Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number NIHR129248. The contractual start date was in January 2021. The draft manuscript began editorial review in March 2023 and was accepted for publication in January 2024. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this article.
Permissions
Copyright statement
Copyright © 2024 Stewart et al. This work was produced by Stewart et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Stewart et al.
Chapter 1 Introduction and background
Response to commissioned call
This report presents the findings from a National Institute for Health and Care Research (NIHR)-funded study that was conducted between January 2021 and December 2022. The study was developed in response to a cross-programme call on ‘Digital Technologies to Improve Health and Care’ in November 2018 and funded through the Health Technology Assessment (HTA) programme. The team was commissioned to explore the acceptability and feasibility of home monitoring for glaucoma and make recommendations about the need for and design of a future definitive evaluative study.
Glaucoma: a brief background
Glaucoma is a chronic disease of the optic nerve and is currently the leading cause of irreversible severe visual loss worldwide and the second most common cause of severe visual loss in the UK. 1,2 Within the UK, there are over 1 million glaucoma-related NHS appointments annually. 3 Glaucoma commonly affects older adults, requires lifelong monitoring and is increasing in prevalence due to the growing ageing population. 4 By 2040, it has been predicted that 111 million people aged 40–80 years will be diagnosed with glaucoma globally. 5
Glaucoma is typically associated with raised intraocular pressure (IOP), leading to characteristic damage of the optic nerve head and visual field (VF) loss. Early glaucoma is asymptomatic but as the pathology progresses, central visual acuity may also be lost, leading to irreversible severe visual loss. 3 Patients impacted by glaucoma-related visual loss have been found to have decreased quality of life (QoL), due to factors including vision-induced limitations, loss of ability to drive, loss of ability to participate in meaningful activities such as hobbies, and an increased risk of falls. 6
Treatment for glaucoma effectively halts or slows disease progression and is achieved by reduction of IOP with medical, laser or surgical therapies. Patients require regular, lifelong monitoring, typically by hospital eye services (HES), where they have their IOP measured and the VF tested. Imaging by scanning the retinal nerve fibre layer of the eye can also be used to monitor glaucoma. Ideally the frequency of monitoring should be individualised to patient needs. Patients need these check-ups for the rest of their lives.
Hospital eye services are very busy, accounting for the highest number of NHS outpatient appointments of any specialty and comprising nearly 10% of all NHS outpatient visits. Glaucoma services are overwhelmed and struggling to accommodate current demands. 7 Providing regular surveillance and treatment is already a major challenge for the NHS. As the prevalence of glaucoma increases with age, the demand for glaucoma care is increasing (and will continue to do so) and more efficient models of care are urgently needed. 7,8
Diagnosis and monitoring of glaucoma
Glaucoma assessment for diagnosis and monitoring is made through combining patient history with objective measures which include assessments of the optic nerve head, retinal nerve fibre layers, VFs, and tonometry. 8 When the diagnosis is confirmed, treatment with pressure-lowering eye drops or laser therapy is commenced. Treatment is escalated (e.g. eye drops added, laser or surgery) if there is evidence of disease progression, defined by worsening of the VF or appearance of the optic nerve over time; or if IOP is above the individualised target level and the risk of progressive loss of vision is high. When treatment is altered, follow-up is arranged to determine response, with success of treatment measured by IOP control and demonstration of lack of change in VF over time. IOP is the only modifiable risk factor for reducing progressive loss of vision due to glaucoma. 9 All patients require lifelong regular assessment to determine disease stability and IOP control and to decide whether further treatment escalation is necessary.
Surveillance of patients with confirmed glaucoma is typically undertaken at HES. 10 Regular monitoring is important as glaucoma is often asymptomatic, and patients are usually unaware that they have worsening VF until the advanced stages;11 however, monitoring is time-consuming, inconvenient for patients and expensive for the NHS. Currently, the NHS is overwhelmed with the demand for glaucoma services and spends over £500M annually on related care. 7,12 Evidence suggests that current lack of capacity will worsen, increasing the risk of appointment delays and inappropriately long monitoring intervals. 7 Delays in follow-up appointments are already recognised as a problem, in some cases leading to irreversible visual loss which could have been prevented with adequate monitoring resources and funding. 7 Reducing demand on hospital-based services will improve the ability to see and treat patients at the highest risk of vision loss. Digital technologies that provide opportunities for home monitoring of glaucoma have the potential to contribute to solve these challenges and, potentially, improve outcomes.
Digital technology for home monitoring chronic conditions
Traditional monitoring methods of many chronic conditions are clinician-led and performed within the hospital. However, recent technological advances allow exploration of patient-led home monitoring or telemonitoring. Interventions for telemonitoring common health conditions such as hypertension, diabetes, heart failure, and stroke have been frequently reported in the literature. 13–18 Some of these conditions are already routinely monitored at home using methods such as blood glucose finger-prick, or sensor technology, or ambulatory blood pressure (BP) monitors.
Research suggests several benefits of home monitoring. Quantitative evaluations indicate greater patient compliance to treatment, shorter hospital stays, reduced frequency of inpatient visits, and faster diagnosis and identification of acute changes. 13–18 It should be noted that systematic reviews of telemonitoring also report that outcomes are variable between studies, particularly in relation to impact upon health resource use outcomes such as the number of healthcare contacts. 13,18 Qualitative evidence synthesis of remote monitoring across a range of chronic diseases (including chronic obstructive pulmonary disease, heart failure, diabetes, hypertension and end-stage kidney disease) highlighted that remote monitoring increased patients’ disease-specific knowledge, enabled early identification of exacerbations and improved self-management and shared decision-making. 19
The 10-year NHS plan has identified chronic condition home monitoring as a priority. 20 This could allow reduced frequency of hospital appointments for stable patients, increase healthcare capacity, potentially lower costs and improve patient convenience and compliance. 20 Implementation of these technologies requires an understanding of the challenges facing the NHS workforce and patients. While the evidence for home monitoring supports opportunities for embedding technologies within routine care, this implementation should ideally be supported by evidence from rigorous evaluation. 21
Current technologies for monitoring glaucoma
Recent advances in technology mean it is now possible for glaucoma patients to monitor IOP and several aspects of visual function, including VFs, in their own home. Utilising digital technologies for glaucoma home monitoring has the potential to contribute to solving a key challenge in glaucoma care: accommodating the increasing glaucoma population with more frequent monitoring assessments against the backdrop of increased wait times, reduced clinic availability and delays in routine monitoring appointments. It is also possible that more frequent monitoring, undertaken at home, may improve health outcomes if used to supplement or replace standard infrequent visits to HES. A novel patient pathway could potentially be used in which home monitoring data would be transferred to the hospital for interpretation by a healthcare professional or artificial intelligence (AI); alternatively, patients could request a hospital appointment if the home tests show their glaucoma has worsened or IOP has increased. Home monitoring could mean patients require fewer hospital visits, while increasing convenience and potentially reducing costs and increasing capacity for healthcare providers.
Home tonometry technologies
It is now possible to collect IOP data for glaucoma patients outside the clinical environment. Prior to 2020, two CE-marked options were available: SENSIMED Triggerfish® (SensiMed, ND, Switzerland) contact lens sensor and the iCare HOME® (iCare Oy, Vantaa, Finland) rebound tonometer.
The SENSIMED Triggerfish (SensiMed, ND, Switzerland) involves wearing disposable contact lenses which transmit data in relation to changes in ocular dimensions to a wearable antenna (worn as a patch). 22 It does not measure IOP directly, but using the ocular dimensions data it can give indications of increases or decreases in IOP. 22 The lens is designed to be inserted and removed by a trained professional, and worn by the patient for a 24-hour period. 22 In the device summary evidence published by the National Institute for Health and Care Excellence (NICE), it appears across 13 trials that the device was well tolerated by patients with few (n = 2) serious adverse events. 22 It obtained CE approval in 2010; however, its use is largely restricted to research purposes due to ongoing concerns regarding how well ocular dimensions relate to IOP, limiting its use in clinical care. 22
The iCare HOME is a handheld device which can record and store IOP measures in the same units (i.e. mmHg) as the gold standard in-clinic Goldmann applanation tonometer (GAT) assessment. 23 Rebound tonometry, developed by iCare, has been in use since 2003, but in 2014 a version, iCare HOME, was released which was specifically designed for patients to use themselves outwith the clinic setting. 23 The device takes six individual readings during each measurement, then disregards the highest and lowest reading and then calculates an average of the remaining four readings, to give a final result. 23 Several studies have compared the IOP readings from the iCare HOME tonometer to GAT. The reported mean differences between the iCare HOME and GAT measurements range from −2.7 to 0.7 mmHg. 24–28 However, variations of 0–5 mmHg are considered acceptable for home monitoring. 24–26,28 Patients are taught to operate this device themselves. Evidence suggests most study participants were able to use the iCare HOME device correctly following training. 25,28–30
Home perimetry technologies
Portable perimeters designed for testing peripheral VF at home are a potentially useful tool for remote home monitoring of glaucoma. They have a theoretical advantage of providing frequent VF data which may help to confirm disease in glaucoma suspects and possibly assess disease progression. 31 Pre-2020, when this study was commissioned, there were two reliable app-based technologies available for the remote monitoring of VFs: the Melbourne Rapid Field (MRF, GLANCE Optical Pty Ltd, Melbourne, Australia) and Moorfields motion displacement test (MMDT). 31
The MRF is an app designed to be used on an iPad® (Apple Inc., Cupertino, CA, USA) and involves the user placing the iPad on a stand and positioning themselves so that they are sitting approximately 33 cm from the screen. 31 The test is conducted in two parts, first asking users to focus on the centre of the screen (central field test) and then asking users to focus on each corner of the screen (peripheral field test). 31 Users are asked to push the space bar on a Bluetooth® (Bluetooth Special Interest Group, Kirkland, WA, USA) keyboard in response to stimuli. 31 Results of the MRF have been found to be consistent with gold standard in-clinic VF testing using the Humphrey Visual Field Analyzer. 32,33 At the point of commissioning, the MRF was yet to be tested out of clinic. 31
The MMDT is designed to be used on a laptop and involves the user resting their head and chin onto a support stand approximately 30 cm from the screen. 31 Users are then asked to focus on a central spot on the screen and click the mouse or press the space bar every time they see a moving line on screen, for a duration of around 5 minutes. 31 Although limited, evidence suggests good diagnostic performance34 and patient engagement35 with this test. Similar to the MRF, out-of-clinic use of the MMDT has not yet been reported. 31
Study rationale
Technological advances have made glaucoma home monitoring possible through the development of innovative portable tonometers and perimeters that use tablet or personal computers, or head-mounted displays. Such devices may be used for assessing IOP, VF loss and other visual parameters, without clinic visits. However, their effectiveness, their ability to detect and quantify the severity of VF damage, and patients’ ability to use them at home are yet to be tested.
At the time of commissioning In-home Tracking of glaucoma: Reliability, Acceptability, and Cost (I-TRAC) there was a lack of detailed exploration with important stakeholders such as patients and clinicians. Collecting these insights is crucial for developing an in-depth understanding of clinicians’ views on home monitoring, particularly regarding which patients would most likely benefit from this approach, and to determine obstacles preventing implementation of these technologies to improve healthcare delivery in glaucoma. From the patients’ perspective, it is important for us to understand how acceptable home monitoring technologies are; will patients engage with the devices and the remote monitoring approach? As part of this, it is important to understand design issues that are important to patients, to ensure the technologies can be used by those they are designed to help. As noted by the NIHR, economic evaluation of these glaucoma home monitoring technologies has not been undertaken. 22,36 A well-designed randomised controlled trial (RCT) is required to answer these questions. However, prior to full trial, we first need to determine the acceptability and feasibility of this approach.
Since commissioning, a small number of studies exploring the acceptability and/or feasibility of these home monitoring interventions for glaucoma have been published, but not within the context of feasibility of a future large-scale evaluative study. 37–39 These will be discussed in detail with relevance to I-TRAC findings in the Discussion (see Chapter 7).
Aims and objectives
The overall aim of this study was to determine the feasibility and acceptability of digital technologies to monitor glaucoma at home and inform the possible need for and design of a definitive evaluative study.
The four specific research objectives were to:
-
identify which glaucoma patients are most appropriate for home monitoring (e.g. all patients, or those with stable disease, or those with severe glaucoma?)
-
understand the views of key stakeholders [patients, clinicians, information technology (IT) personnel, researchers] on whether home monitoring is feasible and acceptable
-
develop a conceptual framework for the economic evaluation of home monitoring for glaucoma
-
explore the need for and provide evidence on the design of a future study to evaluate the clinical and cost-effectiveness of digital technologies for home monitoring of glaucoma.
Study design and overview
The I-TRAC study was a multiphase mixed-methods feasibility study with key components informed by theoretical [i.e. the Theoretical Framework of Acceptability (TFA) and Theoretical Domains Framework (TDF)] and conceptual [A process for Decision-making after Pilot and feasibility Trials (ADePT)] frameworks (see Appendix 1 for a diagrammatic overview of study design). Successful evaluation and future implementation of any new intervention require in-depth understanding of the potential process modifiers. It was recognised that the introduction of home monitoring using digital technology for glaucoma will involve multiple stakeholders (patients, healthcare professionals and researchers with experience of delivering evaluative studies of digital technology used for home monitoring) and various care contexts (home and secondary care) – all of which were important to capture through the study design.
We planned to use two technologies for glaucoma home monitoring, the first being the iCare HOME, a handheld tonometer for measuring IOP. The second, the MRF app, played on an iPad to measure VFs. However, due to the MRF not being CE marked, we had to replace the MRF with the OKKO Visual Health app (OKKO Health, Bristol, UK). A description of the problem and decisions relating to choice of replacement app are reported in Chapter 4. Participants were asked to use both monitoring devices once per week for 12 weeks.
Several methods were utilised to address each of the research objectives mentioned, specifically:
-
A survey regarding glaucoma home monitoring feasibility was designed and distributed among key clinical stakeholders within glaucoma care in the NHS (addresses research objective 1).
-
Glaucoma patients were trained to use the home monitoring technologies and used the technology for a period of 3 months. A sample of patients and the site staff involved in recruitment were interviewed following this to gain insight into each experience (addresses research objective 2).
-
Interviews were conducted with relevant external research teams and ophthalmology consultants (addresses research objective 2).
-
A systematic review to investigate appropriate health economic models for home monitoring of glaucoma was conducted and supplemented with quantitative and qualitative data from clinicians and patients relating to resource use and preferences (addresses research objective 3).
-
A statement was produced regarding the overall feasibility and acceptability of an evaluative study comparing current NHS glaucoma care with home monitoring (addresses research objective 4).
Chapter 2 Identification of which glaucoma patients are most appropriate for home monitoring
There is limited guidance in the literature as to which glaucoma patients would be the ideal candidates for home monitoring using digital technology. Identifying key uncertainties regarding candidate suitability, such as who are the appropriate patients to target for evaluating an intervention, is a critical first step towards evaluating its use. This chapter reports the findings from an online survey with expert glaucoma clinicians to determine which glaucoma patients are most appropriate for home monitoring and to investigate clinical decision-making in relation to patient suitability.
Methods
Study design
Online survey involving expert glaucoma clinicians, who have not been involved in the glaucoma home monitoring intervention component of the study.
Sampling and recruitment
The target population were expert glaucoma clinicians (i.e. ophthalmologists and optometrists). To take part in our study, participants needed to work within the UK NHS, presently deliver care to persons with glaucoma, and agree to take part in the study. Based upon the estimated number of glaucoma clinicians registered with the UK and Eire Glaucoma Society (UKEGS), a non-profit professional society for clinicians with a specialist interest in glaucoma (range n = 69–7240,41) we are accepting our denominator for calculating response rate as n = 72. UKEGS does not currently record the designation of its members, so the exact number of glaucoma consultants surveyed is unknown.
In order to target clinicians with directly relevant experience, the survey was disseminated via UKEGS. A link to the questionnaire with an invitation to participate was e-mailed to members of UKEGS by the UKEGS Communications Manager. In addition to the invitation e-mail, the clinical co-investigators raised awareness of the questionnaire among existing clinical networks. The survey was active from 14 May to 30 October 2021.
Data collection
An online survey including both closed and open-ended questions, informed by the literature and expert opinion within the research [including three clinical Principal Investigators (PIs): AAB, AK and AT], was created through SurveyMonkey® (Palo Alto, CA, USA). 42 A participant information leaflet (containing general information about the I-TRAC study and the home monitoring devices) was included at the start of the survey to support informed consent but also to ensure participants were given contextual insights to promote informed responses. For example, a summary of the NICE guidelines for ocular hypertension (OHT) and primary open-angle glaucoma (POAG) was included alongside an introduction to the technologies being discussed (iCare HOME tonometer and tablet-based apps for measuring visual function).
Participants were asked to decide if they would use the iCare HOME tonometer and/or an app for visual function, for four clinical scenarios. The patient scenarios were developed to reflect fictional patients (but based on real examples) and included hypothetical details on glaucoma severity (mild, moderate, severe), current treatment, risk of visual loss, disease control (apparently well controlled, uncertain, poorly controlled), and management options, as well as demographic details (Table 1). These cases were designed to reflect the NICE guidelines for OHT and POAG. When a clinician did not recommend a patient’s suitability for home monitoring, they were asked to justify this decision. Scenario 5 described a clinical model of care in which a doctor integrates the home monitoring devices within their routine glaucoma care due to reduced clinic capacity. Clinicians were asked to determine whether this model of care was acceptable to them, why it was/was not acceptable, and to tell us about perceived advantages and disadvantages to this model of care.
| Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 | |
|---|---|---|---|---|
| Name | Mr Smith | Ms Adams | Mr Patel | Ms McEwen |
| Age | 63 | 70 | 78 | 55 |
| Gender | Male | Female | Male | Female |
| Brief history | 2-year history of severe bilateral glaucoma No evidence of current progression |
1-year history of bilateral OHT | 3-year history of poorly controlled pseudo exfoliation in R eye and moderate glaucoma in L eye | 5-year history of mild, bilateral normal tension glaucoma No progression noted |
| Intended level of risk | High risk | Low risk | High risk | Low risk |
Survey participants were asked to provide demographic data including age, gender, ethnicity, profession, the number of years’ experience in treating glaucoma, and current or past use of technologies for measuring IOP and/or visual function in patients at home.
The data collected through SurveyMonkey were downloaded and stored in an Excel® worksheet [Microsoft® Excel for Microsoft 365 MSO (Version 2205 Build 16.0.15225.20394); Microsoft Corporation, Redmond, WA, USA]. Participant responses were anonymous and assigned a unique identifier number.
Data analysis
Quantitative data from closed-ended questions were analysed using descriptive statistics (e.g. frequencies, percentages). Agreement with clinical scenarios was defined by the study team as being ≥ 60% in either supporting or not supporting the hypothetical patient to be home monitored using the digital technology. Agreement across clinical scenarios was also investigated in a post hoc analysis and reported using frequencies. Free-text responses, such as those asking for clinical reasoning for the monitoring decision, and the frequency and duration of monitoring, were analysed using directed content analysis. 43 Within this, a hybrid approach, combining both inductive (data-driven) and deductive (based on preconceived ideas) methods was followed. Frequency and duration responses were free text and were reviewed across all clinical scenarios (scenarios 1–4) to create categories that could be applied across scenarios for ease of comparison. Reasons behind the perceived unsuitability of each clinical scenario for home monitoring were analysed and reported within each scenario. Similarly, explorations of the acceptability of this model of care were analysed within scenario 5 where several questions regarding perceived barriers and facilitators were presented. Within this project, a barrier was defined as ‘a circumstance or obstacle that keeps people or things apart or prevents communication or progress’. 44 A facilitator was defined as ‘the factors that enable the implementation of evidence-based interventions’. 45
The Microsoft Excel spreadsheet was transferred to NVivo software (version 12 qualitative analysis programme; QSR International, Warrington, UK). 46 Data were reviewed and emerging themes noted. Following several reviews of the data by two reviewers (UA and CS), a coding dictionary describing the themes and categories found within participant responses was developed. Within NVivo, a tree chart was constructed to explore the weighting of each of the themes, subthemes and codes created from the survey responses. This allowed identification of the major themes based on the volume of responses addressing this topic, number of participant comments and relevance as deemed by the researcher.
Results
Sample demographics
A total of 64 clinicians accessed the survey, a response rate of 89% (n = 64/72, 72 being the upper limit in the range of eligible clinicians registered with UKEGS who had a special interest in glaucoma). Four participants were excluded based on lack of experience treating glaucoma (n = 3), as per the exclusion criteria, and one participant did not respond to any of the questions. A further 11 were excluded from the final analysis as they did not respond to the clinical scenario questions and only provided demographic data. Therefore, 49 clinicians who replied to at least one of the questions in relation to the clinical scenarios were included in the final analysis (77%, n = 49/64). Table 2 shows the characteristics of the respondents. The majority of participants were white (59%, n = 29), male (69%, n = 34), consultants (92%, n = 45), aged between 50 and 59 years (45%, n = 22), who have treated glaucoma patients for > 10 years (71%, n = 35). The demographics of those removed from final analysis (n = 11, not presented) showed a similar pattern in that they were mostly of white ethnicity (82%, n = 9), consultants (82%, n = 9) with extensive experience (64%, n = 7, over 10 years’ experience). However, they were younger (64%, n = 7, < 50 years of age) and the gender split was more equal between male and female (male, 36%, n = 4; female, 36%, n = 4, with 3 preferring not to report their gender).
| Variables | Responses, n (%) (N = 49) |
|---|---|
| Duration of experience with treating glaucoma | |
| < 5 years | 3 (6) |
| 5–10 years | 11 (23) |
| > 10 years | 35 (71) |
| Profession | |
| Optometrist | 4 (8) |
| Consultant ophthalmologist | 45 (92) |
| Participant age (years) | |
| < 40 | 7 (14) |
| 40–49 | 16 (33) |
| 50–59 | 22 (45) |
| > 60 | 4 (8) |
| Gender | |
| Male | 34 (69) |
| Female | 13 (27) |
| Non-binary | 1 (2) |
| Prefer not to say | 1 (2) |
| Ethnicity | |
| White | 29 (59) |
| Mixed white and Black African | 3 (6) |
| Asian/Asian British | 16 (33) |
| Black/Black British African | 1 (2) |
Clinician decisions regarding patient suitability for glaucoma home monitoring
Across the scenarios, there were varying rates of agreement regarding the suitability of each patient scenario for home monitoring (Figure 1 and Table 3). Based on the levels of agreement defined (i.e. > 60% reporting a patient as suitable), only one patient, scenario 4, was deemed suitable for home monitoring. In the scenario, 61% (n = 30) of clinicians would refer the patient to use the iCare tonometer and 65% (n = 32) the home visual function app assessment. Scenario 4 reported Ms McEwen, a low-risk patient with normal tension glaucoma (NTG) and mild disease who had not progressed in 5 years.
FIGURE 1.
Clinician agreement on home monitoring using digital technology within patient scenarios.

| Scenario 1 Mr Smith High risk, n(%) |
Scenario 2 Ms Adams Low risk n(%) |
Scenario 3 Mr Patel High risk n(%) |
Scenario 4 Ms McEwen Low risk n(%) |
|
|---|---|---|---|---|
| Would you consider it useful to monitor IOP at home? | ||||
| Yes | 26 (53) | 28 (57) | 26 (52) | 30 (61) |
| No | 23 (47) | 18 (36) | 18 (36) | 14 (9) |
| No response | 0 (0) | 3 (6) | 6 (12) | 5 (10) |
| Recommended frequency of IOP monitoring | ||||
| Every 1–7 days | 19 (73) | 9 (32) | 13 (50) | 7 (23) |
| Monthly | 3 (12) | 3 (11) | 3 (12) | 4 (13) |
| Every 2–6 months | 3 (12) | 12 (43) | 7 (27) | 13 (43) |
| Every 7–12 months | 0 (0) | 0 (0) | 0 (0) | 4 (13) |
| Unclear | 1 (5) | 3 (11) | 3 (12) | 2 (6) |
| No response | 0 (0) | 1 (3) | 0 (0) | 0 (0) |
| Recommended duration of IOP monitoring | ||||
| Daily | 2 (8) | 1 (3) | 2 (8) | 1 (3) |
| Weekly | 4 (15) | 1 (3) | 3 (12) | 2 (6) |
| Monthly | 4 (15) | 0 (0) | 3 (12) | 1 (3) |
| Every 2–6 months | 8 (30) | 3 (11) | 10 (38) | 4 (13) |
| Every 7–24 months | 1 (5) | 13 (46) | 5 (19) | 17 (57) |
| Unclear | 7 (27) | 7 (25) | 2 (8) | 3 (10) |
| No response | 0 (0) | 3 (11) | 0 (0) | 2 (6) |
| Would you consider it useful to monitor visual function at home? | ||||
| Yes | 28 (57) | 26 (53) | 20 (41) | 32 (65) |
| No | 19 (39) | 18 (37) | 24 (49) | 12 (24) |
| No response | 2 (4) | 5 (10) | 5 (10) | 5 (10) |
| Recommended frequency of visual function monitoring | ||||
| Every 1–7 days | 4 (14) | 0 (0) | 2 (10) | 1 (3) |
| Monthly | 11 (39) | 5 (19) | 4 (20) | 5 (16) |
| Every 2–6 months | 8 (29) | 14 (54) | 10 (50) | 20 (63) |
| Every 7–12 months | 0 (0) | 4 (15) | 1 (5) | 3 (9) |
| Unclear | 5 (18) | 3 (12) | 2 (10) | 2 (6) |
| No response | 0 (0) | 0 (0) | 1 (5) | 1 (3) |
| Recommended duration of visual function monitoring | ||||
| Daily | 0 (0) | 0 (0) | 1 (5) | 0 (0) |
| Monthly | 2 (7) | 0 (0) | 0 (0) | 0 (0) |
| Every 2–6 months | 17 (61) | 4 (15) | 10 (50) | 4 (13) |
| Every 7–24 months | 2 (7) | 16 (62) | 3 (15) | 22 (69) |
| Unclear | 3 (11) | 2 (8) | 3 (15) | 5 (16) |
| No response | 4 (14) | 4 (15) | 3 (15) | 1 (3) |
Clinicians were asked to suggest optimal time frames for the frequency and duration of home monitoring (of both IOP and visual function) through open-ended questions in each scenario. Similar to the variation in responses to clinical scenarios, a wide spectrum of monitoring frequencies and durations for each scenario were suggested (Table 3). Despite this, greatest consensus appeared within low-risk scenarios (2 and 4) where for both IOP and visual function monitoring, participants reported opting for reduced frequency of monitoring (every 2–6 months) but over an increased duration (7–24 months). For high-risk scenarios (1 and 3), frequency and duration for visual function monitoring were consistent and similar to low-risk scenarios (2 and 4). However, in relation to IOP monitoring, participants reported increased frequency (every 1–7 days) but for a lower duration (1–6 months).
Explaining clinician glaucoma home monitoring decision-making
The justifications provided by the clinicians when reporting that Ms McEwen, scenario 4, would be suitable for home monitoring stated this was due to the nature of her stable disease and her low risk of progression. They felt that she could be safely monitored at home without high risk of missed progression, which could allow increased clinic capacity for individuals with advanced disease, ‘[f]reeing up capacity in the hospital eye service, allowing better use of resources and enabling better care of high-risk patients. Low-risk patients may prefer not having to come in the hospital’ (Consultant, 5–10 years of experience).
Among the participants who predicted that this patient would be unsuitable for home monitoring, the justification was that it may be a waste of resources due to the stable nature of her current condition. This was a contradiction with other participants who deemed her suitable due to the exact same reasoning, ‘stable for 5 years, waste of effort’ (Consultant, over 10 years of experience).
Table 4 summarises the justifications given by clinicians when deciding a patient described in the clinical scenarios would be unsuitable for home monitoring. When evaluating the rationale behind clinicians deeming the other scenarios unsuitable, the main concerns contradicted each other. However, many clinicians (n = 32) highlighted that they may be more hesitant to recommend home monitoring to high-risk individuals. They predicted that they would rather assess these patients in clinic to confidently determine their IOP and visual function. This was expressed to be important as it would avoid any discrepancies or impaired clinical judgement due to potentially ‘unreliable’ readings. They also felt that these advanced cases may require additional resources such as imaging, ‘[h]igh risk of further vision loss within lifetime – young, advanced VF defects bilaterally. Would prefer to see in clinic and discuss surgery at each visit’ [regarding scenario 1] (Optometrist, > 10 years of experience).
| Scenario | Reasons reported for ‘Unsuitable’ response | Example quote |
|---|---|---|
| 1 Mr Smith High risk |
|
|
| 2 Ms Adams Low risk |
|
|
| 3 Mr Patel High risk |
|
|
| 4 Ms McEwen Low risk |
|
|
In addition to measuring clinical agreement within each clinical scenario regarding home monitoring of glaucoma patients, we also investigated agreement across scenarios to determine whether the disagreement in scenarios is related to the digital technology or to which patients should be monitored. There was a lack of consensus relating to which patients should be monitored using the tonometer, with 23 clinicians (47%) reporting that home monitoring of patients would be useful in at least three of the four clinical scenarios. This was also true for visual function, with 22 clinicians (45%) believing it to be useful for three out of the four scenarios.
Clinicians’ acceptability of home monitoring of glaucoma within NHS Care pathways
The fifth clinical scenario in the questionnaire described a clinical care model that combined home monitoring into the current hospital-based system. Regarding acceptability, no agreement was reached on this scenario, with just over half of clinicians (52%, n = 26) reporting that this model was acceptable and around one-third (37%, n = 18) reporting that it was unsuitable; 10% (n = 5) did not respond. Thematic content analysis of free-text responses in relation to this scenario resulted in the following seven themes: Resources, Patient Characteristics, Clinician Confidence in Home Monitoring, Perception of Risk, Wider Benefits, Accessibility, and Disease Suitability of Patient. Within each theme, anticipated advantages and disadvantages in relation to glaucoma home monitoring were identified, as shown in Table 5.
| Theme | Subtheme | Code | A/D | Frequency (n) | No. of participants | Example quote |
|---|---|---|---|---|---|---|
| Resources | Financial effectiveness | Cost-effective | A | 2 | 1 | ‘In the long term, it may be cheaper too’ |
| Poor value for money | D | 28 | 24 | ‘home tonometry for all patients seems a grandiose waste of resources’ | ||
| Health care/clinic capacity | Increased clinic capacity | A | 71 | 36 | ‘Utilising the limited capacity to see stable patients virtually is helpful to generate more capacity for patients who require more attention’ | |
| Decreased clinic capacity | D | 4 | 4 | ‘home monitoring would need to be well supported, to train and supervise patients, and well planned, to review data’ | ||
| No or inadequate alternatives | – | A | 13 | 11 | ‘Better to get some monitoring than just being a name on the waiting list and losing sight’ | |
| Human resource demands | Staff needed to train/support patients | D | 11 | 11 | ‘Securing the funding and staffing to train patients and to troubleshoot might be a challenge’ | |
| Staff needed to review data | D | 9 | 9 | ‘[there] would be a significant burden in virtually reviewing all these patients which would need to be accounted for in the business case’ | ||
| Patient characteristics | Patient compliance | Increased compliance | A | 4 | 4 | ‘May empower patient and improve adherence as they get direct feedback on the effects of treatment and status of disease’ |
| Decreased compliance | D | 38 | 26 | ‘The governance of non-compliancy with lack of patient involvement would be another challenge’ | ||
| Patients’ cognitive, physical and mental abilities | Cognitive impairment | D | 2 | 2 | ‘Forgetting the original treatment instruction’ | |
| Physical impairment/disability impairing use of clinic testing | A | 49 | 31 | ‘Patients with reduced mobility/health issues making clinic attendance or VF testing difficult’ | ||
| Physical impairment/disability preventing use with self-monitoring technologies | D | 7 | 6 | ‘Also, patients with physical disabilities or learning difficulties/dementia will struggle with home monitoring themselves’ | ||
| Decreased patient anxiety | D | 4 | 3 | ‘Where they are anxious about something and have phoned in to ask for early review’ | ||
| Increased patient anxiety | D | 4 | 4 | ‘They may get very anxious about small changes in results without full understanding’ | ||
| Clinician confidence in home monitoring | Beliefs that increase clinician confidence | Improved clinician trust in quality of care delivered | A | 11 | 10 | ‘In reality glaucoma patients may actually do better with more regular IOP and field testing as will pick up discrepancies sooner and we can’t [do] the tests this often in the clinic’ |
| Concerns that decrease clinician confidence | Reliability issues | D | 83 | 35 | ‘We do not have enough information about effectivity’ | |
| Standardised conditions | D | 6 | 5 | ‘There is a possibility of someone other than the patient performing the home tests and passing it as the patients’ | ||
| Hospital compatibility and consistency | D | 10 | 9 | ‘No consistency between hospital and home care tests’ | ||
| Limitations of current home monitoring technologies | D | 34 | 18 | ‘OCT not done which may be considered important by some for early disease’ | ||
| Perception of risk | Perceived patient safety of home monitoring | Increased clinical safety | A | 21 | 18 | ‘greater number of patients getting timely monitoring’ |
| Fear of patient harm | D | 51 | 29 | ‘Experience tells us that some patients will lose vision in the virtual system, despite best efforts to risk stratify and see virtually’ | ||
| IT concerns | – | D | 9 | 8 | ‘IT works well when it works well, but more than often there are barriers and incomplete data etc’ | |
| Wider benefits | Environmental benefit | – | A | 1 | 1 | ‘Good for the planet – low carbon footprint from not having to travel to the hospital’ |
| Increased patient convenience | – | A | 9 | 9 | ‘More convenient for the patient’ | |
| Assessments for patients previously unable to attend routine monitoring in clinic | – | A | 21 | 12 | ‘Bedbound patients in care homes’ | |
| Accessibility | Language | Language barrier | D | 17 | 16 | ‘Harder to reach patients would still have a low uptake of the technology. Education is more important. Even if given device might not use or not use correctly. [Patient] education empowers them to access medical help’ |
| Technology and internet access | – | D | 2 | 1 | ‘Internet availability for download of test results’ | |
| Disease suitability of patient | Stable disease | NTG | A | 42 | 25 | ‘In established NTG were progression despite good IOP in office measures’ |
| OHT | A | 10 | 7 | ‘Only OHT patients can be managed safely with virtual clinics’ | ||
| Screening | A | 7 | 7 | ‘also useful as screening test’ | ||
| Care change monitoring | A | 11 | 11 | ‘May be particularly useful immediately after diagnosis or after change in treatment to determine rate of progression’ | ||
| Phasing (24-hour monitoring) | A | 17 | 15 | ‘Patients with progressive glaucoma – with apparently “controlled” IOP’ | ||
| Low-risk suitable | A | 22 | 18 | ‘Low–medium risk patients can be monitored virtually’ | ||
| Low-risk unsuitable | D | 26 | 19 | ‘Due to the limited capacity in hospital glaucoma clinics, we should focus our resources in higher risk patients’ | ||
| Unstable disease | High-risk suitable | A | 8 | 7 | ‘concentrating on riskier cases without losing focus on the well-controlled ones’ | |
| High-risk unsuitable | D | 56 | 32 | ‘In person visits slots kept for those with uncontrolled IOP, high risk, post-op’s etc.’ |
Resources
From a resource perspective, many clinicians believe that implementing home monitoring could increase clinic capacity for review of high-risk patients and believe that this model could be better than the current alternative of no or limited monitoring. There was also a belief that access to timely care via home monitoring could prevent irreversible severe visual loss, promoting patient health and well-being. However, many doubted the cost-effectiveness of glaucoma home monitoring. Some felt that this model could reduce clinic capacity for patients by the time this service was staffed in relation to the human resource needed to train patients and review the data collected.
Patient characteristics
Clinicians had concerns about patients’ cognitive, physical and mental capacity for home monitoring. While concerns were broadly shared about physical and cognitive capacity, opinions were divided in relation to patient anxiety; while some felt home monitoring could alleviate anxiety in relation to glaucoma progression, some felt it had potential to increase this. The majority reported concerns that home monitoring could lead to reduced patient compliance with regular testing and concerns about how this would be monitored. For the few, compliance could increase as regular feedback on disease status was anticipated to be motivational for patients.
Clinician confidence in home monitoring
We found that while there was some optimism about more frequent monitoring, home monitoring was largely anticipated to cause a decrease in clinician confidence in the quality of care delivered to patients. This was related to concerns about the reliability, standardisation, and compatibility of the devices, with and against hospital equipment, and concerns about relying on a narrower range of measures, as opposed to additional measures obtained in clinic (e.g. optical coherence tomography). There was some optimism, however, that more frequent monitoring of IOP and visual function, as would be possible with home monitoring, could detect progression sooner.
Perception of risk
While several clinicians reported more timely monitoring being a positive, there was a strong concern about the risks posed by the home monitoring of glaucoma. Risks raised reflected patient harms (e.g. missing glaucoma progression, resulting in loss of vision) and data and technology risks resulting from events such as failing equipment.
Wider benefits
A couple of wider benefits to home monitoring were raised. For example, the reduced travel arising from monitoring at home offers an environmental advantage. For some, home monitoring was expected to be more convenient for patients. Some also raised the advantage of being able to monitor patients who currently are not able to be monitored in clinic, such as those who are bedbound and/or residing in care homes.
Accessibility
Several disadvantages which could be potential barriers to accessing home monitoring were reported, namely language barriers and technology/internet access. These were expected to result in low uptake of home monitoring among some population groups.
Disease suitability of patient
Similar to the variation in responses to the clinical scenarios (1–4) there was little consensus in responses made in relation to a patient’s medical suitability for home monitoring. Most agreed that those at high risk of progression were unsuitable for home monitoring. Certain disease classifications (OHT and NTG) were frequently considered good candidates. The use of these technologies for glaucoma screening and phasing purposes was often frequently suggested as having potential in addition to or instead of regular monitoring.
Chapter summary
This chapter has demonstrated that there is agreement among the expert glaucoma clinicians surveyed that there is a place for home monitoring of glaucoma patients using digital technology. However, based on the scenarios used in this study, there is limited agreement among clinicians about which glaucoma patients are most suitable for home monitoring using digital technologies to measure IOP and visual function. Agreement (> 60%) was achieved for scenario 4 (a stable, low-risk patient), with clinicians supporting monitoring of IOP and visual function at home. Clinicians reported that they were generally not supportive of the home monitoring of high-risk patients, due to the fear of missing disease progression or unreliable readings. However, they were generally supportive of home monitoring having a role within low-risk scenarios, such as NTG monitoring and 24-hour phasing. Clinicians anticipated that the integration of home monitoring into the current healthcare system could act as an adjunct to increase hospital capacity for glaucoma patients who require face-to-face assessment.
This survey has highlighted a range of issues and challenges related to the home monitoring of glaucoma patients using digital technologies. A central theme is clinicians’ lack of trust in home monitoring technologies, related to concerns about the reliability, accuracy and usefulness of these technologies. Clinicians expressed concerns about patient safety, decreased rather than increased glaucoma progression detection and concerns about how resource efficient (time and financial) this approach could be in comparison to current provision. These areas are explored in more detail in Chapter 3.
Chapter 3 Expert glaucoma clinicians’ acceptability of glaucoma home monitoring
Building on the findings from the survey data investigating expert glaucoma clinicians’ views on patient populations suitable for home monitoring using digital technology, as reported in Chapter 2, this chapter presents the findings of semistructured interviews with the same stakeholders to explore intervention acceptability in more detail. Exploring intervention acceptability among a wider group of clinicians, and in particular those not involved in I-TRAC, was important so as to understand broader community perspectives. The aim of this phase was to identify additional insights to enhance trialability of digital technology for home monitoring of glaucoma.
Methods
Study design
Online focus groups and interviews involving expert glaucoma clinicians, who have not been involved in the glaucoma home monitoring intervention component of the study, guided by the TFA.
Sampling and recruitment
Participants were recruited through the clinician survey (see Chapter 2), where respondents could indicate their willingness to be contacted for interview and provide contact details for arranging this. Participants were asked to select their preferred time and date from three available options. Those unable to accommodate focus group date/time were offered an interview as an alternative. We aimed to recruit 16 participants. We invited all clinicians who had agreed to be contacted (n = 25). Of these, 15 (60% response rate) attended a focus group or interview (seven did not respond to the invitation including a reminder e-mail, and three had booked a focus group session or interview but failed to attend). All participants provided verbal (recorded) consent prior to the focus group or interview.
Data collection
Interviews and focus groups were conducted online (MS Teams®; Microsoft Corporation, Redmond, WA, USA) by CS and facilitated by KG. Demographic data were collected via the clinician survey (see Chapter 2). Discussion was guided by a prepared semistructured topic guide. The topic guide questions were framed around the constructs of the TFA: affective attitude, intervention coherence, ethicality, perceived effectiveness, self-efficacy, anticipated costs and burden. Focus groups and interviews were recorded and transcribed verbatim.
Data analysis
Transcripts were uploaded to NVivo 12 Pro (QSR International, Warrington, UK) for analysis. 46 Analysis was conducted such that a deductive TFA analysis was conducted first, followed by inductive analysis to identify themes within the TFA construct findings. Transcripts were first reviewed noting underlying points, ideas or feelings being conveyed throughout each transcript. These were then considered against the TFA criteria and where applicable, organised within the TFA constructs. Data deemed relevant but not fitting TFA were retained and their relationship with TFA constructs explored. Two researchers (CS and KG) coded the first three transcripts concurrently to develop a coding strategy based upon the TFA. The coding guide developed for analyses is presented in Appendix 2. Subsequent transcripts were coded by one researcher (CS) and a random 10% sample (n = 1) was independently double coded (KG).
Results
Sample demographics
Expert glaucoma clinicians (n = 15; 13 consultant ophthalmologists and two specialist optometrists) participated across three focus groups (n = 2, n = 4, n = 4) and five individual interviews (due to clinician availability). Most (n = 12) reported that they were not currently using home monitoring devices to measure IOP, and none were using devices for home perimetry. Focus groups lasted 56–70 minutes and interviews 31–55 minutes. Most expert glaucoma clinicians who participated had over 10 years of clinical experience (n = 14). Age, gender and ethnicity data are shown in Table 6.
| n | ||
|---|---|---|
| Occupation | Consultant | 13 |
| Specialist optometrist | 2 | |
| Experience of treating glaucoma (years) | < 5 | 1 |
| 5–10 | 0 | |
| > 10 | 14 | |
| Age (years) | < 40 | 2 |
| 40–49 | 3 | |
| 50–59 | 9 | |
| 60+ | 1 | |
| Gender | Male | 6 |
| Female | 8 | |
| Non-binary | 1 | |
| Ethnicity | White (all) | 7 |
| Asian (all) | 7 | |
| Black (all) | 1 |
Findings
Data from the interviews were coded into all seven constructs of the TFA, and inductive analysis within the TFA constructs resulted in 19 themes, under a global theme of cautious optimism. Table 7 presents a summary of the interview findings.
| TFA construct | Theme |
|---|---|
| Affective attitude | Enthusiasm tempered with uncertainty |
| Concerns about negative affect for patients and staff | |
| Ethicality | Ethical ‘risks’ of remote and commercial data collection technologies |
| Intervention fit with principles of ‘good’ care | |
| Managing equity in patient selection | |
| Intervention coherence | Autonomy to determine the who, when, and where of home monitoring |
| Data relevance and integration | |
| Support for patients | |
| Anticipated costs | Beliefs about cost-effectiveness |
| Adjustments to address affordability | |
| Burden | Clinician burden |
| Patient burden | |
| Perceived effectiveness | Anticipated outcomes |
| Balancing benefits and harms | |
| Impact of perceived data and technology limitations upon intervention effectiveness | |
| Patient characteristics impacting intervention effectiveness | |
| Impact of service levels factors on effectiveness | |
| Self-efficacy | Low self-confidence and confidence in patients to use tech |
Affective attitude
Enthusiasm tempered with uncertainty
When asked how they felt about the I-TRAC home monitoring interventions for glaucoma patients, expert glaucoma clinicians were generally enthusiastic about the opportunities this intervention presented, described through terms such as ‘excited’, ‘delighted’, and ‘glad’.
I think it’s a great idea, I think we need to know this. We’ve got real problems with capacity in glaucoma and if we can have a trial that helps us better understand the patient acceptability, clinicians’ views on acceptability and the meaningfulness of the data and so on and so forth then it’s to be welcomed.
P002, Optometrist, Focus Group 1
These feelings were often reported in relation to home monitoring being a potential solution to national capacity problems across HES. However, enthusiasm was tempered with caution – as indicated by a number of conditional statements such as ‘I think it would be a great thing if it worked well and it was reliable’ (P008, Consultant, Focus Group 2) – demonstrating the connection between affective attitude and perceived effectiveness.
Concerns about negative affect for patients and staff
Expert glaucoma clinicians also reported that they felt many patients would be welcoming of this approach, highlighting how this could reduce patient stress and provide comfort, ‘some patients genuinely like writing the numbers down and they like to know what they were before and they get great comfort in knowing there are numbers written down that they have control over’ (P018, Consultant, Interview).
However, among the positive feelings, some clinicians reported feelings of anxiety and frustration as a risk of such interventions, which could be experienced by both staff and patients.
Digital data, I mean it does sound like this panacea and it’s wonderful, but actually when it doesn’t go quite so right it can be extremely difficult and frustrating, and I’m thinking about the age group of our patients, how hard it might be for them.
P004, Consultant, Focus Group 1
Ethicality
When exploring the ethical construct of acceptability, responses generated three linked categories: ethical risks of remote and commercial data collection technologies, how the intervention fits with principles of ‘good’ care and managing equity in patient selection. These are discussed in turn below.
Ethical risks of remote and commercial data collection technologies
When asked about ethical issues this intervention may pose, many clinicians discussed aspects of data security. There were concerns about how to protect these systems from external threats, and the need for secure data exchange. Many felt that NHS systems of governance for IT should be adequate to adopt this; however, one clinician raised the need to consider how commercial entities also manage data governance:
[O]ne thing we’ve not necessarily quite touched on is the link with the commercial sector. So OKKO Health are a commercial sector organisation, whoever’s come up with [home visual field intervention] presumably is as well. So who owns the data, who’s responsible for the governance of the data, all of that.
