really positive. The main contact that I have, she’s great [the Researcher]. She’s really friendly, and she’s always made me feel really at ease. I actually feel like when I’m talking to her she’s listening to me, which maybe doesn’t sound a lot, but when you’ve been to the doctor so many times and just been passed off, for somebody to actually listen to you is nice in itself, really. I feel like she is genuinely interested in how the study is going.
P31, female aged 35–44 years with moderate IBS, 6-month interview
They were always contactable. As I say, when I had a problem entering the survey that one week, I just texted [Researcher’s Name] and they took it from there, so I’ve got no problems with the way it’s been dealt with.
P3, female aged over 65 years with moderate IBS, 6-month interview
Delays and glitches
Trial participants reported common glitches in trial processes around medicine supplies and receiving online questionnaires and reminders. These were generally viewed as managed well (i.e. quickly resolved) by the trial team. Early in the trial, some participants described a long start-up period. This seemed to be due to complications with blood tests and was limited to the first few participants recruited into the trial.
We had one minor issue where there was some confusion and I needed more tablets, and there was a cock-up on your end and they didn’t get sent, but it was soon resolved and they got it to me well in time so I didn’t have any days without. It was fine … Even when there was an error, it was resolved. So fantastic.
P37, male aged 35–44 years with severe IBS, 6-month interview
I thought it was very slow at first. I thought, God, I thought I was supposed to be going on this trial and it seemed to take months. It did take months, I think. I just seemed to think am I ever going to start this, you know?
P1, male aged over 65 years with severe IBS, 6-month interview
Frustrations of converting complexities of symptom fluctuations into a binary response option
One common concern about the trial processes related to the perceived inadequacy of binary response options in some of the questionnaires, in particular the weekly question that asked, ‘Have you had adequate relief of your IBS symptoms?’, with response options Yes or No. Trial participants commonly expressed frustration and concerns about whether such response options accurately captured their experiences. For example, participants described finding it difficult to give a global judgement on whether relief had been ‘adequate’ over the course of a whole week, when the meaning of ‘adequate’ was open to interpretation, and when symptoms fluctuate considerably within a short space of time.
I thought the weekly one, the kind of just yes or no response, maybe it should have been more detailed to catch any different changes.
P21, male aged 25–34 years with moderate IBS, 6-month interview
I do think that the questionnaire that we get every week, which I think the question is, ‘Have you had adequate relief of your IBS symptoms this week?’ I find that really, really hard to answer yes or no. I do answer it yes, but it’s subjective, isn’t it, that ‘adequate’, and also in a whole week. Some days, not other days. So that I don’t know if it’s been the most helpful question.
P23, female aged 45–54 years with severe IBS, 6-month interview
Comparison between participants allocated to placebo and low-dose amitriptyline
Experiences of taking part in the ATLANTIS trial were broadly similar in many ways across participants allocated to placebo and participants allocated to low-dose amitriptyline. The exception to this pattern was in the description of experiences of IBS symptoms during the trial. While participants in both trial arms reported symptomatic improvements during the trial, these were more commonly expressed by participants in the low-dose amitriptyline arm. While participants in both trial arms reported little change or possibly worsening symptoms during the trial, these were more commonly expressed by participants in the placebo arm. Overall, participants in both arms of the trial volunteered for similar reasons and were generally positive about their experiences in the trial, they found the tablets and questionnaires convenient and not unduly burdensome, they experienced a few glitches, they found it difficult to answer binary questions about IBS symptoms, and they valued the support received from the researchers.
Reflections on managing IBS during the coronavirus disease discovered in 2019 pandemic
Trial participants predominantly felt that the COVID-19 pandemic had had minimal impact on their IBS as their symptoms had been easier to manage with social restrictions imposed by lockdowns and measures to reduce social contact.
I suppose to some extent managing my IBS in general, maybe, was a little bit easier, because I didn’t have the stress of going out to dinner or meeting people, because you couldn’t do any of those things, so being sat at home and feeling bloated was easier than being out.
P31, female aged 35–44 years with moderate IBS, 6-month interview
I think it’s much improved because I don’t have the pressure of being in an alien environment, or travelling, or having to eat food that I have no choice but to eat if I go to a meeting or out to lunch with a client. You’re kind of restricted, whereas when you’re in lockdown and at home, you can eat what you want to eat, and you’re not embarrassed by the consequences because it’s only you. So, it’s made it much easier to cope with the symptoms.
P32, female aged 55–54 years with mild IBS, 6-month interview
Despite often experiencing increased stress/anxiety due to the pandemic, for example, financial worries, general anxiety about pandemic, participants typically reported that their symptoms were easier to manage due to reduced worries about going out (home working, not eating out, not having to find public toilets) and eating better (more home-cooked food).
I suppose with the pandemic it has caused things to make my IBS worse because of the stress, but then I can’t go out, so in a way it’s easier being at home near the toilet anyway. So, I haven’t got the normal stresses of planning days out or anything like that, but I’ve got a lot of other new stresses about money and things like that. So, I suppose it’s been a good test case really, because I’ve had some less stresses, but some more stresses as well.
P2, female aged 45–54 years with severe IBS, 6-month interview
Comparison between participants allocated to placebo and low-dose amitriptyline
Talk about the impact of the COVID-19 pandemic on IBS was very similar among trial participants who had been allocated to placebo and those who had been allocated to low-dose amitriptyline.
Reflections on treatment allocation
Trial participants appeared to construct narratives around their treatment-arm allocation based on their early and ongoing experiences of their treatment, around their experience (or lack of experience) of symptom improvement and side effects. Four themes captured participants’ talk about treatment allocation – clues to low-dose amitriptyline allocation; clues to placebo allocation; curiosity and concerns about finding out one’s treatment allocation; and disappointment about one’s treatment allocation. Comparisons between participants across the two trial arms are integrated within the presentation of each of these themes.
Clues to low-dose amitriptyline allocation
Trial participants thought they had been allocated to low-dose amitriptyline if they noticed changes in their health. Some participants expressed the thought that they were taking low-dose amitriptyline because they had noticed improvements in their IBS symptoms, such as pain and bowel function.
I think I’ve received real pills. I’m seeing less symptoms. Not getting the stomach cramps quite as often. When I do go to the bathroom, it’s more of a solid consistency, rather than runnier.
P13, female aged 35–44 years with moderate IBS, 6-month interview
Well, initially I must admit I thought, because it cleared up, I didn’t have any problems after about a fortnight into the study, I felt that it was getting better every day, I didn’t have any and then it come to the point where I didn’t have anything for weeks and weeks and I thought I must be on the amitriptyline because why otherwise have my symptoms all stopped?
P29, female aged over 65 years with mild IBS, 6-month interview
Some trial participants thought they were taking low-dose amitriptyline because they experienced side effects associated with amitriptyline such as dry mouth and sleepiness.
Well, you know, if I was a betting man, I would bet on the fact that I’m on an actual drug. I’m going to feel really cheated if I find out I am not. It will show that placebo produces symptoms that you imagine. I really have got a very dry throat. I’ve had that since straight after the first week, and that’s one of the symptoms that they mention. Also, I found that they were making me slightly constipated at first, that’s why I didn’t double up immediately to two, but there we go. No, I mean, yes, I would say that I’m taking amitriptyline.
P5, male aged over 65 years with moderate IBS, 6-month interview
Well, I think I’m taking the antidepressants or whatever they are because I sleep like a log which I really, really like! I’ve never slept as well in years as I do at the moment, right from the start. So that’s the only thing I can put it down to, is the tablets.
P8, female aged 55–64 years with mild IBS, 12-month interview
Some trial participants seemed to be less confident when asked to guess their treatment allocation and talked about hoping that the changes they had noticed meant that they were taking low-dose amitriptyline. This more tentative hope seemed to be related to the challenges of interpreting symptom changes given the fluctuating nature of IBS symptoms and the multiple possible influences on them.
I hoped I was because I genuinely believed that there had been a difference. I did wonder whether the improvement had been because I hadn’t been at work, and I was less stressed. So that could have been part of the improvement, but I’ve not regressed backwards, so that makes me think, well, perhaps I am on the amitriptyline, because I’ve definitely been stressed since I’ve gone back.
P13, female aged 35–44 years with moderate IBS, 12-month interview
When discussing marked benefits or side effects that they attributed to low-dose amitriptyline, trial participants drew comparisons with placebos that suggested placebos were perceived as unlikely to generate such effects.
It’s been great. I found the amitriptyline really helped. If this is a placebo effect then my mind is way too powerful and men should fear me! Since starting to take it, I would say that my bowel function is overwhelmingly just normal […]. The IBS symptoms are not wholly gone, but overwhelmingly, they’re so much more improved than they were.
P33, female aged 25–34 years with moderate IBS, 6-month interview
Straightaway it did what I was warned, one of the side effects, which was the dry mouth. I do have a dry mouth. […]. The only good thing, really good thing that I think that I’ve noticed, is that – and again, I’ll feel very silly if I’ve just been on the placebo, is I don’t sleep well, and I can sleep for England now!
P9, female aged over 65 years with moderate IBS, 6-month interview
Talk about experiencing symptom improvements and/or side effects and interpreting this to mean one was taking low-dose amitriptyline was slightly more common among participants who had actually been allocated to low-dose amitriptyline compared with those allocated to placebo.
Clues to placebo allocation
Consistent with interpreting symptom changes as meaning one was taking low-dose amitriptyline, trial participants interpreted a lack of symptom change to mean they were taking placebo. If they were still experiencing IBS symptoms (‘it’s no worse, no better’) and/or they felt that they had not experienced any of the common side effects associated with amitriptyline, participants typically reported with conviction that they must be on placebo tablets.
I’m on, convinced that I’m on placebo, so, because it’s made, and I’m on three a day and it makes no difference whatsoever. I wouldn’t mind putting a few quid on that I’m on the placebo! Either that or the drug doesn’t work, at all. There’s no change.
P1, male aged over 65 years with severe IBS, 6-month interview
I think I’m on the placebo, well I’m 99 […]. Unless you tell me – you probably don’t know anyway, but I’ve decided I’m on placebo because there’s been absolutely no change at all, no side effects and I have had no beneficial effects whatsoever unfortunately.
P3, female aged over 65 years with moderate IBS, 6-month interview
Talk about experiencing no noticeable change in symptoms and interpreting this to mean one was taking placebo was slightly more common among participants who had actually been allocated to placebo. Only participants who had actually been allocated to placebo described thinking they were in the placebo arm because they had not experienced any side effects.
Curiosity and concerns about finding out one’s treatment allocation
Typically, regardless of actual treatment allocation, trial participants were very keen to know their actual treatment allocation. Nevertheless, concerns were expressed around actual trial treatments not matching expectations/constructed narratives. Some trial participants who thought they were taking low-dose amitriptyline because of changes in their IBS and/or side effects expressed concerns that they would feel foolish if told they had actually been taking placebo. This seemed to be based on understandings of health that drew sharp distinctions between physical and mental processes, and assumptions that placebos can’t trigger any physical symptom changes, which in turn implied for participants that any perceived benefit would mean their IBS symptoms had been generated mentally and were somehow less ‘real’ than previously thought.
I shall feel really stupid if it’s the placebo! I’d just feel really stupid if I’ve been taking a placebo and I’ve suddenly felt much better. I would start to question myself then as to why I went through years of, you know, what caused it. It is because then you think, well, it must have been in my head then. I must have been willing it or something, I must be imagining it, but I know I wasn’t imagining it, definitely I wasn’t. I know how much, so much better I do feel now.
P29, female aged over 65 years with mild IBS, 6-month interview
You think if I do get relief from the IBS, then find out, if we ever do find out whether it’s a placebo, then I’ll know it’s just in my head. Then I’ve got to rethink what have I been thinking the cause was over the years? Maybe actually something completely different. Then I’d think that I’d been a fake for all these years! Again, it’s something out of my control and if it happens, it happens. I’ll come to that when it does.
P10, male aged 55–64 years with mild IBS, 6-month interview
Some participants also explicitly expressed concern about being embarrassed for having wrongly thought they were taking low-dose amitriptyline and having shared that with trial staff and others.
I was a bit nervous. I didn’t like the thought that I would react to something, and it would turn out to be a placebo, because I thought oh it’s a bit weird. Then it makes you feel a bit stupid. So, I thought, what if I say, ‘Oh it’s amazing’ and then I find out I’ve had the placebo? I’d feel a bit of an idiot.
P2, female aged 45–54 years with severe IBS, 6-month interview
If it’s not the drug I’m going to feel a bit stupid because I’ve told you [interviewer] and [the research nurse] without reservation that I’m convinced it is the drug and that the drug has worked. Obviously, if that was not to be the case, well, the other line is that in some way my mind must think that that placebo is doing me good. As I say, I’m convinced that won’t be the case, but I might be proved wrong … If it’s negative, if it’s not the drug I shall feel very silly talking to the nurse having told her that I’m taking something! I shall just hang my head in shame.
P5, male aged over 65 years with moderate IBS, 12-month interview
However, not all participants were concerned about possibly having experienced benefit from a placebo.
Well, I suppose regardless of whether I’m on the placebo and it’s had a psychological impact or whether I’m on the real thing that’s had a physical impact, I do think it has made some improvement to my symptoms, so I would say that I have a positive view of it!
P7, female, aged 45–54 years with moderate IBS, 12-month interview
Fewer concerns were expressed about the other possible mismatch between perceived and actual treatment allocations, that is thinking one had been receiving placebo and then finding out it had been low-dose amitriptyline. The main concern in this scenario was about the implications for the effectiveness of low-dose amitriptyline for IBS. Given that participants interpreted a lack of symptom change as indicating they were taking placebo, if it turned out they were actually taking low-dose amitriptyline then that would suggest this was not an effective treatment for them and their search for IBS relief would continue.
If they’re not [amitriptyline], it means that I can stop taking the damn things, and if they are the real things, well, you’re back to square one. They clearly don’t work!
P1, male aged over 65 years with severe IBS, 12-month interview
Disappointment about one’s treatment allocation
A few participants expressed slight disappointment after finding out their treatment allocation. Participants who were disappointed to find out that they had been taking low-dose amitriptyline were disappointed not to have experienced benefit from the medicine and/or disappointed that this was another treatment that had not helped them.
Yes, interesting because I thought I was taking the placebo. Yes, it was interesting that I was actually taking the real thing. I mean, it was disappointing for me that it didn’t work for me, but I mean, that’s not to say it didn’t work for other people. I’m still glad that I took part and did it. I think just because it wasn’t working for me, I assumed that it was the placebo, I think.
P18, female aged 25–34 years with moderate IBS, 12-month interview
No, because you know it’s a new trial, you know you can be on the placebo, so there were no expectations there, except that you were hoping. You were hoping that this was going to make it all go away, or at least alleviate an awful lot of it, but it didn’t. It didn’t.
P9, female aged over 65 years with moderate IBS, 12-month interview
I can’t see it getting any better. I just can’t see how – I feel like I’ve tried a lot of things, so apart from perhaps pursuing more like a medical route, pushing my GP to maybe see a gastro doctor, I don’t really know. I think it’s hard because you feel a bit pathetic going to your doctor saying, ‘I’m bloated’, because they’ll be like, ‘So?’ [Both laugh] ‘You’re not going to die, you’re fine’. So I don’t feel like I really want to push for anything extra because – it’s annoying, but it’s not like I’ve got diabetes or anything really serious. It’s just one of those things. I can’t really see it changing. I can’t really see it getting any better. I’ll just have to live like this forever.
P42, female aged 18–24 years with severe IBS, 6-month interview
Participants who were disappointed to find out that they had been taking placebo were mainly disappointed that they had not had the opportunity to try low-dose amitriptyline as part of the main ATLANTIS trial. However, they understood that their treatment had been allocated at random.
Fine. It’s part of the study. I understand these things. That’s fine, no problem. It would have been nice to have known if I’d had the real ones whether it had any effect or not.
P27, female aged over 65 years with moderate IBS, 12-month interview
Interestingly, one participant was disappointed not to have benefitted from taking placebo suggesting they appreciated the potential for placebo effects more than those participants who talked about placebos in more negative ways.
I’m a very positive person and I expected it to work, and that’s why I’m jolly disappointed nothing is happening.
P14, female aged over 65 years with severe IBS, 6-month interview
Relating the qualitative and quantitative findings
Table 41 presents a mapping of selected key quantitative findings against the qualitative findings. Quantitatively, both arms showed some improvements in IBS symptoms over time. The qualitative findings position this within the context of participants’ ongoing desire for symptom relief. The qualitative findings further suggest that the increased ease of managing IBS during the pandemic lockdowns and the valued support received from researchers in the trial might explain some of the symptom improvements in both arms.
| Quantitative finding | |||||
|---|---|---|---|---|---|
| Both arms showed improved IBS symptoms over time | Low-dose amitriptyline improved IBS symptoms significantly more than placebo | High rates of adherence to trial medications | High rates of questionnaire completion | ||
| Qualitative theme/topic | Barriers and facilitators | Participants in both arms entered the trial with some concerns; these were outweighed by facilitators and/or wanting relief from IBS symptoms. | Amitriptyline was seen as a familiar drug with familiar side effects. | ||
| Experiences in the trial | Participants in both arms valued the accessible support received from the researchers. | Participants receiving low-dose amitriptyline were more likely to report symptom improvements and less likely to report no change compared with those receiving placebo. | Participants in both arms found the trial medications convenient to take. | Participants in both arms found the trial questionnaires convenient. Participants in both arms found binary questions frustrating and difficult to answer due to the fluctuating nature of IBS symptoms. | |
| Reflections on the pandemic | Some participants in both arms felt the pandemic eased the impact of IBS. | ||||
| Reflections on treatment allocation | Participants attributed symptomatic improvements or side effects to low-dose amitriptyline; participants attributed no changes to placebo. Not all participants who received low-dose amitriptyline felt their symptoms had improved. Participants who received placebo were disappointed not to have tried low-dose amitriptyline. |
||||
Quantitatively, low-dose amitriptyline improved IBS symptoms significantly more than placebo. This pattern was also observed in participants’ qualitative talk about symptom changes during the trial. Participants’ reflections on treatment allocation suggest some, but not all, participants may have accurately guessed their treatment allocation; these qualitative data are insufficient to suggest whether or how much such guessing may have contributed to between-arm differences.
Quantitatively, rates of adherence to trial medications were high. This maps to the qualitative data on the acceptability of amitriptyline as a familiar, known, drug and the convenience of taking small tablets on a once-daily basis.
Quantitatively, rates of questionnaire completion were high. Qualitatively, participants typically found the questionnaires convenient and straightforward and accepted the need for these as part of the trial. However, they also found it frustrating to have to give a binary response in the context of fluctuating symptoms, and support from researchers may have been important in overcoming this.
Discussion
Overview
This qualitative study conducted and thematically analysed 77 semistructured interviews with 42 participants and 16 GPs taking part in the ATLANTIS trial. A multidisciplinary team including patient collaborators has explored multiple aspects of participants’ and GPs’ experiences of treatments and participating in the ATLANTIS trial: barriers and facilitators to uptake of low-dose amitriptyline for IBS; experiences of taking part in the ATLANTIS trial; reflections on managing IBS during the COVID-19 pandemic; reflections on treatment allocation. Each set of findings is summarised and discussed in turn below, drawing out implications for theory, research, and/or future efforts to promote wider use of amitriptyline for IBS where appropriate. The qualitative findings are related to the trial findings; strengths and limitations are considered.
Barriers and facilitators
Among participants and GPs who took part in the ATLANTIS trial, potential barriers that could hinder prescribing and uptake of low-dose amitriptyline for IBS included: participants’ and GPs’ concerns about possible stigma associated with a medication commonly known as antidepressant; medicalising IBS and the associated administrative burdens for GPs of increased prescribing; and side effects including possible mental health effects (expressed by patients and linked to seeing amitriptyline as an antidepressant) and potentially contributing to anticholinergic burden in some (older) patients (expressed by GPs).
Stigma associated with mental illness has decreased in England in the past decade,49 but stigma related to the use of antidepressants may be distinct from stigma related to depression. 50 Notably, participant interviewees’ concerns about the stigma of taking antidepressants did not deter them from volunteering for the ATLANTIS trial, although of course other patients with stronger concerns may have been deterred.
Participants and GPs were encouraged to try/prescribe amitriptyline for IBS by the low and flexible recommended dosage, its potential to offer benefits beyond IBS symptom relief including for example, its effects on sleep, and perceived ease of treatment (once daily dosing and small tablets) including the participant self-titration process, which most participants found acceptable and empowering. Simple treatment regimens may facilitate adherence to medication,51 where adherence to complex dietary regimens such as FODMAP can be poor52 and difficult for patients to manage within the context of daily life. 46,53 Being able to reframe minor ‘adverse’ effects as potential benefits (such as effects on sleep) could help reduce concerns and thus might increase adherence. 47,54 Empowering patients to self-titrate their dose, with the support of a dose titration document carefully developed with PPI and clinician input, is consistent with increasing patient engagement55 and participation within patient-centred care. 56
Consistent with the common-sense model of illness perception44 and previous work in IBS,46,57 participant-perceived ongoing need for symptom relief facilitated their uptake of a novel treatment, in this case low-dose amitriptyline for IBS. Some participants expressed this in terms of wanting a ‘cure’, suggesting that they may perceive IBS as an acute condition to be cured instead of a long-term condition to be managed. 58 Our findings were also broadly consistent with the necessity-concerns framework, according to which patients adhere to a specific medication for a specific condition when their perceived need for the treatment is greater than their concerns about it. 47 While participant interviewees expressed concerns about low-dose amitriptyline for IBS, these were outweighed by the perceived need and desire for symptom relief and the benefits that participants hoped for (at the start of the trial) and experienced (during the trial).
Our findings map to three key concepts from normalisation process theory. 59 The intervention was ‘coherent’ to GPs, in that low-dose amitriptyline for IBS made sense to GP interviewees. GPs demonstrated ‘cognitive participation’ in the intervention, in that they understood the potential benefits of low-dose amitriptyline for people with IBS, appreciated its ease of use and therefore committed to it. In order to facilitate ‘collective action’, GPs would value additional patient-facing resources to support prescribing low-dose amitriptyline for IBS.
Barriers and facilitators to low-dose amitriptyline for IBS were identified in the context of participants’ desire for a novel approach to IBS: GPs were keen to offer more options for IBS and patients sought a cure for their symptoms. This is consistent with previous work examining the wider impact of IBS on patients’ lives and highlighting the challenges of treatment-seeking. 46,60,61 It suggests GPs and primary care patients with IBS may consider low-dose amitriptyline for IBS despite concerns and/or after their concerns are appropriately addressed.
Overall, the analysis of barriers and facilitators suggests that low-dose amitriptyline for IBS is likely to be acceptable to and, in some cases, welcomed by GPs and participants as an additional treatment option. The familiarity of amitriptyline may both hinder uptake (given its association with depression) and facilitate it (given that it is known and taken in a low dose, distinct from the antidepressant dose, by others for a range of conditions and has comparatively mild and in some cases potentially beneficial side effects such as on sleep). GPs’ and participants’ desire for another treatment option for IBS does not obviate the need to address concerns about amitriptyline. Addressing concerns and promoting facilitators could in turn promote wider use of low-dose amitriptyline for IBS and might be achieved through:
-
Clear communication to clinicians, for example in clinical guidelines, that distinguishes low-dose amitriptyline for IBS from amitriptyline for other conditions (especially depression).
-
Resources to support GP–patient communication to distinguish low-dose amitriptyline for IBS from amitriptyline for other conditions (especially depression). This might include, for example, tips for GPs discussing amitriptyline for IBS with patients, online materials to support or reinforce messages given during consultations, tailored packaging and patient inserts, and education for pharmacists.
-
Clear guidance about low-dose amitriptyline for IBS and anticholinergic burden. This should highlight that low-dose is lower risk and that, currently, anticholinergic burden risk scores do not account for this, thus they can overinterpret risk with low-dose amitriptyline.
-
Guidance and resources for GPs and patients to support patients managing their own dose titration.
Experiences of the trial
Similar to other trial participants, participants described volunteering for ATLANTIS in the hope that an effective treatment could be found for them and/or others with IBS, often in the context of support from significant others. 62 Similar to previous studies of patients’ experiences of IBS, participants described experiencing and trying to monitor their fluctuating IBS symptoms. 60,63 Participants found it difficult to answer binary outcome measures that did not permit them to communicate these symptom fluctuations; qualitative studies nested in other IBS trials have also observed the challenges for participants of providing confident global evaluations of their condition. 63 Future studies in IBS should move away from binary outcome measures given the difficulties faced by participants completing them and the potential for bias and/or measurement error that such difficulties may introduce. Participants particularly valued the convenience of the tablets and brief, online, questionnaires, and the accessibility and support received from the researchers. This valued support from the researchers, both in relation to completing outcome measures and providing reassurance, may have contributed to the improvements seen in the placebo arm64 and reinforces the importance of good support for patients in clinical settings when initiating amitriptyline.
Irritable bowel syndrome during the coronavirus disease discovered in 2019 pandemic
Several quantitative survey studies have examined the impact of the COVID-19 pandemic on IBS symptoms. Findings have highlighted negative impacts of the COVID-19 pandemic on IBS. For example, compared with those seen in the previous 12 months, people seen in one tertiary care centre in England for refractory IBS during the pandemic had higher IBS symptom severity and other symptoms. 65 In comparison, an international survey of 305 people self-reporting IBS found that the majority reported no change in their IBS symptoms, while 27% reported symptom improvements and 12% reported symptom deterioration. 66 Our findings suggest a nuanced picture in which some people with IBS found it easier to cope with their symptoms and experienced less stress and concern about their symptoms during COVID-19 lockdowns (in the absence of eating outside the home) compared with before COVID-19.
