Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as award number 16/152/01. The contractual start date was in November 2019. The draft report began editorial review in February 2023 and was accepted for publication in August 2023. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ manuscript and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this manuscript.
Permissions
Copyright statement
Copyright © 2024 Brown et al. This work was produced by Brown et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
2024 Brown et al.
Chapter 1 Introduction
Background and rationale
In critically ill children, healthcare-associated infections (HCAIs) are a major cause of morbidity and mortality, with an incidence of 7–14%. 1–5 HCAIs can develop either as a direct result of healthcare interventions such as medical or surgical treatment or from being in contact with a healthcare setting. HCAIs can be caused by opportunistic microorganisms, residing in the oral cavity and gastrointestinal tract directly or haematogenously spreading to other organ systems. 1,2 Critical illness affects immune competence, and in those requiring prolonged organ support, this, along with the presence of invasive devices such as urinary catheters, vascular lines and endotracheal tubes, places them at risk of secondary infection. Evidence from adult intensive care studies suggests that using selective decontamination of the digestive tract (SDD) alongside standard infection control measures may reduce mortality and ventilator-associated pneumonia (VAP), although results are mixed in terms of clinical benefit. 1,6 There are no data to suggest a significant increase in antimicrobial resistance following use of SDD; in fact, a recent multicentre clinical trial found a significant reduction in positive blood cultures and cultures of antibiotic-resistant organisms and no significant increase in new Clostridiodes difficile infections in patients who received SDD. Overall antibiotic use was not increased in patients receiving SDD. 7 A meta-analysis of 32 randomised controlled trials (RCTs) including 24,389 participants suggests that use of SDD compared with standard care or placebo was associated with reduced hospital mortality. 8
Despite these data, SDD has not been routinely adopted due to concerns that it may promote antimicrobial resistance. 9,10 Recent ecological studies conducted in adult intensive care have found that SDD was associated with a reduction in antimicrobial utilisation. 11–15
SDD has not been compared directly with modern infection control protocols in the paediatric intensive care unit (PICU) population, with only single-centre and small observational studies reporting its implementation as part of infection control regimens. 16
A clinical trial comparing SDD with standard infection control methods to establish safety and clinical utility is needed in the PICU setting, but the paucity of available data in children suggested a need to establish whether a large, multicentre cluster RCT (cRCT) is feasible, and if so, what components of trial design, safety monitoring and clinical outcomes are of importance to patients and clinical staff caring for critically ill children.
Infection Control in Paediatric Intensive Care (PICnIC) is a feasibility study commissioned by the National Institute for Health and Care Research (NIHR) Health Technology Assessment (HTA) programme, which aims to determine whether it is possible to conduct a cRCT of SDD in critically ill children who are likely to be ventilated for > 48 hours, and to explore and test the acceptability of key components of the study to healthcare professionals and families of patients.
Aim
The main aim of the PICnIC Feasibility Study was to determine whether it was feasible to conduct a multicentre trial in critically ill children comparing SDD with standard infection control procedures by undertaking a pilot cRCT with integrated mixed-methods study exploring the views of patients, their families and clinical staff both within the study PICUs and more broadly across the NHS England PICU setting.
Objectives
The Infection Control in Paediatric Intensive Care pilot randomised controlled trial
-
To test the ability to randomise PICUs to either control or intervention.
-
To test the willingness and ability of healthcare professionals to screen and recruit eligible children.
-
To estimate the recruitment rate of eligible children.
-
To test adherence to the SDD protocol.
-
To test the procedures for assessing and collecting selected clinical and ecological outcomes and for adverse event (AE) reporting.
-
To assess the generalisability of the study results to all PICUs using the Paediatric Intensive Care Audit Network (PICANet).
The Infection Control in Paediatric Intensive Care mixed-methods study
Perspectives of PICU healthcare professionals:
-
To assess the acceptability of implementation of the SDD intervention, recruitment and consent procedures.
-
To assess the acceptability of collecting data to assess the selected clinical and ecological data.
-
To assess the acceptability of the SDD intervention and confirm interest in participation in a definitive trial in the wider PICU community.
Perspectives of parents/guardians of recruited patients:
-
To review and explore the acceptability of a definitive trial that includes the SDD intervention.
-
To test the acceptability of the recruitment and consent procedures for the definitive trial, including all proposed information materials.
-
To review and explore selection of important, relevant, patient-centred primary and secondary outcomes for a definitive trial.
Research governance
An ethics application was made to the West Midlands – Black Country Research Ethics Committee (20/WM/0061) and received a favourable opinion on 3 November 2020, with approval granted by the Health Research Authority (HRA) on 20 November 2020. The PICnIC pilot RCT was registered on the ClinicalTrials.gov database. Registration was confirmed on 30 October 2020 (reference number: ISRCTN40310490). The protocol is available at https://bmjopen.bmj.com/content/12/3/e061838 (accessed 26 January 2023).
Local confirmation of capacity and capability was obtained from each of the six PICU sites participating in the PICnIC pilot RCT. The statement of activities and agreements for non-commercial research in the health service were signed by each participating hospital trust, the Clinical Trials Unit (CTU) and the sponsor (The Masters and Scholars of the University of Cambridge and Cambridge University Hospitals NHS Foundation Trust).
Clinical data collection on patients enrolled into the PICnIC pilot RCT was embedded in the PICANet, which has approval to collect patient-identifiable and personal data without consent. The release of non-identifiable patient data was requested through a customised data collection request to the Healthcare Quality Improvement Partnership for access to unidentifiable routine PICANet data and to collect the additional data required to assess the wider UK feasibility of a definitive PICnIC study. Approval was received for this purpose on 16 August 2022.
Study management
The PICnIC Study was sponsored jointly by the University of Cambridge and Cambridge University Hospitals NHS Foundation Trust. The PICnIC pilot cRCT was led by chief investigator NP and coinvestigator PRM. It was coordinated by the Intensive Care National Audit and Research Centre (ICNARC) CTU (UK Clinical Research Collaboration ID number: 42). The PICnIC mixed-methods study was led by coinvestigator KW. An experienced research associate (MP) was employed to organise, conduct and analyse data from this study. A Study Management Group (SMG) was convened, comprising the chief investigator (NP) and coinvestigators (JP, BHC, RF, TG, RS, LNT, IS, KW, DAH, PRM, KR), and was responsible for overseeing management of the entire study. The SMG met regularly throughout the duration of the study to monitor the conduct and progress. One coinvestigator (IS) has experience of a critical illness and PICU admission, providing valuable input into the design and conduct of the PICnIC Study.
The study co-ordinators (AB, GM, LD) were responsible for day-to-day management of the PICnIC feasibility study, with support from the ICNARC CTU and the SMG.
Network support
To maintain the profile of the PICnIC study, including the mixed-methods work, updates were provided at national meetings, such as the biannual Paediatric Critical Care Society Study Group meetings.
Patient and public involvement
Caregivers of children admitted to PICU and a former patient were involved in prioritising the outcomes and designing the study protocol. Their input continued with patient and public involvement (PPI) representatives on the study oversight panel and development of the topic guide for the qualitative study. A patient representative (a former PICU patient) is a coinvestigator and is an author of this manuscript. IS undertook a review of the study protocol, participant documentation and giving patient perspectives to shape the approach to study recruitment. IS also reviewed documents for parent interviews and made suggestions that were integrated into the final version of the documents, such as follow-up questions on what did or did not influence parents’ decisions to participate in the pilot. IS continued to remain involved over the course of the study, reviewing progress and findings at the Trial Management Group (TMG) meetings.
Chapter 2 Methods for the Infection Control in Paediatric Intensive Care pilot cluster-randomised controlled trial
Aim and objectives
-
To test the ability to randomise PICUs to either control or intervention.
-
To test the willingness and ability of healthcare professionals to screen and recruit eligible children.
-
To estimate the recruitment rate of eligible children.
-
To test adherence to the SDD protocol.
-
To test the procedures for assessing and collecting selected clinical and ecological outcomes and for AE reporting.
-
To assess the generalisability of the study results to all PICUs using the PICANet.
Methods
Study design
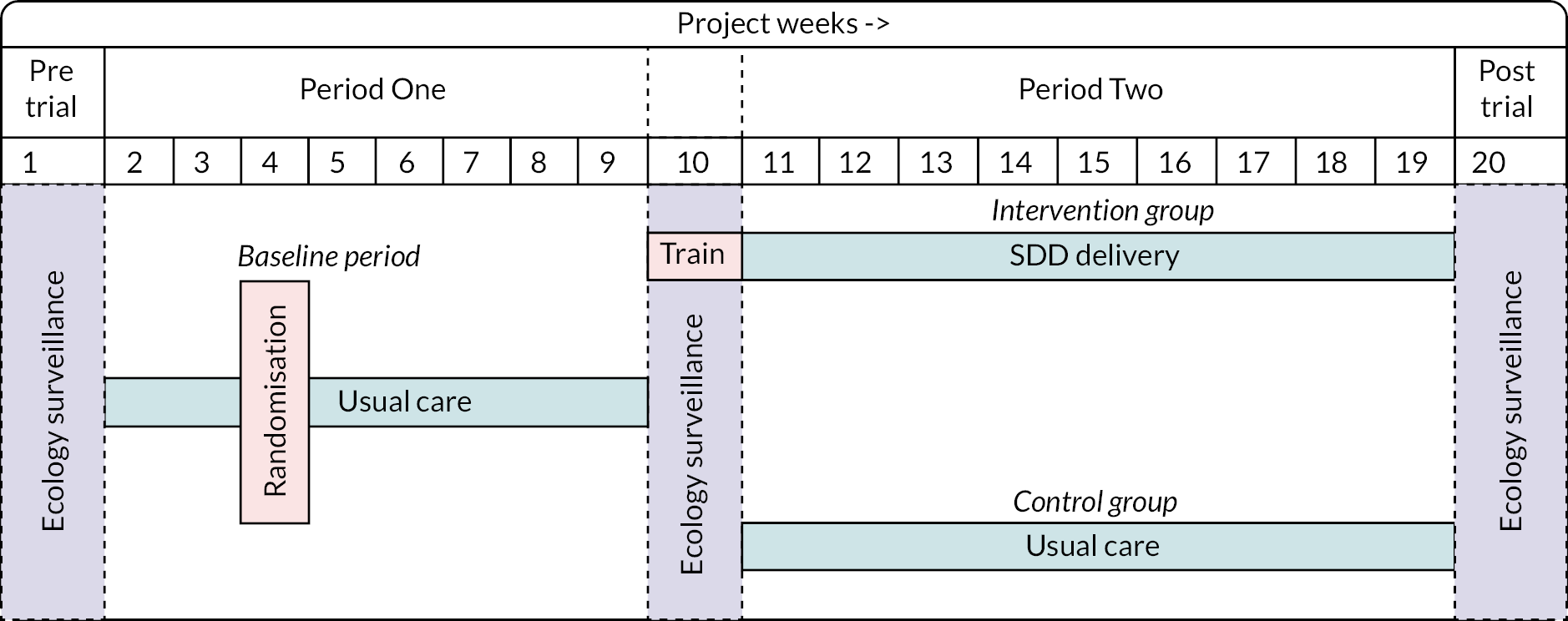
The PICnIC pilot cRCT was an external pilot, parallel-group cRCT, with recruitment for a period of 18 weeks. There were three ecology periods to monitor unit ecology before (week 1), during (week 10) and after (week 20) the two study periods.
Data collection was streamlined by integrating patient-level data collection with existing unit data entry into the national PICANet database. By nesting the study within the national clinical audit of paediatric critical care units in the UK, we ensured a cost-effective, time-efficient design with respect to data collection and trial management within the participating PICUs.
Period One consisted of usual care at all sites (from weeks 1 to 8) as per each unit’s own local practice. This was the baseline period. During this period, the six sites were randomised to either continue their routine infection control practice (usual care sites, n = 3) or incorporate SDD into their local infection control regime for eligible children (intervention sites, n = 3) in Period Two.
In Period Two, after a week of training and transition (week 11), sites were split into usual care and intervention sites from weeks 12 to 19 as per randomisation. The intervention period continued for 8 weeks, during which time intervention sites delivered the SDD intervention using research without prior consent (RWPC), while control sites continued to deliver usual care.
The study flow is demonstrated in Figure 1.
FIGURE 1.
Trial schema pilot cRCT.
Handling of missing data
The proportion of variables included in the analyses that were missing were reported. No measures were taken to replace missing values.
Study management
The study co-ordinator at the ICNARC CTU was responsible for the day-to-day management of the pilot RCT with support from the SMG.
Patient and public involvement
Engagement with patients was vital to the successful conduct of the PICnIC pilot RCT and included the mixed-methods approaches (see Chapter 4), along with involvement of patient stakeholders in the development and oversight of the PICnIC RCT. A parent of a child who was admitted to a PICU with severe infection provided input to the PICnIC pilot RCT design. A former patient on a PICU (IS) is a coinvestigator and provided patient input for development of the pilot RCT, including reviewing the study literature given to patients and their families [e.g. participant information sheets (PISs)]. In addition to this, patients and members of the public were engaged through the PICnIC qualitative study, which has provided invaluable insight that has been incorporated into the recommendations arising from the overall study.
Design and development of the protocol
The PICnIC pilot RCT protocol was designed to inform key parameters and to test the design and possible conduct of the proposed definitive PICnIC RCT.
Amendments to the Infection Control in Paediatric Intensive Care pilot randomised controlled trial protocol
Following receipt of approval from the HRA on 20 November, 12 non-substantial amendments were approved and categorised.
National Health Service support costs
The NHS support costs were agreed prior to the submission of the research grant to include screening to identify eligible patients and obtaining informed consent from parents. These included costs for microbiology swab tests and the administration of SDD at sites allocated to intervention in Period Two of the study.
Setting
The setting for the pilot cRCT was six NHS PICUs in England. For the pilot RCT, each site was obliged to:
-
meet the responsibilities stated in the PICnIC pilot RCT clinical trial site agreement
-
identify and sign up a local principal investigator (PI)
-
identify a responsible research nurse
-
agree to incorporate SDD in infection control if randomised to intervention in Period Two
-
agree to adhere to the study protocol
-
agree, where possible, to recruit all eligible patients and to maintain a screening log.
Site selection
Six sites that had previously expressed an interest in participating in the PICnIC cRCT were selected. Potential sites were asked to complete a site feasibility questionnaire to confirm eligibility by the ICNARC CTU. None of the sites had experience of delivery of SDD in clinical care. Two sites expressed interest as reserve sites but did not need to participate. The reasons for selection included a good research track record, a display of a high level of enthusiasm and ensuring a good diversity of sites.
Site initiation
Site teams from all six participating sites attended a site initiation meeting prior to the commencement of patient screening. These were held online to minimise the risk of infection transmission during the COVID-19 pandemic. During the first meeting, the chief investigator presented the background and rationale for the PICnIC pilot RCT. The meetings included discussion of the protocol, screening and recruitment of patients, data collection and microbiology screening procedures.
Following randomisation, a second site initiation visit was undertaken for the three sites randomised to SDD, where the local PIs and pharmacists attended and were given information on the protocol for prescribing and administering the SDD oral solution and paste. The operational challenges of conducting the PICnIC pilot RCT at sites were discussed, including strategies for communicating the study to all PICU staff. The PI and trial manager maintained close contact with these sites to address any questions that arose as the clinical teams integrated SDD into their infection control procedures.
Investigator site file
An investigator site file was provided to all participating sites. This contained all essential documents for the conduct of the PICnIC pilot RCT and included the approved protocol; all relevant approvals (e.g. local confirmation of capacity and capability); a signed copy of the clinical trial site agreement; the delegation of trial duties log; and copies of the approved PISs, parent/legal representative consent forms and all standard operating procedures (e.g. for screening participants, delivery of the intervention, obtaining informed consent and entering data onto the secure, dedicated eCRF). The site PI was responsible for maintaining the investigator site file.
Randomisation procedure
During week 4, the six participating PICUs were randomised by the trial statistician using computer-based randomisation to either usual care or intervention. Sites were informed of the randomisation outcome on week 8.
Screening log
To enable full and transparent reporting, brief details of all patients who met the inclusion criteria were recorded in the screening log. If the patient was deemed ineligible because they met one or more of the exclusion criteria, this was recorded in the screening log. The reasons for eligible patients not being recruited were recorded under the following categories: limited research capacity, missed patients, clinical decision, nearly or reached recruitment target, anal atresia and no rectum.
Consent
Informed consent for routine microbiology and administration of SDD in sites randomised to intervention was not required, as these were undertaken as part of the routine PICU infection control procedures. Where the study protocol required additional swab samples, these were collected following consent. Staff members who had received training on the background, rationale and purpose of PICnIC and on the principles of good clinical practice were authorised by the PI to take informed consent from parents/legal representatives.
The method used for the PICnIC pilot RCT was RWPC. In line with guidance,17 once notified of the recruitment of a participant to the trial, a delegated member of the site research team approached the parents/legal representatives as soon as practical and appropriate following confirmation of eligibility (usually within 24–48 hours) to discuss consent for ongoing participation. Information about the PICnIC pilot RCT was provided to the parents/legal representatives, which included the purpose of the trial, the reasons why informed consent prior to randomisation could not be sought from parents/legal representatives, what participation in the trial meant (i.e. permission for the use of data already collected and/or for their child to continue to take part in the trial procedures when applicable), participant confidentiality, future availability of the results of the trial and funding of the study. It also provided information on completing a questionnaire and/or taking part in a telephone interview as part of the mixed-methods study. This information was provided in the PIS, along with the name and contact details of the local PI and research nurse(s), which was given to the parents/legal representatives to read before making their decision for their child’s data to be used, or not, and for their child to continue to take part, or not, in the trial. Once the staff member taking informed consent was satisfied that the parents/legal representatives had read and understood the PIS and that all their questions about the trial had been answered, the parents/legal representatives were invited to sign the consent form.
