Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR131021. The contractual start date was in January 2021. The draft report began editorial review in April 2022 and was accepted for publication in February 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Davis et al. This work was produced by Davis et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Davis et al.
Chapter 1 Introduction
The clinical need and current uncertainties
Venous thromboembolism (VTE) remains the leading cause of direct maternal death in the UK, with the most recent Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK (MBRRACE-UK) report highlighting its importance. 1 While uncommon, VTE can occur at a rate of 1–2 per 1000 deliveries and can develop at any time during pregnancy and the puerperium (up to 6 weeks after delivery). 2–4 Deep-vein thrombosis (DVT) has an incidence of 1.1 per 1000 pregnancies, whereas pulmonary embolism (PE) has an incidence of 0.3% per 1000 pregnancies. 5 The maternal mortality rate for thrombosis and thromboembolism is 1 per 100,000 maternities. 1 Thromboprophylaxis with low-molecular-weight heparin (LMWH) is known to reduce VTE risk in medical and surgical patients, but it is also associated with an increased risk of bleeding in these groups. 6 In the UK, the Royal College of Obstetricians and Gynaecologists (RCOG) Guideline recommends LMWH for prophylaxis to prevent VTE in women at higher risk, assessed using a variety or risk factors, during pregnancy and the puerperium. 7 However, the evidence about the benefits and potential harms of offering LMWH to prevent VTE in women who are pregnant or in the puerperium is very uncertain due to a lack of high-quality trials of sufficient size. 8 This evidence gap has resulted in inconsistent recommendations for prophylaxis across international guidelines, with many recommendations based on observational research or findings extrapolated from other populations. 9
Risk assessment models (RAMs) have been developed to help stratify the risk of VTE during pregnancy and the early postnatal period. These models use clinical information from the patient’s history and patient characteristics [such as parity and body mass index (BMI)] to identify those with an increased risk of developing VTE who are most likely to benefit from pharmacological thromboprophylaxis. The use of appropriate RAMs to select high-risk patients for prophylaxis is clearly important as the balance of risks and harms varies according to whether the woman is at high or low risk of a VTE. 10 In addition, guidelines used in different countries, using different RAMs, have been shown to result in significantly different numbers of patients being eligible for LMWH,11,12 which will result in significantly different costs for preventing VTE.
The current National Institute for Health and Care Excellence (NICE) Guideline on the prevention of VTE in hospitalised women who are pregnant or who are in the puerperium recommends that clinicians use a RAM published by a national UK body, professional network or peer-reviewed journal. 6 The NICE Guideline states that the most commonly used RAM is the RCOG guideline. In Wales, the All-Wales maternity risk assessment tool has also been used as an alternative to the RCOG guideline. 6,7,13
A cross-sectional survey to estimate the impact of implementing the 2009 RCOG recommendations for thromboprophylaxis found that 41% of postnatal women and 7% of antenatal women would have qualified for thromboprophylaxis. 14 A more recent estimate, obtained by applying the 2015 RCOG guidance retrospectively to a large, longitudinal primary care database, suggests that 35% of postpartum women (without prior VTE) would have qualified for at least 10 days of postpartum thromboprophylaxis. 11 A retrospective analysis comparing the All-Wales maternity risk assessment to the RCOG guidelines suggests that there may be scope for reducing the numbers receiving thromboprophylaxis without increasing preventable VTE events, although the authors recommend that a prospective study should be conducted. 13 Various other international guidelines on preventing pregnancy-associated VTE have been shown to result in differing proportions of women being offered postpartum prophylaxis ranging from 7% to 37%. 15 We do not currently know whether using an alternative RAM with a higher or lower threshold for offering prophylaxis than RCOG would offer greater benefits on balance when taking into account risks, benefits and costs.
Chapter 2 Rationale and objectives
Rationale
Decision-analytic modelling is particularly useful in this situation, as it allows us to explore the optimal cut-off for thromboprophylaxis intervention in terms of the balance of risks, benefits and costs. For example, a higher threshold for providing thromboprophylaxis may result in more pregnancy-associated VTE, with an associated increase in long-term morbidity and mortality, but this must be balanced against the benefits of exposing fewer women to the risk of major bleeding during thromboprophylaxis which can itself have significant ongoing morbidity. In addition, fewer women receiving thromboprophylaxis will result in lower thromboprophylaxis costs and lower costs for managing thromboprophylaxis related major bleeding. These may somewhat offset the additional costs of short- and long-term VTE management from any increase in pregnancy-related VTE. Decision-analytic modelling could therefore be used to assess whether the current approach to thromboprophylaxis based on the RCOG guidelines is effective and cost-effective compared to the use of alternative RAMs, all of which will have a different balance of benefits, harms and costs. This assessment is dependent on data assessing the performance of the various RAMs which can be identified, and the quality assessed using systematic review methods.
Expected value of perfect information (EVPI) analysis is a form of decision analysis that provides a framework for synthesising the best available evidence at the current time to assess not only the optimal strategy given the current evidence, but also the areas of uncertainty where further research would be worthwhile. 16 Expected value of sample information (EVSI) analysis allows researchers to determine the value of conducting different research studies in the future, by simulating the potential outcomes of those studies. 17 It this context, decision-analytic modelling can be used to determine which factors contribute the most to uncertainty regarding the optimal prophylaxis strategy in women at risk of VTE during pregnancy or the puerperium and what future research would be most worthwhile.
The balance of risk, benefits and costs of alternative VTE prophylaxis strategies will be dependent on the effectiveness of prophylaxis in this population, among other factors. A 2021 Cochrane systematic review concluded that, ‘further high-quality very large-scale randomised trials are needed to determine effects of currently used treatments in women with different VTE risk factors’. 8 However, several pilot studies have been unable to recruit sufficient high-risk patients to such a trial. 18,19 This highlights the need for researchers planning future studies to ensure that they are both feasible to conduct and acceptable to patients, the public and clinicians. This can be achieved by engaging with patients and clinicians through qualitative research to ask whether future research studies assessed as being worthwhile through decision-analytic modelling would actually be acceptable and feasible in practice.
Objectives
Our aim was to determine whether further primary research would be worthwhile to inform NHS practice on the use of RAMs for the prediction of VTE and appropriate provision of thromboprophylaxis for women in pregnancy and in the puerperium. Our specific objectives were as follows:
-
to estimate the expected costs, health benefits [quality-adjusted life-years (QALYs)] and incremental net monetary benefit (INMB) for providing thromboprophylaxis using current and alternative RAMs and to quantify the uncertainty around those estimates, given current evidence
-
to determine which factors are the most important drivers of uncertainty when trying to determine the optimal RAM and thromboprophylaxis treatment strategy in this population
-
to identify one or more potential future studies to gather additional evidence that would reduce the current decision uncertainty, while being acceptable to patients and clinicians
-
to evaluate the value of the potential future research studies in terms of the net health benefits to patients and the cost of the research.
Objectives 1 and 2 are addressed by the cost-effectiveness and EVPI analysis (see Chapter 4), which is informed by the systematic review of RAMs (see Chapter 3). Objective 3 is informed by the findings of the EVPI analysis (see Chapter 4) and further addressed by the qualitative research (see Chapter 5). Objective 4 is addressed by the EVSI analysis (see Chapter 6).
Chapter 3 Systematic review of risk assessment models
A systematic review of the literature was undertaken to determine the comparative accuracy of individual RAMs that identify pregnant and postpartum women at increased risk of developing VTE who could be selected for thromboprophylaxis.
This review was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement20 and was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42020221094). The systematic review of RAMs was conducted in accordance with the review protocol registered with PROSPERO and the methods outlined in the project protocol (version 1.0) which can be accessed on https://fundingawards.nihr.ac.uk/award/NIHR131021 (accessed February 2023).
Methods
Eligibility criteria
All studies evaluating the accuracy (e.g. sensitivity, specificity, c-statistic) of a multivariable RAM (or scoring system) for predicting the risk of developing VTE were eligible for inclusion. We primarily sought and selected studies that included validation of the model in a group of patients that were not involved in the development of the prediction model. Although the included studies could have reported derivation of the model (for internal validation), we only used the external validation data to estimate accuracy, where appropriate. The study population of interest in our review consisted of pregnant and postpartum (within 6 weeks post delivery) women who are at increased risk of developing a VTE and receiving care in hospital, community and primary care settings. Studies that focused on non-pregnant women were excluded as these patient groups have VTE risk profiles that differ markedly from the obstetric population.
Data sources and searches
Potentially relevant studies were identified by searching the following electronic databases and research registers:
-
Ovid MEDLINE(R) Epub Ahead of Print, In-Process and Other Non-Indexed Citations, Ovid MEDLINE(R) Daily, MEDLINE and Versions(R) (OvidSP) 1946 to February 2021
-
EMBASE (OvidSP) 1974 to February 2021
-
Cochrane Database of Systematic Reviews (www.cochranelibrary.com/) Inception to February 2021
-
Cochrane Central Register of Controlled Trials (www.cochranelibrary.com/) Inception to February 2021
-
ClinicalTrials.gov (US National Institutes of Health) 2000 to February 2021
-
International Clinical Trials Registry Platform (World Health Organisation) 1990 to February 2021.
The search strategy used free-text and thesaurus terms and combined synonyms relating to the condition (e.g. VTE in pregnant and postpartum women) with risk prediction modelling terms. 21 No language or date restrictions were used. Searches were supplemented by hand-searching the reference lists of all relevant studies (including existing systematic reviews); forward citation searching of included studies (using the Web of Science Citation Index Expanded and Conference Proceedings Citation Index – Science) to identify articles that cite the relevant articles; contacting key experts in the field; and undertaking targeted searches of the World Wide Web using the Google search engine. Further details on the search strategy are provided (see Appendix 1).
Study selection process
The inclusion of potentially relevant articles was undertaken using a two-step process. First, all titles were examined for inclusion by one reviewer (GR) and any citations that clearly did not meet the inclusion criteria (e.g. non-human, unrelated to VTE in pregnancy and the puerperium) were excluded (for quality assurance a random subset of 20% was checked by a second reviewer). All abstracts and full-text articles were then examined independently by two reviewers (GR and AP). Any disagreements in the selection process were resolved through discussion or, if necessary, arbitration by a third reviewer (JD) or the wider group (BH, CNP, SG) and included by consensus.
Data abstraction and quality-assessment strategy
For eligible studies, data relating to study design, methodological quality and outcomes were extracted by one reviewer (GR) into a standardised data extraction form and independently checked for accuracy by a second reviewer (AP). Any discrepancies were resolved through discussion, or if this was unsuccessful, a third reviewer’s opinion was sought (JD). Where multiple publications of the same study were identified, data were extracted and reported as a single study.
The methodological quality of each included study was assessed using Prediction model Risk Of Bias ASsessment Tool (PROBAST). 22,23 This instrument includes four key domains: participants (e.g. study design and patient selection), predictors (e.g. differences in definition and measurement of the predictors), outcome (e.g. differences related to the definition and outcome assessment) and statistical analysis (e.g. sample size, choice of analysis method and handling of missing data). Each domain is assessed in terms of risk of bias and the concern regarding applicability to the review (first three domains only). To guide the overall domain-level judgement about whether a study is at high, low or an unclear (in the event of insufficient data in the publication to answer the corresponding question) risk of bias, subdomains within each domain include several signalling questions to help judge bias and applicability concerns. An overall risk of bias for each individual study was defined as low risk when all domains were judged as low and high risk of bias when one or more domains were considered as high. Studies were assigned an unclear risk of bias if one or more domains were unclear, and all other domains were low.
The methodological quality of each included study was independently evaluated by two reviewers (GR and AP). Any discrepancies were resolved through discussion or, if necessary, with involvement of a third reviewer (JD). Blinding of the quality assessor to author, institution or journal was not considered necessary.
Data synthesis and analysis
We were unable to perform meta-analysis due to significant levels of heterogeneity between studies (study design, participants, inclusion criteria) and variable reporting of items. As a result, a pre-specified narrative synthesis approach24,25 was undertaken, with data being summarised in tables with accompanying narrative summaries that included a description of the included variables, statistical methods and performance measures [e.g. sensitivity, specificity and c-statistic (a value between 0.7 and 0.8 and > 0.8 indicated good and excellent discrimination, respectively; and values < 0.7 were considered weak)],26 where applicable. All analyses were conducted using Microsoft Excel 365 (Microsoft Corporation, Redmond, WA, USA).
Patient and public involvement
Patients and the public were not involved in the design or conduct of this systematic review.
Results
Quantity and quality of research available
The literature searches identified 2268 citations. Of these, 16 studies11,13,27–40 investigating 19 unique externally validated RAMs met the inclusion criteria. Only one of these studies11 presented data on model development and external validation [this study used UK Clinical Practice Research Data (CPRD) linked to Hospital Episodes Statistics (HES) to develop a risk prediction model and externally validated it using Swedish medical birth registry data]. The remaining studies focused on external validation with no description of the initial derivation methodology. 13,27–40 Due to the lack of model derivation studies with external validation, we also identified and included one internal validation study for completeness (i.e. prediction model development without external validation). 41 This study used a bootstrap validation approach to capture optimism in model performance42,43 when applied to similar future patients. Most of the full-text articles (n = 97) were excluded primarily based on not using a RAM for predicting the risk of developing VTE during pregnancy or the puerperium, having no useable or relevant outcome data or an inappropriate study design (e.g. reviews, commentaries or study protocols). A full list of excluded studies with reasons for exclusion is provided on https://fundingawards.nihr.ac.uk/award/NIHR131021 (accessed February 2023). Figure 1 summarises the study identification process.
Description of included studies (design and patient characteristics)
The design and participant characteristics of the 17 included studies that provided data on the comparative accuracy of RAMs for predicting the risk of developing VTE in women during pregnancy and the puerperium periods are summarised in Table 1. All studies were published between 2000 and 2020 and were undertaken in North America (n = 4),28,39–41 Southeast Asia (n = 1),37 Europe (n = 10),13,27,29–34,36,38 South America (n = 1)35 and one study was multicountry. 11 Sample sizes ranged from 5235 to 662,38711 patients in 14 observational cohort studies [6 prospective29,31,32,35,37,38 (all single-centre) and 8 retrospective11,13,28,30,33,34,39,41 (2 of which were multicentre) in design]. Sample sizes in 2 single-centre case–control studies36,40 ranged from 7640 to 242136 patients and 1 study used a non-randomised multicentre study design. 27 The mean age ranged from 27.841 to 34 years29,33 (not reported in 7 studies). 13,28,31,36,38–40
| Author, year | Country | Design | Single/multicentre | Sample size | Population | Period | Mean age (years) | VTE prophylaxis (%) | RAMs evaluated | Target condition, definition (risk period) | Incidence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antepartum and postpartum following vaginal and caesarean delivery | |||||||||||
| Bauersachs et al., 200727 | Germany | P, NRS | Multi | 810 | Women at increased risk of VTE (due to thromboembolic status and prior VTE) | March 1999–December 2002 | 30.8 | 100 | EThIG | Antepartum and postpartum VTE, symptomatic (NR) | 0.62% (antepartum: 0.25%; postpartum: 0.37%) |
| Chauleur et al., 200831 | France | P, CS | Single | 2685 | All women who delivered | July 2002–June 2003 | NR (median, 29) |
NR | STRATHEGE | Antepartum and postpartum VTE (NR) | 0.34% (antepartum: 0.19%; postpartum: 0.15%) |
| Dargaud et al., 201732 | France | P, CS | Single | 445 | Women at increased risk of VTE (due to thromboembolic status and prior VTE) | January 2005–January 2015 | 33 | 100 | Lyon | Antepartum and postpartum VTE, not defined (pregnancy and 3 months postpartum) | 1.35% |
| Dargaud et al., 200533 | France | R, CS | Single | 116 | Women at increased risk of VTE (due to thromboembolic status and prior VTE) | 2001–3 | 34 | 53 | Lyon | Antepartum and postpartum VTE, not defined (NR) | 0.86% (antepartum only) |
| Hase et al., 201835 | Brazil | P, CS | Single | 52 | Hospitalised pregnant women with cancer | 1 December 2014–31 July 2016 | 31 | 57.7 | RCOG (modified) | Antepartum and postpartum VTE, not defined (pregnancy and 3 months postpartum) | Unable to estimate – no VTE |
| Shacaluga et al., 201913 (correspondence) | Wales | R, CS | Single | 42,000 | All managed pregnancies | 2009–15 | NR | NR | All Wales RCOG |
Antepartum and postpartum VTE, not defined, (NR) | 0.08% (ante partum: 0.04%; postpartum: 0.04%) |
| Testa et al., 201538 | Italy | P, CS | Single | 1719 | All pregnant women enrolled in Pregnancy Healthcare Program | January 2008–December 2010 | NR (median 33) | 4.6 | Novel (Testa) | Antepartum and postpartum VTE (NR) | Unable to estimate – no VTE |
| Weiss et al., 200040 | USA | CC | Single | 19 cases: 57 controla | Women with (confirmed cases) and without (unmatched control) VTE | 1987–98 | NR | NR | Novel (Weiss) | Antepartum and postpartum VTE, not defined (pregnancy and 6 weeks postpartum) | - |
| Postpartum only following vaginal and caesarean delivery | |||||||||||
| Chau et al., 201930 | France | R, CS | Single | 1069 (time period 2012: 557; 2015: 512) |
All women who delivered | February–April 2012 and February–April 2015 | 2012: 29 2015: 29 |
NR | Novel (Chau) | Postpartum VTE, not defined (8 weeks) | 2012: 0.18% 2015: 0.20% |
| Ellis-Kahana et al., 202041,b | USA | R, CS | Multi | 83,500 | All obese women (BMI > 30 kg/m2) who delivered | 2002–8 | 27.8 | NR | Novel (Ellis-Kahana) | Postpartum VTE (NR) | 0.13% |
| Gassmann et al., 202034 | Switzerland | R, CSc | Single | 344 | All women who delivered | 1–31 January 2019 | 32.2 | 24 | RCOG ACOG ACCP ASH |
Postpartum VTE, not defined (3 months) | Unable to estimate – no VTE |
| Lindqvist et al., 200836 | Sweden | CC | Single | 37 cases: 2384 control | All women with (confirmed cases) and without (unselected population-based control) VTE | 1990–2005 | NR | NR | SFOG (Swedish guidelines) | Postpartum VTE (NR) | – |
| Sultan et al., 201611 | England (derivation)d and, Sweden (validation) | R, CS | Multi | 662,387 (validation cohort)d | All women (with no history of VTE) who delivered | 1 July 2005–31 December 2011 | 30.32 | 3 | Novel (Sultan) RCOGd SFOG (Swedish Guidelines) |
Postpartum VTE (6 weeks) |
0.08% (validation cohort) |
| Tran et al., 201939 | USA | R, CS | Single | 6094 | All women who delivered after 14 weeks | 01 January 2015–31 December 2016 | NR | NR | RCOG Padua Caprini |
Postpartum VTE (6 months) | 0.05% |
| Postpartum following caesarean delivery | |||||||||||
| Binstock and Larkin, 2019 (abstract)28 | USA | R, CS | Single | 2875 | Postpartum women following caesarean section | 2011 | NR | NR | Novel (Binstock) RCOG |
Postpartum VTE, not defined (NR) | 0.38% |
| Cavazza et al., 201229 | Italy | P, CS | Single | 501 | Postpartum women following caesarean section | 2007–9 | 34 | 53.5 | Novel (Cavazza) | Postpartum VTE, symptomatic, not defined (90 days) | 0.20% |
| Lok et al., 201937 | Hong Kong | P, CS | Single | 859 | Postpartum women following caesarean section | May 2017–April 2018 | 32.9 | 3.3 | Novel (Lok) R COG ACOG |
Postpartum VTE, symptomatic, not defined (NR) | Unable to estimate – no VTE |
The majority of studies were conducted across antenatal and postnatal periods,13,27,31–33,35,38,40 or postpartum period only11,28–30,34,36,37,39,41 and generally included women at increased risk of VTE. 27–29,32,33,35–37,40,41 One study excluded women with a history of VTE11 and six studies13,30,31,34,38,39 included all pregnant women who delivered. Thromboprophylaxis was employed in about half (n = 9)11,27,29,32–35,37,38 of the studies, with the proportion receiving thromboprophylaxis ranging from 3%11 to 100%. 27,32 The remaining studies did not report data on thromboprophylaxis use.
Only a few studies27,31,36,38 defined the VTE end point (DVT and or PE) as being confirmed by objective testing. Of the remainder, 3 studies11,39,41 had no objective confirmation of VTE and 10 studies13,28–30,32–35,37,40 did not report the methods for diagnosis confirmation. Although nine studies13,27,28,31,33,36–38,41 did not report the VTE risk period, the majority of the remaining studies utilised the RAMs to predict the occurrence of VTE up to 3 months after delivery. 29,32,34,35 Despite differences in study design, study participants, definitions, different criteria for the use of thromboprophylaxis and differences between doses of LMWH, the reported overall incidence of VTE in pregnancy and the puerperium was < 1.3%.
The studies included in this review evaluated 19 externally validated RAMs11,13,27–40 and one internally validated risk model. 41 While most RAMs focused solely on the estimate of thromboembolic risk, RAMs varied in design, structure, threshold, dosage and duration for pharmacological prophylaxis. In addition, the individual predictors and their weighting varied markedly between RAMs. The most commonly used tools were the RCOG guidelines (six studies),11,13,28,34,37,39 American College of Obstetricians and Gynaecologists (ACOG) guidelines (two studies),34,37 Swedish Society of Obstetrics and Gynecology (SFOG) guidelines (two studies)11,36 and the Lyon score (two studies). 32,33 A simplified summary of their associated characteristics and composite clinical variables are provided (see Appendix 2).
Risk of bias and applicability assessments of included studies
The overall methodological quality of the 17 included studies is summarised in Table 2 and Figure 2. The methodological quality of the included studies was variable, with most studies having high or unclear risk of bias in at least one item of the PROBAST tool. The main risk of bias limitations was related to patient selection factors (arising from retrospective data collection,13,28,30,33,34,36,39–41 unclear exclusions/incomplete patient enrolment13,28,30,31,35–38,40,41 or unclear criteria for patients receiving VTE prophylaxis);11,27,34 predictor and outcome bias (due to a general lack of details on the definition13,28–30,32–35,37,40 and methods of outcome determination13,28,30,32–35,37,39–41 and whether all predictors were available at the models intended time of use13,27,28,33,35,36,38–41 or influenced by the outcome measurement)11,13,27–32,34–41 and analysis factors (low event rates,11,13,27–35,37–39,41 unclear handling of missing data13,27–33,35–41 and failure in reporting relevant performance measures such as calibration and discrimination). 13,27–40
| Author, year | Risk of bias | Concern regarding applicability | Overall | Overall | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1. Participant selection | 2. Predictors | 3. Outcome | 4. Analysis | 1. Participant selection | 2. Predictors | 3. Outcomes | Risk of bias | Applicability | |
| Bauersachs et al., 200727 | Unclear | Unclear | Low | High | Unclear | Unclear | Low | High | Unclear |
| Chauleur et al., 200831 | Unclear | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Dargaud et al., 201732 | Unclear | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Dargaud et al., 200533 | High | Unclear | Unclear | High | Unclear | Low | Unclear | High | Unclear |
| Hase et al., 201835 | Unclear | Unclear | Unclear | High | High | Unclear | Unclear | High | High |
| Shacaluga et al., 201913 | High | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Testa et al., 201538 | Unclear | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Weiss and Bernstein, 200040 | High | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Chau et al., 201930 | Unclear | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Ellis-Kahana et al., 202041 | High | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Gassmann et al., 202034 | Unclear | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Lindqvist et al., 200836 | High | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Sultan et al., 201611 | High | Unclear | Low | Low | Low | Unclear | Low | High | Unclear |
| Tran et al., 201939 | High | Unclear | Unclear | High | Unclear | Unclear | Unclear | High | Unclear |
| Binstock and Larkin, 201928 | Unclear | Unclear | Unclear | High | High | Unclear | Unclear | High | High |
| Cavazza et al., 201229 | High | Unclear | Unclear | High | High | Low | Unclear | High | High |
| Lok et al., 201937 | Unclear | Unclear | High | High | High | Low | Unclear | High | High |
FIGURE 2.
PROBAST assessment summary graph – review authors’ judgements.
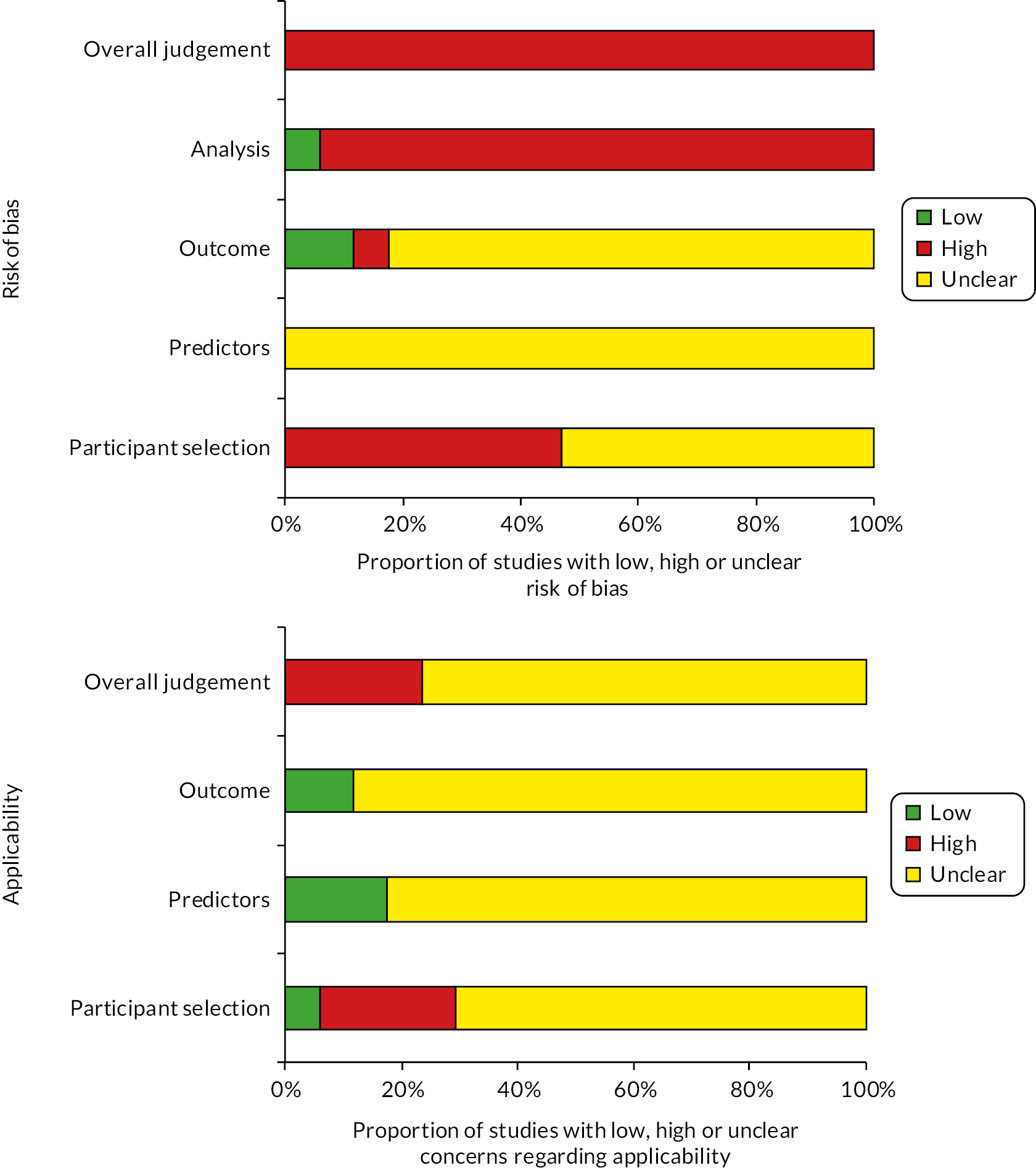
Assessment of applicability to the review question led to the majority of studies being classed either as unclear (n = 13)11,13,27,30–34,36,38–41 or high (n = 4)28,29,35,37 risk of inapplicability. These assessments were generally related to patient selection (highly selected study populations, for example, selected women at increased risk of VTE, caesarean delivery only, single disease pathologies, single-site settings), predictors (inconsistency in definition, assessment or timing of predictors) and outcome determination.
Quantitative data synthesis (summary of results)
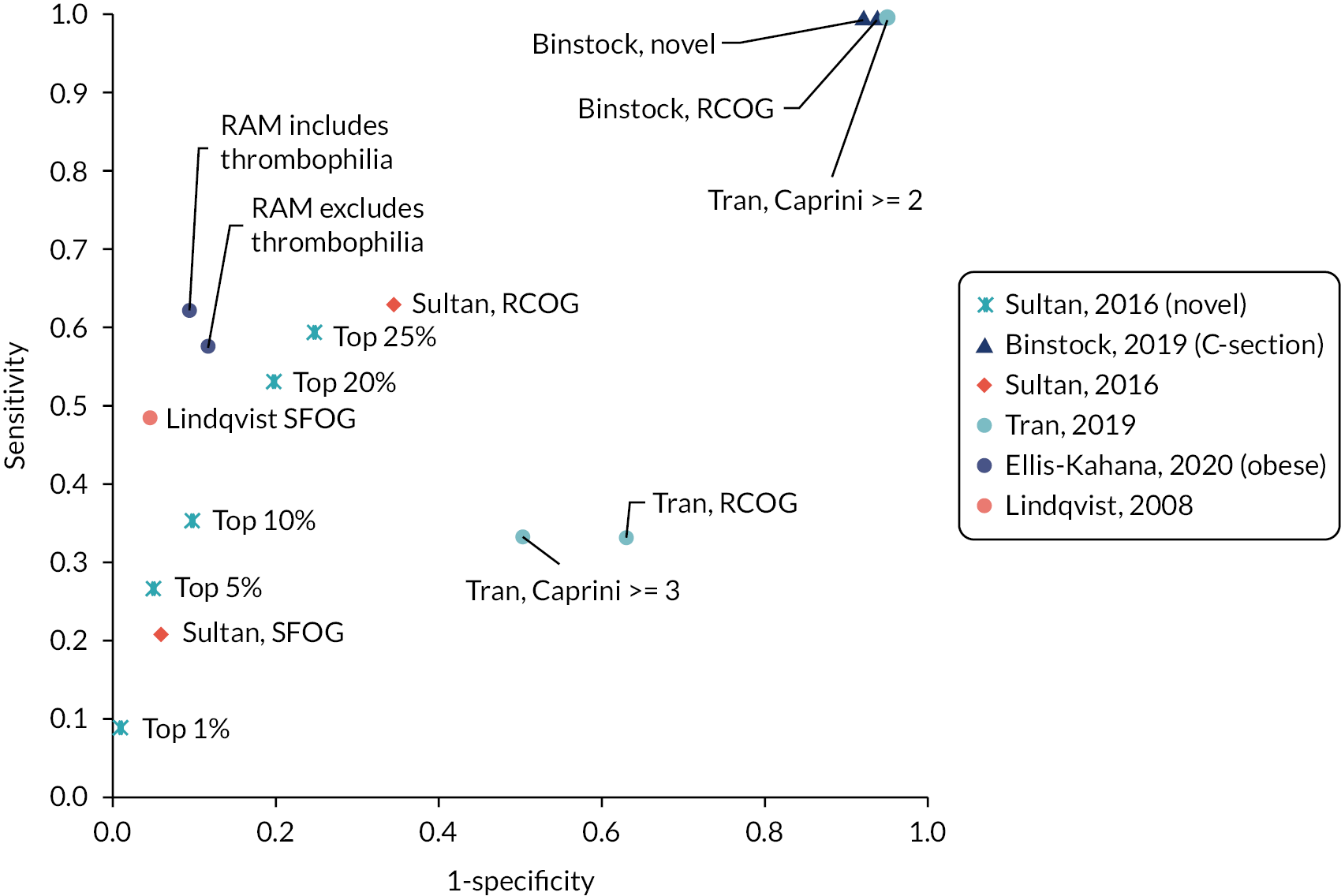
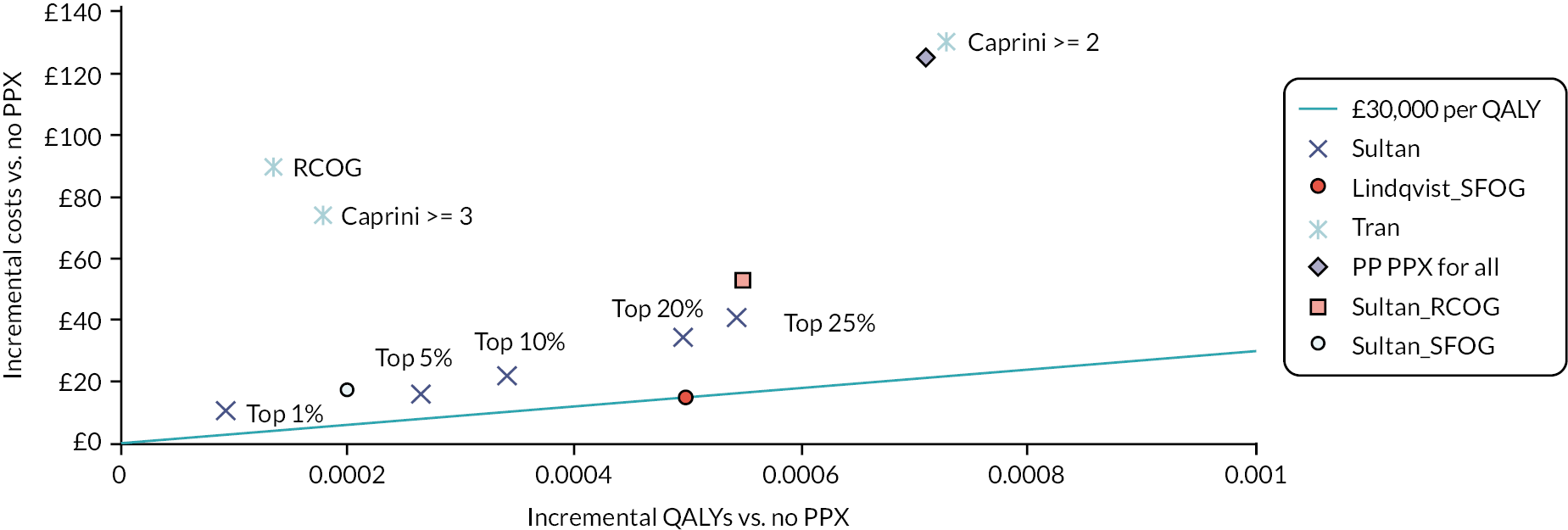
A summary of the sensitivity and specificity of RAMs that were applied to antepartum women to predict antepartum or postpartum VTE or applied postpartum (PP) to predict postpartum VTE, respectively, is presented in Tables 3 and 4, with the results grouped by RAM. However, any meaningful comparisons between these alone is difficult, without considering the models’ corresponding discrimination and calibration metrics, which were not universally reported. Only one external validation study considered model discrimination and calibration. In this study by Sultan et al.,11 their recalibrated novel risk prediction model (also known as the Maternity Clot Risk) provided good discrimination and was able to discriminate postpartum women with and without VTE in the external Swedish cohort with a c-statistic of 0.73 [95% confidence interval (CI) 0.71 to 0.75], and calibration, of observed and predicted VTE risk, close to ideal [calibration slope of 1.11 (95% CI 1.01 to 1.20)]. In the remaining studies, interpretation was further limited by marked heterogeneity, which was exacerbated when different thresholds were reported by different studies evaluating the same model. In general, model accuracy was generally poor, with high sensitivity usually reflecting a threshold effect, as indicated by corresponding low specificity values (and vice versa).
| RAMs | Threshold or cut-off | End point | Data source | Performance measures | |||||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | ||||
| Predicting either antepartum or postpartum VTE | |||||||||
| All Wales (1 study) | NR | VTE | Shacaluga et al., 201913 | 25 | NR | 9 | NR | 0.74 (0.57 to 0.85) | NR |
| EThIG (1 study) | High/very high risk | VTE | Bauersachs et al., 200727 | 5 | 580 | 0 | 225 | 1.00 (0.57 to 1) | 0.28 (0.25 to 0.31) |
| Lyon (2 studies) | Risk score ≥ 3 | VTE | Dargaud et al., 201732 | 5 | 282 | 1 | 157 | 0.83 (0.44 to 0.97) | 0.36 (0.31 to 0.4) |
| Lyon | Risk score ≥ 3 | VTE | Dargaud et al., 200533 | 1 | 56 | 0 | 59 | 1.00 (0.21 to 1) | 0.51 (0.42 to 0.6) |
| RCOG (modified) (1 study) | Risk score ≥ 3 | VTE | Hase et al., 201835 | 0 | 34 | 0 | 18 | Unable to estimate – no VTE | 0.35 (0.23 to 0.48) |
| STRATHEGE (1 study) | Risk score ≥ 3 | VTE | Chauleur et al., 200831 | 0 | 54 | 9 | 2622 | 0.00 (0 to 0.3) | 0.98 (0.97 to 0.99) |
| Testa 2015 (1 study) | Risk score ≥ 2.5 | VTE | Testa et al., 201538 | 0 | 85 | 0 | 1634 | Unable to estimate – no VTE | 0.95 (0.94 to 0.96) |
| Predicting antepartum VTE | |||||||||
| EThIG (1 study) | High/very high risk | VTE | Bauersachs et al., 200727 | 2 | 583 | 0 | 225 | 1.00 (0.34 to 1) | 0.28 (0.25 to 0.31) |
| Lyon (1 study) | Risk score ≥ 3 | VTE | Dargaud et al., 201732 | 1 | 286 | 1 | 157 | 0.50 (0.09 to 0.91) | 0.35 (0.31 to 0.4) |
| STRATHEGE (1 study) | Risk score ≥ 1 | VTE | Chauleur et al., 200831 | 0 | 54 | 4 | 2627 | 0.00 (0 to 0.49) | 0.98 (0.97 to 0.99) |
| Weiss 2000 (1 study) | Risk score ≥ 2 | VTE | Weiss et al., 200040 | 4 | 3 | 15 | 54 | 0.21 (0.09 to 0.43) | 0.95 (0.86 to 0.98) |
| Predicting postpartum VTE | |||||||||
| EThIG (1 study) | High/very high risk | VTE | Bauersachs et al., 200727 | 3 | 582 | 0 | 225 | 1.00 (0.44 to 1) | 0.28 (0.25 to 0.31) |
| Lyon (1 study) | Risk score ≥ 3 | VTE | Dargaud et al., 201732 | 4 | 283 | 0 | 158 | 1.00 (0.51 to 1) | 0.36 (0.31 to 0.4) |
| STRATHEGE (1 study) | Risk score ≥ 1 | VTE | Chauleur et al., 200831 | 0 | 54 | 5 | 2626 | 0.00 (0 to 0.43) | 0.98 (0.97 to 0.98) |
| RAMs | Threshold or cut-off | End point | Data source | Performance measures | |||||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) | ||||
| Predicting postpartum VTE following vaginal and caesarean delivery | |||||||||
| ACCP (1 study) | NR | VTE | Gassmann et al., 202034 | 0 | 34 | 0 | 310 | Unable to estimate – no VTE | 0.90 (0.86 to 0.93) |
| ACOG (1 study) | NR | VTE | Gassmann et al., 202034 | 0 | 30 | 0 | 314 | Unable to estimate – no VTE | 0.91 (0.88 to 0.94) |
| ASH (1 study) | NR | VTE | Gassmann et al., 202034 | 0 | 0 | 0 | 344 | Unable to estimate – no VTE | 1.00 (0.99 to 1) |
| Caprini (1 study) | Risk score ≥ 2 | VTE | Tran et al., 201939 | 3 | 5780 | 0 | 311 | 1.00 (0.44 to 1) | 0.05 (0.05 to 0.06) |
| Caprini | Risk score ≥ 3 | VTE | Tran et al., 201939 | 1 | 3066 | 2 | 3025 | 0.33 (0.06 to 0.79) | 0.50 (0.48 to 0.51) |
| Caprini | Risk score ≥ 4 | VTE | Tran et al., 201939 | 0 | 1257 | 3 | 4834 | 0.00 (0 to 0.56) | 0.79 (0.78 to 0.80) |
| Padua (1 study) | Risk score ≥ 4 | VTE | Tran et al., 201939 | 0 | 50 | 3 | 6041 | 0.00 (0 to 0.56) | 0.99 (0.99 to 0.99) |
| RCOG (3 studies) | NR | VTE | Gassmann et al., 202034 | 0 | 138 | 0 | 206 | Unable to estimate – no VTE | 0.60 (0.55 to 0.65) |
| RCOG | Risk score ≥ 2 | VTE | Tran et al., 201939 | 1 | 3837 | 2 | 2254 | 0.33 (0.06 to 0.79) | 0.37 (0.36 to 0.38) |
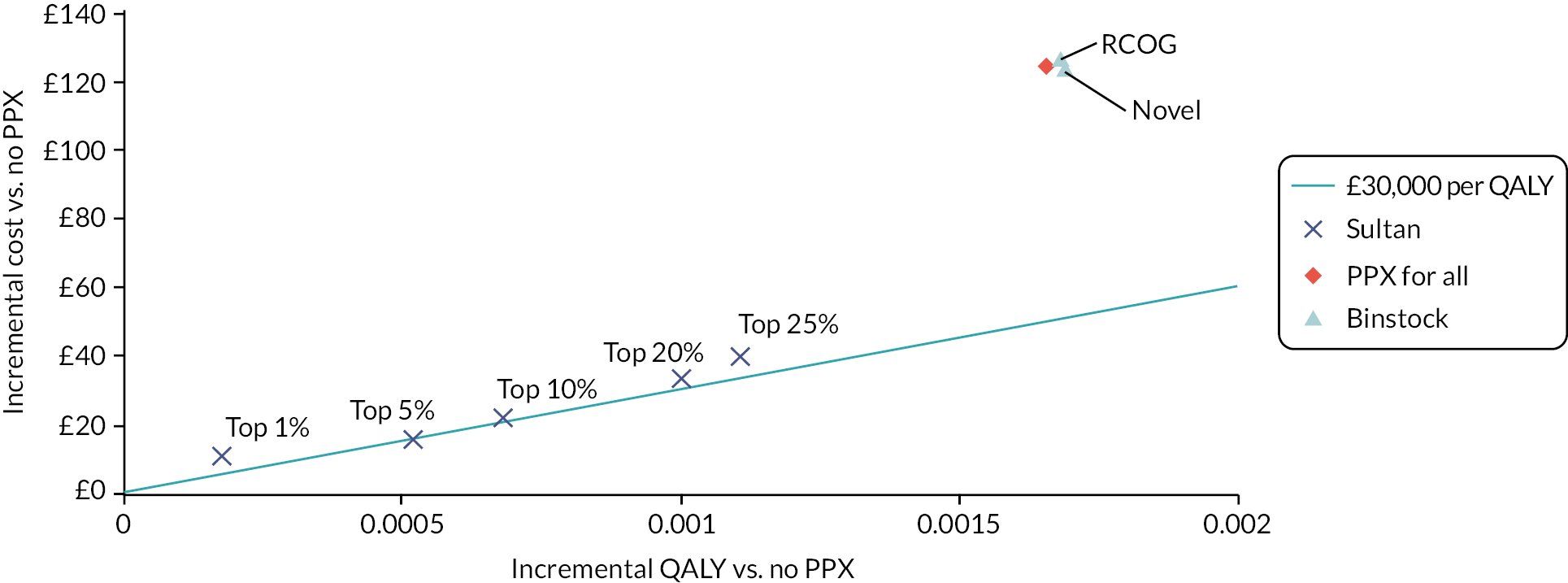
| RCOG | ≥ 2 low risk factors or 1 high risk factor | VTE | Sultan et al., 201611 | 197 | 149,205 | 115 | 283,836 | 0.63 (0.58 to 0.68) | 0.66 (0.65 to 0.66) |
| SFOG (2 studies) | Risk score ≥ 2 | VTE | Lindqvist et al., 200836 | 18 | 111 | 19 | 2273 | 0.49 (0.33 to 0.64) | 0.95 (0.94 to 0.96) |
| SFOG | ≥ 2 risk factors | VTE | Sultan et al., 201611 | 109 | 41,145 | 412 | 620,721 | 0.21 (0.18 to 0.25) | 0.94 (0.94 to 0.94) |
| Chau, 2019 (1 studya) | Risk score ≥ 3 (2012 data set) | VTE | Chau et al., 201930 | 0 | 101 | 1 | 456 | 0.00 (0 to 0.79) | 0.82 (0.78 to 0.85) |
| Chau, 2019 | Risk score ≥ 3 (2015 data set) | VTE | Chau et al., 201930 | 0 | 113 | 1 | 393 | 0.00 (0 to 0.79) | 0.78 (0.74 to 0.81) |
| Ellis-Kahana, 2020 (full model) (1 studyb) | Risk score > 3 (high risk) | VTE | Ellis-Kahana et al., 202041 | 68 | 7942 | 41 | 75,449 | 0.62 (0.53 to 0.71) | 0.90 (0.90 to 0.91) |
| Ellis-Kahana, 2020 (without antepartum thromboembolic disorder) | Risk score > 3 (high risk) | VTE | Ellis-Kahana et al., 202041 | 63 | 9926 | 46 | 73,465 | 0.58 (0.48 to 0.67) | 0.88 (0.88 to 0.88) |
| Sultan, 2016 (1 studyc) | ≥ 2 risk factors: top 35% (threshold: 7.2 per 10,000 deliveries) | VTE | Sultan et al., 201611 | 355 | 231,480 | 166 | 430,386 | 0.68 (0.64 to 0.72) | 0.65 (0.65 to 0.65) |
| Sultan, 2016 | ≥ 2 risk factors: top 25% (threshold: 8.7 per 10,000 deliveries) | VTE | Sultan et al., 201611 | 310 | 164,976 | 211 | 496,890 | 0.60 (0.55 to 0.64) | 0.75 (0.75 to 0.75) |
| Sultan, 2016 | ≥ 2 risk factors: top 20% (threshold: 9.8 per 10,000 deliveries) | VTE | Sultan et al., 201611 | 278 | 131,921 | 243 | 529,945 | 0.53 (0.49 to 0.58) | 0.80 (0.80 to 0.80) |
| Sultan, 2016 | ≥ 2 risk factors: top 10% (threshold: 14 per 10,000 deliveries) | VTE | Sultan et al., 201611 | 185 | 66,053 | 336 | 595,813 | 0.36 (0.32 to 0.40) | 0.90 (0.90 to 0.90) |
| Sultan, 2016 | ≥ 2 risk factors: top 6% (threshold: 18 per 10,000 deliveries) | VTE | Sultan et al., 201611 | 158 | 41,096 | 363 | 620,770 | 0.30 (0.27 to 0.34) | 0.94 (0.94 to 0.94) |
| Sultan, 2016 | ≥ 2 risk factors: top 5% (threshold: 19.7 per 10,000 deliveries) | VTE | Sultan et al., 201611 | 139 | 32,980 | 382 | 628,886 | 0.27 (0.23 to 0.31) | 0.95 (0.95 to 0.95) |
| Sultan, 2016 | ≥ 2 risk factors: top 1% (threshold: 41.2 per 10,000 deliveries) | VTE | Sultan et al., 201611 | 47 | 6576 | 474 | 655,290 | 0.09 (0.07 to 0.12) | 0.99 (0.99 to 0.99) |
| Predicting postpartum VTE following caesarean delivery only | |||||||||
| ACOG (1 study) | Risk score ≥ 3 | VTE | Lok et al., 201937 | 0 | 0 | 0 | 859 | Unable to estimate – no VTE | 1.00 (1 to 1) |
| RCOG (2 studies) | NR | VTE | Binstock and Larkin, 2019 (abstract)28 | 11 | 2692 | 0 | 172 | 1.00 (0.74 to 1) | 0.06 (0.05 to 0.07) |
| RCOG | Risk score ≥ 3 | VTE | Lok et al., 201937 | 0 | 649 | 0 | 210 | Unable to estimate – no VTE | 0.24 (0.22 to 0.27) |
| Binstock, 2019 (1 study) | NR | VTE | Binstock and Larkin, 2019 (abstract)28 | 11 | 2635 | 0 | 229 | 1.00 (0.74 to 1) | 0.08 (0.07 to 0.09) |
| Cavazza, 2012 (1 study) | Moderate/high/very high | VTE | Cavazza et al., 201229 | 0 | 268 | 1 | 232 | 0.00 (0 to 0.79) | 0.46 (0.42 to 0.51) |
| Lok, 2019 (1 study) | Risk score ≥ 3 | VTE | Lok et al., 201937 | 0 | 28 | 0 | 831 | Unable to estimate – no VTE | 0.97 (0.95 to 0.98) |
Summary of key findings
-
Several RAMs for VTE in pregnancy and the puerperium have been developed using a variety of methods and based on a variety of predictor variables.
-
This systematic review provides a comprehensive review of RAMs for predicting the risk of developing VTE in women who are pregnant or in the puerperium (within 6 weeks post delivery).
-
In general, external validation studies have poor designs and limited generalisability.
-
Available data suggest that external validation studies have weak designs and limited generalisability, and so estimates of prognostic accuracy are very uncertain.
Chapter 4 Decision-analytic modelling
Decision problem
Aim
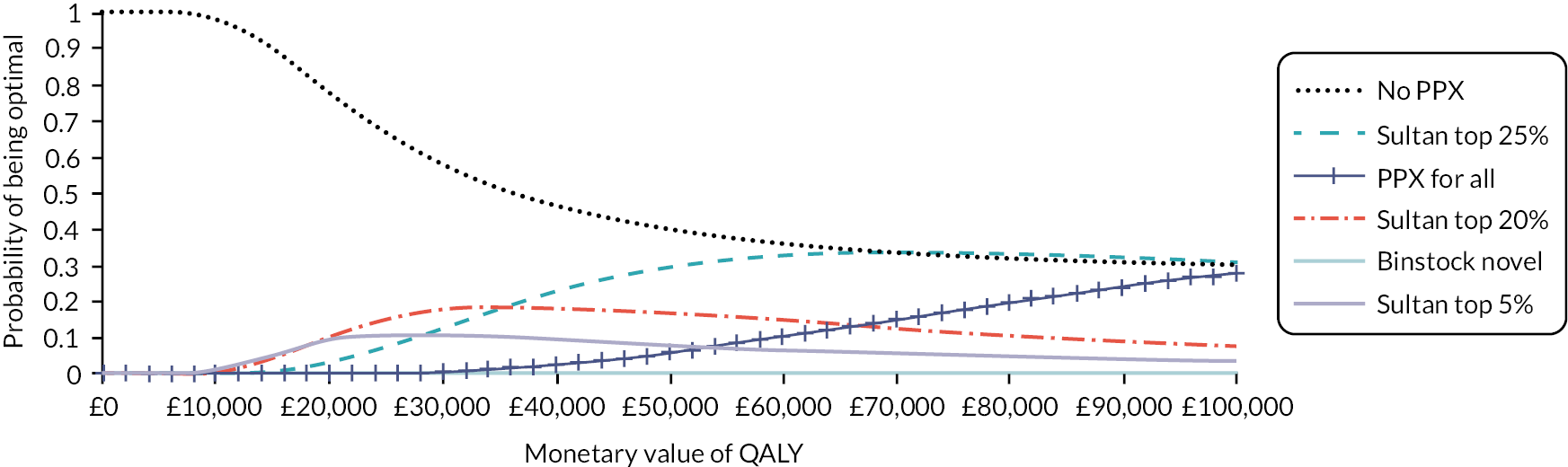
The cost-effectiveness analysis aims to estimate the expected costs, health benefits (QALYs) and INMB of providing thromboprophylaxis, to women who are pregnant or who are in the puerperium, using current and alternative risk stratification tools. The EVPI analysis aims to quantify the uncertainty around those estimates, given current evidence, and to determine which factors are the most important drivers of uncertainty when trying to determine the optimal risk-based thromboprophylaxis strategy in this population. The outcomes of the EVPI analysis are then used alongside the qualitative research (see Chapter 5) to identify potential future studies to gather additional evidence that would reduce the current decision uncertainty, while being feasible and acceptable to patients and clinicians. The EVSI aims to evaluate the value of the potential future research studies in terms of the net health benefits to patients and the cost of the research (see Chapter 6).
Population
The target population for the decision-analytic modelling is women who are pregnant or in the puerperium (within 6 weeks post delivery) receiving care in both hospital and primary care settings. The antenatal and postnatal populations are considered separately. In addition, the systematic review (see Chapter 3) identified RAMs that are specifically targeted at antenatal women at high risk of VTE due to either prior VTE and/or known thrombophilia, and RAMs that are specifically targeted at obese postpartum women and postpartum women following caesarean section. One RAM was identified for use in an unselected antepartum population, but the performance data for this RAM were poor. Therefore, the analysis in the unselected antepartum population was limited to exploratory analysis to determine the range of sensitivity and specificity values that would be required for a RAM in this population. As women who have a prior VTE or known thrombophilia are likely to have received antepartum risk assessment, the postpartum modelling excludes these groups. Therefore, the following subgroups are considered in the decision-analytic model:
-
antepartum women identified as being at high risk (prior VTE or known thrombophilia)
-
unselected postpartum women (excluding those with prior VTE or known thrombophilia)
-
postpartum women identified due to specific risk factors (caesarean section, obesity)
-
unselected antepartum women (exploratory analysis only).