Treatment allocation
Trial participants appeared to construct narratives around their treatment-arm allocation based on their early and ongoing experiences of their treatment, around their experience (or lack of experience) of symptom improvement and side effects. Participants thought, or hoped, that they had been allocated to low-dose amitriptyline when they perceived symptom benefit (though found this challenging to interpret in the context of typical fluctuations) and/or side effects such as dry mouth or sleepiness. Conversely, participants thought they had been allocated to placebo when they perceived ongoing IBS symptoms with no noticeable improvements and/or they felt that they had not experienced any of the common side effects associated with amitriptyline. This is consistent with evidence from other trials in IBS, suggesting common-sense attributions of symptom changes based at least in part on patients’ beliefs about the (beneficial and adverse) effects of the active drug compared with placebo in the context of their illness and symptom perceptions. 67–69
Only participants allocated to the placebo arm discussed experiencing a lack of side effects and interpreting this to mean they were in the placebo arm. This is consistent with evidence suggesting medication side effects may contribute to patient unblinding in IBS trials;70 patient unblinding may in turn bias effect size estimates in favour of the active drug, although effective blinding of investigators is also very important. 71
As in previous trials, participants expressed curiosity about finding out their own personal treatment allocation in the trial and sometimes expressed concerns that their perceived treatment allocation may not match their actual treatment allocation. 63,72,73 Some participants were disappointed on finding out they were taking low-dose amitriptyline because they had not experienced benefit from the medicine and so their search for symptom relief had to resume. Some participants were disappointed to find out that they had been taking placebo because they had not had the opportunity to try low-dose amitriptyline as part of the ATLANTIS trial. It is unsurprising that some participants felt disappointed in these ways, given the refractory nature of ATLANTIS participants’ IBS and the desire for symptom relief that underpinned decisions to volunteer for ATLANTIS. Participants’ curiosity, concerns, and reactions to being unblinded to treatment allocation support our development and use in ATLANTIS of a novel information leaflet to support sensitive participant unblinding to treatment allocation that addresses common concerns (to be reported separately).
Strengths and limitations
Despite using convenience sampling instead of the planned purposive sampling, a diverse sample of participants from each trial arm were included in the qualitative study, ensuing that our findings are not based on a narrow subset of the people who took part in the trial. Although we achieved a diverse range of views, participants willing to undertake the qualitative interviews may not hold the same views as all the participants in the study. Unfortunately, no GPs from the West Yorkshire hub agreed to be interviewed: one declined the invitation without giving a reason and one was part of the qualitative team; no other responses were received. There were fewer participating practices and GPs in West Yorkshire compared with the other two hubs, and West Yorkshire practices were more severely impacted by longer periods in lockdown, limiting capacity for research activity.
Conducting interviews with the same participants repeatedly over time permitted the development of rapport and the elicitation of experiences over a longer time period than is often achieved. Future trials might benefit from including a very early interview time point (e.g. at 2–4 weeks) to explore participants’ initial impressions and experiences of trial interventions; in the case of ATLANTIS, this would have enabled us to capture participants’ perspectives on titrating closer in time to when they were engaged in this aspect of the trial.
Interviewing participants and GPs involved in ATLANTIS permitted a more comprehensive analysis of barriers and facilitators to low-dose amitriptyline for IBS than would have been possible by including only one of these groups. This was essential for developing insights and recommendations for future widespread implementation of low-dose amitriptyline for IBS.
The multidisciplinary nature of the qualitative team including input from patient collaborators meant that we approached the data from diverse perspectives, achieving richer insights than would otherwise have been possible.
Including stand-alone objectives for the qualitative component, as well as relating the qualitative findings to the quantitative findings, maximised the value of adding a qualitative component to a major RCT. Remaining blinded to treatment allocation and not knowing the main trial results while conducting the primary qualitative data analysis ensured interpretations were not shaped by researchers’ perspectives on treatment allocation and efficacy. 74 Subsequent unblinding of researchers facilitated deeper qualitative analysis and mapping of qualitative to quantitative findings, thus enhancing the value of the nested qualitative work and increasing integration during the interpretation phase. 75
Conclusion
This qualitative study has explored participants’ and GPs’ experiences of treatments and participating in the ATLANTIS trial. Findings build on previous work examining participants’ experiences of IBS in the community and in other clinical trials. Facilitators and barriers to prescribing and uptake of low-dose amitriptyline in IBS have been identified and explored within the broader context of participants’ and GPs’ experiences of living with and seeking treatment for IBS. This analysis has generated recommendations to support dissemination activities to enable wider use of low-dose amitriptyline for IBS which in turn should provide more management options and patient benefit. Analysis of participants’ experiences of the trial including outcome reporting, researcher support, and treatment allocation has also generated insights that can inform future research. Future work should develop resources to implement recommended actions to support wider use of low-dose amitriptyline for IBS.
Chapter 5 Discussion
ATLANTIS was a pragmatic, double-blind randomised trial to assess the effectiveness and cost-effectiveness of low-dose amitriptyline as a second-line treatment for IBS in primary care. To our knowledge, it is the largest trial of a TCA in IBS ever undertaken and the first to be based entirely in primary care. The trial was designed to address a key research priority identified by NICE guidance for management of IBS in primary care in 2015. 16 The trial recruited participants who had ongoing troublesome symptoms despite trying first-line therapies, with very similar characteristics to those with a diagnosis of IBS on GP records who were invited by letter to participate, meaning the results are relevant and generalisable to usual clinical practice in primary care. The median duration of symptoms was 10 years, and more than 80% had moderate to severe symptoms.
In this population, low-dose amitriptyline was shown to be superior for the trial’s primary outcome, with a significant difference in mean IBS-SSS score between arms of 27.0, and a mean decrease in IBS-SSS of almost 100 points at both 3 and 6 months, compared with baseline. Low-dose amitriptyline was also superior to placebo for the key secondary outcome for effectiveness, with an OR for SGA of relief of global IBS symptoms of 1.78. Amitriptyline also improved other IBS symptom outcomes, including adequate relief of IBS symptoms and a ≥ 30% decrease in abdominal pain on the IBS-SSS at 6 months. However, there was no significant effect of low-dose amitriptyline on abdominal distention, somatoform symptom-reporting scores, or anxiety or depression scores, nor was there any impact on work and social activities during 6-month follow-up.
In the subset of participants recruited to 12 months of follow-up, and with the choice to continue treatment beyond 6 months, 44% of participants completed 12 months treatment. Despite the mixed sample, weak evidence of a significant effect in favour of low-dose amitriptyline remained on the IBS-SSS, with a mean difference of 22.6 and on the SGA of relief of global IBS symptoms with an OR of 1.58. In contrast to the 6-month results, there was a statistically significant effect in favour of low-dose amitriptyline on HADS-depression scores, with a mean difference of −0.88, and on WSAS scores, with a mean difference of −2.14.
Significantly more participants randomised to low-dose amitriptyline found it acceptable to take than placebo and almost three-quarters adhered to the drug during the 6-month trial. Although AEs were more frequent with low-dose amitriptyline, the drug was generally safe and well-tolerated. The AEs reported in participants receiving amitriptyline in excess of those reported by the placebo arm mainly related to its known anticholinergic effects, including drowsiness and dry mouth, but most participants reported these as mild, and SAEs and SARs were rare. However, withdrawals due to AEs were slightly more frequent with low-dose amitriptyline, occurring in 12.9% assigned to amitriptyline compared with 8.7% in the placebo arm.
The 6-month duration of treatment in ATLANTIS is in line with European Medicines Agency (EMA) recommendations for IBS treatment trials76 and is longer than most drug trials in IBS, where efficacy is usually assessed over 12 weeks. The results of the trial are, therefore, likely to be more representative of the effectiveness of low-dose amitriptyline in IBS, a condition that, for many people, is a relapsing and remitting disorder. 15 The outcomes studied are ones that are accepted widely in pragmatic trials conducted in IBS, including a mean change in the total IBS-SSS and adequate relief of symptoms of IBS. Follow-up rates for participant-reported outcomes at 6 months were high (over 85%). As the analyses were conducted on all participants, irrespective of adherence, and with imputation of missing data, it is unlikely that the effectiveness of low-dose amitriptyline for IBS in primary care has been overestimated.
Limitations
The primary outcomes in the ATLANTIS trial differed from those recommended for drug trials in IBS by the Food and Drug Administration (FDA) in the USA and the EMA in Europe. 76,77 It would have been impractical to adhere to these in a pragmatic 6-month trial recruiting participants in primary care for the following reasons. First, these are IBS subtype-specific, which would have meant using different outcome measures in different groups of participants. Second, we recruited participants with IBS of all subtypes, including IBS-M or IBS-U, but there is no clear consensus on recommended end points for these two subtypes. Third, completion of a daily diary, which would be required to assess FDA or EMA end points, would have been too burdensome for participants for a 6-month period. However, the secondary outcome of adequate relief of IBS symptoms, which allows relief for 50% of weeks of the trial to be assessed, and the exploratory outcome of a ≥ 30% improvement in abdominal pain on the IBS-SSS, were both significantly higher with low-dose amitriptyline. These approximate to the FDA and EMA recommended end points and are more stringent without requiring completion of a daily diary outcome specific to IBS subtype.
Over 80% of recruited participants had either IBS-D or IBS-M. This means it may be harder to judge the effectiveness of low-dose amitriptyline definitively in those with IBS-C or IBS-U. However, although exploratory moderator analysis found no statistical evidence of a moderating effect, larger treatment effects, in favour of amitriptyline, were observed in participants with IBS-C or IBS-D compared with those with IBS-M or IBS-U on the IBS-SSS, and in participants with IBS-D compared with those with IBS-C, IBS-M or IBS-U on the SGA of relief of IBS symptoms. As we were not powered for the moderator analysis, the trial results cannot be used to make inferences about effectiveness of amitriptyline according to IBS subtype. Similarly, we are also likely to be underpowered for some of our secondary end points, including effect on anxiety and depression scores. These may be expected to improve as symptoms of IBS improve but, perhaps, to a lesser degree, and therefore the differences we detected were not statistically significant.
Although the primary outcome was at 6 months, we had planned to offer all participants the option to continue blinded trial medication until 12 months and follow up all participants until 12 months post randomisation. However, the COVID-19 pandemic interrupted the delivery of the trial, and a funded extension was required to complete recruitment. This meant that follow-up after 7 October 2021 was curtailed to 6 months for new participants to minimise additional funding required to complete the trial and to prioritise funds for participant recruitment. Thus, interpretation of the 12-month outcomes is difficult, as there were fewer participants able to choose to continue trial medication than anticipated. For similar reasons, the health economic analyses were removed from the trial. This means that we could not perform a cost-effectiveness analysis as part of the trial. However, given that amitriptyline is cheap, we do not feel this should be a barrier to the implementation and uptake of the findings of the trial. In addition, we cannot exclude that the COVID-19 pandemic itself may have had an impact on trial results, and that participants in both arms experienced a larger improvement in IBS symptoms than expected due to the reduced ability to work and socialise for long periods of time.
Another limitation is that participants entering the trial were not ethnically diverse, which may limit generalisability of the findings to these populations – see Equality, diversity and inclusion for more detail. Finally, because we used the Rome IV criteria to define IBS, the generalisability of the results to patients with a GP’s diagnosis of IBS, who may not meet these criteria, is uncertain.
Generalisability
The definition of IBS used consisted of the current recommended symptom-based criteria, the Rome IV criteria,34 together with limited diagnostic testing to exclude known organic ‘mimics’ of IBS in all participants. This is in line with current UK guidance for the diagnosis of IBS. 16,78 Participants were recruited irrespective of IBS subtype, with symptoms ranging from mild to severe, and these came from a broad range of general practices in three different geographical regions in the UK. Amitriptyline was provided in addition to usual care and current first-line IBS medications, and participants were able to self-titrate their dose between 10 mg on alternate days and 30 mg every day according to symptom response and side effects. This is how amitriptyline is commonly managed for other conditions in primary care and is pragmatic, reflecting usual clinical practice outside a trial setting.
The results of the trial are, therefore, likely to be generalisable to many patients with IBS in UK primary care who have not experienced adequate improvement in their symptoms with first-line therapies. The qualitative findings on guidance and resources needed for improved clinician-patient communication to distinguish low-dose amitriptyline for IBS from use as an antidepressant and to support patients managing their own dose titration are also likely to be generalisable to clinical interactions in secondary care settings.
Interpretation
The Rome IV criteria are known to select a group of patients with higher symptom severity than previous iterations. 79 This is borne out by the mean IBS-SSS scores seen at baseline, which were in the moderate to severe range in more than 80% of participants, and the median duration of IBS among participants was 10 years. Given this, and the relatively long treatment duration of 6 months, the placebo response rates observed in ATLANTIS may appear relatively high, with 36% of those assigned to the placebo arm reporting that they thought the trial medication had worked. However, the placebo response in trials in IBS is known to be high,80,81 and there is evidence that patients with IBS are more likely to respond to a placebo than to a no-treatment control intervention. 82,83 Other possible explanations for this within the trial design include the fact that all participants were provided with the NICE-approved BDA dietary advice sheet, that regular follow-up and usual GP care were allowed throughout the trial, and the regular supportive telephone calls from a research nurse to assist with dose titration and re-supply of trial medication. In addition, as demonstrated by the nested qualitative study results, participants may have felt a sense of empowerment and being more in control of their symptoms because they were able to self-titrate their medication dose during the trial in response to symptoms and side effects. Finally, a regression towards the mean effect during follow-up is well-recognised in clinical trials. However, all of this makes the highly statistically significant benefit seen across almost all IBS outcome measures particularly noteworthy.
Several prior meta-analyses of TCAs in IBS have demonstrated that these drugs, as a class, are superior to placebo. 7,9,84 However, there are limitations of the trials that have been included in these meta-analyses. Most trials, to date, have been relatively small. None of the trials have exceeded a maximum treatment duration of 3 months, nor have any previous trials been conducted entirely in primary care. Finally, many have used definitions of IBS that are no longer in active use and have assessed efficacy using outdated or non-rigorous outcomes. The largest RCT conducted to date recruited 216 female patients with various functional bowel disorders in the USA, 172 of whom had IBS. 85 This trial used the TCA desipramine, commencing at a dose of 50 mg o.d. for 1 week, titrated up to 150 mg o.d. by week 3. As in ATLANTIS, the commonest side effects related to the anticholinergic effects of the drug, including dry mouth in 48% and drowsiness in 20% of participants. Rates of discontinuation of desipramine due to AEs were higher than observed in ATLANTIS, at 20%. This may relate to the higher dosage of desipramine used, compared with the low dose of amitriptyline studied here. In this trial, the primary outcome, which was a composite of patient satisfaction, improvement in symptoms, and increased engagement in social activities, was not met. There was a 60% response rate with desipramine versus 47% with placebo (p = 0.128). However, in subgroup analysis desipramine was superior to placebo in those with moderate, rather than severe, symptoms and in those with IBS-D. Interestingly, despite the high dose of desipramine used, the presence of abnormal depression scores at baseline had no impact on treatment response. In another 8-week trial recruiting 54 patients with IBS-D in secondary care in Iran,20 the response rate with amitriptyline 10 mg o.d. was 70%, compared with 41% with placebo, but this difference was not statistically significant at the 5% level (p = 0.054). Based on our required sample size, it is likely the trial was underpowered. AE rates in this trial were similar between treatment arms. Treatment effects in ATLANTIS on our primary outcome, the IBS-SSS, were generally larger in those with IBS-C or IBS-D, those with lower baseline HADS-anxiety scores, and those with higher baseline IBS-SSS scores.
The magnitude of the observed difference in treatment effect on the IBS-SSS increased between the 3- and 6-month follow-up points from 23 points to 27 points. In addition, the difference in rates of a ≥ 30% improvement in abdominal pain on the IBS-SSS between amitriptyline and placebo was only statistically significant at 6 months, as the placebo response in terms of abdominal pain fell from 46.9% to 42.6% and increased in the amitriptyline arm from 52.3% to 55.7%. These observations highlight the importance of allowing adequate time for low-dose amitriptyline to have a beneficial effect on symptoms in IBS and are compatible with reports of a decrease in placebo response rates as trial duration increases. 80
There was no significant effect of low-dose amitriptyline on somatoform symptom-reporting, anxiety, or depression scores during the 6 months of the trial. This supports previous hypotheses that the benefit of low-dose amitriptyline in IBS is arising from a combination of its peripheral actions on gastrointestinal motility and pain sensation,86,87 rather than via a reduction in extra-intestinal symptom-reporting, or an improvement in symptoms of anxiety or depression, all of which are well-recognised to associate strongly with a diagnosis of IBS. 88,89 Additionally, there was no significant impact on either ability to work or social functioning, according to WSAS scores. However, the reduction in scores was generally greater among participants randomised to low-dose amitriptyline. Although IBS can have substantial impacts on these measures of activities of daily living,90,91 the treatment duration may have been too short to detect any meaningful improvements, as supported by superior effects detected on the WSAS and HADS-D with amitriptyline at 12, but not 3 or 6, months. Additionally, we cannot exclude the possibility that the COVID-19 pandemic had an impact on this measure.
The flexible dosing schedule allowed participants to increase or reduce their dose according to symptom response and side effects. It is interesting to note that by 3 months 57.0% of participants in the placebo arm had already titrated to 30 mg o.d., whereas only 37.8% of those randomised to amitriptyline had reached this. Although 42.8% were taking 30 mg o.d. of amitriptyline by 6 months, almost one-in-four participants were only taking 10 mg o.d. This flexible dosing may also have contributed to tolerability. Amitriptyline at low dose was safe and well-tolerated by participants. There were few SAEs or SARs, no SUSARs or deaths, no pregnancies, and no emergency unblinding events. Although treatment-emergent AEs were generally higher among those receiving amitriptyline most of these were mild, and the overall rates of AEs fell between 3 and 6 months. However, to some extent this is not unexpected as participants reporting AEs may have been more likely to stop treatment before 6 months and ASEC data were only collected for those still on treatment. Some of the side effects reported, such as constipation and diarrhoea, are known symptoms of IBS, and many of the other possible side effects on the ASEC were reported at a similar, or higher, frequency by the placebo arm. In any case, participants were significantly more likely to report that they found amitriptyline acceptable to take, compared with placebo, at 6 months. Finally, adherence rates were high in the amitriptyline arm: 83.2% at 3 months and 74.1% at 6 months. This underlines that if the rationale for the use of a TCA for IBS is explained clearly, as in the information materials provided to participants in this trial, with appropriate support, the majority of people are willing and able to take the drug.
Overall evidence
The ATLANTIS trial provides strong evidence for the effectiveness of low-dose amitriptyline, at a dose of between 10 mg o.d. and 30 mg o.d., as a second-line treatment for IBS in primary care, when first-line treatment has not led to an adequate improvement in symptoms. Amitriptyline was more effective than placebo across a range of IBS symptom measures, and was safe and well-tolerated, when titrated according to symptom response and side effects. Therefore, GPs should offer low-dose amitriptyline to patients who have ongoing troublesome symptoms despite trying first-line therapies. Management guidelines for IBS in primary care need to change to reflect the findings of this definitive trial. Additionally, as mentioned in the qualitative chapter, guidance and resources are needed to support GP–patient communication to distinguish low-dose amitriptyline for IBS from use as an antidepressant and to support patients managing their own dose titration.
Recommendations for future research
It is important to assess the health economic benefits of low-dose amitriptyline for IBS, especially in light of the positive trial results. Although health economic data were collected during the ATLANTIS trial, unfortunately the analysis was unable to be conducted due to the impact of COVID-19 on the trial, with funding being redirected to participant recruitment. Further funding is needed to make use of these valuable data.
ATLANTIS has demonstrated that low-dose amitriptyline is superior to placebo as a second-line treatment for IBS in secondary care. However, it is unclear whether there are particular groups of patients who are more likely to respond to the drug. It is also unclear whether other low-dose antidepressant drugs, such as selective serotonin reuptake inhibitors or serotonin norepinephrine reuptake inhibitors, are also effective for IBS in primary care. In addition, the question remains whether using low-dose amitriptyline as a first-line treatment, potentially earlier in the course of IBS symptoms, is an effective strategy in primary care.
Equality, diversity and inclusion
The participants recruited were not ethnically diverse, despite considerable efforts to reach out to ethnic minorities with IBS during the trial. Given that a recent global survey demonstrated that the prevalence of IBS is similar across multiple countries,2 this likely represents under-sampling of this population due to inequities in, or barriers to, accessing health care and research, rather than differences in the epidemiology of IBS. However, unlike many treatment trials in IBS, where often 80–90% of participants are female, more than 30% of recruited participants were male, there were a wide range of ages and educational backgrounds among participants, and different deprivation indices were well-represented. In addition, the trial was conducted in three distinct geographical regions and those recruited were very similar in age and gender to those invited after searching for an IBS diagnosis on GP lists.
Patient and public involvement
Patient and public involvement representatives were involved prior to the grant application stage and throughout the trial, analysis, and interpretation of the results. They will continue to play a very important role in dissemination of the results.
The trial team includes a public co-investigator who leads ‘Let’sCureIBS’, a local patient support group. He provided valuable, personal experience of IBS and facilitated wider engagement with group members at key points throughout the study. The CTRU PPI lead developed and implemented PPI plans and provided support to public contributors. Examples of PPI input and impact include:
-
Study design – a workshop was held with patients at the grant application development stage, which informed key design and operational decisions: for example, participants being given the choice to cease or continue treatment at 6 months, the use of electronic questionnaires, additional participant support from GPs and researchers, and medication being sent to people’s homes via post.
-
Project management – our public co-investigator joined the TMG and qualitative subgroup, enabling us to seek a patient perspective on emerging issues throughout the study.
-
Project oversight – an additional public contributor joined the TSC, ensuring that the patient perspective was included in high-level decisions about the study.
-
Site set-up – one hub conducted a workshop with research staff who would be recruiting to the study. The workshop was attended by a public contributor and included role-play scenarios co-developed by patients, which enabled staff to rehearse recruitment conversations.
-
Participant communication – PPI was an important part of shaping all participant communication, including invitation and thank you letters, dose titration guidance, and unblinding information. PPI feedback was particularly valuable during development of the participant information sheets. For example, people highlighted the need to explain clearly why amitriptyline might be used for IBS: that is, due to its impact on pain and bowel function rather than mood. This is important due to the potential stigma associated with a drug also used as an antidepressant.
-
Data collection – the online system used for PROM completion was user-tested and PPI helped shape the instructions given to participants.
-
Nested qualitative study – PPI input guided the development of GP and patient interview topic guides. Public contributors also provided general advice to interviewers; for example, how to approach the topic of IBS in a sensitive manner, and the importance of being clear about the boundaries of their role and the fact they are not able to provide medical advice. A public co-investigator was also involved in qualitative data analysis, contributing to the development of a coding framework, themes, and interpretation.
-
Interpretation and dissemination of results – an interpretation workshop was held, where preliminary results were presented to public contributors. They were asked to consider what the results mean for patients and the NHS and how they should be shared. That workshop has influenced the conclusions presented in this monograph, as well as our ongoing dissemination plans. The group highlighted some important dissemination messages for patients and GPs:
-
Titration is useful and needs to be supported by GPs and good quality information.
-
Patients need to be made aware that it may take some time to get the right dose for them and feel the benefit of amitriptyline.
-
GP contact and support are an important part of this intervention.
-
The fact that HADS scores did not improve is very relevant to patients, as it supports the fact that this is not a psychological intervention.
-
Conclusions
This rigorously conducted, pragmatic trial is the largest trial of amitriptyline for IBS undertaken to date, worldwide and the first in primary care. It provides definitive results indicating that GPs should offer low-dose amitriptyline to patients with IBS whose symptoms do not improve with first-line therapies. This recommendation should be widely disseminated to clinical settings in primary and secondary care and incorporated into guidelines for IBS management. We will publicise the results to participants, and other people with IBS, via the ATLANTIS trial website (https://ctru.leeds.ac.uk/atlantis/) and X (formerly Twitter) account (@ATLANTISTrial).
Guidance and resources are needed to support GP–patient communication to distinguish low-dose amitriptyline for IBS from its use as an antidepressant and to support patients managing their own dose titration. The dose titration document successfully used by participants in this trial is included in Report Supplementary Material 1.
Additional information
CRediT contribution statement
Alexandra Wright-Hughes (https://orcid.org/0000-0001-8839-6756): Data curation (lead), Formal analysis, Methodology, Resources, Software, Validation, Visualisation (lead), Writing – original draft, Writing – editing and reviewing.
Alexander C Ford (https://orcid.org/0000-0001-6371-4359): Conceptualisation (lead), Funding acquisition (lead), Investigation, Methodology, Resources, Supervision, Visualisation, Writing – original draft, Writing – editing and reviewing.
Sarah L Alderson (https://orcid.org/0000-0002-5418-0495): Conceptualisation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – editing and reviewing.
Pei Loo Ow (https://orcid.org/0000-0001-6025-6372): Data curation, Formal analysis, Methodology, Resources, Software, Visualisation, Writing – editing and reviewing.
Matthew J Ridd (https://orcid.org/0000-0002-7954-8823): Funding Acquisition, Investigation, Methodology, Resources, Supervision, Writing – editing and reviewing.
Robbie Foy (https://orcid.org/0000-0003-0605-7713): Conceptualisation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – editing and reviewing.
Felicity L Bishop (https://orcid.org/0000-0002-8737-6662): Conceptualisation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Visualisation, Writing – original draft, Writing – editing and reviewing.
Matthew Chaddock: Funding acquisition, Methodology, Writing – editing and reviewing; PPI activities.
Heather Cook (https://orcid.org/0000-0003-0223-417X): Investigation, Methodology, Project administration, Resources, Writing – editing and reviewing.
Deborah Cooper: Investigation, Resources, Writing – editing and reviewing.
Catherine Fernandez (https://orcid.org/0000-0001-8561-5642): Investigation, Methodology, Project administration, Resources, Writing – editing and reviewing.
Elspeth A Guthrie (https://orcid.org/0000-0002-5834-6616): Conceptualisation, Funding acquisition, Methodology, Writing – editing and reviewing.
Suzanne Hartley (https://orcid.org/0000-0003-2346-9461): Funding acquisition, Methodology, Project administration, Supervision, Writing – editing and reviewing.
Amy Herbert (https://orcid.org/0009-0008-6109-6006): Investigation, Resources, Writing – editing and reviewing.
Daniel Howdon (https://orcid.org/0000-0001-8052-2893): Methodology, Resources, Writing – editing and reviewing.
Delia P Muir (https://orcid.org/0000-0003-1136-3416): Funding acquisition, Methodology, Writing – editing and reviewing, PPI activities.
Sonia Newman (https://orcid.org/0009-0007-6827-2861): Investigation, Resources.
Christopher A Taylor (https://orcid.org/0009-0009-8220-4923): Methodology, Project administration, Resources, Software, Writing – editing and reviewing.
