Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 15/130/07. The contractual start date was in May 2017. The draft report began editorial review in June 2021 and was accepted for publication in March 2022. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
Copyright © 2023 Ridd et al. This work was produced by Ridd et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2023 Ridd et al.
Chapter 1 Introduction
Eczema
Around 20% of children in the UK have eczema (also known as atopic eczema/atopic dermatitis). 1 It is most commonly diagnosed in the first 2 years of life. 2 The majority of children have a mild or moderate disease and are diagnosed and managed exclusively in primary care. 3
The main symptoms of eczema are dry and inflamed, itchy skin. The condition can have a significant impact on the quality of a child’s life and on their family. 4 It can adversely influence the affected child’s emotional and social development5 and may lead to psychological difficulties. 6 Parents report loss of sleep and stress, and families can become socially isolated. 7 Impairment in health-related quality of life is comparable to that of many other long-term childhood conditions, including diabetes and asthma. 8
In 1995–6, the total annual UK cost of eczema in children aged ≤ 5 years was estimated to be £47M (or £79.59 per child), of which 64% was NHS costs. 9
Emollients for eczema
Treatments are usually tailored to disease severity (see Figure 1). Emollients as a ‘leave-on’ treatment are recommended for all disease severities, with topical corticosteroids (or sometimes topical calcineurin inhibitors) used alongside to treat or prevent ‘flares’. Emollients may be also used as a soap substitutes, but bath additive products do not confer any additional benefit. 10
FIGURE 1.
Stepped approach for managing atopic eczema in children (adapted from the NICE guidelines). 12 Reproduced from ‘Exacerbation of atopic eczema in children’, Ridd M and Purdy S, 339, b2997, 2009, with permission from BMJ Publishing Group Ltd. [License no 5444140896947].
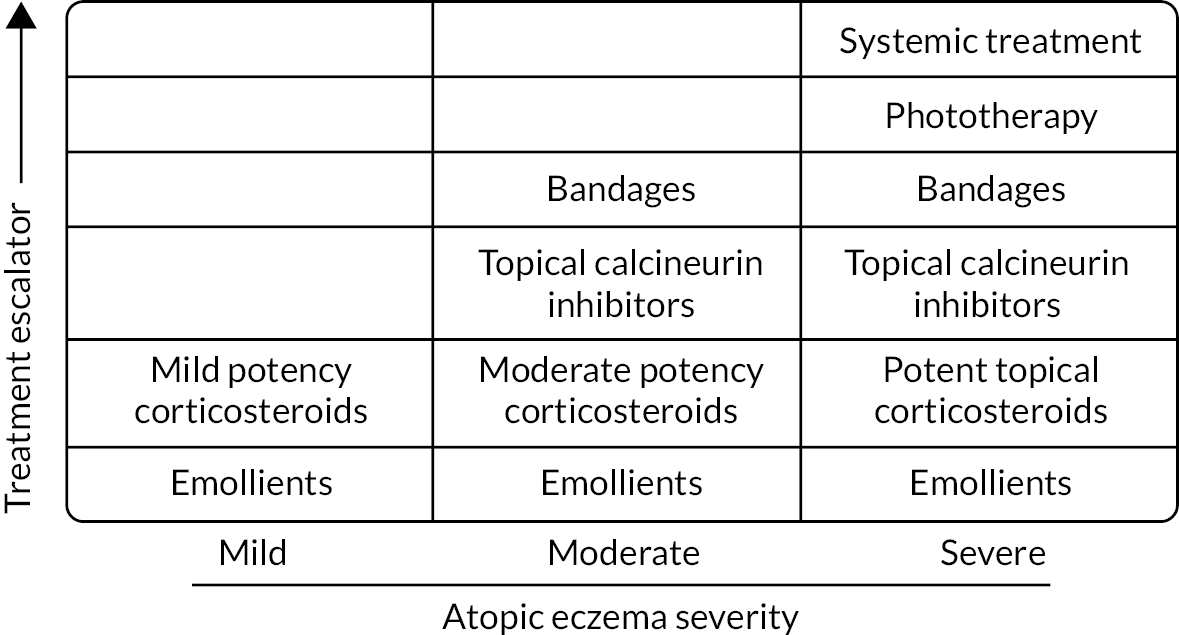
Applied directly to the skin, emollients treat symptoms by directly adding water to the dry outer layers of the skin and reducing water loss by occlusion. 11 They can also act as a barrier to irritants, especially for the hands and around the mouth, and have mild anti-inflammatory properties, thereby reducing reliance on topical corticosteroids.
Multiple emollients are available, but there is limited evidence that any one emollient is better than another. The main types are lotions, creams, gels and ointments. The differences in their consistency (from ‘light’ to ‘heavy’) mainly reflect differences in oil (lipid) to water ratios. Some products contain humectants, which may help retain moisture. Emollients that contain urea or antimicrobial compounds are usually sanctioned for more severe disease only.
There are many emollient formularies, developed by medicine management teams across England and Wales,13 which vary widely in their recommendations. A simplistic approach, prescribing emollients on a cheapest ‘per gram or millilitre’ basis,14 is wrong for two reasons. First, it assumes that all products are equally acceptable and effective. Second, it ignores wider health-care costs associated with repeated consultations for alternative products.
Parents and carers often end up trying many different emollients, seeking one that works for them. 15–17 This ‘trial and error’ approach takes time, causes frustration18 and can cause some families to ‘give up’ using emollients altogether, resulting in suboptimal eczema care.
Development of research priority
The National Institute for Health and Care Excellence (NICE) (2007) and a James Lind Alliance research priority-setting partnership for eczema (2013) have both recommended research comparing the clinical effectiveness and cost-effectiveness, and safety, of emollients in eczema. 19 van Zuuren et al. ,20 in a Cochrane review of randomised controlled trials of emollients and moisturisers for eczema, identified 77 studies, 70 of which were at an unclear or a high risk of bias. Reporting of adverse events was limited and only 13 studies assessed participant satisfaction with treatment. The authors were unable to conclude whether some emollients, or their ingredients, were better than others.
Aims and objectives
Pragmatic randomised trial
The aim of the trial was to compare the effectiveness and acceptability of four commonly used types of emollients for the treatment of childhood eczema.
The objectives of the trial were to compare the four types of emollients at both 16 weeks (medium term) and 52 weeks (long term) in respect of:
-
parent-reported eczema symptoms
-
objective assessment of eczema signs
-
quality of life for the child
-
impact of eczema on the family
-
adverse effects
-
parent satisfaction with study emollient
-
frequency and quantity of study emollient and other emollient use
-
use of topical corticosteroids
-
number of well-controlled weeks.
Nested qualitative study
The aim of the nested qualitative study was to complement, explain and aid understanding of the quantitative findings regarding the delivery/receipt of the intervention, its acceptability and perceived or experienced benefits or harms.
The objectives of the nested qualitative study were to:
-
explore facilitators of or barriers to use and follow up with participants who stopped treatment early
-
explore carers’ and children’s experiences of study emollient use and their views about perceived effectiveness and/or acceptability of study emollients
-
contextualise the trial findings, as an aid to interpreting the results and their potential impact on clinical practice.
Chapter 2 Methods
Trial design
The Best Emollients for Eczema (BEE) study was a multicentre, pragmatic, parallel-group superiority trial of four types of emollient in children with eczema, with a nested qualitative study. 21,22
It was a type A clinical trial of an investigational medicinal product because some of the study emollients were classed as medicines (others were classed as medical devices or cosmetic products). It was classed as low risk because the use of the medicinal product was not higher than the risk of standard medical care.
Changes to trial protocol
The final study protocol (version 7.0, 19 November 2019) is available via the study website (www.bristol.ac.uk/bee-study; accessed 10 September 2022). Amendments are listed in Appendix 1.
The trial design was based on the feasibility trial Choice of Moisturiser in Eczema Treatment (COMET). 16 Like this study, in the original approved BEE protocol (version 1.0, 21 March 2017) participants were to be randomised to four specific named emollients [Aveeno® (Johnson & Johnson, Brunswick, NJ, USA) lotion, Diprobase® (Bayer, Reading, UK) cream, Doublebase™ (Dermal Laboratories Limited, Hitchin, UK) gel or Epaderm® (Mölnlycke Health Care Limited, Oldham, UK) ointment]. However, when participant recruitment was due to start, we submitted a substantial amendment (version 4.0, 3 November 2017) that changed the intervention to four types of emollient, with a list of approved emollients for each category. This was because between applying for funding and trial authorisation, several integrated care boards (ICBs) in the recruiting areas implemented significant changes to their local formularies that did not include the original proposed specific emollients; because the trial intervention was prescribed by participants’ general practitioners (GPs), there was the risk that participants would not receive their allocated emollient.
Protocol amendments to change the duration of the recruitment period of the study were made twice: first to extend the participant recruitment period by 6 months to 26 months (version 6.0, 10 June 2019), and second (after an increase in recruitment rate) to reduce the participant recruitment period back to 22 months total (version 7.0, 19 November 2019).
Other changes to the protocol during the study have been minor, involving clarifications or minor corrections.
Terminology and definitions
Unless otherwise stated, we use the following terms in the ways described.
For the sake of brevity, we use the term parent to include the child’s main carer/legal guardian. In the trial, participants are the children, whereas in the qualitative study, this term could refer to both parents and children. We refer to trial groups (rather than arms), to avoid potential for confusion between trial arms and participants’ upper limbs. Centres are the regional hubs (Bristol, Nottingham – including Lincolnshire and Southampton) through which GP surgeries and participants were recruited, with GP surgeries being the participant identification centres. We prefer the term masking to blinding, although this term is used for the Bang blinding index.
The terms emollient and moisturiser are commonly used interchangeably, but, as explained in Chapter 3, we consistently used moisturiser for patient-facing materials. In lay speak, as captured in the qualitative findings, ‘cream’ is commonly used in a generic sense to describe all the different types of emollients, but in the report we use ‘type’ to refer to lotions, creams, gels and ointments, or their actual group label, as appropriate.
Regarding the use of allocated and non-allocated study emollients, in our protocol and statistical analysis plan we refer to adherence and contamination. We have retained the same definitions, as below, but for clarity refer throughout this report to allocated and non-allocated study emollient use:
-
Allocated emollient use (or ‘adherence’) was defined as the number of days of a non-missing week of emollient use data in which the emollient used was the allocated emollient type.
-
Non-allocated emollient use (or ‘contamination’) was defined as the number of days of a non-missing week of emollient use data in which an emollient other than the allocated emollient type was used.
Participant recruitment and follow-up
Participants were recruited between 19 January 2018 and 31 October 2019.
Identification of potentially eligible children
Participant recruitment was via GP surgeries in the three clinical research network (CRN) areas of West of England (Bristol), Wessex (Southampton) and East Midlands (Nottingham and Lincoln).
General practitioner surgeries
General practitioner surgeries were recruited via CRNs, direct approaches from the study team and promotion at meetings/events. Practices that submitted an expression of interest form had a study set-up meeting, where the study was explained in full and practices were to sign a contract if they were happy to proceed. Research leads at participating surgeries were required to have good clinical practice (GCP) certification.
Participant screening
Practices were sent a specific electronic search that identified all children registered at the practice aged between 6 months and 12 years, who had an eczema diagnosis and had been prescribed an eczema treatment in the past 12 months. They then screened the list, removing any participants who did not fit the inclusion/criteria or should not be approached for social or medical reasons. An anonymised copy (with reasons for exclusion) was submitted to the research team, and invitation letters were sent out to potentially eligible children via DocMail, a NHS-approved mail-out service.
Participant eligibility criteria
To be eligible, children and the responsible adult had to meet the following inclusion criteria:
-
Child
-
aged between 6 months and 12 years
-
have eczema diagnosed by an appropriately qualified health-care professional (registered doctor, nurse or health visitor)
-
mild eczema or worse (POEM score of > 2 points within previous 28 days). At the point of randomisation, at least two POEM scores were available for all participants – one recorded at the time of expression of interest and one recorded at baseline. The expression of interest POEM score was used to determine eligibility and for randomisation; a further POEM score was recorded if the baseline visit was > 28 days later.
-
-
The person giving consent must
-
have parental responsibility for the participant
-
be willing to use the randomly allocated emollient type as the only leave-on emollient for 16 weeks.
-
Exclusion criteria for the child and the responsible adult were as follows:
-
Child
-
known sensitivity to study emollients or their constituents
-
participating in another research study currently or in the last 4 months
-
any other known adverse medical or social circumstance that would make invitation to the study inappropriate (as determined by GP practice staff).
-
-
The person giving consent
-
was unable to give informed consent
-
had insufficient written English to complete outcome measures.
-
Participant recruitment
Mail-out invitation
Invitation packs included (see Report Supplementary Materials 1 and 2):
-
a covering letter
-
a two-page summary, which gave an overview of the study and outlined how to find more information
-
a ‘reply to invitation form’, asking those not interested to give a reason why, and, for interested families, their contact details and POEM questionnaire.
Parents could either complete and return the response form using the enclosed pre-paid envelope or complete an identical online version.
Self-referral
Participating GP surgeries were asked to display posters and put promotional flyers in their waiting rooms, and the study was promoted at various events. If the child was registered at a participating GP surgery, parents could contact the team directly to register their interest via the online form.
Screening telephone call
Interested parents of potentially eligible children were sent participant information sheets (adult and child versions; see Report Supplementary Materials 3 and 4) by e-mail or post and were telephoned by the researcher. During the telephone call, the researcher outlined what taking part would involve and answered any initial questions. If the parent was happy to proceed, a baseline study visit was arranged.
If the visit was > 28 days after the expression of interest, POEM was repeated over the telephone in advance of the visit, either during the main screening telephone call or by means of a further telephone call prior to the visit.
Baseline study visit
Baseline visits took place in the participant’s home, or in a suitable room at the GP practice. Visits lasted approximately 1 hour.
A sample of baseline visits were recorded to help with training and to inform recruitment strategy. With multiple emollients of each type and no control group, this was a complex trial to recruit to. Equipoise was important to establish to minimise the contamination (use of non-allocated emollients) and to maximise participant retention. At these visits, parent were asked to give verbal permission for audio-recording, followed up by written consent at the end of the encounter. We sought at least one recording for each recruiting researcher. The recordings were listened to by the qualitative Senior Research Associate. Any issues identified in the recruitment process were fed back to researchers to improve communication between researchers and participants.
At the baseline visit, the researcher summarised the study and shared a sheet listing which study-approved emollients were available of each type in their local area. They explained what taking part would involve, and demonstrated how the weekly/monthly questionnaires should be completed. They checked that the parent had read and understood the participant information leaflet (and, when relevant, the child their equivalent), and questions were invited and answered. Parents and children were reminded that study researchers were masked and, in any future contact with them, they must not disclose which emollient they were using.
If the parent was happy to continue, written informed consent was taken, with the option for older child to complete an assent form (see Report Supplementary Materials 5 and 6).
Participant follow-up
Parents were telephoned by an unmasked member of the research team with their allocation after randomisation, and told to collect their corresponding prescription from their GP surgery. This was followed up with another telephone call 7 days later to confirm that they had received the study emollient and started using it.
Thereafter, with the exception of the week 16 visit, follow-up was remote, by means of parent-completed questionnaires (see Chapter 2, Data collection, Follow-up questionnaires).
The final week 16 follow-up visit was on 17 February 2020. The trial finished after the last participant’s week 52 follow-up questionnaire was returned.
Participant withdrawals
Participants were free to withdraw at any time, without any consequences for their usual care or follow-up. We analysed any data already collected and obtained their electronic medical record (EMR) data, unless the participant expressly withdrew their consent prior to the database being locked. Participants who actively withdrew were asked to give reason(s).
Participant engagement
Parents were thanked for their time in the study with a £10 voucher at the baseline visit and a £10 voucher on completion of the week 16 visit, and a final £10 voucher was sent on completion of the week 52 survey.
At the baseline visit, children were offered a small thank you of a bee soft toy or a bouncy ball. In addition, children were given an A4 bee colouring template to colour in/decorate while the researcher talked to their parent. Once complete, families were encouraged to share pictures with the BEE research team. Submitted colourings were shared via the study website and Twitter (Twitter, Inc., San Francisco, CA, USA; www.twitter.com), and used in dissemination of the study findings, for example in presentations. At the week 16 visit, children were given a certificate to congratulate them on their participation in the study.
Newsletters were sent out to families via e-mail approximately three times per year and e-cards were sent at Christmas. Newsletters contained information on recruitment, reminders on how to complete the questionnaires, seasonal information relating to eczema, and profiles of research team members.
Parents were encouraged to follow the study Twitter account, which charted progress and shared children’s coloured-in bees. Finally, a study website was maintained, containing study information and links to relevant documents.
Intervention
Participants were randomised to receive a study-approved emollient of one of the following types: lotion, cream, gel or ointment. Which study-approved emollients were available for GPs to prescribe varied by ICB, but all the options are listed in Table 1. Study-approved emollients were all paraffin based and member types shared the characteristics as detailed in the table. Although emollients were allowed to ‘join or leave’ the study as eligible emollients were introduced or removed from local formularies, the list presented remained stable over the life of the trial.
| Treatment | Emollient type | |||
|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | |
| Study emollients | Cetraben® (Thornton & Ross Ltd, Linthwaite, UK) | AproDerm® (Fontus Health Ltd, Walsall, UK) | AproDerm (Fontus Health Ltd, Walsall, UK) | Diprobase (Bayer UK Ltd, Reading, UK) |
| Diprobase (Bayer UK Ltd, Reading, UK) | Aquamax® (Intrapharm Laboratories, Maidenhead, UK) | Doublebase (Diomed Developments, Hitchin, UK) | Emulsifying ointment BP | |
| QV™ (QC Skincare, Melbourne, VIC, Australia) | Diprobase (Bayer UK Ltd, Reading, UK) | Epimax Isomol gel (Aspire Pharma, Petersfield, UK) | Paraffin White soft | |
| Epimax (Aspire Pharma, Petersfield, UK) | MyriBase™ (Penlan Healthcare, Weybridge, UK) | Paraffin Yellow soft | ||
| Zerobase® (Zeroderma®, Thornton & Ross Ltd, Linthwaite, UK) | Zerodouble® (Zeroderma®; Thornton & Ross Ltd, Linthwaite, UK) | White soft/Liquid paraffin 50/50 | ||
General practitioners were instructed to issue the prescription with the directions to apply twice per day and as required, and parents were asked to agree to use the study emollient as their child’s only leave-on emollient for 16 weeks.
Outcomes
Primary outcome
The primary outcome was the Patient Orientated Eczema Measure (POEM) score, measured weekly for 16 weeks. POEM scores were calculated by summing the seven questions, with a possible range of 0–28 points.
The POEM is a patient-reported outcome measure that can be completed by proxy (carer report) and captures symptoms of importance to parents and patients over the previous week. 23 It demonstrates good validity, repeatability and responsiveness to change,24,25 and was favoured as the main outcome by patient contributors.
Secondary outcomes
The following secondary outcomes (time period) were collected and/or calculated:
-
Eczema symptoms (POEM), once every 4 weeks for 52 weeks.
-
Eczema signs [Eczema Area Severity Index (EASI)] at 16 weeks. The total EASI score is a weighted sum of the four EASI scores for each body area (head and neck, upper limbs, trunk and lower limbs). The weights allocated to each body area differ according to the age of the child (≤ 7 or ≥ 8 years). The final EASI scores range between 0 and 72.
-
Masking of researcher at the week 16 visit, using the Bang blinding index. 26
-
Use of study emollient/topical corticosteroids.
-
Participant quality of life [disease-specific Atopic Dermatitis Quality of Life (ADQoL)27 and generic Child Health Utility 9-Dimension (CHU-9D)28,29 scores], coded according to the developer’s instructions. The CHU-9D is validated for children aged ≥ 7 years. The pilot versions for younger children were used with additional guidance notes.
-
Impact of the participant’s eczema on the family [Dermatitis Family Impact (DFI) questionnaire]. 30 The DFI was obtained by summing responses to the 10 items to give a score of 0–30 points.
-
Satisfaction with study emollient.
-
Adverse events – localised skin reactions, slips and falls, and unplanned hospital admissions.
-
Number and quantity of study emollients and other emollients used.
-
Proportion of well-controlled weeks between weeks 1 and 16.
All the above were collected by parent-completed questionnaires, except b, which was assessed by a masked researcher; c, which was obtained by researcher-completed questionnaire at the week 16 visit; i, which was obtained from participants’ EMRs; and j, which was derived from POEM scores, in which each week was classified as well-controlled (POEM score ≤ 2) or not (POEM score > 2), with the proportion of weeks with well-controlled symptoms calculated as the number of well-controlled weeks divided by the number of weeks with non-missing POEM scores.
The format of questions for d was changed in month 12 of recruitment from a numbered (day 1, day 2, etc.) to a named day (Monday, Tuesday, etc.), to improve ease of completion (see Chapter 3, Parent and public involvement during the study).
Items in the final questionnaire asked about trial participation, specifically about following directions on study emollient use.
With a view to carrying out economic analyses in the future, we also asked questions about personal costs related to eczema and health-care consultations (4-weekly).
Data collection
Table 2 sets out what data were collected and when. Parents were given the option to complete questionnaires after the baseline visit online (default) or on paper when this was preferred.
| Data collection | S | V0 | Participant questionnaires | V1 | Participant questionnaires | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Week | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 16 | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 | EMR | |
| Eligibility checks | • | ||||||||||||||||||||||||||||
| Demographics (and consent) | • | ||||||||||||||||||||||||||||
| UK diagnostic criteria for atopic dermatitis | • | ||||||||||||||||||||||||||||
| Opinion about study emollients | • | ||||||||||||||||||||||||||||
| POEM | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||
| Use of treatments for eczema | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | |||
| Adverse events | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||
| Consultations (non-EMR) | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||||||||||||||
| Personal costs | • | • | • | • | • | • | • | • | • | • | • | • | • | ||||||||||||||||
| DFI | • | • | • | ||||||||||||||||||||||||||
| ADQoL | • | • | • | • | |||||||||||||||||||||||||
| CHU-9D | • | • | • | • | |||||||||||||||||||||||||
| Satisfaction with study emollient | • | ||||||||||||||||||||||||||||
| EASI | • | • | |||||||||||||||||||||||||||
| EMR review | • | ||||||||||||||||||||||||||||
Baseline visit
Once consent was received, the following baseline data were collected:
-
participant contact details and demographics
-
eczema history and treatment, POEM score, DFI score, parent opinions about different types of emollients, quality of life (ADQoL and CHU-9D scores)
-
skin assessment by researcher (EASI score and UK diagnostic criteria for atopic dermatitis).
Parents choosing to complete online were offered a laminated aide memoire to enable them to keep a note of their child’s daily emollient use in-between completing the surveys.
Follow-up questionnaires
Participants completed follow-up questionnaires weekly for 16 weeks, then 4-weekly until 52 weeks. Weekly questionnaires comprised POEM and questions about the use of eczema treatments (study emollient, other leave-on emollients and topical corticosteroids) and adverse events. Parents were asked to report eczema treatment use over the previous week, with the first week commencing the Monday after randomisation.
In addition to the above, the 4-weekly questionnaires asked about consultations with health-care professionals (health visitor, pharmacist, dermatologist, and dermatology nurse) and costs (out-of-pocket expenses for eczema-related purchases, private/alternative treatments, travel costs to appointments).
Quality-of-life measures (ADQoL, CHU-9D) were administered at 6, 16 and 52 weeks, and the impact of the condition on the family (DFI) was determined at 16 and 52 weeks. Participants were asked about their satisfaction with their study emollient at 16 weeks.
Parents received e-mail and/or text reminders to complete the questionnaires, with telephone calls if needed.
Follow-up visit
The follow-up visit was scheduled for 16 weeks (± 10 days) after the baseline visit. Participants’ skin was assessed, usually in their own home, by a masked researcher. To maximise data collection, from month 10 of recruitment, researchers were asked to collect POEM scores at 16 weeks as well as EASI scores. When both parent-completed and researcher-collected week 16 POEM scores were available, the scores were compared and sensitivity analysis undertaken (see Statistical methods, Primary outcome, Sensitivity analyses including imputation of missing data).
Electronic medical record review
Consultation and prescription data (from 4 weeks prior to and 52 weeks after randomisation for each participant) were extracted from participants’ EMR remotely, using reports written for SystmOne (The Phoenix Partnership, Leeds, UK) and EMIS Web (EMIS Health, Leeds, UK). After the last participant from each GP surgery had completed their week 52 follow-up questionnaire, their practice was sent the appropriate electronic search strategy, instructions and list of patients recruited into the study. It was not possible to obtain data on quantity (in grams or millilitres) for items prescribed in SystmOne practices.
Data management and validation
Each participant was assigned a trial participant identification number, which was used on questionnaires and the electronic database. All data relating to participants were stored securely in locked cabinets and/or password-protected file stores. Patient identifiers were separate from clinical data. The trial database was locked on 11 December 2020.
Data for a random sample of 10% of participant questionnaires were checked for quality purposes.
Sample size
We sought to recruit 520 participants. This was based on detecting a minimum clinically important difference in POEM scores of 3.0 points between any two treatment groups with 90% power and a significance level of 0.05 (after adjustment for multiple pairwise comparisons). We based our sample size on a standard deviation (SD) of 5.5 and allowed for 20% loss to follow-up.
Participant randomisation, notification and receipt of allocation
Participants were randomised using a validated web-based randomisation system supplied by the Bristol Randomised Trials Collaboration. Using computerised randomly generated numbers, participants were randomised in a 1 : 1 : 1 : 1 ratio to one of the four groups, stratified by centre and minimised by expression of interest POEM score [3–7 points (mild) vs. 17–28 points (severe to very severe)] and participant age (aged < 2 years vs. > 2 years).
Unmasked members of the Bristol-based research team undertook randomisation, notified the parent and asked the GP surgery to prescribe accordingly. Consequently, there was a delay between the baseline visit, randomisation, and the participant starting their study emollient. The study-approved emollient(s) of each type varied among different ICBs, and emollients were issued by participants’ local pharmacist.
Masking
Participants, their parents and clinicians involved in their care were not masked to allocation. They were asked not to disclose their allocation to masked members of the research team.
The trial management group (TMG) were masked to allocation. The trial manager/co-ordinator and administrator were unmasked to undertake randomisation process above, and the qualitative research team (ES, JB and Alison Heawood) were unmasked for the purposes of interview data collection and analysis. The trial statistician was masked until the first version of the statistical analysis plan was approved. They were unmasked after this point, to allow for reporting of contamination so they could discuss unmasked data with the Data Monitoring Committee (DMC) as needed.
Researchers undertaking the objective skin assessments were masked. Masking at the 16-week EASI assessment was assessed by means of a self-complete question, which asked ‘[w]hich moisturiser type do you think the child is using?’ (lotion, cream, gel, ointment or ‘don’t know’). The Bang blinding index26 was estimated for each treatment group comparing correct treatment responses with incorrect or ‘don’t know’ responses. Indices can range from –1 (opposite guesses potentially caused by unmasking) to 1 (complete lack of masking). An index of 0 indicates perfect blinding.
Statistical methods
The analysis and presentation of trial data were in accordance with Consolidated Standards of Reporting Trials guidelines. 31,32 Stata® (StataCorp LP, College Station, TX, USA) version 16 was used for all statistical analyses. A full statistical analysis plan was developed and approved by the independent statistician from the study’s Trial Steering Committee (TSC) ahead of analysis of post-randomisation data and is available via the study website.
Characteristics of non-study patients
We compared the age and sex of study participants with non-study patients using means and SDs (age) and frequencies and proportions (sex). The purpose of this was to investigate whether or not the study participants differed from those who were excluded at earlier stages of the recruitment and screening process.
Baseline characteristics
Baseline sociodemographic and clinical characteristics of study participants were described by treatment group. Any imbalances informed additional adjustment of the primary analyses as appropriate. Continuous variables were summarised using the mean and SD [or median and interquartile range (IQR) if the distribution was skewed] and categorical data were summarised as frequencies and proportions.
Primary outcome
Primary statistical analyses between the randomised groups were conducted on an intention-to-treat basis, defined as analysing participants as randomised, regardless of the adherence to their allocated group and without imputation for missing data. For the primary outcome, linear mixed models (weekly observations, level 1; nested within participants, level 2) were used to explore whether or not there were differences in mean POEM scores between treatment groups after adjusting for baseline scores and all stratification and minimisation variables used in the randomisation.
This approach allowed incomplete cases (i.e. participants who did not complete all of their weekly scores) to contribute to the analysis. Therefore, all participants who contributed at least two POEM scores (one baseline and another between weeks 1 and 16) were included in the primary outcome analysis.
Pairwise comparisons were conducted to identify which intervention groups differed and were presented as mean differences with 95% confidence intervals (CIs) and p-values. To account for multiple testing, we used a modified alpha of 0.0083 (0.05/6 pairwise comparisons equivalent) as a threshold when interpreting p-values.
Sensitivity analyses including imputation of missing data
To assess the robustness of the primary analysis to model selection and data collection, the following sensitivity analyses for the primary outcome were carried out:
-
adjustments for sex imbalance at baseline, because the sex of participants differed by more than 10% between two groups (cream and gel), as per our statistical analysis plan
-
16-week POEM researcher-collected rather than by parent self-report
-
imputation for missing data, using ‘best’ and ‘worst’ case scenarios, and multiple imputation by chained equations (MICE).
For the ‘best’ case scenario, missing POEM scores were replaced by the mean –1 SD for that treatment group. For the ‘worst’ case scenario, missing scores were replaced by the mean +1 SD for that treatment group.
Subgroup analyses
Four prespecified subgroup analyses investigated whether or not treatment effectiveness (POEM) was modified by the below factors measured at randomisation. These were carried out by introducing appropriate interaction terms in the regression models, and likelihood ratio tests were used to compare the model with the interaction term with the model without the interaction term:
-
Parent expectation – parents were asked to score on a scale of 1 (‘very poor’) to 5 (‘very good’) or ‘don’t know’ their thoughts on how effective they thought different moisturisers were for treating the dry skin of eczema. For the purpose of subgroup analysis, the variable was classified as ‘poor’ (score of 1 or 2), ‘average or unsure’ (score of 3 or ‘don’t know’) or ‘good’ (score of 4 or 5). Analysis was based on an individual’s pre-randomisation expectations of effectiveness of the emollient to which they were later allocated.
-
Age – younger (< 2 years) versus older patients (≥ 2 years).
-
Disease severity – mild eczema (POEM score of 3–7 points) versus moderate/severe eczema (POEM score of ≥ 8 points).
-
Diagnosis of eczema – children who did and did not fulfil the UK diagnostic criteria for atopic dermatitis.
Per-protocol analysis
In the statistical analysis plan, we wrote that in the event of ‘substantial contamination’ (undefined), we would undertake a per-protocol analysis (also not specified). Contamination (or use of non-allocated emollient) during the primary outcome period was low, but on the basis of variable allocated emollient use (adherence), we repeated the primary analysis, restricted to participants who had reported using their allocated emollient at least 1 week in every 4 (i.e. in weeks 1–4, 5–8, 9–12 and 13–16) and for at least 60% of days in a reported week.
Secondary outcomes
Analyses of secondary outcomes were conducted on an intention-to-treat basis, according to the data type and frequency of recording. Continuous outcomes measured at multiple time points (POEM, ADQoL, DFI and CHU-9D scores) were analysed similarly to that described in Primary outcome above.
The EASI scores measured at baseline and 16 weeks were analysed using a linear regression model, adjusting for baseline values when available. A sensitivity analysis for the collection of EASI scores at baseline and 16 weeks by the same and different researchers was also undertaken.
The EASI and DFI scores at follow-up were found to be highly skewed and contained values of 0; therefore, scores were transformed by taking the natural log of the score plus 1. The results of these analyses are presented as the ratio of the geometric means of the two groups being compared.
Patterns of the use eczema treatments over the primary outcome period (weeks 1–16) are summarised using descriptive statistics. The number of days in a week that participants used their allocated emollient was analysed using a mixed-effects Poisson regression model adjusting for all stratification and minimisation variables. A mixed-effects negative binomial model was used for the number of days in a week that participants used a non-study emollient.
Parental satisfaction with the study emollient at 16 weeks was analysed using an ordered logistic regression model adjusting for all stratification and minimisation variables.
The proportion of weeks with well controlled symptoms was analysed using a linear regression model adjusting for stratification and minimisation variables.
Impact of COVID-19
On 11 March 2020, the UK Government issued public health guidance recommending that residents wash hands with soap and water, or use hand sanitiser to protect against COVID-19 transmission. 33 A concern was raised by the TMG that increased hand washing and use of sanitiser gels might worsen eczema symptoms and reduce the effectiveness of the intervention.
To explore the possible impact this guidance had on symptoms, a sensitivity analysis was conducted on the repeated-measures POEM scores, before and after the handwashing advice was issued. A binary variable classifying the follow-up period as pre- or post-COVID-19 handwashing advice was generated. A linear mixed model [weekly observations (level 1) nested within participants (level 2)] including a COVID group interaction term was used to explore whether or not the differences in mean POEM scores between treatment groups differed between the periods before and after handwashing advice. The model also adjusted for baseline POEM scores and variables used in the randomisation.
Analysis of safety
Descriptive analysis of safety end points are presented by allocation. The number of events and the number of participants having at least one are tabulated.
Nested qualitative study
Design
We conducted semistructured interviews with parents of participants from each trial group at 2–4 weeks (hereafter termed week 4 interviews) and 16 weeks after randomisation. The week 4 interviews aimed to capture initial experiences with, and opinions of, the study emollient, along with any early deviations from allocation and the reasons for change. The week 16 interviews aimed to capture the longer-term experiences of using the allocated emollients or reasons for stopping or changing, along with parents’ future intentions around emollient use.
Recruitment and consent of parents/assent of children for interviews
All parents received brief information about the qualitative interview study at the time of recruitment into the trial and were asked for consent to be contacted for interview. Following the sampling framework, potential interviewees were invited to take part by means of an e-mailed invitation letter with accompanying qualitative study information sheet (see Report Supplementary Materials 7 and 8).
Informed written consent was received from the parent (see Report Supplementary Material 9). At the discretion of the parent and the preference of their child, children (usually aged ≥ 7 years) using the emollient were invited to participate in the interview and, if they agreed, written assent (see Report Supplementary Material 6) was obtained from the child.
Recruitment stopped when there was agreement that inductive thematic saturation had been reached for the main themes. 34,35
Sampling
Our original design was cross-sectional, in that we anticipated sampling different participants at the week 4 and week 16 time points. However, we revisited a small number of participants from week 4 at week 16. Their data were analysed, as with all the data, cross-sectionally. We sampled from the characteristics of the participants but primarily interviewed parents. At the preference and with the assent of parents/carers and their children, children also sometimes participated in the interviews.
At week 4, we sampled parents across the four trial groups, with variation according to recruiting centre (Bristol, Southampton and Nottingham), age of child, eczema severity (mild, moderate or severe, determined by categorised baseline POEM score)36 and length of use of study emollient, to include some who had stopped using their allocated treatment (determined from patient contact and information from Clinical Studies Officer/Research Nurses). As secondary sampling criteria we included parents/carers whose children represented a range of different ethnicities to capture the experiences of using emollients for different skin types.
The week 16 interviews were conducted from each group of the trial as soon as possible after participants’ primary outcome had been collected or their week 16 visit conducted by the Clinical Studies Officer, whichever was later. Sampling criteria were the same as the week 4 interviews, with the addition of participant intentions regarding future use of the allocated emollient (e.g. intending to continue, stop or switch to another type of emollient, or already stopped).
Qualitative data collection
Data were collected by an experienced qualitative researcher based in Bristol (ESu) using semi-structured face-to-face or telephone interviews. For the week 4 interviews, participants in the Bristol area were interviewed face to face in their home on all but one occasion. Interviews with participants from the other research sites and the repeat interviews were conducted via telephone.
The interview process followed an agreed study protocol using NHS Research Ethics Committee approved topic guides. The topic guides (see Appendix 3, Topic guide) were informed by the qualitative study aims, the feasibility study16 and the wider literature. They were used flexibly to allow unanticipated issues to emerge and to incorporate new topics that were developing from ongoing analysis. For example, following discussion between the qualitative research team and the chief investigator at analysis meetings, and also as a result of input from the study patient and public involvement (PPI) group, some additional prompts were added during the week 4 interviews. We used a range of prompts building on the topics covered with parents/carers, to enable the child to contribute their views and experience.
The week 4 interviews focused on the initial acceptability of the assigned emollient to families; their prior experiences and beliefs about emollient use; their experiences of early use of the assigned emollient; whether, and how, their use of emollient was consistent with, or differed from, recommended use and anticipated adherence to the emollient during the trial. We elicited barriers to and facilitators of use, particularly examining these with participants who stopped using the emollient early or switched to another type of emollient.
At the week 16 interviews, participants reflected on their emollient use over the full trial period to that date. The topics covered were similar to the week 4 interviews, with additional prompts relating to their overall experience, acceptability and effectiveness of the assigned emollient, and planned future use of emollients. These interviews were also informed by themes emerging from the week 4 interviews.
When parents and children were interviewed together, telephone interviews were conducted via speakerphone. A subtopic guide was created (see Appendix 3, Topic guide), adapting questions to enable children to participate.
All interviews were audio-recorded using an encrypted digital recorder. The qualitative researcher (ESu) met the senior qualitative co-applicants (ARGS, JB) on a fortnightly basis to share progress and discuss issues arising in interviews regarding process and content.
Qualitative data analysis
The interviews were transcribed verbatim by a University of Bristol (Bristol, UK) member of staff and fully anonymised by Eileen Sutton. Coding and data management were aided by NVivo12 software (QSR International, Warrington, UK).
Analysis took place alongside data collection in an iterative process, to allow insights from earlier interviews to shape future interviews. 37 Analysis was led by the qualitative researcher (ESu) with detailed input from the senior qualitative researchers (ARGS, JB), who read and independently conducted preliminary coding on a subset of transcripts from both time points (week 4 and week 16). The chief investigator (MJR) also contributed, but only had sight of redacted transcripts and data excerpts to preserve masking.
Using a thematic approach drawing on Braun and Clarke,34 analysis incorporated a combination of deductive (based on the qualitative study aim) and inductive (data-driven) coding strategies, from which a preliminary coding framework was developed (see Appendix 3, Coding framework). The coding framework was repeatedly refined through discussion within the qualitative team and incorporated feedback from the PPI panel, the TMG and the TSC. The codes used for the week 4 and week 16 interviews were broadly the same, with the addition of some codes for the week 16 data. Eileen Sutton flexibly applied the coding framework to the whole data set, with Alison R G Shaw, Jonathan Banks and Matthew J Ridd checking a subset of the coding for coherence and completeness. Alison R G Shaw, Jonathan Banks and Matthew J Ridd read and independently coded a subset of interview transcripts.
Following coding of the whole data set, the coded data were examined and compared both across and within trial groups. Themes and subthemes were identified and refined through continual comparison of data elements with each other in an iterative manner. A narrative summary of the findings from the interviews was produced (by ESu, JB and ARGS), attending to areas of divergence and convergence in the data sets and the different perspectives represented.
Chapter 3 Patient and public involvement
This chapter reports on how the involvement of patients and the public shaped the design, delivery and interpretation of the study. Its format observes the GRIPP2 recommendations for reporting. 38
Public involvement in this study can be traced back to 2012 when ‘which emollients are the most effective and safe in treating eczema?’ emerged as one of the top four uncertainties in the patient-led James Lind Alliance eczema research priority-setting exercise. 19 We adopted the National Institute for Health and Care Research (NIHR) definition of PPI in our trial, as being ‘research being carried out “with” or “by” members of the public rather than “to”, “about” or “for” them’39 (© NIHR 2022, contains public sector information licensed under the Open Government Licence v3.0).
Aim and objectives
The overall aim was to involve parents of children with eczema throughout the study to help us deliver a study in a topic that is not easy to research and generate answers that were meaningful to patients. Specifically, the objectives were to:
-
ensure that all aspects of trial design were relevant and suitable for recruitment and retention of the target population of children with eczema and their parents
-
ensure that the patient perspective was included in the management and oversight of the trial
-
plan effectively for the dissemination of results such that key messages would make sense to parents in the light of the daily challenges of managing childhood eczema
-
provide GPs and commissioners with data that were most relevant and useful in guiding treatment decisions that would be in the best interests of patients.
Although the clinicians on the research team had extensive experience and expertise in the treatment of children with eczema, we were keen to avoid professional ‘blind spots’ or unintended consequences.
Method
To achieve our aim and objectives, we worked to create and maintain mutual understanding and trust between the researchers and the lay contributors, and to ensure that there were opportunities for parent involvement throughout the research process.
Parent and public involvement before the study
The design of the study to answer the James Lind Alliance question was informed by face-to-face discussions and a ‘tweet chat’ with parents of children with eczema who supported the COMET study, the feasibility trial that preceded this study. We also undertook an online survey of 176 parents/carers of children with eczema, which we have previously reported. 17
Parent and public involvement during the study
Co-applicant Amanda Roberts, a mother of children with eczema and who also has the condition herself, fully participated in study development, delivery and dissemination planning. She brought her experience from involvement in this capacity on previous primary care eczema trials (CREAM,40 BATHE41) by attending TMG, lay advisory group and other ad hoc meetings as appropriate.
We convened a group of parents of children with eczema who met six times over the course of the trial (in person, except for the final meeting owing to COVID-19 restrictions). We also involved members of the UK Dermatology Controlled Trials Network patient panel, mainly by e-mail but also through two online meetings towards the end of the project. Members had lived experience of different skin diseases, but were not necessarily parents of children with eczema.
The TSC included lay contributors, initially Abbi Gutierrez (2017–8) and then Sariqa Wagley (2019–21). They commented on trial progress and findings and provided oversight from a parent perspective.
The remit of different parent and public contributors was agreed at the beginning of the study, aligned with the aim and objectives above. Their collective focus was (but not restricted to) assisting with protocol development and design of all patient-facing study materials, identifying potential problems and helping to troubleshoot unanticipated difficulties, and helping with dissemination and planning pathways to meaningful impact.
Study materials
All public contributors were invited to review and comment on study materials, including the study logo design, study flyer and invitation letter, patient information sheets and consent/assent forms for parents and children, and questionnaires. They also tested draft versions of the online questionnaires.
During the internal pilot, it became evident that there were some issues with participants’ interpretation of the section of the weekly/4-weekly questionnaires that asked about the use of eczema treatments. In the first version (see Report Supplementary Material 10, completed between January 2018 and February 2019), days of the week were numbered 1–7 and parents were asked to complete it with day 1 as the day their child was randomised (‘the day you were told which treatment you had been given’). We received feedback that some parents were confusing the day of the week on which emollients were used with the number of days of emollient use, and were unsure which questions to answer if the child had changed from their allocated emollient.
Two potential versions of the adapted BEE questionnaire were shown to four volunteers from the advisory group. They were invited to work through the questions, reading and ‘thinking aloud’ as they completed the questionnaires, with prompts from the accompanying researcher (a cognitive interviewing technique known as verbal probing).
Nested qualitative study
During three of the meetings with the public advisory group over the course of the study, the qualitative researcher (ES) presented a summary of qualitative work and invited the group to comment on the qualitative study topic guide and suggest additional questions (2017); an update of work to date, including early findings on effectiveness and acceptability, and invited comments on a draft qualitative coding framework (2018); and progress on analysis of findings and invited comments on these (2019).
Pathways to impact
Prior to the results being available, we discussed various potential trial findings with the public advisory group, and how these may be interpreted by different stakeholders. This helped us to think through how some of these findings could be communicated.
Support for parent and public involvement
All parent and public activity was supported by a dedicated co-ordinator, Julie Clayton. She liaised with public contributors, facilitated meetings, and dealt with queries and administration of payments. Dr Clayton developed role descriptions and terms of reference for the public advisory group, and provided support and training opportunities (e.g. FutureLearn Research Ethics and Clinical Research in Health Care online courses).
All public contributors were sent copies of the newsletter, which was sent to study participants on an approximately 4-monthly basis.
Other public engagement activity
The lead trial team undertook three public engagement events, led by Shoba Dawson and Anna Gilbertson, supported by two public engagement grants from the NIHR Schools for Primary Care Research. These took place in different venues close to communities that were under-represented in health research and involved two University of Bristol (Bristol, UK) medical students (Jonathan Chan and Alisha Bhanot) in planning and delivering the events.
The first event was in October 2019, at Barton Hill Settlement in East Bristol, as part of ‘Fun Palaces’, a national initiative designed to support people to co-create cultural and community events across the UK and worldwide, with local communities co-developing and hosting events. It involved art and other activities designed to engage with parents and children about eczema. Co-applicant Amanda Roberts attended and shared her experiences of living with eczema, being a mother of children with eczema and being involved in research as a public contributor.
The second event was in January 2020, at St. Werburgh’s Community Centre, East/Central Bristol, in partnership with a South Asian community group. It was more structured, with short presentations and plenty of opportunities for attendees to ask questions. Community group organisers acted as interpreters throughout the event when needed.
The third event was in September 2020 during National Eczema Awareness Week, ‘hosted’ by East Bristol Children’s Centre (a group of four children’s centres); owing to COVID-19 restrictions, this event was online and so was advertised more widely.
In addition to regular tweets via the study Twitter account (@bee_study), we published a series of blogs by different team members on the study website.
Results
Parent and public involvement before the study
Discussions with parents face to face in groups and online confirmed that the question ‘Which emollients are the most effective and safe in treating eczema?’ remained highly relevant. They also supported comparing the four main types of emollient (lotion, cream, gel or ointment) for treating eczema but that a ‘no emollient’ group would not be acceptable. Parents thought that the evaluation should be driven by patient-reported outcomes. They also advocated for qualitative research to understand the trade-offs described between effectiveness and acceptability.
We discussed issues of masking with public contributors, including the influence of brand and packaging on use and the perceived effectiveness of different products. In common with participants in the feasibility trial and respondents to our public pre-grant application survey,17 public advisory group members and their children had used many different emollients. Although some disliked the ‘medicalised’ nature of the emollients and may have initially valued products that look more attractive and ‘cosmetic’, they also told us that the proof was in their use – that is, whether it helped with eczema symptoms and did not cause any harm. Attempts to mask users to the intervention would have required repackaging or overpackaging of products, and public contributors were concerned about the potential effects of on the usability and portability of the emollients. For these, and the other reasons listed in Chapter 10, Strengths and limitations, Pragmatic randomised trial, Internal validity, we decided not to try to mask participants to the emollients.
Parent and public involvement during the study
Co-applicant Amanda Roberts attended most TMG meetings and acted as a ‘critical friend’, giving sufficient challenge and support as needed over time. She also liaised with patient stakeholder groups, such as the National Eczema Society (London, UK) and the Nottingham Support Group for Carers of
Children with Eczema (Nottingham, UK). Online, she helped to promote the study through her @eczemasupport Twitter feed (with over 6500 followers).
We initially established a group local to the lead centre of four parents of children with eczema, who were variously involved in the feasibility study and in developing the BEE study application and had expressed interest in continuing involvement. We expanded membership of the group to nine, and recruited new members at later stages, as some parents were unable to continue for the full duration of the study owing to work and other commitments. For example, new contributors joined following public engagement work in 2019/2020.
General principles
Early on, we agreed with parents that we should be consistent in all patient-facing materials and refer to the study treatments as either emollients or moisturisers. Public contributors favoured the latter, as it was less medical and it explained the action of the treatment.
We were encouraged by parents’ support for the research, because ‘choice can be overwhelming’. They welcomed evidence that informed a ‘start here’ approach (especially for newly diagnosed eczema). However, from the beginning there were shared concerns that the findings should inform (not restrict) choice, and families should not be forced to ‘change to this’ if they were happy with their current emollient.
The public-facing name for the study was originally ‘Best Emollient for Eczema’, but we amended this to ‘Best Emollients for Eczema’ on contributor feedback, in recognition of the fact that no one type of emollient is likely to suit everyone. Although the BEE study acronym and logo was child-friendly and easy to remember, it was also a source of confusion for some people, who assumed that the emollients under investigation were products of bees. Consequently, we agreed that all dissemination material should refer to the Best Emollients for Eczema, without the bee logo.
Study materials
Of the two alternative versions of the treatment use questionnaire, one (see Report Supplementary Material 11) was thought by all the public contributors consulted to be superior to the other, with instructions to complete it from the first Monday following randomisation. However, there were still some potential points that could be misinterpreted in this version, such as:
-
how to complete if you were allocated an emollient already being used
-
completing the questionnaire before starting the study emollient
-
how to distinguish problems related to different emollients (if using more than one).
The public contributors felt that a clear explanation by the consenting researcher, and using a laminated aide memoire, could help to overcome these problems. An amendment was submitted to approve these changes to the questionnaire, and these were subsequently approved.
Nested qualitative study
Early feedback from public contributors on the qualitative study highlighted the need to explore issues of emollient acceptability with both parents and children whenever possible, the ways that topical corticosteroids were used in conjunction with emollients; and any variations according to ethnic or other backgrounds. Subsequent meetings prompted exploration of the time given by families to test the efficacy of emollients, the impact of study participation on emollient use and prior sources of information on emollient use.
Pathways to impact
We met consecutively with the UK Dermatology Controlled Trials Network patient panel and the local Bristol PPI group in October 2020 to consider four different hypothetical scenarios around different findings with respect to effectiveness and acceptability:
-
One type of emollient is found more effective/acceptable than others.
-
One or more type of emollient is more effective/others are more acceptable.
-
None of the emollient types differ in effectiveness/acceptability.
-
One type of emollient is less effective/acceptable than all the others.
The groups made many helpful suggestions, although it was mainly those with eczema who contributed from the patient panel. From this we generated the following list of potential messages:
-
Having information around the use of emollients is important, such as what the choices are and how long to persevere with a new emollient.
-
Findings offering choice (e.g. comparable effectiveness and acceptability between two or more types) could be empowering to families.
-
The key message of the study should be ‘where to start’ as opposed to ‘change to this’; people who are happy with their treatments do not want to be forced to change.
-
There may be trade-offs between effectiveness and acceptability according to age, severity, body site and local climate. A ‘stronger’ emollient may be better if it means less frequent application.
-
As the results may be specific to the study moisturisers and not to other lotions, creams, gels or ointments, care needs to be taken to ensure that the results are not applied to non-study moisturisers.
The findings of the trial were revealed to our public advisory group in a confidential online meeting in March 2021. Contributors welcomed the findings, because ‘as a parent you can sometimes feel very helpless when you are trying to support your child’. They felt that the study provided ‘a real opportunity to offer reassurances to parents that there was no need to be worried that what you are prescribed is the “best”’ and to communicate to parents that it is fine to continue what they have been using or ‘find something that works for you’.
Public contributors said that the qualitative findings also concurred with their own experiences. They suggested that most suitable messages to parents might be as follows:
-
‘You have a choice’ – choice is good and finding what works for you is important.
-
None of the emollients is perfect and problems are common.
-
‘Give it time’ to find out if the emollient prescribed works for you.
-
Keeping a diary of emollient use and symptoms during the first weeks of trying a new emollient may be helpful.
However, the group also raised concerns that if all emollients are as effective as each other then choice could be restricted to the cheapest. They recommended that summaries for GPs and prescribers needed to be clear, so that they can help parents to understand the implications, and also cautioned that extra attention needs to be paid to how children describe symptoms; for example, children may not be able to communicate their symptoms (e.g. ‘stinging’). The group suggested an animation and an infographic to effectively share the key findings.
Other public engagement activity
Only 10, predominantly white, families attended the first public engagement ‘Fun Palaces’ event. Discussions with attendees revealed a low awareness of the different types of emollients available or how to use them safely and effectively. After the event, we shared our experiences and lessons learned with the public advisory group and their suggestions (reducing the number of activities and adopting a more structured approach) shaped the second event. This was attended by approximately 20 people, primarily of South Asian origin, and led to new members joining the public advisory group. More detail on both the events can be found in a subsequently published paper. 42
The third, 1-hour Zoom (Zoom Video Communications, San Jose, CA, USA), event was attended by 16 people, including student nurses, mothers and grandmothers of children with eczema, and a health visitor. Eight attendees returned online evaluation forms, with an overall score of 4 out of 5, and statements that the event was either ‘useful’ or ‘very useful’. One responded that the event was ‘[v]ery well planned and executed. Was left in awe of [those] who are doing so much for children suffering eczema. The practical tips were invaluable.’
By June 2021, we had published four blogs on the study website: ‘Including the views and experiences of parents and children in a clinical trial’ [Eileen Sutton, May 2019, URL: https://capcbristol.blogs.bristol.ac.uk/2019/05/20/including-the-views-and-experiences-of-parents-and-children-in-a-clinical-trial-the-best-emollient-for-eczema-bee-study/ (accessed 10 September 2022)], ‘Finding the best moisturiser for eczema’ [Zoe Wilkins, May 2020, URL: https://capcbristol.blogs.bristol.ac.uk/2020/05/20/finding-the-best-moisturiser-for-eczema-the-impact-research-can-have-on-everyday-lives/ (accessed 10 September 2022)], ‘Why does the type of moisturiser matter to a child with eczema?’ [Sue Davis-Jones, July 2020, URL: https://capcbristol.blogs.bristol.ac.uk/2020/07/29/why-does-the-type-of-moisturiser-matter-to-a-child-with-eczema-a-research-nurses-perspective/ (accessed 10 September 2022)], and ‘A for planning, BEE for impact’ [Amanda Roberts, November 2020, URL: https://www.bristol.ac.uk/primaryhealthcare/researchthemes/bee-study/blogs/ (accessed 10 September 2022)]. Further blogs to accompany the publication of the trial results are planned.
Discussion and conclusions
We were successful in recruiting public contributors and facilitating their input throughout the study. Lay colleagues inspired and encouraged us in our interactions with them, reminding us of the confusion and frustration they had experienced in not knowing which emollient(s) to use for their children. Parent and public involvement influenced the study at all stages but was particularly important in:
-
supporting participant recruitment and data collection, by road-testing written and verbal participant information about a study with complex elements, and helping us improve the eczema treatment use questions
-
interpretation and dissemination of the results, by highlighting from the start concerns about the findings being misinterpreted or misused, to restrict rather inform emollient choice.
Reflections/critical perspective
Retaining members in the public advisory group over > 4 years was challenging, with three parents declining to continue after the first year because they had taken on new work roles or schedules, which meant that they were no longer able to attend meetings. Similarly, the study was supported by two PPI co-ordinators, prior to Dr Clayton taking on the role. Ongoing engagement work provided opportunities to invite new parent members to join, which was a positive development in helping to maintain enthusiasm for the study and broaden the experiences shared by advisory group members. The drawback, however, was that new members did not have full knowledge of the trial history, or first-hand experience of influencing the trial design or materials. We took care at all meetings to ensure that there was time to revise the purpose and aims of the trial and to make new contributors feel welcome.
Another challenge was the transition to an online meeting format during COVID-19 lockdown. This meant needing to have shorter meetings of 1–1.5 hours rather than 2-hour face-to-face meetings, to avoid ‘Zoom fatigue’. Because of the limitations of online meetings, discussions may have been less rich or dynamic than in person. However, there were definite advantages to the research and to public contributors, as we were able to easily involve people beyond the Bristol locality for no extra monetary, time or carbon cost. For example, a joint meeting with the UK Dermatology Controlled Trials Network patient advisory group based in Nottingham took place with public contributors in London and Exeter.
Consequently, future PPI is likely to take a blended approach, with some meetings face to face but also offering videoconference as an option, to enable researchers and members of the public to join who might not otherwise be able to because of time or travel constraints.
Chapter 4 Trial results
Participant recruitment
Searches of EMRs identified 12,417 potentially eligible children, of whom GPs excluded 2980 (see Figure 2). Reasons for GP exclusion are presented in Figure 2. The age and sex of potentially eligible children of those excluded by their GP were similar (see Appendix 2, Table 22).
FIGURE 2.
Participant recruitment and follow-up.
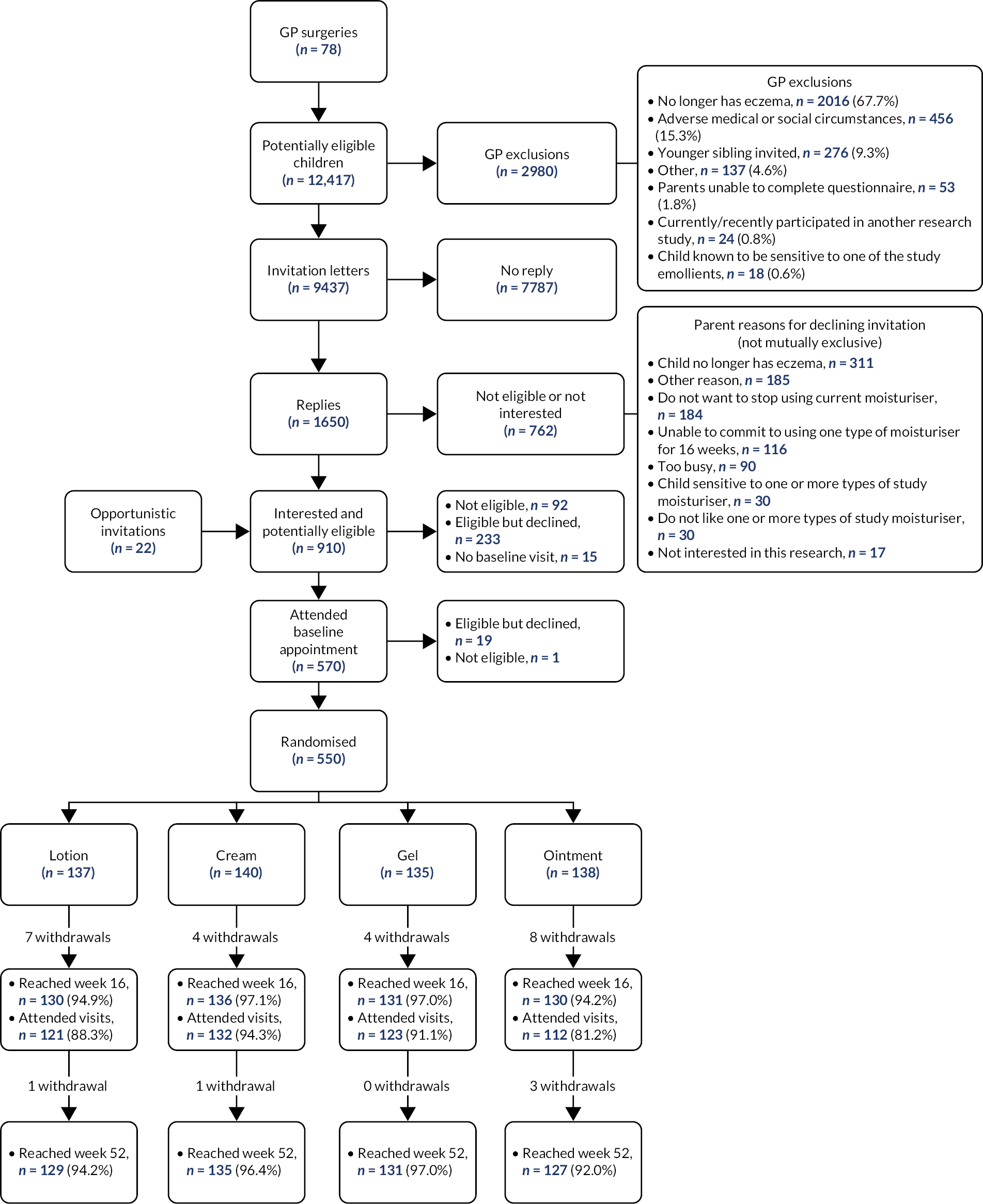
Invitation letters were sent to 9437 families from 78 GP surgeries, with 1650 replies. Of these, 762 declined to take part and 7787 did not reply. Reasons for parent non-participation are listed in Figure 2. Of those potentially eligible children identified by searches of EMRs, those who were screened by the research study were slightly younger than those who did not respond or declined (see Appendix 2, Table 23).
In total, 888 expressions of interest were received from the mail-out, with a further 22 families expressing an interest in participating via opportunistic recruitment (n = 910 in total). Of those who expressed an interest in participating, 92 were found not to be eligible, 233 were eligible yet declined to participate, 15 were eligible yet had no baseline visit booked and 570 attended a baseline visit. A further 20 either declined or were not eligible. Therefore, 550 children were randomised from 77 GP surgeries (no participants were recruited from one practice that sent invitation letters).
The 340 children excluded before the baseline visit were similar in respect of sex to those attending the baseline visit, although they tended to be slightly older (see Appendix 2, Table 24). The age and sex of children who did not attend a baseline visit or give consent were similar to those who did, but mean POEM scores were lower among those who attended but did not give consent (see Appendix 2, Table 25).
Participant characteristics
The variables on which participants were stratified (centre) and minimised (categorised age and POEM scores) were balanced at baseline (see Appendix 2, Table 26).
The characteristics of participants at baseline were balanced at baseline (see Table 3) apart from sex, as there were more girls in the cream group than in the gel group (55% vs. 40%, respectively). The median age of participants was 4 years (IQR 2–8 years), with the majority being white (86.0%). Most (81.3%) of the children met the UK diagnostic criteria for atopic dermatitis, with moderate severity eczema (mean POEM scores of 9.32 points, SD 5.46 points; see Appendix 2, Figure 8 and Table 27). Appendix 2, Table 28 presents the socioeconomic characteristics of the main carer. Almost 50% of parents were self-employed and reported ‘other’ qualifications.
| Characteristics | Treatment group | Total (N = 550) | Overall, N | |||
|---|---|---|---|---|---|---|
| Lotion (N = 137) | Cream (N = 140) | Gel (N = 135) | Ointment (N = 138) | |||
| Demographics | ||||||
| Age (years), median (IQR) | 4 (2–7) | 5 (2–8) | 4 (2–8) | 4 (2–7) | 4 (2–8) | 550 |
| Number female, n (%) | 64 (46.72) | 77 (55.00) | 54 (40.00) | 60 (43.48) | 255 (46.36) | 550 |
| Ethnic group, n (%) | ||||||
| White | 119 (86.86) | 126 (90.00) | 112 (82.96) | 116 (84.06) | 473 (86.00) | |
| African/Caribbean/Black British | 1 (0.73) | 4 (2.86) | 4 (2.96) | 9 (6.52) | 18 (3.27) | |
| Asian/Asian British | 3 (2.19) | 4 (2.86) | 7 (5.19) | 2 (1.45) | 16 (2.91) | |
| Mixed | 14 (10.22) | 6 (4.29) | 12 (8.89) | 11 (7.97) | 43 (7.82) | |
| IMD score, median (IQR) | 13.75 (7.58–20.24) | 11.74 (5.84–21.09) | 13.20 (6.39–20.63) | 11.80 (5.89–20.84) | 12.50 (6.30–20.63) | 503 |
| History of atopy, n (%) | ||||||
| Self-reported food allergy | ||||||
| No | 103 (76.30) | 109 (77.86) | 111 (82.84) | 112 (82.35) | 435 (79.82) | |
| Yes | 26 (19.26) | 20 (14.29) | 15 (11.19) | 17 (12.50) | 78 (14.3) | |
| Unsure/not diagnosed | 6 (4.44) | 11 (7.86) | 8 (5.97) | 7 (5.15) | 32 (5.87) | |
| Meet UK diagnostic criteria for atopic dermatitis | 113 (82.48) | 108 (77.14) | 109 (80.74) | 117 (84.78) | 447 (81.27) | 550 |
| Eczema severity | ||||||
| POEM score, mean (SD) | 8.67 (5.15) | 9.34 (5.25) | 9.80 (5.42) | 9.50 (5.97) | 9.32 (5.46) | 549 |
| EASI score, median (IQR) | 3.3 (2–7.2) | 3.15 (2–6.3) | 4 (2.35–8) | 3.3 (1.58–6.5) | 3.45 (1.9–6.9) | 543 |
| Quality of life, median (IQR) | ||||||
| DFI sore | 3 (1–6) | 3 (1–6) | 3 (1–8) | 2 (0–6) | 3 (1–6) | 543 |
| ADQoL | 0.36 (0.36–0.50) | 0.36 (0.36–0.50) | 0.36 (0.36–0.56) | 0.36 (0.36–0.50) | 0.356 (0.356–0.5) | 540 |
| CHU-9D score | 0.90 (0.80–0.97) | 0.91 (0.78–0.97) | 0.90 (0.78–0.97) | 0.89 (0.78–0.97) | 0.90 (0.78–0.97) | 533 |
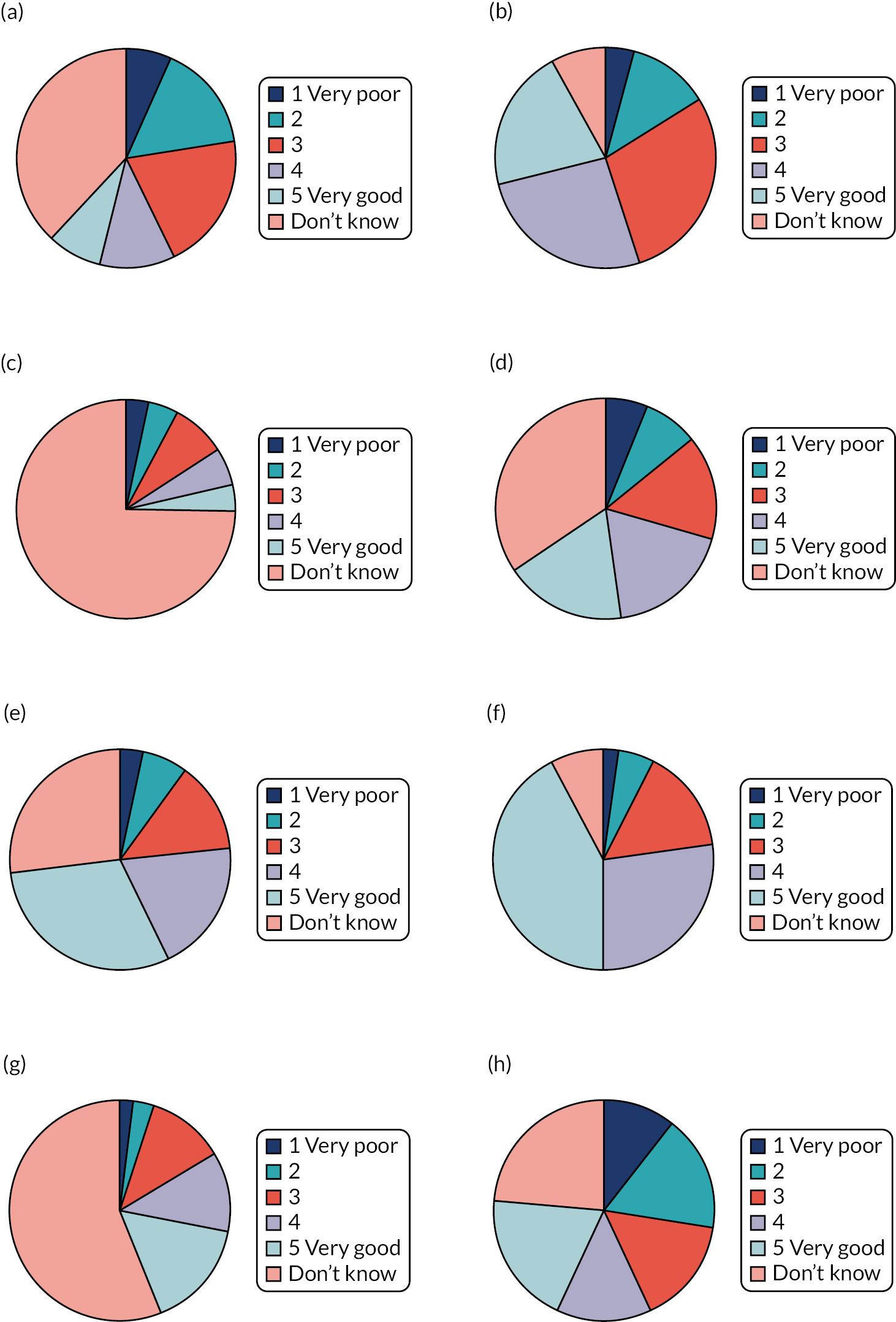
Parent-reported use of the topical corticosteroids, bath additives and the four emollient types of lotion, cream, gel and ointment was balanced across the groups at baseline (see Appendix 2, Table 29). The most used (current or past) type of emollient was cream (94.5%), followed by ointment (66.0%), lotion (63.0%) and gel (25.0%). The lower levels of prior ‘exposure’ to gels are reflected in parent perception of the effectiveness and acceptability of different emollient types (see Figure 3), with half to three-quarters of parents reporting ‘Don’t know’ for gels.
FIGURE 3.
Parent-reported opinion of effectiveness and acceptability of different emollient types at baseline. (a) Lotion effectiveness (n = 548); (b) cream effectiveness (n = 549); (c) gel effectiveness (n = 545); (d) ointment effectiveness (n = 548); (e) lotion acceptability (n = 549); (f) cream acceptability (n = 549); (g) gel acceptability (n = 548); and (h) ointment acceptability (n = 548).
Allocation and receipt of intervention
The 550 participants were randomly allocated to the lotion (n = 137), cream (n = 140), gel (n = 135) and ointment (n = 138) groups. The median number of days between baseline visit and randomisation was 0 (IQR 0–1; minimum 0, maximum 5 days). The median number of days between randomisation and self-reported first use of emollient was 4 (IQR 3–7; minimum 0, maximum 45), with 80% reporting first use within 7 days of randomisation. Breakdown by recruiting centre is shown in Appendix 2, Table 31.
A review of available prescribing data from EMRs (n = 522 participants) confirmed that 97.5% (509/522) were issued with a study-approved emollient of the correct type in the first 10 days after randomisation. The name of the specific emollients and number of participants prescribed these at baseline are presented in Table 4.
| Type (prescription data available/number randomised) | Specific emollient | n (%) |
|---|---|---|
| Lotion (132/137) | QV lotion | 77 (59.1) |
| Cetraben lotion | 37 (28.0) | |
| Diprobase lotion | 15 (11.4) | |
| Non-lotiona | 2 (1.5) | |
| Cream (132/140) | Epimax cream | 72 (54.5) |
| Zerobase cream | 40 (31.1) | |
| Diprobase cream | 10 (6.8) | |
| Aquamax cream | 8 (6.0) | |
| AproDerm cream | 0 (0.0) | |
| Non-study creamb | 2 (2.3) | |
| Gel (129/135) | Isomol gel | 96 (74.4) |
| Zerodouble gel | 21 (16.3) | |
| Doublebase gel | 9 (7.0) | |
| AproDerm gel | 1 (0.8) | |
| MyriBase gel | 0 (0.0) | |
| Non-gelc | 2 (1.6) | |
| Ointment (129/138) | White soft/Liquid paraffin 50/50 ointment | 88 (68.2) |
| Emulsifying ointment BP | 29 (22.5) | |
| Diprobase ointment | 9 (7.0) | |
| Paraffin White soft ointment | 0 (0.0) | |
| Paraffin Yellow soft ointment | 0 (0.0) | |
| Non-ointmentd | 3 (2.3) |
Reported behaviours regarding study emollient use
At the end of their time in the study, parents were asked about their use of the study emollients during the first 16 weeks (see Table 5). Adherence to directions on use and use of only the study emollient were similar across the different groups, although almost half reported more persistent use than normal.
| Response item | Treatment group, n (column %) | |||
|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | |
| How difficult was it for you/your child to follow the recommendation of applying your study moisturiser at least twice every day? | ||||
| Do not know | 4 (4.4) | 0 | 2 (2.5) | 5 (6.0) |
| Not at all (1) | 38 (41.3) | 38 (38.4) | 31 (38.3) | 31 (37.4) |
| (2) | 21 (22.8) | 27 (27.3) | 19 (23.5) | 12 (14.5) |
| (3) | 16 (17.4) | 17 (17.2) | 13 (16.1) | 10 (12.1) |
| (4) | 10 (10.9) | 12 (12.1) | 9 (11.1) | 11 (13.3) |
| Extremely (5) | 3 (3.3) | 5 (5.1) | 7 (8.6) | 14 (16.9) |
| How difficult was it to only use your assigned study moisturiser for the first 16 weeks of the study? | ||||
| Do not know | 0 | 1 (1.0) | 1 (1.23) | 3 (3.6) |
| Not at all (1) | 56 (60.9) | 48 (49.0) | 49 (60.5) | 38 (45.8) |
| (2) | 9 (9.8) | 17 (17.4) | 10 (12.4) | 6 (7.2) |
| (3) | 9 (9.8) | 10 (10.2) | 4 (4.9) | 6 (7.2) |
| (4) | 6 (6.5) | 7 (7.1) | 8 (9.9) | 11 (13.3) |
| Extremely (5) | 12 (13.0) | 15 (15.3) | 9 (11.1) | 19 (22.9) |
| Do you think that you used your assigned study moisturiser for a longer period of time than you would normally? | ||||
| No | 32 (34.8) | 37 (38.1) | 31 (38.8) | 33 (39.8) |
| Yes | 44 (47.8) | 45 (46.4) | 34 (42.5) | 41 (49.4) |
| Not sure | 16 (17.4) | 15 (15.5) | 15 (18.8) | 9 (10.8) |
Participant follow-up and withdrawals
Participant allocation and follow-up at the key time points of 16 and 52 weeks are shown in Figure 2. There were 29 withdrawals across the groups as follows (proportion randomised): ointment group, n = 12 (8.7%); lotion group, n = 8 (5.8%); cream group n = 5 (3.6%); and gel group, n = 4 (3.0%). The most common reasons given for withdrawal were ‘adverse reaction to moisturiser’ (n = 6), ‘insufficient time’ (n = 6) and ‘other’ (n = 6) (see Appendix 2, Table 32).
Data collection and completeness
Most parents (n = 507, 92.2%) chose to complete their questionnaires online, with 43 (7.8%) choosing paper versions.
Patient Orientated Eczema Measure scores were available from 549 out of 550 participants at baseline. Post baseline, 95% (524/550) of participants provided at least one further POEM score (lotion group, 131/137; cream group, 137/140; gel group, 130/135; and ointment group, 126/138) and therefore contributed to the primary outcome analysis (see Appendix 2, Figure 9). Completeness of data on secondary outcomes is presented in Appendix 2, Table 33.
Of the 77 GP surgeries that recruited at least one participant, 75 supplied EMR data from 524 out of 550 (95.3%) participants (see Figure 4). Reasons for missing EMR participant data were non-return of data by GP surgery (n = 17), moved practice (n = 8) and no consent for EMR (n = 1). Prescription data were obtained for 522 patients in the correct time period: 310 from EMIS and 214 from SystmOne.
FIGURE 4.
Electronic medical record review flow chart.
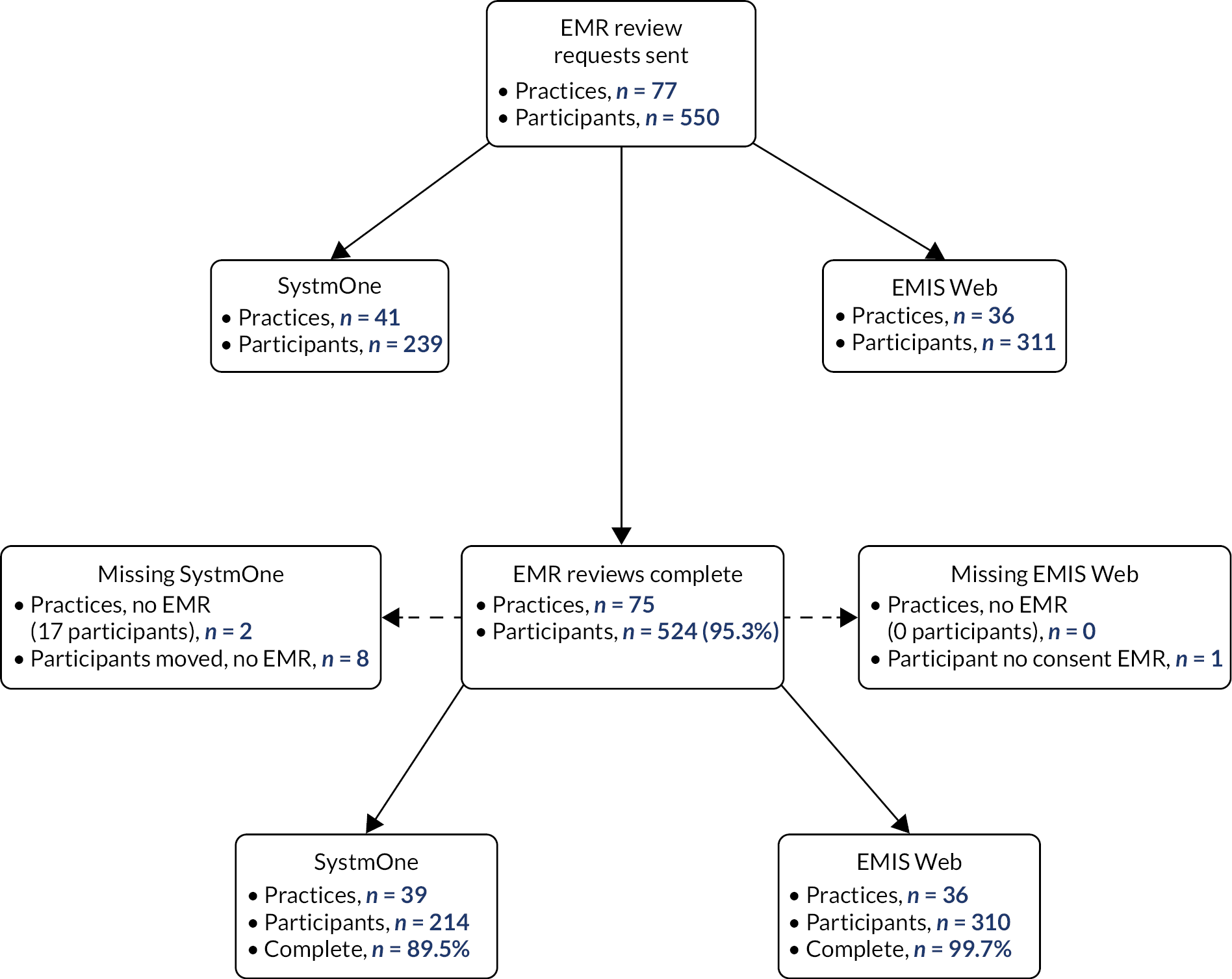
Protocol deviations
There were a total of 101 protocol deviations. One related to the one item of study paperwork; the others, relating to 89 individual participants, are listed in Table 6. The most common problems were the week-16 visit occurring outside the ± 10-day window (n = 41) and errors related to randomisation (n = 28).
| Deviation | Allocation, n | Overall | |||
|---|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | ||
| Week-16 visita | 7 | 13 | 15 | 6 | 41 |
| Randomisation | 6 | 10 | 6 | 6 | 28 |
| Unmasking | 2 | 4 | 4 | 2 | 12 |
| Paperwork | 1 | 2 | 1 | 2 | 6 |
| Interventionb | 4 | 0 | 0 | 0 | 4 |
| Datac | 2 | 0 | 0 | 2 | 4 |
| Eligibilityd | 2 | 1 | 0 | 0 | 3 |
| Prescription | 0 | 0 | 1 | 1 | 2 |
| Total | 24 | 30 | 27 | 19 | 100 |
Masking of researcher at week-16 visit
Most researchers undertaking the skin assessments were masked to participant allocation at week 16 (see Table 7, 97.4% ‘don’t know’). Only 7 out of 13 instances where blinding was thought to be compromised were correct, meaning researcher blinding was maintained for 98.6% of participants.
| Researcher response | Participant allocation | Overall | |||
|---|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | ||
| Lotion, n (%) | 1 (0.82) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (0.20) |
| Cream, n (%) | 1 (0.82) | 1 (0.76) | 0 (0.00) | 2 (1.77) | 4 (0.81) |
| Gel, n (%) | 0 (0.00) | 0 (0.00) | 3 (2.40) | 0 (0.00) | 3 (0.61) |
| Ointment, n (%) | 1 (0.82) | 1 (0.76) | 1 (0.80) | 2 (1.77) | 5 (1.02) |
| Don’t know, n (%) | 119 (97.54) | 130 (98.48) | 121 (96.80) | 109 (96.46) | 479 (97.36) |
| Total | 122 | 132 | 125 | 113 | 492 |
| Bang blinding indexa (95% CI); p-value | –0.008 (–0.032 to 0.015); 0.718 | 0 (–0.018 to 0.018); 0.500 | 0.016 (–0.010 to 0.042); 0.158 | 0 (–0.029 to 0.029); 0.500 | |
The Bang blinding index did not differ from zero for any of the treatment groups, indicating excellent masking.
Outcomes
Primary outcome
Across the treatment groups, mean (SD) POEM scores at baseline were lotion 8.7 (5.2) points, cream 9.3 (5.3) points, gel 9.8 (5.4) points and ointment 9.5 (6.0) points, with a range of 0 (no eczema symptoms) to 28 (very severe eczema symptoms) points. By week 16, scores had fallen slightly and the mean (SD) POEM scores at 16 weeks were lotion 5.7 (4.5) points, cream 6.7 (5.1) points, gel 6.7 (5.8) points and ointment 6.1 (5.9) points.
No differences in mean POEM scores were seen between the different groups over the 16-week primary outcome period (repeated-measures analysis of POEM scores, p = 0.765; see Table 8). (POEM scores by week and group are given in Appendix 2, Table 34). There was also no evidence of a difference in mean POEM scores over the first 16 weeks of the study between treatment groups in any of the pairwise comparisons (see Table 8).
| Allocated emollient (number of participants included in model/number of participants randomised) | Univariate difference in mean POEM (95% CI) | Adjusteda difference in mean POEM (95% CI) | p-valuea | ||||
|---|---|---|---|---|---|---|---|
| Lotion (n = 131/137) | Cream (n = 137/140) | Gel (n = 130/135) | Ointment (n = 126/138) | ||||
| Mean POEM score (SD) (points) | 6.79 (5.14) | 7.62 (5.37) | 7.45 (5.81) | 7.04 (6.13) | 0.765 | ||
| Pairwise comparisons | |||||||
| Lotion vs. cream |

