Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/86/03. The contractual start date was in December 2014. The draft report began editorial review in December 2021 and was accepted for publication in August 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Donoghue et al. This work was produced by Donoghue et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Donoghue et al.
Chapter 1 Introduction
Material throughout the chapter has been adapted from the trial protocol. 1
Background and rationale
Alcohol is a significant risk factor for morbidity and mortality, and is causally related to over 200 non-communicable diseases and injuries. 2,3 The World Health Organization reports that 3 million deaths were caused by harmful alcohol use worldwide in 2018. 4 In the UK, there has been a consistent, year-on-year increase in harm related to alcohol. There were almost 1.3 million NHS hospital admissions related to alcohol in 2018–19, an 8% increase from the previous year. 2 Chronic physical health conditions related to alcohol use have increased in prevalence in the UK, including certain cancers, neuropsychiatric conditions, cardiovascular and digestive diseases, alcoholic liver disease and alcohol use disorders. 5,6 Chronic, heavy alcohol use is also associated with an increased risk of mental health disorders7 and contributes to social problems such as unemployment, poor quality of life, marital breakdown and domestic violence. 8–11 The cost to the UK economy due to the physical, mental and social problems associated with alcohol is estimated at £21B annually, of which the NHS costs are estimated at £3.5B. 3,12
In the UK, the proportion of adults drinking more than the recommended unit guidelines (14 units a week) varied between age groups, with men and women aged 55–64 years being the most common (38% and 19%, respectively). 2 Across all age groups, men were more likely than women to drink at increasing and higher risk levels. 2 The number of adults entering specialist alcohol treatment has fallen since a peak in 2013–14, decreasing each year until present day 2019–20. 13 In this same period, there have been substantial cuts to public health budgets, including alcohol services. 14,15 Frequent episodes of relapse and resumption of drinking are common in those dependent on alcohol, as many as 70% of service users relapse in the first 12 months post treatment. 16,17 Reducing alcohol-related hospital admissions and NHS costs has been identified as a priority in the UK Government’s Alcohol Strategy. 3 The combined benefits of drug and alcohol treatment amount to £2.4B every year, resulting in savings from areas including crime and health and social care. Alcohol treatment has been shown to reflect a return on investment of £3 for every £1 invested. 18–20 Providing effective treatment for alcohol dependence to reduce relapse rates, and therefore alcohol-associated harms, will help to achieve this.
Acamprosate for relapse prevention in alcohol dependence
Acamprosate and naltrexone have been recommended by the National Institute for Health and Care Excellence (NICE), in combination with a psychological intervention, as first-line treatments to support relapse prevention in alcohol dependence. 21 Disulfiram is regarded by NICE as a second-line treatment due to a more limited evidence base and potential adverse effects. In the past 10 years, there has been a 58% increase in prescriptions for these medications issued, however, in the past 3 years, there has been a slight decrease. 2 Naltrexone has been licensed for use in the UK to support relapse prevention in alcohol dependence since May 2013 and can also be used to treat opioid dependence. As the reason for prescription is not recorded in the NHS prescription service, the prescribing data for this in relation to alcohol dependence cannot be directly compared against acamprosate. 2 However, Prescription Cost Analysis data produced by the NHS Business Service Authority for 2012 shows that there were 117,417 acamprosate prescription items compared to just 17,790 prescription items for naltrexone. 13,22 Since naltrexone has been granted marketing authorisation for alcohol dependence, this difference seems not to have changed. 23 As these data include prescriptions for naltrexone to treat opiate dependence, the number of prescription items for alcohol dependence is likely to be fewer than indicated. Due to this difference in prescribing practices and differences in dosing regimens and side effects between medications, which may affect adherence, acamprosate was the focus for the current research.
Acamprosate modulates the glutamatergic system and attenuates the imbalance between inhibitory (GABA) and excitatory (glutamate) neurotransmitters in the brain during alcohol withdrawal, reducing the conditioned effect of alcohol and the negative reinforcement of the addictive behaviour. 24–27 A meta-analysis found acamprosate to have a moderate effect on maintenance of abstinence in people with alcohol dependence. 26 These results have been supported by a more recent systematic review and meta-analysis that found acamprosate to be the only alcohol relapse prevention intervention with enough evidence to conclude that it is better than placebo in supporting detoxification and for alcohol-dependent patients to maintain abstinence for up to 12 months in primary care settings. There was additional evidence that acamprosate might be effective longer term but the evidence was weak. 28
Adherence to acamprosate
Poor adherence to medication is a common problem, particularly in chronic conditions, a greater risk of poor adherence has also been associated with substance use disorder. 29,30 Although the available evidence supports the efficacy of acamprosate in clinical trials, poor adherence to the medication may pose a problem for effectiveness in clinical practice. We conducted a systematic review of the rates of adherence to acamprosate reported in clinical trials. We found that the mean adherence rate reported ranged from 54.2%31 to 95%. 32 This variation in adherence may be partially explained by differences in the definition of medication adherence (e.g. percentage of prescribed medication taken or percentage of those taking 80% or more of prescribed medication) and measurement of adherence. Several different methods are used in clinical trials to monitor medication adherence, with variation in the confidence in their accuracy. For example, counting returned medication and self-report of adherence may be considered ‘low’ confidence measures, electronic monitoring of pill bottle opening ‘medium confidence’ and supervised dosing ‘high confidence’. 33 Medication non-adherence in clinical practice is likely to be significantly greater than that seen in clinical trials that often offer payment for participation, adherence support and frequent monitoring appointments.
The median duration of acamprosate pharmacotherapy has been found to be only 2.1 months with just 27.7% of those prescribed acamprosate persisting for 6 months as recommended by NICE. 34 Therefore, patients may not be gaining the maximum benefits from the medication. 21 Medication effectiveness can be further limited by underdosing, overdosing or taking medication at incorrect intervals. 35 The Testing Combined Pharmacotherapies and Behavioral Interventions in Alcohol Dependence trial, a large US-based trial, found an association between poor adherence to both acamprosate and naltrexone and lower percentage days abstinent and higher percentage days heavy drinking. 36,37 Furthermore, poorer alcohol outcomes were also identified in those who were non-adherent early in the Testing Combined Pharmacotherapies and Behavioral Interventions in Alcohol Dependence trial compared to those who were non-adherent later in the trial. 36,37
Reasons for non-adherence
Poor adherence to a medication may be due to multiple and complex reasons. 29 The complexity of the dose regimen, with greater dose frequency and complexity of instructions, has been associated with poorer adherence. 38–40 Side effects were commonly reported as impacting on adherence in the Testing Combined Pharmacotherapies and Behavioral Interventions in Alcohol Dependence trial. 36,37 Poorer adherence was also found for the trials combined therapy group compared to the single active therapy group, which may be explained by an increase in side effects experienced by participants taking both acamprosate and naltrexone. 30
Horne has proposed that patients weigh up the potential costs and benefits of medication when making adherence decisions. 41,42 Greater adherence to medications in chronic health conditions such as asthma, diabetes, cardiac disease and cancer has been associated with a greater perception of the benefits of the medication. Conversely, greater concern about potential side effects of medication has been associated with poorer adherence. 42,43 The development of effective interventions to address the uncertainty that some patients feel towards the benefits of medication and their concerns about the potential adverse effects is a priority to improve adherence in the treatment of chronic health conditions. 35,43
Medication Management
The NICE alcohol treatment guidelines21 recommend monthly supervision for the first 6 months while taking acamprosate. There is a wide variation in the type and frequency of support received in clinical practice with support usually delivered by a combination of primary care and specialist addiction services.
Psychosocial interventions to maximise adherence to medications for alcohol relapse prevention, including acamprosate, have been used successfully in clinical trials. A six-stage, manualised intervention called BRENDA (Biopsychosocial evaluation, Report, Empathy, Needs assessment/goals, Direct advice, and Assessment)44 has been found to be beneficial for improving adherence to medication for alcohol relapse prevention. 45,46
The manual-based psychosocial intervention, Medical Management, was developed from BRENDA and other Medication Management (MM) interventions47 for the Testing Combined Pharmacotherapies and Behavioral Interventions in Alcohol Dependence trial48 and it has since been used in clinical trials of other medications. 49,50 Medical Management provides education, support and practical advice to service users about their alcohol drinking behaviour and medication to support adherence. An initial session, lasting up to 60 minutes, identifies the rationale for taking the prescribed medication, provides an overview of the dosing regimen, highlights the importance of taking the medication as it has been prescribed and an individualised plan to support adherence is developed. In addition, the service users’ diagnosis, treatment goals and participation in support groups are discussed. The initial session acts as a foundation for the preceding shorter Medical Management sessions that last up to 30 minutes. Despite the successful inclusion of the MM interventions BRENDA and Medical Management in clinical trials, research to support their use in clinical practice is lacking.
The role of the pharmacist
The community pharmacist’s role has extended beyond medication supply to improve public health,51–55 HIV prevention in opioid dependence56–58 and supporting adherence to medication. 59–61 A joint statement by the Royal College of General Practitioners and the Royal Pharmaceutical Society in 2011 identified a role for suitably qualified community pharmacists to contribute to care planning and treatment interventions in substance use disorder (RCGP and RPS, 2011). This has been followed by a recent report of the commission on the future models of NHS care delivered through pharmacy. 62
Healthy Living Pharmacies (HLPs) aim to provide a range of health promotion interventions, with alcohol dependence as one of the conditions targeted. 55,63,64 Community pharmacists and support staff have expressed positive attitudes towards providing extended services in alcohol and substance use disorder when adequate training is provided. 65–67
Contingency Management
Engagement and retention in treatment for alcohol dependence are often suboptimal and are related to poor treatment outcomes. 68,69 Contingency Management (CM) has been found to improve substance use disorder treatment retention and engagement70–74 as well as increase adherence to prescribed pharmacotherapies such as naltrexone for opiate relapse prevention75 and improve prevention, diagnosis and treatment outcomes for HIV, tuberculosis and hepatitis control in substance use disorders. 76 There is also evidence of an increased rate of abstinence of cannabis use over and above evidenced-based treatment (individual Motivational Enhancement Therapy/Cognitive Behavioural Therapy),77 reduced tobacco and alcohol use among adults not in treatment for substance use disorders,78 and it is effective for cocaine, tobacco, opiates and cocaine, and polysubstance use. 79,80
Research on the use of CM in the treatment of alcohol dependence is limited. 21,80 A systematic review81 concluded that CM continues to be a highly effective intervention for a range of substance use disorder treatment and follow-up outcomes, showing sustained growth and high treatment efficacy, and recommends further dissemination and implementation of CM to increase its impact.
Petry et al. 82 examined the use of CM and the common concerns of its implementation and recommend that more research needs to be done to promote a better understanding of CM and its benefits. It is this lack of understanding that leads to CM being underutilised despite its clearly effective results in substance use disorder treatment outcomes. Subsequently, a systematic review of the dissemination and implementation of CM has been conducted. 83 The findings report the importance of organisational input and ongoing supervision and consultation to optimise the effects of CM as well as including barriers to implementing CM, the main one being cost despite evidence of CM’s cost-effectiveness. 84
Studies can use a prize-based protocol with incentives of variable magnitude based on abstinence and/or treatment participation. 85,86 Alternatively, fixed monetary incentives or monetary incentives on an escalating scale may also be used, for example, to improve substance use disorder treatment retention and drug use and post-traumatic stress disorder outcomes,87 improve retention and abstinence with stimulant users in outpatient psychosocial treatment programs,88 improve hepatitis B vaccination adherence and completion in injecting drug users and to reduce heavy alcohol consumption. 89
Why this research is needed now
The effectiveness of acamprosate to support alcohol relapse prevention has been well documented. 21,26,28,46,48 The full benefit of acamprosate in clinical practice has not been maximised due to poor adherence and insufficient duration of its use. Supporting patients in taking acamprosate as prescribed through the application of MM has the potential to help improve adherence and increase the clinical effectiveness of acamprosate. However, there is currently insufficient evidence to determine the most effective form of intervention to support adherence.
The delivery of MM within the pharmacy setting is aligned with the development and expansion of the role of the pharmacist. 55,62–66,90,91 However, the effectiveness of interventions to increase medication adherence for alcohol dependence delivered by pharmacists is currently not known.
Research into the application of CM in alcohol dependence treatment has been recommended by NICE21 to build on the existing small, but growing, body of evidence. 70,78,92 The financial cost of delivering CM is low (< £10 per session) and extensive training to deliver the intervention is not required. If shown to be effective, CM has considerable potential to be adopted within NHS services and community pharmacy to enhance alcohol dependence treatment.
Aims and objectives
The current trial, ADAM (Alcohol Dependence and Adherence to Medicine), aimed to evaluate the effectiveness and cost-effectiveness of adjunctive MM with and without CM in improving adherence to acamprosate for relapse prevention in alcohol dependence.
Objectives
-
To conduct a definitive three-arm, randomised controlled trial (RCT) of the effectiveness of MM with and without CM compared to Standard Support (SS) alone in enhancing adherence to acamprosate in alcohol dependence relapse prevention.
-
To estimate the cost-effectiveness of MM with and without CM compared to SS alone in enhancing adherence to acamprosate in alcohol dependence relapse prevention.
-
To assess the impact of adherence to acamprosate for alcohol dependence relapse prevention on abstinence and reduced alcohol consumption.
Primary hypothesis
The primary null hypothesis was:
-
MM and MM + CM will be no more effective than SS alone in terms of per cent adherence to acamprosate, 6 months post randomisation.
Secondary hypotheses
The secondary null hypotheses were:
-
MM and MM + CM will be no more cost-effective than SS alone at 6 months post randomisation.
-
MM and MM + CM will be no more effective than SS in terms of the percentage of possible doses of acamprosate taken, at 12 months post randomisation.
-
MM and MM + CM will be no more cost-effective than SS alone in terms of quality-adjusted life-years (QALYs) at 12 months post randomisation.
-
No greater adherence to acamprosate will be associated with improved alcohol outcomes, namely a higher percentage of days abstinent, fewer units of alcohol per drinking day, reduced relapse to any drinking and reduced relapse to heavy drinking at 6 and 12 months post randomisation.
-
Service user beliefs about medication, and therapeutic relationship with care providers, will not be moderated by medication adherence at 6 and 12 months post randomisation.
Chapter 2 Intervention development and pharmacist training
A manualised version of MM has been designed for use in clinical trials48,93 and a shortened version of this manual has been produced for use in routine clinical practice. 94 However, the content and structure of the manual required some adaptation for use with UK community pharmacists. CM has been extensively used to enhance treatment engagement and medication adherence in substance use disorder, and to a lesser extent in alcohol dependence. Adaptation of the CM procedures was also required for the UK community pharmacy setting to support attendance at MM sessions. We aimed to adapt the standardised MM manual and develop a suitable CM protocol to be delivered by trained pharmacists to improve adherence to acamprosate for alcohol relapse prevention.
This chapter contains four elements supporting the development of MM and CM interventions and pharmacist training:
-
focus groups with service users and pharmacists
-
patient and public involvement
-
manual development and adaptation (based on 1–2)
-
pharmacist training.
Focus groups with service users and pharmacists
To support this aim, we conducted focus groups with service users and pharmacists/pharmacy support staff to explore the following:
-
service users’ past experiences of taking acamprosate, the support received while taking the medication and any perceived benefits and concerns about acamprosate
-
service users’ views on factors influencing medication adherence and specifically adherence to acamprosate
-
service users’ views on MM and its delivery in the pharmacy setting/being pharmacist delivered
-
service users’ views on the optimal incentive schedule for CM to improve attendance at MM sessions
-
pharmacist/pharmacy support staff beliefs, attitudes and knowledge of alcohol dependence
-
pharmacists/pharmacy support staff perceptions of factors that may influence adherence to acamprosate
-
pharmacist/pharmacy support staff views on the barriers and facilitators to delivering MM and CM within the pharmacy setting
-
pharmacist/pharmacy support staff previous experiences of delivering health interventions, their self-perceived training needs, and the support that they would like/feel that they need to deliver MM and CM in relation to alcohol dependence
Methods
The full methods and results have been published elsewhere. 95
The study received local NHS approvals, and NHS research ethics approval from the West of Scotland Research Ethics Committee (Ref: 15/WS/0048).
Setting
Eight focus groups were conducted, four with service users with experience in treatment for alcohol dependence and four with pharmacists/pharmacy support staff. Focus groups took place in four different geographical locations (London, Birmingham, Southampton, Hull) in line with the study sites of the main ADAM trial, so that any issues relating to geographical location and differences in treatment practices could be considered. Focus groups were used to allow a range and depth of ideas to be explored among individuals with a shared experience. 96,97
Participants
There is no optimum number of participants for a focus group but it has been suggested that between six and ten participants enable a discussion with varied perspectives. 98 Purposive sampling was used to identify up to 12 participants to invite to each focus group to achieve a recruitment rate of between 6 and 10 participants per focus group. 99 Service users were identified through service user involvement and recovery groups in each locality. Service users were included if they had received treatment for alcohol dependence. In an attempt to ensure that all participants felt comfortable expressing their views, we aimed for a 50/50 composition of those who had and those who had not had previous experience with acamprosate. Pharmacists/pharmacy support staff were identified through Pharmacy Area Managers for the pharmacy locations where the focus groups were taking place. Participants were recruited irrespective of age, gender and ethnicity. People unable to understand verbal explanations given in English were excluded given that focus groups rely on verbal interaction between participants to gain a rich data set. People who were unable to adequately understand verbal English were considered unlikely to be able to participate adequately in focus groups where ideas and topics of conversation could move at a fast pace. The use of an interpreter was also discounted on the grounds that it would interfere with the group dynamic.
Procedure
All potential participants were given written information about the research and a minimum of 24 hours to consider their participation and ask any questions before providing written informed consent. The Participant Information Sheet highlighted participants’ right to withdraw from the research at any time without giving reason (see Project Webpage Document for Participant Information Sheet and Consent Form). Focus groups were facilitated by a member of the research team (see Project Webpage Document for the topic guide). A second facilitator took notes on the session and gave a brief presentation at the start of the focus group to explain the purpose of the study and to describe the interventions of MM and CM. A visual summary of the ADAM study, an example of a CM schedule, and actual adherence recording bottles with the electronic Medication Event Monitoring System (MEMS) cap were shown to participants at the start of each focus group to aid their understanding. All groups lasted 60–90 minutes and were audio-recorded and transcribed in full. All participants were reimbursed their travel expenses and given £20 cash to thank them for their time. Data were anonymised and stored securely (password-protected computers/laptops, locked filing cabinets in lockable offices in buildings with swipe assess and security presence) in accordance with Good Clinical Practice and King’s College London’s Standard Operating Procedures.
Analysis
A modified framework analysis was used to analyse the data thematically. 100,101 Codes were developed inductively from the transcripts as well as deductively from the topic guide by a researcher allocated to analyse each focus group. The transcripts were coded line by line using NVivo (version 11) (QSR International, Warrington, UK) program.
Results
Service user focus groups
Please see Table 1 for an overview of the participant characteristics.
| Characteristics | Focus group Site 1 (n = 10) |
Focus group Site 2 (n = 6) |
Focus group Site 3 (n = 5) |
Focus group Site 4 (n = 5) |
Total (n = 26)a |
|---|---|---|---|---|---|
| Age years: mean (SD), range | 50 (7.91), 35–60a | 45 (2.80), 42–50 | 45 (9.26), 30–54 | 52 (12.17), 35–69 | 48 (8.36), 30–69b |
| Gender | |||||
| Female | 5 (50) | 2 (33) | 1 (20) | 2 (40) | 10 (38) |
| Male | 5 (50) | 4 (67) | 4 (80) | 3 (60) | 16 (62) |
| Ethnicity | |||||
| Asian/Asian British | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 1 (4) |
| Black/African/Caribbean/Black British | 2 (20) | 0 (0) | 0 (0) | 0 (0) | 2 (8) |
| Mixed/multiple ethnicity | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| White British | 7 (70) | 4 (67) | 5 (100) | 5 (100) | 21 (81) |
| White Irish | 1 (10) | 1 (17) | 0 (0) | 0 (0) | 2 (7) |
| Currently receiving relapse prevention treatment (psychosocial or pharmaceutical) for alcohol dependence | |||||
| Yes | 4 (40) | 5 (85) | 3 (60) | 3 (60) | 15 (58) |
| No | 6 (60) | 1 (17) | 2 (40) | 2 (40) | 11 (42) |
| Ever been prescribed acamprosate | |||||
| Yes | 2 (20) | 5 (83) | 4 (80) | 2 (40) | 13 (50)c |
| No | 8 (80) | 1 (17) | 1 (20) | 3 (60) | 13 (50) |
Four themes were identified:
-
Concerns about support and availability of alcohol relapse prevention medication
Participants reported difficulties accessing relapse prevention treatment, including acamprosate, and limited professional support following alcohol withdrawal. Participants reported accessing alternative types of support, other than with health-care professionals including mutual aid groups (e.g. alcoholics anonymous and SMART groups).
-
Perceptions of acamprosate treatment
There were conflicting views on the benefit of acamprosate. Many participants expressed that acamprosate could help them reduce their cravings and remain abstinent for longer. However, others appeared to doubt the effectiveness of acamprosate. Participants from all groups expressed concerns about the side effects of acamprosate (e.g. gastrointestinal problems and nausea) and it appeared that participants were not made aware of the potential side effects and were unsure how to seek help if they were experienced. Participants from all groups expressed difficulties with taking acamprosate, particularly remembering to take six tablets a day. Few expressed that the number and frequency of tablets created an additional barrier as they were taking multiple medications for other health concerns.
-
Perceptions of acamprosate adherence telephone support role for pharmacists
Participants from all focus groups expressed positive views of pharmacists being able to help resolve their concerns of acamprosate. Participants agreed that intervention calls should be structured, take place regularly, have flexibility in the length of the call according to need, and be led by the service user. Participants also expressed a need for greater support during more difficult times in their recovery, in particular, the initial period following alcohol withdrawal. There was some concern expressed by participants in each of the focus groups that telephone support could be impersonal and face-to-face was preferred. Some participants expressed some uncertainty with how knowledgeable and skilled pharmacists would be to provide telephone support for acamprosate and they also highlighted the need for good communication skills for this role.
-
Perceptions of CM to support acamprosate adherence
Participants initially expressed strong negative views towards CM, expressing that individuals should not be rewarded for engaging in treatment. Participants had reservations related to the effectiveness of CM and held the belief that the money would be used to buy alcohol. However, during the focus group, initial negative views became more positive when considering CM in the context of rewarding someone in recovery. The type of CM incentive was discussed by participants and it was suggested that it should be practical and relevant to the needs of the service user. Furthermore, participants indicated a preference for the payment of the CM incentive to be given at intervals throughout the trial. Participants suggested alternatives to cash expressing that they wanted the incentives to not only impact them financially but also personally and make them feel valued.
Pharmacist focus groups
Please see Table 2 for an overview of the participant characteristics.
| Characteristics | Focus group Site 1 (London) (n = 6) |
Focus group Site 2 (West Midlands) (n = 5) |
Focus group Site 3 (Wessex) (n = 4) |
Focus group Site 4 (Yorkshire and The Humber) (n = 3) |
Total (n = 18) |
|---|---|---|---|---|---|
| Age years: mean (SD), range | 42 (11.68), 29–60a | 47 (11.28), 29–58 | 34 (5.26), 28–37a | 52 (9.71), 44–63 | 44 (11.21), 28–63 |
| Gender | |||||
| Female | 1 (17) | 1 (20) | 1 (25) | 1 (33) | 4 (22) |
| Male | 5 (83) | 4 (80) | 3 (75) | 2 (67) | 14 (78) |
| Ethnicity | |||||
| Asian/Asian British | 6 (100) | 2 (40) | 2 (50) | 0 (0) | 10 (56) |
| White British | 0 (0) | 3 (60) | 2 (50) | 3 (100) | 8 (44) |
| Years registered as a pharmacist: mean (SD), range | 20 (12.48), 2–36 | 25 (12.98), 4–37 | 11 (5.26), 5–15 | 30 (8.94), 21–39 | 21 (11.96), 2–39 |
| Years practiced as a community pharmacist: mean (SD), range | 20 (12.48), 2–36 | 25 (12.98), 4–37 | 11 (5.26), 5–15 | 30 (8.94), 21–35 | 20 (11.52), 2–37 |
| Drug misuse training since registration (hours): mean (SD), range | 25 (18.33), 29–60 | 218 (313.54), 0–750 | 14 (8.64), 4–23 | 29 (8.78), 20–38 | 77 (177.17), 0–750 |
| Smoking cessation training since registration (hours): mean (SD), range | 90 (115.02), 8–42 | 24 (17.10), 0–45 | 13 (14.59), 3–35 | 28 (20.46), 10–50 | 44 (71.97), 0–50 |
| Alcohol misuse training since registration (hours): mean (SD), range | 10 (10.71), 0–30 | 34 (39.27), 0–90 | 1 (0.96), 0–2 | 6 (8.14), 0–15 | 14 (24.14), 0–90 |
Five themes were identified:
-
Challenges affecting medication adherence
This theme refers to the barriers and incentives to patients’ adherence to medications, including the importance of adherence to treatment effectiveness as well as ways to support adherence.
-
Assumptions about patients with alcohol problems
Participants in the focus groups expressed some assumptions made about those with alcohol dependence that related to their socioeconomic status, mistrust of health-care professionals, inevitability of relapse and comorbid problems. Pharmacists also expressed some negative attitudes and perceptions towards patients with alcohol problems. They separated the patient group as being ‘other’ or different to professionals, and implied that this patient group would be particularly motivated by money.
-
Pharmacists’ training and support needs
Pharmacists expressed concerns about the existing burden and work pressures they face, and highlighted challenges to combining their clinical work with research activities within a pharmacy setting. They expressed there was lack of alcohol-specific training as well as time to undertake the training.
-
Practical considerations on the delivery of an alcohol relapse medication adherence service
Pharmacists appeared inquisitive about the ADAM study and the proposed health intervention; this included querying the rationale to establish their understanding of how the study would work. They showed interest with the study and its proposed methods. Pharmacists had an overall positive view of research, deeming it to be worthwhile and something pharmacists would engage with.
-
Unique professional characteristics of pharmacists
The pharmacists expressed the uniqueness of their role as pharmacists in the community to provide health-care advice and services to their patients and the public. They highlighted the benefits of involving pharmacists in research and this was an untapped resource.
The themes that arose from the focus groups with service users and pharmacists were incorporated into the development of the MM and CM interventions, as discussed in subsequent sections of this chapter.
Patient and public involvement
Following the focus groups with service users, we met with the King’s College London Addiction Department Service User Research Group (SURG) to discuss the MM and CM interventions in more detail. We presented the group with examples of three different CM schedules to initiate discussions of the monetary value and type of schedule (fixed vs. escalating schedule). The group expressed the importance of the simplicity of the CM schedule, finding CM schedules that used an escalating reset design too complex and difficult to follow. However, the group did see the value in receiving an increasing amount of incentive for completion of each session, suggesting it would enhance motivation through feeling more valued. The idea of including a bonus for completion of a set number of calls was discussed, with the group believing it would also help with motivation. The group expressed concern with including a reset of the monetary value for missed calls. They believed that it may be detrimental for those who are already vulnerable, with them feeling they were being punished, which could lead to treatment dropout or relapse. The type of incentive was discussed and the group stressed the importance of the incentive being of value to the individual. The group suggested the incentive could be something personal to the individual and that this could be identified at the start of treatment as a goal to work towards. The term ‘Contingency Management’ was not favoured by service users who participated in the focus groups. This term was discussed with the SURG and the idea of calling it a ‘Personal Achievement Award’ was raised. The idea of text messages to reinforce the CM value obtained and as a practical reminder of future MM sessions was discussed. The frequency and length of the MM calls were discussed. The group were happy with the number of calls and agreed with the more intensive support at the start when a person may need it most. There was a preference for continuity in the pharmacists delivering the intervention to allow rapport and trust to be built.
Developing the interventions
Medication Management
The focus groups with service users and pharmacists highlighted the importance of clear guidance but with room for the conversation to be led by the needs of the service user. The importance of excellent communication skills and knowledge of alcohol dependence was also noted. This was incorporated into the guidance documents produced and the pharmacist training (see Project Webpage Document). Treatment goals were identified during the welcome call to help tailor the intervention to the participants’ needs. A printed summary letter was sent to participants that they were encouraged to edit and goals were revised during the subsequent calls. We developed a partnership with Celesio/Lloyds Pharmacies who provided essential input into how the MM calls were delivered. They suggested using a call centre with a small pool of specially trained pharmacists to deliver the intervention. This allowed continuity of the pharmacist delivering the intervention for participants, which had been highlighted as important during both the focus groups and meeting with SURG for building rapport. Text message reminders were also incorporated as suggested by SURG. The length and number of MM sessions were guided by the original48,93 MM manual as well as conversations with SURG and Celesio/Lloyds pharmacies, with a longer initial ‘welcome call’ (~30 minutes) followed by shorter (10–15 minutes) follow-up calls weekly for 6 weeks, fortnightly for 6 weeks and monthly for 3 months (12 calls in total). More frequent calls were completed at the start of treatment when the greatest support may be required with calls becoming less frequent over the 6-month period.
Contingency Management
To maximise the effectiveness of CM, three key principles have been identified in the research literature: clear and objective verification of the treatment goal, immediacy of the reinforcement and significant magnitude of the reinforcement. 102,103 These principles were taken into consideration in conjunction with the results of the focus group and patient and public involvement work when developing the CM schedule (see Chapter 3, Table 3).
Clear and objective verification of the treatment goal
For the current study, the treatment goal to be incentivised using CM was completing the MM telephone calls. A standard text message was developed that was sent on completion of each of the MM calls to all participants in the MM or the MM + CM group. The text message sent to those in the MM + CM group included information on the amount of incentive awarded for completion of that particular call and the total amount of CM achieved to date. Through speaking with the SURG group, it was clear that in order to maintain motivation of participants avoiding penalising participants was key. For example, there was concern that if calls were missed due to reasons out of participants’ control, they would be unfairly penalised. To help mitigate this, a clear missed call decision tree was developed that allowed some flexibility in completion of the calls while maintaining the core components of the CM (see Project Webpage Document for the missed call decision tree). This procedure was made clear to participants in the initial welcome call and text messages were used to alert participants when calls were missed and when the next call attempt would be made.
Immediacy of the reinforcement
During the focus groups, service users expressed mixed opinions on when the incentive should be given, after each call, periodically throughout the study or all at the end. Due to the discussions with service users attending the focus groups and the SURG about working towards a goal, and for practical reasons, it was decided that the full voucher incentive would be given to participants following the final MM call. However, participants were given the option to receive the CM incentive that they had achieved earlier if preferred. To reinforce achievement of the CM incentive, participants were informed by the intervention pharmacists of the CM incentive achieved for completing that particular call and the total incentive achieved, and they additionally received a text message as described above.
Significant magnitude of the reinforcement
Through the focus groups and our discussions with the SURG the importance of the personal value, as opposed to financial value, of the CM incentive was highlighted. Due to practicalities, we could not tailor the CM incentive to each participant. Love to Shop vouchers were chosen as the incentive as they have a wide choice of shops as well as family days out. In the initial welcome call discussion, the intervention pharmacists encouraged participants to set personal goals for the CM incentive, identifying what they may wish to purchase with the voucher. This was also reinforced by the use of the term ‘Personal Achievement Award’ that was used in all communication with participants in line with the suggestions made in the focus groups and SURG group meeting. The monetary value of completing each MM call was based on the discussions had with the SURG and included an escalating bonus (see Chapter 3, Table 3).