Emma J Teasdale (https://orcid.org/0000-0001-9147-193X): Formal Analysis, Investigation, Resources, Visualisation, Writing – original draft, Writing – editing and reviewing.
Ruth Thornton (https://orcid.org/0009-0006-4041-0233): Investigation, Resources, Writing – editing and reviewing.
Hazel A Everitt (https://orcid.org/0000-0001-7362-8403): Conceptualisation (lead), Funding acquisition (lead), Investigation, Methodology, Resources, Supervision, Visualisation, Writing – original draft, Writing – editing and reviewing.
Amanda J Farrin (https://orcid.org/0000-0002-2876-0584): Conceptualisation (lead), Data curation, Formal analysis (lead), Funding acquisition (lead), Methodology (lead), Resources, Supervision, Validation, Visualisation, Writing – original draft, Writing – editing and reviewing.
Sandy Tubeuf: Funding acquisition, Methodology, Investigation.
Gina Bianco: Investigation.
Taposhi Nath: Investigation.
Amy West: Investigation.
Sarah T Brown: Methodology.
Acknowledgements
Trial Steering Committee
Dr Tim Holt, TSC Chair (University of Oxford), Professor David Sanders (Sheffield Teachings Hospitals NHS Foundation Trust), Professor Kerry Hood (Cardiff University), Dr Maureen Twiddy (University of Hull), Dr Peter Paine (Salford Royal NHS Foundation Trust), Asociate Professor Mara Violato (University of Oxford), Dr Iryna Schlackow (University of Oxford), Ms Jill Durnell (PPI Representative).
Data monitoring ethics committee
Professor Peter Whorwell DMEC Chair (Wythenshawe Hospital), Miss Natalie Rowland (University of Birmingham), Professor Christopher Burton (University of Sheffield), Professor Peter Bower (University of Manchester).
Leeds Institute of Clinical Trials Research
Florence Day, Catriona Parker, Sarah Brown, Tom Morris, Richard Brindle, Alasdair Fellows, Jake Emmerson, Taposhi Nath, Thomas Smith, Amy West, Aaron Dowse, Aimee Christodoulou, Sandra Lopes Goncalves Graca, Gina Bianco, Maggie Barratt, Aminah Malik, and Damien Hindmarch.
University of Leeds
Sandy Tubeuf and Roberta Longo.
Clinical Research Networks
With thanks to the staff at the Clinical Research Networks who supported the hub research staff with the collection of trial data across the three locations:
Wessex Clinical Research Network;
West of England Clinical Research Network;
Yorkshire and Humber Clinical Research Network.
Recruiting general practitioners
With thanks to all the research staff at the participating centres who provided and cared for trial participants and collected trial data:
Wessex
Friarsgate Practice, Dr Stephen Fowler and Dr Stephanie Hughes
Swanage Medical Practice, Dr Claire Hombersley
Highcliffe Medical Centre, Dr Zelda Cheng
Gratton Surgery, Dr Laila Nicholson
Park and St Francis Surgery, Dr Boon Yong
Chawton Park Surgery, Dr Emma Bowen-Simpkins
Liphook and Liss surgery, Dr Anna Lalonde
Oaks Healthcare, Dr Nicola Millen
Highlands Practice, Dr Rem Lee
Westlands Medical Practice, Dr Helen Pandya
The Adam Practice, Dr Patrick Moore
Trafalgar Medical Group Practice, Dr Shruti Singh
The Bosmere Medical Practice, Dr Dirk Konig
Emsworth Surgery, Dr Andrew Slingsby
Abbeywell Surgery, Dr Nicola Lester
Solent GP Surgery, Dr Tara Watson
Wareham Surgery, Dr Nathan Francis
Raymond Road surgery, Dr Stuart Robinson
Brook House Surgery, Dr Stuart Robinson
Three Chequers Medical Practice, Dr Craig Kyte
West of England
Portishead Medical Group, Dr Matthew Ridd
Tyntesfield Medical Group, Dr Edward Mann
Greenway Community Practice, Dr Liz Grimshaw
Mendip Vale Medical Practice, Dr Richard Reed
Westbury on Trym Primary Care Centre, Dr Jenny Eachus
Nightingale Valley Practice, Dr Katharine Alsop
Fishponds Family Practice, Dr Simon Atkins
Whiteladies Medical Group, Dr Stephen Granier
The Family Practice, Dr James Baker
Beechwood Medical Practice, Dr Justine de Mink
Fallodon Way Medical Centre, Dr Katia Chapman
Clevedon Medical Centre, Dr Ann-Marie Streeton
Pembroke Road Surgery, Dr Rohan Perera
Horfield Health Centre, Dr Farida Ahmad
Monks Park (PIC to Mendip Vale Medical Practice), Dr Richard Reed
Frome Valley Medical Centre, Dr Jane Goram
Student Health Services, Dr Bushra Shahid
Trowbridge Health Centre, Dr Tobias Cookson
Phoenix Health Group, Dr Naomi Vernon
Hathaway Surgery, Dr Phillip Grimmer
Close Farm Surgery, Dr Ashutosh Singh
CONCORD Medical Centre, Dr Alastair Hay
West Yorkshire
Craven Road Medical Practice and Hollybank Surgery, Dr Robbie Foy
Blackburn Road Medical Centre, Dr Nitish Singh
Croft Medical Centre (formerly Bradford Road Medical Centre), Dr MB Ahmed
Oaklands Health Centre, Dr Sarah Alderson
Meltham Road Surgery, Dr Naim Hasanie
Slaithwaite Health Centre (accruals to go through Oaklands Health Centre), Dr Sarah Alderson
Haworth Medical Practice, Dr Tessa Mayo
Affinity Care Bradford – Shipley Medical Practice, Dr Ikechukwuka Nwachukwu
Affinity Care Bradford – Willows Medical Centre, Dr Ikechukwuka Nwachukwu
Affinity Care Bradford – Cowgill Surgery, Dr Ikechukwuka Nwachukwu
Affinity Care Bradford – Haigh Hall Medical Centre, Dr Ikechukwuka Nwachukwu
Affinity Care Bradford – Thornton Medical Practice, Dr Ikechukwuka Nwachukwu
Affinity Care Bradford – Sunnybank Medical Centre, Dr Ikechukwuka Nwachukwu
Patient and public involvement
Mr Matthew Chaddock, Ms Jill Durnell, and Ms Emma-Jane Williamson.
Participants
We are grateful to all the trial participants for their essential contribution to the trial.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration and would be subject to review by a subgroup of the trial team, which will include the data guarantor. Access to anonymised data may be granted following this review. All data-sharing activities would require a data-sharing agreement.
Ethics statement
Ethical approval for the study was given by the Yorkshire and The Humber Sheffield Research Ethics Committee on 5 August 2019 (reference number 19/YH/0150). Confirmation of capacity and capability was obtained from the recruiting centres as well as the PICs in primary care. The trial was registered with the International Standard Randomised Controlled Trial Register under the reference number ISRCTN48075063.
Information governance statement
The University of Leeds organisation/institution is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, the University of Leeds is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://dataprotection.leeds.ac.uk/.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/BFCR7986.
Primary conflicts of interest: Alexandra Wright-Hughes: NIHR grant funding paid to her institution, Data Monitoring and Ethics Committee and Trial Steering Committee member of NIHR and MRC funded projects, travel reimbursement for expert Committee membership of the Yorkshire and Northeast Regional Advisory Committee for NIHR Research for Patient Benefit, and payment to her institution for role as protocol editor for Trials journal. Alexander C Ford: NIHR grant funding paid to his institution. Sarah L Alderson: NIHR, YCR, and Health Data Research UK grant funding paid to her institution, consulting fees from West Yorkshire Integrated Care Board paid to her institution, speaker’s payments from Xytal, member of an HS&DR Funding Committee, and Data Monitoring and Ethics Committee member for an NIHR-funded study. Pei Loo Ow: none. Matthew J Ridd: NIHR grant funding paid to his institution, Co-Chair for SAPC and NIHR SPCR skin/allergy research groups, committee member for NIHR In Practice Fellowships, member of an HTA General Committee, an ESP – Evidence Synthesis Programme Advisory Group, an ESP – Evidence Synthesis Programme Grants Committee, and an ESP – NIHR Incentive Awards Committee. Robbie Foy: NIHR (HTA, PGfAR and HSDR) and YCR grant funding paid to his institution, Chair of NICE Implementation Strategy Group, member of an NIHR Dissemination Centre Advisory Group, member of UK Harkness Fellowship selection Committee, and Chair of Independent Steering Groups and Data Monitoring and Ethics Committee member for NIHR-funded studies. Felicity L Bishop: none. Matthew Chaddock: none. Heather Cook: none. Deborah Cooper: none. Catherine Fernandez: none. Elspeth A Guthrie: NIHR and Leeds Hospitals Charity grant funding paid to her institution. Suzanne Hartley: none. Amy Herbert: none. Daniel Howdon: NIHR and ESRC funding paid to his institution, consulting fees from Organisation for Economic Co-operation and Development, and United Nations Asia Pacific Region, speaker’s payment paid to institution from University of Lucerne. Delia P. Muir: none. Sonia Newman: member of an HTA MNCH Methods Group. Christopher M Taylor: none. Emma J Teasdale: none. Ruth Thornton: none. Hazel A Everitt: NIHR grant funding paid to her institution, personal/institutional income received as a result of a licence agreement with Mahana Therapeutics, and consulting fees/share options paid from Mahana Therapeutics. Amanda J Farrin: NIHR grant funding (HTA, EME, PGfAR, HS&DR, NIHR/MRC) paid to her institution, Data Monitoring and Ethics Committee and Trial Steering Committee member of NIHR and BHF funded projects, NIHR senior investigator, and member of an NIHR CTU Standing Advisory Committee, an HTA Funding Committee Policy Group (formerly CSG), an HTA Clinical Evaluation and Trials Committee, and a Prophylaxis Platform Study Funding Committee.
Publications
Alderson SL, Wright‑Hughes A, Ford AC, Farrin A, Hartley S, Fernandez C, et al. Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment (the ATLANTIS trial): protocol for a randomised double-blind placebo-controlled trial in primary care. Trials 2022;23:552. https://doi.org/10.1186/s13063-022-06492-6
Ford AC, Wright-Hughes A, Alderson SL, Ow P-L, Ridd MJ, Foy R, et al.; ATLANTIS trialists. Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment in primary care (ATLANTIS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023;402:1773–85. https://doi.org/10.1016/S0140-6736(23)01523-4.
Conferences
-
6th International Clinical Trials Methodology Conference – ‘Methodological considerations in the unblinding of participants in a double-blinded randomised controlled trial: why, when, and how’. Poster presented, 3–6 October 2022.
-
British Society of Gastroenterology – Amitriptyline at Low-dose and Titrated for Irritable Bowel Syndrome as Second-line Treatment in primary care (ATLANTIS): A Double-blind Placebo-controlled Trial (main results). Oral presentation, 21 June 2023, Liverpool, UK. Awarded prize for ‘Best Neuro-gastroenterology Oral Presentation’.
-
Society of Academic Primary Care Conference – Amitriptyline at Low-dose and Titrated for Irritable Bowel Syndrome as Second-line Treatment in Primary Care (The ATLANTIS Study): A Double-blind Placebo-controlled Trial (main results). Oral presentation, 18–20 July 2023, Brighton, UK.
-
Society of Academic Primary Care Conference – Low-dose amitriptyline for irritable bowel syndrome (IBS): patients’ and GPs’ views on barriers and facilitators of prescribing and uptake. Oral presentation, 18–20 July 2023, Brighton, UK.
-
Society of Academic Primary Care Conference – Unblinding to treatment allocation in randomised placebo-controlled trials: a new process and analysis of patient perspectives from a trial of low-dose amitriptyline for irritable bowel syndrome (IBS) in primary care. Oral presentation, 18–20 July 2023, Brighton, UK.
-
United European Gastroenterology conference – Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment (ATLANTIS): a randomised double-blind placebo-controlled trial in primary care. Oral presentation, 14–17 October 2023, Copenhagen.
-
Royal College of General Practitioners Conference – Amitriptyline at Low-dose and Titrated for Irritable Bowel Syndrome as Second-line Treatment in primary care (ATLANTIS): A Double-blind Placebo-controlled Trial. Oral and e-poster presentation, 19–20 October 2023, Glasgow, UK. Awarded the winning ePoster in the ‘Clinical’ category.
-
North American Primary Care Research Group (NPCRG) – Amitriptyline at Low-dose and Titrated for Irritable Bowel Syndrome in Primary Care: ATLANTIS: Randomised Controlled Trial, Oral Presentation, 30 October to 3 November 2023, San Francisco, USA. Awarded Distinguished Paper Award.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet 2020;396:1675-88.
- Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome Foundation global study. Gastroenterology 2021;160:99-114.e3.
- Thompson WG, Heaton KW, Smyth GT, Smyth C. Irritable bowel syndrome in general practice: prevalence, characteristics, and referral. Gut 2000;46:78-82.
- Goodoory VC, Ng CE, Black CJ, Ford AC. Direct healthcare costs of Rome IV or Rome III – defined irritable bowel syndrome in the United Kingdom. Aliment Pharmacol Ther 2022;56:110-20.
- Pace F, Molteni P, Bollani S, Sarzi-Puttini P, Stockbrügger R, Bianchi Porro G, et al. Inflammatory bowel disease versus irritable bowel syndrome: a hospital-based, case-control study of disease impact on quality of life. Scand J Gastroenterol 2003;38:1031-8.
- Shivaji UN, Ford AC. Prevalence of functional gastrointestinal disorders among consecutive new patient referrals to a gastroenterology clinic. Frontline Gastroenterology 2014;5:266-71.
- Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochr Datab Syst Rev 2011;2011.
- Ford AC, Quigley EM, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 2014;109:1350-65; quiz 1366.
- Ford AC, Lacy BE, Harris LA, Quigley EM, Moayyedi P. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol 2019;114:21-39.
- Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol 2005;96:399-40.
- Talley NJ, Locke GR, Saito YA, Almazar AE, Bouras EP, Howden CW, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multi-center, randomized, controlled study. Gastroenterology 2015;149:340-9.e2.
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochr Datab Syst Rev 2007;2007.
- Wong J, Motulsky A, Abrahamowicz M, Eguale T, Buckeridge DL, Tamblyn R. Off-label indications for antidepressants in primary care: descriptive study of prescriptions from an indication based electronic prescribing system. BMJ 2017;356.
- Gorard DA, Libby GW, Farthing MJ. Influence of antidepressants on whole gut orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther 1994;8:159-66.
- Ford AC, Forman D, Bailey AG, Axon ATR, Moayyedi P. Irritable bowel syndrome: a 10-year natural history of symptoms, and factors that influence consultation behavior. Am J Gastroenterol 2008;103:1229-39; quiz 1240.
- Hookway C, Buckner S, Crosland P, Longson D. Irritable bowel syndrome in adults in primary care: summary of updated NICE guidance. BMJ 2015;350.
- Shivaji UN, Ford AC. Beliefs about management of irritable bowel syndrome in primary care: cross-sectional survey in one locality. Prim Health Care Res Dev 2014;16:263-9.
- Everitt H, McDermott L, Leydon G, Yules H, Baldwin D, Little P. GPs’ management strategies for patients with insomnia: a survey and qualitative interview study. Br J Gen Pract 2014;64:e112-9.
- Nigam P, Kapoor KK, Rastog CK, Kumar A, Gupta AK. Different therapeutic regimens in irritable bowel syndrome. J Assoc Physicians India 1984;32:1041-4.
- Vahedi H, Merat S, Momtahen S, Kazzazi AS, Ghaffari N, Olfati G, et al. Clinical trial: the effect of amitriptyline in patients with diarrhea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2008;27:678-84.
- Thoua NM, Murray CD, Winchester WJ, Roy AJ, Pitcher MCL, Kamm MA, et al. Amitriptyline modifies the visceral hypersensitivity response to acute stress in the irritable bowel syndrome. Aliment Pharmacol Ther 2009;29:552-60.
- Alderson SL, Wright-Hughes A, Ford AC, Farrin A, Hartley S, Fernandez C, et al. Amitriptyline at low-dose and titrated for irritable bowel syndrome as second-line treatment (The ATLANTIS trial): protocol for a randomised double-blind placebo-controlled trial in primary care. Trials 2022;23.
- Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395-402.
- Muller-Lissner S, Koch G, Talley NJ, Drossman D, Rueegg P, Dunger-Baldauf C, et al. Subject’s global assessment of relief: an appropriate method to assess the impact of treatment on irritable bowel syndrome-related symptoms in clinical trials. J Clin Epidemiol 2003;56:310-6.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258-66.
- Spiller RC, Humes DJ, Campbell E, Hastings M, Neal KR, Dukes GE, et al. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther 2010;32:811-20.
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361-70.
- Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4.
- Kennedy T, Jones R, Darnley S, Seed P, Wessely S, Chalder T. Cognitive behaviour therapy in addition to antispasmodic treatment for irritable bowel syndrome in primary care: randomised controlled trial. BMJ 2005;331:435-7.
- Moss-Morris R, McAlpine L, Didsbury LP, Spence MJ. A randomized controlled trial of a cognitive behavioural therapy-based self-management intervention for irritable bowel syndrome in primary care. Psychol Med 2010;40:85-94.
- Uher R, Farmer A, Henigsberg N, Rietschel M, Mors O, Maier W, et al. Adverse reactions to antidepressants. Br J Psychiatry 2009;195:202-10.
- EuroQol G. EuroQol – a new facility for the measurement of health-related quality of life. Health Pol 1990;16:199-208.
- Bushnell DM, Martin ML, Ricci JF, Bracco A. Performance of the EQ-5D in patients with irritable bowel syndrome. Value Health 2006;9:90-7.
- Lacy BE, Mearin F, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology 2016;150:1393-407.
- NICE . Suspected Cancer: Recognition and Referral 2015. www.nice.org.uk/guidance/NG12/chapter/1-Recommendations-organised-by-site-of-cancer#lower-gastrointestinal-tract-cancers (accessed 29 June 2023).
- Everitt H, Landau S, Little P, Bishop FL, McCrone P, O’Reilly G, et al. ACTIB Trial Team . Assessing Cognitive behavioural Therapy in Irritable Bowel (ACTIB): protocol for a randomised controlled trial of clinical-effectiveness and cost-effectiveness of therapist delivered cognitive behavioural therapy and web-based self-management in irritable bowel syndrome in adults. BMJ Open 2015;5.
- Everitt HA, Landau S, O’Reilly G, Sibelli A, Hughes S, Windgassen S, et al. ACTIB Trial Group . Assessing telephone-delivered cognitive-behavioural therapy (CBT) and web-delivered CBT versus treatment as usual in irritable bowel syndrome (ACTIB): a multicentre randomised trial. Gut 2019;68:1613-23.
- British Dietetic Association . Irritable Bowel Syndrome and Diet n.d. www.bda.uk.com/resource/irritable-bowel-syndrome-diet.html (accessed 29 June 2023).
- Sisson G, Ayis S, Sherwood RA, Bjarnason I. Randomised clinical trial: a liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome – a 12 week double-blind study. Aliment Pharmacol Ther 2014;40:51-62.
- Peckham EJ, Relton C, Raw J, Walters C, Thomas K, Smith C, et al. Interim results of a randomised controlled trial of homeopathic treatment for irritable bowel syndrome. Homeopathy 2014;103:172-7.
- Braun V, Clarke V. One size fits all? What counts as quality practice in (reflexive) thematic analysis?. Qual Res Psychol 2021;18:328-52.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Strauss A, Corbin J. Basics of Qualitative Research. Thousand Oaks, CA: SAGE; 1998.
- Leventhal H, Phillips LA, Burns E. The Common-Sense Model of Self-Regulation (CSM): a dynamic framework for understanding illness self-management. J Behav Med 2016;39:935-46.
- May C, Finch T, Mair F, Ballini L, Dowrick C, Eccles M, et al. Understanding the implementation of complex interventions in health care: the normalization process model. BMC Health Serv Res 2007;7:1-7.
- Harvey JM, Sibelli A, Chalder T, Everitt H, Moss-Morris R, Bishop FL. Desperately seeking a cure: treatment seeking and appraisal in irritable bowel syndrome. Br J Health Psychol 2018;23:561-79.
- Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the necessity-concerns framework. PLOS ONE 2013;8.
- QSR International Pty Ltd . NVivo. Version 12 Ed. 2018.
- Gagné T, Henderson C, McMunn A. Is the self-reporting of mental health problems sensitive to public stigma towards mental illness? A comparison of time trends across English regions (2009–19). Soc Psychiatry Psychiatr Epidemiol 2023;58:671-80.
- Castaldelli-Maia JM, Scomparini LB, Andrade AG, Bhugra D, de Toledo Ferraz Alves TC, D’Elia G. Perceptions of and attitudes toward antidepressants stigma attached to their use: a review. J Nerv Ment Dis 2011;199:866-71.
- Ingersoll K, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med 2008;31:213-24.
- Mari A, Hosadurg D, Martin L, Zarate-Lopez N, Passananti V, Emmanuel A. Adherence with a low-FODMAP diet in irritable bowel syndrome: are eating disorders the missing link?. Eur J Gastroenterol Hepatol 2019;31:178-82.
- Tuck CJ, Reed DE, Muir JG, Vanner SJ. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: a real-world experience. Neurogastroenterol Motil 2020;32.
- Stewart S-JF, Moon Z, Horne R. Medication nonadherence: health impact, prevalence, correlates and interventions. Psychol Health 2023;38:726-65.
- Timmermans S. The engaged patient: the relevance of patient–physician communication for twenty-first-century health. J Health Soc Behav 2020;61:259-73.
- NHS England . The NHS Long Term Plan 2019. www.longtermplan.nhs.uk/publication/nhs-long-term-plan/ (accessed 29 June 2023).
- Schwille-Kiuntke J, Rüdlin SL, Junne F, Enck P, Brenk-Franz K, Zipfel S, et al. Illness perception and health care use in individuals with irritable bowel syndrome: results from an online survey. BMC Fam Pract 2021;22.
- Tonkin-Crine S, Bishop FL, Ellis M, Moss-Morris R, Everitt H. Exploring patients’ views of a cognitive behavioral therapy-based website for the self-management of irritable bowel syndrome symptoms. J Med Internet Res 2013;15.
- Murray E, Treweek S, Pope C, MacFarlane A, Ballini L, Dowrick C, et al. Normalisation process theory: a framework for developing, evaluating and implementing complex interventions. BMC Med 2010;8.
- Shorey S, Demutska A, Chan V, Siah KTH. Adults living with irritable bowel syndrome (IBS): a qualitative systematic review. J Psychosom Res 2021;140.
- Sibelli A, Moss-Morris R, Chalder T, Bishop FL, Windgassen S, Everitt H. Patients’ perspectives on GP interactions after cognitive behavioural therapy for refractory IBS: a qualitative study in UK primary and secondary care. Br J Gen Pract 2018;68:e654-62.
- Sheridan R, Martin-Kerry J, Hudson J, Parker A, Bower P, Knapp P. Why do patients take part in research? An overview of systematic reviews of psychosocial barriers and facilitators. Trials 2020;21.
- Kaptchuk TJ, Shaw J, Kerr CE, Conboy LA, Kelley JM, Csordas TJ, et al. ‘Maybe I made up the whole thing’: placebos and patients’ experiences in a randomized controlled trial. Cult Med Psychiatry 2009;33:382-411.
- Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ (Clin Res Ed) 2008;336:999-1003.
- Noble H, Hasan SS, Whorwell PJ, Vasant DH. The symptom burden of irritable bowel syndrome in tertiary care during the COVID-19 pandemic. Neurogastroenterol Motil 2022;34.
- Quek SXZ, Loo EXL, Demutska A, Chua CE, Kew GS, Wong S, et al. Impact of the coronavirus disease 2019 pandemic on irritable bowel syndrome. J Gastroenterol Hepatol 2021;36:2187-97.
- Stone DA, Kerr CE, Jacobson E, Conboy LA, Kaptchuk TJ. Patient expectations in placebo-controlled randomized clinical trials. J Eval Clin Pract 2005;11:77-84.
- Bishop FL, Jacobson EE, Shaw JR, Kaptchuk TJ. Debriefing to placebo allocation: a phenomenological study of participants’ experiences in a randomized clinical trial. Qual Health Res 2012;22:1138-49.
- Bishop FL, Jacobson EE, Shaw JR, Kaptchuk TJ. Scientific tools, fake treatments, or triggers for psychological healing: how clinical trial participants conceptualise placebos. Soc Sci Med 2012;74:767-74.
- Shah E, Triantafyllou K, Hana AA, Pimentel M. Adverse events appear to unblind clinical trials in irritable bowel syndrome. Neurogastroenterol Motil 2014;26:482-8.
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359:696-700.
- Shalowitz DI, Miller FG. Disclosing individual results of clinical research: implications of respect for participants. JAMA 2005;294:737-40.
- Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: attitudes, practices, and future directions. PLOS Med 2008;5.
- Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: Medical Research Council guidance. BMJ (Clin Res Ed) 2015;350.
- Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs – principles and practices. Health Serv Res 2013;48:2134-56.
- European Medicines Agency . Guideline on the Evaluation of Medicinal Products for the Treatment of Irritable Bowel Syndrome 2014. www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-medicinal-products-treatment-irritable-bowel-syndrome-revision-1_en.pdf (accessed 29 June 2023).
- Food and Drug Administration . Guidance for Industry: Irritable Bowel Syndrome – Clinical Evaluation of Drugs for Treatment 2012. www.fda.gov/media/78622/download (accessed 29 June 2023).
- Vasant DH, Paine PA, Black CJ, Houghton LA, Everitt HA, Corsetti M, et al. British Society of Gastroenterology guidelines on the management of irritable bowel syndrome. Gut 2021;70:1214-40.
- Black CJ, Yiannakou Y, Houghton LA, Ford AC. Epidemiological, clinical, and psychological characteristics of individuals with self-reported irritable bowel syndrome based on the Rome IV vs Rome III criteria. Clin Gastroenterol Hepatol 2020;18:392-398.e2.
- Ford AC, Moayyedi PM. Factors affecting placebo response rate in irritable bowel syndrome. Aliment Pharmacol Ther 2010;32:144-58.
- Barberio B, Savarino EV, Black CJ, Ford AC. Placebo response rates in trials of licensed drugs for irritable bowel syndrome with constipation or diarrhea: meta-analysis. Clin Gastroenterol Hepatol 2022;20:e923-44.
