Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/34/64. The contractual start date was in July 2016. The draft report began editorial review in April 2021 and was accepted for publication in February 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Little et al. This work was produced by Little et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaption in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – Journals Library, and the DOI of the publication must be cited.
2023
Chapter 1 Introduction
The problem of antimicrobial drug resistance
Acute respiratory tract infections (RTIs) are among the commonest conditions managed in primary care. The World Health Organization1 and the UK Department of Health and Social Care2 recognise that antibiotic resistance is an increasingly serious public health problem, with rising rates of resistance to a range of antibiotics and a clear relationship between primary care antibiotic prescribing (constituting 80% of prescribing) and antibiotic resistance at national3 and individual4–6 levels. The costs of resistance are also often not included in current estimates of cost-effectiveness, and these can have an important impact on estimates. 7 Although consultation rates and antibiotic prescription rates for upper RTIs or chest infections declined sharply from the late 1990s until the early 2000s (consultation rate of 160 out of 1000 for females and 120 out of 1000 for males8), antibiotic use rose again and then fell 15% between 2015 and 2019,9 and increased again during the COVID-19 pandemic. 10
Antibiotic prescribing for children presenting with respiratory tract infections in primary care
Children have higher consultation rates than adults for RTI, and, even when antibiotic prescribing was at its lowest, most children labelled as having upper RTIs or chest infection still were prescribed antibiotics. 11 Data from our observational study in lower-prescribing practices confirmed that at least 40% of children are prescribed antibiotics for chest infections,12 which translates to 2 million prescriptions for antibiotics for cough in this age group in the UK,11–13 or approximately £30M annually in direct consultation and dispensing costs, let alone the indirect costs incurred by ‘medicalising’ illness in the family and wider social networks. 14,15
Although trials among adults suggest only modest benefit, even among important clinical subgroups,16 little evidence from randomised placebo-controlled trials exists to support or dispute the common use of antibiotics in children with chest infections: only one trial in a Cochrane review of antibiotic prescribing included young children aged ≥ 3 years. 15–17 It may be that antibiotics in children also have limited benefit; however, the differences in immunity and anatomy between adults and children prohibit simply applying evidence derived from adults to the management of children. 18
Parent and clinician concerns
Parents want help to improve the course of illness and are concerned about significant adverse outcomes,19,20 and prescribing antibiotics could potentially reduce societal costs. Clinicians also face the difficulty of knowing whether or not a patient presenting is an ‘average’ patient, given the variation in pathophysiology and disease severity, and prescribing decisions are made by general practitioners (GPs) using traditional but non-evidence-based clinical signs such as sputum production, fever, chest signs and being unwell. 21–24
We report a trial, with qualitative, observational and economic studies, that aimed to estimate the effectiveness of amoxicillin overall and in key clinical subgroups of children presenting with uncomplicated (non-pneumonic) lower respiratory tract infection (LRTI) in primary care.
Chapter 2 Aims and objectives
Objective
The objective was to undertake a trial of antibiotics for children presenting with LRTI in primary care, with an observational study.
Aims
The aims were to:
-
estimate the effectiveness of amoxicillin overall and in key clinical subgroups of children presenting with uncomplicated (non-pneumonic) LRTI in primary care
-
estimate the cost-effectiveness of antibiotics overall in children presenting with uncomplicated (non-pneumonic) LRTI in primary care
-
explore the estimates of effectiveness according to key pathophysiological subgroups (the presence of bacterial pathogens)
-
explore which variables predict poor prognosis and develop a prediction model for poor prognosis
-
explore the views of parents and clinicians regarding management of children and of participation in the trial.
Chapter 3 Main trial
Main trial methods
Summary of trial design
This was a randomised, placebo-controlled, parallel-group trial of amoxicillin for children presenting in primary care with uncomplicated LRTI, powered to detect benefits important enough to be worth prescribing antibiotics for, both overall and among key clinical subgroups in whom antibiotic prescribing is very common. We also included qualitative (see Chapter 3), microbiological (see Chapter 4) and economic (see Chapter 4) studies. Patients who were not randomised as a result of patient or clinician beliefs or preference were invited to participate in an observational study in which the same measures and outcomes were collected (see Chapter 2).
Ethics
The trial protocol was approved by the South West – Central Bristol Research Ethics Committee (reference 15/SW/0300), and the protocol can be found on the project web page of the NIHR Journals Library website (URL: www.journalslibrary.nihr.ac.uk/programmes/hta/133464/#/).
Intervention
The intervention was 50 mg/kg/day oral amoxicillin in three divided doses for 7 days, or placebo.
Amoxicillin was chosen as it is the first-choice antibiotic for LRTI and, at current levels of intermediate resistance, should cover most susceptible organisms. 25,37 The rationale for the dose is in line with guidance from the British National Formulary for children, and this was supported by a Monte Carlo simulation to achieve a minimal inhibitory concentration of around 1.5 mg/l to cover Haemophilus influenzae as well as intermediate-resistant pneumococci for 90% of the intended population. 25 We estimated that, to achieve bacterial eradication, the blood concentration of amoxicillin needs to be above the minimal inhibitory concentration for at least 5 days. A 7-day course was chosen to allow for poor adherence26 and on pragmatic grounds to match current practice at the time the study commenced to achieve greater clinician and parent acceptability; similar consensus had been required for the previous trial in adults. 25
Inclusion criteria
Included were children aged between 6 months and 12 years presenting to primary care with an acute LRTI, defined in several previous cohorts and trials as an acute cough as the predominant symptom and judged by the GP to be infective in origin, lasting < 21 days, and with other symptoms or signs localising to the lower respiratory tract (e.g. shortness of breath, sputum, pain). 27–30 These inclusion criteria reflect the clinical criteria used in daily practice to diagnose acute bronchitis31 and were used in the Cochrane review;17 they are also the key drivers of prescribing. 2,11,22
Exclusion criteria
Exclusion criteria were cough as judged by the clinician to have a non-infectious aetiology (e.g. hay fever or asthma) or to be almost certain viral aetiology (croup, for which antibiotics are not commonly prescribed); immune compromise; and antibiotic use in previous 30 days. Children with suspected pneumonia based on clinical examination or who were very severely ill as judged by the GP were also excluded from the trial, but they were eligible to enter a parallel observational study.
Consent
The parent or guardian of the child provided written consent. Children who were able to understand the study read an age-appropriate patient information leaflet and signed an age-appropriate assent form.
Randomisation
Parents and children who consented to the study and agreed to randomisation received oral suspension of either amoxicillin or placebo, randomised in a 1 : 1 ratio. Investigational Medicinal Product (IMP) packs were indistinguishable in appearance and packaging, and each pack was labelled with a unique identification number to maintain allocation concealment. A computer-generated random number list was provided by an independent statistician and kept only by the IMP manufacturer. Random block sizes of two to four packs were used, with practice sites receiving whole blocks. Investigators randomised and dispensed by selecting the next sequentially numbered IMP pack.
Data collection
Measurements and follow-up
The recruiting clinician completed a case report form of comorbidities, clinical signs and severity of baseline symptoms reported by the patient (rating each symptom ‘no problem’, ‘mild problem’, ‘moderate problem’ or ‘severe problem’). 25 Patients and clinicians also each rated how unwell they judged the child to be (on a scale of 0 = well to 10 = very unwell). Comorbidity and the number of RTIs in the previous year were also documented, and pulse oximetry was performed.
We chose throat swabs for microbiological sampling owing to our experience of their having both high pick-up rates for and acceptability to children. 32 For parents and children willing to have a throat swab, a swab was taken and analysed in a central laboratory using multiplex polymerase chain reaction (PCR).
Symptom diary
Parents kept a diary of symptoms and daily activities (including, for parents, days away from work) using a validated daily diary for at least 1 week and after that for as long as symptoms persisted, up to 4 weeks after inclusion. The diary items recorded the severity of the following symptoms: cough, phlegm, shortness of breath, wheeze, blocked/runny nose, disturbed sleep, feeling general unwell, fever and interference with normal activities. Each symptom was scored from 0 to 6 (0 = no problem, 1 = very little problem, 2 = slight problem, 3 = moderately bad, 4 = bad, 5 = very bad and 6 = as bad as it could be). 25,33 All patients were asked to return the diary at 1 month with medication bottles. When the diary was not returned, the data for the primary outcome were collected using a brief postal questionnaire or telephone call as necessary; we have shown that telephone data are a reasonable proxy for diary data. 34
Outcomes
Primary outcome
The primary outcome was the mean duration of symptoms rated moderately bad or worse, as used in previous studies on acute LRTI25, recorded for up to 28 days in a validated daily diary until symptoms settled. This matches parental concerns about more severe symptoms. 28,29 The diary has previously been validated and was shown to be sensitive to change in both adults and children, and internally reliable (Cronbach’s alpha 0.75, i.e. in the optimal range). 15,33
Secondary outcomes
Severity of symptoms
We chose severity in the first 2–4 days after seeing the doctor, as this is typically when symptoms are the most severe15 and antibiotics might make a difference.
Total symptom duration
This was the duration of symptoms until very little or no problem.
Reconsultation with new or worsening symptoms
This was documented based on a structured notes review.
Progression of illness requiring hospital assessment
These outcomes were documented based on a structured notes review, which we have shown to be feasible and reliable,35,36 and demonstrated antibiotic effectiveness in the previous large trial in adults. 25
Side effects
Diarrhoea, rash or nausea are common side effects of treatment and were recorded in the daily diary if they occurred.
Quality of life
Quality of life was measured using the EQ-5D-Y (EuroQol-5 Dimensions Youth), collected weekly by self-report in the diary. Its results were translated into utility scores based on the UK tariff (EQ-5D-Y user guide 2015). QALYs were derived using area under the curve based on 5 points of utility scores (baseline, week 1–week 4).
Health-care resource use
Information on NHS resource use was collected by notes review at the end of the trial, including resource use for major adverse events (e.g. anaphylaxis, hospital attendance, hospital admissions) at baseline and at 28 days. This was used to assess NHS and social services use [primary care visits, community service, hospital inpatient and outpatient visits, and accident and emergency (A&E) attendances]. Data on purchases of remedies and time off work or care were recorded in the symptom diaries to calculate out-of-pocket spending and parents’/carers’ time off work to take care of children.
Cost-effectiveness
The outcomes of the economic analysis were expressed as the incremental cost per unit of the primary outcome (mean duration of symptoms rated moderately bad or worse) and per quality-adjusted life-year (QALY) gained.
Adherence
The number of doses of medication taken was documented in the daily diary.
Sample size calculation
The trial was designed to have sufficient power for the key clinical subgroups for whom GPs are most likely to prescribe. Balancing the problems of antibiotic resistance, a 3-day difference in symptom resolution [hazard ratio (HR) 1.7] for an illness lasting 14–21 days (i.e. reducing the duration by 15–20%) was judged to be clinically important enough to warrant treatment. We originally estimated that 938 children were required (for alpha = 0.01, 90% power, 80% follow-up) to detect a HR of 1.7 for the primary outcome among any one of five clinical subgroups (chest signs, fever, physician rating of unwell, sputum/rattly chest, shortness of breath), assuming that any subgroup constituted ≥ 30% of the sample. Following discussions with the Data Management Committee, the Trial Steering Committee and the funder (agreed in a variation to contract on 12 April 2019), we prioritised the subgroup with chest signs (alpha of 0.05; 0.01 for the other subgroups) based on evidence from systematic reviews that abnormal chest signs are the most important driver of antibiotic prescriptions;21 from six studies the odds ratio for prescribing antibiotics ranged from 3 to 20. For the primary imputed analysis for the chest subgroup, assuming that 40% of the trial cohort had chest signs (based on the study data at the time calculations were revised), we estimated we would need a total trial sample of 298 participants for 80% power and 398 participants for 90% power. For the imputed analysis in other subgroups, assuming that at least 50% had those characteristics, we estimated that we would need 377 participants for 80% power. Multiple imputation was chosen for the primary analysis, and complete cases for a sensitivity analysis, as multiple imputation is generally more efficient than complete-case analysis. 38
Statistical analysis
Cox regression was used for the primary outcome and for total symptom duration, adjusting for age, baseline symptom severity, prior duration of illness and comorbidity. The proportional hazards assumptions was assessed visually using the Kaplan–Meier curve. Linear regression was used for symptom severity, and logistic regression was used for reconsultation, progression of illness and side effects, adjusting for the same baseline covariates as in the primary analysis. To aid interpretation, risk ratios were also calculated for the binary outcomes using a log-binomial model. Analysis was by intention to treat, as randomised regardless of non-adherence or protocol deviations. Multiple imputation was used as the primary analysis, agreed with the funder and documented in the statistical analysis plan, which superseded the protocol (it should be noted that the protocol incorrectly stated that multiple imputation was a secondary analysis). Multiple imputation comprised all variables from the analysis model and any predictors of missingness, and used 100 imputations. A complete-case analysis was performed as a sensitivity analysis. Prespecified subgroup analyses were carried out on chest signs (alpha = 0.05), sputum/rattly chest, history of fever, physician rating of unwell, shortness of breath, oxygen saturation below 95% and STARWAVe clinical prediction rule for hospitalisation39 (all alpha = 0.01). Analyses were carried out in Stata® 16 (StataCorp LP, College Station, TX, USA) and IBM SPSS Statistics 26 (IBM Corporation, Armonk, NY, USA), with analysts blinded to the randomisation group.
Role of funder
The funder had no role in data collection, analysis, data interpretation, report writing or the decision to submit for publication.
Changes to methods and protocol from original funding application
-
The main change to the study was the modification to the sample size requirements (see Sample size calculation).
-
The remit of the qualitative work was expanded to include the view of parents and clinicians on the wider issues of the decision to attend and management.
-
We had planned to carry out chest radiography and blood tests for the majority of children but on a voluntary basis so that we did not impact recruitment. As it turned out, the refusal rate was such that these data became meaningless, and in the end the request to parents to carry out these tests was dropped.
-
We had initially thought that a health economic analysis would be valuable for each subgroup, but the subgroups were, in the end, too small for that to be sensible. The lack of difference by subgroup meant that such an analysis would not add value. We also suggested that a decision-analytic model could be developed to explore the cost-effective implication over the longer term and investigate the potential implication of the costs associated with antibiotic resistance, but we did not do this for several reasons, including the overall conclusion that antibiotics are not clinically worthwhile for children, the lack of data on the costs of antibiotic resistance and the incompleteness of the EQ-5D data.
-
One of the secondary outcomes was described in the protocol as ‘complications’, but the descriptions we had, based on the medical notes and SAE reports, were much more detailed, so we have provided a more accurate description of what happened and described this as ‘progression of illness requiring hospital assessment and/or admission’.
-
The protocol (version 10) incorrectly stated that the primary analysis was complete cases, and this was superseded by the statistical analysis plan (see above); the protocol and statistical analysis plan are available on the project web page on the NIHR Journals Library website (URL: www.journalslibrary.nihr.ac.uk/programmes/hta/133464/#/).
Main trial results
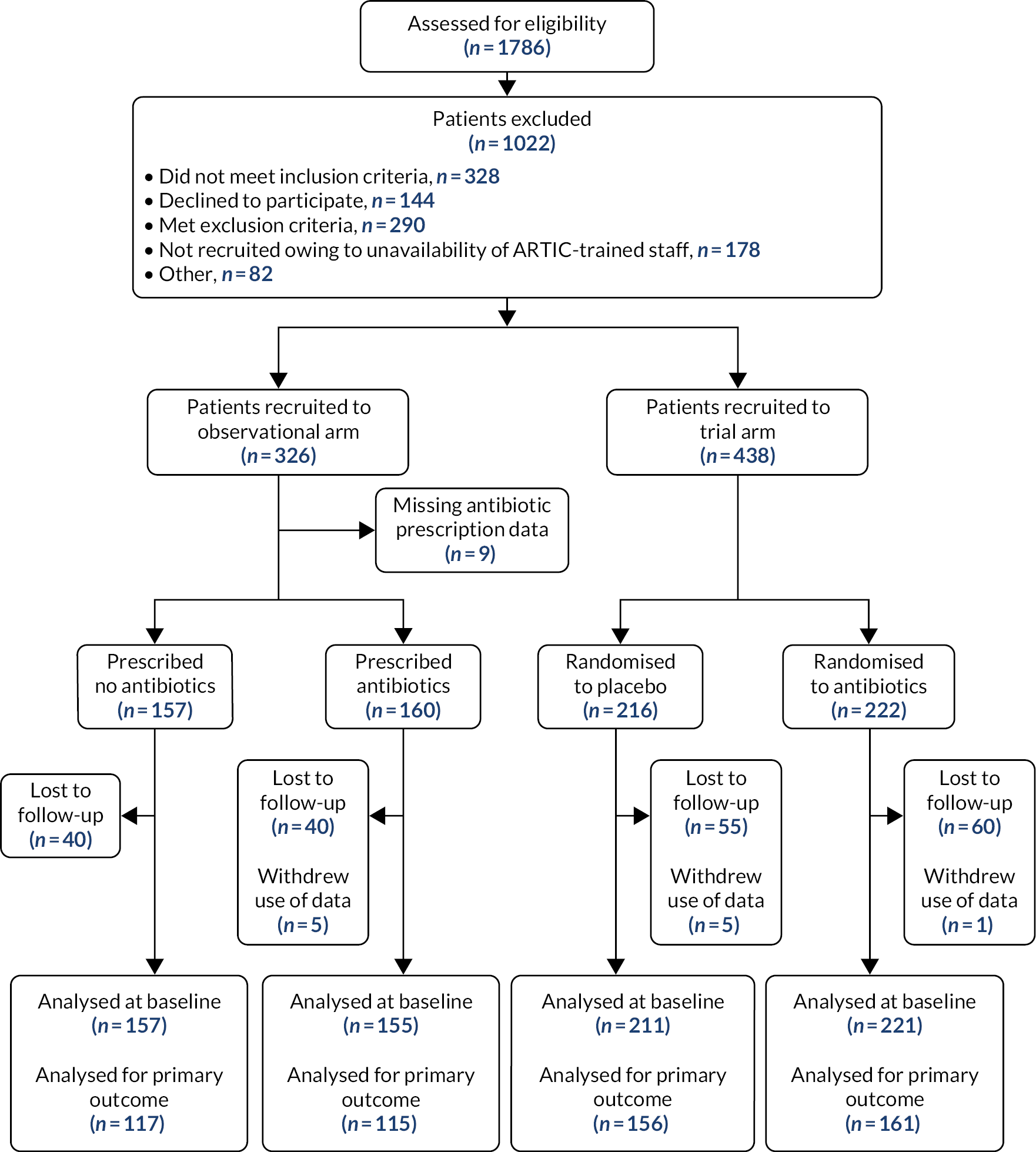
Participants were recruited from 56 general practices between 9 November 2016 and 17 March 2020; recruitment was stopped by the COVID-19 pandemic. A total of 438 patients, out of the 664 eligible children for whom informed consent could have been obtained, were randomised, and six withdrew the use of their data, meaning that these could not be used in the intention-to-treat analysis. A total of 432 participants were then analysed in the randomised trial: 221 were randomised to antibiotics and 211 were randomised to placebo (see Figure 1). A total of 233 participants (53.9%) were male; the median age of participants was 3.2 years [interquartile range (IQR) 1.6–5.7 years] and 55 (12.7%) had a comorbidity (see Table 1). Regarding the prespecified key clinical subgroups, 34.7% of participants had abnormal chest signs, 75.8% had sputum/rattly chest, 78.2% had a fever during the illness, 65.7% were categorised as unwell according to the physician rating (rating of ≥ 5 on a scale of 1–10) and 46.1% had shortness of breath (see Table 2); 6.6% had oxygen saturation below 95%. In accordance with the STARWAVe prediction rule,39 53.9% of participants were at very low risk of hospital admission, 43.8% were at normal risk and 2.3% were at high risk. The key baseline characteristics were similar between the treatment groups (see Tables 1 and 2).
FIGURE 1.
The Consolidated Standards of Reporting Trials (CONSORT) flow diagram. a, Not meeting inclusion: 61 too young; 13 too old; 181 GP judged not LRTI; 65 ≥ 21 days; 8 other. b, Exclusion: 25 asthma/allergy-related cough; 83 GP suspected viral infection; 26 croup; 79 prior antibiotics; 3 penicillin-allergic; 9 already enrolled, or sibling already enrolled; 39 admitted to hospital/too unwell; 26 other. c, Informed consent not possible.

| Characteristic | Number | Placebo (N = 211) | Antibiotics (N = 221) | Overall (N = 432) |
|---|---|---|---|---|
| Male, n (%) | 432 | 112 (53.1) | 121 (54.8) | 233 (53.9) |
| Age in years, median (IQR) | 432 | 3.1 (1.4–5.6) | 3.2 (1.7–5.8) | 3.2 (1.6–5.7) |
| Comorbidity, n (%) | 432 | 31 (14.7) | 24 (10.9) | 55 (12.7) |
| Asthma, n (%) | 431 | 27 (12.8) | 18 (8.1) | 45 (10.4) |
| Long-term illness, n (%) | 233 | 7 (6.3) | 13 (10.7) | 20 (8.6) |
| Hay fever/eczema, n (%) | 232 | 39 (35.1) | 44 (36.4) | 83 (35.8) |
| Family history of asthma, n (%) | 229 | 66 (58.9) | 81 (69.2) | 147 (64.2) |
| Breastfed at 3 months, n (%) | 230 | 49 (44.6) | 65 (54.2) | 114 (49.6) |
| Mother’s age, mean (SD) | 219 | 34.8 (6.4) | 34.9 (7.2) | 34.9 (6.8) |
| Number of times had cough in last 12 months, mean (SD) | 222 | 2.5 (2.3) | 2.8 (2.8) | 2.6 (2.6) |
| Prior influenza vaccine in last 12 months, n (%) | 401 | 55 (27.5) | 59 (29.4) | 114 (21.4) |
| Prior pneumococcal vaccine (booster) in last 12 months, n (%) | 401 | 61 (30.5) | 64 (31.8) | 125 (31.2) |
| Smoker in household, n (%) | 432 | |||
| Yes | 44 (20.9) | 50 (22.6) | 94 (21.8) | |
| No | 165 (78.2) | 166 (75.1) | 331 (76.6) | |
| Don’t know | 2 (1.0) | 5 (2.2) | 7 (1.6) | |
| Number of children in home, n (%) | 432 | |||
| 1 | 87 (41.2) | 86 (38.9) | 173 (40.1) | |
| 2 | 73 (34.6) | 95 (43.0) | 168 (38.9) | |
| 3 | 35 (16.6) | 25 (11.3) | 60 (13.9) | |
| 4 | 13 (6.2) | 7 (3.2) | 20 (4.6) | |
| ≥ 5 | 3 (1.4) | 8 (3.6) | 11 (2.5) | |
| Parent highest qualification, n (%) | 432 | |||
| Degree | 78 (37.0) | 81 (36.7) | 159 (36.8) | |
| Diploma | 27 (12.8) | 23 (10.4) | 50 (11.6) | |
| A level | 23 (10.9) | 16 (7.2) | 39 (9.0) | |
| GCSE/O level | 20 (9.5) | 27 (12.2) | 47 (10.9) | |
| None | 10 (4.7) | 7 (3.2) | 17 (3.9) | |
| Not given | 42 (19.9) | 53 (24.0) | 95 (22.0) | |
| Other | 11 (5.2) | 14 (6.3) | 25 (5.8) | |
| Ethnic group, n (%) | 432 | |||
| British/Irish/other white | 182 (86.3) | 189 (85.5) | 371 (85.9) | |
| Mixed | 8 (3.8) | 11 (5.0) | 19 (4.4) | |
| South Asian | 15 (7.1) | 14 (6.3) | 29 (6.7) | |
| Other | 4 (1.9) | 5 (2.3) | 9 (2.1) | |
| Prefer not to say | 1 (0.5) | 2 (0.9) | 3 (0.7) |
| Number | Placebo (N = 211) | Antibiotics (N = 221) | Overall (N = 432) | |
|---|---|---|---|---|
| Baseline severity,a mean (SD) | 432 | 1.6 (0.3) | 1.6 (0.3) | 1.6 (0.3) |
| Cough severity, mean (SD) | 432 | 1.9 (1.1) | 2.0 (1.1) | 1.9 (1.1) |
| Duration of illness in days, median (IQR) | 432 | 6 (3–10) | 5 (3–10) | 5 (3–10) |
| Abnormal chest signs,a n (%) | 432 | 72 (34.1) | 78 (35.3) | 150 (34.7) |
| Sputum/rattly chest, n (%) | 429 | 155 (73.8) | 170 (77.6) | 325 (75.8) |
| Fever during illness, n (%) | 428 | 161 (76.3) | 177 (80.1) | 338 (78.2) |
| Unwell (according to physician), n (%) | 432 | 141 (66.8) | 143 (64.7) | 284 (65.7) |
| Shortness of breath (yes/no), n (%) | 432 | 95 (45.0) | 104 (47.1) | 199 (46.1) |
| Oxygen saturation low, n (%) | 336 | 9 (5.4) | 13 (7.7) | 22 (6.6) |
| STARWAVe,a n (%) | 432 | |||
| Very low risk | 110 (52.1) | 123 (55.7) | 233 (53.9) | |
| Normal risk | 95 (45.0) | 94 (42.5) | 189 (43.8) | |
| High risk | 6 (2.8) | 4 (1.8) | 10 (2.3) | |
| Physician rating of unwell,a mean (SD) | 432 | 5.5 (1.7) | 5.5 (1.6) | 5.5 (1.6) |
| Parent rating of unwell,a mean (SD) | 432 | 3.8 (1.7) | 3.7 (1.7) | 3.7 (1.7) |
| Temperature (°C), mean (SD) | 428 | 37.3 (0.8) | 37.2 (0.8) | 37.3 (0.8) |
| Oxygen saturation, mean (SD) | 336 | 97.3 (1.6) | 97.3 (1.6) | 97.3 (1.6) |
| Heart rate (beats per minute), mean (SD) | 420 | 112.0 (20.3) | 111.8 (17.9) | 111.9 (19.1) |
| Respiratory rate (breaths per minute), mean (SD) | 411 | 24.8 (6.8) | 25.4 (7.1) | 25.1 (7.0) |
| Tachypnoea, n (%) | 411 | 25 (12.6) | 30 (14.1) | 55 (13.4) |
| Capillary refill > 3 seconds, n (%) | 422 | 3 (1.5) | 2 (0.9) | 5 (1.2) |
| Consciousness, n (%) | 431 | |||
| Normal | 203 (96.2) | 214 (97.3) | 417 (96.8) | |
| Irritable | 8 (3.8) | 5 (2.3) | 13 (3.0) | |
| Drowsy | 0 (0.0) | 1 (0.5) | 1 (0.2) | |
| Ill appearance, n (%) | 431 | 48 (22.9) | 47 (21.3) | 95 (22.0) |
| Number of days unwell before seeing general practitioner, median (IQR) | 227 | 5 (3–9) | 5 (3–7) | 5 (3–9) |
| Treated with OTC medication, n (%) | 232 | 105 (94.6) | 107 (88.4) | 212 (91.4) |
Among parents who reported which medication they thought their child had received, 47 out of 101 (46.5%) in the antibiotics group and 33 out of 84 (39.3%) in the placebo group thought that their child had received antibiotics.