Developing pharmacist training
Detailed training manuals were developed for the MM and CM interventions, including welcome call and the weekly/fortnightly/monthly calls (see Project Webpage Document). An initial two-day face-to-face training was completed with the intervention pharmacists. The training consisted of a general overview of the ADAM trial, Good Clinical Practice, discussion of the pharmacist’s experience of supporting those with an alcohol problem and a demonstration of how to administer the MM calls (with feedback and questions). The intervention pharmacists were then given the opportunity to practice delivering the MM calls, which were audio-recorded to allow peer and research team assessment and feedback. At the end of the two-day training, the intervention pharmacists completed an assessment where they role-played completion of an MM and an MM + CM call. The scenarios were identical for each pharmacist and were assessed by members of the ADAM study team using an assessment tool developed by the ADAM study team (see Project Webpage Document). This was a summative assessment, with feedback and guidance given to the intervention pharmacists. Two further refresher training sessions were completed with the intervention pharmacists during the trial, and additional training sessions with a clinical psychologist specialising in addictions were completed. These were informal sessions that were led by the needs of the intervention pharmacists. In addition, each month 10% of the completed MM calls were assessed by trained members of the research team for fidelity to the intervention. Verbal and written feedback was given to the intervention pharmacists to support intervention fidelity.
Chapter 3 Trial methods
Material throughout the chapter has been adapted from the trial protocol. 1
Design and theoretical/conceptual framework
The study was a three-arm, parallel-group pragmatic, randomised controlled trial. The trial began with an internal pilot phase to demonstrate that recruitment, randomisation and the interventions were implemented as planned. The methodology of the pilot phase and the full trial were identical allowing for data collected during the pilot trial to be included in the statistical analyses of the primary and secondary outcomes on completion of the full trial.
Participants were prescribed acamprosate by participating in local specialist alcohol treatment services as soon as possible after alcohol abstinence was achieved. Follow-up contacts with the research team took place 6 (+60 days follow-up window) and 12 months (+60 days follow-up window) post randomisation. Participants collected their medication monthly from the community pharmacy, dispensed from designated pharmacies in bottles fitted with MEMS Caps. Eligible and consenting participants were randomised to receive either SS or SS plus MM (SS + MM) or SS plus MM and CM (SS + MM + CM). Allocation was conducted in the ratio of 2 : 1 : 1, SS : SS + MM : SS + MM + CM, respectively.
Treatment arm 1: Standard Support
All participants in the trial were prescribed acamprosate (two 333 mg tablets morning, afternoon and evening if the service user’s body weight was 60 kg or above, or two 333 mg tablets in the morning and one 333 mg tablet in the afternoon and evening if the service users body weight was below 60 kg, according to the manufacturer’s Summary of Product Characteristics) as soon as possible following alcohol abstinence in addition to the psychosocial care normally provided. The decision to initiate acamprosate was determined by the treating clinician in the specialist alcohol service in conjunction with the service user.
Based on the current service provision of the five study centres, SS comprised monthly dispensing of prescribed acamprosate from the Lloyds community pharmacy, monthly monitoring of the service user for 3 months by the specialist alcohol service, and then returned to the care of their GP for monthly monitoring in accordance with the NICE guidelines104,105 and current NHS clinical practice.
Treatment arm 2: Standard Support plus Medication Management (SS + MM)
Participants followed the same care pathway as those in the SS arm of the trial with the addition of MM, which was delivered by a central telephone support service by trained pharmacists. The MM intervention was adapted from the Medical Management intervention developed by Pettinati et al.,106 for the Testing Combined Pharmacotherapies and Behavioral Interventions in Alcohol Dependence study, a randomised controlled clinical trial of naltrexone and acamprosate for alcohol dependence. A freely available comprehensive manual was published by the Testing Combined Pharmacotherapies and Behavioral Interventions in Alcohol Dependence research group. This was used as a basis for the MM intervention for the proposed research. Adaptation was made in consultation with service users and pharmacists to ensure that it is suitable and acceptable in the context of a UK central pharmacy telephone support service, delivered by trained pharmacists (see Chapter 2).
MM was delivered once a week for the first 6 weeks, reducing to once a fortnight for the following 6 weeks, and then monthly for 3 months, following the same schedule as the Testing Combined Pharmacotherapies and Behavioral Interventions in Alcohol Dependence study. MM was delivered by telephone by a trained pharmacist based in a central telephone support service in the UK provided by Celesio/Lloyds Pharmacy. The initial MM session lasted approximately 30–45 minutes and acted as a foundation for the subsequent sessions, which lasted approximately 10–15 minutes each. Each participant was assigned a specific pharmacist based in the central telephone support service to deliver each of the MM sessions for that participant where possible. The pharmacist sent a text message reminder the day before the appointment and called the participant to deliver the MM session at an agreed time.
MM provided support in developing strategies to help participants to manage their medication including the rationale for taking acamprosate, adhering to the dose regimen and managing side effects, education about their medication and alcohol dependence, and supporting participants’ efforts to change their drinking behaviours. Treatment goals were identified to tailor the intervention to the participant and an individual plan for maintaining adherence was developed with the participant in the initial session to guide the successive MM sessions. Over the 6-month period, the pharmacist sent four summary letters to the participant highlighting the participant’s individual aims, goals and key information regarding their MM plan.
Treatment arm 3: Standard Support plus Medication Management with Contingency Management (SS + MM + CM)
Evidence shows that there can be barriers to participation in MM sessions. 107 To optimise attendance, participants in this arm followed the same care pathway as those in the SS + MM arm of the trial but with the addition of CM. Incentives in the form of Love to Shop vouchers (not redeemable for alcohol) were provided to reinforce attendance at MM sessions by telephone with the pharmacist. The CM procedure and value of the incentives have been informed by the available literature on CM in substance use disorder80 and alcohol dependence104 and focus groups with service users with experience with treatment services for alcohol dependence (see Chapter 2).
Participants received £5 in the form of a voucher for each MM session completed. In addition, they received a £10 bonus voucher for completing four calls in succession, a £20 bonus for completing eight calls and a £30 bonus for completing all 12 calls, with a total value of up to £120 for completing all support sessions (see Table 3). After each MM session, a SMS text message was sent to the participant to inform them that they had been awarded a voucher, the magnitude of the voucher and the total voucher value received to date. Vouchers were given on completion of the 6-month follow-up visit by the research team unless the participant withdrew from the trial or requested part payment earlier.
| Sessions completed | Incentive | Bonus | Running total |
|---|---|---|---|
| 1 | £5 | £5 | |
| 2 | £5 | £10 | |
| 3 | £5 | £15 | |
| 4 | £5 | £10 | £30 |
| 5 | £5 | £35 | |
| 6 | £5 | £40 | |
| 7 | £5 | £45 | |
| 8 | £5 | £20 | £70 |
| 9 | £5 | £75 | |
| 10 | £5 | £80 | |
| 11 | £5 | £85 | |
| 12 | £5 | £30 | £120 |
| Total | £60 | £60 | £120 |
Study setting/context
Participants were recruited from specialist alcohol treatment services based in England. SS was provided by these specialist services and the participants’ General Practitioners as per current standard practice. MM and CM were administered by pharmacists via a central telephone support service in the UK.
Target population
Abstinent alcohol-dependent adults within the first month of prescription of acamprosate and who were both willing and able to provide informed consent to take part in the research.
Inclusion/exclusion criteria
Inclusion and exclusion criteria were selected to recruit a sample that is broadly representative of target population as a whole. The decision to prescribe acamprosate was made by the service users treating clinician in conjunction with the service user; the research team was not part of this decision.
Inclusion criteria
-
Adult, aged 18 years or older.
-
An International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, diagnosis of alcohol dependence.
-
Abstinent from alcohol at baseline assessment.
-
In receipt of a prescription for acamprosate.
-
Willing and able to provide informed consent.
Exclusion criteria
-
Diagnosis of a severe physical/mental illness likely to preclude active participation in treatment or follow-up.
-
Unable to understand verbal English at a level necessary to engage in the intervention and follow-up assessments.
-
Concurrent dependence on an illicit substance (other than cannabis).
Study entry and baseline assessment
Potential participants were initially contacted by a member of their specialist alcohol service, who sought permission for a member of the research team to contact the patient. The research team member subsequently contacted the patient to provide details of the nature and purpose of the research and an information sheet. Informed consent and inclusion/exclusion criteria were reviewed by a trained researcher at an initial assessment appointment that took place at least 24 hours after the study information sheet was given, to allow time to consider the information and ask any questions. Participants were then randomised by an independent party using a secure randomisation system, following consent and baseline assessment.
Withdrawal of participants
It was made clear to participants that the SS that they receive would not be affected by their decision whether or not to take part in the research and they were free to withdraw at any time for any reason. Data collected up to the time of withdrawal would be used as appropriate unless the participant specifically requested that the data already collected was destroyed. Withdrawn subjects were replaced as far as possible within the constraints of the duration of recruitment. If the decision not to continue prescribing/taking acamprosate was made at any stage of the trial, participants were withdrawn from the treatment and scheduled outcomes collected.
Alcohol abstinence
If participants resumed alcohol consumption during the trial period, this did not exclude them from any aspect of taking part in the trial.
Randomisation
Randomisation was in the order of 2 : 1 : 1 to maximise the utility of resources with twice as many being allocated to the SS group than the two intervention groups. Randomisation was carried out after consent had been gained and the initial baseline assessment had been conducted. A remote randomisation procedure was used through an online system developed and maintained by the company Codeface Ltd to generate the treatment allocation, which was initiated by a trained researcher. Allocation employed a stratified random permuted block method with stratification by severity of alcohol dependence [Severity of Alcohol Dependence Questionnaire (SADQ) score of ≤ 30 or > 30], research site and the prescription of other relapse prevention medication. These variables are known to be related to clinical outcomes and were collected at the baseline assessment. Due to the nature of the trial, participants and research staff were not blind to treatment allocation. The trial statistician was blind to treatment allocation.
Data collection and management
Table 2 outlines the study outcome measures and timing of their administration during the study. Research and personal data were collected using electronic data capture, specifically designed for this research study, using a laptop computer. Laptop computers were password protected. Data were entered and saved on a secure server with a 256-bit encryption (SSL/https) connection; no data were saved directly onto the laptop computer. Research data were anonymised by assigning each participant a unique ID number, with personal data stored separately from the research data to maintain participant anonymity. The Chief Investigator, Trial Manager and Trial Statistician have access to the final full data set.
Outcome measures
The primary outcome measure was the self-reported percentage of medication taken as prescribed during the 6-month target phase of prescribing, post randomisation.
Secondary outcome measures
Secondary, process and economic outcome measures are detailed in Table 4. At baseline assessment, participant demographics were collected as well as a history of use of acamprosate, other relapse prevention medication use, and previous medically assisted detoxification using a medical history checklist devised specifically for the trial. The substance use section of the Alcohol, Smoking and Substance Involvement Screening Test-Lite (ASSIST-Lite)104 was administered to obtain a history of any substance use.
| Baseline | Months 2 and 4 | Month 6 | Month 12 | |
|---|---|---|---|---|
| Clinical outcomes | ||||
| MEMSCap | ✓ | ✓ | ✓ | |
| Pharmacist pill count | ✓ | ✓ | ||
| Past 28 days adherence self-report | ✓ | ✓ | ✓ | |
| MMAS-8 | ✓ | ✓ | ✓ | |
| SADQ | ✓ | ✓ | ✓ | |
| AUQ | ✓ | ✓ | ✓ | |
| TLFB 28 days | ✓ | ✓ | ✓ | |
| Economic outcomes | ||||
| Health-related quality of life (EQ-5D-5L) | ✓ | ✓ | ✓ | |
| AD-SUS | ✓ | ✓ | ✓ | |
| Prognostic outcomes | ||||
| Demographics | ✓ | |||
| BMQ | ✓ | ✓ | ✓ | |
| APQ | ✓ | |||
| STAR | ✓ | |||
Severity of dependence was measured at the baseline assessment and at 6- and 12-month follow-ups using the SADQ. 108 The SADQ is a 20-item self-complete questionnaire containing items representing five domains of the alcohol dependence syndrome: (1) physical withdrawal signs, (2) psychological withdrawal signs, (3) withdrawal relief drinking, (4) tolerance and (5) reinstatement following a period of abstinence.
Alcohol consumption was measured using the TLFB Form 90,109 administered at initial baseline assessment and at 6- and 12-month follow-ups after initiation of acamprosate. Percentage days abstinent, units of alcohol per drinking day (1 UK unit = 8 g alcohol), relapse to any drinking and relapse to heavy drinking (8 +/6 + units for males/females on a single occasion) were derived.
Participants’ beliefs about medications were assessed using the BMQ. 110 The BMQ assesses an individual’s beliefs about medication specific to them and their health, as well as their general beliefs about medication. This questionnaire was administered at baseline assessment and at 6-month follow-up. The measure was also used to evaluate the impact of MM on beliefs and concerns about medication and the association with adherence to acamprosate. The MMAS-8 assesses non-adherence and was administered at months 2, 4, 6 and 12. 111–113
Participants in the SS + MM and SS + MM + CM groups were asked to rate their therapeutic relationship with the telephone pharmacist at 6 months using the STAR rating scale. 114 Therapeutic relationship (or alliance) has been found to predict clinical outcome across a range of mental disorders115 including alcohol dependence. 116 This was used as an additional process measure to explore the impact of therapeutic relationship on medication adherence and clinical outcome.
Alcohol-related problems were assessed at baseline assessment using the APQ. 8 The APQ is a 46-item questionnaire assessing potential problems with psychological, physical, social, legal, employment, relationships and parenting that may be experienced due to alcohol. The AUQ117 assesses current urge for alcohol using eight items which cover three factors: desire for a drink (four items); expectation of positive effect from drinking (two items) and inability to avoid drinking if alcohol was available (two items). This questionnaire was administered at baseline assessment and at 6- and 12-month follow-ups.
Measures for the economic evaluation, collected at baseline, 6- and 12-month follow-ups, included the EQ-5D-5L measure of health-related quality of life118 suitable for the calculation of QALYs and the AD-SUS, based on a version designed for use in alcohol and drug populations119 and adapted for the purpose of the ADAM trial. These measures are described in more detail in Chapter 5.
Participants were asked at each bi-monthly research visit whether they have experienced any side effects from the medication. In addition, reasons for non-adherence were recorded.
Fidelity of intervention delivery
The fidelity of delivery of MM and CM and its impact on acamprosate adherence and clinical outcomes was assessed. All MM sessions were audio-recorded. A random sample, stratified by pharmacists delivering the intervention, of 10% of all audio recordings of each of the MM and MM + CM interventions were rated by at least two trained raters who were members of the research team, using a checklist of required elements. The raters were supervised by the postdoctoral research pharmacist and the trial manager through regular meetings. The postdoctoral research pharmacist and trial manager checked 10% of the fidelity ratings completed. The information gained from checking the fidelity ratings was fed back to the raters during the regular supervision meetings to ensure as much accuracy as possible of the fidelity assessments. The information from the fidelity assessments was fed back to the pharmacists delivering the MM and CM to improve intervention fidelity.
Sample size
A clinically important difference in adherence to medication was estimated as an effect size of the order of 0.3, equivalent to a 12% difference in medication adherence between the groups and a number needed to treat of 8 for drinking outcomes, in that if any intervention strategy is found to be superior eight participants would need to be treated to create an additional participant who is abstinent. A meta-analysis120 identified a larger effect size for acamprosate versus placebo when converted to drinking outcomes of the order of 0.4, with a number of studies reporting larger effects. As with all pragmatic studies final interpretation was based on actual effects observed and the integration of economic outcomes, but an effect size of < 0.3 is unlikely to be clinically important.
To estimate this difference using power of 80%, alpha 0.05 with a two-sided tested and differential allocation of 2 : 1 : 1 required 524 analysed at the primary end point, 6 months across the three groups. Allowing for attrition of 30%, less than observed in other trials in similar populations, required a total sample size at allocation of 748; 374 allocated to SS, 187 to SS + MM and 187 to SS + MM + CM. In addition to addressing the primary outcome, the sample size was sufficient to identify a clinically important difference effect size of 0.3 in alcohol consumption measures at 6 months post randomisation.
Statistical analysis
The primary analysis is based on the analysis by treatment allocated (ATA) where participants are analysed as members of their allocated group irrespective of the treatment they receive; this provides a conservative estimate of effect. Secondary analyses examined treatment effects under different scenarios for compliance with allocation/treatment: complier average causal effects (CACE). Two scenarios of compliance were defined in this trial. In the first compliance, those in the SS + MM and SS + MM + CM group was defined as adhering to at least 50% of the MM calls. In the second, the threshold was increased to 100%. Both scenarios are modelled in the analysis.
The different scenarios of compliance with allocation for this trial are shown in Table 5. There are two scenarios according to the number of calls received. Under the first scenario those receiving 6 or more calls are considered ‘compliers’ in the active treatment groups and those receiving fewer than 6 calls are considered non compliers. Under scenario 2, those in the active treatment groups receiving 12 or more calls are considered compliers and those who receive fewer than 12 calls are considered non-compliers. (2) As all particpants in the active tretament groups also received the control treatment, all non-compliers in the treatment group are regarded as being ‘contaminated’. For the control group, there is no option for control participants to access the intervention, so there cannot be non-compliance, cell C.
| Allocated | Received | |
|---|---|---|
| SS + MM/SS + MM + CM | Control | |
| SS + MM SS + MM + CM |
A. Treatment complier Scenario 1: Received 6 or more calls Scenario 2: Received 12 or more calls |
B. Treatment non-complier Received < 6 calls Received < 12 calls |
| Control | C. Control non-complier N/A |
D. Control complier Control group participant |
Analysis by treatment allocated
Analyses all available data for participants who were randomised, regardless of whether they complied with allocation. This data set includes participants who were withdrawn from the trial post randomisation. These analyses are a lower bound estimate of treatment effects as they represent the effect of offering an intervention, rather than the effect of receiving that intervention.
Complier average causal effects
We assessed treatment effects in the presence of non-compliance, with compliance measured at the individual level and including all those allocated as part of the trial. Our approach for assessing treatment effects under non-compliance was via the instrumental variables (IV) framework. 121 The benefit of using an IV approach is that randomisation is maintained in the analysis, which is crucial for estimating unbiased treatment effects. 122 CACE weights the ATA treatment effect by the proportion of compliers (see Equation 1):
If the proportion compliant is 1.0 (i.e. perfect compliance), then the CACE estimate is the same as the ATA estimate, but otherwise the impact of this approach is to increase the magnitude of the treatment effect.
Complier average causal effects use a two-stage least squares (2SLS). The first stage model uses treatment received (T) as the outcome, with random allocation (Z) as the independent variable (see Equation 2):
Based on the stage 1 model, we then calculate predicted values of treatment received (T^) for use in stage 2. The second stage model predicts the substantive outcome (Y, e.g. days abstinent) using the predicted values of treatment received (T^) based on the stage 1 model (see Equation 3):
The CACE analysis was conducted using 2SLS estimation with the ivregress command in Stata® (StataCorp LP, College Station, TX, USA). A linear regression approach was used to assess per cent adherence with medication and per cent days abstinent from alcohol at 6 months post randomisation.
Missing data
The proportion of missing data and patterns of missingness were examined for the primary outcome, per cent adherence to medication at month 6. Levels of missing data are reported along with any systematic occurrences of missing data observed in the data sets. Where outcomes are derived scores, individual item scores were checked for systematic missingness by comparing the proportion of missing values by SADQ category (≤ 30 and > 30), current prescription of relapse prevention medication, community alcohol treatment service and allocated group.
To avoid loss of efficiency, we imputed missing primary outcome values using multiple imputation using the MI commands of Stata; we employed 50 iterations of a multiple imputation model and combined these using MIcombine. We then tested the extent to which the imputed model deviates from the model generated using the observed data. This approach makes an underlying assumption that the missing data are missing at random (MAR). To test a potential assumption that data were not missing at random (MNAR), we conducted a sensitivity analysis using a pattern mixture approach and multiple imputation to explore the sensitivity of the primary outcome results to departures from the MAR assumption, and this was implemented using the rctmiss command in Stata. 123,124
Statistical analysis methods
Analysis of the study is presented in accordance with CONSORT guidelines. 125 The primary analysis is an ATA and is based on all available data for participants who were randomised, irrespective of whether they complied with their allocation or not.
Diagnostic tests and plots were conducted to assess the assumptions of normality for per cent adherence to acamprosate at month 6. There were significant departures from normality (Project Webpage Document) and the distribution overall and by group was a bimodal distribution with a zero-inflation. As the primary outcome is a percentage, it can be recalculated as a proportion between 0 and 1 by dividing the per cent adherence by 100. The fractional nature of the outcome allows for a fractional logistic model126 to be fitted, assuming the variance in response is proportional to a binomial distribution, and employing a logit link function to maintain bonds between 0 and 1. Fixed effects were included for allocation (SS/SS + MM/SS + MM + CM) and stratification covariates (SADQ; ≤ 30 or > 30, other relapse prevention medication; yes or no, site). Results are initially presented as odds ratios, but marginal effects can be derived to present the mean difference in per cent adherence between the groups and the associated 95% CI. Secondary analysis of the primary outcome encompasses multiple imputation to assess missing data as MAR, sensitivity analysis to assess missing data being MNAR and inclusion of data on adherence to allocated intervention using two pre-specified scenarios incorporated into a CACE analysis. Analysis was undertaken to explore differences between SS + MM + CM versus SS and then to explore differences between SS + MM versus MM. As significant effects were observed on the primary outcome measure, an analysis exploring SS + MM + CM versus SS + MM was then undertaken.
Secondary outcomes are analysed in a similar manner by assessing distributional assumptions and conducting an appropriate regression with the inclusion of the same covariates as the primary analysis. Where baseline values for the secondary outcome are available, these are also included as covariates.
Stepwise regression was conducted to model the between pre-randomisation factors and per cent adherence to Acamprosate and per cent days abstinent at month 6. Interaction terms with treatment allocation were included in the model and a significance level of 0.1 was used as a threshold to determine which variables were maintained in the final model reported. Baseline variables included initially in the model include age, gender, marital status, ethnicity, employment status, number of children, age of first drink, weekly and daily drinking, frequency and quantity of alcohol consumption, severity of alcohol dependence, alcohol urges and alcohol-related problems. This analysis was augmented with an additional analysis for the SS + MM and SS + MM + CM groups where the same dependent variable was assessed with the same independent variables with the addition of therapeutic alliance.
Economic analysis
Please see Chapter 5 for full details of the health economic evaluation.
Safety reporting
A serious adverse event (SAE) is defined as an untoward occurrence that:
-
results in death
-
is life-threatening
-
requires hospitalisation or prolongation of an existing hospital stay
-
results in persistent or significant disability or incapacity
-
consists of a congenital anomaly or birth defect, or
-
is otherwise considered medically significant by the investigator.
The Chief Investigator reported any SAE to the Research Ethics Committee (REC) who provided ethical opinion within 15 days of becoming aware of the event if the event was related (i.e. resulted from administration of any of the research procedures), and unexpected (i.e. the type of event is not listed in the protocol as an expected occurrence). The Chief Investigator reported on the safety of participants in the annual progress report to the REC.
Ethics and dissemination
The trial was conducted in compliance with the principles of the Declaration of Helsinki (1996) and Good Clinical Practice (GCP), and in accordance with all applicable regulatory requirements including, but not limited to, the Research Governance Framework (Department of Health, 2008). At each research appointment (baseline assessment, 6- and 12-month follow-ups), all participants were given £10 cash to compensate them for their time, plus travel expenses.
Participants’ anonymity was preserved throughout using code numbers for all data collection. Data were anonymised and stored by secure means (password-protected computers/laptops, locked filing cabinets in lockable offices in buildings with swipe access and security presence).
Ethical approval has been granted by the East of England – Cambridge South Research Ethics Committee (Ref: 15/EE/0308). All participants gave written informed consent to take part in the research.
Monitoring
An independent Steering Committee was set up and approved by the National Institute for Health Research. An independent Data Monitoring Committee was set up and included an independent statistician who is not otherwise involved in the project. A trial management group met regularly to monitor the progress of the trial. This group included the Chief Investigator, the Principal Co-Investigators, the researchers, clinicians and service user representative.
Amendments
During the trial, six substantial amendments were made to the trial protocol, Table 6 details these amendments and the rationale for each. No amendments affected participant safety.
| Version | Date | Amendment | Rationale for amendment |
|---|---|---|---|
| 1.0 | 21 July 2015 | N/A | N/A |
| 2.0 | 18 November 2015 | Inclusion of a summary letter to participants receiving MM | The summary letter provided a visual reminder of the aims, goals and key information of the participant’s MM plan |
| 3.0 | 05 September 2016 | Additional acamprosate supply reporting questions | To capture instances when participants receive additional emergency supplies of acamprosate – e.g. when admitted to hospital or an emergency supply from a pharmacy |
| 4.0 | 02 May 2017 | Extension of data collection window to 60 days at 6- and 12-month follow-up | To maximise data collection by extending the time frame for follow-up |
| 5.0 | 20 June 2017 | Removal of current dependence on cannabis use as an exclusion criterion | To maximise participant recruitment. Following advice from the Trial Management Group, it was felt that cannabis use would not hinder the efficacy of the trial interventions but was negatively impacting on recruitment |
| 6.0 | 13 May 2019 | Removal of the 12-month follow-up assessment | To maximise participant recruitment. The 12-month follow-up assessment was removed to extend the recruitment period without extending the trial end-date |
| 7.0 | 21 July 2020 | Clarification of the 12-month data analysis | Removal of the 12-month follow-up period was not intended to alter the data analysis plans. Clarification to the working of protocol V6 to reflect this was therefore made |
Deviations from the statistical analysis plan
The APQ was not collected at 6 and 12 months, which is a deviation from the trial protocol. In addition, two key changes were implemented that deviated from the statistical analysis plan. These changes were agreed with the trial steering committee.
-
Measuring the primary outcome
Our primary outcome was per cent adherence to acamprosate at month 6. At the design stage, we explored the current literature on measuring adherence and settled on a hierarchy of measure. Our first measure was to be the MEMS, where medication is placed in a pill bottle and a cap measures every time the cap was opened. As acamprosate is taken in three daily doses (666 mg three times per day or 666 mg once followed by 333 mg twice a day), we planned to calculate the denominator as the number of days in the period between baseline and month 6 multiplied by three and the numerator the number of MEMS registered openings; this would allow an objective measure of adherence. At month 6, MEMS data were available for 514 participants, 70%, yet only one participant was 100% adherent and the majority, 383 (75%) had zero adherence with a mean adherence of 23% (95% CI 21% to 26%). Exploration of qualitative notes taken at the time of follow-up suggested large numbers of participants complied with their medication but found the MEMS device inconvenient and cumbersome, rather than taking medication out of the device many either stopped using it at all or took their daily medication out on a weekly basis. An alternative objective measure was pill counts conducted by the pharmacist when participants came to collect their prescribed medication, but it was clear early in the trial that this method was fraught with complications. Many participants did not actually attend the pharmacy to collect medications, those that did often did not bring their remaining pills for counting and when pill counts were conducted, they often failed to include details of what medication had been previously dispensed. Pharmacy pill counts were not considered a reliable source of adherence data. Our third source of adherence data was self-report, where we asked participants at month 6 to estimate the per cent of days they had been adherent to medication in the past 28 days. At month 6, self-report data were available for 514 participants, 70%, of whom 257 (50%) stated they had been completely non-adherent, 70 (15%) had been 100% adherent and the mean adherence was 42% (95% CI 38% to 46%). There was a mean difference between self-report and MEMS of 12.3% (95% CI 8.7% to 15.9%). Considering the known issues with the MEMS data, we decided to use the self-report adherence as the primary outcome. Our reasoning was based on evidence that in the trial, the MEMS device underestimated adherence, evidence that self-report is a valid and reliable method for estimating adherence127 and that self-report estimates over shorter period are reliable proxies for adherence over longer periods for those with chronic conditions. 128
-
Exploring the impact of participants being followed up late at the month 6 primary end point
In our trial protocol, we stated that the primary end point would be 6 months after randomisation. As we are dealing with a relatively hard-to-reach group, we allowed a window of + 60 days around the 6-month follow-up point. It became apparent prior to analysis that some participants had 6-month follow-up assessments conducted later than 6 months plus 60 days. We identified 45 participants that fell into this group, 9% of those followed up at month 6. Exploring baseline variables for those followed up late versus those not, identified no differences in terms of demographics, allocation, or outcomes (Project Webpage Document). We did note that the majority of those followed up late indicated zero adherence to acamprosate at month 6, 34 out of 45 (76%). To place maximum confidence on our findings, we proposed to include an additional sensitivity analysis of the primary outcome where we include and exclude these participants to explore any impact of late follow-up on the outcomes observed.
Chapter 4 Trial statistical analysis
A full CONSORT diagram is presented in Figure 1. Participant recruitment took place between July 2016 and December 2019, and participant follow-ups were completed in July 2020. A total of 1459 potential participants were approached of whom 1019 (70%) were assessed. Of these, 739 (73%) were eligible and consented to participate in the study. Allocation was in the ratio of 2 : 1 : 1, 372 (50%) were allocated to SS, 182 (25%) to SS + MM and 185 (25%) to SS + MM + CM. At the primary end point, 6 months post randomisation, 518 (70%) were successfully followed up. Seventy-five serious adverse events were reported during the trial (including 16 deaths), none of which were considered to be related to the trial interventions.
FIGURE 1.
Alcohol Dependence and Adherence to Medicine trial CONSORT diagram.