- Lembo A, Kelley JM, Nee J, Ballou S, Iturrino J, Cheng V, et al. Open-label placebo vs double-blind placebo for irritable bowel syndrome: a randomized clinical trial. Pain 2021;162:2428-35.
- Kaptchuk TJ, Friedlander E, Kelley JM, Sanchez MN, Kokkotou E, Singer JP, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLOS ONE 2010;5.
- Black CJ, Yuan Y, Selinger CP, Camilleri M, Quigley EMM, Moayyedi P, et al. Efficacy of soluble fibre, antispasmodic drugs, and gut-brain neuromodulators in irritable bowel syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol 2020;5:117-31.
- Drossman DA, Toner BB, Whitehead WE, Diamant NE, Dalton CB, Duncan S, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology 2003;125:19-31.
- Gorard DA, Libby GW, Farthing MJ. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea-predominant irritable bowel syndrome. Dig Dis Sci 1995;40:86-95.
- Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut 2005;54:601-7.
- Patel P, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P, et al. Irritable bowel syndrome is significantly associated with somatisation in 840 patients, which may drive bloating. Aliment Pharmacol Ther 2015;41:449-58.
- Zamani M, Alizadeh-Tabari S, Zamani V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2019;50:132-43.
- Goodoory VC, Ng CE, Black CJ, Ford AC. Impact of Rome IV irritable bowel syndrome on work and activities of daily living. Aliment Pharmacol Ther 2022;56:844-56.
- Frandemark A, Tornblom H, Jakobsson S, Simren M. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem. Am J Gastroenterol 2018;113:1540-9.
Appendix 1 Additional clinical results tables and figures
Study summary and baseline characteristics
| West Yorkshire | Wessex | West of England | Total | |
|---|---|---|---|---|
| Mailed out | ||||
| Patients | 3862 (24.6%) | 6218 (39.7%) | 5592 (35.7%) | 15,672 (100%) |
| GP practices | 13 | 20 | 22 | 55 |
| Responded to mail-out | 153 (4.7%) | 1688 (52.3%) | 1387 (43.0%) | 3228 (100%) |
| Interested | 116 (9.3%) | 525 (41.9%) | 612 (48.8%) | 1253 (100%) |
| Screening call | 113 (10.2%) | 501 (45.3%) | 491 (44.4%) | 1105 (100%) |
| Eligible | 108 (18.7%) | 256 (44.2%) | 215 (37.1%) | 579 (100%) |
| Consented | 103 (19.5%) | 223 (42.2%) | 202 (38.3%) | 528 (100%) |
| Registered | 101 (19.2%) | 222 (42.3%) | 202 (38.5%) | 525 (100%) |
| Randomised | ||||
| Patients | 87 (18.8%) | 192 (41.5%) | 184 (39.7%) | 463 (100%) |
| GP practicesa | 11 | 20 | 21 | 52 |
| Amitriptyline (n = 232) (%) | Placebo (n = 231) (%) | Total (n = 463) (%) | |
|---|---|---|---|
| Protocol violation identified? | 3 (1.3) | 3 (1.3) | 6 (1.3) |
| Reason for protocol violation | |||
| Breached eligibility criteria | 1 (33.3) | 0 (0.0) | 1 (16.7) |
| Unplanned treatment error | 1 (33.3) | 3 (100) | 4 (66.7) |
| Other protocol violation | 1 (33.3) | 0 (0.0) | 1 (16.7) |
| Type of protocol violation | |||
| Major | 2 (66.7) | 2 (66.7) | 4 (66.7) |
| Minor | 1 (33.3) | 1 (33.3) | 2 (33.3) |
FIGURE 8.
Time from general practice mail-out to randomisation. N refers to the number of randomised participants in each practice.

FIGURE 9.
Timing of questionnaire completion.
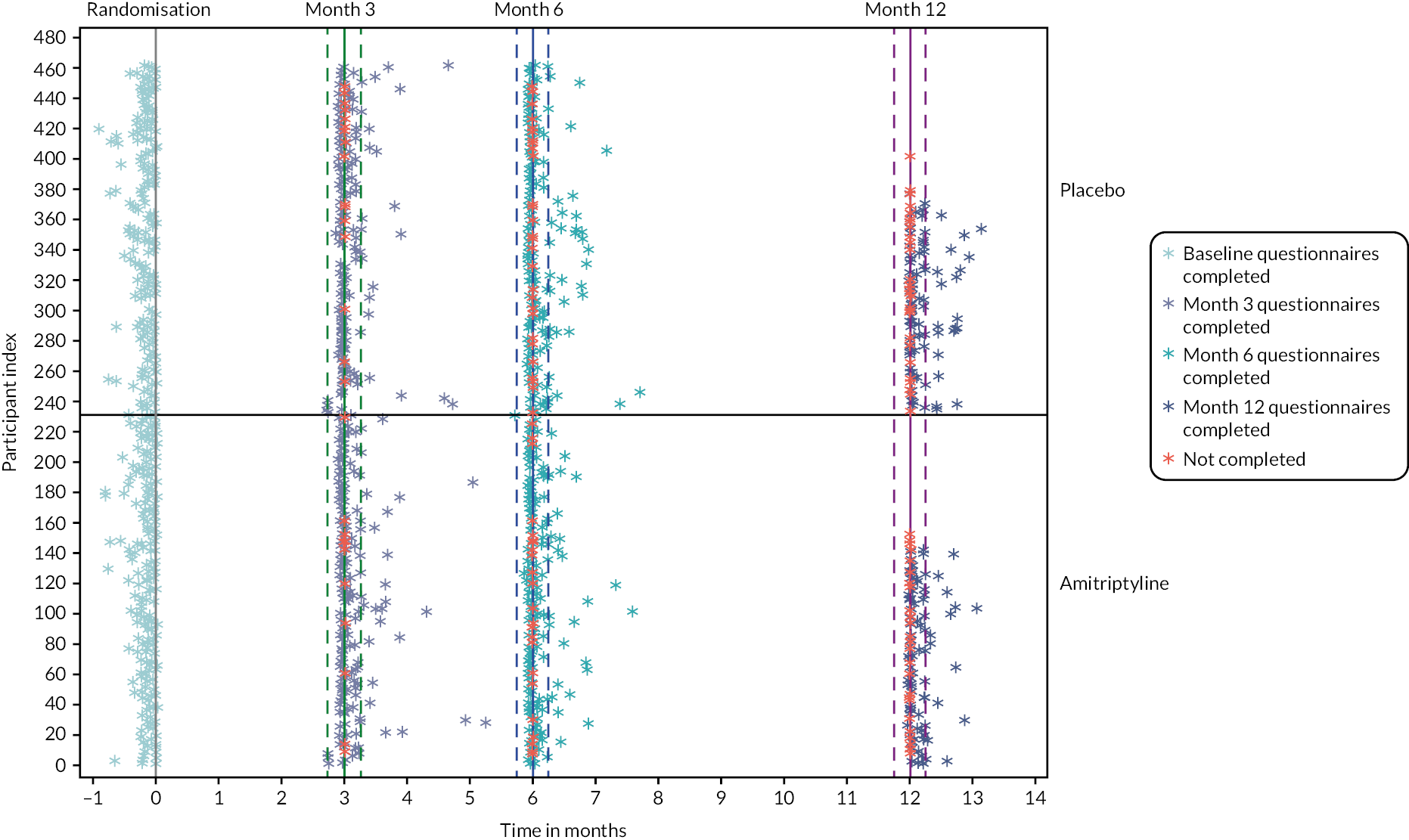
FIGURE 10.
Weekly adequate relief question completion.
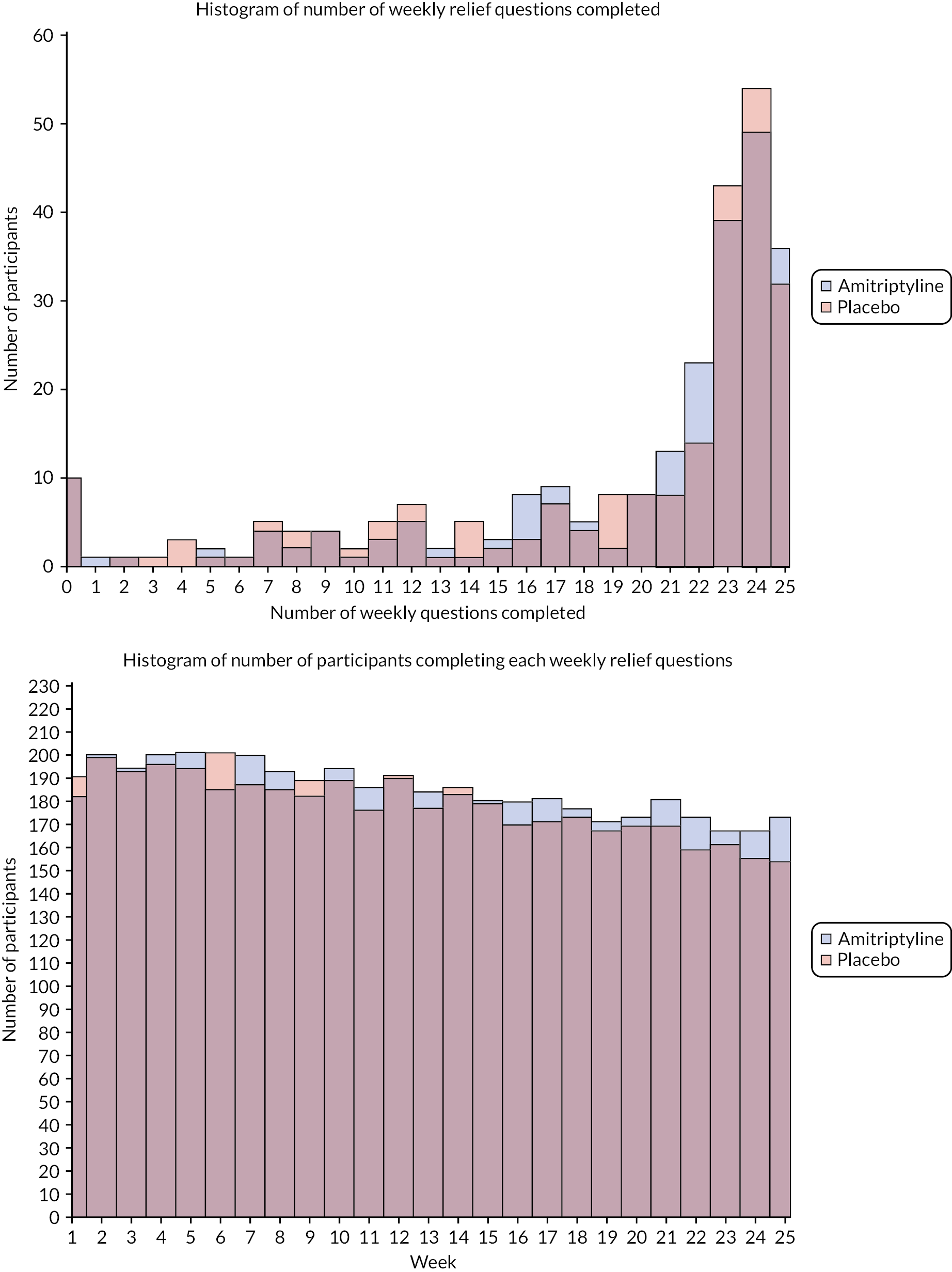
| Decilea | Treatment allocation | Recruitment hub | Total (n = 463) | |||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | West Yorkshire (n = 87) | Wessex (n = 192) | West of England (n = 184) | ||
| 1 | 3 (1.3%) | 3 (1.3%) | 1 (1.1%) | 2 (1.0%) | 3 (1.6%) | 6 (1.3%) |
| 2 | 10 (4.4%) | 10 (4.3%) | 9 (10.6%) | 4 (2.1%) | 7 (3.8%) | 20 (4.4%) |
| 3 | 13 (5.7%) | 14 (6.1%) | 8 (9.4%) | 12 (6.3%) | 7 (3.8%) | 27 (5.9%) |
| 4 | 21 (9.2%) | 13 (5.7%) | 13 (15.3%) | 13 (6.8%) | 8 (4.4%) | 34 (7.4%) |
| 5 | 19 (8.3%) | 19 (8.3%) | 8 (9.4%) | 15 (7.9%) | 15 (8.2%) | 38 (8.3%) |
| 6 | 19 (8.3%) | 14 (6.1%) | 10 (11.8%) | 17 (8.9%) | 6 (3.3%) | 33 (7.2%) |
| 7 | 38 (16.6%) | 38 (16.5%) | 14 (16.5%) | 35 (18.3%) | 27 (14.8%) | 76 (16.6%) |
| 8 | 37 (16.2%) | 36 (15.7%) | 12 (14.1%) | 30 (15.7%) | 31 (16.9%) | 73 (15.9%) |
| 9 | 36 (15.7%) | 39 (17.0%) | 9 (10.6%) | 29 (15.2%) | 37 (20.2%) | 75 (16.3%) |
| 10 | 33 (14.4%) | 44 (19.1%) | 1 (1.2%) | 34 (17.8%) | 42 (23.0%) | 77 (16.8%) |
| Missing | 3 | 1 | 2 | 1 | 1 | 4 |
FIGURE 11.
Years from IBS diagnosis to trial randomisation. (a) Overall, (b) by IBS-SSS severity.

Treatment delivery and receipt
FIGURE 12.
Reported side effects as a reason for treatment discontinuation.

FIGURE 13.
Distribution of time from randomisation to treatment end date.

FIGURE 14.
Treatment adherence reported at each follow-up time point (for participants on trial medication).
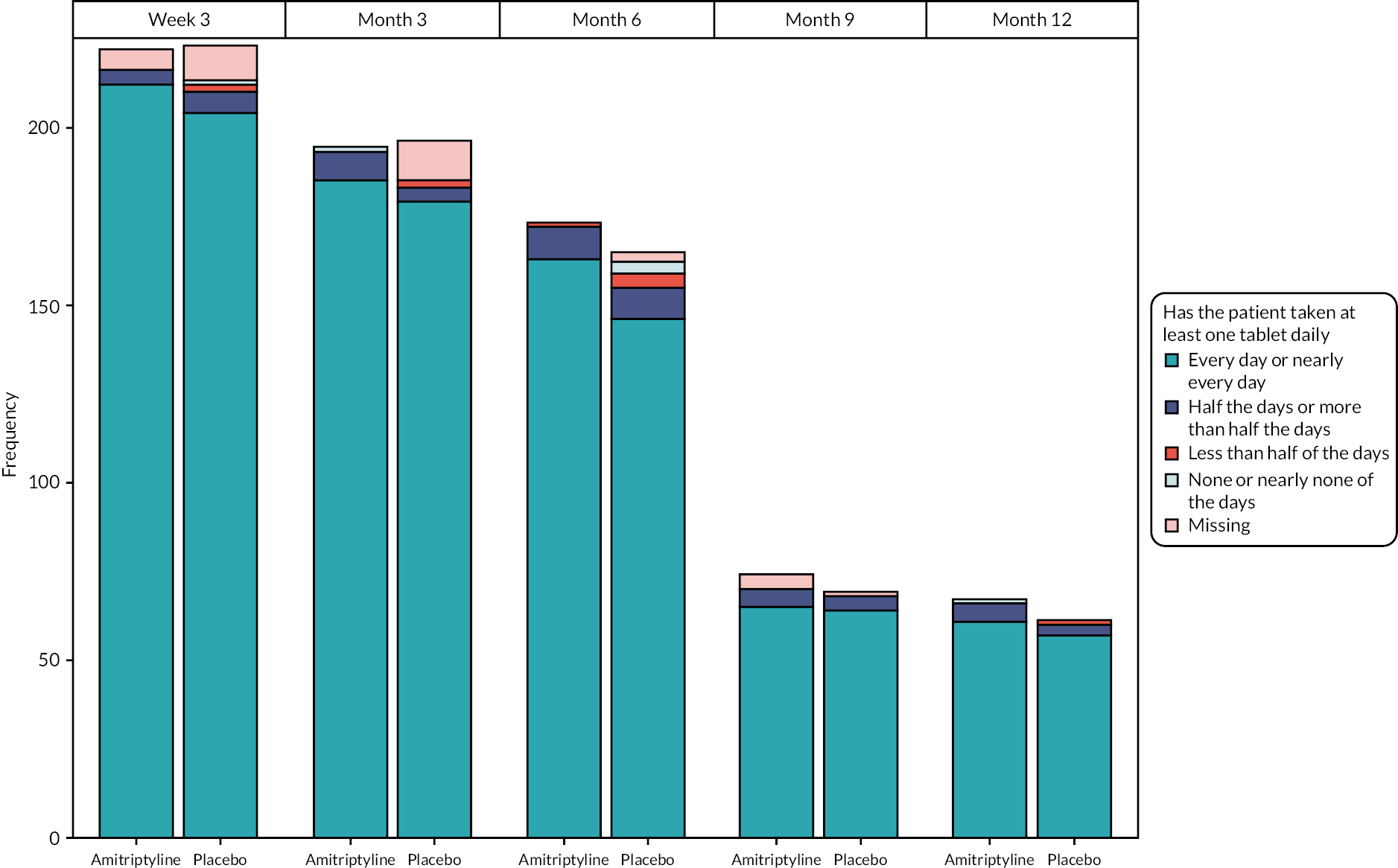
FIGURE 15.
Treatment dose reported at each follow-up time point (for participants on trial medication).

| Before month 3 | Between month 3 and month 6 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 32) | Placebo (n = 31) | Total (n = 63) | Amitriptyline (n = 14) | Placebo (n = 28) | Total (n = 42) | |
| Has the patient taken at least one tablet daily? | ||||||
| Every/nearly every day | 22 (91.7%) | 19 (86.4%) | 41 (89.1%) | 13 (92.9%) | 23 (85.2%) | 36 (87.8%) |
| ≥ half the days | 2 (8.3%) | 2 (9.1%) | 4 (8.7%) | 1 (7.1%) | 3 (11.1%) | 4 (9.8%) |
| < half of the days | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (3.7%) | 1 (2.4%) |
| None/nearly none of the days | 0 (0.0%) | 1 (4.5%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Missing | 8 | 9 | 17 | 0 | 1 | 1 |
| Current dose of trial treatment | ||||||
| 1 × 10 mg every other day | 0 (0.0%) | 1 (3.2%) | 1 (1.7%) | 0 (0.0%) | 1 (3.6%) | 1 (2.4%) |
| 1 × 10 mg daily | 10 (37.0%) | 18 (58.1%) | 28 (48.3%) | 6 (42.9%) | 6 (21.4%) | 12 (28.6%) |
| 2 × 10 mg daily | 11 (40.7%) | 7 (22.6%) | 18 (31.0%) | 6 (42.9%) | 10 (35.7%) | 16 (38.1%) |
| 3 × 10 mg daily | 6 (22.2%) | 5 (16.1%) | 11 (19.0%) | 2 (14.3%) | 11 (39.3%) | 13 (31.0%) |
| Missing | 5 | 0 | 5 | 0 | 0 | 0 |
| After month 6 | At point of discontinuation | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 16) | Placebo (n = 11) | Total (n = 27) | Amitriptyline (n = 62) | Placebo (n = 70) | Total (n = 132) | |
| Has the patient taken at least one tablet daily? | ||||||
| Every/nearly every day | 14 (87.5%) | 11 (100.0%) | 25 (92.6%) | 38 (79.2%) | 40 (70.2%) | 78 (74.3%) |
| ≥ half the days | 2 (12.5%) | 0 (0.0%) | 2 (7.4%) | 6 (12.5%) | 12 (21.1%) | 18 (17.1%) |
| < half of the days | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 3 (6.3%) | 3 (5.3%) | 6 (5.7%) |
| None/nearly none of the days | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.1%) | 2 (3.5%) | 3 (2.9%) |
| Missing | 0 | 0 | 0 | 14 | 13 | 27 |
| Current dose of trial treatment | ||||||
| 1 × 10 mg every other day | 1 (6.3%) | 2 (18.2%) | 3 (11.1%) | 2 (4.3%) | 3 (5.2%) | 5 (4.8%) |
| 1 × 10 mg daily | 5 (31.3%) | 0 (0.0%) | 5 (18.5%) | 28 (59.6%) | 13 (22.4%) | 41 (39.0%) |
| 2 × 10 mg daily | 2 (12.5%) | 5 (45.5%) | 7 (25.9%) | 7 (14.9%) | 18 (31.0%) | 25 (23.8%) |
| 3 × 10 mg daily | 8 (50.0%) | 4 (36.4%) | 12 (44.4%) | 10 (21.3%) | 24 (41.4%) | 34 (32.4%) |
| Missing | 0 | 0 | 0 | 15 | 12 | 27 |
| Week 3 | Month 3 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (n = 222) | Placebo (n = 223) | Total (n = 445) | Amitriptyline (n = 194) | Placebo (n = 196) | Total (n = 390) | |
| Month 6 | Month 9 | |||||
| Amitriptyline (n = 173) | Placebo (n = 165) | Total (n = 338) | Amitriptyline (n = 74) | Placebo (n = 69) | Total (n = 143) | |
| Bottles requested | ||||||
| 0 | 9 (4.1%) | 19 (8.5%) | 28 (6.3%) | 20 (10.3%) | 26 (13.3%) | 46 (11.8%) |
| 1 | 14 (6.3%) | 14 (6.3%) | 28 (6.3%) | 33 (17.0%) | 19 (9.7%) | 52 (13.3%) |
| 2 | 127 (57.2%) | 95 (42.6%) | 222 (49.9%) | 37 (19.1%) | 28 (14.3%) | 65 (16.7%) |
| 3 | 70 (31.5%) | 91 (40.8%) | 161 (36.2%) | 48 (24.7%) | 49 (25.0%) | 97 (24.9%) |
| 4 | 2 (0.9%) | 4 (1.8%) | 6 (1.3%) | 47 (24.2%) | 60 (30.6%) | 107 (27.4%) |
| 5 | 0 | 0 | 0 | 9 (4.6%) | 14 (7.1%) | 23 (5.9%) |
| Bottles requested | ||||||
| 0 | 89 (51.4%) | 89 (53.9%) | 178 (52.7%) | 8 (10.8%) | 5 (7.2%) | 13 (9.1%) |
| 1 | 14 (8.1%) | 8 (4.8%) | 22 (6.5%) | 7 (9.5%) | 1 (1.4%) | 8 (5.6%) |
| 2 | 12 (6.9%) | 15 (9.1%) | 27 (8.0%) | 14 (18.9%) | 11 (15.9%) | 25 (17.5%) |
| 3 | 23 (13.3%) | 24 (14.5%) | 47 (13.9%) | 19 (25.7%) | 24 (34.8%) | 43 (30.1%) |
| 4 | 27 (15.6%) | 22 (13.3%) | 49 (14.5%) | 19 (25.7%) | 23 (33.3%) | 42 (29.4%) |
| 5 | 8 (4.6%) | 7 (4.2%) | 15 (4.4%) | 7 (9.5%) | 5 (7.2%) | 12 (8.4%) |
| Month 6 | Discontinued treatment before month 6 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline (%) | Placebo (%) | Total (%) | Amitriptyline (%) | Placebo (%) | Total (%) | |
| New diet during the studya | ||||||
| Low carbohydrate | 6 (18.2) | 5 (14.7) | 11 (16.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gluten-free or low gluten | 5 (15.2) | 3 (8.8) | 8 (11.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Weight loss programme | 1 (3.0) | 5 (14.7) | 6 (9.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Low FODMAP | 0 (0.0) | 5 (14.7) | 5 (7.5) | 0 (0.0) | 1 (16.7) | 1 (16.7) |
| Increase vegetables or fruit intake | 5 (15.2) | 0 (0.0) | 5 (7.5) | 0 (0.0) | 1 (16.7) | 1 (16.7) |
| Reduce meat intake | 5 (15.2) | 0 (0.0) | 5 (7.5) | 0 (0.0) | 1 (16.7) | 1 (16.7) |
| Reduce portion sizes | 3 (9.1) | 1 (2.9) | 4 (6.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Dairy-free or low diary | 2 (6.1) | 1 (2.9) | 3 (4.5) | 0 (0.0) | 2 (33.3) | 2 (33.3) |
| Fermented food or drinks | 2 (6.1) | 1 (2.9) | 3 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Low fibre | 1 (3.0) | 2 (5.9) | 3 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Low lactose or lactose-free | 1 (3.0) | 1 (2.9) | 2 (3.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Paleo | 0 (0.0) | 1 (2.9) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mediterranean | 0 (0.0) | 1 (2.9) | 1 (1.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vegetarian | 1 (3.0) | 0 (0.0) | 1 (1.5) | 0 (0.0) | 1 (16.7) | 1 (16.7) |
| Other | 7 (21.2)b | 14 (41.2)c | 21 (31.3) | 0 (0.0) | 1 (16.7) | 1 (16.7)d |
| Total | 33 (100) | 34 (100) | 67 (100) | 0 | 6 (100) | 6 (100) |
| Other treatments for IBS symptoms during the studye | ||||||
| Antispasmodics | 6 (33.3) | 2 (12.5) | 8 (23.5) | 1 (20.0) | 0 (0.0 | 1 (14.3) |
| Probiotics | 3 (16.7) | 3 (18.8) | 6 (17.6) | 1 (20.0) | 1 (50.0) | 2 (28.6) |
| Peppermint oil/tablets/tea | 2 (11.1) | 2 (12.5) | 4 (11.8) | 0 (0.0) | 1 (50.0) | 1 (14.3) |
| Prebiotics | 2 (11.1) | 1 (6.3) | 3 (8.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anti-diarrhoeal | 2 (11.1) | 1 (6.3) | 3 (8.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Laxatives | 1 (5.6) | 1 (6.3) | 2 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Homeopathy | 0 (0.0) | 1 (6.3) | 1 (2.9) | 1 (20.0) | 0 (0.0) | 1 (14.3) |
| Other | 6 (33.3)f | 6 (37.5)g | 12 (35.3) | 2 (40.0)h | 0 (0.0) | 2 (28.6) |
| Missing | 0 (0.0) | 1 (6.3) | 1 (2.9) | 0 | 0 | 0 |
| Total | 18 (100) | 16 (100) | 34 (100) | 5 (100) | 2 (100) | 7 (100) |
| What attributed to improved IBS symptomsi | ||||||
| ATLANTIS medication | 103 (87.3) | 75 (84.3) | 178 (86.0) | 11 (84.6) | 7 (70.0) | 18 (78.3) |
| Changes in diet | 15 (12.7) | 11 (12.4) | 26 (12.6) | 0 (0.0) | 2 (20.0) | 2 (8.7) |
| Changes in exercise | 5 (4.2) | 3 (3.4) | 8 (3.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Uncertain | 4 (3.4) | 4 (4.5) | 8 (3.9) | 2 (15.4) | 0 (0.0) | 2 (8.7) |
| Less stress | 4 (3.4) | 2 (2.2) | 6 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Changes in work/personal environments | 2 (1.7) | 3 (3.4) | 5 (2.4) | 0 (0.0) | 1 (10.0) | 1 (4.3) |
| Changes in other treatments | 2 (1.7) | 0 (0.0) | 2 (1.0) | 0 (0.0) | 1 (10.0) | 1 (4.3) |
| Other treatment/therapies | 0 (0.0) | 2 (2.2) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 2j (1.7) | 3k (3.4) | 5 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 118 (100) | 89 (100) | 207 (100) | 13 (100) | 10 (100) | 23 (100) |
Primary end point
FIGURE 16.
