Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 14/08/60. The contractual start date was in April 2016. The draft manuscript began editorial review in February 2023 and was accepted for publication in September 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 James et al. This work was produced by James et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 James et al.
Chapter 1 Introduction and background literature
Bladder cancer (BC) is the fifth most common cancer in Western society. In the UK there are approximately 10,000 new cases and 5000 deaths attributed to BC annually. 1 In Western populations, over 90% of BCs are urothelial cell carcinoma (UCC). Standard management follows a pattern established during the 1950s with the development of the rigid cystoscope. Improvements in this pathway have high priority from patient Delphi consensus work. 2 Prognosis has not improved in the last 30 years. 3–5
Standard management involves a pathway of diagnostic flexible cystoscopy followed by a transurethral resection of the bladder tumour (TURBT) with a rigid cystoscope. 6,7 TURBT has the multiple purposes of diagnosis, staging and treatment of non-muscle-invasive BC (NMIBC), that is removal of the tumour. Further treatments, such as neoadjuvant chemotherapy, radical cystectomy or chemoradiotherapy, are then necessary for muscle-invasive BC (MIBC). 8
For MIBC, this initial TURBT often understages invasion (up to 30% of MIBCs are initially staged as high-grade NMIBC at first TURBT5) and may contribute to extravesical tumour dissemination through bladder perforation or venous emboli generated through the high-pressure resection process. 9 Cross-sectional pelvic imaging after TURBT impedes the accuracy of staging due to surgical artefacts (such as perivesical inflammation and reactive lymph nodes). 10–13
Typically, the need for a TURBT, histopathological review and multidisciplinary team (MDT) decision-making adds at least 6–12 weeks to the pathway, prolonging the delay to commencing (the most appropriate) correct radical treatment for patients with MIBC. 5,14–17
An ideal pathway would separate NMIBC patients from MIBC patients at the time of initial macroscopic diagnosis. Faster and more accurate application of established technologies would then streamline therapy, potentially improving outcomes and saving clinical costs.
For the 75–80% of BC patients who present with NMIBC, tumour recurrence and progression following TURBT are significant issues, compelling current guidelines to recommend intense long-term surveillance by cystoscopy and urine cytology. With the UK prevalence of BC estimated at 46,500, at any one time there will be 35,000–37,000 patients with NMIBC requiring such surveillance, performed as often as every 3–6 months at an estimated cost of at least £533 per flexible cystoscopy/cytology ‘episode’ (as costed in 201018). Around 30% of NMIBC cases will progress to MIBC and require additional therapy.
Around 20–25% of new BC patients present with de novo MIBC. 19,20 Survival with MIBC remains poor (27–50% 5-year survival) and has not improved in the past 30 years. 1 The present pathway is largely geared to the treatment of NMIBC patients and actively delays effective MIBC treatment, which is often carried out in a different hospital to initial diagnosis and TURBT, increasing handovers and therefore delays. In Birmingham, for example, many NHS Trusts run haematuria clinics, a smaller number offer systemic chemotherapy, but only two carry out major pelvic surgery, and only one radiotherapy. Early clarity on staging and diagnosis would facilitate more coordinated planning and treatment delivery. Similar considerations exist in all major healthcare systems worldwide, especially in North America where BC patients are frequently diagnosed in an ‘office urology’ setting21 and then need referral into the hospital setting for TURBT and definitive therapy.
This fragmented care with complicated staging and follow-up leads to the cumulative cost of treating BC exceeding all other forms of human cancer.
The current shared patient pathway thus delays therapy for MIBC patients. There is a growing body of opinion that such pathways should separate earlier in order to more appropriately and expeditiously treat MIBC patients,3 and this is what we evaluated in BladderPath.
Staging and treatment
The pros and cons of staging and treatment techniques for BC are summarised in Table 1, including the aim of TURBT in the settings of NMIBC and MIBC.
| Aim of TURBT | NMIBC | MIBC |
|---|---|---|
| Diagnosis | ✓ | ✓ |
| Staging | ✓ | Sometimes – under-staging in up to 30% |
| Treatment | ✓ | No – may be harmful |
| Palliation of symptoms | Sometimes in cases of heavy bleeding | Sometimes in cases of heavy bleeding |
From the above, it is clear that the main functions of TURBT in MIBC are histological diagnosis of cancer and staging. Diagnosis does not require large quantities of tissue – very small amounts are sufficient to confirm the presence of high-grade malignant cells to ascertain grade (as exemplified by the almost ubiquitous use of urine cytology). The main function of TURBT in MIBC therefore is to assess stage. Where muscle is adequately sampled and is found to contain tumour, a diagnosis of MIBC is correct by definition (although not a more comprehensive nodal or metastasis stage). The issue is the understaging of high-grade tumours due to inadequate sampling of muscle that subsequently turns out to be involved by tumour. As cystectomy is a recognised treatment for high-risk NMIBC,12 either at diagnosis or after the failure of treatments such as bacillus Calmette–Guérin (BCG), the false-negative rate with respect to distinguishing NMIBC from MIBC in the highest risk cases can be estimated – this appears to be as high as 30%,1,6 although will clearly vary depending upon the surgeon and referred case mix. Within this context, we can assess the accuracy of magnetic resonance imaging (MRI) for the same purpose. The key factor here is the split between tumours of stage pT1 and lower versus pT2 and higher.
Thus, the diagnostic function of TURBT can be substituted by a smaller biopsy obtained during outpatient flexible cystoscopy. For staging, multiparametric MRI (mpMRI) has performance characteristics that exceed those reported for TURBT, are less subject to operator variability and are amenable to external review. 22–24 Furthermore, in most cases, the therapeutic benefit for TURBT in MIBC patients remains unproven, particularly if cystectomy is the preferred definitive treatment option. The literature on staging BC has been recently reviewed by Bouchelouche and co-workers. 25–27
A role for TURBT as palliation of severe symptoms from MIBC pending a definitive treatment decision will remain. Its precise magnitude will be quantified in this study but is likely to be limited as, in most cases, symptoms such as haematuria are intermittent (one of the factors leading to delayed presentation).
Hypothesis
The purpose of the BladderPath study is to evaluate a new pathway that would eliminate TURBT from the initial staging of MIBC patients. This allows more expeditious treatments for both MIBC (by eliminating delays and improved targeting of subsequent therapy) and NMIBC (by reducing demand for TURBT in the system). Our approach integrates flexible cystoscopy, urine cytology, biopsy and detailed imaging to confirm the diagnosis and stage of disease. Appropriate definitive radical therapy can then be rapidly commenced. This could include TURBT if indicated for reasons such as diagnostic uncertainty, assessment of carcinoma in situ (CIS; e.g. for planning cystectomy) or debulking prior to radiotherapy. For the purposes of the trial, TURBT in patients with MIBC did not count as part of their definitive treatment. This is a paradigm shift in the context of BC but is standard practice in virtually every other solid tumour setting (e.g. prostate, breast, lung). Although TURBT is considered a standard part of care for NMIBC, for MIBC it is less obviously essential, particularly for patients undergoing subsequent radical surgery (cystectomy). This study tested the utility of TURBT and mpMRI as components of the initial care for MIBC patients in a randomised fashion.
Rationale
The prognosis for MIBC remains poor and has not changed for three decades. 1,3,4 Modern MRI approaches now have the ability to accurately stage bladder tumours11,28,29 and experimental urinary biomarkers show great promise in identifying MIBC from a urine test. 30,31 The platforms therefore exist to improve patient pathways, potentially leading to improved outcomes.
In order to change the current pathway, we need to show that alternatives to TURBT exist for staging, and that faster treatment will improve outcomes:
-
Do we need TURBT for histology?
-
Flexible cystoscopy and biopsy can give accurate tumour histological diagnosis and grading but does not assess stage or muscle invasion.
-
-
Can we replace TURBT for detailed assessment of the bladder tumour?
-
TURBT is frequently inaccurate and operator dependent – up to 30% of tumours assessed as high-grade NMIBC at TURBT are subsequently diagnosed as invasive (MIBC) on repeat TURBT or at cystectomy. 5,32
-
Guidelines recommend repeat TURBT for patients staged G3pT1 because of the high incidence of understaging – further delaying correct treatment in some patients with MIBC. 5,32
-
Sensitivity and specificity of mpMRI for separating NMIBC from MIBC are 94% and 100%, respectively. 11,27–29,33,34
-
Introducing mpMRI ahead of TURBT (if indicated) should not compromise staging and may improve it.
-
-
Is TURBT an essential component of treatment?
-
There are no randomised data on this topic – this is one of the aims of this study.
-
Evidence exists that TURBT may increase local tumour dissemination35 and lead to increases in circulating tumour cells. 9
-
In most other oncology settings, imaging and biopsy are sufficient for correct treatment; in some cases, imaging alone is sufficient (e.g. kidney cancer and upper tract urothelial cancer). Few tumour sites use an intermediate piecemeal debulking ahead of definitive therapy. 36
-
-
Does delaying the correct definitive treatment affect prognosis?
Aims and objectives
The aims of the BladderPath study are to evaluate whether it is possible to expedite radical treatment for patients with MIBC using MRI rather than TURBT to diagnose and more accurately and rapidly stage their cancer. We hypothesise this may improve outcomes from MIBC by reducing the time from diagnosis to radical treatment.
Outcome measures
The primary and secondary outcomes change as we go through the study. These are summarised in Table 2.
| Primary outcomes | Secondary outcomes | |
|---|---|---|
| Feasibility stage | The proportion of possible MIBC patients randomised to Pathway 2 who correctly follow pathway protocol |
|
| Intermediate stage | The TTCT for patients who were initially classified as possible MIBC and then were confirmed to have MIBC |
|
Methods
BladderPath is a randomised trial comparing risk-stratified image-directed (mpMRI) care with TURBT for patients with newly diagnosed BC. Patients with symptoms suspicious of a new diagnosis of BC were identified via haematuria clinics and provided written informed consent for study participation. Ineligible patients were those who were unable or unwilling to undergo MRI, those with a previous BC diagnosis and those who had previously entered the study. Participants were randomised to the standard clinical pathway (Pathway 1: all patients undergo TURBT) or the investigational pathway (Pathway 2) whereby those participants with possible MIBC (Likert 3–5 as visually assessed on a 5-point Likert scale at flexible cystoscopy) undergo initial mpMRI-based assessment with flexible cystoscopy tumour biopsy.
Pathway 2 probable NMIBC (Likert 1–2) participants underwent TURBT. Primary outcomes: feasibility phase – proportion of Pathway 2 possible MIBC participants who correctly followed protocol (target: 80%); intermediate stage – time to first correct treatment (chemotherapy, radiotherapy, surgery, decision for palliative care) for participants with confirmed MIBC (target: 30-day improvement). Randomisation was achieved by using a computerised allocation program; stratification variables included participants’ sex, age and clinician’s initial visual assessment of muscle invasiveness of the tumour. Blinding of participants, caregivers and outcome assessors was not possible.
Study design
The Image Directed Redesign of BC Treatment Pathways (‘BladderPath’) study was an open-label, multistage, randomised controlled study with three overlapping stages: feasibility, intermediate and final efficacy stage. BladderPath was conducted by the Urology units in 15 UK hospitals and was sponsored by the University of Birmingham, UK, with NHS Research ethics approval (17/LO/1819, ISRCTN 35296862), funded by the UK National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) scheme. 14,38 The study protocol is available online: www.birmingham.ac.uk/research/crctu/trials/bladder-path/index.aspx.
Participants
Following the provision of dedicated patient information sheets, participants were recruited by Urology teams at hospital outpatient haematuria clinics. 39 A two-stage written informed consent process was adopted to allow prospective collection of urine samples before initial cystoscopy for a diagnostic urinary biomarker substudy (first stage),35 with confirmatory written informed consent undertaken following cystoscopy (second stage). Hence, inclusion criteria were: patients attending clinic for the investigation of symptoms suspicious of BC (initial consent process), and patients given a diagnosis of suspected BC and requiring TURBT based on visual cystoscopic examination of the bladder (confirmatory consent process following outpatient flexible cystoscopy). Excluded patients were those unable or unwilling to undergo MRI, those with a previous diagnosis of BC and previous entry in the present study.
Randomisation and masking
Participants were randomised by computer using minimisation on a 1 : 1 basis to Pathway 1 (standard of care: TURBT) or Pathway 2 (investigational: mpMRI): minimisation factors used were patient sex (male/female), age (< 75/≥ 75 years old) and clinician assessment at outpatient flexible cystoscopy (probable NMIBC/possible MIBC). A random element was incorporated into the minimisation algorithm at 20% ensuring it was not predictable. Randomisation was not blinded, with both participants and healthcare teams knowing which pathway had been allocated to participants.
Procedures
The study compared TURBT with mpMRI for the initial assessment of possible MIBC. The current SOC pathway comprises flexible cystoscopy in outpatient clinics combined with upper urinary tract imaging and, potentially, cross-sectional imaging of the bladder/pelvis followed by TURBT for participants with lesions suspicious for BC.
Participants were randomised to either Pathway 1 or 2 following visual assessment of the suspicion of NMIBC or MIBC at the time of outpatient flexible cystoscopy. The definition of likelihood of MIBC was based on a 5-point Likert scale: (1) strongly agree or (2) agree that the lesion is NMIBC, or (3) equivocal, or (4) agree or (5) strongly agree that the lesion is MIBC. Likert 1 and 2 were considered probable NMIBC and 3, 4 and 5 were considered possible MIBC.
Pathway 1
For lesions suspicious for BC, inpatient TURBT was subsequently undertaken. TURBT was conducted as recommended by the European Association of Urology (EAU) and British Association of Urological Surgeons (BAUS):6 resection of the exophytic component, sampling of underlying detrusor muscle, recording of clinical stage post TURBT (complete/incomplete resection, semi-fixed/fixed mass, etc.), bladder neck or urethral sampling for patients suitable for neobladder reconstruction, and sampling of areas suspicious of CIS. Separate biopsies of the tumour base were taken, with all samples sent for histopathological reporting and multidisciplinary review.
Pathway 2
In the investigational pathway, participants visually identified as probable NMIBC (Likert 1–2) underwent TURBT as SOC; participants identified as possible MIBC underwent mpMRI instead of TURBT. The criteria for diagnosing patients as possible MIBC were as follows: by appearance on flexible cystoscopy (Likert 3–5), by examination (the presence of a semi-fixed mass within the bladder before or after flexible cystoscopy), by cytology (the presence of high-grade urothelial cells in either urine or flexible cystoscopy biopsy) or by cross-sectional imaging when used [e.g. computerised tomography (CT) urography]. Where possible, biopsy of suspicious lesions was carried out during initial outpatient flexible cystoscopy; if not achieved, then a confirmatory tissue sample was taken at a subsequent outpatient flexible cystoscopy.
For possible MIBC participants, mpMRI was conducted and reported locally according to the BladderPath Imaging Manual. With the subsequent development of the ‘Vesical Imaging-Reporting and Data System (VI-RADS)’ protocol,22 later patients were assessed using this system. Two of the VI-RADS authors are also part of the BladderPath team and hence both the systems were similar. Following mpMRI, TURBT was permitted for possible MIBC participants for the following indications: to ascertain the presence of histological variants; to debulk the tumour prior to radical therapy (e.g. prior to chemoradiotherapy); lack of confidence that the MRI showed MIBC; to perform examination under anaesthesia in order to assess operability; to assess for CIS; to obtain prostatic urethral biopsies when considering neobladder reconstruction; to re-stage after neo-adjuvant chemotherapy; or for the management of symptoms (e.g. haematuria).
All participants
Non-muscle-invasive bladder cancer participants underwent TURBT. In accordance with national and international guidelines, radical treatment with chemotherapy, surgery or (chemo) radiation was offered to all participants with MIBC where appropriate, based upon the results of either TURBT or mpMRI staging or both. For both groups, if unsuited to radical treatment, participants were referred for palliative care. All treatment decisions were made by the treating MDT. The study schema is shown in Figure 1.
FIGURE 1.
BladderPath Trial schema.
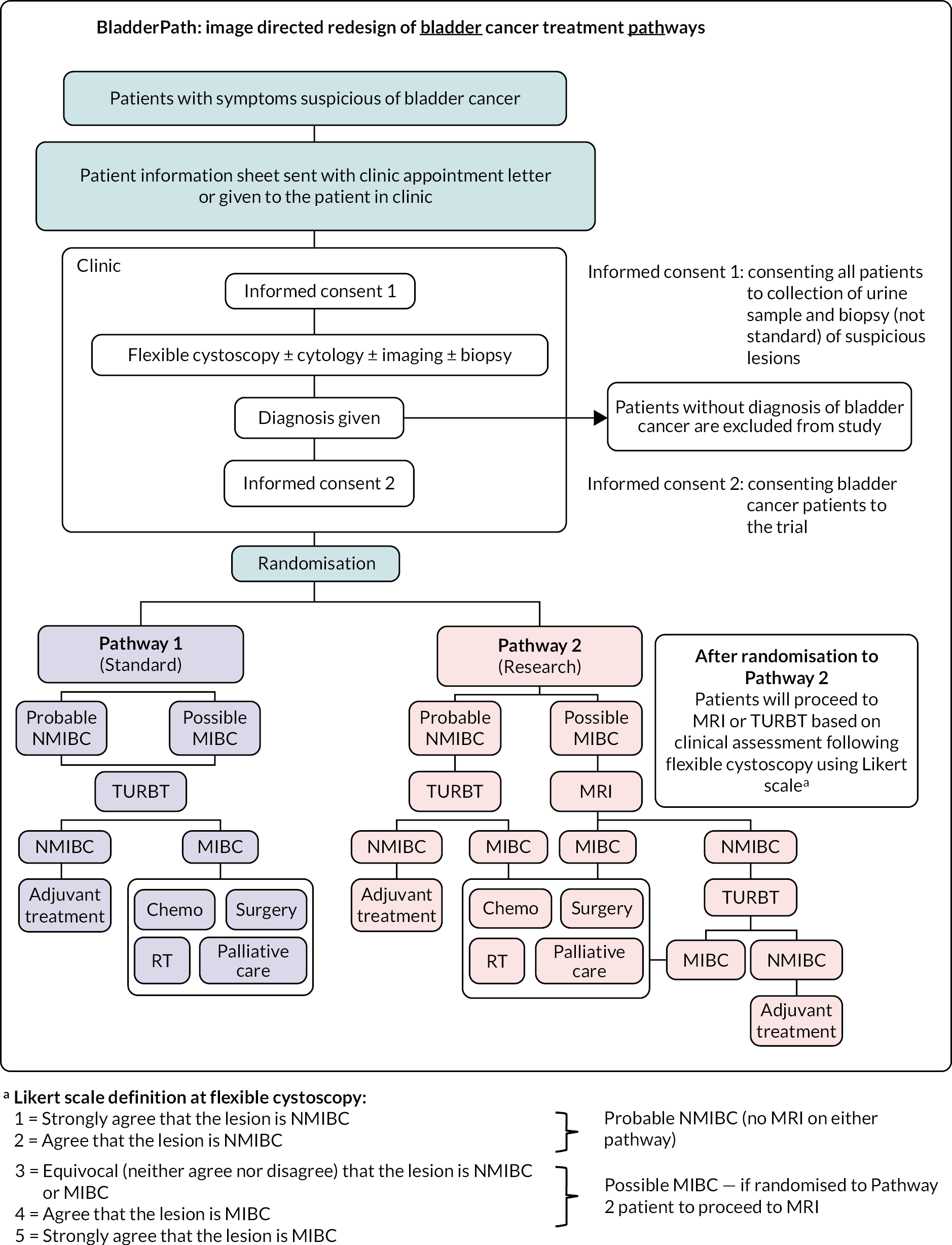
Assessments
Initial clinical assessments (visit 1), potentially split over more than one visit depending on local practice) comprised: medical history (including concomitant medication), full blood count (FBC), liver function tests (LFTs), urea and electrolytes (U+Es), collection of urine samples for translational research, tumour biopsy (either via flexible cystoscopy at initial visit or at a subsequent visit if randomised to pathway 2 and considered possible MIBC) and completion of a participant reported outcomes quality of life booklet. At the time of the study procedure (visit 2, for either TURBT or mpMRI), a review of adverse events (AEs) was undertaken. At the decision to treat (DTT) (visit 3, following TURBT or mpMRI and multidisciplinary review), assessments comprised: medical history, FBC, LFTs, U+Es, and review of AEs. Adjuvant therapy and follow-up were according to SOC dependent upon NMIBC risk category6 or MIBC treatment strategy. 14
Outcomes
The aims of the BladderPath study were to evaluate whether it was possible to expedite radical treatment for participants with MIBC using mpMRI rather than TURBT to more accurately and rapidly stage their cancer. We hypothesised that this may improve outcomes from MIBC by reducing the time from diagnosis to the correct (radical) treatment. The study was conceived as three stages with the primary outcomes of feasibility, time to correct therapy (TTCT) for MIBC and clinical progression-free survival.
Feasibility stage
Primary outcome: the proportion of possible MIBC participants randomised to Pathway 2 who correctly follow pathway protocol. Secondary outcomes: overall proportion of participants who correctly follow protocol on each pathway for all randomised participants, recruitment and retention rates at each study site, counts of each type of correct treatment.
Intermediate stage
Primary outcome: TTCT for participants initially classified as possible MIBC and then confirmed to have MIBC. Secondary outcomes: TTCT for all randomised participants, TTCT for probable NMIBC participants confirmed as NMIBC, time to definitive treatment (TTDT) for all randomised participants.
Correct treatment was defined as TURBT for all confirmed NMIBC participants. For confirmed MIBC participants, the correct treatment may have included systemic chemotherapy, radiotherapy, cystectomy and/or palliative care. The final result of MIBC/NMIBC was based on cystectomy/TURBT pathological tumour staging. For participants who had MRI-diagnosed MIBC and were treated as MIBC (i.e. received at least one of the correct treatments for MIBC), their final result was MIBC. For participants who had MRI-diagnosed NMIBC, their correct treatment was TURBT.