|
0.87 (–0.27 to 2.00) | 0.42 (–0.48 to 1.32) | 0.360 | |||
| Lotion vs. gel |

|
0.84 (–0.31 to 1.99) | 0.17 (–0.75 to 1.09) | 0.718 | |||
| Lotion vs. ointment |

|
0.42 (–0.74 to 1.58) | –0.01 (–0.93 to 0.91) | 0.983 | |||
| Cream vs. gel |

|
–0.02 (–1.16 to 1.11) | –0.25 (–1.15 to 0.65) | 0.586 | |||
| Cream vs. ointment |

|
–0.45 (–1.59 to 0.70) | –0.43 (–1.34 to 0.48) | 0.354 | |||
| Gel vs. ointment |

|
–0.42 (–1.59 to 0.74) | –0.18 (–1.11 to 0.75) | 0.704 | |||
Secondary outcomes
Patient-reported eczema severity over 52 weeks
As observed in the primary outcome analysis, there was no evidence of a difference in mean POEM scores between the different groups over the 52-week follow-up period (repeated-measures analysis of POEM scores, p = 0.909; see Figure 5). (POEM scores by week and group are given in Appendix 2, Table 35). Pairwise comparisons also showed no evidence of differences between treatment groups (see Table 9).
| Allocated emollient (number of participants included in model/number of participants randomised) | Univariate difference in mean POEM (95% CI) | Adjusteda difference in mean POEM (95% CI) | p-valuea | ||||
|---|---|---|---|---|---|---|---|
| Lotion (n = 131/137) | Cream (n = 137/140) | Gel (n = 130/135) | Ointment (n = 126/138) | ||||
| Mean POEM score (SD) (points) | 6.72 (5.44) | 7.22 (5.41) | 7.15 (5.71) | 6.69 (6.06) | 0.909 | ||
| Pairwise comparisons | |||||||
| Lotion vs. cream |

|
0.60 (–0.49 to 1.68) | 0.19 (–0.69 to 1.06) | 0.677 | |||
| Lotion vs. gel |

|
0.64 (–0.45 to 1.74) | 0.01 (–0.88 to 0.90) | 0.987 | |||
| Lotion vs. ointment |

|
0.24 (–0.87 to 1.35) | –0.15 (–1.04 to 0.75) | 0.751 | |||
| Cream vs. gel |

|
0.05 (–1.04 to 1.13) | –0.18 (–1.06 to 0.70) | 0.690 | |||
| Cream vs. ointment |

|
–0.36 (–1.46 to 0.74) | –0.33 (–1.22 to 0.55) | 0.463 | |||
| Gel vs. ointment |

|
–0.40 (–1.52 to 0.71) | –0.15 (–1.06 to 0.75) | 0.739 | |||
FIGURE 5.
Mean POEM scores over 52 weeks by group.

Objective, masked assessment of eczema at week 16
Eczema Area Severity Index scores were collected by masked assessors at week 16 from 488 children with a slightly lower response rate in the ointment group (112/138) than other treatment groups (lotion, 121/137; cream, 132/140; gel, 123/135; see Appendix 2, Table 36). As with the patient-reported measures of eczema severity, no evidence of a difference in mean EASI scores was seen between the different groups at week 16 (p = 0.419; see Table 10).
| Allocated emollient (number of participants included in model/number of participants randomised) | Univariate ratio of geometric meansa in EASI (95% CI) | Adjusteda,b ratio of geometric means in EASI (95% CI) | p-valuea,b | ||||
|---|---|---|---|---|---|---|---|
| Lotion (n = 121/137) | Cream (n = 132/140) | Gel (n = 123/135) | Ointment (n = 112/138) | ||||
| Median EASI scores (IQR), points | |||||||
| Baseline | 3.3 (2, 7.2) | 3.15 (2, 6.3) | 4 (2.35, 8) | 3.3 (1.58, 6.5) | |||
| Week 16 | 2.15 (0.6, 3.6) | 2.3 (0.9, 4.7) | 2.25 (0.9, 5.15) | 2.2 (0.8, 4.8) | 0.420 | ||
| Pairwise comparisons | |||||||
| Lotion vs. cream |

|
1.17 (0.95 to 1.43) | 1.14 (0.97 to 1.35) | 0.123 | |||
| Lotion vs. gel |

|
1.14 (0.93 to 1.40) | 1.07 (0.90 to 1.26) | 0.464 | |||
| Lotion vs. ointment |

|
1.12 (0.38 to 1.38) | 1.12 (0.94 to 1.33) | 0.205 | |||
| Cream vs. gel |

|
0.98 (0.80 to 1.20) | 0.93 (0.79 to 1.10) | 0.424 | |||
| Cream vs. ointment |

|
0.96 (0.78 to 1.18) | 0.98 (0.83 to 1.16) | 0.828 | |||
| Gel vs. ointment |

|
1.17 (0.95 to 1.43) | 1.05 (0.88 to 1.25) | 0.579 | |||
Participants’ quality of life and impact on family
Quality of life was assessed using the eczema-specific ADQoL and the generic CHU-9D tools. There was no evidence of differences between the different treatment groups for either of these outcomes (see Tables 11 and 12). Impact on the family was measured using the DFI questionnaire at week 16 or week 52 (see Table 13) and there was no evidence of a difference between treatment arms (p-value = 0.0829).
| Data collection | Allocated emollient (number of participants included in model/number of participants randomised), median ADQoL scores (IQR); number of participants with non-missing data | Univariate difference in mean ADQoL scores (95% CI) | Adjusteda difference in mean ADQoL scores (95% CI) | p-valuea | |||
|---|---|---|---|---|---|---|---|
| Lotion (n = 121/137) | Cream (n = 128/140) | Gel (n = 119/135) | Ointment (n = 108/138) | ||||
| Baseline | 0.36 (0.36–0.50); 136 | 0.36 (0.36–0.50); 137 | 0.36 (0.36–0.56); 134 | 0.36 (0.36–0.50); 133 | |||
| Week 6 | 0.36 (0.36–0.50); 121 | 0.36 (0.36–0.50); 126 | 0.36 (0.36–0.50); 110 | 0.36 (0.36–0.49); 98 | |||
| Week 16 | 0.36 (0.36–0.49); 109 | 0.36 (0.36–0.56); 110 | 0.36 (0.36–0.49); 108 | 0.36 (0.36–0.36); 89 | |||
| Week 52 | 0.36 (0.36–0.49); 92 | 0.36 (0.36–0.50); 101 | 0.36 (0.36–0.49); 83 | 0.36 (0.36–0.49); 84 | |||
| Repeated-measures analysis (weeks 6–52) | 0.36 (0.36–0.49); 122 | 0.36 (0.36–0.50); 131 | 0.36 (0.36–0.49); 120 | 0.36 (0.36–0.49); 111 | 0.630 | ||
| Pairwise comparisons | |||||||
| Lotion vs. cream |

|
0.02 (−0.05 to 0.04) | 0.01 (−0.01 to 0.03) | 0.200 | |||
| Lotion vs. gel |

|
0.01 (−0.01 to 0.04) | 0.01 (−0.01 to 0.03) | 0.434 | |||
| Lotion vs. ointment |

|
0.003 (−0.02 to 0.03) | 0.01 (−0.02 to 0.03) | 0.651 | |||
| Cream vs. gel |

|
−0.01 (−0.03 to 0.02) | −0.01 (−0.03 to 0.02) | 0.631 | |||
| Cream vs. ointment |

|
−0.02 (−0.04 to 0.01) | −0.01 (−0.03 to 0.01) | 0.439 | |||
| Gel vs. ointment |

|
−0.01 (−0.04 to 0.01) | −0.003 (−0.03 to 0.02) | 0.761 | |||
| Data collection | Allocated emollient (number of participants included in model/number of participants randomised), median CHU-9D scores (SD) | Univariate difference in mean CHU-9D scores (95% CI) | Adjusteda difference in mean CHU-9D scores (95% CI) | p-valueb | |||
|---|---|---|---|---|---|---|---|
| Lotion (n = 119/137) | Cream (n = 127/140) | Gel (n = 112/135) | Ointment (n = 108/138) | ||||
| Baseline | 0.86 (0.15) | 0.86 (0.15) | 0.85 (0.14) | 0.85 (0.16) | 0.254 | ||
| Week 6 | 0.82 (0.20) | 0.81 (0.19) | 0.80 (0.20) | 0.80 (0.19) | |||
| Week 16 | 0.87 (0.15) | 0.80 (0.22) | 0.82 (0.18) | 0.84 (0.22) | |||
| Week 52 | 0.86 (0.17) | 0.81 (0.21) | 0.83 (0.20) | 0.88 (0.17) | |||
| Pairwise comparisons (week 16) | |||||||
| Lotion vs. cream |

|
−0.04 (–0.08 to 0.00) | –0.03 (–0.07 to 0.00) | 0.076 | |||
| Lotion vs. gel |

|
–0.03 (–0.08 to 0.01) | –0.03 (–0.07 to 0.01) | 0.144 | |||
| Lotion vs. ointment |

|
–0.02 (–0.06 to 0.03) | –0.01 (–0.05 to 0.03) | 0.644 | |||
| Cream vs. gel |

|
0.00 (–0.04 to 0.04) | 0.01 (–0.03 to 0.04) | 0.798 | |||
| Cream vs. ointment |

|
0.02 (–0.02 to 0.06) | 0.02 (–0.01 to 0.06) | 0.213 | |||
| Gel vs. ointment |

|
0.02 (–0.03 to 0.06) | 0.02 (–0.02 to 0.06) | 0.337 | |||
| Data collection | Allocated emollient (number of participants included in model/number of participants randomised), median DFI scores (IQR); number of participants with non-missing data | Univariate per cent differencea in DFI scores between groups (95% CI) | Adjusteda,b per cent difference in DFI scores between groups (95% CI) | p-valuea,b | |||
|---|---|---|---|---|---|---|---|
| Lotion (n = 112/137) | Cream (n = 115/140) | Gel (n = 112/135) | Ointment (n = 97/138) | ||||
| Baseline | 3 (1–6); 135 | 3 (1–6); 138 | 3 (1–8); 135 | 2 (0–6); 136 | |||
| Week 6 | 1 (0–3); 107 | 1 (0–5); 111 | 2 (0–5); 106 | 0 (0–5); 90 | |||
| Week 52 | 1 (0–4); 89 | 1 (0–4); 102 | 1 (0–4); 83 | 0 (0–2); 81 | |||
| Repeated measures | 1 (0–3); 112 | 1 (0–5); 115 | 2 (0–5); 112 | 0 (0–3); 97 | 0.0829 | ||
| Pairwise comparisons (repeated measures) | |||||||
| Lotion vs. cream |

|
1.20 (0.96 to 1.49) | 1.15 (0.95 to 1.38) | 0.143 | |||
| Lotion vs. gel |

|
1.31 (1.05 to 1.63) | 1.15 (0.95 to 1.38) | 0.154 | |||
| Lotion vs. ointment |

|
1.01 (0.81 to 1.28) | 0.93 (0.77 to 1.13) | 0.477 | |||
| Cream vs. gel |

|
1.09 (0.88 to 1.36) | 1.00 (0.83 to 1.20) | 0.993 | |||
| Cream vs. ointment |

|
0.85 (0.68 to 1.06) | 0.81 (0.67 to 0.98) | 0.033 | |||
| Gel vs. ointment |

|
0.78 (0.62 to 0.98) | 0.81 (0.67 to 0.99) | 0.037 | |||
Well-controlled weeks
The mean proportion of weeks with well-controlled symptoms ranged from 17.5% in the cream group to 23.8% in the lotion group. Consistent with these results and those of patient-reported eczema symptoms, there was no evidence that the proportion of weeks with well-controlled symptoms differed between treatment groups (p = 0.182; see Table 14).
| Data collection | Allocated emollient (number of participants included in model/number of participants randomised) | Univariate difference in proportion of weeks with well-controlled symptoms (95% CI) | Adjusteda difference in proportion of weeks with well-controlled symptoms (95% CI) | p-valuea | |||
|---|---|---|---|---|---|---|---|
| Lotion (n = 128/137) | Cream (n = 134/140) | Gel (n = 135/124) | Ointment (n = 123/138) | ||||
| Proportion of weeks with well controlled symptoms; mean (SD) | 23.79 (29.43) | 17.54 (25.06) | 22.89 (30.25) | 22.36 (28.29) | 0.182 | ||
| Pairwise comparisons | |||||||
| Lotion vs. cream |

|
–6.25 (–13.04 to 0.54) | –6.58 (–13.13 to –0.03) | 0.049 | |||
| Lotion vs. gel |

|
–0.91 (–7.78 to 5.96) | –0.79 (–7.42 to 5.83) | 0.814 | |||
| Lotion vs. ointment |

|
–1.43 (–8.37 to 5.50) | –1.71 (–8.15 to 5.22) | 0.667 | |||
| Cream vs. gel |

|
5.34 (–1.45 to 12.13) | 5.79 (–0.76 to 12.34) | 0.083 | |||
| Cream vs. ointment |

|
4.82 (–2.04 to 11.68) | 5.11 (–1.50 to 11.73) | 0.129 | |||
| Gel vs. ointment |

|
–0.52 (–7.46 to 6.41) | –0.67 (–7.36 to 6.02) | 0.843 | |||
Use of eczema treatments
Data on emollient use was provided by 494 (90%) participants (see Table 15). Figures 6 and 7 show reported allocated and non-allocated emollient and topical corticosteroid use by week and group, respectively.
| Patient-reported use | Lotion (n = 137) | Cream (n = 140) | Gel (n = 135) | Ointment (n = 138) | Overall | p-valuea |
|---|---|---|---|---|---|---|
| Number (%) of participants with any emollient use data | 115 (84) | 116 (83) | 110 (82) | 114 (83) | 455 (83) | |
| Study emollient use: median (IQR) number of days of use per participant week | 5 (0–7) | 6 (0–7) | 4 (0–7) | 3 (0–7) | 4 (0–7) | 0.481 |
| Non-study emollient use: median (IQR) number of days of use per participant week | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0 (0–2) | 0 (0–7) | 0.112 |
| Topical corticosteroid use: median (IQR) number of days of use per participant-week | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0 (0–2) | 0.819 |
FIGURE 6.
Parent-reported daily use of allocated and non-allocated emollients. (a) Allocated lotion use; (b) allocated cream use; (c) allocated gel use; (d) allocated ointment use; (e) non-allocated lotion use; (f) non-allocated cream use; (g) non-allocated gel use; and (h) non-allocated ointment use. (continued)