P002, Optometrist, Focus Group 1
Also related to the commercial side were concerns about what would happen to data should a private enterprise withdraw from service, posing the risk that patient data could be lost and no longer governed by NHS data governance. There were concerns that these data could then be misused. There were also some concerns about safety, with calls for regular data auditing being required to establish the safety of this service. One issue raised by a number of clinicians connected to data control is the risk that patients may allow the device to be used by others. The potential risks from this include poor treatment decisions from non-patient data, issues about consent if researchers hold data that are not from the consented patients, and the potential for detecting eye diseases in others (not the patient). However, having user logins to prevent non-patients from using the devices was suggested as an approach that could overcome this, suggesting addressing this concern was not insurmountable.
I wonder… the temptation would be to try it on your family and friends. I mean, I don’t know. But they might say, ‘Have a go. This is what I have. Look at what the hospital’s given me’ which will mess up your data.
P010, Consultant, Interview
Intervention fit with principles of good care
There were a number of references to how this intervention fits with the principles of good care. For some, home monitoring fits well within the self-management framework, where it is considered appropriate to empower patients to manage their own health care. For others, there was concern that home monitoring could lead to reducing glaucoma clinics to little more than data monitoring hubs, leading to a loss of personalised and holistic care.
. . . there’s the reduction potentially of… it could be that glaucoma clinics are seen to be reduced down to data collection on instruments. We know that many of these patients have things they want to talk to us about, we know that they have things wrong with their eyes other than glaucoma and there’s a loss of the holistic approach to patients when things are reduced down to maybe a few questions on a proforma and some data supplied intermittently versus a face-to-face interaction.
P002, Optometrist, Focus Group 1
For others, limitations in the current system are contributing to the overtreatment of glaucoma, whereby clinicians, knowing it could be some time before they review a patient again, tend to provide medical treatment, on a ‘just in case’ basis. Being able to offer home monitoring was viewed as a solution to this and could reduce unnecessary medical treatment.
I think actually virtual clinics you often overtreat and also I guess many of you have these, what we call follow up pending lists, and certainly COVID’s made them go much higher, and you think a patient may have a problem and you say, ‘Okay, we’ll see you again in six months’, but six months maybe 18 months, and, so on that basis I think you sometimes overtreat because you just don’t know when you’re going to see this patient again. So the fact you could have a facility which can help with monitoring patients that you haven’t got capacity to see within the hospital eye service may enable you to treat less patients and therefore get less morbidity from their treatments.
P005, Consultant, Focus Group 2
For one clinician, while supportive of home monitoring, they wondered if this focus upon already diagnosed patients was missing a bigger problem in glaucoma care, specifically, the low level of detection of the disease in its earlier stage.
I think it’s fine this sort of study, but there is a bigger picture that there are lots of people in the UK and particularly in the developing world who don’t get picked up in the community and there has to be solutions to that that could involve these mobile technologies or whatever.
P009, Consultant, Focus group 2
Managing equity in patient selection
Clinicians frequently reported concerns related to equity and equality, particularly in relation to making decisions as to who should obtain home monitoring equipment, and what fallout there may be from those decisions. Frequent references to factors which may make it difficult for some to participate include accessibility, language, education, and technical abilities (discussed in detail in Perceived effectiveness). However, these led to ethical consequences: how to select and prioritise patients for home monitoring (particularly where there will be resource constraints), impact upon those who are not selected for home monitoring and the risk of creating a two-tier system. The latter was in relation to an expectation that patients may have to contribute financially towards equipment, which opens the door to one system for those who can afford and one for those who cannot.
Well actually, prioritisation. If say someone’s neighbour got this and someone else didn’t get it, word goes around in the glaucoma community: ‘Why did she get it? How come I didn’t get it. Am I more less important? Are you more important?’ That’s not going to go well, I suppose. Ethically how do you choose because you have a limited resource, so that limited resource ethic problem. Even if you gave them the option to buy it, then it’s also another ethical dilemma, because the rich are getting better monitoring. But, having said that, it frees up space, frees up one iCare for another person who can’t afford it, so I mean it’s that ethical dilemma of privatisation versus, you know, but… so that same story. But if they want to buy it, I think they should be able to buy it.
P010, Consultant, Interview
However, some argued that such a two-tier system would be inevitable and necessary to accommodate those for whom home monitoring is not suitable regardless of affordability. Clinicians’ accounts report the need for clear guidance as to how to select patients for home monitoring.
I suppose the other ethical issue is, and we’ve kind of touched on it with patients that are able to use these devices and patients that aren’t able to use these devices, if this becomes the gold standard of treatment for patients and then you have a patient that is unable to do it, how can you ethically not let them have treatment, do you then pass(?) that on to go and take the measurements as regularly in-house. That’s obviously years down the line.
P015, Consultant, Focus Group 3
Intervention coherence
Autonomy to determine the who, when and where of home monitoring
When asked about the advantages and disadvantages of this intervention, several attributes became apparent. The first was that there was a desire among clinicians for autonomy to decide how best to integrate home monitoring into usual care.
So I don’t think we could decide or should decide as to who can use it. I guess if the underpinning evidence is that these are meaningful measurements, then it’s about the training and accreditation and ability to autonomously make decisions by whoever is looking after the case mix of patients where that service is being commissioned, and that could be primary care or secondary care.
P002, Optometrist, Focus Group 1
Clinicians discussed varied purposes and patient scenarios (varied clinical situations and parameters) where these technologies could be helpful, and it became apparent there was no consensus among clinicians as to the ideal clinical scenario or patient for glaucoma home monitoring. Suggested clinical scenarios included phasing, reducing overtreatment, monitoring for progression, risk-stratifying/referral strategies, promoting patient self-management, increasing localised care (reducing hospital visits) and increasing clinic testing capacity for higher-risk patients.
[T]he way I envisaged it is more as a trigger to find the patients that need intervention. So this isn’t going to be the be all [and] end all care of a patient’s pathway, it’ll be a service where you can gather whatever data you can regularly that gives you a trigger to say, ‘Well actually that patient’s doing absolutely fine, don’t need to get involved’, whereas another patient, ‘Oh, they need to come in hospital, we need to have a proper look at them’.
P015, Consultant, Focus Group 3
It could also be valuable across patient groups as some felt the increase in data collected would be relevant and beneficial for all groups.
[A]ny data points you can get will enable you to have a better picture and diagnosis of what’s going on with a patient, but I think the more points you can get, the more data you can get, the better. I think that could be taken to any patient group.
P015, Consultant, Focus Group 3
Clinicians generally agreed with the use of home monitoring technologies for patients considered at low risk of progressing to significant visual loss, but some could see value in considering these technologies for higher-risk patients in specific circumstances.
[C]hecking your pressure having changed treatment in somebody who’s relatively low risk and your next clinical decision probably isn’t going to be surgery. Then again, you’ve got your ocular hypertensive, in fact we discharge ours to community optometrists, but if you haven’t managed that service with your local optometry committee then you could argue very low risk glaucomas could be monitored quite long term with fields and IOP.
P005, Consultant, Focus Group 2
I think the patient… the people who we see in the hospital services are high risk that need to be seen, because I think the addition of… additional pathology, the very nature of the discussions we have to have with the patients that a home monitoring system isn’t ideal. However, for… even with our high-risk patients, I think again it comes down to how accurate the data is. This might help with anxiety for some patients who are always worried about what their pressures are doing and obviously you can’t see patients frequently, but if you get a baseline with this then patients being able to measure their own pressure at home a couple of… two or three times a week, it might ease that.
P004, Consultant, Focus Group 1
There were several statements suggesting that defining an ideal patient was perhaps not the right approach and that there should be flexibility afforded to individual services as to for whom and how home monitoring is used.
So I suppose each local… well it will have to be very individual to each place and how they work isn’t it as to who looks at the data and which group of patients, that kind of thing as well.
P016, Consultant, Focus Group 3
Some mentioned that age may be a deciding factor. Partly this was in relation to ability to use the devices, partly this was in relation to those considered at risk of the greatest QoL losses from losing visual function at younger age, and partly this was in relation to a subgroup of glaucoma patients who may still be working, and therefore for whom attending clinics for testing is perceived as being more burdensome.
I’m thinking of young normal tension glaucoma patients, so I mean 50. They’re the ones I worry about because they’ve got a lot of life to live and so they’ve got higher likelihood of going blind. And the normal tension glaucomas have trans-central scotomas, so they’re very sensitive. As soon as they lose one decibel that’s it, the whole vision is really bad, so those might be the ones.
P010, Consultant, Interview
Some clinicians felt that home monitoring may not be able to replace usual care for complex cases (e.g. multimorbidities) where information gathered from face-to-face observations is really important.
[T]he people who we see in the hospital services are high risk that need to be seen, because I think the addition of… additional pathology, the very nature of the discussions we have to have with the patients that a home monitoring system isn’t ideal.
P004, Consultant, Focus Group 1
Data relevance and integration
Distinct but complementary to concerns about data ‘ethicality’ was the intervention coherence concerns about the reliability and relevance of the data collected, and how these data will be integrated with electronic medical records. Those claiming to be less familiar with the evidence appeared sceptical that these technologies would produce reliable data to make meaningful decisions about patients’ care. There was disagreement about what aspects of visual health need to be measured; some agreed with both IOP and VF, others felt only IOP is required, others VF only and others worried about the lack of data in relation to other visual health domains, such as disc imaging and acuity, often measured in clinic. While it was agreed that data must be meaningful to clinicians (i.e. the measures being assessed are useful to make clinical judgements), there was little agreement regarding exactly what measures are relevant to make home monitoring meaningful, ‘[u]nless you can make the disc images cheaper and the pressures cheaper, then you can’t just rule out fields. I mean, fields itself are known as not very useful, if you catch my drift’ (P010, Consultant, Interview).
Some were so enthused by the evidence for glaucoma home monitoring that they were keen to see what future technology developments could achieve and made suggestions as to what else this home monitoring intervention could look to incorporate and overcome limitations from just assessing IOP and VF (data-related opportunities to improve for the future). For example, several clinicians discussed a contact lens device which can measure IOP or technologies permitting home disc imaging.
I was just wondering if when we’re talking about disc photographs, there are a few devices now that you can attach to smartphones to give you a disc photograph, I’m not sure would it be worth expanding to that you know, and you get your disc photograph.
P019, Consultant, Interview
Some participants reported being worried about how these data would integrate with existing medical records and any associated time required to input the data into existing systems. This links with perceived burden, discussed below. A solution put forward by many was to make the intervention software compatible with electronic patient records so that results can be immediately integrated without additional effort. A further solution proposed by some participants is the future use of AI for helping review the data as it comes in, making it quicker for clinicians to interpret the data collected remotely.
I’m pretty sure with artificial intelligence, we may be able to even define that what has changed has really change[d] or not, and then we can fast-track that… the results can be interpreted with the AI, I think I can see in five, ten years’ time, this is the way we do.
P001, Consultant, Interview
Support for patients
There was also significant importance placed upon the need to ensure the intervention is supportive for patients to address potential concerns. Participants felt many patients would require professional reassurance in understanding this intervention, particularly in terms of educating patients to understand that readings can fluctuate day to day and that it is the overall trend that the clinicians are concerned with rather than daily readings. Clinicians felt this would be important to prevent unnecessary worry.
You know, as long as they know that someone’s going to tell them I want a report of it or something, then all that hard work’s not for nothing, then I think that’s fine… then you could tell them that everyone’s pressure varies with… there’s a variation every day etc, and warn them not to worry about it, and the whole point is that we gather data over time so that we can make a judgement at the end of the day, so don’t worry about that. If you give them a clause, that will be fine….
P010, Consultant, Interview
Limiting access to results or reconsidering how results are presented to patients was one area of patient-related opportunities to improve in the future suggested by several participants. For example, more generic feedback, such as informing the patient that they had completed the tests correctly, was suggested to be a compromise to maintain engagement and prevent worry from results that may not be fully understood by the patient, causing unnecessary worry.
[W]e should just say in the app, ‘You have correctly answered 75% and you are among the top grade who have done the test very nicely’, or ‘You’ve done test reasonably, but it could be improved if you pay attention to these things’, or, ‘Your test needs to be repeated’. That is the only feedback going to the patient, and then we say, ‘Okay, now, you’ve done the test, it will be reviewed by the doctor… medical team, and we will come back to you.’ So that when we inform them that the situation is getting worse or the visual function is getting worse, we give them the solution there and then that, ‘Okay, we reviewed your results, your results shows deterioration, we have looked at your management plan, and we suggest you should be changing this, and after this you will be reviewed again by somebody.’
P001, Consultant, Interview
Another solution proposed is to offer a simpler intervention in the community for those struggling with the technology, for example facilities at shopping centres, etc.
This could even be done by a trained station in a supermarket, and we can just tell that person to go… you can’t do the technology for somebody, maybe there to help you guys, they don’t need to come to the hospital. They could go for their weekly shopping, and they go to a booth where there is one trained person who could have these appointments for these eighty, eight five… and those people who cannot cope with technology. And it is then outside the hospital, so we basically take it to the community in real sense.
P001, Consultant, Interview
In addition to clinician-provided support, participants felt this intervention would likely require utilisation of social support, making use of family and friends. For example, reminding patients to perform their assessments or physical support to use the devices.
[I]n the same way that we’re quite content to have, a lot of our elderly patients have their partner put their eye drops in, well it can be a team effort. Sometimes elderly people cope rather better when, ‘Oh, yes, my husband always remembers to do this’, who locks the door, there’s a certain way, people survive together don’t they and I just wonder whether there’s something in that for some of our patients. It goes beyond this technology really, but yes, you’d probably need to involve the younger generation.
P002, Optometrist, Focus Group 1
Anticipated costs
Beliefs about cost-effectiveness
Two clinicians reported that they believed the proposed intervention would be good value for money.
Well, it could potentially reduce the cost. If you’re not having to bring patients in, you don’t require transports, you won’t need technicians to gather the data of the patients as they can gather the data themselves. It would be an actual virtual data collection, and fewer staff costs.
P018, Consultant, Interview
Several clinicians specifically stated that this approach is not good value for money, and many were concerned about the cost of the equipment, particularly the home tonometer. Additional costs raised were maintenance of this equipment, having spares in case of damages, and the staffing to train patients and review data.
[W]e could be looking at wasting our resources looking after too much data points from patients who are well and were not really at risk of going blind with their glaucoma and are we diverting our resources be it instrumentation, time looking at the data and collecting the data which could be used more effectively for other patients.
P017, Consultant, Focus Group 3
I think I prefer something which would be a bit even easier than Home iCare tonometer at the moment, and perhaps less expensive as well, because we give it to such a big population and if you can just give it to one person they will take it for a week, so just calculate for one person the population how many Home Care eye tonometer you need, and then if you extrapolate that cost and the overall benefit you are going to get from that, I’m not convinced that it is for everyone.
P001, Consultant, Interview
Some felt this would make it difficult to obtain management or clinical commissioners’ support for such a service.
I think the distribution and the (inaudible) the devices and persuading the health and social care service that we want to spend thousands of pounds on purchasing these things, I think that could potentially be an issue.
P019, Consultant, Interview
Adjustments to address affordability
However, several clinicians perceived that it could be made more affordable, firstly by looking at app services and dropping the iCare tonometer or asking patients to purchase their own equipment. One clinician felt that the costs would reduce as the technology improved and became more mainstream.
Yeah, that’s where your visual field app may be more cost effective in terms of economics and access to the piece of equipment that the patient has to take home, perhaps an app or something that they can download on to a tablet with a licence, it may be much more generalisable than to take home an iCare HOME.
P009, Consultant, Focus Group 2
Burden
Burden was discussed in relation to the clinicians themselves, the services they operate within and upon patients.
Clinician burden
Burden upon clinicians was perceived to stem from several aspects, most commonly from concerns about finding the time to manage services and review and action patient data.
[S]o you’ve got all of these other notes and scans and things to look at from virtual clinics and I think this will add to it, even though these patients aren’t clogging up your clinic they are sending you lots of data which you need to be on top of, and if you don’t action it then they’re going to miss out anyway. So for example, if someone’s in trouble you need to be able to action that and find a space in your clinic for them.
P011, Consultant, Interview
There were also concerns about the potential to increase patient caseload and unscheduled care. This was mostly in relation to spurious results and anxious patients making contact concerned about their results. One clinician raised the burden of adapting to change and learning new skills, likening it to when electronic medical records were introduced, and discussing the time and effort it took to learn a new way of working.
But I suspect what will happen, potentially, is that as the numbers go up, we’re able to deal with more patients and you’ll just end up with a bigger workload, more patients under your care, I guess.
P018, Consultant, Interview
Conversely to these many concerns, one clinician felt that there would not be additional burden from this service, but instead a change in burden, away from the footfall of patients towards an increase in administrative burden, ‘It’ll lighten the burden in terms of footfall perhaps, but it increases the burden in terms of additional or admin work that you have to do’ (P011, Consultant, Interview).
Several clinicians discussed burden more in terms of its impact upon services rather than themselves as individuals. This was often qualified with statements such as ‘I’d have to delegate’ as clinicians felt they did not have time to take on these roles. Tasks to be delegated included maintaining and distributing equipment, training patients, and reviewing data.
Then you have to find the people that are suitable and then you have to train someone who’s able to train the patients on how to use it and someone to distribute the devices, to maintain them, to chase them up when they’re not brought back, to clean them, and then someone to look at the information. So probably quite a lot of work actually.
P019, Consultant, Interview
Patient burden
Several references were made in relation to burden upon patients. While many felt this service could reduce patient burden (linked to and discussed in Intervention coherence), there was some concern about the psychological burden patients may experience, particularly in relation to those who may worry about their results (see Perceived effectiveness). There was also a suggestion about the burden of additional tasks this would involve for the patient, such as collecting equipment from the clinic. One clinician wondered whether, by the time patients collect equipment and learn how to use it, they could have just had their tests done in the clinic, ‘So you’re kind of thinking if they have to come and pick up an iCare [tonometer] you might as well do it’ (P019, Consultant, Interview).
Perceived effectiveness
Anticipated outcomes
In line with different perceptions of the purpose of home monitoring, several expected outcomes were reported: improved management from increased data collected, detecting progression quicker and preventing sight loss, either directly from the monitoring patients or indirectly as home monitoring allowed clinicians to spend more time with high-risk patients in clinic. Several discussed how this intervention would be more convenient and less stressful and burdensome for patients. However, one clinician felt that some patients would demand in-clinic services regardless.
Balancing benefits and harms
There was mixed certainty across the clinicians’ accounts as to the degree of clinical effectiveness that could be achieved. Some clinicians were confident that patients would benefit from this intervention. However, a number of clinicians focused upon the potential unintended consequence of increased patient anxiety arising from monitoring. The impact of this was unclear, but clinicians were concerned that rather than empowering patients with responsibility and knowledge, it could add psychological burden. Some were concerned that any benefits in terms of clinic capacity could be eliminated by increase in patient contacts from the ‘worried well’ or ‘unreliable data’. Statements about expected outcomes were often tempered with ‘potentially’ reflecting uncertainty.
This might help with anxiety for some patients who are always worried about what their pressures are doing and obviously you can’t see patients frequently, but if you get a baseline with this then patients being able to measure their own pressure at home a couple of… two or three times a week it might ease that. But at the same time sometimes it can make it worse (over speaking) get phone calls unnecessarily coming back to you so it’s going to be a double-edged sword, I think.
P004, Consultant, Focus Group 1
A number of additional factors which could enhance or limit effectiveness were raised, falling under categories of data and technology limitations, patient demographics and health, and service support and culture.
Impact of perceived data and technology limitations upon intervention effectiveness
An overarching theme was doubt as to whether current technologies are reliable enough to home monitor safely and effectively. Specifically, many clinicians were not convinced that they or their patients would find these technologies acceptable, adversely impacting effectiveness through low engagement.
I still don’t know how reliable these technologies are at the moment so I can’t really comment whether we are going to have meaningful data from this at the moment, certainly with the visual field at home I don’t know personally, yeah… So I think yes, certainly there is a role for this in future but I think there is still a way to go before it comes common practice based mainly on the technology.
P017, Consultant, Focus Group 3
We have to be clear about how meaningful it is, they’re measuring visual function as has been suggested in patients who’ve got a range of other conditions, and clinicians as I say can disagree on routine perimetry. So I’m going to take some persuading, I feel, that the visual function measures are meaningful and valid for glaucoma care personally. That doesn’t mean I’m saying that… I just feel that the jury’s out and we need that data to be confident.
P002, Optometrist, Focus Group 1
Patient characteristics impacting intervention effectiveness
Several patient demographic and health characteristics were stated to have potential impact upon how effective these home monitoring interventions could be. Clinicians were concerned about the physical and cognitive abilities required for this intervention, fearing that a substantial proportion of the glaucoma population would either be excluded from this intervention or would find it unacceptable and would not engage in the first place. This would limit its impact.
Yeah, they have to have the manual dexterity for it as well. So I suppose that will exclude a few people from the clinic able to do that, particularly for the elderly patients.
P019, Consultant, Interview
There are groups of people who are frail, vulnerable, who are serial DNA’ers [Do Not Attend] or whatever and they might be tricky to persuade to do these home-based tests….
P002, Optometrist, Focus Group 1
Impact of service levels factors on effectiveness
There were also several references to effectiveness being related to wider service issues, such as support from NHS Trusts to staff such a service appropriately and the culture of the work environment. One clinician discussed how adoption of this intervention would likely be gradual, requiring time for people to learn and adapt.
So certainly there’ll be a learning curve and I’m guessing after the learning curve people might abandon in it or… like in anything that’s implemented. Certainly like electronic patient records, people hated them to start with. I think now we’re in a situation I really can’t bear to look at paper notes, but it did take us a while.
P016, Consultant, Focus Group 3
Self-efficacy
Low self-confidence and confidence in patients to use technology
There were fewer statements reported in relation to self-efficacy, likely due to the hypothetical nature of the interviews in relation to the use of these interventions. Clinicians were generally not confident about delivering these home monitoring interventions. Confidence concerns were often related to low confidence in the technologies and doubt regarding service support.
. . . have to say our Trust is very… anything would need a lot of planning and a lot of business plans and those kind of things. I don’t know whether it’s just my Trust, but I’ve certainly seen making anything happen in this Trust is really hard.
P016, Consultant, Focus Group 3
I’d have to say without having all the data, the validity data to hand I’d have to say not very at the moment.
P004, Consultant, Focus Group 1
Several clinicians discussed concerns about the self-efficacy of patients to participate in this intervention. Several aspects were perceived to be quite challenging for patients’ confidence, including worries about using the equipment, risks of damaging equipment, and some patients really struggling with other aspects of eye care.
I don’t know how much an iCare HOME costs, but I can imagine that there’d be some patients who’d be frightened of breaking it or damaging it and then having to kind of pay for it.
P018, Consultant, Interview
I’m really quite select(?) who I give it to because it’s amazing actually how many people just don’t like anything to do with their eyes and it’s all they can do to put their eye drop in.
P019, Consultant, Interview
Impact of COVID-19 pandemic
While not a TFA construct, the coronavirus disease discovered in 2019 (COVID-19) pandemic was mentioned frequently by clinicians, perhaps unsurprisingly, and so is important to report. Contextually, clinicians discussed their current service situation, lengthy waiting lists of patients to be seen in clinic, and staffing shortages. They also spoke of how this intervention would have been helpful during the pandemic when clinicians were encouraged to see as many patients as possible remotely, over the phone. These statements link with the enthusiastic affective attitude described earlier and overlap with the issue of burden. While there were concerns about additional burden resulting from this intervention, there was also support for something which could alleviate the current burden upon health services from lengthy waiting lists due to the COVID-19 pandemic, ‘if anything, COVID has shown us that we cannot manage glaucoma virtually with the current equipment that we have, and if we can do that then we would be able to increase the numbers that we could process’ (P018, Consultant, Interview).
For some, COVID-19 triggered a need to explore new ways of working, and this intervention could be one solution. It has also provided reassurance for some that gaps between monitoring in clinic were not resulting in significant visual loss as would have been anticipated prior to the pandemic, ‘I agree that in COVID when you see some of the reviews that are delayed it’s amazing how many people don’t actually go bad over what we would consider quite a long gap in their coming to the hospital’ (P009, Consultant, Focus Group 2).
However, there were concerns about resourcing this post COVID.
Yeah, I think if you’d asked me that question two years ago I think it would’ve been implementable, but right now with COVID recovery our services are really, really stretched so trying to bring in a new thing is difficult.
P008, Consultant, Focus Group 2
Chapter summary
Our findings from the clinicians suggest there is cautious optimism – they are interested and enthused by the potential of glaucoma home monitoring, but there are several areas of concern that need to be addressed before they would feel reassured to buy-in to this approach. They believe home monitoring could meet an existing clinical need, addressing the present difficulties (current imbalance in capacity/demand) by monitoring an increasing glaucoma population. They can see the potential patient and service benefits, but they require reassurances about the technologies and the implementation of such a service into routine care.
Contextually, the influence of the COVID-19 pandemic was evident: clinicians’ experiences throughout the pandemic have prompted a need to adapt and change the way they monitor glaucoma patients – it highlighted that non-clinic monitoring is possible and, in many ways, can be done safely – and the current care backlog post COVID-19 is driving enthusiasm for solutions. Another seemingly important contextual factor was hands-on experience with the technologies. As noted in the results, while three participants had some experience with the iCare HOME tonometer, most experiences of handheld tonometry were in relation to in-clinic use. The context of these experiences was somewhat different from the model proposed by the I-TRAC study. Experiences involved collecting patient IOP with the iCare tonometers in the clinic and this was undertaken by a trained member of staff, not undertaken by the patient nor in the patient’s home environment. Experience in the clinic led to doubts about the accuracy of these devices through experienced in-clinic differences between the measures produced by the iCare tonometer and Goldmann. This led to concerns that this difference in measures may widen when used by patients at home, a current evidence gap that needs to be addressed.
Chapter 4 Patient participant and site staff perspectives on acceptability and feasibility of digital technology for home monitoring glaucoma
This chapter presents findings from the mixed-methods intervention-focused component of the study. This phase investigated the use of home monitoring technologies across three sites and explored patients’ (herein referred to as patient participants) and site staff’s perspectives of acceptability and feasibility of the use of digital technologies for glaucoma home monitoring. The analysis focused on how patient participants and site staff perceived the intervention, how they engaged with the interventions and study processes, and reporting of aspects of study design and/or delivery that would require amendment for a future clinical trial. Two main frameworks were used to guide this phase of the study: the TDF, which was used to investigate patient participant behaviour in relation to the home monitoring technology and the ADePT guidance to identify challenges for a future large-scale evaluation.
Methods
Study design
A mixed-methods design involving concurrent quantitative and qualitative data collection and analysis.
Setting
Three secondary care ophthalmology glaucoma clinics across the UK (one each within Scotland, England and Northern Ireland). All sites host both a face-to-face glaucoma clinic and a virtual glaucoma clinic, both of which include a mix of patients with mild to moderate glaucoma.
Intervention
Patient participants were asked to use two home monitoring technologies to measure IOP and visual function (through a contrast sensitivity assessment) on a weekly basis. In the original project plan, the duration of monitoring was planned for 16 weeks; however, due to delays in opening sites [linked to a change in the app-based technology and delays to research and development (R&D) approval], the monitoring period was reduced to 12 weeks for all participants in order to deliver all project activities.
Participants were provided with an iCare HOME 2 handheld tonometer to measure IOP. This device is CE marked and approved for use in the UK. Chapter 1 provides further technical and clinical details about the tonometer. The devices had batteries inserted by site staff. To use the device, participants had to attach a new probe for each measurement. Participants were provided with a surplus of probes for the required duration of monitoring to allow for damage or compromised sterility. Participants were required to hold the device steady in a specified position (by lining themselves up with a fixation target on the device) and push a button to complete measurement.
The original project plan proposed to measure VF using home monitoring technology. The MRF has been evaluated longitudinally in a cohort of patients with glaucoma to measure VFs. Several peer-reviewed publications have reported that the MRF is reliable and has shown strong agreement with standard automated perimetry. This was the preferred and planned intervention for I-TRAC. However, during R&D approvals, one of the study sites identified that the MRF was not CE marked [it has now obtained UK Conformity Assessed (UKCA) marking] at the time. Following discussions with the Medicines and Healthcare products Regulatory Agency (MHRA), the funder, and the Study Steering Committee (SSC), it was agreed that MRF was not a viable option. As such, a decision was made by the I-TRAC study team (supported by the SSC and funder) for the MRF to be replaced with the OKKO Visual Health app. An evidence scan of existing app-based VF or visual function apps was conducted. A total of 15 alternative apps were identified but only one had a CE mark at the time (OKKO Visual Health app), which was also the only app to measure visual function rather than VF (i.e. not a perimeter) (see Appendix 3). The OKKO Visual Health app has been designed to measure visual function on portable devices, such as smartphones, tablets, and iPads. Developed using video game technologies, it tests several aspects of visual function (e.g. visual acuity, contrast sensitivity) using interactive games which are designed to be entertaining and engaging for the participant, so as to increase user adherence. Data regarding visual indicators (in our case, contrast sensitivity) are stored on the OKKO Health portal. It is UKCA marked and approved for use in the UK. However, there are currently no published studies evaluating OKKO app reliability and/or compliance, and it had not been designed as a glaucoma test. The I-TRAC study seeks to explore feasibility through the lens of ‘will patients use and adhere to these devices’ rather than addressing clinical effectiveness. The OKKO app, like the MRF, required patient participants to complete a task on a tablet that measures an aspect of visual function and as such could be used to determine feasibility of home monitoring of glaucoma using a different technology and platform, for example a tablet. The app was installed onto an iPad (by University of Aberdeen IT support staff) and provided to the participant by the site staff. The iPad was managed using remote software and limited to use the OKKO app only (i.e. all other functions and features were switched off with the exception of the power on/off button and WiFi/4G connections).
Participants received a prompt each week to remind them to use their home monitoring equipment; they could opt between receiving e-mail or text message electronic reminders.
Site staff and patient participant training in intervention use
Site staff received training from Mainline, the UK distributor of iCare HOME 2 tonometers, as to how to use and teach patient participants to use the home tonometers. This training was in person and delivered on site to each site individually. The study Research Fellow (CS) was given a demonstration of the OKKO Health app (by the OKKO Chief Technology Officer) and then instructed site staff verbally during an online site initiation visit, where site staff were encouraged to use the app on the iPad as CS instructed them on its use. An electronic training manual was also produced as a visual aid for site staff, using a combination of text and screenshots to demonstrate how to use the tonometer and the OKKO app on the iPad.
Site staff delivered the training on how to use both devices to the patient participants in the clinic setting. This was delivered immediately after consent and baseline data collection. We standardised patient participant training through production and provision of a site staff manual detailing each stage of the training process. Training was predicted to take 30–45 minutes to complete per patient participant.
Report Supplementary Material 1 presents a completed TIDieR reporting checklist for the digital home monitoring intervention.
Sample size
We aimed to recruit 45 patient participants (15 from each site) across three cohorts for the home monitoring intervention. The sample size of 45 was in line with previously proposed sample sizes of between 24 and 50 for feasibility/pilot studies. 47,48 We planned to recruit in three cohorts so that each site would recruit five participants at any time, and once the home monitoring period was complete, the next cohort of five participants would be recruited.
For the interviews with I-TRAC patient participants, a purposive sample (identified at the end of the 3-month monitoring) was selected based on site, age, gender and adherence levels (adherers defined as completing ≥ 80% of home monitoring sessions). We also aimed to recruit a convenience sample of around 10 research site staff to participate in focus groups to discuss their experiences of conducting the I-TRAC study. This sample was to include PIs, Research Nurses and other trained members of research staff involved in running this study. A sample of 10 participants was proposed based on the principles of information power. 49 This was deemed appropriate given the aim was relatively narrow, with highly specified participants in relation to the aim, informed (through collection and analysis) by a theoretical framework, with anticipated good-quality dialogue combined with the exploratory nature of this analysis.
Sampling and recruitment
Patient participants were identified from clinical caseload lists in the three recruiting centres, referred to as site 1, site 2, and site 3 to protect identity. Research Nurses, Consultants, and other suitably qualified persons at each site identified potentially eligible patient participants to be recruited into I-TRAC and participate in the home monitoring. All patient participants were approached at their regular scheduled glaucoma follow-up appointment within the clinic. Recruitment approaches varied across sites. Patient participant selection criteria were broad, informed by the findings from the survey in Chapter 2 which were unable to confirm clinical parameters for suitable home monitoring candidates. Therefore, our eligibility criteria were any patients with glaucoma who were being treated at one of the three NHS sites, with exclusions based on an inability to provide consent or understand English. One of the sites had a substantial delay in obtaining R&D approval and thus recruitment start was delayed.
Patient participants who had completed their 3-month monitoring period from recruitment cohorts 1 and 2 and had consented (at baseline) to be contacted for an interview were screened according to the sampling criteria. Participants were then contacted by the I-TRAC Research Fellow on their preferred contact e-mail or phone number and invited to participate in an interview. If in agreement, an appropriate time for a phone or online interview was scheduled.
All site staff involved in the patient-facing delivery of I-TRAC were e-mailed an invitation to participate in a focus group or interview and asked to indicate a date and time from a selection of three proposed.
Consent
For both the home monitoring study and the interviews, patient participants were provided with an information sheet at least 24 hours prior to providing consent. All patient participants in the home monitoring study provided written informed consent, which was sought by site staff trained in good clinical practice. Consent for both the home monitoring and involvement in follow-up interview was sought at baseline. Consent for those patient participants who agreed to be interviewed was then reconfirmed verbally, by the Research Fellow, immediately prior to the interview.
Site staff were sent an information sheet about the purpose of the discussion along with the e-mail invitation and at least 3 weeks prior to the first discussion group. Site staff provided written informed consent in advance of the interview and consent was reconfirmed by the Research Fellow at the start of the focus group/interview.
Procedures for patient home monitoring
Upon confirming their interest in the study, site staff arranged a time for the patient participant to attend the clinic to provide consent, collect baseline data, receive the training on how to use both devices, and receive the equipment ready to commence home monitoring. After being trained and issued with both devices, participants were provided with training manuals which included contact details of the site staff and Research Fellow. Patient participants were then contacted towards the end of their home monitoring period to arrange a time to return to the clinic for follow-up data collection and to return the equipment.
Data collection
Home monitoring participants
Baseline case report forms collected demographic information (age, gender, ethnicity, employment status), current use of technologies (smartphones, tablets/iPads, laptops), preference for text or e-mail reminders, clinical information about their glaucoma (e.g. glaucoma type and severity, treatment, surgeries), and baseline IOP and VF measurements. Data were collected by a trained member of site staff through participant report (demographics and technology use) and clinical information recorded in medical records. IOP and VF tests were only performed if they had not been conducted in clinic (with results available in medical records) within the last 3 months.
Site staff collected the follow-up case report form data on IOP and VF measurements conducted at follow-up and information on any changes in treatment. Data were also collected by site staff through participant report relating to satisfaction with the home monitoring intervention, satisfaction with training received, and overall preference between home and clinic monitoring. Participants were also asked to complete questions covering health resource use during the home monitoring period, which asked about their actual use of health services during that time, and if they considered contacting/using health services, to measure whether home monitoring led to any change in use of health services. Again, data were collected verbally from the participant by a trained member of site staff. IOP and VF tests were performed by a trained member of site staff at the follow-up visit.
Data were downloaded from the tonometer device via USB cable to dedicated software (iCare LINK) when the device was returned at the follow-up assessment. The data were transferred as an Excel sheet (.csv file) and added to the secure study database. The OKKO app data were stored on the OKKO Health portal and provided to the research team on request.
Site staff were asked to record any contact they had from the participant throughout the home monitoring period. Data collected included: date, method of communication, a brief description of the reason for the contact, and the approximate time the site staff member spent to resolve this issue.
Interviews
Data collection for the interviews with patient participants recruited to the home monitoring study was informed by a topic guide with questions framed around the constructs of the TDF. The TDF is an established behavioural framework that integrates 33 theories of behaviour into 14 domains that inhibit or enable behaviour (Knowledge, Skills, Social/Professional Role and Identity, Beliefs about Capabilities, Beliefs about Consequences, Optimism, Reinforcement, Intentions, Goals, Memory/Attention/Decision-making Processes, Environmental Context and Resources, Social Influences, Emotion, Behavioural Regulation). 50 For the purposes of I-TRAC, the target behaviour of interest was broad in its definition and was considered as use of the digital technologies for monitoring their glaucoma at home. Interviews were conducted by the Research Fellow over the phone.
Site staff focus groups and interviews were informed by a topic guide framed around key questions from the ADePT framework to assess pragmatics of running the study and the feasibility of progressing this study to a full-size trial. 51 Focus groups and interviews were conducted via MS Teams.
All focus groups and interviews were recorded for verbatim transcription by an external transcription company.
Data analysis
Data on participant demographics, clinical descriptors of disease status and measurements, and data relating to satisfaction and preferences for future were summarised using mean [standard deviation (SD)] or median [interquartile range (IQR)] for continuous variables. Categorical variables were summarised with numbers and percentages.
Home monitoring
Frequency of patient participant contacts throughout the study period (for assessing resource impact) was collected via staff self-report and presented using frequencies. Adherence to intervention was calculated for each participant and for each device. This was calculated based on the number of weekly measurements they performed over the 12-week monitoring period. The start of the monitoring period was classified as the date the participant was due to perform their first measure plus each week for the subsequent 11 weeks (12 measures in total). A reported measurement was considered adherent to schedule if it was conducted ± 3 days of the scheduled measure. Overall adherence for each device was calculated as a percentage based on the number of measurements adherent to schedule, divided by 12 (total number of possible measures). Those scoring ≥ 80% were considered ‘adherers’ to the device. Combined adherence scores were categorised as ‘adherent’ based on ≥ 80% adherence to both devices individually with all other variations (i.e. adherence to only one device or to neither) classified as non-adherent or combined adherence.
Participant contact records were collated by the Research Fellow and the total number of contacts, mode of contact, reason for contact, and total time spent by site staff resolving any questions, for each participant, summarised.
Interviews
Interviews with patient participants were analysed using the TDF. A coding guide was developed to describe relevant data to be coded under each domain (see Appendix 4). Each transcript was reviewed for data relevant to any of the TDF codes but also concurrently for any utterances relevant to patient experience of glaucoma home monitoring but that did not fit TDF domains. Three members of the research team (KG, TC, CS) reviewed and double coded transcripts (KG n = 2, TC and CS n = 3). Coders met to review coding decisions with any disagreements resolved through discussion with an independent TDF expert for arbitrating unresolved disagreements. The coding guide was updated to reflect these discussions and then applied to the remaining transcripts.
Site staff focus groups and interviews were analysed using the items from the ADePT framework to help directly inform decisions about progression to full trial. A coding framework for the ADePT items was developed to describe the relevant data to be coded under each ADePT item (see Appendix 5). A second member of the research team checked coding of two of the four transcripts and disagreements were resolved through discussion between the two coders (CS and KG). Data under each ADePT item were reviewed to identify themes, subthemes and relationships between these. Analysis was further refined during writing with both coders reviewing and agreeing the final thematic framework.
Any changes from inception to the design of this phase of the research (and linked phases) are reported in Appendix 6.
Results
Home monitoring
Sample characteristics
Across the 3 sites, 42 patient participants were recruited. Differences in recruitment approaches were apparent in the screening logs received from each site. In sites 1 and 2, one strategy used was to prescreen clinic lists in advance to identify potentially suitable participants for the clinician to discuss with the patient at their clinic appointment. Another strategy involved the Research Nurses/other suitably qualified persons approaching patients in the clinic waiting area. Due to delays in opening and the requirement for time-efficient recruitment, site 3 purposively selected glaucoma patients who were known to the research team as being research active (e.g. having participated in one or more previous research studies). With regard to participation rates, for site 1, all patients approached by the PI at their clinic appointment agreed to participate in the study. For site 3, all patients approached by Research Optometrists agreed to participate. For site 2, where the Research Assistant approached patients waiting in clinic waiting rooms, the participation rate was 16% (18/116). While most did not provide a reason for declining participation, of those who did, 13 patients declined due to a reported lack of confidence with technologies, 10 due to a lack of interest in technologies, 5 due to the travel requirements to attend additional clinic visits and 4 due to concerns about burden related to multiple comorbidities. One person declined due to disagreement with the concept of home monitoring and another declined due to glaucoma testing being anxiety provoking.
Figure 2 provides an overview of participant recruitment and retention.
FIGURE 2.
In-home Tracking of glaucoma: Reliability, Acceptability, and Cost participant flow chart.

The patient participant-level characteristics shown in Table 8 indicate a mean age of 67 years in our cohort; the majority were white, and there was equal gender representation. The majority were familiar with some form of electronic device.
| Baseline characteristics | N = 42 (%) | |
|---|---|---|
| Age (years) | Median (IQR) | 69.0 (59.0–76.0) |
| (min, max) | (31.0, 86.0) | |
| Sex | Male | 20 (48) |
| Female | 22 (52) | |
| Ethnicity | White | 39 (94) |
| Asian | 1 (2) | |
| Caribbean | 1 (2) | |
| Missing | 1 (2) | |
| Current employment status | Full-time employment | 9 (21) |
| Part-time employment | 6 (14) | |
| Homemaker/carer | 1 (2) | |
| Retired | 25 (60) | |
| Missing | 1 (2) | |
| Use of glasses for distance | Yes | 33 (79) |
| No | 9 (21) | |
| Use of reading glasses or varifocals | Yes | 36 (86) |
| No | 6 (14) | |
| Use of laptop/PC | Regular | 24 (57) |
| Occasional | 9 (21) | |
| Missing | 9 (21) | |
| Use of smartphone | Regular | 34 (81) |
| Occasional | 5 (12) | |
| Missing | 3 (7) | |
| Use of tablet/iPad | Regular | 24 (57) |
| Occasional | 8 (19) | |
| Missing | 10 (24) | |
| No devices used | 2 (5) |
Baseline clinical characteristics
Median baseline IOP using GAT was 17 mmHg (IQR 12–20) and 16 mmHg (IQR 12–20) in right and left eyes respectively. Among the 42 patient participants, 26 (62%) had glaucoma in the right eye and 30 (71%) had glaucoma in the left eye. Other diagnoses are summarised by eye in Table 9.
| N (%) | ||
|---|---|---|
| IOP (mmHg): right | Mean (SD); N | 16.5 (5.3); N = 42 |
| Median (IQR) | 17.0 (12.0–20.0) | |
| (min, max) | (4.0, 33.0) | |
| IOP (mmHg): left | Mean (SD); N | 16.3 (5.2); N = 42 |
| Median (IQR) | 16.0 (12.0–20.0) | |
| (min, max) | (8.0, 29.0) | |
| Visual field test (dB): right | Mean (SD); N | −3.4 (4.4); N = 41 |
| Median (IQR) | −1.8 (−5.0 to −0.1) | |
| (min, max) | (−15.2, 1.2) | |
| Visual field test (dB): left | Mean (SD); N | −5.9 (7.2); N = 42 |
| Median (IQR) | −3.8 (−8.4 to −1.1) | |
| (min, max) | (−30.5, 0.8) | |
| Reliability of visual field test: right | Yes | 40 (95) |
| No | 1 (2) | |
| Missing | 1 (2) | |
| Reliability of visual field test: left | Yes | 41 (98) |
| No | 1 (2) | |
| Current glaucoma treatment: right | Prostaglandin | 17 (40) |
| Beta-blocker | 3 (7) | |
| Topical CAI | 7 (17) | |
| α2 agonist | 2 (5) | |
| Combination of prostaglandin and beta-blocker | 5 (12) | |
| Combination of beta-blocker and topical CAI | 5 (12) | |
| Combination of topical CAI and α2 agonist | 1 (2) | |
| Other treatment | 1 (2) | |
| Current glaucoma treatment: left | Prostaglandin | 20 (48) |
| Beta-blocker | 4 (10) | |
| Topical CAI | 8 (19) | |
| α2 agonist | 1 (2) | |
| Combination of prostaglandin and beta-blocker | 6 (14) | |
| Combination of beta-blocker and topical CAI | 4 (10) | |
| Combination of topical CAI and α2 agonist | 2 (5) | |
| Other treatment | 1 (2) | |
| Past history of eye surgery: right | Laser trabeculoplasty | 9 (21) |
| Trabeculectomy | 4 (10) | |
| Other | 12 (29) | |
| Past history of eye surgery: left | Laser trabeculoplasty | 10 (24) |
| Trabeculectomy | 5 (12) | |
| Other | 15 (36) | |
| Diagnosis: right | Glaucoma | 26 (62) |
| Disc suspect | 3 (7) | |
| VF suspect | 1 (2) | |
| OHT (normal disc and field) | 6 (14) | |
| PAC suspect (normal disc and field) | 1 (2) | |
| Missing | 5 (12) | |
| Diagnosis: left | Glaucoma | 30 (71) |
| Disc suspect | 1 (2) | |
| VF suspect | 1 (2) | |
| OHT (normal disc and field) | 5 (12) | |
| PAC (normal disc and field) | 1 (2) | |
| PAC suspect (normal disc and field) | 2 (5) | |
| No glaucoma-related | 1 (2) | |
| Missing | 1 (2) | |
| Severity of glaucoma: right | Mild | 15 (36) |
| Moderate | 8 (19) | |
| Severe | 3 (7) | |
| Missing | 16 (38) | |
| Severity of glaucoma: left | Mild | 20 (48) |
| Moderate | 4 (10) | |
| Severe | 7 (17) | |
| Missing | 11 (26) | |
| For glaucoma and suspects: right | Open-angle | 24 (57) |
| Angle closure | 4 (10) | |
| Other | 3 (7) | |
| Missing | 11 (26) | |
| For glaucoma and suspects: left | Open-angle | 26 (62) |
| Angle closure | 5 (12) | |
| Other | 3 (7) | |
| Missing | 8 (19) | |
| Comorbidity: right | Cataract | 5 (12) |
| Other | 3 (7) | |
| Comorbidity: left | Age-related macular degeneration | 1 (2) |
| Cataract | 4 (10) | |
| Other | 3 (7) |
Median baseline VF mean deviation (MD) was −1.8 dB and −3.8 dB in right and left eyes. Median MD in the worse eye (by baseline MD) was −4.65 dB (IQR −10.2 to −1.5, range −30.5 to 0.75). Median MD in the better eye was −1.3 dB (IQR −4.2 to −0.1, range −30.5 to 1.2). The patient participant with a MD of −30.5 dB had VF data entered for only one eye. No other patient participants had VF data entered for only one eye. For almost all patient participants the VF test was deemed reliable by the clinicians (for 40 of 41 tests and 41 of 42 tests in right and left eyes, respectively).
