Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number NIHR131224. The contractual start date was in January 2021. The draft report began editorial review in February 2022 and was accepted for publication in August 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Wade et al. This work was produced by Wade et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Wade et al.
Chapter 1 Background
Over the last decade, liver cancer incidence has increased by 45% in the UK and is projected to rise further to 15 cases per 100,000 people by 2035. 1 Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer. 2 Between 1997 and 2017 the incidence of HCC in the UK increased by 5.9% a year on average. 3 Primary liver cancer frequently arises on a background of chronic liver disease, and around 90% of cases of HCC are associated with a known underlying aetiology. 2 Globally, hepatitis B virus (HBV) infection is the most common cause of primary liver cancer, but aetiology varies between regions and countries. 4 In the UK, the majority of HCC is associated with the development of cirrhosis, which is most often a consequence of alcohol-related liver disease or non-alcoholic fatty liver disease. Around one-third of patients with cirrhosis develop HCC. 2 Risk increases with the severity of the underlying liver disease in cirrhotic patients,2 such that patients developing HCC often have advanced liver disease and a significant risk of developing liver failure.
Hepatocellular carcinoma is often asymptomatic until late in its disease course, and the prognosis of HCC patients presenting with symptoms is poor. 5 Recognising the importance of early HCC diagnosis in patients with cirrhosis, the National Institute for Health and Care Excellence (NICE) recommends regular surveillance ultrasound scans intended to diagnose small HCCs so that they can be treated. 6 The Barcelona Clinic Liver Cancer (BCLC) staging system defines very early-stage HCC as a single tumour < 2 cm, preserved liver function and performance status of 0; early-stage disease is defined as a single tumour of any size or up to three tumours ≤ 3 cm, preserved liver function and performance status of 0. Patients with multinodular disease and/or larger tumours would be categorised as having intermediate, advanced or terminal-stage disease (also depending on liver function and performance status). 2 Patients with good liver function who are diagnosed with HCC at an early stage can be offered surgical and non-surgical interventions with curative intent; in general, these patients have favourable 5-year survival rates. 2 However, if patients have signs of advanced cirrhosis with the development of portal hypertension, this restricts the use of liver resection as a treatment option. 7 While liver transplantation is associated with reduced HCC recurrence compared with other treatments, transplantation is limited by availability. 8 Consequently, ablative therapies are frequently used in patients with small HCCs.
A range of ablative and non-surgical therapies is available for treating small HCC tumours in patients with very early or early-stage disease and preserved liver function. The main methods used are microwave ablation (MWA) and radiofrequency ablation (RFA). Alternative methods of ablation include percutaneous ethanol injection (PEI) or percutaneous acetic acid injection (PAI), irreversible electroporation (IRE), laser ablation and cryoablation. Stereotactic ablative radiotherapy [SABR; the term stereotactic body radiotherapy (SBRT) is also used for this technology, but for simplicity SABR is used throughout this report] is emerging as an alternative to invasive ablation and has recently been commissioned as a treatment option by NHS England. 9 Non-ablative approaches, which achieve cure much less frequently, include transarterial (chemo-) embolisation [TA(C)E] and selective internal radiation therapy (SIRT).
However, there has been no definitive assessment of these therapies. NICE guidance comprises overviews of interventional procedures based on rapid reviews, rather than a full systematic assessment of the different treatment options. 10–12 Scoping searches identified four Cochrane Reviews of ablative and minimally invasive therapies that appeared to have populations relevant to this research question; these generally found few or no randomised controlled trials (RCTs), low-quality evidence and a high risk of bias (RoB). 13–16 While some network meta-analyses (NMAs) have been completed, these did not include all relevant therapies and could not assess all relevant outcomes. 17–19 The evidence base is large, but the majority of studies are small and of poor quality. It is also important to consider the applicability of the research evidence to the UK population, since the aetiology of HCC differs between European and Asian populations;20 many primary studies of interventions for HCC have been undertaken in Asia. Therefore, a thorough systematic evaluation of the existing research evidence was required to inform UK clinical practice and the design of future effectiveness and cost-effectiveness studies of emerging treatments.
Chapter 2 Aim and objectives
The aim of this project was to evaluate and compare the effectiveness of ablative and non-surgical therapies for patients with HCC whose tumours are small (up to 3 cm).
The key objectives were:
-
to systematically identify all RCTs of ablative and non-surgical therapies for HCC (including registered, unpublished and ongoing trials)
-
to evaluate their quality and applicability to UK populations
-
to determine the comparative effectiveness of therapies using NMA techniques
-
where the evidence base is insufficient, to supplement the RCT evidence with targeted systematic reviews of high-quality, non-randomised, prospective comparative studies of specific therapies
-
to identify priority areas where additional high-quality evidence is required (in collaboration with patients and clinicians)
-
to assess whether future economic analysis based on the findings would be feasible and worthwhile.
Chapter 3 Methods
The systematic reviews were conducted following the Centre for Reviews and Dissemination (CRD) guidance on undertaking systematic reviews21 and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. 22,23 The protocol is registered on PROSPERO, the international prospective register of systematic reviews in health and social care; registration number CRD42020221357.
Systematic review of randomised controlled trials
Search strategy for identification of randomised controlled trials
A comprehensive, systematic search of bibliographic databases and trial registers was undertaken to identify RCTs of ablative and non-surgical therapies for the treatment of early/small (≤ 3 cm diameter) HCCs. The search strategy was developed in Ovid MEDLINE by an information specialist (MH) with input from the review team. The strategy combined relevant text word searches for terms that appear in the titles or abstracts of database records, with relevant subject headings (e.g. MeSH terms). The strategy consisted of a set of terms for early/small HCC combined with terms for each of the ablative and non-surgical therapies. The MEDLINE search strategy was adapted for use in all other resources searched.
Searches were limited to RCTs using validated study design search filters where available. Retrieval was restricted to articles published from 2000 onwards, as clinical advice confirmed that practice has evolved over the past 20 years and techniques have changed over time. In addition, the natural history and treatment of the underlying liver disease have also changed over the last 20 years, including antiviral therapies for HBV/hepatitis C virus (HCV); therefore, overall outcomes will have changed over this period. Language limits were not applied to the strategy.
The following databases were searched on 3 February 2021:
-
MEDLINE ALL (Ovid)
-
Embase (Ovid)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley)
-
Science Citation Index (Web of Science).
Relevant systematic reviews were also sought, in order to check their reference lists for additional relevant studies. The following systematic review databases were searched on 3 February 2021:
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley)
-
Database of Abstracts of Reviews of Effects (DARE) (CRD databases)
-
International Health Technology Assessment database
-
Epistemonikos
-
International Prospective Register of Systematic Reviews (PROSPERO).
At our first advisory group meeting on 15 February 2021, a few additional non-surgical therapies were suggested for inclusion in the review: electrochemotherapy (ECT), histotripsy and wider radiotherapy techniques. Therefore, all of the databases listed above were searched again on 17–18 March 2021 using terms for the condition taken from the original searches (devised by MH), with further terms for additional therapies (devised by HF). The records retrieved from these searches were deduplicated against the original search results in EndNote™ 20 (Clarivate Analytics, Philadelphia, PA, USA).
Information on studies in progress and unpublished research was sought by searching ClinicalTrials.Gov and the European Union Clinical Trials Register on 27 April 2021, using terms for early/small HCC only. These searches were devised and performed by an information specialist (HF). As trial registers have limited search interfaces which are not designed for expert searches, terms for the condition were searched for without listing any of the interventions, to capture as many relevant records as possible. The search of ClinicalTrials.Gov was limited to ‘interventional studies’, and both registers were limited to trials first posted from 2010 onwards, since the main purpose of searching clinical trial registers was to identify ongoing trials. Clinical advisors were consulted about relevant studies they were aware of.
Search results were imported into EndNote 20 and deduplicated. MEDLINE search strategies are presented in Appendix 1.1. Search strategies for other databases are presented in Report Supplementary Material 1.
Inclusion criteria
Participants
Patients diagnosed with HCC with tumour size up to 3 cm (studies with mixed populations were considered if the data for patients with tumour size up to 3 cm could be extracted separately), who were suitable for treatment with ablative or non-surgical therapies. Key participant subgroups considered included:
-
size of tumour
-
number of tumours (single or multiple lesions)
-
disease stage
-
cirrhosis and severity (Child–Pugh A or B)
-
liver disease (HBV/HCV, other)
-
prior HCC treatment
-
study location.
Interventions
Any ablative or non-surgical therapy, including:
-
RFA
-
MWA
-
laser ablation
-
high-intensity focused ultrasound (HIFU)
-
cryoablation
-
PEI
-
PAI
-
IRE
-
TACE
-
transarterial embolisation
-
SIRT
-
ECT
-
histotripsy
-
SABR
-
wider radiotherapy techniques.
Comparators
The project aimed to evaluate the comparative effectiveness of all of the therapies listed above, so no specific comparator therapy was considered; any comparator was eligible for inclusion, including ablative, minimally invasive or more invasive interventions. Studies comparing a relevant therapy versus surgical resection were also included. Studies comparing different methods of undertaking the same intervention were not eligible for inclusion (e.g. conventional temperature control RFA vs. impedance control RFA, RFA under ultrasound guidance vs. RFA under computed tomography guidance); studies had to compare two different therapies.
Outcomes
The outcomes of interest were:
-
overall survival (OS)
-
progression-free survival (PFS)
-
time to progression (TTP)
-
recurrence
-
serious adverse events (AEs)
-
intervention-specific AEs (e.g. pneumothorax, post-ablation syndrome, post-embolisation syndrome, thermoablative injury, pain, haemorrhage or bile leak)
-
quality of life.
Where reported, outcomes of economic relevance were recorded, including healthcare costs and duration of hospital stay.
Study design
Randomised controlled trials were eligible for inclusion.
Study selection and data extraction
Studies were initially assessed for relevance using titles and abstracts. As the database searches were expected to be extensive, a single reviewer screened each identified title/abstract, and 10% of records were checked by another reviewer. Full-text articles were independently screened by two reviewers for final inclusion. Any disagreements were resolved through discussion and, where necessary, consultation with a third reviewer. Foreign-language studies were translated and assessed for inclusion. Studies only available as conference abstracts were identified and attempts were made to contact authors for further data to enable them to be assessed for inclusion in the review.
A data extraction form was developed using Microsoft Excel® (Microsoft Corporation, Redmond, WA, USA), piloted on a sample of studies and refined. Data on intervention, comparator and patient characteristics and results were extracted by one reviewer (SS-H or ES) and checked by a second reviewer (RW). Any discrepancies were resolved by discussion. Foreign-language studies were data extracted by a native speaker and discussed at a meeting with a second reviewer (RW). Authors of conference abstracts were contacted for further information; data were extracted using only the abstract when authors did not respond.
For all outcomes, data were extracted from publications either as hazard ratios (HRs) for survival outcomes, or as relative risks (RRs) for dichotomous outcomes, and in all cases with their corresponding 95% confidence intervals (CIs) or standard errors (SEs).
For survival outcomes, where studies did not report HRs and their variances, Kaplan–Meier (KM) data, including the numbers at risk, were extracted using methods reported by Guyot et al. 24 and HRs were computed using the reconstructed individual patient data. If a study did not report the numbers at risk, the p-value for the log-rank test was used to calculate the HR and its corresponding variance using methods described by Irvine et al. 25
In the instance where neither HRs were reported nor KM plots were provided, HRs and SEs were back-calculated using the reported survival rates and the p-value of the log-rank test with the log-rank test. 26
Critical appraisal
Risk of bias in RCTs was assessed using the latest version of the Cochrane RoB tool. 27 RoB assessment was undertaken by one reviewer (SS-H or ES) and independently checked by a second reviewer (RW). Any disagreements were resolved through consensus.
Network meta-analysis
Feasibility assessment
Randomised controlled trials were mapped according to interventions included, outcomes reported, trial size and quality, to determine the overall extent of the RCT evidence. Trials were grouped according to identified subgroups (e.g. tumour size and stage), where appropriate. Key interventions and comparisons of interventions where existing RCT data are absent, limited or of poor quality were identified. The mapping was used to determine whether NMA of the RCTs was feasible.
Networks of treatment comparisons were drawn for each outcome to check that they were connected. Not all RCTs reported data that could be used; only studies with usable data were included in the networks.
Included data
Network meta-analyses were conducted for four outcomes: OS, PFS, overall recurrence, and local recurrence. For OS and PFS, only contrast-level data were available in the form of HRs. For overall recurrence and local recurrence, both contrast-level and arm-level data were available. Data for both HRs and RRs were synthesised on the log scale, by log-transforming estimates and their CIs from studies.
For OS and PFS, summary effect estimates from the NMAs were presented as HRs and their corresponding 95% credible intervals (CrIs), whereas overall and local recurrence estimates were presented as RRs and their corresponding 95% CrIs.
Any deviation from proportional hazards was tested for, and the Schoenfeld residuals, survival curves and piecewise hazards visually inspected. If there is strong evidence that the proportional hazards assumption does not hold, or the simpler models initially considered do not fit the data well, more complex, time-varying models that account for non-proportional hazards should be considered, if sufficient data are available. However, data were limited, so this was not possible. Consequently, appropriate caution with the results is expressed, where appropriate.
Network meta-analysis
Network meta-analyses were conducted in a Bayesian framework using Markov chain Monte Carlo techniques. For the aggregate RCT data (HRs and RRs), contrast-based models proposed by Dias et al., which appropriately account for correlations in trials with more than two arms, were used. 28–30 All four outcomes were modelled using a normal likelihood with an identity link. 30 Where arm-level data were available for overall and local recurrence, the binomial likelihood, logit link model suggested by Warn et al. 31 was also fitted to prove comparability of the results.
All analyses were carried out using the GeMTC package32 in R (version 4.1.2).
To account for the correlation between the relative effects in three-arm trials33 the covariance between differences taken with respect to the same control arm was calculated using the equation:
Fixed-effect (FE) and random-effects (RE) models were fitted. Models were sampled for 100,000 iterations over four chains after an initial burn-in of 50,000 iterations. Model convergence was assessed through visual inspection of Brook–Gelman–Rubin diagnostic and history plots. 34
For the RE models, the choice of prior distributions for the between-study standard deviation (SD) was explored. A half-normal (0, 0.192) and a uniform (0, 3) prior distribution were considered. As a sensitivity analysis, a half-normal (0, 0.502) prior was also used for the between-study heterogeneity. 35
Models were compared based on their deviance information criteria (DIC), and the model with the smallest DIC was selected as the base-case analysis. 36,37 Differences < 3 were not considered meaningful, and the simplest model was selected. Where a FE model was selected, results for the RE models were also presented as a sensitivity analysis.
In networks with loops formed by independent studies (i.e. where different studies provided direct and indirect evidence for the same comparison), inconsistency (i.e. conflict between the direct and indirect evidence) was checked by comparing the model fit and between-study heterogeneity from the NMA models versus the corresponding unrelated mean effects (inconsistency) models. 28,38 Where inconsistency was identified, it was explored by inspecting the characteristics of the included studies (participant and design characteristics) that may contribute to inconsistency. Where feasible, node-split models were fitted to provide further evidence of the location and impact of potential inconsistency. 39
Where judged appropriate, NMA was used to assess and rank interventions by comparative effectiveness. Where feasible, the potential impact of additional evidence on the NMAs was investigated using threshold analysis. 40,41
Threshold analysis
Threshold analysis40,41 was conducted at the contrast level to examine the impact of potential changes to the evidence on each treatment contrast to identify which treatment comparisons lacked robust RCT evidence. Threshold analysis represents a robust statistical alternative to qualitative assessment of the robustness of evidence. It is a novel statistical approach that can be used to investigate which comparisons in a NMA have estimated relative effects which might not be robust to changes in the observed evidence due to either possible bias, sampling variation or relevance. 40,41 Threshold analysis uses formal statistical methods to quantify precisely how much the results of a NMA could vary (due to changes in the amount of data, or due to potential bias) before any conclusion changes (e.g. changes to the ranking of an intervention), by examining what the smallest changes to the available data required to alter a conclusion are. It can therefore be used to identify which interventions, or comparisons of interventions, have the most robust evidence, and which interventions would benefit from further trials.
Threshold analysis was carried out using the nmathresh package40 in R (version 4.1.2). Results of the threshold analysis are presented graphically as forest plots and threshold tables. The results have been used to identify interventions and comparisons where non-randomised evidence should be sought for further review, based on the sensitivity shown by the comparison with potential additional evidence.
Following clinical advice, comparisons that included PAI and PEI were excluded from the threshold analysis to restrict attention to interventions considered relevant to current practice.
Systematic review of non-randomised evidence
Results of the mapping exercise, NMAs and threshold analyses were used to identify interventions or comparisons where non-randomised evidence might usefully add to the RCT evidence or potentially resolve uncertainty (see Systematic review of RCTs, Network meta-analysis results and Threshold analysis of RCT networks). This identified and classified evidence for interventions:
-
with no RCT evidence
-
with limited RCT evidence (e.g. only one or two trials, or <50 or <100 patients in total)
-
where RCT evidence is very heterogeneous (e.g. very different results across trials)
-
where RCT evidence is highly uncertain (e.g. wide CIs or uncertain ranking in NMAs, as identified by the threshold analysis)
-
where RCT evidence is of low or uncertain quality, or at ROB.
The advisory group was consulted to identify interventions of particular practical interest where RCT evidence was lacking. A distinction was made between comparisons without any current RCT evidence (i.e. where an intervention of interest was disconnected from the main network) and comparisons with imprecise or non-robust RCT evidence.
This targeted approach was used because preliminary searches suggested that the quantity of non-randomised evidence was too large to be fully reviewed within the time and resource available for this project; furthermore, this would be of limited value as much of the non-randomised evidence is likely to be of insufficient quality for inclusion in any analysis.
For the interventions identified for further investigation by our classification and by the advisory group, targeted database searching and screening were performed.
Search strategy for identification of non-RCTs
Searches were undertaken to identify non-randomised studies of selected interventions for early/small (≤ 3 cm diameter) HCC, where RCT evidence was not available. The search strategy consisted of terms for small or early HCC combined with terms for the selected interventions (HIFU, cryoablation, IRE, ECT, histotripsy, SABR and wider radiotherapy techniques). Relevant subject headings alongside text word searches in the title and abstracts of records were included in the search strategy. To allow comprehensive retrieval of non-randomised studies, the search was not restricted by study type. 42 The strategy was limited to articles published from the year 2000 onwards. Language limits were not applied.
The searches were carried out on 28 July 2021. The following databases were searched: MEDLINE (Ovid), Embase (Ovid), CENTRAL (Wiley) and the Science Citation Index (Web of Science, Clarivate). EndNote 20 was used to manage and deduplicate the search results.
Although conference abstracts were due to be identified via a search of the Conference Proceedings Citation Index – Science, a pragmatic decision to not search this database was taken due to a lack of time and resources to screen and follow up ongoing studies reported as conference abstracts. Similarly, conference abstracts were removed from the search results retrieved in Embase.
MEDLINE search strategies are presented in Appendix 1.2. Search strategies for other databases are presented in Report Supplementary Material 1.
Searches were also undertaken to identify studies of selected interventions for comparisons where additional evidence could plausibly change the NMA conclusions, as identified by the threshold analysis. The search strategy consisted of terms for small or early HCC combined with terms for the selected interventions (RFA, MWA and laser ablation, compared with each other or with surgical resection). Relevant subject headings alongside text word searches in the title and abstracts of records were included in the search strategy. The strategy was limited to articles published from the year 2000 onwards, and animal studies were removed where possible. Language limits were not applied.
The searches were carried out on 24 August 2021. The following databases were searched: MEDLINE (Ovid), Embase (Ovid), CENTRAL (Wiley) and the Science Citation Index (Web of Science, Clarivate). EndNote 20 was used to manage and deduplicate the search results.
MEDLINE search strategies are presented in Appendix 1.3. Search strategies for other databases are presented in Report Supplementary Material 1.
Inclusion criteria
Participants
Patients diagnosed with HCC with tumour size up to 3 cm (studies with mixed populations were considered if the data for patients with tumour size up to 3 cm could be extracted separately), who were suitable for treatment with ablative or non-surgical therapies. Studies of patients with recurrent HCC were excluded, as clinical advisors confirmed that it was not appropriate to synthesise the results of these studies with the studies of HCC patients included in the networks.
Interventions
Informed by the systematic review of RCTs and results of the NMAs and threshold analyses (see Systematic review of RCTs, Network meta-analysis results and Threshold analysis of RCT networks), ablative or non-surgical therapies of particular practical interest where RCT evidence was lacking were sought; these were interventions where either RCT evidence was not available, or where additional evidence could plausibly change the NMA result, as identified by the threshold analysis. The specific interventions were:
-
RFA
-
MWA
-
laser ablation
-
HIFU
-
cryoablation
-
IRE
-
ECT
-
histotripsy
-
SABR
-
wider radiotherapy techniques.
Comparators
The project aimed to evaluate the comparative effectiveness of all the therapies listed above, so no specific comparator therapy was considered; any comparator was eligible for inclusion, including ablative, minimally invasive or more invasive interventions. Studies comparing a relevant therapy versus surgical resection were also included. Studies comparing different methods of undertaking the same intervention were not eligible for inclusion (e.g. conventional temperature control RFA vs. impedance control RFA; RFA under ultrasound guidance vs. RFA under computed tomography guidance); studies had to compare two different therapies.
Outcomes
The outcomes of interest were:
-
OS
-
PFS
-
TTP
-
quality of life.
Studies only reporting response and AE results were excluded from the review of non-RCTs as these outcomes were not relevant for the NMAs.
Study design
Only prospective non-randomised studies that compared two or more eligible therapies were included; studies of single therapies were excluded.
Study selection and data extraction
Consistent with the review of RCTs, titles and abstracts were screened by a single reviewer, with 10% of records checked by another reviewer. Full-text articles were independently screened by two reviewers for final inclusion. Any disagreements were resolved through discussion and, where necessary, consultation with a third reviewer. Foreign-language studies were translated and assessed for inclusion. Studies only available as conference abstracts were assessed based on the limited data available and were included if there were sufficient data reported on the relevant outcomes.
The data extraction form developed using Microsoft Excel for the review of RCTs was modified for the review of non-RCTs. Data on intervention, comparator and patient characteristics and results were extracted by one reviewer (RW or ES) and independently checked by a second reviewer (ES or RW). Any discrepancies were resolved by discussion. Foreign-language studies were data extracted by a native speaker and discussed at a meeting with a second reviewer (RW). Where studies were only reported as conference abstracts, data were extracted using the limited data available. Where possible, HRs and their variances were extracted by one reviewer and checked by a second reviewer. When the HRs were not available, KM data were extracted using methods reported by Guyot et al. 24 If neither HRs nor KM data were available, survival rates and p-values for the log-rank test were extracted.
Critical appraisal
A validity assessment tool was developed, piloted on a sample of studies and refined. Validity assessment was undertaken by one reviewer (RW or ES) and independently checked by a second reviewer (ES or RW). Any disagreements were resolved through consensus. The most important quality assessment criteria were selected, based on their potential impact on the overall validity of the studies, and an overall RoB judgement was made for each study; important criteria were those relating to the participant inclusion criteria, appropriateness of treatment allocation, similarity of treatment groups at baseline and whether missing outcome data were balanced across treatment groups.
Updated network meta-analysis
For non-randomised studies that were of sufficient quality, the NMA and threshold analyses were repeated after pooling (without any adjustments) the non-randomised evidence with the RCT evidence, to assess whether estimates were improved.
The updated NMA was conducted using the methods detailed in Network meta-analysis.
Updated threshold analysis
A threshold analysis was conducted on the results for the updated NMAs using both RCT and non-randomised evidence to explore the robustness of the updated results.
The updated threshold analysis was conducted using methods detailed in Threshold analysis.
Chapter 4 Results
Systematic review of RCTs
The electronic searches identified a total of 7550 records after deduplication between databases; 6774 records were identified from the original searches of bibliographic databases undertaken on 3–4 February 2021, 655 from the searches for studies of ECT, histotripsy and wider radiotherapy techniques undertaken on 17–18 March 2021, and 121 from the trial register searches undertaken on 27 April 2021. One additional record was identified from screening reference lists of relevant systematic reviews. Clinical advisors were not aware of any additional RCTs not identified in the electronic searches.
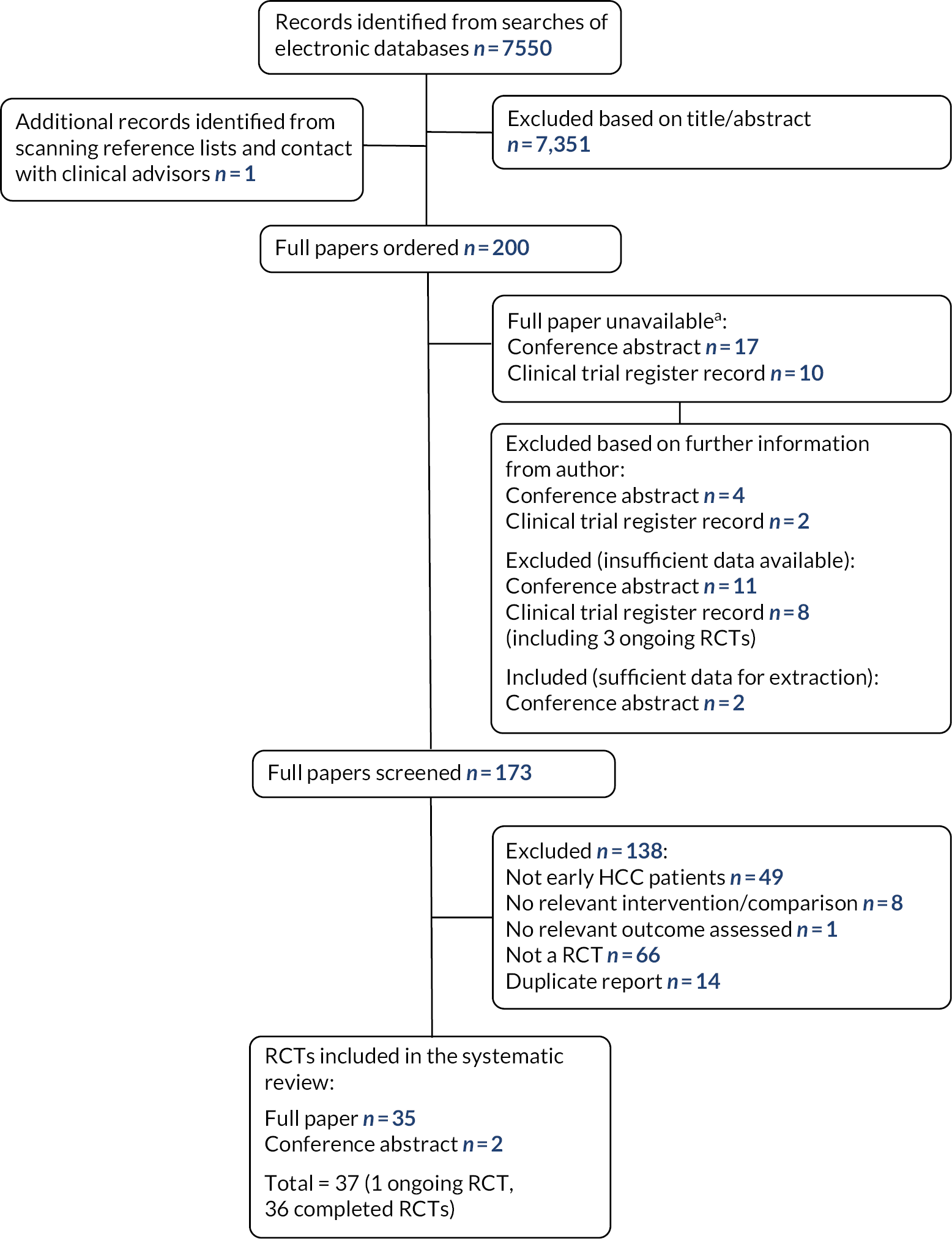
Two hundred potentially relevant studies were ordered for full paper screening. Twenty-seven full papers were unavailable as they were only reported as conference abstracts or clinical trial register records; study authors were e-mailed (where contact details could be found) and authors of six records confirmed that they were either duplicate reports or did not meet our inclusion criteria. One hundred and seventy-three full papers were screened; 138 were excluded at the full paper stage and are listed in Appendix 2, along with the reasons for their exclusion. Figure 1 presents the flow of studies through the study selection process.
FIGURE 1.
Flow diagram of the study selection process (RCTs).
Characteristics of RCTs included in the review
Details of the 37 RCTs that were included in the systematic review are presented in Table 1. One RCT was ongoing and therefore no results were available for data extraction. The characteristics and results of the other 36 RCTs were extracted into an Excel spreadsheet.
| Study | Location | Participant information | Intervention | Comparator |
|---|---|---|---|---|
| Abdelaziz, 201464 | Egypt | 111 patients (with 128 tumours) ≤ 5 cm; subgroup of 87 tumours ≤ 3 cm | RFA | MWA |
| Aikata, 2006 (abstract)43 | Not reported (authors from Japan) | 44 patients with tumours < 3 cm | RFA + TACE | RFA alone |
| Azab, 201165 | Egypt | 90 patients (with 98 tumours) ≤ 5 cm; subgroup of 48 tumours ≤ 3 cm | PEI + RFA | RFA alone PEI alone |
| Bian, 201478 | China | 127 patients with BCLC stage 0–B; subgroup of 78 patients with tumours < 3 cm | RFA + iodine-131 metuximab | RFA alone |
| Brunello, 200844 | Italy | 139 patients with tumours ≤ 3 cm | RFA | PEI |
| Chen, 2005 (reported in Chinese)66 | China | 86 patients with tumours ≤ 5 cm; subgroup of 47 patients with tumours ≤ 3 cm | RFA + PEI | RFA alone |
| Chen, 2005 (reported in Chinese)67 | China | 132 patients with tumours ≤ 5 cm; subgroup of 55 patients with tumours ≤ 3 cm | Resection | RFA |
| Chen, 200668 | China | 180 patients with tumours ≤ 5 cm; subgroup of 79 patients with tumours ≤ 3 cm | Percutaneous local ablative therapy | Partial hepatectomy |
| Chen, 201445 | China | 136 patients with tumours ≤ 3 cm | RFA + iodine-125 | RFA alone |
| Fang, 201446 | China | 120 patients with tumours ≤ 3 cm | RFA | Hepatectomy |
| Feng, 201258 | China | 168 patients with tumours < 4 cm; subgroup of 56 patients with tumours ≤ 2 cm | RFA | Surgical resection |
| Ferrari, 200777 | Not reported (authors from Italy) | 81 patients with tumours ≤ 4 cm; subgroup of 28 patients with tumours ≤ 2.5 cm | Laser ablation | RFA |
| Gan, 2004 (reported in Chinese)47 | China | 38 patients with tumours ≤ 3 cm | RFA alone | RFA + chemotherapy |
| Giorgio, 201148 | Italy | 285 patients with tumours ≤ 3 cm | RFA | PEI |
| Huang, 200549 | Taiwan | 82 patients with tumours ≤ 3 cm | PEI | Resection |
| Huang, 201069 | China | 230 patients with tumours ≤ 5 cm; subgroup of 159 patients with tumours ≤ 3 cm | RFA | Resection |
| Huo, 200370 | Taiwan | 108 patients with tumours ≤ 5 cm; subgroup of 55 patients with tumours ≤ 3 cm | Sequential TACE and PAI | PAI alone |
| Izumi, 2019 (abstract)50 | Japan | 308 patients with tumours ≤ 3 cm | RFA | Surgery |
| Kim, 202051 | South Korea | 144 patients with recurrent/residual tumours < 3 cm | Proton beam radiotherapy | RFA |
| Koda, 200152 | Japan | 52 patients with tumours < 3 cm | TACE + PEI | PEI alone |
| Lencioni, 200371 | Not reported (authors from Italy and Germany) | 104 patients with tumours ≤ 5 cm (large proportion had tumours ≤ 3 cm) | PEI | RFA |
| Lin, 200459 | Not reported (authors from Taiwan) | 157 patients with tumours ≤ 4 cm; subgroup of 114 patients with tumours ≤ 3 cm | RFA | Low-dose PEI High-dose PEI |
| Lin, 200553 | Taiwan | 187 patients with tumours ≤ 3 cm | RFA | PEI PAI |
| Liu, 201672 | China | 200 patients with tumours ≤ 5 cm; subgroup of 135 patients with tumours ≤ 3 cm | Partial hepatectomy | TACE + RFA |
| Mizuki, 201060 | Japan | 30 patients with tumours ≤ 4 cm (large proportion had tumours ≤ 3 cm) | PEI alone | TACE + PEI |
| Ng, 201779 | China | 218 patients with tumours ≤ 5 cm; subgroup of 55 patients with tumours ≤ 2 cm | Resection | RFA |
| Orlacchio, 201461 | Italy | 30 patients with tumours ≤ 4 cm (mean tumour size 2.4 cm) | Laser ablation | RFA |
| Peng, 201274 | China | 139 patients with recurrent HCC tumours ≤ 5 cm; subgroup of 87 patients with tumours ≤ 3 cm | RFA + TACE | RFA alone |
| Shibata, 200262 | Japan | 72 patients (with 94 tumours) < 4 cm; subgroup of 88 tumours ≤ 3 cm | RFA | MWA |
| Shibata, 200954 | Japan | 89 patients with tumours ≤ 3 cm | RFA + TACE | RFA alone |
| Shiina, 200555 | Japan | 232 patients with tumours ≤ 3 cm | RFA | PEI |
| Vietti Violi, 201863 | Switzerland and France | 152 patients with tumours ≤ 4 cm (mean tumour size 1.8 cm, < 8% patients had tumours > 3 cm) | MWA | RFA |
| Xia, 202075 | China | 240 patients with recurrent HCC tumours ≤ 5 cm; subgroup of 159 patients with tumours ≤ 3 cm | RFA | Repeat hepatectomy |
| Yan, 201656 | China | 120 patients with tumours ≤ 3 cm | Resection | MWA + sorafenib |
| Zhang, 200776 | China | 133 patients with tumours ≤ 7 cm; subgroup of 60 patients with tumours ≤ 3 cm | RFA + PEI | RFA alone |
| Zhu, 2021 (protocol)80 | China | Ongoing RCT | RFA | Laparoscopic hepatectomy |
| Zou, 201757 | China | 74 patients with tumours ≤ 3 cm | Laser ablation | RFA |
Fifteen of the 36 completed RCTs restricted inclusion criteria to HCC patients with tumour size up to 3 cm in diameter. 43–57 Six RCTs included patients with tumours up to 4 cm in diameter,58–63 12 RCTs included patients with tumours up to 5 cm in diameter64–75 and one RCT included patients with tumours up to 7 cm in diameter. 76 One RCT did not report specific tumour size criteria but included patients with small HCCs,77 and one RCT included patients within BCLC stages 0–B. 78 The RCTs that included patients with larger tumours (>3 cm diameter) were included in the review if they reported separate results for the subgroup of patients with a tumour diameter up to 3 cm or, in the case of three RCTs, if a clear majority of patients had tumours < 3 cm in diameter. 60,63,71 Three RCTs included patients with recurrent/residual tumours ≤ 3 cm. 51,74,75 Sample sizes ranged from 30 to 308 patients.
The majority of RCTs were conducted in Asian countries, which has implications for the generalisability of results to the UK population. HCC in European patients is more likely to be caused by alcohol or hepatitis C, whereas in Asia it is more likely to be caused by hepatitis B. The natural history of these diseases is different and treatment options for the underlying liver disease differ. RCTs were conducted in China (n = 17), Japan (n = 7), Taiwan (n = 4), South Korea (n = 1), Egypt (n = 2), Italy (n = 4), Italy and Germany (n = 1) and Switzerland and France (n = 1).
The most frequently assessed ablative/non-surgical therapy was RFA, either alone or in combination with TACE, PEI, iodine-131 metuximab, iodine-125 or chemotherapy. Table 2 shows the comparisons made in the included RCTs. The majority of RCTs assessed OS, progression-/disease-free survival and/or recurrence, along with response and AEs. A few RCTs presented economic outcomes. Only one RCT assessed patient satisfaction.
| RFA | ||||||||||||||
| MWA | 3 | MWA | ||||||||||||
| PEI | 7 | PEI | ||||||||||||
| PAI | 1 | 1 | PAI | |||||||||||
| Laser | 3 | Laser | ||||||||||||
| Resection | 8 | 1 | Resection | |||||||||||
| Proton beam radiotherapy | 1 | Proton beam radiotherapy | ||||||||||||
| Local ablative therapy | 1 | Local ablative | ||||||||||||
| RFA + TACE | 3 | 1 | RFA + TACE | |||||||||||
| RFA + PEI | 3 | 1 | RFA + PEI | |||||||||||
| RFA + 131I or 125I | 2 | RFA + 131Ior 125I | ||||||||||||
| RFA + Chemo | 1 | RFA+Chemo | ||||||||||||
| TACE + PAI | 1 | TACE+PAI | ||||||||||||
| TACE + PEI | 2 | TACE+PEI | ||||||||||||
| MWA + Sorafenib | 1 |
Quality of RCTs included in the review
Risk of bias was assessed for each of the main study outcomes using the Cochrane RoB tool, resulting in 58 assessments for the 35 included RCTs for which RoB could be assessed; two RCTs did not have a RoB assessment as they were either ongoing80 or did not report any relevant outcomes for the subgroup of patients with tumours ≤ 3 cm. 58 Results of the RoB assessment for the most relevant outcome assessed are presented in Table 3. Results for each of the main study outcomes are presented in Appendix 3. Two RCTs were only reported as conference abstracts; therefore, some questions had a ‘no information’ response owing to the limited reporting, resulting in a high RoB for the domain and the study overall. 43,50
| Trial | ROB arising from the randomisation process | ROB due to deviations from the intended intervention | ROB due to missing outcome data | ROB in measurement of the outcome | ROB in selection of the reported result | Overall judgement of ROB |
|---|---|---|---|---|---|---|
| Abdelaziz, 201464 | High | Low | Low | Low | Low | High |
| Aikata, 2006 (abstract)43 | Some concerns | High | High | Low | Low | High |
| Azab, 201165 | Some concerns | Low | Low | Low | Low | Some concerns |
| Bian, 201478 | Some concerns | Low | Low | Low | Low | Some concerns |
| Brunello, 200844 | Low | Low | Low | Low | Low | Low |
| Chen, 200567 | Some concerns | Low | Low | Low | Low | Some concerns |
| Chen, 200566 | Some concerns | Low | High | Low | Low | High |
| Chen, 200668 | Low | High | Low | Low | Low | High |
| Chen, 201445 | Low | Low | Low | Low | Low | Low |
| Fang, 201446 | Some concerns | Low | Low | Low | Low | Some concerns |
| Ferrari, 200777 | Some concerns | Low | Low | Low | Low | Some concerns |
| Gan, 200447 | Some concerns | Low | High | Low | Low | High |
| Giorgio, 201148 | Low | High | High | Low | Low | High |
| Huang, 200549 | High | High | High | Low | Low | High |
| Huang, 201069 | Low | Low | High | Low | Low | High |
| Huo, 200370 | High | Low | High | Low | Low | High |
| Izumi, 2019 (abstract)50 | Some concerns | High | High | Low | Some concerns | High |
| Kim, 202051 | Some concerns | Low | Low | Low | Low | Some concerns |
| Koda, 200152 | Low | Low | Low | Low | Low | Low |
| Lencioni, 200371 | Low | Low | Low | Low | Low | Low |
| Lin, 200459 | Low | Low | Low | Low | Low | Low |
| Lin, 200553 | Some concerns | Low | Low | Low | Low | Some concerns |
| Liu, 201672 | Some concerns | Low | Low | Low | Low | Some concerns |
| Mizuki, 201060 | Low | Some concerns | Some concerns | Low | Low | Some concerns |
| Ng, 201779 | Low | Low | Low | Low | Low | Low |
| Orlacchio, 201461 | Some concerns | Low | Some concerns | Low | Low | Some concerns |
| Peng, 201274 | Low | Low | Low | Low | Low | Low |
| Shibata, 200262 | Low | Low | Some concerns | Low | Low | Some concerns |
| Shibata, 200954 | High | Low | Low | Low | Low | High |
| Shiina, 200555 | Some concerns | Low | Low | Low | Low | Some concerns |
| Vietti Violi, 201863 | Low | Some concerns | Low | Low | Low | Some concerns |
| Xia, 202075 | Low | Low | Low | Low | Low | Low |
| Yan, 201656 | High | Some concerns | Low | Low | Low | High |
| Zhang, 200776 | Low | Low | Low | Low | Low | Low |
| Zou, 201757 | Some concerns | Low | Low | Low | Low | Some concerns |
| Total |
High: 5
Some concerns: 15 Low: 15 |
High: 5
Some concerns: 3 Low: 27 |
High: 8
Some concerns: 3 Low: 24 |
Low: 35 |
Some concerns: 1
Low: 34 |
High: 12
Some concerns: 14 Low: 9 |
Generally, methods were poorly reported. There was either a high RoB or some concerns arising from the randomisation process in 20/35 of the RCTs assessed. Most RCTs had a low RoB for domains relating to deviations from the intended intervention (27/35), missing outcome data (24/35) and selective outcome reporting (34/35 had a low RoB for the most relevant outcome). All RCTs had a low RoB relating to measurement of the outcome, using computerised tomography (CT) (or magnetic resonance imaging) for assessment of tumour response, progression and recurrence. The overall judgement of RoB was low for 9 RCTs and high for 12 RCTs, and there were some concerns for 14 RCTs.
Results of RCTs included in the review
A table of study characteristics and results is presented in Appendix 4.
Radiofrequency ablation versus microwave ablation
Three RCTs compared RFA with MWA. One was assessed as having a high RoB64 and the other two as having some concerns. 62,63 One RCT included 152 participants with tumours up to 4 cm but only a small minority of patients had tumours > 3 cm. 63 The other two RCTs only reported the number of tumours ≤ 3 cm (n = 87 and n = 88) rather than the number of patients. 62,64
Only one RCT (with some RoB concerns) reported OS and recurrence outcomes. 63 OS was similar between the two treatment groups at 2 years (RFA 84% vs. MWA 86%). More patients in the RFA group had experienced recurrence (local tumour progression) at 2 years (12% vs. 6%; RR 1.62, 95% CI 0.66 to 3.94), but the median TTP was longer after RFA than after MWA (16 months vs. 12 months; HR 0.72, 95% CI 0.44 to 1.18).
There was a high rate of complete response or complete ablation of tumours in both the RFA and MWA arms in all three RCTs. A slightly higher proportion of HCC nodules showed complete response after RFA in one RCT (96% vs. 89%),62 whereas in the other two RCTs the rates were similar between treatment arms.
One RCT reported a higher rate of major complications with MWA than with RFA (RFA 3% vs. MWA 11%). 62 Another RCT reported that grade IV AEs only occurred in the MWA arm (0 vs. 2%), but more grade III (3% vs. 0%) and grade I–II (11.5% vs. 5%) AEs occurred in the RFA arm. 63 The RCT at high RoB reported that there were no major complications in either group. 64
Radiofrequency ablation versus percutaneous ethanol injection
Seven RCTs compared RFA with PEI (n = 1061 patients in six RCTs; the other RCT included 48 tumours). Three RCTs had a low RoB,44,59,71 three were judged to have some concerns53,55,65 and one had a high RoB. 48 One RCT included two different PEI arms with either a low dose or a high dose of PEI. 59 One RCT compared RFA versus PEI versus RFA in combination with PEI; the results of the combined RFA + PEI group are reported in the relevant sections below. 65 One RCT included patients with tumours ≤ 5 cm, but a large proportion had tumours ≤ 3 cm. 71
Six of the seven RCTs reported OS (see Table 4). 44,48,53,55,59,71 OS was better after treatment with RFA in four of the RCTs, which were at low RoB59,71 or had some concerns. 53,55 OS was similar between groups in one high-quality RCT44 and one low-quality RCT. 48
| RFA | PEI | High-dose PEI | Study | |
|---|---|---|---|---|
| 1 year | 95% | 95% | - | Giorgio, 2011 |
| 100% | 96% | - | Lencioni, 2003 | |
| 1–2 cm: 96% / 2.1–3 cm: 89% | 1–2 cm: 94% / 2.1–3 cm: 84% | 1–2 cm: 93% / 2.1–3 cm: 83% | Lin, 2004 | |
| 93% | 88% | - | Lin, 2005 | |
| 2 years | 90% | 83% | - | Giorgio, 2011 |
| 98% | 88% | - | Lencioni, 2003 | |
| 1–2 cm: 84% / 2.1–3 cm: 78% | 1–2 cm: 78% / 2.1–3 cm: 70% | 1–2 cm: 80% / 2.1–3 cm: 71% | Lin, 2004 | |
| 81% | 66% | - | Lin, 2005 | |
| 3 years | 26 deaths/70 patients | 28 deaths/69 patients | - | Brunello, 2008 |
| 83% | 78% | - | Giorgio, 2011 | |
| 1–2 cm: 78% / 2.1–3 cm: 73% | 1–2 cm: 70% / 2.1–3 cm: 62% | 1–2 cm: 72% / 2.1–3 cm: 64% | Lin, 2004 | |
| 74% | 51% | - | Lin, 2005 | |
| 4 years | 73% | 70% | - | Giorgio, 2011 |
| 74% | 57% | - | Shiina, 2005 | |
| 5 years | 70% | 68% | - | Giorgio, 2011 |
Event-free survival (survival free of local recurrence, new HCC and extrahepatic metastases) was also higher after RFA than after PEI in one high-quality RCT (2-year rate: 64% vs. 43%). 71 Two RCTs (one high quality,59 one with some concerns53) reported that cancer-free survival was higher after RFA than after PEI at 1, 2 and 3 years [e.g. 3-year rate (tumours 2.1–3 cm): RFA 40% vs. low-dose PEI 30% vs. high-dose PEI 32%59].
Five RCTs reported recurrence44,48,53,55,71 or local tumour progression. 59 The outcome measures reported differed between RCTs (e.g. distant intrahepatic recurrence,44 local recurrence,48,71 etc). In the five better-quality RCTs (low RoB or some concerns), recurrence or local tumour progression occurred in more patients in the groups that received PEI,44,53,55,59,71 although the difference was only small in one RCT (distant intrahepatic recurrence: RFA 32/70 vs. PEI 35/6944). One of these RCTs reported results by tumour size. Local tumour progression was similar between groups for smaller tumours (1–2 cm diameter) (3-year rate: RFA 9% vs. low-dose PEI 13% vs. high-dose PEI 12%), but it occurred in more patients with larger tumours (2.1–3 cm) after PEI treatment (RFA 18% vs. low-dose PEI 37% vs. high-dose PEI 33%). 59 In one low-quality RCT, the rate of local recurrence was similar between the two arms (5-year rate: RFA 11.7% vs. PEI 12.8%). 48
Four RCTs reported a higher proportion of patients achieving complete response or complete ablation with RFA treatment than with PEI treatment. 44,53,65,71
Findings on AEs were mixed, with some RCTs reporting worse AEs after RFA53,55,59,71 and others reporting similar rates between treatment groups. 44,48 One high-quality RCT44 and one low-quality RCT48 reported a similar rate of major complications in each arm (RFA 2/70 vs. PEI 2/69;44 RFA 0.9% vs. PEI 1.9%48). The rate of treatment-emergent AEs was also similar in the high-quality RCT (RFA 14.3% vs. PEI 17.4%). 44 In two RCTs, serious AEs were uncommon but only occurred in the RFA group (1.9% vs. 0;59 4.8% vs. 053). AEs were also worse in the RFA group in the other two RCTs (RFA 32 vs. PEI 19 events;71 RFA 5.1% vs. PEI 2.6% grade ≥ III events55). One RCT reported only that there were no mortalities related to either treatment. 65
Five RCTs reported economic outcomes. 44,48,53,55,59 Two RCTs reported the direct medical costs of the procedures (see Appendix 4 for details). 44,48 Three RCTs reported average length of hospital stay, which was considerably longer for patients who received RFA in two RCTs (RFA 4.2 days vs. PEI 1.7 days;53 RFA 4.4 days vs. low-dose PEI 1.6 days vs. high-dose PEI 2.1 days59), but considerably longer for patients who received PEI in one RCT (RFA 10.8 days vs. PEI 26.1 days55).
Radiofrequency ablation versus percutaneous acid injection
Only one RCT compared RFA with PAI, and it was judged to have some RoB concerns (n = 187 patients). 53 OS was better in the RFA arm than the PAI arm (3-year survival: 74% vs. 53%; 10/62 deaths vs. 15/63 deaths). Cancer-free survival (3-year rate: 43% vs. 23%) and recurrence (3-year rate: 14% vs. 31%; 8 vs. 17 local recurrence events) were also better after treatment with RFA. Complete response was achieved in a similar proportion of tumours in each group (RFA 96.1% vs. PAI 92.4%). However, three serious AEs occurred in the RFA group (4.8% of patients) and none in the PAI group. Mean length of hospital stay was longer for patients who received RFA than for those receiving PAI (4.2 days vs. 2.2 days).
Radiofrequency ablation versus laser ablation
Three RCTs compared RFA with laser ablation, with all three assessed as having some RoB concerns (n = 132 patients). 57,61,77 One RCT included patients with tumours ≤ 4 cm, but the mean tumour size was 2.4 cm. 61 One RCT included a subgroup of patients with tumours ≤ 2.5 cm. 77
Only one of the RCTs reported survival or progression outcomes. 61 There were no deaths in either treatment group, but PFS (1-year rate: RFA 86% vs. laser ablation 54%) and local disease progression (2/15 patients vs. 6/15 patients) were better in the RFA group than the laser ablation group.
Two RCTs reported complete response or complete ablation. In one RCT the proportion of tumours with complete ablation was higher in the RFA arm after both one procedure (86.7% vs. 66.7% of nodules) and two procedures (93% vs. 87%). 61 In the other RCT the complete response rate was similar between arms (RFA 92.3% vs. laser ablation 88.6%). 57
One RCT measured patient satisfaction, using a self-made satisfaction questionnaire that included intraoperative discomfort, postoperative therapy effects, adverse reactions and physical recovery. 57 There was greater satisfaction with the laser ablation treatment than with RFA [great satisfaction (score 61–100 out of 100): RFA 64.1% vs. laser ablation 85.7%]. 30.8% of patients were dissatisfied (score < 60) after RFA, compared with just 5.7% of patients who received laser ablation.
All three RCTs reported AE results. One reported considerably more AEs (intra- or post-procedural) in patients who received RFA (93.3% vs. 13.3%), although there were no major complications in either arm. 61 In one RCT, postoperative rates of fever, nausea, vomiting, diarrhoea, abdominal pain and skin rash were similar between the two treatments. 57 The other RCT reported no major or minor complications during the procedures in either group. 77
Radiofrequency ablation versus resection
Seven completed RCTs compared RFA with surgical resection (n = 912 patients). 46,50,58,67,69,75,79 One ongoing RCT was also identified. 80 One RCT did not report any data for the relevant subgroup (HCC ≤ 2 cm; the full population included patients with tumours < 4 cm, and the proportion with tumours ≤ 3 cm was not stated) and so RoB was not assessed. 58 Another RCT, which was judged to have some RoB concerns, did not report any relevant data for the ≤ 3 cm subgroup other than a KM curve. 67 Of the remaining RCTs, two were judged to have low RoB,75,79 two had high RoB50,69 and one had some concerns. 46 One of the RCTs recruited patients with recurrent HCC. 75
Four RCTs reported OS,46,69,75,79 with mixed findings. In one high-quality RCT79 and one low-quality RCT,69 OS at 1, 3 and 5 years was better after surgical resection [5-year rate: RFA 69% vs. resection 76%;79 5-year rate: RFA 61.4%/45.2% (solitary tumours/multifocal tumours) vs. resection 82.2%/69.2%69]. However, the RCT with some RoB concerns reported slightly better OS at 1, 2 and 3 years in the group that received RFA (3-year rate: 82.5% vs. 77.5%). 46 The high-quality RCT of recurrent HCC found that the two treatments were similar [HR (RFA vs. resection) 1.05, 95% CI 0.67 to 1.65]. 75
There were also mixed findings from the four RCTs that measured disease- or recurrence-free survival. 46,50,75,79 Recurrence-free survival was similar between treatment groups in one low-quality RCT (3-year rate: RFA 47.7% vs. surgery 49.8%; HR 0.96)50 and the high-quality RCT of recurrent HCC patients (repeat-recurrence-free survival: HR 1.07, 95% CI 0.71 to 1.675). The other high-quality RCT reported better disease-free survival after resection (5-year rate: 46% vs. 52%). 79 However, the disease-free survival rate was higher for patients who received RFA in the RCT with some RoB concerns (3-year rate: 55.4% vs. 41.3%). 46
Only the RCT with some RoB concerns reported recurrence of HCC, with a similar proportion of patients experiencing recurrence in the RFA group as in the hepatectomy group (22/60 vs. 21/60). 46 This was also the only RCT to report on response, with a similar rate of complete tumour treatment after RFA as after surgery (57/60 vs. 58/60).
There were limited data on AEs reported. One RCT reported that postoperative complications (RFA 2/60 vs. resection 17/60), major complications (1/60 vs. 14/60) and serious pain requiring analgesia (3/60 vs. 43/60) were all more common after surgery than after RFA. 46 Four RCTs reported that there was no mortality related to the treatment or within the hospital admission period in either arm. 46,50,58,69
Two RCTs reported average length of hospital stay, which was shorter for patients receiving RFA than resection in both RCTs (4 days vs. 7 days;79 4.3 days vs 11.8 days46). Length of intensive care unit (ICU) stay was also shorter after RFA (0 days vs. 6 days). 46
Radiofrequency ablation versus proton beam radiotherapy
One RCT of patients with recurrent or residual tumours compared RFA with proton beam radiotherapy and was judged to have some RoB concerns (n = 144 patients). 51 OS was similar between the treatment groups (4-year rate: RFA 77.0% vs. proton beam radiotherapy 75.4%; HR at 2 years 1.07, 95% CI 0.58 to 1.98). PFS was also similar between treatment groups, with a median of 13.4 months after proton beam radiotherapy and 13.7 months after RFA. The rate of PFS was the same at 2 years (31.9% vs. 31.9%; HR 0.99, 95% CI 0.70 to 1.41), slightly higher after proton beam therapy at 3 years (17.9% vs. 26.3%), with a smaller difference between groups at 4 years (12.6% vs. 18.7%). The total number of progression events was greater in the RFA group (62/72 vs. 56/72). There were nine (16%) AEs at grade III or above in the RFA group compared with none in the proton beam radiotherapy group.
Radiofrequency ablation versus radiofrequency ablation + transarterial chemoembolisation
Three RCTs compared RFA alone versus RFA combined with TACE (n = 220 patients). 43,54,74 One included patients with recurrent HCC and was judged to be at low RoB. 74 The other two RCTs were at high RoB,43,54 although one was only reported as a conference abstract, with very limited reporting of methods. 43
All three RCTs reported OS. In the high-quality RCT of patients with recurrent HCC, OS was better at 1 and 3 years after RFA combined with TACE (3-year rate: RFA 60% vs. RFA + TACE 70%), but was the same in both arms at 5 years (50% vs. 50%). 74 Similarly, in one low-quality RCT, survival was better in the combined treatment arm at 2 years (88.8% vs. 100%) but similar by 3 and 4 years (4-year rate: RFA 74% vs. RFA + TACE 72.7%). 54 Overall the total number of deaths was similar between treatment arms in this RCT (5/46 vs. 6/43). However, in the other low-quality RCT, OS was better after treatment with RFA combined with TACE at 2 and 3 years (3-year rate: 73.9% vs. 84%), but similar at 1 year (RFA: 100% vs. RFA + TACE: 95.2%). 43
Recurrence-free survival was higher after RFA combined with TACE in the high-quality RCT of patients with recurrent HCC (5-year rate: 26% vs. 48%). 74 One low-quality RCT reported higher local PFS in the combined treatment group at 2 years (74.1% vs. 81.1%) but a higher rate in the RFA-alone group at 3 and 4 years (4-year rate: 61.7% vs. 55.8%). 54 However, event-free survival (time from the beginning of treatment to last follow-up CT examination, local tumour progression, new lesions in the liver, distant metastasis, or death) was better after the combined treatment at 2, 3 and 4 years (4-year rate: 29.7% vs. 36.6%).
The two low-quality RCTs43,54 both reported a similar rate of local tumour progression in both treatment groups (3-year rate: 8.7% vs. 9.5%;43 3-year rate: 14.4% vs. 17.6%54). Only one RCT reported response, with 100% of patients achieving complete response in both arms. 54 The rate of major complications was the same between treatment groups in the two low-quality RCTs. 43,54
Radiofrequency ablation versus radiofrequency ablation + percutaneous ethanol injection
Three RCTs compared RFA treatment alone versus RFA combined with PEI (n ≥ 147 patients). 65,66,76 One was judged to have a low RoB,76 one some concerns65 and one a high RoB. 66
Overall survival was reported by two RCTs (one high quality and one low quality). 66,76 Both reported higher OS after treatment with RFA combined with PEI than after RFA alone (5-year rate: RFA 50.2% vs. RFA + PEI 55.3%;76 2-year rate: RFA 64.9% vs. RFA + PEI 79.0%66). In the low-quality RCT there was also more HCC recurrence after RFA treatment alone (2-year rate: 34.1% vs. 20.9%). 66
Two RCTs reported data on response. 65,76 In both RCTs the rate of complete ablation was higher after one treatment of RFA combined with PEI than after one session of RFA alone. After two sessions of treatment (if necessary), the rate was similar between groups in the high-quality RCT76 but remained higher in the RFA + PEI group in the RCT with some concerns (87.5% vs. 100%65).
Very limited data on AEs were reported. The two lower-quality RCTs reported that there were no serious AEs66 or mortalities related to treatment65 in either arm.
Radiofrequency ablation versus radiofrequency ablation + iodine-131 metuximab
One RCT with some RoB concerns compared RFA alone with RFA and iodine-131 metuximab but reported limited data for the relevant subgroup of patients with tumours < 3cm (n = 78 patients). 78 There was less recurrence in the group that received RFA combined with iodine-131 metuximab (HR 0.46, 95% CI 0.21 to 1.01). There were no serious AEs or treatment-related deaths in either group.
Radiofrequency ablation versus radiofrequency ablation + iodine-125
One RCT with a low RoB compared RFA alone versus RFA and iodine-125 (n = 136 patients). 45 OS was better after the combined treatment than after RFA alone (RFA: mean 70.8 months vs. RFA + iodine-125: 95.8 months; 36/68 vs. 23/68 deaths; HR 0.502, 95% CI 0.313 to 0.806). There was also less recurrence in patients who received the combined treatment (39/68 patients vs. 27/68 patients; HR 0.508, 95% CI 0.317 to 0.815; mean time to recurrence 66.8 vs. 93 months). Complete ablation was achieved in more patients with one treatment of RFA + iodine-125 than with one treatment of RFA alone, although after two treatments all participants in both arms had achieved complete ablation. There were more AEs at grade III or above after RFA combined treatment than after RFA alone (11 vs. 15 events; patient numbers not reported), although there were no procedure-related mortalities and no iodine-125 seed migration from the liver to the heart or other organs.
Radiofrequency ablation versus radiofrequency ablation + chemotherapy
One RCT with a high RoB compared RFA alone versus RFA combined with chemotherapy (n = 38 patients). 47 Recurrence was higher in the RFA group than in the RFA + chemotherapy group at 1 year (50% vs. 27%). There were no serious AEs in either group.
Radiofrequency ablation + transarterial chemoembolisation versus resection
One RCT compared RFA combined with TACE versus partial hepatectomy, and it was judged to have some RoB concerns (n = 135 patients). 72 The paper did not report any relevant efficacy data for the subgroup of patients with tumours ≤ 3 cm. However, KM curves for OS and recurrence showed that hepatectomy was more effective than RFA + TACE. There was no 30- or 90-day mortality in either arm.
Percutaneous ethanol injection versus percutaneous acid injection
One RCT compared PEI with PAI and was judged to have some RoB concerns (n = 187 patients). 53 OS (3-year rate: PEI 51% vs. PAI 53%; number of deaths 17/62 vs. 15/63), cancer-free survival (3-year rate: 21% vs. 23%), recurrence (3-year rate 34% vs. 31%; number of events 19/55 vs. 17/58) and complete response (88.1% vs. 92.4%) were all similar between arms. No serious AEs were reported in either arm. The average length of hospital stay was also similar between PEI and PAI groups (1.7 days vs. 2.2 days).
Percutaneous ethanol injection versus resection
One RCT with high RoB compared PEI with resection (n = 82 patients). 49 There was a higher rate of OS in the PEI arm at 2 and 3 years (3-year rate 96.7% vs. 88.1%) but by 4 years it was similar (92.1% vs. 88.1%) and at 5 years it was higher in the resection arm (46.0% vs. 81.8%). PFS was higher after resection at 1, 2, 3 and 4 years (4-year rate: 44.6% vs. 56.2%) but was similar by 5 years (44.6% vs. 48.2%). There was more recurrence of HCC in the PEI group (18/40 vs. 15/42 patients). Three patients had adverse effects after PEI, but for the resection arm the paper only reported that there were no significant complications.
Percutaneous ethanol injection versus radiofrequency ablation + percutaneous ethanol injection
One RCT compared PEI alone versus RFA combined with PEI and was judged to have some RoB concerns (n = 48 tumours). 65 The only relevant data reported were on complete response. After both one and two treatment sessions, no tumours in the PEI arm had been completely ablated, compared with 93.8% and 100%, respectively, in the RFA + PEI arm. Only 81.25% of tumours in the PEI group achieved complete ablation after all sessions. There were no mortalities related to either treatment.
Percutaneous ethanol injection versus percutaneous ethanol injection + transarterial chemoembolisation
Two RCTs compared PEI alone with PEI combined with TACE (n = 82 patients). One had a low RoB52 and one had some bias concerns. 60 The two RCTs differed in their results. The high-quality RCT reported higher OS rates in the PEI + TACE arm at 1, 2 and 3 years (3-year rate: PEI 65.9% vs. PEI + TACE 80.8%), although it was similar between groups at 5 years (37.7% vs. 40.4%). 52 Rates of local residual disease (5-year rate 39.3% vs. 19.3%) and new nodular recurrence (5-year rate 100% vs. 50.2%) were lower after the combined PEI and TACE treatment. However, the lower-quality RCT reported a longer mean OS (57.2 vs. 42.4 months) and fewer deaths (6/14 vs. 8/13) in the PEI-alone arm. 60 Recurrence was also higher in the combined treatment arm (71.4% vs. 84.6%). However, the mean length of cancer-free survival was longer after PEI + TACE (16.7 vs. 22.9 months). 60
The high-quality RCT reported two major complications (among 26 patients) in the combined treatment group and none in the PEI-alone group. Fever, continuous abdominal pain and transient increases in C-reactive protein were common AEs in both treatment groups. 52 The other RCT reported that no serious adverse effects or complications were related to either treatment. 60
Percutaneous acid injection versus percutaneous acid injection + transarterial chemoembolisation
One RCT with a high RoB compared PAI versus sequential TACE and PAI treatment (n = 55 patients). 70 The rate of OS was 100% in both groups at 1 year, but at 3 years it was higher in the group that had received the combined treatment (49% vs. 73%). Data on cancer-free survival were not reported for the subgroup of patients with tumours ≤ 3 cm (other than that there were no significant differences between treatment groups). There were no serious complications necessitating intensive care in either group.
Percutaneous local ablative therapy versus resection
One RCT with high RoB compared percutaneous local ablative therapy (RFA, followed by RFA/PEI for any residual tumour, and TACE if residual tumour still remained) with partial hepatectomy (n = 79 patients). 68 The paper did not report any relevant data for the subgroup of patients with tumours ≤ 3 cm, other than a KM curve. However, it reported that there were no significant differences in OS or disease-free survival between the two treatment groups for the ≤ 3 cm subgroup.
Microwave ablation + sorafenib versus resection
One RCT with a high RoB compared treatment with MWA combined with sorafenib versus surgical resection (n = 120 patients). 56 Rates of OS and tumour-free survival were similar between the two treatments at 1, 3 and 5 years, but mean OS was longer in the MWA + sorafenib group than the resection group (64.6 vs. 51.2 months). However, at 5 years there had been more recurrence of HCC in the MWA + sorafenib group (38.3% vs. 18.3%). Pain, fever, abdominal bleeding and infection were all experienced by considerably more patients in the resection arm than the MWA + sorafenib arm (pain: MWA + sorafenib 23.3% vs. resection 63.3%; fever: 25% vs. 48.3%; abdominal bleeding: 3.3% vs. 11%; infection: 1.7% vs. 30%).
Ongoing trials
The electronic searches for RCTs undertaken on 3 February 2021 identified four potentially relevant ongoing RCTs: the published protocol by Zhu et al. 80 and three clinical trial register records, for which no further information was available. The searches for studies in progress and unpublished research, undertaken on 27 April 2021, identified 121 records in ClinicalTrials.Gov and 64 records in the European Union Clinical Trials Register; there was only one further potentially relevant ongoing RCT, after deduplication between databases. Further details are presented in Table 5.
| Study | Further details |
|---|---|
| Ongoing studies identified from searches of bibliographic databases for RCTs | |
| Zhu, 202180 | Published protocol for a single centre (The Ninth People’s Hospital of Chongqing, China) RCT comparing RFA vs. laparoscopic hepatectomy for small HCC (three or fewer tumours ≤ 3 cm in diameter). |
| ClinicalTrials.gov: NCT04727307 | Clinical trial register record describing a multicentre RCT comparing atezolizumab + bevacizumab combined with RFA vs. RFA alone for small HCC (one to three nodules < 3 cm). Sponsor: University Hospital, Montpellier, France. Actual study start date: 26 January 2021. Estimated primary completion date: January 2025. Estimated study completion date: July 2027. |
| ClinicalTrials.gov: NCT03790059 | Clinical trial register record describing a multicentre RCT comparing RFA combined with recombinant human adenovirus Type 5 (H101) injection vs. RFA alone for small HCC (single lesion ≤ 3 cm in diameter). Sponsor: Southwest Hospital, China. Study start date: October 2016. Estimated primary completion date: September 2020. Estimated study completion date: September 2020. |
| ClinicalTrials.gov: NCT04235660 | Clinical trial register record describing a single-centre pilot RCT comparing Y90 radioembolisation vs. stereotactic body radiation therapy for solitary early-stage (≤ 3 cm) HCC. Sponsor: Indiana University. Actual study start date: 22 July 2020. Estimated primary completion date: May 2024. Estimated study completion date: May 2024. |
| Studies identified from searches of ClinicalTrials.Gov and the European Union Clinical Trials Register for ongoing RCTs | |
| ClinicalTrials.gov: NCT04663035 | Clinical trial register record describing a single-centre RCT comparing ablation followed by tislelizumab (immunotherapy) vs. ablation alone for early recurrent HCC. Sponsor: Sun Yat-sen University. Actual study start date: 21 December 2020. Estimated primary completion date: December 2023. Estimated study completion date: December 2025. |
Network meta-analysis results
Randomised controlled trials assessing the clinical effectiveness of ablative and non-surgical therapies for patients with early or very early HCC have been discussed and summarised in Systematic review of RCTs. Four NMA models were produced, for the outcomes OS, PFS and overall recurrence and local recurrence.
Of the 37 RCTs described in Systematic review of RCTs, six did not report any relevant data for the subgroup of patients with tumours ≤ 3 cm that could be included in the NMA57,58,62,64,65,78 and one was ongoing, so no results were available. 80 A further three RCTs of patients with recurrent/residual HCC were not included. 51,74,75 Not all the resulting 27 RCTs included in the NMAs reported data for all four NMA outcomes; which RCTs reported for each outcome, as well as the type of data reported, are presented in Report Supplementary Material 3.
Due to the small number of RCTs in each network, there was little evidence to inform the between-study heterogeneity. The uniform (0,3) prior distribution was considered in exploratory analyses and found to be too influential on the results. The half-normal (0, 0.192) was used instead, as it expresses the prior belief that 95% of trials will give HRs within a factor of 2 from the estimated mean HR. 28 Results estimated using the half-normal (0, 0.502) prior distribution are also reported.
Results for checks on the proportional hazards assumption are presented in Report Supplementary Material 2. Schoenfeld residuals81 were calculated for RCTs that reported the numbers at risk for the included KM curve. For RCTs that did not report the numbers at risk,46,77,82 the proportional hazards assumption was assessed by visual inspection of the KM curves. For two trials (Aikata et al. 43 and Izumi et al. 50) the proportional hazards assumption could not be tested as there were no KM curves available.
There were four RCTs for which the KM curves for OS crossed over,46,48,52,70 which suggests that there may be some concerns about the proportional hazards assumption; however, for all other RCTs there was no statistical evidence that the assumption was violated. The validity of the NMAs depends on the proportional hazards assumption being correct, and more complex models with non-proportional hazards could not be fitted due to limitations of the data. Therefore, results should be interpreted with caution.
Overall survival
Data
Of the 27 RCTs that reported relevant data, 16 were included in the NMA for OS. Eleven RCTs were excluded from the NMA: two47,50 did not report OS data, and eight49,56,59,60,66–68,71 reported data that would require strong assumptions to be made in order to calculate log-HRs required for the NMA; Orlacchio et al. (2014)61 was also not included in the NMA as both arms in the trial reported zero deaths. Further details about the inclusion/exclusion of studies and how the evidence reported in the studies was transformed to a form suitable for NMA are summarised in the Report Supplementary Material 3.
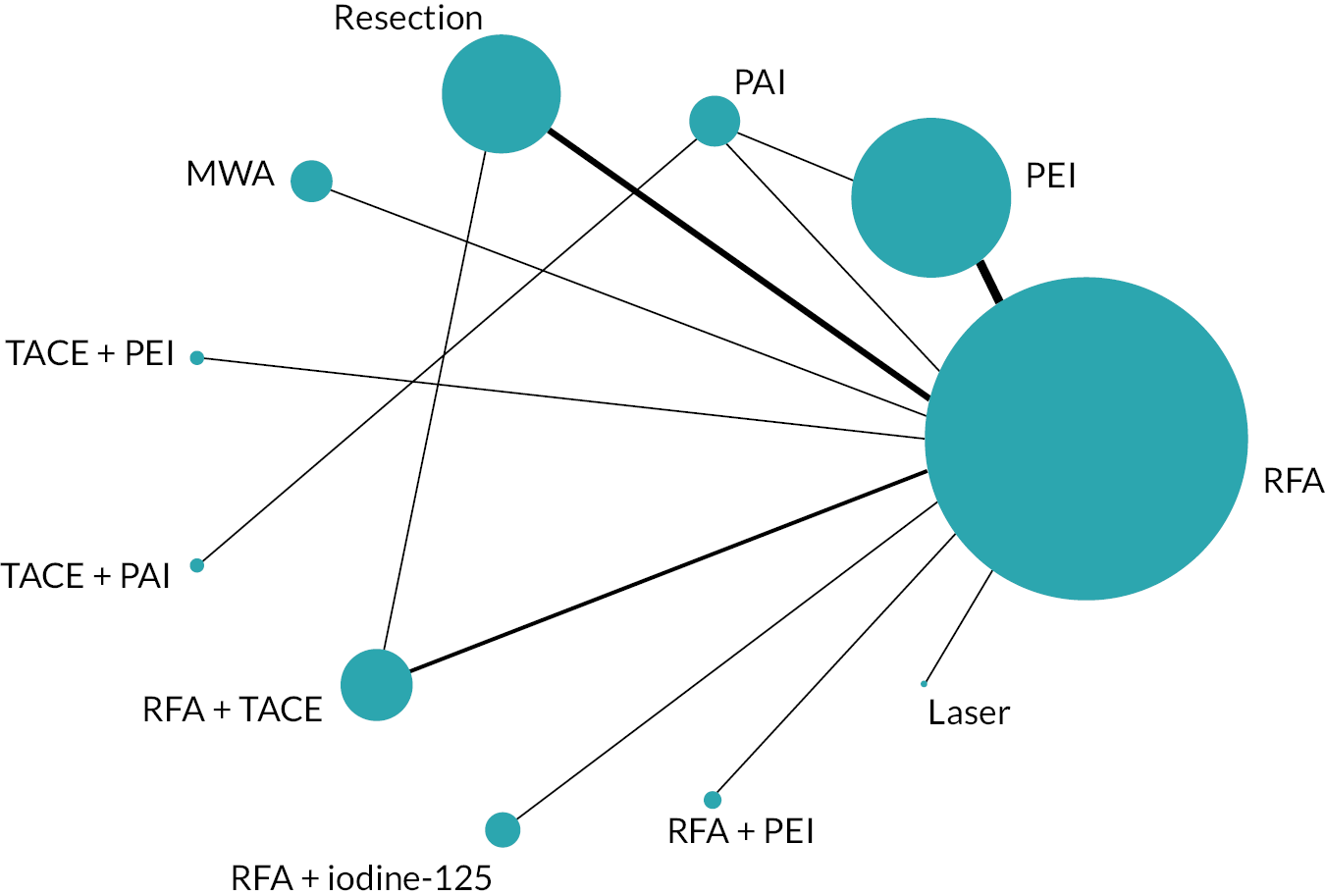
The network diagram for OS is presented in Figure 2. Fifteen two-arm trials and one three-arm trial provided evidence on 11 interventions. A summary of the data used for the NMA is provided in Report Supplementary Material 4.
FIGURE 2.
Network diagram for OS.
Model selection and consistency checking
Model fit parameters for the FE and RE models are presented in Report Supplementary Material 5. All three models fitted the data well, but as the difference in the DICs between the FE and RE models was < 3, the simpler FE model was chosen.
The 95% CrI for the RE model using the half-normal (0, 0.502) prior was almost twice as wide as the 95% CrI for the model using half-normal (0, 0.192), evidence that the priors for heterogeneity are influential due to few studies being included for each comparison in the network. Plots for the prior and posterior distributions of the between-study heterogeneity for the RE models are presented in Report Supplementary Material 5.
There was no evidence to suggest inconsistency in the network. Details of the inconsistency check and node-splitting results are presented in Report Supplementary Material 5.
Model results
Hazard ratios for OS for all treatments compared with RFA are presented in Figure 3.
FIGURE 3.
Plot of HRs for OS compared with RFA for the FE model.
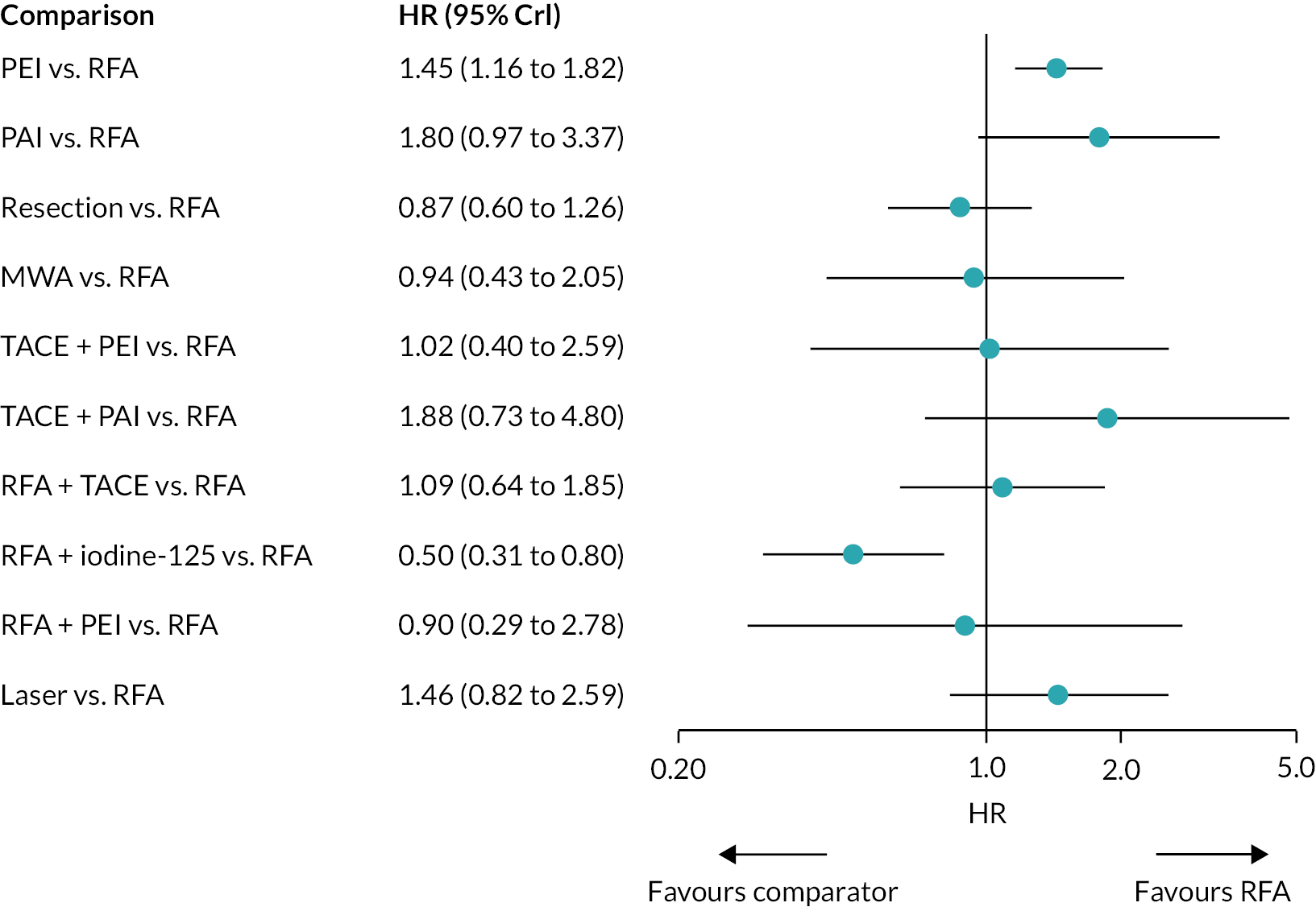
There was evidence to suggest that PEI worsens OS compared with RFA, and that RFA + iodine-125 improves OS compared with RFA (see Figure 3). There was also evidence to suggest that PEI worsens OS compared with resection, and that RFA + iodine-125 improves survival compared with PEI, PAI, TACE + PAI, RFA + TACE, and laser. There was insufficient evidence to suggest a difference in OS for all other treatment comparisons.
Hazard ratios comparing all treatment groups against each other for FE and RE models are reported in Report Supplementary Material 5. Results for RE models displayed more uncertainty than the FE model, where results estimated using the wider half-normal (0, 0.502) prior were more uncertain compared with results estimated using a half-normal (0, 0.192) prior.
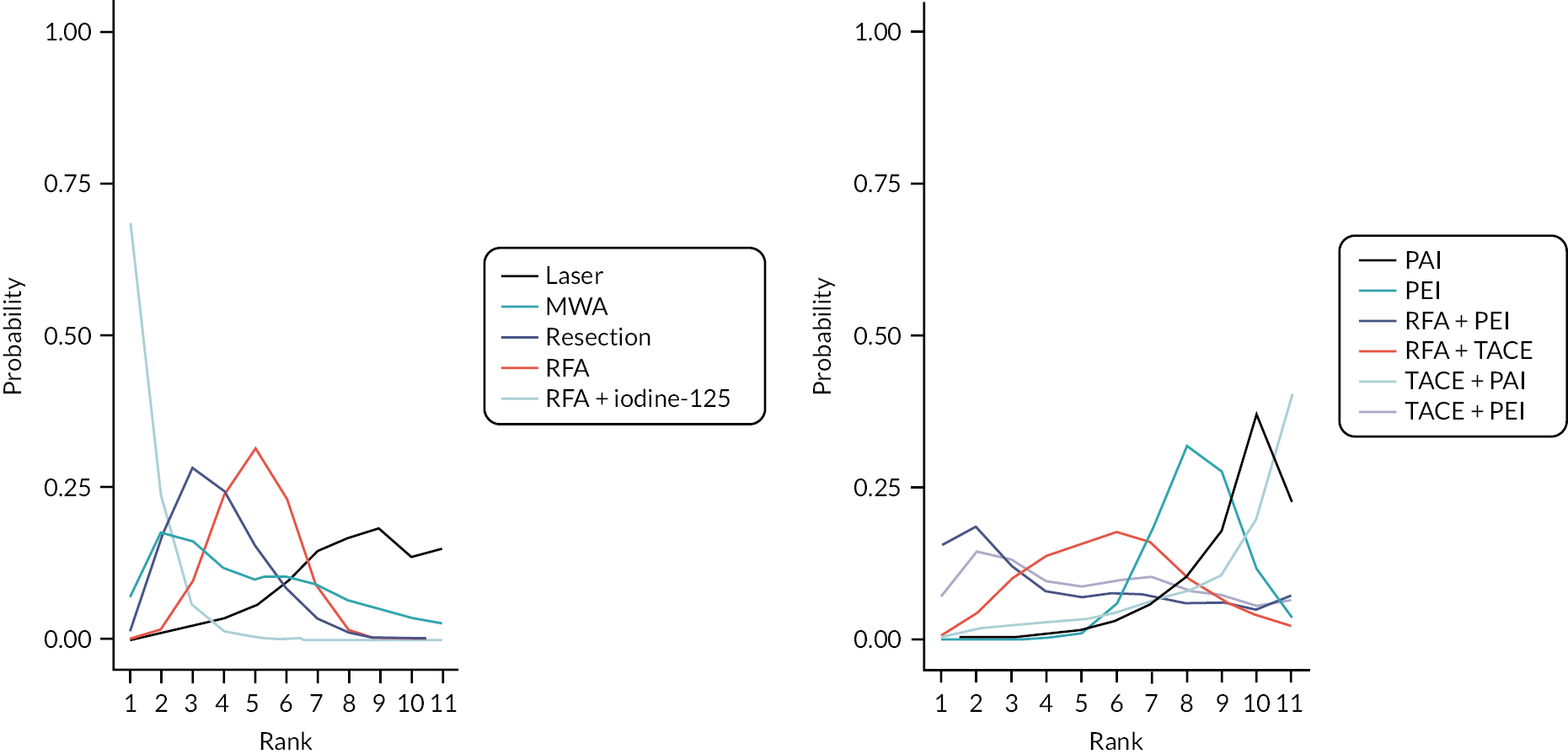
The mean and median ranks for each treatment, with their corresponding 95% CrIs, are presented in Table 6. RFA + iodine-125 had the highest probability of being ranked the best treatment. However, there was a high level of uncertainty in treatment rankings; all treatments apart from RFA + iodine-125 displayed very wide CrIs. In fact, MWA, RFA + PEI, and TACE + PEI had 95% CrIs that included all 11 potential treatment ranks.
| Treatments | Mean rank | Median rank | 95% CrI for the rank |
|---|---|---|---|
| RFA + iodine-125 | 1.42 | 1 | (1.00 to 3.00) |
| Resection | 3.84 | 4 | (2.00 to 7.00) |
| MWA | 4.81 | 4 | (1.00 to 11.00) |
| RFA + PEI | 4.82 | 4 | (1.00 to 11.00) |
| RFA | 4.98 | 5 | (3.00 to 7.00) |
| TACE + PEI | 5.42 | 5 | (1.00 to 11.00) |
| RFA + TACE | 5.90 | 6 | (2.00 to 10.00) |
| Laser | 8.07 | 8 | (3.00 to 11.00) |
| PEI | 8.28 | 8 | (6.00 to 11.00) |
| TACE + PAI | 9.11 | 10 | (3.00 to 11.00) |
| PAI | 9.34 | 10 | (5.00 to 11.00) |
The treatment rank plot for OS (see Figure 4) also shows that RFA + iodine-125 had the highest probability of being the best treatment; however, the uncertainty in treatment ranks is also evident, as the probability of all other treatment ranks is < 50%.
FIGURE 4.
Rank plot for OS for the FE model.
Progression-free survival
Data
Of the 27 RCTs that reported relevant data, six were included in the NMA for PFS. Twenty-one RCTs were excluded from the NMA: 1443–45,47,48,52,55,66,67,69,70,72,76,77 did not report PFS data, and five49,56,59,60,68 reported data that would require strong assumptions to be made in order to calculate log-HRs required for the NMA; a further two61,63 were excluded as they only reported local disease-free survival/PFS. Details about the inclusion/exclusion of studies and how the evidence reported in the studies was transformed into a form suitable for NMA are summarised in Report Supplementary Material 3.
The network diagram for PFS is presented in Figure 5. Five two-arm trials and one three-arm trial provided evidence on six interventions. A summary of the data used for the NMA is provided in Report Supplementary Material 4.
FIGURE 5.
Network diagram for PFS.