Strategies for prophylaxis
Strategies for prophylaxis in women having antepartum risk assessment
The current NICE Guideline on the prevention of VTE in hospitalised women who are pregnant or who are in the puerperium recommends that clinicians use a tool published by a national UK body, professional network or peer-reviewed journal. 6 The NICE Guideline states that the most commonly used tool is the RCOG guideline;6,7 this is considered to represent current practice in the decision analysis. No data were identified to assess the sensitivity and specificity of RCOG in predicting VTE in women having antepartum risk assessment. (The only study assessing the use of RCOG in antepartum women was not suitable for inclusion in the modelling because it was in a small cohort of hospitalised pregnant women with cancer and no sensitivity data were available.) However, two RAMs for antepartum VTE risk assessment [Lyon32,33 and Efficacy of Thromboprophylaxis as an Intervention during Gravidity (EThIG)27] in women at high risk of VTE (prior VTE and/or thrombophilia) were identified in the systematic review (see Chapter 3). Therefore, in the high-risk antepartum population, the Lyon and EThIG RAMs are compared against each other and against strategies of prophylaxis for all and prophylaxis for none. Table 5 summarises current RCOG guidance on antepartum thromboprophylaxis for high-risk women (prior VTE and/or thrombophilia),7 and compares these with the two RAMs for high-risk patients. 27,32
| Risk factors | RCOG7 | Lyon32 | EThIG27 |
|---|---|---|---|
| Prior pregnancy-related VTE | LMWH from booking | LMWH from booking | LMWH from booking |
| Prior VTE which was unprovoked | LMWH from booking | LMWH from 28 weeks gestation | LMWH from booking |
| Prior VTE associated with major surgery | LMWH from 28 weeks gestation | Postnatal LMWH only | Postnatal LMWH only |
| Thrombophilia without prior VTE | Consider antenatal LMWH (depends on type of thrombophilia) | LMWH from 28 weeks gestation or postnatal only depending on type of thrombophilia | From booking or postnatal only depending on type of thrombophilia |
As the RAMs vary in their recommendations regarding the timing of prophylaxis for some groups, the base-case analysis assumes that risk assessment occurs at the time of the antenatal booking appointment and LMWH is offered from booking to women identified as being high risk using the RAM. Scenario analysis is then used to explore whether the conclusions are sensitive to prophylaxis being deferred to 28 weeks. In scenarios where antepartum prophylaxis is offered, it is assumed that prophylaxis is also continued for 6 weeks after delivery.
In the high-risk antepartum scenario, the model estimates outcomes for prophylaxis for the following strategies:
-
antepartum prophylaxis followed by postpartum prophylaxis for all [prophylaxis (PPX) from booking]
-
antepartum prophylaxis based on a RAM (Lyon/EThIG)27,32 followed by postpartum prophylaxis for all
-
postpartum prophylaxis for all but no antepartum prophylaxis [postpartum (PP) PPX only]
-
no prophylaxis, either antepartum or postpartum (no PPX).
The exploratory analysis for unselected antepartum women makes similar assumptions regarding the timing of risk assessment (antenatal booking appointment) and the duration of prophylaxis offered to those identified as high risk (from booking until 6 weeks postpartum); however, the comparator strategy of postpartum prophylaxis for all (PP PPX only) is not included as unselected women not receiving antepartum prophylaxis are likely to receive a further risk assessment after delivery.
Strategies for prophylaxis in women having postpartum risk assessment
In the postpartum population model, the strategies compared are:
-
postpartum prophylaxis for all (PP PPX for all)
-
postpartum prophylaxis based on a RAM
-
no postpartum prophylaxis (no PPX).
In each case, postpartum prophylaxis is assumed to be offered for 10 days. This is because for the majority of women receiving postpartum thromboprophylaxis, they would fit the criteria for short-term VTE prevention strategies based on their transient risk factors in line with the RCOG guidance. Extended postnatal prophylaxis lasting 6 weeks is mainly offered to those having antepartum prophylaxis, who are excluded from this analysis, and some women with multiple or persistent risk factors. For the unselected postpartum population, the RAMs compared are RCOG,11,39 SFOG,11,36 Caprini39 and the novel RAM reported by Sultan et al. 11 In the postpartum subgroups selected based on specific risk factors, the RAMs compared are RCOG and the novel RAM reported by Binstock et al. 28 in the post-caesarean section population and the novel RAM reported by Ellis-Kahana et al. 41 in the obese population.
Modelling methods
Context
The model estimates lifetime costs and QALYs for the different thromboprophylaxis strategies and the comparator of no thromboprophylaxis under an NHS and Personal Social Services (PSS) perspective. Future costs and benefits are both discounted to their net present value at a rate of 3.5% per annum in accordance with the 2013 NICE guide to the methods of technology appraisal. 44 Costs are reported in Great British pounds based on 2020 prices. To achieve this, historical prices used as model inputs were uplifted using the hospital and community health services pay and prices index up to 2016 and the NHS Cost Inflation Index thereafter. 45
Conceptual model for antepartum women
The conceptual model has been developed in collaboration with the project management group (which included both clinical and patient experts). The group provided guidance on the selection of model outcomes based on clinical importance and assessed the appropriateness of data sources and model assumptions. An existing published model that has been used to evaluate RAM-based thromboprophylaxis strategies in other populations was used as a starting point for discussion. 46,47 Other models that were excluded from the systematic review of published economic evaluations, which addressed similar but not identical decision problems (see Appendix 3), were also used to inform discussions regarding relevant clinical outcomes for inclusion.
The model consists of a decision-tree phase, summarised in Figure 3, to capture short-term outcomes followed by a lifetime state-transition (Markov) model, summarised in Figure 4, to capture the impactof outcomes that result in death or ongoing morbidity. For women being assessed for antepartum prophylaxis, the decision-tree phase of the model is repeated to capture the antepartum and postpartum periods separately. Those patients who are well at the end of the antepartum decision tree remain at riskof postpartum VTE and enter into a postpartum decision tree with the same structure. Those patients who have experienced a symptomatic VTE or a non-fatal intracerebral haemorrhage (ICH) in the antepartum model are assumed to have ongoing costs and utility decrements [reductions in health-related quality of life (HRQoL)] driven mainly by these events, so they remain in the same health state in the postpartum phase.
FIGURE 3.
Short-term decision-tree model structure (repeated for antepartum and postpartum phases).
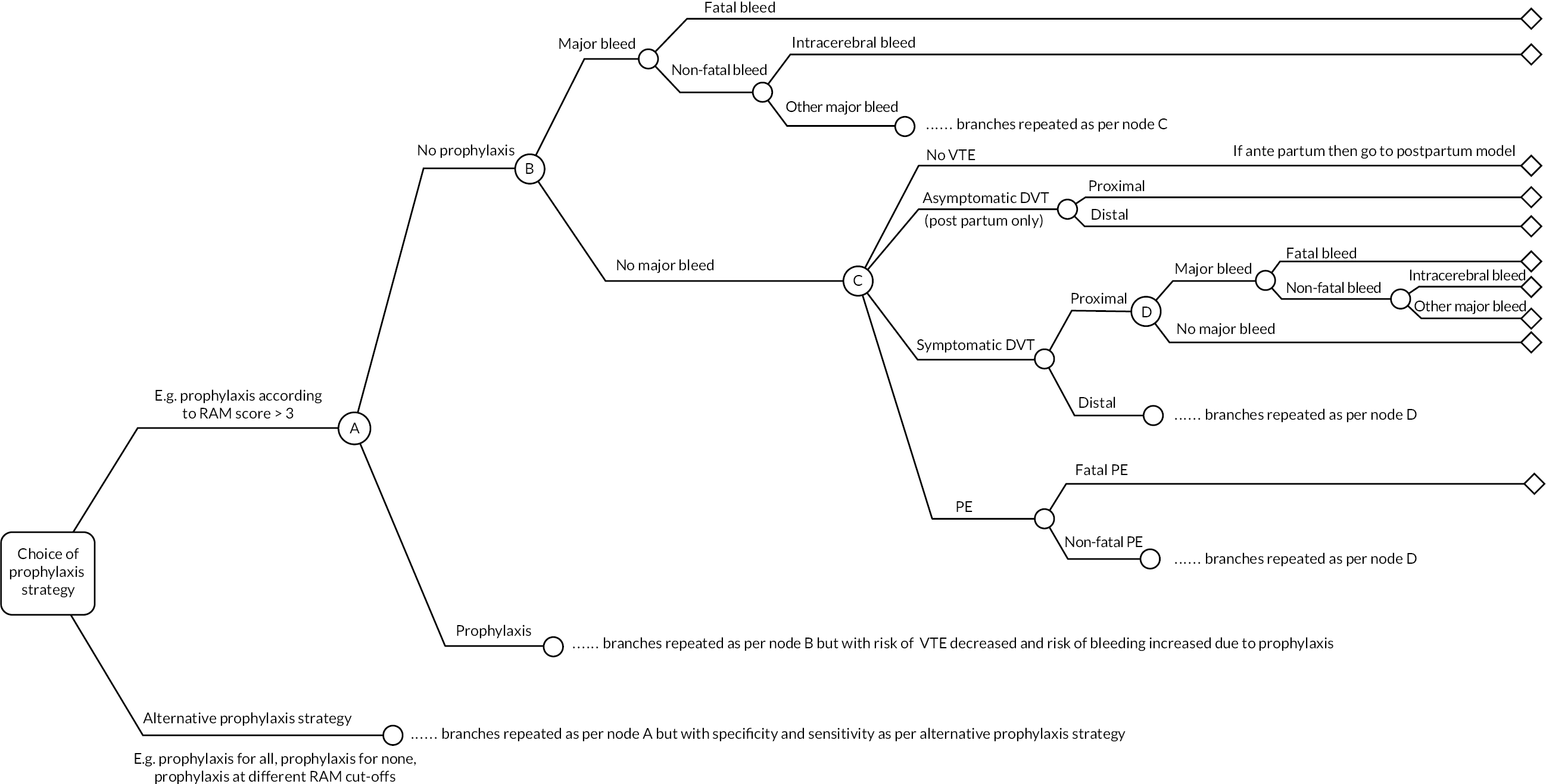
FIGURE 4.
Long-term state-transition model. CTEPH, chronic thromboembolic pulmonary hypertension.

The decision tree is used to estimate for each strategy: the number of patients receiving thromboprophylaxis; the impact of thromboprophylaxis on VTE outcomes (PEs and DVTs); and the incidence of major bleeds during either thromboprophylaxis or VTE treatment with anticoagulants. PEs were divided into fatal and non-fatal events. DVTs were divided first into symptomatic and asymptomatic DVTs and then into proximal and distal DVTs. Symptomatic DVTs and non-fatal PEs are assumed to result in 3 months of anticoagulant treatment, which should be continued until at least 6 weeks post delivery.
In our previous analysis of thromboprophylaxis strategies in patients having lower limb immobilisation following injury, we found that the prevention of post-thrombotic syndrome (PTS) following asymptomatic DVT was an important driver of both cost effectiveness and decision uncertainty due to asymptomatic DVTs being more common than symptomatic DVTs but their long-term consequences being more uncertain. 46 So while asymptomatic DVTs are assumed to remain undetected and untreated, it is important to capture these DVTs in the decision-tree phase of the model in order to capture any ongoing morbidity due to PTS in the long-term state-transition model. However, asymptomatic DVTs are only included in the postpartum model as this ensures that women without symptomatic VTE at delivery progress to the postpartum model where they remain at risk of symptomatic DVT. The risk of asymptomatic DVT is therefore only applied to those not experiencing symptomatic VTE in either the antepartum or postpartum periods. The total period covered by the decision-tree model is 1 year, with the first 30 weeks (from booking appointment at 10 weeks to delivery) covered by the antepartum model, and the remainder (155 days) covered by the postpartum model. This is considered sufficient to capture both the periods at risk of VTE (pregnancy and the 6 weeks after delivery) in addition to the period of VTE treatment if this occurs at the end of the period of risk. Diagnosis of PTS and chronic thromboembolic pulmonary hypertension (CTEPH) is assumed not to occur until the end of the decision-tree phase of the model, as it is difficult to distinguish these chronic complications from acute symptoms during the first 3 months after VTE. Major bleeding can occur both with and without prophylaxis. Major bleeds were considered to be those meeting the criteria proposed by the International Society on Thrombosis and Haemostasis (ISTH) subcommittee on the control of anticoagulation (Tardy et al. 2019). 48 Major bleeds were divided into fatal bleeds, non-fatal ICHs and other major bleeds (referred to as non-fatal non-ICH major bleeds). These other major bleeds were assumed to have no impact on costs or quality-of-life implications after 1 month, whereas ICHs are assumed to have long-term morbidity which is captured in the state-transition model. Wound haematomas can result in delayed discharge from hospital or women consulting at general practice (GP) surgeries or emergency departments (EDs) and they can also impact on HRQoL. These are included in the model as a form of clinically relevant non-major bleeding (CRNMB). Heparin-induced thrombocytopenia (HIT) was not included in the model because in a systematic review of 2777 pregnancies, there were no cases of HIT. 49 Heparin-related osteoporosis was not included as an adverse event in the model because use of LMWH in pregnancy has not been found to be associated with reduced bone mineral density. 50
The model estimates outcomes for a cohort of identical patients with average characteristics. In reality, the application of RAMs may lead to treated and untreated patients having different characteristics. This could lead to the cost effectiveness being over- or underestimated if the consequences of VTE are different for those selected for prophylaxis according to the RAMs. For example, if those being selected for prophylaxis by the RAMs are older, then any deaths prevented by prophylaxis will result in fewer life-years gained than for the model estimates based on women with an average age. Similarly, if the women offered prophylaxis have higher BMI than those not being offered prophylaxis, then the costs of prophylaxis will be higher than estimated based on average BMI. While the impact of these factors was expected to be small, this was checked by varying the starting age and BMI in scenario analysis to determine if the optimal prophylaxis strategy was sensitive to these characteristics. Age was found to have a bigger impact than BMI, but overall the cohort approach using average characteristics was considered a reasonable approximation for determining the optimal prophylaxis strategy.
The key model assumptions for the decision-tree phase are as follows:
-
Patients who are well at the end of the antepartum decision-tree progress to the postpartum decision tree, while those experiencing an antepartum VTE event or ICH remain in their current state until entering the long-term state-transition model.
-
No patient experiences an asymptomatic DVT in the antepartum decision tree as this ensures that they continue to be at risk of a symptomatic DVT in the postpartum model.
-
Bleeding events are possible in both those having thromboprophylaxis and those having no thromboprophylaxis.
-
VTE associated with pregnancy is assumed to occur within 6 weeks of delivery.
-
Patients who stop or have a pause in prophylaxis due to major bleeding are assumed to have the same reduction in VTE risk as those who completed treatment.
-
All patients with symptomatic DVT receive accurate diagnosis and initiate treatment with anticoagulants (LMWH until 6 weeks after delivery or a minimum or 3 months).
-
Asymptomatic DVTs are not detected and are not treated.
-
All PEs are symptomatic and lead to detection and treatment (LMWH until 6 weeks after delivery or a minimum or 3 months).
-
Patients treated for symptomatic DVT and PE have a bleed risk associated with treatment, which is assumed to occur during the 3 months treatment period.
-
Chronic complications of VTE (CTEPH following PE and PTS following DVT) are assumed to be diagnosed at least 3 months after VTE and therefore occur after any bleeds associated with VTE treatment.
-
Patients having fatal PE are not at risk of other adverse outcomes prior to death (e.g. bleeding due to anticoagulant treatment).
-
Risk of bleeding during treatment for VTE is independent of whether the patient bled during prophylaxis.
-
Risk of VTE, risk of bleeding and risk of PTS/CTEPH are based on average patient characteristics (e.g. age and BMI) for the cohort being risk assessed.
A state-transition model (see Figure 4) was then used to extrapolate lifetime outcomes, including overall survival and ongoing morbidity related to either bleeds or VTE. The health states included within the state-transition model capture the risk of PTS following VTE and the risk of CTEPH following PE. The risk of PTS is modelled separately according to whether the DVT is asymptomatic or symptomatic and also whether the DVT is proximal or distal. All patients with PTS are combined in a single health state as costs, utilities (a measure of HRQoL on a scale of 0 to 1) and survival are not expected to be affected by whether PTS occurred following proximal or distal DVT. The PTS health state is not split into different severity levels as the utility estimates are based on the average utility across severity levels and the costs are not expected to differ by severity. The CTEPH health state is divided according to whether patients receive medical or surgical management to allow for differential costs and survival between these groups. There is also a post-ICH state to capture ongoing morbidity following ICHs. Further adverse outcomes (PTS, CTEPH) are not modelled following ICH, as lifetime costs and QALYs are assumed to be predominantly determined by morbidity related to ICH. The state-transition model has annual cycles. All-cause mortality during the first year is applied before patients enter the state-transition model. Health state occupancy is half-cycle corrected such that all transitions between states, including mortality, is assumed to occur mid-cycle. The key model assumptions during the state-transition phase are as follows:
-
All symptomatic DVTs are associated with a risk of PTS, but the rate is allowed to differ depending on whether the DVT is distal or proximal and whether it is symptomatic or asymptomatic.
-
There is no risk of PTS following PE, and CTEPH is possible only after PE.
-
Further outcomes (i.e. VTE, CTEPH and PTS) are not modelled for those who experience ICH as lifetime cost and QALYs will be determined predominantly by disability related to the ICH.
-
All-cause mortality is applied to all transition states except CTEPH and post ICH which have state-specific mortality rates.
-
Recurrent VTE (that is a second VTE occurring after the first VTE during the index pregnancy) is not modelled.
Conceptual model for postpartum women
The conceptual model for postpartum women is identical except that it starts at the point that women deliver and therefore no events occur during the antepartum phase of the model described above. Therefore, women spend 155 days in the postpartum model before progressing to the long-term state-transition model.
Data sources
The input parameters used in a previous analysis of thromboprophylaxis during hospitalisation were examined to identify any that were less relevant to women at risk of VTE during pregnancy and the puerperium. 47 The following data were updated to use data specific to our target population: data related to population characteristics (age, BMI and life expectancy); incidence of VTE; incidence of bleeding; incidence of PTS; costs of prophylaxis and cost of VTE treatment. Other data were generally based on the same sources used in the previous analysis with costs updated to reflect changes in prices. These included incidence of CTEPH following VTE; costs following PTS, CTEPH and ICH; the utility values for patients experiencing adverse outcomes and mortality risks following CTEPH and ICH.
A systematic review of published economic evaluations was conducted, which failed to identify any full economic evaluations that directly addressed the research question (see Appendix 3). However, several full-text articles that addressed similar research questions were examined to identify relevant data sources. These were supplemented with ad hoc searches for relevant literature, focusing where possible on systematic reviews.
When identifying data sources to populate the antepartum model for women at high risk of VTE, we focused on sources related to women with a prior VTE. Two-way scenario analysis was then used to explore whether the conclusions would differ if the target group had a higher or lower risk of VTE or major bleeding. When identifying data sources to populate the postpartum model, we focused first on sources related to women who have had a caesarean section as this is one of the most common risk factors that results in women requiring postpartum prophylaxis. O’Shaughnessy et al. estimated that the RCOG guideline would result in 85% of women having caesarean delivery receiving prophylaxis compared with only 15% of women having vaginal delivery. 15 Therefore, the consequences of postpartum prophylaxis are likely to be best represented by outcomes estimated in women having caesarean delivery even when modelling an unselected postpartum population. However, VTE risks have been estimated specifically for each of the postpartum populations and drug dosages, which are dependent on weight, have been adjusted for the obese postpartum population. In addition, two-way scenario analyses have been conducted to explore whether the conclusions would differ if the target group had a higher or lower risk of VTE or major bleeding than assumed in the base case.
Clinical input parameters are described below with a summary of the key parameters for each of the different populations provided in Table 6 (for reference all parameters are provided in Appendix 4, Tables 22–28).
| Parameter | High-risk antepartum women (e.g. prior VTE) |
Postpartum women (unselected, C-section or obese) |
Report section |
|---|---|---|---|
| Age (years) | 30 | 30 | Population characteristics |
| BMI (kg/m2) | 27 | 27 36 (obese subgroup) |
Population characteristics |
| Duration of prophylaxis | From booking until 6 weeks PP | 10 days | Strategies for prophylaxis in women having antepartum risk assessment and Strategies for prophylaxis in women having postpartum risk assessment |
| Absolute risk of PE without prophylaxis | 1.40% AP and 1.65% PP | 0.017% (unselected) 0.029% (C-section) 0.037% (obese) |
Risk of venous thromboembolism in antepartum women with a prior venous thromboembolism, Risk of venous thromboembolism in postpartum women and Proportion of venous thromboembolism that is deep-vein thrombosis without pulmonary embolism |
| Absolute risk of symptomatic DVT without prophylaxis | 4.41% AP and 5.20% PP | 0.055% (unselected) 0.092% (C-section) 0.116% (obese) |
Risk of venous thromboembolism in antepartum women with a prior venous thromboembolism, Risk of venous thromboembolism in postpartum women and Proportion of venous thromboembolism that is deep-vein thrombosis without pulmonary embolism |
| Absolute risk of asymptomatic DVT without prophylaxis | 0% APa 20.80% PP |
0.229% (unselected) 0.370% (C-section) 0.460% (obese) |
Ratio of asymptomatic deep-vein thrombosis to symptomatic deep-vein thrombosis in postpartum women |
| RR of VTE for prophylaxis (LMWH) vs. no prophylaxis | 0.33 | 0.53b |
Relative risk of venous thromboembolism in women having antepartum prophylaxis and Relative risk of venous thromboembolism in women having postpartum prophylaxis |
| Absolute risk of major bleeding with prophylaxis (LMWH) | 0.24% AP and 5.49% PP | 4.58% | Risk of major bleeding in women having antepartum and postpartum prophylaxis and Risk of major bleeding in women having postpartum prophylaxis |
| RR of bleeding for prophylaxis (LMWH) vs. no prophylaxis | 1.53 | 1.53 | Relative risk of major bleeding in women having antepartum prophylaxis compared to no antepartum prophylaxis and Relative risk of major bleeding for postpartum prophylaxis compared to no postpartum prophylaxis |
| Absolute risk of fatal major bleeding (without LMWH) | 0.5 in 100,000 AP 0.6 in 100,000 PP |
0.6 in 100,000 | Risk of fatal bleeding and non-fatal intracerebral haemorrhage |
| Absolute risk of non-fatal ICH (without LMWH) | 0.9 in 100,000 AP 1.1 in 100,000 PP |
1.1 in 100,000 | Risk of fatal bleeding and non-fatal intracerebral haemorrhage |
| Increased risk of wound haematoma for LMWH | 2.1% | 0.6% | Risk of wound haematoma in women having antepartum and postpartum prophylaxis and Risk of wound haematoma in women having postpartum prophylaxis |
Population characteristics
The average age in the cohort (30 years) is based on the mean age reported by Sultan et al. (2016) from a large UK longitudinal primary care database (CPRD). 11 The cohort included 433,353 women, without a history of VTE, whose pregnancy ended in a live birth or still birth between 1997 and 2014 and who had at least 6 weeks of postpartum follow-up. The average weight, which is required for estimating LMWH dosing, is based on the average BMI of 27.4 kg/m2 for 25- to 44-year-olds reported in the 2019 Health Survey for England. 51 For the obese subgroup, we have assumed a BMI of 35.8 kg/m2, based on the average BMI in the RAM study in an obese cohort reported by Ellis-Kahana et al. 41
Risk of venous thromboembolism in antepartum women with a prior venous thromboembolism
De Stefano et al. report 19 VTE events in 155 pregnancies where the women had a history of VTE prior to pregnancy but did not receive prophylaxis during pregnancy. 52 This gives an overall probability of 12.3% of having a VTE associated with the current pregnancy. The antepartum VTE risk was 5.8%, and the risk of postpartum VTE was of 6.9% (conditional on not having an antepartum VTE). Pabinger et al. reported similar VTE risks of 4% during pregnancy and 5% postpartum. 53 Brill-Edwards et al. reported a lower risk of VTE during pregnancy (2%), but their cohort excluded women with known thrombophilia and women could be recruited up to 20 weeks gestation, meaning that those having VTE early in pregnancy may have been excluded. 54 The risks from De Stefano et al. have been applied in the model. Higher and lower VTE risks have been explored in scenario analyses.
Risk of venous thromboembolism in unselected antepartum women
The risk of VTE in unselected antepartum women is based on the risk reported by Chauleur et al. in the cohort risk assessed using the STRATHEGE RAM. 31 There were nine VTE events in 2685 women (0.34%), of which four were antepartum and five were postpartum, giving absolute risks of 0.15% and 0.19% for antepartum and postpartum VTE, respectively. These data were only used in the exploratory analysis for unselected antepartum women.
Risk of venous thromboembolism in postpartum women
The risk of VTE in women following caesarean section has been estimated from an earlier analysis of the CPRD database (data from 1997 to 2010) reported by Sultan et al. (2014) in which women with prior VTE were excluded from the analysis. 55 For comparison, the risk of VTE within 6 weeks was 0.071% in this earlier cohort (158 in 222,334 deliveries) compared with 0.072% in the later cohort used to derive the Sultan RAM. 11,55 In this earlier study, the incidence of VTE within 6 weeks of any caesarean delivery was estimated to be 0.137% (74 VTEs occurring within 6 weeks across 31,843 emergency and 22,341 elective caesarean sections). 55 The risk of postpartum VTE within 6 weeks of delivery in women with obesity (BMI ≥ 30 kg/m2) was 0.153% (37 VTEs in 24,141 women). The risk of VTE over 6 weeks in unselected postpartum women was taken to be 0.072% based on the later study by Sultan et al. 11
Ratio of asymptomatic deep-vein thrombosis to symptomatic deep-vein thrombosis in postpartum women
A review by Blondon et al. 56 examining the incidence of VTE following caesarean section or vaginal delivery identified six studies which screened women postnatally to identify asymptomatic DVT. Over the 6 studies, we identified 1 symptomatic and 4 asymptomatic cases in a combined cohort of 717 patients. 57–62 Therefore, a ratio of 4:1 is applied in the base case. All of the asymptomatic cases of DVT identified were distal calf DVTs. A zero rate of asymptomatic DVT is explored in scenario analyses as the clinical significance of asymptomatic distal calf DVTs is unclear.
Proportion of venous thromboembolism that is deep-vein thrombosis without pulmonary embolism
The proportion of symptomatic VTE that is PE compared with DVT without PE has been estimated from studies included in the systematic review by Meng et al., which reported the incidence of PE and DVT without PE (24% of VTE is PE based on ratio of 17,035 DVT without PE to 5401 PE). 5 The review included both antepartum and postpartum VTE and the same ratio is applied to both the antepartum and postpartum incidences of VTE.
Proportion of deep-vein thrombosis that is distal
Data from the Computerized Registry of Patients with VTE (RIETE) were used to determine the proportion of symptomatic DVTs that are distal versus proximal. RIETE is an ongoing prospective registry of patients with objectively confirmed VTE and Elgendy et al. describe clinical characteristics for the subset of women who were pregnant or postpartum (within 2 months of delivery) at the time of VTE presentation. 63 Elgendy et al. report that 71% of postpartum DVTs (215 of 301) were proximal, whereas 78% of antepartum DVTs (342 of 438) were proximal. 63
We assumed that all asymptomatic DVTs are distal as none of the asymptomatic DVTs identified through systematic screening of postnatal women in the six studies described in section Ratio of asymptomatic deep-vein thrombosis to symptomatic deep-vein thrombosis in postpartum women were proximal.
Relative risk of venous thromboembolism in women having antepartum prophylaxis
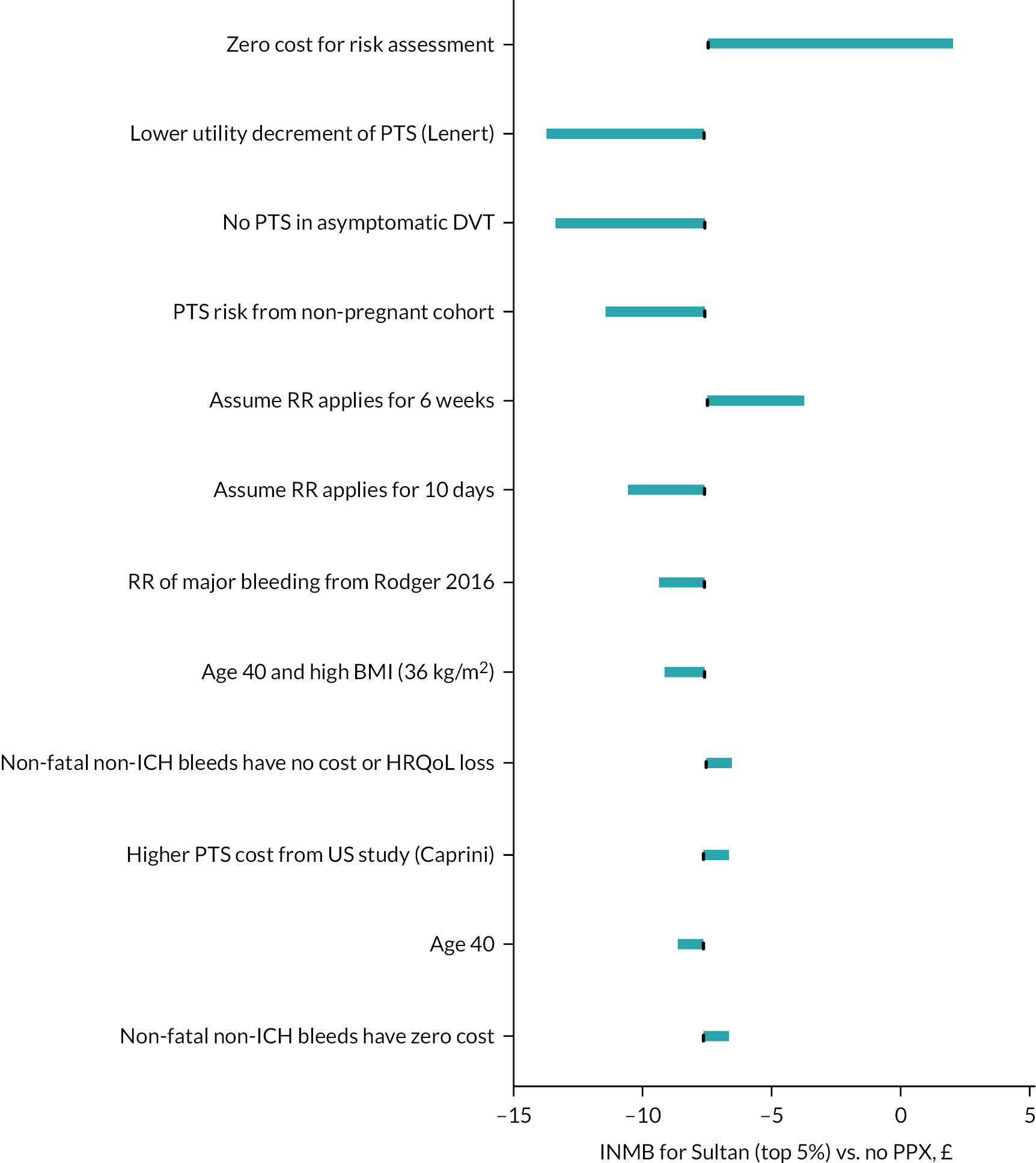
The relative risk (RR) of symptomatic VTE for antenatal LMWH (with or without postnatal prophylaxis) compared with no prophylaxis is reported as being 0.39 (95% CI 0.08 to 1.98) based on four randomised controlled trials (RCTs) included in the updated Cochrane review by Middleton et al. 8 However, three of the RCTs included in this meta-analysis were considered by our clinical experts to be less applicable to the modelled population of high-risk women with a prior VTE. Two of the papers related to the LMWH (FRagmin®) in pregnant women with a history of Uteroplacental Insufficiency and Thrombophilia (FRUIT) trial, which aimed to investigate LMWH combined with aspirin to prevent recurrent early-onset pre-eclampsia. This trial specifically excluded women at high risk of VTE due to prior history of VTE. 64,65 The third study was the Thrombophilia in Pregnancy Prophylaxis Study (TIPPS), in which LMWH was not given specifically for the indication of reducing VTE risk and less than half of the cohort had risk factors for VTE. 66 The dose of LMWH used in the TIPPS study was also higher than recommended for prophylaxis by RCOG. 7,66 The remaining RCT by Gates et al. was the only study included in the previous Cochrane review, and this had a RR of 0.33 (95% CI 0.02 to 7.14). 67 It should be noted that this was in fact a pilot study and the numbers recruited were small (n = 8 in each arm), and only one VTE event was observed, hence the wide CIs. The RR from this single pilot RCT was used in the base-case analysis due to the indirectness of the populations recruited in the FRUIT and TIPPS RCTs and also because of concerns regarding the dose used in the TIPPS study and the use of aspirin in the FRUIT study. However, scenario analysis was conducted using the meta-analysed estimate from all four papers reported by Middleton et al. 8
Relative risk of venous thromboembolism in women having postpartum prophylaxis
The updated Cochrane review reports a RR for VTE of 2.97 (95% CI 0.31 to 28.03) for LMWH versus no prophylaxis following caesarean section based on two studies. 67,68 Both of these were pilot studies. The dose used in one was lower than recommended in the RCOG guideline [2500 IU of dalteparin (Fragmin, Roche) daily for 4–5 days]. 68 For symptomatic DVT, an estimate of 1.40 (95% CI 0.17 to 11.55) is reported by Middleton et al. 8 based on two RCTs. 68,69 Two feasibility studies on postnatal prophylaxis in higher-risk postpartum women (low-risk thrombophilia, immobilisation or two or more risk factors) were also included in the updated Cochrane review (Rodger et al. 2015, 2016),18,19 but neither of these reported any VTE outcomes and both struggled to recruit. There is therefore a paucity of data on the efficacy of LMWH when used as postpartum prophylaxis and those studies that do exist estimate a higher risk of VTE compared with no LMWH, which is the opposite of what is expected based on studies in medical (RR = 0.49, 95% CI 0.37 to 0.67) and surgical cohorts [odds ratio (OR) = 0.26, 95% CI 0.09 to 0.87]. 47 To conduct EVPI analysis to estimate the value of future research, it is necessary to have some prior estimate of treatment efficacy even if that is based on indirect sources or expert consensus. In order to capture both our experience from other populations that LMWH is expected to reduce VTE, and the high degree of uncertainty in the efficacy of LMWH when used as postpartum prophylaxis, we decided to use the RR applied for antepartum prophylaxis (0.33, 95% CI 0.02 to 7.14).
It is unclear whether giving 10 days of LMWH provides protection from VTE for 10 days or for a longer period. Studies in general medical and surgical patients usually involve patients being offered LMWH during admission, or for a defined period such as 7 or 10 days and then they report the RR for VTE over a longer period such as 90 days post admission. Therefore, in these studies, the RR attributed to a short period of LMWH has been estimated over a longer time. Therefore, in previous models of thromboprophylaxis in medical and surgical in patients, the RR estimated from the meta-analyses of RCTs has been applied to the whole period at risk. 47 However, in this case, the RR has been taken from a study of antenatal prophylaxis in which LMWH was continued over the whole period at risk. It is therefore unclear whether the RR estimated in this setting should be applied to the whole 6 weeks over which patients are at risk of VTE, or just to the 10 days during which they received treatment. It was considered that giving 10 days of postpartum thromboprophylaxis would provide a risk reduction of VTE for longer than 10 days. This is because the development of clots occurs in the early postpartum period but may present symptomatically after 10 days, but not beyond 6 weeks postpartum. Given this uncertainty, we have assumed in the base-case scenario that risk falling in the first 3 weeks has the full treatment effect and risk falling beyond this has no treatment effect. This gives an average RR of 0.53 across the 6 weeks when applying a RR of 0.33 to risk falling in the first 3 weeks and a RR of 1 to risk falling from then up to 6 weeks. We have also conducted scenarios exploring the two extreme scenarios of having the RR apply to all 6 weeks and only the first 10 days. The proportion of the 6-week VTE risk falling within each time frame was estimated from the data provided by Sultan et al. 55 The RRs applied when assuming that the efficacy applies for 3 weeks (base case), 10 days (pessimistic scenario) and 6 weeks (optimistic scenario) are 0.53, 0.69 and 0.33, respectively.
Risk of major bleeding in women having antepartum and postpartum prophylaxis
A paper by Nelson-Piercy et al. reports the incidence of serious antepartum bleeds within their reporting of adverse events in a cohort of women having antenatal tinzaparin (Innohep®, LEO Pharma). 70 Serious was defined as ‘clinical events that: resulted in death; were life-threatening; required inpatient hospitalisation or prolongation of existing hospitalisation; resulted in persistent or significant disability/incapacity; were congenital anomalies/birth defects; were other medically important conditions’. The incidence was 3 in 1267 (0.24%), but this included 1013 women having LMWH as prophylaxis and 254 having LMWH as treatment for VTE. 70 Therefore, this may overestimate the risk of major antepartum bleeding for prophylaxis doses of LMWH as some women were having higher doses of LMWH for VTE treatment. All three of the serious bleeds were recorded as possibly, but not probably, related to LMWH. In a UK cohort, reported by Schoenbeck et al., one of the 91 women who received both antepartum and postpartum prophylaxis experienced major obstetric haemorrhage (placental abruption requiring caesarean section at full term) giving a major antepartum bleeding risk of 1%. 71 In contrast, Cox et al. reported four severe antepartum bleeds requiring urgent delivery in 98 pregnancies (4.08%) exposed to LMWH in a New Zealand cohort. 72 Therefore, the risks of antepartum major bleeding appear to vary greatly (0.24–4.08%). Some of this variation is likely to be due to inconsistent definitions of what constitutes major bleeding and some due to differences in the cohorts of women described.
Tardy et al. conducted a review of RCTs of pregnant women having heparin to identify how RCTs have reported bleeding complications from heparin in the past. 48 Tardy et al. conclude, ‘at present it is impossible to estimate the rates and severities of either antepartum bleeding or primary PPH occurring during prophylactic treatment with heparin’. 48 They propose a definition for major bleeding in antepartum women that includes both the standard definition for major bleeding applied in medical inpatients and risks specific to pregnancy. 48,73 Although the definition proposed by the ISTH includes outcomes such as placenta previa requiring delivery and placenta abruption, it is unclear if these are likely to be causally related to the use of LMWH. Tardy et al. also state that antepartum bleeding due to placenta previa and placenta abruption is observed in 2–5% of all pregnancies in the absence of thromboprophylaxis. 48 We have applied the risk of serious antepartum bleeding from Nelson-Piercy et al. in the base case (0.24%), because it has been estimated in a large cohort of women receiving antenatal LMWH (N = 1267) and the definition of serious antepartum bleeding is specified. 70 However, given the uncertainty associated with this parameter, a range of major antepartum bleeding risk up to 4.08% is explored in sensitivity analysis to determine the significance of this parameter for decision-making.
The rate of postpartum major bleeding in women having both antepartum and postpartum prophylaxis has been estimated from a cohort reported by Schoenbeck et al. 71 In this cohort, that 5 of 91 women (5.5%) having antepartum prophylaxis followed by postpartum prophylaxis required a postpartum transfusion due to bleeding. This is higher than the risk of serious postpartum haemorrhage (PPH) (3.2%) reported by Nelson-Piercy et al., which was estimated across both treatment and prophylaxis doses of LMWH. 70 Nelson-Piercy et al. also report that 2.9% of women having prophylaxis doses of LMWH had bleeding requiring intervention, although this is not divided into antepartum and postpartum bleeding. 70 Given that postpartum bleeding requiring transfusion clearly meets the ISTH criteria for major PPH, we have applied the risk of 5.5% from Schoenbeck et al. in the base case, but higher and lower bleeding risks have been explored in sensitivity analysis.
Relative risk of major bleeding in women having antepartum prophylaxis compared to no antepartum prophylaxis
The only RCT reporting the RR of major bleeding in women having antepartum prophylaxis was the TIPPS study (RR = 1.48, 95% CI 0.25 to 8.72). 66 However, this population was considered somewhat indirect because not all women were included due to their VTE risk, and the dose used in this study was higher than the dose usually given to prevent VTE (women had the RCOG recommended dose of 5000 IU of dalteparin daily from randomisation until 20 weeks gestation but the dose was then doubled to 5000 IU twice daily until at least 37 weeks). 66 Furthermore, the timing of the major bleeding was not reported in the TIPPS study, so it is unclear if the bleeding occurred during the antepartum prophylaxis or during the postpartum period when both study arms received LMWH for 6 weeks. The RR of major bleeding in medical patients has been previously reported as 1.53 (95% CI 0.80 to 2.92), based on a meta-analysis of three RCTs. 6 Although it is expected that the absolute risk of bleeding is likely to differ between pregnant women and general medical inpatients, it was decided that the RR of bleeding from the medical cohort could be applied to women having antepartum prophylaxis, as the dose of LMWH used in medical in patients is consistent with that recommended by RCOG. Use of the alternative estimate provided by the TIPPS study is explored in scenario analysis.
Risk of wound haematoma in women having antepartum and postpartum prophylaxis
Lindqvist et al. recorded a risk of 2.5% for haematoma in women who had a prior VTE who were having LMWH during and after pregnancy and a risk of 0.4% in controls not having LMWH (p < 0.001). 74 We have therefore assumed an increased risk of wound haematoma attributable to LMWH of 2.1% in women having antepartum and postpartum prophylaxis.
Risk of major bleeding in women having postpartum prophylaxis
Although the severity of PPH is often defined according to volume of blood loss, with major PPH usually defined as blood loss > 1000 ml,75 the definition of major bleeding for postpartum women proposed by Tardy et al. includes blood loss < 1000 ml leading to transfusion. 48 In addition, blood loss of > 1000 ml is not defined as major bleeding by Tardy et al. unless it is combined with the need for transfusion or other intervention (e.g. second-line uterotonics or surgical intervention). Therefore, we tried to identify a study which reported the incidence of major postpartum bleeding that is consistent with the definition proposed by Tardy et al. rather than one based purely on volume of blood loss.
The risk of major bleeding in women having postpartum prophylaxis following a caesarean section is taken from a study by Gizzo et al. 76 In this study, the incidence of bleeding requiring transfusion after starting prophylaxis (12 hours after caesarean section) was reported as 4.6% (16/349). In this study, haemoglobin levels both post caesarean section and pre transfusion are reported, along with units of blood transfused, and transfusion was considered necessary if the haemoglobin fell below 8 g/dl. Based on these data, it was considered that the outcome of requiring transfusion in this study was reasonably consistent with one of the definitions of major bleeding proposed by ISTH, which is transfusion of two or more units of whole blood or red cells to maintain a haemoglobin level > 7–9 g/dl. 48,76 However, it is acknowledged that there is significant uncertainty regarding the incidence of PPH meeting the ISTH proposed definition and therefore both higher and lower incidence of major bleeding are explored in sensitivity analysis.
Relative risk of major bleeding for postpartum prophylaxis compared to no postpartum prophylaxis
There were no useful data on major bleeding in women having postpartum prophylaxis following a caesarean section from studies included in the Cochrane review. 8 Although some data were reported for bleeding-related adverse events, none of the studies reported any cases of major bleeding using a definition consistent with that proposed by the ISTH. The open-label pilot study by Rodger et al. (2016) reported one major bleeding episode in the LMWH arm and none in the prophylaxis arm giving a RR for major bleeding of 3.53 (95% CI 0.15 to 81.11). 19 However, the wide CIs produced by this single-pilot RCT were considered to be unrepresentative of the broader evidence on the safety of LMWH in indirect populations (i.e. medical and surgical patients who are not pregnant or in the puerperium). Therefore, the RR of major bleeding for medical inpatients receiving LMWH for VTE prophylaxis was used in the base-case analysis and the data from Rodger et al. were applied in a sensitivity analysis.
Risk of wound haematoma in women having postpartum prophylaxis
Ferres et al. reported an incidence of wound haematoma of 1.7% (11/653) in women at high risk of VTE after caesarean section who had received enoxaparin (Clexane®, Sanofi) and an incidence of 1.1% in those who were eligible but who were not offered enoxaparin (11/1042). 77 Although this difference was not statistically significant, we were keen to capture the potential for increased wound haematoma as this was considered an important side effect of LMWH for women. We therefore assumed in the base case that postpartum prophylaxis increases the risk of wound haematoma by 0.6%.
Risk of fatal bleeding and non-fatal intracerebral haemorrhage
From the report published by MBRRACE-UK, we estimated that the rate of fatal bleeds, including either obstetric haemorrhages or ICH, was 0.53 per 100,000 maternities in the antepartum period and 0.57 per 100,000 maternities in the postpartum period. 78
The incidence of ICH has been estimated from a study by Ban et al. which used routine hospital (HES) and GP records (CPRD) to estimate the incidence of strokes in the antepartum, peripartum (1 day prior to 1 day after delivery) and early postpartum (within 6 weeks of delivery) periods. 79 This study reported the incidences separately for ischaemic stroke, ICH, subarachnoid haemorrhage and unspecified strokes. We used the data reported to estimate an incidence of 0.9 per 100,000 pregnancies and 1.1 per 100,000 pregnancies for non-fatal ICH in the antepartum and postpartum groups, respectively. As it is unclear whether the risk of fatal bleeding and non-fatal ICH is causally linked to women receiving LMWH as prophylaxis for VTE, we have also conducted a scenario analysis in which these outcomes are excluded from the model.
Risk of major bleeding during treatment for venous thromboembolism
The Global Anticoagulant Registry in the FIELD (GARFIELD) and RIETE registries report subgroup analyses for women who are pregnant or postpartum at the time of their acute VTE diagnosis. 63,80 They report the incidence of major bleeding during treatment for pregnancy-associated VTE. The RIETE registry provides a larger sample than the GARFIELD registry. The incidence of major bleeding reported in the RIETE registry is 0.8% for women having pregnancy-related VTE and this has been applied in the model. 63
No fatal bleeds on VTE treatment doses are reported for the postpartum VTE subgroup in the RIETE cohort and only one was reported in the pregnancy-associated VTE subgroup of the RIETE cohort. 63 However, data are also presented for a subgroup of younger women (aged under 50) with non-pregnancy-associated VTE who were used as a comparative cohort for the pregnant/postpartum subgroups. As this provided a larger sample size (N = 8084), the data from the non-pregnant cohort were used to estimate the proportion of major bleeds that are fatal (6.3% = 4/63). 63 When combined with the 1.1% absolute risk of major bleeding, this gives an absolute risk of fatal bleeds during VTE treatment of 0.07% (7 in 10,000), which is similar to the lowest risk category for fatal bleeding identified from a separate analysis of the whole RIETE cohort. 81 There are two limitations with this: the non-pregnant cohort diagnosed with VTE may have other risk factors for VTE that place them at increased risk of death such as cancer; and there is a higher rate of direct oral anticoagulant (DOAC) use for long-term anticoagulation in the non-pregnant cohort (9.4% for non-pregnancy-related VTE vs. 4.7% for postpartum VTE). 63 Given the uncertainty regarding the risk of fatal bleeding during treatment of pregnancy-related VTE, we have conducted a scenario analysis exploring a zero rate of fatal bleeding to see how important this parameter is to the decision analysis.
Data on the incidence of non-fatal ICH for VTE treatment doses of LMWH for women having pregnancy-associated VTE are sparse. The RIETE registry does not report the site of major bleeding. 63 The GARFIELD registry does report the site of any major or minor bleed and none of these are reported to be intracranial, although 11 bleeds in the non-pregnancy-associated VTE group and 1 in the pregnancy-associated VTE group are reported to as being ‘other’. 80 The GARFIELD registry does report 2 events as stroke/transient ischaemic attack in 29 women having major bleeding during treatment of non-pregnancy-related VTE, but these could be ischaemic events. 80 If we assume that half of these strokes were haemorrhagic events,79 then the proportion of major bleeding events in GARFIELD which were ICH would be 3.4% (= 1/29) in the non-pregnancy-associated VTE subgroup. 80 This is slightly lower than the proportion of non-fatal major bleeding events which are ICH in the RIETE registry as a whole (9%). 81 This gives an absolute risk of non-fatal ICH on treatment doses of LMWH of 0.04% (4 in 10,000), which is applied in the base-case analysis. A scenario analysis exploring a zero rate of non-fatal ICH is also explored to determine how important this parameter is to the decision analysis.
Risk of chronic complications
The risk of CTEPH in patients having PE was taken from a systematic review by Ende-Verhaar et al. 82 A cumulative incidence of 3.2% (95% CI 2.0 to 4.4) over 2 years was reported in those who survived the initial 3-month period after PE and this was applied to the model giving a risk of 1.6% per annum. We assumed no risk of new CTEPH beyond 2 years based on a study with a median follow-up of 94 months, which reported no new cases of CTEPH after 2 years. 83
A study describing the risk of PTS following VTE related to pregnancy was identified from a review by Kourlaba et al. 84 This paper by Wik et al. reported that the risk of PTS, defined as a Villalta score ≥ 5 was 42% following DVT. 85 Wik et al. also report that age, smoking, timing of VTE (postnatal rather than antenatal) and location (proximal rather than distal) were significant predictors of the risk of PTS in pregnancy-related VTE. Wik et al. also found a significant interaction between timing and location of DVT such that proximal DVT occurring postpartum gave the highest risk of PTS. However, this association between timing and location did not occur in women having antenatal DVT. We, therefore, used the data from Wik et al. to estimate separate risks of PTS for antenatal DVT (34%), distal postpartum DVT (31%) and proximal postpartum DVT (66%). In the base-case scenario, asymptomatic DVTs are assumed to carry the same risk of PTS as symptomatic DVTs, but in a scenario analysis we have assumed no risk of PTS from asymptomatic DVTs. The proportion of cumulative PTS risk falling in years 1–5 after DVT was based on a study by van Dongen et al. which followed up patients with a DVT (not specifically pregnancy-related DVT) every 6 months for a maximum of 5 years (median follow-up 4.9 years) to assess them for signs and symptoms of PTS. 86
To explore how sensitive the model is to the risk of PTS, we have also conducted a scenario analysis in which the risk of PTS (15.6% and 32.4% in distal and proximal DVT, respectively) were estimated from a non-pregnant population. 46
Mortality risks
Patients who survive the first 28 days after having an ICH have an increased risk of mortality in the long term. Fogelholm et al. report a standardised mortality ratio (SMR) of 4.5 in the first year and 2.2 in years two to six for patients who survived 28 days after ICH, compared to age- and sex-matched controls. 87 These SMRs have been applied as multipliers to the risks of all-cause mortality in women having non-fatal ICH. This means that women having ICH have an increased risk of death in the long term compared to women not having an ICH, but the absolute risk of death in women having ICH is much lower than in the study reported by Fogelholm, where the average age was > 65 years in both men and women recruited to the study. Given that the SMRs have been calculated in a predominantly older cohort, and the relationship between ICH and increased mortality may not translate to younger patients, we have conducted a scenario analysis in which women who have a non-fatal ICH (i.e. survive 28 days after an ICH) do not have any increased mortality in the long term.
A review by Kourlaba et al. reports a case fatality rate for PE of 2% (95% CI 1.44 to 2.56), which was based on a meta-analysis of four studies. 84 This estimate was largely dependent on the rates from two large studies based on discharge records which reported case-fatality rates of 1.73% (Liu et al.) and 2.43% (James et al. ), respectively. 3,88 Case-fatality rates from registry studies such as RIETE were lower (< 1%),63 but these may be biased because women who die shortly after PE would not necessarily be recruited into registry studies of anticoagulant treatment. A case-fatality rate of 2% has therefore been applied in the model.
Mortality risks in patients with CTEPH having either medical or surgical management were based on survival curves reported by Goodacre et al., which were estimated from an international prospective registry of patients with CTEPH. 89 Deaths related to PE occurring within 1 year of PE are already accounted for in the model. 90 For this reason, the hazard of death for patients with CTEPH are only applied from 1 year onwards. To ensure that the risk of death in the CTEPH group was not artificially low compared with the risk of death in the general population, general population mortality risks were applied whenever these were higher than the risk in the CTEPH population, based on the survival curves.
All-cause mortality is estimated from age- and sex-specific general population mortality estimates and is applied to all women not experiencing CTEPH, ICH, fatal bleeds or fatal PE. 91 These have not been adjusted to account for any increased risk of mortality associated with pregnancy.
Cost of prophylaxis
We have assumed that the pharmacological prophylaxis used is LMWH as the use of oral anticoagulants including vitamin K antagonists, such as warfarin, and DOACs should be avoided in women who are pregnant. 7 Although warfarin can be used postpartum in women who are breastfeeding, clinical expert advice was that most women preferred to use LMWH postnatally due to the monitoring requirements associated with warfarin.
Women having antepartum prophylaxis are assumed to receive prophylaxis from 10 weeks as this is the typical time of the antenatal booking appointment. It is acknowledged that some women with a prior VTE may be on anticoagulant treatment prior to pregnancy and may immediately switch to LMWH as soon as pregnancy is confirmed, and some women may take longer than 10 weeks to have their risk assessed and start prophylaxis, but 10 weeks was considered a reasonable average starting time. Prophylaxis started antenatally is assumed to continue until 6 weeks after delivery.
As some women are recommended to have antepartum prophylaxis only from 28 weeks under the RCOG guidance, a scenario analysis exploring the impact of delaying antepartum prophylaxis until 28 weeks in this population is described in the section Exploratory deterministic analysis for antepartum women with three risk factors. In addition, the Lyon score recommends that some women having antepartum prophylaxis from booking and some from 28 weeks. Therefore, a scenario analysis exploring the impact of delaying prophylaxis to 28 weeks is described in the section Deterministic scenario analyses for antepartum women with a prior venous thromboembolism.
Women starting prophylaxis postnatally are assumed to be offered 10 days of postpartum prophylaxis in line with current RCOG guidance for those at intermediate risk. Six weeks of postpartum prophylaxis is only offered to those at high risk of VTE, many of whom will already be identified as requiring antepartum prophylaxis.
Patients having a major bleed while taking antenatal prophylaxis would be likely to stop antepartum prophylaxis and restart postnatally. We assumed a 66% reduction in their antepartum prophylaxis cost in such cases. The cost of postpartum prophylaxis is not assumed to reduce if a major bleed occurs postnatally, because even if treatment is discontinued, it is likely that the rest of the 10 days or 6 weeks course would be wasted.