Intervention
The intervention selected was the addition of SDD to the standard infection control strategy of the participating PICU. The intervention consisted of three topical antimicrobial agents – colistin, tobramycin and nystatin – prepared according to international standards for Good Manufacturing Procedures and manufactured in a Therapeutic Goods Administration-approved facility (Verita Pharma®, Sydney, under licence from the George Institute for Global Health, Sydney; see Appendix 1). The SDD preparations were manufactured specifically for the Selective Decontamination of the Digestive Tract in the Intensive Care Unit (SuDDICU) RCT conducted in Australia and Canada between 2017 and 2023. 7 The SDD preparations were prepared as a topical oral paste and gastric suspension that underwent extensive temperature and stability testing. The SDD preparations were imported under temperature-controlled conditions to the UK for this pilot trial. The products were assigned a shelf life of 12 months, being stored at 4 °C. After reconstitution by the bedside nurse, the enteric suspension was stable at room temperature for up to 1 week. SDD paste and suspension were administered every 6 hours to all eligible patients as follows:
-
topical application of a pea-sized (0.5 g) SDD paste containing 2% polymyxin E (colistin), 2% tobramycin and 2% nystatin to the buccal mucosa and oropharynx
-
enteric administration via feeding tube of SDD liquid suspension on an age-based dosing schedule (Table 1) containing polymyxin E (colistin), tobramycin and nystatin.
| 0–4 years | 5–12 years | ≥ 13 years | |
|---|---|---|---|
| Polymyxin E (Colistin) | 25 mg | 50 mg | 100 mg |
| Tobramycin | 20 mg | 40 mg | 80 mg |
| Nystatin | 0.5 × 106 IU | 1 × 106 IU | 2 × 106 IU |
| Volume | 2.5 ml | 5 ml | 10 ml |
Selective decontamination of the digestive tract treatment was protocolised to be started within 6 hours of the patient being identified as eligible and continued for a maximum of 30 days (defined as the treatment period). Treatment continued until the patient was extubated or no longer mechanically ventilated (in tracheostomised patients). The intervention was restarted if patients were subsequently re-intubated (either during this PICU admission or re-admission to PICU from another inpatient area) during the treatment period. All other usual care was provided at the discretion of the treating clinical team.
In all sites during all trial periods, nasopharyngeal and faecal/rectal swab samples were taken from all patients at admission as part of routine care and then where consent was obtained these were repeated twice weekly. The nasopharyngeal swab was plated for methicillin-resistant Staphylococcus aureus (MRSA) detection. Faecal/rectal swabs were plated for:
-
extended-spectrum beta-lactamase (ESBL) and AmpC producing organisms
-
carbapenemase producing Enterobacteriaceae (CPE)
-
vancomycin-resistant Enterococcus (VRE)
-
Candida auris.
During the ecology surveillance weeks, where consent was obtained for additional study specific samples, patients had an additional sample taken at least every 48 hours during the course of the week if they remained an inpatient. Samples were not taken after the end of the ecology screening week, regardless of when the patient had been admitted. During Periods One and Two, where consent was obtained for additional study specific samples, these were taken at least twice weekly. Patients who did not consent for additional study samples had samples taken only if clinically indicated as part of routine care, according to local practice. At all time points, microbiology data were obtained from samples taken for clinical reasons, including blood, nasopharyngeal, stool/rectal swabs, urine, sputum/secretions from the endotracheal tube and wound swabs.
Participants
Pilot cluster-randomised controlled trial eligibility criteria
Inclusion criteria
-
> 37 weeks corrected gestational age to < 16 years.
-
Receiving mechanical ventilation.
-
Expected to remain on mechanical ventilation for ≥ 48 hours (from the time of screening).
Exclusion criteria
-
Known allergy, sensitivity or interaction to polymyxin E (colistin), tobramycin or nystatin.
-
Known to be pregnant.
-
Death perceived as imminent.
Ecology period eligibility criteria
Inclusion criteria
-
All patients admitted to the PICU, regardless of ventilation status, during any of the three ecological surveillance periods.
There were no exclusion criteria for the ecology periods.
Data collection
Baseline patient characteristics
Baseline data were collected at critical care admission via data linkage with PICANet, and directly via trial case report forms (CRFs). The following baseline demographic and clinical data were summarised overall and by allocated treatment group and study period:
-
age (years) – median [interquartile range (IQR)]
-
age group (< 1 year, 1 year, 2 – 4 years, 5 – 9 years, 10 – 16 years) – number and %
-
sex (male, female) – number and %
-
weight (kg) – median (IQR)
-
ethnic group
-
Paediatric Index of Mortality (PIM) 3 score18 – median (IQR)
-
main reason for PICU admission.
Outcome definitions
The outcomes of this study were selected to measure the respective objectives as previously stated and were focused on assessing the feasibility of a larger-scale definitive study. Specifically, these were as follows:
-
The ability to randomise PICUs to either control or intervention was assessed by the successful random assignment of three PICUs to the intervention without delay to subsequent phases of the trial.
-
The willingness and ability of healthcare professionals to screen and recruit eligible children were assessed by the proportion of eligible children recorded on study screening logs successfully recruited to the pilot cRCT as a percentage of total eligible admissions, and the reported reasons for non-recruitment.
-
The potential recruitment rate for a future definitive cRCT trial of SDD-enhanced infection control in eligible children was estimated by combining the proportion of eligible children recruited to the pilot cRCT with the size of the potentially eligible population (estimated from nesting the screening log data from participating PICUs with the national UK PICU data from PICANet).
-
Adherence to the SDD protocol was assessed using the proportion of eligible children allocated to the intervention receiving (1) both elements and (2) each individual element of the SDD intervention, the number of days on which these elements were received relative to days eligible for the SDD intervention and the reported reasons for non-adherence.
-
Procedures for assessing and collecting selected clinical and ecological outcomes and for AE reporting were assessed using the proportion of children with complete data for these outcomes (as listed later) including, for ecological outcomes, the proportion consenting to additional study specific sample collection.
-
Generalisability of the study results to all UK PICUs was assessed by comparing baseline characteristics (as listed earlier) and potential outcome measures (as listed later) for children recruited to the pilot cRCT with data from all potentially eligible children (receiving invasive mechanical ventilation for at least three calendar days) within participating PICUs and within all UK PICUs (from PICANet).
With the aim of understanding potential patient-centred primary and secondary outcome measures for the definitive cRCT, the following potential outcome measures were reported by arm and trial period (during the ecology surveillance weeks, only HCAIs and positive microbiology results were recorded):
-
HCAI (confirmed/presumed) – as defined by a local microbiology and clinical team as infections acquired during the PICU stay (presented as number and % of enrolled patients)
-
any positive microbiology swab or sample – result, n/N (% of enrolled patients)
-
duration of invasive ventilation (days) – mean [standard deviation (SD)] and median (IQR)
-
days alive and free of ventilation to day 28 – median (IQR)
-
length of hospital stay days from confirming eligibility to hospital discharge – mean (SD) and median (IQR)
-
length of stay in PICU: hours from confirming eligibility to PICU discharge – mean (SD) and median (IQR)
-
PICU survival: status (alive or dead) before PICU discharge – number and %
-
hospital mortality: status (alive or dead) before hospital discharge – number and %.
-
mortality within 30 days post enrolment (alive or dead): from the patients’ survival status as of 30 days post enrolment on the CRF – number and %.
Safety monitoring and adverse events
Adverse event reporting followed the HRA guidelines on safety reporting in non-clinical trial investigational medicinal product studies.
The ICNARC CTU monitored data for documented AEs that are not considered to be related to the trial treatment. In the event that any trial procedure appeared to be resulting in AEs, the TMG was contacted for their opinion.
Data management
Participant data were entered onto a secure web-based data entry system. The site PI oversaw and was responsible for data collection, quality and recording. Collection of data could be delegated by the site PI to qualified members of the research team recorded on the Delegation Log. All data were collected and processed in line with General Data Protection Regulation (GDPR). Data entered onto the secure trial database underwent validation checks for completeness, accuracy and consistency of data. Queries on incomplete, inaccurate or inconsistent data were sent to the research team at participating sites for resolution. During the conduct of the trial, all electronic participant data were encrypted, and all trial documents stored securely at the site or the ICNARC CTU, as appropriate. On completion of the trial, all participant data (electronic and paper) and other trial documents were archived securely and will be retained for 10 years at the site, the sponsor or at the ICNARC CTU, as appropriate.
Sample size
The PICnIC pilot study was set up to test the feasibility of the protocol to recruit eligible patients. Therefore, there was no primary outcome to be compared between the two groups and, hence, a power calculation to determine sample size was not appropriate. Instead, the sample size has been determined to be adequate to estimate critical parameters to be tested to a necessary degree of precision. Based on available data from PICANet, it was anticipated that the participating sites would see approximately 4.5 eligible children per week; therefore, the anticipated recruitment rate was three children per PICU per week providing a total of approximately 324 children in 18 weeks, of which 90 would receive the intervention. Assuming an intracluster correlation coefficient (ICC) of 0.05 (average ICC for binary process measures in implementation studies, reported by the Health Services Research Unit at the University of Aberdeen),19 this sample size would enable rate parameters (recruitment, adherence, follow-up) with an observed value of 80% or greater to be estimated with a precision of ± 10% or less.
Statistical methods
The analyses were conducted using Stata/MP™ version 16.1 (StataCorp LP, College Station, TX, USA). All recruited patients were included with the trial population, excluding only those who withheld or withdrew consent to data collection. Children were analysed according to the group they were randomised to (based on site and date of recruitment), irrespective of whether the treatment allocated was received.
Screening and eligibility
The numbers of participants screened and deemed eligible are reported overall and by site.
Recruitment
The numbers of participants enrolled, consented (for study-specific procedures and use of their clinical data) are reported by site and treatment summarised as a Consolidated Standards of Reporting Trials (CONSORT) flow diagram. 20 The numbers of patients enrolled are reported per site and period of the study. When reported, the reasons for exclusion and non-consent are also summarised. Recruitment to the pilot cRCT was summarised graphically and presented as a rate of patients per week, overall and by site and study period.
Baseline demographic and clinical data were summarised for the trial population overall and by treatment group and study period. In addition, using PICANet data, patients’ characteristics are reported for potentially eligible children within participating PICUs, and within all UK PICUs, by study period. There was no statistical testing for any of the summary measures while comparing the baseline variables.
Adherence and protocol deviations
The number of screened patients, and the number and percentage of patients found to have been ineligible, together with the reasons for ineligibility (inclusion criteria not met or exclusion criteria met), were reported by treatment group and study period.
The number and percentage of patients with at least one protocol deviation in the SDD intervention group was reported, and the total number of such deviations. A deviation was defined as any of the following:
-
SDD treatment starts > 6 hours after being identified as eligible
-
SDD treatment starts within 6 hours of being identified as eligible but continues for more than 30 days (treatment period) or finishes before the patient is extubated/no longer mechanically ventilated
-
dose of one or both SDD treatments were not given, and patient was mechanically ventilated
-
dose was wrongly administered (four per day, every 6 hours while intubated).
Analysis principles
Data completeness of clinical and ecological outcomes and for AE reporting are summarised using counts and percentages, by intervention group and treatment period.
Potential outcome measures were summarised using counts and percentages (for binary outcomes), median and IQR (for all continuous outcomes), and means and SDs (for length of stay and duration of treatment), and they were reported by intervention group and study period.
To account for cluster randomisation, multilevel logistic or generalised linear regressions were used to estimate potential treatment effects. The effect estimate was calculated as the interaction between treatment group and time period and reported either as an odds ratio with 95% confidence interval (CI; for binary outcomes) or as difference in means with 95% CIs (for continuous outcomes). These calculations were done only to inform planning of a definitive trial and should not be used to draw any conclusions regarding differences in outcomes between treatment groups.
To determine the most appropriate primary outcome for a definitive trial, for all potential outcome measures the number of patients with complete data in each treatment group are reported. For measures requiring data linkage with routine data sources (PICANet), the proportion of successfully linked records are reported.
Confidence intervals and p-values
As this was a pilot cRCT and not powered to detect differences in outcomes, analyses were treated as exploratory and were mainly descriptive. p-values were not calculated or quoted. Effect estimates were reported with a 95% CI.
Missing data
To assess the follow-up procedures, the number (%) of participants with complete follow-up data for each of the potential outcome measures for the definitive RCT, overall and by treatment group, is reported. All analyses were undertaken in the trial population. There was no imputation of missing data.
Harms
The number and percentage of participants experiencing each prespecified AE (plus any other AEs as reported) while in PICU were collected for each treatment group. Numbers of serious AEs (SAEs), severity and reported relatedness SDD administration in the intervention sites during Period Two are also reported.
Chapter 3 Results of the Infection Control in Paediatric Intensive Care pilot cluster-randomised controlled trial
Screening and recruitment
Sites
The six participating PICU sites obtained local NHS permissions/approvals and opened to recruitment in parallel on 19 September 2021. Site initiation visits were carried out at all participating sites prior to start of patient screening and recruitment. Sites were randomised in week 6 of recruitment (during the baseline period) on 25 October 2021. The flow of sites (clusters) through the cRCT is presented in Figure 2, according to the CONSORT extension for cluster trials. 21
FIGURE 2.
CONSORT flow of sites (clusters) and patients. a, Duplicate record.

As planned, six sites participated in the PICnIC pilot cRCT. Intervention site initiation visits were carried at all intervention group sites ahead of the delivery of the SDD during the first week of the transition period. The transition period was delayed by 1 week due to delays in delivery of the SDD to sites. One intervention site had a further delay of a week due to local pharmacy sign-off issues and was only able to start the intervention from week 12.
One site from the intervention group of sites closed to recruitment on 30 January 2022, earlier than the planned completion date due to reaching their recruitment target of 30 children and cited the need to direct research capacity to other ongoing studies. The remaining five sites remained open to screening and recruitment until the end of recruitment on 13 February 2022.
Participants
Screening and recruitment
In total, 539 children were screened across the six sites during Periods One and Two combined, of which 434 (80.5%) were eligible. The rates of eligibility were higher across periods in the control sites, compared to the intervention sites. Of the 434 eligible children, 351 (80.9%) children were recruited (207 in Period One; 161 in Period Two). This was higher than the pre-trial expected patient recruitment of 306 children across the course of both Period One and Period Two (Table 2).
| Study period | Week 1 | Weeks 2–9 (Period One) |
Week 10 | Weeks 11–19 (Period Two) |
Week 20 | ||
|---|---|---|---|---|---|---|---|
| Sites | All | Intervention | Control | All | Intervention | Control | All |
| Screened, n | 89 | 142 | 125 | 70 | 125 | 147 | 74 |
| Eligible, n (% of screened) |
87 (97.8) | 111 (78.2) | 112 (89.6) | 65 (92.9) | 87 (69.6) | 123 (83.7) | 71 (95.9) |
| Recruited, n (% of eligible) |
73 (83.9) | 104 (93.7) | 103 (92.0) | 60 (92.3) | 67 (77.0) | 94 (76.4) | 58 (81.7) |
| Consented, n (% of recruited) |
22 (30.1) | 32 (30.8) | 46 (44.7) | 14 (23.3) | 38 (56.7) | 46 (48.9) | 19 (32.8) |
| Refused consent, n (% of recruited) | 7 (9.6) | 40 (38.5) | 17 (16.5) | 12 (20.0) | 16 (23.9) | 16 (17.0) | 17 (29.3) |
| Unable to approach for consent, n (% of recruited) | 44 (60.3) | 32 (30.8) | 40 (38.8) | 33 (55.0) | 11 (16.4) | 30 (31.9) | 22 (37.9) |
Overall recruitment was similar between intervention and control sites during Period One. During Period Two, a reduction in recruitment was observed across both groups, but due to the early cessation of recruitment at week 17 of the largest recruiting intervention site (Birmingham Children’s Hospital), this was more marked in the intervention sites (Figure 3).
FIGURE 3.
Cumulative number of patients recruited over time and by treatment group. a, Week 11 was treated as a transition period.
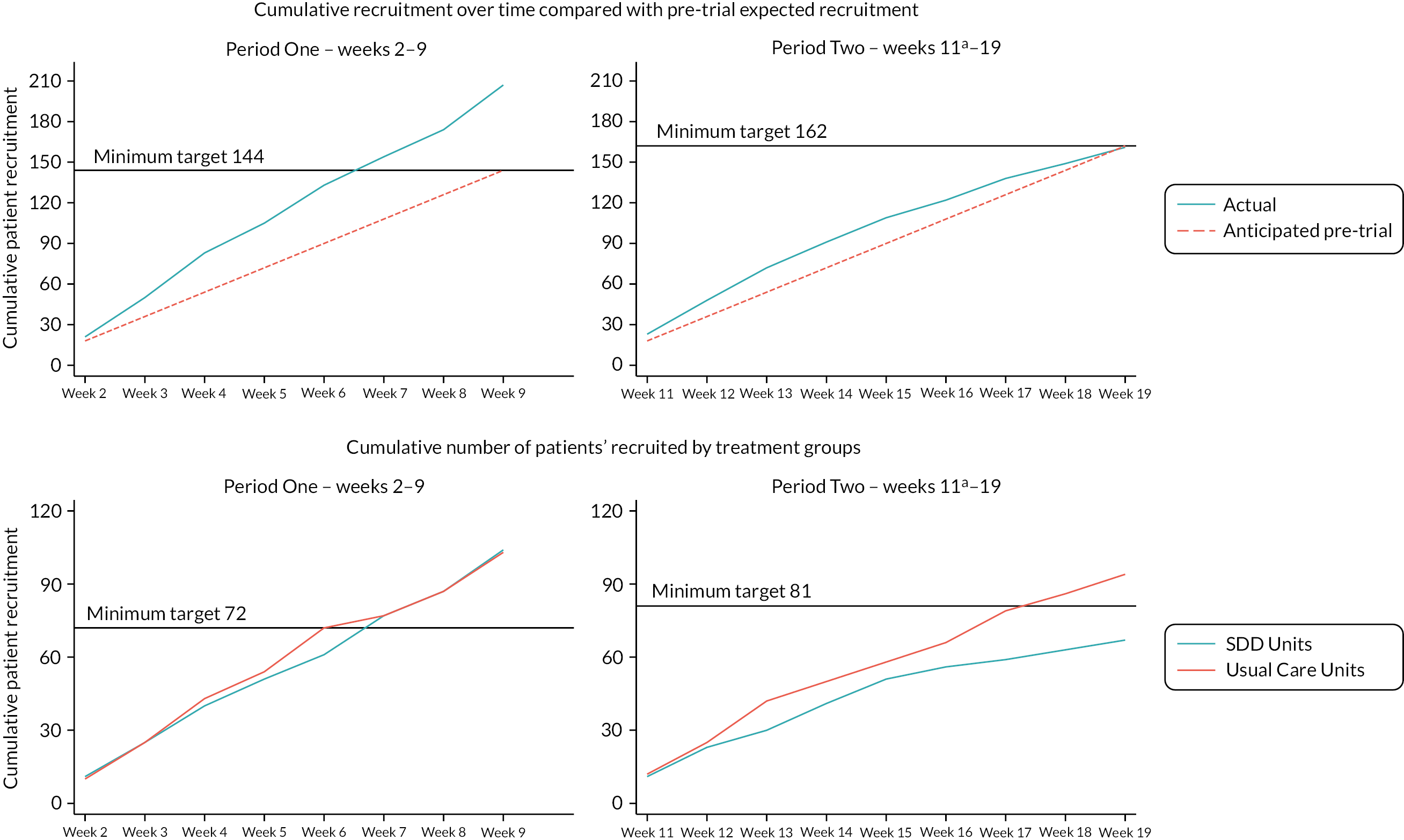
Recruitment varied across sites (Table 3, Figure 4). In Period One, with the exception of St George’s Hospital, the recruitment rate (patients per week) of each site met or exceeded the pre-trial estimates of three children per week. Within Period Two, there was a slight drop in recruitment across all sites, due to a seasonal reduction in eligible patients.
| Site | Period One | Period Two | ||||||
|---|---|---|---|---|---|---|---|---|
| Screened | Recruited | Screened | Recruited | |||||
| Total | Meana | Total n (%)b |
Meana | Total | Meana | Total n (%)b |
Meana | |
| Addenbrooke’s Hospital | 29 | 3.6 | 25 (100.0) | 3.1 | 37 | 4.1 | 23 (100.0) | 2.6 |
| Birmingham Children’s Hospital | 70 | 8.8 | 61 (93.8) | 7.6 | 57 | 6.3 | 29 (59.2) | 3.2 |
| St George’s Hospital, London | 43 | 5.4 | 18 (90.0) | 2.3 | 31 | 3.4 | 15 (88.2) | 1.7 |
| Total intervention | 142 | 17.8 | 104 (94.5) | 13.0 | 125 | 13.9 | 67 (75.3) | 7.4 |
| Bristol Royal Hospital for Children | 42 | 5.3 | 24 (77.4) | 3.0 | 55 | 6.1 | 20 (44.4) | 2.2 |
| John Radcliffe Hospital, Oxford | 28 | 3.5 | 26 (96.3) | 3.3 | 38 | 4.2 | 29 (93.5) | 3.2 |
| Southampton Children’s Hospital | 55 | 6.9 | 53 (96.4) | 6.6 | 54 | 6.0 | 45 (97.8) | 5.0 |
| Total control | 125 | 15.6 | 103 (91.2) | 12.9 | 147 | 16.3 | 94 (77.0) | 10.4 |
FIGURE 4.