Model selection and consistency checking
Model fit parameters for the FE and RE models are presented in Report Supplementary Material 5. All three models fit the data well, but as the difference in DICs between the fixed and RE models was < 3, the simpler FE model was chosen.
The between-study heterogeneity was low for the two RE models; however, the 95% CrI for the model using the half-normal (0, 0.502) prior was almost twice as wide as the 95% CrI for the model using the half-normal (0, 0.192) prior, evidence that the priors for heterogeneity are influential due to few studies being included in the network. Plots for the prior and posterior distributions of the between-study heterogeneity for the RE models are presented in Report Supplementary Material 5.
There is no potential for inconsistency in this network as there is no independent, indirect evidence for any of the comparisons – the single loop is formed by a three-arm study. 53
Model results
Hazard ratios for PFS for all treatments compared with RFA are presented in Figure 6.
FIGURE 6.
Plot of HRs for PFS compared with RFA for the FE model.

There was evidence to suggest that PEI and PAI are associated with worse PFS compared with RFA (see Figure 6). There was insufficient evidence to suggest a difference in PFS for all other treatment comparisons.
Hazard ratios comparing all treatment groups against each other for FE and RE models are reported in Report Supplementary Material 5. Results for RE models displayed more uncertainty compared with the FE model, where results estimated using the wider half-normal (0, 0.502) prior were more uncertain compared with results estimated using a half-normal (0, 0.192) prior.
The treatment rank plot for PFS is presented in Figure 7, and the mean and median ranks for each treatment, with their corresponding 95% CrIs are presented in Table 7. RFA + TACE had the highest probability to be ranked the best treatment. However, there was a high level of uncertainty in the treatment ranking – all treatments displayed wide CrIs for ranks.
FIGURE 7.
Rank plot for PFS for the FE model

| Treatments | Mean rank | Median rank | 95% CrI for the rank |
|---|---|---|---|
| RFA + TACE | 1.53 | 1 | (1.00 to 4.00) |
| RFA | 2.24 | 2 | (1.00 to 3.00) |
| Resection | 2.38 | 2 | (1.00 to 4.00) |
| PEI | 4.12 | 4 | (3.00 to 5.00) |
| PAI | 4.73 | 5 | (3.00 to 5.00) |
Overall recurrence
Data
Of the 27 RCTs that reported relevant data, seven were included in the NMA for overall recurrence. Twenty RCTs were excluded from the NMA: 1943,48,50,52–54,59,61,63,66–72,76,77,79 did not report overall recurrence data, and one reported distant recurrence. 44 Details about the inclusion/exclusion of RCTs and how the evidence reported was transformed into a form suitable for NMA are summarised in Report Supplementary Material 3.
The network diagram for overall recurrence is presented in Figure 8. Seven two-arm RCTs provided evidence on seven interventions. A summary of the data used for the NMA is provided in Report Supplementary Material 4.
FIGURE 8.
Network diagram for overall recurrence.

Model selection and consistency checking
Model fit parameters for the FE and RE models are presented in Report Supplementary Material 5. All three models fit the data well, but as the difference in DICs between the fixed and RE models was < 3, the simpler FE model was chosen.
The between-study heterogeneity was low for the two RE models; however, the 95% CrI for the model using the half-normal (0, 0.502) prior was almost twice as wide as the 95% CrI for the model using the half-normal (0, 0.192) prior, evidence that the priors for heterogeneity are influential due to few studies being included in the network. Plots for the prior and posterior distributions of the between-study heterogeneity for the RE models are presented in Report Supplementary Material 5.
There was no evidence to suggest inconsistency in the network. Details of the inconsistency check and node-splitting results are presented in Report Supplementary Material 5.
Model results
Relative risks for overall recurrence for all treatments compared with RFA are presented in Figure 9.
FIGURE 9.
Plot of RRs for overall recurrence compared with RFA for the FE model.
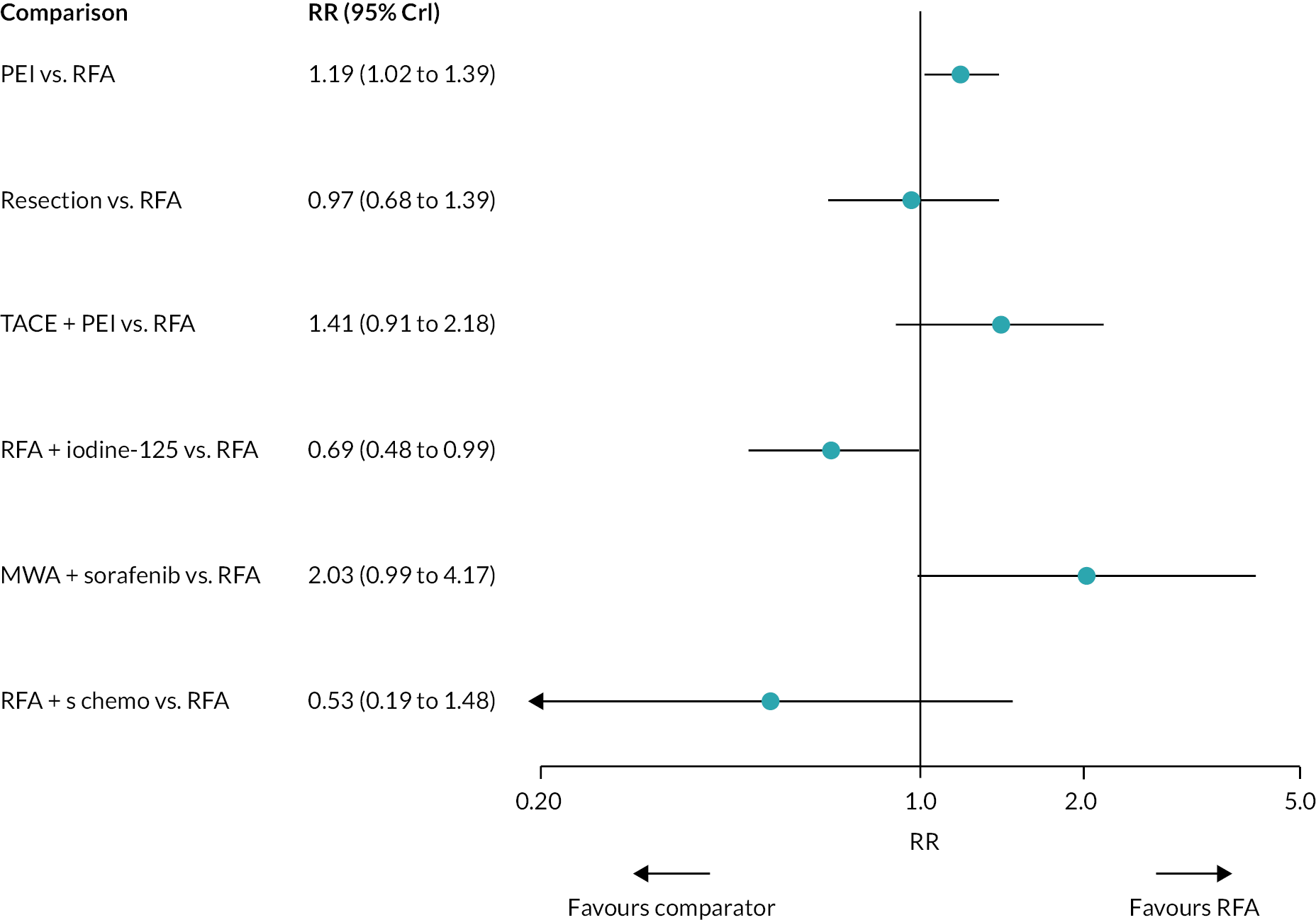
There was evidence to suggest that PEI increases the risk of overall recurrence compared with RFA (see Figure 9), and that RFA + iodine-125 decreases the risk of overall recurrence compared with RFA. The 95% CrIs of these estimates are very close to the ‘null’ effect.
There was evidence to suggest that RFA + iodine-125 decreases the risk of overall recurrence compared with PEI and TACE + PEI.
There was evidence to suggest that MWA + sorafenib increases the risk of overall recurrence compared with resection, and that RFA + iodine-125 and RFA + systemic chemotherapy decrease the risk of overall recurrence compared with MWA + sorafenib. There was insufficient evidence to suggest a difference in overall recurrence for all other treatment comparisons.
Relative risks comparing all treatment groups against each other for FE and RE models are reported in Report Supplementary Material 5. Alternative models using arm-level data gave similar results. Results for RE models displayed more uncertainty compared with the FE model, where results estimated using the wider half-normal (0, 0.502) prior were more uncertain compared with results estimated using a half-normal (0, 0.192) prior.
The treatment rank plot for overall recurrence is presented in Figure 10, and the mean and median ranks for each treatment, with their corresponding 95% CrIs, are presented in Table 8. RFA + systemic chemotherapy had the highest probability of being ranked the best. There was a high level of uncertainty in treatment rankings – all treatment ranks displayed wide CrIs.
FIGURE 10.
Rank plot for overall recurrence for the FE model.

| Treatments | Mean rank | Median rank | 95% CrI for the rank |
|---|---|---|---|
| RFA + systemic chemotherapy | 1.70 | 1 | (1.00 to 6.00) |
| RFA + iodine-125 | 1.81 | 2 | (1.00 to 3.00) |
| Resection | 3.44 | 3 | (2.00 to 6.00) |
| RFA | 3.52 | 4 | (2.00 to 5.00) |
| PEI | 5.06 | 5 | (4.00 to 6.00) |
| TACE + PEI | 5.79 | 6 | (3.00 to 7.00) |
| MWA + sorafenib | 6.67 | 7 | (4.00 to 7.00) |
Local recurrence
Data
Of the 27 RCTs that reported relevant data, 10 were included in the NMA for local recurrence. Seventeen44–47,49,50,55,56,60,67–70,72,76,77,79 did not report local recurrence data and were therefore excluded from the NMA. Details about the inclusion/exclusion of RCTs and how the evidence reported was transformed into a form suitable for NMA are summarised in Report Supplementary Material 3.
The network diagram for overall recurrence is presented in Figure 11. Eight two-arm and two three-arm RCTs provided evidence on nine interventions. A summary of the data used for the NMA is provided in Report Supplementary Material 4.
FIGURE 11.
Network diagram for local recurrence.

Model selection and inconsistency checking
Model fit parameters for the FE and RE models are presented in Report Supplementary Material 5. All three models fit the data well, but as the difference in DICs between the FE and RE models was < 3, a simpler FE model was chosen.
The between-study heterogeneity was low and consistent for the two RE models. However, the 95% CrI for the model using the half-normal (0, 0.502) prior was twice as wide as the 95% CrI for the model using the half-normal (0, 0.192). Plots for the prior and posterior distribution of the between-study heterogeneity for the RE models are presented in Report Supplementary Material 5.
There is no potential for inconsistency in this network as there is no independent, indirect evidence for any of the comparisons – the two loops in the network are formed by two separate three-arm studies. 53,59
Model results
Relative risks for local recurrence for all treatments compared with RFA are presented in Figure 12.
FIGURE 12.
Plot of RRs for local recurrence compared with RFA for the FE model.

There was evidence to suggest that PEI increases the risk of local recurrence compared with RFA (see Figure 12), and that RFA + PEI decreases the risk of local recurrence compared with PEI. There was insufficient evidence to suggest a difference in local recurrence for all other treatment comparisons.
Relative risks comparing all treatment groups against each other for FE and RE models are reported in Report Supplementary Material 5. Alternative models using arm-level data gave similar results. Results for RE models displayed more uncertainty compared with the FE model, where results estimated using the wider half-normal (0, 0.502) prior were more uncertain compared with results estimated using a half-normal (0, 0.192) prior.
The treatment rank plot for local recurrence is presented in Figure 13, and the mean and median ranks for each treatment, with their corresponding 95% CrIs, are presented in Table 9. RFA + PEI had the highest probability of being ranked the best, although this probability was < 50%. The level of uncertainty in the treatment ranks was high – all treatments, with the exception of laser for the ninth rank, had rank probabilities below 50%. All treatments also had very wide CrIs for their rank.
FIGURE 13.
Rank plot for local recurrence for the FE model.

| Treatments | Mean rank | Median rank | 95% CrI of the rank |
|---|---|---|---|
| RFA + PEI | 1.96 | 2 | (1.00 to 6.00) |
| TACE + PEI | 2.27 | 2 | (1.00 to 7.00) |
| RFA | 3.33 | 3 | (2.00 to 5.00) |
| RFA + TACE | 4.59 | 4 | (1.00 to 9.00) |
| MWA | 5.98 | 6 | (2.00 to 9.00) |
| High-dose PEI | 6.01 | 6 | (2.00 to 9.00) |
| PAI | 6.33 | 6 | (3.00 to 9.00) |
| PEI | 6.78 | 7 | (4.00 to 9.00) |
| Laser ablation | 7.75 | 9 | (2.00 to 9.00) |
Threshold analysis of RCT networks
Overall survival
The forest plot for the threshold analysis is presented in Figure 14.
FIGURE 14.
Forest plot for threshold analysis results for OS.
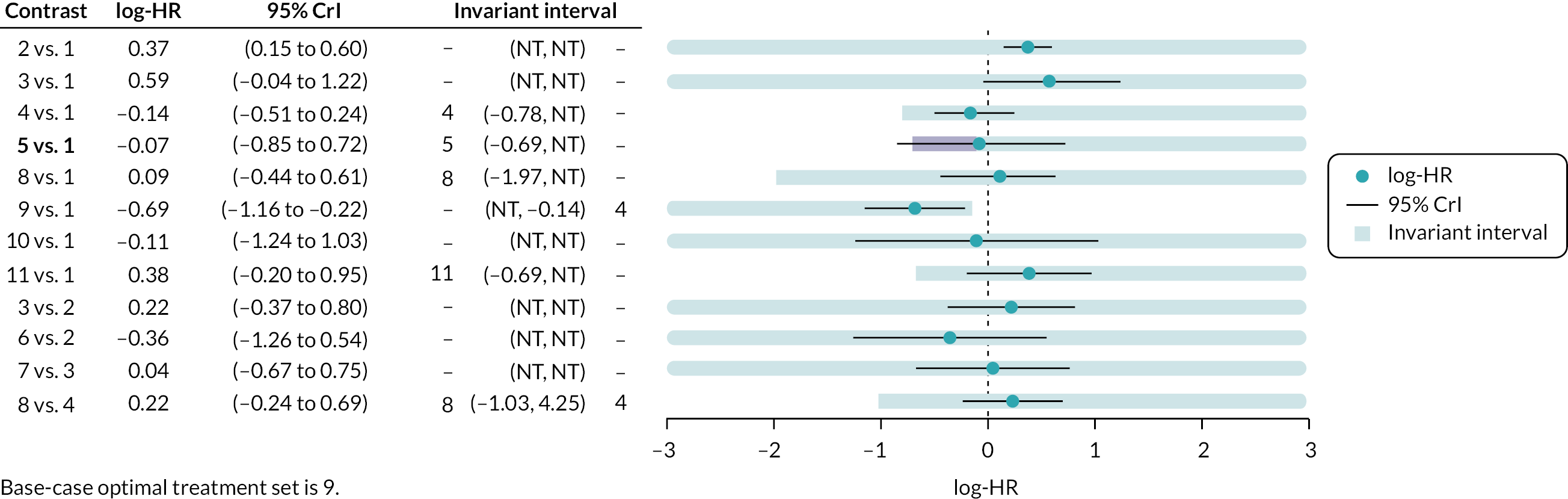
Credible intervals for the MWA versus RFA (5 vs. 1) comparison extend beyond the limits of the invariance intervals, suggesting that the recommended treatment is sensitive to the uncertainty in the data.
As interventions that included PEI and PAI were not considered in the threshold analysis, comparisons including those interventions – PEI versus RFA (2 vs. 1), PAI versus RFA (3 vs. 1), RFA + PEI versus PEI (10 vs. 1), PAI versus PEI (3 vs. 2), TACE + PEI versus RFA (6 vs. 2), and TACE + PAI versus PAI (6 vs. 3) – had large thresholds on the log scale. None of the comparisons had thresholds that would be sensitive to small changes in log-HRs. The thresholds and new optimum treatments, based only on relative effects, are presented in Report Supplementary Material 5.
Progression-free survival
The forest plot for the threshold analysis is presented in Figure 15.
FIGURE 15.
Forest plot for the threshold analysis for PFS.
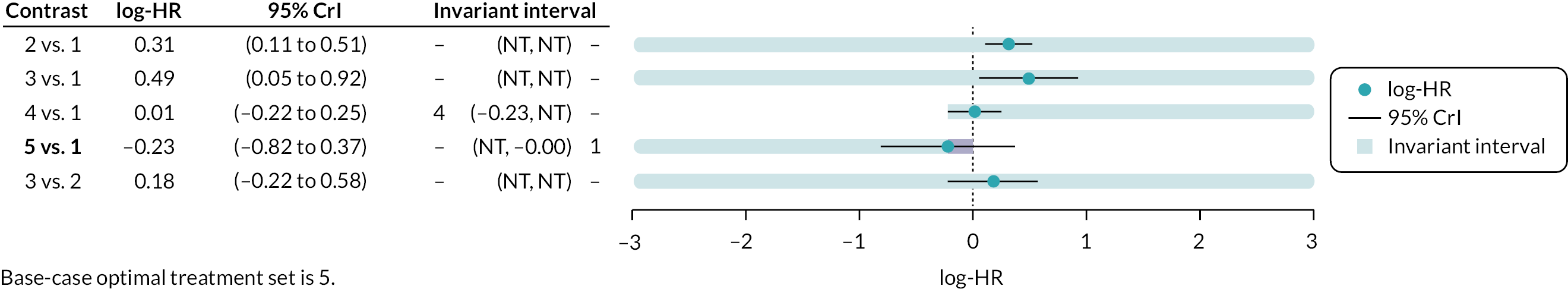
Credible intervals for the RFA + TACE versus RFA (5 vs. 1) comparison extend beyond the limits of the invariance intervals, suggesting that the recommended treatment is sensitive to the uncertainty in the data, changing the optimum treatment to RFA.
Comparisons including PEI and PAI – PEI versus RFA (2 vs. 1), PAI versus RFA (3 vs. 1), and PAI versus PEI (3 vs. 2) – had very large thresholds on the log scale. However, the negative threshold for the resection versus RFA (4 vs. 1) comparison was very small, and a change of 0.24 units on the log-HR scale in the negative direction changes the optimum treatment to resection. Thresholds and new optimum treatments, based only on relative effects, are presented in Report Supplementary Material 5.
Overall recurrence
The forest plot for the threshold analysis is presented in Figure 16.
FIGURE 16.
Forest plot for threshold analysis results for overall recurrence.
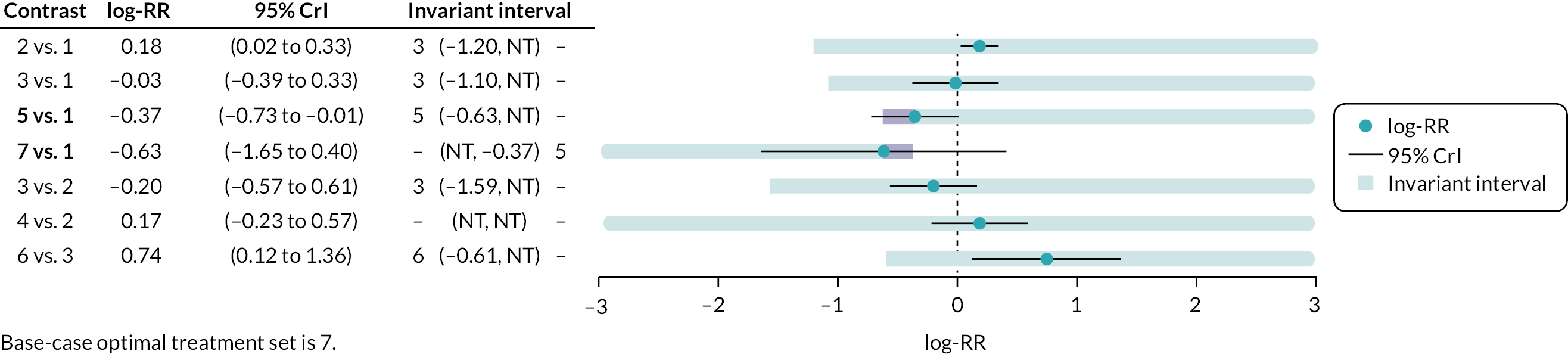
Credible intervals for the RFA + iodine-125 versus RFA (5 vs. 1) and RFA + systemic chemotherapy versus RFA (7 vs. 1) comparisons extend beyond the limits of the invariance intervals, suggesting that the recommended treatment is sensitive to the uncertainty in the data.
Three comparisons – PEI versus RFA (2 vs. 1), resection versus PEI (3 vs. 2), and MWA + sorafenib versus resection (6 vs. 3) – had very large thresholds on the log scale. On the other hand, the negative threshold for the RFA + iodine-125 versus RFA (5 vs. 1) comparison was very small, and a change of 0.26 units on the log-RR scale in the negative direction changes the optimum treatment to RFA + iodine-125. Additionally, the positive threshold for RFA + systemic chemotherapy versus RFA (7 vs. 1) was very small, and a change of 0.26 units on the log-RR scale in the positive direction also changes the optimum treatment to RFA + iodine-125. Thresholds and new optimum treatments, based only on relative effects, are presented in Report Supplementary Material 5.
Local recurrence
The forest plot for the threshold analysis is presented in Figure 17.
FIGURE 17.
Forest plot for results of threshold analysis for local recurrence.