It is assumed that the lowest cost preparation of LMWH is prescribed. Therefore, drug costs for LMWH were based on the cost of dalteparin (5000 units given every 24 hours by subcutaneous injection, based on a weight of 73 kg) as this had a lower cost per day (£2.82) than either enoxaparin or tinzaparin (based on NHS drug tariff prices for lowest cost preparation). 92 The cost was increased to £4.23 per day in the obese cohort (7500 units per day for a 95 kg woman). 92 The cost for administration of LMWH has been estimated by adjusting the costs estimated by Menakaya et al. for outpatient LMWH. 93 This includes the cost of counselling women to self-inject when they first start prophylaxis with LMWH and the cost of administration by a district nurse in the small minority of women (4%) who are unable to self-inject. The cost of administration is £74.94 for 10 days of postnatal LMWH and £321.64 for those starting LMWH antenatally and continuing until 6 weeks after delivery (see Appendix 4, Table 23). In addition, for women having antepartum prophylaxis, it is assumed that they will receive one additional outpatient appointment (£205 for a multiprofessional face-to-face follow-up appointment)94 in late pregnancy to discuss the need to discontinue LMWH at the onset of labour or prior to any planned delivery.
In the strategies examining the use of prophylaxis based on RAMs, we have assumed that the risk assessment will require 5 minutes of time spent by a hospital consultant, which is a cost of £9.92 when applying a unit cost per hour of £119. 45 This cost is applied to all strategies involving the use of a RAM, but not to the comparator strategies of prophylaxis for all and prophylaxis for none. A sensitivity analysis has also been conducted to explore whether the optimal strategy would be different if it could be assumed that the risk assessment would result in no additional cost, with the aim of exploring whether the cost of the risk assessment itself is a significant source of decision uncertainty.
Costs of treating venous thromboembolism
Treatment for VTE (PE or DVT) in the model is assumed to consist of 3 months of LMWH, because warfarin and DOACs are not safe during pregnancy. Although warfarin is a possible alternative treatment for postnatal women, it is less commonly used because of the need for regular blood tests and many mothers would prefer subcutaneous injections without monitoring. Equally, although DOACs can be offered postnatally, these are limited to women who are not breastfeeding. The recommended doses for each of the three available LMWHs (enoxaparin, dalteparin and tinzaparin) have been based on Table 1a–c of the RCOG guideline on the acute management of thromboembolic disease (Green-top guideline no. 37b). 75 A weight of 73 kg has been assumed based on the average weight of women aged 25–44 years in the 2019 Health Survey for England. 51 Where the dose required does not match exactly one of syringe sizes provided by manufacturers, it is assumed that the next size up is used and any excess is discarded so that a new syringe is used each time. The proportion of patients receiving each of the three LMWH preparations (enoxaparin 65.2%, dalteparin 21.6%, tinzaparin 13.2%) was based on data from a recent survey of clinicians by McFarlane et al.,95 which also reported that 56.5% used once daily rather than twice daily dosing. This was used to estimate the proportion using once daily dosing (51%) for dalteparin and enoxaparin after accounting for the fact that tinzaparin is only recommended for once daily dosing. The total cost for drug acquisition for 91 days of treatment is £887.21. This was increased to £1155.32 when using the higher weight of 95 kg for the obese postpartum population.
Women having VTE treatment with LMWH who experience major bleeding are assumed to stop LMWH while actively bleeding, but it is assumed that treatment dose LMWH will be started as soon as it is safe to do so. Therefore, no reduction in VTE drug treatment cost is assumed in the base-case analysis. However, a scenario analysis is conducted assuming that LMWH is stopped for 4 weeks to see whether this factor is an important driver of cost-effectiveness. This reduces the costs of VTE treatment by 11% for those having antepartum VTE and by 18% for those having postpartum VTE.
The costs for administering LMWH, including the cost of training patients to self-administer and the cost of administration by a district nurse for a small minority of patients (4%), are based on the costs reported by Menakaya et al.,93 but these were adjusted to reflect the longer duration of treatment (91 days compared with 42 days in Menakaya), giving a cost of £157.51 for administration. In the base-case analysis, it is assumed that women having treatment dose LMWH for either antepartum or postpartum VTE will have monthly joint outpatient clinic appointments with a haematologist and obstetrician while receiving treatment dose LMWH [£205; Healthcare resource group (HRG) WF02A Service code 303]. 94 However, it was noted that many women experiencing antepartum VTE will already be having regular clinical appointments to manage the comorbidities that put them at increased risk of VTE and not all of the monthly appointments will be required solely due to the LMWH treatment. To explore this, a scenario analysis was conducted where it was assumed that women having antepartum LMWH have three additional clinic appointments and those having postpartum LMWH have one additional clinic appointment. The total cost of drug treatment for VTE is £1659.79 for those having postpartum VTE and £2748.29 for those having antepartum VTE (see Appendix 4, Table 24). This is because women having antepartum VTE are assumed to experience VTE on average at 24 weeks gestation (see section Timing and duration of utility decrements applied in the decision tree), resulting in 154 days of VTE treatment.
Resource use for management of acute VTE is provided in a summary table (see Appendix 4, Table 25). The costs of diagnosing DVT and PE in patients having these outcomes postnatally have been based on the costs used previously in non-pregnant populations having DVT or PE in an outpatient setting. 46 These previous analyses assumed that 10% of patients having proximal DVTs and 60% of patients having PEs would be admitted but none having distal DVTs would be admitted. In addition, it was assumed that 10% of those having PE would be admitted to critical care. For women having an antenatal DVT or PE, we would expect a greater likelihood of admission compared to a non-pregnant population. We have assumed that the likelihood of admission for proximal DVT is double that assumed in the non-pregnant population (20% vs. 10%) but remains zero for distal DVT. We have assumed that the likelihood of admission increases from 60% to 90% in women having antenatal PE. We have also assumed that the risk of admission to critical care is double for pregnant women having a PE compared to non-pregnant people having a PE (20% vs. 10%). We assumed maximum resource use for people having fatal PE (i.e. ambulance transfer to the ED leading to a short-stay admission including a critical care unit stay) but have excluded the long-term cost of VTE drug treatment.
For patients having antenatal PE, we have taken the diagnostic tests used from the Diagnosis of Pulmonary Embolism in Pregnancy (DiPEP) study. 96 We have assumed a 50 : 50 split between ventilation/perfusion (V/Q) single photon emission computed tomography (SPECT) and V/Q planar as Goodacre et al. only report the frequency of ventilation-perfusion scanning and not the specific type. As the DiPEP study did not report on the use of echocardiogram, we have taken the proportion from a previous UK Obstetric Surveillance System (UKOSS) data set reported by Knight et al. 97 (Appendix 4, Table 25). The total cost for acute management of VTE (i.e. excluding long-term drug treatment) ranged from £311.32 for symptomatic distal DVT to £3261.24 for fatal PE (details provided in Appendix 4, Table 25). Given that many assumptions have been employed to estimate resource use associated with diagnosis and management of VTE, the importance of these costs is explored in a scenario analysis. In this scenario, we assume that all antenatal VTE results in admission and 50% of antenatal PE also results in a critical care stay.
Costs of treating major bleeding
The cost over 90 days of fatal haemorrhagic stroke provided by Luengo-Fernandez et al. was uplifted to current prices and applied as the cost of fatal bleeds in the decision-tree phase of the model. 98 This paper also provided costs over 90 days for non-fatal haemorrhagic stroke stratified by the level of disability. A weighed average cost was calculated across non-disabling, moderately disabling and totally disabling haemorrhagic strokes. 98 This was then uplifted to current prices and applied as the cost of non-fatal ICH in the decision-tree phase of the model. Luengo-Fernandez et al. also report the average costs per annum from 90 days to 5 years post stroke, but these are not reported separately for haemorrhagic stroke. The costs of GP care and emergency care are reported to be statistically significantly higher post stroke compared to the year before stroke. In addition, they report the cost of residential care in patients not living in residential care prior to their stroke. The total post-acute (beyond 90 days) costs for primary care, emergency care and residential care were calculated and uplifted to current prices and applied in the state-transition phase of the model to those having non-fatal ICH. A pro rata cost is also applied to those having stroke more than 90 days before the end of the decision-tree phase of the model.
There are limited data available to determine the cost of managing non-fatal, non-ICH bleeds in pregnant women. The cost of managing non-fatal non-ICH major bleeds was assumed to be similar to the cost of managing a gastrointestinal (GI) bleed, despite these being different pathologies. This is consistent with the approach that has been taken in previous published models of VTE prevention covering both patients having outpatient lower limb immobilisation and patients at risk of hospital acquired VTE. 46,47 It was also the approach taken in a US economic evaluation of heparin use in pregnant women reported by Johnston et al. (2005). 99 The cost of GI bleeding was estimated based on a weighted average cost for non-elective inpatient and non-elective short-stay management of GI bleeds using NHS reference costs for bleeds requiring single, multiple or no interventions (HRG codes FD03A to FD03H). 94 The average length of stay was 3 days for the most common HRG code for GI bleeding which comprised 46% of the spells for GI bleeding, suggesting that the median length of stay is around 3 days. Costs estimated using GI bleeding as a proxy are probably more applicable to women having a major non-obstetric bleed, where the bleed itself would be the reason for admission. This is because it is assumed that many women having major bleeding at the time of delivery (i.e. a PPH) are likely to already be receiving inpatient care. A cost-effectiveness model examining the use of uterotonics to prevent PPH estimated that the average length of stay would be 1.5 days in women without PPH (< 500 ml blood loss), increasing to 3 days in women who have > 1500 ml of blood loss, who are also assumed to require transfusion of two units of blood,100 in which case the additional length of stay attributable to major PPH would be closer to 1.5 days. An international survey of midwives (N = 100) estimated that women having a major PPH would have an increased length of stay of 1 day compared to women not having a PPH. However, when UK-specific midwives were questioned (N = 25), it was suggested the estimated additional length of stay attributable to a major PPH would be longer at 2.3 days. 101 Based on these data, it is possible that the cost of an admission for GI bleeding is higher than the additional cost of managing a major PPH in a woman already admitted for delivery. Given the likely heterogeneity associated with the costs of major bleeding in this population, we have conducted a scenario analysis in which we assume no additional cost, and a scenario analysis in which we assume the cost is twice that expected for GI bleeding, to determine how sensitive the results are to this parameter.
Costs of wound haematoma
Wound haematomas can lead to a delay in discharge for women after delivery. Therefore, we assumed that a wound haematoma would result in a long-stay admission instead of a short-stay admission using the reference costs for normal delivery (cost difference of £1372 between non-elective and short-stay admission NZ30C). 94 We explored a more conservative scenario in which a wound haematoma only leads to one ED attendance in a scenario analysis.
Costs of managing post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension
The management of PTS is assumed to involve one first and one follow-up vascular surgery outpatient appointment in the first year after diagnosis and two follow-up GP appointments every year thereafter. This is consistent with the assumption applied in a previous analysis. 46 An alternative cost based on the burden estimated in a US cohort is considered in a sensitivity analysis. 102
Drug costs for medical management of CTEPH were based on the costs used in CG92, which were uplifted to give a cost of £18,980 per year. The costs for medically managed patients are applied each year to those surviving with CTEPH. The proportion of patients having surgical management for CTEPH (59%) is based on data from Delcroix et al. 90 The cost for surgical management of CTEPH is based on a weighted average of the reference costs for complex thoracic procedures (DZ02H/J/K) giving an average cost of £8175. 94 A proportion of patients having surgical management (29%) are assumed to require medical treatment as a bridging therapy (average of 4.6 months). Including these costs brings the total cost in the first year to £10,282 for surgical management. No costs are applied beyond the first year for those managed surgically.
Utility values
A recent systematic review of utility values by Etxeandia-Ikobaltzeta et al. was identified which included utilities values in studies published up to April 2018. 103 This review identified only one study of relevance to pregnant/postpartum women, but this study used the EuroQol-5 Dimensions (EQ-5D) visual analogue scale (VAS) which is not a preference-based measure of utility. Estimates of utility following DVT and PE in the general population from the Prevention of Thromboembolic Events – European Registry in VTE (PREFER-VTE) study (not specific to pregnancy or puerperium)104,105 have been used in a previous model of thromboprophylaxis in hospitalised patients. 46,47 As no additional utility values were identified from the review by Etxeandia-Ikobaltzeta et al., the utility values from the PREFER-VTE study applied in previous VTE prevention models were maintained in this model. These gave utility multipliers of 0.962 and 0.960 when averaging the reported utility values over the first 6 months after DVT and PE, respectively (see Appendix 4, Table 26), and long-term utility multipliers of 1.00 and 0.99, respectively (see Appendix 4, Table 27).
One of the key assumptions in previous models was that the utility decrement of PE and non-ICH major bleeding is similar in the month following these events. 46,47 This assumption is somewhat supported by Etxeandia-Ikobaltzeta et al., who found that the EQ-5D VAS score was 30 for both PE and major obstetric bleeding. 103 Therefore, it seemed reasonable to maintain this assumption in the model for pregnant women. However, it should be noted that the utility decrement for major non-ICH bleeding is applied for only 1 month, whereas an ongoing utility decrement is applied for PE, so PE has a larger impact on QALYs than major bleeding. Given the lack of utility values measured directly in women following major bleeding, a scenario analysis in which no utility decrement is applied for those having major non-ICH bleeding has been conducted to explore the importance of this parameter. For wound haematoma, we have assumed a utility decrement equivalent to major non-ICH bleeding for 1 week to capture any adverse impact on HRQoL. A scenario analysis removing this utility decrement for wound haematoma was also explored.
No pregnancy-specific estimates of utility following PTS or CTEPH were identified in the review by Etxeandia-Ikobaltzeta et al.,103 so the sources applied in previous models were maintained. 46,47 In our previous analysis of prophylaxis during lower limb injury, we used utility data from the Catheter-Directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis (CaVenT) study to estimate the utility decrement in patients with PTS. 106 An estimate of 10% was applied from diagnosis onwards which was obtained from the CaVenT study by comparing the EQ-5D scores in those with and without PTS at 2 years. The CaVenT study did not stratify the utility estimates by severity of PTS, so this estimate was applied to all patients with PTS in the model regardless of severity. This may overestimate the utility decrement if the proportion of patients having severe PTS is lower in the modelled population than in the CaVenT study which recruited patients with acute iliofemoral DVT. A study by Lenert and Soetikno reported utility estimates for mild and severe PTS (0.98 and 0.93, respectively) obtained by using health state descriptions and a standard gamble valuation technique in a sample of volunteers. 107 These were not used in the base case as utility measured using the EQ-5D in patients with the condition is preferable to utility measured using standard gamble in volunteers based on descriptions of the condition. However, a scenario analysis was conducted in which the data from Lenert and Soetikno107 were combined with data on the proportion of PTS that is severe (6%) from a registry study in outpatients having VTE,108 to estimate a utility decrement of 2% across all patients with PTS. In the current analysis, we have taken the same approach and have applied a 10% decrement in the base case and a 2% decrement in a scenario analysis.
The utility decrement in patients with CTEPH was estimated from a study by Meads et al. by comparing the utility in patients having CTEPH (0.56) and the utility in patients with disease categorised as New York Heart Association (NYHA) class 1 (0.89) in which the HRQoL impact of symptoms would be expected to be minimal. 109 This gave a utility multiplier of 0.63 or a 37% decrement. This decrement is applied lifelong in the model to those having medical management of CTEPH, but only for 1 year in those having surgical management who have the utility multiplier for PE applied thereafter.
The values applied following non-fatal ICH were also taken from those used in previous VTE prevention models. Utility values following ICH were based on data from 5-year follow-up of the Oxford Vascular Study (OXVASC) study as these data were applied in a previous analysis of thromboprophylaxis after lower limb injury. 110 An absolute decrement of 0.22 was assumed in the decision-tree part of the model where time since stroke was < 1 year and a decrement of 0.09 was assumed in the long-term part of the model. This study was chosen as the source of utility values previously as the duration of follow-up allowed time since stroke to be accounted for, and a comparison was made against general population norms.
In the previous analysis of thromboprophylaxis after lower limb injury, we identified several sources which estimated the utility decrement associated with VTE prophylaxis or VTE treatment. 46 The study selected for use in the previous model was a study by Marchetti et al. which reported that patients would be willing to trade 2.7 of 365 days to avoid treatment with LMWH. 111 These data were previously used to estimate a utility decrement of 0.007 for LMWH. These same decrements have been applied in this model. However, it is noted that the utility decrement may differ for women during pregnancy or the puerperium. Therefore, to determine how sensitive the model results are to this parameter, we conducted scenario analyses in which we assumed that the utility decrement is either double the value assumed in the base case or zero.
Utility values for patients not experiencing any utility decrement due to prophylaxis, treatment, symptomatic VTE events, bleeding events (ICH or other major bleeds), long-term sequelae following VTE (PTS or CTEPH) or death are based on general population norms for a cohort of the same age and this is allowed to vary as the cohort ages during the model. 112
Timing and duration of utility decrements applied in the decision tree
To calculate the QALYs gained by patients having different paths through the decision tree, it is necessary to make some assumptions regarding the timing of events as these are not explicitly modelled in a decision tree. The average timing of postpartum VTE is 21 days based on the timings reported by Sultan et al. 55 However, we are interested in the timing of the VTE that is prevented by prophylaxis. If you assume that the VTE events prevented by prophylaxis occur on average halfway through the prophylaxis, then that would mean assuming they occur at 5 days. However, it is also possible that early prophylaxis for 10 days prevents VTEs that would have been diagnosed later in the puerperium after prophylaxis has ended. Therefore, in our base-case analysis, we have assumed that the VTEs being prevented occur on average at 21 days, and we explore the impact of varying this from 5 to 42 days in scenario analysis.
The average timing of VTE occurring during pregnancy was estimated using data from Voke et al. which provides a scatter plot of timing of VTE events. 113 From this it was estimated that the average timing of VTE was 24 weeks.
Timing of postpartum bleeds during prophylaxis (3 days) was based on the average timing reported by Gizzo et al. 76 Bleeds occurring during antepartum prophylaxis are assumed to occur 28 days before the timing of VTE. Bleeds occurring during treatment for VTE are assumed to occur at 13, 32 and 12 days post diagnosis of VTE for fatal, ICH and other major bleeds, respectively (based on data from the RIETE registry reported by Nieto et al. ). 81
We made the following assumptions when estimating QALYs in the decision tree:
-
Baseline utilities using general population utility values for the starting age are applied to those not having treatment and not having any clinical events (e.g. VTE, bleeds).
-
A disutility for ICH is applied lifelong, but separate values are applied in decision-tree and state-transition phases of the model.
-
Disutility of non-fatal non-ICH major bleeding is assumed to last a maximum of 28 days.
-
Disutility of prophylaxis applies for the duration of prophylaxis.
-
Disutility of treatment for VTE applies for the duration of treatment.
We made the following assumptions when estimating QALYs in the state-transition model:
-
Utility values for patents without any long-term sequelae (ICH, CTEPH, PTS) are taken from general population values and decrease as patients age in the model.
-
All other utility values are applied as multipliers such that the absolute utility value decreases due to ageing in all patients.
-
Utility decrements continue in the state-transition model for the remainder of the patients’ lifetime for PE but not for DVT where patients are assumed to return to general population utility values at 1 year.
-
Patients with CTEPH who are treated medically have a lifelong utility decrement, whereas those treated surgically return after 1 year to the same utility as those surviving PE without CTEPH.
-
Patients with PTS have the same utility decrement from diagnosis to death.
-
Patients with ICH have the same utility decrement from the start of state-transition model to death.
Sensitivity and specificity of risk assessment models
Estimates of sensitivity and specificity are based on the data presented in the systematic review (see Chapter 3). Only those studies that reported both sensitivity and specificity could be included in the modelling and any study reporting a sensitivity of 0% was excluded from the modelling. In the antepartum model for high-risk women, only the EThIG and Lyon RAMs had data suitable for inclusion. The modelling for high-risk women assumes that patients classified as low risk by the EThIG and Lyon RAMs will receive postpartum prophylaxis and those categorised as high risk based on the RAMs will receive antepartum prophylaxis in addition to postpartum prophylaxis. Therefore, it is the sensitivity and specificity of the RAMs in predicting antepartum VTE that are relevant to the economic analysis. The data available for the high-risk antepartum women for the EThIG and Lyon RAMs are summarised in Figure 5 as a receiver operating characteristics (ROC) curve.
In the unselected postpartum population, data were available for the RCOG,11,39 SFOG11,36 and Caprini39 RAMs and the novel RAM reported by Sultan et al.,11 for which performance data were reported for multiple cut-offs (defined according to those falling in the top 1%, 5%, 10%, 20% and 25% of absolute risk). In the postpartum population with obesity, only the novel RAM reported by Ellis-Kahana et al.,41 is available, but performance data are provided for two versions of this RAM, in which thrombophilia was either included or excluded from the risk algorithm. In the post-caesarean section population, a novel RAM is reported by Binstock et al. along with data for the RCOG RAM. 28 The data for postpartum RAMs across the three populations are summarised in Figure 6.
In the unselected antepartum population, the only RAM identified with available performance data was the STRATHEGE RAM,31 but the data suggested that it had poor performance (sensitivity of 0% and specificity of 98%). Therefore, the analysis in the unselected antepartum population was limited to exploratory analysis. In this analysis, various theoretical combinations of sensitivity and specificity values were tested to determine the range of sensitivity and specificity values that would be required for a RAM to be cost-effective in this population.
Model inputs for secondary scenarios where antepartum prophylaxis is offered at 28 weeks
In the main analysis for antepartum prophylaxis in women at high risk of VTE, we have assumed that women are offered prophylaxis from booking if they are identified as being at high risk by either the EThIG or Lyon RAMs. However, for the Lyon RAM, some women are offered antepartum prophylaxis from 28 weeks only. 32,33 Therefore, we have conducted a scenario analysis where prophylaxis is only offered from 28 weeks in the high-risk subgroup to explore whether this results in a different strategy being most cost-effective. For this analysis, the efficacy of prophylaxis is adjusted so that it only applies to the 40% of antepartum VTE risk occurring after 28 weeks gestation and the average timing of VTE is moved from 24 to 34 weeks, with consequent impacts on the cost of VTE treatment. The average timing of VTE occurring after 28 weeks was based on the scatterplot provided by Voke et al. 113 Other risks, such as the risks of major antepartum bleeding, are not adjusted.
In the main analysis for women being offered antepartum prophylaxis, we have focused on women at high risk being offered prophylaxis from booking. However, within the RCOG guidance, antepartum prophylaxis is also recommended from 28 weeks in women having three risk factors. 7 A secondary scenario has been conducted to explore whether this is cost-effective. Some data on the risk of antepartum VTE for specific risk factors and for some combinations of risk factors were available from an analysis of a GP database by Sultan et al. (2013). 114 This used a different GP database (The Health Improvement Network) from that used to generate the postpartum RAM (CPRD),11 but again it excluded women with a prior history of VTE. This provided the risk of VTE for women with any 2 or more risk factors as being 95 antepartum events per 100,000 pregnancies and 111 postpartum events per 100,000 risk factors (0.20% VTE risk overall). 114 The risk for women with three or more risk factors is not provided; however, if we applied the RR for the strongest individual risk factor, which was varicose veins (RR of 2.21 for antepartum VTE and 3.90 for postpartum VTE), to the absolute risks for two or more risk factors, then this would suggest an upper limit for the absolute risk of 217 antepartum VTEs per 100,000 pregnancies and 433 postpartum VTEs per 100,000 pregnancies. This suggests an upper limit for women with three risk factors of around 0.6%. As an exact risk cannot be identified for women with three or more risk factors, we have used the model to identify the level of risk that would be required in this group for prophylaxis at 28 weeks to be cost-effective.
Approach to quantifying decision uncertainty
A probabilistic sensitivity analysis (PSA) has been conducted to incorporate uncertainty regarding the model inputs and determine how this uncertainty propagates through the model to translate into uncertainty in the incremental costs and QALYs and therefore decision uncertainty regarding the optimal prophylaxis strategy. The PSA is based on 10,000 parameter samples (probability distributions are provided, see Appendix 4, Tables 22 and 28). In the PSA, the OR for VTE was sampled using the event rates from the study by Gates et al. 67 (OR 0.29, 95% CI 0.01 to 8.37), and this was used to calculate the expected RR given the sampled absolute risk of VTE in the model for people not receiving prophylaxis.
In addition, the decision uncertainty associated with not having perfect information on all model parameters is estimated by the EVPI analysis. The overall EVPI provides an estimate of the increase in net monetary benefit that could be achieved by having perfect information on all model parameters simultaneously. The increase in net monetary benefit that can be achieved by obtaining perfect information on individual parameters or groups of parameters is known as the expected value of perfect parameter information (EVPPI). We have estimated EVPPI using the online Sheffield Accelerated Value of Information (SAVI) tool which uses a regression-based approach to obtain estimates of EVPPI directly from the outcomes of the PSA and the set of parameter inputs that generated those PSA outputs. 115,116 We provide EVPI and EVPPI estimates per patient and we also estimate the EVPI and EVPPI over 5 years of births, assuming 640,370 live births per annum in England and Wales,117 and discounting of future costs and benefits at 3.5%.
Aspects of structural uncertainty such as the choice of one data source over another to inform the parameter distribution, or the impact of various model assumptions, are explored within scenario analyses using the mean estimates for the parameter inputs (referred to as the deterministic model).
Results – cost-effectiveness and value of perfect information
Clinical outcomes predicted by the model with and without prophylaxis
Table 7 shows the clinical outcomes predicted by the model with and without prophylaxis in each of the modelled populations when using the deterministic model (i.e. mean parameter inputs).
| Outcomes at 6 months per 100,000 patients | Outcomes at 5 years per 100,000 patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatal PE | Fatal bleed | Non-fatal ICH | Other major bleeda | Non-fatal PE | Symptomatic DVT | Asymptomatic DVT | PTS | PE survivor with CTEPH | PE survivor without CTEPH | ICH survivor | Dead (any cause) | |
| High-risk antepartum women (e.g. prior VTE) | ||||||||||||
| No prophylaxis | 59 | 7 | 5 | 3423 | 2890 | 9300 | 19,593 | 10,824 | 74 | 2790 | 5 | 321 |
| Prophylaxisb | 20 | 4 | 4 | 5400 | 974 | 3136 | 6733 | 3696 | 25 | 941 | 4 | 266 |
| Unselected postpartum women | ||||||||||||
| No prophylaxis | 0 | 1 | 1 | 2996 | 17 | 55 | 219 | 101 | 0 | 16 | 1 | 238 |
| Prophylaxisc | 0 | 1 | 2 | 4582 | 9 | 29 | 116 | 53 | 0 | 9 | 2 | 238 |
| Obese postpartum women | ||||||||||||
| No prophylaxis | 1 | 1 | 1 | 2996 | 36 | 116 | 465 | 215 | 1 | 35 | 1 | 238 |
| Prophylaxisc | 0 | 1 | 2 | 4582 | 19 | 62 | 246 | 114 | 0 | 18 | 2 | 238 |
| Postpartum women following caesarean section | ||||||||||||
| No prophylaxis | 1 | 1 | 1 | 2996 | 32 | 104 | 415 | 192 | 1 | 31 | 1 | 238 |
| Prophylaxisc | 0 | 1 | 2 | 4582 | 17 | 55 | 219 | 101 | 0 | 16 | 2 | 238 |
In the population of high-risk antepartum women, prophylaxis reduces serious adverse outcomes (fatal PEs, fatal bleeds and non-fatal ICHs) from 71 per 100,000 to 28 per 100,000. Prophylaxis reduces the risk of fatal bleeding and non-fatal ICH because it reduces the risk of VTE and therefore the risk of requiring anticoagulant treatment, which itself has a risk of fatal bleeding and non-fatal ICH. The reduction in symptomatic DVTs is higher than the increase in other major bleeds. In the long-term outcomes, presented in Table 7 at 5 years, there are also reductions in both PTS (7127 per 100,000) and CTEPH (49 per 100,000).
The absolute risks of VTE are much lower in the unselected postpartum population, but the bleeding risks are of the same order of magnitude. Prophylaxis for all would result in one additional serious adverse outcome (1 additional ICH per 100,000) but would reduce symptomatic VTE by 34 per 100,000. However, the risk of other major bleeding is significant at 1586 per 100,000. In the postpartum subgroups selected for specific risk factors (obesity, post caesarean section), the benefits of prophylaxis are slightly higher, because the risks of VTE are slightly higher, but these are still outweighed by the increased risks of major bleeding.
Antepartum women with a prior venous thromboembolism
Deterministic base-case results for antepartum women with a prior venous thromboembolism
The deterministic base-case results obtained when applying the midpoint parameters estimates to the base-case scenario for antepartum women with a prior VTE are shown in Figure 7. It can be seen that all of the strategies have an incremental cost-effectiveness ratio (ICER) under £30,000 per QALY when compared to a strategy of offering no antepartum or postpartum prophylaxis (no PPX). The strategy of offering only postpartum prophylaxis (PP PPX only) is cost saving compared to no PPX and generates additional QALYs and therefore dominates no PPX. The EThIG RAM has the highest QALY gains and it has lower costs that offer prophylaxis to all from booking (PPX from booking). Therefore, PPX from booking is said to be dominated by the EThIG RAM. The ICER for the Lyon RAM compared to PP PPX only is £53,757 per QALY, whereas the ICER for the EThIG RAM compared to the Lyon RAM is less at £1468 per QALY. Therefore, the Lyon RAM is extendedly dominated because it would never be preferable when the EThIG RAM and PP PPX only strategies are available. The ICER for the EThIG RAM compared to PP PPX for all is £24,982. Therefore, based on the deterministic analysis, PP PPX only would be most cost-effective when applying a cost per QALY threshold of £20,000 and the EThIG RAM would be most cost-effective when applying a cost per QALY threshold or £30,000.
Probabilistic base-case results for antepartum women with a prior venous thromboembolism
The results based on the mean outcomes from 10,000 probabilistic model runs are summarised in Table 8. The broad conclusions are the same, in that all of the strategies are cost-effective compared to no prophylaxis, and the optimal strategy when valuing a QALY at £20,000 is PP PPX only. However, the ICER for the EThIG RAM compared to PP PPX only is £56,761 per QALY in the probabilistic analysis, whereas the ICER for this comparison was under £30,000 in the deterministic analysis. This means that the optimal strategy when valuing a QALY at £30,000 is the PP PPX only strategy based on the probabilistic analysis.
| % AP PPX (%) | Sensitivity for predicting AP VTE (%) | Specificity for predicting AP VTE (%) | Absolute costs, (£) | Absolute QALYs | Cost vs. no PPX, (£) | QALYs vs. no PPX | ICER vs. no PPX, (£) | ICER vs. next least effective strategy, (£) | INMB vs. no PPX at £20K, (£)a | INMB vs. no PPX at £30K, (£)a | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No PPX | 0 | 0 | 100 | 729.26 | 20.802 | NA | NA | NA | NA | NA | NA |
| PP PPX only | 0b | 0c | 100d | 757.52 | 20.877 | 28.26 | 0.075 | 375 | 375 | 1477.17 | 2229.88 |
| Lyon ≥ 3 | 64 | 50 | 35 | 1388.52 | 20.884 | 659.27 | 0.082 | 7994 | Extendedly dominated | 990.22 | 1814.96 |
| EThIG | 74 | 100 | 28 | 1449.76 | 20.889 | 720.51 | 0.087 | 8237 | 56,761 | 1028.84 | 1903.52 |
| PPX from booking | 100 | 100 | 0 | 1709.13 | 20.893 | 979.88 | 0.091 | 10,796 | 78,722 | 835.37 | 1742.99 |
However, there is significant uncertainty in the incremental costs and QALYs as demonstrated by Figure 8, which shows the spread of incremental costs and QALYs for antepartum prophylaxis based on the EThIG RAM compared to a strategy of PP PPX only, with 42% of the PSA samples providing an ICER of under £30,000 per QALY for the EThIG RAM compared to PP PPX only. The cost-effectiveness acceptability curve (CEAC) is presented in Figure 9. This shows that the PP PPX only strategy has the highest probability of being most cost-effective (36%), when valuing a QALY at £30,000. However, no PPX, and PPX according to the EThIG RAM both have a > 20% probability of being optimal.
FIGURE 8.
Probabilistic base-case results for antepartum prophylaxis according to the EThIG RAM vs. postpartum prophylaxis only (PP PPX only).
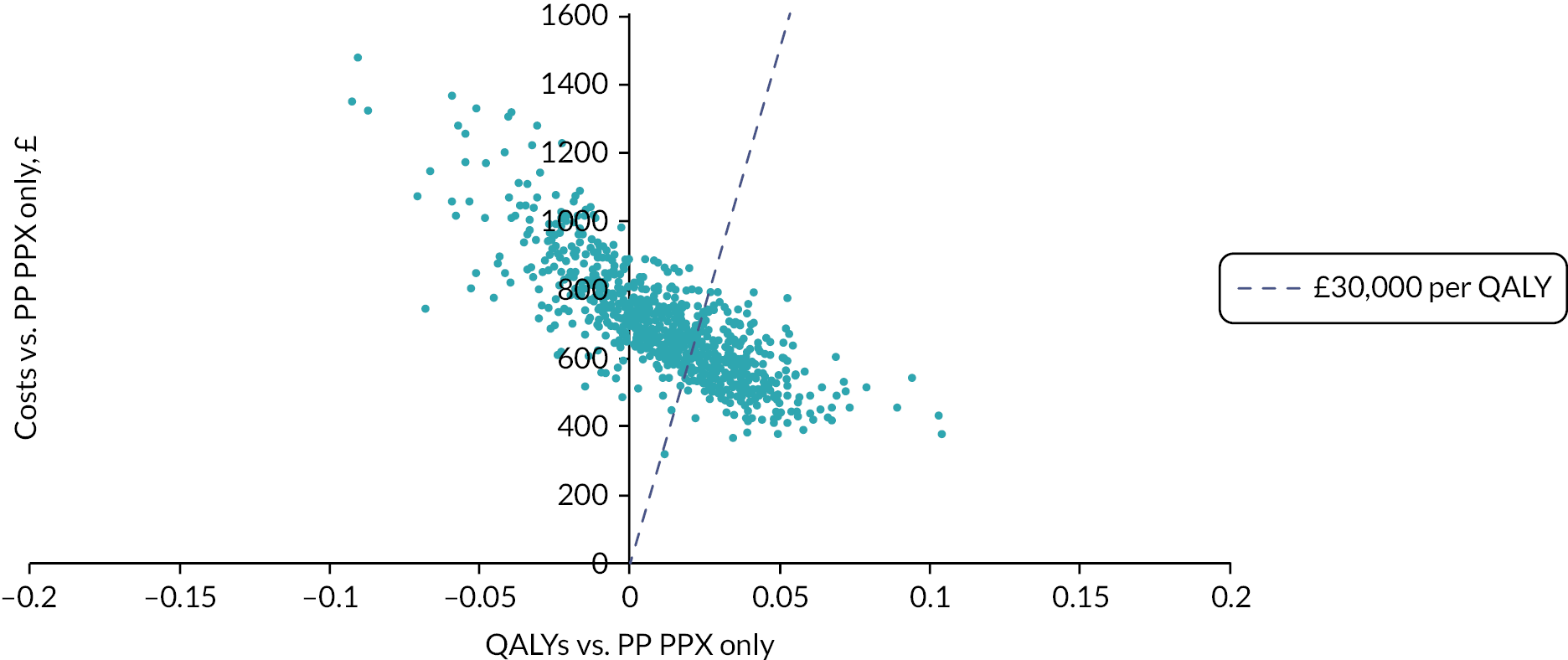
Due to this high degree of uncertainty regarding the optimal strategy, the overall EVPI associated with all parameters included in the PSA, when valuing a QALY at £30,000, was £1454 per patient. Therefore, the population EVPI over 5 years of births would be £21.8 million when taking into account that there are 640,000 births per year, and 0.5% of these are in women with a prior history of VTE. 15,117
Expected value of perfect parameter information was used to determine which individual parameters and groups of parameters were the greatest drivers of uncertainty regarding the optimal strategy. Full results for individual parameters are provided (see Appendix 5, Table 29), but the single most important parameter was the RR of VTE which accounted for 94% of the overall EVPI. Given that any study which provides additional evidence on the RR of VTE could also be used to capture additional information on the RR of bleeding, we estimated the EVPI for these two parameters, which was £1363 per patient, or £20.4 million over 5 years of births (see Appendix 5, Table 30). The remaining groups of parameters examined all had an EVPPI that was less than 1% of the total EVPI.
Deterministic scenario analyses for antepartum women with a prior venous thromboembolism
The deterministic scenario analyses were conducted to explore which model assumptions and inputs were key drivers of decision uncertainty. To do this, deterministic results were generated using midpoint parameter inputs when varying individual model inputs or assumptions. In the base-case deterministic analysis, the optimal strategy, when valuing a QALY at £30,000, was using the EThIG RAM to determine antepartum prophylaxis. The sensitivity of the model results to the various alternative assumptions and data inputs are expressed using INMB benefit for EThIG RAM compared to no prophylaxis. A negative INMB would mean that antepartum prophylaxis using the EThIG RAM has an ICER over £30,000 per QALY compared to a strategy of no prophylaxis.
Figure 10 shows the results for the scenario analyses that had the greatest impact on the INMB when comparing antepartum prophylaxis according to the EThIG RAM against a strategy of no PPX. (Full results for the deterministic scenario analyses are provided, see Appendix 6, Table 40.) It can be seen that none of the scenario analyses result in a negative INMB. Also, it can be seen that the factors that had the greatest impact were those related to PTS, patient characteristics, efficacy and safety of LMWH and the utility decrement associated with daily LMWH injections.
Figure 11 shows the deterministic scenario analyses when comparing antepartum prophylaxis according to the EThIG RAM against a strategy of postpartum prophylaxis only (PP PPX only). In this comparison, many of the same factors are important, but for three scenario analyses, the INMB was negative meaning the optimal strategy switched from antepartum prophylaxis according to the EThIG RAM to PP PPX only. This was true when assuming a lower utility decrement for PTS and in the two scenarios which assumed higher BMI (36 kg/m2), which affects the dosage and therefore the costs of prophylaxis.
In the base-case analysis for high-risk antepartum women, we have assumed that those identified as high risk according to the RAM receive antepartum prophylaxis from their booking appointment. However, the Lyon RAM actually recommends antepartum prophylaxis from 28 weeks for those with a score 3–6 and only recommends prophylaxis from booking in those with a score of 6 or more. As this will affect both the costs and efficacy of offering antepartum prophylaxis using the Lyon RAM, we have therefore conducted a scenario analysis to determine the impact of assuming that antepartum prophylaxis is deferred until 28 weeks. The results for this scenario analysis are provided in Figure 12. In this scenario, the Lyon RAM has an ICER of £83,144 per QALY compared to PP PPX only, which is less favourable than the deterministic ICER in the base case (£53,757 per QALY). This is because, although delaying prophylaxis to 28 weeks reduces the costs of prophylaxis, it also reduces the period of effective prophylaxis and therefore lowers the QALY gains and the cost savings. This scenario analysis suggests that the benefits of the Lyon RAM may be overestimated in the base-case scenario because it recommends a mixture of prophylaxis from booking and prophylaxis from 28 weeks gestation, but the base-case analysis assumes prophylaxis is given from booking in any patient with a Lyon score ≥ 3 as this is the cut-off for offering any antepartum prophylaxis.
FIGURE 12.
Deterministic scenario analysis for the Lyon RAM in women at high risk of VTE when assuming that antepartum prophylaxis is delayed until 28 weeks. (Reference for figure: Lyon. 32)
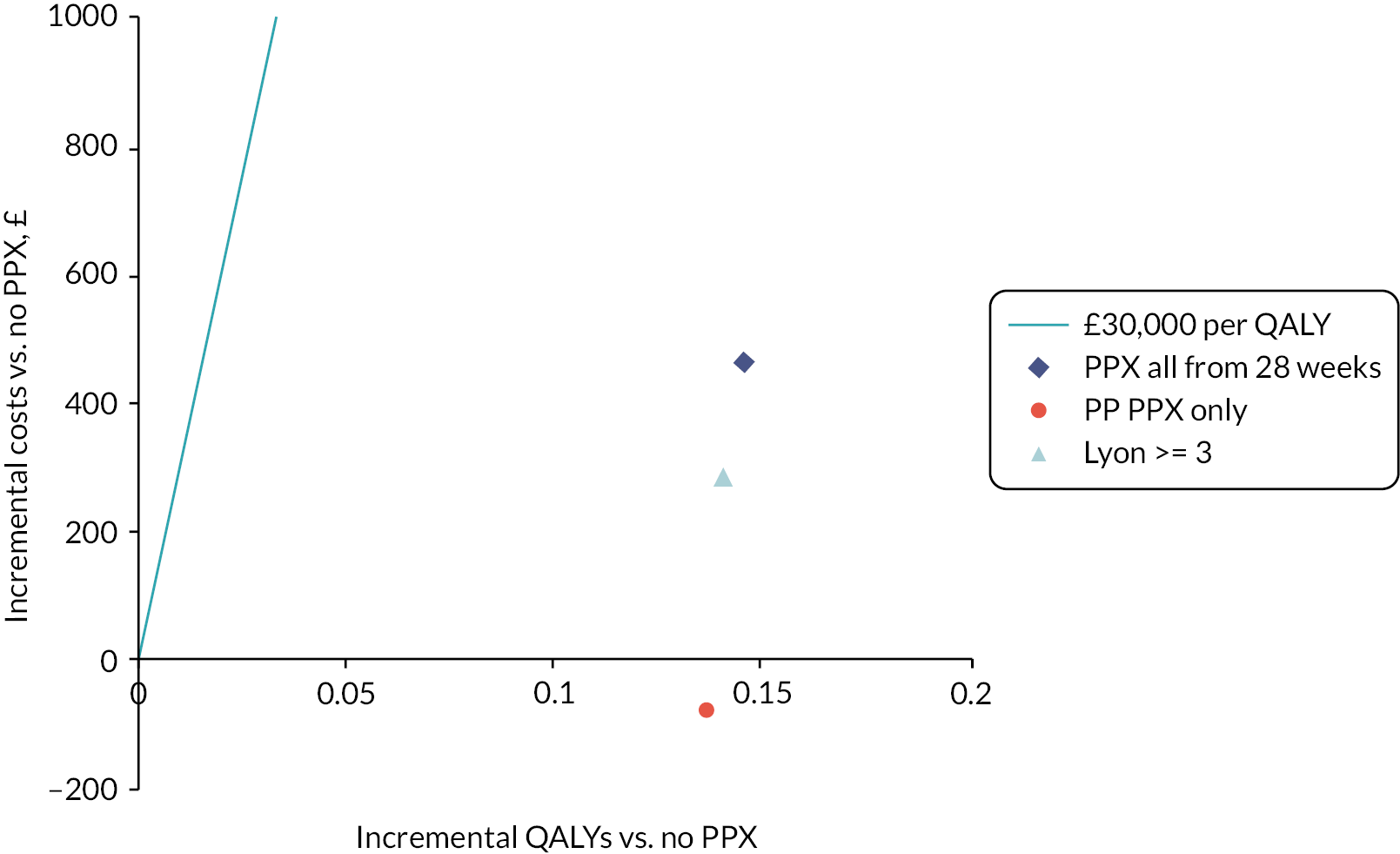
Given that there were uncertainties in the evidence used to determine the risk of VTE and major bleeding in the cohort of high-risk antepartum patients, a two-way scenario analysis was conducted to explore whether the optimal prophylaxis strategy would vary if the average risks of VTE and major bleeding were higher or lower. The results (see Appendix 6, Table 35) show that the optimal strategy would be to offer only postpartum prophylaxis if the VTE risk was under 10%. The results are not particularly sensitive to the risks of major bleeding, although the optimal strategy does change for higher bleeding risks (> 7%) when the VTE risk is in the range of 11–12%.
Unselected postpartum women
Deterministic base-case results for unselected postpartum women
The deterministic base-case results obtained when applying the midpoint parameter estimates to the base-case scenario for unselected postpartum women are presented in Figure 13. For the Sultan RAM, results are presented for various cut-offs defined according to the proportion of women defined as being high risk (e.g. top 1%) when using the Sultan calculator to determine absolute risk. The ICER for the SFOG RAM when using the sensitivity and specificity data reported by Lindqvist et al. 36 is £29,777 compared to no prophylaxis. However, this was a low-quality study, and this RAM did not perform as well in the higher-quality study conducted by Sultan et al. and using this performance data resulted in a deterministic ICER of £86,142 compared to no prophylaxis.
Probabilistic base-case results for unselected postpartum women
The PSA was run to compare the RCOG, SFOG and Sultan RAMs using performance data from the Sultan paper. This was chosen as it was the highest-quality study and was considered to provide the most robust estimates of performance for these three RAMs. Also, it had the benefit of estimating the performance of all three RAMs in the same cohort which minimises the risk of bias due to difference in the cohort characteristics or differences in the methods employed.
The results based on the mean outcomes from 10,000 probabilistic model runs are summarised in Table 9. It can be seen that the average QALY gains for all RAM-based strategies are now negative. This means that no PPX is the dominant strategy when incorporating uncertainty regarding the parameter inputs. Figure 14 shows the incremental costs and QALYs for using the Sultan RAM to offer prophylaxis to the top 1% of VTE risk versus no PPX. It can be seen that there is significant uncertainty regarding the incremental QALYs, with a 95% CI of −0.0011 to 0.0003 and 24% of PSA samples resulting in a negative incremental QALY gain.
| % PPX | Sensitivity (%) | Specificity (%) | Absolute costs, (£) | Absolute QALYs | Cost vs. no PPX, (£) | QALYs vs. no PPX | ICER vs. no PPX, (£) | ICER vs. next least effective strategy, (£) | INMB vs. no PPX at £20K, (£)a | INMB vs. no PPX at £30K, (£)a | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No PPX | 0 | 0 | 100 | 43.66 | 20.5549 | NA | NA | NA | NA | NA | NA |
| Sultan top 1% | 1 | 9 | 99 | 54.60 | 20.5549 | 10.94 | −0.0000 | Dominated | Dominated | −11.71 | −12.09 |
| Sultan top 5% | 5 | 27 | 95 | 59.84 | 20.5548 | 16.17 | −0.0001 | Dominated | Dominated | −18.59 | −19.79 |
| SFOG | 6 | 21 | 94 | 61.29 | 20.5548 | 17.63 | −0.0001 | Dominated | Dominated | −19.71 | −20.75 |
| Sultan top 10% | 10 | 36 | 90 | 66.24 | 20.5547 | 22.57 | −0.0002 | Dominated | Dominated | −26.01 | −27.73 |
| Sultan top 20% | 20 | 53 | 80 | 79.04 | 20.5546 | 35.38 | −0.0003 | Dominated | Dominated | −40.89 | −43.65 |
| Sultan top 25% | 25 | 60 | 75 | 85.41 | 20.5546 | 41.75 | −0.0003 | Dominated | Dominated | −48.08 | −51.24 |
| RCOG | 35 | 63 | 66 | 97.32 | 20.5545 | 53.65 | −0.0004 | Dominated | Dominated | −61.02 | −64.71 |
| PPX for all | 100 | 100 | 0 | 170.86 | 20.5542 | 127.20 | −0.0007 | Dominated | Dominated | −141.75 | −149.02 |
FIGURE 14.
Probabilistic base-case results for using the Sultan RAM (top 1%) vs. no prophylaxis (no PPX) in unselected postpartum women.
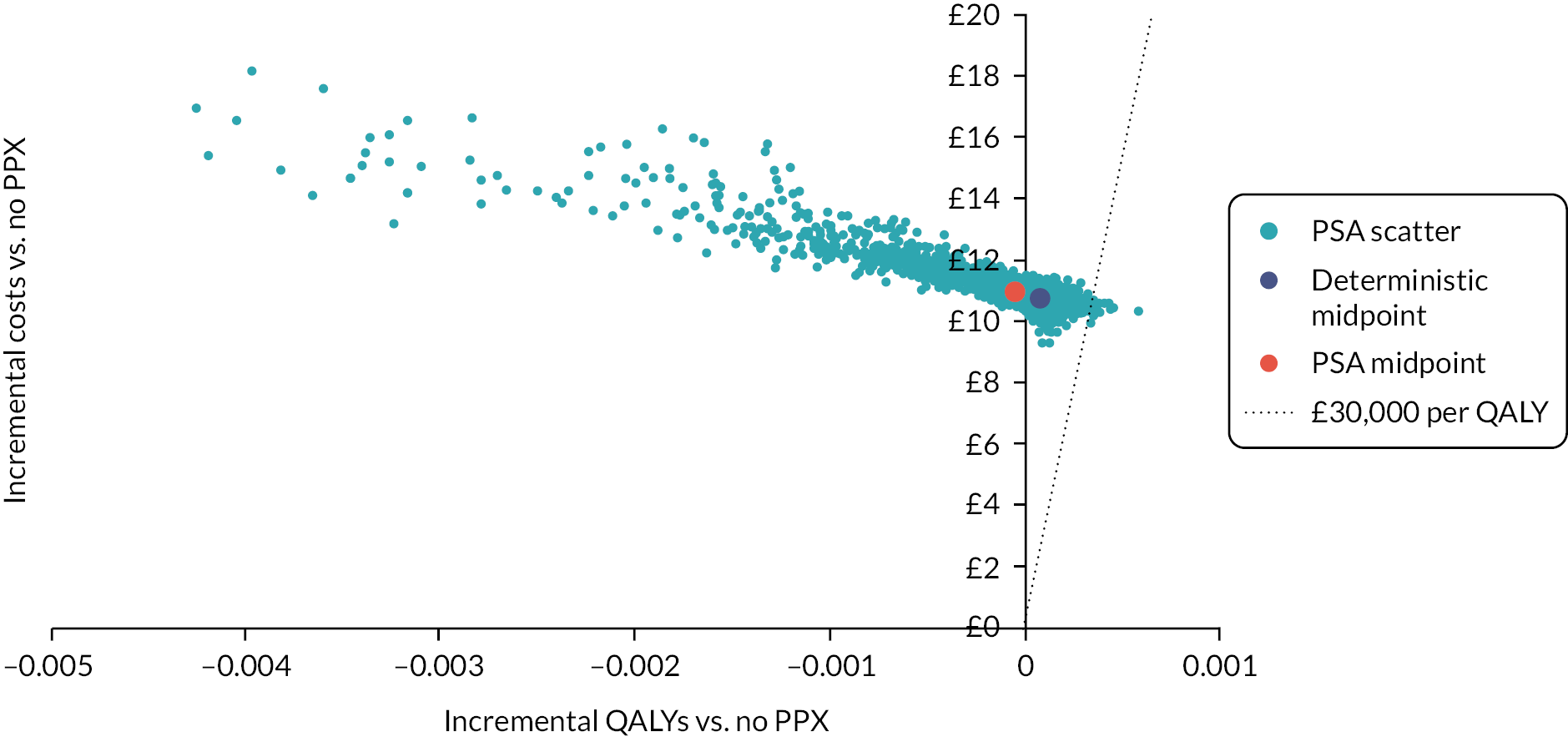
The CEAC for postpartum prophylaxis in unselected women is presented in Figure 15. When valuing a QALY at £30,000, a strategy of offering no prophylaxis has an 89% probability of being optimal, whereas all of the remaining strategies have less than a 10% chance of being optimal.
FIGURE 15.
Cost-effectiveness acceptability curve for prophylaxis strategies in unselected postpartum women.

The overall EVPI associated with all parameters included in the PSA when valuing a QALY at £30,000 was £0.68 per person. Although this is a small amount of EVPI per person, the amount across 5 years of births, assuming 640,000 births per annum,117 would be £2.0 million. No individual parameter had an EVPPI of more than £0.01 per person.
The broad spread of incremental QALY estimates appears to be driven by the uncertainty in the RR of VTE. However, in this case, there is not a large EVPI associated with this parameter as only 11% of the PSA samples resulted in a strategy other than no PPX being optimal (defined as having the maximum INMB when valuing a QALY at £30,000).
Deterministic scenario analyses for unselected postpartum women
Deterministic scenario analyses were conducted to explore which model assumptions and inputs were key drivers of decision uncertainty. To do this, deterministic results were generated using midpoint parameter inputs when varying individual model inputs or assumptions. In the base-case deterministic analysis, the optimal strategy, when valuing a QALY at £30,000, was no prophylaxis, but the strategy with the second highest INMB was offering prophylaxis to patients in the top 5% of VTE risk using the Sultan RAM. Therefore, the sensitivity of the model results to the various alternative assumptions and data inputs are expressed using INMB benefit for Sultan (top 5%) compared to no prophylaxis. A positive INMB would mean Sultan (top 5%) has an ICER of under £30,000 per QALY compared to a strategy of no prophylaxis, whereas a negative INMB would mean that no prophylaxis remains the optimal strategy as in the base case.
The 10 scenario analyses that had the greatest impact on the INMB are presented in Figure 16. (Full results for the deterministic scenario analyses are provided, see Appendix 6, Table 41.) It can be seen that the only one that resulted in Sultan (top 5%) having a positive INMB, and therefore an ICER under £30,000 per QALY, was when we assumed no cost for conducting the risk assessment. It should be noted that this also resulted in a positive INMB for Sultan (top 1%) but the INMB for Sultan (top 5%) was higher meaning that the latter would be the optimal strategy in this scenario. Other factors that appear to be important based on the deterministic scenario analyses were those related to PTS, patient characteristics, safety and efficacy of LMWH and finally the cost and utility impact of non-fatal non-ICH bleeding. The assumption regarding the duration of efficacy applied for 10 days of LMWH was fairly influential, but even assuming a full 6 weeks of efficacy, instead of the 3 weeks assumed in the base case, did not result in a positive INMB for Sultan (top 5%) compared to no prophylaxis.
A two-way scenario analysis was also conducted to determine how sensitive the conclusions are to the absolute risks of VTE and major bleeding. The results (see Appendix 6, Table 36) show that using a RAM to select patient for postpartum prophylaxis would be cost-effective (when valuing a QALY at £30,000) if the risks of VTE were higher than assumed in the base-case analysis. For example, an increase in VTE risk from 0.07% to 0.14% would mean that offering prophylaxis using the Sultan (top 5%) would be most cost-effective, but only if the risks of bleeding were 2–7%. Offering prophylaxis to a broader group, using Sultan (top 20%), would be optimal if the risks of VTE were 0.14% and the bleeding risks were under 2%. However, at the level of VTE risk assumed in the base-case scenario, the optimal strategy is not sensitive to the risks of major bleeding.