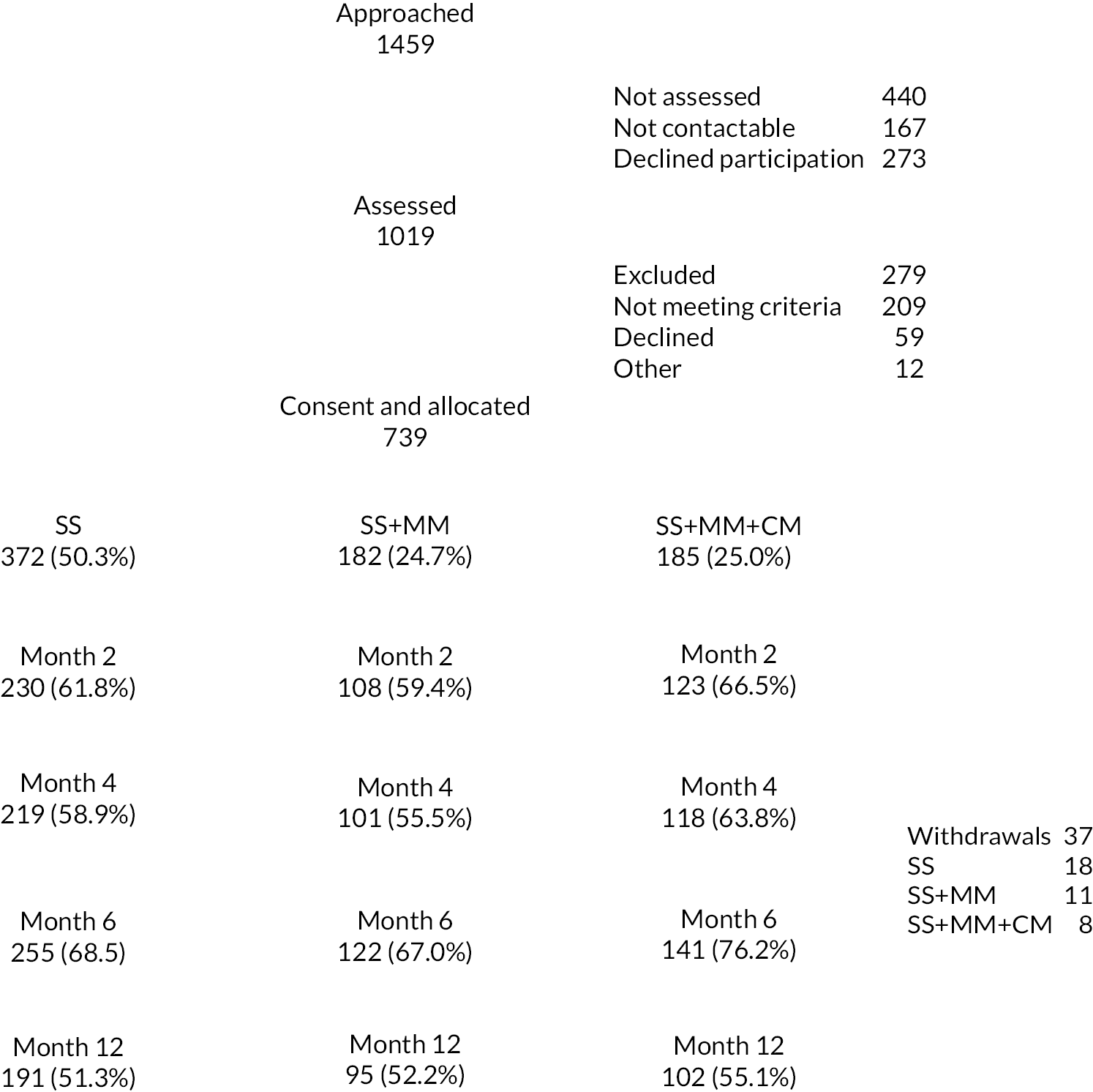
Of those allocated to one of the intervention groups, 105 (58%) adhered to 50% of the MM calls in the SS + MM group and 128 (69%) in the SS + MM + CM group. Compliance with 100% of MM calls also differed by allocated group with 20 (11%) complying in the SS + MM group and 73 (39%) complying in the SS + MM + CM group. The allocation method appears to have worked with no observed differences in demographics (see Table 7) or baseline outcomes (see Table 8) between the groups at baseline.
| SS + MM + CM (n = 186) |
SS + MM (n = 182) |
SS alone (n = 372) |
Overall (n = 740) |
|
|---|---|---|---|---|
| Site, n (%) | ||||
| Birmingham | 57 (30.7) | 55 (30.2) | 111 (29.8) | 223 (30.1) |
| C&NW London | 38 (20.4) | 40 (22.0) | 78 (21.0) | 156 (21.1) |
| South East London | 19 (10.2) | 16 (8.8) | 38 (10.2) | 73 (9.9) |
| Southampton | 22 (11.8) | 24 (13.2) | 48 (12.9) | 94 (12.7) |
| Yorks & Humber | 50 (26.9) | 47 (25.8) | 97 (26.1) | 194 (26.2) |
| Mean age [standard error (SE)] | 45.9 (0.74) | 46.7 (0.75) | 46.2 (0.55) | 46.2 (0.38) |
| Male, n (%) | 114 (61.3) | 109 (60.2) | 226 (60.8) | 449 (60.8) |
| Ethnicity, n (%) | ||||
| White | 153 (82.3) | 154 (84.6) | 317 (85.2) | 624 (84.3) |
| Black | 13 (7.0) | 9 (5.0) | 14 (3.8) | 36 (4.9) |
| Asian | 13 (7.0) | 13 (7.1) | 23 (6.2) | 49 (6.6) |
| Other | 7 (3.7) | 6 (3.3) | 18 (4.8) | 31 (4.2) |
| Main occupation ever, n (%) | ||||
| Professional | 45 (24.9) | 53 (29.4) | 109 (30.6) | 207 (28.9) |
| Skilled | 57 (31.5) | 53 (29.4) | 107 (30.1) | 217 (30.3) |
| Semi-skilled | 32 (17.7) | 37 (20.6) | 64 (18.0) | 133 (18.5) |
| Unskilled | 41 (22.6) | 32 (17.8) | 67 (18.8) | 140 (19.5) |
| Unemployed | 2 (1.1) | 4 (2.2) | 3 (0.8) | 9 (1.3) |
| Other | 4 (2.2) | 1 (0.6) | 6 (1.7) | 11 (1.5) |
| Current employ status, n (%) | ||||
| Full time | 45 (24.3) | 40 (22.0) | 85 (22.8) | 170 (23.0) |
| Part time | 11 (5.9) | 6 (3.3) | 22 (5.9) | 39 (5.3) |
| Unemployed/incapacity | 118 (63.4) | 123 (67.6) | 238 (64.0) | 479 (64.7) |
| Student | 1 (0.5) | 1 (0.5) | 1 (0.3) | 3 (0.4) |
| Other | 11 (5.9) | 12 (6.6) | 26 (7.0) | 49 (6.6) |
| Accom. status, n (%) | ||||
| Owner | 63 (33.9) | 44 (24.2) | 104 (28.0) | 211 (28.6) |
| Tenant | 95 (51.1) | 108 (59.3) | 187 (50.4) | 390 (52.8) |
| Homeless | 3 (1.6) | 7 (3.9) | 17 (4.6) | 27 (3.6) |
| Other | 25 (13.4) | 23 (12.6) | 63 (17.0) | 111 (15.0) |
| Mean number of children (SE) | 1.22 (0.06) | 1.21 (0.06) | 1.14 (0.05) | 1.18 (0.03) |
| Mean age first drink (SE) | 14.57 (0.35) | 14.41 (0.35) | 14.30 (0.22) | 14.4 (0.16) |
| Mean age week drink (SE) | 20.22 (0.53) | 20.24 (0.63) | 20.16 (0.44) | 20.19 (0.30) |
| Mean age daily drink (SE) | 29.12 (0.83) | 29.78 (0.87) | 28.58 (0.61) | 29.01 (0.43) |
| Stratification | ||||
| SADQ ≤ 30, n (%) | 73 (39.2) | 75 (41.2) | 149 (40.0) | 297 (40.1) |
| Relapse medication, n (%) | 16 (8.6) | 14 (7.7) | 30 (8.1) | 60 (8.1) |
| SS + MM + CM (n = 186) |
SS + MM (n = 182) |
SS alone (n = 372) |
Overall (n = 740) |
|
|---|---|---|---|---|
| ASSIST low risk, n (%) | ||||
| Tobacco | 58 (31.2) | 64 (35.2) | 130 (35.0) | 252 (34.0) |
| Cannabis | 156 (83.9) | 151 (83.0) | 300 (80.9) | 607 (82.1) |
| Stimulants | 178 (95.7) | 175 (96.2) | 356 (95.7) | 709 (95.8) |
| Sedatives | 185 (99.5) | 179 (98.4) | 364 (97.8) | 728 (98.4) |
| Opioids | 181 (97.3) | 179 (98.4) | 370 (99.5) | 730 (98.6) |
| Novel psychoactive | 186 (100) | 181 (99.4) | 369 (99.2) | 736 (99.5) |
| Mean APQ (SE) | ||||
| Friends | 2.06 (0.19) | 1.86 (0.27) | 2.22 (0.21) | 2.09 (0.13) |
| Finances | 1.44 (0.26) | 0.78 (0.26) | 0.96 (0.21) | 1.05 (0.14) |
| Police | 0.31 (0.18) | 0.14 (0.14) | 0.33 (0.12) | 0.28 (0.08) |
| Physical health | 3.51 (0.50) | 3.43 (0.49) | 4.67 (0.27) | 4.04 (0.24) |
| Affective | 2.50 (0.43) | 2.78 (0.32) | 2.67 (0.29) | 2.65 (0.20) |
| Common | 9.84 (1.06) | 9.02 (0.92) | 10.85 (0.78) | 10.12 (0.53) |
| Marital | 3.69 (0.54) | 3.93 (0.50) | 4.18 (0.44) | 3.98 (0.28) |
| Children | 1.06 (0.32) | 1.93 (0.42) | 2.11 (0.31) | 1.77 (0.21) |
| Work | 3.00 (0.48) | 2.14 (0.37) | 2.59 (3.63) | 2.60 (0.24) |
Number abstinent, n (%) |
2 (1.08) | 0 | 7 (1.92) | 9 (1.24) |
| Mean TLFB drinks (SE) | 1958 (117) | 1999 (195) | 1831 (87) | 1903 (71) |
| Mean PDA (SE) | 33.2 (2.27) | 29.9 (2.14) | 32.1 (1.47) | 31.8 (1.07) |
| Mean APD (SE) | 32.8 (2.00) | 31.2 (2.47) | 28.8 (1.22) | 30.2 (1.0) |
| Mean % days 6 + units (SE) | 65.4 (2.31) | 67.8 (2.22) | 66.30 (1.52) | 66.4 (1.10) |
| Mean % days 15 + units (SE) | 60.2 (2.70) | 57.6 (2.76) | 57.3 (1.84) | 58.1 (1.33) |
| Mean AUQ (SE) | 1.85 (0.09) | 1.84 (0.09) | 1.64 (0.05) | 1.74 (0.04) |
| Mean BMQ (SE) | ||||
| Specific | 26.5 (0.41) | 26.2 (0.45) | 26.1 (0.29) | 26.2 (0.21) |
| General | 20.7 (0.39) | 21.2 (0.38) | 20.8 (0.26) | 20.8 (0.19) |
| Mean SADQ (SE) | 33.7 (0.96) | 32.9 (1.02) | 33.5 (0.71) | 33.4 (0.50) |
| SADQ status, n (%) | ||||
| Mild | 19 (10.2) | 24 (13.2) | 42 (11.3) | 85 (11.5) |
| Moderate | 54 (29.0) | 51 (28.0) | 107 (28.8) | 212 (28.6) |
| Severe | 113 (60.8) | 107 (58.8) | 223 (59.9) | 443 (59.9) |
| Mean EQ-5D-5L utility (SE) | 0.79 (0.01) | 0.80 (0.01) | 0.81 (0.01) | 0.80 (0.01) |
| Mean EQ-5D-5L visual analogue scale (SE) | 64.7 (1.41) | 65.3 (1.39) | 65.5 (1.04) | 65.3 (0.72) |
Primary outcome analysis
Table 9 presents the outcomes at months 6 and 12 by allocated group (see Appendix 1, Table 23 for outcomes by alloctaded group at 2 and 4 months). To conduct a fractional regression using per cent adherence to acamprosate at month 6, a fractional outcome was generated by dividing the outcome by 100. Fractional regression, with a logit link function, was conducted using the fracreg logit command in Stata, allocation and stratification variables were included as fixed effect covariates, as adherence was not available at baseline, this covariate was omitted. The odds ratio and 95% CI can be difficult to interpret so these were converted to marginal effects representing mean differences in per cent adherence to acamprosate at month 6.
| Month 6 | Month 12 | |||||
|---|---|---|---|---|---|---|
| SS + MM + CM (n = 141) |
SS + MM (n = 122) |
SS alone (n = 255) |
SS + MM + CM (n = 104) |
SS + MM (n = 96) |
SS alone (n = 193) |
|
| Prescribed acamprosate, n (%) | 88 (62.4) | 63 (51.64) | 130 (51.0) | 27 (36.0) | 21 (21.9) | 54 (28.0) |
| Mean % adherence (SE) | 49.1 (3.7) | 41.2 (4.1) | 37.9 (2.7) | 19.5 (3.7) | 17.9 (3.7) | 21.6 (2.7) |
| Mean MMAS-8 (SE) | 5.5 (0.16) | 5.3 (0.18) | 5.0 (0.2) | 5.4 (0.2) | 5.6 (0.3) | 5.2 (0.2) |
| Number abstinent, n (%) | 59 (42.5) | 51 (42.9) | 96 (39.5) | 39 (37.9) | 42 (43.8) | 76 (41.3) |
| Mean TLFB drinks (SE) | 375.3 (60.0) | 544.2 (84.6) | 527.1 (56.2) | 553.0 (106) | 537.9 (97.2) | 528.6 (64.7) |
| Mean PDA (SE) | 74.1 (3.1) | 72.4 (3.5) | 69.6 (2.4) | 73.6 (3.6) | 71.0 (4.0) | 68.8 (2.9) |
| Mean APD (SE) | 8.8 (1.1) | 10.5 (1.2) | 10.8 (0.8) | 12.7 (1.6) | 9.8 (1.3) | 10.4 (0.9) |
| Mean % days 6 + units (SE) | 21.0 (3.0) | 25.2 (3.4) | 27.8 (2.4) | 23.7 (3.5) | 26.6 (3.9) | 28.6 (2.8) |
| Mean % days 15 + units (SE) | 13.3 (2.8) | 19.3 (3.3) | 19.2 (2.3) | 17.0 (3.1) | 14.9 (3.2) | 16.7 (2.5) |
| Mean AUQ (SE) | 2.0 (0.1) | 2.2 (0.2) | 2.1 (0.1) | 2.2 (0.2) | 1.9 (0.2) | 1.9 (0.1) |
| Mean BMQ (SE) | ||||||
| Specific | 20.7 (0.4) | 20.7 (0.5) | 21.1 (0.3) | 20.1 (0.6) | 20.0 (0.6) | 21.3 (0.4) |
| General | 22.8 (0.5) | 21.1 (0.6) | 22.2 (0.4) | 19.7 (0.7) | 19.0 (0.6) | 20.9 (0.6) |
| Mean SADQ (SE) | 21.2 (1.8) | 25.9 (2.1) | 23.1 (1.3) | 21.6 (2.2) | 21.1 (2.3) | 24.0 (1.5) |
| SADQ status, n (%) | ||||||
| Mild | 30 (21.0) | 20 (16.4) | 60 (23.2) | 26 (23.8) | 26 (25.7) | 41 (21.1) |
| Moderate | 23 (16.1) | 20 (16.4) | 37 (14.3) | 12 (11.0) | 10 (9.9) | 24 (12.4) |
| Severe | 90 (62.9) | 82 (67.2) | 162 (62.5) | 71 (65.2) | 65 (64.4) | 129 (66.5) |
| Mean EQ-5D-5L (SE) | 0.78 (0.02) | 0.80 (0.02) | 0.78 (0.02) | 0.83 (0.02) | 0.83 (0.02) | 0.80 (0.02) |
| Mean EQ-5D-5L VAS (SE) | 67.5 (1.7) | 67.1 (2.4) | 63.8 (1.5) | 68.6 (2.2) | 66.4 (2.3) | 65.0 (1.8) |
The distribution of the primary outcome by allocated group is presented in Table 10 and the overall distribution presented in Appendix 1, Figure 20. The results of the ATA are presented in Table 11.
| SS + MM + CM (n = 141) |
SS + MM (n = 122) |
SS (n = 255) |
|
|---|---|---|---|
| Mean (SD) | 49.1 (44.4) | 41.2 (45.7) | 37.9 (43.7) |
| Percentiles | |||
| 1% | 0 | 0 | 0 |
| 5% | 0 | 0 | 0 |
| 10% | 0 | 0 | 0 |
| 25% | 0 | 0 | 0 |
| Median | 70 | 0 | 0 |
| 75% | 95 | 95 | 90 |
| 90% | 100 | 100 | 100 |
| 95% | 100 | 100 | 100 |
| 99% | 100 | 100 | 100 |
| SS + MM + CM vs. SS | SS + MM vs. SS | SS + MM + CM vs. SS + MM | |
|---|---|---|---|
| Marginal effect | |||
| Allocated group | |||
| SS | −0.106 (−0.196; −0.016)* | −0.031 (−0.128; 0.065) | - |
| MM | – | – | −0.079 (−0.187; 0.028) |
| Odds ratio | |||
| Allocated group | |||
| SS | 0.642 (0.442; 0.934)* | 0.874 (0.580; 1.317) | – |
| SS/MM | – | – | 0.719 (0.459; 1.123) |
| SS/MM/CM | – | – | – |
| Sitea | |||
| Central and North West London | 0.714 (0.430; 1.186) | 0.625 (0.364; 1.075) | 1.428 (0.764; 2.670) |
| South East London | 0.306 (0.142; 0.662) | 0.381 (0.180; 0.808)* | 0.592 (0.215; 1.634) |
| Southampton | 0.897 (0.502; 1.602) | 0.978 (0.538; 1.778) | 1.406 (0.679; 2.913) |
| Yorks & Humber | 0.912 (0.552; 1.508) | 0.815 (0.482; 1.378) | 1.349 (0.740; 2.457) |
| SADQb | 1.088 (0.745; 1.589) | 1.090 (0.735; 1.616) | 1.589 (1.000; 2.525)* |
| Current prescriptionc | 0.815 (0.373; 1.780) | 0.857 (0.382; 1.921) | 0.816 (0.335; 1.984) |
The mean difference in per cent adherence to acamprosate at month 6 between those allocated to SS and SS + MM + CM is 10.6% (95% CI 19.6% to 1.6%) lower in the SS group than the SS + MM + CM group; this difference is statistically significant. When the SS + MM group is compared to SS alone, the SS + MM group has a lower per cent days adherent than the SS group, mean difference 3.1% (95% CI 12.8% to −6.5%), but this difference is not significant. A similar non-significant finding is seen when we compared the SS + MM and SS + MM + CM groups, mean difference 7.9% (95% CI 18.7% to −2.8%).
Late follow-up sensitivity analysis
Our first sensitivity analysis incorporates those who followed up on time and those who followed up late at month 6 (please see Appendix 1, Table 24). Table 12 presents the results of this sensitivity analysis. There are no significant deviations from the primary analysis conducted so all participants are included in the analysis.
| All participants | Followed up on time | |
|---|---|---|
| CM vs. SS | −10.59 (−19.57; −1.62) | −12.77 (−22.10; −3.43) |
| MM vs. SS | −3.14 (−12.80; 6.51) | −3.94 (−14.08; 6.19) |
| CM vs. MM | −7.95 (−18.69; 2.79) | −9.19 (−20.37; 1.99) |
Missing primary outcome data
To explore whether missing primary outcome data are MAR we conducted a logistic regression analysis to explore what variables might predict missing outcome data. Variable selection was undertaken by exploring associations at the 0.10 p-level. The analysis identified age, site, gender and per cent days abstinent at baseline as being predictive of missing primary outcome data. Using these predictive variables, we imputed 50 iterations of a multiple imputation model and pooled these using the micombine command in Stata.
We used a similar fractional regression approach as the primary analysis to generate odds ratios comparing SS + M + CM versus SS, SS + MM + versus SS and SS + MM + CM versus SS + MM. As the mi command set does not allow for the generation of marginal effect, we plotted odds ratios and 95% CIs for each of the comparisons in order to visually explore whether there are significant deviations between the observed and imputed values of the primary outcome. Appendix 1, Figures 21–23 presents the plots and it is confirmed that the influence of missing data, based on a MAR assumption, does not influence our interpretation of the results.
To explore the influence of missing data being MNAR, we employed a sensitivity analysis to explore departures from the MAR assumption. Varying deltas of 0.1 ranging from −0.5 to 0 were employed in intervention group alone, control group alone and both intervention and control group. Analysis was undertaken using the rctmiss command in Stata. Visual inspection of the data indicates even at large departures from the MAR assumption the impact on the treatment effect is small; these are presented in Appendix 1, Figures 24–26.
We can be confident that the treatment effects are robust under different interpretations of the nature of missing data.
Complier average causal effects analysis
We defined our compliance criteria as two separate scenarios: scenario 1 where participants in the intervention groups adhered to at least 6 (50%) MM calls and scenario 2 where they adhered to 12 (100%). Of those allocated to one of the intervention groups, 105 (58%) adhered to 50% of the MM calls in the SS + MM group and 128 (69%) in the SS + MM + CM group. Compliance with 100% of MM calls also differed by allocated group with 20 (11%) complying in the SS + MM group and 73 (39%) complying in the SS + MM + CM group.
We conducted a CACE analysis using the ivregress 2sls command in Stata. The dependent variable was per cent adherence to acamprosate at month 6 adjusted for stratification covariates. Table 13 presents a comparison of ATA effects and CACE effects for each of our scenarios.
| ATA | CACE | |
|---|---|---|
| 100% adherent | ||
| CM vs. SS | −10.59 (−19.57; −1.62)* | −22.19 (−29.71; −14.66)* |
| MM vs. SS | −3.14 (−12.80; 6.51) | −31.78 (−60.46; −3.10)* |
| 50% adherent | ||
| CM vs. SS | −10.59 (−19.57; −1.62)* | −12.45 (−17.76; −7.14)* |
| MM vs. SS | −3.14 (−12.80; 6.51) | −13.2 (−25.38; −1.15)* |
Under scenario 1, 50% adherence, the mean difference in per cent adherence increases from 10.6% (95% CI 19.6% to 1.62%) to 12.45% (95% CI 17.8%) in the SS + MM + CM versus SS comparison. This indicated that at 50% compliance, those allocated to SS + MM + CM have a mean of 12.4% more adherence to acamprosate at month 6 than those allocated to SS alone. When comparing the SS + MM versus the SS group, a previous non-significant difference of 3.14% (95% CI 12.8% to −6.5%) becomes a significant difference of 13.2% (25.4% to 1.15%). Under scenario 2, the differences are larger in magnitude, the SS + MM + CM group having 22.2% (95% CI 29.7% to 14.7%) more adherent days than the SS group alone. While the magnitude of difference is larger for the SS + MM versus SS comparison 31.8% (95–60.5% vs. 3.10%), this comparison is based on a small number of participants (n = 20), and should be interpreted with caution.
Secondary outcome analysis
A similar analytical approach was employed to assess secondary outcomes. Table 14 presents the distribution, mean and median of per cent days abstinent from alcohol at month 6 by allocated group and Table 15 presents the ATA and CACE analysis, under scenario 2, for per cent days abstinent at month 6.
| SS + MM + CM (n = 139) |
SS + MM (n = 119) |
SS (n = 243) |
|
|---|---|---|---|
| Mean (SD) | 74.1 (37.1) | 72.4 (37.9) | 69.6 (38.3) |
| Percentiles | |||
| 1% | 0 | 0 | 0 |
| 5% | 0 | 0 | 0 |
| 10% | 0 | 0 | 0 |
| 25% | 46.67 | 40 | 38.9 |
| Median | 97.78 | 97.78 | 94.4 |
| 75% | 100 | 100 | 100 |
| 90% | 100 | 100 | 100 |
| 95% | 100 | 100 | 100 |
| 99% | 100 | 100 | 100 |
| ATA | CACE | |
|---|---|---|
| 100% adherent | ||
| CM vs. SS | −3.76 (−12.0; 4.50) | −12.5 (−21.1; −3.90)* |
| MM vs. SS | −4.40 (−11.9; 3.16) | −15.1 (−26.10; −4.20)* |
The ATA indicates lower per cent days abstinent at month 6 in the SS compared to both SS + MM + CM and SS + MM, but these are not significant; when compliance is incorporated, those who are 100% compliant have a significantly lower per cent days abstinent at month 6 in the SS group compared to both the SS + MM + CM and SS + MM groups.
Analysis of other secondary variables; per cent adherence to acamprosate at month 12, per cent days abstinent at month 12, standard drinks consumed, per cent days heavy drinking, drinks per drinking day, severity of alcohol dependence, alcohol urge, beliefs in medication and MMAS-8 showed no significant differences between the groups at either month 6 or 12 post randomisation. The results of these analyses are presented in Appendix 1.
Table 15 presents the results of a CACE analysis of compliance with MM and outcomes in terms of per cent days abstinent at month 6. Those who comply with MM are significantly more likely to have improved alcohol use outcomes, 12.5% more per cent days abstinent in the SS + MM + CM group and 15.1% more in the SS + MM group compared to SS alone, although the differences in the SS + MM group should be taken with caution considering the small numbers involved.
Figure 2 presents error bars of median and interquartile range (IQR) of per cent days abstinent at month 6 categorised into first, second and third terciles. It is clear overall and for those still drinking that there is an association between increased adherence to acamprosate and better drinking outcomes. A significant Spearman correlation coefficient of 0.3944 (p < 0.001).
FIGURE 2.
Median and IQR for per cent days abstinent at month 6 by tercile of adherence to acamprosate for all participants and those still drinking at month 6.
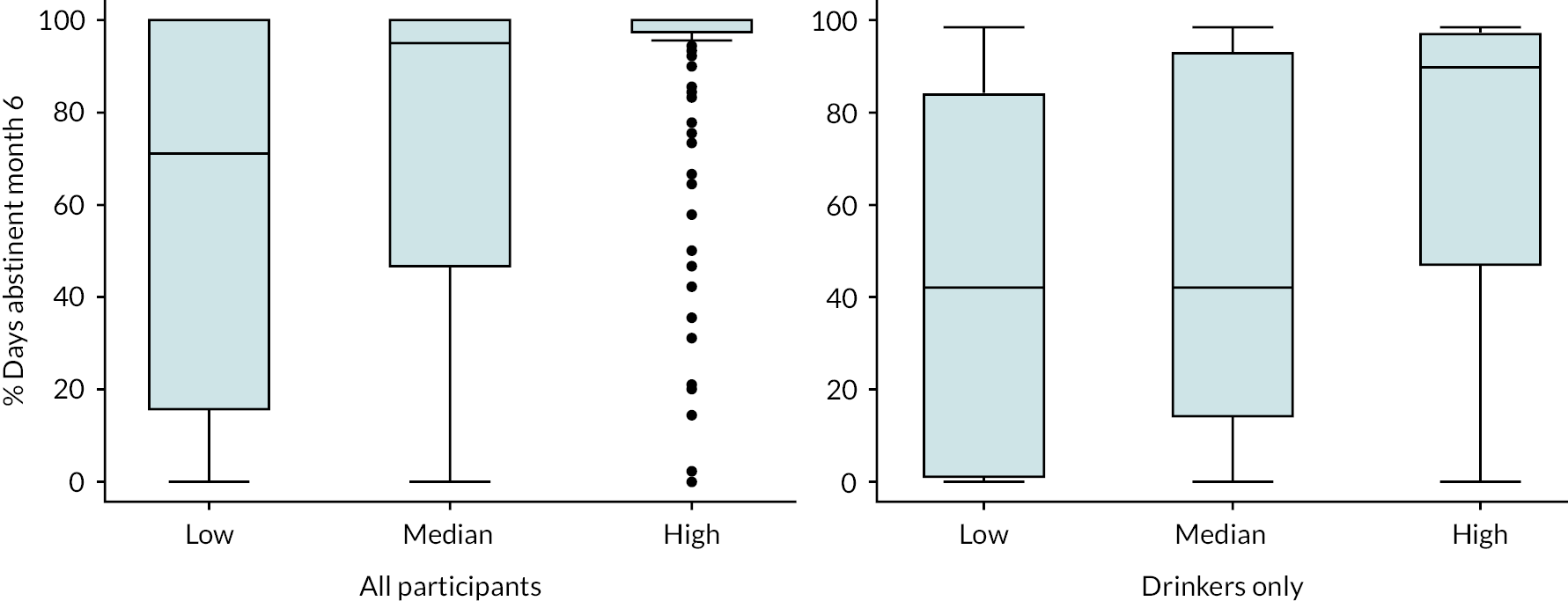
Stepwise regression was conducted to model the between pre-randomisation factors and per cent adherence to acamprosate and per cent days abstinent at month 6. Interaction terms with treatment allocation were included in the model and a significance level of 0.1 was used as a threshold to determine which variables were maintained in the final model reported. Baseline variables included initially in the model include age, gender, marital status, ethnicity, current employment status, number of children, age of first drink, weekly and daily drinking, frequency and quantity of alcohol consumption, severity of alcohol dependence, alcohol urges and alcohol-related problems. This analysis was augmented with an additional analysis for the SS + MM and SS + MM + CM groups where the same dependent variable was assessed with the same independent variables with the addition of therapeutic alliance.
With the dependent variable per cent adherence to acamprosate at month 6, item reduction analysis indicated the following baseline independent variables should be included in the model: allocation, site, having children, age, age of first alcoholic drink, alcohol-related problems, affective and common domains and alcohol urges. The full model specification is presented in Appendix 1, Table 25. SS alone predicted lower adherence, and lower alcohol urge predicted lower adherence; no other variables were significant prognostic indicators of outcome. With per cent days abstinent at month 6 as the baseline dependent variables included in the model were allocated arm, site, alcohol urge, per cent days abstinent and per cent days heavy drinking, only alcohol urges were significant with lower alcohol urges predicting lower per cent days abstinent at month 6. The full model specification is presented in Appendix 1, Table 26. For the two intervention groups SS + MM and SS + MM + CM, the prognostic analysis was augmented with an analysis including a measure of therapeutic alliance, and therapeutic alliance did not predict per cent adherence to acamprosate at month 6 for either of the allocated groups.
Summary of results
In sum, we set out to recruit 748 participants and allocate them in a ratio of 2 : 1 : 1 to SS, SS + MM and SS + MM + CM, respectively. To address our sample size requirements, we aimed to follow up 70% of these at the primary end point, 6 months post randomisation. We achieved this aim.
Our primary outcome was per cent adherence to acamprosate at month 6. When comparing SS + MM + CM versus SS alone, we observed a significantly higher per cent adherence to acamprosate in the SS + MM + CM group; the differences were of the magnitude that would indicate a clinically important difference. Differences were also observed when comparing SS + MM versus SS and SS + MM + CM versus SS + MM, but these were not significant. We explored how robust these findings were to assumptions about the nature of any missing data and we explored a missing data imputation model to explore the impact of data that may be MAR and a sensitivity analysis to explore data that may not be MAR. Neither of these analyses found any significant deviation from the analysis based on observed values, so we can be confident that our findings from the ATA analysis are robust. To explore the effect of compliance to MM, we conducted a CACE analysis using two scenarios of compliance, 50% and 100% compliance. In both scenarios, we found greater benefits associated with SS + MM + CM and SS + MM versus SS alone and these benefits were significant and clinically important.
Our secondary analysis included per cent days abstinent from alcohol at month 6. The ATA found differences favouring both intervention groups over SS alone but neither of these was significant. When a CACE analysis was conducted using scenario 2, both intervention groups had significantly greater per cent days abstinent than SS alone. This analysis also highlighted the relationship between adherence to acamprosate and better outcomes something additionally highlighted by a medium significant correlation between adherence to acamprosate and increased per cent days abstinent at month 6.
Our prognostic analysis found little evidence of baseline predictors for either adherence to medication or per cent days abstinent at month 6. There was no evidence that increased therapeutic alliance in the intervention groups was associated with increased adherence.
Chapter 5 Health economic evaluation
Methods
Method of economic evaluation
The primary economic analysis was a cost-utility analysis where outcomes were expressed as QALYs, as recommended by NICE. 129 A secondary analysis explored cost-effectiveness in terms of the primary clinical outcome which was adherence to relapse prevention medication.
Perspective
The primary economic perspective was the NHS and personal social services (NHS/PSS) perspective preferred by NICE. This covers all hospital, community health and social services. A sensitivity analysis based on a broader perspective including impact on the criminal justice sector was planned, however, this became redundant because criminal activity was not reported by any patient at any time point.
Time horizon
The primary time horizon of the economic analyses using both QALYs and relapse medication adherence was the 6-month follow-up, consistent with the primary clinical analysis. A secondary analysis was carried out at the 12-month follow-up using QALYs.
Outcomes
The primary economic outcome was QALYs calculated using the EQ-5D-5L measure of health-related quality-of-life scores at baseline, 6- and 12-month follow-ups. The EuroQol-5 Dimensions (EQ-5D) is a non-disease-specific measure for describing and valuing health-related quality of life. 118 The measure covers five health domains (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) which are rated on five levels (e.g. no, slight, moderate, severe, extreme problems).
The EQ-5D-5L (five levels) is a revised version of the EuroQol-5 Dimensions, three-level version (EQ-5D-3L). It has been established that the EQ-5D-3L can be used with confidence in economic evaluations for alcohol dependence. 114 The health states from the EQ-5D-5L were given a utility score using responses from a representative sample of adults in the UK. 125 These utility scores, however, have not been accepted by NICE which instead recommends using EQ-5D-3L values based on a cross-walk algorithm. 126 Accordingly, a cost-utility analysis based on the EQ-5D-3L values is presented as a sensitivity analysis.
Economic model
The costs and benefits of interventions for alcohol problems, such as alcohol-related complications and mortality, extend well beyond the usual time horizons of clinical trials. Economic modelling can be utilised to project costs and QALYs beyond the trial end points. A de novo patient level, multistate life table model was built using published risk equations130 to estimate the effects of changing alcohol consumption on costs and QALYs over a 20-year time horizon.
Measurement of resources
Intervention resource use
The clinical contact centre hosted by Celsio/Lloyds pharmacy recorded the number of telephone calls and the number of vouchers earned for each study participant and these were used as the basis for the calculation of the total cost of the intervention.