Baseline topical IOP-lowering medications are summarised in Table 9. Out of 42 patient participants, 11 (26.2%) were not using regular eye drops for glaucoma; 9 patient participants had previously undergone laser trabeculoplasty in their right eye and 10 in their left eye; 16 patient participants had had previous surgery to their right eye and 20 previous surgery to their left eye. The majority of patient participants had a diagnosis of glaucoma (26 right eyes and 30 left eyes), but patient participants with suspected glaucoma, OHT and primary angle closure or primary angle suspect were also included in the study (Table 9). A total of 34 patient participants had glaucoma in at least 1 eye.
The majority of patients had mild to moderate glaucoma severity. The VF MD values also allow severity of glaucomatous VF loss to be determined. In all, 23 of 42 patient participants had mild to moderate glaucoma in their right eye and 24 mild to moderate glaucoma in the left eye. Ocular comorbidities including cataract and age-related macular degeneration were present in eight right eyes and eight left eyes.
Table 10 shows the clinical data obtained at the 3-month follow-up visit. Median Goldmann IOP was 17.0 mmHg in the right eye (IQR 13.5–19.0) and 17.0 mmHg in the left eye (IQR 13.0–19.5). VF MD was −2.0 dB (IQR −4.8 to −0.6dB) and −3.2 dB (IQR −7.3 to −1.2) in right and left eyes, respectively. Only 2 of 42 patient participants had a change in glaucoma treatment instigated by their clinician while participating in the study.
| N (%) | ||
|---|---|---|
| IOP (mmHg): right | Mean (SD); N | 16.2 (5.0); N = 40 |
| Median (IQR) | 17.0 (13.5–19.0) | |
| (min, max) | (3.0, 27.0) | |
| IOP (mmHg): left | Mean (SD); N | 16.4 (4.2); N = 40 |
| Median (IQR) | 17.0 (13.0–19.5) | |
| (min, max) | (8.0, 24.0) | |
| Visual field test (dB): right | Mean (SD); N | −3.3 (4.0); N = 37 |
| Median (IQR) | −2.0 (−4.8 to −0.6) | |
| (min, max) | (−14.9, 1.4) | |
| Visual field test (dB): left | Mean (SD); N | −5.1 (5.9); N = 38 |
| Median (IQR) | −3.2 (−7.3 to −1.2) | |
| (min, max) | (−24.4, 0.9) | |
| Reliability of visual field test: right | Yes | 34 (81) |
| No | 3 (7) | |
| Missing | 5 (12) | |
| Reliability of visual field test: left | Yes | 37 (88) |
| No | 1 (2) | |
| Missing | 4 (10) | |
| Change in glaucoma treatment | Yes | 2 (5) |
| No | 38 (90) | |
| Missing | 2 (5) |
Three patient participants withdrew from the study during the monitoring period, two from site 2 and one from site 1. One was due to health reasons (unrelated to glaucoma) and two were due to difficulties with the technology, with one withdrawing from the home monitoring intervention only and completing follow-up data collection and the post-intervention interview.
Adherence to intervention
Adherence to the tonometer alone was 67% (n = 28) compared to 60% (n = 25) of participants being adherent to the OKKO app (Table 11). Overall adherence to both devices (i.e. ≥ 80% adherence to both devices) was 55% (n = 23), with 31% (n = 13) considered as non-adherent to both devices and 14% adherent to only one device.
| Measure | N = 42 | |
|---|---|---|
| iCare tonometer | Adherence ≥ 80% | 28 (67) |
| Mean overall % adherence (SD) | 74% (34) | |
| Mean number of recorded measures (out of 12) per participant (SD) | 9 (4) | |
| Median | 11 | |
| OKKO app | Adherence ≥ 80% | 26 (62) |
| Mean overall % adherence (SD) | 72% (34) | |
| Mean number of recorded measures (out of 12) per participant (SD) | 9 (4) | |
| Median | 10 | |
| Combined | Both ≥ 80% (‘adherent’) | 23 (55) |
| iCare only (‘non-adherent’) | 4 (9) | |
| OKKO only (‘non-adherent’) | 2 (5) | |
| Neither (‘non-adherent’) | 13 (31) |
Additional patient participant contact with site
Of the 42 participants, 20 (48%) contacted their respective study site during their home monitoring period, averaging 1.7 contacts per participant who required support, and were spread across sites (Table 12). In total, 503 minutes (average of 15 minutes per contact) were estimated by site staff as being required to answer or resolve issues. The most common reasons for contact were problems with usernames and passwords for the OKKO app (n = 5), and patient participants having difficulties using both devices (n = 5) (Table 13).
| No. of participants who contacted | No. of contacts from participants | No. of phone calls from participants | No. of e-mails from participants | No. of other contacts from participant (e.g. walk-in/visit clinic unscheduled) | Site staff time to resolve (minutes) | |
|---|---|---|---|---|---|---|
| Total | 20 (N = 42, 48%) | 34 | 17 | 8 | 8 | 503 |
| Average contacts or time per participant who contacted (n = 20) | 1.7 | 0.9 | 0.4 | 0.4 | 15 |
| Reason | No. of related contacts |
|---|---|
| Username and/or password problems | 5 |
| Difficulties using either device | 5 |
| Uncertainties about data transmission process | 3 |
| Concern relating to tonometer reading that patient felt required clinical opinion | 2 |
| Physical health problem preventing use of devices/withdrawal from study | 2 |
| Not receiving electronic text reminders | 1 |
| Difficulty accessing patient training materials (YouTube videos) | 1 |
| Tonometer ran out of battery | 1 |
When asked about satisfaction with the home monitoring, the most frequent response (40%, n = 17) was that patient participants were ‘very satisfied’, and similarly, a majority (45%, n = 19) reported being ‘very satisfied’ with the training they received on the digital technologies (Table 14). When asked to report their preference for future monitoring, 50% of patient participants reported that they would prefer to be monitored at home.
| N (%) | ||
|---|---|---|
| Satisfaction with monitoring | Very dissatisfied | 2 (5) |
| [scale from 0 (very dissatisfied) to 10 (very satisfied)] | 4 | 1 (2) |
| Neither dissatisfied or satisfied | 2 (5) | |
| 6 | 1 (2) | |
| 8 | 9 (21) | |
| 9 | 4 (10) | |
| Very satisfied | 17 (40) | |
| Missing | 6 (14) | |
| Satisfaction with training | Neither dissatisfied or satisfied | 4 (10) |
| [scale from 0 (very dissatisfied) to 10 (very satisfied)] | 7 | 1 (2) |
| 8 | 8 (19) | |
| 9 | 7 (17) | |
| Very satisfied | 19 (45) | |
| Missing | 3 (7) | |
| Future monitoring preference | Home | 21 (50) |
| Clinic/hospital | 14 (33) | |
| Missing | 7 (17) |
Patient participant interviews
Patient participant demographics
A total of 13 patient participants were identified from the first two cohorts for interview, and 11 of these were invited to participate in an interview. A total of 10 participants were interviewed to gain their perspectives on the feasibility and acceptability of digital technology for home monitoring of glaucoma within I-TRAC. Participants were sampled from three sites within the trial, with an even proportion of men and women. The mean age of the participants was 64.5 (SD = 17.4) years, and all participants identified as white from either British, Scottish or Northern Irish backgrounds (Table 15).
| N = 10 (%) | ||
|---|---|---|
| Gender | Male | 5 (50) |
| Female | 5 (50) | |
| Age (years) | < 40 | 1 (10) |
| 40–59 | 2 (20) | |
| 60–79 | 4 (40) | |
| 80 + | 3 (30) | |
| Ethnicity | White (all) | 10 (100) |
| Adherence OKKO ≥ 80% | Yes | 7 (70) |
| Adherence iCare ≥ 80% | Yes | 7 (70) |
| Adherence (combined) ≥ 80% | Both devices | 6 (60) |
| Neither device | 2 (20) | |
| OKKO only | 1 (10) | |
| iCare only | 1 (10) |
Findings from patient participant interviews
Overarching ‘global’ themes on the use of the home monitoring devices were generated from the interview findings, which included the perceived advantages and disadvantages of home monitoring, and potential improvements to either the devices themselves or the process of home monitoring within further trials.
Participants engaged with home monitoring through the I-TRAC study to varying degrees. Interviews revealed certain participant-level characteristics that seemed to correlate with more active engagement in monitoring. For succinctness, those characteristics are summarised in Table 16, comparing those ‘more likely to engage’ and those ‘less likely to engage’. These participant archetypes have not been correlated to the available quantitative evidence on monitoring use but rely on the qualitative impressions of barriers and facilitators to monitoring offered during the interviews.
| Beliefs or behaviours reported by participants more likely to engage | Beliefs or behaviours reported by participants less likely to engage |
|---|---|
| Conversant with technology or willing to acquire skills through directed engagement | Prior life experiences divested from technology; disinterest in acquiring experience |
| Actively seeks out novel experiences/challenges that are relevant to their health and/or interests | Frequent boredom/frustration with monitoring |
| Prior/current experiences in monitoring some aspect of their health | Doubts on the accuracy of home monitoring compared to clinics |
| Desire for information about control over their health | Lack of confidence in ability to accurately perform measures |
| Belief in ability to navigate emergent issues | Anxieties about separating monitoring from guidance/reassurances of healthcare providers |
In addition, the analysis revealed several important themes relevant to the use of, and satisfaction with, the home monitoring process. As there are several distinct aspects to the monitoring process and challenges and opportunities associated with the devices and the study processes, each of these overarching themes and associated findings are presented below, with TDF domains relevant for each theme presented in parentheses and belief statement and illustrative quotes presented in each section. Further illustrative quotes are given as examples in Table 17 presented with belief statements, assigned to relevant domains, for each overarching theme.
| Overarching theme | Belief statements | TDF domain | Sample quote |
|---|---|---|---|
| Decision to participate | Others have helped convince me that I should participate in home monitoring | Social influences | ‘Well, I think if you have glaucoma and you’ve been told by your consultant, or nurse, or whatever, the impacts it can have on your sight, you should be pretty motivated to want to make sure that you’re not getting … going out of control…’ (Pt 12001) |
| My own personal or family history of glaucoma motivated me to do home monitoring | Intentions | ||
| I wanted more regular information about my eye health | Goals | ‘For me as I say, I’m a bit younger, I want to keep my eyes, I’ve a young family, I want to see them grow up so it’s very important to me that the pressures are low and that I am getting the right treatment and keeping on top of things, whereas somebody that’s maybe older it’s maybe not as important to them’ (Pt 14003) | |
| I’m the type of person that will engage with new challenges or technology | Social professional role and identity | ‘You know, the other story is that I was brought up on a telex machine, so an iPhone is quite a challenge, but I have overcome the challenge and I quite enjoy all these new technologies, yes’ (Pt 12001) | |
| I was interested in home monitoring because it was novel/interesting technology | Intentions | ||
| I understand the rationale behind home monitoring | Knowledge | ‘R – In terms of your confidence, it sounds like you felt quite confident at that first contact about the study that this would be something that you would be able to do. P – Yes, but I probably didn’t quite understand what you’re trying to achieve. I thought what you were trying to do was sort of find out… not quite sure. I didn’t realise that it was actually sort of testing the equipment. I thought it was more the idea of regular pressure checks, that sort of emphasis. I suppose I got the emphasis wrong’ (Pt 12007) |
|
| Use of devices | Training should be delivered near as possible to the start of use | Behavioural regulation | ‘Well, I just had met up with this one nurse and she was struggling to find a room where we could do it, so… but then found one, and we had a limited amount of time, but it probably wasn’t ideal for her. [… ] I think it’s somehow sort of a… I think in order to go from a situation where you’re shown something to you’re doing it, you need a sort of continuity, and I don’t think that was there in the session, and because of the holiday break, I wasn’t following it through so there wasn’t a sort of trans-… a smooth transition from trying it to using it’ (Pt 12007) |
| I found the manual/videos helpful in figuring out what to do | Skills | ‘I didn’t really need any help with it to be honest, there was no real times I’d come to it apart from that time it didn’t work in the dim light. I actually did have the manual actually open to see if there was something wrong with it but once I caught on that it was the light… but there was no really other time that I couldn’t get it to work, it was all pretty straightforward’ (Pt 14003) | |
| I was able to eventually perform the process after practice | Skills | ‘To start with, I found it difficult because I couldn’t… you know, I was following the instructions… reading the instructions rather than following it in my brain but as I… so to start with, the time it took me to register the pressures was a bit frustrating, but within, I don’t know, two… three weeks maximum, it was just routine. I must be… I think I was doing it in five minutes easy peasy, yes’ (Pt 12001) | |
| I was able to do the monitoring by doing it in a particular place/with particular tools | Environment, context, and resources | ‘Some people might have benefited from – I had a stand, so if you didn’t have a stand… I found the books… thing is, books are actually quite good but the iPad would slide, so you’d have a multitude of books. I suppose maybe a wee stand or something would be good’ (Pt 12011) | |
| I incorporated the monitoring as part of a routine | Behavioural regulation | ‘R – How easy or difficult was it to remember to do your weekly monitoring each week? P – I found it pretty easy but I’ve got a reasonably decent memory. And I also put it near the eye drops so when I was doing my eye drops, I would see it and think, “Is it Tuesday? No, it’s not.” But it would be there to be seen, it was somewhere it could be seen and not just away in a cupboard out of sight’ (Pt 13005) |
|
| Challenges to home monitoring using digital technology | The tonometer was complicated to use | Environment, context, and resources | ‘Then the tonometer. I normally looked at the instructions to make sure I was doing the thing correctly. I found it very difficult to have the piece of equipment correctly on the eye because it was so sensitive to every little movement, but I did eventually manage to get the green circle of light, but then often when I went to press the button to record the measurement, the piece of equipment moved, so you lost the [measurement]. That was one of the little difficulties. Then it went on. I can think of all sorts of things that didn’t happen that shouldn’t have done’ (Pt 13001) |
| I could not reliably get the tonometer to work | Skills | ||
| Older adults would be less likely to be able to use the device due to general technology or dexterity issues | Environment, context, and resources | ‘I think for people who have things like arthritis, it is very difficult. Let’s say people who have got problems with their mobility in their hands, fingers. I’ve got arthritis in one, my index finger, and it’s bent so it’s not in line. It causes problems like opening that wretched packet’ (Pt 12001) | |
| The device did not give me any feedback as to whether I got an accurate measurement | Reinforcement | ‘R – You mentioned about your not getting beeps. I think that was quite early on in the interview there was no beeping, there was no alerts. P – No. No, it never beeped at all. R – Would that be something that would be useful? P – Well, yes because it indicates that you have at least made an either successful or unsuccessful measurement. I mean it never even beeped to say I’d been unsuccessful, which I find a bit odd’ (Pt 13001) |
|
| Familiarity with iPads or similar devices useful | Skills | ‘Well, most people are used to using the iPad, so I don’t think that would cause any difficulty at all; that was very straightforward’ (Pt 13001) | |
| It was difficult to focus on the monitoring at times | Memory, attention, decision-making | ‘Because, I mean, the peripheral vision test is very… is slightly arbitrary because it depends also on personal concentration, and so… I don’t know. I mean it’s one that feels as if it depends on the day you’re doing it more than they eye pressure or the state of your eye. Your mental state, probably’ (Pt 12007) | |
| Advantages of home monitoring | It takes pressure off eye-care providers | Beliefs about consequences | ‘Well, it’s time-saving, isn’t it, for the hospital. If it goes to the hospital, it’s time-saving them to be able to just sit there and look at results that are put in front of them, rather than spend two or three minutes getting those results and then looking at them. That doesn’t sound much but if you’re doing that for, maybe five people an hour, that’s quarter of an hour. If you’re doing that all day, that’s quite a lot of time you could save’ (Pt 13005) |
| It provides me regular information about my eye health | Knowledge | ‘I think it brings your focus much more to your particular problem. Instead of going once every six months, or may so in some cases only every year, to have your check-up, this is a week by week, month by month knowledge of what’s happening to your pressure in this particular instance, yes. You know, it makes it live, not something that is… you have to wait months to be told you’ve got a big problem. [… ] I think you’re in control, that’s how I would describe it. Are actually in control of your glaucoma from the point of view… monitoring. Yes, which in to certain individuals is a great feeling, you know’ (Pt 12001) | |
| It allows me more control over my eye health | Beliefs about consequences | ||
| It saves me from having to go to clinic | Environment, context, and resources | ‘It’s certainly much more convenient to sit here than have to trundle up to the eye clinic. I know that’s necessary because they need to see you as well, but maybe not just as often. I think it’s a good idea and having undertaken it, I would recommend it’ (Pt 14002) | |
| Disadvantages of home monitoring | I’m not sure the tonometer gave as accurate readings as clinic equipment | Beliefs about consequences | ‘P – Well, obviously, if it’s not giving up accurate results that’s quite dangerous, yes. But, well, also whether people are actually doing it, or whether they say they’re doing it and they’re not doing it. I mean, in a sense it’s like any sort of drug – you give it out, but you don’t actually know whether somebody’s taking it, or whether they’re doing it in the way prescribed. R – Yes. What would be the disadvantage of that, then? Well, what does that potentially risk? P – Well, it risks the pressure not being under control [… ], your eyesight deteriorating from the glaucoma’ (Pt 12007) |
| Home monitoring is useful as long as it’s accurate | Beliefs about consequences | ||
| If I don’t do the monitoring reliably or accurately, my eye health could deteriorate without me knowing | Beliefs about consequences | ||
| High readings can cause anxiety, particularly if care is unavailable/delayed | Emotion | ‘I think for me at first, it was just because I’d be scared, thinking it’s really high. Say it was like eight o’clock at night when you measured and for me, it would be something like 37 and I would have to massage my eye. I would then think, “Now I’ve got to wait until nine in the morning to contact them if it goes up.” It just affects you psychologically that way. Sometimes I’d think I’d rather not know. I mean, you could phone a doctor at the hospital, but you know it’s not an acute emergency so you’re actually then very anxious. That was probably the bit that scared me the most at the beginning’ (Pt 12011) | |
| You no longer have the reassurance of an HCP | Social, professional role identity | ||
| Potential improvements | Positioning of the tonometer needs to be streamlined | Environment, context, and resources | ‘I think one of the things if it could be designed so that you could lock it into… once you’ve achieved that green circle, if something’s coming to lock it into that, that would be good because it… you know, it just doesn’t stay in place. [… ] It was not the easiest of equipment to manoeuvre with one hand while you were trying to press the measurement button with another, so it was awkward’ (Pt 13001) |
| Feedback on readings should be more easily available | Reinforcement | ‘This is a personal observation, but it… probably a more reassuring sound or sight on the tonometer to actually tell you that the information has been recorded, it… but of course this would involve the design of the tonometer, wouldn’t it? [… ] A visual probably, just to… or a flashing light and something like that to just to get over this nagging doubt that am I doing it right and has it been recorded’ (Pt 13002) | |
| Training could have been improved | Behavioural regulation | P – Well, I think it would… well, personally, I would have perhaps benefited on a bit more detailed training on the tonometer. [… ] Yes, to be satisfied that it was working correctly, that it was beeping when it should have beeped. I mean, I wasn’t aware during this training about the beeping; it wasn’t until I came home and read the instructions that I realised it should have been making noises and giving me messages on the screen. I mean, that should have been explained, or at least gone through at the training session. R – Then the face-to-face training, do you want longer sessions or more sessions? P – Well, probably a longer session would suffice. R – Is there anything else, training-wise, that might have helped? P – Well, I think the person doing the training ought to be satisfied that the person they are training is able to use the piece of equipment adequately before they disappear and not say, ‘Your experience is part of the trial’ (Pt 13001) |
|
| ‘R – So, one suggestion that’s come in from a couple of participants is that it might be useful to have a lead-in period, so you get maybe a week or two just to practise at your leisure with the equipment and then commence the study. What would you think of that suggestion? P – I think that’s a very good idea because when you’re only doing it weekly, from one week to the next, you can forget how you managed to do it correctly. So, if you had a week where you could just use it anytime, say every day, just to get into the swing of it, I think that would be a good idea’ (Pt 13009) |
|||
| It would have been helpful to have more support from others | Social influences | ‘Well, I would have certainly appreciated some extra help when I e-mailed him to say I was having problems. I mean I was fully prepared to go back to the hospital and spend some more time with the tonometer and getting a bit more advice, but that wasn’t offered. [… ] It would have been useful to speak to somebody else who was also involved in the trial to find out what experience they were having, but there was no… I had no idea who else was doing it and I think he did say there were 15 people in the area, but I didn’t get to meet or see anyone else’ (Pt 13001) | |
| The frequency of monitoring should be restricted | Behavioural regulation | ‘I think it would be valuable if you could do it monthly or something like that, just once, no more than that if it was stable. Then that way it could be fed into some computer to see the trends or something. But any more, I’m just not sure, just like my blood pressure. Psychologically, you just might get neurotic’ (Pt 12011) |
Decision to participate (goals; intentions; knowledge; social influences; social professional role and identity)
Patient participants reported that entry into the I-TRAC study was often precipitated by a discussion with their glaucoma specialist to participate in a research study that would explore the potential of home monitoring as an alternative to traditional monitoring. Participants did not report basing their decision to participate on the input of others, such as friends and family. Instead, they were often motivated by their own history of glaucoma to join the study or were interested in engaging with a new technology or research project, generally. Participants typically understood the rationale behind the trial (i.e. to test the feasibility of home monitoring as an alternative/supplement to in-clinic monitoring). However, some participants struggled with the concept that the devices themselves, particularly the tonometer, were also being refined through the I-TRAC study before they could be implemented into standard clinical practice.
R – In terms of your confidence, it sounds like you felt quite confident at that first contact about the study that this would be something that you would be able to do.
P – Yes, but I probably didn’t quite understand what you’re trying to achieve. I thought what you were trying to do was sort of find out… not quite sure. I didn’t realise that it was actually sort of testing the equipment. I thought it was more the idea of regular pressure checks, that sort of emphasis. I suppose I got the emphasis wrong.
Pt 12007
Use of the devices (behavioural regulation; environmental context and resources; skills)
When considering participants’ experiences using the devices, several patient participants discussed their impressions of the intervention training. Most patient participants reported that training for the use of the device was generally well received and believed to instil the skills necessary for participants to monitor on their own. However, delivery of training was not consistent across the sample, with some participants stating that the session was ‘rushed’ and did not cover all aspects of monitoring to their satisfaction. At times, this appeared to be attributable to time and space pressures within the I-TRAC sites, which either did not allow sufficient time or a suitable environment to conduct training. Some participants also voiced concerns about the difficulties staff conducting the training were having aligning the tonometer to take a measurement. Lastly, some participants felt that the interval between the training session and active monitoring was too long, leading to them forgetting many of the skills they had acquired.
Well, I just had met up with this one nurse and she was struggling to find a room where we could do it, so … but then found one, and we had a limited amount of time, but it probably wasn’t ideal for her. […] I think it’s somehow sort of a … I think in order to go from a situation where you’re shown something to you’re doing it, you need a sort of continuity, and I don’t think that was there in the session, and because of the holiday break, I wasn’t following it through so there wasn’t a sort of trans- … a smooth transition from trying it to using it.
Pt 12007
Regardless of training, many participants felt that the devices were accessible enough to engage with through step-by-step, trial-and-error perseverance, assisted by the manual and demonstration videos, until they were reasonably confident they were conducting the monitoring as intended. Engaging with the devices through practice also allowed participants to individualise their process, such as performing it in certain rooms or with additional materials (e.g. stacks of books), so that they could reliably achieve readings in less time and/or fewer attempts. Remembering to monitor was not described as difficult, with participants successfully incorporating the process into their established routines, most often in tandem with taking medications or self-care activities.
R – How easy or difficult was it to remember to do your weekly monitoring each week?
P – I found it pretty easy but I’ve got a reasonably decent memory. And I also put it near the eye drops so when I was doing my eye drops, I would see it and think, ‘Is it Tuesday? No, it’s not.’ But it would be there to be seen, it was somewhere it could be seen and not just away in a cupboard out of sight.
Pt 13005
Potential challenges to home monitoring (environmental context and resources; memory, attention, and decision processes; reinforcement; skills)
Challenges to using the devices were predominantly focused on the use of the tonometer. Difficulties opening and mounting probes, as well as positioning the tonometer in front of the eye, were described often and appeared to be a persistent frustration for some, particularly those with dexterity issues. Feedback from the tonometer on accurate positioning and the pressure readings themselves was deemed insufficient or believed to be absent. Issues performing the visual function tests on the iPad were comparatively fewer, as use of a tablet was more familiar to most participants. However, a frequent criticism of the VF testing was that it was ‘boring’ or ‘tedious’ and required efforts to maintain concentration on the task. Overall, however, use of the devices appeared to be a surmountable challenge for most participants.
Then the tonometer. I normally looked at the instructions to make sure I was doing the thing correctly. I found it very difficult to have the piece of equipment correctly on the eye because it was so sensitive to every little movement, but I did eventually manage to get the green circle of light, but then often when I went to press the button to record the measurement, the piece of equipment moved, so you lost the [measurement]. That was one of the little difficulties. Then it went on. I can think of all sorts of things that didn’t happen that shouldn’t have done.
Pt 13001
Perceived advantages and disadvantages of home monitoring (beliefs about consequences; emotion; environmental context and resources; knowledge; social professional role and identity)
Participants could readily volunteer both potential advantages and disadvantages to home monitoring. Speaking first of advantages, there were perceived benefits to the individual as well as to the larger healthcare system. The most frequently mentioned benefit of home monitoring was its promise to relieve pressures on clinics and eye-care providers for providing all aspects of routine monitoring. Participants could appreciate that taking on some aspects of that monitoring could free up appointments for those with more acute health concerns or otherwise allow providers to reallocate their time to improve standards of care. Having control over how often one could monitor was also seen as advantageous, particularly when waiting times exceeded several months in clinic settings. Participants believed that more frequent monitoring could be beneficial to catch any early indicators of advancing disease and seek treatment before their condition worsened. Finally, the logistics of travelling to in-person appointments were described as a practical motivator to engage in home monitoring to reduce the number of clinic visits necessary.
It’s certainly much more convenient to sit here than have to trundle up to the eye clinic. I know that’s necessary because they need to see you as well, but maybe not just as often. I think it’s a good idea and having undertaken it, I would recommend it.
Pt 14002
Conversely, several of the perceived disadvantages were related to deviating from the standard treatment pathway. Firstly, several participants were concerned about the overall accuracy of the home monitoring device and how it compared to monitoring within professional settings. These concerns appeared to be predicated either on the accuracy of the home monitoring devices themselves or in the participant’s belief in their capability to use the equipment as intended. In either case, the potential for inaccurate results drew concerns that an individual’s eye health could be deteriorating without their, or their provider’s, knowledge. Even when concerns over accuracy were not as relevant, delays between recording high pressures and being able to access care were seen as a source of anxiety. Even nominal readings were felt to be of little value if they were not contextualised by the knowledge of the healthcare provider, who could provide reassurance that values were trending normally or that a plan of care needed to be enacted.
I think for me at first, it was just because I’d be scared, thinking it’s really high. Say it was like eight o’clock at night when you measured and for me, it would be something like 37 and I would have to massage my eye. I would then think, ‘Now I’ve got to wait until nine in the morning to contact them if it goes up.’ It just affects you psychologically that way. Sometimes I’d think I’d rather not know. I mean, you could phone a doctor at the hospital, but you know it’s not an acute emergency so you’re actually then very anxious. That was probably the bit that scared me the most at the beginning.
Pt 12011
Recommendations to improve monitoring (behavioural regulation; environmental context and resources; reinforcement; social influences)
Several aspects of the devices and the home monitoring process were identified from this sample. The coordination required to align the tonometer for readings was cited as a barrier for those with dexterity or other hand–eye coordination issues. Participants believed that this could be streamlined in some way to promote easier alignment of the tonometer with the eye that was not as susceptible to fluctuations in hand steadiness. Some participants also mentioned difficulties in accessing their pressure readings from the device, possibly due to a display time-out. It was suggested that readings should be available in an easier-to-read format that preserves the history of recordings, as well.
Potential improvements to training were mentioned by several participants. As mentioned above, some participants felt there was too long an interval between the training session and the first ‘live’ monitoring session. They suggested that training be held as near as possible to this first session to increase the likelihood that skills practised during training will transfer to real-world use. ‘Top-up sessions’ (i.e. condensed training focused on reiterating or improving skills already learned) were seen as a useful addition to the course of home monitoring if participants were finding it difficult to apply their skills from one session. Additionally, some participants suggested that ongoing technical support would have been helpful, but their attempts to solicit that help within this feasibility trial were not addressed. As for any other additional content of the training, some participants were unaware of the demonstration videos and suggested those be emphasised as an available resource in the course of training. There were also mentions that the training manual provided, while helpful for most, should be reviewed for readability, as some participants complained of not being able to utilise it.
R – So, one suggestion that’s come in from a couple of participants is that it might be useful to have a lead-in period, so you get maybe a week or two just to practise at your leisure with the equipment and then commence the study. What would you think of that suggestion?
P – I think that’s a very good idea because when you’re only doing it weekly, from one week to the next, you can forget how you managed to do it correctly. So, if you had a week where you could just use it anytime, say every day, just to get into the swing of it, I think that would be a good idea.
Pt 13009
Finally, some improvements to the process of monitoring were suggested. In order to help build confidence in their ability to conduct monitoring, participants suggested the inclusion of a ‘lead-in’ period. This period would be an opportunity for participants to practise with the device without fear of ‘getting it wrong’ and affecting trial results. Once in active monitoring, as mentioned above, participants would have also liked easier-to-read and easier-to-understand feedback from the devices so they could track the progress of their readings. Further resources on how to interpret results may also prove valuable in this sense. A final comment on the active monitoring period was the anxiety that may be precipitated by too-frequent measurements. To pre-empt this, participants suggested that monitoring should be restricted to only what is necessary, for example once a week.
I think it would be valuable if you could do it monthly or something like that, just once, no more than that if it was stable. Then that way it could be fed into some computer to see the trends or something. But any more, I’m just not sure, just like my blood pressure. Psychologically, you just might get neurotic.
Pt 12011
Site staff interviews
Site staff demographics
We interviewed 9 site staff members (from a total of 11 site staff members delegated to support I-TRAC study at their site) from across the three study sites. This comprised three PIs (one from each site), two Research Nurses, one Research Assistant and three Research Optometrists, balanced across genders and from a range of ages but all identifying as white. Table 18 provides site staff demographics.
| N = 9 (%) | ||
|---|---|---|
| Gender | Male | 4 (44) |
| Female | 5 (56) | |
| Age (years) | < 40 | 4 (44) |
| 40–49 | 1 (11) | |
| 50–59 | 4 (44) | |
| Ethnicity | White (all) | 9 (100) |
| Current role | Research Associate | 1 (11) |
| Research Nurse | 2 (22) | |
| Research Optometrist | 3 (33) | |
| PI | 3 (33) |
Findings from site staff interviews
Site staff were asked about what they thought had worked well or less well for I-TRAC within their site, in response to which they talked about a range of influences as impacting on the successful delivery of the study. A focus of the findings was to consider what may need to be changed in study design or conduct in order for a future large-scale trial to be feasible. These findings are discussed in detail below, with supportive illustrative quotes presented.
Site staff knowledge, beliefs and feelings towards home monitoring
All site staff reported positively when asked to reflect upon how they felt when first approached to help deliver the I-TRAC study. All staff were optimistic about a novel approach for glaucoma care which they believed could improve patient care.
Always positive about doing the stuff you know, there’s something new coming it’s always nice to have something new isn’t it. But particularly I think the it’s the fact it’s a device study and you’re interested in how the devices will actually improve patient care. So it’s about excitement of being able to introduce something new that’s going to hopefully make a difference really, yeah.
P037, Research Nurse, Site 2
Several site staff members reported having one or two concerns prior to the site opening. One concern related to patient participants asking about clinical implications of data collected, as this was not something being assessed within I-TRAC. Another found some of the flexibilities in the protocol (e.g. eligibility criteria, what actual tests were done for assessing IOP and VFs in clinic prior to the study) a bit worrying to start, due to past experiences on tightly controlled trials (compared to the I-TRAC feasibility study).
Yeah, there is also the fear, going back to feelings or thoughts, that if you’re doing this as part of a study as well, the patient wants to know, ‘All that effort I put in taking the measurements, what does it mean? Am I okay?’ that creates a challenge as well particularly when you’re just looking at feasibility and you’re actually not that interested in the actual data they’re collecting.
P036, PI, Site 1
Site staff perceived a number of benefits, particularly for patients, arising from home monitoring. These included a sense of control of their glaucoma through self-monitoring, greater convenience from not having to attend clinic as often, and increased chances of detecting significant changes more quickly, leading to better outcomes in terms of vision. Across site staff accounts, there was a strong sense of buy-in and a desire to see the home monitoring interventions work for patients.
Well that’s the thing, I mean it could be useful for people who live far from the hospital where there is a remote… access issue, it might be helpful, people perhaps with some mobility issues about them getting to the hospital but not stopping them from using the home monitoring device. Yeah, I think there are niches where it would be helpful, but I’ve always had in my mind that this would be a device for continuous monitoring for some reason, and that ultimately the aim is to keep the patient out of the hospital.
P035, PI, Site 2
The ease of running the I-TRAC study and the compatibility of its components
The findings around study conduct were themed in relation to ease of running the study and were composed of two subthemes related to study deliverability: (1) easy-to-run and low-burden study; and (2) quality and usefulness of training.
-
Low-burden, easy-to-run study
Overall, all participating site staff felt that I-TRAC was easy to run and its components (i.e. study processes) worked well together. This was qualified by reports that the I-TRAC study was well explained, supported and was perceived to be relatively low burden for participants and sites.
A lot easier than I thought it was going to be. I think as well, I did sit down the day before and go through the paperwork and just have it in my head, ‘This is what I’m going to say, this is how I’m going to do it’, and have it laid out. I think you have to do that to seem confident in front of the patient. Otherwise, if I’m faffing about, they’ll be like, ‘You don’t know what you’re doing’, and I’ll not know what I’m doing. I think the combination of the training plus the written material that was there too, it was fine.
P038, Research Optometrist, Site 3
Site staff reflected on I-TRAC being surprisingly easy to recruit for and felt this was due to patient participants having a keen interest in something that was novel and that had a clear benefit for them in terms of reassurance, control and burden (being able to monitor from home instead of travelling to clinics).
think maybe for this particular study, home monitoring, I don’t know if that’s such a big issue because we found that recruiting was really easy and we recruited just from our face-to-face clinic, and really we recruited the majority of the patients for each phase in a day. It was literally asking consecutive patients and most people said, ‘Yes, I’d be very interested in this’, and that was surprising, that’s certainly not an experience that is very common in studies. I know there were… I mean it was a very wide inclusion criteria, but nevertheless you still expect perhaps a fair few to decline.
P036, PI, Site 1
Site staff reported few challenges in terms of study conduct. Those challenges raised involved logistics (equipment return and appointments), an issue relating to accessing data from the tonometers (i.e. one site forgot to download the data from the tonometers before reissuing the devices to the next set of participants), and research and information governance issues prior to site opening.
I think we may have had some challenges in getting the equipment back and how the patients… how we got patients back into the system, because I think that was potentially an issue in terms of clinicians’ time because there’s also the idea of getting… picking up all the other data like the visual fields and things like that, and these things all… the organisation of those things and organising when the patients were coming back about picking suitable times where clinicians were available and the actual patient was available, the logistics of that with lots of people.
P037, Research Nurse, Site 2
-
Quality and usefulness of training
The training of patient participants to use both pieces of home monitoring equipment was a critical and central component of this intervention. The consensus among site staff was that the time to train patient participants reduced with practice, initially requiring more time than had been allocated (sometimes up to 2 hours) but reducing to the allocated time (30–45 minutes) after they had trained their first few patient participants. Site staff reflected that they felt the quality of their training improved after the first few participants, as they gained in confidence and developed the skills and tips to help patient participants learn to use the devices. They reported that the patient participant-facing training materials (a step-by-step handbook with images, and several YouTube videos) were a good resource for patient participants. However, some site staff reported that they felt not all participants were using the provided support, resulting in a number of contacts from patient participants requiring additional support during the monitoring period. Site staff reported that they found the site training materials and site initiation visit training (protocol and study instruction manuals) to be useful, coherent and generally to cover all that was needed, ‘Yeah, the step-by-step guide was very, very detailed, the fact that there was one for the patient and one for the practitioner as well was really handy’ (P033, Research Optometrist, Site 3).
However, one aspect of site training, the instructional training for using the tonometer, was delivered by a representative from the commercial partner. The consensus across site staff was that this training did not help them to teach patient participants how to use the tonometers. Several mentioned that they felt the representative was there in a sales capacity, not to deliver training, and had no practical experience of using the device so could not answer a number of questions the site staff had.
We need somebody who’s thorough, who knows all about it, who knows from a patient perspective as well. I’ve had some fantastic training in the past with other companies and real trainers but I kind of feel there was a sales element there. So no, that wasn’t very good for explaining.
P039, Research Nurse, Site 1
Many mentioned that the YouTube videos and written instructions were more helpful than the commercial partner training in terms of helping them master how to use the equipment and train patient participants to use the equipment.
Yes, because I mean I actually learned more from watching that YouTube training than I did actually from the sale rep side of things, so, yeah. Yeah, certainly the YouTube thing helped once we’d got to grips with the YouTube address.
P037, Research Nurse, Site 2
The feedback from sites was that equipment training should be developed and delivered by those with experience of using the equipment and training others (preferably patients) to use the equipment, so that they have knowledge and awareness of common problems patients have and how to overcome these.
Eligibility, recruitment and representativeness of sample
All three sites reported that they found it easier to recruit than they had anticipated at the outset of the I-TRAC study. Across the sites, staff generally felt, however, that their samples were not representative of the typical glaucoma population. The three sites reported how they approached recruitment differently.
Site 1 recruited from a general face-to-face clinic and participants were first approached by the clinician/PI and if agreeable, referred to the Research Nurse. The PI reported that the selected participants were really keen to participate. The Research Nurse in one site, however, noted a trend where they felt older participants appeared more determined to master the technology, and the younger participants, particularly those still working, had other priorities. This was echoed by the PI from site 2. Conversely, some staff from other sites believed younger participants were easier to train, perceiving older participants as being more time-intensive to train and support with new technologies.
I must say though, when I was phoning some people, maybe one or two of the younger ones, they said, ‘We’ve not been doing it, just not got round to it’, etc., so that was a wee bit of a disappointment… They were more elderly in the first cohort but they were determined they were going to do it. I don’t know if it’s that generation, I’m not sure.
P039, P039, Research Nurse, Site 1
Site 2 recruited participants through their virtual clinic, which typically deals with straightforward glaucoma cases (i.e. glaucoma patients considered at low risk of progression due to stable disease and few comorbidities which may complicate glaucoma management). Site 2 staff reported that while recruitment was easy given the access to a large pool of suitable participants, the approach to recruitment involved the Research Associate spending time in the waiting area of the clinic with patients not pre-notified about the study by the clinician/PI. The PI discussed how while time-efficient (a high volume of patients to speak with in one half-day session), recruiting from this clinic only, as they did, would not represent all glaucoma patients. This is because those whose condition may be more complex would be seen in a different clinic and, conversely, those patients with straightforward glaucoma and OHT would be discharged to community services.
[W]e have discharged quite a few of our patients to the community, the very, very straightforward ones like ocular hypertensions, so they weren’t… there might’ve been one or two but they’re usually… if they’re ocular hypertensions in the hospital they’re usually there for a reason. So actually quite a lot of them… I’m sorry, that group would be missing.
P035, PI, Site 2
The Research Assistant discussed the difficulties of having around half of participants decline participation, with reasoning related to the person approached having no interest in a technology study.
But yeah, generally I think my group is diverse-ish, but obviously there’s that inherent lack of diversity you get by just sitting down outside a clinic saying, ‘We want you to take some technology home’, and then half of the patients say, ‘Oh, I don’t do tech’, and just don’t want to talk to you ever again.
P031, Research Assistant, Site 2
The Research Assistant also discussed how they had found it challenging to recruit a diverse sample of participants, especially in relation to ethnicity. An issue reported by several members of site staff was that of language, perceiving language issues as being a significant barrier not just to accessing the information leaflets but also learning to use the devices in a second language.
Yeah, I did recruit, I did very happily manage to recruit a lovely I think Caribbean lady who’s part of the study, but other than that I have approached a lot of people and I think sometimes there have been language issues, the fact that it’s all in English, the information sheet’s in English, that’s been an obstacle I think a couple of times, although it hasn’t always been explicitly stated that that is the issue, yeah.
P031, Research Assistant, Site 2
The PI from this site questioned whether it would be more helpful to have a more diverse study team, or at least a more diverse team of recruiters, as he felt a shared ethnicity may go some way towards the study having a more inclusive approach to recruitment of glaucoma patients.
Well I think there is a certain racial thing, I mean if you had a black guy, black doctor asking black people to participate in a study I think that’s much better than some old white fella like me asking them to do it, and I think that just makes it much more accessible.
P035, PI, Site 3
For site 3 the seven participants were specifically targeted as they were known as individuals with the educational and technology familiarity needed to participate and would enhance recruitment efficiency. Site 3 staff reported they felt their participants were younger than typical glaucoma patients. The following excerpt presents this site’s views when asked about how representative their sample was.
Well I don’t… well I expected ours [when considering the I-TRAC sample of patients] to be older….
P032, Research Optometrist, Site 3
Yeah, not in terms of age and probably also not in terms of familiarity with electronic devices.
P033, Research Optometrist, Site 3
But then I suppose it’s good to have a range and different types of glaucoma as well as part of the study, but on the whole they’re not.
P032, Research Optometrist, Site 3
Unintended consequences arising from the intervention
Three members of site staff (one from each site) discussed how they had initially been concerned about the intervention causing patient participant anxiety arising from increased focus on their disease. Despite these concerns, there were no reports from any site staff of any actual or suspicions of induced anxiety within their patient participants. One site reported two patient participants who required treatment, an outcome that was triggered by patient concerns relating to the tonometer readings which resulted in contact with their consultant. In both cases, the site staff member reported that the patient participants did require treatment and that both patient participants were glad they had participated so it could be detected.
There were two participants who actually had to come in as a result of the pressure that they measured. One of them went to the optometrist and then she came in to us, and the other one just came directly to us because… if they hadn’t had the equipment, they wouldn’t have known their pressure had gone up…. Both these people liked the equipment, they liked using it, they thought it was worthwhile.
P039, Research Nurse, Site 1
Adaptations or considerations required for a future trial
Site staff reflected on several aspects of study conduct that would require careful review prior to progressing to a full trial. This included: (1) the additional resources that would be required; (2) aspects of study design to be improved (i.e. determining the ideal follow-up duration); (3) participant selection criteria; and (4) improvements to intervention training. These subthemes are presented below.
-
Increased human and financial resources
In relation to resource use, sites reported that to feasibly run I-TRAC as a full trial with more participants, increased logistical and technical support would be required to manage the devices and assist participants with any queries. There were also concerns about the perceived (significant) costs of acquiring a large number of devices and accessories (e.g. batteries), and how a large trial would operate if equipment had to be rotated between participants in an effort to reduce technology costs.
Does it mean you’re going to follow patients for a year? Does that mean you’re going to follow patients for… if you have to follow them for a year, well essentially you need equipment for every patient in the trial, that’s costly I expect, and you need backup to manage the technical aspects that they may have, repairs, all of those sorts of things. I suppose it’s all manageable, it’s just understanding it before you do it, which I guess is what this is about.
P035, PI, Site 2
While it was not conducted as part of the I-TRAC study, it was recognised that the human resource requirements for reviewing and acting on the data received from the devices in a future trial would have implications for delivery. Site staff were concerned about the substantial time required to process this amount of data. Suggestions to make this achievable included exploring methods for efficient data review (e.g. making use of automated algorithms that would trigger a message to the clinician only if a test is abnormal, or AI technologies) and integrating data into electronic patient records. There were also concerns about the impact on resources required to address readings from the home monitoring devices that suggest the need for further intervention.
I guess at the moment it would be… perhaps it’s not very efficient because it’s all very innovative. But I guess, you know, you can incorporate this into electronic records and there will be, let’s say, the potential of having the information displayed in a different way, that makes the clinicians’ tasks easier. It depends, you know? At the moment it will be time-consuming because we don’t have a good system to process this information. It would need to be well thought to make it efficient.
P034, PI, Site 3
-
Changes to study design
Most suggestions related to study design were improvements to the intervention, such as involving carers/family members in the monitoring, offering home visits instead of patients coming to the clinic for training, improving the electronic reminders to avoid spam filters, and to consider restricting the study to evaluating only one technology rather than multiple technologies at the same time.
One way that might help simplify it, and also perhaps help identify the key research question would be to just test one thing rather than testing visual fields and pressure. I don’t know which would be best. The technology for pressure is more developed but visual fields are probably more important.
P036, PI, Site 1
Exploring the potential for utilising participants’ social support within both the training and use of the devices to be more inclusive of those at greatest risk of vision loss was also suggested.
The other thing I suppose is that we focused on self-measurement, but I think that looking at carers and how they can help and support the patient is really important too because I find it hard to put eye drops in my own eyes, now if I had glaucoma I’d be asking my partner to do it for me, I’m terrible, and I think, well patients do that too, and I think having someone to help you and support you really to take the measurements at home would help because one of my worries about this sort of technology is it’s the vulnerable groups, the people who are probably at highest risk of losing vision, the people who can’t put eye drops in themselves, and they’re the people who might need it most and they might not be able to do it. So I think looking at, if there was further work with this, I think looking at carers as well would be useful.
P036, PI, Site 1
Within I-TRAC, digital reminders for home monitoring devices (such as e-mails and text messages) were provided to enhance adherence. Site staff highlighted the need to ensure these reminders are received as intended and not filtered out by spam filters or blocked by mobile phone providers. One site staff member reported that the need for patient participants to travel to the clinic for the training and provision of equipment acted as a barrier for several participants, undermining the potential benefit for patients who cannot travel. For this site staff participant, they suggested that providing study visits at home may improve recruitment of those who are often less represented in research.
I think it would be entirely possible. I think you… I think definitely within the capacity of going and visiting patients there’s no reason it couldn’t be done at the patient’s home, it’s just whether that’s a reasonable use of time I think.