Obese postpartum women
Deterministic base-case analysis for obese postpartum women
Figure 17 shows the incremental costs and QALYs versus no prophylaxis (no PPX) for the subgroup of postpartum women with obesity as a specific risk factor. It can be seen that prophylaxis for all (PPX for all) would not be the optimal strategy in this population as the ICER is above £30,000 per QALY. In comparison, both versions of the RAM developed by Ellis-Kahana et al. have ICERs under £30,000 per QALY. The two RAMs presented by Ellis-Kahana differ in that one included thromboembolic disorder within the risk score (referred to as the full RAM) and the other excluded this specific risk factor.
FIGURE 17.
Deterministic base-case results for Ellis-Kahana RAM vs. no prophylaxis (no PPX) in obese postpartum women. (References for figure: Ellis-Kahan 2020. 41)
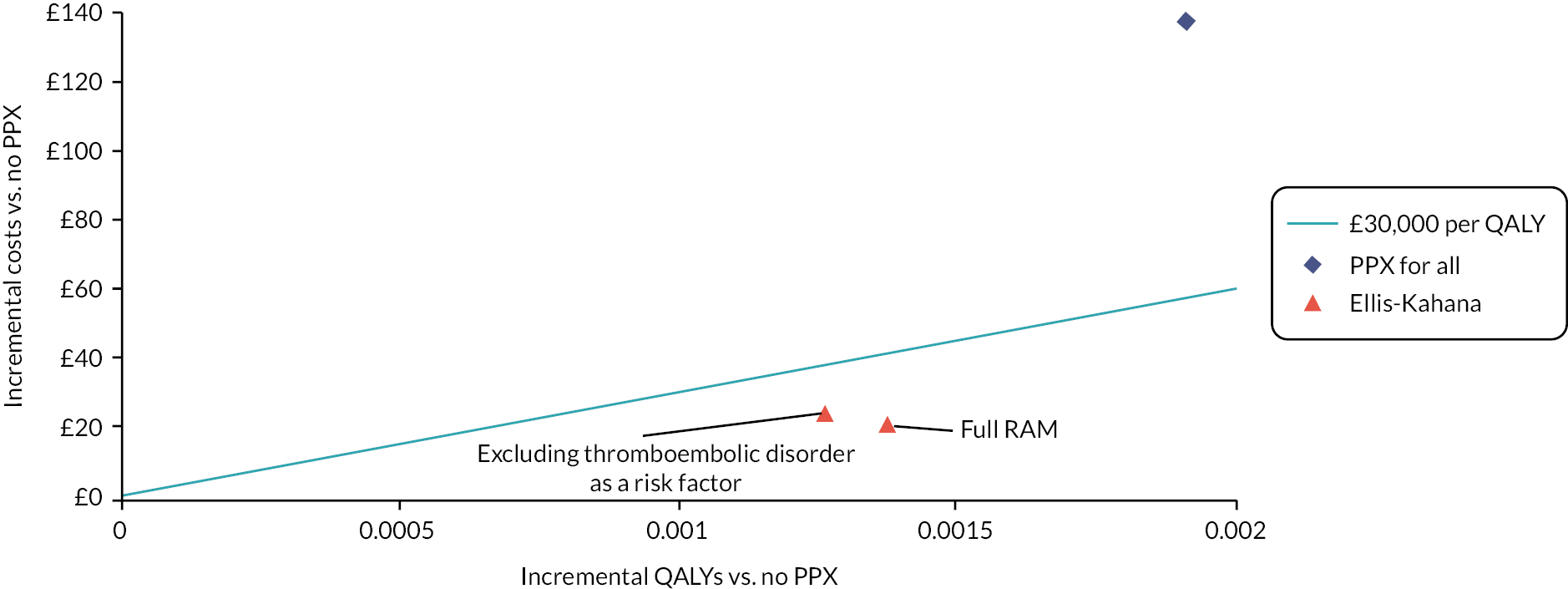
Probabilistic base-case analysis for obese postpartum women
In the PSA, we have compared the Ellis-Kahana RAM (full RAM) with a strategy of no prophylaxis. Results based on mean costs and QALYs are summarised in Table 10. Figure 18 shows the spread of incremental costs and QALYs on the cost-effectiveness plane for the Ellis-Kahana RAM (full RAM) compared to no prophylaxis. The mean QALY gain is negative (−0.0001), but there is a wide spread of incremental QALY estimates, with a 95% CI of −0.013 to 0.004. Therefore, although using the Ellis-Kahana RAM (full RAM) has an ICER under £30,000 versus no prophylaxis in the deterministic analysis, this strategy is dominated by no prophylaxis when using the mean outputs of the PSA as on average it has lower QALYs and higher costs (see Table 10). This is despite the fact that the ICER falls under £30,000 for 64% of the PSA samples. The CEAC for the alternative prophylaxis strategies in obese postpartum women is shown in Figure 19. This shows that the strategy of using the Ellis-Kahana RAM (full RAM) to determine postpartum prophylaxis in obese women has the highest probability (64%) of being the optimal strategy, when valuing a QALY at £30,000.
| % PPX | Sensitivity (%) | Specificity (%) | Absolute costs, (£) | Absolute QALYs | Cost vs. no PPX, (£) | QALYs vs. no PPX | ICER vs. no PPX, (£) | ICER vs. next least effective strategy, (£) | INMB vs. no PPX at £20K, (£)a | INMB vs. no PPX at £30K, (£)a | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No PPX | 0 | 0 | 100 | 49.01 | 20.552 | NA | NA | NA | NA | NA | NA |
| Ellis-Kahana (full RAM) | 10 | 62 | 90 | 73.20 | 20.552 | 24.20 | −0.0004 | Dominated | Dominated | −33.17 | −37.65 |
| Ellis-Kahana (excluding thrombophilia) | 12 | 58 | 88 | 76.44 | 20.552 | 27.43 | −0.0004 | Dominated | Dominated | −36.13 | −40.48 |
| PPX for all | 100 | 100 | 0 | 190.62 | 20.551 | 141.61 | −0.0010 | Dominated | Dominated | −162.27 | −172.59 |
FIGURE 18.
Probabilistic results for Ellis-Kahana RAM (full RAM including thromboembolic disorder) vs. no prophylaxis (no PPX) in obese postpartum women.
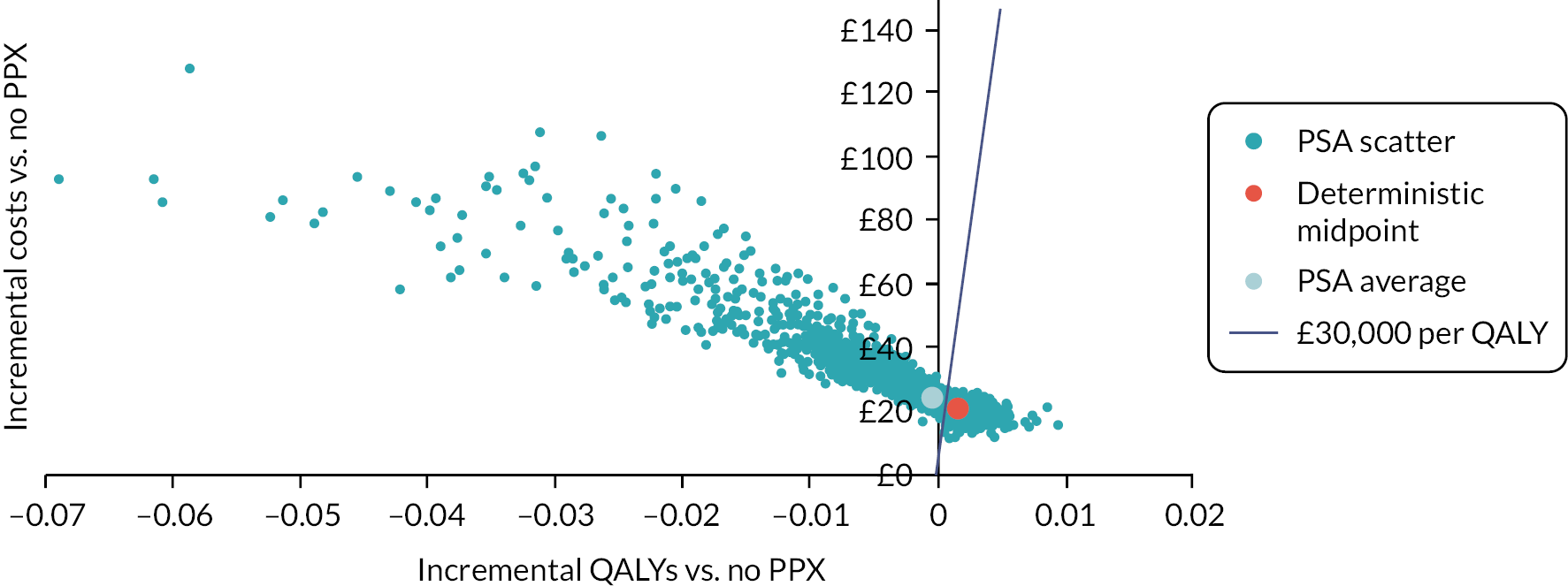
FIGURE 19.
Cost-effectiveness acceptability curve for prophylaxis strategies in obese postpartum women. (References for figure: Ellis-Kahana. 41)

The overall EVPI for this population when comparing these three prophylaxis strategies is £22.35 per patient. This would mean an overall EVPI of £13.4 million over 5 years of births117 when assuming that around 20% of pregnant women are obese. 118 The single most important individual parameter in the EVPPI analysis was the RR of VTE which had an EVPPI that was 99% of the overall EVPI, meaning that obtaining perfect information on this individual parameter would lead to an expected gain of £13.4 million over 5 years of births (see Appendix 5, Table 31). The EVPPI for both the RR of VTE and the RR of bleeding combined was similar (see Appendix 5, Table 32).
The broad spread of incremental QALY estimates appears to be driven by the uncertainty in the RR of VTE. In the population of obese postpartum women, there is a large EVPI associated with this parameter as the wide spread of incremental QALY gains, which is driven by uncertainty in the efficacy of LMWH to prevent VTE, results in the optimal prophylaxis strategy (defined as having the maximum INMB when valuing a QALY at £30,000) being uncertain.
Deterministic scenario analysis for obese postpartum women
The deterministic scenario analyses were conducted to explore which model assumptions and inputs were key drivers of decision uncertainty. To do this, deterministic results were generated using midpoint parameter inputs when varying individual model inputs or assumptions. In the base-case deterministic analysis, the optimal strategy when valuing a QALY at £30,000, was using the Ellis-Kahana RAM (full RAM). Therefore, the sensitivity of the model results to the various alternative assumptions and data inputs are expressed using INMB for Ellis-Kahana RAM (full RAM) compared to no prophylaxis. A negative INMB would mean that the Ellis-Kahana RAM has an ICER of over £30,000 per QALY compared to a strategy of no prophylaxis in that scenario, whereas a positive INMB would mean that using the RAM remains optimal as in the base case.
It can be seen from Figure 20 that the factors that have the largest impact on the INMB were related to PTS and the efficacy of 10 days of LMWH to prevent VTE over 6 weeks. However, only two of these scenarios result in a negative INMB and these were assuming a lower utility decrement for PTS and assuming no risk of PTS in asymptomatic DVT. Other factors that are moderately important are patient characteristics, the RR of major bleeding and cost and QALY implications of non-fatal, non-ICH bleeds, although the impact of these is smaller. Results for the deterministic scenario analyses are provided in full (see Appendix 6, Table 42).
The two-way scenario analysis (see Appendix 6, Table 37) demonstrates that the choice of optimal strategy is not particularly sensitive to the risk of major bleeding when the VTE risk is below 0.6%. However, a strategy of no PPX would be optimal if the VTE risk was 0.07%, similar to that in unselected postpartum women. This suggests that the difference in optimal strategy between this specific at-risk subgroup and the general postpartum population is the level of VTE risk.
Postpartum women after caesarean section
Deterministic base-case results for postpartum women after caesarean section
It can be seen from Figure 21 that neither the RCOG RAM nor the novel RAM reported by Binstock et al. 28 is cost-effective when used in postpartum women after caesarean section. This is because both these RAMs had poor specificity in the cohort reported by Binstock et al. and therefore result in over 90% of women being offered prophylaxis after caesarean section in the model. However, given that the risks of VTE are similar in the post-caesarean section group and the obese postpartum group, we considered it likely that a RAM with a better performance would be cost-effective in this subgroup. Therefore, we decided to include the Sultan RAM in the analysis for post-caesarean section women to explore whether a RAM with similar performance to the Sultan RAM would be cost-effective. The results should be interpreted with caution as the novel RAM reported by Sultan et al. 11 has been validated in a broad population of postpartum women and the sensitivity and specificity may be different in the specific subgroup of postpartum women with obesity. However, it can be seen that a RAM with performance similar to the Sultan RAM would need to select the 5% of patients with the highest risk of VTE for prophylaxis to have an ICER under £30,000.
Probabilistic base-case results for postpartum women after caesarean section
Based on the results of the deterministic analysis, we decided to include the Sultan RAM in the probabilistic analysis along with the RCOG and novel RAMs reported by Binstock et al. 28 This was to explore whether a RAM with performance similar to the Sultan RAM would be cost-effective in the post-caesarean section population. The mean outputs of the PSA are shown in Table 11, where it can be seen that no PPX dominates (i.e., has lower costs and higher QALYs than) all alternative strategies. This is due to the wide spread of incremental QALYs on the cost-effectiveness plane, which is shown in Figure 22 for the Binstock novel RAM and for the Sultan RAM (top 5%). For the Binstock RAM, 41% of PSA samples resulted in a negative incremental QALY gain compared to no prophylaxis, whereas for the Sultan RAM (top 5%) this occurred in only 24% of PSA samples. The CEAC in Figure 23 shows that no PPX had the highest probability of being optimal (57%) in the post-caesarean section population when valuing a QALY at £30,000.
| % PPX | Sensitivity (%) | Specificity (%) | Absolute costs, (£) | Absolute QALYs | Cost vs. no PPX, (£) | QALYs vs. no PPX | ICER vs. no PPX, (£) | ICER vs. next least effective strategy, (£) | INMB vs. no PPX at £20K, (£)a | INMB vs. no PPX at £30K, (£)a | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No PPX | 0 | 0 | 100 | 47.29 | 20.5527 | NA | NA | NA | NA | NA | NA |
| Sultan top 1%b | 1 | 9 | 99 | 58.28 | 20.5527 | 10.99 | −0.0000 | Dominated | Dominated | −11.93 | −12.40 |
| Sultan top 5%b | 5 | 27 | 95 | 63.59 | 20.5526 | 16.30 | −0.0001 | Dominated | Dominated | −19.06 | −20.44 |
| Sultan top 10%b | 10 | 36 | 90 | 70.05 | 20.5525 | 22.75 | −0.0002 | Dominated | Dominated | −26.62 | −28.55 |
| Sultan top 20%b | 20 | 53 | 80 | 82.95 | 20.5524 | 35.66 | −0.0003 | Dominated | Dominated | −41.72 | −44.75 |
| Sultan top 25%b | 25 | 60 | 75 | 89.36 | 20.5524 | 42.07 | −0.0003 | Dominated | Dominated | −49.01 | −52.48 |
| Binstock novel | 92 | 100 | 8 | 173.89 | 20.5522 | 126.60 | −0.0005 | Dominated | Dominated | −136.51 | −141.46 |
| RCOG | 94 | 100 | 6 | 176.40 | 20.5522 | 129.11 | −0.0005 | Dominated | Dominated | −139.66 | −144.94 |
| PPX for all | 100 | 100 | 0 | 175.21 | 20.5519 | 127.92 | −0.0008 | Dominated | Dominated | −143.40 | −151.14 |
The overall EVPI was £7.74 per patient, which is equivalent to £5.6 million over 5 years of births117 taking into account that 24% of births are by elective or emergency caesarean section. 55 The parameter with the highest EVPPI was the RR of VTE, which has an EVPPI equivalent to 68% of the overall EVPPI (see Appendix 5, Table 33). This is equivalent to £3.8 million over 5 years of births. No other individual parameter had significant EVPPI (i.e. none >£1 per person). An analysis exploring the EVPPI for various groups of parameters is reported in full (see Appendix 5, Table 34). The EVPPI for both the RR of VTE and the RR of bleeding is estimated to be £5.47 per person or £4.0 million over 5 years of births. All other groups of parameters had an EVPPI that was much lower (≤£1 per person).
If the Sultan RAM is excluded from the analysis, the overall EVPI is lower at £2.06 per person, but none of the individual parameters have significant EVPPI (i.e. none >£1 per person). This is because the optimal strategy is less uncertain with no PPX having a 93% probability of being optimal (i.e. maximising INMB when valuing a QALY at £30,000) when compared to PPX for all and prophylaxis using either the novel Binstock RAM or the RCOG RAM.
In the population of postpartum women who have had a caesarean section, there is significant EVPPI associated with the RR of VTE but only when assuming that a RAM that performs similarly to the Sultan RAM is available. When assuming that only the RCOG or Binstock novel RAMs are available in the post-caesarean section population, the uncertainty regarding the optimal strategy (defined as having the maximum INMB when valuing a QALY at £30,000) is much lower and choice of optimal strategy is less sensitive to the uncertainty regarding the efficacy of LMWH in preventing VTE.
Deterministic scenario analyses for postpartum women after caesarean section
We decided to use the Sultan RAM (top 5%) to explore the sensitivity of the model to the various assumptions and data sources as this strategy was the only strategy with an ICER under £30,000 per QALY in the deterministic analysis. Therefore, in the scenario analyses, if the INMB becomes negative, this means that the Sultan RAM (top 5%) has an ICER over £30,000 per QALY compared to no prophylaxis and the optimal strategy has changed to become no prophylaxis.
The optimal strategy is sensitive to many assumptions because the ICER for using the Sultan RAM (top 5%) compared to no prophylaxis is £29,281 per QALY meaning that factors that have a small impact on the costs and benefits have the potential to change the optimal strategy (see Appendix 6, Table 43 for full deterministic scenario analysis results). However, it can be seen in Figure 24 that factors related to PTS are again important drivers of the INMB with a lower utility decrement for PTS and a lower incidence of PTS resulting in no prophylaxis becoming the optimal strategy. The results are also particularly sensitive to the assumptions regarding whether the efficacy of LMWH is applied for 10 days or 6 weeks rather than the 3 weeks assumed in the base case.
We also conducted the deterministic scenario analyses for the Binstock Novel RAM compared to no prophylaxis, but none of the scenarios explored resulted in the Binstock novel RAM having an ICER under £30,000 per QALY. The same was true when we used the data from Binstock for the RCOG RAM in the post-caesarean section population.
The two-way scenario analysis (see Appendix 6, Table 38) demonstrates that the VTE risk would need to be much higher for the Binstock novel RAM to be more cost-effective (when valuing a QALY at £30,000), than using a RAM with performance similar to the Sultan RAM. In addition, the risk of VTE would need to be similar to that observed in the unselected postpartum population (0.07%) before no prophylaxis became the optimal strategy. Also, if the risk of VTE was above 0.5%, then the optimal strategy would depend on the risk of major bleeding. For example, prophylaxis for all would be optimal if the risk of bleeding was lower than assumed in the base case and prophylaxis using the Binstock RAM would be optimal if the bleeding risk was similar to that assumed in the base case.
Exploratory analyses for antepartum women
Exploratory deterministic analysis for unselected antepartum women
The deterministic results for the STRATHEGE RAM31 are shown in Figure 25 alongside various theoretical combinations of sensitivity and specificity. These theoretical combinations are provided to explore the trade-off between sensitivity and specificity that would be required for a RAM to achieve a cost per QALY under £30,000, when being used to determine antepartum prophylaxis in an unselected cohort. It can be seen from Figure 25 that the poor sensitivity (0%) of the STRATHEGE RAM,31 which results in 2% of women having antepartum prophylaxis, results in negative QALYs compared with no prophylaxis but at additional cost and it is therefore dominated by a strategy of offering no prophylaxis. From the theoretical combinations of sensitivity and specificity explored, it can be seen that a high degree of specificity would be required for a RAM used in this population, with a specificity of 90–95% being required for a RAM whose sensitivity is between 100% and 53%, respectively.
FIGURE 25.
Deterministic results for using the STRATHEGE RAM or a theoretical RAM (shown for various combinations of sensitivity and specificity) compared to no prophylaxis (no PPX) in an unselected antepartum cohort. (References for figure: STATHEGE. 31)
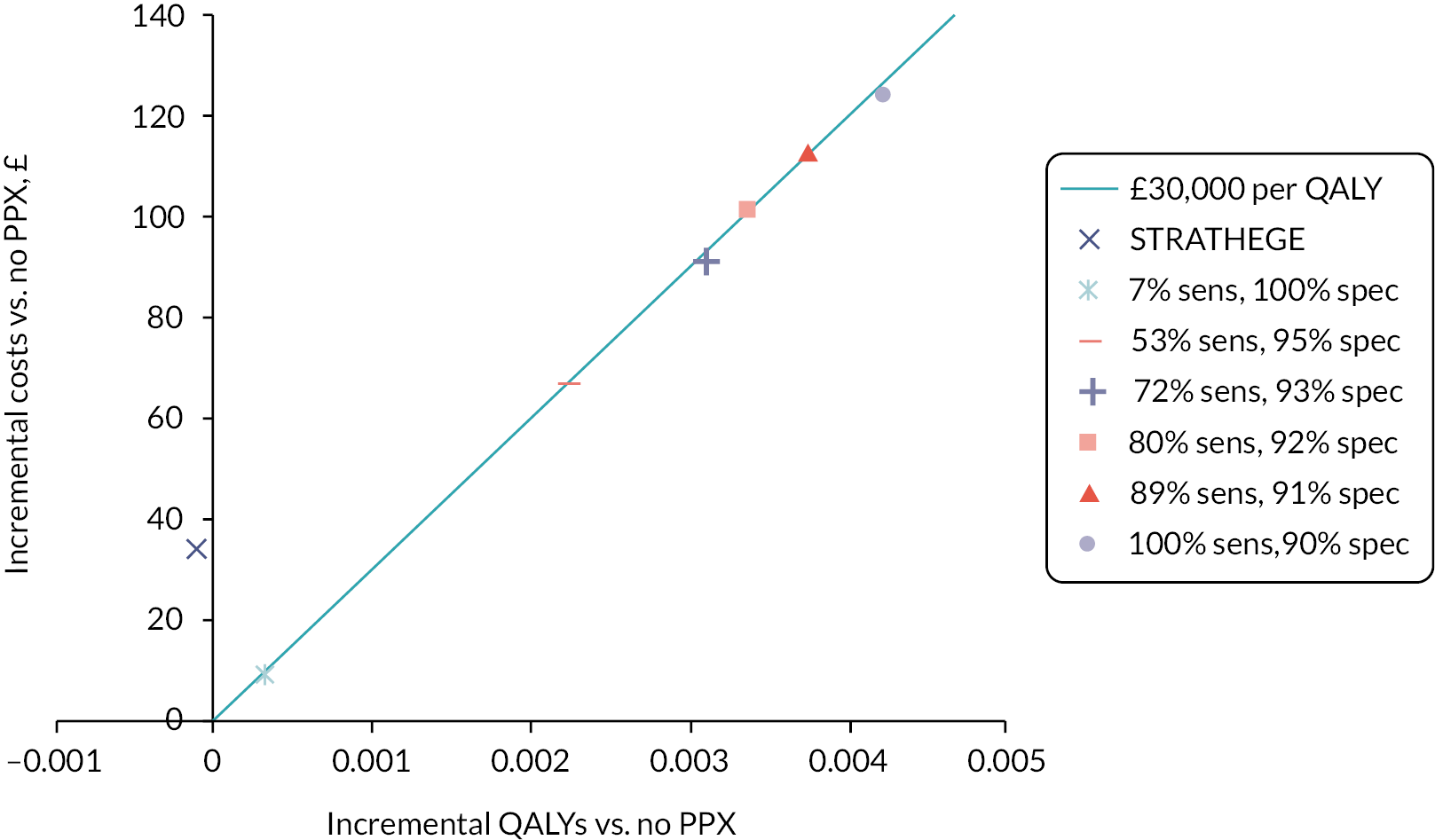
Exploratory deterministic analysis for antepartum women with three risk factors
Antepartum women with three risk factors are currently offered antepartum prophylaxis from 28 weeks gestation according to the RCOG guidance (provided none of the risk factors is prior VTE or another risk factor that warrants earlier prophylaxis). 7 Any woman offered antepartum prophylaxis within RCOG is then eligible for 6 weeks of postpartum prophylaxis. As we were unable to obtain an exact estimate for absolute VTE risk in the group with three antepartum risk factors (see Model inputs for secondary scenarios where antepartum prophylaxis is offered at 28 weeks), we have conducted an exploratory analysis to determine the optimal strategy in this group across differing levels of VTE and bleeding risk. The strategy of prophylaxis from 28 weeks gestation (followed by postpartum prophylaxis for 6 weeks) is compared against a strategy of offering no prophylaxis at all and a strategy of offering no antepartum prophylaxis but assuming that all women will receive 6 weeks of postpartum prophylaxis. The results (see Appendix 6, Table 39) show that for the level of bleeding risk assumed in the base case (4.58%), an absolute risk of VTE of > 0.5% would be required for 6 weeks of postpartum prophylaxis for all to be optimal. A more precise threshold analysis identified that 6 weeks of postpartum prophylaxis had an ICER under £30,000 compared to thromboprophylaxis for none only when the risk of VTE was > 0.57%. We estimated in section Model inputs for secondary scenarios where antepartum prophylaxis is offered at 28 weeks that the upper limit of VTE risk in antepartum women with three risk factors (excluding a prior VTE) was likely to be around 0.6%. This exploratory analysis suggests that offering 6 weeks of postpartum prophylaxis to women with three antepartum risk factors would only be cost-effective if the absolute risk in this group is at the higher end of the expected range. In addition, offering antepartum prophylaxis from 28 weeks is unlikely to be cost-effective in this group. These findings only apply to women where none of the three risk factors are a prior VTE or another risk factor that currently qualifies the woman for prophylaxis from booking under the RCOG guideline as these women were excluded when calculating the absolute risks.
Summary of key findings
-
In high-risk antepartum women, such as those with a prior VTE, prophylaxis with LMWH reduces the risk of both symptomatic VTE and a serious adverse outcome (fatal PE, fatal bleed, ICH). These benefits outweigh the increased risks of other major bleeding even when offering antepartum prophylaxis from booking to all.
-
In high-risk antepartum women, there is considerable uncertainty regarding the most cost-effective prophylaxis strategy, and this is largely due to uncertainty in the effectiveness of LMWH for preventing VTE in this population (i.e. the RR of VTE).
-
In unselected postpartum women, the risks of VTE are low and the benefits of preventing VTE are not clearly outweighed by the additional risks of major bleeding.
-
In unselected postpartum women, none of the prophylaxis strategies compared were likely to be cost-effective compared to offering no prophylaxis, and the choice of optimal prophylaxis strategy is not particularly sensitive to any of the uncertainties in the parameter inputs.
-
In the subgroup of obese postpartum women, the uncertainty regarding the optimal prophylaxis strategy is greater than in the unselected group, because the risks of VTE are slightly higher than in the unselected postpartum group and because the RAM developed for obese postpartum women (Ellis-Kahana) performs slightly better than the RAMs available for unselected postpartum women (Sultan, RCOG, SFOG).
-
In the subgroup of obese postpartum women, the majority of the uncertainty regarding the most cost-effective prophylaxis strategy is related to the uncertainty in the RR of VTE for LMWH compared to no prophylaxis.
-
In postpartum women who have had a caesarean section, the available RAMs with performance data in this population (RCOG and Binstock novel) have poor specificity and the most cost-effective strategy is likely to be prophylaxis for none when considering only those RAMs validated in women having caesarean section.
-
If we assume that a RAM can be developed for women who have had caesarean section, which performs similarly to the Sultan RAM in the unselected postpartum population, then there would be significant uncertainty regarding the most cost-effective prophylaxis strategy in women following caesarean section and most of that uncertainty would relate to the RR of VTE.
-
The deterministic scenario analyses suggest the impact of PTS on quality of life is fairly important in determining the optimal prophylaxis strategy for both antepartum and postpartum prophylaxis.
-
The deterministic scenario analyses also suggest that assumptions regarding the risk of PTS in those with asymptomatic DVT, the cost of risk assessment and the duration of efficacy assumed for 10 days of LMWH are important in the postpartum population.
-
For a RAM to be cost-effective for use in an unselected antepartum population, it would need to have high specificity (specificity of 90–95% for sensitivity of 100–53%).
-
Offering antepartum prophylaxis from 28 weeks to women with three antepartum clinical risk factors (excluding prior VTE) as per current RCOG guidance is unlikely to be cost-effective.
Chapter 5 Stakeholder perspectives of recruitment to future trials of thromboprophylaxis
Introduction
The VTEP study aims to identify potential future studies that may help reduce decision uncertainty when prescribing thromboprophylaxis in pregnancy and the puerperium. However, previous studies have struggled to recruit pregnant patients to trials, and there are a number of factors that affect whether pregnant patients are willing to participate in research studies, including perceptions of risk and inconvenience factors. 119,120 In order to increase the value of information from the literature review and modelling phase of the study, we explored stakeholder perspectives of potential future studies. We aimed to understand the views of pregnant women with experience of being offered thromboprophylaxis and clinicians managing these patients to understand the acceptability of any potential future primary research. More specifically, we aimed to understand how clinicians and pregnant women would feel about recruiting to and being recruited to future RCTs, barriers and enablers to recruitment and views on different trial designs (individual vs. cluster RCTs).
Workshops with women with experience of venous thromboembolism or prophylaxis in pregnancy or the puerperium
We undertook workshops with two groups of people who had been offered LMWH in pregnancy or the puerperium:
-
women who have experienced DVT or PE during pregnancy or within 6 weeks after delivery;
-
women who have been offered thromboprophylaxis during pregnancy or within 6 weeks after delivery but have no prior VTE.
The workshops were conducted in accordance with the methods outlined in the project protocol (version 1.0), which can be accessed https://fundingawards.nihr.ac.uk/award/NIHR131021 (accessed February 2023).
Ethical approval
We obtained University of Sheffield Ethics approval (University of Sheffield 038511) in March 2021 to undertake the workshops and survey. Due to recruitment being via special interest groups or professional organisations rather than recruitment via the NHS, we did not require NHS ethics approval.
Workshop recruitment
We approached a number of national special interest groups that represent diverse cultural and socioeconomic backgrounds to try to recruit a wide range of participants for workshops, with a particular focus on identifying people from a range of ethnic backgrounds. We initially approached Thrombosis UK, FiveXMore, Katie’s Team (an East London women’s health research patient and public advisory group), Maternity Voices Partnership (Bristol) and the Public Health Inequalities Group research group at City University. Groups sent out invitations via social media or e-mail distribution lists. We also advertised the study on Twitter™, tagging in the above organisations.
We received 28 initial responses for the prior VTE group (principally through Thrombosis UK) and 18 initial responses for the no prior VTE group in total. We e-mailed information sheets and consent forms to respondents, along with a list of proposed dates and a survey of basic demographic details to enable us to select as wide a group of participants as possible. We initially selected a group of 12 participants to invite to the prior VTE workshop but then expanded the invitation to the whole group due to participants not responding further. Despite reminders, we only received enough responses for an initial low-risk group workshop of six participants. We undertook this workshop in December 2021, and then after discussion with the project management group, we decided to run a further study with people who were at lower risk and who would not necessarily require anticoagulants in future pregnancies. We advertised the study further with Action on Pre-Eclampsia, National Childbirth Trust and the hyperemesis gravidarum charity Pregnancy Sickness Support.
We initially intended to offer an option of face-to-face or online workshops, but due to the ongoing COVID-19 pandemic, we offered only online workshops. Workshops took place via the online Google MeetTM video conferencing system in November 2021 to January 2022 and lasted between 1.5 and 2 hours. Participants were sent a £50 shopping voucher after the workshop.
The workshop topic guide was developed after discussions with the project management group, particularly the patient and public involvement (PPI) lead (RC). Workshops were run by a single facilitator, with another member of the research team present to monitor the recording, take notes and let people in and out of the workshop.
The facilitator explained the background of the project and then asked questions using a broad topic guide. Participants were asked to talk about their background, how they were told they would need blood thinners, how risks and benefits were communicated, their experiences of taking blood thinners and asked for their thoughts about being recruited to a trial of blood thinners or no blood thinners during pregnancy (see Appendix 7 for topic guide). The facilitator tried to ensure every respondent addressed each of the broad topics where time allowed. The facilitator summarised findings throughout the workshop to clarify understanding and allow participants to correct misunderstandings. Workshops were recorded so that the research team could take detailed notes/transcripts. Transcripts were read and reread, then analysed using a broad thematic approach according to the principles of Braun and Clarke,121 with a focus on understanding the influences on future trial participation.
We recruited a total of 22 women over 4 workshops: 2 high risk (n = 7, n = 3), 2 low risk (n = 6, n = 6). Participants are detailed in Table 12.
| Participant ID | Age group, years | Ethnicity | Education | Employment | Previous DVT/PE | Reason for attending |
|---|---|---|---|---|---|---|
| W1P1 | 34 | White/Caucasian | Full-time employment (currently on maternity leave) | Professional qualification after bachelor’s degree | Both | PE and DVT during pregnancy. No known risk factors |
| W1P2 | 55+ | White/Caucasian | Full-time employment | Doctorate degree | PE | VTE after pregnancy. Factor V Leiden |
| W1P3 | 28 | White/Caucasian | Student | Bachelor’s degree | Both | Previous recurrent VTE |
| W1P4 | 35–44 | White/Caucasian | Student | Master’s degree | Both | Previous recurrent VTE and recurrent miscarriage. First-degree relative previous DVT |
| W1P5 | 30 | White/Caucasian | Part-time employment | Associate degree | Both | PE and DVT during pregnancy. No known risk factors |
| W1P6 | 35–44 | White/Caucasian | Part-time employment | High school/college graduate, diploma or equivalent | PE | PE during pregnancy no 3. No known risk factors |
| W1P7 | 35–44 | Asian/Asian British | Part-time employment | Bachelor’s degree | PE | Bilateral PE during pregnancy with second pregnancy. No known risk factors |
| W2P1 | 32 | White/Caucasian | Full-time employment (currently on maternity leave) | Bachelor’s degree | PE | Previous PE, factor V Leiden diagnosedpre pregnancy |
| W2P2 | 35–44 | Asian/Asian British | Full-time employment | Professional qualification after bachelor’s degree | DVT | Thromboprophylaxis post caesarean section for first pregnancy. DVT during second pregnancy |
| W2P3 | 34 | White/Caucasian | Part-time employment | Bachelor’s degree | DVT | DVT during pregnancy |
| W3P1 | 35–44 | White/Caucasian | Part-time employment | High school/college graduate, diploma or equivalent | None | Prescribed thromboprophylaxis for recurrent miscarriage |
| W3P2 | N/A | N/A | N/A | N/A | None | Caesarean section. High BMI |
| W3P3 | N/A | N/A | N/A | N/A | None | Caesarean section due to gestational diabetes. High BMI |
| W3P4 | 35–44 | White/Caucasian | Unemployed (not looking for work) | Bachelor’s degree | None | Blood loss, pre-eclampsia |
| W3P5 | N/A | N/A | N/A | N/A | None | Blood loss |
| W3P6 | 45–54 | White/Caucasian | Full-time employment | Doctorate degree | None | Factor V Leiden |
| W4P1 | 35–44 | White/Caucasian | Part-time employment | Master’s degree | None | Postpartum thromboprophylaxis. Pre-eclampsia, caesarean section with twins |
| W4P2 | 35–44 | White/Caucasian | Full-time employment | Master’s degree | None | Given thromboprophylaxis during and after pregnancy |
| W4P3 | 45–54 | White/Caucasian | Full-time employment | Doctorate degree | None | Postpartum thromboprophylaxis after caesarean sections (1 emergency and pre-eclampsia, 1 elective) |
| W4P4 | 35–44 | White/Caucasian | Full-time employment | Master’s degree | None | IVF, significant blood loss and sepsis |
| W4P5 | 45–54 | White/Caucasian | Part-time employment | Bachelor’s degree | None | Postpartum thromboprophylaxis due to age > 40 |
| W4P6 | 35–44 | White/Caucasian | Part-time employment | Master’s degree | None | No details. Given thromboprophylaxis for both pregnancies |
Workshop findings
We identified six themes that may impact on future recruitment to clinical trials.
-
Pregnant women receive limited information about VTE or risks and benefits of thromboprophylaxis during pregnancy or postpartum.
Participants described receiving little information about VTE or thromboprophylaxis in terms of either risk of VTE during pregnancy, understanding why they had been given anticoagulants (particularly for low-risk participants) or the risks and benefits of treatment. For participants who had pre-existing conditions, some had investigated their treatment and identified the need for thromboprophylaxis prior to being asked to take them by a healthcare professional. However, although the majority of participants had some general awareness about DVT and PE, it was not considered to be something that was spoken about or discussed as part of their maternity care and participants without pre-existing conditions that increased their risk of VTE could recall little or no discussion of the increased risk of VTE during or after pregnancy. This lack of information was perceived to be a potential barrier to participation in a trial.
W1P6: I had no idea, I didn’t really understand any of it, it was ticked off in your book, it wasn’t really spoken about openly and I think if more people knew more about it and the risks involved, they would much more likely take part in a trial.
W1P1: I mean there is some talk of VTE, but in pregnancy I don’t think, it didn’t come across as a real risk to me and I definitely didn’t know that I’d be almost permanently disabled because of the clot that wasn’t treated properly.
W2P3: No-one has the time to talk to you in pregnancy and I hardly had no more than 10 minutes with the midwife during pregnancy before the DVT. [ … ] I didn’t even know blood clots could happen during pregnancy at that point.
Participants in the low-risk groups in particular recalled little to no discussion of risks and benefits of anticoagulants. Participants took anticoagulants because they had been told to, but often did not understand why they had been prescribed them and made their own assumptions about the rationale behind their treatment. Notably, few participants recalled any discussion of risks associated with thromboprophylaxis.
W2P2: After surgery, they just said ‘Here’s a Paracetamol and here’s an injection’. And sent me home with a bag of injections and told to do it.
W3P3: I’m not aware of the risks, so no one’s explained it to me, and I didn’t feel that there was much point searching for it after being on it for a while, so that was it.
W3P4: I was on the blood-thinning injections for ten days after giving birth, and I, to me it was because I lost, I lost 1.5 litres of blood and I had pre-eclampsia, so they’re the reasons I believe that I was put on it, but I wasn’t actually told why I needed them, I was just discharged with them, and I didn’t really question it.
This lack of understanding of risk factors or rationale behind treatment may affect compliance with treatment. Some participants described stopping treatment early because they did not understand why they had been prescribed thromboprophylaxis, or because they did not understand why their treatment duration differed from previous pregnancies or from peers.
W2P2: The first time round I just took the injections because I was told to, but if I missed I wasn’t really bothered.
W3P4: […] I probably did about 7 injections in total, including the two in hospital. So I missed three basically. (Int: Right. And did you understand why you were doing it?) W3P4: No. I think that’s probably why I didn’t really continue.
W4P6: But, for this time, it seemed much, much longer and it seemed like it was they’d said almost like a month for the blood thinners and the injections which I was really upset out because you know ten days is bad enough but to keep injecting yourself for a month without any kind of explanation as to why the time had increased. On reflection, I had lost a lot of blood during the birth, during the surgery so I was put two together myself there. However, I stopped after ten days because just the stress of having to it with two little ones running round was just too much and I didn’t have any blood pressure issues.
Women described information seeking and doing their own independent research to understand why they had been given thromboprophylaxis. Others described how they sought information on the internet or using forums such as Thrombosis UK.
W2P3: I was sent home with injections and after I went home and it all sunk in, I realized what the impact of that was, not understanding what that meant for the pregnancy. It took a lot of researching you know by myself.
-
Pregnant women who had previously received thromboprophylaxis accepted current prescribing practice and perceived potential future trials to be withholding treatment.
Some high-risk participants, such as those who were on long-term thromboprophylaxis, felt that taking part in a trial with a placebo option was not an option for them as a placebo was not a feasible option; ‘I couldn’t choose not to take the blood thinners’ (W1P7). Although reporting limited knowledge about risks and benefits associated with thromboprophylaxis, they perceived the risk factors to be a reason why they needed treatment and would not welcome taking part in a trial.
W1P1: I had a risk factor in that Dad had DVT but if I didn’t have enough risk factors to qualify then I would have been happy to partake in a trial because I wouldn’t have had it anyway so I’d have been happy to take part in a trial to reduce my risk. If I have placebo I’m no worse off than I would have been. But now, knowing what I know now there’s no way that I wouldn’t be taking clexane.
W1P2: How ethically can you deny someone a treatment if they have an identified risk factor?
W3P5: So from my perspective because of what happened because of how traumatic it was, I wouldn’t have said I’d take a trial, and maybe have it, I think just accepted that what they were giving me was what I needed to have.
Thromboprophylaxis was perceived as potentially life-saving and the prescription of thromboprophylaxis accepted as best practice. Participants from all groups struggled to understand the concept of a poor evidence base underpinning current guidelines and perceived the introduction of a trial as removing current best practice rather than offering a choice of treatments where the current evidence is unclear. They perceived receiving the placebo as a risk and would take part in a trial when they saw it as an opportunity to obtain a treatment that would otherwise be withheld.
W4P1: Of course I took that [thromboprophylaxis], but if somebody mentioned the word trial to me, I would have said no, cos I would not have put anything at risk for me or my children. And I say, that feels quite uncomfortable for me to say a flat out no cos I’m not usually a no person, but I feel in this situation I would have said no.
W3P2: I would definitely take it (LMWH rather than be in a trial), 100%. I think if it can’t harm you what’s the harm in doing it?
W3P6: I’ve been really anxious. If it had been a choice of you know, ‘you’re not going to get them, but if we put you on a trial there is a fifty-fifty chance you’ll get them, or placebo’, then I’d have gone for it, but if it was a case of ‘you can have them or you can go into a trial’, I would definitely have wanted them, because of my anxiety around being in a you know, over coagulated state not having the anticoagulants.
Even when not fully understanding reasons for needing the treatment, most participants complied with the treatment and did not question whether it had been prescribed appropriately. They appeared to be passive recipients of the treatment and even when they did not fully understand the rationale behind why they had been prescribed thromboprophylaxis, they complied despite being unhappy about it.
W4P3: I don’t really understand the mechanisms other than, you know, ok a blood clot can be very serious so, you know, there wasn’t, I didn’t really feel around that point that I knew enough to challenge or to refuse. But, I also I didn’t really feel like I wanted to, I just sort of was resigned to have to do this, you know, unpleasant thing for a while.
W4P6: [ … ] the resentment and resignation are the two sort of words that really spring out to me in my experience, the kind of resentment of feeling sort of done to and the just being resigned to just having to do it, so they just resonated with me.
W3P5: I prefer not to have done it, I didn’t really like having to have to do injections, I didn’t enjoy it, but you know, I just did it. Got on with it.
-
Negative experiences associated with injections were minimised by healthcare practitioners but may increase likelihood of attrition.
Participants spent a significant amount of time discussing the side effects of the injections which they felt would prevent adherence to active treatment for people who had not had previous experience of VTE. They felt that healthcare professionals greatly underestimate the negative impact of undertaking the injections and minimise the problems associated with the pain and discomfort of the injections themselves, as well as the significant bruising or lumps on injection sites.
W1P3: My experience has been that clinical staff involved don’t necessarily understand what the injections are so the midwives have been ‘oh yes, it’s just a bit stingy’.
W1P4: I found a lot of people who administer these injections have got no idea. They just go (mimics giving injection) and then you get that burn. It takes a while to know how to do it and you don’t need to have that burn at all if you know how to do it.
For some high-risk participants who valued the LMWH injections as a more acceptable alternative to warfarin due to the difficulties in moderating international normalised ratio (INR), the injections were difficult but welcomed as an opportunity to ‘keep them safe’. For others, although a minority did not struggle with the injections (notably those who had to inject for other reasons), many described feelings of resentment and hating the experience of doing the injections, finding the process of injecting to be difficult both physically and psychologically. One high-risk participant said she would welcome participating in a trial as an opportunity not to have the injections and other high-risk patients reported choosing not to have another child due to the impact of the injections.
W4P2: I actually ended up with some physical lumps on my stomach from it, but I in the end had to inject for 18 weeks which was so, so painful and sore in my stomach and I just resented it, I really hated it.
W2P2: I think my bruising hurt more than my caesarean section [ … ] I certainly would never consider having another baby now because of the thought of injecting myself.
W1P2: I decided not to have a second baby because I couldn’t face the idea of taking those injections twice a day.
Again, a lack of information was felt to be a contributor to the anxiety surrounding the injections due to ‘not knowing what was normal’ (W2P2) and participants would have valued information from health-care professionals about what to expect with regard to the injections, particularly the potential for lumps and advice about how to inject less painfully. Without being shown how to do the injections, they were unclear about the most appropriate place to place the needle (particularly during the latter stages of pregnancy) and were unsure whether the pain meant they were not doing the injections correctly. In the absence of information from clinicians, they obtained information about how to undertake the injections from Thrombosis UK, social media, internet sites or watching others do the injection.
W2P1: I absolutely freaked [at the pain], I thought something was wrong.
W3P1: I was so happy that I’d actually seen somebody do it, it gave me a lot more confidence, to know what I’m doing, like how much fat do I need to grab on my stomach.
Negative experiences associated with the injections also included difficulty in undertaking the injections while looking after a newborn (potentially alongside other young children), and practical issues such as being able to dispose of sharps bins safely. These factors were all felt to potentially impact on attrition rates within future trials but may be addressed by improved information and understanding of the rationale behind the trial.
W1PM. I don’t know how you could recruit normal people who didn’t have that trauma and that personal history to actually inject themselves because it’s such a massive thing to do. For a normal person that doesn’t have diabetes or other reasons to inject themselves when they haven’t had to inject themselves that I just don’t know how you could recruit thousands of people to get enough evidence.
W1P4: I think you’d have to make it really clear what the benefit to the person is and their case, because for the greater good to have an injection every day that stings and really awkward to do, and this may be not necessary, I think that would be hard to sell.
W3P3: I think if you understand fully why you’re doing it, and also how to do it and how to do it safely, and all the rest of it, I think it then becomes easier and actually you know why you’re doing it, so even if it hurts you’re more likely to carry on and do it, for a period of time.
-
Participants saw RCTs as an opportunity to access improved care and information, as well as improving future care for others.
A number of participants had previously taken part in clinical trials either while pregnant or at other times and spoke favourably about participating in clinical trials as a way of helping future pregnant women. They understood the potential benefits to future patients, and even specified that they would have preferred to take treatment as part of a clinical trial as it would provide wider benefit. While accepting some level of risk to themselves, they were clear that they would be unwilling to take part in a clinical trial that may cause any level of risk to the baby.
W3P6: So I feel like if I could help people in the future so that their post-recovery, was better, I would like to take part. [ … ] I’d like to feel that I would take part in a trial for that reason. Not specifically just to benefit myself, but to help with the research as well going forward for other women in the future.
W1P1: Yes, just going to say that I would view the risks to the baby would be different to me, so I would probably be happy to take some risks to me, but have a very low tolerance to having risks to the baby.
Overall concerns about being dismissed, not listened to and offered little information about their care meant that participants saw a benefit to enrolling in a trial as a way to receive a better standard of care and discussion of the risks and benefits of thromboprophylaxis. Participants felt that taking part in a clinical trial would offer additional monitoring and access to health care, and the offer of additional scans or appointments may encourage people to participate in a clinical trial.
W1P2: I think if you’re doing it as a trial, then you get a lot more contact with health professionals, specifically about the injections. Whereas if it was standard care [ … ] you probably don’t have so much contact with somebody, specifically about the anticoagulation, so you probably get better yeah, better adherence if you were in a trial than if you just had it in bog standard of care.
W3P3: I think for me it’s, it would be about clarity of information and actually, almost providing ‘okay this what we’re trying to research, but if you join this trial, we’ll give you xyz’. So you get extra check-ups, extra scans, extra, if you were doing it obviously pre-, during your pregnancy so that you were confident that regardless of whether you were or you weren’t, your standard of care is almost raised up another level. so you weren’t just being looked after, you were being like gold standard, you know you were getting check-ups, you know, once a month.
W3P6: I think if you had [ … ] perhaps you had a midwife or somebody from the trial team, who are checking in on you. If you’re finding the injections okay, or if you’re on a no treatment arm, just checking in that you’re psychologically okay with that, I think again having somebody checking in on you in those early weeks particularly if you might not have other support at home, that could be a benefit for some people.
The provision of additional care may also provide reassurance for people who perceived the trial as introducing additional risk. Again, concerns related mainly to the potential risks associated with not receiving LMWH, rather than potential risks of LMWH and participants felt that clear information about potential benefits of the trial would be needed.
W2P1: I think for me it would be how closely you’re going to monitor it, you know, like what sort of tests I suppose, would you be doing to monitor if I’m getting a blood clot anywhere? You know, how closely are you going to be looking after me kind of thing? For me personally. I’d want to know are you going to see me quite often?
W4P3: If someone had said to me (at planned caesarean section) you could be prescribed this drug because of factors age and planned caesarean section, but you know if you had the opportunity not to and we would monitor your situation, I think I’d be more inclined.
W4P6: But, I think one thing that could have helped me [ … ], if you were going to have to help me get the risk of an unexpected bleed or something like that we might have you in more regularly for blood pressures checks, or that we might have you go to a particular clinic just to keep an eye on you, that kind of thing.
There were mixed views about whether participation in a trial would increase or decrease their likelihood of continuing with injections. While some felt that they would feel a moral obligation to continue with uncomfortable or painful treatment due to the wider contribution to research, others perceived being offered treatment as part of a trial as evidence that they did not really need the treatment.
W4P1: But, yeah if it was part of the trial I probably would have been much more likely to continue because I would have had the rationalised reasons why it needed to be for that period of time.
W4P5: I would feel like I was obliged, that I would be letting people down or affecting their research and outcome if I weren’t to, if I was to give up.
W3P5: I think your mindset changes if you’re doing it for a trial, you don’t necessarily need it, so you kind of feel like you can opt in or out, if you’re finding it too difficult, if you don’t feel like you’re getting anything out of doing this.
-
Consent for future trials should be undertaken antenatally rather than postnatally. Information provision and understanding are key.
Within these workshops, participants who had received their thromboprophylaxis antenatally or had their risk factors explained antenatally [e.g. in vitro fertilisation (IVF) births] were more likely to have had the risks and benefits of treatment explained to them. Participants strongly supported antenatal recruitment to trials, with information provision and provisional consent provided at a time when they had ‘headspace’ (W4P1) and time to understand the information given, and to discuss with their partners.
W2P1: I think like [W2P2] said, it would be good if they were given information before giving birth, just so they’ve got the capacity to understand it, and process it and everything.
W3P1: obviously it’s not something you would want to have pounced on you, just as you’ve given birth, ‘do you want to take part in this trial?’, I think it would definitely have to be, you know, something that you talk about at least in the last maybe three months of your pregnancy, with your midwife.
W4P6: It was only just to sort of say that I agree with the sort of consensus there, that part of information, like I say high quality of information with a trusted person during pregnancy so you had the chance to have that some form of better clarity of decision making and then I say the opportunity to probably opt-out just depending on kind of how the birth went, how it felt.
Postnatal recruitment and provision of full informed consent were considered unfeasible and impractical, particularly following emergency caesarean section or a difficult labour. Participants described the confusion and feelings of being overwhelmed after giving birth, which would make them unwilling or unable to consider participating in a trial if they had not already undertaken to do so.
W3P3: I think if someone had sat down, if the option was a trial or nothing, I would have been up for doing a trial, but I wouldn’t have wanted to make that decision after birth. I think once you’ve just given birth it’s that kind of, there’s a lot going on, and you’ve got a lot to process, and for me I was a first-time Mum, so it was a lot to take in, sort of first baby, I’m like ‘oh my god, what do I need to do?’,
W3P2: No, not at all, if someone said to me about a trial I’d say ‘sorry I’m not interested at the moment, I’m trying to find my feet at the moment being a parent’, and I think that would even be the same with a second child because you’re adding another child into the mix, kind of balance, having another one at home with you I feel like I wouldn’t be interested at all, personally.
W3P6: And I think what you were saying about communication and understanding is going to be key, particularly given the difficulties and how overwhelmed you can be in that immediate post birth period, both kind of physically and emotionally. A slow burn in terms of awareness of the trial and in sort of mid to late pregnancy would be the way to do it, I think.
-
Cluster randomisation was felt to provide greater buy-in from clinicians, and lead to quicker identification of any problems than individual randomisation.
When describing management of prior VTE, participants described a lack of consistency of advice between different departments of the hospital and felt that, for example, midwives and ED staff had different understanding of how to manage patients with VTE during pregnancy. Participants generally favoured the concept of cluster randomisation as they perceived that the treatment arm provided would be more acceptable to all clinicians, which would lead to improved consistency of management throughout the hospital. They felt that support would be improved and that any problems arising would be more visible and picked up quicker than within individual randomisation.
W4P3: Then, yeah the kind of security of knowing that there are other people, other women in that situation [ … ] you’d feel like you’re monitored as group, not just as an individual, and individuals, you know, sometimes you don’t want to feel like you’re the one that gets overlooked or falls through the cracks.
W1P4: I think it would be easier to provide support because the whole hospital would be going the same direction, I think I would actually be reassured as a patient, because if something was going really wrong I think the doctor would pick it up faster,
W1P6: I think I’d be much more likely to take something that was done as a hospital, you know that everyone else is doing the same thing, and it’s not sort of just a one person thing, if that makes sense? I think I’d feel more comfortable. [ … ] I think just because that’s what they’re doing, they’re all doing the same thing, rather than it just being you know it’s just a certain amount of people. It would be everybody that’s in that same care setting as you.