Use of all other health and social care services
Data on use of other health and social care services included in the study were collected using the AD-SUS, modified for application to alcohol-dependent populations. 131 Information about study participants’ use of services was collected in interview with a researcher at baseline and at 6- and 12-month follow-up interviews. The AD-SUS asks participants to report the number and duration of contacts with a range of health and social care professionals. At baseline, participants were asked to report services used in the previous 6 months. At follow-up, participants were asked to report services used since their last interview.
Crime
The AD-SUS was also used to collect information about crimes committed by and against (victims of crime) participants. Responses were recorded by type of crime.
Productivity
Changes in productivity were not measured as it was expected that < 10% of detoxified dependent drinkers would be employed or able to return to work. However, 28% of the sample were in full or part-time employment at baseline. Accordingly, productivity gains from reduced absenteeism, presenteeism and unemployment may represent an important economic benefit from treatment that was not recorded.
Valuation of resources
A unit cost was applied to each resource used to calculate the total cost of resources used by each study participant (summarised in Table 16). All unit costs are for the financial year 2018–19, uprated where necessary using the GDP deflator. 137
| Item | Source | Unit cost |
|---|---|---|
| Intervention | ||
| Initial session | Celsio/Lloyds Pharmacy | £89 per session |
| Follow-up sessions | Celsio/Lloyds Pharmacy | £30 per session |
| Voucher administration | Celsio/Lloyds Pharmacy | £10 per session |
| Vouchers | Celsio/Lloyds Pharmacy | £5 per voucher |
| Accommodation | ||
| Staffed accommodation | Residential rehabilitation for people who misuse drugs/alcohol132 | £1040 per week |
| Hospital services | ||
| Alcohol admissions | Alcohol services133 | £424 per bed-day |
| Inpatient ≥ 5 days | NHS reference costs133 | £3053 per episode |
| Inpatient < 5 days | NHS reference costs133 | £631 per episode |
| Outpatient | NHS reference costs133 | £135 per attendance |
| Accident and emergency | NHS reference costs (NHS Reference Costs 2018–19) | £166 per attendance |
| Ambulance | NHS reference costs133 | £125 per attendance |
| Community health and social care services | ||
| Alcohol keyworker | Alcohol health worker133 | £76 per contact houra |
| Psychiatrist | Psychiatric consultant133 | £336 per contact hourb |
| Psychologist | Agenda for Change Band 7133 | £180 per contact hourc |
| Nurse specialist | Agenda for Change Band 6133 | £84 per contact houra |
| Counsellor | Alcohol health worker133 | £76 per contact houra |
| Structured day program | Local authority day care133 | £38 per client attendance |
| Recovery groups | Not valued | Zero rated |
| Alcoholics Anonymous | Not valued | Zero rated |
| Self-help lines and apps | Based on cost reported by the Samaritans134 | £4.24 per call |
| Pharmacist | Celesio/Lloyds Pharmacy | £75 per contact hourd |
| Psychiatric nurse | Agenda for Change Band 5133 | £92 per contact houra |
| Community Mental Health Team | Mental health specialist team (Improving Access to Psychological Therapies (IAPT),adult and elderly) NHS reference costs133 | £96 per client attendance |
| General practitioner | General practitioner133 | £34 per visit |
| Practice nurse | Nurse (GP practice)133 | £50 per contact houre |
| District nurse | Assumed equivalent to practice nurse | £50 per contact houre |
| Occupational therapist | Community occupational therapist133 | £80 per contact hourf |
| Accommodation worker | Assumed equivalent to Social Work Assistant133 | £33 per contact houra |
| Social worker | Social worker133 | £255 per contact hourg |
| Day centre | Assumed equivalent to structured day program | £38 per client attendance |
| Advice service | Assumed equivalent to social work assistant133 | £33 per contact houra |
| Medications | ||
| Acamprosate 333 mg | Drug Tariff (Part VIII)135 | Pack of 168 tablets £37.70 |
| Naltrexone 50 mg | Drug Tariff (Part VIII)135 | Pack of 28 tablets £57.95 |
| Nalmefene 18 mg | Drug Tariff (Part VIII)135 | Pack of 14 tablets £42.42 |
| Disulfiram 200 mg | Drug Tariff (Part VIII)135 | Pack of 50 tablets £111.53 |
Intervention cost
Medication Management
The unit cost per session was estimated by dividing the clinical call centre budget from Celsio/Lloyds pharmacy for the study by the total number of scheduled sessions. The call centre budgeted for telephone costs per session, pharmacist time per session, set up costs (phone lines, standard operating procedures, data management), pharmacist training costs and management costs. On this basis, the total cost for the initial session of 45 minutes was £89, and for each follow-up session of 15 minutes, it was £30.
Contingency Management
In addition to MM, participants in the CM group received vouchers based on the number of sessions they attended. Participants were given vouchers at the 6-month follow-up interview according to a CM reward schedule based on completed sessions. The CM schedule was a £5 voucher per completed session plus a £10 bonus for completing 4 calls, £20 for completing 8 calls and £30 for completing 12 calls.
Hospital and community services
Unit costs for inpatient and outpatient hospital services, Accident & Emergency attendances and use of ambulance services were taken from estimates based on NHS Reference Costs contained in the Unit Costs of Health and Social Care. 133 For NHS primary care services, social workers and social services support workers, we used costs contained in the Unit Costs of Health and Social Care. 133 Self-help groups without paid involvement of health-care professionals, such as Alcoholics Anonymous and SMART recovery meetings, were not valued.
Alcohol relapse prevention medication
Medication costs were calculated using daily dose information, the cost of the generic drug and controlled drug dispensing costs as per the NHS Business Services Authority website. 135
Costs of crime
Participants did not report committing crimes at any time point. A small number of participants reported being victims of crime at the 6-month follow-up (n = 28, 5%) and the 12-month follow-up (n = 33, 8%) – these reports were evenly distributed across groups (see results for more detail). A secondary analysis taking a broader perspective for costs from the NHS/PSS based on these few data was considered to be uninformative, and, accordingly, victim of crime data are presented descriptively and are not valued.
Data analysis
Resource use
Resource use by study participants is reported as the mean (SD) by group and as a percentage of the group who had at least one contact. Differences in the use of services between randomised groups are reported descriptively and are not compared statistically to avoid problems associated with multiple testing, and because the focus of the economic evaluation was on the quantitative analysis of cost and cost-effectiveness.
Difference in costs and outcomes
We initially present the unadjusted observed data on costs and outcomes. We imputed missing cost and outcome data using multiple imputation using chained equations (MICE) under the assumption that these data were MAR. 138 Multiple imputation in the economic analysis included baseline costs and baseline utilities as covariates in the chained equations which led to slightly different imputed estimates to the clinical analysis. Multiple imputation indicates the sensitivity of results to missing data under the assumption that the data are MAR. It is generally accepted that multiple imputation provides less biased estimates of costs and effects than complete case analysis unless data are MNAR. Differences in the imputed data on costs and outcomes were adjusted for baseline costs or outcomes, as relevant, and baseline stratification variables and covariates pre-specified in the statistical analysis plan [severity of alcohol dependence (SADQ score of ≤ 30 or > 30), study site, prescription of other relapse prevention medication (yes/no) and gender].
Cost-effectiveness and cost-utility analyses
The primary economic analysis was composed of two separate comparisons: (1) SS + MM + CM versus SS alone; and (2) SS + MM versus SS alone, both at 6 months post randomisation, and assessed cost-effectiveness in terms of cost per QALY using the EQ-5D measure of quality of life. QALYs were calculated using the area under the curve approach as defined by the utility values at baseline and each follow-up. It was assumed that changes in utility score over time followed a linear path. 139 Two secondary economic evaluations were carried out, a cost-utility analysis at 12 months post randomisation using QALYs as the measure of effect and a cost-effectiveness analysis using adherence to relapse medication which was the primary clinical outcome.
Initially, incremental cost-effectiveness ratios (ICERs) were calculated, which are the difference in mean cost divided by the difference in mean effect. 140 Repeat resampling (bootstrapping) from the cost and outcomes data was used to generate a distribution of mean costs and effects. 141 The probability that each of the two treatments, SS + MM + CM and SS + MM, were cost-effective compared to the comparison group, SS alone, was explored using cost-effectiveness acceptability curves (CEACs) which are graphical representations of the probability that one treatment is cost-effective compared to another for a range of possible levels of willingness to pay for unit improvements in outcome (in this case, either QALYs or adherence to medication). 141
Sensitivity analyses
Sensitivity analyses were carried out to test the impact of varying methods and assumptions on the relative cost-effectiveness of the interventions being compared. We planned three sensitivity analyses:
-
A broader analytical perspective to include the cost of crime (which was not completed as no crimes were reported).
-
A complete case analysis for comparison with the results that used multiple imputation for missing data.
-
A cost-utility analysis using QALYs calculated from EQ-5D-3L tariffs, currently the approach preferred by NICE.
Economic model
The decision-analytic model methods, assumptions and parameter inputs are specified in the Project Webpage Document.
Results
Data completeness
Full service use data at the 6-month follow-up point were available for 76% (141/185) in the SS + MM + CM group, 67% (122/182) in the SS + MM group and 69% (255/372) in the SS alone group, which was 70% of the total number randomised. Full service use data for the 6 months prior to the 12-month follow-up were available for 56% (104/185) in the SS + MM + CM group, 53% (96/182) in the SS + MM group and 52% (193/372) in the SS alone group, which was 53% of the total number randomised.
Resource use
Resource use reported over the periods from baseline to 6-month follow-up and from 6-month to 12-month follow-up is summarised in Table 17.
| Baseline to 6-month follow-up | SS + MM + CM | SS + MM | SS (TAU) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | |
| (n = 141) | (n = 122) | (n = 255) | ||||
| MM (sessions) | 7.7 (4.8) | 83.2 | 6.2 (4.3) | 80.2 | 0.0 (0.0) | 0.0 |
| CM (vouchers) | 9.2 (3.7) | 98.2 | 0.0 (0.0) | 0.0 | 0.0 (0.0) | 0.0 |
| Supported accommodation (weeks) | 3.1 (20.1) | 5.0 | 15.1 (47.4) | 12.6 | 10.4 (39.2) | 7.9 |
| Alcohol inpatient (nights) | 1.8 (9.6) | 10.6 | 2.1 (9.3) | 12.3 | 1.3 (6.2) | 11.0 |
| Other inpatient (nights) | 1.2 (4.9) | 17.7 | 0.4 (1.7) | 9.8 | 0.8 (4.3) | 9.8 |
| Alcohol outpatient (appointments) | 0.2 (1.0) | 4.3 | 0.1 (0.4) | 2.5 | 0.2 (0.9) | 5.5 |
| Other outpatient (appointments) | 0.9 (1.6) | 38.3 | 1.1 (2.3) | 32.8 | 1.0 (3.2) | 28.2 |
| Accident and emergency (visits) | 0.3 (0.6) | 26.2 | 0.3 (0.6) | 27.9 | 0.4 (0.8) | 31.4 |
| Ambulance (calls) | 0.2 (0.5) | 14.2 | 0.2 (0.5) | 12.3 | 0.3 (0.8) | 16.5 |
| Alcohol keyworker (contact) | 6.8 (17.3) | 66.7 | 5.5 (8.0) | 68.8 | 5.8 (11.8) | 62.7 |
| Psychiatrist (contact) | 0.5 (2.2) | 13.4 | 0.3 (0.9) | 13.9 | 0.4 (1.9) | 13.4 |
| Psychologist (contact) | 0.1 (0.6) | 5.6 | 0.1 (0.5) | 2.5 | 0.3 (2.0) | 5.1 |
| Nurse specialist (contact) | 1.1 (5.4) | 16.9 | 2.1 (7.0) | 25.4 | 2.5 (8.8) | 21.6 |
| Counsellor (contact) | 1.4 (4.4) | 15.5 | 2.9 (6.9) | 23.8 | 1.4 (4.3) | 13.8 |
| Structured day program (visits) | 8.3 (21.9) | 21.8 | 5.6 (16.2) | 19.7 | 9.7 (27.9) | 23.6 |
| Recovery groups (meetings) | 7.8 (15.3) | 37.3 | 9.8 (17.5) | 43.8 | 9.2 (19.9) | 39.0 |
| Alcoholics Anonymous (meetings) | 6.5 (22.9) | 18.3 | 6.8 (18.7) | 29.5 | 5.7 (17.9) | 23.6 |
| Self-help telephone or app (calls) | 4.7 (24.3) | 5.6 | 5.2 (26.5) | 9.0 | 8.0 (38.8) | 9.8 |
| Pharmacists (contact) | 5.0 (10.7) | 67.6 | 4.3 (5.7) | 66.4 | 3.9 (6.5) | 63.1 |
| Mental health team (visits) | 0.4 (2.3) | 7.0 | 0.1 (0.3) | 4.1 | 0.3 (1.6) | 5.1 |
| GP (visits) | 3.3 (4.0) | 73.9 | 3.0 (2.8) | 79.5 | 2.8 (3.3) | 76.8 |
| Practice nurse (contact) | 0.9 (1.6) | 42.3 | 0.7 (1.1) | 40.2 | 0.8 (1.8) | 39.8 |
| Acamprosate (days prescribed) | 120.0 (71.0) | 80.8 | 101.0 (75.0) | 73.8 | 100.0 (75.0) | 75.3 |
| Naltrexone/disulfiram (days prescribed) | 4.1 (24.0) | 3.5 | 9.4 (37.0) | 8.2 | 2.0 (18.2) | 2.0 |
| Criminal activity (offences) | 0.0 (0.0) | 0.0 | 0.0 (0.0) | 0.0 | 0.0 (0.0) | 0.0 |
| Six-month to twelve-month follow-up | (n = 104) | (n = 96) | (n = 193) | |||
| Supported accommodation (weeks) | 9.1 (45.0) | 4.8 | 14.5 (47.9) | 11.6 | 8.5 (36.4) | 7.3 |
| Alcohol inpatient (nights) | 2.7 (20.5) | 10.7 | 0.5 (3.1) | 7.3 | 1.2 (4.3) | 10.9 |
| Other inpatient (nights) | 1.1 (6.2) | 12.6 | 0.7 (3.5) | 10.4 | 0.7 (2.9) | 11.9 |
| Alcohol outpatient (appointments) | 0.0 (0.1) | 1.0 | 0.0 (0.1) | 1.0 | 0.0 (0.4) | 2.0 |
| Other outpatient (appointments) | 0.8 (1.7) | 31.1 | 1.9 (4.7) | 47.9 | 1.1 (2.6) | 32.2 |
| Accident and emergency (visits) | 0.4 (0.7) | 27.2 | 0.4 (0.6) | 27.2 | 0.4 (0.6) | 28.1 |
| Ambulance (calls) | 0.2 (0.5) | 16.5 | 0.3 (0.8) | 17.7 | 0.3 (0.6) | 21.8 |
| Alcohol keyworker (contact) | 3.5 (10.4) | 46.6 | 3.0 (6.0) | 42.1 | 3.8 (7.2) | 46.1 |
| Psychiatrist (contact) | 0.4 (1.6) | 14.4 | 0.3 (0.9) | 11.5 | 0.3 (1.8) | 7.8 |
| Psychologist (contact) | 0.2 (1.1) | 4.9 | 0.1 (0.3) | 3.1 | 0.3 (1.6) | 4.1 |
| Nurse specialist (contact) | 0.2 (0.7) | 10.8 | 0.3 (1.2) | 8.3 | 0.5 (2.5) | 13.0 |
| Counsellor (contact) | 0.9 (3.7) | 8.4 | 2.6 (6.3) | 20.9 | 1.1 (3.5) | 12.9 |
| Structured day program (visits) | 2.5 (11.2) | 7.4 | 0.1 (1.2) | 1.2 | 2.7 (12.8) | 8.8 |
| Recovery groups (meetings) | 3.8 (11.9) | 22.3 | 3.6 (10.7) | 22.9 | 4.7 (13.3) | 28.5 |
| Alcoholics Anonymous (meetings) | 6.1 (22.5) | 20.7 | 5.1 (15.5) | 22.9 | 6.7 (31.6) | 16.7 |
| Self-help telephone or app (calls) | 0.9 (7.5) | 2.9 | 5.2 (27.2) | 5.3 | 5.7 (32.4) | 10.9 |
| Pharmacists (contact) | 3.1 (6.2) | 46.6 | 3.1 (6.5) | 42.7 | 2.8 (4.1) | 50.8 |
| Mental health team (visits) | 0.4 (2.4) | 5.8 | 0.1 (0.7) | 3.1 | 0.6 (3.9) | 9.3 |
| GP (visits) | 2.1 (2.9) | 64.1 | 3.1 (4.3) | 71.6 | 2.7 (4.0) | 74.1 |
| Practice nurse (contact) | 0.7 (1.8) | 29.2 | 1.0 (3.0) | 37.5 | 1.0 (2.3) | 45.1 |
| Acamprosate (days prescribed) | 43.6 (74.0) | 26.0 | 36.8 (69.8) | 21.9 | 47.0 (75.6) | 28.0 |
| Naltrexone/disulfiram (days prescribed) | 3.2 (23.2) | 2.0 | 6.7 (30.5) | 6.3 | 1.7 (17.1) | 1.0 |
| Criminal activity (offences) | 0.0 (0.0) | 0.0 | 0.0 (0.0) | 0.0 | 0.0 (0.0) | 0.0 |
Interventions
Patients in the SS + MM + CM group attended more MM sessions than the SS + MM group (mean 7.7 sessions per participant vs. 6.1). Patients in the SS + MM + CM group received an average of around nine vouchers out of a possible 12; 98% received at least one voucher.
Health and social care services
Apart from less reported use of supported accommodation in the SS + MM + CM group (mean 3.1 weeks per participant) compared to the SS + MM group (mean 15.1 weeks) and the SS group (mean 10.4 weeks) over the 6-month follow-up, the use of other health and social care services was broadly similar across the three randomised groups over this period.
Over the period from the 6-month to the 12-month follow-up, the use of supported accommodation was highest in the SS + MM group (mean 14.5 weeks per participant), compared to the SS + MM + CM group (mean 9.1 weeks) and the SS group (mean 8.5 weeks). The use of all other health and social care services was broadly comparable across all three groups.
Alcohol relapse prevention medication
The SS + MM + CM group were prescribed acamprosate for a longer period on average (mean 120 days) compared to the SS + MM group (mean 101 days) and the SS group (mean 100 days) over the 6-month follow-up period. The pattern remained similar when including use of other relapse medication, with the highest use in the SS + MM + CM (mean 124 days) compared to the SS + MM group (mean 110 days) and the SS group (mean 102 days). Over the 6- to 12-month follow-up period, the SS group were prescribed acamprosate for a longer period on average (mean 47 days) compared to the SS + MM + CM group (mean 44 days) and the SS + MM group (mean 37 days). However, these differences reduced when all relapse medications were combined (mean 49 days SS; 47 SS + MM + CM; 44 SS + MM).
Crime
Participants did not report committing any criminal offences over the 6-month or 12-month follow-up periods. This is consistent with data suggesting that most alcohol-related crimes, such as assault and domestic abuse, are due to alcohol abuse rather than alcohol dependence. 142 At 6 months, 28 (5%) of participants followed up (SS + MM + CM n = 5 (4%), SS + MM n = 8 (7%), SS n = 15 (6%)) reported being victims of crime including fraud (n = 2), personal theft (n = 7), house burglary (n = 4), criminal damage (n = 1), assault (n = 9), harassment (n = 4) and domestic violence (n = 1). At 12 months, 33 (8%) of participants followed up [SS + MM + CM n = 9 (9%), SS + MM n = 10 (10%) SS n = 14 (7%)] reported being victims of crime including fraud (n = 2), personal theft (n = 6), house burglary (n = 6), criminal damage (n = 2), assault (n = 9), harassment (n = 5) and indecent assault (n = 3).
Costs
Disaggregated costs
Table 18 summarises health and social care costs from the NHS/PSS perspective over the baseline to 6-month and 6-month to 12-month follow-up periods. Disaggregated costs are based on complete case data as data imputation was conducted at the aggregate level. Over the baseline to 6-month follow-up period, the SS + MM + CM group had the lowest total costs per participant (mean £3628), due primarily to lower supported accommodation costs. The SS + MM group had the highest costs (mean £5068), due to higher costs in supported accommodation. For the 6-month to 12-month follow-up period, cost differences between the groups were smaller, with the SS + MM + CM group having the lowest total costs (mean £2926) and the SS + MM having the highest costs (mean £3721). In total, over the full period from baseline to 12-month follow-up, total costs were similar for the SS + MM + CM group (mean £6372) and the SS group (mean £6258) but were higher in the SS + MM group (mean £8264).
| Baseline (previous 6 months) | Baseline to 6-month follow-up | Baseline to 12-month follow-up | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SS + MM + CM (n = 185) |
SS + MM (n = 182) |
SS (TAU) (n = 372) |
SS + MM + CM (n = 104) |
SS + MM (n = 96) |
SS (TAU) (n = 193) |
SS + MM + CM (n = 104) |
SS + MM (n = 96) |
SS (TAU) (n = 193) |
|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| MM | 0 (0) | 0 (0) | 0 (0) | 280 (162) | 233 (145) | 0 (0) | 280 (162) | 233 (145) | 0 (0) |
| CM vouchers | 0 (0) | 0 (0) | 0 (0) | 67 (50) | 0 (0) | 0 (0) | 67 (50) | 0 (0) | 0 (0) |
| Acamprosate | 0 (0) | 0 (0) | 0 (0) | 162 (96) | 137 (102) | 135 (101) | 221 (196) | 187 (196) | 198 (203) |
| Naltrexone/disulfiram | 0 (0) | 0 (0) | 0 (0) | 9 (52) | 21 (81) | 5 (40) | 16 (100) | 36 (149) | 9 (78) |
| Supported accommodation | 755 (3825) | 2170 (10086) | 1451 (5191) | 453 (2991) | 2246 (7035) | 1542 (5828) | 1533 (6849) | 4094 (12096) | 2044 (8984) |
| Hospital-based alcohol services | 2123 (2195) | 1908 (2385) | 2156 (3328) | 880 (4103) | 968 (3957) | 681 (2651) | 1321 (4719) | 1287 (4601) | 1098 (2327) |
| Hospital-based other services | 496 (1046) | 485 (1231) | 608 (1444) | 486 (1019) | 306 (725) | 363 (1182) | 875 (1539) | 699 (1190) | 596 (1409) |
| Community-based alcohol services | 1214 (1755) | 1069 (1259) | 1009 (1116) | 860 (1432) | 794 (1121) | 981 (1584) | 1327 (1738) | 1157 (1392) | 1591 (2331) |
| Community-based other services | 522 (901) | 430 (674) | 509 (1189) | 431 (923) | 363 (598) | 375 (765) | 732 (1310) | 571 (778) | 722 (1331) |
| Total costs | 5110 (5352) | 6062 (10672) | 5733 (7454) | 3628 (7384) | 5068 (8397) | 4082 (7008) | 6372 (10841) | 8264 (13661) | 6258 (10399) |
Differences in costs
The results of tests of differences in imputed and adjusted health and social care costs from the NHS/PSS perspective at the primary 6-month follow-up and the secondary 12-month follow-up are presented in Table 19. The SS + MM + CM group was £467 less costly on average than SS over the baseline to 6-month follow-up period but £276 more costly over the full follow-up period from baseline to 12-month follow-up. The SS + MM group was £380 more costly on average than SS over the 6-month follow-up period and £362 more costly over the full 12-month follow-up period. Differences in costs were not statistically significant at any follow-up time point.
| Costs | SS + MM + CM vs. SS | SS + MM vs. SS | ||||
|---|---|---|---|---|---|---|
| Mean diff (SE) | 95% CI | p | Mean diff (SE) | 95% CI | p | |
| Baseline to 6 months (£) | −467 (717) | −1874 to 941 | 0.515 | 380 (366) | −338 to 1098 | 0.299 |
| Baseline to 12 months (£) | 276 (1144) | −1976 to 2527 | 0.810 | 362 (552) | −722 to 1446 | 0.512 |
Primary cost-utility analysis
EQ-5D-5L scores
EQ-5D-5L health states are summarised in Figure 3 based on the complete data. The graph shows that the average health state values at baseline were similar for all groups (range 0.786–0.809). At the 6-month follow-up point, the average health state values were also similar for all groups (range 0.778–0.805), although with small declines seen in the SS + MM + CM and SS groups. At the 12-month follow-up, the SS + MM + CM and SS + MM treatment groups recorded small gains compared to baseline of + 0.044 and + 0.026, respectively, while the SS group recorded a slight decline (−0.014).
FIGURE 3.
Mean EQ-5D-5L health state scores over time by group.
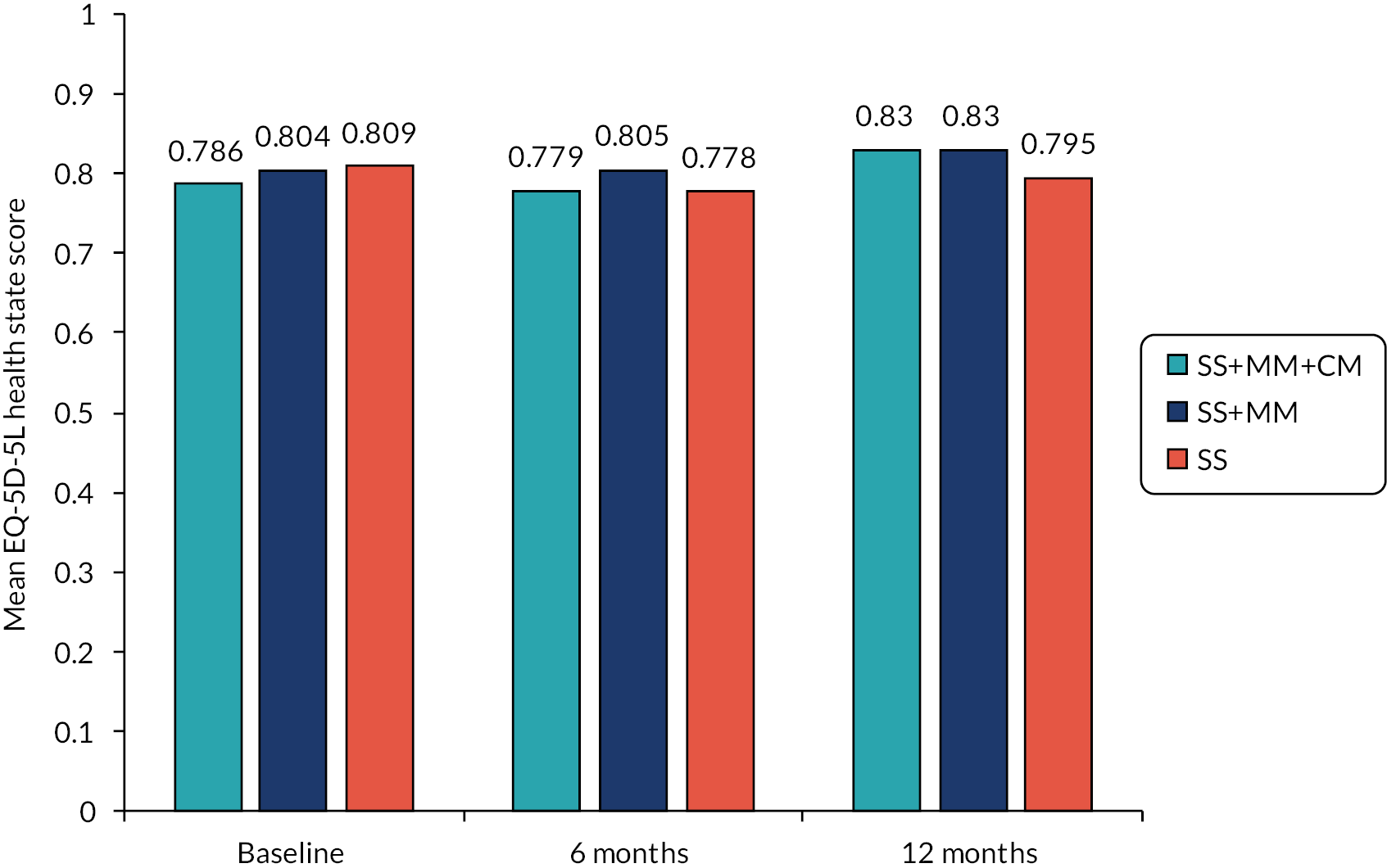
QALYs
The results of tests on differences in imputed and adjusted QALYs at the primary 6-month follow-up and the secondary 12-month follow-up are presented in Table 20. Differences between groups at the 6-month follow-up were small, with patients in the SS + MM + CM and SS + MM groups on average having slightly more QALYs than patients in the SS group, but these differences were not statistically significant. However, differences between groups were greater at the 12-month follow-up and were statistically significant for SS + MM versus SS (mean diff 0.015; p = 0.032), but not for SS + MM + CM versus SS (mean diff 0.023; p = 0.151).
| Follow-up | SS + MM + CM vs. SS | SS + MM vs. SS | ||||
|---|---|---|---|---|---|---|
| Mean diff (SE) | 95% CI | p | Mean diff (SE) | 95% CI | p | |
| 6 months | 0.005 (0.005) | −0.006 to 0.015 | 0.384 | 0.004 (0.003) | −0.001 to 0.010 | 0.125 |
| 12 months | 0.029 (0.017) | −0.006 to 0.063 | 0.102 | 0.015 (0.009) | 0.001 to 0.032 | 0.032 |
Cost-utility analysis at 6-month follow-up
In the primary economic evaluation, a cost-utility analysis at the 6-month follow-up point, the SS + MM + CM group achieved small gains in QALYs compared to the SS group at a lower cost per participant. Thus, the SS + MM + CM group dominated the SS group (better outcomes, lower costs). The SS + MM group also achieved small gains in QALYs but at an additional cost per participant compared to the SS group. The ICER (additional cost per additional QALY) for the SS + MM group was £95,000 per QALY, which is well above the NICE £20,000–30,000 per QALY threshold.
The scatterplots in the cost-effectiveness planes in Figures 4 and 5 illustrate uncertainty in the joint distribution of costs and outcomes generated using non-parametric bootstrap methods for SS + MM + CM versus SS and SS + MM versus SS, respectively, at the 6-month follow-up point. For SS + MM + CM (see Figure 4), the scatterplot illustrates that for the majority of replications, total costs were lower and total QALYs were higher for SS + MM + CM than SS. For SS + MM (see Figure 5), the scatterplot illustrates that for the majority of replications, total costs and QALYs were higher for SS + MM when compared to SS.
FIGURE 4.
Scatterplot showing the bootstrapped mean differences in imputed costs and imputed QALYs for SS + MM + CM compared to SS at 6 months.