Cumulative number of patients recruited by sites (solid lines indicate Period Two). Hospitals: Br, Bristol Royal Hospital for Children; Jr, John Radcliffe Hospital, Oxford; So, Southampton Children’s Hospital; Ad, Addenbrooke’s Hospital; Bi, Birmingham Children’s Hospital; Sg, St George’s Hospital, London. Period: p1, Period One (weeks 2–9); p2, Period Two (weeks 11–19).

Consent
Consent for the collection of additional samples for study-specific ecology monitoring was obtained in 162 patients (44% of those recruited), with differences seen between intervention and control sites. Intervention sites showed a higher rate of refused consent, but this difference was reduced during Period Two (see Table 2). This was therefore not deemed to be related to the delivery of the intervention. In over 30% of the recruited patients (43 in the intervention group and 70 in the control group), the research teams stated that they were unable to approach the parents for consent, of which in 25 cases (22%) staff reported it was not appropriate to approach them.
Patient characteristics
Patients were well balanced across treatment groups and time periods (Table 4). The mean age of patients was similar in Period One (33.3 months in the usual care sites and 31.5 months in the intervention sites), and in Period Two (28.0 months in the usual care sites and 25.0 months in the intervention sites), with most of the recruited children being < 1 year old. Over 62% of the patients were male, although this proportion was slightly lower in the intervention group during Period One. The recruited children were predominantly white in ethnicity, with a slightly higher proportion of Asian ethnicity at intervention group sites. The PIM3 score was similar across treatment groups in both time periods but slightly lower during the intervention Period Two (than during the usual care Period One), with a median predicted risk of 2%. The most common primary diagnostic group of the recruited children was respiratory (43%), followed by cardiac (23%), with a slightly higher proportion of both diagnosis in the intervention group during Period One (49% and 27%, respectively) and a higher proportion with infection as primary diagnostic group in the intervention group during Period One (16%), and in the usual care group during Period Two (20%).
| Variables | Recruited patients | |||
|---|---|---|---|---|
| Intervention sites | Control sites | |||
| Period One | Period Two | Period One | Period Two | |
| N = 104 | N = 56 | N = 103 | N = 82 | |
| Age at admission (months) | ||||
| Median (IQR) | 7 (48) | 5 (18) | 7 (43) | 5 (25) |
| Mean (SD) | 31.5 (46.5) | 25.0 (45.3) | 33.3 (52.5) | 28.0 (49.0) |
| Age category at admission | ||||
| < 1 year | 55/101 (54.5%) | 34/55 (61.8%) | 55/100 (55.0%) | 53/79 (67.1%) |
| 1 years | 13/101 (12.9%) | 9/55 (16.4%) | 15/100 (15.0%) | 5/79 (6.3%) |
| 2–4 years | 8/101 (7.9%) | 3/55 (5.5%) | 9/100 (9.0%) | 7/79 (8.9%) |
| 5–9 years | 15/101 (14.9%) | 5/55 (9.1%) | 9/100 (9.0%) | 6/79 (7.6%) |
| 10–16 years | 10/101 (9.9%) | 4/55 (7.3%) | 12/100 (12.0%) | 8/79 (10.1%) |
| Sex | ||||
| Male | 58/101 (57.4%) | 38/55 (69.1%) | 64/100 (64.0%) | 49/79 (62.0%) |
| Female | 43/101 (42.6%) | 17/55 (30.9%) | 36/100 (36.0%) | 30/79 (38.0%) |
| Ethnic category | ||||
| Asian | 12/101 (11.9%) | 8/55 (14.5%) | 5/100 (5.0%) | 4/79 (5.1%) |
| Black | 9/101 (8.9%) | 1/55 (1.8%) | 2/100 (2.0%) | 3/79 (3.8%) |
| Chinese | 0/101 (0.0%) | 1/55 (1.8%) | 0/100 (0.0%) | 0/79 (0.0%) |
| Mixed | 6/101 (5.9%) | 1/55 (1.8%) | 3/100 (3.0%) | 6/79 (7.6%) |
| White | 55/101 (54.5%) | 36/55 (65.5%) | 63/100 (63.0%) | 41/79 (51.9%) |
| Other | 8/101 (7.9%) | 3/55 (5.5%) | 2/100 (2.0%) | 0/79 (0.0%) |
| Unknown | 11/101 (10.9%) | 5/55 (9.1%) | 25/100 (25.0%) | 25/79 (31.6%) |
| PIM3 predicted risk of PICU mortality (%) | ||||
| Median (IQR) | 4 (7) | 3 (5) | 2 (4) | 2 (5) |
| Mean (SD) | 5.8 (7.5) | 6.3 (12.6) | 4.2 (7.8) | 5.7 (11.9) |
| Primary diagnosis group | ||||
| Neurological | 5/101 (5.0%) | 3/55 (5.5%) | 7/100 (7.0%) | 4/79 (5.1%) |
| Cardiac | 22/101 (21.8%) | 15/55 (27.3%) | 25/100 (25.0%) | 16/79 (20.3%) |
| Respiratory | 42/101 (41.6%) | 27/55 (49.1%) | 43/100 (43.0%) | 33/79 (41.8%) |
| Oncology | 1/101 (1.0%) | 2/55 (3.6%) | 1/100 (1.0%) | 0/79 (0.0%) |
| Infection | 16/101 (15.8%) | 2/55 (3.6%) | 9/100 (9.0%) | 16/79 (20.3%) |
| Musculoskeletal | 0/101 (0.0%) | 0/55 (0.0%) | 0/100 (0.0%) | 1/79 (1.3%) |
| Gastrointestinal | 2/101 (2.0%) | 0/55 (0.0%) | 3/100 (3.0%) | 0/79 (0.0%) |
| Other | 5/101 (5.0%) | 2/55 (3.6%) | 6/100 (6.0%) | 7/79 (8.9%) |
| Blood and lymph | 2/101 (2.0%) | 1/55 (1.8%) | 1/100 (1.0%) | 1/79 (1.3%) |
| Trauma | 2/101 (2.0%) | 0/55 (0.0%) | 1/100 (1.0%) | 1/79 (1.3%) |
| Endocrine/metabolic | 2/101 (2.0%) | 1/55 (1.8%) | 3/100 (3.0%) | 0/79 (0.0%) |
| Multisystem | 0/101 (0.0%) | 0/55 (0.0%) | 0/100 (0.0%) | 0/79 (0.0%) |
| Body wall and cavities | 2/101 (2.0%) | 0/55 (0.0%) | 1/100 (1.0%) | 0/79 (0.0%) |
| Unknown | 0/101 (0.0%) | 2/55 (3.6%) | 0/100 (0.0%) | 0/79 (0.0%) |
Adherence to sampling
The vast majority of patients (91–99% across both periods) had samples taken at admission to the PICU. Consent for repeat samples was low (44%).
When consent was obtained for the additional sampling, adherence to the sampling regimen was good, with samples collected for more than 90% of eligible patients at each time point as shown in Table 5. Repeat samples were not taken (missed) for consented patients on a total of 11 occasions.
| Ecology surveillance (week 1) | Period One | Ecology surveillance (week 10) | Period Two | Ecology surveillance (week 20) | |
|---|---|---|---|---|---|
| Admission sample | |||||
| Enrolled, n | 73 | 207 | 60 | 138 | 58 |
| Sample taken, n (%) | 71 (97.3) | 204 (98.6) | 55 (91.7) | 137 (99.3) | 53 (91.4) |
| Sample not taken, n (%) | 2 (2.7) | 3 (1.4) | 5 (8.3) | 1 (0.7) | 5 (8.6) |
| Sample taken on day of admission, n (%) | n/aa | 184 (88.9) | n/aa | 123 (89.1) | n/aa |
| First repeat sample | |||||
| Consented and still in PICU, n | 8 | 56 | 9 | 43 | 5 |
| Research sample taken, n (%) | 7 (87.5) | 54 (96.4) | 6 (66.7) | 42 (97.7) | 5 (100.0) |
| Research sample not taken, n (%) | 1 (12.5) | 2 (3.6) | 3 (33.3) | 1 (2.3) | 0 (0.0) |
| Did not consent and still in PICU,a n | 50 | 22 | |||
| Did not consent and routine sample taken, n | 11 | 9 | |||
| Second repeat sample | |||||
| Consented and still in PICU, n | 42 | 26 | |||
| Research sample taken, n (%) | 40 (95.2) | 26 (100.0) | |||
| Research sample not taken, n (%) | 2 (4.8) | 0 (0.0) | |||
| Did not consent and still in PICU,a n | 30 | 15 | |||
| Did not consent and routine sample taken, n | 10 | 7 | |||
| Third repeat sample | |||||
| Consented and still in PICU, n | 20 | 15 | |||
| Research sample taken, n (%) | 19 (95.0) | 14 (93.3) | |||
| Research sample not taken, n (%) | 1 (5.0) | 1 (6.7) | |||
| Did not consent and still in PICU,a n | 19 | 8 | |||
| Did not consent and routine sample taken, n | 1 | 2 | |||
Adherence to selective decontamination of the digestive tract intervention
After excluding week 11 (transition week), a total of 56 children were recruited in the intervention sites, and 55 of them (98%) received SDD treatment (Table 6). Around 68% of eligible children received SDD within the first 6 hours as per protocol. The median (IQR) number of doses administered per patient was 14 (9–32) for the SDD oral paste and 14 (9–32) for the gastric suspension. Of the expected doses, 9.2% of the oral paste and 9.1% of the gastric suspension were not administered. The main reasons were being nil by mouth (29.5% and 31.1%, respectively) and dose missed (26.2% and 24.6%, respectively).
| SDD treatment method | |||
|---|---|---|---|
| Any | Oral paste | Gastric suspension | |
| Patients | |||
| Enrolled, n | 56 | ||
| Received SDD, n (%) | 55 (98.2) | ||
| Received SDD within 6 hours of enrolment, n (%) | 38 (67.9) | ||
| Doses | |||
| Total expected,a n | 1330 | 1330 | |
| Total administered, n (%) | 1208 (90.8) | 1209 (90.9) | |
| Total not administered, n (%) | 122 (9.2) | 121 (9.1) | |
| Median (IQR) per patient | 14.0 (9.0–32.0) | 14.0 (9.0–32.0) | |
| Reasons for doses not given | |||
| Not applicable, n (%) | 13 (10.7) | 4 (3.3) | |
| Nil by mouth, n (%) | 36 (29.5) | 38 (31.1) | |
| Unable to administer via prescribed route, n (%) | 4 (3.3) | 5 (4.1) | |
| Dose missed, n (%) | 32 (26.2) | 30 (24.6) | |
| Omitted on clinician’s instruction, n (%) | 6 (4.9) | 6 (4.9) | |
| SDD not available, n (%) | 10 (8.2) | 13 (10.7) | |
| Prescription issues, n (%) | 9 (7.4) | 14 (11.5) | |
| Parent decision, n (%) | 11 (9.0) | 10 (8.2) | |
Potential outcome measures
Completeness of the ecology outcome measures was excellent in intervention sites and very good in the usual care sites (Table 7). Table 8 summarises the outcome data by ecology week.
| Outcome measure | Intervention sites | Control sites | ||||
|---|---|---|---|---|---|---|
| Week 1 | Week 10 | Week 20 | Week 1 | Week 10 | Week 20 | |
| Patients enrolled, n | 41 | 31 | 39 | 32 | 29 | 19 |
| Data completed, n (%) | 40 (97.6) | 31 (100.0) | 39 (100.0) | 30 (93.8) | 27 (93.1) | 19 (100.0) |
| Any microbiology positive result, n (%) | 40 (97.6) | 31 (100.0) | 39 (100.0) | 30 (93.8) | 27 (93.1) | 19 (100.0) |
| Site of positive sample, n (%) | 17/17 (100.0) | 10/10 (100.0) | 17/17 (100.0) | 8/8 (100.0) | 4/4 (100.0) | 6/6 (100.0) |
| Organism, n (%) | 17/17 (100.0) | 10/10 (100.0) | 17/17 (100.0) | 8/8 (100.0) | 4/4 (100.0) | 6/6 (100.0) |
| Outcome measure | Intervention sites | Control sites | ||||
|---|---|---|---|---|---|---|
| Week 1 | Week 10 | Week 20 | Week 1 | Week 10 | Week 20 | |
| Patients enrolled, n | 41 | 31 | 39 | 32 | 29 | 19 |
| Patients with positive microbiology result, n (%) | 17/40 (42.5) | 10/31 (32.3) | 17/39 (43.6) | 8/30 (26.7.0) | 4/27 (14.8) | 6/19 (31.6) |
| Total microbiology positive results, n | 22 | 12 | 32 | 13 | 7 | 9 |
| Site of positive sample | ||||||
| Nasopharyngeal, n (%) | 13 (59.1) | 4 (33.3) | 13 (40.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Stool/rectal, n (%) | 2 (9.1) | 1 (8.3) | 2 (6.3) | 5 (38.5) | 0 (0.0) | 2 (22.2) |
| Urine, n (%) | 0 (0.0) | 0 (0.0) | 2 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| ETT secretions, n (%) | 5 (22.7) | 6 (50.0) | 12 (37.5) | 8 (61.5) | 6 (85.7) | 5 (55.6) |
| Wound, n (%) | 0 (0.0) | 1 (8.3) | 3 (9.4) | 0 (0.0) | 1 (14.3) | 0 (0.0) |
| Blood, n (%) | 2 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (22.2) |
| Organism(s) of positive samplea | ||||||
| Gram-negative bacteria, n (%) | 8 (36.4) | 2 (16.7) | 14 (43.8) | 6 (46.2) | 4 (57.1) | 6 (66.7) |
| Gram-positive bacteria, n (%) | 8 (36.4) | 3 (25.0) | 7 (21.9) | 6 (46.2) | 3 (42.9) | 3 (33.3) |
| Virology, n (%) | 9 (40.9) | 12 (100.0) | 17 (53.1) | 4 (30.8) | 3 (42.9) | 1 (11.1) |
| Fungal, n (%) | 2 (9.1) | 0 (0.0) | 2 (6.3) | 1 (7.7) | 1 (14.3) | 0 (0.0) |
| Other, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Overall, patient-centred potential outcomes measures had an excellent completion rate (Table 9), which was similar between groups with a range between 96.3% and 100%.
| Outcome measure | Intervention sites | Control sites | ||
|---|---|---|---|---|
| Period One (n = 104) | Period Twoa (n = 56) | Period One (n = 103) | Period Twoa (n = 82) | |
| Patients enrolled, n | 104 | 56 | 103 | 82 |
| HCAI, n (%) | 104 (100.0) | 56 (100.0) | 102 (99.0) | 82 (100.0) |
| Any positive microbiology result, n (%) | 104 (100.0) | 56 (100.0) | 103 (100.0) | 82 (100.0) |
| Duration of invasive ventilation (days), n (%) | 104 (100.0) | 56 (100.0) | 102 (99.0) | 82 (100.0) |
| Days alive and free of ventilation to day 28, n (%) | 101 (97.1) | 55 (98.2) | 100 (97.0) | 79 (96.3) |
| Length of PICU stay (days), n (%) | 104 (100.0) | 56 (100.0) | 102 (99.0) | 82 (100.0) |
| Length of hospital stay (days), n (%) | 104 (100.0) | 54 (96.4) | 98 (95.1) | 82 (100.0) |
| PICU mortality, n (%) | 104 (100.0) | 56 (100.0) | 102 (99.0) | 82 (100.0) |
| Hospital mortality, n (%) | 104 (100.0) | 54 (96.4) | 100 (97.1) | 82 (100.0) |
| 30-day mortality, n (%) | 103 (99.0) | 56 (100.0) | 101 (98.1) | 82 (100.0) |
Characteristics of the patient-centred potential outcomes among all patients receiving standard care (all children in control PICUs and children in intervention PICUs in Period One) are reported in Table 10.
| Outcome measure | Patient receiving standard care,a n (%) [N] | ICC (CI) |
|---|---|---|
| HCAI | 34 (11.8) [288] | 0.316 (0.060 to 0.771) |
| Any positive microbiology result | 172 (59.7) [288] | 0.374 (0.129 to 0.707) |
| Duration of invasive ventilation, days | 7.6 (7.9) [289] | 0.012 (0.001 to 0.177) |
| Days alive and free of ventilation to day 28 | 19.5 (8.1) [280] | 0.018 (0.001 to 0.188) |
| Length of PICU stay, days | 10.1 (10.6) [288] | 0.014 (0.001 to 0.164) |
| Length of hospital stay, days | 23.3 (26.9) [284] | 0.006 (0.000 to 0.296) |
| PICU mortality | 21 (7.3) [288] | 0.099 (0.007 to 0.629) |
| Hospital mortality | 27 (9.4) [286] | 0.068 (0.002 to 0.684) |
| 30-day mortality | 22 (7.7) [286] | 0.041 (0.000 to 0.803) |
Although the study has not been powered to compare outcomes between groups, a summary of the potential outcome effects is shown in Table 11 to allow consideration of plausible ranges of treatment effects for a definitive trial. As anticipated for a pilot study, the CIs do not rule out either substantial benefit or harm on any of the potential outcomes.
| Outcome measure | Intervention sites | Control sites | Effect estimate (95% CI) | ||
|---|---|---|---|---|---|
| Period One | Period Two | Period One | Period Two | ||
| HCAI, n/N (%) | 6/104 (5.8) | 4/56 (7.1) | 16/102 (15.7) | 12/82 (14.6) | 1.31a (0.27 to 6.38) |
| Any positive microbiology result, n/N (%) | 62/104 (59.6) | 30/56 (53.6) | 61/102 (59.8) | 49/82 (59.8) | 0.92a (0.34 to 2.46) |
| Duration of invasive ventilation (days), [N] | [104] | [56] | [102] | [82] | |
| Mean (SD) | 8.7 (10.8) | 8.5 (13.2) | 7.7 (6.3) | 6.0 (4.3) | 1.51 (−2.37 to 5.38) |
| Median (IQR) | 5 (3–8) | 5 (4–10) | 5 (3–9) | 4 (3–7) | |
| Days alive and free of ventilation to day 28b | [101] | [55] | [100] | [79] | |
| Mean (SD) | 19.0 (8.8) | 19.9 (7.3) | 18.8 (8.3) | 21.0 (6.6) | −1.57 (−5.05 to 1.91) |
| Median (IQR) | 23 (17–25) | 23 (18–25) | 23 (15–25) | 24 (20–25) | |
| Length of PICU stay (days), [N] | [104] | [56] | [102] | [82] | |
| Mean (SD) | 11.5 (13.6) | 11.5 (16.8) | 10.1 (9.3) | 8.3 (7.2) | 1.95 (−3.18 to 7.08) |
| Median (IQR) | 7 (4–13) | 6 (4–13) | 6 (5–13) | 6 (4–10) | |
| Length of hospital stay (days), [N] | [104] | [54] | [98] | [82] | |
| Mean (SD) | 26.4 (29.0) | 25.9 (25.2) | 21.7 (28.4) | 21.4 (21.5) | −0.11 (−11.76 to 11.55) |
| Median (IQR) | 16 (8–31) | 15 (8–39) | 12 (8–25) | 13 (8–26) | |
| PICU mortality, n/N (%) | 7/104 (6.7) | 3/56 (5.4) | 10/102 (9.8) | 4/82 (4.9) | 1.75a (0.27 to 11.17) |
| Hospital mortality, n/N (%) | 8/104 (7.7) | 3/54 (5.6) | 13/100 (13.0) | 6/82 (7.3) | 1.41a (0.25 to 7.85) |
| 30-day mortality, n/N (%) | 6/103 (5.8) | 3/56 (5.4) | 11/101 (10.9) | 5/82 (6.1) | 1.76a (0.29 to 10.72) |
The proportions of children who received any antimicrobial therapy were similar across groups (Figure 5), with a reduction during the second week following eligibility.