Definitive treatment is defined under NHS guidelines, and the definitive treatment for BC was as TURBT at study inception. In this study, TURBT was used for diagnosis and treatment, and termed definitive treatment for all participants initially classified as probable NMIBC and for Pathway 1 participants classified as possible MIBC. For MIBC-classified participants randomised into Pathway 2, the definitive treatment at study inception included TURBT, systemic chemotherapy, radiotherapy, cystectomy and/or palliative care. Subsequently, NHS guidelines changed to align with our study definitions and TURBT was removed from the list of definitive treatments for MIBC and hence the TTDT end point became superfluous as it become identical to our TTCT end point and hence is not reported.
Final result of MIBC/NMIBC was based on cystectomy/TURBT pathological tumour staging if available. For participants who had MRI-staged MIBC and were treated as MIBC (i.e. received at least one of the correct treatments for MIBC), their final result was MIBC. Participants who were MRI-diagnosed NMIBC were to undergo TURBT as their correct treatment.
Sample size calculation
In the feasibility stage, the target sample size was 150 participants (approximately 38 possible MIBC participants in Pathway 2). If the proportion of possible MIBC participants randomised to Pathway 2 who correctly follow pathway protocol exceeded 80%, the image-directed Pathway 2 was considered feasible in clinical practice. 38
For the Intermediate stage, the primary outcome was TTCT. We assumed that the TTCT in standard care had a median of 100 days and the effects of mpMRI would be to reduce the median TTCT to 70 days for MIBC participants. If the distribution of the TTCT for participants undergoing mpMRI followed a Weibull distribution with the same shape parameter as those receiving standard care, and that the usual proportional hazards assumption held, then a ‘hazard ratio (HR)’ of 3.6 was the effect size we wished to detect. More specifically, on average, it would be 3.6 times quicker to receive correct treatment for MIBC participants who underwent mpMRI compared to those underwent TURBT. To have 80% power to detect a HR of 3.6 using a Cox model required 20 MIBC participants. Around 20–25% of new BC participants present with de novo MIBC; hence, to recruit 20 MIBC participants, approximately 80–100 participants were required.
In the intermediate stage, the power was event driven and depended upon the number of observed events.
Due to slow recruitment (affected by COVID-19 the pandemic), it was unfeasible to reach the sample size required for a final clinical stage. A decision was made by the Trial Management Group (TMG), in discussion with the NIHR HTA, to close recruitment after sufficient participants for the first two stages had been recruited. The trial closed to recruitment on 31 December 2021. The database was locked on 20 September 2022 to allow a period of follow-up for the time-to-event outcomes. Longer-term follow-up to 2 years for all patients will be carried out via NHS digital records using methodology piloted during the study and will be reported once available in a separate publication.
Statistical analysis
The statistical analyses were carried out on an intention-to-treat basis, retaining patients in their randomised pathway groups and including patients who were protocol deviations and ineligible patients.
Proportions were calculated using the exact method and presented with 95% confidence intervals (CIs). Time-to-event estimates were assessed using Kaplan–Meier method and presented with 95% CIs. Time-to-event outcomes were analysed using a Cox regression model with stratification factors of age and sex included as covariates and study centre included as a random effect. Proportional hazards assumptions were investigated using Schoenfeld residuals and log–log plots. For instances when the Cox regression method was not appropriate due to small sample sizes, a mixed-effect Weibull survival model was utilised.
Health economics
Quality of life and health economics were outcomes for the final stage which was not completed. A basic health economic section has been added. Quality-of-life questionnaires from baseline assessment and subsequent follow-up time points were requested from consenting randomised participants. The returned data will be analysed and reported alongside the long-term follow-up data.
EuroQol-5 Dimensions data were collected but a formal health economic analysis was not carried out, as this would require the long-term outcomes data which are not yet available. We have however carried out some simple cost modelling using tariffs from NHS England40 to estimate the crude cost impact of introducing MRI into the pathway for possible muscle-invasive disease.
Oversight
Trial Management Group: The TMG consisted of the Chief Investigator (Professor Nicholas James), Co-investigator Urology (Professor James Catto, Mr Prashant Patel and Mr Kieran Jefferson), Co-investigator Patient Involvement (Ms Jean Gallagher), Co-investigator Biomarker Research (Dr Richard Bryan), Co-investigator Qualitative substudy (Dr Veronica Nanton), Co-investigator Health Informatics (Ms Alicia Jakeman), Biology Systems Co-investigator, Statistics Co-investigator, Imaging Co-investigators, Medical Oncology Co-investigator, Trial Management Team Leader, Senior Trial Coordinator, Trial Coordinator/Administrator, Lead Statistician and Trial Statistician. Notwithstanding the legal obligations of the Sponsor and Chief Investigator, the TMG were responsible for the day-to-day running and management of the trial.
Data analyses were supplied in confidence to an independent Data Monitoring Committee (DMC) who monitored patient safety and advised on whether the accumulated data justified continuation of recruitment. DMC meetings were scheduled at least annually until the study closed to recruitment.
Chapter 2 Results
Recruitment
Between 31 May 2018 and 31 December 2021, 15 of the 17 centres open to the BladderPath study recruited at least 1 participant; 638 patients were screened as potentially eligible, of which 309 were registered and 143 randomised (72 to Pathway 1, 71 to Pathway 2); 166 registered patients not randomised were not found to have BC during initial cystoscopy. Figure 2 shows cumulative accrual versus target recruitment during the course of the study. The graph clearly shows the impact of the COVID-19 pandemic with cessation of recruitment for most of 2020, as required by the NHS pandemic response. Post pandemic, the recruitment rate was clearly much slower than prior to the event.
FIGURE 2.
Recruitment between June 2018 and December 2021.
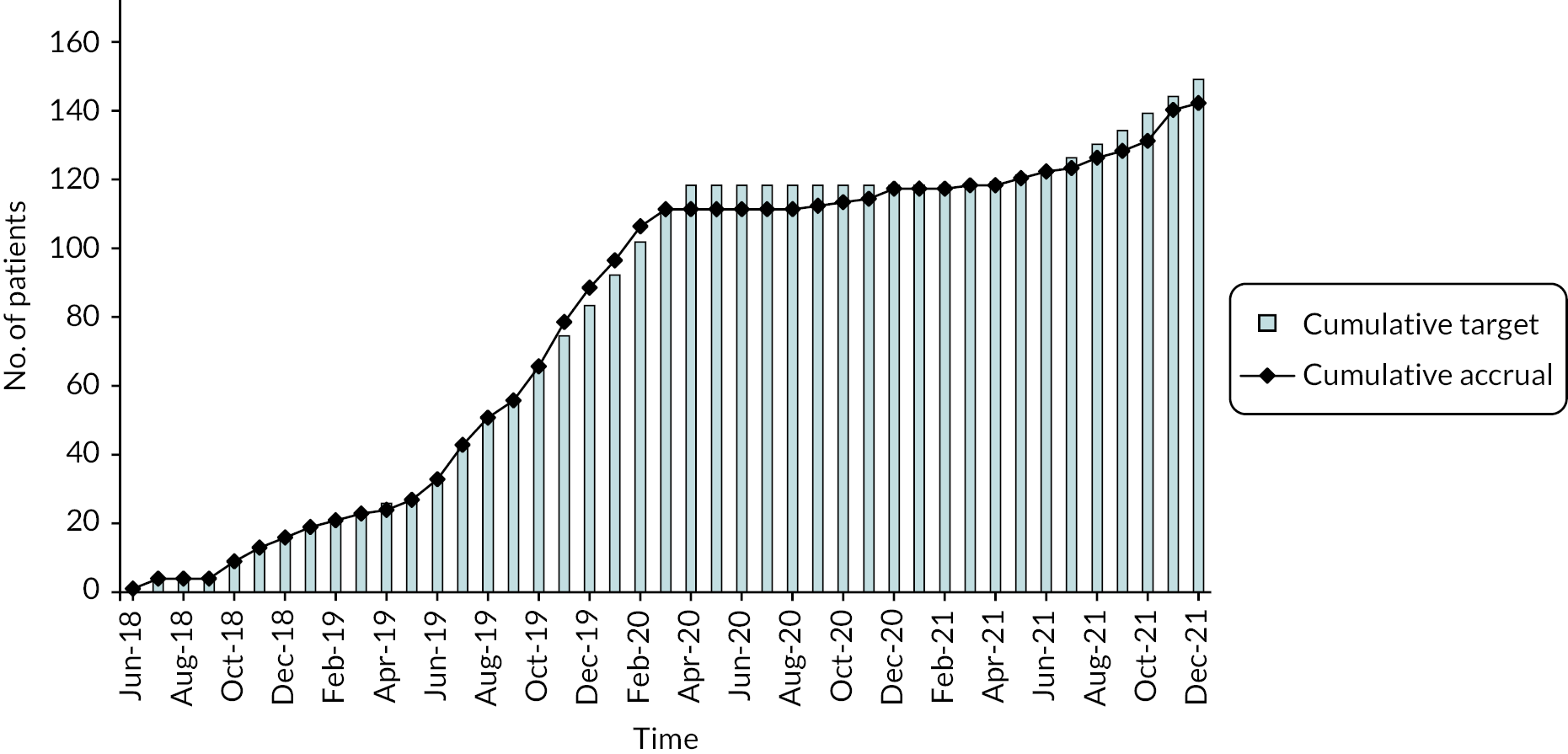
Recruitment targets were adjusted several times, initially aiming to improve recruitment (notably between June 2019 and February 2020) and, subsequently, to account for the devastating effect of the COVID-19 pandemic on recruitment from March 2020 onwards. The study eventually recruited 143 participants, summarised in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 3), close to the feasibility stage target of 150. Despite most sites having reopened to recruitment following the pandemic, fewer patients were able or willing to consider taking part in the study, and one site was unable to re-open.
FIGURE 3.
Consolidated Standards of Reporting Trials flow chart – showing recruitment through to randomised allocation.

Outcomes following randomisation are shown in a separate diagram (Figure 4).
FIGURE 4.
Illustration of the flow of participants through the study. aPopulation in primary outcome analysis (i.e. possible MIBC participants confirmed MIBC by TURBT/cystectomy or treated with MIBC therapy, 14 in Pathway 1 and 12 in Pathway 2). NAC, neo-adjuvant chemotherapy; Pal Care, Palliative care; SCR, synchronous chemo-radiotherapy.

Losses and exclusions
No patients were reported as being lost to follow-up during the study.
Ineligibilities
Three participants were subsequently found to be ineligible post randomisation: one in Pathway 1 and one in Pathway 2 due to estimated glomerular filtration rate (eGFR) below the accepted range and one participant in Pathway 2 due to ineligibility for MRI scanning.
Protocol deviations
Nine protocol deviations were reported by nine participants (five in Pathway 1, four in Pathway 2), mainly due to administrative error, summarised in Table 3.
| Category of deviation | Pathway 1 | Pathway 2 |
|---|---|---|
| Participant underwent MRI rather than TURBT due to an administrative error | 1 | 2 |
| Patient incorrectly randomised as possible MIBC instead of probably NMIBC as per initial flexible cystoscopy – clinician’s error | 1 | 0 |
| Randomised after having had MRI | 1 | 0 |
| Participant had ad hoc mpMRI prior to protocol stipulated TURBT – clinician decision | 1 | 0 |
| MRI performed for superficial looking disease – misunderstanding of protocol | 0 | 1 |
| Initial flexible cystoscopy form returned Likert score of 2; however, at time of randomisation, clinician’s assessment was possible MIBC based on available information at the time | 0 | 2 |
Patient withdrawal of consent
Seven patients withdrew from the main trial (of which five also withdrew consent from all substudies). Of the seven withdrawals, three were found not to have cancer on histopathology, two participants felt unable to continue including one with severe dementia, one experienced delays in patient care timeline and one withdrew due to the complex nature of the diagnosis.
Six out of the seven participants who wished to withdraw from trial were not willing for further data to be supplied to the Trials Office (Table 4). One patient did not specify their wish to allow further data collection, not being able to remember consenting to take part – the clinical team withdrew the patient on discovering the participant had dementia.
| Pathway 1 (N = 72) | Pathway 2 (N = 71) | Overall (N = 143) | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Full withdrawal of consent | 3 | 4.16 | 4 | 5.63 | 7 | 4.89 |
| Withdrawal from: | ||||||
|
1 | 4 | 5 | |||
|
1 | 4 | 5 | |||
|
1 | 4 | 5 | |||
Stratification factors
Participants were stratified by three factors at randomisation (Table 5).
| Pathway 1 (N = 72) | Pathway 2 (N = 71) | Overall (N = 143) | ||||
|---|---|---|---|---|---|---|
| Stratifying variable | N | % | N | % | N | % |
| Sex | ||||||
|
55 | 76.4 | 53 | 74.6 | 108 | 75.5 |
|
17 | 23.6 | 18 | 25.4 | 35 | 24.5 |
| Age | ||||||
|
48 | 66.7 | 49 | 69.0 | 97 | 67.8 |
|
24 | 33.3 | 22 | 31.0 | 46 | 32.2 |
| Clinician’s initial assessment | ||||||
|
34 | 47.2 | 32 | 45.1 | 66 | 46.2 |
|
38 | 52.8 | 39 | 54.9 | 77 | 53.8 |
Participant characteristics
Characteristics of the 143 randomised participants are shown in Table 6.
| Pathway | Pathway 1 (n = 72) | Pathway 2 (n = 71) | Overall (n = 143) |
|---|---|---|---|
| Height (cm) | |||
| N | 66 | 60 | 126 |
| Mean (SD) | 171.2 (8.9) | 171.5 (8.8) | 171.3 (8.9) |
| Median | 173.0 | 172.5 | 173.0 |
| IQR | 165.0–178.0 | 163.5–179.0 | 165.0–178.0 |
| Range | 147.0–187.0 | 152.0–191.0 | 147.0–191.0 |
| Weight (kg) | |||
| N | 65 | 61 | 126 |
| Mean (SD) | 83.6 (16.2) | 85.3 (17.8) | 84.4 (16.9) |
| Median | 82.0 | 82.0 | 82.0 |
| IQR | 71.8–91.4 | 72.0–97.8 | 72.0–96.0 |
| Range | 55.2–137.4 | 50.7–127.0 | 50.7–137.4 |
| WHO performance status | |||
| 0 | 54 (79.4) | 52 (78.8) | 106 (79.1) |
| 1 | 8 (11.8) | 10 (15.2) | 18 (13.4) |
| 2 | 3 (4.4) | 2 (3.0) | 5 (3.7) |
| 3 | 3 (4.4) | 2 (3.0) | 5 (3.7) |
| Not known | 4 | 5 | 9 |
| eGFR (value used for eligibility assessment ≥ 40 ml/minute/1.73 m2) | |||
| N | 72 | 71 | 143 |
| Mean (SD) | 74.0 (16.2) | 72.7 (16.2) | 73.4 (16.1) |
| Median | 79.0 | 78.0 | 78.0 |
| IQR | 60.5–89.0 | 60.0–88.0 | 60.0–88.0 |
| Range | 30.0–99.0 | 39.0–113.0 | 30.0–113.0 |
| Smoking history | |||
| Non-smoker | 19 (27.1) | 25 (37.3) | 44 (32.1) |
| Ex-smoker | 40 (57.1) | 35 (52.2) | 75 (54.7) |
| Smoker | 11 (15.7) | 7 (10.4) | 18 (13.1) |
| Not known | 2 | 4 | 6 |
| Number of cigarettes per day | |||
| N | 34 | 28 | 62 |
| Mean (SD) | 17.5 (10.8) | 15.5 (8.4) | 16.6 (9.7) |
| Median | 20.0 | 16.5 | 20.0 |
| IQR | 10.0–20.0 | 10.0–20.0 | 10.0–20.0 |
| Range | 1.0–40.0 | 1.0–40.0 | 1.0–40.0 |
Research sites were asked to provide baseline biochemistry and haematology results if tested as part of standard care at the time of screening, or within a short period prior to study entry. These results, as summarised in Tables 7 and 8, were only recorded at baseline with a view to forming a baseline picture. Blood tests were not repeated at subsequent time points for analysis purposes.
| Pathway | Pathway 1 (n = 72) | Pathway 2 (n = 71) | Overall (n = 143) |
|---|---|---|---|
| Serum creatinine (μmol/L) | |||
| N | 71 | 68 | 139 |
| Mean (SD) | 85.8 (24.8) | 87.3 (21.4) | 86.6 (23.1) |
| Median | 76.0 | 82.5 | 80.0 |
| IQR | 70.0–95.0 | 71.5–98.0 | 71.0–96.0 |
| Range | 57.0, 193.0 | 56.0–166.0 | 56.0–193.0 |
| Urea (mmol/L) | |||
| N | 64 | 62 | 126 |
| Mean (SD) | 6.1 (2.0) | 6.2 (2.0) | 6.2 (2.0) |
| Median | 5.8 | 5.9 | 5.8 |
| IQR | 5.2–7.0 | 5.0–6.9 | 5.0–6.9 |
| Range | 2.5–14.7 | 2.6–13.6 | 2.5–14.7 |
| Albumin (g/L) | |||
| N | 40 | 38 | 78 |
| Mean (SD) | 40.7 (6.5) | 43.8 (3.3) | 42.2 (5.4) |
| Median | 42.0 | 44.0 | 43.0 |
| IQR | 37.5–44.5 | 42.0–46.0 | 40.0–46.0 |
| Range | 14.0–51.0 | 33.0–50.0 | 14.0–51.0 |
| Total protein (g/L) | |||
| N | 32 | 34 | 66 |
| Mean (SD) | 68.6 (4.4) | 71.8 (5.0) | 70.2 (4.9) |
| Median | 68.0 | 71.5 | 70.0 |
| IQR | 65.5–71.0 | 69.0–75.0 | 67.0–73.0 |
| Range | 61.0–78.0 | 59.0–84.0 | 59.0–84.0 |
| Bilirubin (μmol/L) | |||
| N | 40 | 37 | 77 |
| Mean (SD) | 8.3 (4.9) | 8.5 (3.1) | 8.4 (4.1) |
| Median | 7.0 | 8.0 | 8.0 |
| IQR | 5.0–10.0 | 7.0–10.0 | 6.0–10.0 |
| Range | 3.0–29.0 | 4.0–21.0 | 3.0–29.0 |
| AST or ALT (IU/L) | |||
| N | 41 | 36 | 77 |
| Mean (SD) | 22.5 (10.1) | 22.8 (9.9) | 22.6 (10.0) |
| Median | 22.0 | 20.0 | 21.0 |
| IQR | 14.0–29.0 | 15.0–30.5 | 14.0–29.0 |
| Range | 5.0–43.0 | 7.0–48.0 | 5.0–48.0 |
| Alk phos (IU/L) | |||
| N | 43 | 35 | 78 |
| Mean (SD) | 93.4 (74.3) | 77.1 (20.3) | 86.1 (57.1) |
| Median | 80.0 | 75.0 | 77.0 |
| IQR | 65.0–102.0 | 64.0–91.0 | 65.0–97.0 |
| Range | 38.0–543.0 | 36.0–119.0 | 36.0–543.0 |
| Sodium (mmol/L) | |||
| N | 70 | 65 | 135 |
| Mean (SD) | 139.6 (4.1) | 140.0 (2.6) | 139.8 (3.4) |
| Median | 140.5 | 140.0 | 140.0 |
| IQR | 137.0–142.0 | 139.0–142.0 | 138.0–142.0 |
| Range | 122.0–149.0 | 133.0–145.0 | 122.0–149.0 |
| Potassium (mmol/L) | |||
| N | 67 | 64 | 131 |
| Mean (SD) | 4.5 (0.4) | 4.4 (0.4) | 4.5 (0.4) |
| Median | 4.5 | 4.4 | 4.5 |
| IQR | 4.2, 4.8 | 4.2, 4.8 | 4.2, 4.8 |
| Range | 3.4, 5.7 | 3.4, 5.2 | 3.4, 5.7 |
| PSA (males only) (ng/ml) | |||
| N | 26 | 34 | 60 |
| Mean (SD) | 6.6 (11.0) | 8.8 (16.5) | 7.9 (14.3) |
| Median | 2.3 | 3.0 | 2.5 |
| IQR | 0.9–5.0 | 1.7–8.0 | 1.4–6.7 |
| Range | 0.0–43.0 | 0.1–79.0 | 0.0–79.0 |
| Pathway | Pathway 1 (n = 72) | Pathway 2 (n = 71) | Overall (n = 143) |
|---|---|---|---|
| Hb (g/L) | |||
| N | 65 | 68 | 133 |
| Mean (SD) | 138.5 (16.7) | 142.2 (13.7) | 140.4 (15.3) |
| Median | 141.0 | 143.5 | 142.0 |
| IQR | 127.0–151.0 | 135.0–151.0 | 134.0–151.0 |
| Range | 101.0–172.0 | 104.0–173.0 | 101.0–173.0 |
| WBC (x109/L) | |||
| N | 66 | 68 | 134 |
| Mean (SD) | 8.4 (3.5) | 7.9 (2.5) | 8.1 (3.0) |
| Median | 8.0 | 7.5 | 7.6 |
| IQR | 6.3–9.3 | 6.2–8.4 | 6.3–8.9 |
| Range | 4.5–29.0 | 3.5–16.7 | 3.5–29.0 |
| Neutrophils (× 109/L) | |||
| N | 66 | 68 | 134 |
| Mean (SD) | 5.6 (3.1) | 4.9 (1.9) | 5.3 (2.6) |
| Median | 4.8 | 4.4 | 4.5 |
| IQR | 4.1–6.0 | 3.9–5.3 | 3.9–5.8 |
| Range | 2.4–24.0 | 1.8–11.4 | 1.8–24.0 |
| Platelets (× 109/L) | |||
| N | 66 | 68 | 134 |
| Mean (SD) | 264.2 (78.7) | 249.8 (74.5) | 256.9 (76.7) |
| Median | 250.0 | 247.5 | 250.0 |
| IQR | 221.0–298.0 | 200.0–281.5 | 213.0–293.0 |
| Range | 117.0–667.0 | 113.0–528.0 | 113.0–667.0 |
Initial flexible cystoscopy
One hundred and forty-two participants underwent initial flexible cystoscopy; one participant underwent CT chest abdomen pelvis, so all flexible cystoscopy data for that patient are missing (Table 9).
