Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 16/167/56. The contractual start date was in June 2018. The draft report began editorial review in April 2023 and was accepted for publication in September 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Kearney et al. This work was produced by Kearney et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Kearney et al.
Chapter 1 Introduction
Background
The shoulder is the most frequently dislocated joint; dislocation occurs in 8.2–23.9 per 100,000 people per year, and 95% of these are anterior dislocations. 1 They occur when excessive forces during a traumatic event displace the humeral head frontwards, out of the shoulder socket (glenoid fossa), resulting in the joint surfaces completely losing contact. 1–3
Traumatic anterior shoulder dislocation (TASD) predominantly affects males under 25 years during high-impact incidents and females over 80 years during low-impact incidents. This is an increasing health problem in this group because of our ageing population. 1
Regardless of their age, people sustaining TASD may have ongoing pain, disability and substantial morbidity linked to high recurrence rates and subsequent need for repeated episodes of management. 1–3 Re-dislocation following a first-time traumatic event typically occurs within 12 months of the index dislocation. 4 Common reasons leading to re-dislocation include soft-tissue damage surrounding the shoulder, such as a Bankart lesion in which there is damage to the glenoid rim, and bony injuries such as Hill–Sachs lesions whereby the humeral head sustains a compression fracture during the index event. 5,6
Rehabilitation may reduce ongoing re-dislocations and restore a functional, painless and stable shoulder through early restoration of joint movement and promotion of exercises to retrain muscles to maintain stability. 2 However, prior to the start of this trial a 2014 Cochrane review did not find an evidence base to support this. 2 Dutch national guidelines explicitly state no referral to physiotherapy should be made,3 while UK guidelines state that referral ‘may be helpful’. 1 Thus, the nature and extent of physiotherapy required for the management of patients following TASD are unclear.
A typical course of six physiotherapy sessions costs around £378; a single assessment and advice session costs £63 (lead centre costs). Hence, the choice of physiotherapy package after TASD has large resource implications for the NHS. Assuming, conservatively, an incidence of 10/100,000 of first TASD, by 2020 there will be around 67,000 TASDs annually treated by the NHS.
In addition to the cost of providing physiotherapy services, there was a clear message from our patient workshop that attending a typical course of six sessions of physiotherapy is burdensome. Younger people may need to take time from work or arrange care for dependents, while older people may find travel challenging, particularly if unable to drive following the dislocation. For both groups this can be time-consuming and costly. If a single advice session were all that is required, it would have a positive impact on patient experience after TASD, lessening the burden on patients and their friends and families.
Consequently, a course of supervised, tailored physiotherapy needs to be of clear additional benefit, when compared to a single session and an advice leaflet, if it is to be implemented as standard care in the NHS. There is no clinical consensus or high-quality evidence on how best to manage TASDs. 1 With increasing numbers, because of an ageing population and the need to remain active in older age through continued participation in sporting activities, there is a need for evidence regarding the nature and extent of what physiotherapy is required for the management of patients following TASD.
Existing knowledge
Joint British Elbow and Shoulder Society (BESS) and British Orthopaedic Association (BOA) guidelines, and two Cochrane reviews, advocate non-operative management for people with a first TASD who are aged 25 years or over and suggest further research on the possible benefits of surgery in those under 25. 1,2,7 Despite non-operative care being the predominant first-line strategy, at the start of this study there was no randomised controlled trial (RCT) evidence regarding what to do once the decision not to operate has been made. 1–3 A 2014 Cochrane review on methods of non-operative management concluded that there were no published RCTs comparing rehabilitation methods after the initial 2 weeks of immobilisation. 2 The review also found no evidence of any ongoing studies. 1–3
With more people receiving first-line non-operative management, combined with the large personal and societal cost associated with this injury, the evidence gap in rehabilitation was a clear priority. Crucially, we needed to know whether resourcing an intensive physiotherapy package was clearly superior to a single advice session.
An updated review in 2019 identified one ongoing study, in addition to this study, that has since completed (n = 56). 8,9 The researchers randomised 56 participants, across three orthopaedic shoulder units in Denmark, to either a home-based exercise intervention or a supervised 12-week intervention, led and supervised by a physiotherapist. No further ongoing studies were identified in an updated search of trial registries.
Intervention development
The Acute Rehabilitation following Traumatic anterior shoulder dISlocAtioN (ARTISAN) trial intervention was developed following the Medical Research Council (MRC) guidance for developing and evaluating complex interventions. 10 Using an iterative process, based on research evidence, clinical guidelines, current practice, clinicians’ and patients’ opinions, we developed a rehabilitation intervention following a TASD. The intervention had four phases: (1) education, (2) range of movement exercises, (3) strengthening exercises and (4) returning to sports. We developed an intervention manual for physiotherapists, and patient materials consisting of paper booklets and web-based materials.
Our aim was to ensure that the single-session ARTISAN intervention was scientifically grounded, acceptable to patients and clinicians, and deliverable in the UK NHS setting.
Clinical guidelines
In the UK, the BESS and the BOA published joint guidelines advocating conservative management for TASD for those aged 25 years and over, alongside early referral to physiotherapy. 1 They did not make any recommendations regarding the content of rehabilitation. Outside of the UK, only one further set of national guidelines was identified. Dutch Orthopaedic Association guidelines state that physiotherapy is not recommended after a TASD. 3
Literature review of best practice prior to trial
We obtained full papers included in a second Cochrane review entitled ‘Surgical versus non-surgical treatment for acute anterior shoulder dislocation’. 7 The aim was to collate and summarise rehabilitation protocols following conservatively managed TASD from these RCTs. However, rehabilitation protocols were either absent from the research papers or not sufficiently detailed to replicate.
Within the limited literature identified, there was a consensus on a phased approach to rehabilitation based on the underlying mechanism of injury and recovery timescales: beginning with simple range of movement exercises and progressing to strengthening exercises that are manipulated to be easier or more challenging by altering load, frequency and repetitions.
Consultation and national survey of practice
A synthesis of clinical guidelines and current evidence was used as a basis for consultation exercises at five physiotherapy departments. The findings from these were used to inform a national survey, administered to 43 NHS sites which had expressed an interest in taking part in the ARTISAN RCT, to establish (1) what protocols are in use across the UK and (2) current care pathways. Of the 43 responders, 7 used locally developed physiotherapy protocols. Sites were consistent with an educational component and phased exercise approach for rehabilitation.
Patient and public involvement
We presented the intervention to a patient group who framed the intervention around their experiences and expectations of physiotherapy after TASD. The patient and public involvement (PPI) group discussed that although the content was relevant, it lacked information to help them understand their injury more fully and aid with adherence to the programme, which the group all agreed was difficult at times.
Subsequently, the intervention was further refined to include behavioural components to facilitate self-management and aid with adherence. This included additional information to improve understanding of the injury and expected length of recovery, goal-setting and an exercise log. Following these refinements, the intervention was presented back to both our patient group and clinicians for final feedback prior to clinical implementation.
Research objectives
Our primary objective was to quantify and draw inferences about observed differences in Oxford Shoulder Instability Score (OSIS) between the trial treatment groups 6 months post randomisation, for adults with first-time TASD managed non-operatively. Our secondary objectives were:
-
To estimate comparative cost-effectiveness [cost/quality-adjusted life-year (QALY)] of the two trial treatments, from an NHS and personal social services (PSS) perspective.
-
To determine the complication rate (i.e. shoulder re-dislocation) in the first 12 months between the trial treatment groups.
-
To quantify and draw inferences between the functional status (OSIS) of the trial treatment groups at 6 weeks and at 3 and 12 months.
-
To quantify and draw inferences on observed differences in the functional status (QuickDASH) between the trial treatment groups at 6 weeks and at 3, 6 and 12 months.
-
To quantify and draw inferences on observed differences of health-related quality of life [EuroQol-5 Dimensions, five-level version (EQ-5D-5L)] between the trial treatment groups at 6 weeks and at 3, 6 and 12 months.
-
To qualitatively explore participants’ experience of receiving the trial treatments and facilitators and obstacles to adhering to them.
Chapter 2 Trial methods
Summary of trial design
ARTISAN was a multicentre, randomised, pragmatic trial. People presenting at 41 UK hospitals in the NHS with a TASD for non-surgical management were randomised 1 : 1 to receive advice or advice and a programme of physiotherapy (see Appendix 1).
Ethics, registration and oversight
The National Research Ethics Committee approved this study on 26 July 2018 (18/WA/0236), with each trial site granting individual NHS Trust approval prior to recruitment at each site. The ARTISAN protocol was accepted for publication on 13 October 2020 and first published on 19 November 2020. 11
The trial was conducted in accordance with the principles of the Declaration of Helsinki and the MRC good clinical practice guidelines as well as all applicable UK legislation and University of Warwick standard operating procedures (SOPs). Trial oversight was provided by a Trial Management Group (TMG) and had independent oversight from a Data Monitoring Committee (DMC) and Trial Steering Committee (TSC).
Settings and locations
There were 41 trauma research teams at UK NHS Trust sites who screened adults with a first-time TASD confirmed radiologically, being managed non-operatively.
Participants
Participant screening
Adults with a primary (first-time) TASD were screened against the eligibility criteria to take part in the trial. Broad eligibility criteria ensured that the results of the study could readily be generalised to the wider patient population.
Inclusion criteria
-
Provision of written informed consent.
-
Aged 18 years or over.
-
They have a primary (as reported by the potential participant) traumatic acute shoulder dislocation, confirmed radiologically.
Exclusion criteria
-
Bilateral shoulder dislocation at time of injury.
-
Having first-line surgical treatment (indications include a displaced greater tuberosity fracture, for example).
-
Cannot receive first session of physiotherapy within 6 weeks of injury.
-
In the opinion of the assessing clinician there is a significant neurovascular complication associated with TASD (e.g. brachial plexus injury).
-
Unable to adhere to trial procedures or complete questionnaires (e.g. a history of permanent cognitive impairment).
-
Previous randomisation in the present trial.
If a trial participant were to sustain a contralateral TASD during the trial period, the second TASD would not be included in the study because the outcomes of this intervention would not be independent from the first intervention.
All potential participants meeting the entry criteria were checked for eligibility and entered on the monthly screening log. Potential participants who were willing to be approached by a suitably trained member of the research team were provided with verbal and written information about the study. They were then asked if they wished to take part in the study. All new non-operatively managed potential participants had a maximum of 6 weeks from date of injury to make a final decision and be randomised. If a patient was eligible and consenting, a member of the local research team completed the informed consent process, enrolment, baseline and pre-injury data collection.
Participants were placed on the waiting list for physiotherapy, with a typical wait of up to 2 weeks. The eligibility was reconfirmed by the treating physiotherapist at the first appointment, and potential participants were excluded at this stage if there had been a change in status. This allowed someone who had consented to the study to be deemed not eligible at the point of randomisation and to be excluded, using the predefined exclusion criteria.
Informed consent
Written informed consent was obtained by a suitably trained member of the research team at each site as per the delegation log, after allowing sufficient time for the potential participant to consider their decision and ask questions about the trial. If participants were identified through a virtual fracture clinic setting or had left the face-to-face clinical setting before being approached, verbal consent was gained in the first instance by telephone communication, and the participant was then posted a paper informed consent document to be completed and handed to the research team prior to randomisation.
The principal investigator (PI) or co-PIs (an orthopaedic consultant and/or physiotherapy lead) retained overall responsibility for informed consent at their site and ensured that any person delegated responsibility to participate in the informed consent process was duly authorised, trained, qualified and competent.
As there is a delay of a number of weeks between consent and randomisation (due to the waiting list for physiotherapy), people who had entered the study had the option to withdraw before treatment started if for any reason they changed their mind.
The participants remained free to withdraw at any time without giving reasons and without prejudice to any further treatment and were provided with a contact point where they could obtain further information about the trial if required.
Randomisation
Pre-randomisation eligibility checks were carried out to ensure that potential participants met the eligibility criteria. Written informed consent for entry into the trial and baseline assessment were obtained prior to randomisation. Participants were randomised once they had been registered as eligible for randomisation on the web-based system and completed their physiotherapy advice session. Allocation concealment was maintained by an independent randomisation team who were responsible for generation of the sequence and had no role in the allocation of participants.
The treatment group were allocated by computer using a minimisation algorithm with a random element and stratification by participant age (≤ 39 years old or ≥ 40 years old), hand dominance and treating centre. The physiotherapist, following delivery of the control intervention, randomised all trial participants. Physiotherapists were only able to obtain the randomisation code after verifying that the initial control advice session had been complete. Because participants were only made aware of their group allocation after attendance at the control treatment, the impact of resentful demoralisation was minimised to avoid participants failing to engage with the control intervention.
Minimisation was a better option than stratification with variable block sizes, due to the relatively small number of participants expected in some strata. The randomisation service was available 24 hours a day, 7 days a week to facilitate the inclusion of all eligible participants. A confirmation e-mail was automatically generated to the research site containing the randomisation details.
In an open trial of this nature, it was not possible to control for the effects of demoralisation bias on engagement with the interventions. Failure to engage with an intervention is part of the reality of clinical practice regardless of the trial being conducted, meaning that the effect size observed is likely to reflect real-world effectiveness. We were cognisant of the risk of differential loss to follow-up between the two groups. We monitored this closely.
Post-randomisation withdrawals
Unless a participant explicitly withdrew consent, data were collected until trial end. For those withdrawing consent for follow-up procedures, trial data obtained up until the point of withdrawal were included. Participants could withdraw from follow-up but continue to provide routine NHS data for the purposes of the trial.
Participants who withdrew were not replaced in the trial, and a corresponding withdrawal case report form (CRF) was completed. Participants could be withdrawn from treatment and, if necessary, the trial at the discretion of the investigator and/or TSC due to safety concerns.
Participants could also be withdrawn post randomisation by the TMG if participants were found during routine site quality assurance checks not to have had ‘radiological confirmation’ of the primary traumatic dislocation on checking source data. In these cases, participants were withdrawn from the randomised total and replaced, but were still followed up and analysed as part of a preplanned sensitivity analysis.
Trial interventions
Details of the intervention development were first published in December 2021. 12 The written trial materials can be found in Appendices 2 and 3. The website reflected the same material content but in a series of short animations.
Control
All participants had an initial period where the injured arm was supported in a sling, and then received an appointment for a physiotherapy advice session within 6 weeks of their injury. At this first encounter, consenting participants were provided with a web link to phase 1 of the advice materials and provided with a paper-based booklet version of the same content. 12 [Reproduced with permission from Liew et al. 12 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.]
-
Phase 1 covered the below information:
-
What has happened to me?
-
What can go wrong?
-
How do I stop this happening again?
-
How long do I have to wear my sling?
-
Should I move my arm?
-
How do I control my pain?
-
When can I return to usual activities?
-
What if something goes wrong?
-
The expectation was that all participants would receive an appointment for physiotherapy within 2 weeks of injury (i.e. the time at which the immobilisation would be expected to be removed). However, to reflect that some clinicians/sites may recommend immobilisation to be worn for greater or lesser time, and to offer achievable time frames for all physiotherapy services, an upper time limit of 6 weeks from date of injury was accepted; 6 weeks was chosen as the upper period limit to reflect the time point at which a soft-tissue injury is no longer considered acute.
All participants received a single session of advice to aid self-management. This lasted up to 1 hour and was administered by an ARTISAN-trained physiotherapist. Following routine assessment, the physiotherapist delivered a core set of phase 2 intervention components that included education and discussion reiterating points a–h above and on the following:
-
points of contact if complications occur or expected recovery times are not achieved
-
a core set of progressive phase 2 range of movement exercises and what they aim to achieve
-
enhancing self-management behaviours through the addition of goal-setting, exercise planning and diaries.
The outlined participant information (a–i) aimed to improve understanding of the condition and its management, to counter any participant misconceptions. Points j and k aimed to agree with the participant an exercise (or other) goal (e.g. repetition, duration, frequency) and to prompt them to think of possible factors (obstacles and facilitators) influencing the behaviour (e.g. controlling the pain) and come up with strategies to overcome them. They were also prompted to make detailed planning of performance of the behaviour or behaviours (e.g. exercise or pain management) to include at least one of context, frequency, duration or intensity; for example, they were encouraged to complete one set of exercises every day after work and as soon as they returned home.
The physiotherapist provided details of web-based materials, which included all the core components above in written and video format, and included a dedicated area for participants to set goals and keep diaries. The physiotherapist also discussed with the participant that the website resources also contained progression to a core set of progressive phase 3 strengthening exercises and what they aimed to achieve, and later-stage information in phase 4 on how to return to sports. Participants were offered paper-based alternatives. Offering different formats (e.g. written and digital resources) enhanced adherence, as it adapts to a variety of individual needs. Table 1 summarises the advice materials received by all participants.
| Component | Method of administration | When |
|---|---|---|
| Phase 1 advice | Website and booklet | Consent |
| Phase 1 advice | Verbally by physiotherapist | At first physiotherapy appointment |
| Phase 2 range of movement exercises | Verbally by physiotherapist, website and booklet | At first physiotherapy appointment |
| Goal-setting and exercise diaries | Verbally by physiotherapist, website and booklet | At first physiotherapy appointment |
| Phase 3 strengthening exercises | Website and booklet | Following first physiotherapy appointment at home |
| Phase 4 return to sport advice | Website and booklet | Following first physiotherapy appointment at home |
Following completion of the advice appointment, the participant was randomised, allocating them to this advice session alone or to this advice session plus the offer of additional physiotherapy.
Participants randomised to advice only were provided with a contact point to self-refer back to the clinical team if recovery did not occur. Participants who self-referred back to the clinical team were considered to be per protocol.
Table 2 summarises the key components of the ARTISAN intervention according to the Template for Intervention Description and Replication (TIDieR) criteria, as described in the published intervention development paper. 12
| TIDieR criteria | Description | |
|---|---|---|
| Brief name | ARTISAN | |
| Why | Referral to a course of physiotherapy is a common conservative management for TASD. However, the evidence is lacking and there are conflicting clinical guidelines. | |
| What | The ARTISAN intervention comprises a standardised, single session up to an hour long with self-management materials. All participants in the study receive this session. | |
| Materials: participant |
Website with animated videos covering contents based on the phase 1–4 booklets. Also contains an online goal-setting page and exercise log. Website is password protected and participants can obtain the password from the booklets |
|
| Materials: physiotherapist |
Training: face-to-face training of the ARTISAN intervention conducted by the ARTISAN-trained physiotherapist. Sessions are up to 2 hours long. Therapist manual: detailing all components of the study and the study intervention. Also contains a list of coded exercises as a reference for the online additional physiotherapy form. Post-injury questionnaire: contains the inclusion/exclusion criteria revalidation checklist, OSIS, QuickDASH, EQ-5D-5L, randomisation form and quality assurance check form. |
|
| Procedure: single physiotherapy session (ARTISAN intervention) | At the single physiotherapy appointment, the physiotherapists will:
|
|
Topics included are:
|
||
|
||
| Procedure: group allocation | ARTISAN session only: Participants receive the single physiotherapy (ARTISAN) session. Discharge from physiotherapy. Participants can contact their GP/orthopaedic team if recovery is not as expected. |
ARTISAN session with follow-up: Participants receive the initial physiotherapy session and follow-up physiotherapy sessions within 4 months post randomisation. The frequency, duration and content of the follow-up sessions are based on the discretion of the treating physiotherapist, as per normal physiotherapy follow-up sessions. The physiotherapist records the contents of each follow-up session in the additional physiotherapy online form. |
| Who provides | Physiotherapist working within an existing NHS musculoskeletal service in the UK ARTISAN does not exclude any physiotherapist based on the number of years qualified or experience in treating shoulder conditions. |
|
| How | Face-to-face, virtual or telephone-delivered session | |
| Where | The ARTISAN session is delivered in a UK NHS physiotherapy outpatient setting. For physiotherapists who work as part of the orthopaedic team, the session is delivered within a UK NHS orthopaedic clinic setting. | |
| When and how much | The initial physiotherapy session is delivered within 6 weeks post injury. The session is up to 1 hour long. | |
| Tailoring | To standardise the sessions across all recruiting sites, all physiotherapists deliver the same set of advice, exercises and their progressions. Physiotherapists can tailor the progression of exercises based on participants’ ability during the initial appointment. The repetitions for each exercise and goals set are tailored based on the participant’s ability at initial appointment. | |
| Modification | Minor language and image clarifications to patient-facing booklets were made prior to the main phase. | |
| Intervention fidelity | Monitored centrally via the quality assurance check form and the quality assurance checks conducted by a member of the research team, external to the site research team. If sites are found to deviate from the standards required by the protocol further training, either face-to-face or through the phone, is arranged by the study team. | |
Intervention
The additional course of physiotherapy consisted of the offer of at least one additional physiotherapy session after the pre-randomisation session. Each additional session lasted for up to 30 minutes, over a maximum duration of 4 months from date of randomisation; no upper limit on the number of sessions was set. It was tailored, supervised and taught incorporating common methods to increase adherence by a physiotherapist trained by the trial team or local lead trial therapist. The course of physiotherapy involved teaching and supervising the ‘core set’ of progressive exercises offered to the control arm and published on the web-based resources, in addition to being able to tailor through offering additional exercise components from a trial manual menu which provided a range of exercises at differing levels which the physiotherapist could then choose and set specified frequency, loads and number of repetitions at their discretion.
Monitoring intervention delivery and compliance
Following site set-up, the trial team implemented mechanisms to ensure treatment fidelity. This was based on a standardised approach of evaluating fidelity. 13
-
Direct observations: with additional permissions, a member of the trial team observed trial-related procedures and the delivery of the two intervention arms (permission was sought from the trial participants to observe treatment sessions). An adherence evaluation form consisting of items that reflect the occurrence or non-occurrence of an event formed the basis of the assessment.
-
Audio recordings: with additional permissions, and in addition to the adherence form, the interactions between the therapist and trial participant were recorded during the above observation (additional permission was sought from trial participants to record treatment sessions). This was used to assess success or failure of the therapist to introduce the aims/rationale of each component and consolidate participant learning at the end of each component. Assessments were given as ‘yes/demonstrated’, ‘no/not demonstrated’ or ‘unsure’.
-
Therapist self-report: the adherence evaluation form was also self-reported by the site therapist. CRFs were collected on intervention delivery including number of treatment sessions attended, materials provided and exercise components prescribed. This was completed for every trial participant.
Points (1) and (2) were evaluated twice annually for the duration of recruitment and intervention delivery. Any issues identified were discussed on a case-by-case basis by the TMG, who were responsible for recommending appropriate action. If issues with individual sites were not resolved following the recommendations, they were escalated to the TSC.
Changes to the intervention
Recruitment and trial delivery took place prior to and during the COVID pandemic. Prior to the pandemic the control and intervention were delivered face to face. During the pandemic some NHS Trusts switched to virtual delivery of physiotherapy services. No protocol amendment was required to accommodate this change in service delivery.
Outcome measures
Primary
Oxford Shoulder Instability Score
The OSIS is a self-completed outcome measure containing 12 questions (0–4 points each), with possible scores from 0 (worst function) to 48 (best function). 14,15 These questions relate to activities of daily living particularly relevant to patients exhibiting shoulder instability. The OSIS has been specifically designed to assess outcome of therapy (both surgical and non-surgical) by measuring activities of daily living and pain of patients exhibiting shoulder instability. The development of the score demonstrated reproducibility and internal consistency and was shown to correlate well with existing related clinical and generic patient-reported outcome measures (PROMs).
Secondary
QuickDASH
The QuickDASH is a self-completed shortened version of the Disabilities of the Arm, Shoulder and Hand (DASH) questionnaire. Instead of 30 items, the QuickDASH uses 11 items to measure physical function and symptoms in people with any or multiple musculoskeletal disorders of the upper limb. The questionnaire was designed to help describe the disability experienced by people with upper-limb disorders and also to monitor changes in symptoms and function over time. 16
EQ-5D-5L
The EQ-5D-5L is a well-validated, generic health-related quality-of-life measure consisting of five dimensions each with five levels of response. Each combination of answers can be converted into a health utility score. It has good test–retest reliability, is simple for participants to use, and gives a single preference-based index value for health status that can be used for broader cost-effectiveness comparative purposes. 17
Complications
Serious adverse events (SAEs) were reported through the following mechanisms: (1) participant reported during routine collection of follow-up data; (2) local research teams reported any additional investigations or treatment of participants; (3) local physiotherapists delivering the trial interventions reported any events occurring during treatment sessions; and (4) medical records of non-responding participants were retrieved by local research teams at site.
Serious adverse events not related to the intervention or TASD event were recorded on the SAE form but were not formally analysed or reported. SAEs that were predefined complications directly related to the trial interventions or directly caused by the primary TASD event were recorded as complications and not reported.
Resource use questionnaires
The primary health economic analysis concentrated on direct intervention and healthcare/PSS costs, while wider impact (societal) costs were included within the sensitivity analyses. Participants completed resource use questionnaires at baseline and all follow-up points, to collect resource use data associated with the interventions under examination.
Qualitative interviews
One of the secondary objectives for ARTISAN was to qualitatively explore the participant experiences of receiving the trial treatments, and facilitators and obstacles to adhering to them.
At a point soon after the return of the 12-month follow-up questionnaire, a purposive sample informed by treatment allocation, gender, age and outcome of up to 50 participants was invited for a one-off face-to-face interview [by telephone or via Microsoft Teams® (Microsoft Corporation, Redmond, WA, USA)]. The aim of the interviews was to explore the participant experience of receiving the trial treatments, and facilitators and obstacles to adhering to them.
Follow-up
Core outcomes were completed over the telephone or by e-mail if postal copies were not returned. Text messages were also sent to participants; text messages were only sent to participants who gave prior consent.
Multiple contact details were recorded, such as addresses and telephone numbers, mobile telephone numbers and e-mail addresses, and contact details of next of kin, to prevent loss to follow-up. This information was held separately from the trial data, on a password-protected database, to uphold anonymisation, in line with current regulations. If the participant was lost to follow-up at a certain time point, reasonable efforts (e.g. phone calls, mobile text messaging, post) were used to acquire outcome data at each time point.
Changes to trial outcomes
Outcome collection took place prior to and during the COVID pandemic. Prior to the pandemic, postal data collection was the primary method of data collection. During the pandemic, due to the mandate to work from home and not leave the house, the primary method of data collection became telephone. Once the national restrictions were lifted, the team reverted back to postal mechanisms as the primary collection method. A protocol amendment was made to clarify the different methods of acceptable data collection.