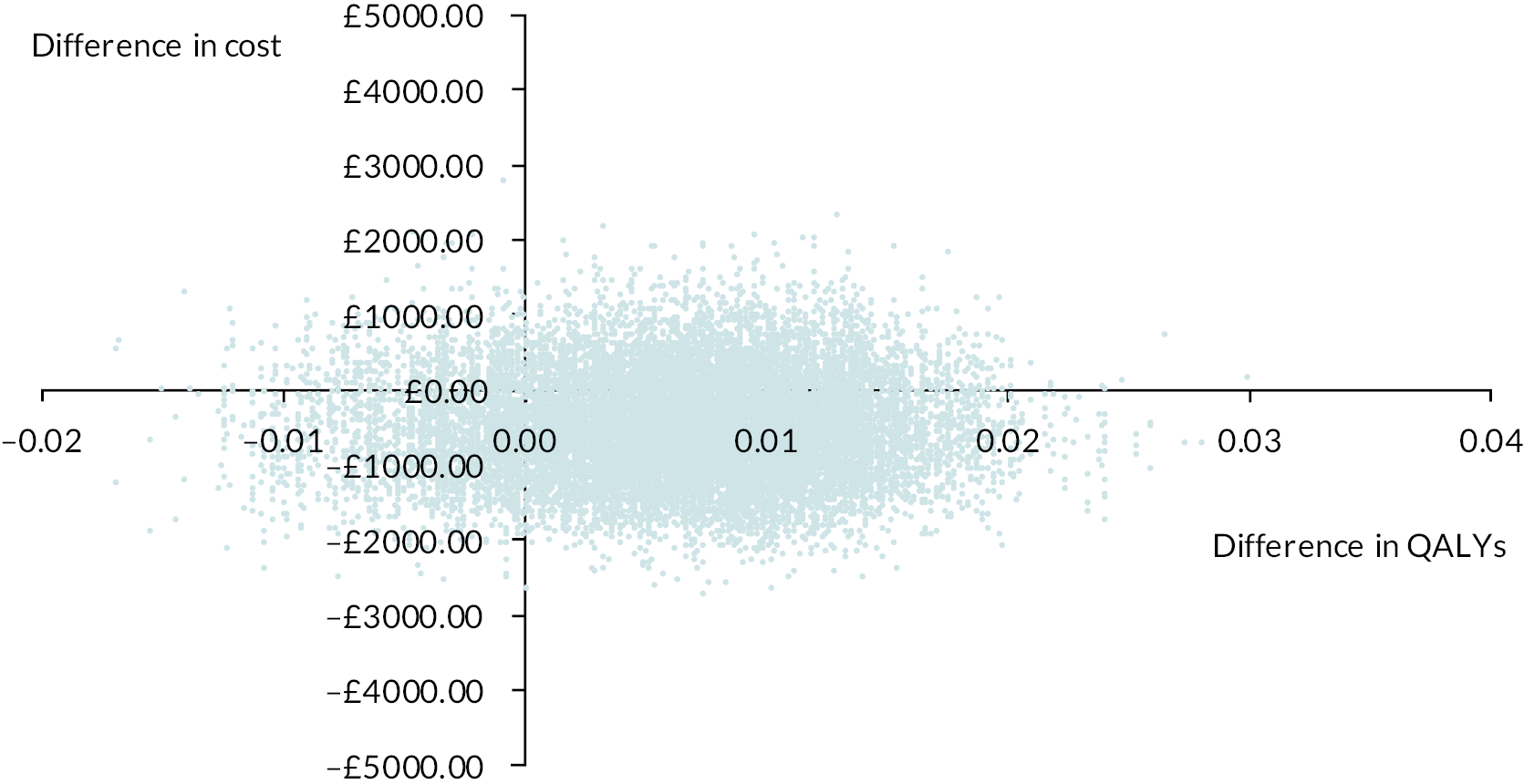
FIGURE 5.
Scatterplot showing the bootstrapped mean differences in imputed costs and imputed QALYs for SS + MM compared to SS at 6 months.

The CEAC in Figure 6 shows that there was a 78% to 80% probability that SS + MM + CM was cost-effective compared to SS, using the NICE willingness-to-pay thresholds of £20,000–30,000 per QALY at the 6-month follow-up point. The CEAC in Figure 7 shows that there was a 22% to 27% probability that SS + MM was cost-effective compared to SS, using the NICE willingness-to-pay thresholds of £20,000–30,000 per QALY at the 6-month follow-up point.
FIGURE 6.
Cost-effectiveness acceptability curve showing the probability that SS + MM + CM is cost-effective compared to SS alone in terms of QALYs at 6 months.

FIGURE 7.
Cost-effectiveness acceptability curve showing the probability that SS + MM is cost-effective compared to SS alone in terms of QALYs at 6 months.
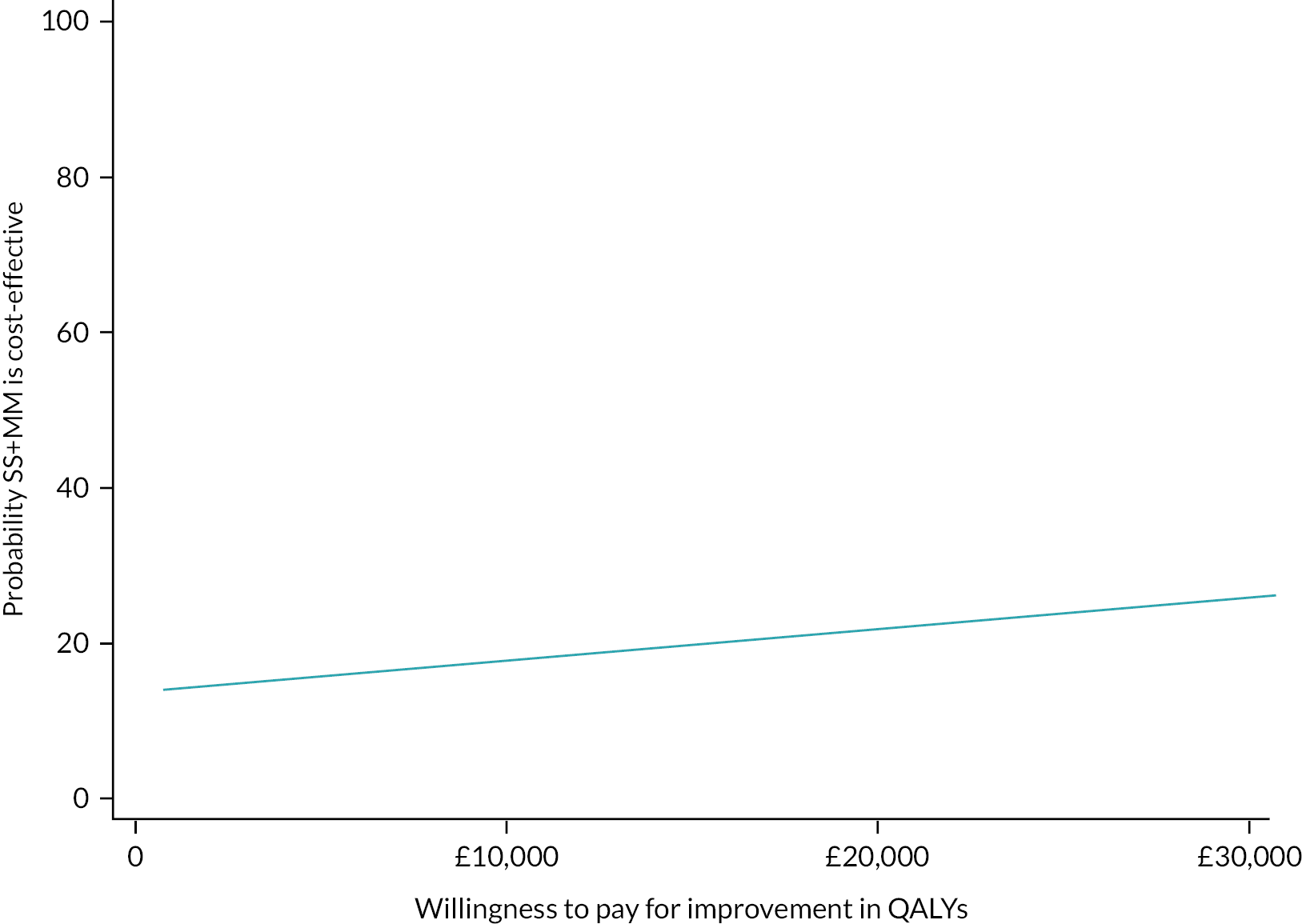
Secondary cost-utility analysis at 12 months
At the secondary 12-month follow-up point, the SS + MM + CM group made gains in QALYS but at a higher cost per participant compared to the SS group. The ICER (additional cost per additional QALY) was £9517 per QALY, which is below the NICE £20,000–30,000 per QALY threshold. The SS + MM group also achieved gains in QALYs with an additional cost per participant compared to the SS group. The ICER (additional cost per additional QALY) for the SS + MM group compared to the SS group was £24,133 per QALY, also below the NICE threshold.
For SS + MM + CM, the 12-month scatterplot (see Figure 8) illustrates that in the majority of replications, total costs were greater and QALYs also greater compared to SS. For SS + MM, the 12-month scatterplot (see Figure 9) is similar to the SS + MM + CM versus SS comparison, with the majority of replications also showing higher costs and QALYs for SS + MM compared to SS.
FIGURE 8.
Scatterplot showing the bootstrapped mean differences in imputed costs and imputed QALYs for SS + MM + CM compared to SS at 12 months.

FIGURE 9.
Scatterplot showing the bootstrapped mean differences in imputed costs and imputed QALYs of SS + MM compared to SS at 12 months.
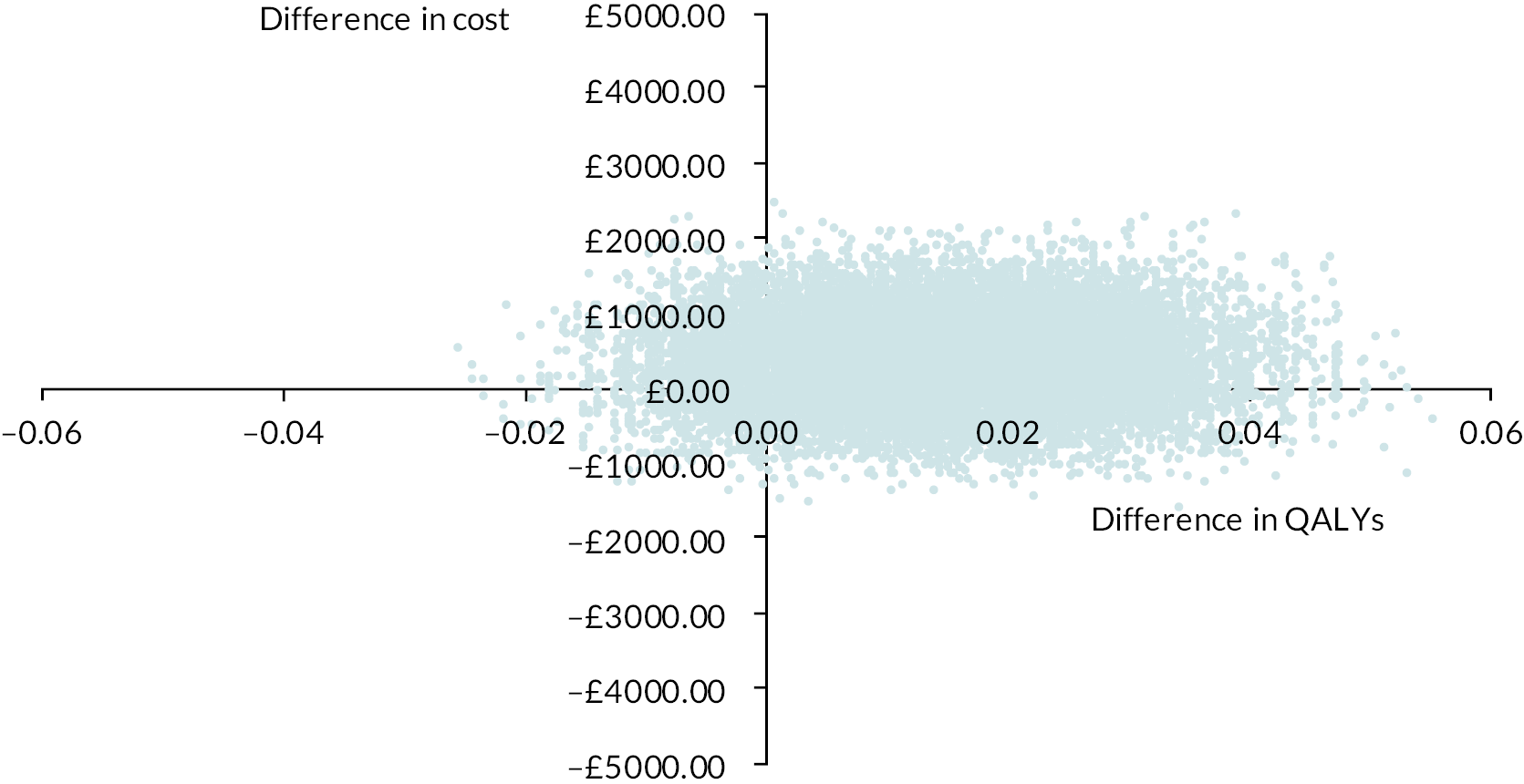
The CEAC in Figure 10 shows that the probability that SS + MM + CM was cost-effective compared to SS ranged between 61% and 68% using the NICE willingness-to-pay threshold of £20,000–30,000 per QALY. The CEAC in Figure 11 shows that the probability that SS + MM was cost-effective compared to SS ranged between 48% and 581%, using the same thresholds.
FIGURE 10.
Cost-effectiveness acceptability curve showing the probability that SS + MM + CM is cost-effective compared to SS alone in terms of QALYs at 12 months.
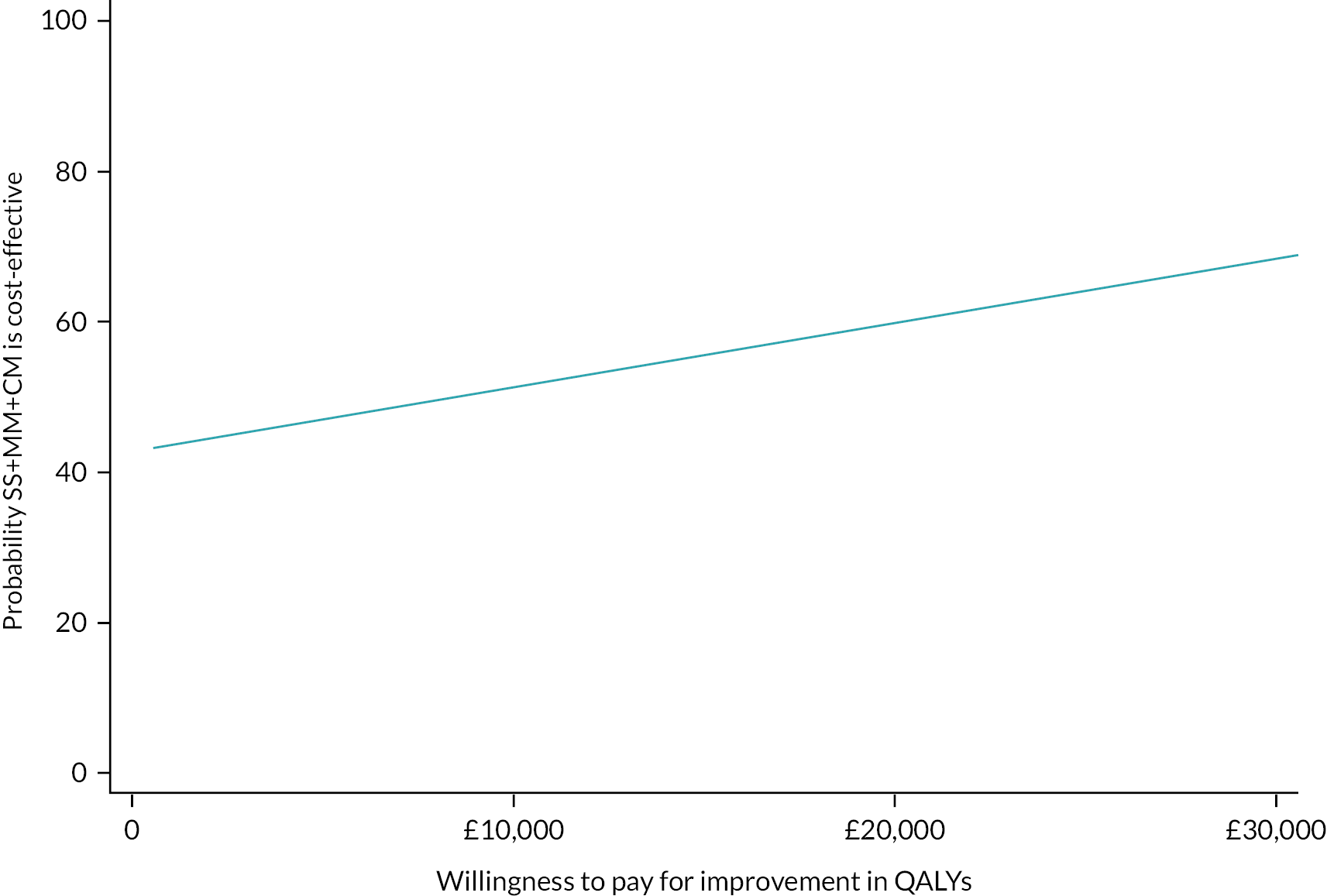
FIGURE 11.
Cost-effectiveness acceptability curve showing the probability that SS + MM is cost-effective compared to SS alone in terms of QALYs at 12 months.
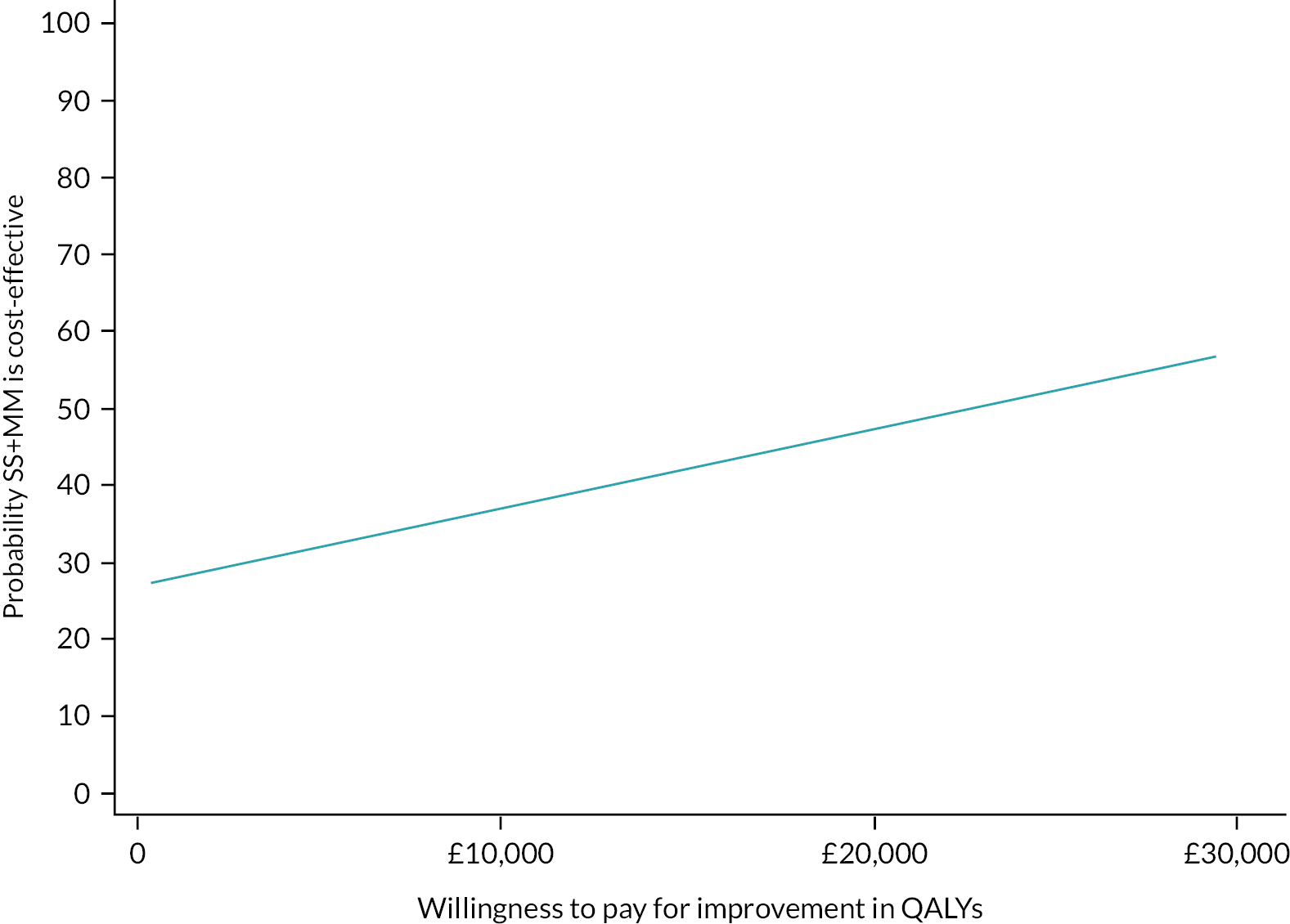
Secondary cost-effectiveness analysis at 6 months
The primary clinical outcome was adherence to acamprosate medication expressed as percentage of days adherent at the 6-month follow-up. Adherence was highest in the SS + MM + CC group (47%, n = 141,) followed by SS + MM group (41%, n = 122) and lowest in the SS group (38%, n = 255).
The results of tests of differences in imputed and adjusted percentage of adherent days at the 6-month primary clinical follow-up point are presented in Table 21. Note that multiple imputation in the economic analysis included baseline costs and baseline utilities as covariates in the chained equations leading to slightly different imputed estimates to the clinical analysis. Adherence was better in both treatment groups compared to control, reaching statistical significance in the SS + MM + CM group.
| Outcome | SS + MM + CM vs. SS | SS + MM vs. SS | ||||
|---|---|---|---|---|---|---|
| Mean diff (SE) | 95% CI | p | Mean diff (SE) | 95% CI | p | |
| Adherence (%) | 10.39 (4.52) | 1.52 to 19.27 | 0.022 | 1.84 (2.32) | −2.72 to 6.41 | 0.428 |
In terms of the primary clinical outcome at 6-month follow-up (acamprosate medication adherence), costs were lower and outcomes significantly better in the SS + MM + CM group compared to the SS group, so SS + MM + CM again dominated SS alone. For the SS + MM group, outcomes were better but costs were higher compared to the SS group, meaning that assessment of cost-effectiveness depends upon the maximum threshold value decision-makers would be prepared to pay for a unit change in outcome.
The scatterplots in the cost-effectiveness planes in Figures 12 and 13 illustrate uncertainty in the joint distribution of costs and outcomes generated using non-parametric bootstrap methods, for SS + MM + CM versus SS and SS + MM versus SS, respectively. For SS + MM + CM (see Figure 12), the scatterplot clearly illustrates that for the majority of replications, total costs are lower for SS + MM + CM versus SS and effectiveness is higher. For the SS + MM group (see Figure 13), most replications fall in the north-east quadrant where both costs and effects are higher compared to the SS group.
FIGURE 12.
Scatterplot showing the bootstrapped mean differences in imputed costs and imputed medication adherence for SS + MM + CM compared to SS at 6 months.

FIGURE 13.
Scatterplot showing the bootstrapped mean differences in imputed costs and imputed medication adherence for SS + MM compared to SS at 6 months.

The CEAC in Figure 14 shows that the probability that SS + M + CM is cost-effective compared to SS is between 89% and 95% when decision-makers are willing to pay between £0 and £50 per percentage point improvement in medication adherence over 6 months. However, the probability that SS + MM is cost-effective compared to SS was only 18% to 22% across the same range of willingness-to-pay values (see Figure 15).
FIGURE 14.
Cost-effectiveness acceptability curve showing probability that SS + MM + CM is cost-effective compared to SS in terms of acamprosate adherence at 6 months.
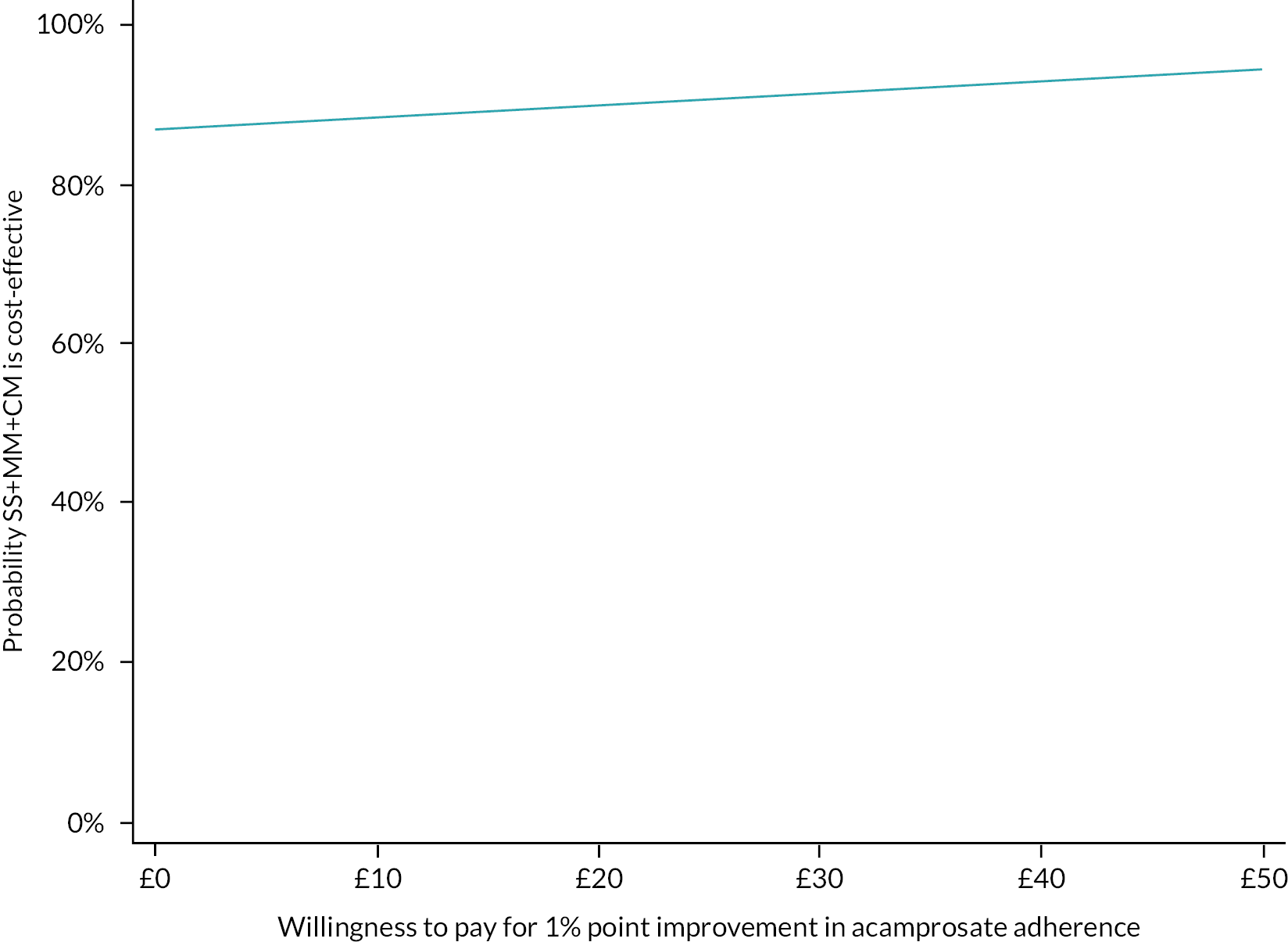
FIGURE 15.
Cost-effectiveness acceptability curve showing the probability that SS + MM is cost-effective compared to SS in terms of acamprosate adherence at 6 months.
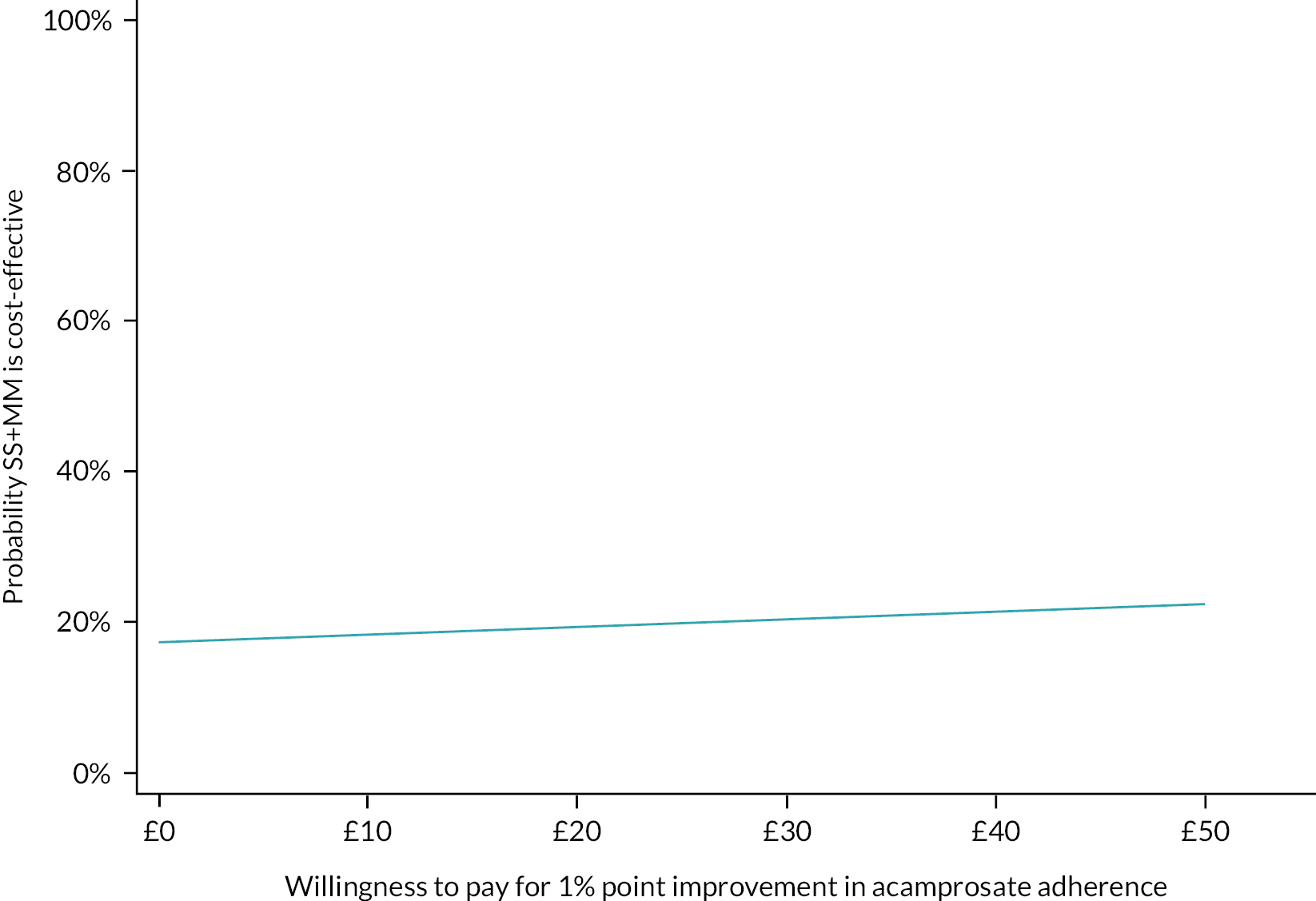
Sensitivity analysis
Results of the sensitivity analyses are reported in Table 22. Both the complete case analysis (rather than imputation of missing data) and the analysis using the EQ-5D-3L value set (rather than the EQ-5D-5L) produced similar results to the primary analysis at 6-month follow-up, indicating that the results of the primary analysis were robust to variation of assumptions.
| SS + MM + CM vs. SS | SS + MM vs. SS | |||||
|---|---|---|---|---|---|---|
| Mean difference in costs (£) | Mean difference in QALYs | ICER (cost per QALY) | Mean difference in costs (£) | Mean difference in QALYs | ICER (cost per QALY) | |
| Primary analysis | −467 | 0.005 | Dominant | 380 | 0.004 | £95,000 |
| Complete case | −752 | 0.004 | Dominant | 295 | 0.003 | £98,333 |
| EQ-5D-3L | −467 | 0.004 | Dominant | 380 | 0.006 | £63,000 |
Cost-effectiveness outcomes modelled over 20 years
Running the patient-level, multistate life table model over 20 years after the end of the trial produced results in favour of both SS + MM + CM and SS + MM compared to SS alone. The SS + MM + CM group dominated SS achieving 0.03 more discounted QALYs at a lower discounted cost of −£401 per participant. The SS + MM group achieved QALY gains of 0.15 discounted QALYS but at a higher discounted cost of £64 per participant compared to the SS group. The ICER (additional cost per additional QALY) was £427 per QALY, well below the NICE £20,000–30,000 threshold.