FIGURE 5.
Proportion of children who received any antimicrobial therapy during PICU stay.

Adverse events
Among the 67 patients, there was only one AE reported during Period Two in the intervention sites, which was not assessed as serious. No SAEs were reported (Table 12).
| Intervention sites | Control sites | |
|---|---|---|
| Number of patients | 67 | 94 |
| Total number of AEs | 1 | 0 |
| Number (%) of patients experiencing one or more AEs | 1 (1.5) | 0 (0.0) |
| Specified AEs, n (%) | ||
| NG tube blockage | 0 (0.0) | 0 (0.0) |
| Choking on paste | 1 (100.0) | 0 (0.0) |
| Allergic reaction to SDD | 0 (0.0) | 0 (0.0) |
| Total number of SAEs | 0 | 0 |
| Number (%) of patients experiencing one or more SAEs | 0 (0.0) | 0 (0.0) |
Generalisability to the wider paediatric intensive care unit population
Characteristics of participating sites
The characteristics of the six PICUs that participated in the PICnIC pilot cRCT compared with all PICUs in PICANet (n = 29) are presented in Table 13. Overall, the sites participating in the study were a representative mix of small and large PICUs with a broad case mix of cardiac and general admissions drawn from across the NHS England geographical region.
| Participating sites | UK PICUs | ||
|---|---|---|---|
| Type of PICU | |||
| General | 3 (50%) | 18 (62%) | |
| General and cardiac | 3 (50%) | 7 (24%) | |
| Cardiac | 0 (0%) | 4 (14%) | |
| PICU beds (intensive care and high dependency) | |||
| < 8 | 0 (0%) | 5 (17%) | |
| 8–11 | 1 (17%) | 1 (3%) | |
| 12–15 | 2 (33%) | 8 (28%) | |
| ≥ 16 | 3 (50%) | 15 (52%) | |
| Annual PICU admissions | |||
| < 550 | 2 (33%) | 18 (62%) | |
| 550–749 | 3 (50%) | 6 (21%) | |
| 750–999 | 0 (0%) | 3 (10%) | |
| ≥ 1000 | 1 (17%) | 2 (7%) | |
Characteristics of participating patients
Table 14 shows patient characteristics, for recruited patients by treatment group and study period, and for all potentially eligible patients in study PICUs, and in all UK PICUs over the period the study was recruiting.
| Variables | Overall | Potentially eligible patients | |
|---|---|---|---|
| N = 345 | Study PICUs (n = 6) | All UK PICUs (n = 29) | |
| N = 464 | N = 1730 | ||
| Age at admission (months) | |||
| Median (IQR) | 6 (32) | 7.0 (42.5) | 5.0 (32.0) |
| Mean (SD) | 30.1 (48.7) | 31.4 (48.7) | 30.2 (49.5) |
| Age category at admission, n/N (%) | |||
| < 1 year | 197/335 (58.8%) | 269/464 (58%) | 1062/1730 (61%) |
| 1 years | 42/335 (12.5%) | 47/464 (10%) | 168/1730 (10%) |
| 2–4 years | 27/335 (8.1%) | 54/464 (12%) | 184/1730 (11%) |
| 5–9 years | 35/335 (10.4%) | 50/464 (11%) | 138/1730 (8%) |
| 10–16 years | 34/335 (10.1%) | 44/464 (9%) | 178/1730 (10%) |
| Sex | |||
| Male | 209/335 (62.4%) | 280/464 (60%) | 976/1730 (56%) |
| Female | 126/335 (37.6%) | 184/464 (40%) | 754/1730 (44%) |
| Ethnic category, n/N (%) | |||
| Asian | 29/335 (8.7%) | 40/464 (9%) | 202/1730 (12%) |
| Black | 15/335 (4.5%) | 27/464 (6%) | 98/1730 (6%) |
| Chinese | 1/335 (0.3%) | 1/464 (0%) | 11/1730 (1%) |
| Mixed | 16/335 (4.8%) | 24/464 (5%) | 68/1730 (4%) |
| White | 195/335 (58.2%) | 262/464 (56%) | 1028/1730 (59%) |
| Other | 13/335 (3.9%) | 23/464 (5%) | 67/1730 (4%) |
| Unknown | 66/335 (19.7%) | 87/464 (19%) | 256/1730 (15%) |
| PIM3 predicted risk of PICU mortality (%) | |||
| Median (IQR) | 3 (5) | 3.2 (5.3) | 2.2 (4.9) |
| Mean (SD) | 5.4 (9.7) | 6.0 (10.5) | 5.2 (9.0) |
| Primary diagnosis group, n/N (%) | |||
| Neurological | 19/335 (5.7%) | 41/464 (9%) | 138/1730 (8%) |
| Cardiac | 78/335 (23.3%) | 109/464 (23%) | 440/1730 (25%) |
| Respiratory | 145/335 (43.3%) | 178/464 (38%) | 738/1730 (43%) |
| Oncology | 4/335 (1.2%) | 8/464 (2%) | 29/1730 (2%) |
| Infection | 43/335 (12.8%) | 48/464 (10%) | 120/1730 (7%) |
| Musculoskeletal | 1/335 (0.3%) | 3/464 (1%) | 20/1730 (1%) |
| Gastrointestinal | 5/335 (1.5%) | 21/464 (5%) | 68/1730 (4%) |
| Other | 20/335 (6.0%) | 29/464 (6%) | 72/1730 (4%) |
| Blood and lymph | 5/335 (1.5%) | 6/464 (1%) | 12/1730 (1%) |
| Trauma | 4/335 (1.2%) | 4/464 (1%) | 21/1730 (1%) |
| Endocrine/metabolic | 6/335 (1.8%) | 9/464 (2%) | 43/1730 (2%) |
| Multisystem | 0/335 (0.0%) | 0/464 (0%) | 3/1730 (0%) |
| Body wall and cavities | 3/335 (0.9%) | 3/464 (1%) | 21/1730 (1%) |
| Unknown | 2/335 (0.6%) | 5/464 (1%) | 5/1730 (0%) |
| Died in PICU during admission event, n/N (%) | |||
| No | 311/335 (92.8%) | 422/464 (91%) | 1605/1730 (93%) |
| Yes | 24/335 (7.2%) | 42/464 (9%) | 125/1730 (7%) |
| Length of stay (days) | |||
| Median (IQR) | 7 (8) | 8.4 (9.8) | 8.0 (9.7) |
| Mean (SD) | 12.0 (18.4) | 14.4 (20.3) | 14.7 (20.9) |
| Days free from invasive ventilation | |||
| Median (IQR)a | 1 (2) | 1 (3) | 1 (4) |
To assess generalisability of the study results, baseline characteristics and outcomes for children recruited to the pilot cRCT are displayed alongside with data from PICANet for all potentially eligible children (receiving invasive mechanical ventilation for at least three calendar days) within participating PICUs and within all UK PICUs. Children who were recruited to the PICnIC study were representative of similar potentially eligible patients in the study PICUs and all UK PICUs, but they were more likely to be male and with a primary diagnosis of infection when compared with those in all UK PICUs.
Statistical approach to a definitive clinical trial of selective decontamination of the digestive tract in the paediatric intensive care unit setting
The potential recruitment rate for a future definitive cRCT was 2.98 children/site/week and was estimated by combining a potentially eligible population of 1730 children (estimated from nesting the screening log data from participating PICUs with the national UK PICU data from PICANet) and the overall proportion of eligible children recruited of 85%. It was extremely similar to the pre-trial estimated rate value of 3.0. The number of eligible children identified from screening logs in the six recruiting PICUs (434) was very similar to the estimate of potentially eligible children from PICANet for these PICUs (464).
Assuming a parallel-arm cluster-randomised design with a baseline period, and with only 29 PICUs in the UK, Table 15 shows the number of clusters per arm and overall sample size required to detect alternative treatment effects with 90% power and a significance level of 0.05. The number of children per cluster is set at 190 (based on 1 year of recruitment, including the baseline period), using the ICC observed and mean/proportion among all patients receiving usual care during the pilot cRCT. After considering different scenarios, none of the binary outcomes are feasible with the number of clusters in the UK, except maybe positive microbiology. However, we should note that it is not very patient-centred and corresponds to an unlikely large reduction. Continuous outcomes are feasible for small to moderate treatment effects. We are assuming a simple approach of difference in means here for illustration and a final definitive sample size calculation would require more in-depth work on analysis approach. Further modifications to study design may also be considered; for example, the use of a cluster-crossover design. The cluster sample size application (https://clusterrcts.shinyapps.io/rshinyapp/) was used for sample size calculations.
| Study outcome (% or mean) | Relative risk/effect sizes SD | Absolute difference | Clusters per arm (sample size per arm) | |
|---|---|---|---|---|
| HCAI | 11.8% | 0.8 | 2.4% | 434 (82,460) |
| 11.8% | 0.6 | 4.4% | 120 (22,800) | |
| Any positive microbiology result | 59.7% | 0.8 | 11.9% | 53 (10,070) |
| 59.7% | 0.6 | 23.9% | 13 (2470) | |
| Duration of invasive ventilation, days | 7.6 | 0.2 SD | 1.6 | 12 (2280) |
| 7.6 | 0.3 SD | 2.4 | 5 (950) | |
| Days alive and free of mechanical ventilation at 28 days | 19.5 | 0.2 SD | 1.6 | 12 (2280) |
| 19.5 | 0.3 SD | 2.4 | 5 (950) | |
| Length of PICU stay, days | 10.1 | 0.2 SD | 2.0 | 12 (2280) |
| 10.1 | 0.3 SD | 3.2 | 5 (950) | |
| Length of hospital stay, days | 23.3 | 0.2 SD | 5.4 | 8 (1520) |
| 23.3 | 0.3 SD | 8.1 | 4 (760) | |
| PICU mortality | 7.3% | 0.8 | 1.5% | 290 (55,100) |
| 7.3% | 0.6 | 2.9% | 70 (13,300) | |
| Hospital mortality | 9.4% | 0.8 | 1.9% | 179 (34,010) |
| 9.4% | 0.6 | 3.8% | 40 (7600) | |
| 30-day mortality | 7.7% | 0.8 | 1.54 | 182 (34,580) |
| 7.7% | 0.6 | 3.1% | 38 (7220) |
Chapter 4 Infection Control in Paediatric Intensive Care mixed-methods study to assess trial design and processes
Research exploring family and practitioner perspectives can help identify whether a clinical trial is acceptable and feasible. Importantly, such insight, from qualitative or mixed-methods work, can help ensure that trials are designed in a way that is patient- and family-centred. Trials in paediatric intensive care can be challenging to conduct with the need to carefully consider recruitment and consent processes so they do not cause burden or delay clinical care.
Methods
Study design
The PICnIC pilot cRCT included an embedded mixed-methods study. This involved a questionnaire and interviews with parents/legal representatives of children involved in the pilot cRCT, as well as focus groups, interviews and an online survey with PICU practitioners.
Aims and objectives
To assess with input from PICU healthcare professionals:
-
the acceptability of implementation of the SDD intervention, recruitment and consent procedures
-
the acceptability of collecting data to assess the selected clinical and ecological data
-
the acceptability of the SDD intervention and confirm interest in participation in a definitive trial in the wider PICU community.
To review, explore and test with input from parents/guardians of recruited patients:
-
the acceptability of a definitive trial that includes the SDD intervention
-
the acceptability of the recruitment and consent procedures for the definitive trial, including all proposed information materials
-
the selection of important, relevant, patient-centred primary and secondary outcomes for a definitive trial.
Design and development of the protocol
The design and development of the protocol, including sample estimation, recruitment strategy and interview topic guide (see Appendix 2 and 3), were informed by previous feasibility studies22,23 and reviewed by a PPI member. A list of outcomes to explore with parents during interviews (see Appendix 4) was developed from a review of relevant literature22,23 (and through a discussion with the larger team involved in the PICnIC pilot study) and reviewed by a PPI member.
Participants
Based on previous studies,22,23 we anticipated recruiting 15–25 parents/legal representatives to an interview to reach the point of information power, and a balance of parents of children in each trial arm was reached. Information power24 is the point at which data address the study aims, including sample specificity, participants’ experience relevant to the study aims and sample diversity.
Based on previous studies and the cRCT sample size, we aimed to receive approximately 100 parent questionnaires if both parents were present at the time of recruitment. For practitioners, we aimed to include approximately 8 to 10 practitioners in each of the two focus groups and up to 10 interviews (for those who could not attend a focus group), as well as an online survey using snowball sampling to involve wider PICU staff not involved in the pilot cRCT.
Parents/legal representatives were recruited via the same process and information materials used in the pilot cRCT. During the recruitment discussion, practitioners invited parents to complete the questionnaire and/or provide contact details if they wished to take part in an interview. Due to the COVID-19 pandemic, parents were offered interviews online [via Zoom (Zoom Video Communications, San Jose, CA, USA)] or via telephone. Parents were asked to complete the questionnaire before they left the PICU and return it in the envelope provided, which was addressed to the University of Liverpool team.
Pilot cRCT practitioners were recruited to focus groups and the online survey via an e-mail invitation and Twitter [now X (X Corp., San Francisco, CA, USA); www.twitter.com] advertising. To boost survey recruitment, MP attended the Paediatric Critical Care Society Study Group (PCCS-SG) meeting in May 2022 and presented a study recruitment update with link to the online survey and QR code.
Eligibility criteria
Inclusion criteria:
-
parents/legal representatives of children involved in the pilot cRCT, including those who withdraw from data collection.
Exclusion criteria:
-
parents/legal representatives who do not speak English.
We use the term parents to include legal representatives from this point forward for brevity.
Screening and conduct of interviews and focus groups interviews
Expressions of interest to participate in an interview were responded to in sequential order. Once eligibility was confirmed, an interview date and time were scheduled.
The draft pilot RCT PIS and list of potential outcomes were e-mailed to parents to read prior to the interview. Screening and interview conduct stopped when data saturation and sample variation (recruitment of parents via multiple recruitment routes) were achieved.
Informed consent
The University of Liverpool researcher (MP) contacted parents/legal representatives to arrange an interview within 1 month of consent (or withdrawn consent) for the pilot cRCT. Parents were offered either a telephone or online video conference interview via Zoom. Informed consent for participation, for audio recording and to receive a copy of the mixed-methods study findings was sought verbally before the interview commenced. This involved the researcher (MP) reading each aspect of the consent form to parents. Each box was initialled by the researcher on the consent form when verbal consent was provided by the parent. Informed consent discussions were audio-recorded for auditing purposes.
Conduct of the interviews
All parent interviews were conducted by MP using the parent/legal representative interview topic guide (see Appendix 2 and 3). Respondent validation was used so that previously unanticipated topics will be added to the topic guide and discussed with participants as interviewing and analyses progress. An example was additional questions to explore parental understanding about whether or not their child would have received the intervention if the pilot cRCT was not being conducted. A distress protocol was available but did not need to be used. After the interview, participants were sent a copy of the consent form and a thank you letter, including a £30 Amazon (Amazon.com, Inc., Bellevue, WA, USA) voucher to thank them for their time.
Focus groups
Focus groups took place towards the end of the pilot cRCT recruitment period. All were conducted online, facilitated by MP and KW and included a voting system using Poll Everywhere (Poll Everywhere, San Francisco, CA, USA) to gain quantitative data on key questions (e.g. ‘Overall how would you rate the PICnIC site training?’ and ‘How acceptable is the proposed PICnIC trial to conduct?’). The practitioner focus group topic guide (see Appendix 3) was developed using relevant literature and early findings from parent/legal representative questionnaires and interviews. Additional focus groups were arranged to accommodate practitioners’ availability so that all were able to attend and no individual interviews were needed.
Informed consent
Informed consent for participation, for audio recording and to receive a copy of the mixed-methods study findings was sought verbally before the focus group commenced. This involved a member of the University of Liverpool team reading each aspect of the consent form to individual practitioners in Zoom breakout rooms. Each box was initialled by the researcher on the consent form when verbal consent was provided. Informed consent discussions were audio-recorded for auditing purposes.
Conduct of the focus groups
Focus groups began with introductions and were semistructured, informed by the topic guide. Unanticipated topics were added as data collection and analysis progressed. Voting took place via Poll Everywhere throughout each focus group on topics as they were discussed. Once the focus group was complete, participants were thanked for their time.