Due to the national instruction to close non-COVID research to recruitment for a period of 3 months in 2020, there was delay to achieving recruitment milestones. A request was submitted to the funder and it was agreed that the date for the end of the trial would be extended to 30 November 2022, the impact being that all patients randomised after 1 August 2021 were only consented for 6-month follow-up and not 12 months. This allowed the primary research question to be answered, while minimising cost to the funder.
Adverse events and serious adverse events
Adverse events
An adverse event (AE) was defined as any untoward medical occurrence in a participant which did not necessarily have a causal relationship with this treatment/intervention. Foreseeable AEs related to the management of TASD occurring as a result of the trial intervention(s) were not recorded as part of the trial because advice and physiotherapy are part of normal clinical practice, with a good safety profile. Examples of such AEs include pain and reduced shoulder movement.
Serious adverse events
An AE was considered a SAE if it was an untoward medical occurrence that fulfilled one or more of the following criteria:
-
resulted in death
-
was immediately life-threatening
-
required hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability or incapacity
-
was a congenital abnormality or birth defect
-
was an important medical condition.
In the context of this protocol, ‘hospitalisation’ referred to any hospital event including day surgery and single A&E attendances. SAEs that may be expected as part of the interventions were predefined and recorded on the participant’s CRF for routine return to the ARTISAN central office and reported to the relevant oversight committees, but not recorded on a SAE form; they were instead defined as a complication. AEs/SAEs that were expected as part of the TASD and defined as a complication were: damage to nerves or blood vessels, fractures, re-dislocation, torn ligaments or muscles, persistent exacerbation of shoulder pain, restriction of range of movement, adhesive capsulitis (frozen shoulder) and persistent instability.
All participants experiencing non-predefined SAEs related to the intervention or TASD injury were entered onto the appropriate reporting form and reported to Warwick Clinical Trials Unit (WCTU) using a dedicated ARTISAN and quality assurance resource account within 24 hours of the investigator becoming aware of them.
Reporting serious adverse events
Serious adverse events and the associated management of them that may be expected as part of the interventions, and which were predefined, were recorded on the participant’s CRF only for routine return to the ARTISAN central office and reported to the relevant oversight committees. SAEs were entered onto a SAE form and once received, causality and expectedness were confirmed by either the PI or Chief Investigator.
Serious adverse events that were deemed to be unexpected and possibly, probably or definitely related to the trial interventions were notified to the Research Ethics Committee (REC) within 15 days. All such events were reported to the TMG at their next meeting. All SAEs that occurred between the date of randomisation and the end of 12-month follow-up for the participant were reported. For each SAE the following information was collected:
-
details of event that occurred from participant CRF or direct from site.
If the event was identified on the participant CRF, this resulted in the site being contacted to collect:
-
full details in medical terms and case description
-
event duration (start and end dates, if applicable)
-
action taken
-
outcome
-
seriousness criteria
-
causality (i.e. relatedness to intervention), in the opinion of the investigator
-
whether the event would be considered expected or unexpected.
If the event was identified by site, this resulted in the site contacting WCTU with the above details via a SAE form.
Any change of condition or other follow-up information was communicated to the sponsor as soon as it was available. Events were followed up until the event had resolved or a final outcome was reached. The research team liaised with the investigator to compile all the necessary information. The trial co-ordinating centre was responsible for reporting any related and unexpected SAEs to the sponsor and REC within required timelines.
Blinding
Following randomisation it was not possible to blind participants or treating clinicians to treatment allocation. However, both the treating clinician and the participant were blind to treatment allocation during the initial advice session. All staff involved in follow-up data collection were blind to treatment allocation; any unblinding was reported to the TMG. The central research team members were blinded until after data analysis was complete, with the exception of the trial statisticians, who had access to treatment assignment for the purposes of data monitoring and safety, and data-entry personnel who entered data from questionnaires, including some details of treatments received.
Statistical methods
All data have been analysed and reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines. 18,19 A detailed statistical analysis plan and a data-sharing plan were agreed with the DMC prior to any formal analyses being conducted. These are publicly available. All statistical analyses were carried out using R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Power and sample size
The OSIS is the only PROM recommended by UK BESS/BOA guidelines and was used by the most recent Cochrane review,1,2 so was chosen as the primary outcome for the study. The standard deviation (SD) of the OSIS 6 months after injury is around 10 points;20,21 however, the literature has predominantly included a younger population. Given that we planned to recruit a wider range of ages, the SD for this study might be expected to be larger. We estimated a required sample size with two-sided significance set at 5% for various scenarios of difference, power and SD (Table 3). The bolded figure of 191 participants per treatment arm represented the most likely scenario, based on a SD of 12, and our current knowledge when beginning the study, for 90% power to detect the selected, worthwhile, difference. This corresponds to a small standardised mean difference of 0.3. This represents a conservative evaluation of the sample size required based on the above literature.
| Difference | 80% power | 90% power | |||||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 3 | 4 | 5 | ||
| SD | 10 | 176 | 100 | 64 | 235 | 133 | 86 |
| 12 | 253 | 143 | 92 | 338 | 191 | 123 | |
| 14 | 343 | 194 | 125 | 459 | 259 | 166 | |
Allowing a SD of 12 and for 20% loss during follow-up, this gives a figure of 478 participants in total. Therefore, 239 participants randomised to each group would provide 90% power to detect a difference of 4 points (corresponding to a small standardised mean difference of 0.3) in OSIS at 6 months at the 5% level. 15,20
To address the possibility that therapist effects might adversely affect our statistical power, we planned an interim analysis to estimate the intracluster correlation coefficients (ICCs) for the pooled study data when we had 3-month data available on around 200 participants. We chose to use the earlier follow-up point because if there were therapist effect present, we would expect it would be maximal soon after the end of the treatment phase and to attenuate over longer-term follow-up, allowing us to conduct the analysis earlier. As the analysis used pooled data, adjustments to control the type I error were not needed. 22
We modelled the ICC using the same mixed-effects model; we used the fixed effects planned for the primary analysis, with the term for treatment allocation removed and the physiotherapist conducting the initial physiotherapy session and subsequent randomisation added as a random effect. The 95% confidence interval (CI) of the calculated ICC was then estimated using bootstrap methods. 23
This adaptive design had the additional advantage that we had actual data on the SD of our primary outcome at 3 months, which allowed us to further refine our sample size estimate.
Summary of baseline data and flow of participants
Baseline data were summarised to check comparability between treatment arms, and screening data checked to highlight any characteristic differences between those individuals in the study, those ineligible and those eligible but withholding consent. Standard statistical summaries were constructed for the primary outcome measure (i.e. OSIS) and all secondary outcome measures.
Primary outcome analysis
The main analysis investigated differences in the primary outcome measure (OSIS), 6 months after randomisation, between the two treatment groups. Unadjusted and adjusted regression analyses were used to estimate the between-group difference. The adjusted analyses accounted for the stratification variables (intervention, age group, dominant arm injured) and the baseline score. More specifically, adjusted mixed-effects modelling was used where the recruiting centre was included as a random effect to allow for possible heterogeneity in participant outcomes due to the recruiting centre. This adjusted model was predefined as the primary efficacy analysis of the study.
Secondary outcome analyses
Descriptive statistics of each PROM data set (i.e. QuickDASH and EQ-5D-5L) at each time point were constructed, and between-group analyses were performed following the method set out for the primary analysis. The patterns of recovery were also explored.
The secondary analyses carried out chi-squared tests to compare the number of dislocations and other complications between allocation groups. It was specified in the statistical analysis plans that Kaplan–Meier curves would be constructed for important complications; however, as there were only a few complications, this was omitted. The secondary outcomes were also modelled at each time point. Temporal effects on the intervention effects were also investigated using a multilevel model of all follow-up data. A mixed-effects regression model was fitted with an interaction term (treatment allocation group and time point), controlling for age group and dominant arm injured. This was analysed and reported for each of the primary and secondary outcomes.
Subgroup and exploratory analyses
Two pre-specified subgroup analyses were undertaken to measure whether there was any difference in intervention effects for hand dominance (injured shoulder in dominant arm vs. injured shoulder in non-dominant arm) and age group (younger participants vs. older participants). The analyses followed the methods described for the primary analyses, with additional interaction terms incorporated into the mixed-effects regression model.
The age group distribution was also analysed to define the best cut-off point between younger and older participants. We did this by fitting a Gaussian mixture model with a fixed support size of two, using an expectations maximisation algorithm. 24 We predefined the cut point to be when the probability of membership on either distribution was 0.5. If this cut point was found to be more than 10 years different to our initial age cut point (40 years), we would use the new boundary as a sensitivity analysis.
Furthermore, we suspected the events of the COVID epidemic may have influenced the study. Therefore, we conducted exploratory analyses to investigate the effects this had on the trial follow-up rates as well as participants’ anxiety levels pre and post the date when the UK Government enforced lockdown (20 March 2020).
Criteria for the premature termination of the trial
The incidence of complications, AEs and SAEs in each group was also analysed for the interim analyses and presented to the DMC. If there were concerns regarding the patient-reported incidences of complications, the DMC could decide that further investigations needed to be made. The trial team contacted study recruiting centres to obtain confirmed complications, which were reported in the primary analyses.
Participant population
The primary analysis and secondary analyses were conducted on an intention-to-treat basis on the randomised population. That is, they included any participant randomised into the study, regardless of whether they received study intervention and regardless of protocol deviations, unless specified above.
Procedures to account for missing data
Every effort was made to ensure compliance and return of questionnaires. The impact of COVID created new challenges to obtain participant data; however, steps were taken to minimise the loss of participants’ follow-up questionnaires. Also, due to the population demographics, there were a number of participants lost to follow-up. The missingness and crossovers were carefully monitored and reported. As a result, multiple imputation was used to account for the missing data. The multiple imputation by chained equations (MICE) method using predictive-mean-matching technique was used to impute missing outcomes, with variables chosen in conjunction with the health economic analysis (see below). The baseline scores of the primary outcome (post-injury OSIS scores), and the randomisation strata (dominant arm injured, age group), were used as predictor variables for the missing values. Twenty-five imputed data sets were generated and pooled using Rubin’s rules and then a fixed-effects linear regression model was fitted, adjusting for treatment allocation, age group, dominant arm injured and baseline OSIS score, with site fitted as a fixed effect.
Health economic evaluation
Overview
The health economic objective was to assess the comparative cost-effectiveness of ARTISAN compared to ARTISAN Plus in the management of patients presenting with TASD. Resources associated with each trial arm were collected alongside information on quality of life at 6 weeks, 3 months, 6 months and 12 months. Quality-of-life information was collected using the EQ-5D-5L at baseline, 6 weeks, 3 months, 6 months and 12 months. The primary analysis adopted a NHS and PSS perspective in line with the recommendations from the National Institute of Health and Care Excellence (NICE). 25 A societal perspective was used for the sensitivity analysis, and this included private costs incurred by patients because of the interventions and loss of earnings due to work absences. Outcomes were analysed using cost–utility analysis and expressed in terms of incremental costs per QALY gained. The time horizon covered the period from randomisation to 12 months post randomisation. Cost and outcomes were not discounted due to the 12-month time horizon.
Resource use
Data on health and social care services were recorded when used during the study time horizon. Societal costs included private medical costs and productivity losses due to injury. All costs were expressed in GBP using 2020/2021 prices. If necessary, costs were inflated or deflated to current prices using the NHS Cost Inflation Index. 26 Resources used were collected at each follow-up point over the time horizon using questionnaires. The questionnaires captured details of the following resource use categories: medication, outpatient and emergency attendances, encounters with primary or community health and social services, inpatient and day case admission, walking and adaptive aids related to injury, and number of days off work due to injury.
It was anticipated that hospital physiotherapy visits would be key to the analysis of cost as these formed the core of the ARTISAN Plus intervention. Hospital physiotherapy visits were recorded by two methods in both groups: site records and patient recall. Neither method is perfect, since site records cannot differentiate trial-related and unrelated physiotherapy visits and patient recall might be vulnerable to under-reporting. Participant recall was used as the primary method of analysis, given its consistent use across all resource items. Site-reported hospital physiotherapy use was substituted in a sensitivity analysis.
Intervention costs
The difference between the trial arms was the offer of at least one course of physiotherapy (ARTISAN Plus) following a single advice session (ARTISAN). The recall period of the 6-week resource use questionnaire covered the period during which at least a single course of physiotherapy was offered to participants. Physiotherapy contacts reported in CRFs could not be distinguished from those that formed part of the intervention; hence intervention costs were not applied, to avoid double counting.
However, a sensitivity analysis was conducted by replacing all patient-reported physiotherapy contacts with site-reported physiotherapy visits over the follow-up period.
Valuation of resource use
Resources used were valued in accordance with methods recommended by the NICE Guide to Methods of Technology Appraisal. 25 Unit costs were derived for each resource use item from national databases. The key databases used to derive unit costs for resource use items include: Department of Health and Social Care Reference Costs, Personal Social Services Research Unit’s unit cost compendium, 2021 NHS Prescription Cost Analysis database for England, 2021 volumes of the British National Formulary, and the NHS Supply Chain Catalogue 2019. 27–30 Data from the Office for National Statistics were used to estimate loss of earnings due to time off work. 31
Summary statistics were generated for resource use variables by treatment allocation and assessment point. Mean differences in costs between treatment groups for patients with complete data were compared using the two-sample t-test. Statistical significance was assessed at a 5% significance level.
Measuring outcomes
The primary health outcome for economic evaluation was the QALY, in accordance with NICE guidelines. 25 The health-related quality of life of trial participants was assessed at baseline (pre and post injury), 6 weeks, 10 weeks, 16 weeks, 24 weeks and 12 months post randomisation using the EQ-5D-5L instrument. 17 Responses to the EQ-5D-5L instrument were converted to utilities by mapping responses from the EQ-5D-5L to the EQ-5D-3L valuation set using a mapping function as recommended by NICE. 32,33 QALYs were generated for each participant using the area under the curve assuming linear interpolation across each temporal measurement point. QALYs accrued over the follow-up period were summarised across each time point and reported by trial group. Between-group differences in QALYs for patients with complete data were compared using a two-sample t-test.
Missing data
Missing data are common in RCTs. Participants are likely to be lost to follow-up for various reasons. Due to trial-related systematic differences in costs and outcomes, participants with missing data may systematically differ from those with fully observed data. Hence, missing data need to be handled in a principled way, underpinned by the missing data mechanism. Missing costs and health utility data were imputed under the missing at random (MAR) assumption, at each time point, using fully conditional MICE implemented through the MICE package in Stata® 17 (StataCorp LP, College Station, TX, USA). 34 The appropriateness of using the MAR assumption was evaluated by investigating the missing data patterns and comparing attributes with and without missing cost and health-related quality-of-life data at each follow-up time point. Predictors of missingness were identified using a stepwise logistic regression model at each follow-up time point, adjusting for baseline covariates. The multiple imputation model used baseline covariates (age, gender and dominant arm; i.e. whether the injured shoulder was the dominant arm).
Unobserved costs and QALYs were imputed separately by trial arm at each time point using observed values. The imputation model was assessed for convergence, and the distributions of observed, imputed and completed data for costs and QALYs were compared graphically. 35 [Reproduced with permission from Faria et al. 35 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.] It ran 50 times following methodological guidance that the number of imputations be determined by the fraction of missing information rather than the proportion of missing data. 36 Bivariate regression using the seemingly unrelated regression model (sureg), within the Stata MI (multiple imputation) framework, was used to estimate the costs and QALYs over the time horizon, controlling for baseline covariates (age and gender) and baseline utility. There were no significant interactions between the interventions and any of the baseline covariates. Joint distributions of costs and outcomes from the original data set were generated through non-parametric bootstrapping of the MI model and incremental costs and QALYs were calculated. 34
Presentation of cost-effectiveness results
Cost-effectiveness was presented as an incremental cost-effectiveness ratio (ICER), calculated as the mean difference in costs and QALYs, with ARTISAN as the reference (control) treatment and ARTISAN Plus as the comparator (intervention). Bootstrapped replicates of incremental costs and QALYs were used to populate the ICER plane. Cost-effectiveness acceptability curves showed the probability that ARTISAN Plus was cost-effective compared to ARTISAN at different cost-effectiveness thresholds ranging from £0 to £100,000 per QALY. The net monetary benefit (NMB) of using ARTISAN Plus was calculated at the different cost-effectiveness thresholds. A positive incremental NMB would show that ARTISAN Plus is cost-effective when compared to ARTISAN at the specified cost-effectiveness threshold. The expected value of perfect information was calculated at willingness-to-pay thresholds and represented graphically. The expected value of perfect information reflects the monetary value of removing uncertainty from the cost-effectiveness estimates at different willingness-to-pay thresholds.
Sensitivity and secondary analysis
Sensitivity analyses were conducted to test the robustness of the cost-effectiveness estimates. This included re-estimating the cost-effectiveness estimates under the following scenarios: (1) using complete data; (2) adopting a wider societal perspective that included private costs incurred by the trial participant and productivity losses due to work absences; and (3) using data up to 6-month follow-up due to relatively lower levels of completion rates at 12 months compared to earlier time points.
Due to difficulties in distinguishing physiotherapy visits that were part of the intervention and routine visits that were requested by patients, cost-effectiveness estimates were re-evaluated using site-reported physiotherapy data. The costs of missed appointments were included (assumed to be half the price of an NHS physio visit) and the cost-effectiveness estimates were re-evaluated.
Qualitative data analysis
Interviews were digitally recorded, subject to permission of each participant, and were transcribed verbatim. Data were analysed as follows [Reproduced with permission from Ellard et al. 37 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) license, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/. The text below includes minor additions and formatting changes to the original text.]:
-
Data familiarisation: reading of complete interview transcripts, listening to original audio recordings and use of field notes.
-
Identifying a thematic framework: key issues, concepts and themes identified and an index of codes developed.
-
Indexing: whereby the index generated through identification of the thematic framework was applied to all data.
-
Charting: a summary of each passage of text was transferred into a chart to allow more overall and abstract consideration of index codes across the data set and by each individual.
-
Mapping and interpretation: understanding the meaning of key themes, dimensions and broad overall picture of the data and identifying and understanding the typical associations between themes and dimensions.
The charting process provided an opportunity to code data from numerous vantage points: by demographic factors, such as gender or age; by personality characteristics, such as looking specifically at people who are highly anxious compared to those who are not; or by medical aspects, such as those with a particular condition compared to those without.
The computer package NVivo 7 (QSR International, Warrington, UK) was used to facilitate this process. Researcher bias was minimised through regular cross-checking of data and findings by the members of the team. In addition, transcripts were returned to participants (where necessary), providing them with the opportunity to check the transcripts for accuracy and authenticity and to offer any subsequent reflections.
Data management
Personal data collected during the trial were handled and stored in accordance with applicable UK data protection law. Personal identifying information was brought to WCTU for follow-up purposes. Handling of personal data was documented in the participant information sheet and consent obtained.
Disclosure of confidential information was only considered if there was an issue which may jeopardise the safety of the participant or another person, according to Warwick SOPs and the UK regulatory framework.
Data collection and management
The CRFs were developed by the trial manager in consultation with the chief investigator, statistician, health economist and other relevant members of the trial team to the required trial data. A suitably trained member of the research team completed and returned the CRFs to the ARTISAN trial office. The co-ordinating team checked and entered the data onto a secure trial database held at WCTU, in accordance with the WCTU SOPs.
Various methods were used to chase missing data/unreturned questionnaires, including post, phone, text, mobile app and e-mail. Appropriate consent was sought to contact participants.
Database
The database was developed by the Programming Team at WCTU and all specifications (i.e. database variables, validation checks, screens) were agreed between the programmer and appropriate trial staff.
Data storage
All essential documentation and trial records were stored at WCTU in conformance with the applicable regulatory requirements, and access to stored information (paper and electronic) was restricted to authorised personnel. All data were stored in a designated storage facility within the University Hospitals Coventry and Warwickshire and/or WCTU. Electronic data were stored on password-protected university computers in a restricted-access building.
Data access and quality assurance
All data collected were pseudonymised after the collection of the baseline demographic data for each participant. Confidentiality was strictly maintained, and names or addresses were not disclosed to anyone other than the staff involved in running the trial and collecting follow-up information when necessary. Participants were identified by ID number, initials and date of birth only where necessary. Identifiable participant data were held in a locked filing cabinet and coded with the trial number to tag identifiable data to the outcome data. Direct access to source data/documents was available for trial-related monitoring or audit.
Summary of changes to the trial protocol
Changes to the protocol via substantial amendments are summarised in Table 4.
| Amendment number | Details of amendment | Status |
|---|---|---|
| SA1 | Following feedback from TSC from last meeting, the protocol and all CRFs were amended. Protocol: a paragraph added to ‘Trial Summary and Flow Design’ section to clarify that in addition to the pilot and main trial we will also be conducting studies within a trial aimed at improving the way we conduct clinical trials. This will not impact on the main trial recruitment procedures, interventions, follow-up time points or outcome measures collected as outlined for the main trial. Participant age group was also clarified in the new version of the protocol. Patient information sheet: a sentence added to clarify that in addition to the main study we will also be evaluating ways to improve the way we do studies. Looking at how to improve the way we do things will not affect the main study. Additionally, three new forms were created. These include File Note, First Physiotherapy Session Quality Assurance Checklist, and Protocol Deviation Form | Health Research Authority (HRA) approval: 9 October 2018 REC approval: 1 October 2018 |
| SA2 | Two new documents created which include the Physiotherapy Manual and Physiotherapy Booklet | HRA approval: 14 November 2018 REC approval: 09 November 2018 |
| SA3 |
|
HRA approval: 04 December 2018 REC approval: 03 December 2018 |
| SA4 |
|
HRA approval: 15 March 2019 REC approval: 14 March 2019 |
| SA5 | Following feedback from sites and quality assurance during the pilot phase, CRFs and protocol were amended in preparation for the main phase. Study Within a Trial (SWAT) was also added | HRA approval: 7 August 2019 REC approval: 14 August 2019 |
| SA6 | Protocol safety reporting and clarification on exclusion criteria. Amend consent form and follow-up questionnaires with number of calls to hospital physiotherapy department. Amend posters and CRF with exclusion criteria. | HRA and REC approval: 2 October 2019 |
| SA7 | Protocol update from v5.0 to v6.0: clarification throughout that sites can have an individual PI or co-PIs, dependent on local arrangements. Clarification that if the participant is unable to attend face to face with the research team, then verbal consent can be gained and a paper consent form will be handed to the research team prior to randomisation. Clarification that the core outcome set will be collected by telephone, text or e-mail if postal questionnaires are not returned. Removal of personnel names in Appendix 4 plus the addition of SWAT registration number in Appendix 4 | Approved by REC and HRA. Date of implementation 9 July 2020 |
| SA8 | ARTISAN extension, change to timelines and contacts in protocol: end of recruitment 31 January 2022, end of trial 30 November 2022. 12th month follow-up for patients randomised after 1 August 2021 removed. Participant contact details form v3.0, participant information sheet v5.0, summary information sheet v5.0, SWAT postcard v2.0 | Approved by REC and HRA. Date of implementation 12 December 2021 |
Chapter 3 Clinical results
Recruitment
Forty-one NHS Trusts screened 1551 adults with a traumatic shoulder dislocation, from whom 1069 were not randomised. Between 14 November 2018 and 14 March 2022 we randomised 482 of into the study, 101% of target (Figure 1). One Trust was unable to randomise any participants prior to closure of the trial.
FIGURE 1.
CONSORT flow diagram.