P031, Research Assistant, Site 2
The importance of selecting research sites and staff purposively was also commented upon, due to the importance of confidence with technologies and the patience required to help teach others. A further recommendation in relation to design was the potential for a further study to determine the duration of follow-up.
I wonder if you almost need another… a pilot study to see how long do you need to… do patients need to keep them for to get full results, because that would be a key question in terms of how many devices you need and then the cost of the study….
P036, PI, Site 1
-
Recruitment of participants
When asked about their expectations for recruiting a larger number of participants for a future trial, there were mixed views. Although site staff felt recruitment for this feasibility study, with very broad criteria for participant selection, had been easy to recruit to, there were concerns about how easy recruitment of a more tightly defined eligible population might be.
I think the fact that we have a virtual clinic where there is a large pool of what would’ve been considered suitable patients for this all coming to the same place in a non-stressful environment made a massive difference to our ability to recruit patients, and that has two real implications for me. Going ahead to a randomised controlled trial, which I guess is what you’re hoping to do, I would be very much looking at recruiting centres which run virtual clinic services because the patients are quite easy to access or more easy to access. But it also creates a barrier as well because in a way they are generally more straightforward patients and it depends who you’re trying to evaluate these devices on. If you want more advanced patients then you’re going to have to look elsewhere and then it will be harder to recruit. This was particularly in relation to the typical age demographic of glaucoma patients, and assumptions about older patients abilities to use the technologies.
P035, PI, Site 2
Especially at the moment, we didn’t have any restrictions, the availability was anyone with glaucoma, so that was basically… if in the context of a trial, you have a particular population, it might be more difficult… I can imagine from my impression, this technology might not be for everybody. I guess if there is a trial, there will be an inclusion and exclusion criteria, there would be a particular population of glaucoma people, people with glaucoma. I guess, you know, I’m just thinking that perhaps recruitment will be difficult.
P034, PI, Site 3
Site staff discussed how there were uncertainties about which glaucoma patients home monitoring would best meet the needs of in terms of usefulness for the patient, usefulness for the clinician, and from a cost-effectiveness perspective.
Then yeah, maybe those ones who find it difficult, who are stable but also find it difficult to come to appointments, and I agree with [other participant] that those younger ones that are familiar with technology and have busy lives.
P032, Research Optometrist, Site 3
That’s the biggest thing because I think… yeah, it’s a bit… it’s a chicken and egg situation isn’t it because you don’t really know what sort of patients it might benefit… But yeah, I think it’s really hard because it’s just speculating and guessing because we don’t really know what kind of patients or what scenarios it would be of use.
P036, PI, Site 1
For one PI, the difficulty arose because the two technologies being utilised together as the home monitoring intervention in the I-TRAC study are measuring different outcomes. For them, each of those outcomes independently would help very different subgroups of glaucoma patients, and those who would benefit from both were a very exclusive group. Clarifying who this intervention would be most appropriate for was indicated to be an important priority prior to any full-size trial.
But I think separating the pressure and the visual fields and looking at the different indications for those is really important because it’s very different situations where you might do one or the other. So yeah, I think there’s almost three situations, there’s three questions, how can we use home glaucoma monitoring, but how can we use home fields and home IOP because they’re different, different groups.
P036, PI, Site 1
-
Improvements to intervention training (for staff and patient participants)
Many site staff participants reported suggestions as to how the training for patient participants could be improved. The first was to identify and develop ‘good trainers’ at research sites – those who are patient and able to promote confidence and reassurance among patient participants. It was considered helpful if the person delivering the training had some awareness of the patients’ clinical and disease status, as this provided insightful information as to how best to help the patient learn to use the devices.
I think also the other thing that I noticed was that it’s helpful if the person teaching the patient has an understanding of the patient’s condition, because we had a patient where they had poor vision on one eye and it was really hard to get them to measure the pressure in that eye, but they could do the other eye, and we didn’t realise initially, and then once the notes were opened and you’re like, ‘Oh, the vision’s quite poor in that eye’, it became apparent why there was that big difference. I think just having some… I think if you just dump a patient with a technician who knows nothing about that patient, that might not be the best way to teach them.
P036, PI, Site 1
In terms of the specific tips and stuff, it’s generally been stuff like knowing that some patients find it far easier with one eye closed or knowing that actually sometimes I just need to go in and adjust the device a bit myself and help them get it in the right position, and then once they know how that should feel, a lot of them just… they can remember that and it helps a lot.
P031, Research Assistant, Site 2
A number of site staff wondered if group training for patient participants would be helpful.
For me, they’d be in an environment where the equipment would be there for them to play with. As I say, internally here, there was issues about getting space so I didn’t think that initially helped. If I had a conference room for them, get the videos up where they can see it, huge, not make it too long because they’ll maybe get a wee bit tired, provide a wee provision of some fluids and what-have-you, cups of tea, they’d like that, it would be a social thing for them. Have the equipment there that they can play with at the time, each one get a wee shot themselves, see how they feel with it, ‘How do you feel? Go to that table, have a wee shot’. Somebody like myself always there on hand to explain to them and also demonstrate. I think a huge presentation behind us, like the videos you get on YouTube showing them, big enough for them to see, take questions, play with it, I’m always there to help them.
P039, Research Nurse, Site 1
This group approach was perceived to be a potentially efficient approach of delivering training for sites, but the additional social aspect of the training environment was also expected to be helpful for patient participants.
It would probably be easier if we just had an I-TRAC demo and just brought everybody on the same day to do training, or maybe did group training with the patients rather than individually. That would be something that would save time, so one person can maybe teach three or four people at the same time and maybe those people have a bit of support as well because they’re all part of the same study… People with conditions can feel isolated sometimes if they think they’re alone, but if they have a group where maybe they come to the clinic once a month, like you say, it’s a social thing. I know older patients love coming, getting tea and biscuits and a chat, that’s why it takes so much time!
P038, Research Optometrist, Site 3
Several aspects of training for site staff, directly related to the intervention devices themselves, were also suggested as areas of improvement for the future. Increased provision of more detailed technical information on the devices, what they are measuring and how they work, were suggested as ways of improving site staff confidence not just in the devices but in their ability to answer patient participant questions about the technologies being explored: ‘I think more clarity in what that test is actually doing would be good, there maybe wasn’t that much information on that’ (P032, Research Optometrist, Site 3).
Site staff were generally in agreement that they would like to see more training opportunities to increase the time they could spend practising using the devices and practising training another person to use the device, so as to increase their familiarity and confidence with the devices. Site staff also suggested several benefits of having spare equipment on site, such as that staff can continue to practise with the device while participants are home monitoring, and it would be helpful in supporting participants when they contact for help with their device over the phone.
I don’t know if this is doable in terms of resources, but to have a device, one of the [tonometers] on site that we get to keep to solely use as a practice device, and then if we’ve got new staff coming on board or whatever we’ve always got something there that we can do in-house training for people.
P033, Research Optometrist, Site 3
Pragmatic considerations for the delivery of site training also included ensuring a suitable training environment is made available at site for the training session; as was reflected on by one site, space can be limited at NHS sites.
There wasn’t much space for them [a suitable room for the commercial trainers to deliver training at site] here. It didn’t really hit off very well initially. Do you know what I would have liked? If we could have all gone to a wee training course, you know, meeting up with the other people. I know the budget is only so much but if we’d done a training course with the other people where we could all ask questions, take our time and been shown how to use it, we could take notes and things like that as well and then try and start a wee bit earlier.
P039, Research Nurse, Site 1
Multisite training days, where staff from across sites get together off-site to complete their device training, was mentioned as a preference by several site staff participants.
I think it’s something I wouldn’t necessarily think would need to be an organised event, but it’s something I’d recommend for future sites is familiarising themselves with the devices, maybe just letting them use some of the probes, just actually go through, ‘This is how the device works. If I’m going to use this, this is how I use it’, show it, get all of the members of the team used to how it works so that then they can explain it to patients quickly, because when I learnt how to use it, it was just the one meeting, I think was the only person who had it demonstrated on, and then from then it was just, ‘Now you’ve got to show patients how this works, and actually having a bit more experimentation in the meantime might’ve been useful, yeah’.
P031, Research Assistant, Site 2
Impact and influence of COVID-19 pandemic on perceptions of acceptability to patients and clinicians
Site staff were asked to discuss whether they believed the COVID-19 pandemic had any influence on recruitment to I-TRAC. No instances were reported where the pandemic appeared to influence a participant’s decision to participate. It was noted, however, that the pandemic may have made some patients more amenable to the use of technologies, through increased familiarity gained by using them during the pandemic to stay in touch with friends and family and use of remote health consultation services. Our PI in site 2 reported that more patients were now being seen in virtual clinics post pandemic, as patient care pathways have changed due to the pandemic, and as a result this could be said to have made recruitment easier as the more stable patients were available to be recruited from one clinic, as opposed to searching multiple clinics.
I wonder, just add to that, whether patients might be more happy to try technology and might be… I think there were certainly some people that had never used an iPad and Zoom before and now they do to talk to relatives and maybe, so maybe it helped, and people might’ve been worried about coming to clinics and, so the idea to monitor disease at home might, they might’ve been more open to that than they would’ve been before the pandemic.
P036, PI, Site 1
Table 19 provides a summary of the strengths and weaknesses of I-TRAC perceived by site staff. Table 20 reports a summary of site staff suggestions regarding a future evaluation of digital technology for home monitoring glaucoma.
| Strengths: things that went well | Weaknesses: things that could be better |
|---|---|
| Study protocol and supporting documents (e.g. training manuals) allowed effective delivery of the study | Commercial partner training did not prepare staff for reality of training patient participants to use the handheld tonometers |
| Many patient participants were accepting of and willing to try the technologies for home monitoring, making recruitment straightforward | Logistical issues in returning equipment and organising follow-up appointments |
| Strong buy-in and acceptability from site staff across the three selected sites | Representativeness of sample: glaucoma patients of ethnic backgrounds other than white and those less familiar with technologies |
| No evidence of increased patient participant anxiety in relation to study contacts | Research governance issues delaying opening in two out of three sites |
| Study perceived as low burden in terms of visit schedule | |
| Patient participant training generally went well once the staff member had completed two or three sets of training |
| Key design and conduct changes suggested by site staff for future RCT |
|---|
| Design |
| Clarify eligibility criteria – who is the ‘ideal’ patient for home monitoring of IOP and VFs together |
| Intervention – consider evaluating only one device at a time |
| Consider trade-off in maximum duration of follow-up and adherence |
| Software/algorithms for managing the volume of data collected by the devices |
| Conduct |
| Tech-confident team member at site |
| Increased site staff training in terms of time |
| Patient training – seek to involve carers in this process, explore making training sessions more social (group training), ensure trainer understands the patient/their disease and health status |
| Home visits – increase inclusion for those who could benefit most as they cannot attend clinic |
| Ensure electronic reminders not blocked by spam filters |
Chapter summary
The findings from the mixed-methods study of intervention acceptability demonstrate that for both patient participants and site staff tasked with delivering I-TRAC there were many positives and the intervention was deemed broadly acceptable. Overall, the I-TRAC study recruited well, recruiting 95% of its proposed sample size (42 of 45) in the planned recruitment period (November 2021 to August 2022, 10 months). Retention and completion of follow-up procedures was also successful, with 95% (n = 40) completing the 3-month follow-up clinic visits. Adherence to the interventions was generally high, especially considering our predetermined adherence levels were above 80%, and satisfaction with the process and the training were also scored highly by patient participants. However, 48% (n = 20) of patient participants contacted site staff at least once when at home, resulting in additional input from site staff regarding study process or intervention delivery.
The qualitative data from the interviews and focus groups with patient participants and site staff did corroborate some of these findings (e.g. stating recruitment worked well and study processes were easy to follow and low burden). Yet the qualitative data also highlighted important areas not identified through the quantitative pilot, such as: the need for a refinement of eligibility criteria and associated recognition of limited sample diversity in I-TRAC; issues relating to inadequate training (for both site staff and patients); a lack of confidence in the technology (and their ability) in relation to purpose of home monitoring; familiarity with the device and physical dexterity issues; and some anxieties in relation to a lack of clinical oversight when monitoring at home. These findings highlight that several key factors need to be taken into account when considering the feasibility of future trials evaluating digital technology for home monitoring of glaucoma; these are discussed in more detail in Chapter 7.
Chapter 5 Researchers’ experiences of conducting evaluations of digital technologies for home monitoring health conditions
There was a requirement to identify key challenges and considerations for the delivery of a future large-scale evaluation of digital technology for home monitoring of glaucoma. In order to answer this research objective, we engaged with research teams involved in carrying out research studies or trials of digital home monitoring technologies (DHTs) for both ophthalmology and a range of other health conditions.
Methods
Study design
Online semistructured qualitative interviews with researchers who had been involved in conducting evaluative studies or trials of DHTs across a range of clinical specialties, including ophthalmology but also other health conditions.
Sampling and recruitment
Our sample size was informed by the five key principles of information power: broad or narrow study aim; dense or sparse sample specificity; application or not of established theory; quality of the dialogue; and finally, whether case or cross-case analysis. 49 Informed by literature and our Project Management Group, it was agreed that three to four studies and six to eight participants would be sufficient to address our research objectives.
Chief Investigators and Trial/Study Managers of study/trial teams that have been involved in evaluating digital technologies for home monitoring of eye disease or other diseases (either as feasibility, pilot or full-size trials) were invited to participate in an interview to discuss their experiences of running such studies/trials. We first sampled purposively by contacting Chief Investigators of studies known to the authors or identified in the literature, with attempts to match study types to those identified in the literature review in Chapter 6. We invited Chief Investigators to participate, and then identified additional participants through snowball sampling by asking them to nominate the relevant Trial Manager/research fellow who may also provide important insights. We aimed for two representatives per trial and a total of six participants. We invited several research teams from within ophthalmology (n = 3 teams) and outside of ophthalmology (n = 3 teams) to consider challenges common to DHTs, and others which may be unique to ophthalmology. All participants provided verbal consent prior to the interview.
We had originally planned to also interview IT staff (n = 3) within NHS organisations to explore organisational IT issues in relation to DHT. However, evidence of issues relating to IT that are relevant for DHTs was generated through insights from existing stakeholder groups. Therefore, we chose to interview additional participants from research teams involved in delivering evaluative DHT studies or trials to provide further evidence relating directly to the broad issues of future evaluation challenges.
Data collection
Interviews were conducted online (Microsoft Teams)52 by CS and transcribed verbatim. Demographic data were collected at the start of the interviews. Discussion was guided by a semistructured topic guide developed within the team and included input from a researcher with expertise in digital technology, to cover broad questions about challenges and solutions for running trials of digital technology for home monitoring. Participants were also asked about their opinions about how easy it is to scale up DHT studies or trials, either to definitive trials or for implementation into the real-world setting. We had originally proposed that these interviews would be guided by the Consolidated Framework for Implementation Research (CFIR). However, following further review of the CFIR and through I-TRAC team discussions (supported by the SSC), it was deemed to be more appropriate to design less directive topic guides to capture all relevant data around the pragmatics of running evaluative studies of digital technology for home monitoring.
Data analysis
The six-phase Braun and Clarke approach to thematic analysis was adopted to analyse and code the data. 53 The transcripts were reviewed several times by two reviewers, noting underlying points, ideas or feelings being conveyed. Transcripts were then transferred to NVivo 12 Pro46 for further analysis. The underlying points, ideas or feelings noted in first review were noted as codes. These codes were reviewed for themes – broader ideas linking the individual codes. Themes were constructed inductively (e.g. what types of challenges and solutions were reported) and then deductively (e.g. what did participants say about a given facilitator or challenge), noting potential relationships between themes, until a clear framework of inter-related themes, that could explain much of the data, was proposed. A coding dictionary describing the themes was developed and updated throughout the analysis (see Appendix 7).
Results
Sample characteristics
Twelve researchers were invited, with eight researchers interviewed (66.7% response rate): three Chief Investigators, four Study/Trial Managers and one Study Coordinator (who worked under one of the recruited Trial Managers). Of the four who either declined or did not respond, three were Chief Investigators and one a Trial Manager. All researcher participants had experience in DHT studies or trials within the UK, with some researchers working across a range of disease areas. The trials they worked on involved using digital technology to home monitor a range of health (mostly long-term chronic) conditions, including diabetes, hypertension, chronic obstructive pulmonary disease, COVID-19, age-related macular degeneration, glaucoma, alcohol addiction, and skin cancer. Table 21 shows further demographic information.
| n | ||
|---|---|---|
| Occupation | Chief Investigator | 3 |
| Trial/Study Manager | 4 | |
| Study Coordinator | 1 | |
| Age | < 40 years | 3 |
| 40–49 years | 3 | |
| 50–59 years | 1 | |
| 60 + years | 1 | |
| Gender | Male | 5 |
| Female | 3 | |
| Ethnicity | White (all) | 7 |
| Asian (all) | 1 |
Findings
Despite the challenges researchers reported they encountered during the trials, the majority (n = 7) had positive attitudes towards digital technology research. Researchers emphasised the possible solutions to address the challenges they encountered rather than focusing on the challenges themselves.
All researchers described a number of challenges they had experienced in setting up and conducting DHT home monitoring studies or trials. However, they also presented examples of good practice (solutions) that helped, or could help, mitigate these challenges. These challenges and solutions fell under six major themes: stakeholder acceptance of technologies; understanding the devices and predicting potential technical problems; resource planning; peripheral infrastructure – institution ethics and funding; committed and supportive commercial partners; and digital exclusion. These themes, and the associated challenges and solutions, are reported in detail below and illustrated in Figure 3.
FIGURE 3.
Challenges and solutions for DHT trials.

Stakeholder acceptance of technologies
Similar to trials evaluating other interventions, most researcher participants (n = 7) reported challenges recruiting and/or retaining participants. This was described in relation to recruiting both patients and site staff/clinicians and concerned the acceptability of the digital technologies being studied by these central stakeholders. The key challenge for DHT studies or trials related to recruiting and retaining representative samples. Researchers frequently reported non-representative samples, over-represented by individuals who were highly educated and had previous access to technology and the technological confidence to participate.
[T]here was positivity from a lot of participants and a willingness to take part, but when we look at our demographics we’re obviously looking for evidence of inequalities and the ones that you would expect are there, sort of higher education, white, British as opposed to those that chose not to take part and in particular with respect to exposure to previous technology, about 66% had a smartphone, about 85% had internet in the home, so all of those for that demographic are much higher than the national average.
P021, Chief Investigator
Patient acceptability
Low digital literacy skills, anxieties about using technologies, difficulties understanding how to use technologies, and no or low support at home to assist participation were commonly reported by researchers as reasons for patients choosing not to engage with DHT studies or trials.
So we got quite a lot of, ‘No, sorry, I can’t’, or, ‘Sorry, I tried but I can’t manage this, it’s just giving me too much stress and hassle’, or, ‘Sorry, I need my son to help me but he’s not around because I can’t use a computer’. So there was a lot of… I would say probably, you could probably say about a third of them were very happy and capable and able, a third were willing and needed assistance and the other third were probably like you don’t even ask.
P028, Trial Manager
Researchers described how these issues reflected low acceptability of DHT from the patients’ perspective, resulting in a lack of willingness to participate in studies or trials of this type. Several researchers reported how DHT study or trial populations were over-represented by younger, more educated, white participants – a finding that, while not dissimilar to issues across research fields, researchers felt was more difficult to overcome in evaluating DHTs due to generational differences in ability and perceptions of technologies. A further impediment to recruiting patients was the assumptions made by clinicians or site staff about patients’ willingness and/or ability to take part in DHT studies or trials, often making judgements based upon age (i.e. perceiving their patient group as too old to use technologies and anticipating barriers to participation prior to discussing with patients). Researchers frequently reported how sites varied in their attempts to offer DHT study/trial participation to as a great a number of participants as possible. Some researchers highlighted selective approaches and offers, stating that many of their patients would not be able to use technologies or would not be interested.
. . . well identifying patients that were eligible wasn’t necessarily a problem because that’s irrelevant whether or not they wanted to take part in the study or not, it’s I think the sites, some sites were more selective with who they approached depending on how they felt the patient would cope with it, and some sites were… would approach anyone who was eligible and try get them on board. In a lot of ways the latter was better because you get a better picture, but we still had a lot of rejection because of the technology.
P023, Trial Manager
Clinician and site staff acceptability
It also became apparent that there were a number of barriers put up by clinicians in agreeing to host DHT research at their site. Researchers discussed how a lack of supportive evidence on the effectiveness and benefits of the technology being tested contributed to the lack of staff/clinician buy-in. In some circumstances, clinicians challenged the benefit of the intervention, questioning what value the digital intervention had for them personally, and at times for their patients.
And the other beauty was that we were able to search the records to look at the number of face to face consultations, and we were able to demonstrate that there was a significant fall in face to face consultations in the group that were being managed that way, a 25% reduction in all face to face consultations. Once you have this sort of data and you can show this to people, they start to get interested…’
P026, Chief Investigator
If clinicians did not see that the digital technology would make their life easier – that is, if they perceived the technology to be cumbersome, time-consuming and not leading to a clear improvement for the patient – they would be reluctant to participate.
. . . the main thing is that the equipment works. That’s really, really important that it works and is reliable. If you’re a busy ophthalmologist, a busy GP and it just doesn’t work a couple of times, you’ll think, ‘Stuff this’, and you just won’t use it, you know? Even an enthusiast, after a while, they might like you and say, ‘We’re doing our best here but I’ve got a busy life and I can’t be bothered with this’. It’s really important that it works.
P026, Chief Investigator
Researchers reported that many of these acceptability issues, believed by the researchers to inhibit recruitment and retention, could be enhanced in DHT studies or trials through several mechanisms. Ensuring the technology has a clear purpose and is as simple to use as possible, with minimal steps and actions, and that this is communicated clearly to clinicians, can aid buy-in. The importance of simplicity was also echoed in relation to improving the acceptability of DHT for patients.
For the [study name] the patient population, the average age was, I think it was 74 and, so that hugely impacts how that patient population should be approached for the trial because the main reason for not being interested in taking part was being put off by the technology. So that… you will need to consider how best to approach the patient population without putting them off. We had training sessions for an hour long where the site would demonstrate the technology, go through it, but it’s still a lot to take in and obviously we weren’t just testing one device, we had a paper journal plus two apps, plus a MiFi device, it was a lot to take on. So I feel like yeah, considering the patient population is really important, and also not to overwhelm them. I appreciate if a trial’s being set up and you want to measure as much as possible, but keeping it to one device or one test is the easiest way from a patient perspective, yeah.
P023, Trial Manager
Exploring the needs of stakeholders early in the development of the technology, and building these into the design, was considered important. Many researchers felt using technologies they (clinicians and patients) are already familiar with aids acceptability. However, this issue may be a double-edged sword, linking with ‘digital exclusion’, discussed later in this chapter. Also, many believed that recruiting patients face to face in a clinical setting, demonstrating the technologies, helps with training coherence. In relation to the training to be delivered, multiple pilots of training materials were considered a good approach to ensuring the final training programme is effective and acceptable to patients and clinicians. Contextually, many considered that the COVID-19 pandemic helped with both clinician and patient acceptability through increased interest, motivation, and confidence in using technologies. The value of digital technologies was also perceived by researchers to have increased for patients and clinicians; DHTs have offered a lifeline for remote monitoring when hospital visits were not possible or were undesirable due to COVID-19 risk: ‘So basically have a system where you only have one click option so they can’t click on the wrong thing, that’s ultimate necessity’ (P028, Trial Manager).
Some researchers reported how selecting a research site experienced in running DHT studies/trials may be beneficial. One participant described this as identifying research champions, those who are motivated and enthusiastic enough to see new DHTs through the early testing period where many problems are anticipated. These champions were less likely to give up at the first hurdle and set the path for future implementation. This was considered good practice for facilitating feasibility or pilot studies of DHT. As was stated, busy clinicians often drop challenging studies at the first hurdle. Additionally, researchers reported that the involvement of researchers from communities and ethnicities that were under-represented in recruitment helped to balance the representation.
It’s really good if you can get champions, so if you can get a few people who are tech enthusiasts who will get this thing going, who don’t mind if it’s going to be difficult in the first – there’ll be bumps in the road. We always did this, we would pick a few practices, for example, where we knew the people there were reasonable folk that understood technology, that understood that this was not perfect, you know? We would say that to them at the start, ‘You are helping us develop this. We know there are going to be bumps in the road and the reason we have chosen you is because you’ve got that approach and that you will be prepared to put up with this and you will feed back. You know that we will take what you say and make changes as a result of that’. I think that’s really, really important. Try to as much as possible involve the early people in this as much as you can in the actual design and running of this, continually feed back to them. We always kept feeding back to people, ‘Has anyone come up with an idea? We’ve heard that this works, this is another way of recruiting people’, you know, this sort of stuff. ‘Here are the numbers, these people have managed to recruit this number of people. Let us know what trouble you’re having, we’ll see if we can sort it out’, and respond quickly every time… .
P026, Chief Investigator
Understanding the devices and predicting potential problems with the technologies
A number of problems related to the technologies researchers had tested were reported, typically in relation to hardware, software, accessories (e.g. chargers), and technical issues (e.g. passwords).
There were some issues with passwords which we thought we had solved at the start. There were some of the laptops that had password renewals that came into play after the first six laptops that were issued. We had to give instructions about how they could change their password. So although we thought we had turned that off we hadn’t done so, so for the final 14 or so we were able to ensure that they didn’t have that password notification come up on their laptops.
P025, Trial Manager
While problems are specific to individual technologies, a number of issues are likely applicable to many DHT studies/trials. Common issues reported by researchers included compatibility of software with different devices, outdated applications requiring frequent updates, managing mass number of remote devices, and connectivity issues (internet, Bluetooth to submit and receive data).
[T]here was an Apple update then that it messed with the apps and then there would be a period when they couldn’t use them and that sort of tech stuff, there was a few issues throughout the study follow up time where there was just periods where the participants weren’t able to test due to tech problems.
P021, Chief Investigator
Researchers reflected on how a number of these technical problems encountered could be mitigated through utilising familiar devices and simplifying technologies (as discussed in Stakeholder acceptability above), such as minimising opportunity for error by reducing the number of options participants can choose. However, a recurring subtheme was the lack of foresight as to what DHT studies/trials would involve to set up and deliver.
I think when we first… we got the iPods and we thought it would be a case of just turning them on, downloading two apps and giving it to the patient, but it turns out it’s not as simple as that.
P023, Trial Manager
It became evident across the interviews that researchers felt they had opened DHT studies or trials without really understanding how the technologies worked.
I think anything that’s tech based has to be very closely checked first of all for the potential for breakages or software errors or problems with the device… I would think that from a tech versus non tech perspective you would have to have all your options covered for the unknowns which are more so than other non tech based studies.
P025, Trial Manager
Several statements from researchers reported that they felt many issues would have been prevented, or resolved more efficiently, if they, or another on the research team, had more knowledge of technologies. This led to a suggestion from most that research teams need to have digital expertise within the team from the very beginning, concept development stage, whether provided internally (within the institution) or externally (such as commercial partners).
In terms of leadership I think it would be very useful to have someone with experience in the trial group, some who are experienced in setting up devices and all that because the trial team consists of a trial manager, a statistician, a database manager, a qualitative researcher and none of these people are particularly clued up on how to set up Apple devices for example. So I think yeah, you would want to have someone like that within the trial team.
P023, Trial Manager
It was also suggested that feasibility studies have an important role to play in ensuring all stakeholders understand the technology and that potential problems with the digital technologies are identified and resolved at a very early stage, prior to progressing to trial. Below is a quote from one Trial Co-ordinator discussing this when reflecting upon the numerous problems they encountered with the devices being studied in their trial.
I think some kind of pilot study or feasibility study would’ve been very useful in identifying some of the issues and they’re addressed before we go into a full trial or a full study; hindsight.
P023, Trial Manager
Resource planning
Across researcher accounts, the under-resourcing of DHT studies/trials was repeatedly mentioned. The complexities of setting up and distributing technologies placed significant time demands upon trial managers and research fellows who were often unfamiliar with technologies, which likely worsened the time-consuming nature of these activities.
The intervention took a lot of setting up, you know? I think we were probably under resourced, I think we probably needed more resource than we actually had to set up the technical infrastructure and architecture of the intervention. So the complexity in setting that up.
P027, Chief Investigator
For some, these activities were delegated to sites; however, research site staff were frequently unable to accommodate this around their routine clinical research roles and often pushed back to the central research site. Another significant demand upon trial managers’ time was the provision of support to both site staff and participating patients.
[W]e had a single mobile phone included to act as a patient helpline and the calls to that were considerable and that was a huge massive resource but I don’t think we had fully anticipated how much it would be used and how necessary it was.
P021, Chief Investigator
Most reported feeling as though they were running a support helpdesk, which in addition to time demands was at times frustrating for study/trial staff as they generally felt unprepared to provide this support. Researchers reported feeling as though they did not know the technologies any better than the site staff or patient participants, and resources for ensuring adequate support were required.
As a trial manager you’re quite busy and then to then have to deal with a technical support helpline, it was actually quite time consuming and difficult because you’re trying to resolve issues over the phone with elderly patients and it can be quite difficult to resolve issues in that way. So I think if you’re implementing a digital technology you have to have factored in support from the start and that can be with a helpline, with regular calls going to patients. We tried to… every now and then we would try and call patients for whom we haven’t received any data after a certain amount of time, and, so that was another thing that we hadn’t particularly considered but was time consuming, and also support to the sites, they’re the ones training the patients and, so they also needed technical support. Then from the trial management point of view, I personally didn’t set up the devices because they were based in [city name], but there was someone there who’s actually the qualitative researcher, the responsibility fell to them to set up the devices, which was completely out of their job description. So yeah, factoring in costs and resources for device set up and device support.
P023, Trial Manager
The challenge of providing this support depended upon the study/trial population. For example, it was difficult to provide support to technologically inexperienced users remotely over the phone.
I think sufficient support is really important. It depends on I think obviously the patient population and my experience is with age related disease so they’re an elderly bunch and, so they need technical support, well a lot more than the normal population, and also providing support to the sites. I think yeah, any trial using digital technology will require some level of support, some more than others, so I think that’s really important.
P023, Trial Manager
Balancing providing this support with time demands for addressing unexpected technical issues (as discussed in Understanding the devices and potential problems) and more routine study/trial management activities was a frequent challenge. In addition to the time and human resource demands, several issues were reported in relation to the financial resourcing of DHT studies/trials. Researchers often reported unexpected costs connected with the technologies, many of which were related to lack of researcher experience with technologies (as discussed in Understanding the devices and potential problems). For example, the need for multi-device management licences – which, while beneficial for the study/trial as they allowed important functions like sending out updates automatically, were an unexpected significant cost which went beyond planned budgets – led to the use of less optimal alternatives.
. . . so the Apple, the fact that we were using Apple devices so they needed multiple device management system, which we hadn’t costed for in the grant, so we then had to use the standard Apple one, which had particular constraints
P021, Chief Investigator
Factoring in requirements for spare equipment, particularly accessories (such as replacement chargers) that were expected to get lost or broken, was also cited by researchers. A further complexity of resource planning was reported as the unpredictable changes in technology costs and availability. One researcher highlighted that while technology costs are expected to reduce over time, this is not always the case, ‘I think technology gets cheaper over time but I guess costing is a factor because certain things get cheaper but certain things get more expensive’ (P025, Trial Manager).
Researchers recommended that future DHT studies/trials carefully plan resource requirements, especially in relation to the time and human resource to manage technical support required by participants and sites. There was also a belief among researchers that more resource-efficient ways of delivering technical support be explored. For example, apps could have inbuilt help sections that are easy to access and understand.
I’m just trying to think of ways around the support without burdening the clinicians further, it’s having patients help themselves, it’s self-taught. A little practice or a little demo that’s in there, so again the app itself, make a game of it; take people through and show them, okay, you want to enter these data here. Where do you go? It’s got a little button flashing and boink, and we reiterate, now where do you go? But this time the button doesn’t flash and it’s all about inbuilt training of getting people used to the layout and where the steps are. Little videos, for the trials we often try and have little, short ones, almost like Twitter snapshot training videos. Anything from little cartoons with a voice-over to handheld actual physical videos. Then maybe testimonials from patients who have used it. Top tips from people who have actually used the blooming thing.
P022, Trial Manager
Ensuring adequate feasibility testing of technologies, as suggested in Understanding the devices and potential problems, was reported as one approach to improving resource planning for DHT pilot studies and full-size trials in the future, allowing researchers to monitor resource needs on a smaller scale, prior to scaling up.
Peripheral infrastructure: institution, regulation and funding
Researchers discussed several challenges imposed by, or made more problematic through, insufficient support from peripheral infrastructure systems such as academic institutions, regulatory bodies (e.g. research ethics) and research funders. For example, several researchers reported that their academic institutions were not set up to provide the technical expertise required to run DHT studies/trials.
[W]e worked with the computer scientists and result of enthusiasm about getting that going, but the actual support provided to those of us working in digital health, there’s not a great deal about actual practical go-to sought-out support. We saw… this sounds a bit moany but we visited a unit in [city name] that were developing digital applications and, to be quite honest, the actual applications that they were developing were no better than anything I’ve seen around here. They’ve just got that team of their own programmers that they can access and can help them with that sort of stuff.
P027, Chief Investigator
The additional and often complex movement and storage of patient data which frequently occurs in DHT studies/trials posed challenges when navigating regulatory bodies. For example, several researchers reported having ethics approval difficulties due to the monitoring devices collecting data in patients’ homes and transmitting data via third parties, potentially breaching patients’ safety, data security and confidentiality.
GDPR, the data protection impact assessment, the risk assessment around personal data was a huge thing for our sponsor… and so it took a long time to show them that’s it’s okay… because of the personal data aspect it’s all being collected on a widget that’s outside of their control, your control, it’s in a patient’s home
P022, Trial Manager
This was reported as becoming even more problematic for multisite studies, where each geographic regional approval body may have different information governance, research and development processes for studies/trials involving DHTs.
The IT governance thing is a bit of a nightmare, you know? And it’s absolutely bizarre that a country like Scotland, how you have to go through this 13 or 14 times, everyone has different questions, everyone has different ideas, everyone has different ways of using the software. We did this recently with a piece of work we did on tele Covid, which is a method of monitoring Covid at home. Oh, it took forever, you know? The epidemic was almost over by the time we got permissions, you know?
P026, Chief Investigator
A fundamental problem for DHT studies/trials expressed by researchers was the clash between the fast pace of technologies and the inflexible and time-consuming research infrastructure, which resulted in lengthy delays awaiting approval for changes, and as reported by some, contributions to research waste. As reported by two researchers, the duration between research grant application, research grant award, regulatory approvals and opening a trial could be several years, by which time the technologies proposed for evaluation are no longer current.
I think the other challenge was, again, the technical side of things, it wasn’t a particularly mature intervention that we were trying to get set up, it was kind of out of date by the time we were setting it up and it needed to be updated…
P027, Chief Investigator
Another reported contribution towards waste in DHT research is the lack of stopping rules, which are common in other trials (e.g. drug trials). These rules give clear criteria to stop when there either appears to be no benefit, or in the event that a better treatment becomes available. One researcher argued that this should also apply to DHT studies/trials if a new and better technology becomes available. The solution proposed by several researchers is to develop a more agile and responsive funding and regulatory system for DHT studies/trials, one in which research protocols can be changed and implemented quickly and where minor changes in technologies no longer necessitate significant resource to approve and implement.
So I think there’s bound to be a more efficient way of getting the answers that you need quickly and making sure that research resources et cetera, et cetera, aren’t squandered in the process and people’s time and effort and all of that. So yeah, that’s kind of… and the amount of time that it takes from an HTA… ours was a commissioned call and it went through various iterations and then by the time contracting(?), all the desperately long drawn-out processes that are necessary in a big NIHR trial it’s just the complete opposite of how this kind of digi-health thing works.
P021, Chief Investigator
If Apple decide to make a change and therefore the provider of the app has to make a change in accordance with Apple’s change, then do you have to go back to the start again? These questions need to be sorted out. Your phone is updating itself every three months, you know? You can’t be going back to the MHRA every time.
P027, Chief Investigator
A more streamlined approach to approving data management and assessing data risks and security is also necessitated. From an academic institution perspective, more networking of DHT researchers alongside IT and technical specialists was proposed as an important step towards improving the quality of DHT studies/trials and would help prevent and resolve a number of technical issues (as discussed in Understanding the devices and potential problems).
Effective relationships with commercial partners
For researchers involved in delivering DHT studies/trials with commercially developed technologies, relationships with commercial partners led to several challenges. These were, namely, delays in receiving technical help to resolve problems, delays in access to data, removal of support following a company decision to withdraw a product from market, and support withdrawal following the commercial partner being acquired by another company.
[O]ne of the companies got bought by another company… it made things a bit complicated for a while. The other app, the company that owned it discontinued it. So they continued running it for our trial but support was absolutely minimal because they were discontinuing it.
P023, Trial Manager
One researcher also raised the challenges of maintaining good relationships with commercial partners when results fail to demonstrate evidence of benefit of the technology under evaluation.
But in COPD [chronic obstructive pulmonary disease], it was very clear it didn’t work at all, it just doesn’t work, you know? Didn’t reduce the number of admissions to hospital, it increased workload, you know? It was no good. I’ll tell you, you’re never very popular when you come out with a result like that, particularly with tech companies, they are really cheesed off.
P026, Chief Investigator
For researchers, these problems emerged due to having poor relationships and communication plans with commercial companies, and a lack of, or poorly termed, contractual agreement with commercial partners. For researchers who felt commercial partnerships had been beneficial, clear contracts, enthusiasm, willing support and investment from the commercial partner were critical components of this relationship. It was also highlighted by some researchers that large commercial partners have significant human resource dedicated to supporting DHT studies/trials, not just in relation to technical support, but on the regulatory support. For example, one researcher reported how their commercial partner facilitated all ethics and R&D approvals, using their own experience and expertise to navigate this efficiently and freeing up the research team to focus on their own expertise, trial design and set-up.
. . . just even from working more closely with them you’re getting more of an insight of how a health type start up works and the processes and the agile situation that they have and ways of doing things quickly and efficiently.
P021, Chief Investigator
One researcher reported how she felt commercial technology companies are more motivated than pharmaceutical companies, as often the product is their main focus for business development due to their limited portfolio of products.
They seem to be much more engaged and enthusiastic and interested than, I don’t know, a thumping great pharmaceutical company that seems to begrudge giving their tablet for an academic site to go and test. But I think some of it is also that the company relies on the outcome of this. That’s where it’s different to a thumping great pharmaceutical company who, they’ve got loads of products, they’ll keep the money rolling in, whereas this company… I think they do have other apps and things, but this is the one that’s their newest I think, they want to test it properly.
P022, Trial Manager
The relationship the research team has with commercial partners appears to be an important one, and one in which researchers reported that having a clear contractual agreement outlining agreed expectations and requirements from both parties was key in reducing some of the burdens they encountered.
. . . some form of agreement with the third parties or the app developers to make sure that if certain things need to be changed that they are there… they will be willing to provide support and make certain, obviously they can’t change everything, but to make some minor changes.
P023, Trial Manager
Digital exclusion
Frequent concerns were raised by researchers about study or trial design leading to digital exclusion of participants, which was perceived to lead to recruitment difficulties and unrepresentative samples that fail to reflect the disease population being targeted. Among researchers interviewed, this arose from participants lacking personal devices such as smartphones, and/or lacking access to the internet.
So again, it’s that thing of then how you think hard about preventing the digital exclusion because if I was redoing [study name], there is part of me would say you don’t even attempt to give people new devices, you just use their own smartphone or, and then you cut out so much of the… there isn’t issues about somebody with familiarisation to a new device. But yet then on the other hand then you still then have that whole big problem of the digital exclusion. So I don’t have an easy answer as to how that works or whether you just accept that there is a batch of people that you can remove from your follow ups or see less and that will help everybody else and just make sure that the people that then aren’t part of the home monitoring thing are still being seen by a speciality. So yeah, it’s a tricky balance with that.
P021, White, Chief Investigator
It struck me, it was actually our Steering Committee, so our Chair there pointed out that 80% of the problem with alcohol related liver disease is in the 20% lower socioeconomic group, and who’s less likely to have a smartphone? You can’t say that they won’t, but it’s just that on average, so there is something about still being able to reach everybody, our NHS, free at the point of care for everyone and I know that… because we did discuss providing smartphones if some didn’t have their own but that was quickly vetoed because people need to be familiar with the technology.
P022, Trial Manager
While researchers agreed that device familiarity was important and allowing participants to use their own devices as far as possible was seen as helpful for engagement, the concern about excluding those who did not possess such technologies was evident. Providing devices was the obvious and frequently reported potential solution. However, as reported by many, lack of familiarity replaces the problem of lack of technology, and many doubted whether it would help. This option was also reported as including significant financial costs.
. . . because if they’ve got the tech then they’re used to that tech and they know how to use… and we’re all the same, I know how to use my phone, it’s an iPhone, whereas if you give me your Samsung I’m lost, I don’t know what to click, what to do with it. So if you’re signing me up to the study and then you give me a new laptop or a new tablet on top of that I’m like, ‘Okay, firstly, I don’t know what I’m doing, and secondly now you’ve given me something else new to play with’, which again, makes matters worse, so I don’t think that’s going to help in that aspect. The ones that didn’t have the tech, I don’t think giving them the tech would help because I would say they don’t have the tech for a reason, as in they’ve chosen not to…
P023, Trial Manager
Access to the internet, so often required in DHT studies, can also be a problem. One researcher reported how they tried to overcome this by providing a second device allowing the primary device to connect to the internet regardless of what the participant has available. However, rather than reduce digital exclusion, this seemed to increase it; the second device only added more complexity and resulted in a number of participants dropping out of the study. The agreed problem of digital exclusion, as yet, remains without recommendation.
. . . even with us trying really hard, because we provided not just the device… but we also provided a MiFi device with a mobile contract for internet access, so that was our attempt at trying to avoid the digital exclusion, but you can see that it’s still there. So I think one of the biggest challenges is addressing that in whatever way you do a bigger study.
P021, Chief Investigator
For some, digital exclusion was anticipated to be less of a problem in the future.
Well I think as we move forward in time it’ll be a lot easier for a patient to do it on their own device and as the population becomes more technologically literate that will be more and more acceptable, and I think patients will be more open to that.
P023, Trial Manager
For now, the challenge of overcoming digital exclusion remains, without solution, for many of the researchers.
Chapter summary
Although researchers reported multiple challenges encountered while carrying out DHT studies/trials, they also had many suggestions as to how many could be prevented or overcome. The common barriers were low stakeholder acceptability, lack of understanding of digital technologies, poor resource planning, insufficient peripheral infrastructure, problematic relationships with commercial partners, and the unsolved dilemma of digital exclusion. The findings illustrate that researchers in the UK carrying out DHT studies/trials encounter a number of challenges impacting on the successful design, conduct, and delivery of DHT studies/trials and potentially leading to wasted research efforts. This broader exploration of feasibility issues surrounding DHT studies/trials highlights significant agreement about critical trial design and conduct issues that require consideration in DHT studies/trials.
Chapter 6 Developing a conceptual framework for the economic evaluation of home monitoring glaucoma
This chapter reports on the work conducted to address the research objective to developing a conceptual framework for the economic evaluation of home monitoring for glaucoma. Three areas were explored to define the framework: (A) the resource use implications of introducing home monitoring for glaucoma; (B) the drivers of patient preferences as sources of patient utilities; (C) the feasibility of the decision modelling approaches to incorporate home monitoring as a comparative strategy using a structured literature review.
Economic evaluation is the comparative analysis of two or more healthcare strategies in terms of both their cost and consequences. As this is a comparative analysis, the interest of the analyst is focused on the drivers of the difference in cost and consequences. This explains the exploration of areas (A) and (B). Moreover, decision-analytic models are usually needed to synthetise all the available economic evidence; this explains exploratory area (C). The next sections present the work conducted, with methods and findings. These are followed by a synthesis of the findings (Section D) using the framework proposed by Gomes et al. for the economic evaluation of digital health interventions (DHIs). 54
Resource use implications of introducing home monitoring for glaucoma
We explored the resource use implications of home monitoring by obtaining the views of: (1) clinical experts not directly involved in I-TRAC; (2) clinical staff from the I-TRAC sites; and (3) I-TRAC participants.
Methods
Data collection
Previous chapters described the focus group discussions with clinical staff not involved in I-TRAC, expert glaucoma clinicians (see Chapter 3), and I-TRAC site staff (see Chapter 4). Patient participant interviews were conducted alongside the staff interviews to understand the feasibility of glaucoma home monitoring from patients’ perspectives (see Chapter 4). As part of their interviews, I-TRAC participants were asked to verbally answer questions relevant to healthcare resource use during the glaucoma home monitoring, which included: (1) whether they had contacted the HES about glaucoma monitoring and, if so, the number of times they had contacted HES; (2) if the patient had not contacted HES, a follow-up question was asked regarding whether the respondent thought about contacting HES; and (3) other health service use, such as being admitted to hospital or visiting their GP. In addition, site staff were asked to record any contact they had from the participant throughout the 3-month home monitoring period, including the reason for the contact.
Finally, IOP measurements from the iCare HOME tonometer were retrieved and discussed with the I-TRAC clinical collaborators in an online meeting. The aim was to identify how clinicians would use home monitoring data, and the IOP thresholds that would trigger further assessments.
Data analysis
Full details of expert glaucoma clinicians, site staff focus groups and interviews, and patient participant interviews can be found in Chapters 3 and 4. For the purposes of this section, the analysed data in Chapters 3 and 4 were re-examined and considered for any findings relevant for resource use and costs.
The tonometer data were extracted on 29 November 2022 (cohort 1 and cohort 2, 27 participants in total). The original data were aggregated into a mean daily IOP measure per individual. A decision table, including mean, median, minimum and maximum IOP measurement from the home tonometers as well as the baseline characteristics (e.g. age, diagnosis and medical history) of the participants, was created and sent to three I-TRAC clinical colleagues. The clinical colleagues initially made individual decisions on which participants they would like to put under additional clinical observation and eventually see earlier than planned. A discussion meeting was held on 7 December 2022, during which clinicians were asked to make decisions regarding whether the IOP measurements from the home monitoring tonometers would trigger earlier-than-planned visits. In cases of disagreement after discussion, a consensus agreement was achieved for each case.