Participants saw benefits to randomising as a unit (hospital) in terms of having a higher likelihood of meeting other people who were on the same treatment, and one participant who had previously taking part in clinical research felt that randomising as a unit (hospital) would prevent discussions or concerns about which was the ‘best’ treatment between patients in hospital (e.g. if on a ward together). However, there were some concerns that cluster randomisation may lead to a ‘postcode lottery’ that would result in you being offered treatment depending on where you lived, and participants expressed the need to ensure that hospitals were well matched in terms of populations. (Again, concerns centred around hospitals delivering the placebo as offering a higher-risk option.)
W1P4: I would want to be reassured that it wasn’t like all the hospitals in the North are doing it one way, and all the hospitals in the South [waved gesture 34m 56s] so that they actually do take into account populations and make it you know, really sort of correct [Int: Yeah], just age, and socioeconomic, and race, all that.
W2P3: I would definitely, no it depends on the occasion, you say some hospitals offer and some don’t, not all of them might be you know convenient for you to access, so if the nearest hospital to me didn’t offer it, I’d probably not want to be there, so, yeah...
W3P6: I think it kind of the postcode lottery pops kind of into your head then [ … ] I do think that if you were told if you were giving birth in Plymouth you’re going to have a different post-birth care than your giving birth in Exeter or wherever. I think if you could opt out of that and just be guided by your individual consultant or midwife lead, and they would decide based on current evidence what is best for you, I think that would be okay. But if you were kind of put in the situation where that was what was going to happen just because of where you are, I think that would probably be less acceptable.
Clinician survey
The survey of clinicians was conducted in accordance with the methods outlined in the project protocol (version 1.0), which can be accessed on https://fundingawards.nihr.ac.uk/award/NIHR131021 (accessed February 2023).
Development of the survey
When the first draft of modelling results was available (September/October 2021), we developed potential questions for both the survey and workshops with the project management group members and piloted the survey with project management group members and wider colleagues. The main survey questions (see Appendix 8) were intended to understand how likely clinicians would be to recruit groups of patients into future clinical trials, that were indicated as having potential value based on the findings of the modelling. The results of the literature search and modelling indicated that there were two groups where further information from clinical (open label) trials of LMWH would be valuable: low risk (no prior VTE) with BMI > 30 kg/m2 and high risk (prior VTE).
We developed and piloted the survey in Qualtrics so that it could be used on both computer and mobile phone platforms. The initial consent questions were mandatory, but in order to increase the response rate, we did not require other questions to be answered and collected results for partially completed questionnaires. Due to the short timescale between development of the survey and distribution of the survey in time for the MBRRACE launch in November 2021, we were unable to undertake iterative piloting with people outside the research group.
In order to encourage respondents not to refer solely to the RCOG guidance when responding, we explained that we had identified groups of patients for whom further evidence from clinical trials would reduce the uncertainty in current VTE RAMs, and that guidance from other parts of the world differs from the RCOG guidance.
Survey recruitment
We wrote to the following groups to ask for help with circulating the survey to their members: British Maternal Fetal Medicine Society, British Society for Haematology Obstetric Haematology Group, Obstetric Anaesthetist Association, MacDonald Obstetric Medicine Society, RCOG. Organisations shared links to the survey on social media pages, added the link to their website research pages and sent out direct links to members where they had a specific research participant list.
Members of the project management group also shared details of the research via social media pages and the survey was introduced at the MBRRACE conference on the week of 12 November 2021. A reminder e-mail was sent after 2 weeks to ask organisations to send a reminder, and the survey was recirculated on social media. The survey was closed on 6 December 2021.
Survey findings
We received 115 responses to the first section of the survey, with 82 people completing the demographic data at the end of the survey.
Who would clinicians be prepared to randomise in a future trial of low-molecular-weight heparin or no low-molecular-weight heparin?
The questions and results for the patient scenarios for patients who were not eligible for antepartum prophylaxis, and patients who were eligible for antepartum prophylaxis are reported in Tables 13 and 14 respectively. We asked participants to state whether they would randomise the patient, not randomise and prescribe LMWH, not randomise and not prescribe LMWH for each scenario, assuming the patient has no other risk factors. For the patients who were eligible for antepartum prophylaxis, we asked them to specify each option (1) from booking, (2) from 28 weeks or (3) postnatally.
| Yes, would randomise (%) | Not randomise, would prescribe (%) | Neither randomise nor prescribe (%) | Do not know/other (%) | N | ||
|---|---|---|---|---|---|---|
| A | Emergency caesarean section (BMI ≤ 30 kg/m2) | 60 (53) | 46 (40) | 3 (3) | 4 (4) | 114 |
| B | Elective caesarean section and age 36 years (BMI ≤ 30 kg/m2) | 84 (74) | 20 (18) | 6 (5) | 4 (4) | 114 |
| C | BMI ≥ 40 kg/m2 | 34 (30) | 77 (68) | 0 (0) | 2 (2) | 113 |
| D | BMI 32 kg/m2 and PPH requiring blood transfusion | 49 (45) | 51 (46) | 4 (4) | 6 (5) | 110 |
| E | BMI 32 kg/m2 and elective caesarean section | 78 (69) | 27 (24) | 2 (2) | 5 (4) | 113 |
| F | BMI 32 kg/m2 and emergency caesarean section | 38 (34) | 69 (62) | 0 (0) | 4 (4) | 111 |
| G | BMI 32 kg/m2 and age 36 years | 85 (75) | 18 (16) | 6 (5) | 4 (4) | 113 |
| Yes, would randomise (%) | Not randomise, would prescribe (%) | Neither randomise nor prescribe (%) | Do not know/other (%) | N | |||
|---|---|---|---|---|---|---|---|
| A | Age < 35 years, BMI < 30 kg/m2, prior unprovoked VTE | Booking | 16 (21) | 60 (78) | 0 | 1 (1) | 77 |
| 28 weeks | 8 (11) | 67 (88) | 0 | 1 (1) | 76 | ||
| Postnatally | 8 (10) | 68 (88) | 0 | 1 (1) | 77 | ||
| B | Age < 35 years, BMI < 30 kg/m2, prior VTE associated with major abdominal surgery | Booking | 47 (68) | 16 (23) | 4 (6) | 2 (3) | 69 |
| 28 weeks | 37 (55) | 26 (39) | 2 (3) | 2 (3) | 67 | ||
| Postnatally | 19 (28) | 45 (67) | 0 | 3 (4) | 67 | ||
| C | Age < 35 years, BMI < 30 kg/m2, prior pregnancy-related VTE | Booking | 10 (13) | 65 (84) | 0 | 2 (3) | 77 |
| 28 weeks | 4 (5) | 67 (92) | 0 | 2 (3) | 73 | ||
| Postnatally | 4 (5) | 69 (93) | 0 | 1 (2) | 74 | ||
| D | Age 36 years, BMI 32 kg/m2, para 3 | Booking | 52 (81) | 1 (2) | 9 (14) | 2 (3) | 64 |
| 28 weeks | 52 (78) | 8 (12) | 6 (9) | 1 (1) | 67 | ||
| Postnatally | 40 (62) | 21 (32) | 3 (5) | 1 (1) | 65 | ||
| E | Age < 35 years, BMI < 30 kg/m2, antiphospholipid antibodies without prior VTE | Booking | 40 (56) | 24 (33) | 2 (3) | 6 (8) | 72 |
| 28 weeks | 30 (46) | 33 (48) | 2 (3) | 4 (6) | 69 | ||
| Postnatally | 29 (41) | 38 (54) | 1 (1) | 3 (4) | 71 | ||
| F | Age < 35 years, BMI < 30 kg/m2, Protein C deficiency without prior VTE | Booking | 44 (63) | 18 (26) | 4 (6) | 4 (6) | 70 |
| 28 weeks | 31 (46) | 31 (46) | 2 (2) | 4 (6) | 68 | ||
| Postnatally | 22 (32) | 41 (60) | 2 (3) | 3 (4) | 68 | ||
| G | Age < 35 years, BMI < 30 kg/m2, factor V Leiden homozygous without prior VTE | Booking | 38 (53) | 26 (36) | 4 (6) | 4 (6) | 72 |
| 28 weeks | 30 (43) | 32 (46) | 3 (4) | 4 (6) | 69 | ||
| Postnatally | 24 (34) | 43 (61) | 2 (3) | 1 (1) | 70 |
Concerns about recruiting patients in the scenarios listed into randomised controlled trials
We also asked clinicians to explain any concerns they may have about recruiting any of the patients listed above into a RCT (36 responses). Free-text comments indicated that clinicians were reluctant to randomise for women with high BMI (some said > 30 kg/m2, others > 40 kg/m2) or previous VTE but more support for the groups who were perceived to be lower risk; age 35–40 years, BMI 30–35 kg/m2.
This was reflected in the scenario results, which suggested lower support for randomisation with higher BMI [e.g. 30% (34/113) willing to prescribe for BMI > 40 kg/m2 vs. 75% (85/113) willing to prescribe for BMI 32 kg/m2and age 36 years]. Similarly, willingness to randomise for patients with previous VTE was low antenatally, although 68% said they would randomise from booking for prior VTE associated with major abdominal surgery and no other risk factors. Four people commented that emergency caesarean section has a higher risk than elective caesarean section, which was reflected in the scenarios. While 69% (38/113) would be willing to randomise patients with BMI 32 kg/m2 and elective caesarean section, only 34% (38/111) would be willing to do so for a patient with BMI 32 kg/m2 and emergency caesarean section.
Some free-text comments indicated that the lack of detail within the scenarios made it difficult to provide a response (e.g. ‘depends on the mode of delivery’ or ‘I would want to consider other risk factors, how long they were in labour, other confounding factors before I was happy for them to be randomised’). While some explained the rationale that underpinned their understanding of risk (e.g. ‘emergency caesarean section patients are usually less mobile and take much longer to recover’, ‘really difficult with BMI over 40 kg/m2 as often also very sedentary’), most comments related to ‘risk’ as currently perceived within the existing RCOG guidelines. Some recognised the lack of evidence base and the need for further interventional trials, whereas others suggested a high level of trust in existing guidelines and reluctance to deviate from guidelines or understanding of the need for further evidence. Free-text comments as well as question responses suggest that clinicians would be unwilling to randomise patients who are currently assessed as high risk within the RCOG guidance and that there is a low level of clinical equipoise in this population.
In your preamble you suggest that I should not use clinical guidelines to influence my choice however my practice is entirely formed by the clinical guidelines! I trust the professionals and processes behind the guidelines. All the patients listed meet criteria for postnatal LMWH: they all have at least 2 risk factors.
As far as I can make out there is virtually no intervention-based study is, regional and national guidelines are based on observational and uncertain population studies and clinical opinion. Much better interventional data required at all levels for thrombosis treatment and prevention.
For all of the women listed I would consider that they have sufficient risk factors to warrant prophylaxis which is my current practice and is well tolerated and I would not deny them this by entering into a trial.
Clinician perspectives of acceptability of recruitment to cluster randomised controlled trials
We asked clinicians whether they felt that it would be acceptable to randomly allocate hospitals or NHS Trusts to provide LMWH or no LMWH for the specified patient groups, rather than the traditional approach of randomly allocating each individual person to either LMWH or no LMWH.
Table 15 shows that two-thirds of the clinicians felt that it was only acceptable to allocate treatment at an individual level, but few provided further details explaining their answer. Some participants questioned how treatment groups could be matched when using cluster randomisation (i.e. ensuring similar populations at different hospitals), and one participant was concerned that hospitals/NHS Trusts who were randomised to no LMWH would have to report higher rates of VTE. One clinician who felt that it was acceptable to allocate treatment at hospital/NHS Trust level commented that cohort randomisation would simplify the trial.
If it was felt that the treatment groups could be appropriately matched i.e. similar district generals given treatment and not. Otherwise would have to be same centre with the 2 options.
In my view I 100% agree on cohort randomisation to simplify it.
Not blinded and may change other management within trust if all patients managed the same way therefore better to randomise each individual patient for more meaningful results.
The trust allocated no LMWH would presumably have higher rates of VTE to be reported which would be unfair.
| Response | N |
|---|---|
| Yes, acceptable to allocate treatment at hospital/NHS Trust level | 20 |
| No, only acceptable to allocate treatment at individual level | 46 |
| Unsure/don’t know | 0 |
| Don’t understand the question | 2 |
| Other | 2 |
What guidelines do clinicians use to support decision-making?
We asked what guidelines clinicians use to support decision-making, to understand what influenced their perspectives. RCOG were most commonly referenced, but clinicians also referenced American Society of Hematology (ASH) guidelines (n = 2), ACOG (n = 2) other local regional or hospital-based guidelines (n = 7). Their responses are summarised in Table 16.
| Response | N (tick as many as applies, N = 83) |
|---|---|
| RCOG guideline | 72 |
| All-Wales policy | 3 |
| NICE antenatal care risk assessment | 22 |
| Other | 13 |
Which groups of patients would benefit from improved evidence from clinical trials?
We asked clinicians whether there were any particular groups of patients who they felt would benefit from improved evidence from clinical trials. Although 24 clinicians responded, the groups were disparate and there was no single group who was highlighted more than others, suggesting a wide range of conditions for which clinicians felt further evidence would be valued. Respondents indicated a range of combinations of risk factors, as well as the following: family history of VTE/thrombophilia, low-risk thrombophilias, IVF-assisted reproductive technology (ART) patients, advanced maternal age, elective caesarean section, emergency caesarean section, blood loss 500–1000 ml, first trimester pregnancy loss, BMI < 35 kg/m2, e-cigarette use, non-Caucasian patients, pre-term birth, hyperemesis severe dehydration (temporary), hypertriglyceridaemia, hypothyroid, previous history of transient ischaemic attack or stroke of unknown aetiology while on birth control pill.
Demographic information (n = 82)
The clinician role and demographic information for survey respondents are detailed in Table 17.
| Role | Consultant/trainee (n = 115) |
|---|---|
| Obstetrician | 36/2 |
| Obstetrician and gynaecologist | 16/14 |
| Consultant midwife/other midwife | 2/2 |
| Haematologist | 15/1 |
| Obstetric physician | 10/1 |
| Consultant anaesthetist | 4 |
| Consultant obstetrician and haematologist | 6 |
| Other | 6 |
| Length of time in current role | N = 82 |
| < 2 years | 10 |
| 2–5 years | 21 |
| 5–10 years | 20 |
| 10+ years | 30 |
| Prefer not to say | 1 |
| Female | 65 |
| Male | 14 |
| Other/prefer not to say | 3 |
| Ethnic background | N = 82 |
| Asian/Asian British | 16 |
| Black/African/Caribbean/black British | 1 |
| White/Caucasian | 60 |
| Other | 2 |
| Prefer not to say | 3 |
| How did you hear about the survey? (Tick as many as applies) | N = 80 |
| British Maternal Fetal Medicine Society | 2 |
| MacDonald Obstetric Medicine Society | 43 |
| Obstetric Anaesthetist Association | 0 |
| British Society for Haematology Obstetric Haematology Group | 15 |
| Other | 21 |
Summary of key findings
-
Clinicians demonstrated low support for randomising patients with high BMI (> 40 kg/m2) or previous VTE and were more likely to support randomisation for patients with elective caesarean section than emergency caesarean section. Clinical equipoise and perceptions of risk may be linked more to current guidelines (notably RCOG) than awareness of underlying evidence.
-
Pregnant women receive limited information about VTE or risks and benefits of thromboprophylaxis during pregnancy or postnatally and those without prior VTE often do not understand why they have been given the treatment. Clearer information about the risks and benefits of treatment and an understanding of the rationale behind treatment may improve recruitment and compliance.
-
Pregnant women who had previously received thromboprophylaxis accepted current prescribing practice and perceived potential future trials to be withholding treatment. Some level of reluctance to participate in future trials appeared to stem from a perception of future RCTs as withholding treatment according to current best practice and women would need to be given clear information about existing treatment uncertainty in order to accept the no-treatment arm.
-
Negative experiences associated with injections were minimised by healthcare practitioners but may increase likelihood of attrition. Women wanted improved patient information about how to undertake the injections and an understanding of what side effects are normal.
-
Participants saw RCTs as an opportunity to access improved care and information, as well as improving future care for others. In order to maximise recruitment for future trials, consent procedures should be undertaken antenatally, and trials may wish to offer additional healthcare checks to provide reassurance to both clinicians and patients.
-
Patients supported cluster randomisation which they felt may provide greater buy-in from clinicians, more consistent management and lead to quicker identification of any problems than individual randomisation. However, there were also some concerns that cluster randomisation may lead to differences in care in different geographic locations. Clinicians favoured individual randomisation, but there was no clear indication of whether cluster randomisation may be more acceptable if explained more clearly and units were matched appropriately.
-
In order for future trials to recruit appropriately, clear explanation of existing evidence, risks and benefits of treatment will need to be made available to both clinicians and patients, particularly for patients with prior VTE where clinical equipoise is lower.
Chapter 6 Estimating the expected value of future research
Introduction
The aim of the EVSI analysis was to determine how much additional net monetary benefit could be achieved by further research to reduce uncertainty in those parameters that are associated with significant decision uncertainty, as identified in the EVPPI analysis (see Chapter 4). While the EVPPI analysis estimated the maximum net monetary benefit that could be achieved by having perfect information on a particular parameter or set of parameters, the EVSI analysis acknowledges that no future study could realistically obtain perfect information. It, therefore, estimates the maximum net monetary benefit that could be achieved by a study with a particular design and sample size, which is conducted with the intention of providing additional information to reduce the uncertainty in a particular set of parameters.
Methods
The EVPPI analysis (see Chapter 4) suggested that the majority of the decision uncertainty was related to uncertainty around the RR of VTE. Therefore, we decided to estimate the EVSI of obtaining sample information on the RR of VTE using a RCT design. As any RCT to determine the risk of VTE would also be likely to record major bleeding episodes as a safety outcome, we assumed that our RCT would update both the RR of VTE and the RR of major bleeding.
In the high-risk antepartum population, we acknowledge that a RCT of LMWH compared to no LMWH was not considered likely to be acceptable or feasible based on the findings of the qualitative research. However, given the high degree of uncertainty in the RR of VTE, and the high EVPPI associated with this parameter, we have decided to estimate the EVSI for a RCT of LMWH versus no LMWH in order to quantify the opportunity cost of not conducting a RCT in this group.
As there was minimal EVPPI in the unselected postpartum population, we decided to focus our EVSI on the subgroups of postpartum women selected according to risk factors (obesity and caesarean section delivery) where the EVPPI was higher. However, for the post-caesarean section population, it should be noted that the calculations assume that a RAM is available in the post-caesarean section population that performs similarly to the Sultan RAM in the unselected population. This is because there was minimal EVPI for individual parameters when considering only the RAMs validated in a post-caesarean section population (RCOG/Binstock).
The EVSI was calculated using the regression-based approach described by Strong et al. 122 This approach was implemented using the online SAVI tool. 116,122 In this method, for each set of parameter samples used in the PSA, it is necessary to simulate the summary statistics we would expect in a future trial. 122 In this case, we used a binominal distribution to sample the expected number of patients having VTE in each arm conditional on the sampled absolute risk in patients having no LMWH and conditional on the sampled RR of VTE for LMWH compared to no LMWH. The proportion of patients having events in each arm was then used to estimate the RR for a trial with those VTE outcomes. A similar approach was used for the risk of major bleeding but conditional on the absolute risk of major bleeding for those having LMWH and the RR of major bleeding for LMWH compared to no LMWH. Due to the similarity between the EVPPI calculation and the EVSI calculation, the SAVI tool can be used to provide an estimate of the EVSI, by including these sampled estimates of the RRs expected from future trials as two additional sets of parameters, and then using the SAVI tool to calculate the EVPPI for these two additional parameters. 116 This process was repeated for trials of different sizes.
To provide some context as to whether the research benefits are likely to outweigh the research, an informal review of National Institute for Health and Care Research (NIHR)-funded projects was conducted to identify clinical trials of pharmacological interventions in women who are pregnant or who have recently given birth (see Appendix 9). Twenty relevant studies were identified with numbers recruited ranging from 200 to 11,020. The median cost was £1.4 million with an interquartile range (IQR) of £1.1–2.0 million.
Results
Antepartum women with a prior venous thromboembolism
The overall EVPI was £1454 per patient in high-risk antepartum women, and this is therefore the most EVSI per patient that can be obtained from any study design. Figure 26 presents the EVSI per patient for a RCT of LMWH versus no LMWH, which updates both the RR of VTE and the RR of major bleeding for various trial sizes [assuming the same number of participants (N) per arm]. It can be seen that the EVSI increases as the size of the proposed trial increases but with diminishing returns rising from £874 per patient for a trial with 30 patients per arm to £1318 per patient for a trial with 500 patients per arm. The population-level EVSI over 5 years of births is estimated to be £13.1 million for a RCT with 30 patients per arm, rising to £19.7 million for a trial with 500 patients per arm.
FIGURE 26.
Patient-level and population-level EVSI for a RCT of LMWH vs. no prophylaxis in high-risk antepartum women.

It should be noted that from a frequentist hypothesis test-based perspective, to detect a difference of 3.83% in VTE risk, with 80% power and a two-sided significance level of 5%, a RCT would need to recruit 616 patients per arm. (This calculation assumes both arms are given postpartum LMWH and uses the incidences of VTE predicted by the economic model; 4.14% for antepartum LMWH from booking followed by postpartum LMWH, 7.94% for no antepartum prophylaxis followed by postpartum LMWH.)
Obese postpartum women
In the obese postpartum subgroup, the patient EVPI was £22.35. The results of the EVSI analysis are summarised in Figure 27. The EVSI analysis found that a RCT which updated the RR of VTE and the RR of major bleeding would result in EVSI of £4.73 per patient for 300 patients per arm, rising to £19.33 per patient for a RCT with 10,000 patients per arm. This corresponds with a population-level EVSI of £2.8 million over 5 years of births for a RCT of 300 patients per arm, rising to £11.6 million over 5 years of births for a RCT of 10,000 patients per arm.
FIGURE 27.
Patient-level and population-level EVSI for a RCT of LMWH vs. no prophylaxis in obese postpartum women.
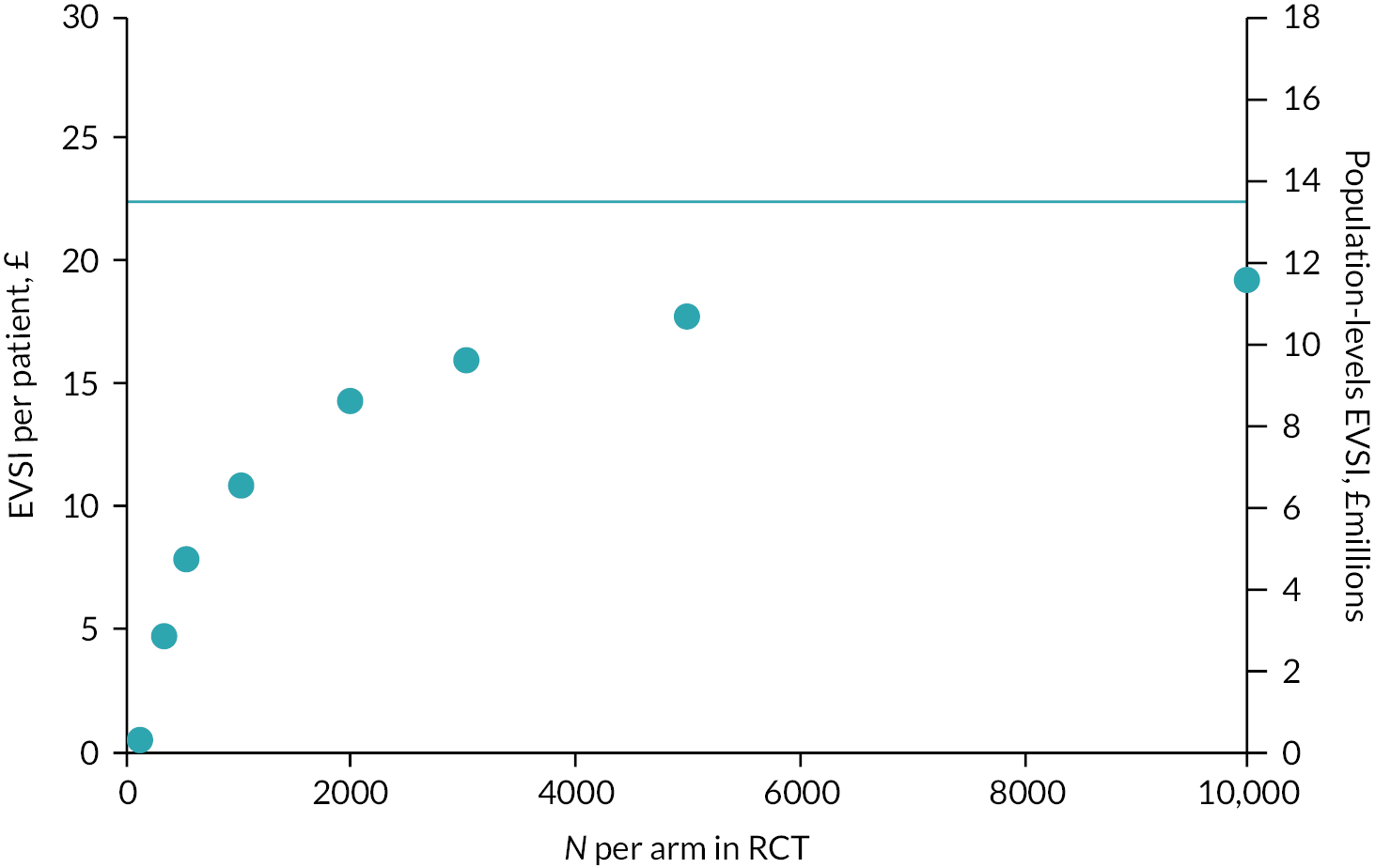
It should be noted that from a frequentist hypothesis test-based perspective, to detect a difference in VTE risk of 0.07%, with 80% power and a two-sided significance level of 5%, a RCT would need to recruit 36,798 patients per arm. (NB: This calculation uses the incidences of VTE predicted by the economic model; 0.08% for postpartum LMWH, 0.15% for no prophylaxis.)
Postpartum women following caesarean section
The EVSI analysis in postpartum women following caesarean section assumes that a RAM with similar performance to the Sultan RAM is available for women following caesarean section. This is because there was minimal EVPPI associated with the decision regarding the optimal prophylaxis strategy when assuming that the only RAMs available were the ones validated in cohorts of women who have had a caesarean section (i.e. the RCOG RAM and novel Binstock RAM). These findings should therefore be considered to be exploratory.
It can be seen from Figure 28 that the EVSI rises sharply from £0.62 per patient for a trial with 1000 patients per arm, to £2.20 per patient for a RCT of 3000 patients per arm. However, it only reaches 49% of the overall EVPI, even when the N per arm is increased to 10,000 patients. The population EVSI over 5 years of births is £1.1 million for a RCT of 2000 patients per arm, rising to £2.2 million for a RCT of 5000 patients per arm.
FIGURE 28.
Patient-level and population-level EVSI for a RCT of LMWH vs. no prophylaxis in women recruited following caesarean section.

It should be noted that from a frequentist hypothesis test-based perspective, to detect a difference of 0.08% in VTE risk, with 80% power and a two-sided significance level of 5%, a RCT would need to recruit 24,502 patients per arm when comparing LMWH with no prophylaxis in postpartum women following caesarean section. (NB: This calculation uses the incidences of VTE predicted by the economic model; 0.06% for postpartum LMWH, 0.14% for no prophylaxis.)
Summary of key findings
-
The per patient EVSI is high in high-risk antepartum women leading to a high population-level EVSI despite the fact that only 0.5% of births occur in women with a history of prior VTE.
-
A small RCT of only 30 high-risk antepartum women per arm would be sufficient to generate a substantial population-level EVSI of £13.1 million.
-
In the obese postpartum population, the per patient EVSI is much lower but a RCT of 300 patients per arm would generate a population-level EVSI of £2.8 million over 5 years of births as around 128,000 pregnancies per annum are in people with high BMI (> 30 kg/m2).
-
In the post-caesarean section population, a larger RCT of 5000 patients per arm would be required to generate a population-level EVSI of £2.2 million over 5 years of births, but this level of EVSI would only be achieved if a RAM was available for the post-caesarean section population which performed similarly to how the Sultan RAM performed in unselected postpartum women.
-
Trials designs comparing LMWH with no prophylaxis which are underpowered from a frequentist hypothesis-testing perspective, would still have substantial value compared to the typical cost of trials in these populations which is £1.1–2.0 million.
Chapter 7 Discussion
Statement of principal findings
Systematic review of risk assessment models
The systematic review identified 19 externally validated RAMs (and one internally validated risk model) that aimed to predict the risk of VTE in pregnant and postpartum women and who could be selected for thromboprophylaxis. Although various risk models (based on a variety of predictor variables) are being used, most of these lacked rigorous development and evaluation. The predictive accuracy of the RAMs was highly variable, and the substantial risk of bias concerns and the general lack of methodological clarity and unclear applicability make meaningful comparisons of the evidence difficult.
Cost-effectiveness and value of perfect information
In high-risk antepartum women, such as those with a prior VTE or thrombophilia, there is considerable uncertainty regarding the cost effectiveness of using RAMs to select women for antepartum prophylaxis, with none of the strategies having more than a 36% probability of being optimal (when valuing a QALY at £30,000). The overall EVPI is £1454 per patient with 94% of this attributable to the uncertainty in the RR of VTE for LMWH compared to no prophylaxis. This conclusion was fairly robust in the sensitivity and scenario analyses, although the optimal strategy varied when assuming a lower utility loss attributable to PTS and when assuming that the average patient has a BMI of 36 kg/m2 instead of 27 kg/m2.
In unselected postpartum women, the combination of poor RAM performance and low absolute risks of VTE meant that a strategy of offering no prophylaxis had a high probability (89%) of being optimal (when valuing a QALY at £30,000) compared to RAM-based prophylaxis strategies. This conclusion was fairly robust in the sensitivity and scenario analyses, although using the Sultan RAM to offer prophylaxis to 5% of patients with the highest VTE risk would be optimal if the risk of VTE was double that assumed in the base case or if the RAM could be administered for zero cost.
In the subgroup of obese postpartum women, the uncertainty regarding the optimal prophylaxis strategy is greater than in the unselected group, because the risks of VTE are slightly higher than in the unselected postpartum group and because the RAM developed for obese postpartum women (Ellis-Kahana) performs slightly better than the RAMs available for unselected postpartum women (Sultan, RCOG, SFOG). Using the Ellis-Kahana RAM to select obese postpartum women for prophylaxis has a 64% probability of being the optimal strategy, when valuing a QALY at £30,000. The EVPI is £22.35 per patient, with 99% of this attributable to uncertainty regarding the RR of VTE for LMWH compared to no prophylaxis.
In postpartum women who have had a caesarean section, the available RAMs with performance data in this population (RCOG and Binstock novel) have poor specificity, and a strategy of no prophylaxis has a high probability of being optimal (93%) when considering only those RAMs validated in women having caesarean section (and when valuing a QALY at £30,000). However, if we assume that a RAM can be developed for women who have had a caesarean section, which performs similarly to the Sultan RAM in the unselected postpartum population, then the probability of no prophylaxis being optimal would reduce to 57%. In this scenario, the EVPI would be £7.74 per patient, with 68% of that related to the RR of VTE.
The exploratory analyses suggest that for a RAM to be cost-effective for use in an unselected antepartum population, it would need to have high specificity (90–95% for sensitivity of 100–53%). In addition, offering antepartum prophylaxis from 28 weeks to women with three antepartum clinical risk factors (excluding prior VTE) as per current RCOG guidance is unlikely to be cost-effective.
Workshops
The workshops indicated that a study randomising women to LMWH or placebo would be less acceptable to women who have had a prior VTE or thrombophilia than for other groups of women. Workshop participants reported receiving limited information about VTE or risks and benefits of thromboprophylaxis during pregnancy and the puerperium and those without prior VTE often did not understand why they had received treatment. However, women with experience of a prior VTE felt that it would not be ethical to randomise women to placebo given the perceived risk of VTE and the perceived effectiveness of LMWH in this group. The workshop participants generally favoured using a cluster randomisation approach over individual randomisation, to allocate women to LMWH or no LMWH in future trials, as they perceived that providing consistency in care across a hospital would have benefits, although some expressed concerns that cluster randomisation may lead to differences in care in different geographic locations.
Survey
Healthcare professionals surveyed most commonly reported using the RCOG guidelines to support decision-making and reported lower clinical equipoise for women with prior VTE, thrombophilia, or BMI > 40 kg/m2. Healthcare professionals who would be responsible for recruiting women into the study felt that randomisation to a RCT of LMWH or placebo would be less acceptable to women who have had prior VTE or thrombophilia than for other groups of women. The survey also suggests that healthcare professionals have greater clinical equipoise for a study determining the effectiveness of thromboprophylaxis in antepartum women with three clinical risk factors (other than prior VTE or thrombophilia) who are currently eligible for prophylaxis from 28 weeks. The survey results also suggest that in postpartum women there is greater clinical equipoise in women whose risk factors are an elective caesarean section combined with either age over 35 years or obesity, and women whose only clinical risk factors are age and a BMI between 30 and 40 kg/m2. The majority of healthcare professionals surveyed felt that, in a future trial of LMWH compared to placebo in women who are pregnant or who have recently given birth, it would only be acceptable to allocate treatment at an individual level, as opposed to using cluster randomisation at the hospital or NHS trust level.
Expected value of future research
The EVSI analysis found that a RCT of 30 patients per arm comparing LMWH with no prophylaxis would have a value of £13.1 million over 5 years of births, rising to £19.7 million for a RCT of 500 patients per arm. This suggests that further research would have substantial benefits relative to the typical costs for an NIHR-funded RCT in this population which are estimated to be £1.1–2.0 million. The EVSI analysis found that a RCT of LMWH versus no prophylaxis in obese postpartum women would have a value of £2.8 million, over 5 years of births, if it enrolled 300 patients per arm, rising to £11.6 million if enrolling 10,000 patients per arm. In the post-caesarean section group, a RCT of 2000 patients per arm would be needed to generate an EVSI of £1.1 million over 5 years of births, when assuming that a RAM which performs similarly to the Sultan RAM is available. Trials designs which are underpowered from a frequentist hypothesis-testing perspective would still have substantial value compared to the typical cost of trials in these populations, which is £1.1–2.0 million, assuming that decision-makers are willing to use the estimates of efficacy obtained, to make better informed decisions about prophylaxis in this population, without requiring them to meet a formal hypothesis test.
Strengths and limitations
Systematic review of risk assessment models
Our systematic review work has a number of strengths. This is the first systematic review to evaluate RAMs for predicting the risk of developing VTE in women during pregnancy and in the puerperium period. It was conducted with robust methodology in accordance with the PRISMA statement20 and the protocol was registered with the PROSPERO register. Clinical experts, in addition to the core review team, were involved and consulted throughout as advisors and to assess the validity and applicability of research findings during the review processes.
The main limitations of this study related to the observational nature of the studies reviewed and their own limitations. Most of the included risk prediction studies were retrospective cohorts. Retrospective cohort studies of large health database registries are limited by poor data quality and failure to accurately ascertain outcomes and case-control designs are prone to bias including uncontrolled confounding, temporal and selection bias. 123 Conversely, better-quality data may be obtained with prospective cohorts, but smaller sample sizes will lack statistical power. In addition, most of the external validation studies evaluated predictive performance of risk models that were not statistically derived (i.e. without model development and internal validation). This process is vital, as risk models with only external validation may be subject to overfitting and optimism. 42 Similarly, the absence of model performance measures such as calibration or discrimination hinders the full appraisal of models. 43
Due to the high levels of heterogeneity between studies, we were unable to undertake any meta-analysis or statistical examination of the causes of heterogeneity due to the small number of external validation studies per risk model. Potential sources of heterogeneity include variation in study design, the study population, risk model implementation, outcome definition and measurement and the use of thromboprophylaxis. As a result, we reported descriptive statistics to provide a better understanding of the evidence base applicable to the subject matter, and shortcomings regarding reliability and validity of the data. Finally, assessments on study relevance, information gathering and validity of articles were unblinded and could potentially have been influenced by pre-formed opinions. However, masking is resource-intensive with uncertain benefits in protecting against bias decisions. 124
Cost-effectiveness and value of perfect information
A strength of the decision-analytic modelling is that we have been able bring together the available evidence to explore whether prophylaxis is cost-effective in different groups of women at differing levels of VTE risk and to identify which factors are associated with significant decision uncertainty when trying to determine the optimal prophylaxis strategy. This is important because much of the current guidance on prophylaxis in women who are pregnant or who have recently given birth is based on expert consensus that the effectiveness would be similar to that seen in other populations: medical and surgical patients who are not pregnant or in the puerperium. This is because there is minimal RCT evidence to quantify the safety and efficacy of LMWH in women who are pregnant or who have recently given birth. Assuming that the effectiveness of thromboprophylaxis is similar in pregnant and non-pregnant populations did not seem clinically reasonable given the pro-thrombotic physiological changes during pregnancy. Therefore, rather than relying on the assumption that efficacy is equivalent to that seen in other populations, we have instead been able to explore the decision uncertainty associated with having broad CIs around the estimates of treatment efficacy. This led to the conclusion that there would be substantial net benefits (cost savings or QALY gains) from having better information on the efficacy of LMWH in pregnant women and women who have recently given birth.
The main limitations in the analysis relate to areas where data were lacking entirely. For example, we were unable to assess the cost effectiveness of using the RCOG RAM in an unselected antepartum population due to an absence of studies reporting both sensitivity and specificity for RCOG in this population. In addition, for some parameters, we had to rely on data that had been estimated in non-pregnant populations. In many cases, such as the risk of fatal bleeding during VTE treatment and the costs of major bleeding, these factors were not found to be significant drivers of decision uncertainty in the scenario analyses. However, for some parameters, the optimal prophylaxis strategy was different when plausible alternatives were explored, such as when assuming that PTS is associated with a 2% decrement in utility instead of a 10% decrement in utility. Another limitation is that we used a cohort-level modelling approach which assumes that everyone in the model has average characteristics. We found that this may have affected the choice of optimal strategy but only in the high-risk antepartum population where it adds to the decision uncertainty between offering antepartum prophylaxis using EThIG and offering only postpartum prophylaxis. In addition, the results for the Lyon RAM should be interpreted with some caution as we have assumed in the base case that patients identified as requiring antepartum prophylaxis using the Lyon score will have prophylaxis from booking, whereas, in fact, some will have prophylaxis delayed until 28 weeks gestation if their Lyon score is between 3 and 6. This is likely to have overestimated the cost effectiveness of using the Lyon score, as delaying prophylaxis until 28 weeks reduces the incremental QALYs more than it reduces the incremental costs.
The EVPI and EVSI analyses use a regression-based approach with a generalised additive model (GAM) and therefore examination of the residuals is useful for assessing the robustness of the regression assumptions. While checking the regression assumptions, we noted that there was some heteroskedasticity in the plot of residuals against fitted values, but no structure (e.g. a U-shaped or S-shaped pattern) which would suggest any bias in the fitted values. In addition, the normal Q–Q plot had tails showing deviation from the assumption of normality at extreme values. As the calculation of EVPI/EVSI using the GAM regression approach only requires an estimate of the posterior mean net benefits, the calculation of the EVPI/EVSI is not biased by unequal variance of errors. 122 However, the estimation of the standard error of the EVPI/EVSI does rely on the net benefits having approximately equal variance and approximate normality. 122 Therefore, the standard errors for the EVPPI estimates provided in Appendix 5 should be treated with caution.
Furthermore, although the EVPI and EVSI analyses capture the CIs around various parameters that inform the model, these CIs mainly reflect uncertainty related to the sample size in the study and they may not adequately capture uncertainty related to study quality. Where possible, we have used sensitivity analyses to explore the uncertainties in the evidence base and any assumptions made in the model due to a lack of evidence. We have also highlighted where the conclusions rely on evidence from studies where there are quality issues, such the lack of an external validation study for the Ellis-Kahana RAM.
Workshops
Workshops were designed to understand potential perspectives of future trial engagement and were not intended as consensus events or to provide in-depth qualitative analysis of patient experience. However, they did highlight aspects of the patient experience that had potentially hitherto been underestimated and that would likely have an impact on future trial recruitment and retention.
Many of our findings were reflected in wider studies exploring the patient perspective of randomisation to clinical trials during pregnancy. We identified that women appear to have a high level of trust in treatment decisions made on their behalf during pregnancy and the puerperium and were involved in limited discussions of risks and benefits of treatment. Smyth et al. reported that high levels of trust in clinicians made pregnant women more likely to take part in trials, which suggests that clinicians may have a key role in information provision and influencing decisions about whether to take part in trials. 125
Questions about pregnant women’s decisional capacities have been highlighted in previous studies. 126,127 Women in our study reported concerns about their ability to make informed choices immediately after birth, but not during pregnancy and were strongly in favour of antepartum recruitment to non-emergency trials. Smyth et al. found that women reported recruitment to clinical trials to be better earlier in pregnancy, although some expressed concerns that being aware of potential complications in pregnancy may create anxiety. 125
We reported that participants saw RCTs as an opportunity to access improved care and information, as well as improving care for others and emphasised the need to protect their baby in treatment decisions. In a review of factors influencing recruitment to maternal and perinatal trials, Tooher et al. identified that women will prioritise their responsibility to the unborn child over their own health or altruistic reasons for participation. 128 van der Zande et al. similarly reported that pregnant women would be more likely to participate in research if they perceived there to be ‘collateral benefits’ such as access to additional services and enhanced maternity care. 119 They similarly identified that barriers to research included discomfort due to tests such as needle pricks, which reflects the findings from our study that suggested that the impact of injections may affect recruitment or retention to a trial.
Although we tried to include a range of participants, particularly those who had different educational backgrounds, our sample was disproportionately highly educated. Participants were, by definition, interested in taking part in research and so may not offer a view about participation in research that was representative of the general population. Respondents for the high-risk workshop were principally identified via an advert circulated via Thrombosis UK and could therefore be considered to be a selected group of patients with a higher level of health literacy and engagement.
Similarly, due to non-dominant ethnic groups being under-represented in health studies and due to the clear ethnic disparities in maternity outcomes,1,129 we were keen to include women from different ethnic backgrounds. We selected diverse organisations with access to a range of under-represented populations to help with the recruitment, as well as specifying that we wanted to speak to people from ethnic minority groups in some of our recruitment materials. However, none of the respondents who provided demographic details identified themselves as black or mixed/multiple ethnic groups and only two participants were Asian/Asian British with the rest being white/Caucasian. We offered payment at a rate of double minimum wage, which has been suggested as a potential enabler to encouraging diversity of engagement. 129,130 However, our approaches were entirely impersonal (partly due to the COVID-19 pandemic) and materials were not made available in other languages, with workshops conducted in English, which have been highlighted as potential barriers to successful recruitment for minority ethnic groups. 130 Given that communication of risk and paucity of information was highlighted as a significant issue in understanding clinical equipoise and potential future trial involvement by the mainly white/Caucasian research participants, it is likely that these perceptions would be amplified in a more diverse population. 129,130
Survey
We were unable to calculate a survey response rate for the online survey due to the lack of denominator; survey respondents were recruited via professional organisations’ research networks and social media pages. The survey response rate was low and should not be used to indicate sample size for potential future trials (i.e. results may not be representative of the broader clinical population). However, although survey response numbers overall were low, they provided an indication of clinician perspectives on the evidence base for thromboprophylaxis in pregnancy and thereby likely support for recruitment to future RCTs in this population.
We did not try to understand clinicians’ views of recruitment to clinical trials in depth, but to understand which patient groups they would be most likely to be willing to recruit. Our findings suggested that clinicians would be risk averse in recruiting groups of women who may currently be considered high risk according to existing guidelines. Other recent studies have reported that clinicians are protective advocates for pregnant women and play a strong gatekeeping role in recruitment to clinical trials. 126,127 Hanrahan et al. identified that clinicians were uncomfortable recruiting for trials that ‘moved them away established clinical practice’127 and that intervention needs to align with their professional opinion,131 suggesting that clinicians are unlikely to recruit without altering their perceptions of clinical equipoise. Similarly, Tooher et al. identified that doctors with strong preference for one or other of the trial options are less likely to recruit to clinical trials. 128
Expected value of future research
The EVSI analysis suggests that substantial net benefits (cost savings or QALY gains) could be generated by conducting further research and using this to make better decisions on when to offer prophylaxis to women who are at risk of VTE during pregnancy or in the puerperium. Overall, the EVSI analysis is supportive of further research to estimate the RR of VTE for LMWH compared to no LMWH. However, this information should not be acted on in isolation, but must also take into account the acceptability and feasibility of randomising women to receive no LMWH, particularly in the high-risk antepartum population.
The EVSI analysis in the post-caesarean section population should be interpreted with caution because the analysis assumes that a RAM is available that performs better than the available RAMs with performance data in this population (RCOG/novel Binstock). In addition, the EVSI analysis for the obese subgroup uses performance data from the Ellis-Kahana RAM which has not yet been evaluated in an external cohort.
The EVSI analysis does not rely on estimating the size of trial that would be required to meet a formal hypothesis test of whether there is a difference in VTE risk between LMWH and no prophylaxis. Instead, it simulates the expected outcomes from trials of various sizes and estimates the net benefits (cost savings or QALY gains) that would be achieved from using that additional evidence to make better informed decisions about prophylaxis in this population. Therefore, a trial would not need to be adequately powered from a frequentist hypothesis-testing perspective to provide valuable information. However, for the value of the future research studies estimated by the EVSI analysis to be realised in practice, there would need to be a willingness to use the updated estimates of efficacy obtained, to make better informed decisions about prophylaxis in this population, without requiring them to meet a formal hypothesis test.
Equality, diversity and inclusion
Participant representation
We specifically developed our recruitment strategy for the workshops to obtain a diverse sample of participants, seeking in particular to recruit participants from ethnic minority backgrounds. We selected diverse organisations with access to a range of under-represented populations to help with the recruitment, as well as specifying that we wanted to speak to people from ethnic minority groups in some of our recruitment materials. However, despite offering payment, we were unable to recruit a diverse sample of participants (see Workshops for details). Recruitment was affected by the ongoing COVID-19 pandemic, which meant that we were unable to offer face-to-face workshops and undertook recruitment entirely remotely.
For the clinician survey, we were unable to state whether our sample was representative of the wider population of clinicians as we did not have data about non-respondents. We reported the characteristics of respondents in terms of gender, ethnicity and length of experience.
Research team
The research team was mixed in terms of gender and ethnicity and the project provided a development opportunity for several project team members.
Patient and public involvement
The project team included a PPI representative (RC), from Thrombosis UK, who has relevant personal experience of VTE. She contributed to the design of the study at the application stage. She attended all project management group meetings and contributed to key decisions such as ensuring that the economic model captured outcomes important to women at risk of VTE during pregnancy or in the puerperium. She was instrumental in developing the questions for the workshop and in leading the recruitment strategy for patients with prior VTE. She also contributed to the interpretation and dissemination of the study findings including the lay summary. We recruited two PPI members to join the study steering committee but struggled to maintain engagement from these members after the first meeting.
Chapter 8 Conclusions
Implications for patients, clinicians and policy-makers
The absolute risk of VTE across unselected antepartum patients is low (34 in 10,000). Therefore, any RAM being used in an unselected antepartum population would need to have a high specificity (90–95% for a sensitivity of 100–53%) in order to be used to target prophylaxis in a cost-effective manner. Performance data from studies in unselected antepartum women were limited and no data were available on the performance of the current RCOG guidance across an unselected group of antepartum women. However, exploratory analyses found that offering antepartum prophylaxis from 28 weeks to women who have three clinical risk factors (none of which would qualify them for earlier prophylaxis) as per current RCOG guidance is unlikely to be cost-effective. The survey of clinicians suggests that there is reasonable clinical equipoise about the value of antepartum prophylaxis in women who currently qualify for antepartum prophylaxis from 28 weeks because of a combination of age, BMI and parity (number of previous births).
The absolute risk of VTE in women who have had a prior VTE is 5.81% in the antepartum period and 6.85% in the 6 weeks after delivery. Two RAMs developed specifically for high-risk antepartum women, such as those with a prior VTE or known thrombophilia were identified (Lyon and EThIG). No data were identified on how these performed compared to the RCOG guidelines. However, there is an ongoing study comparing the Lyon RAM with current local practice, which in the UK would be the RCOG guideline. The decision analysis in high-risk antepartum women suggests that offering postpartum prophylaxis for 6 weeks after delivery is likely to be cost-effective compared to no prophylaxis, because the majority of the costs of postpartum prophylaxis are offset by the cost savings of avoiding postpartum VTE. However, the cost-effectiveness of offering antepartum prophylaxis, in those already receiving postpartum prophylaxis, is less certain. This is because the majority of the VTE risk in high-risk women falls in the postpartum period, but the costs of offering antepartum prophylaxis from booking are much higher than the costs of 6 weeks of postpartum prophylaxis.
In postpartum women who have not had a prior VTE, the average absolute risk of VTE in the 6 weeks after delivery is low (7 in 10,000). In women who have had a caesarean delivery, the risks are higher but still low in absolute terms (14 in 10,000). The decision analysis suggests that any RAM used in these groups would need to have high accuracy to provide an appropriate balance of costs, risks and benefits in these groups and would need to perform better than the RAMs identified in the review. This includes the RCOG guideline which is predicted to result in 35% of all postpartum patients receiving prophylaxis for 10 days or more, the proportion being higher (94%) in women who had a caesarean section. The cost effectiveness of RAM-based prophylaxis was more favourable in the subgroup of obese postpartum women (absolute risk of 15 in 10,000), partly because the RAM specifically developed for obese women had a high specificity, meaning that it selected only 10% of obese women for prophylaxis, while achieving a sensitivity of 62%.
Our analysis of the benefits, harms and costs of prophylaxis suggests that it only appears to be cost-effective for selected high-risk groups. However, we acknowledge that decision-making needs to draw upon other factors. For example, the threshold of using prophylaxis to prevent harm in pregnancy may be perceived by some to be lower than the threshold in other clinical areas, such as the use of prophylaxis to prevent hospital-associated VTE. Furthermore, international studies have found that the proportion of women receiving postpartum prophylaxis under the RCOG guideline is higher than when applying equivalent guidance from other countries, suggesting that decision-makers in different countries have come to a different assessment of the balance or benefits, harms and costs. 12,15
The stakeholder workshops identified a need for better information for women about the risks and benefits of prophylaxis with LMWH including better information about how to undertake the injections and what side effects to expect.
Suggested research priorities
Having considered both the value of information analysis and the information on the feasibility and acceptability of potential future studies obtained from the workshops and clinician survey, our suggested research priority is:
-
A RCT comparing LMWH with no prophylaxis in postpartum women who have not had a previous VTE, but who have other risk factors. Obesity is a highly suitable risk factor to study due to its current high prevalence and easy identification.
The main source of decision uncertainty identified in the value of information analysis was related to uncertainty in the RR of thromboprophylaxis for preventing VTE in women who are pregnant or who have recently given birth. This is because there is minimal RCT evidence to quantify the safety and efficacy of LMWH in this group, with the most directly applicable evidence coming from one small pilot study which recruited eight women per arm. For this reason, much of the current guidance on prophylaxis in women who are pregnant or who have recently given birth is based on expert consensus. The data on effectiveness are extrapolated from other populations, such as medical and surgical patients, who are not pregnant or in the puerperium, and are therefore biologically different. Our analysis has incorporated the uncertainty that comes from this minimal evidence base to estimate the value of further research rather than relying on this assumption of similar efficacy.
The analysis suggests that a future RCT comparing antepartum LMWH with no antepartum prophylaxis in high-risk antepartum women would have substantial value even if it was underpowered from a frequentist hypothesis-testing perspective. This is because it would provide a more precise estimate of the efficacy of LMWH in this group and that has the potential to change the choice of thromboprophylaxis strategy. However, the survey and workshops found that a RCT randomising high-risk antepartum women, with a history of prior VTE or known thrombophilia, to LMWH or placebo is unlikely to be acceptable or feasible. This was because healthcare professionals who would be responsible for recruiting women into the study did not feel that there was clinical equipoise in women who are currently assessed as high risk within the RCOG guidance. Similarly, women with experience of a prior VTE felt that it would not be ethical to randomise women to placebo (i.e. no prophylaxis), given the perceived risk of VTE and the perceived effectiveness of LMWH in this group. For this reason, we consider that any future trial of LMWH versus no prophylaxis should recruit women without a prior VTE, but who have other risk factors for VTE.
There was also substantial decision uncertainty regarding the use of RAMs to select obese postpartum women for postpartum prophylaxis. Again, the main source of decision uncertainty was related to uncertainty in the RR of thromboprophylaxis for preventing VTE. There is a paucity of data on the efficacy of LMWH when used as postpartum prophylaxis and meta-analyses of studies that do exist estimate a higher risk of VTE compared with no LMWH, which is the opposite of what is expected based on data from studies in medical and surgical cohorts. For this reason, the decision analysis incorporated the estimate of efficacy from antepartum women to capture both the clinical expectation that LMWH reduces VTE and the high uncertainty based on current evidence. The analysis suggests that a future RCT comparing LMWH with no prophylaxis in obese postpartum women would have substantial value, even if it was underpowered from a frequentist hypothesis-testing perspective, because it would provide a more precise estimate of the efficacy of LMWH in this group. Therefore, such a study has the potential to change the choice of thromboprophylaxis strategy nationally and wider afield. It would also be relatively easy to do because obesity is easy to identify and measure, no blood tests are required and it is highly prevalent in the obstetric population. The survey results suggest that in postpartum women there was greater clinical equipoise in women whose risk factors are an elective caesarean section combined with either age over 35 years or obesity, and women whose only clinical risk factors are age over 35 and a BMI between 30 and 40 kg/m2. However, there was lower support for randomising women with a BMI > 40 kg/m2 and those having emergency caesarean sections.