Baseline characteristics
Two-thirds of participants were male (n = 317/482; 66%), 36% of participants had a sport-related injury (n = 172/482) for their dislocated shoulder and 16% reported a concurrent injury (n = 96). The mean age of participants was 45 years (SD = 20) (Table 5).
| Characteristic | ARTISAN only (n = 240) | ARTISAN + physiotherapy (n = 242) | All participants (n = 482) | |
|---|---|---|---|---|
| Gender (n, % of group total) | Female | 83 (34) | 82 (34) | 165 (34) |
| Male | 159 (66) | 158 (66) | 317 (66) | |
| Age at randomisation in years (mean, SD) | 45 (20) | 45 (19) | 45 (20) | |
| Age group (n, %) | 39 and under | 109 (45) | 112 (46) | 221 (46) |
| 40 and over | 131 (55) | 130 (53) | 261 (54) | |
| Injured arm (n, %) | Dominant | 134 (56) | 136 (56) | 270 (56) |
| Non-dominant | 106 (44) | 106 (44) | 212 (44) | |
| Mechanism of injury (n, %) | Sports | 94 (39) | 78 (32) | 172 (36) |
| Non-sports | 146 (61) | 164 (68) | 310 (64) | |
| Concurrent injury (n, %) | Yes (any) | 50 (21) | 46 (19) | 96 (20) |
| Head | 17 (7) | 9 (4) | 26 (5) | |
| Chest | 10 (4) | 2 (1) | 12 (3) | |
| Abdomen | 0 (0) | 2 (1) | 2 (0) | |
| Pelvis | 2 (1) | 3 (1) | 5 (1) | |
| Spine | 0 (0) | 2 (1) | 2 (0) | |
| Legs | 17 (7) | 19 (8) | 36 (8) | |
| Injury to opposite arm | 8 (3) | 5 (2) | 13 (3) | |
| Injury to same arm | 10 (4) | 21 (9) | 31 (6) | |
| BMI (n valid) | 212 | 212 | 424 | |
| BMI (mean, SD) | 27 (5) | 27 (6) | 27 (6) | |
| Concomitant medication (n, %) | Systemic steroids | 2 (1) | 5 (2) | 7 (2) |
| Pain medication pre-dislocation | 19 (8) | 22 (9) | 41 (9) | |
| Concomitant illness (n, %) | Inflammatory arthritis | 8 (3) | 6 (2) | 14 (3) |
| Diabetes | 9 (4) | 9 (4) | 18 (4) | |
| Smoking status (n, %) | Yes (n, %) | 31 (13) | 44 (18) | 75 (16) |
| If yes, no. per week (mean, SD) | 41 (31) | 40 (39) | 41 (35) | |
| For how many years (mean, SD) | 13 (13) | 15 (15) | 14 (14) | |
| Alcohol units per week (n, %) | 0–7 | 154 (64) | 157 (65) | 311 (65) |
| 8–14 | 57 (24) | 53 (22) | 110 (23) | |
| 15–21 | 14 (6) | 21 (9) | 35 (7.3) | |
| > 21 | 14 (6) | 11 (5) | 25 (5) | |
| Ethnicity (n, %) | White | 205 (85) | 206 (85) | 411 (85) |
| Mixed | 2 (1) | 7 (3) | 9 (2) | |
| Asian | 18 (8) | 19 (8) | 37 (8) | |
| Black/African/Caribbean | 12 (5) | 5 (2) | 17 (4) | |
| Other | 3 (1) | 5 (2) | 8 (2) | |
| Missing | 0 | 0 | 0 | |
| Employment (n, %) | Full-time employed | 119 (50) | 113 (47) | 232 (48) |
| Part-time employed | 26 (11) | 20 (8) | 46 (10) | |
| Self-employed | 14 (6) | 31 (13) | 45 (9) | |
| Retired/looking after home/inactive | 50 (21) | 48 (20) | 98 (20) | |
| Unpaid work | 0 (0) | 1 (0) | 1 (0) | |
| Unemployed | 11 (5) | 8 (3) | 19 (4) | |
| Full-time student | 18 (8) | 21 (9) | 39 (8) | |
| Full-time carer | 2 (1) | 0 (0) | 2 (0) | |
| Missing | 0 | 0 | 0 | |
| Trial allocation preference (n, %) | ARTISAN Plus | 65 (27) | 74 (31) | 139 (29) |
| ARTISAN alone | 46 (19) | 35 (15) | 81 (17) | |
| No preference | 128 (53) | 128 (53) | 256 (53) | |
| Missing | 1 (0) | 5 (2) | 6 (1) | |
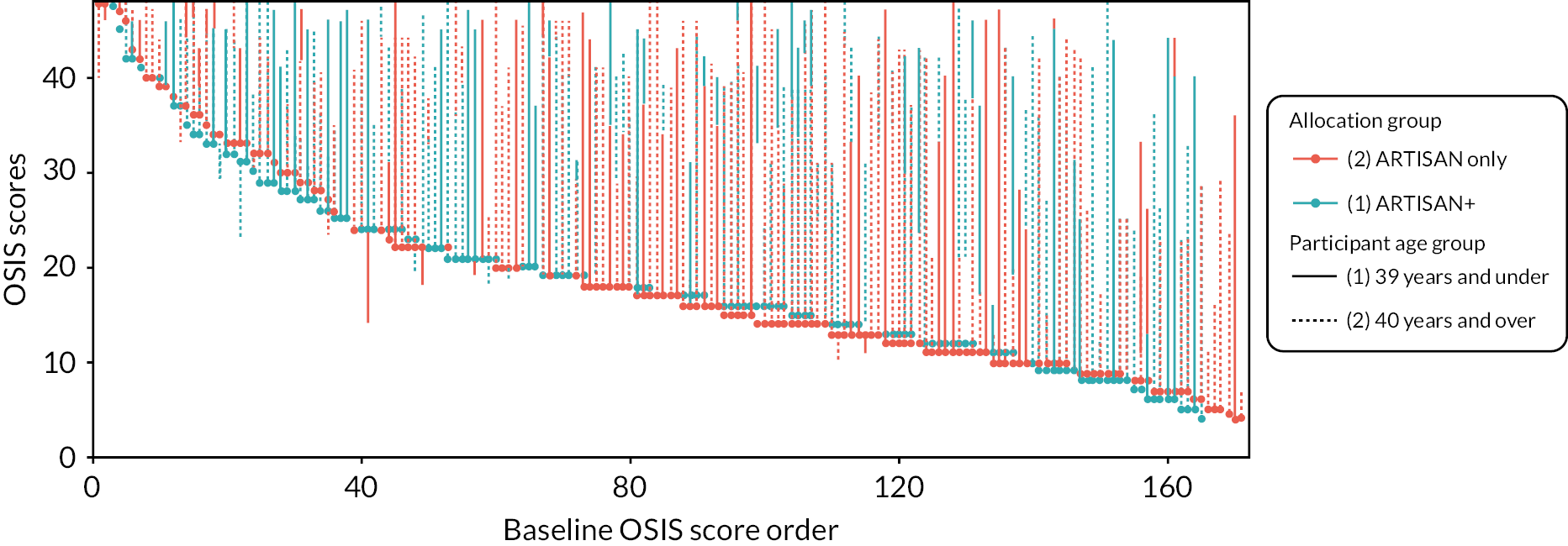
The group allocated to additional physiotherapy had a slightly higher baseline score than the ARTISAN-only group. Figure 2 shows this graphically, where it can also be seen that while the distributions of the two groups are similar, the overwhelming majority of participants have increased from their baseline OSIS score. It is also clear that participants with lower OSIS scores at baseline achieve larger increases in function partly due to regression to the mean.
FIGURE 2.
Oxford Shoulder Instability Score waterfall plot. Participants are ranked by baseline score (dot) and are connected to their 6-month score by a line. Line colour represents allocation group and line type (solid or dashed) represents age group at randomisation.
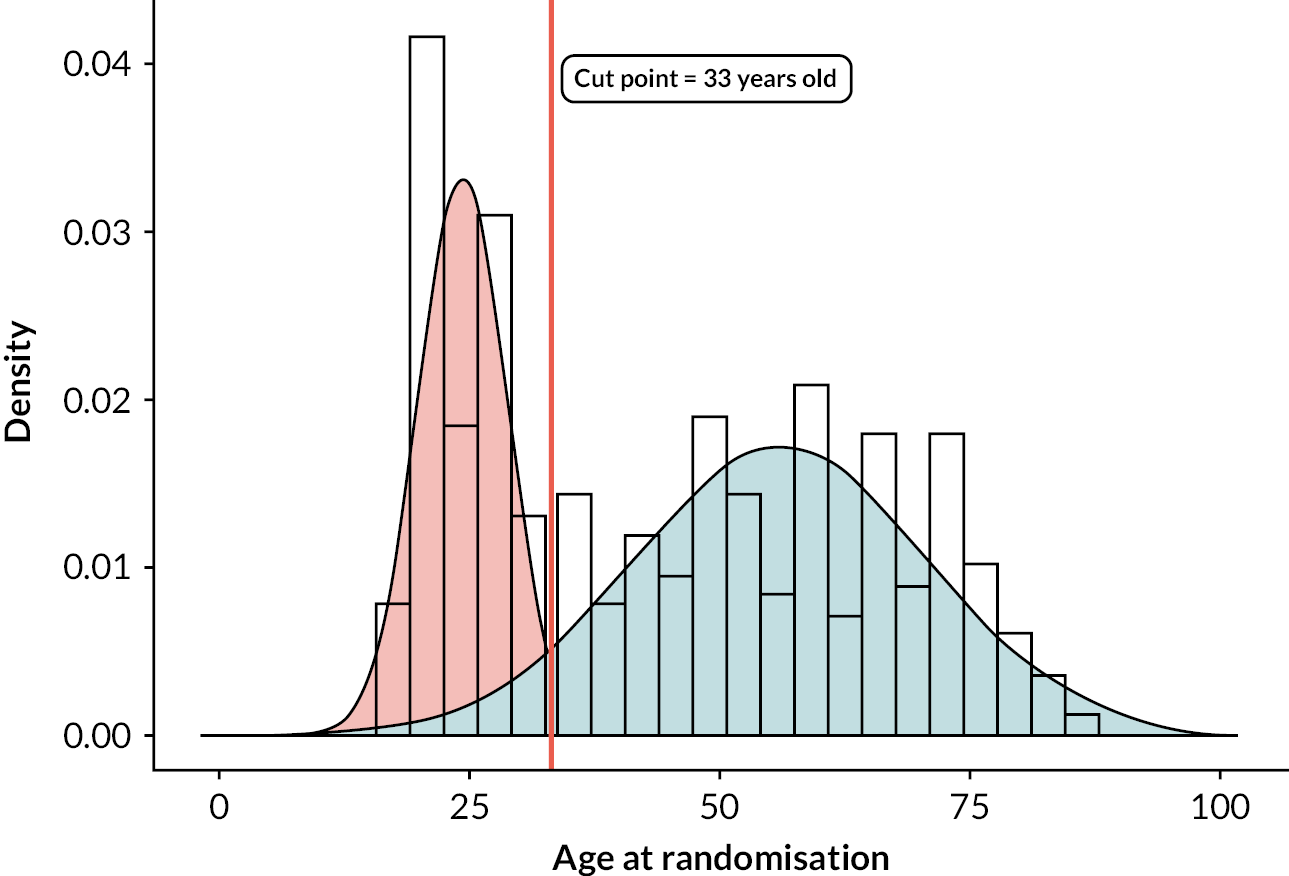
Age cut point
Figure 3 shows the probability density plots of the participant age. Two distributions were fitted, centred at 24 and 56 years old, with the point where probability of membership in both distributions was 50% at 33 years of age. As this was within 10 years of the randomisation stratum (age of 40), no further sensitivity analyses exploring the effects of age were done.
FIGURE 3.
Probability density plots of the participant age.
Intervention delivery
During the ARTISAN study, 96 therapists delivered the core ARTISAN session across the 41 study sites. The number of physiotherapists at each site ranged from one to seven, with a low number of physiotherapists randomising and treating more than three participants in the study.
Compliance
Source physiotherapy data were requested from sites for all 482 participants; only four records were unobtainable, meaning data were available for 478 participants. Table 6 shows the number of participants attending additional physiotherapy sessions. Of those receiving the ARTISAN-only intervention, 15% had extra physiotherapy, whereas 83% of participants in the group allocated to additional physiotherapy received at least one additional physio session.
| Site-reported physiotherapy | ARTISAN only (n = 240) |
ARTISAN Plus (n = 242) |
All participants (n = 482) |
|
|---|---|---|---|---|
| Number of participants with additional physio appointments (n, %) | 44 (19) | 239 (99) | 284 (58.9) | |
| Number of participants completed at least one session of physiotherapy (n, %) | 37 (15) | 201 (83) | 238 (49) | |
| Median number of sessions attended (min–max) | All participants | 0 (0–14) | 2 (0–12) | (0–14) |
| Participants with at least one session | 2 (1–14) | 2 (1–12) | 2 (2–14) | |
Table 7 also shows patients’ clinical treatment status at 6 months in both groups.
| Status at 6 months post randomisation | ARTISAN only (n = 240) |
ARTISAN Plus (n = 242) |
All participants (n = 482) |
|---|---|---|---|
| Did not attend (n, %) | 7 (3) | 54 (22) | 61 (13) |
| Patient discharged (n, %) | 220 (92) | 167 (69) | 387 (80) |
| Ongoing treatment (n, %) | 11 (5) | 18 (7) | 29 (6) |
| Missing or other outcome (n, %) | 2 (1) | 3 (1) | 5 (1) |
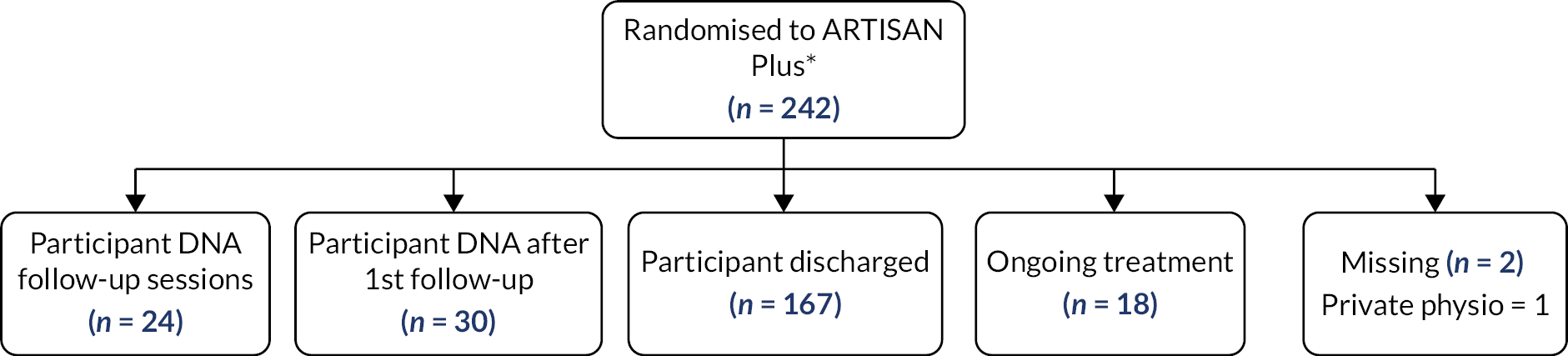
In the ARTISAN-only group, 42 participants opted to have extra physio sessions, 194 received the ARTISAN-only session and 2 participants were referred to physiotherapy by clinicians. These two participants’ treatments were not compliant with the intervention protocol and they were considered to have switched allocation groups for the per-protocol analysis. There were two participants whose data were missing.
In the group allocated to ARTISAN plus additional physiotherapy, 24 participants were confirmed by site records not to have attended any physiotherapy appointments. Seventeen participants attended an additional physiotherapy session but were scheduled to have more and did not attend these sessions. The remaining 185 participants either had been discharged (i.e. completed the physiotherapy course) or were still receiving ongoing treatment. Finally, there were two participants for whom data could not be retrieved in this group.
The treatment patient pathways are demonstrated in the participant treatment flow diagrams for each respective allocation group in Figures 4 and 5.
FIGURE 4.
Diagram of participant pathway for the ARTISAN-only group. a Participants are compliant with protocol. b Participants are not compliant with protocol (i.e. have switched treatment groups).

FIGURE 5.
Diagram of physiotherapy pathways for the ARTISAN Plus group.
Therapist effect
As the study is focused on the effectiveness of physiotherapy, there were concerns at the beginning of the study that there may be a physiotherapist effect which could strongly affect the estimate of efficacy of the ARTISAN intervention. Hence, the study captured the number of therapists who delivered the core (pre-randomisation) session.
There were 96 therapists who delivered the core ARTISAN session across the 41 study sites. The median number of therapists at each site was 2 (range 1–7). The number of participants randomised by each therapist ranged from 1 to 21; of the 96 therapists, only 42 (44%) randomised more than three participants.
At the interim analysis point, we estimated the therapist effect by calculating the ICC using the 3-month follow-up data with a multilevel model. A chi-squared test comparing the likelihood of the full model against the model without the physiotherapist effect was also conducted. The ICC was estimated to be 0.0201 (95% CI 0 to 0.601) (participants n = 138; physiotherapists n = 67). The addition of physiotherapy effects did not improve the model (χ2 = −2.27e-13, df = 1; p = 1).
Due to the low number of physiotherapists randomising more than three participants, the DMC asked for a sensitivity analysis with those physiotherapists randomising three or fewer participants removed. Again, the addition of therapist effects did not improve the model (χ2 = 1.14e-13, df = 1; p = 0.5). The ICC for was estimated to be 0.0019 (95% CI 0 to 0.483) (participants n = 94; physiotherapists n = 23).
The DMC endorsed the recommendation that the sample size did not need to be inflated for therapist effects, but requested that the analysis was repeated using the 6-month follow-up data at the end of the study to check whether the interim analysis was correct. Again, 12 of the 41 sites had a single physiotherapist performing the randomisation of the participants into the ARTISAN study, and many physiotherapists still did not randomise more than three participants.
Repeating the interim analysis using the 6-month follow-up data showed that the physiotherapist effect was small and the ICC was not statistically significant. The model was adjusted for the dominant arm injured, age group and physiotherapist, but did not include the allocation group (354 participants and 80 physiotherapists). An ICC value of 0.026 was observed (95% CI 0 to 0.106). Again, comparing the models with and without the physiotherapy effects had χ2 = 0.638 and p = 0.424. Again, this showed that including physiotherapy effects did not improve the model fit.
Physiotherapy-delivered intervention content
Details of the core (pre-randomisation) ARTISAN session are given in Table 8. A total of 460 participants were given the core session as per protocol; 22 participants had details missing. The mean times taken to deliver the ARTISAN session were similar in both interventions.
| ARTISAN programme (n = 240) |
ARTISAN + physiotherapy (n = 242) |
All participants (n = 482) |
||
|---|---|---|---|---|
| ARTISAN session given as per protocol | 230 | 230 | 460 | |
| Time taken to deliver ARTISAN sessiona (minutes) (mean, SD) | 40 (13) | 38 (13) | 39 (13) | |
| Session longer than 60 minutesa (n, %) | 7 (3) | 4 (2) | 11 (2) | |
| Grade of physiotherapist delivering follow-up sessions (at each session) | Grade 5 | 3 | ||
| Grade 6 | 115 | |||
| Grade 7 | 178 | |||
| Grade 8 | 50 | |||
| Other | 4 | |||
Table 8 also contains the self-reported grade of the physiotherapists who delivered the additional physiotherapy sessions.
Tables 9–11 show the physiotherapy exercises prescribed at home and during the session in the additional physiotherapy group.
| Exercise | Times given at sessions (n) | Times prescribed for home (n) |
|---|---|---|
| ROM1: flexion in lying | 22 | 19 |
| ROM2: flexion with a stick | 12 | 15 |
| ROM3: flexion with a table | 23 | 23 |
| ROM4: flexion with a gym ball | 4 | 7 |
| ROM5: flexion with a wall | 32 | 36 |
| ROM6: flexion with a pulley | 5 | 3 |
| ROM7: abduction in lying | 4 | 3 |
| ROM8: abduction with a stick | 28 | 26 |
| ROM9: abduction with a table | 11 | 11 |
| ROM11: abduction with a gym ball | 4 | 3 |
| ROM12: abduction with a wall | 11 | 11 |
| ROM13: abduction with a pulley | 3 | 3 |
| ROM14: external rotation in lying | 6 | 5 |
| ROM15: external rotation with a stick | 23 | 25 |
| ROM16: rotation with a table | 4 | 5 |
| ROM17: rotation with a gym ball | 1 | 2 |
| ROM18: internal rotation with a stick | 7 | 8 |
| ROM19: internal rotation with a towel | 10 | 12 |
| ROM20: extension with a stick | 2 | 2 |
| ROM21: pendulum exercises | 0 | 1 |
| ROM22: other ROM exercise | 19 | 26 |
| Exercise | Times given at sessions (n) | Times prescribed for home (n) |
|---|---|---|
| Strength1: TheraBand extension | 13 | 16 |
| Strength2: TheraBand external rotation (standing) | 21 | 24 |
| Strength3: TheraBand external rotation (sitting) | 14 | 16 |
| Strength4: TheraBand internal rotation (standing) | 20 | 19 |
| Strength5: TheraBand internal rotation (sitting) | 7 | 8 |
| Strength6: TheraBand flexion (stable surface) | 14 | 13 |
| Strength7: TheraBand flexion (standing) | 17 | 23 |
| Strength8: TheraBand abduction | 24 | 22 |
| Strength9: Scapula setting | 3 | 2 |
| Stregnth10: Glenohumeral control | 22 | 21 |
| Strength11: Standing weight drop | 16 | 23 |
| Stength12: Lying weight drop | 3 | 4 |
| Strength13: Other strength exercise | 99 | 109 |
| Exercise | Times given at sessions (n) | Times prescribed for home (n) |
|---|---|---|
| Adv1: floor push-ups | 22 | 20 |
| Adv2: wall push-ups | 40 | 35 |
| Adv3: gym ball push-ups | 3 | 4 |
| Adv4: gym ball weight transfer | 5 | 11 |
| Adv5: gym ball proprioception | 0 | 1 |
| Adv6: proprioception | 1 | 2 |
| Adv7: sport-specific drills | 5 | 5 |
| Adv8: falling press-up, waist level | 6 | 7 |
| Adv9: falling press-up, standing height | 0 | 2 |
| Adv10: other advice | 33 | 49 |
Follow-up
Follow-up at 6 months post randomisation was less than expected, with 354/482 (73%) returning their questionnaires. Comparing the baseline demographics of the two groups, participants returning their questionnaires were more likely to be female (n = 138/354, 39% returned vs. n = 27/128, 21% lost to follow-up), aged 40 and over (n = 227/354, 64% returned vs. n = 34/128, 27% lost to follow-up) and to have had their dislocation via a non-sporting-related incident (n = 246/354, 70% returned vs. n = 64/128, 50% lost to follow-up). However, mean baseline OSIS scores were similar (mean scores: 19.1 returned vs. 19.1 lost) and there was no correlation with whether or not the participant’s dominant arm was injured (n = 199/354, 56% returned vs. n = 71/128, 55% lost).
Despite a larger than anticipated loss to follow-up, the baseline characteristics predominantly remained similar between intervention groups. For example, the percentage of the younger population (39 years and under) who returned their questionnaires was 36% in both intervention groups; similarly for gender, females comprised 39% of those who returned questionnaires in the ARTISAN only group and 40% in the ARTISAN Plus group. The imbalance between the interventions for mechanism of injury also remained the same as the baseline figures. This provides reassurance that the mechanism of missingness was not related to the trial interventions or processes. Therefore, we do not expect a large risk of bias due to the participants lost to follow-up.
Primary outcome
The primary outcome was the OSIS. The possible scores range from 0 (worst function) to 48 (best function). The trial was designed to detect a difference of 4 points between allocation groups at 6 months post randomisation. Descriptive statistics for the OSIS at all time points are shown in Table 12 and Figure 6.
| Time point | Statistic | ARTISAN + physiotherapy (n = 242) |
ARTISAN only (n = 240) |
All participants (n = 482) |
Mean differencea (unadjusted) | p-value |
|---|---|---|---|---|---|---|
| Pre-injury | N (%) | 242 (100) | 240 (100) | 482 (100) | 0.5 (−0.7 to 1.8) | 0.394 |
| Mean (SD) | 45.9 (6.5) | 45.4 (7.4) | 45.6 (7.0) | |||
| Missing | 0 (0) | 0 (0) | 0 (0) | |||
| Baseline | N (%) | 231 (95.5) | 232 (96.7) | 463 (96.1) | 0.5 (−1.4 to 2.3) | 0.624 |
| Mean (SD) | 19.3 (9.7) | 18.8 (10.0) | 19.0 (9.9) | |||
| Missing | 11 (4.5) | 8 (3.3) | 19 (3.9) | |||
| 6 weeks | N (%) | 173 (71.5) | 166 (69.2) | 339 (70.3) | 1.1 (−1.1 to 3.2) | 0.334 |
| Mean (SD) | 24.4 (9.9) | 23.3 (10.4) | 23.9 (10.1) | |||
| Missing | 69 (28.5) | 74 (30.8) | 143 (29.7) | |||
| 3 months | N (%) | 182 (75.2) | 170 (70.8) | 352 (73) | 2.2 (−0.1 to 4.5) | 0.064 |
| Mean (SD) | 32.2 (10.4) | 30 (11.4) | 31.2 (11) | |||
| Missing | 60 (24.8) | 70 (29.2) | 130 (27) | |||
| 6 months | N (%) | 174 (71.9) | 180 (75) | 354 (73.4) | 2.2 (0.1 to 4.3) | 0.040 |
| Mean (SD) | 38.4 (9.2) | 36.2 (10.7) | 37.3 (10) | |||
| Missing | 68 (28.1) | 60 (25) | 128 (26.6) | |||
| 12 months | N (%) | 135 (55.8) | 129 (53.8) | 264 (54.8) | 1.7 (−0.4 to 3.7) | 0.115 |
| Mean (SD) | 41.6 (7.8) | 39.9 (9.2) | 40.8 (8.5) | |||
| Missing | 107 (44.2) | 111 (46.2) | 218 (45.2) |
FIGURE 6.
Oxford Shoulder Instability Score box plot: median and interquartile range (IQR), min and max represented by line.
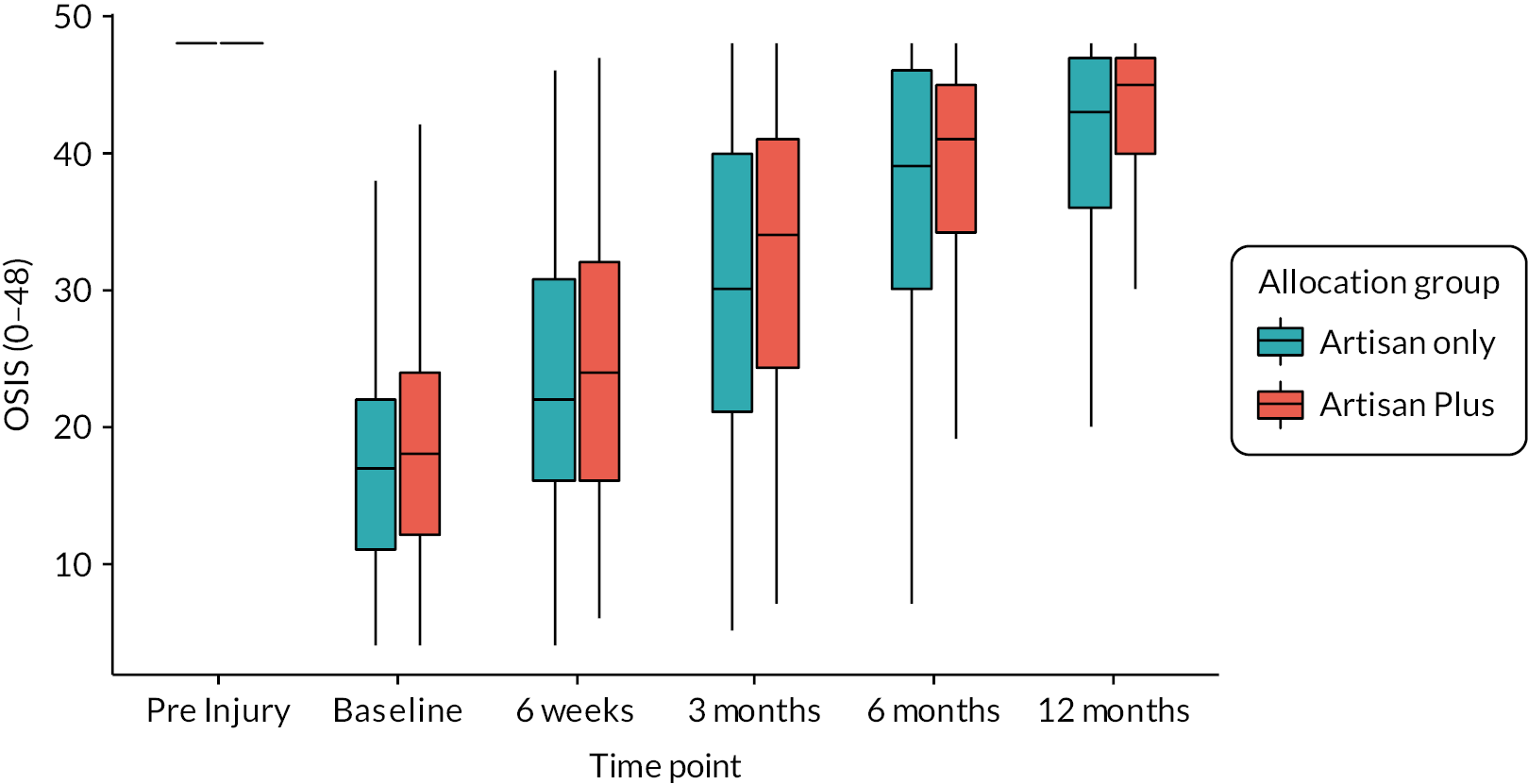
The mean difference (unadjusted) at the 6-month follow-up showed that the ARTISAN Plus group had a better outcome and was statistically significant. The ARTISAN Plus group had a positive mean difference of 2.2 (95% CI 0.1 to 4.3). The point estimate was just over half of our target difference of 4.0.