Findings
Resource use findings from site staff and glaucoma expert clinicians
Expert glaucoma clinicians not involved in In-home Tracking of glaucoma: Reliability, Acceptability, and Cost
While some clinicians believed that home monitoring could free up staff time, others stressed a number of situations where additional hospital resources would be needed. First, extra staff time will be needed to review the device data and take relevant actions. Second, unreliable data from the tonometer or data incompatibility with the existing medical records can increase the burden on NHS staff. Third, signs of disease progression from the device data may trigger contacts to HES if device readings are visible to home-monitored patients.
Regarding data collected from home monitoring, as the device data are currently not well integrated with the existing medical records, software development may be needed to resolve the issue. Clinicians also mentioned the potential use of AI, for example to interpret data and only highlight to clinicians any abnormal findings. AI may help to conduct some primary review of home monitoring data, making clinical decision-making more efficient.
Clinicians viewed the current home monitoring device used in the I-TRAC study as an expensive technology due to the equipment cost and the maintenance and patient training needed; however, technology advancement might help to reduce these costs. Home monitoring was also seen as a way to manage overtreatment and thus reduce unnecessary costs of treatment. In the current post-pandemic NHS environment, clinicians are uncertain on the time of the next patient assessment. Therefore, clinicians might opt to treat patients instead of maintaining them under no-treatment and observation.
In-home Tracking of glaucoma: Reliability, Acceptability, and Cost site staff
Several issues which may trigger further resource use were mentioned in the interviews with site staff. First, issues with logistics were reported, including challenges with the equipment return and booking appointments with patients after they finished their home monitoring period. Moreover, additional devices might be needed if these get lost or delays are experienced during the return process (e.g. due to the time needed to get the devices ready for the next patient). While these issues are important for any home monitoring service provision, they are particularly relevant to a future study.
Second, the resource use implications on patient training were mixed. On the one hand, site staff reported a decrease in staff time on patient training due to increased experience. In addition, site staff brought in the idea of group training, which can be more time-efficient and reduce staff time devoted to training. On the other hand, as participants might not fully use the provided support at the site, additional technical support might be needed during the monitoring period. In addition to the devices being used by patients, extra devices are required to allow site staff to train the next cohort of patients using the monitoring devices. These requirements imply additional equipment and staff cost.
Resource use findings of patient participants’ contacts to hospital eye service
In Table 22, the findings of patient participants’ contacts to HES are summarised. Of 39 patient participants, 31% contacted the HES. Notably, one patient participant visited the hospital four times and phoned the service twice during the study period. Of those who contacted the HES, patient participants either chose to visit the hospital in person or phoned the HES, with a similar frequency. Four had other health service use in relation to their glaucoma, two of whom were routine check-ups at the local optician or optometrist. For site staff contacts, a considerable number of patient participants (i.e. 48% of total) contacted the local site during the study period. The main reason for the contact was about technical issues encountered when using the device or inability to use the provided device, with only 2 contacts (of the 20 who contacted) concerned about the high readings on the tonometer, which have potential to trigger contacts with the clinical staff instead of the technical support. During the patient participant interviews, some pointed out issues with the training received for the use of the home monitoring devices such as insufficient training time, space pressures, and difficulties in using the tonometer. This suggests that additional resources on staff time and venues for patient training may be needed to manage the provision of a home monitoring service for glaucoma.
| Total number of patient participants, n | 39 |
| Total patient participants who contacted HES, n (%) | 12 (31) |
| Frequency of visits per person (who contacted the HES) | |
| Hospital visit | 1.7 |
| Phone the hospital | 1.2 |
| Visited at home | 0 |
| Thought about contacting HES, n (%) | 4 (10) |
| Accessed other health service related to glaucoma, n (%) | 4 (10) |
Resource use findings linked to triggers for clinical action in response to home monitoring measurement
From inspection of the decision tables, clinicians agreed that an IOP level of 30 mmHg or higher from the home tonometer would trigger clinical action, likely a review of the patient’s medical records, to inform a decision as to whether or not to see this patient earlier than planned. Using a rule of majority, it is suggested that for 26% of participants in our sample, clinicians would request an earlier appointment due to increased risk of progression (indicated by the distribution of IOP measurements during the 3-month study period). However, clinicians mentioned that in clinical practice a full review of patients’ medical history, age and other aspects of their glaucoma would be considered, and decisions would be based on the individual’s IOP targets instead of a general rule.
Echoing the findings from the interviews and focus groups with clinical staff in Chapters 3 and 4, clinicians expressed concerns on how the tonometer data would fit into the existing medical system, given the extensive volume of data points generated from the device. They suggested that an algorithm would be needed to signify possible increased risk and facilitate decision-making of further clinical management, for example a warning message sent to clinicians when the IOP measurement of the tonometer exceeds a certain threshold.
The drivers of patient preferences as sources of patient utilities
Methods
The perceived advantages and disadvantages of home monitoring were explored during the patient participant interviews as a way to identify the drivers of patient preferences for glaucoma home monitoring. The methods are reported in Chapter 4. The findings in Chapter 4 were re-examined to identify key sources of patient utilities categorised according to whether the perceived advantage or disadvantage directly led to gains in health-related quality of life (HRQoL) or not (i.e. non-HRQoL). The standard economic evaluation approach used for HTA in the UK usually incorporates only HRQoL. 55
Findings related to patient preferences
The major HRQoL-related benefit of home monitoring is having control over frequency of monitoring, which helps to catch any early sign of disease progression, resulting in better clinical outcomes in the long term, such as less vision loss. Non-HRQoL-related benefits of home monitoring include convenience (i.e. no travelling and waiting needed compared with face-to-face consultation) and reduced HES visits if disease appears to be well controlled (i.e. allowing better resource allocation to improve the standards of health care, a type of other-regarding preference). Speaking of disadvantages of home monitoring, participants were concerned about the inaccuracy of the device data due to the devices themselves and/or participants not being able to use them properly. In either case, inaccurate data can lead to information asymmetry between health providers and patients, and finally cause delayed diagnosis of disease progression or delayed treatment. In addition, participants worried that they would have little chance to receive reassurance from the clinicians compared with traditional face-to-face consultation (a non-HRQoL-related effect on utility).
Feasibility of modelling approaches: the structured literature review
In order to determine the feasibility of modelling approaches for home monitoring of glaucoma using digital technologies, two literature reviews were conducted. The first review was a systematic review of economic evaluations assessing monitoring strategies for glaucoma. In particular, we were interested in the methods used to translate relevant clinical outcomes from the home monitoring devices into long-term health economic outcomes. Also of interest were the economic modelling approaches used, as the particularities of home monitoring might need modelling approaches not often used in healthcare evaluation (e.g. condition-based Markov models). For example, a service-based model might be more appropriate when the proposed change has profound implications for the way the healthcare service will be organised (e.g. running a remote and face-to-face service simultaneously). In order to complement the original review of economic evaluations of home monitoring technologies for glaucoma, a scoping review of the literature evaluating home or remote monitoring in other (chronic) health conditions was conducted (the second review was not a systematic review and therefore the two parts were titled as a structured literature review). The next two sections present the methods and findings of the literature reviews.
Application of economic evaluations in glaucoma monitoring studies
Methods
Eligibility criteria
Population
The population included are adults (age ≥ 18 years) with diagnosed glaucoma or OHT (i.e. IOP > 21 mmHg).
Intervention
We included studies conducting economic evaluation of any glaucoma monitoring strategies in any setting; that is, in or out of hospital.
Comparator
Eligible studies must state the group comparator (e.g. standard care or usual care) against the intervention group. Eligible studies can also include a situation in which no active or organised monitoring service is available.
Outcomes
The primary outcome of interest was HRQoL outcomes, costs and quality-adjusted life-years (QALYs).
Study type
The qualified studies must be full economic evaluations [i.e. cost-minimisation analysis (CMA), cost–consequence analysis (CCA), cost-effectiveness analysis (CEA), cost–utility analysis (CUA) or cost–benefit analysis (CBA)]; that is to say, both health outcomes and costs for the intervention and comparator should be considered.
Search strategy
The search was undertaken in December 2021 covering two databases: MEDLINE and EMBASE. Only studies published after 2000 were included in the search. Only articles written in English were included, and editorials and letters were excluded. Reference lists from extracted articles were hand-searched to complement the review. See Appendix 8 for the search strategy.
Article screening
Initial screening of titles and abstracts and subsequent full-text screening were conducted by two reviewers independently (HW and RH). Any disagreements between the reviewers were resolved through discussion within the project team. Reasons for exclusion were noted and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 56
Data extraction and analysis
The following data were extracted from all the studies included: reference, authors, year of publication, studied country, patient population, study perspective, intervention and comparator, time horizon of the trial, analysis type, method of evaluation, the role of clinical outcomes (VF and IOP) in the economic evaluation, primary results [incremental cost-effectiveness ratio (ICER), willingness-to-pay (WTP) thresholds] and main conclusion (authors’ determined cost-effectiveness). One reviewer independently performed the data extraction, and another reviewed each item extracted. Any disagreements between the reviewers were resolved through discussion within the project team. A narrative synthesis was undertaken with presentation of tables and graphics.
Results
We identified 644 records from MEDLINE and EMBASE. After initial title/abstract screening and deduplication, 617 were excluded, leaving 27 for full-text screening. Finally, a total of four studies were deemed eligible for inclusion in the final review. 57–60 The selection process is summarised in Figure 4 using the PRISMA chart. Note that in the screening process, two pairs of similar articles were identified: Burr et al. 57 and Hernández et al. , and61 Crabb et al. 58 and Boodhna and Crabb. 62 The articles in each pair are the same regarding the main methodology and data inputs used and main conclusions made. Burr et al. 57 and Crabb et al. 58 were finally included in our analysis, as sufficient modelling details have been reported in these articles. A list of studies selected for full-text review and reasons for exclusion is provided in Appendix 9.
FIGURE 4.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram for the search of economic evaluation studies on glaucoma and OHT.
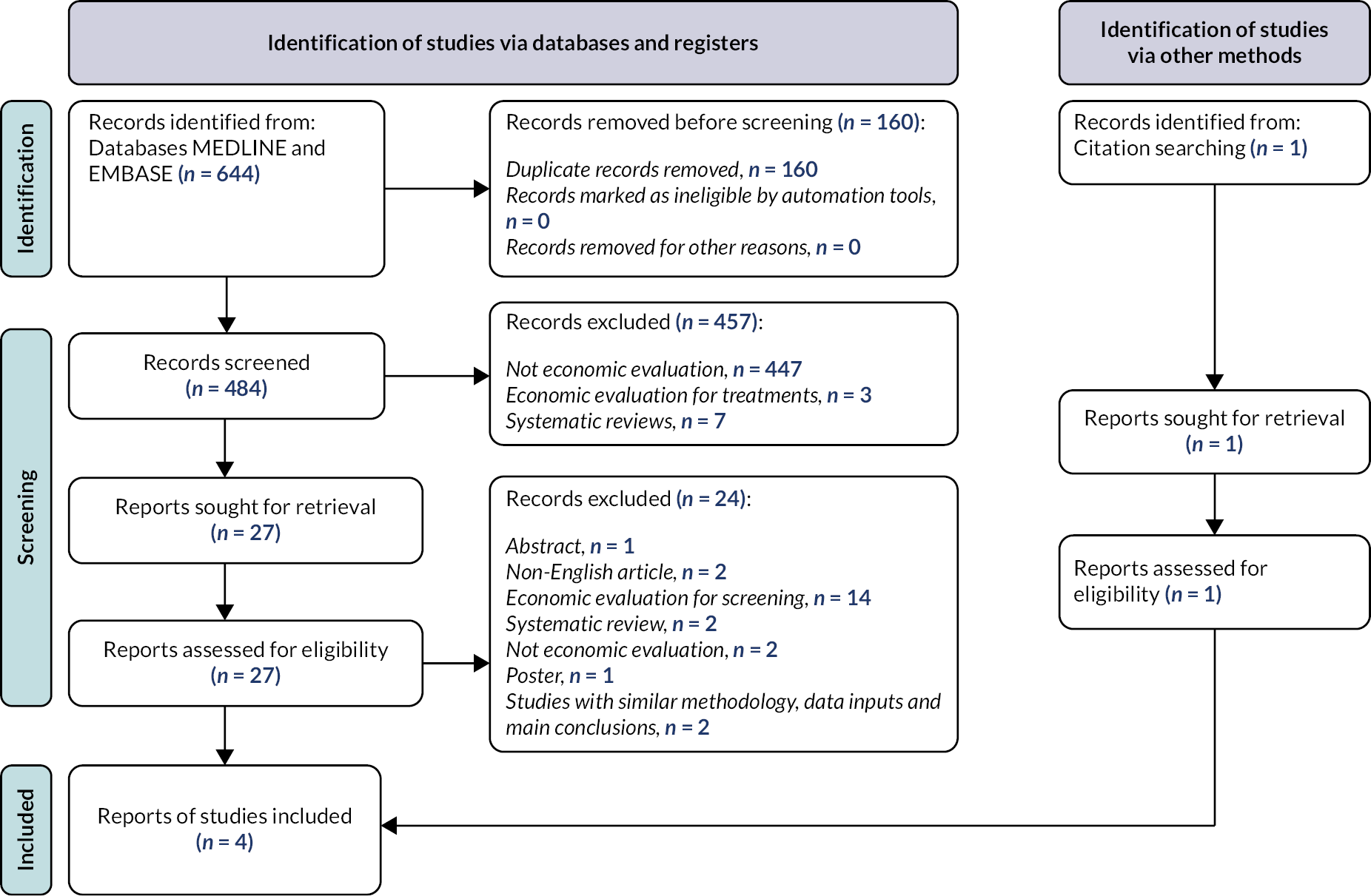
General characteristics of included studies
Two studies were based on the UK population,57,58 while the other two studies were based on the Netherlands population. 59,60 One UK-based study investigated patients with OHT,57 while all other studies focused on patients with chronic open-angle glaucoma (COAG). 58–60 As for the setting of the care pathways, one UK-based study considered both primary and secondary care in the economic evaluation from the perspective of the healthcare system,57 while the other three studies were based on secondary care only, but also provided analysis from the societal perspective. 58–60 Holtzer-Goor et al. 60 is the only study that conducted a comprehensive analysis from the perspectives of all involved parties (i.e. patient, healthcare system and society). In terms of the types of intervention, three studies explored the cost-effectiveness of alternative monitoring frequencies,57–59 while Holtzer-Goor et al. 60 focused on the maintenance of stable COAG patients by alternative (less expensive) health professionals. One study investigated a number of alternative monitoring pathways for OHT patients which differed in the setting (community vs. hospital), treatment decision and reassessment frequency (see Table 23 for details). 57
| Author (year) | Country | Targeted population | Setting (perspective) | Intervention | Comparator | Analysis type | Method of evaluation | Time horizon |
|---|---|---|---|---|---|---|---|---|
| Holtzer-Goor et al. (2010)60 | Netherlands | Diagnosed COAG | Secondary care (health system, patient and societal perspective) | Patients monitored by optometrists and ophthalmic technicians in a glaucoma follow-up unit in hospital. Optic disc assessment will not be conducted | Usual care provided by glaucoma specialists in hospital. Optic disc assessment will be conducted at every visit, but some visual function tests only conducted as required by the specialists | CMA | Trial (RCT, 410 vs. 405) | 30 months |
| Burr et al. (2012)57 | UK | OHT and suspected glaucoma | Primary and secondary care (health system perspective) | Four interventions are investigated: (1) SOH – community pathway: initial treatment decision is based on a risk calculator; check IOP within 2 months after the start of a treatment, monitored every 2 years in primary care; referral if converted or IOP off target. | Individuals are advised to attend a community optometrist annually for IOP check; all individuals with IOP > 21 mmHg are treated with PGAs, and referral if IOP off target. | CUA; CEA; CBA | Model (DES) | 20 years |
| (2) SOH – hospital pathway: check IOP within 2 months after treatment start; monitored every 2 years in secondary care; initial treatment decision is based on a risk calculator. | ||||||||
| (3) NICE intensive pathway: initial treatment decision is based on the NICE guidelines; check IOP after 2 months of start of treatment; full assessment intervals are based on the NICE guidelines and the lower bounds are adopted for this intensive pathway. | ||||||||
| (4) NICE conservative: initial treatment decision is based on the NICE guidelines; check IOP after 2 months of start of treatment; full assessment intervals are based on the NICE guidelines and the upper bounds are adopted for this conservative pathway | ||||||||
| van Gestel et al. (2012)59 | Netherlands | Diagnosed COAG | Secondary care (societal perspective) | Two interventions are investigated which differ in the intervals of VF test: (1) every 6 months; (2) every 24 months |
VF test every 12 months | CUA | Model (DES) | Lifetime |
| Crabb et al. (2014)58 | UK | Diagnosed COAG | Secondary care (health system perspective) | VF test 3 times a year in the first 2 years after diagnosis, and then test annually | Annual VF test | CUA | Model (Markov) | 25 years |
Regarding the types of economic evaluation, three studies conducted a CUA,57–59 and one study conducted a CMA based on a 30-month RCT. 60 Note that both costs and effectiveness are considered in Holtzer-Goor et al. , but effectiveness of intervention and comparator is assumed to be equal. 60 In a HTA report, Burr et al. also included a CBA and a CEA in addition to CUA. 57 Among the three CUA studies, one conducted a Markov model assuming a time horizon of 25 years,58 while two studies conducted a discrete event simulation,57,59 with one study assuming a 20-year time horizon57 and one study assuming a lifelong horizon. 60
The role of clinical outcomes in economic evaluation of glaucoma monitoring
As shown in Appendix 10, Table 30, MD of VF data is usually linked with glaucomatous stages, which is subsequently associated with different utility values representing different levels of QoL. 57,58 One study directly used VF (MD) as one of the predictors of utility values. 59 One study used pattern standard deviation of VF to inform the distribution of the baseline characteristics. 57 Two studies associated IOP outcomes with the subsequent treatment a patient would receive. 57,59 This is achieved by evaluating whether a patient’s IOP is on target after the last treatment, and then deciding whether an alternative treatment is needed. Two studies directly linked IOP with progression rate. 58,59 In Van Gestel et al. ,59 progression rate is a function of the difference between current IOP and the average IOP of the reference COAG population, while the reduction of IOP is translated to a constant rate of improvement in MD by Crabb et al. 58 Burr et al. 57 used IOP as a baseline characteristic of the model and one of the predictors of OHT patients’ risk profile, which affects the time to conversion. As only costs of interventions were compared in Holtzer-Goor et al. ,60 clinical outcomes were not used in the economic evaluation.
Overall, clinical outcomes such as VF and IOP are commonly integrated into economic models to inform simulated monitoring and treatment decisions, which finally affect the costs and QoL of different care pathways. The I-TRAC study also measured patients’ visual acuity and contrast sensitivity using the app. However, no significant link between these clinical outcomes and QoL has been established based on experts’ views. We also did not find such links in the reviewed studies.
Economic evaluations applied in glaucoma monitoring studies
As shown in Table 24, most interventions in the extracted studies were found to be more costly but yielded higher QALYs (Figure 5), with ICERs ranging from £21,392 to £186,805 per QALY gained. 57–59 Of the two studies in which WTP thresholds were explicitly specified, interventions in one study were cost-effective (i.e. ICER below the specified WTP threshold),58 while the other study was not cost-effective. 57 No definitive WTP threshold was specified in van Gestel et al. ;59 however, for the VF 6 months intervention, the probabilistic sensitivity analysis results suggest that only 14% of the ICERs were below the €80,000/QALY threshold reportedly recommended by the Council for Public Health and Health Care in the Netherlands. 63 In a CMA study, Holtzer-Goor et al. found evidence that monitoring of stable glaucoma patients in a follow-up unit by a group of less costly health professionals is cost-effective, yet the conclusion only holds under the assumption of equal quality of care between the alternative and usual care (i.e. the care provided by optometrists compared to that provided by ophthalmologists). 60 The authors justify the appropriateness of the assumption by discussing evidence from the literature.
| Study | ICER | WTP threshold | Author determined cost-effective |
|---|---|---|---|
| Burr et al. (2012)57 | (1) Biennial monitoring (Secondary care): £105,437 (2) Biennial monitoring (Primary care): dominateda (3) NICE conservative: dominated (4) NICE intensive: dominated |
£37,077 (£30,000) | N |
| van Gestel et al. (2012)59 | (1) VF 6 months: £186,805 (2) VF 24 months: £23,168 |
NS | NS |
| Crabb et al. (2014)58 | £25,884 | £35,819 (£30,000) | Y |
FIGURE 5.
Cost–utility analyses mapped on the cost-effectiveness plane (n= 3).
Overall, the cost-effectiveness of alternative monitoring frequencies (with either hospital or community care) investigated in these studies is not supported by strong evidence.
Economic evidence for home and (or) remote monitoring beyond glaucoma
The first review of economic evaluations of glaucoma monitoring did not identify any relevant studies of glaucoma home monitoring reported in the literature to date. We, therefore, conducted a scoping search for systematic review articles on the economic evaluation of home monitoring for other chronic diseases in order to identify lessons from economic evaluations in other home monitoring contexts that could inform decision-making for glaucoma. The interest was in economic evaluation approaches (i.e. what type of economic evaluation techniques were used, what decision-analytic models were used – intervention-based or system-based model), home monitoring cost categories and possible resource use changes (relative to the comparator) in the identified studies.
Methods
Eligibility criteria
Population
Individuals of any age who received remote patient monitoring (RPM) for any chronic disease, such as heart failure, hypertension and chronic obstructive pulmonary disease.
Intervention
Any remote monitoring interventions at home, including devices that allow for remote consultation.
Comparator
Standard or usual care where patients were monitored at a clinic or hospital.
Outcomes
Costs and health outcomes for home monitoring of chronic diseases.
Study type
Systematic review of any types of economic evaluation studies, including those using CMA, CCA, CEA, CUA or CBA. Simple cost analyses were excluded.
Search strategy
The search was undertaken in May 2022 covering two databases: MEDLINE and EMBASE. Only articles written in English were included. Editorials and letters were excluded. Reference lists from extracted articles were hand-searched to complement the review. See Appendix 8 for the search strategy.
Article screening
Initial screening of titles and abstracts and subsequent full-text screening was conducted by two reviewers independently (HW and RH). Any disagreements that arose between the reviewers were resolved through discussion within the reviewer group and with the project team. Reasons for exclusion were noted and reported using the PRISMA statement. 56
Data extraction and analysis
The following data were extracted from all the studies included: reference, authors, year of publication, studied country, patient population, study perspective, intervention, sample size, method of evaluation, time horizon of the trial, functionalities of RPM device, resource use and cost categories analysis type, primary results (incremental QALY and cost, ICER, WTP thresholds) and main conclusion (authors’ determined cost-effectiveness). One reviewer independently performed the data extraction, and another reviewed each item extracted. Any disagreements between the reviewers were resolved through discussion within the project team. A narrative synthesis was undertaken with presentation of tables and graphics.
Results
General characteristics of included studies
We identified 27 records from MEDLINE and EMBASE. After initial title/abstract screening and deduplication, 22 were excluded, leaving 5 for full-text screening. 65–69 A variety of health conditions were included in these systematic reviews. Following advice from the clinicians in the project team and with support of the SSC, we decided to focus the review only on home monitoring of hypertension due to the comparability between hypertension and glaucoma in disease progression, that is, slow, with no immediate action required on the part of patients post monitoring. For this reason, four systematic review studies that focused on non-hypertension diseases were excluded,65–68 leaving the study by De Guzman et al. as the only systematic review deemed eligible for inclusion (Figure 6 for the PRISMA chart). This is a recent systematic review that includes economic evaluation studies of remote monitoring for several chronic diseases (including hypertension) published from inception until September 2021. 69 Studies on hypertension remote monitoring from this systematic review were extracted and analysed. A list of initially excluded studies and further excluded studies in full-text review, and reasons for exclusion, is presented in Appendix 9.
FIGURE 6.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses diagram for the search of systematic reviews of economic evaluation studies on home monitoring.
Table 25 provides the general characteristics of the primary studies deemed eligible from the identified systematic review. In total, eight primary studies were included for data extraction,70–77 seven70–74,76,77 of which were published within the latest 10 years. Five studies targeted patients with hypertension (with or without age limits) and were conducted in Europe,71–73,75,76 two were conducted in North America,74,77 and one70 was conducted in Asia. In all included studies, the standard care of BP monitoring for hypertension patients was reported as a primary care setting. For the interventions, telemonitoring using a RPM device was the main intervention component reported in all studies, but some interventions also included remote counselling services. 72,74,77
| Author (year) | Country | Targeted population | Setting (perspective) | Intervention | Method of evaluation | N | Time horizon | RPM device | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Device description | Allow remote BP reading by doctors? | Allow remote communication with doctors? | ||||||||
| Ionov et al. (2021)72 | Russia | Uncontrolled hypertension | Primary care (healthcare system) | Telemonitoring with remote counselling | Trial (RCT); model (Markov) | Trial: 160 RPM, 80 UC | 3 months; 10 years | BP measurement device, smartphone app and web page | Y | Y |
| Teo et al. (2021)70 | Singapore | Hypertension (with or without hyperlipidemia); age ≥ 21 years | Primary care (healthcare system) | Telemonitoring | Trial (quasi-experiment) | 103 RPM, 115 UC | 6 months | BP measurement device, mobile network device | Y | Y |
| Monahan et al. (2019)73 | UK | Hypertension | Primary care (healthcare system and personal social services) | Self-monitoring only; telemonitoring | Model (Markov) | Based on 1182 from trial | Lifetime | BP measurement device, SMS, and website | (1) Self-monitoring only: N (2) Telemonitoring: Y |
(1) Self-monitoring only: N (2) Telemonitoring: N/R |
| Padwal et al. (2019)74 | Canada | High-risk hypertension patients | Primary care (healthcare payer perspective) | Telemonitoring with pharmacist case management | Model (Markov) | Based on 279 from trial | Lifetime | BP measurement device, web portal | N/R | N/R |
| Dehmer et al. (2018)77 | USA | Hypertension; age ≥ 21 years | Primary care (healthcare system) | Telemonitoring with pharmacist case management | Trial (RCT) | 148 RPM, 150 UC | 1 year | BP measurement device, modem | Y | N |
| Kaambwa et al. (2014)76 | UK | Hypertension; age ≥ 66 years (in the model) | Primary care (healthcare system) | Self-monitoring with self-titration of antihypertensives and BP telemonitoring | Model (Markov) | N/A | 35 years | Automated sphygmomanometer and equipment to transmit readings | Y | N/R |
| Stoddart et al. (2013)71 | UK | Uncontrolled hypertension | Primary care (healthcare system) | Telemonitoring | Trial (RCT) | 195 RPM, 188 UC | 6 months | Bluetooth automated sphygmomanometer, and mobile phone | Y | Y |
| Madsen et al. (2011)75 | Denmark | Uncontrolled hypertension | Primary care (healthcare system) | Telemonitoring | Trial (RCT) | 105 RPM, 118 UC | 6 months | BP measurement device and personal digital assistant | Y | Y |
Most studies attempted to explore the cost-effectiveness of the interventions from the perspective of the healthcare system or payer, while one study also analysed this issue from the perspective of personal social services. 73 In terms of the analytical model used, four studies conducted a trial-based economic evaluation only,70,71,75,77 three were extrapolation studies using a Markov model,73,74,76 and one72 conducted a trial-based economic evaluation together with an extrapolation analysis. However, the trial periods were generally not long (i.e. all ≤ 1 year), implying that a trial extrapolation is needed in conjunction with the trial to estimate the long-term effect of the intervention. No study used a system-based model for home monitoring for hypertension. Regarding the functionality of the RPM device used in the interventions, the majority of studies reported that the devices allowed remote BP reading by health professionals, while patients in one study19 had to write down the readings from a self-monitoring BP device and post them to the doctors for review. The functionality of the device used by Padwal et al. 74 was not detailed. Four studies reported that the BP devices used in their interventions enabled remote communication with doctors,70–72,75 yet the device used by Dehmer et al. 77 did not allow this functionality. Information is not available in the other three studies. 73,74,76
Resource use and cost categories
Table 26 presents the changes in resource use and cost categories for the included studies. Three studies reported the differences in resource use between the intervention and usual care;70,71,77 among them one71 found higher resource use in GP/nurse surgery consultation and nurse phone consultation for the telemonitoring group and no differences in other reported resource use, while two studies70,77 observed no difference between the intervention and usual care groups. However, results were not reported in the rest of the four studies reviewed, making it extremely challenging to draw any conclusions on which intervention is more costly.
| Author (year) | Resource use (impact of intervention) | Equipment cost (amount) | Human resource cost (amount) | Other costs (amount) | Total programme cost |
|---|---|---|---|---|---|
| Ionov et al. (2021)72 | N/R | N/R | N/R | N/R | £223 |
| Teo et al. (2021)70 | Incremental hyper-related face-to-face visit (no effect) | (1) Tele-vital signs monitoring subscription (£37) (2) Bluetooth BP device (£15) |
N/R | N/R | N/R |
| Monahan et al. (2019)73 | N/R | (1) BP monitoring (N/R) (2) Telemonitoring server – only for telemonitoring intervention (N/R) |
Self-monitoring training (N/R) | Forms and envelope (N/R) | (1) Self-monitoring (£64 for 6 months). (2) Self-monitoring and telemonitoring (£80 for 6 months) |
| Padwal et al. (2019)74 | N/R | (1) BP device: first 3 months (£74/year) + subsequent replacement (£74/year) (2) Data: first 3 months (£19/3months) + subsequent annual (£76/year) |
(1) Pharmacist consultation: first 3 months (£89) + subsequent annual (£35) (2) Physician: first 3 months (£11) + subsequent annual (£22) |
N/R | First 3 months (£193) + subsequent annual (£207) |
| Dehmer et al. (2018)77 | Office visits (no diff); hospital encounters (no effect) | N/R | N/R | N/R | £1121 |
| Kaambwa et al. (2014)76 | N/R | Equipment and training (£286/5 years) | N/R | £587 | |
| Stoddart et al. (2013)71 | (1) GP surgery consultation (higher). (2) Practice nurse surgery consultation (higher). (3) Practice nurse phone consultation (higher). No effect in other resource use reported. |
(1) BP device (£66 + £1/month) (2) Mobile phone (£60 + £2/month) (3) Server hosting (£1/month) (4) Web hosting (£3/month) (5) Sim card (£2/month) |
(1) Patient training (£15) (2) Nurse time checking HBPM data (£3/month) |
N/R | £88 for 6 months |
| Madsen et al. (2011)75 | N/R | BP device + PDA + mobile phone + SD card + BP measuring interface software (£113/6 months) | Training (N/R) | N/R | N/R |
Next, we focused on the cost breakdown analysis for home BP monitoring. The main reported cost categories were equipment costs and human resource costs. We defined equipment costs as the cost of BP equipment and its replacement, server and web hosting, data management, phone and sim cards used for remote monitoring. Human resource costs included the cost for patient training and cost for the time of health professionals used for monitoring and consultation. Six studies reported the total programme cost, which varied from £64 to £1121, reflecting differences in the contexts and length of trials. 71–74 Equipment cost accounted for the majority of the programme cost in two studies where both data were reported. 71,74 For example, Padwal et al. reported that the BP device and data management accounted for 48.2% of the total programme cost in the first 3 months of the trial, and accounted for 72.5% in the subsequent year. 74 Stoddart et al. also reported that the cost of BP devices, mobile phones, server and web hosting, and sim cards accounted for over 50% of the total programme cost. 71 Caution needs to be taken in the interpretation of the overall result as data were unavailable in the other six studies. 70,72,73,75–77 However, even in these six studies, four (i.e. 67%) acknowledged the relatively short length of trials they were based on as a limitation and expected that a longer home monitoring period would dilute the impact of equipment cost on total intervention cost and increase the cost-effectiveness ratio. 70,72,75,77 Padwal et al. (2019) recommended reducing the telemonitoring cost by gradually reducing the frequency of monitoring after BP is controlled. 74
Overall, there is mixed evidence on the impact of BP home monitoring on healthcare resource use. For the cost disaggregation analysis, equipment cost seems to account for a large proportion of the total programme cost, and a longer period of home monitoring may help to flatten the total annual cost, potentially improving the cost-effectiveness of home monitoring.
Economic evaluations applied in hypertension home monitoring studies
Economic evaluation findings are reported in Table 27. All five CUA studies reported that telemonitoring and/or teleconsultation is cost-effective compared with usual care,70,72–74,76 yet four of them found the interventions being more costly (Figure 7),70,72,73,76 suggesting that the main benefit lies in the QALYs gained from these interventions. Four studies conducted a CEA using systolic BP reduction as the effectiveness outcome. 71,72,75,77 The reported ICERs were £22.68,72 £115 [95% confidence interval (CI) £38 to £288],77 £32 (95% CI £20 to £58)71 and £31 (95% CI −£103 to £544)75 per mmHg reduction in systolic BP, which are quite similar except Dehmer et al. 77 All these studies reported higher total costs for the intervention group compared with usual care. Given the absence of a WTP threshold in these CEA studies, justification regarding the cost-effectiveness cannot be made.
| Author (year) | Analysis type | Incremental QALY (RPM vs. UC)a | Incremental cost (RPM vs. UC) | ICER | WTP threshold | Author determined cost-effectiveness of RPM |
|---|---|---|---|---|---|---|
| Ionov et al. (2021)72 | CEA; CUA | 0.490 | £202 | CUA: £8537 CEA: £22.68 per mmHg reduction in systolic BP |
£3102 (RUB 100,000) | Y |
| Teo et al. (2021)70 | CUA | 0.004 | £20 | £20,908 | £68,134 (SGD 78,000) | Y |
| Monahan et al. (2019)73 | CUA | (1) Self-monitoring only: 0.041 (2) Self and telemonitoring: 0.058 |
(1) Self-monitoring only: 139 (2) Self and telemonitoring: 479 |
(1) Self-monitoring only: £3412 (2) Self and telemonitoring: £8243 |
£22,484 (GBP 20,000) | Y |
| Padwal et al. (2019)74 | CUA | 0.830 | −£1222 | Dominant | Y | |
| Dehmer et al. (2018)77 | CEA | N/A | −£233 | £115 (£38 to 288)b per mmHg reduction in systolic BP | N/A | |
| Kaambwa et al. (2014)76 | CUA | 0.240 | £473 | £2007 | £24,718 (GBP 20,000) | Y |
| Stoddart et al. (2013)71 | CEA | N/A | £142 | £32 (£20 to £58)b per mmHg reduction in systolic BP | N/A | |
| Madsen et al. (2011)75 | CEA | N/A | £85 | £31 (−£103 to £544)b per mmHg reduction in systolic BP | N/A |
FIGURE 7.
Cost–utility analyses mapped on the cost-effectiveness plane (n = 5).

Overall, all extracted CUA studies reported that telemonitoring and/or teleconsultation is cost-effective against usual care. Most CEAs, although conducted in different country settings, reported similar ICERs in systolic BP reduction, yet the cost-effectiveness of these interventions cannot be confirmed without definitive WTP criteria.
Synthesis of findings
A number of studies have discussed the particularities of conducting economic evaluations of digital technologies, which argues that different from economic evaluations of pharmaceutical interventions, the distinctive features of DHIs may raise several substantial challenges to economic evaluation. 54,78 Gomes et al. provided a thorough discussion on key features of DHI and its impact on the design, analysis and reporting of an economic evaluation. 54 Following Gomes et al. ’s framework on the key differences between DHIs and pharmaceuticals/medical devices, we discuss the typical features of glaucoma home monitoring intervention and their implications on economic analysis of a future study. 54 Note that the discussion of product and user involvement are combined to save space. We also exclude the discussion on the impacts of home monitoring outside the health sector (e.g. impacts on productivity), as it is beyond the scope of this study.
Choice of comparators
Home monitoring can be complementary to or a substitute for existing face-to-face hospital consultation. This is an important distinction that will have implications for the care pathways and associated costs and consequences for the compared strategies. For example, home monitoring can be an additional part of hospital care for the high-risk group whose disease progression is uncertain, which would mean higher cost, but also help to provide better disease control. In this case, more intensive VF and IOP measurements from the home monitoring can be synchronised and shared with clinicians. Clinicians would be alerted, for example, whenever these measurements suggest uncontrolled IOP or disease progression. Alternatively, home monitoring could be implemented for low-risk patients, substituting the need for or reducing the frequency of any further face-to-face monitoring.
The length of time kept in home monitoring is also likely to differ by risk groups. For example, a short period of monitoring in addition to normal clinic visits could be useful to determine whether a change in therapy is required for the high-risk patients, while a long-term monitoring is more likely to be needed for low-risk patients. The implications of what glaucoma monitoring strategies are being compared expand beyond the care pathways, for example, to the decision-analytic model selected for the evaluation. If home monitoring is replacing face-to-face monitoring, the standard disease-based modelling approach might be suitable to assess cost-effectiveness. However, if home monitoring plus traditional face-to-face monitoring is compared with traditional face-to-face monitoring, the potential service bottlenecks might become an important feature of the evaluation. In this case, a service system model might be more appropriate to assess cost-effectiveness.
Product and user involvement
Economic evaluation needs to account for the rapid advancement of the device and more user-friendly apps and technical services provided by the product supplier, which will also have implications for costs and patients’ benefits. A considerable number of I-TRAC participants have reported difficulties in using the tonometer or app as well as a lack of technical support. Therefore, a more user-friendly interface of the device and more ready technical support in the future could increase adherence and reduce dropout rates. A more efficient system also implies a reduction in patients’ time (and thus cost), which can be important if a patient’s or societal perspective is taken into consideration.
Intervention cost
Results from the meeting with I-TRAC clinicians to discuss the data retrieved from the home tonometers suggest that clinicians will look at the whole history and clinical details of patients’ record rather than the device data alone when deciding on a further monitoring and treatment plan. Concerns on the incompatibility between device data and medical records from the existing digital system in the hospitals were shared by I-TRAC and non-I-TRAC clinicians. This suggests that additional cost on data storage and integration will be needed before home monitoring could be implemented at scale. In addition, the applicability issue of the device data itself was mentioned, as a large data set will be generated due to frequent monitoring. Therefore, an algorithm and the possible use of AI may be needed to aggregate the measurement data from the devices and send warning message to the clinicians in case of worrisome outcomes, further increasing the cost on software development and application. Rules for such communication would have to be developed and validated.
For DHI (e.g. the app used in I-TRAC), initial investment on the fixed costs, such as development of apps, may account for a large share of total cost, yet the average cost can decrease as scale increases – this has also been found in the reviewed studies for hypertension home monitoring (variable costs such as subsequent cost on upgrading and maintaining the products are often small and sometimes negligible). However, the scale effect is likely to be ignored when estimating an intervention effect using a short trial and/or a trial with a small sample size. Therefore, alongside a trial-based economic evaluation (assuming a future trial is needed), allowing for a sufficiently long period of time in an extrapolation model is recommended in order to fully assess the long-term costs of home monitoring.
Benefit assessment
One of the main drivers of glaucoma home monitoring is that it allows patients to have control over the number of times they can be monitored for disease progression. This is particularly important for those in employment who may have limited time for a hospital visit. Home monitoring may provide timely information outside the need for a normal hospital visit, which may be particularly useful when NHS clinic capacity and waiting time are increasing. However, caution needs to be taken here, as glaucoma is a slowly progressing condition for the majority of patients. Therefore, the likelihood of finding HRQoL differences compared with standard care is small. Non-HRQoL benefits can be important to glaucoma patients monitored at home. For example, participants mentioned convenience as an advantage, with home monitoring leading to decreased travel and waiting times and need to take time off work. They also mentioned that the convenience can be even more beneficial for those who physically cannot travel. Overall, 50% of participants preferred home monitoring compared with standard visits according to the interview results.
The characteristic identification exercise conducted in Section B is a good start, but an expanded investigation of benefits relevant to patients using qualitative or mixed-methods approaches, such as the nominal group technique, would be welcomed. 79,80
Economic analysis: concluding considerations for economic evaluation approaches
What clinical outcomes can be used in economic evaluation of home monitoring for glaucoma?
In the economic evaluation literature, clinical outcomes from the trials are often used as important inputs in the economic model. 81,82 In the systematic review of economic evaluation studies on glaucoma monitoring, we find that VF (the main marker of disease severity) and IOP (the main marker of risk of progression) are commonly used to inform baseline characteristics of simulated patients, link utility and model glaucoma progression. In van Gestel et al. , progression rate, represented by the deterioration rate of MD of VF, was modelled as a function of IOP. 83 This is sensible, as raised IOP can accelerate the speed of glaucoma progression. However, more IOP check-ups are needed to obtain a meaningful relationship between the two variables, which can be difficult to achieve in clinics due to increased cost. Home monitoring can be a promising alternative to obtain more IOP point estimates using a home tonometer. However, clinicians as well as choice of comparator of the home monitoring devices, which includes the reading itself and whether patients are able to use them correctly. The accuracy of the home monitoring devices will need to be accounted for if home monitoring data, such as IOP measurements, are going to be used in economic evaluation models.
While several app-based technologies have been developed to measure VF (such as the MRF), none of them had undergone CE marking at the time of the study opening and therefore were unable to be included in this feasibility study. Instead, the OKKO Visual Health app, which is CE marked and is approved for use in the UK, was used to measure visual function in this study. This app allows contrast sensitivity to be tested on portable devices such as smartphones, tablets and iPads using interactive games.
Types of economic evaluation approach for home monitoring glaucoma
Different economic evaluation approaches have been proposed and applied in the literature to investigate cost-effectiveness of a treatment or diagnostic intervention. 81 CUA is the standard approach suggested by NICE for HTA. 84 More than half of the included studies on home monitoring for hypertension applied a CUA, implying that utility difference between the intervention and the comparator were expected from HRQoL by the authors. However, CUA fails to account for non-health benefits of home monitoring such as convenience, which can be an important source of patient utility in home monitoring of glaucoma.
Several recent systematic reviews on economic evaluation of DHI recommended CCA as a reference case due to the special nature of DHIs. 54,78 CCA allows analysts to assess different types of costs and benefits and compare them in separate domains, which provides a comprehensive comparison between the intervention and comparator. This exercise encourages thorough identification, facilitates separate comparisons across different care sectors, and gives more flexibility to decision-makers to choose and weight the costs and effects that are important to them from their local context. 54 This is especially important as it empowers decision-making at local level, given the current heterogeneous HES models across different UK countries. CCA also requires disaggregating cost categories, allowing researchers to easily accommodate rapid technology change (e.g. a sudden reduction in equipment cost due to technology advancement). This is also echoed by the articles we have reviewed for hypertension home monitoring, in which detailed cost categories were under-reported, making them less informative in informing the types of economic evaluation that should be conducted for future studies. CCA is also recommended by NICE when high-quality economic evaluation studies are unavailable. 55
An alternative way of measuring benefits is CBA, which allows researchers to incorporate important non-health benefits of home monitoring. Stated preference methods, such as contingent valuation and discrete choice experiment (DCE), have been applied in healthcare studies. In the case of I-TRAC, results from patient interviews suggest that non-health benefits of home monitoring, such as convenience or reduction of travel and waiting time, can form a significant part of the total benefits of home monitoring.
Overall, given that key research questions have not been clearly defined at this stage, we recommend using a step-by-step approach leading to a meaningful economic evaluation; that is, an evaluation that provides information on the costs and benefits of the interventions in a way that allows decision-makers to make informed decisions about resource allocation. Conducting an impact inventory exercise is an essential first step to further identify and define cost categories in detail. CUA is a standard approach for economic evaluation but cannot reflect utility of non-HRQoL benefits. Alternatively, a CCA is recommended to determine the cost-effectiveness of glaucoma home monitoring in the absence of adequate economic evaluation studies on this topic. This suggestion is also consistent with recent methodological frameworks of economic evaluation of DHI. 54,78 For the consequences/benefits of home monitoring, more systematic qualitative research needs to be conducted to explore further sources of utility. This can be followed by a quantitative analysis, such as DCE, to quantify patients’ preference. By then, researchers can decide whether a CBA is a suitable economic evaluation tool on it, or as a supplement of other approaches. In the reviewed hypertension studies, several conducted CEA and reported ICERs regarding systolic BP reduction. However, stakeholders in different fields may have divergent views on what health outcomes are important and should be reported, whist utility data used in CUA tend to provide a broader measure of overall health benefits. In addition, compared with CUA, there is no widely used threshold for the ICERs from CEA outcomes; it is therefore difficult to inform policy decisions about the cost-effectiveness of the intervention, although cross-study comparisons are possible to inform decision-making. CMA assumes the equality of health outcomes and focuses on the comparison of total costs between the intervention and comparator. In other words, the use of CMA needs to be validated with strong evidence regarding the belief of insignificant differences in health outcomes.
To conclude, this chapter develops a conceptual framework for the economic evaluation of home monitoring for glaucoma by discussing the potential drivers of the difference in cost and consequences between standard glaucoma care and glaucoma home monitoring from results in previous chapters. A structured review is also conducted to explore the feasibility of using decision modelling approaches in the economic evaluation of glaucoma home monitoring. These are followed by a further discussion on typical features of glaucoma home monitoring intervention and their implications on economic analysis of a future study. Overall, the key categories of intervention costs of glaucoma home monitoring identified in this study include equipment cost, patient training, ongoing patient support during home monitoring, potential spill-over costs (e.g. high readings triggering hospital visits) and costs of data integration (to the existing medical records) and evaluation by AI, while key sources of patient utilities of glaucoma home monitoring are categorised as HRQoL related (e.g. more frequent disease monitoring and faster identification of disease progression) and non-HRQoL related (e.g. convenience). Given the complexity and scarcity of relevant evidence in the literature, it is recommended that a number of further qualitative/quantitative studies need to be conducted to better understand the study population, care pathways (i.e. of the interventions and comparators), cost categories and benefits of home monitoring and the appropriate time horizon for the analysis before a formal economic evaluation can be conducted. A step-by-step approach is then recommended to carefully explore what economic evaluation approach can be suitable in the context of glaucoma home monitoring.
Chapter 7 Discussion and conclusions
The overall aim of the I-TRAC study was to determine the feasibility and acceptability of digital technologies to monitor glaucoma at home and inform the possible need and design of a definitive evaluative study. Through a multiphase mixed-methods study design, I-TRAC sought to answer four interlinked research objectives, and the data addressing this have been presented in the preceding chapters. This chapter brings together the key learning from the study in the form of a statement of feasibility for a future evaluative study. We will draw largely on the empirical data generated from the study findings; however, we will also include important ‘lessons learned’ captured as notes and reflections throughout the duration of I-TRAC.