Total IBS-SSS score severity at each time point.
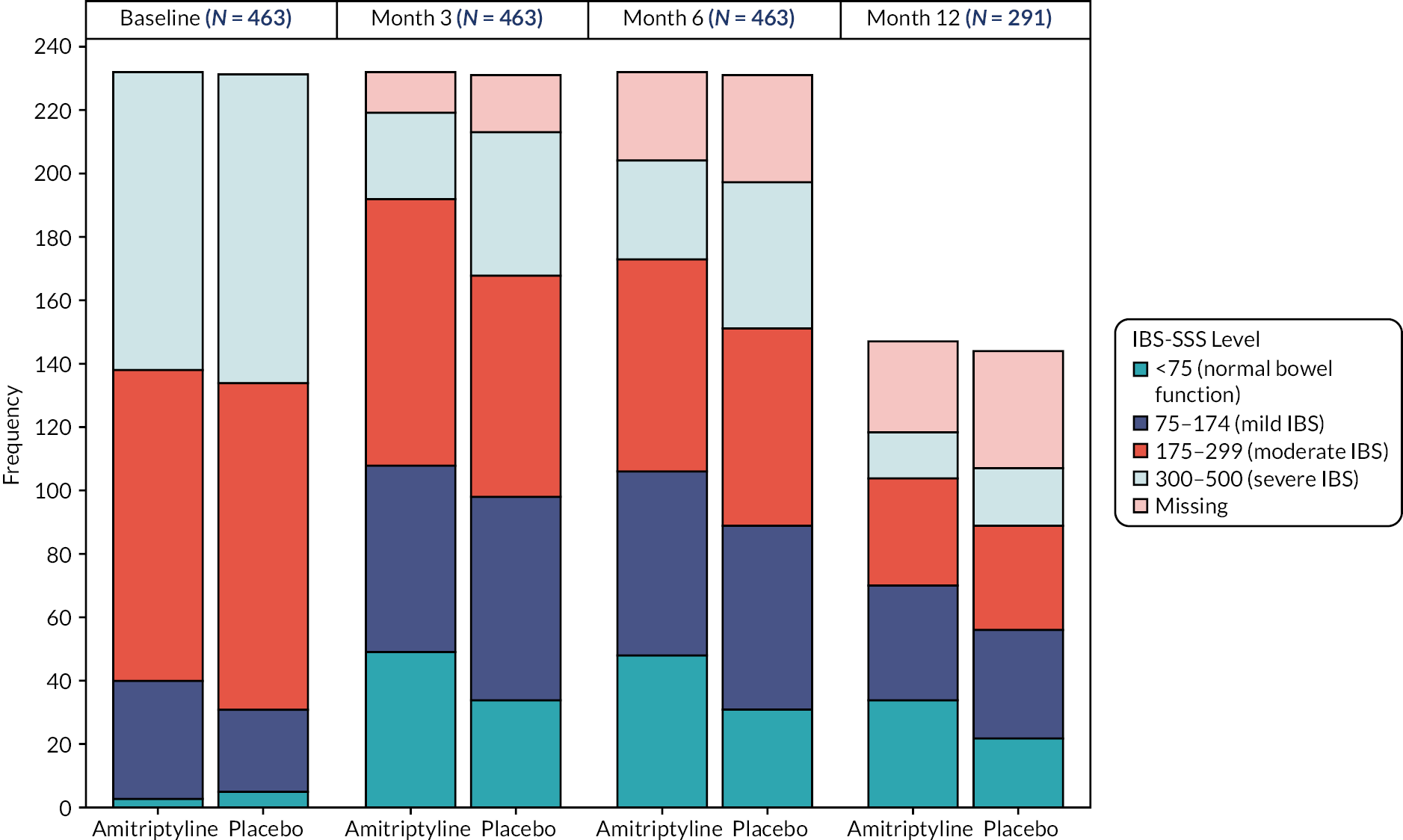
FIGURE 17.
Unadjusted total IBS-SSS score and item level scores with 95% CIs based on available data.
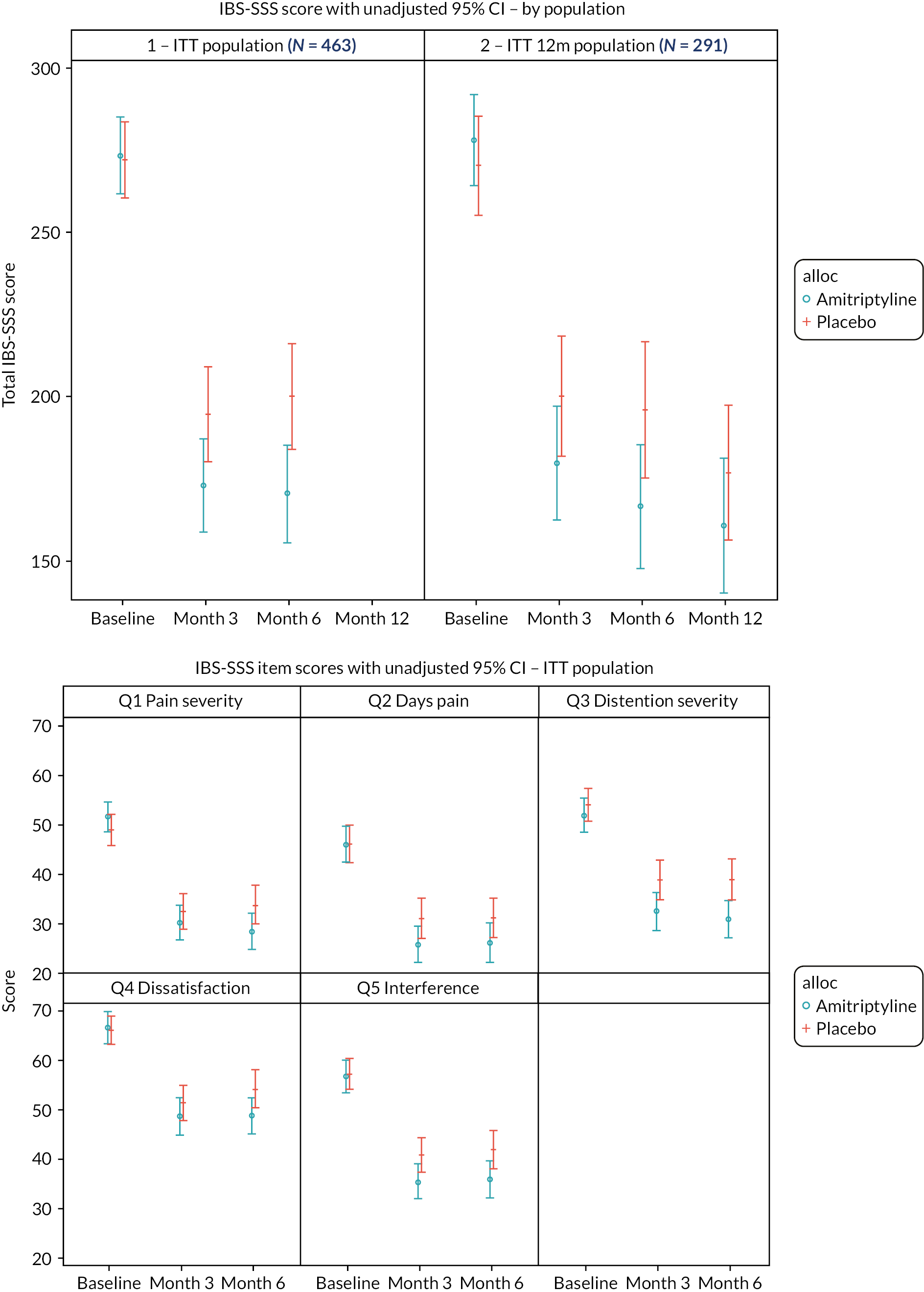
| Baseline | Month 3 | Month 6 | Month 12 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 232) | Placebo (n = 231) | Total (n = 463) | Amitriptyline (n = 147) | Placebo (n = 144) | Total (n = 291) | |
| 1a. Current (past 10 days) abdominal pain? | ||||||||||||
| No | 13 (5.6%) | 21 (9.1%) | 34 (7.3%) | 73 (33.3%) | 63 (29.6%) | 136 (31.5%) | 76 (37.3%) | 59 (29.9%) | 135 (33.7%) | 50 (42.4%) | 40 (37.4%) | 90 (40.0%) |
| Yes | 219 (94.4%) | 210 (90.9%) | 429 (92.7%) | 146 (66.7%) | 150 (70.4%) | 296 (68.5%) | 128 (62.7%) | 138 (70.1%) | 266 (66.3%) | 68 (57.6%) | 67 (62.6%) | 135 (60.0%) |
| Missing | 13 | 18 | 31 | 28 | 34 | 62 | 29 | 37 | 66 | |||
| 1b. Severity of abdominal pain | ||||||||||||
| 0 – No pain | 13 (5.6%) | 21 (9.1%) | 34 (7.4%) | 73 (33.3%) | 64 (30.0%) | 137 (31.7%) | 76 (37.3%) | 59 (29.9%) | 135 (33.7%) | 51 (43.2%) | 40 (37.4%) | 91 (40.4%) |
| 10 | 4 (1.7%) | 1 (0.4%) | 5 (1.1%) | 5 (2.3%) | 7 (3.3%) | 12 (2.8%) | 3 (1.5%) | 5 (2.5%) | 8 (2.0%) | 3 (2.5%) | 2 (1.9%) | 5 (2.2%) |
| 20 | 10 (4.3%) | 17 (7.4%) | 27 (5.8%) | 12 (5.5%) | 12 (5.6%) | 24 (5.6%) | 14 (6.9%) | 12 (6.1%) | 26 (6.5%) | 8 (6.8%) | 8 (7.5%) | 16 (7.1%) |
| 30 | 28 (12.1%) | 29 (12.6%) | 57 (12.3%) | 34 (15.5%) | 25 (11.7%) | 59 (13.7%) | 28 (13.7%) | 21 (10.7%) | 49 (12.2%) | 13 (11.0%) | 8 (7.5%) | 21 (9.3%) |
| 40 | 27 (11.7%) | 26 (11.3%) | 53 (11.5%) | 30 (13.7%) | 34 (16.0%) | 64 (14.8%) | 27 (13.2%) | 26 (13.2%) | 53 (13.2%) | 10 (8.5%) | 10 (9.3%) | 20 (8.9%) |
| 50 | 37 (16.0%) | 31 (13.4%) | 68 (14.7%) | 17 (7.8%) | 19 (8.9%) | 36 (8.3%) | 14 (6.9%) | 23 (11.7%) | 37 (9.2%) | 9 (7.6%) | 15 (14.0%) | 24 (10.7%) |
| 60 | 42 (18.2%) | 34 (14.7%) | 76 (16.5%) | 18 (8.2%) | 22 (10.3%) | 40 (9.3%) | 20 (9.8%) | 21 (10.7%) | 41 (10.2%) | 5 (4.2%) | 8 (7.5%) | 13 (5.8%) |
| 70 | 35 (15.2%) | 41 (17.7%) | 76 (16.5%) | 22 (10.0%) | 19 (8.9%) | 41 (9.5%) | 14 (6.9%) | 15 (7.6%) | 29 (7.2%) | 8 (6.8%) | 11 (10.3%) | 19 (8.4%) |
| 80 | 24 (10.4%) | 22 (9.5%) | 46 (10.0%) | 7 (3.2%) | 10 (4.7%) | 17 (3.9%) | 4 (2.0%) | 13 (6.6%) | 17 (4.2%) | 10 (8.5%) | 4 (3.7%) | 14 (6.2%) |
| 90 | 7 (3.0%) | 7 (3.0%) | 14 (3.0%) | 1 (0.5%) | 1 (0.5%) | 2 (0.5%) | 2 (1.0%) | 0 (0.0%) | 2 (0.5%) | 0 (0.0%) | 1 (0.9%) | 1 (0.4%) |
| 100 – Very severe pain | 4 (1.7%) | 2 (0.9%) | 6 (1.3%) | 2 (1.0%) | 2 (1.0%) | 4 (1.0%) | 1 (0.8%) | 0 (0.0%) | 1 (0.4%) | |||
| Missing | 1 | 0 | 1 | 13 | 18 | 31 | 28 | 34 | 62 | 29 | 37 | 66 |
| 2. N days abdominal pain | ||||||||||||
| 0 days | 13 (5.6%) | 21 (9.1%) | 34 (7.4%) | 73 (33.3%) | 64 (30.0%) | 137 (31.7%) | 76 (37.3%) | 59 (29.9%) | 135 (33.7%) | 50 (42.4%) | 40 (37.4%) | 90 (40.0%) |
| 1 | 13 (5.6%) | 8 (3.5%) | 21 (4.5%) | 20 (9.1%) | 11 (5.2%) | 31 (7.2%) | 11 (5.4%) | 4 (2.0%) | 15 (3.7%) | 9 (7.6%) | 6 (5.6%) | 15 (6.7%) |
| 2 | 32 (13.9%) | 25 (10.8%) | 57 (12.3%) | 37 (16.9%) | 26 (12.2%) | 63 (14.6%) | 32 (15.7%) | 29 (14.7%) | 61 (15.2%) | 16 (13.6%) | 14 (13.1%) | 30 (13.3%) |
| 3 | 37 (16.0%) | 43 (18.6%) | 80 (17.3%) | 27 (12.3%) | 33 (15.5%) | 60 (13.9%) | 25 (12.3%) | 26 (13.2%) | 51 (12.7%) | 13 (11.0%) | 12 (11.2%) | 25 (11.1%) |
| 4 | 25 (10.8%) | 26 (11.3%) | 51 (11.0%) | 18 (8.2%) | 19 (8.9%) | 37 (8.6%) | 15 (7.4%) | 26 (13.2%) | 41 (10.2%) | 6 (5.1%) | 10 (9.3%) | 16 (7.1%) |
| 5 | 34 (14.7%) | 30 (13.0%) | 64 (13.9%) | 9 (4.1%) | 16 (7.5%) | 25 (5.8%) | 14 (6.9%) | 14 (7.1%) | 28 (7.0%) | 8 (6.8%) | 8 (7.5%) | 16 (7.1%) |
| 6 | 23 (10.0%) | 17 (7.4%) | 40 (8.7%) | 10 (4.6%) | 13 (6.1%) | 23 (5.3%) | 5 (2.5%) | 14 (7.1%) | 19 (4.7%) | 5 (4.2%) | 7 (6.5%) | 12 (5.3%) |
| 7 | 17 (7.4%) | 14 (6.1%) | 31 (6.7%) | 7 (3.2%) | 9 (4.2%) | 16 (3.7%) | 7 (3.4%) | 6 (3.0%) | 13 (3.2%) | 2 (1.7%) | 3 (2.8%) | 5 (2.2%) |
| 8 | 7 (3.0%) | 18 (7.8%) | 25 (5.4%) | 6 (2.7%) | 8 (3.8%) | 14 (3.2%) | 5 (2.5%) | 6 (3.0%) | 11 (2.7%) | 2 (1.7%) | 2 (1.9%) | 4 (1.8%) |
| 9 | 5 (2.2%) | 8 (3.5%) | 13 (2.8%) | 1 (0.5%) | 0 (0.0%) | 1 (0.2%) | 4 (2.0%) | 2 (1.0%) | 6 (1.5%) | 0 (0.0%) | 1 (0.9%) | 1 (0.4%) |
| 10 days = pain every day | 25 (10.8%) | 21 (9.1%) | 46 (10.0%) | 11 (5.0%) | 14 (6.6%) | 25 (5.8%) | 10 (4.9%) | 11 (5.6%) | 21 (5.2%) | 7 (5.9%) | 4 (3.7%) | 11 (4.9%) |
| Missing | 1 | 0 (0.0%) | 1 | 13 | 18 | 31 | 28 | 34 | 62 | 29 | 37 | 66 |
| 3a. Current (past 10 days) abdominal distention? | ||||||||||||
| No | 25 (10.8%) | 18 (7.8%) | 43 (9.3%) | 77 (35.2%) | 51 (23.9%) | 128 (29.6%) | 67 (32.8%) | 51 (25.9%) | 118 (29.4%) | 39 (33.1%) | 32 (29.9%) | 71 (31.6%) |
| Yes | 207 (89.2%) | 213 (92.2%) | 420 (90.7%) | 142 (64.8%) | 162 (76.1%) | 304 (70.4%) | 137 (67.2%) | 146 (74.1%) | 283 (70.6%) | 79 (66.9%) | 75 (70.1%) | 154 (68.4%) |
| Missing | 13 | 18 | 31 | 28 | 34 | 62 | 29 | 37 | 66 | |||
| 3b. Severity of abdominal distention | ||||||||||||
| 0 – No distention | 25 (10.8%) | 18 (7.8%) | 43 (9.3%) | 77 (35.2%) | 51 (23.9%) | 128 (29.6%) | 68 (33.3%) | 51 (25.9%) | 119 (29.7%) | 39 (33.1%) | 32 (29.9%) | 71 (31.6%) |
| 10 | 3 (1.3%) | 3 (1.3%) | 6 (1.3%) | 2 (0.9%) | 6 (2.8%) | 8 (1.9%) | 5 (2.5%) | 3 (1.5%) | 8 (2.0%) | 4 (3.4%) | 0 (0.0%) | 4 (1.8%) |
| 20 | 11 (4.7%) | 4 (1.7%) | 15 (3.2%) | 11 (5.0%) | 11 (5.2%) | 22 (5.1%) | 18 (8.8%) | 11 (5.6%) | 29 (7.2%) | 6 (5.1%) | 5 (4.7%) | 11 (4.9%) |
| 30 | 17 (7.3%) | 23 (10.0%) | 40 (8.6%) | 18 (8.2%) | 30 (14.1%) | 48 (11.1%) | 19 (9.3%) | 18 (9.1%) | 37 (9.2%) | 22 (18.6%) | 16 (15.0%) | 38 (16.9%) |
| 40 | 29 (12.5%) | 30 (13.0%) | 59 (12.7%) | 30 (13.7%) | 22 (10.3%) | 52 (12.0%) | 24 (11.8%) | 22 (11.2%) | 46 (11.5%) | 10 (8.5%) | 13 (12.1%) | 23 (10.2%) |
| 50 | 24 (10.3%) | 33 (14.3%) | 57 (12.3%) | 31 (14.2%) | 23 (10.8%) | 54 (12.5%) | 25 (12.3%) | 31 (15.7%) | 56 (14.0%) | 11 (9.3%) | 10 (9.3%) | 21 (9.3%) |
| 60 | 39 (16.8%) | 39 (16.9%) | 78 (16.8%) | 16 (7.3%) | 25 (11.7%) | 41 (9.5%) | 20 (9.8%) | 19 (9.6%) | 39 (9.7%) | 8 (6.8%) | 11 (10.3%) | 19 (8.4%) |
| 70 | 35 (15.1%) | 35 (15.2%) | 70 (15.1%) | 15 (6.8%) | 17 (8.0%) | 32 (7.4%) | 14 (6.9%) | 20 (10.2%) | 34 (8.5%) | 8 (6.8%) | 10 (9.3%) | 18 (8.0%) |
| 80 | 32 (13.8%) | 22 (9.5%) | 54 (11.7%) | 14 (6.4%) | 18 (8.5%) | 32 (7.4%) | 6 (2.9%) | 11 (5.6%) | 17 (4.2%) | 5 (4.2%) | 6 (5.6%) | 11 (4.9%) |
| 90 | 10 (4.3%) | 10 (4.3%) | 20 (4.3%) | 2 (0.9%) | 6 (2.8%) | 8 (1.9%) | 3 (1.5%) | 7 (3.6%) | 10 (2.5%) | 1 (0.8%) | 2 (1.9%) | 3 (1.3%) |
| 100 – Very severe distention | 7 (3.0%) | 14 (6.1%) | 21 (4.5%) | 3 (1.4%) | 4 (1.9%) | 7 (1.6%) | 2 (1.0%) | 4 (2.0%) | 6 (1.5%) | 4 (3.4%) | 2 (1.9%) | 6 (2.7%) |
| Missing | 13 | 18 | 31 | 28 | 34 | 62 | 29 | 37 | 66 | |||
| 4. Dissatisfaction with bowel functioning in past 10 days? | ||||||||||||
| 0 – Not dissatisfied | 3 (1.3%) | 2 (0.9%) | 5 (1.1%) | 14 (6.4%) | 7 (3.3%) | 21 (4.9%) | 9 (4.4%) | 8 (4.1%) | 17 (4.2%) | 7 (5.9%) | 3 (2.8%) | 10 (4.4%) |
| 10 | 2 (0.9%) | 6 (2.6%) | 8 (1.7%) | 15 (6.8%) | 11 (5.2%) | 26 (6.0%) | 15 (7.4%) | 9 (4.6%) | 24 (6.0%) | 8 (6.8%) | 12 (11.2%) | 20 (8.9%) |
| 20 | 14 (6.0%) | 4 (1.7%) | 18 (3.9%) | 24 (11.0%) | 28 (13.1%) | 52 (12.0%) | 25 (12.3%) | 18 (9.1%) | 43 (10.7%) | 18 (15.3%) | 10 (9.3%) | 28 (12.4%) |
| 30 | 12 (5.2%) | 10 (4.3%) | 22 (4.8%) | 25 (11.4%) | 19 (8.9%) | 44 (10.2%) | 19 (9.3%) | 18 (9.1%) | 37 (9.2%) | 9 (7.6%) | 11 (10.3%) | 20 (8.9%) |
| 40 | 15 (6.5%) | 13 (5.7%) | 28 (6.1%) | 19 (8.7%) | 25 (11.7%) | 44 (10.2%) | 20 (9.8%) | 15 (7.6%) | 35 (8.7%) | 13 (11.0%) | 10 (9.3%) | 23 (10.2%) |
| 50 | 26 (11.2%) | 25 (10.9%) | 51 (11.0%) | 28 (12.8%) | 25 (11.7%) | 53 (12.3%) | 32 (15.7%) | 26 (13.2%) | 58 (14.5%) | 10 (8.5%) | 10 (9.3%) | 20 (8.9%) |
| 60 | 28 (12.1%) | 40 (17.4%) | 68 (14.7%) | 24 (11.0%) | 17 (8.0%) | 41 (9.5%) | 23 (11.3%) | 26 (13.2%) | 49 (12.2%) | 18 (15.3%) | 11 (10.3%) | 29 (12.9%) |
| 70 | 35 (15.1%) | 39 (17.0%) | 74 (16.0%) | 28 (12.8%) | 37 (17.4%) | 65 (15.0%) | 21 (10.3%) | 28 (14.2%) | 49 (12.2%) | 16 (13.6%) | 18 (16.8%) | 34 (15.1%) |
| 80 | 37 (15.9%) | 52 (22.6%) | 89 (19.3%) | 21 (9.6%) | 24 (11.3%) | 45 (10.4%) | 22 (10.8%) | 26 (13.2%) | 48 (12.0%) | 11 (9.3%) | 15 (14.0%) | 26 (11.6%) |
| 90 | 20 (8.6%) | 20 (8.7%) | 40 (8.7%) | 7 (3.2%) | 8 (3.8%) | 15 (3.5%) | 8 (3.9%) | 12 (6.1%) | 20 (5.0%) | 3 (2.5%) | 4 (3.7%) | 7 (3.1%) |
| 100 – Very dissatisfied | 40 (17.2%) | 19 (8.3%) | 59 (12.8%) | 14 (6.4%) | 12 (5.6%) | 26 (6.0%) | 10 (4.9%) | 11 (5.6%) | 21 (5.2%) | 5 (4.2%) | 3 (2.8%) | 8 (3.6%) |
| Missing | 0 (0.0%) | 1 | 1 | 13 | 18 | 31 | 28 | 34 | 62 | 29 | 37 | 66 |
| 5. Interference of abdominal pain, discomfort, altered bowel function | ||||||||||||
| 0 – Not at all | 4 (1.7%) | 3 (1.3%) | 7 (1.5%) | 24 (11.0%) | 14 (6.6%) | 38 (8.8%) | 22 (10.8%) | 16 (8.1%) | 38 (9.5%) | 14 (11.9%) | 13 (12.1%) | 27 (12.0%) |
| 10 | 7 (3.0%) | 8 (3.5%) | 15 (3.2%) | 32 (14.6%) | 29 (13.6%) | 61 (14.1%) | 39 (19.1%) | 28 (14.2%) | 67 (16.7%) | 22 (18.6%) | 20 (18.7%) | 42 (18.7%) |
| 20 | 17 (7.3%) | 17 (7.4%) | 34 (7.4%) | 32 (14.6%) | 26 (12.2%) | 58 (13.4%) | 25 (12.3%) | 23 (11.7%) | 48 (12.0%) | 21 (17.8%) | 10 (9.3%) | 31 (13.8%) |
| 30 | 28 (12.1%) | 22 (9.6%) | 50 (10.8%) | 28 (12.8%) | 28 (13.1%) | 56 (13.0%) | 25 (12.3%) | 22 (11.2%) | 47 (11.7%) | 16 (13.6%) | 20 (18.7%) | 36 (16.0%) |
| 40 | 20 (8.6%) | 18 (7.8%) | 38 (8.2%) | 37 (16.9%) | 21 (9.9%) | 58 (13.4%) | 21 (10.3%) | 17 (8.6%) | 38 (9.5%) | 10 (8.5%) | 9 (8.4%) | 19 (8.4%) |
| 50 | 30 (12.9%) | 24 (10.4%) | 54 (11.7%) | 20 (9.1%) | 29 (13.6%) | 49 (11.3%) | 25 (12.3%) | 20 (10.2%) | 45 (11.2%) | 12 (10.2%) | 4 (3.7%) | 16 (7.1%) |
| 60 | 34 (14.7%) | 48 (20.9%) | 82 (17.7%) | 13 (5.9%) | 17 (8.0%) | 30 (6.9%) | 11 (5.4%) | 22 (11.2%) | 33 (8.2%) | 10 (8.5%) | 9 (8.4%) | 19 (8.4%) |
| 70 | 31 (13.4%) | 36 (15.7%) | 67 (14.5%) | 11 (5.0%) | 29 (13.6%) | 40 (9.3%) | 12 (5.9%) | 21 (10.7%) | 33 (8.2%) | 4 (3.4%) | 12 (11.2%) | 16 (7.1%) |
| 80 | 28 (12.1%) | 23 (10.0%) | 51 (11.0%) | 11 (5.0%) | 12 (5.6%) | 23 (5.3%) | 13 (6.4%) | 16 (8.1%) | 29 (7.2%) | 7 (5.9%) | 9 (8.4%) | 16 (7.1%) |
| 90 | 12 (5.2%) | 19 (8.3%) | 31 (6.7%) | 3 (1.4%) | 5 (2.3%) | 8 (1.9%) | 4 (2.0%) | 9 (4.6%) | 13 (3.2%) | 1 (0.8%) | 1 (0.9%) | 2 (0.9%) |
| 100 – Completely | 21 (9.1%) | 12 (5.2%) | 33 (7.1%) | 8 (3.7%) | 3 (1.4%) | 11 (2.5%) | 7 (3.4%) | 3 (1.5%) | 10 (2.5%) | 1 (0.8%) | 0 (0.0%) | 1 (0.4%) |
| Missing | 0 (0.0%) | 1 | 1 | 13 | 18 | 31 | 28 | 34 | 62 | 29 | 37 | 66 |
FIGURE 18.