| Allocation | Pathway 1 (72) | Pathway 2 (71) | Overall (143) |
|---|---|---|---|
| Number of lesions | |||
| N | 61 | 63 | 124 |
| Mean (SD) | 1.8 (1.8) | 1.7 (1.6) | 1.8 (1.7) |
| Median | 1.0 | 1.0 | 1.0 |
| IQR | 1.0–2.0 | 1.0–2.0 | 1.0–2.0 |
| Range | 1.0–10.0 | 1.0–10.0 | 1.0–10.0 |
| Largest dimension | |||
| N | 55 | 59 | 114 |
| Mean (SD) | 2.9 (1.9) | 3.2 (1.7) | 3.0 (1.8) |
| Median | 2.5 | 3.0 | 3.0 |
| IQR | 1.5–3.0 | 2.0–4.0 | 2.0–4.0 |
| Range | 0.2–10.0 | 0.5–10.0 | 0.2–10.0 |
| Random biopsies | |||
| No | 53 (76.8) | 54 (78.3) | 107 (77.5) |
| Yes | 16 (23.2) | 15 (21.7) | 31 (22.5) |
| Unknown | 3 | 2 | 5 |
| Poor views | |||
| No | 57 (83.8) | 60 (88.2) | 117 (86.0) |
| Yes | 11 (16.2) | 8 (11.8) | 19 (14.0) |
| Unknown | 4 | 3 | 7 |
| Describe the significant lesion | |||
| Flat and solid | 1 (1.6) | 0 (0.0) | 1 (0.8) |
| Papillary | 32 (50.0) | 41 (64.1) | 73 (57.0) |
| Papillary and solid | 9 (14.1) | 9 (14.1) | 18 (14.1) |
| Solid | 22 (34.4) | 14 (21.9) | 36 (28.1) |
| Not reported | 8 | 7 | 15 |
| Estimated bladder capacity (ml) | |||
| N | 27 | 23 | 50 |
| Mean (SD) | 422.2 (118.8) | 434.8 (110.2) | 428.0 (113.9) |
| Median | 400.0 | 450.0 | 400.0 |
| IQR | 400.0–500.0 | 400.0–500.0 | 400.0–500.0 |
| Range | 200.0–700.0 | 200.0–700.0 | 200.0–700.0 |
Transurethral resection of bladder tumour
One hundred and thirty participants had 1 TURBT, 43 had 2, 7 had 3 and 2 had 4. In total, 182 TURBT procedures were carried out (Table 10).
| Pathway | Pathway 1 (97) | Pathway 2 (85) | Overall (182) |
|---|---|---|---|
| Number of tumours visible | |||
| N | 77 | 73 | 150 |
| Mean (SD) | 1.8 (1.8) | 1.6 (1.4) | 1.7 (1.6) |
| Median | 1.0 | 1.0 | 1.0 |
| IQR | 1.0–2.0 | 1.0–1.0 | 1.0–2.0 |
| Range | 0.0–10.0 | 0.0–7.0 | 0.0–10.0 |
| Size of largest tumour (cm) | |||
| N | 65 | 59 | 124 |
| Mean (SD) | 3.4 (2.6) | 2.6 (1.8) | 3.0 (2.3) |
| Median | 3.0 | 2.0 | 2.9 |
| IQR | 1.9–4.0 | 1.0–4.0 | 1.5–4.0 |
| Range | 0.4–12.0 | 0.0–10.0 | 0.0–12.0 |
| Random biopsies | |||
| No | 73 (79.3) | 65 (84.4) | 138 (81.7) |
| Yes | 19 (20.7) | 12 (15.6) | 31 (18.3) |
| Not known | 5 | 8 | 13 |
| Location of tumour(s) present | |||
| Anterior | 4 (4.1) | 1 (1.2) | 5 (2.7) |
| Dome | 4 (4.1) | 2 (2.4) | 6 (3.3) |
| Dome, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Left lateral | 13 (13.4) | 17 (20.0) | 30 (16.5) |
| Left lateral, anterior | 2 (2.1) | 2 (2.4) | 4 (2.2) |
| Left lateral, anterior, dome | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Left lateral, dome | 1 (1.0) | 1 (1.2) | 2 (1.1) |
| Left lateral, posterior | 4 (4.1) | 1 (1.2) | 5 (2.7) |
| Left lateral, posterior, anterior, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Left lateral, posterior, dome | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Left lateral, posterior, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Left lateral, prostatic urethra | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Left lateral, trigone | 2 (2.1) | 4 (4.7) | 6 (3.3) |
| Left lateral, trigone, prostatic urethra | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| N/A | 11 (11.3) | 13 (15.3) | 24 (13.2) |
| Posterior | 8 (8.2) | 4 (4.7) | 12 (6.6) |
| Posterior, anterior | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Posterior, dome | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Posterior, trigone, prostatic urethra | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral | 21 (21.6) | 22 (25.9) | 43 (23.6) |
| Right lateral, anterior | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral, anterior, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral, dome, trigone | 1 (1.0) | 1 (1.2) | 2 (1.1) |
| Right lateral, left lateral | 2 (2.1) | 0 (0.0) | 2 (1.1) |
| Right lateral, left lateral, anterior, dome | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral, left lateral, posterior, anterior | 2 (2.1) | 1 (1.2) | 3 (1.6) |
| Right lateral, left lateral, posterior, anterior, dome | 2 (2.1) | 0 (0.0) | 2 (1.1) |
| Right lateral, left lateral, posterior, anterior, dome, trigone | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Right lateral, left lateral, posterior, anterior, trigone | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Right lateral, posterior | 4 (4.1) | 1 (1.2) | 5 (2.7) |
| Right lateral, posterior, dome | 1 (1.0) | 1 (1.2) | 2 (1.1) |
| Right lateral, posterior, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral, prostatic urethra | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Right lateral, trigone | 1 (1.0) | 3 (3.5) | 4 (2.2) |
| Trigone | 4 (4.1) | 4 (4.7) | 8 (4.4) |
| Initial clinician assessment | |||
| Probable NMIBC | 50 (51.5) | 41 (48.2) | 91 (50.0) |
| Possible MIBC | 47 (48.5) | 44 (51.8) | 91 (50.0) |
| Location(s) of tumour resected/diathermied | |||
| Anterior | 3 (3.1) | 1 (1.2) | 4 (2.2) |
| Dome | 4 (4.1) | 1 (1.2) | 5 (2.7) |
| Dome, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Left lateral | 10 (10.3) | 17 (20.0) | 27 (14.8) |
| Left lateral, anterior | 2 (2.1) | 2 (2.4) | 4 (2.2) |
| Left lateral, anterior, dome | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Left lateral, dome | 1 (1.0) | 1 (1.2) | 2 (1.1) |
| Left lateral, posterior | 3 (3.1) | 1 (1.2) | 4 (2.2) |
| Left lateral, posterior, anterior, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Left lateral, posterior, dome | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Left lateral, posterior, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Left lateral, prostatic urethra | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Left lateral, trigone | 1 (1.0) | 2 (2.4) | 3 (1.6) |
| N/A | 24 (24.7) | 22 (25.9) | 46 (25.3) |
| Posterior | 7 (7.2) | 4 (4.7) | 11 (6.0) |
| Posterior, anterior | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Posterior, dome | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Posterior, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Posterior, trigone, prostatic urethra | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral | 21 (21.6) | 19 (22.4) | 40 (22.0) |
| Right lateral, anterior | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral, anterior, trigone | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral, dome, trigone | 1 (1.0) | 1 (1.2) | 2 (1.1) |
| Right lateral, left lateral | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral, left lateral, anterior, dome | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral, left lateral, anterior, trigone | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Right lateral, left lateral, posterior, anterior | 2 (2.1) | 0 (0.0) | 2 (1.1) |
| Right lateral, left lateral, posterior, anterior, dome | 1 (1.0) | 0 (0.0) | 1 (0.5) |
| Right lateral, left lateral, posterior, anterior, trigone | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Right lateral, posterior | 3 (3.1) | 1 (1.2) | 4 (2.2) |
| Right lateral, posterior, dome | 1 (1.0) | 1 (1.2) | 2 (1.1) |
| Right lateral, prostatic urethra | 0 (0.0) | 1 (1.2) | 1 (0.5) |
| Right lateral, trigone | 1 (1.0) | 3 (3.5) | 4 (2.2) |
| Trigone | 2 (2.1) | 2 (2.4) | 4 (2.2) |
| Post-resection examination under anaesthetic | |||
| No mass | 40 (60.6) | 32 (56.1) | 72 (58.5) |
| Mobile mass | 5 (7.6) | 4 (7.0) | 9 (7.3) |
| Fixed mass | 3 (4.5) | 4 (7.0) | 7 (5.7) |
| Uncertain | 7 (10.6) | 7 (12.3) | 14 (11.4) |
| Not done | 11 (16.7) | 10 (17.5) | 21 (17.1) |
| N/A | 31 (32.0) | 28 (32.9) | 59 (32.4) |
Transurethral resection of bladder tumour pathology
Details from 172 TURBT histology reports were available (Table 11).
| Pathway | Pathway 1 (90) | Pathway 2 (82) | Overall (172) |
|---|---|---|---|
| Histological composition | |||
| Adenocarcinomatous elements | 0 (0.0) | 1 (1.2) | 1 (0.6) |
| None | 1 (1.1) | 1 (1.2) | 2 (1.2) |
| Other | 10 (11.1) | 6 (7.3) | 16 (9.3) |
| Squamous elements | 1 (1.1) | 1 (1.2) | 2 (1.2) |
| Squamous elements, other | 1 (1.1) | 0 (0.0) | 1 (0.6) |
| Transitional cell carcinoma | 71 (78.9) | 68 (82.9) | 139 (80.8) |
| Transitional cell carcinoma, other | 1 (1.1) | 1 (1.2) | 2 (1.2) |
| Transitional cell carcinoma, sarcomatous elements | 0 (0.0) | 1 (1.2) | 1 (0.6) |
| Transitional cell carcinoma, squamous elements | 3 (3.3) | 3 (3.7) | 6 (3.5) |
| Transitional cell carcinoma, squamous elements, sarcomatous elements | 2 (2.2) | 0 (0.0) | 2 (1.2) |
| Detrusor muscle (tumour base) | |||
| No | 36 (41.4) | 42 (51.9) | 78 (46.4) |
| Yes | 51 (58.6) | 39 (48.1) | 90 (53.6) |
| Not known | 3 | 1 | 4 |
| Tumour present in muscle | |||
| No | 40 (80.0) | 30 (81.1) | 70 (80.5) |
| Yes | 10 (20.0) | 7 (18.9) | 17 (19.5) |
| Not known | 40 | 45 | 85 |
| Random bladder biopsy | |||
| No | 79 (89.8) | 73 (91.3) | 152 (90.5) |
| Yes | 9 (10.2) | 7 (8.8) | 16 (9.5) |
| Not known | 2 | 2 | 4 |
| Cytology | |||
| No | 82 (95.3) | 78 (97.5) | 160 (96.4) |
| Yes | 4 (4.7) | 2 (2.5) | 6 (3.6) |
| Not known | 4 | 2 | 6 |
| Other sampling | |||
| Adjacent flat urothelium sampled no cancer found | 1 (1.1) | 0 (0.0) | 1 (0.6) |
| Cystitis glandularis sighted in the background | 1 (1.1) | 0 (0.0) | 1 (0.6) |
| Left ureteric biopsy | 0 (0.0) | 1 (1.2) | 1 (0.6) |
| N/A | 87 (96.7) | 81 (98.8) | 168 (97.7) |
| Ureteric biopsy grade 2 pTa | 1 (1.1) | 0 (0.0) | 1 (0.6) |
| Bladder carcinoma | |||
| No | 10 (11.1) | 7 (8.5) | 17 (9.9) |
| Yes | 80 (88.9) | 75 (91.5) | 155 (90.1) |
| Grade (WHO 1973) | |||
| Grade 1 | 3 (3.9) | 4 (5.9) | 7 (4.9) |
| Grade 2 | 25 (32.9) | 34 (50.0) | 59 (41.0) |
| Grade 3 | 47 (61.8) | 27 (39.7) | 74 (51.4) |
| Unable to determine | 1 (1.3) | 3 (4.4) | 4 (2.8) |
| Not known | 14 | 14 | 28 |
| Grade (WHO 2004) | |||
| High | 53 (69.7) | 33 (47.1) | 86 (58.9) |
| Low | 23 (30.3) | 37 (52.9) | 60 (41.1) |
| Not known | 14 | 12 | 26 |
| pT stage | |||
| Ptx | 2 (2.4) | 4 (5.0) | 6 (3.7) |
| T2 | 9 (11.0) | 5 (6.3) | 14 (8.6) |
| T2 or higher | 2 (2.4) | 0 (0.0) | 2 (1.2) |
| T3 | 0 (0.0) | 2 (2.5) | 2 (1.2) |
| Unable to specify | 3 (3.7) | 0 (0.0) | 3 (1.9) |
| pT1 | 21 (25.6) | 20 (25.0) | 41 (25.3) |
| pTa | 45 (54.9) | 45 (56.3) | 90 (55.6) |
| pTis | 0 (0.0) | 4 (5.0) | 4 (2.5) |
| Not known | 8 | 2 | 10 |
| Concomitant flat in situ carcinoma | |||
| No | 40 (51.3) | 50 (64.9) | 90 (58.1) |
| Yes | 18 (23.1) | 7 (9.1) | 25 (16.1) |
| Not known | 20 (25.6) | 20 (26.0) | 40 (25.8) |
| Not known (missing) | 12 | 5 | 17 |
| Total tumour volume (cm3) | |||
| N | 10 | 9 | 19 |
| Mean (SD) | 3.8 (3.0) | 11.0 (17.9) | 7.2 (12.7) |
| Median | 3.0 | 2.5 | 2.7 |
| IQR | 1.5–5.0 | 0.5–14.0 | 1.3–8.0 |
| Range | 0.7–10.0 | 0.2–54.0 | 0.2–54.0 |
| Total biopsy or tumour dimension (mm) | |||
| N | 36 | 30 | 66 |
| Mean (SD) | 21.7 (20.1) | 20.1 (21.2) | 21.0 (20.5) |
| Median | 17.0 | 12.0 | 14.5 |
| IQR | 8.5–25.5 | 8.0–25.0 | 8.0–25.0 |
| Range | 0.7–85.0 | 0.5–104.0 | 0.5–104.0 |
The number (and proportion) of participants in Pathway 2 who underwent TURBT after MRI-diagnosed MIBC was also monitored. (Note: participants who were diagnosed NMIBC by MRI and then underwent TURBT as the correct treatment, or where MRI diagnosis was considered inconclusive, were excluded.) Seventeen participants were diagnosed MIBC by MRI, of which eight had TURBT afterwards; two had TURBT procedures twice (including one for TURBT biopsy only). Clinician intention for carrying out TURBT following MRI for Pathway 2 participants with confirmed MIBC is summarised in Table 12 for each procedure.
| Procedure and intention | Number of participants |
|---|---|
| Formal TURBT | 13 |
| Lack of confidence that the MRI shows MIBC | 3 |
| To ascertain presence of histological variants | 4 |
| To check for CIS | 1 |
| To debulk the tumour prior to radical therapy | 4 |
| To perform examination under anaesthesia in order to assess resectability | 1 |
| TURBT biopsy | 1 |
| To ascertain presence of histological variants | 1 |
| Grand total | 14 |
Magnetic resonance imaging
Table 13 summarises the numbers of participants who underwent MRI. In all, 42 participants underwent MRI, including 6 in error (4 in Pathway 1 who were possible MIBC; 2 in Pathway 2 who were probable NMIBC).
| Pathway | Pathway 1 (n = 4) | Pathway 2 (n = 38) |
|---|---|---|
| Number of tumours visible | ||
| 0 | 1 (33.3) | 1 (3.0) |
| 1 | 1 (33.3) | 30 (90.9) |
| 2 | 1 (33.3) | 0 (0.0) |
| 3 | 0 (0.0) | 1 (3.0) |
| 10 | 0 (0.0) | 1 (3.0) |
| Not reported | 1 | 5 |
| Size of largest tumour (cm) | ||
| N | 4 | 33 |
| Mean (SD) | 4.0 (3.3) | 3.2 (1.6) |
| Median | 4.0 | 3.5 |
| IQR | 1.6–6.4 | 1.8–4.4 |
| Range | 0.0–8.0 | 0.0–6.3 |
| Location of tumour(s) | ||
| Anterior | 1 (25.0) | 2 (5.3) |
| Anterior, dome | 0 (0.0) | 1 (2.6) |
| Dome | 0 (0.0) | 1 (2.6) |
| Left lateral | 1 (25.0) | 7 (18.4) |
| Left lateral, posterior | 0 (0.0) | 1 (2.6) |
| Left lateral, trigone | 0 (0.0) | 1 (2.6) |
| N/A | 1 (25.0) | 2 (5.3) |
| Posterior | 0 (0.0) | 2 (5.3) |
| Posterior and trigone | 0 (0.0) | 2 (5.3) |
| Right lateral | 1 (25.0) | 11 (28.9) |
| Right lateral, dome | 0 (0.0) | 1 (2.6) |
| Right lateral, left lateral, posterior, anterior | 0 (0.0) | 1 (2.6) |
| Right lateral, left lateral, posterior, anterior | 0 (0.0) | 1 (2.6) |
| Dome, trigone, prostatic urethra | ||
| Right lateral, posterior | 0 (0.0) | 2 (5.3) |
| Trigone | 0 (0.0) | 3 (7.9) |
| VI-RADS score | ||
| 1 | 1 (50.0) | 2 (10.5) |
| 2 | 0 (0.0) | 7 (36.8) |
| 3 | 0 (0.0) | 3 (15.8) |
| 4 | 1 (50.0) | 5 (26.3) |
| 5 | 0 (0.0) | 2 (10.5) |
| Not reported | 2 | 19 |
| Diagnosis | ||
| MIBC | 3 (75.0) | 17 (44.7) |
| NMIBC | 1 (25.0) | 18 (47.4) |
| Inconclusive | 0 (0.0) | 3 (7.9) |
| Lymph node involvement | ||
| No | 3 (100.0) | 34 (89.5) |
| Yes | 0 (0.0) | 3 (7.9) |
| Unclear | 0 (0.0) | 1 (2.6) |
| Not reported | 1 | 0 |
Definitive and correct treatments
Overall, 137 (95.8%) patients received their definitive treatment. Of the six participants who did not receive definitive treatment, one did not have cancer, four were early withdrawals and one was due to an administrative error in which a Pathway 1 participant underwent MRI (but not TURBT) with MRI confirming MIBC.
As summarised in Table 14, 130 (90.9%) participants received correct treatment. Of the 13 participants who did not, 3 did not have cancer, 3 withdrew early (˂ 100 days), 1 died, 2 were probable NMIBC who had TURBT-diagnosed MIBC and were awaiting a correct treatment, and 4 were probable NMIBC participants who had no confirmed MIBC, NMIBC or were awaiting a correct treatment.
| Arm | Pathway 1 (n = 72) | Pathway 2 (n = 71) | Overall (n = 143) |
|---|---|---|---|
| Was definitive treatment received | |||
| No | 3 (4.2) | 3 (4.2) | 6 (4.2) |
| Yes | 69 (95.8) | 68 (95.8) | 137 (95.8) |
| Definitive treatment | |||
| Chemotherapy | 0 (0.0) | 3 (4.4) | 3 (2.2) |
| Cystectomy | 0 (0.0) | 1 (1.5) | 1 (0.7) |
| Palliative care | 0 (0.0) | 1 (1.5) | 1 (0.7) |
| Radiotherapy | 0 (0.0) | 2 (2.9) | 2 (1.5) |
| TURBT | 69 (100.0) | 61 (89.7) | 130 (94.9) |
| Not known | 3 | 3 | 6 |
| Was correct treatment received | |||
| No | 9 (12.5) | 4 (5.6) | 13 (9.1) |
| Yes | 63 (87.5) | 67 (94.4) | 130 (90.9) |
| First correct treatment | |||
| Chemotherapy | 4 (6.3) | 5 (7.5) | 9 (6.9) |
| Cystectomy | 2 (3.2) | 3 (4.5) | 5 (3.8) |
| Palliative care | 4 (6.3) | 3 (4.5) | 7 (5.4) |
| Radiotherapy | 3 (4.8) | 2 (3.0) | 5 (3.8) |
| TURBT | 50 (79.4) | 54 (80.6) | 104 (80.0) |
| Not known | 9 | 4 | 13 |
Swimmer plots: initial clinical assessment of probable non-muscle-invasive bladder cancer and possible muscle-invasive bladder cancer across arms
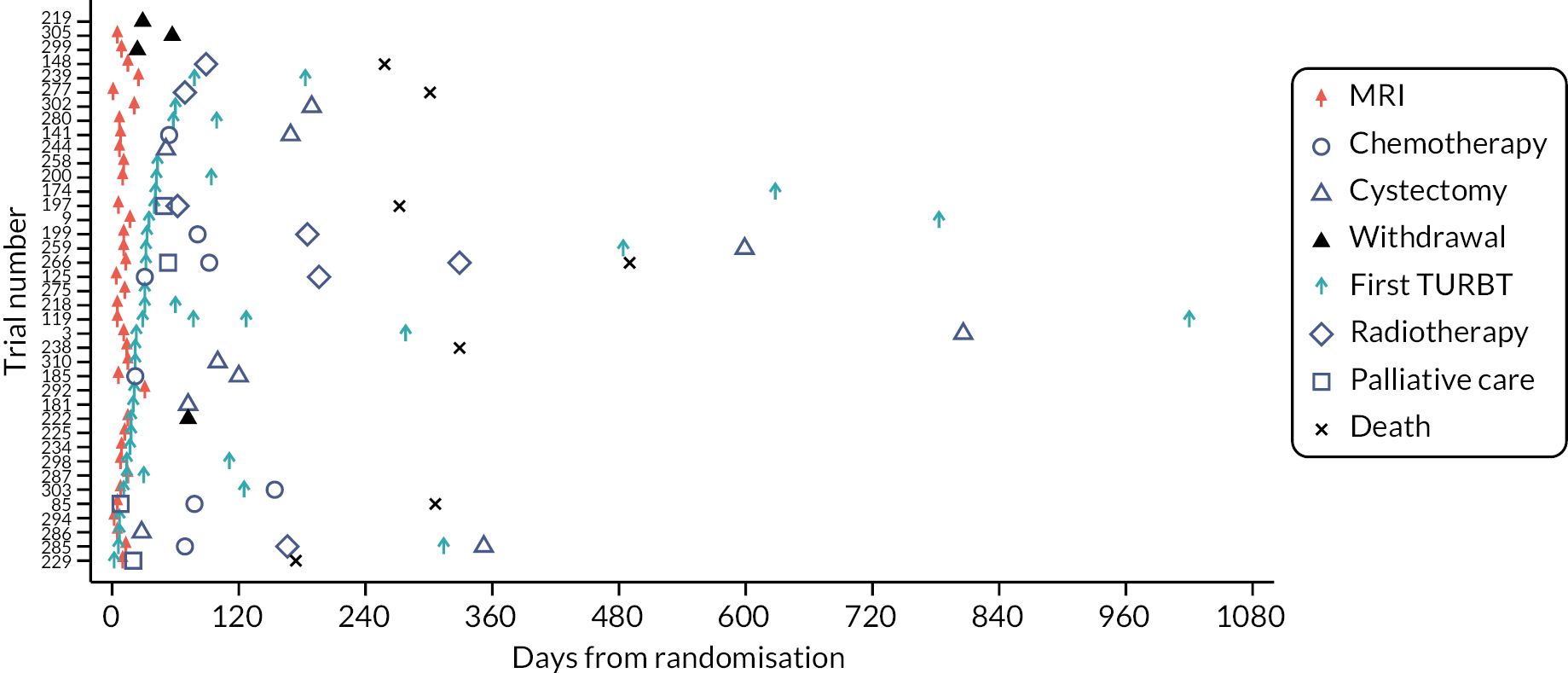
Four swimmer plots sorted by TTDT: 34 Pathway 1 probable NMIBC participants Figure 5), 38 Pathway 1 possible MIBC (Figure 6), 32 Pathway 2 probable NMIBC (Figure 7) and 39 Pathway 2 possible MIBC (Figure 8). Two participants in Figure 6 and four participants in Figure 7 received MRI in error.