Credible intervals for the MWA versus RFA (4 vs. 1), RFA + TACE versus RFA (6 vs. 1), and laser versus RFA (7 vs. 1) comparisons extend beyond the limits of the invariance intervals, suggesting that the recommended treatment is sensitive to the uncertainty in the data.
Comparisons including PEI and PAI – PEI versus RFA (2 vs. 1), PAI versus RFA (3 vs. 1), RFA + PEI versus RFA (8 vs. 1), high-dose PEI versus RFA (9 vs. 1), PAI versus PEI (3 vs. 2), TACE + PEI versus PEI (5 vs. 2), and high-dose PEI versus PEI (9 vs. 2) – had very large thresholds on the log-RR scale, as did the laser versus RFA (7 vs. 1) comparison. On the other hand, the negative threshold for the MWA versus RFA (4 vs. 1) and RFA + TACE versus RFA (6 vs. 1) comparisons was small, and changes of 0.48 and 0.19 units on the log-RR scale in the negative direction would change the optimum treatment to MWA and RFA + TACE, respectively. Thresholds and new optimum treatments, based only on relative effects, are presented in Report Supplementary Material 5.
Systematic review of non-randomised evidence
The electronic searches for non-randomised studies of selected interventions, where RCT evidence was not available (HIFU, cryoablation, IRE, ECT, histotripsy, SABR and wider radiotherapy techniques) or for comparisons where the threshold analysis suggested that additional evidence could plausibly change the NMA result (RFA, MWA and laser ablation, compared with each other or surgical resection), identified a total of 8009 records after deduplication between databases. One additional record was identified from screening reference lists of relevant systematic reviews. Clinical advisors were not aware of any additional studies, other than those already identified from the electronic searches. However, clinical advisors were aware of additional unpublished data from a prospective registry of patients undergoing treatment for HCC at Leeds Teaching Hospitals NHS Trust. These data were made available for use in the updated NMAs (see Updated network meta-analyses using RCT and non-RCT evidence).
Two hundred and thirty-four potentially relevant studies were ordered for full paper screening. Eight papers were unavailable as they were only reported as conference abstracts or clinical trial register records. Two hundred and twenty-six full papers were screened; 218 were excluded at the full paper stage and are listed in Appendix 5, along with the reasons for their exclusion. Figure 18 presents the flow of non-RCT studies through the study selection process.
FIGURE 18.
Flow diagram of the study selection process (non-RCTs).
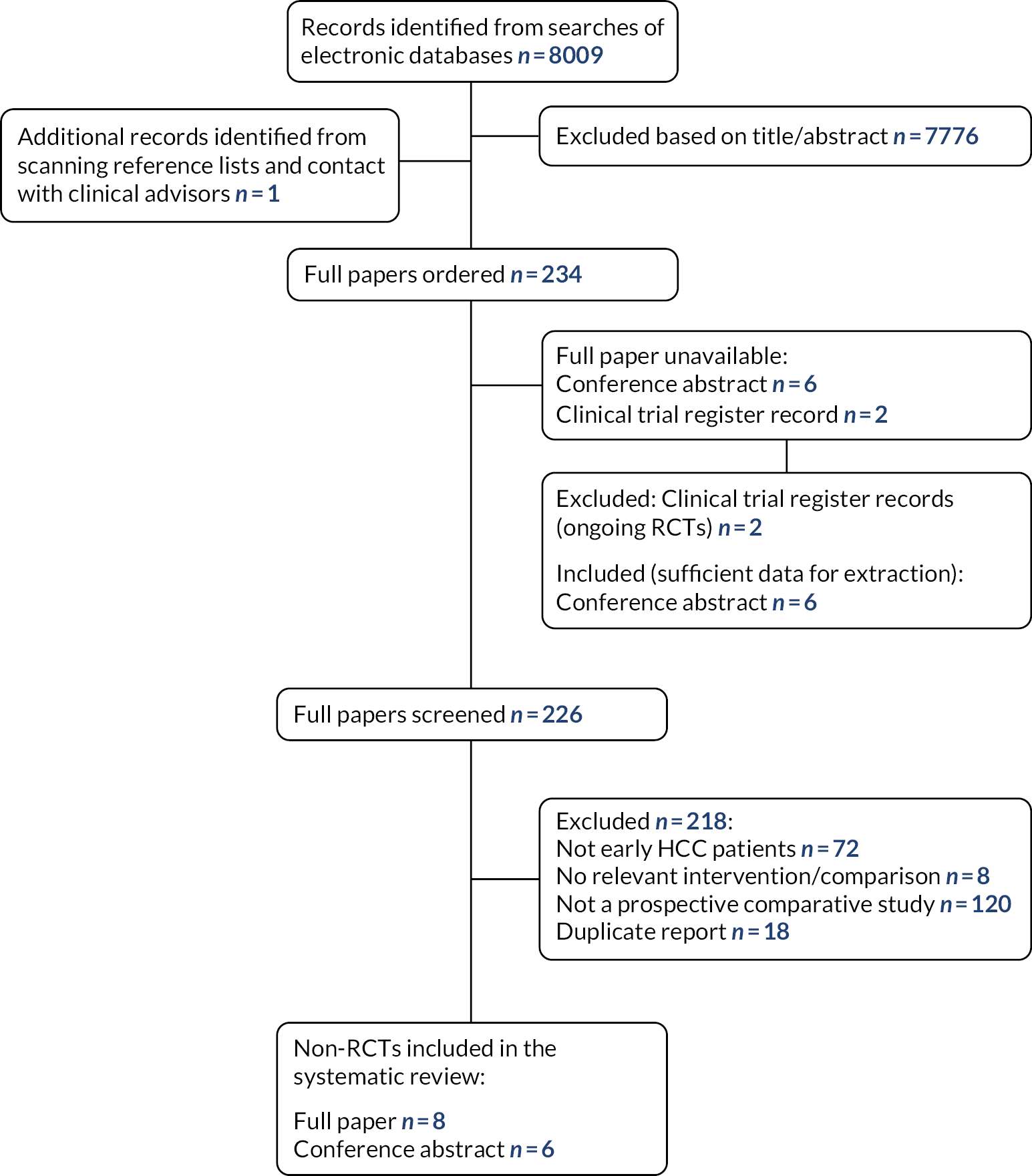
Characteristics of non-randomised studies included in the review
Details of the 14 non-randomised comparative studies that were included in the systematic review are presented in Table 10. Eight of the 14 studies restricted inclusion criteria to HCC patients with tumour size up to 3 cm in diameter. 83–90 One study restricted inclusion criteria to HCC patients with tumour size up to 2 cm in diameter. 91 One study included patients with tumours up to 5 cm in diameter, but reported separate results for the subgroup of patients with tumours up to 3 cm in diameter. 92 Two studies did not report specific tumour size inclusion criteria, but in one study average tumour size was 2.15 (±0.53) cm in one arm and 1.92 (± 0.5) cm in the other,93 and the other study reported median tumour size of 2.03 (SD 0.44) cm in one arm and 1.73 (SD 0.67) cm in the other. 94 Two studies included patients with tumours up to 5 cm, but a clear majority of patients had tumours < 3 cm in diameter. 95,96 In three of the included studies the patients had tumours unsuitable for percutaneous treatment,85,91,93 and one study included patients with primary or first recurrent HCC. 83 Study sample sizes ranged from 21 to 740 patients.
| Study | Location | Participant information | Intervention | Comparator |
|---|---|---|---|---|
| Barabino, 201693 (abstract) | Italy | 154 patients with HCC unsuitable for percutaneous treatments or hepatic resection (average tumour size 2.15 (± 0.53) cm in one arm and 1.92 (± 0.5) cm in the other) | Laparoscopic RFA | Laparoscopic MWA |
| Cheung, 201383 | China | 106 patients (with 119 tumours) with < 3 cm tumours (primary or first recurrence) | HIFU | RFA |
| Choi, 200484 (abstract) | Korea | 164 patients with ≤ 3 cm tumours | RFA | Hepatic resection |
| Du, 201292 (reported in Chinese) | China | 116 patients with tumours ≤ 5 cm; subgroup of 60 patients with tumours ≤ 3 cm | RFA | Surgical resection |
| Ei, 201595 | Japan | 119 patients with < 5 cm tumours, included a few patients with tumours > 3 cm; median 2.5 cm in cryoablation group (maximum 4 cm), median 1.9 cm in RFA/MWA group (maximum 4.5 cm) | Cryoablation | RFA or MWA |
| Elgendi, 201485 (abstract) | Egypt | 51 patients with < 3 cm tumours in locations not amenable for percutaneous route | Intraoperative RFA | Surgical resection |
| Elgendi, 201591 (abstract) | Egypt | 92 patients with < 2 cm tumours in locations not amenable for percutaneous route | Intraoperative RFA | Surgical resection |
| Harada, 201696 | Japan | 121 patients with < 5 cm tumours and portal hypertension, included a few patients with tumours > 3 cm in the resection group; mean 2.1 cm (range 0.7–5 cm) | RFA | Liver resection |
| Horigome, 200086 | Japan | 105 patients with ≤ 3 cm tumours | Resection | MWA PEI |
| Huang, 201487 | China | 346 patients with ≤ 3 cm tumours | RFA | Surgical resection |
| Peng, 201088 (abstract) | China | 195 patients with ≤ 3 cm tumours | RFA (n = 79), surgical resection (n = 24) | Surgical resection (n = 75), RFA (n = 17) |
| Qian, 201289 | China | 42 patients with < 3 cm tumours | MWA | RFA |
| Sugimoto, 201994 | Japan | 21 patients (with 24 tumours; median tumour size 2.03 (SD 0.44) cm in one arm and 1.73 (SD 0.67) cm in the other) | IRE | RFA |
| Tateishi, 202090 (abstract) | Japan | 740 patients with ≤ 3 cm tumours | RFA | Surgery |
The majority of studies were conducted in Asian countries, which has implications for the generalisability of results to the UK population, as discussed in Characteristics of RCTs included in the review. Studies were conducted in China (n = 5), Japan (n = 5), Egypt (n = 2), Korea (n = 1) and Italy (n = 1). Six of the included studies were only reported as conference abstracts, and therefore limited data were available. 84,85,88,90,91,93 While the inclusion criteria stated that only prospective studies were eligible for inclusion, for six studies it was not possible to determine whether patients were recruited prospectively or retrospectively; these studies were included to ensure that no relevant data were missed. 83–85,91,93,95
Table 11 shows the comparisons made in the included studies, 13 of which assessed RFA. While RFA was usually delivered via the percutaneous route, three studies assessed laparoscopic93 or intraoperative RFA85,91 in patients with tumours unsuitable for percutaneous treatment. It should also be noted that several of the studies allocated patients to treatment groups depending on their tumour characteristics. Cheung et al. offered HIFU to patients with poor liver function or decompensated cirrhosis or tumours located at sites considered difficult for RFA;83 Ei et al. allocated patients to cryoablation if tumours were in close vicinity to major veins or organs;95 both studies by Elgendi et al. allocated patients depending on the location and depth of the tumour from the liver capsule;85,91 Harada et al. allocated patients depending on Child–Pugh class, tumour location and indocyanine green retention tests;96 and Sugimoto et al. allocated patients depending on operator preference, tumour size, geometry and location. 94 In the study by Peng et al., patients were allocated to RFA or surgical resection as the first choice, but the actual treatment received depended on the tumour location. 88
| RFA | RFA | ||||||
| MWA | 2 | MWA | |||||
| PEI* | 1 | PEIa | |||||
| Resection | 8 | 1 | 1 | Resection | |||
| HIFU | 1 | HIFU | |||||
| Cryoablation | Cryoablation | ||||||
| RFA/MWA | 1 | RFA/MWA | |||||
| IRE | 1 |
Quality of non-randomised studies included in the review
Results of the quality assessment of the non-randomised comparative studies are presented in Table 12. Six of the included studies were only reported as conference abstracts, and therefore there are a few ‘Unclear’ responses to some of the quality assessment criteria owing to the limited reporting.
| Trial | Inclusion criteria clearly defineda | Allocation to treatment groups adequately described/appropriatea | Groups similar at baselinea | Clearly described and consistently delivered intervention | Clearly described and consistently delivered comparator | Outcome assessors blinded | Missing outcome data balanced across groupsa | Free from suggestion of selective reporting | Overall judgement of ROB |
|---|---|---|---|---|---|---|---|---|---|
| Barabino, 201693 (abstract) |
No | No | No | No | No | Unclear | Unclear | Unclear | High |
| Cheung, 201383 | Yes | No | No | Yes | Yes | Unclear | Unclear | Yes | High |
| Choi, 200484 (abstract) |
No | No | Unclear | No | No | Unclear | Unclear | Unclear | High |
| Du, 201292 (Chinese) | Yes | No | Unclear | Yes | Yes | Unclear | Yes | Yes | High |
| Ei, 201595 | Yes | No | No | Yes | No | Unclear | Yes | Yes | High |
| Elgendi, 201485 (abstract) |
No | No | Unclear | No | No | Unclear | Unclear | Unclear | High |
| Elgendi, 201591 (abstract) |
No | No | Unclear | No | No | Unclear | Unclear | Unclear | High |
| Harada, 201696 | Yes | No | No | Yes | Yes | Unclear | Yes | Yes | High |
| Horigome, 200086 | No | No | No | Yes | Yes | Unclear | Yes | Yes | High |
| Huang, 201487 | Yes | Yes | Yes | Yes | Yes | Yes for HRQoL, unclear for survival/AE | Unclear | Yes | Unclear |
| Peng, 201088 (abstract) |
No | No | Unclear | No | No | Unclear | Unclear | Unclear | High |
| Qian, 201289 | Yes | Yes | Yes | Yes | Yes | Unclear | Yes | Yes | Low |
| Sugimoto, 201994 | Yes | No | No | Yes | Yes | Unclear | Unclear | Yes | High |
| Tateishi, 202090 (abstract) |
Yes | No | No | No | No | Unclear | Unclear | Yes | High |
| Total |
Yes: 8
No: 6 Unclear: 0 |
Yes: 2
No: 12 Unclear: 0 |
Yes: 2
No: 7 Unclear: 5 |
Yes: 8
No: 6 Unclear: 0 |
Yes: 7
No: 7 Unclear: 0 |
Yes: 0
No: 0 Unclear: 14 |
Yes: 5
No: 0 Unclear: 9 |
Yes: 9
No: 0 Unclear: 5 |
High: 12
Low: 1 Unclear: 1 |
Generally, methods were poorly reported. Inclusion criteria were clearly defined in 8/14 studies. The intervention was clearly described and consistently delivered in 8/14 studies and the comparator was clearly described and consistently delivered in 7/14 studies. None of the studies reported whether outcome assessors were blinded to treatment group.
Allocation to treatment groups was adequately described and appropriate in only two studies, resulting in patients having similar baseline characteristics between groups. 87,89 As discussed in Overall survival, several of the studies allocated patients to treatment groups depending on their tumour characteristics. Because appropriateness of treatment allocation and similarity of treatment groups at baseline were two of the important quality assessment criteria, this resulted in the other 12 studies having a high overall RoB judgement. The study by Qian et al. was the only study to have a low overall RoB judgement89 and the study by Huang et al. had an unclear overall RoB judgement, as it was unclear whether missing outcome data were balanced across treatment groups. 87
Results of non-randomised studies included in the review
A table of study characteristics and results is presented in Appendix 6. In view of the high RoB of 12 of the 14 included studies – differences in baseline characteristics between treatment groups and treatment allocation being dependent on tumour characteristics for several studies – the results below should be interpreted with caution. The non-randomised nature of these studies and the possibility that some studies may have been undertaken retrospectively mean that these results are less reliable than those of the RCTs described in Results of RCTs included in the review.
Radiofrequency ablation versus microwave ablation
Two non-randomised studies compared RFA with MWA. One study was assessed as having a low RoB (n = 42 patients). 89 The other study was only reported as a conference abstract and had a high RoB (n = 154 patients). 93 The conference abstract did not report participant inclusion criteria relating to tumour size or the maximum tumour size of the included participants, but the mean size was 2.15 cm in the MWA arm and 1.92 cm in the RFA arm. Patients were unsuitable for percutaneous treatments; the interventions assessed were laparoscopic RFA and laparoscopic MWA.
Only the low-quality study reported OS and disease-free survival rates, which were both higher after laparoscopic RFA than after laparoscopic MWA at 5 years (OS 50% vs. 37%; disease-free survival 19% vs. 12%). 93 However, local tumour progression occurred in more patients in the RFA group than in the MWA group in the low-quality study (21.2% vs. 8.3%) and was similar between groups in the high-quality study (RFA 15% vs. MWA 18.2%). The proportion of patients with a new intrahepatic tumour was also higher in the RFA group than in the MWA group in the high-quality study (20% vs. 4.5%).
Both studies reported that around 95% of patients achieved complete ablation in both arms. After a second treatment, 100% of patients achieved complete ablation in the high-quality study. 89
The low-quality study reported a similar rate of major complications in both arms (RFA 1% vs. MWA 2%) and no treatment-related deaths in either group. 93 The high-quality study reported only that there were no skin burns, tumour seeding or treatment-related deaths in either group. 89
Radiofrequency ablation versus resection
Eight non-randomised studies compared RFA with resection (n = 1769 patients). Seven of the studies had a high RoB84,85,88,90–92,96 and one had an unclear RoB. 87 Five of the studies with a high RoB were only reported as conference abstracts. 84,85,88,90,91 One study included tumours up to 5 cm in the resection group, but the mean tumour size in this group was 2.1 cm. 96 In four of the studies the treatment received was decided on the basis of patient characteristics (e.g. tumour location) and either there were baseline differences between groups or it was not clearly reported whether this was the case. 85,88,91,96 In one of these studies, group allocation determined which of the two treatments was given as the first choice, but the final decision was based on tumour location. 88 In three of the other studies, allocation to treatment groups was not adequately described and either there were baseline differences between groups or it was unclear whether this was the case. 84,90,92 Only one study reported similar baseline characteristics between treatment groups. 87
Five of the eight studies reported 1- and 3-year OS rates. In most of these studies, survival was similar between groups at 1 year,84,85,91,96 although it was slightly higher in the RFA group in one study (RFA 95.9% vs. resection 90.1%). 88 At later time points, findings were more mixed. Three studies reported a higher OS rate in the resection arm at 3 years (RFA vs. resection: 73.9% vs. 83.0%,84 74% vs 81%85 and 76% vs. 83%91), but it remained similar in one study (84.5% vs. 84.1%96) and was higher in the RFA arm in the other study (75.8% vs. 63.7%88). Two studies also reported higher survival rates after RFA at 4 (70.7% vs. 55.5%88) or 5 years (50.6% vs. 37.1%96).
Two studies reported recurrence-free survival. In one study it was higher after resection at 1, 3 and 5 years (5-year rate 4.8% vs. 42.9%). 96 In the other study it was similar at 1 year (RFA 74.1% vs. resection 75.9%) but higher after resection at 3 years (40.2% vs. 54.7%). 84
Findings on recurrence were also mixed. In two studies, recurrence (local and distant/remote) was experienced by more patients in the RFA group (local or distant 85% vs. 42%;96 local 11.3% vs. 2.0%; remote 53.7% vs. 45.3%84). Another two studies reported similar relapse or recurrence rates between groups (1-year relapse rate: RFA 12.9% vs. resection 13.8%;92 3-year recurrence rate: RFA 61.7% vs. resection 66%; adjusted HR 0.89, 95% CI 0.72 to 1.190). Two studies reported that no tumours showed local progression or recurrence during the follow-up period in either group. 85,91
Only two studies reported the complete ablation/resection rate, which was 100% in both treatment arms. 85,91 One study reported quality-of-life outcomes, measured using the FACT-Hep questionnaire. 87 Patients in the RFA group had significantly better HRQoL total scores than those in the resection group after 3, 6, 12, 24 and 36 months.
Data on AEs were limited. Two studies reported a considerably higher rate of total AEs and AEs at grade III or above on the Clavien–Dindo scale after resection. 87,96 While one study reported no hospital deaths in either group,87 one study reported one hospital death occurring secondary to sepsis in the resection group (RFA 0/40 vs. resection 1/8196), and another study reported two cases of treatment-related mortality in the resection group (RFA 0/103 vs. resection 2/9288). Two conference abstracts reported only that complication rates were ‘comparable’ between groups. 85,91
The average length of hospital stay was approximately twice as long after resection as after RFA in two studies. 87,96 One of the studies also reported that the RFA group experienced a shorter procedure (RFA 44.0 vs. resection 166.5 minutes) and lower blood transfusion rates. 96
Microwave ablation versus resection
One non-randomised study with a high RoB compared MWA with resection (n = 105 patients). 86 It also included a treatment arm that received PEI. Fewer patients experienced recurrence after MWA than after resection (MWA 38% vs. resection 72%). No data were reported on survival outcomes or AEs.
High-intensity focused ultrasound versus radiofrequency ablation
One non-randomised study with a high RoB compared HIFU with RFA (n = 106 patients). 83 Included patients had primary HCC or first recurrence. Treatment was allocated on the basis of patient characteristics (liver function, decompensated cirrhosis or tumour location), so the groups were not similar at baseline. OS was similar between arms at 1 and 3 years (1 year: HIFU 97.4% vs. RFA 94.6%; 3 years: 81.2% vs. 79.8%). Disease-free survival was also similar at 1 year (HIFU 63.6% vs. RFA 62.4%) but lower in the HIFU group at 3 years (25.9% vs. 34.1%). Complete response was slightly higher in the RFA arm (87.2% vs. 94.9%). More patients in the HIFU group than the RFA group experienced AEs (21.3% vs. 8.5%). However, the rates of AEs at grade III or above on the Clavien–Dindo scale was similar in both groups (HIFU 3/47 vs. RFA 4/59). Patients in the HIFU group had a shorter length of hospital stay than those in the RFA group (median 4 vs. 6 days).
Cryoablation versus radiofrequency ablation or microwave ablation
One non-randomised study with a high RoB compared cryoablation with a group that received either RFA or MWA (n = 119 patients). 95 Results were not reported separately for patients receiving RFA and those receiving MWA. Patients with HCCs up to 5 cm were eligible, but the median tumour size was 2.5 cm in the cryoablation group and 1.9 cm in the RFA/MWA group. Treatment was allocated based on tumour location, so there were baseline differences between the groups.
Overall survival and local recurrence were reported separately for patients with tumours up to 2 cm and patients with tumours over 2 cm. In the ≤ 2 cm subgroup, the 2-year OS rate was slightly higher in the RFA/MWA group (88% vs. 95%) and the 2-year local recurrence rate was similar in both groups (cryoablation 19% vs. RFA/MWA 23%). OS was similar between groups in the > 2 cm subgroup (cryoablation 86% vs. RFA/MWA 85%), but local recurrence occurred in considerably more patients who underwent RFA or MWA than patients who underwent cryoablation (21% vs. 56%).
The 2-year local recurrence-free survival rate (for all tumour sizes) was higher in the cryoablation group (80% vs. 68%). Initial recurrence at other sites of the liver was similar between groups (cryoablation 38% vs. RFA/MWA 34%). Two patients suffered distant metastases in the bone or lung; both were in the cryoablation group.
There was a similar total rate of AEs in the two groups (cryoablation 6/55 vs. RFA/MWA 7/64) and a similar proportion of patients had AEs at grade III or above on the Clavien–Dindo scale (3/55 vs. 3/64). There was no in-hospital mortality in either group. Operative time was longer in the cryoablation group (median 180 vs. 132 minutes). The median length of hospital stay was 8 days in both groups.
Irreversible electroporation versus radiofrequency ablation
One non-randomised study with a high RoB compared IRE with RFA (n = 21 patients). 94 The maximum tumour size was not reported, but the median size was 2.03 cm in the IRE group and 1.73 cm in the RFA group. Treatment was allocated based on operator preference, tumour size, geometry and location, so there were baseline differences between groups. This study aimed to assess temporal changes in systemic immune responses between these two different types of ablation, and the only relevant data reported were on local tumour progression at 6 months. Local tumour progression was experienced by 1 of 10 patients in the IRE group and 0 of 11 patients in the RFA group.
Ongoing trials
The electronic searches for non-randomised trials identified two potentially relevant ongoing RCTs that were not identified in the RCT searches (described in Ongoing trials). Further details are presented in Table 13.
| Study | Further details |
|---|---|
| ChiCTR2000039404 | Clinical trial register record describing a single-centre RCT comparing SBRT vs. RFA for ≤ 2 cm small HCC. Registered 2020. |
| ClinicalTrials.gov: NCT04891874 | Clinical trial register record describing a single-centre RCT comparing adjuvant SBRT after surgery vs. surgery alone for early-stage HCC with microvascular invasion and narrow resection margin. Sponsor: Eastern Hepatobiliary Surgery Hospital, China. Trial status: Completed, last update posted 10 September 2021. |
Updated network meta-analyses using RCT and non-RCT evidence
Of the 14 non-randomised studies that were included in the systematic review, two87,89 could be included in the updated NMAs. Huang (2014) reported data that could be incorporated in the NMAs for OS and PFS,87 while Qian (2012) reported data that could be incorporated in the NMA for PFS. 89
Data from a prospective registry of patients undergoing treatment for HCC were made available to the research team by a research group at Leeds Teaching Hospitals NHS Trust (Dr Tze Wah, Leeds Teaching Hospitals NHS Trust, 5 October 2021, personal communication). This contained data for 303 patients who had received either RFA, MWA, IRE or cryoablation for primary HCC. Most patients received RFA, with a smaller number receiving MWA. Very few patients received IRE or cryoablation. Data were unpublished at the time of our analysis, but have been submitted for publication.
Data from the Leeds patients were reported for numerous outcomes. There were sufficient data for inclusion in NMAs for OS, PFS, and local recurrence.
As there was no new evidence for overall recurrence, no updated NMAs or threshold analyses were conducted for this outcome.
Overall survival
Data
The network diagram for OS is presented in Figure 19. In addition to the randomised studies included in the NMA in Overall survival, one two-arm and one three-arm study provided non-randomised evidence for one new intervention in addition to three interventions already included in the network. A summary of the additional non-randomised evidence included in the NMA is provided in Report Supplementary Material 4.
FIGURE 19.
Network diagram for OS.
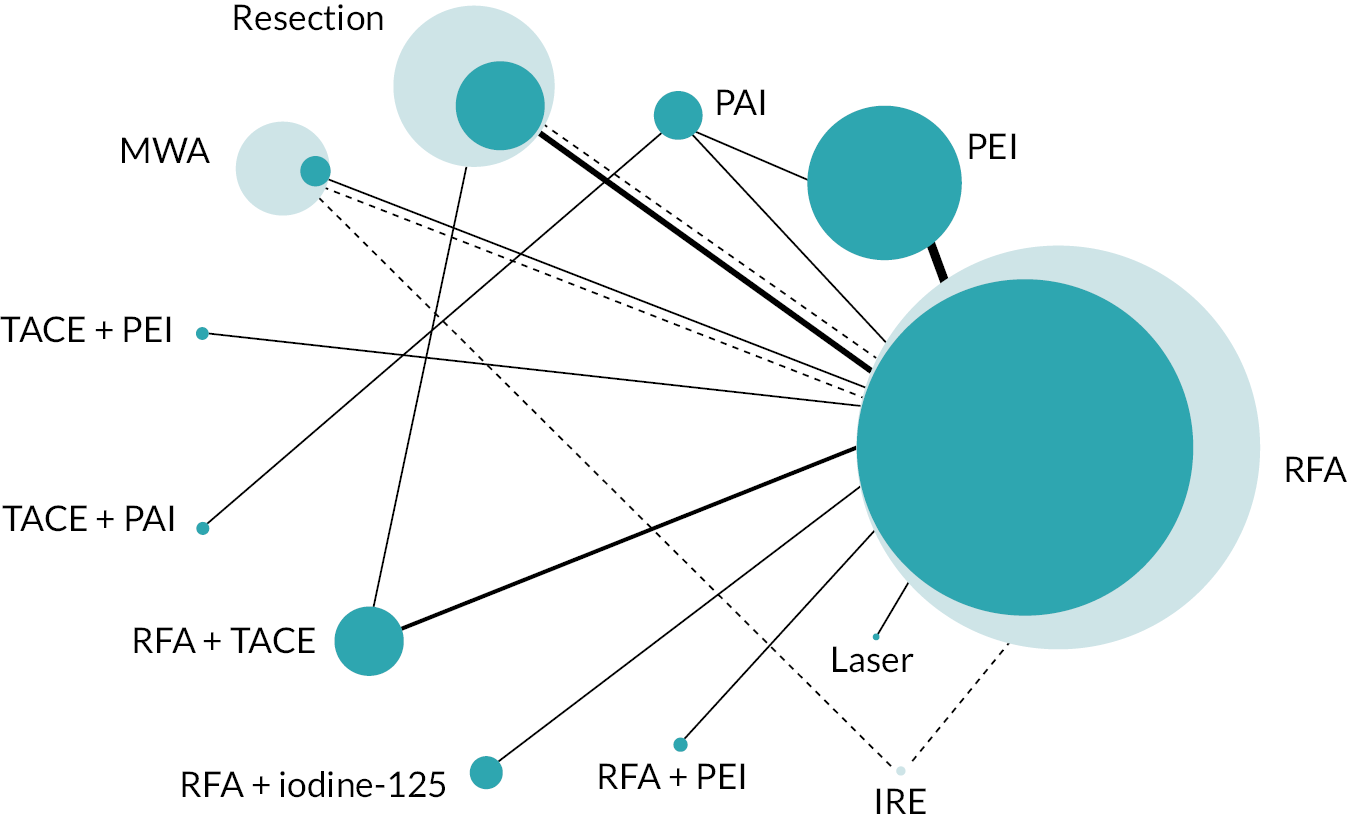
Model selection and inconsistency checking
Model fit parameters for the FE and RE models are presented in Report Supplementary Material 5. All three models fit the data well, but as the difference in the DICs between the FE and RE models was < 3, the simpler FE model was chosen.
The 95% CrI for the model using the half-normal (0, 0.502) prior was wider than the 95% CrI for the model using the half-normal (0, 0.192). This shows that the estimate of between-study heterogeneity is sensitive to the level of prior heterogeneity assumed due to few studies being included for each comparison in the network. Plots for the prior and posterior distributions of the between-study heterogeneity for the RE models are presented in Report Supplementary Material 5. There was no evidence to suggest inconsistency in the network. Details of the inconsistency check and node-splitting results are presented in Report Supplementary Material 5.
Model results
Hazard ratios for OS for all treatments compared with RFA are presented in Figure 20.
FIGURE 20.
Plot of HRs for OS compared with RFA for the FE model.
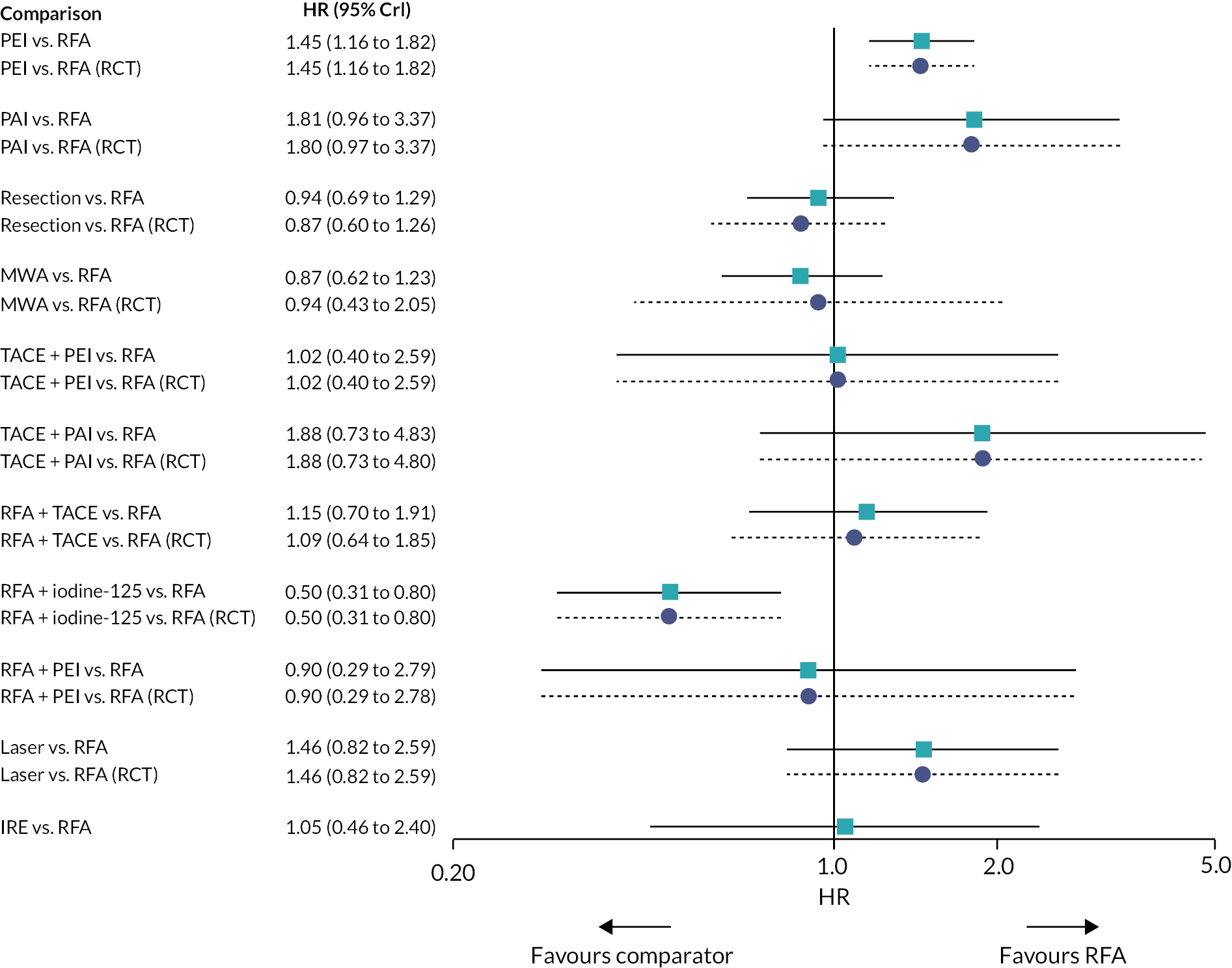
The results for the NMA were not very different from the results from the NMA comparing only randomised evidence (see Model results). With the addition of non-randomised studies there was also evidence to suggest that RFA + iodine-125 improves survival compared with resection. There was also evidence to suggest that MWA improves survival compared with PEI and PAI. HRs comparing all treatment groups against each other for FE and RE models are reported in Report Supplementary Material 5.
The mean and median ranks for each treatment, with their corresponding 95% CrIs, are presented in Table 14. RFA + iodine-125 had the highest probability of being ranked the best treatment. However, as seen for NMAs including only randomised evidence (see Model results), there was a high level of uncertainty in treatment rankings, also visible in the treatment rank plots (see Figure 21).
| Treatments | Mean rank | Median rank | 95% CrI of the rank |
|---|---|---|---|
| RFA + iodine-125 | 1.40 | 1 | (1.00 to 3.00) |
| MWA | 4.10 | 4 | (2.00 to 8.00) |
| Resection | 4.76 | 5 | (2.00 to 8.00) |
| RFA + PEI | 5.19 | 4 | (1.00 to 12.00) |
| RFA | 5.46 | 5 | (3.00 to 8.00) |
| TACE + PEI | 5.88 | 6 | (1.00 to 12.00) |
| IRE | 6.08 | 6 | (1.00 to 12.00) |
| RFA + TACE | 6.94 | 7 | (2.00 to 12.00) |
| Laser | 8.86 | 9 | (3.00 to 12.00) |
| PEI | 9.14 | 9 | (7.00 to 12.00) |
| TACE + PAI | 9.95 | 11 | (3.00 to 12.00) |
| PAI | 10.23 | 11 | (5.00 to 12.00) |
FIGURE 21.
Rank plot for OS for the FE model

Progression-free survival
Data
The network diagram for PFS is presented in Figure 22. In addition to the randomised studies included in the NMA in Progression-free survival, two two-arm and one three-arm study provided non-randomised evidence for two new interventions in addition to two interventions already included in the network. A summary of the additional non-randomised evidence included in the NMA is provided in Report Supplementary Material 4.
FIGURE 22.
Network diagram for PFS.
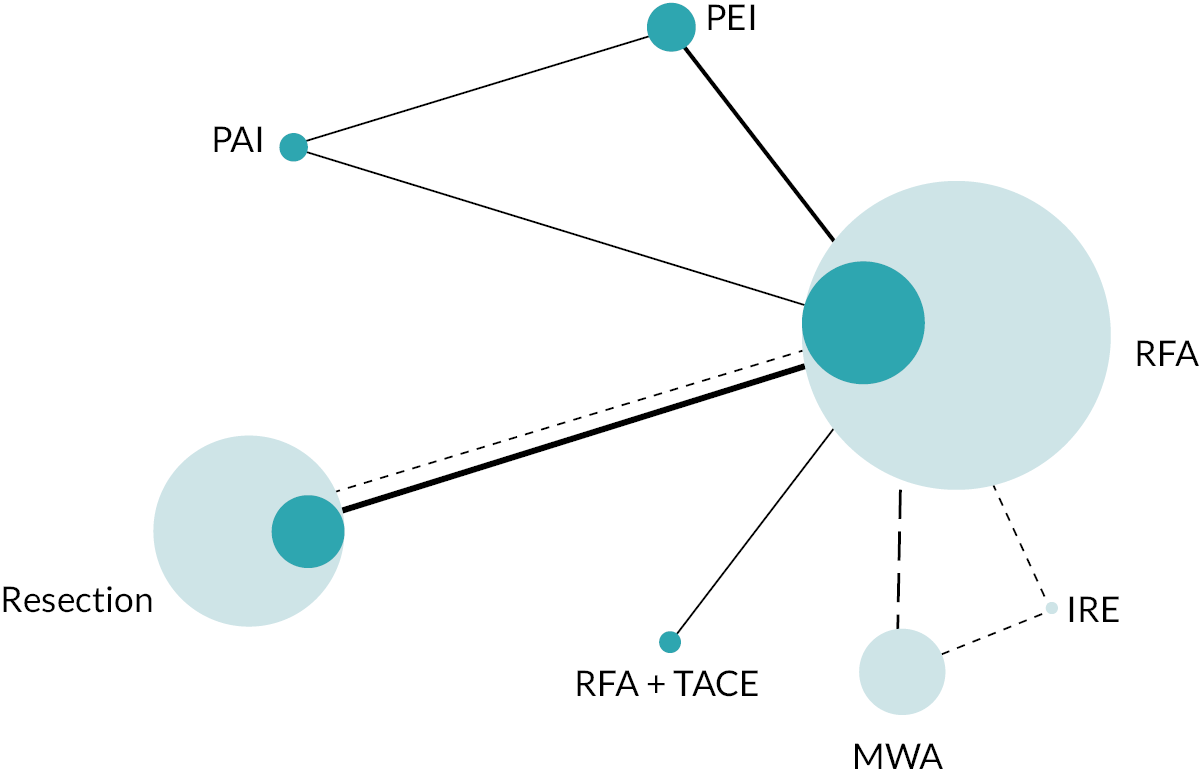
Model selection and consistency checking
Model fit parameters for the FE and RE models are presented in Report Supplementary Material 5. All three models fit the data well, but as the difference in the DICs between the FE and RE models was < 3, the simpler FE model was chosen.
The between-study heterogeneity was low for the two RE models. However, the 95% CrI for the model using the half-normal (0, 0.502) prior was wider than the 95% CrI for the model using the half-normal (0, 0.192) indicating that the estimate of between-study heterogeneity is sensitive to the level of prior heterogeneity assumed due to few studies being included for each comparison in the network.
There is no potential for inconsistency in this network as there is no independent, indirect evidence for any of the comparisons – the two loops are formed by two three-arm studies, one of which is the work at Leeds Teaching Hospitals NHS Trust described above (Dr Tze Wah, personal communication). 53
Model results
HRs for PFS for all treatments compared with RFA are presented in Figure 23.
FIGURE 23.
Plot of HRs for PFS compared with RFA for the FE model.
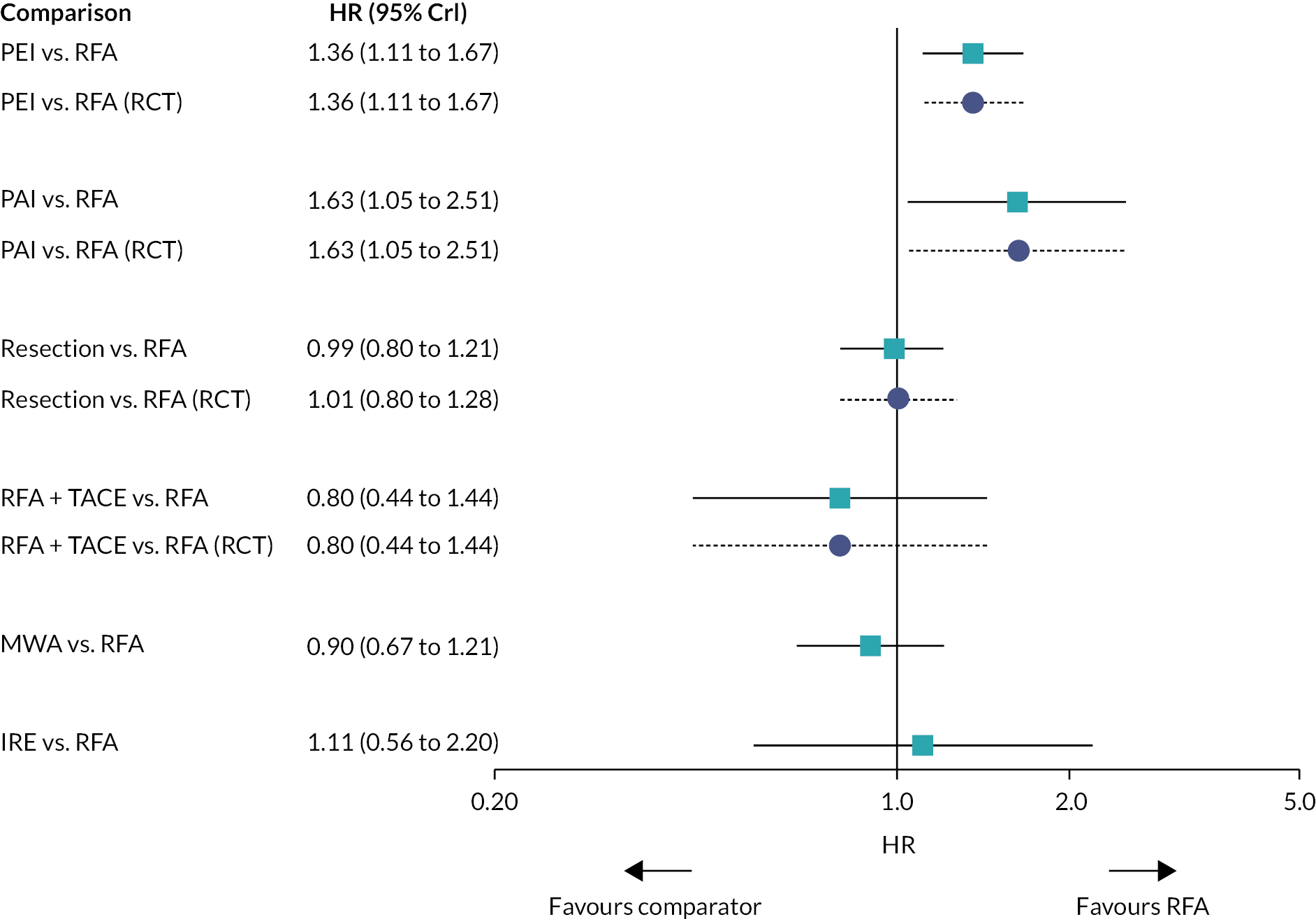
Similar to the NMA using only RCT evidence (see Model selection and consistency checking), there was evidence to suggest that PEI and PAI worsen PFS compared with RFA. However, with the addition of the non-randomised studies, there was also evidence to suggest that resection and MWA improved PFS compared with PEI and PAI. HRs comparing all treatment groups against each other for FE and RE models are reported in Report Supplementary Material 5.
The treatment rank plot for PFS is presented in Figure 24, and the mean and median ranks for each treatment, with their corresponding 95% CrIs, are presented in Table 15. RFA + TACE had the highest probability to be ranked the best treatment. However, there was a high level of uncertainty in the treatment ranking – all treatments displayed wide CrIs for ranks.
FIGURE 24.
Rank plot for PFS for the FE model.

| Treatments | Mean rank | Median rank | 95% CrI |
|---|---|---|---|
| RFA + TACE | 2.14 | 1 | (1.00 to 6.00) |
| MWA | 2.50 | 2 | (1.00 to 5.00) |
| Resection | 3.28 | 3 | (1.00 to 5.00) |
| RFA | 3.48 | 4 | (2.00 to 5.00) |
| IRE | 4.21 | 5 | (1.00 to 7.00) |
| PEI | 5.83 | 6 | (4.00 to 7.00) |
| PAI | 6.55 | 7 | (4.00 to 7.00) |
Local recurrence
Data
The network diagram for local recurrence is presented in Figure 25. In addition to the randomised studies included in the NMA in Overall recurrence, one three-arm study provided non-randomised evidence for one new intervention in addition to two interventions already included in the network. A summary of the additional non-randomised evidence included in the NMA is provided in Report Supplementary Material 4.
FIGURE 25.
Network diagram for local recurrence.
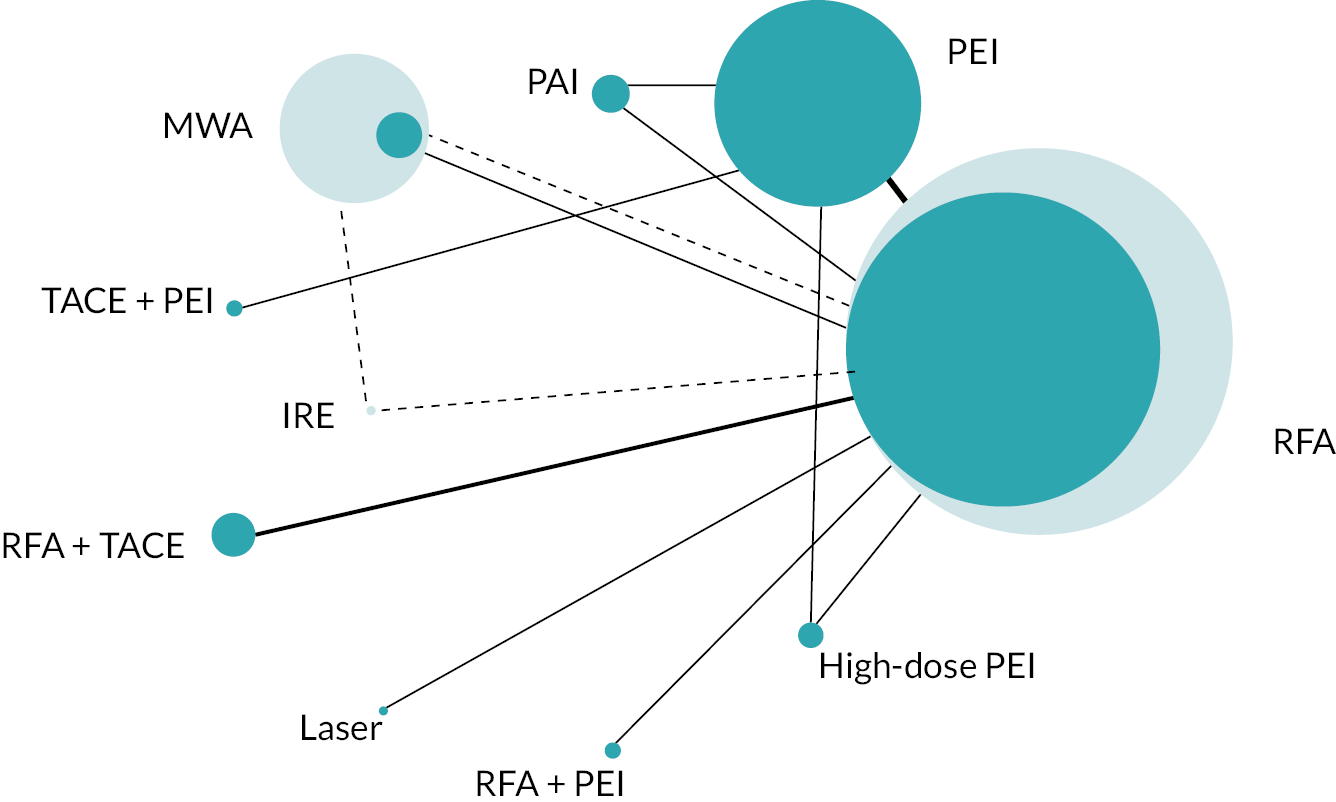
Model selection and inconsistency checking
Model fit parameters for the three models are reported in Report Supplementary Material 5. All three models fit the data well. The between-study heterogeneity was low and consistent for the two RE models. However, the 95% CrI for the model using the half-normal (0, 0.502) prior was wider than the 95% CrI for the model using the half-normal (0, 0.192).
As the difference in the DICs between the FE and RE models was < 3, the simpler FE model was chosen. Plots for the prior and posterior distributions of the between-study heterogeneity for the RE models are presented in Report Supplementary Material 5.
There is no potential for inconsistency in this network as there is no independent, indirect evidence for any of the comparisons; the three loops in the network are formed by three separate three-arm studies.
Model results
Relative risks for local recurrence for all treatments compared with RFA are presented in Figure 26.
FIGURE 26.
Plot of RRs for local recurrence compared with RFA for the FE model.

Similar to the NMA using only RCT evidence (see Model results), there was evidence to suggest that PEI increased the risk of local recurrence compared with RFA, and that RFA + PEI decreased the risk of local recurrence compared with PEI. However, with the addition of non-randomised studies, there was also now evidence to suggest that IRE increased the risk of local recurrence compared with RFA and RFA + PEI, although the CrIs for both comparisons were very wide. RRs comparing all treatment groups against each other for FE and RE models are reported in Report Supplementary Material 5.
The treatment rank plot for local recurrence is presented in Figure 27, and the mean and median ranks for each treatment, with their corresponding 95% CrIs, are presented in Table 16. There was a high level of uncertainty in treatment ranks; all treatments had rank probabilities below 50% for all treatment ranks.
FIGURE 27.
Rank plot for local recurrence for the FE model.

| Treatments | Mean rank | Median rank | 95% CrI |
|---|---|---|---|
| RFA + PEI | 2.03 | 2 | (1.00 to 6.00) |
| TACE + PEI | 2.38 | 2 | (1.00 to 7.00) |
| RFA | 3.53 | 3 | (2.00 to 5.00) |
| MWA | 4.23 | 4 | (2.00 to 8.00) |
| RFA + TACE | 4.90 | 5 | (1.00 to 9.00) |
| High-dose PEI | 6.45 | 7 | (2.00 to 10.00) |
| PAI | 6.78 | 7 | (3.00 to 10.00) |
| PEI | 7.25 | 7 | (5.00 to 9.00) |
| Laser | 8.40 | 9 | (2.00 to 10.00) |
| IRE | 9.05 | 9 | (6.00 to 10.00) |
Updated threshold analysis
Overall survival
The forest plot for the threshold analysis is presented in Figure 28.
FIGURE 28.
Forest plot for threshold analysis results for OS for the updated NMA.

Interventions that included PEI and PAI were not considered in the threshold analysis, and therefore comparisons including those interventions – PEI versus RFA (2 vs. 1), PAI versus RFA (3 vs. 1), RFA + PEI versus RFA (10 vs. 1), PAI versus PEI (3 vs. 2), TACE + PEI versus PEI (6 vs. 2), and TACE + PAI versus PAI (7 vs. 3) – had very large thresholds on the log scale. The following comparisons also had very large thresholds on the log scale: RFA + TACE versus RFA (8 vs. 1), IRE versus RFA (11 vs. 1). None of the other comparisons have thresholds that indicate estimates are sensitive to small changes in log-HRs. The thresholds and new optimum treatments, based only on relative effects, are presented in Report Supplementary Material 5.
Progression-free survival
The forest plot for the threshold analysis is presented in Figure 29.
FIGURE 29.
Forest plot for the threshold analysis for PFS for the updated NMA.
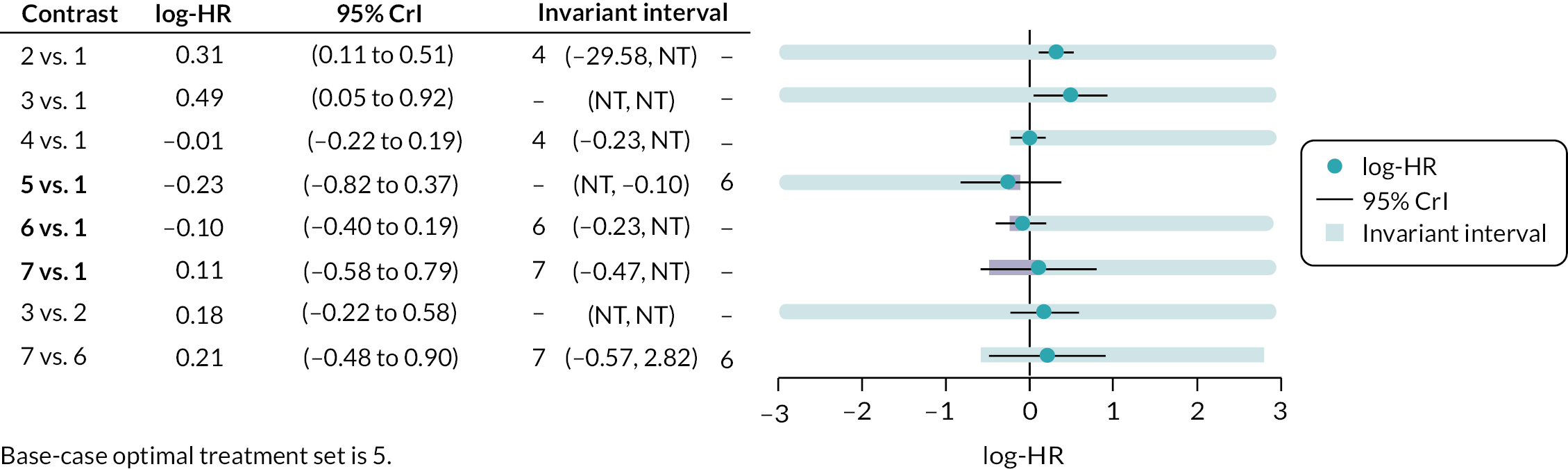
Credible intervals for the RFA + TACE versus RFA (5 vs. 1), MWA versus RFA (6 vs. 1), and IRE versus RFA (7 vs. 1) comparisons extend beyond the limits of the invariance intervals, suggesting that the recommended treatment is sensitive to the uncertainty in the data, changing the optimum treatment to MWA for the RFA + TACE versus RFA and MWA versus RFA comparisons and to IRE for the IRE versus RFA comparison.
Three comparisons that included PEI and PAI – PEI versus RFA (2 vs. 1), PAI versus RFA (3 vs. 1), and PAI versus PEI (3 vs. 2) – had very large thresholds on the log scale. The negative threshold for the resection versus RFA comparison (4 vs. 1) was very small, and a change of 0.21 units on the log-HR scale in the negative direction changes the optimum treatment to resection. The positive threshold for the RFA + TACE versus RFA comparison (5 vs. 1) was very small, and a change of 0.12 units in the positive direction changes the optimum treatment to MWA. Similarly, the negative threshold for the MWA versus RFA comparison (6 vs. 1) was very small, and a change of 0.13 units in the negative direction changes the optimum treatment to MWA.
Thresholds and new optimum treatments, based only on relative effects, are presented in Report Supplementary Material 5.
Local recurrence
The forest plot for the threshold analysis is presented in Figure 30.
FIGURE 30.
Forest plot for results of threshold analysis for local recurrence for the updated NMA.