The adjusted intention-to-treat model was the primary analysis for the study. A mixed-effects model was fitted with the following (pre-specified) variables as fixed effects: allocation group, whether the participant’s dominant arm was injured, age group, and baseline score. Site of randomisation was fitted as a random effect. The model coefficients are reported in Table 13. The mean adjusted difference was 1.5 points (95% CI −0.3 to 3.5) in favour of the ARTISAN Plus group. This shows that there was no significant difference between the intervention groups. The limits of the 95% CI excluded our target difference of 4.0. Age group was found to be significant, with participants aged 40 years and over performing worse by −2.7 points. The reason why the unadjusted result was different to the adjusted result was that the baseline score was a significant predictor of the participants’ outcome. Since this was not accounted for in the unadjusted analysis, the slight imbalance in scores at baseline caused a different result to that of the adjusted model.
| Model variable (n = 337) |
Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| Intervention group | ARTISAN only | 0 | – | 0.111 |
| ARTISAN Plus | 1.5 | −0.3 to 3.5 | ||
| Age group | 39 and under | 0 | – | 0.009 |
| Over 40 | −2.7 | −4.7 to −0.7 | ||
| Dominant arm injured | Non-dominant | 0 | – | 0.306 |
| Dominant | −1.0 | −2.9 to 0.9 | ||
| Oxford shoulder instability | Baseline score | 0.4 | 0.3 to 0.5 | < 0.001 |
Secondary outcomes
QuickDASH
Table 14 and Figure 7show the results of the QuickDASH outcomes at each time point. The results at the 6-month primary end point show a mean difference of −1.7 (95% CI −5.4 to 2.0) in favour of the ARTISAN Plus group; therefore the unadjusted results show that the effect of the intervention is not statistically significant.
| Time point | Statistic | ARTISAN + physiotherapy (n = 242) |
ARTISAN only (n = 240) |
All participants (n = 482) |
Mean difference (unadjusted) | p-value |
|---|---|---|---|---|---|---|
| Pre-injury | N | 242 (100) | 239 (99.6) | 481 (99.8) | 0.5 (−3.2 to 4.3) | 0.775 |
| Mean (SD) | 9.4 (22) | 8.8 (19.9) | 9.1 (21) | |||
| Missing | 0 (0) | 1 (0.4) | 1 (0.2) | |||
| Baseline | N | 230 (95) | 230 (95.8) | 460 (95.4) | −1.5 (−5.8 to 2.9) | 0.507 |
| Mean (SD) | 46.1 (23.6) | 47.6 (23.7) | 46.8 (23.6) | |||
| Missing | 12 (5) | 10 (4.2) | 22 (4.6) | |||
| 6 weeks | N | 168 (69.4) | 154 (64.2) | 322 (66.8) | −5.2 (−10.1 to −0.3) | 0.038 |
| Mean (SD) | 27.6 (21.4) | 32.8(23.2) | 30.1 (22.4) | |||
| Missing | 74 (30.6) | 86 (35.8) | 160 (33.2) | |||
| 3 months | N | 177 (73.1) | 162 (67.5) | 339 (70.3) | −3.5 (−8.0 to 0.9) | 0.121 |
| Mean (SD) | 19.3 (19.9) | 22.8 (21.7) | 21 (20.8) | |||
| Missing | 65 (26.9) | 78 (32.5) | 143 (29.7) | |||
| 6 months | N | 169 (69.8) | 169 (70.4) | 338 (70.1) | −1.7 (−5.4 to 2.0) | 0.372 |
| Mean (SD) | 12.7 (16.9) | 14.4 (17.5) | 13.5 (17.2) | |||
| Missing | 73 (30.2) | 71 (29.6) | 144 (29.9) | |||
| 12 months | N | 133 (55) | 126 (52.5) | 259 (53.7) | −1.8 (5.6 to 2.0) | 0.352 |
| Mean (SD) | 9.2 (15.2) | 11 (16) | 10.1 (15.6) | |||
| Missing | 109 (45) | 114 (47.5) | 223 (46.3) |
FIGURE 7.
QuickDASH score box plot: median and IQR, min and max represented by line.
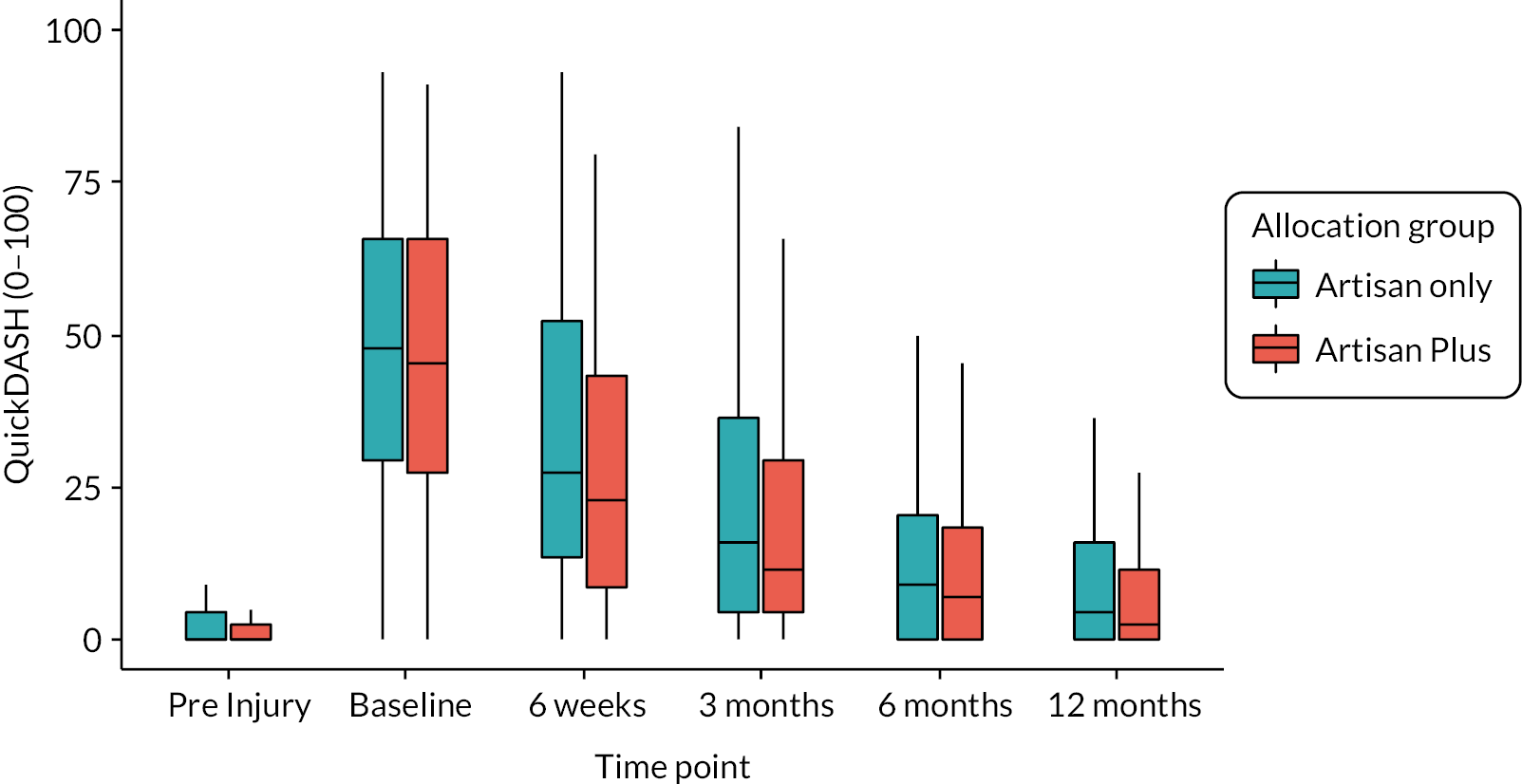
QuickDASH adjusted model results
Table 15 presents the adjusted results for the QuickDASH at the primary end point of 6 months. The results also agree with the previous unadjusted result; there was no significant difference observed between the interventions. However, the ARTISAN Plus group had slightly higher levels of disability than the ARTISAN-only group. Participant age group was a statistically significant variable, with the older age group having 7.5 points more disability than the younger group. The models for the early end points also followed this pattern.
| Model variable (N = 319) |
Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| Intervention group | ARTISAN only | 0 | – | 0.646 |
| ARTISAN Plus | −0.8 | (−4.0 to 2.5) | ||
| Age group | 39 and under | 0 | – | < 0.001 |
| Over 40 | 7.5 | (3.9 to 11.1) | ||
| Dominant arm injured | Non-dominant | 0 | – | 0.272 |
| Dominant | 1.8 | (−1.4 to 5.1) | ||
| QuickDASH | Baseline score | 0.7 | – (0.6 to 0.9) |
< 0.001 |
Quality of life (EuroQol 5-dimension score)
Table 16 and Figure 8 show the unadjusted results for the EQ-5D-5L at each time point.
| Time point | Statistic | ARTISAN + physiotherapy (n = 242) |
ARTISAN only (n = 240) |
All participants (n = 482) |
Mean difference (unadjusted) | p-value |
|---|---|---|---|---|---|---|
| Pre-injury | N | 242 (100) | 239 (99.6) | 481 (99.8) | −0.024 (−0.065 to 0.018) | 0.267 |
| Mean (SD) | 0.824 (0.239) | 0.848 (0.227) | 0.836 (0.233) | |||
| Missing | 0 (0) | 1 (0.4) | 1 (0.2) | |||
| Baseline | N | 231 (95.5) | 232 (96.7) | 463 (96.1) | −0.007 (−0.048 to 0.034) | 0.738 |
| Mean (SD) | 0.588 (0.212) | 0.595 (0.233) | 0.591 (0.222) | |||
| Missing | 11 (4.5) | 8 (3.3) | 19 (3.9) | |||
| 6 weeks | N | 173 (71.5) | 166 (69.2) | 339 (70.3) | 0.019 (−0.023 to 0.061) | 0.370 |
| Mean (SD) | 0.711 (0.189) | 0.692 (0.201) | 0.702 (0.195) | |||
| Missing | 69 (28.5) | 74 (30.8) | 143 (29.7) | |||
| 3 months | N | 182 (75.2) | 170 (70.8) | 352 (73) | 0.046 (0.005 to 0.088) | 0.030 |
| Mean (SD) | 0.787 (0.179) | 0.741 (0.215) | 0.765 (0.198) | |||
| Missing | 60 (24.8) | 70 (29.2) | 130 (27) | |||
| 6 months | N | 173 (71.5) | 179 (74.6) | 352 (73) | 0.018 (−0.024 to 0.060) | 0.410 |
| Mean (SD) | 0.815 (0.183) | 0.797 (0.217) | 0.806 (0.2) | |||
| Missing | 69 (28.5) | 61 (25.4) | 130 (27) | |||
| 12 months | N | 135 (55.8) | 129 (53.8) | 264 (54.8) | 0.022 (−0.018 to 0.062) | 0.273 |
| Mean (SD) | 0.870 (0.16) | 0.848 (0.169) | 0.859 (0.165) | |||
| Missing | 107 (44.2) | 111 (46.2) | 218 (45.2) |
FIGURE 8.
Quality-of-life EQ-5D-5L box plot: median and IQR, min and max represented by line.

EuroQol 5-dimension score adjusted model results
Initially, the adjusted model did not converge. However, replacing the site random effect as a fixed effect showed that the outcome at 6 months did not differ significantly between the intervention groups. The other follow-up points also showed no significant between-group differences (Table 17).
| Model variable (n = 335) |
Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| Intervention group | ARTISAN only | 0 | – | 0.585 |
| ARTISAN Plus | 0.010 | (−0.026 to 0.047) | ||
| Age group | 40 and under | 0 | – | < 0.001 |
| Over 40 | −0.074 | (−0.113 to −0.035) | ||
| Dominant arm injured | Non-dominant | 0 | – | 0.147 |
| Dominant | −0.028 | (−0.065 to 0.009) | ||
| OSIS | Baseline score | 0 | – | < 0.001 |
| 0.405 | (0.321 to 0.488) | |||
Complications and serious adverse events
Table 18 reports the number of complications (pre-specified AEs and SAEs) reported by participants at any follow-up. There were 27 reported SAEs during the trial that were unrelated to the TASD or TASD treatment, 13 in the ARTISAN-only group and 14 in the ARTISAN Plus additional sessions group (Table 19).
| Complication | Allocation group | Patient-reported events | Confirmed by site | Not confirmed | Unknowna | Unobtainable |
|---|---|---|---|---|---|---|
| Torn muscle or tendon | ARTISAN Plus | 32 | 21 | 9 | 2 | |
| ARTISAN only | 37 | 22 | 15 | |||
| Shoulder redislocation | ARTISAN Plus | 8 | 3 | 2 | 2 | 1 |
| ARTISAN only | 19 | 7 | 7 | 4 | 1 | |
| Frozen shoulder | ARTISAN Plus | 12 | 7 | 5 | ||
| ARTISAN only | 7 | 3 | 4 | |||
| Fractured bone | ARTISAN Plus | 6 | 4 | 2 | ||
| ARTISAN only | 12 | 8 | 4 | |||
| Blood vessel damage | ARTISAN Plus | 0 | ||||
| ARTISAN only | 1 | 1 |
| SAE | ARTISAN only (n = 240) | ARTISAN + physiotherapy (n = 242) | All participants (n = 482) | |
|---|---|---|---|---|
| All reported SAEs | 13 | 14 | 27 | |
| SAEs per participant | 1 SAE | 11 | 12 | 23 |
| 2 SAEs | 1 | 1 | 2 | |
| Death (n, % of SAEs) | 0 | 0 | 0 | |
| Life-threatening (n, % of SAEs) | 0 | 1 (7) | 1 (3) | |
| Hospitalisation or prolongation of existing hospitalisation (n, % of SAEs) | 12 (92) | 11 (79) | 23 (79) | |
| Persistent or significant disability or incapacity (n, % of SAEs) | 1 (8) | 1 (7) | 2 (7) | |
| Other reason (n, % of SAEs) | 0 | 2 (14) | 2 (7) | |
| Relatedness to intervention (n, % of SAEs) | Related | 0 | 0 | 0 |
| Unrelated | 13 (100) | 14 (100) | 27 (100) | |
| If related, was the SAE expected (n, % of related SAEs)? | Expected | N/A | N/A | N/A |
| Unexpected | N/A | N/A | N/A | |
Temporal effects
It was pre-specified that temporal effects would be assessed to observe whether participants in one treatment group had faster recovery than those in the other.
The model results for each of the outcome measures (OSIS, QuickDASH and EQ-5D) had shown that time point is a significant predictor in determining the outcome of participants; in other words, people do make statistically significant improvements at each time point. However, when analysing the interactions, there were no significant results between the time points and treatment groups (no interaction term in any model crossed the significance threshold for the p-value of 0.05). This means that the recovery trajectories are non-significant between the allocation groups.
Sensitivity and subgroup analyses
Sensitivity analyses: predefined per-protocol analysis
There were two participants randomised to the ARTISAN-only allocation group for whom the treating clinician prescribed further physio sessions. These participants were included in the intention-to-treat primary analysis. Also, four participants were incorrectly randomised into the study as they had no radiological evidence of their shoulder dislocation.
To assess the effects of the treatment group switching and inclusion of ineligible participants, the treatment-switching participants were removed and those randomised in error were added to their randomisation groups. It was found that this did not affect the primary analysis model. The adjusted mean difference was 1.4 points for the ARTISAN Plus physio group (95% CI −0.5 to 3.3; p = 0.150).
Sensitivity analysis: post hoc ‘as treated’ analysis
During the analysis, it was noted that there were a large proportion of participants who did not attend any physiotherapy. Hence, we did a post hoc ‘as-treated’ analysis. In this scenario, we considered participants who received at least one physiotherapy session (as defined by the recruitment site), regardless of their initial allocation, to be part of the ‘additional physiotherapy’ programme. Participants who did not have any additional physiotherapy were considered as the ARTISAN-only arm.
We found that 238 participants had additional physiotherapy and 239 had no additional physiotherapy after randomisation. We fitted a random-effects model with the same covariates as in the primary analysis. The results in Table 20 show that the models were similar and there was no significant difference between the two intervention groups.
| Model variable (n = 334) |
Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| Intervention group | ARTISAN only | 0 | – | 0.774 |
| ARTISAN Plus | −0.3 | (−2.2 to 1.6) | ||
| Age group | 39 and under | 0 | – | 0.005 |
| Over 40 | −2.9 | (−4.9 to −0.9) | ||
| Dominant arm injured | Non-dominant | 0 | 0.362 | |
| Dominant | −0.9 | (−2.8 to 1.0) | ||
| OSIS | Baseline score | 0.4 | (0.3 to 0.5) | < 0.001 |
Sensitivity analysis: post hoc per protocol (physiotherapy received)
In this scenario, we only considered the intervention to be the receipt or not of additional physiotherapy sessions. That is, we compared those participants in the ARTISAN-only group who did not have a further physiotherapy session with participants in the ARTISAN Plus group who received at least one further session of physiotherapy.
Of the 478 participants who had their physiotherapy details confirmed by recruitment site, 200 participants allocated to the ARTISAN-only group received only the core ARTISAN session, and 201 participants in the additional physiotherapy arm received at least one additional physiotherapy visit. Repeating the primary analysis with these participants yielded a model which did not converge. However, including site as a fixed effect showed no significant differences between the two interventions, and broadly similar model coefficients, as shown in Table 21.
| Model variable (n = 292) |
Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| Intervention group | ARTISAN only | 0 | – | 0.431 |
| ARTISAN Plus | 0.8 | (−1.2 to 2.7) | ||
| Age group | 39 and under | 0 | – | 0.024 |
| Over 40 | −2.4 | (−4.5 to −0.3) | ||
| Dominant arm injured | Non-dominant | 0 | – | 0.308 |
| Dominant | −1.0 | (−3.0 to 0.9) | ||
| OSIS | Baseline score | 0.4 | (0.3 to 0.5) | < 0.001 |
Imputation of missing data
As the study had a sizeable amount of missing follow-up data, the missing scores for the primary outcome were imputed using MICE in R. The results showed no significant differences in intervention effects. The mean difference was 0.9 (95% CI −0.8 to 2.6; p = 0.309) in favour of the ARTISAN Plus physiotherapy programme.
Pre-specified subgroups: dominant arm
The study pre-specified a subgroup analysis to explore whether there was evidence of differences in the intervention effects between participants who injured their dominant arm and those who injured the non-dominant arm. This was explored by adding an interaction term between the allocation group and dominant arm injury term in the intention-to-treat model (Table 22). The results show that there were no significant differences, nor was the model largely altered. The overall estimates of the treatment effects are shown in Figure 9.
| Model variable (n = 337) |
Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| Intervention group | ARTISAN only | 0 | – | 0.663 |
| ARTISAN Plus | 0.6 | (2.2 to 3.5) | ||
| Age group | 39 and under | 0 | – | 0.007 |
| Over 40 | −2.8 | (−4.8 to −0.8) | ||
| Dominant arm injured | Non-dominant | 0 | 0.918 | |
| Dominant | −0.1 | (−2.9 to 2.6) | ||
| Interaction term | ARTISAN Plus and dominant arm injured | −1.7 | (−5.6 to 2.2) | 0.405 |
| OSIS | Baseline score | 0.4 | (0.3 to 0.5) | < 0.001 |
FIGURE 9.
Interaction plot between arm of injured shoulder and intervention for OSIS.

Pre-specified subgroups: age group
The study also pre-specified a subgroup analysis to explore whether there was evidence of differences in the intervention effects between the two participant age groups. Again, this was explored by adding an interaction term between the allocation and age groups (Table 23 and Figure 10).
| Model variable (n = 337) |
Coefficient | 95% CI | p-value | |
|---|---|---|---|---|
| Intervention group | ARTISAN only | 0 | – | 0.021 |
| ARTISAN Plus | 3.8 | (0.6 to 6.9) | ||
| Age group | 39 and under | 0 | – | 0.484 |
| Over 40 | −1.0 | (−3.8 to 1.8) | ||
| Dominant arm injured | Non-dominant | 0 | – | 0.404 |
| Dominant | −0.8 | (−2.7 to 1.1) | ||
| Interaction term | ARTISAN Plus and age group 40 and over | −3.44 | (−7.4 to 0.5) | 0.091 |
| OSIS | Baseline score | 0.4 | (0.3 to 0.5) | < 0.001 |
FIGURE 10.
Interaction plot between age group and intervention for OSIS.

The interaction model for age group showed that older participants who were in the ARTISAN Plus arm were overall better by 0.64 points compared to younger counterparts in the ARTISAN-only arm. This is not a statistically significant or clinically meaningful effect. The results for the primary outcome split by age group are also presented in Table 24.
| Subgroup | ARTISAN only (n = 240) |
ARTISAN Plus (n = 242) |
Total (n = 482) |
|---|---|---|---|
| Age 39 and under | 37.5 (11.1) | 41.6 (7.7) | 39.5 (9.8) |
| Age 40 and over | 35.6 (10.5) | 36.6 (9.5) | 36.1 (10) |
| Non-dominant arm injured | 37.9 (9.5) | 38.2 (9.9) | 38 (9.6) |
| Dominant arm injured | 34.9 (11.5) | 38.6 (8.6) | 36.8 (10.3) |
| Female | 34.4 (10.5) | 36.7 (9.7) | 35.6 (10.2) |
| Male | 37.4 (10.7) | 39.5 (8.6) | 38.4 (9.8) |
Impact of coronavirus disease
A sensitivity analysis was planned to investigate whether the COVID pandemic had an impact on the ARTISAN trial patients who were recruited during the COVID pandemic where the trial and physiotherapy sessions were paused for a period of time.
The results for the primary outcome (OSIS at 6 months) showed that the participants recruited prior to COVID and those recruited after the trial had restarted had no difference in their outcomes. The mean OSIS score of pre-COVID patients was 36.8 and the mean score for patients after COVID was 37.8, a difference of 1 point in favour of the patients recruited post COVID (95% CI −1.0 to 3.1).
The results after adjusting for the effect of the interventions and the randomisation variables showed a difference of 0.7 points (95% CI −1.3 to 2.6; p = 0.490). As this is not statistically or clinically significant, we conclude that COVID did not impact on the outcome of the trial.
Chapter 4 Health economic results
Missingness of data at each follow-up time point
Table 25 shows missingness of data for both groups per resource use category at each follow-up time point. Data were missing in a non-monotonic pattern, as the proportion of missingness decreased between 6 weeks and 3 months and increased at subsequent time points. The percentage of missing data at 12 months was significantly higher than for other time periods, mainly due to the impact of the COVID pandemic on research. However, there was no significant interaction among participants randomised before and after the UK’s pandemic lockdown (i.e. 23 March 2020). Data were considered missing if a trial participant failed to complete a resource use section at a follow-up point despite all other resource use categories being completed. For example, data were classified as missing if outpatient care visits were not completed at 6-week follow-up period, but all other resource use categories were completed. Imputation was done at the aggregate resource use level at each follow-up time point. Similarly, an EQ-5D-5L response needed to be complete in all five domains at a time point to be classified as non-missing.
| ARTISAN Plus n = 242 |
ARTISAN n = 240 |
Total n = 482 |
|||||
|---|---|---|---|---|---|---|---|
| Number complete | % complete | Number complete | % complete | Number complete | % complete | ||
| Medication | 0–6 weeks | 167 | 69% | 154 | 64% | 321 | 67% |
| 7–13 weeks (3 months) | 175 | 72% | 161 | 67% | 336 | 70% | |
| 14–26 weeks (6 months) | 166 | 69% | 167 | 70% | 333 | 69% | |
| 27–52 weeks (12 months) | 132 | 55% | 123 | 51% | 255 | 53% | |
| Follow-up | 99 | 41% | 90 | 38% | 189 | 39% | |
| Outpatient visits | 0–6 weeks | 168 | 69% | 154 | 64% | 322 | 67% |
| 7–13 weeks (3 months) | 177 | 73% | 162 | 68% | 339 | 70% | |
| 14–26 weeks (6 months) | 169 | 70% | 168 | 70% | 337 | 70% | |
| 27–52 weeks (12 months) | 134 | 55% | 126 | 53% | 260 | 54% | |
| Follow-up | 104 | 43% | 92 | 38% | 196 | 41% | |
| Community care visits | 0–6 weeks | 168 | 69% | 155 | 65% | 323 | 67% |
| 7–13 weeks (3 months) | 176 | 73% | 161 | 67% | 337 | 70% | |
| 14–26 weeks (6 months) | 169 | 70% | 167 | 70% | 336 | 70% | |
| 27–52 weeks (12 months) | 134 | 55% | 126 | 53% | 260 | 54% | |
| Follow-up | 103 | 43% | 91 | 38% | 194 | 40% | |
| Inpatient admission | 0–6 weeks | 168 | 69% | 155 | 65% | 323 | 67% |
| 7–13 weeks (3 months) | 177 | 73% | 162 | 68% | 339 | 70% | |
| 14–26 weeks (6 months) | 169 | 70% | 166 | 69% | 335 | 70% | |
| 27–52 weeks (12 months) | 134 | 55% | 126 | 53% | 260 | 54% | |
| Follow-up | 104 | 43% | 92 | 38% | 196 | 41% | |
| Aids and adaptations | 0–6 weeks | 166 | 69% | 152 | 63% | 318 | 66% |
| 7–13 weeks (3 months) | 174 | 72% | 161 | 67% | 335 | 70% | |
| 14–26 weeks (6 months) | 169 | 70% | 168 | 70% | 337 | 70% | |
| 27–52 weeks (12 months) | 133 | 55% | 125 | 52% | 258 | 54% | |
| Follow-up | 98 | 40% | 92 | 38% | 190 | 39% | |
| PSS | 0–6 weeks | 167 | 69% | 154 | 64% | 321 | 67% |
| 7–13 weeks (3 months) | 177 | 73% | 162 | 68% | 339 | 70% | |
| 14–26 weeks (6 months) | 168 | 69% | 168 | 70% | 336 | 70% | |
| 27–52 weeks (12 months) | 134 | 55% | 126 | 53% | 260 | 54% | |
| Follow-up | 102 | 42% | 93 | 39% | 195 | 40% | |
| Time off work | 0–6 weeks | 173 | 71% | 166 | 69% | 339 | 70% |
| 7–13 weeks (3 months) | 182 | 75% | 170 | 71% | 352 | 73% | |
| 14–26 weeks (6 months) | 174 | 72% | 180 | 75% | 354 | 73% | |
| 27–52 weeks (12 months) | 135 | 56% | 129 | 54% | 264 | 55% | |
| Follow-up | 112 | 46% | 107 | 45% | 219 | 45% | |
| EQ-5D-5L | Baseline | 231 | 95% | 232 | 97% | 463 | 96% |
| 0–6 weeks | 173 | 71% | 166 | 69% | 339 | 70% | |
| 7–13 weeks (3 months) | 182 | 75% | 170 | 71% | 352 | 73% | |
| 14–26 weeks (6 months) | 173 | 71% | 179 | 75% | 352 | 73% | |
| 27–52 weeks (12 months) | 135 | 56% | 129 | 54% | 264 | 55% | |
| Aggregate resource use | 0–6 weeks | 164 | 68% | 150 | 63% | 314 | 65% |
| 7–13 weeks (3 months) | 171 | 71% | 159 | 66% | 330 | 68% | |
| 14–26 weeks (6 months) | 165 | 68% | 164 | 68% | 329 | 68% | |
| 27–52 weeks (12 months) | 132 | 55% | 122 | 51% | 254 | 53% | |
| Follow-up | 91 | 38% | 86 | 36% | 177 | 37% | |
Resource use
The mean number of NHS and PSS resource uses at each follow-up time point are shown in Appendix 4. Resource use was similar comparing groups except for the use of hospital physiotherapy visits.