FIGURE 5.
Swimmer plot for pathway 1 probable NMIBC participants.
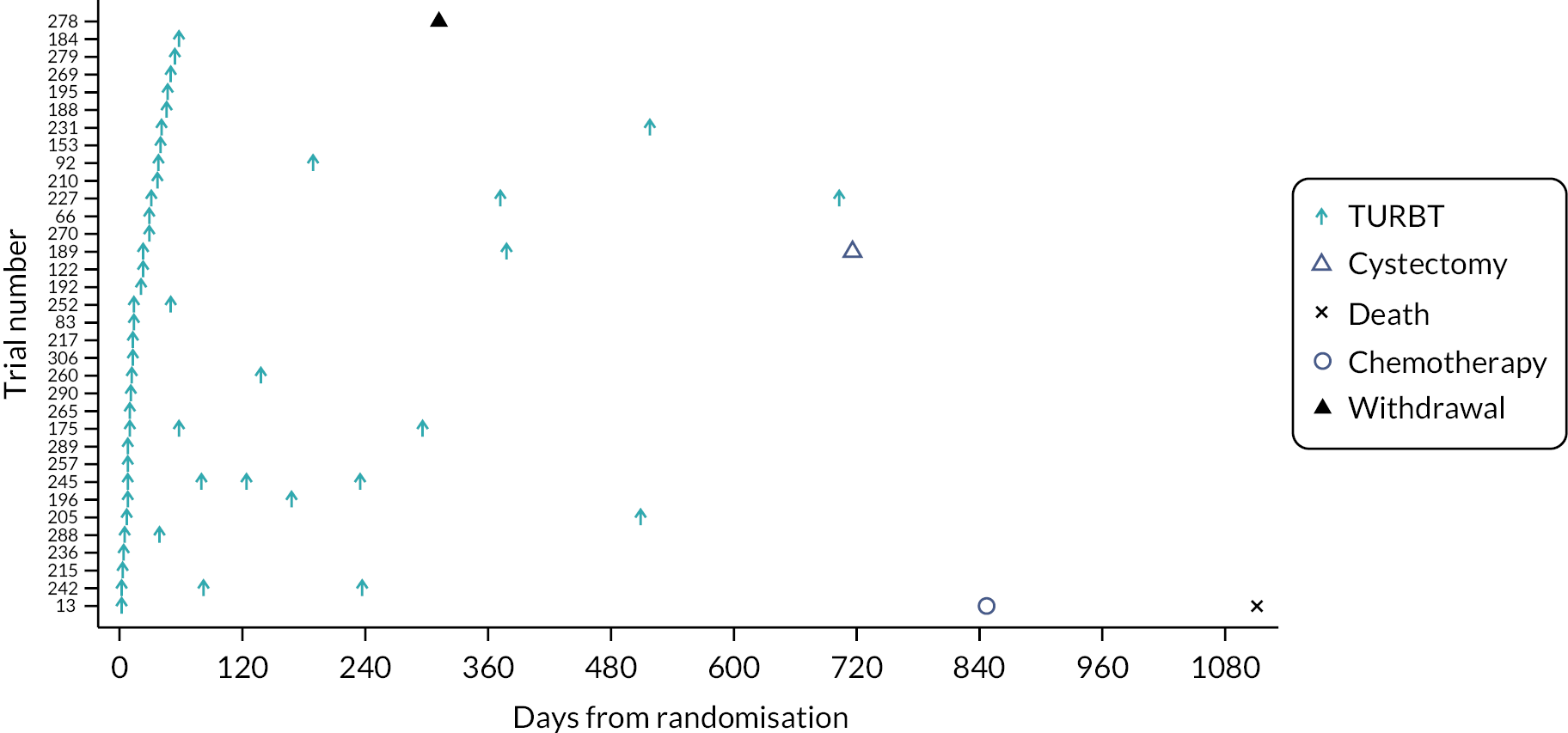
FIGURE 6.
Swimmer plot for Pathway 2 probable NMIBC participants.
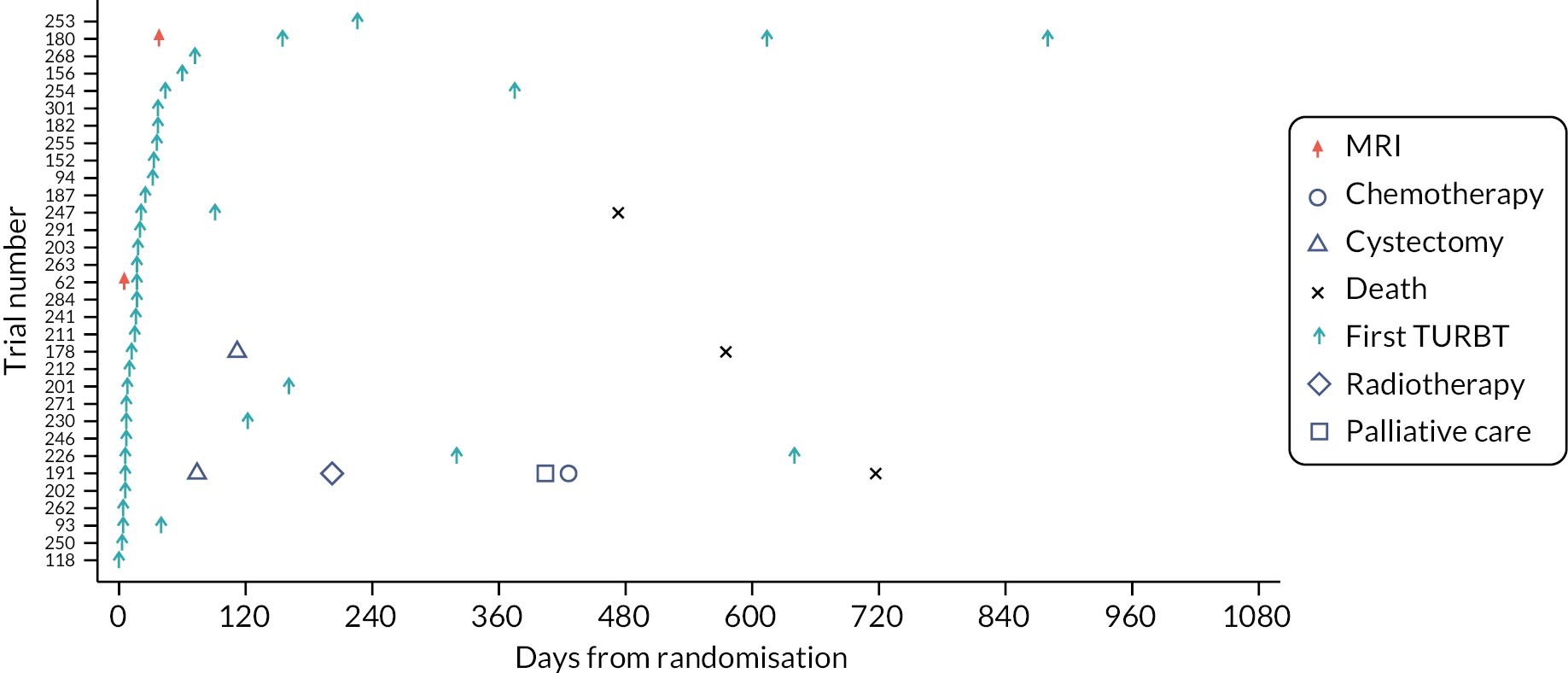
FIGURE 7.
Swimmer plot for Pathway 1 possible MIBC participants.

FIGURE 8.
Swimmer plot for Pathway 2 possible MIBC participants.
Swimmer plots: final assessment of non-muscle-invasive bladder cancer and muscle-invasive bladder cancer
Four swimmer plots sorted by TTCT. Of the 143 participants, 133 (93.0%) had a confirmed NMIBC/MIBC, including 51 NMIBC in Pathway 1 (Figure 9), 55 NMIBC in Pathway 2 (Figure 10), 14 MIBC in Pathway 1 (Figure 11) and 13 MIBC in Pathway 2 (Figure 12). Of note, 7/14 MIBC patients on Pathway 2 avoided the need for TURBT.
FIGURE 9.
Swimmer plot for Pathway 1 NMIBC participants.
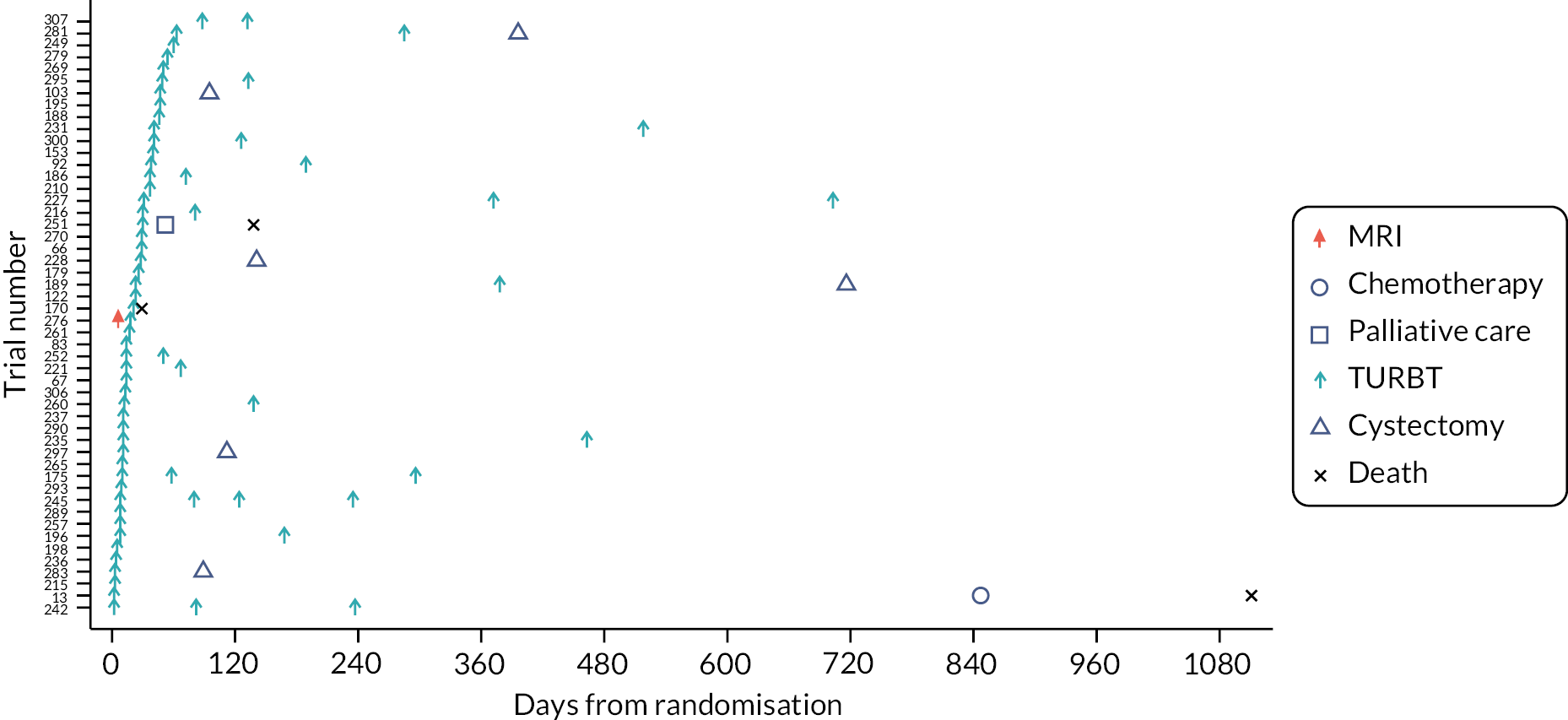
FIGURE 10.
Swimmer plot for Pathway 2 NMIBC participants.

FIGURE 11.
Swimmer plot for Pathway 1 MIBC participants.
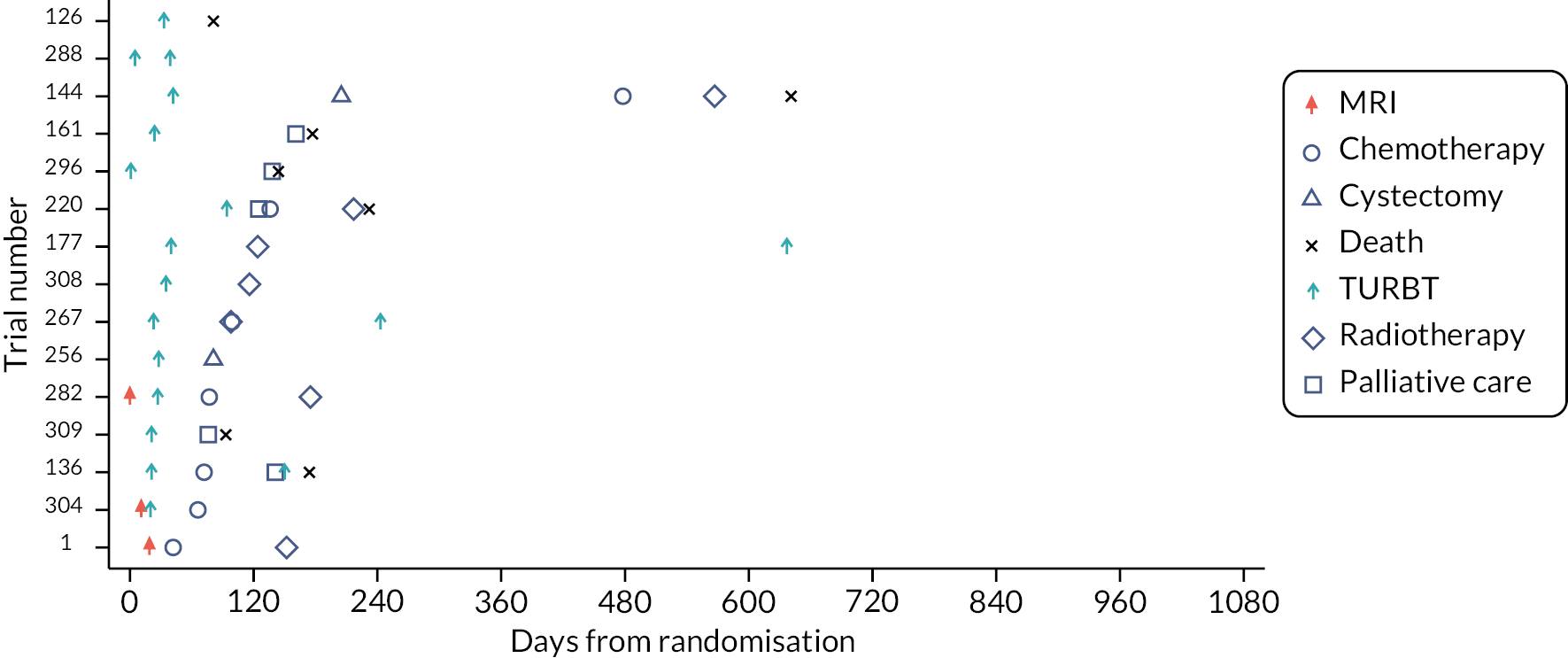
FIGURE 12.
Swimmer plot for Pathway 2 MIBC participants.

Summaries of types of treatment received
Intravesical therapy
Thirty-four participants received at least one intravesical BCG (Table 15); 11 participants received intravesical chemotherapy (Table 16).
| Pathway | Pathway 1 (20) | Pathway 2 (14) | Overall (34) |
|---|---|---|---|
| Induction course | |||
| Yes | 20 (100.0) | 14 (100.0) | 34 (100.0) |
| Number of induction doses | |||
| N | 20 | 14 | 34 |
| Mean (SD) | 6.2 (1.1) | 6.0 (0.0) | 6.1 (0.8) |
| Median | 6.0 | 6.0 | 6.0 |
| IQR | 6.0–6.0 | 6.0–6.0 | 6.0–6.0 |
| Range | 4.0–9.0 | 6.0–6.0 | 4.0–9.0 |
| Maintenance course | |||
| No | 8 (40.0) | 5 (35.7) | 13 (38.2) |
| Yes | 12 (60.0) | 9 (64.3) | 21 (61.8) |
| Number of maintenance doses | |||
| N | 12 | 9 | 21 |
| Mean (SD) | 4.8 (4.6) | 7.7 (7.8) | 6.0 (6.2) |
| Median | 3.0 | 3.0 | 3.0 |
| IQR | 3.0–5.0 | 3.0–9.0 | 3.0–6.0 |
| Range | 1.0–18.0 | 3.0–21.0 | 1.0–21.0 |
| Pathwaya | Initial clinician assessment | Within 24 hours, single dose | Single drug used | Chemotherapy course of intravesical | Course drug used | Number of cycles |
|---|---|---|---|---|---|---|
| Pathway 1 | Probable NMIBC | – | – | Yes | Mitomycin C | 6 |
| Pathway 1 | Probable NMIBC | – | – | Yes | Mitomycin C | 7 |
| Pathway 1 | Probable NMIBC | Yes | Mitomycin C | Yes | Mitomycin C | 12 |
| Pathway 1 | Possible MIBC | Yes | Epirubicin | – | – | – |
| Pathway 2 | Possible MIBC | Yes | Mitomycin C | Yes | Mitomycin C | 6 |
| Pathway 2 | Possible MIBC | – | – | Yes | Epirubicin | 8 |
| Pathway 2 | Probable NMIBC | Yes | Mitomycin C | Yes | Mitomycin C | 6 |
| Pathway 2 | Possible MIBC | – | – | Yes | Epirubicin | 6 |
| Pathway 2 | Probable NMIBC | – | – | Yes | Epirubicin | 8 |
| Pathway 2 | Probable NMIBC | Yes | Epirubicin | – | – | – |
| Pathway 2 | Possible MIBC | – | – | Yes | Other | 6 |
Chemotherapy
Eighteen chemotherapy treatments were received by 17 participants (one received both neo-adjuvant and synchronous chemotherapy with radiotherapy; Table 17).
| Pathway | Pathway 1 (n = 8) | Pathway 2 (n = 10) | Overall (n = 18) |
|---|---|---|---|
| Type | |||
| Neoadjuvant | 4 (50.0) | 5 (50.0) | 9 (50.0) |
| Synchronous with radiotherapy | 1 (12.5) | 2 (20.0) | 3 (16.7) |
| Palliative chemotherapy | 3 (37.5) | 3 (30.0) | 6 (33.3) |
| Regimen | |||
| Fluorouracil and mitomycin | 0 (0.0) | 1 (10.0) | 1 (5.6) |
| Gemcitabine Carboplatin | 2 (25.0) | 2 (20.0) | 4 (22.2) |
| Gemcitabine Cisplatinum | 4 (50.0) | 7 (70.0) | 11 (61.1) |
| Gemcitabine | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Pembrolizumab flat dose 6 weekly | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Number of cycles | |||
| N | 8 | 9 | 17 |
| Mean (SD) | 2.9 (1.0) | 5.3 (3.2) | 4.2 (2.7) |
| Median | 3.0 | 4.0 | 4.0 |
| IQR | 2.5–3.5 | 4.0–6.0 | 3.0–4.0 |
| Range | 1.0–4.0 | 1.0–12.0 | 1.0–12.0 |
Radiotherapy
Fifteen participants received radiotherapy (Table 18).
| Pathway | Initial clinician assessment | Intention of treatment | Intention of treatment field | Number of fractions given | Total dose given (Gy) | Radiotherapy completed as planned | Was chemotherapy given synchronously | Synchronous chemotherapy |
|---|---|---|---|---|---|---|---|---|
| Pathway 1 | Possible MIBC | Radical | Bladder | 20 | 55 | Yes | Yes | Mitomycin |
| Pathway 2 | Possible MIBC | Radical | Bladder | – | – | Yes | No | N/A |
| Pathway 1 | Possible MIBC | Palliative | Metastatic state | 5 | 20 | Yes | – | N/A |
| Pathway 2 | Possible MIBC | Radical | Bladder | 20 | 55 | Yes | No | N/A |
| Pathway 1 | Possible MIBC | Radical | Bladder | 20 | 50 | Yes | No | N/A |
| Pathway 2 | Probable NMIBC | – | Bladder, upper renal tract | 27 | 55 | Yes | No | N/A |
| Pathway 2 | Possible MIBC | – | Metastatic site | 5 | 20 | Yes | No | N/A |
| Pathway 2 | Possible MIBC | Radical | Bladder | 55 | 20 | Yes | Yes | Cisplatin, gemcitabine |
| Pathway 1 | Possible MIBC | Palliative | Bladder | 1 | 8 | Yes | No | N/A |
| Pathway 2 | Possible MIBC | Palliative | Metastatic site | 5 | 30 | Yes | No | N/A |
| Pathway 1 | Possible MIBC | Radical | Bladder | 20 | 55 | Yes | Yes | Gemcitabine |
| Pathway 2 | Possible MIBC | Radical | Bladder | 20 | 55 | Yes | Yes | Other |
| Pathway 1 | Possible MIBC | Radical | Bladder | 20 | 55 | Yes | Yes | 5-Fluorouracil, mitomycin |
| Pathway 2 | Possible MIBC | Radical | Bladder | 20 | 55 | Yes | Yes | Mitomycin, other |
| Pathway 1 | Possible MIBC | Radical | Bladder | 20 | 55 | Yes | Yes | Mitomycin, capecitabine |
Cystectomy
Twenty participants underwent cystectomy (Table 19). One participant underwent surgery which was immediately abandoned upon anaesthetic induction due to the participant experiencing heart arrhythmia.