Credible intervals for the MWA versus RFA (4 vs. 1), RFA + TACE versus RFA (6 vs. 1), and laser versus RFA (7 vs. 1) comparisons extend beyond the limits of the invariance intervals, suggesting that the recommended treatment is sensitive to the uncertainty in the data, changing the optimum treatment to MWA, RFA + TACE and laser, respectively.
Seven comparisons that included PEI and PAI – PEI versus RFA (2 vs. 1), PAI versus RFA (3 vs. 1), RFA + PEI versus RFA (8 vs. 1), high-dose PEI versus RFA (9 vs. 1), PAI versus PEI (3 vs. 2), TACE + PEI versus PEI (5 vs. 2), high-dose PEI versus PEI (9 vs. 2) – had very large thresholds on the log scale.
The negative thresholds for the MWA versus RFA (4 vs. 1) and RFA + TACE versus RFA (6 vs. 1) comparisons were very small, and changes of 0.09 and 0.19 units in the negative direction change the optimum treatments to MWA and RFA + TACE, respectively.
Thresholds and new optimum treatments, based only on relative effects, are presented in Report Supplementary Material 5.
Chapter 5 Feasibility of economic modelling
This section considers the feasibility of developing a de novo economic model to inform a cost-effectiveness and value of information (VOI) analysis considering ablative and non-surgical therapies for the treatment of small HCC tumours. In considering the feasibility of an appropriate economic evaluation, it is assumed that the developed model will be consistent with the NICE reference case,97 adopting a UK perspective and using a cost–utility approach accounting for both the relevant costs and benefits of the assessed technology.
Approach
Assessment of the feasibility of undertaking economic evaluation and VOI analysis was considered by conducting a targeted review exploring previous economic analyses evaluating technologies for the treatment of HCC; see Review methods below for details of methods used. Studies identified in the review were then summarised to consider key features and what data are typically required to support these models. Based on these previous evaluations and in consultation with clinical experts, a conceptual model was then developed to consider an appropriate model structure that could be used in any future economic analysis.
The availability of data to inform an economic analysis was considered. This assessment covered the availability of relevant clinical evidence (based principally on the clinical effectiveness review). The availability of evidence concerning quality of life, resource use and costs was also considered; this was informed by evidence identified as part of the clinical effectiveness review, the identified cost-effectiveness studies and established sources of relevant data.
Cost-effectiveness review
Review methods
Targeted literature searches were adapted from the search strategies used to identify RCTs (see Appendix 1) and included terms for small or early HCC and a broad set of terms aimed at identifying any economic evidence. The following databases were searched in May 2021:
-
Ovid MEDLINE(R) ALL: 1946 to 12 May 2021
-
Embase: 1974 to 12 May 2021
-
NHS Economic Evaluation Database
-
Econlit: 1886 to 29 April 2021.
Study design search filters for economic papers were applied to Ovid MEDLINE and Ovid Embase only. The Canadian Journal of Health Technologies (CADTH)’s98 narrow economic filter was used on MEDLINE and was adapted for use on Embase. No language or geographical restrictions were applied to the searches across any of the databases. A date limit of 2000 onwards was applied to the searches to align with the clinical effectiveness review. Details of the search strategies used are reported in Report Supplementary Material 1.
Study selection was conducted in two stages: (1) titles and abstracts were examined and screened for any study potentially relevant to the cost-effectiveness review; and (2) full texts were then obtained and screened for inclusion. A single reviewer screened all studies.
Studies were included in the review if they assessed the cost-effectiveness of any technology for the treatment of very early/early HCC; note that this is broader than the inclusion criteria for the clinical effectiveness review. A broad range of studies was considered for inclusion in the review, including economic evaluations conducted alongside trials, modelling studies, and analyses of administrative databases. Only full economic evaluations comparing two or more options and including both costs and consequences (cost-effectiveness, cost–utility or cost–benefit analyses) were included.
Studies meeting the inclusion criteria were summarised, noting key features including the model structure adopted, key assumptions and any data reported that may be relevant to undertaking an economic evaluation of ablative and non-surgical therapies for early HCC. As this was not intended to be a formal review of cost-effectiveness studies, study quality was not assessed.
Results
A flow diagram describing study selection is presented in Figure 31. Searches of the literature for economic evidence identified 496 papers following the removal of duplicates, with 38 identified for full text review. A further 21 papers were identified for full text review as part of the clinical effectiveness review, making a total of 59 papers. Following the selection process, seven studies reported in 11 publications were found to meet the eligibility criteria and were included in the review.
FIGURE 31.
Flow diagram of the study selection process (cost-effectiveness review).