The ARTISAN Plus group had a higher number of physiotherapy contacts compared to the ARTISAN-only group. However, this difference gradually reduced over time, and use was similar at 12 months. Both the level and difference in physiotherapy visits comparing treatment arms were higher when reported by sites than when recalled by patients.
Economic costs
NHS and PSS costs were similar at each time point, except for outpatient services costs, which were higher in the ARTISAN Plus group, as shown in Table 26. The higher costs in the outpatient services were largely driven by the higher number of physiotherapy contacts. However, outpatient services costs gradually reduced and were similar when comparing groups at the 6-month follow-up point. Total NHS and PSS costs were higher in the ARTISAN Plus group at the 6-week time point (Table 27) but converged over subsequent time points, as shown in Figure 11.
| ARTISAN Plus | ARTISAN | ARTISAN Plus − ARTISAN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (£) | SD | n | Mean (£) | SD | Mean difference (£) | 95% CI | p-value | |
| 6 weeks | |||||||||
| Medication | 167 | 0.73 | 2.57 | 154 | 1.07 | 4.09 | −0.34 | −0.1.10 to 0.42 | 0.38 |
| Outpatient services | 168 | 152.30 | 182.48 | 154 | 106.12 | 199.48 | 46.17 | 4.13 to 88.22 | 0.03 |
| Community care | 168 | 15.48 | 53.33 | 155 | 11.35 | 46.18 | 4.13 | −6.77 to 15.03 | 0.46 |
| Inpatient admission | 168 | 24.54 | 226.98 | 155 | 2.10 | 16.83 | 22.44 | −12.23 to 57.12 | 0.20 |
| Aids | 166 | 1.25 | 4.48 | 152 | 2.73 | 10.89 | −1.48 | −3.35 to 0.39 | 0.12 |
| PSS | 167 | 0.45 | 5.83 | 154 | 0.00 | 0.00 | 0.45 | −0.44 to 1.34 | 0.32 |
| 3 months | |||||||||
| Medication | 175 | 0.70 | 2.99 | 161 | 0.56 | 2.10 | 0.14 | −0.41 to 0.69 | 0.61 |
| Outpatient services | 177 | 80.43 | 153.84 | 162 | 52.17 | 162.41 | 28.26 | −5.62 to 62.13 | 0.10 |
| Community care | 176 | 9.25 | 37.43 | 161 | 7.74 | 29.68 | 1.51 | −5.70 to 8.71 | 0.68 |
| Inpatient admission | 177 | 14.51 | 179.57 | 162 | 10.74 | 136.71 | 3.77 | −30.16 to 37.71 | 0.83 |
| Aids | 174 | 1.05 | 5.62 | 161 | 0.21 | 1.99 | 0.84 | −0.05 to 1.74 | 0.07 |
| PSS | 177 | 0.00 | 0.00 | 162 | 0.20 | 2.51 | −0.20 | −0.59 to 0.19 | 0.32 |
| 6 months | |||||||||
| Medication | 169 | 53.06 | 110.96 | 167 | 0.40 | 1.67 | 1.22 | −1.10 to 3.54 | 0.30 |
| Outpatient services | 169 | 53.06 | 110.96 | 168 | 52.44 | 144.62 | 0.62 | −27.02 to 28.26 | 0.97 |
| Community care | 169 | 7.30 | 34.33 | 167 | 12.57 | 72.78 | −5.27 | −17.53 to 6.98 | 0.40 |
| Inpatient admission | 169 | 38.49 | 290.04 | 166 | 39.92 | 292.67 | −1.43 | −64.06 to 61.20 | 0.96 |
| Aids | 169 | 1.01 | 6.38 | 168 | 0.36 | 1.92 | 0.65 | −0.36 to 1.66 | 0.21 |
| PSS | 168 | 0.00 | 0.00 | 168 | 0.00 | 0.00 | 0.00 | . | |
| 12 months | |||||||||
| Medication | 132 | 0.70 | 4.48 | 123 | 0.50 | 2.56 | 0.19 | −0.70 to 1.08 | 0.67 |
| Outpatient services | 134 | 23.31 | 81.46 | 126 | 74.68 | 326.81 | −51.37 | −110.59 to 7.85 | 0.09 |
| Community care | 134 | 2.32 | 17.53 | 126 | 31.51 | 160.51 | −29.20 | −57.65 to 0.75 | 0.04 |
| Inpatient admission | 134 | 30.77 | 253.97 | 126 | 46.81 | 304.40 | −16.05 | −84.75 to 52.66 | 0.65 |
| Aids | 133 | 16.54 | 189.72 | 125 | 0.36 | 2.00 | 16.55 | −16.37 to 48.72 | 0.33 |
| PSS | 134 | 0.34 | 3.97 | 126 | 0.37 | 4.10 | −0.02 | −1.01 to 0.97 | 0.97 |
| Follow-up (12 months) | |||||||||
| Medication | 99 | 2.07 | 6.24 | 90 | 1.33 | 4.27 | 0.74 | −0.78 to 2.26 | 0.34 |
| Outpatient services | 104 | 340.40 | 399.15 | 92 | 322.37 | 685.76 | 18.02 | −143.10 to 179.715 | 0.83 |
| Community care | 103 | 32.47 | 85.70 | 91 | 59.72 | 195.69 | −27.25 | −71.71 to 16.68 | 0.22 |
| Inpatient admission | 104 | 87.25 | 432.91 | 92 | 130.68 | 556.92 | −43.42 | −185.4 to 98.55 | 0.55 |
| Aids | 98 | 2.25 | 7.92 | 92 | 4.35 | 14.84 | −2.10 | −5.55 to 1.34 | 0.23 |
| PSS | 102 | 0.74 | 7.46 | 93 | 0.84 | 5.78 | −0.10 | −1.98 to 1.78 | 0.92 |
| ARTISAN Plus | ARTISAN | ARTISAN Plus − ARTISAN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (£) | SD | n | Mean (£) | SD | Mean difference (£) | 95% CI | p-value | |
| 0–6 weeks | 164 | 195.57 | 324.84 | 150 | 121.31 | 209.75 | 74.27 | 14.02 to 134.51 | 0.02 |
|
7–13 weeks
(3 months) |
171 | 103.35 | 257.56 | 159 | 72.90 | 221.57 | 30.45 | −21.47 to 82.38 | 0.25 |
|
14–26 weeks
(6 months) |
165 | 102.34 | 340.60 | 164 | 106.12 | 371.11 | −3.78 | −81.05 to 73.50 | 0.92 |
|
25–52 weeks
(12 months) |
132 | 74.95 | 385.00 | 122 | 159.28 | 522.10 | −84.33 | −198.52 to 29.86 | 0.15 |
FIGURE 11.
Unadjusted NHS and PSS costs and EQ-5D-5L utility estimates at each time point per trial group.
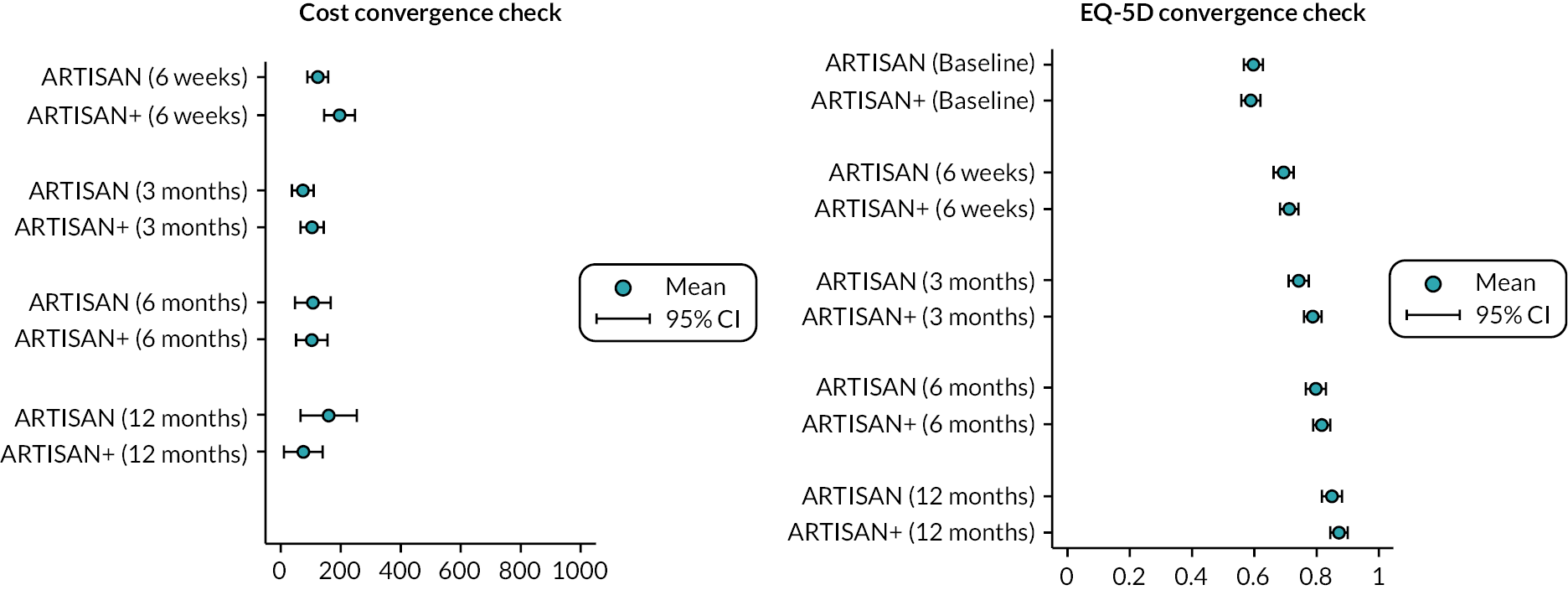
Health outcomes
Table 28 summarises the unadjusted EQ-5D-5L utility estimates and between-group mean differences at each time point and across the follow-up period for cases with complete data. There was a statistically significant increase in utility for the ARTISAN Plus group at the 3-month follow-up time point. Utility estimates were similar at subsequent time points and converged over time (Figure 11).
| ARTISAN Plus | ARTISAN | ARTISAN Plus − ARTISAN | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Mean difference | 95% CI | p-value | |
| Baseline | 231 | 0.588 | 0.212 | 232 | 0.595 | 0.233 | −0.007 | −0.048 to 0.034 | 0.74 |
| 6 weeks | 173 | 0.711 | 0.189 | 166 | 0.692 | 0.201 | 0.019 | −0.023 to 0.061 | 0.37 |
| 3 months | 182 | 0.787 | 0.179 | 170 | 0.741 | 0.215 | 0.046 | 0.005 to 0.088 | 0.03 |
| 6 months | 173 | 0.815 | 0.183 | 179 | 0.797 | 0.217 | 0.018 | −0.024 to 0.060 | 0.41 |
| 12 months | 135 | 0.870 | 0.160 | 129 | 0.848 | 0.169 | 0.022 | −0.018 to 0.062 | 0.27 |
Cost-effectiveness analysis
Cost-effectiveness results are presented in Table 29 with ARTISAN as the reference treatment and ARTISAN Plus as the comparator. Analytic time horizon covers the period from randomisation to 6 months post randomisation. Point estimates of the incremental costs and QALYs are shown with 95% CIs. The probability of ARTISAN Plus being cost-effective is presented at three willingness-to-pay thresholds (£15,000, £20,000 and £30,000). The mean NMB is similarly presented at these thresholds.
| Incremental cost (95% CI) | Incremental QALY (95% CI) | ICER (£/QALY) | P1 | P2 | P3 | NMB1 (95% CI) | NMB2 (95% CI) | NMB3 (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Base casea,b | 64 (−61 to 191) | 0.0190 (−0.0005 to 0.0375) | 3373 | 0.896 | 0.925 | 0.946 | 221 (−125 to 556) | 316 (−115 to 740) | 506 (−111 to 1102) |
| Base casea,c | 69 (−88 to 226) | 0.0157 (−0.0061 to 0.0374) | 4409 | 0.795 | 0.832 | 0.867 | 166 (−229 to 561) | 244 (−253 to 741) | 401 (−306 to 1107) |
| Complete cased | −61 (−357 to 201) | 0.0189 (−0.0157 to 0.0546) | ARTISAN dominated | 0.849 | 0.855 | 0.858 | 352 (−299 to 1005) | 447 (−364 to 1257) | 638 (−521 to 1796) |
| Societal perspectivea | 422 (−240 to 1099) | 0.0191 (0.0002 to 0.0373) | 22,073 | 0.367 | 0.470 | 0.638 | −140 (−934 to 612) | −45 (−892 to 780) | 145 (−846 to 1060) |
| Using 6-month dataa,b | 120 (33 to 207) | 0.0131 (−0.0032 to 0.0293) | 9163 | 0.704 | 0.779 | 0.845 | 76 (−221 to 354) | 142 (−236 to 493) | 273 (−263 to 777) |
| Using site-reported physio dataa | 95 (−41 to 231) | 0.0188 (−0.0041 to 0.0417) | 5084 | 0.842 | 0.889 | 0.921 | 176 (−174 to 513) | 267 (−169 to 692) | 449 (−170 to 1043) |
| Including cost of missed appointmentsa,c | 104 (−31 to 240) | 0.0176 (−0.006 to 0.0415) | 5932 | 0.794 | 0.837 | 0.873 | 160 (−222 to 542) | 248 (−248 to 743) | 424 (−304 to 1151) |
Base-case analysis
Patients in the ARTISAN Plus group had a small, non-significant increase in quality of life of 0.019 QALYs (95% CI −0.0005 to 0.0375) at a small, significant increased cost of £64 (95% CI 33 to 207) over the follow-up period. The ICER for the base-case analysis of £3373/QALY suggests that ARTISAN Plus is a cost-effective alternative: the probability of ARTISAN Plus being cost-effective at a willingness-to-pay threshold of £30,000 is 0.946.
Figure 12 shows a graphical representation of the cost-effectiveness estimates on the ICER plane, the probability that ARTISAN Plus is cost-effective at different cost-effectiveness thresholds and the NMB of ARTISAN Plus at different cost-effectiveness thresholds (£0 to £100,000/QALY).
FIGURE 12.
ARTISAN Plus vs. ARTISAN: cost-effectiveness estimates on the ICER plane.
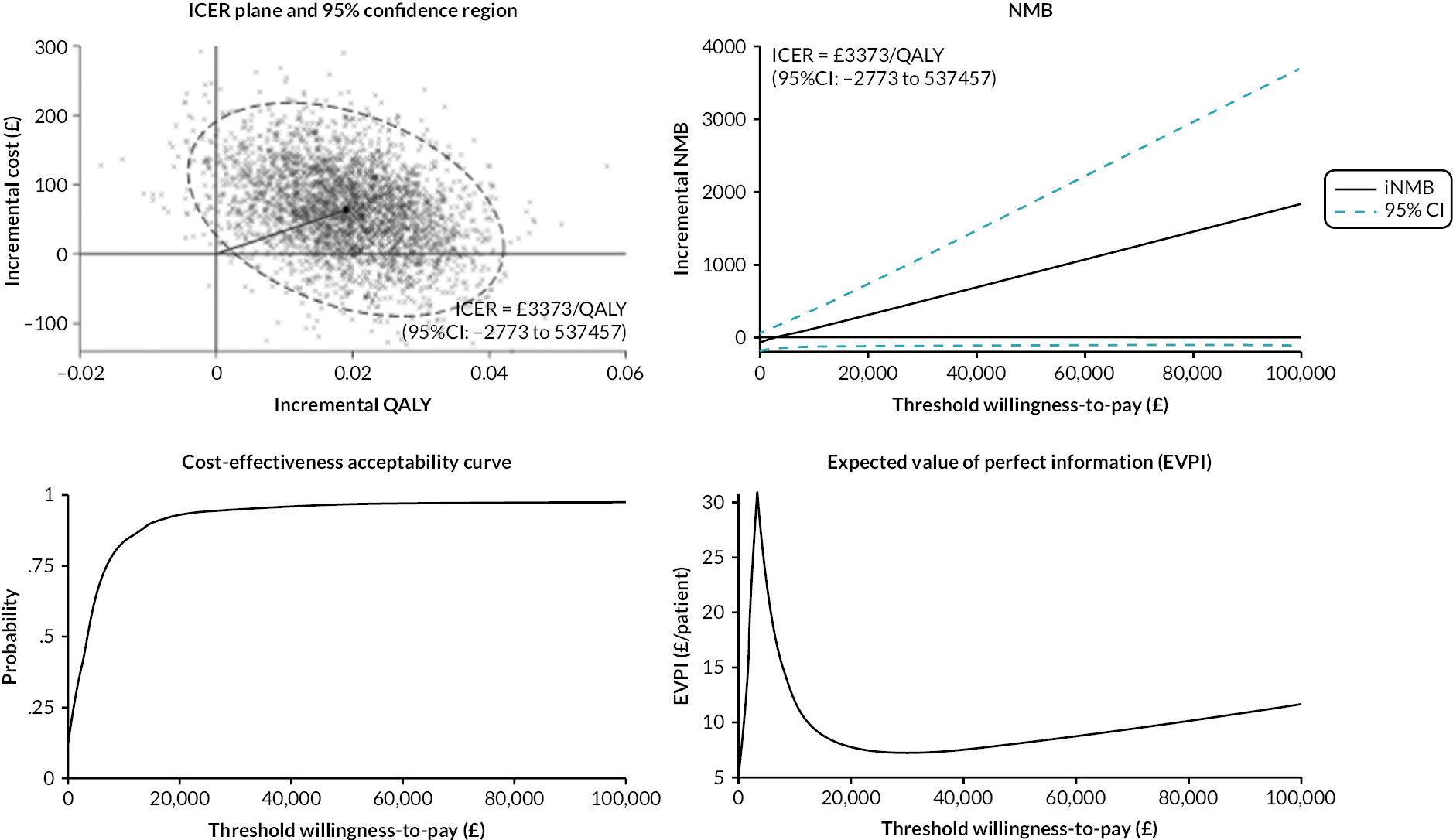
At a cost-effectiveness threshold of £30,000/QALY, the expected value or information38 (EVPI) is £7 per participant. Given an incidence of 8.2–23.9 per 100,000 people per year in the UK, the monetary value of removing all uncertainty from the cost-effectiveness results ranges from £39,032 to £113,764 per annum. However, the technological lifespan of the evidence for ARTISAN Plus (in years) is uncertain and so the total EVPI has not been estimated.
Sensitivity analysis
Incremental costs and QALYs were compared under different analytic scenarios. These sensitivity analyses support the base-case findings. In the complete case analysis, the ARTISAN Plus group had a reduction in costs of £61 (95% CI −357 to 201) and an increase in QALYs of 0.0189 (95% CI −0.0157 to 0.0546). An ICER of −£3245/QALY indicates dominance of the ARTISAN Plus group over the ARTISAN-alone group. While the reductions in costs and QALYs were not statistically significant, the probability of ARTISAN Plus being cost-effective at a £30,000 per QALY threshold is 0.846. For the societal perspective, the ICER increased to £22,073/QALY. ARTISAN Plus had a non-significant increase in costs of £422 (95% CI −240 to 1099) and a significant increase in quality of life of 0.0191 (95% CI 0.0002 to 0.0373). The probability of ARTISAN Plus being cost-effective at a £30,000/QALY threshold is 0.638. Limiting the analysis time window to 6 months’ follow-up, the ICER increased to £9163/QALY. The ARTISAN Plus group had a non-significant increase in quality of life of 0.013 QALYs (95% CI −0.0032 to 0.0293) at an increased cost of £120 (95% CI 33 to 207). The probability of ARTISAN Plus being cost-effective at a £30,000/QALY threshold is 0.845.
Findings are similar when site-reported physiotherapy data were used in place of patient-reported data. The ARTISAN Plus group had a non-significant increased cost of £95 (95% CI −41 to 231) and QALYs of 0.0188 (95% CI 0.0041 to 0.0417). The ICER increased to £5084/QALY. The probability of ARTISAN Plus being cost-effective at a £30,000/QALY threshold is 0.921. Including the costs of missed appointments in site-reported physiotherapy data, the ARTISAN Plus group had increased costs of £104 (95% CI −31 to 240) and QALYs of 0.0176 (95% CI −0.006 to 0.0415). The ICER increased to £5932 and the probability of ARTISAN Plus being cost-effective at a £30,000/QALY threshold is 0.873. Findings were also similar when the EQ-5D-5L responses were valued using the mapping algorithm developed by Alava et al. The ARTISAN Plus group had non-significant incremental costs of £69 (95% CI −88 to 226) and incremental QALYs of 0.0157 (95% CI −0.0061 to 0.0374). The probability of ARTISAN Plus being cost-effective at a threshold of £30,000/QALY is 0.867.
It is notable (Tables 27 and 28) that ARTISAN Plus is generally characterised by small and imprecise increases in cost and health (measured as QALYs). Examining the NMB values at any threshold, it is apparent that all sensitivity analyses are statistically similar to the base case.
Longer-term economic modelling
The protocol allowed for decision-analytic modelling to estimate longer-term cost-effectiveness if costs and outcomes do not converge over the analytic time horizon. As shown in Figure 11, Tables 28 and 29, incremental costs and QALYs converged at between 3 and 6 months. Due to their convergence within the follow-up time horizon, longer-term extrapolation of cost-effectiveness is unlikely to provide further insight or understanding.
Chapter 5 Qualitative results
Sample
A total of 102 participants, who had expressed an interest in involvement in the qualitative study at the time of consenting to be in the ARTISAN trial, were contacted. Of these, 17 declined to participate and 54 did not respond.
A total of 31 participants consented and were interviewed from both arms of the trial, ARTISAN and ARTISAN Plus (Table 30). The participants were from 17 different trial sites in the UK; 16 participants were allocated to the ARTISAN arm, and 15 participants were allocated to the ARTISAN Plus arm. Most of the participants in the ARTISAN Plus arm were male (11 out of 15), while there was a balance between participants in the ARTISAN arm (8 male and 8 female). The interview participants’ median ages were 51 years (IQR 36) and 65 years (IQR 24.5) for the ARTISAN and ARTISAN Plus arms, respectively. Five participants in the ARTISAN arm and four in the ARTISAN Plus arm stated they were involved in sporting activities that needed high levels of physical activity.
| ARTISAN participants | ARTISAN Plus participants | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Aa (ID) |
Age | Gender | Occupation | High-level contact sports? | APb (ID) |
Age | Gender | Occupation | High-level contact sports? |
| 2 | 56 | F | Shopkeeper | No | 1 | 27 | M | Healthcare worker | Yes (rugby, tennis) |
| 6 | 33 | F | Desk job | No | 3 | 65 | M | Retired | No |
| 8 | 49 | M | Desk job | No | 5 | 71 | F | Retired | No |
| 9 | 24 | M | Desk job | Yes (football) | 7 | 37 | M | Police officer | Yes (squash, jujitsu) |
| 11 | 31 | M | Desk job | Yes (football) | 10 | 57 | F | Retired | No |
| 14 | 74 | F | Retired | No | 12 | 62 | F | Bartender | No |
| 16 | 19 | M | Desk job | Yes (football) | 13 | 59 | M | Distance learning co-ordinator | No |
| 17 | 83 | M | Unknown | No | 15 | 33 | M | No | Yes (rock climbing) |
| 18 | 77 | F | Runs a farm B&B | No | 20 | 81 | M | Retired | No |
| 19 | 48 | M | Unknown | No | 22 | 67 | M | Retired | No |
| 21 | 65 | F | Cleaner | No | 24 | 74 | M | Retired | No |
| 23 | 53 | F | Speech and language therapist | Yes (paddle boarding, gym, obstacle course) | 25 | 72 | M | Retired | No |
| 26 | 68 | F | Retired | No | 27 | 72 | M | Retired | No |
| 29 | 26 | M | Site builder | No | 28 | 36 | M | Office worker | Yes (gym, cycling, running) |
| 31 | 23 | M | Student | Yes (competitive rowing, WLc and S+C4d) | 30 | 70 | F | Retired | No |
| 32 | 59 | F | Office worker | No | |||||
| Median (IQR) | 51 (36) | 8 (M) | Median (IQR) | 65 (24.5) | 11 (M) | ||||
| 8 (F) | 4 (F) | ||||||||
Our findings illustrate participants’ experiences of receiving and interacting with rehabilitation services and participating in the ARTISAN trial.