The workshop participants felt that recruitment for future trials would be maximised if consent for enrolment was undertaken antenatally. They also generally favoured cluster randomisation over individual randomisation, as they perceived that this would lead to more consistent management within a hospital which would have benefits, although some expressed concerns that cluster randomisation may lead to differences in care in different geographic locations. Clinicians favoured individual randomisation, but there was no clear indication of whether cluster randomisation may be more acceptable if explained more clearly and units were matched appropriately. Clinical equipoise and perceptions of risk may be linked more to current guidelines (notably RCOG) than awareness of underlying evidence. Therefore, in order for future trials to recruit appropriately, a clear explanation of existing evidence, and the risks and benefits of treatment will need to be made available to both clinicians and patients.
Acknowledgements
The authors are grateful to Andrew Metry, who assisted with model verification and validation, and Donna Davis for administration and project management support. The authors would also like to thank all members of Study Steering Committee for their advice.
Contributions of authors
Sarah Davis (https://orcid.org/0000-0002-6609-4287) (Senior Research Fellow, Health Economics) contributed to the design of the study, led the Project Management Group, conducted the cost effectiveness and value of information analyses, drafted sections of the monograph and critically revised the work for important intellectual content.
Abdullah Pandor (https://orcid.org/0000-0003-2552-5260) (Senior Research Fellow, Systematic Reviewing) conducted the systematic review of risk assessment models, including data acquisition, analysis and interpretation, contributed to the design of the study, drafted sections of the monograph and critically revised the work for important intellectual content.
Fiona C Sampson (https://orcid.org/0000-0003-2321-0302) (Senior Research Fellow, Qualitative Research) designed, conducted and analysed the patient workshops and clinician survey, drafted sections of the monograph and critically revised the work for important intellectual content.
Jean Hamilton (https://orcid.org/0000-0003-3326-9842) (Research Fellow, Statistics) contributed to the design of the study, provided statistical support to the systematic review of risk assessment models and critically revised the work for important intellectual content.
Catherine Nelson-Piercy (https://orcid.org/0000-0001-9311-1196) (Professor and Consultant, Obstetric Medicine) provided expert clinical advice including contributing to the design of the study, the project management group, the interpretation of the data and critically revising the report for important intellectual content.
Beverley J Hunt (https://orcid.org/0000-0002-4709-0774) (Professor and Consultant, Thrombosis and haemostasis) provided expert clinical advice including contributing to the design of the study, the project management group, the interpretation of the data and critically revising the report for important intellectual content.
Jahnavi Daru (https://orcid.org/0000-0001-5816-2609) (NIHR Clinical Lecturer, Obstetrics and Gynaecology) provided expert clinical advice including contributing to the design of the study, the project management group, the interpretation of the data and critically revising the report for important intellectual content.
Steve Goodacre (https://orcid.org/0000-0003-0803-8444) (Professor, Emergency Medicine) provided expert clinical advice including contributing to the design of the study, the project management group, the interpretation of the data and critically revising the report for important intellectual content.
Rosie Carser (https://orcid.org/0000-0002-1456-868X) (Patient Expert, Patient and Public Involvement) contributed to the design of the study, contributed to the project management group throughout the study and provided patient and public involvement throughout the study.
Gill Rooney (https://orcid.org/0000-0002-8388-9444) (Programme Manager, Systematic Reviewing) conducted the systematic review of risk assessment models, including data acquisition, analysis and interpretation.
Mark Clowes (https://orcid.org/0000-0002-5582-9946) (Information Specialist, Literature searching) designed and conducted the searches to inform the systematic review of risk assessment models and critically revised the work for important intellectual content.
All authors were involved in the final approval of the version to be published.
All authors agree to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Publication(s)
Davis S, Pandor A, Sampson FC, Hamilton J, Nelson-Piercy C, Hunt BJ, et al. Estimating the value of future research into thromboprophylaxis for women during pregnancy and after delivery: a value of information analysis. J Thromb Haemost 2024; in press. https://doi.org/10.1016/j.jtha.2023.12.035
Data-sharing statement
All available data can be obtained from the corresponding author. Due to a requirement of ethical approval, qualitative research data related to the workshops and survey cannot be shared.
Ethics statement
We obtained University of Sheffield Ethics approval (University of Sheffield 038511) in March 2021 for the workshops and survey detailed in Chapter 5.
Disclaimers
This manuscript presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Knight M, Bunch K, Tuffnel D, Patel R, Shakespeare J, Kotnis R, et al. Saving Lives, Improving Mother’s Care: Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2017–19. Oxford: National Perinatal Epidemiology Unity, University of Oxford; 2021.
- Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med 2005;143:697-706. https://doi.org/10.7326/0003-4819-143-10-200511150-00006.
- James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006;194:1311-5. https://doi.org/10.1016/j.ajog.2005.11.008.
- Lindqvist P, Dahlbäck B, Marŝál K. Thrombotic risk during pregnancy: a population study. Obstet Gynecol 1999;94:595-9. https://doi.org/10.1016/S0029-7844(99)00308-7.
- Meng K, Hu X, Peng X, Zhang Z. Incidence of venous thromboembolism during pregnancy and the puerperium: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2015;28:245-53. https://doi.org/10.3109/14767058.2014.913130.
- National Institute for Health and Care Excellence (NICE) . Venous Thromboembolism in Over 16s: Reducing the Risk of Hospital-Acquired Deep Vein Thrombosis or Pulmonary Embolism – NICE Guideline 89 2019. www.nice.org.uk/guidance/ng89/evidence (accessed 20 February 2022).
- Royal College of Obstetricians & Gynaecologists . Thrombosis and Embolism During Pregnancy and the Puerperium, Reducing the Risk (Green-Top Guideline No. 37a) 2015.
- Middleton P, Shepherd E, Gomersall JC. Venous thromboembolism prophylaxis for women at risk during pregnancy and the early postnatal period. Cochrane Database Syst Rev 2021;3. https://doi.org/10.1002/14651858.CD001689.pub4.
- Zheng J, Chen Q, Fu J, Lu Y, Han T, He P. Critical appraisal of international guidelines for the prevention and treatment of pregnancy-associated venous thromboembolism: a systematic review. BMC Cardiovasc Disord 2019;19. https://doi.org/10.1186/s12872-019-1183-3.
- Kotaska A. Postpartum venous thromboembolism prophylaxis may cause more harm than benefit: a critical analysis of international guidelines through an evidence-based lens. BJOG 2018;125:1109-16. https://doi.org/10.1111/1471-0528.15150.
- Sultan AA, West J, Grainge MJ, Riley RD, Tata LJ, Stephansson O, et al. Development and validation of risk prediction model for venous thromboembolism in postpartum women: multinational cohort study. BMJ 2016;355. https://doi.org/10.1136/bmj.i6253.
- Palmerola KL, D’Alton ME, Brock CO, Friedman AM. A comparison of recommendations for pharmacologic thromboembolism prophylaxis after caesarean delivery from three major guidelines. BJOG 2016;123:2157-62. https://doi.org/10.1111/1471-0528.13706.
- Shacaluga A, Rayment R. Venous thromboembolic risk assessment in pregnancy: comparison of the All-Wales maternity risk assessment tool with guidance from the Royal College of Obstetrics and Gynaecology. Br J Haematol 2019;185:162-5. https://doi.org/10.1111/bjh.15417.
- Revell BJ, Smith RP. Thrombosis and embolism in pregnancy and the puerperium, reducing the risk: what proportion of patients reach the threshold for thromboprophylaxis?. Obstet Med 2011;4:12-4. https://doi.org/10.1258/om.2010.100042.
- O’Shaughnessy F, Donnelly JC, Bennett K, Damkier P, Ainle FN, Cleary BJ. Prevalence of postpartum venous thromboembolism risk factors in an Irish urban obstetric population. J Thromb Haemost 2019;17:1875-85. https://doi.org/10.1111/jth.14568.
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8:1-103. https://doi.org/10.3310/hta8310.
- Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modeling. Med Decis Making 2004;24:207-27. https://doi.org/10.1177/0272989X04263162.
- Rodger MA, Phillips P, Kahn SR, James AH, Konkle BA. PROSPER Investigators Low-molecular-weight heparin to prevent postpartum venous thromboembolism. A pilot randomised placebo-controlled trial. Thromb Haemost 2015;113:212-6. https://doi.org/10.1160/TH14-06-0485.
- Rodger MA, Phillips P, Kahn SR, Bates S, McDonald S, Khurana R, et al. PROSPER Investigators: Tim Ramsay, Dean Fergusson, Anne McLeod, Wee Shian Chan, Rshmi Khurana, Kara Narenberg, Haim Abenhaim, John Heit, Ghada Bourjeilly, Paul Gibson, Kent Bailey . Low molecular weight heparin to prevent postpartum venous thromboembolism: a pilot study to assess the feasibility of a randomized, open-label trial. Thromb Res 2016;142:17-20. https://doi.org/10.1016/j.thromres.2016.04.004.
- Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9. https://doi.org/10.7326/0003-4819-151-4-200908180-00135.
- Wilczynski N. McMaster University – HiRU’s Approach to Search Filter Development. Ontario, Canada: Health Information Research Unit; n.d.
- Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1-33. https://doi.org/10.7326/M18-1377.
- Wolff RF, Moons KGM, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST Group† . PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51-8. https://doi.org/10.7326/M18-1376.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, . Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. London: Cochrane; 2021.
- Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: John Wiley & Sons; 2000.
- Bauersachs RM, Dudenhausen J, Faridi A, Fischer T, Fung S, Geisen U, et al. EThIG Investigators . Risk stratification and heparin prophylaxis to prevent venous thromboembolism in pregnant women. Thromb Haemost 2007;98:1237-45. https://doi.org/10.1160/th07-05-0329.
- Binstock AB, Larkin JC. Development of a venous thromboembolism prevention tool in the postpartum period. Obstet Gynecol 2019;133. https://doi.org/10.1097/01.AOG.0000558997.74246.16.
- Cavazza S, Rainaldi MP, Adduci A, Palareti G. Thromboprophylaxis following cesarean delivery: one site prospective pilot study to evaluate the application of a risk score model. Thromb Res 2012;129:28-31. https://doi.org/10.1016/j.thromres.2011.06.028.
- Chau C, Campagna J, Vial M, Rambeaud C, Loundou A, Bretelle F. Use of a personalized iterative score to evaluate risk of venous thromboembolism during pregnancy and puerperium. Int J Gynaecol Obstet 2019;144:277-82. https://doi.org/10.1002/ijgo.12754.
- Chauleur C, Quenet S, Varlet MN, Seffert P, Laporte S, Decousus H, et al. Feasibility of an easy-to-use risk score in the prevention of venous thromboembolism and placental vascular complications in pregnant women: a prospective cohort of 2736 women. Thromb Res 2008;122:478-84. https://doi.org/10.1016/j.thromres.2007.12.020.
- Dargaud Y, Rugeri L, Fleury C, Battie C, Gaucherand P, Huissoud C, et al. Personalized thromboprophylaxis using a risk score for the management of pregnancies with high risk of thrombosis: a prospective clinical study. J Thromb Haemos JTH 2017;15:897-906. https://doi.org/10.1111/jth.13660.
- Dargaud Y, Rugeri L, Ninet J, Negrier C, Trzeciak MC. Management of pregnant women with increased risk of venous thrombosis. Int J Gynaecol Obstet 2005;90:203-7. https://doi.org/10.1016/j.ijgo.2005.05.003.
- Gassmann N, Viviano M, Righini M, Fontana P, Martinez de Tejada B, Blondon M. Estimating the risk thresholds used by guidelines to recommend postpartum thromboprophylaxis. J Thromb Haemos 2020;19:452-9. https://doi.org/10.1111/jth.15166.
- Hase EA, de Barros VIPVL, Igai AMK, Francisco RPV, Zugaib M. Risk assessment of venous thromboembolism and thromboprophylaxis in pregnant women hospitalized with cancer: preliminary results from a risk score. Clinics (Sao Paulo) 2018;73. https://doi.org/10.6061/clinics/2018/e368.
- Lindqvist PG, Torsson J, Almqvist A, Bjorgell O. Postpartum thromboembolism: severe events might be preventable using a new risk score model. Vasc Health Risk Manag 2008;4:1081-7. https://doi.org/10.2147/vhrm.s2831.
- Lok WY, Kong CW, To WWK. A local risk score model for venous thromboembolism prophylaxis for caesarean section in Chinese women and comparison with international guidelines. Taiwan J Obstet Gynecol 2019;58:520-5. https://doi.org/10.1016/j.tjog.2019.05.016.
- Testa S, Passamonti SM, Paoletti O, Bucciarelli P, Ronca E, Riccardi A, et al. The ‘Pregnancy Health-care Program’ for the prevention of venous thromboembolism in pregnancy. Intern Emerg Med 2015;10:129-34. https://doi.org/10.1007/s11739-014-1111-6.
- Tran JP, Stribling SS, Ibezim UC, Omere C, McEnery KA, Pacheco LD, et al. Performance of risk assessment models for peripartum thromboprophylaxis. Reprod Sci 2019;26:1243-8. https://doi.org/10.1177/1933719118813197.
- Weiss N, Bernstein PS. Risk factor scoring for predicting venous thromboembolism in obstetric patients. Am J Obstet Gynecol 2000;182:1073-5. https://doi.org/10.1067/mob.2000.105441.
- Ellis-Kahana J, Sparks AD, Gimovsky AC, James AH, Ahmadzia HK. Developing a model for predicting venous thromboembolism in obese pregnant women in a national study. Thromb Res 2020;191:42-9. https://doi.org/10.1016/j.thromres.2020.03.025.
- Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMC Med 2015;13. https://doi.org/10.1186/s12916-014-0241-z.
- Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG, et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLOS Med 2014;11. https://doi.org/10.1371/journal.pmed.1001744.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013. www.nice.org.uk/process/pmg9/chapter/foreword (accessed 23 November 2020).
- Curtis L, Burns A. Unit Costs of Health and Social Care. Canterbury: University of Kent; 2020.
- Pandor A, Horner D, Davis S, Goodacre S, Stevens JW, Clowes M, et al. Different strategies for pharmacological thromboprophylaxis for lower-limb immobilisation after injury: systematic review and economic evaluation. Health Technol Assess 2019;23:1-190. https://doi.org/10.3310/hta23630.
- Horner D, Davis S, Pandor A, Shulver H, Goodacre S, Hind D, et al. An evidence synthesis and economic evaluation of venous thromboembolism risk assessment models for hospital inpatients: the VTEAM project. Health Technol Assess 2021.
- Tardy B, Chalayer E, Kamphuisen PW, Ni Ainle F, Verhamme P, Varlet MN, et al. SSC Subcommittee on Control of Anticoagulation of the ISTH . Definition of bleeding events in studies evaluating prophylactic antithrombotic therapy in pregnant women: a systematic review and a proposal from the ISTH SSC. J Thromb Haemost 2019;17:1979-88. https://doi.org/10.1111/jth.14576.
- Greer IA, Nelson-Piercy C. Low-molecular-weight heparins for thromboprophylaxis and treatment of venous thromboembolism in pregnancy: a systematic review of safety and efficacy. Blood 2005;106:401-7. https://doi.org/10.1182/blood-2005-02-0626.
- Galambosi P, Hiilesmaa V, Ulander VM, Laitinen L, Tiitinen A, Kaaja R. Prolonged low-molecular-weight heparin use during pregnancy and subsequent bone mineral density. Thromb Res 2016;143:122-6. https://doi.org/10.1016/j.thromres.2016.05.016.
- Moody A. Health Survey for England 2019 Overweight and Obesity in Adults and Children. Leeds: NHS Digital; 2020.
- De Stefano V, Martinelli I, Rossi E, Battaglioli T, Za T, Mannuccio Mannucci P, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006;135:386-91. https://doi.org/10.1111/j.1365-2141.2006.06317.x.
- Pabinger I, Grafenhofer H, Kaider A, Kyrle PA, Quehenberger P, Mannhalter C, et al. Risk of pregnancy-associated recurrent venous thromboembolism in women with a history of venous thrombosis. J Thromb Haemost 2005;3:949-54. https://doi.org/10.1111/j.1538-7836.2005.01307.x.
- Brill-Edwards P, Ginsberg JS, Gent M, Hirsh J, Burrows R, Kearon C, et al. Recurrence of Clot in This Pregnancy Study Group . Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med 2000;343:1439-44. https://doi.org/10.1056/NEJM200011163432002.
- Sultan AA, Grainge MJ, West J, Fleming KM, Nelson-Piercy C, Tata LJ. Impact of risk factors on the timing of first postpartum venous thromboembolism: a population-based cohort study from England. Blood 2014;124:2872-80. https://doi.org/10.1182/blood-2014-05-572834.
- Blondon M, Casini A, Hoppe KK, Boehlen F, Righini M, Smith NL. Risks of venous thromboembolism after cesarean sections: a meta-analysis. Chest 2016;150:572-96. https://doi.org/10.1016/j.chest.2016.05.021.
- Sia WW, Powrie RO, Cooper AB, Larson L, Phipps M, Spencer P, et al. The incidence of deep vein thrombosis in women undergoing cesarean delivery. Thromb Res 2009;123:550-5. https://doi.org/10.1016/j.thromres.2008.06.004.
- Macklon NS, Barry J, Greer IA. Duplex ultrasound screening for deep venous thrombosis in the puerperium. Br J Obstet Gynaecol 1995;102:255-6. https://doi.org/10.1111/j.1471-0528.1995.tb09104.x.
- Kalro BN, Davidson RA, Owen P. Low incidence of asymptomatic deep venous thrombosis following caesarean section: a colour Doppler study. Health Bull (Edinb) 1999;57:418-21.
- Jacobsen AF, Drolsum A, Klow NE, Dahl GF, Qvigstad E, Sandset PM. Deep vein thrombosis after elective cesarean section. Thromb Res 2004;113:283-8. https://doi.org/10.1016/j.thromres.2004.03.008.
- Goto M, Yoshizato T, Tatsumura M, Takashima T, Ogawa M, Nakahara H, et al. Safety and efficacy of thromboprophylaxis using enoxaparin sodium after cesarean section: a multi-center study in Japan. Taiwan J Obstet Gynecol 2015;54:248-52. https://doi.org/10.1016/j.tjog.2014.09.008.
- Chan LYS, Lam KYM, Metreweli C, Lau TK. Duplex ultrasound screening for deep vein thrombosis in Chinese after cesarean section. Acta Obstet Gynecol Scand 2005;84:368-70. https://doi.org/10.1111/j.0001-6349.2005.00591.x.
- Elgendy IY, Fogerty A, Blanco-Molina A, Rosa V, Schellong S, Skride A, et al. Clinical characteristics and outcomes of women presenting with venous thromboembolism during pregnancy and postpartum period: findings from the RIETE registry. Thromb Haemost 2020;120:1454-62. https://doi.org/10.1055/s-0040-1714211.
- van Hoorn ME, Hague WM, van Pampus MG, Bezemer D, de Vries JIP. FRUIT Investigators Low-molecular-weight heparin and aspirin in the prevention of recurrent early-onset pre-eclampsia in women with antiphospholipid antibodies: the FRUIT-RCT. Eur J Obstet Gynecol Reprod Biol 2016;197:168-73. https://doi.org/10.1016/j.ejogrb.2015.12.011.
- de Vries JIP, van Pampus MG, Hague WM, Bezemer PD, Joosten JH. FRUIT Investigators Low-molecular-weight heparin added to aspirin in the prevention of recurrent early-onset pre-eclampsia in women with inheritable thrombophilia: the FRUIT-RCT. J Thromb Haemost 2012;10:64-72. https://doi.org/10.1111/j.1538-7836.2011.04553.x.
- Rodger MA, Hague WM, Kingdom J, Kahn SR, Karovitch A, Sermer M, et al. TIPPS Investigators . Antepartum dalteparin versus no antepartum dalteparin for the prevention of pregnancy complications in pregnant women with thrombophilia (TIPPS): a multinational open-label randomised trial. Lancet 2014;384:1673-83. https://doi.org/10.1016/S0140-6736(14)60793-5.
- Gates S, Brocklehurst P, Ayers S, Bowler U. Thromboprophylaxis in Pregnancy Advisory Group . Thromboprophylaxis and pregnancy: two randomized controlled pilot trials that used low-molecular-weight heparin. Am J Obstet Gynecol 2004;191:1296-303. https://doi.org/10.1016/j.ajog.2004.03.039.
- Burrows RF, Gan ET, Gallus AS, Wallace EM, Burrows EA. A randomised double-blind placebo controlled trial of low molecular weight heparin as prophylaxis in preventing venous thrombolic events after caesarean section: a pilot study. BJOG 2001;108:835-9. https://doi.org/10.1111/j.1471-0528.2001.00198.x.
- Algahtani FH, Al-Dohami H, Abo-Harbesh S, Gader AGA, Aamer A. Thromboembolism prophylaxis after cesarean section (PRO-CS) trial. Thromb Res 2015;130. https://doi.org/10.1016/j.thromres.2012.08.253.
- Nelson-Piercy C, Powrie R, Borg JY, Rodger M, Talbot DJ, Stinson J, et al. Tinzaparin use in pregnancy: an international, retrospective study of the safety and efficacy profile. Eur J Obstet Gynecol Reprod Biol 2011;159:293-9. https://doi.org/10.1016/j.ejogrb.2011.08.005.
- Schoenbeck D, Nicolle A, Newbegin K, Hanley J, Loughney AD. The use of a scoring system to guide thromboprophylaxis in a high-risk pregnant population. Thrombosis 2011;2011. https://doi.org/10.1155/2011/652796.
- Cox S, Eslick R, McLintock C. Effectiveness and safety of thromboprophylaxis with enoxaparin for prevention of pregnancy-associated venous thromboembolism. J Thromb Haemost 2019;17:1160-70. https://doi.org/10.1111/jth.14452.
- Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-4. https://doi.org/10.1111/j.1538-7836.2005.01204.x.
- Lindqvist PG, Bremme K, Hellgren M. Working Group on Hemostatic Disorders (Hem-ARG), Swedish Society of Obstetrics and Gynecology . Efficacy of obstetric thromboprophylaxis and long-term risk of recurrence of venous thromboembolism. Acta Obstet Gynecol Scand 2011;90:648-53. https://doi.org/10.1111/j.1600-0412.2011.01098.x.
- Royal College of Obstetricians & Gynaecologists . Thromboembolic Disease in Pregnancy and the Puerperium: Acute Management: Green-Top Guideline No. 37b 2015.
- Gizzo S, Noventa M, Anis O, Saccardi C, Zambon A, Di Gangi S, et al. Pharmacological anti-thrombotic prophylaxis after elective caesarean delivery in thrombophilia unscreened women: should maternal age have a role in decision making?. J Perinat Med 2014;42:339-47. https://doi.org/10.1515/jpm-2013-0160.
- Ferres MA, Olivarez SA, Trinh V, Davidson C, Sangi-Haghpeykar H, Aagaard-Tillery KM. Rate of wound complications with enoxaparin use among women at high risk for postpartum thrombosis. Obstet Gynecol 2011;117:119-24. https://doi.org/10.1097/AOG.0b013e3182029180.
- Knight M, Bunch K, Tuffnel D, Shakespeare J, Kotnis R, Kenyon S, et al. Saving Lives, Improving Mother’s Care: Lessons Learned to Inform Maternity Care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2016–18. Oxford: National Perinatal Epidemiology Unity, University of Oxford; 2020.
- Ban L, Sprigg N, Abdul Sultan A, Nelson-Piercy C, Bath PM, Ludvigsson JF, et al. Incidence of first stroke in pregnant and nonpregnant women of childbearing age: a population-based cohort study from England. J Am Heart Assoc 2017;6. https://doi.org/10.1161/JAHA.116.004601.
- Jerjes-Sanchez C, Rodriguez D, Farjat AE, Kayani G, MacCallum P, Lopes RD, et al. GARFIELD-VTE Investigators . Pregnancy-associated venous thromboembolism: insights from GARFIELD-VTE. TH Open 2021;5:e24-34. https://doi.org/10.1055/s-0040-1722611.
- Nieto JA, Solano R, Ruiz-Ribo MD, Ruiz-Gimenez N, Prandoni P, Kearon C, et al. Riete Investigators . Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE registry. J Thromb Haemost 2010;8:1216-22. https://doi.org/10.1111/j.1538-7836.2010.03852.x.
- Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, Delcroix M, Pruszczyk P, Mairuhu ATA, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017;49. https://doi.org/10.1183/13993003.01792-2016.
- Pengo V, Lensing AW, Prins MH, Marchiori A, Davidson BL, Tiozzo F, et al. Thromboembolic Pulmonary Hypertension Study Group . Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257-64. https://doi.org/10.1056/NEJMoa032274.
- Kourlaba G, Relakis J, Kontodimas S, Holm MV, Maniadakis N. A systematic review and meta-analysis of the epidemiology and burden of venous thromboembolism among pregnant women. Int J Gynaecol Obstet 2016;132:4-10. https://doi.org/10.1016/j.ijgo.2015.06.054.
- Wik HS, Jacobsen AF, Sandvik L, Sandset PM. Prevalence and predictors for post-thrombotic syndrome 3 to 16 years after pregnancy-related venous thrombosis: a population-based, cross-sectional, case-control study. J Thromb Haemost 2012;10:840-7. https://doi.org/10.1111/j.1538-7836.2012.04690.x.
- van Dongen CJJ, Prandoni P, Frulla M, Marchiori A, Prins MH, Hutten BA. Relation between quality of anticoagulant treatment and the development of the postthrombotic syndrome. J Thromb Haemost 2005;3:939-42. https://doi.org/10.1111/j.1538-7836.2005.01333.x.
- Fogelholm R, Murros K, Rissanen A, Avikainen S. Long term survival after primary intracerebral haemorrhage: a retrospective population based study. J Neurol Neurosurg Psychiatry 2005;76:1534-8. https://doi.org/10.1136/jnnp.2004.055145.
- Liu S, Rouleau J, Joseph KS, Sauve R, Liston RM, Young D, et al. Epidemiology of pregnancy-associated venous thromboembolism: a population-based study in Canada. J Obstet Gynaecol Can 2009;31:611-20. https://doi.org/10.1016/S1701-2163(16)34240-2.
- Goodacre S, Horspool K, Shephard N, Pollard D, Hunt BJ, Fuller G, et al. Selecting pregnant or postpartum women with suspected pulmonary embolism for diagnostic imaging: the DiPEP diagnostic study with decision-analysis modelling. Health Technol Assess 2018;22:1-230. https://doi.org/10.3310/hta22470.
- Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D’Armini AM, Snijder R, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an International Prospective Registry. Circulation 2016;133:859-71. https://doi.org/10.1161/CIRCULATIONAHA.115.016522.
- Office for National Statistics . National Life Tables, England: 2016–18 2019.
- Joint Formulary Committee . British National Formulary (online) 2021.
- Menakaya CU, Pennington N, Muthukumar N, Joel J, Ramirez Jimenez AJ, Shaw CJ, et al. The cost of outpatient venous thromboembolism prophylaxis following lower limb injuries. Bone Joint J 2013;95-B:673-7. https://doi.org/10.1302/0301-620X.95B5.30555.
- National Cost Collection . National Schedule of NHS Costs – Year 2018–19: NHS Trust and NHS Foundation 2020.
- McFarlane SQ, Patel JP, Auyeung V, Arya R. An international survey of low molecular weight heparin prescribing in the context of antenatal venous thromboembolism. Thromb Res 2021;200:99-101. https://doi.org/10.1016/j.thromres.2021.01.024.
- Goodacre S, Nelson-Piercy C, Hunt BJ, Fuller G. Accuracy of PE rule-out strategies in pregnancy: secondary analysis of the DiPEP study prospective cohort. Emerg Med J 2020;37:423-8. https://doi.org/10.1136/emermed-2019-209213.
- Knight M. UKOSS . Antenatal pulmonary embolism: risk factors, management and outcomes. BJOG 2008;115:453-61. https://doi.org/10.1111/j.1471-0528.2007.01622.x.
- Luengo-Fernandez R, Yiin GS, Gray AM, Rothwell PM. Population-based study of acute- and long-term care costs after stroke in patients with AF. Int J Stroke 2013;8:308-14. https://doi.org/10.1111/j.1747-4949.2012.00812.x.
- Johnston JA, Brill-Edwards P, Ginsberg JS, Pauker SG, Eckman MH. Cost-effectiveness of prophylactic low molecular weight heparin in pregnant women with a prior history of venous thromboembolism. Am J Med 2005;118:503-14. https://doi.org/10.1016/j.amjmed.2004.12.009.
- Pickering K, Gallos ID, Williams H, Price MJ, Merriel A, Lissauer D, et al. Uterotonic drugs for the prevention of postpartum haemorrhage: a cost-effectiveness analysis. PharmacoEconomics Open 2019;3:163-76. https://doi.org/10.1007/s41669-018-0108-x.
- Richardson J, Hollier-Hann G, Kelly K, Chiara Alvisi M, Winter C, Cetin I, et al. A study of the healthcare resource use for the management of postpartum haemorrhage in France, Italy, the Netherlands, and the UK. Eur J Obstet Gynecol Reprod Biol 2022;268:92-9. https://doi.org/10.1016/j.ejogrb.2021.11.432.
- Caprini JA, Botteman MF, Stephens JM, Nadipelli V, Ewing MM, Brandt S, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health 2003;6:59-74. https://doi.org/10.1046/j.1524-4733.2003.00204.x.
- Etxeandia-Ikobaltzeta I, Zhang Y, Brundisini F, Florez ID, Wiercioch W, Nieuwlaat R, et al. Patient values and preferences regarding VTE disease: a systematic review to inform American Society of Hematology guidelines. Blood Adv 2020;4:953-68. https://doi.org/10.1182/bloodadvances.2019000462.
- Chuang LH, Gumbs P, van Hout B, Agnelli G, Kroep S, Monreal M, et al. Health-related quality of life and mortality in patients with pulmonary embolism: a prospective cohort study in seven European countries. Qual Life Res 2019;28:2111-24. https://doi.org/10.1007/s11136-019-02175-z.
- Monreal M, Agnelli G, Chuang LH, Cohen AT, Gumbs PD, Bauersachs R, et al. Deep vein thrombosis in Europe-Health-Related Quality of Life and Mortality. Clin Appl Thromb Hemost 2019;25. https://doi.org/10.1177/1076029619883946.
- Enden T, Wik HS, Kvam AK, Haig Y, Klow NE, Sandset PM. Health-related quality of life after catheter-directed thrombolysis for deep vein thrombosis: secondary outcomes of the randomised, non-blinded, parallel-group CaVenT study. BMJ Open 2013;3. https://doi.org/10.1136/bmjopen-2013-002984.
- Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. J Am Med Inform Assoc 1997;4:49-56. https://doi.org/10.1136/jamia.1997.0040049.
- Hach-Wunderle V, Bauersachs R, Gerlach HE, Eberle S, Schellong S, Riess H, et al. Post-thrombotic syndrome 3 years after deep venous thrombosis in the Thrombosis and Pulmonary Embolism in Out-Patients (TULIPA) PLUS Registry. J Vasc Surg Venous Lymphat Disord 2013;1:5-12. https://doi.org/10.1016/j.jvsv.2012.07.003.
- Meads DM, McKenna SP, Doughty N, Das C, Gin-Sing W, Langley J, et al. The responsiveness and validity of the CAMPHOR Utility Index. Eur Respir J 2008;32:1513-9. https://doi.org/10.1183/09031936.00069708.
- Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Oxford Vascular Study . Quality of life after TIA and stroke: ten-year results of the Oxford vascular study. Neurology 2013;81:1588-95. https://doi.org/10.1212/WNL.0b013e3182a9f45f.
- Marchetti M, Pistorio A, Barone M, Serafini S, Barosi G. Low-molecular-weight heparin versus warfarin for secondary prophylaxis of venous thromboembolism: a cost-effectiveness analysis. Am J Med 2001;111:130-9. https://doi.org/10.1016/s0002-9343(01)00793-8.
- Ara R, Brazier JE. Using health state utility values from the general population to approximate baselines in decision analytic models when condition-specific data are not available. Value Health 2011;14:539-45. https://doi.org/10.1016/j.jval.2010.10.029.
- Voke J, Keidan J, Pavord S, Spencer NH, Hunt BJ. British Society for Haematology Obstetric Haematology Group . The management of antenatal venous thromboembolism in the UK and Ireland: a prospective multicentre observational survey. Br J Haematol 2007;139:545-58. https://doi.org/10.1111/j.1365-2141.2007.06826.x.
- Sultan AA, Tata LJ, West J, Fiaschi L, Fleming KM, Nelson-Piercy C, et al. Risk factors for first venous thromboembolism around pregnancy: a population-based cohort study from the United Kingdom. Blood 2013;121:3952-61. https://doi.org/10.1182/blood-2012-11-469551.
- Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making 2014;34:311-26. https://doi.org/10.1177/0272989X13505910.
- Kunst N, Wilson ECF, Glynn D, Alarid-Escudero F, Baio G, Brennan A, et al. Collaborative Network for Value of Information . Computing the expected value of sample information efficiently: practical guidance and recommendations for four model-based methods. Value Health 2020;23:734-42. https://doi.org/10.1016/j.jval.2020.02.010.
- Office for National Statistics . Births in England and Wales: 2019 2020.
- Ziauddeen N, Wilding S, Roderick PJ, Macklon NS, Alwan NA. Is maternal weight gain between pregnancies associated with risk of large-for-gestational age birth? Analysis of a UK population-based cohort. BMJ Open 2019;9. https://doi.org/10.1136/bmjopen-2018-026220.
- van der Zande ISE, van der Graaf R, Hooft L, van Delden JJM. Facilitators and barriers to pregnant women’s participation in research: a systematic review. Women Birth 2018;31:350-61. https://doi.org/10.1016/j.wombi.2017.12.009.
- Rodger MA, Makropoulos D, Walker M, Keely E, Karovitch A, Wells PS. Participation of pregnant women in clinical trials: will they participate and why?. Am J Perinatol 2003;20:69-76. https://doi.org/10.1055/s-2003-38318.
- Braun V, Clarke V. One size fits all? What counts as quality practice in (reflexive) thematic analysis?. Qual Res Psychol 2021;18:328-52. https://doi.org/10.1080/14780887.2020.1769238.
- Strong M, Oakley JE, Brennan A, Breeze P. Estimating the expected value of sample information using the probabilistic sensitivity analysis sample: a fast, nonparametric regression-based method. Med Decis Making 2015;35:570-83. https://doi.org/10.1177/0272989X15575286.
- Reps JM, Ryan PB, Rijnbeek PR, Schuemie MJ. Design matters in patient-level prediction: evaluation of a cohort vs. case-control design when developing predictive models in observational healthcare datasets. J Big Data 2021;8. https://doi.org/10.1186/s40537-021-00501-2.
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021). Cochrane; 2021.
- Smyth RMD, Jacoby A, Elbourne D. Deciding to join a perinatal randomised controlled trial: experiences and views of pregnant women enroled in the Magpie Trial. Midwifery 2012;28:E478-85. https://doi.org/10.1016/j.midw.2011.08.006.
- van der Zande ISE, van der Graaf R, Oudijk MA, van Vliet-Lachotzki EH, van Delden JJM. A qualitative study on stakeholders’ views on the participation of pregnant women in the APOSTEL VI study: a low-risk obstetrical RCT. BMC Pregnancy Childbirth 2019;19. https://doi.org/10.1186/s12884-019-2209-7.
- Hanrahan V, Gillies K, Biesty L. Recruiters’ perspectives of recruiting women during pregnancy and childbirth to clinical trials: a qualitative evidence synthesis. PLOS ONE 2020;15. https://doi.org/10.1371/journal.pone.0234783.
- Tooher RL, Middleton PF, Crowther CA. A thematic analysis of factors influencing recruitment to maternal and perinatal trials. BMC Pregnancy Childbirth 2008;8. https://doi.org/10.1186/1471-2393-8-36.
- Renert H, Russell-Mayhew S, Arthur N. Recruiting ethnically diverse participants into qualitative health research: lessons learned. Qual Rep 2013;18:1-13. https://doi.org/10.46743/2160-3715/2013.1542.
- Rooney LK, Bhopal R, Halani L, Levy ML, Partridge MR, Netuveli G, et al. Promoting recruitment of minority ethnic groups into research: qualitative study exploring the views of South Asian people with asthma. J Public Health (Oxf) 2011;33:604-15. https://doi.org/10.1093/pubmed/fdq100.
- Hanrahan V, Biesty L, Lawrie L, Duncan E, Gillies K. Theory-guided interviews identified _behavioral barriers and enablers to healthcare professionals recruiting participants to maternity trials. J Clin Epidemiol 2022;145:81-9. https://doi.org/10.1016/j.jclinepi.2022.01.015.
- James A, Birsner M, Kaimal A. Collaboration with American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins-Obstetrics . ACOG Practice Bulletin No. 196 Summary: thromboembolism in pregnancy. Obstet Gynecol 2018;132:243-8. https://doi.org/10.1097/AOG.0000000000002707.
- Lindqvist PG, Hellgren M. Obstetric thromboprophylaxis: the Swedish guidelines. Adv Hematol 2011;2011. https://doi.org/10.1155/2011/157483.
- Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al. Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study. Health Technol Assess 2006;10:1-110. https://doi.org/10.3310/hta10110.
- Wu O, Robertson L, Twaddle S, Lowe G, Clark P, Walker I, et al. Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) Study . Screening for thrombophilia in high-risk situations: a meta-analysis and cost-effectiveness analysis. Br J Haematol 2005;131:80-9. https://doi.org/10.1111/j.1365-2141.2005.05715.x.
- Wu O, Greer IA. Is screening for thrombophilia cost-effective?. Curr Opin Hematol 2007;14:500-3. https://doi.org/10.1097/MOH.0b013e32825f5318.
- Wormer KC, Jangda AA, El Sayed FA, Stewart KI, Mumford SL, Segars JH. Is thromboprophylaxis cost effective in ovarian hyperstimulation syndrome: a systematic review and cost analysis. Eur J Obstet Gynecol Reprod Biol 2018;224:117-24. https://doi.org/10.1016/j.ejogrb.2018.03.028.
- Westhoff G, Yanit K, Volpe KA, Pilliod R, Doss A, Rodriguez M, et al. The cost-effectiveness of thromboprophylaxis with low-molecular weight heparin or unfractionated heparin after cesarean delivery. Am J Obstet Gynecol 2012;206. https://doi.org/10.1016/j.ajog.2011.10.854.
- Sievert A, Hernandez M, Hersh AR, Harmon D, Caughey AB. Pregnancy-adapted years algorithm for diagnosing suspected pulmonary embolism: a cost-effectiveness analysis. Obstet Gynecol 2020;135. https://doi.org/10.1097/01.AOG.0000664664.88371.1c.
- Sabol BA, Rosenbloom JI, Cahill AG, Macones GA. 832: Universal screening of inherited thrombophilias to reduce venous thromboembolism risk in pregnancy: a decision analysis. Am J Obstet Gynecol 2019;220:S543-4. https://doi.org/10.1016/j.ajog.2018.11.855.
- Rizvi AH, Smith KJ, Ragni MV. Cost-effectiveness of thromboprophylaxis in pregnant women with sickle cell disease. Blood 2013;122. https://doi.org/10.1182/blood.V122.21.426.426.
- Quinones JN, James DN, Stamilio DM, Cleary KL, Macones GA. Thromboprophylaxis after cesarean delivery: a decision analysis. Obstet Gynecol 2005;106:733-40. https://doi.org/10.1097/01.AOG.0000178792.51401.3a.
- Pollard D, Goodacre S, Stevenson M, Fuller G. Decision analysis modelling of diagnostic strategies for suspected pulmonary embolism in pregnancy: the DiPEP economic evaluation. Emerg Med J 2017;34:A867-8. https://doi.org/10.1136/emermed-2017-207308.10.
- Lee VR, Westhoff GL, Pilliod RA, Yanit KE, Caughey AB. Cost-effectiveness of post-cesarean pharmacologic VTE prophylaxis in obese women. Am J Obstet Gynecol 2017;216:S232-3. https://doi.org/10.1016/j.ajog.2016.11.645.
- Iroz CB, Dahl CM, Cassimatis IR, Wescott AB, Miller ES. Prophylactic anticoagulation for preterm premature rupture of membranes: a decision analysis. Am J Obstet Gynecol MFM 2021;3. https://doi.org/10.1016/j.ajogmf.2021.100311.
- Houlihan M, Higgins J, Ismail S, Murphy A. Prospective cost analysis of low molecular weight heparin thromboprophylaxis post-planned caesarean section. Ir J Med Sci 2017;186. https://doi.org/10.1007/s11845-017-1629-5.
- Eckman MH, Alonso-Coello P, Guyatt GH, Ebrahim S, Tikkinen KAO, Lopes LC, et al. Women’s values and preferences for thromboprophylaxis during pregnancy: a comparison of direct-choice and decision analysis using patient specific utilities. Thromb Res 2015;136:341-7. https://doi.org/10.1016/j.thromres.2015.05.020.
- Dahl CM, Iroz C, Wescott A, Cassimatis I, Miller ES. Prophylactic anticoagulation while hospitalized for premature prelabor rupture of membranes: a decision analysis. Obstet Gynecol 2020;135. https://doi.org/10.1097/01.AOG.0000664672.29477.41.
- Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol 2006;108:535-40. https://doi.org/10.1097/01.AOG.0000227780.76353.05.
- Bunce E, Sheperd JP, Paruchuri Y, Simhan HN. Optimal Strategy for Venous Thromboembolism Prophylaxis Following Cesarean Delivery: A Decision Analysis. Am J Obstet Gynecol 2017;216:S379-80.
- Blondon M, Perrier A, Nendaz M, Righini M, Boehlen F, Boulvain M, et al. Thromboprophylaxis with low-molecular-weight heparin after cesarean delivery: a decision analysis. Thromb Haemost 2010;103:129-37. https://doi.org/10.1160/TH09-06-0349.
- Becker DA, Einerson BD, Wetta LL, Kuper SG, Casey BM, Subramaniam A. 742: Postpartum venous thromboembolism prophylaxis: a cost-effectiveness analysis. Am J Obstet Gynecol 2019;220:S487-8. https://doi.org/10.1016/j.ajog.2018.11.765.
- Bajaj PS, Veenstra DL. A risk-benefit analysis of factor V Leiden testing to improve pregnancy outcomes: a case study of the capabilities of decision modeling in genomics. Genet Med 2013;15:374-81. https://doi.org/10.1038/gim.2012.139.
- National Clinical Guideline Centre . Venous Thromboembolism: Reducing the Risk for Patients in Hospital 2012.
Appendix 1 Literature search strategy
| Database searched: | Ovid MEDLINE(R) Epub Ahead of Print, In-Process and Other Non-Indexed Citations, Ovid MEDLINE(R) Daily, Ovid MEDLINE and Versions(R) |
| Platform or provider used: | Ovid SP |
| Date of coverage: | 1946–February 2021 |
| Search undertaken: | February 2021 |
-
Pregnant Women/or exp Pregnancy Complications/or exp Maternal Health Services/or exp Fetal Monitoring/or exp Prenatal Diagnosis/or Perinatal Care/or Labor pain/or Analgesia, Obstetric/or exp Obstetric Surgical Procedures/or exp Postpartum Period/
-
(pregnan* or antenatal* or ante-natal* or prenatal* or pre-natal* or gestational* or matern* or perinatal* or peri-natal* or postnatal* or post-natal* or postpartum or post-partum or puerper* or obstetric).mp.
-
1 or 2
-
pulmonary embolism/or thromboembolism/or venous thromboembolism/or venous thrombosis/or upper extremity deep vein thrombosis/
-
(((venous or vein) adj (thrombosis or thromboses or thrombus or thromboemboli*)) or (dvt or vte) or ((pulmonary or lung) adj3 (embolism or emboli or embolus or emboliz* or thromboemboli*))).ti,ab.
-
4 or 5
-
editorial/or news/or exp historical article/or anecdotes as topic/or comment/or case report/or (letter or comment).ti.
-
randomized controlled trial/or random*.ti,ab.
-
7 not 8
-
animals/not humans/
-
exp animals, laboratory/
-
exp animal experimentation/
-
exp models, animal/
-
exp rodentia/
-
(rat or rats or mouse or mice).ti.
-
9 or 10 or 11 or 12 or 13 or 14 or 15
-
6 not 16
-
(risk* adj2 assess*).ti,ab.
-
((score* or scoring) adj2 (tool* or system*)).ti,ab.
-
((risk* or predict* or prognos*) adj4 (tool* or rule* or index* or indices or score* or scoring or scale* or model* or system* or algorithm* or stratif* or criteria or calculat*)).ti,ab.
-
department of health.ti,ab,au.
-
(guidance or guideline*).ti,hw,pt.
-
18 or 19 or 20 or 21 or 22
-
17 and 23
-
3 and 24
| Databases searched: | EMBASE |
| Platform or provider used: | Ovid SP |
| Date of coverage: | 1974–February 2021 |
| Search undertaken: | February 2021 |
-
exp pregnancy/or maternal health service/or exp pregnancy complication/or exp fetus monitoring/or exp prenatal diagnosis/or exp perinatal care/or exp obstetric analgesia/or exp labor pain/or exp obstetrics/or obstetric analgesia/or exp obstetric operation/or puerperium/
-
(pregnan* or antenatal* or ante-natal* or prenatal* or pre-natal* or gestational* or matern* or perinatal* or peri-natal* or postnatal* or post-natal* or postpartum or post-partum or puerper* or obstetric or labo?r).mp.
-
1 or 2
-
lung embolism/or exp venous thromboembolism/or exp vein thrombosis/or upper extremity deep vein thrombosis/
-
(((venous or vein) adj (thrombosis or thromboses or thrombus or thromboemboli*)) or (dvt or vte) or ((pulmonary or lung) adj3 (embolism or emboli or embolus or emboliz* or thromboemboli*))).ti,ab.
-
4 or 5
-
editorial/or comment/or case report/or (letter or comment).ti.
-
randomized controlled trial/or random*.ti,ab.
-
7 not 8
-
exp animal/not exp human/
-
(rat or rats or mouse or mice).ti.
-
9 or 10
-
6 not 12
-
(risk* adj2 assess*).ti,ab.
-
((score* or scoring) adj2 (tool* or system*)).ti,ab.
-
((risk* or predict* or prognos*) adj4 (tool* or rule* or index* or indices or score* or scoring or scale* or model* or system* or algorithm* or stratif* or criteria or calculat*)).ti,ab.
-
department of health.ti,ab,au.
-
(guidance or guideline*).ti,hw,pt.
-
14 or 15 or 16 or 17 or 18
-
13 and 19
-
3 and 20
| Databases searched: | Cochrane CENTRAL Register of Randomised Controlled Trials and Cochrane Database of Systematic Reviews |
| Platform or provider used: | www.thecochranelibrary.com |
| Date of coverage: | Inception to February 2021 |
| Search undertaken: | February 2021 |
-
MeSH descriptor: [Pregnancy] explode all trees
-
MeSH descriptor: [Pregnancy Complications] 1 tree(s) exploded
-
MeSH descriptor: [Maternal Health Services] explode all trees
-
MeSH descriptor: [Fetal Monitoring] explode all trees
-
MeSH descriptor: [Perinatal Care] explode all trees
-
MeSH descriptor: [Labor Pain] explode all trees
-
MeSH descriptor: [Analgesia, Obstetrical] explode all trees
-
MeSH descriptor: [Obstetric Surgical Procedures] explode all trees
-
MeSH descriptor: [Postpartum Period] explode all trees
-
(pregnan* or antenatal* or ‘ante-natal*’ or prenatal* or ‘pre-natal*’ or gestational* or matern* or perinatal* or ‘peri-natal*’ or postnatal* or ‘post-natal*’ or postpartum or ‘post-partum’ or puerper* or obstetric):ti,ab,kw (Word variations have been searched)
-
MeSH descriptor: [Pulmonary Embolism] explode all trees
-
MeSH descriptor: [Venous Thromboembolism] explode all trees
-
MeSH descriptor: [Venous Thrombosis] explode all trees
-
MeSH descriptor: [Upper Extremity Deep Vein Thrombosis] explode all trees
-
((venous or vein) near/2 (thrombosis or thromboses or thrombus or thromboemboli*)):ti,ab,kw OR ((dvt or vte)):ti,ab,kw OR ((pulmonary or lung) near/2 (embolism or emboli or embolus or emboliz* or thromboemboli*)):ti,ab,kw (Word variations have been searched)
-
#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10
-
#11 or #12 or #13 or #14 or #15
-
#16 and #17
-
(risk* or predict* or prognos*):ti,ab,kw AND (tool* or rule* or index* or indices or score* or scoring or scale* or model* or system* or algorithm* or stratif* or criteria or calculat*):ti,ab,kw OR ((pulmonary or lung) near/3 (embolism or emboli or embolus or emboliz* or thromboemboli*)):ti,ab,kw (Word variations have been searched)
-
(score* or scoring) near/2 (tool* or system*)
-
guidance or guideline* or ‘department of health’
-
#19 or #20 or #21
-
#18 and #22
Appendix 2 Summary of widely evaluated generic risk assessment models
| Characteristics | Name of VTE RAM | |||
|---|---|---|---|---|
| RCOG | ACOG | SFOG | Lyon score | |
| General | ||||
| Author, year | Royal College of Obstetricians and Gynaecologists, 2015 7 |
James et al., 2018132 | Lindquist et al., 200836 and Lindqvist and Hellgren, 2011133 | Dargaud et al., 201732 |
| Applicable cohort | All pregnant and postpartum women | All pregnant and postpartum women at risk | Pregnant women with moderate-high risk of VTE | Pregnant women with high risk of thrombosis |
| Design | Risk factor based with cumulative score | Risk factor based | Risk factor based with cumulative score | Risk factor based with cumulative score |
| Number of VTE risk variables | 26 | Not specified | 23 | 15 |
| When is pharmacological thromboprophylaxis recommended? |
|
|
|
|
| Pre-existing risk factors | ||||
| Previous VTE (personal) | Yes (except a single event related to major surgery) | Yes | Yes | Yes [pregnancy related, DVT or massive PE or VTE in childhood (< 16 years); unprovoked or oestrogen related; transient risk factor induced] |
| Recurrent VTE | No | Yes | Yes | Yes (personal history; residual venous thrombi with clinical signs of PTS, recent < 2 years) |
| Previous VTE provoked by specific event | Yes (major surgery) | Yes (surgery, trauma or immobility AND additional major thrombotic risk factors)b | No | No |
| Family history of VTE | Yes (unprovoked or oestrogen related) | Yes (first degree with thrombophilia) | Yes (first degree < 60 years) | Yes (severe or recurrent) |
| Thrombophilia, for example factor V Leiden and factor II mutations; protein C, protein S and antithrombin deficiency; antiphospholipid syndrome (with or without VTE) | Yes (various forms) | Yes (various forms) | Yes (various forms) | Yes (various forms) |
| Medical comorbidities | Yes (3 points for any individual comorbidities) | No | Yes (inflammatory bowel disease) | No |
| Age | Yes (> 35 years) | No | Yes (> 40 years) | Yes (> 35 years) |
| Obesity | Yes (≥ 30 kg/m2; ≥ 40 kg/m2) | No | Yes (> 28 kg/m2 in early pregnancy) | Yes (≥ 30 kg/m2) |
| Parity | Yes (≥ 3) | No | No | No |
| Smoker | Yes | No | No | No |
| Varicose veins | Yes (gross) | No | No | No |
| Hyperhomocysteinaemia | No | No | Yes (homocysteine > 8 μmol/l in pregnancy) | No |
| Mechanical heart prosthesis | No | No | Yes | No |
| Chronic warfarin prophylaxis | No | No | Yes | No |
| Obstetric | ||||
| Pre-eclampsia | Yes (current pregnancy) | No | Yes | No |
| ART/IVF | Yes (antenatal only) | No | No | No |
| Multiple pregnancy | Yes | No | No | Yes |
| Caesarean section | Yes (elective/in labour) | No | Yes | No |
| Mid-cavity or rotational operative delivery | Yes | No | No | No |
| Prolonged labour (> 24 hours) | Yes | No | No | No |
| PPH | Yes (> 1 l or transfusion) | No | No | No |
| Preterm birth | Yes (< 37 weeks, current pregnancy) | No | No | No |
| Stillbirth | Yes (current pregnancy) | No | No | No |
| Abruptio placenta | No | No | Yes | No |
| Transient factors | ||||
| Any surgical procedure | Yes (pregnancy or puerperium except immediate repair of the perineum) | No | No | No |
| Hyperemesis | Yes | No | No | No |
| Ovarian hyperstimulation syndrome | Yes (first trimester only) | No | No | No |
| Systemic infection | Yes (current) | No | No | No |
| Immobility | Yes (current and dehydration) | No | Yes | Yes |
| Other | ||||
| ‘Other risk factors’ | No | No | Yes (according to clinical decision) | No |
Appendix 3 Review of relevant published cost-effectiveness analyses
A systematic review was undertaken to identify any existing published studies on the cost-effectiveness of thromboprophylaxis with LMWH during pregnancy or in the puerperium. The research question being addressed here is whether risk assessment tools can be used to identify women for thromboprophylaxis LMWH. Therefore, studies which used alternative methods of thromboprophylaxis such as unfractionated heparin or mechanical prophylaxis were not considered relevant. The inclusion/exclusion criteria were as follows:
-
Population: Women at risk of VTE who are either pregnant or within 6 weeks of the end of pregnancy.
-
Intervention: Thromboprophylaxis for all or thromboprophylaxis given according to a RAM.
-
Comparators: No thromboprophylaxis or thromboprophylaxis given according to an alternative RAM.
-
Study design: Full economic evaluation, that is not resource use or cost–consequences study.
-
Outcomes: Expected costs and QALYs for each thromboprophylaxis strategy and incremental cost-effectiveness ratio for the comparison(s) of interest.
-
Setting/Perspective: UK NHS or NHS and PSS.