Probabilistic sensitivity analysis was used to vary model key parameters to estimate model uncertainty. For SS + MM + CM, the 20-year scatterplot (see Figure 16) shows that 999 out of 1000 simulations fell in the south-east quadrant, indicting more QALY gains at lower cost compared to SS. For SS + MM, the 20-year scatterplot (see Figure 17) shows relatively greater gains in QALYs compared to SS but with over half of these at a higher cost.
FIGURE 16.
Scatterplot showing the bootstrapped mean differences in imputed costs and imputed QALYs for SS + MM + CM compared to SS modelled over 20 years.

FIGURE 17.
Scatterplot showing the bootstrapped mean differences in imputed costs and imputed QALYs for SS + MM compared to SS modelled over 20 years.

The CEAC in Figure 18 shows that the probability that SS + MM + CM was cost-effective compared to SS was 100% across all values of the NICE willingness-to-pay per QALY threshold plotted (£0–30,000). The CEAC in Figure 19 shows that the probability that SS + MM was cost-effective compared to SS was 100% across the range of the NICE willingness-to-pay threshold of £20,000–30,000 per QALY.
FIGURE 18.
Cost-effectiveness acceptability curve showing the probability that SS + MM + CM is cost-effective compared to SS in terms of QALYs modelled over 20 years.
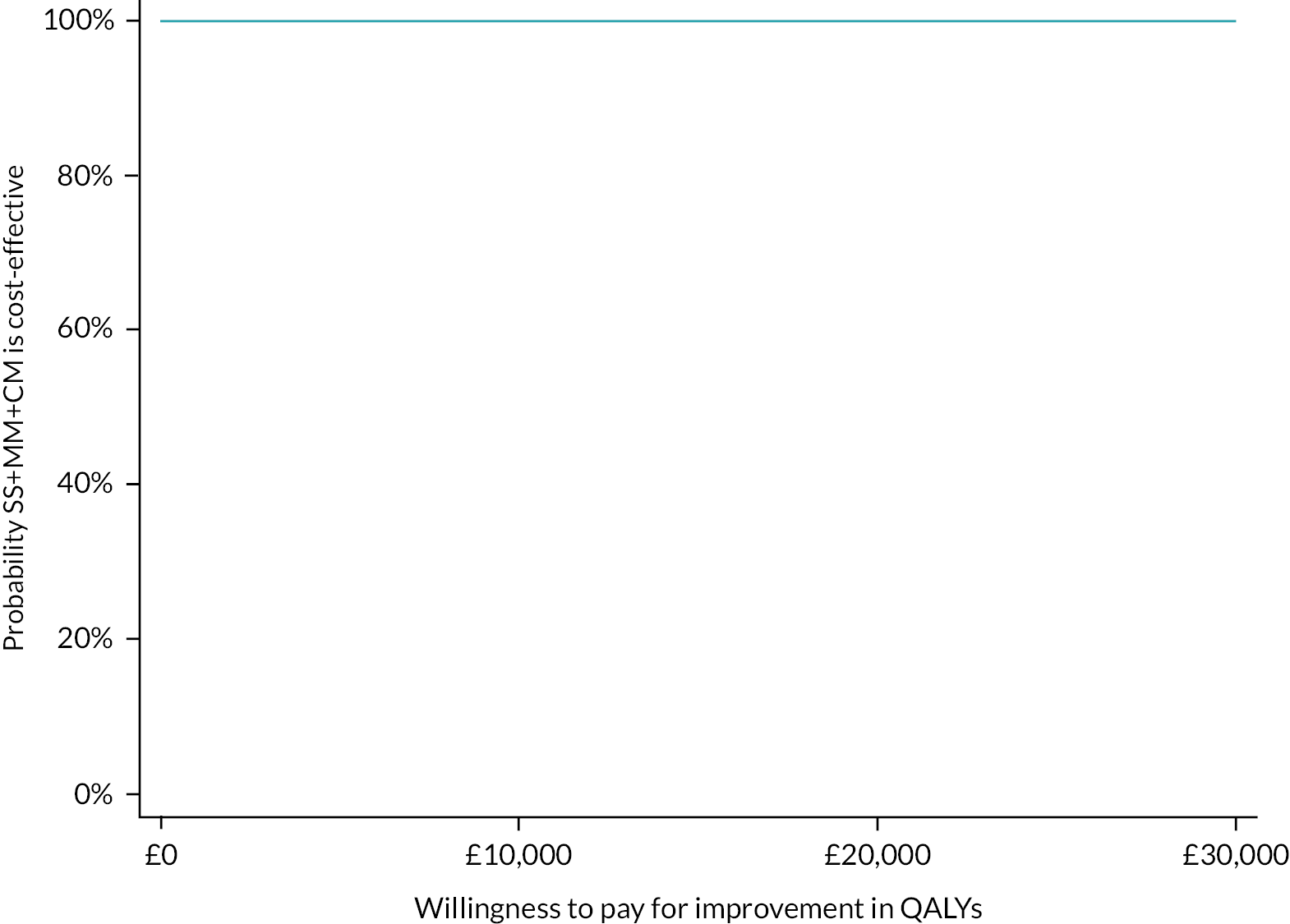
FIGURE 19.
Cost-effectiveness acceptability curve showing the probability that SS + MM is cost-effective compared to SS in terms of QALYs modelled over 20 years.
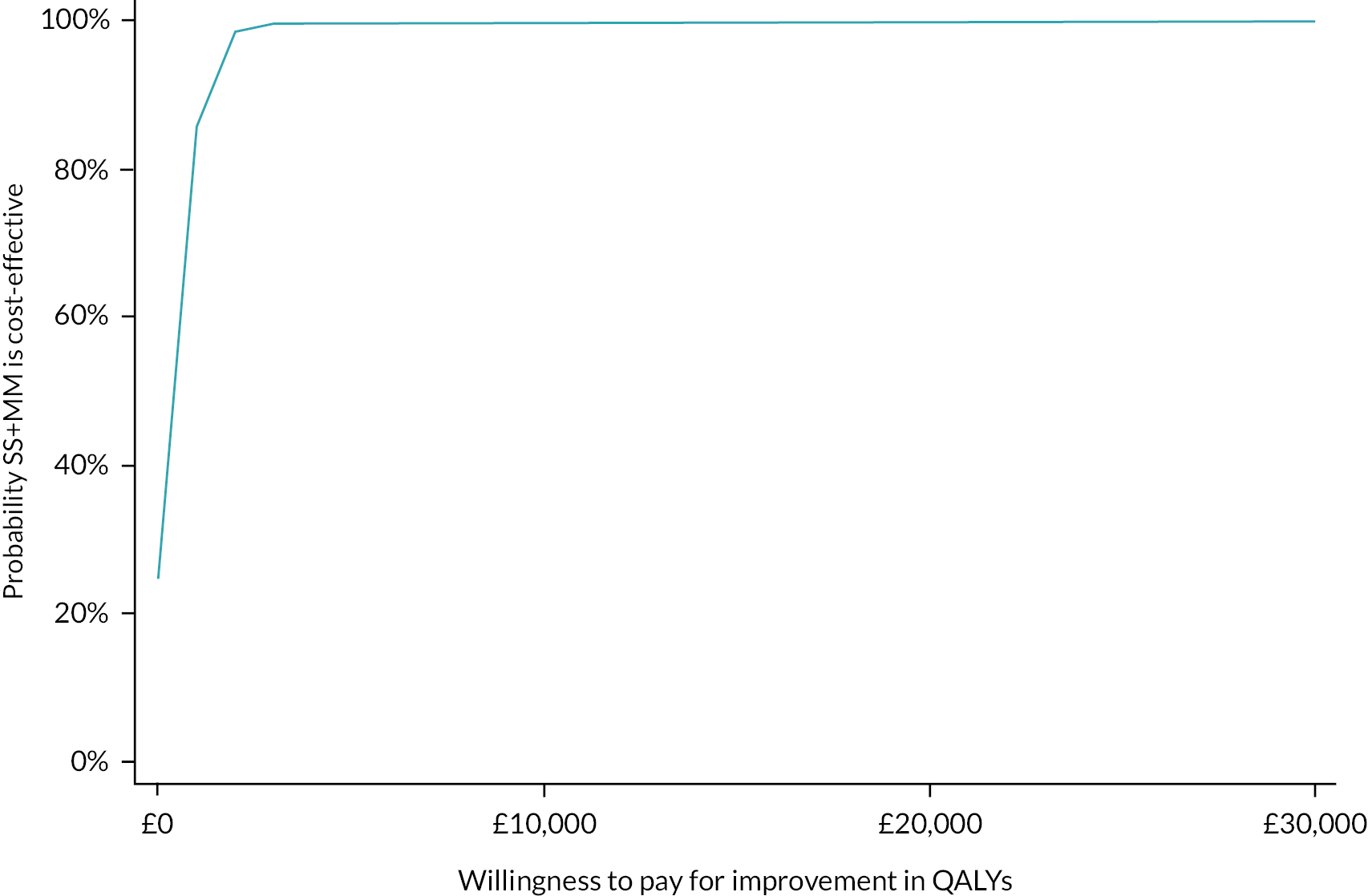
Summary of findings
SS + MM + CM versus SS
For SS + MM + CM versus SS, the primary economic analysis at 6-month follow-up using QALYs, the secondary economic analysis at 6-month follow-up using medication adherence and the economic modelling over a 20-year time horizon using QALYs all found SS + MM + CM to dominate SS (better outcomes at lower cost). At 12-month follow-up, although SS + MM + CM was not dominant, it generated more QALYs at an additional cost that was below the NICE cost per QALY threshold. There was, therefore, a higher probability of SS + MM + CM being cost-effective compared to SS alone in all analyses and at all time points.
SS + MM versus SS
For SS + MM versus SS, at 6- and 12-month follow-up and when modelled over 20 years, SS + MM achieved better outcomes at higher cost compared to SS. In terms of cost-effectiveness, SS + MM was not found to be cost-effective at 6-month follow-up but had a higher probability of being cost-effective compared to SS at both the 12-month follow-up (using the higher £30,000 per QALY NICE threshold) and when modelled over 20 years (over the full £20,000–30,0000 cost per QALY threshold).
Implications
The results of the primary economic analysis at the 6-month follow-up point suggest that MM was only cost-effective when supported by voucher incentives to encourage support session uptake. This finding was heavily influenced by lower total costs in the SS + MM + CM group as a result of lower use of residential rehabilitation facilities compared to both SS + MM and SS alone, which may be related to the significantly higher medication adherence seen in the CM group. Over the medium (12 months) and longer term (20 years), SS + MM + CM remained cost-effective compared to SS and there was a higher probability of SS + MM being cost-effective compared to SS. These results support the addition of MM to SS for alcohol dependence, with or without CM. However, the economic benefit was stronger when CM was included.
Chapter 6 Discussion
Contingency Management has an extensive evidence base showing effectiveness in the treatment of substance use disorder, but less was known about its application in the treatment of alcohol dependence. 21,81 There is greater evidence of CM reinforcing adherence to a range of interventions for substance use disorder, including medications, than for changes in consumption per se. However, there is evidence of effectiveness of CM targeted at reduced use of tobacco, cocaine, opiates and polysubstance use. 143
Relapse prevention medications also have a strong evidence base, and acamprosate and naltrexone are recommended as first-line treatments for people with alcohol dependence in order to reduce the high relapse rate following initial detoxification. 21 However, despite the evidence of effectiveness from clinical trials, medication adherence is a common problem in clinical practice, with wide variation between trials, and adherence rates as low as 27% over 12 weeks in the only UK clinical trial of acamprosate. 26,144 There is also evidence from routine prescription data that many patients discontinue relapse prevention medication before the recommended 6-month course is completed; manufacturer’s data suggest the average duration of acamprosate use is around 6 weeks. Both of these factors will limit the potential of this treatment to impact upon relapse, as poor medication adherence is associated with worse clinical outcomes. This led NICE to recommend further research on methods to increase medication adherence in alcohol dependence and on the potential of CM in alcohol dependence treatment more widely. 21
We report here on a multicentre RCT of the effectiveness and cost-effectiveness of CM and another intervention, MM, to increase adherence to acamprosate in patients who have undergone detoxification from alcohol, in community addiction services in England. This was conducted and reported in accordance with international guidelines for research excellence. 145
The MM intervention was adapted from the Medical Management intervention used to support medication adherence in a US NIH-funded Testing Combined Pharmacotherapies and Behavioral Interventions in Alcohol Dependence trial of naltrexone and acamprosate for alcohol dependence. 44 Medical Management was originally designed to be used by physicians in the Testing Combined Pharmacotherapies and Behavioral Interventions in Alcohol Dependence trial and subsequent studies. 146–151 However, we took advantage of recent developments in the UK of the extended clinical role of community pharmacists, including the HLP programme, which includes alcohol dependence as a key target population for pharmacist interventions. 55,63,64,90,152 We developed an effective partnership with Lloyds Pharmacy in order to implement a national pharmacist-delivered adaptation of the Medical Management intervention, known as MM. This was efficiently delivered by specially trained pharmacists from an established centralised call centre, where a wide range of pharmacist interventions across a range of clinical conditions was already being delivered by telephone. As MM was delivered by a small team of pharmacists, we were able to train and supervise the clinicians more effectively than in a dispersed model in community pharmacies across all the participating sites in four regions in England, as had been envisaged in our initial plan. In addition, this centralised model allowed routine recording of all telephone-delivered MM sessions, and we monitored and rated a random sample of calls, stratified by pharmacist, in order to assess fidelity to the intervention and feedback into supervision where necessary. This meant that not only did the pharmacists develop a high level of skill in delivering the intervention, but we were also able to achieve a high level of fidelity to the planned intervention although it was being delivered to a large number of participants across a wide geographical area.
The CM intervention was also adapted from previous protocols that had been designed for use in treatment of substance use disorder, as well as published evidence from clinical trials in the alcohol field. 70,71,89,92,153 In the case of both the MM and CM interventions, we conducted extensive patient and clinician involvement in developing the manuals to ensure that both were acceptable and feasible to implement.
We were also able to develop an excellent working partnership with the manufacturers of acamprosate, Merck Serono, who supplied the medications for participants in this trial, dispensed through the network of Lloyds Pharmacies across the participating sites.
We developed a large network of participating community addiction treatment services across four regions in England (London, Yorkshire and Humber, West Midlands and South East), where all recruitment of trial participants took place. During the trial, addiction services nationally were under extreme pressure with reducing funding from local authorities and a considerable turnover in changes of contracts and providers. 15 This proved extremely challenging for participation in clinical research and resulted in delays in achieving full recruitment to the trial, and resulted in an extension to the trial of a year which was funded by NIHR.
Acamprosate prescriptions were commenced by community addiction teams, but there were also challenges in persuading general practitioners to continue to prescribe acamprosate for participants as intended in the protocol. This was in spite of the fact that acamprosate has been recommended as a first-line treatment for alcohol dependence for over 10 years. 21 We found that attitudes towards prescribing acamprosate were not as positive as we had expected, and NHS authorities in some local areas did not support acamprosate prescribing. In these instances, the community addiction services had to continue prescribing for participants, but this may have provided a disincentive to both clinicians and participants to continue prescribing up to and beyond the recommended 6-month period.
Key findings
Recruitment and randomisation
This study aimed to compare the effects of pharmacist-delivered MM, with and without incentivisation provided by CM, compared to SS. The primary hypothesis was that MM plus CM plus SS (SS + MM + CM), and MM plus SS without CM (SS + MM), would result in greater medication adherence than SS alone. We also hypothesised that SS + MM + CM would result in greater adherence to MM, as well as greater adherence to medication than SS + MM.
We were able to recruit 739 participants, of whom 372 (50%) were randomly allocated to the SS group, 182 (25%) to SS + MM group and 185 (25%) to SS + MM + CM group, achieving our planned recruitment target, although due to changes in service provider contracts, this took longer to achieve than planned. We also managed to successfully follow up 518 (70%) participants at the primary end point, achieving the planned statistical power. Our follow-up rate at 12 months was lower than planned but this had no influence on the primary outcome analysis.
Comparing the characteristics of participants across study arms at baseline showed no differences between allocated groups, suggesting that the randomisation method minimised bias.
Primary outcome analysis and sensitivity analyses
As discussed in Chapter 3, we were unable to use the primary outcome measure as intended due to various limitations of the MEMS and pill count data. We therefore used self-reported adherence as our primary outcome measure. The potential limitations of this are discussed below.
Our primary ATA showed that the mean difference in per cent adherence to acamprosate was 10.6% lower in the SS group compared to the SS + MM + CM group which was statistically significant. This was also close to the pre-specified clinically important difference. Although the mean adherence was 3.1% lower in the SS group compared to the SS + MM group, this was not statistically significant, and a similar non-significant finding was seen comparing SS + MM and SS + MM + CM groups, with SS + MM 7.9% lower than SS + MM + CM.
We found that 45 (6.1%) participants had been followed up outside of the 2-month follow-up window at the primary end point. A sensitivity analysis, however, showed that this had no impact on the primary outcome analysis. Similarly, both multiple imputation and a sensitivity analysis to explore the impact of missing data indicated that we can be confident that the observed treatment effects are robust under different interpretations of the nature of missing data.
A CACE analysis was conducted under two scenarios: scenario 1 where participants adhered to at least 6 (50%) of the MM calls, and scenario 2 where they adhered to 12 (100%). In scenario 1, 58% in the SS + MM group adhered to 50% of the MM calls, compared to 69% in the SS + MM + CM group. In scenario 2, 11% in the SS + MM group adhered to 100% of MM calls compared to 39% in the SS + MM + CM group. The CACE analysis showed that compared to our intention-to-treat analysis, under scenario 1, the difference between SS + MM + CM and SS increased from 10.6% to 12.4% greater adherence to acamprosate at month 6. Comparing SS + MM to SS, the previously non-significant difference (3.1%) became statistically significant within the CACE analysis at a difference of 13.2%.
Under scenario 2, the differences became larger in magnitude at 22.2% comparing SS + MM + CM with SS, and 31.8% comparing SS + MM with SS (although this latter analysis needs to be interpreted cautiously given the small number of participants in the analysis). So, in both scenarios 1 and 2, the benefits of SS + MM + CM were significant and clinically important. This was the case for SS + MM only when compliance was incorporated into the analysis.
Secondary outcome analyses
The ATA analysis showed lower per cent days abstinent in SS compared to both SS + MM + CM and SS + MM, but these were not significant. However, when assessed under the scenario 2 CACE analysis, per cent days abstinent were significantly lower in the SS group compared with both SS + MM + CM and SS + MM groups. ATA analysis of other secondary outcome measures at both 6 and 12 months showed no significant differences between groups.
The analysis of compliance with MM calls showed that those who complied were significantly more likely to have improved alcohol use outcomes: 12.5% more per cent days abstinent in the SS + MM + CM group and 15.1% more in the SS + MM group compared to SS alone (although the differences in the SS + MM group should be taken with caution considering the small numbers involved). There was also a significant correlation between increased adherence to acamprosate and better drinking outcomes (r = 0.39; p < 0.001).
Health economic analysis
The primary economic analysis was a cost-utility analysis where outcomes were expressed as QALYs, as recommended by NICE. 129 A secondary analysis explored cost-effectiveness in terms of the primary clinical outcome, which was adherence to relapse prevention medication. The primary economic perspective was the NHS/PSS perspective preferred by NICE. This covers all hospital, community health and social services. A sensitivity analysis based on a broader societal perspective, including impact on the criminal justice sector was planned. However, this became redundant because criminal activity was not reported by any patient at any time point.
Full service use data at the primary follow-up point of 6 months were available for 70% of the sample randomised, and 53% at the 12-month follow-up point. We found that SS + MM + CM participants attended more MM calls than the SS + MM group (mean of 7.7 vs. 6.1), and participants in the SS + MM + CM group received an average of 9 vouchers out of a possible 12, with 98% receiving at least one voucher. The SS + MM + CM group were prescribed acamprosate for a longer period compared to the SS + MM and SS groups at month 6, although this reduced and was broadly similar between groups by month 12.
The main difference in health and social care costs at month 6 was the SS + MM + CM group reported much less use of supported accommodation (primarily residential rehabilitation) than either the SS + MM or SS groups. The use of all other health and social care services was broadly similar across groups at month 6. At month 12, the SS + MM group reported greater use of supported accommodation than SS + MM + CM and SS, but the use of all health and social care services was similar across groups.
Differences in quality of life between groups and across time points were small, but favoured the SS + MM + CM and SS + MM groups over the SS group. This translated into small differences in QALYs which were significantly higher only for the SS + MM group over SS at 12 months.
The primary economic analysis found that SS + MM + CM was cost-effective compared to SS alone, but that SS + MM was not cost-effective compared to SS alone. SS + MM + CM dominated SS alone, achieving more QALYs at lower cost, with an 89% probability that SS + MM + CM was more cost-effective compared to SS over the NICE £20,000–30,000 cost per QALY threshold range. The cost per QALY benefit of SS + MM versus SS was at an additional cost of £151,250 per QALY which is well over the NICE threshold. The results were robust in the planned sensitivity analyses.
Secondary analysis based on the main clinical outcome of medication adherence showed that SS + MM + CM dominated SS, and SS + MM also achieved better medication adherence but at an additional cost of £329 per one additional per cent of medication adherence. At 12 months, both SS + MM + CM and SS + MM had a higher probability of being cost-effective than SS alone at the NICE cost-per-QALY thresholds of £20,000–30,000 while SS + MM had a higher probability of being cost-effective than SS alone at the £30,000 cost-per-QALY threshold. An analysis of cost-effectiveness modelled over 20 years amplified these findings and showed that the probability both SS + MM + CM and SS + MM were cost-effective compared to SS was 100% across all values of the NICE willingness-to-pay threshold of £20,000–30,000 per QALY.
Consideration of possible explanations
While we found a clinically significant effect of SS + MM + CM compared to SS in terms of our primary outcome measure, medication adherence, we did not find a significant effect on alcohol consumption outcomes in the ATA, although this was the case in the CACE analysis. Arguably, from a clinical perspective, the benefit of increased medication adherence is only relevant if it translates into improved drinking outcomes. This is where our secondary analyses become important in understanding the potential mechanisms involved.
The CACE analyses showed that those participants who engaged with the MM calls not only had greater medication adherence but also had better drinking outcomes. These favoured both SS + MM and SS + MM + CM groups. So, our hypothesis is that MM has a positive impact on drinking outcomes in people who engage with it, through improved medication adherence. 144 CM has a positive impact on adherence with MM calls and, by doing so, an impact on both medication adherence and reduced drinking. This supports the hypotheses of the trial.
Furthermore, these clinical benefits translated into economic benefits. The analysis of service use data showed that SS + MM + CM group both attended more MM sessions, and took acamprosate for longer than the SS group. Also, the SS + MM + CM group used less residential rehabilitation than either the SS + MM or SS groups. This was also reflected in improvements in quality of life in the SS + MM + CM group compared to the SS group, and modelling the data over a 20-year time horizon, SS + MM + CM and SS + MM were both found to be cost-effective compared to SS alone.
Taking these findings together, this suggests that SS + MM + CM is the better intervention as a result of supporting better adherence with MM, which translates into improved acamprosate adherence, improved drinking outcomes, and probably as a consequence, improved quality of life and reduced need for high-cost residential care. It is possible that MM as an intervention may have had benefits, in terms of reduced drinking and other outcomes, not exclusively because of its impact on medication adherence. Although medication adherence was the primary target of MM sessions, it was also designed to include other aspects of clinical support from the pharmacists, including encouragement to remain abstinent from alcohol and/or seek and engage with help from primary care and specialist addiction services where needed. So, the benefits of MM may be broader than simply improving medication adherence.
Comparison with previous research
Our findings are broadly consistent with previous research on CM in both drug and alcohol dependence. 143 CM is strongly grounded in behavioural science and is one of the most widely studied intervention techniques in substance use disorder research. 82,154 CM is recommended by several international clinical guidelines as a key evidence-based intervention in substance use disorder, particularly for stimulant users. 80,155,156 Published systematic reviews have shown consistently larger effects of CM on treatment adherence than on abstinence outcomes in both drug and alcohol dependence, with effect sizes that are consistently greater for adherence than for abstinence. 156 One recent systematic review found the effect size across 10 CM studies to be ‘moderate’ (d = 0.47; CI: 0.25 to 0.69) for adherence directed, and ‘small’ (d = 0.22; CI: 0.12 to 0.33) for abstinence directed, CM. 156 This is consistent with our results, although our secondary analyses suggested that although the CM was, in our case, targeted at increasing attendance at MM sessions, it also had an effect on drinking outcomes. An intriguing possibility, and a suggestion of a recent review, is that it would be useful to consider combining CM directed at attendance with CM directed at abstinence, and to compare the effects of combined CM with individually directed CM and care as usual. 156
Another target for CM in previous studies has been directly incentivising medication adherence rather than adherence to interventions directed at improving medication adherence, as in the ADAM trial. For example, there is evidence of CM directed at improving adherence to both methadone maintenance treatment and hepatitis B vaccination in opiate-dependent patients having a significant and cost-effective impact on outcomes. 153,155 We considered this application in the ADAM trial, but the challenge was to find a reliable method of measuring adherence to acamprosate. In the case of methadone and hepatitis B vaccination adherence, there are simple routine methods of measuring adherence which made this possible in these trials. Also, in those with substance use disorder, the availability and relatively low cost of urine and saliva drug testing lend itself to routine use in CM incentivising abstinence.
Some early clinical trials of acamprosate used urine analysis to detect acamprosate adherence, but this was considered too costly and impractical for routine clinical use, and hence, in this pragmatic clinical trial. 26 We also considered using the MEMS data as a way of measuring adherence to acamprosate. However, we considered this would also have some limitations, particularly in routine clinical care, and indeed the lack of reliable data from MEMS over an extended period of monitoring in this trial vindicated that decision.
The reliable measurement of abstinence is also challenging in alcohol-dependent patients due to the short half-life of ethanol, and the low reliability of existing blood markers of alcohol consumption. 157 Therefore, using CM to directly incentivise abstinence from alcohol is not yet technically possible. But the development of wearable transdermal alcohol sensors may allow the development of this approach in the future. 89 Taken together, because of these limitations, we decided to use CM to incentivise adherence to MM sessions which could be reliably measured, rather than having medication adherence itself or alcohol abstinence as the CM target.
Previous reviews have pointed to evidence that the effects of CM often decay once they are discontinued and indeed we found that in this trial. 158 CM was only applied up to the 6-month point, where its beneficial effects were found. Thereafter there was some regression to the mean across groups. This is by no means unique to CM; trials of other psychological and pharmacological interventions in the drug and alcohol dependence field report a similar decay over time. Stitzer159 and others72 have raised the possibility of longer-term CM interventions, including the possibility of an ‘incentives maintenance model, outreach efforts designed to monitor, detect and retreat relapsed clinicals or a combination of CM with cognitive-behavioural therapies’. 159
Previous research has shown that psychosocial interventions to increase medication adherence are effective across a range of clinical conditions and medications. 158,160 While the ‘medical management’ approach, adapted for this trial in the form of MM, has been used in several trials of pharmacotherapy for alcohol dependence, its effectiveness and cost-effectiveness were unknown. 146–151 Our results show that MM enhanced with CM was more effective than SS in increasing relapse prevention medication adherence and had a higher probability of being cost-effective (at 6- and 12-month follow-up and when modelled over 20 years). However, MM alone was not more effective than SS. We did find evidence that patients who engaged with MM had better adherence than those who did not, and the cost-effectiveness analyses favoured MM compared to SS at 12-month follow-up and when modelled over 20 years.
Strengths and limitations
The strengths of this study include that it was a pragmatic, multicentre RCT conducted in a diverse population across four regions in England and had adequate statistical power to detect treatment effects. There was a relatively low level of exclusions compared to other alcohol trials, and withdrawals were few. We were able to train a relatively small and stable team of pharmacists to deliver the MM intervention across a wide geographical area cost-effectively, and we were able to maintain a high level of fidelity to the intervention manual through monitoring of intervention calls and regular supervision.
The baseline data show that our randomisation process created equal groups and we used validated outcome measures to assess outcomes. We were able to achieve our target follow-up rate at the primary outcome end point. Further, the trial included an evaluation of cost-effectiveness, which has been the case in few previous studies of relapse prevention medications, and the bespoke economic model was able to assess impacts across a 20-year time horizon, far longer than is common in other alcohol clinical trials which typically are restricted to a 1-year time frame. 21 Given that for many people, alcohol dependence is a chronic relapsing condition, this longer time frame is arguably more meaningful than shorter time frames in terms of likely real-world impact.
The trial also had a number of weaknesses. We intended to use a hierarchy of medication adherence measures to assess the primary outcome, with MEMS as a ‘medium’, and pill count and self-report as ‘low’, confidence measures of adherence. In implementation, we found that it was not possible to obtain reliable MEMS measures for the majority of participants. This was likely due to a number of factors, principally the large number of pills that needed to be taken each day, making the process unwieldy for participants, resulting in poor compliance with the recommended process – for example, we became aware that some participants were taking a day’s or a week’s supply from the MEMS bottles at a time rather than twice or three times per day, or stopped using MEMS altogether, meaning that the MEMS data most likely underestimated the true level of adherence.
Similarly, we had problems in implementing the pill count measure because of the large number of retail pharmacies involved in dispensing the medication across multiple sites, and consequent difficulties in ensuring pharmacist adherence to the protocol in relation to pill count. We also had difficulties with participants failing to bring used medication packs to collect new prescriptions, or receiving medications by mail rather than in-person.
Therefore, both MEMS and pill count were not considered a reliable source of data on adherence, and we relied on the self-report measure as our primary outcome measure as being the most reliable and consistent measure available across participants. However, it is worth noting that although self-report is a ‘low’ confidence measure in terms of medication adherence, it is the preferred method of measuring the other main primary outcome in most alcohol clinical trials, namely self-reported alcohol consumption, where it has been shown to have a high level of reliability compared to other outcome measures. 161 Also, there is evidence that self-report is a valid and reliable method for estimating adherence, and that self-report estimates of shorter periods are reliable proxies for adherence over longer periods for people with chronic conditions. 127,128
Another limitation was the follow-up of a small proportion (6.1%) of participants beyond the 60-day follow-up time frame at 6 and 12 months. This had the potential to introduce bias into the analysis of outcome, in that participants followed up later than planned may have differed from those followed up within the time window. However, a sensitivity analysis showed that this had no impact on the outcome result, and so all participants were included in the primary analysis. We also had a lower than planned follow-up rate at the 12-month outcome end point, which may have reduced the statistical power to detect differences in the secondary analyses, while having no impact on the primary outcome findings.
Patient and public involvement
Please see Chapter 2 for a description of the involvement of patients and the public in the development of the trial interventions. There has not been service user involvement in the interpretation of the main trial results. We worked with a service user representative to write the plain language summary of this report.
Conclusions
Generalisability
The study had minimal exclusion criteria compared to some other trials of relapse prevention medications and a large proportion of those approached to participate were both eligible and gave consent to take part. Given the large and diverse range of community addiction services in four regions across England from which participants were recruited, including both NHS and third sector treatment providers in urban, metropolitan and suburban/semi-rural areas, we are confident that our participants were representative of patients attending typical services in England at the time of the study. Participants had to be recently detoxified from alcohol in order to be eligible, so the sample will not be representative of all patients attending addiction services. However, they will be generally representative of people for whom initiation of acamprosate prescribing is recommended by NICE.
Implications for health care
Importance of adherence
Alcohol relapse prevention medications such as acamprosate have a strong evidence base and are recommended for routine use in clinical practice in the UK and many other countries. 21,162–165 However, it is also known that adherence to these medications is suboptimal, which limits their potential to reduce relapse and improve treatment outcomes. Yet, until now, little attention has been paid to finding effective and cost-effective methods to enhance mediation adherence in alcohol dependence. We and others have shown that better outcomes are achieved with greater medication adherence. We have also shown a relationship between adherence to MM and medication adherence, and MM is more effective when it is reinforced by CM. Both MM and MM combined with CM are cost-effective compared to standard care within NICE parameters at 12-month follow-up and when modelled across a 20-year time horizon. Our findings suggest that addiction services should incorporate CM and MM methods to enhance medication adherence into routine clinical practice to improve patient outcomes.