Transcription
Digital audio-recordings were transcribed verbatim by a professional transcription company (UK Transcription) in accordance with the Data Protection Act 1998. Twenty-three transcripts were anonymised and checked for accuracy. All identifiable information, such as names (e.g. of patients, family members or the hospital their child was admitted to), were removed.
Data analysis
Qualitative data analysis of the interviews and focus groups was interpretive and iterative (Table 16). Utilising a thematic analysis approach, the aim was to provide an accurate representation of parental views on trial design and acceptability. Thematic analysis is a method for identifying, analysing and reporting patterns (or themes) within data. Analysis was informed by the work of Braun and Clarke and their guide to thematic analysis (see Table 16). 25 The NVivo 10 software (QSR International, Warrington, UK) was used to assist the coding of data. Survey and voting data from focus groups were analysed using SPSS version 27 (IBM SPSS Statistics, Armonk, NY, USA) for descriptive statistics. Qualitative and quantitative data were analysed separately and then synthesised through constant comparative analysis. 26 MP (female, a social scientist) led the analysis with assistance from KW (female, a social scientist).
| Phase | Description |
|---|---|
| 1. Familiarising with data | Mariana Popa (MP) read and re-read transcripts, noting down initial ideas |
| 2. Generating initial codes | Initially, a data-coding framework was developed using a priori codes identified from the study’s objectives and the interview topic guide. During the familiarisation stage, MP identified additional data-driven codes and concepts not previously captured in the initial coding frame |
| 3. Developing the coding frame | Kerry Woolfall (KW) coded 20% of the transcripts using the initial coding frame and made notes on any new themes identified and how the framework could be refined |
| 4. Defining and naming themes | Following review and reconciliation by MP and KW, a revised coding frame was subsequently developed and ordered into themes (nodes) within the NVivo database |
| 5. Completing coding of transcripts | KW and MP met regularly on Zoom to discuss developing themes, sample variance and potential data saturation by looking at the data and referring back to the study aims. When saturation was achieved, data collection stopped. MP completed coding of all transcripts in preparation for write-up |
| 6. Producing the report | KW and MP developed the manuscript using themes to relate back to the study aims, ensuring that key findings and recommendations were relevant to the PICnIC cRCT design and site staff training (i.e. catalytic validity27). Final discussion and development of selected themes took place during the write-up phase |
Results
Participants: paediatric intensive care unit staff
A total of 44 PICU staff completed the survey representing 11 UK PICU. Of these, 36 (81%) were involved in the PICnIC pilot and were from other PICUs representing six units. Six focus groups with 25 staff were conducted, which was an additional four than originally planned to ensure staff from all PICnIC pilot sites had an opportunity to participate. Within focus groups 24/25 (96%) used the voting system, because one of the practitioners arrived late and had difficulties accessing the platform. MP led facilitation of all focus groups; KW co-facilitated the first focus group to help inform the initial development of the topic guide.
Paediatric intensive care unit staff characteristics
Survey participants included nurses (23/44, 52%), doctors (19/44, 43%) and pharmacists (2/44, 5%). The focus group sample included nurses (17/26, 65%), doctors (8/26, 30%) and one pharmacist (1/26, 5%). Those who took part in the focus group may also have taken part in the survey.
The majority of participants were involved in the clinical care of children. All units were research active and the majority of participants had been actively involved in previous trials. Only six survey participants indicated they were new to research and had no prior clinical trial experience. None of the units were using the SDD intervention before the PICnIC pilot trial.
Focus groups took, on average, 70 minutes (range 46–100 minutes).
Participants: parents
A total of 65 parents (44 mothers, 21 fathers) completed the survey across five PICU pilot sites. Of these, 15 (23%) were approached when the child was in the intervention group, 24 (36%) in the control group and 24 (36%) during ecology week (missing n = 2).
Telephone interviews were conducted with 23 parents (7 of which also completed the survey), including 15 mothers (2 during ecology week, 7 during the control phase and 6 during the intervention phase) and 8 fathers (1 during ecology week, 4 during the control phase and 3 during the intervention phase) across 5 out of the 6 PICU pilot sites in the UK (3 intervention and 2 control sites).
Parent characteristics
The majority of parents identified their ethnic group as being white (British n = 13, other n = 3), with the rest identifying as African (n = 1), black British (n = 1) and Pakistani (n = 2; missing = 3). The majority of parents had non-medically related backgrounds (n = 22) and most parents were in employment (n = 18).
Parent interviews related to 23 children (17 boys) between the ages of 4 weeks and 12 years (average 2.6 years). Parents were non-bereaved, while one child had been transferred to a child’s hospice, but was back home at the time of the interview. The main reasons that children were admitted to PICU were viral infections or conditions caused by it (rhinovirus, respiratory syncytial virus, bronchiolitis, COVID-19, meningitis), conditions affecting the airway (laryngomalacia, tracheomalacia) and bacterial pneumonia. Other reasons included trauma, cardiac arrest and neurosurgery.
Interviews took, on average, 54 minutes (range 16–70 minutes).
Paediatric intensive care unit staff perspectives
Focus groups with site staff began with MP providing an outline of the aims of the focus group aims and testing of the voting handsets before moving on to participants’ involvement in the PICnIC pilot trial and whether or not SSD was used in their unit before the trial. Most had multiple roles including screening to identify eligible patients, enrolment, recruitment, administration, data collection and promoting the trial ‘as much as I can amongst the doctor folk’ (P14, FG4, control site, doctor). Others were bedside nurses who delivered the SDD at intervention sites or ‘purely research’ nurses (P04, FG2, intervention site, research nurse) involved in recruitment and consent processes and follow-up with other staff, such as ‘nagging people to take swabs’ (P11, FG4, control, research nurse). Variance in roles was also apparent in the survey for pilot site staff with 13 (13/36, 36%) involved in the clinical care of children without any research duties, while the majority (23/36, 64%) had multiple roles in conducting the pilot trial including administering the SDD.
Acceptable screening process and inclusion/exclusion criteria
Most survey and focus group participants indicated they were satisfied with the screening process and ‘quite happy with the inclusion and exclusion’ (P17, FG5, control, PI/doctor) criteria. However, three nurses and multiple focus group discussions suggested that site staff found aspects of checking patient eligibility challenging, which led to eligible patients being missed or excluded unnecessarily. Discussion centred upon the need for staff to decide whether or not the patient would be mechanically ventilated for at least 48 hours in order for them to be eligible. As the quotations below illustrate, this was difficult to predict with any certainty at the point of PICU admission, which led to potentially eligible patients being excluded:
I think the main issue with recruitment was this wobbly window about they’ve got to be ventilated for 48 hours. If you ask any of them they’d say, ‘We haven’t got a crystal ball, we don’t know’.
P10, FG3, intervention group, research nurse
It’s hard to predict the future. We could have a good idea, but sometimes you think they were going to walk out the door the next day but it changed.
P03, FG2, intervention part 2, research nurse
Although some patients did become eligible the day after admission, they often were missed as screening only took place at admission.
We think, ‘This person came in, they’ll be extubated tomorrow’. Then, the day after, it’s like, ‘Great, they didn’t’. Then, if I’m not here and I’m like, ‘Guys, it’s okay, we can screen them today’, they’ve already forgotten about it. Because if they don’t do it for admission, then it’s gone … I find that the team then struggles to have that click of, ‘Yes, we can include them now’.
P01, FG1, intervention site, doctor
One unit described how screening would take place on multiple occasions for the same patient, which would help identify children who became eligible sometime after admission. For others, ‘re-screening’ was not possible due to staffing constraints. Research nurses also described how some patients were no longer eligible when ventilation was removed fairly soon after admission, or the time window to administer the SDD intervention had passed by the time they had screened and found a clinician to prescribe the SDD.
Not user friendly for clinical staff (i.e. ability to rescreen the patient if unexpectedly remains ventilated).
P20, research nurse, pilot site survey
It was very much a research led, chasing the doctor-screening and chasing doctors to prescribe, sort of thing. It wasn’t the clinical team, and we sort of explained their load and the fact that it was just such a short period of time that we were doing it, that obviously they were probably never going to catch on, because by the time people had started saying, ‘Oh, actually this patient–’ you’re like, ‘Oh, we’re not doing it anymore, sorry’. That sort of thing.
P04, FG2 part 2, intervention group, research nurse
Research nurses used free-text responses in the survey to highlight the need to clarify the screening process. Some suggested widening the inclusion criteria to all patients, or all ventilated patients and re-screen, to help prevent potentially eligible patients being excluded at the point of admission.
Perhaps include all ventilated patients at admission? Patients rescreened daily – if not expected to be ventilated ‘day after tomorrow’ then they were repeatedly excluded.
P21, research nurse, pilot site survey
I guess the only question would be whether or not it would be all patients, irrespective of whether they were invasively ventilated, if you were doing it across the whole unit, would you just give it to everybody who was admitted if you really wanted to look at embedding it in practise. Whenever you’re wanting something to happen, it’s easier if you say you do it for everybody rather than having subsets of patients. Even if they’re a large, you know, let’s say 70% + of all our patients are intubated at some point. It’s still potentially easier if you just say, ‘Let’s do it for everybody’.
P17, FG5, control group, PI/doctor
Support for cluster randomisation in the proposed trial
During focus groups, there was a general consensus that cluster randomisation was acceptable for the proposed PICnIC trial and ‘I think there’s no other, that I can think of, way that you’d do it’ (P05, FG2 part 2, intervention group, PI/doctor). Three survey participants used open text responses to state that ‘Cluster/step wedge RCT [is the] way to go’ (P29, doctor, pilot site survey). When asked to consider whether individual randomisation would be preferable, staff described how this may have caused confusion:
I think the choice to cluster was a good plan. I think that would have caused a lot of confusion if we were randomised to that within each unit, so I think the clustering seemed to work very well. It would be a good idea for this sort of study.
P17, FG5, control group, PI/doctor
Approach to recruitment and consent including samples to assess clinical and ecological data
All but one practitioner involved in recruitment to the pilot trial (22/44 survey participants) stated the PICnIC recruitment process was acceptable (16/22, 70%), or very acceptable (6/22, 26%). The one recruiter who did not find the process acceptable ‘disliked assumed consent’ for the SDD intervention.
Across focus groups, practitioners commented on how parents did not comment or react negatively to their child being given SDD, or to having routine care samples taken, without their prior consent. Many implied that consent did not need to be sought for the intervention or routine sample aspects of the study as parents were not dissatisfied that they had been taken.
I don’t think, as a general feeling, I don’t feel that we had any parents who have been particularly outspoken about it.
P06, FG2 part 1, intervention site, research nurse
I found that they were never unhappy about us collecting routine samples, and particularly if you said that this is our standard practise. They were very happy with that.
P16, FG5, control site, research nurse
Some staff at intervention sites were surprised that parents had not expressed concern about their child being given antimicrobials in a research study without their prior consent. They reflected on how it may have been how they had presented study information to families. As the following quotation highlights, accessing SDD may have been presented and therefore viewed in a positive light, with parents hoping that the intervention may help their child:
I don’t know if it’s the way we explain the medication to them, as well? I make a point of telling them some centres are doing it, others aren’t, because we want to see if it helps them, in a way, to give this as part of infection control, prophylactics. I think maybe they see it in a positive way? Like, ‘We’re getting it, so hopefully we won’t grow any bugs’. I don’t know if that’s the way they see it.
P01, FG1, intervention site, research nurse
Those involved in pilot trial recruitment described how parents were not concerned that prior consent had not been sought as they had been informed that samples were part of standard care in their unit, or that other sites were using SDD.
I don’t think many parents minded because we hadn’t really done anything to their child that was out of standard care anyway. So I think they’d have probably had more – they’d have been more upset about it if we’d have done things to the child, if that makes sense.
P20, FG6, control site, research nurse
However, for some sites, admission samples, referred to as ‘routine or standard samples’ in the study, were not part of standard infection control measures for their unit. Nurses described how they felt uncomfortable collecting these samples, particularly for older children or those who were awake, as they were not routine for their unit:
They were awake patients, often older and they were difficult to approach. Also, it felt a bit wrong that we were asking for all of these admission swabs to be done on them that would not normally be done on them ...
P12, FG4, control site, research nurse
After admission samples, additional samples were taken twice weekly until discharge from the nose, rectum and wound (if present). Practitioners spoke of how parents’ responses to collection of these additional samples varied, which was in contrast to the collection of admission samples, or administration of the SDD intervention. Some parents did not consent for this aspect of the pilot trial as ‘they just did not want to put their child through anything extra’ (P14, FG4, control site, PI/Doctor), or had declined at a later date when parents decided the swabs were upsetting their child.
I’d say I’ve had a few parents who when going through the consent form after reading the information sheet, they have said that they just point blank refuse any rectal swabs unless they’re needed, because … adding more additional tests and things that their child doesn’t clinically need. That distresses the parents.
P15, FG5, control site, research nurse
Not the initial ones, they know, when we explain, say, ‘They’re samples that are taken as part of their routine care. They would have the regardless of the PICnIC study or not’. The second, the extra samples, very varied. Some are very anti it, some were willing.
P04, FG2 part 2, intervention site, research nurse
As much, you know, the PIS sort of states that it’s – they won’t harm your child or they won’t cause any pain. Well, actually, you know, putting a swab up your bottom and a swab up your nostrils, I mean even though you’re a bit sedated, I don’t think that’s the most pleasant thing … we’d go back maybe like the third time, the third set of swabs, or something, they were like, ‘Actually, the child’s quite scared now of people coming up and doing things’. We’re like, ‘That’s absolutely fine, thank you so much for agreeing to other things’.
P06, FG2 part 2, intervention site, research nurse
Practitioners at one control site described how they did not to approach parents and take rectal samples due to how critically ill the patient was, or the ‘parents’ personality’:
If they’re in a really critical situation, I don’t want to then go and speak to them about rectal swabs because in the grand scheme of things, it’s just not that important.
P16, FG5, control site, research nurse
The sample processing increased workload for microbiology labs. At one site, the staffing and pandemic pressures meant additional discussion was undertaken at local level to allow set-up.
You know, the microbes. I do know that there are many. Say, for our unit, staph aureus … Candida was not a part of our routine. And vancomycin resistance was also not part of our routine swabs … With COVID and the lab running to almost 300% of its capacity, it took a lot of negotiation to agree to get them to even do this.
P02, FG1, intervention site, PI/doctor
Such discussions might have been better before rather than after the site was included in the study and this will need to be part of the set-up discussion in any future definitive study. Microbiologist discussion will need to include the specific expectations of the study to allow appropriate ecology screening and the resources to enable this within the local laboratories.
Not an opt-out approach
Focus group discussions and a few open responses in the survey indicated that there was some confusion about the consent process being described as an ‘opt-out’ approach in the protocol as ‘they can’t opt out of having the drug’ (P06, FG2 part 2, intervention site, research nurse). Some staff stated there was a need to clarify what parents were consenting for:
Clarity on what they are actually consenting for. The consent form talks about consent for data collection but that was done anyway. The consent was purely for the follow up.
P21, research nurse, pilot site survey
Some voiced their confusion about studies that use an opt-out approach as its ‘“opt-out consent” is an oxymoron. It doesn’t make sense’ and others described how they did not use the term during recruitment conversations with parents.
Clinician 1:I don’t know that I necessarily used the words opt out.
Clinician 2:No, I’ve never used that.
FG3, intervention group, research nurses
Approaching every parent for consent for specific elements of the study meant that the PICnIC consent process differed to a traditional opt-out consent model, which often does not involve a formal consent form. However, there was support for the same approach to consent used in the PICnIC pilot trial to be used in the proposed PICnIC trial. This support related to not seeking consent for the intervention and samples at the point of admission with consent sought for additional samples, which was referred to as ‘a combination approach’ (P04, FG2 part 1, intervention site, research nurse).
Approaching parents to discuss the pilot trial – the importance of timing
As consent did not need to be sought prior to SDD delivery, and/or samples collected at the point of admission, staff were able to assess when was the most appropriate time to approach parents to discuss the study. Many emphasised how ‘timing is key’ given the PICU context, which is ‘a very intense environment for them [parents] to come into unexpectedly’ (P10, FG3, intervention group, research nurse). Those involved in recruitment discussions with parents described how they observed parents and patients to establish the most appropriate time to approach, which was never the ‘first day that a parent arrives to the PICU because I feel like that’s the day that they’re most stressed out’ (P01, FG1, intervention site, research nurse). Practitioners would commonly wait until the child’s condition had stabilised or improved, which was checked with the bedside nurse, to establish timing. This checking helped to ensure parents would be ready and receptive to research discussions:
I think the difference here is its intensive care, isn’t it? If they’re really unwell, they’re less likely to consent as well. Because the last thing parents want is more things added on to that. We do see that if we go day two, if the child is improving, then they’re quite happy. They’re quite receptive to listen.
P02, FG1, intervention site, research nurse
Some we’ve approached within 24 hours and others we’ve left for a number of weeks, because it’s just not been something to add to their mental load. It’s really varied.
P03, FG2 part 2, intervention site, research nurse
We would generally talk to the bedside nurses, the clinicians, to know what situation they’re in at that point.
P03, FG2 part 2, intervention site, research nurse
Not all parents were not approached about the study, or given the SDD intervention. Some practitioners described how a decision was made by the clinical team due to concerns about how parents would react to finding out that something had changed in their child’s clinical care and something ‘new’ had been given.
There were some patients that we specifically excluded or clinicians wouldn’t necessarily start it on, because we knew how this family can behave and react to new things. If someone is extremely hospitalised, they’re very aware of medicines, and something new, it just doesn’t sit well with them, sort of thing. We kind of excluded a couple of patients before even starting it. Not many. I think it was maybe like three. Yes, the clinicians were sort of like, ‘I don’t really think I want to give this to this patient because of what repercussions – the family are just going to kick off’. That sort of thing. We pre-empted patients that we already knew would have an issue, potentially, to try and avoid that issue.
P04, FG2 part 2, intervention site, research nurse
Patients were missed due to sites not having research cover over weekends, or if the research team were not based in a PICU: ‘I think if the research team were based on PICU, it would be a lot easier … probably would not miss as many as we missed’ (P11, FG4, control site, research nurse). One doctor described how they would provide brief information to parents of patients who arrived on a Friday, as they were aware it would be a few days before the research team would be available to speak to them: ‘The research team will come and speak to you in more detail, but this is just to sew the seed’ (P09, FG3, intervention site, research nurse) as sometimes families were ready to be discharged at the point that the team approached them on the Monday. One site described how some parents were not provided with any information about the study as their child’s condition did not improve and care was withdrawn:
For some PICnIC patients we haven’t approached at all because if they come in and it’s been a very difficult couple of days and then the focus has changed to palliative care and then they’ve moved towards withdrawal of care, then we’ve made that decision that actually it, it doesn’t feel appropriate to – because in theory it should be opt-out, like you say. We haven’t felt that for all patients it is appropriate to burden them with that information when realistically they’re not going to be – it’s not going to change their management.