Follow-up
Data were available on symptom duration for 317 participants (73.3%), on symptom severity for 298 participants (69.0%), on reconsultation with new or worsening symptoms for 401 participants (92.8%), on children who required hospital assessment for 415 participants (96.1%) and on side effects for 310 participants (71.8%) (see Table 3). Most of the symptom data came from the diaries, and the estimates of median duration of illness were a day longer for both diary (placebo, 6 days; antibiotic, 5 days) and telephone (placebo, 7 days; antibiotic, 6 days). The missingness of diary data (including adherence data) was associated particularly with parental qualification (those with higher qualifications were less likely to have missing data), and hence this variable was included in the imputation model.
| Placebo | Antibiotics | |||
|---|---|---|---|---|
| N | N | |||
| Duration of moderately bad or worse symptoms in days, median (IQR) | 156 | 6 (4–15) | 161 | 5 (4–11) |
| Symptom severity, mean (SD) | 149 | 2.1 (1.1) | 149 | 1.8 (1.0) |
| Duration of symptoms until very little problem in days, median (IQR) | 156 | 8 (5–20) | 161 | 7 (4–17) |
| Return with new or worsening symptoms, n (%) | 199 | 76 (38.2) | 202 | 60 (29.7) |
| Progression of illness,a n (%) | 204 | 4 (2.0) | 211 | 5 (2.4) |
| Side effects, n (%) | 153 | 52 (34.0) | 157 | 60 (38.2) |
| Diarrhoea | 88 | 26 (29.6) | 98 | 24 (24.5) |
| Nausea | 92 | 32 (34.8) | 102 | 35 (34.3) |
| Rash | 91 | 20 (22.0) | 99 | 25 (25.3) |
Primary outcome
The duration of moderately bad or worse symptoms was similar in both groups [antibiotic, median 5 (IQR 4–11) days; placebo, median 6 (IQR 4–15) days; HR 1.13, 95% confidence interval (CI) 0.90 to 1.42)] (see Table 4 and Figure 2). None of the prespecified clinical subgroups or the additional post hoc exploratory subgroups (low oxygen saturation; STARWAVe categories) modified the effect of treatment on the duration of moderately bad or worse symptoms (see Table 5).
| Placebo (n = 211) | Antibiotics (n = 221) | Unadjusted treatment estimate (95% CI) | Adjusteda treatment estimate (95% CI) | |
|---|---|---|---|---|
| Duration of moderately bad or worse symptoms in days, median (IQR) | 6 (4–15) | 5 (4–11) | HR 1.15 (0.93 to 1.44) | HR 1.13 (0.90 to 1.42) |
| Symptom severity, mean (SD) | 2.1 (1.1) | 1.8 (1.1) | Difference –0.25 (–0.49 to –0.01) | Difference –0.28 (–0.51 to –0.04) |
| Duration of symptoms until very little problem in days, median (IQR) | 8 (5–19) | 7 (4–17) | HR 1.12 (0.88 to 1.42) | HR 1.09 (0.86 to 1.38) |
| Return with new or worsening symptoms | 38% | 30% | OR 0.69 (0.45 to 1.04); RR 0.78 (0.59 to 1.02) | OR 0.71 (0.46 to 1.09); RR 0.80 (0.58 to 1.05) |
| Assessment or admission needed in hospitalb | 2% | 2% | OR 1.02 (0.25 to 3.54); RR 1.02 (0.30 to 3.43) | OR 1.24 (0.32 to 4.78); RR 1.23 (0.32 to 4.44) |
| Side effects | 33 | 39 | OR 1.28 (0.82 to 2.01); RR 1.17 (0.88 to 1.57) | OR 1.33 (0.81 to 2.17); RR 1.20 (0.87 to 1.55) |
| Subgroup | Placebo | Antibiotics | Interaction term (99% CI)a | Unadjusted HR (99% CI) | Adjustedb HR (99% CI)a | ||
|---|---|---|---|---|---|---|---|
| n | Median (IQR) | n | Median (IQR) | ||||
| Abnormal chest signs | |||||||
| Yes | 54 | 6 (4–16) | 52 | 6 (4–15) | 0.84 (0.52 to 1.36) | 1.04 (0.71 to 1.51) | 0.97 (0.65 to 1.43) |
| No | 102 | 7 (4–15) | 109 | 5 (3–11) | 1.23 (0.93 to 1.61) | 1.21 (0.91 to 1.60) | |
| Sputum | |||||||
| Yes | 115 | 7 (4–16) | 124 | 5 (4–14) | 1.11 (0.55 to 2.26) | 1.18 (0.84 to 1.65) | 1.16 (0.83 to 1.64) |
| No | 41 | 5 (4–14) | 36 | 5 (3–10) | 1.11 (0.62 to 1.99) | 0.99 (0.52 to 1.90) | |
| Fever | |||||||
| Yes | 115 | 6 (4–16) | 131 | 5 (3–10) | 1.45 (0.71 to 2.98) | 1.25 (0.90 to 1.72) | 1.23 (0.88 to 1.73) |
| No | 41 | 7 (4–13) | 30 | 7 (4–26) | 0.90 (0.47 to 1.72) | 0.78 (0.40 to 1.53) | |
| Physician rating of unwell | |||||||
| Yes | 104 | 6 (4–15.5) | 101 | 5 (3–10) | 1.32 (0.71 to 2.46) | 1.28 (0.89 to 1.86) | 1.25 (0.85 to 1.83) |
| No | 52 | 8 (4–14.5) | 60 | 6 (4–16) | 0.97 (0.59 to 1.59) | 0.96 (0.58 to 1.58) | |
| Shortness of breath | |||||||
| Yes | 77 | 6 (4–11) | 71 | 5 (3–14) | 0.96 (0.54 to 1.73) | 1.08 (0.70 to 1.66) | 1.13 (0.72 to 1.77) |
| No | 79 | 7 (4–18.5) | 90 | 5.5 (4–11) | 1.20 (0.81 to 1.79) | 1.17 (0.78 to 1.75) | |
| Oxygen saturation low | |||||||
| Yes | 7 | 11 (6–18) | 8 | 8 (4–20) | 0.95 (0.23 to 3.94) | 0.98 (0.25 to 3.80) | 1.20 (0.24 to 5.93) |
| No | 119 | 6 (4–15) | 116 | 5 (3.5–10) | 1.17 (0.83 to 1.63) | 1.11 (0.78 to 1.57) | |
| STARWAVe39 | |||||||
| Very low risk | 78 | 7 (4–17) | 93 | 5 (4–10) | 1.29 (0.86 to 1.91) | 1.27 (0.84 to 1.91) | |
| Normal risk | 72 | 6 (4–11.5) | 65 | 6 (3–14) | 0.77 (0.45 to 1.30) | 1.08 (0.69 to 1.67) | 1.06 (0.67 to 1.66) |
| High risk | 6c | – | 3c | – | – | – | – |
FIGURE 2.
Kaplan–Meier curve of duration of moderately bad or worse symptoms in days.

Secondary outcomes
There was a small but statistically significant difference between the groups in symptom severity on days 2–4 after seeing the doctor (antibiotics 1.8, placebo 2.1; mean difference–0.28, 95% CI –0.51 to –0.04) (see Table 4), equivalent to less than 1 in 3 children rating symptoms as slight rather than very little problem.
The duration of symptoms until very little problem was also similar between the groups (antibiotics median 7, IQR 4–17; placebo median 8, IQR 5–20), with no significant difference between the groups (HR 1.09, 95% CI 0.86 to 1.38) (see Table 4).
The number of participants reconsulting with new or worsening symptoms was 60 (29.7%) in the antibiotics group compared with 76 (38.2%) in the placebo group (risk ratio 0.80, 95% CI 0.58 to 1.05) (see Table 4). Few children needed hospital assessment (antibiotics, n = 5, 2.4%, placebo, n = 4, 2.0%; risk ratio 1.23, 95% CI 0.32 to 4.44) (see Table 4). The number of participants experiencing side effects was similar (antibiotics, n = 60, 38.2%, placebo, n = 52, 34.0%; risk ratio 1.20, 95% CI 0.87 to 1.55) (see Table 4).
Serious adverse events
The number of children requiring hospital assessment in both groups was small and similar (see Table 6), including when they were categorised using the STARWAVe prediction rule (see Table 7).
| Placebo | Antibiotics |
|---|---|
| Hospital admission –worsening of symptoms/‘viral-induced’ wheeze | Hospital admission – stridor, floppy episode and febrile convulsion, croup, shortness of breath |
| Hospital admission –shortness of breath. Bronchiolitis, increased work of breathing requiring high-flow therapy and nasogastric feeding | Persistent symptoms of fever, increasing breathlessness, oxygen saturation 91%, respiratory rate 32/minute, expiratory wheeze; admission to hospital (not overnight) |
| Exacerbation of cough and phlegm/cough getting worse, vomiting overnight, decreased feeding –not admitted overnight | Hospital admission –bronchiolitis |
| Hospital admission –no further information available | Gastrointestinal symptoms: hospital admission –not overnight |
| Temperature 38.5 °C, ‘rattle in throat/chest’, stomach pain |
| STARWAVe | Placebo | Antibiotics |
|---|---|---|
| Very low risk | 1/104 (1.0%) | 0/118 (0.0%) |
| Normal risk | 2/94 (2.1%) | 2/89 (2.2%) |
| High risk | 0/6 (0.0%) | 0/4 (0.0%) |
Sensitivity analyses
The main analyses (see Tables 4 and 5) were calculated based on 100 multiply imputed data sets. Complete-case analyses gave very similar results (see Tables 8 and 9).
| Outcome | Number analysed | Placebo | Antibiotics | Unadjusted treatment effect (95% CI) | Adjusteda treatment estimate (95% CI) |
|---|---|---|---|---|---|
| Duration of moderately bad or worse symptoms in days, median (IQR) | 317 | 6 (4–15) | 5 (4–11) | HR 1.21 (0.95 to 1.53) | HR 1.15 (0.91 to 1.46) |
| Symptom severity on days 2–4, mean (SD) | 298 | 2.1 (1.1) | 1.8 (1.0) | Difference –0.28 (–0.53 to –0.03) | Difference –0.29 (–0.53 to –0.04) |
| Duration of symptoms until very little problem in days, median (IQR) | 317 | 8 (5–20) | 7 (4–17) | HR 1.15 (0.90 to 1.47) | HR 1.10 (0.85 to 1.40) |
| Return with new or worsening symptoms, n (%) | 401 | 76 (38.2) | 60 (29.7) | OR 0.68 (0.45 to 1.04); RR 0.78 (0.59 to 1.03) | OR 0.71 (0.46 to 1.08); RR 0.78 (0.61 to 1.04) |
| Progression of illness,b n (%) | 415 | 4 (2.0) | 5 (2.4) | OR 0.97 (0.28 to 3.39); RR 0.97 (0.28 to 3.29) | OR 1.21 (0.31 to 4.67); RR 1.18 (0.33 to 4.26) |
| Side effects, n (%) | 310 | 52 (34.0) | 60 (38.2) | OR 1.20 (0.76 to 1.91); RR 1.12 (0.84 to 1.51) | OR 1.32 (0.81 to 2.15); RR 1.19 (0.64 to 1.56) |
| Subgroup | n | Placebo, median (IQR) | Antibiotics, median (IQR) | Interaction term (99% CI)a | Unadjusted HR (99% CI)a | Adjustedb HR (99% CI)a |
|---|---|---|---|---|---|---|
| Abnormal chest signs | ||||||
| Yes | 106 | 6 (4–16) | 6 (4–15) | 0.82 (0.49 to 1.36) | 1.05 (0.69 to 1.60) | 0.91 (0.59 to 1.41) |
| No | 211 | 7 (4–15) | 5 (3–11) | 1.29 (0.97 to 1.73) | 1.25 (0.93 to 1.68) | |
| Sputum | ||||||
| Yes | 239 | 7 (4–16) | 5 (4–14) | 1.20 (0.58 to 2.49) | 1.26 (0.88 to 1.80) | 1.22 (0.85 to 1.75) |
| No | 77 | 5 (4–14) | 5 (3–10) | 1.10 (0.59 to 2.06) | 0.95 (0.47 to 1.90) | |
| Fever | ||||||
| Yes | 246 | 6 (4–16) | 5 (3–10) | 1.63 (0.74 to 3.58) | 1.33 (0.94 to 1.89) | 1.28 (0.90 to 1.83) |
| No | 71 | 7 (4–13) | 7 (4–26) | 0.87 (0.43 to 1.75) | 0.65 (0.30 to 1.42) | |
| Physician rating of unwell | ||||||
| Yes | 205 | 6 (4–15.5) | 5 (3–10) | 1.43 (0.74 to 2.76) | 1.42 (0.96 to 2.09) | 1.33 (0.89 to 1.98) |
| No | 112 | 8 (4–14.5) | 6 (4–16) | 0.94 (0.55 to 1.59) | 0.92 (0.54 to 1.57) | |
| Shortness of breath | ||||||
| Yes | 148 | 6 (4–11) | 5 (3–14) | 1.07 (0.56 to 2.02) | 1.17 (0.74 to 1.84) | 1.24 (0.78 to 1.99) |
| No | 169 | 7 (4–18.5) | 5.5 (4–11) | 1.23 (0.80 to 1.89) | 1.16 (0.75 to 1.81) | |
| Oxygen saturation low | ||||||
| Yes | 15 | 11 (6–18) | 8 (4–20) | 0.80 (0.18 to 3.55) | 0.83 (0.19 to 3.56) | 1.45 (0.25 to 8.46) |
| No | 235 | 6 (4–15) | 5 (3.5–10) | 1.22 (0.85 to 1.74) | 1.12 (0.78 to 1.61) | |
| STARWAVe | ||||||
| Very low risk | 171 | 7 (4–17) | 5 (4–10) | 1.35 (0.88 to 2.07) | 1.24 (0.89 to 1.99) | |
| Normal risk | 137 | 6 (4–11.5) | 6 (3–14) | 0.88 (0.46 to 1.66) | 1.13 (0.70 to 1.82) | 1.09 (0.67 to 1.78) |
| High risk | 9 | –c | – | – | – | – |
Exploratory post hoc analyses for clinical subgroups
The treatment effects for all outcomes were similar for most subgroups (none of the interaction terms was significant), but the impact of antibiotics was slightly greater (albeit not significantly) among those with fever or those being unwell (see Tables 10 and 11). Reconsultations with antibiotics were slightly greater among the less unwell children (see Table 12).
| Subgroup | n | Placebo, median (IQR) | Antibiotics, median (IQR) | Interaction term (99% CI)a | Unadjusted HR (99% CI)a | Adjustedb HR (99% CI)a |
|---|---|---|---|---|---|---|
| Abnormal chest signs | ||||||
| Yes | 106 | 6 (4–16) | 6 (4–15) | 0.83 (0.50 to 1.40) | 1.05 (0.69 to 1.60) | 0.92 (0.59 to 1.42) |
| No | 211 | 7 (4–15) | 5 (3–11) | 1.29 (0.97 to 1.73) | 1.27 (0.94 to 1.71) | |
| Sputum | ||||||
| Yes | 239 | 7 (4–16) | 5 (4–14) | 1.24 (0.60 to 2.58) | 1.26 (0.88 to 1.80) | 1.23 (0.86 to 1.78) |
| No | 77 | 5 (4–14) | 5 (3–10) | 1.10 (0.59 to 2.06) | 0.93 (0.45 to 1.89) | |
| Fever | ||||||
| Yes | 246 | 6 (4–16) | 5 (3–10) | 1.62 (0.74 to 3.57) | 1.33 (0.94 to 1.89) | 1.30 (0.91 to 1.87) |
| No | 71 | 7 (4–13) | 7 (4–26) | 0.87 (0.43 to 1.75) | 0.66 (0.30 to 1.44) | |
| Physician rating of unwell | ||||||
| Yes | 205 | 6 (4–15.5) | 5 (3–10) | 1.49 (0.76 to 2.89) | 1.42 (0.96 to 2.09) | 1.35 (0.91 to 2.02) |
| No | 112 | 8 (4–14.5) | 6 (4–16) | 0.94 (0.55 to 1.59) | 0.87 (0.50 to 1.50) | |
| Shortness of breath | ||||||
| Yes | 148 | 6 (4–11) | 5 (3–14) | 1.07 (0.56 to 2.03) | 1.17 (0.74 to 1.84) | 1.27 (0.79 to 2.04) |
| No | 169 | 7 (4–18.5) | 5.5 (4–11) | 1.23 (0.80 to 1.89) | 1.20 (0.76 to 1.88) | |
| Oxygen saturation low | ||||||
| Yes | 15 | 11 (6–18) | 8 (4–20) | 0.80 (0.18 to 3.53) | 0.83 (0.19 to 3.56) | 1.20 (0.24 to 5.93) |
| No | 235 | 6 (4–15) | 5 (3.5–10) | 1.22 (0.85 to 1.74) | 1.15 (0.79 to 1.66) | |
| STARWAVe | ||||||
| Very low risk | 171 | 7 (4–17) | 5 (4–10) | 1.35 (0.88 to 2.07) | 1.32 (0.86 to 2.04) | |
| Normal risk | 137 | 6 (4–11.5) | 6 (3–14) | 0.88 (0.65 to 1.19) | 1.13 (0.70 to 1.82) | 1.12 (0.67 to 1.88) |
| High risk | 9 | –c | – | – | – | – |
| Subgroup | Placebo, mean (SD) | Antibiotics, mean (SD) | Interaction term (99% CI) | Unadjusted mean difference (99% CI) | Adjusteda mean difference (99% CI) |
|---|---|---|---|---|---|
| Abnormal chest signsb –yes | 2.2 (1.2) | 2.0 (0.9) | –0.01 (–0.70 to 0.69) | –0.17 (–0.73 to 0.39) | –0.21 (–0.80 to 0.38) |
| Abnormal chest signsb –no | 2.0 (1.1) | 1.7 (1.1) | –0.32 (–0.73 to 0.09) | –0.27 (–0.67 to 0.13) | |
| Sputum – yes | 2.1 (1.1) | 1.8 (1.0) | –0.04 (–0.79 to 0.73) | –0.31 (–0.67 to 0.05) | –0.30 (–0.65 to 0.05) |
| Sputum – no | 2.0 (1.2) | 1.8 (1.3) | –0.23 (–1.00 to 0.56) | –0.29 (–1.16 to 0.59) | |
| Fever – yes | 2.2 (1.1) | 1.8 (1.0) | –0.31 (–1.10 to 0.48) | –0.39 (–0.74 to –0.03) | –0.36 (–0.71 to –0.01) |
| Fever – no | 1.6 (1.2) | 1.6 (1.2) | –0.02 (–0.82 to 0.78) | –0.07 (–0.92 to 0.79) | |
| Unwell – yes | 2.2 (1.1) | 1.8 (1.1) | –0.22 (–0.90 to 0.46) | –0.35 (–0.76 to 0.06) | –0.35 (–0.76 to 0.07) |
| Unwell – no | 1.8 (1.2) | 1.7 (0.9) | –0.13 (–0.67 to 0.40) | –0.12 (–0.67 to 0.42) | |
| Shortness of breath – yes | 2.2 (1.1) | 2.0 (1.1) | 0.10 (–0.56 to 0.76) | –0.23 (–0.72 to 0.26) | –0.14 (–0.64 to 0.34) |
| Shortness of breath – no | 1.9 (1.2) | 1.6 (1.0) | –0.29 (–0.74 to 0.15) | –0.31 (–0.76 to 0.14) | |
| STARWAVe | |||||
| Very low risk | 2.0 (1.2) | 1.7 (1.1) | –0.25 (–0.72 to 0.22) | –0.25 (–0.71 to 0.21) | |
| Normal risk | 2.2 (1.1) | 1.8 (1.0) | –0.11 (–0.78 to 0.55) | –0.32 (–0.79 to 0.16) | –0.37 (–0.85 to 0.11) |
| High risk | 2.2 (1.2) | 2.4 (1.4) | 0.65 (–1.38 to 2.68) | 0.24 (–2.80 to 3.28) | 1.73 (–4.71 to 8.16) |
| Subgroup | Placebo, n (%) | Antibiotics, n (%) | Interaction term (99% CI) | Adjusteda odds ratio (99% CI) | Adjusteda risk ratio (99% CI) | NNT (99% CI) |
|---|---|---|---|---|---|---|
| Abnormal chest signsb – yes | 31 (44.9) | 26 (38.2) | 1.37 (0.42 to 4.48) | 0.81 (0.31 to 2.10) | 0.89 (0.45 to 1.40) | –15 (7 to –4) |
| Abnormal chest signsb – no | 45 (34.6) | 34 (25.4) | 0.62 (0.30 to 1.28) | 0.72 (0.40 to 1.17) | –9 (16 to –4) | |
| Sputum – yes | 57 (39.3) | 50 (32.7) | 1.60 (0.56 to 4.62) | 0.77 (0.40 to 1.46) | 0.85 (0.52 to 1.24) | –15 (13 to –5) |
| Sputum – no | 18 (34.0) | 9 (18.8) | 0.45 (0.13 to 1.58) | 0.55 (0.18 to 1.32) | –6 (17 to –3) | |
| Fever – yes | 52 (34.2) | 48 (29.8) | 1.97 (0.70 to 5.53) | 0.82 (0.43 to 1.56) | 0.87 (0.53 to 1.30) | –23 (11 to –6) |
| Fever – no | 24 (51.2) | 12 (29.3) | 0.41 (0.12 to 1.41) | 0.59 (0.22 to 1.17) | –5 (20 to –2) | |
| Unwell – yes | 52 (38.8) | 40 (30.8) | 1.25 (0.38 to 4.12) | 0.75 (0.37 to 1.51) | 0.83 (0.49 to 1.26) | –12 (14 to –4) |
| Unwell – no | 24 (36.9) | 20 (27.8) | 0.64 (0.24 to 1.71) | 0.74 (0.33 to 1.35) | –11 (9 to –3) | |
| Shortness of breath – yes | 33 (36.7) | 37 (38.9) | 2.81 (0.89 to 8.92) | 1.15 (0.51 to 2.61) | 1.09 (0.62 to 1.64) | 44 (5 to –6) |
| Shortness of breath – no | 43 (39.4) | 23 (21.5) | 0.42 (0.18 to 0.95) | 0.54 (0.26 to 0.97) | –6 (–56 to –3) | |
| STARWAVe | ||||||
| Very low risk | 28 (27.2) | 30 (26.6) | 1.04 (0.47 to 2.36) | 1.03 (0.55 to 1.72) | –157 (7 to –6) | |
| Normal risk | 46 (51.1) | 28 (32.9) | 0.44 (0.14 to 1.40) | 0.43 (0.19 to 1.01) | 0.61 (0.32 to 1.00) | –6 (99 to –3) |
| High risk | 2 (33.3) | 2 (50.0) | c | c | c | c |
Adherence
A limitation of the study is that only 232 (53.7%) participants provided data on adherence to medication. Among those who reported adherence, over the first 5 days it was most common to report 14 or 15 doses being taken (out of a maximum of 15; Figure 3), and the majority (95%) started taking their medication on day 1 (see Figure 3). Adherence was maintained in the antibiotics group over days 1–5 but decreased gradually to 84% by day 5 in the placebo group. A total of 98 out of 119 (82.4%) participants in the antibiotics group and 87 out of 113 (77.0%) participants in the placebo group took at least 11 doses of medication over days 1–5. A per-protocol analysis suggested no statistically significant difference in the duration of moderately bad or worse symptoms (HR 1.06, 95% CI 0.77 to 1.46). Reported adherence was higher for longer prior duration of illness (odds ratio 1.08, 95% CI 1.01 to 1.16), adjusting for group and other prespecified covariates. Among children whose parents thought they were receiving antibiotics, 68 (85%) adhered to their medication, and among children whose parents thought they were receiving placebo, 82 (82%) adhered to their medication. There was little evidence for clustering by GP practice (intracluster correlation 0.01, 95% CI 0.00 to 0.99).
FIGURE 3.
Number of doses of medication taken in total over days 1–5 by treatment group: (a) placebo; and (b) antibiotics.

Adherence sensitivity analyses
To provide a lower bound to the adherence, we assumed that all of those who completed the diary but did not fill in medication dosage did not adhere to their medication. Under this assumption, 98 out of 161 participants (60.9%) in the antibiotics group and 87 out of 156 participants (55.8%) in the placebo group adhered to their medication. To provide an upper bound to the adherence, we assumed that all of those who completed the diary but did not fill in medication dosage did adhere to their medication. Under this assumption, 140 out of 161 participants (87.0%) in the antibiotics group and 130 out of 156 participants (83.3%) in the placebo group adhered to their medication. If adherence is low, then the intention-to-treat effect of antibiotics on duration will be diluted. Therefore, as a sensitivity analysis, assuming all those who completed the diary but did not fill in medication dosage did not adhere in the antibiotics group, a complier-average causal effect analysis gave an unadjusted HR for the duration of moderately bad or worse symptoms of 1.31 (95% CI 0.90 to 1.89; cf. unadjusted HR for primary analysis 1.21, 95% CI 0.95 to 1.53). This provides an upper bound to the treatment effect of antibiotics among those who would have complied.
Discussion
This trial documents that for children presenting to primary care with uncomplicated acute LRTI where pneumonia was not suspected there is little difference in symptom burden both overall and for the key subgroups in whom antibiotics are commonly prescribed, and no evidence of additional complications when antibiotics are not prescribed.
Strengths and limitations
The study is one of the very few to report on the effectiveness of prescribing antibiotics among younger children presenting with chest infections in primary care, and the parents of two-thirds of eligible children agreed to be randomised in the trial. The study was designed to detect a 3-day improvement in the duration of more severe symptoms in key clinical subgroups. Our patient and public involvement and engagement (PPIE) group regarded the duration of illness as the most important outcome, and they judged that, given the problem of antibiotic resistance, a duration of 3 days was important enough to warrant prescribing an antibiotic. 2,3 Our prior data suggested an illness duration of 21 days, and so 3 days would represent a 15% improvement,15 but from the current study 3 days represents a 25% improvement for an illness lasting 12 days. Although the IQR was slightly wider in the placebo group, the Kaplan–Meier curves did not separate significantly. We used the most patient-relevant outcome (parent-reported symptoms) and documented illness progression. The study confirmed that illness progression is uncommon, but the study was not specifically powered to assess the progression of illness or reconsultations. In the final sample, imputed and complete cases analyses were adequately powered overall and for the subgroups, but slightly underpowered for the complete-case analysis in the chest signs subgroup, owing to, in part, slightly fewer children than expected having chest signs and the COVID-19 pandemic prematurely ending recruitment. However, the estimates for the primary outcome for complete and imputed cases in the chest signs subgroup were very similar (6 days in antibiotics and placebo groups in both analyses) and the HRs were near unity (0.91 and 0.97, respectively), and the upper 95% CIs of the HRs (1.41 and 1.43) suggest that the ‘true’ benefit for children with chest signs is unlikely to be more than 2 days, that is, not likely to be very important clinically. Although the study was placebo controlled, it was at the pragmatic end of the spectrum in that there was no close monitoring of parents and children; parents behaved as they might in practice as to whether or not they gave their child medication, and, although we had limited data on medication adherence, a per-protocol analysis provided similar estimates to those for the total trial population. The antibiotic (amoxicillin) was chosen as it is the first-choice antibiotic in UK national guidance for use in LRTIs among children. 37 Compared with representative observational cohorts, our trial population had a more severe clinical presentation,39 so we may have overestimated the impact of antibiotics.