An overview of the study characteristics for each included study is presented in Table 17. The majority of studies evaluated two treatment alternatives. Interventions evaluated included liver transplant, resection, RFA, SIRT, and TACE. In UK clinical practice, the use of SIRT, TACE and liver transplant for the treatment of small HCCs is limited; their inclusion in the identified studies reflects the broad inclusion criteria and national differences in clinical practice.
| Cucchetti (2013)99–101 | |
| Model structure | The modelling approach is not fully clear; described as a Markov model, but potentially adopts a semi-Markov or simulation approach. Model considers survival, recurrence and Child–Pugh status. |
| Time horizon, perspective and discounting | Time horizon was not stated. Perspective and setting were not stated, though the majority of the costs were drawn from Italian national health system. Costs and benefits were discounted at a rate of 3%. |
| Population | Patients within the Milan criteria up to three tumours < 3 cm, or one tumour up to 5 cm. |
| Intervention and comparators | Resection vs. RFA. |
| Clinical evidence | Parametric extrapolation of survival data (OS and disease-free survival) appears to have been undertaken though the specific approach adopted is unclear. Hazard rates applied to model treatment efficacy were based on the proportion of patients achieving 3-year survival/3-year disease-free survival and were drawn from a meta-analysis of relevant studies. The model also drew on evidence of hospital length of stay which was drawn from the meta-analysis and parameterised in the model. |
| HRQoL | Health state utilities were based on values reported in the literature, including a review by McLemon et al.110 Values did not vary by treatment received and were not specific to HCC. |
| Resources and costs | Cost categories modelled included procedure costs, length of stay, costs of subsequent treatments and patient follow-up costs. Costs applied in the model were obtained from Medicare and Italian national health system sources. |
| Lai (2014) 103 | |
| Model structure | Markov model with the following health states: small HCC < 3 cm tumour, cancer-free, progressive HCC and death. Additional tunnel states were also used to count the number of ablation procedures, with a maximum of three permitted. |
| Time horizon, perspective and discounting | Time horizon appeared to be lifetime horizon (until 99% of patients were dead). A Chinese health-care setting was considered, but the perspective was not stated formally. Costs and benefits were discounted at a rate of 3%. |
| Population | Patients with a solitary, small tumour < 3 cm and Child–Pugh class A or B. |
| Intervention and comparators | Real-time virtual sonography-guided ablation vs ultrasound-guided ablation. |
| Clinical evidence | Probabilities for each outcome were drawn from the literature, with the majority of inputs drawn from Cho et al.111 Efficacy was not determined using comparator estimates of effect. Outcomes considered included mortality rates (with separate rates applied to cirrhotic patients, tumour-free patients, progressed HCC), ablation success rate, rate of local recurrence, distant recurrence, probability of seeding tumour (RFA only), liver transplant rate, procedure-related mortality and procedure-related complications. |
| HRQoL | Health state utilities were based on values reported in the literature, including McLemon et al.110 Values did not vary by treatment received and were not specific to HCC. |
| Resources and costs | Cost categories considered included procedure costs, inpatient administration costs associated with RFA, disease management and follow-up care costs, terminal care costs, and AE costs. Values were drawn from the literature and did not consider any UK relevant sources. |
| Lim (2015) 109 | |
| Model structure | Markov cohort model with alternative model structures applied according to treatment received. In the liver resection arm the following health states were modelled: compensated cirrhosis, decompensated cirrhosis, HCC recurrence, dead. In the liver transplant arm the following states were modelled: waiting list compensated cirrhosis, waiting list decompensated cirrhosis, liver transplant contraindicated, post liver transplant, dead. |
| Time horizon, perspective and discounting | Time horizon was not reported; a payer perspective was adopted though setting was not clear. Costs and benefits discounted at a rate of 3%. |
| Population | Patients within the Milan criteria up to three tumours < 3 cm, or one tumour up to 5 cm. |
| Intervention and comparators | Liver resection vs. liver transplant. |
| Clinical evidence | Evidence was drawn from multiple sources identified in the literature and did not rely on comparative assessment of effectiveness. Outcomes modelled included: decompensation risk, decompensated cirrhosis-related survival, postoperative risks (liver resection and liver transplant), post liver resection recurrence rate, wait list time, dropout risk and survival. |
| HRQoL | Health state utilities were based on values reported in the literature, though the specific studies used were not reported. Values did not vary by treatment received. |
| Resources and costs | Cost categories considered included: procedure costs, and disease management and follow-up costs. Costs were drawn from a systematic review of values reported in the literature and the median reported value used. Where data were unavailable, clinical expert opinion was used. Costs used were not directly relevant to the UK. |
| Naugler (2010) 106 | |
| Model structure | Markov model using two distinct structures for each arm. In the watchful waiting arm the following health states were modelled: monitoring without therapy, tumour progression inside Milan criteria, tumour progression outside Milan criteria, liver decompensation, and death. In the immediate treatment arm the following health states were modelled: HCC therapy, tumour progression inside Milan criteria, liver decompensation, and death. |
| Time horizon, perspective and discounting | Time horizon was 10 years. Perspective and setting were not stated; costs were however, drawn from the US health system. Costs and benefits were discounted at a rate of 3%. |
| Population | Patients with tumours < 2 cm, not eligible for resection but eligible for transplant with compensated cirrhosis. |
| Intervention and comparators | Watchful waiting vs. immediate treatment with TACE vs. immediate treatment with RFA. |
| Clinical evidence | Probabilities for each outcome were drawn from the literature using multiple sources. Efficacy was not determined using comparator estimates of effect. Outcomes modelled in the watchful waiting arm included: tumour progression inside/outside Milan criteria, and survival inside/outside Milan criteria. Outcomes modelled in the immediate treatment arm included: survival within Milan criteria, survival without progression, tumour progression inside Milan criteria. In both arms the model also considered liver decompensation risk, liver transplant rate, and post-transplant survival. |
| HRQoL | Not considered. |
| Resources and costs | Cost categories modelled included procedure costs, disease management and patient follow-up costs, drug acquisition costs. Costs applied in the model were obtained from Medicare and were not relevant to a UK setting. |
| Rostambeigi (2014)107,108 | |
| Model structure | The model used a simulation approach. The structure adopted was not clearly reported, but appeared to allow for disease recurrence, mortality, and liver transplant. |
| Time horizon, perspective and discounting | Time horizon was not stated. Perspective and setting were not stated; costs were however, drawn from the US health system. Discounting of future costs and benefits does not appear to have been applied. |
| Population | BCLC A. |
| Intervention and comparators | SIRT vs. TACE. |
| Clinical evidence | Probabilities for each outcome were drawn from exponential curves and used to estimate survival based on reported survival rates. Other outcomes considered include recurrence and re-treatment of HCC, and transplant rates. |
| HRQoL | Not considered. |
| Resources and costs | Cost categories modelled included procedure costs, AEs, and patient follow-up costs. Costs applied in the model were obtained from Medicare reimbursement costs and were not directly relevant to the UK. |
| Sarasin (2001) 102 | |
| Model structure | A Markov model was developed that accounted for wait time for transplant. Modelled health states included: cirrhosis, HCC, no contraindications to CLT, cured HCC and cirrhosis, contraindications to CLT/palliative care, and death. |
| Time horizon, perspective and discounting | Time horizon was not stated. A US payer perspective was adopted using 1998 prices. Costs and benefits were discounted at a rate of 3%. |
| Population | Early HCC – single HCC not exceeding 5 cm in diameter, or up to three tumours up to 3 cm in size, in the absence of vascular or extrahepatic involvement. |
| Intervention and comparators | CLT vs. LDLT. |
| Clinical evidence | Parameter inputs were identified via searches of the literature. Outcomes were determined by wait time (2 months for LDLT, 6 months for CLT), probability of developing contraindications, donor mortality, palliative care mortality and post-transplant mortality. Transplant outcomes between CLT and LDLT were assumed to be the same. |
| HRQoL | Utility values were informed by the literature and did not vary by treatment received. |
| Resources and costs | Cost categories modelled included chemoembolisation costs incurred while waiting for transplant, transplant-related costs (assumed to be the same for CLT and LDLT), donor assessment (accounting for failures to proceed), and disease management and patient follow-up costs. Costs used were not directly relevant to the UK. |
| Spolverato (2015)104,105 | |
| Model structure | Multistate model with alternative model structures applied according to treatment received. The model considered the following states: undergoing liver resection or radiofrequency treatment (liver resection/RFA only), liver decompensation (liver resection/RFA only), HCC recurrence (liver resection/RFA only), progression of disease within Milan criteria (liver resection/RFA only), progressive disease outside Milan criteria, transplant waiting list, (post) liver transplant, and death. |
| Time horizon, perspective and discounting | Time horizon was not reported. Italian and US healthcare settings were considered using a payer perspective. Costs and benefits were discounted at a rate of 3%. |
| Population | Patients within the Milan criteria up to three tumours < 3 cm, or one tumour up to 5 cm. |
| Intervention and comparators | Liver transplant vs. liver resection or RFA with salvage liver transplantation. |
| Clinical evidence | Evidence was drawn from multiple sources identified in the literature and did not rely on comparative assessment of effectiveness. Outcomes modelled included: transplant wait time, post-transplant mortality, wait list dropout rate, liver decompensation, disease recurrence. |
| HRQoL | Health state utilities were based on data reported in Lim et al.109 and did not vary by treatment received. |
| Resources and costs | Cost categories modelled included procedure costs, drug acquisition costs, disease management and patient follow-up costs. Resource data were drawn from two previous reviews of the literature: Cucchetti et al.99–101 was used for Italian healthcare costs and Lim et al.109 for US costs. Costs used were not directly relevant to the UK. |
None of the identified studies considered a UK NHS perspective. One study considered an Italian setting only,99–101 one a US setting only,102 and one a Chinese setting only. 103 One further study considered both an Italian and a US setting. 104,105 In three studies the setting was not formally stated. In two of these studies,106–108 costs were reported for a US setting, while a third study109 reported costs for three alternative settings: USA, Singapore and Switzerland. All studies considered a payer perspective, where stated. As no study considered a UK setting, costs utilised are not relevant to the UK perspective. The identified studies are therefore unlikely to represent an informative source of resource data for any future economic evaluation adopting a UK perspective.
The model structures adopted in the identified studies varied significantly, with several alternative underlying approaches adopted. These included Markov models,103–106,109 semi-Markov models,99–101 and simulation approaches. 107,108 Model structures adopted were typically highly complex, with several using a large number of health states. Importantly, model structures did not conform to the three-state models commonly used in cancer evaluations. Despite a lack of consistency in the approach adopted across models, several features were common to the included studies. These included the modelling of recurrence of disease and the competing risks of declining liver function. Both of these features were uniquely associated with locoregional therapies such as RFA and resection and were not considered relevant to patients receiving a liver transplant. In several models, this meant that the structure adopted differed substantially between treatment arms. 104–106,109
Because of the novel model structures adopted, treatment effects were often modelled using several parameters typically drawn from multiple studies. While this approach reflects the complex treatment pathways and allows a broader evidence base to be drawn upon, it comes with significant disadvantages. Namely, in this approach treatment effects are not based on comparative evidence and are highly likely to be subject to confounding biases. Further, while many models considered multiple outcomes, it is clear from model results that survival is the principal driver of benefits. An important consideration for future economic evaluations will therefore be how to best integrate available comparative evidence while also accounting for the divergent treatment pathways. In an ideal scenario this is likely to mean drawing directly on comparative evidence of survival. However, given the potentially curative nature of the evaluated treatments, such comparative evidence may be uninformative due to lack of maturity and developments in care for progressed HCC. It may therefore be necessary to draw on external data sources potentially linked to intermediate outcomes or events like transplant or recurrence of disease to populate an economic model.
Model scope and availability of comparative data
Based on the systematic review of clinical effectiveness evidence and clinical advice, it is anticipated that there is a wide range of relevant comparators. These include established treatments such as resection and MWA, treatments that have more recently become available to UK patients such as SABR, and treatments that are no longer/rarely used in clinical practice (PEI and laser ablation). In principle, all of these therapies could be considered by a future cost-effectiveness analysis. However, clinical advice suggests that many of these newer technologies are rarely used in routine practice (e.g. ECT) owing to a lack of evidence/approval, while older technologies such as PEI and PAI have largely been discontinued due to lack of efficacy and concerns regarding AEs. Further, clinical advice suggests that some technologies such as IRE and SABR would not be used in the whole small-HCC population but instead would be reserved for patients with tumours in locations that are either difficult to treat or patients who are otherwise medically unsuitable for RFA. Any future economic analysis will therefore need to carefully consider the decision problem being addressed and which comparators are likely most relevant to decision-makers. Further, given the absence of evidence for some potentially relevant comparators, including many of the newer technologies, it may be necessary for a future economic analysis to focus only on a subset of all relevant comparators. This may limit the feasibility of implementing an informative economic analysis and is likely to impact on the strength of conclusions that can be drawn.
Model structure and clinical data availability
The model structure typically adopted in economic evaluations of treatments for cancer uses a partition survival model (PSM) based around three health states: (1) pre progression, (2) post progression and (3) death. In a PSM the proportion of patients in each health state is determined directly from the survival curves, typically PFS and OS. Under this approach the proportion of patients in the ‘pre-progression’ state is determined by the PFS curve while the proportion in the ‘post progression’ state is determined by the difference between the modelled OS and PFS survival curves. Theoretically this approach could be adopted in the context of early HCC, but it may need adaptation to account for specific features of the indication. For example, as highlighted above, many models account for the competing risk associated with liver decompensation and potential for recurrence but not progressed disease. These complications may undermine the feasibility of a PSM approach, and the adaptations necessary may be easier to accommodate in a state transition model where it is often easier to explicitly acknowledge competing risks.
An alternative to the PSM approach would be to use a state transition model focused on utilising comparative evidence on recurrence and disease-free survival. This approach aligns with much of the previous cost-effectiveness literature and would more readily recognise the surrogate role that recurrence and disease-free survival play in determining OS. Under such an approach, post-recurrence survival would likely be modelled using a common set of assumptions for all treatments. While notionally this is a disadvantage as it assumes a consistent surrogate relationship between recurrence and OS, it would allow external data to be levied; this may provide improved estimates relative to the available trial data, which may be limited due to the short follow-up in many studies. This approach also allows post-recurrence survival to reflect recent developments in the treatment and care of patients with intermediate and advanced-stage HCC. This may be important given the more recent (post 2009) availability of sorafenib and other agents for the treatment of advanced HCC and the fact that the majority of the currently available clinical evidence is not from a UK setting.
Clinical advice on the aims of treatment emphasised the importance of recurrence, and particularly local recurrence, as a marker of treatment success. The importance of local recurrence as a determinant of mortality was also emphasised. It was, however, also emphasised that other factors are also important determinants of survival and may confound any relationship between local recurrence and OS. These included both intrahepatic and extrahepatic recurrence, which may lead to cancer progression regardless of local disease control. Further, clinicians noted the importance of liver function as a competing mortality risk, as well as its significance in determining patient quality of life.
This advice would appear to broadly support the use of a recurrence-focused approach but also emphasises the complexity of very early/early HCC and the need to account for the competing risks of disease progression and liver decompensation. The clinical data available to inform a recurrence-focused approach are, however, limited. Few studies identified in the clinical review reported recurrence, with only 10 of 27 identified studies reporting recurrence outcomes. This may impact on the feasibility of developing a robust economic model based around recurrence of disease, as it means that the totality of the evidence cannot be considered.
More broadly, inherent uncertainties in the clinical evidence, as well as concerns about the quality of included evidence, will have important consequences for any future economic analysis. As presented in Updated network meta-analyses using RCT and non-RCT evidence, current clinical evidence is insufficient to make recommendations about the relative effectiveness of the majority of treatments. An economic analysis cannot resolve these uncertainties and will necessarily be limited by them. Importantly, these uncertainties are likely to undermine the ability of any future economic analysis to make recommendations about which treatments are most cost-effective. This may undermine the value of implementing an economic analysis. An economic analysis may, however, still be worthwhile because of its ability to quantify the uncertainty associated with implementation decisions. In doing so, an economic analysis can help provide information about the value of future research; see Value of information below for further discussion.
Utilities and quality of life
In the literature identified as part of the clinical effectiveness review, no RCTs and only one non-RCT collected quality-of-life data,87 and no study reported utility data. Any new economic evaluation will therefore have to identify alternative sources of relevant utility data. The identification of relevant utility data is likely to require detailed searches of the literature. Based on the cost-effectiveness evidence identified in our review, several studies reported utility values that may be relevant to any future analysis. 99–103,106,109 However, the provenance of some of the values reported is unclear. 109 In other cases, it is also apparent that the values obtained are taken from patients with liver disease rather than specifically from patients with HCC. 102,103 Further, several of the evaluations identified in the cost-effectiveness review highlighted limitations in the available quality-of-life data. 102,104,105,109 Identifying relevant utility data is likely to represent a significant challenge and source of uncertainty for any new economic evaluation in early HCC.
Resource use and costs
Resource use and costs should include treatment costs (acquisition, procedures, and monitoring), changes in health service utilisation driven by disease status (i.e. progression-free, progressed disease, and death), and AE management. Costing data from previous economic analyses in early HCC are unlikely to be informative due to differences in perspective; no study was conducted from a UK perspective. Further, few studies reported relevant resource-use estimates associated with specific treatments. Previous economic evaluations are therefore unlikely to provide resource inputs for a new model.
Several of the studies identified in the clinical effectiveness review reported on useful economic outcomes such as length of hospital stay. Assuming these studies are generalisable to a UK setting, these outcomes could be used to support inputs regarding acute care and monitoring following treatment. The majority of resource-use inputs will, however, need to be identified in further research. This may be in the form of a clinician survey to elicit resource utilisation or identification of relevant costing studies. Alternatively, health state management costs may be informed by previous UK economic evaluations in advanced HCC, and adapted to account for the target early HCC population. Costing data for the UK are readily available from several commonly used sources. These include NHS reference costs,112 Personal Social Services Research Unit,113 and the British National Formulary. 114 While further research is necessary, the availability of resource-use and costing data is unlikely to represent a significant barrier to implementing a future economic evaluation.
Value of information
The construction of a de novo economic analysis in which uncertainty is fully parameterised would allow the implementation of a VOI analysis. A VOI analysis permits the value of reducing decision uncertainty to be quantified in monetary terms. The VOI can then be compared with the costs of further studies and used to assess whether additional research should be conducted to reduce decision uncertainty.
In the context of the current evidence, a VOI analysis may be particularly helpful, as there are currently several treatment alternatives for which there is limited evidence on effectiveness. A VOI analysis could help prioritise which of these treatments should be assessed in future trials, accounting for both the degree of clinical uncertainty and the economic case for a specific treatment. This may be of particular relevance in considering treatments that are currently rarely used in NHS practice but may be effective; for example, laser ablation and RFA. Moreover, a VOI analysis may help to provide clearer guidance on where research is not worthwhile despite the presence of clinical uncertainty. For example, VOI may be able to rule out particularly expensive technologies on cost grounds alone despite the potential for clinical benefit.
Chapter 6 Patient and public involvement
Aim
The aim of patient and public involvement was to ensure that the patient’s perspective was captured at all stages, from protocol development through to interpreting the results of the project and drawing conclusions and recommendations for further research.
Methods
A patient collaborator was recruited to the project at the proposal writing stage via ‘Involvement@York’, the patient and public involvement network at the University of York. The patient collaborator attended all advisory group meetings and provided ongoing advice throughout the project. The patient collaborator was also consulted when producing materials in ‘plain English’, such as materials used when recruiting additional patients to the advisory group and the plain English summary section of the final report. The patient collaborator will be consulted during further dissemination activities.
Four additional patients were identified by our clinical advisors and recruited as members of the advisory group. With help from the patient collaborator, a lay summary of the project was produced describing the project, the role of advisory group members and details of how patients would be compensated for their time. This was circulated to patients who had expressed an interest in being a member of the advisory group. Patients were also provided with a lay summary of the different interventions included in the systematic review.
One member of the project team (RW) was the main contact for all patient advisors and held individual meetings with patients at the protocol development stage. During this initial meeting, patients were given background information to the project and a rudimentary description of the protocol and were asked for their comments, specifically whether any patient-relevant outcomes or aspects of treatment were missing from the protocol. Owing to the COVID-19 pandemic, all advisory group meetings were held via the Zoom™ online videoconferencing platform (Zoom Video Communications, San Jose, CA, USA), rather than in person. Patients were invited to attend the next advisory group meeting and the end-of-project workshop (see Workshop). Patients were also asked to comment on the final report.
Results
All four patients were available at the beginning of the project to advise on the protocol. The patient collaborator and three of the patient advisory group members attended the second advisory group meeting held midway through the project to discuss the interim findings, prioritise interventions for further review and prioritise the most relevant patient outcomes. Patients provided helpful information about the outcomes most important to them, such as length of hospital stay and disruption to life (interventions requiring multiple appointments or repeat treatments) and level of pain involved. Non-recurrence of disease was another important outcome to patients. The patient collaborator and two patient advisory group members attended one of the end-of-project workshops. Unfortunately the other two patients were unavailable around the time of the workshops; in view of the reasons for their lack of availability, they were not pursued to attend at a different time. Patients were surprised by the lack of data on patient preference and quality-of-life outcomes in the existing evidence base. The patient collaborator and two patients commented on the final report.
Discussion and conclusions
The patient and public involvement aspect of the project highlighted the outcomes most important to patients, which informed the development of the data extraction form. Their views added context to the review findings and their input was valuable when drawing conclusions and making recommendations for further research. The initial meeting with patients was informative to help the researchers understand the experience of patients, their concerns and preferences.
Reflective/critical perspective
Patient involvement was a valuable part of this project, enabling researchers to understand important aspects of the different treatment options from a patient’s perspective. One drawback was that meetings had to be held via the Zoom online videoconferencing platform, owing to the COVID-19 pandemic, which constrained the interactions with patients.
The feedback from patients was positive; they commented that information was presented clearly and that they found the meetings interesting and enjoyed being involved in the project.
Chapter 7 Workshop
Two workshops were held with clinical and patient advisory group members and additional clinicians with an interest in HCC (identified by advisory group members) in order to discuss the project findings and identify key priorities for future research. Due to the COVID-19 pandemic, the workshops were held using the Zoom online videoconferencing platform, on 29 November and 2 December 2021. Prior to the workshops, the attendees were sent a short summary of the findings of the project to date, including a summary of the methods and results of the systematic reviews, NMAs and the assessment of the feasibility of economic modelling.
Members of the project team presented a summary of the findings of the project and responded to clarification questions. There was a general discussion of the interpretation of the project findings, and workshop participants were asked about the key priorities for future research, including interventions, patient groups, and outcomes.
The lack of evidence for many interventions, low quality of the available evidence and uncertainty of the findings was highlighted. The generalisability of the findings of studies from East Asia (where the underlying aetiology of the liver disease differs from that in the West) was discussed, since most of the RCTs assessed RFA, which is more widely used in the East, whereas MWA has become the standard of care in most centres in the West. It was agreed that differences in underlying liver disease are likely to affect the absolute OS of patients, rather than the relative survival when comparing one treatment against another.
The progression in the West from RFA to MWA as the standard of care has been driven by technological advances and ease of use of MWA (which only requires single needle placement, so is both faster and simpler to deliver) rather than data on improved clinical effectiveness. MWA gives a more predictable ablation zone up to 3 cm, whereas the RFA ablation zone is less predictable towards the periphery. However, it was considered that, moving forward, it would not be appropriate to compare the clinical effectiveness of RFA versus MWA, as many interventional radiologists in the UK only know how to use MWA, not RFA, and clinicians believe that MWA is the superior treatment, so it may be difficult to recruit patients to a trial comparing the two treatments. In addition, RFA is only used for tumours up to 2 cm (owing to increased local recurrence after RFA in lesions larger than 2 cm), whereas MWA can be used for larger tumours; therefore, any trial comparing both technologies would have to restrict recruitment to patients with tumours up to 2 cm in order for patients to be eligible for both treatment arms. Lesions close to hepatic vessels are also less amenable to RFA, reducing the eligible patient cohort further.
RFA was used as the baseline treatment in the NMA (for comparison against other treatments) because it was the most widely assessed technology in the RCTs. Historically, surgery was considered to be the gold standard, before RFA became available. However, it would not be appropriate to compare the effectiveness of surgery versus ablation, as the risks of surgery for many patients are too high. Resection is not suitable for cirrhotic patients with marginal liver function or patients with clinically significant portal hypertension. Tumour location is also important; resection would not be suitable for patients with central tumours, particularly in patients with cirrhosis, as the risks of resection are much higher than those of ablation. At the second workshop, the comparison of MWA versus resection was discussed further, as some centres are still quite ‘surgery heavy’ and may want to see more trial evidence on MWA versus resection, although most centres are moving towards MWA owing to the complications of resection making it less acceptable than ablation.
Specific effectiveness outcomes and the association between liver decompensation (liver failure) and mortality were discussed. Registry data suggest that around half of patients who undergo ablation will die following liver decompensation, and half will die without liver decompensation; therefore, the risk between recurrence and mortality is important, but there is substantial competing risk according to the severity of underlying disease. At the second workshop it was highlighted that some HCC treatments have a risk of causing liver decompensation, although patients with early HCC have good Child–Pugh scoring and good performance status.
It is difficult to demonstrate treatment benefit in a trial when there are competing risks of both liver disease progression to decompensation in addition to the risks of recurrent or new HCC. Recurrence can be local (near the site of the previously ablated lesion) or distant (a new lesion elsewhere within the liver); therefore, treatment of the original tumour may not impact on OS. Rates of recurrent and new HCC are very high; up to half of patients will have a new metachronous cancer within 3 years of treatment of the index lesion, which is a further driver of poor outcomes in this patient group. Rates of metachronous disease would be expected to be similar between different treatment arms in a trial, unless one of the interventions treats the whole liver. This is why transplantation is theoretically the best treatment for early-stage HCC, because it replaces the liver that has malignant potential with a new one, so there is no longer the risk of metachronous disease or decompensation. However, liver transplant is not normally the primary intervention for the population of patients with early-stage HCC.
Quality of life was only reported in one included study, a non-randomised study undertaken in China. It is important to assess quality-of-life outcomes and patient acceptability in any future trial; there is a lack of evidence on these important outcomes in the existing literature.
The problem of patient recruitment was discussed, as there are not many patients with early-stage (≤ 3 cm) HCC in the UK. The marginal benefit of novel treatments compared with the existing standard of care is likely to be small, so future studies would need to be large to demonstrate a significant difference in outcomes. Therefore, an international multicentre RCT may be more appropriate than a UK-based trial.
At the first workshop, clinicians said that SABR and proton beam therapy are interventions of interest and that a trial of SABR or proton beam therapy versus MWA would be useful, although use of proton beam therapy is limited by geographical availability. Local control rates with both treatments are very high; therefore, undertaking a trial that was sufficiently powered to show a survival benefit would be difficult, since neither deals directly with recurrence (metachronous or extrahepatic disease) and neither has an impact on rates of decompensation. Local recurrence would have to be the primary outcome in such a trial, with OS and PFS as secondary outcomes. At the second workshop it was also agreed that local recurrence and overall recurrence are both important outcomes.
It is internationally recognised that there needs to be more trial-based evidence for SABR in the treatment of patients with early-stage HCC. The availability of such evidence is limited by the fact that ablation techniques such as MWA and RFA are usually employed first for patients with early HCC and SABR reserved for recurrent, refractory or more advanced disease. SABR can usually only be delivered once because of the radiation dose, whereas ablation can be repeated; therefore, there is also the question of when it should be used – should it be saved until later in the treatment pathway? There was discussion around assessing different treatment sequences. Although treatment sequencing is an important question, the difficulty is the heterogeneity of recurrence, which has implications for the next treatment choice; therefore, it may not be possible to predetermine the second line in the sequence. Both MWA and SABR can be used for patients with tumours ≤ 3 cm, and both interventions can be used to treat more than one lesion at once; therefore, trial eligibility criteria would have to reflect this. There would also need to be eligibility criteria limitations based on liver function, as patients with advanced/moderately advanced liver disease are not suitable for SABR but could possibly be suitable for ablation; patients recruited to a trial would have to be eligible for both treatments.
At the second workshop, clinicians considered that a trial of SABR versus MWA may be less appropriate; MWA is a good treatment for small tumours, while SABR is usually reserved for tumours that do not respond or are unsuitable for ablation or are in a difficult location. Therefore, SABR and other radiotherapy techniques would not replace MWA. In addition, MWA can be repeated, while SABR can usually only be used once. This positioning of SABR reflects current NHS England commissioning guidance which suggests SABR and MWA for different patient populations; for patients with very early/early-stage HCC, ablation is the first choice, while if the lesion cannot be clearly visualised for ablation or is in a location that cannot be reached with a needle, then TACE would be offered. If TACE is contraindicated (e.g. for cardiac reasons or if the patient has had TACE previously and failed), then SABR would be offered. The clinicians also noted there is a study in North America comparing SABR versus proton beam therapy; proton beam therapy may be the preferred modality for patients depending on disease location.
At the first workshop, clinicians said that IRE can be used for lesions that are very central; therefore, a trial of IRE versus MWA for the subgroup of patients with central lesions may be useful, although SABR could also be used. At the second workshop it was agreed that IRE is sometimes used for more challenging tumours, but owing to the evidence base being very limited for IRE, SABR is the preferred option. In addition, IRE is quite costly; therefore, MWA would be used when suitable; they would not be comparable in a trial.
ECT is very similar to IRE but with the addition of bleomycin. It is beginning to feature in Europe, so may be of interest.
Cryoablation was not considered to be of interest as it is a high-risk treatment. It has not been widely adopted in the West. At the second workshop, it was stated that more evidence is being published on cryoablation, especially for lesions that are difficult to treat with MWA, such as those that are near the dome of the liver or close to the heart, where freezing therapy is slightly less damaging than heat treatment; thus it is mostly used for those lesions that are difficult to treat because of nearby vital structures. However, if IRE is available, that would be used rather than cryoablation, so cryoablation has a lower priority.
Laser has also not been widely adopted in the West. It involves multiple needle placement, whereas MWA only requires single needle placement so is both faster and simpler to deliver. However, there are no clinical effectiveness data comparing it with MWA, so the comparative effectiveness is unknown. However, ease of use is an important consideration in treatment choice; any intervention with a substantial learning curve barrier is going to be less easily accepted from a clinical perspective. HIFU has also been around for several years but has not been widely adopted.
Histotripsy is currently being evaluated as an investigational product; therefore, it should not be assessed further until efficacy has been demonstrated. However, it appears to be very promising and may be of interest further down the line.
At the second workshop, it was considered that the questions to answer in early HCC are more in the setting of challenging locations, less fit patients and in the setting of incomplete response to primary therapy, rather than a comparison with the current preferred first treatment option. There is probably some variation between multidisciplinary teams on whether they would offer TACE and whether they have SABR and/or IRE available. It may be difficult to define the population and ensure that a trial was acceptable to multidisciplinary teams that might have slight variations in practice and also looking at what technologies are available locally to a patient.
Chapter 8 Discussion
Summary of findings
The aim of this research was to evaluate and compare the effectiveness of ablative and non-surgical therapies for patients with small (up to 3 cm) HCC tumours. The key objectives were: to systematically identify all RCTs of ablative and non-surgical therapies for HCC; to evaluate their quality and applicability to UK populations; to determine the comparative effectiveness of therapies using NMA techniques; to supplement the RCT evidence with non-randomised prospective comparative studies of specific therapies where the evidence base was insufficient; to identify priority areas where additional high-quality evidence is required; and to assess whether future economic analysis based on the findings would be feasible and worthwhile.
Thirty-seven RCTs (one ongoing, 36 completed) were included in the systematic review. Several included patients with tumours larger than 3 cm, but reported separate results for the subgroup of patients with tumours up to 3 cm, although often the data reported for the subgroup were limited to response and/or AE outcomes. The RCT evidence was limited; most studies were small and at a high RoB (12 RCTs) or had some bias concerns (14 RCTs). The vast majority of RCTs were conducted in China or Japan, which has implications for the generalisability of results to the UK population, owing to differences in HCC aetiology and the different treatment options for the underlying liver disease. The most frequently assessed ablative therapy was RFA, which is widely used in Asia. However, in the UK and Europe MWA has been more widely adopted because of advances in microwave technology; MWA gives a more predictable ablation zone and is easier and faster to use, requiring single needle placement. Many interventional radiologists in the UK do not have experience of using RFA.
The results of many of the included RCTs were heterogeneous, particularly for the comparison of RFA versus surgical resection, with some RCTs favouring surgical resection and others favouring RFA or reporting similar OS and disease-free survival rates between treatment groups. However, AE rates were higher after resection. There was no evidence to suggest a difference between treatment with RFA and resection in the NMA.
Data comparing RFA with MWA, laser ablation or proton beam therapy were limited, with few RCTs and very small sample sizes. RCTs assessing RFA in combination with other treatments were also limited by small sample sizes. The uncertainty associated with the available data is demonstrated in the NMA results, where CrIs were generally wide and most crossed the line of no effect. The estimated treatment effectiveness ranking was also very uncertain, with very wide CrIs for most interventions.
The only firm conclusion that can be drawn from the available RCT data is that RFA appears to be better than PEI in terms of OS, PFS and recurrence. However, AEs appear to be more frequent after RFA than PEI, although this outcome could not be evaluated in a NMA. PAI appears to have similar effectiveness to PEI and had marginally worse PFS than RFA in the NMA, although data for this comparison were more limited.
One trial assessed RFA in combination with iodine-125, which appeared to be superior to RFA in terms of OS and overall recurrence; however, clinical advisors stated that this is only used in selected centres in China, and very few centres outside of China have used this combination.
No RCT evidence was identified for several of the interventions of interest: HIFU, cryoablation, IRE, ECT, histotripsy, SABR and wider radiotherapy techniques. As highlighted at the project workshop, histotripsy is currently being evaluated as an investigational product; therefore, it is unlikely that randomised evidence will be available within the next few years. Cryoablation, IRE, ECT and SABR are generally reserved for the subgroup of patients with lesions that are more challenging to treat because of their location. SABR is also reserved in the treatment pathway for patients with recurrent, refractory or more advanced disease, or patients with comorbidities that make them unsuitable for ablative therapies. This makes it more difficult to undertake a randomised trial, as recruited patients would have to be eligible for both treatment arms. In addition, some of these technologies are less widely available and have a higher cost than ablative technologies such as RFA and MWA.
The threshold analysis suggested that additional evidence could plausibly change the NMA result for comparisons including RFA, MWA or laser ablation, as well as RFA in combination with TACE, systemic chemotherapy or iodine-125. Therefore, a systematic review of non-randomised prospective comparative studies was undertaken to identify evidence on RFA, MWA, laser ablation, HIFU, cryoablation, IRE, ECT, histotripsy, SABR and wider radiotherapy techniques, compared with each other or with surgical resection.
The systematic review of non-randomised evidence included 14 studies, although only two studies did not have a high RoB. Several studies allocated patients to treatment groups based on tumour characteristics (such as tumour location), meaning that there were differences in baseline characteristics between treatment groups that could be prognostic. This has implications for the interpretation of the non-randomised evidence; in addition, included patients may not have been eligible for both of the treatments assessed. Again, the vast majority of studies were conducted in China or Japan, with implications for the generalisability of results to the UK HCC patient population. In view of the significant limitations of the non-randomised studies, the studies with a high RoB were not included in the updated NMAs, leaving only the two studies that had a low RoB or some bias concerns. Additional non-randomised comparative data from Leeds Teaching Hospitals NHS Trust were made available by one of the clinical advisors, prior to publication; these data were also included in the updated NMAs.
The results of the updated NMAs including the non-randomised evidence were largely consistent with those of the NMAs of RCTs. As with the NMAs of randomised evidence, the findings were highly uncertain. However, the results suggested that MWA appears to be better than PEI and PAI in terms of OS and PFS. Resection appears to be better than PEI in terms of OS, and better than PEI and PAI in terms of PFS. In addition, IRE appears to be worse than RFA and RFA + PEI in terms of local recurrence.
The feasibility of developing an economic analysis to inform decision-makers on the cost-effectiveness of alternative treatments for small HCCs was assessed. This included a targeted literature review, which was undertaken to identify previous economic evaluations in very early/early HCC. The key features of the identified studies were summarised and used to inform the development of a conceptual model and to consider the data needed to develop a robust economic analysis. The review identified that previous economic evaluations have used recurrence events and liver function to predict long-term outcomes. This approach is likely to be the most appropriate way to model early HCC given the current evidence. Limitations in the available clinical data are, however, likely to impact on the feasibility of developing a robust economic analysis and limit any conclusions that could be drawn. Specifically, uncertainties in the clinical effectiveness will pervade any future economic analysis.
Given these uncertainties, a VOI analysis may be helpful and could help prioritise which of these treatments should be assessed in future trials, accounting for both the degree of clinical uncertainty and the economic case for a specific treatment. This may be of particular relevance in considering effective treatments that are currently rarely used in NHS practice.
There are considerable limitations to the existing evidence base on ablative and non-surgical therapies for early HCC. Two workshops were held to discuss the project findings and identify key priorities for future research; three patients and six clinicians provided expert advice. In view of the wide adoption of MWA as the standard of care within the UK and Europe, it was agreed that MWA would be the most appropriate comparator in any future trials. Clinicians considered that ablative technologies that are more complex and take longer to deliver than MWA (e.g. laser and RFA, which require multiple needle placement) are unlikely to displace MWA as the preferred ablative therapy, despite a lack of clinical effectiveness evidence demonstrating better outcomes.
Specific interventions considered to be of particular interest to the HCC community were SABR and proton beam therapy, although these radiotherapy-based treatments can usually only be delivered once, whereas ablation can be repeated. In addition, there would need to be limitations to trial eligibility criteria, as patients with advanced/moderately advanced liver disease are not suitable for SABR because of the radiotherapy dose delivered to the surrounding liver. SABR and other radiotherapy techniques are unlikely to replace MWA as the first treatment choice; these techniques are generally reserved for a subgroup of patients depending on their suitability for ablation, tumour location and other patient and disease characteristics. A trial of IRE versus MWA for the subgroup of patients with central lesions may be useful, although again IRE would be unlikely to replace MWA in patients suitable for ablation. A trial of ECT versus MWA was also considered to be of interest. For early HCC, further research may be most relevant in the setting of challenging locations, less fit patients and incomplete response to primary therapy, rather than a comparison with the current preferred first treatment option (MWA).
Histotripsy was identified as an investigational product that may be promising in the future; however, it is at an early stage of regulatory approval, so should not be assessed until efficacy has been demonstrated.
Because of the low number of patients in the UK with early-stage HCC who would be eligible for all treatments within a trial, particularly for those interventions reserved for the subgroup of patients with more challenging tumours, it is likely to be more feasible to undertake an international multicentre RCT than a UK-based trial, in terms of recruiting sufficient patients to demonstrate a significant difference in outcomes. However, patients’ disease characteristics, such as aetiology of liver disease and prior treatments received, would need to be similar to those of HCC patients in the UK to ensure that trial results were generalisable to the UK HCC population. Unfortunately, there were insufficient data on specific patient subgroups (i.e. tumour size and number, severity of cirrhosis and underlying liver disease) to enable subgroup analysis to be undertaken within the review. Therefore, it is unclear whether these characteristics are effect modifiers.
Local recurrence, overall recurrence, OS, PFS and HRQoL are important outcomes that should be assessed in any future trials. The definition of specific outcomes, such as recurrence and PFS, should be consistent in future trials to allow results to be compared and synthesised in the future.
The 2022 update of the BCLC strategy for prognosis prediction and treatment recommendation states that further prospective studies are needed to define the role of SABR for very early HCC. 7
Strengths and limitations
The key strengths of this assessment are the comprehensive searches for relevant RCT evidence, the systematic data extraction and assessment of the quality and applicability of the included studies, and the inclusion of relevant data in NMAs of four important clinical effectiveness outcomes in an attempt to draw indirect comparisons of the therapies and rank them from best to worst in terms of the relevant outcomes.
The systematic review of RCTs was supplemented with a targeted review of non-randomised evidence in an attempt to fill gaps in the RCT evidence base and strengthen the evidence where data on specific comparisons were considered to be weak. Attention was focused on those interventions with current clinical relevance and those comparisons sensitive to potential changes in the evidence, as determined using novel threshold analysis techniques.
The project benefited from the expertise of several patient and clinical advisors, with meetings held at key stages of the project. However, the absence of ‘in person’ meetings, owing to the COVID-19 pandemic, constrained the interactions with patients. In addition, two of the patient advisory group members were unfortunately unavailable for the workshops at the end of the project.
The assessment was limited by the weaknesses in the clinical evidence base. There was no evidence on several of the interventions of interest, and the evidence was extremely weak (in terms of size and quality) for most of the other therapies, limiting our ability to draw any firm conclusions. Because of the significant gaps in the evidence base, the recommendations for prioritising specific therapies and comparisons for future research were primarily made based on expert advice received during the end-of-project workshop.
Chapter 9 Conclusions
Implications for practice
The evidence on ablative and non-surgical therapies for early and very early HCC is very limited. The only firm conclusions that can be drawn from the available data are that PEI and PAI are inferior to RFA, and that they also appear to be inferior to MWA and resection, for certain survival outcomes. There is insufficient evidence to draw any conclusions on quality-of-life outcomes.
The uptake of specific ablative therapies in the UK appears to be based more on technological advancements and ease/speed of use (and NHS England commissioning policies) than on high-quality evidence demonstrating superior clinical effectiveness of one therapy over another.
Recommendations for research
There are currently no comparative data on several ablative and non-surgical therapies, particularly those treatments reserved for the subgroup of patients with more challenging tumours. However, owing to the small number of such patients who would be eligible for both treatment arms within a trial, it is likely to be difficult to recruit sufficient numbers of patients to demonstrate a significant survival benefit, particularly in the presence of a competing risk of recurrence from the underlying liver disease.
Future studies should assess local recurrence, overall recurrence, OS, PFS, HRQoL and patient acceptability, using clear and consistent definitions, in order to allow results to be compared across studies.
It is difficult to make firm recommendations for research based on our findings. The current evidence suggests a trial of MWA versus RFA versus resection could address uncertainty about the standard of care; however, clinicians consider this unlikely to be helpful as RFA is no longer widely used in NHS practice.
Clinical experts suggest that SABR is a promising intervention and could be compared with MWA; this may have international relevance, allowing for wider patient recruitment through multinational trials. However, SABR can usually only be used once because it is limited by the radiotherapy dose received by the surrounding liver, so further research is needed to identify where it should sit in the treatment pathway.
There were insufficient data on specific patient subgroups (i.e. relating to tumour size and number, severity of cirrhosis and underlying liver disease) to enable subgroup analysis to be undertaken. Therefore, further research to assess whether certain disease characteristics may modify treatment effect could be beneficial.
Feasibility studies could address these potential issues and complexities in undertaking research in this area prior to undertaking a trial. This would enable investigation of: the acceptability of the intervention (and comparator) to both clinicians and patients and their willingness to participate in a trial; the practicality of delivering the intervention; and the ability to measure relevant outcomes.
Acknowledgements
We would like to thank the members of our advisory group: Dr Rebecca Goody, Consultant Clinical Oncologist, Leeds Teaching Hospitals NHS Trust; Dr Jai Patel, Consultant Vascular and Interventional Radiologist, Leeds Teaching Hospitals NHS Trust; Professor Ajith Siriwardena, Consultant Hepatobiliary and General Surgeon, Manchester University NHS Foundation Trust; Dr Tze Wah, Consultant Interventional Radiologist, Leeds Teaching Hospitals NHS Trust; and the patient advisors: Dave Clarke, Ian Doyle, Richard McCabe and Ian Teunion. In addition, we would like to thank the other clinicians who attended the workshop: Dr Salil Karkhanis, Consultant Interventional Radiologist, University Hospitals Birmingham NHS Foundation Trust; and Professor Derek Manas, Professor of Hepatobiliary and Transplant Surgery, Newcastle Hospitals NHS Foundation Trust. We would also like to thank Dr Tze Wah for providing additional unpublished registry data that were included in the updated network meta-analyses. We are grateful for the input of CRD staff early in the life of the project who contributed to the proposal or protocol, namely Lindsay Claxton and Kath Wright. We would also like to thank Jinshuo Li and Nick Meader for assistance translating Chinese and Japanese publications.
Contributions of authors
Ros Wade (https://orcid.org/0000-0002-8666-8110) (Research Fellow) contributed to the protocol, study selection, data extraction, validity assessment and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report.
Emily South (https://orcid.org/0000-0003-2187-4762) (Research Fellow) contributed to the selection, data extraction, validity assessment and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report.
Sumayya Anwer (https://orcid.org/0000-0002-1740-0399) (Research Fellow) undertook the threshold analysis and incorporated non-randomised evidence into the network meta-analyses. She also contributed to the interpretation of the results and the writing of the report.
Sahar Sharif-Hurst (https://orcid.org/0000-0001-6885-0456) (Research Fellow) contributed to the protocol, study selection, data extraction and validity assessment. She developed the synthesis models and undertook the analyses. She also contributed to the interpretation of the results and the writing of the report.
Melissa Harden (https://orcid.org/0000-0003-2338-6869) (Information Specialist) contributed to the protocol, developed search strategies, conducted a range of searches to locate studies and wrote the sections of the report relating to the literature searches.
Helen Fulbright (https://orcid.org/0000-0002-1073-1099) (Information Specialist) developed search strategies, conducted a range of searches to locate studies and contributed to the sections of the report relating to the literature searches.
Robert Hodgson (https://orcid.org/0000-0001-6962-2893) (Research Fellow in Health Economics) undertook the review of economic studies. He was responsible for assessing the feasibility of economic modelling and contributed to the writing of the report.
Sofia Dias (https://orcid.org/0000-0002-2172-0221) (Professor in Health Technology Assessment) provided methodological expertise throughout the project and contributed to the protocol, interpretation of the results and the writing of the report.
Mark Simmonds (https://orcid.org/0000-0002-1999-8515) (Senior Research Fellow) provided methodological expertise throughout the project and contributed to the protocol, interpretation of the results and the writing of the report.
Ian Rowe (https://orcid.org/0000-0003-1288-0749) (Honorary Consultant Hepatologist) provided expert clinical advice, contributed to the protocol and interpretation of the results, and commented on drafts of the report.
Patricia Thornton (https://orcid.org/0000-0002-8814-0790) (patient collaborator) provided advice as a patient expert, contributed to the protocol and interpretation of the results, and commented on drafts of the report.
Alison Eastwood (https://orcid.org/0000-0003-1079-7781) (Professor of Research) contributed to the protocol, study selection and synthesis of the included studies. She also contributed to the interpretation of the results and the writing of the report. Alison had overall responsibility for the project.
Publications
South E, Wade R, Anwer S, Sharif S, Harden M, Fulbright HA, et al. The effectiveness of ablative and non-surgical therapies for early hepatocellular carcinoma: systematic review and network meta-analysis of randomised controlled trials. Cancer Medicine 2023. https://doi.org/10.1002/cam4.6643
Data-sharing statement
The majority of data are available in the appendices to the report; any additional information can be obtained from the corresponding author.
Ethics statement
This systematic review did not require ethical approval.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Cancer Research UK . Liver Cancer Statistics n.d. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/liver-cancer (accessed 7 December 2021).
- European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182-236.