FIGURE 7.
Parent-reported daily use of topical corticosteroids. (a) Lotion; (b) cream; (c) gel; and (d) ointment.
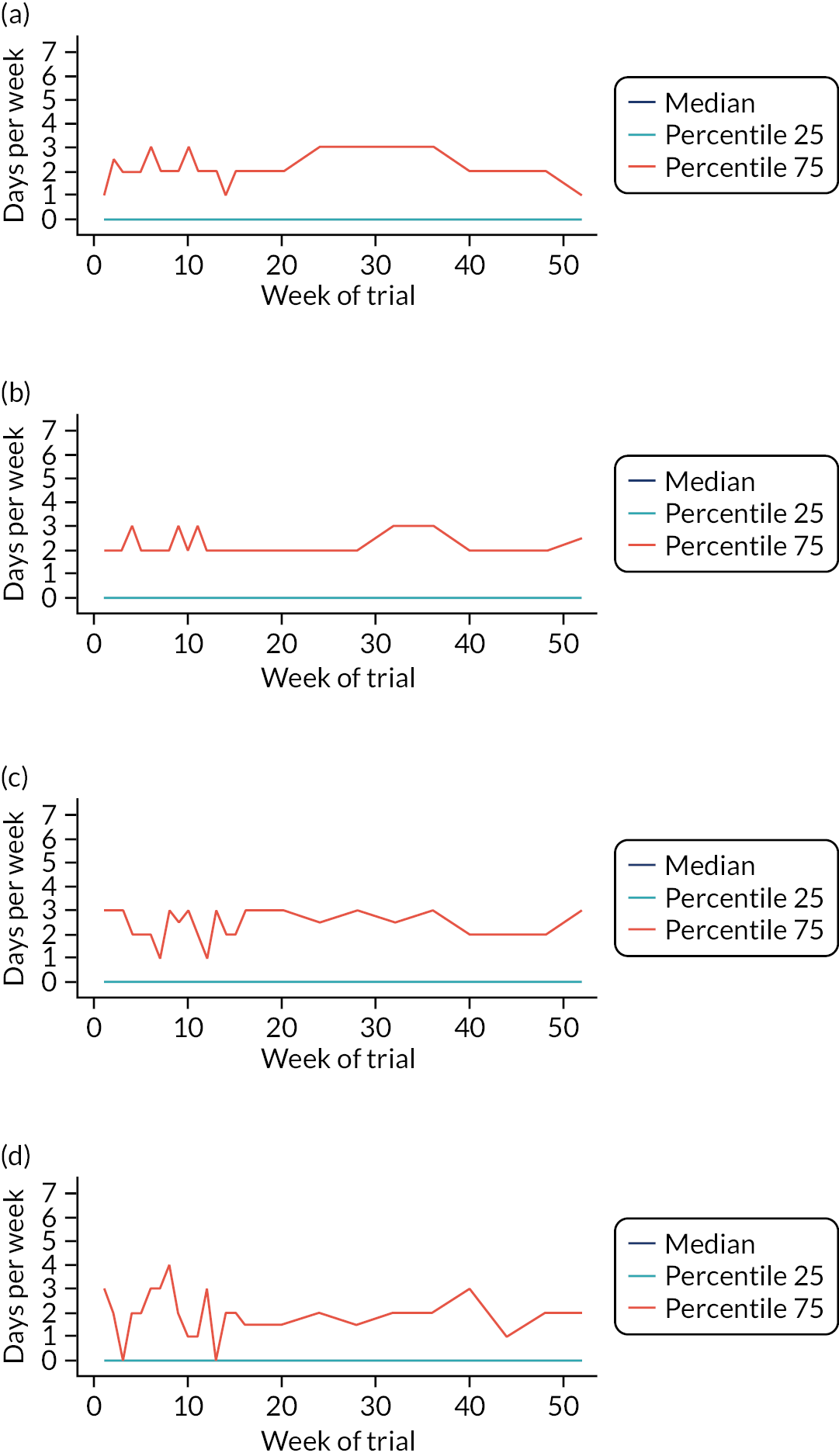
The median number of days per week that allocated emollient were used ranged between 3 (ointment) and 6 (lotion, cream and gel) days (p = 0.481), and was 0 in all treatment groups for non-allocated emollient use (p = 0.112; see Table 15). Similarly, the median number of days per week of reported topical corticosteroid use was the same for all emollient groups (p = 0.819; see Table 15).
Overall satisfaction with study emollient and future emollient use intentions
At week 16, 414 participants gave their overall satisfaction with their emollient (on a five-point scale, from very dissatisfied to very satisfied) and intentions regarding continued use (see Table 16). Responses were lower in the ointment group (64.5%); differences were observed (p = 0.003) between the treatment groups, with satisfaction highest with lotions and gels (67.9% and 64.5% very or mostly satisfied, respectively) and dissatisfaction highest with creams and ointments (34.2% and 40.5% dissatisfied/very dissatisfied, respectively) (see Appendix 2, Table 37). Participants in the lotion (61.7%) and gel (57.0%) groups were most likely to continue, with most uncertainty in the cream group (24.3%). Opinions in the ointment group were divided (39.3% each for yes and no).
| Rating | Number (%) | |||
|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | |
| n/N (%) | 107/137 (78.1) | 111/140 (79.3) | 107/135 (79.3) | 89/138 (64.5) |
| Very satisfied | 38 (35.5) | 24 (21.6) | 31 (29.0) | 10 (11.2) |
| Mostly satisfied | 34 (31.8) | 33 (29.7) | 38 (35.5) | 29 (32.6) |
| Neither satisfied/dissatisfied | 15 (14.0) | 16 (14.4) | 15 (14.0) | 14 (15.7) |
| Dissatisfied | 15 (14.0) | 21 (18.9) | 14 (13.1) | 19 (21.4) |
| Very dissatisfied | 5 (4.7) | 17 (15.3) | 9 (8.4) | 17 (19.1) |
| Will you carry on using this moisturiser? | ||||
| Yes | 66 (61.7) | 44 (39.6) | 61 (57.0) | 35 (39.3) |
| No | 27 (25.2) | 40 (36.0) | 26 (24.3) | 35 (39.3) |
| Not sure | 14 (13.1) | 27 (24.3) | 20 (18.7) | 19 (21.3) |
‘Per-protocol’ analysis
Participants were consistent in returning POEM scores over the follow-up period, with at least one POEM score reported monthly by 458 out of 550 (83.3%) participants. In contrast, only 276 (50.2%) participants reported emollient use every month, with 152 (27.6%) reporting emollient use on 60% of days (see Appendix 2, Table 38).
When restricting the analysis of the primary outcome to these 152 ‘per-protocol’ participants, there was no evidence of a difference in mean POEM scores over the first 16 weeks between treatment groups or in any of the pairwise comparisons (p = 0.238; see Appendix 2, Table 39).
Sensitivity analyses
Patient Orientated Eczema Measure sensitivity analyses
Estimates of pairwise differences between treatment groups in the primary outcome were not altered by adjustment for sex, which was imbalanced at baseline (p = 0.785; see Appendix 2, Table 40).
We adopted three approaches to assess the robustness of the primary analysis to missing data. Using MICE yielded results in line with the primary analysis (p = 0.696; see Appendix 2, Table 41). In the unlikely scenario that all missing data came from participants with worse eczema symptoms (‘worst case scenario’), the pairwise differences were slightly larger, but there remained no evidence that these differed from the null (p = 0.351; see Appendix 2, Table 42). Only when assuming the equally unlikely scenario that missing data came from patients with milder symptoms (‘best case scenario’) the pairwise differences were larger and was there evidence of a difference between ointments and creams (p = 0.002; see Appendix 2, Table 43).
Excluding the three ineligible patients who were still randomised yielded similar results to the primary analysis (see Appendix 2, Table 44) as did using researcher-completed POEM scores for missing parent-reported POEM scores at week 16 (see Appendix 2, Table 45).
Eczema Area Severity Index sensitivity analysis
The proportion of baseline and week-16 visits being done by the same researcher was similar across all treatment groups (see Appendix 2, Table 36). After adjusting for different researchers collecting EASI scores at baseline and week 16, there was still no difference in mean EASI scores between the different treatment groups over the primary outcome period (p = 0.486; see Appendix 2, Table 46).
Impact of COVID-19
The majority (n = 9628) of POEM scores were collected before the COVID-19 pandemic reached the UK (taken as before or on 11 March 2020) and 890 POEM scores were collected after this time point (see Appendix 2, Figure 10). All participants had completed their primary outcome period. There was no evidence of any difference in POEM scores before and after the COVID-19 pandemic reached the UK (likelihood ratio test comparing the model without the COVID-19 variable and a model including an interaction term with treatment group and COVID-19 time period, p = 0.1819).
Subgroup analyses
We planned four subgroup analyses to assess whether or not treatment group differences were modified by different baseline characteristics: parental expectation of effectiveness, age, eczema severity and meeting the UK diagnostic criteria for atopic dermatitis. The findings of these analyses are presented in Table 17. There was no evidence that treatment effects differed by any of the subgroups studied.
| Hypothesis | Subgroup: number of participants in each level of subgroup (%) | p-valuea |
|---|---|---|
| Effectiveness of emollient in treatment eczema symptoms (as determined by POEM, completed by an unmasked parent) influenced by prior expectation | Poor: 95 (17.34) | 0.935 |
| Average or unsure: 295 (53.83) | ||
| Good: 158 (28.83) | ||
| Emollient effectiveness varies with age | Under 2 years old: 94 (17.09) | 0.343 |
| Over 2 years old: 456 (82.91) | ||
| Emollient effectiveness varies with disease severity, determined by POEM at baseline | Mild eczema: 225 (41.0) | 0.042 |
| Moderate/severe eczema: 324 (59.0) | ||
| Emollient effectiveness varies according to whether participants meet formal criteria for diagnosis | Criteria not met: 103 (18.73) | 0.291 |
| Criteria met: 447 (81.27) |
Adverse events
Patient-reported adverse reactions are presented in Tables 18 and 19. Table 18 presents the total number and type of adverse reactions, and Table 19 presents the number of patients and type of adverse reactions. Overall, 37% (205/550) of participants reported at least one adverse reaction, but there was no evidence that the proportion of children reporting adverse reactions in the first 16 weeks differed by treatment group (p = 0.794).
| Adverse event | Lotion (n = 137) | Cream (n = 140) | Gel (n = 135) | Ointment (n = 138) | Overall (n = 550) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Worsening of eczema | 28 | 20 | 35 | 25 | 36 | 27 | 32 | 23 | 131 | 24 |
| Itching | 24 | 18 | 33 | 24 | 38 | 28 | 29 | 21 | 124 | 23 |
| Redness/inflammation | 25 | 18 | 28 | 20 | 32 | 24 | 27 | 20 | 112 | 20 |
| Dryness | 19 | 14 | 22 | 16 | 27 | 20 | 21 | 15 | 89 | 16 |
| Stinging | 28 | 20 | 24 | 17 | 25 | 19 | 12 | 9 | 89 | 16 |
| Burning sensation | 12 | 9 | 14 | 10 | 9 | 7 | 5 | 4 | 40 | 7 |
| Pain | 6 | 4 | 10 | 7 | 14 | 10 | 10 | 7 | 40 | 7 |
| Peeling of the skin | 6 | 4 | 6 | 4 | 8 | 6 | 6 | 4 | 26 | 5 |
| Tingling | 7 | 5 | 7 | 5 | 2 | 1 | 2 | 1 | 18 | 3 |
| Swelling | 1 | 1 | 4 | 3 | 4 | 3 | 6 | 4 | 15 | 3 |
| Other - rash | 2 | 1 | 1 | 1 | 1 | 1 | 4 | 3 | 8 | 1 |
| Skin infection | 3 | 2 | 1 | 1 | 4 | 3 | 4 | 3 | 12 | 2 |
| Slip or fall | 1 | 1 | 4 | 3 | 0 | 0 | 4 | 3 | 9 | 2 |
| Other – grease | 2 | 1 | 1 | 1 | 1 | 1 | 8 | 6 | 12 | 2 |
| Other – disliked emollient | 2 | 1 | 0 | 0 | 2 | 1 | 3 | 2 | 7 | 1 |
| Other – allergic reaction | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Total | 49 | 36 | 54 | 39 | 54 | 40 | 48 | 35 | 205 | 37 |
| Number (% of allocated participants) of participants reporting at least one adverse reaction in weeks 1–16 | |||||
|---|---|---|---|---|---|
| Adverse event | Lotion | Cream | Gel | Ointment | Overall |
| Worsening of eczema | 28 (20) | 35 (25) | 36 (27) | 32 (23) | 131 (24) |
| Itching | 24 (18) | 33 (24) | 38 (28) | 29 (21) | 124 (23) |
| Dryness | 19 (14) | 22 (16) | 27 (20) | 21 (15) | 89 (16) |
| Redness/inflammation | 25 (18) | 28 (20) | 32 (24) | 27 (20) | 112 (20) |
| Stinging | 28 (20) | 24 (17) | 25 (19) | 12 (9) | 89 (16) |
| Burning sensation | 12 (9) | 14 (10) | 9 (7) | 5 (4) | 40 (7) |
| Pain | 6 (4) | 10 (7) | 14 (10) | 10 (7) | 40 (7) |
| Peeling of the skin | 6 (4) | 6 (4) | 8 (6) | 6 (4) | 26 (5) |
| Tingling | 7 (5) | 7 (5) | 2 (1) | 2 (1) | 18 (3) |
| Swelling | 1 (1) | 4 (3) | 4 (3) | 6 (4) | 15 (3) |
| Other – rash | 2 (1) | 1 (1) | 1 (1) | 4 (3) | 8 (1) |
| Skin infection | 3 (2) | 1 (1) | 4 (3) | 4 (3) | 12 (2) |
| Slip or fall | 1 (1) | 4 (3) | 0 (0) | 4 (3) | 9 (2) |
| Other – grease | 2 (1) | 1 (1) | 1 (1) | 8 (6) | 12 (2) |
| Other – disliked emollient | 2 (1) | 0 (0) | 2 (1) | 3 (2) | 7 (1) |
| Other – allergic reaction | 0 (0) | 1 (1) | 0 (0) | 0 (0) | 1 (0) |
| Total | 49 (36) | 54 (39) | 54 (40) | 48 (35) | 205 (37) |
The most reported adverse reactions were ‘application site reactions’. Overall, the ‘top five’ reported problems were worsening of eczema (19.6% of adverse reactions and 24% of participants), itching (17.5% and 23%, respectively), dryness (15.0% and 16%, respectively), redness/inflammation (14.2% and 20.0%, respectively) and stinging (13.1% and 16%, respectively).
Skin infections appeared to be more common with gels and ointments (3% of participants) and slips or falls with creams (3%) and ointments (3%). However, it should be noted that the number of events (12 skin infections in 12 participants and 12 slips or falls in nine participants) is small.
There were no significant adverse events.
Chapter 5 Nested qualitative study results
This chapter reports on the findings of the nested qualitative interview study within BEE. The first interview took place on 21 February 2018 (month two of participant recruitment) and the last interview on 17 September 2019 (month 19 of 20).
Characteristics of interview participants
In total, 44 parents were interviewed: 20 at week 4 and 24 (including five repeat interviews) at week 16. Interviews lasted between 15 and 61 minutes (average 28 minutes). The characteristics of their children are shown in Tables 20 and 21.
| Characteristic | Treatment group, n | |||
|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | |
| Participant | ||||
| Parents | 5 | 5 | 5 | 5 |
| Participating children | 3 | 4 | 4 | 1 |
| Eczema severity | ||||
| Mild | 1 | 2 | 1 | 2 |
| Moderate | 3 | 3 | 3 | 2 |
| Severe | 1 | 1 | 1 | |
| Age | ||||
| 6 months to < 7 years | 2 | 2 | 1 | 4 |
| ≥ 7 years to < 12 years | 3 | 3 | 4 | 1 |
| Stopped emollient before primary outcome | 1 | 1 | 1 | 3 |
| Ethnicity | ||||
| White | 5 | 5 | 2 | 4 |
| Black | 1 | |||
| Asian | 1 | |||
| Mixed | 2 | |||
| Recruiting centre | ||||
| Bristol | 3 | 2 | 2 | 3 |
| Nottingham | 1 | 1 | 1 | 1 |
| Southampton | 1 | 2 | 2 | 1 |
| Characteristic | Treatment group, n | |||
|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | |
| Participant | ||||
| Parents | 6 | 6 | 7 | 5 |
| Participating children | 3 | 3 | 3 | 3 |
| Eczema severity | ||||
| Mild | 2 | 3 | 2 | 1 |
| Moderate | 2 | 2 | 4 | 3 |
| Severe | 2 | 1 | 1 | 1 |
| Age | ||||
| 6 months to < 7 years | 3 | 2 | 3 | 2 |
| ≥ 7 years to < 12 years | 3 | 4 | 4 | 3 |
| Study emollient use | ||||
| Stopped | 2 | 3 | 0 | 0 |
| Continue | 4 | 3 | 4 | 3 |
| Change | 3 | 2 | ||
| Ethnicity | ||||
| White | 3 | 6 | 5 | 4 |
| Black | ||||
| Asian | 1 | 1 | ||
| Mixed | 2 | 1 | 1 | |
| Recruiting centre | ||||
| Bristol | 3 | 1 | 2 | 3 |
| Nottingham | 2 | 2 | 2 | 1 |
| Southampton | 1 | 3 | 3 | 1 |
Parents were sampled by recruiting centre (Bristol, n = 19; Southampton, n = 14; Nottingham, n = 11), severity of child’s eczema (mild, n = 14; moderate, n = 22; severe, n = 8), age of child (aged 6 months to < 7 years, n = 19; aged 7 years to < 12 years, n = 25) and study emollient use (stopped before 16 weeks, n = 11; intended to continue study emollient after 16 weeks, n = 14; planned to change from study emollient after 16 weeks, n = 5). The majority of children were white (34), with six of mixed, three of Asian and one of black ethnicity. A total of 24 children took part in the interviews.
The quotations are tagged by participant’s study identifier, eczema severity, age group, emollient type, interview time point, if a child interviewee and if they stopped or changed using study emollient (‘stopped’ indicates participant stopped using study emollient before primary outcome at week 16; ‘changed’ indicates participant had reverted to previous or alternative emollient at week 16).
In addition to the study emollients (see Table 1), the following products (by type) were referred to by interviewees: lotions – Aveeno, Child’s Farm and Dermol; creams – Balneum, Aveeno, Oilatum, Dermol and Zeroveen; and ointments – Hydromol, Epaderm, Vaseline. No non-study gels were mentioned.
Perceptions of effectiveness of emollients
Participants judged the effectiveness of emollients across the four different types by comparing their study emollient to others they had used in the past, the level of hydration provided, the appearance of the skin, the extent of skin area affected, the feel of their skin after using, a change in symptoms such as cracked or bleeding skin, the level of itching and number of flare-ups experienced, and the need for topical corticosteroid use. Participants recognised that the effectiveness of an emollient was also affected by several factors, such as the time of year (heat/cold/wind), how regularly the emollient was applied and how much was necessary to apply to moisturise the skin:
It’s always climate change – if the climate is always same then his skin is better but when the climate change – one day rain, one day hot, one day cold, and it’s [eczema] all over his skin.
N08, Mild, ≥ 7 years, (study lotion) SL, week 16
Even if I moisturise her – ‘cos the doctor said I should use like apply heavily, especially when it’s very dry – but when you do it it’s still, it’s almost like her skin sucks in the moisture.
B09, Mild, 0–7 years, (study ointment) SO, week 4
Study lotions
Most interviewees felt that in terms of effectiveness the lotion they were allocated was ‘about the same’ as their pre-study emollient and had ‘maintained’ rather than improved the skin:
Well I’d say his skin generally is probably about the same … I don’t know whether it maybe is taking slightly longer for the dry patches to clear up, but I just make sure that I moisturise him regularly.
B04, Mild, ≥ 7 years, SL, week 4
One parent reported a decrease in the number of flare-ups while using their study lotion, but most felt that this and other eczema-related symptoms had not lessened:
Doesn’t always necessarily get rid of the eczema but sometimes it just stops it getting any worse, stops her getting any flare ups.
N06, Mild, 0–7 years, SL week 16
His scratching is probably about the same to be honest.
S03, Mild, 0–7 years, SL, week 4
Some parents noted that they were having to apply the lotion more frequently than emollients of other types for it to be effective:
I feel like if we just applied it twice a day, I don’t know that his skin would be quite as good … it’s gone so quickly and then his skin gets quite – with the cream his skin just, it didn’t feel rough again. It would feel rough again before you applied it again in the evening but not a couple of hours after. You do a nappy change couple of hours after and you think that’s already getting a bit dry and rough again.
SO3, Mild, 0–7 years, week 4
Only one of the parents interviewed reported a noticeable improvement with their child’s skin while using their study lotion, with both the parent and child confirming this at the week 16 interview:
I’d say it was better with the new one.
B06 child, Mod, ≥ 7 years, SL, week 16
Yeah, it seems to have improved, yes, ‘cos there were a couple of quite bad sore patches, quite oozy. Now the worst ones seem to have cleared up completely.
B06, Mod, ≥ 7 years, SL, week 16
One of the children believed that their skin had improved with the lotion, whereas their parent saw it as of a similar efficacy:
I think it’s better than the one I had before. Because the rest of my itchy spots have gone.
B04 child, Mild, ≥ 7 years, SL, week 4
Well I’d say his skin generally is probably about the same.
B04, Mild, ≥ 7 years, SL, week 4
Most participants who felt that lotion was equivalent or better than their pre-study emollient intended to keep using the lotion after the 16-week study period.
Adverse effects such as stinging on application of lotion were reported by some, but this tended to be an issue when applying to a ‘raw’ or ‘fresh’ patch of eczema:
It’s only like sometimes when this one [points to raw patch of eczema on arm] is like worse, then I put it on and it stinged.
B06 child, Mod, ≥ 7 years, SL, week 16
Flare-ups, itching and worsening of eczema were also reported. Some participants with these symptoms carried on using their study lotion and the situation improved. However, for others, the problems they experienced led them to stop using their lotion. Its perceived lack of effectiveness, for a number of families, was linked to it being too ‘thin’ and unable to sufficiently moisturise dry skin:
It was so thin and watery, it had no effect whatsoever and we had to go … can’t remember if we went to the doctors and got a different like better emollient ‘cos it just wasn’t effective. I just don’t think it was effective enough to keep a flare-up at bay.
B16, Mod, ≥ 7 years, SL, week 16
Two of the participants who had reported issues with using their study lotion and had stopped using it revealed existing preferences that may have had an impact on their assessment, although one had never used a prescribed lotion previously:
It was like she had the first-time round. When we got randomised to that Certraben [lotion] I thought ‘Oh no!’ [laughs], I’ll give it a go but I’ve done this before so I expected the same kind of results [itching, child scratching until skin bled] which did happen.
N02, Mod, 0–7 years, SL, week 4
I’d already got a bit of a preconceived idea of what it was going to be like … [but] I wanted to give it a go because again ‘cos it was a prescribed one as well, it was potentially different to the shop-bought lotions so I was happy to see if it made any difference.
B15, Sev, 0–7 years, SL, week 16
Study creams
Some participants reported that their study cream was more effective than their previous emollient. One parent was dubious about changing from their pre-study emollient and had not previously used the cream they were allocated, but reported that they were very happy and saw it as a positive change:
Still using it, yeah, and I prefer it to Diprobase [pre-study lotion]. I don’t know, it just seems to have a better effect on his skin so I don’t know if it’s ‘cos it’s a little … well I think it’s oily by comparison but, yeah, he seems to have done really well with it … I was really worried, sort of got used to Diprobase ‘cos we’ve tried a few different ones and ‘cos we’d got used to Diprobase, I was a bit worried about changing it but it changed for the positive actually.
S07, Mod, ≥ 7 years, (study cream) SC, week 16
Another parent had been wary about the effectiveness of their study cream when they were first using it, describing their child’s skin as ‘sandpaper rough’, but they persevered and reported that ‘her skin is better, it seems to be working well’. They recognised that the time of year may also have had an impact:
I don’t know if it’s the cream, I don’t know if it’s the sun, I don’t know if it’s the warmth but her skin has suddenly become really good so we actually went over the 16 weeks and there was times in that I was thinking this is not doing anything to suddenly really good.
B12, Mod, 0–7 years, SC, week 16
Reductions in flare-ups and symptoms were also reported:
Certainly in terms of patches of like flary-uppy eczema we’ve hardly had any at all, even when she’s kind of been away with the Brownies [Girlguiding, London, UK] and stuff where we know she’s perhaps not as thorough about applying [emollient] as she could be. Normally that would be a trigger for a flare-up and that hasn’t happened at all, so yeah, it’s a lot better … It felt a bit like we were fighting a losing battle of just sorting one flare-up out and then you were getting another one whereas now we’re hardly – I mean you’ve not had a bad flare since you’ve been on it have you? It’s been brilliant.
N03, Mod, ≥ 7 years, SC, week 4
I fill in those weekly surveys and you’re surprised how quickly that goes, but probably maybe once or twice we’d had to use hydrocortisone but it’s never been really bad so we haven’t had cracked or bleeding and it’s gone quite well.
S07, Mod, ≥ 7 years, SC, week 16
One family reported that despite experiencing issues at first, they persevered with their study cream because they were committed to the trial, and things had improved. Similarly, another felt that it was important to give the skin time as it adjusts to a different emollient (see also Study participation and emollient use, Using the study emollient for longer – ‘persevering’):
I think just recently we’ve not been putting it on so much so we’ve been weaning off a bit ‘cos his skin’s gradually improving. I think the first few weeks his skin was quite bad with the Epimax [study cream] but its slowly getting better. It’s adjusting to a different cream [generic].
B05, Mod, 0–7 years, SC, week 4
A few were neutral, some reporting that their study cream was maintaining but not improving their child’s skin, that it ‘kept it at bay’ or was ‘keeping things going’:
I’d say it’s kept it at bay … I can remember as a child the creams never do away with the eczema completely, but it helps soothe it. It’s one of those things that happens.
S05, Mod, 0–7 years, SC, week 4
Stinging on application was reported by a number of participants. Although this was mainly related to applying the cream to ‘raw’ patches of eczema, it did have an impact on families’ ongoing use of cream. One family was in the process of arranging to see their GP to request an alternative emollient as they felt that in addition to stinging on application the cream was ineffective, leading to a worsening of eczema that had then become infected:
It stings where the main part is on my arm, so like the bend.
B10 child, Mod, ≥ 7 years, SC, week 4
So it’s never stung before when we’ve put previous creams [generic] on. So this one’s done straight away and some days it’s a lot worse than others. I think it depends how raw her eczema is … ‘cos I filled out my form and said there was just this really gross thing on her arm and it kind of burst and a load of yellow pus came out and then there was something else there and my husband sort of give it a bit of a like a … hard white lump just came out.
B10, Mod, ≥ 7 years, SC, week 4
Three families who had already stopped using their study cream at the time of the interview reported ‘stinging’ and symptoms such as ‘itching’ and the appearance of ‘spots all over my body’. Another parent reported that it could not control their child’s eczema. One parent reported several attempts at using it, deciding to stop before the week 4 interview as their child’s skin ‘… went all bobbly and he was really, really itching’ (S01, Sev, ≥ 7 years, SC, week 4).
Another of these parents reported that they did not expect the cream to be effective owing to prior negative experiences, but was surprised at how rapidly their child’s skin had deteriorated:
So as soon as I was told that was the one I knew that it wouldn’t work anyway but I didn’t expect it to – I think within 2 or 3 weeks his skin was worse than it had ever been … the Zerobase [study cream] obviously wasn’t good enough to then control it and it went nuts …
S08, Mild, 0–7 years, SC, week 16
One participant had to use topical corticosteroids for the first time in a while and another reported that they had to use these more frequently over the 4 weeks:
During the first week. It was quite quick unfortunately. Yeah, so we had to go back to using steroids which we hadn’t used for quite a while.
B05, Mod, 0–7 years, SC, week 4
It was wrecked.
B05 child, Mod, 0–7 years, SC, week 4
Study gels
The study gel was unusual in that participants who were happy with this emollient tended to report using it alongside other treatments, including other emollients and topical corticosteroids, particularly on certain areas of the body:
It were brilliant to be honest with you. It’s the Isomol gel [study gel]. So it were brilliant to be fair. It’s a combination, we’ve been using a combination if I’m honest, we’ve been using the Isomol gel with the Oilatum [non-study cream], with a bit of the [bath emollient]. To be honest with you I believe it’s a combination of those three things that’s cleared her eczema up. She’s not had a breakout since I’d say the second week that we started the study.
N09, Sev, 0–7 years, (study gel) SG, week 16
Like I say, our magic cream [generic] is the steroid … to maintain it well. But when it flares up, because he’s just finished Year 6 SATs [Standard Assessment Tests] as well and the weather’s been changing, so I think all that as well, we still need actually use steroid cream [generic] unfortunately in combination … but I think of the normal regular ones Isomol [study gel] seems to work.
S04, Mod, ≥ 7 years, SG, week 16
We carried on using it because on the skin that wasn’t broken it was fine and it seems to do a decent enough job. I would say it’s just as good as the Cetraben [pre-study lotion] that we’ve been using. It seems to give the same kind of level of moisturising and its kinder to his clothes, it’s not really greasy, it seems to soak in well. So we carried on using it and then just literally would put something else on his face and hands when he needed it.
B18, Mild, 0–7 years, SG, week 16
A number reported adverse effects including stinging, particularly when applied to broken skin, as well as soreness and redness. Some persevered despite adverse effects and the skin improved:
Initially when we started applying, he almost straightaway got a slight flare up and we were a bit concerned … but we persevered and it was fine.
N07, Mod, 0–7 years, SG, week 16
Others continued but reported negative effects:
It’s got like a bit more of a cracked, bleeding.
S04 child, Mod, ≥ 7 years, SG, week 4
It stings, sometimes, you know, she cries and she says, ‘oh I want my ointment back’.
S04, Mod, ≥ 7 years, SG, week 4
Two families who had already stopped using their study gel at time of interview reported that it was not effective in treating eczema, with one reporting an immediate adverse reaction to using it:
I didn’t think it made any difference to her eczema. It didn’t, you know, she still had like really red dry bits, so I don’t think it really helped, considering she was putting it on probably … twice a day, so sometimes even more.
B14, Mod, ≥ 7 years, SG, week 16
Terribly from the off to be honest … by the end of that day it was red and really bad and within a couple of days she was screaming and crying in pain whenever we were putting it on her … so we were probably only on it 3 or 4 days I think.
B07, Sev, 0–7 years, SG, week 4
A further participant continued using their study gel until week 16 but decided to change as they had not found it effective in hydrating the skin:
Most of it was the fact that his body had gone back to being like the sandpapery effect when he was a baby … no it had been like 9, 10 years.
S11, Mild, ≥ 7 years, SG, week 16
Study ointments
Some parents and children reported an improvement using study ointment with a decrease in symptoms, such as itching, and a reduction in flare-ups:
We currently use an [study] ointment and we have used it and since then it has cleared up beautifully, like round her neck and on her arms you can barely tell she’s [got eczema] … massively [improved].
B11, Mod, ≥ 7 years, SO, week 16
The chest and the back of her neck has cleared up a lot. Obviously it’s still there and noticeable but it’s not as bad as it was before.
B08, Mild, 0–7 years, SO, week 4
It was quite good. It helped.
S09 child, Sev, ≥ 7 years, SO, week 16
One of the children thought that their study ointment was more effective than their pre-study emollient, but their mother disagreed, believing it to be comparable. Nevertheless, this parent conceded that they preferred using it as it was easier to apply (see Acceptability of emollients, Study ointments). A further parent was unsure that it was as effective as their pre-study emollient:
I don’t think it’s worse, but I don’t think it improves it ‘cos she does still get the flare up of the very fine bumps that comes with her eczema. I don’t know if it’s as effective.
B09, Mild, 0–7 years, SO, week 4
Adverse effects such as stinging on application, redness and itching were reported by some along with a worsening of eczema symptoms:
Within a couple of days I would say he was starting to have a flare … He did say it was stinging but whether that’s stinging or just that tingly sensation that you get from a different emollient or that his skin was particularly sensitive that day, though … he never complains about the cream I would say. I would say that is a big difference.
B17, Mod, 0–7 years, SO, week 16
We had to stop using it after two days because she just broke out in a horrible, itchy rash all over her body.
N01, Mod, 0–7 years, SO, week 4
All the families who had stopped using their study ointment at the time of their interview reported experiencing adverse effects and a worsening of eczema, although some had persevered with using it for the 16-week period but then changed back to their previous emollient:
So I thought it felt lovely and I was hoping it would work because when we put it on it felt really lovely on her skin when you were putting it on, but then it just seemed to make her skin worse so her skin went really red with it.
N05, Mod, 0–7 years, SO, week 16
Not too great. Like I said its one of the ones with the texture that she hates and it’s … spread her eczema now, it’s kind of all over her back, all over her belly and its down on her bum and things where she’s never had it before.
S06, Sev, ≥ 7 years, SO, week 4
One of these parents who changed after 16 weeks indicated an existing preference:
[Research nurse] had obviously gone through them with me and she said were there any that you wouldn’t do and I said at the time, I said, ‘well I don’t get on with the ointment so I wouldn’t be particularly pleased to get that, but that I would do whichever one that we got’.
B17, Mod, 0–7 years, SO, week 16
Temperature change/heat was mentioned by three of those who had stopped using their study ointment at time of interview and one whose skin had improved (see also Acceptability of emollients, Study ointments and Participant characteristics and perceived effectiveness and acceptability):
It was OK for a few weeks, first three weeks, 2 to 3 weeks it was fine, then I think with the temperature change as well ‘cos it dropped quite a lot … between October and November it’s dropped quite a lot. I didn’t really notice till [son] said, ‘my skin’s itching again,’ … I don’t know whether it’s the temperature change or – you don’t know do you?
B02, Mod, ≥ 7 years, SO, week 4
Acceptability of emollients
Acceptability was discussed in several different ways by participants and was usually considered alongside, or as part of, the effectiveness of an emollient. Absorbency was reported as an important factor across all four types of emollient, although there appeared to be a tension between participants preferring an emollient that absorbs into the skin quickly, is not sticky and does not mark clothing, with the desire for providing a protective ‘barrier’, for example before swimming. Perceptions of the way an emollient felt on the skin was also important, along with the ease of its application and the type of container it was dispensed from. The way an emollient smelled was not an issue, as participants acknowledged that having a slightly unpleasant smell was usual for most prescribed emollients. This contrasts with over-the-counter products that contain perfume, which one of the older girls would have liked to use, but who recognised that it could have been detrimental to her skin.
Study lotions
The study lotion was generally reported as easy to apply and as cooling to the skin:
It goes on well, it’s just a bit thin, a bit runny …
B01, Sev, ≥ 7 years, SL, week 4
I quite like it … and it’s cold.
B01 child, Sev, ≥ 7 years, SL, week 4
It is cold which [I] quite like that.
B01, Sev, ≥ 7 years, SL, week 4
It was reported to soak into the skin well, which was a plus for most participants, but some felt that the lotion was too thin and watery to sufficiently moisturise the skin and preferred an emollient that left a visible residue or protective barrier. As previously noted (see Perceptions of effectiveness of emollients, Study lotions), participants reported that they needed to apply it more often or use a greater amount:
I think the lotion that he’s got now soaks in a lot better. Obviously it’s a lot thinner so … specially the dry patches that he’s got at the moment, I’ll put some on, just a little bit, rub it in and almost make sure it’s soaked in and then I tend to put some more on whereas with the thicker one you could put one lot on, you could kind of see it all and know it was going to stay on, although back then I think probably sometimes it rubbed off on clothes anyway.
B04, Mild, ≥ 7 years, SL, week 4
It’s gone so quickly and then his skin gets quite – with the cream his skin just, it didn’t feel rough again. It would feel rough again before you applied it again in the evening but not a couple of hours after. You do a nappy change couple of hours after and you think that’s already getting a bit dry and rough again.
S03, Mild, < 7 years, SL, week 4
I think I had to use it more because it wasn’t moisturising his skin as much as I’d hoped it would.
B15, Severe, 0–7 years, SL, week 16
Participants generally found the smell of their study lotion to be acceptable.
Study creams
There were differing opinions on how well the study cream was absorbed into the skin, with some reporting that it was easy to apply and absorbed well and others reporting that it took a while to ‘rub in’. The time taken for the study cream to dry was an important factor for some in the context of busy family life:
The Epimax [study cream] I found isn’t sticky, it seems to absorb quite quickly, ‘cos even – Daddy can put it on you and then say I’m sorting out rooms for bedtime then I can come in and feel her arm and I don’t know if he’s put it on or not.
B12, Mod, 0–7 years, SC, week 16
It’s almost on the skin for a while. You can see it and it doesn’t quite absorb as quickly as maybe the Balneum [pre-study cream] did so maybe need to wait a while to put his pyjamas on or his clothes on.
B05, Mod, 0–7 years, SC, week 4
It’s pretty hard to rub in and it takes like 3 or 4 minutes to rub in.
B10, Mod, ≥ 7 years, SC, week 4
Participants described the consistency of their study cream as ‘thick’ and ‘gloopy’, one child commenting that their cream was ‘disgusting’. Those who saw it in these terms also described it as being difficult to apply:
If I was honest and had to describe it [laughs] I would say it’s really, really like, oh, paint, what’s the paint, the thick … it’s like gloss paint. It is like, you know, the really thick stuff, so not the one-coat.
B10, Mod, ≥ 7 years, SC, week 4
It’s quite sort of gloopy and I don’t know whether applying it is that easy whereas with the Balneum [pre-study cream] it was a lot smoother and more easier to soak in.
B05, Mod, 0–7 years, SC, week 4
The thickness of the cream was thought by some to be a good attribute as it could provide a protective barrier on the skin, or it meant that less needed to be applied in comparison with other types of emollients they had used, particularly lotions:
I think it seems to be slightly thicker so she’s still putting the same amount on that she would have done with the Cetraben [pre-study lotion] but it’s actually more appropriate, so you don’t actually need as much.
N03, Mod, ≥ 7 years, SC, week 16
The smell of their study cream was not a significant issue, with just two families commenting that ‘it smells like paint’ or that ‘it doesn’t smell great’.
Study gels
A number of participants described the study gel as absorbing into the skin quickly, which was seen as a positive attribute:
I think just the ease of the emollient, you know, being absorbed by the skin is quite helpful because when it’s … just sitting on top is not something very attractive to use.
S02, Mod, ≥ 7 years, SG, week 16
However, one of the children reported that ‘white bits’ remained visible on the skin despite rubbing it in well.
Some participants felt that as it was thinner, they needed to use more, in comparison with creams and ointments they had previously used. Others reported that they were not happy with the way the gel made their skin feel, commenting that it ‘felt cold’, ‘like clay’ or was a little ‘sticky’:
Well it kind of stuck a little bit when I like put my clothes on and when you put it on it was like all cold on your skin and it felt really cold a little bit.
B14 child, Mod, ≥ 7 years, SG, week 16
It’s like clay. I don’t really like the feeling and like what it does to my skin. It kind of makes my skin sticky and then it feels weird, when I put it on my skin inside my skin it feels like really weird. When I itch or when it becomes itchy it does start to sting as well.
S04 child, Mod, ≥ 7 years, SG, week 4
Participants had less experience of using a gel prior to the study and some were surprised at its appearance, expecting it to be translucent rather than white, or thinner, and these pre-conceptions may have had an impact on their views:
It’s quite creamy, it’s called a gel for some reason … I thought a gel was like – I thought it was translucent.
S02, Mod, ≥ 7 years, SG, week 4
I always thought a gel should be a bit more like watery content … But I think its content is almost exactly the same as Zeroveen [non-study cream], which … is a cream so, yeah, but fine, if they want to call it a gel its fine. When I found out it’s a gel, because I had never heard of this product before, try the gel, let’s see what happens. But as far as I was concerned, as I said because gels tend to be more watery and like thinner, thought it might not be as effective at hydrating his skin.
N07, Mod, 0–7 years, SG, week 16
Participants generally found the smell of their study gel to be acceptable.
Study ointments
The perceived thickness of ointment meant that, for some, it ‘seems to last longer on the skin’ and therefore could provide a protective barrier:
So, I thought it felt lovely and I was hoping it would work because when we put it on it felt really lovely on her skin when you were putting it on.
N05, Mod, 0–7 years, SO, week 16
Participants generally felt that as it was thick, their study ointment took longer to absorb into the skin than other types of emollient they had used. Some found this difficult in the context of a busy family life, whereas others found ways of coping if they found their ointment effective:
It does absorb into the skin though not as quickly and I found it was particularly difficult because – so on a morning we need to be out of the house by 07.20, quite early, three of us. Dad goes at 05.30 so he’s long gone. It was adding extra time to – I had to get [son] up early ‘cos the cream it sunk in, you turn around and its sunk in, the ointment takes what felt like quite a lot longer so I almost had to put a towel, do him head to toe, let him lie on the towel while I was getting … baby ready or whatever and then come back, check, and then if we were putting steroid on then we’ve got to apply the steroids and then go off again and then OK, right, well you can get dressed now.
B17, Mod, 0–7 years, SO, week 16
It makes a huge difference how well it absorbs and with him, when his skin’s really bad … like getting into the habit of putting a towel on the bed, putting the telly on, a programme that he likes, and then after he’s had a nice warm soak in the bath, drying him off sort of partially, then putting it all over him and just letting him lie on the towel with nothing on while it soaks in before I put his pyjamas on. It is all about having a technique, about how we put it on, when we put it on.
B13, Mild, ≥ 7 years, SO, week 16
However, the ‘greasy’, ‘oily’ or ‘waxy’ qualities led to problems with clothing for some participants as the ointment stuck to, or stained, clothing:
I was going to say it’s nice, isn’t it, it doesn’t sting your legs, she hasn’t complained ever about it and so that’s, you know, really good from her point of view. I suppose the worst thing about it is the fact that it does mark her clothes.
B11, Mod, ≥ 7 years, SO, week 16
Obviously it’s a bit slippery on the kind of flooring that we’ve got and the fact that you have to put clothes on over the top of it because of what it is and they do kind of stick so when it’s a hot day she’s got clothes like stuck to her.
S06, Sev, 0–7 years, SO, week 4