Plots of residuals for 6-month total IBS-SSS score.

Missing data exploration
We prespecified imputation by treatment arm including planned analysis covariates (data informing stratification: recruitment hub, stool pattern, HADS-D score; and baseline score). Missing data exploration was performed to identify additional auxiliary variables to include in the multiple imputation models based on variables found to be predictive of missingness (see Table 7) or outcome for the primary 6-month total IBS-SSS score. Auxiliary variables explored were: baseline HADS-A, baseline WSAS, and baseline PHQ-12 scores, age, sex and treatment indicators for whether patients were still on treatment at 6 months.
Other potential baseline variables were not considered due to sparsity of the cells (marital status, ethnicity, education level) or due to their value of information compared with other included auxiliary variables (previous treatment for anxiety or depression, time since IBS diagnosis).
Univariable logistic and linear regression were used to identify auxiliary variables predictive of missingness and outcome respectively (see Table 49). Overall, across trial arms, univariable analysis found all auxiliary variables, except for sex, to be predictive (p < 0.05) of missing data status, outcome, or both. Recruitment hub was predictive of missing data status; baseline HADS-D, baseline HADS-A, baseline and PHQ-12 scores were predictive of outcome; and age, baseline IBS-SSS, and baseline WSAS scores, and 6-month treatment status were predictive of both missing data status and outcome. Although sex was not found to be predictive of missing data status or outcome for the 6-month total IBS-SSS score, as sex was a covariate in the PHQ-12 analysis model (due to differences in available total scores for male and females), we included sex in the multiple imputation models for all outcomes to ensure consistency.
Missing data were, therefore, imputed by treatment arm via multiple imputation by chained equations with 25 imputations, including recruitment hub, IBS subtype, sex, age, baseline questionnaire scores (IBS-SSS, PHQ-12, HADS and WSAS), 3-month IBS-SSS score, and 6-month treatment status in the model. The 3-month IBS-SSS score was imputed within the same model in a preliminary step, incorporating 3-month (rather than 6-month) treatment status. The 12-month IBS-SSS score was imputed in a further separate imputation model including the 12-month intention-to-treat population, incorporating 12-month treatment status and 3- and 6-month IBS-SSS scores (also imputed in preliminary steps based on 3- and 6-month treatment status, respectively).
The same imputation variables were incorporated into multiple imputations models for SGA or relief, HADS-A, HADS-D, WSAS, PHQ-12 and acceptability outcomes.
Key secondary end point
Primary analysis
Residual plots (see Figure 20) show that assumptions for logistic regression hold; residuals fell within −2 to 2 and no extreme outliers were identified.
Secondary analysis
The score test for the proportional odds assumption [p = 0.903 (from complete case ordinal logistic regression of SGA of relief of IBS symptoms)] indicated that the assumption holds and the ORs for the treatment effect can be interpreted as constant across all possible cut points of the outcome.
Secondary end points: 3-month irritable bowel syndrome Severity Scoring System and subjective global assessment
| 6-month total IBS-SSS score | ||
|---|---|---|
| Predictive of missingness, p-value | Predictive of outcome, p-value | |
| Covariates | ||
| Recruitment hub | 0.0068 | 0.2626 |
| Baseline HADS-D score | 0.8202 | 0.0462 |
| IBS subtype | 0.8896 | 0.5023 |
| Baseline IBS-SSS score | 0.0140 | < 0.0001 |
| Potential auxiliary variables | ||
| Baseline HADS-A score | 0.8471 | 0.0004 |
| Baseline PHQ-12 score | 0.2396 | 0.0003 |
| Baseline WSAS score | 0.0024 | < 0.0001 |
| Age | 0.0489 | 0.0012 |
| Sex | 0.4370 | 0.4654 |
| On treatment at 6 months | < 0.0001 | 0.0003 |
FIGURE 19.
Subjective global assessment of relief of IBS symptoms at 3 months, 6 months and 12 months.
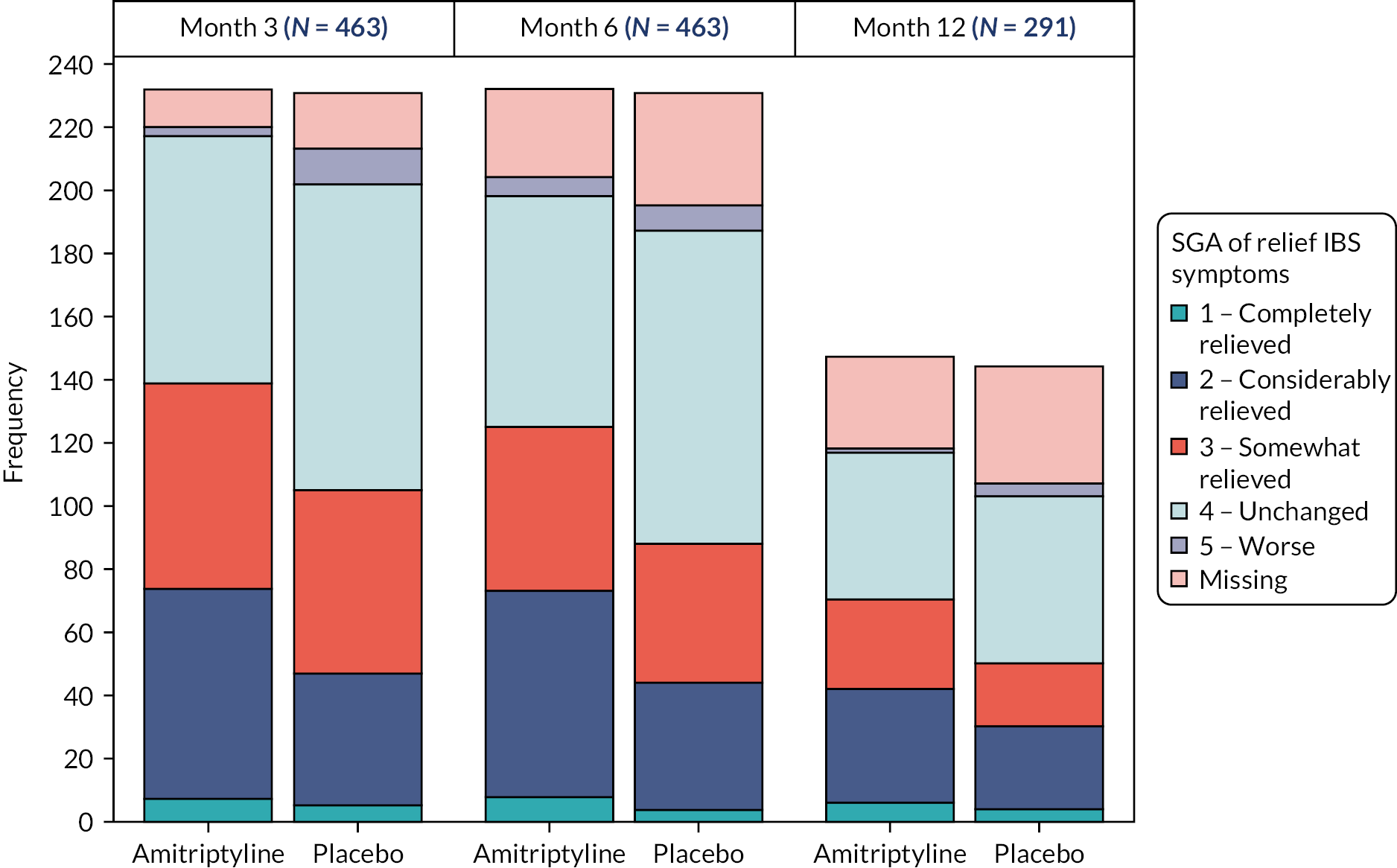
FIGURE 20.
Plots of residuals for logistic regression of the 6-month SGA. From complete case logistic regression of SGA of relief of IBS symptoms (symptoms completely, considerably, or somewhat relieved vs. unchanged or worse).
| Primary analysis (n = 463) | Complete case (n = 433) | Per protocol (n = 373) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimates (95% CI) | Std Error | p-value | Parameter estimates (95% CI) | Std Error | p-value | Parameter estimates (95% CI) | Std Error | p-value | |
| Intercept | 53.91 (19.01 to 88.82) | 17.80 | 0.002 | 52.24 (17.67 to 86.81) | 17.59 | 0.003 | 45.33 (8.24 to 82.42) | 18.92 | 0.017 |
| Treatment: amitriptyline (vs. placebo) | −23.30 (−41.96 to −4.64) | 9.52 | 0.014 | −23.95 (−42.35 to −5.56) | 9.36 | 0.011 | −27.70 (−47.23 to −8.17) | 9.96 | 0.005 |
| Baseline IBS-SSS score | 0.49 (0.38 to 0.59) | 0.05 | < 0.001 | 0.49 (0.38 to 0.59) | 0.05 | < 0.001 | 0.50 (0.39 to 0.61) | 0.06 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | 0.278 | ||||||||
| IBS-C | 21.92 (−4.53 to 48.38) | 13.50 | 0.104 | 21.06 (−5.50 to 47.63) | 13.52 | 0.120 | 25.09 (−3.35 to 53.53) | 14.51 | 0.084 |
| IBS-D | 2.35 (−17.85 to 22.55) | 10.31 | 0.820 | 2.17 (−18.03 to 22.36) | 10.27 | 0.833 | 13.85 (−7.54 to 35.25) | 10.92 | 0.204 |
| Baseline HADS-D score | 2.14 (−0.60 to 4.87) | 1.40 | 0.126 | 1.86 (−0.90 to 4.63) | 1.41 | 0.185 | 1.39 (−1.50 to 4.28) | 1.48 | 0.346 |
| Recruitment hub (vs. Wessex) | 0.119 | ||||||||
| West of England | 4.94 (−15.29 to 25.18) | 10.32 | 0.632 | 5.49 (−14.82 to 25.81) | 10.34 | 0.595 | −0.65 (−22.07 to 20.77) | 10.93 | 0.953 |
| West Yorkshire | −20.83 (−47.31 to 5.66) | 13.50 | 0.123 | −21.86 (−48.03 to 4.32) | 13.32 | 0.102 | −24.57 (−53.02 to 3.89) | 14.52 | 0.091 |
| Primary analysis (responder 1–3 vs. 4–5) (n = 463) | Sensitivity analysis | Secondary analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Complete case (responder 1–3 vs. 4–5) (n = 433) | Alternative responder definition (responder 1–2 vs. 3–5) (n = 463) | Ordinal regressiona (n = 463) |
||||||||||||||
| P. est. | SE | p-value | Odds ratio (95% CI) | P. est. | SE | p-value | Odds ratio (95% CI) | P. est. | SE | p-value | Odds ratio (95% CI) | P. est. | SE | p-value | Odds ratio (95% CI) | |
| Intercept | 0.16 | 0.24 | 0.517 | 0.19 | 0.24 | 0.442 | −1.14 | 0.28 | < 0.0001 | |||||||
| 1 – Completely relieved | −3.69 | 0.36 | < 0.0001 | |||||||||||||
| 2 – Considerably relieved | −1.07 | 0.23 | < 0.0001 | |||||||||||||
| 3 – Somewhat relieved | 0.14 | 0.22 | 0.546 | |||||||||||||
| 4 – Unchanged | 3.28 | 0.34 | < 0.0001 | |||||||||||||
| Treatment: amitriptyline (vs. placebo) | 0.53 | 0.20 | 0.008 | 1.70 (1.15 to 2.53) | 0.59 | 0.20 | 0.003 | 1.81 (1.23 to 2.67) | 0.59 | 0.22 | 0.008 | 1.81 (1.17 to 2.79) | 0.59 | 0.18 | 0.001 | 1.80 (1.26 to 2.58) b |
| IBS subtype (vs. IBS-M or IBS-U) | 0.178 | |||||||||||||||
| IBS-C | −0.51 | 0.28 | 0.071 | 0.60 (0.34 to 1.05) | −0.53 | 0.28 | 0.064 | 0.59 (0.34 to 1.03) | 0.07 | 0.31 | 0.829 | 1.07 (0.58 to 1.97) | −0.34 | 0.26 | 0.188 | 0.71 (0.43 to 1.18) |
| IBS-D | −0.14 | 0.21 | 0.503 | 0.87 (0.57 to 1.32) | −0.16 | 0.22 | 0.461 | 0.85 (0.56 to 1.30) | 0.02 | 0.24 | 0.936 | 1.02 (0.64 to 1.63) | −0.11 | 0.19 | 0.549 | 0.89 (0.61 to 1.30) |
| Baseline HADS-D score | −0.02 | 0.03 | 0.432 | 0.98 (0.92 to 1.03) | −0.02 | 0.03 | 0.451 | 0.98 (0.92 to 1.04) | −0.05 | 0.03 | 0.152 | 0.95 (0.89 to 1.02) | −0.04 | 0.03 | 0.139 | 0.96 (0.91 to 1.01) |
| Recruitment hub (vs. Wessex) | 0.643 | |||||||||||||||
| West of England | −0.05 | 0.21 | 0.833 | 0.96 (0.63 to 1.46) | −0.05 | 0.22 | 0.810 | 0.95 (0.62 to 1.45) | −0.11 | 0.24 | 0.650 | 0.90 (0.56 to 1.44) | −0.04 | 0.19 | 0.851 | 0.96 (0.66 to 1.41) |
| West Yorkshire | 0.13 | 0.27 | 0.628 | 1.14 (0.67 to 1.95) | 0.21 | 0.28 | 0.452 | 1.24 (0.71 to 2.15) | 0.28 | 0.29 | 0.341 | 1.32 (0.74 to 2.34) | 0.20 | 0.25 | 0.426 | 1.22 (0.75 to 1.98) |
Secondary end points: HADS-A, HADS-D, WSAS and PHQ-12
FIGURE 21.
Unadjusted HADS-A and HADS-D scores with 95% CIs based on available data.
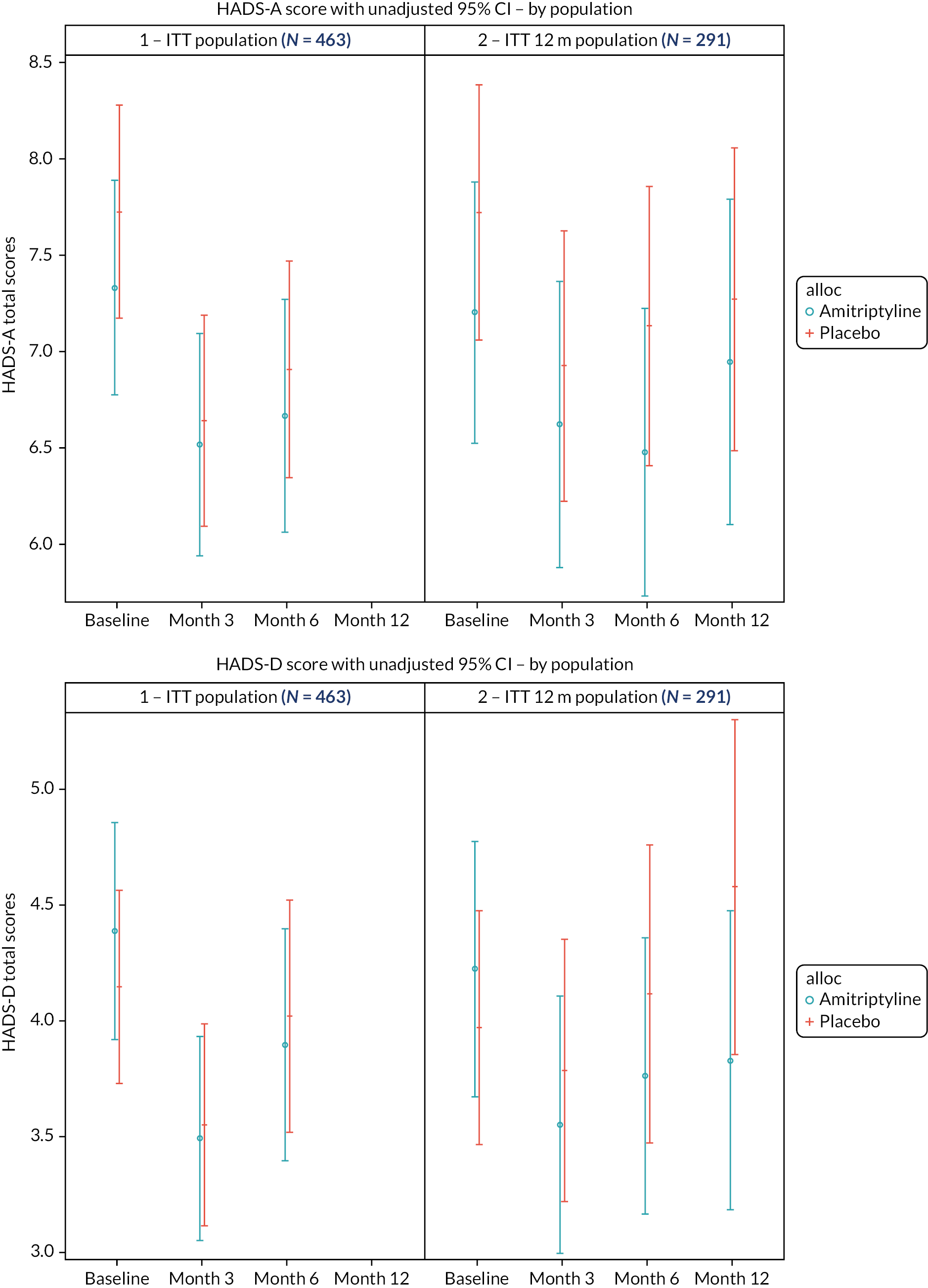
FIGURE 22.
Unadjusted WSAS scores with 95% CIs based on available data.