| Pathway | Pathway 1 (n = 8) | Pathway 2 (n = 12) | Overall (n = 20) |
|---|---|---|---|
| Treatment intent | |||
| Curative | 8 (100.0) | 12 (100.0) | 20 (100.0) |
| Resection type | |||
| Radical cystectomy | 7 (100.0) | 10 (83.3) | 17 (89.5) |
| Partial cystectomy | 0 (0.0) | 2 (16.7) | 2 (10.5) |
| Not known | 1 | 0 | 1 |
| Technique | |||
| Pure open | 5 (62.5) | 9 (90.0) | 14 (77.8) |
| Pure robotic | 2 (25.0) | 1 (10.0) | 3 (16.7) |
| Mixed | 1 (12.5) | 0 (0.0) | 1 (5.6) |
| Not known | 0 | 2 | 2 |
| Lymph nodes | |||
| Sampled | 5 (62.5) | 3 (25.0) | 8 (40.0) |
| Clearance | 3 (37.5) | 9 (75.0) | 12 (60.0) |
| Lymph nodes clinically suspicious | |||
| No | 3 (50.0) | 7 (58.3) | 10 (55.6) |
| Yes | 3 (50.0) | 5 (41.7) | 8 (44.4) |
| Not known | 2 | 0 | 2 |
| Other viscera removed | |||
| None | 1 (12.5) | 0 (0.0) | 1 (5.0) |
| Other | 0 (0.0) | 2 (16.7) | 2 (10.0) |
| Ovaries | 0 (0.0) | 1 (8.3) | 1 (5.0) |
| Prostate | 2 (25.0) | 4 (33.3) | 6 (30.0) |
| Prostate, other | 0 (0.0) | 3 (25.0) | 3 (15.0) |
| Prostate, urethra | 1 (12.5) | 0 (0.0) | 1 (5.0) |
| Urethra, uterus | 1 (12.5) | 0 (0.0) | 1 (5.0) |
| Urethra, vagina | 1 (12.5) | 0 (0.0) | 1 (5.0) |
| Urethra, vagina, uterus, ovaries | 2 (25.0) | 0 (0.0) | 2 (10.0) |
| Uterus, ovaries | 0 (0.0) | 1 (8.3) | 1 (5.0) |
| Vagina, uterus, other | 0 (0.0) | 1 (8.3) | 1 (5.0) |
| Reconstruction | |||
| Conduit and urostomy | 8 (100.0) | 8 (100.0) | 16 (100.0) |
| Not known | 0 | 4 | 4 |
Cystectomy histology
Histopathology reports were provided for all participants who underwent cystectomy. Table 20 summarises the findings. The commonest histological tumour type in this subset was transitional cell carcinoma (50%). One participant’s pathology report related to a colorectal resection involving partial cystectomy of the bladder; however, the report stated ‘no evidence of malignant neoplasm’ in the bladder.
| Pathway | Pathway 1 (n = 8) | Pathway 2 (n = 12) | Overall (n = 20) |
|---|---|---|---|
| Histological composition | |||
| Adenocarcinomatous elements | 0 (0.0) | 2 (16.7) | 2 (10.0) |
| None | 0 (0.0) | 1 (8.3) | 1 (5.0) |
| Squamous elements, other | 0 (0.0) | 1 (8.3) | 1 (5.0) |
| Transitional cell carcinoma | 4 (50.0) | 6 (50.0) | 10 (50.0) |
| Transitional cell carcinoma, adenocarcinomatous elements | 0 (0.0) | 1 (8.3) | 1 (5.0) |
| Transitional cell carcinoma, other | 2 (25.0) | 0 (0.0) | 2 (10.0) |
| Transitional cell carcinoma, squamous elements | 2 (25.0) | 1 (8.3) | 3 (15.0) |
| Detrusor muscle (tumour base) | |||
| No | 3 (60.0) | 5 (50.0) | 8 (53.3) |
| Yes | 2 (40.0) | 5 (50.0) | 7 (46.7) |
| Not known | 3 | 2 | 5 |
| Tumour present in muscle | |||
| Yes | 2 (100.0) | 5 (100.0) | 7 (100.0) |
| Not known | 6 | 7 | 13 |
| Random bladder biopsy | |||
| No | 6 (100.0) | 11 (100.0) | 17 (100.0) |
| Not known | 2 | 1 | 3 |
| Cytology | |||
| No | 6 (100.0) | 11 (100.0) | 17 (100.0) |
| Not known | 2 | 1 | 3 |
| Other sampling | |||
| Abdominoperineal resection (APER) | 0 (0.0) | 1 (8.3) | 1 (5.0) |
| N/A | 8 (100.0) | 10 (83.3) | 18 (90.0) |
| Uterus, cervix, adnexa | 0 (0.0) | 1 (8.3) | 1 (5.0) |
| Bladder carcinoma | |||
| No | 0 (0.0) | 2 (16.7) | 2 (10.0) |
| Yes | 8 (100.0) | 10 (83.3) | 18 (90.0) |
| Grade (WHO 1973) | |||
| Grade 2 | 0 (0.0) | 1 (11.1) | 1 (6.3) |
| Grade 3 | 5 (71.4) | 7 (77.8) | 12 (75.0) |
| Unable to determine | 2 (28.6) | 1 (11.1) | 3 (18.8) |
| Not known | 1 | 3 | 4 |
| Grade (WHO 2004) | |||
| High | 4 (100.0) | 8 (88.9) | 12 (92.3) |
| Low | 0 (0.0) | 1 (11.1) | 1 (7.7) |
| Not known | 4 | 3 | 7 |
| pT stage | |||
| T2 | 0 (0.0) | 2 (18.2) | 2 (10.5) |
| pT0 | 1 (12.5) | 1 (9.1) | 2 (10.5) |
| pT1 | 1 (12.5) | 0 (0.0) | 1 (5.3) |
| pT1a (pCIS) | 3 (37.5) | 1 (9.1) | 4 (21.1) |
| pT1b (pCIS) | 0 (0.0) | 2 (18.2) | 2 (10.5) |
| pT3a | 0 (0.0) | 1 (9.1) | 1 (5.3) |
| pT3b | 1 (12.5) | 1 (9.1) | 2 (10.5) |
| pT4a | 1 (12.5) | 0 (0.0) | 1 (5.3) |
| pTa | 0 (0.0) | 2 (18.2) | 2 (10.5) |
| pTis | 1 (12.5) | 1 (9.1) | 2 (10.5) |
| Not known | 0 | 1 | 1 |
| pN stage | |||
| N0 | 6 (85.7) | 10 (100.0) | 16 (94.1) |
| N2 | 1 (14.3) | 0 (0.0) | 1 (5.9) |
| Not known | 1 | 2 | 3 |
| Margins/status | |||
| Positive | 0 (0.0) | 1 (9.1) | 1 (5.6) |
| Negative | 7 (100.0) | 7 (63.6) | 14 (77.8) |
| Unknown | 0 (0.0) | 3 (27.3) | 3 (16.7) |
| Not known | 1 | 1 | 2 |
| Concomitant flat in situ carcinoma | |||
| No | 2 (25.0) | 5 (50.0) | 7 (38.9) |
| Yes | 6 (75.0) | 5 (50.0) | 11 (61.1) |
| Not known | 0 | 2 | 2 |
| Total tumour volume (cm3) | |||
| N | 0 | 1 | 1 |
| Mean (SD) | 1.5 (.) | 1.5 (.) | |
| Median | 1.5 | 1.5 | |
| IQR | 1.5–1.5 | 1.5–1.5 | |
| Range | 1.5–1.5 | 1.5–1.5 | |
| Total biopsy or tumour dimension (mm) | |||
| N | 3 | 6 | 9 |
| Mean (SD) | 164.3 (120.2) | 111.8 (122.0) | 129.3 (116.6) |
| Median | 100.0 | 64.5 | 90.0 |
| IQR | 90.0–303.0 | 40.0–125.0 | 54.0–125.0 |
| Range | 90.0–303.0 | 26.0–350.6 | 26.0–350.6 |
A total of nine patients had prostate cancer diagnosed upon pathological examination of the cystoprostatectomy specimen, three in pathway 1 and six in Pathway 2, as summarised in Table 21.
| Pathway | Pathway 1 (3) | Pathway 2 (6) | Overall (9) |
|---|---|---|---|
| pT | |||
| TX | 1 (50.0) | 0 (0.0) | 1 (16.7) |
| T2 | 1 (50.0) | 4 (100.0) | 5 (83.3) |
| Not known | 1 | 2 | 3 |
| pN | |||
| NX | 1 (50.0) | 0 (0.0) | 1 (16.7) |
| N0 | 1 (50.0) | 4 (100.0) | 5 (83.3) |
| Not known | 1 | 2 | 3 |
| pM | |||
| MX | 1 (100.0) | 1 (33.3) | 2 (50.0) |
| M0 | 0 (0.0) | 2 (66.7) | 2 (50.0) |
| Not known | 2 | 3 | 5 |
| Margin positive | |||
| No | 1 (50.0) | 5 (100.0) | 6 (85.7) |
| Yes | 1 (50.0) | 0 (0.0) | 1 (14.3) |
| Not known | 1 | 1 | 2 |
| Gleason sum score | |||
| N | 1 | 5 | 6 |
| Mean (SD) | 7.0 (.) | 6.2 (0.4) | 6.3 (0.5) |
| Median | 7.0 | 6.0 | 6.0 |
| IQR | 7.0–7.0 | 6.0–6.0 | 6.0–7.0 |
| Range | 7.0–7.0 | 6.0–7.0 | 6.0–7.0 |
Participants whose cystectomy specimens showed non-muscle-invasive bladder cancer
Thirteen participants were confirmed as NMIBC (and not MIBC) by pathological examination of the cystectomy specimen, summarised in Table 22. There was no statistical difference in the number of cystectomies undertaken for NMIBC between the two pathways, Fisher’s exact test (p = 0.337). One participant experienced two recurrences of locoregional disease (on 15 April 2019 and 21 December 2019) and a new primary tumour site (prostate) confirmed (on 9 October 2020). All patients who had no invasive disease at cystectomy had prior TURBT in addition, so no patient had cystectomy due to incorrect MRI staging. It should be noted that it is well documented that post TURBT with MIBC, around 10–15% of cystectomy specimens will then show no invasive disease, presumably due to the prior endoscopic resection.
| Pathway | Initial assessment | MRI diagnosis | First TURBT stage | Second TURBT stage | Chemotherapy Y/N | Radiotherapy Y/N | Cystectomy stage |
|---|---|---|---|---|---|---|---|
| 2 | Possible MIBC | NMIBC | pT1 | pTa | pTa | ||
| 1 | Possible MIBC | pT1 | pT1 | ||||
| 2 | Possible MIBC | pT1 | pTa | ||||
| 1 | Probable NMIBC | pT1 | pT0 | ||||
| 1 | Possible MIBC | pT1 | pTa | ||||
| 2 | Possible MIBC | NMIBC | pT1 | pT0 | |||
| 1 | Possible MIBC | pTa | pTa | ||||
| 1 | Possible MIBC | pT1 | pTis | ||||
| 2 | Possible MIBC | MIBC | pTa | pTa | Y | Y | pTis |
| 2 | Possible MIBC | MIBC | pTa | pT1 | |||
| 1 | Possible MIBC | pT1 | pTis | ||||
| 2 | Possible MIBC | Inconclusive | pT1 | pT1 | |||
| 2 | Possible MIBC | MIBC | T2 | pTis |
Accuracy of magnetic resonance imaging
Nine Pathway 2 participants underwent both MRI diagnosis and subsequent cystectomy with available histology (Table 23).
| MRI diagnosis | MIBC (n = 6) | NMIBC (n = 2) | Inconclusive (n = 1) | Overall (n = 9) |
|---|---|---|---|---|
| Tumour stage based on cystectomy pathology | ||||
| pT0 | 0 (0.0) | 1 (50.0) | 0 (0.0) | 1 (11.1) |
| pTa | 0 (0.0) | 1 (50.0) | 0 (0.0) | 1 (11.1) |
| pTis | 2 (33.3) | 0 (0.0) | 0 (0.0) | 2 (22.2) |
| pT1 | 1 (16.7) | 0 (0.0) | 1 (100.0) | 2 (22.2) |
| T2 | 2 (33.3) | 0 (0.0) | 0 (0.0) | 2 (22.2) |
| T3 | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (11.1) |
| Total | 6 (100.0) | 2 (100.0) | 1 (100.0) | 9 (100.0) |
Accuracy of transurethral resection of bladder tumour
Eight participants in each pathway had both TURBT and cystectomy tumour staging available (see Tables 24 and 25).
| Tumour stage based on TURBT pathology | pTa (1) | pT1 (5) | T2 (1) | T2 or higher (1) | Overall (8) |
|---|---|---|---|---|---|
| Tumour stage based on cystectomy pathology | |||||
| pT0 | 0 (0.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) |
| pTa | 1 (100.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 2 (25.0) |
| pTis | 0 (0.0) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 2 (25.0) |
| pT1 | 0 (0.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) |
| T3 | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) | 2 (25.0) |
| Total | 1 (100.0) | 5 (100.0) | 1 (100.0) | 1 (100.0) | 8 (100.0) |
| Tumour stage based on TURBT pathology | pTa (2) | pT1 (4) | T2 (2) | Overall (8) |
|---|---|---|---|---|
| Tumour stage based on cystectomy pathology | ||||
| pT0 | 0 (0.0) | 1 (25.0) | 0 (0.0) | 1 (12.5) |
| pTa | 0 (0.0) | 2 (50.0) | 0 (0.0) | 2 (25.0) |
| pTis | 1 (50.0) | 0 (0.0) | 1 (50.0) | 2 (25.0) |
| pT1 | 1 (50.0) | 1 (25.0) | 0 (0.0) | 2 (25.0) |
| T3 | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (12.5) |
| Total | 2 (100.0) | 4 (100.0) | 2 (100.0) | 8 (100.0) |
Outcomes
Feasibility stage
Primary outcome: proportion of possible muscle-invasive bladder cancer participants randomised to Pathway 2 who correctly followed the protocol pathway
In total, there were 39 possible MIBC participants in Pathway 2, of which 36 (92%, 95% CI 79% to 98%) received MRI as per protocol. Three Pathway 2 possible MIBC participants did not undergo MRI after randomisation, including one who was found to have a metal fragment in his eye prior to undergoing the MRI examination, one who cancelled their MRI as the participant withdrew from the trial (29 days post randomisation) and one who underwent MRI prior to being entered into the trial (the scan was requested by the surgeon independently of the study). Of the 36 participants who underwent MRI, 17 were diagnosed as having MIBC, 16 were NMIBC and for 3 the mpMRI images were inconclusive.
Secondary outcome: overall proportion of all randomised participants who correctly followed the protocol pathway in their respective pathways
For Pathway 1, this was defined as the number of probable NMIBC and possible MIBC participants randomised to the pathway who received a TURBT at the appropriate pathway stage as a proportion of all participants randomised to Pathway 1.
For Pathway 2, it was defined as the number of probable NMIBC participants in the pathway who had a TURBT plus the number of possible MIBC participants in the pathway who underwent MRI as a proportion of all participants randomised to Pathway 2.
The overall proportion of participants who correctly followed their respective pathway protocol was 96% CI (88% to 99%) in each pathway. No statistical difference between the pathways was found.
Intermediate stage
Primary outcome: time to correct treatment for participants who were initially classified as possible muscle-invasive bladder cancer and then were confirmed to have muscle-invasive bladder cancer
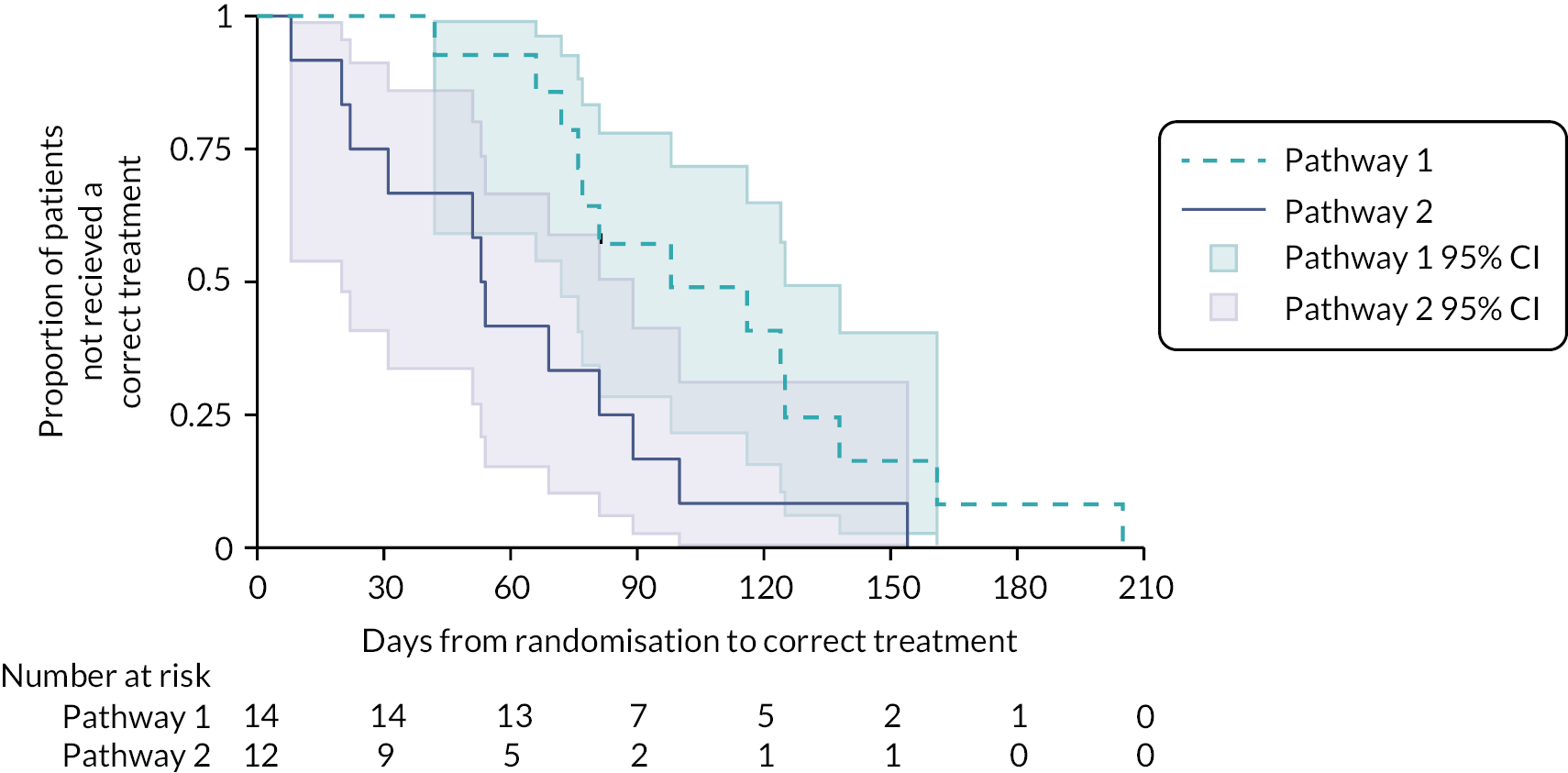
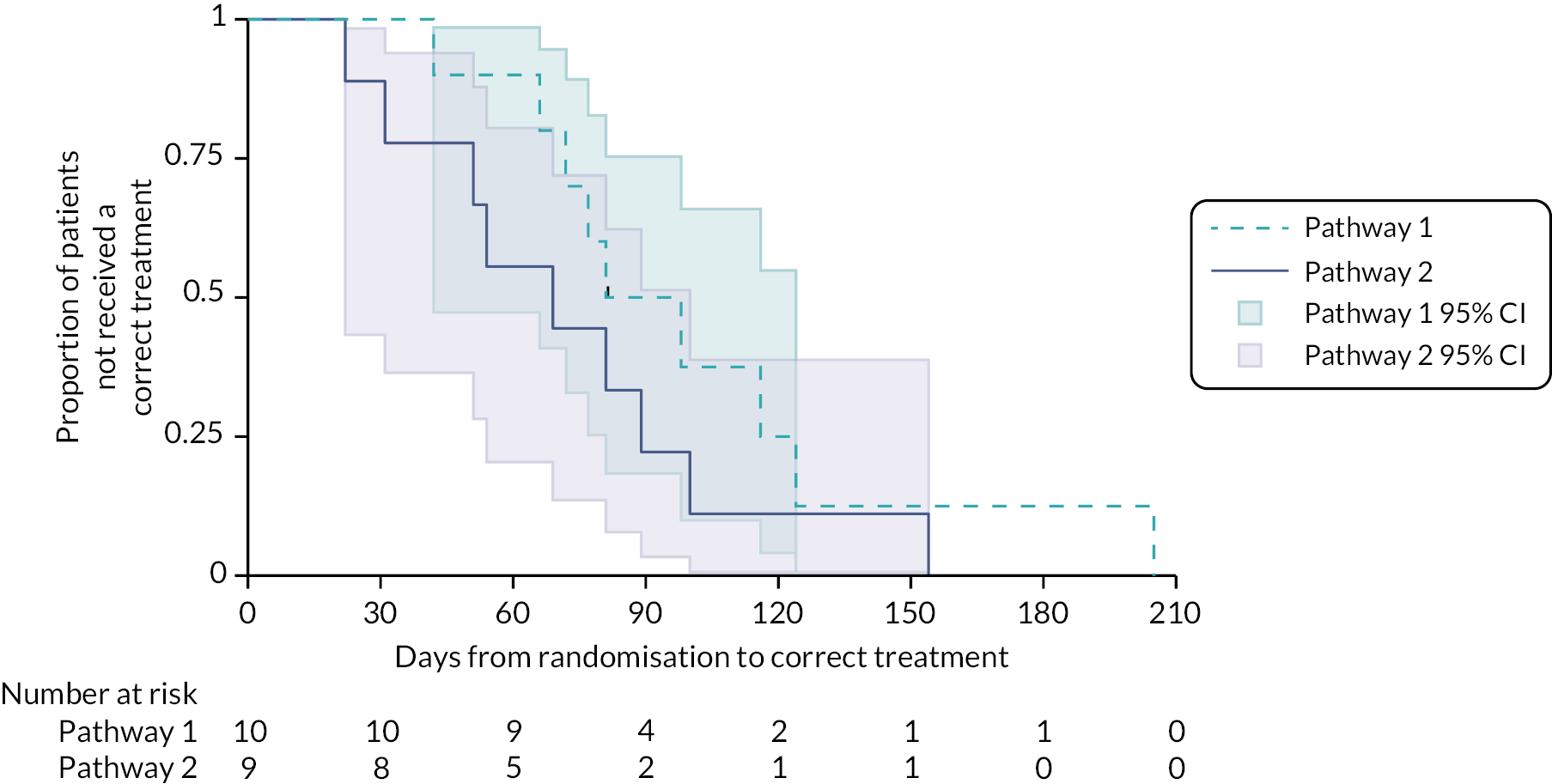
Of the 26 participants who were initially classified as possible MIBC and then were confirmed MIBC (14 in Pathway 1 and 12 in Pathway 2; Figure 13), 25 received a correct treatment and 1 participant did not due to death 81 days post randomisation. For this latter participant, the date last seen was used in the time-to-event analysis to account for the length of time they had waited to start treatment. Median TTCT for all participants who were initially classified as possible MIBC and then were confirmed to have MIBC (N = 26) was 77 days (95% CI 54 to 100). Median TTCT for Pathway 1 (N = 14) was 98 days (95% CI 72 to 125). Median TTCT for Pathway 2 (N = 12) was 53 days (95% CI 20 to 89, p = 0.0201), suggesting a statistical difference in TTCT between the two pathways. A Cox model, adjusted for the stratification factors of sex and age with study centre included as a random effect in the model, showed that the HR of an event for Pathway 2 versus Pathway 1 was 2.9 (95% CI 1.0 to 8.1, p = 0.04). An event in this model relates to a participant receiving a correct treatment; therefore, a HR of 2.9 indicates that participants in Pathway 2 received correct treatment 2.9 times quicker than those in Pathway 1.