Interpretation and comparison with previous literature
Only one trial in the Cochrane review of antibiotics for acute bronchitis included young children aged ≥ 3 years presenting with uncomplicated acute chest infections. 15,17 In that trial there were only 100 children aged ≤ 12 years, and the estimate of immediate antibiotics compared with no offer of antibiotics on symptom duration (HR 1.00) and symptom severity (a mean reduction of –0.3 points on a scale of 0–6 points) was similar to the non-significant result of the whole trial cohort. 15 These results are consistent with the results of the current study. As in the data from adults,15 the duration of illness in the current study is dominated by the duration of cough. A Cochrane review40 found inconclusive evidence of the effect of antibiotics in preventing RTIs, but a more recent trial of azithromycin used in early infections was effective in preventing severe illness among preschool children with recurrent infection41 (from 8% to 5%), although concern remains about the longer-term effects on antibiotic resistance from the use of long-acting macrolides. 6 A placebo-controlled trial42 of pneumonia in young children in a developing country found low failure rates in both the placebo (4.9%) and antibiotics (2.6%) groups.
Our results suggest that antibiotics do not provide a clinically important benefit, on average, for symptom reduction or symptom severity. The question of there potentially being children who receive a meaningful benefit that is imperceptible among the large numbers of children who receive no benefit remains. We explored this hypothesis by conducting subgroup analyses in five prespecified subgroups. Our subgroup analyses results suggest that none of the groups we specified is likely to achieve substantial benefits in terms of symptomatic improvement from antibiotics, although we did not have the power to exclude more moderate benefits. On the other hand, the average benefit from antibiotics in the general population may be even lower than our findings suggest. We had significantly fewer children with a very low risk STARWAVe score in this study than did the STARWAVe observational study, which recruited a representative sample of children with RTIs from the population (children with a very low risk score 67%, current trial 54%; see Table 1). 39 This suggests that the present trial successfully recruited more unwell children, in whom antibiotics might be expected to be more effective, and that the average impact of antibiotics in a more generalisable low-risk population is likely to be even lower than reported here. There was no evidence of selective benefit among children in whom pathogenic bacteria were isolated, which could be due to high carriage rates among children rather than true infection. The estimates of resource use suggest that not only are consultations, referral and hospitalisation costs significant,43 but societal costs are high. Antibiotic prescribing was not associated with health or societal resource savings and, if anything, resulted in slightly higher costs. If the costs of antibiotic resistance were included, then the adverse impact on health and societal resource use would be even greater. 7
Conclusion
Antibiotics make little difference to the symptom burden for uncomplicated LRTI in children, both overall and for the key clinical subgroups in whom antibiotic prescribing is common. Unless pneumonia is suspected, although it is important to provide ‘safety-netting’ advice to cover any deterioration in illness, clinicians should not prescribe antibiotics for most children presenting with chest infections.
Chapter 4 Observational study
Background
Children presenting with RTI are frequent attenders; almost all are managed in primary care and most still receive antibiotics. 11,44–46 However, both clinicians and patients worry about more severe or prolonged illness and complications, and clinicians fear medicolegal consequences. 47–49 Evidence to reassure clinicians is, however, limited: the Cochrane review did not document the impact of antibiotics on complications,17 and in the GRACE cohorts25,50 hospital admission or death occurred in < 1% of patients who consulted.
The Cochrane review of antibiotics for bronchitis comprised 17 trials, but only two of these included children: one with children aged ≥ 3 years,15 and a small trial (n = 140) that included one study with children aged ≥ 8 years. 51 Trial data can be limited by external validity and substantially greater drug compliance than in observational settings. 26 Thus, observational evidence can provide important data to complement trials. Conversely, observational data have the disadvantage of confounding by indication, so the impact of antibiotics should be assessed using techniques to control for the propensity to prescribe. 52,54
We report the characteristics of children not recruited to the trial, the estimates of effectiveness of antibiotics when controlling for propensity to prescribe in the observational data alone and also when combined with the trial data, and a prognostic model to predict significant illness deterioration or illness sufficient to require assessment in hospital settings.
Methods
When clinicians or patients were not willing or able to randomise children into the trial, they were invited to consider joining an observational study that used the same data collection methods used in the trial. The sites that participated in the trial and the observational data sets were not comparable: the observational data sets included more children recruited after assessment in hospital A&E or paediatric assessment, which could only recruit as part of the observational study [most commonly owing to the issues of a CTIMP (Controlled Trial of Investigational Medical Product) and managing study IMP].
Sample size: developing a new prognostic model
The standard rules used by statisticians suggest that the number of variables that can be assessed robustly in a prognostic model is 1 variable per 10 cases, so the three-variable model should be adequately powered. 53 However, the traditional rule of thumb has been questioned, and making the recommended assumptions53 (a margin of error of ≤ 0.05, mean absolute prediction error of ≤ 0.05, shrinkage factor of < 10% and expected optimism factor of 0.05) with an expected outcome event rate of 5% suggests that 566 participants were required.
Statistical analysis
We planned to control for confounding by indication by using inverse probability of treatment weighting using propensity scores in each of the regression models. However, the inverse probability of treatment weighting approach did not achieve good balance on the key covariates, whereas stratification by propensity score improved balance and, therefore, was used in analysing both the observational data and the combined data set that included both observational and trial data. Given the numbers of missing data, we imputed missing data using a chained equations model with 100 imputations.
We used a backwards-fitting approach to model the predictors of progression of illness. For the first, most inclusive, model, we retained in the multivariable model those variables with a p-value of < 0.20. Given the danger of overfitting, we also assessed another model for the variables that were significant at a p-value of < 0.05 in multivariate analysis in the first model, and finally for the three most significant variables. We explored whether or not progression of illness could be predicted from the following baseline characteristics: age, baseline severity, heart rate, respiratory rate, temperature, duration of illness prior to consultation, gender, comorbid conditions, history of asthma, chest signs, feeling generally unwell, oxygen saturation, sputum/rattly chest, vomiting, dry cough, chills, diarrhoea, disturbed sleep and passing urine less often. We present the discrimination of the model in the area under the receiver operator curve (AUROC), bootstrapping the estimates to limit overly optimistic estimates due to overfitting, and as a more efficient approach to internal validation rather than using split samples. 55 Model calibration was assessed with a Hosmer–Lemeshow test. We also tested the STARWAVE score using the three STARWAVe classifications of low, normal or high risk. 39
Results
A total of 326 patients were recruited to the observational study (see Figure 4), 312 with data documenting whether or not antibiotics were prescribed: 157 received no antibiotic and 160 received an antibiotic (141 immediate, 14 delayed; 5 patients withdrew consent).
FIGURE 4.
Flow diagram for observational study and trial.
As the numbers of patients with a delayed prescription were so small, these have been combined with the numbers with an immediate prescription for the purposes of analysis. Combined with the trial data, there were 744 participants, of whom 368 received no antibiotic and 376 received an antibiotic. In the observational cohort, 52 out of 312 (16.7%) were recruited via A&E/paediatric assessment compared with 260 out of 312 recruited via GP practices. In the trial, 5 out of 432 participants (1.1%) were recruited via A&E/paediatric assessment compared with 427 out of 432 recruited via practices.
Proportions followed up for key outcome measures
In the observational study, the duration of illness and illness severity in days 2–4 following the consultation were recorded for 232 out of 312 (74.4%) participants. Reconsultation was available for 271 (86.9%), progression of illness for 290 (92.9%) and side effects for 228 (73.1%) participants.
In the combined data set, 549 out of 744 participants (73.8%) reported the duration and severity of illness. Reconsultation was recorded for 672 (90.3%), progression of illness for 705 (94.8%) and side effects for 538 (72.3%) participants.
Clinical characteristics
As expected, the number of children with more severe clinical features (see Table 13) was much larger in the antibiotics group than in the no-antibiotics group. Children in the antibiotics group had more severe baseline symptoms (1.8 score and 1.5 score, respectively), more abnormal chest signs (81% vs. 24%), sputum production (87% vs. 70%), history of fever (91% vs. 64%), cases of feeling unwell (81% vs. 51%), shortness of breath (70% vs. 36%) and low oxygen saturation (21% vs. 7%) than children in the no-antibiotics group.
| Characteristic | Observational study | Trial only | Combined | |||
|---|---|---|---|---|---|---|
| No antibiotics (N = 157) | Antibiotics (N = 155) | Placebo (N = 211) | Antibiotics (N = 221) | No antibioticsa (N = 368) | Antibiotics (N = 376) | |
| Male, n (%) | 82 (52.2) | 86 (55.5) | 112 (53.1) | 121 (54.8) | 194 (52.7) | 207 (55.1) |
| Age in years, median (IQR) | 3.0 (1.4–4.9) | 3.1 (1.8–5.2) | 3.1 (1.4–5.6) | 3.2 (1.7–5.8) | 3.1 (1.4–5.4) | 3.2 (1.7–5.5) |
| Comorbidity, n (%)b | 17 (10.8) | 18 (11.6) | 31 (14.7) | 24 (10.9) | 48 (13.0) | 42 (11.2) |
| Asthma, n (%) | 9 (5.7) | 10 (6.5) | 19 (9.0) | 13 (5.9) | 28 (7.6) | 23 (6.1) |
| Long-term illness that limits activity, n (%)b | 12 (11.8) | 7 (7.5) | 7 (6.3) | 13 (10.7) | 19 (8.9) | 20 (9.3) |
| Baseline severity, mean (SD)c | 1.5 (0.3) | 1.8 (0.4) | 1.6 (0.3) | 1.6 (0.3) | 1.6 (0.3) | 1.7 (0.3) |
| Duration of illness in days, median (IQR) | 5 (3–7) | 4 (2–7) | 6 (3–10) | 5 (3–10) | 6 (3–10) | 5 (3–8) |
| Abnormal chest signs, n (%)c | 38 (24.2) | 126 (81.3) | 72 (34.1) | 78 (35.3) | 110 (29.9) | 204 (54.3) |
| Sputum/rattly chest, n (%) | 108 (69.7) | 135 (87.1) | 155 (73.8) | 170 (77.6) | 263 (71.5) | 305 (81.6) |
| Fever during illness, n (%) | 100 (63.7) | 141 (91.0) | 161 (76.3) | 177 (80.1) | 261 (70.9) | 318 (84.6) |
| Unwell, n (%) | 79 (51.0) | 125 (80.7) | 141 (66.8) | 143 (64.7) | 220 (60.1) | 268 (71.3) |
| Shortness of breath, n (%) | 57 (36.3) | 109 (70.3) | 95 (45.0) | 104 (47.1) | 152 (41.3) | 213 (56.7) |
| Oxygen saturation low, n (%) | 7 (6.6) | 28 (21.2) | 9 (5.4) | 13 (7.7) | 16 (5.9) | 41 (13.6) |
| STARWAVe, n (%)c | ||||||
| Very low risk | 94 (59.9) | 60 (38.7) | 110 (52.1) | 123 (55.7) | 204 (55.4) | 183 (48.7) |
| Normal risk | 60 (38.2) | 77 (49.7) | 95 (45.0) | 94 (42.5) | 155 (42.1) | 171 (45.5) |
| High risk | 3 (1.9) | 18 (11.6) | 6 (2.8) | 4 (1.8) | 9 (2.5) | 22 (5.9) |
| Physician rating of unwell, mean (SD)c | 4.9 (1.9) | 6.3 (1.6) | 5.5 (1.7) | 5.5 (1.6) | 5.3 (1.8) | 5.9 (1.7) |
| Parent rating of unwell, mean (SD)c | 3.3 (1.6) | 5.3 (1.7) | 3.8 (1.7) | 3.7 (1.7) | 3.6 (1.7) | 4.3 (1.8) |
| Temperature, mean (SD) | 37.1 (0.7) | 37.5 (0.9) | 37.3 (0.8) | 37.2 (0.8) | 37.2 (0.8) | 37.3 (0.8) |
| Oxygen saturation, mean (SD) | 97.6 (1.5) | 96.1 (2.3) | 97.3 (1.6) | 97.3 (1.6) | 97.4 (1.6) | 96.8 (2.0) |
| Heart rate (beats per minute), mean (SD) | 110.8 (19.0) | 124.5 (21.3) | 112.0 (20.3) | 111.8 (17.9) | 111.6 (19.8) | 117.1 (20.3) |
| Respiratory rate (breaths per minute), mean (SD) | 24.0 (7.4) | 30.7 (10.3) | 24.8 (6.8) | 25.4 (7.1) | 24.4 (7.0) | 27.6 (8.9) |
| Capillary refill > 3 seconds, n (%) | 1 (0.7) | 3 (2.0) | 3 (1.5) | 2 (0.9) | 4 (1.1) | 5 (1.4) |
| Consciousness, n (%) | ||||||
| Normal | 154 (98.7) | 138 (90.2) | 203 (96.2) | 214 (97.3) | 357 (97.3) | 352 (94.4) |
| Irritable | 1 (0.6) | 11 (7.2) | 8 (3.8) | 5 (2.3) | 9 (2.5) | 16 (4.3) |
| Drowsy | 1 (0.6) | 4 (2.6) | 0 (0.0) | 1 (0.5) | 1 (0.3) | 5 (1.3) |
| Ill appearance, n (%) | 17 (10.8) | 71 (45.8) | 48 (22.9) | 47 (21.3) | 65 (17.7) | 118 (31.4) |
Propensity scores
The differences between the antibiotics group and no-antibiotics group are shown in Figure 5 before and after adjustment using propensity scores; this demonstrates that, although there was a major impact of adjustment, there is nevertheless likely to be some residual confounding.
FIGURE 5.
Standardised differences in baseline characteristics between antibiotics and no-antibiotics groups in the observational data set and after adjusting for confounding by indication using the propensity score.

Primary and secondary outcomes
For the combined trial and observational data set, the estimates for primary and secondary outcomes were similar to the trial outcomes (see Table 14). The effect of antibiotic treatment on severity of symptoms was not significant. The apparent non significant increase in the progression of illness in the antibiotics group is very likely to be due to inadequate control of confounding by indication. The only outcome for which there was a statistically significant comparison between the antibiotics and no-antibiotics groups was side effects, which were higher in the antibiotics group.
| Observational study only | Combined trial and observational data | |||||
|---|---|---|---|---|---|---|
| No antibiotics | Antibiotics | Unadjusted/adjusted treatment estimate | No antibiotics | Antibiotics | Unadjusted/adjusted treatment estimate | |
| Duration of moderately bad or worse symptoms in days, median (IQR)a | 6 (4–10) | 5 (3–7) | 1.19 (0.80 to 1.76); 1.23 (0.83 to 1.82) | 6 (4–12) | 5 (3–9) | 1.21 (0.99 to 1.47); 1.16 (0.95 to 1.41) |
| Symptom severity at days 2–4, mean (SD) | 1.6 (0.99) | 1.9 (1.12) | 0.25 (–0.14 to 0.65); 0.27 (–0.12 to 0.67) | 1.8 (1.11) | 1.8 (1.08) | –0.13 (–0.33 to 0.07); –0.14 (–0.34 to 0.07) |
| Duration of symptoms until very little/no problem in days, median (IQR) | 8 (5–14) | 6 (5–11) | 1.27 (0.84 to 1.91); 1.33 (0.88 to 2.00) | 8 (5–17) | 7 (5–14) | 1.23 (1.00 to 1.51); 1.16 (0.95 to 1.51) |
| Return with new or worsening symptoms, n (%) | 45/142 (31.7) | 43/129 (33.3) | 1.12 (0.53 to 2.35); 1.10 (0.53 to 2.32) | 121/341 (35.5) | 103/331 (31.1) | 0.78 (0.54 to 1.11); 0.79 (0.55 to 1.13) |
| Progression of illness necessitating hospital attendance or admission, n (%) | 5/150 (3.3) | 15/140 (10.7) | 1.79 (0.37 to 8.57) | 9/354 (2.5) | 20/351 (5.7) | 1.64 (0.68 to 3.95) |
| Side effects, n (%) | 37/114 (32.5) | 58/114 (50.9) | 3.18 (1.40 to 7.19); 3.11 (1.38 to 7.03) | 89/267 (33.3) | 118/271 (43.5) | 1.61 (1.10 to 2.34); 1.62 (1.08 to 2.43) |
Subgroups
After controlling for confounding with propensity scores, none of the prespecified subgroups had statistically significant interaction terms in either the observational data set or the combined data set (see Table 15). When selecting each subgroup, we found a suggestion of benefit among both children with productive sputum and children with fever.
| Subgroup | n | No antibiotics | Antibiotics | Interaction term, HR (99% CI)** | Unadjusted HR (99% CI) | Adjusted *HR (99% CI)** |
|---|---|---|---|---|---|---|
| Abnormal chest signs | ||||||
| Yes | 314 | 5 (4, 14) | 5 (3, 8) | 1.02 (0.68 to 1.52) | 1.10 (0.80 to 1.51) | 1.06 (0.77 to 1.46) |
| No | 430 | 7 (5, 12) | 5 (3, 10) | 1.23 (0.95 to 1.58) | 1.19 (0.94 to 1.52) | |
| Sputum | ||||||
| Yes | 568 | 7 (4, 14) | 5 (3, 9) | 1.46 (0.91 to 2.34) | 1.34 (1.07 to 1.67) | 1.29 (1.03 to 1.61) |
| No | 171 | 5 (3, 10) | 5 (3, 9) | 0.85 (0.54 to 1.34) | 0.86 (0.54 to 1.38) | |
| Fever | ||||||
| Yes | 579 | 6 (4, 13) | 5 (3, 8) | 1.49 (0.90 to 2.44) | 1.32 (1.00 to 1.77) | 1.28 (1.03 to 1.60) |
| No | 165 | 6 (4, 12) | 6 (3, 23) | 0.87 (0.55 to 1.39) | 0.72 (0.44 to 1.18) | |
| Physician rating of unwell | ||||||
| Yes | 488 | 6 (4, 11) | 5 (3, 8) | 1.24 (0.82 to 1.86) | 1.32 (1.03 to 1.71) | 1.26 (0.98 to 1.63) |
| No | 254 | 7 (4, 14) | 5 (3, 14) | 1.04 (0.74 to 1.45) | 1.03 (0.74 to 1.47) | |
| Shortness of breath | ||||||
| Yes | 365 | 6 (4, 11) | 5 (3, 9) | 0.97 (0.66 to 1.42) | 1.29 (0.97 to 1.71) | 1.26 (0.94 to 1.68) |
| No | 379 | 6 (4, 14) | 5 (4, 10) | 1.21 (0.91 to 1.59) | 1.20 (0.90 to 1.59) | |
| Oxygen saturation < 95% | ||||||
| Yes | 57 | 6 (4.5, 14.5) | 6 (3, 10) | 1.03 (0.51 to 2.10) | 0.96 (0.33 to 2.75) | 1.20 (0.24 to 5.93) |
| No | 516 | 6 (4, 13) | 5 (3, 9) | 1.20 (0.89 to 1.62) | 1.16 (0.94 to 1.43) | |
| STARWAVe | ||||||
| Very low risk | 387 | 6 (4, 13.5) | 5 (3,9) | Reference | 1.26 (0.96 to 1.65) | 1.21 (0.92 to 1.59) |
| Normal risk | 326 | 6 (4, 11) | 5 (3, 8) | 1.03 (0.70 to 1.52) | 1.26 (0.93 to 1.72) | 1.20 (0.88 to 1.64) |
| High risk | 31 | 5 (3, 20) | 6.5 (4, 11) | – | – | – |
Prognostic model
Twenty-nine children (4%) in the cohort had illness progression necessitating attendance or admission to hospital (details of the illnesses are in Table 18). We tested the STARWAVe score using the three classifications. The calibration was excellent, with a Hosmer–Lemeshow test p-value of 0.9847, but the AUROC was modest, at 0.66 (95% CI 0.50 to 0.77). As STARWAVe was developed to predict the progression of illness requiring overnight hospital admission (11 of the children in the current data set), we separately tested test performance for this outcome; the AUROC for STARWAVe in predicting the need for overnight hospital admission was 0.70 (0.56 to 0.84; Hosmer–Lemeshow p-value 1.00).
Given the modest discrimination of the STARWAVe model, we developed new models. The predictors of the progression of illness are shown in Table 16. We retained 8 variables: baseline severity, difference in respiratory rate from normal for age, duration of illness prior to consultation, low oxygen saturation, sputum/rattly chest, passing urine less often and diarrhoea as variables in the model. This model had an AUROC of 0.83 (95% CI 0.74 to 0.92); model calibration was good, with a Hosmer–Lemeshow test p-value of 0.22. A reduced model using only the three significant predictors from the eight variate model (difference in respiratory rate from normal for age, sputum and low oxygen saturation) had an AUROC of 0.81 (95% CI 0.71 to 0.91; Hosmer–Lemeshow statistic p-value 0.86) (see Table 17). We did not include antibiotic prescription in the first predictive model owing to the observed inverse association between antibiotic prescribing and the progression of illness (very likely to be due to confounding by indication); nevertheless, we also assessed the model after including antibiotic prescription and the same variables were included. We also looked at the discrimination of the five-item model for the 11 children requiring overnight admissions: the AUROC was 0.88 (95% CI 0.78 to 0.97; Hosmer–Lemeshow p-value 0.74).
| No progression of illness | Progression of illness | Univariable RR (95% CI) | Multivariable RR (95% CI) | |
|---|---|---|---|---|
| Female, n (%) | 316/676 (46.8) | 10/29 (34.5) | 0.57 (0.27 to 1.24) | |
| Age, mean (SD) | 3.79 (4.89) | 3.59 (2.75) | 1.00 (0.93 to 1.06) | |
| Baseline severity, mean (SD) | 1.64 (0.33) | 1.73 (0.38) | 2.29 (0.96 to 6.13) | 0.34 (0.09 to 1.35) |
| Longer duration of illness in days prior to consultation, n (%) | 419/676 (62.0) | 13/29 (44.8) | 0.57 (0.27 to 1.17) | 0.43 (0.17 to 1.07) |
| At least one comorbid condition, n (%) | 83/676 (12.3) | 4/29 (13.8) | 1.03 (0.35 to 3.00) | |
| Asthma, n (%) | 66/676 (9.8) | 3/29 (10.3) | 0.98 (0.29 to 3.30) | |
| Abnormal chest signs, n (%) | 271/676 (40.1) | 20/29 (69.0) | 3.72 (1.67 to 8.29) | |
| Sputum/rattly chest, n (%) | 404/670 (60.3) | 23/28 (82.1) | 2.62 (1.05 to 6.50) | 3.79 (1.36 to 10.56) |
| Unwell, n (%) | 442/676 (65.5) | 24/29 (82.8) | 1.89 (0.77 to 4.61) | |
| Oxygen saturation < 95%, n (%) | 43/509 (8.5) | 10/28 (35.7) | 5.87 (2.64 to 13.09) | 2.51 (0.98 to 6.45) |
| Dry cough, n (%) | 368/676 (54.4) | 12/29 (41.4) | 0.64 (0.31 to 1.31) | |
| Runny nose, n (%) | 550/676 (81.4) | 24/29 (82.8) | 1.17 (0.44 to 3.11) | |
| Diarrhoea, n (%) | 89/676 (13.2) | 5/29 (17.2) | 1.28 (0.48 to 3.42) | 2.26 (0.80 to 6.44) |
| Chills, n (%) | 165/676 (24.4) | 7/29 (24.1) | 1.02 (0.44 to 2.40) | |
| Vomiting, n (%) | 217/676 (32.1) | 14/29 (48.3) | 2.22 (1.07 to 4.61) | |
| Taking less fluid, n (%) | 284/676 (42.0) | 16/29 (55.2) | 1.72 (0.83 to 3.56) | |
| Disturbed sleep, n (%) | 575/676 (85.1) | 27/29 (93.1) | 2.10 (0.50 to 8.71) | |
| Passing urine less often, n (%) | 165/676 (24.4) | 15/29 (53.3) | 3.71 (1.79 to 7.72) | 2.68 (1.01 to 7.10) |
| Temperature (mean, SD) | 37.26 (0.78) | 37.5 (1.08) | 1.35 (0.90 to 2.03) | |
| Heart rate (beats per minute), mean (SD) | 113.5 (19.69) | 125.8 (22.96) | 1.03 (1.01 to 1.05) | |
| Difference between respiratory rate and normal respiratory rate for agea in breaths per minute, mean (SD) | –1.8 (7.8) | 6.0 (11.1) | 1.07 (1.05 to 1.10) | 1.06 (1.02 to 1.11) |
| Antibiotics, n (%) | 331/676 (49.0) | 20/29 (69.0) | 2.64 (1.20 to 5.81) | N/A |
| STARWAVe, n (%) | ||||
| • Very low risk | 363/676 (53.3) | 8/29 (27.6) | Reference | N/A |
| • Normal risk | 292/676 (42.9) | 17/29 (58.6) | 2.70 (1.16 to 6.28) | |
| • High risk | 26/676 (3.9) | 4/29 (13.8) | 7.60 (2.34 to 24.59) | |
| No progression of illness | Progression of illness | Three-item model RR (95% CI) | |
|---|---|---|---|
| Difference between respiratory rate and normal respiratory rate for age in breaths per minute, mean (SD) | –1.8 (7.8) | 6.0 (11.1) | 1.06 (1.03 to 1.10) |
| Sputum/rattly chest,a n (%) | 404/670 (60.3) | 23/28 (82.1) | 3.11 (1.32 to 7.37) |
| Oxygen saturation < 95%, n (%) | 43/509 (8.5) | 10/28 (35.7) | 4.29 (1.58 to 11.62) |
| Passing urine less often,a n (%) | 165/676 (24.4) | 15/29 (53.3) | |
| Diarrhoea,a n (%) | 89/676 (13.2) | 5/29 (17.2) | |
| Baseline severity,a mean (SD) | 1.64 (0.33) | 1.73 (0.38) | |
| Longer duration of illness in days prior to consultation, n (%) | 419/676 (62.0) | 13/29 (44.8) |
| Placebo | Antibiotics |
|---|---|
| Trial | |
| Hospital admission –worsening of symptoms/‘viral-induced’ wheeze | Hospital admission –stridor, floppy episode and febrile convulsion, croup, shortness of breath |
| Hospital admission –shortness of breath. Bronchiolitis, increased work of breathing requiring optiflow and nasogastic feeding | Persistent symptoms of fever, increasing breathlessness, oxygen saturation 91%, respiratory rate of 32 breaths per minute, expiratory wheeze; admission to hospital (not overnight) |
| Exacerbation of cough and phlegm/cough getting worse, vomiting overnight, decreased feeding –not admitted overnight | Hospital admission –bronchiolitis |
| Hospital admission –no further information available | Gastrointestinal symptoms: hospital admission –not overnight |
| Temperature 38.5 °C, ‘rattle in throat/chest’, stomach pain | |
| Observational study | |
| No antibiotics | Antibiotics |
| Hospital admission –overheating | Hospital admission –upper RTI |
| Hospital admission –bronchiectasis in middle of right lobe | Bronchopneumonia |
| Hospital admission –stiff limbs, possible febrile convulsion | Hospital admission –infection |
| Hospital admission –LRTI | Hospital admission –abscess on right lung |
| Hospital admission –no further information | Hospital admission –urticarial rash |
| Hospital admission –right lower lobe pneumonia | |
| Hospital admission –viral induced wheeze | |
| Hospital admission –three nights | |
| Hospital admission –overnight but no information | |
| Hospital admission –three nights, chest infection | |
| Hospital admission –overnight, pyrexia of unknown origin | |
| Hospital admission –13 nights, no further information | |
| Hospital admission –ongoing | |
| Hospital admission –overnight, no further information | |
| Hospital admission –no further information | |
We also converted the three-item model [respiratory rate (breaths per minute), oxygen saturation < 95%, sputum] to a score by multiplying the beta-coefficients by 10 and rounding to the nearest integer (score = 46 + difference in respiratory rate from normal for age + 14 × low oxygen saturation + 18 × sputum). Scores range from 19 to 102, and the AUROC was 0.78 (95% CI 0.68 to 0.88) for progression of illness and 0.86 (95% CI 0.72 to 1.00) for hospital admission.