| 3 months | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimates (95% CI) | SE | p-value | Parameter estimates (95% CI) | SE | p-value | Parameter estimates (95% CI) | SE | p-value | |
| Primary analysis | |||||||||
| Intercept | 1.31 (0.49 to 2.14) | 0.42 | 0.002 | 1.33 (0.52 to 2.14) | 0.41 | 0.001 | 2.45 (1.15 to 3.74) | 0.66 | < 0.001 |
| Treatment: amitriptyline (vs. placebo) | 0.05 (−0.53 to 0.63) | 0.30 | 0.861 | 0.08 (−0.49 to 0.65) | 0.29 | 0.775 | −0.38 (−1.22 to 0.47) | 0.43 | 0.385 |
| Baseline HADS-A score | 0.71 (0.62 to 0.79) | 0.04 | < 0.001 | 0.69 (0.61 to 0.78) | 0.04 | <0.001 | 0.71 (0.59 to 0.83) | 0.06 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | |||||||||
| IBS-C | 0.02 (−0.82 to 0.86) | 0.43 | 0.970 | −0.17 (−1.00 to 0.67) | 0.43 | 0.698 | −0.90 (−2.08 to 0.29) | 0.60 | 0.139 |
| IBS-D | −0.61 (−1.24 to 0.01) | 0.32 | 0.055 | −0.60 (−1.24 to 0.04) | 0.33 | 0.068 | −1.04 (−1.94 to −0.15) | 0.46 | 0.022 |
| Baseline HADS-D score | −0.03 (−0.13 to 0.08) | 0.05 | 0.597 | 0.04 (−0.07 to 0.14) | 0.05 | 0.485 | −0.00 (−0.17 to 0.16) | 0.08 | 0.956 |
| Recruitment hub (vs. Wessex) | |||||||||
| West of England | 0.48 (−0.15 to 1.12) | 0.32 | 0.135 | 0.61 (−0.02 to 1.25) | 0.32 | 0.059 | 0.45 (−0.48 to 1.38) | 0.47 | 0.345 |
| West Yorkshire | 1.10 (0.27 to 1.94) | 0.43 | 0.010 | 0.86 (0.03 to 1.69) | 0.42 | 0.041 | 0.84 (−0.35 to 2.03) | 0.60 | 0.165 |
| Complete case | |||||||||
| Intercept | 1.22 (0.42 to 2.03) | 0.41 | 0.003 | 1.32 (0.50 to 2.15) | 0.42 | 0.002 | 2.07 (0.82 to 3.31) | 0.63 | 0.001 |
| Treatment: amitriptyline (vs. placebo) | 0.07 (−0.50 to 0.64) | 0.29 | 0.815 | −0.07 (−0.66 to 0.51) | 0.30 | 0.808 | −0.18 (−1.02 to 0.65) | 0.42 | 0.662 |
| Baseline HADS-A score | 0.71 (0.63 to 0.80) | 0.04 | < 0.001 | 0.68 (0.60 to 0.77) | 0.04 | < 0.001 | 0.74 (0.61 to 0.86) | 0.06 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | 0.130 | 0.167 | 0.023 | ||||||
| IBS-C | −0.01 (−0.83 to 0.81) | 0.42 | 0.974 | −0.11 (−0.94 to 0.73) | 0.42 | 0.800 | −1.01 (−2.20 to 0.18) | 0.60 | 0.097 |
| IBS-D | −0.60 (−1.23 to 0.02) | 0.32 | 0.058 | −0.60 (−1.24 to 0.04) | 0.33 | 0.066 | −1.23 (−2.15 to −0.32) | 0.46 | 0.008 |
| Baseline HADS-D score | −0.03 (−0.14 to 0.07) | 0.05 | 0.522 | 0.05 (−0.05 to 0.16) | 0.05 | 0.322 | −0.02 (−0.18 to 0.15) | 0.08 | 0.847 |
| Recruitment hub (vs. Wessex) | 0.034 | 0.063 | 0.421 | ||||||
| West of England | 0.48 (−0.15 to 1.11) | 0.32 | 0.131 | 0.59 (−0.05 to 1.23) | 0.33 | 0.073 | 0.54 (−0.39 to 1.46) | 0.47 | 0.252 |
| West Yorkshire | 1.05 (0.24 to 1.85) | 0.41 | 0.011 | 0.89 (0.05 to 1.73) | 0.43 | 0.038 | 0.67 (−0.56 to 1.89) | 0.62 | 0.285 |
| 3 months | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimates (95% CI) | SE | p-value | Parameter estimates (95% CI) | SE | p-value | Parameter estimates (95% CI) | SE | p-value | |
| Primary analysis | |||||||||
| Intercept | 1.13 (0.53 to 1.73) | 0.31 | < 0.001 | 1.10 (0.44 to 1.77) | 0.34 | 0.001 | 2.16 (1.14 to 3.18) | 0.52 | < 0.001 |
| Treatment: amitriptyline (vs. placebo) | −0.22 (−0.71 to 0.26) | 0.25 | 0.369 | −0.20 (−0.75 to 0.34) | 0.28 | 0.462 | −0.88 (−1.71 to −0.06) | 0.42 | 0.036 |
| Baseline HADS-D score | 0.62 (0.55 to 0.69) | 0.04 | < 0.001 | 0.72 (0.64 to 0.80) | 0.04 | < 0.001 | 0.66 (0.54 to 0.79) | 0.06 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | |||||||||
| IBS-C | −0.13 (−0.83 to 0.58) | 0.36 | 0.724 | −0.27 (−1.00 to 0.47) | 0.38 | 0.476 | −0.59 (−1.69 to 0.51) | 0.56 | 0.293 |
| IBS-D | −0.40 (−0.93 to 0.13) | 0.27 | 0.139 | −0.33 (−0.94 to 0.28) | 0.31 | 0.292 | −0.50 (−1.38 to 0.37) | 0.44 | 0.261 |
| Recruitment hub (vs. Wessex) | |||||||||
| West of England | −0.01 (−0.54 to 0.52) | 0.27 | 0.962 | 0.32 (−0.25 to 0.90) | 0.29 | 0.274 | 0.03 (−0.85 to 0.92) | 0.45 | 0.945 |
| West Yorkshire | 0.56 (−0.14 to 1.25) | 0.36 | 0.119 | 0.28 (−0.48 to 1.05) | 0.39 | 0.467 | 1.27 (0.23 to 2.30) | 0.53 | 0.016 |
| Complete case | |||||||||
| Intercept | 1.17 (0.58 to 1.76) | 0.30 | < 0.001 | 1.05 (0.41 to 1.70) | 0.33 | 0.001 | 2.04 (1.01 to 3.07) | 0.52 | < 0.001 |
| Treatment: amitriptyline (vs. placebo) | −0.27 (−0.75 to 0.21) | 0.24 | 0.264 | −0.37 (−0.89 to 0.15) | 0.27 | 0.161 | −0.85 (−1.63 to −0.07) | 0.39 | 0.032 |
| Baseline HADS-D score | 0.62 (0.55 to 0.69) | 0.04 | < 0.001 | 0.71 (0.64 to 0.79) | 0.04 | < 0.001 | 0.68 (0.55 to 0.80) | 0.06 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | 0.311 | 0.630 | 0.306 | ||||||
| IBS-C | −0.11 (−0.80 to 0.58) | 0.35 | 0.750 | −0.22 (−0.97 to 0.52) | 0.38 | 0.558 | −0.73 (−1.85 to 0.38) | 0.57 | 0.195 |
| IBS-D | −0.40 (−0.93 to 0.12) | 0.27 | 0.131 | −0.27 (−0.84 to 0.30) | 0.29 | 0.356 | −0.54 (−1.39 to 0.32) | 0.43 | 0.215 |
| Recruitment hub (vs. Wessex) | 0.493 | 0.386 | 0.368 | ||||||
| West of England | −0.01 (−0.53 to 0.52) | 0.27 | 0.985 | 0.40 (−0.17 to 0.97) | 0.29 | 0.170 | 0.10 (−0.77 to 0.96) | 0.44 | 0.823 |
| West Yorkshire | 0.38 (−0.30 to 1.06) | 0.34 | 0.275 | 0.24 (−0.52 to 0.99) | 0.38 | 0.537 | 0.79 (−0.35 to 1.94) | 0.58 | 0.175 |
| 3 months | 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimates (95% CI) | SE | p-value | Parameter estimates (95% CI) | SE | p-value | Parameter estimates (95% CI) | SE | p-value | |
| Primary analysis | |||||||||
| Intercept | 2.46 (1.00 to 3.93) | 0.75 | 0.001 | 2.17 (0.67 to 3.66) | 0.76 | 0.005 | 2.33 (−0.09 to 4.75) | 1.23 | 0.059 |
| Treatment: amitriptyline (vs. placebo) | −0.27 (−1.36 to 0.83) | 0.56 | 0.633 | −0.38 (−1.48 to 0.72) | 0.56 | 0.499 | −2.14 (−3.80 to −0.49) | 0.84 | 0.011 |
| Baseline WSAS score | 0.46 (0.38 to 0.54) | 0.04 | < 0.001 | 0.49 (0.41 to 0.56) | 0.04 | < 0.001 | 0.45 (0.32 to 0.58) | 0.06 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | |||||||||
| IBS-C | −0.63 (−2.20 to 0.95) | 0.80 | 0.435 | −0.62 (−2.22 to 0.98) | 0.81 | 0.435 | 0.38 (−2.00 to 2.75) | 1.21 | 0.757 |
| IBS-D | 0.24 (−0.99 to 1.47) | 0.63 | 0.698 | 0.22 (−0.98 to 1.43) | 0.61 | 0.698 | 0.39 (−1.40 to 2.18) | 0.91 | 0.669 |
| Baseline HADS-D score | 0.36 (0.20 to 0.53) | 0.08 | < 0.001 | 0.38 (0.22 to 0.55) | 0.09 | < 0.001 | 0.34 (0.07 to 0.60) | 0.13 | 0.013 |
| Recruitment hub (vs. Wessex) | |||||||||
| West of England | 0.95 (−0.23 to 2.12) | 0.60 | 0.115 | 0.97 (−0.23 to 2.17) | 0.61 | 0.114 | 2.11 (0.32 to 3.90) | 0.91 | 0.021 |
| West Yorkshire | 0.17 (−1.39 to 1.72) | 0.79 | 0.832 | 0.06 (−1.55 to 1.67) | 0.82 | 0.943 | 2.79 (0.46 to 5.13) | 1.19 | 0.019 |
| Complete case | |||||||||
| Intercept | 1.73 (−0.01 to 3.46) | 0.88 | 0.051 | 1.57 (−0.21 to 3.35) | 0.90 | 0.083 | 1.78 (−0.51 to 4.07) | 1.16 | 0.127 |
| Treatment: amitriptyline (vs. placebo) | −1.04 (−2.30 to 0.23) | 0.64 | 0.108 | −1.29 (−2.61 to 0.02) | 0.67 | 0.054 | −1.70 (−3.24 to −0.15) | 0.78 | 0.031 |
| Baseline WSAS score | 0.46 (0.37 to 0.55) | 0.05 | < 0.001 | 0.49 (0.40 to 0.58) | 0.05 | < 0.001 | 0.45 (0.34 to 0.57) | 0.06 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | 0.594 | 0.993 | 0.910 | ||||||
| IBS-C | −0.32 (−2.13 to 1.49) | 0.93 | 0.729 | 0.05 (−1.87 to 1.97) | 0.98 | 0.957 | −0.32 (−2.61 to 1.96) | 1.16 | 0.782 |
| IBS-D | −0.10 (−1.46 to 1.27) | 0.70 | 0.890 | 0.09 (−1.35 to 1.52) | 0.73 | 0.905 | 0.19 (−1.49 to 1.87) | 0.85 | 0.825 |
| Baseline HADS-D score | 0.44 (0.24 to 0.64) | 0.10 | < 0.001 | 0.42 (0.22 to 0.62) | 0.10 | < 0.001 | 0.37 (0.12 to 0.62) | 0.13 | 0.004 |
| Recruitment hub (vs. Wessex) | 0.246 | 0.227 | 0.013 | ||||||
| West of England | 1.27 (−0.10 to 2.64) | 0.70 | 0.069 | 1.26 (−0.18 to 2.70) | 0.73 | 0.085 | 2.58 (0.86 to 4.29) | 0.87 | 0.003 |
| West Yorkshire | 1.04 (−0.78 to 2.86) | 0.93 | 0.264 | 0.65 (−1.28 to 2.57) | 0.98 | 0.508 | 1.14 (−1.19 to 3.48) | 1.18 | 0.336 |
| 6 months | |||
|---|---|---|---|
| Parameter estimates (95% CI) | SE | p-value | |
| Primary analysis | |||
| Intercept | 2.47 (1.68 to 3.25) | 0.40 | < 0.001 |
| Treatment: amitriptyline (vs. placebo) | −0.04 (−0.58 to 0.49) | 0.27 | 0.877 |
| Baseline PHQ-12 score | 0.50 (0.41 to 0.59) | 0.04 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | |||
| IBS-C | −0.26 (−1.01 to 0.48) | 0.38 | 0.488 |
| IBS-D | −0.54 (−1.11 to 0.02) | 0.29 | 0.061 |
| Baseline HADS-D score | 0.16 (0.08 to 0.25) | 0.04 | < 0.001 |
| Recruitment hub (vs. Wessex) | |||
| West of England | −0.13 (−0.69 to 0.43) | 0.29 | 0.639 |
| West Yorkshire | −0.31 (−1.08 to 0.46) | 0.39 | 0.427 |
| Sex: males vs. females | −0.11 (−0.68 to 0.46) | 0.29 | 0.704 |
| Complete case | |||
| Intercept | 2.46 (1.67 to 3.25) | 0.40 | < 0.001 |
| Treatment: amitriptyline (vs. placebo) | −0.22 (−0.73 to 0.29) | 0.26 | 0.400 |
| Baseline PHQ-12 score | 0.51 (0.42 to 0.60) | 0.04 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | 0.180 | ||
| IBS-C | −0.26 (−0.99 to 0.47) | 0.37 | 0.489 |
| IBS-D | −0.54 (−1.11 to 0.03) | 0.29 | 0.064 |
| Baseline HADS-D score | 0.17 (0.08 to 0.25) | 0.04 | 0.000 |
| Recruitment hub (vs. Wessex) | 0.557 | ||
| West of England | −0.20 (−0.76 to 0.36) | 0.29 | 0.483 |
| West Yorkshire | −0.39 (−1.13 to 0.35) | 0.38 | 0.306 |
| Sex: males vs. females | −0.15 (−0.72 to 0.42) | 0.29 | 0.601 |
Secondary end point: weekly relief
FIGURE 23.
Number and proportion of participants reporting adequate relief each week.

| Week | Observed N (%) satisfactory relief | Model estimatesa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 232) | Placebo (n = 231) | Proportion satisfactory relief (95% CI)b | Odds ratio (95% CI) | p-valuec | ||||||||
| N | % of completed | % of randomised | Missing | N | % of completed | % of randomised | Missing | Amitriptyline | Placebo | |||
| 1 | 50 | 27.5 | 21.6 | 50 | 50 | 26.2 | 21.6 | 40 | 0.26 (0.20 to 0.35) | 0.25 (0.19 to 0.33) | 1.06 (0.67 to 1.68) | 0.805 |
| 2 | 70 | 35.0 | 30.2 | 32 | 58 | 29.1 | 25.1 | 32 | 0.33 (0.26 to 0.41) | 0.28 (0.21 to 0.36) | 1.30 (0.84 to 1.99) | 0.238 |
| 3 | 98 | 50.5 | 42.2 | 38 | 59 | 30.6 | 25.5 | 38 | 0.49 (0.40 to 0.57) | 0.30 (0.23 to 0.38) | 2.20 (1.45 to 3.34) | < 0.001 |
| 4 | 76 | 38.0 | 32.8 | 32 | 69 | 35.2 | 29.9 | 35 | 0.38 (0.30 to 0.47) | 0.35 (0.27 to 0.43) | 1.17 (0.78 to 1.75) | 0.448 |
| 5 | 90 | 44.8 | 38.8 | 31 | 78 | 40.2 | 33.8 | 37 | 0.44 (0.36 to 0.53) | 0.39 (0.31 to 0.47) | 1.26 (0.85 to 1.87) | 0.255 |
| 6 | 100 | 54.1 | 43.1 | 47 | 79 | 39.3 | 34.2 | 30 | 0.54 (0.46 to 0.62) | 0.39 (0.31 to 0.47) | 1.87 (1.26 to 2.78) | 0.002 |
| 7 | 96 | 48.0 | 41.4 | 32 | 76 | 40.6 | 32.9 | 44 | 0.48 (0.39 to 0.56) | 0.40 (0.32 to 0.48) | 1.37 (0.92 to 2.04) | 0.116 |
| 8 | 102 | 52.8 | 44.0 | 39 | 63 | 34.1 | 27.3 | 46 | 0.51 (0.43 to 0.60) | 0.34 (0.26 to 0.42) | 2.09 (1.41 to 3.12) | < 0.001 |
| 9 | 95 | 52.2 | 40.9 | 50 | 78 | 41.3 | 33.8 | 42 | 0.50 (0.41 to 0.59) | 0.39 (0.31 to 0.48) | 1.56 (1.05 to 2.32) | 0.029 |
| 10 | 96 | 49.5 | 41.4 | 38 | 76 | 40.2 | 32.9 | 42 | 0.49 (0.40 to 0.57) | 0.39 (0.31 to 0.47) | 1.46 (0.99 to 2.16) | 0.054 |
| 11 | 99 | 53.2 | 42.7 | 46 | 68 | 38.6 | 29.4 | 55 | 0.52 (0.43 to 0.61) | 0.37 (0.29 to 0.46) | 1.84 (1.24 to 2.73) | 0.003 |
| 12 | 101 | 53.2 | 43.5 | 42 | 77 | 40.3 | 33.3 | 40 | 0.53 (0.44 to 0.61) | 0.39 (0.31 to 0.48) | 1.77 (1.19 to 2.63) | 0.005 |
| 13 | 98 | 53.3 | 42.2 | 48 | 71 | 40.1 | 30.7 | 54 | 0.53 (0.45 to 0.62) | 0.39 (0.31 to 0.48) | 1.76 (1.18 to 2.61) | 0.005 |
| 14 | 99 | 54.1 | 42.7 | 49 | 72 | 38.7 | 31.2 | 45 | 0.53 (0.45 to 0.61) | 0.36 (0.28 to 0.44) | 2.03 (1.36 to 3.04) | < 0.001 |
| 15 | 102 | 56.7 | 44.0 | 52 | 70 | 39.1 | 30.3 | 52 | 0.54 (0.46 to 0.63) | 0.37 (0.29 to 0.45) | 2.05 (1.37 to 3.06) | < 0.001 |
| 16 | 95 | 52.8 | 40.9 | 52 | 67 | 39.4 | 29.0 | 61 | 0.51 (0.42 to 0.60) | 0.38 (0.30 to 0.46) | 1.71 (1.14 to 2.58) | 0.010 |
| 17 | 101 | 55.8 | 43.5 | 51 | 76 | 44.4 | 32.9 | 60 | 0.54 (0.45 to 0.63) | 0.45 (0.36 to 0.54) | 1.45 (0.97 to 2.17) | 0.068 |
| 18 | 93 | 52.5 | 40.1 | 55 | 81 | 46.8 | 35.1 | 58 | 0.52 (0.44 to 0.61) | 0.44 (0.35 to 0.52) | 1.43 (0.96 to 2.13) | 0.080 |
| 19 | 87 | 50.9 | 37.5 | 61 | 76 | 45.5 | 32.9 | 64 | 0.50 (0.41 to 0.59) | 0.41 (0.33 to 0.50) | 1.41 (0.94 to 2.12) | 0.100 |
| 20 | 85 | 49.1 | 36.6 | 59 | 71 | 42.0 | 30.7 | 62 | 0.47 (0.39 to 0.56) | 0.40 (0.32 to 0.49) | 1.33 (0.89 to 2.01) | 0.167 |
| 21 | 91 | 50.3 | 39.2 | 51 | 78 | 46.2 | 33.8 | 62 | 0.49 (0.40 to 0.57) | 0.42 (0.34 to 0.51) | 1.30 (0.87 to 1.94) | 0.194 |
| 22 | 92 | 53.2 | 39.7 | 59 | 75 | 47.2 | 32.5 | 72 | 0.53 (0.44 to 0.61) | 0.44 (0.36 to 0.53) | 1.42 (0.95 to 2.13) | 0.084 |
| 23 | 82 | 49.1 | 35.3 | 65 | 70 | 43.5 | 30.3 | 70 | 0.50 (0.42 to 0.59) | 0.40 (0.31 to 0.48) | 1.56 (1.03 to 2.36) | 0.035 |
| 24 | 90 | 53.9 | 38.8 | 65 | 73 | 47.1 | 31.6 | 76 | 0.51 (0.42 to 0.60) | 0.41 (0.33 to 0.50) | 1.50 (1.00 to 2.26) | 0.052 |
| 25 | 85 | 49.1 | 36.6 | 59 | 57 | 37.0 | 24.7 | 77 | 0.48 (0.39 to 0.56) | 0.32 (0.24 to 0.41) | 1.92 (1.25 to 2.95) | 0.003 |
| Overall | 1.56 (1.20 to 2.03) | < 0.001 | ||||||||||
Secondary end point: tolerability
| Month 3 | Month 6 | Month 12 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Amitriptyline (n = 193) (%) | Placebo (n = 192) (%) | Total (n = 385) (%) | Amitriptyline (n = 166) (%) | Placebo (n = 152) (%) | Total (n = 318) (%) | Amitriptyline (n = 61) (%) | Placebo (n = 56) (%) | Total (n = 117) (%) | |
| 1. Dry mouth | 122 (63.2) | 87 (45.3) | 209 (54.3) | 90 (54.2 | 56 (36.8) | 146 (45.9) | 33 (54.1) | 24 (42.9) | 57 (48.7) |
| Linked to trial medication | 84 (43.5) | 45 (23.4) | 129 (33.5) | 57 (34.3) | 26 (17.1) | 83 (26.1) | 22 (36.1) | 9 (16.1) | 31 (26.5) |
| 2. Drowsiness | 128 (66.3) | 67 (34.9) | 195 (50.6) | 88 (53.0) | 52 (34.2) | 140 (44.0) | 28 (45.9) | 25 (44.6) | 53 (45.3) |
| Linked to trial medication | 95 (49.2) | 27 (14.1) | 122 (31.7) | 56 (33.7) | 13 (8.6) | 69 (21.7) | 16 (26.2) | 8 (14.3) | 24 (20.5) |
| 3. Insomnia (difficulty sleeping%) | 78 (40.4) | 108 (56.3) | 186 (48.3) | 77 (46.4) | 96 (63.2) | 173 (54.4) | 25 (41.0) | 35 (62.5) | 60 (51.3) |
| Linked to trial medication | 10 (5.2) | 13 (6.8) | 23 (6.0) | 4 (2.4) | 5 (3.3) | 9 (2.8) | 5 (8.2) | 2 (3.6) | 7 (6.0) |