P04, FG2 part 1, intervention site, research nurse
PICnIC pilot trial recruitment took place at a time when multiple other studies had started, or restarted after the COVID-19 national lockdowns. As a result, research teams were approaching families about multiple studies during their PICU stay. As the following quote illustrates, this required careful consideration so that parents were not burdened with too much paperwork and decisions to make:
Normally it would be the following day, at least, really. I think the problem we had, not with your study, but at the time that PICnIC was running is we’ve got a raft of other studies. The timing became more and more crucial because these patients could have been eligible for four or five different studies and you can’t give the parents masses of paper and just say, ‘If you just read these and I’m going to sign here’. We had to do it gradually.
P10, FG3, intervention group, research nurse
Challenges in communicating the pilot trial to parents
During focus groups, the researcher asked those involved in recruitment about how they explained the study to parents, including cluster randomisation. At control sites, staff described how they often provided a broad overview of the study aims and how their unit was not taking part in the intervention side of the study. Cluster randomisation or the SDD intervention was not always mentioned in the recruitment discussion, with a focus on the sample aspect of the pilot trial that their child would be participating in.
Well, basically say that it is a study, an infection control study that is looking at introducing a new measure, compared to just our standard infection control, to see whether that made a difference to [hospital-wide] infections. Depending on the family, I would obviously go into more or less detail about what those things all mean. Then I would say that they are looking at introducing medication to treat gut bacteria. But our unit are not doing that. Then discuss the sampling.
P14, FG4, control site PI/doctor
I think I would mention the PICU was randomised but I didn’t go much into what SSD was for our patients.
P20, FG6, control site, research nurse
At sites delivering the SDD intervention, recruiters had also simplified their explanations as much as possible for parents and avoided terms such as cluster randomisation.
I have to be honest, I try to simplify it as much as I can when I’m explaining it to parents. Because then, if you use those terms with parents, then it’s just making your life harder.
P01, FG1, intervention site, research nurse
One intervention site clinician described how a cRCT design was easier to explain to parents than individual randomisation as all children would receive the same treatment:
PICnIC, then, is actually probably the easiest one to explain, because we don’t randomise them in two. I think it’s going to be more difficult to say if, in the same unit, children got randomised to treatment and some didn’t. I think it was because the whole unit has the same thing, it’s easier.
P01, FG1, intervention site, research nurse
However, there appeared to be subtle differences in how staff described the nature of the intervention at sites administering SDD, which may have led to parental misconceptions reported under the ‘SDD acceptability’ subtheme. Across examples of recruitment descriptions provided by staff, many had used the term ‘standard practice’ when explaining the trial and the nature of intervention. While some staff at intervention sites gave the example of SDD being used in other hospitals, or ‘in (the) adult world’ (P10, FG3, intervention group, research nurse) as their rationale for using the term ‘standard practice’, they emphasised how they were careful to clarify this was a research study. Others stated that admission swabs were standard practice in their PICU before the pilot trial, which appeared to underpin the use of this term in their discussions with parents.
Because I like to make sure that they know that this is part of our practice anyway, to do the admission swabs anyway. It would be something that we would have done anyway. I don’t think parents question me because of that.
P01, FG1, intervention site, research nurse
Although SDD was not used at any of the intervention sites prior to the pilot trial, there were also examples given of staff informing parents that SDD was their current standard practice, without explaining how SDD was not used in their PICU before the research.
So that everybody – this is now our standard practice as a unit, this is our standard practice.
P04, FG2 part 1, intervention site, research nurse
Yes, it’s just our standard treatment for patients with a breathing tube in. That’s what our PICU is doing for these ten weeks, it is part of our standard care. Every patient with a tube that meets inclusion, sort of thing.
P03, FG2 part 2, intervention site, research nurse
Staff at two intervention sites spoke of the challenge of communicating the study to parents who did not speak the English language. Although interpreters had been used, staff described how they were unsure how the study had been explained and how these parents would often not provide consent for their child’s participation. They felt the challenge was compounded by consent forms only being available in English, which meant that these parents did not know what they were signing. Recommendations were made to have information sheets and consent forms available in multiple languages for the proposed trial.
You never know what the translator’s saying. People always don’t consent there because it’s better not to consent than to commit to something which they’re not entirely sure of … I remember this very well, of one of the doctors translating for me for one of the families from Egypt. And mum was looking at the form like, ‘Yes, you’re telling me, but is it actually what’s written here?’ Because how will she know? I wouldn’t want to sign something without knowing what it says.
P02, FG1, intervention site, PI/doctor
Acceptability of the selective decontamination of the digestive tract intervention – support for the proposed trial
Overall, focus group participants were mainly positive about the SDD intervention and its use in the proposed trial. Many supported the study as they wished to know the answer to the research question in the hope that it would help their patients in the future.
I’m generally pro [use of SDD in the proposed trial]. I’m very aware of healthcare-associated infections, and I think anything that can reduce that has to be good. I would love this pilot to work and this trial to go ahead, to see whether there’s the evidence to back up my hope for it to be of use in reducing healthcare-associated infections. I think it has to be in a way which is acceptable clinically and for families.
P05, FG2 part 2, intervention site, PI/doctor
I was interested in the fact that it can reduce the amount of antimicrobials used in that patient, but also in other patients on the unit. That’s what interested me quite a bit was the effect on the microbiological sort of flora within the unit.
P18, FG5, control group, PIC consultant
Discussions about the potential risks and benefits of SDD suggested many clinicians were in equipoise: ‘We just do not know. We need to know’ (P12, FG4, control site, research nurse).
I don’t have any ethical convictions or anything … VAPs are not good. It prolongs ventilation, which then weakens … It just prolongs recovery for a child. I can see the advantage of it, but I can also see that you’re giving an antimicrobial that they don’t necessarily need. If they never develop a VAP, then they never needed it.
P09, FG3, intervention site, research nurse
There were a few focus group participants at one intervention site who expressed concern about the use of SDD without evidence in children. Their concerns related to the potential impact on the gut flora and whether this would lead to allergies in individual children, or negatively affect the infection profile on the unit. After discussion, two of these clinicians stated they would find it acceptable to deliver SDD in the proposed trial, although one remained uncertain: ‘this is something new that has come in. I don’t have to say no or yes yet’ (P07, FG2 part 1, intervention site, bedside nurse) and had additional concerns about the ability to deliver the trial unless there was sufficient nurse support (see Concerns about delivering the selective decontamination of the digestive tract to paediatric intensive care unit patients).
Others spoke of how they were aware of colleagues who had polarised views on SDD: ‘Some of them are very pro. Some of them are very anti. It is a bit Marmite’ (P14, FG4, control site, PI/doctor).
Is there the risk for altering your unit flora in an adverse way as well?
P17, FG5, control site, PI/doctor
One of my only concerns would be there is another study in one of the neonatal units that I was involved in the recruiting team that was giving prophylactic antimicrobials. And one of the concerns about that trial appeared in neonates was how this was going to affect, as [Doctor/PI] said, the gut flora long term and whether this was going to lead to further down the line increases in allergies and essentially it just wasn’t known. I guess the same concerns would therefore apply to this as well.
P19, FG6, control site, doctor
One clinician was concerned about the sample size needed to answer the research question:
In principle, I don’t have an issue with it. I can see the logic behind it, but I would want to see more evidence which obviously is why we’re doing the trial we’re going to need a huge, huge number of patients because actually in children, VAP is not a huge problem.
P04, FG2 part 2, intervention site, research nurse
The practitioner survey (pilot site and wider PICU versions) included questions to access views on the acceptability of the SDD intervention and its use in the proposed trial. As shown in Table 17, a series of statements were proposed, with participants asked to indicate how strongly they agreed with each statement using a Likert scale (strongly agree to strongly disagree). Survey data reflected findings from focus group discussions, with the majority (31/44, 71%) indicating they were not opposed to SDD (statement 11). A quarter (11/44, 25%) did not agree nor disagree with this statement, while a minority (2/44, 4%) were opposed to SDD. Most (38/44, 86%) agreed that ‘the decision to adopt SDD requires consensus between my colleagues’ (statement 2).
| Statement | Responses n (%) | ||||
|---|---|---|---|---|---|
| Strongly agree | Agree | Neither agree nor disagree | Disagree | Strongly disagree | |
| 1. SDD increases antibiotic resistance | 0 (0) | 5 (11) | 33 (75) | 6 (14) | 0 (0) |
| 2. The decision to adopt SDD requires consensus between my colleagues | 16 (36) | 22 (50) | 5 (11) | 1 (2) | 0 (0) |
| 3. Research to date has not adequately addressed concerns about antibiotic resistance and SDD | 4 (9) | 22 (50) | 16 (36) | 2 (4.5) | 0 (0) |
| 4. The decision to adopt SDD requires a review and appraisal of the current best evidence | 21 (48) | 17 (39) | 5 (11) | 1 (2) | 0 (0) |
| 5. My hospital tries to reduce antibiotic use | 16 (36) | 24 (54) | 4 (9) | 0 (0) | 0 (0) |
| 6. Part of the decision to adopt SDD requires agreement about which patients will receive it | 12 (27) | 26 (59) | 5 (11) | 1 (2) | 0 (0) |
| 7. SDD would increase ICU Clostridium difficile infections | 0 (0) | 3 (7) | 34 (77) | 7 (16) | 0 (0) |
| 8. I know the SDD evidence base well enough to have an informed opinion regarding its use | 5 (11) | 7 (16) | 9 (21) | 16 (37) | 7 (16) |
| 9. We are addressing hospital-acquired infections using other strategies | 4 (9) | 22 (50) | 17 (39) | 1 (2) | 0 (0) |
| 10. We are addressing VAP using other strategies | 4 (9) | 24 (54.5) | 13 (29.5) | 3 (7) | 0 (0) |
| 11. I am opposed to SDD | 1 (2) | 1 (2) | 11 (25) | 21 (48) | 10 (23) |
| 12. SDD is not on my unit’s list of clinical priorities | 3 (7) | 9 (21) | 20 (45) | 8 (18) | 4 (9) |
Responses to other statements in Table 17 suggested that pilot site and wider PICU staff were in equipoise, as the majority did not have strong feelings (neither agree nor disagree) about the effectiveness of SDD in increasing hospital antimicrobial resistance (statement 1) or intensive care unit (ICU) Clostridium difficile infections (statement 7). Although many indicated their unit did have other strategies to address hospital-acquired infections (statement 9) and VAP (statement 10), over half (32/44, 53%) disagreed or were uncertain (9/44, 21%) they knew the SDD evidence base well enough to have an informed opinion regarding its use (statement 8).
Most staff (40/44, 90%) were working in hospitals that tried to reduce antimicrobial use (statement 5), yet over half (26/44, 59%) agreed that research to date has not adequately addressed concerns about antimicrobial resistance and SDD and agreed (38/44, 86%) that a review and appraisal of current best practice was needed to inform a decision about whether to adopt SDD (statement 4).
Concerns about delivering the selective decontamination of the digestive tract to paediatric intensive care unit patients – the look and texture of the formulation
In the survey, PICU pilot site and wider PICU staff were asked about how acceptable they did or would find delivering the SDD intervention to patients. Of those who stated this question was applicable to them (33/44, 75%), the majority stated it was acceptable (19/33, 57%) or very acceptable (8/33, 24%) across both groups. Four of the six staff that did not find delivering the SDD acceptable were from pilot sites.
Both survey and focus group findings highlighted some concerns and challenges regarding the delivery of SDD to patients.
Some survey participants had doubts over clinical equipoise, while one stated they ‘can’t see how this has a favourable risk–benefit ratio’ (Pox, 872699-872681-93590253, male wider PICU survey). Another raised concerns about the size of the sample needed and how findings of a trial in adult critical care setting may mean this study is not needed:
This is a waste of time, money and energy. The power of your study to show a difference will need to be massive and it will not affect outcome. This has been shown in adults who have stopped doing SDD.
P10, doctor, male, pilot site
More commonly there were concerns raised about the look and taste of SDD paste, which was yellow in a child’s mouth, which ‘[j]ust looks vile’ (P10, FG3, intervention site, research nurse) and ‘looks like they haven’t had any mouth care’ (P05, FG2 part 2, intervention site, PI/doctor). Intervention site staff described how parents would question whether or not their child had been sick due to the look of the paste and how some children had become unsettled. This became more of a concern when mouth care could not be done for two hours after SDD delivery, as per protocol.
To leave that in a child’s mouth or in any patient’s mouth I think would be quite unsettling. The parents when they see their children, they want to see that they’re comfortable and they’re settled. If they’re thrashing their head around because they clearly have got this stuff in their mouth, I think that was a bit of a problem – vile.
P09, FG3, intervention site, research nurse
The mouth care, the protocol says that mouth care is supposed to be not done for two hours and that really is very difficult, impossible, pretty much. If that’s seen as a deviation from the protocol, then that’s an issue.
P03, FG2 part 2, intervention site, research nurse
As a complex intervention that leaves a gunk in the mouth of kids, will be hard to convince families of its role.
P28, doctor, male, pilot site survey
Those involved in the clinical care of children commented on the texture of the paste and how its thick sticky texture interfered with tapes that were securing tubes on ventilated children and made them look dirty.
This thing [SDD paste] is very nasty. And even talking with my colleagues, I think the form of the paste is too thick and to try and dissipate a little bit, it’s just so sticky that it is just going on one place. I found it very difficult dealing with the paste physically.
P07, FG2 part 1, intervention site, bedside nurse
There is definitely a degree of concern, so concern about the look, and concern – there’s been concern about the tapes, and whether or not it affects the security of the tape that’s securing the ET [endotracheal] tube.
P04, FG2 part 2, intervention site, research nurse
A concern at one control site was that the paste would block nasogastric tubes as ‘we do have that problem with small NG tubes in kids’ (P18, FG5, control site, PICU consultant). Two intervention sites described how the paste blocked the suction tubes during mouth care: ‘The nurses would say when they do the mouth care and the suction it blocks up the suction tubing they use. They had to keep chucking it away, the Yankauer sucker’ (P09, FG3, intervention site, research nurse). This clinician also stated that their unit’s four-hourly mouth care at some sites ‘meant that throughout 24 hours they’re going to be suctioning it pretty much straight out because they’re doing their next mouth care’.
As the following quotation illustrates, having a generic amount of the SDD paste prescribed to children regardless of size or age was also discussed by two intervention sites who felt that the amount was too much for smaller babies.
I think it would be difficult to be standardised for every patient. Because, obviously, in paediatrics, you might get a zero-month one and you can get a 16-year-old. And the syringes being the same size for both of the patients is very different. Is there some way of changing it depending on the weight? Rather than have a generic amount for everybody. Because the technique of giving a 3-kilo baby, as opposed to a 60-kilo child is very different, isn’t it?
P01, FG1, intervention site, research nurse
Across focus group and survey data there were descriptions of how research nurse teams had ‘done the vast majority of the work’ (P06, FG2 part 2, intervention site, research nurse) and would remind doctors about the elements they would need to do. There was agreement that the proposed trial would need to be a nurse-led study. Supporting nurse research staff to run this trial was highlighted as important to ensuring trial success.
This study was very much a nursing-led study from our perspective … it’s about the nurses administering it and trying to fit it into their routine of all the other things they’re having to do in terms of observations and other medications, etc., etc. It just becomes another piece of the jigsaw of what they have to do for the care for their patients.
P17, FG5, control site, PI/doctor
Protocol adherence – issues to consider for the proposed trial
Five research nurses (5/27, 19%) who took part in the survey noted difficulties in adhering to the protocol. During focus groups, there were examples provided of how the clinical teams had decided not to take additional swabs for a variety of reasons, including perceived burden on the patient, and therefore ‘it was not appropriate’, and staff capacity issues, particularly during nights shifts, which meant that swabs became less of a priority or forgotten.
Getting the swabs taken twice weekly is a very big challenge. We did not adhere properly to the protocol for that for a lot of patients … The unit went through a very busy period in October/November time and it is not the priority for them to do that when they have got so much else to do.
P14, FG4, control site, PI/doctor
We’re finding that if something’s happening at night, it’s missed because people just aren’t at their best at those times.
P16, FG5, control site, research nurse
Intervention sites described difficulties accessing drugs, or delayed access, which meant that the administration of the first dose was delayed or missed as not all staff knew where it was stored or the nurse was busy when the intervention should have been given.
We had one child, for example, they had the drug, it was prescribed, but they couldn’t find it. It was in the fridge marked ‘PICnIC study drug’, just in here, this one here. They just couldn’t find it …. I think that delayed the start of the medicine on many occasions, that I personally saw. The doctor would write it up. We’d tap the doctor on the shoulder, ‘Can you just write the SSD up?’ They’d write it up. Then the nurse was busy and because the drug was as is, it was then delayed, they’d missed the first dose and it would be done for the second dose.
P10, FG3, intervention site, research nurse
Yes, sure, we had missed doses and for varying different reasons and it was hard at some points to figure it out. if things weren’t done perfectly, maybe if someone hadn’t maybe written a date on it – obviously no one is going to give a drug that they don’t really know anything about if the date was missing. So then obviously missed dose is until the next. Because it’s obviously something new and people don’t have a lot of knowledge on, if it wasn’t set up perfectly, it just creates questions and it’s safer just not to give it.
P06, FG2 part 1, intervention site, research nurse
In one focus group, staff stated that the relatively short shelf life of the SDD contributed to protocol adherence issues. They suggested some of these issues could be rectified by the addition of a time of drug preparation added to the bottle label.
[B]ecause the drug had a very short shelf life, it was only 5 days once you’d made it up, if they’d made it up on the 5th at 10:00 at night, when it got to the 10th, say, and it had expired use before the 10th they would bin it and not give it. But actually there wasn’t space on the bottle to say what time they’d made it up, so actually you could give it until 10:00 that night. So you’d have missed doses where people perhaps thought that it had expired, hadn’t necessarily gone to make up a new bottle.
P04, FG2 part 1, intervention site, research nurse
Logistics and packaging
Sites described a number of logistical issues such as temperature deviations with storage of the intervention in fridges and the work involved in making up the drug for each individual child, which was described as a burden on nurse time, delaying the intervention being administered. Some commented on how it was wasteful to have one per child rather than one bottle per unit.
… in the fridge before it’s made up and then it sits in the unit. It was all a bit faffy ... I understand because it was a pilot study. The fact that each of these children had to have their own kit, which seems wasteful. A huge amount of work, which also delayed the making up of the drug. Obviously, the nurse that’s looking after the patient has got other things to do, she’s now to got to make this drug up in order to give it to the patient. If it was just a bottle in the cupboard she would have done it, but because of the way it’s been dispensed in the fact that each patient has to have their own kit.
P10, FG3, intervention site, research nurse
Others commented on how the SDD packaging was fragile and difficult to store, which lead to bottles being smashed and wasted:
P03, research nurse: I think the box, the bottle and individual syringes in the box, storage-wise, is really tricky. If the paste could come in like a tube, like Daktarin that squeeze into your syringe, for storage that would be so much better. The box wouldn’t stay shut. It had to be stored upright but kept falling over, so the bottle would fall out–
P06, research nurse: And smash. I smashed four.