Discussion
The observational data provided a useful addition to the trial data, providing more power and facilitating the generation of a prognostic model to predict the progression of illness. For the main outcome (symptom resolution), the combined data set demonstrated similar estimates of effect of antibiotic treatment to the ‘pure’ trial data, despite representing a much more severely affected group. The prognostic model used clinical variables that are all readily available in consultations and was effective in predicting the progression of illness.
Limitations
The method of controlling for confounding by indication needed to be adapted to improve the balance between the groups, and there was evidence of some residual confounding by indication for some of the outcomes, particularly progression of illness. It is also likely that residual confounding by indication contributes to the much higher rate of apparent ‘side effects’ seen in the antibiotics group, as we know that diarrhoea, vomiting and skin rash occur commonly in the placebo group (i.e. as part of the illness for both children and adults). 25 Therefore, the more common ‘side effects’ in the antibiotics group in the observational data may reflect illness severity rather than side effects per se. It is also possible that there is greater attribution and monitoring of known side effects when parents know that their child is receiving antibiotics. The differences in clinical characteristics between observational and trial data sets cannot be attributed just to clinical decision-making, as the range of primary care settings was different: many of the observational patients came from primary care sites that were less typical of routine general practice and were not able to recruit to the trial (e.g. A&E). The prognostic model was limited by the relatively few children in whom illness progressed (and so we may not have had sufficient power to include all of the relevant variables), although having more significant variables would increase the discriminatory power of the model. Although bootstrapping was used to limit the problem of overfitting, and the reduced model with five or three variables also provided reasonable estimates of discrimination, external validation of these models in a separate sample will be needed.
Main results in context of other literature
Children given antibiotics in the observational study had much more severe clinical presentations than children not given antibiotics, matching the trends in the much larger STARWAVe cohort. 12,39 The children in our trial cohort were more severely affected than the children in the STARWAVe cohort, and that trend is even more apparent in the children contributing to the observational data: there were high percentages of children given antibiotics with sputum production (87% of children, compared with 63% in STARWAVe), fever (91% of children, compared with 75% in STARWAVe) and shortness of breath (70% of children, compared with 46% in STARWAVe).
Despite major differences in clinical presentation between children given and children not given antibiotics, we found that, when controlling for the propensity to prescribe antibiotics, the main outcomes of the combined trial and observational data were very similar to those of the ‘pure’ trial data; overall, there were 1–2 days’ difference between the groups, both for the duration of moderately bad or worse symptoms and the duration of symptoms until very little or no problem. In the subgroup analyses, although there was some suggestive evidence of differences for some subgroups, the interaction terms were not statistically significant. Among children with productive sputum or fever, the lower CIs for the HRs were only just above the null, so it is possible that these are chance findings. The estimates of benefit for both of the above subgroups were also not clinically important (neither subgroup had a difference in symptom duration of more than 2 days).
The final prognostic model to predict the progression of illness included eight variables and demonstrated excellent discrimination (AUROC 0.85) and good calibration. However, it included rather different variables from the STARWAVe model:12,39 the models had only age and prior illness duration in common (STARWAVe included age < 2 years, current asthma, illness duration of ≤ 3 days, parent-reported moderate or severe vomiting in the previous 24 hours, parent-reported severe fever in the previous 24 hours or a body temperature of ≥ 37.8 °C at presentation, clinician-reported intercostal or subcostal recession, and clinician-reported wheeze on auscultation). Thus, it is perhaps not surprising that the STARWAVe model had lower discrimination in the current population (AUROC 0.65), and the difference probably reflects the more severely affected group of children recruited to the current study. Another difference from STARWAVe is that data on pulse oximetry were available for only < 50% of children in the STARWAVe cohort, and if more had been available pulse oximetry might have been included in STARWAVe. In addition, intercostal recession was not measured in the current study, and so it is possible the estimated discrimination might be improved further; and if the outcome is overnight hospital admission the discrimination of both the new model and STARWAVe improve further. The prognostic model could have clinical utility in that all of the variables are easily documented in routine consultations, so they could possibly be used by clinicians to predict adverse outcome when seeing more unwell children, potentially guiding management decisions for antibiotic prescribing and/or for follow-up. 57
Conclusion
The observational study included children from a slightly different range of settings from the trial so direct comparisons are not possible. Although children given antibiotics in the observational settings were much more severely affected, there was no evidence of greater effectiveness of antibiotics in modifying symptom resolution. We have developed simple clinical scores to identify the minority of children whose illness is more likely to progress sufficiently to require hospital assessment. A simple clinical score requires external validation but could potentially be used to guide clinical management.
Chapter 5 Qualitative studies
Introduction and methods
Introduction
A large proportion of consultations in primary care involve parents consulting with children with symptoms of a RTI, and antibiotics are frequently prescribed. 45 The overuse of antibiotics is a cause for concern in terms of both the growing threat of antimicrobial resistance (AMR)2 and the cost burden on the NHS. 11,13,34
Research has shown that parents vary in their confidence and self-efficacy when it comes to assessing the symptoms of a RTI58 and consult a primary care service owing to perceptions of illness severity, uncertainty about appropriate management and a desire for a clinical examination and reassurance from a trusted medical professional. 19,20,58–60 Although some parents may consult with an expectation for antibiotics, studies have shown that these expectations tend to be overestimated by clinicians during the consultation and are a significant contributor to unnecessary prescribing. 61,62 Furthermore, many parents are said to be accepting of a ‘no-antibiotic’ treatment outcome as long as they leave the consultation feeling reassured and have a plan for symptomatic relief at home. 59,63,64 Clinician decision-making to prescribe antibiotics is influenced by perceived clinical need,19,62,65 but more significantly by clinical uncertainty about future illness deterioration and fears of missing something more serious in a child. 19,20,58,66–68
Two qualitative studies with parents and clinicians were incorporated into ARTIC-PC to explore their views and experiences of managing a child with symptoms of LRTI, as well as their views of participating overall.
Methods
Two qualitative studies using semistructured telephone interviews were carried out with parents and clinicians who participated in ARTIC-PC. Parents who had given written informed consent to take part in an interview at the time of recruitment to ARTIC-PC were invited to participate in a telephone interview. Participants were purposively sampled by their participation either in the randomised controlled trial (RCT) or in the observational study, to represent a range of views/experiences following participation, to explore parental reasons for taking part in both aspects of the research and use the findings to optimise study procedures and improve recruitment as part of the ongoing trial. The topic guide included questions about parental motivations for taking part in the research.
Parent interviews took place between November 2016 and July 2017. Clinicians taking part in recruiting participants into the study were also invited to take part in a telephone interview. We aimed to recruit 10–15 clinicians from different practices taking part in the trial. The interviews took place between August 2017 and February 2018.
Catherine Woods conducted semistructured telephone interviews with parents and clinicians. Telephone interviews were deemed suitable for both types of participants, and it was thought that this format would encourage participation by making it easier for parents and clinicians to fit the interview around childcare responsibilities and clinical practice, respectively. The parent interview guide explored views and experiences of managing a child with RTI, reasons for consulting, and views of the study procedures and why they chose to take part in the trial or the observational study. Interviews with parents lasted between 20 and 50 minutes, with an average duration of 33 minutes. The clinician interview guide explored views and experiences of managing children with symptoms of a RTI in primary care as well their views of taking part in the trial. The interviews with clinicians lasted between 25 and 50 minutes, with an average duration of 35 minutes.
All interviews were audio-recorded, transcribed verbatim and analysed using inductive thematic analysis. 69 The parent interviews were analysed by Catherine Woods and second-coded by a medical student (Zöe Morrice) on a 6-week summer placement in the Primary Care Research Centre in Southampton. The codes were compared and refined in meetings with Catherine Woods and Zöe Morrice and the qualitative work package lead Geraldine Leydon; and the final thematic framework which captured salient themes across the entire data set was developed by Catherine Woods. The clinician interviews were coded by Catherine Woods. Analyses were facilitated by NVivo 11 software.
Results
Sixteen parents took part in the interviews: 15 mothers and one father from six GP practices. All of the children were under 6 years of age at the time they were recruited to the study; the youngest was 9 months and the eldest had recently turned 6. The sample was evenly split between first-time parents and parents who had one or more other children. Seven of the parents interviewed took part in the trial and nine took part in the observational study. Fourteen practitioners (9 GPs, 4 nurses and 1 research nurse) agreed to take part from 14 practices involved in the study.
We identified seven themes related to parent and clinician views of managing a child with symptoms of a LRTI, antibiotic use, and their experiences of taking part in the trial (see Table 19).
| Themes | Subthemes |
|---|---|
| Parent interviews | |
| Theme 1: reasons for consulting |
|
| Theme 2: parent perspectives and understandings about antibiotic use and antibiotic resistance |
|
| Theme 3: views of taking part in the trial and its procedures |
|
| Clinician interviews | |
| Theme 1: antibiotic prescribing for children with LRTI and other common infections |
|
| Theme 2: views about AMR |
|
| Theme 3: positive views of taking part in the trial and its procedures |
|
| Theme 4: factors affecting recruitment |
|
The following will summarise the main themes across parent and clinician interviews in turn.
Parent interviews
Theme 1: reasons for consulting
Perceptions of symptom severity and cause
The severity of the cough was ascertained by parents based on its sound, with many parents describing their child’s cough as ‘rattly’, ‘hacking’, ‘barking’ and ‘wheezy’. Children often had other symptoms in combination with the cough, including cold-like symptoms (e.g. runny nose, disturbed sleep) and a high temperature. Several of the parents reported that they had tried to self-manage the child’s illness at home prior to consulting a clinician (as they would usually), mainly with pain relief (e.g. Calpol) to try to alleviate the symptoms and help the child sleep. The perceived severity of the cough, duration of the illness and persistence of the cough despite attempts at self-care were key factors that led some of the parents to conclude that their child had a chest infection. Other parents were uncertain about the exact cause of the cough, which was also a cause for concern:
Yeah, like an illness smell and then it was this sort of, it was like that rattly, kind of quite tight cough that you could tell was sort of hurting her, and then when she woke up on the morning after the doctor’s appointment, erm, she was able to [eat]. She was able to tell me that her chest was hurting, so, well she didn’t actually say her chest but she pointed, so, er, I knew, I knew that actually, that’s probably what it was.
Parent 13
Rather than say chesty, not really. It sounded rattly, is possibly the way of putting it.
Parent 11
Previous experiences of lower respiratory tract infection or other respiratory issues
Several parents reported that their child (or a sibling of their child) had previously experienced symptoms of a LRTI, which ranged from a previous instance of a chest infection or a similar cough, to more serious experiences that involved pneumonia or hospitalisation. Parents also reported the difficulties of interpreting the signs and symptoms of a LRTI in children, especially younger children, because of their inability to verbalise how they are feeling; and knowing the difference between mild symptoms that are part of a routine illness and symptoms that could be indicative of something more serious:
Interviewer: OK. So, erm, w- what made you book an e- emergency appointment?
Parent 13: Because, in the past when she had chest infections, if I didn’t sort of jump on it quite quickly, it then took about 2 weeks to get rid of it. So, if, you know, the faster I sort of solved it, the easy, you know, the sort of less extreme it was for her. Erm, but before, when we kind of left it a couple of days it had then taken a while and it was quite hard to get rid of it.
Parent 13
Interviewer: Did you have any idea what might be wrong with her since she’s got this cough and she’s had it before?
Parent 1: I worried it was going to be on the bottom of her lung again, but with the trial I think we went for an X-ray, and I don’t think it shows up as being anything on her . . . Obviously, now I’m always going to think that because it’s been not so long a time before and it felt a bit like we were just told at the doctors, ‘Oh no it’s clear, oh no it’s clear’. Then when we finally went and there was something wrong. I thought it was going to be that again and that’s why I went quicker and pushed.
Parent 1
Expectations of the consultation
Many parents said that the main reason they had wanted an appointment was because they wanted a clinical assessment of the child’s cough/chest. Parents described feeling reassured by clinical assessments, as a clinician could determine if the symptoms indicated a viral or bacterial infection and if the child’s chest was clear and could also confirm that the symptoms were not indicative of something more serious. A few parents said that they wanted a prescribed medical treatment, which did include antibiotics but also inhalers. Others said that they were happy to be told to continue to self-management of their child’s illness, but that they wanted a clinician to confirm this. The assessment and diagnosis seemed more important to the parents than expectations for specific treatments:
I just wanted them to check her chest really and make sure it was definitely clear.
Parent 4
Just kind of listening to, especially with the cough, sometimes just listening and saying, ‘OK’, either, ‘I can hear noises here’, or ‘I can’t hear noises here. It’s all up her airway’, and just checking if there is anything else in there.
Parent 2
Theme 2: parent perspectives and understandings about antibiotic use and antibiotic resistance
Perspectives about antibiotics and when they should be used
Many of the parents acknowledged that antibiotics should be used only when needed and associated this necessity with having an infection. Some parents conveyed their understanding of how they could differentiate between viral and bacterial infections, for example that a bacterial infection will not clear up by itself, causes more severe symptoms and is associated with a high temperature or the child acting out of character. Many parents thought that antibiotics were effective and generally worked straight away or within a few days if prescribed correctly:
Interviewer: And so you said bacterial and viral infections, can you tell me a little about the difference between those and if you know the difference?
Parent 14: Well, a viral infection is something that you generally pick up, like for example a cold would be a viral infection. Something that you take paracetamol for, you just rest up and generally runs its course and goes by itself. On the other hand, a bacterial infection is something that will give you a temperature. Erm, and it will, you know, unfortunately cause, you know, erm – how can I explain it? Something that’s, erm, yeah, going to cause infection within the body, therefore it’s going to give you a temperature and it’s not going to go unless it’s, it’s treated with something in order to get rid of it.
Parent 14
Yes, well antibiotics are if it’s a bacterial infection and one which is having quite significant symptoms, or signs that the body is not fighting it off or that it’s likely to develop into something else, then yes.
Parent 3
Understandings of antibiotic resistance
Many parents had an understanding of antibiotic resistance and said that this referred to when antibiotics no longer work, which some attributed to overuse and unnecessary prescribing. References were made to ‘immunity’, ‘tolerance’, ‘superbugs’ and ‘bacteria evolving’. A few parents pointed to the causes of overprescribing, such as clinicians being more likely to prescribe antibiotics if they were time constrained or erring on the side of caution with children. One parent suggested a possible solution to the overuse of antibiotics and thought that it would be helpful to have more diagnostic tests so GPs could determine what might be wrong and if antibiotics are needed more precisely.
Interviewer: You mentioned the word resistance there. Can you just tell me a little bit more about what that means to you?
Parent 11: To me, I assume that it’s where the antibiotics begin to lose any effect. I don’t know enough about it, but from what I understand, it’s to do with the fact that if you take them as often as you can do, then the effectiveness of the antibiotics will start to diminish and your body will build a resistance to it, and that’s about as much as I know.
Parent 11
Antibiotics are perhaps being given out for things that they’re not needed, or it could be a case of people not finishing the course then the infections are mutating slightly so that some of them can actually survive the antibiotics, which means that as they’re evolving you’re then needing stronger antibiotics to treat the same kind of things. I think the thought is if we gave out antibiotics less often then this wouldn’t happen as much.
Parent 7
Theme 3: views on taking part in the trial and its procedures
Motivations for taking part in the study
Many parents said that they were happy to join both the trial and observational studies, once they had been introduced by the clinician. Reasons for participating included wanting to improve care owing to previous experiences of LRTI, that the research involved children and might benefit their own child (or other children) in the future, and curiosity regarding whether or not antibiotics were always warranted. A few parents said they had taken part in research studies before and were always happy to help. Parents reported that their trust in the judgement of the clinician or university was an additional reason for taking part (as neither party would want to harm children):
Interviewer: I’m just going to move on now to your participation in the trial. So when the GP initially introduced it, what do you think?
Parent 4: Yes, I was quite interested in doing it and seeing if it did make a difference or not.
Interviewer: What in particular made you interested in participating?
Parent 4: The fact that it was to do with kids, younger children, to see if there was any way of going round it rather than giving them antibiotics all the time.
Parent 4
Erm, I thought it was quite a good idea . . . and I was quite willing to take part, so – and I went to university, I know how hard it was to be a participant to take part in . . . studies and stuff, so I’ve always kind of been a bit aware of that . . . to try to sort of, and I thought yeah, I can just fill out the, fill out the book and stuff, so it’s not that hard, it won’t take up too much time, we can do that, so . . .
Parent 13
Positive aspects of taking part overall
Several parents reported that they had a positive experience of taking part overall and would consider taking part in a trial again in the future. Some simply said that they enjoyed taking part, while others listed specific reasons for this, such as their being able to see how long it took for their child to recover and make sure that their child’s cough went away. A couple of parents said that they would consider taking part in a trial again, but that their decision would be contingent on the circumstances, such as what the doctor thought at the time and their perception of whether or not the trial would harm the child:
Interviewer: Have you ever taken part in a trial before?
Parent 4: No.
Interviewer: Do you think you would again in the future?
Parent 4: Yes.
Interviewer: Why is that?
Parent 4: More if it was for the kids again because with children it’s a bit different to adults. They can’t actually tell you what’s wrong.
Parent 4
Interviewer: Yes, sure. I guess, just the last question is, if you were asked to participate in a trial again, do you think you would?
Parent 11: Yes. It all comes down to the circumstances and the opinion of the doctors as to whether . . . What I would never want to do is do something that could potentially harm him. Yes, circumstances-dependent, would be the answer.
Parent 11
The study materials were easy to follow or administer
Nearly all parents reported that the study materials including the participant information sheets, consent forms and symptom diary were clear and easy to follow. Some parents said that the diary was useful for monitoring their child’s illness and that since using it they had a better understanding about how the symptoms of a suspected LRTI might progress (i.e. that their child might display signs of wellness and then worsen, and that this would be considered normal). A few parents said that they would consider using a symptom diary again in their daily life for this reason. Some parents, however, reported a few problems or contrasting views about the symptom diary, namely that they forgot to fill it out, they thought it was too long because their child had recovered within the first week of taking part (the symptom diary was supposed to be filled out for 4 weeks), and that they had problems filling it out for younger children who could not verbalise how they were feeling. All parents who took part in the trial reported that they found the medication easy to administer and store:
Yes, absolutely. You could see it as it was written down over the course of the 10 days, you could, you could see what was deteriorating and what was actually peaking, and yes. It was very, very interesting. Yeah . . . I think that actually, that actually I would consider doing it myself for monitoring illness. Because it was that, it was that informative.
Parent 14
Interviewer: And what about taking the medication, how did [name] find it? How did you find it?
Parent 2: He was fine with it, so with the syringe he was, yes, he was fine. Yes, I think he quite liked the taste of it, which is always a bonus.
Parent 2
Perspectives on and understandings about placebos
Fourteen of the parents interviewed described their understanding of a placebo. All but two had heard of a placebo before taking part in the trial. The most common understanding was that a placebo is the same as ‘real’ medicine in appearance and taste but does not have ‘active’ ingredients. Some participants thought that the purpose of a placebo was psychological, to determine if the idea of a medication working was more significant than the medication itself. Three of the parents were unsure about the use of placebos, either within ARTIC-PC or as a treatment generally. One said that she did not think a placebo would ‘work’ if a patient had a ‘serious bacterial infection’ because placebos do not affect the blood; another appreciated the idea of a placebo-controlled trial but said that they would prefer to receive the active medication if enrolled in one; and the third displayed a strong negative stance and said that they would be unhappy if they received a placebo for a chest infection and that placebo-controlled trials are unnecessary for children:
It’s something that’s got no medicine in it at all, like a Smartie almost or a Tic-Tac but it looks like the real thing and tastes – is almost labelled the same, so that the patient is unaware whether they’re taking the real medication or not. I guess mind over matter in a way, because they believe they’re taking the medication, they might get better but it might turn out they never had any medicine and they’ve got better on their own.
Parent 5
Interviewer: Do you think they’re effective, the placebo effect?
Parent 6: I don’t know, I think psychology plays a huge part in ill health, so maybe it can work with some symptomatology, but I think if someone’s got a serious bacterial infection then no they don’t do anything to the blood, to the infection within the bloodstream. So I think maybe they can help with elements of it, but I don’t think placebo can cure a serious bacterial infection, whatever it may be.
Parent 6
Parents’ willingness for their child to have the trial medication
The degree of parents’ willingness for their child receiving an antibiotic or a placebo shaped their choice to participate in the trial or the observational study. The parents who took part in the trial were more optimistic about participation and weighed the perceived benefits of taking part (e.g. recovery) with the perceived risks (e.g. illness deterioration). A few parents discussed the dilemma of wanting to do the best for their child but also not wanting the child to take antibiotics unnecessarily. Being asked to take part in the trial was considered positive as it removed some of the parental responsibility attached to the treatment decision. The parents who took part in the observational study were more centred on the risks associated with taking a placebo, which were related either to the concept of a placebo itself (e.g. ingesting a medication that might not be ‘ready’ or ‘thoroughly tested’) or to preferences for immediate antibiotics. A couple of parents said that the GP had not asked them to join the trial, and that this was related to the child not being ill enough at the time of the consultation:
Erm, to be honest, we didn’t, we didn’t mind, because we just thought there was the potential for it to be, for it to be the antibiotic, so we were just, you know, we were just hoping we could clear whatever she had up, you know, do if it was a fake medicine, it was fake. If not, you know, we had nothing to lose, really. Just wanted to get her better, really.
Parent 16
It’s not fair on [name] if she is feeling poorly and I don’t get the medicine to fix her, so I didn’t want to risk it.
Parent 5
Perceptions of recovery from illness in children who took part in the trial
Of the seven parents interviewed who took part in the trial, most were unsure about whether their child had taken a placebo or an antibiotic. Two simply stated that they had ‘no idea’, whereas others offered tentative theories about the plausibility of the child having received the placebo or the antibiotic based on when their child had started displaying signs of improvement and how long the child’s recovery had taken overall. Two parents were more certain that their child had received a placebo because it had taken several days for their child to recover, and they thought that antibiotics would have worked within 48 hours:
Yeah, I, I, we thought she got better quite quickly after having whatever, if it was antibiotics or not, I don’t know, but we thought she got better fairly quickly after she was taking the medicine, but then I don’t know whether that was because it was an antibiotic, or because she was on, like, day 3 or 4 of the illness, and it would have cleared up on its own, as well, if you see what I mean, because I’d only just taken, because I’d taken her to the doctor’s maybe a bit later, it would have cleared up anyway, but it seemed to be that after we’d been, you know, if it was antibiotics, then it did clear it up first, or it felt like it did! Maybe it was just, you know, a placebo (laughs).
Parent 16
Parent 4: I think it was about 3 or 4 days. Normally with antibiotics they say give it 24 to 48 hours to start working, but I didn’t see any drastic changes for 4 days.
Interviewer: So you said before it was 3 days before you took her to the GP and then was it 3 or 4 days to take to recover so she had it for about a week?
Parent 4: About a week, yes.
Interviewer: You’ve mentioned that she didn’t get better straight away so did that lead you to think that she had the placebo?
Parent 4: Yes.
Parent 4
Clinician interviews
Theme 1: antibiotic prescribing for children with lower respiratory tract infection and other common infections
Decision to prescribe antibiotics is based on clinical indicators and risk factors
Clinicians emphasised that a patient would have to have more than one clinical indication of an infection for them to consider prescribing immediate antibiotics. In addition to clinical signs, GPs mentioned the longevity of symptoms, the patient’s medical history and if the symptoms had persisted despite attempts at self-care. This was summarised by one GP as ‘one takes the whole picture’, which was also apparent in other clinician narratives. Additionally, participants also reported that their decision to prescribe antibiotics would be influenced by whether the patient had any underlying risk factors or comorbidities that might result in them deteriorating quickly if an infection progressed:
Well, an isolated high temperature wouldn’t make me prescribe antibiotics. It’s in conjunction with other findings, so if a child had never had a temperature and had a tachycardia, then you’d be questioning why else would they have a tachycardia rather than it being an infectious effective process, I think that’s what I mean, so it’s a high temperature in conjunction with other clinical signs?
GP 9
Nurse 2: It would be good if they are. Being a nurse prescriber, as (. . .), when I was working as a nurse practitioner, I wouldn’t give antibiotics to a child with a cold, as I would call it, unless they had a proper chest infection.
Interviewer: I know it might sound like a silly question, but how can you tell if they’ve got a proper chest infection? What kinds of things are you looking out for?
Nurse 2: Have they got any chest sounds when you’re listening to their chest? Are they pyrexial? How well are they? You know, are they interacting?
Nurse 2
Prescribing alternatives to immediate antibiotics
Clinicians said that they regularly offered self-care advice when they did not prescribe immediate antibiotics and sometimes when they gave delayed prescriptions. In terms of forms of self-care, simple analgesics were recommended most frequently, although some clinicians said that they also recommend spending time in steamy rooms and speaking with a pharmacist. In terms of delayed antibiotic prescriptions, clinicians generally had favourable views, and many used them within their consultations. The scripts were said to be useful for giving patients a sense of ‘control’ and ‘empowering’ them to manage their own or their child’s health, and also if the clinician was unable to review the patient again in person (e.g. because it was the weekend). However, some clinicians offered contrasting views and reported that they did not like using delayed prescriptions because they would prefer to review the patient in person or for the patient to use another primary care service (e.g. out of hours). A concern related to symptoms of a LRTI specifically was that a patient might need to be referred to hospital and this would be missed if a delayed prescription was used as the patient would not be reviewed in person. Additionally, several clinicians thought that although the strategy of a delayed prescriptions might be useful in theory, they might not work as intended because of patient behaviour (e.g. redeeming a prescription immediately after the consultation or giving the prescription away to others). For both alternative prescribing strategies – self-care and delayed prescriptions – clinicians reported that they also provide safety-netting advice to ensure that the parent/patient knows what to do should their/their child’s illness deteriorate after leaving the consultation. Clinicians reported that patients and parents accepted alternatives to immediate antibiotics:
Interviewer: How do parents usually react to being given a delayed script?