- Burton A, Tataru D, Driver RJ, Bird TG, Huws D, Wallace D, et al. Primary liver cancer in the UK: incidence, incidence-based mortality, and survival by subtype, sex, and nation. JHEP Rep 2021;3. https://doi.org/10.1016/j.jhepr.2021.100232.
- Global Burden of Disease Liver Cancer Collaboration . The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease study 2015. JAMA Oncol 2017;3:1683-91. https://doi.org/10.1001/jamaoncol.2017.3055.
- Balogh J, Victor D, Asham EH, Burroughs SG, Boktour M, Saharia A, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma 2016;3:41-53. https://doi.org/10.2147/jhc.S61146.
- National Institute for Health and Care Excellence . Cirrhosis in Over 16s: Assessment and Management 2016.
- Reig M, Forner A, Rimola J, Ferrer-Fábrega J, Burrel M, Garcia-Criado A, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2021;76:681-93. https://doi.org/10.1016/j.jhep.2021.11.018.
- Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ 2020;371. https://doi.org/10.1136/bmj.m3544.
- NHS England . Clinical Commissioning Policy Stereotactic Ablative Radiotherapy (SABR) for Hepatocellular Carcinoma (Adults) 2019.
- National Institute for Health and Care Excellence . Irreversible Electroporation for Primary Liver Cancer. Interventional Procedures Guidance 2019.
- National Institute for Health and Care Excellence . Radiofrequency Ablation of Hepatocellular Carcinoma. Interventional Procedures Guidance 2003.
- National Institute for Health and Care Excellence . Microwave Ablation of Hepatocellular Carcinoma. Interventional Procedures Guidance 2007.
- Awad T, Thorlund K, Gluud C. Cryotherapy for hepatocellular carcinoma. Cochrane Database Syst Rev 2009;4. http://dx.doi.org/10.1002/14651858.CD007611.pub2.
- Majumdar A, Roccarina D, Thorburn D, Davidson BR, Tsochatzis E, Gurusamy KS. Management of people with early‐ or very early‐stage hepatocellular carcinoma. Cochrane Database Syst Rev 2017;3. http://dx.doi.org/10.1002/14651858.CD011650.pub2.
- Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev 2013;12. http://dx.doi.org/10.1002/14651858.CD003046.pub3.
- Weis S, Franke A, Berg T, Mössner J, Fleig WE, Schoppmeyer K. Percutaneous ethanol injection or percutaneous acetic acid injection for early hepatocellular carcinoma. Cochrane Database Syst Rev 2015;1. http://dx.doi.org/10.1002/14651858.CD006745.pub3.
- Kamarajah SK, Bundred JR, Littler P, Reeves H, Manas DM, White SA. Treatment strategies for early stage hepatocellular carcinoma: a systematic review and network meta-analysis of randomised clinical trials. HPB (Oxford) 2020;11:1-11.
- Tian G, Yang S, Yuan J, Threapleton D, Zhao Q, Chen F, et al. Comparative efficacy of treatment strategies for hepatocellular carcinoma: systematic review and network meta-analysis. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-021269.
- Lan T, Chang L, Rahmathullah MN, Wu L, Yuan YF. Comparative efficacy of interventional therapies for early-stage hepatocellular carcinoma: a PRISMA-compliant systematic review and network meta-analysis. Medicine (Baltim) 2016;95. https://dx.doi.org/10.1097/MD.0000000000003185.
- Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int 2015;35:2155-66. https://doi.org/10.1111/liv.12818.
- Centre for Reviews and Dissemination . Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care 2009.
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339. https://doi.org/10.1136/bmj.b2535.
- Hutton B, Catalá-López F, Moher D. The PRISMA statement extension for systematic reviews incorporating network meta-analysis: PRISMA-NMA. Med Clin (Barc) 2016;147:262-6. https://doi.org/10.1016/j.medcli.2016.02.025.
- Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012;12. https://doi.org/10.1186/1471-2288-12-9.
- Irvine AF, Waise S, Green EW, Stuart B. A non-linear optimisation method to extract summary statistics from Kaplan–Meier survival plots using the published P value. BMC Med Res Methodol 2020;20. https://doi.org/10.1186/s12874-020-01092-x.
- Bland JM, Altman DG. The logrank test. BMJ 2004;328. https://doi.org/10.1136/bmj.328.7447.1073.
- Higgins JP, Savovic J, Page MJ, Sterne JA. ROB2 Development Group . Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2) 2019. https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/current-version-of-rob-2 (accessed 27 November 2019).
- Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Network Meta-analysis for Decision Making. Hoboken, NJ: Wiley; 2018.
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making 2013;33:607-17.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-analysis of Randomised Controlled Trials. London: NICE; 2014.
- Warn DE, Thompson SG, Spiegelhalter DJ. Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Stat Med 2002;21:1601-23. https://doi.org/10.1002/sim.1189.
- van Valkenhoef G, Kuiper J. R package. CRAN; 2021.
- Franchini A, Dias S, Ades A, Jansen J, Welton NJ. Accounting for correlation in mixed treatment comparisons with multi-arm trials. Res Synth Methods 2012;3:142-60.
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-55. https://doi.org/10.1080/10618600.1998.10474787.
- Röver C, Bender R, Dias S, Schmid CH, Schmidli H, Sturtz S, et al. On weakly informative prior distributions for the heterogeneity parameter in Bayesian random-effects meta-analysis. Res Synth Methods 2021;12:448-74. https://doi.org/10.1002/jrsm.1475.
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B Stat Methodol 2002;64:583-616.
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A. The deviance information criterion: 12 years on. J R Stat Soc Ser B Stat Methodol 2014;76:485-93.
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. London: NICE; 2011.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932-44.
- Phillippo DM, Dias S, Ades AE, Didelez V, Welton NJ. Sensitivity of treatment recommendations to bias in network meta-analysis. J R Stat Soc Ser A Stat Soc 2018;181:843-67. https://doi.org/10.1111/rssa.12341.
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses. Ann Intern Med 2019;170:538-46. https://doi.org/10.7326/M18-3542.
- Hausner E, Metzendorf MI, Richter B, Lotz F, Waffenschmidt S. Study filters for non-randomized studies of interventions consistently lacked sensitivity upon external validation. BMC Med Res Methodol 2018;18. https://doi.org/10.1186/s12874-018-0625-4.
- Aikata H, Shirakawa H, Takaki S, Uka K, Miki D, Yamashina K. Radiofrequency ablation combined with transcatheter arterial chemoembolization for small hepatocellular carcinomas. Hepatology 2006;44.
- Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: a randomized controlled trial. Scand J Gastroenterol 2008;43:727-35. https://dx.doi.org/10.1080/00365520701885481.
- Chen K, Chen G, Wang H, Li H, Xiao J, Duan X, et al. Increased survival in hepatocellular carcinoma with iodine-125 implantation plus radiofrequency ablation: a prospective randomized controlled trial. J Hepatol 2014;61:1304-11. https://dx.doi.org/10.1016/j.jhep.2014.07.026.
- Fang Y, Chen W, Liang X, Li D, Lou H, Chen R, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol 2014;29:193-200. https://dx.doi.org/10.1111/jgh.12441.
- Gan YH, Yie SL, Ren ZG, Xia JL, Zhang BH, Wang YH, et al. Prospective randomized trial of RFA and chemotherapy for unresectable small hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 2004;26:496-8.
- Giorgio A, Di Sarno A, De Stefano G, Scognamiglio U, Farella N, Mariniello A, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma compared to percutaneous ethanol injection in treatment of cirrhotic patients: an Italian randomized controlled trial. Anticancer Res 2011;31:2291-5.
- Huang GT, Lee PH, Tsang YM, Lai MY, Yang PM, Hu RH, et al. Percutaneous ethanol injection versus surgical resection for the treatment of small hepatocellular carcinoma: a prospective study. Ann Surg 2005;242:36-42.
- Izumi N, Hasegawa K, Nishioka Y, Takayama T, Yamanaka N, Kudo M, et al. A multicenter randomized controlled trial to evaluate the efficacy of surgery vs. radiofrequency ablation for small hepatocellular carcinoma (SURF trial). J Clin Oncol 2019;37.
- Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial. J Hepatol 2020;74:603-12. https://dx.doi.org/10.1016/j.jhep.2020.09.026.
- Koda M, Murawaki Y, Mitsuda A, Oyama K, Okamoto K, Idobe Y, et al. Combination therapy with transcatheter arterial chemoembolization and percutaneous ethanol injection compared with percutaneous ethanol injection alone for patients with small hepatocellular carcinoma: a randomized control study. Cancer 2001;92:1516-24.
- Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut 2005;54:1151-6.
- Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment?. Radiology 2009;252:905-13. https://dx.doi.org/10.1148/radiol.2523081676.
- Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology 2005;129:122-30.
- Yan SY, Zhang Y, Sun C, Cao HX, Li GM, Wang YQ, et al. The clinical effect and relevant mechanism of combined sorafenib and radiofrequency ablation in the treatment of early small hepatocellular carcinoma. Oncol Lett 2016;12:951-5.
- Zou D, Pan D, Deng L, Mao W, Zhang F, Miao J. Comparison of ultrasound-guided laser ablation and radiofrequency ablation in the treatment of small hepatocellular carcinoma. Int J Clin Exp Med 2017;10:9562-8.
- Feng K, Yan J, Li X, Xia F, Ma K, Wang S, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794-802. https://dx.doi.org/10.1016/j.jhep.2012.05.007.
- Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology 2004;127:1714-23.
- Mizuki A, Tatemichi M, Tsukada N, Nagamatsu R, Kawaguchi M, Itoshima T, et al. Addition of transcatheter arterial chemoembolization decreased local recurrence but had no survival benefit to percutaneous ethanol injection therapy for patients with small hepatocellular carcinoma: a multicenter randomized control study. Oncol Lett 2010;1:855-9.
- Orlacchio A, Bolacchi F, Chegai F, Bergamini A, Costanzo E, Del Giudice C, et al. Comparative evaluation of percutaneous laser and radiofrequency ablation in patients with HCC smaller than 4 cm. Radiol Med 2014;119:298-30. https://dx.doi.org/10.1007/s11547-013-0339-y.
- Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002;223:331-7.
- Vietti V, Duran R, Guiu B, Cercueil JP, Aube C, Digklia A, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol 2018;3:317-25. https://dx.doi.org/10.1016/S2468-1253(18)30029-3.
- Abdelaziz A, Elbaz T, Shousha HI, Mahmoud S, Ibrahim M, Abdelmaksoud A, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc 2014;28:3429-34. https://dx.doi.org/10.1007/s00464-014-3617-4.
- Azab M, Zaki S, El-Shetey AG, Abdel-Moty MF, Alnoomani NM, Gomaa AA, et al. Radiofrequency ablation combined with percutaneous ethanol injection in patients with hepatocellular carcinoma. Arab J Gastroenterol 2011;12:113-8. https://dx.doi.org/10.1016/j.ajg.2011.07.005.
- Chen MS, Zhang YJ, Li JQ, Liang HH, Zhang YQ, Zheng Y. Randomized clinical trial of percutaneous radiofrequency ablation plus absolute ethanol injection compared with radiofrequency ablation alone for small hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 2005;27:623-5.
- Chen MS, Li JQ, Liang HH, Lin XJ, Guo RP, Zheng Y, et al. Comparison of effects of percutaneous radiofrequency ablation and surgical resection on small hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi 2005;85:80-3.
- Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321-8.
- Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-12. https://dx.doi.org/10.1097/SLA.0b013e3181efc656.
- Huo TI, Huang YH, Wu JC, Chiang JH, Lee PC, Chang FY, et al. Sequential transarterial chemoembolization and percutaneous acetic acid injection therapy versus repeated percutaneous acetic acid injection for unresectable hepatocellular carcinoma: a prospective study. Ann Oncol 2003;14:1648-53.
- Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology 2003;228:235-40.
- Liu H, Wang ZG, Fu SY, Li AJ, Pan ZY, Zhou WP, et al. Randomized clinical trial of chemoembolization plus radiofrequency ablation versus partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br J Surg 2016;103:348-56. https://dx.doi.org/10.1002/bjs.10061.
- Ng KK, Chok KS, Chan AC, Cheung TT, Wong TC, Lo CM. Long-term analysis of a prospective randomized trial of hepatic resection versus radiofrequency ablation for early stage hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2017;24.
- Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo RP, Chen MS. Recurrent hepatocellular carcinoma treated with sequential transcatheter arterial chemoembolization and RF ablation versus RF ablation alone: a prospective randomized trial. Radiology 2012;262:689-700. https://doi.org/10.1148/radiol.11110637.
- Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, et al. Long-term effects of repeat hepatectomy vs percutaneous radiofrequency ablation among patients with recurrent hepatocellular carcinoma: a randomized clinical trial. JAMA Oncol 2020;6:255-63. https://dx.doi.org/10.1001/jamaoncol.2019.4477.
- Zhang YJ, Liang HH, Chen MS, Guo RP, Li JQ, Zheng Y, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology 2007;244:599-607.
- Ferrari FS, Megliola A, Scorzelli A, Stella A, Vigni F, Drudi FM, et al. Treatment of small HCC through radiofrequency ablation and laser ablation. Comparison of techniques and long-term results. Radiol Med 2007;112:377-93.
- Bian H, Zheng JS, Nan G, Li R, Chen C, Hu CX, et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J Natl Cancer Inst 2014;106. https://dx.doi.org/10.1093/jnci/dju239.
- Ng KK, Chok KS, Chan AC, Cheung TT, Wong TC, Fung JY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg 2017;104:1775-84. https://dx.doi.org/10.1002/bjs.10677.
- Zhu F, Chang Q, Duan S, Leng W. Efficacy and safety of radiofrequency ablation versus laparoscopic hepatectomy for small hepatocellular carcinoma: a protocol for a randomized controlled trial. Medicine (Baltim) 2021;100. https://dx.doi.org/10.1097/MD.0000000000023678.
- Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239-41. https://doi.org/10.1093/biomet/69.1.239.
- Huo TI, Huang YH, Wu JC, Lee PC, Chang FY, Lee SD. Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for hepatocellular carcinoma in cirrhotic patients: a prospective study. Scand J Gastroenterol 2003;38:770-8.
- Cheung TT, Fan ST, Chu FS, Jenkins CR, Chok KS, Tsang SH, et al. Survival analysis of high-intensity focused ultrasound ablation in patients with small hepatocellular carcinoma. HPB (Oxford) 2013;15:567-73. https://dx.doi.org/10.1111/hpb.12025.
- Choi MS, Hong SN, Lee JH, Koh KC, Paik SW, Yoo BC, et al. Radiofrequency ablation vs. surgical resection in the treatment of small hepatocellular carcinoma – a comparative study. J Hepatol 2004;40:75-6. https://doi.org/10.1016/s0168-8278(04)90238-3.
- Elgendi A, Elshafei M, Abdel-Azizi F, Bedewy E. Intraoperative ablation for small HCC not amenable for percutaneous radio frequency ablation in Child A cirrhotic patients. HPB (Oxford) 2014;16:227-8.
- Horigome H, Nomura T, Nakao H, Saso K, Takahashi Y, Akita S, et al. Treatment of solitary small hepatocellular carcinoma: consideration of hepatic functional reserve and mode of recurrence. Hepatogastroenterology 2000;47:507-11.
- Huang G, Chen X, Lau WY, Shen F, Wang RY, Yuan SX, et al. Quality of life after surgical resection compared with radiofrequency ablation for small hepatocellular carcinomas. Br J Surg 2014;101:1006-15. https://dx.doi.org/10.1002/bjs.9539.
- Peng ZW, Chen MS, Lin XJ, Zhang YJ. A prospective study on radiofrequency ablation versus surgical resection for small solitary hepatocellular carcinoma. Hepatol Int 2010;4. http://dx.doi.org/10.1007/s12072-010-9169-3.
- Qian GJ, Wang N, Shen Q, Sheng YH, Zhao JQ, Kuang M, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol 2012;22:1983-90. https://dx.doi.org/10.1007/s00330-012-2442-1.
- Tateishi R, Hasegawa K, Kawaguchi Y, Takayama T, Izumi N, Yamanaka N, et al. A multicenter nonrandomized controlled trial to evaluate the efficacy of surgery versus radiofrequency ablation for small hepatocellular carcinoma (SURF cohort trial). J Clin Oncol 2020;38. http://dx.doi.org/10.1200/JCO.2020.38.15_suppl.4581.
- Elgendi AM, Elshafey M, Bdeawey E. Intraoperative radiofrequency ablation versus surgical resection in solitary small HCC. HPB (Oxford) 2015;17. http://dx.doi.org/10.1111/hpb.12399_8/abstract.
- Du J. The curative effect of percutaneous RFA and radical resection to small hepatocellular carcinoma. Clin J Med Offic 2012;40:570-2.
- Barabino M, Santambrogio R, Chiang J, Meloni F, Bruno S, Zuin M, et al. Laparoscopic thermal ablation of hepatocellular carcinoma unsuitable for percutaneous treatments or hepatic resection: a comparative study. Dig Liver Dis 2016;48. http://dx.doi.org/10.1016/j.dld.2015.12.109.
- Sugimoto K, Kakimi K, Takeuchi H, Fujieda N, Saito K, Sato E, et al. Irreversible electroporation versus radiofrequency ablation: comparison of systemic immune responses in patients with hepatocellular carcinoma. J Vasc Interv Radiol 2019;30:845-53. https://dx.doi.org/10.1016/j.jvir.2019.03.002.
- Ei S, Hibi T, Tanabe M, Itano O, Shinoda M, Kitago M, et al. Cryoablation provides superior local control of primary hepatocellular carcinomas of >2 cm compared with radiofrequency ablation and microwave coagulation therapy: an underestimated tool in the toolbox. Ann Surg Oncol 2015;22:1294-300. https://dx.doi.org/10.1245/s10434-014-4114-7.
- Harada N, Shirabe K, Maeda T, Kayashima H, Takaki S, Maehara Y. Comparison of the outcomes of patients with hepatocellular carcinoma and portal hypertension after liver resection versus radiofrequency ablation. World J Surg 2016;40:1709-19. https://dx.doi.org/10.1007/s00268-016-3465-6.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013.
- Canadian Agency for Drugs and Technologies in Health . Strings Attached: CADTH’s Database Search Filters - Narrow Economic Filter - OVID MEDLINE, Embase 2021. https://www.cadth.ca/strings-attached-cadths-database-search-filters#narrow (accessed 13 May 2021).
- Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Bolondi L, Pinna AD. Cost-effectiveness of hepatic resection versus percutaneous ablation for hepatocellular carcinoma within the Milan criteria. J Hepatol 2013;58:S112-3. http://dx.doi.org/10.1016/S0168-8278%2813%2960267-6.
- Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 2013;59:300-7. http://dx.doi.org/10.1016/j.jhep.2013.04.009.
- Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Radiofrequency ablation versus hepatic resection for early hepatocellular carcinoma: a cost-effectiveness perspective. Dig Liver Dis 2013;45.
- Sarasin FP, Majno PE, Llovet JM, Bruix J, Mentha G, Hadengue A. Living donor liver transplantation for early hepatocellular carcinoma: a life-expectancy and cost-effectiveness perspective. Hepatology 2001;33:1073-9.
- Lai Y, Li K, Li J, Liu SX. Cost-effectiveness of navigated radiofrequency ablation for hepatocellular carcinoma in China. Int J Technol Assess Health Care 2014;30:400-8. https://dx.doi.org/10.1017/S0266462314000452.
- Spolverato G, Vitale A, Bagante F, Connolly R, Pawlik TM. Liver resection for breast cancer liver metastases: a cost–utility analysis. Ann Surg 2017;265:792-9. https://dx.doi.org/10.1097/SLA.0000000000001715.
- Spolverato G, Vitale A, Ejaz A, Kim Y, Maithel SK, Cosgrove DP, et al. The relative net health benefit of liver resection, ablation, and transplantation for early hepatocellular carcinoma. World J Surg 2015;39:1474-84. https://dx.doi.org/10.1007/s00268-015-2987-7.
- Naugler WE, Sonnenberg A. Survival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinoma. Liver Transpl 2010;16:1186-94. https://dx.doi.org/10.1002/lt.22129.
- Rostambeigi N, Dekarske A, Austin E, Golzarian J, Cressman E. Simulation study on cost-effectiveness of radioembolization compared with trans-arterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol 2014;25. http://dx.doi.org/10.1016/j.jvir.2013.12.292.
- Rostambeigi N, Dekarske AS, Austin EE, Golzarian J, Cressman EN. Cost effectiveness of radioembolization compared with conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Vasc Interv Radiol 2014;25:1075-84. https://dx.doi.org/10.1016/j.jvir.2014.04.014.
- Lim KC, Wang VW, Siddiqui FJ, Shi L, Chan ES, Oh HC, et al. Cost-effectiveness analysis of liver resection versus transplantation for early hepatocellular carcinoma within the Milan criteria. Hepatology 2015;61:227-37. https://dx.doi.org/10.1002/hep.27135.
- McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making 2008;28:582-92. https://doi.org/10.1177/0272989x08315240.
- Cho SK, Hay JW, Barzi A. Cost-effectiveness analysis of regorafenib and TAS-102 in refractory metastatic colorectal cancer in the United States. Clin Colorectal Cancer 2018;17:e751-e61. https://dx.doi.org/10.1016/j.clcc.2018.08.003.
- National Health Service . National Schedule of NHS Costs 2019. https://www.england.nhs.uk/national-cost-collection/ (accessed 1 December 2021).
- Curtis LA, Burns A. Unit Costs of Health and Social Care 2020. Canterbury: Personal Social Services Resarch Unit (PSSRU), University of Kent; 2020.
- Joint Formulary Committee . British National Formulary 2021. https://bnf.nice.org.uk/ (accessed 1 December 2021).
- An C, Li WZ, Huang ZM, Yu XL, Han YZ, Liu FY, et al. Small single perivascular hepatocellular carcinoma: comparisons of radiofrequency ablation and microwave ablation by using propensity score analysis. Eur Radiol 2021;31:4764-73. https://doi.org/10.1007/s00330-020-07571-5.
- Chang W, Lee JM, Lee DH, Kim YJ, Yoon JH, Han JK. Comparison of switching bipolar ablation with multiple cooled wet electrodes and switching monopolar ablation with separable clustered electrode in treatment of small hepatocellular carcinoma: a randomized controlled trial. PLOS ONE 2018;13:1-16. http://dx.doi.org/10.1371/journal.pone.0192173.
- Cho YK. A comparison of surgical resection and radiofrequency ablation for the treatment of single small hepatocellular carcinoma ≤ 2 cm. Hepatology 2014;59. http://dx.doi.org/10.1002/hep.26692.
- Chong C, Fong A, Cheung S, Wong J, Lee KF, Lai P. Microwave versus radiofrequency ablation for hepatocellular carcinoma: a randomized controlled trial. Hepatol Int 2017;11. https://doi.org/10.1007/s12072-016-9783-9.
- Chong C, Fong A, Chu C, Yu S, Fung A, Lok HT, et al. Microwave versus radiofrequency ablation for hepatocellular carcinoma: a randomized controlled trial. J Hepatobiliary Pancreat Sci 2017;24.
- Chong CCN, Lee KF, Fong AKW, Chu CCM, Yu SCH, Fung AKY, et al. Microwave versus radiofrequency ablation for hepatocellular carcinoma: a prospective randomized controlled trial. Liver Cancer 2018;7:118-9. https://doi.org/10.1159/000490877.
- Chong CCN, Lee KF, Cheung SYS, Chu CCM, Fong AKW, Wong J, et al. Prospective double-blinded randomized controlled trial of microwave versus radiofrequency ablation for hepatocellular carcinoma (McRFA trial). HPB (Oxford) 2020;22:1121-7. https://dx.doi.org/10.1016/j.hpb.2020.01.008.
- Crocetti L. Radiofrequency ablation versus resection for small hepatocellular carcinoma: are randomized controlled trials still needed?. Radiology 2018;287:473-5. http://dx.doi.org/10.1148/radiol.2018172822.
- Di Costanzo GG, Tortora R, D’Adamo G, Galeota Lanza A, Carannante N, Lampasi F, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma: a randomized controlled trial. Dig Liver Dis 2011;43. https://doi.org/10.1016/s1590-8658(11)60029-7.
- Di Costanzo GG, Tortora R, D’Adamo G, Galeota Lanza A, Carannante N, Lampasi F, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma: a randomized controlled trial. Dig Liver Dis 2011;43. https://doi.org/10.1016/s1590-8658(11)60207-7.
- Di Costanzo GG, Tortora R, D’Adamo G, Lanza AG, Carannante N, Lampasi F, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma: a randomized controlled trial. J Hepatol 2011;54:S254-S5.
- Di Costanzo GG, Tortora R, D’Adamo G, Galeota Lanza A, Lampasi F, Addario L, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma: a randomized controlled trial. Dig Liver Dis 2013;45.
- Di Costanzo GG, Tortora R, D’Adamo G, De Luca M, Lampasi F, Addario L, et al. Radiofrequency ablation versus laser ablation for the treatment of small hepatocellular carcinoma in cirrhosis: a randomized trial. J Gastroenterol Hepatol 2015;30:559-65. https://dx.doi.org/10.1111/jgh.12791.
- Duan J, Yue H, Liu K, Wu M, Yang J. Percutaneous radiofrequency ablation versus repeat hepatectomy for recurrent hepatocellular carcinoma: retrospective randomized control study. J Med Colleges PLA 2011;26:316-23. http://dx.doi.org/10.1016/S1000-1948%2812%2960027-6.
- DuBay DA, Sandroussi C, Kachura JR, Ho CS, Beecroft JR, Vollmer CM, et al. Radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. HPB (Oxford) 2011;13:24-32. https://dx.doi.org/10.1111/j.1477-2574.2010.00228.x.
- Fan J, He S, Zheng Y. Analyses of clinical efficacy of ultrasound-guided radiofrequency ablation in liver cancer adjacent to the gallbladder and its prognosis. J BUON 2019;24:2411-7.
- Fang WQ, Li SP, Zhang CQ, Xu L, Shi M, Chen MS, et al. Prophylaxis and clinical treatment for surgical margin recurrence of small primary hepatocellular carcinoma. Ai Zheng 2005;24:834-6.
- Ferrer Puchol M, la Parra C, Esteban E, Vano M, Forment M, Vera A, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma. Radiologia 2011;53:246-53. https://dx.doi.org/10.1016/j.rx.2010.07.010.
- Filippiadis D, Mazioti A, Velonakis G, Tsochantzis A, Tosoratti N, Kelekis A, et al. Continuous versus pulsed microwave ablation in the liver: any difference in intraoperative pain scores?. Ann Gastroenterol 2021;34:80-4. https://dx.doi.org/10.20524/aog.2020.0557.
- Fong AK, Chong CC, Cheung SY, Wong J, Lee KF, Lai PB. Preliminary results on microwave versus radiofrequency ablation for hepatocellular carcinoma: a randomized controlled trial. Surg Pract 2016;20. https://doi.org/10.1111/1744-1633.12205.
- Frangakis C, Geschwind JF, Kim D, Chen Y, Koteish A, Hong K, et al. Chemoembolization decreases drop-off risk of hepatocellular carcinoma patients on the liver transplant list. Cardiovasc Intervent Radiol 2010;34:1-8. http://dx.doi.org/10.1007/s00270-010-0077-7.
- Fukushima T, Ikeda K, Kawamura Y, Sorin Y, Hosaka T, Kobayashi M, et al. Randomized controlled trial comparing the efficacy of impedance control and temperature control of radiofrequency interstitial thermal ablation for treating small hepatocellular carcinoma. Oncology (Huntingt) 2015;89:47-52. https://dx.doi.org/10.1159/000375166.
- Gerunda GE, Neri D, Merenda R, Barbazza F, Zangrandi F, Meduri F, et al. Role of transarterial chemoembolization before liver resection for hepatocarcinoma. Liver Transpl 2000;6:619-26. http://dx.doi.org/10.1053/jlts.2000.8312.
- Giorgio A, De Stefano G, Di Sarno A, Scognamiglio U, Farella N, Sabino G, et al. Three cm or less single hepatocellular carcinoma: a randomized controlled trial comparing percutaneous ethanol injection versus percutaneous radiofrequency ablation. Dig Liver Dis 2010;42. https://doi.org/10.1016/s1590-8658(10)60479-3.
- Giorgio A, De Stefano G, Di Sarno A, Scognamiglio U, Nunzia F, Sabino G, et al. Comparison between PEI and RF in the treatment of HCC in cirrhotic patients with 3 cm or less single nodule: a prospective randomized controlled trial. J Hepatol 2010;52. https://doi.org/10.1016/s0168-8278(10)60215-2.
- Guo J, Sun XN, Huang M. Evaluation of the efficacy for alternated treatment on primary liver cancer by interventional therapy in combination with fractionated stereotactic conformal radiotherapy. Chin J Clin Oncol 2005;32:1418-20.
- Guo WX, Sun JX, Cheng YQ, Shi J, Li N, Xue J, et al. Percutaneous radiofrequency ablation versus partial hepatectomy for small centrally located hepatocellular carcinoma. World J Surg 2013;37:602-7. https://dx.doi.org/10.1007/s00268-012-1870-z.
- Ha TY, Hwang S, Lee YJ, Kim KH, Ko GY, Gwon DH, et al. Absence of benefit of transcatheter arterial chemoembolization (TACE) in patients with resectable solitary hepatocellular carcinoma. World J Surg 2016;40:1200-10. https://doi.org/10.1007/s00268-015-3373-1.
- Hayes . Radioactive Yttrium-90 Microspheres for Treatment of Primary Liver Cancer 2008.
- He HJ, Lv MY, Wang LP. Value of contrast-enhanced ultrasonography in microwave ablation of liver cancer. Shi Jie Hua Ren Xiao Hua Za Zhi 2018;26:195-8. http://dx.doi.org/10.11569/wcjd.v26.i3.195.
- Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol 2005;39:247-52.
- Hsiao CY, Hu RH, Ho CM, Wu YM, Lee PH, Ho MC. Surgical resection versus radiofrequency ablation for Barcelona Clinic Liver Cancer very early stage hepatocellular carcinoma: long-term results of a single-center study. Am J Surg 2020;220:958-64. http://dx.doi.org/10.1016/j.amjsurg.2020.03.017.
- Hsu KF, Chu CH, Chan DC, Yu JC, Shih ML, Hsieh HF, et al. Superselective transarterial chemoembolization vs hepatic resection for resectable early-stage hepatocellular carcinoma in patients with Child–Pugh class A liver function. Eur J Radiol 2012;81:466-71. https://dx.doi.org/10.1016/j.ejrad.2010.12.058.
- Huang XL, Yang HY, Liu BY, Guo YW, Zhan-Sen E. Ultrasound guided percutaneous ethanol injection and hyperthermal distilled-water injection in treatment for HCC. Chin J Intervent Imag Therapy 2009;6:309-13.
- Huang ZM, Zuo MX, Gu YK, Gu HF, Lai CX, Zhang TQ, et al. Computed tomography-guided radiofrequency ablation combined with transarterial embolization assisted by a three-dimensional visualization ablation planning system for hepatocellular carcinoma in challenging locations: a preliminary study. Abdom Radiol (NY) 2020;45:1181-92. https://dx.doi.org/10.1007/s00261-020-02426-5.
- Hung HH, Chiou YY, Hsia CY, Su CW, Chou YH, Chiang JH, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. J Clin Gastroenterol Hepatol 2011;9:79-86. https://doi.org/10.1016/j.cgh.2010.08.018.
- Hyun D, Cho SK, Shin SW, Park KB, Park HS, Choo SW, et al. Early stage hepatocellular carcinomas not feasible for ultrasound-guided radiofrequency ablataion: comparison of transarterial chemoembolization alone and combined therapy with transarterial chemoembolization and radiofrequency ablation. Cardiovasc Intervent Radiol 2016;39:417-25. https://dx.doi.org/10.1007/s00270-015-1194-0.
- Iida H, Aihara T, Ikuta S, Yamanaka N. Comparative study of percutaneous radiofrequency ablation and hepatic resection for small, poorly differentiated hepatocellular carcinomas. Hepatol Res 2014;44:E156-62. https://dx.doi.org/10.1111/hepr.12264.
- Ikeda M, Okada S, Ueno H, Okusaka T, Kuriyama H. Radiofrequency ablation and percutaneous ethanol injection in patients with small hepatocellular carcinoma: a comparative study. Jpn J Clin Oncol 2001;31:322-6.
- Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Hayashi H, et al. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res 2013;43:853-64. https://dx.doi.org/10.1111/hepr.12035.
- Jiang L, Yan L, Wen T, Li B, Zeng Y, Yang J, et al. Comparison of outcomes of hepatic resection and radiofrequency ablation for hepatocellular carcinoma patients with multifocal tumors meeting the Barcelona-Clinic Liver Cancer stage A classification. J Am Coll Surg 2015;221:951-61. http://dx.doi.org/10.1016/j.jamcollsurg.2015.08.009.
- Jiang FQ, Lu W, Yang C, Du P, Ma JP, Yang J, et al. Curative effect of transcatheter arterial chemoembolization combined with radiofrequency ablation in treating hepatic cell carcinoma and its effect on serum markers. Cancer Biomark 2017;20:17-22. http://dx.doi.org/10.3233/CBM-160508.
- Jiang T, Zeng ZC, Yang P, Hu Y. Exploration of superior modality: safety and efficacy of hypofractioned image-guided intensity modulated radiation therapy in patients with unresectable but confined intrahepatic hepatocellular carcinoma. Can J Gastroenterol Hepatol 2017;2017. https://dx.doi.org/10.1155/2017/6267981.
- Kaibori M, Tanigawa N, Kariya S, Ikeda H, Nakahashi Y, Hirohara J, et al. A prospective randomized controlled trial of preoperative whole-liver chemolipiodolization for hepatocellular carcinoma. Dig Dis Sci 2012;57:1404-12. https://dx.doi.org/10.1007/s10620-012-2029-3.
- Kaibori M, Ishizaki M, Matsui K, Kwon AH. A prospective randomized controlled trial of preoperative whole-liver chemolipiodolization for hepatocellular carcinoma. Hepatol Int 2012;6. https://doi.org/10.1007/s12072-011-9333-4.
- Kayali Z, Slater J, Bush D. Randomized controlled trial for proton beam radiotherapy versus transarterial chemoembolization for the treatment of hepatocellular carcinoma; preliminary results. J Hepatol 2013;58. https://doi.org/10.1016/s0168-8278(13)60262-7.
- Kim W, Cho SK, Shin SW, Hyun D, Lee MW, Rhim H. Combination therapy of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) for small hepatocellular carcinoma: comparison with TACE or RFA monotherapy. Abdom Radiol (NY) 2019;44:2283-92. https://dx.doi.org/10.1007/s00261-019-01952-1.
- Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B, et al. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: a randomized phase III trial. J Hepatol 2021;74:603-12. https://doi.org/10.1016/j.jhep.2020.09.026.
- Kitamoto M, Imagawa M, Yamada H, Watanabe C, Sumioka M, Satoh O, et al. Radiofrequency ablation in the treatment of small hepatocellular carcinomas: comparison of the radiofrequency effect with and without chemoembolization. AJR Am J Roentgenol 2003;181:997-1003. http://dx.doi.org/10.2214/ajr.181.4.1810997.
- Kiyoshi H, Norihiro K, Shuichiro S, Ryosuke T, Masatoshi M. Surgery versus radiofrequency ablation for small hepatocellular carcinoma: start of a randomized controlled trial (SURF trial). Hepatol Res 2010;40:851-2. http://dx.doi.org/10.1111/j.1872-034X.2010.00696.x.
- Kobayashi M, Ikeda K, Kawamura Y, Hosaka T, Sezaki H, Yatsuji H, et al. Randomized controlled trial for the efficacy of hepatic arterial occlusion during radiofrequency ablation for small hepatocellular carcinoma – direct ablative effects and a long-term outcome. Liver Int 2007;27:353-9.
- Koda M, Murawaki Y, Kawasaki H. The combination therapy of transcatheter arterial embolization and percutaneous ethanol injection for small hepatocellular carcinoma: randomized controlled study. J Hepatol 2000;32.
- Koh PS, Chan AC, Cheung TT, Chok KS, Dai WC, Poon RT, et al. Efficacy of radiofrequency ablation compared with transarterial chemoembolization for the treatment of recurrent hepatocellular carcinoma: a comparative survival analysis. HPB (Oxford) 2015;16. https://dx.doi.org/10.1111/hpb.12495.
- Kong QF, Jiao JB, Chen QQ, Li L, Wang DG, Lv B. Comparative effectiveness of radiofrequency ablation with or without transarterial chemoembolization for hepatocellular carcinoma. Tumour Biol 2014;35:2655-9.
- Lai C, Jin RA, Liang X, Cai XJ. Comparison of laparoscopic hepatectomy, percutaneous radiofrequency ablation and open hepatectomy in the treatment of small hepatocellular carcinoma. J Zhejiang Univ Sci B 2016;17:236-46. https://dx.doi.org/10.1631/jzus.B1500322.
- Lambert B, Dhondt E, Hermie L, Verhelst X, Defreyne L. Transarterial radioembolization versus drug-eluting beads chemoembolization for treatment of inoperable early and intermediate hepatocellular carcinoma: interim results of the randomized controlled TRACE trial. Eur J Nucl Med Mol Imaging 2020;47:S57-S8.
- Lee J. Hypofractionated radiotherapy for small-sized hepatocellular carcinoma as salvage therapy: sustained local control and safety. Int J Radiat Oncol Biol Phys 2009;75. https://doi.org/10.1016/j.ijrobp.2009.07.606.
- Lee HW, Lee JM, Yoon JH, Kim YJ, Park JW, Park SJ, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res 2018;94:74-82. https://dx.doi.org/10.4174/astr.2018.94.2.74.
- Lee H, Yoon CJ, Seong NJ, Jeong SH, Kim JW. Comparison of combined therapy using conventional chemoembolization and radiofrequency ablation versus conventional chemoembolization for ultrasound-invisible early-stage hepatocellular carcinoma (Barcelona Clinic Liver Cancer stage 0 or A). Korean J Radiol 2018;19:1130-9. https://dx.doi.org/10.3348/kjr.2018.19.6.1130.
- Li XY, Yang LZ. Analysis of transcatherter arterial chemoembolization combined with percutaneous ethanol injection for hepatoma. Chin J Interv Imaging Ther 2007;4:269-72.
- Li J. Intraarterial chemotherapy combined with microwave ablation in treatment of hepatocellular carcinoma. J Pract Oncol 2011;26:386-9.
- Li H, Hu Y, Li N, Zhou Y. Liver fibrosis and five year survival of hepatocellular cancer cases undergoing transcatheter arterial chemo embolization using small doses. Asian Pac J Cancer Prev 2012;13:1589-93.
- Liao M, Zhong X, Zhang J, Liu Y, Zhu Z, Wu H, et al. Radiofrequency ablation using a 10-mm target margin for small hepatocellular carcinoma in patients with liver cirrhosis: a prospective randomized trial. J Surg Oncol 2017;115:971-9. https://dx.doi.org/10.1002/jso.24607.
- Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Erratum: randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less (Gut (August 2005) 54 (1151–1156)). Gut 2005;54. http://dx.doi.org/10.1136/gut.2004.045203corr1.
- Lin SM, Chu CM. Percutaneous tumor ablation or surgical resection for small hepatocellular carcinoma?. J Gastroenterol Hepatol 2007;22:1561-4.
- Lin SM, Lin CC, Chen WT, Chen YC, Hsu CW. Radiofrequency ablation for hepatocellular carcinoma: a prospective comparison of four radiofrequency devices. J Vasc Interv Radiol 2007;18:1118-25.
- Liu YS, Chuang MT, Tsai YS, Tsai HM, Lin XZ. Nitroglycerine use in transcatheter arterial (chemo)embolization in patients with hepatocellular carcinoma and dual-energy CT assessment of Lipiodol retention. Eur Radiol 2012;22:2193-200. https://dx.doi.org/10.1007/s00330-012-2484-4.
- Liu B, Huang JW, Li Y, Hu BS, He X, Zhao W, et al. Single-agent versus combination doxorubicin-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a single-blind, randomized, phase II trial. Oncology (Huntingt) 2015;89:23-30. https://dx.doi.org/10.1159/000371522.
- Liu R, Sun M, Shi L, Yu H. The efficacy assessment of radiofrequency ablation treatment on primary hepatocellular carcinoma by contrast enhanced ultrasonography. Int J Clin Exp Med 2016;9:16218-23.
- Liu Z, Wang X, Xing L, Pan Y, Huang S. The application comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography in radiofrequency ablation treatment for hepatocellular carcinoma. Cancer Biother Radiopharm 2019;34:621-5. https://dx.doi.org/10.1089/cbr.2019.2845.
- Liu E, Li B, Huang Y, Zhang H. Clinical Study of the Beneficial and Harmful Effects of Transcatheter Arterial Chemoembolization Combined With Radiofrequency Ablation Versus Transcatheter Arterial Chemoembolization Alone for Primary Liver Cancer Inadults 2020. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42020207801 (accessed 3 February 2021).
- Lu MD, Kuang M, Liang LJ, Xie XY, Peng BG, Liu GJ, et al. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi 2006;86:801-5.
- Lu LG, Hu BS, Li Y, Luo PF, Chen XM. Research of high frequency induced thermotherapy efficiency for treatment of hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi 2008;88:1618-20.
- Luo BM, Wen YL, Yang HY, Zhi H, Xiao XY, Ou B, et al. Percutaneous ethanol injection, radiofrequency and their combination in treatment of hepatocellular carcinoma. World J Gastroenterol 2005;11:6277-80.
- Ma Y, Zhao C, Zhao H, Li H, Chen C, Xiang H, et al. Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients. Am J Transl Res 2019;11:7456-70.
- Maeda S, Shibata J, Fujiyama S, Tanaka M, Noumaru S, Sato K, et al. Long-term follow-up of hepatic arterial chemoembolization with cisplatin suspended in iodized oil for hepatocellular carcinoma. Hepatogastroenterology 2003;50:809-13.
- Masuda T, Beppu T, Ishiko T, Horino K, Toyama E, Tanaka H, et al. Local ablation therapy combined with liver resection for multiple hepatocellular carcinomas. Gan To Kagaku Ryoho 2007;34:2077-9.
- Mbalisike EC, Vogl TJ, Zangos S, Eichler K, Balakrishnan P, Paul J. Image-guided microwave thermoablation of hepatic tumours using novel robotic guidance: an early experience. Eur Radiol 2015;25:454-62. https://dx.doi.org/10.1007/s00330-014-3398-0.
- Meniconi RL, Komatsu S, Perdigao F, Boelle PY, Soubrane O, Scatton O. Recurrent hepatocellular carcinoma: a Western strategy that emphasizes the impact of pathologic profile of the first resection. Surgery 2015;157:454-62. https://dx.doi.org/10.1016/j.surg.2014.10.011.
- Meyer T, Kirkwood A, Roughton M, Beare S, Tsochatzis E, Yu D, et al. A randomised phase II/III trial of 3-weekly cisplatin-based sequential transarterial chemoembolisation vs embolisation alone for hepatocellular carcinoma. Br J Cancer 2013;108:1252-9. https://dx.doi.org/10.1038/bjc.2013.85.
- Mohamed B, Emara M, Tai C. Percutaneous injection of ethanol and mitoxantrone versus radiofrequency ablation in the treatment of hepatocellular carcinoma. Hepatology 2018;68. https://doi.org/10.1002/hep.30257.
- Mornex F, Wautot V, Kubas A, Serres A, Maillard E, Trepo C, et al. High-dose 3D-conformal radiation therapy (CRT): a new curative treatment for patients with small hepatocellular carcinomas (HCC). Mature results of a French phase II trial (RTF1). EJC Suppl 2007;5.
- Mornex F, Wautot V, Kubas A, Maillard E, Trepo C, Merle P. High-dose 3D-conformal radiation therapy (CRT): a new curative treatment for patients with small hepatocellular carcinomas (HCC). Mature results of a French phase II trial (RTF1). Ann Oncol 2007;18:VII1-02.
- Murakami T, Ishimaru H, Sakamoto I, Uetani M, Matsuoka Y, Daikoku M, et al. Percutaneous radiofrequency ablation and transcatheter arterial chemoembolization for hypervascular hepatocellular carcinoma: rate and risk factors for local recurrence. Cardiovasc Intervent Radiol 2007;30:696-704.
- Ni LD, Fu QC, Chen CW, Wang XJ, Zhou F. The comparative study on treatment for hepatocellular carcinoma with decompensated hepatocirrhosis by different interventional modes. J Interv Radiol 2007;16:249-52.
- National Institute for Health Research . Doxorubicin-Eluting Beads (DC Bead, DC Bead M1 and Radiopaque DC Bead) for Hepatocellular Carcinoma 2016.
- Nouso K, Oonishi A, Wakuta A, Kariyama K. Modified radiofrequency ablation for the treatment of hepatocellular carcinoma. Hepatol Res 2016;46:1158-61. https://dx.doi.org/10.1111/hepr.12683.
- Ohmoto K, Yamamoto S. Comparison between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. Clin Radiol 2006;61:800-1. http://dx.doi.org/10.1016/j.crad.2006.04.015.
- Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, et al. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. JGH Open 2009;24:223-7. http://dx.doi.org/10.1111/j.1440-1746.2008.05596.x.
- Olschewski M, Lencioni R, Allgeier H, Cioni D, Deibert P, Frings H, et al. A randomized comparison of radiofrequencythermal ablation and percutaneous ethanol injection for the treatment of small hepatocellular carcinoma (abstract). Proc Am Soc Clin Oncol 2002;20.
- Paik EK, Kim MS, Jang WI, Seo YS, Cho CK, Yoo HJ, et al. Benefits of stereotactic ablative radiotherapy combined with incomplete transcatheter arterial chemoembolization in hepatocellular carcinoma. Radiat Oncol 2016;11. https://dx.doi.org/10.1186/s13014-016-0597-7.
- Panaro F, Ramos J, Gallix B, Mercier G, Herrero A, Niampa H, et al. Hepatic artery complications following liver transplantation. Does preoperative chemoembolization impact the postoperative course?. Clin Transplant 2014;28:598-605. https://dx.doi.org/10.1111/ctr.12358.
- Park SJ, Cho EJ, Lee JH, Yu SJ, Kim YJ, Yoon JH, et al. Switching monopolar no-touch radiofrequency ablation using octopus electrodes for small hepatocellular carcinoma: a randomized clinical trial. Liver Cancer 2020;10:72-81. http://dx.doi.org/10.1159/000512338.
- Peng ZW, Zhang YJ, Chen MS, Liang HH, Li JQ, Zhang YQ, et al. Risk factors of survival after percutaneous radiofrequency ablation of hepatocellular carcinoma. Surg Oncol 2008;17:23-31. http://dx.doi.org/10.1016/j.suronc.2007.08.002.
- Petrowsky H, Busuttil RW. Resection or ablation of small hepatocellular carcinoma: what is the better treatment?. J Hepatol 2008;49:502-4. http://dx.doi.org/10.1016/j.jhep.2008.07.018.
- Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤ 3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89-97. https://doi.org/10.1016/j.jhep.2013.03.009.
- Riaz A, Ryu RK, Kulik LM, Mulcahy MF, Lewandowski RJ, Minocha J, et al. Alpha-fetoprotein response after locoregional therapy for hepatocellular carcinoma: oncologic marker of radiologic response, progression, and survival. J Clin Oncol 2009;27:5734-42. https://doi.org/10.1200/jco.2009.23.1282.
- Roche AJ. Results and complications of selective chemoembolization in limited HCC. Cardiovasc Intervent Radiol 2002;25:S123-5.
- Ryu T, Takami Y, Wada Y, Tateishi M, Matsushima H, Mikagi K, et al. Double- and triple-positive tumor markers predict early recurrence and poor survival in patients with hepatocellular carcinoma within the Milan criteria and Child–Pugh class A. J Gastrointest Surg 2017;21:957-66. https://dx.doi.org/10.1007/s11605-017-3394-1.
- Santambrogio R, Opocher E, Zuin M, Selmi C, Bertolini E, Costa M, et al. Surgical resection versus laparoscopic radiofrequency ablation in patients with hepatocellular carcinoma and Child–Pugh class A liver cirrhosis. Ann Surg Oncol 2009;16:3289-98. https://dx.doi.org/10.1245/s10434-009-0678-z.
- Shen H, Zhou S, Lou Y, Gao Y, Cao S, Wu D, et al. Microwave-assisted ablation improves the prognosis of patients with hepatocellular carcinoma undergoing liver resection. Technol Cancer Res Treat 2018;17. https://dx.doi.org/10.1177/1533033818785980.
- Sherman M. What is the long-term efficacy of percutaneous ethanol injection for small hepatocellular carcinoma?. Nat Clin Pract Gastroenterol Hepatol 2006;3:78-9.
- Shi J, Sun Q, Wang Y, Jing X, Ding J, Yuan Q, et al. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol 2014;29:1500-7. https://dx.doi.org/10.1111/jgh.12572.
- Shibata T, Shibata T, Maetani Y, Isoda H, Hiraoka M. Radiofrequency ablation for small hepatocellular carcinoma: prospective comparison of internally cooled electrode and expandable electrode. Radiology 2006;238:346-53.
- Sun Q, Shi J, Ren C, Du Z, Shu G, Wang Y. Survival analysis following microwave ablation or surgical resection in patients with hepatocellular carcinoma conforming to the Milan criteria. Oncol Lett 2020;19:4066-76. https://dx.doi.org/10.3892/ol.2020.11529.
- Tashiro H, Aikata H, Waki K, Amano H, Oshita A, Kobayashi T, et al. Treatment strategy for early hepatocellular carcinomas: comparison of radiofrequency ablation with or without transcatheter arterial chemoembolization and surgical resection. J Surg Oncol 2011;104:3-9. https://dx.doi.org/10.1002/jso.21745.
- Toyoda H, Kumada T, Kaneoka Y, Osaki Y, Kimura T, Arimoto A, et al. Prognostic value of pretreatment levels of tumor markers for hepatocellular carcinoma on survival after curative treatment of patients with HCC. J Hepatol 2008;49:223-32. https://dx.doi.org/10.1016/j.jhep.2008.04.013.
- Tsai WL, Cheng JS, Lai KH, Lin CP, Lo GH, Hsu PI, et al. Clinical trial: percutaneous acetic acid injection vs. percutaneous ethanol injection for small hepatocellular carcinoma – a long-term follow-up study. Aliment Pharmacol Ther 2008;28:304-11.
- Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, et al. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg 2004;240:102-7.
- Wang C, Wang H, Yang W, Hu K, Xie H, Bai W, et al. A multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation therapy in patients with hepatocellular carcinoma. Hepatology 2014;60:282A-3A. https://doi.org/10.1002/hep.27475.
- Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015;61:1579-90. https://dx.doi.org/10.1002/hep.27548.
- Wu J, Song ZJ. Long-term efficacy of hepatic artery chemoembolization combined with cryosurgical for the treatment of primary liver cancer. Int J Clin Exp Med 2016;9:16797-801.
- Xu F, Huang YQ, Li YS, Wu L, Yang JM. Is postoperative adjuvant transchatheter arterial chemoembolization necessary for small hepatocellular carcinoma patients: a randomized controlled trial. Ti Erh Chun I Ta Hsueh Hsueh Pao 2012;33:274-9. http://dx.doi.org/10.3724/SP.J.1008.2012.00274.
- Xu F, Huang YQ, Li YS, Wu L, Yang JM. Role of adjuvant TACE in prevention of early recurrence for HCC in BCLC stage A1 after radical hepatectomy, a RCT. HPB (Oxford) 2013;15:131-2. https://doi.org/10.1111/hpb.12093.
- Xu C, Lv PH, Huang XE, Wang SX, Sun L, Wang FA. Efficacy of transarterial chemoembolization combined with radiofrequency ablation in treatment of hepatocellular carcinoma. Asian Pac J Cancer Prev 2015;16:6159-62.
- Xu J, Zhao Y. Comparison of percutaneous microwave ablation and laparoscopic resection in the prognosis of liver cancer. Int J Clin Exp Pathol 2015;8:11665-9.
- Yamamoto J, Okada S, Shimada K, Okusaka T, Yamasaki S, Ueno H, et al. Treatment strategy for small hepatocellular carcinoma: comparison of long-term results after percutaneous ethanol injection therapy and surgical resection. Hepatology 2001;34:707-13.
- Yamasaki T, Hamabe S, Saeki I, Harima Y, Yamaguchi Y, Uchida K, et al. A novel transcatheter arterial infusion chemotherapy using iodized oil and degradable starch microspheres for hepatocellular carcinoma: a prospective randomized trial. J Gastroenterol 2011;46:359-66. http://dx.doi.org/10.1007/s00535-010-0306-5.
- Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J Hepatol 2014;61:82-8. https://dx.doi.org/10.1016/j.jhep.2014.03.012.
- Yin L, Zhou W, Lau W, Lai E, Wu M. Partial hepatectomy versus transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. HPB (Oxford) 2015;17.
- Yin X, Hua T, Liang C, Chen Z. Efficacy of re-resection versus radiofrequency ablation for recurrent Barcelona Clinic Liver Cancer stage 0/A hepatocellular carcinoma (HCC) after resection for primary HCC. Transl Cancer Res 2019;8:1035-45. http://dx.doi.org/10.21037/tcr.2019.06.11.
- Yu J, Liang P, Yu X, Cheng Z, Han Z, Liu F. Comparison of cooled-probe microwave and radiofrequency ablation treatment in incipient hepatocellular carcinoma: a phase III randomized controlled trial with 6-year follow-up. J Clin Oncol 2016;34.
- Yuan G, Zeng CL, Zhu DD, Shi XJ. Influences of RFA combined with TACE on the HIF-1alpha and EGR level of patients with primary hepatic carcinoma. Eur Rev Med Pharmacol Sci 2017;21:1738-45.
- Yuen MF, Chan AO, Wong BC, Hui CK, Ooi GC, Tso WK, et al. Transarterial chemoembolization for inoperable, early stage hepatocellular carcinoma in patients with Child–Pugh grade A and B: results of a comparative study in 96 Chinese patients. Am J Gastroenterol 2003;98:1181-5.
- Yun WK, Choi MS, Choi D, Rhim HC, Joh JW, Kim KH, et al. Superior long-term outcomes after surgery in Child–Pugh class A patients with single small hepatocellular carcinoma compared to radiofrequency ablation. Hepatol Int 2011;5:722-9. http://dx.doi.org/10.1007/s12072-010-9237-8.
- Zeng JY, Piao XH, Zou ZY, Yang QF, Qin ZL, Chen JB, et al. Cryoablation with drug-loaded bead embolization in the treatment of unresectable hepatocellular carcinoma: safety and efficacy analysis. Oncotarget 2018;9:7557-66. http://dx.doi.org/10.18632/oncotarget.24029.
- Zhang L, Wang N, Shen Q, Cheng W, Qian GJ. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0076119.
- Zhang K, Jiang L, Jia Z, Zhang Y, He R, Ding Z, et al. Radiofrequency ablation plus devascularization is the preferred treatment of hepatocellular carcinoma with esophageal varices. Dig Dis Sci 2015;60:1490-501. https://dx.doi.org/10.1007/s10620-014-3455-1.
- Zhang B, Zhou CJ, Liu QH, Yang W, Liu YL. Percutaneous cryoablation versus hepatectomy for hepatocellular carcinoma treatment. Int J Clin Exp Med 2016;9:10354-63.
- Zhao M, Wang JP, Li W, Huang ZL, Zhang FJ, Fan WJ, et al. Comparison of safety and efficacy for transcatheter arterial chemoembolization alone and plus radiofrequency ablation in the treatment of single branch portal vein tumor thrombus of hepatocellular carcinoma and their prognosis factors. Zhonghua Yi Xue Za Zhi 2011;91:1167-72.
- Zhi Luo BM, Wen YL, Yang HY, Ou B, Ma JH. Comparative study on treatment of small hepatocellular carcinoma by radiofrequency ablation and percutaneous ethanol injection. Chin J Interv Imaging Ther 2006;3:291-3.
- Zhou P, Liu X, Li R, Nie W. Percutaneous coagulation therapy of hepatocellular carcinoma by combining microwave coagulation therapy and ethanol injection. Eur J Radiol 2009;71:338-42. http://dx.doi.org/10.1016/j.ejrad.2008.04.010.
- Zhou Z, Lei J, Li B, Yan L, Wang W, Wei Y, et al. Liver resection and radiofrequency ablation of very early hepatocellular carcinoma cases (single nodule <2 cm): a single-center study. Eur J Gastroenterol Hepatol 2014;26:339-44. https://dx.doi.org/10.1097/MEG.0000000000000012.
- Zhou GH, Sun JH, Zhang YL, Zhou TY, Nie CH, Zhu TY, et al. Transcatheter embolization of hepatocellular carcinoma with epirubicin-loaded DC beads in Chinese patients. Transl Cancer Res 2019;8:279-89. http://dx.doi.org/10.21037/tcr.2019.01.36.
- Zhu W, Wang S, Zhang J. Combination treatment of unresectable hepatic carcinoma with percutaneous cryotherapy and ethanol injection therapy. Technol Cancer Res Treat 2007;6:564-7.
- Zhu D, Yuan D, Wang Z, Chen S. Efficacy of drug-eluting bead transarterial chemoembolization (DEB-TACE) combined with radiofrequency ablation versus DEB-TACE alone in Chinese hepatocellular carcinoma patients. Medicine (Baltim) 2019;98. https://dx.doi.org/10.1097/MD.0000000000015682.
- Aikata H, Hiramatsu A, Tsuge M, Kawaoka T, Imamura M, Murakami E, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. Hepatology 2018;68:544A-5A.
- Al-Judaibi B, Dokus MK. Surgical resection vs radiofrequency ablation in older adults with early stage hepatocellular carcinoma: where do we stand?. Saudi J Gastroenterol 2018;24:309-10. https://dx.doi.org/10.4103/sjg.SJG_501_18.
- Anand AK, Punnakal AU, Chaudhoory AR, Garg C, Munjal RK, Bansal AK, et al. Stereotactic body radiation therapy (SBRT) for lung and liver tumours. J Indian Med Assoc 2012;110:462-4.
- Barabino M, Santambrogio R, Giovenzana M, Polizzi M, Torri F, Opocher E. Clinical outcomes of laparoscopic radio-frequency or microwave ablation for hepatocellular carcinoma. Surg Endosc 2018;32. http://dx.doi.org/10.1007/s00464-018-6180-6.
- Bassanello M, Cillo U, Vitale A, Lumachi F, Ciarleglio FA, Boccagni P, et al. Multimodal approach and its impact on survival for patients with hepatocellular carcinoma. Anticancer Res 2003;23:4047-53.
- Beyer LP, Lurken L, Verloh N, Haimerl M, Michalik K, Schaible J, et al. Stereotactically navigated percutaneous microwave ablation (MWA) compared to conventional MWA: a matched pair analysis. Int J Comput Assist Radiol Surg 2018;13:1991-7. https://dx.doi.org/10.1007/s11548-018-1778-7.
- Bhutiani N, Philips P, Scoggins CR, McMasters KM, Potts MH, Martin RCG. Evaluation of tolerability and efficacy of irreversible electroporation (IRE) in treatment of Child–Pugh B (7/8) hepatocellular carcinoma (HCC). HPB (Oxford) 2016;18:593-9. https://dx.doi.org/10.1016/j.hpb.2016.03.609.
- Borzio M, Colloredo G, Pioltelli P, Quagliuolo M, Gruppo E. Lombardo . Epidemiology and outcome of hepatocellular carcinoma in Lombardy. Dig Liver Dis 2007;39:1011-7.
- Bouda D, Barrau V, Raynaud L, Dioguardi Burgio M, Paulatto L, Roche V, et al. Factors associated with tumor progression after percutaneous ablation of hepatocellular carcinoma: comparison between monopolar radiofrequency and microwaves. Results of a propensity score matching analysis. Cardiovasc Intervent Radiol 2020;43:1608-18. https://dx.doi.org/10.1007/s00270-020-02549-8.
- Bu X, Ge Z, Ma J, Guo S, Wang Y, Liu J. Long-term efficacy of radiofrequency ablation compared to surgical resection for the treatment of small hepatocellular carcinoma. J BUON 2015;20:548-54.
- Bujold A, Massey C, Kim JJ, Brierley JD, Cho C, Wong R, et al. Outcomes of stereotactic body radiotherapy (SBRT) for hepatocellular carcinoma (HCC). Int J Radiat Oncol Biol Phys 2011;81:S70-S1.
- Casaccia M, Santori G, Bottino G, Diviacco P, Andorno E. Laparoscopic resection vs laparoscopic radiofrequency ablation for the treatment of small hepatocellular carcinomas: a single-center analysis. World J Gastroenterol 2017;23:653-60. https://dx.doi.org/10.3748/wjg.v23.i4.653.
- Chagnon S. Prospective randomized trial of radiofrequency ablation versus surgical resection for small hepatocellular carcinomas. J Radiol 2007;88:1842-4. http://dx.doi.org/10.1016/S0221-0363%2807%2978361-0.
- Chen KY, Xiang GA, Wang HN, Xiao FL. Intraoperative radiofrequency ablation and 125I therapy for preventing local recurrence in hepatocellular carcinoma after hepatectorny. Zhonghua Zhong Liu Za Zhi 2007;29:626-8.
- Chen LC, Chiou WY, Lin HY, Lee MS, Lo YC, Huang LW, et al. Comparing stereotactic ablative radiotherapy (SABR) versus re-trans-catheter arterial chemoembolization (re-TACE) for hepatocellular carcinoma patients who had incomplete response after initial TACE (TASABR): a randomized controlled trial. BMC Cancer 2019;19. http://dx.doi.org/10.1186/s12885-019-5461-3.
- Cheung TT, Tp PR, Tat F, Lo CM. Survival analysis of high intensity focused ultrasound ablation in patients with small HCC. HPB (Oxford) 2012;14. http://dx.doi.org/10.1111/j.1477-2574.2012.00510.x.
- Cheung TT, Fan ST, Chan SC, Chok KS, Chu FS, Jenkins CR, et al. High-intensity focused ultrasound ablation: an effective bridging therapy for hepatocellular carcinoma patients. World J Gastroenterol 2013;19:3083-9. https://dx.doi.org/10.3748/wjg.v19.i20.3083.
- Cho HJ, Kim JK, Nam JS, Wang HJ, Lee JH, Kim BW, et al. High circulating microRNA-122 expression is a poor prognostic marker in patients with hepatitis B virus-related hepatocellular carcinoma who undergo radiofrequency ablation. Clin Biochem 2015;48:1073-8. http://dx.doi.org/10.1016/j.clinbiochem.2015.06.019.
- Chong CC, Lee KF, Chu CM, Chan AW, Yu SC, Lai PB. Laparoscopic hepatectomy (with or without robotic assistance) versus radiofrequency ablation as a minimally invasive treatment for very early-stage or early-stage hepatocellular carcinoma. Dig Surg 2020;37:65-71. https://dx.doi.org/10.1159/000497112.
- Cillo U, Noaro G, Vitale A, Neri D, D’Amico F, Gringeri E, et al. Laparoscopic microwave ablation in patients with hepatocellular carcinoma: a prospective cohort study. HPB (Oxford) 2014;16:979-86. https://dx.doi.org/10.1111/hpb.12264.
- Costa M, Santambrogio R, Barabino M, Zuin M, Bertolini E, Opocher E. Microinvasive intraoperative ultrasound pattern in predicting outcomes for single small (<3 cm) hepatocellular carcinoma. J Hepatol 2015;62:S319-20.
- de Geus SW, Kasumova G, Kent TS, Ng SC, McAneny D, Sachs T, et al. Ablation versus hepatectomy for early-stage hepatocellular carcinoma: a matched nationwide review. Gastroenterology 2018;154. http://dx.doi.org/10.1016/S0016-5085%2818%2934201-X.
- Denecke T, Stelter L, Schnapauff D, Steffen I, Sinn B, Schott E, et al. CT-guided interstitial brachytherapy of hepatocellular carcinoma before liver transplantation: an equivalent alternative to transarterial chemoembolization?. Eur Radiol 2015;25:2608-16. http://dx.doi.org/10.1007/s00330-015-3660-0.
- Di Sandro S, Benuzzi L, Lauterio A, Botta F, De Carlis R, Najjar M, et al. Single hepatocellular carcinoma approached by curative-intent treatment: a propensity score analysis comparing radiofrequency ablation and liver resection. Eur J Surg Oncol 2019;45:1691-9. https://dx.doi.org/10.1016/j.ejso.2019.04.023.
- Ding W, Yu J, Liu F, Yu X, Cheng Z, Han Z, et al. Percutaneous microwave ablation versus robot-assisted hepatectomy for early hepatocellular carcinoma: a real-world single-center study. Dig Liver Dis 2021;06. https://dx.doi.org/10.1016/j.dld.2021.04.008.
- Ei S, Tanabe M, Hibi T, Itano O, Shinoda M, Kitago M, et al. Cryoablation compared with radiofrequency ablation and microwave coagulation therapy for the local control of primary hepatocellular carcinoma. J Clin Oncol 2013;31.
- Eloubeidi MA, Provenzale D. CT-guided treatment of ultrasonically invisible hepatocellular carcinoma. Am J Gastroenterol 2000;95:2102-6. http://dx.doi.org/10.1016/S0002-9270%2800%2901067-4.
- Freeman E, Cheung W, Ferdousi S, Kavnoudias H, Majeed A, Kemp W, et al. Irreversible electroporation versus radiofrequency ablation for hepatocellular carcinoma: a single centre propensity-matched comparison. Scand J Gastroenterol 2021;56:942-7. https://dx.doi.org/10.1080/00365521.2021.1930145.
- Gannon CJ, Izzo F, Aloia TA, Pignata S, Nasti G, Vallone P, et al. Can hepatocellular cancer screening increase the proportion of long-term survivors?. Hepatogastroenterology 2009;56:1152-6.
- Garavoglia M, Oldani A, Gentilli S, Portigliotti L, D’Agostino G. Percutaneous radiofrequency ablation versus surgical radiofrequency-assisted nodulectomy in treatment of small single nodes of hepatocellular carcinoma: our experience. Minerva Chir 2013;68:367-75.
- Ghweil A, Osman H. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a comparative study. Hepatol Int 2019;13:S161-2. http://dx.doi.org/10.1007/s12072-019-09936-5.
- Guibal A, Bertin C, Egels S, Savier E, Grenier PA, Lucidarme O. Contrast-enhanced ultrasound (CEUS) follow-up after radiofrequency ablation or cryoablation of focal liver lesions: treated-area patterns and their changes over time. Eur Radiol 2013;23:1392-400. https://dx.doi.org/10.1007/s00330-012-2702-0.
- Guo WX, Zhai B, Lai EC, Li N, Shi J, Lau WY, et al. Percutaneous radiofrequency ablation versus partial hepatectomy for multicentric small hepatocellular carcinomas: a nonrandomized comparative study. World J Surg 2010;34:2671-6. https://dx.doi.org/10.1007/s00268-010-0732-9.
- Hara K, Takeda A, Tsurugai Y, Sanuki N, Saigusa Y, Maeda S, et al. Clinical outcomes after treatment for hepatocellular carcinoma, stereotactic body radiotherapy vs radiofrequency ablation: a propensity score-matched analysis. Hepatology 2018;68. http://dx.doi.org/10.1002/hep.30257.
- Hara K, Takeda A, Tsurugai Y, Saigusa Y, Sanuki N, Eriguchi T, et al. Radiotherapy for hepatocellular carcinoma results in comparable survival to radiofrequency ablation: a propensity score analysis. Hepatology 2019;69:2533-45. https://dx.doi.org/10.1002/hep.30591.
- Hasegawa K, Kokudo N, Makuuchi M, Izumi N, Ichida T, Kudo M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 2013;58:724-9. https://dx.doi.org/10.1016/j.jhep.2012.11.009.
- He Q, Jiang JJ, Jiang YX, Wang WT, Yang L, Liver S, et al. Health-related quality of life comparisons after radical therapy for early-stage hepatocellular carcinoma. Transplant Proc 2018;50:1470-4. https://dx.doi.org/10.1016/j.transproceed.2018.04.041.
- Helmberger T, Dogan S, Straub G, Schrader A, Jungst C, Reiser M, et al. Liver resection or combined chemoembolization and radiofrequency ablation improve survival in patients with hepatocellular carcinoma. Digestion 2007;75:104-12.
- Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi K, Yamamoto Y, et al. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology 2008;55:2171-4.
- Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Takaguchi K, et al. Clinical features of hemodialysis patients treated for hepatocellular carcinoma: comparison between resection and radiofrequency ablation. Mol Clin Oncol 2017;6:455-61. https://dx.doi.org/10.3892/mco.2017.1192.
- Ho EY, Lerrigo R, Arunkumar A, Shen H, Trimble EL, Corvera CU, et al. Hepatocellular carcinoma with a plan for surgical resection is associated with improved survival compared to a plan for liver transplantation. Gastroenterology 2012;142.
- Hsu CY, Lee YH, Hsia CY, Huang YH, Su CW, Lin HC, et al. Performance status enhances the selection of treatment for patients with hepatocellular carcinoma within the Milan criteria. Ann Surg Oncol 2013;20:2035-42. https://dx.doi.org/10.1245/s10434-012-2847-8.
- Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2012;84:355-61. https://dx.doi.org/10.1016/j.ijrobp.2011.11.058.
- Ikeda K, Kobayashi M, Saitoh S, Someya T, Hosaka T, Sezaki H, et al. Cost-effectiveness of radiofrequency ablation and surgical therapy for small hepatocellular carcinoma of 3cm or less in diameter. Hepatol Res 2005;33:241-9.
- Ikeda K, Seki T, Umehara H, Inokuchi R, Tamai T, Sakaida N, et al. Clinicopathologic study of small hepatocellular carcinoma with microscopic satellite nodules to determine the extent of tumor ablation by local therapy. Int J Oncol 2007;31:485-91.
- Imai K, Beppu T, Chikamoto A, Doi K, Okabe H, Nitta H, et al. Radiofrequency ablation versus hepatic resection as an initial treatment for solitary small-sized hepatocellular carcinoma. J Clin Oncol 2012;30.
- Ito T, Tanaka S, Iwai S, Takemura S, Agihara A, Uchida S, et al. Local control of laparoscopic hepatic resection for surface hepatocellular carcinoma: comparison with percutaneous radiofrequency ablation. Liver Cancer 2015;4:169-70. http://dx.doi.org/10.1159/000367745.
- Ito T, Tanaka S, Iwai S, Takemura S, Hagihara A, Uchida-Kobayashi S, et al. Outcomes of laparoscopic hepatic resection versus percutaneous radiofrequency ablation for hepatocellular carcinoma located at the liver surface: a case–control study with propensity score matching. Hepatol Res 2016;46:565-74. http://dx.doi.org/10.1111/hepr.12592.
- Jianyong L, Jinjing Z, Lunan Y, Jingqiang Z, Wentao W, Yong Z, et al. Preoperative adjuvant transarterial chemoembolization cannot improve the long term outcome of radical therapies for hepatocellular carcinoma. Sci Rep 2017;7. https://dx.doi.org/10.1038/srep41624.
- Juloori A, Liao CY, Lemons JM, Singh AK, Iyer R, Robbins JR, et al. Phase I study of stereotactic body radiotherapy followed by ipilimumab with nivolumab vs. nivolumab alone in unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2020;108:S149-50. https://dx.doi.org/10.1016/j.ijrobp.2020.07.900.
- Kang TW, Rhim H. Radiofrequency ablation versus non-anatomical resection: long-term results in 580 patients with a single small hepatocellular carcinoma of ≤ 3cm. Cardiovasc Intervent Radiol 2014;37.
- Kanwal F, Befeler A, Chari RS, Marrero J, Kahn J, Afdhal N, et al. Potentially curative treatment in patients with hepatocellular cancer – results from the Liver Cancer Research Network. Aliment Pharmacol Ther 2012;36:257-65. https://dx.doi.org/10.1111/j.1365-2036.2012.05174.x.
- Katsoulakis E, Riaz N, Cannon DM, Goodman K, Spratt DE, Lovelock M, et al. Image-guided radiation therapy for liver tumors: gastrointestinal histology matters. Am J Clin Oncol 2014;37:561-7. https://dx.doi.org/10.1097/COC.0b013e318282a86b.
- Kawamura Y, Ikeda K, Shindoh J, Kobayashi Y, Kasuya K, Fujiyama S, et al. No-touch ablation in hepatocellular carcinoma has the potential to prevent intrasubsegmental recurrence to the same degree as surgical resection. Hepatol Res 2019;49:164-76. https://dx.doi.org/10.1111/hepr.13254.
- Kawaoka T, Aikata H, Morio K, Nakahara T, Murakami E, Tsuge M, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. Hepatology 2019;70.
- Kennedy JE, Wu F, ter Haar GR, Gleeson FV, Phillips RR, Middleton MR, et al. High-intensity focused ultrasound for the treatment of liver tumours. Ultrasonics 2004;42:931-5. https://dx.doi.org/10.1016/j.ultras.2004.01.089.
- Kim GA, Shim JH, Kim SY, Won HJ, Shin YM, Kim PN, et al. Surgical resection versus radiofrequency ablation in the treatment of patients with solitary hepatocellular carcinoma without portal hypertension: a 10-year outcome study. J Hepatol 2014;60. http://dx.doi.org/10.1016/S0168-8278%2814%2960130-6.
- Kim JM, Kang TW, Choi JY, Lee S, Cho W, Kwon CHD, et al. Single HCC ≤3cm in left lateral segment: liver resection or radiofrequency ablation?. J Hepatol 2014;60. https://doi.org/10.1016/S0168-8278(14)61123-5.
- Kim DS, Jung SW, Yu YD, Han JH, Suh SO. Intention-to-treat analysis of various treatment outcomes for hepatocellular carcinoma defined BCLC stage a with portal hypertension. HPB (Oxford) 2016;18.
- Kim HJ, Park S, Kim KJ, Seong J. Clinical significance of soluble programmed cell death ligand-1 (sPD-L1) in hepatocellular carcinoma patients treated with radiotherapy. Radiother Oncol 2018;129:130-5. https://dx.doi.org/10.1016/j.radonc.2017.11.027.
- Kim TH, Park JW, Kim BH, Kim H, Moon SH, Kim SS, et al. Does risk-adapted proton beam therapy have a role as a complementary or alternative therapeutic option for hepatocellular carcinoma?. Cancers 2019;11. https://dx.doi.org/10.3390/cancers11020230.
- Kimura T, Aikata H, Doi Y, Imano N, Takeuchi Y, Takahashi I, et al. Comparison of stereotactic body radiation therapy combined with or without transcatheter arterial chemoembolization for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Technol Cancer Res Treat 2018;17:1-11. https://dx.doi.org/10.1177/1533033818783450.
- Kimura T, Doi Y, Aikata H, Imano N, Takeuchi Y, Takahashi I, et al. Comparison of stereotactic body radiation therapy combined with or without transcatheter arterial chemoembolization for patients with small hepatocellular carcinoma ineligible for resection or ablation therapies. Int J Radiat Oncol Biol Phys 2018;102. https://dx.doi.org/10.1016/j.ijrobp.2018.07.481.
- Ko SE, Lee MW, Rhim H, Kang TW, Song KD, Cha DI, et al. Comparison of procedure-related complications between percutaneous cryoablation and radiofrequency ablation for treating periductal hepatocellular carcinoma. Int J Hyperthermia 2020;37:1354-61. https://dx.doi.org/10.1080/02656736.2020.1849824.
- Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer 2011;117:4890-904. https://dx.doi.org/10.1002/cncr.26134.
- Komatsu S, Terashima K, Matsuo Y, Takahashi D, Suga M, Nishimura N, et al. Validation of combination treatment with surgical spacer placement and subsequent particle radiotherapy for unresectable hepatocellular carcinoma. J Surg Oncol 2019;120:214-22. https://dx.doi.org/10.1002/jso.25495.
- Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol 2010;21:224-30. http://dx.doi.org/10.1016/j.jvir.2009.10.013.
- Kuang M, Xie XY, Huang C, Wang Y, Lin MX, Xu ZF, et al. Long-term outcome of percutaneous ablation in very early-stage hepatocellular carcinoma. J Gastrointest Surg 2011;15:2165-71. https://dx.doi.org/10.1007/s11605-011-1716-2.
- Kudithipudi V, Day E, Thai N, Kirichenko A. Liver stereotactic radiotherapy (SRT) with functional treatment planning for patients with intermediate stage hepatocellular carcinoma (HCC). J Radiat Oncol 2017;6:371-7. http://dx.doi.org/10.1007/s13566-017-0325-4.
- Kuo YH, Lu SN, Chen CL, Cheng YF, Lin CY, Hung CH, et al. Hepatocellular carcinoma surveillance and appropriate treatment options improve survival for patients with liver cirrhosis. Eur J Cancer 2010;46:744-51. http://dx.doi.org/10.1016/j.ejca.2009.12.018.
- Kuo MJ, Mo LR, Chen CL. Factors predicting long-term outcomes of early-stage hepatocellular carcinoma after primary curative treatment: the role of surgical or nonsurgical methods. BMC Cancer 2021;21. https://dx.doi.org/10.1186/s12885-021-07948-9.
- Kuromatsu R, Takata A, Fukushima N, Sumie S, Nakano M. DCP is an important risk factor for recurrence after radiofrequency ablation of single hepatocellular carcinoma -< 3cm in diameter. Hepatol Int 2009;3. http://dx.doi.org/10.1007/s12072-009-9123-4.
- Kwon JH, Lee JH, Cho EJ, Lee YB, Cho Y, Lee DH, et al. The efficacy of transarterial chemoembolization for small hepatic nodules with atypical enhancement on contrast-enhanced ct but typical enhancement on gadoxetic acid-enhanced MRI in chronic liver disease patients: a post hoc analysis of prospective study. Hepatology 2012;56. http://dx.doi.org/10.1002/hep.26040.
- Lapinski TW, Tarasik A, Januszkiewicz M, Flisiak R. Clinical aspects and treatment of hepatocellular carcinoma in north-eastern Poland. Clin Exp Hepatol 2021;7:79-84. http://dx.doi.org/10.5114/ceh.2021.104631.
- Lee G, Choi MS, Song L, Min YW, Gwak GY, Paik YH, et al. Optimal treatment policy for elderly patients with single small hepatocellular carcinoma: comparison with young patients after propensity score matching. J Gastroenterol Hepatol 2012;27. http://dx.doi.org/10.1111/jgh.12006.
- Lee G, Choi MS, Sinn DH, Gwak GY, Paik YH, Lee JH, et al. Long-term outcomes after treatment of single small hepatocellular carcinoma in elderly patients with well-preserved liver function and good performance status. United Eur Gastroenterol J 2014;2. http://dx.doi.org/10.1177/2050640614548980.
- Lee HA, Lee YR, Kim T, Young YS, Lee YS, Suh SJ, et al. Surgical resection versus radiofrequency ablation in single small hepatocellular carcinoma: data from Korea Central Cancer Registry for Hepatocellular Carcinoma database. Hepatology 2018;68:841A-2A. http://dx.doi.org/10.1002/hep.30257.
- Lee DH, Lee JM, Kim JW, Lee MW, Kim JM. Laparoscopic liver resection vs. percutaneous radiofrequency ablation for small single nodular HCC: comparison of treatment outcome. J Hepatol 2019;70:611-3. http://dx.doi.org/10.1016/S0168-8278%2819%2930204-1.
- Lee DH, Kim JW, Lee JM, Kim JM, Lee MW, Rhim H, et al. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small single nodular hepatocellular carcinoma: comparison of treatment outcomes. Liver Cancer 2021;10:25-37. https://dx.doi.org/10.1159/000510909.
- Lei JY, Wang WT, Yan LN, Wen TF, Li B. Radiofrequency ablation versus surgical resection for small unifocal hepatocellular carcinomas. Medicine (Baltim) 2014;93. https://dx.doi.org/10.1097/MD.0000000000000271.
- Li S, Wang Y, Jun D, Wu P. Comparison of the effectiveness of percutaneous microwave ablation versus hepatectomy for hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 2015;37:301-7. http://dx.doi.org/10.3760/cma.j.issn.0253-3766.2015.04.0I3.
- Li XL, Guo WX, Hong XD, Yang L, Wang K, Shi J, et al. Efficacy of the treatment of transarterial chemoembolization combined with radiotherapy for hepatocellular carcinoma with portal vein tumor thrombus: a propensity score analysis. Hepatol Res 2016;46:1088-98. https://dx.doi.org/10.1111/hepr.12657.
- Li IF, Huang JC, Chen JJ, Wang TE, Huang SS, Tsay SL. Factors related to the quality of life in liver cancer patients during treatment phase: a follow-up study. Eur J Cancer Care 2019;28. https://dx.doi.org/10.1111/ecc.13146.
- Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤ 2 cm in a propensity score model. Ann Surg 2016;263:538-45. https://dx.doi.org/10.1097/SLA.0000000000001178.
- Liu PH, Huo TI. Treating very early-stage HCC: have we found the holy grail?. Ann Surg 2017;266:e31-2. http://dx.doi.org/10.1097/SLA.0000000000001349.
- Liu W, Zheng Y, He W, Zou R, Qiu J, Shen J, et al. Microwave vs radiofrequency ablation for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Aliment Pharmacol Ther 2018;48:671-81. https://dx.doi.org/10.1111/apt.14929.
- Liu W, Li B, Yuan Y. Letter: is microwave ablation superior to radiofrequency ablation for early stage hepatocellular carcinoma? Authors’ reply. Aliment Pharmacol Ther 2018;48:1326-7. https://dx.doi.org/10.1111/apt.15048.
- Liu H, Lee D, McLean K, Leggett D, Hodgkinson P, Fawcett J, et al. Australian experience of SBRT in early and advanced stage hepatocellular carcinoma. Radiother Oncol 2019;141:S19-20.
- Loo KF, Woodman R, Bogatic D, Muller K, Chandran V, Chinnaratha MA, et al. Treatment stage migration for early hepatocellular carcinoma and its impact on outcomes: a multicenter study. JGH Open 2020;35:67-8. https://dx.doi.org/10.1111/jgh.15269.
- Lu SN, Wang JH, Chen CL. Survival comparison between surgical resection and radiofrequency ablation in hepatocellular carcinoma patients with BCLC (very) early stage. J Hepatol 2010;52:S94-S5. http://dx.doi.org/10.1016/S0168-8278%2810%2960225-5.
- Maezawa K, Midorikawa T, Kigawa G, Ishibashi K, Hatakeyama T, Miyakawa K, et al. A role of laparotomic microwave coagulation therapy for hepatocellular carcinoma as a less invasive surgery. Nihon Shokaki Geka Gakkai Zasshi 2005;38:279-88. http://dx.doi.org/10.5833/jjgs.38.279.
- Mariani NM, Santambrogio R, Barabino M, Scifo G, Giovenzana M, Opocher E. Laparoscopic liver resection vs radiofrequency ablation in cirrhotic patients with hepatocellular carcinoma: towards the ‘gold standard’. Surg Endosc 2017;31. http://dx.doi.org/10.1007/s00464-017-5540-y.
- Martin AN, Das D, Johnston LE, Bauer TW, Adams RB, Zaydfudim VM. Management strategies for patients with solitary hepatocellular carcinoma ≤ 3cm. Ann Surg Oncol 2016;23:S104-5. http://dx.doi.org/10.1245/s10434-015-5010-5.
- Merle P, Beziat C, Wautot V, Trepo C, Mornex F. Tolerance and efficacy of high-dose 3D-conformal radiation therapy (CRT) in cirrhotic patients with small hepatocellular carcinomas (HCC) not suitable for curative therapies. Hepatology 2005;42:394A-5A.
- Ming W. Survival comparison between surgical resection and percutaneous radiofrequency ablation for patients in BCLC early stage hepatocellular carcinoma. Hepatol Int 2012;6. http://dx.doi.org/10.1007/s12072-011-9333-4.
- Mizumoto M, Okumura T, Hashimoto T, Fukuda K, Oshiro Y, Fukumitsu N, et al. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys 2011;81:1039-45. https://dx.doi.org/10.1016/j.ijrobp.2010.07.015.
- Mo QG, Liang AM, Yang NW, Zhao YN, Yuan WP. Surgery-predominant comprehensive therapy for 134 patients with small hepatocellular carcinoma. Ai Zheng 2003;22:189-91.
- Mohnike K, Steffen IG, Seidensticker M, Hass P, Damm R, Peters N, et al. Radioablation by image-guided (HDR) brachytherapy and transarterial chemoembolization in hepatocellular carcinoma: a randomized phase II trial. Cardiovasc Intervent Radiol 2019;42:239-49. http://dx.doi.org/10.1007/s00270-018-2127-5.
- Molinari M, Walsh MJ, Renfrew PD, Kneteman NM, Helton W. Treatment trade-off for ablation or hepatic resection for small HCC. Hepatology 2009;50. http://dx.doi.org/10.1002/hep.23307.
- Molinari M, De Coutere S, Krahn M, Helton S, Urbach DR. Patients’ preferences and trade-offs for the treatment of early stage hepatocellular carcinoma. J Surg Res 2014;189:57-6. https://dx.doi.org/10.1016/j.jss.2014.02.015.
- Mornex F, Merle P, Beziat C, Wautot V, Kubas A, Khodri M, et al. Tolerance and efficacy of high-dose 3D-conformal radiation therapy (CRT) in cirrhotic patients with small hepatocellular carcinomas (HCC) not suitable for curative therapies. Int J Radiat Oncol Biol Phys 2005;63. https://dx.doi.org/10.1016/j.ijrobp.2005.07.032.
- Nahon P, Seror O, Oberti F, Dimby SF, Aube C, Blanc JF, et al. Radiological and pathological response to neoadjuvant nivolumab in patients with BCLC A HCC treated by curative percutaneous irreversible electroporation: preliminary report from the NIVOLEP trial (NCT03630640). J Hepatol 2021;75.
- Nanashima A, Yamaguchi H, Omagari K, Nakazaki T, Aritomi T, Hatano K, et al. A comparison of hepatic resection and ablative therapy regarding the survival of hepatocellular carcinoma patients in Nagasaki. Acta Med Nagasaki 2004;49:87-91.
- Nanashima A, Tobinaga S, Masuda J, Miyaaki H, Taura N, Takeshita H, et al. Selecting treatment for hepatocellular carcinoma based on the results of hepatic resection and local ablation therapy. J Surg Oncol 2010;101:481-5. https://dx.doi.org/10.1002/jso.21523.
- Nathan H, Segev DL, Bridges JF, Massie AB, Cameron AM, Hirose K, et al. Influence of nonclinical factors on choice of therapy for early hepatocellular carcinoma. Ann Surg Oncol 2013;20:448-56. https://dx.doi.org/10.1245/s10434-012-2619-5.
- Ogiso S, Seo S, Eso Y, Yoh T, Kawai T, Okumura S, et al. Laparoscopic liver resection versus percutaneous radiofrequency ablation for small hepatocellular carcinoma. HPB (Oxford) 2021;23:533-7. https://dx.doi.org/10.1016/j.hpb.2020.08.009.
- Oh JH, Sinn DH, Kang W, Gwak GY, Paik YH, Lee JH, et al. Long-term outcome according to initial treatment modality in multiple hepatocellular carcinoma within the Milan criteria. J Hepatol 2019;70. http://dx.doi.org/10.1016/S0168-8278%2819%2930204-1.
- Oh JH, Sinn DH, Choi GS, Kim JM, Joh JW, Kang TW, et al. Comparison of outcome between liver resection, radiofrequency ablation, and transarterial therapy for multiple small hepatocellular carcinoma within the Milan criteria. Ann Surg Treat Res 2020;99:238-46. https://dx.doi.org/10.4174/astr.2020.99.4.238.
- Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, et al. Thermal ablation therapy for hepatocellular carcinoma: comparison between radiofrequency ablation and percutaneous microwave coagulation therapy. Hepatogastroenterology 2006;53:651-4.
- Pan YX, Long Q, Yi MJ, Chen JB, Chen JC, Zhang YJ, et al. Radiofrequency ablation versus laparoscopic hepatectomy for hepatocellular carcinoma: a real world single center study. Eur J Surg Oncol 2020;46:548-59. https://dx.doi.org/10.1016/j.ejso.2019.10.026.
- Park KW, Park JW, Kim TH, Choi JI, Kim SH, Park HS, et al. Five-year survival analysis of a cohort of hepatocellular carcinoma patients who treated at the National Cancer Center, Korea. Korean J Hepatol 2007;13:530-42.
- Park S, Lee EJ, Rim CH, Seong J. Plasma cell-free DNA as a predictive marker after radiotherapy for hepatocellular carcinoma. Yonsei Med J 2018;59:470-9. https://dx.doi.org/10.3349/ymj.2018.59.4.470.
- Park JW, Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, et al. Proton beam radiotherapy versus radiofrequency ablation treatment in patients with recurrent hepatocellular carcinoma: a randomized controlled phase 3 non-inferiority APROH trial. J Hepatol 2020;73. https://dx.doi.org/10.1016/s0168-8278(20)30756-x.
- Peng ZW, Zhang YJ, Chen MS. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinoma small than 2 cm. HPB (Oxford) 2011;13. http://dx.doi.org/10.1111/j.1477-2574.2011.00383.x.
- Peng Z, Chen M. Radiofrequency ablation versus liver resection for elderly patients with hepatocellular carcinoma (HCC) within the Milan criteria. J Vasc Interv Radiol 2012;23:853.e3-4. http://dx.doi.org/10.1016/j.jvir.2012.04.029.
- Peng ZW, Liu FR, Ye S, Xu L, Zhang YJ, Liang HH, et al. Radiofrequency ablation versus open hepatic resection for elderly patients (> 65 years) with very early or early hepatocellular carcinoma. Cancer 2013;119:3812-20. https://dx.doi.org/10.1002/cncr.28293.
- Peng JR, Zhu WH, Xu QQ, Gan LY, Zheng SM, Zhang DF, et al. Comparison of the therapeutic effects of microwave ablation and surgical resection for small hepatocellular carcinoma. J Clin Oncol 2014;32.
- Praktiknjo M, Krabbe V, Pohlmann A, Sampels M, Jansen C, Meyer C, et al. Evolution of nodule stiffness might predict response to local ablative therapy: a series of patients with hepatocellular carcinoma. PLOS ONE 2018;13. http://dx.doi.org/10.1371/journal.pone.0192897.
- Pryor D, Liu H, Hodgkinson P, Leggett D, McLean K, Lee D, et al. Efficacy and toxicity of stereotactic radiation therapy in early and advanced stage hepatocellular carcinoma. J Gastroenterol Hepatol 2019;34:34-5.
- Rong W, Yu W, Wang L, Wu F, Zhang K, Chen B, et al. Adjuvant radiotherapy in central hepatocellular carcinoma after narrow-margin hepatectomy: a 10-year real-world evidence. Chin J Cancer Res 2020;32:645-53. http://dx.doi.org/10.21147/j.issn.1000-9604.2020.05.09.
- Ruzzenente A, Guglielmi A, Sandri M, Campagnaro T, Valdegamberi A, Conci S, et al. Surgical resection versus local ablation for HCC on cirrhosis: results from a propensity case-matched study. J Gastrointest Surg 2012;16:301-11. https://dx.doi.org/10.1007/s11605-011-1745-x.
- Ryu T, Takami Y, Wada Y, Tateishi M, Hara K, Yoshitomi M, et al. Hepatic resection versus surgical microwave ablation for single hepatocellular carcinoma ≤ 5cm in a propensity score matching. Hepatology 2018;68. http://dx.doi.org/10.1002/hep.30257.
- Ryu T, Takami Y, Wada Y, Hara T, Sasaki S, Saitsu H. Hepatic resection versus operative microwave ablation for single hepatocellular carcinoma ≤ 5 cm: a propensity score-matched analysis. Surgery 2019;166:254-62. https://dx.doi.org/10.1016/j.surg.2019.05.007.
- Sako K, Komorizono Y, Oketani M, Hasegawa S, Yamasaki N, Shibatou T, et al. Outcome of patients with hepatitis C virus-related single, small hepatocellular carcinoma. Anticancer Res 2003;23:4191-6.
- Santambrogio R, Costa M, Conti M, Zuin M, Opocher E, Bruno S. The impact of radiofrequency ablation and liver resection on hepatocellular carcinoma (HCC) recurrence and survival in cirrhotic patients with early and very early HCC: a retrospective-prospective cohort study. Dig Liver Dis 2009;41:A8-9. https://doi.org/10.1016/j.dld.2009.02.025.
- Santambrogio R, Chiang J, Barabino M, Meloni FM, Bertolini E, Melchiorre F, et al. Comparison of laparoscopic microwave to radiofrequency ablation of small hepatocellular carcinoma (≤ 3 cm). Ann Surg Oncol 2017;24:257-63. https://dx.doi.org/10.1245/s10434-016-5527-2.
- Santambrogio R, Barabino M, Bruno S, Mariani N, Maroni N, Bertolini E, et al. Surgical resection vs. ablative therapies through a laparoscopic approach for hepatocellular carcinoma: a comparative study. J Gastrointest Surg 2018;22:650-60. https://dx.doi.org/10.1007/s11605-017-3648-y.
- Santambrogio R, Barabino M, D’Alessandro V, Iacob G, Opocher E, Gemma M, et al. Micronvasive behaviour of single small hepatocellular carcinoma: which treatment?. Updates Surg 2021;73:1359-69. http://dx.doi.org/10.1007/s13304-021-01036-0.
- Schaible J, Lurken L, Wiggermann P, Verloh N, Einspieler I, Zeman F, et al. Primary efficacy of percutaneous microwave ablation of malignant liver tumors: comparison of stereotactic and conventional manual guidance. Sci Rep 2020;10. https://dx.doi.org/10.1038/s41598-020-75925-6.
- Sheng L, Wang Y, Jun D, Peihong W. Comparison of the effectiveness of percutaneous microwave ablation versus hepatectomy for hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi 2015;37:301-7.
- Shibata T, Maetani Y, Ametani F, Kubo T, Itoh K, Konishi J. Tumor ablation therapy for small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology 2001;221:248-9.
- Shiozawa K, Watanabe M, Ikehara T, Matsukiyo Y, Kogame M, Kishimoto Y, et al. Comparison of percutaneous radiofrequency ablation and CyberKnife for initial solitary hepatocellular carcinoma: a pilot study. World J Gastroenterol 2015;21:13490-9. http://dx.doi.org/10.3748/wjg.v21.i48.13490.
- Simo KA, Sereika SE, Newton KN, Gerber DA. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol 2011;104:822-9. https://doi.org/10.1002/jso.21933.
- Song KD, Lim HK, Rhim H, Lee MW, Kang TW, Paik YH, et al. Hepatic resection vs percutaneous radiofrequency ablation of hepatocellular carcinoma abutting right diaphragm. World J Gastrointest Oncol 2019;11:227-37. http://dx.doi.org/10.4251/wjgo.v11.i3.227.
- Spangenberg HC. Resection or ablation in hepatocellular carcinoma: pro ablation. Dtsch Med Wochenschr 2008;133. http://dx.doi.org/10.1055/s-2008-1081113.
- Stuart KA, Liu H, McLean K, Pryor D. Early Australian experience with stereotactic body radiation therapy in the management of hepatocellular carcinoma: efficacy and toxicity. J Gastroenterol Hepatol 2018;33:23-4.
- Su TS, Liang P, Shixiong L, Zhou Y, Huang Y, Cheng T. Stereotactic body radiation therapy as a radical or salvage treatment for early-stage hepatocellular carcinoma: a prospective phase II study. Int J Radiat Oncol Biol Phys 2020;108.
- Suh SW, Lee KW, Lee JM, Yoo T, Choi YR, Yi NJ, et al. Prediction of aggressiveness in early-stage hepatocellular carcinoma for selection of surgical treatment. Hepatology 2013;58. http://dx.doi.org/10.1002/hep.26883.
- Sutter O, Fihri A, Ourabia-Belkacem R, Sellier N, Diallo A, Seror O. Real-time 3D virtual target fluoroscopic display for challenging hepatocellular carcinoma ablations using cone beam CT. Technol Cancer Res Treat 2018;17:1-7. https://dx.doi.org/10.1177/1533033818789634.
- Takamatsu S, Yamamoto K, Kawamura M, Asahi S, Satoh Y, Tameshige Y, et al. Early experience with proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2014;90:S370-1.
- Takami Y, Wada Y, Saitsu H. Strategy for treatment of hepatocellular carcinoma - Hr and MCN. Hepatol Int 2009;3. http://dx.doi.org/10.1007/s12072-008-9120-z.
- Takami Y, Ryu T, Wada Y, Saitsu H. Evaluation of microwave coagulo-necrotic therapy (MCN) for hepatocellular carcinoma (HCC): a single center experience of 650 consecutive cases. Hepatology 2010;52. http://dx.doi.org/10.1002/hep.23996.
- Takayama T, Makuuchi M, Hasegawa K. Single HCC smaller than 2 cm: surgery or ablation?. J Hepatobiliary Pancreat Sci 2010;17:422-4. https://dx.doi.org/10.1007/s00534-009-0239-7.
- Takayasu K, Arii S, Sakamoto M, Matsuyama Y, Kudo M, Kaneko S, et al. Impact of resection and ablation for single hypovascular hepatocellular carcinoma ≤ 2 cm analysed with propensity score weighting. Liver Int 2018;38:484-93. https://dx.doi.org/10.1111/liv.13670.
- Takeda A, Takahashi M, Kunieda E, Takeda T, Sanuki N, Koike Y, et al. Hypofractionated stereotactic radiotherapy with and without transarterial chemoembolization for small hepatocellular carcinoma not eligible for other ablation therapies: preliminary results for efficacy and toxicity. Hepatol Res 2008;38:60-9.
- Takeda A, Sanuki N, Tsurugai Y, Iwabuchi S, Matsunaga K, Ebinuma H, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 2016;122:2041-9. https://dx.doi.org/10.1002/cncr.30008.
- Tanaka S, Iwai S, Takemura S, Hagiwara A, Uchida S, Ito T, et al. Laparoscopic hepatic resection versus radiofrequency ablation for local control of small hepatocellular carcinoma located on the surface of the liver. HPB (Oxford) 2015;17.
- Tanguturi S, Kambadakone A, Niemierko A, Nguyen KN, Wo JY, Blaszkowsky LS, et al. Early response assessment after hypofractionated proton radiation therapy for primary liver tumors. Int J Radiat Oncol Biol Phys 2015;93.
- Tatineni T, Kataki K, Madhavan R, Das R, Dutta D. Prognostication of HCC with PVT treated with SBRT: early results from a prospective study in India. Radiother Oncol 2019;133:S759-60. https://dx.doi.org/10.1016/s0167-8140(19)31814-6.
- Teramoto K, Kawamura T, Takamatsu S, Nakamura N, Kudo A, Noguchi N, et al. Laparoscopic and thoracoscopic approaches for the treatment of hepatocellular carcinoma. Am J Surg 2005;189:474-8.
- Toro A, Pulvirenti E, Palermo F, Di Carlo I. Health-related quality of life in patients with hepatocellular carcinoma after hepatic resection, transcatheter arterial chemoembolization, radiofrequency ablation or no treatment. Surg Oncol 2012;21:e23-30. https://dx.doi.org/10.1016/j.suronc.2011.10.005.
- Trotschel H. Hepatocellular carcinoma: early MR imaging and monitoring of progression in irreversible electroporation. Rofo 2016;188:910-1. http://dx.doi.org/10.1055/s-0036-1585605.
- Ueno M, Takabatake H, Kayahara T, Morimoto Y, Mizuno M. Stereotactic body radiotherapy versus radiofrequency ablation for single small hepatocellular carcinoma: a propensity score matching analysis. Hepatology 2020;72:708A-9A.
- Utsunomiya T, Shimada M, Kudo M, Ichida T, Matsui O, Izumi N, et al. Nationwide study of 4741 patients with non-B non-C hepatocellular carcinoma with special reference to the therapeutic impact. Ann Surg 2014;259:336-45. http://dx.doi.org/10.1097/SLA.0b013e31829291e9.
- Vietti Violi N, Duran R, Guiu B, Aube C, Bize PE, Pache I, et al. Prospective randomized controlled trial comparing efficacy of microwave ablation and radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with a chronic liver disease. Cardiovasc Intervent Radiol 2017;40. http://dx.doi.org/10.1007/s00270-017-1725-y.
- Vietti VN, Duran R, Guiu B, Aube C, Cercueil JP, Bize P, et al. Microwave ablation and radiofrequency ablation for the treatment of hepatocellular carcinoma: result of the first prospective randomized controlled trial. Ann Oncol 2017;28. http://dx.doi.org/10.1093/annonc/mdx263.013.
- Vitale A, D’Amico F, Gringeri E, Zanus G, Cillo U. Laparoscopic ablation and salvage transplantation in patients with hepatocellular carcinoma. Transplantation 2012;94.
- Vitali GC, Laurent A, Terraz S, Majno P, Buchs NC, Rubbia-Brandt L, et al. Minimally invasive surgery versus percutaneous radio frequency ablation for the treatment of single small (≤ 3 cm) hepatocellular carcinoma: a case-control study. Surg Endosc 2016;30:2301-7. https://dx.doi.org/10.1007/s00464-015-4295-6.
- Wang S, Huang J, Zhang J, Hu X, Zhu W, Hu L, et al. Combination treatment of hepatic carcinoma with transcatheter hepatic arterial chemoembolization and percutaneous hepatic cryoablation. Technol Cancer Res Treat 2007;6:524-7.
- Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol 2012;56:412-8. https://dx.doi.org/10.1016/j.jhep.2011.05.020.
- Wang WH, Wang Z, Wu JX, Zhang T, Rong WQ, Wang LM, et al. Survival benefit with IMRT following narrow-margin hepatectomy in patients with hepatocellular carcinoma close to major vessels. Liver Int 2015;35:2603-10. https://dx.doi.org/10.1111/liv.12857.
- Wang J, Sun W, Liu Q. Comparative study on effect of radiofrequency ablation and operation for the treatment of small hepatocellular carcinoma. Cancer Res Clin 2015;27:620-3. http://dx.doi.org/10.3760/cma.j.issn.1006-9801.2015.09.012.
- Wang G, Zhang W, Tan Y, Jiang L, Yang J, Yang J, et al. The risk factors for long-term survival outcome in solitary hepatocellular carcinoma up to 2cm: propensity score matching analysis in a population cohort with a high rate of HBV infection. Int J Surg 2019;72:1-6. https://dx.doi.org/10.1016/j.ijsu.2019.10.006.
- Wang L, Deng D, Deng C. Clinical efficacy and long-term prognosis of laparoscopic liver resection and radiofrequency ablation for small hepatocellular carcinoma. Int J Clin Exp Med 2020;13:7349-56.
- Wei J, Li J, Fan W. Unresectable hepatocellular carcinoma: transcatheter arterial chemoembolization combined with microwave ablation versus combined with cryoablation. Gut 2020;69:A76-7. https://dx.doi.org/10.1136/gutjnl-2020-IDDF.146.
- Wigg AJ, Muller KR, Teo M, Rodgers A, Mounkley D, Chinnaratha MA, et al. Stereotactic ablative body radiotherapy for early hepatocellular carcinoma: a promising early South Australian experience. J Gastroenterol Hepatol 2017;32.
- Wiggermann P, Puls R, Vasilj A, Sieron D, Schreyer AG, Jung EM, et al. Thermal ablation of unresectable liver tumors: factors associated with partial ablation and the impact on long-term survival. Med Sci Monit 2012;18:CR88-92.
- Wong TC, Lee VH, Law AL, Pang HH, Lam KO, Lau V, et al. Prospective study of stereotactic body radiation therapy for hepatocellular carcinoma on waitlist for liver transplant. Hepatology 2021;74:2580-94. https://dx.doi.org/10.1002/hep.31992.
- Wu D, Yang Y, Chen J, Cai H, Duan Y, Sun D. Three different ways of treating primary hepatocellular carcinoma at an early stage: a prospective comparative study. Gastroenterol Res Pract 2020;2020. https://dx.doi.org/10.1155/2020/7802498.
- Xie L, Cao F, Qi H, Song Z, Shen L, Chen S, et al. Efficacy and safety of CT-guided percutaneous thermal ablation for hepatocellular carcinoma adjacent to the second porta hepatis. Int J Hyperthermia 2019;36:1122-8. https://dx.doi.org/10.1080/02656736.2019.1684575.
- Xu KC, Niu LZ, Zhou Q, Hu YZ, Guo DH, Liu ZP, et al. Sequential use of transarterial chemoembolization and percutaneous cryosurgery for hepatocellular carcinoma. World J Gastroenterol 2009;15:3664-9.
- Xu W, Kim BW, Park YK, Cheong JY, Cho SW, Wang HJ. Comparison of hepatic resection and radiofrequency ablation for solitary hepatocellular carcinoma smaller than 4 cm. HPB (Oxford) 2014;16.
- Xu XS, Chen W, Miao RC, Zhou YY, Wang ZX, Zhang LQ, et al. Survival analysis of hepatocellular carcinoma: a comparison between young patients and aged patients. Chin Med J (Engl) 2015;128:1793-800. https://dx.doi.org/10.4103/0366-6999.159356.
- Yamao T, Imai K, Yamashita YI, Kaida T, Nakagawa S, Mima K, et al. Surgical treatment strategy for hepatocellular carcinoma in patients with impaired liver function: hepatic resection or radiofrequency ablation?. HPB (Oxford) 2018;20:244-50. https://dx.doi.org/10.1016/j.hpb.2017.08.031.
- Yamashita S, Katagiri S, Ariizumi SI, Ohmori A, Takahashi Y, Namoto S, et al. Hepatectomy vs. radio frequency ablation as first-line treatment for a small hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2017;24.
- Yamashita S, Ariizumi S, Otera YK, Omori A, Kato T, Nemoto S, et al. Clinical outcome by tumor diameter in initial treatment for small hepatocellular carcinoma. HPB (Oxford) 2019;21:S371-S2. http://dx.doi.org/10.1016/j.hpb.2019.10.2009.
- Yamazaki O, Oka H, Manabe T, Kioka K, Kurai O, Murata K, et al. Comparison of the long-term results between surgical resection and local ablation for Child–Pugh A patients with a single hepatocellular carcinoma of 5 cm or less in size: a single center study. Kanzo 2009;50:173-84. http://dx.doi.org/10.2957/kanzo.50.173.
- Yang ZX, Wang D, Wang G, Zhang QH, Liu JM, Peng P, et al. Clinical study of recombinant adenovirus-p53 combined with fractionated stereotactic radiotherapy for hepatocellular carcinoma. J Cancer Res Clin Oncol 2010;136:625-30. https://dx.doi.org/10.1007/s00432-009-0701-6.
- Ye X, Ge Z, Fei X, Wang S, Cheng Y, Chen X, et al. Clinical study on HIFU combined with Jinlong capsules in treating 54 cases of primary liver cancer. Chin J Clin Oncol 2008;35:372-4.
- Yi Y, Zhang Y, Wei Q, Zhao L, Han J, Song Y, et al. Radiofrequency ablation or microwave ablation combined with transcatheter arterial chemoembolization in treatment of hepatocellular carcinoma by comparing with radiofrequency ablation alone. Chin J Cancer Res 2014;26:112-8. https://doi.org/10.3978/j.issn.1000-9604.2014.02.09.
- Yohji H, Aikata H, Kimura T, Kakizawa H, Kenjo M, Awai K, et al. Pilot study of stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. Hepatology 2012;56.
- Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, et al. Efficacy and safety of transarterial chemoembolization plus external beam radiotherapy vs sorafenib in hepatocellular carcinoma with macroscopic vascular invasion a randomized clinical trial. JAMA Oncol 2018;4:661-9. https://dx.doi.org/10.1001/jamaoncol.2017.5847.
- Yu WB, Wang WH, Rong WQ, Wang LM, Xu Q, Wu F, et al. Adjuvant radiotherapy in centrally located hepatocellular carcinomas after hepatectomy with narrow margin (<1 cm): a prospective randomized study. J Am Coll Surg 2014;218:381-92. https://dx.doi.org/10.1016/j.jamcollsurg.2013.11.030.
- Yun WK, Choi MS, Yoo BC, Lee JH, Koh KC, Paik SW, et al. Long-term outcome after surgery is superior to that after radiofrequency ablation in Child–Pugh class A patients with single small hepatocellular carcinoma. Hepatology 2009;50. http://dx.doi.org/10.1002/hep.23307.
- Zhang J, Zhang C, Chen S, Lai J, Zheng W, Wang X. Effect of stereotactic radiotherapy (SRT) combined with percutaneous ethanol injection therapy (PEIT) for small hepatocarcinoma. Chin J Clin Oncol 2008;35:852-4.
- Zheng L, Zhang CH, Lin JY, Song CL, Qi XL, Luo M. Comparative effectiveness of radiofrequency ablation vs. surgical resection for patients with solitary hepatocellular carcinoma smaller than 5 cm. Front Oncol 2020;10. https://dx.doi.org/10.3389/fonc.2020.00399.
Appendix 1 Search strategies
The MEDLINE search strategies can be found in Appendix 1.1–1.4, along with a list of further databases and resources searched. All other search strategies can be found in Report Supplementary Material 1.
The terms used in all search strategies build upon those used in the searches to inform a previous systematic review on SIRT therapies for hepatocellular carcinoma:
Walton M, Wade R, Claxton L, Sharif-Hurst S, Harden M, Patel J, et al. Selective internal radiation therapies for unresectable early-, intermediate- or advanced-stage hepatocellular carcinoma: systematic review, network meta-analysis and economic evaluation. Health Technol Assess 2020;24(48)
Appendix 1.1 Search strategies for identification of randomised controlled trials
The following databases were searched:
-
MEDLINE ALL (Ovid)
-
Embase (Ovid)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley)
-
Science Citation Index (Web of Science)
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley)
-
Database of Abstracts of Reviews of Effects (DARE) (CRD databases)
-
International Health Technology Assessment database
-
Epistemonikos
-
International Prospective Register of Systematic Reviews (PROSPERO)
-
ClinicalTrials.gov
-
European Union Clinical Trials Register.
The MEDLINE search strategy can be found below. See Report Supplementary Material 1 for all other search strategies.
MEDLINE ALL
(includes: epub ahead of print, in-process and other non-indexed citations, Ovid MEDLINE Daily and Ovid MEDLINE)
via Ovid http://ovidsp.ovid.com/
Date range: 1946 to 1 February 2021
Date searched: 3 February 2021
Records retrieved: 2303
The MEDLINE strategy below includes a search filter to limit retrieval to RCTs using the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity-maximising version (2008 revision); Ovid format.
Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, Noel-Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from: www.training.cochrane.org/handbook.
1 Carcinoma, Hepatocellular/ (86,979)
2 Liver Neoplasms/ (151,355)
3 ((liver or hepatocellular or hepato-cellular or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (150,762)
4 (hepatocellularcarcinoma$ or hepatocarcinoma$ or hepato-carcinoma$).ti,ab. (4183)
5 hepatoma$.ti,ab. (28,611)
6 HCC.ti,ab. (58,929)
7 or/1-6 (234,592)
8 Neoplasm Staging/ (177,611)
9 (small$ or early or earlystage?).ti,ab. (3,163,695)
10 (((BCLC or Barcelona-Clinic Liver Cancer) adj3 (“0” or A or A1 or A2 or A3 or A4)) or BCLC0-A).ti,ab. (578)
11 ((“1” or “2” or “3” or one or two or three) adj (cm$ or centimet$)).ti,ab. (77,157)
12 (1cm$ or 2cm$ or 3cm$).ti,ab. (4783)
13 ((carcinoma$ or tumor$ or tumour$ or lesion$ or nodule$) adj6 (size$ or diameter$)).ti,ab. (124,495)
14 (eHCC or sHCC).ti,ab. (251)
15 or/8-13 (3,424,647)
16 14 or (7 and 15) (50,618)
17 Radiofrequency Ablation/ (1071)
18 Catheter Ablation/ (33,067)
19 Radiofrequency Therapy/ (1098)
20 ((radiofrequenc$ or radio frequenc$) adj3 ablat$).ti,ab. (20,597)
21 RFA.ti,ab. (6924)
22 RF ablation.ti,ab. (2496)
23 RTA.ti,ab. (2494)
24 RFTA.ti,ab. (62)
25 or/17-24 (45,285)
26 16 and 25 (3125)
27 Microwaves/ (17,445)
28 (microwave$ or micro wave$).ti,ab. (38,436)
29 (MWA or MCT or PMCT or PMWA).ti,ab. (7692)
30 or/27-29 (47,890)
31 16 and 30 (715)
32 Laser Therapy/ (38,202)
33 (laser$ adj2 ablat$).ti,ab. (10,008)
34 LTA.ti,ab. (3499)
35 or/32-34 (48,883)
36 16 and 35 (142)
37 High-Intensity Focused Ultrasound Ablation/ (1728)
38 Ultrasonic Therapy/ (9714)
39 High intensity focus?ed ultrasound.ti,ab. (3137)
40 HIFU.ti,ab. (2449)
41 or/37-40 (12,933)
42 16 and 41 (132)
43 Cryosurgery/ (13,169)
44 Cryotherapy/ (5214)
45 (cryoablat$ or cryo-ablat$ or cryotherap$ or cryo-therap$ or cryosurg$ or cryo-surg$).ti,ab. (14,455)
46 or/43-45 (23,464)
47 16 and 46 (317)
48 Ethanol/ (88,324)
49 ((alcohol or ethanol) adj2 (inject$ or ablat$)).ti,ab. (5254)
50 (PEI or PEIT).ti,ab. (8408)
51 or/48-50 (98,037)
52 16 and 51 (975)
53 Acetic Acid/ (10,269)
54 Acetates/ (39,925)
55 (acetic acid adj2 (inject$ or ablat$)).ti,ab. (408)
56 PAI.ti,ab. (14,810)
57 PAAI.ti,ab. (19)
58 or/53-57 (62,615)
59 16 and 58 (132)
60 Electroporation/ (8033)
61 electroporation.ti,ab. (10,705)
62 IRE.ti,ab. (2151)
63 or/60-62 (15,275)
64 16 and 63 (122)
65 ((stereotactic or stereotaxic) adj3 ablat$).ti,ab. (1271)
66 ((stereotactic or stereotaxic) adj3 (radiotherap$ or radiation)).ti,ab. (9612)
67 (SABR or SABRT).ti,ab. (803)
68 SBRT.ti,ab. (4238)
69 SABER.ti,ab. (356)
70 or/65-69 (10,729)
71 16 and 70 (428)
72 Ablation Techniques/ (2918)
73 (ablat$ adj2 (therap$ or intervention$ or treatment$ or technique$ or method$ or procedure$)).ti,ab. (16,748)
74 (ablat$ adj2 (chemical$ or thermal$)).ti,ab. (4065)
75 (ablat$ adj2 (tumour$ or tumor$)).ti,ab. (4083)
76 or/72-75 (24,549)
77 16 and 76 (1788)
78 Chemoembolization, Therapeutic/ (5983)
79 (chemo-emboli$ or chemoemboli$).ti,ab. (8271)
80 TACE.ti,ab. (5534)
81 cTACE.ti,ab. (144)
82 (DEBTACE or DEB-TACE).ti,ab. (243)
83 (eluting adj2 bead$).ti,ab. (624)
84 DC bead$.ti,ab. (108)
85 or/78-84 (11,130)
86 16 and 85 (3538)
87 Embolization, Therapeutic/ (32,829)
88 (embolization$ or embolisation$ or embolize$ or embolise$ or embolizing$ or embolising$ or embolotherap$).ti,ab. (52,479)
89 TAE.ti,ab. (2435)
90 or/87-89 (63,331)
91 16 and 90 (2015)
92 ((locoregional or loco-regional) adj2 (therap$ or intervention$ or treatment$ or technique$ or method$ or procedure$)).ti,ab. (3346)
93 16 and 92 (531)
94 (Therasphere$ or Thera-sphere$).ti,ab. (79)
95 (SIR-Sphere$ or SIRSphere$).ti,ab. (119)
96 (QuiremSphere$ or Quirem-Sphere$).ti,ab. (4)
97 or/94-96 (167)
98 16 and 97 (44)
99 Microspheres/ (28,670)
100 (microsphere$ or sphere$).ti,ab. (76,678)
101 (microbead$ or bead$).ti,ab. (56,354)
102 or/99-101 (139,820)
103 Yttrium Radioisotopes/ (3105)
104 Yttrium/ (3157)
105 Yttrium Isotopes/ (709)
106 (Yttrium$ or 90Yttrium$ or Y90 or Y-90 or 90Y or 90-Y).ti,ab. (9775)
107 Holmium/ (904)
108 (Holmium$ or 166Holmium$ or Ho-166 or Ho166 or 166Ho or 166-Ho).ti,ab. (3496)
109 Radiopharmaceuticals/ (51,067)
110 or/103-109 (66,136)
111 102 and 110 (1871)
112 ((radioactiv$ or radio-activ$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$ or radiolabel$ or radio-label$ or radiopharmaceutic$ or radio-pharmaceutic$) adj2 (sphere$ or microsphere$ or bead$ or microbead$)).ti,ab. (4168)
113 (radiomicrosphere$ or radio-microsphere$).ti,ab. (33)
114 or/111-113 (5932)
115 16 and 114 (315)
116 Brachytherapy/ (19,954)
117 (brachytherap$ or brachy-therap$ or microbrachytherap$).ti,ab. (18,064)
118 or/116-117 (25,595)
119 118 and (110 or 112 or 113) (1048)
120 16 and 119 (85)
121 (radioemboli$ or radio-emboli$ or radioembolotherap$ or radio-embolotherap$).ti,ab. (1791)
122 TARE.ti,ab. (276)
123 (internal$ adj3 (radiation$ or radiotherap$ or radio therap$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$)).ti,ab. (2446)
124 ((intra-arterial$ or intraarterial$) adj3 (radiation$ or radiotherap$ or radio therap$ or radionuclide$ or radio-nuclide$ or radioisotope$ or radio-isotope$)).ti,ab. (284)
125 ((intra-arterial$ or intraarterial$) adj2 (brachytherap$ or brachy-therap$)).ti,ab. (20)
126 SIRT.ti,ab. (1519)
127 (SIR adj2 (therap$ or treatment$)).ti,ab. (88)
128 (radiation adj2 (segmentectom$ or lobectom$)).ti,ab. (53)
129 or/121-128 (5699)
130 16 and 129 (617)
131 26 or 31 or 36 or 42 or 47 or 52 or 59 or 64 or 71 or 77 (5352)
132 86 or 91 or 93 or 98 or 115 or 120 or 130 (5241)
133 randomized controlled trial.pt. (521,951)
134 controlled clinical trial.pt. (94,049)
135 randomized.ab. (510,387)
136 placebo.ab. (215,580)
137 drug therapy.fs. (2,274,478)
138 randomly.ab. (351,559)
139 trial.ab. (541,682)
140 groups.ab. (2,157,357)
141 133 or 134 or 135 or 136 or 137 or 138 or 139 or 140 (4,916,502)
142 exp animals/ not humans.sh. (4,782,806)
143 141 not 142 (4,274,490)
144 131 and 143 (1485)
145 132 and 143 (1633)
146 144 or 145 (2615)
147 limit 146 to yr=“2000 -Current” (2303)
Key:
/ = subject heading (MeSH heading)
sh = subject heading (MeSH heading)
exp = exploded subject heading (MeSH heading)
$ = truncation
? = optional wild card character – stands for zero or one characters
ti,ab = terms in title or abstract fields
adj3 = terms within three words of each other (any order)
pt = publication type
fs = floating subheading
Search strategies for identification of randomised controlled trials of wider radiotherapy techniques (March 2021)
MEDLINE ALL
(includes: epub ahead of print, in-process and other non-indexed citations, Ovid MEDLINE Daily and Ovid MEDLINE)
via Ovid http://ovidsp.ovid.com/
Date range: 1946 to 16 March 2021
Date searched: 17 March 2021
Records retrieved: 399
The MEDLINE strategy below includes a search filter to limit retrieval to RCTs using the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity-maximising version (2008 revision); Ovid format.
Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, Noel-Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from: www.training.cochrane.org/handbook.
1 Carcinoma, Hepatocellular/ (87,724)
2 Liver Neoplasms/ (152,378)
3 ((liver or hepatocellular or hepato-cellular or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (151,424)
4 (hepatocellularcarcinoma$ or hepatocarcinoma$ or hepato-carcinoma$).ti,ab. (4202)
5 hepatoma$.ti,ab. (28,603)
6 HCC.ti,ab. (59,303)
7 or/1-6 (235,478)
8 Neoplasm Staging/ (178,539)
9 (small$ or early or earlystage?).ti,ab. (3,174,425)
10 (((BCLC or Barcelona-Clinic Liver Cancer) adj3 (“0” or A or A1 or A2 or A3 or A4)) or BCLC0-A).ti,ab. (588)
11 ((“1” or “2” or “3” or one or two or three) adj (cm$ or centimet$)).ti,ab. (77,490)
12 (1cm$ or 2cm$ or 3cm$).ti,ab. (4785)
13 ((carcinoma$ or tumor$ or tumour$ or lesion$ or nodule$) adj6 (size$ or diameter$)).ti,ab. (125,055)
14 (eHCC or sHCC).ti,ab. (254)
15 or/8-13 (3,436,575)
16 14 or (7 and 15) (50,862)
17 Electrochemotherapy/ (673)
18 (electrochemotherap* or electro-chemotherap* or electro chemotherap* or electropermeabili?ation).ti,ab. (1115)
19 (electric* adj2 stimulat* adj2 (therap* or chemotherap* or chemo-therap* or chemo therap* or treat*)).ti,ab. (1260)
20 or/17-19 (2670)
21 histotripsy.ti,ab. (209)
22 Radiotherapy/ or Radiotherapy, Conformal/ or Radiotherapy, Intensity-Modulated/ or Radiotherapy, High-Energy/ or Radiotherapy, Image-Guided/ (72,234)
23 (radiotherap* or radiation-therap* or radiation therap*).ti,ab. (237,815)
24 ((intensity-modulat* or intensity modulat* or volumetric-modulat* or volumetric modulat*) adj4 (arc therap* or arc-therap*)).ti,ab. (2469)
25 (helical* adj4 tomotherap*).ti,ab. (1214)
26 or/22-25 (267,755)
27 Proton Therapy/ (3960)
28 (proton* adj4 therap*).ti,ab. (7126)
29 or/27-28 (8349)
30 20 or 21 or 26 or 29 (275,176)
31 16 and 30 (1887)
32 randomized controlled trial.pt. (525,223)
33 controlled clinical trial.pt. (94,097)
34 randomized.ab. (512,974)
35 placebo.ab. (216,151)
36 drug therapy.fs. (2,290,533)
37 randomly.ab. (353,254)
38 trial.ab. (543,763)
39 groups.ab. (2,167,571)
40 or/32-39 (4,942,795)
41 31 and 40 (496)
42 exp animals/ not humans.sh. (4,800,681)
43 41 not 42 (478)
44 limit 43 to yr=“2000 -Current” (399)
Key:
/ = subject heading (MeSH heading)
sh = subject heading (MeSH heading)
exp = exploded subject heading (MeSH heading)
$ = truncation
? = optional wild card character – stands for zero or one characters
ti,ab = terms in title or abstract fields
adj3 = terms within three words of each other (any order)
pt = publication type
fs = floating subheading
Appendix 1.2 Search strategies for identification of non-randomised studies where randomised controlled trial evidence was not available
The following databases were searched:
-
MEDLINE ALL (Ovid)
-
Embase (Ovid)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley)
-
Science Citation Index (Web of Science).
The MEDLINE search strategy can be found below. See Report Supplementary Material 1 for all other search strategies.
MEDLINE ALLw
(includes: epub ahead of print, in-process and other non-indexed citations, Ovid MEDLINE Daily and Ovid MEDLINE)
via Ovid http://ovidsp.ovid.com/
Date range: 1946 to 27 July 2021
Date searched: 28 July 2021
Records retrieved: 1139
1 Carcinoma, Hepatocellular/ (90,761)
2 Liver Neoplasms/ (156,595)
3 ((liver or hepatocellular or hepato-cellular or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (156,050)
4 (hepatocellularcarcinoma$ or hepatocarcinoma$ or hepato-carcinoma$).ti,ab. (4256)
5 hepatoma$.ti,ab. (28,811)
6 HCC.ti,ab. (61,752)
7 or/1-6 (241,089)
8 Neoplasm Staging/ (182,051)
9 (small$ or early or earlystage?).ti,ab. (3,247,367)
10 (((BCLC or Barcelona-Clinic Liver Cancer) adj3 (“0” or A or A1 or A2 or A3 or A4)) or BCLC0-A).ti,ab. (617)
11 ((“1” or “2” or “3” or one or two or three) adj (cm$ or centimet$)).ti,ab. (79,017)
12 (1cm$ or 2cm$ or 3cm$).ti,ab. (4905)
13 ((carcinoma$ or tumor$ or tumour$ or lesion$ or nodule$) adj6 (size$ or diameter$)).ti,ab. (128,094)
14 (eHCC or sHCC).ti,ab. (265)
15 or/8-13 (3,515,054)
16 14 or (7 and 15) (52,166)
17 High-Intensity Focused Ultrasound Ablation/ (1845)
18 Ultrasonic Therapy/ (9862)
19 High intensity focus?ed ultrasound.ti,ab. (3223)
20 HIFU.ti,ab. (2524)
21 or/17-20 (13,202)
22 16 and 21 (133)
23 Cryosurgery/ (13,402)
24 Cryotherapy/ (5359)
25 (cryoablat$ or cryo-ablat$ or cryotherap$ or cryo-therap$ or cryosurg$ or cryo-surg$).ti,ab. (14,771)
26 or/23-25 (23,935)
27 16 and 26 (323)
28 Electroporation/ (8248)
29 electroporation.ti,ab. (10,923)
30 IRE.ti,ab. (2244)
31 or/28-30 (15,583)
32 16 and 31 (133)
33 ((stereotactic or stereotaxic) adj3 ablat$).ti,ab. (1364)
34 ((stereotactic or stereotaxic) adj3 (radiotherap$ or radiation)).ti,ab. (10,149)
35 (SABR or SABRT).ti,ab. (859)
36 SBRT.ti,ab. (4543)
37 SABER.ti,ab. (421)
38 or/33-37 (11,398)
39 16 and 38 (456)
40 Electrochemotherapy/ (698)
41 (electrochemotherap* or electro-chemotherap* or electro chemotherap* or electropermeabili?ation).ti,ab. (1153)
42 (electric* adj2 stimulat* adj2 (therap* or chemotherap* or chemo-therap* or chemo therap* or treat*)).ti,ab. (1304)
43 40 or 41 or 42 (2757)
44 16 and 43 (25)
45 histotripsy.ti,ab. (230)
46 16 and 45 (7)
47 Radiotherapy, Conformal/ or Radiotherapy, Intensity-Modulated/ or Radiotherapy, High-Energy/ or Radiotherapy, Image-Guided/ (30,927)
48 ((radiotherap* or radiation-therap* or radiation therap*) adj3 (conformal or intensity-modulat* or intensity modulat* or high-energy or high energy)).ti,ab. (14,403)
49 ((intensity-modulat* or intensity modulat* or volumetric-modulat* or volumetric modulat*) adj4 (arc therap* or arc-therap*)).ti,ab. (2595)
50 (helical* adj4 tomotherap*).ti,ab. (1237)
51 47 or 48 or 49 or 50 (36,559)
52 16 and 51 (274)
53 Proton Therapy/ (4266)
54 (proton* adj4 therap*).ti,ab. (7420)
55 53 or 54 (8702)
56 16 and 55 (106)
57 22 or 27 or 32 or 39 or 44 or 46 or 52 or 56 (1292)
58 exp animals/ not humans.sh. (4,866,074)
59 57 not 58 (1226)
60 limit 59 to yr=“2000-Current” (1139)
Key:
/ = subject heading (MeSH heading)
sh = subject heading (MeSH heading)
exp = exploded subject heading (MeSH heading)
$ = truncation
* = truncation
? = optional wild card character – stands for zero or one characters
ti,ab = terms in title or abstract fields
adj3 = terms within three words of each other (any order)
Appendix 1.3 Search strategies for identification of non-randomised studies where additional evidence could plausibly change the network meta-analysis result, as identified by the threshold analysis
The following databases were searched:
-
MEDLINE ALL (Ovid)
-
Embase (Ovid)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley)
-
Science Citation Index (Web of Science)
The MEDLINE search strategy can be found below. See Report Supplementary Material 1 for all other search strategies.
MEDLINE ALL
(includes: epub ahead of print, in-process and other non-indexed citations, Ovid MEDLINE Daily and Ovid MEDLINE)
via Ovid http://ovidsp.ovid.com/
Date range: 1946 to 23 August 2021
Date searched: 24 August 2021
Records retrieved: 2539
1 Carcinoma, Hepatocellular/ (91,350)
2 Liver Neoplasms/ (157,448)
3 ((liver or hepatocellular or hepato-cellular or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (156,958)
4 (hepatocellularcarcinoma$ or hepatocarcinoma$ or hepato-carcinoma$).ti,ab. (4266)
5 hepatoma$.ti,ab. (28,844)
6 HCC.ti,ab. (62,236)
7 or/1-6 (242,196)
8 Neoplasm Staging/ (182,692)
9 (small$ or early or earlystage?).ti,ab. (3,261,749)
10 (((BCLC or Barcelona-Clinic Liver Cancer) adj3 (“0” or A or A1 or A2 or A3 or A4)) or BCLC0-A).ti,ab. (628)
11 ((“1” or “2” or “3” or one or two or three) adj (cm$ or centimet$)).ti,ab. (79,327)
12 (1cm$ or 2cm$ or 3cm$).ti,ab. (4920)
13 ((carcinoma$ or tumor$ or tumour$ or lesion$ or nodule$) adj6 (size$ or diameter$)).ti,ab. (128,670)
14 (eHCC or sHCC).ti,ab. (268)
15 or/8-13 (3,530,492)
16 14 or (7 and 15) (52,397)
17 Laser Therapy/ (38,954)
18 (laser$ adj2 ablat$).ti,ab. (10,374)
19 LTA.ti,ab. (3598)
20 or/17-19 (49,955)
21 Radiofrequency Ablation/ (1620)
22 Catheter Ablation/ (34,863)
23 Radiofrequency Therapy/ (1141)
24 ((radiofrequenc$ or radio frequenc$) adj3 ablat$).ti,ab. (21,317)
25 RFA.ti,ab. (7252)
26 RF ablation.ti,ab. (2534)
27 RTA.ti,ab. (2555)
28 RFTA.ti,ab. (63)
29 or/21-28 (47,522)
30 Microwaves/ (18,128)
31 (microwave$ or micro wave$).ti,ab. (39,869)
32 (MWA or MCT or PMCT or PMWA).ti,ab. (8015)
33 or/30-32 (49,569)
34 Carcinoma, Hepatocellular/su (13,662)
35 Liver Neoplasms/su (27,831)
36 Hepatectomy/ (31,584)
37 Surgical Procedures, Operative/ (56,264)
38 ((surgical$ or surger$ or operat$ or resect$) adj6 (carcinoma$ or tumor$ or tumour$ or lesion$ or nodule$ or neoplasm$ or liver$ or lobe$)).ti,ab. (257,196)
39 (hepatectom$ or hemi-hepatectom$ or hemihepatectom$ or lobectom$ or microlobectom$ or micro-lobectom$ or segmentectom$ or trisegmentectom$).ti,ab. (46,091)
40 ((carcinoma$ or tumor$ or tumour$ or lesion$ or nodule$ or neoplasm$ or liver$ or lobe$) adj4 (excis$ or remov$ or dissect$)).ti,ab. (71,760)
41 or/34-40 (396,793)
42 20 and (29 or 33 or 41) (3589)
43 29 and (20 or 33 or 41) (8135)
44 33 and (20 or 29 or 41) (2905)
45 41 and (20 or 29 or 33) (9295)
46 16 and (42 or 43 or 44 or 45) (2696)
47 exp animals/ not humans/ (4,877,412)
48 46 not 47 (2619)
49 limit 48 to yr=“2000-Current” (2539)
Key:
/ = indexing term (Medical Subject Heading: MeSH)
/su = indexing term with subheading for surgery
exp = exploded indexing term (MeSH)
$ = truncation
ti,ab = terms in either title or abstract fields
adj3 = terms within three words of each other (any order).
Appendix 1.4 Search strategies for identification of economic studies
The following databases were searched:
-
MEDLINE ALL (Ovid)
-
Embase (Ovid)
-
EconLit (Ovid)
-
NHS Economic Evaluations Database (CRD databases).
The MEDLINE search strategy can be found below. See Report Supplementary Material 1 for all other search strategies.
Ovid MEDLINE(R) ALL
(includes epub ahead of print, in-process and other non-indexed citations, Ovid MEDLINE Daily and Ovid MEDLINE)
via Ovid http://ovidsp.ovid.com/
Date range searched: 1946 to 12 May 2021
Date searched: 13 May 2021
Records retrieved: 181
The MEDLINE strategy below (lines 17–24) includes a narrow search filter to limit retrieval to economic studies. The filter was designed by the Canadian Journal of Health Technologies (CADTH).
Strings attached: CADTH database search filters [Internet]. Ottawa: CADTH; 2016. [cited 2021 05 13]. Available from: https://www.cadth.ca/resources/finding-evidence/strings-attached-cadths-database-search-filters#narrow
1 Carcinoma, Hepatocellular/ (88,831)
2 Liver Neoplasms/ (153,869)
3 ((liver or hepatocellular or hepato-cellular or hepatic$) adj3 (carcinoma$ or cancer$ or neoplas$ or tumour$ or tumor$ or malign$)).ti,ab. (153,407)
4 (hepatocellularcarcinoma$ or hepatocarcinoma$ or hepato-carcinoma$).ti,ab. (4221)
5 hepatoma$.ti,ab. (28,694)
6 HCC.ti,ab. (60,357)
7 or/1-6 (237,841)
8 Neoplasm Staging/ (179,919)
9 (small$ or early or earlystage?).ti,ab. (3,206,080)
10 (((BCLC or Barcelona-Clinic Liver Cancer) adj3 (“0” or A or A1 or A2 or A3 or A4)) or BCLC0-A).ti,ab. (604)
11 ((“1” or “2” or “3” or one or two or three) adj (cm$ or centimet$)).ti,ab. (78,139)
12 (1cm$ or 2cm$ or 3cm$).ti,ab. (4828)
13 ((carcinoma$ or tumor$ or tumour$ or lesion$ or nodule$) adj6 (size$ or diameter$)).ti,ab. (126,376)
14 (eHCC or sHCC).ti,ab. (257)
15 or/8-13 (3,470,534)
16 14 or (7 and 15) (51,398)
17 *economics/ (10,739)
18 exp *“costs and cost analysis”/ (74,182)
19 (economic adj2 model*).mp. (13,712)
20 (cost minimi* or cost-utilit* or health utilit* or economic evaluation* or economic review* or cost outcome or cost analys?s or economic analys?s or budget* impact analys?s).ti,ab,kf,kw. (35,068)
21 (cost-effective* or pharmacoeconomic* or pharmaco-economic* or cost-benefit or costs).ti,kf,kw. (76,616)
22 (life year or life years or qaly* or cost-benefit analys?s or cost-effectiveness analys?s).ab,kf,kw. (32,546)
23 (cost or economic*).ti,kf,kw. and (costs or cost-effectiveness or markov).ab. (61,298)
24 or/17-23 (187,991)
25 16 and 24 (199)
26 exp animals/ not humans.sh. (4,823,832)
27 25 not 26 (199)
28 limit 27 to yr=“2000-Current” (181)
Key:
/ or.sh. = indexing term (Medical Subject Heading: MeSH)
exp = exploded indexing term (MeSH)
$ or * = truncation
ti,ab = terms in either title or abstract fields
kw,kf = terms in keyword or keyfield field
adj3 = terms within three words of each other (any order).
mp = multipurpose field
? = replaces or adds up to one additional character
Appendix 2 Studies excluded at full paper stage with rationale (randomised controlled trial searches)
| Study | Reason for exclusion |
|---|---|
| An, 2021115 | Not a RCT |
| Chang, 2018116 | No relevant intervention/comparison |
| Cho, 2014117 | Not a RCT |
| Chong, 2017a118 | Not early HCC patients (≤ 3 cm tumour) |
| Chong, 2017b119 | Not early HCC patients (≤ 3 cm tumour) |
| Chong, 2018120 | Duplicate report |
| Chong, 2020121 | Not early HCC patients (≤ 3 cm tumour) |
| Crocetti, 2018122 | Not a RCT |
| Di Costanzo, 2011a123 | Not early HCC patients (≤ 3 cm tumour) |
| Di Costanzo, 2011b124 | Not early HCC patients (≤ 3 cm tumour) |
| Di Costanzo, 2011c125 | Not early HCC patients (≤ 3 cm tumour) |
| Di Costanzo, 2013126 | Not early HCC patients (≤ 3 cm tumour) |
| Di Costanzo, 2015127 | Not early HCC patients (≤ 3 cm tumour) |
| Duan, 2011128 | Not early HCC patients (≤ 3 cm tumour) |
| DuBay, 2011129 | Not a RCT |
| Fan, 2019130 | Not a RCT |
| Fang, 2005131 | Not a RCT |
| Ferrer Puchol, 2011132 | Not a RCT |
| Filippiadis, 2021133 | Not early HCC patients (≤ 3 cm tumour) |
| Fong, 2016134 | Not early HCC patients (≤ 3 cm tumour) |
| Frangakis, 2010135 | Not a RCT |
| Fukushima, 2015136 | No relevant intervention/comparison |
| Gerunda, 2000137 | Not early HCC patients (≤ 3 cm tumour) |
| Giorgio, 2010a138 | Duplicate report |
| Giorgio, 2010b139 | Duplicate report |
| Guo, 2005140 | Not early HCC patients (≤ 3 cm tumour) |
| Guo, 2013141 | Not a RCT |
| Ha, 2016142 | Not a RCT |
| Hayes, 2008143 | Not a RCT |
| He, 2018144 | Not early HCC patients (≤ 3 cm tumour) |
| Hong, 2005145 | Not a RCT |
| Hsiao, 2020146 | Not a RCT |
| Hsu, 2012147 | Not a RCT |
| Huang, 2009148 | Not early HCC patients (≤ 3 cm tumour) |
| Huang, 2020149 | Not a RCT |
| Hung, 2011150 | Not a RCT |
| Huo, 200382 | Not a RCT |
| Hyun, 2016151 | Not a RCT |
| Iida, 2014152 | Not a RCT |
| Ikeda, 2001153 | Not a RCT |
| Imai, 2013154 | Not a RCT |
| Jiang, 2015155 | Not a RCT |
| Jiang, 2017a156 | Not a RCT |
| Jiang, 2017b157 | Not early HCC patients (≤ 3 cm tumour) |
| Kaibori, 2012a158 | Not early HCC patients (≤ 3 cm tumour) |
| Kaibori, 2012b159 | Not early HCC patients (≤ 3 cm tumour) |
| Kayali, 2013160 | Not early HCC patients (≤ 3 cm tumour) |
| Kim, 2019161 | Not a RCT |
| Kim, 2021162 | Duplicate report |
| Kitamoto, 2003163 | Not a RCT |
| Kiyoshi, 2010164 | Duplicate report |
| Kobayashi, 2007165 | No relevant intervention/comparison |
| Koda, 2000166 | Duplicate report |
| Koh, 2015167 | Not early HCC patients (≤ 3 cm tumour) |
| Kong, 2014168 | Not a RCT |
| Lai, 2016169 | Not a RCT |
| Lambert, 2020170 | Not early HCC patients (≤ 3 cm tumour) |
| Lee, 2009171 | Not a RCT |
| Lee, 2018a172 | Not early HCC patients (≤ 3 cm tumour) |
| Lee, 2018b173 | Not a RCT |
| Li, 2007174 | Not early HCC patients (≤ 3 cm tumour) |
| Li, 2011175 | Not early HCC patients (≤ 3 cm tumour) |
| Li, 2012176 | Not early HCC patients (≤ 3 cm tumour) |
| Liao, 2017177 | No relevant intervention/comparison |
| Lin, 2005178 | Duplicate report |
| Lin, 2007a179 | Not a RCT |
| Lin, 2007b180 | Not a RCT |
| Liu, 2012181 | Not early HCC patients (≤ 3 cm tumour) |
| Liu, 2015182 | Not early HCC patients (≤ 3 cm tumour) |
| Liu, 2016183 | Not early HCC patients (≤ 3 cm tumour) |
| Liu, 2019184 | No relevant intervention/comparison |
| Liu, 2020185 | Not a RCT |
| Lu, 2006186 | Not early HCC patients (≤ 3 cm tumour) |
| Lu, 2008187 | Not early HCC patients (≤ 3 cm tumour) |
| Luo, 2005188 | Not a RCT |
| Ma, 2019189 | Not early HCC patients (≤ 3 cm tumour) |
| Maeda, 2003190 | Not early HCC patients (≤ 3 cm tumour) |
| Masuda, 2007191 | Not a RCT |
| Mbalisike, 2015192 | Not early HCC patients (≤ 3-cm tumour) |
| Meniconi, 2015193 | Not a RCT |
| Meyer, 2013194 | Not early HCC patients (≤ 3 cm tumour) |
| Mohamed, 2018195 | Not early HCC patients (≤ 3 cm tumour) |
| Mornex, 2007a196 | Not a RCT |
| Mornex, 2007b197 | Duplicate report |
| Murakami, 2007198 | Not a RCT |
| Ng, 201773 | Duplicate report |
| Ni, 2007199 | Not early HCC patients (≤ 3 cm tumour) |
| NIHR Horizon Scanning Centre200 | Not a RCT |
| Nouso, 2016201 | No relevant intervention/comparison |
| Ohmoto, 2006202 | Not a RCT |
| Ohmoto, 2009203 | Not a RCT |
| Olschewski, 2002204 | Duplicate report |
| Paik, 2016205 | Not a RCT |
| Panaro, 2014206 | Not a RCT |
| Park, 2020207 | No relevant intervention/comparison |
| Peng, 2008208 | Not a RCT |
| Petrowsky, 2008209 | Not a RCT |
| Pompili, 2013210 | Not a RCT |
| Riaz, 2009211 | Not a RCT |
| Roche, 2002212 | Not a RCT |
| Ryu, 2017213 | Not a RCT |
| Santambrogio, 2009214 | Not a RCT |
| Shen, 2018215 | Not early HCC patients (≤ 3 cm tumour) |
| Sherman, 2006216 | Not a RCT |
| Shi, 2014217 | Not a RCT |
| Shibata, 2006218 | No relevant intervention/comparison |
| Sun, 2020219 | Not a RCT |
| Tashiro, 2011220 | Not a RCT |
| Toyoda, 2008221 | Not a RCT |
| Tsai, 2008222 | Not a RCT |
| Vivarelli, 2004223 | Not a RCT |
| Wang, 2014224 | Duplicate report |
| Wang, 2015225 | No relevant outcome assessed |
| Wu, 2016226 | Not a RCT |
| Xu, 2012227 | Not early HCC patients (≤ 3 cm tumour) |
| Xu, 2013228 | Duplicate report |
| Xu, 2015a229 | Not a RCT |
| Xu, 2015b230 | Not early HCC patients (≤ 3 cm tumour) |
| Yamamoto, 2001231 | Not a RCT |
| Yamasaki, 2011232 | Not early HCC patients (≤ 3 cm tumour) |
| Yin, 2014233 | Not early HCC patients (≤ 3 cm tumour) |
| Yin, 2015234 | Duplicate report |
| Yin, 2019235 | Not a RCT |
| Yu, 2016236 | Duplicate report |
| Yuan, 2017237 | Not early HCC patients (≤ 3 cm tumour) |
| Yuen, 2003238 | Not early HCC patients (≤ 3 cm tumour) |
| Yun, 2011239 | Not a RCT |
| Zeng, 2018240 | Not early HCC patients (≤ 3 cm tumour) |
| Zhang, 2013241 | Not a RCT |
| Zhang, 2015242 | Not a RCT |
| Zhang, 2016243 | Not a RCT |
| Zhao, 2011244 | Not early HCC patients (≤ 3 cm tumour) |
| Zhi, 2006245 | Not a RCT |
| Zhou, 2009246 | Not early HCC patients (≤ 3 cm tumour) |
| Zhou, 2014247 | Not a RCT |
| Zhou, 2019248 | Not early HCC patients (≤ 3 cm tumour) |
| Zhu, 2007249 | Not early HCC patients (≤ 3 cm tumour) |
| Zhu, 2019250 | Not early HCC patients (≤ 3 cm tumour) |
Appendix 3 Risk of bias assessment results (randomised controlled trials)
| Trial | ROB arising from the randomisation process | ROB due to deviations from the intended intervention | ROB due to missing outcome data | ROB in measurement of the outcome | ROB in selection of the reported result | Overall judgement of ROB |
|---|---|---|---|---|---|---|
| Abdelaziz, 2014 – Complete response64 | High | Low | Low | Low | Low | High |
| Aikata, 2006 – OS (abstract)43 | Some concerns | High | High | Low | Low | High |
| Aikata, 2006 – PFS (abstract)43 | Some concerns | High | High | Low | Some concerns | High |
| Azab, 2011 – Complete response65 | Some concerns | Low | Low | Low | Low | Some concerns |
| Bian, 2014 – Recurrence78 | Some concerns | Low | Low | Low | Low | Some concerns |
| Brunello, 2008 – OS 44 | Low | Low | Low | Low | Low | Low |
| Chen, 2005 – OS 67 | Some concerns | Low | Low | Low | Low | Some concerns |
| Chen, 2005 – OS 66 | Some concerns | Low | High | Low | Low | High |