FIGURE 13.
Kaplan–Meier curves of TTCT by pathway for possible MIBC participants who were confirmed MIBC and received a correct treatment.
Exploratory sensitivity analysis: the primary outcome in the intermediate stage but excluding participants whose correct treatment was palliative care
Some participants were declared as requiring palliative care, but the date of that decision depended upon on the sites’ clinical teams. Hence, careful consideration was made to account for these participants appropriately within the time-to-treatment analysis, while avoiding misleading results. This section shows a sensitivity analysis for the primary outcome, excluding participants with palliative care as their correct treatment.
There were 19 participants who were initially classified and then confirmed as having MIBC, where their correct treatment was not palliative care alone (10 in Pathway 1, and 9 in Pathway 2; Figure 14). Median TTCT for this subset of participants (N = 19) was 81 days (95% CI 54 to 100). Median TTCT for Pathway 1 (N = 10) was 81 days (95% CI 42 to 124) and median TTCT for Pathway 2 (N = 9) was 54 days (95% CI 22 to 100), log-rank p = 0.2366. Hence, the difference in TTCT between pathways became smaller when excluding participants whose correct treatment was palliative care only. In this post hoc subgroup analysis, a Cox model adjusted for the stratification factors of sex and age shows that the HR for Pathway 2 versus Pathway 1 was 1.9 (95% CI 0.6 to 5.9).
FIGURE 14.
Kaplan–Meier curves of TTCT by pathway for possible MIBC participants with confirmed MIBC and received correct treatment, excluding participants who received palliative care as their correct treatment.
It should be noted in this context that the decision to offer palliative care was made often very early in the MRI pathway, whereas it was often made very late in the standard pathway (in one case after the patient had died). This should be viewed as a very positive advantage for early MRI as patients will have likely been offered more appropriate palliative care support and are potentially spared the morbidity of a diagnostic TURBT if, for example, they are found to have locally advanced or metastatic disease.
Secondary outcome: TTCT for probable non-muscle-invasive bladder cancer participants confirmed as non-muscle-invasive bladder cancer
There were 58 participants who were initially classified as having probable NMIBC which was then confirmed (28 in Pathway 1 and 30 in Pathway 2; Figure 15); all such participants received their correct treatment of TURBT. Median TTCT for probable NMIBC participants confirmed as NMIBC (N = 58) was 16 days (95% CI 11 to 23). Median TTCT for Pathway 1 (N = 28) was 14 days (95% CI 10 to 29) and median TTCT for Pathway 2 (N = 30) was 17 days (95% CI 8 to 25, p = 0.6677). A Cox model adjusted for the stratification factors of sex and age showed that the HR for Pathway 2 versus Pathway 1 was 0.8 (95% CI 0.5 to 1.5).
FIGURE 15.
Kaplan–Meier curves of TTCT by pathway for probable NMIBC participants who were confirmed NMIBC and received a correct treatment.

Secondary outcome: TTCT for all randomised participants
Of the 143 randomised participants (72 in Pathway 1 and 71 in Pathway 2; Figure 16), 131 received a correct treatment. Participants who did not receive a correct treatment were censored at their date last seen and were included in the time-to-treatment analysis. Median TTCT for all randomised participants (N = 143) was 31 days (95% CI 22 to 37). Median TTCT for Pathway 1 (N = 72) was 37 days (95% CI 23 to 47) and median TTCT for Pathway 2 (N = 71) was 25 days (95% CI 18 to 35, p = 0.0295). A Cox model adjusted for the stratification factors of sex and age showed that the HR for Pathway 2 versus Pathway 1 was 1.4 (95% CI 0.9 to 2.0).
FIGURE 16.
Kaplan–Meier curves of TTCT by pathway for all randomised participants.

Health economics
Pre-trial costing suggested that the pathway may be cost-saving depending on the numbers of TURBT procedures that were removed by the earlier use of MRI and biopsy. The NHSE (NHS England) tariff cost of TURBT is £2183 (LB13D) and the tariff cost of mpMRI is £200 (RD05Z). There may be a small additional charge if biopsy is carried out during flexible cystoscopy but pre trial we ascertained that in many sites this was already common standard practice.
Based on these tariffs, we can estimate that bypassing TURBT in cases where it is not required is likely to be cost saving if only > 1 : 10 MRI scans lead to this outcome. Our data show that 7/36 patients had either definitive therapy or palliative care that did not require TURBT, a saving of approximately £8000–9000 on the £78,000 that would have been spent had all these patients had a TURBT. There are thus unlikely to be significant cost barriers to implementing the pathway. Separate issues apply within trusts concerning the relative availability of scanning capacity and operating theatre or surgical capacity which are likely to vary from hospital to hospital.
Follow-up
Length of follow-up
Overall, the median length of follow-up was 23.7 months (95% CI 23.7 to 24.0), 23.7 months (95% CI 23.7 to 24.0) for Pathway 1 (N = 72) and 24.0 months (95% CI 23.7 to 24.1) for Pathway 2 (N = 71), illustrated in Figure 17.
FIGURE 17.
Proportion of participants followed up.

Follow-up cystoscopy results are summarised in Table 26.
| Follow-up (month) | 3 (131) | 6 (123) | 9 (115) | 12 (105) | 18 (97) | 24 (90) | 36 (22) | Overall (683) |
|---|---|---|---|---|---|---|---|---|
| Has the participant had a cystoscopy as part of their follow-up | ||||||||
| No | 107 (82.9) | 73 (60.8) | 61 (55.5) | 56 (57.1) | 28 (31.5) | 35 (40.7) | 7 (33.3) | 367 (56.2) |
| Yes | 22 (17.1) | 47 (39.2) | 49 (44.5) | 42 (42.9) | 61 (68.5) | 51 (59.3) | 14 (66.7) | 286 (43.8) |
| Not known | 2 | 3 | 5 | 7 | 8 | 4 | 1 | 30 |
| Cystoscopy type | ||||||||
| Flexible cystoscopy | 14 (66.7) | 41 (87.2) | 45 (91.8) | 35 (83.3) | 55 (91.7) | 50 (98.0) | 13 (92.9) | 253 (89.1) |
| Rigid cystoscopy | 7 (33.3) | 6 (12.8) | 4 (8.2) | 7 (16.7) | 5 (8.3) | 1 (2.0) | 1 (7.1) | 31 (10.9) |
| Not known | 110 | 76 | 66 | 63 | 37 | 39 | 8 | 399 |
| Tumour biopsies taken at the time of cystoscopy | ||||||||
| No | 13 (59.1) | 41 (87.2) | 43 (87.8) | 33 (78.6) | 45 (73.8) | 40 (78.4) | 9 (75.0) | 224 (78.9) |
| Yes | 9 (40.9) | 6 (12.8) | 6 (12.2) | 9 (21.4) | 16 (26.2) | 11 (21.6) | 3 (25.0) | 60 (21.1) |
| Not known | 109 | 76 | 66 | 63 | 36 | 39 | 10 | 399 |
| Random biopsies of mucosa taken at the time of cystoscopy | ||||||||
| No | 15 (78.9) | 41 (91.1) | 43 (95.6) | 36 (90.0) | 49 (86.0) | 44 (88.0) | 12 (92.3) | 240 (89.2) |
| Yes | 4 (21.1) | 4 (8.9) | 2 (4.4) | 4 (10.0) | 8 (14.0) | 6 (12.0) | 1 (7.7) | 29 (10.8) |
| Not known | 112 | 78 | 70 | 65 | 40 | 40 | 9 | 414 |
| Cystoscopy findings | ||||||||
| No evidence of disease | 14 (66.7) | 36 (76.6) | 32 (65.3) | 31 (73.8) | 41 (67.2) | 33 (64.7) | 10 (71.4) | 197 (69.1) |
| No tumour seen but suggestive of CIS | 2 (9.5) | 1 (2.1) | 0 (0.0) | 1 (2.4) | 3 (4.9) | 3 (5.9) | 0 (0.0) | 10 (3.5) |
| Evidence of tumour | 5 (23.8) | 8 (17.0) | 10 (20.4) | 9 (21.4) | 12 (19.7) | 12 (23.5) | 3 (21.4) | 59 (20.7) |
| Equivocal (tumour suspected but not confirmed) | 0 (0.0) | 2 (4.3) | 7 (14.3) | 1 (2.4) | 5 (8.2) | 3 (5.9) | 1 (7.1) | 19 (6.7) |
| Not known | 110 | 76 | 66 | 63 | 36 | 39 | 8 | 398 |
Follow-up data reporting cytology and imaging results on suspicion of recurrence are summarised in Table 27.
| Follow-up (month) | 3 (131) | 6 (123) | 9 (115) | 12 (105) | 18 (97) | 24 (90) | 36 (22) | Overall (683) |
|---|---|---|---|---|---|---|---|---|
| Clinical examination findings suspicious of recurrence | ||||||||
| No | 95 (93.1) | 87 (91.6) | 64 (83.1) | 58 (82.9) | 61 (82.4) | 65 (86.7) | 18 (94.7) | 448 (87.5) |
| Yes | 7 (6.9) | 8 (8.4) | 13 (16.9) | 12 (17.1) | 13 (17.6) | 10 (13.3) | 1 (5.3) | 64 (12.5) |
| Not known | 29 | 28 | 38 | 35 | 23 | 15 | 3 | 171 |
| Cytology | ||||||||
| No | 99 (96.1) | 90 (92.8) | 71 (94.7) | 63 (91.3) | 67 (90.5) | 67 (89.3) | 15 (78.9) | 472 (92.2) |
| Yes | 4 (3.9) | 7 (7.2) | 4 (5.3) | 6 (8.7) | 7 (9.5) | 8 (10.7) | 4 (21.1) | 40 (7.8) |
| Not known | 28 | 26 | 40 | 36 | 23 | 15 | 3 | 171 |
| Cytology result | ||||||||
| Abnormal cells suspicious | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 1 (1.3) | 1 (5.3) | 3 (0.6) |
| Malignant cells present | 2 (1.9) | 0 (0.0) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 1 (1.3) | 0 (0.0) | 4 (0.8) |
| N/A | 99 (96.1) | 90 (92.8) | 71 (95.9) | 63 (91.3) | 67 (90.5) | 67 (89.3) | 15 (78.9) | 472 (92.4) |
| No malignant cells | 2 (1.9) | 6 (6.2) | 3 (4.1) | 4 (5.8) | 7 (9.5) | 6 (8.0) | 3 (15.8) | 31 (6.1) |
| Not done | 0 (0.0) | 1 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Not known | 28 | 26 | 41 | 36 | 23 | 15 | 3 | 172 |
| Further imaging | ||||||||
| No | 82 (71.3) | 90 (81.1) | 79 (84.9) | 71 (84.5) | 68 (77.3) | 59 (72.8) | 16 (76.2) | 465 (78.4) |
| Yes | 33 (28.7) | 21 (18.9) | 14 (15.1) | 13 (15.5) | 20 (22.7) | 22 (27.2) | 5 (23.8) | 128 (21.6) |
| Not known | 16 | 12 | 22 | 21 | 9 | 9 | 1 | 90 |
| Further imaging detail | ||||||||
| CT scan | 28 (24.3) | 19 (17.1) | 13 (14.0) | 12 (14.3) | 16 (18.2) | 19 (23.5) | 5 (23.8) | 112 (18.9) |
| MRI scan | 2 (1.7) | 2 (1.8) | 0 (0.0) | 1 (1.2) | 2 (2.3) | 1 (1.2) | 0 (0.0) | 8 (1.3) |
| N/A | 82 (71.3) | 90 (81.1) | 79 (84.9) | 71 (84.5) | 68 (77.3) | 59 (72.8) | 16 (76.2) | 465 (78.4) |
| Ultrasound | 2 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0 (0.0) | 3 (0.5) |
| X-ray | 1 (0.9) | 0 (0.0) | 1 (1.1) | 0 (0.0) | 1 (1.1) | 2 (2.5) | 0 (0.0) | 5 (0.8) |
| Not known | 16 | 12 | 22 | 21 | 9 | 9 | 1 | 90 |
| Disease recurrence/progression | ||||||||
| N/A | 82 (71.9) | 90 (81.8) | 79 (84.9) | 71 (84.5) | 68 (77.3) | 59 (72.8) | 16 (76.2) | 465 (78.7) |
| No | 26 (22.8) | 15 (13.6) | 11 (11.8) | 8 (9.5) | 15 (17.0) | 18 (22.2) | 2 (9.5) | 95 (16.1) |
| Yes | 6 (5.3) | 5 (4.5) | 3 (3.2) | 5 (6.0) | 5 (5.7) | 4 (4.9) | 3 (14.3) | 31 (5.2) |
| Not known | 17 | 13 | 22 | 21 | 9 | 9 | 1 | 92 |
Recurrence/progression/new primary/death
There were 70 recurrence, progression or new primary events from 47 participants (26 in Pathway 1 and 21 in Pathway 2), summarised in Table 28.
| Pathway | Pathway 1 (n = 39) | Pathway 2 (n = 31) | Overall (n = 70) |
|---|---|---|---|
| Locoregional disease | |||
| No | 7 (18.4) | 4 (13.3) | 11 (16.2) |
| Yes | 31 (81.6) | 26 (86.7) | 57 (83.8) |
| Not known | 1 | 1 | 2 |
| Upper tract urothelial cancer or urethra | |||
| N/A | 7 (18.9) | 4 (13.8) | 11 (16.7) |
| No | 26 (70.3) | 23 (79.3) | 49 (74.2) |
| Yes | 4 (10.8) | 2 (6.9) | 6 (9.1) |
| Not known | 2 | 2 | 4 |
| Was the recurrence in the same location as the primary tumour | |||
| N/A | 7 (20.0) | 4 (14.8) | 11 (17.7) |
| No | 7 (20.0) | 9 (33.3) | 16 (25.8) |
| Yes | 21 (60.0) | 14 (51.9) | 35 (56.5) |
| Not known | 4 | 4 | 8 |
| Metastatic disease | |||
| No | 27 (73.0) | 25 (89.3) | 52 (80.0) |
| Yes | 10 (27.0) | 3 (10.7) | 13 (20.0) |
| Not known | 2 | 3 | 5 |
| Metastatic site | |||
| Brain | 1 (2.6) | 0 (0.0) | 1 (1.4) |
| Liver | 1 (2.6) | 0 (0.0) | 1 (1.4) |
| Lung | 3 (7.7) | 2 (6.5) | 5 (7.1) |
| Lung, bone | 1 (2.6) | 0 (0.0) | 1 (1.4) |
| Lung, pelvic nodal, metastatic nodal | 1 (2.6) | 0 (0.0) | 1 (1.4) |
| N/A | 27 (69.2) | 25 (80.6) | 52 (74.3) |
| Not known | 2 (5.1) | 3 (9.7) | 5 (7.1) |
| Other | 1 (2.6) | 1 (3.2) | 2 (2.9) |
| Pelvic nodal | 1 (2.6) | 0 (0.0) | 1 (1.4) |
| Pelvic nodal, metastatic nodal | 1 (2.6) | 0 (0.0) | 1 (1.4) |
| New primary tumour | |||
| No | 37 (97.4) | 25 (86.2) | 62 (92.5) |
| Yes | 1 (2.6) | 4 (13.8) | 5 (7.5) |
| Not known | 1 | 2 | 3 |
| Site of new primary tumour | |||
| N/A | 37 (94.9) | 25 (80.6) | 62 (88.6) |
| Not known | 1 (2.6) | 5 (16.1) | 6 (8.6) |
| Other | 0 (0.0) | 1 (3.2) | 1 (1.4) |
| Renal | 1 (2.6) | 0 (0.0) | 1 (1.4) |
Twenty participant deaths were reported (10 in each pathway), summarised in Table 29.
| Pathway | Pathway 1 (n = 10) | Pathway 2 (n = 10) | Overall (n = 20) |
|---|---|---|---|
| Cause of death | |||
| Disease related | 7 (70.0) | 3 (30.0) | 10 (50.0) |
| Not known | 1 (10.0) | 3 (30.0) | 4 (20.0) |
| Other cancer – metastatic prostate Ca + metastatic oropharyngeal Ca | 1 (10.0) | 0 (0.0) | 1 (5.0) |
| Other cancer – leukaemia | 0 (0.0) | 1 (10.0) | 1 (5.0) |
| Other non-cancer – COVID-19 | 0 (0.0) | 1 (10.0) | 1 (5.0) |
| Other non-cancer – COVID-19 pneumonitis | 1 (10.0) | 0 (0.0) | 1 (5.0) |
| Other non-cancer – multiorgan failure | 0 (0.0) | 1 (10.0) | 1 (5.0) |
| Other non-cancer – pulmonary thromboembolism | 0 (0.0) | 1 (10.0) | 1 (5.0) |
Substudies
Urinary DNA substudy
The trial included a translational study evaluating the use of a urinary DNA test in the haematuria clinic. The aim was to test the ability of mutational analysis of urinary DNA to non-invasively detect BC within the context of haematuria investigations and NMIBC surveillance. The initial BladderPath screening patients were offered entry into this biomarker study which had separate funding and eventually 176 participants were recruited. It should be noted that these patients only partially overlap with the main trial patients as most did not have BC and hence did not proceed to the main study. The DNA substudy was also supplemented with other patients from haematuria clinics, separate from BladderPath recruitment. The results summarised below have been separately published. 30
In brief, pre-cystoscopy mid-stream urine specimens (up to 50 ml) were collected in Norgen Urine Collection and Preservation Tubes (Norgen Biotek, Thorold, ON, Canada) on the day of clinic attendance and transferred to the Human Biomaterials Resource Centre (HBRC) at the University of Birmingham at ambient temperature by post (in UN3373 packaging). On receipt at HBRC, samples were centrifuged at 1000 g for 10 minutes; cell pellets and supernatants were then separated and frozen at –80 °C. The non-BC patients were determined to be ‘normal’ or with diagnoses including calculi, benign prostatic hyperplasia, cystitis, inflammation, urinary tract infection, prostate cancer and kidney cancer. A ‘panel of normals’ and ‘confirmatory controls’ were randomly selected from this cohort.
Deoxyribonucleic acid was extracted from cell pellets (cp) using Quick-DNA Urine Kits (Zymo Research, Irving, CA, USA) and quantitated using high-sensitivity double-stranded DNA Qubit kits (Thermo Fisher, Waltham, MA, USA). Laboratory staff were unaware of patient diagnoses. Libraries were prepared from 25 ng urine cell pellet DNA (cpDNA; extracted from an average of 23 ml of urine) using Nonacus Cell3 Target enrichment. DNA was enzymatically sheared, end-repaired and A-tailed, and adapters (including Unique Molecular Identifiers) ligated to the fragments. Libraries were amplified and pooled in batches of 12 prior to overnight hybridisation with biotinylated probes and subsequent capture and final amplification of the next generation sequencing libraries. The probes targeted hotspots or regions of 23 genes. All libraries were 2 × 150 bp sequenced on a NovaSeq (Illumina, San Diego, CA, USA).