GP 2: They like it, because it gives them a little bit of empowerment I think, and allows them to feel a bit more confident of the fact that they don’t have to give antibiotics now and they can wait and see and they often quite like it. That seems to be the rationale! Certainly sometimes if their child had bronchiolitis severely when they were a baby and they’re now 4 and presenting with a cough, and their child had to go into hospital when they were a baby or whatever, they think back to that time. So actually giving them antibiotics, giving them a plan as to what to do over the next few days if things do deteriorate, they’re very happy and they like that.
GP 2
Interviewer: If you’re not going to prescribe antibiotics what kind of things do you suggest to parents?
GP 6: We do safety-netting, which involves talking about the natural history of the disease, telling people what to look out for and when I want to see them again. So if it’s a chest infection, since we’re talking about ARTIC, I’d talk about increasing breathlessness, temperature that’s hard to control, lethargy, off food; anything like that we have another look at them. I say, ‘I can’t hear anything right now, but things might have changed and therefore I’ll review the child because they get ill quickly and get better quickly so there be new signs that I can’t see now, but will have developed in 1 or 2 days’ time’. So it’s just telling them what to look out for so that we don’t miss anything.
Interviewer: Are parents OK with this usually?
GP 6: Yes.
GP 6
Managing expectations and the importance of communication
Many clinicians said that parent/patient attitudes had changed over time and most now consulted for a clinical examination and reassurance rather than for antibiotics. Clinicians stated the importance of communicating clearly with patients/parents to ensure that they are informed about how symptoms from an infection should be managed, when antibiotics should and should not be used and that their symptoms are in keeping with how illness usually progresses. Within these narratives, clinicians provided details of their experiences of communicating with patients/parents during their consultations. Some clinicians said that they used antibiotic prescribing tools (such as the Centor criteria or leaflets) to help them explain signs and symptoms; others said that if a clinician thinks that a parent or patient is hinting that they want to receive antibiotics, it is important to ask them about this outright as eliciting their expectations will help manage them accordingly. Explaining what the clinician could see from their clinical assessments also played a big part in communicating ‘clearly’:
Interviewer: How do you feel about explaining these signs and symptoms to parents, so, what you’ve found during the examination, or what you haven’t found?
GP 5: Yes, I would hope to think that I’m quite clear when I’m speaking to parents. Typically, they often come in saying, ‘I just want to check they don’t need antibiotics any more’, which I think’s been quite a cultural shift over the last few years or so, rather than coming in demanding medication.
GP 5
Interviewer: How would you go about managing patient expectations in your own consultation?
GP 7: I think you’ve got to find out what they want really and I think allowing the patient to speak, but also exploring it directly, so asking about their expectations is good communication really . . . so to find out whether they want antibiotics or not is . . . powerful piece of information. Interestingly lots, I have found myself forgetting to do that sometimes recently and explaining to patients antibiotics are unlikely to work for their viral bronchitis or whatever infection, they’ve actually said to me they weren’t expecting antibiotics!
GP 7
Theme 2: views about antimicrobial resistance
Antimicrobial resistance is caused by overprescribing antibiotics
Clinicians acknowledged that AMR is a national problem. A couple of GPs described instances of AMR in their daily practice, including noticing urine infections becoming more resistant, patients not getting better as quickly when taking antibiotics and sometimes needing to prescribe a second or third course. Clinicians recognised that antibiotics have been overprescribed in primary care and sometimes unnecessarily. The main difficulties in reducing antibiotic prescribing were attributed to the amount of variation between clinicians across primary care (e.g. that each clinician has their own reasons for prescribing, habits that are hard to break) and factors related to patients (e.g. expectations for antibiotics, lack of knowledge about when antibiotics are needed or knowledge about the usual duration of infections). Some clinicians just described the challenges associated with AMR, whereas others suggested what might be needed to reduce unnecessary prescribing, such as educating patients about when antibiotics are needed and how long the symptoms of a common infection can last. One GP also used the example of sepsis and pointed out the ‘double-edged sword’ of AMR and antibiotic prescribing, namely the difficulty of not prescribing unnecessarily (and increasing resistance) while not missing something serious and potentially fatal:
Interviewer: That’s really helpful. Again you touched on this before but could you just tell me a little about your views of antibiotic resistance?
GP 4: So I think that it’s going to become more and more of a problem. So I’m finding myself giving – if I do give somebody antibiotics, that they’re not getting better as quickly. We’re having to sometimes give second and third courses of antibiotics.
GP 4
Interviewer: Then are there any other challenges you can think of about reducing antibiotic resistance overall, other than the education that you were talking about?
Nurse 2: No, and education shouldn’t be a challenge, either.
Nurse 1: No, it’s about awareness. It’s about people being aware. It’s not until they’re aware that they can actually change their behaviour. If they don’t know something’s not right, they won’t change. So I think that is the key, isn’t it?
Nurses 2 and 3
Strategies for combating antimicrobial resistance
Many participants said that clinicians and practices must work together to reduce unnecessary antibiotic prescribing. In terms of practice-level strategies, many clinicians reported that their practice conducts regular audits to monitor the type and frequency of antibiotics being prescribed. Audits were said to be useful for checking that clinicians are prescribing ‘appropriately’ and to flag which clinicians might be overprescribing or overusing a certain type of antibiotic. The clinicians acknowledged that audits are necessary and most clinicians are accepting of them. The two GPs quoted below described audits as opportunities for learning rather than avenues for blame, which ultimately serve to ensure that the appropriate medicines are being used to treat (and benefit) patients. Additionally, some clinicians emphasised the importance of a ‘practice message’ or ‘ethos’ that involved working together to try to prescribe antibiotics only when necessary and providing patients with a consistent message about when antibiotics are needed. A few clinicians also said that they ‘keep up to date’ with antibiotic prescribing and what is appropriate through, for example, knowledge of current guidelines (e.g. NICE) and attendance at relevant updates:
Interviewer: Are the clinicians in your practice quite happy with that, with the auditing?
GP 3: . . . but I think people, overall people see the clinical benefit of having those internal discussions and auditing. But it’s not – you know, it’s not – no one gets told off if they’ve prescribed the wrong thing; it’s just, ‘Actually, just to remind you that these are the latest guidelines. They have changed; we all forget stuff. Don’t worry’.
GP 3
Interviewer: Then you just touched on overprescribing antibiotics, so can you just tell me a little bit about your views of that?
GP 2: I think we have been overprescribing, particularly for throat. When I started, throats and ears, and that’s what I was saying, I think there’s been a massive sea change here. I think there’s been a massive sea change, certainly within the practice, for a long time we’ve been trying not to and that’s been the ethos. I think I definitely prescribe less antibiotics, particularly in children. Ears, throats and coughs, definitely much, much less and it’s more acceptable.
GP 2
Theme 3: positive views of taking part in the trial and its procedures
The topic of the trial is important
The clinicians said that they were happy to take part in ARTIC-PC for several reasons, including that they thought the topic was important (determining if antibiotics are warranted for LRTI), that more research had been conducted with adults than with children and that there was more research about upper RTIs than LRTIs:
I think it’s a very important piece of information, because we are constantly telling patients that they don’t need antibiotics for upper respiratory tract infections, but the research really isn’t there that lower respiratory tract infections don’t need an antibiotic to prevent important admissions to hospital and complications. That piece of work needs to be done, and as far as I’m aware it hasn’t been yet.
GP 1
When I saw the trial I thought it was an interesting trial. It’s answering an important clinical question which is – because we see a lot of kids with fevers and the parents say that they they’re coughing up phlegm and that they think it’s lower and we might think it’s upper and occasionally hear some noise in the lungs, but this can often be a viral induced wheeze of funny noises so it’s hard to differentiate. So the fact that through trial has – actually the three arms, but the antibiotics versus placebo could answer an important question about whether these kids need antibiotics more than we think or whether actually we’re giving them the right treatment.
GP 6
The trial procedures were clear and easy to follow
The clinicians thought that all of the trial procedures, including the study packs, paperwork (i.e. participant information sheets and consent forms) and criteria for identifying suitable children, were clear and easy to follow. The clinicians also felt comfortable explaining the concept of a placebo to parents at the time of recruitment:
Interviewer: How have you found things like the information sheets and things like that that we’ve given?
Nurse 4: I think they’re fine. I’ve not had a problem. I think they do explain things clearly.
Nurse 4
Interviewer: How easy is it to explain this [concept of placebo] to patients?
GP 1: I haven’t really found a particular problem, you just have to explain that, in order to compare whether something’s working or not, you have to compare it with something else, and that something else has to not be influenced by either them or me, making any judgements because we know for sure what they’re having. I think most people grasp that concept quite quickly.
GP 1
Clinicians felt supported by the study team
The clinicians felt that the study team were supportive and available to answer queries when needed. They also found the reminders about recruitment to the trial in e-mails and newsletters useful:
I think the amount of support from you guys has been really good, compared to other trials that we’ve been involved in.
GP 5
Interviewer: No? OK, and is there anything that we can be doing to help support you as you carry on in the trial, do you think?
GP 9: No, I mean, again, I’d like the updates, not quite weekly, but the fortnightly update e-mails about how the trial is going I think is useful to remind people that you’re quite a long way off getting your total numbers and any effort is still appreciated. I mean really and truly the input from you guys is fantastic.
GP 9
Many parents were willing to take part
Some clinicians reported that the parents approached had been willing for their child to be recruited to the trial and randomised and that they seemed to understand the concept of a placebo:
I think most people are seemingly quite amenable. Once you’ve seen them and examined them, made sure that they’re not clinically ill enough to need treatment immediately, I find the majority are amenable to having a 50/50 shot at antibiotics or the randomisation shot at antibiotics. They usually accept it.
GP 1
Some parents, most parents we’ve been involved with and people put in have been super, really receptive and understand why it needs to be done. Also, they’ve all been happy to sign up, although it takes ages to go through it all, sign up for the interviews as well.
GP 2
However, although many of the clinicians acknowledged that the trial was important and well designed, the majority reported numerous factors that affected recruitment to the trial.
Theme 4: factors affecting recruitment
The recruitment process is too time-consuming
Nearly all clinicians said that they thought there was too much paperwork involved when recruiting parents and their children. Clinicians said that the recruitment process was not ‘easy’ as they needed to ensure that parents understood what would happen to their child if randomised and that it was hard to recruit to the trial during a busy day in general practice. The successful recruiters described adopting a teamwork approach to recruitment, which involved, for example, a research nurse identifying suitable children and consenting them to the study and the GP conducting the clinical examinations. Others said that they had been able to allocate an entire day to ARTIC-PC or create longer appointment slots. However, this was not possible for all clinicians, which had an impact on their ability to recruit:
I recruited someone yesterday or 2 days ago, but actually it is incredibly time-consuming and there’s a large amount of paperwork.
GP 6
But there’s no doubt that the pressures of general practice make it difficult to find the time, so if you do get someone that might be eligible, it’s very difficult to fit that in a busy clinic when you’re seeing, you know, 25, 30 patients in the morning, between, spend 40 minutes or half an hour, which is about the time that I found particularly to do the recruitment process properly, it’s not really practical. Yes, so the logistics has been, it would definitely be a factor, I would say.
GP 7
Opportunistic recruitment is challenging
In addition to the recruitment process being time-consuming, many of the clinicians reported the challenges associated with opportunistic recruitment and gave these as a key reason for their finding recruitment difficult. The challenges included being unable to screen patients in advance, being unable to fund a research nurse to wait all day for eligible patients and being unable to give parents more time to think about whether or not they wanted to take part because a decision had to be made on the day:
Yes, so obviously opportunistic recruitment in primary care is really tricky, and the way that these patients present into the surgery is also difficult, because we can’t predict so well who’s going to present, if you see what I mean.
GP 3
Interviewer: How do you find your role?
Nurse 4: Yes,> well, I’m enjoying it. I think the opportunistic research is a challenge because obviously the doctors only have 10 minutes to see a patient. They don’t really have time to tell the patients about the studies and that’s where the problems arise really.
Nurse 4
Children not eligible to participate
Several clinicians reported that they did not encounter many children who were suitable to take part in the main trial. ‘Suitability’ commonly referred to the child not fitting the eligibility criteria; for example, they were either unwell with a chest infection and ‘needed’ antibiotics immediately; or in opposite circumstances, they were not ill enough, or the practice was not seeing as many children with cough (e.g. the practice did not encounter as many children as adults generally, it was the wrong time of year, other infections were more common):
It’s just a real struggle to find the right patients and that’s certainly something we’ve had this winter interestingly and we haven’t actually had anyone that’s been eligible at this stage.
GP 7
I think it’s been more from our point of view that they weren’t suitable. That the doctor felt actually, yes, no they really have got a chest infection and then they want them to have, erm, immediate antibiotics or, the ones that they didn’t really feel were ill enough to warrant the possibility of antibiotics, erm, which is probably more of them than, well it is I’m sure, m-, much of them.
Nurse 1
Parents were unwilling to take part
Although some clinicians reported that parents were willing to take part in and were accepting of the trial, the majority said that they had experience of parents declining. Clinicians reported parents saying ‘no’ shortly after the trial had been explained or offering reasons for refusing, such as wanting their child to have antibiotics immediately, or in other cases, not wanting their child to receive unnecessary medication or investigations. A few clinicians said that the latter reason was related to the culture they had fostered in their practice of not giving antibiotics unnecessarily and a change in parental attitudes in terms of wanting a clinical assessment and reassurance rather than antibiotics. Some parents did not want to go through a lengthy recruitment process with an ill child:
The other concern I have is the fact that most parents, if they’ve got an ill child, especially if they’re young, they do seem to want to have antibiotics, want them to have antibiotics, and they don’t seem willing to potentially . . . If they take part in the study, they may get the placebo, they don’t really want to risk that, despite explaining that in previous studies antibiotics have not proven to be that effective in children with chest infections.
Nurse 4
I think that’s been a big sea change, so a couple of parents who I’ve talked to about taking part in the actual study have said, ‘I don’t want my child to have antibiotics unless they really need it. So I’m happy to do the observational part, but I don’t want them to have antibiotics unless they need it’.
GP 2
Clinician concerns and phrasing of the trial
Finally, within clinicians’ narratives, some alluded to behaviour of their own that might have influenced the recruitment process. These included descriptions of how the clinician phrased the trial and clinicians who displayed concerns about aspects of the trial (e.g. recruiting children, whether or not to offer the investigations). These views might have dissuaded some parents from taking part or meant that they had not been asked in the first place:
That made it a limitation, I think, on the number of children that were recruited. I think certain partners were anxious about recruiting children compared with adults. Especially younger ones, where you couldn’t really explain it to the child themselves. I think clinicians tended to be more happy to recruit older people, and tended to play safe with younger children.
GP 1
And I suppose if I’m entirely honest just their age, the . . . Are you doing, just watching your own back I suppose is, I-, is part of it. And again, with a big strapping adult, erm, you don’t know. So, I, I think that is probably a, a bit of a reluctance to, to try and persuade a parent to put their child into a research project which, which is something I would think most of our patients don’t, er, very infrequently get asked to put children into a, a research project which involves actually swallowing, er, medication or dummy medication.
Nurse 1
Discussion
This qualitative process evaluation shows that parents’ concerns about the severity of their child’s cough, difficulty in assessing severity at home and previous experiences of RTI are key drivers of consulting in primary care when their child has symptoms of RTI, with a perceived need for a clinical assessment and reassurance. The parents were aware that antibiotics should be prescribed only when ‘necessary’ and had an understanding of AMR, but they also had some misconceptions about identifying bacterial infections and the role played by antibiotics. Clinicians reported a general reduction in expectations and pressure for antibiotics over time, and that parents were generally satisfied with a clinical examination, reassurance and alternatives to immediate antibiotics, such as self-care advice and delayed prescriptions. Clinicians reported that they would prescribe immediate antibiotics only when clinically necessary, which was determined by more than one clinical indicator of RTI, as well as risk factors such as predisposition to or underlying respiratory issues (e.g. asthma). Chest signs were prominent in their decision-making, highlighting the importance of the current main trial results. Clinicians emphasised the importance of ‘clear communication’, especially when implementing ‘no antibiotic’ prescribing strategies, to ensure that parents understood how to manage the symptoms of a RTI at home and recognise signs that would indicate illness deterioration.
Parents and clinicians had positive views of taking part in the trial and its procedures, and many parents were willing for their child to be randomised. Parents’ decisions about participating either in the trial or in the observational study were influenced by their perceptions of the risks related to taking antibiotics unnecessarily, taking a placebo or not receiving immediate antibiotics. Parents’ perceptions of these risks reported across both the parent and the clinician interviews.
Strengths and limitations
The two interview studies provided insights into how perceptions of illness severity and management can affect parent and clinician decision-making regarding the appropriate treatment for a child with an LRTI and whether or not that child should participate in a placebo-controlled trial. However, the sample of each type of participant was small. All of the parents interviewed apart from one were mothers, and so the views of fathers have not been fully captured. Additionally, we did not capture the views of children. The parents interviewed were those who agreed to take part in the trial or observational study, and so we did not explore the views of parents who declined to take part in ARTIC-PC and how these compared. It should also be noted that parents might have expressed less concern about their child not receiving antibiotics because they were enrolled in a research study and would have ready access to care if their child’s illness deteriorated. This attitude may differ in usual consultations, although the clinicians in the study did report a decline in parents’ expectations for antibiotics.
Comparisons with existing literature
A study by Ingram et al. 58 found that parents judged the threat from their child’s cough through a combination of the severity of the illness, whether the cough appeared alongside other symptoms such as fever or a croupy cough, and whether or not the child had experienced a RTI previously. Parents consulted primary care because they did not feel confident caring for a child with a persistent cough or assessing the severity of the illness at home. 58 A more recent study, by Halls et al. ,59 found that although several parents reported at the consultation that their child had a cough, they were more concerned if their child had breathing difficulties and placed great value on obtaining a chest examination in that situation. Our study adds that parents judge the severity of a cough by its sound, in addition to whether or not it appears in combination with other symptoms and the child’s susceptibility to RTIs. Similar to Halls et al. ,59 our findings show that parents consult primary care because they do not feel able to assess illness severity at home, and they place great value on a clinician conducting a chest examination to rule out a chest infection or indications of serious illness. 58,60,61 The persistence of these findings over several years may indicate a need for better information and support for parents that will enable them to assess illness severity at home.
In terms of clinicians’ decisions to prescribe immediate antibiotics for symptoms of RTI, research has shown that perceived clinical need,19,62,65 uncertainty about future illness deterioration and fear of missing something more serious are significant drivers of prescribing antibiotics for children. 19,20,58,61,66,67,70 Additionally, clinicians’ perceptions of parents’ preferences for antibiotics have been found to impact prescribing more than parents’ actual wishes or communicative behaviours during the consultation. 61,62,71 The clinicians in our study displayed confidence both in their decisions not to prescribe antibiotics and their ability to communicate ‘no antibiotic’ prescribing outcomes to parents. They emphasised the importance of ‘clear’ communication during the consultation, especially in terms of safety-netting advice. The provision of safety-netting information is recommended in clinical guidelines,72 but there is evidence that such information might not always be delivered in useful ways that parents can understand. 73–75 Further research on the optimum delivery of safety-netting advice when implementing ‘no antibiotic’ prescribing strategies may, thus, be warranted.
Conclusion
This study found that parents primarily consult primary care services because of particular concerns about interpreting a cough and assessing the severity of the illness, as well as a desire for reassurance. Most parents are willing to accept ‘no antibiotic’ prescribing strategies, including self-care advice and delayed prescriptions, as long as their concerns have been addressed. The clinicians we interviewed displayed confidence in their ability to decide when not to prescribe antibiotics, but other research has shown that fear of illness deterioration and concerns about safety can lead to unnecessary prescribing for children. Our findings indicate a need for better information and support for parents that will enable them to assess illness severity at home and a need for further research on the optimum delivery of safety-netting advice to support the implementation of ‘no antibiotic’ prescribing strategies.
Chapter 6 Microbiology
Introduction and methods
Lower respiratory tract infection in children presenting to general practitioners is common, and children are frequently treated with antibiotics. 11,12,39 Both uncomplicated and complicated LRTI are commonly associated with a range of respiratory viruses and, less commonly, bacteria. 32,39,76–78 Dual bacterial/viral infections may be more significant; among adult patients with LRTI and dual bacterial/viral infections, antibiotics significantly reduced the likelihood of reconsultation for ongoing or progressive illness, but there is no such evidence to date for children. 30,79
Point-of-care tests could potentially be used to target the use of antibiotics to treat bacterial infections. Although in routine primary care clinical practice collecting sputum from very young children is not straightforward, throat swabs have a high yield and could be used to target treatment, and a multiplex PCR analysis of throat swabs would detect bacteria as well as viruses. 32
The level of pathogen load may be as important as detection and may correlate with symptom severity in viral illnesses80 and with severity of pulmonary involvement in both S. pneumoniae81 and H. influenzae. 82 We are aware of no randomised trial to date in children that has explored the impact of pathogen detection or pathogen load on the effectiveness of antibiotics.
We report the microbiological findings from PCR testing of throat swab samples in children included in the ARTIC-PC-trial and when combined with data from the accompanying observational study. We explored whether detection of the presence of bacterial, viral or dual bacterial/viral pathogens, or the pathogen load, was associated with greater benefit from antibiotic treatment.
Methods
For description of participant recruitment, see Chapters 3 and 4.
When parents and children were willing for a sample to be taken, a single sweep dual viral/bacterial throat swab was taken and analysed using multiplex PCR. A throat swab was chosen because it has high yield and was acceptable in previous large primary care cohorts. 32
Sample processing for multiplex polymerase chain reaction
Throat swabs in viral transport medium were stored at –80 °C until they were required for testing. Batches of samples for extraction were allowed to thaw at room temperature and 200 µl of viral transport medium was extracted using the QIAsymphony SP (QIAGEN, Manchester, UK) along with an internal process control containing bacteriophages T4 and MS2. Extraction was carried out using the QIAsymphony DSP Virus/Pathogen Mini Kit (QIAGEN) and the 60 µl elution protocol. Real-time PCR samples were amplified and analysed using a Life Technologies Custom TaqManTM Low Density Array (TLDA) system on the ViiA™ 7 Real-Time PCR System. Reaction mixes (104 µl) were prepared containing 26 µl of TaqMan Fast Virus 1-Step Master Mix (Life Technologies), 58 µl of molecular grade water and 20 µl of nucleic acid extract. Samples were vortex mixed and pulse centrifuged briefly before 100 µl of the reaction mix was loaded into the chamber of the TLDA card. TLDA cards were centrifuged twice at 1200 r.p.m. (336 x g) for 1 minute to load the wells with reaction mix. TLDA cards were sealed twice with the staking device before they were loaded into the ViiA 7 and amplification was initiated (50 °C for 5 minutes and 95 °C for 20 seconds followed by 45 cycles of 95 °C for 1 second and 60 °C for 20 seconds). On completion of the amplification reactions, fluorescent traces were inspected and analysed for sigmoidal curves. Baselines and thresholds were set automatically using the software algorithms or, where necessary, by manual adjustment to avoid background fluorescence noise. The cycle threshold (Ct) of positive samples was recorded as the point at which the fluorescent trace rises above background and passes through the threshold.
Statistical analyses
The initial plan was to use Cox regression for the primary outcome (the duration of symptoms rated moderately bad or worse). However, for some subgroups (particularly the dual bacterial/viral subgroup) the proportional hazards assumption was not met, so quantile regression was used. Linear regression was used for symptom severity, and logistic regression was used for reconsultations with ongoing, new or worsening symptoms. We assessed whether or not antibiotics had an effect among subgroups with bacterial infections where there was a potentially sensitive organism (H. influenzae, M. catarrhalis, S. pneumoniae) or a viral or dual infection. We report both adjusted analyses (adjusting for age, duration of illness, baseline severity and comorbidity) and unadjusted analyses (as microbiological status may be linked to prior duration and severity and so controlling for these could be controlling for microbiological status).
Potential pathogens were categorised according to Ct value as bacterial pathogens, or viruses, that could potentially respond to amoxicillin. When detected, Neisseria meningitidis and coagulase-negative staphylococci were classified as commensal carriage organisms. 83 We assumed that indeterminate Ct values were negative, but we also performed a sensitivity analysis assuming that indeterminate Ct t values were positive. We also looked at the interaction between the mean Ct value (inversely related to bacterial/viral load) and antibiotic group. We performed a Cox regression of symptom duration on antibiotic group, bacterial Ct value and their interaction, adjusting for baseline covariates. We repeated this analysis using mean viral Ct value. We then repeated these analyses for symptom severity and reconsultation outcomes.
These analyses were repeated when the observational data were included to increase power, and propensity scores were used to control for confounding by indication, but, as noted with the effectiveness data, there is still likely to be residual confounding by indication.
Results
Trial results
The flow diagram of samples in both the trial data and the observational data is shown in Figure 6.
FIGURE 6.
Flow diagram of throat swabs.