Feasibility of a future evaluative study of clinical and cost-effectiveness of digital technologies for home monitoring of glaucoma
The data from across all phases of the I-TRAC study were mapped to the AdePT framework in order to establish the feasibility of a future evaluative study of the clinical and cost-effectiveness of digital technologies for home monitoring of glaucoma. The AdePT framework contains 14 methodological ‘issues’ to be evaluated in feasibility studies or pilot trials. 51 The results of mapping the data from I-TRAC to the 14 methodological ‘issues’ in AdePT are presented in Table 28.
| AdePT methodological issue | Findings relevant for issue |
|---|---|
| 1. Did the feasibility/pilot study allow a sample size calculation for the main trial? | N/A – outcome data not used to calculate effect size |
| 2. What factors influenced eligibility and what proportion of those approached were eligible? | Clinical status of patient (perception of low-risk vs. high risk). Majority approached were eligible |
| 3. Was recruitment successful? | Yes. Recruited 42/45 (93%) within original recruitment window. Recruitment varied across three sites. Site staff interviews report ease of process. Largely white population |
| 4. Did eligible participants consent? | Yes. Variation across sites due to methods used to identify patients – need to consider impact of randomisation on future decision |
| 5. Were participants successfully randomised and did randomisation yield equality in groups? | N/A – not randomised |
| 6. Were masking procedures adequate? | No masking. Participants and site staff could not be masked in a future study |
| 7. Did participants adhere to the intervention? | Good – 67% adhered to tonometer and 60% to app – need to consider duration and frequency for future trial |
| 8. Was the intervention acceptable to the participants? | Yes. Generally acceptable to all stakeholders ‘cautiously optimistic’ Patients involved in I-TRAC home monitoring – interviews and 3-month assessment Site staff involved in I-TRAC home monitoring – interviews/focus groups Clinicians not involved in I-TRAC – survey and interviews |
| 9. Was it possible to calculate intervention costs and duration? | Yes. Decision on model for economic evaluation in future trial plus consideration of resource use and patient preferences |
| 10. Were outcome assessments completed? | Yes. 40/42 (95%) attended clinic for final outcome assessment 10 of 11 approached for interview were interviewed |
| 11. Were outcomes measured those that were the most appropriate outcomes? | Yes – for acceptability and feasibility Consideration of relevant outcomes for a future trial re clinical (e.g. IOP) and cost (e.g. healthcare contacts) effectiveness (e.g. QALYs and patient preferences) outcomes |
| 12. Was retention to the study good? | Yes. Retained majority of participants, n = 39 (93%) Three participants stopped using the intervention; of those, two also declined follow-up |
| 13. Were the logistics of running a multicentre trial assessed? | Yes. Lessons from running feasibility across three centres and from the interviews with research teams and site staff |
| 14. Did all components of the protocol work together? | Overall, yes but some key areas (i.e. training) need attention |
| Item not relevant for I-TRAC feasibility. | |
| Evidence from I-TRAC suggests this item has been partially met. | |
| Evidence from I-TRAC suggests this item has been met. |
We did not design I-TRAC to inform sample size calculation, randomisation procedure, or masking procedures – the latter because masking would not be possible in a future study due to the nature of the intervention. The issues relevant for I-TRAC will be discussed below.
Eligibility
Findings from the survey (see Chapter 2) and interviews and focus groups with clinicians (see Chapters 3 and 4) highlight a lack of agreement on which patient population would be most suitable for using digital technology at home to monitor their glaucoma. While generally there was agreement that high-risk patients would not be appropriate for home monitoring as a replacement for face-to-face examination, there was support for home monitoring with digital technology of low-risk patients. The possible benefits of adding home monitoring to current face-to-face clinic visits in high-risk patients were mentioned by some clinicians. This variation in agreement among clinicians was also evident from the way in which recruitment was approached across the three sites involved in the I-TRAC intervention study, with some staff approaching higher-risk patients for inclusion while others only approached those who are already being monitored through virtual clinics. It is also worth considering that different populations may be best suited to one or other of the interventions. For a future trial to be feasible, further clarification and definition of the patient population, and whether home monitoring would be used as a replacement for or an addition to the current model of hospital-based eye care, would be required such that there was agreement among the clinical community.
Findings from the qualitative interviews with expert glaucoma clinicians (see Chapter 3) also highlighted concerns in relation to patient characteristics that may influence eligibility and/or opportunities to participate. These included: accessibility, language, education, and technical abilities, which could lead to ethical consequences; how to select and prioritise patients for home monitoring (particularly where there will be resource constraints); impact upon those who are not selected for home monitoring; and the risk of creating a two-tier system (as some may have to contribute financially).
Recruitment
In-home Tracking of glaucoma: Reliability, Acceptability, and Cost recruited 93% (42/43) of its proposed sample size within the original recruitment window (1 October 2021 – 31 August 2022). The recruitment approach varied across each of the three sites. Originally it had been proposed that each site would recruit 15 patient participants each across three home monitoring cohort periods, with 5 participants in each of the three cohorts. However, given delays in approvals for one of the sites, recruitment was predominantly across two sites with the third recruiting to the last two cohorts. Site staff reported in interviews that I-TRAC was easy to recruit for due to significant patient buy-in to the interventions. However, staff did report perceptions about the samples recruited not being representative of the typical glaucoma population. This was predominantly linked to disease stage, age and ethnicity.
The representativeness of patients in DHT trials was raised in the external researcher team interviews; they believed that trial populations often over-represented individuals who were white, younger, highly educated and had previous access to technology and the technological confidence to participate (see Chapter 5). Linked to this, it is important to note that 94% of patient participants in I-TRAC identified as white and only two (5%) reported not being a current user of a smartphone or tablet. Any trial evaluating home monitoring technologies should use strategies to target eligible patient groups that are not currently recruited to these trials.
Consent
Two centres prescreened participants before approaching, which resulted in 100% of participants consenting to participate. In the third site, there was a much lower proportion of participants who consented (16%). This was likely due to this site not prescreening but rather approaching all potentially eligible glaucoma patients in clinic waiting areas – which may be the case for a future large evaluative study. Where provided, reasons for non-participation were linked to a lack of confidence or interest in technologies. This was supported by findings from external researcher interviews which highlighted low digital literacy and a lack of social support as barriers to participation in trials of DHT more broadly (see Chapter 5). Patient participants reported reasons for participating in I-TRAC to be based on recommendations of their clinician but also were often motivated by their own history of glaucoma and interested in engaging with new technology and/or the research project. These motivations may also translate to decisions to participate in a future evaluative study; however, the influence of randomisation and how it may modify those decisions should also be considered.
Adherence to intervention
Patient participant adherence to the interventions was good overall, with 67% (n = 28) and 63% (n = 26) adhering to the tonometer and app, respectively (i.e. > 80% of measurements completed), and 55% (n = 23) adhering to both interventions. In the interviews, patient participants stated that remembering to conduct the monitoring was not difficult and they incorporated it into their established routines, pairing it up with taking medications or self-care activities. As the monitoring period progressed, they individualised the process (e.g. location to ensure light levels, or aid to enable steady motion) such that they could reliably achieve reading in less time or with fewer attempts. The ability to access the readings from the devices may also have enhanced adherence due to the positive feedback on the behaviour. A familiarity with technology, prior experiences in monitoring health, and control over their health were factors that influenced engagement with the intervention. As part of the study design, I-TRAC included reminder e-mails or texts (for which the patient participants expressed their preference) to be sent weekly to participants to remind them to monitor to further enhance adherence. In some cases, the e-mails and texts were being filtered into spam folders or blocked by providers. This would need to be addressed for a future evaluative study.
The overall adherence rate is especially positive given the high prespecified definition of adherence that the study implemented: > 80% of all weekly measures conducted across the 3-month monitoring period. Definitions of adherence for complex interventions such as these DHTs may be expected to be lower than our prespecified definition of adherence. Further consideration of what may be an appropriate adherence level for interventions of this type is critically important. For any future evaluative study, it would be important to consider the likely trade-off between duration of monitoring and intervention adherence. Patient participants suggested that to minimise anxiety through frequent measurement, the monitoring should be restricted to the necessary requirements – which they deemed to be once a week.
Acceptability of intervention
Overall affective attitudes of patient participants, site staff, and expert glaucoma clinicians in relation to beliefs about the interventions were positive; they cited reduced demand on the hospital-based clinical service as a main benefit. From a patient participant perspective, 71% stated they were highly or very satisfied with the home monitoring. In interviews, they reported that overall, they were able to engage with the new home monitoring technology and appreciated its potential impact on their health and the eye healthcare system. A notable subset of participants had persistent difficulties with the device (the tonometer in particular) that limited their engagement and resulted in some of them doubting the feasibility of implementing home monitoring going forward, whereas engagement with the app seemed more acceptable, reportedly due to familiarity. Additionally, there was a large proportion (48%, n = 20) of the intervention study participants who required at least one additional contact with the site staff to resolve issues that largely related to app access issues or difficulties using the device. Linked to the difficulties using the devices, both patients/participants and site staff interviewed raised the issue of a need to improve training, both of staff and of patients, for any future study. Yet on the whole, relevant stakeholders were cautiously optimistic about the interventions and their potential for benefit.
It is worth noting that the broader global context for I-TRAC was its delivery within the COVID-19 pandemic and related lockdowns. This meant that many patients were being advised to stay away from hospitals but were also becoming more familiar with technology. This may have also enhanced perceptions of acceptability of this type of intervention as a mechanism, to support recovery of the health service post lockdown.
A key consideration for a future trial is to further define the intervention. Within I-TRAC, the digital technologies were considered collectively as the intervention. However, findings from the qualitative data suggest there were preferences for one of the technologies regarding ease of use and readiness for use in practice. Some reported that only one intervention should be evaluated in a future study, which may have been linked to the OKKO app not being exactly fit for purpose for glaucoma. Other aspects relating to intervention maturity were linked to concerns about the volume of data this type of activity will generate and how this will be managed. The use of AI to support systems that can manage this volume of information and identify troubling readings and trigger additional alerts would also be required. Linked to this, obtaining clinical agreement to define the ‘trigger’ would also be required.
Cost and duration of intervention
In-home Tracking of glaucoma: Reliability, Acceptability, and Cost did not conduct an economic evaluation within the intervention study; however, it did develop a conceptual framework for the future economic evaluation through a review of existing models and combined this with data collected from participants and staff on resource use and patient preferences. Overall, given the complex care pathway and insufficient studies in the literature on glaucoma home monitoring, we recommend using a step-by-step approach leading to a meaningful economic evaluation in a future study. Categories of intervention costs of glaucoma home monitoring identified include equipment cost, patient training, ongoing patient support during home monitoring, potential spill-over costs such as hospital visits triggered by high IOP readings, costs of data integration to the existing medical records, and evaluation by AI. Glaucoma is a chronic condition, and relatively high initial costs can be balanced by long-term benefits. Therefore, long-term follow-up or modelling extrapolation beyond the evaluative study follow-up should be considered.
Perceptions of intervention cost-effectiveness were also explored in interviews with expert glaucoma clinicians (see Chapter 3). These clinicians had mixed views: some believed the interventions would be cost-effective but a majority believed this approach would not be good value for money, with concerns about initial capital cost of equipment and associated maintenance and support (e.g. staff time for training) costs. While these concerns were raised within the context of service use, they are also valid for a future evaluative study.
Outcome assessment
We did not attempt to analyse in detail data on clinical outcomes, as our goal was to focus on feasibility and acceptability issues. The majority (40/42, 95%) of patient participants attended follow-up data collection at the final clinic visit at 3 months. Travel costs for patients to attend this follow-up visit, which was not part of routine clinical care, were provided and this may also have acted as an incentive to return. For any future trial it is worth considering the duration and timing of follow-up, and any necessary co-interventions that may be required to maintain data completion and return. I-TRAC asked patient participants to monitor their glaucoma at home using the digital technology for 3 months. This allowed assessment of acceptability and feasibility. It would be likely that a future trial may expect a longer duration of follow-up, with 6- or 12-month or longer time points to complement the clinical guidelines for in-hospital monitoring of glaucoma. 4
Selection of most appropriate outcomes
The objectives of I-TRAC were to assess the outcomes of feasibility and acceptability regarding digital technology for home monitoring glaucoma. The clinical outcomes collected at baseline and 3-month follow-up centred on IOP and VFs. We collected IOP and visual function data weekly during the home monitoring period. Clinical outcome data were collected and reported for descriptive purposes only, with no statistical testing undertaken. One of the site staff interviews felt that the outcomes being collected are of benefit to different subgroups of glaucoma patients and those who would benefit from both were in the minority. This links back to eligibility and who the patient population are. It is worth noting that IOP is the most frequently selected primary outcome in glaucoma trials, with over 90% of all glaucoma trials registered reporting this outcome as the primary end point, and is considered a core outcome. 82,85
It is also worth considering the secondary outcomes required for a future evaluative study. The qualitative research generated data from clinicians that identified a range of important additional outcomes, including: improved management from increased data collected, detecting progression quicker and preventing sight loss (either directly from monitoring or indirectly due to clinicians spending more time with high-risk patients in clinic); patient anxiety; and reduced travel. In addition to the resource use categories such as time for patient training, ongoing patient support, and healthcare contacts triggered by information from the home monitoring devices, a future study should consider health-related (e.g. QALYs) and non-health-related QoL outcome measures. Patient participants stated convenience as a benefit of home monitoring and this is a possible source of non-HRQoL utility; however, they also mentioned concerns about the potential lack of reassurance from clinicians under home monitoring. These are examples of possible sources of utility that should be explored further when deciding the outcome measures for an economic evaluation within an evaluative study.
Retention
The I-TRAC study retained 39 (93%) patient participants across the 3-month monitoring period. One of these participants had withdrawn from completing the monitoring but completed all data collection. Two participants withdrew from all study procedures due to events external to the study (one had a stroke and was unable to continue to complete the study and the other Bell’s palsy). As mentioned previously in relation to intervention adherence, if the monitoring period were to be extended, it is important to consider the trade-off between duration and completion of follow-up. In I-TRAC patient participants were asked to return to the clinic (a research visit not part of standard routine care) at 3 months at which they had clinical measurements taken and completed additional follow-up data collection. As such, timing and mode of follow-up require further consideration for implementation in a future evaluative study. In addition, it would be important to consider any negative affect from the ‘control’ group and how this would need to be mitigated during design to ensure there is no differential retention between trial arms.
Logistics of a multicentre trial
All three I-TRAC sites were keen and engaged, and this would need to be replicated for the scale-up of implementation of I-TRAC across multiple centres. External researcher interviews (see Chapter 5) highlighted that a lack of evidence of effectiveness of technology can contribute to a lack of staff buy-in at sites. Therefore, it would be important to ensure that any evidence that is available on efficacy of interventions is made available to sites to support buy-in. Having a DHT champion with experience of trials of this type, and selecting sites with confidence in technologies, would also be ideal for supporting delivery.
Suggestions for improvement to training that would be relevant for a multicentre trial included: identification of a dedicated experienced clinical site trainer; more detailed training for site staff such that they could better address patient concerns; multisite training days for staff to get together to complete training; and for patients, group training as an efficient approach for sites but also to provide social opportunities for patients which may be motivating. Having a technical support line available for patients such that they do not have to use site staff time with technical queries about the interventions was also suggested as an improvement.
One of the key logistical considerations for a future trial relates to the large volume of data that would be generated from a weekly (or similar) data collection schedule. Consideration would be required for how to review and act on the data (i.e. what measurement would trigger a patient to be seen in hospital) received from the devices in a future trial. In addition, the time required for review of data processing etc. would also need to be factored in. It was suggested that it would be valuable to have an automated algorithm that would highlight abnormal findings, to minimise the need for clinicians’ time to review data. The additional and often complex movement and storage of patient data which frequently occurs in digital home monitoring studies can provide challenges, with each geographic regional approval body having different information governance, research, and development processes which required factoring into delivery timelines and resource requests. The impact of differing policies regarding regulatory approvals did cause delays in opening sites and additional administration workload for the I-TRAC study team. For a future evaluative study, exploring local policies before recruiting sites is essential. These challenges with approvals, and specifically slow systems not built for DHT trials, were also noted in the external research team’s interviews (see Chapter 5). Solutions proposed were the need for more agile regulatory systems with the use of standardised approaches for ethical issues posed by DHT trials, and institutional digital support.
All components of the protocol work together
There were a number of protocol amendments during the study, and these are documented in Appendix 6. Overall, the I-TRAC study as delivered was fit for purpose and, as site staff reported in the interviews, the components worked well together. The study was perceived by site staff as low burden for both staff and patients, and largely easy to run and deliver. There were, however, some aspects that could be improved. The main one of these, a recurring theme, was the training of staff and patients to use the interventions. Additional opportunities for site staff to learn about the technologies and how to train patients in their use, possibly through a ‘see one, do one, teach one’ model, would be favourable.
Several key features of the interventions also arose. The first, and most notable, was the change in the app-based intervention from the MRFs VF technology to the OKKO visual function app. This was due to the MRF not being CE marked at the time of study approval (it has since obtained a UKCA mark) and as such being deemed a medical device by the MHRA, timeline for approval of which would have threatened the overall delivery of I-TRAC. Therefore, after a review of existing app-based methods for measuring VF or visual function (see Appendix 3), the CE-marked OKKO app was deemed fit for purpose. Our clinical experts suggested that the OKKO app would not be suitable for monitoring disease progression in glaucoma as it is measuring central visual function. Clinically, the MRF or another app designed to measure peripheral vision is the better suited technology, and while I-TRAC did not specifically assess acceptability, it did assess the general acceptability of tablet-based apps (which is also the MRF mode of delivery). In addition, findings from the patient participant interviews suggested they perceived the OKKO app to be less relevant to their glaucoma, which may also explain the slightly lower adherence to the OKKO app compared to the tonometer, which was reported as harder to use.
Additional unforeseen intervention issues with delivery also arose related to delays in iPad supply due to chip shortage and a surge in demand (due to remote working) which also resulted in an increase in cost. In one site, chargers for devices were lost, and due to not having replacements immediately available this led to delays in recruiting patients. Finally, there were two issues in relation to use of batteries. The first related to batteries running out quickly in the tonometers, which then required a specialist screwdriver to open the battery compartment (that had to be ordered from the distributor), and only specific branded batteries could be used. The other issue was mailed devices being returned due to batteries being included inside the device that were required to be sent separately, again leading to slight delays with recruitment.
As highlighted in interviews with the external research teams (see Chapter 5), I-TRAC also faced some minor challenges with commercial partners. At study outset, establishing realistic expectations regarding timely access to data and whose responsibility it was within the partner organisation to provide would have been helpful.
These lessons will be incorporated into any future evaluative study design.
Research findings in context: relevant evidence published since I-TRAC was commissioned
Since the commissioning of I-TRAC in 2019, a small number of relevant papers reporting on acceptability testing of digital technologies for home monitoring of glaucoma have been published. Unlike I-TRAC, these studies were not designed to explore the feasibility of a future evaluative study; however, the studies’ findings were mapped against the AdePT issues to allow any additional insights to supplement the findings from I-TRAC and inform future decision-making. The studies were conducted in four geographical settings: Slovenia, UK, Australia and USA. 37–39,86 They used a range of methods to determine acceptability and feasibility of measurement for a single intervention across various technologies measuring IOP (iCare ONE HOME) and VF (Virtual Field, Eyecatcher, MRFs), with only one of the studies evaluating both IOP and VF technologies. One study did not require patients to monitor at home but rather assessed acceptability within a hospital setting. 37 Details of each of these studies are provided in Table 29. Other studies investigating the accuracy and reliability of home monitoring technologies for glaucoma have also been published. 87–90 These studies are not discussed here as they did not explore patient and/or clinician acceptability.
| AdePT issue | Cvenkel et al. 201937 (Slovenia) |
Jones et al. 202138 (UK) |
Prea et al. 202186 (Australia) |
Hu et al. 202239 (USA) |
|---|---|---|---|---|
| Intervention |
NB: this study explored self-measurement within an eye clinic and did not require patients to monitor at home |
|
|
|
| 1. Did the feasibility/pilot study allow a sample size calculation for the main trial? | N/A | N/A | N/A | N/A |
| 2. What factors influenced eligibility and what proportion of those approached were eligible? | Patients (≥ 18 years) with suspected glaucoma, OAG and OHT | Diagnosis of glaucoma – including OAG and NTG | Diagnosis of OHT or stable glaucoma in at least one eye, visual acuity better than 20/40 (6/12), and the ability to understand English instructions. | POAG, OAG, OHT, or suspected glaucoma In interviews, patients expressed that those who would benefit most from home monitoring would be patients who are underserved, busy, have flexibility in their schedules, older, committed to their eye care, tech-savvy, immobile, open-minded, interested in fine-tuning their disease management, children or glaucoma suspects |
| 3. Was recruitment successful? | 117 Mean age 57, 63% female |
20 Median age 71 (62–78), 50% female |
101 Mean age 64.6 (21–89), 32% female |
20 Mean age 55.4 (range 25–83), 65% female |
| 4. Did eligible participants consent? | N/R | N/R | N/R | N/R |
| 5. Were participants successfully randomised and did randomisation yield equality in groups? | N/A | N/A | N/A | N/A |
| 6. Were masking procedures adequate? | N/A | N/A | N/A | N/A |
| 7. Did participants adhere to the intervention? | 103 of 117 patients (88%) measured IOP and 96 (82%) fulfilled the requirements | 19 of 20 (95%) individuals completed the full regimen of six home monitoring sessions* | 78 of 101 (77%) patients were analysed based on providing more than one home monitoring measurement | 100% of 65 patients obtained an acceptable IOP and completed a VF test at home |
| 8. Was the intervention acceptable to the participants? | After training, 96 out of 117 (82%) subjects were able to perform self-tonometry. 73 of 93 (79%) felt that self-tonometry was easy to use and 75 patients (81%) responded that they would use the device at home. Majority agreed the device was easy to use, comfortable and would use it at home. The most common listed advantage was being able to measure IOP at home [13 out of 40 (32.5%)]. Sixteen (40%) had problems with alignment and taking measurements in their left eye, 12 (30%) perceived as a disadvantage not seeing the results of measurements |
Findings suggest overall acceptability. Main findings included:
|
36 patients provided reasons for withdrawal and noncompliance which related to:
|
74% felt that the home tonometer was easy to use. 100% felt that Vfi was easy to use
|
|
|
|||
|
||||
|
||||
| 9. Was it possible to calculate intervention costs and duration? | N/R | N/R | N/R | N/R |
| 10. Were outcome assessments completed? | Once in hospital | Once per month for 6 months | Once per week for 6 weeks | Monitor for 1 week: home tonometer four times/day for 4 days and Vfi three times total |
| 11. Were outcomes measured those that were the most appropriate outcomes? | IOP | VF | VF | IOP and VF |
| 12. Was retention to the study good? | 93 of 96 (97%) filled out the questionnaire | One participant discontinued home testing after four sessions/months after consultation with the study investigators. This was due to the test exacerbating chronic symptoms of vertigo | 12 (12%) participants provided no data and a further 7 (7%) provided only 1 measurement. | One patient was initially recruited for this study, but was excluded upon training due to an inability to demonstrate competence with the home tonometer. 19 out of 20 (95%) completed the survey |
| 13. Were the logistics of running a multicentre trial assessed? | N/R | No but did explore implementation into service
|
N/R | N/R |
| 14. Did all components of the protocol work together? | N/R | N/R | N/R | N/R |
Studies were broad in their eligibility criteria, including patients with POAG, OAG, OHT, and glaucoma suspects. Sample sizes varied, ranging from 20 to 117, with no information provided about number of eligible patients approached or reasons for declining participation. Studies reported participant age and sex, but not ethnicity. Durations of home monitoring included 1 week, 14 days, 6 weeks, and 6 months. Adherence to the home monitoring interventions under investigation was good, ranging from 77% up to 100%. Retention to the studies was also good, with the lowest level being 81%. 86
The UK-based study monitoring VF at home reported that patients were widely accepting of home monitoring, and some found it empowering to be involved in their care. 38 Home-based VF monitoring in glaucoma patients was tested and indicated many potential benefits. This included improved patient focus due to calmer home testing environments, increased compliance with testing, and burden removal of attending appointments. 38 However, there was an associated concern with performance pressure and anxiety surrounding constant awareness of symptoms. 38 The study which explored the use of the MRF app did not directly explore acceptability, but it did investigate reasons for non-adherence. These included reasons related to: the technology being too difficult to use; logistical IT reasons; too much effort to participate; no interest or motivation to participate; a deterioration in health; and competing life demands. 86 Hu et al. , conducted a mixed-methods feasibility study in which the acceptability of home tonometry and home perimetry was assessed within glaucoma care in the USA. 39 They demonstrated that the majority of patients found the devices easy to use and acceptable. 39 Patients in the study reported a range of potential personal advantages such as convenience, enhancing the relationship with physicians, disease prevention and empowerment. Patients also recognised personal advantages of the intervention, such as the feedback to ensure appropriate use; and advantages for the overall service, such as additional data for disease management. 39 However, there were also challenges or concerns in relation to using the home monitoring technologies, such as concerns about accuracy, technical difficulties, test fatigue and frustration and, more broadly, the impacts on the relationship with their physician. Patients also raised that, when considering future application, there may be patient groups who are particularly suited to home monitoring, such as those who are underserved, older, glaucoma suspects, or children. 39
In addition to these studies exploring acceptability, a systematic review of glaucoma home monitoring interventions was published in 2022. 91 The review indicated that self-led monitoring has promising potential for the future but found that current literature provides insufficient relevant evidence to fully support a home monitoring model – indicating room for further work. 91 These findings were primarily due to a small total sample of conducted studies that were performed with a highly specific target patient group, decreasing the overall generalisability of results. Additionally, technical standardisation issues regarding home monitoring measurements, such as control of screen distance from patient and calibration requirements, were identified across numerous studies, adding a supplementary barrier to overcome for generalisability. This review demonstrated that patient-led home monitoring may not replace clinic care but may provide great value in certain situations, such as pandemics.
There are additional studies worth highlighting with findings that are also relevant when considering the overall results of I-TRAC. Firstly, Ballouz et al. explored whether glaucoma screening could be conducted remotely in two Michigan community clinics. 92 The study found that patients from poorer demographics identified transportation, cost, trust, and lack of personal knowledge as barriers to glaucoma care. 92 This was particularly prominent among those from ethnic minority groups. These findings from glaucoma screening research may also translate to a home monitoring setting, and further support the need to explore acceptability among the glaucoma populations not represented in I-TRAC and who are underserved by research. It is also important to note the MONARCH study, which has evaluated the diagnostic accuracy of self-led monitoring within the management of neurovascular age-related macular degeneration, another chronic ophthalmology condition. 93 The study team introduced two digital (iPod)-based home monitoring tests and one paper-based journal to patients. In addition, the MONARCH team conducted interviews with participants, close family members, and healthcare professionals to explore acceptability and adherence. 94 As with the home monitoring studies in glaucoma, the findings indicated that patients were largely accepting of home monitoring in this context and viewed it as relatively straightforward and low burden, citing the benefits to reduce clinical visits as a motivator. There was also recognition of the essential requirement for effective training and ongoing support to ensure those less digitally adept were supported. 94
Finally, it is worth highlighting evidence from a recent overview of qualitative reviews that synthesised evidence from studies of digital health experiences reported in chronic disease management. 95 Evidence was synthesised from 22 systematic reviews from across mental health, cancer, diabetes, chronic obstructive pulmonary disorder, chronic pain, cardiovascular disease, irritable bowel disease and combinations of chronic disease reviews. Several common themes across the conditions were identified and categorised through nine domains:
-
Participation and engagement – references strong usability and engagement balanced against a reluctance to use digital technologies when the former is not considered.
-
Trust, confidence, and competence – users felt reassured, but for those with technology illiteracy there was a perceived lack of control.
-
Perceived value, perceived effectiveness, transaction cost – in respect of gains afforded by digital technology but could also be lost due to burden of data entry requirements.
-
Perceived care quality – which required tailoring and motivation.
-
Barriers and threats – of the technologies’ risks and challenges.
-
Health outcomes – improved capabilities about self-management.
-
Relationships – improved healthcare professional relationships, but interpersonal aspects of in-person care lacking.
-
Unplanned benefits – patient empowerment.
-
Diversity of experiences – highlighting condition-specific experiences or ambivalence of experiences.
Overall, this overview of reviews highlights the need for digital technologies to be developed through a co-design model, ensuring the ‘consumer’ has meaningful involvement in the planning and design phases of products and developments. 95 This could also extend to the planning and design of evaluative studies of these technologies, ensuring patients are actively involved in the design of trials that plan to evaluate these interventions.
Findings from these primary studies and literature reviews echo many of the findings from I-TRAC and suggest that home monitoring of glaucoma is largely acceptable, adherence is adequate, and delivery within the context of the study was feasible. However, there are several unknowns in relation to scalability and sustainability for a future evaluative study. A recently developed conceptual framework to support implementability of healthcare interventions recommends starting with assessing acceptability (across a range of stakeholders), then moving to explore fidelity (which can include adherence but also whether the intervention was delivered as intended), and then assessing feasibility of delivery. 96 These factors should be investigated, iteratively, with stakeholders during intervention development and early evaluation. Then, when initial evidence of effectiveness has been established, scalability and sustainability should be considered. It is important to note that acceptability, fidelity, and feasibility are dynamic concepts that likely require reconsideration when scaling to different settings (e.g. research to healthcare practice) or populations (e.g. adults to children) over time. 96
Strengths and limitations
One of the main strengths of I-TRAC was its ability to recruit across three geographically distinct locations, with varied patient pathways and routes to patient recruitment. This allowed ‘testing’ of study processes in a range of contexts, providing greater reassurance of acceptability of study processes when considering scale-up to a larger study. The inclusion of perspectives from a range of stakeholders (expert glaucoma clinicians, patient participants, site staff, and external research teams with experience in DHT) was also a key strength to the study – and not evidenced in the literature to date. Encouraging a plurality of perspectives should ensure the main opportunities and challenges of future research and delivery of DHT for glaucoma are evidenced and acted upon. Lastly, the use of guiding theoretical frameworks (TFA and the TDF) to ensure interview questions were comprehensive in assessing possible barriers and facilitators to engaging with home monitoring was also a strength. Responses were also coded using this same framework to produce deductive themes centred around the behavioural domains thought to drive behaviours, such as engagement with health monitoring, and provided rich sources of data to help feed into overall assessments. This will facilitate the development of future behaviour change strategies to help address the key challenges identified.
A number of key weaknesses of I-TRAC stemmed from study design. Firstly, the lack of assessment of the original VF MRF app was not ideal. While we would anticipate that – given the MRF, like the OKKO app, is also delivered on a tablet – many of the findings on acceptability would be transferable, this remains to be demonstrated. Secondly, there are some limitations in design of the survey described in Chapter 2, albeit the accounts and responses across the study were insightful. Within the survey of expert glaucoma clinicians, we did not specify whether home monitoring would be in addition to or a replacement for existing services. This means some responses may have considered service replacement and others addition of a service, which makes interpretations of the findings less clear. However, this might explain why there was considerable lack of agreement across the four clinical scenarios. It is also important to acknowledge that the survey represents only a sample of expert glaucoma clinicians’ views on which glaucoma patients are most appropriate for home monitoring. Understanding the views and opinions across the wider profession, including community optometrists, would also add value to this work.
Equality, diversity and inclusion
Another important limitation of the study was the patient population: largely white, experienced with technology, and generally research motivated. While we did not capture data on socioeconomic status, education or health literacy, given the types of patients who tend to participate in research it is likely that some categories of these other characteristics were also under-represented. This is of particular importance when considering that some ethnic groups are at much higher risk of advanced vision loss after a glaucoma diagnosis (e.g. six times more likely in black patients than white patients) and as such, monitoring may be more relevant for these higher-risk patients. 97 In addition, given many studies have reported that disadvantaged groups who experience health inequalities are also more likely to be digitally excluded, it will be critical to ensure people from these groups are not excluded from future research or evaluation. 98 In order to ensure representativeness across the sample, any future trial should use the INCLUDE Ethnicity framework (plus broader INCLUDE frameworks) and associated guidelines to help inform recruitment and retention of underserved populations. 99–101
Recommendations for future research
Given the evidence generated from the I-TRAC study, we believe there are key unknowns that need to be addressed before moving to an evaluative study. These are outlined below using the population, intervention, comparator, outcome (PICO) framework. Once these unknowns related to the PICO framework and the specification of the research question have been elucidated, decisions about type of study design could be determined.
-
Population
Determining precisely which patient group would be most suitable for home monitoring requires further attention; for example, whether home monitoring using digital technology in patients with high risk of progression or those with low risk of progression should be the priority. Ensuring research teams engage with underserved communities to support recruitment to future studies such that there is broad representation across future populations is also required. Different populations may benefit from home monitoring if either or both tests are used. Linked to this, considering potential unconscious bias at sites with regard to participant selection should also be explored and mitigated against for any future study.
Also linked to population is whether home monitoring is considered as an additional service (i.e. in addition to routine monitoring through HES) or as a replacement service (i.e. patients would not attend HES and instead would be monitored at home). This may be directly linked to the eligible population, as high-risk patients may require an additional service whereas low-risk patients would be able to use home monitoring as a replacement.
-
Intervention
A single test or a combination of tests may potentially be useful for home monitoring of glaucoma. The interventions for home monitoring are complex interventions, and need to be conceptualised as such to ensure effective assessment and implementation. 102 Understanding how these complex interventions operate within the complex system is also key, and using a systems perspective during evaluation would be important. The complexity could also extend to consideration of these interventions as behaviour change interventions, and findings from the behavioural theory-informed components of I-TRAC could be used to develop behaviour change techniques to embed within patient information, training etc. to enhance intervention uptake and engagement.
Given the trade-offs, mentioned earlier in this chapter, that may be required in intervention adherence, duration, and follow-up for implementation and evaluation in a future study, it is also important to base these adjustments on evidence. This could be achieved through conducting a DCE to determine design and delivery components of the home monitoring interventions. There are examples in the literature of how DCEs have been used to inform the design of complex interventions, which could in turn improve user uptake and adherence. 103 In addition, this DCE approach has been used to develop a digital self-management intervention for chronic kidney disease. 104 Further exploratory work to assess intervention acceptability would then be required, again ensuring engagement from all appropriate populations.
Lastly, further research to obtain agreement from clinicians around what level of adherence would be deemed acceptable for these home monitoring interventions would also be important both for future evaluative studies and for clinical practice.
-
Comparator
While the comparator in a future evaluative study would likely be standard care, there is variation across (and within) the devolved nations in the UK regarding patient care pathways. For example, there is an increasing but variable use of: (1) ‘virtual clinics’ where patients attend the HES and have a series of glaucoma tests, and then a clinician reviews and reports the findings at a later date; and (2) community optometry-based glaucoma clinics for patients at low risk of visual impairment, for example those with OHT. Understanding these contextual differences would be important for planning and analysis of any future study to identify how the intervention would be situated and any differences in results from a site or country setting.
-
Outcome(s)
Further research is needed to determine the most appropriate outcomes for evaluation of digital technologies for home monitoring, which could consider structure, process and outcome outcomes. The DCE approach to intervention development, mentioned earlier, could also be used to identify which outcomes are highly valued by patients, healthcare professionals, service managers, and commissioners of services.
In addition to the glaucoma-specific outcomes, a future evaluative study may also need to consider whether there are overarching outcomes of importance that could be informative for other trials of home monitoring technologies. There may be scope for a generic core outcome set (defined as the minimum set of outcomes that should be collected and reported)105 for digital technology interventions used to monitor chronic conditions at home. There are examples of core outcome set development for telehealth in particular clinical contexts which could provide a starting point for the development of linked sets. 106
Patient and public involvement
Research to ‘improve early diagnosis of sight-threatening glaucoma’ has been identified as a top priority (for patients and clinicians) for funding (James Lind Alliance Priority Setting Partnership). 107 Therefore, patients have been directly involved in identifying and prioritising the broader research question. We have worked closely with two people living with glaucoma (patient partners DS and BL) and have also had representation of a patient advocacy group (Glaucoma UK) throughout the duration of the study to ensure consideration of wider members of the community. One patient partner, DS, was engaged at grant development stage, and, through discussion with their local clinician and the Chief Investigator, agreed that I-TRAC was aiming to answer an important research question and agreed to act as patient co-applicant (having input into the development of the applications and specifically the plain language summary) and member of the SSC. DS has provided input into the overall plan of activities. Through another clinical collaborator, we identified one further patient partner (BL) who also contributed to the study as a member of the SSC.
Support for engaging in activities and meetings was provided by the I-TRAC Research Fellow (CS) and the Chief Investigator (KG) and ensured patient partners had received the necessary information, in preferred mode (e.g. paper over electronic) to support their contributions to meetings. The two patient partners were reimbursed for their time in line with NIHR recommendations. 108
Our patient partners actively contributed to all SSC meetings, inputting to study decisions (including the change of digital technologies and decision to apply for an extension for the final cohort of participants), and providing feedback on provisional findings. In addition to the standard inputs into patient-facing documentation such as patient information leaflets and questionnaires, activities where specific patient input has been sought during I-TRAC included: contributing to the content and writing of the training materials for participants, ensuring they were clear and covered a range of possible problems that participants may encounter; and patient partners helped to develop the topic guide for patient participant interviews through reviewing the initial draft, making recommendations for improving the questions and structure of the topic guide, and reviewing and approving the revised version. The patient partners also made important contributions to the plain language summary and contributed to a discussion of a dissemination strategy for communicating findings to people living with glaucoma. Having the benefit of insights from persons with a lived experience of glaucoma had a positive effect on patient-facing materials, in terms of both content and display, with our patient partner representatives identifying poor word choices and poor layout that may have deterred volunteers from participating. This likely contributed to the positive feedback from patient participants as to the clarity and ease of use of study materials. There were no obvious negative effects of their contributions, but important lessons were learned in terms of how to ensure patient partner input is as effective as possible; that is, patient partners actually being familiar with the technologies and having lived experience of learning to use the technologies.
Reflecting critically on the process of involvement, two key items arose. Firstly, the involvement of I-TRAC patient partners in developing training manuals without them having lived experience with the technologies was not ideal. One way to have combatted this would have been to have sent the technologies to the patient partners and asked them to use them in line with the training manuals, as this would have highlighted critical aspects. The other area that proved challenging for our patient partners (on occasion) was joining the SSC meeting online using MS Teams. The Chief Investigator’s host institution only supports the use of MS Teams as a platform for online meetings. Even with support from the I-TRAC Research Fellow in advance of meetings to join the Teams call, on one occasion this meant a patient partner was not able to join (which was resolved through an opportunity to meet after the fact).
Before submission of the final report, we invited our patient partners to complete a feedback form. The feedback was largely positive. They felt information about the study was communicated well and that meetings had a clear purpose, were well managed, and were of an appropriate duration. When asked what they would have changed, suggestions were: being provided with a tonometer to monitor their own glaucoma at home and reducing the use of unfamiliar abbreviations. Overall, patient partners felt involved, valued, and that their involvement made a difference to the study.
Conclusion
The I-TRAC study has demonstrated ‘cautious optimism’ when considering patients’ and healthcare professionals’ views on the acceptability of digital technologies for home monitoring patients with glaucoma. The study also evidenced sufficient fidelity, good adherence to the interventions, and feasibility of delivery of both the interventions and the study processes, but this must be considered with reference to the narrow patient population included. However, I-TRAC also highlighted several unknowns relating to the PICO framework of a future evaluative study that require addressing before progression to a RCT. The I-TRAC study has also considered the wider ecosystem challenges of running digital health technology trials through evidencing the views of external research teams experienced in DHT delivery. Given the high system demand for digital solutions, in a space where innovation happens at pace, generating evidence to evaluate digital health technologies is challenging. Yet the potential promise for the health system more generally, and HES more specifically, provides justification for further research in this area.
Additional information
Contributions of authors
Carrie Stewart (https://orcid.org/0000-0002-2325-3380) (Research Fellow) was the Research Fellow recruited for the study and oversaw day-to-day study management. She conducted the data collection of clinician survey; all interviews and focus groups; and the home monitoring intervention study. She led or supported the analysis of these components. She supported the patient partners contributions to the study and led a significant amount of the write-up of the report.
Hangjian Wu (https://orcid.org/0000-0001-8377-3276) (Research Fellow, Health Economics) conducted the literature review, resource use data analysis and overall synthesis for the health economics in Chapter 6. He was a member of the Project Management Group and supported decision-making throughout the study through attendance at meetings and e-mail correspondence and commented on the final report.
Uma Alagappan (https://orcid.org/0000-0001-5279-6088) (Undergraduate Medical Student) contributed to the analysis of the clinician survey and led the writing of the initial drafts of Chapters 1 and 2.
Augusto Azuara-Blanco (https://orcid.org/0000-0002-4805-9322) (Professor of Ophthalmology) was a co-applicant and led the recruitment of patient participants into the home monitoring intervention phase of the study. He was a member of the Project Management Group and supported decision-making throughout the study through attendance at meetings and e-mail correspondence and commented on the final report.
Anthony J King (https://orcid.org/0000-0002-3091-911X) (Professor of Ophthalmology) led the recruitment of patient participants into the home monitoring intervention phase of the study. He was a member of the Project Management Group and supported decision-making throughout the study through attendance at meetings and e-mail correspondence and commented on the final report.
Andrew J Tatham (https://orcid.org/0000-0003-0372-3100) (Consultant Ophthalmic Surgeon) led the recruitment of patient participants into the home monitoring intervention phase of the study. He was a member of the Project Management Group and supported decision-making throughout the study through attendance at meetings and e-mail correspondence and commented on the final report.
Rodolfo Hernández (https://orcid.org/0000-0003-2619-8230) (Research Fellow, Health Economics) was a co-applicant and led the design of the health economics components in the original grant application. He oversaw the conduct and analysis of the health economics components in Chapter 6. He was a member of the Project Management Group and supported decision-making throughout the study through attendance at meetings and e-mail correspondence and commented on the final report.
Bruce Lowe (Patient Partner) was a member of the Project Management Group; contributed to the project meetings; advised on information leaflets, intervention training documents, interview topic guides; and reviewed and commented on the plain language summary of the final report. He supported decision-making throughout the study through attendance at meetings and e-mail correspondence.
Darian Shotton (Patient Partner) was a co-applicant; contributed to the original grant application, project meetings; advised on information leaflets, intervention training documents, interview topic guides; and reviewed and commented on the plain language summary of the final report. She supported decision-making throughout the study through attendance at meetings and e-mail correspondence.
Nana Appiah (https://orcid.org/0000-0002-7232-2733) (Masters Student) contributed to the analysis of the external researcher interviews and focus groups in Chapter 5.
Taylor Coffey (https://orcid.org/0000-0002-6921-8230) (Research Fellow) contributed to the analysis of the patient interviews in Chapter 4.
Thenmalar Vadiveloo (https://orcid.org/0000-0001-5531-6289) (Research Fellow – Statistics) conducted the analysis of the patient participant data from the home monitoring intervention phase of the study in Chapter 4.
Graeme MacLennan (https://orcid.org/0000-0002-1039-5646) (Professor of Medical Statistics and Director of the Centre for Healthcare Randomised Trials) was a co-applicant and supported the development of the grant application and study delivery. He provided trial expertise and advised on the critical study components to inform the design of a future evaluative study. He was a member of the Project Management Group and supported decision-making throughout the study through attendance at meetings and e-mail correspondence and commented on the final report.
Katie Gillies (https://orcid.org/0000-0001-7890-2854) (Reader and Director of the Healthcare Assessment Programme) was the Chief Investigator, with overall responsibility for the design, coordination and delivery of the study. With the co-investigators, she conceived, designed and led the original grant application. She facilitated some of the focus groups. She led on drafting report chapters and made substantial contributions to synthesis and write-up of chapters in the report and commented on drafts and outputs of the study. She is responsible for overall content of this report.
Acknowledgements
The authors would like to thank all of the participants in the study who contributed to the survey, interviews, focus groups and the intervention study.
We are very grateful to our Study Steering Committee members whose support and contributions enhanced the design and delivery of I-TRAC, specifically, Professor Daniel Hind (Chair, University of Sheffield), Professor Jennifer Burr (St Andrews University), Professor Luke Vale (Newcastle University), Karen Osbourne and Phillipa Mason (both Glaucoma UK).
We would also like to acknowledge the contributions of several colleagues during the delivery of the study and/or the final report: Dr Ruth Thomas for her work on the original grant application; Mark Forrest for IT support; Dr Heather Morgan for advice on digital health interventions; Beverley Smith for providing administrative support for the study; Janice Cruden for support with production of the final report and Paul Manson for referencing support on the final report.
The Health Services Research Unit receives funding from the Chief Scientist Office of the Scottish Government’s Health and Social Care Directorate.
The University of Aberdeen sponsored this study. The feasibility study was run under the auspices of the Health Services Research Unit (HSRU), University of Aberdeen. HSRU has internationally recognised expertise in the design, conduct, analysis, and reporting of multicentre trials and surgical trials in particular. The Study Office was based in HSRU who provided day-to-day support for the clinical centres. The Research Fellow in HSRU in Aberdeen took responsibility for the day-to-day transaction of study activities. The programmer created, maintained and updated all administrative and analysis databases. The HSRU Quality Assurance Manager was responsible for overseeing and advising on assessments of quality in the study, ensuring that staff received appropriate training in the principles of good clinical practice (GCP), and was available to assist with any external monitoring and auditing.
A Project Management Group (PMG) and an independent SSC were convened. The study was supervised by a PMG comprising grant holders and representatives from HSRU. The PMG met monthly. The role of the SSC was to monitor and supervise study progress. SSC membership consisted of an independent Chair, two other independent members, a patient and public involvement representative and the Chief Investigator. The SSC met bi-annually.
Patient data statement
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data is used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Ethics statement
The study was conducted in accordance with the principles of good clinical practice (GCP). In addition to Sponsorship approval, a favourable ethical opinion was obtained from the appropriate REC (ref id: 20/EM/0244) and appropriate NHS R&D approval(s) were obtained prior to commencement of the study. A separate favourable opinion was granted by the University of Aberdeen School Ethics Research Committee (CERB/2020/5/1963) for the clinician survey.