| Chen, 2006 – OS 68 | Low | High | Low | Low | Low | High |
| Chen, 2006 – PFS 68 | Low | High | Low | Low | Some concerns | High |
| Chen, 2014 – OS 45 | Low | Low | Low | Low | Low | Low |
| Chen, 2014 – Recurrence45 |
Low | Low | Low | Low | Low | Low |
| Fang, 2014 – OS 46 | Some concerns | Low | Low | Low | Low | Some concerns |
| Fang, 2014 – Disease-free survival46 | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Fang, 2014 – Recurrence46 | Some concerns | Low | Low | Low | Low | Some concerns |
| Ferrari, 2007 – OS 77 | Some concerns | Low | Low | Low | Low | Some concerns |
| Gan, 2004 – Recurrence47 | Some concerns | Low | High | Low | Low | High |
| Giorgio, 2011 – OS 48 | Low | High | High | Low | Low | High |
| Giorgio, 2011 – Recurrence48 | Low | High | High | Low | Low | High |
| Huang, 2005 – OS 49 | High | High | High | Low | Low | High |
| Huang, 2005 – PFS 49 | High | High | High | Low | Low | High |
| Huang, 2005 – Recurrence49 | High | High | High | Low | Some concerns | High |
| Huang, 2010 – OS 69 | Low | Low | High | Low | Low | High |
| Huo, 2003 – OS 70 | High | Low | High | Low | Low | High |
| Izumi, 2019 – PFS (abstract)50 | Some concerns | High | High | Low | Some concerns | High |
| Kim, 2020 – OS 51 | Some concerns | Low | Low | Low | Low | Some concerns |
| Kim, 2020 – PFS 51 | Some concerns | Low | Low | Low | Low | Some concerns |
| Koda, 2001 – OS 52 | Low | Low | Low | Low | Low | Low |
| Koda, 2001 – Recurrence52 | Low | Low | Low | Low | Low | Low |
| Lencioni, 2003 – OS 71 | Low | Low | Low | Low | Low | Low |
| Lencioni, 2003 – PFS 71 | Low | Low | Low | Low | Low | Low |
| Lencioni, 2003 – Recurrence71 | Low | Low | Low | Low | Low | Low |
| Lin, 2004 – OS 59 | Low | Low | Low | Low | Low | Low |
| Lin, 2004 – PFS 59 | Low | Low | Low | Low | Low | Low |
| Lin, 2004 – Recurrence59 | Low | Low | Low | Low | Low | Low |
| Lin, 2005 – OS 53 | Some concerns | Low | Low | Low | Low | Some concerns |
| Lin, 2005 – PFS 53 | Some concerns | Low | Low | Low | Low | Some concerns |
| Liu, 2016 – OS 72 | Some concerns | Low | Low | Low | Low | Some concerns |
| Mizuki, 2010 – OS 60 | Low | Some concerns | Some concerns | Low | Low | Some concerns |
| Mizuki, 2010 – PFS 60 | Low | Some concerns | Some concerns | Low | Low | Some concerns |
| Ng, 2017 – OS 79 | Low | Low | Low | Low | Low | Low |
| Ng, 2017 – PFS 79 | Low | Low | Low | Low | Low | Low |
| Orlacchio, 2014 – OS 61 | Some concerns | Low | Some concerns | Low | Low | Some concerns |
| Peng, 2012 – OS 74 | Low | Low | Low | Low | Low | Low |
| Peng, 2012 – Recurrence-free survival74 | Low | Low | Low | Low | Low | Low |
| Shibata, 2002 – Recurrence62 | Low | Low | Some concerns | Low | Low | Some concerns |
| Shibata, 2009 – OS 54 | High | Low | Low | Low | Low | High |
| Shibata, 2009 – PFS 54 | High | Low | Low | Low | Low | High |
| Shiina, 2005 – OS 55 | Some concerns | Low | Low | Low | Low | Some concerns |
| Vietti Violi, 2018 – OS 63 | Low | Some concerns | Low | Low | Low | Some concerns |
| Vietti Violi, 2018 – Progression63 | Low | Some concerns | Low | Low | Low | Some concerns |
| Vietti Violi, 2018 – TTP 63 | Low | Some concerns | Low | Low | Low | Some concerns |
| Xia, 2020 – OS 75 | Low | Low | Low | Low | Low | Low |
| Xia, 2020 – Recurrence-free survival75 | Low | Low | Low | Low | Low | Low |
| Yan, 2016 – OS 56 | High | Some concerns | Low | Low | Low | High |
| Yan, 2016 – PFS 56 | High | Some concerns | Low | Low | Low | High |
| Zhang, 2007 – OS 76 | Low | Low | Low | Low | Low | Low |
| Zou, 2017 – Response57 | Some concerns | Low | Low | Low | Low | Some concerns |
| Total |
High: 9
Some concerns: 20 Low: 29 |
High: 10
Some concerns: 7 Low: 41 |
High: 12
Some concerns: 4 Low: 42 |
Low: 58 |
Some concerns: 5
Low: 53 |
High: 19
Some concerns: 21 Low: 18 |
Appendix 4 Characteristics and results of randomised controlled trials included in the review
| Study name and location | Participant information | Intervention | Comparator | Main results | ROB |
|---|---|---|---|---|---|
| RFA vs. MWA | |||||
| Abdelaziz, 201464 Egypt |
111 patients (with 128 tumours) ≤ 5 cm; subgroup of 87 tumours ≤ 3 cm | RFA (n = 32 tumours ≤ 3 cm) | MWA (n = 55 tumours ≤ 3 cm) | Response: RFA: Complete ablation: 30/32 (93.8%) tumours; partial ablation: 2/32 (6.2%) tumours. MWA: Complete ablation: 54/55 (98.2%) tumours; partial ablation: 1/55 (1.8%) tumours. Adverse events: There were no major complications or deaths in either group. No other results reported for ≤ 3 cm tumour subgroup. |
High |
| Shibata, 200262 Japan |
72 patients (with 94 tumours) < 4 cm; subgroup of 88 tumours ≤ 3 cm | RFA (n = 36 patients, 3 of whom had tumours 3–4cm) | MWA (n = 36 patients, 3 of whom had tumours 3–4 cm) | Response: RFA: 46/48 (96%) nodules showed complete response and 2 (4%) had residual lesions or incomplete response. MWA: 41/46 (89%) nodules showed complete response and 5 (11%) showed incomplete response. Adverse events: RFA: one major complication (3%) (segmental hepatic infarction). MWA: four major complications (11%) (liver abscess, cholangitis, subcutaneous abscess with skin burn and subcapsular hematoma). No other results reported, other than Kaplan–Meier curve. |
Some concerns |
| Vietti Violi, 201863 Switzerland and France |
152 patients with tumours ≤ 4 cm (mean tumour size 1.8 cm, <8% patients had tumours > 3 cm) | MWA (n = 76 patients, 6 of whom had tumours 3–4 cm) | RFA (n = 76 patients, 5 of whom had tumours 3–4 cm) | OS: MWA: 2 years: 86%. RFA: 2 years: 84%. Recurrence: MWA: 2 years: 6% (local tumour progression). RFA: 2 years: 12% (local tumour progression). Comparison between groups: RR 1.62, 95% CI 0.66 to 3.94. TTP: MWA: Median 12 months (95% CI 5 to 28). RFA: Median 16 months (95% CI 4 to 24). Comparison between groups: HR 0.72, 95% CI 0.44 to 1.18. Response: MWA: 95% achieved complete response after one treatment; 5% achieved complete response after two treatments. RFA: 96% achieved complete response after one treatment; 4% achieved complete response after two treatments. Adverse events: MWA: 2 (2%) grade IV AEs; 5 (5%) grade I–II AEs. RFA: 3 (3%) grade III AEs; 12 (11.5%) grade I–II AEs. No treatment-related deaths in either group. |
Some concerns |
| RFA vs. PEI | |||||
| Azab, 201165 Egypt |
90 patients (with 98 tumours) ≤ 5 cm; subgroup of 48 tumours ≤ 3 cm | PEI + RFA (n = 16 tumours ≤ 3 cm) | RFA alone (n = 16 tumours ≤ 3 cm) PEI alone (n = 16 tumours ≤ 3 cm) |
Response: PEI + RFA: 15/16 (93.8%) nodules had complete ablation and 1/16 (6.2%) had partial ablation after one session; 16/16 (100%) nodules had complete ablation after two sessions. RFA alone: 12/16 (75%) nodules had complete ablation and 4/16 (25%) had partial ablation after one session; 14/16 (87.5%) nodules had complete ablation and 2/16 (12.5%) had partial ablation after two sessions. PEI alone: 0% nodules had complete ablation after two sessions; 13/16 (81.25%) nodules had complete ablation and 3/16 (18.75%) had partial ablation after all sessions. Adverse events: There were no mortalities related to any of the techniques. No other results reported for ≤ 3cm tumour subgroup. |
Some concerns |
| Brunello, 200844 Italy |
139 patients with tumours ≤ 3 cm | RFA (n = 70 patients) | PEI (n = 69 patients) | OS: RFA: Number of events (death): 26. PEI: Number of events (death): 28. Comparison between groups: HR 0.82, 95% CI 0.48 to 1.41. Adjusted HR 0.88, 95% CI 0.50 to 1.53. Distant intrahepatic recurrence: RFA: Number of events: 32. PEI: Number of events: 35. Response: RFA: 1-year response: 46/70 (65.7%). Early complete response (30-50 days): 67/70 (95.7%). PEI: 1-year response: 25/69 (36.2%). Early complete response (30–50 days, patients with 1-year follow-up only): 42/64 (65.6%). Adverse events: RFA: Treatment-emergent AEs: 10 (14.3%) patients; major complications: 2 patients. PEI: Treatment-emergent AEs: 12 (17.4%) patients; major complications: 2 patients. Economic outcomes: Mean direct medical costs were €4097 for PEI and €6540 for RFA. ICER for using RFA instead of PEI was €8286 (95% CI €2742 to €20,917). |
Low |
| Giorgio, 201148 Italy |
285 patients with tumours ≤ 3 cm | RFA (n = 142 patients) | PEI (n = 143 patients) | OS: RFA: 1 year: 95%; 2 years: 90%; 3 years: 83%; 4 years: 73%; 5 years: 70%. PEI: 1 year: 95%; 2 years: 83%; 3 years: 78%; 4 years: 70%; 5 years: 68%. Comparison between groups: HR 0.81, 95% CI 0.46 to 1.39. Local recurrence: RFA: 1 year: 4.1%; 2 years: 5.7%; 3 years: 7.8%; 4 years: 8.9%; 5 years: 11.7%. PEI: 1 year: 5.2%; 2 years: 6.7%; 3 years: 9.4%; 4 years: 11.5%; 5 years: 12.8%. Adverse events: RFA: Major complication: 0.9%. PEI: Major complication: 1.9%. No deaths related to the procedure in either group and no cases of seeding. Economic outcomes: In Italy the medical costs of both procedures were the same so cost calculated only on basis of technical material. The overall cost for PEI needles plus ethanol was €1359 plus VAT, the cost of the generator plus the RFA electrode needles was €171,000 plus VAT, with a statistically significant difference (p < 0.001). |
High |
| Lencioni, 200371 Authors from Italy and Germany |
104 patients with tumours ≤ 5 cm (large proportion had tumours ≤ 3 cm) | PEI (n = 50 patients with mean tumour size 2.8 cm; 84% had tumours ≤ 3 cm) | RFA (n = 54 patients with mean tumour size 2.8 cm; 88% had tumours ≤ 3 cm) | OS: PEI: 1 year: 96%; 2 years: 88%. RFA: 1 year: 100%; 2 years: 98%. PEI: Number of events (death): 5. RFA: Number of events (death): 1. Event-free survival: PEI: 1 year: 77%; 2 years: 43%. RFA: 1 year: 86%; 2 years: 64%. Local recurrence: PEI: Number of events: 13. RFA: Number of events: 3. Response: PEI: 60/73 (82%) tumours had complete response after one cycle; 69/73 (94.5%) tumours had complete response after two cycles. RFA: 63/69 (91%) tumours had complete response after one session; 68/69 (98.6%) tumours had complete response after two sessions. Adverse events: PEI: No procedure-related death, haemorrhage, infection, needle-track seeding or hepatic failure. Mild to moderate pain requiring analgesics: 13; fever: 5; chemical thrombosis of a portal venous branch: 1. RFA: No procedure-related death, haemorrhage, infection, needle-track seeding or hepatic failure. Mild to moderate pain requiring analgesics: 15; fever: 10; pleural effusions: 4 lesions; asymptomatic arteriovenous shunts: 3. |
Low |
| Lin, 200459 Authors from Taiwan |
157 patients with tumours ≤ 4 cm; subgroup of 114 patients with tumours ≤ 3 cm | RFA (n = 37 patients with tumours ≤ 3 cm) | Low-dose PEI (n = 38 patients with tumours ≤ 3 cm) High-dose PEI (n = 39 patients with tumours ≤ 3 cm) |
OS: RFA in patients with tumours 1–2 cm: 1 year: 96%; 2 years: 84%; 3 years: 78%. RFA in patients with tumours 2.1–3 cm: 1 year: 89%; 2 years: 78%; 3 years: 73%. Low-dose PEI in patients with tumours 1–2 cm: 1 year: 94%; 2 years: 78%; 3 years: 70%. Low-dose PEI in patients with tumours 2.1–3 cm: 1 year: 84%; 2 years: 70%; 3 years: 62%. High-dose PEI in patients with tumours 1–2 cm: 1 year: 93%; 2 years: 80%; 3 years: 72%. High-dose PEI in patients with tumours 2.1–3 cm: 1 year: 83%; 2 years: 71%; 3 years: 64%. Cancer-free survival: RFA in patients with tumours 1–2 cm: 1 year: 84%; 2 years: 64%; 3 years: 49%. RFA in patients with tumours 2.1–3 cm: 1 year: 76%; 2 years: 53%; 3 years: 40%. Low-dose PEI in patients with tumours 1–2 cm: 1 year: 73%; 2 years: 57%; 3 years: 41%. Low-dose PEI in patients with tumours 2.1–3 cm: 1 year: 68%; 2 years: 57%; 3 years: 30%. High-dose PEI in patients with tumours 1–2 cm: 1 year: 71%; 2 years: 61%; 3 years: 43%. High-dose PEI in patients with tumours 2.1–3 cm: 1 year: 63%; 2 years: 51%; 3 years: 32%. Local tumour progression: RFA in patients with tumours 1–2 cm: 1 year: 4%; 2 years: 9%; 3 years: 9%. RFA in patients with tumours 2.1–3 cm: 1 year: 11%; 2 years: 18%; 3 years: 18%. Low-dose PEI in patients with tumours 1–2 cm: 1 year: 8%; 2 years: 13%; 3 years: 13%. Low-dose PEI in patients with tumours 2.1–3 cm: 1 year: 18%; 2 years: 37%; 3 years: 37%. High-dose PEI in patients with tumours 1–2 cm: 1 year: 7%; 2 years: 12%; 3 years: 12%. High-dose PEI in patients with tumours 2.1–3 cm: 1 year: 21%; 2 years: 33%; 3 years: 33%. Adverse events: RFA: One patient (1.9%) had transient pleural effusion, although not reported whether their tumour was < 3cm or not. No other severe adverse effect was observed. Low-dose PEI: No severe adverse effect was observed. High-dose PEI: No severe adverse effect was observed. Economic outcomes: RFA: Mean hospital stay: 4.4 days (range 3–15). Low-dose PEI: Mean hospital stay: 1.6 days (range 2–3). High-dose PEI: Mean hospital stay: 2.1 days (range 2–4). |
Low |
| Lin, 200553 Taiwan |
187 patients with tumours ≤ 3 cm | RFA (n = 62 patients) | PEI (n = 62 patients) PAI (n = 63 patients) |
OS: RFA: 1 year: 93%; 2 years: 81%; 3 years: 74%. PEI: 1 year: 88%; 2 years: 66%: 3 years: 51%. PAI: 1 year: 90%; 2 years: 67%; 3 years: 53%. RFA: Number of events (death): 10 (16.1%). PEI: Number of events (death): 17 (27.4%). PAI: Number of events (death): 15 (23.8%). Cancer-free survival: RFA: 1 year: 74%; 2 years: 60%; 3 years: 43%. PEI: 1 year: 70%; 2 years: 41%: 3 years: 21%. PAI: 1 year: 71%; 2 years: 43%; 3 years: 23%. Recurrence: RFA: 1 year: 10%; 2 years: 14%; 3 years: 14%. PEI: 1 year: 16%; 2 years: 34%: 3 years: 34%. PAI: 1 year: 14%; 2 years: 31%; 3 years: 31%. RFA: Number of local recurrence events: 8/60 (13.3%). PEI: Number of local recurrence events: 19/55 (34.5%). PAI: Number of local recurrence events: 17/58 (29.3%). Complete response: RFA: 96.1% (75/78 tumours). PEI: 88.1% (67/76 tumours). PAI: 92.4% (73/79 tumours). Adverse events: RFA: 4.8% (3/62 patients) had serious AEs (2 patients with haemothorax and 1 with gastric bleeding and perforation). PEI: No serious AEs. PAI: No serious AEs. Economic outcomes: RFA: Mean hospitalisation: 4.2 days (range 3–18). PEI: Mean hospitalisation: 1.7 days (range 2–3). PAI: Mean hospitalisation: 2.2 days (range 2–5). |
Some concerns |
| Shiina, 200555 Japan |
232 patients with tumours ≤3cm | RFA (n = 118 patients) | PEI (n = 114 patients) | OS: RFA: 4 years: 74% (95% CI 65% to 84%). PEI: 4 years: 57% (95% CI 45% to 71%). RFA: Number of events (death): 25 (21.2%). PEI: Number of events (death): 40 (35.1%). Comparison between groups: RR 0.54, 95% CI 0.33 to 0.89. Recurrence: RFA: Number of events: 78 (66.1%) (new lesion: 74; local tumour progression: 2; extrahepatic recurrence: 2). PEI: Number of events: 90 (78.9%) (new lesion: 73; local tumour progression: 13; extrahepatic recurrence: 4). Comparison between groups: RR 0.57, 95% CI 0.41 to 0.80. Local tumour progression: RR 0.12, 95% CI 0.03 to 0.55. Adverse events: RFA: AEs grade ≥3: 6 (5.1%) (1 transient jaundice, 1 skin burn, 1 hepatic infarction and 3 seeding of malignant cells). PEI: AEs grade ≥3: 3 (2.6%) (1 liver abscess and 2 neoplastic seeding). Economic outcomes: RFA: Length of hospitalisation: 10.8 ± 5.5 days. PEI: Length of hospitalisation: 26.1 ± 9.9 days. |
Some concerns |
| RFA vs. PAI | |||||
| Lin, 200553 Taiwan |
187 patients with tumours ≤3cm | RFA (n = 62 patients) | PEI (n = 62 patients) PAI (n = 63 patients) |
OS: RFA: 1 year: 93%; 2 years: 81%; 3 years: 74%. PEI: 1 year: 88%; 2 years: 66%: 3 years: 51%. PAI: 1 year: 90%; 2 years: 67%; 3 years: 53%. RFA: Number of events (death): 10 (16.1%). PEI: Number of events (death): 17 (27.4%). PAI: Number of events (death): 15 (23.8%). Cancer-free survival: RFA: 1 year: 74%; 2 years: 60%; 3 years: 43%. PEI: 1 year: 70%; 2 years: 41%: 3 years: 21%. PAI: 1 year: 71%; 2 years: 43%; 3 years: 23%. Recurrence: RFA: 1 year: 10%; 2 years: 14%; 3 years: 14%. PEI: 1 year: 16%; 2 years: 34%: 3 years: 34%. PAI: 1 year: 14%; 2 years: 31%; 3 years: 31%. RFA: Number of local recurrence events: 8/60 (13.3%). PEI: Number of local recurrence events: 19/55 (34.5%). PAI: Number of local recurrence events: 17/58 (29.3%). Complete response: RFA: 96.1% (75/78 tumours). PEI: 88.1% (67/76 tumours). PAI: 92.4% (73/79 tumours). Adverse events: RFA: 4.8% (3/62 patients) had serious AEs (2 patients with haemothorax and 1 with gastric bleeding and perforation). PEI: No serious AEs. PAI: No serious AEs. Economic outcomes: RFA: Mean hospitalisation: 4.2 days (range 3–18). PEI: Mean hospitalisation: 1.7 days (range 2–3). PAI: Mean hospitalisation: 2.2 days (range 2–5). |
Some concerns |
| RFA vs. laser ablation | |||||
| Ferrari, 200777 Authors from Italy |
81 patients with tumours ≤ 4 cm; subgroup of 28 patients with tumours ≤ 2.5 cm | Laser ablation (n = 12 patients with tumours ≤ 2.5 cm) | RFA (n = 16 patients with tumours ≤ 2.5 cm) | Adverse events: No deaths or major or minor complications occurred during the procedures in either group and no cases of neoplastic seeding observed. No other results reported for ≤ 2.5 cm subgroup, other than Kaplan–Meier curve. |
Some concerns |
| Orlacchio, 201461 Italy |
30 patients with tumours ≤ 4 cm (mean tumour size 2.4 cm) | Laser ablation (n = 15 patients with mean tumour size 2.34 cm) | RFA (n = 15 patients with mean tumour size 2.41 cm) | OS: Laser ablation: Number of events (death): 0 RFA: Number of events (death): 0 PFS: Laser ablation: 3 months: 85%; 6 months: 62%; 1 year: 54%. RFA: 3 months: 92%; 6 months: 86%; 1 year: 86%. Recurrence: Laser ablation: Number of events (local disease progression): 6 (40%). RFA: Number of events (local disease progression): 2 (13.3%). Complete response: Laser ablation: 66.7% nodules (10/15) after first procedure; 3/5 patients after a second procedure (87% in total). RFA: 86.7% nodules (13/15) after first procedure; 1/2 patients after a second procedure (93% in total). Adverse events: Laser ablation: 2 (13.3%) (1 vasovagal reaction and 1 postablation syndrome). RFA: 14 (93.3%) (2 vasovagal reactions and 12 postablation syndrome). No major complications in either arm. |
Some concerns |
| Zou, 201757 China |
74 patients with tumours ≤ 3 cm | Laser ablation (n = 35 patients) | RFA (n = 39 patients) | Response: Laser ablation: 31 (88.6%) had a complete response; 4 (11.4%) had a partial response. RFA: 36 (92.3%) had a complete response; 3 (7.7%) had a partial response. Patient satisfaction: Self-made satisfaction questionnaire including intraoperative discomfort, postoperative therapy effects, adverse reactions and physical recovery. Maximum score of 100, 81–100 = great satisfaction, 61–80 = general satisfaction, < 60 = dissatisfaction. Laser ablation: great satisfaction: 30 (85.7%), general satisfaction: 3 (8.6%), dissatisfaction: 2 (5.7%). RFA: great satisfaction: 25 (64.1%), general satisfaction: 2 (5.1%), dissatisfaction: 12 (30.8%). Adverse events: Laser ablation: Fever: 4 (11.4%); nausea: 17 (48.6%); vomiting: 10 (28.6%); diarrhoea: 1 (2.9%); abdominal pain: 26 (74.3%); skin rash: 1 (2.9%). RFA: Fever: 5 (12.8%); nausea: 19 (48.7%); vomiting: 12 (30.8%); diarrhoea: 1 (2.6%); abdominal pain: 29 (74.4%); skin rash: 3 (7.7%). |
Some concerns |
| RFA vs. resection | |||||
| Chen, 2005 (reported in Chinese)67 China |
132 patients with tumours ≤ 5 cm; subgroup of 55 patients with tumours ≤ 3 cm | Resection (n = 31 patients with tumours ≤ 3 cm) | RFA (n = 24 patients with tumours ≤ 3 cm) | No results reported for ≤ 3 cm subgroup, other than Kaplan–Meier curve. | Some concerns |
| Fang, 201446 China |
120 patients with tumours ≤ 3 cm | RFA (n = 60 patients) | Hepatectomy (n = 60 patients) | OS: RFA: 1 year: 97.5%; 2 years: 91.2%; 3 years: 82.5%. Hepatectomy: 1 year: 93.7%; 2 years: 86.2%; 3 years: 77.5%. Disease-free survival: RFA: 1 year: 91.6%; 2 years: 87.4%; 3 years: 55.4%. Hepatectomy: 1 year: 90.4%; 2 years: 85.2%; 3 years: 41.3%. Recurrence: RFA: Number of events: 22. Hepatectomy: Number of events: 21. Response: RFA: Complete tumour treatment: 57/60 (95.0%). Hepatectomy: Complete tumour treatment: 58/60 (96.7%). Adverse events: RFA: Postoperative complications: 2. Major complications: 1. Hospital mortality: 0. Serious pain requiring analgesic (not included in postoperative complications): 3. Hepatectomy: Postoperative complications: 17. Major complications: 14. Hospital mortality: 0. Serious pain requiring analgesic (not included in postoperative complications): 43. Economic outcomes: RFA: ICU stay: 0. Hospital days (mean): 4.3 (SD 1.5). Hepatectomy: ICU stay: 6 (10%). Hospital days (mean): 11.8 (SD 3.1). |
Some concerns |
| Feng, 201258 China |
168 patients with tumours < 4 cm; subgroup of 56 patients with tumours ≤ 2 cm | RFA (n = 31 patients with tumours ≤ 2 cm) | Surgical resection (n = 25 patients with tumours ≤ 2 cm) | No results reported for ≤ 2 cm subgroup. Adverse events: No treatment-related mortality. |
N/A |
| Huang, 201069 China |
230 patients with tumours ≤ 5 cm; subgroup of 159 patients with tumours ≤ 3 cm | RFA (n = 88 patients with tumours ≤ 3 cm) | Resection (n = 71 patients with tumours ≤ 3 cm) | OS: RFA in patients with solitary tumours ≤ 3 cm (n = 57): 1 year: 91.2%; 2 years: 84.2%; 3 years: 77.2%; 4 years: 71.9%; 5 years: 61.4%. RFA in patients with multifocal tumours ≤ 3 cm (n = 31): 1 year: 77.4%; 2 years: 64.5%; 3 years: 58.1%; 4 years: 58.1%; 5 years: 45.2%. Resection in patients with solitary tumours ≤ 3 cm (n = 45): 1 year: 100%; 2 years: 97.8%; 3 years: 95.6%; 4 years: 86.7%; 5 years: 82.2%. Resection in patients with multifocal tumours ≤ 3 cm (n = 26): 1 year: 92.3%; 2 years: 88.5%; 3 years: 80.8%; 4 years: 73.1%; 5 years: 69.2%. Adverse events: No deaths within same hospital admission. No other results reported for ≤ 3 cm subgroup, other than separate Kaplan–Meier curves for solitary tumours ≤ 3 cm and multifocal tumours ≤ 3 cm. |
High |
| Izumi, 201950 (conference abstract) Japan |
308 patients with tumours ≤ 3 cm | RFA (n = 148 analysed) | Surgery (n = 145 analysed) | Recurrence-free survival: RFA: 3 years: 47.7%. Surgery: 3 years: 49.8%. Comparison between groups:HR 0.96. Adverse events: No perioperative mortality in either group. |
High |
| Ng, 201773 China |
218 patients with tumours ≤ 5 cm; subgroup of 55 patients with tumours ≤ 2 cm | Resection (n = 29 patients with tumours ≤ 2 cm) | RFA (n = 26 patients with tumours ≤ 2 cm) | OS: Resection: 1 year: 100%; 3 years: 93%; 5 years: 76%. RFA: 1 year: 100%; 3 years: 89%: 5 years: 69%. Disease-free survival: Resection: 1 year: 83%; 3 years: 66%; 5 years: 52%. RFA: 1 year: 77%; 3 years: 62%: 5 years: 46%. Economic outcomes: Resection: Median hospital stay: 7 days. RFA: Median hospital stay: 4 days. |
Low |
| Xia, 202075 China |
240 patients with recurrent HCC tumours ≤ 5 cm; subgroup of 159 patients with tumours ≤ 3 cm | RFA (78 patients with tumours ≤ 3 cm) | Repeat hepatectomy (81 patients with tumours ≤ 3 cm) | OS: Comparison between group: In HR 0.05, 95% CI -0.4 to 0.5. HR 1.05, 95% CI 0.67 to 1.65 (calculated by CRD). Repeat-recurrence-free survival: Comparison between group: In HR 0.07, 95% CI -0.34 to 0.47. HR 1.07, 95% CI 0.71 to 1.6 (calculated by CRD). |
Low |
| Zhu, 2021 (protocol)80 China |
Ongoing RCT | RFA | Laparoscopic hepatectomy | N/A | N/A |
| RFA vs. proton beam radiotherapy | |||||
| Kim, 2021162 South Korea |
144 patients with recurrent/residual tumours < 3 cm | Proton beam radiotherapy (n = 72 patients) | RFA (n = 72 patients) | OS: Proton beam radiotherapy: 2 years: 91.7%; 3 years: 80.8%; 4 years: 75.4%. RFA: 2 years: 90.3%; 3 years: 86.0%; 4 years: 77.0%. Comparison between groups: HR (2 years) 1.07, 95% CI 0.58 to 1.98. PFS: Proton beam radiotherapy: Median 13.4 months (90% CI 7.69 to 16.76 months). RFA: Median 13.7 months (90% CI 9.86 to 18.89 months). Proton beam radiotherapy: 2 years: 31.9%; 3 years: 26.3%; 4 years: 18.7%. RFA: 2 years: 31.9%; 3 years: 17.9%; 4 years: 12.6%. HR (2 years) 0.99, 95% CI 0.70 to 1.41. Proton beam radiotherapy: Number of events (progression): 56 RFA: Number of events (progression): 62 Adverse events: Proton beam radiotherapy: AEs grade ≥ 3: 0. RFA: AEs grade ≥ III: 9 (16%). No treatment-related late hepatic failure and death without evidence of disease progression and/or subsequent treatment. |
Some concerns |
| RFA vs. RFA + TACE | |||||
| Aikata, 2006 (conference abstract)43 Authors from Japan |
44 patients with tumours < 3 cm | RFA + TACE (n = 21 patients) | RFA alone (n = 23 patients) | OS: RFA + TACE: 1 year: 95.2%; 2 years: 95.2%; 3 years: 84%. RFA alone: 1 year: 100%; 2 years: 82.6%; 3 years: 73.9%. Local tumour progression: RFA + TACE: 1 year: 9.5%; 2 years: 9.5%; 3 years: 9.5%. RFA alone: 1 year: 4.3%; 2 years: 8.7%; 3 years: 8.7%. Adverse events: There were no major complications in either group. |
High |
| Peng, 201274 China |
139 patients with recurrent HCC tumours ≤ 5 cm; subgroup of 87 patients with tumours ≤ 3 cm | RFA + TACE (n = 41 patients with tumours ≤ 3 cm) | RFA alone (n = 46 patients with tumours ≤ 3 cm) | OS: RFA + TACE: 1 year: 98%; 3 years: 70%; 5 years: 50%. RFA alone: 1 year: 83%; 3 years: 60%: 5 years: 50%. Recurrence-free survival: RFA + TACE: 1 year: 90%; 3 years: 48%; 5 years: 48%. RFA alone: 1 year: 86%; 3 years: 26%: 5 years: 26%. |
Low |
| Shibata, 200954 Japan |
89 patients with tumours ≤ 3 cm | RFA + TACE (n = 46 patients) | RFA alone (n = 43 patients) | OS: RFA + TACE: 1 year: 100%; 2 years: 100%; 3 years: 84.8%; 4 years: 72.7%. RFA alone: 1 year: 100%; 2 years: 88.8%: 3 years: 84.5%; 4 years: 74%. RFA + TACE: Number of events (death): 5 (10.9%). RFA alone: Number of events (death): 6 (13.9%). Event-free survival: RFA + TACE: 1 year: 71.3%; 2 years: 59.9%; 3 years: 48.8%; 4 years: 36.6%. RFA alone: 1 year: 74.3%; 2 years: 52.4%; 3 years: 29.7%; 4 years: 29.7%. Local PFS RFA + TACE: 1 year: 84.6%; 2 years: 81.1%; 3 years: 69.7%; 4 years: 55.8%. RFA alone: 1 year: 88.4%; 2 years: 74.1%; 3 years: 74.1%; 4 years: 61.7%. Local tumour progression: RFA + TACE: 1 year: 14.4%; 2 years: 17.6%; 3 years: 17.6%; 4 years: 17.6%. RFA alone: 1 year: 11.4%; 2 years: 14.4%; 3 years: 14.4%; 4 years: 14.4%. RFA + TACE: Number of recurrence events: 8 (17.4%). RFA alone: Number of recurrence events: 6 (13.9%). Complete response: RFA + TACE: 100%. RFA alone: 100%. Adverse events: RFA + TACE: 1 major complication (2%) (segmental hepatic infarction). RFA alone: 1 major complication (2%) (pseudoaneurysm of the anterosuperior branch of the right hepatic artery). |
High |
| RFA vs. RFA + PEI | |||||
| Azab, 201165 Egypt |
90 patients (with 98 tumours) ≤ 5 cm; subgroup of 48 tumours ≤ 3 cm | PEI + RFA (n = 16 tumours ≤ 3 cm) | RFA alone (n = 16 tumours ≤ 3 cm) PEI alone (n = 16 tumours ≤ 3 cm) |
Response: PEI + RFA: 15/16 (93.8%) nodules had complete ablation and 1/16 (6.2%) had partial ablation after one session; 16/16 (100%) nodules had complete ablation after two sessions. RFA alone: 12/16 (75%) nodules had complete ablation and 4/16 (25%) had partial ablation after one session; 14/16 (87.5%) nodules had complete ablation and 2/16 (12.5%) had partial ablation after two sessions. PEI alone: 0% nodules had complete ablation after two sessions; 13/16 (81.25%) nodules had complete ablation and 3/16 (18.75%) had partial ablation after all sessions. Adverse events: There were no mortalities related to any of the techniques. No other results reported for ≤ 3 cm tumour subgroup. |
Some concerns |
| Chen, 2005 (reported in Chinese)66 China |
86 patients with tumours ≤ 5 cm; subgroup of 47 patients with tumours ≤ 3 cm | RFA + PEI (n = 24 patients with tumours ≤ 3 cm) | RFA alone (n = 23 patients with tumours ≤ 3 cm) | OS: RFA + PEI: 1 year: 86.8%; 2 years: 79.0%. RFA alone: 1 year: 81.2%; 2 years: 64.9%. Recurrence: RFA + PEI: 1 year: 4.3%; 2 years: 20.9%. RFA alone: 1 year: 16.8%; 2 years: 34.1%. Adverse events: There were no serious AEs in either group. |
High |
| Zhang, 200776 China |
133 patients with tumours ≤ 7 cm; subgroup of 60 patients with tumours ≤ 3 cm | RFA + PEI (29 patients with tumours ≤ 3 cm) | RFA alone (31 patients with tumours ≤ 3 cm) | OS: RFA + PEI: 1 year: 96.5%; 2 years: 92.9%; 3 years: 83.6%; 4 years: 77.4%; 5 years: 55.3%. RFA alone: 1 year: 93.5%; 2 years: 80.7%; 3 years: 76.6%; 4 years: 70.2%; 5 years: 50.2%. Response: RFA + PEI: 52/66 (78.8%) had complete tumour ablation after one treatment, 14 (21.2%) had complete tumour ablation after a second treatment. RFA alone: 48/67 (71.6%) had complete tumour ablation after one treatment, 18 (26.9%) had complete tumour ablation after a second treatment and 1 patient (1.5%) had viable tumour cells after two treatment sessions. Tumour diameter was a significant prognostic factor for overall recurrence, intrahepatic recurrence and local recurrence. |
Low |
| RFA vs. RFA + iodine-131 metuximab | |||||
| Bian, 201478 China |
127 patients with BCLC stage 0–B; subgroup of 78 patients with tumours < 3 cm | RFA + iodine-131 metuximab (n = 38 patients with tumours < 3 cm) | RFA alone (n = 40 patients with tumours < 3 cm) | Recurrence: Comparison between groups: HR 0.46, 95% CI 0.21 to 1.01. Adverse events: There were no serious AEs or treatment-related deaths in either group. No other results reported for < 3 cm tumour subgroup. |
Some concerns |
| RFA vs. RFA + iodine-125 | |||||
| Chen, 201445 China |
136 patients with tumours ≤ 3 cm | RFA + iodine-125 (n = 68 patients) | RFA alone (n = 68 patients) | OS: RFA + iodine-125: Mean 95.8 months. RFA alone: Mean 70.8 months. RFA + iodine-125: 1 year: 100%; 2 years: 95.6%; 3 years: 86.7%; 4 years: 73.5%; 5 years: 66.1%. RFA alone: 1 year: 95.6%; 2 years: 85.2%; 3 years: 75.0%; 4 years: 58.8%; 5 years: 47.0%. RFA + iodine-125: Number of events (death): 23. RFA alone: Number of events (death): 36. Comparison between groups: HR 0.502, 95% CI 0.313 to 0.806. Recurrence: RFA + iodine-125: 1 year: 4.5%; 2 years: 11.8%; 3 years: 22.1%; 4 years: 32.4%; 5 years: 39.8%. RFA alone: 1 year: 14.8%; 2 years: 25.0%; 3 years: 35.3%; 4 years: 47.1%; 5 years: 57.4%. RFA + iodine-125: Number of events: 27. RFA alone: Number of events: 39. Comparison between groups: HR 0.508, 95% CI 0.317 to 0.815. RFA + iodine-125: Mean time to recurrence: 93 months. RFA alone: Mean time to recurrence: 66.8 months. Response: RFA + iodine-125: Complete ablation after one treatment: 56/68; complete response after two treatments: 12/68. RFA alone: Complete ablation after one treatment: 49/68; complete response after two treatments: 19/68. Adverse events: RFA + iodine-125: AEs grade ≥ 3: 15 events (not patient numbers) RFA alone: AEs grade ≥ 3: 11 events (not patient numbers) No procedure-related mortalities and no iodine-125 seed migration from the liver to the heart or other organs. |
Low |
| RFA vs. RFA + chemotherapy | |||||
| Gan, 2004 (reported in Chinese)47 China |
38 patients with tumours ≤ 3 cm | RFA alone (n = 18 patients) | RFA+ chemotherapy (n = 20 patients) |
Recurrence: RFA alone: 1 year: 50% (6/12 analysed). RFA + chemotherapy: 1 year: 27% (4/15 analysed). RFA: Number of events: 6 (1 original location recurrence + 5 other location recurrence). RFA + chemotherapy: Number of events: 4 (4 other location recurrence). Adverse events: There were no serious AEs in either group. |
High |
| RFA + TACE vs. resection | |||||
| Liu, 201672 China |
200 patients with tumours ≤ 5 cm; subgroup of 135 patients with tumours ≤ 3 cm | Partial hepatectomy (n = 66 patients with tumours ≤ 3 cm) | TACE + RFA (n = 69 patients with tumours ≤ 3 cm) | No results reported for ≤ 3 cm subgroup, other than Kaplan–Meier curve. Adverse events: No 30- or 90-day mortality in either group. |
Some concerns |
| PEI vs. PAI | |||||
| Lin, 200553 Taiwan |
187 patients with tumours ≤ 3 cm | RFA (n = 62 patients) | PEI (n = 62 patients) PAI (n = 63 patients) |
OS: RFA: 1 year: 93%; 2 years: 81%; 3 years: 74%. PEI: 1 year: 88%; 2 years: 66%: 3 years: 51%. PAI: 1 year: 90%; 2 years: 67%; 3 years: 53%. RFA: Number of events (death): 10 (16.1%). PEI: Number of events (death): 17 (27.4%). PAI: Number of events (death): 15 (23.8%). Cancer-free survival: RFA: 1 year: 74%; 2 years: 60%; 3 years: 43%. PEI: 1 year: 70%; 2 years: 41%: 3 years: 21%. PAI: 1 year: 71%; 2 years: 43%; 3 years: 23%. Recurrence: RFA: 1 year: 10%; 2 years: 14%; 3 years: 14%. PEI: 1 year: 16%; 2 years: 34%: 3 years: 34%. PAI: 1 year: 14%; 2 years: 31%; 3 years: 31%. RFA: Number of local recurrence events: 8/60 (13.3%). PEI: Number of local recurrence events: 19/55 (34.5%). PAI: Number of local recurrence events: 17/58 (29.3%). Complete response: RFA: 96.1% (75/78 tumours). PEI: 88.1% (67/76 tumours). PAI: 92.4% (73/79 tumours). Adverse events: RFA: 4.8% (3/62 patients) had serious AEs (2 patients with haemothorax and 1 with gastric bleeding and perforation). PEI: No serious AEs. PAI: No serious AEs. Economic outcomes: RFA: Mean hospitalisation: 4.2 days (range 3–18). PEI: Mean hospitalisation: 1.7 days (range 2–3). PAI: Mean hospitalisation: 2.2 days (range 2–5). |
Some concerns |
| PEI vs. resection | |||||
| Huang, 200549 Taiwan |
82 patients with tumours ≤ 3 cm | PEI (n = 40 patients) | Resection (n = 42 patients) | OS: PEI: 1 year: 100%; 2 years: 100%; 3 years: 96.7%; 4 years: 92.1%; 5 years: 46.0%. Resection: 1 year: 97.4%; 2 years: 91.3%; 3 years: 88.1%; 4 years: 88.1%; 5 years: 81.8%. PFS: PEI: 1 year: 76.1%; 2 years: 64.5%; 3 years: 49.1%; 4 years: 44.6%; 5 years: 44.6%. Resection: 1 year: 89.5%; 2 years: 71.3%; 3 years: 60.9%; 4 years: 56.2%; 5 years: 48.2%. Recurrence: PEI: Number of events: 18. Resection: Number of events: 15. Adverse events: PEI: 3 patients had adverse effects (1 decreased blood pressure, 2 wound pain). Resection: No significant complications. |
High |
| PEI vs. RFA + PEI | |||||
| Azab, 201165 Egypt |
90 patients (with 98 tumours) ≤ 5 cm; subgroup of 48 tumours ≤ 3 cm | PEI + RFA (n = 16 tumours ≤ 3 cm) | RFA alone (n = 16 tumours ≤ 3 cm) PEI alone (n = 16 tumours ≤ 3 cm) |
Response: PEI + RFA: 15/16 (93.8%) nodules had complete ablation and 1/16 (6.2%) had partial ablation after one session; 16/16 (100%) nodules had complete ablation after two sessions. RFA alone: 12/16 (75%) nodules had complete ablation and 4/16 (25%) had partial ablation after one session; 14/16 (87.5%) nodules had complete ablation and 2/16 (12.5%) had partial ablation after two sessions. PEI alone: 0% nodules had complete ablation after two sessions; 13/16 (81.25%) nodules had complete ablation and 3/16 (18.75%) had partial ablation after all sessions. Adverse events: There were no mortalities related to any of the techniques. No other results reported for ≤ 3 cm tumour subgroup. |
Some concerns |
| PEI vs. PEI + TACE | |||||
| Koda, 200152 Japan |
52 patients with tumours < 3 cm | TACE + PEI (n = 26 patients) | PEI alone (n = 26 patients) | OS: TACE + PEI: 1 year: 100%; 2 years: 94.4%; 3 years: 80.8%; 5 years: 40.4%. PEI alone: 1 year: 91.3%; 2 years: 81.6%; 3 years: 65.9%; 5 years: 37.7%. TACE + PEI: Number of events: 4. PEI alone: Number of events: 8. Recurrence: TACE + PEI local residual disease: 1 year: 3.7%; 2 years: 19.3%; 3 years: 19.3%, 5 years: 19.3%. TACE + PEI new nodular recurrence: 1 year: 8.7%; 2 years: 41.9%; 3 years: 50.2%; 5 years: 50.2%. PEI alone local residual disease: 1 year: 34.2%; 2 years: 39.3%; 3 years: 39.3%, 5 years: 39.3%. PEI alone new nodular recurrence: 1 year: 26.9%; 2 years: 60.1%; 3 years: 80.1%; 5 years: 100%. TACE + PEI local residual disease: Number of events: 4 nodules (of 31). PEI alone local residual disease: Number of events: 11 nodules (of 34). TACE + PEI new nodular recurrence: Number of events: 9. PEI alone new nodular recurrence: Number of events: 17. Adverse events: TACE + PEI: Major complications: 2. PEI alone: Major complications: 0. TACE + PEI: After TACE: continuous abdominal pain: 6; severe abdominal pain: 6; fever: 19; high-grade fever: 10; liver dysfunction: 3; leukocytosis: 11; C-reactive protein: 22. After PEI: continuous abdominal pain: 16; severe abdominal pain: 7; fever: 20; high-grade fever: 10; liver dysfunction: 3; leukocytosis: 4; C-reactive protein: 20. PEI alone: continuous abdominal pain: 11; severe abdominal pain: 5; fever: 22; high-grade fever: 5; liver dysfunction: 3; leukocytosis: 5; C-reactive protein: 15. |
Low |
| Mizuki, 201060 Japan |
30 patients with tumours ≤ 4 cm (large proportion had tumours ≤ 3 cm) | PEI alone (n = 14 patients with average tumour size 2.64 cm) | TACE + PEI (n = 16 patients with average tumour size 2.65 cm) | OS: PEI alone: Mean 57.2 months, 95% CI 37.2 to 77.2 months. TACE + PEI: Mean 42.4 months, 95% CI 29.2 to 55.6 months. PEI alone: Number of events (death): 6/14 (44%). TACE + PEI: Number of events (death): 8/13 (61.5%). Cancer-free survival: PEI alone: Mean 16.7 months, 95% CI 7.3 to 26.0 months. TACE + PEI: Mean 22.9 months, 95% CI 12.4 to 33.4 months. Recurrence: PEI alone: Number of events: 10 (71.4%). TACE + PEI: Number of events: 11 (84.6%). Adverse events: In all 30 cases, serious adverse effects or complications were not related to treatment with TACE and/or PEI. |
Some concerns |
| PAI vs. PAI + TACE | |||||
| Huo, 200370 Taiwan |
108 patients with tumours ≤ 5 cm; subgroup of 55 patients with tumours ≤ 3 cm | Sequential TACE and PAI (n = 24 patients with tumours ≤ 3 cm) | PAI alone (n = 31 patients with tumours ≤ 3 cm) | OS: Sequential TACE and PAI: 1 year: 100%; 3 years: 73%. PAI alone: 1 year: 100%; 3 years: 49%. Cancer-free survival: There were no significant differences in cancer-free survival between the two groups of patients with tumour sizes ≤ 3 cm (p = 0.217). Adverse events: Sequential TACE and PAI: Transient fever, abdominal pain and elevation of liver enzymes were present in the majority of patients (in whole sample) after TACE. No serious complications that necessitated intensive care. PAI alone: Side effects relatively mild. Most patients (in whole sample) experienced transient mild to moderate local pain during or after acetic acid injection, which could be controlled with additional analgesics. No serious complications that necessitated intensive care. |
High |
| Percutaneous local ablative therapy vs. resection | |||||
| Chen, 200668 China |
180 patients with tumours ≤ 5 cm; subgroup of 79 patients with tumours ≤ 3 cm | Percutaneous local ablative therapy (initial RFA followed by RFA/PEI if residual tumour, and TACE if residual tumour remained) (n = 37 patients with tumours ≤ 3 cm) | Partial hepatectomy (n = 42 patients with tumours ≤ 3 cm) | No results reported for ≤ 3 cm subgroup, other than Kaplan–Meier curve. No significant difference in overall and disease-free survival between the two treatment groups in the ≤ 3 cm subgroup. |
High |
| MWA + sorafenib vs. resection | |||||
| Yan, 201656 China |
120 patients with tumours ≤ 3 cm | Resection (n = 60 patients) | MWA+ sorafenib (n = 60 patients) |
OS: Resection: Mean 51.2 ± 1.5 months. MWA + sorafenib: Mean 64.6 ± 2.4 months. Resection: 1 year: 90.7%; 3 years: 71.5%; 5 years: 56.7%. MWA + sorafenib: 1 year: 91.1%; 3 years: 72.8%; 5 years: 57.5%. Tumour-free survival: Resection: 1 year: 87.8%; 3 years: 44.3%; 5 years: 33.2%. MWA + sorafenib: 1 year: 86.2%; 3 years: 48.3%; 5 years: 34.6%. Recurrence: Resection: 5 years: 18.3%. MWA + sorafenib: 5 years: 38.3%. Resection: Number of events: 11 (2 local recurrence, 9 distant recurrence). MWA + sorafenib: Number of events: 23 (7 local recurrence, 16 distant recurrence). Adverse events: Resection: Pain: 38 (63.3%); fever: 29 (48.3%); abdominal bleeding: 7 (11%); infection: 18 (30%). MWA + sorafenib: Pain: 14 (23.3%); fever: 15 (25%); abdominal bleeding: 2 (3.3%); infection: 1 (1.7%). |
High |
Appendix 5 Studies excluded at full paper stage with rationale (non-randomised controlled trial searches)
| Study | Reason for exclusion |
|---|---|
| Abdelaziz, 201464 | Duplicate report |
| Aikata, 2018251 | Not a prospective comparative study |
| Al-Judaibi, 2018252 | Not a prospective comparative study |
| Anand, 2012253 | Not early HCC patients (≤ 3 cm tumour) |
| Barabino, 2018254 | Not a prospective comparative study |
| Bassanello, 2003255 | Not early HCC patients (≤3 cm tumour) |
| Beyer, 2018256 | Not early HCC patients (≤ 3 cm tumour) |
| Bhutiani, 2016257 | Not early HCC patients (≤ 3 cm tumour) |
| Borzio, 2007258 | No relevant intervention/comparison |
| Bouda, 2020259 | Not a prospective comparative study |
| Bu, 2015260 | Not a prospective comparative study |
| Bujold, 2011261 | Not early HCC patients (≤ 3 cm tumour) |
| Casaccia, 2017262 | Not early HCC patients (≤ 3 cm tumour) |
| Chagnon, 2007263 | Not a prospective comparative study |
| Chen, 2007264 | No relevant intervention/comparison |
| Chen, 2019265 | Not early HCC patients (≤ 3 cm tumour) |
| Cheung, 2012266 | Not a prospective comparative study |
| Cheung, 2013267 | Not early HCC patients (≤ 3 cm tumour) |
| Cho, 2015268 | Not a prospective comparative study |
| Chong, 2020269 | Not a prospective comparative study |
| Cillo, 2014270 | Not a prospective comparative study |
| Costa, 2015271 | Not a prospective comparative study |
| De Geus, 2018272 | Not a prospective comparative study |
| Denecke, 2015273 | Not early HCC patients (≤ 3 cm tumour) |
| Di Costanzo, 2015127 | Not early HCC patients (≤ 3 cm tumour) |
| Di Sandro, 2019274 | Not a prospective comparative study |
| Ding, 2021275 | Not a prospective comparative study |
| Ei, 2013276 | Duplicate report |
| Eloubeidi, 2000277 | Not a prospective comparative study |
| Ferrari, 200777 | Duplicate report |
| Freeman, 2021278 | Not a prospective comparative study |
| Gannon, 2009279 | Not early HCC patients (≤ 3 cm tumour) |
| Garavoglia, 2013280 | No relevant intervention/comparison |
| Ghweil, 2019281 | Not early HCC patients (≤ 3 cm tumour) |
| Guibal, 2013282 | Not early HCC patients (≤ 3 cm tumour) |
| Guo, 2005140 | Duplicate report |
| Guo, 2010283 | Not a prospective comparative study |
| Guo, 2013141 | Not a prospective comparative study |
| Hara, 2018284 | Not a prospective comparative study |
| Hara, 2019285 | Not a prospective comparative study |
| Hasegawa, 2013286 | Not a prospective comparative study |
| He, 2018287 | Not a prospective comparative study |
| Helmberger, 2007288 | No relevant intervention/comparison |
| Hiraoka, 2008289 | Not a prospective comparative study |
| Hiraoka, 2017290 | Not a prospective comparative study |
| Ho, 2012291 | Not early HCC patients (≤ 3 cm tumour) |
| Hong, 2005145 | Not early HCC patients (≤ 3 cm tumour) |
| Hsiao, 2020146 | Not a prospective comparative study |
| Hsu, 2013292 | Not a prospective comparative study |
| Huang, 2012293 | Not early HCC patients (≤ 3 cm tumour) |
| Hung, 2011150 | Not a prospective comparative study |
| Iida, 2014152 | Not a prospective comparative study |
| Ikeda, 2005294 | Not a prospective comparative study |
| Ikeda, 2007295 | Not a prospective comparative study |
| Imai, 2012296 | Duplicate report |
| Imai, 2013 | Not a prospective comparative study |
| Ismailova, 2017 | Not early HCC patients (≤ 3 cm tumour) |
| Ito, 2015297 | Duplicate report |
| Ito, 2016298 | Not a prospective comparative study |
| Jiang, 2015 | Not a prospective comparative study |
| Jianyong, 2017299 | Not a prospective comparative study |
| Juloori, 2020300 | Not early HCC patients (≤ 3 cm tumour) |
| Kang, 2014301 | Not a prospective comparative study |
| Kanwal, 2012302 | Not early HCC patients (≤ 3 cm tumour) |
| Katsoulakis, 2014303 | Not early HCC patients (≤ 3 cm tumour) |
| Kawamura, 2019304 | Not a prospective comparative study |
| Kawaoka, 2019305 | Not a prospective comparative study |
| Kennedy, 2004306 | Not early HCC patients (≤ 3 cm tumour) |
| Kim, 2014a307 | Not early HCC patients (≤ 3 cm tumour) |
| Kim, 2014b308 | Not a prospective comparative study |
| Kim, 2016309 | Not early HCC patients (≤ 3 cm tumour) |
| Kim, 2018310 | Not early HCC patients (≤ 3 cm tumour) |
| Kim, 2019311 | Not early HCC patients (≤ 3 cm tumour) |
| Kim, 2021162 | Duplicate report |
| Kimura, 2018a312 | Not early HCC patients (≤ 3 cm tumour) |
| Kimura, 2018b313 | Duplicate report |
| Ko, 2020314 | Not a prospective comparative study |
| Komatsu, 2011315 | Not early HCC patients (≤ 3 cm tumour) |
| Komatsu, 2019316 | Not early HCC patients (≤ 3 cm tumour) |
| Kooby, 2010317 | Not early HCC patients (≤ 3 cm tumour) |
| Kuang, 2011318 | Not a prospective comparative study |
| Kudithipudi, 2017319 | Not early HCC patients (≤ 3 cm tumour) |
| Kuo, 2010320 | Not a prospective comparative study |
| Kuo, 2021321 | Not a prospective comparative study |
| Kuromatsu, 2009322 | Not a prospective comparative study |
| Kwon, 2012323 | No relevant intervention/comparison |
| Lai, 2016169 | Not a prospective comparative study |
| Lapinski, 2021324 | Not early HCC patients (≤ 3 cm tumour) |
| Lee, 2012325 | Not a prospective comparative study |
| Lee, 2014326 | Not a prospective comparative study |
| Lee, 2018327 | Not a prospective comparative study |
| Lee, 2019328 | Duplicate report |
| Lee, 2021329 | Not a prospective comparative study |
| Lei, 2014330 | Not a prospective comparative study |
| Li, 2015331 | Not early HCC patients (≤ 3 cm tumour) |
| Li, 2016332 | Not a prospective comparative study |
| Li, 2019333 | Not early HCC patients (≤ 3 cm tumour) |
| Lin, 2007179 | Not a prospective comparative study |
| Liu, 2016334 | Not a prospective comparative study |
| Liu, 2017335 | Not a prospective comparative study |
| Liu, 2018a336 | Not a prospective comparative study |
| Liu, 2018b337 | Not a prospective comparative study |
| Liu, 2019338 | Not a prospective comparative study |
| Loo, 2020339 | Not early HCC patients (≤ 3 cm tumour) |
| Lu, 2010340 | Not a prospective comparative study |
| Maezawa, 2005341 | Not early HCC patients (≤ 3 cm tumour) |
| Mariani, 2017342 | Not a prospective comparative study |
| Martin, 2016343 | Not a prospective comparative study |
| Merle, 2005344 | Not early HCC patients (≤ 3 cm tumour) |
| Ming, 2012345 | Not a prospective comparative study |
| Mizumoto, 2011346 | Not early HCC patients (≤ 3 cm tumour) |
| Mo, 2003347 | Not early HCC patients (≤ 3 cm tumour) |
| Mohnike, 2019348 | Not early HCC patients (≤ 3 cm tumour) |
| Molinari, 2009349 | Duplicate report |
| Molinari, 2014350 | Not a prospective comparative study |
| Mornex, 2005351 | Not early HCC patients (≤ 3 cm tumour) |
| Mornex, 2007197 | Not a prospective comparative study |
| Murakami, 2007198 | Not a prospective comparative study |
| Nahon, 2021352 | Not early HCC patients (≤ 3 cm tumour) |
| Nanashima, 2004353 | Not a prospective comparative study |
| Nanashima, 2010354 | Not a prospective comparative study |
| Nathan, 2013355 | Not a prospective comparative study |
| Ogiso, 2021356 | Not a prospective comparative study |
| Oh, 2019357 | Not a prospective comparative study |
| Oh, 2020358 | Not a prospective comparative study |
| Ohmoto, 2006359 | Not a prospective comparative study |
| Paik, 2016205 | Not a prospective comparative study |
| Pan, 2020360 | Not a prospective comparative study |
| Park, 2007361 | Not a prospective comparative study |
| Park, 2018362 | Not early HCC patients (≤ 3 cm tumour) |
| Park, 2020363 | Duplicate report |
| Peng, 2011364 | Not a prospective comparative study |
| Peng, 2012365 | Duplicate report |
| Peng, 2013366 | Not a prospective comparative study |
| Peng, 2014367 | Not a prospective comparative study |
| Pompili, 2013210 | Not a prospective comparative study |
| Praktiknjo, 2018368 | Not early HCC patients (≤ 3 cm tumour) |
| Pryor, 2019369 | Not early HCC patients (≤ 3 cm tumour) |
| Rong, 2020370 | Not early HCC patients (≤ 3 cm tumour) |
| Ruzzenente, 2012371 | Not a prospective comparative study |
| Ryu, 2018372 | Not a prospective comparative study |
| Ryu, 2019373 | Not a prospective comparative study |
| Sako, 2003374 | Not a prospective comparative study |
| Santambrogio, 2009a214 | Not early HCC patients (≤ 3 cm tumour) |
| Santambrogio, 2009b375 | Not a prospective comparative study |
| Santambrogio, 2017376 | Not a prospective comparative study |
| Santambrogio, 2018377 | Not a prospective comparative study |
| Santambrogio, 2021378 | Not a prospective comparative study |
| Schaible, 2020379 | Not early HCC patients (≤ 3 cm tumour) |
| Sheng, 2015380 | Not a prospective comparative study |
| Shi, 2014217 | Not a prospective comparative study |
| Shibata, 2001381 | Duplicate report |
| Shiozawa, 2015382 | Not early HCC patients (≤ 3 cm tumour) |
| Simo, 2011383 | Not a prospective comparative study |
| Song, 2019384 | Not a prospective comparative study |
| Spangenberg, 2008385 | Not a prospective comparative study |
| Stuart, 2018386 | Not a prospective comparative study |
| Su, 2020387 | Not early HCC patients (≤ 3 cm tumour) |
| Suh, 2013388 | Not a prospective comparative study |
| Sun, 2020219 | Not a prospective comparative study |
| Sutter, 2018389 | Not early HCC patients (≤ 3 cm tumour) |
| Takamatsu, 2014390 | Not early HCC patients (≤ 3 cm tumour) |
| Takami, 2009391 | Not early HCC patients (≤ 3 cm tumour) |
| Takami, 2010392 | Not a prospective comparative study |
| Takayama, 2010393 | Not a prospective comparative study |
| Takayasu, 2018a394 | Not a prospective comparative study |
| Takayasu, 2018b394 | Duplicate report |
| Takeda, 2008395 | Not early HCC patients (≤ 3 cm tumour) |
| Takeda, 2016396 | Not early HCC patients (≤ 3 cm tumour) |
| Tanaka, 2015397 | Not a prospective comparative study |
| Tanguturi, 2015398 | Not early HCC patients (≤ 3 cm tumour) |
| Tashiro, 2011220 | Not a prospective comparative study |
| Tatineni, 2019399 | Not early HCC patients (≤ 3 cm tumour) |
| Teramoto, 2005400 | Not a prospective comparative study |
| Toro, 2012401 | Not early HCC patients (≤ 3 cm tumour) |
| Toyoda, 2008221 | Not a prospective comparative study |
| Trotschel, 2016402 | Not a prospective comparative study |
| Ueno, 2020403 | Not a prospective comparative study |
| Utsunomiya, 2014404 | Not a prospective comparative study |
| Vietti Violi, 2017a405 | Duplicate report |
| Vietti Violi, 2017b406 | Duplicate report |
| Vitale, 2012407 | Not early HCC patients (≤ 3 cm tumour) |
| Vitali, 2016408 | Not a prospective comparative study |
| Vivarelli, 2004223 | Not a prospective comparative study |
| Wang, 2007409 | Not early HCC patients (≤ 3 cm tumour) |
| Wang, 2012410 | Not a prospective comparative study |
| Wang, 2015a411 | Not early HCC patients (≤ 3 cm tumour) |
| Wang, 2015b412 | Not a prospective comparative study |
| Wang, 2019413 | Not a prospective comparative study |
| Wang, 2020414 | Not early HCC patients (≤ 3 cm tumour) |
| Wei, 2020415 | Not early HCC patients (≤ 3 cm tumour) |
| Wigg, 2017416 | Not early HCC patients (≤ 3 cm tumour) |
| Wiggermann, 2012417 | Not a prospective comparative study |
| Wong, 2021418 | Not early HCC patients (≤ 3 cm tumour) |
| Wu, 2020419 | Not early HCC patients (≤ 3 cm tumour) |
| Xie, 2019420 | Not early HCC patients (≤ 3 cm tumour) |
| Xu, 2009421 | Not early HCC patients (≤ 3 cm tumour) |
| Xu, 2014422 | Not early HCC patients (≤ 3 cm tumour) |
| Xu, 2015423 | Not a prospective comparative study |
| Yamao, 2018424 | Not a prospective comparative study |
| Yamashita, 2017425 | Not a prospective comparative study |
| Yamashita, 2019426 | Not a prospective comparative study |
| Yamazaki, 2009427 | Not a prospective comparative study |
| Yang, 2010428 | Not early HCC patients (≤ 3 cm tumour) |
| Ye, 2008429 | No relevant intervention/comparison |
| Yi, 2014430 | No relevant intervention/comparison |
| Yohji, 2012431 | Not a prospective comparative study |
| Yoon, 2018432 | Not early HCC patients (≤ 3 cm tumour) |
| Yu, 2014433 | Not early HCC patients (≤ 3 cm tumour) |
| Yun, 2009434 | Duplicate report |
| Yun, 2011239 | Not a prospective comparative study |
| Zhang, 2008435 | No relevant intervention/comparison |
| Zhang, 2013241 | Not a prospective comparative study |
| Zhang, 2016243 | Not a prospective comparative study |
| Zheng, 2020436 | Not a prospective comparative study |
| Zhou, 2014247 | Not a prospective comparative study |
| Zhu, 2007249 | Not early HCC patients (≤ 3 cm tumour) |
| Zhu, 202180 | Duplicate report |
Appendix 6 Characteristics and results of non-randomised studies included in the review
| Study name and location | Participant information | Intervention | Comparator | Main results | ROB |
|---|---|---|---|---|---|
| RFA vs. MWA | |||||
| Barabino, 201693 (conference abstract) Italy |
154 patients with HCC unsuitable for percutaneous treatments or hepatic resection | Laparoscopic RFA [n = 94 patients with average tumour size 1.92 (±0.5) cm] | Laparoscopic MWA [n = 60 patients with average tumour size 2.15 (±0.53) cm] | OS: Laparoscopic RFA: 5 years: 50%. Laparoscopic MWA: 5 years: 37%. Disease-free survival: Laparoscopic RFA: 5 years: 19%. Laparoscopic MWA: 5 years: 12%. Laparoscopic RFA: Local tumour progression rate: 21.2%. Laparoscopic MWA: Local tumour progression rate: 8.3%. Response: Laparoscopic RFA: Complete ablation: 95%. Laparoscopic MWA: Complete ablation: 95%. Adverse events: Laparoscopic RFA: 1% major complications. Laparoscopic MWA: 2% major complications. No deaths related to the procedure in either group. |
High |
| Qian, 201289 China |
42 patients with < 3 cm tumours | MWA (n = 22 patients) | RFA (n = 20 patients) | PFS: MWA: 4/22 (18.2%) had local tumour progression, 1/22 (4.5%) had a new intrahepatic tumour. RFA: 3/20 (15%) had local tumour progression, 4/20 (20%) had a new intrahepatic tumour. Response: MWA: Complete ablation: 21/22 (95.5%) after the first ablation, 100% after the second ablation. RFA: Complete ablation: 19/20 (95%) after the first ablation, 100% after the second ablation. Adverse events: No skin burns, tumour seeding or treatment-related death in either group. |
Low |
| RFA vs. resection | |||||
| Choi, 200484 (conference abstract) Korea |
164 patients with ≤ 3 cm tumours | RFA (n = 62 patients) | Hepatic resection (n = 102 patients) | OS: RFA: 1 year: 100%; 3 years: 73.9%. Resection: 1 year: 97.1%; 3 years: 83.0%. Recurrence-free survival: RFA: 1 year: 74.1%; 3 years: 40.2%. Resection: 1 year: 75.9%; 3 years: 54.7%. RFA: Local recurrence rate: 11.3%; Remote recurrence rate: 53.7%. Resection: Local recurrence rate: 2.0%; Remote recurrence rate: 45.3%. |
High |
| Du, 201292 China |
116 patients with tumours ≤ 5 cm; subgroup of 60 patients with tumours ≤ 3 cm | RFA (n = 31 patients with tumours ≤ 3 cm) | Surgical resection (n = 29 patients with tumours ≤ 3 cm) | Relapse: RFA: 1 year: 12.9%. Resection: 1 year: 13.8%. RFA: Number of events: 4. Resection: Number of events: 4. No other results reported for ≤ 3cm tumour subgroup. |
High |
| Elgendi, 201485 (conference abstract) Egypt |
51 patients with < 3 cm tumours in locations not amenable for percutaneous route | Intraoperative RFA (n = 24 patients) | Surgical resection (n = 27 patients) | OS: Intraoperative RFA: 1 year: 92%; 3 years: 74%. Resection: 1 year: 93%; 3 years: 81%. Recurrence: No tumours showed local recurrence after median 37 months follow-up in either group. Response: Intraoperative RFA: Complete ablation: 100%. Resection: Complete resection: 100%. Adverse events: Complication rate was comparable between treatment groups. |
High |
| Elgendi, 201591 (conference abstract) Egypt |
92 patients with < 2 cm tumours in locations not amenable for percutaneous route | Intraoperative RFA (n = 44 patients) | Surgical resection (n = 48 patients) | OS: Intraoperative RFA: 1 year: 91%; 3 years: 76%. Resection: 1 year: 92%; 3 years: 83%. Recurrence: No tumours showed local recurrence after median 46 months follow-up in either group. Response: Intraoperative RFA: Complete ablation: 100%. Resection: Complete resection: 100%. Adverse events: Complication rate was comparable between treatment groups. |
High |
| Harada, 201696 Japan |
121 patients with < 5 cm tumours and portal hypertension (large proportion had tumours ≤ 3 cm) | RFA (n = 40 patients) | Surgical resection [n = 81 patients, a few had tumours > 3 cm; mean 2.1 cm (range 0.7–5 cm)] | OS: RFA: 1 year: 97.2%; 3 years: 84.1%; 5 years: 50.6%. Resection: 1 year: 93.4%; 3 years: 84.5%; 5 years: 37.1%. HR (RFA vs. resection) 0.86, 95% CI 0.58 to 1.74. Recurrence-free survival: RFA: 1 year: 36.1%; 3 years: 22.2%; 5 years: 4.8%. Resection: 1 year: 74.1%; 3 years: 48.8%; 5 years: 42.9%. RFA: Number of events: 34/40 (85%); 27 intrahepatic distant recurrences and 7 local recurrences. Resection: Number of events: 34/81 (42%); 33 intrahepatic distant recurrences and one local recurrence. HR (RFA vs. resection) 2.74, 95% CI 1.70 to 4.43. Adverse events: RFA: 5 (12.5%) Resection: 38 (46.9%) RFA: Clavien–Dindo ≥ grade III AEs: 1 (2.5%). Resection: Clavien–Dindo ≥ grade III AEs: 13 (16.1%). One in-hospital death occurred in the resection group, secondary to sepsis. Economic outcomes: RFA: Median postoperative hospital stay: 8 days (range 2–29). Resection: Median postoperative hospital stay: 15 days (range 7–80). |
High |
| Huang, 201487 China |
346 patients with ≤ 3 cm tumours | RFA (n = 121 patients) | Surgical resection (n = 225 patients) | Health-related quality of life: FACT-Hep: Patients treated with RFA had significantly better HRQoL total scores after 3, 6, 12, 24 and 36 months than those who had resection (further details reported in supplementary tables/figures). Adverse events: RFA: 6 Clavien–Dindo grade III (2 pleural effusion requiring tapping, 2 pneumothorax or haemothorax, 1 liver abscess, 1 intra-abdominal haemorrhage). No grade I, II, IV or V AEs. Resection: 10 Clavien–Dindo grade I, 3 Clavien–Dindo grade II, 59 Clavien–Dindo grade III (9 delayed gastric emptying, 14 pleural effusion requiring tapping, 10 biliary leakage, 5 intra-abdominal abscess, 15 moderate/severe ascites, 6 intra-abdominal haemorrhage), 3 grade IV (2 liver failure, 1 renal insufficiency), 0 grade V AEs. No hospital death in either group. Economic outcomes: RFA: Average duration of procedure: 44 minutes. Resection: Average duration of procedure: 166.5 minutes. RFA: Average length of hospital stay: 7.1 days. Resection: Average length of hospital stay: 14.5 days. Blood transfustion rates were higher in the resection group. No other results reported, other than Kaplan–Meier curves. |
Unclear |
| Peng, 201088 (conference abstract) China |
195 patients with ≤ 3 cm tumours | RFA (n = 79) as first choice, otherwise surgical resection (n = 24) (total n = 103 patients) | Surgical resection (n = 75) as first choice, otherwise RFA (n = 17) (total n = 92 patients) | OS: RFA: 1 year: 95.9%; 3 years: 75.8%; 4 years: 70.7%. Resection: 1 year: 90.1%; 3 years: 63.7%; 4 years: 55.5%. Adverse events: RFA: No cases of treatment-related mortality. Resection: Two cases of treatment-related mortality. |
High |
| Tateishi, 2020 (conference abstract)90 Japan |
740 patients with ≤ 3 cm tumours | RFA (n = 369 patients) | Surgery (n = 371 patients) | Recurrence: RFA: 3 years: 61.7%. Surgery: 3 years: 66.0%. RFA: Number of events: 218. Surgery: Number of events: 192. Adjusted HR using inversed probability of treatment weighting 0.89, 95% CI 0.72 to 1.1. |
High |
| MWA vs. resection | |||||
| Horigome, 200086 Japan |
105 patients with ≤ 3 cm tumours | Hepatic resection (n = 43 patients) | MWA (n = 29 patients) PEI (n = 33 patients) |
Recurrence: Resection: Number of events: 31 (72%); 13 had intrahepatic metastases, 18 had multicentric occurrence. MWA: Number of events: 11 (38%); 7 had intrahepatic metastases, 4 had multicentric occurrence. PEI: Number of events: 22 (67%); 16 had intrahepatic metastases, 6 had multicentric occurrence. |
High |
| HIFU vs. RFA | |||||
| Cheung, 201383 China |
106 patients (with 119 tumours) with < 3 cm tumours (primary or first recurrence) | HIFU (n = 47 patients, 52 tumours) | RFA (n = 59 patients, 67 tumours) | OS: HIFU: 1 year: 97.4%, 3 years: 81.2%. RFA: 1 year: 94.6%, 3 years: 79.8%. Disease-free survival: HIFU: 1 year: 63.6%, 3 years: 25.9%. RFA: 1 year: 62.4%, 3 years: 34.1%. Response: HIFU: Complete response: 87.2%. RFA: Complete response: 94.9%. Adverse events: HIFU: 10 (21.3%); 2 pneumothorax, 1 third degree skin burn, 7 relatively minor complications. RFA: 5 (8.5%); 2 pleural effusion requiring tapping, 1 liver abscess, 1 oesophageal variceal bleeding, 1 mild wound infection. HIFU: Clavien–Dindo ≥ grade III AEs: 3. RFA: Clavien–Dindo ≥ grade III AEs: 4. Economic outcomes: HIFU: Median hospital stay: 4 days (range 2–18). RFA: Median hospital stay: 6 days (range 1–31). |
High |
| Cryoablation vs. RFA or MWA | |||||
| Ei, 201595 Japan |
119 patients with < 5 cm tumours (large proportion had tumours ≤ 3 cm) | Cryoablation (n = 55 patients with median tumour size 2.5 cm; maximum 4 cm) | RFA (n = 27) or microwave (n = 37) (Total n = 64 patients with median tumour size 1.9 cm; maximum 4.5 cm) | OS: Cryoablation: 2 years: 88% in ≤ 2 cm subgroup; 86% in > 2 cm subgroup. RFA/MWA: 2 years: 95% in ≤ 2 cm subgroup; 85% in > 2 cm subgroup. Local recurrence-free survival: Cryoablation: 2 years: 80%. RFA/MWA: 2 years: 68%. Local recurrence: Cryoablation: 2 years: 19% in ≤ 2 cm subgroup; 21% in > 2 cm subgroup. RFA/MWA: 2 years: 23% in ≤ 2 cm subgroup; 56% in > 2 cm subgroup. Initial recurrence at other sites of the liver: Cryoablation: 2 years: 38%. RFA/MWA: 2 years: 34%. Distant metastases (bone/lung): Cryoablation: 2 years: 2 patients. Adverse events: Cryoablation: 6; 3 Clavien–Dindo grade III (2 pleural effusion, 1 intraabdominal bleeding), 3 grade I/II. RFA/MWA: 7; 3 Clavien–Dindo grade III (haemothorax, wound infection, pneumonia), 4 grade I/II. No in-hospital mortality in either group. Economic outcomes: Cryoablation: Median operative time: 180 minutes. RFA/MWA: Median operative time: 132 minutes. Length of hospital stay: median 8 days (IQR 6–11 days) in both groups. |
High |
| IRE vs. RFA | |||||
| Sugimoto, 201994 Japan |
21 patients (with 24 tumours) | IRE [n = 10 patients (13 tumours) with median tumour size 2.03 (SD 0.44) cm] | RFA [n = 11 patients (11 tumours) with median tumour size 1.73 (SD 0.67) cm] | Recurrence: IRE: 1 patient had local tumour progression at 6 months. RFA: 0 patients had local tumour progression at 6 months. No other results reported, other than systemic immune responses. |
High |
List of abbreviations
- BCLC
- Barcelona Clinic Liver Cancer
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- CrI
- credible interval
- CT
- computerised tomography
- DIC
- deviance information criteria
- ECT
- electrochemotherapy
- FE
- fixed-effect
- HBV
- hepatitis B virus
- HCC
- hepatocellular carcinoma
- HCV
- hepatitis C virus
- HIFU
- high-intensity focused ultrasound
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICU
- intensive care unit
- IRE
- irreversible electroporation
- KM
- Kaplan–Meier
- MWA
- microwave ablation
- NICE
- National Institute for Health and Care Excellence
- NMA
- network meta-analysis
- OS
- overall survival
- PAI
- percutaneous acetic acid injection
- PEI
- percutaneous ethanol injection
- PFS
- progression-free survival
- PSM
- partition survival model
- RCT
- randomised controlled trial
- RE
- random-effects
- RFA
- radiofrequency ablation
- RoB
- risk of bias
- RR
- relative risk
- SABR
- stereotactic ablative radiotherapy (this is the same technology as SBRT, for simplicity SABR is used throughout this report)
- SBRT
- stereotactic body radiotherapy (this is the same technology as SABR, see comment above)
- SD
- standard deviation
- SE
- standard error
- SIRT
- selective internal radiation therapy
- TACE
- transarterial chemoembolisation
- TTP
- time to progression
- VOI
- value of information
Notes
-
NMA results
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/GK5221).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