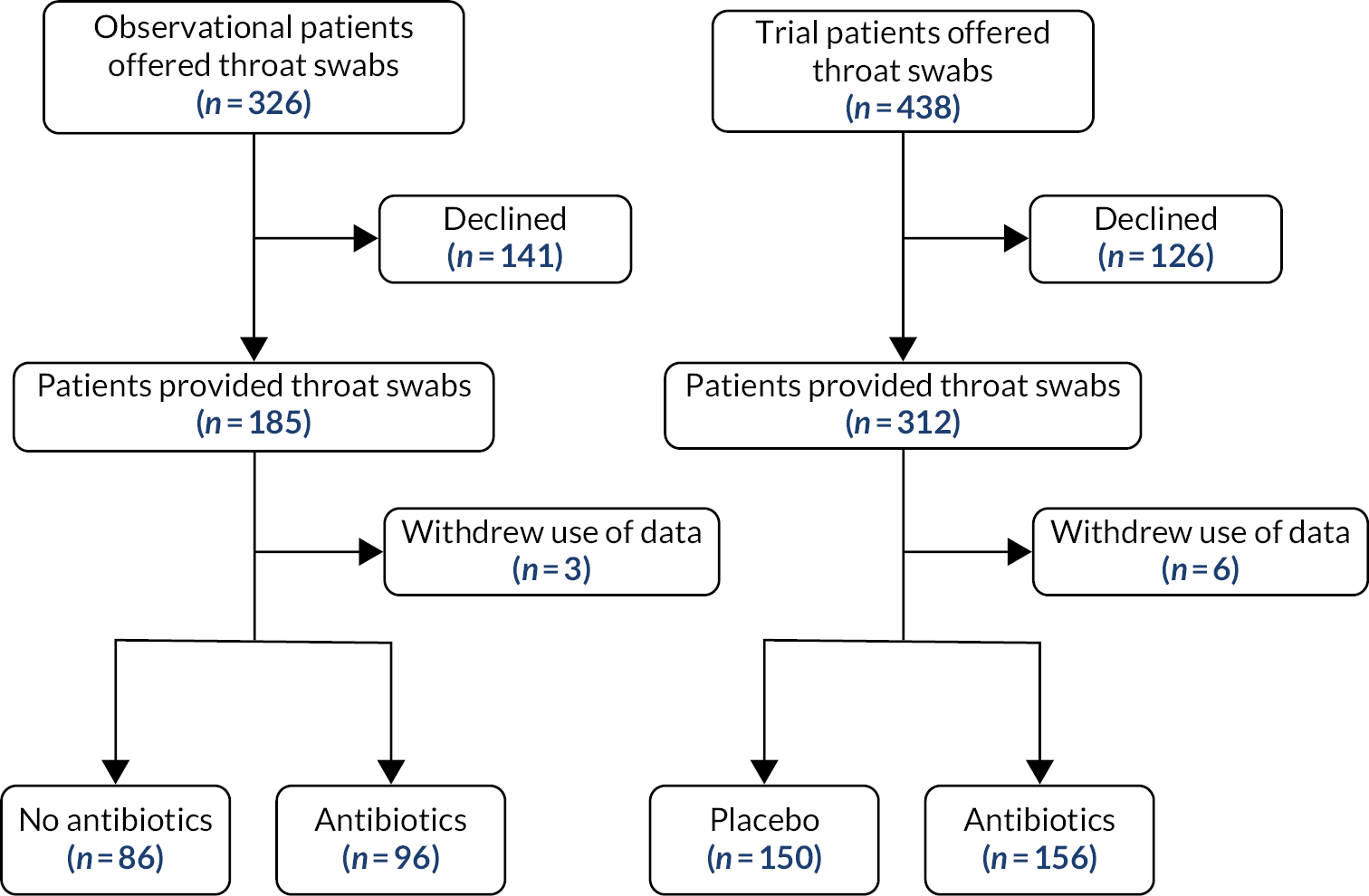
A total of 306 throat swabs were analysed in the RCT. Potential pathogens categorised as bacterial pathogens, viruses or carriage organisms were balanced between the treatment groups (see Table 20; for individual organisms, see Table 30). Bacterial pathogens that were potentially sensitive to amoxicillin (H. influenzae, M. catarrhalis, S. pneumoniae) were found in 51% (76/150) of the trial placebo group and 49% (76/156) of the trial antibiotics group.
| Bacteriaa | Viruses | Carriage organisms | Placebo (N = 150), n (%) | Antibiotics (N = 156), n (%) | Total (N = 306), n (%) |
|---|---|---|---|---|---|
| Yes | No | Yes | 7 (4.7) | 13 (8.3) | 56 (18.3) |
| Yes | No | No | 20 (13.3) | 16 (10.3) | |
| No | Yes | Yes | 12 (8.0) | 12 (7.7) | 64 (20.9) |
| No | Yes | No | 19 (12.7) | 19 (12.2) | |
| Yes | Yes | Yes | 28 (18.7) | 19 (12.2) | 96 (31.4) |
| Yes | Yes | No | 21 (14.0) | 28 (18.0) | |
| No | No | Yes | 16 (10.7) | 17 (10.9) | 90 (29.4) |
| Nob | No | No | 25 (16.7) | 32 (20.5) |
Results assuming that intermediate cycle threshold values were negative
There were no statistically significant interactions of bacterial, viral or dual bacterial/viral subgroups with antibiotic group for any of the outcomes, and the adjusted effect of antibiotics on the primary outcome (duration of symptoms rated moderately bad or worse) for those with potential bacterial pathogens was similar to that for those without, as it was for viral infections (see Table 21). The only subgroup with potentially important differences in duration of illness with antibiotics was the dual bacterial/viral subgroup, where quantile regression suggested that the estimate of the effect of antibiotics in this group was a non-significant reduction in the median duration of illness of 3 days (99% CI –9.9 to 3.9 days).
| Subgroup | n | Placebo | Antibiotics | Unadjusted interaction term (99% CI) | Unadjusted median difference (99% CI) | Adjusteda interaction term (99% CI) | Adjusteda median difference (99% CI) |
|---|---|---|---|---|---|---|---|
| Bacteria b | |||||||
| Yes | 40 | 5 (3.5, 14) | 4 (3.5, 9.5) | 0 (–6.1 to 6.1) | –1 (–12.3 to 10.3) | 0.1 (–7.7 to 7.9) | –0.9 (–10.4 to 8.6) |
| No | 189 | 7 (4, 17) | 5.5 (3,10) | –1 (–4.5 to 2.5) | –1.2 (–4.7 to 2.2) | ||
| Virus | |||||||
| Yes | 52 | 5 (4, 17) | 6 (4, 11) | 3 (–3.3 to 9.3) | 1 (–8.1 to 10.0) | 2.4 (–5.6 to 10.4) | 0.7 (–10.1 to 11.4) |
| No | 177 | 6.5 (4, 16) | 5 (3, 10) | –2 (–5.3 to 1.3) | –1.7 (–5.4 to 2.1) | ||
| Dual | |||||||
| Yes | 74 | 8 (4, 18) | 5 (3, 10) | –3 (–8.9 to 2.9) | –3 (–9.9 to 3.9) | –3.1 (–10.3 to 4.2) | –2.7 (–9.2 to 3.8) |
| No | 155 | 6 (4, 16) | 6 (4, 10) | 0 (–3.6 to 3.6) | –0.2 (–4.2 to 3.9) | ||
The adjusted effect of antibiotics on symptom severity was also similar whether there were bacteria, viruses or dual infections, albeit non-significantly greater when no viruses were detected or with dual infections (see Table 22). The adjusted effect of antibiotics on reducing reconsultation documented a non-significantly greater effect when no viruses were present (see Table 23).
| Subgroup | n | Placebo | Antibiotics | Unadjusted interaction term (99% CI) | Unadjusted mean difference (99% CI) | Adjusted interaction term (99% CI) | Adjusteda mean difference (99% CI) |
|---|---|---|---|---|---|---|---|
| Bacteria b | |||||||
| Yes | 39 | 1.9 (1.1) | 1.8 (1.3) | 0.25 (–0.80 to 1.31) | –0.05 (–1.09 to 0.98) | 0.18 (–0.84 to 1.23) | –0.19 (–1.23 to 0.85) |
| No | 102 | 2.1 (1.2) | 1.8 (1.1) | –0.31 (–0.76 to 0.14) | –0.29 (–0.74 to 0.16) | ||
| Virus | |||||||
| Yes | 48 | 2.2 (1.0) | 2.4 (1.4) | 0.59 (–0.37 to 1.56) | 0.18 (–0.78 to 1.15) | 0.53 (–0.42 to 1.48) | 0.16 (–0.87 to 1.19) |
| No | 167 | 2.1 (1.2) | 1.8 (0.8) | –0.41 (–0.85 to 0.03) | –0.40 (–0.82 to 0.03) | ||
| Dual | |||||||
| Yes | 69 | 2.2 (1.3) | 1.8 (0.8) | –0.29 (–1.16 to 0.59) | –0.45 (–1.15 to 0.25) | –0.30 (–1.16 to 0.56) | –0.40 (–1.10 to 0.31) |
| No | 146 | 2.0 (1.1) | 1.8 (1.2) | –0.17 (–0.67 to 0.34) | –0.19 (–0.69 to 0.30) | ||
| Subgroup | N | Placebo | Antibiotics | Unadjusted interaction term (99% CI) | Unadjusted RR (99% CI) | Interaction term (99% CI) | Adjusteda RR (99% CI) |
|---|---|---|---|---|---|---|---|
| Dual | |||||||
| Bacteria b | |||||||
| Yes | 51 | 6 (24.0) | 8 (30.8) | 1.89 (0.53 to 6.77) | 1.28 (0.39 to 4.21) | 1.64 (0.47 to 5.77) | 1.01 (0.30 to 3.37) |
| No | 238 | 51 (42.5) | 34 (28.8) | 0.68 (0.43 to 1.08) | 0.74 (0.47 to 1.17) | ||
| Virus | |||||||
| Yes | 60 | 10 (31.3) | 10 (35.7) | 1.72 (0.60 to 4.95) | 1.14 (0.45 to 2.92) | 1.57 (0.47 to 4.07) | 0.88 (0.43 to 1.53) |
| No | 229 | 47 (41.6) | 32 (27.6) | 0.66 (0.41 to 1.07) | 0.65 (0.27 to 1.24) | ||
| Yes | 90 | 25 (53.2) | 17 (39.5) | 0.98 (0.42 to 2.26) | 0.74 (0.41 to 1.35) | 0.99 (0.55 to 1.22) | 0.83 (0.38 to 1.34) |
| No | 199 | 32 (32.7) | 25 (24.8) | 0.76 (0.42 to 1.36) | 0.79 (0.40 to 1.34) | ||
The regression of symptom duration on antibiotic group, bacterial Ct value and their interaction, adjusting for baseline covariates, is shown in Table 24. There was no evidence that antibiotics had a greater effect on symptom resolution when high bacterial loads were present; if anything, a non-significant trend was seen for the impact of antibiotics to increase as bacterial load decreased, i.e. as Ct value increased (HR 1.05, 95% CI 0.96 to 1.13), and no evidence of an interaction with virus Ct value. There was also no clear evidence of treatment benefit from the interaction with Ct value for symptom severity or reconsultations for either bacteria or viruses (see Table 24, and shown graphically for symptom severity in Figures 7 and 8).
| Bacterial concentration (Ct valuea) | Viral concentration (Ct valuea) | |||||
|---|---|---|---|---|---|---|
| n | Unadjusted interaction term | Adjustedb interaction term | n | Unadjusted interaction term | Adjustedb interaction term | |
| Duration, HR (95% CI) | 146 | 1.05 (0.96 to 1.13) | 1.06 (0.97 to 1.15) | 151 | 1.04 (0.97 to 1.13) | 1.02 (0.94 to 1.10) |
| Severity, mean difference (95% CI) | 141 | –0.07 (–0.20 to 0.05) | –0.07 (–0.20 to 0.05) | 141 | 0.01 (–0.11 to 0.13) | –0.01 (–0.13 to 0.11) |
| Reconsultation, OR (95% CI) | 181 | 0.93 (0.75 to 1.16) | 0.90 (0.72 to 1.13) | 184 | 1.01 (0.84 to 1.22) | 1.01 (0.84 to 1.22) |
FIGURE 7.
Symptom severity at days 2–4 vs. bacterial Ct value.

FIGURE 8.
Symptom severity at days 2–4 vs. viral Ct value.

Results assuming that intermediate cycle threshold values are positive
Potential bacterial, viral pathogens and carriage organisms were balanced between the trial groups (see Table 25).
| Bacterial pathogens | Viruses | Carriage organisms | Placebo (N = 150), n (%) | Antibiotics (N = 156), n (%) | Total (N = 306), n (%) |
|---|---|---|---|---|---|
| Yes | No | Yes | 16 (10.7) | 21 (13.5) | 60 (19.6) |
| Yes | No | No | 12 (8.0) | 11 (7.1) | |
| No | Yes | Yes | 21 (14.0) | 15 (9.6) | 59 (19.3) |
| No | Yes | No | 11 (7.3) | 12 (7.7) | |
| Yes | Yes | Yes | 50 (33.3) | 39 (25.0) | 133 (43.5) |
| Yes | Yes | No | 19 (12.7) | 25 (16.0) | |
| No | No | Yes | 12 (8.0) | 19 (12.2) | 54 (17.7) |
| No | No | No | 9 (6.0) | 14 (9.0) |
There were no statistically significant interactions between any of the pathogen subgroups and antibiotic group for any of the outcomes, and no consistent trends. There was no evidence of an increased effect of antibiotics on the duration and severity of symptoms or on reconsultations for those with potential bacterial pathogens (see Tables 26–28). When viruses were not present, there was a trend for increased impact on symptom severity, reconsultation and duration of symptoms. For dual infections, there was a trend for an impact on symptom severity and duration but not on reconsultation.
| Subgroup | n | Placebo | Antibiotics | Unadjusted interaction term (99% CI) | Unadjusted median difference (99% CI) | Adjustedb interaction term (99% CI) | Adjustedb median difference (99% CI) |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Yes | 45 | 5 (4, 17) | 5 (4, 10) | 2 (–5.0 to 9.0) | 0 (–12.0 to 12.0) | 0.2 (–8.1 to 8.4) | 0.1 (–8.6 to 8.7) |
| No | 185 | 6.5 (4, 16) | 5 (3, 10) | –2 (–5.0 to 1.0) | –0.4 (–4.0 to 3.2) | ||
| Virus | |||||||
| Yes | 46 | 6 (4, 13.5) | 6 (4, 11) | 1 (–5.6 to 7.6) | 0 (–7.4 to 7.4) | 2.3 (–5.9 to 10.5) | 0.7 (–8.0 to 9.4) |
| No | 183 | 6 (4, 18) | 5 (3, 10) | –1 (–4.7 to 2.7) | –1.5 (–5.6 to 2.7) | ||
| Dual | |||||||
| Yes | 101 | 6.5 (4, 20.5) | 5 (3, 9) | –2 (–7.6 to 3.6) | –2 (–7.5 to 3.5) | –1.8 (–8.1 to 4.6) | –1.2 (–6.1 to 3.6) |
| No | 127 | 6 (4, 14) | 6 (4, 10.5) | 0 (–3.9 to 3.9) | –0.2 (–4.7 to 4.3) | ||
| Subgroup | n | Placebo | Antibiotics | Unadjusted interaction term (99% CI) | Unadjusted mean difference (99% CI) | Adjusteda interaction term (99% CI) | Adjusteda mean difference (99% CI) |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Yes | 42 | 1.8 (1.2) | 1.6 (1.0) | 0.06 (–1.00 to 1.09) | –0.21 (–1.13 to 0.72) | 0.10 (–0.93 to 1.12) | –0.19 (–1.18 to 0.80) |
| No | 173 | 2.1 (1.2) | 1.9 (1.1) | –0.27 (–0.73 to 0.19) | –0.28 (–0.74 to 0.18) | ||
| Virus | |||||||
| Yes | 43 | 1.9 (0.8) | 2.6 (1.4) | 1.15 (0.15 to 2.13) | 0.66 (–0.26 to 1.59) | 1.12 (0.14 to 2.09) | 0.66 (–0.29 to 1.60) |
| No | 172 | 2.1 (1.3) | 1.7 (1.0) | –0.48 (–0.93 to –0.04) | –0.48 (–0.91 to –0.04) | ||
| Dual | |||||||
| Yes | 95 | 2.2 (1.2) | 1.8 (1.0) | –0.32 (–1.14 to 0.50) | –0.43 (–1.04 to 0.17) | –0.36 (–1.17 to 0.46) | –0.46 (–1.08 to 0.17) |
| No | 120 | 2.0 (1.1) | 1.9 (1.2) | –0.11 (–0.67 to 0.45) | –0.12 (–0.66 to 0.43) | ||
| Subgroup | n | Placebo | Antibiotics | Unadjusted interaction term (99% CI) | Unadjusted RR (99% CI) | Interaction term (99% CI) | Adjusteda RR (99% CI) |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Yes | 55 | 9 (34.6) | 7 (24.1) | 0.92 (0.28 to 3.04) | 0.70 (0.23 to 2.09) | 0.76 (0.23 to 2.48) | 0.41 (0.11 to 1.56) |
| No | 234 | 48 (40.3) | 35 (30.4) | 0.75 (0.47 to 1.20) | 0.84 (0.53 to 1.33) | ||
| Virus | |||||||
| Yes | 55 | 10 (32.2) | 8 (33.3) | 1.50 (0.50 to 4.55) | 1.03 (0.38 to 2.81) | 1.14 (0.59 to 1.38) | 0.82 (0.19 to 2.07) |
| No | 234 | 47 (41.2) | 34 (28.3) | 0.69 (0.43 to 1.10) | 0.76 (0.42 to 1.19) | ||
| Dual | |||||||
| Yes | 126 | 30 (44.8) | 20 (33.9) | 0.96 (0.26 to 3.51) | 0.63 (0.24 to 1.63) | 1.02 (0.60 to 1.25) | 0.85 (0.41 to 1.39) |
| No | 163 | 27 (34.6) | 22 (25.9) | 0.66 (0.27 to 1.60) | 0.73 (0.34 to 1.33) | ||
Analyses including the observational data
A total of 488 throat swabs were analysed, with a further 182 in the observational cohort. Both in the observational cohort and overall these potential pathogens were fairly balanced between the antibiotics and no-antibiotics groups in the observational, combined and trial samples (see Tables 29 and 30).
| Bacteria | Viruses | Carriage organisms | Observational, n (%) | Combined, n (%) | |||
|---|---|---|---|---|---|---|---|
| No antibiotics (N = 86) | Antibiotics (N = 96) | No antibiotics (N = 236) | Antibiotics (N = 252) | Total (N = 488) | |||
| Yes | No | Yes | 5 (7.0) | 2 (2.1) | 12 (5.1) | 15 (6.0) | 91 (18.7) |
| Yes | No | No | 15 (17.4) | 13 (13.5) | 35 (14.8) | 29 (11.5) | |
| No | Yes | Yes | 7 (8.1) | 7 (7.3) | 19 (8.1) | 19 (7.5) | 102 (20.9) |
| No | Yes | No | 10 (11.6) | 16 (16.7) | 29 (12.3) | 35 (13.9) | |
| Yes | Yes | Yes | 14 (16.3) | 13 (13.5) | 42 (17.8) | 32 (12.7) | 165 (33.8) |
| Yes | Yes | No | 17 (19.8) | 25 (26.0) | 38 (16.1) | 53 (21.0) | |
| No | No | Yes | 5 (7.0) | 3 (3.1) | 21 (8.9) | 20 (7.9) | 130 (26.6) |
| No | No | No | 15 (17.4) | 17 (17.7) | 40 (17.0) | 49 (19.4) | |
| Organism | Result | Treatment group | Total | ||||
|---|---|---|---|---|---|---|---|
| Placebo | Antibiotics | ||||||
| n | % | n | % | n | % | ||
| Bacteria | |||||||
| Bordetella pertussis | Negative | 148 | 98.7 | 155 | 99.4 | 303 | 99.0 |
| Positive | 2 | 1.3 | 1 | 0.6 | 3 | 1.0 | |
| H. influenzae | Negative | 81 | 54.0 | 89 | 57.1 | 170 | 55.6 |
| Positive | 61 | 40.7 | 61 | 39.1 | 122 | 39.9 | |
| Indeterminate | 8 | 5.3 | 6 | 3.8 | 14 | 4.6 | |
| S. pneumoniae | Negative | 106 | 70.7 | 111 | 71.2 | 217 | 70.9 |
| Positive | 25 | 16.7 | 26 | 16.7 | 51 | 16.7 | |
| Indeterminate | 19 | 12.7 | 19 | 12.2 | 38 | 12.4 | |
| Mycoplasma pneumoniae | Negative | 147 | 98.0 | 155 | 99.4 | 302 | 98.7 |
| Positive | 3 | 2.0 | 0 | 0.0 | 3 | 1.0 | |
| Indeterminate | 0 | 0.0 | 1 | 0.6 | 1 | 0.3 | |
| Chlamydia pneumoniae | Negative | 149 | 99.3 | 156 | 100.0 | 305 | 99.7 |
| Positive | 1 | 0.7 | 0 | 0.0 | 1 | 0.3 | |
| Fusobacterium necrophorum | Negative | 145 | 96.7 | 153 | 98.1 | 298 | 97.4 |
| Positive | 5 | 3.3 | 3 | 1.9 | 8 | 2.6 | |
| Streptococcus pyogenes | Negative | 135 | 90.0 | 142 | 91.0 | 277 | 90.5 |
| Positive | 11 | 7.3 | 9 | 5.8 | 20 | 6.5 | |
| Indeterminate | 4 | 2.7 | 5 | 3.2 | 9 | 2.9 | |
| Moraxella catarrhalis | Negative | 107 | 71.3 | 108 | 69.2 | 215 | 70.3 |
| Positive | 28 | 18.7 | 37 | 23.7 | 65 | 21.2 | |
| Indeterminate | 15 | 10.0 | 11 | 7.1 | 26 | 8.5 | |
| Bacteria (any) total | Negative | 49 | 32.7 | 52 | 33.3 | 101 | 33.0 |
| Positive | 82 | 54.7 | 81 | 51.9 | 163 | 53.3 | |
| Indeterminate | 19 | 12.7 | 23 | 14.7 | 42 | 13.7 | |
| Viruses | |||||||
| Enterovirus | Negative | 139 | 92.7 | 148 | 94.9 | 287 | 93.8 |
| Positive | 5 | 3.3 | 2 | 1.3 | 7 | 2.3 | |
| Indeterminate | 6 | 4.0 | 6 | 3.8 | 12 | 3.9 | |
| Rhinovirus | Negative | 103 | 68.7 | 126 | 80.8 | 229 | 74.8 |
| Positive | 28 | 18.7 | 19 | 12.2 | 47 | 15.4 | |
| Indeterminate | 19 | 12.7 | 11 | 7.1 | 30 | 9.8 | |
| HMPV | Negative | 139 | 92.7 | 141 | 90.4 | 280 | 91.5 |
| Positive | 9 | 6.0 | 15 | 9.6 | 24 | 7.8 | |
| Indeterminate | 2 | 1.3 | 0 | 0.0 | 2 | 0.7 | |
| Influenza virus | Negative | 137 | 91.3 | 136 | 87.2 | 273 | 89.2 |
| Positive | 8 | 5.3 | 14 | 9.0 | 22 | 7.2 | |
| Indeterminate | 5 | 3.3 | 6 | 3.8 | 11 | 3.6 | |
| Parainfluenza virus | Negative | 131 | 87.3 | 147 | 94.2 | 278 | 90.8 |
| Positive | 15 | 10.0 | 8 | 5.1 | 23 | 7.5 | |
| Indeterminate | 4 | 2.7 | 1 | 0.6 | 5 | 1.6 | |
| Coronavirus | Negative | 143 | 95.3 | 148 | 94.9 | 291 | 95.1 |
| Positive | 6 | 4.0 | 6 | 3.8 | 12 | 3.9 | |
| Indeterminate | 1 | 0.7 | 2 | 1.3 | 3 | 1.0 | |
| RSV | Negative | 131 | 87.3 | 135 | 86.5 | 266 | 86.9 |
| Positive | 19 | 12.7 | 19 | 12.2 | 38 | 12.4 | |
| Indeterminate | 0 | 0.0 | 2 | 1.3 | 2 | 0.7 | |
| Adenovirus | Negative | 141 | 94.0 | 152 | 97.4 | 293 | 95.8 |
| Positive | 9 | 6.0 | 3 | 1.9 | 12 | 3.9 | |
| Indeterminate | 0 | 0.0 | 1 | 0.6 | 1 | 0.3 | |
| Bocavirus | Negative | 142 | 94.7 | 149 | 95.5 | 291 | 95.1 |
| Positive | 5 | 3.3 | 5 | 3.2 | 10 | 3.3 | |
| Indeterminate | 3 | 2.0 | 2 | 1.3 | 5 | 1.6 | |
| Parechovirus | Negative | 146 | 97.3 | 155 | 99.4 | 301 | 98.4 |
| Positive | 3 | 2.0 | 1 | 0.6 | 4 | 1.3 | |
| Indeterminate | 1 | 0.7 | 0 | 0.0 | 1 | 0.3 | |
| Viruses (any) total | Negative | 49 | 32.7 | 65 | 41.7 | 114 | 37.3 |
| Positive | 82 | 54.7 | 78 | 50.0 | 160 | 52.3 | |
| Indeterminate | 19 | 12.7 | 13 | 8.3 | 32 | 10.5 | |
| Carriage organisms | |||||||
| Coagulase-negative staphylococci | Negative | 69 | 46.0 | 78 | 50.0 | 147 | 48.0 |
| Positive | 47 | 31.3 | 47 | 30.1 | 94 | 30.7 | |
| Indeterminate | 34 | 22.7 | 31 | 19.9 | 65 | 21.2 | |
| S. aureus Nuc | Negative | 130 | 86.7 | 143 | 91.7 | 273 | 89.2 |
| Positive | 19 | 12.7 | 13 | 8.3 | 32 | 10.5 | |
| Indeterminate | 1 | 0.7 | 0 | 0.0 | 1 | 0.3 | |
| PVL-Staphylococcus aureus | Negative | 124 | 82.7 | 134 | 85.9 | 258 | 84.3 |
| Positive | 1 | 0.7 | 3 | 1.9 | 4 | 1.3 | |
| Indeterminate | 25 | 16.7 | 19 | 12.2 | 44 | 14.4 | |
| N. meningiditis | Negative | 150 | 100.0 | 154 | 98.7 | 304 | 99.3 |
| Positive | 0 | 0.0 | 1 | 0.6 | 1 | 0.3 | |
| Indeterminate | 0 | 0.0 | 1 | 0.6 | 1 | 0.3 | |
| mecA resistance | Negative | 144 | 96.0 | 153 | 98.1 | 297 | 97.1 |
| Positive | 6 | 4.0 | 2 | 1.3 | 8 | 2.6 | |
| Indeterminate | 0 | 0.0 | 1 | 0.6 | 1 | 0.3 | |
There were no statistically significant interactions of any of the pathogen subgroups with antibiotics for any of the outcomes, no evidence of important clinical benefits from antibiotics in subgroups (see Tables 31–34) and no impact of Ct values (see Figures 9 and 10).
| Subgroup | n | No antibiotics | Antibiotics | Unadjusteda interaction term (99% CI) | Unadjusteda median difference (99% CI) | Adjustedb interaction term (99% CI) | Adjustedb median difference (99% CI) |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Yes | 70 | 5 (3, 14) | 4 (3, 8) | 1 (–3.0 to 5.0) | –1 (–5.6 to 3.6) | 0.7 (–4.2 to 5.5) | –1.3 (–7.5 to 4.9) |
| No | 302 | 7 (4, 14) | 5 (3, 9) | –2 (–3.9 to –0.1) | –1.5 (–3.5 to 0.5) | ||
| Virus | |||||||
| Yes | 70 | 6 (4, 13.5) | 5 (3, 8) | 0 (–3.8 to 3.8) | –1 (–5.5 to 3.5) | 0.08 (–4.5 to 4.6) | –0.5 (–5.4 to 4.4) |
| No | 292 | 6 (4, 14) | 5 (3, 9) | –1 (–3.1 to 1.1) | –1.5 (–3.8 to 0.8) | ||
| Dual | |||||||
| Yes | 125 | 6 (4, 13) | 5 (3, 10) | 0 (–3.2 to 3.2) | –1 (–4.7 to 2.7) | –0.7 (–4.5 to 3.2) | –1.8 (–6.1 to 2.5) |
| No | 247 | 6 (4, 14) | 5 (3, 8) | –1 (–2.8 to 0.8) | –1.1 (–3.3 to 1.1) | ||
| Subgroup | n | Placebo | Antibiotics | Unadjusteda interaction term (99% CI) | Unadjusteda mean difference (99% CI) | Adjustedb interaction term (99% CI) | Adjustedb mean difference (99% CI) |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Yes | 69 | 1.5 (1.1) | 1.6 (1.1) | 0.18 (–0.65 to 1.00) | –0.01 (–0.93 to 0.92) | 0.05 (–0.77 to 0.87) | –0.12 (–0.99 to 0.76) |
| No | 283 | 2.0 (1.1) | 1.9 (1.1) | –0.28 (–0.66 to 0.10) | –0.21 (–0.59 to 0.17) | ||
| Virus | |||||||
| Yes | 75 | 2.2 (1.0) | 2.2 (1.4) | –0.04 (–0.85 to 0.77) | –0.29 (–1.11 to 0.54) | –0.06 (–0.87 to 0.74) | –0.16 (–1.02 to 0.70) |
| No | 277 | 1.8 (1.1) | 1.7 (1.0) | –0.20 (–0.58 to 0.18) | –0.17 (–0.54 to 0.21) | ||
| Dual | |||||||
| Yes | 117 | 2.0 (1.2) | 2.0 (1.1) | 0.13 (–0.56 to 0.81) | –0.06 (–0.72 to 0.61) | 0.19 (–0.49 to 0.87) | 0.01 (–0.68 to 0.69) |
| No | 217 | 1.8 (1.1) | 1.8 (1.2) | –0.29 (–0.69 to 0.12) | –0.28 (–0.67 to 0.12) | ||
| Subgroup | n | Placebo | Antibiotics | Unadjusteda interaction term (99% CI) | Unadjusteda OR (99% CI) | Adjustedb interaction term (99% CI) | Adjustedb OR (99% CI) |
|---|---|---|---|---|---|---|---|
| Bacteria | |||||||
| Yes | 75 | 9 (23.7) | 14 (37.8) | 3.0 (0.7 to 13.6) | 2.1 (0.3 to 14.2) | 2.8 (0.6 to 13.0) | 1.6 (0.2 to 13.2) |
| No | 370 | 71 (38.6) | 55 (29.6) | 0.6 (0.3 to 1.2) | 0.7 (0.3 to 1.3) | ||
| Virus | |||||||
| Yes | 93 | 19 (40.4) | 17 (37.0) | 1.0 (0.3 to 3.7) | 0.9 (0.2 to 3.8) | 0.9 (0.2 to 3.5) | 0.9 (0.2 to 3.9) |
| No | 352 | 61 (34.9) | 52 (29.4) | 0.7 (0.3 to 1.3) | 0.7 (0.4 to 1.4) | ||
| Dual | |||||||
| Yes | 152 | 32 (41.6) | 25 (33.3) | 0.7 (0.2 to 2.2) | 0.6 (0.2 to 1.6) | 0.8 (0.2 to 2.5) | 0.6 (0.20 to 1.9) |
| No | 293 | 48 (33.1) | 44 (29.7) | 0.8 (0.4 to 1.6) | 0.8 (0.4 to 1.7) | ||
| Bacteria Ct value | Virus Ct value | |||||
|---|---|---|---|---|---|---|
| n | Unadjusteda interaction term | Adjustedb interaction term | n | Unadjusteda interaction term | Adjustedb interaction term | |
| Duration, HR (95% CI) | 221 | 1.03 (0.96 to 1.11) | 1.04 (0.96 to 1.12) | 229 | 1.03 (0.96 to 1.10) | 1.01 (0.94 to 1.08) |
| Severity, mean difference (95% CI) | 211 | –0.05 (–0.17 to 0.07) | –0.06 (–0.17 to 0.06) | 216 | 0.02 (–0.08 to 0.11) | 0.01 (–0.06 to 0.09) |
| Reconsultation, OR (95% CI) | 265 | 1.02 (0.84 to 1.22) | 1.00 (0.83 to 1.22) | 276 | 1.03 (0.89 to 1.20) | 1.04 (0.92 to 1.17) |
FIGURE 9.