Information governance statement
University of Aberdeen is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under the Data Protection legislation, University of Aberdeen is the Data Controller, and you can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for our Data Protection Officer here: https://www.abdn.ac.uk/staffnet/governance/data-protection-6958.php.
Disclosure of interests
Full disclosure of interests: Completed ICMJE forms for all authors, including all related interests, are available in the toolkit on the NIHR Journals Library report publication page at https://doi.org/10.3310/GTWD6802.
Primary conflicts of interest: Augusto Azuara-Blanco is a member of HTA Prioritisation Committee B.
Anthony J King has received funding from the pharmaceutical industry for contributions to Advisory Boards and conference organisers for attendance/speaking at conferences. He is also Chairman/trustee of Glaucoma UK.
Andrew J Tatham has received funding from the pharmaceutical industry for contributions to consulting, lectures and/or presentations, and Advisory Board participation. He is also a member of the European Glaucoma Society executive committee.
Katie Gillies is a member of the NIHR HTA Clinical Evaluations and Trials Committee.
Disclaimers
This article presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Parihar JK. Glaucoma: the ‘black hole’ of irreversible blindness. Med J Armed Forces India 2016;72:3-4.
- Quartilho A, Simkiss P, Zekite A, Xing W, Wormald R, Bunce C. Leading causes of certifiable visual loss in England and Wales during the year ending 31 March 2013. Eye (Lond) 2016;30:602-7.
- King A, Azuara-Blanco A, Tuulonen A. Glaucoma. BMJ 2013;346.
- National Institute for Health and Care Excellence . Glaucoma: Diagnosis and Management [NG81] 2017. www.nice.org.uk/guidance/ng81 (accessed 29 July 2022).
- Barkana Y, Dorairaj S. Re: Tham et al.: Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis (Ophthalmology 2014;121:2081-90). Ophthalmol 2015;122:e40-1.
- Cesareo M, Ciuffoletti E, Ricci F, Missiroli F, Giuliano MA, Mancino R, et al. Visual disability and quality of life in glaucoma patients. Prog Brain Res 2015;221:359-74.
- Buchan J. The Way Forward: Age-related Macular Degeneration and Diabetic Retinopathy. London: The Royal College of Ophthalmologists; 2017.
- McMonnies CW. Glaucoma history and risk factors. J Optom 2017;10:71-8.
- McMonnies CW. The importance of and potential for continuous monitoring of intraocular pressure. Clin Exp Optom 2017;100:203-7.
- Kotecha A, Longstaff S, Azuara-Blanco A, Kirwan JF, Morgan JE, Spencer AF, et al. Developing standards for the development of glaucoma virtual clinics using a modified Delphi approach. Br J Ophthalmol 2018;102:531-4.
- Lusthaus J, Goldberg I. Current management of glaucoma. Med J Aust 2019;210:180-7.
- Winyard S, McLaughlan B. Cost Oversight? The Cost of Eye Disease and Sight Loss in the UK Today and in the Future. London: Royal National Institute for the Blind; 2009.
- Bashshur RL, Shannon GW, Smith BR, Alverson DC, Antoniotti N, Barsan WG, et al. The empirical foundations of telemedicine interventions for chronic disease management. Telemed J E Health 2014;20:769-800.
- Farmer A, Gibson OJ, Tarassenko L, Neil A. A systematic review of telemedicine interventions to support blood glucose self-monitoring in diabetes. Diabet Med 2005;22:1372-8.
- Jayaram NM, Khariton Y, Krumholz HM, Chaudhry SI, Mattera J, Tang F, et al. Impact of telemonitoring on health status. Circ Cardiovasc Qual Outcomes 2017;10.
- Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med 2012;156:416-24.
- Omboni S, Ferrari R. The role of telemedicine in hypertension management: focus on blood pressure telemonitoring. Curr Hypertens Rep 2015;17.
- Purcell R, McInnes S, Halcomb EJ. Telemonitoring can assist in managing cardiovascular disease in primary care: a systematic review of systematic reviews. BMC Fam Pract 2014;15.
- Walker RC, Tong A, Howard K, Palmer SC. Patient expectations and experiences of remote monitoring for chronic diseases: systematic review and thematic synthesis of qualitative studies. Int J Med Inform 2019;124:78-85.
- NHS England . The NHS Long Term Plan 2019. www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf (accessed 25 October 2023).
- Topol EJ. A decade of digital medicine innovation. Sci Transl Med 2019;11.
- National Institute for Health and Care Excellence . The SENSIMED Triggerfish Contact Lens Sensor for Continuous 24-Hour Recording of Ocular Dimensional Changes in People With or at Risk of Developing Glaucoma [MIB14] 2014. www.nice.org.uk/advice/mib14 (accessed 28 July 2022).
- National Institute for Health and Care Excellence . ICare Rebound Tonometer to Measure Intraocular Pressure [MIB57] 2014. www.nice.org.uk/advice/mib57 (accessed 18 July 2022).
- Barbour-Hastie CC, Tatham AJ. Teaching home tonometry using a remote video link. Eye (Lond) 2022;37:501-5.
- Chen E, Quérat L, Åkerstedt C. Self-tonometry as a complement in the investigation of glaucoma patients. Acta Ophthalmol 2016;94:788-92.
- Kim KN, Jeoung JW, Park KH, Yang MK, Kim DM. Comparison of the new rebound tonometer with Goldmann applanation tonometer in a clinical setting. Acta Ophthalmol 2013;91:e392-6.
- Noguchi A, Nakakura S, Fujio Y, Fukuma Y, Mori E, Tabuchi H, et al. A pilot evaluation assessing the ease of use and accuracy of the new self/home-tonometer IcareHOME in healthy young subjects. J Glaucoma 2016;25:835-41.
- Pronin S, Brown L, Megaw R, Tatham AJ. Measurement of intraocular pressure by patients with glaucoma. JAMA Ophthalmol 2017;135:1030-6.
- Mudie LI, LaBarre S, Varadaraj V, Karakus S, Onnela J, Munoz B, et al. The iCare HOME (TA022) study: performance of an intraocular pressure measuring device for self-tonometry by glaucoma patients. Ophthalmol 2016;123:1675-84.
- Takagi D, Sawada A, Yamamoto T. Evaluation of a new rebound self-tonometer, iCare HOME: comparison with Goldmann applanation tonometer. J Glaucoma 2017;26:613-8.
- Che Hamzah J, Daka Q, Azuara-Blanco A. Home monitoring for glaucoma. Eye (Lond) 2020;34:155-60.
- Folgar FA, de Moraes CGV, Prata TS, Teng CC, Tello C, Ritch R, et al. Glaucoma Surgery Decreases the Rates of Localized and Global Visual Field Progression. Am J Ophthalmol 2010;149:258-264.e2. https://doi.org/10.1016/J.AJO.2009.09.010.
- Prea SM, Kong YXG, Mehta A, He M, Crowston JG, Gupta V, et al. Six-month longitudinal comparison of a portable tablet perimeter with the Humphrey Field Analyzer. Am J Ophthalmol 2018;190:9-16.
- Ong EL, Zheng Y, Aung T, Tan L, Cheng CY, Wong TY, et al. Performance of the Moorfields motion displacement test for identifying eyes with glaucoma. Ophthalmol 2014;121:88-92.
- Loughman J, Gonzalez Alvarez C, Verdon-Roe GM, Anderson R, Manuel RA, Naidoo K. Impact of computer experience on the viability and repeatability of the Moorfields motion displacement test in a developing and underserved African setting. J Clin Exp Ophthalmol 2013;4.
- National Institute for Health and Care Excellence . Icare Rebound Tonometer to Measure Intraocular Pressure [MIB57]: Evidence Review 2016. www.nice.org.uk/advice/mib57/chapter/Evidence-review (accessed 18 July 2022).
- Cvenkel B, Velkovska MA, Jordanova VD. Self-measurement with iCare HOME tonometer, patients’ feasibility and acceptability. Eur J Ophthalmol 2020;30:258-63.
- Jones L, Callaghan T, Campbell P, Jones PR, Taylor DJ, Asfaw DS, et al. Acceptability of a home-based visual field test (Eyecatcher) for glaucoma home monitoring: a qualitative study of patients’ views and experiences. BMJ Open 2021;11.
- Hu GY, Prasad J, Chen DK, Alcantara-Castillo JC, Patel VN, Al-Aswad LA. Home monitoring of glaucoma using a home tonometer and a novel virtual reality visual field device: acceptability and feasibility. Ophthalmol Glaucoma 2022;6:121-8.
- Mercieca K, Drury B, Bhargava A, Fenerty C. Trabeculectomy bleb needling and antimetabolite administration practices in the UK: a glaucoma specialist national survey. Br J Ophthalmol 2018;102:1244-7.
- Khan SA, Whittaker K, Razzaq MA, Arain UR. National survey of intraoperative mitomycin C use during trabeculectomy surgery in the UK. Int Ophthalmol 2021;41:1309-16.
- Momentive Inc . SurveyMonkey n.d. www.surveymonkey.co.uk/ (accessed 17 August 2022).
- Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277-88.
- Elite Cafemedia . Encyclopedia.Com 2019. www.encyclopedia.com/ (accessed 9 March 2022).
- Cambridge University Press . Cambridge Dictionary 2022. https://dictionary.cambridge.org/ (accessed 9 March 2022).
- QSR International . NVivo 12 2020.
- Browne RH. On the use of a pilot sample for sample size determination. Stat Med 1995;14:1933-40.
- Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat 2005;4:287-91.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60.
- Cane J, O’Connor D, Michie S. Validation of the Theoretical Domains Framework for use in behaviour change and implementation research. Implement Sci 2012;7.
- Bugge C, Williams B, Hagen S, Logan J, Glazener C, Pringle S, et al. A process for Decision-making after Pilot and feasibility Trials (ADePT): development following a feasibility study of a complex intervention for pelvic organ prolapse. Trials 2013;14.
- Microsoft Corporation . MS Teams 2022.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Gomes M, Murray E, Raftery J. Economic evaluation of digital health interventions: methodological Issues and recommendations for practice. PharmacoEcon 2022;40:367-78.
- National Institute for Health and Care Excellence 2014. www.nice.org.uk/process/pmg20/chapter/introduction (accessed 22 December 2022).
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350.
- Burr JM, Botello-Pinzon P, Takwoingi Y, Hernández R, Vazquez-Montes M, Elders A, et al. Surveillance for ocular hypertension: an evidence synthesis and economic evaluation. Health Technol Assess 2012;16:1iii-271iv.
- Crabb DP, Russell RA, Malik R, Anand N, Baker H, Boodhna T, et al. Frequency of visual field testing when monitoring patients newly diagnosed with glaucoma: mixed methods and modelling. Health Serv Deliv Res 2014;2. https://doi.org/10.3310/hsdr02270.
- van Gestel A, Webers CA, Severens JL, Beckers HJ, Jansonius NM, Hendrikse F, et al. The long-term outcomes of four alternative treatment strategies for primary open-angle glaucoma. Acta Ophthalmol 2012;90:20-31.
- Holtzer-Goor KM, van Sprundel E, Lemij HG, Plochg T, Klazinga NS, Koopmanschap MA. Cost-effectiveness of monitoring glaucoma patients in shared care: an economic evaluation alongside a randomized controlled trial. BMC Health Serv Res 2010;10.
- Hernández R, Burr JM, Vale L, Azuara-Blanco A, Cook JA, Banister K, et al. Surveillance of Ocular Hypertension Study group . Monitoring ocular hypertension, how much and how often? A cost-effectiveness perspective. Br J Ophthalmol 2016;100:1263-8.
- Boodhna T, Crabb DP. More frequent, more costly? Health economic modelling aspects of monitoring glaucoma patients in England. BMC Health Serv Res 2016;16.
- Raad Voor Volksgezondheid En Zorg Zinnige En Duurzame Zorg (Sensible and Sustainable Care) 2006.
- Shemilt I, James T, Marcello M. A web-based tool for adjusting costs to a specific target currency and price year. Evid Policy 2010;6:51-9.
- Polisena J, Coyle D, Coyle K, McGill S. Home telehealth for chronic disease management: a systematic review and an analysis of economic evaluations. Int J Technol Assess Health Care 2009;25:339-49.
- Morris T, Aspinal F, Ledger J, Li K, Gomes M. The impact of digital health interventions for the management of type 2 diabetes on health and social care utilisation and costs: a systematic review. PharmacoEcon Open 2022;7:163-73.
- Ben-Assuli O. Measuring the cost-effectiveness of using telehealth for diabetes management: a narrative review of methods and findings. Int J Med Inform 2022;163.
- Nguyen NH, Martinez I, Atreja A, Sitapati AM, Sandborn WJ, Ohno-Machado L, et al. Digital health technologies for remote monitoring and management of inflammatory bowel disease: a systematic review. Am J Gastroenterol 2022;117:78-97.
- De Guzman KR, Snoswell CL, Taylor ML, Gray LC, Caffery LJ. Economic evaluations of remote patient monitoring for chronic disease: a systematic review. Value Health 2022;25:897-913.
- Teo VH, Teo SH, Burkill SM, Wang Y, Chew EA, Ng DW, et al. Effects of technology-enabled blood pressure monitoring in primary care: a quasi-experimental trial. J Telemed Telecare 2024;30:121-30. https://doi.org/10.1177/1357633X211031780.
- Stoddart A, Hanley J, Wild S, Pagliari C, Paterson M, Lewis S, et al. Telemonitoring-based service redesign for the management of uncontrolled hypertension (HITS): cost and cost-effectiveness analysis of a randomised controlled trial. BMJ Open 2013;3.
- Ionov MV, Zhukova OV, Yudina YS, Avdonina NG, Emelyanov IV, Kurapeev DI, et al. Value-based approach to blood pressure telemonitoring and remote counseling in hypertensive patients. Blood Press 2021;30:20-3.
- Monahan M, Jowett S, Nickless A, Franssen M, Grant S, Greenfield S, et al. Cost-effectiveness of telemonitoring and self-monitoring of blood pressure for antihypertensive titration in primary care (TASMINH4). Hypertension 2019;73:1231-9.
- Padwal RS, So H, Wood PW, McAlister FA, Siddiqui M, Norris CM, et al. Cost-effectiveness of home blood pressure telemonitoring and case management in the secondary prevention of cerebrovascular disease in Canada. J Clin Hypertens (Greenwich) 2019;21:159-68.
- Madsen LB, Christiansen T, Kirkegaard P, Pedersen EB. Economic evaluation of home blood pressure telemonitoring: a randomized controlled trial. Blood Press 2011;20:117-25.
- Kaambwa B, Bryan S, Jowett S, Mant J, Bray EP, Hobbs FD, et al. Telemonitoring and self-management in the control of hypertension (TASMINH2): a cost-effectiveness analysis. Eur J Prev Cardiol 2014;21:1517-30.
- Dehmer SP, Maciosek MV, Trower NK, Asche SE, Bergdall AR, Nyboer RA, et al. Economic evaluation of the home blood pressure telemonitoring and pharmacist case management to control hypertension (Hyperlink) trial. J Am Coll Clin Pharm 2018;1:21-30.
- Benedetto V, Filipe L, Harris C, Spencer J, Hickson C, Clegg A. Analytical frameworks and outcome measures in economic evaluations of digital health interventions: a methodological systematic review. Med Decis Making 2023;43:125-38.
- Somers C, Chimonas S, McIntosh E, Kaltenboeck A, Briggs A, Bach P. Using nominal group technique to identify key attributes of oncology treatments for a discrete choice experiment. MDM Policy Pract 2019;4.
- Hiligsmann M, van Durme C, Geusens P, Dellaert BG, Dirksen CD, van der Weijden T, et al. Nominal group technique to select attributes for discrete choice experiments: an example for drug treatment choice in osteoporosis. Patient Prefer Adherence 2013;7:133-9.
- Drummond M, Sculpher M, Claxton K, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015.
- Ismail R, Azuara-Blanco A, Ramsay CR. Consensus on outcome measures for glaucoma effectiveness trials: results from a Delphi and nominal group technique approaches. J Glaucoma 2016;25:539-46.
- van Gestel A, Severens JL, Webers CA, Beckers HJ, Jansonius NM, Schouten JS. Modeling complex treatment strategies: construction and validation of a discrete event simulation model for glaucoma. Value Health 2010;13:358-67.
- National Institute for Health and Care Excellence . Evidence Standards Framework for Digital Health Technologies 2018. www.nice.org.uk/about/what-we-do/our-programmes/evidence-standards-framework-for-digital-health-technologies (accessed 15 September 2022).
- Storgaard L, Tran TL, Freiberg JC, Hauser AS, Kolko M. Glaucoma clinical research: trends in treatment strategies and drug development. Front Med (Lausanne) 2021;8.
- Prea SM, Kong GYX, Guymer RH, Vingrys AJ. Uptake, persistence, and performance of weekly home monitoring of visual field in a large cohort of patients with glaucoma. Am J Ophthalmol 2021;223:286-95.
- Jones PR, Campbell P, Callaghan T, Jones L, Asfaw DS, Edgar DF, et al. Glaucoma home monitoring using a tablet-based visual field test (Eyecatcher): an assessment of accuracy and adherence over 6 months. Am J Ophthalmol 2021;223:42-5.
- Prea SM, Vingrys AJ, Kong GYX. Test reliability and compliance to a twelve-month visual field telemedicine study in glaucoma patients. J Clin Med 2022;11.
- Rosenfeld E, Rabina G, Barequet D, Mimouni M, Fischer N, Kurtz S. Role of home monitoring with iCare ONE rebound tonometer in glaucoma patients management. Int J Ophthalmol 2021;14:405-8.
- Levin AM, McGlumphy EJ, Chaya CJ, Wirostko BM, Johnson TV. The utility of home tonometry for peri-interventional decision-making in glaucoma surgery: case series. Am J Ophthalmol Case Rep 2022;28.
- Daka Q, Mustafa R, Neziri B, Virgili G, Azuara-Blanco A. Home-based perimetry for glaucoma: where are we now?. J Glaucoma 2022;31:361-74.
- Ballouz D, Cho J, Woodward MA, Elam AR, Musch DC, Zhang J, et al. Facilitators and barriers to glaucoma screening identified by key stakeholders in underserved communities: a community-engaged research approach. J Glaucoma 2021;30:402-9.
- Ward E, Wickens RA, O’Connell A, Culliford LA, Rogers CA, Gidman EA, et al. Monitoring for neovascular age-related macular degeneration (AMD) reactivation at home: the MONARCH study. Eye (Lond) 2021;35:592-600.
- O’Connor SR, Treanor C, Ward E, Wickens RA, O’Connell A, Culliford LA, et al. Monarch Study Group . Patient acceptability of home monitoring for neovascular age-related macular degeneration reactivation: a qualitative study. Int J Environ Res Public Health 2022;19.
- Taylor ML, Thomas EE, Vitangcol K, Marx W, Campbell KL, Caffery LJ, et al. Digital health experiences reported in chronic disease management: an umbrella review of qualitative studies. J Telemed Telecare 2022;28:705-17.
- Klaic M, Kapp S, Hudson P, Chapman W, Denehy L, Story D, et al. Implementability of healthcare interventions: an overview of reviews and development of a conceptual framework. Implement Sci 2022;17.
- Kang JH, Wang M, Frueh L, Rosner B, Wiggs JL, Elze T, et al. Cohort study of race/ethnicity and incident primary open-angle glaucoma characterized by autonomously determined visual field loss patterns. Transl Vis Sci Technol 2022;11.
- Honeyman M, Maguire d, Evans H, Davies A. Digital Technology and Health Inequalities: A Scoping Review. Cardiff: Public Health Wales NHS Trust; 2020.
- Dawson S, Banister K, Biggs K, Cotton S, Devane D, Gardner H, et al. Trial Forge Guidance 3: randomised trials and how to recruit and retain individuals from ethnic minority groups-practical guidance to support better practice. Trials 2022;23.
- Treweek S, Banister K, Bower P, Cotton S, Devane D, Gardner HR, et al. Developing the INCLUDE Ethnicity Framework-a tool to help trialists design trials that better reflect the communities they serve. Trials 2021;22.
- Witham MD, Anderson E, Carroll C, Dark PM, Down K, Hall AS, et al. INCLUDE writing group . Developing a roadmap to improve trial delivery for under-served groups: results from a UK multi-stakeholder process. Trials 2020;21.
- Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374.
- Terris-Prestholt F, Neke N, Grund JM, Plotkin M, Kuringe E, Osaki H, et al. VMMC study team . Using discrete choice experiments to inform the design of complex interventions. Trials 2019;20.
- Gc VS, Iglesias CP, Erdem S, Hassan L, Peek N, Manca A. Using discrete-choice experiments to elicit preferences for digital wearable health technology for self-management of chronic kidney disease. Int J Technol Assess Health Care 2022;38.
- Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials 2017;18.
- Harris Y, Gilman B, Ward MM, Ladinsky J, Crowley J, Warren C, et al. Building the evidence base for tele-emergency care: efforts to identify a standardized set of outcome measures. Telemed J E Health 2017;23:561-6.
- James Lind Alliance . Glaucoma Top 10 2013. www.jla.nihr.ac.uk/priority-setting-partnerships/sight-loss-and-vision/top-10-priorities/glaucoma-top-10.htm (accessed 28 January 2023).
- National Institute for Health and Care Research . Payment Guidance for Researchers and Professionals 2022. www.nihr.ac.uk/documents/payment-guidance-for-researchers-and-professionals/27392 (accessed 10 February 2023).
Appendix 1 I-TRAC study design overview highlighting mixed-methods contributions within and across research objectives
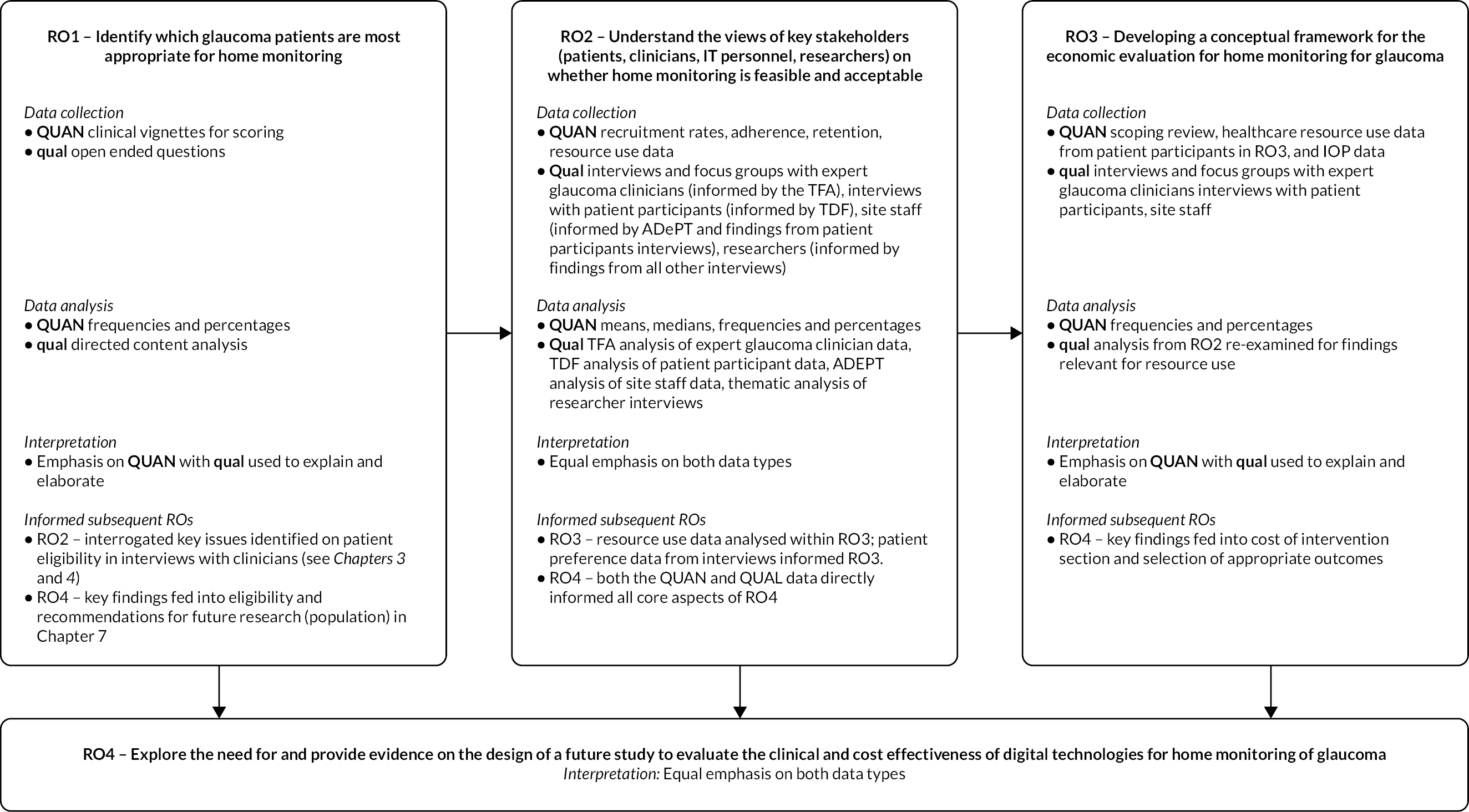
Appendix 2 Coding guide for interviews with expert glaucoma clinicians (see Chapter 3)
| TFA construct | Theme | Description |
|---|---|---|
| Affective attitude References as to how the clinician feels about the intervention. Include only feelings and emotions, for example likes, dislikes, supports, does not support, preferences, agreement, acceptance, indifference, importance. May be positive or negative or neutral/indifferent or mixed |
|
References to how clinician feels about home monitoring of glaucoma |
|
References to how the clinician feels about potential outcomes/benefits/impact on care from home monitoring | |
|
References as to where the clinician states that feelings are conditional, for example IF the technologies work OR where there is preference for one component over another for the intervention OR if there are concerns about negative impact on patients. | |
|
All references reported by clinicians in relation to how they believe patients will feel about home monitoring of glaucoma. This can be positive (e.g. welcome, glad) and negative (e.g. scared, anxious). This is most commonly in relation to handling/use of equipment but can be broader, for example convenience | |
|
References where clinicians report beliefs that patients may have positive emotional responses to the home monitoring of glaucoma | |
|
References where clinicians report beliefs that patients may have negative emotional responses (affective states) to the home monitoring of glaucoma | |
| Ethicality References where the clinician reports how the intervention fits with the individual’s values and/or is morally good or correct, including participants’ values towards patient care. Includes content in relation to possible ethical issues the intervention could pose and participants’ acceptance of those issues. Includes content in relation to equality, diversity and inclusion issues and how the participant views this as acceptable or unacceptable |
|
References where the clinician reports concerns or questions about ethical risks and challenges of remote data collection; its security, governance, protection, fidelity (knowing you are looking at the correct person’s data and that this person has consented for you to review their data). Include references to whether these challenges can or cannot be accommodated within NHS. Include statements suggesting concerns related to commercial aspects of technological devices |
|
References where the clinician reports how they perceive this intervention fits with existing principles of care; promoting self-care/self-management, concerns about glaucoma clinics becoming reduced to managing data and losing their holistic approach. Include statements where it could or could not be a solution to a current ethical issues of glaucoma care such as overtreatment | |
|
References where the clinician reports perceived equity and equality issues in relation to this intervention. Include statements about the impact of issues such as accessibility, language, education, and technical abilities upon the ethicality of home monitoring. Include statements about the perceived impact of equity and equality issues such as creating a two-tier healthcare system | |
| Intervention coherence References where the clinician reports content in relation to the extent to which they understand the components of the intervention and how it works. To include understanding of the purpose, or benefit finding, from the perspective of clinician, service and patient. Include content in relation to seeing opportunities to improve the intervention |
|
References where the clinician reports content in relation to the need for autonomy in how home monitoring is implemented in terms of deciding which patients, which services (e.g. community or secondary care), to integrate with existing services (e.g. virtual clinics), and for what purposes |
|
References where the clinician reports their perceptions of what the point of glaucoma home monitoring is; what it could or should achieve. Purposes included reducing overtreatment, refining referrals to ensure patient seen by appropriate service, improving management of glaucoma, phasing, improving detection of progression, reducing sight loss, increasing clinic patient capacity, allowing clinics to catch up on COVID-induced waiting lists, promoting patient self-management and introducing more localised care. | |
Minor subcodes:
|
||
|
References where the clinician reports which patients could be targeted with this intervention. This includes targeting younger patients, those with spurious clinic results, high-risk progressors, low risk of progression patients, those with NTG, those suspected to have glaucoma, those requiring phasing, those who are physically able, those who need to be risk assessed, those with clinic access issues. This also includes perceptions that describing the ideal patient is not possible. Minor subcodes: |
|
|
||
|
References where the clinician reports content regarding the data obtained from home monitoring in relation to the need for it to integrate with existing electronic records, be easy to access and read, and have alerts/notifications to highlight any problems in the data received | |
|
References where the clinician reports ways that data limitations could be overcome to include simpler reporting, use of AI, and the addition of disc imaging. Minor subcodes: |
|
|
||
|
References where the clinician reports knowing or not knowing how well devices work. Include reports of knowledge of scientific evidence and clinical experience in relation to how the devices work, and how satisfied participant is with the known evidence or experience. Provides contextual information that may explain perceived limitations of the home monitoring technologies. Minor subcodes: |
|
|
||
|
References where the clinician reports content in relation to the work of providing patient reassurance and exploring social support if required for additional reassurance | |
|
References where the clinician reports statements in relation to the importance of or requirement for clinician to deliver reassurance, or to commence or continue home monitoring | |
|
References where the clinician reports any statements specifically ways that patient-related limitations could be overcome, to include making technology more accessible to all, keeping technologies simpler and minimising risk of unintended consequences (e.g. how to reduce anxiety risk). Minor subheadings: |
|
|
||
| Anticipated costs References where the clinician reports the extent to which benefits, profits or values must be given up to engage with the intervention. Include content in relation to cost–benefit/value considerations. Include content in relation to whether it is believed that the intervention is economically possible/not possible |
|
References where the clinician reports suggestions as to how they think glaucoma home monitoring could be cost-effective |
|
References where the clinician reports beliefs that glaucoma home monitoring could offer value for money or be cost-neutral | |
|
References where the clinician reports doubts or concerns that home monitoring of glaucoma will not be good value for money | |
|
References where the clinician reports concerns about management, NHS or CCG buy-in related to costs and value | |
|
References where the clinician reports expectations that the intervention will be too costly to implement due to cost of the technologies | |
|
References where the clinician reports other (non-capital) types of costs they believe this intervention will impose. Minor subcodes: |
|
|
||
|
References where the clinician reports suggestions as to how they think glaucoma home monitoring could be made more affordable | |
|
References where the clinician suggests patients purchase or co-purchase equipment for home monitoring | |
|
References where the clinician suggests that app-based approaches to home monitoring will be more cost-effective | |
|
References where the clinician reports believing that in the future the costs of home monitoring technologies will reduce | |
| Burden References where the clinician reports the perceived amount of extra effort to participate in intervention. Include perceived burden upon others as well as upon self |
|
References where the clinician reports perceived burden upon clinicians |
|
References where the clinician reports burden of learning new skills and changing practice | |
|
References where the clinician reports beliefs that burden will not increase but change in its nature | |
|
References where the clinician reports that finding time to review and action home monitoring data will be burdensome | |
|
References where the clinician reports that home monitoring will result in increased patient numbers | |
|
References where the clinician reports home monitoring will result in increased appointments from patients who have become anxious due to home monitoring | |
|
References where the clinician reports the time to implement and manage a home monitoring service would be burdensome | |
|
References where the clinician reports that home monitoring would impose burden upon the health service/organisation | |
|
References where the clinician reports that home monitoring would impose burden upon the patient. This was in relation to the both the physical tasks home monitoring would involve and psychological burden (e.g. impact of the affective states of worry or anxiety). Minor subcodes: |
|
|
||
| Perceived effectiveness References where the clinician reports the extent to which the intervention is expected to achieve its intended purpose (anticipated effectiveness). Include content regarding expected outcomes, expected quantifiable changes, and factors perceived to potentially limit effectiveness (e.g. reliability of technologies, patient ability, breadth/scope of visual health aspects being measured e.g. no disc imaging). Include unintended consequences |
|
References where clinicians report expected outcomes of glaucoma home monitoring. These include detecting progression quicker (reducing sight loss), freeing up time in clinic to see higher-risk patients, increased data from which to make better treatment decisions, and increased convenience for patients. Potential negative outcomes include some patients demanding in-clinic services as home monitoring may be perceived as lower-quality care |
|
||
|
References where the clinicians report strong confidence that home monitoring will be effective | |
|
References where clinicians report uncertainties about whether possible benefits will be realised with glaucoma home monitoring. Doubts included whether potential increase in capacity could be realised, and doubts whether the effort to run such a service would be less than current provisions | |
|
Data and technology factors perceived to limit the effectiveness of glaucoma home monitoring include their confidence in the data produced by the technologies and patients’ ability to use these technologies. References where the clinician reports any perceived limitation of the data and technologies. To include missing or unnecessary components, and time and complexity to manage resulting volume of data |
|
|
Patient, sociodemographic and health factors perceived to limit the effectiveness of home monitoring. Includes patient age, compliance, physical and cognitive abilities, language and ethnicity. References where the clinician reports any perceived limitation related to the patient. To include unanticipated outcomes such as increased patient anxiety, technological limitations, and loss of useful information from the loss of face-to-face contact |
|
|
Health service/organisational culture factors which could limit effectiveness of home monitoring included staffing available, support from Trusts and CCGs, and being able to adapt to change. Minor subheadings: Home monitoring is not a replacement for in-person care and lose holistic value of face-to-face care |
|
| Self-efficacy (clinician) References where clinicians report their confidence in performing the behaviours necessary to participate in the intervention. This also includes their perceptions of the confidence of other staff that they work with |
|
References where clinicians report their beliefs about patients’ confidence to perform behaviours necessary to participate in the intervention. This includes beliefs that patients would or would not have the confidence to perform home monitoring |
|
References where clinicians report impacts on confidence to deliver home monitoring. Can include current service set-up/organisation, care pathways, and shared care where different patient scenarios result in patients being seen by different healthcare staff | |
| COVID (not TFA) Code any statement mentioning COVID to explore how this may have impacted on clinician perspectives or acceptance of these technologies or changes to service delivery |
|
References where the clinician reports how COVID changed the way in which care for glaucoma patients is/was being delivered. Includes changes in clinic attendance, changes in frequency of testing, and increased use of virtual clinics |
|
References where the clinician reports how COVID has created a need to explore new approaches to monitoring glaucoma and how home monitoring could be the solution for current challenges such as restricted patient numbers in clinic, performing tests without face masks, increasing adherence with tests through a COVID-safe approach (home not clinic) |
Appendix 3 Alternative app-based visual field or visual function measures
| App name | PC/iPad/Android tablet | Developer | CE marked (March 2021) |
|---|---|---|---|
| visualFields easy | iPad | George Kong softwares | No |
| MyVisualFields | Smartphone, iPhone or Android | MAGNETICA Development SRL | No |
| VF Fast | iPad | Leonard Yip | No |
| http://www.specvis.pl/index.html | PC (but working on a mobile app) | Piotr Dzwiniel | No |
| Visual Field (Google Play) | Android device (5.0) | FLM | No |
| Anxiety, Visual Field Test app – Glaucoma/Stroke | Desktop or Android | VCE-VSchoener | No |
| Eyecatcher | Tablet with eye tracking device | David Crabb (Open Source) | No |
| C3 fields analyser (virtual reality headset) | Unclear | Alfaleus | No |
| GearVision: a smartphone-based head- mounted perimeter (VR) | Smartphone | T Sircar | No |
| Stylianos Tsapakis (no given name for device/set-up) | The software uses the web camera as a ‘virtual photometer’ in order to detect room luminosity and allows self-testing using a computer monitor or virtual reality glasses using an Android smartphone with a 6-inch display | Stylianos Tsapakis | No |
| Peristat online perimetry | Peristat online perimetry testing was conducted within 3 months of HVF testing on a 17-inch monitor in a darkened room in the clinic with guidance by a trained investigator | Lowry EA 2016 | No |
| Damato Multifixation Campimetry Online (DMCO), a free-of-charge internet-based VF test. | Laptop | TestVision./org | No |
| PERformance CEntered Portable Test (PERCEPT) | App iPad | Peter Rosen | No |
| Virtual Eye | VR headset, unsure of device | Unclear | No |
| OKKO | Smartphone, tablet, iPad | OKKO Health | Yes |
Appendix 4 Coding guide for interviews with patient participants (see Chapter 4)
| Behaviour: Engagement with/use of home monitoring intervention for glaucoma. Two devices: a handheld tonometer to measure eye pressure, and a game in the form of an app on an iPad to measure visual function. Both to be used together, once per week, for 12 weeks. Training session given by Research Nurse and provided with manuals and YouTube videos for support at home, in addition to being able to contact study team for help. Behaviour involves use of both devices; however, attention to be paid to different responses between devices. |
|||
|---|---|---|---|
| TDF domain | Description | Decision rule(s) | Example quote |
| Knowledge | An awareness of the existence of something. Demonstrated by: Knowledge (knowledge of condition/scientific rationale for intervention); Procedural knowledge (Knowledge of how to use the devices/undertake the intervention); Knowledge of task environment (e.g. how home monitoring fits within NHS/care environment) | Awareness of what glaucoma monitoring is, what is involved and why it is performed (Knowledge of condition/rationale for intervention); Knowledge of how to use the home monitoring devices (procedural); Knowledge of what the study was trying to achieve; Knowledge of how home monitoring fits with NHS environment. Include a subcode for others – what is perceived that others need to know or should know to engage in home monitoring. Inappropriate coding/potential for crossover: Knowledge of glaucoma in general can be added as a subcode of knowledge for contextual information but knowledge should be specific to monitoring, not the disease. For example, quotes discussing how glaucoma is a progressive eye disease that results in pressure changes in the eyes should be coded as knowledge (other – contextual information, and NOT knowledge – monitoring) |
Glaucoma is a condition of the eye whereby it increases the pressure within the eye… it causes the pressure to increase, and unless it’s controlled it can eventually move to a catastrophic blindness problem. It’s something that lasts your whole life once you have been diagnosed with it, that’s my experience, and it requires continual dedication to taking your eye drops and attending your appointments at the hospital (12001) |
| Skills | An ability or proficiency acquired through practice. Skills development; Competence; Ability; Interpersonal skills; Practice; Skill assessment | Include information here on skills perceived to be required to successfully use the home monitoring equipment; include information on adequacy of training provided in relation to these skills and if participant feels their skills could be improved (specifically, which skills could be improved). Inappropriate coding/potential for crossover: Distinguish between Skills, Beliefs About Capabilities and Memory, attention, and decision processes: Comments about confidence in those skills should be coded under Beliefs and Capabilities. Comments about memory skills such as remembering how to perform home monitoring, remembering to do home monitoring each week or the mental complexity of using home monitoring devices should be coded under Memory, attention, and decision processes |
I suppose… you know, it may be a matter… you know, finding the optimum commit the decision for people using it, or something like that, rather than saying, ‘Oh, you could try this way, you could try that way’. Yes, I think the nurse and I, at that point, tried a number of different ways and I think… I’m struggling to remember now, but I think… there were a number of attempts were made and maybe only got a reading that seemed as if it was accurate about once. 12007 You would need to log it on a computer or an iPad, I suppose, if you weren’t familiar. My mother’s got glaucoma and probably would never do it because she’s 88. She would never do it (12011) |
| Social/professional role and identity | A coherent set of behaviours and displayed personal qualities of an individual in a social or work setting. Social identity; Group identity | Include information here about whether the participant sees themselves as someone who already monitors their health/uses technologies for monitoring their health away from usual care/clinic care (e.g. at home). Include previous experiences of home monitoring for other conditions (e.g. BP), use of technologies for tracking activity levels. Include any statements reporting views towards patients’ role in managing glaucoma |
I’m the sort of person who would persevere, been part of my culture. I’ve spent my life commercially travelling the world and you have to persevere to succeed (12001) |
| Beliefs about capabilities | Acceptance of the truth, reality or validity about an ability, talent or facility that a person can put to constructive use. Self-confidence; Perceived competence; Self-efficacy; Perceived behavioural control; Self-esteem; Empowerment | Include comments about how confident the participant states they are with using the home monitoring technologies. Also include comments about how easy or difficult using the home monitoring technologies was for them. Capture participant views towards their abilities to perform home monitoring. Capture differences in confidence levels between different steps involved in home monitoring. Can include sense of control if statement suggests self-efficacy. IF participant talks about their beliefs about capabilities of OTHERS, code as Beliefs about capabilities of others. Inappropriate coding/potential for crossover: Be aware of potential crossover with skills and MADP. Specific skills should be coded under Skills/MADP (if memory based skills). Beliefs about capabilities should focus on confidence and ease/y of these skills |
To start with, I found it difficult because I couldn’t… you know, I was following the instructions… reading the instructions rather than following it in my brain but as I… so to start with, the time it took me to register the pressures was a bit frustrating, but within, I don’t know, two… three weeks maximum, it was just routine. I must be… I think I was doing it in five minutes easy peasy, yes
(12001) No, I don’t think so. I think that… as I say, it’s just going back to the fact that I think the equipment itself wasn’t at the stage where you’re getting a sort of more positive result (12007) |
| Optimism | The confidence that things will happen for the best, or that desired goals will be attained. Optimism: The attitude that outcomes will be positive and that people’s wishes or aims will be ultimately fulfilled Pessimism: The attitude that things will go wrong and that people’s wishes or aims are unlikely to be fulfilled |
Include comments where participants describe their level of optimism that home monitoring can make a positive difference for them OR describe their level of pessimism that home monitoring cannot make a positive difference for them. Capture their attitude towards this approach of glaucoma monitoring – can this approach make a difference? Inappropriate coding to this domain: Descriptions of the harms/disadvantages/benefits/advantages associated with glaucoma home monitoring should be coded under Beliefs about consequences. Could cross over with beliefs about capabilities |
Well, I was pretty… I was very switched on, yes. Here’s the equipment, I knew ultimately I’d get it to work efficiently, so let’s do it week by week, yes
(12001) I mean, it was a mix. If you said, ‘Out of 10, how do you think the experience was at home?’ Probably a seven in that it wouldn’t be good to be monitoring all the time, but equally it would be a very handy tool to have, especially on my holiday. I’ve still got my stitches in my eye, I had COVID and I’m coughing lots so I have unusual pain, so having the monitor and it just saying that my pressure was fine, I was fine. So from that it would be great, but equally… if I had pre all this going on and I was just going about quite the thing with my yearly checks… I’m not sure that would have been beneficial because I would just get neurotic. So yeah, there is definitely a use for it and I think that is the way forward, but yeah, just correct use of it (12011) |
| Beliefs about consequences | Acceptance of the truth, reality or validity about outcomes of a behaviour in a given situation. Outcome expectancies; Characteristics of outcome expectancies; Anticipated regret; Consequents | Include comments that describe the participants’ expected or experienced outcomes or consequences from home monitoring, both for them and for others/services, and the extent that they expect these outcomes to be realised. Include positive and negative outcomes/consequences. Include mention of barriers and enablers that can inhibit or promote the extent to which consequences can be achieved. Include statements such as ‘makes you focus on problems’. Include statements such as ‘tech is not fit for purpose’ but this may double code with environmental context or resources if there is some interaction between the intervention and the environment that is affecting perceptions about readiness for use (e.g. something like without extra time being allocated for training this intervention is not fit for purpose). Can include references about self-control if statement suggests this is an outcome/consequence of home monitoring. IF participant talks about their beliefs about consequences upon OTHERS, code as Beliefs about consequences for others. Inappropriate coding to this domain: References inferring attitude towards consequences of home monitoring should be coded under optimism |
firstly I could see the rationale taking time away from serious hospital appointments to moving it to home where the individual could carry out and have the information transferred… you know, Wi-Fied through to the hospital department. The benefit was to take pressure off the hospital to do other things, more important things, yes… (12001) I guess, you would need to be convinced that it was accurate and reliable (12007) |
| Reinforcement | Increasing the probability of a response by arranging a dependent relationship or contingency, between the response and a given stimulus. Rewards (proximal/distal, valued/not valued, probable/improbable) Incentives; Punishment; Consequents; Reinforcement; Contingencies; Sanctions | Include comments about reinforcement/reward for home monitoring of glaucoma. Include positive feelings arising from perceived altruism/benefit of their home monitoring upon others/services (social rewards). Include here app/tonometer feedback (readings/no readings) and impact upon behaviour to continue to home monitor. Separate actualised reinforcement from hypothetical reinforcement, for example seeing results or wishing to see the results, and its impact on behaviour/engagement with home monitoring. Inappropriate coding to this domain: Feelings/emotions should only be coded here if described as an outcome/consequence of home monitoring that is motivating the participant to engage or disengage in home monitoring, for example ‘I knew that by home monitoring I was allowing those who need clinic time to be seen quicker, and that made me feel good and that is why I wanted to do the home monitoring’. Here focus should be placed on the link between emotion and increase/decrease in behaviour (use of home monitoring). Other references to feelings/emotions should be coded under emotions, for example ‘home monitoring made me feel anxious at the time as I worried if my vision was getting worse’ <there is no stated impact of worry/anxiety upon behaviour change> |
I’m not sure if this is right, but I think I thought that I would know what my eye pressure was so would be seeing for myself how it was working. Whereas, in fact, I think I was slightly… I think I… as it was explained to me, I don’t think I was going to have that information. I’m not sure on that. I thought that was quite disappointed [sic] because I thought if you were doing… if you were monitoring you’d sort of wanted to see the results, though I could also be aware that you might not do it accurately so the results might not be accurate, so it might be considered that you’re worrying yourself when you didn’t need to. But I think it was some motivation in understanding what you’re doing and seeing the results…. What would have probably encouraged you is actually seeing the measures each week as you take them
(12007) I’ve had a very unusual last 4 months with a lot of problems, so for me, yes, it was actually hugely beneficial. Whereas for if you were just a steady… I’m not sure how beneficial it would be, but for me, I was able to phone in and say that my pressure was 50 and then I would phone and say my pressure was five and get immediately seen. So from that point of view, it was very good (12011) |
| Intentions | A conscious decision to perform a behaviour or a resolve to act in a certain way. Stability of intentions; Stages of change model; Transtheoretical model and stages of change | Include comments here that reflect participants’ descriptions of how motivated they are/were to do home glaucoma monitoring, and whether this motivation changed at all from pre-monitoring period, during monitoring and after. Include content in relation to preferences; would they now prefer home monitoring or clinic monitoring? Inappropriate coding to this domain: Be careful not to code the reasons for the intention (focus on statements that directly reflect their intention and motivation) |
Only the knowledge that I have to monitor my eye pressures and field of vision. You know, that’s the motivation because it’s a number one priority. I’ve got a… I have a cousin who’s… she’s what, three years younger than me, who’s in the advance stages of glaucoma, going blind. Very sad. I know what it can do to one’s life in terms of looking… seeing pictures, seeing… reading, etc, etc. Terrible, terrible (12001) Well, I suppose because I’ve got a family history of glaucoma and can see the benefit of the changes in treatment that anything that helped that seemed worthwhile (12007) Well, at the moment, I think I’d go with monitoring in a clinic because there is equipment available that does it, that is easy to use, and you feel reassured it’s doing it accurately (12007) |
| Goals | Mental representation of outcomes or end states that an individual wants to achieve. Goals (distal/proximal); Goal priority; Goal/target setting; Goals (autonomous/controlled); Action planning; Implementation intention | Include comments here that report how important home monitoring is to them and if that influenced their decision to home monitor. Include comments in relation to how much they prioritised home monitoring over other activities, for example ‘I got really busy with family things and I just didn’t get around to taking it out of the box’. Consider statements where participant discusses any specific goals they had/set for home monitoring |
I wouldn’t say it was very important because I’m at the stage where I’m… tend to be going to have a check-up every 6 months, which seems to be working in terms of keeping the pressure under control, so it’s not… I didn’t feel it’s crucial (12007) |
| Memory, attention and decision processes | The ability to retain information, focus selectively on aspects of the environment and choose between two or more alternatives. Memory Attention; Attention control; Decision-making; Cognitive overload/tiredness | Include statements that discuss how easy or difficult it was to remember to do weekly home monitoring, and to remember the steps involved in home monitoring. Include any specific aspects that were difficult or easier to remember. Include references to the mental complexity of the task of home monitoring, for example the level of concentration needed to complete the game on the app. For memory, focus on how they remember to do it. Included statements about it becoming simpler/easier to deal with cognitively. Inappropriate coding to this domain: If participants simply recite their awareness of how to use the equipment, without the mental processes, this should be coded under Knowledge. May cross over with Behaviour regulation– this should focus on what it involves (e.g. having a daily routine, vs. MADP of adding it to their daily routine) |