Sequencing data were demultiplexed and aligned to hg19 using bwa (version 0.7.15-r1140). Consensus reads were built using fgbio (version 1.1.0) requiring ≥ 3 reads to produce a consensus as described previously36 and re-aligned to the reference. Average raw and consensus read depths were 27,100 × and 3000 ×, respectively. Samples with consensus read depth < 500 at ≥ 10 loci were excluded (35 out of 919, 3.8%). Base calls with quality ≥ 30 were extracted using bam-readcount and used to calculate VAFs at 443 predefined genomic coordinates (a refined set of single nucleotide variants from our study of 956 BCs36). An optimal variant calling strategy was developed based on the maximum variant allele frequencies observed in a panel of 100 BC-negative haematuria patients and confirmed on a further 62 BC-negative haematuria patients.
A ‘positive test’ was defined as detection of any one of the 443 mutations in a cpDNA sample at > 0.9% VAF for chr5:129528A/G or > 0.5% VAF at all other coordinates. This combination provided 89.9% specificity in the ‘panel of normals’ and 91.2% specificity in a further 62 ‘confirmatory controls’ (non-BC haematuria clinic cpDNAs).
Applying the assay to the prospectively collected haematuria clinic cohort, including BladderPath patients, achieved 86.8% sensitivity at 81.0% specificity. 30 Combining two haematuria clinic cohorts to derive test positivity and VAFs across grades and stages of disease, 144/165 BCs tested positive [87.3% (95% CI 81.2 to 92.0) sensitivity] and 223/264 non-BCs tested negative [84.8% (95% CI 79.9 to 89.0) specificity]. 30 Mutations were detected significantly more commonly (and with higher VAFs) in cpDNA from patients with all stages and grades of BC compared with non-BC patients (p < 0.001). Sensitivity was 97.4% (95% CI 91.4 to 99.7) for grade 3 BC, 86.5% (95% CI 74.2 to 94.4) for grade 2 BC and 70.8% (95% CI 48.9 to 87.4) for grade 1 BC. Sensitivity was 79.3% (95% CI 69.3 to 87.2) for pTa, 100% (95% CI 90.0 to 100.0) for pT1 and 91.7% (95% CI 78.1 to 98.3) for MIBC; all three cases of solitary CIS were detected. The median maximum VAF in incident BCs was 18.7%, versus 0.28% in non-BC patients (p < 0.001), with a median of three mutations per BC cpDNA. The most commonly detected mutations were TERT, TP53, FGFR3, PIK3CA, ERCC2, ERBB2 and RHOB, mirroring previous tumour tissue data. 36,41,42
The study concluded that ultra-deep sequencing of somatic mutations in a 23 gene panel in urinary DNA had the potential to detect new cases of BC with high sensitivity and specificity and could reduce reliance on cystoscopy in the haematuria clinic setting. The test also has a potential role in post-diagnosis surveillance, and this is being evaluated further. 35
Routine data use
The trial initially intended to use access to routine data to reduce the need for conventional data collection via case record forms. The methodology was developed as part of a PhD thesis and the findings have been separately published. 43 This data model could be used as part of a future Commissioning through Evaluation (CTE)-based evaluation. It was not unfortunately possible to use the data collected in this way for the primary trial analysis as NHS Digital wished to charge around £3000 for each data extraction, even though these data extractions were multiple repeats of the same query, rather than each being a new bespoke query. As this was potentially monthly for up to 5 years, these costs were prohibitive. 44 The Chief Investigator met with the Chair of NHS Digital to explore ways of reducing these costs. While he agreed that it made no sense to levy these high charges for simple repeat queries, there did not seem to be any mechanism to waive them. In the end, we were forced to collect data the ‘traditional’ way via local case record forms, research nurses and data managers, thereby depleting valuable local trial resources within trusts. A positive end note to this is that the HTA have agreed to fund a one-off NHS Digital data sweep to correspond with the last entered patient completing 2 years of follow-up. This has allowed us to close all the trial sites to further follow-up and still to perform an event-driven and survival analysis using this methodology as a future analysis.
Conclusions
The mpMRI-directed pathway (Pathway 2) led to a substantial reduction in TTCT for MIBC participants without detriment to the TTCT for NMIBC participants. The initial Likert scale assessment at flexible cystoscopy accurately identified lower-risk NMIBC who all required TURBT and suggest no benefit from MRI in this lower-risk setting. Higher-risk patients identified at flexible cystoscopy benefited from MRI prior to any further intervention. Consideration should be given to the incorporation of mpMRI ahead of TURBT in the standard pathway for all patients with suspected MIBC. The improved decision-making accelerated time to treatment, even though many patients subsequently needed TURBT as part of their treatment plan. It also allowed around half of the MIBC patients to avoid TURBT completely and to proceed, with combined histological (for tissue diagnosis) and radiological confirmation of invasion or metastasis, to correct treatment for their MIBC, saving resources and reducing patient morbidity.
Discussion
We undertook the BladderPath study to investigate whether suspected MIBC participants may be safely expedited to correct treatment using initial mpMRI for initial local staging rather than TURBT. We have shown that it is feasible to introduce mpMRI for a proportion of participants visually diagnosed with possible MIBC at outpatient diagnostic flexible cystoscopy45 and, in doing so, MIBC participants receive their correct treatment significantly (over 6 weeks) quicker. We also show that deploying mpMRI in this way also allowed NMIBC participants to receive their correct treatment (TURBT) more rapidly, perhaps by reducing operating theatre demand through avoiding inappropriate TURBT for MIBC. As noted, many of these higher-risk patients also required TURBT, for example to resolve diagnostic uncertainty, to debulk patients prior to chemoradiation or to assess factors such as CIS or variant histology. Despite this, the Pathway 2 patients still received their definitive treatment faster than those assessed solely with TURBT.
Delays in administering the correct treatment for MIBC participants are internationally widespread and contribute to worsening prognosis. 14,21,45–47 The shortcomings of TURBT are well-reported and delay the correct treatment for MIBC participants by radical therapies, lead to incorrect therapy choices and possibly contribute to tumour spread. 45 Over the last decade, multiple studies have confirmed that mpMRI has higher sensitivity and specificity for more accurate discrimination of NMIBC and MIBC than TURBT,22,23 and so potentially offering a safer and faster route to staging and hence correct treatment. Although the relationship between delay and survival in BC is complex,48 it is reasonable to suggest that administering correct treatment to MIBC participants more than 6 weeks earlier than the current SOC can only be beneficial. Several studies report adverse outcomes associated with delays of over 3 months between BC diagnosis and RC;49,50 the mpMRI-guided pathway (Pathway 2) undercut this by a considerable margin (median TTCT 53 days), whereas the standard pathway did not (median TTCT 98 days).
Transurethral resection of bladder tumour has been the cornerstone of the MIBC pathway for nearly 100 years. 51 Advocates suggest it is important to resect the luminal BC component to allow full histological categorisation (including the identification of variant histology) to obtain pathological proof of muscle invasion prior to commencing systemic chemotherapy/radical treatment and for reduction in tumour volume (perhaps to facilitate bladder sparing radical treatment). 52 However, the literature clearly shows that TURBT and cystectomy histology are often discordant with respect to variant and subtyping,53,54 understaging of muscle invasion in TURBT samples is common,11,55 and there are few data to suggest that tumour debulking is a necessary component of radiotherapy regimens. It should be noted that debulking is prognostic in many series, with incomplete debulking being associated with worse outcomes. However, this is likely to reflect completeness of debulking as being a surrogate for stage rather than a direct therapeutic effect. Indeed, the operator dependency of TURBT56 may confuse the identification of complete chemotherapy response (stage ypT0) and so mean that cystectomy is used in many participants where bladder sparing would be possible. 57 Although not yet routine, modern technologies allow complex RNA expression profiling and DNA mutation analysis to be undertaken on small biopsies or on tumour DNA from urine. 35,58 There is thus no need for large excision biopsies for detailed molecular subtyping. Small tumour biopsies obtained during flexible cystoscopy yield enough material to permit both histopathological diagnosis of cancer and molecular subtyping (cf. prostate cancer59); liquid biopsy approaches may contribute additional risk stratification. 35,60,61
An important component of this new pathway is the ability of urologists to accurately triage patients as probable NMIBC or possible MIBC at the time of outpatient diagnostic flexible cystoscopy based upon the macroscopic appearances of suspicious bladder lesions. Building upon previous evidence,62 we have shown that such initial triage is accurate, demonstrating that 89% of lesions where the initial cystoscopic assessor strongly agreed or agreed the likely diagnosis was NMIBC were subsequently confirmed as NMIBC and accounted for around 50% of all cases. For the remaining 50%, where the initial urological assessment was less certain, the addition of mpMRI provided rapid, accurate triage with an approximately equal split between NMIBC and MIBC. Such an approach dovetails neatly with standard practice for prostate cancer diagnostics in the same units. 63 With initial outpatient cystoscopic triage, plus the lower numbers of cases of BC versus prostate cancer, any impact on departmental MRI workload would be relatively modest. Notwithstanding, it should be noted that a proportion of participants in Pathway 2 underwent TURBT for a range of reasons post MRI (e.g. to ascertain presence of histological variants, debulking pre-radiotherapy) without compromising TTCT. Of 14 TURBTs undertaken after mpMRI scanning in Pathway 2, only 3 (21%) were undertaken due to ‘lack of confidence that the MRI shows MIBC’, all from the same hospital. Hence, for the majority of patients, mpMRI provided staging information such that these TURBT procedures could be assigned to the correct surgeon for the correct indication (e.g. to debulk tumour prior to radical therapy) and be given high priority to reflect the needs of MIBC participants.
Thirteen participants underwent cystectomy but were later diagnosed with NMIBC after histopathological examination of the cystectomy specimen (six in Pathway 1 and seven in Pathway 2); there was no significant difference in the number of cystectomies undertaken for NMIBC between the two pathways. All 13 of these participants underwent TURBT prior to cystectomy, with TURBT diagnosing NMIBC in all but 1 participant. This latter participant (Pathway 2, possible MIBC) had MIBC confirmed at TURBT and then pTis in the cystectomy specimen, indicating a complete resection of tumour at the initial TURBT. Hence, there was no evidence that early use of MRI for staging led to NMIBC patients undergoing ‘unnecessary’ cystectomy. As detailed in the introduction, cystectomy is a potentially appropriate treatment for high-grade or extensive NMIBC and the similar numbers in both pathways reflect this. We were unable to determine in more detail the decision-making process at the MDT meeting following first TURBT. We suggest that future studies in this setting should set out to capture such data.
Experience with the introduction of MRI into the prostate cancer pathway has been that, after initial scepticism, there has been widespread acceptance of the paradigm that more accurate imaging can improve clinical decision-making. 64 Notably, this adoption has occurred in the absence of randomised studies linked to clinical outcomes beyond the initial biopsy findings. To apply similar considerations to the BC pathway, generally managed via the same teams, is likely to be potentially more straightforward. Experience with setting up BladderPath suggests that clinicians rapidly become comfortable with using MRI to guide downstream decisions.
Regarding the cost associated with Pathway 2, a simple analysis demonstrates that saving 1 : 10 patients from needing a TURBT pays the crude costs of the additional nine MRI scans. Around 1 : 6 patients avoided a MRI scan in BladderPath making it likely that the pathway can be self-funding at the very least. The faster time to definitive therapy for NMIBC, but also for MIBC, is likely to improve outcomes which in itself may be cost saving. A full health economic analysis to examine these effects will require access to the long-term follow-up data which will become available in around 18 months’ time and will be analysed separately. We conclude that it is feasible to add mpMRI for those participants visually diagnosed with possible MIBC at outpatient diagnostic cystoscopy. By doing so, possible MIBC participants receive their correct therapy significantly quicker, potentially leading to improved long-term outcomes, even if these patients require TURBT as part of their further assessments prior to definitive treatment. The initial radiological staging data which MRI adds over and above the urinary tract imaging that is routinely carried out in haematuria clinics thus leads to accelerated access to correct treatment. Anecdotally, where patients are undergoing TURBT post MRI, the procedure may be different and more targeted than the standard full diagnostic procedure on Pathway 1. Additionally, separating the lower-risk NMIBC from the higher-risk NMIBC and MIBC allows triage of cases to appropriate levels of surgical expertise. This is more difficult on the standard pathway where all patients need to have a TURBT for staging in order to plan further therapy.
The BC diagnostic and staging pathway has followed a largely unchanged pattern for nearly a century with rigid cystoscopy forming the mainstay of both histological diagnosis and initial staging. For patients with NMIBC, TURBT is also the mainstay of treatment. Unfortunately, TURBT is not the main treatment for the most lethal form of BC and may even be pro-metastatic. In the main, most cancers are staged with a biopsy to confirm histology and imaging to determine stage ahead of definitive treatment. For patients with MIBC, the TURBT pathway is frequently inaccurate with the need for repeat TURBT to assess muscle invasion with consequent delay – a minimum delay of 6 weeks between initial and re-do TURBT is standard.
Interpretation
A modified pathway with initial triage based on appearance at flexible cystoscopy correctly identified lower-risk NMIBC patients requiring TURBT. For higher-risk patients, use of a MRI scan allowed identification of NMIBC patients for TURBT while accelerating the identification of very high-risk patients requiring more complex therapy, which may include TURBT but equally allowed some patients to bypass this stage and proceed to definitive treatment more rapidly. The impact of the MRI on TTCT for these patients is substantial with no evidence of a detrimental effect on NMIBC care.
Limitations
There are various limitations to the current study. Unfortunately, there were substantial interruptions to recruitment due to COVID-19 and so we were unable to enrol sufficient participants to evaluate our a priori progression free survival-based outcomes.
Secondly, the exact pathological stage in participants who underwent systemic chemotherapy, radiotherapy or palliation for mpMRI-diagnosed MIBC was unknown and so it is impossible to conclusively know whether these were correct treatments. However, TURBT has been shown to have substantial error rates, with many NMIBC patients upstaged to MIBC at subsequent cystectomy. 11,65 Hence, there is no perfect ‘ground truth’ on either side of the randomisation – this would require a trial in which all participants undergo cystectomy. Thirdly, the development of a mpMRI grading system, VI-RADS,22 occurred during the conduct of our study (two of the trial team were part of the VI-RADS group) and provides a separate, peer-reviewed classification system for implementation. Work to validate the VI-RADS system with the BladderPath images is ongoing. Notwithstanding, the VI-RADS mpMRI sequences are analogous to those in widespread use for prostate cancer diagnosis by the same clinical teams. Hence, roll-out of a mpMRI-based pathway incorporating VI-RADS should be straightforward.
Further work to cross-correlate with the VI-RADS system22 will improve accuracy and aid dissemination. Longer follow-up to examine the effect of the pathway on outcomes is also required.
A comprehensive health economic analysis was not feasible in the absence of longer-term outcomes data. However, a simple cost: consequence analysis shows that the MRI-based pathway is likely to be cost saving.
Generalisability
The MRI sequences used are similar to those in widespread use for prostate cancer diagnosis by the same teams and hence roll out should be relatively straightforward. The trial was carried out in a range of NHS units and thus broader roll out should be feasible.
Overall evidence
The trial was carried out at a time when image-directed treatment decisions have become standard practice in prostate cancer. The development of a MRI grading system, VI-RADS,22 occurred during the conduct of the trial and provides a separate, peer-reviewed, classification system for implementation. Taken with the work on VI-RADS, a move to the pathway examined in the BladderPath trial appears to be both feasible and desirable.
Research recommendations
Key areas for further research:
-
A large, randomised trial to look at failure-free survival is unlikely to be feasible given the issues faced by BladderPath and the current very difficult research and broader NHS environment. However, part of the BladderPath programme included the development of tools to extract the key end points of interest from NHS Digital records. This would allow broader evaluation of a MRI-based pathway via the CTE programme. As our costings indicate, this is likely to be cost saving and will free up around 10% of TURBT slots, participation in such a programme may be attractive to Trusts.
-
A CTE programme looking at MRI should also evaluate the role of VI-RADS specifically as a decision-making tool. Studies examining the role of biomarkers, particularly liquid DNA biomarkers in blood and urine, are required. These may further improve the diagnostic accuracy and therapeutic decision-making provided by MRI (± TURBT) as well as providing non-invasive tools for follow-up and response assessment reducing the need for invasive check cystoscopy.
Patient participation and involvement
Patient participation and involvement in this study are summarised in Table 30.
| Section and topic | Item |
|---|---|
| 1: Aim | To work with clinicians and researchers to ensure that the study remained patient-centred, had public accountability and the findings are shared with the general public in appropriate and accessible ways |
| 2: Methods | The BladderPath study has had strong patient representative involvement from the protocol development and grant application stages, through to active engagement as members of both the TMG (two members, male and female) and Trial Steering Committee (one member) As members of the TMG, patient representative members have regularly attended TMG meetings throughout the duration of the study, ensuring the patient perspective was properly considered |
| 3: Study results | The lived experience of patients as active contributors supported the development and delivery of the study. In particular:
|
| 4: Discussion and conclusions | It was helpful to have more than one patient representative member at the TMG – where one might not be able to attend, the other usually did. However, having only three contributors may have limited the breadth and diversity of public perspective into the study |
| 5: Reflections/critical perspective | The strong collaboration with patient/public contributors has added relevance and value to the study and highlights the importance of collaboration throughout the trial cycle |
Equality, diversity and inclusion
Registration into the study was open to any adult patient of 18 years or more attending haematuria clinic for investigation of blood in their urine. If initial examination found evidence of potential BC, then randomised entry into the main study was offered to the patient. During the course of the trial only one registered participant was under the age of 20 years, and they were not found to have cancer during initial examination. Lowering the age of entry to 16 years was considered in 2020, but with the approaching end of recruitment and the unlikely prospect of a 16- to 17-year-old presenting with BC, this was decided against.
One recruiting site reported that sending out the invitation letter and participant information sheet to patients due to attend the haematuria clinic for initial investigation was found to be upsetting to some of the patients, which also upset the research staff. The site’s research team explained that the biggest barrier and upset for potential patients was the term ‘Cancer’ in the information sent to them prior to clinic, when approximately 80% of patients attending for investigation of their haematuria do not typically have cancer. At the same time, an investigator at another recruiting site pointed out that eligible patients might be discovered through alternative routes, such as inpatient investigations. Further to these early comments from sites, the protocol was amended to allow sites to approach patients about the study after their being given an initial diagnosis of suspicious tumours suitable for TURBT, which would in turn permit other patients found to be eligible for TURBT and treatment of BC to be recruited.
The difference between participants being registered prior to their initial bladder examination or registered and randomised into the study after the examination was that a biopsy of any tumour found might be obtained. Most of the sites were not able to obtain a biopsy during the initial examination (flexible cystoscopy) due to the procedure being carried out by a clinical nurse specialist rather than a urologist or that the surgical kit required to obtain the biopsy was not available. Participants who were both registered and randomised after flexible cystoscopy and found to have possible MIBC and randomised to Pathway 2 had to return for a repeat cystoscopy with biopsy in order that a histological diagnosis could be made.
These changes made it easier both for the recruiting sites to recruit, and reduced some of the stress experienced by patients awaiting examination.
Pictures and diagrams used in the participant information sheet were of body parts common to all, rather than having any link to a particular part of society.
The study management group is composed of clinicians, academics and academic and support research staff and patient representatives of various ethnicities, ages and abilities – as is the University of Birmingham in general.
Additional information
Contributions of authors
Nicholas James (https://orcid.org/0000-0002-7314-8204) [Chief Investigator, Institute of Cancer Research (ICR) and Principal Investigator (PI) at The Royal Marsden Hospital, London] substantially contributed to the conception and design of the work, revising this report critically for important intellectual content and had final approval of the version to be published. NDJ is also accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Sarah Pirrie (https://orcid.org/0000-0002-2894-2230) [Principal Statistician at the Cancer Research UK Clinical Trials Unit (CRCTU), Institute of Cancer and Genomic Sciences, University of Birmingham] substantially contributed to the design of the work and analysis of trial data.
Wenyu Liu (https://orcid.org/0000-0002-6682-2053) [Senior Statistician at the Cancer Research UK Clinical Trials Unit (CRCTU), Institute of Cancer and Genomic Sciences, University of Birmingham] substantially contributed to the design of the work and analysis of trial data.
James Catto (https://orcid.org/0000-0003-2787-8828) (Co-investigator, Consultant Urological Surgeon and PI at Department of Urology, Sheffield Teaching Hospitals NHS Foundation Trust, UK) substantially contributed to the conception and design of the work, drafting and revising this report critically for important intellectual content.
Kieran Jefferson (https://orcid.org/0000-0002-0029-8359) (Co-investigator, Consultant Urological Surgeon and PI at Department of Urology, University Hospitals Coventry and Warwickshire NHS Trust, UK) substantially contributed to the conception and design of the work, drafting and revising this report critically for important intellectual content.
Prashant Patel (https://orcid.org/0000-0002-2882-5990) (Co-investigator, Consultant Urological Surgeon and PI at University Hospitals Birmingham recruiting site) substantially contributed to the conception and design of the work, drafting and revising this report critically for important intellectual content.
Ana Hughes (https://orcid.org/0000-0001-7274-9422) (Senior Trial Coordinator at CRCTU, Institute of Cancer & Genomic Sciences, University of Birmingham) contributed to the drafting and revising this report critically for important intellectual content.
Ann Pope (https://orcid.org/0000-0001-7458-3294) (Trial Coordinator at CRCTU, Institute of Cancer & Genomic Sciences, University of Birmingham) contributed to the drafting and revising this report critically for important intellectual content.
Veronica Nanton (https://orcid.org/0000-0002-9553-822X) (Co-investigator, based at the Medical School, University of Warwick) contributed to the qualitative research section.
Harriet P Mintz (https://orcid.org/0000-0003-1583-6268) (Research Associate) contributed to the design of the work.
Allen Knight (https://orcid.org/0000-0001-8123-9547) (Patient and Public Involvement Representative on the Trial Management Group) contributed to the design and concept and has been involved in drafting the report.
Jean Gallagher (https://orcid.org/0009-0007-6308-2292) (Patient and Public Involvement Representative on the Trial Management Group) contributed to the design and concept and has been involved in drafting the report.
Richard T Bryan (https://orcid.org/0000-0003-2853-4293) (Co-investigator and Director of the Bladder Cancer Research Centre, Institute of Cancer & Genomic Sciences, University of Birmingham) substantially contributed to the conception and design of the work, drafting and revising this report critically for important intellectual content. He also obtained funding for and led the diagnostic urinary biomarker sub-study of BladderPath.