Symptom severity at days 2–4 vs. bacterial Ct value in combined trial and observational data.
FIGURE 10.
Symptom severity at days 2–4 vs. viral Ct value in combined trial and observational data.
Discussion
The current study is one of the few trials of antibiotics for LRTI to document the impact of microbiological diagnosis on outcomes. Both bacteria and viral pathogens were frequently detected, but for both symptomatic outcomes (duration or severity of symptoms) and reconsultations there were no clearly demonstrated differences in the effectiveness of antibiotics according to microbiological subgroups.
Limitations
The analyses were not powered specifically to assess the interaction of microbiological diagnosis with outcome, and the power was reduced by some children or their parents not wanting swabs to be taken. Nevertheless, the estimates largely indicate no clear evidence of differential impact of antibiotics on bacterial or viral subgroups. In cases in which microbiological diagnosis may demonstrate an impact (the impact of antibiotics where viral pathogens were absent in the combined data set on symptoms duration), given the number of secondary analyses, a chance effect is quite possible, and the estimate was of only borderline significance. The detection of Chlamydophila pneumoniae, Bordetella pertussis and Mycoplasma pneumoniae implies a role of those micro-organisms in the disease, as they are seldom carried asymptomatically. However, pathobionts such as S. pneumoniae, M. catarrhalis and H. influenzae may also be detected by PCR testing, but because these organisms are frequently detected in throat swabs from normal children,84–86 their detection does not necessarily imply a role in the disease process, and the presence of asymptomatic carriage will dilute the apparent effectiveness of antibiotics in bacterial subgroups. Similarly, we did not assess the antibiotic resistance of individual strains, and the presence of resistant strains will also dilute the apparent effectiveness of antibiotics, but most strains are susceptible to amoxicillin in community samples of children. 84–86 Similarly, the study is not powered to detect the impact of antibiotics on infection with particular viruses such as influenza. 87 It is also possible that lower airway samples would be better but are not possible in routine primary care.
Relationship to other studies
The trial data set shows that the prevalence of both potential viral and bacterial pathogens using similar sampling methods is similar to that in very large observational data sets of children presenting in primary care,32 suggesting that the impact of microbiological diagnosis should be generalisable. As in a previous study, we have not shown that microbiological diagnosis improves the estimation of prognosis in children79 and we found a similar finding to studies in adults of no impact on symptom duration. 80
For the dual infection subgroup, we found no clear impact of dual infections on reconsultations, unlike in the trial in adults,30 but the current study had much less power than the adult trial. We did find a potentially important, albeit non-significant, 3-day reduction in the median duration of symptoms with antibiotics for the dual infection subgroup, but the analysis was underpowered and, given the exploratory nature of subgroup analyses, should be viewed with some caution. Assuming that the effect in this subgroup might be real, three children would have to be assessed to identify one child who might benefit, and the cost-effectiveness of such an approach would need to be demonstrated.
Conclusion
Both viral and bacterial pathogens are frequently detected among children presenting with uncomplicated LRTI in primary care, but there is no clear evidence that the impact of antibiotics is likely to be different according to the presence of bacteria or viruses. This calls into question if microbial diagnosis using point-of-care tests to detect pathogens in the upper airway should be used for children presenting with uncomplicated LRTI in primary care and suggests that, prior to introduction, microbiological point-of-care tests should be subject to rigorous trials of effectiveness.
Chapter 7 Health economic analysis
Aim
The aim was to estimate the incremental cost per symptom-free day and per QALY of the intervention compared with the control.
Methods and description of available data
Two perspectives were adopted: one from the NHS and the other quasi-societal based on including privately purchased remedies and days off work. Only respiratory-related service use was included. The base case was from the perspective of the NHS within a time frame of 4 weeks. National-level unit costs were employed to express resource use data in monetary terms. Uncertainty was explored using CEACs. QALYs were based on the EQ-5D-Y, but VAS scores (part of the EQ-5D tool) are also reported. Different results are presented for children aged < 5 years and children aged ≥ 5 years.
Costs
The service use data were collected from the patient records held by each practice and from patient self-reported questionnaires. The main resource use included RTI-related primary care consultations, prescriptions, referral and hospitalisation during the period of the study.
Four main types of NHS resource use related to RTI were recorded based on reviews of patients’ notes in primary care at the end of the trial:
-
primary care consultations
-
drugs prescribed in those primary care consultations
-
referrals from primary care to A&E, outpatients and out-of-hours service, and use of NHS Direct
-
hospital admissions (both day-case and inpatient episodes).
In addition, data were collected from parent’s diaries about ‘remedies’ purchased, time taken off work or college or having to pay for additional care owing to the child’s illness.
Units of resources used were costed using national unit costs based mainly on those provided by the Personal Social Services Research Unit (PSSRU)88 and supplemented by the National Drugs Tariff89 and National Reference Costs90 for hospital services. Remedies bought over the counter were priced using Boots online (www.boots.com). Time off work or care was costed based on the national minimum wage for adults. The base year was 2019. Discounting was not necessary as the trial included patients for only 4 weeks.
Intervention cost
In the trial, both amoxicillin and placebo were specially prepared in identical formats. This meant that the cost of the intervention drug did not reflect its cost in normal practice, in which it is priced as a prescription-only drug. Among the amoxicillin variants available, the NHS-funded drug most similar to the intervention drug was 100 ml oral suspension of amoxicillin in 20-dose vials, which is priced to the NHS at £1.75. A dispensing cost of £1.26 was added, giving a cost of £3.01 per patient in the intervention group.
The placebo cost was set at zero as this was specific to the study. The cost of the initial consultation was also set at zero as it was specific to the study and the same in both groups.
Primary care consultations
A total of 129 patients in the trial had a primary care consultation in the 4 weeks following randomisation. Consultations were classified by who they were with (GP, practice nurse) and how they took place (face to face or over the telephone). Most patients saw a GP and most consultations were face to face. A few patients (< 5) who did not have a record of who saw them were assumed to have been seen by a GP and costed accordingly. All were costed using PSSRU 2019/20 financial year values (see Table 35).
The PSSRU cost of a GP was for an average consultation lasting just over 9 minutes. The only cost available from PSSRU for nurse consultations was per hour. The duration of nurse consultations was assumed to be the same as that of consultations with GPs, giving an estimated cost of £5.99 per consultation. The cost of a telephone consultation with a GP is given by PSSRU as £8, and it was assumed that a telephone consultation with a nurse cost the same. The alternative of pro-rating the nurse cost to that of the GP would have led to an unreasonably low unit cost. As the number of telephone consultations with nurses was very small, this assumption makes minimal difference to the total costs.
Drugs prescribed
A total of 51 antibiotic drug prescriptions were recorded (see Table 36) in primary care consultations, classified as penicillin, macrolides and other antibiotics. These were costed using the National Drugs Tariff for January 2020. 89
| Antibiotic | Cost (£) |
|---|---|
| Penicillin | 2.91 |
| Other antibiotic | By name |
| Macrollides | 3.94 |
The unit cost of penicillin was £1.75, based on a 125 mg/5 ml oral solution of phenoxymethylpenicillin.
The unit cost of macrolides was £3.94, based on the average of the three drugs and so defined based on the same oral solution. The cost of other antibiotics was that of the named drug, with the dose assumed to be that of the most commonly prescribed.
Referrals
The note review provided data on 24 patients referred by primary care to one of the following services: A&E, out of hours, NHS Direct, and outpatients and other.
Of the two under ‘other’, based on the text, one was allocated to outpatients (‘hospital’ was recorded) and one had zero cost attributed (‘no referral’).
These were costed using the unit costs in Table 37, taken from the NHS Reference Costs. 90
| Referral | Unit cost (£) |
|---|---|
| A&E | 133 |
| Out of hours | 74.05 |
| NHS Direct | 8.13 |
| Outpatients | 216 |
Admissions
Data collected included hospital admissions by reason. These are shown in Table 38, which indicates that six of the seven were directly related to respiratory problems and the seventh, ‘gastrointestinal’, was possibly related to respiratory problems. The dates of admission and discharge were used to distinguish between inpatient and day cases.
| Reason(s) | Date seen | Date discharged | Admission/day case | |
|---|---|---|---|---|
| 04237 | Bronchiolitis | 2 March 2019 | 4 March 2019 | Admission |
| 04306 | Bronchiolitis, increased work of breathing requiring optiflow | 2 March 2019 | 4 March 2019 | Admission |
| 04312 | Cough getting worse, vomiting overnight, decreased feeding | 20 February 2019 | 20 February 2019 | Day case |
| 05528 | Gastrointestinal | 14 December 2019 | 14 December 2019 | Day case |
| 06075 | Temperature 38.5 °C, rattle in throat and chest, breathing in, sucking in | 16 February 2020 | 16 February 2020 | Day case |
| 01188 | Viral-induced wheeze | 13 December 2016 | 14 December 2016 | Admission |
| 01282 | VIW, croup, shortness of breath | 10 November 2017 | 11 November 2017 | Admission |
These cases were costed using the NHS National Tariff (https://improvement.nhs.uk/documents/6486/2_-_National_schedule_of_NHS_costs_V2.xlsx), which distinguishes between elective and non-elective and by Healthcare Resource Group (HRG). If referral was indicated in primary care notes, then the elective cost was used; otherwise, the non-elective was employed. The four admissions were assumed to fall under a paediatric respiratory HRG.
The HRGs used (see Table 39) were as follows. PD15D Paediatric Acute Bronchiolitis (with CC Score 0, that is with zero complications) was assigned to those two admissions that mentioned bronchiolitis. PD12C Paediatric Lower Respiratory Tract Disorders without Acute Bronchiolitis (with CC Score 0) was assigned to those with wheezing or cough as well as those not fitting readily into the other two HRGs below. PD14F Paediatric, Asthma or Wheezing (with CC Score 0) was assigned to the patient admitted with gastrointestinal, as the most appropriate respiratory related condition paediatric HRG. In a sensitivity analysis this patient was omitted.
| HRG code | Description |
|---|---|
| PD15D | Paediatric Acute Bronchiolitis (with CC Score 0) |
| PD14F | Paediatric Lower Respiratory Tract Disorders without Acute Bronchiolitis (with CC Score 0) |
| PD12C | Paediatric, Asthma or Wheezing (with CC Score 0) |
The hospital unit costs ranged from £396 to £562. The relatively small number of patients and cost differences involved imply that the allocation to a particular HRG has little effect on the overall costing.
Remedies
Thirty-two participants reported buying over-the-counter remedies for their children. Most of these remedies were low-priced items such as Calpol, but a few were more expensive; notably, one parent reported the nightly use of a humidifier. Over-the-counter remedy costs were based on information from Boots online, the largest UK retail pharmacy. A small number of prescription drugs recorded under remedies were costed using the National Drugs Tariff. 89
Time off work
Both time off work and additional care purchased because of the child’s illness were recorded weekly in patient diaries; 79 parents reported having to take time off work or college or to pay for child care. Most parents reported this in terms of days but a few reported it in terms of hours. These items were aggregated and costed at minimum wage for adults of £8.21 per hour (the value in April 2019).
Additional care purchased
Nineteen parents reported that they had had to purchase additional care for their children. This was again recorded mainly in days but sometimes in hours. As with time off work, this was costed at the national adult minimum wage.
The cost of remedies plus that of time off work and any additional care comprised the non-NHS costs which, added to the NHS costs, comprised the societal costs.
Results
Cost results
Table 40 summarises the NHS cost differences between the treatment groups. The mean NHS cost per patient was slightly higher in the antibiotics group at £29.40, than in the placebo group at £25.80: a difference of £3.50. This was largely because of the cost of the intervention (£3.01). The overall cost difference was not statistically significant and neither were any of the more detailed elements shown in Table 40.
| Treatment group | Service | Mean cost (£) (SD) |
|---|---|---|
| Placebo (n = 211) | Reconsultation | 13.2 (25) |
| Medication | 0.6 (1.6) | |
| Referral | 5.7 (28.1) | |
| Hospitalisation | 7 (58.5) | |
| Total NHS | 25.8 (78.8) | |
| Non-NHS | 32.7 (105.8) | |
| Societal | 58.6 (128.9) | |
| Antibiotics (n = 221) | Reconsultation | 9.4 (20.4) |
| Medication | 0.3 (1.2) | |
| Referral | 7.7 (33.2) | |
| Hospitalisation | 9.1 (67.9) | |
| Intervention | 3 (0) | |
| Total NHS | 29.4 (86.2) | |
| Non-NHS | 32.9 (93) | |
| Societal | 62.3 (130.2) |
Societal costs, that is NHS costs plus remedies purchased and time off work and care, were, on average, roughly double NHS costs, mainly owing to time off work. Societal costs were slightly lower in the placebo group at £58.60, than in the antibiotics group at £62.30. This difference of £3.70 was almost entirely owing to the NHS cost difference, indicating that the groups incurred fairly similar non-NHS costs.
Quality of life and quality-adjusted life-years
The EQ-5D-Y questionnaire scores were translated into utility scores based on the UK tariff (EQ-5D-Y user guide 2015). QALYs were derived using area under the curve based on five points of utility scores (baseline and weeks 1–4).
The means of the overall EQ-5D scores and VAS scale of all patients at baseline and follow-up are shown in Table 41. The completion level was poor, with around two-thirds of the data missing. Completion rates fell from just under 40% at baseline to just over 20% at week 4. There were no differences in completion rates and mean EQ-5D scores between the groups at baseline or at follow-up. The EQ-5D scores and VAS scale increased over time in both groups. The completion rates and scales for VAS in the antibiotics group are similar to those in the placebo group at baseline and follow-up (see Table 41). The utility scores are shown in Table 42; these increased over time in both groups and in both children aged ≥ 5 years and children aged < 5 years (see Figure 11).
| Treatment group | Collection time | EQ-5D score | VAS scale | ||
|---|---|---|---|---|---|
| Completion, n (%) | Mean (SD) | Completion, n (%) | Mean (SD) | ||
| Placebo (N = 211) | Baseline | 76 (36) | 0.605 (0.306) | 106 (50) | 55.9 (17.2) |
| Week 1 | 80 (38) | 0.88 (0.157) | 97 (46) | 78.3 (19.3) | |
| Week 2 | 64 (30) | 0.937 (0.133) | 81 (38) | 86 (15.1) | |
| Week 3 | 47 (22) | 0.945 (0.201) | 56 (27) | 88.2 (15.7) | |
| Week 4 | 38 (18) | 0.928 (0.2) | 52 (25) | 88.4 (15) | |
| Antibiotics (N = 221) | Baseline | 80 (36) | 0.61 (0.316) | 107 (48) | 54.6 (17.9) |
| Week 1 | 84 (38) | 0.882 (0.234) | 102 (46) | 79.7 (19.7) | |
| Week 2 | 69 (31) | 0.927 (0.195) | 87 (39) | 87.4 (17.3) | |
| Week 3 | 61 (28) | 0.953 (0.214) | 72 (33) | 89.9 (15.9) | |
| Week 4 | 49 (22) | 0.932 (0.172) | 59 (27) | 87.2 (21.4) | |
| Age group (years) | Treatment group | Utility score | n | % with complete data | Mean | SD |
|---|---|---|---|---|---|---|
| ≥ 5 | Placebo (N = 63) | Week 0 | 37 | 59 | 0.62 | 0.31 |
| Week 1 | 38 | 60 | 0.89 | 0.13 | ||
| Week 2 | 29 | 46 | 0.92 | 0.17 | ||
| Week 3 | 24 | 38 | 0.92 | 0.27 | ||
| Week 4 | 18 | 29 | 0.9 | 0.26 | ||
| Antibiotics (N = 66) | Week 0 | 38 | 58 | 0.64 | 0.29 | |
| Week 1 | 37 | 56 | 0.85 | 0.27 | ||
| Week 2 | 32 | 48 | 0.92 | 0.2 | ||
| Week 3 | 26 | 39 | 0.98 | 0.07 | ||
| Week 4 | 24 | 36 | 0.9 | 0.22 | ||
| < 5 | Placebo (N = 148) | Baseline | 39 | 26 | 0.59 | 0.31 |
| Week 1 | 42 | 28 | 0.87 | 0.18 | ||
| Week 2 | 35 | 24 | 0.95 | 0.09 | ||
| Week 3 | 23 | 16 | 0.97 | 0.08 | ||
| Week 4 | 20 | 14 | 0.95 | 0.13 | ||
| Antibiotics (N = 155) | Baseline | 42 | 27 | 0.58 | 0.34 | |
| Week 1 | 47 | 30 | 0.9 | 0.2 | ||
| Week 2 | 37 | 24 | 0.93 | 0.19 | ||
| Week 3 | 35 | 23 | 0.93 | 0.28 | ||
| Week 4 | 25 | 16 | 0.96 | 0.11 |
FIGURE 11.
Utility scores at different time points for antibiotics (dark blue) and placebo (light blue) groups for children aged > 4 years.

Table 43 and Figure 12 document the VAS results. In line with the EQ-5D results, these indicated only minimal differences between the groups.
| Treatment group | Variable | n | % with completed data | Mean | SD |
|---|---|---|---|---|---|
| Placebo (N = 211) | VAS 0 | 106 | 50 | 55.86 | 17.23 |
| VAS W1 | 97 | 46 | 78.32 | 19.31 | |
| VAS W2 | 81 | 38 | 85.99 | 15.12 | |
| VAS W3 | 56 | 27 | 88.22 | 15.73 | |
| VAS W4 | 52 | 25 | 88.44 | 14.96 | |
| Antibiotics (N = 221) | VAS 0 | 107 | 48 | 54.62 | 17.93 |
| VAS W1 | 102 | 46 | 79.66 | 19.67 | |
| VAS W2 | 87 | 39 | 87.37 | 17.27 | |
| VAS W3 | 72 | 33 | 89.86 | 15.85 | |
| VAS W4 | 59 | 27 | 87.23 | 21.43 |
FIGURE 12.
The VAS score at different time points for antibiotics (dark blue) and placebo (light blue) for all patients in the treatment groups.
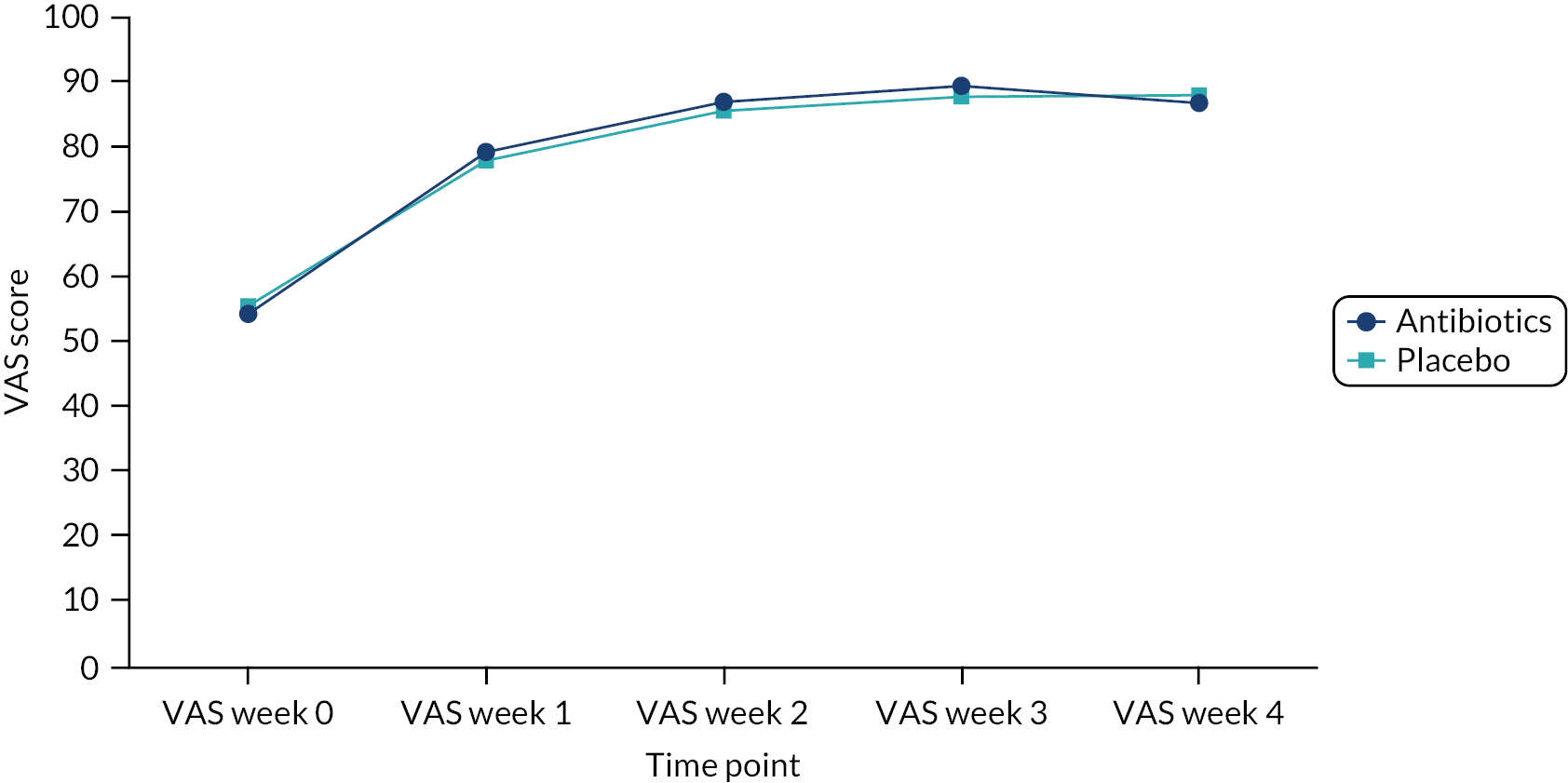
Incremental cost-effectiveness analysis
In terms of cost per illness-day (i.e. a day rated moderately bad or worse), the incremental cost of £3.50 in the antibiotics group combined with a difference in symptom-free days of 0.5 yielded an incremental cost per illness-day of £22 (95% CI –£63 to £48). The lack of a willingness-to-pay threshold makes the interpretation of this estimate difficult.
Incremental cost, incremental quality-adjusted life-years and incremental cost-effectiveness analysis
Using the EQ-5D data based on the mean scores between the groups based on the available EQ-5D scores at baseline and follow-up (see Table 44) led to a QALY difference that was very small, at 0.0001, and not significant. The incremental cost-effectiveness ratio (ICER) was estimated at £30,851, with a wide credible interval from –£73,639 to £109,429 (see Table 45). However, as noted above, EQ-5D scores were available for less than half of all participants.
| Treatment group | Cost (£) | Incremental cost (£) | Duration of illness | Incremental day of illness | ICER (day of illness) (£) |
|---|---|---|---|---|---|
| Placebo | 26 (18 to 35.6) | 7.6 (7.0 to 8.3) | |||
| Antibiotics | 29.4 (20.2 to 38.9) | 3.5 (–9.4 to 16.3) | 7.1 (0.064 to 0.070) | –0.5 (–1.4 to 0.4) | –22 (–63 to 48) |
| Treatment group | Cost (£) | Incremental cost (£) | QALYs | Incremental QALY | ICER (QALY) (£) |
|---|---|---|---|---|---|
| Placebo | 26 (18 to 35.6) | 0.0667 (0.0672 to 0.0682) | |||
| Antibiotics | 29.4 (20.2 to 38.9) | 3.5 (–9.4 to 16.3) | 0.0668 (0.0671 to 0.0684) | 0.0001 (–0.0008 to 0.0009) | 30,851 (–73,639 to 109,429) |
Multiple imputation was explored as a means of dealing with the missing EQ-5D data. Applying the same imputation method as for the primary outcome led to a larger but not statistically significant QALY difference of 0.002 and an ICER of £6417 (95% CI £12,240 to –£20,535) (see Table 46). The exploration of other imputation methods led to a wide variety of estimates that were not considered reliable. Details of these are available from the authors.
| Treatment group | Cost (£) | Incremental cost (£) | QALYs | Incremental QALY | ICER (QALY) (£) |
|---|---|---|---|---|---|
| Placebo | 26 (18 to 35.6) | 0.065 (0.062 to 0.068) | |||
| Antibiotics | 29.4 (20.2 to 38.9) | 3.5 (–9.4 to 16.3) | 0.067 (0.064 to 0.070) | 0.002 (–0.002 to 0.006) | 6417 (–12,240 to 20,535) |
Exploration of the uncertainty around this estimated incremental cost per QALY can be shown in terms of a scatterplot (see Figure 13) and cost-effectiveness acceptability curves (see Figure 14).