It’s worked really well. I think she finds it a bit strange ‘cos the texture, ‘cos its quite waxy isn’t it, and she’s used to creams so she’s a bit like, ‘oh, what’s that on me’.
B08, Mild, 0–7 years, SO, week 4
Participants reported issues with ointment and the weather or temperature. One parent raised concerns about using it in hot weather. There were also problems highlighted for cold temperatures, whereby the emollient became thicker and harder to apply:
I have to be like mindful, especially because the weather’s so hot, so like ‘cos I cover her skin with it so it’s like I don’t want to use it on her just before she goes in the sun ‘cos its very oily and I don’t know if it’s going to have an impact on the skin.
B09, Mild, 0–7 years, SO, week 4
Participants also disagreed on the ease of application of their study ointment, and again, temperature change/heat were important factors here:
They get quite thick so it’s hard, especially during winter when it’s like cold so they get harder … [laughs] so you kind of almost melt them or get them somewhere that’s quite soft because otherwise when you apply it to the skin in winter it’s almost like a brick … so like now during the warmer times it tends to be a bit more softer but during winter it’s like rock hard so applying it is quite tricky actually, ‘cos getting it out and like I’ll use – I’ll put it in the airing cupboard ‘cos its gets a bit warm so it softens a little bit. Other than that it’s very difficult to get out.
B09, Mild, 0–7 years, SO, week 4
As noted earlier, a parent and child disagreed on effectiveness (see Perceptions of effectiveness of emollients, Study ointments), but the parent preferred using it as it was easier to apply:
It’s better than the old one.
B13 child, Mild, ≥ 7 years, SO, week 16
I don’t think there’s a change but I don’t think that’s a bad thing ‘cos I think that we were happy with the Hydromol [pre-study ointment]. Hydromol was maintaining like an optimum level for him so it just seems to have maintained what I felt was already working to some degree … do you know actually there is improvement, not necessarily in his skin but I think more like what [son] was saying about its easier to apply, I think that’s where the improvement is.
B13, Mild, ≥ 7 years, SO, week 16
There was a tension around ointment use, more than other emollients, between perceived effectiveness and acceptability. The perception of it as a barrier, as good for the skin, was set against the difficulty in applying it and problems associated with it sitting on the skin more than other emollients:
To be honest it’s not the type of emollient that I’d go for again because it’s just too greasy.
N01, Mod, 0–7, SO, week 4
It’s not very nice to have all over your hands ‘cos it doesn’t – like with the cream, you put the cream on your hand and I can rub it into him say and then I can rub my hands together and it’s gone but with the ointment, ‘cos it’s got like a, I don’t know if it is, but it feels like it’s almost water repellent, it’s got that kind of texture … it feels more like a barrier cream. It’s got that Vaseline kind of feel about it.
B16, Mod, ≥ 7 years, SL, week 16
Epaderm [pre-study ointment] does help her so I can cope with skanky pyjamas for it [laughs].
B07, Severe, 0–7 years, SG, week 4
The latter parent reverted to using their pre-study emollient ointment in favour of the gel they were allocated because it was more effective, despite the effect it had on the child’s clothing. A few participants mentioned the ‘oily’ or slightly unpleasant smell of their study ointment, but this did not put off those who found it effective.
Emollient containers
The type of container the study emollient was dispensed from had some impact on perceptions of acceptability, in particular on ease of application. Containers varied across different emollient types and included pumps, squeezy bottles, tubs and a tube. Overall, participants were positive about pumps and squeezy bottles:
It’s just so easy. You just leave it on the surface, quick pump and then you’re kind of done whereas that one [bottle], its opening it up then squeezing it onto your hand then putting it down then putting it on. I know it sounds silly but when you’ve got two children with eczema and you’ve got to get everybody ready for bed or ready for school in the morning that kind of thing does make a difference ‘cos it’s just quicker.
B14, Mod, ≥ 7 years, SG, week 16
Because of the shape of the bottle, like even [daughter] can do it if I say [daughter] go and pop some cream on, [daughter] can go and squirt, you know, it’s easy enough for her to squirt a bit out and rub it on. So yeah, we’ve found that fine.
B12, Mod, 0–7 years, SC, week 16
However, participants expressed negative views about tubs, which were used for ointments. This related to the advised method of application, which was to use a spoon to scoop out the required amount. This was thought to be time-consuming by some, who used their hands instead, which raised the risk of infection:
When I went to see the dermatologist she said that the best way, or what the advice should be that you scoop it out with a spoon … I did use my hands … but I think that adding a spoon to that just, it’s something else for, it sounds awful, it’s a spoon isn’t it at the end of the day, but for me it’s like adding an extra, if you think like a mum who works with a baby and a child and dad’s, obviously they’ve got a dad but he works incredibly long hours, he’s not here, he’s not here in the morning, he’s not here at night so it just – it’s adding some, you know, it’s another something to do isn’t it, something else to clean up, whereas that pump is so [easy].
B16, Mod, ≥ 7 years, SL, week 16
Participants believed that it was important for them to be able to access containers of different sizes to make emollients easier to transport:
I probably think if I get a smaller one when I go away in the summer and we’ve got to go on an aeroplane … obviously we’re not going to need to take a big one … and that will be easier.
B04, Mild, ≥ 7 years, SL, week 4
A number of participants specifically mentioned that effectiveness of the emollient they were using was more important than its packaging:
The design of the packaging isn’t ideal I don’t think. I don’t think that necessarily impacts the product itself.
N04, Mod, ≥ 7 years, SG, week 4
None of the study emollients came in a spray format, but some participants commented that they found this a useful way of getting older children to apply emollient, for example after participating in sport activities and had bought these over the counter if they were not prescribed by their GP.
Decision-making at week 16: the effectiveness and acceptability trade-off
In this section we look at participant intentions at week 16 to see whether or not they would be continuing with their study emollient and to consider their reasons for continuing or switching to a different emollient. We purposively sampled participants so that we spoke with those who were still using their study emollient, those who were intending to change and those who had already stopped before the 16-week point.
There was no clear pattern or differentiation between the study emollients in terms of continued use. The reasons for stopping/staying with an emollient reflect those factors outlined in Perceptions of effectiveness of emollients and Acceptability of emollients. Parents and children based their decision on whether effectiveness and/or acceptability were either equal to or better than the previous emollient they were using before the trial started.
Decisions to continue with study emollient
For some participants there was a clear improvement, and it was therefore an easy decision for them to carry on using their allocated emollient:
So it seems to be keeping it under control better … the fact that it seems to be helping [was a] good incentive, so we weren’t counting down the weeks until the end of the study, having to decide whether or not it was worth carrying on … there was nothing really to think about.
B06, Mod, ≥ 7 years, SL, week 16
The data indicated that effectiveness was the primary driver of decision-making. This was evident in cases in which parents recognised the improved acceptability of their assigned emollient but were unable to keep using it because it did not control eczema in the way that their previous emollient had done:
It went on really nicely and she was quite excited about having this new cream [generic] … It just didn’t solve, yeah, if anything it made it so worse. I think if it had just kept it the same I would have probably carried on using it but I felt it was making it worse and so couldn’t then carry on.
N05, Mod, 0–7 years, SO, week 16
However, the data also highlighted the value placed on acceptability by participants, especially when the effectiveness of the emollient was negligible in comparison with emollients they had previously used:
Actually, there is improvement, not necessarily in his skin but I think like [son] was saying about it’s easier to apply, I think that’s where the improvement is. It’s easier to apply, it’s not as sticky … and uncomfortable as the other one.
B13, Mild, ≥ 7 years, SO, week 16
Trading off did not just happen at the 16-week point, for those that stopped using the study emollient at an earlier stage also had to grapple to obtain balance between effectiveness and acceptability:
I remember he liked the smell and I remember he liked the feel and him saying I like the smell of it. But in terms of effectiveness, I don’t think we remember but it just didn’t do anything … it just wasn’t rich enough. I don’t know, just didn’t have enough moisture in it.
B16, Mod, ≥ 7 years, SL, week 16
Decisions to discontinue study emollient
Some participants had persisted with their study emollient owing to their commitment to the study, but admitted that they reverted to their preferred pre-study emollient as soon as they reached the 16-week point, with the daughter commenting here that primary outcome period felt long:
We used it for the whole study … and the day after the study finished she went back to the other one [Zeroveen cream].
B14, Mod, ≥ 7 years, SG, week 16
This is really funny, I know from the BEE study that 16 weeks is a really, really, really, really long time [laughter].
B14 child, Mod, ≥ 7 years, SG, week 16
This family had an older sibling with very severe eczema and had found that Zeroveen (non-study cream) had worked best for them so reverted to using this for both of their children.
Another family who decided to change at weeks 16 explained that they had experienced issues with their study ointment and had reverted to using a combination of emollients that they already had at home. This was partly from convenience, but mainly because they were still searching for an effective emollient:
It really just seemed to flare-up and her arms seemed to go really red and ever drier … I just thought I can’t carry on just for the sake of the study and let it get worse … I’ve got three on the go, so I’ve got Aveeno, Cetraben and the Child’s Farm are kind of in the bathroom and in her bedroom and whatever’s nearest I get on her ‘cos I think well its better than nothing … I kind of hope that one of them works and I mean like I say if someone could tell me what the magic one was then I’d just use that but it’s – and I would pay anything for it, I don’t even mind what it costs but I just – I kind of think well I’ll use it till it finishes, you know, until I’ve finished it and then maybe I’ll buy a different one and see if that has a better effect … so that’s the way we’re going to play it I think.
N05, Mod, 0–7 years, SO, week 16
Another participant had decided to swap based on reasons related to acceptability, preferring the type of container of their pre-study emollient (see Emollient containers), as their study gel had not proved to be more effective than their pre-study emollient.
As noted in Acceptability of emollients, a number of participants had already stopped using their study emollient at the time of interview. Most had stopped at the early stages of the study, even before the week-4 interview. Those who stopped at this early stage tended to do so because of adverse reactions such as stinging, worsening of eczema and/or increasing flare-ups. Some stopped as soon as they experienced difficulties, whereas a few paused and then tried again (see also Study participation and emollient use, Using the study emollient for longer – ‘persevering’).
Participant characteristics and perceived effectiveness and acceptability
Severity of eczema
We found no clear preference for a particular type of emollient across the different levels of severity (mild, moderate and severe). However, participants whose children had very dry and/or rough skin tended to prefer an emollient with a thicker consistency, such as a cream or an ointment, because they were seen as better at keeping the skin hydrated or moisturised. All types of emollient were reported to sting on application to cracked or broken skin but there were slightly more reports of this occurring while using a lotion or gel.
Age of child
Parents reported that because it was easier to regularly apply emollients in younger children, for example at nappy change times; consequently, they may have been applied more frequently. As they became older, some children were less co-operative, with one parent describing it as a ‘battle’to persuade their child to have the emollient applied. With increasing independence and becoming more self-conscious of their body, some of the older children had started to apply their own emollients, but parents varied in how far they trusted their child to do this. Parents reported allowing this ‘if his skin is under control’ or stepping in if they thought their child was ‘struggling’:
He’s nearly seven so he doesn’t want mum putting cream [generic] on his legs and his back but if I look at it and think it’s bad I’ll do it, but generally he does it himself.
S10, Sev, 0–7 years, SL week 16
Some of the children in the older group reported a slight preference for types that were lighter and more easily absorbed, and hence quicker to apply, with two of these mentioning a preference for non-study emollients in a spray format, which made them easy to transport for use at school or when participating in physical activity.
Ethnicity
Our sample included 10 participants from different ethnic backgrounds (see Tables 20 and 21). Overall, we found that there was no clear pattern around emollient preference when taking ethnicity into consideration. There was evidence that participants related their child’s eczema and the way that an emollient worked or did not work to their ethnicity and skin type. In the example below, the participant explained that they had found ointments, including their study ointment, to be generally more effective for their child’s ‘skin type’ (white and black Caribbean), relating this to their ethnicity:
That would be an ointment I think … I mean everyone’s different but in our experience … like I’ve got friends who’ve got children who’ve got eczema and I don’t know if it makes a difference as well … in terms of skin type. I think personally potentially, ‘cos as a child I had eczema but my eczema’s completely different to his, I think ‘cos his mix is different to mine and he’s sort of a bit more Afro-Caribbean than I am.
B13, Mild, ≥ 7 years, SO, week 16
Study participation and emollient use
Regular use of study emollient
At the baseline visits participants were provided with an emollient information sheet (see Report Supplementary Material 11), which included information on how emollients should be applied. Participants were directed to use their study emollient at least twice per day, and during the interviews they compared this usage with that prior to participation.
Many participants reported that this was similar to their usual routine, but there were also some who acknowledged that having to fill in their study surveys had acted as a reminder to help them to apply their emollient regularly, rather than using it for an exacerbation of eczema:
We probably haven’t been as diligent in terms of applying it on a daily basis as we have been with the study … because we said we would, we’ve probably only used the [pre-study emollient] when we’ve needed to, when he’s had really dry skin.
N04, Mod, ≥ 7 years, SG, week 4
Participating in the study had reoriented some parents’ approach to emollient use. Many reflected on their experiences of using different emollients to find one that suited them best:
I think the problem with chopping and changing all the time is that you don’t really know … you don’t give it a chance and that’s why I didn’t really want to just try the study moisturiser for a couple of days and go ‘it’s not working’.
B15, Sev, 0–7 years, SL, week 16
Some participants commented that more regular application may have contributed to an improvement in their child’s skin as before participation they could sometimes be ‘slack’ or ‘lazy’:
Probably, like religiously and I think because we’ve had the form to fill in and keep an eye on it, I think it’s definitely helped us manage it better … If you were to look at him now and compared to last summer … he was at school in long-sleeved tops and trousers because of his eczema and he didn’t want to have sun cream on, he’s actually in shorts and t-shirt this year and it’s actually under control.
S07, Mod, ≥ 7 years, SC, week 16
There were also participants who reported that participating in the study had improved their knowledge of eczema management and treatments by engaging with the study information about emollients:
I’ve only ever had that from the BEE study actually, advice about baths and things like that, about how to put on [emollients]. That’s the first time I’d ever heard of that.
S07, Mod, ≥ 7 years, SC, week 16
However, there were also cases in which participants deviated from best practice and/or study guidance. Few participants adhered to instructions for using a spoon to avoid contamination from the hands when applying their study ointment (see Emollient containers).
Using the study emollient for longer – ‘persevering’
Committing to using the study emollient for 16 weeks led some to see benefits in using regularly over a longer period. In the quote below a parent describes the emollient’s effectiveness in equivocal terms but recognises a benefit in sticking with an emollient:
I don’t think it’s improved things massively but I am going to continue using it because I think, like I was saying before about not giving things a long enough go, I think especially with the winter coming up and chances are he’s probably going to get – it’s probably going to get worse for him a little bit, so we’ve ordered more of it … on prescription to continue using it. I mean apart from the fact that it’s thin and it’s more difficult for him to apply, it’s certainly not a bad [emollient].
S10, Sev, 0–7 years, SL, week 16
This parent, like many, had not found the study lotion to be a significant improvement but it had made enough of an impact to outweigh the negative aspects associated with acceptability and this was a pattern found across the study emollients.
Commitment to the study encouraged participants to use their emollient for a longer period and ‘persevering’ with the same emollient, despite experiencing periods of worsening eczema during the 16 weeks. Overall, participants could see a positive impact:
I think if I wasn’t on the study when it was really bad I would have gone back to the doctor and possibly seen if we could try something else so yes, so I don’t know whether the persevering and having it a set length of time has done us good … so this is the longest we’ve ever tried something … maybe just to persevere [laughs] and to give it like – giving something 6 weeks isn’t long enough, maybe 16 weeks isn’t long enough, maybe like 6 months, a year, you know, even though you’ve got rough times through that.
B12, Mod, 0–7 years, SC, week 16
I think because it was so bad I don’t think we ever gave it long enough in terms of, you know, we’d get this [emollient] and then we’d go back to the doctors and they’d say, ‘oh it’s not really working so change it,’ and I think in hindsight … and also based on this experiment that he’s done with the cream [generic] and we’ve obviously had it for, what are we … so we would never have given [an emollient] this long to work.
S10, Sev, 0–7 years, SL, week 16
Persisting with an emollient was novel for participants who had previously tended to swap frequently. Participation in the trial had contributed to a change whereby they recognised it was important to continue with their study emollient for longer, and sticking with an emollient beyond the main study period of 16 weeks gave an opportunity to assess its performance throughout the year:
I think it would be really interesting to see if we give it more time whether it would improve things for him even just a little bit, if his legs weren’t dry all the time or they weren’t … so they feel rough quite a lot as well. If it stopped maybe just a little bit of that I think it’s worth a go.
S10, Sev, 0–7 years, SL, week 16
Interviews with children
Children participated in the interviews when acceptable to families (12/20, week 4; 12/24, week 16). Those who contributed to discussions were mostly children in the ≥ 7 years age group. During the interviews they were encouraged to provide their own opinions.
The possible impact of parent presence on children when conducting paired qualitative interviews is recognised. 43 Although we did not find that parents dominated conversations, the level of the children’s contribution during the interviews varied. Some of the children were more talkative than others while others provided short answers. A few children provided contrasting opinions on effectiveness and acceptability to their parents, which we have highlighted in Perceptions of effectiveness of emollients, Acceptability of emollients and Participant characteristics and perceived effectiveness and acceptability, Age of child.
Chapter 6 Discussion
Main findings
Pragmatic randomised trial
In a primary care trial comparing four types of emollient for the treatment of eczema in children aged 6 months to 12 years, we found no evidence of a difference in eczema symptoms between lotions, creams, gels or ointments over the primary outcome period of 16 weeks. In our prespecified subgroup analyses, effectiveness did not vary by parent expectation, participant eczema severity, age or whether or not they met the UK diagnostic criteria for atopic dermatitis. Similarly, our secondary outcomes and per-protocol and sensitivity analyses showed no evidence of a difference between treatments. Over one-third of participants reported at least one adverse reaction, mainly application site reactions, with a similar number of problems across all emollient types. Reported daily use of allocated emollient and topical corticosteroids was similar for all four groups. At 16 weeks, overall satisfaction and intention to continue using the study emollient was highest for lotions and gels, whereas opinion on cream and ointments was more divided.
Nested qualitative study
The nested qualitative study was highly informative in understanding the findings of the main trial. No clear ‘winners’ or ‘losers’ were identified, yet problems were reported with all the different types of emollient.
We explored how families got on with emollients mainly through the lenses of perceived effectiveness and acceptability. Some interviewees positioned these aspects in opposition to each other, with an emollient judged to be useful but difficult to use or apply, whereas others deemed their emollient to be both effective and acceptable. However, for most participants, opinion was somewhere in between – for example, the study emollient might be as effective as their previous emollient but not stain clothing, making it more acceptable.
In terms of effectiveness, a number of participants using creams and ointments felt that their child’s eczema improved whereas those using lotions and gels tended to report their emollients as only controlling or maintaining their child’s eczema. Peculiarly to gel, a number of users found it useful only when using it on certain areas of the body or alongside other emollients (or sometimes topical corticosteroids).
Regarding acceptability, participants reported a wider range of views (positive and negative) on the acceptability of creams and ointments than for lotions and gels. A key consideration was the absorbency. Creams and ointments ‘sat’ on the skin, which for some was a positive, acting as a protective barrier, keeping out environmental factors that could irritate the skin, requiring fewer applications, hence reducing the time devoted to eczema management. However, others disliked the staining of clothes, and complained that the higher viscosity of these emollients made them more difficult to apply and uncomfortable for the child. In contrast, lotions and gels were described as lighter emollients that were easier to apply and ‘disappeared’ into the skin. Some parents thought that this indicated moisturising properties, whereas others perceived this as not as giving sufficient protection and so applied them more frequently.
Participants also reported factors, notably climate, which affected severity of their child’s eczema and the perceived effectiveness and acceptability of an emollient. The ways in which ointments were perceived and used were linked to the weather, with complaints of them being difficult to apply in colder weather and concerns about skin safety in hot sunny conditions.
As a consequence of taking part, some parents reported improved knowledge about emollients and how to use them, which led to their more regular use. This change was partly attributed to the ‘emollient information sheet’, given to all participants at baseline and partly from trial-related activities. Parents variously said they were more likely to ‘persist’ with a study emollient, more than they might normally, because they were taking part in a study, and study questionnaires served both as a reminder to use the study emollient and also as a self-monitoring tool, enabling parents to see the benefit of regular emollient use.
Strengths and limitations
Pragmatic randomised trial
To the best of our knowledge, this is the first head-to-head randomised trial of the four main types of emollients as a leave-on treatment for childhood eczema.
Internal validity
The characteristics of participants at baseline were balanced, with the exception of sex, but findings were unchanged when we adjusted for this in sensitivity analyses.
Parents were not masked to their allocated emollient for the following reasons. Foremostly, differences in the appearance and consistency of different emollient types means that they are readily identifiable. We considered trying to mask parents at the level of emollient type by ‘over-packaging’ into identical containers. However, it would have been technically difficult and expensive to do; the ‘shelf-life’ may have been problematic; and emollients may not have been dispensed as well from a universal pump as their original device, thus potentially influencing use of, and satisfaction with, the product. Our approach of having study-approved emollients prescribed by the participant’s GP and issued by their usual pharmacy had the advantage of being logistically easier to deliver, cheaper and closer to usual care, with attendant advantages for a planned health economic analysis.
We sought to minimise the potential for performance bias by ensuring that at the point of consent parents were willing to use any of the four emollients for the first 16 weeks. We also measured parent opinion regarding the four different study emollients at baseline, and in a subgroup analysis did not find any evidence that reported that effectiveness was linked to high/low prior expectations. The collection of an objective measure of eczema severity (EASI) by a masked researcher as a secondary outcome allowed us to examine outcomes in relation to signs of eczema.
We chose the parent-reported POEM as our primary outcome because it captures symptoms of importance to parents and patients over the previous week, and it has good validity, repeatability and responsiveness to change. 24,44 Eczema is a relapsing and remitting long-term condition, so collecting POEM scores weekly during the 16-week primary outcome period, combined with our repeated analysis measures approach, enabled us to both make the most of the available data (95% contributed to the primary analysis) and also capture the effectiveness of treatments better than comparing outcomes at a single time point. Hitherto, short-term follow-up has been a limitation of previous studies of emollients. 20 Repeated analysis measures of 4-weekly POEM scores means we have also been able to comment on the long-term (52-week) effectiveness of emollients. Stuart et al. 45 have recommended that monthly (4-weekly) is probably adequate in terms of striking the balance between statistical advantage from more measures versus patient burden and effects from data collection on the outcome of interest itself.
It could be argued that POEM, or indeed any measure of eczema severity, is not specific enough to detect the benefits of emollients that include skin hydration, comfort and possibly prevention of flares. However, POEM is recommended as the core symptom outcome by Harmonising Outcome Measures for Eczema (HOME46), was favoured as the main outcome by lay advisors and its validation has only been shown for the full seven-item scale rather than modified versions that ask questions only about skin dryness.
We have also collected and reported EASI scores, the other HOME-recommended outcome for clinical signs. Masking of the researchers undertaking the EASI assessments at 16 weeks to allocation was excellent (98.6% ‘Don’t know’ or incorrect guesses of participant allocation). During the life of the trial, HOME subsequently recommended that IDQoL47 (Infants’ Dermatitis Quality of Life index) and CDLQI48 (Children’s Dermatology Quality of Life Index) be collected for infants and children respectively in the domain of quality of life and that RECAP49 (Recap of atopic eczema) or ADCT50 (Atopic Dermatitis Control Tool) be collected for control. We have, however, collected and reported disease (ADQoL) and generic (CHU-9D) quality-of-life measures, and explored control through the concept of ‘well-controlled weeks’. 51 Lastly, in respect of outcomes, we have also collected and present data on reported treatment use and patient satisfaction: important secondary outcomes that were often missing in trials included in the 2017 Cochrane review. 20
Collecting data on use of eczema treatments (study emollient, other emollients and topical corticosteroids) was challenging. Despite work with parent contributors in developing the original question items that asked about this, we made changes part-way through the study to try to improve completion (see Chapter 3, Results, Parent and public involvement during the study, Study materials). It was not possible, without making the questionnaires overly complex or burdensome, to collect data on a weekly basis on the names (and, in the case of topical corticosteroids, strength) of treatments, number of applications per day and/or quantity of treatment used. The resulting questionnaire (capturing daily or more frequent use, by emollient type and any topical corticosteroid) was therefore a compromise between feasibility and granularity of data desired, without compromising completion of the primary outcome. In retrospect, to avoid ambiguity in interpretation, the questionnaire could have been improved with the addition of a ‘None (this week)’ option for each treatment. Consequently, we have taken a conservative approach and taken no record of treatment use as missing data, whereas it may genuinely represent no use of any treatments that week.
There is no agreed ways to define and report use of topical treatments. For the purposes of the internal pilot, and in our statistical analysis plan, we defined ‘adherence’ and ‘contamination’ as stated in Chapter 2, Terminology and definitions. However, as presented in this report, we prefer the labels ‘allocated emollient use’ and ‘non-allocated emollient use’, for the following reasons. First, this was a pragmatic trial comparing the effectiveness and acceptability of different types of emollient, not one designed to improve adherence. Second, there is a lack of evidence on how frequently emollients should be applied to be effective, and how this may differ between emollient types. Therefore, although we asked GPs to issue the allocated emollient type with directions ‘Twice daily and when required’ (reflecting the most commonly used approach in primary care), this study represented an opportunity to also capture reported frequency of use in relation to outcomes.
As stated in our statistical analysis plan, we recognised from the outset the limitations of defining ‘allocated emollient use’ and ‘non-allocated emollient use’ this way, and, based on the patterns of reported emollient use we agreed a definition for ‘per-protocol’ analysis. As with all per-protocol analyses, caution should be made when interpreting the results, especially when, because of missing data, only a proportion of participants contributed to the finding, but the results were consistent with the primary intention-to-treat and other secondary outcomes.
All participants completed their primary outcome period, and the majority of POEM scores (91.5%) were collected, before public health measures were widely introduced in the UK in response to the COVID-19 pandemic. Therefore, not only were the main findings not affected by this event, but we did not find any evidence of any difference in POEM scores before and after this time point.
External validity
The study was pragmatic in design, so aside from the participant’s emollient being randomised, all other eczema care of the child was as usual. Participants received their initial prescription, and requested any subsequent prescriptions, from their own GP. All participants received a short information sheet about emollient use and skin care, but no attempt was made to enhance emollient use. The emollients of each type compared are in everyday use in the UK and were similar in respect of their key constituents: all paraffin based, lotions with glycerol, creams with no humectant or lanolin; gels with no povidine and ointments with no additives.
In designing the trial, we acknowledged a tension recognised between pragmatism and concerns about ‘adherence’. That is, we wanted to mimic normal use as much as possible while encouraging participants to use only their allocated emollient so as to avoid significant ‘contamination’ that would render any comparisons meaningless. This was addressed by careful attention to the consent process, asking parents to take part only if they were willing to be randomised to, and use, any of the study emollients as their main emollient during the primary outcome period. However, this did not preclude them or their clinicians from stopping or changing because of adverse reactions or perceived clinical need. After the 16-week primary outcome period, families were at liberty to change emollients if they wanted, providing further ‘real world’ insight into how families evaluated the study emollients. During the primary outcome period, there was no evidence of any difference in use of allocated or non-allocated emollient types.
Children who took part in the trial are representative of children with eczema in the community generally, in respect of both the distribution of disease severity and also ethnicity. However, this does not mean that possible differences between ethnic groups have been excluded. The response rate (5.8%, 550 children randomised from 9437 invitation letters) to the mail-out invitation of children with a history of eczema is comparable with that in previous primary care trials (5.8%, 107/1849, COMET study;18 3.9%, 483/12,504, BATHE study43). The most common reason for GPs to exclude children from the mail-out invitation or for respondents to decline participation was because the eczema had been resolved. We think that this, or the misdiagnosis of eczema, is the most likely explanation for the majority of parents not replying to the invitation letter. We are unable to comment on how those who responded compared with those who did not in respect of ethnicity or deprivation. It could be argued that willingness to be randomised to all types of emollient as an inclusion criterion may reduce the generalisability of the findings, yet less-than-complete satisfaction with emollients previously or currently used is common. 19 That is, the findings almost certainly apply to parents of similar participants who are open to changing their child’s emollient, and some of those who are satisfied with what they are using, just because they have not tried an alternative type.
Participants in eczema studies20,52 have been shown to respond to study information and other aspects of study participation, with their use of emollients becoming more regular and in line with best practice recommendations. Also, committing to the study and regularly completing study questionnaires promoted regular long-term use of emollients, which led to positive results for some participants.
Although there were more withdrawals in the ointment group compared with the other groups, 5 out of 12 reasons given were ‘other’, of which only one was related to problems with the study emollient. In addition, there were more missing data from the ointment group than might be attributable to participants withdrawals (completed POEM from week 1 onwards: < 85% for the ointment group compared with > 90% for the lotion, cream and gel groups). The explanation for this is unclear, but imputation analyses are reassuring in terms of any effect on the primary outcome.
We originally proposed and were funded to undertake a trial comparing one emollient of each type, as per the design of the forerunner feasibility study, COMET. 15 However, restrictions in prescribing formularies across the different ICBs in England meant that we were required to amend the study at a late stage to compare similar emollients of each type, which can be considered to be both a strength and a weakness. It strengthens the generalisability of the findings, in that it increases confidence in the findings as a ‘class’ effect, albeit in relation to emollients that meet the group criteria. Another advantage of this approach was a practical one, for the delivery of the trial, as it allowed specific emollients to ‘leave’ or ‘join’ the trial, should ICBs make further changes during the study.
We sought medicine management team engagement with the study, asked that they notify us of any changes and monitored the emollient formularies for all the areas on a quarterly basis. No relevant changes occurred, and although not all of the emollients were available in all of the formularies, at least one product of each type was and the combined ‘picking list’ remained stable during recruitment and follow-up. A weakness of the ‘multiple versus single emollient per type’ approach is the assumption that emollients that are similar in terms of their major constituents are experienced as the same by the user, for example in appearance (including packaging) and feel. A series of studies have been published by Djokic-Gallagher et al. ,53–55 suggesting that generic copies (‘identikit’) of brands are experienced differently by different patients. Another criticism is that differences between emollients within each type may have masked differences between emollients of each type. Finally, it cannot be assumed that a trial comparing different types of non-study emollients would have similar findings, in particular non-paraffin-based emollients (e.g. Aveeno lotion, Aproderm cream) and ointments with emulsifiers (e.g. Epaderm, Hydromol).
In the light of medical consensus that emollients are a key part of eczema management, we thought that it would be considered unethical to run, and that parents would be reluctant to join, a trial with a ‘no emollient’ group (and it is impossible to implement a placebo) so we cannot say from this study whether or not emollients are effective per se. The improvement seen in POEM scores, particularly during the first 4 weeks, may be an example of ‘regression to the mean’. 56 Instead, our study was of different types of emollients in addition to usual care, which includes use of topical corticosteroids when required. As parents and any health-care professionals that they saw during the study were unmasked, it is possible, but unlikely, that knowledge of their study emollient could have biased prescribing, advice and use of other treatments.
As evident in the qualitative findings, parents commonly evaluate their study emollient by comparing them with one(s) previously used, so parents with a wider range of experience may have made different judgements from those who took part in our trial. Although all participants had used one or more emollient previously, awareness of different types, gels in particular, was low, and participants were randomised to try only one type of emollient. However, this is similar to the current arrangement in primary care, in which parents may have no or limited knowledge about emollients, and are often prescribed one emollient at a time.