Acknowledgements
The study was sponsored by University of Birmingham and run by the Cancer Research UK Clinical Trials Unit (CRCTU). Staff at the CRCTU are supported by a core funding grant from Cancer Research UK (C22436/A25354). The trial was initiated and conducted independently by the trial investigators. We thank the participants who took part in the trial and their families; the principal investigators and co-investigators from the 15 recruiting centres, and their research staff. We would also like to acknowledge the contribution of the Trial Steering Committee and Data Monitoring Committee. Additional biomarker research on samples derived from the trial was funded by Cancer Research UK.
Other contributors
Sophia Magwaro, Trial Management Team Leader, CRCTU.
Hiede Doyle, Trial Data Manager, CRCTU.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it is important that there are safeguards to make sure that they are stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Data-sharing statement
Participant data and the associated supporting documentation will be available within 6 months after the publication of the study results in a peer-reviewed journal. Details of our data request process are available on the CRCTU website. Only scientifically sound proposals from appropriately qualified research groups will be considered for data sharing. All data requests should be submitted to the corresponding author for consideration.
Ethics statement
The BladderPath study was conducted in accordance with the World Medical Association (WMA) Declaration of Helsinki (1964) amended by the 48th WMA General Assembly, Somerset West, Republic of South Africa, 1996 [World Medical Association. WMA Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. 6 September 2022. URL: www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed 14 July 2023)]. The study was also conducted in accordance with applicable UK Statutory Instruments (including the Data Protection Act 1998 and Human Tissue Act 2004 and Good Clinical Practice (GCP). The study protocol and patient information and consent documents were peer reviewed and ethically approved prior to the study opening (NIHR London Bridge Research Ethics Committee, UK project reference 17/LO/1819; approval date 4 December 2017). It was the responsibility of Principal Investigators at the recruiting sites that local approvals were in place and maintained, and to take immediate clinical action if thought necessary to protect the health and interest of individual patients.
Information governance statement
The University of Birmingham is the data controller and its Cancer Research Clinical Trials Unit (CRCTU) the data processor of all study participants’ personal data handled throughout the BladderPath study. As per the EU General Data Protection Regulation and the UK Data Protection Act 2018, all trial/study participants were provided with additional details regarding the legal basis that allows us to hold and process their personal data, available on the study’s web page [bladderpath-patient-info-sheet-v5.0a-10-sep-2020.pdf (birmingham.ac.uk)].
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR journals Library report publication page at https://doi.org/10.3310/DEHT5407.
Primary conflicts of interest: Nicholas James received funding for this project from the National Institute of Health and Care Research (NIHR) Health Technology Assessment programme and was a member of HTA General Committee. He is also a NIHR Senior Investigator. Richard T Bryan received research funding for this project from the National Institute of Health and Care Research Health Technology Assessment program. His research is also funded by grants from Cancer Research UK (Early Detection & Diagnosis), Cancer Research UK (Data Innovation Award), Janssen, University Hospitals Birmingham Charity (UK), Cancer Research UK (Biospecimen Collection), UroGen Pharma (USA). He receives consulting fees from Cystotech (Denmark) and is an unpaid charity trustee for Action Bladder Cancer UK. James Catto receives consulting fees from Astra Zeneca, BMS, Gilead, QED Therapeutics, Roche, Ferring, Steba biotech, UroGen, Jansenn, Photocure, and also received honoraria payments from BMS, Astra Zeneca and Roche. He is an unpaid trustee for Fight Bladder Cancer UK and member of BMS advisory board. Allen Knight was a member of EME Strategy Group, PHR Research Funding Board and EME Funding Committee Member, he is an unpaid Trustee of Action Bladder Cancer UK, unpaid Director of the World Bladder Cancer Patient Coalition and unpaid Birmingham Bladder Cancer Research Centre advisory board member.
Publications
Articles
Mintz HP. Can Routinely Collected Data be Used to Inform Randomised Controlled Trial Outcomes in Oncology? PhD thesis. Coventry: University of Warwick; 2019.
Mintz HP, Dosanjh A, Hughes A, Jakeman A, James ND, Parsons HM, et al. Development and validation of a follow-up methodology for a randomised controlled trial, utilising routine clinical data as an alternative to traditional designs: a pilot study to assess the feasibility of use for the BladderPath trial. Pilot Feasibil Stud J 2020;6:165. https://doi.org/10.1186/s40814-020-00713-y
Bryan RT, Liu W, Pirrie SJ, Amir R, Gallagher J, Hughes AI, et al. Comparing an imaging-guided pathway with the standard pathway for staging muscle-invasive bladder cancer: preliminary data from the BladderPath study. Eur Uro J 2021;80(1). https://doi.org/10.1016/j.eururo.2021.02.021
Ward DG; The BladderPath Trial Management Group, James ND, Bryan RT, et al. Highly sensitive and specific detection of bladder cancer via targeted ultra-deep sequencing of urinary DNA. Eur Urol Oncol 17 March 2022. https://doi.org/10.1016/j.euo.2022.03.005
Mintz HP, Dosanjh A, Parsons, H, Sydes, M, James, N, Patel P. Making administrative healthcare systems clinical data the future of clinical trials: lessons from BladderPath. BMJ Oncol 17 July 2023. https://doi.org/10.1136/bmjonc-2023-000038
Meetings
BAUS December 2018 Featured in debate involving Prashant Patel.
FIGHT magazine Published by Fight Bladder Cancer, UK (Registered Charity No. 1157763).
AI Hughes. BladderPath study: Re-designing the current diagnostic and management pathway for bladder cancer. FIGHT magazine article, 8th Edition, 2019.
12th Atlantic Canadian Uro-Oncology Group (ACUOG) Annual Meeting, November 2019, Halifax, UK.
Featured in presentation given by Nick James
ASCO GU 2020 (13–15 February 2020) – San Francisco, California (UroToday.com).
Nick James presented a poster entitled: Replacing TURBT with mpMRI for Staging MIBC: Pilot Data from the BladderPath study (source: www.urotoday.com/conference-highlights/asco-gu-2020/asco-gu-2020-bladder-cancer/119353-asco-gu-2020-replacing-turbt-with-mpmri-for-staging-mibc-pilot-data-from-the-bladderpath-study.html) EAU March 2020.
Bryan RB, Pirrie SJ, Liu W, Amir R, Gallagher G, Hughes AI, et al. Replacing TURBT with mpMRI for staging MIBC: pilot data from the BladderPath study. (Poster) July 2020. Eur Urol Open Sci 2020;19(Suppl. 2):e824. https://doi.org/10.1016/S2666-1683(20)33133-5
ESMO September 2022, London Mini oral presentation (Abstract 5806).
N.D. James, S. Pirrie, W. Liu, K. Jefferson, J. Gallagher, A. Hughes, et al. 1733MO First results from BladderPath: A randomised trial of MRI versus cystoscopic staging for newly diagnosed bladder cancer, Ann Oncol 2022;33(Suppl. 7):S1330. https://doi.org/10.1016/j.annonc.2022.07.1811
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- CRUK Cancerstats: Bladder Cancer 2014. www.cancerresearchuk.org/cancer-info/cancerstats/types/bladder/ (accessed 22 April 2014).
- Bessa A, Maclennan S, Enting D, Bryan R, Josephs D, Hughes S, et al. Consensus in Bladder Cancer Research Priorities Between Patients and Healthcare Professionals Using a Four-stage Modified Delphi Method. Eur Urol 2019;76:258-9.
- Bryan RT, Kirby R, O’Brien T, Mostafid H. So much cost, such little progress. Eur Urol 2014;66:263-4.
- Kaplan AL, Litwin MS, Chamie K. The future of bladder cancer care in the USA. Nat Rev Urol 2014;11:59-62.
- Kulkarni GS, Hakenberg OW, Gschwend JE, Thalmann G, Kassouf W, Kamat A, et al. An updated critical analysis of the treatment strategy for newly diagnosed high-grade T1 (previously T1G3) bladder cancer. Eur Urol 2010;57:60-7.
- Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Dominguez Escrig JL, et al. EAU guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol 2022;81:75-94.
- Woldu SL, Bagrodia A, Lotan Y. Guideline of guidelines: non-muscle-invasive bladder cancer. BJU Int 2017;119:371-80.
- Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol 2021;79:82-104.
- Engilbertsson H, Aaltonen KE, Bjornsson S, Kristmundsson T, Patschan O, Rydén L, et al. Transurethral bladder tumor resection can cause seeding of cancer cells into the bloodstream. J Urol 2014;193:53-7.
- Maurer T, Souvatzoglou M, Kubler H, Opercan K, Schmidt S, Herrmann K, et al. Diagnostic efficacy of [11C]choline positron emission tomography/computed tomography compared with conventional computed tomography in lymph node staging of patients with bladder cancer prior to radical cystectomy. Eur Urol 2012;61:1031-8.
- Rajesh A, Sokhi HK, Fung R, Mulcahy KA, Bankart MJ. Bladder cancer: evaluation of staging accuracy using dynamic MRI. Clin Radiol 2011;66:1140-5.
- Swinnen G, Maes A, Pottel H, Vanneste A, Billiet I, Lesage K, et al. FDG-PET/CT for the preoperative lymph node staging of invasive bladder cancer. Eur Urol 2010;57:641-7.
- Vargas HA, Akin O, Schoder H, Olgac S, Dalbagni G, Hricak H, et al. Prospective evaluation of MRI, (1)(1)C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur J Radiol 2012;81:4131-7.
- Russell B, Liedberg F, Khan MS, Nair R, Thurairaja R, Malde S, et al. A systematic review and meta-analysis of delay in radical cystectomy and the effect on survival in bladder cancer patients. Eur Urol Oncol 2020;3:239-49.
- Fahmy NM, Mahmud S, Aprikian AG. Delay in the surgical treatment of bladder cancer and survival: systematic review of the literature. Eur Urol 2006;50:1176-82.
- Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Longer wait times increase overall mortality in patients with bladder cancer. J Urol 2009;182:1318-24.
- Mahmud SM, Fong B, Fahmy N, Tanguay S, Aprikian AG. Effect of preoperative delay on survival in patients with bladder cancer undergoing cystectomy in Quebec: a population based study. J Urol 2006;175:78-83; discussion 83.
- Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TRL, et al. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol Assess 2010;14:1-331, iii.
- Boustead GB, Fowler S, Swamy R, Kocklebergh R, Hounsome L. Section of Oncology, BAUS . Stage, grade and pathological characteristics of bladder cancer in the UK: British Association of Urological Surgeons (BAUS) Urological Tumour Registry. BJU Int 2014;113:924-30.
- Bryan RT, Zeegers MP, van Roekel EH, Bird D, Grant MR, Dunn JA, et al. A comparison of patient and tumour characteristics in two UK bladder cancer cohorts separated by 20 years. BJU Int 2013;112:169-75.
- Meeks JJ, Herr HW. Office-based management of nonmuscle invasive bladder cancer. Urol Clin North Am 2013;40:473-9.
- Panebianco V, Narumi Y, Altun E, Bochner BH, Efstanthiou JA, Hafeez S, et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur Urol 2018;74:294-306.
- Del Giudice F, Flammia RS, Pecoraro M, Moschini M, D’Andrea D, Messina E, et al. The accuracy of Vesical Imaging-Reporting and Data System (VI-RADS): an updated comprehensive multi-institutional, multi-readers systematic review and meta-analysis from diagnostic evidence into future clinical recommendations. World J Urol 2022;40:1617-28.
- Del Giudice F, Barchetti G, De Berardinis E, Pecoraro M, Salvo V, Simone G, et al. Prospective assessment of Vesical Imaging Reporting and Data System (VI-RADS) and its clinical impact on the management of high-risk non-muscle-invasive bladder cancer patients candidate for repeated transurethral resection. Eur Urol 2020;77:101-9.
- Bouchelouche K. PET/CT in bladder cancer: an update. Semin Nucl Med 2022;52:475-85.
- Bouchelouche K, Turkbey B, Choyke PL. PET/CT and MRI in bladder cancer. J Cancer Sci Ther 2012;S14.
- Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, et al. Urinary bladder cancer: diffusion-weighted MR imaging: accuracy for diagnosing T stage and estimating histologic grade. Radiology 2009;251:112-21.
- Donaldson SB, Bonington SC, Kershaw LE, Cowan R, Lyons J, Elliott T, et al. Dynamic contrast-enhanced MRI in patients with muscle-invasive transitional cell carcinoma of the bladder can distinguish between residual tumour and post-chemotherapy effect. Eur J Radiol 2013;82:2161-8.
- Takeuchi M, Sasaki S, Naiki T, Kawai N, Kohri K, Hara M, et al. MR imaging of urinary bladder cancer for T-staging: a review and a pictorial essay of diffusion-weighted imaging. J Magnet Reson Imaging 2013;38:1299-309.
- Ward DG, Baxter L, Ott S, Gordon NS, Wang J, Patel P, et al. BladderPath Trial Management Group . Highly sensitive and specific detection of bladder cancer via targeted ultra-deep sequencing of urinary DNA. Eur Urol Oncol 2023;6:67-75.
- Ward DG, Gordon NS, Boucher RH, Pirrie SJ, Baxter L, Ott S, et al. Targeted deep sequencing of urothelial bladder cancers and associated urinary DNA: a 23-gene panel with utility for non-invasive diagnosis and risk stratification. BJU Int 2019;124:532-44.
- Stenzl A, Cowan NC, De Santis M, Jakse G, Kuczyk MA, Merseburger AS, et al. The updated EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 2009;55:815-25.
- Rosenkrantz AB, Haghighi M, Horn J, Naik M, Hardie AD, Somberg MB, et al. Utility of quantitative MRI metrics for assessment of stage and grade of urothelial carcinoma of the bladder: preliminary results. AJR Am J Roentgenol 2013;201:1254-9.
- Rosenkrantz AB, Mussi TC, Melamed J, Taneja SS, Huang WC. Bladder cancer: utility of MRI in detection of occult muscle-invasive disease. Acta Radiol 2012;53:695-9.
- Bryan RT, Collins SI, Daykin MC, Zeegers MP, Cheng KK, Wallace DMA, et al. Mechanisms of recurrence of Ta/T1 bladder cancer. Ann Royal Coll Surg Engl 2010;92:519-24.
- Wilby D, Thomas K, Ray E, Chappell B, O’Brien T. Bladder cancer: new TUR techniques. World J Urol 2009;27:309-12.
- Begum G, Dunn JA, Bryan RT, Bathers S, Wallace DM. West Midlands Urological Research Group . Socio-economic deprivation and survival in bladder cancer. BJU Int 2004;94:539-43.
- Bryan RT, Liu W, Pirrie SJ, Amir R, Gallagher J, Hughes AI, et al. Comparing an imaging-guided pathway with the standard pathway for staging muscle-invasive bladder cancer: preliminary data from the BladderPath study. Eur Urol 2021;80:12-5.
- Lynch TH, Waymont B, Dunn JA, Hughes MA, Wallace DM. Rapid diagnostic service for patients with haematuria. Br J Urol 1994;73:147-51.
- NHSEngland . NHS Payment System 2020. www.england.nhs.uk/publication/national-tariff-payment-system-documents-annexes-and-supporting-documents/ (accessed 21 July 2023).
- Bellmunt J, Kim J, Reardon B, Perera-Bel J, Orsola A, Rodriguez-Vida A, et al. Genomic predictors of good outcome, recurrence or progression in High grade T1 non-muscle invasive bladder cancer. Cancer Res 2020;80:4476-86.
- Pietzak E, Bagrodia A, Cha E, Drill EN, Iyer G, Isharwal S, et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur Urol 2017;72:952-9.
- Mintz HP, Dosanjh A, Parsons HM, Hughes A, Jakeman A, Pope AM, et al. Development and validation of a follow-up methodology for a randomised controlled trial, utilising routine clinical data as an alternative to traditional designs: a pilot study to assess the feasibility of use for the BladderPath trial. Pilot Feasibil Stud 2020;6.
- Mintz HP, Dosanjh A, Parsons HM, Matthew S, Bryan RT, James NJ, et al. Making administrative healthcare systems clinical data the future of clinical trials: lessons from BladderPath. BMJ Oncol 2023;2.
- Chu AT, Holt SK, Wright JL, Ramos JD, Grivas P, Yu EY, et al. Delays in radical cystectomy for muscle-invasive bladder cancer. Cancer 2019;125:2011-7.
- Cancer Alliance Data Evidence and Analysis Service (CADEAS) . Median Pathway Analysis by Patient Demographics, Stage at Diagnosis, Route to Diagnosis, and Geography 2018. www.cancerdata.nhs.uk/median_pathways/tool (accessed 9 February 2023).
- Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey-Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020;371.
- Wallace DM, Bryan RT, Dunn JA, Begum G, Bathers S. Delay and survival in bladder cancer. BJU Int 2002;89:868-78.
- Gore JL, Lai J, Setodji CM, Litwin MS, Saigal CS. Urologic Diseases in America Project . Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a surveillance, epidemiology, and end results-Medicare analysis. Cancer 2009;115:988-96.
- Lee CT, Madii R, Daignault S, Dunn RL, Zhang Y, Montie JE, et al. Cystectomy delay more than 3 months from initial bladder cancer diagnosis results in decreased disease specific and overall survival. J Urol 2006;175:1262-7; discussion 1267.
- Beer E. Landmark article May 28, 1910: removal of neoplasms of the urinary bladder By Edwin Beer. JAMA 1983;250:1324-5.
- Kulkarni GS, Hermanns T, Wei Y, Bhindi B, Satkunasivam R, Athanasopoulos P, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol 2017;35:2299-305.
- Cai T, Tiscione D, Verze P, Pomara G, Racioppi M, Nesi G, et al. Concordance and clinical significance of uncommon variants of bladder urothelial carcinoma in transurethral resection and radical cystectomy specimens. Urology 2014;84:1141-6.
- Lonati C, Baumeister P, Ornaghi PI, Di Trapani E, De Cobelli O, Rink M, et al. EAU-YAU Urothelial Cancer Working Party . Accuracy of transurethral resection of the bladder in detecting variant histology of bladder cancer compared with radical cystectomy. Eur Urol Focus 2022;8:457-64.
- Catto JWF, Gordon K, Collinson M, Poad H, Twiddy M, Johnson M, et al. BRAVO Study Group . Radical cystectomy against intravesical BCG for high-risk high-grade nonmuscle invasive bladder cancer: results from the randomized controlled BRAVO-feasibility study. J Clin Oncol 2021;39:202-14.
- Brausi M, Collette L, Kurth K, van der Meijden AP, Oosterlinck W, Witjes JA, et al. EORTC Genito-Urinary Tract Cancer Collaborative Group . Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol 2002;41:523-31.
- Schober JP, Plimack E, Geynisman DM, Zibelman M. The past, present, and future of pT0 in bladder cancer clinical trials. Curr Opin Urol 2022;32:495-9.
- Lindskrog SV, Prip F, Lamy P, Taber A, Groeneveld CS, Birkenkamp-Demtröder K, et al. An integrated multi-omics analysis identifies prognostic molecular subtypes of non-muscle-invasive bladder cancer. Nat Commun 2021;12.
- Awasthi S, Grass GD, Torres-Roca J, Johnstone PAS, Pow-Sang J, Dhillon J, et al. Genomic testing in localized prostate cancer can identify subsets of African-Americans with aggressive disease. J Natl Cancer Inst 2022;114:1656-64.
- Christensen E, Birkenkamp-Demtroder K, Sethi H, Shchegrova S, Salari R, Nordentoft I, et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J Clin Oncol 2019;37:1547-57.
- Vandekerkhove G, Lavoie JM, Annala M, Murtha AJ, Sundahl N, Walz S, et al. Plasma ctDNA is a tumor tissue surrogate and enables clinical-genomic stratification of metastatic bladder cancer. Nat Commun 2021;12.
- During VA, Sole GM, Jha AK, Anderson JA, Bryan RT. Prediction of histological stage based on cytoscopic appearances of newly diagnosed bladder tumours. Ann R Coll Surg Engl 2016;98:547-51.
- Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer – 2020 Update Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2021;79:243-62.
- Eldred-Evans D, Connor MJ, Bertoncelli Tanaka M, Bass E, Reddy D, Walters U, et al. The rapid assessment for prostate imaging and diagnosis (RAPID) prostate cancer diagnostic pathway. BJU Int 2023;131:461-70.
- Dyer T, Siemens DR, Nippak P, Meyer J, Booth CM. Histology at transurethral resection of bladder tumor and radical cystectomy for bladder cancer: insights from population-based data. Can Urol Assoc J 2021;15:138-40.
Appendix 1 Protocol amendments
| Amendment | Amendment date | Description | Documents approved |
|---|---|---|---|
| SA1 | 19 February 2019 | Clarification of different methods of referral and communication between hospitals and patients, sources of referral and order of consent/initial investigation process | Protocol, V3.0, 19 February 2019 |
| SA2 | 17 September 2019 | Addition of qualitative substudy details. | Protocol, V4.0, 17 September 2019 |
List of abbreviations
- AE
- adverse event
- BAUS
- British Association of Urological Surgeons
- BC
- bladder cancer
- BCG
- Bacillus Calmette–Guerin
- CIS
- carcinoma in situ
- CTE
- Commissioning through Evaluation
- CONSORT
- Consolidated Standards of Reporting Trials
- cp
- cell pellet
- CRCTU
- Cancer Research Clinical Trials Unit
- CTE
- Commissioning through Evaluation
- DMC
- Data Monitoring Committee
- DTT
- decision to treat
- EAU
- European Association of Urology
- eGFR
- estimated glomerular filtration rate
- FBC
- full blood count
- GCP
- good clinical practice
- HBRC
- Human Biomaterials Resource Centre
- HTA
- Health Technology Assessment
- ISRCTN
- International Standard Randomised Controlled Trial Number
- LFT
- liver function test
- MDT
- multidisciplinary team
- MIBC
- muscle-invasive bladder cancer
- mpMRI
- multiparametric magnetic resonance imaging
- MRI
- magnetic resonance imaging
- NIHR
- National Institute for Health and Care Research
- NMIBC
- non-muscle-invasive bladder cancer
- REC
- Research Ethics Committee
- SOC
- standard of care
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TTCT
- time to correct treatment
- TTDT
- time to definitive treatment
- TURBT
- transurethral resection of bladder tumour
- UCC
- urothelial cell carcinoma
- VI-RADS
- Vesical Imaging-Reporting and Data System
- WHO
- World Health Organization