Four dominant and inter-related topics were constructed based on the interview questions: (1) feelings about their shoulder rehabilitation outcome; (2) judgement of ARTISAN rehabilitation materials; (3) assessment of shoulder rehabilitation services provision; and (4) experiences of involvement in ARTISAN. Themes and subthemes emerged from the data in each of the four inter-related topics.
These themes and their subthemes are reported below, comparing and contrasting responses from both the experimental arm (ARTISAN Plus) and those from the control arm (ARTISAN) of the trial. Quotations are used as exemplars of themes, with each quote linked to a particular participant denoted by the arm of the trial they were involved in (AP = ARTISAN Plus arm; A = ARTISAN-only arm) followed by their ID number, their gender [male (m) or female (f)] and age in years (e.g. ‘A11, m, 31’).
Results
Topic 1: feelings about their shoulder rehabilitation outcome
This topic breaks down into a number of themes/subthemes and provides an insight into the assessment of participants’ feelings about their recovery, including movement and use of their shoulder and being able to get back to their previous activities or not.
Shoulder status (how they feel their shoulder is post rehabilitation)
Participants who received ARTISAN Plus reported improvement in returning to their normal life and working activities. Almost all of them expressed the status of their shoulder as ‘better’:
I feel good now, and then I can … I’m back to doing all the jobs I could do before.
AP13, m, 59
I think I’m back at 100 now.
AP25, m, 72
Probably getting up to 100%, I guess.
AP27, m, 72
A few did still feel that they still had a little way to go:
I would say yeah, probably 70% now. Yeah, around about that. Yes, yeah.
AP3, m, 65
Like, maybe more 60 than 50 in terms of movements.
AP28, m, 36
This compared to the ARTISAN arm, where there were fewer participants who believed their shoulder was at a good level of movement:
… had almost complete range of movement.
A21, f, 65
It’s absolutely fine …
A26, f, 68
Satisfaction
Interestingly, most participants who had received ARTISAN Plus apparently liked to recommend it to the others with the same problem because they were very satisfied with the result from having multi-physiotherapy sessions.
Yeah, I would recommend, I would definitely recommend anybody to go do that what I did, yeah, definitely. Definitely, yeah.
AP12, f, 62
The other source of satisfaction from the result for most of them was receiving positive reinforcement from the physiotherapist about outcomes and being told that they were doing the exercise correctly.
Only the reassurance that everything was going fine.
AP22, m, 67
So, I suppose I needed those sorts of reassurances before I got into heavier physiotherapy.
AP12, f, 62
On the other hand, most people in the ARTISAN arm have found the exercises as the most helpful part of rehabilitation to get back their abilities.
Yeah. I would … I would tell them, you need that physio exercise, yeah.
A19, m, 48
Setting short-term goals and identifying milestones was noted as helpful by one participant who received more physio sessions, helping them have a realistic view of their shoulder progression.
the goals were … because I think it kind of helps you set a realistic view of you know, going on to next session that if you could do this.
A19, m, 48
Topic 2: participants’ judgement about ARTISAN rehabilitation materials
All of the participants had the opportunity to use the provided rehabilitation training materials in addition to the support given by the physiotherapist in the session they all had with them. These materials seemed to be more important for the ARTISAN participants as they generally only had the one in-person session with the physiotherapist. However, it is also important to know to what extent the participants have used the materials and what their experience is in using them.
Material usefulness
Almost all participants in the ARTISAN arm reported using the booklets, while only half of the ARTISAN Plus arm reported using them. In addition, none of the ARTISAN Plus participants reported having used the videos and website, compared to around half of those in the ARTISAN arm. The ARTISAN Plus participants who had not used the materials believed that they had gained enough from their in-person visits with the physiotherapist; therefore, they did not feel the need to refer to the training materials:
Just after the first [physio] session, I had the chance to have a very quick look at the booklets, so, it was enough exercise and guidance from physiotherapist over the sessions … didn’t feel need them!
AP7, m, 37
I had time, was off from work, almost at home, and was keen on going through the booklets, but only booklets, no website, and videos, so I think it was quite helpful.
AP15, m, 33
I got those stuff [training materials] … but yeah! I don’t remember using them! Yeah, didn’t look at them at all.
A18, f, 72
[I] got the booklet and everything, I went on the website, saw the videos and, yeah.
A32, F, 59
Problems with study materials provided
The main reported source of difficulty in using the materials provided was in the ARTISAN arm, where there were problems reported accessing the video and internet-based materials. A few of the participants from ARTISAN also reported some problems with the booklets provided. These problems were very low in the ARTISAN Plus arm.
There was but I don’t have the internet at my house …
A21, f, 65
I couldn’t quite find … I couldn’t follow the instructions on those. And I couldn’t log into the website. So, I couldn’t get any additional information. I had a booklet. I didn’t find them all easy to follow. There was a couple … I mean they did run through it with me, the fracture clinic, but they more demonstrated it I would say. But I didn’t find everything in the booklet that easy to follow. I did with the hospital physiotherapist, I actually had to do it because my arm was in better shape.
A32, f, 59
I think there was one [picture] that wasn’t quite clear, but I checked it out with her the next time I went.
AP22, m, 67
I suppose the booklet, umm, is in some ways misinterpreted because it is not in 3D, you know, so surely for a picture … a picture does not always give you the right angle or the right, umm, motion to use
AP5, f, 71
Material comprehensiveness and consistency
The content of the materials was consistent with what had been provided in face-to-face rehabilitation sessions, in the view of most participants. The materials were also reported to be comprehensive and to meet the needs of the participants in both arms.
From the ARTISAN participants:
It was very good because you get … further on, you’ve sort of got more exercises to do. So, you really need that leaflet to show you the different exercises you need to do.
A26, f, 68
Yeah, well, the exercises we did were the exercises in the pamphlet that I was given which I said to you, and, hmmm, the physio, she did them all with me … two or three times.
A2, m, 56
From the ARTISAN Plus participants:
It was just giving me alternatives to do. It’s the same kind of stress on the shoulder, but it was a different exercise. But more or less, there were those like, you know…
AP28, m, 36
She [the physiotherapist] did. She did go through the booklet with it and marked certain things, you know, like she wrote in the book the few exercises that I should be doing
AP1, m, 27
Topic 3: participants’ assessment of rehabilitation service provision
This topic reflects participants’ feelings about the provision of physiotherapy/rehabilitation in terms of the accessibility to the providing rehab centres, physiotherapist performance and then the form of sessions provided. This part of the participants’ experiences reflects insights about the obstacles that can influence the participants getting to the centres, and to what extent participants believe the physiotherapists have been engaged in their rehabilitation/treatment process. Finally, it has an implication about the impact of participating in group physiotherapy sessions as a possible form of rehabilitation provision.
Accessibility to the rehabilitation services
Several of the participants in ARTISAN Plus experienced some physical difficulties in attending the rehabilitation sessions, such as problems with driving, finding a car parking space and a considerable distance to the physio clinic or hospital from their home.
Taking into account quite a long distance to travel and the roads aren’t that great, I have to be taken in by my wife – obviously I was not driving.
AP3, m, 62
Sort of two hours right around the hospital so if I wasn’t close it would be more difficult
AP1, m, 27
Most of the people in this group stated that there were no difficulties to attend the physio sessions. They also mentioned that the health centres were flexible in providing suitable slots for appointments. In addition, there were positive comments about public transport and employers who were supportive.
Nothing whatsoever because I was able to get a time slot that suited me, which was earlyish morning. And there was no delay, you know. There was … it was all perfectly right for me.
AP27, m, 72
No, no. Well, they were quite supportive at work.
AP28, m, 36
No, you can get a bus to the hospital.
AP15, m, 33
Most of the participants in the ARTISAN group reported not having experienced any obstacles or obstacle to accessing rehabilitation services:
I didn’t [have trouble to attend], but I walked (there).
A23, f, 53
That’s fine.
A26, f, 68
However, some obstacles remained for a small number of ARTISAN participants:
I couldn’t drive. I got some free transport from the transport people. And then … and that stopped when I was told that … they had to tell me I must get on a bus because they have to keep it for people that are more seriously ill than me. And I was, therefore, trying to sort that out, and eventually, I got a friend to take me, across the road … (After requiring additional physio sessions for frozen shoulder.)
A21, f, 65
Parking. It’s terrible up there.
A29, m, 26
The physiotherapists’ performance
The physiotherapist was considered a valuable source of motivation in improving the injured shoulder by participants in both arms of the trial. All participants expressed that their physiotherapists went through the exercises and correct movement in detail as much as they could. Giving feedback and setting short- and long-term goals by physiotherapists had a positive impact on the participants’ feelings, especially for ARTISAN Plus:
It gave us confidence.
AP10, f, 25
I don’t know who he [the physiotherapist] was, but it was a good job.
AP13, m, 59
She [the physiotherapist] pretty much answered the questions anyway as we went along, you know.
AP20, m, 81
The participants in the ARTISAN arm also found an excellent experience with their physiotherapists:
She [the physiotherapist] was a really, really, really good physio. Very straightforward, practical, on to work, which I really like.
A19, m, 48
He’s … he [the physiotherapist] was very, very positive attitude so that’s about all really. No, they’re all very positive about what to do next. (Participant who was given additional physio session by the clinician.)
A21, f, 65
Single, group and multiple physio sessions
Almost all participants in the ARTISAN group found the physio session helpful and informative; a few expressed that they may have preferred having multiple physio sessions. A number noted that they felt they benefited from a single session:
Yes, was helpful. He [the physiotherapist] gave me a booklet, he gave me lots of things and did say that if I had problems, then I was to go back.
A14, f, 74
It was informative. They gave me a few exercises to do, and they scanned through all different movements to see where I was sort of suffering with it.
A29, m, 26
One ARTISAN participant noted that she experienced group-based physio sessions (outside of that provided by the trial). She was keen on attending these sessions because she thought it was an excellent opportunity to get peer support, to understand the limitations and develop coping strategies for dealing with issues that may arise:
Although everybody is different, it was nice to talk to other people and other people have different things, but it was still nice to speak to someone in the same situation as you.
A2, f, 56
Others in the ARTISAN arm had sought out additional treatments or programmes apart from the training materials to increase their chance of returning to their previous sports activities:
I’m lucky enough that the club that I play rugby for has a physio that they employ as well, he recommended the [name of a local programme], so I went along, did some of those exercises on that, and that was good, got to all progressions that I was trying to get back to, and the exercise that was provided from like the ARTISAN from the hospital was kind of due to get me back to general life, it probably would’ve been perfectly fine, but yeah, I want to go back to rugby so I know I needed to get my shoulder back to like full strength.
A9, m, 24
Almost to the year, it was almost to the year and my shoulder was absolutely killing. I couldn’t drive. It almost felt like, well, I didn’t know what was wrong with it. It felt like a frozen shoulder, and I ended up in the … I ended up going to a chiropractor.
A23, f, 53
Most participants with ARTISAN Plus multi-physio sessions pointed out that they had enough chances to visit their physiotherapist to assess their shoulder movement, achieve their goals and receive instructions on doing the exercises correctly:
If I was doing something wrong, they could correct me.
AP15, m, 33
The most emphasised point in this group was getting reassurance and feedback from the physiotherapist:
Well, I suppose it’s … the fact that you can do a certain exercise over a period of time, then you get some feedback with a consultant to tell you how you’re doing.
AP13, m, 59
Several of them expressed that communication with a professional member such as a physiotherapist psychologically impacts the rehabilitation journey:
It certainly helped mentally. It gave you support. You felt that somebody was interested in trying to help, which was as much benefit as the physical side of it.
AP20, m, 81
A participant from the ARTISAN arm who received additional physiotherapy sessions highlighted again the reassurance of having the contact with a therapist:
The other benefit really was the reassurance because I think one of the things that you did … that you worry about is, you know, it’s going to dislocate again.
A19, m, 48
Topic 4: ARTISAN trial processes from the participants’ viewpoint
This topic addresses the experiences of participants’ involvement in the ARTISAN trial, exploring their experiences of trial procedures and materials.
Questionnaires
Comments on the questionnaire were mixed, with some participants noting that they were clear and easy to complete and others noting problems such as confusing terminology, difficulty in understanding, and from some there were recall issues related to remembering their status in a few previous weeks for both arms:
That was fine.
AP1, m, 27
It was straightforward.
AP12, f, 62
This was … some of it was a bit confusing, some of the terminologies around the, before your accident and after your accident, a little bit unclear.
A19, m, 48
I think that there is a problem in how the questions are asked because it’s not very clear at which timescale they have directed the questions. I think there is a problem there.
AP28, m, 36
I wasn’t quite sure, you know, like, it was saying it would say, like, in the last … and I think sometimes because your pain goes up and down, doesn’t it.
A23, f, 53
Comments to improve the study
A number of the younger interviewees from the ARTISAN Plus arm liked the idea of virtual rehabilitation sessions:
I think [virtual physio sessions] would be a lot less time-consuming for both sides… and it’s just reassuring that you know that what you are actually doing, you are doing it correctly.
AP30, f, 70
Some participants who had a single session raised a few remarks regarding home-based physio sessions and developing an online format of questionnaires to improve the process because posting them needs more time:
I know, I mean, something I appreciate that if people can come up with even with the tiniest suggestion of what could have been easier for them and that is the only thing that could have made it easier for me was having it online rather than, well, it wasn’t inconvenient on paper, but it was just that it would have been a bit quicker
A6, f, 33
Participants’ satisfaction with the ARTISAN trial
The study’s introduction and familiarisation with the process have been identified as a factor in the participants’ satisfaction. Almost all participants appreciated a chance to ask for details about the study from ARTISAN members since the participants stated:
She was very clear.
AP10, m, 57
I was able to ask questions, but I didn’t need to.
A26, f, 68
Participants in the ARTISAN arm were aware of having the right to go back to the usual care as an interviewee detailed
… you know, it’s not a punishment to … to not get help if you feel like you don’t … if … even though you’ve been randomised to one session only, you’re more than welcome to do so if you feel like you need more than that. That … is that … if that’s OK.
A29, m, 26
Participants’ motivation
All participants, regardless of their background and allocated arm, expressed that the most important motivating factor to attend the study was the excellent sense of the applicability of the study for helping injured people in the future:
Well, I think if it can help other people experiencing that thing.
AP15, m, 33
Summary
The overall aim of the interview study was to explore the participants’ experience of receiving the trial treatments and facilitators and obstacles to adhering to them, and this has been achieved. We have interviewed a representative sample of trial participants from both treatment arms of the trial who have openly shared their experiences. These experiences include participants’ feelings about their recovery after their rehabilitation journey.
More of the ARTISAN Plus participants reported having had a better experience, based on their expectations after the rehabilitation sessions, compared to the ARTISAN participants. It appears that the main route of the discrepancy in this regard relates to the levels of physical activities that the participant wanted to return to (e.g. sports). Participants in the ARTISAN group did seem to want more physiotherapy sessions. However, it is important to know that, even among those who do engage in sporting activities, in the ARTISAN Plus group, some are still reporting that they are not reaching their pre-injury levels of activity.
A number of the younger participants in the ARTISAN group have sought out additional treatment options (e.g. exercise programmes, chiropractic sessions) in addition to what was provided to increase their chance of returning to pre-injury levels of activities. ARTISAN Plus participants, it seems, were less likely to go for additional treatments outside of those provided.
The interviews give us an insight into the participants’ motivations and experiences in their rehabilitation journey. Involving them in the improvement journey seems to have a positive influence on their sense of improvement. Regardless of the participant’s allocated group, gender, age and history of doing exercise, the physiotherapist’s advice and efforts to give reassurance appear to play a crucial role in the participants’ mindset and confidence.
Alongside the sense of reassurance, communicating with the participants and giving them some ideas about their rehabilitation journey ahead would be motivating for the participants to be more involved in the treatment process, subsequently giving a better experience for the treatment outcomes. This is more obvious among participants of older ages. This also could be as easy as telling the participants about the physiotherapy session length, durations and expectations from each single and whole session.
Those participants who have consulted with the materials have found them helpful regardless of their background factors (sex, age and history of doing sport) and allocated arms. Both groups of participants have used the booklets and expressed good experiences with them.
The participants raised some minor points regarding the difficulty with the booklets and that the online materials were sometimes difficult to access. However, the participants in the ARTISAN Plus group seem to have had a better chance to resolve this kind of problem because of having more contact with their therapist. Participants in both groups report using the videos and website less than the booklets. This is more obvious among the senior groups and females. Lack of internet access was an issue for some. However, for those who have viewed the video, it has given them a better idea of how to do the exercises correctly. The latter is more apparent among those participants at younger ages and with less severe injuries.
In terms of the trial process, almost all participants, across both groups, have had a positive experience regarding the staff communications, responsiveness and explaining the ambiguities. Some do report that paper-based questionnaires are not very convenient to deal with due to their injuries and the volume of the questions. Younger participants seem to have found the questionnaires more convenient and easier to do regardless of their group allocation.
While it is a strength of this qualitative study that it includes participants representing both arms of the trial from multiple sites across the UK, there are a number of limitations. This was a one-off single interview undertaken with consenting participants 12 months after they received their treatment. Recall at these times was an issue with some participants. While the interviewers mitigated this by restructuring questions and using prompts, it may have impacted on the quality of the data. During the analysis of the data, it became very clear that the qualitative sample as a whole were responding very positively. In addition, in both groups there were those who liked things and those who did not. We can only interview those who are willing to participate, and it may be that the sample who agreed were very positive overall about the trial. Therefore, we took a very cautious approach to reporting the findings descriptively, comparing and contrasting outcomes within and across the groups.
Chapter 6 Discussion
Aim and overview of trial findings
For the primary outcome, there was no evidence of a difference in the OSIS at the primary 6-month time point between participants randomised to receive advice only and those who received one session of advice and the offer of additional physiotherapy. At all time points the direction of change favoured a programme of physiotherapy; however, the 95% CI at each time point excluded the target difference of 4 points. There were no statistically significant differences in the QuickDASH or consistent differences in the EQ-5D-5L or complication secondary outcomes. Secondary unadjusted and per-protocol analyses, and a sensitivity analysis accounting for missingness, were not materially different.
Predefined subgroup analyses were undertaken to assess whether there was evidence that the intervention effect differed between age group (≤ 39 years old and ≥ 40 years old) or arm dominance. Our predefined subgroup analyses showed no evidence of clinically relevant effects from either age or arm dominance.
The probability of being cost-effective at a willingness-to-pay threshold of £30,000 for advice and the offer of additional physiotherapy was 0.946. Sensitivity analyses, including complete cases only and including broader societal costs, provided similar findings.
Qualitative experiences of involvement in ARTISAN were generally positive across both arms. There was a trend towards a more positive outcome reported by those in the ARTISAN Plus arm of the trial. There were some areas where there were differences related to age and participants’ requirements to return to sporting activities.
Generalisability
The ARTISAN trial was a pragmatic, multicentre RCT. People presenting at 41 UK hospitals in the NHS with a TASD for non-surgical management were randomised 1 : 1 to receive advice or advice and a programme of physiotherapy.
Two-thirds of participants were male, with the most common mechanism of injury being sport related. The mean age of participants was 45 years, and two age distributions centred at 24 and 56 years old, with the point where probability of membership in both distributions was 50% at 33 years of age. This was within 10 years of the randomisation stratum (age of 40) and therefore no further sensitivity analysis was required. These baseline demographics and two age distributions are reflective of the wider literature on first-time shoulder dislocation demographics.
Limitations
Among those screened and not meeting the eligibility criteria, the main reasons were that the patient was to undergo surgery (17%), the patient could not see a physiotherapist within 6 weeks (28%) or the patient could not adhere to trial procedures (39%).
A proportion of people with a first TASD having surgery is normal practice, and this trial was not addressing this surgical population. There was a higher than expected percentage of participants not being able to see a physiotherapist within 6 weeks, which on further discussion with sites was a result of added COVID pressures in some cases. The trial team did not collect further detailed information on the precise reasons why patients could not adhere to trial procedures. However, as this trial recruited before and during the pandemic there were changes in care pathways that needed to be accommodated in real time, such as a change to virtual clinics, which resulted in teams using this criterion in some cases.
The ARTISAN trial did have a loss to follow-up of 27%; however, the observed SD was much smaller than anticipated. This change in parameters reduced the number of participants required to observe the planned target difference of 4 points. The loss in participants was MAR, as it could be explained by our data. It was seen that the majority of the participants lost to follow-up were male (78%); however, this did not affect the balance between the intervention groups at the follow-up.
As the study was focused on the effectiveness of physiotherapy, there were concerns at the beginning of the study that there may be a physiotherapist effect which could strongly affect the estimate of efficacy of the ARTISAN intervention.
There were 96 therapists who delivered the core ARTISAN session across the 41 study sites. Only 42 therapists (44%) randomised more than three participants. An interim analysis demonstrated that the sample size did not need to be inflated for therapist effects. The analysis was repeated using the 6-month follow-up data at the end of the study to check whether the interim analysis was correct, which was confirmed.
Physiotherapists were trained to deliver the advice session and trial-related procedures. In the advice-only group, two participants did not adhere to the protocol due to a clinician mandating additional physiotherapy, and subsequently crossed over to receive a programme of physiotherapy. In the advice-only arm, all participants received the intervention.
Of those receiving the ARTISAN-only intervention, 15% subsequently had extra physiotherapy, whereas 83% of participants in the group allocated to additional physiotherapy received the additional physio sessions, demonstrating good levels of adherence.
Equality, diversity and inclusion
In preparation for this trial, patient and public groups were consulted who had experience of sustaining and being treated for a shoulder dislocation. These groups initially focused on local groups to the host organisation. Once an initial draft of trial materials and patient-facing documents were produced, these were presented to groups from a wider geographical area.
One of the areas of focus specific to the population who sustain a shoulder dislocation was the age range. Recognising that there are two dominant groups (young males who injure during sports and older women who injure during a fall), it felt inappropriate to produce materials that featured either young males or older females. Consequently, an approach using animations was chosen in an attempt to make the materials to apply to everyone equally.
In designing the trial materials, we ensured that plain simple language was used, there was a mix of media to access the same content (e.g. diagrams, videos, websites, booklets and text), and access to the materials was available regardless of access to electronic devices, to not exclude those who do not have regular access.
The qualitative study found that the use of animations was well received; however, some participants did comment on the difficulty of relating to some of the content because it was not three-dimensional or it was not clear exactly what was being moved and how to achieve the target exercise.
On reflection, although steps were taken to address the main area of ensuring that both key demographic groups felt the materials were appropriate, further work may be required to refine the animations or use a mixed medium of animations and real-life images to exemplify the rehabilitation content.
The trial population were broadly representative of the ethnic diversity of the UK. The trial materials were only available in the English language, so this is also an area for further development. Intervention materials will be made freely available to those who want to translate them as part of implementation of the findings.
Patient and public involvement
Patient and public involvement was at the heart of the ARTISAN study. Prior to the study, clinical co-applicants consulted with patients during appointments to ascertain whether the research gaps highlighted in the literature were of high importance to them. These same people were asked whether they would be interested in a consultation role for further development of the study and future roles in management of the research and dissemination of findings.
Subsequently, two patient representatives agreed, and discussed experiences and expectations of services and the plans for the trial. These perspectives were key in the development of the protocol to ensure trial processes, materials and interventions were feasible and acceptable. Key inputs from this group into the protocol were to ensure that the intervention employed a holistic approach, not just focusing on physical well-being.
One patient representative was included as a lay co-applicant who, in addition to contributing during our development work, was a member of the TMG. They contributed to trial processes and paperwork, such as patient information leaflets. They took a lead in the development of the information, training materials and resources used within the study. As the trial progressed, in the later stages our PPI team members changed, but continued in the same role with the same aims. Going forwards, they will be key to us ensuring that we disseminate our findings to a wider audience. A second PPI member agreed to an oversight role on the independent TSC for the duration of the trial and advised on the final report and dissemination plans.
Overall evidence
ARTISAN is the largest RCT that has investigated two different two rehabilitation interventions in adults with a first-time traumatic shoulder dislocation. We did not find any statistically significant differences in the primary outcome (OSIS) or other secondary outcomes. Advice and a programme of physiotherapy was cost-effective at a £30,000/QALY willingness-to-pay threshold.
While the economic interpretation is supportive of ARTISAN plus additional physiotherapy, the clinical interpretation may be more nuanced: where health gains appear small and uncertain and physiotherapy resources are fully stretched meeting current service demands, a programme of physiotherapy may not be perceived by practitioners as a service priority.
A 2014 Cochrane Review did not find any RCTs that compared different rehabilitation methods after the initial 2 weeks of supporting the arm in a sling. An updated review in 2019 had the same conclusion, but identified one ongoing study that has since completed. 8,9 No further ongoing studies were identified in an updated search of trial registries.
The now completed single RCT randomised 56 participants, across three orthopaedic shoulder units in Denmark, to a home-based exercise intervention or a supervised 12-week intervention. This trial included participants under the age of 40 years only and compared two different rehabilitation approaches (neuromuscular exercises vs. strengthening exercises), with one being delivered in a physiotherapy clinic and the other being home-based. Additionally, the physiotherapy-based programme was a prescriptive 14 sessions of 45 minutes each. ARTISAN included all adults and compared the same rehabilitation approach, with the only difference being delivery by a physiotherapist versus delivery at home; the ARTISAN protocol also allowed a tailored approach determined by the physiotherapist, rather than being prescriptive. This heterogeneity means it is not appropriate to consider pooling results from the two studies.
The authors reported a statistically significant improvement in the primary outcome (Western Ontario Shoulder Instability Index) from clinic-based neuromuscular exercise when compared to home exercises. The point estimate was less than their predefined clinically relevant between-group difference of 250, although a clinically relevant difference was not excluded (between-group mean difference, –228.1, 95% CI –430.5 to –25.6; p = 0.028). In ARTISAN, although the point estimate favoured physiotherapy, this was not statistically significant, and the limits of the 95% CI excluded our target difference. Overall, these findings, taken in conjunction with the ARTISAN health economic analysis, mean the possibility that there is a small mean benefit from additional physiotherapy after a first shoulder dislocation has not been totally excluded. However, any such benefit is likely to be very small and not of clinical importance or relevance for most people with a shoulder dislocation.