Nested qualitative study
To our knowledge, this is the first qualitative study nested in a trial comparing the four main types of emollients for treating childhood eczema, the analysis of which was completed before the quantitative data were available. The purpose of the nested qualitative study was not to make overall recommendations in terms of effectiveness or acceptability. Instead, it was to explore and better understand the quantitative findings, with which it is consistent.
Our sampling framework encompassed a wide range of participant characteristics across the four trial groups. We included those who continued to use and those who had stopped using their study emollient at different time points and questioned participants regarding their intentions moving forward after the primary outcome period. Nevertheless, we recognise that owing to the wide range of our sampling criteria, the numbers within each category were small.
We also endeavoured to capture the views of children during the paired interviews. The level of their contribution varied considerably, so we are unable to draw firm conclusions regarding differing views and opinions with their parents and carers, but we have noted where their views on effectiveness and acceptability diverged. When considering our findings, it is important to recognise the potential impact of research participation on emollient use.
Data were collected by means of face-to-face and telephone interviews. We recognise that these modes of interviewing can shape the depth of data generated. 57 However, we did not find any substantive differences between the two modes of interviewing in terms of depth of data, although telephone interviews were more challenging when including children.
Parents and public involvement
A considerable strength of our research was our involvement of parent and public contributors. We listened to, and incorporated, many of their suggestions in the design of study recruitment materials and questionnaires, as well as the interpretation and development of key messages from the trial results. The commitment of our public co-applicant throughout the study was particularly valuable, providing continuity of memory across the life of the study.
Findings in the context of the literature
Clinical guidelines and formularies
There are many guidelines and review articles about on how eczema should be managed. Although emollient use, including as maintenance therapy, is recommended by all guidelines, there is minimal consensus between them on which emollient(s) children with eczema should use, or the frequency or quantity that should be applied. 58 In 2016–7, Chan et al. 13 identified 102 unique emollient formularies across all 209 ICBs and seven local health boards (LHBs) in England and Wales. They generally supported the use of the four main types (recommended at least one cream, 99%; ointment, 98%; lotion, 85%; and gel, 85%) but poor agreement over which emollient should be used ‘first line’, with three of the ‘top five’ being ointments (White Soft/Liquid Paraffin 50/50, Emulsifying Ointment BP, Hydromol ointment), followed by Dermol 500 Lotion and Cetraben Cream. The justification for multiple different, conflicting, formularies is unclear and reflects the prior lack of good research in this area.
Randomised trials comparing emollients in eczema
The best synthesis of existing evidence to date is the 2017 Cochrane review by van Zureen et al. ,22 which included 77 trials, comprising 6603 participants, evaluating the effectiveness of emollients. The majority of these trials (70/77) were classified as ‘unclear’ or at ‘high’ risk of bias. The authors were unable to conclude whether some of the emollients or their ingredients were better than others, as most head-to-head comparisons had been evaluated in single studies, which generally had small sample sizes. It did, however, support the value of emollient compared with no emollient or placebo, with statistically (but not clinically) significant difference in reduction of disease severity, something that our study does not add to, because all participants received an active treatment.
We are not aware of any on-going trials like the BEE study, comparing commonly used emollients in a head-to-head fashion in children recruited from a primary-care setting. Of trials included in the Cochrane review, the study of 80 children aged 1–12 years with mild to moderate eczema by Hlela et al. 59 is most similar. They found no differences between emulsifying ointment, cetomacrogol, white petroleum jelly and glycerine/petroleum, although the results are difficult to interpret because another trial (comparing two different emollients as soap substitutes) is reported in the same paper. Since the Cochrane review literature searches were done, the closest comparable published study is COMET,15 which was the feasibility forerunner to this main trial. Children who took part in it had eczema, were aged 1-month to < 5-years, and were randomised to Aveeno lotion, Diprobase cream, Doublebase gel or Hydromol ointment for 12 weeks. It was not powered to compare the effectiveness of the interventions, but eczema severity improved in all groups. 16
Djokic-Gallagher et al. 54 compared Doublebase Dayleve gel (similar to Doublebase gel, with the addition of povidone, a film forming agent) and Diprobase cream in double-blind, concurrent bi-lateral comparison in 34 women with eczema. They compared their effectiveness using corneometry, a non-invasive method for accurate determination of skin hydration by measuring changes in electrical capacitance of the stratum corneum, and physical acceptability by completion of an unvalidated patient questionnaire (likeability, willingness to use again and preference). Hydration was greater with the gel than the cream, the clinical significance of which is unknown, and participants appeared to prefer the gel. Tiplica et al. 60,61 randomised 335 children aged 2–6 years with mild to moderate atopic dermatitis to Dexeryl® [(V0034CR) Pierre Fabre Dermatology, Castres, France] cream, Atopiclair® (Alliance Pharmaceuticals Limited, Chippenham, UK) cream or no emollient. Children in emollient groups had fewer flares, with Dexeryl cream appearing to perform better than Atopiclair cream.
Other studies of emollient awareness and satisfaction
Parents’ awareness of the different types of emollients was highest for creams and lowest for gels. We did not ask specifically about their understanding of the purpose of emollients or how they worked, but poor knowledge has been associated with low or no treatment use. 62 In a survey63 of parents of 350 children with mostly mild–moderate eczema attending a hospital dermatology clinic in China (2014–15), only 50.0% knew that moisturisers can restore the skin barrier and 15.4% knew that emollients can improve eczema. This was evident in interviews in which participants described how the study information improved their knowledge about emollient use.
An alternative approach to trials, to compare emollients, is the use of self-complete ‘satisfaction’ questionnaires. Unfortunately, many studies adopting this approach have been small53,54 and until recently all have been in adults and used unvalidated tools. 53,54,64–66 Rowley et al. 67 have recently validated a seven-item ‘Emollient Evaluation Questionnaire’, which was completed by participants in the COMET study. 15 Analysing data from parents of 152 children, they reported highest satisfaction with Aveeno lotion and lowest for Hydromol® ointment, yet the number of participants intending to continue using their emollient was highest in the Hydromol ointment group, which suggests a trade-off between effectiveness and acceptability.
Unwanted and adverse effects
There is limited research into how common emollients are associated with adverse events and what implications this may have for adherence and use of other therapy. In an online survey of 210 patients and carers in 2016, Oakley and Lawson68 reported that 68% had experienced ‘unwanted effects’, of which 71% said that as a consequence they stopped using the leave-on emollient. The most common problem was stinging, followed by greasiness that interfered with everyday life.
‘Unwanted’ or ‘adverse effects’ affect treatment use, and studies over several years have reported low adherence to topical dermatological treatments in general and specifically in eczema. 62 In an online survey of 86 parents of children with mostly moderate or severe eczema, just over half reported taking or applying eczema medications as directed. 69 The top reasons for low adherence included worry about side effects, symptom resolution and perception that the medication was not working: 71% (90/126) of respondents stopped a leave-on emollient because of unwanted effects.
Although over half of the studies (41/77) in the 2017 Cochrane review20 included some data on adverse reactions, detail to enable comparison of the frequency and nature of adverse reactions across different emollients is absent. Bhanot et al. 70 conducted a ‘restricted’ review of published data on adverse events associated with emollient use in eczema. Interpretation of the results and comparison of the emollients across the 24 papers reporting on 29 different emollients was difficult owing to poor reporting and missing data. However, 2–59% of participants experienced treatment-related adverse events, most of which were skin related and mild. The data presented in our study, both in terms of overall frequency and by emollient type, are therefore unique.
Frequency of application and quantity of emollients and topical corticosteroids
Parent-reported use of emollients can be considered as both a mediator (‘Did participants use their allocated emollient?’) and an outcome (‘Is there a difference in the frequency of application of different types of emollients for the same effect?’). Although the findings from our study suggested that lotions and creams require more frequent application for the same effect than gels or ointments, the differences were not statistically significant.
Published data to support the amount of emollient needed to maintain disease control, and how this varies by age, severity and type of emollient, are scarce. Hon et al. ,71 in a study of 67 children with eczema using Cetaphil Moisturizing Cream (supplied by Galderma) correlated the quantity of emollient used with different measures of eczema severity (Nottingham Eczema Severity Scale, SCORing Atopic Dermatitis, Children’s Dermatology Life Quality Index), trans-epidermal water loss and mid-forearm skin hydration. They proposed that the quantity of emollient needed can be based on body surface area (between 73 and 136 g/m2/week) rather than disease severity. ‘Real world’ emollient use in moderate to severe disease is reported to be much lower (median of 17.5 g/day, 25th and 75th percentiles 12 g and 30 g/day, respectively, for children). 72 Choi et al. ’s72 interpretation of this finding is that many patients use emollients reactively rather than proactively. Our findings point to less than daily use, even in the context of a clinical trial that requested participants to apply their emollients ‘twice daily and when required’.
The van Zureen et al. 20 Cochrane review also found low-quality evidence that emollients prolong the time to flare, decrease the number of flares and reduce the amounts of topical corticosteroid needed to control eczema. What causes and how best to define an eczema ‘flare’ are both uncertain63,73 but we did not find any difference in ‘well-controlled weeks’ between the four types of emollient. Although we found no difference in the reported number of days of topical corticosteroid use, there may have been differences in total number of applications or quantity used. Two previous trials, by Giordano-Labadie et al. 74 and Grimalt et al. ,75 reported that the use of emollients decreased the use for topical corticosteroids. This may be desirable in respect of simplifying treatment burden and minimising the risk of adverse effects, but should not distract from the appropriate use of topical corticosteroids to treat inflamed skin.
Qualitative research
There is an established body of qualitative research literature around childhood eczema,76 which looks at the lived experience of eczema for children and families,8,18,77 beliefs around eczema and its causes,78,79 and the management and treatment of eczema. 18,80 Our research cuts across all these but engages mostly with the latter aspect.
Although many qualitative studies among people with eczema have highlighted confusion and concern about different emollient types and some concern about different emollient constituents,76 only one paper has specifically focused on perceptions of emollients among patients or carers with eczema. Santer et al. 52 drew on data from two studies, totalling 54 interviews with carers of children aged ≤ 5-years with eczema. This research identified the same kind of trade-offs that we saw in our work, with participants weighing up effectiveness and acceptability when choosing an emollient. Our results resonate with and add to these findings because we were able to assess experiences across the main four types of emollients.
There has been limited qualitative work with children81–84 and adolescents/young adults85–87 with eczema before. In a recently published study,88 Teasdale et al. interviewed 14 children (predominantly girls, with mild to moderate eczema) aged from 6 to 12 years. 88 They found that applying ‘creams’ (also used generically to describe creams, lotions, gels or ointment type of emollients) was helpful in relieving eczema symptoms. As in our interviews, participants had mixed views about the texture, viscosity and odour of some topical treatments, yet reported using topical treatments even if they disliked its texture or odour, supporting any trade-off favouring effectiveness over acceptability. Participants also wanted treatments that soaked into the skin quickly without having to reapply emollients, for example during the school day.
Emollients for primary prevention of eczema
Finally, ours was a study of emollients for the treatment of eczema, but it should be noted that during its lifetime two large trials (BEEP89 and PreventADALL90) and a recently published meta-analysis91 have found no evidence that their prophylactic use prevents the onset of disease. These findings have been a surprise, because prior pilot studies suggested a relative risk reduction of 50%. Indeed, the Cochrane review91 by Kelleher et al. concluded that their use increases the risk of skin infections and may increase the risk of food allergy. One explanation is that the application of emollients with unclean hands may introduce foods to the immune system via the defective skin barrier they were seeking to enhance. Questions have been raised previously about systemic absorption of some constituents of emollients via the same route,92 with concerns about food allergy induced by oatmeal-based emollients in particular. 93
Chapter 7 Conclusions
In a trial of the four main types for children with eczema, we found no difference in symptoms, signs or quality-of-life outcomes over the medium and long term. These findings were robust to sensitivity analyses and no differences were seen by parent expectation, age, disease severity or diagnostic criteria. Creams and gels may cause more local skin reactions and require more frequent application. Overall, satisfaction and intention to continue was highest for lotions and gels.
These findings are supported by the qualitative study, which found ‘no clear winner’, but widespread discussions of trade-offs between effectiveness and acceptability, with perceived effectiveness generally winning. The variation in opinions about the same types of emollients reflects the individual preferences and circumstances of parents and older children. For practical reasons, participants generally favoured pumps and tubes over tubs. We identified knowledge gaps, with parents reporting educational benefits and changes in behaviour and attitude as a result of taking part in the trial.
Recommendations for research
Interpretation of our findings will be strengthened by a formal health economic analysis, currently under way. In addition, we are developing a decision aid, summarising the merits and problems of different types of emollients. This should help parents, children and clinicians understand and discuss the issues in a way that helps users identify the best emollient(s) for them more quickly than before.
Our findings apply to children with eczema who are similar to those who took part in the study. Future studies ought to establish whether or not the findings also apply to other age groups (adolescents and adults) and people of different cultures and ethnicities.
The emollients evaluated in our trial were ‘first line’ treatments available on prescription,15 and all were paraffin-based emollients. Future trials could directly compare:
-
‘first line’ with ‘second line’ emollients that contain urea94 or antimicrobials95
-
‘conventional’ with ‘novel’ emollients, which claim to have superior skin barrier enhancement properties or which contain ingredients or live bacteria to help restore the skin microbiome and thus skin barrier function. 97,98
Participants evaluated their allocated type in parallel to each other, often drawing on their prior experience of emollients, which was most commonly creams. Future research could ask participants to directly compare different emollient types, perhaps by serially randomising them to different types, which may further elucidate the trade-offs between different types. An alternative approach would be to randomise children to usual care or ‘home tester’ samples of different types.
Although the clinical consensus strongly favours emollients for everyone with eczema, data on the overall effectiveness of any type of emollient are limited. This supports a trial of standard eczema treatments that compares the use of emollients with a ‘no emollient’ group to substantiate the claim that ‘any emollient is better than no emollient’.
Studies of effects of emollients on transepidermal water loss or similar can be useful in the development of new emollients, but any changes seen do not necessarily correlate with, or translate into, clinically meaningful differences. 99 Therefore, head-to-head comparisons of different emollients should focus on clinical outcomes, and all trials of emollients must embrace the HOME initiative by including the core outcome measures recommended for the four domains of symptoms, signs, quality of life and control. 46,100–102
An ‘ideal emollient’ is one that is both effective and acceptable for the user. Although it is probably a fallacy that one emollient will suit everyone all the time, there is room to further discern what characteristics users are willing to trade-off against one another, or indeed how this varies between different groups of users, perhaps through the use of discrete choice experiment methodology.
Studies ought to establish how emollients best fit into an overall ‘care package’. This could include combinations of either emollients (for different times of day, body sites or season) or formulations, for example sprays, ‘best bathing advice’ and/or application of topical treatments (e.g. emollients or topical corticosteroids first). 103 There is uncertainty as to whether or not written action plans improve the use of emollients alongside other treatments, although the ECO trial104 could help us better understand whether or not online education improves patient outcomes for children and young adults.
Implications for health care
Successful treatment of eczema relies on the avoidance of irritants, regular use of emollients and use topical corticosteroids for flare ups. Non-use, or irregular use, of emollients is reported to be the biggest reason for treatment failure. 52,80,105 It is also known that parents and GPs are confused by the wide array of eczema treatment products available. 62,76 Health-care professionals play a pivotal role in helping patients to select an emollient that they will accept and use. 106 Parents have said that they feel passive in primary care consultations and that they are assigned treatments without understanding why any particular one is assigned over another. 107 Trust in the physician and their recommendation have been reported to be the main determinants of emollient used. 108–110
Our study supports the use of lotion, cream, gel and ointment emollients for the treatment of childhood eczema. We found that the different types are similarly effective, challenging conventional wisdom that ointments are better, particularly in more severe disease. However, opinion on acceptability varies and so informed choice is required for all users to be able to find an emollient that suits them. We therefore recommend that all emollient formularies include at least one of each type, with the caveat that our findings may not apply to emollients of a type that are very different from those prescribed in this trial. (Aqueous cream is a special case in point, and our study does not change the advice of not using it as a leave-on treatment.)111,112
Our findings advocate for patient involvement and shared decision-making, which in turn may improve treatment use. Prescribing clinicians need to take a patient-centred approach to emollient prescribing113 and share decisions about which emollient(s) to start with, or change to, with the users. The hitherto ‘trial and error’ approach to emollient prescribing can be reduced by sharing information about the different emollient types and their different characteristics, to help parents and children choose what is most likely to suit them. Clinicians should start by finding out what families think about emollients, what they have used before, what their expectations are and what their concerns or priorities might be. When possible, children should be included in these discussions, as they have opinions about what they put on their skin. 88
Agreeing a management strategy, perhaps in the form of a written action plan,114 can help build trust and support treatment use. This should include education on how to apply emollients, advice on giving a ‘new’ emollient a reasonable trial period of 1–2 weeks and a warning that localised skin reactions are common. Emollient use may be further supported by recording POEM scores and planned reviews. Evidence to guide what are appropriate quantities of emollient is limited and will vary between and within people over time. Therefore, a shift in emphasis from measuring patients’ ‘adherence’ in terms number of prescriptions to what matters to individual(s) is likely to be more productive.
Chapter 8 Knowledge mobilisation
Knowledge mobilisation is about sharing knowledge between different communities to catalyse change. To understand what the potential knowledge mobilisation opportunities were for the study, an initial stakeholder analysis was conducted to identify the key target stakeholders, identify the main knowledge mobilisation approaches and design an effective approach for catalysing change. This stakeholder analysis was then further developed to understand how the different stakeholders may be interested in the results of the study and influence their impact.
Key stakeholders
Medicine management teams, policy-makers and guideline developers
In the UK, emollient formularies are determined by medicine management teams, which in turn are influenced by policy-makers and guideline developers, mainly NICE, the Scottish Intercollegiate Guideline Network (SIGN) and NHS in the devolved nations. They are the primary audience in terms of ensuring that new knowledge is incorporated into guidelines and formularies, to support effective prescribing by clinicians.
Policy-makers (national or local) are always mindful of costs. As all four emollient types were found equally effective but not equally acceptable to everyone, we recommend that they should be available to all children with eczema. However, there is a risk that ‘all equally effective’ may be taken out of context with respect to acceptability, and misused to restrict patient choice to the cheapest type(s) only.
Patients and families
Eczema is common in children yet their care varies across the UK, and emollient formularies in adjacent ICBs and LHBs can make contradictory recommendations. In the absence of consistent clinical advice, the emollient a family uses is often decided through a process of trial and error, which can be confusing and frustrating. Families who relocate may be forced to change from tried and trusted emollient(s). Effectiveness, acceptability (because of properties such as feel or absorbency) and use are interlinked, because a ‘less effective but more acceptable’ emollient used regularly is likely to be more beneficial than an infrequently used ‘more effective but less acceptable’ emollient.
Given that the results of the trial support all four types of emollient for use, the results of the trial are likely to be acceptable to families, as they support patient choice. However, if the results of the trial are used to suggest that all four emollient products are equally effective and there is no need to have all four available on the formularies, then this may cause difficulties for patients and families. It could result in distrust in the research as it is balanced against their own personal experience. Although this ‘end user’ group has little weight on policy decision-making, they may be able to influence policy through support groups.
Support groups (including voluntary sector and patient interest groups)
The main charities in the UK are the National Eczema Society, Eczema Outreach Support, Nottingham Eczema Support Group for Carers of Children with Eczema and the British Skin Foundation (London, UK). These groups consist of patients and health-care professionals, who are supported by experts in, or are specialists themselves, in eczema. Use of topic experts to inform these groups may be an effective strategy to ensure that knowledge is communicated accurately.
Skin specialists (dermatologists and dermatology specialist nurses)
Dermatologists, dermatology specialist nurses and GPs with extended roles in dermatology often influence local formularies that cover primary and secondary care. A change in their prescribing practice based on the study’s findings may be reflected in follow-on prescriptions in primary care. However, the findings may be less applicable to the children they see with more severe eczema and their decision-making may also be restricted by local formularies.
Specialists are likely to be supportive of new evidence, especially from a randomised controlled trial. Reaching this group can be done via scientific journals and special conferences. We discussed our findings with key opinion leaders, or those likely to be approached by the press, prior to publication to aim for consistent messaging. Careful explanation of the findings within the context of previous work can ensure that topic experts are available to support other audiences with accurate translation of new knowledge.
Primary care clinicians (including general practitioners and community pharmacists)
Most children with eczema are diagnosed and managed by their GP surgery. Historically, undergraduate and postgraduate GP training in dermatology has been limited, with skin problems such as eczema overshadowed by other long-term conditions. Community pharmacists sell emollients directly or give advice to families, based on the prescription issued by the GP surgery.
Prescribing clinicians in primary care (GPs, nurse practitioners, physician associates, pharmacists) will have varying interest in the results. They are most likely to be influenced by changes in local prescribing guidelines, which should reflect the evidence such as that from our randomised trial. Some community pharmacists will have relationships with medicines management teams (made up of pharmacists) so may be able to influence decisions.
Manufacturers of emollients
There are many manufacturers of emollients, with the main suppliers in the UK being Alliance Pharmaceuticals Limited (Chippenham, UK), Aspire Pharma Limited (Petersfield, UK), Bayer UK Ltd (Reading, UK), Crawford Healthcare (Knutsford, UK), Dermal Laboratories Ltd (Hitchin, UK), Fontus Health Ltd (Walsall, UK), Galen Limited (Craigavon, UK), Mölnlycke Health Care Limited (Milton Keynes, UK), and Thornton & Ross (Huddersfield, UK). Many advertise directly to parents, with some placing emphasis in their promotional material on claims about clinical effectiveness whereas others focus on cost-effectiveness. All have vested financial interests in their product(s) being portrayed in the best light.
Academics
Academics have an important role in reviewing the basis of the recommendations we make, and in supporting their uptake. Careful explanation of the findings within the context of previous work can support the accurate translation of new knowledge to different audiences.
Relationships between key stakeholders
The relationships between the key stakeholders are complex. Previous research107,115 has highlighted the differences in the views of family doctors and families, with GPs tending to focus on the appearance of the skin, whereas parents are often more concerned about symptoms of eczema or their psychosocial impact. Emollient-prescribing decisions of primary care clinicians are guided (or restricted) by area formularies, which may be influenced by local dermatology opinion, which in turn may be affected by pharmaceutical marketing. In the absence of robust evidence on the effectiveness of different emollients, the range in the formularies may be restricted to those in a lower cost bracket.
Knowledge mobilisation and dissemination strategy
Our strategy included mechanisms to both inform target audiences (breadth approaches) and actively engage them with the research and findings (depth approaches) in a two-way process. It is thought that by incorporating the multiple perspectives of stakeholders as the study progressed, this will increase both the relevance of research and its use within practice. 116–118
Breadth approaches (dissemination)
These approaches focus on disseminating the key knowledge findings with target audiences and other stakeholders. Breadth approaches were chosen to target each stakeholder group, but they assume a more linear approach to the way knowledge is shared. However, these approaches have the advantage of reaching a far larger number of individuals than the depth approaches.
Policy-makers
An animation and executive summary were chosen as key approaches to target policy-makers. This decision was based on evidence that policy-makers prefer conversations and brief forms of written communication. 119 It was considered that policy-makers may use the study website if social media was used to drive traffic and an active Twitter account was established to support communication with target audiences. All outputs, both academic and non-academic, with key findings produced for policy-makers and NHS audiences, will be held on the website, which will act as a repository for such materials.
Clinicians
Findings will be shared nationally and internationally through conferences, meetings and workshops, and through peer-reviewed publications. Associated summaries will be produced specifically for NHS audiences as well as for peer-reviewed publications. We have previously published an ‘uncertainty’ in the British Medical Journal to highlight the research question and trial,120 with further publications targeted for general medical, primary care and dermatology journals. Presentations are planned to communicate the findings to clinicians at primary care, dermatology and allergy conferences.
Academic
Publications in academic journals are the primary means for widely communicating our findings to academic colleagues. In addition, conferences will be attended for dissemination purposes and abstracts have been submitted to academic primary care meetings.
Members of the public, patients and families
We have previously described our study in Exchange, the magazine of the National Eczema Society. The study website is accessible by the general public, with attention to it drawn by regular tweets on the study Twitter account; regular newsletters have been produced for participating families and public contributors. The blogs hosted on the study target a wide audience to generate interest and to increase the accessibility of the study’s findings. We anticipate that the animation, summarising the study and its findings, will be particularly useful to participants and the wider public.
Depth approaches (knowledge mobilisation)
The depth approaches were targeted for the key stakeholders identified as having high power in the stakeholder analysis, in terms of future impact on policy and practice. Previous research has illustrated the importance of personal relationships and face-to-face interactions to maximise knowledge sharing and mobilisation with clinicians and policy-makers. Although restrictions related to the COVID-19 pandemic have limited physical meetings, virtual ‘face-to-face’ discussions have been possible.
The depth approaches aimed to target individuals and key organisations to achieve maximum opportunities to share and co-produce knowledge with stakeholders. It was only possible to conduct these on a small scale and at a local level, but it was intended that this learning could then be used to inform the wider-reach breadth approaches at a later stage.
Two theories of knowledge mobilisation (communities of practice and socialisation121 and externalisation, combination and internalisation theory122) were drawn on to inform the approach and strategy for knowledge mobilisation during the study. Both theories explain how knowledge is shared through everyday interaction and highlight where opportunities to introduce new knowledge may lie. By adopting the knowledge mobilisation approaches likely to be most effective for the key stakeholder groups identified (policy-makers and clinicians), the impact of the study findings on practice should be maximised, as well as the opportunities for practice to influence the potential study outputs.
Connections with commissioning organisations
The knowledge mobilisation co-applicant drew on existing relationships with the local medicine optimisation team and sought their advice and insight into where the study may have influence and impact on practice. Discussions with teams based in other ICBs were sought, but only one person from another area attended a meeting with the research team.
Contact with the medicines optimisation teams was enriched by information from the pharmacist co-applicant who had previous experience of working within both ICBs and general practice. This information was fed back to the study team to provide a steer into how the end results might be interpreted.
Contact with other stakeholders
We sought to access and connect with stakeholders’ wider networks in the field of eczema and dermatology to understand further the impact and potential interpretation of the final study results. Stakeholders attended three online meetings, at which the key findings were shared in confidence. Representatives from academia, the National Eczema Society, Eczema Outreach, Primary Care Dermatology Society (Rickmansworth, UK), general practice, dermatology, medicine optimisation teams and the Bristol, North Somerset and South Gloucestershire ICB attended.
The aim of these meetings was to glean from stakeholders their interpretation of the findings and the implications of the results for practice. There was a particular focus on how best to communicate the results to the different groups who have an interest in the study findings. All the meetings consisted of a brief presentation from the research team followed by an open discussion. With the potential risk of the results being used to restrict, rather than support, patient choice of different emollient types [see Knowledge mobilisation and dissemination strategy, Breadth approaches (dissemination), Policy-makers], we specifically asked questions of policy-makers and other stakeholders for their interpretation of the findings and how best to present the findings to mitigate this risk. Stakeholders were also asked how they might describe the results to a colleague to avoid misinterpretations. This information has been fed into the dissemination strategy for the study.
After the series of meetings, we reworked and refined the key messages coming from the study, which were used to inform the dissemination strategy for the study going forward.
Acknowledgements
Host and partner organisations
The BEE study was:
-
hosted by Bristol, North Somerset and South Gloucestershire ICB
-
developed with support from UK Dermatology Clinical Trials Network. The UK Dermatology Clinical Trials Network is grateful to the British Association of Dermatologists and the University of Nottingham for financial support
-
designed and delivered in collaboration with the Bristol Randomised Trials Collaboration, a UKCRC registered clinical trials unit which, as part of the Bristol Trials Centre, is in receipt of NIHR Clinical Trials Unit support funding
-
supported by NIHR Clinical Research Networks West of England, Wessex and East Midlands.
Other members of research team
The research team would like to acknowledge and thank the following people who have helped deliver the study:
-
Bristol, North Somerset and South Gloucestershire ICB – Paul Roy and Rachel Avery
-
University of Bristol – Ms Anna Gilbertson, Dr Shoba Dawson, Dr Louisa Edwards, Dr Sandra Hollinghurst, Dr Grace Boyd, Ms Kingsley Powell, Mai Baquedano, David Carmichael, Martin House, Nancy Horlick, Rachel Davies, Birgit Whitman, Adam Taylor
-
University of Nottingham – Ms Victoria Maddox, Ms Sue Davies-Jones, Faye Shelton
-
University of Southampton – Dr Martina Prude, Ms Kate Martinson
-
Rosalia Hawkins
-
Clinical Research Network Wessex – Andrea Jarman, Chantelle Moorbey, Lesley-Ann Castle, Ruth Gibbins, Ruth Jackson, Clinical Studies Officer for the Clinical Research Network Wessex
-
Clinical Research Network East Midlands – Ms Valentina Lazarevic, Ms Joanne Llewellyn, Stephanie Townsend, Janet Beecham, Karen Duff, Stephen Bosel-Doyle, Katy Ward.
Oversight committees
We are grateful to the following members of the trial oversight committees:
-
Trial Steering Committee – Professor Richard McManus (chairperson), Dr Ben Carter (medical statistician), Professor Joanne Protheroe, Dr Sariqa Wagley and Dr Andrew Moore (non-independent member)
-
Data Monitoring Committee – Dr John Ingram (chairperson), Dr Catriona Keerie (medical statistician) and Dr Chin Whybrew.
Contributions of authors
Matthew J Ridd (https://orcid.org/0000-0002-7954-8823) (GP and Chief Investigator) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter; led qualitative analysis; and drafted the report, with all authors revising it critically for important intellectual content.
Sian Wells (https://orcid.org/0000-0002-5334-7599) (Trial Manager) operationalised the study.
Stephanie J MacNeill (https://orcid.org/0000-0001-6553-1433) (Medical Statistician) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter; and did trial analysis.
Emily Sanderson (https://orcid.org/0000-0003-2268-4194) (Medical Statistician) did trial analysis.
Douglas Webb (https://orcid.org/0000-0001-6818-1838) (Trial Manager) operationalised the study.
Jonathan Banks (https://orcid.org/0000-0002-3889-6098) (Qualitative Research Fellow) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter; led qualitative analysis; and drafted the report, with all authors revising it critically for important intellectual content.
Eileen Sutton (https://orcid.org/0000-0003-4105-8471) (Qualitative Researcher and Trial Co-ordinator) operationalised the study; led qualitative analysis; and drafted the report, with all authors revising it critically for important intellectual content.
Alison RG Shaw (https://orcid.org/0000-0002-5907-4608) (Co-lead Qualitative Research) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter; and led qualitative analysis.
Zoe Wilkins (https://orcid.org/0000-0002-9150-8250) (Trial Administrator) operationalised the study.
Julie Clayton (https://orcid.org/0000-0002-2808-3552) (PPI Co-ordinator) operationalised the study; and drafted the report, with all authors revising it critically for important intellectual content.
Amanda Roberts (https://orcid.org/0000-0003-0370-3695) (Patient Representative) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter; and drafted the report, with all authors revising it critically for important intellectual content.
Kirsty Garfield (https://orcid.org/0000-0002-8301-3602) (Health Economist) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter.
Lyn Liddiard (https://orcid.org/0000-0003-0162-0757) (Clinical Studies Officer) operationalised the study.
Tiffany J Barrett (https://orcid.org/0000-0003-1756-696X) (Pharmacist) helped Matthew J Ridd, Stephanie J MacNeill, Jonathan Banks, Alison RG Shaw, Amanda Roberts, Kirsty Garfield, Alastair D Hay, Hywel C Williams, Kim S Thomas, and Miriam Santer with input for designing the study.
J Athene Lane (https://orcid.org/0000-0002-7578-4925) (Trialist) helped Matthew J Ridd, Stephanie J MacNeill, Jonathan Banks, Alison RG Shaw, Amanda Roberts, Kirsty Garfield, Alastair D Hay, Hywel C Williams, Kim S Thomas, and Miriam Santer with input for designing the study.