Partnerships with pharmacy
There has been an increasing focus on the extension of the role of pharmacists beyond medication supply to improve public health, and this has been endorsed in the UK by several Government reports and initiatives, including the HLP and Public Health England. We managed to develop an effective working partnership with Lloyds Pharmacy, capitalising on existing expertise and infrastructure to implement the pharmacist-delivered MM intervention. That we were able to do so efficiently through an existing pharmacy call centre team across a wide geographical area, cost-effectively, points to the potential to expand this provision beyond this multicentre trial. This will require the development of greater partnerships between pharmacy, NHS and third sector addiction treatment providers. Advances have already been made in integrating pharmacy-delivered interventions for substance use disorder and smoking cessation, although these have recently been inhibited by changes in commissioning arrangements and cuts to the national public health budget. Nevertheless, this trial should encourage greater partnership working, training and new initiatives to extend the role of pharmacists in addiction treatment.
Partnerships with primary care
Although relapse prevention medications, including acamprosate, have been recommended by NICE for over a decade, uptake and coverage have been slow, such that only a small proportion of patients who could benefit from pharmacotherapy actually receive these effective medications. Part of this may be a lack of awareness among clinicians in both primary and secondary care of the benefits to patients of acamprosate and naltrexone, at relatively low cost. We also became aware during this trial that some general practitioners are unwilling to prescribe acamprosate even if it has been initiated and recommended by a specialist addiction service, and in some areas, acamprosate was not approved by local NHS commissioners even although it is cost-effective and approved by NICE. For some participants, the specialist addiction service had to continue prescribing medication beyond the usual treatment period due to the reluctance of general practitioners to take on prescribing. This may have limited the length of prescribing for some participants, and at a minimum, a lack of endorsement by patients’ GPs may have affected patient attitudes to the value of continuing for the duration recommended by NICE. We feel there is a need for greater education and awareness of the benefits of relapse prevention medications, such as acamprosate, and it should be routinely available and funded by the NHS and local authority commissioning across the UK.
Implications for research
The importance and feasibility of alcohol clinical trials
Relatively few trials of clinical interventions in alcohol dependence have been funded or conducted in the UK, with the consequence that many of the current treatment guidelines rely on evidence from other countries, mostly the USA, where the health systems may differ in important ways. This may be partly because alcohol use disorders are not seen as a sufficiently important public health area to research. However, there is ample evidence that while morbidity and mortality from most non-communicable diseases are decreasing in the UK, alcohol-attributable diseases are increasing, as are alcohol-related hospital admission rates. 166 One-fifth of all hospital admissions in England are alcohol related. 167 Therefore, greater research, and in particular clinical trials, are needed to find effective and cost-effective ways of reducing this increasing burden on society.
Another reason for the lack of alcohol clinical trials in the UK may be due to a perception that it is not possible to conduct clinical trials in alcohol-dependent populations. It is certainly true that we encountered many challenges in conducting this trial. However, these were mostly due to structural problems in the commissioning of clinical services, with frequent tendering of services and changes of provider through the course of the trial. This had an impact on our ability to recruit to the trial. There were also challenges in accessing relevant NHS research funding streams, such as research support costs to conduct research in third sector organisations. Even in relation to NHS providers, we were unable to obtain any excess treatment costs for this trial, in spite of repeated efforts, which delayed recruitment and provided a disincentive for providers to participate. Given the changing landscape of health-care provision in England including greater private and third sector provision, not only in the addiction field, but consideration should also be given to facilitating clinical research across the health system beyond simply the NHS.
Nevertheless, we have been able to demonstrate that it is possible to deliver a large multicentre, multisector clinical trial in alcohol dependence with adequate statistical power, and of relevance to clinical practice. There is a need to commission more clinical trials in alcohol dependence to develop the evidence base necessary to guide clinical and policy decision-making in the UK health system.
Potential of CM in alcohol dependence
Since we started developing this trial, the evidence base on CM in addictions has continued to grow both in the UK and internationally. 148,164 However, there remain relatively few trials of CM in alcohol dependence compared with substance use disorder, which is surprising given the large and growing findings in relation to its effectiveness in substance use disorder. We were also able to implement CM relatively easily in routine clinical practice. It does not require a high level of skill to deliver and it can be done cost-effectively and at relatively low cost compared to most of the recommended psychosocial interventions for alcohol dependence. This current trial should prompt greater investigation of the potential of CM in alcohol dependence, including its use in reinforcing both adherence to psychosocial interventions and in promoting abstinence or reduced alcohol consumption. There is evidence for its effectiveness for both applications in the substance use disorder field. Given our findings in relation to CM enhancing MM adherence, future trials should be developed to explore its effectiveness with other alcohol interventions where there is evidence of poor adherence.
Acknowledgements
Merck Serono Ltd supplied the acamprosate for all participants in this trial. The views and opinions expressed are those of the authors and do not necessarily reflect those of Merck Serono Ltd.
The MM intervention was delivered by pharmacists employed by Lloyds Pharmacies at a central Clinical Contact Centre, and pharmacists were trained in intervention delivery by researchers at King’s College London. We would like to thank the Clinical Contact Centre pharmacists for their work delivering the MM intervention. We would also like to thank all the pharmacists employed by Lloyds Pharmacies who undertook the dispensing of acamprosate to participants in bottles with MEMS caps.
We would like to thank all of the specialist addiction services across our five sites in London (South and Central and North West), West Midlands, Wessex and Yorkshire and Humber who participated in the trial, assisting with identification and recruitment of participants. We would also like to thank all of the participants who took part in the research.
We would like to thank our Trial Steering Committee and Data Monitoring Committee for their expert advice and oversight during the course of the trial. Trial Steering Committee: Professor Elizabeth Murray (Chair), Professor Alan Brennan, Dr Lauren Bell, Dr Sarah Welch. Data Monitoring Committee: Professor Eilish Gilvarry (Chair), Professor Lee Shepstone, Dr Chris Daly.
Contributions of authors
Kim Donoghue (https://orcid.org/0000-0002-7316-1716) (Senior Research Fellow, Mental Health) involved in the conception and design of the trial, trial manager and led the writing of the final report.
Sadie Boniface (https://orcid.org/0000-0003-3492-6402) (Head of Research and Visiting Researcher) was interim trial manager. Reviewed and commented on the final report.
Eileen Brobbin (https://orcid.org/0000-0001-8464-4847) (Research Assistant and PhD Student) responsible for recruitment and data collection for the London site. Involved in the writing and review of the final report.
Sarah Byford (https://orcid.org/0000-0001-7084-1495) (Professor, Health Economics) was a co-applicant involved in the conception and design of the trial, was responsible for the health economic component of the trial and line management of the health economist, contributed to the drafting of the economic sections of the final report and commented on the full report.
Rachel Coleman (https://orcid.org/0000-0003-3404-0839) (PhD Student) involved in Yorkshire and Humber site set up, participant recruitment and data collection. Reviewed and commented on the final report.
Simon Coulton (https://orcid.org/0000-0002-7704-3274) (Professor, Health Research) was a PI and took responsibility for the methodological aspects of the trial, including the design, conduct, analysis and interpretation.
Edward Day (https://orcid.org/0000-0002-7106-7013) (Clinical Reader, Addiction Psychiatry) was the Principal Investigator for the West Midlands site involved in the conception and design of the trial.
Ranjita Dhital (https://orcid.org/0000-0002-4504-7318) (Lecturer, Interdisciplinary Health Studies and registered pharmacist) led the focus group study conducted with service users and pharmacists; design and piloting of the MM and CM interventions; and the design and delivery of trial intervention pharmacists’ and community pharmacists training and support.
Anum Farid (https://orcid.org/0000-0002-7658-1804) (Programme Manager) responsible for recruitment and data collection for the London site.
Laura Hermann (https://orcid.org/0000-0002-4688-6615) (NIHR Pre-doctoral Fellow) responsible for recruitment and data collection for the Yorkshire and Humber site. Involved in the writing and review of the final report.
Amy Jordan (https://orcid.org/0000-0002-9403-7706) (Clinical Psychologist) responsible for recruitment and data collection for the West Midlands site.
Andreas Kimergård (https://orcid.org/0000-0002-2080-9865) (Senior Research Fellow) involved as trial manager (maternity cover).
Maria-Leoni Koutsou (https://orcid.org/0000-0002-4599-9853) (Project Manager) responsible for recruitment, data collection at baseline and follow-up time points.
Anne Lingford-Hughes (https://orcid.org/0000-0003-4512-3453) (Professor, Addiction Biology) involved in the conception and design of the trial, Principal Investigator for the Central and North West London site and reviewed of the final report.
John Marsden (https://orcid.org/0000-0002-1307-2498) (Professor, Addiction Psychology) involved in the conception and design of the trial (linked to development and delivery of Contingency Management) and the review of the final report.
Joanne Neale (https://orcid.org/0000-0003-1502-5983) (Professor, Addictions Qualitative Research) advised on the design and conduct of the focus groups (but did not participate in their conduct, coding or analyses).
Aimee O’Neill (https://orcid.org/0000-0002-5358-6944) (PhD Student) responsible for the Wessex site recruitment and data collection.
Thomas Phillips (https://orcid.org/0000-0001-8020-4510) (Professor, Nursing (Addictions)) involved in the conception and design of the trial, Principal Investigator for the Yorkshire and Humber site and reviewed the final report.
James Shearer (https://orcid.org/0000-0002-1658-9767) (Lecturer, Health Economics) was a co-applicant involved in the conception and design of the trial, completed the economic analysis and model development, drafted the economic sections of the final report and commented on the full report.
Julia Sinclair (https://orcid.org/0000-0002-1905-2025) (Professor, Addiction Psychiatry) was the Principal Investigator for the Wessex site involved in the conception and design of the trial.
Joanna Smith (https://orcid.org/0000-0001-9306-9397) (Research assistant) responsible for the recruitment and data collection at the Wessex site.
John Strang (https://orcid.org/0000-0002-5413-2725) (Professor, Addictions Medicine) involved in the conception and design of the trial (Contingency Management).
John Weinman (https://orcid.org/0000-0002-6786-0166) (Professor, Psychology as applied to Medicines) – primarily involved in design, choice and interpretation of the adherence-related measures.
Cate Whittlesea (https://orcid.org/0000-0002-4951-2272) (Professor, Pharmacy Practice) involved in the conception and design of the trial (linked to development and delivery of the MM intervention and acamprosate supply by community pharmacists) and involved in the writing and review of the final report.
Kideshini Widyaratna (https://orcid.org/0000-0001-6783-776X) (Trainee Clinical Psychologist) involved in the design of the trial, London site set up, recruitment and data collection as a research assistant.
Colin Drummond (https://orcid.org/0000-0001-9379-5452) (Professor, Addiction Psychiatry) Chief Investigator, led the conception and design of the trial, chair of the Trial Management Group, involved in the writing and review of the final report.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
Ethical approval has been granted by the East of England – Cambridge South Research Ethics Committee (Ref: 15/EE/0308). All participants will give written informed consent to take part in the research.
Permissions
The MMAS-8 Scale, content, name and trademarks are protected by US copyright and trademark laws. Permission for use of the scale and its coding is required. A license agreement is available from: MMAR, LLC. Donald E. Morisky, ScD, ScM, MSPH, 294 Lindura Ct., Las Vegas, NV USA; dmorisky@gmail.com.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Drummond C. Protocol (Version 7.0): NIHR-HTA Funded Study: 13 86 03. Alcohol Dependence and Adherence to Medication (ADAM) 2020. www.fundingawards.nihr.ac.uk/award/13/86/03 (accessed 9 June 2022).
- National Health System (NHS) . Statistics on Alcohol, England 2020. 2020.
- Government’s Alcohol Strategy . Government’s Alcohol Strategy: Third Report of Session 2012–13 2012. https://publications.parliament.uk/pa/cm201213/cmselect/cmhealth/132/132.pdf (accessed 21 June 2022).
- WHO . Global Status Report on Alcohol and Health 2018 2018. www.who.int/publications/i/item/9789241565639 (accessed 21 June 2022).
- Shield KD, Parry C, Rehm J. Chronic diseases and conditions related to alcohol use. Alcohol Res 2014;35:155-71.
- Degenhardt L, Charlson F, Ferrari A, Santomauro D, Erskine H, Mantilla-Herrara A, et al. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 2018;5:987-1012. https://doi.org/10.1016/S2215-0366(18)30337-7.
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997;349:1436-42. https://doi.org/10.1136/bmjno-2019-000014.
- Drummond C. The relationship between alcohol dependence and alcohol-related problems in a clinical population. Br J Addict 1990;85:357-66. https://doi.org/10.1111/j.1360-0443.1990.tb00652.x.
- Henkel D. Unemployment and substance use: a review of the Literature (1990–2010). Curr Drug Abuse Rev 2011;4:4-27. https://doi.org/10.2174/1874473711104010004.
- Leonard K. Domestic violence and alcohol: what is known and what do we need to know to encourage environmental interventions?. J Subst Use 2001;6:235-47.
- Foster JH, Powell JE, Marshall EJ, Peters TJ. Quality of life in alcohol-dependent subjects – a review. Qual Life Res 1999;8:255-61. https://doi.org/10.1023/A:1008802711478.
- Home Office . A Minimum Unit Price for Alcohol Impact Statement 2012. www.gov.uk/government/uploads/system/uploads/attachment_data/file/157763/ia-minimum-unit-pricing.pdf (accessed 21 June 2022).
- National Health System (NHS) . Statistics on Alcohol, England 2020. 2020.
- Elwell-Sutton T, Tinson A, Greszczuc C, Finch D, Holt-White E, Everest G, et al. Creating Healthy Lives: A Whole-Government Approach to Long-Term Investment in the Nation’s Health 2019.
- Drummond C. Cuts to addiction services are a false economy. BMJ 2017;357:2-3. https://doi.org/10.1136/bmj.j2704.
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol 1971;27:455-6.
- Brandon TH, Vidrine JI, Litvin EB. Relapse and relapse prevention. Annu Rev Clin Psychol 2007;3:257-84. https://doi.org/10.1146/annurev.clinpsy.3.022806.091455.
- Public Health England . Alcohol and drugs prevention, treatment and recovery: why invest? n.d.
- Masters R, Anwar E, Collins B, Cookson R, Capewell S. Return on investment of public health interventions: a systematic review. J Epidemiol Community Health 2017;71:827-34. https://doi.org/10.1136/jech-2016-208141.
- Public Health England . 2016–17 Alcohol and Drugs Treatment Commissioning Tool: Guidance Document 2017. https://www.gov.uk/government/publications/alcohol-and-drug-prevention-treatment-and-recovery-why-invest/alcohol-and-drug-prevention-treatment-and-recovery-why-invest (accessed 13 July 2023).
- National Institute for Health and Care Excellence (NICE) . Diagnoses Assessment and Management of Harmful Drinking and Alcohol Dependence. National Clinical Practice Guideline 115 2011. www.nice.org.uk/guidance/cg115/evidence/full-guideline-136423405 (accessed 21 June 2022).
- NHS Business Services Authority . Annual Report and Accounts. Post Medieval Archaeology 2011:466-70. https://doi.org/10.1179/pma.2011.45.2.466.
- NHS Business Services Authority . Prescription Cost Analysis (PCA) data n.d. https://www.nhsbsa.nhs.uk/statistical-collections/prescription-cost-analysis-england/prescription-cost-analysis-england-2019 (accessed 20 July 2023).
- Cole JC, Littleton JM, Little HJ. Acamprosate, but not naltrexone, inhibits conditioned abstinence behaviour associated with repeated ethanol administration and exposure to a plus-maze. Psychopharmacology (Berl) 2000;147:403-11. https://doi.org/10.1007/s002130050009.
- Littleton JM. Acamprosate in alcohol dependence: how does it work?. Addiction 1995;90:1179-88. https://doi.org/10.1046/j.1360-0443.1995.90911793.x.
- Rösner S, Hackl-Herrwerth A, Leucht S, Lehert P, Vecchi S, Soyka M. Acamprosate for alcohol dependence. Cochrane Database Syst Rev 2010;8. https://doi.org/10.1590/S1516-31802010000600014.
- Guglielmo R, Kobylinska L, de Filippis R. NeuroPsychopharmacotherapy. Cham: Springer; n.d.
- Cheng HY, McGuinness LA, Elbers RG, MacArthur GJ, Taylor A, McAleenan A, et al. Treatment interventions to maintain abstinence from alcohol in primary care: systematic review and network meta-analysis. BMJ 2020;371. https://doi.org/10.1136/bmj.m3934.
- Weiss RD. Adherence to pharmacotherapy in patients with alcohol and opioid dependence. Addiction 2004;99:1382-92. https://doi.org/10.1111/j.1360-0443.2004.00884.x.
- Zweben A, Pettinati HM, Weiss RD, Youngblood M, Cox CE, Mattson ME, et al. Relationship between medication adherence and treatment outcomes: The COMBINE study. Alcohol Clinical Exp Res 2008;32:1661-9. https://doi.org/10.1111/j.1530-0277.2008.00743.x.
- Paille F, Guelfi J, Perkins A, Royer R, Steru L, Parot P. Double-blind randomized multicentre trial of acamprosate in maintaining abstinence from alcohol. Alcohol 1995;30:239-47. https://doi.org/10.1016/S0140-6736(96)91682–7.
- Pelc I, Verbanck P, le Bon O, Gavrilovic M, Lion K, Lehert P. Efficacy and safety of acamprosate in the treatment of detoxified alcohol-dependent patients. Br J Psychiatry 1997;171:73-7.
- Swift R, Oslin D, Alexander M, Forman R. Adherence monitoring in naltrexone pharmacotherapy trials: a systematic review. J Stud Alcohol Drugs 2011;72:1012-8. https://doi.org/10.1111/adb.12012.
- Thompson A, Ashcroft DM, Owens L, van Staa TP, Pirmohamed M. Drug therapy for alcohol dependence in primary care in the UK: a Clinical Practice Research Datalink study. PLOS ONE 2017;12. https://doi.org/10.1371/journal.pone.0173272.
- National Institute for Health and Care Excellence (NICE) . Alcohol use disorders: diagnosis and clinical management of alcohol-related physical complications. NICE Guidelines 2009. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0047849/ (accessed 21 June 2022).
- Gueorguieva R, Wu R, Krystal JH, Donovan D, O’Malley SS. Temporal patterns of adherence to medications and behavioral treatment and their relationship to patient characteristics and treatment response. Addict Behav 2013;38:2119-27. https://doi.org/10.1016/j.addbeh.2013.01.024.
- Gueorguieva R, Wu R, Fucito LM, O’Malley SS. Predictors of abstinence from heavy drinking during follow-up in COMBINE. J Stud Alcohol Drugs 2015;76:935-41. https://doi.org/10.15288/jsad.2015.76.935.
- Haynes RB, Devereaux PJ, Guyatt GH. Clinical expertise in the era of evidence-based medicine and patient choice. BMJ Evid-Based Med 2002;7:36-8. https://doi.org/10.1111/j.1423-0410.2002.tb05339.x.
- Ammassari A, Trotta MP, Murri R, Castelli F, Narciso P, Noto P, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr 2002;31:123-7. https://doi.org/10.1097/00126334-200212153-00007.
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001;23:1296-310. https://doi.org/10.1016/S0149-2918(01)80109-0.
- Horne R. Perceptions of Health and Illness: Current Research and Applications. Harwood Academic Publishers Reading; 1997.
- Horne R, Weinman J. Adherence to Treatment in Medical Conditions. Harwood Academic Publishers Reading; 1998.
- Horne R, Chapman SCE, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLOS ONE 2013;8. https://doi.org/10.1371/journal.pone.0080633.
- Volpicelli J, Pettinati H, McLellan A, O’Brien C. Combining Medication and Psychosocial Treatments for Addictions: The BRENDA Approach. New York: Guildford Press; 2001.
- Pettinati HM, Volpicelli JR, Pierce JD, O’Brien CP. Improving naltrexone response. J Addict Dis 2000;19:71-83.
- Aron S, Leeman RF, Volpicelli JR. The BRENDA model: integrating psychosocial treatment and pharmacotherapy for the treatment of alcohol use disorders. J Psychiatry Pract 2006;12:80-9.
- Pettinati HM, Weiss RD, William W, Miller WR, Donovan D, Ernst DB, et al. A structured approach to medical management: a psychosocial intervention to support pharmacotherapy in the treatment of alcohol dependence. J Stud Alcohol 2005;15:170-8.
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. J Am Med Assoc 2006;295:2003-17. https://doi.org/10.1001/jama.295.17.2003.
- O’Malley SS, Robin RW, Levenson AL, GreyWolf I, Chance LE, Hodgkinson CA, et al. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska natives and non-natives residing in rural settings: a randomized controlled trial. Alcohol Clin Exp Res 2008;32:1271-83. https://doi.org/10.1111/j.1530-0277.2008.00682.x.
- O’Malley SS, Zweben A, Fucito LM, Wu R, Piepmeier ME, Ockert DM, et al. Effect of varenicline combined with medical management on alcohol use disorder with comorbid cigarette smoking: a randomized clinical trial. JAMA Psychiatry 2018;75:129-38. https://doi.org/10.1001/jamapsychiatry.2017.3544.
- van Eikenhorst L, Salema NE, Anderson C. A systematic review in select countries of the role of the pharmacist in consultations and sales of non-prescription medicines in community pharmacy. Res Social Adm Pharm 2017;13:17-38. https://doi.org/10.1016/j.sapharm.2016.02.010.
- Anderson AC, Brodin H, Nilsson JLG. Pharmacist interventions in relation to patient drug-related problems. J Soc Admin Pharm 2003;20:82-91.
- Cooper RJ, Anderson C, Avery T, Bissell P, Guillaume L, Hutchinson A, et al. Nurse and pharmacist supplementary prescribing in the UK – A thematic review of the literature. Health Policy 2008;85:277-92. https://doi.org/10.1016/j.healthpol.2007.07.016.
- Eades CE, Ferguson JS, O’Carroll RE. Public health in community pharmacy: a systematic review of pharmacist and consumer views. BMC Public Health 2011;11. https://doi.org/10.1186/1471-2458-11-582.
- Donovan GR, Paudyal V. England’s Healthy Living Pharmacy (HLP) initiative: facilitating the engagement of pharmacy support staff in public health. Res Social Adm Pharm 2016;12:281-92. https://doi.org/10.1016/j.sapharm.2015.05.010.
- Sheridan J, Strang J, Barber N, Glanz A. Role of community pharmacies in relation to HIV prevention and drug misuse: findings from the 1995 national survey in England and Wales. BMJ 1996;313.
- Sheridan J, Manning V, Ridge G, Mayet S, Strang J. Community pharmacies and the provision of opioid substitution services for drug misusers: changes in activity and attitudes of community pharmacists across England 1995–2005. Addiction 2007;102:1824-30. https://doi.org/10.1111/j.1360-0443.2007.02016.x.
- Strand MA, Eukel H, Burck S. Moving opioid misuse prevention upstream: a pilot study of community pharmacists screening for opioid misuse risk. Res Social Adm Pharm 2019;15:1032-6. https://doi.org/10.1016/j.sapharm.2018.07.011.
- Vermeire E, Wens J, van Royen P, Biot Y, Hearnshaw H, Lindenmeyer A. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;2.
- Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Self-monitoring and other non-pharmacological interventions to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract 2010;60:e476-88. https://doi.org/10.3399/bjgp10X544113.
- Lowrie R, Johansson L, Forsyth P, Bryce SL, McKellar S, Fitzgerald N. Experiences of a community pharmacy service to support adherence and self-management in chronic heart failure. Int J Clin Pharm 2014;36:154-62. https://doi.org/10.1007/s11096-013-9889-2.
- Smith J, Picton C, Dayan M. Now or Never: Shaping Pharmacy for the Future. The Report of the Commission on Future Models of Care Delivered through the Pharmacy 2013. www.rpharms.com/resources/reports/now-or-never-shaping-pharmacy-for-the-future (accessed 21 June 2022).
- Pharmaceutical Services Negotiating Committee (PSNC) . NHS Community Pharmacy Services – a Summary 2013. http://psnc.org.uk/wp-content/uploads/2013/08/CPCF-summary-July-2013.pdf (accessed 21 June 2022).
- Brown D, Portlock J, Rutter P, Nazar Z. From community pharmacy to healthy living pharmacy: positive early experiences from Portsmouth, England. Res Social Adm Pharm 2014;10:72-87. https://doi.org/10.1016/j.sapharm.2013.04.014.
- MacKridge AJ, Scott J. Experiences, attitudes and training needs of pharmacy support staff providing services to drug users in Great Britain: a qualitative study experiences and training needs of UK pharmacy support staff. J Subst Use 2009;14:375-84. https://doi.org/10.3109/14659890802695840.
- Dhital R, Norman I, Whittlesea C, McCambridge J. Effectiveness of alcohol brief intervention delivered by community pharmacists: study protocol of a two-arm randomised controlled trial. BMC Public Health 2013;13. https://doi.org/10.1186/1471-2458-13-152.
- Matheson C, Thiruvothiyur M, Robertson H, Bond C. Community pharmacy services for people with drug problems over two decades in Scotland: implications for future development. Int J Drug Policy 2016;27:105-12. https://doi.org/10.1016/j.drugpo.2015.11.006.
- Graff FS, Morgan TJ, Epstein EE, McCrady BS, Cook SM, Jensen NK, et al. Engagement and retention in outpatient alcoholism treatment for women. Am J Addict 2009;18:277-88. https://doi.org/10.1080/10550490902925540.
- Simpson DD, Joe GW, Rowan-Szal GA. Drug abuse treatment retention and process effects on follow-up outcomes. Drug Alcohol Depend 1997;47:227-35. https://doi.org/10.1016/S0376-8716(97)00099-9.
- Fitzsimons H, Tuten M, Borsuk C, Lookatch S, Hanks L. Clinician-delivered contingency management increases engagement and attendance in drug and alcohol treatment. Drug Alcohol Depend 2015;152:62-7. https://doi.org/10.1016/j.drugalcdep.2015.04.021.
- Pedersen MU, Hesse M, Thylstrup B, Jones S, Pedersen MM, Frederiksen KS. Vouchers versus reminders to prevent dropout: findings from the randomized youth drug abuse treatment project (youthDAT project) (E-Pub). Drug Alcohol Depend 2021;218. https://doi.org/10.1016/j.drugalcdep.2020.108363.
- Rash CJ, Maxine S, Jeremiah W. Contingency management: new directions and remaining challenges for an evidence-based intervention. J Subst Abuse Treat 2017;72:10-8. https://doi.org/10.1016/j.jsat.2016.09.008.
- Rash CJ, Alessi SM, Petry NM. Substance abuse treatment patients in housing programs respond to contingency management interventions. J Subst Abuse Treat 2017;72:97-102. https://doi.org/10.1016/j.jsat.2016.07.001.
- Stitzer ML, Natalie G, Matheson T, Sorensen JL, Feaster DJ, Duan R, et al. Enhancing patient navigation with contingent financial incentives for substance use abatement in persons with HIV and substance use. Psychol Addict Behav 2020;34:23-30. https://doi.org/10.4324/9780429465666-17.
- Jarvis BP, Holtyn AF, DeFulio A, Dunn KE, Everly JJ, Leoutsakos JS, et al. Effects of incentives for naltrexone adherence on opiate abstinence in heroin-dependent adults. Addiction 2017;112:830-7. https://doi.org/10.1111/add.13724.
- Herrmann E, Matusiewicz A, Stitzer M, Higgins S, Sigmon S, Heil S. Contingency management interventions for HIV, tuberculosis, and hepatitis control among individuals with substance use disorders: a systematized review. J Subst Abuse Treat 2017;72:117-25. https://doi.org/10.1016/j.jsat.2016.06.009.
- Catherine S, Stacy R, Emily S, Gray N, Alan B. Clinic- and home-based contingency management plus parent training for adolescent cannabis use disorders. J Am Acad Child Adolesc Psychiatry 2015;54:445-53. https://doi.org/10.1016/j.jaac.2015.02.009.
- Getty CA, Morande A, Lynskey M, Weaver T, Metrebian N. Mobile telephone-delivered contingency management interventions promoting behaviour change in individuals with substance use disorders: a meta-analysis. Addiction 2019;114:1915-25. https://doi.org/10.1111/add.14725.
- Ainscough TS, McNeill A, Strang J, Calder R, Brose LS. Contingency management interventions for non-prescribed drug use during treatment for opiate addiction: a systematic review and meta-analysis. Drug Alcohol Depend 2017;178:318-39. https://doi.org/10.1016/j.drugalcdep.2017.05.028.
- National Institute for Health and Care Excellence (NICE) . Drug Misuse in over 16s: Psychosocial Interventions Clinical Guideline 2007. www.nice.org.uk/guidance/cg51/chapter/1-Guidance#formal-psychosocial-interventions%0Ahttps://www.nice.org.uk/guidance/cg51/resources/drug-misuse-in-over-16s-psychosocial-interventions-pdf-975502451653 (accessed 21 June 2022).
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Prevent Med 2016;92:36-4. https://doi.org/10.1016/j.ypmed.2016.08.008.
- Petry NM, Alessi SM, Olmstead TA, Rash CJ, Kristyn Z. Contingency management treatment for substance use disorders: how far has it come, and where does it need to go?. Psychol Addict Behav 2017;31:897-906. https://doi.org/10.1037/adb0000287.
- Oluwoye O, Kriegel L, Alcover K, McPherson S, McDonell M, Roll J. The dissemination and implementation of contingency management for substance use disorders: a systematic review. Psychol Addict Behav 2020;34:99-110. https://doi.org/10.1037/adb0000487.
- Murphy SM, McDonell MG, McPherson S, Srebnik D, Angelo F, Roll JM, et al. An economic evaluation of a contingency-management intervention for stimulant use among community mental health patients with serious mental illness. Drug Alcohol Depend 2015;153:293-9. https://doi.org/10.1016/j.drugalcdep.2015.05.004.
- DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of Contingency Management (CM) in the Department of Veterans Affairs: attendance at CM sessions and substance use outcomes. Drug Alcohol Depend 2018;185:367-73. https://doi.org/10.1016/j.drugalcdep.2017.12.020.
- Miguel AQC, Madruga CS, Cogo-Moreira H, Yamauchi R, Simões V, da Silva CJ, et al. Contingency management is effective in promoting abstinence and retention in treatment among crack cocaine users in Brazil: a randomized controlled trial. Psychol Addict Behav 2016;30:536-43. https://doi.org/10.1037/adb0000192.
- Schacht RL, Brooner RK, King VL, Kidorf MS, Peirce JM. Incentivizing attendance to prolonged exposure for PTSD with opioid use disorder patients: a randomized controlled trial. J Consult Clin Psychol 2017;85:689-701. https://doi.org/10.1037/ccp0000208.
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry 2005;62:1148-56. https://doi.org/10.1001/archpsyc.62.10.1148.
- Barnett NP, Celio MA, Tidey JW, Murphy JG, Colby SM, Swift RM. A preliminary randomized controlled trial of contingency management for alcohol use reduction using a transdermal alcohol sensor. Addiction 2017;112:1025-35. https://doi.org/10.1111/add.13767.
- Public Health England . Pharmacy: A Way Forward for Public Health – Opportunities for Action through Pharmacy for Public Health 2017. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/643520/Pharmacy_a_way_forward_for_public_health.pdf (accessed 21 June 2022).
- Dhital R, Whittlesea CM, Norman IJ, Milligan P. Community pharmacy service users’ views and perceptions of alcohol screening and brief intervention. Drug Alcohol Rev 2010;29:596-602. https://doi.org/10.1111/j.1465-3362.2010.00234.x.
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 2000;68:250-7. https://doi.org/10.1037/0022-006X.68.2.250.
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol 2006;26:610-25. https://doi.org/10.1097/01.jcp.0000245566.52401.20.