Chapter 7 Conclusion
Implications for health care
The ARTISAN trial found no evidence of a difference between referring patients to a programme of physiotherapy and referring patients for a single session of advice with a physiotherapist. However, a programme of physiotherapy is cost-effective and could be considered for patients who are not experiencing recovery as expected or who express a strong preference for supervised physiotherapy, who could self-refer for a supervised programme of physiotherapy. This will provide a balance between best use of NHS resources, empowering patients and reducing unnecessary appointments for those who can self-manage.
Implications for research
Further research should be directed towards optimising self-management strategies in this population. The ARTISAN trial was designed to address all patients who sustain a first-time shoulder dislocation; further research may also be directed towards populations who sustain recurrent shoulder dislocations.
Additional information
Acknowledgements
Data Monitoring Committee members
Mike Bradburn, Catey Bunce and Anju Jaggi
Trial Steering Committee members
Melinda Cairns, Alex McConnachie, Susan Jowett, Rachel Chester and Alwin McGibbon
Recruitment centres
-
University Hospital Coventry and Warwickshire
-
Royal Free London NHS Foundation Trust
-
Yeovil District Hospital NHS Foundation Trust
-
Southmead Hospital Bristol
-
Harrogate and District NHS Foundation Trust
-
University Hospital of North Tees
-
Norfolk & Norwich University Hospital NHS Foundation Trust
-
Royal Devon and Exeter Hospital
-
Addenbrookes Hospital
-
Royal Derby Hospital
-
Leicester Royal Infirmary
-
Royal Victoria Infirmary
-
Calderdale and Huddersfield NHS Foundation Trust
-
North West Anglia NHS Foundation Trust
-
Airedale NHS Foundation Trust
-
Ashford and St Peter’s Hospitals NHS Foundation Trust
-
Oxford University Hospital NHS Foundation Trust
-
Milton Keynes University Hospital
-
Blackpool Teaching Hospital NHS Foundation Trust
-
Royal Cornwall Hospital and Cornwall Partnership Trust
-
Royal Berkshire Hospital
-
Sheffield Teaching Hospital
-
York Teaching Hospital NHS Foundation Trust
-
Doncaster & Bassetlaw Teaching Hospitals NHS Trust
-
United Lincolnshire Hospitals NHS Trust
-
Grampian Health Board
-
Warrington and Halton Hospitals NHS Trust
-
East Suffolk and North Essex NHS Foundation Trust
-
Somerset NHS Foundation Trust
-
Glasgow Royal Infirmary
-
Royal Blackburn Teaching Hospital
-
Sunderland Royal Hospital
-
West Suffolk Hospital
-
King’s College London
-
St George’s University Hospitals NHS Foundation Trust
-
University Hospitals of Morecambe Bay NHS Foundation Trust
-
North Cumbria University Hospital NHS Trust
-
East Cheshire NHS Trust
-
Pilgrim Hospital
-
Grantham and District Hospital.
Contributions of authors
Rebecca Kearney (https://orcid.org/0000-0002-8010-164X) (Professor) was responsible for study conception, trial design, obtaining grant funding, trial management and acquisition of data.
David Ellard (https://orcid.org/0000-0002-2992-048X) (Professor) was responsible for study conception, trial design, obtaining grant funding and trial management.
Helen Parsons (https://orcid.org/0000-0002-2765-3728) (Associate Professor Statistics) was responsible for study conception, trial design, obtaining grant funding, trial management and the statistical analysis.
Aminul Haque (https://orcid.org/0000-0003-3589-6751) (Research Associate, Statistician) was responsible for the statistical analysis.
James Mason (https://orcid.org/0000-0001-9210-4082) (Professor, Health Economics) was responsible for study conception, trial design, obtaining grant funding, trial management and the health economics analysis.
Henry Nwankwo (https://orcid.org/0000-0001-7401-1923) (Assistant Professor Health Economics) was responsible for the health economics analysis.
Helen Bradley (https://orcid.org/0009-0003-1663-4462) (Senior Project Manager) was responsible for leading trial management operations.
Steve Drew (https://orcid.org/0000-0002-9523-682X) (Orthopaedic Consultant) were responsible for study conception, trial design, obtaining grant funding and trial management.
Chetan Modi (https://orcid.org/0009-0008-3337-4419) (Orthopaedic Consultant) were responsible for study conception, trial design, obtaining grant funding and trial management.
Howard Bush (https://orcid.org/0000-0001-9360-0504) (Physiotherapist) were responsible for study conception, trial design, obtaining grant funding and trial management.
David Torgerson (https://orcid.org/0000-0002-1667-4275) (Professor) were responsible for study conception, trial design, obtaining grant funding and trial management.
Martin Underwood (https://orcid.org/0000-0002-0309-1708) (Professor) were responsible for study conception, trial design, obtaining grant funding and trial management.
All authors were responsible for the interpretation of the data and for drafting and approving the final monograph.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/CMYW9226.
Primary conflicts of interest: All authors have read and concur with the content of this monograph. All authors meet the uniform requirements for manuscripts submitted to biomedical journals criteria for authorship. All authors confirm that they have approved the monograph for submission.
Rebecca Kearney is a previous member of the UK NIHR HTA CET board, NIHR ICA Doctoral panel and chair of the NIHR RfPB panel. Rebecca Kearney is current subcommittee chair for NIHR PGfAR. James Mason is a member of NIHR HTA Efficient Study Designs, HTA End of Life Care and Add-on Studies and HS&DR Funding Committee Member. David Torgerson was a member of HTA Medicines for Children Themed Call and is currently a member of CTUs funded by NIHR. Martin Underwood was a member of NIHR Journals Library Editors Group.
Rebecca Kearney, David Ellard, Helen Parsons, Ainul Haque, James Mason, Henry Nwamkwo, Steve Drew, Chetan Modi, Helen Bradley, David Torgerson and Martin Underwood have all been awarded current and previous NIHR research grants.
Martin Underwood and Rebecca Kearney are co-investigators on grants funded by the Australian NHMRC, and Helen Parsons, Martin Underwood and Rebecca Kearney have been co-applicants on NIHR funded studies receiving additional support from Stryker Ltd.
Martin Underwood is a co-investigator on grants funded by Norwegian Medical Research Council. He is a director and shareholder of Clinvivo Ltd that provides electronic data collection for health services research. He is part of an academic partnership with Serco Ltd, funded by the European Social Fund, related to return-to-work initiatives.
Data-sharing statement
All data requests should be submitted to WCTUDataAccess@warwick.ac.uk for consideration. Access to anonymised data may be granted following review.
Ethics statement
The National Research Ethic Committee approved this study on 26 July 2018 (18/WA/0236), with each trial site granting individual NHS Trust approval prior to recruitment at each site.
Information governance statement
The University of Warwick is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, University of Warwick and University Hospitals Coventry and Warwickshire are the Data Controller.
Department of Health and Social Care disclaimer
This publication presents independent research commissioned by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, MRC, NIHR Coordinating Centre, the HTA programme or the Department of Health and Social Care.
This monograph was published based on current knowledge at the time and date of publication. NIHR is committed to being inclusive and will continually monitor best practice and guidance in relation to terminology and language to ensure that we remain relevant to our stakeholders.
Disclaimers
This manuscript presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Brownson P, Donaldson O, Fox M, Rees JL, Rangan A, Jaggi A, et al. BESS/BOA Patient Care Pathways: traumatic anterior shoulder instability. Shoulder Elbow 2015;7:214-26.
- Hanchard NC, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev 2014.
- Berendes TD, Pilot P, Nagels J, Vochteloo AJ, Nelissen RG. Survey on the management of acute first-time anterior shoulder dislocation amongst Dutch public hospitals. Arch Orthop Trauma Surg 2015;135:447-54.
- Zacchilli MA, Owens BD. Epidemiology of shoulder dislocations presenting to emergency departments in the United States. J Bone Joint Surg Am 2010;92:542-9.
- Calandra JJ, Baker CL, Uribe J. The incidence of Hill–Sachs lesions in initial anterior shoulder dislocations. Arthroscopy 1989;5:254-7.
- Taylor DC, Arciero RA. Pathologic changes associated with shoulder dislocations. Arthroscopic and physical examination findings in first-time, traumatic anterior dislocations. Am J Sports Med 1997;25:306-11.
- Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev 2004;2004.
- Eshoj HR, Rasmussen S, Frich LH, Hvass I, Christensen R, Boyle E, et al. Neuromuscular exercises improve shoulder function more than standard care exercises in patients with a traumatic anterior shoulder dislocation: a randomized controlled trial. Orthop J Sports Med 2020;8.
- Braun C, McRobert CJ. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev 2019;5.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Medical Research Council Guidance . Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337.
- Kearney RS, Dhanjal G, Parsons N, Ellard D, Parsons H, Haque A, et al. Acute Rehabilitation following Traumatic anterior shoulder dISlocAtioN (ARTISAN): protocol for a multicentre randomised controlled trial. BMJ Open 2020;10.
- Liew Z, Mazuquin B, Ellard DR, Karasouli E, Drew S, Modi C, et al. Development of a single-session physiotherapy and self-management intervention for the treatment of primary traumatic anterior shoulder dislocation for the ‘Acute Rehabilitation following Traumatic anterior shoulder dISlocAtioN (ARTISAN)’ multi centre RCT. Physiotherapy 2021;113:80-7.
- Mars T, Ellard D, Carnes D, Homer K, Underwood M, Taylor SJ. Fidelity in complex behaviour change interventions: a standardised approach to evaluate intervention integrity. BMJ Open 2013;3.
- Dawson J, Fitzpatrick R, Carr A. The assessment of shoulder instability the development and validation of a questionnaire. J Bone Joint Surg Br 1999;81:420-6.
- Dawson J, Rogers K, Fitzpatrick R, Carr A. The Oxford shoulder score revisited. Arch Orthop Trauma Surg 2009;129:119-23.
- Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord 2006;7.
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36.
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4.
- Dimairo M, Coates E, Pallmann P, Todd S, Julious SA, Jaki T, et al. Development process of a consensus-driven CONSORT extension for randomised trials using an adaptive design. BMC Med 2018;16.
- van der Linde JA, van Kampen DA, van Beers LW, van Deurzen DF, Terwee CB, Willems WJ. The Oxford Shoulder Instability Score; validation in Dutch and first-time assessment of its smallest detectable change. J Orthop Surg Res 2015;10.
- Moser JS, Barker KL, Doll HA, Carr AJ. Comparison of two patient-based outcome measures for shoulder instability after nonoperative treatment. J Shoulder Elbow Surg 2008;17:886-92.
- FDA . Adaptive Design Clinical Trials for Drugs and Biologics Guidance for Industry 2020. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM201790.pdf (accessed 3 July 2023).
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005;2:152-62.
- Schlattmann P. Medical Applications of Finite Mixture Models. Springer; 2009.
- NICE . Guide to the Methods of Technology Appraisal 2008.
- Jones K, Burns A. Unit Costs of Health and Social Care. Kent: Personal Social Services Research Unit; 2021.
- NHS Digital . National Cost Collection for the NHS. National Schedule of NHS Costs 2021.
- NHSBSA . Prescription Cost Analysis – England 2021.
- BNF . British National Formulary 2021. https://bnf.nice.org.uk/ (accessed 3 July 2023).
- NHS Supply Chain . NHS Supply Chain Catalogue 2019. https://my.supplychain.nhs.uk/catalogue (accessed January 2020).
- Employee Earnings in the UK: 2021 2021. www.ons.gov.uk/employmentandlabourmarket/peopleinwork/earningsandworkinghours/bulletins/annualsurveyofhoursandearnings/2021 (accessed 3 July 2023).
- Van Hout B, Janssen M, Feng Y-S, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012;15:708-15.
- NICE . Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019) 2019. www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l (accessed 3 July 2023).
- StataCorp 2021.
- Faria R, Gomes M, Epstein D, White IR. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. PharmacoEcon 2014;32:1157-70.
- Madley-Dowd P, Hughes R, Tilling K, Heron J. The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol 2019;110:63-7.
- Ellard D, Simkiss D, Quenby S, Davies D, Kandala N, Kamwendo F, et al. The impact of training non-physician clinicians in Malawi on maternal and perinatal mortality: a cluster randomised controlled evaluation of the enhancing training and appropriate technologies for mothers and babies in Africa (ETATMBA) project. BMC Pregnancy Childbirth 2016;12.
- Hernández Alava M, Pudney S, Wailoo A. Estimating the relationship between EQ-5D-5L and EQ-5D-3L: Results from a UK population study. Pharmacoeconomics 2023;41:199-207. https://doi.org/10.1007/s40273-022-01218-7.
Appendix 1 Acute Rehabilitation following Traumatic anterior shoulder dISlocAtioN collaborators
-
University Hospital Coventry and Warwickshire: Kerri McGowan, Joanna Brough, Howard Bush, Chetan Modi
-
Yeovil District Hospital NHS Foundation Trust: Oliver Donaldson, Michael Jones, Alison Lewis, Kate Beesley
-
Harrogate and District NHS Foundation Trust: Cheryl McDermott, Caroline Bennett, Joyce Guy, Jospeh Askew, Charlie Talbot, Dave Copas, Su Leeming, Angela Sheldon, Venkata Vakamallu, Erin Demoulin, Tom Bradshaw
-
University Hospital of North Tees: Deborah Wilson, Emma Tindall, Andrea Meddes, Rajesh Nanda
-
Southmead Hospital Bristol: Jane Evans, Steven Barnfield, Kirsty Mapstone, Rhian Witham, Ian Packham, Paula Brock, Josephine Morley, Fionnaula Palmer, Ruth Halliday, Katherine Coates, Laura Lunny, Vicki Stoneman
-
Norfolk & Norwich University Hospital NHS Foundation Trust: Nicola Ward, James Kennedy, Celia Whitehouse, Carol Payne, Emmet Griffiths, Andrew Lee
-
Addenbrookes Hospital: Leila Dehghani, Aileen Nacorda, Natasha Woodbine, Niel Kang, Kelly Marie Grant, Laura Fabb, Rebecca Lewis
-
Royal Derby Hospital: Marcus Bateman, Evelina Siwerska, Louise Amandini, Caroline Clarkson, Kaite Walstow, Emma Whitby, Rebecca Smith, David Clark, Mona Mohamed, Kathleen Holding
-
Leicester Royal Infirmary: Kathryn Robinson, Tracey Lee, Helen Fort, Alison Armstrong, Amber Bery, Paul Lewis, Dileep Kumar, Helen Tunnicliffe
-
Royal Victoria Infirmary: Karen Smith, Jenny Baron, Lisa Robinson, John Williams, Heather Hunter, Anne Marie Ginnever, John Paul Gowland, Sophie Collins, Nicola Rose, Kate Graham, Helen Watson, Stephen Aldridge, Steven Galloway, Stuart Watson
-
Calderdale and Huddersfield NHS Foundation Trust: Hannah Riley, Kathryn Hanson, Simon Fogerty, Laura Hedley, Tash Maher, Siobhan Currie, Kayleigh Leatham
-
North West Anglia NHS Foundation Trust: Andrew White, James Wheble, Susan O’Sullivan, Stephanie Diaz, Paula Goodyear, Janki Bhayani, Deborah Butcher, Nicola Parker, Richard Cooper
-
Airedale NHS Foundation Trust: Lisa Armstrong, Emma Dooks, Stacey Lalande, James Tyler, Sally Mitchell, Chantel McParland, Natalie Rose Craven, Lauren Harker, Jessica Holdsworth, Martin Thomas, Zanib Taj, Daniel Kantas, Justin Murr, Rob Hunter
-
Ashford and St Peter’s Hospitals NHS Foundation Trust: Rohit Gupta, Sebastian Prentice, Leon Palmer-Wilson, Caroline Coulthard, Nicky Holland, Isaac John
-
Oxford University Hospital NHS Foundation Trust: Kathryn Lewis, Warren Sheehan, Colin Forde, Tessa Sedwin, Chandramohan Panneerselvam, Caroline Partner, Pablo Llorian, Georgina Taylor, Maria Mestre, Andrew Titchener, Sangeetha Prasath
-
Blackpool Teaching Hospital NHS Foundation Trust: Ella Riedel, Alex Maley, Emma Stoddard, Charalambos P. Charalambos, Suzanne Lane, Clare Blundell, Conor Wilkinson, Gemma Brown, Sarah Melling, Marin Sirbu, Sam Remnant, Shamina Hankinson, Denise Bennett, Emma Ward
-
Royal Cornwall Hospital and Cornwall Partnership Trust: Sean Dixon, Sharon Botfield, Cathal Murphy, Suzanne Dean, Eve Fletcher, Deanne De Beer, Annie Rae, Andrew McCarley, Hayley Potter, Damian Warner, Tamsin Ranson, Steven Cromey, Nicole Toomey
-
Royal Berkshire Hospital: Jennifer Armistead, Kelly Brooks, Leanne Dymott, Chloe Brown, Georgie Parsons, Salma Chaudhury, Terence Campbell, Emma Craig, Joanna Pinto, Rashmi Easow, Amar Malhas, Catherine Anderson
-
York Teaching Hospital NHS Foundation Trust: Greg Quinn, John Whitwell, Nadine Siddle, Mary Wragg, Hywell Williams, Paula Strider, Rebecca Tait, Clive Nicholson, Liz Johnson, Pearl Clark Brown
-
Doncaster & Bassetlaw Teaching Hospitals NHS Trust: Scott MacInnes, David Bailey, Gemma Kirkman, Lisa Warren, Julie Bury
-
Cwm Taf Morgannwg University Health Board: Lisa Mellish, John Rice, Rhys Owen, Christopher Greenfield, Ajay Sharma, Joanna Wilson, Caroline Hamilton
-
Grampian Health Board: Scott Barker, Carol Carnegie, Ann Quirk, Claire Stewart
-
East Suffolk and North Essex NHS Foundation Trust: Bally Purewal, Deborah Beeby, Stephanie Bell, Carol Buckman, Stephanie Garnham, Chris Roberts, Marie Welch
-
Glasgow Royal Infirmary: Fiona Carleton, James Doonan, Katharine Duffy, Valerie Forgie, Paul Jenkins, Jennifer Love, Linda Mercer, Gary Semple
-
West Suffolk Hospital: Angharad Williams, Thomas Lockwood
-
St George’s University Hospitals NHS Foundation Trust: Yemi Pearse, Sarah Latham, Gemma Shearer, Susan Nickle, Teresa Cepelkova, Andrew Wright, Kirsty Harwood, Michael Flatman, Miles Benjamin, Alexandros Haris, Oliver Brown, Prodrollos Tsinaslendis, Mathew Facey, Bethany Hedges, Abrar Gani, Selma Al-Ahmad, Nicholas Judkins, Judith Burnham, Andrew Gaukroger, Tomisin Omogbehin, Conor McDermott, Cleo Dobson, Charlotte Brookes, Sara Dardak, Roshan Rupra, Rhody Asirvatham, Maria Dadabhoy, Jennifer Rees, Sally Fisher, Simon Nicole, Lynn Smyth
-
University Hospitals of Morecambe Bay NHS Foundation Trust: Kathryn Allison, Ellie Higham, Jayne Craig, Jack Burke, Sarah Hart, Allie Menzies, Deborah Power, Holly Davies, Alisha Walters, Kerry Simpson, Karen Burns, Laura Dodd, Gautam Talawadekar
-
North Cumbria University Hospital NHS Trust: Syed Mannan, Scott Smith, Helen Wilson, Melanie Clapham, Jane Gregory, Steven Wright, Una Poultney, Polly Rice, Nicci Kelsall, Katherine Davidson, Fran Armstrong, Diane Armstrong, David Mackay, Hannah Craig
-
East Cheshire NHS Trust: Natalie Keenan, Matt Hurst, Maureen Holland, Jessica Clitheroe, Jochen Fischer, Victoria Austin, Jack Mullins
-
United Lincolnshire Hospitals NHS Trust: Charles Corbin, Sarah Shephardson, Alison Wilkinson, Rosaline Keane, Carol Lockwood, Helen Morris, Lynn Osborne, Alun Yewlett, Jo Fletcher, Eleanor Gates, Grace Homer, Kimberley Netherton, Munya Tsuro
-
Sheffield Teaching Hospital: Simon Booker, Valerie Jones, Grahame Clark, Bonito Tsong, Abiola Alli, Rachel Sellars, Julie Walker, Elizabeth Hurditch, Pene Fati, Karne Robinson, Harjinder Kaur, Sandra Guzaviciute
-
Milton Keynes University Hospital: Louise Mew, Lee How, Annie Rose, Louise Kelly, Louise Heylen, James Underwood, Aabid Sanaullah, Sarah Overall
-
Sunderland Royal Hospital: Yan Cunningham, Julie Doughty, Yusuf Michla, Debbie Watson
-
Royal Devon and Exeter Hospital: Peter Hamer
-
King’s College London: Adel Tavakkolizadeh, Nicky Wilson, Victoria Rowe, Christopher Ratcliffe, Scarlett McCalla, Ines Reichert, Kerim Gokturk, Hazel Giles, Victoria Withers, Christy Walkin, Harshinder Virdee, Raye Marinas, Laarni Bonganay, Sheena Mendoza, Christopher Beggs, Gary Chan, Jordan Bethel, Silvana Skotiniyadis, Ross Moorely
-
Somerset NHS Foundation: Barnaby Sheridan, Simon Ingram, Kimberley Gillman, Alison Whitcher, Claire Gutherie
-
Warrington and Halton Hospitals NHS Trust: Lynne Connell, Carole Cummings, Pascal De Feyter, Nicola De Jong, Gary Dobson, Amy Moreton, Layla Morrissey, Nasir Shah, Suzi Thompson
-
Royal Blackburn Teaching Hospital: Rachel Dean, Saif Hadi, Karen Marsden, Helen Thompson
-
Royal Free London NHS Foundation Trust: Philip Ahrens, Andrea Francis, Francesca Gowing, Rebecca Portwood, Nikunji Depala, Stamatios Tsamados, Adam Yasen, Lara Beaman, Sarah Flynn, Sangna Unadkat, Aiden Gallagher
-
Cwm Taf Morgannwg University Health Board: Lisa Mellish, John Rice, Rhys Owen, Christopher Greenfield, Ajay Sharma, Joanna Wilson, Caroline Hamilton.