Helen Baxter (https://orcid.org/0000-0002-3320-2915) (Knowledge Mobilisation) helped Matthew J Ridd, Stephanie J MacNeill, Jonathan Banks, Alison RG Shaw, Amanda Roberts, Kirsty Garfield, Alastair D Hay, Hywel C Williams, Kim S Thomas, and Miriam Santer with input for designing the study; operationalised the study; and drafted the report, with all authors revising it critically for important intellectual content.
Laura Howells (https://orcid.org/0000-0003-4157-7394) (Research Fellow) operationalised the study.
Jodi Taylor (https://orcid.org/0000-0001-7171-8923) (Senior Trial Manager) operationalised the study.
Alastair D Hay (https://orcid.org/0000-0003-3012-375X) (GP) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter.
Hywel C Williams (https://orcid.org/0000-0002-5646-3093) (Consultant Dermatologist) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter.
Kim S Thomas (https://orcid.org/0000-0001-7785-7465) (Professor of Applied Dermatology Research) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter.
Miriam Santer (https://orcid.org/0000-0001-7264-5260) (GP and Principal Investigator) designed the study, with input from Tiffany J Barrett, J Athene Lane and Helen Baxter.
All authors have seen and approved the report and are accountable for all aspects of the work.
Publications
Ridd MJ, Santer M, MacNeill SJ, Sanderson E, Wells S, Webb D. Effectiveness and safety of lotion, cream, gel, and ointment emollients for childhood eczema: a pragmatic, randomised, phase 4, superiority trial. Lancet Child Adolesc Health 2022;6:522–32.
Sutton E, Shaw ARG, Ridd MJ, Santer M, Roberts A, Baxter H, et al. How parents and children evaluate emollients for childhood eczema: a qualitative study. Br J Gen Pract 2022;72:e390–7.
Ridd MJ, Wells S, Edwards L, Santer M, MacNeill S, Sanderson E, et al. Best emollients for eczema (BEE) – comparing four types of emollients in children with eczema: protocol for randomised trial and nested qualitative study. BMJ Open 2019;9:e033387.
Ridd MJ, Roberts A, Grindlay D, Williams HC. Which leave-on emollients are effective and acceptable for children with eczema? BMJ 2019;367:I5882.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to anonymised data may be granted following review.
Patient data
This work uses data provided by patients and collected by the NHS as part of their care and support. Using patient data is vital to improve health and care for everyone. There is huge potential to make better use of information from people’s patient records, to understand more about disease, develop new treatments, monitor safety, and plan NHS services. Patient data should be kept safe and secure, to protect everyone’s privacy, and it’s important that there are safeguards to make sure that it is stored and used responsibly. Everyone should be able to find out about how patient data are used. #datasaveslives You can find out more about the background to this citation here: https://understandingpatientdata.org.uk/data-citation.
Disclaimers
This report presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004;113:832-6. https://doi.org/10.1016/j.jaci.2003.12.591.
- Williams HC. Atopic Dermatitis: The Epidemiology, Causes and Prevention of Atopic Eczema. Cambridge: Cambridge University Press; 2000.
- Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. Br J Dermatol 1998;139:73-6. https://doi.org/10.1046/j.1365-2133.1998.02316.x.
- Ben-Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. Br J Dermatol 2004;150:284-90. https://doi.org/10.1111/j.1365-2133.2004.05776.x.
- Barnetson RS, Rogers M. Childhood atopic eczema. BMJ 2002;324:1376-9. https://doi.org/10.1136/bmj.324.7350.1376.
- Absolon CM, Cottrell D, Eldridge SM, Glover MT. Psychological disturbance in atopic eczema: the extent of the problem in school-aged children. Br J Dermatol 1997;137:241-5. https://doi.org/10.1046/j.1365-2133.1997.18121896.x.
- Levy ML. Atopic dermatitis: understanding the disease and its management. Curr Med Res Opin 2007;23:3091-103. https://doi.org/10.1185/030079907X242593.
- Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract 2006;60:984-92. https://doi.org/10.1111/j.1742-1241.2006.01047.x.
- Emerson RM, Williams HC, Allen BR. What is the cost of atopic dermatitis in preschool children?. Br J Dermatol 2001;144:514-22. https://doi.org/10.1046/j.1365-2133.2001.04077.x.
- Santer M, Rumsby K, Ridd MJ, Francis NA, Stuart B, Chorozoglou M, et al. Adding emollient bath additives to standard eczema management for children with eczema: the BATHE RCT. Health Technol Assess 2018;22. https://doi.org/10.3310/hta22570.
- Moncrieff G, Cork M, Lawton S, Kokiet S, Daly C, Clark C. Use of emollients in dry-skin conditions: consensus statement. Clin Exp Dermatol 2013;38:231-8. https://doi.org/10.1111/ced.12104.
- Ridd M, Purdy S. Exacerbation of atopic eczema in children. BMJ 2009;339.
- Chan JP, Boyd G, Quinn PA, Ridd MJ. Emollient prescribing formularies in England and Wales: a cross-sectional study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2018-022009.
- Wilson L, Smith K. Cost Effective Prescribing of Emollients. PrescQIPP: East Anglia Medicines Information Service; 2015.
- Ridd MJ, Redmond NM, Hollinghurst S, Ball N, Shaw L, Guy R, et al. Choice of Moisturiser for Eczema Treatment (COMET): study protocol for a randomized controlled trial. Trials 2015;16. https://doi.org/10.1186/s13063-015-0830-y.
- Ridd MJ, Garfield K, Gaunt DM, Hollinghurst S, Redmond NM, Powell K, et al. Choice of Moisturiser for Eczema Treatment (COMET): feasibility study of a randomised controlled parallel group trial in children recruited from primary care. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-012021.
- Ridd MJ, Santer M, Thomas KS, Roberts A, MacNeill SJ. How do carers and children with eczema choose their emollient?. Clin Exp Dermatol 2017;42. https://doi.org/10.1111/ced.12982.
- Santer M, Burgess H, Yardley L, Ersser S, Lewis-Jones S, Muller I, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. Br J Gen Pract 2012;62:e261-7. https://doi.org/10.3399/bjgp12X636083.
- Batchelor JM, Ridd MJ, Clarke T, Ahmed A, Cox M, Crowe S, et al. The Eczema Priority Setting Partnership: a collaboration between patients, carers, clinicians and researchers to identify and prioritize important research questions for the treatment of eczema. Br J Dermatol 2013;168:577-82. https://doi.org/10.1111/bjd.12040.
- van Zuuren EJ, Fedorowicz Z, Christensen R, Lavrijsen APM, Arents BWM. Emollients and moisturisers for eczema. Cochrane Database Syst Rev 2017;2. https://doi.org/10.1002/14651858.CD012119.pub2.
- National Institute for Health and Care Research n.d. www.journalslibrary.nihr.ac.uk/programmes/hta/1513007 (accessed 1 November 2021).
- Ridd MJ, Wells S, Edwards L, Santer M, MacNeill S, Sanderson E, et al. Best emollients for eczema (BEE) – comparing four types of emollients in children with eczema: protocol for randomised trial and nested qualitative study. BMJ Open 2019;9. http://doi.org/10.1136/bmjopen-2019-033387.
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol 2004;140:1513-19. https://doi.org/10.1001/archderm.140.12.1513.
- Gaunt DM, Metcalfe C, Ridd M. The Patient-Oriented Eczema Measure in young children: responsiveness and minimal clinically important difference. Allergy 2016;71:1620-5. https://doi.org/10.1111/all.12942.
- Schmitt J, Langan S, Williams HC. European Dermato-Epidemiology Network . What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol 2007;120:1389-98. https://doi.org/10.1016/j.jaci.2007.08.011.
- Bang H, Ni L, Davis CE. Assessment of blinding in clinical trials. Controlled Clinical Trials 2004;25:143-56. https://doi.org/10.1016/j.cct.2003.10.016.
- Stevens KJ, Brazier JE, McKenna SP, Doward LC, Cork MJ. The development of a preference-based measure of health in children with atopic dermatitis. Br J Dermatol 2005;153:372-7. https://doi.org/10.1111/j.1365-2133.2005.06736.x.
- Stevens K. Assessing the performance of a new generic measure of health-related quality of life for children and refining it for use in health state valuation. Appl Health Econ Health Policy 2011;9:157-69. https://doi.org/10.2165/11587350-000000000-00000.
- Stevens K. Valuation of the Child Health Utility 9D Index. PharmacoEconomics 2012;30:729-47. https://doi.org/10.2165/11599120-000000000-00000.
- Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. Br J Dermatol 1998;138:107-13. https://doi.org/10.1046/j.1365-2133.1998.02034.x.
- Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337. https://doi.org/10.1136/bmj.a2390.
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. CONSORT PRO Group . Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309:814-22. https://doi.org/10.1001/jama.2013.879.
- Department of Health and Social Care 2020. www.gov.uk/government/news/public-information-campaign-focuses-on-handwashing (accessed 12 September 2022).
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101. https://doi.org/10.1191/1478088706qp063oa.
- Saunders B, Sim J, Kingstone T, Baker S, Waterfield J, Bartlam B, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893-907. https://doi.org/10.1007/s11135-017-0574-8.
- Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br J Dermatol 2013;169:1326-32. https://doi.org/10.1111/bjd.12590.
- Barbour RS, Barbour M. Evaluating and synthesizing qualitative research: the need to develop a distinctive approach. J Eval Clin Pract 2003;9:179-86. https://doi.org/10.1046/j.1365-2753.2003.00371.x.
- Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. Res Involv Engagem 2017;3. https://doi.org/10.1186/s40900-017-0062-2.
- National Institute for Health and Care Research 2021. www.nihr.ac.uk/documents/briefing-notes-for-researchers-public-involvement-in-nhs-health-and-social-care-research/27371 (accessed 1 November 2021).
- Francis NA, Ridd MJ, Thomas-Jones E, Butler CC, Hood K, Shepherd V, et al. Oral and topical antibiotics for clinically infected eczema in children: a pragmatic randomized controlled trial in ambulatory care. Ann Fam Med 2017;15:124-30. https://doi.org/10.1370/afm.2038.
- Santer M, Ridd MJ, Francis NA, Stuart B, Rumsby K, Chorozoglou M, et al. Emollient bath additives for the treatment of childhood eczema (BATHE): multicentre pragmatic parallel group randomised controlled trial of clinical and cost effectiveness. BMJ 2018;361. https://doi.org/10.1136/bmj.k1332.
- Gilbertson A, Ridd MJ, Sutton E, Liddiard L, Clayton J, Roberts A, et al. Engaging with diverse audiences to raise awareness about childhood eczema: reflections from two community events. Res Involv Engagem 2021;7. https://doi.org/10.1186/s40900-021-00251-8.
- Huang X, O’Connor M, Ke LS, Lee S. Ethical and methodological issues in qualitative health research involving children: a systematic review. Nurs Ethics 2016;23:339-56. https://doi.org/10.1177/0969733014564102.
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67:99-106. https://doi.org/10.1111/j.1398-9995.2011.02719.x.
- Stuart B, Howells L, Chalmers J, . How Often Should Outcomes Be Measured in Eczema Clinical Trials? n.d.
- Spuls PI, Gerbens LAA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. POEM a core instrument to measure symptoms in clinical trials: a HOME statement. Br J Dermatol 2016;176:979-84. https://doi.org/10.1111/bjd.15179.
- Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol 2001;144:104-10. https://doi.org/10.1046/j.1365-2133.2001.03960.x.
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 1995;132:942-9. https://doi.org/10.1111/j.1365-2133.1995.tb16953.x.
- Howells LM, Chalmers JR, Gran S, Ahmed A, Apfelbacher C, Burton T, et al. Development and initial testing of a new instrument to measure the experience of eczema control in adults and children: Recap of atopic eczema (RECAP). Br J Dermatol 2020;183:524-36. https://doi.org/10.1111/bjd.18780.
- Pariser DM, Simpson EL, Gadkari A, Bieber T, Margolis DJ, Brown M, et al. Evaluating patient-perceived control of atopic dermatitis: design, validation, and scoring of the Atopic Dermatitis Control Tool (ADCT). Curr Med Res Opin 2020;36:367-76. https://doi.org/10.1080/03007995.2019.1699516.
- Langan SM, Stuart B, Bradshaw L, Schmitt J, Williams HC, Thomas KS. Measuring long-term disease control in patients with atopic dermatitis: a validation study of well-controlled weeks. J Allergy Clin Immunol 2017;140:1580-6. https://doi.org/10.1016/j.jaci.2017.02.043.
- Santer M, Muller I, Yardley L, Lewis-Jones S, Ersser S, Little P. Parents’ and carers’ views about emollients for childhood eczema: qualitative interview study. BMJ Open 2016;6. https://doi.org/10.1136/bmjopen-2016-011887.
- Djokic-Gallagher J, Rosher P, Walker J, Hart V. Objective and subjective in vivo comparison of two emollient products. Clin Cosmet Investig Dermatol 2012;5:85-91. https://doi.org/10.2147/CCID.S32642.
- Djokic-Gallagher J, Rosher P, Walker J, Sykes K, Hart V. Emollient efficacy and acceptability in the treatment of eczematous dry skin: a double-blind, randomised comparison of two UK-marketed products. J Dermatolog Treat 2016;27:461-6. https://doi.org/10.3109/09546634.2015.1136384.
- Djokic-Gallagher J, Rosher P, Oliveira G, Walker J. A double-blind, randomised study comparing the skin hydration and acceptability of two emollient products in atopic eczema patients with dry skin. Dermatol Ther 2017;7:397-406. https://doi.org/10.1007/s13555-017-0188-z.
- Bland JM, Altman DG. Statistics notes: some examples of regression towards the mean. BMJ 1994;309. https://doi.org/10.1136/bmj.309.6957.780.
- Sturges JE, Hanrahan KJ. Comparing telephone and face-to-face qualitative interviewing: a research note. Qualitat Res 2004;4:107-18. https://doi.org/10.1177/1468794104041110.
- LePoidevin LM, Lee DE, Shi VY. A comparison of international management guidelines for atopic dermatitis. Pediatr Dermatol 2019;36:36-65. https://doi.org/10.1111/pde.13678.
- Hlela C, Lunjani N, Gumedze F, Kakande B, Khumalo NP. Affordable moisturisers are effective in atopic eczema: A randomised controlled trial. S Afr Med J 2015;105:780-4. https://doi.org/10.7196/SAMJnew.8331.
- Tiplica GS, Kaszuba A, Malinauskienė L, Konno P, Boralevi F, Garrigue E, et al. Prevention of flares in children with atopic dermatitis with regular use of an emollient containing glycerol and paraffin: a randomized controlled study. Pediatr Dermatol 2017;34:282-9. https://doi.org/10.1111/pde.13113.
- Tiplica GS, Boralevi F, Konno P, Malinauskiene L, Kaszuba A, Laurens C, et al. The regular use of an emollient improves symptoms of atopic dermatitis in children: a randomized controlled study. J Eur Acad Dermatol Venereol 2018;32:1180-7. https://doi.org/10.1111/jdv.14849.
- Sokolova A, Smith SD. Factors contributing to poor treatment outcomes in childhood atopic dermatitis. Australas J Dermatol 2015;56:252-7. https://doi.org/10.1111/ajd.12331.
- Li Y, Zheng H, Li Y, Li W, Guo X, Lv Z. Parental knowledge of moisturizers and their application to infants with eczema in Hangzhou, China. Medicine 2020;99. https://doi.org/10.1097/MD.0000000000020329.
- Kawakami T, Soma Y. Questionnaire survey of the efficacy of emollients for adult patients with atopic dermatitis. J Dermatol 2011;38:531-5. https://doi.org/10.1111/j.1346-8138.2010.01052.x.
- Lee JH, Jung KE, Lee YB, Kim JE, Kim HS, Lee KH, et al. Use of emollients in atopic dermatitis: a questionnaire survey study. Ann Dermatol 2014;26:528-31. https://doi.org/10.5021/ad.2014.26.4.528.
- Antonijević MD, Owusu-Ware S, Sanchon-Lopez B. Emollient product design: objective measurements of formulation structure, texture and performance, and subjective assessments of user acceptability. Clin Exp Dermatol 2018;43:423-9. https://doi.org/10.1111/ced.13364.
- Rowley GG, MacNeill SJ, Ridd MJ. Emollient satisfaction questionnaire: validation study in children with eczema. Clin Exp Dermatol 2022;47:1337-45. https://doi.org/10.1111/ced.15189.
- Oakley R, Lawson S. Views on unwanted effects of leave-on emollients and experiences surrounding their incidence. Dermatol Nurs 2016;15:38-43.
- Capozza K, Schwartz A. Does it work and is it safe? Parents’ perspectives on adherence to medication for atopic dermatitis. Pediatr Dermatol 2020;37:58-61. https://doi.org/10.1111/pde.13991.
- Bhanot A, Huntley A, Ridd MJ. Adverse events from emollient use in eczema: a restricted review of published data. Dermatol Ther 2019;9:193-208. https://doi.org/10.1007/s13555-019-0284-3.
- Hon KLE, Ching GK, Leung TF, Choi CY, Lee KKC, Ng PC. Estimating emollient usage in patients with eczema. Clin Exp Dermatol 2010;35:22-6. https://doi.org/10.1111/j.1365-2230.2009.03341.x.
- Choi JY, Dawe R, Ibbotson S, Fleming C, Doney A, Foerster J. Quantitative analysis of topical treatments in atopic dermatitis: unexpectedly low use of emollients and strong correlation of topical corticosteroid use both with depression and concurrent asthma. Br J Dermatol 2020;182:1017-25. https://doi.org/10.1111/bjd.18265.
- Langan SM, Schmitt J, Williams HC, Smith S, Thomas KS. How are eczema ‘flares’ defined? A systematic review and recommendation for future studies. Br J Dermatol 2014;170:548-56. https://doi.org/10.1111/bjd.12747.
- Giordano-Labadie F, Cambazard F, Guillet G, Combemale P, Mengeaud V. Evaluation of a new moisturizer (Exomega milk) in children with atopic dermatitis. J Dermatolog Treat 2006;17:78-81. https://doi.org/10.1080/09546630600552216.
- Grimalt R, Mengeaud V, Cambazard F. Study Investigators’ Group. The steroid-sparing effect of an emollient therapy in infants with atopic dermatitis: a randomized controlled study. Dermatology 2007;214:61-7. https://doi.org/10.1159/000096915.
- Teasdale E, Muller I, Sivyer K, Ghio D, Greenwell K, Wilczynska S, et al. Views and experiences of managing eczema: systematic review and thematic synthesis of qualitative studies [published online ahead of print August 14 2020]. Br J Dermatol 2020. https://doi.org/10.1111/bjd.19299.
- Magin P, Adams J, Heading G, Pond D. ‘Perfect skin’, the media and patients with skin disease: a qualitative study of patients with acne, psoriasis and atopic eczema. Aust J Prim Health 2011;17:181-5. https://doi.org/10.1071/PY10047.
- Gore C, Johnson RJ, Caress AL, Woodcock A, Custovic A. The information needs and preferred roles in treatment decision-making of parents caring for infants with atopic dermatitis: a qualitative study. Allergy 2005;60:938-43. https://doi.org/10.1111/j.1398-9995.2005.00776.x.
- Noerreslet M, Jemec GB, Traulsen JM. Involuntary autonomy: patients’ perceptions of physicians, conventional medicines and risks in the management of atopic dermatitis. Soc Sci Med 2009;69:1409-15. https://doi.org/10.1016/j.socscimed.2009.08.036.
- Krejci-Manwaring J, Tusa MG, Carroll C, Carmacho F, Kaur M, Carr D, et al. Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol 2007;56:211-6. https://doi.org/10.1016/j.jaad.2006.05.073.
- Iio M, Hamaguchi M, Nagata M, Yoshida K. Stressors of school-age children with allergic diseases: a qualitative study. J Pediatr Nurs 2018;42:e73-78. https://doi.org/10.1016/j.pedn.2018.04.009.
- Roje M, Rezo I, Flander GB. Quality of life and psychosocial needs of children suffering from chronic skin diseases. Alcohol Psychiatry Res 2016;52:133-48. https://doi.org/10.20471/apr.2016.52.02.04.
- Xie QW, Chan CL, Chan CH. The wounded self-lonely in a crowd: a qualitative study of the voices of children living with atopic dermatitis in Hong Kong. Health Soc Care Community 2020;28:862-73. https://doi.org/10.1111/hsc.12917.
- Wake EV, Batchelor J, Lawton S, Thomas KS, Harrison EF, Cowdell FC. U.K. Dermatology Clinical Trials Network’s CLOTHES Trial Team. The views of children and young people on the use of silk garments for the treatment of eczema: a nested qualitative study within the CLOTHing for the relief of Eczema Symptoms (CLOTHES) randomized controlled trial. Br J Dermatol 2018;178:183-90. https://doi.org/10.1111/bjd.15909.
- Kosse RC, Bouvy ML, Daanen M, de Vries TW, Koster ES. Adolescents’ perspectives on atopic dermatitis treatment-experiences, preferences, and beliefs. JAMA Dermatol 2018;154:824-7. https://doi.org/10.1001/jamadermatol.2018.1096.
- Ghio D, Muller I, Greenwell K, . ‘It’s like the bad guy in a movie who just doesn’t die’: a qualitative exploration of young people’s adaptation to eczema and implications for self-care. Br J Dermatol 2020;182:112-8. https://doi.org/10.1111/bjd.18046.
- Greenwell K, Ghio D, Muller I, Roberts A, McNiven A, Lawton S, et al. Taking charge of eczema self-management: a qualitative interview study with young people with eczema. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2020-044005.
- Teasdale E, Sivyer K, Muller I, Ghio D, Roberts A, Lawton S, et al. Children’s views and experiences of treatment adherence and parent/child co-management in eczema: a qualitative study. Children 2021;8. https://doi.org/10.3390/children8020158.
- Chalmers JR, Haines RH, Bradshaw LE, Montgomery AA, Thomas KS, Brown SJ, et al. Daily emollient during infancy for prevention of eczema: the BEEP randomised controlled trial. Lancet 2020;395:962-72. https://doi.org/10.1016/S0140-6736(19)32984-8.
- Skjerven HO, Rehbinder EM, Vettukattil R, LeBlanc M, Granum B, Haugen G, et al. Skin emollient and early complementary feeding to prevent infant atopic dermatitis (PreventADALL): a factorial, multicentre, cluster-randomised trial. Lancet 2020;395:951-61. https://doi.org/10.1016/S0140-6736(19)32983-6.
- Kelleher MM, Cro S, Cornelius V, Lodrup Carlsen KC, Skjerven HO, Rehbinder EM, et al. Skin care interventions in infants for preventing eczema and food allergy. Cochrane Database Syst Rev 2021;2. https://doi.org/10.1002/14651858.CD013534.pub2.
- Halling-Overgaard AS, Kezic S, Jakasa I, Engebretsen KA, Maibach H, Thyssen JP. Skin absorption through atopic dermatitis skin: a systematic review. Br J Dermatol 2017;177:84-106. https://doi.org/10.1111/bjd.15065.
- Radhakrishna N, Prickett S, Phan T, Rolland JM, Puy R, O’Hehir RE. Anaphylaxis to oats after cutaneous sensitization by oatmeal in skin products used for the treatment of atopic dermatitis. J Allergy Clin Immunol Pract 2016;4:152-3. https://doi.org/10.1016/j.jaip.2015.07.005.
- Ho VPY, Ma E, Liew HM, Ng MSY, Koh MJA. Comparing the potential for irritation of a ceramide-based moisturizer with a urea-based moisturizer for pediatric atopic dermatitis. Dermatol Ther 2020;10:807-13. https://doi.org/10.1007/s13555-020-00388-6.
- Tan WP, Suresh S, Tey HL, Chiam LY, Goon AT. A randomized double-blind controlled trial to compare a triclosan-containing emollient with vehicle for the treatment of atopic dermatitis. Clin Exp Dermatol 2010;35:e109-12. https://doi.org/10.1111/j.1365-2230.2009.03719.x.
- Johnston GA, Bilbao RM, Graham-Brown RA. The use of complementary medicine in children with atopic dermatitis in secondary care in Leicester. Br J Dermatol 2003;149:566-71. https://doi.org/10.1046/j.1365-2133.2003.05471.x.
- Yu Y, Dunaway S, Champer J, Kim J, Alikhan A. Changing our microbiome: probiotics in dermatology. Br J Dermatol 2020;182:39-46. https://doi.org/10.1111/bjd.18088.
- Chandan N, Rajkumar JR, Shi VY, Lio PA. A new era of moisturizers. J Cosmetic Dermatol 2021;20:2425-30. https://doi.org/10.1111/jocd.14217.
- Ridd MJ, Gaunt DM, Guy RH, Redmond NM, Garfield K, Hollinghurst S, et al. Comparison of patient (POEM), observer (EASI, SASSAD, TIS) and corneometry measures of emollient effectiveness in children with eczema: findings from the COMET feasibility trial. Br J Dermatol 2018;179:362-70. https://doi.org/10.1111/bjd.16475.
- Schmitt J, Spuls P, Boers M, Thomas K, Chalmers J, Roekevisch E, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012;67:1111-7. https://doi.org/10.1111/j.1398-9995.2012.02874.x.
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014;134:800-7. https://doi.org/10.1016/j.jaci.2014.07.043.
- Thomas KS, Apfelbacher CA, Chalmers JR, Simpson E, Spuls PI, Gerbens LAA, et al. Recommended core outcome instruments for health-related quality of life, long-term control and itch intensity in atopic eczema trials: results of the HOME VII consensus meeting [published online ahead of print November 11 2020]. Br J Dermatol 2020. https://doi.org/10.1111/bjd.19673.
- Ng SY, Begum S, Chong SY. Does order of application of emollient and topical corticosteroids make a difference in the severity of atopic eczema in children?. Pediatr Dermatol 2016;33:160-4. https://doi.org/10.1111/pde.12758.
- Muller I, Stuart B, Sach T, Hooper J, Wilczynska S, Steele M, et al. Supporting self-care for eczema: protocol for two randomised controlled trials of ECO (Eczema Care Online) interventions for young people and parents/carers. BMJ Open 2021;11. https://doi.org/10.1136/bmjopen-2020-045583.
- Jacquet L, Gaunt DM, Garfield K, Ridd MJ. Diagnosis, assessment, and treatment of childhood eczema in primary care: cross-sectional study. BJGP Open 2017;1. https://doi.org/10.3399/bjgpopen17X100821.
- Hon KL, Kung JSC, Tsang KYC, Yu JWS, Lee VW, Leung TF. Emollient acceptability in childhood atopic dermatitis: not all emollients are equal. Curr Pediatr Rev 2018;14:117-22. https://doi.org/10.2174/1573396313666170605080034.
- Powell K, Le Roux E, Banks J, Ridd MJ. GP and parent dissonance about the assessment and treatment of childhood eczema in primary care: a qualitative study. BMJ Open 2018;8. https://doi.org/10.1136/bmjopen-2017-019633.
- Hon KL, Wang SS, Pong NH, Leung TF. The ideal moisturizer: a survey of parental expectations and practice in childhood-onset eczema. J Dermatolog Treat 2013;24:7-12. https://doi.org/10.3109/09546634.2012.672713.
- Joergensen KM, Jemec GBE. Use of moisturizers among Danish atopic dermatitis patients-which perceived product characteristics associate with long-term adherence?. J Dermatol Treat 2018;29:116-22. https://doi.org/10.1080/09546634.2017.1358803.
- Xu S, Kwa M, Lohman ME, Evers-Meltzer R, Silverberg JI. Consumer preferences, product characteristics, and potentially allergenic ingredients in best-selling moisturizers. JAMA Dermatol 2017;153:1099-105. https://doi.org/10.1001/jamadermatol.2017.3046.
- BNF for Children 2021–2022. London: BMJ Group; 2021.
- Aqueous Cream: May Cause Skin Irritation, Particularly in Children with Eczema, Possibly due to Sodium Lauryl Sulfate Content. London: MHRA; 2014.
- Granger BB, Britten N, Swedberg K, Ekman I. Dumping adherence: a person-centred response for primary care. Fam Pract 2020;37:862-4. https://doi.org/10.1093/fampra/cmaa060.
- Powell K, Le Roux E, Banks JP, Ridd MJ. Developing a written action plan for children with eczema: a qualitative study. Br J Gen Pract 2018;68:e81-e89. https://doi.org/10.3399/bjgp17X693617.
- Le Roux E, Powell K, Banks JP, Ridd MJ. GPs’ experiences of diagnosing and managing childhood eczema: a qualitative study in primary care. Br J Gen Pract 2018;68:e73-e80. https://doi.org/10.3399/bjgp18X694529.
- Buick F, Blackman D, O’Flynn J, O’Donnell M, West D. Effective practitioner–scholar relationships – lessons from a coproduction partnership. Public Admin Rev 2016;76:35-47. https://doi.org/10.1111/puar.12481.
- Batalden M, Batalden P, Margolis P, Seid M, Armstrong G, Opipari-Arrigan L, et al. Coproduction of healthcare service. BMJ Qual Saf 2016;25:509-17. https://doi.org/10.1136/bmjqs-2015-004315.
- Heaton J, Day J, Britten N. Collaborative research and the co-production of knowledge for practice: an illustrative case study. Implement Sci 2016;11. https://doi.org/10.1186/s13012-016-0383-9.
- Checkland K, Harrison S, Snow S, Coleman A, McDermott I. Understanding the work done by NHS commissioning managers: an exploration of the microprocesses underlying day-to-day sensemaking in UK primary care organisations. J Health Organ Manag 2013;27:149-70. https://doi.org/10.1108/14777261311321752.
- Ridd MJ, Roberts A, Grindlay D, Williams HC. Which emollients are effective and acceptable for eczema in children?. BMJ 2019;367. https://doi.org/10.1136/bmj.l5882.
- Wenger E. Communities of Practices: Learning, Meaning and Identity. Cambridge: Cambridge University Press; 2000.
- Nonaka I. A dynamic theory of organizational knowledge creation. Organ Sci 1994;5:14-37. https://doi.org/10.1287/orsc.5.1.14.
- legislation.gov.uk n.d. www.legislation.gov.uk/ukpga/2018/12/contents/enacted (accessed 11 September 2022).
Appendix 1 Protocol amendments
Record of protocol version numbers and amendments.
| Version | Notes | |
|---|---|---|
| Number | Date | |
| 1.0 | 21 March 2017 | Submitted for approval (March 2017) and approval received from REC, MHRA and HRA |
| 2.0 | 27 June 2017 | Title page: ISRCTN, NHS REC and NIHR portfolio numbers added |
| 10.3: clarification of eligibility confirmation | ||
| 10.6: ‘blinding to treatment allocation’ table amended to reflect changes in research team/processes to minimise un-blinding of TMG members, in accordance with TSC/DMC recommendation | ||
| 12.3: clarification that first set of interviews will be with participants during their first 4 weeks in the study, not during the first 4 weeks of the life of the trial itself | ||
| 19.2: clarity to TSC/DMC composition/roles | ||
| 14.3: clarification about who makes decisions regarding causality of adverse events/reactions | ||
| 3.0 | 3 August 2017 | Clarification that screening POEM must be within 28 days of recruitment. Removal of signature page to separate document |
| 4.0 | 3 November 2017 | Amendment to the intervention, from four specific emollient, to type of emollient. Correction of minor typos. Clarification of safety reporting section. Update to ‘Timetable and milestones’ to reflect delayed start to internal pilot |
| 5.0 | 1 August 2018 | Change ‘Bristol CCG’ to ‘Bristol, North Somerset and South Gloucestershire CCG’ to reflect merger/name change |
| Changes to blinding arrangements and removal of reference to ‘Ms Jameson’, former CAPC PPI&E co-ordinator who was never a TMG member and has subsequently left | ||
| Update to section 19.2 (Oversight committees) to describe separate TSC and DMC created at request of funder after approval of protocol 4.0 | ||
| Other minor grammatical/style changes/corrections | ||
| 6.0 | 10 June 2019 | Updated references to timelines throughout to reflect 38-month recruitment and follow-up/50-month total study duration. Insertion of paragraph on participant communication (section 10.8, Participant stipends and communication). Replace Avon Primary Care Research Collaboration logo with Bristol, North Somerset and South Gloucestershire CCG logo. Replace any reference to blind, blinded or blinding with masked or masking. Extra information for parents of study participants in order to bring study in-line with the EU General Data Protection Regulations 2018. 123 Minor changes to titles/postal addresses |
| 7.0 | 19 November 2019 | Updated references to timelines throughout to reflect 34-month recruitment and follow-up/46-months total study duration. Additional £10 voucher for participants around 52 weeks. Updated Clinical Trials Unit from Bristol Randomised Trial Collaboration to Bristol Trials Centre, following merger and new umbrella name; also updated logo |
Appendix 2 Trial supplementary tables and figures
Participant recruitment and baseline characteristics
| Age and sex | Eligible (N = 9437) | Not eligible (N = 2980) |
|---|---|---|
| Mean (SD) age, years | 5.32 (3.17) | 6.12 (3.16) |
| Female, n (%) | 4432 (47.0) | 1395 (46.8) |
| Age and sex | Responded to invitation (N = 1650) | Did not respond (N = 7787) | |
|---|---|---|---|
| Agreed to further screening (N = 888) | Declined further screening (N = 762) | ||
| Mean (SD) age, years | 4.91 (3.15) | 5.50 (3.20) | 5.35 (3.17) |
| Female, n (%) | 405 (45.6) | 348 (45.7) | 3679 (47.3) |
| Age and sex | Potentially eligible and attended baseline visit (n/N = 570/570) | Expressed an interest in the study but were excluded before baseline visit (N = 340) | ||
|---|---|---|---|---|
| Ineligible (n/N = 92/92) | Declined (n/N = 231/233) | Eligible but visit not booked (n/N = 15/15) | ||
| Mean (SD) age, years | 4.86 (3.24) | 5.22 (3.18) | 5.11 (3.20) | 5.93 (2.66) |
| Female, n (%) | 259 (45.44) | 43 (46.74) | 107 (47.19) | 7 (46.67) |
| Characteristics | Agreed to baseline visit (n/N = 570/570) | Declined baseline visit (n/N = 231/233) | Consented at baseline visit (n/N = 550/550) | Did not consent at baseline visit (n/N = 20/20) |
|---|---|---|---|---|
| Mean (SD) age, years | 4.86 (3.24) | 5.11 (3.20) | 4.87 (3.25) | 4.75 (3.04) |
| Female, n (%) | 259 (45.44) | 107 (47.19) | 253 (46.00) | 6 (30.00) |
| POEM scores (points), mean (SD) | 11.05 (5.43) | 11.56 (6.35) | 11.09 (5.44) | 9.75 (5.08) |
FIGURE 8.
Distribution of POEM scores at baseline.
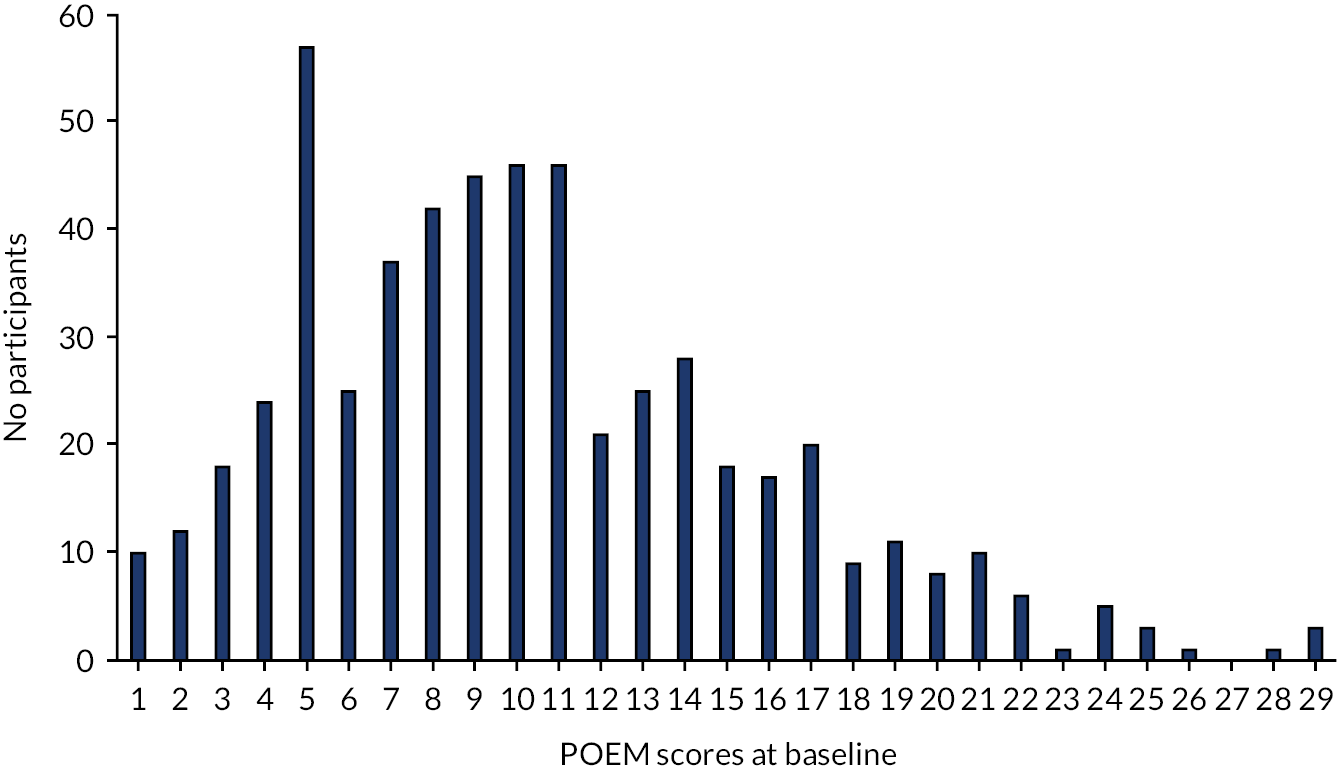
| Variable | Treatment group, n (%) | ||||
|---|---|---|---|---|---|
| Lotion (N = 137) | Cream (N = 140) | Gel (N = 135) | Ointment (N = 138) | Total (N = 550) | |
| Stratification variable | |||||
| Centre | |||||
| Bristol | 54 (39.42) | 57 (40.71) | 56 (41.48) | 54 (39.13) | 221 (40.18) |
| Nottingham/Lincoln | 45 (32.85) | 47 (33.57) | 42 (31.11) | 46 (33.33) | 180 (32.73) |
| Southampton | 38 (27.74) | 36 (25.71) | 37 (27.41) | 38 (27.54) | 149 (27.09) |
| Minimisation variables | |||||
| Age (years) | |||||
| < 2 | 22 (16.06) | 26 (18.57) | 22 (16.30) | 24 (17.39) | 94 (17.09) |
| ≥ 2 | 115 (83.94) | 114 (81.43) | 113 (83.70) | 114 (82.61) | 456 (82.91) |
| POEM scores (points) | |||||
| Mild (3–7) | 43 (31.39) | 46 (32.86) | 40 (29.63) | 42 (30.43) | 171 (31.09) |
| Moderate to very severe (8–28) | 94 (68.61) | 94 (67.14) | 95 (70.37) | 96 (69.57) | 379 (68.91) |
| Categorised scores | Treatment group, n (%) | Overall, N | ||||
|---|---|---|---|---|---|---|
| Lotion (N = 137) | Cream (N = 140) | Gel (N = 135) | Ointment (N = 138) | Total (N = 550) | ||
| POEM | ||||||
| Clear or almost clear (0–2) | 13 (9.49) | 8 (5.71) | 10 (7.46) | 9 (6.52) | 40 (7.29) | 549 |
| Mild (3–7) | 49 (35.77) | 53 (37.86) | 39 (29.10) | 44 (31.88) | 185 (33.70) | |
| Moderate (8–16) | 66 (48.18) | 62 (44.29) | 69 (51.49) | 69 (50.00) | 266 (48.45) | |
| Severe (17–24) | 8 (5.84) | 17 (12.14) | 15 (11.19) | 13 (9.42) | 53 (9.65) | |
| Very severe (25–28) | 1 (0.73) | 0 (0) | 1 (0.75) | 3 (2.17) | 5 (0.91) | |
| EASI | ||||||
| Clear or almost clear | 15 (11.11) | 16 (11.51) | 13 (9.77) | 20 (14.71) | 64 (11.79) | 543 |
| Mild | 86 (63.70) | 94 (67.63) | 80 (60.15) | 87 (63.97) | 347 (63.90) | |
| Moderate | 31 (22.96) | 26 (18.71) | 39 (29.32) | 23 (16.91) | 119 (21.92) | |
| Severe or very severe | 3 (2.22) | 3 (2.16) | 1 (0.75) | 6 (4.41) | 13 (2.39) | |
| Characteristics | Treatment group, n (%) | Overall, N | ||||
|---|---|---|---|---|---|---|
| Lotion (N = 137) | Cream (N = 140) | Gel (N = 135) | Ointment (N = 138) | Total (N = 550) | ||
| Employment status | ||||||
| Full-time paid work (≥ 30 hours each week) | 1 (0.73) | 6 (4.29) | 5 (3.70) | 4 (2.90) | 16 (2.91) | 550 |
| Part-time paid work (< 30 hours each week) | 7 (5.11) | 21 (15.00) | 10 (7.41) | 8 (5.80) | 46 (8.36) | |
| Full-time education at school, college or university | 21 (15.33) | 0 (0) | 7 (5.19) | 20 (14.49) | 48 (8.73) | |
| Unemployed | 1 (0.73) | 0 (0) | 0 (0) | 0 (0) | 1 (0.18) | |
| Permanently sick or disabled | 0 (0) | 0 (0) | 1 (0.74) | 1 (0.72) | 2 (0.36) | |
| Fully retired from work | 1 (0.73) | 0 (0) | 3 (2.22) | 3 (2.17) | 7 (1.27) | |
| Looking after the home | 1 (0.73) | 1 (0.71) | 2 (1.48) | 1 (0.72) | 5 (0.91) | |
| Self-employed | 67 (48.91) | 70 (50.00) | 71 (52.59) | 64 (46.38) | 272 (49.45) | |
| Doing something else | 38 (27.74) | 42 (30.00) | 36 (26.67) | 37 (26.81) | 153 (27.82) | |
| Highest qualification or training | ||||||
| Degree (or equivalent) | 4 (2.92) | 2 (1.43) | 6 (4.48) | 4 (2.90) | 16 (2.91) | 549 |
| Diploma (or equivalent) | 2 (1.46) | 2 (1.43) | 1 (0.75) | 7 (5.07) | 12 (2.19) | |
| A Level | 2 (1.46) | 5 (3.57) | 2 (1.49) | 2 (1.45) | 11 (2.00) | |
| GCSE/O level | 5 (3.65) | 3 (2.14) | 7 (5.22) | 1 (0.72) | 16 (2.91) | |
| Do not wish to answer | 20 (14.6) | 12 (8.57) | 20 (14.93) | 15 (10.87) | 67 (12.20) | |
| NVQ Levels 1–3/GNVQ | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| NVQ Levels 4–5, HNC, HND | 10 (7.30) | 10 (7.14) | 10 (7.46) | 14 (10.14) | 44 (8.01) | |
| Other vocational/work-related qualifications (e.g. apprenticeship, RSA/OCR, BTEC/Edexcel) | 10 (7.30) | 9 (6.43) | 15 (11.19) | 15 (10.87) | 49 (8.93) | |
| None | 16 (11.68) | 18 (12.86) | 9 (6.72) | 17 (12.32) | 60 (10.93) | |
| Other (not specified) | 68 (49.64) | 79 (56.43) | 64 (47.76) | 63 (45.65) | 274 (49.91) | |
| Home ownership | ||||||
| No | 31 (22.63) | 35 (25.00) | 31 (22.96) | 34 (24.64) | 131 (23.82) | 550 |
| Own it outright | 0 (0) | 1 (0.71) | 1 (0.74) | 5 (3.62) | 7 (1.27) | |
| Buying it with the help of a mortgage or loan | 95 (69.34) | 93 (66.43) | 94 (69.63) | 85 (61.59) | 367 (66.73) | |
| Pay part rent and part mortgage (shared ownership) | 11 (8.03) | 11 (7.86) | 9 (6.67) | 14 (10.14) | 45 (8.18) | |
| Treatments | Treatment group, n (%) | Overall, N | ||||
|---|---|---|---|---|---|---|
| Lotion (N = 137) | Cream (N = 140) | Gel (N = 135) | Ointment (N = 138) | Total (N = 550) | ||
| Topical corticosteroids | 108 (78.83) | 98 (70.00) | 103 (76.30) | 106 (76.81) | 415 (75.45) | 550 |
| Bath additives | 59 (43.07) | 67 (47.86) | 61 (45.19) | 61 (44.20) | 248 (45.09) | 550 |
| Emollients | ||||||
| Lotions | 548 | |||||
| Never used | 36 (26.47) | 36 (25.90) | 46 (34.07) | 44 (31.88) | 162 (29.56) | |
| Currently using | 28 (20.59) | 26 (18.71) | 29 (21.48) | 28 (20.29) | 111 (20.26) | |
| Used previously | 62 (45.59) | 61 (43.88) | 52 (38.52) | 59 (42.75) | 234 (42.70) | |
| Don’t know | 10 (7.35) | 16 (11.51) | 8 (5.93) | 7 (5.07) | 41 (7.48) | |
| Cream | 549 | |||||
| Never used | 5 (3.68) | 7 (5.00) | 4 (2.96) | 4 (2.90) | 20 (3.64) | |
| Currently using | 90 (66.18) | 93 (66.43) | 80 (59.26) | 93 (67.39) | 356 (64.85) | |
| Used previously | 38 (27.94) | 40 (28.57) | 49 (36.30) | 36 (26.09) | 163 (29.69) | |
| Don’t know | 3 (2.21) | 0 (0.00) | 2 (1.48) | 5 (3.62) | 10 (1.82) | |
| Gels | 543 | |||||
| Never used | 84 (62.22) | 79 (56.83) | 88 (65.67) | 96 (71.11) | 347 (63.90) | |
| Currently using | 14 (10.37) | 11 (7.91) | 7 (5.22) | 10 (7.41) | 42 (7.73) | |
| Used previously | 24 (17.78) | 29 (20.86) | 28 (20.90) | 13 (9.63) | 94 (17.31) | |
| Don’t know | 13 (9.63) | 20 (14.39) | 11 (8.21) | 16 (11.85) | 60 (11.05) | |
| Ointments | 548 | |||||
| Never used | 28 (20.59) | 40 (28.78) | 40 (29.63) | 37 (26.81) | 145 (26.46) | |
| Currently using | 41 (30.15) | 35 (25.18) | 40 (29.63) | 39 (28.26) | 155 (28.28) | |
| Used previously | 57 (41.91) | 51 (36.69) | 50 (37.04) | 52 (37.68) | 210 (38.32) | |
| Don’t know | 10 (7.35) | 13 (9.35) | 5 (3.70) | 10 (7.25) | 38 (6.93) | |
| Emollient type | Treatment group, mean (SD), points | Overall, N | ||||
|---|---|---|---|---|---|---|
| Lotion (N = 137) | Cream (N = 140) | Gel (N = 135) | Ointment (N = 138) | All groups (N = 550) | ||
| Opinions about moisturiser effectiveness | ||||||
| Lotions | 2.90 (1.23) | 2.84 (1.24) | 2.95 (1.06) | 3.16 (1.19) | 2.96 (1.18) | 548 |
| Creams | 3.54 (1.09) | 3.62 (1.09) | 3.37 (1.15) | 3.51 (1.11) | 3.51 (1.11) | 459 |
| Gels | 3.20 (1.35) | 3.15 (1.21) | 2.93 (1.10) | 3.04 (1.33) | 3.08 (1.23) | 545 |
| Ointments | 3.45 (1.35) | 3.62 (1.07) | 3.57 (1.22) | 3.40 (1.42) | 3.50 (1.28) | 548 |
| Opinions about moisturiser acceptability | ||||||
| Lotions | 3.78 (1.23) | 3.85 (1.25) | 4.07 (1.01) | 3.93 (1.17) | 3.91 (1.17) | 549 |
| Creams | 4.05 (1.13) | 4.13 (0.96) | 4.10 (1.06) | 4.14 (1.00) | 4.10 (1.04) | 549 |
| Gels | 3.70 (1.12) | 3.80 (1.18) | 3.91 (1.08) | 3.98 (1.17) | 3.84 (1.14) | 548 |
| Ointments | 3.22 (1.42) | 3.17 (1.35) | 3.09 (1.41) | 3.31 (1.44) | 3.20 (1.40) | 548 |
Intervention receipt
| Number of days | Recruiting centre | Total (N = 550) | ||
|---|---|---|---|---|
| Bristol (N = 223) | Nottingham (N = 178) | Southampton (N = 149) | ||
| Time between baseline and randomisation, days | ||||
| Median (IQR) | 0 (0–1) | 1 (0–1) | 1 (1–1) | 1 (0–1) |
| Minimum, maximum | 0, 5 | 0, 4 | 0, 5 | 0, 5 |
| Time between randomisation and reported first use of emollient, days | ||||
| Median (IQR) | 5 (3–7) | 5 (2–7) | 4 (3–6) | 4 (3–7) |
| Minimum, maximum | 0, 43 | 0, 31 | 0, 45 | 0, 45 |
| > 7 days between randomisation and first reported use of emollient, n (%) | 51 (23) | 30 (17) | 27 (18) | 108 (20) |
Participant follow-up
| Group (number of withdrawals) | Daya | Main reason given for withdrawal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Study moisturiser not working/effective | Adverse reaction to study moisturiser | Disliked moisturiser given | Just simply changed my mind | Do not have enough time | My child’s skin has improved | Reason not given | Other | ||
| Lotion (8) | 26 | • | |||||||
| 28 | • | ||||||||
| 32 | • | ||||||||
| 41 | • | ||||||||
| 77 | b | ||||||||
| 104 | • | ||||||||
| 104 | c | ||||||||
| 218 | d | ||||||||
| Cream (5) | 50 | • | |||||||
| 97 | • | ||||||||
| 105 | • | ||||||||
| 111 | • | ||||||||
| 181 | • | ||||||||
| Gel (4) | 50 | • | |||||||
| 50 | • | ||||||||
| 55 | • | ||||||||
| 70 | • | ||||||||
| Ointment | 24 | • | |||||||
| (12) | 35 | • | |||||||
| 43 | • | ||||||||
| 86 | • | ||||||||
| 81 | e | ||||||||
| 91 | • | ||||||||
| 99 | • | ||||||||
| 103 | f | ||||||||
| 105 | • | ||||||||
| 119 | g | ||||||||
| 290 | • | ||||||||
| 221 | • | ||||||||
| Total | 3 | 6 | 2 | 4 | 6 | 1 | 1 | 6 | |
FIGURE 9.
Patient Orientated Eczema Measure data completeness up to 16 weeks by group.