FG2 part 2, intervention site
Training and the importance of sufficient set-up time
Of those who had completed the pilot site survey, just over half (15/27, 56%) had been trained by a member of their site team, while the remainder (11/27, 40.7) had been trained by the study team (ICNARC). Only one member of staff had received training from both groups. Training received was rated highly (good or excellent) regardless of the provider.
In free-text responses, staff recommended that more time to prepare in advance of the proposed trial starting would help ensure ‘it will be read by clinical staff and busy team’ (P18, research nurse, female, pilot site survey). Suggestions were made to clarify guidance for sample collection and to conduct training in groups (as opposed to individually) or in an online format for the proposed trial. A number of wider PICU and pilot site staff suggested that a 2-month lead in time would be needed for training and preparation to engage as many staff as possible including microbiologists. Part of this training should include how to access the intervention at each site.
Clearer guidance at times re the swabbing timings at the start of the study and data input of both consented and non-consented patients.
P8, research nurse, female, pilot site survey
The biggest stumbling blocks are the hospital microbiologists ... need to get them on board first.
P01, clinical academic nurse, female, PICU-wide survey
The importance of ‘getting the doctors on board’ (P14, FG4, control site, PI/doctor) was emphasised by two sites. Due to doctor rotations, it became challenging to ensure all staff were familiar with the protocol, which suggests the need for frequent training to help ensure patients, samples or doses of the intervention are not missed.
I think the underlying thing is still there, which is the cohort of doctors on PICU is ever-changing. People are on a rolling rota of people coming in for three or four months at a time and then going off again and then coming in. So, actually, the time to keep people up to date and trained, and enough people so that there is always somebody on shift that has got PICnIC at the front of their mind is just relatively hard.
P17, FG5, control site, PI/doctor
Overall acceptability of the proposed trial
Towards the end of focus groups, staff were asked to consider discussions that had taken place and conclude whether or not they felt the proposed trial was acceptable to conduct. They were also asked to use a link to a voting system (Poll Everywhere) to vote on acceptability using a five-point Likert scale (very acceptable, acceptable, neutral, not acceptable and very unacceptable). Both verbal responses and handset voting suggested that staff involved in the PICnIC pilot trial sites found the proposed trial acceptable. All of those who voted on trial acceptability stated they would be interested in participating in the proposed PICnIC trial and no staff used the voting poll system to indicate the trial was unacceptable; most indicated it was acceptable (17/24, 71%), while four (4/24, 17%) stated the proposed trial was very acceptable. Two (2/24, 8%) provided a neutral response. The wider PICU survey responses also indicated support and trial acceptability. Wider pilot site and wider PICU staff feedback highlighted that the challenges described in this chapter, such as delivering the SDD, collection of additional samples, sufficient support and engagement, or nurse research teams, and time for trial set-up, should be taken into consideration and changes made to help improve trial acceptability and ultimately trial success.
I think acceptable. I would say acceptable with, clearly, the feedback we have given. It needs a bit of finetuning.
P01, FG1, intervention site, research nurse
It depends how the proposal is going to work, especially who is going to randomise, if it’s going to involve the clinical team, like, the doctors, then really there will be some challenges.
P07, FG2 part 1, intervention site, bedside nurse
I think the pilot has shown that, yes, we can definitely – Well, from the control side anyway, it was possible. It was acceptable to parents and, yes, the only issue was getting all of the swabs. I do not know what kind of leeway of how – What percentage of swabs you need to get, to get the information you need. So, that is the only sticking point, I think.
P11, FG4, control site, research nurse
Parents’ views
A professional approach to recruitment and consent
Parents described how staff were professional and ‘respectful’ (P15, father, control) of their situation, explaining ‘the key points of the study’ and ‘what the treatment involved’ (P07, father, SDD). Across each of the control, ecology and intervention groups, parents described how they appreciated the time and care taken by staff when explaining the study.
I think also the people coming round are obviously very knowledgeable about it, and they’re talking to the parents up front, ahead of reading the material. So, normally, if there was something that wasn’t covered, that they generally would get a common question about, they normally bring that to your attention at the beginning. So, I think the approach is very good.
P07, father, SDD
Most had been approached about the study by a research nurse. Views on whether it should be research nurses or medical staff who discuss research with families were supportive of a nurse-led approach as ‘these women were highly specialist’ (P11, mother, control) and had knowledge of the trial as well as the clinical care of their child’s condition. Views on whether or not staff broaching the trial should be involved in clinical care were mixed, some suggesting independence was important so that clinical care remains focused on their child, while others highlighted that staff involved in care would have the best knowledge of their child’s condition.
Someone involved would be better because they know, and they have all the background, and they have more information. Rather than someone from outside, who doesn’t have so much knowledge of what’s going on with their care.
P18, mother, SDD
I think it was nice to have somebody separate come and explain it. Because, from my personal view, if you’ve got a nurse that is looking after your child in intensive care, you want their focus to be 100% on just looking after your child.
P08, father, SDD
The importance of appropriate timing
The majority of survey respondents (n = 63, missing = 1) agreed that staff had checked whether it was a convenient time to discuss research before discussing the PICnIC trial (96.9%; Table 18). During the interview, the majority of parents emphasised the importance of appropriate timing when approaching parents, which is when the child is in a stable condition:
| Statement | N (missing) | Agree, n (%) | Neither agree nor disagree, n (%) | Disagree, n (%) |
|---|---|---|---|---|
| 1. The doctor or nurse checked that it was a convenient time to discuss research before discussing PICnIC | 64 (1) | 63 (96.9%) | 1 (1.5%) | |
| 2. I was initially surprised to find out that my child had already been entered into PICnIC | 63 (2) | 10 (15.4%) | 21 (41.5%) | 26 (40.0%) |
| 3. The information I received about PICnIC was clear and straightforward to understand | 64 (1) | 63 (96.9%) | 1 (1.5%) | |
| 4. I understood why consent was not sought for my child’s participation in PICnIC | 62 (3) | 30 (46.2%) | 25 (38.5%) | 7 (10.8%) |
| 5. I had enough opportunity to ask questions about PICnIC | 64 (1) | 57 (87.7%) | 7 (10.8%) | |
| 6. I was satisfied with the recruitment process for PICnIC | 65 | 59 (90.8%) | 6 (9.3%) | |
| 7. It was difficult to take in the information I was given about PICnIC | 64 (1) | 5 (7.7%) | 14 (21.5%) | 45 (69.2%) |
I don’t think there’s ever a great time to approach anyone with a child that’s that ill about it. But the best time is I think when the diagnosis has settled down a bit and is not all fresh in the mind.
P22, father, ecology period 3
However, appropriate timing can depend on how sick the child is, and ‘how big a shock things have been, which may affect how willing people are to have those sorts of discussions about research’ (P19, father, control), but usually parents reported that a day or two into the child being admitted to hospital would be acceptable.
Nevertheless, one parent suggested that for the units that are randomised to the treatment arm, it may be more important to tell the parents ‘then and there’ in order to provide an early opportunity to opt out of receiving treatment rather than only a decision about using data:
If it’s a ward where everyone on that ward is being given it, maybe as close to the time that the medicine is given or just before the medicine is given, maybe speak to the parent then and there because that is what you are doing and this is the reason why. There is a choice for them to opt out. Not giving them, ‘Do you mind if we do this to your child?’ Go, ‘This is what we’re doing. This is why we’re doing it but you have an option for us not to use the data and to opt out.’ But obviously that’s a tricky decision to make I suppose.
P16, father, control
PICnIC participant information was positively viewed
The majority of parents (96%) who completed the survey agreed that the information they received about the study was clear and straightforward to understand (see Table 18).
Parents were asked to reflect on the written information they were given about the PICnIC study during their child’s hospital visit. A copy of the intervention or control information leaflet was also sent to parents so they had an opportunity to see the alternative version of PICnIC information. For example, parents whose children were in a control or ecology weeks were sent the intervention PICU leaflet.
Being provided with an information leaflet by a research nurse or doctor was commonly the first-time parents were aware of the study. Only two parents (1 ecology, 1 SDD group) remembered seeing posters about the PICnIC study in the PICU waiting room but did not focus on it too much due to what was happening to their child.
Parent:As you’re waiting in ICU just to … As soon as we got sent into ICU, I wasn’t allowed to go in while they ventilated him and stuff. I was, obviously, sitting in the ICU waiting room. Automatically, your eyes wander to the boards and stuff so I did see something there. […] I wasn’t thinking, if I’m honest. I was more concerned about the fact that I was told we were going to HDU and now we’re being moved to ICU and what they were doing.
P02, mother, ecology
Many recalled how staff providing them with the information leaflet, which was ‘a couple of pages’, was ‘pretty much to the point’ and ‘providing the information that was needed’ (P2, mother, ecology). The leaflet was viewed positively, with parents describing the information as being clear, short and jargon-free.
I think it was really informative, actually. So we did reflect back it on later on when we were discussing it, but I think it was quite informative really. It had the key points on there, and I think it had someone to contact if we needed, had any questions. I’m sure what there was a point of contact on there.
P03, mother, control
It had the main points in, how they were going to do the study. That’s all that I thought, at that time, was needed.
P02, mother, ecology
They were really helpful. They were definitely very helpful. And they worded it in, like, a way that was easy to, kind of, comprehend and understand, without trying to have to understand that medical jargon. It was really – it was well-written, do you know what I mean? It was easy to understand why, and what was happening.
P21, mother, SDD
All confirmed that there was opportunity for any questions, yet few could recall asking any as ‘there was just so much going on. It didn’t seem like a big deal once we’d had time to think about it’ (P05, mother, control). Those that did have questions about potential risks of the SDD paste, or how the samples would be returned, felt their queries had been addressed sufficiently.
Because she came to me and gave me some specimen … There was an envelope with some specimen containers. So, I just asked her to clarify to me as and when they want the specimens, and how I should post them back to them.
P20, mother, SDD
I did ask about the paste, I guess, and she did answer my question about the paste. I said, ‘If there is any kind of risks to her involved?’ and she answered that saying, ‘No, there wasn’t’.
P18, mother, SDD
While most parents stated that they appreciated being given a leaflet and/or a PIS that they could read in their own time, in order to understand more about the study, some mentioned that, as they were being approached at a very difficult time, it was difficult to read the study information and they therefore read the leaflet at a later point. Some had poor recall of the study yet were still satisfied with their consent decision having read the PIS again prior to the interview. They emphasised the importance of keeping the information brief and the benefit of receiving a full explanation from staff to aid decision-making in such stressful circumstances:
I couldn’t read the leaflet and all the information that she gave me because, until now, I’m handling him. I spend a lot of time with my kid because he is still not very well […]. So, I think, if that information could be a bit brief, or if someone can explain it a bit better, rather than the long leaflets, for the information.
P04, mother, SDD
I can’t really remember much of it because it was such an emotional time. But I knew that it was quite simple and it wasn’t going to stop our lives.
P17, mother, control
Parents suggested creating an animation or a video that explains the study or adding diagrams to the PIS to make it more appealing than text. They suggested this may help parents engage with proposed trial information, particularly during such emotional and stressful times.
So I think it was just two sides of fairly full-on text, I seem to remember. I don’t know if there are ways to use diagrams or anything to make it visually as well more appealing.
P19, father, control
Some people cannot do just text, so it is good to see the information on a video or something.
P13, mother, control
Time to decide
The amount of time it took parents to decide to consent or not varied from a very quick decision, taking less than an hour, to decisions that took a few days, which was often due to wanting to discuss the study with family members, or the research nurse not returning for a consent decision until after the weekend. Most suggested that 24–48 hours would be an optimal time frame to allow for decision-making in the proposed PICnIC trial.
I didn’t sign straightaway, I said that I would have to discuss it with dad and then come back. I think, a couple of days later, they came back and we had agreed to be a part of the study.
P2, mother, ecology
I knew in the first 20 minutes but it took my partner a little longer to come to terms with it. I think four hours is a good amount of time.
P15, father, control
Parents’ understanding of the rationale
Parents were then asked to recall how staff had explained the PICnIC study and if they could tell the researcher (MP) what the study was looking at in their own terms. The level of parental understanding of the PICnIC pilot trial and proposed trial varied. Some parents had no recall of what the study was about, which they attributed to the stress they were under when the PICnIC pilot study was discussed. A few confused the study with other trials that were being conducted at the same time: ‘It’s to do with the ventilation, isn’t it, the diseases?’ (P08, father, SDD). For this reason, fairly early in the research process the research team began to send the PICnIC pilot PIS to parents prior to interviews to assist parental recall.
To be honest, I can’t remember word for word what they said, not if I’m totally honest with you.
P03, mother, SDD
I can’t remember, to be honest with you. There has been so much going on.
P08, father, SDD
However, most parents interviewed had a general understanding that the study was exploring infection control measures or those in the intervention group could describe the SDD intervention:
It was the paste that they had to use for antibacterial, the gut
P18, mother, SDD
I cannot remember now, but I think it was to do with babies that are chosen, or babies that are put forward for the trial, to test to see about any infections.
P13, mother, control
Few could recall staff explaining any risks or potential benefits of their child’s participation in the pilot trial, but felt they had received sufficient information through the leaflet provided, and did not recall being concerned about participation posing a risk to their child’s safety:
I don’t remember them talking about any risks or benefits, to be perfectly honest with you, but then I don’t really remember a whole lot of it. Again, I walked away from it feeling as though I knew everything that I needed to know.
P15, father, control
Yes. It was all in the handouts that they gave us at the time. They didn’t actually talk us through it, but they did give us a full leaflet, that explained all of the drawbacks to it as well.
P07, father, SDD
Parents’ perspectives on the acceptability of a definitive trial that includes the selective decontamination of the digestive tract intervention
All survey respondents (n = 65) indicated that they thought it was acceptable to conduct a larger PICnIC study in hospitals across the UK. Despite supporting the proposed cRCT, two parents from the same site decided to opt out of the study describing how ‘I was happy to go ahead at first, I then decided that my child already has plenty of people poking him all day and didn’t want any more if not needed’ (survey, P42, father, control).
Similarly, after the discussion during the interview about their experience of taking part in the study, most parents who had experienced randomisation to control, intervention and ecology aspects of the pilot trial were in favour of the proposed cRCT:
I said to the nurse at the time as well when I read it, ‘You should definitely roll this out into different intensive care units around the UK or further afield’. Like I say, it can only benefit.
P9, father, SDD
I think it’s absolutely necessary. To be honest, I think the pilot study you’re going to get a good idea of the accuracy but you won’t necessarily get as accurate data as you would if you did the whole of the UK. It just makes sense to do the study across the whole of the UK, increase the accuracy of the study. Yes, I think it’s a good idea.
P15, father, control
Reasons for the acceptability of the proposed study were linked to the nature of the intervention, which was viewed as non-invasive, as well as the provision of information and reassurance by staff on site, particularly about the low-risk nature of the trial, which was seen as safe for the child to be involved in.
And the drawbacks were very unlikely and very minimal in their impact anyway. So, I think it was fine, as far as I was concerned, that that had already been started. And the reassurance was given that, if there were any concerns that it would have affected her in any way, they wouldn’t have started her on the treatment.
P7, father, SDD
I think the acceptability depends on the information that is given to mothers or care-givers. If whoever is doing the study explains the same way that they did to me, I think it will be very much acceptable to parents and care-givers. It all depends on the information dissemination. If it’s good, then I think you can have good results out of that.
P20, mother, SDD
On the basis that it is 100% safe to children, then I’m all for it. Medical research is an important part of development and in evolution of protection against viruses and bacteria, so, yes, I’m, in principle, for it.
P10, father, control
Some parents (two control, four intervention) hoped or held the misconception that their child would directly benefit from the study:
Like I say, even if nothing came from it, nothing developed from it, but … You wouldn’t know obviously, if he hadn’t had the medicine, if he then also didn’t get anything. You wouldn’t know if there was a benefit to it. But from a point of having it, being in his system, there, ready, it’s just another thing, isn’t it? It’s like another protective layer almost, for him to have.
P23, mother, SDD
I was fine with that. I mean, you know, if this is going to help keep our child safe, and especially with his injury, if there was a risk of him getting an infection that could cause further damage, or a longer stay in hospital, or further complications, then I would have been happy to take part in that study.
P12, mother, control
While others spoke of how the trial was presented to them as something that may help others in the future rather than having direct benefit for their own child:
Yes, of course. So, when I got to PICU, they gave me a leaflet with what PICnIC is, and they explained, you know, it doesn’t actually benefit or change anything for your child, but it’s for future, like, future researches.
P21, mother, SDD
Acceptability and understanding of randomisation
Overall, parents acknowledged the importance of randomisation in a study such as PICnIC and trusted that the practitioners would ‘treat the study in an ethical manner’ (P15, father, control).
I think it’s acceptable, like I said, because you’re not going to have [any comparison, no outcome]. And if [they] weren’t doing the study, they wouldn’t be giving it to [them] anyway. So, it’s not something they’re removing from them, the standard practice. It’s an additional – as a measure outcome really.
P3, mother, Period One control
Two parents thought the question of randomisation was hard to answer, as it would depend on how sick the child is and whether the benefits outweigh the risks.
For me, personally, if my son was really very ill, which he was at one point, to find out that there could be a medication out there that could help him to not get worse by catching an infection, then I would want him to have it. So, it’s a tricky one, isn’t it?
P5, mother, Period One control
That’s a hard one. I don’t know because it’s [outweighing] the benefits and the risks, at the end of the day, isn’t it? It’s difficult. I think, because it’s something we don’t routinely use here, yes, I wouldn’t ask questions if they weren’t to receive it, to be honest. […] But obviously for those that are using it, hopefully the benefits outweigh the risks, at the end of the day. So yes. I suppose all trials and things have got to start somewhere.
P6, mother, SDD
When asked about staff explanations of how PICnIC sites had been randomised, the majority of parents reported that the process of cluster randomisation was not explained to them:
I don’t think so. I wasn’t under the impression that everybody in [PICU] would be in a control … I think I knew there were other hospitals involved, but I hadn’t grasped that one hospital would be giving all the treatment and we were a control.
P19, father, control
Only one parent reporting remembering having this process explained to them.
Some parents wanted to know that the hospitals were comparing the two methods; however; one parent explained that they were not bothered by the lack of knowledge of the technical word: ‘Yes. It’s more important to know that than the name. The name would have been nice information to know beforehand, but at the same time we still knew what the words meant, just without the words’ (P15, father, control). Parents in both groups wanted to know more about the actual intervention, rather than the trial arm the child had been randomised to:
I don’t think it’s as important as explaining what the actual treatment involves and what that means for your child and what the drawbacks could be to the treatment actually. I think that’s more important than knowing whether your child is actually getting the treatment or not for it.