Searches for economic evidence were conducted in two phases in February 2021. Searches used the population terms from the systematic review of RAMs (see Appendix 1) and included a facet for pregnancy as well as the conditions (DVT, PE, thrombosis). These were combined with the cost and economic filters developed by the McMaster University Health Information Research Unit (‘best balance’ of sensitivity and specificity). After validating this approach against known studies, one further term (‘decision’, in titles or keywords) was added to the filters. A cut-off date of 2017 (the date of the searches used to inform the NICE guideline update) was initially applied; however, a decision was later taken to remove this limit and backdate the searches to database inception. In total, 1013 unique records were retrieved (after removal of duplicates). An example search strategy (from MEDLINE) is reproduced in Table 19 – similar searches were run on Embase and the Cochrane Library.
| Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review and Other Non-Indexed Citations, Daily and Versions < 1946 to 9 February 2021> | |
|---|---|
| 1 | Pregnant Women/or exp Pregnancy Complications/or exp Maternal Health Services/or exp Fetal Monitoring/or exp Prenatal Diagnosis/or Perinatal Care/or Labor pain/or Analgesia, Obstetric/or exp Obstetric Surgical Procedures/or exp Postpartum Period/ |
| 2 | (pregnan* or antenatal* or ante-natal* or prenatal* or pre-natal* or gestational* or matern* or perinatal* or peri-natal* or postnatal* or post-natal* or postpartum or post-partum or puerper* or obstetric).mp. |
| 3 | 1 or 2 |
| 4 | pulmonary embolism/or thromboembolism/or venous thromboembolism/or venous thrombosis/or upper extremity deep vein thrombosis/ |
| 5 | (((venous or vein) adj (thrombosis or thromboses or thrombus or thromboemboli*)) or (dvt or vte) or ((pulmonary or lung) adj3 (embolism or emboli or embolus or emboliz* or thromboemboli*))).ti,ab. |
| 6 | 4 or 5 |
| 7 | (cost: or cost benefit analys: or health care costs).mp. or (exp ‘costs and cost analysis’/or costs.tw. or cost effective:.tw.) or decision*.ti,hw,kw. |
| 8 | 3 and 6 and 7 |
Twenty-two papers were identified as being potentially relevant during the sift of titles and abstracts, summarised in Figure 29. 89,99,134–153 Two papers reported the same economic evaluation and were therefore considered as one study. 89,143 Similarly, three papers reported the same economic evaluation and were therefore considered as one study. 134–136 Nine of the 20 citations were for conference abstracts. 138–141,144,146,148,150,152 In one case, an abstract was included but the later full-text publication of the same analysis was identified and the abstract was excluded for this reason. 145,148 For the remaining eight abstracts,138–141,144,146,150,152 no full-text paper was identified so the application of the inclusion criteria was based on the limited information presented in the abstract. Therefore, there were 19 unique economic evaluations reported across the 22 papers.
FIGURE 29.
Flow chart for identification of economic evaluations.
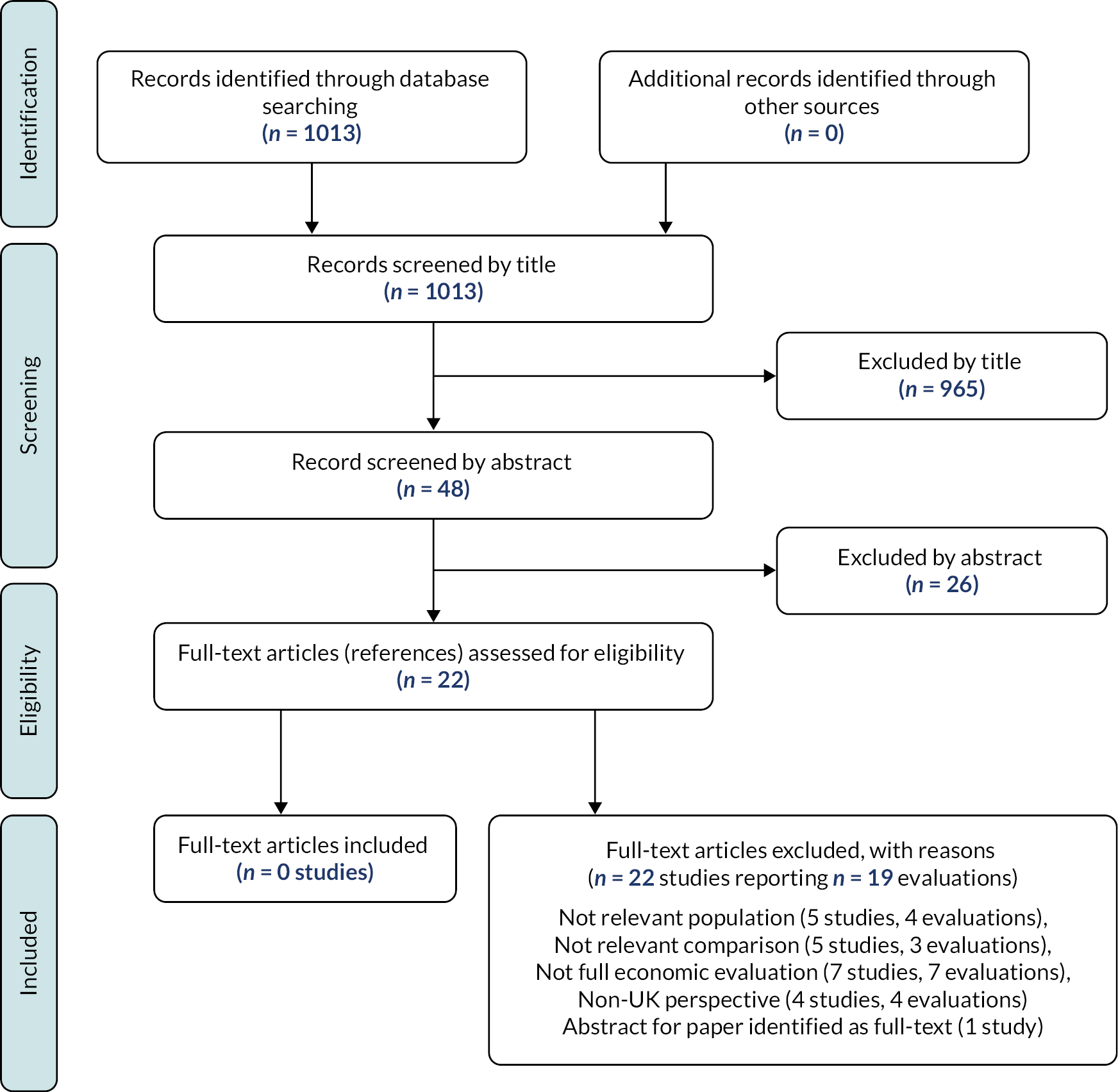
None of the economic evaluations identified in the sift of titles and abstracts met all of the inclusion criteria. The key reasons for exclusion are provided in Table 20. Four analyses were not in relevant populations. 137,140,143,153 One was in patients with ovarian hyperstimulation syndrome (OHSS) and only a proportion of this population were pregnant. 137 Two studies compared alternative diagnostic strategies for pregnant women with suspected PE and were therefore neither relevant comparisons nor a relevant population. 139,143 One paper considered screening for thrombophilia in patients with recurrent pregnancy loss, but the population was not limited to women already pregnant. 153 This and two additional studies compared screening strategies for inherited thrombophilia and were therefore not relevant comparisons. 135,140,153 One paper reported intermittent pneumatic compression versus expectant management and was therefore not a relevant comparison as it did not consider pharmacological prophylaxis. 149 Seven papers reported a relevant comparison in a relevant population but were not full economic evaluations. 142,145–147,150–152 One reported clinical outcomes alone,142 one was a cost-consequence analysis152 and the rest reported either costs alone146 or QALYs alone. 147,150,151 All four of the papers that reported a full economic evaluation of a relevant comparison in a relevant population were assumed to be non-UK based as they reported costs in US$. 99,138,141,144 In addition, all of these papers except that by Johnston et al. 99 were reported only in abstract form which meant they provided only limited information and relevant data sources could not be extracted.
| Study | Reason(s) for not meeting inclusion criteria |
|---|---|
| Bajaj 2013153 | Not relevant population (recurrent pregnancy loss), not relevant comparison (testing for thrombophilia), not full economic evaluation (no costs) |
| Becker 2019152 | Not full economic evaluation – cost-consequence (e.g. cost per VTE prevented), US healthcare perspective (abstract only) |
| Blondon 2010151 | Not full economic evaluations (no costs only QALYs) |
| Bunce 2017150 | Not full economic evaluation – no costs only QALYs, perspective not stated (abstract only) |
| Casele 2006149 | Not relevant comparison (intermittent pneumatic compression vs. no prophylaxis), US perspective |
| Dahl 2020148 | Excluded as abstract only and subsequent full paper identified |
| Eckman 2015147 | Not a full economic evaluation (no costs only QALYs) |
| Iroz 2021145 | Not full economic evaluation – no costs only QALYs, perspective not stated |
| Houlihan 2017146 | Not full economic evaluation – cost only (abstract only) |
| Johnston 200599 | Non-UK perspective (same model updated in Eckman 2015) |
| Lee 2017144 | Perspective not explicitly stated but costs reported in US$ (abstract only) |
| Pollard 2017143 and Goodacre 201889 | Not relevant population (pregnant and suspected PE), not relevant comparison (comparing diagnostic strategies for PE) |
| Quiñones 2005142 | Not a full economic evaluation as only clinical outcomes reported |
| Rizvi 2013141 | US perspective (abstract only) |
| Sabol 2019140 | Not relevant comparison (screening for thrombophilia), not full economic evaluation – number needed to screen to prevent 1 VTE (abstract only) |
| Sievert 2020139 | Not relevant population (pregnant with suspected PE), not relevant comparison (comparing diagnostic strategies for PE), US perspective (abstract only) |
| Westhoff 2012138 | US perspective (abstract only) |
| Wormer 2018137 | Not relevant population (OHSS with only a proportion of these being pregnant), not full economic evaluation – costs only, perspective unclear – US costs converted to Euros |
| Wu 2005,135 Wu 2006134 and Wu 2007136 | Relevant population (pregnant women is one of the subpopulations considered), not relevant comparison (screening vs. no screening), not full economic evaluation |
Although none of the papers identified during the title/abstract sift met all of the inclusion criteria for this review, in most cases there was similarity between the decision problem addressed in these studies and the target decision problem for the review. Therefore, those papers reported as full-text articles99,135,137,142,143,145,147,149,151,153 were examined to identify whether the clinical outcomes included in the models were relevant for the de novo economic evaluation (see Chapter 4) or whether they contained data that might be relevant for that analysis.
The key clinical outcomes that appeared to be commonly included across the 10 economic evaluations summarised in Table 21 were:
-
fatal VTE (DVT or PE)
-
non-fatal VTE (DVT and PE)
-
fatal bleeding
-
non-fatal major bleeding.
| Reference | Population | Comparison | Design | Clinical outcomes | Economic outcomes |
|---|---|---|---|---|---|
| Bajaj 2013153 | Women with recurrent pregnancy loss who had no personal or family history of VTE (who are not pregnant at the time of testing) | Testing for thrombophilia vs. no testing (LMWH prescribed during any subsequent pregnancy if thrombophilia detected) | Decision tree | Test outcome Pregnancy VTE (fatal/non-fatal) Major bleed (fatal/non-fatal) Pregnancy loss |
Clinical outcomes and QALYs gained over 1 year and over lifetime |
| Blondon 2010151 | Pregnant women after having caesarean delivery | LMWH No prophylaxis |
Decision tree over 3 months | DVT PE Non-gynaecological major bleeding (fatal, ICH and other) PPH (fatal, non-fatal with and without hysterectomy) HIT (with or without VTE) Recurrent VTE |
QALYs at 3 months (based on either utilities or disutilities) |
| Caseles 2006149 | Women having caesarean delivery | Intermittent pneumatic compression No prophylaxis |
Markov model | DVT (symptomatic and treated or asymptomatic and untreated) PE (fatal or non-fatal) Major bleeding during treatment (fatal or non-fatal ICH) Minor bleeding during treatment DVT recurrent within 1 year PTS |
Cost per QALY |
| Eckman 2015147 (NB: update of Johnston 2005) |
Pregnant women with a history of VTE (for decision model, broader group for HRQoL valuations) | LMWH No prophylaxis |
Lifetime Markov model with 6-week cycle lengths (covers both antenatal and postnatal VTE risk) | VTE (DVT/PE) Recurrent VTE Major obstetric bleed during prophylaxis Bleed during treatment Death from DVT, PE or major bleeding Morbidity after major bleed Vena cava filter |
Quality-adjusted life expectancy (VAS-based preferences) No costs |
| Iroz 2021145 | Women with a singleton pregnancy who are hospitalised for premature rupture of membranes | LMWH Unfractionated heparin (UFH) No prophylaxis |
Markov model tracking clinical outcomes from 24 to 34 weeks assuming induction at 34 weeks in those not already delivered | VTE (fatal and non-fatal) PPH (fatal, non-fatal with or without complications) Route of delivery (vaginal or caesarean) Pain relief during delivery Spontaneous or induced labour |
Utilities are combined to estimate the strategy with the highest ‘expected value’ but unclear if is this is the QALY gain over 10 weeks or some other measure No long-term morbidity considered. Unclear how utility loss from death captured given short time horizon. |
| Johnston 200599 | Pregnant women with a history of prior VTE (unselected and then also subgroups for high/low risk) | LMWH from 16 weeks to delivery No prophylaxis (both groups assumed to have 6 weeks of postpartum warfarin) |
Markov model | VTE (DVT and PE, both fatal and non-fatal) Recurrent VTE Major bleed (with or without long-term morbidity) Minor bleed Lifelong warfarin Vena cava filter Vertebral fracture |
Cost per QALY |
| Pollard 2017/Goodacre89,143 2018 | Pregnant or postpartum (up to 6 weeks after birth) women presenting with PE | Alternative diagnostic strategies | Decision tree | Fatal PE major bleeding (fatal and non-fatal) including ICH CTEPH Recurrent VTE (fatal and non-fatal) Imaging induced adverse effects |
Cost per QALY |
| Quiñones 2005142 | Pregnant women who have had a caesarean delivery | Heparin for all (LMWH or UFH) Heparin only for those with thrombophilia Pneumatic compression stockings No prophylaxis |
Decision tree | DVT HIT from prophylaxis HIT from VTE treatment Major bleeding from either Prophylaxis or treatment HIT-related VTE Recurrent VTE/PE |
Clinical outcomes (i.e. strategy that minimises number experiencing VTE or severe drug effects is optimal) |
| Wormer 2018137 | Women with OHSS (42% pregnancy rate assumed) | LMWH No prophylaxis |
Decision tree Time horizon unclear but includes some long-term complications of VTE and costs from premature death estimated based on expected lifetime earnings |
VTE (upper and lower limb DVT, PE) Recurrence/re-admission PTS CTEPH Bleeds (fatal and non-fatal) HIT (fatal and non-fatal) |
Costs with and without prophylaxis |
| Wu 2005135 | Pregnant women | Screening for thrombophilia at 6 weeks with LMWH in those testing positive. Universal or targeting (VTE history) screening compared to no screening | Decision tree | VTE (PE or DVT) Early and late pregnancy loss Pre-eclampsia (mild or severe) Abruption Intrauterine growth restriction PPH (not included in final model due to lack of data) |
Cost per adverse consequence avoided |
The non-fatal major bleeding outcomes were sometimes described more specifically as PPH, ICH, non-gynaecological major bleeding, major obstetric bleeding or major bleeding with or without long-term morbidity. The project management group, which included clinical experts and patient experts, were consulted regarding the type of bleeding events that should be includes in the model to inform the conceptual model (described in Conceptual model for antepartum women and Conceptual model for postpartum women).
The following outcomes were included less frequently across the evaluations:
-
recurrent VTE
-
HIT
-
minor bleeding
-
vertebral fractures
-
pregnancy or pregnancy loss
-
pre-eclampsia
-
abruption
-
intrauterine growth restriction
-
delivery characteristics (e.g. vaginal vs. caesarean, spontaneous vs. induced, pain relief during delivery)
-
PTS
-
CTEPH
-
imaging-induced adverse events
-
treatment given after VTE, for example vena cava filter, warfarin.
Many of these outcomes were clearly more relevant to the decision problem in the study in question than to the decision problem addressed in the de novo economic analysis (see Chapter 4). For example, outcomes such as pregnancy loss, pre-eclampsia, intrauterine growth restriction and abruption are more relevant when LMWH is given in women with thrombophilia to prevent pregnancy complications that are separate from their risk of VTE. The outcomes of PTS, CTEPH, recurrent VTE, HIT, minor bleeding, heparin-related osteoporotic fractures, pregnancy loss and abruption were considered to be potentially relevant and were discussed with the clinical experts, but of these only PTS and CTEPH were included (see conceptual model discussion in Conceptual model for antepartum women and Conceptual model for postpartum women). Minor bleeding was not included as a general outcome, but the more specific outcome of wound haematoma was included as a form of CRNMB.
Appendix 4 Inputs for decision-analytic modelling
| Parameter description | Mid-point value | Uncertainty measure | Distribution | Source | ||
|---|---|---|---|---|---|---|
| Sensitivity and specificity of decision tools | See Tables 3 and 4 | See Tables 3 and 4 | Normally distributed on the logit scale | Systematic review of RAMs (see Chapter 3) | ||
| Probability of VTE in unselected postpartum women (6 weeks) | 0.072% | 95% CI 0.64% to 0.80% | Beta(312, 422041) | Sultan 201611 | ||
| Probability of VTE in obese postpartum women (6 weeks) | 0.153% | 95% CI 0.108% to 0.206% | Beta(37, 24104) | Sultan 201455 | ||
| Probability of VTE in following caesarean section (6 weeks) | 0.137% | 95% CI 0.107% to 0.169% | Beta(74, 54110) | Sultan 201455 | ||
| Antepartum VTE risk in high-risk women | 5.81% | 95% CI 2.71% to 9.98% | Beta(9, 146) | De Stefano 200652 | ||
| Postpartum VTE risk in high-risk women | 6.85% | 95% CI 3.36% to 11.5% | Beta(10, 136) | De Stefano 200652 | ||
| Antepartum VTE risk in unselected antepartum women | 0.15% | 95% CI 0.04% to 0.33% | Beta(4, 2681) | Chauleur 200831 | ||
| Postpartum VTE risk in unselected antepartum women | 0.19% | 95% CI 0.06% to 0.38% | Beta(5, 2680) | Chauleur 200831 | ||
| Proportion of VTE that is PE | 24.1% | 95% CI 23.5% to 24.6% | Beta(5401, 11634) | Meng 20155 | ||
| Proportion of antepartum VTE that occurs prior to 28 weeks | 60.1% | Fixed | Not applicable | Voke 2007113 | ||
| Proportion of 6-week postpartum VTE risk falling in first 3 weeks | 70.3% | Fixed | Not applicable | Sultan 201455 | ||
| Ratio of asymptomatic to symptomatic DVT | 4 : 1 | 95% CI of 2.8 : 1 to 6.7 : 1 | Beta(40, 10) : Beta(10 : 40) | Ratio estimated from events pooled across 6 RCTs.57–62 Number of events increased when sampling to limit unrealistic samples. | ||
| Proportion of antepartum symptomatic DVTs that are proximal | 78% | 95% CI 74% to 82% | Beta(342, 96) | Elgendy 202063 | ||
| Proportion of postpartum symptomatic DVTs that are proximal | 71% | 95% CI 66% to 76% | Beta(215, 86) | Elgendy 202063 | ||
| OR of VTE for LMWH vs. no LMWH | 0.29 | 95% CI 0.01 to 8.37 | Lognormal(−1.22, 1.71) | Gates 200467 | ||
| Risk of antepartum major bleeding during antepartum LMWH | 0.24% | 95% CI 0.05% to 0.57% | Beta(3, 1264) | Nelson-Piercy 201170 | ||
| Risk of postpartum major bleeding in women having antepartum and postpartum LMWH | 5.49% | 95% CI 1.83% to 11.0% | Beta(5, 86) | Schoenbeck 200171 | ||
| Risk of major bleeding for in patients having postpartum LMWH | 4.58% | 95% CI 2.66% to 7.01% | Beta(16, 333) | Gizzo 201476 | ||
| Antepartum incidence of fatal major bleeding | 0.5 per 100,000 | Fixed | NA | MBRRACE 202078 | ||
| Postpartum incidence of fatal major bleeding | 0.6 per 100,000 | Fixed | NA | MBRRACE 202078 | ||
| Antepartum incidence of non-fatal ICH | 0.9 per 100,000 | Fixed | NA | Ban 201779 | ||
| Postpartum incidence of non-fatal ICH | 1.1 per 100,000 | Fixed | NA | Ban 201779 | ||
| RR of bleeding for prophylaxis vs. none | 1.53 | 95% CI 0.90 to 2.53 | Lognormal(0.43, 0.33) | Meta-analysis of VTE events in the three RCTs included in NICE Guideline for LMWH (standard dose/standard duration) vs. placebo in acutely ill medical patients6 | ||
| Risk of bleeding during 3-month anticoagulant treatment for VTE | 0.8% | 95% CI 0.4% to 1.4% | Beta(9, 1110) | Elgendy 202063 | ||
| Proportion of major bleeds during VTE treatment that are fatal | 6.3% | 95% CI 1.7% to 13.5% | Beta(4, 59) | Jerjes-Sanchez 202180 | ||
| Proportion of non-fatal major bleeds during VTE treatment that are ICH | 3.4% | 95% CI 0.1% to 12.3% | Beta(1, 28) | Jerjes-Sanchez 202180 | ||
| Wound haematoma without antepartum LMWH | 0.4% | 95% CI 0.2% to 0.6% | Beta(12, 2088) | Lindqvist 201174 | ||
| Wound haematoma with antepartum LMWH | 2.5% | 95% CI 1.1% to 4.3% | Beta(8, 318) | Lindqvist 201174 | ||
| Wound haematoma without postpartum LMWH | 1.1% | 95% CI 0.8% to 2.8% | Beta(11, 652) | Ferres 201177 | ||
| Wound haematoma with postpartum LMWH | 1.7% | 95% CI 0.5% to 1.8% | Beta(11, 1031) | Ferres 201177 | ||
| All-cause (non-VTE-related) mortality for general population not in hospital | Varies by age | Assumed fixed | Not applicable | Office for National Statistics lifetables91 Risk applied each year is based on current age and is not adjusted to account for contribution of VTE to population mortality. |
||
| SMR for patients surviving ICH compared with general population | SMR from Fogelholm et al.87 applied for years 1–6 and then assumed no increased mortality risk CIs around SMR not reported so have assumed ± 20% on the log scale |
|||||
|
|
95% CI 1.28 to 1.69 | Log(SMR) = norm(1.5, 0.1) | |||
|
|
95% CI 1.8 to 2.7 | Log(SMR) = norm(0.8, 0.1) | |||
| Probability of PE being fatal in general medical inpatients | 2% | 95% CI 1.4% to 2.6% | Norm(0.02, 0.003) | Kourlaba 201684 | ||
| Cumulative risk of PTS for DVT | Wiks 201285 | |||||
|
|
|
Beta(11, 24) | |||
|
|
|
Beta(41, 79) | |||
| Proportion of cumulative PTS risk falling in | Fixed | Fixed | van Dongen86 | |||
|
72% | |||||
|
89% | |||||
|
95% | |||||
|
100% | |||||
| OR for PTS in proximal vs. distal DVT for postpartum DVT | 3.5 | 95% CI 1.8 to 7.0 | Log(OR) = norm(1.25, 0.35) | Wik 201285 | ||
| Incidence of CTEPH at 2 years (converted to annual risk of 1.6%) | 3.2% | 95% CI 2.0 % to 4.4% | Beta(32, 967) | Ende-Verhaar et al.82 based on incidence in those surviving the initial treatment period of 3–6 months Assumed no risk beyond 2 years based on Pengo et al. |
||
| Proportion of CTEPH treated surgically | 59.5% | 95% CI 55.8% to 63.2% | Beta(404, 275) | Delcroix et al.90 | ||
| Proportion of CTEPH that are surgically treated who also received bridging medical care | 30.0% | 95% CI 24.6% to 33.5% | Beta(117, 287) | Delcroix et al.90 | ||
| Mean hazard for exponential survival curve in medically treated patients with CTEPH | 0.1168 | SE = 0.0123 | Norm(0.1168, 0.0123) | Original data from Delcroix et al.90 but curves taken from Goodacre et al.89 (If the death hazard falls below general population values, then general population values apply) |
||
| Mean and SD for lognormal survival curve in surgically treated patients with CTEPH | Mean = 5.08 SD = 3.34 |
SE of mean = 0.574 SE of SD = 0.399 |
Multivariate normal | Original data from Delcroix et al.90 but curves taken from Goodacre et al.89 (If the death hazard falls below general population values, then general population values apply) Variance–covariance matrix |
||
| Mean log | SD log | |||||
| Mean log | 0.017708 | −0.05572 | ||||
| SD log | −0.05572 | 0.230935 | ||||
| Parameter description | Mean value | 95% CIa | Source | Notes |
|---|---|---|---|---|
| Application of RAM to patient | £9.92 | Fixed | Curtis and Burns45 | Cost for 5 minutes of hospital consultant time |
| Prophylaxis drug cost per day | £2.82 for 73 kg woman £4.23 for 95 kg woman |
NA | Admin costs from Curtis et al.45 Drug costs based on Drug Tariff92 |
Dalteparin is lowest cost formulation of LMWH based on current Drug Tariff prices. 5000 units daily for 73kg 7500 units daily for 95kg |
Administration costs
|
|
|
Menakaya93 | Adapted to adjust for duration of prophylaxis. Assumes 4% (95% CI 1% to 8%) require district nurse administration |
| Monitoring for antepartum prophylaxis | £205.03 | Fixed | NHS reference costs 2018–994 | |
| Treatment of VTE | See Tables 24 and 25 | |||
| Fatal bleed | £1865.51 | £685.90–3736.50 | Luengo-Fernandez et al.98 | Costs of fatal haemorrhagic stroke from OXVASC subgroup with atrial fibrillation. Uplifted to current prices using inflation indices |
| Non-fatal non-ICH bleed | £1209.75 | £1199.79–1220.07 | NHS reference costs 2018–994 | Weighted average of reference costs for GI bleed (HRG codes FZ38G – FZ38P) |
| Post non-fatal ICH – first 90 days | £22,005.18 | £17,427.88–27,325.03 | Luengo-Fernandez et al.98 | Weighted average of costs for non-fatal haemorrhagic strokes Uplifted to current prices using inflation indices |
| Post non-fatal ICH – post acute (beyond 90 days) costs per annum | £8378.91 | £5492.17–11,462.82 | Luengo-Fernandez et al.98 | Average costs across all stroke types (haemorrhagic not reported separately). Includes GP and ED costs and long-term care cost Uplifted to current prices using inflation indices |
| Cost of wound haematoma | £1372 | Fixed | NHS reference costs 2018–994 | Difference between cost of short-stay and long-stay admissions for normal delivery (NZ30C) |
| PTS cost per annum – year 1 | £293.16 in year 1 | £279.90–306.40 | NHS reference costs 2018–994 | One first and one follow-up vascular surgery outpatient appointments Weighted average of consultant-led and non-consultant-led outpatient appointments for non-admitted face-to-face first attendance (WF01B) and follow-up (WF01A) for vascular surgery (service code 107) |
| PTS cost per annum – year 2 | £78.00 in each subsequent year | Fixed | Curtis and Burns45 | 2 × GP surgery consultations with qualification costs including direct care staff costs at £39 per appointment |
CTEPH cost per annum
|
£18,979.91 each year | Fixed | NICE CG92154 | Cost in CG92 was £1219 per 4 weeks in 2008/9 prices. This was uplifted to 2019–20 prices using inflation indices. Assume treatment lifelong |
CTEPH cost per annum
|
£10,236.60 in year 1 and 0 in Y2 onwards | £9976.73–10,604.19 | NHS reference costs 2018–994 | Average of DZ02H, DZ02J and DZ02K ‘Complex thoracic procedures’ relating to procedure code L041 ‘Pulmonary thromboendodartectomy’ for elective inpatients including excess bed-days In addition, 29% of surgically treated patients require medical bridging therapy for 4.6 months |
| Drug | Dosing and delivery | Product and cost | Drug cost per course92 | Proportion using treatment95 |
|---|---|---|---|---|
| Enoxaparin | 120 mg once daily | 120 mg/0.8 ml solution for injection pre-filled syringes (Techdow Pharma England Ltd, Guildford, UK/Sanofi/Rovi Biotech Ltd, Croydon, UK) – £87.93 for 10 pre-filled syringes | £800.16 per 13-week course for once daily | 33.3% |
| 80 mg twice daily | 80 mg/0.8 ml solution for injection pre-filled syringes (Sanofi/Rovi Biotech Ltd/Techdow Pharma England Ltd) – £55.13 per 10 | £1003.37 per 13-week course of twice daily | 31.9% | |
| Dalteparin | 16,000 units once daily | Dalteparin sodium 18,000 units/0 ml solution (Pfizer Ltd, Sandwich, UK) – £50.82 for 5 pre-filled syringes | £924.92 per 13-week course for once daily | 11.0% |
| 8000 twice daily | Dalteparin sodium 10,000 units/1 ml solution (Pfizer Ltd) – £51.22 for 10 pre-filled syringes | £932.20 per 13-week course for twice daily | 10.6% | |
| Tinzaparin | 175 units/kg once daily (i.e. 12,705 units daily assuming 73 kg) | Innohep 14,000 units/0.7 ml solution (Leo Pharma) – £83.30 for 10 pre-filled syringes | £758.03 per 13-week course | 13.2% |
| Average for drug acquisition | £887.21 for postnatal VTE; £1501.44 for antenatal VTE | |||
| Administration | £157.51b for postnatal | |||
| £221.74b for antenatal | ||||
| Monitoring | £615.07c for postnatal | |||
| £1025.11c for antenatal | ||||
| Total | Postnatal: £1659.79 for 13 weeks treatment | |||
| Antenatal: £2748.29 treatment with average duration of 22 weeks | ||||
| Proportion using resource | Unit cost per patient using this resource | Description | |||
|---|---|---|---|---|---|
| Non-fatala PE | Symptomatic proximal DVT | Symptomatic distal DVT | |||
| Healthcare contacts/admission | |||||
| GP visit | 20% | 50% | 50% | £39 | GP cost per surgery consultation with qualification costs including direct care staff costs45 |
| Ambulance transfer to ED | 60% postnatal | 10% postnatal | 0% | £257 | NHS Schedule for Reference Costs 2018–9 |
| 90% antenatal | 20% antenatal | ‘See and treat and convey’, code ASS0294 | |||
| ED visit leading to admission | 60% postnatal | 10% postnatal | 0% | £279 | NHS Schedule for Reference Costs 2018–9 |
| 90% antenatal | 20% antenatal | VB05Z Type 01 Admitted (Category 2 investigation with Category 3 treatment)94 | |||
| ED without admission | 40% postnatal | 90% postnatal | 100% | £239 | NHS Schedule for Reference Costs 2018–9 |
| 10% antenatal | 80% antenatal | VB05Z Type 01 Non-admitted (Category 2 investigation with Category 3 treatment)94 | |||
| Short-stay admission for PE | 60% postnatal | 0% | 0% | £1410 | NHS Schedule for Reference Costs 2018–9 |
| 90% antenatal | Weighted average cost of non-elective inpatient (short and long stay with excess bed-days) for ‘Pulmonary Embolus with Interventions’, codes DZ09J to DZ09N and DZ09P and DZ09Q94 | ||||
| Short-stay admission for DVT | 0% | 10% postnatal | 0% | £904 | NHS Schedule for Reference Costs 2018–9 |
| 20% antenatal | Weighted average cost of non-elective inpatient (short- and long-stay with excess bed-days) for ‘DVT’ complication or comorbidity score 0–12+, codes YQ51A to YQ51E94 | ||||
| Critical care unit stay | 10% postnatal | 0% | 0% | £1028 | NHS Schedule for Reference Costs 2018–9 |
| 20% antenatal | Weighted average cost of adult Critical Care, 0–6 or more organs supported, codes XC01Z to XC01Z94 | ||||
| Subtotal for healthcare contacts | £1374 postnatal | £379 postnatal | £259 | ||
| £1989 antenatal | £499 antenatal | ||||
| Diagnostic costs | |||||
| Chest X-ray | Included in ED visit | ||||
| Proximal leg vein Ultrasound | 0% | 100% | 100% | £53 | NHS Schedule for Reference Costs 2018–9. RD40Z Outpatient ultrasound scan with duration of less than 20 minutes, without contrast £5594 |
| CTPA | 90% postnatal | 0% | 0% | £108 | NHS Schedule for Reference Costs 2018–9. RD21A Outpatient computerised tomography scan of one area, with post contrast only, 19 years and over94 |
| 28% antenatal | |||||
| V/Q SPECT | 5% postnatal | 0% | 0% | £287 | NHS Schedule for Reference Costs 2018–9. RN08A Outpatient SPECT, 19 years and over94 |
| 40% | |||||
| V/Q planar | 5% postnatal | 0% | 0% | £321 | NHS Schedule for Reference Costs 2018–9. RN18A Outpatient lung ventilation or perfusion scan, 19 years and over94 |
| 40% antenatal | |||||
| Echocardiogram | 20% postnatal | 0% | 0% | £76 | NHS Schedule for Reference Costs 2018–9. RD51A Outpatient simple echocardiogram94 |
| 16% antenatal | |||||
| Subtotal for unbundled diagnostics | £143 postnatal | £53 | £53 | ||
| £287 antenatal | |||||
| Subtotal for drug treatment b | £1660 postnatal | £1660 postnatal | £1660 postnatal | See Table 24 | |
| £2748 antenatal | £2748 antenatal | £2748 antenatal | |||
| Total | £3321 postnatal | £2092 postnatal | £1972 postnatal | ||
| £5024 antenatal | £3300 antenatal | £3060 antenatal | |||
| Absolute utility value | Absolute utility value | Range | Source | Notes |
|---|---|---|---|---|
| Well/asymptomatic DVT without prophylaxis | 0.923 | 0.922–0.923 | Ara and Brazier 2010112 | Population mean utility values based on average age at baseline |
| Symptomatic proximal or distal DVT | 0.888 | 0.872–0.899 | Monreal 2019105 | 3.8% reduction relative to well patients based on comparison of average utility over 6 months for DVT (0.820) vs. PE vs. utility of matched population norms (0.852) |
| Non-fatal PE | 0.886 | 0.873–0.899 | Chuang 2019104 | 4.0% reduction relative to well patients based on comparison of average utility over 6 months (0.804) for PE vs. utility of matched population norms (0.838) |
| Non-fatal ICH | 0.703 | 0.663–0.742 | Luengo-Fernandez 2013110 | Absolute decrement of 0.22 measured at 1 month |
| Non-fatal non-ICH bleed | 0.790 | 0.789–0.791 | Chuang 2019104 | Assumed same utility decrement for PE and GI bleeds at 1 month. 14% reduction based on utility for PE at 1 month (0.718) vs. utility of matched population norms (0.838) from Chuang 2019 |
| LMWH as treatment or prophylaxis – absolute decrement applied to utility values of well/asymptomatic DVT | 0.007 | 0.000–0.050 | Marchetti 2001111 | Patients willing to trade average of 2.7 days per year to avoid treatment with LMWH |
| Fatal PE/fatal bleed | 0 | NA | Assumption |
| Health state (s) | Utility multiplier relative to well | Range | Source | Notes |
|---|---|---|---|---|
| PE survivor without CTEPH and PE survivor more than 1 year after surgery for CTEPH | 1.000 | 0.998–1.000 | Chuang 2019104 | Average over 6–12 months following PE compared to matched general population norms |
| Any DVT without PTS | 1 | NA | Assumption | Supported by Lubberts et al. systematic review finding no significant HRQoL decrement in nine long-term studies based on SF-36 outcomes |
| Non-fatal ICH | 0.902 | 0.859–0.946 | Luengo-Fernandez 2013110 | Multiplier calculated based on absolute decrement of 0.09 at 5 years (utility values stable from 6 months to 5 years) relative to absolute utility for well state |
| PTS | 0.895 | 0.816–0.954 | Enden 2013106 | Multiplier calculated based on absolute decrement of 0.09 relative to absolute utility for well state of 0.86 |
| CTEPH – first year for surgically managed and every year for medically managed | 0.629 | 0.579–0.690 | Meads 2008109 | Multiplier calculated based on comparison of utility for CTEPH (0.56) vs. utility for NYHA class I (0.89) |
| Dead | 0 | Assumption |
| Parameter description | Mid-point value | Uncertainty measure | Distribution | Source | |||
|---|---|---|---|---|---|---|---|
| Ambulance transfer to ED | £257 | SE = £11 | Gamma(551, 0.47) | NHS Schedule for Reference Costs 2018–9. HRG code, ASS02 See and treat and convey94 |
|||
| ED visit leading to admission | £279 | SE = £6 | Gamma(2210, 0.15) | NHS Schedule for Reference Costs 2018–9. HRG code: Type 01, leading to admission, VB05Z Emergency Medicine, Category 2 Investigation with Category 3 Treatment94 |
|||
| ED visit not leading to admission | £239 | SE = £4 | Gamma(3204, 0.07) | NHS Schedule for Reference Costs 2018–9. HRG code: Type 01, not leading to admission, VB05Z Emergency Medicine, Category 2 Investigation with Category 3 Treatment94 |
|||
| DVT admission – weighted average of following HRG costs: | NHS Schedule for Reference Costs 2018–9. NEI and NESS costs for HRG codes covering DVT with complication or comorbidity scores ranging from 0 to 12+94 |
||||||
| YQ51A – NEI (N = 1377) | £4017 | SE = £198 | Gamma(412, 9.7) | ||||
| YQ51A – NESS (N = 492) | £564 | SE = £33 | Gamma(288, 2.0) | ||||
| YQ51B – NEI (N = 1183) | £2873 | SE = £129 | Gamma(495, 5.8) | ||||
| YQ51B – NESS (N = 895) | £470 | SE = £13 | Gamma(1237,0.4) | ||||
| YQ51C – NEI (N = 1665) | £2433 | SE = £78 | Gamma(973, 2.5) | ||||
| YQ51C – NESS (N = 2391) | £418 | SE = £11 | Gamma(1433, 0.3) | ||||
| YQ51D – NEI (N = 1686) | £2020 | SE = £46 | Gamma(1903, 1.1) | ||||
| YQ51D – NESS (N = 6249) | £384 | SE = £9 | Gamma(1822, 0.2) | ||||
| YQ51E – NEI (N = 908) | £1772 | SE = £42 | Gamma(1814, 1.0) | ||||
| YQ51E – NESS (N = 11,731) | £320 | SE = £9 | Gamma(1330, 0.2) | ||||
| PE admission – weighted average of following HRG costs; | NHS Schedule for Reference Costs 2018–9. NEI costs and NESS costs for HRG codes covering pulmonary embolus with and without interventions with complication or comorbidity scores from 0 to 12+94 |
||||||
| DZ09J – NEI (N = 888) | £5450 | SE = £277 | Gamma(338, 14) | ||||
| DZ09J – NESS (N = 62) | £1280 | SE = £168 | Gamma(58, 22) | ||||
| DZ09K – NEI (N = 585) | £3384 | SE = £130 | Gamma(676, 5.0) | ||||
| DZ09K – NESS (N = 65) | £790 | SE = £56 | Gamma(199, 4.0) | ||||
| DZ09L – NEI (N = 3160) | £3522 | SE = £140 | Gamma(663, 5.5) | ||||
| DZ09L – NESS (N = 1181) | £667 | SE = £21 | Gamma(1026, 0.7) | ||||
| DZ09M – NEI (N = 3716) | £2671 | SE = £75 | Gamma(1255, 2.1) | ||||
| DZ09M – NESS (N = 2197) | £577 | SE = 18 | Gamma(1054, 0.6) | ||||
| DZ09N – NEI (N = 5105) | £2201 | SE = £45 | Gamma(2358, 0.9) | ||||
| DZ09N – NESS (N = 4374) | £533 | SE = £12 | Gamma(2091, 0.3) | ||||
| DZ09P – NEI (N = 6126) | £1845 | SE = £38 | Gamma(2417, 0.8) | ||||
| DZ09P – NESS (N = 8768) | £488 | SE = £12 | Gamma(1595, 0.3) | ||||
| DZ09Q – NEI (N = 3226) | £1584 | SE = £29 | Gamma(2989, 0.5) | ||||
| DZ09Q – NESS (N = 9048) | £448 | SE = £9 | Gamma(2376, 0.2) | ||||
| Critical care – weighted average of HRG costs for codes: | NHS Schedule for Reference Costs 2018–9. HRG codes for Adult Critical Care for 0–6 organs supported94 |
||||||
| XC01Z | £1673 | N = 1 | Fixed | ||||
| XC02Z | £1574 | SE = £152 | Gamma(107, 14.7) | ||||
| XC03Z | £1655 | SE = £114 | Gamma(211, 7.9) | ||||
| XC04Z | £1640 | SE = £67 | Gamma(605, 2.7) | ||||
| XC05Z | £1450 | SE = £49 | Gamma(884, 1.7) | ||||
| XC06Z | £792 | SE = £78 | Gamma(104, 7.6) | ||||
| XC07Z | £516 | SE = £129 | Gamma(16.0, 32.2) | ||||
| Proximal leg vein ultrasound | £53 | SE = £1 | Gamma(2135, 0.03) | NHS Schedule for Reference Costs 2018–994 | |||
| CTPA | £108 | SE = £4 | Gamma(635, 0.17) | NHS Schedule for Reference Costs 2018–9 RD21A outpatient computerised tomography scan of one area, with post contrast only, 19 years and over94 |
|||
| V/Q SPECT | £287 | SE = £20 | Gamma(202, 1.42) | NHS Schedule for Reference Costs 2018–9 RN08A, outpatient SPECT, 19 years and over94 |
|||
| V/Q planar | £321 | SE = £10 | Gamma(1045, 0.31) | NHS Schedule for Reference Costs 2018–9 RN18A outpatient lung ventilation or perfusion scan, 19 years and over94 |
|||
| Echocardiogram | £76 | SE = £6 | Gamma(146, 0.52) | NHS Schedule for Reference Costs 2018–9 RD51A outpatient simple echocardiogram, 19 years and over94 |
|||
| Proportion receiving LMWH who need district nurse administration | 4% | 95% CI 1.3% to 7.8% | Beta(5, 123) | Menakaya et al.93 | |||
| Fatal bleed | £1592 | SD = £1886, N = 8 | Gamma(5.70, 279) | Luengo-Fernandez et al.98 (cost before inflation) | |||
| Acute costs for non-fatal ICH (first 90 days) – weighted average of: | Luengo-Fernandez et al.98 (cost before inflation) | ||||||
| Non-disabling non-fatal stroke | £9903 | SD = £4510, N = 5 | Gamma(24, 411) | ||||
| Moderately disabling non-fatal stroke | £25,442 | SD = £9635, N = 3 | Gamma(21, 1216) | ||||
| Totally disabling non-fatal stroke | £43,036 | SD = NA, N = 1 | Fixed | ||||
| Residential costs for non-fatal ICH (first 90 days) | £6880 | SD = £15,600, N = 136 | Gamma(26, 260) | Luengo-Fernandez et al.98 (cost before inflation) | |||
| GP costs for non-fatal ICH (first 90 days) | £98 | 95% CI £27 to £169 | Norm(98, 36) | Luengo-Fernandez et al.98 (cost before inflation) | |||
| Emergency care costs for non-fatal ICH (first 90 days) | £99 | 95% CI £56 to £141 | Norm (99, 22) | Luengo-Fernandez et al.98 (cost before inflation) | |||
| Non-fatal non-ICH bleed (weighted average of HRG costs): | NHS Schedule for Reference Costs 2018–9 HRG codes for GI bleed without interventions, with single interventions and with multiple interventions94 |
||||||
| FD03A – NEI (N = 1110) | £5377 | SE = £201 | Gamma(714, 7.5) | ||||
| FD03A – NESS (N = 30) | £2360 | SE = £310 | Gamma(58, 41) | ||||
| FD03B – NEI (N = 885) | £3510 | SE = £131 | Gamma(722, 4.9) | ||||
| FD03B – NSS (N = 16) | £2088 | SE = £1109 | Gamma(3.6, 590) | ||||
| FD03C – NEI (N = 1642) | £3866 | SE = £171 | Gamma(514, 7.5) | ||||
| FD03C – NSS (N = 41) | £1345 | SE = £105 | Gamma(166, 8.1) | ||||
| FD03D – NEI (N = 2329) | £2796 | SE = £92 | Gamma(913, 3.0) | ||||
| FD03D – NSS (N = 46) | £2360 | SE = £156 | Gamma(229, 10) | ||||
| FD03E – NEI (N = 5481) | £2247 | SE = £47 | Gamma(2331, 1.0) | ||||
| FD03E – NEI (N = 108) | £1089 | SE = £82 | Gamma(178, 6.1) | ||||
| FD03F – NEI (N = 2891) | £2818 | SE = £100 | Gamma(792, 3.6) | ||||
| FD03F – NEI (N = 2213) | £591 | SE = £19 | Gamma(1000, 0.6) | ||||
| FD03G – NEI (N = 7278) | £2198 | SE = £41 | Gamma(2931, 0.8) | ||||
| FD03G – NEI (N = 8830) | £541 | SE = £15 | Gamma(1221,0.4) | ||||
| FD03H – NEI (N = 16,290) | £1575 | SE = £27 | Gamma(3523, 0.8) | ||||
| FD03H – NEI (N = 40,167) | £438 | SE = £11 | Gamma(1640, 0.3) | ||||
| Vascular surgery first appointment face-to-face, consultant-led | £165 | SE = £6 | Gamma(759,0.22) | NHS Schedule for Reference Costs 2018–9 Service code 107 – WF01B non-admitted94 |
|||
| Vascular surgery follow-up appointment face to face, consultant led | £134 | SE = £4 | Gamma(942, 0.14) | NHS Schedule for Reference Costs 2018–9 Service code 107 – WF01A non-admitted94 |
|||
| Vascular surgery first appointment face-to-face, non-consultant-led | £132 | SE = £11 | Gamma(132, 1.0) | NHS Schedule for Reference Costs 2018–9 Service code 107 – WF01B non-admitted94 |
|||
| Vascular surgery follow-up appointment face-to-face, non-consultant-led | £121 | SE = £14 | Gamma(79, 1.53) | NHS Schedule for Reference Costs 2018–9 Service code 107 – WF01A non-admitted94 |
|||
| Surgical management of CTEPH – average of following HRG costs: | NHS Schedule for Reference Costs 2018–9 HRG codes for complex thoracic procedures, 19 years and over, with CC Score ranging from 0 to 6+94 |
||||||
| DZ02H | £9782 | SE = £363 | Gamma(723, 13.5) | ||||
| DZ02J | £7500 | SE = £300 | Gamma(627, 12.0) | ||||
| DZ02K | £6506 | SE = £270 | Gamma(579, 11.2) | ||||
| Disutility for stroke up to 6 months | −0.22 | 95% CI −0.26 to −0.18 | Norm(−0.22, 0.02) | Luengo-Fernandez et al. (2013)110 | |||
| Disutility for stroke from 6 months | −0.09 | 95% CI −0.13 to −0.05 | Norm(−0.09, 0.02) | Luengo-Fernandez et al. (2013)110 | |||
| Utility immediately after DVT | 0.72 | SE = 0.006 | Beta(3977, 1565) | Monreal (2019)105 | |||
| Utility immediately after PE | 0.72 | SE = 0.007 | Beta(2741, 1080) | Chuang (2019)104 (assumed same SD as observed for patients having DVT in Monreal 2019) |
|||
| Utility for DVT without PTS | 0.86 | 95% CI 0.823 to 0.903 | Beta(248, 40.3) | Enden et al. (2013)106 | |||
| Disutility for PTS vs. no PTS after DVT | 0.09 | 95% CI 0.03 to 0.15 | Beta(7.78, 78.6) | Enden et al. (2013)106 | |||
| Utility for CTEPH | 0.56 | SD = 0.29, N = 308 | Beta(505, 397) | Meads et al. (2008)109 | |||
| Utility for NYHA class 1 | 0.86 | SD = 0.17,N = 35 | Beta(105, 12.9) | Meads et al. (2008)109 | |||
| Utility for LMWH | 0.993 | SD = 0.016 | Beta(27.5, 0.205) | Marchetti et al. (2001)111 | |||
| Utility regression for age-related decrement – coefficients for: | Ara and Brazier (2011)112 Variance–covariance matrix |
||||||
| Age | −0.0001728 | SE = 0.0003737 | Multivariate normal | ||||
| Age × age | −0.000034 | SE = 3.96 × 10–6 | Age | Age × age | constant | ||
| constant | 0.9584588 | SE = 0.0077431 | Age | 1.4 × 10–7 | |||
| Age × age | −1.5 × 10–9 | 1.6 × 10–11 | |||||
| constant | −2.80 × 10–6 | 2.8 × 10–8 | 6 × 10–5 | ||||
Appendix 5 Expected value of perfect parameter information results for individual parameters and groups of parameters
| Parametera | Per person EVPPI (£) | Standard error of per person EVPPI | Indexed to overall EVPI = 1.00 | Population-level EVPPI over 5 years of birthsb (£) |
|---|---|---|---|---|
| RR of symptomatic VTE | 1363.93 | 24.63 | 0.94 | 20,407,801 |
| Parametersa | Per person EVPPI (£) | Standard error of per person EVPPI | Indexed to overall EVPI = 1.00 | Population-level EVPPI over 5 years of birthsb (£) |
|---|---|---|---|---|
| RR of VTE and RR of major bleeding | 1363.20 | 23.18 | 0.94 | 20,396,877 |
| Parametera | Per person EVPPI (£) | Standard error of per person EVPPI | Indexed to overall EVPI = 1.00 | Population-level EVPPI over 5 years of birthsb (£) |
|---|---|---|---|---|
| RR of symptomatic VTE | 22.38 | 0.55 | 0.99 | 13,394,429 |
| Parametersa | Per person EVPPI (£) | Standard error of per person EVPPI | Indexed to overall EVPI = 1.00 | Population-level EVPPI over 5 years of birthsb (£) |
|---|---|---|---|---|
| RR of VTE and RR of major bleeding | 22.30 | 0.57 | 0.99 | 13,347,392 |
| Absolute risk of VTE without PPX | 0.35 | 0.38 | 0.02 | 211,980 |
| Parameterb | Per person EVPPI (£) | Standard error of per person EVPPI | Indexed to overall EVPI = 1.00 | Population-level EVPPI over 5 years of birthsc (£) |
|---|---|---|---|---|
| RR of symptomatic VTE | 5.28 | 0.23 | 0.68 | 3,839,497 |
| Parametersb | Per person EVPPI (£) | Standard error of per person EVPPI | Indexed to overall EVPI = 1.00 | Population-level EVPPI over 5 years of birthsc (£) |
|---|---|---|---|---|
| RR of VTE and RR of major bleeding | 5.47 | 0.22 | 0.70 | 3,974,135 |
| Sensitivity and specificity of RAMs | 0.94 | 0.42 | 0.12 | 680,745 |
| Absolute risks of VTE without PPX | 0.79 | 0.54 | 0.10 | 577,398 |
| Costs of major bleeds | 0.10 | 0.08 | 0.01 | 72,155 |
Appendix 6 Deterministic scenario analyses
| VTE risk without PPX | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.00% | 2.00% | 3.0% | 4.0% | 5.0% | 6.0% | 7.0% | 8.0% | 9.0% | 10% | 11% | 12.0% | 12.7% | 14.0% | 15.% | 17% | 20% | ||||
| Risk of major bleeding with PPX | 0.01% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 0.01% | Risk of major bleeding without PPX |
| 0.10% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 0.07% | ||
| 0.20% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 0.13% | ||
| 0.40% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 0.26% | ||
| 0.50% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 0.33% | ||
| 1.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 0.65% | ||
| 2.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 1.31% | ||
| 3.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 1.96% | ||
| 4.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 2.61% | ||
| 4.82% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 3.15% | ||
| 5.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 3.27% | ||
| 6.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | E | 3.92% | ||
| 7.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | 4.58% | ||
| 8.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | 5.23% | ||
| 9.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | 5.88% | ||
| 10.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | 6.54% | ||
| 20.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | E | 13.1% | ||
| 30.00% | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | E | E | E | E | E | 19.6% | ||
| 0.3% | 0.7% | 1.0% | 1.3% | 1.7% | 2.0% | 2.3% | 2.6% | 3.0% | 3.3% | 3.6% | 4.0% | 4.2% | 4.6% | 5.0% | 5.6% | 6.6% | ||||
| VTE risk with PPX | ||||||||||||||||||||
| VTE risk without PPX | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01% | 0.07% | 0.14% | 0.20% | 0.30% | 0.35% | 0.40% | 0.45% | 0.5% | 0.6% | 0.8% | 1.0% | 1.2% | 1.4% | 1.5% | 5.0% | 15% | ||||
| Risk of major bleeding with PPX | 0.01% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.01% | Risk of major bleeding without PPX |
| 0.05% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.03% | ||
| 0.10% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.07% | ||
| 0.20% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.13% | ||
| 0.30% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.20% | ||
| 0.40% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.26% | ||
| 0.50% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.33% | ||
| 1.00% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.65% | ||
| 2.00% | N | N | S5 | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | 1.31% | ||
| 3.00% | N | N | S5 | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | 1.96% | ||
| 4.00% | N | N | S5 | S20 | S25 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | 2.61% | ||
| 4.58% | N | N | S5 | S20 | S25 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | 3.00% | ||
| 5.00% | N | N | S5 | S20 | S25 | S25 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | 3.27% | ||
| 6.00% | N | N | S5 | S20 | S25 | S25 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | 3.92% | ||
| 7.00% | N | N | N | S20 | S25 | S25 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | 4.58% | ||
| 8.00% | N | N | N | S20 | S25 | S25 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | 5.23% | ||
| 9.00% | N | N | N | S5 | S25 | S25 | S25 | S25 | S25 | S25 | A | A | A | A | A | A | A | 5.88% | ||
| 10.0% | N | N | N | S5 | S20 | S25 | S25 | S25 | S25 | S25 | A | A | A | A | A | A | A | 6.54% | ||
| 20.0% | N | N | N | S5 | S5 | S20 | S20 | S25 | S25 | S25 | S25 | A | A | A | A | A | A | 13.1% | ||
| 30.0% | N | N | N | N | S5 | S5 | S20 | S20 | S20 | S25 | S25 | S25 | A | A | A | A | A | 19.6% | ||
| 0.01% | 0.04% | 0.07% | 0.11% | 0.16% | 0.19% | 0.21% | 0.24% | 0.26% | 0.32% | 0.42% | 0.5% | 0.6% | 0.7% | 0.8% | 2.6% | 7.9% | ||||
| VTE risk with PPX | ||||||||||||||||||||
| VTE risk without PPX | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01% | 0.07% | 0.15% | 0.20% | 0.30% | 0.35% | 0.45% | 0.5% | 0.6% | 0.8% | 1.0% | 1.2% | 1.4% | 1.5% | 2% | 10% | 15% | ||||
| Risk of major bleeding with PPX | 0.01% | N | N | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | A | 0.01% | Risk of major bleeding without PPX |
| 0.05% | N | N | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | A | 0.03% | ||
| 0.10% | N | N | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | A | 0.07% | ||
| 0.20% | N | N | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | A | 0.13% | ||
| 0.30% | N | N | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | A | 0.20% | ||
| 0.40% | N | N | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | A | 0.26% | ||
| 0.50% | N | N | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | A | 0.33% | ||
| 1.00% | N | N | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | A | 0.65% | ||
| 2.00% | N | N | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | 1.31% | ||
| 3.00% | N | N | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | 1.96% | ||
| 4.00% | N | N | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | 2.61% | ||
| 4.58% | N | N | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | 3.00% | ||
| 5.00% | N | N | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | 3.27% | ||
| 6.00% | N | N | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | 3.92% | ||
| 7.00% | N | N | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | A | 4.58% | ||
| 8.00% | N | N | EK | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | 5.23% | ||
| 9.00% | N | N | EK | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | 5.88% | ||
| 10.00% | N | N | EK | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | A | A | 6.54% | ||