- Pettinati HM, Mattson ME. Medical Management Treatment Manual: A Clinical Guide for Researchers and Clinicians Providing Pharmacotherapy for Alcohol Dependence (Generic Version; 2010 Edition). Maryland: National Institutes of Health; 2004.
- Dhital R, Coleman R, Day E, Drummond C, Lingford-Hughes A, Marsden J, et al. Service users’ views and experiences of alcohol relapse prevention treatment and adherence: new role for pharmacists?. Alcohol Alcohol 2022;57:602-8. https://doi.org/10.1093/alcalc/agac011.
- Finch H, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: SAGE; 2009.
- Krueger RA, Casey MA. Focus Groups: A Practical Guide for Applied Research. London: SAGE; 2000.
- Rabiee F. Focus-group interview and data analysis. Proc Nutr Soc 2004;63:655-60. https://doi.org/10.1079/pns2004399.
- Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Admin Policy Mental Health Mental Health Services Res 2015;42:533-44. https://doi.org/10.1007/s10488-013-0528-y.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Gale NK, Heath G, Cameron E, Rashid S, Redwood S. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol 2013;13. https://doi.org/10.1186/1471-2288-13-117.
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction 2006;101:1546-60. https://doi.org/10.1111/j.1360-0443.2006.01581.x.
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192-203. https://doi.org/10.1111/j.1360-0443.2006.01311.x.
- Ali R, Meena S, Eastwood B, Richards I, Marsden J. Ultra-rapid screening for substance-use disorders: the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST-Lite). Drug Alcohol Depend 2013;132:352-61. https://doi.org/10.1016/j.drugalcdep.2013.03.001.
- Alcohol-Use Disorders: Diagnosis, Assessment and Management of Harmful Drinking and Alcohol Dependence. The British Psychological Society and The Royal College of Psychiatrists; 2011.
- Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence. NIAAA COMBINE Monograph Series, Vol. 2, DHHS Publication No. (NIH) 04-5289. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004.
- Reid SC, Teesson M, Sannibale C, Matsuda M, Haber PS. The efficacy of compliance therapy in pharmacotherapy for alcohol dependence: a randomized controlled trial. J Stud Alcohol 2005;66:833-41. https://doi.org/10.15288/jsa.2005.66.833.
- Stockwell T, Sitharthan T, McGrath D, Lang E. The measurement of alcohol dependence and impaired control in community samples. Addiction 1994;89:167-84. https://doi.org/10.1111/j.1360-0443.1994.tb00875.x.
- Sobell L, Sobell M. Measuring Alcohol Consumption. Totowa, NJ: Humana Press; 1992.
- Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999;14:1-24. https://doi.org/10.1080/08870449908407311.
- Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens 2008;10:348-54.
- Berlowitz DR, Foy CG, Kazis LE, Bolin LP, Conroy MB, Fitzpatrick P, et al. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med 2017;377:733-44. https://doi.org/10.1056/nejmoa1611179.
- Bress AP, Bellows BK, King JB, Hess R, Beddhu S, Zhang Z, et al. Cost-effectiveness of intensive versus standard blood-pressure control. N Engl J Med 2017;377:745-55. https://doi.org/10.1056/nejmsa1616035.
- McGuire-Snieckus R, McCabe R, Catty J, Hansson L, Priebe S. A new scale to assess the therapeutic relationship in community mental health care: STAR. Psychol Med 2007;37:85-9. https://doi.org/10.1017/S0033291706009299.
- Flückiger C, del Re AC, Wampold BE, Symonds D, Horvath AO. How central is the alliance in psychotherapy? A multilevel longitudinal meta-analysis. J Couns Psychol 2012;59:10-7. https://doi.org/10.1037/a0025749.
- Ernst DB, Pettinati HM, Weiss RD, Donovan DM, Longabaugh R. An intervention for treating alcohol dependence: relating elements of medical management to patient outcomes with implications for primary care. Ann Fam Med 2008;6:435-40. https://doi.org/10.1370/afm.884.
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res 1995;19:600-6. https://doi.org/10.1111/j.1530-0277.1995.tb01554.x.
- Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. https://doi.org/10.1007/s11136-011-9903-x.
- Byford S, Barrett B, Metrebian N, Groshkova T, Cary M, Charles V, et al. Cost-effectiveness of injectable opioid treatment v. Oral methadone for chronic heroin addiction. Br J Psychiatry 2013;203:341-9. https://doi.org/10.1192/bjp.bp.112.111583.
- Maisel N, Blodgett J, Wilbourne P, Humphreys K, Finney J. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful?. Addiction 2013;108:275-93. https://doi.org/10.1111/j.1360-0443.2012.04054.x.
- Sussman JB, Hayward RA. Using instrumental variables to adjust for treatment contamination in randomised controlled trials. BMJ (Online) 2010;340:1181-4. https://doi.org/10.1136/bmj.c2073.
- Ye C, Beyene J, Browne G, Thabane L. Estimating treatment effects in randomised controlled trials with non-compliance: a simulation study. BMJ Open 2014;4. https://doi.org/10.1136/bmjopen-2014.
- White IR, Kalaitzaki E, Thompson SG. Allowing for missing outcome data and incomplete uptake of randomised interventions, with application to an Internet-based alcohol trial. Stat Med 2011;30:3192-207. https://doi.org/10.1002/sim.4360.
- White I. RCTMISS: Stata Module to Analyse a Randomised Controlled Trial (RCT) Allowing for Informatively Missing Outcome Data 2018.
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol 2010;63:e1-37. https://doi.org/10.1016/j.jclinepi.2010.03.004.
- UKATT Research Team. Effectiveness of treatment for alcohol problems: findings of the randomised United Kingdom Alcohol Treatment Trial (UKATT) . BMJ 2005;331:541-4.
- Stirratt MJ, Dunbar-Jacob J, Crane HM, Simoni JM, Czajkowski S, Hilliard ME, et al. Self-report measures of medication adherence behavior: recommendations on optimal use. Transl Behav Med 2015;5:470-82. https://doi.org/10.1007/s13142-015-0315-2.
- Jerant A, Dimatteo R, Arnsten J, Moore-Hill M, Franks P. Self-report adherence measures in chronic illness retest reliability and predictive validity. Med Care 2008;46:1134-9.
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 2013. www.nice.org.uk/process/pmg9 (accessed 16 August 2022).
- Angus C, Henney M, Webster L, Gillespie D, Sheffield CA. Alcohol-Attributable Diseases and Dose-Response Curves for the Sheffield Alcohol Policy Model Version 4.0. Sheffield; 2018.
- Barrett B, Byford S, Crawford MJ, Patton R, Drummond C, Henry JA, et al. Cost-effectiveness of screening and referral to an alcohol health worker in alcohol misusing patients attending an accident and emergency department: a decision-making approach. Drug Alcohol Depend 2006;81:47-54.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2009. Personal Social Services Research Unit, University of Kent; 2009.
- Curtis L, Burns A. PSSRU: Unit Costs of Health and Social Care 2019 n.d. https://www.pssru.ac.uk/project-pages/unit-costs/ (accessed 16 August 2022).
- Samaritans . Analysis: Counting the Cost of Reform at Samaritans n.d. www.thirdsector.co.uk/analysis-counting-cost-reform-samaritans/management/article/1175711 (accessed 5 November 2021).
- NHSBSA . Drug Tariff Part VIII n.d. https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff/drug-tariff-part-viii (accessed 20th July 2023).
- Curtis L, Burns A. Unit Costs of Health and Social Care 2007 n.d. https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2007/ (accessed 20 July 2023).
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey 1995.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. https://doi.org/10.1002/sim.4067.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. https://doi.org/10.1002/hec.901.
- Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Barber JA, Thompson SG. Analysis of Cost Data in Randomized Trials: An Application of the Non-Parametric Bootstrap 2000;19.
- National Institute for Health and Care Excellence (NICE) . Alcohol-Use Disorders: Prevention 2010. https://www.nice.org.uk/guidance/ph24 (accessed 16 August 2022).
- Pfund RA, Ginley MK, Rash CJ, Zajac K. Contingency management for treatment attendance: a meta-analysis. J Subst Abuse Treat 2022;133. https://doi.org/10.1016/j.jsat.2021.108556.
- Chick J, Howlett H, Morgan MY, Ritson B. United Kingdom Multicentre Acamprosate Study (UKMAS): a 6-month prospective study of acamprosate versus placebo in preventing relapse after withdrawal from alcohol. Alcohol Alcohol 2000;35:176-87. https://doi.org/10.1093/alcalc/35.2.176.
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18-27. www.consort-statement.org (accessed 16 August 2022).
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O’Brien CP, et al. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict 2001;10:258-68. https://doi.org/10.1080/105504901750532148.
- Oslin DW, Lynch KG, Pettinati HM, Kampman KM, Gariti P, Gelfand L, et al. A placebo-controlled randomized clinical trial of naltrexone in the context of different levels of psychosocial intervention. Alcohol Clin Exp Res 2008;32:1299-308. https://doi.org/10.1111/j.1530-0277.2008.00698.x.
- Pettinati HM, Kampman KM, Lynch KG, Suh JJ, Dackis CA, Oslin DW, et al. Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. J Subst Abuse Treat 2008;34:378-90. https://doi.org/10.1016/j.jsat.2007.05.011.
- van den Brink W, Aubin HJ, Bladström A, Torup L, Gual A, Mann K. Efficacy of as-needed nalmefene in alcohol-dependent patients with at least a high drinking risk level: results from a subgroup analysis of two randomized controlled 6-month studies. Alcohol Alcohol 2013;48:570-8. https://doi.org/10.1093/alcalc/agt061.
- Gual A, He Y, Torup L, van den Brink W, Mann K. A randomised, double-blind, placebo-controlled, efficacy study of nalmefene, as-needed use, in patients with alcohol dependence. Eur Neuropsychopharmacol 2013;23:1432-42. https://doi.org/10.1016/j.euroneuro.2013.02.006.
- van den Brink W, Sørensen P, Torup L, Mann K, Gual A. Long-term efficacy, tolerability and safety of nalmefene as-needed in patients with alcohol dependence: a 1-year, randomised controlled study. J Psychopharmacol 2014;28:733-44. https://doi.org/10.1177/0269881114527362.
- EY . Impacts of Current Funding, Policy and Economic Environment on Independent Pharmacy in England 2020. www.npa.co.uk/wp-content/uploads/2020/09/EY-NPA-Impacts-of-current-funding-policy-and-economic-environment-on-pharmacy-in-England-FINAL-1.pdf (accessed 21 June 2022).
- Weaver T, Metrebian N, Hellier J, Pilling S, Charles V, Little N, et al. Use of contingency management incentives to improve completion of hepatitis B vaccination in people undergoing treatment for heroin dependence: a cluster randomised trial. Lancet 2014;384:153-63. https://doi.org/10.1016/S0140-6736(14)60196-3.
- Bigelow GE, Silverman K. Motivating Behavior Change among Illicit-Drug Abusers: Research on Contingency Management Interventions. Washington: American Psychological Association; 1999.
- European Monitoring Centre for Drugs and Drug Addiction . How can contingency management support treatment for substance use disorders? A systematic review. EMCDDA Papers 2016. www.emcdda.europa.eu/publications/papers/contingency-management-systematic-review_en (accessed 21 June 2022).
- Principles of Drug Addiction Treatment – A Research-Based Guide. Bethesda: National Institutes of Health, US Department of Health and Human Services; 2018.
- Coulton S, Drummond C, James D, Godfrey C, Bland JM, Parrott S, et al. Opportunistic screening for alcohol use disorders in primary care: comparative study. Br Med J 2006;332:511-4. https://doi.org/10.1136/bmj.38743.421574.7C.
- Rains LS, Steare T, Mason O, Johnson S. Improving substance misuse outcomes in contingency management treatment with adjunctive formal psychotherapy: a systematic review and meta-analysis. BMJ Open 2020;10. https://doi.org/10.1136/bmjopen-2019–034735.
- Stitzer M. Contingency management and the addictions. Addiction 2007;101:1536-7.
- Petry NM. Contingency management: what it is and why psychiatrists should want to use it. Psychiatrist 2011;35:161-3. https://doi.org/10.1192/pb.bp.110.031831.
- del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction 2003;98:1-12. https://doi.org/10.1046/j.1359-6357.2003.00586.x.
- Work Group on Substance Use Disorders . Practice Guideline for the Treatment of Patients With Substance Use Disorders 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/substanceuse.pdf (accessed 8 November 2021).
- The Management of Substance Use Disorders Working Group . VA/DoD/Practice/Guideline/for/Management/of/Substance/Use/Disorders/(SUD) 2009. www.healthquality.va.gov/guidelines/MH/sud/sud_full_601f.pdf (accessed 8 November 2021).
- Paul H, Nick L, Elizabeth P, Olga L. Guidelines for the Treatment of Alcohol Problems. Commonwealth of Australia; 2009.
- Mann K, Batra A, Fauth-Bühler M, Hoch E. German guidelines on screening, diagnosis and treatment of alcohol use disorders. Eur Addict Res 2017;23:45-60. https://doi.org/10.1159/000455841.
- Murray CJL, Richards MA, Newton JN, Fenton KA, Anderson HR, Atkinson C, et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet 2013;381:997-1020. https://doi.org/10.1016/S0140-6736(13)60355-4.
- Roberts E, Morse R, Epstein S, Hotopf M, Leon D, Drummond C. The prevalence of wholly attributable alcohol conditions in the United Kingdom hospital system: a systematic review, meta-analysis and meta-regression. Addiction 2019;114:1726-37. https://doi.org/10.1111/add.14642.
Appendix 1 Additional statistical output
| Month 2 | Month 4 | |||||
|---|---|---|---|---|---|---|
| SS + MM + CM (n = 123) |
SS + MM (n = 109) |
SS alone (n = 231) |
SS + MM + CM (n = 118) |
SS + MM (n = 102) |
SS alone (n = 221) |
|
| Prescribed acamprosate, n (%) | 113 (91.8) | 93 (85.3) | 230 (99.6) | 88 (74.6) | 67 (65.7) | 138 (62.4) |
| Mean % adherence (SE) | 84.6 (2.4) | 80.7 (2.9) | 74.6 (2.3) | 82.7 (2.7) | 74.6 (4.1) | 72.7 (2.6) |
| Mean MMAS-8 (SE) | 5.6 (0.14) | 5.3 (0.16) | 5.2 (0.11) | 5.7 (0.15) | 5.3 (0.18) | 5.1 (0.13) |
| Within tolerance n = 695 (93.9%) |
Without tolerance n = 45 (6.1%) |
|
|---|---|---|
| Site, n (%) | ||
| Birmingham | 206 (92.4) | 17 (7.6) |
| Central and North West London | 153 (98.1) | 3 (1.9) |
| South East London | 69 (94.5) | 4 (5.5) |
| Southampton | 88 (93.6) | 6 (6.4) |
| Yorks | 179 (92.3) | 15 (7.7) |
| SADQ, n (%) | ||
| ≤ 30 | 279 (93.9) | 18 (6.1) |
| 30 plus | 416 (93.9) | 27 (6.1) |
| Allocation, n (%) | ||
| SS | 347 (93.3) | 25 (6.7) |
| SS + MM | 173 (95.0) | 9 (5.0) |
| SS + MM + CM | 175 (94.1) | 11 (5.9) |
| Per cent adherent at month 6 | ||
| Mean (SD) | 0.44 (0.45) | 0.21 (0.38) |
| Median (IQR) | 0.25 (0; 0.95) | 0 (0; 0) |
| PDA at month 6 | ||
| Mean (SD) | 0.72 (0.38) | 0.65 (0.40) |
| Median (IQR) | 0.97 (0.43; 1.00) | 0.93 (0.24; 1.00) |
FIGURE 20.
Distribution of primary outcome, per cent adherence to acamprosate at month 6.

FIGURE 21.
SS + MM + CM vs. SS comparison of observed and imputed odds ratio and 95% CI for per cent days adherence at month 6.

FIGURE 22.
SS + MM vs. SS comparison of observed and imputed odds ratio and 95% CI for per cent days adherence at month 6.
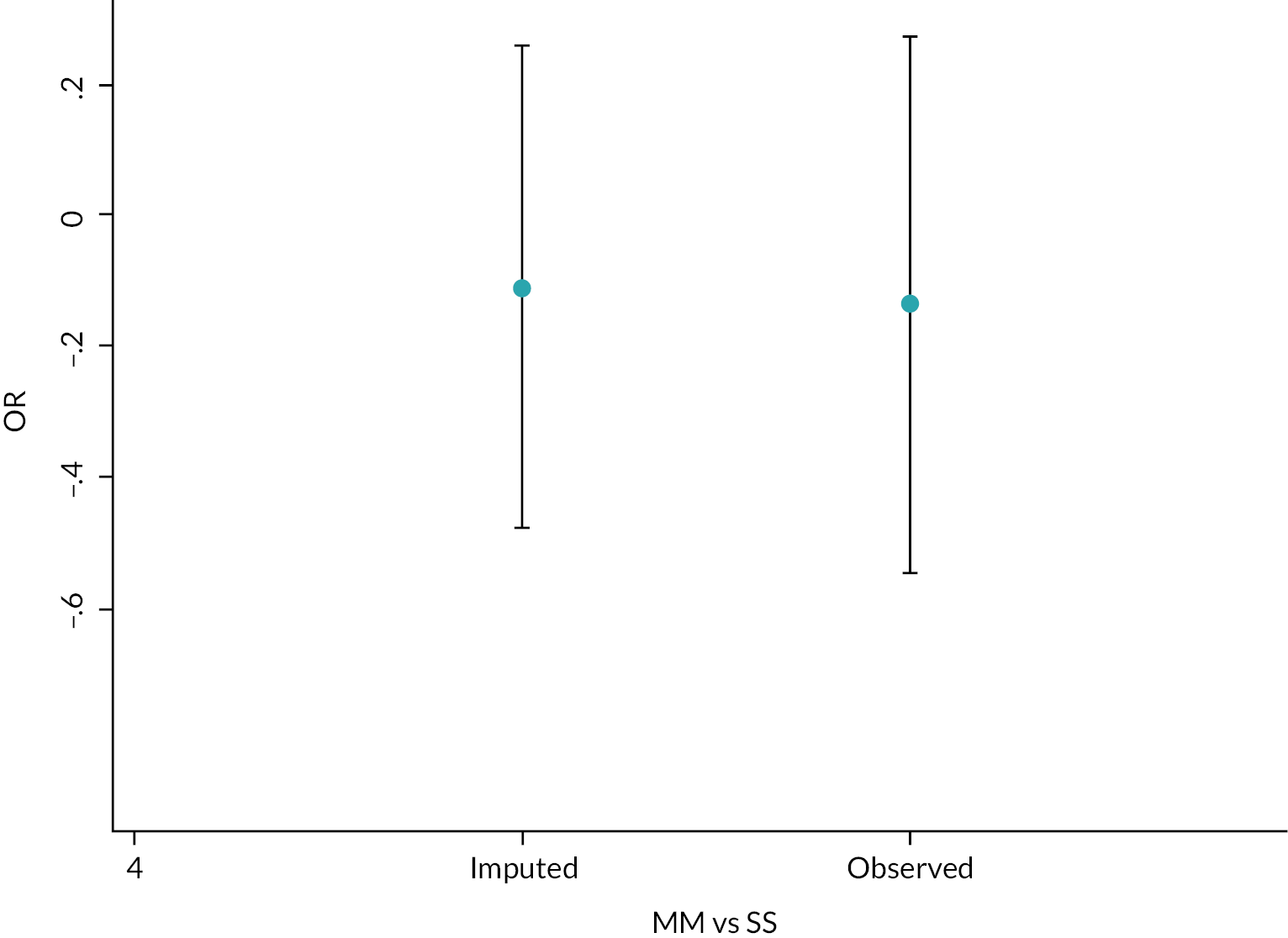
FIGURE 23.
SS + MM + CM vs. SS + MM comparison of observed and imputed odds ratio and 95% CI for per cent days adherence at month 6.

FIGURE 24.
SS + MM + CM vs. SS sensitivity analysis of missing data modelled by varying delta between −0.5 and 0.
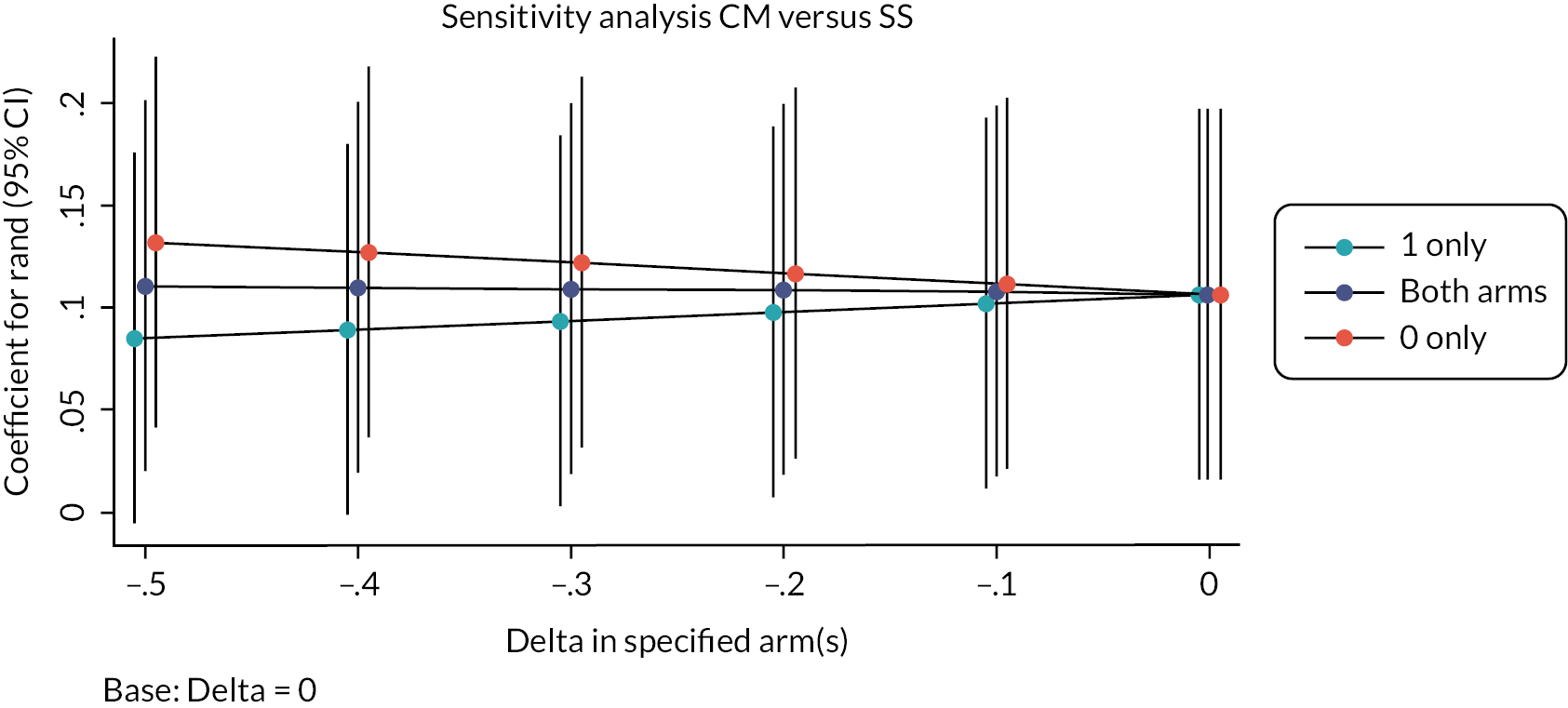
FIGURE 25.
SS + MM vs. SS sensitivity analysis of missing data modelled by varying delta between −0.5 and 0.

FIGURE 26.
SS + MM + CM vs. SS + MM sensitivity analysis of missing data modelled by varying delta between −0.5 and 0.
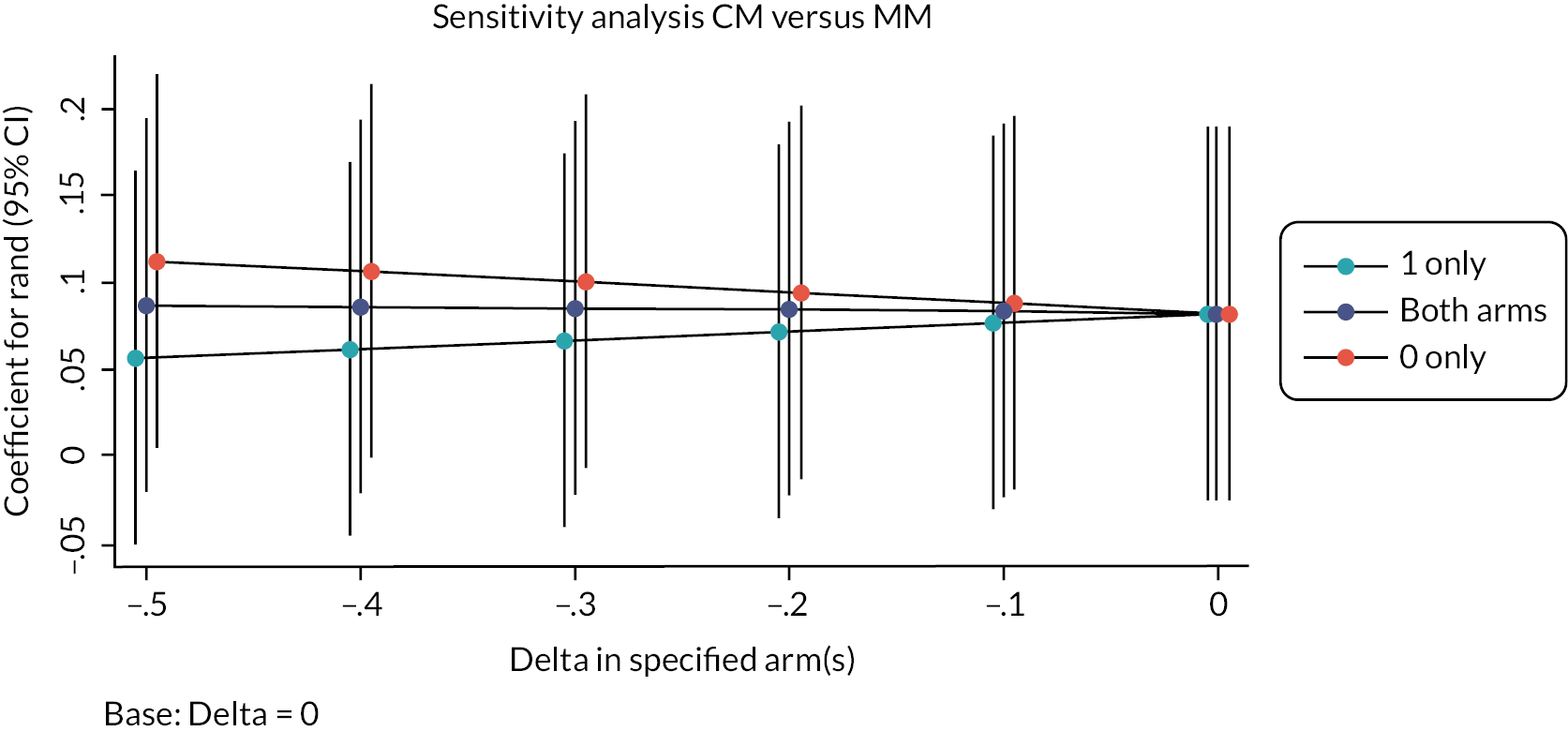
Analysis indicating odds ratio, marginal effects (95% CI) of predictors of % adherence at month 6 derived from a fractional regression. Variables excluded if no evidence of association.
| M6_frac_pcad | Odds ratio | Robust Std. Err. | Z | p > |z| | 95% CI | |
|---|---|---|---|---|---|---|
| StudyArm | ||||||
| SSMM | 0.723 | 0.168 | −1.39 | 0.164 | 0.458 | 1.140 |
| SS | 0.600 | 0.115 | −2.66 | 0.008 | 0.412 | 0.875 |
| Site | ||||||
| Central and North West London | 0.848 | 0.199 | −0.70 | 0.483 | 0.535 | 1.344 |
| South East London | 0.438 | 0.151 | −2.40 | 0.017 | 0.223 | 0.861 |
| Southampton | 0.919 | 0.251 | −0.31 | 0.757 | 0.538 | 1.570 |
| Yorkshire and Humber | 0.950 | 0.214 | −0.23 | 0.820 | 0.611 | 1.477 |
| Children | ||||||
| Yes-resident | 0.759 | 0.178 | −1.18 | 0.239 | 0.479 | 1.202 |
| Yes-non-resident | 1.250 | 0.265 | 1.05 | 0.292 | 0.825 | 1.894 |
| Age | 1.014 | 0.009 | 1.48 | 0.140 | 0.996 | 1.032 |
| Age_first_drink | 1.034 | 0.019 | 1.80 | 0.072 | 0.997 | 1.072 |
| n0apqaffect | 0.851 | 0.070 | −1.95 | 0.052 | 0.724 | 1.001 |
| n0apqcommo | 1.016 | 0.032 | 0.52 | 0.604 | 0.956 | 1.081 |
| n0auq | 0.816 | 0.071 | −2.34 | 0.019 | 0.688 | 0.967 |
| _cons | 0.674 | 0.429 | −0.62 | 0.536 | 0.194 | 2.346 |
Analysis indicating odds ratio, marginal effects (95% CI) of predictors of PDA at month 6 derived from a fractional regression. Variables excluded if no evidence of association.
| M6_frac_pda | Odds ratio | Robust Std. Err. | z | p > |z| | 95% CI | |
|---|---|---|---|---|---|---|
| StudyArm | ||||||
| SSMM | 0.975 | 0.265 | −0.09 | 0.927 | 0.572 | 1.662 |
| SS | 0.781 | 0.174 | −1.11 | 0.269 | 0.504 | 1.210 |
| Site | ||||||
| Central and North West London | 1.068 | 0.278 | 0.25 | 0.801 | 0.641 | 1.780 |
| South East London | 0.672 | 0.224 | −1.19 | 0.232 | 0.50 | 1.290 |
| Southampton | 1.171 | 0.349 | 0.53 | 0.596 | 0.653 | 2.100 |
| Yorkshire and Humber | 1.468 | 0.421 | 1.34 | 0.181 | 0.837 | 2.574 |
| n0auq | 0.848 | 0.070 | −1.99 | 0.047 | 0.721 | 0.998 |
| n0PDA | 1.008 | 0.006 | 1.27 | 0.203 | 0.996 | 1.020 |
| n0PD15plus | 0.992 | 0.005 | −1.64 | 0.100 | 0.982 | 1.002 |
| _cons | 4.208 | 2.477 | 2.44 | 0.015 | 1.328 | 13.336 |
List of abbreviations
- ADAM
- Alcohol Dependence and Adherence to Medicine
- AD-SUS
- Adult Service Use Schedule
- APQ
- Alcohol Problems Questionnaire
- ASSIST
- Alcohol, Smoking and Substance Involvement Screening Test-Lite
- ATA
- analysis by treatment allocated
- AUQ
- Alcohol Urge Questionnaire
- BMQ
- Beliefs About Medications Questionnaire
- CACE
- complier average causal effect
- CEAC
- cost-effectiveness acceptability curve
- CM
- Contingency Management
- EQ-5D-5L
- EuroQol-5 Dimensions, five-level version
- HLP
- Healthy Living Pharmacy
- MAR
- missing at random
- MEMS
- Medication Event Monitoring System
- MM
- Medication Management
- MMAS-8
- Morisky Medication Adherence Scale
- MNAR
- missing not at random
- NICE
- National Institute for Health and Care Excellence
- QALY
- quality-adjusted life-years
- RCT
- randomised controlled trial
- SADQ
- Severity of Alcohol Dependence Questionnaire
- SS
- Standard Support
- STAR
- Scale to Assess Therapeutic Relationship
- SURG
- Service User Research Group
- TLFB
- Time-Line Follow-Back