| Outcome measure | Week, n (%) of those randomised | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 | 6 | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 | |
| Questionnaires returned | 550 (100) | 474 (86.18) | 459 (83.45) | 451 (82.00) | 431 (78.36) | 426 (77.45) | 398 (72.36) | 386 (70.18) | 363 (66.00) | 369 (67.09) | 361 (65.64) | 355 (64.55) | 350 (63.64) | 358 (65.09) | 363 (66.00) |
| POEM | 549 (99.82) | 474 (86.18) | 459 (83.45) | 451 (82.00) | 431 (78.36) | 426 (77.45) | 398 (72.36) | 386 (70.18) | 363 (66.00) | 369 (67.09) | 361 (65.64) | 355 (64.55) | 349 (63.45) | 356 (64.73) | 362 (65.82) |
| Eczema treatments | 472 (85.82) | 456 (82.91) | 451 (82.00) | 429 (78.00) | 422 (76.73) | 395 (71.82) | 385 (70.00) | 362 (65.82) | 365 (66.36) | 357 (64.91) | 352 (64.00) | 349 (63.45) | 355 (64.55) | 363 (66.00) | |
| Eczema pain and bother | 549 (99.82) | 474 (86.18) | 450 (81.82) | 430 (78.18) | 418 (76.00) | ||||||||||
| Data on personal costs (non-EMR) | 472 (85.82) | 450 (81.82) | 428 (77.82) | 419 (76.18) | 397 (72.18) | 385 (70.00) | 361 (65.64) | 366 (66.55) | 359 (65.27) | 354 (64.36) | 350 (63.64) | 358 (65.09) | 362 (65.82) | ||
| DFI | 543 (98.73) | 419 (76.18) | 362 (65.82 | ||||||||||||
| ADQoL | 550 (100) | 459 (83.45) | 417 (75.82) | 360 (65.45) | |||||||||||
| CHU-9D | 550 (100) | 458 (83.27) | 415 (75.45) | 359 (65.27 | |||||||||||
| EASI | 548 (99.64) | 496 (90.18) | |||||||||||||
| Satisfaction | 414 (75.27) | ||||||||||||||
Primary outcome
| Week | Treatment group, n, mean (SD) | ||||
|---|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | Overall | |
| Baseline | 137, 8.67 (5.15) | 140, 9.34 (5.25) | 134, 9.80 (5.42) | 138, 9.50 (5.97) | 549, 9.32 (5.46) |
| 1 | 124, 7.74 (4.72) | 132, 9.36 (5.27) | 123, 10.05 (5.70) | 117, 8.62 (5.91) | 496, 8.95 (5.46) |
| 2 | 126, 7.39 (4.61) | 132, 9.60 (5.33) | 118, 8.31 (5.50) | 113, 8.40 (5.72) | 489, 8.44 (5.34) |
| 3 | 119, 7.55 (4.96) | 136, 8.76 (5.27) | 120, 8.36 (5.53) | 109, 8.60 (6.80) | 484, 8.32 (5.65) |
| 4 | 121, 7.17 (5.16) | 128, 7.95 (5.02) | 121, 7.60 (5.34) | 104, 7.78 (6.42) | 474, 7.63 (5.46) |
| 5 | 120, 6.76 (4.90) | 127, 7.58 (5.19) | 111, 7.19 (5.68) | 104, 7.31 (6.08) | 462, 7.21 (5.44) |
| 6 | 121, 6.76 (5.28) | 128, 7.77 (5.47) | 111, 7.38 (5.90) | 99, 7.30 (6.16) | 459, 7.31 (5.68) |
| 7 | 119, 6.93 (5.38) | 131, 7.36 (5.19) | 112, 6.90 (5.62) | 98, 7.21 (6.29) | 460, 7.11 (5.58) |
| 8 | 118, 6.97 (5.33) | 129, 7.10 (5.40) | 105, 7.32 (6.09) | 99, 6.93 (6.11) | 451, 7.08 (5.69) |
| 9 | 114, 7.01 (5.54) | 123, 6.68 (5.03) | 113, 7.27 (5.94) | 97, 6.58 (5.93) | 447, 6.89 (5.59) |
| 10 | 109, 7.25 (5.39) | 122, 6.89 (5.07) | 105, 6.93 (5.74) | 95, 6.32 (5.90) | 431, 6.87 (5.50) |
| 11 | 115, 6.78 (5.60) | 121, 7.23 (5.43) | 101, 6.72 (5.73) | 101, 6.36 (6.43) | 438, 6.79 (5.78) |
| 12 | 117, 6.35 (5.56) | 117, 7.24 (5.72) | 104, 7.04 (5.89) | 93, 6.09 (5.72) | 431, 6.70 (5.72) |
| 13 | 107, 6.34 (5.04) | 117, 7.09 (5.44) | 107, 7.01 (5.97) | 93, 6.08 (5.71) | 424, 6.66 (5.54) |
| 14 | 107, 5.98 (4.78) | 120, 6.83 (5.47) | 108, 7.21 (6.21) | 95, 5.65 (5.71) | 430, 6.46 (5.57) |
| 15 | 109, 5.69 (4.88) | 112, 7.19 (5.55) | 102, 6.46 (5.83) | 94, 6.29 (6.35) | 417, 6.41 (5.65) |
| 16 | 109, 5.67 (4.69) | 112, 6.73 (5.10) | 110, 6.73 (5.77) | 95, 6.11 (5.85) | 426, 6.32 (5.36) |
Secondary outcomes
| Week | Treatment group, mean (SD) | |||
|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | |
| Baseline | 8.67 (5.15) | 9.34 (5.25) | 9.80 (5.42) | 9.50 (5.97) |
| 4 | 7.17 (5.16) | 7.95 (5.02) | 7.60 (5.34) | 7.78 (6.42) |
| 8 | 6.97 (5.33) | 7.10 (5.40) | 7.32 (6.09) | 6.93 (6.11) |
| 12 | 6.35 (5.56) | 7.24 (5.72) | 7.04 (5.89) | 6.09 (5.72) |
| 16 | 5.67 (4.69) | 6.73 (5.10) | 6.73 (5.77) | 6.11 (5.85) |
| 20 | 6.46 (5.44) | 6.90 (5.53) | 6.57 (5.61) | 5.10 (4.97) |
| 24 | 7.05 (6.46) | 6.37 (5.53) | 6.90 (6.20) | 5.57 (5.57) |
| 28 | 6.83 (6.64) | 5.57 (5.14) | 6.79 (5.36) | 5.58 (5.74) |
| 32 | 6.67 (5.94) | 6.44 (5.33) | 5.76 (5.13) | 5.90 (6.03) |
| 36 | 6.87 (6.21) | 6.30 (5.34) | 6.33 (4.88) | 6.82 (5.69) |
| 40 | 6.14 (5.85) | 6.48 (5.52) | 6.95 (5.35) | 6.10 (5.81) |
| 44 | 6.42 (6.06) | 6.71 (5.62) | 6.33 (4.97) | 6.18 (6.52) |
| 48 | 6.47 (6.38) | 6.79 (5.45) | 6.56 (5.13) | 6.43 (6.41) |
| 52 | 5.86 (5.59) | 5.94 (5.39) | 6.26 (5.93) | 5.82 (5.82) |
| Group | Number of participants (% of randomised) | Number of participants with same researcher at baseline and 16 weeks (% of identified researcher) | ||||
|---|---|---|---|---|---|---|
| Randomised | EASI at baseline | EASI at 16 weeks | EASI at baseline and 16 weeks | Identified researcher at baseline and 16 weeks | ||
| Lotion | 137 (100.0) | 135 (98.5) | 123 (89.8) | 121 (88.3) | 121 (88.3) | 113 (93.4) |
| Cream | 140 (100.0) | 139 (99.3) | 133 (95.0) | 132 (94.3) | 132 (94.3) | 117 (88.6) |
| Gel | 135 (100.0) | 133 (98.5) | 125 (92.6) | 123 (91.1) | 123 (91.1) | 115 (93.5) |
| Ointment | 138 (100.0) | 136 (98.6) | 114 (82.6) | 112 (81.2) | 112 (81.2) | 104 (92.9) |
| Comparison | Ratings | Adjusteda difference (95% CI) | p-value |
|---|---|---|---|
| Lotion vs. cream | Very satisfied | −0.45 (−1.15 to 0.26) | 0.211 |
| Mostly satisfied | Reference | ||
| Neither satisfied/dissatisfied | 0.14 (−0.72 to 0.99) | 0.754 | |
| Dissatisfied | 0.40 (−0.42 to 1.23) | 0.335 | |
| Very dissatisfied | 1.30 (0.19 to 2.41) | 0.022 | |
| Lotion vs. gel | Very satisfied | −0.33 (−0.99 to 0.34) | 0.335 |
| Mostly satisfied | Reference | ||
| Neither satisfied/dissatisfied | −0.09 (−0.95 to 0.76) | 0.829 | |
| Dissatisfied | −0.16 (−1.02 to 0.71) | 0.718 | |
| Very dissatisfied | 0.51 (−0.68 to 1.70) | 0.401 | |
| Lotion vs. ointment | Very satisfied | −1.23 (−2.09 to −0.37) | 0.005 |
| Mostly satisfied | Reference | ||
| Neither satisfied/dissatisfied | 0.15 (−0.74 to 1.04) | 0.743 | |
| Dissatisfied | 0.45 (−0.39 to 1.30) | 0.296 | |
| Very dissatisfied | 1.45 (0.32 to 2.57) | 0.012 | |
| Cream vs. gel | Very satisfied | 0.12 (−0.59 to 0.83) | 0.738 |
| Mostly satisfied | Reference | ||
| Neither satisfied/dissatisfied | −0.23 (−1.08 to 0.62) | 0.593 | |
| Dissatisfied | −0.56 (−1.39 to 0.26) | 0.18 | |
| Very dissatisfied | −0.79 (−1.73 to 0.15) | 0.099 | |
| Cream vs. ointment | Very satisfied | −0.78 (−1.68 to 0.11) | 0.087 |
| Mostly satisfied | Reference | ||
| Neither satisfied/dissatisfied | 0.01 (−0.87 to 0.89) | 0.979 | |
| Dissatisfied | 0.05 (−0.75 to 0.85) | 0.908 | |
| Very dissatisfied | 0.15 (−0.70 to 0.99) | 0.734 | |
| Gel vs. ointment | Very satisfied | −0.90 (−1.77 to −0.04) | 0.041 |
| Mostly satisfied | Reference | ||
| Neither satisfied/dissatisfied | 0.24 (−0.64 to 1.12) | 0.588 | |
| Dissatisfied | 0.61 (−0.24 to 1.46) | 0.158 | |
| Very dissatisfied | 0.94 (−0.01 to 1.89) | 0.053 |
Per-protocol analysis
| Emollient use | Treatment group, n (%) | ||||
|---|---|---|---|---|---|
| Lotion (N = 137) | Cream (N = 140) | Gel (N = 135) | Ointment (N = 138) | Overall (N = 550) | |
| Monthly POEM | 117 (85.4) | 126 (90.0) | 113 (83.7) | 102 (73.9) | 458 (83.3) |
| Monthly emollient use | 70 (51.1) | 76 (54.3) | 66 (48.9) | 64 (46.4) | 276 (50.2) |
| Use on ≥ 60% days per week | 43 (31.4) | 47 (33.6) | 35 (25.9) | 27 (19.6) | 152 (27.6) |
| Number (%) of observations contributing to analysis | 664 (28.7) | 719 (31.0) | 534 (23.0) | 400 (17.3) | 2317 (100.0) |
| Allocated emollient (number of participants included in model/number of participants randomised) | Univariate difference in mean POEM scores (95% CI) | Adjusteda difference in mean POEM scores (95% CI) | p-valuea | ||||
|---|---|---|---|---|---|---|---|
| Lotion (n = 43/137) | Cream (n = 47/140) | Gel n = 35/135) | Ointment (n = 27/138) | ||||
| Mean POEM (SD) | 6.07 (4.41) | 7.25 (5.37) | 7.19 (5.95) | 9.25 (7.11) | 0.238 | ||
| Pairwise comparisons | |||||||
| Lotion vs. cream |