Well, it became part of a routine. I didn’t worry about the fact that it was going to take me ultimately 10 minutes. You know, it is just a routine. I take a lot… I have a lot pills because I’ve had heart problems and all the rest of it, and diabetes, and it’s just part of the routine. Easy-peasy, yes (12001) I think with the iPad, I got bored doing it. It was just the same week on week. I was expecting it to get harder and harder. Too simplistic (12001) |
| Environmental context and resources | Any circumstance of a person’s situation or environment that discourages or encourages the development of skills and abilities, independence, social competence and adaptive behaviour. Environmental stressors; Resources/material resources; Organisational culture/climate; Salient events/critical incidents; Person × environment interaction; Barriers and facilitators | Include statements that discuss whether and how the environment (social, cultural, personal circumstances, including other health issues) was suitable/unsuitable for home monitoring or made home monitoring suitable/unsuitable. Include any statements regarding any adjustments participants had to make to allow them to home monitor, for example using a pile of books to hold the iPad in the right position. Include any references made by participants about their views towards the costs of equipment and any accessories needed that could influence engagement with home monitoring. Include any comments in relation to COVID-19 pandemic and home monitoring which suggest greater acceptability/engagement of home monitoring as a consequence. Include statements about how the physical body interacts with environment and home monitoring, for example if a health condition limits use of home monitoring technologies. This may be in relation to specific aspects or features of the devices, for example handling the probes for the tonometer is tricky due to arthritic finger joints. Include statements about wider influences of physical complexity (not mental/cognitive complexity) Inappropriate coding to this domain: If participants advocate the need for training/more training on how to perform home monitoring, this should be coded under Skills. |
No. I just had a nice desk… The iPad I propped up against a book
(12001) Yeah, the iPad was absolutely fine. You couldn’t really do much with it other than what it’s programmed to do (12011) |
| Social influences | Those interpersonal processes that can cause an individual to change their thoughts, feelings or behaviours. Social pressure; Social norms; Group conformity; Social comparisons; Group norms; Social support; Power; Intergroup conflict; Alienation; Group identity; Modelling | Include any statements where participants report the opinions of others and whether/how it influenced their decision to home monitor their glaucoma. Includes influence of other healthcare professionals/trial staff/patients/families. Separate between ‘participation in study’ and ‘engagement in home monitoring’ as these are two different behaviours. Inappropriate coding: Specific descriptions of the roles of others – this should be coded under Social Role and Identity |
Well, [Consultant] has been my consultant for many years. I’ve also been with him privately to have cataract operations on both eyes. I’ve built up quite a nice rapport with him; I find him very approachable and nice to chat to and so when he asked me during a session at the [hospital name] would like to take part, I said, ‘Yes, no problem’ (12001) |
| Emotion | A complex reaction pattern, involving experiential, behavioural and physiological elements, by which the individual attempts to deal with a personally significant matter or event. Fear; Anxiety; Affect; Stress; Depression; Positive/negative affect; Burn-out | Focus on emotional experience of home monitoring rather than emotional consequences which are outcomes of home monitoring or motivating home monitoring (which should be coded under Consequences or Reinforcement). Include statements which refer to how the participant felt emotionally about home monitoring before, during and after their home monitoring period. Inappropriate coding to this domain/potential crossover: Comments that report that how a participant felt changed their use/performance of home monitoring, for example ‘I was getting frustrated with it and I just stopped using it’, are examples of emotional reinforcement or punishment. In emotion, focus on the experience of the emotion and how it was arising, and focus on its impact in reinforcement |
I got a bit frustrated to start with. It was taking me a little… you know, what I felt was too long to actually get the blue light rather that the red light to be able to push the button. But within days, rather than… you know, days not weeks, I got the hang of it, and it then became a very simple process (12001) At first I was very scared because my pressures were 50, so in actual fact they came down, so I was scared to take it in case it was high again. Just because I was going through such an acute stage. But once it stabilised, it didn’t bother me either way. In fact, the more it stabilised, I actually became quite laid back and didn’t use the monitor other than when I was asked to. (12011) |
| Behavioural regulation | Anything aimed at managing or changing objectively observed or measured actions. Self-monitoring; Breaking habit; Action planning | Include references to strategies/processes reported by participants to help do home monitoring at home. Include strategies used to remember to do home monitoring. Include strategies used to remember how to perform home monitoring. Include strategies such as self-monitoring, for example writing down scores to keep track of their glaucoma. Include hypothetical statements about what would be helpful or improvements for the future |
I think as I got better, I didn’t need to use it as much. You know, I think I just did the weekly. Then when I knew it was going to go back, I actually used it more, just more as… like, I would go out for a fast walk or, just because I hadn’t gone back to the gym by that point. I would go for a fast walk or just do something a bit more strenuous, and just see if that made a difference to the pressure. Just more as a trial for me, just to say, ‘Okay, that made no difference to my pressure by doing such and such strenuous’. I just thought I would try that out before it goes back (12011)
I just thought that in actual fact, to be measuring all the time wasn’t good for me either, so I tried to come away from it so you weren’t getting too reliant on it, especially when I knew it had to go back (12011) |
| Nature of behaviour | Additional domain taken from TDF version 1 | Include references to potential changes in behaviour, for example how someone believes their behaviour would change if… | |
Appendix 5 Coding guide for interviews and focus groups with site staff (see Chapter 4)
| ADePT item/theme | Description | Example |
|---|---|---|
| Site staff knowledge, beliefs and feelings towards home monitoring | ||
| ADePT Acceptability: Staff emotions and knowledge – beliefs towards I-TRAC and home monitoring | Code data relevant to: Acceptability – Was the intervention acceptable to clinicians (and site staff)? How did they feel about it? Did it make sense? | Always positive about doing the stuff you know, there’s something new coming it’s always nice to have something new isn’t it. But particularly I think the it’s the fact it’s a device study and you’re interested in how the devices will actually improve patient care. So it’s about excitement of being able to introduce something new that’s going to hopefully make a difference really, yeah (P037) |
|
Code any reference stating an emotion or feelings towards home monitoring or the I-TRAC study. DO NOT include statements about how it sits with current care or alongside other chronic conditions unless an emotion is stated alongside this | I think my initial thoughts were obviously, ‘This seems really interesting’, but I think I’m probably not alone in thinking, ‘Oh, yeah, how are our patients going to deal with this?’ (P031) |
|
Include data relevant to how site staff perceive home monitoring of glaucoma in its wider context, for example does it make sense to them? Does it have a place/fit with NHS usual care? DO NOT include affect – emotions can be coded under positive and negative emotions towards home monitoring or I-TRAC study in other codes | So in the context of having a chronic disease in the current model does not make much sense because people have to travel for things that potentially can be done at home (P034) |
| The ease of running the I-TRAC study and the compatibility of its components | ||
| ADePT Study Conduct: Delivered as planned, components working together and unintended consequences | Code statements in relation to: Study Conduct – Was the intervention delivered as planned? Were the logistics of running a multicentre trial assessed? Did all components of the protocol work together? Were there any unintended consequences? | I think it was rolled out just as exactly as we would’ve hoped it would’ve been because we recruited quickly, we had good support in our research team, we had a very able and IT-savvy individual who was able to help with those aspects of it, which actually are quite challenging for the age group that we’re recruiting from and I think… (P035) |
|
Code statements where site staff state how easy or difficult the study was to run, and specific difficulties or challenges encountered. DO NOT include data in relation to recruitment or representativeness as this is coded under ADePT Eligibility, recruitment and consent. Challenges faced by site staff not related to training [Include data here where site staff report challenges or difficulties implementing the study. DO NOT include anything connected with site or patient participant training (code instead under Training Quality and Usefulness)]. I-TRAC was easy to run due to being well explained and low burden for patients and sites in terms of scheduled visits and activities |
Yes, because I mean I actually learned more from watching that YouTube training than I did actually from the sale rep side of things, so, yeah. Yeah, certainly the YouTube thing helped once we’d got to grips with the YouTube address which was (overspeaking) P037 |
|
Code any data related to the quality and usefulness of both the site training/SIV training and patient training. Include statements about problems with or resulting from the site or patient training | Yeah, the step-by-step guide was very, very detailed, the fact that there was one for the patient and one for the practitioner as well was really handy (P033) |
| ADePT Adherence | Code data related to participant adherence. This was not a question directly asked in topic guide but several participants reflected on this | . . . found that, potentially, because they were working and things like that, they maybe didn’t spend the time on it. I might be wrong but this is the impression I got (P039) |
| Eligibility, recruitment and representativeness of sample | ||
| ADePT Eligibility, recruitment and consent: Recruitment and Representativeness of sample | Code data relevant to: Eligibility – Was the resulting sample representative of target population? What factors influenced eligibility and what proportion of those approached were eligible? Recruitment – Was recruitment successful? What might the impact be of change upon recruitment, for example evolving IT literacy, pandemic? Consent – Did eligible participants consent? |
The fact that the virtual clinics were there as a good pot of people that we could actually approach, it made it easier for us to be able to just then blanket sort of coordinate and collect patients in those virtual clinics, it worked really well (P037) |
|
Code data here when site staff state the ease of recruiting and where this was due to positive response from patients. DO NOT include suggestions for the future recruitment as this is coded under ADePT What needs to be changed or improved | I know one of the patients we have, she’s 70 next month or something and I thought, ‘Oh, she might be a bit tricky’, you know? But she was just class, straight away, quicker than I picked it up! She was so good and I was like, oh, it just shows you. She wanted to see the numbers and she was really interested in it (P038) |
|
Include data statements where site staff report their perceptions of how representative their participants are of glaucoma population | . . . we have discharged quite a few of our patients to the community, the very, very straightforward ones like ocular hypertensions, so they weren’t… there might’ve been one or two but they’re usually… if they’re ocular hypertensions in the hospital they’re usually there for a reason. So actually quite a lot of them… I’m sorry, that group would be missing (P035) |
|
Code statements that refer to site staff comments in relation to the recruitment process (including how to identify patients) and what they perceived to work well or less well. DO NOT include suggestions for the future as this should be coded in ADePT What needs to be changed | I have approached a lot of people and I think sometimes there have been language issues, the fact that it’s all in English, the information sheet’s in English, that’s been an obstacle I think a couple of times, although it hasn’t always been explicitly stated that that is the issue, yeah (P031) |
| Unintended consequences | ||
| ADePT unintended consequences | Code data in relation to study conduct – were there any unintended consequences (e.g. adverse events). This can include concerns about potential unintended consequences as well as unintended consequences that did happen/site staff were aware of. Include any outcome that was not expected or planned for | . . . oh, it depends on the person. Their personality just… they know too much or find out too much and it gives them anxiety… (P038) |
| Adaptations of considerations for future evaluation | ||
| ADePT what needs to be changed or improved or resourced to run as full-size trial | Code data here in relation to suggestions for what and how any aspect of the study design or conduct could be changed or improved to run as a full-size trial. This can include perceptions about what resources would be required if it has a direct link to study design or conduct (e.g. include the need for technical support resources, additional equipment such as spares or replacements to run smoothly) | It would probably be easier if we just had an I-TRAC demo and just brought everybody on the same day to do training, or maybe did group training with the patients rather than individually. That would be something that would save time, so one person can maybe teach three or four people at the same time and maybe those people have a bit of support as well because they’re all part of the same study (P038) |
|
Code data here in relation to financial and human resource costs perceived to be required for a larger study | Again, you know, just to have a system that captures that information and also that is able to attend potential emergencies or calls or if the patients are… if they have a high or abnormal reading, that they know how to implement that pathway (P034) |
|
Code data here related to site staff suggestions for changes to study design that they believe would be helpful for a larger study. Where possible include why they think this (which may overlap with a challenge or difficulty faced). DO NOT include recruitment and participant selection issues as these will be coded under future participant selection | I guess at the moment it would be… perhaps it’s not very efficient because it’s all very innovative. But I guess, you know, you can incorporate this into electronic records and there will be, let’s say, the potential of having the information displayed in a different way, that makes the clinicians’ tasks easier. It depends, you know? At the moment it will be time-consuming because we don’t have a good system to process this information. It would need to be well thought to make it efficient (P034) |
|
Code data here in relation to recruiting and selecting patient participants in a bigger trial where the site staff member feels this would be different in some way from what they did and experienced in the feasibility study, I-TRAC. Include concerns about clarifying the purpose of home monitoring to tighten eligibility criteria. DO NOT include perceptions on how recruitment ran in the feasibility study as this should be coded under ADePT Eligibility, recruitment and consent | Well, because I can imagine from my impression, this technology might not be for everybody. I guess if there is a trial, there will be an inclusion and exclusion criteria, there would be a particular population of glaucoma people, people with glaucoma (P034) |
|
Code data here in relation to how site staff perceive the staff/site training (for both conduct of the study and how to train patient participants to use the home monitoring technologies) could be improved in a future trial and any explanations offered to explain why these suggestions might be better | There wasn’t much space for them here. It didn’t really hit off very well initially. Do you know what I would have liked? If we could have all gone to a wee training course, you know, meeting up with the other people. I know the budget is only so much but if we’d done a training course with the other people where we could all ask questions, take our time and been shown how to use it, we could take notes and things like that as well and then try and start a wee bit earlier (P039) |
|
Include data that are relevant to how site staff perceive the patient participants’ training could be improved in a future trial and any explanations offered to explain why these suggestions might be better. Explanations may overlap with codes under Training quality and usefulness (linking experience to perception of what needs to change in relation to patient training) | I think also the other thing that I noticed was that it’s helpful if the person teaching the patient has an understanding of the patient’s condition, because we had a patient where they had poor vision on one eye and it was really hard to get them to measure the pressure in that eye, but they could do the other eye, and we didn’t realise initially, and then once the notes were opened and you’re like, ‘Oh, the vision’s quite poor in that eye’, it became apparent why there was that big difference. I think just having some… I think if you just dump a patient with a technician who knows nothing about that patient, that might not be the best way to teach them (P036) |
| Impact and influence of COVID-19 pandemic on perceptions of acceptability | ||
| Any aspects of the data where site staff reflected on advantages and disadvantages that the COVID-19 pandemic offered for home monitoring of glaucoma of a future evaluation of the intervention | Maybe with patients maybe wanting to do stuff more at home, that would be more the impact rather than the other way around. You know? They’d be like, ‘Ooh, don’t have to go to hospital, great!’ You know? (P038) | |
Appendix 6 Changes to protocol
| Version number | Revision date | Summary of changes |
|---|---|---|
| 1 | 16 September 2020 | Approval of V1 |
| 2 | 27 November 2020 | Inclusion of details for prompting participants to complete eye measurement, and, inclusion of PPI activity as requested by funder for Protocol upload |
| 3 | 16 December 2020 | Edit to state ethic approval had been confirmed rather than submitted and include associated REC reference number |
| 4 | 10 May 2021 | Following discovery that the MRF VF app was not CE marked and cannot be CE marked in sufficient time to avoid a significant delay to the study, we changed the app we used for monitoring participants engagement with the visual testing aspect of home monitoring for glaucoma. We used the OKKO Visual Health App (OKKO Health) which was CE marked |
| 5 | 1 June 2021 | Edit to add that clinical measures for IOP and visual function may be obtained from medical records if taken within 3 months of baseline assessment |
| 6 | 30 July 2021 | Approval for use of Android tablets instead of iPads which are also compatible with the OKKO Health app. Allows use of alternative technology which may be quicker to resource. Also proposed reducing the home monitoring period from 4 months to 3 months. E-mail/text reminders content also submitted for REC approval |
| 7 | 28 October 2021 | Addition of collecting demographic data on qualitative interview participants. Addition of an e-mail verification which was sent to verify patient participants e-mail address for weekly electronic reminders while undergoing home monitoring. Collection of time of the follow-up appointment on baseline CRF |
| 8 | 16 December 2021 | Amendment to Appendix 1, SSC Charter, to remove KO and add PM from member list |
| 9 | 27 May 2022 | Updated Study Flow Diagram (Appendix 3) plan in accordance with 4 month NIHR funded extension |
| 10 | 27 May 2022 | Update our patient participant PIL to advise patients that they will see their measurements while they are home monitoring – no change to protocol |
Appendix 7 Coding guide for interviews regarding researchers’ experiences of conducting evaluations of digital technologies for home monitoring health conditions (see Chapter 5)
| Theme | Subtheme | Code | Subcode | Description | Example (mix of generic examples and verbatim quotes) |
|---|---|---|---|---|---|
| Challenges | |||||
| Recruitment challenges | Lack of patient acceptability (issues with agreeing to partake in study or staying in study till the end) | Lack of interest/dislike | – | Mention of dislike or non-interest in technology being the reason for patients’ refusal to partake in the study OR continue the study |
‘I am not interested in technology’
‘I’m not convinced that technology is for me’ |
| Lack of home support | – | Mention of patients’ refusal to partake in or continue study due to lack of support with technology | ‘My son who will assist me is currently away’ | ||
| Ageism | – | References of the patients’ age perceived to bar their acceptance of the study | ‘I am too old for studies involving technology’ | ||
| Study or technology anxiety | – | References of the uncertainties that interfere with participants’ participation in the study, for example anxiety, stress, worry, concern, frustrations | ‘No, no, sorry, it stresses me out, I can’t do it’ | ||
| Technology phobia | – | References that relate to potential participants’ fear of technology | ‘Patient was afraid of technology’ | ||
| Digital literacy issues | – | Expressions by the researchers that relate to patients’ inability or difficulty navigating/utilising technology |
‘I am not good with computers’
‘Oh, I don’t know what to do in terms of I can check my e-mails and that’s about it’ |
||
| Instruction/training incoherence | – | References to difficulties related to patients’ understanding of or following instructions or content related to patients’ retraining |
‘Study was not clear to me’
‘Retraining of patients had to be done’ |
||
| Lack of representative sample (any expression about hindrance in getting a sample with minimal inequalities) | Digital exclusion | Content that references lack of equipment needed to partake in study |
‘I have an iPad but not a computer’
‘I do not have internet at home’ |
||
| Recruitment bias | Content referencing selective recruitment of participants |
‘we only advertised in the Glaucoma Bulletin and so only had access to patients who read the bulletin’
‘recruitment was done in a white predominant community’ |
|||
| Geographic limitation | Expressions about location of study preventing potential participants from being recruited | ‘our study was in London but we received calls from people in Wales who were interested. The fortnight clinic visits did not permit that’ | |||
| Surgical appointment or routine | Content that relates to the surgical or recovery status of participants | ‘some people were not recruited because they had a recent surgery or had been booked for surgery’ | |||
| Lack of clinician/staff acceptability | Lack of buy-in | References difficulties participants encountered in getting clinicians/staff interested in the technology under study |
‘this technology will not benefit my patients’
‘how is this making my life easier?… there is too much hassle using it’ |
||
| Assumptions by staff/clinicians | Mention of staff/clinicians’ assumptions about patients’ willingness or ability to partake in study | ‘there was a site where the clinicians thought their patients were too old to be part of the study…’ | |||
| Site enthusiasm | Quotes that relate to enthusiasm of site staff that led to participant drop-out or low recruitment |
‘We had one site that they were hugely enthusiastic and maybe a bit trigger-happy than the recruited patients, because that site there had a really high drop-out rate, so about twice the rest of the sites’
‘there’s the role of the anti-champion as well, and you’ll get… some people set their minds against these things and then you’re in trouble, you know?… You’ll get one partner sitting around the table that says, “No, I think this is rubbish”, and that’s it gone’ |
|||
| Equipment challenges | Hardware issues | – | – | Any comment that references problems with devices used in the study. Expressions related to delays in device acquisition. These problems are specific and restricted to the device |
‘delays with bulk shipment of devices’
‘for some patients, the device stopped working abruptly while in use’ ‘COVID delayed hardware shipment because of freight issues during the pandemic’ |
| Software/technology problem | – | – | References to difficulties using the software or technology OR limitations brought on by the software |
‘constant update of the app was problematic’
‘software was only compatible with Apple’ ‘tech was complex to use’ |
|
| Technical issues | – | – | Any mention of a problem that warranted IT expertise or issue with the IT team | ‘reluctant IT personnel’ | |
| Accessory issues | – | – | Contents relating to problems associated with the accessories such as charging cables and MiFi | ‘faulty charging cables that had to be replaced’ | |
| Resource challenges | Staffing issues | Low staff numbers | – | Expressions about issues relating to inadequate staff numbers | ‘there were no staff to man the helpdesk’ |
| Expertise of team | – | Quotes about the expertise of members of the team barring the trial | ‘not having a tech-savvy person on the team meant we had to contact IT often’ | ||
| Study design problems | – | – | Comments that reference under-allocation, underestimating or non-anticipation by researcher. Also includes expressions that relate to limitations of the study method/design such as RCTs taking too long, not piloting the technology before trial |
‘we thought the study would be over in 4 months but it was a push getting to 100, we had underestimated the time needed to recruit and complete the study’
‘I mean, this is the thing about randomised control trials. There’s a lot of people, you’ve probably come across this, in tech that do not believe in randomised control trials at all. They’ll say they take too long… By the time you’ve got the answer, the technology has moved on’. ‘But if you’ve got a positive RCT… it’s actually quite hard to fight against. But even so, something like NICE, NICE still does not say there’s a proven case for telemonitoring. That’s because of the way that NICE works. NICE has described every paper in telemonitoring of blood pressure as medium to low quality… It’s non-blinding, you know?’ |
|
| Financial problems | – | – | Contents referencing any difficulties in securing funds to start or maintain the research OR difficulties financing miscellaneous |
‘funding for additional telephone at the help desk was not available’
‘securing funds for feasibility is difficult’ |
|
| Regulation challenges | Ethical issues | – | – | Expressions about concerns regarding safety, data confidentiality, storage and transfer | ‘ethics committee raised issue about patients doing these tests at home’ |
| IT governance issues | – | Expressions about concerns regarding safety, data confidentiality, storage and transfer | ‘there were concerns about where the results are stored and who sees them… our data went straight to servers of the tech company…’ | ||
| Regulatory problems | – | – | Any expression that relates to limitations from regulatory bodies or new legislation |
‘it’s absolutely bizarre that a country like Scotland, how you have to go through this 13 or 14 times… Oh, it took forever, you know? The epidemic was almost over by the time we got permissions, you know?’
‘there were delays in getting nod to start the trial… and by the time trial started, the technology was outdated’ ‘the new legislation because of Brexit meant we needed a new approval’ |
|
| Challenges with internal and external partners | Issues with commercial company | Lack of contracts | – | Expressions referencing restrictions, limitations, or issues that relate to the commercial companies OR complications/difficulties caused by commercial companies |
‘In the end one of the companies got bought by another company… things a bit complicated for a while’
‘The other app, the company that owned it discontinued it.… support was absolutely minimal because they were discontinuing it ’. ‘the company that produced the tech was unresponsive’ |
| Problems with other institutions | Procurement challenges | – | Expressions about procurement hindering trial | ‘we had to change our device of choice to one to suited the university’s procurement standard’ | |
| Opportunities | |||||
| Recruitment facilitators | Patient acceptability (factors that enhance agreeing to partake in study or staying in study till the end) | Familiarity | – | Mention of familiarity in study or technology enhancing participation |
‘Patient was OK with study because he had done a similar thing’
‘Patient uses technology well’ |
| Perceived benefit | – | Mention of patients’ acceptance to partake in or continue study due to the potential benefit they believe the technology offers | ‘The technology addressing an identified need enhanced acceptance’ | ||
| Clinician/staff acceptability | Good relationship | – | Content that references relationship between staff/clinician and patients OR between researcher and staff/clinicians that promotes acceptability | ‘I probably was lucky because I knew a lot of people… a lot of my people who took part were people who I was probably pals with which always helps, you know?’ | |
| Perceived benefits | – | Content referencing to enhanced buy-in due to reliability of the technology, and how it benefits both clinicians and patients |
‘if it genuinely isn’t going to cause them much hassle, they’ll do it’
‘if the technology is accurate, reliable and beneficial to their clients, they will do it’ |
||
| Enthusiasm | – | Expressions about enthusiasm promoting acceptability | ‘It’s really good if you can get champions, so if you can get a few people who are tech enthusiasts who will get this thing going’ | ||
| Familiarity | – | Mention of familiarity in study or technology enhancing participation | ‘staff found it easy to get on board because they had done technology trials before’ | ||
| Equipment facilitators | Hardware factors | Device familiarity | – | Any comment that references factors that eases use of devices for the study | ‘patients used their own phones to help minimise difficulty navigating a new device’ |
| Software/technology factors | Compatibility | – | References to software or technology being compatible with more than one device | ‘software could be used on most smartphones’ | |
| Simplicity | – | Any mention of a factor that eliminates complexities using the software or technology | ‘So basically have a system where you only have one click option so they can’t click on the wrong thing, that’s ultimate necessity’ | ||
| Address identified need | – | Contents relating to problems associated with the accessories such as charging cables and MiFi | ‘faulty charging cables that had to be replaced’ | ||
| Accessory factors | References any factor relating to accessories and how it helps minimise complexities |
‘we provided a MiFi to provided connectivity needed to easily upload data’
‘no internet access stopped all the updates which were a nuisance to participants’ |
|||
| Resource facilitators | Research team | Expertise of team | – | Expressions about skills of team members that benefited study | ‘my team was good, we had statistician, qualitative researchers…’ |
| Study design | Feasibility studies | – | Comments that reference piloting DHT studies | ‘I think some kind of pilot study or feasibility study would’ve been very useful in identifying some of the issues and they’re addressed before we go into a full trial or a full study; hindsight’. | |
| Patient support | Help desks | – | Contents referencing benefits of helpdesk | ‘the helplines helped in monitoring patients and also assist those who needed technical support’ | |
| Regulation facilitators | Regulatory evaluation | Safety | – | Any expression that relates to factors that ensure safety of the DHT | ‘we got CE markings and all other markings before trial?’ |
| Support from internal and external partners | Resource support | Technical support | – | Expressions referencing any support received from professional IT personnel | ‘the university IT team helped us set up the management systems on the devices and set up passwords’ |
| Equipment support | – | Content about equipment provided by external agent that helped study | ‘we got the testing device from the university which we could not have obtained by ourselves for this study’ | ||
| Clinical setting | – | References to the use of partner’s clinical setting for recruitment | ‘Having a facility where the participants can attend in a clinical setting, we had the university eye clinic, was helpful to use the resources there for their initial visit and as a point of contact when the participant arrives. There was a reception area so it felt like a regular eye appointment. That wasn’t a challenge but it potentially might be’ | ||
| Financial support | References about receiving funds from partners | ‘Glaucoma Foundation provided funds’ | |||
| Regulatory support | – | Expressions about assistance received to solve issues with regulatory or ethical bodies | ‘the pharmaceutical company the trial was for resolved the ethical issues’ | ||
Appendix 8 Search strategies for the literature review in Chapter 6
Review of articles on the economic evaluation of glaucoma monitoring: December 2021
Database searched: MEDLINE and EMBASE
-
“Costs and Cost Analysis”/
-
Cost-Benefit Analysis/
-
(cost* or economic*).ti.
-
1 or 2 or 3
-
exp Glaucoma/
-
Ocular Hypertension/
-
glaucoma.tw.
-
“ocular hypertension”.tw.
-
5 or 6 or 7 or 8
-
4 and 9
-
monitor*.tw.
-
Mass Screening/
-
exp Vision Tests/
-
screen*.tw.
-
population surveillance/or public health surveillance/
-
surveillance.tw.
-
check*.tw.
-
11 or 12 or 13 or 14 or 15 or 16 or 17
-
4 and 9 and 18
-
(editorial or letter).pt.
-
19 not 20
-
limit 21 to (english language and yr=“2000 -Current”)
-
“randomized controlled trial”.pt.
-
(random$ or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab.
-
(retraction of publication or retracted publication).pt.
-
23 or 24 or 25
-
(animals not humans).sh.
-
((comment or editorial or meta-analysis or practice-guideline or review or letter) not “randomized controlled trial”).pt.
-
(random sampl$ or random digit$ or random effect$ or random survey or random regression).ti,ab. not “randomized controlled trial”.pt.
-
26 not (27 or 28 or 29)
-
exp cohort studies/
-
cohort$.tw.
-
controlled clinical trial.pt.
-
31 or 32 or 33
-
30 or 34
-
10 and 35
-
10 not 18
-
35 and 37
-
38 not (editorial or letter).pt.
-
limit 39 to (english language and yr=“2000 -Current”)
Review of systematic review articles on the economic evaluation of home monitoring studies for any chronic conditions: May 2022
Database searched: MEDLINE and EMBASE
-
(“Remote monitoring” or “Remote patient monitoring” or “In-home monitoring” or “Inhome monitoring” or “Home telehealth” or Telemonitoring or Telecare or RPM or telemetric or “remote sens*”).ti,ab,kw.
-
exp Chronic Disease/
-
chronic.ti,ab.
-
2 or 3
-
Cost-Benefit Analysis/
-
“economic evaluation”.ti,ab,kw.
-
(cost adj (effect* or utilit* or benefit*)).ti,ab,kw.
-
5 or 6 or 7
-
1 and 8
-
4 and 9
-
limit 10 to “systematic review”
-
limit 11 to english language
Appendix 9 Lists of excluded studies and reasons for exclusion for the literature review in Chapter 6
A list of studies selected for full-text review for the systematic review of application of economic evaluations in glaucoma monitoring studies
Economic evaluation studies for glaucoma screening (14)
-
Liu Q, Davis J, Mackey DA, Hewitt AW, Han X, Macgregor S, et al. Cost-effectiveness of polygenic risk profiling for primary open-angle glaucoma in the United Kingdom and Australia. Invest Ophthalmol Vis Sci 2021;62(8).
-
Congdon NG, Kee F, O’Neill C, Tang J, Liang Y. Cost-effectiveness and cost-utility of population-based glaucoma screening in China: a decision-analytic Markov model. Invest Ophthalmol Vis Sci 2019;60(9).
-
Tang J, Liang Y, O’Neill C, Kee F, Jiang J, Congdon N. Cost-effectiveness and cost-utility of population-based glaucoma screening in China: a decision-analytic Markov model. Lancet Glob Health 2019;7(7):e968–78.
-
John D, Parikh R. Cost-effectiveness of community screening for glaucoma in rural India: a decision analytical model. Public Health 2018;155:142–51.
-
Thomas S, Hodge W, Malvankar-Mehta M. The cost-effectiveness analysis of teleglaucoma screening device. PLOS ONE 2015;10(9):137913.
-
Blumberg DM, Vaswani R, Nong E, Al-Aswad L, Cioffi GA. A comparative effectiveness analysis of visual field outcomes after projected glaucoma screening using SD-OCT in African American communities. Invest Ophthalmol Vis Sci 2014;55(6):3491–500.
-
Burr J, Hernández R, Ramsay C, Prior M, Campbell S, Azuara-Blanco A, et al. Is it worthwhile to conduct a randomized controlled trial of glaucoma screening in the United Kingdom? J Health Serv Res Policy 2014;19(1):42–51.
-
John D, Ashton T, Nirmalan P, Parikh R. Cost effectiveness analysis of community screening for glaucoma in India. Value Health 2012;15(7):A643.
-
Guertin JR, Rhame E, Lelorier J, Kamdeu Fansi AA, Li G, Harasymowycz PJ. Cost-effectiveness of different modes of screening for open angle glaucoma in glaucoma high-risk populations. J Popul Ther Clin Pharmacol 2010;17(1):e107.
-
Hernández RA, Burr JM, Vale LD, Group O.a.G.S.P. Economic evaluation of screening for open-angle glaucoma. Int J Technol Assess Health Care 2008;24(2):203–11.
-
Burr JM, Mowatt G, Siddiqui MaR, Cook J, Lourenco T, Ramsay C, et al. The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation. Health Technol Assess 2007;11(41).
-
Vaahtoranta-Lehtonen H, Tuulonen A, Kovanen N, Malmivaara A, Aronen P, Sintonen H, et al. Cost effectiveness and cost utility of an organized screening programme for glaucoma. Acta Ophthalmol Scand 2007;85(5):508–18.
-
Hitzl W, Ortner C, Hornykewycz K, Grabner G, Reitsamer HA. Resource use and costs for a glaucoma screening program in Austria: an 8-year review: a cost-consequence analysis based on the Salzburg–Moorfields Collaborative Glaucoma Study. Eur J Ophthalmol 2006;16(1):92–9.
-
Xiao X, Xue L, Ye L, Li H, He Y. Health care cost and benefits of artificial intelligence-assisted population-based glaucoma screening for the elderly in remote areas of China: a cost-offset analysis. BMC Public Health 2021;21(1):1065.
Studies with similar methodology, data inputs and main conclusions as the included studies (2)
-
Boodhna T, Crabb DP. More frequent, more costly? Health economic modelling aspects of monitoring glaucoma patients in England. BMC Health Serv Res 2016;16(1):611.
-
Hernández R, Burr JM, Vale L, Azuara-Blanco A, Cook JA, Banister K, et al. ; Surveillance of Ocular Hypertension Study, G. Monitoring ocular hypertension, how much and how often? A cost-effectiveness perspective. Br J Ophthalmol 2016;100(9):1263–8.
Abstract (1)
-
Hernández R, Ryan M, Vale L, Burr J. Broadening the valuation space in health technology assessment: the case of monitoring individuals with ocular hypertension. Value Health 2017;20(9):A736.
Systematic review studies (2)
-
Azuara-Blanco A, Piyasena P, O’Neill C, Olawoye O, Chan VF, Crealey GE, Congdon N. A review to populate a proposed cost-effectiveness analysis of glaucoma screening in sub-Saharan Africa. Ophthalmic Epidemiol 2021.
-
Singh K, Doshi A. Cost-effective evaluation of the glaucoma suspect. Curr Opin Ophthalmol 2007;18(2):97–103.
Non-economic evaluation studies (2)
-
Lazcano-Gomez G, Ramos-Cadena MDLA, Torres-Tamayo M, Hernandez De Oteyza A, Turati-Acosta M, Jimenez-Roman J. Cost of glaucoma treatment in a developing country over a 5-year period. Medicine 2016;95(47):e5341.
-
Wittenborn JS, Zhang X, Shrestha S, Saaddine JB, Feagan CW, Crouse WL, et al. The economic burden of vision loss and eye disorders among the United States population younger than 40 years. Ophthalmology 2013;120(9):1728–35.
Poster (1)
-
Crane GJ, Karnon J, Casson R, Metcalfe A, Hiller JE, Kymes S. A discrete event simulation to optimise the allocation of constrained hospital resources for glaucoma. Value Health 2011;14(3):A55.
Non-English articles (2)
-
Hirneis C, Kernt M, Kampik A, Neubauer AS, Niedermaier A. Health-economic aspects of glaucoma screening. Ophthalmologe 2010;107(2):143–9.
-
Hirneis C, Kampik A, Neubauer AS. Value-based medicine for glaucoma. Ophthalmologe 2010;107(3):223–7.
A list of initially excluded studies and further excluded studies in full-text scoping review for economic evidence for home and (or) remote monitoring beyond glaucoma
Not systematic review of economic evaluation studies (22)
-
Chan AHY, Pleasants RA, Dhand R, Tilley SL, Schworer SA, Costello RW, Merchant R. Digital inhalers for asthma or chronic obstructive pulmonary disease: a scientific perspective. Pulm Ther 2021;7(2):345–76.
-
Eberle C, Stichling S. Effect of telemetric interventions on glycated hemoglobin A1c and management of type 2 diabetes mellitus: systematic meta-review. J Med Internet Res 2021;23(2):e23252.
-
Jang S, Kim Y, Cho WK. A systematic review and meta-analysis of telemonitoring interventions on severe COPD exacerbations. Int J Environ Res Public Health 2021;18(13):23.
-
Eurlings CGMJ, Boyne JJ, De Boer RA, Brunner-La Rocca HP. Telemedicine in heart failure – more than nice to have? Neth Heart J 2019;27(1):5–15.
-
Smith SM, Holland AE, McDonald CF. Beyond forest plots: clinical gestalt and its influence on COPD telemonitoring studies and outcomes review. BMJ Open 2019;9(12):e030779.
-
Bech B, Primdahl J, Scholte-Voshaar M, Zangi HA, Van Tubergen A, Van Eijk-Hustings Y. Eular recommendations for the role of the nurse in the management of chronic inflammatory arthritis: 2018 update. Ann Rheum Dis 2018;77(Suppl. 2):1817–8.
-
Clark RA. Telehealth in the elderly with chronic heart failure: what is the evidence? Stud Health Technol Inform 2018;246:18–23.
-
Peretz D, Arnaert A, Ponzoni NN. Determining the cost of implementing and operating a remote patient monitoring programme for the elderly with chronic conditions: a systematic review of economic evaluations. J Telemed Telecare 2018;24(1):13–21.
-
Singh N, Lazkani M, Desai S, Feringa H. Home telemonitoring to improve disease management and clinical outcomes in patients with heart failure: an updated meta analysis of randomized controlled trials. Catheter Cardiovasc Interv 2017;89(Suppl. 2):S132.
-
Hofer F, Achelrod D, Stargardt T. Cost–utility analysis of telemonitoring interventions for patients with chronic obstructive pulmonary disease (COPD) in Germany. Appl Health Econ Health Policy 2016;14(6):691–701.
-
Kotb A, Cameron C, Hsieh S, Wells G. Comparative effectiveness of different forms of telemedicine for individuals with heart failure (HF): a systematic review and network meta-analysis. PLOS ONE 2015;10(2):e0118681.
-
Koivunen M, Saranto K. Nursing professionals’ experiences of the facilitators and barriers to the use of telehealth applications: – a systematic review of qualitative evidence. JBI Database System Rev Implement Rep 2014;10(57):3894–906.
-
Pericas JM, Aibar J, Soler N, Lopez-Soto A, Sanclemente-Anso C, Bosch X. Should alternatives to conventional hospitalisation be promoted in an era of financial constraint? Eur Eur J Clin Invest 2013;43(6):602–15.
-
Ekeland AG, Bowes A, Flottorp S. Effectiveness of telemedicine: a systematic review of reviews. Int J Med Inform 2010;79(11):736–71.
-
Gaikwad R, Warren J. The role of home-based information and communications technology interventions in chronic disease management: A systematic literature review. Health Inform J 2009;15(2):122–46.
-
Inglis SC, Clark RA, Cleland JGF, McAlister F, Stewart S. Structured telephone support or telemonitoring programs for patients with chronic heart failure. Cochrane Database Syst Rev 2008;(3) (no pagination):CD007228.
-
Barlow J, Singh D, Bayer S, Curry R. A systematic review of the benefits of home telecare for frail elderly people and those with long-term conditions. J Telemed Telecare 2007;13(4):172–9.
-
Jaana M, Pare G. Home telemonitoring of patients with diabetes: a systematic assessment of observed effects. J Eval Clin Pract 2007;13(2):242–53.
-
Mair FS. Does remote monitoring improve outcome in patients with chronic heart failure? Commentary. Nat Clin Pract Cardiovasc Med 2007;4(11):588–9.
-
Pare G, Jaana M, Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. J Am Med Inform Assoc 2007;14(3):269–77.
-
Rychlik R, Rulhoff H. Socioeconomic relevance of selected treatment strategies in patients with chronic heart failure. Expert Rev Pharmacoecon Outcomes Res 2005;5(3):277–86.
-
Currell R, Urquhart C, Wainwright P, Lewis R. Telemedicine versus face to face patient care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2000;(2):CD002098.
Non-hypertension studies (2)
-
Nguyen NH, Martinez I, Atreja A, Sitapati AM, Sandborn WJ, Ohno-Machado L, Singh S. Digital health technologies for remote monitoring and management of inflammatory bowel disease: a systematic review. Am J Gastroenterol 2022;117(1):78–97.
-
Ben-Assuli O. Measuring the cost-effectiveness of using telehealth for diabetes management: A narrative review of methods and findings. Int J Med Inform 2022;163 (no pagination):104764.
Abstract (1)
-
Miller R, Fox D, Ioannou P. PUK27 The economic impact of digital health interventions in the management of type 2 diabetes: a systematic review. Value Health 2019;22(Suppl. 3):S918.
Outdated systematic review (1)
-
Polisena J, Coyle D, Coyle K, McGill S. Home telehealth for chronic disease management: a systematic review and an analysis of economic evaluations. Int J Technol Assess Health Care 2009;25(3):339–49.
Appendix 10 The role of clinical outcomes in the economic evaluation
| Study (year) | VF | IOP |
|---|---|---|
| Burr et al. (2012)57 | (1) VF (PSD): as one of the baseline characteristics shown to be predictors of conversion to OAG. Once converted, QoL will be reduced. (2) VF (MD): VF-based staging system is used to link VF with disease severity, which subsequently linked with QoL |
(1) As one of the baseline characteristics shown to be predictors of conversion to OAG. Once converted, QoL will be affected. (2) To determine the subsequent treatment received. For example, if the IOP reduction from the last treatment < 15%, the subsequent treatment plan was considered; otherwise maintain the current treatment plan. In this case, IOP affects QoL by deterring disease progression |
| van Gestel et al. (2012)59 | VF (MD) was directly linked with utility values such as VFQ-25 and HUI3 [see Equation 1 and Equation 2 in van Gestel et al. (2012)]. The coefficients of the equations were derived from a regression analysis using 537 individuals with OHT or OAG in Netherlands. The details are revealed in Van Gestel et al. (2010)83 | (1) To determine the subsequent treatment received. In this case, IOP affects QoL by deterring disease progression (2) Directly linked with progression rate (measured by MD), which is subsequently linked with QoL. The higher the IOP, the higher the rate of progression, except for IOP < 13 mmHg when progression rate equals 0 [see Equation 3 in Van Gestel et al. (2010)83 for details] |
| Crabb et al. (2014)58 | (1) VF (MD): VF-based staging system is used to link VF with disease severity, which subsequently linked with QoL (2) VF (MD): treatment effectiveness is represented by improvement in MD rate from clinical evidence. For example, argon laser trabeculoplasty was found to be equal to MD improvement of 0.74 dB/year and trabeculectomy equal to improvement of 1.22 dB/year |
Linked with progression rate (a 1 mmHg reduction in IOP translated to a 0.1 dB/year improvement in MD rate). The coefficient is obtained from Folgar et al. (2010)32 in which a regression analysis with 28 glaucoma patients was conducted to link IOP reduction due to glaucoma surgery and disease progression rate |
List of abbreviations
- ADePT
- A process for Decision-making after Pilot and feasibility Trials
- AI
- artificial intelligence
- BP
- blood pressure
- CBA
- cost–benefit analysis
- CCA
- cost–consequence analysis
- CEA
- cost-effectiveness analysis
- CMA
- cost-minimisation analysis
- COVID-19
- coronavirus disease discovered in 2019
- COAG
- chronic open-angle glaucoma
- CUA
- cost–utility analysis
- DCE
- discrete choice experiment
- DHI
- digital health intervention
- DHT
- digital home monitoring technologies
- GAT
- Goldmann applanation tonometer
- HES
- hospital eye services
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IOP
- intraocular pressure
- IQR
- interquartile range
- IT
- information technology
- I-TRAC
- In-home Tracking of glaucoma: Reliability, Acceptability, and Cost
- MD
- mean deviation
- MHRA
- Medicines and Healthcare Products Regulatory Agency
- MMDT
- Moorfields motion displacement test
- MRF
- Melbourne Rapid Field
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- NTG
- normal tension glaucoma
- OAG
- open-angle glaucoma
- OHT
- ocular hypertension
- PI
- principal investigator
- PICO
- population, intervention, comparator, outcome
- POAG
- primary open-angle glaucoma
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- RPM
- remote patient monitoring
- SD
- standard deviation
- SSC
- Study Steering Committee
- TDF
- Theoretical Domains Framework
- TFA
- Theoretical Framework of Acceptability
- UKCA
- UK Conformity Assessed
- UKEGS
- UK and Eire Glaucoma Society
- VF
- visual field
- WTP
- willingness-to-pay
Notes
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/GTWD6802).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