Appendix 2 Web-based rehabilitation materials
Phase 1: Outline of voiceover script
| Script text | (animation of Jess walking into physio department wearing a sling and going in to meet Martin) |
| Narrator: You have dislocated your shoulder, and you probably have a few questions, but don’t worry, we have put together some information about these early stages. So grab a cup of tea, sit back and let us help you understand the steps to recovery. | |
| We’d like you to meet Jess, who dislocated her shoulder a few days ago, and she is on her way to meet Martin, her physiotherapist. | |
| (animation of Jess sitting opposite Martin in a physiotherapy consultation room, wearing a sling) | |
| Jess: What has happened to me? | |
| Martin: You’ve had a shoulder dislocation, which is when the bone in the upper arm is forced out of its joint at the shoulder. | |
| (animation of shoulder joint dislocating) | |
| Your shoulder joint is now back in place, and you will have had an X-ray to check it is OK. | |
| (animation of shoulder going back in) | |
| Jess: What can go wrong? | |
| (animation of Jess sitting opposite Martin in a physiotherapy consultation room, wearing a sling) | |
| Martin: Usually once the shoulder joint is back in place there are no major problems. Occasionally your physiotherapist or doctor might identify damage to the nerves, muscles or bone around the shoulder that might need further investigation. | |
| Jess: How do I stop this happening again? | |
| Martin: Whilst things are healing you should avoid holding your arm in a ‘surrender’ position or putting your hands behind your head. But sometimes it will happen again, whatever you do. | |
| (animation of surrender position – arm away from side with fingers pointing to ceiling and/or hand behind head) | |
| Jess: How long do I have to wear my sling? | |
| (animation of Jess sitting opposite Martin in a physiotherapy consultation room, wearing a sling) | |
| Martin: The sling is to keep you comfortable in the early days. You should remove it from time to time as soon as is comfortable. It is usually not used for more than 2 weeks. | |
| Jess: Should I move my arm? | |
| Martin: Yes. You should move your hand, wrist and elbow frequently to prevent stiffness. | |
| (animation of taking sling off and moving elbow up and down and moving wrist up and down) | |
| You should also practise moving your shoulder forwards and out to the side. If it is difficult to move, you can use your other arm to support it. | |
| (animation of moving arm out to side and forwards) | |
| Jess: How do I control my pain? | |
| Martin: You can use ice packs wrapped in a cloth around your shoulder, but no more than 20 minutes at a time and do not put ice directly on the skin. Common painkillers like paracetamol or ibuprofen may also help with pain. It’s best to talk to your pharmacist to get advice on how much and how often. | |
| (animation of frozen peas in tea towel on shoulder) | |
| Even if your shoulder is uncomfortable, it is important to keep it moving. If you are having a lot of trouble with pain, talk to your GP who may give you a prescription for stronger painkillers, but these are not usually needed. | |
| (animation of Jess moving shoulder forwards and out to side) | |
| Jess: When can I get back to doing my usual stuff? | |
| Martin: You can get back to doing most activities as soon as you feel comfortable. If you need to do anything heavier, like helping a mate move some heavy boxes or a burning desire to rugby tackle someone on a grassy pitch, then you should usually wait until after 6 weeks. | |
| (animation of Jess rugby tackling) | |
| Jess: What if something goes wrong? | |
| Martin: If you think your shoulder has come out of joint again or if you experience a sudden change in symptoms, then you should get that checked out urgently by the emergency department. | |
| (animation of Jess feeling sudden pain and going back to A&E) | |
| If your shoulder is just not getting better, then contact the clinic you attended when you first injured it or see your GP. | |
| Jess: Thank you for your time, your advice has been very useful, and I will make sure I follow it because I want to get back to my usual self as soon as possible. | |
| (animation of Jess leaving the physiotherapy consultation room) | |
| Narrator: We have given you some top tips about your shoulder. To help with your recovery, there are some shoulder exercises that your physiotherapist will show you at your first appointment. Doing these exercises will help with your recovery. |
Phases 2 and 3: Outline of animated exercises
| Title of exercise | Animation required | Descriptive text to accompany the animation on the webpage |
|---|---|---|
| Insert relevant text for scapula setting advice for all exercises on website | ||
| Exercise 1 | Animation of standing character moving their arm forwards (flexion). | Forwards movement:
|
| If forwards movement is too difficult, repeat as above, but you can use your unaffected arm to help support your affected arm during the forward movement. | ||
| Exercise 2 | Animation of standing character moving their arm out to the side (abduction). | Out to the side movement:
|
| If moving your arm out to the side is too difficult, repeat as above, but you can use your unaffected arm to help support your affected arm during the movement. | ||
| Exercise 3 | Animation of standing character moving their arm out to the side and back across tummy with the elbow bent (internal and external rotation) | Rotation movement:
|
| If this movement is too difficult you can repeat as above, but you can use your unaffected arm to help support your affected arm during the movement. | ||
| Exercise 4 | Animation of standing character moving their arm into internal and external rotation against a door but not moving (isometric rotation exercise) | Static strengthening for rotation movement:
|
| Exercise 5 | Animation of standing character pushing their fist into a wall and standing with back to wall pushing backwards (isometric flexion and extension exercise) | Static strengthening forwards and backwards movement:
|
| Exercise 6 | Animation of standing character pushing their whole arm into a wall with elbow bent and repeating the movement into the body (isometric abduction and adduction) | Static strengthening out to the side movement:
|
| Exercise 7 | Animation of standing character moving their arm forwards (flexion) and backwards (extension) with a tin of beans in their hand. | Dynamic strengthening for forwards movement:
|
| To make the exercise more difficult, progressively increase the weight (you can use heavier tins/bottles) and gradually increase the number of repetitions. | ||
| Exercise 8 | Animation of standing character moving their arm out to the side (abduction) with a tin of beans in their hand. | Dynamic strengthening for out to the side movement:
|
| To make the exercise more difficult, progressively increase the weight (you can use heavier tins/bottles) and gradually increase the number of repetitions. | ||
| Exercise 9 | Animation of standing character moving their arm out to the side and back across tummy with the elbow bent (internal and external rotation) with a tin of beans in their hand | Dynamic strengthening for rotation movement:
|
| To make the exercise more difficult, progressively increase the weight (you can use heavier tins/bottles) and gradually increase the number of repetitions. | ||
Phase 4: Outline of voiceover script
| Script text | (animation of Jess entering a physiotherapy consultation room) |
| Narrator: Not all of you will need to take this last step; it is just for those of you who like to do a bit of sport. Sports that involve overhead activities or contact carry a higher risk of further re-dislocation; however, many people wish to continue with what they enjoy, and this next information will help you get there. | |
| I’ll reintroduce you to Jess – she’s off back to meet her physio Martin because she enjoys playing rugby and is keen to get back to her former sporting self. | |
| (animation of Jess playing rugby) | |
| Jess: I’m really looking forward to getting back to my sports but I’m really worried that my shoulder may come out again. | |
| (animation of Jess sitting in consultation room opposite Martin) | |
| Martin: The exercises you have been doing will help you get back to full activities. There is always a chance it might come out again, especially if you take part in high-risk sports that involve contact, like rugby, or overhead force, like tennis. | |
| (animation of Jess playing rugby again) | |
| Jess: I want to go back to these sports, so how do I phase back in? | |
| (animation of Jess sitting in consultation room opposite Martin) | |
| Martin: Go back to some training sessions first and gradually introduce more contact and overhead activities. For example, in rugby you would first do passing drills, footwork, kicking and agility and then progress to overhead throwing, tackling, scrums and line-outs. Once you’ve practised the full range of skills in a training environment, you can progress to a competitive environment. When you do this, it would be good to plan to do the last part as a substitute towards the end of the game and then build up each week depending on how you feel. | |
| (animation of drills in rugby) | |
| Jess: How will I know if I am ready for each new progression? | |
| (animation of Jess sitting in consultation room opposite Martin) | |
| Martin: We usually expect a full return within 6 months of the injury. If you feel you are not progressing, then we advise that you get back in touch with your GP or treating hospital site. | |
| Jess: What should I do if I take the next steps and it becomes painful? | |
| Martin: You can expect the shoulder to ache a little bit as you gradually increase the demands you place on it. If it becomes painful, then you should step back to the stage before until it settles and then gradually phase forwards again. | |
| Jess: What should I do if it comes out of the joint again? | |
| Martin: You should go back to the emergency department. Once relocated, you will usually be referred back the orthopaedic team for review. | |
| (animation of Jess to going to A&E) | |
| Jess: Thanks a lot for all your help, you have been great. Hopefully I will not see you again! | |
| (animation of Jess leaving the physiotherapy department) | |
| Narrator: Well, that is it! All done! Back to your usual self! Nothing more to say other than all the best and well done for seeing it through to the end. |
Appendix 3 Outline of paper-based materials
Phase 1: Outline of booklet
| Front cover | Logos Images: Still image of Jess sitting opposite Martin in physiotherapy consulting room, wearing a sling Title: Your Recovery Begins Here |
| Page 1 | Images: Still image of shoulder dislocating and still image of shoulder going back in Title: Introduction Text: You have dislocated your shoulder, and you probably have a few questions, but don’t worry, we have put together some information about these early stages to help you understand the steps to recovery. |
| Page 2 | Images: Still image of surrender position – arm away from side with fingers pointing to ceiling and/or hand behind head under ‘how do I stop this happening again’ Title: Common Questions Answered Text: What has happened to me? You’ve had a shoulder dislocation, which is when the bone in the upper arm is forced out of its joint at the shoulder. Your shoulder joint is now back in place and you will have had an X-ray to check it is OK. What can go wrong? Usually once the shoulder joint is back in place there are no major problems. Occasionally your physiotherapist or doctor might identify damage to the nerves, muscles or bone around the shoulder that might need further investigation. How do I stop this happening again? Whilst things are healing you should avoid holding your arm in a ‘surrender’ position or putting your hands behind your head. But sometimes it will happen again, whatever you do. |
| Page 3 | Images: Still image with sling off and moving elbow up and down and moving wrist up and down and still image of arm out to side and forwards under heading ‘should I move my arm’ Title: More Common Questions Answered Text: How long do I have to wear my sling? The sling is to keep you comfortable in the early days. You should remove it from time to time as soon as is comfortable. It is usually not used for more than 2 weeks. Should I move my arm? Yes. You should move your hand, wrist and elbow frequently to prevent stiffness. You should also practise moving your shoulder forwards and out to the side. If it is difficult to move, you can use your other arm to support it. |
| Page 4 | Images: Still image of frozen peas in tea towel on shoulder under heading ‘how do I control my pain’ Title: Even More Common Questions Answered Text: How do I control my pain? You can use ice packs wrapped in a cloth around your shoulder, but no more than 20 minutes at a time and do not put ice directly on the skin. Common painkillers like paracetamol or ibuprofen may also help with pain. It’s best to talk to your pharmacist to get advice on how much and how often. Even if your shoulder is uncomfortable, it is important to keep it moving. If you are having a lot of trouble with pain, talk to your GP who may give you a prescription for stronger painkillers, but these are not usually needed. When can I get back to doing my usual stuff? You can get back to doing most activities as soon as you feel comfortable. If you need to do anything heavier, like helping a mate move some heavy boxes or a burning desire to rugby tackle someone on a grassy pitch, then you should usually wait until after 6 weeks. |
| Page 5 | Images: Still image of feeling sudden pain and going back to A&E under heading ‘What if something goes wrong’ Title: Last Common Question Answered What if something goes wrong? If you think your shoulder has come out of joint again or if you experience a sudden change in symptoms, then you should get that checked out urgently by the emergency department. If your shoulder is just not getting better, then contact the clinic you attended when you first injured it or see your GP. |
| Page 6 | Images: Title: Final Words Text: We have given you some top tips about your shoulder. To help with your recovery, there are some shoulder exercises that your physiotherapist will show you at your first appointment. Doing these exercises will help with your recovery. |
| Back page | Logos |
Phases 2 and 3: Outline of booklet
| Front cover | Logos Images: Still image of character at home doing an exercise Title: Your ARTISAN Exercise Programme |
| Page 1 | Images: Still of shoulder in joint Title: Moving your shoulder Text: The exercises on the next pages will help you get your shoulder moving. To help you keep track of what you have done, there are pages at the end of this section to record your goals and how many and how often you do these exercises. |
| Page 2 | Title: Moving your arm forwards Image: Still image of standing character moving their arm forwards (flexion). |
Text: Forwards Movement:
|
|
| If forward movement is too difficult, repeat as above, but you can use your unaffected arm to help support your affected arm during the forward movement. | |
| Page 3 | Title: Moving your arm out to the side Image: Still image of standing character moving their arm out to the side (abduction). |
Text: Out to the side movement:
|
|
| If moving your arm out to the side is too difficult, repeat as above, but you can use your unaffected arm to help support your affected arm during the movement. | |
| Page 4 | Title: Rotating your arm Image: Still image of standing character moving their arm out to the side and back across tummy with the elbow bent (internal and external rotation) |
Text: Rotation movement:
|
|
| If this movement is too difficult, you can repeat as above, but you can use your unaffected arm to help support your affected arm during the movement. | |
| Page 5 | Title: My Goals Text: In this section, please write down your goals during these early phases; an example might be to be able to brush your hair or reach into a cupboard. It’s important that your goal is personal to you and you acknowledge when you have achieved it. Image: Insert a text box with dotted lines for goals to be recorded. |
| Page 6 | Title: Record of Progress Text: In this section, please keep a record of the movement exercises you have done. Once you are comfortable moving your arm, you can progress to the exercises on the next page that will begin to strengthen your shoulder. Image: Insert of exercise diary |
| Page 7 | Images: Still of shoulder in joint Title: Starting to Strengthen Your Shoulder Text: The exercises on the next pages will help you start getting your shoulder stronger. To help you keep track of what you have done, there are pages at the end of this section to record your goals and how many and how often you do these exercises. It would be good if you could do the next three exercises (p.8–p.10) comfortably before moving onto the more dynamic strengthening exercises (p.11 onwards). |
| Page 8 | Title: Static Strengthening for Shoulder Rotation Image: Still image of standing character moving their arm into internal and external rotation against a door but not moving (isometric rotation exercise) |
Text: Static strengthening for rotation movement:
|
|
| Page 9 | Title: Static strengthening forwards and backwards movement Image: Still image of standing character pushing their fist into a wall and standing with back to wall pushing backwards (isometric flexion and extension exercise) |
Text: Static strengthening forwards and backwards movement:
|
|
| Page 10 | Title: Static strengthening out to the side movement Image: Still image of standing character pushing their whole arm into a wall with elbow bent and repeating the movement into the body (isometric abduction and adduction) |
Text: Static strengthening out to the side movement:
|
|
| Page 11 | Title: Dynamic strengthening for forwards movement Image: Still animation of standing character moving their arm forwards (flexion) and backwards (extension) with a tin of beans in their hand. |
Text: Dynamic strengthening for forwards movement:
|
|
| To make the exercise more difficult, progressively increase the weight (you can use heavier tins/bottles) and gradually increase the number of repetitions. | |
| Page 12 | Title: Dynamic strengthening for out to the side movement Image: Still image of standing character moving their arm out to the side (abduction) with a tin of beans in their hand. |
Text: Dynamic strengthening for out to the side movement:
|
|
| To make the exercise more difficult, progressively increase the weight (you can use heavier tins/bottles) and gradually increase the number of repetitions. | |
| Page 13 | Title: Dynamic strengthening for rotation movement Image: Still image of standing character moving their arm out to the side and back across tummy with the elbow bent (internal and external rotation) with a tin of beans in their hand |
Text: Dynamic strengthening for rotation movement:
|
|
| To make the exercise more difficult, progressively increase the weight (you can use heavier tins/bottles) and gradually increase the number of repetitions. | |
| Page 14 | Title: My Goals Text: In this section, please write down your goals during these late phases; an example might be to be able to lift your child or lift bags of shopping. It’s important that your goal is personal to you and you acknowledge when you have achieved it. Image: Insert a text box with dotted lines for goals to be recorded. |
| Page 15 and 16 | Title: Record of Progress Text: In this section, please keep a record of the movement exercises you have done. Once you are comfortable moving your arm, you can progress to the exercises on the next page that will begin to strengthen your shoulder. Image: Insert of exercise diary |
| Back page | Logos |
Phase 4: Outline of booklet
| Front cover | Logos Images: Still image of Jess playing rugby Title: Your Recovery Ends Here |
| Page 1 | Images: Still image of rugby drills Title: Introduction Text: Not all of you will need to take this last step; it is just for those of you who like to do a bit of sport. Sports that involve overhead activities or contact carry a higher risk of further re-dislocation; however, many people wish to continue with what they enjoy, and this next information will help you get there. |
| Page 2 | Images: Still image of consultation room with physiotherapist Title: Common Questions Answered Text: I’m really looking forward to getting back to my sports but I’m really worried that my shoulder may come out again. The exercises you have been doing will help you get back to full activities. There is always a chance it might come out again, especially if you take part in high-risk sports that involve contact, like rugby, or overhead force, like tennis. I want to go back to these sports, so how do I phase back in? Go back to some training sessions first and gradually introduce more contact and overhead activities. For example, in rugby you would first do passing drills, footwork, kicking and agility, and then progress to overhead throwing, tackling, scrums and line-outs. Once you’ve practised the full range of skills in a training environment, you can progress to a competitive environment. When you do this, it would be good to plan to do the last part as a substitute, towards the end of the game and then build up each week depending on how you feel. |
| Page 3 | Images: Still image of playing rugby Title: More Common Questions Answered Text: How will I know if I am ready for each new progression? We usually expect a full return within 6 months of the injury. If you feel you are not progressing, then we advise that you get back in touch with your GP or treating hospital site. What should I do if I take the next steps and it becomes painful? You can expect the shoulder to ache a little bit as you gradually increase the demands you place on it. If it becomes painful, then you should step back to the stage before until it settles and then gradually phase forwards again. What should I do if it comes out of the joint again? You should go back to emergency department. Once relocated, you will usually be referred back the orthopaedic team for review. |
| Page 4 | Image: Still image of brand characters and ARTISAN logo Title: Final words Text: Well, that is it! All done! Back to your usual self! Nothing more to say other than all the best and well done for seeing it through to the end. |
| Back page | Logo |
Appendix 4 Mean NHS and personal social services resource use and productivity losses by trial group among patients reporting use
| ARTISAN Plus | ARTISAN | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Community care services | |||||||||
| 0–6 weeks | |||||||||
| GP visits in surgery | 6 | 1.00 | 0.00 | 7 | 1.29 | 0.76 | 13 | 1.15 | 0.55 |
| GP home visits | 0 | – | – | 0 | – | – | 0 | – | – |
| GP telephone contacts | 6 | 2.17 | 2.40 | 6 | 1.17 | 0.41 | 12 | 1.67 | 1.72 |
| Practice nurse contacts | 0 | – | – | 1 | 1.00 | – | 1 | 1.00 | – |
| District nurse contacts | 0 | – | – | 1 | 3.00 | – | 1 | 3.00 | – |
| Community physiotherapy contacts | 10 | 2.30 | 1.70 | 5 | 2.20 | 2.17 | 15 | 2.27 | 1.79 |
| Calls to NHS Direct | 1 | 1.00 | . | 1 | 1.00 | – | 2 | 1.00 | 0.00 |
| Calls to hospital physiotherapist | 5 | 1.40 | 0.55 | 4 | 1.50 | 0.58 | 9 | 1.44 | 0.53 |
| Calls for ambulance or paramedic | 1 | 1.00 | – | 1 | 1.00 | – | 2 | 1.00 | 0.00 |
| Occupational therapy contacts | 1 | 2.00 | – | 1 | 1.00 | – | 2 | 1.50 | 0.71 |
| 7–13 weeks (3 months) | |||||||||
| GP visits in surgery | 3 | 1.30 | 0.58 | 4 | 1.00 | 0.00 | 7 | 1.14 | 0.38 |
| GP home visits | 0 | – | – | 0 | – | – | 0 | – | – |
| GP telephone contacts | 4 | 1.50 | 0.58 | 4 | 1.50 | 0.58 | 8 | 1.50 | 0.53 |
| Practice nurse contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| District nurse contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Community physiotherapy contacts | 4 | 2.25 | 1.50 | 5 | 1.60 | 0.89 | 9 | 1.89 | 1.17 |
| Calls to NHS Direct | 1 | 2.00 | – | 0 | – | – | 1 | 2.00 | – |
| Calls to hospital physiotherapist | 5 | 2.80 | 0.84 | 1 | 3.00 | – | 6 | 2.83 | 0.75 |
| Calls for ambulance or paramedic | 0 | – | – | 0 | – | – | 0 | – | – |
| Occupational therapy contacts | 0 | – | – | 4 | 1.25 | 0.50 | 4 | 1.25 | 0.50 |
| 14–26 weeks (6 months) | |||||||||
| GP visits in surgery | 2 | 1.50 | 0.71 | 1 | 2.00 | – | 3 | 1.67 | 0.58 |
| GP home visits | 0 | – | – | 0 | – | – | 0 | – | – |
| GP telephone contacts | 2 | 1.00 | 0.00 | 2 | 3.50 | 2.12 | 4 | 2.25 | 1.89 |
| Practice nurse contacts | 0 | – | – | 3 | 1.00 | 0.00 | 3 | 1.00 | 0.00 |
| District nurse contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Community physiotherapy contacts | 7 | 1.71 | 0.76 | 2 | 4.50 | 0.71 | 9 | 2.33 | 1.41 |
| Calls to NHS Direct | 0 | – | – | 0 | – | – | 0 | – | – |
| Calls to hospital physiotherapist | 1 | 1.00 | – | 3 | 2.00 | 1.00 | 4 | 1.75 | 0.96 |
| Calls for ambulance or paramedic | 0 | – | – | 0 | – | – | 0 | – | – |
| Occupational therapy contacts | 3 | 1.00 | 0.00 | 1 | 4.00 | – | 4 | 1.75 | 1.50 |
| 27–52 weeks (12 months) | |||||||||
| GP visits in surgery | 2 | 1.00 | 0.00 | 1 | 2.00 | – | 3 | 1.33 | 0.58 |
| GP home visits | 0 | – | – | 0 | – | – | 0 | – | – |
| GP telephone contacts | 1 | 1.00 | – | 1 | 1.00 | – | 2 | 1.00 | 0.00 |
| Practice nurse contacts | 1 | 1.00 | – | 3 | 1.33 | 0.58 | 4 | 1.25 | 0.50 |
| District nurse contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Community physiotherapy contacts | 0 | – | – | 5 | 10.00 | 5.00 | 5 | 10.00 | 5.00 |
| Calls to NHS Direct | 0 | – | – | 0 | – | – | 0 | – | – |
| Calls to hospital physiotherapist | 1 | 5.00 | – | 3 | 5.00 | 4.36 | 4 | 5.00 | 3.56 |
| Calls for ambulance or paramedic | 0 | – | – | 0 | – | – | 0 | – | – |
| Occupational therapy contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Outpatient visits | |||||||||
| 0–6 weeks | |||||||||
| Orthopaedics | 31 | 1.29 | 0.59 | 39 | 1.28 | 0.56 | 70 | 1.28 | 0.57 |
| Pathology | 1 | 1.00 | – | 0 | – | – | 1 | 1.00 | – |
| Radiology | 22 | 1.27 | 0.55 | 23 | 1.17 | 0.49 | 45 | 1.22 | 0.52 |
| Physiotherapy (NHS) | 114 | 1.87 | 0.81 | 56 | 1.16 | 0.85 | 170 | 1.64 | 0.89 |
| Physiotherapy (private) | 2 | 2.50 | 2.12 | 8 | 2.50 | 2.73 | 10 | 2.50 | 2.51 |
| Physiotherapy telephone advice | 12 | 7.08 | 9.26 | 4 | 18.75 | 13.15 | 16 | 10.00 | 11.17 |
| Emergency department | 6 | 1.83 | 2.04 | 5 | 1.00 | 0.71 | 11 | 1.45 | 1.57 |
| 7–13 weeks (3 months) | |||||||||
| Orthopaedics | 17 | 1.29 | 0.59 | 22 | 1.32 | 1.17 | 39 | 1.31 | 0.95 |
| Pathology | 1 | 0.00 | – | 0 | – | – | 1 | 0.00 | – |
| Radiology | 8 | 1.13 | 0.35 | 9 | 1.67 | 1.66 | 17 | 1.41 | 1.23 |
| Physiotherapy (NHS) | 62 | 1.85 | 1.29 | 17 | 1.82 | 1.47 | 79 | 1.85 | 1.32 |
| Physiotherapy (private) | 8 | 2.00 | 0.53 | 6 | 1.03 | 1.67 | 14 | 1.86 | 0.77 |
| Physiotherapy telephone advice | 8 | 9.13 | 9.92 | 6 | 3.17 | 3.87 | 14 | 6.57 | 8.25 |
| Emergency department | 2 | 1.00 | 0.00 | 3 | 1.00 | 0.00 | 5 | 1.00 | 0.00 |
| 14–26 weeks (6 months) | |||||||||
| Orthopaedics | 11 | 1.64 | 0.67 | 19 | 1.16 | 0.50 | 30 | 1.33 | 0.61 |
| Pathology | 0 | – | – | 0 | – | – | 0 | – | – |
| Radiology | 4 | 1.25 | 0.50 | 8 | 1.00 | 0.00 | 12 | 1.08 | 0.29 |
| Physiotherapy (NHS) | 41 | 2.10 | 1.28 | 21 | 2.43 | 1.57 | 62 | 2.21 | 1.38 |
| Physiotherapy (private) | 5 | 3.20 | 1.92 | 4 | 8.50 | 7.94 | 9 | 5.56 | 5.77 |
| Physiotherapy telephone advice | 3 | 1.67 | 1.15 | 5 | 7.40 | 12.66 | 8 | 5.25 | 10.03 |
| Emergency department | 0 | – | – | 3 | 1.00 | 0.00 | 3 | 1.00 | 0.00 |
| 27–52 weeks (12 months) | |||||||||
| Orthopaedics | 8 | 1.13 | 0.35 | 10 | 1.40 | 1.17 | 18 | 1.28 | 0.89 |
| Pathology | 0 | – | – | 1 | 1.00 | – | 1 | 1.00 | – |
| Radiology | 4 | 1.00 | 0.00 | 5 | 1.60 | 0.55 | 9 | 1.33 | 0.50 |
| Physiotherapy (NHS) | 9 | 2.33 | 1.94 | 11 | 7.09 | 12.33 | 20 | 4.95 | 9.36 |
| Physiotherapy (private) | 1 | 1.00 | – | 2 | 5.00 | 2.83 | 3 | 3.67 | 3.06 |
| Physiotherapy telephone advice | 1 | 3.00 | – | 3 | 15.67 | 25.40 | 4 | 12.50 | 21.69 |
| Emergency department | 2 | 1.00 | 0.00 | 0 | – | – | 2 | 1.00 | 0.00 |
| Site-reported physio contacts a | 239 | 2.54 | 2.26 | 237 | 0.57 | 1.83 | 476 | 1.56 | 2.28 |
| Aids and adaptations | |||||||||
| 0–6 weeks | |||||||||
| Sling | 17 | 1.00 | 0.00 | 24 | 1.00 | 0.00 | 41 | 1.00 | 0.00 |
| 7–13 weeks (3 months) | |||||||||
| Sling | 1 | 1.00 | – | 4 | 1.00 | 0.00 | 5 | 1.00 | 0.00 |
| 14–26 weeks (6 months) | |||||||||
| Sling | 2 | 1.00 | 0.00 | 5 | 1.00 | 0.00 | 7 | 1.00 | 0.00 |
| 27–52 weeks (12 months) | |||||||||
| Sling | 1 | 1.00 | – | 5 | 1.00 | 0.00 | 6 | 1.00 | 0.00 |
| PSS | |||||||||
| 0–6 weeks | |||||||||
| Meals on Wheels | 0 | – | – | 0 | – | – | 0 | – | – |
| Laundry services | 0 | – | – | 0 | – | – | 0 | – | – |
| Social worker contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Care worker contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| 7–13 weeks (3 months) | |||||||||
| Meals on Wheels | 0 | – | – | 0 | – | – | 0 | – | – |
| Laundry services | 0 | – | – | 0 | – | – | 0 | – | – |
| Social worker contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Care worker contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| 14–26 weeks (6 months) | |||||||||
| Meals on Wheels | 0 | – | – | 0 | – | – | 0 | – | – |
| Laundry services | 0 | – | – | 0 | – | – | 0 | – | – |
| Social worker contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Care worker contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| 27–52 weeks (12 months) | |||||||||
| Meals on Wheels | 0 | – | – | 0 | – | – | 0 | – | – |
| Laundry services | 0 | – | – | 0 | – | – | 0 | – | – |
| Social worker contacts | 1 | 1.00 | – | 0 | – | – | 1 | 1.00 | – |
| Care worker contacts | 0 | – | – | 0 | – | – | 0 | – | – |
| Time off work (number of days off work) | |||||||||
| 0–6 weeks | 71 | 11.55 | 13.03 | 64 | 11.53 | 13.08 | 135 | 11.54 | 13.01 |
| 7–13 weeks (3 months) | 65 | 12.18 | 11.97 | 62 | 9.35 | 11.15 | 127 | 10.8 | 11.62 |
| 14–26 weeks (6 months) | 73 | 15.55 | 20.51 | 79 | 14.73 | 20.82 | 152 | 15.13 | 20.61 |
| 27–52 weeks (12 months) | 44 | 20.52 | 30.58 | 47 | 17.23 | 28.89 | 91 | 18.82 | 29.60 |
List of abbreviations
- AE
- adverse event
- ARTISAN
- Acute Rehabilitation following Traumatic anterior shoulder dISlocAtioN
- BESS
- British Elbow and Shoulder Society
- BOA
- British Orthopaedic Association
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- DASH
- Disabilities of the Arm, Shoulder and Hand
- DMC
- Data Monitoring Committee
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- EVPI
- estimated value of perfect information
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- MAR
- missing at random
- MICE
- multiple imputation by chained equations
- MRC
- Medical Research Council
- NMB
- net monetary benefit
- OSIS
- Oxford Shoulder Instability Score
- PI
- principal investigator
- PPI
- patient and public involvement
- PROM
- patient-reported outcome measure
- PSS
- personal social services
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- SOP
- standard operating procedure
- TASD
- traumatic anterior shoulder dislocation
- TIDieR
- Template for Intervention Description and Replication
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- WCTU
- Warwick Clinical Trials Unit