|
1.24 (−0.71 to 3.19) | 0.24 (−1.30 to 1.79) | 0.758 | |||
| Lotion vs. gel |

|
1.13 (−0.98 to 3.24) | −0.49 (−2.15 to 1.17) | 0.562 | |||
| Lotion vs. ointment |

|
3.03 (0.76 to 5.31) | 1.42 (−0.39 to 3.23) | 0.124 | |||
| Cream vs. gel |

|
−0.11 (−2.17 to 1.96) | −0.73 (−2.33 to 0.87) | 0.369 | |||
| Cream vs. ointment |

|
1.79 (−0.44 to 4.03) | 1.18 (−0.57 to 2.92) | 0.186 | |||
| Gel vs. ointment |

|
1.90 (−0.47 to 4.27) | 1.91 (0.06 to 3.76) | 0.043 | |||
Sensitivity analyses
| Comparison | Difference in mean POEM scores | |
|---|---|---|
| Adjusting for baseline scores and all stratification and minimisation variables used in the randomisation (95% CI); p-value | Adjusting for baseline scores and all stratification and minimisation variables used in the randomisation and sex (95% CI); p-value | |
| Global test across all four arms | p = 0.765 | p = 0.785 |
| Lotion vs. cream | 0.42 (−0.48 to 1.32); 0.360 | 0.41 (−0.50 to 1.31); 0.377 |
| Lotion vs. gel | 0.17 (−0.74 to 1.09); 0.717 | 0.18 (−0.74 to 1.10); 0.697 |
| Lotion vs. ointment | −0.01 (−0.93 to 0.91); 0.983 | −0.01 (−0.93 to 0.92); 0.990 |
| Cream vs. gel | −0.25 (−1.15 to 0.65); 0.586 | −0.22 (−1.13 to 0.68); 0.628 |
| Cream vs. ointment | −0.43 (−1.34 to 0.48); 0.354 | −0.41 (−1.33 to 0.50); 0.376 |
| Gel vs. ointment | −0.18 (−1.11 to 0.75); 0.704 | −0.19 (−1.12 to 0.74); 0.690 |
| Comparison | Complete-case analysis (primary intention-to-treat analysis) | Missing data imputed using MICE | ||
|---|---|---|---|---|
| Difference in mean POEM scores using a linear mixed model adjusting for baseline POEM scores, minimisation and stratification variables (95% CI) | p-value | Difference in mean POEM scores using a linear mixed model adjusting for baseline POEM scores, minimisation and stratification variables (95% CI) | p-value | |
| Global test across all four arms | 0.765 | 0.696 | ||
| Lotion vs. cream | 0.42 (−0.48 to 1.32) | 0.360 | 0.36 (−0.42 to 1.15) | 0.363 |
| Lotion vs. gel | 0.17 (−0.74 to 1.09) | 0.717 | −0.09 (−0.88 to 0.70) | 0.821 |
| Lotion vs. ointment | −0.01 (−0.93 to 0.91) | 0.983 | −0.06 (−0.85 to 0.73) | 0.883 |
| Cream vs. gel | −0.25 (−1.15 to 0.65) | 0.586 | −0.46 (−1.25 to 0.34) | 0.263 |
| Cream vs. ointment | −0.43 (−1.34 to 0.48) | 0.354 | −0.42 (−1.22 to 0.37) | 0.297 |
| Gel vs. ointment | −0.18 (−1.11 to 0.75) | 0.704 | 0.03 (−0.77 to 0.83) | 0.939 |
| Comparison | Complete-case analysis (primary intention-to-treat analysis) | Worst-case scenario – missing values replaced with mean + 1 SD of study arm | ||
|---|---|---|---|---|
| Difference in mean POEM scores using a linear mixed model adjusting for baseline POEM scores, minimisation and stratification variables (95% CI) | p-value | Difference in mean POEM scores using a linear mixed model adjusting for baseline POEM scores, minimisation and stratification variables (95% CI) | p-value | |
| Global test across all four arms | – | 0.765 | – | 0.351 |
| Lotion vs. cream | 0.42 (−0.48 to 1.32) | 0.360 | 0.40 (−0.45 to 1.25) | 0.352 |
| Lotion vs. gel | 0.17 (−0.74 to 1.09) | 0.717 | 0.45 (−0.41 to 1.31) | 0.306 |
| Lotion vs. ointment | −0.01 (−0.93 to 0.91) | 0.983 | 0.79 (−0.07 to 1.64) | 0.071 |
| Cream vs. gel | −0.25 (−1.15 to 0.65) | 0.586 | 0.05 (−0.81 to 0.90) | 0.917 |
| Cream vs. ointment | −0.43 (−1.34 to 0.48) | 0.354 | 0.38 (−0.46 to 1.26) | 0.376 |
| Gel vs. ointment | −0.18 (−1.11 to 0.75) | 0.704 | 0.34 (−0.52 to 1.19) | 0.441 |
| Comparison | Complete-case analysis (primary intention-to-treat analysis) | Best-case scenario – missing values replaced with mean –1 SD of study arm | ||
|---|---|---|---|---|
| Difference in mean POEM scores using a linear mixed model adjusting for baseline POEM scores, minimisation and stratification variables (95% CI) | p-value | Difference in mean POEM scores using a linear mixed model adjusting for baseline POEM scores, minimisation and stratification variables (95% CI) | p-value | |
| Global test across all four arms | – | 0.765 | – | 0.002 |
| Lotion vs. cream | 0.42 (−0.48 to 1.32) | 0.360 | 0.77 (−0.10 to 1.65) | 0.084 |
| Lotion vs. gel | 0.17 (−0.74 to 1.09) | 0.717 | −0.01 (−0.90 to 0.88) | 0.986 |
| Lotion vs. ointment | −0.01 (−0.93 to 0.91) | 0.983 | −0.94 (−1.82 to −0.06) | 0.036 |
| Cream vs. gel | −0.25 (−1.15 to 0.65) | 0.586 | −0.78 (−1.66 to 0.10) | 0.082 |
| Cream vs. ointment | −0.43 (−1.34 to 0.48) | 0.354 | −1.71 (−2.59 to −0.84) | < 0.001 |
| Gel vs. ointment | −0.18 (−1.11 to 0.75) | 0.704 | −0.93 (−1.82 to −0.05) | 0.038 |
| Treatment group, mean (SD) | Univariate difference in mean POEM scores (95% CI) | Adjusteda difference in mean POEM scores (95% CI) | p-valuea | ||||
|---|---|---|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | ||||
| POEM scores | 6.84 (5.14) | 7.65 (5.37) | 7.45 (5.81) | 7.04 (6.13) | 0.768 | ||
| Pairwise comparisons | |||||||
| Lotion vs. cream |

|
0.83 (−0.31 to 1.98) | 0.42 (−0.49 to 1.33) | 0.365 | |||
| Lotion vs. gel |

|
0.78 (−0.37 to 1.93) | 0.17 (−0.76 to 1.09) | 0.722 | |||
| Lotion vs. ointment |

|
0.36 (−0.81 to 1.52) | −0.01 (−0.94 to 0.92) | 0.978 | |||
| Cream vs. gel |

|
−0.06 (−1.19 to 1.08) | −0.25 (−1.16 to 0.65) | 0.585 | |||
| Cream vs. ointment |

|
−0.48 (−1.63 to 0.67) | 0.43 (−1.35 to 0.48) | 0.354 | |||
| Gel vs. ointment |

|
−0.42 (−1.59 to 0.74) | −0.18 (−1.10 to 0.75) | 0.704 | |||
| Treatment group | Univariate difference in mean POEM scores (95% CI) | Adjusteda difference in mean POEM scores (95% CI) | p-valuea | ||||
|---|---|---|---|---|---|---|---|
| Lotion | Cream | Gel | Ointment | ||||
| Number of researcher POEM scores | 9 | 12 | 8 | 11 | |||
| POEM scores, mean (SD) | 6.80 (5.15) | 7.62 (5.36) | 7.44 (5.81) | 7.04 (6.14) | 0.766 | ||
| Pairwise comparisons | |||||||
| Lotion vs. cream |

|
0.85 (−0.28 to 1.98) | 0.40 (−0.50 to 1.30) | 0.378 | |||
| Lotion vs. gel |

|
0.83 (−0.32 to 1.97) | 0.15 (−0.76 to 1.06) | 0.747 | |||
| Lotion vs. ointment |

|
0.39 (−0.77 to 1.55) | 0.04 (−0.96 to 0.88) | 0.934 | |||
| Cream vs. gel |

|
−0.02 (−1.15 to 1.11) | −0.25 (−1.15 to 0.65) | 0.580 | |||
| Cream vs. ointment |

|
−0.46 (−1.60 to 0.68) | −0.44 (−1.35 to 0.47) | 0.340 | |||
| Gel vs. ointment |

|
−0.44 (−1.60 to 0.72) | −0.19 (−1.11 to 0.73) | 0.688 | |||
| Treatment group, median (IQR) | Adjusteda per cent change in EASI scores (95% CI) | p-valuea | ||||
|---|---|---|---|---|---|---|
| Lotion (n = 121/137) | Cream (n = 132/140) | Gel (n = 123/135) | Ointment (n = 112/138) | |||
| Baseline | 3.3 (2–7.2) | 3.15 (2–6.3) | 4 (2.35–8) | 3.3 (1.58–6.5) | ||
| 16 weeks | 2.15 (0.6–3.6) | 2.3 (0.9–4.7) | 2.25 (0.9–5.15) | 2.2 (0.8–4.8) | 0.486 | |
| Pairwise comparisons | ||||||
| Lotion vs. cream |

|
1.13 (0.95 to 1.33) | 0.163 | |||
| Lotion vs. gel |

|
1.06 (0.99 to 1.26) | 0.405 | |||
| Lotion vs. ointment |

|
1.12 (0.94 to 1.33) | 0.211 | |||
| Cream vs. gel |

|
0.95 (0.80 to 1.12) | 0.511 | |||
| Cream vs. ointment |

|
0.99 (0.84 to 1.17) | 0.925 | |||
| Gel vs. ointment |

|
0.95 (0.88 to 1.24) | 0.590 | |||
FIGURE 10.
Number of and mean POEM scores before and after public health measures introduced in the UK in response to the COVID-19 pandemic. (a) Mean POEM score; and (b) number of questionnaires completed.

Appendix 3 Nested qualitative study
Topic guide
Topics for Best Emollients for Eczema qualitative interviews
The overall aim of the interviews with families at around 4 and 16 weeks post randomisation is to understand families’ experiences of emollient use, focusing on acceptability and perceived effectiveness of the study emollient, including facilitators of and barriers to use. The exact topics and questions asked may evolve during the interviews in response to issues raised by participants, but will cover the below key topics to address the qualitative study objectives.
-
Thank you for agreeing to take part in this interview. The interview should take less than an hour to complete.
-
Do you have any questions before we begin?
-
Can you confirm that you are happy for the interview to be recorded?
Topics to cover in both week 4 and week 16 interviews
Introduction
Ask about child’s history of eczema, parent/carer understanding of eczema, beliefs about causes or reasons for eczema (e.g. food allergies, other), how such beliefs shape their views about treatment.
Perceived impact of eczema on child and family
Probe for experiences relating to impact of eczema on the child and family, especially on quality of life and any personal ‘costs’ e.g. sleep, social activities, emotional well-being. Seek child’s perspective as appropriate, depending on age and engagement with interview.
Previous use of treatments for eczema
Probe for prior use of treatments for eczema (both prescribed and bought), focusing on topical treatments, especially moisturisers/emollients, and beliefs about emollients [how they work, when and how to use them (e.g. what makes a good one? Importance of using one even when the skin looks ‘clear’? Is switching regularly important depending on weather/time of year/to avoid building up some sort of imagined ‘tolerance’ to the emollient?)]. Ask about types used, how they accessed these (all ‘trial and error’ or did health-care professionals give advice guidance?), successes, difficulties, satisfaction with care and treatment.
Experiences of using the study emollient, including ease of use
Probe for how they have found using the study emollient, how acceptable the emollient is to child and carer(s), any ‘adverse effects’, any consideration of stopping the emollient and why.
Facilitators of and barriers to using the study emollient
Probe for things that have enabled or encouraged them to use the study emollient and things that have hindered or caused difficulties in using the study emollient.
Perceptions of effectiveness of study emollient
Probe for satisfaction/dissatisfaction with emollient and reasons for this, how well they think it is working, how helpful or otherwise acceptable emollient is, reasons why they think it is working/not (what ‘evidence’ are they drawing on), list best and worst thing about their emollient.
Experiences of using other treatments (concurrently) for eczema
Probe for types of treatments used, reasons for using, when they started using, whether previously used, satisfaction with use, experiences of and reasons for using alongside (or instead of) study emollient.
Initial experiences of taking part in the study
Probe for thoughts about randomisation process/allocation of study emollient [e.g. initial expectations of their allocated emollient around effectiveness and acceptability (happy/disappointed/indifferent regarding their allocation – where did any preconceptions come from? Previous experience, family/friends, healthcare professionals, something they read about online?)], reasons for taking part and/or withdrawing (if applicable), expectations of participation going forward, ease/difficulty following the recommendation of applying the emollient at least twice a day/only using study emollient for 16 weeks.
Any other issues
Additional topics to cover in week 16 interviews (post primary outcome)
Experiences of change in eczema symptoms during the study period
Probe for what (if any) changes for better or worse, how they noticed any changes.
Perceptions of reasons for changes in eczema symptoms
Probe for factors they think have contributed to changes (improvements or worsening) during the study period.
Changes in experiences of using the study emollient
Probe for whether their experiences of ease of use and views about acceptability of the emollient have changed during the course of the study and why. (NB: If this is a repeat interview with a participant interviewed at 4 weeks, refer back to what they said as a reminder and prompt, especially probing if changed views).
Views about future use of study and other emollients
Ask whether they will continue using the study emollient after the end of the study, reasons why/why not. Probe for future use of other emollients and topical treatments and reasons why/why not.
Views and experiences of study procedures and participation
Probe for experiences of randomisation, feelings about allocated emollient, experiences of questionnaire completion (and whether this impacted adherence to allocated or any other treatment), reasons for taking part, any points at which considered stopping their participation in the trial and why, what factors encouraged them to continue participation in the trial and why, did they use their study emollient for longer than they would have in ‘normal’ life (if not part of a study) and did the skin improve because of this, which might not have happened if they had switched sooner, did they feel free to switch/stop using their study emollient if they wanted to, what did they learn from taking part in the study (anything they were not previously aware of regarding emollients)?
Sub-topic guide (for use in paired interviews with children)
Start off with some ice-breaker questions (e.g. what’s your favourite colour/T.V. character/food/subject at school, etc.) for the children.
-
Can you tell me about your eczema?
-
What’s it like having eczema?
-
How does it affect you? At home, school, at play and/or at night?
-
-
What treatments have you used/do you use for eczema?
-
What do you think makes a good moisturiser? Why?
-
What’s it like using the moisturiser you were given to use for the BEE study?
-
Is there anything you particularly like or do not like about your study moisturiser?
-
Do you think it is helping your skin? How can you tell?
-
-
What has it been like taking part in the BEE study for you?
-
Do you use a moisturiser more/less often than before joining the BEE study? Probe for any other changes in their behaviour as a result of taking part in the study.
-
Have you learned anything from taking part in the BEE study about eczema or treating eczema?
-
-
Anything else that you would like to tell us about living with your eczema or moisturisers for eczema?
Coding framework
| Code name | Definition | Notes |
|---|---|---|
| 1.0 History and experience of eczema | ||
| 1.1 Diagnosis | When the child was diagnosed, experiences of diagnosis | |
| 1.2 Family experience_ecz or other | Other family members with eczema, asthma, allergies | |
| 1.3 Specialist_dermatology | Have they been referred/requested/would like an appointment | Experiences of appointment, advice given |
| 1.4 Understanding_beliefs re eczema | Beliefs about causes/reasons for eczema | |
| 1.4.1 Allergies | Does the child have any diagnosed allergies and if so what are the parent’s/child’s beliefs about allergies | |
| 1.4.2 Makes it worse | Have they noticed anything that makes their child’s eczema worse e.g. swimming | |
| 1.4.3 Climate_Heat_Cold | Impact of temperature/climate on child’s eczema and using sun cream | (Will include comments about climate etc. here when talking about study emollient) |
| 2.0 Impact of eczema | ||
| 2.1 Physical impact | Physical impact of eczema on child – how/where it presents, severity, scratching, lack of sleep, tiredness etc. | Including physical location of eczema |
| 2.2. Infections | Skin infections and use of antibiotics | |
| 2.3 Social impact | ||
| 2.3.1 Child_Social | Social impact for child: making friends, missing out on social activities, trips, sleepovers | |
| 2.3.2 Family_Social | Social impact for family impact including relationships | |
| 2.4 Quality of life | ||
| 2.4.1 Child_QoL_emotional | Perceived impact of eczema on child’s general quality of life, child’s mood, sleep, missing out | |
| 2.4.2 Family_QoL_emotional | Perceived impact of eczema on family’s general quality of life, seeing child scratching/upset, sleep, perceptions of child missing out | |
| 2.5 Practical | Greasy/sticky emollients and clothing/bedding, holidays/travel, getting prescriptions | |
| 3.0 Previous use of treatments | ||
| 3.1 Prescribed treatments | Experience of treatments prescribed by GP/dermatologist | |
| 3.2 Purchased treatments | Over the counter emollients, or anything else purchased e.g. clothing, food | |
| 3.3 How they are used | General information on the way that emollients are used | Plus how they are used on different areas of the body |
| Control: how do they judge? | ||
| 3.3.1 Trial and error | Experiences of trying out different emollients until they find one that works for their child | |
| 3.3.2 Swapping_tolerance | Do they regularly change treatments, do certain emollients become less effective over time | |
| 3.3.3 Coping with flare ups _steroid use | How they cope with flare ups, use of topical steroids and attitudes to steroid use. How do they use (before/after emollient, mix together). Size of tube/packaging and impact on use | Do they try not to use steroids |
| 3.3.4 Daily routine_emollient use | What was their routine before they joined the study | |
| 3.4 Advice on treatments | Advice from health professionals, family, friends, other parents on treating eczema, online. Perceptions need/want more information? | Where advice comes from |
| 3.4.1 Satisfaction with care | Are they happy with the care provided by their GP/dermatologist, ease of getting appointments/ease of getting prescriptions for emollients/tcs | |
| 3.5 Perceived effectiveness | ||
| 3.5.1 Child_Effec-nonStudy | How effective are the emollients they have used before the study – child view | |
| 3.5.2. Family_Effec-nonStudy | How effective are the emollients they have used before the study – parent/carer’s view | |
| 3.6 Acceptibility_what’s a good one | ||
| 3.6.1 Child_Accep-nonStudy | What kind of things do children/young people like/do not like about the emollients they have used before the study | |
| 3.6.2 Family_Accep_nonStudy | What kind of things do parents/carers like/do not like about the emollients they have used before the study | |
| 3.7 Facilitators | Things that help them use a emollient | |
| 3.8 Barriers | Things that put them off using a emollient | |
| 4.0 Study emollient | ||
| 4.1 Study Lotion | ||
| 4.1.1 Perceived effectivenessSL | How effective have they found the study lotion – does it work for them | |
| 4.1.2 AcceptibilitySL | Ease of use, absorption, texture etc, container | |
| 4.1.3 Adverse effectsSL | Any problems | |
| 4.1.4. Considered stopping_stoppedSL | Did they ever consider stopping using it or did they stop and reasons why | |
| 4.1.5 SL Best Worst | What are the best and worst things about the study lotion | |
| 4.1.6 Other used_SL | Have they used any other leave on emollients, steroid use, bath | |
| 4.2 Study Cream | ||
| 4.2.1 Perceived effectivenessSC | How effective have they found the SC – does it work for them | |
| 4.2.2 AcceptibilitySC | Ease of use, absorption, texture etc, container | |
| 4.2.3 Adverse effectsSC | Any problems | |
| 4.2.4. Considered stopping_stoppedSC | Did they ever consider stopping using it or did they stop and reasons why | |
| 4.2.5 SC Best Worst | What are the best and worst things about the SC | |
| 4.2.6 Other used_SC | Have they used any other leave on emollients, steroid use, bath | |
| 4.3 Study Gel | ||
| 4.3.1 Perceived effectivenessSG | How effective have they found the SG – does it work for them | |
| 4.3.2 AcceptibilitySG | Ease of use, absorption, texture etc, container | |
| 4.3.3 Adverse effectsSG | Any problems | |
| 4.3.4. Considered stopping_stoppedSG | Did they ever consider stopping using it or did they stop and reasons why | |
| 4.3.5 SG Best Worst | What are the best and worst things about the SG | |
| 4.3.6 Other used_SG | Have they used any other leave on emollients, steroid use, bath | |
| 4.4. Study Ointment | ||
| 4.4.1 Perceived effectivenessSO | How effective have they found the SO – does it work for them | |
| 4.4.2 AcceptibilitySO | Ease of use, absorption, texture etc, container | |
| 4.4.3 Adverse effectsSO | Any problems | |
| 4.4.4. Considered stopping_stoppedSO | Did they ever consider stopping using it or did they stop and reasons why | |
| 4.4.5. SO Best worst | What are the best and worst things about the SO | |
| 4.4.6 Other used_SO | Have they used any other leave on emollients, steroid use, bath | |
| 5.0 Study participation | ||
| 5.1 Randomisation_allocation | Thoughts about the randomisation process. What did they think of their allocation e.g. in the light of prior preferences | |
| 5.2 Following study recommendations | How easy/hard has it been sticking to using study emollient twice a day as sole leave-on emollient | |
| 5.3 Use it more_less | Have they used the study emollient more regularly than they used their pre-study emollient | |
| 5.4 Anything else study participation | E.g. how did they get on with questionnaire/diary completion, collecting study prescription | Including reasons for participating |
| 5.4.1 Expectations of participation | Hopes on taking part – symptom improvement, less flares, trying something new etc. | |
| 5.5 Learnt anything | Have they learnt anything by taking part in BEE e.g. treatment/management of eczema, causes | |
| 5.5.1 Learnt parent | ||
| 5.5.2. Learnt child | ||
| 6.0 Anything else | Anything that they think it is important for us to note | |
| 7.0 Future emollient use_16week | What are their intentions at the 16-week point | |
| 7.1 Stay on study emollient | Will they carry on with the study emollient | |
| 7.2 Swap to pre-study emollient | Will they swap back to their pre-study emollient | |
| 7.3 Other | Will they try another | |
| 7.2 Unsure | Unsure/do not know | |
List of abbreviations
- ADQoL
- Atopic Dermatitis Quality of Life
- BEE
- Best Emollients for Eczema
- CHU-9D
- Child Health Utility 9-Dimension
- CI
- confidence interval
- CRN
- clinical research network
- DFI
- Dermatitis Family Impact
- DMC
- Data Monitoring Committee
- EASI
- Eczema Area Severity Index
- EMR
- electronic medical record
- GP
- general practitioner
- HOME
- Harmonising Outcome Measures for Eczema
- ICB
- Integrated care board
- IQR
- interquartile range
- LHB
- local health board
- MICE
- multiple imputation by chained equations
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health and Care Research
- POEM
- Patient Orientated Eczema Measure
- PPI
- patient and public involvement
- SD
- standard deviation
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
Notes
-
BEE flyer
Supplementary material can be found on the NIHR Journals Library report page (https://doi.org/10.3310/GZQW6681).
Supplementary material has been provided by the authors to support the report and any files provided at submission will have been seen by peer reviewers, but not extensively reviewed. Any supplementary material provided at a later stage in the process may not have been peer reviewed.