| 20.00% | N | N | EK | EK | EK | EK | EK | EK | EK | EK | EK | EK | A | A | A | A | A | 13.1% | ||
| 30.00% | N | N | N | EK | EK | EK | EK | EK | EK | EK | EK | EK | EK | EK | A | A | A | 19.6% | ||
| 0.01% | 0.04% | 0.07% | 0.11% | 0.16% | 0.19% | 0.24% | 0.3% | 0.3% | 0.4% | 0.5% | 0.6% | 0.7% | 0.8% | 1% | 5% | 8% | ||||
| VTE risk with PPX | ||||||||||||||||||||
| VTE risk without PPX | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.01% | 0.07% | 0.14% | 0.20% | 0.30% | 0.35% | 0.4% | 0.50% | 0.6% | 0.8% | 1.0% | 1.5% | 2.0% | 3.0% | p | 10% | 15% | ||||
| Risk of major bleeding with PPX | 0.01% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.01% | Risk of major bleeding without PPX |
| 0.10% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.07% | ||
| 0.20% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.13% | ||
| 0.40% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.26% | ||
| 0.50% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.33% | ||
| 1.00% | N | N | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | A | 0.65% | ||
| 2.00% | N | N | S5 | S20 | S25 | S25 | S25 | A | A | A | A | A | A | A | A | A | A | 1.31% | ||
| 3.00% | N | N | S5 | S20 | S25 | S25 | S25 | B | B | B | B | A | A | A | A | A | A | 1.96% | ||
| 4.00% | N | N | S5 | S20 | S25 | S25 | S25 | B | B | B | B | B | B | A | A | A | A | 2.61% | ||
| 4.58% | N | N | S5 | S20 | S25 | S25 | S25 | B | B | B | B | B | B | B | A | A | A | 3.00% | ||
| 5.00% | N | N | S5 | S20 | S25 | S25 | S25 | B | B | B | B | B | B | B | A | A | A | 3.27% | ||
| 6.00% | N | N | N | S20 | S25 | S25 | S25 | S25 | B | B | B | B | B | B | A | A | A | 3.92% | ||
| 7.00% | N | N | N | S20 | S25 | S25 | S25 | S25 | B | B | B | B | B | B | B | A | A | 4.58% | ||
| 8.00% | N | N | N | S20 | S25 | S25 | S25 | S25 | B | B | B | B | B | B | B | A | A | 5.23% | ||
| 9.00% | N | N | N | S5 | S25 | S25 | S25 | S25 | B | B | B | B | B | B | B | A | A | 5.88% | ||
| 10.00% | N | N | N | S5 | S20 | S25 | S25 | S25 | S25 | B | B | B | B | B | B | A | A | 6.54% | ||
| 20.00% | N | N | N | S5 | S5 | S20 | S20 | S25 | S25 | S25 | B | B | B | B | B | B | A | 13.1% | ||
| 30.00% | N | N | N | N | S5 | S5 | S20 | S20 | S25 | S25 | S25 | B | B | B | B | B | B | 19.6% | ||
| 0.01% | 0.04% | 0.07% | 0.11% | 0.16% | 0.19% | 0.2% | 0.26% | 0.3% | 0.4% | 0.5% | 0.8% | 1.1% | 1.6% | 2.6% | 5% | 8% | ||||
| VTE risk with PPX | ||||||||||||||||||||
| VTE risk without PPX | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.20% | 0.35% | 0.50% | 1.00% | 1.50% | 1.70% | 2.0% | 3.00% | 4.0% | 5.0% | 6.0% | 8.0% | 10.0% | 15.0% | 16% | 17% | 20% | ||||
| Risk of major bleeding with PPX | 0.01% | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 0.01% | Risk of major bleeding without PPX |
| 0.05% | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 0.03% | ||
| 0.10% | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 0.07% | ||
| 0.20% | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 0.13% | ||
| 0.30% | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 0.20% | ||
| 0.40% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 0.26% | ||
| 0.50% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 0.33% | ||
| 1.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 0.65% | ||
| 2.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 1.31% | ||
| 3.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 1.96% | ||
| 4.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 2.61% | ||
| 4.58% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 3.00% | ||
| 5.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 3.27% | ||
| 6.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 3.92% | ||
| 7.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 4.58% | ||
| 8.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 5.23% | ||
| 9.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 5.88% | ||
| 10.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 6.54% | ||
| 20.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 13.1% | ||
| 30.00% | N | N | N | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | PP | 19.6% | ||
| 0.10% | 0.18% | 0.26% | 0.52% | 0.79% | 0.89% | 1.0% | 1.57% | 2.1% | 2.6% | 3.1% | 4.2% | 5.2% | 7.9% | 8% | 9% | 10% | ||||
| VTE risk with PPX | ||||||||||||||||||||
| Scenario | PP PPX only | Lyon | EThIG | PPX for all | Maximum INMB at £30K | ||||
|---|---|---|---|---|---|---|---|---|---|
| Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | ||
| Base case | −73.98 | 0.136 | 421.15 | 0.156 | 549.68 | 0.161 | 820.43 | 0.160 | EThIG |
| Double utility decrement for PPX | −73.98 | 0.135 | 421.15 | 0.153 | 549.68 | 0.157 | 820.43 | 0.155 | EThIG |
| Zero utility decrement for PPX | −73.98 | 0.137 | 421.15 | 0.159 | 549.68 | 0.165 | 820.43 | 0.165 | EThIG |
| No PTS in asymptomatic DVT | 12.74 | 0.045 | 506.47 | 0.067 | 634.64 | 0.072 | 905.39 | 0.071 | EThIG |
| Higher PTS costs from US study (Caprini) | −586.27 | 0.136 | −154.97 | 0.156 | −43.37 | 0.161 | 227.38 | 0.160 | EThIG |
| Wound haematoma results in ED visit | −73.98 | 0.136 | 407.56 | 0.156 | 532.51 | 0.161 | 797.15 | 0.160 | EThIG |
| LMWH stops for 4 weeks after major bleed in VTE treatment | −73.98 | 0.136 | 421.32 | 0.156 | 549.87 | 0.161 | 820.62 | 0.160 | EThIG |
| All antepartum VTE results in admission and 50% of PE admit to ICU | −73.98 | 0.136 | 395.23 | 0.156 | 517.12 | 0.161 | 787.87 | 0.160 | EThIG |
| Non-fatal, non-ICH bleeds have no cost or HRQoL implications | −94.56 | 0.136 | 398.29 | 0.156 | 526.22 | 0.161 | 796.72 | 0.160 | EThIG |
| PPX results in zero fatal bleeds and zero non-fatal ICH | −75.13 | 0.136 | 419.90 | 0.156 | 548.40 | 0.161 | 819.15 | 0.160 | EThIG |
| RR of major bleeding from TIPPS | −75.43 | 0.136 | 419.62 | 0.156 | 548.12 | 0.161 | 818.83 | 0.160 | EThIG |
| RR of major bleeding from Rodger 2016 | −47.28 | 0.136 | 450.74 | 0.155 | 580.02 | 0.160 | 852.00 | 0.159 | EThIG |
| RR of VTE from Cochrane review | −52.56 | 0.124 | 453.25 | 0.141 | 584.54 | 0.146 | 855.29 | 0.145 | EThIG |
| Lower utility decrement of PTS (Lenert) | −73.98 | 0.036 | 421.15 | 0.043 | 549.68 | 0.045 | 820.43 | 0.044 | PP PPX only |
| Fewer outpatient appointments for treatment dose VTE | −56.94 | 0.136 | 450.13 | 0.156 | 581.71 | 0.161 | 852.46 | 0.160 | EThIG |
| PTS risk from non-pregnant cohort | −14.93 | 0.074 | 479.89 | 0.094 | 608.48 | 0.100 | 879.23 | 0.098 | EThIG |
| Zero risk of fatal bleeds or ICH on treatment dose LMWH | −71.71 | 0.136 | 425.05 | 0.155 | 553.99 | 0.160 | 824.74 | 0.159 | EThIG |
| No increased risk of death in first year after ICH | −73.99 | 0.136 | 421.15 | 0.156 | 549.68 | 0.161 | 820.43 | 0.160 | EThIG |
| Zero costs for risk assessment | −73.98 | 0.136 | 411.57 | 0.156 | 540.10 | 0.161 | 820.43 | 0.160 | EThIG |
| Age 40 years | −64.13 | 0.120 | 432.31 | 0.137 | 561.18 | 0.142 | 831.90 | 0.141 | EThIG |
| Age 20 years | −81.26 | 0.149 | 412.92 | 0.171 | 541.19 | 0.176 | 811.96 | 0.175 | EThIG |
| High BMI (36 kg/m2) | −31.77 | 0.136 | 616.41 | 0.156 | 785.30 | 0.161 | 1131.02 | 0.160 | PP PPX only |
| High BMI (36 kg/m2) and high age (40 years) | −21.92 | 0.120 | 627.57 | 0.137 | 796.79 | 0.142 | 1142.50 | 0.141 | PP PPX only |
| Non-fatal non-ICH bleeds have zero cost | −94.56 | 0.136 | 398.29 | 0.156 | 526.22 | 0.161 | 796.72 | 0.160 | EThIG |
| Non-fatal non-ICH bleeds have double cost | −53.41 | 0.136 | 444.02 | 0.156 | 573.13 | 0.161 | 844.13 | 0.160 | EThIG |
| Antepartum bleed risk of 4% | −73.98 | 0.136 | 415.00 | 0.156 | 541.91 | 0.161 | 809.75 | 0.160 | EThIG |
| PP VTE at 5 days | −75.18 | 0.136 | 419.91 | 0.156 | 548.43 | 0.161 | 819.18 | 0.160 | EThIG |
| PP VTE at 42 days | −72.41 | 0.136 | 422.78 | 0.156 | 551.32 | 0.161 | 822.07 | 0.160 | EThIG |
| Scenario | Sultan (top 1%) | Sultan (top 5%) | SFOG | RCOG | PPX for all | Maximum INMB at £30K | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALYvs. no PPX | ||
| Base case | 10.715 | 0.00009 | 15.546 | 0.00026 | 17.208 | 0.00020 | 52.640 | 0.00055 | 126.215 | 0.00071 | No PPX |
| Double utility decrement for PPX | 10.715 | 0.00009 | 15.546 | 0.00026 | 17.208 | 0.00019 | 52.640 | 0.00048 | 126.215 | 0.00052 | No PPX |
| Zero utility decrement for PPX | 10.715 | 0.00009 | 15.546 | 0.00027 | 17.208 | 0.00021 | 52.640 | 0.00061 | 126.215 | 0.00090 | No PPX |
| No PTS in asymptomatic DVT | 10.777 | 0.00003 | 15.727 | 0.00008 | 17.350 | 0.00005 | 53.069 | 0.00010 | 126.895 | 0.00000 | No PPX |
| Higher PTS costs from US study (Caprini) | 10.358 | 0.00009 | 14.490 | 0.00026 | 16.379 | 0.00020 | 50.140 | 0.00055 | 122.255 | 0.00071 | No PPX |
| Wound haematoma results in ED visit | 10.643 | 0.00009 | 15.186 | 0.00026 | 16.762 | 0.00020 | 50.179 | 0.00054 | 119.089 | 0.00069 | No PPX |
| Treatment dose LMWH restarted 4 weeks after bleed | 10.715 | 0.00009 | 15.546 | 0.00026 | 17.208 | 0.00020 | 52.641 | 0.00055 | 126.216 | 0.00071 | No PPX |
| Non-fatal, non-ICH bleeds have no cost or HRQoL loss | 10.527 | 0.00009 | 14.611 | 0.00027 | 16.047 | 0.00021 | 46.234 | 0.00058 | 107.666 | 0.00080 | No PPX |
| PPX results in zero fatal bleeds and zero non-fatal ICH | 10.716 | 0.00009 | 15.523 | 0.00025 | 17.163 | 0.00019 | 52.320 | 0.00055 | 125.176 | 0.00078 | No PPX |
| RR of major bleeding from TIPPS | 10.702 | 0.00009 | 15.481 | 0.00027 | 17.126 | 0.00020 | 52.192 | 0.00055 | 124.918 | 0.00072 | No PPX |
| RR of major bleeding from Rodger 2016 | 10.964 | 0.00009 | 16.778 | 0.00025 | 18.736 | 0.00018 | 61.072 | 0.00041 | 150.629 | 0.00032 | No PPX |
| RR of VTE from Cochrane review | 10.730 | 0.00008 | 15.591 | 0.00024 | 17.243 | 0.00018 | 52.746 | 0.00049 | 126.383 | 0.00062 | No PPX |
| Lower utility decrement of PTS (Lenert) | 10.715 | 0.00002 | 15.546 | 0.00006 | 17.208 | 0.00004 | 52.640 | 0.00006 | 126.215 | −0.00007 | No PPX |
| Assume RR applies for 10 days | 10.772 | 0.00006 | 15.713 | 0.00017 | 17.338 | 0.00013 | 53.035 | 0.00032 | 126.841 | 0.00036 | No PPX |
| Assume RR applies for 6 weeks | 10.644 | 0.00013 | 15.335 | 0.00038 | 17.042 | 0.00029 | 52.141 | 0.00083 | 125.425 | 0.00116 | No PPX |
| Zero cost for risk assessment | 1.134 | 0.00009 | 5.965 | 0.00026 | 7.626 | 0.00020 | 43.059 | 0.00055 | 126.215 | 0.00071 | Sultan (top 5%) |
| PTS risk from non-pregnant cohort | 10.756 | 0.00005 | 15.666 | 0.00014 | 17.302 | 0.00010 | 52.924 | 0.00025 | 126.664 | 0.00024 | No PPX |
| Fewer outpatient follow-ups during VTE treatment | 10.727 | 0.00009 | 15.582 | 0.00026 | 17.236 | 0.00020 | 52.724 | 0.00055 | 126.349 | 0.00071 | No PPX |
| Zero fatal bleeds or ICH during treatment dose LMWH after VTE | 10.717 | 0.00009 | 15.551 | 0.00026 | 17.211 | 0.00020 | 52.651 | 0.00055 | 126.233 | 0.00071 | No PPX |
| No increased risk of death in first year after ICH | 10.715 | 0.00009 | 15.546 | 0.00026 | 17.208 | 0.00020 | 52.641 | 0.00055 | 126.217 | 0.00071 | No PPX |
| Age 40 years | 10.721 | 0.00008 | 15.561 | 0.00023 | 17.217 | 0.00017 | 52.654 | 0.00047 | 126.191 | 0.00060 | No PPX |
| Age 20 years | 10.711 | 0.00010 | 15.535 | 0.00029 | 17.200 | 0.00022 | 52.630 | 0.00061 | 126.232 | 0.00080 | No PPX |
| High BMI (36 kg/m2) | 10.846 | 0.00009 | 16.211 | 0.00026 | 18.043 | 0.00020 | 57.293 | 0.00055 | 139.761 | 0.00071 | No PPX |
| Age 40 years and high BMI (36 kg/m2) | 10.852 | 0.00008 | 16.226 | 0.00023 | 18.052 | 0.00017 | 57.307 | 0.00047 | 139.736 | 0.00060 | No PPX |
| Non-fatal non-ICH bleeds have zero cost | 10.527 | 0.00009 | 14.611 | 0.00026 | 16.047 | 0.00020 | 46.234 | 0.00055 | 107.666 | 0.00071 | No PPX |
| Non-fatal non-ICH bleeds have double cost | 10.904 | 0.00009 | 16.481 | 0.00026 | 18.368 | 0.00020 | 59.046 | 0.00055 | 144.765 | 0.00071 | No PPX |
| PP VTE at 5 days | 10.715 | 0.00009 | 15.546 | 0.00027 | 17.208 | 0.00021 | 52.640 | 0.00058 | 126.215 | 0.00080 | No PPX |
| PP VTE at 42 days | 10.715 | 0.00009 | 15.546 | 0.00026 | 17.208 | 0.00020 | 52.640 | 0.00053 | 126.215 | 0.00065 | No PPX |
| Scenario | Ellis-Kahana (full RAM) | PPX for all | Maximum INMB at £30K | ||
|---|---|---|---|---|---|
| Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | ||
| Base case | 20.95 | 0.0014 | 137.55 | 0.0019 | Ellis-Kahana (full RAM) |
| Double utility decrement for PPX | 20.95 | 0.0014 | 137.55 | 0.0017 | Ellis-Kahana (full RAM) |
| Zero utility decrement for PPX | 20.95 | 0.0014 | 137.55 | 0.0021 | Ellis-Kahana (full RAM) |
| No PTS in asymptomatic DVT | 21.85 | 0.0004 | 139.00 | 0.0004 | No PPX |
| Higher PTS costs from US study (Caprini) | 15.69 | 0.0014 | 129.12 | 0.0019 | Ellis-Kahana (full RAM) |
| Wound haematoma results in ED visit | 20.24 | 0.0014 | 130.43 | 0.0019 | Ellis-Kahana (full RAM) |
| Treatment dose LMWH restarted 4 weeks after bleed | 20.95 | 0.0014 | 137.56 | 0.0019 | Ellis-Kahana (full RAM) |
| Non-fatal, non-ICH bleeds have no cost or HRQoL loss | 19.12 | 0.0014 | 119.01 | 0.0020 | Ellis-Kahana (full RAM) |
| PPX results in zero fatal bleeds and zero non-fatal ICH | 20.83 | 0.0014 | 136.34 | 0.0020 | Ellis-Kahana (full RAM) |
| RR of major bleeding from TIPPS | 20.82 | 0.0014 | 136.26 | 0.0019 | Ellis-Kahana (full RAM) |
| RR of major bleeding from Rodger 2016 | 23.35 | 0.0013 | 161.97 | 0.0015 | Ellis-Kahana (full RAM) |
| RR of VTE from Cochrane review | 21.18 | 0.0012 | 137.93 | 0.0017 | Ellis-Kahana (full RAM) |
| Lower utility decrement of PTS (Lenert) | 20.95 | 0.0003 | 137.55 | 0.0003 | No PPX |
| Assume RR applies for 10 days | 21.81 | 0.0009 | 138.95 | 0.0012 | Ellis-Kahana (full RAM) |
| Assume RR applies for 6 weeks | 19.85 | 0.0020 | 135.79 | 0.0029 | Ellis-Kahana (full RAM) |
| Zero cost for risk assessment | 11.36 | 0.0014 | 137.55 | 0.0019 | Ellis-Kahana (full RAM) |
| PTS risk from non-pregnant cohort | 21.54 | 0.0007 | 138.51 | 0.0009 | Ellis-Kahana (full RAM) |
| Fewer outpatient follow-ups during VTE treatment | 21.12 | 0.0014 | 137.84 | 0.0019 | Ellis-Kahana (full RAM) |
| Zero fatal bleeds or ICH during treatment dose LMWH after VTE | 20.97 | 0.0014 | 137.59 | 0.0019 | Ellis-Kahana (full RAM) |
| No increased risk of death in first year after ICH | 20.95 | 0.0014 | 137.56 | 0.0019 | Ellis-Kahana (full RAM) |
| Age 40 years | 21.04 | 0.0012 | 137.62 | 0.0017 | Ellis-Kahana (full RAM) |
| Age 20 years | 20.88 | 0.0015 | 137.51 | 0.0021 | Ellis-Kahana (full RAM) |
| Normal BMI | 19.72 | 0.0014 | 124.11 | 0.0019 | Ellis-Kahana (full RAM) |
| Non-fatal non-ICH bleeds have zero cost | 19.12 | 0.0014 | 119.01 | 0.0019 | Ellis-Kahana (full RAM) |
| Non-fatal non-ICH bleeds have double cost | 22.77 | 0.0014 | 156.10 | 0.0019 | Ellis-Kahana (full RAM) |
| PP VTE at 5 days | 20.95 | 0.0014 | 137.55 | 0.0020 | Ellis-Kahana (full RAM) |
| PP VTE at 42 days | 20.95 | 0.0014 | 137.55 | 0.002 | Ellis-Kahana (full RAM) |
| Scenario | Sultan (top 1%) | Sultan (top 5%) | Binstock novel | RCOG | PPX for all | Maximum INMB at £30K | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | Inc costs vs. no PPX, £ | Inc QALY vs. no PPX | ||
| Base case | 10.591 | 0.00018 | 15.172 | 0.00052 | 123.937 | 0.00169 | 126.472 | 0.00168 | 124.540 | 0.00166 | Sultan (top 5%) |
| Double utility decrement for PPX | 10.591 | 0.00018 | 15.172 | 0.00051 | 123.937 | 0.00152 | 126.472 | 0.00150 | 124.540 | 0.00147 | Sultan (top 5%) |
| Zero utility decrement for PPX | 10.591 | 0.00018 | 15.172 | 0.00053 | 123.937 | 0.00186 | 126.472 | 0.00186 | 124.540 | 0.00185 | Sultan (top 5%) |
| No PTS in asymptomatic DVT | 10.707 | 0.00006 | 15.516 | 0.00016 | 125.227 | 0.00034 | 127.762 | 0.00034 | 125.830 | 0.00031 | No PPX |
| Higher PTS costs from US study (Caprini) | 9.913 | 0.00018 | 13.167 | 0.00052 | 116.425 | 0.00169 | 118.960 | 0.00168 | 117.029 | 0.00166 | Sultan (top 5%) |
| Wound haematoma result in ED visit | 10.517 | 0.00018 | 14.808 | 0.00052 | 117.377 | 0.00167 | 119.771 | 0.00167 | 117.414 | 0.00164 | Sultan (top 5%) |
| Treatment dose LMWH restarted 4 weeks after bleed | 10.591 | 0.00018 | 15.172 | 0.00052 | 123.938 | 0.00169 | 126.473 | 0.00168 | 124.542 | 0.00166 | Sultan (top 5%) |
| Non-fatal, non-ICH bleeds have no cost or HRQoL loss | 10.399 | 0.00018 | 14.226 | 0.00052 | 106.865 | 0.00177 | 109.033 | 0.00176 | 105.994 | 0.00174 | Sultan (top 5%) |
| PPX results in zero fatal bleeds and zero non-fatal ICH | 10.578 | 0.00018 | 15.110 | 0.00052 | 122.822 | 0.00176 | 125.333 | 0.00175 | 123.329 | 0.00173 | Sultan (top 5%) |
| RR of major bleeding from TIPPS | 10.578 | 0.00018 | 15.105 | 0.00052 | 122.742 | 0.00170 | 125.252 | 0.00169 | 123.243 | 0.00167 | Sultan (top 5%) |
| RR of major bleeding from Rodger 2016 | 10.844 | 0.00017 | 16.417 | 0.00050 | 146.409 | 0.00133 | 149.427 | 0.00132 | 148.953 | 0.00127 | No PPX |
| RR of VTE from Cochrane review | 10.620 | 0.00016 | 15.256 | 0.00047 | 124.254 | 0.00151 | 126.789 | 0.00150 | 124.857 | 0.00148 | No PPX |
| Lower utility decrement of PTS (Lenert) | 10.591 | 0.00004 | 15.172 | 0.00013 | 123.937 | 0.00022 | 126.472 | 0.00021 | 124.540 | 0.00019 | No PPX |
| Assume RR applies for 10 days | 10.698 | 0.00012 | 15.488 | 0.00034 | 125.123 | 0.00102 | 127.658 | 0.00101 | 125.727 | 0.00099 | No PPX |
| Assume RR applies for 6 weeks | 10.456 | 0.00025 | 14.772 | 0.00074 | 122.438 | 0.00254 | 124.973 | 0.00253 | 123.041 | 0.00251 | Sultan (top 20%) |
| Zero cost for risk assessment | 1.010 | 0.00018 | 5.590 | 0.00052 | 114.355 | 0.00169 | 116.890 | 0.00168 | 124.540 | 0.00166 | Sultan (top 5%) |
| PTS risk from non-pregnant cohort | 10.668 | 0.00010 | 15.399 | 0.00028 | 124.788 | 0.00080 | 127.323 | 0.00079 | 125.392 | 0.00077 | No PPX |
| Fewer outpatient follow-ups during VTE treatment | 10.614 | 0.00018 | 15.239 | 0.00052 | 124.190 | 0.00169 | 126.725 | 0.00168 | 124.794 | 0.00166 | Sultan (top 5%) |
| Zero fatal bleeds or ICH during treatment dose LMWH after VTE | 10.594 | 0.00018 | 15.181 | 0.00052 | 123.971 | 0.00168 | 126.506 | 0.00167 | 124.574 | 0.00165 | Sultan (top 5%) |
| No increased risk of death in first year after ICH | 10.591 | 0.00018 | 15.172 | 0.00052 | 123.938 | 0.00169 | 126.473 | 0.00168 | 124.542 | 0.00166 | Sultan (top 5%) |
| Age 40 years | 10.603 | 0.00016 | 15.205 | 0.00046 | 123.989 | 0.00147 | 126.522 | 0.00146 | 124.585 | 0.00144 | No PPX |
| Age 20 years | 10.582 | 0.00019 | 15.147 | 0.00057 | 123.897 | 0.00187 | 126.433 | 0.00186 | 124.506 | 0.00184 | Sultan (top 5%) |
| High BMI (36 kg/m2) | 10.718 | 0.00018 | 15.823 | 0.00052 | 136.320 | 0.00169 | 139.125 | 0.00168 | 138.007 | 0.00166 | No PPX |
| Non-fatal non-ICH bleeds have zero cost | 10.399 | 0.00018 | 14.226 | 0.00052 | 106.865 | 0.00169 | 109.033 | 0.00168 | 105.994 | 0.00166 | Sultan (top 5%) |
| Non-fatal non-ICH bleeds have double cost | 10.783 | 0.00018 | 16.117 | 0.00052 | 141.008 | 0.00169 | 143.910 | 0.00168 | 143.087 | 0.00166 | No PPX |
Appendix 7 Workshop questions
Suggested script for introduction
Thanks for coming today. (Talk about the workshop and how it will all work. Going to audio record. Request no-one takes photos or video. Need for confidentiality.) If you need to dip in and out, that’s fine. If feel distressed or want to back out at any stage, please do so, don’t need to give an explanation.
(Talk about payment). Going to explain how this workshop will happen. I’m going to give you a brief introduction to the project, then I’m going to ask for your thoughts on various things. There are no right and wrong answers – just want your opinions. COVID – although everything we’ve done recently has been dominated by COVID, would like to avoid talking about it where possible.
Will have a break for 5 minutes after an hour.
You have all responded as you have previous experience of blood clots and have had to take blood thinners during pregnancy. Despite there being national guidance about who should receive blood thinners, the number of people presenting with blood clots during and shortly after pregnancy has not changed much over the years and we are doing a research project to understand where there is a need for clearer evidence. Current guidelines recommend giving blood thinners based on different risk factors, which can include previous clots, pre-existing clotting disorders, BMI, age etc. These recommendations have been based on the results of different clinical trials that have shown how effective blood thinners are for people with different risk factors. However, most of the clinical trials on which these recommendations were based did not include pregnant women, and studies that did include pregnant women struggled to recruit enough people to get meaningful results. Our research study so far has identified areas where it would be most useful to have further evidence from clinical trials to understand how effective blood thinners are for people with different risk factors.
We did a systematic review of the existing research literature to find all existing evidence for how effective treatments are for preventing further clots in pregnancy, or after giving birth and have undertaken mathematical modelling to understand which areas have the highest levels of uncertainty and would benefit most from evidence from RCTs. Before we report this to the funders, we want to understand a bit about whether trials would actually be feasible, and whether pregnant women would be willing to take part in trials. We want to speak to you as you have real-world experience of having been offered blood thinners to help us to understand what people might think if asked to take part in research in future. (For first groups: As you have previously had DVT, your risks and perceptions of risks may be different.)
Questions (cover in any order)
To start off with, can you give us a bit of background and tell me a bit about how you were told you would need blood thinners (prompt – how potential risks and benefits were explained).
Can you tell me a bit about your experience of taking blood thinners (prompt – did you take them as prescribed? Practical issues).
Next, can we talk about how you think you might respond if a doctor or nurse explained that the evidence for blood thinners in your particular group was not very clear, and that they would like you to take part in a RCT where you would be randomly allocated to either receive blood thinners or no treatment.
Would you be willing to take part in a trial? What would your concerns be about taking part?
When do you think would be the best time to make these decisions (prompt – during pregnancy/shortly after giving birth?)
How would you feel about going through pregnancy without taking blood thinners when randomised to a trial?
Is there any further information that might help you make the decision whether to take part in a trial? (Prompt – potential benefits to being in a study, what might make you more willing to take part?).
If instead of being told you needed to take heparin, you were told that there was not yet enough evidence about whether heparin was needed for your population, do you think you would have been willing to take part in a trial?
Some types of trial will involve some hospitals giving blood thinners to a group of patients, and others not, rather than some individuals being given blood thinners. How do you feel about this?
Would you prefer the hospital to be randomised, or the individual? What would influence your decision? Who would influence your decision?
At 2 hours – end discussion. Thank all for attending and remind them about the process for receiving payment.
Appendix 8 Venous thromboembolism in pregnancy survey
We are inviting you to take part in a survey that will help us inform NHS practice on the use of risk stratification tools for the prediction of VTE and appropriate provision of thromboprophylaxis for women in pregnancy and the puerperium.
We have undertaken a systematic review of published literature and undertaken mathematical modelling to identify which factors are key drivers of uncertainty and therefore high value from future research. We would like you to take part in a survey to help us understand whether you would be likely to enrol patients into future trials in this area.
The survey should take between 5 and 10 minutes to complete.
Please read the information sheet, which can be accessed by clicking the link below:
Vtep survey information sheet v1.2
Q2 I have read and understood the information sheet
Yes (1)
Q3 I am happy to participate in the survey
Yes (1)
Q1. Which of the following best describes your role?
| Consultant (1) | Trainee (2) | |
|---|---|---|
| Obstetrician (1) | ○ | ○ |
| Gynaecologist (2) | ○ | ○ |
| Obstetrician and gynaecologist (3) | ○ | ○ |
| Midwife (4) | ○ | ○ |
| Haematologist (5) | ○ | ○ |
| Obstetric physician (6) | ○ | ○ |
| Consultant midwife (7) | ○ | ○ |
| Other (8) | ○ | ○ |
Q1. If ‘other’, please give details_____________________________________________________________________ ___________________________________________________________________________________________________ ___________________________________________________________________________________________________ ___________________________________________________________________________________________________
We would like to understand whether clinicians would be likely to enrol patients who are pregnant or in the puerperium into future trials, particularly in groups where guidance currently suggests that patients should be given thromboprophylaxis. There are currently differences in the patient groups for whom thromboprophylaxis is recommended by RCOG and guidance from other parts of the world. Please answer questions below based upon your current clinical knowledge of the benefits, risks and uncertainties around the use of thromboprophylaxis, rather than what is recommended in any guidelines you might expect to follow in your clinical practice.
The following questions are based on groups of patients for whom we identified that further evidence from clinical trials would reduce the uncertainty in current VTE-RAMs.
Q2. For the following seven patient scenarios who were not eligible for antepartum prophylaxis, please state whether you would be willing to randomise these patients into a study of LMWH versus no LMWH.
| Yes, I would randomise this patient (1) |
No, I wouldn’t randomise and I would prescribe LMWH (2) |
No, I wouldn’t randomise and I would NOT prescribe LMWH (4) |
Don’t know/other (Please comment) (5) |
|
|---|---|---|---|---|
| 2a) Emergency C-section (BMI ≤ 30) (1) | ○ | ○ | ○ | ○ |
| 2b) Elective C-section and age 36 (BMI ≤ 30) (2) | ○ | ○ | ○ | ○ |
| 2c) BMI ≥ 40 (3) | ○ | ○ | ○ | ○ |
| 2d) BMI 32 and PPH requiring blood transfusion (4) | ○ | ○ | ○ | ○ |
| 2e) BMI 32 and elective C-section (5) | ○ | ○ | ○ | ○ |
| 2f) BMI 32 and emergency C-section (6) | ○ | ○ | ○ | ○ |
| 2g) BMI 32 and age 36 (7) | ○ | ○ | ○ | ○ |
Q2. Please explain any concerns you may have about recruiting any of the patients listed above into a RCT: _________________________________________________________________________________________________________________________________________________________________________________________________ ___________________________________________________________________________________________________
Q3: For the following seven patient scenarios, please state whether you would be willing to randomise these patients into a study of LMWH vs. no LMWH. For each patient scenario, please state whether you would be willing to randomise (1) from booking in, (2) from 28 weeks pregnancy, (3) postnatally.
| Please select one answer | ||||
|---|---|---|---|---|
| Yes, I would randomise this patient (1) |
No, I wouldn’t randomise and I would prescribe LMWH (2) |
No, I wouldn’t randomise and I would NOT prescribe LMWH (3) |
Don’t know/other (please comment in box below) (4) |
|
| Q3a: Patient age < 35, BMI < 30, prior unprovoked VTE (1) From booking (5) | ○ | ○ | ○ | ○ |
| (2) From 28 weeks (6) | ○ | ○ | ○ | ○ |
| (3) Postnatally (7) | ○ | ○ | ○ | ○ |
| Q3b: Patient age < 35, BMI < 30, prior VTE associated with major abdominal surgery (1) From booking (9) | ○ | ○ | ○ | ○ |
| (2) From 28 weeks (13) | ○ | ○ | ○ | ○ |
| (3) Postnatally (14) | ○ | ○ | ○ | ○ |
| Q3c: Patient age < 35, BMI < 30, prior pregnancy-related VTE (1) From booking (16) | ○ | ○ | ○ | ○ |
| (2) From 28 weeks (17) | ○ | ○ | ○ | ○ |
| (3) Postnatally (18) | ○ | ○ | ○ | ○ |
| Q3d: Patient age 36, BMI 32, para 3 (1) From booking (20) | ○ | ○ | ○ | ○ |
| (2) From 28 weeks (21) | ○ | ○ | ○ | ○ |
| (3) Postnatally (22) | ○ | ○ | ○ | ○ |
| Q3e: Patient age < 35, BMI < 30, antiphospholipid antibodies without prior VTE (1) From booking (24) | ○ | ○ | ○ | ○ |
| (2) From 28 weeks (25) | ○ | ○ | ○ | ○ |
| (3) Postnatally (26) | ○ | ○ | ○ | ○ |
| Q3f: Patient age < 35, BMI < 30, Protein C deficiency without prior VTE (1) From booking (28) | ○ | ○ | ○ | ○ |
| (2) From 28 weeks (29) | ○ | ○ | ○ | ○ |
| (3) Postnatally (30) | ○ | ○ | ○ | ○ |
| Q3g: Patient age < 35, BMI < 30, Factor V Leiden homozygous without prior VTE (1) From booking (32) | ○ | ○ | ○ | ○ |
| (2) From 28 weeks (33) | ○ | ○ | ○ | ○ |
| (3) Postnatally (34) | ○ | ○ | ○ | ○ |
Q3: Please explain any concerns you may have about recruiting any of the patients listed above into a RCT: ______________________________________________________________________________________________ ___________________________________________________________________________________________________ ___________________________________________________________________________________________________
Q4. In a future RCT in which women who are pregnant or in the puerperium are allocated to receive either LMWH or no LMWH, would it be acceptable to randomly allocate hospitals or NHS Trusts to provide LMWH or no LMWH for the specified patient groups, rather than the traditional approach of randomly allocating each individual person to either LMWH or no LMWH?
Yes, it would be acceptable to allocate treatment at hospital/NHS Trust level (1)
No, it would only be acceptable to allocate treatment at an individual level (2)
Unsure/don’t know (3)
Don’t understand the question (4)
Other (please give details below) (5)
If ‘other’, please give details._________________________________________________________________________ ___________________________________________________________________________________________________ ___________________________________________________________________________________________________
Q5: What guidance do you currently use to help you decide whether to prescribe LMWH in this population? (Tick all that apply)
Royal College of Obstetricians and Gynaecologists guideline Reducing the Risk of VTE during Pregnancy and the Puerperium. (1)
All-Wales Consensus Policy Exemplar Guide (Thromboembolism Prophylaxis in Pregnancy) (2)
National Institute for Health and Care Excellence guidance Antenatal Care Risk Assessment – VTE (3)
Other (please state below) (4)
For Q5 other, please give details: ______________________________________________________________________________________________________________________________________________________________________ ___________________________________________________________________________________________________ ___________________________________________________________________________________________________
Q6. If there are any particular groups of patients who you feel would benefit from improved evidence from clinical trials, please detail below: ________________________________________________________________________________________________________________________________________________________________ ___________________________________________________________________________________________________
Q7. About you
How long have you been in your current role?
< 2 years (1)
Between 2 and 5 years (2)
Between 5 and 10 years (3)
10+ years (4)
Prefer not to say (5)
Q8. Are you:
Male (1)
Female (2)
Other/prefer not to say (3)
Q9. What is your ethnic background?
Asian/Asian British (1)
Black/African/Caribbean/black British (2)
Mixed/Multiple ethnic groups (3)
White/Caucasian (4)
Other ethnic group (5)
Prefer not to say (6)
Q10. How did you hear about this survey?
British Maternal Fetal Medicine Society (1)
Obstetric Anaesthetist Association (2)
British Society for Haematology Obstetric Haematology Group (3)
MacDonald Obstetric Medicine Society (4)
Other (5)
Q10 Other (please detail) __________________________________________________________________________ ___________________________________________________________________________________________________ ___________________________________________________________________________________________________
Thank you for your responding to this survey. If you have any further comments about anything in the survey, please write them here: ________________________________________________________________________________________________________________________________________________________________________ ___________________________________________________________________________________________________
End of Survey
We thank you for your time spent taking this survey.
Your response has been recorded.
Appendix 9 Costs of research in pregnancy and in the puerperium
To obtain an estimate of typical costs for clinical trials in relevant populations, the NIHR funding awards website (https://fundingawards.nihr.ac.uk/) was searched with the following keywords: pregnancy, pregnant, antepartum, antenatal, ante-natal, postpartum, postnatal, post-natal and puerperium. After de-duplication, 554 unique projects were identified using these terms of which 329 were excluded based on the project titles using the criteria in Table 44. The inclusion/exclusion criteria were designed to identify studies comparing pharmacological interventions to either placebo or another pharmacological intervention. A total of 205 were excluded after considering the details provided in abstract and plain language summary. The main reasons for exclusion were non pharmacological interventions (85 studies); research projects that were not controlled trials (61); studies of diagnostic or monitoring interventions (31 studies); feasibility or pilot studies (10 studies); inappropriate population (9 studies), or interventions where the primary outcome was a benefit to the fetus, baby or child (6 studies); complex interventions (2 studies) and projects that covered multiple trials addressing different research questions (1 study). There remained 20 funded projects that are described in Table 45. Of these, one study was in a cohort of women who had recently given birth (within 24 hours of birth), five were for interventions given during delivery (intrapartum) and the rest were for interventions given antenatally. The median cost was £1.4 million with an IQR of £1.1–2.0 million. One study was described as a phase II study, and this had substantially higher costs than the remaining studies (£7.5 million compared to next highest cost of £2.4 million) and was considered an outlier. The numbers to be recruited range from 200 to 11,020, but study size was a poor predictor of cost (R2 = 0.35), even when excluding the high-cost outlier. The largest study, which had the second highest cost, may also not be representative because the RCT described formed one of five work packages, which included the development of a behavioural package to optimise recruitment and adherence that was given in both trial arms.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Population | Women who are pregnant or who have recently given birth | Women who are not pregnant at the time of receiving the intervention Babies or children |
| Intervention | Pharmacological intervention | Non-pharmacological interventions such as psychological interventions, complex interventions (including where some but not all receive a pharmacological treatment), diagnostic/monitoring intervention, method of delivery, interventions to induce or manage labour (except where these are limited to comparisons between two pharmacological interventions for the same indication) |
| Comparator | Placebo or another pharmacological intervention | Expectant management as a comparator to induction of labour |
| Outcome | Primary outcome is women centred | Primary outcome is for fetus, baby or child |
| Design | Controlled clinical trials where patients are allocated to intervention or comparator | Secondary research including systematic reviews, network meta-analyses Cross-sectional and cohort studies Case-control Diagnostic accuracy or prognostic accuracy studies Qualitative research Research grants that cover multiple work packages that address different research questions |
| NIHR project identifier | Dates | Population | Intervention | Comparator | Design | Cost |
|---|---|---|---|---|---|---|
| 06/07/01 | 2006–13 | Pregnant women between 12 and 24 weeks gestation who smoke | Nicotine replacement therapy patches, N = 521 | Placebo patches, N = 529 | Double-blind randomised placebo-controlled trial, multicentre | £1,355,640 |
| PB-PG-0407-13170 | 2008–12 | Women with singleton pregnancy requesting intramuscular analgesia for labour (recruited antenatally) | Intramuscular pethidine, N = 225 | Intramuscular diamorphine, N = 225 | Two-centre double-blind RCT | £276,601 |
| 09/800/27 | 2008–15 | Women with a singleton pregnancy at high risk of preterm labour (appropriate history or a short (< 25 mm) cervix on ultrasound scan and a positive fFN. | Progesterone (vaginal) from 22 weeks to 34 gestation, N = 600 | Placebo from 22 weeks to 34 gestation, N = 600 | RCT, multicentre (double blind) | £2,248,866 |
| 08/38/01 | 2009–14 | Women with a history of recurrent miscarriages with a positive pregnancy test | Progesterone pessaries, N = 404 | Placebo pessaries, N = 432 | RCT (double blind), multicentre | £1,083,873 |
| 08/246/09 | 2010–5 | Obese (BMI > 30 kg/m2) pregnant women between 12 and 16 weeks gestation | Metformin, N = 100 | Placebo, N = 100 | RCT (double blind), multicentre | £1,166,534 |
| 12/29/01 | 2014–8 | Women with retained placenta at risk of needing manual removal of placenta after vaginal birth | Glyceryl trinitrate sublingual spray, N = 543 | Placebo, N = 543 | RCT (double blind), multicentre (including internal pilot study) | £1,341,128 |
| 12/167/26 | 2014–8 | Women presenting with vaginal bleeding in first trimester of pregnancy | Progesterone (vaginal capsules), N = 2075 | Placebo, N = 2075 | RCT (double blind), multicentre | £1,784,983 |
| 13/04/22 | 2014–9 | Women with twin pregnancy and short cervix at < 20 + 6 weeks gestation (N = 2500 to be screened for cervix length) | Arabin cervical pessary, N = 250 | Conventional treatment, N = 250 | RCT (open-label), multicentre | £1,464,994 |
| 13/96/07 | 2015–9 | Pregnant women undergoing delivery by forceps (any type) or ventouse (any type) at 37 + 0 weeks or greater gestation | Co-amoxiclav single dose after cord clamping, N = 1712 | Placebo, N = 1712 | RCT, (double blind), multicentre | £1,427,689 |
| 12/164/16 | 2015–9 | Women with intrahepatic cholestasis of pregnancy between 20 and 40 weeks gestation | Ursodeoxycholic acid, N = 291 | Placebo, N = 291 | RCT, multicentre | £1,242,610 |
| PB-PG-1013-32011 | 2015–22 | Pregnant women with a history of two or more miscarriages with confirmed inherited thrombophilia | LMWH plus standard care, N = 200 | Placebo plus standard care, N = 200 | RCT (open-label with blinded outcome assessment), multicentre (multinational) | £411,473 |
| 14/140/44 | 2016–23 | Nulliparous women with a singleton cephalic pregnancy at term (37 + 0–41 + 6 weeks gestation) with confirmed delay in the first stage of labour (using NICE definitions) | Standard-dose regimen of oxytocin, N = 750 | High-dose regimen of oxytocin, N = 750 | RCT (double blind), multicentre | £2,301,392 |
| PB-PG-0215-36133 | 2016–23 | Pregnant women with antiphospholipid antibodies | Hydroxychloroquine in addition to usual care, N = 164 | Placebo in addition to usual care, N = 164 | RCT, multicentre | £409,838 |
| 16/16/06 | 2017–21 | Women giving birth (vaginally or by caesarean) who require treatment for vaginal bleeding within 24 hours of birth | Oxytocin 10iu by intravenous injection, N = 1974 | Carboprost 250 mcg by intramuscular injection, N = 1974 |
RCT (double blind, double dummy), multicentre | £1,814,109 |
| 16/15/03 | 2017–20 | Women presenting with severe nausea and vomiting in pregnancy before 16 + 6 weeks gestation who have first-line antiemetic treatment | Metoclopramide, N = 300 | Ondanestron, N = 300 | RCT, multicentre (double dummy, double masked) | £1,079,684 |
| 17/137/02 | 2019–22 | Nulliparous women with singleton pregnancy undergoing induction of labour | High-dose Syntocinon, N = 1200 | Low-dose Syntocinon, N = 1200 | RCT (double blind), multicentre | £2,024,936 |
| NIHR200869 | 2020–5 | Pregnant women | Iron supplementation with behavioural intervention, N = 5510 |
Placebo with behavioural intervention N = 5510 |
RCT (part of larger research programme including earlier pilot study) | £2,368,676 |
| NIHR128721 | 2020–4 | Pregnant women (34 + 0 weeks gestation) with hypertension | Nifedipine (calcium channel blocker), N = 1150 | Labetalol (mixed alpha/beta blocker), N = 1150 |
RCT (open-label), multicentre | £1,973,988 |
| NIHR127325 | 2020–5 | Women at high risk of pre-eclampsia deemed eligible for aspirin | Calcium from 12–22 weeks gestation plus usual care (including aspirin), N = 3878 | Placebo plus usual care (including aspirin) N = 3878 |
RCT (triple-masked placebo controlled) multicentre | £1,966,973 |
| NIHR203306 | 2021–4 | Pregnant women at 13–34 weeks gestation | COVID-19 vaccination with short interval (4–6 weeks), N = 100 | COVID-19 vaccination at long interval (8–12 weeks), N = 100 | Randomised, single-blind phase II trial | £7,551,382 |
Glossary
- Clinically relevant non-major bleeding
- Bleeding episodes which are not major, but require clinical assessment and potential intervention, as defined by the International Society for Thrombosis and Haemostasis.
- Cost-effectiveness acceptability curve
- A way of illustrating cost-effectiveness results by plotting the probability that the intervention is cost-effective (y-axis) against the maximum that society is willing to pay for an improvement in health (x-axis).
- Cost-effectiveness plane
- A way of illustrating cost-effectiveness results by plotting the mean incremental cost and effectiveness on a four-quadrant graph. Interventions that are more costly and more effective fall in the north-east quadrant.
- Deep-vein thrombosis
- A blood clot that develops within a deep vein in the body, most commonly in the leg.
- Dominates
- An intervention that provides greater health benefits for lower costs is said to dominate the strategies it is being compared against.
- Expected value of perfect information
- An estimate of the increase in net monetary benefit that could be achieved by having perfect information on all model parameters simultaneously.
- Expected value of perfect parameter information
- An estimate of the increase in net monetary benefit that could be achieved by having perfect information on individual or selected groups of model parameters.
- Expected value of sample information
- An estimate of the increase in net monetary benefit that could be achieved by obtaining additional information about a parameter or group of parameters by conducting further research.
- Incremental cost-effectiveness ratio
- The difference in the mean costs in the population of interest divided by the differences in the mean outcomes in the population of interest.
- Major bleeding
- Serious or fatal bleeding episodes, as defined by the International Society for Thrombosis and Haemostasis.
- Net monetary benefit
- A summary statistic that represents the value of an intervention in monetary terms taking into account both the costs incurred and the value placed on health benefits achieved.
- Post-thrombotic syndrome
- Pain, swelling, itching, skin discolouration and leg ulcers occurring after a deep-vein thrombosis, caused by damage to the valves in the leg veins that prevent backflow of blood.
- Prophylaxis
- A measure taken to prevent a disease.
- Puerperium
- The period of about 6 weeks after childbirth during which the mother’s reproductive organs return to their original non-pregnant condition.
- Pulmonary embolism
- A blood clot that breaks off from the deep veins and travels around the circulation to block the pulmonary arteries (arteries in the lung). Most deaths arising from deep-vein thrombosis are caused by pulmonary embolism.
- Quality-adjusted life-year
- A measure of the benefit of healthcare that combines the impact of both the expected length of life and quality of life.
- Risk assessment models
- A set of criteria which aims to estimate the risk of a particular condition/complication and often used by clinicians to inform individual patient decisions on medical interventions.
- Thromboprophylaxis
- A measure taken to reduce the risk of thrombosis; prophylaxis against thrombosis.
- Venous thromboembolism
- Thrombosis is the blocking of a blood vessel by a blood clot. This clot may be dislodged fully or partly from its site of origin and travel downstream to lodge in a vital organ, a process described as embolisation. Clots formed in the deep veins of the legs are known as deep-vein thromboses and when fragments break off, they travel through the body to block pulmonary arteries. This process is termed pulmonary embolism. Venous thromboembolism is a composite term to describe all the above, including both deep-vein thrombosis and pulmonary embolism.
List of abbreviations
- ACOG
- American College of Obstetricians and Gynaecologists
- AP
- antepartum
- ART
- assisted reproductive technology
- BMI
- body mass index
- CaVenT
- Catheter Directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CPRD
- Clinical Practice Research Data
- CRNMB
- clinically relevant non-major bleeding
- CTEPH
- chronic thromboembolic pulmonary hypertension
- DiPEP
- diagnosis of pulmonary embolism in pregnancy
- DOAC
- direct oral anticoagulant
- DVT
- deep-vein thrombosis
- ED
- emergency department
- EThIG
- efficacy of thromboprophylaxis as an intervention during gravidity
- EQ-5D
- EuroQol-5 Dimensions
- EVPI
- expected value of perfect information
- EVPPI
- expected value of perfect parameter information
- EVSI
- expected value of sample information
- FRUIT
- low-molecular-weight heparin (FRagmin®) in pregnant women with a history of Uteroplacental Insufficiency and Thrombophilia: a randomised trial
- GARFIELD
- Global Anticoagulant Registry in the FIELD
- GI
- gastrointestinal
- GP
- general practice
- HES
- Hospital Episodes Statistics
- HIT
- heparin-induced thrombocytopenia
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- ICH
- intracerebral haemorrhage
- IQR
- interquartile range
- INMB
- incremental net monetary benefit
- ISTH
- International Society on Thrombosis and Haemostasis
- IVF
- in vitro fertilisation
- LMWH
- low-molecular-weight heparin
- MBRRACE
- Mothers and Babies: Reducing Risk through Audits and Confidential Enquiries across the UK
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NYHA
- New York Heart Association
- OHSS
- ovarian hyperstimulation syndrome
- OR
- odds ratio
- OXVASC
- Oxford Vascular Study
- PE
- pulmonary embolism
- PP
- postpartum
- PPI
- patient and public involvement
- PPX
- prophylaxis
- PREFER-VTE
- Prevention of Thromboembolic Events – European Registry in Venous Thromboembolism
- PRISMA
- preferred reporting items for systematic review and meta-analysis
- PROSPERO
- International Prospective Register of Systematic Reviews
- PSA
- probabilistic sensitivity analysis
- PSS
- personal social services
- PTS
- post-thrombotic syndrome
- QALYs
- quality-adjusted life-years
- RAM
- risk assessment model
- RCOG
- Royal College of Obstetricians and Gynaecologists
- RCT
- randomised controlled trial
- RIETE
- The Computerized Registry of Patients with Venous Thromboembolism
- ROC
- receiver operating characteristics
- RR
- relative risk
- SAVI
- Sheffield accelerated value of information
- SFOG
- Swedish Society of Obstetrics and Gynecology
- SMR
- standardised mortality ratio
- SPECT
- single photon emission computed tomography
- TIPPS
- thrombophilia in pregnancy prophylaxis study
- VAS
- visual analogue scale
- V/Q
- ventilation/perfusion
- VTE
- venous thromboembolism