| 4. Blurred vision | 29 (15.0) | 24 (12.5) | 53 (13.8) | 28 (16.9) | 14 (9.2) | 42 (13.2) | 9 (14.8) | 10 (17.9) | 19 (16.2) |
| Linked to trial medication | 5 (2.6) | 2 (1.0) | 7 (1.8) | 4 (2.4) | 1 (0.7) | 5 (1.6) | 4 (6.6) | 0 (0.0) | 4 (3.4) |
| 5. Headache | 74 (38.3) | 85 (44.3) | 159 (41.3) | 78 (47.0) | 80 (52.6) | 158 (49.7) | 21 (34.4) | 23 (41.1) | 44 (37.6) |
| Linked to trial medication | 14 (7.3) | 14 (7.3) | 28 (7.3) | 7 (4.2) | 5 (3.3) | 12 (3.8) | 4 (6.6) | 0 (0.0) | 4 (3.4) |
| 6. Constipation | 110 (57.0) | 89 (46.4) | 199 (51.7) | 93 (56.0) | 78 (51.3) | 171 (53.8) | 30 (49.2) | 31 (55.4) | 61 (52.1) |
| Linked to trial medication | 36 (18.7) | 21 (10.9) | 57 (14.8) | 22 (13.3) | 13 (8.6) | 35 (11.0) | 8 (13.1) | 3 (5.4) | 11 (9.4) |
| 7. Diarrhoea | 117 (60.6) | 126 (65.6) | 243 (63.1) | 98 (59.0) | 103 67.8) | 201 (63.2) | 37 (60.7) | 40 (71.4) | 77 (65.8) |
| Linked to trial medication | 21 (10.9) | 16 (8.3) | 37 (9.6) | 15 (9.0) | 7 (4.6) | 22 (6.9) | 5 (8.2) | 2 (3.6) | 7 (6.0) |
| 8. Increased appetite | 54 (28.0) | 44 (22.9) | 98 (25.5) | 45 (27.1) | 34 (22.4) | 79 (24.8) | 21 (34.4) | 15 (26.8) | 36 (30.8) |
| Linked to trial medication | 20 (10.4) | 17 (8.9) | 37 (9.6) | 16 (9.6) | 4 (2.6) | 20 (6.3) | 6 (9.8) | 1 (1.8) | 7 (6.0) |
| 9. Decreased appetite | 34 (17.6) | 28 (14.6) | 62 (16.1) | 17 (10.2) | 22 (14.5) | 39 (12.3) | 5 (8.2) | 6 (10.7) | 11 (9.4) |
| Linked to trial medication | 10 (5.2) | 5 (2.6) | 15 (3.9) | 3 (1.8) | 2 (1.3) | 5 (1.6) | 1 (1.6) | 1 (1.8) | 2 (1.7) |
| 10. Nausea or vomiting | 35 (18.1) | 26 (13.5) | 61 (15.8) | 26 (15.7) | 26 (17.1) | 52 (16.4) | 9 (14.8) | 6 (10.7) | 15 (12.8) |
| Linked to trial medication | 11 (5.7) | 7 (3.6) | 18 (4.7) | 3 (1.8) | 3 (2.0) | 6 (1.9) | 1 (1.6) | 1 (1.8) | 2 (1.7) |
| 11. Problems with urination | 31 (16.1) | 23 (12.0 | 54 (14.0) | 36 (21.7) | 20 (13.2) | 56 (17.6) | 11 (18.0) | 10 (17.9) | 21 (17.9) |
| Linked to trial medication | 9 (4.7) | 3 (1.6) | 12 (3.1) | 11 (6.6) | 3 (2.0) | 14 (4.4) | 5 (8.2) | 1 (1.8) | 6 (5.1) |
| 12. Problems with sexual function | 29 (15.0) | 23 (12.0) | 52 (13.5) | 24 (14.5) | 16 (10.5) | 40 (12.6) | 9 (14.8) | 8 (14.3) | 17 (14.5) |
| Linked to trial medication | 6 (3.1) | 3 (1.6) | 9 (2.3) | 4 (2.4) | 0 (0.0) | 4 (1.3) | 3 (4.9) | 0 (0.0) | 3 (2.6) |
| 13. Palpitations | 56 (29.0) | 37 (19.3) | 93 (24.2) | 41 (24.7) | 38 (25.0) | 79 (24.8) | 14 (23.0) | 18 (32.1) | 32 (27.4) |
| Linked to trial medication | 18 (9.3) | 4 (2.1) | 22 (5.7) | 6 (3.6) | 1 (0.7) | 7 (2.2) | 3 (4.9) | 2 (3.6) | 5 (4.3) |
| 14. Feeling light-headed on standing | 73 (37.8) | 63 (32.8) | 136 (35.3) | 69 (41.6) | 54 (35.5) | 123 (38.7) | 22 (36.1) | 22 (39.3) | 44 (37.6) |
| Linked to trial medication | 19 (9.8) | 7 (3.6) | 26 (6.8) | 13 (7.8) | 6 (3.9) | 19 (6.0) | 2 (3.3) | 2 (3.6) | 4 (3.4) |
| 15. Feeling like the room is spinning | 29 (15.0) | 24 (12.5) | 53 (13.8) | 20 (12.0) | 19 (12.5) | 39 (12.3) | 6 (9.8) | 6 (10.7) | 12 (10.3) |
| Linked to trial medication | 8 (4.1) | 3 (1.6) | 11 (2.9) | 5 (3.0) | 1 (0.7) | 6 (1.9) | 1 (1.6) | 0 (0.0) | 1 (0.9) |
| 16. Sweating | 71 (36.8) | 60 (31.3) | 131 (34.0) | 54 (32.5) | 49 (32.2) | 103 (32.4) | 19 (31.1) | 23 (41.1) | 42 (35.9) |
| Linked to trial medication | 20 (10.4) | 8 (4.2) | 28 (7.3) | 9 (5.4) | 6 (3.9) | 15 (4.7) | 5 (8.2) | 2 (3.6) | 7 (6.0) |
| 17. Increased body temperature | 56 (29.0) | 48 (25.0) | 104 (27.0) | 35 (21.1) | 36 (23.7) | 71 (22.3) | 13 (21.3) | 10 (17.9) | 23 (19.7) |
| Linked to trial medication | 14 (7.3) | 15 (7.8) | 29 (7.5) | 7 (4.2) | 3 (2.0) | 10 (3.1) | 2 (3.3) | 2 (3.6) | 4 (3.4) |
| 18. Tremor | 17 (8.8) | 13 (6.8) | 30 (7.8) | 13 (7.8) | 11 (7.2) | 24 (7.5) | 8 (13.1) | 2 (3.6) | 10 (8.5) |
| Linked to trial medication | 1 (0.5) | 2 (1.0) | 3 (0.8) | 4 (2.4) | 2 (1.3) | 6 (1.9) | 2 (3.3) | 0 (0.0) | 2 (1.7) |
| 19. Disorientation | 24 (12.4) | 8 (4.2) | 32 (8.3) | 13 (7.8) | 10 (6.6) | 23 (7.2) | 2 (3.3) | 4 (7.1) | 6 (5.1) |
| Linked to trial medication | 10 (5.2) | 1 (0.5) | 11 (2.9) | 4 (2.4) | 1 (0.7) | 5 (1.6) | 2 (3.3) | 0 (0.0) | 2 (1.7) |
| 20. Yawning | 67 (34.7) | 68 (35.4) | 135 (35.1) | 63 (38.0) | 50 (32.9) | 113 (35.5) | 18 (29.5) | 25 (44.6) | 43 (36.8) |
| Linked to trial medication | 19 (9.8) | 10 (5.2) | 29 (7.5) | 13 (7.8) | 3 (2.0) | 16 (5.0) | 3 (4.9) | 1 (1.8) | 4 (3.4) |
| 21. Weight gain | 72 (37.3) | 59 (30.7) | 131 (34.0) | 73 (44.0) | 49 (32.2) | 122 (38.4) | 28 (45.9) | 25 (44.6) | 53 (45.3) |
| Linked to trial medication | 22 (11.4) | 14 (7.3) | 36 (9.4) | 20 (12.0) | 10 (6.6) | 30 (9.4) | 10 (16.4) | 3 (5.4) | 13 (11.1) |
Secondary end points: 12-month irritable bowel syndrome with constipation and subjective global assessment
| Primary analysis (n = 291) | Complete case (n = 225) | |||||
|---|---|---|---|---|---|---|
| Parameter estimates (95% CI) | Std error | p-value | Parameter estimates (95% CI) | Std error | p-value | |
| Intercept | 15.37 (−34.48 to 65.22) | 25.41 | 0.545 | 11.24 (−42.15 to 64.63) | 27.09 | 0.679 |
| Treatment: amitriptyline (vs. placebo) | −22.59 (−49.35 to 4.16) | 13.60 | 0.098 | −24.34 (−50.49 to 1.81) | 13.27 | 0.068 |
| Baseline IBS-SSS score | 0.49 (0.35 to 0.63) | 0.07 | < 0.001 | 0.50 (0.34 to 0.65) | 0.08 | < 0.001 |
| IBS subtype (vs. IBS-M or IBS-U) | 0.893 | |||||
| IBS-C | 8.27 (−26.68 to 43.22) | 17.81 | 0.643 | 4.08 (−33.06 to 41.22) | 18.84 | 0.829 |
| IBS-D | −5.46 (−32.94 to 22.01) | 14.00 | 0.696 | −4.63 (−33.45 to 24.18) | 14.62 | 0.752 |
| Baseline HADS-D score | 7.20 (3.31 to 11.09) | 1.98 | < 0.001 | 7.13 (3.01 to 11.24) | 2.09 | 0.001 |
| Recruitment hub (vs. Wessex) | 0.077 | |||||
| West of England | 22.84 (−6.15 to 51.83) | 14.77 | 0.122 | 26.30 (−2.81 to 55.40) | 14.77 | 0.076 |
| West Yorkshire | −15.79 (−56.90 to 25.32) | 20.78 | 0.449 | −9.95 (−48.24 to 28.35) | 19.43 | 0.609 |
| Primary analysis (n = 291) | Complete case (n = 225) | |||||||
|---|---|---|---|---|---|---|---|---|
| P. est. | SE | p-value | Odds ratio (95% CI) | P. est. | SE | p-value | Odds ratio (95% CI) | |
| Intercept | −0.01 | 0.35 | 0.984 | 0.20 | 0.36 | 0.577 | ||
| Treatment: amitriptyline (vs. placebo) | 0.46 | 0.26 | 0.083 | 1.58 (0.94 to 2.64) | 0.55 | 0.27 | 0.046 | 1.73 (1.01 to 2.95) |
| IBS subtype (vs. IBS-M or IBS-U) | 0.870 | |||||||
| IBS-C | −0.14 | 0.39 | 0.720 | 0.87 (0.40 to 1.88) | −0.13 | 0.39 | 0.732 | 0.88 (0.41 to 1.88) |
| IBS-D | −0.06 | 0.29 | 0.839 | 0.94 (0.53 to 1.67) | −0.15 | 0.30 | 0.619 | 0.86 (0.48 to 1.55) |
| Baseline HADS-D score | −0.05 | 0.04 | 0.216 | 0.95 (0.88 to 1.03) | −0.06 | 0.04 | 0.164 | 0.94 (0.87 to 1.02) |
| Recruitment hub (vs. Wessex) | 0.231 | |||||||
| West of England | −0.14 | 0.29 | 0.635 | 0.87 (0.49 to 1.54) | −0.21 | 0.30 | 0.479 | 0.81 (0.45 to 1.46) |
| West Yorkshire | 0.31 | 0.39 | 0.433 | 1.36 (0.63 to 2.92) | 0.46 | 0.41 | 0.262 | 1.58 (0.71 to 3.52) |
Further exploratory analysis
Reduction in total irritable bowel syndrome with constipation score and item scores at 3 and 6 months
| 3 months | 6 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter estimate | Std error | p-value | Odds ratio (95% CI) | Parameter estimate | Std error | p-value | Odds ratio (95% CI) | |
| Intercept | −1.39 | 0.40 | 0.001 | −1.33 | 0.41 | 0.001 | ||
| Treatment: amitriptyline (vs. placebo) | 0.40 | 0.22 | 0.068 | 1.49 (0.97 to 2.28) | 0.39 | 0.22 | 0.068 | 1.48 (0.97 to 2.27) |
| Baseline total IBS-SSS score | 0.01 | 0.00 | < 0.001 | 1.01 (1.01 to 1.01) | 0.01 | 0.00 | < 0.001 | 1.01 (1.00 to 1.01) |
| IBS subtype (vs. IBS-M or IBS-U) | ||||||||
| IBS-C | −0.67 | 0.31 | 0.028 | 0.51 (0.28 to 0.93) | −0.56 | 0.30 | 0.059 | 0.57 (0.32 to 1.02) |
| IBS-D | 0.03 | 0.23 | 0.912 | 1.03 (0.65 to 1.63) | −0.06 | 0.23 | 0.805 | 0.94 (0.60 to 1.49) |
| Baseline HADS-D score | −0.07 | 0.03 | 0.019 | 0.93 (0.87 to 0.99) | 0.02 | 0.03 | 0.640 | 1.02 (0.95 to 1.08) |
| Recruitment hub (vs. Wessex) | ||||||||
| West of England | −0.09 | 0.23 | 0.684 | 0.91 (0.58 to 1.43) | −0.28 | 0.23 | 0.215 | 0.75 (0.48 to 1.18) |
| West Yorkshire | 0.31 | 0.32 | 0.325 | 1.37 (0.73 to 2.54) | −0.45 | 0.32 | 0.162 | 0.64 (0.34 to 1.20) |
| 3 months | 6 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter estimate | Std error | Odds ratio (95% CI) | p-value | Parameter estimate | Std error | Odds ratio (95% CI) | p-value | |
| Intercept | −0.54 | 0.37 | 0.138 | −0.63 | 0.37 | 0.089 | ||
| Treatment: amitriptyline (vs. placebo) | 0.17 | 0.20 | 1.19 (0.81 to 1.75) | 0.377 | 0.51 | 0.20 | 1.66 (1.12 to 2.46) | 0.012 |
| Baseline IBS-SSS score | 0.00 | 0.00 | 1.00 (1.00 to 1.01) | 0.006 | 0.00 | 0.00 | 1.00 (1.00 to 1.00) | 0.101 |
| IBS subtype (vs. IBS-M or IBS-U) | ||||||||
| IBS-C | 0.00 | 0.28 | 1.00 (0.58 to 1.74) | 0.992 | −0.38 | 0.29 | 0.68 (0.39 to 1.20) | 0.188 |
| IBS-D | 0.22 | 0.22 | 1.25 (0.82 to 1.90) | 0.305 | −0.07 | 0.23 | 0.93 (0.59 to 1.46) | 0.751 |
| Baseline HADS-D score | −0.08 | 0.03 | 0.93 (0.87 to 0.98) | 0.009 | −0.02 | 0.03 | 0.98 (0.93 to 1.04) | 0.559 |
| Recruitment hub (vs. Wessex) | ||||||||
| West of England | −0.36 | 0.22 | 0.70 (0.46 to 1.06) | 0.094 | −0.15 | 0.22 | 0.86 (0.56 to 1.31) | 0.482 |
| West Yorkshire | −0.03 | 0.29 | 0.97 (0.55 to 1.70) | 0.914 | 0.21 | 0.29 | 1.23 (0.70 to 2.18) | 0.472 |
| 3 months | 6 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter estimate | Std error | Odds ratio (95% CI) | p-value | Parameter estimate | Std error | Odds ratio (95% CI) | p-value | |
| Intercept | −0.83 | 0.37 | 0.024 | −0.93 | 0.39 | 0.017 | ||
| Treatment: amitriptyline (vs. placebo) | 0.21 | 0.20 | 1.23 (0.83 to 1.82) | 0.303 | 0.28 | 0.21 | 1.33 (0.89 to 1.99) | 0.171 |
| Baseline IBS-SSS score | 0.00 | 0.00 | 1.00 (1.00 to 1.00) | 0.124 | 0.00 | 0.00 | 1.00 (1.00 to 1.00) | 0.115 |
| IBS subtype (vs. IBS-M or IBS-U) | ||||||||
| IBS-C | 0.23 | 0.28 | 1.26 (0.73 to 2.17) | 0.404 | 0.01 | 0.29 | 1.01 (0.57 to 1.78) | 0.966 |
| IBS-D | −0.09 | 0.22 | 0.91 (0.60 to 1.40) | 0.677 | 0.10 | 0.22 | 1.11 (0.71 to 1.71) | 0.650 |
| Baseline HADS-D score | −0.02 | 0.03 | 0.98 (0.93 to 1.04) | 0.588 | −0.01 | 0.03 | 0.99 (0.94 to 1.05) | 0.819 |
| Recruitment hub (vs. Wessex) | ||||||||
| West of England | −0.04 | 0.22 | 0.96 (0.63 to 1.47) | 0.863 | −0.25 | 0.22 | 0.78 (0.51 to 1.21) | 0.269 |
| West Yorkshire | 0.01 | 0.28 | 1.01 (0.59 to 1.75) | 0.958 | −0.07 | 0.29 | 0.93 (0.52 to 1.65) | 0.805 |
Moderator analysis of 6-month total irritable bowel syndrome with constipation score
| Baseline | Month 6 | |||||
|---|---|---|---|---|---|---|
| Amitriptyline, mean (SD) | Placebo, mean (SD) | Total, mean (SD) | Amitriptyline, mean (SD) | Placebo, mean (SD) | Total, mean (SD) | |
| IBS subtype | ||||||
| IBS-C | 253.5 (98.36) | 298.1 (102.92) | 274.9 (102.40) | 165.3 (102.52) | 239.0 (119.84) | 199.4 (116.08) |
| IBS-D | 269.9 (86.94) | 263.4 (88.65) | 266.7 (87.60) | 163.8 (103.69) | 199.0 (110.84) | 180.9 (108.33) |
| IBS-M or IBS-U | 284.6 (89.80) | 270.3 (86.13) | 277.3 (88.01) | 178.6 (113.99) | 187.5 (113.92) | 183.1 (113.72) |
| Recruitment hub | ||||||
| West Yorkshire | 288.4 (88.29) | 290.4 (104.31) | 289.4 (96.17) | 138.5 (101.39) | 196.1 (127.84) | 166.9 (117.91) |
| Wessex | 260.6 (95.54) | 271.2 (94.51) | 265.8 (94.93) | 168.2 (104.93) | 201.9 (112.10) | 185.1 (109.58) |
| West of England | 279.9 (85.16) | 264.3 (77.66) | 272.1 (81.65) | 185.3 (111.16) | 199.9 (112.58) | 192.3 (111.73) |
FIGURE 24.
Moderating effect of recruitment hub on the total IBS-SSS score treatment effect at 6 months.

FIGURE 25.
Total IBS-SSS score by treatment arm and recruitment hub.
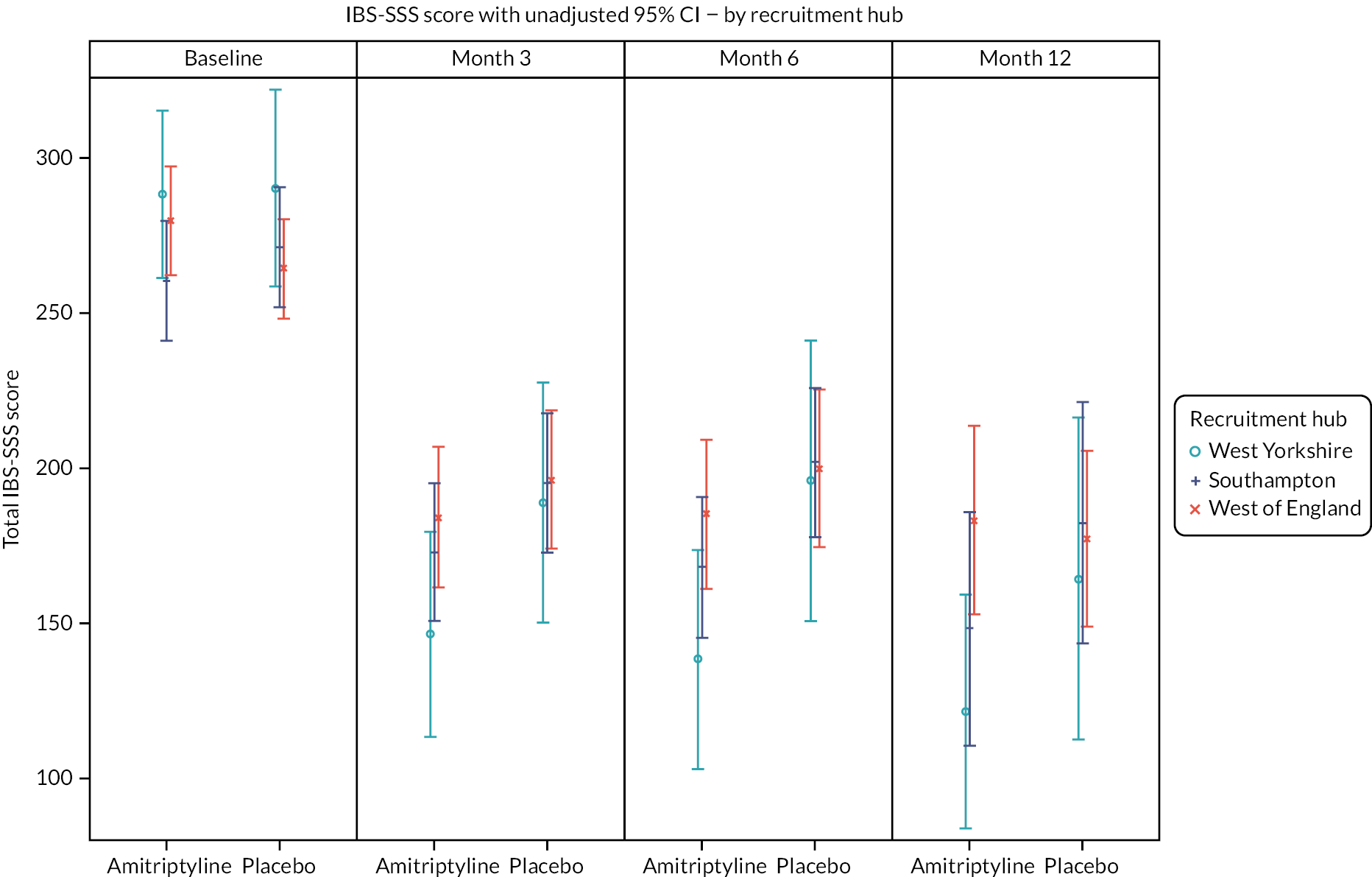
FIGURE 26.
Moderating effect of IBS subtype on the total IBS-SSS score treatment effect at 6 months.

FIGURE 27.
Total IBS-SSS score by treatment arm and IBS subtype.
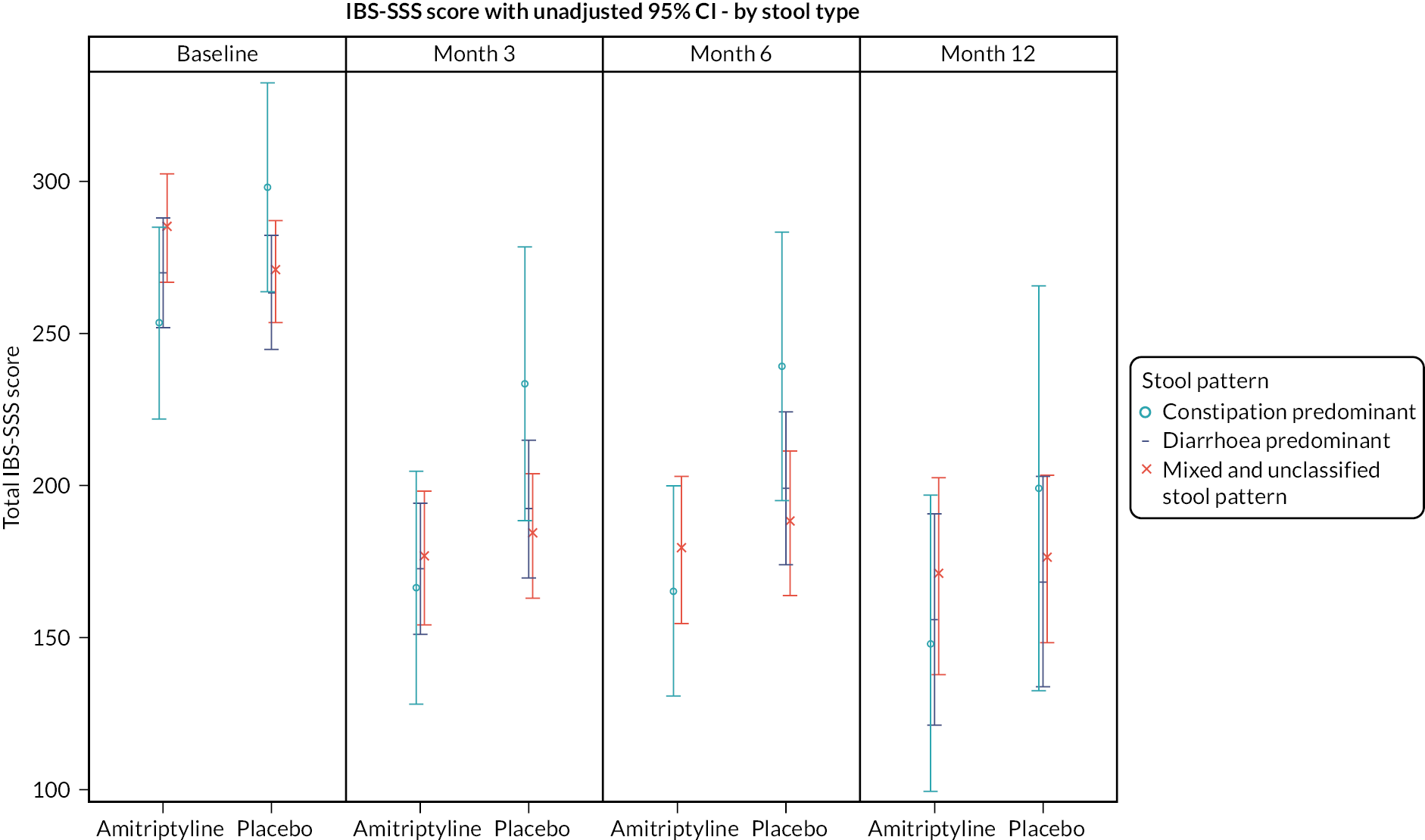
FIGURE 28.
Moderating effect of baseline IBS-SSS score on the total IBS-SSS score treatment effect at 6 months.

FIGURE 29.
Moderating effect of baseline HADS-A score on total IBS-SSS score at 6 months.
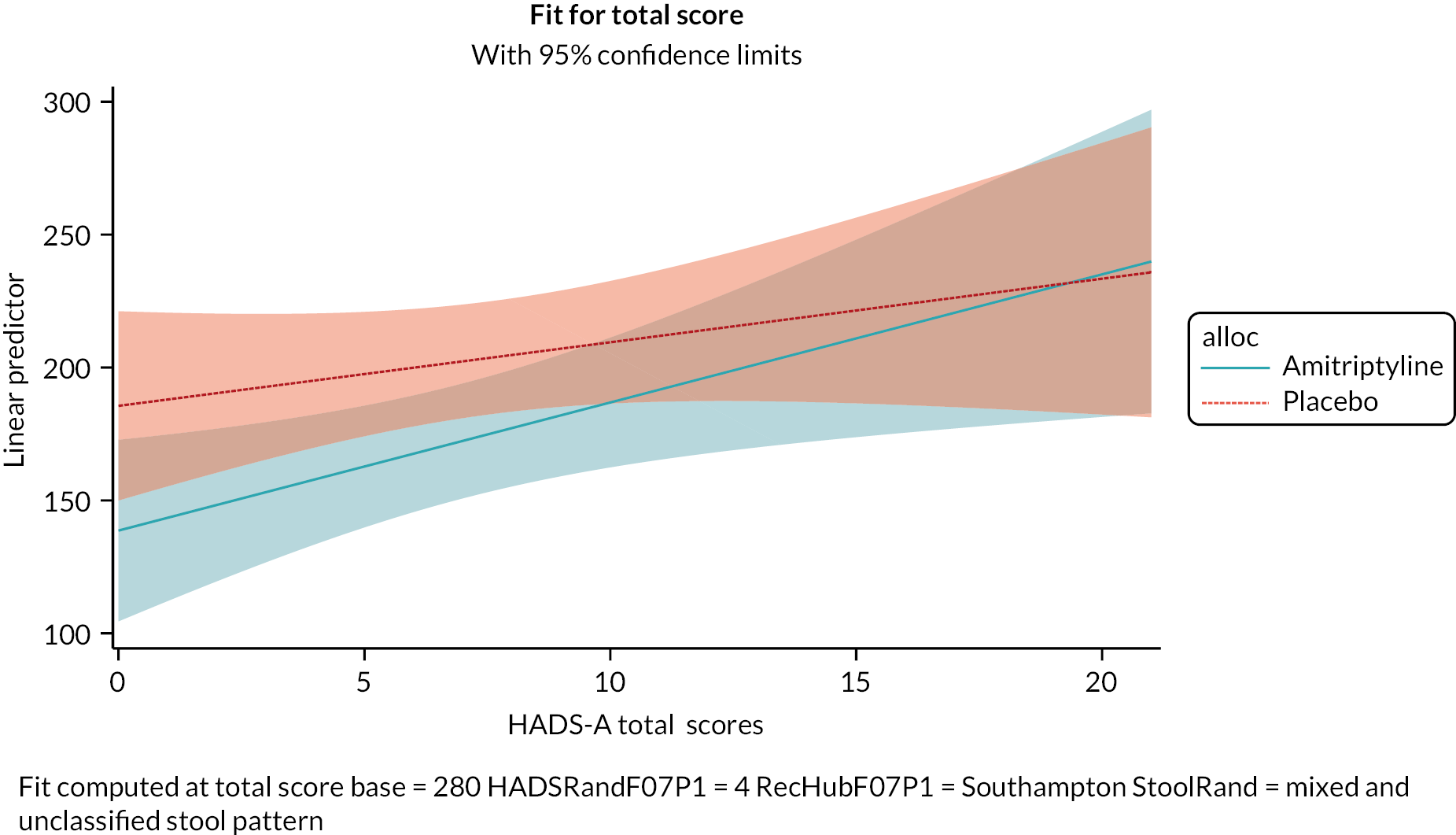
FIGURE 30.
Moderating effect of baseline HADS-D score on total IBS-SSS score at 6 months.

Moderator analysis of 6-month subjective global assessment of relief of irritable bowel syndrome symptoms
FIGURE 31.
Moderating effect of IBS subtype on SGA of relief of IBS symptoms treatment effect at 6 months.
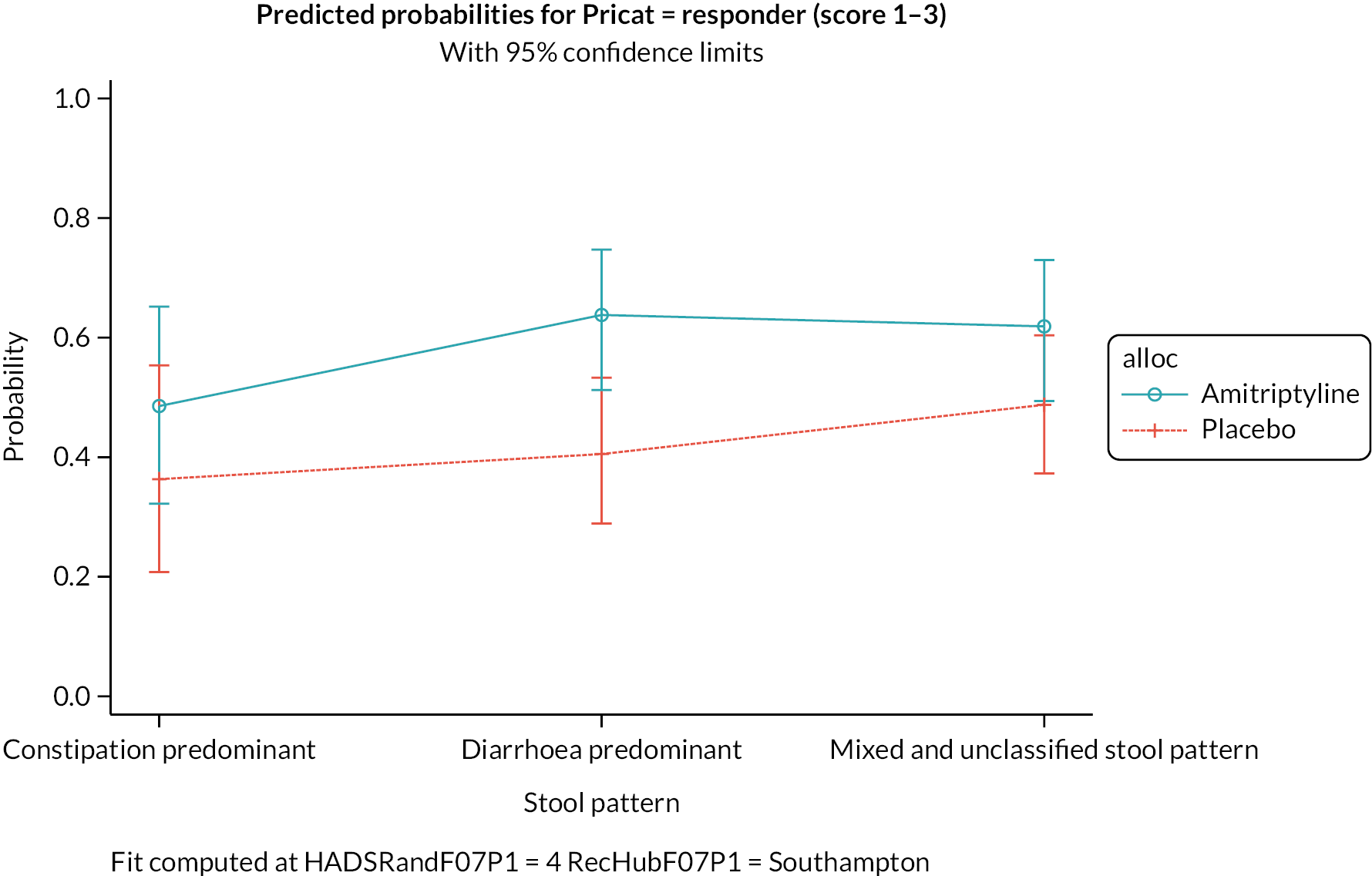
FIGURE 32.
Subjective global assessment of relief of IBS symptoms at 6 months by treatment arm and stool type.

List of abbreviations
- AE
- adverse event
- AR
- adverse reaction
- ASEC
- Antidepressant Side-Effect Checklist
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRP
- C-reactive protein
- CTRU
- Clinical Trials Research Unit
- DMEC
- Data Monitoring and Ethics Committee
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HTA
- Health Technology Assessment
- IBS
- irritable bowel syndrome
- IBS-C
- irritable bowel syndrome with constipation
- IBS-D
- irritable bowel syndrome with diarrhoea
- IBS-M
- irritable bowel syndrome with mixed bowel habits
- IBS-U
- irritable bowel syndrome unclassified
- IBS-SSS
- irritable bowel syndrome Severity Scoring System
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- OR
- odds ratio
- PHQ-12
- Patient Health Questionnaire-12
- PI
- principal investigator
- PIC
- participant identification centre
- PPI
- patient and public involvement
- QALYs
- quality-adjusted life-years
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SAR
- serious adverse reaction
- SD
- standard deviation
- SGA
- subjective global assessment
- SUSAR
- suspected unexpected serious adverse reaction
- TCA
- tricyclic antidepressant
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- tTG
- tissue transglutaminase
- WSAS
- Work and Social Adjustment Scale
- WCC
- white cell count
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/BFCR7986).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