FIGURE 13.
Scatterplot of joint distribution of incremental mean cost from NHS perspective and mean QALY over 1 month: 95% confidence ellipse of antibiotics vs. placebo.
FIGURE 14.
Cost-effectiveness acceptability curve of the antibiotics and placebo groups based on QALY over 1 month.
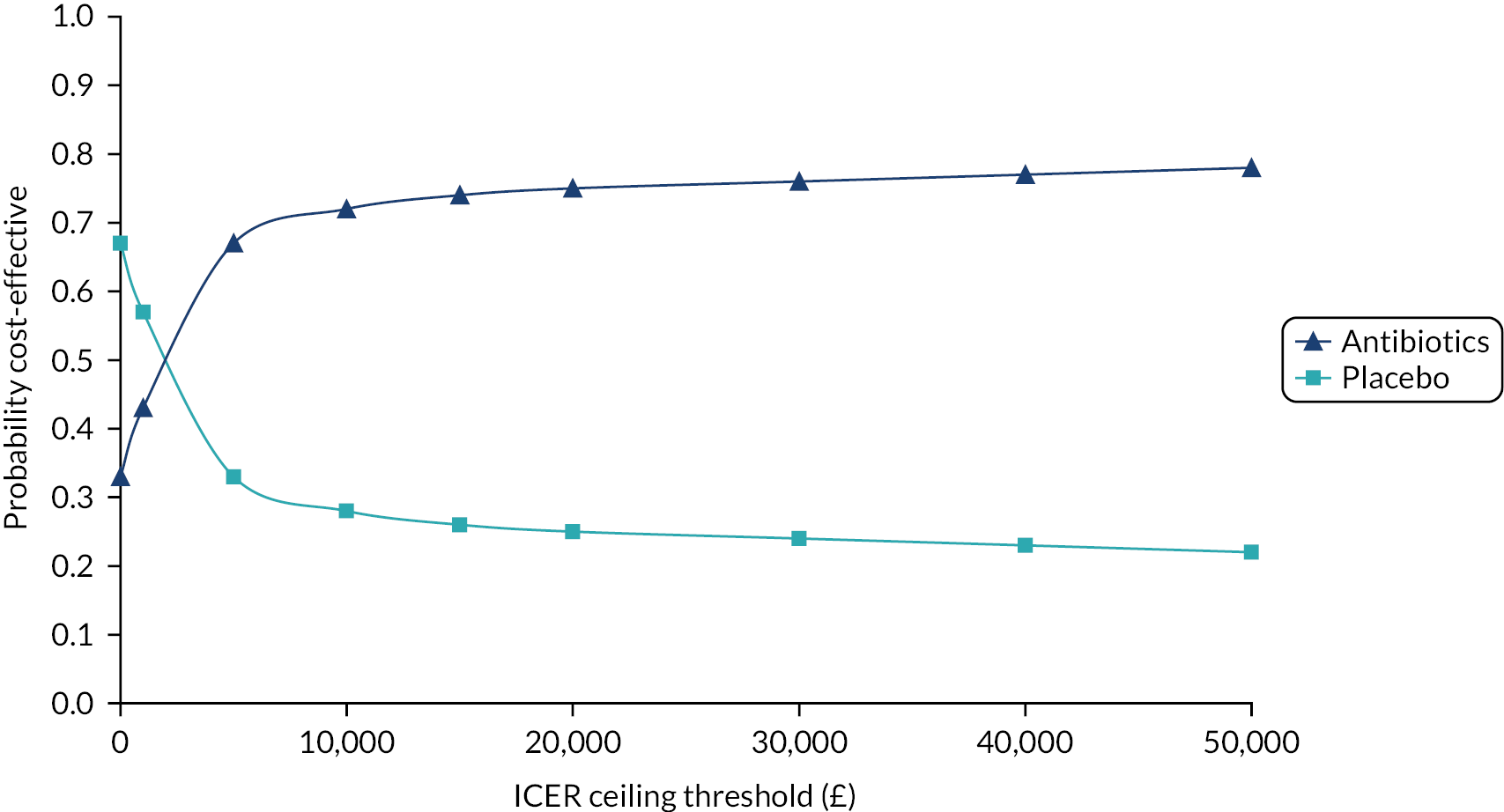
Figure 13 shows a wide spread around the points of zero difference for both cost and QALY increments. The cost-effectiveness acceptability curves (see Figure 14) show that the probabilities that the intervention is cost-effective against the placebo were 75% and 76% at the thresholds of £20,000 and £30,000, respectively.
Non-NHS costs based on private purchases of remedies and on time off work or caring were fairly equally distributed between the groups but led to a higher cost increment in the antibiotics group, with a resulting ICER of just over £40,000.
Discussion
The antibiotics group had marginally better outcomes in terms of both the primary outcome and QALY and also had a slightly higher cost per patient, but none of these differences was statistically significant, and, in relation to QALYs, they were based on large numbers of missing data. The cost per symptom-day avoided was £22 (–£63 to £48), a figure that has a wide CI and is difficult to interpret in the absence of what society or individuals might be willing to pay. The imputed incremental cost per QALY was just over £6500, with a probability of being cost-effective of around 75% at both £20,000 and £30,000 willingness-to-pay thresholds, but this must be viewed with great caution because of missing data.
The interpretation of these results also depends on the minimal differences considered worthwhile.
The trial was designed to detect a 3-day improvement in the duration of more severe symptoms in key clinical subgroups. The difference observed in the trial was much smaller, at around 1 day, which was not statistically significant.
Incremental cost-effectiveness ratios that take no account of the magnitudes of the differences in outcomes disregard the differences that might matter to patients, parents and clinicians. Costs based on the prices charged for antibiotics fail to consider the real but unquantifiable burden of AMR. For these reasons, the incremental cost-effectiveness estimates reported above provide help in relation to policy on antibiotic prescribing for children.
Limitations
No analysis of cost-effectiveness in subgroups was carried out for several reasons, including the lack of any relevant differences in the primary outcome, the extent of missingness in the QALY data, and the lack of cost differences between the groups.
The major limitation of this study is the high (two-thirds) proportion of missing data for the EQ-5D-Y. With so many missing data, imputation involved such major assumptions as to make bias likely.
Conclusion
In conclusion, the intervention is unlikely to be cost-effective as a result of the small and non-statistically significant differences observed, the difficulty of imputing the majority of the QALY data, and the lack of consideration of the societal costs of antibiotics in relation to resistance. 105,106
Chapter 8 Discussion
Main findings of this research
For the main trial in the full sample there was little evidence of a significant difference between antibiotic and placebo, with an estimated 1-day non-significant difference in the median duration of illness rated moderately bad or worse after seeing the doctor, representing around a 13% faster resolution of more severe symptoms. There were very similar findings for all clinical subgroups that were specified a priori: chest signs (antibiotics 6 days vs. placebo 6 days), sputum/rattly chest (antibiotics 5 days vs. placebo 7 days), fever (antibiotics 5 days vs. placebo 6 days), physician rating of unwell (antibiotics 5 days vs. placebo 6 days) and shortness of breath (antibiotics 5 days vs. placebo 6 days). There was also no evidence that the presence of bacteria based on PCR from a swab of the throat mediated antibiotic effectiveness or that the intensity of bacterial colonisation (based on Ct value) was important. Estimates from complete cases were very similar to those from the main trial results, as were estimates from a per-protocol analysis.
The health economic analysis put the NHS cost per child slightly higher with antibiotics (antibiotics, £29; placebo, £26), and societal costs were similar (antibiotics, £33; placebo, £33), but these differences were not statistically significant. As the societal costs were driven mainly by time off work, it is reasonably clear that work-related absence cannot be used to justify antibiotic prescribing. Large numbers of missing QALY data resulted in the estimates of incremental cost per QALY being based largely on utilising imputed QALY values.
Almost as many children were recruited to the observational study as to the trial. While there was very probably some confounding by indication for some outcomes, the estimated benefit of antibiotics for the primary outcome when adding the observational data to the trial data was similar to that in the trial (HR 1.16, 95% CI 0.95 to 1.41). A prognostic model to predict complications consisting of eight variables (age/baseline severity/gender/respiratory rate/duration of prior illness/oxygen saturation/sputum or rattly chest/passing urine less often) had excellent discrimination (bootstrapped AUROC 0.85) and good calibration, and a three-item model (respiratory rate; oxygen saturation; sputum/rattly chest) also performed well (AUROC 0.81).
The qualitative data showed that parents clearly found it very difficult to interpret symptoms and signs, and commonly used the sounds of the cough to judge its severity. The trial results suggest that the features parents use did not predict benefit from antibiotics, which highlights the need to provide better information to parents to support them in their decision-making. Many parents said that their main reason for consulting was for a clinical examination and reassurance regarding illness severity. There also appeared to have been a shift in parents’ perceptions regarding antibiotics; parents acknowledged that antibiotics should be used only when ‘necessary’, which aligns with many clinicians noting a shift in parents’ attitudes and reporting that parents were now largely satisfied with a clinical assessment, reassurance and advice. Parents’ decisions to take part in the trial were, very reasonably, influenced by the perceived risks associated with taking a placebo compared with immediate antibiotics. Parents also had concerns about the unnecessary use of antibiotics, which links with the findings above regarding changes in parent attitudes. Clear communication about the self-management of their child’s illness and safety-netting were identified by clinicians as important when implementing ‘no antibiotic’ prescribing strategies to reassure parents and support prescribing decisions, which links with the findings that parents need good information to support their decisions about when to consult the doctor.
Strengths and limitations
This study is one of the very few to report on the effectiveness of prescribing antibiotics among younger children presenting with chest infections. Among parents of eligible children, two-thirds were willing for their child to participate.
The study was designed to detect a 3-day improvement in symptom duration in key clinical subgroups, which were chosen based on the evidence of the main predictors of GP prescribing. Of particular importance was the subgroup with chest signs, the biggest driver of prescribing. Three days was judged enough to warrant prescribing an antibiotic and represented a 25% improvement in an illness lasting 12 days. Although the full trial sample had power to document smaller differences in symptom duration, the trial was underpowered to detect differences smaller than 3 days in the clinical subgroups and underpowered for differences in complications and reconsultations. The analysis was adequately powered for the primary analysis using imputed data but underpowered for a complete-case analysis, including the per-protocol analysis. However, the estimates for the complete-case analysis were so similar to the imputed data that the per-protocol analysis still has some value. The achieved sample size may also have resulted in insufficient power to detect antibiotic mediating effects of upper respiratory tract microbes. This in part reflects the fact that, as described in Chapter 5, this was a very challenging trial to recruit to, which reflects the difficulty of opportunistic recruitment during surgery time in winter months when practices are stretched, but also parental and clinician concerns about the safety of a vulnerable group. The sample size was also smaller than anticipated, in part because of the COVID-19 pandemic prematurely ending recruitment in the last few months of the winter season of 2019–20.
The study was at the pragmatic end of the spectrum despite being placebo controlled; there was no close monitoring of parents and children, so parents were more likely to have behaved as they might in practice in terms of whether or not they gave their child medication. Thus, the trial estimates are of effectiveness, but conversely we may have underestimated the efficacy of antibiotics. However, it is reassuring that a per-protocol analysis provided very similar estimates to those of the total trial population.
A surprising finding was, that compared with representative observational cohorts, this trial population included children with slightly more severe clinical presentations,39 which suggests that we may have overestimated the impact of antibiotics. The antibiotic (amoxicillin) was chosen as it is the first-choice in UK national prescribing guidance for LRTIs in children,37 but it is possible that other antibiotics could have demonstrated greater efficacy.
The addition of the observational data to the trial data had the advantage of increasing the power of the primary analysis, but there were concerns about residual confounding by indication for some of the outcomes, particularly complications and ‘side effects’. Nevertheless, for the primary outcome, the inclusion of the observational data suggested very little difference in the estimates of benefit compared with the ‘pure’ trial estimates. The different recruitment settings for the observational study (some A&E and paediatric assessment units contributed children to the observational study as they were not able to host the trial, and some GP practices predominantly recruited to the observational study alone) made direct comparison of trial and observational estimates difficult.
As with many studies, there were large numbers of missing QALY data, and so the estimates of incremental cost per QALY based on utilising imputed QALY values (£6417), although well under the putative NICE threshold, must be viewed with caution. This raises the more general issue of whether or not an economic analysis is sensible when clinical effectiveness has not been proven.
The qualitative studies occurred prior to the COVID-19 pandemic, but they provide important insights into how parents judge illness severity and what they consider when deciding whether or not to take part in a RCT. The sample size for both parents and clinicians was small but saturation was reached; however, the findings may not be representative of the wider population. We have limited data from fathers, but it is mothers who usually bring their children to see the GP.
Comparison with other studies
Only one trial in the Cochrane review of antibiotics for acute bronchitis included young children aged ≥ 3 years presenting with uncomplicated acute chest infections. 15,17 This was a pragmatic open trial of antibiotic prescribing strategies and only included 100 children aged ≤ 12 years. 15 The overall results of the current study in children are very similar to those of the Cochrane review and suggest that for both adults and children there is very modest symptomatic benefit from prescribing antibiotics for LRTI.
Comparison of the clinical characteristics of children with the large observational STARWAVe cohort indicated, surprisingly, that the children admitted to the trial were more unwell on average than a more generalisable population of children presenting with chest infections. 39 This suggests that the present trial successfully recruited more unwell children, in whom antibiotics might be expected to be more effective, and that the average impact of antibiotics in a more generalisable low-risk population is likely to be even lower than that reported here.
There was also no evidence of either selective benefit among children in whom pathogenic bacteria were isolated or an impact of viral load measure using Ct values, and this matches the findings in adults. 92
We found that consultation, referral and hospitalisation costs were significant43 but that societal costs were equally high. Importantly, antibiotic prescribing was not associated with health or societal resource savings, and if anything resulted in slightly higher costs, which helps to counter the argument that prescribing antibiotics could reduce time off work for parents and so be efficient for society. The results of the cost-effectiveness analyses suggest a big difference in interpretation depending on whether the complete cases (the minority) or the imputed data are used. In either scenario if the costs of antibiotic resistance were included the adverse impact on health and societal resource use would be even higher,7 let alone the future costs of antibiotic resistance.
Children given antibiotics in the observational study had much more severe clinical presentations than children not given antibiotics, matching the trends in the much larger STARWAVe cohort. 12,39 Despite this, we found that, when controlling for the propensity to prescribe antibiotics, the estimates for the primary outcome were similar when including the observational data and when including the ‘pure’ trial data: an overall difference of 1–2 days between the groups in both moderately bad symptoms and total duration of symptoms. This is consistent with the estimates from the Cochrane review and in predominantly adult studies. 15,17
The final prognostic model to predict complications included a range of different variables from the STARWAVe model;12,39 only age and prior illness duration were in common, which probably reflects the more severely affected group of children recruited for the current study and explains why the discrimination of the STARWAVe model in this cohort was much lower than in the model we developed. The excellent discrimination of the model we developed suggests potential for its use in consultations as a clinical decision tool. The utility of clinical prediction algorithms in reducing antibiotic prescribing by converting them to clinical decision tools to be used in the consultations was demonstrated in the PRISM trial,93 and decision tools are cited as one of the most promising interventions in the most recent systematic review of antimicrobial stewardship interventions by the AHRQ. 94 It should be possible to develop a clinical decision tool based on the algorithm that we developed in the current study, and for a range of other infections, supported by further validation and evidence from systematic reviews and/or large cohorts. 95–97
The qualitative findings confirm that a key driver of consulting is parental perception of illness severity. 58,59,98 Importantly, we found that parents use the sound of the cough as one of the important discriminators, which is not supported by current evidence and suggests a place for improved provision of information to support parents in their decision to consult, but, as in previous studies, parents are very uncertain about relying on their own evaluations and assumptions. 19,20,58 The perceived risk factors that drive the prescribing of antibiotics for children from the perspectives of the clinicians involved were supported by previous literature. 19,20,67,68
Parents weighed potential benefits against perceived risks when deciding whether or not to participate, matching previous studies,58,63,99–103 but the perceived risk of taking an antibiotic reflected changing preferences for antibiotic treatment, although that risk was to their child rather than to public health. 63,104 In addition to that change in attitude of parents, clinicians identified discussions as key to communicating ‘no antibiotic’ outcomes, particularly in terms of natural history, self-management of their child’s illness and safety-netting, to make sure that parents understand. Clinicians reported that the main barrier to recruitment was the lengthy process in a busy and time-constrained clinical environment, and where recruitment worked well this was due to teamwork, such as enlisting a nurse to aid the recruitment process.
Patient and public involvement and engagement
Three PPIE contributors were included at each stage of the project. PPIE input was particularly important in developing the basic concept of the study, as it helped with the decision about the level of benefit that would be considered worthwhile for a child to have antibiotics. The natural history of the illness without antibiotics (15–21 days) was discussed, as was how to balance the danger of antibiotic resistance. It was felt that even if antibiotics shortened the illness by 2 days this would not be clinically significant as it would represent a reduction of less than one-tenth of the total illness duration expected from the natural history. Therefore, the tipping point to justify giving antibiotics was agreed as a 3-day reduction in symptoms. This formed an important basis for the conclusion that the 1-day reduction seen in the antibiotics group was not clinically significant. PPIE collaborators were also involved in deciding key outcomes and developing patient materials (e.g. information leaflets, symptom diaries).
One aspect of the trial that perhaps had not been fully anticipated by the PPIE team was the difficulty in recruiting both parents and clinicians, who for different reasons did not want to be randomised to an unknown treatment group. Some, such as clinicians who were risk averse, especially when young children were involved or there was pressure from parents to prescribe antibiotics, wanted children to be prescribed antibiotics. Others, such as clinicians concerned about overprescribing and parents concerned about their children becoming resistant to antibiotics, did not want children to receive antibiotics. Therefore, another area of PPIE contribution for helping to refine recruitment strategies to overcome GP hesitancy, such as helping to develop a script for GPs to use when recruiting families to the trial.
The PPIE collaborators regularly attended and were active in trial management meetings, and they contributed to the interpretation of study findings, both quantitative and qualitative. They also contributed to the Plain English summary and the final report. Parents of all participating children were sent a summary of the results.
One PPIE collaborator commented that, ‘even if antibiotics only reduced symptoms by 2 days, in the grand scheme of antibiotic resistance, this is a win, and it’s useful to see that antibiotics don’t provide much benefit . . . there isn’t much difference in healing with or without – this provides a potential opportunity of cutting down on antibiotic use’.
Conclusions and recommendations
The use of antibiotics does not make an important difference to the symptom burden for uncomplicated LRTI in children either overall or for the key clinical subgroups in whom antibiotic prescribing is common. Antibiotic prescribing also did not save NHS or societal costs. In view of the major danger of antibiotic resistance, unless pneumonia is suspected clinicians should generally not prescribe antibiotics for most children presenting with uncomplicated chest infection but instead give appropriate ‘safety-netting’ advice to cover any deterioration in illness.
A simple score needs to be externally validated but could potentially identify the minority of children who will develop complications. The prognostic model could have clinical utility in that all the variables are easily documented in routine consultations, so it could potentially be used by clinicians to predict adverse outcome when seeing more unwell children.
The achieved sample size may also have resulted in insufficient power for detecting the antibiotic-mediating effects of upper respiratory tract microbes.
Parents need better access to information to support their decision to consult, as well as clear communication in the consultation about the self-management of their child’s illness and when to reconsult (‘safety-netting’).
Implications for health care
-
Clinicians can reduce their prescribing of antibiotics for children presenting with uncomplicated chest infections without this leading to poor symptom control or high societal costs. The perception that parents’ attitudes to antibiotics are changing should help in antimicrobial stewardship initiatives.
-
A ‘simple’ score that incorporates respiratory rate and low oxygen saturation could potentially identify the minority of children who will develop complications. To be clinically useful it could be developed as an app with automated outputs.
-
Parents need better access to information to support them in their decision to consult and in the self-management of their child’s illness.
Recommendations for research
-
The data can be incorporated in the Cochrane review and in individual patient data meta-analysis.
-
Further work on the incremental QALY gain from antibiotics is needed, assessing a range of models and their implications when imputing missing QALY data, as is better evidence about how to incorporate AMR resource implications in modelling.
-
Further research is necessary to determine if upper respiratory tract microbes mediate the effects of antibiotics and are, therefore, useful targets for future microbial point-of-care tests.
-
The prognostic score and other recently developed clinical prediction rules need to be externally validated, and the score could be developed as an app with automated outputs as a tool to reduce antibiotic prescribing.
Acknowledgements
This project is funded by the NIHR Health Technology Assessment (HTA) programme (study reference 13/34/64). Saul N Faust is part-funded by the Southampton NIHR Biomedical Research Centre. We are very grateful to both the Trial Steering Committee [Elaine Hay (chairperson), Robbie Foy, Reuben Ogollah and Kirsty Samuel (PPI representative)] and the DMSC [Sally Kerry (chairperson), Chris Griffiths, Andrew Hayward and Peter Landman (PPI representative)] for their support and advice. Zöe Morrice (student placement) contributed to the qualitative study.
Contributions of authors
Paul Little (https://orcid.org/0000-0003-3664-1873) and Theo Verheij (https://orcid.org/0000-0002-0166-1498) developed the original idea.
Paul Little led the funding applications with input from Theo Verheij, Beth Stuart (https://orcid.org/0000-0001-5432-7437), Alastair D Hay (https://orcid.org/0000-0003-3012-375X), Kay Wang (https://orcid.org/0000-0002-7195-1730), Michael Sharland (https://orcid.org/0000-0001-8626-8291), Anthony Harnden (https://orcid.org/00000003-0013-9611), Guiqing Yao (https://orcid.org/0000-0002-0591-9636), James Raftery (https://orcid.org/0000-0003-1094-8578), Shihua Zhu (https://orcid.org/0000-0002-1430-713X), Samuel Coenen (https://orcid.org/00000002-1238-8052), Christopher C Butler (https://orcid.org/0000-0002-0102-3453), Saul N Faust (https://orcid.org/0000-0003-3410-7642), Geraldine Leydon (https://orcid.org/0000-0001-5986-3300), Mandy Wan (https://orcid.org/0000-0001-8802-0425), Kerenza Hood (https://orcid.org/0000-0002-5268-8631), Jane Whitehurst (https://orcid.org/0000-0002-2173-9088), Samantha Richards-Hall (https://orcid.org/0000-0002-7665-4268), Peter Smith (https://orcid.org/0000-0003-4423-5410), Michael Thomas (https://orcid.org/0000-0001-5939-1155), Michael Moore (https://orcid.org/0000-0002-5127-4509) and Robert C Read (https://orcid.org/0000-0002-4297-6728), and the protocol was developed and modified by all co-authors.
The study progress was supervised by Paul Little, Theo Verheij, Gilly O’Reilly (https://orcid.org/0000-0001-6141-4360), Natalie Thompson (https://orcid.org/00000002-1880-6438), Joseph Little (https://orcid.org/0000-0003-3456-1626), Taeko Becque (https://orcid.org/0000-0002-0362-3794), Alastair D Hay (https://orcid.org/0000-0003-3012-375X), Charlotte Hookham (https://orcid.org/0000-0002-2034-4383), Kate Rowley (https://orcid.org/0000-0003-4388-5569), Joanne Euden (https://orcid.org/0000-0002-2844-6878), Christopher C Butler, Nick A Francis (https://orcid.org/0000-0001-8939-7312), Mandy Wan, Kerenza Hood, Michael Moore and Robert C Read.
Beth Stuart, Kim Harman, Peter Smith, Taeko Becque, Theo Verheij and Paul Little developed the statistical analysis plan and interpreted the analyses, with input from all authors.
Taeko Becque performed the statistical analysis, supervised by Beth Stuart and Peter Smith.
James Raftery, Guiqing Yao, Shihua Zhu and Joseph Little developed the economic analysis protocol, and the analysis was performed by Shihua Zhu supervised by James Raftery and Guiqing Yao.
Paul Little led the writing of the report and all authors contributed to interpretation of the analyses and to revisions of the report.
Catherine Woods (https://orcid.org/0000-0002-2245-5773) led the qualitative study, supervised by Geraldine Leydon, with input from Nick A Francis, Theo Verheij and Paul Little.
Beth Stuart, Natalie Thompson and Taeko Becque accessed and verified the data, and Paul Little and Theo Verheij were responsible for the decision to submit the report.
Publications
Halls AV, Van’t Hoff CI, Little P, Verheij TJM, Leydon GM. A qualitative interview study of parents’ perspectives, concerns and experiences of the management of lower respiratory tract infections in children in primary care. BMJ Open 2017;7:e015701.
Little P, Francis NA, Stuart B, O’Reilly G, Thompson N, Becque T, et al. Antibiotics for lower respiratory tract infection in childrenpresenting in primary care in England (ARTIC PC): a double-blind, randomised, placebo-controlled trial. Lancet 2021;398:1417–26.
Woods CJ, Morrice Z, Francis NA, Little P, Verheij T, Leydon GM. Parent and clinician views of managing children with symptoms of a lower respiratory tract infection and their influence upon decisions to take part in a placebo-controlled randomised control trial. Antibiotics 2021;10:356.
Little P, Read RC, Becque T, Francis NA, Hay AD, Stuart B, et al. Antibiotics for lower respiratory tract infection in children presenting in primary care (ARTIC-PC): the predictive value of molecular testing. Clin Microbiol Infect 2022;28:1238–44.
Data-sharing statement
De-identified participant data are available for further analyses. Requests for data should be submitted to the corresponding author.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
List of abbreviations
- A&E
- accident and emergency
- AMR
- antimicrobial resistance
- AUROC
- area under the receiver operator curve
- CI
- confidence interval
- Ct
- cycle threshold
- EQ-5D
- EuroQol-5 Dimensions
- EQ-5D-Y
- EuroQol-5 Dimensions Youth
- GP
- general practitioner
- HRG
- Healthcare Resource Group
- ICER
- incremental cost-effectiveness ratio
- IMP
- Investigational Medicinal Product
- IQR
- interquartile range
- ISRCTN
- International Standard Randomised Controlled Trials Number
- LRTI
- lower respiratory tract infection
- NICE
- National Institute for Health and Care Excellence
- PCR
- polymerase chain reaction
- PPIE
- Patient and Public Involvement and Engagement
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RTI
- respiratory tract infection
- SD
- standard deviation