P7, father, SDD
If we were in the intensive care unit that obviously were obviously then yes, maybe it’s probably worth saying to them, ‘Look, this is why we’re giving this to your child because everyone on this unit we’d like to give it because we’re comparing it to another unit who is not having it to see the results.’ Yes, maybe we could have been told that information.
P16, father, control
Following a discussion in which the researcher described the cluster approach to randomisation used in the PICnIC study, parents were then asked how acceptable they found this method, how they felt when they found out which trial arm their child was in (e.g. control or intervention) and how they would have felt had their child been randomised to the other trial arm.
Some parents were under the impression that children would be chosen to be given the medication for a particular reason; however, one of them did not think it was acceptable when it was explained that units were (and would be in a future trial) randomly assigned to give medication to children.
I think she’d have been better off being in the unit she was because at the end of the day certainly no intervention and just leaving wouldn’t have really … That’s pretty much what’s happened for [Name of child] anyway. We knew that she was probably chosen for what was probably the better one for her.
P9, father, intervention
Researcher: How acceptable do you feel it is to give SDD, to give the medication, to only half of the babies who take part in the study?
Parent: I think, obviously, they must be chosen for a reason.
Parent: I would say it is not acceptable.
P11, mother, Period One control
Two parents (one control, one intervention) indicated that they would prefer a cluster design, as that would be beneficial ‘because you’re not going to accidentally give treatment to the wrong person’ (P15, father, control).
If the babies are not in the same unit, I think it’s fine. But it only raises questions if half of them are given and the other half is not given. But if all of them are given or if all of them are not given, I don’t think that becomes an issue.
P20, mother, intervention
Researcher: Okay. Given that your child was in the control group, how do you think you would feel if you were informed that the intensive care unit that your child was in, if you were informed that all children in that unit were given SDD?
Parent: Again, personally, I wouldn’t be upset. I’d maybe ask some more questions regarding it, but I wouldn’t have any adverse feelings.
P15, father, control
Selective decontamination of the digestive tract acceptability and lack of parental understanding
While parents had a rough understanding of the study aims, most of them were not aware of what the SDD medication was. Parents whose children were in the intervention unit remembered their children being given the SDD paste, ‘Yes, it was like a thick yellowy – it was, like, an off-yellow colour paste that they put in’ (P18, mother, SDD), but they did not know that it was an antimicrobial:
No. I mean, they call it, on the ward, concrete, because it’s quite dense and stuff. But I don’t really know what’s in it, no.
P7, father, SDD
Only one parent was concerned about the child being given an antimicrobial: ‘Oh my God, basically stripping the gut of stuff’ (P11, mother, Period One control). Their aversion to their child receiving the SDD medication was based on past experience in ICU when the child had experienced severe adverse events as a result of being given antimicrobials. However, they were open to persuasion if given the right information: ‘it’s difficult I suppose, it depends on how you pitch it anyway doesn’t it? […] However, if I think my son is at greater risk because he’s more vulnerable or this and that then I’m open to persuasion’ (P11, mother, Period One control).
It’s really a tough call isn’t it because I understand that these infections are absolutely horrendous, however [Name of child] received some very aggressive antibiotics while he was in the ICU which then led to his skin breakdown as he was trying to poo and wee. It’s taken me two months to recover, because he’s incontinent so he wears incontinence products so it’s really difficult.
P11, mother, control
Some parents described the practice of giving SDD to children as ‘standard practice’, believing that it was what children would have gotten anyway outside of the trial:
Because when they explained everything, they said that it was all very low risk anyway, because they’re not doing anything more to him than he would be having, other than just giving him the medicine that he was going to be having.
P8, father, SDD
While most parents trusted the medical team and considered their child to be in safe hands [‘It’ll be fine, she’s in the safest hands and everything.’ (P23, mother, SDD)], parents (both who understood that SDD was new, as well as those who were informed about the medication during the interview) indicated that they would want the research team to explain better what medication they were giving their child, but they would have still agreed for their child to participate:
Yes, I definitely think that’s important information, it should be clarified. Especially because it’s not typical standard of care, do you know what I mean? Like, I think you should have all the knowledge, as a parent, going into it, exactly. Especially what is being put into your child; you should know.
P21, mother, SDD
Yes. My initial thought is, ‘Gosh, is this using children to test on?’ but I understand it’s the hospitals that are carrying on in their usual way aren’t doing anything different, so I guess children aren’t being put at any risk by not using the medication. I guess some parents wouldn’t want their children to have medication, so, yes, I guess it’s an important thing to do, definitely.
P5, mother, control
It could have been just nice to know information, but it wouldn’t make any changes in my decision-making.
P20, mother, SDD
No, I don’t think it really – Well no but I didn’t, sort of, know that, that it wasn’t used widely across … It doesn’t really change my view of it, I don’t think, because she didn’t develop any infections or anything.
P23, mother, SDD
Some parents in the intervention group perceived the study as ‘increasing the amount of care that people would have had’, which was seen as a benefit and a reason why it was acceptable and felt ‘a bit sorry for the ones that, obviously, haven’t’ (P7, father, intervention).
Acceptability of sample collection and the importance of being informed
The majority of parents thought that it is acceptable to collect routine samples as ‘they’re taking them anyway’ (P3, mother, control):
Yes, because that is part of established nationwide routine care. That is part and parcel of a routine admission, so you have presumed consent.
P19, father, control
However, as the following quotes highlight, many parents would have preferred to have been informed about the samples and what they would be used for before samples were taken. Few stated that they would have preferred for their prospective consent to have been sought for routine sample collection as they were part of a research study:
So yes, I would not be happy if it was taken without my consent. Again, because my child’s enrolment, I do not know and now you are taking samples, I do not know what you are doing with it, if I knew more of the information.
P13, mother, control
I think it’s best for the clinicians to explain to the care-givers of the babies, ‘That we are going to collect some specimens for the study, or just as routine investigations’, because sometimes you really wonder, Why they are taking that urine, how are they going to use it? But it’s fair if they can explain to you that, ‘We are going to do this, we are doing it routinely’.
P20, mother, SDD
No, I think parents would want to know and would want to give consent on providing samples. Obviously, I guess, it’s all about the protection of their child. Knowing where the blood samples are going, who are they going to, what the bloods are going to be used for, etc. I think it’s important to get parents’ consent around that for any, I guess, fluids or any other tests that result in something taken from a child. I think parental consent is important.
P2, mother, ecology
Some parents held the misconception that routine samples would be destroyed if they decided to opt out:
And it was explained to us at the time that they had already taken the samples. They hadn’t used them, they hadn’t done anything with them, they just took them because they needed to have them within a time frame. That makes complete sense. So, they’d got it there. And they said, ‘If you agree to the study, we’ve got them, we can get them sent off. If you don’t agree to it, they will be destroyed, that’s the end of it.’ ‘Again, that’s no …’
P08, father, SDD
The majority of the parents did not have an issue with the collection of additional samples. However, some have expressed concern about doing so without consent or without being given the relevant information.
If they’re routine, I think that’s fine. If they’re taking the additional samples without consent, then no, not so good.
P5, mother, Period One control
Researcher: […] and then seek consent for additional samples?
Parent: Well, I think it’s just part of being aware of what was going on. Yes, so no issues.
P19, father, control
Two survey respondents declined consent for samples, saying that their children had been through enough while they were ventilated or that the child ‘already has plenty of people poking him all day and didn’t want any more if not needed’ (survey, P42, father, control). Out those who attended the interview, two (one control, one intervention) declined consent mostly because of the condition that their child was in, saying that ‘if her health condition was stable, then I would have said yes, but because she was going through a phase of seizures, so other health issues. That’s probably what made me say no’ (P18, mother, SDD).
Research without prior consent – the importance of communication, perceived risk, and context
Initially, some parents indicated being surprised when they found out that their child had been entered into the study without their consent [‘I think I felt quite shocked to start with’ (P5, mother, Period One control)], whereas others, who had past research experience, were not surprised: ‘No, not at all, because to be honest, my son was born quite prematurely and he was put into a lot of research as well. I think it was just something that doesn’t really surprise me’ (P14, mother, SDD). However, only half of the parents who completed the survey indicated that they understood why consent was not sought for their child’s participation in PICnIC (see Table 18), suggesting there is a need to improve communication about why this approach is used.
Although parents did not want to opt out after being provided with the information on what the study entailed, they were concerned about there being a drug tested on their child and some stated they were initially annoyed that the trial intervention had been given to their child without their knowledge:
I was quite shocked if I’m honest. I did sort of … When I spoke to her and she said, ‘Your child is trialling out one of our drugs from the research called PICnIC.’ I sort of sat there and thought, ‘Well …’ I hadn’t been asked and I didn’t know what this study was about at all. I was a little bit annoyed to start with because I didn’t really know what they’d given her. I thought, ‘My child is trialling out things that haven’t ever been used before and she’s in a really ill position’.
P23, mother, SDD
Again, just because it is important, especially when giving medication, or even to do swabs, that you have parents’ consent.
P13, mother, control
Nevertheless, parents went on to describe how they found the PICnIC approach to consent acceptable as the study was seen as low risk:
I don’t feel there would be any need to opt out of something like this. It’s certainly not something that is going to harm my child. It is going to give information and certainly do her better than any harm.
P9, father, SDD
And it was quite clear that we could opt out if we wanted to at any point.
P12, mother, control
They also indicated that all children being eligible on the unit helped to improve the acceptability of the trial [‘if they are using that method with all the children in there anyway, then they’ve obviously got good reason to be using that method’ (P6, mother, SDD)], and, as mentioned in the section on sample collection, that the collection of samples was part of the unit’s routine.
One mother held a misconception that her child had not been entered into the trial prior to her giving consent.
Researcher: What did you think when you found out that your child had already been entered into the study before you were approached by the research nurse about your consent?
Parent: He had not been already involved.
P13, mother, control
This parent, along with others who did understand that their child had already been entered into the study, said they would have preferred to give informed consent: ‘I did think that it was better for parents to be approached before just giving their child a trial drug that they’re not aware of’ (P23, mother, SDD).
With anything, you would want to know what the risks were between the two. Similar to when he went in to have the lumbar puncture, I was given the risks of having the lumbar puncture and the risks of not having it to then enable me to make my decision on whether he should go ahead with that. I think the same should apply with regards to antibiotics given unless there’re clear medical grounds to be giving the antibiotics that he has this …
P02, mother, ecology
However, most parents acknowledged that PICU is a highly emotive setting and recommended that parents be approached once the child is in a more stable condition and have come to terms with the situation: ‘I appreciate that sometimes you have to do it that way. Otherwise, you might not be able to start things in time’ (P1, mother, ecology).
Reasons for participation or opting out.
As discussed previously, parents often stated that they took part in the PICnIC pilot study to help other children in future. As one following quotation illustrates, one father was not aware of his child’s participation in the pilot trial until it was broached, but felt it was important to consent, even if his child did not recover, as he found comfort in the thought of helping other children in the future:
I didn’t have any knowledge about the trial, I didn’t actually even realise it was something that hospitals did. But my initial reactions to it were, if something bad was to happen with him, it would be nice to know that any data that you could take from him would be used to help someone else. And that was almost a consoling kind of feeling.
P15, father, control
This finding was also supported by survey data with parents (50/61 who consented, 81%) indicating that they did not opt out/provided consent for the study ‘to help other children in the future’ with 11/50 (22%) indicating this was their main reason. This was the most common main reason indicated by parents. Other reasons were because they ‘trusted the doctor or nurse who explained PICnIC’ (41/61, 67%), because ‘medical studies like PICnIC are important’ (49/61, 80%), or to ‘help my child’ (36/61, 59%). Less commonly, parents did not opt out/provided consent because ‘the treatment had already been given’ to their child (8/61, 13%), they ‘didn’t feel comfortable saying no to the nurse or doctor who explained the study’ (1/61, 2%), or because their ‘child recovered’ (5/61, 8%).
During interviews, many parents spoke of the trust they had in the clinical and research staff to act in their child’s best interests and how their children were in ‘the safest hands’ (P23, mother, SDD).
The thing is, the research people for the study is not going to harm your kid, it’s just the information that you are giving to them. So, you can think quite quickly. You have to trust the medical team and medical people.
P04, mother, Period One control
Some spoke about how their child’s condition had improved which meant they were therefore willing for their child to take part to help others and reflected on how they could understand if parents of children who were very unwell were hesitant.
I would say because I knew he was okay, I was happy to speak to somebody who would tell me about the programme, and … But I can see if other parents didn’t want to take part, or didn’t want to speak to someone at the time, because of their child’s condition, it’s very understandable.
P12, mother, control
This was supported by parents who did decline consent for additional samples as they felt that their child was too poorly to take part in the study and did not want any ‘additional tests’.
I guess if her health condition was stable, then I would have said yes, but because she was going through a phase of seizures, so other health issues. That’s probably what made me say no. Had she not had that, I probably would have said yes. I guess it depends on the outcome of health, current health, at that point.
P18, mother, SDD
Although I was happy to go ahead at first, I then decided that my child already has plenty of people poking him all day and didn’t want any more if not needed.
Survey P42, father, control, opt out
Importantly, all parents who completed the questionnaire (65/65, 100%) and were interviewed (23/23, 100%) stated that the proposed PICnIC RCT was acceptable to conduct. This included parents who declined consent for some aspect of the trial. Having the option to decline certain aspects of trial involvement, such as additional samples, appeared to make the pilot trial more acceptable to parents.
Outcomes measures of importance to parents.
Parents were asked to review the list of outcomes that had been sent to them prior to the interview. In the few cases in which parents did not have access to the materials, a definition of each outcome was read to them, including an explanation about why it is important to explore parents’ perspectives about important outcomes (Box 1).
As we have discussed, we want to find out if a study looking at the use of SDD in PICU is feasible, acceptable and what the trial should look like.
In the full trial we will collect information on Outcomes (the list that I sent to you prior to interview). For example, the PICnIC trial may explore whether SDD helps to reduce the number of hospital-acquired infections or incidence of ventilator-acquired pneumonia. These are called outcome measures. We collect data on outcomes to see if a trial medication is effective or not.
However, these outcomes have come from research papers and don’t really give us much information on how children or families feel, or what is important to them. It is important that we include outcome measures that matter to children and their families.
MP then asked parents, ‘Thinking about your experience of your child being admitted to the PICU, what would you hope the study would do to help your child?’ Most parents hoped that their child’s quality of life will improve, that is:
… getting back eating; obviously, not having relapses; I think getting back to where she was, developmentally, before she got poorly.
P19, father, control
[That] it would reduce the risk of an infection and a need for further intervention.
P02, mother, ecology
Overall, parents felt that the list of outcomes presented to them was comprehensive:
I think you’ve got the majority of it covered.
P02, mother, ecology
Most prioritised outcomes that were included in the list they were provided with, such as reduced likelihood of complications or infections:
Less complications because they received the SDD, then I think those would be priority.
P12, mother, control
So, from a parent’s perspective, I’m hoping that this study drives an outcome where secondary infections are less common in children receiving intensive care treatment.
P07, father, SDD
Parents were then asked to rank the outcomes listed in order of importance from the list provided. The top prioritised outcomes can be seen in Box 2. Survival (1) and health complication/AEs (2) were ranked joint as most important for the majority of parents.
Ranked parent outcomes
-
Survival
-
Health complications/AEs.
-
Number of, duration spent, and type of treatment for their child’s vital organs (including mechanical support).
-
Current health status in terms of development, functioning and/or life quality.
-
Re-admission to hospital.
Although many did not initially mention it, the majority of parents considered survival an important outcome. When MP inquired about survival, the most reported reason for not mentioning it was that the death of their child was not something they wished to consider [‘I don’t know, the outcome of survival … I try not to think about that really’ (P9, father, SDD)], while others thought it was obvious that survival would be a top one [‘Survival is obviously quite a big one. (Laughter)’ (P5, mother, Period One control)]. One parent in the control group found it difficult to answer, as it made them wonder whether they would question which treatment arm their child was in and whether being in the intervention arm could have improved their child’s chance of survival:
If I was taking part in the study and he didn’t survive, would I be asking, ‘Could he have survived if he had received SDD?’ And that would obviously depend on, was it because of complications and an infection that they picked up while they were in hospital? So, that’s a difficult one to call.
P12, mother, control
Next, associated with the health complications outcome were the infections acquired in the ICU, tolerance of intervention, number of specific treatments their child received, and looking and behaving like normal self, that is overall feeling of return to health or normality:
‘Oh, this treatment will help [name of child] be her normal self a week quicker,’ I’d jump at it, wouldn’t I? So, I think that is my overarching thing and I want her to be her normal self as soon as possible.
P19, father, control
Although health complications were one of the top prioritised outcomes, most parents agreed that the length of PICU and hospital stay, associated with this outcome, was not a priority for them as,
there is no time limit on the rehabilitation of your child, so to me, it’s not up there in the priority. You would stay for however long it would be required. I think [their] wellbeing would be first, in terms of their behaviour, and their presentation.
P03, mother, Period One control
The number of, duration spent, and type of treatment for their child’s vital organs (including mechanical support) (3) was generally reported as being third most important outcome, ‘Because if they’re on it for longer, that risk of infection is increased. So, I’d put that as number one’ (P10, father, SDD).
Current health status in terms of development, functioning and/or life quality (4) and re-admission to hospital (5) were seen as the least important outcomes for the majority parents:
The returning to good health, that is difficult, it’s really difficult because you don’t know what a new-born should do or when you have a special needs child the knock-on effect is far greater. That’s not something that I would focus on exclusively in the hospital, in the ICU, that would be something then to focus on normal ward.
P11, mother, Period One control
Overall, however, as previous research has shown,22 parents seemed to find the questions on outcomes difficult to answer and there was general consensus that they were all as important as each other:
They’re all quite important facts. If you put one then there is another one you read and you’re like, ‘Well actually that one is as important.’ I think to put them in an actual first, second, third is pretty hard to do. Sorry. I don’t think I can.
P16, father, control
Chapter 5 Discussion and conclusions
Our overall findings suggest that a definitive cRCT in SDD in PICUs is feasible. The pilot cRCT was representative in terms of sites and patients when compared with the whole UK PICU population and can therefore inform the design of a definitive cRCT. This feasibility study, comprising a pilot cRCT and the mixed-methods study, has identified a number of design modifications, which would need to be included in a definitive cRCT to ensure that the efficiency of trial processes is maximised.
Cluster-randomised controlled trial design
The cluster randomisation model was effective and supported by staff and caregivers. PICUs were able to be randomised into groups that were relatively evenly split in terms of unit size, and patient characteristics including age, ethnicity and admitting diagnosis.
The contracted nature of the pilot cRCT meant that staff had to adapt practice more quickly than would be expected in a definitive cRCT, where a more prolonged and intensive support could be planned and instituted. Moving periods more frequently than would be in a definitive cRCT added to this burden, and that is unlikely to be the same situation if the proposed trial takes place.
The potential recruitment rate for a future definitive cRCT trial is similar to the pre-trial estimated rate value of three per site per week. One site from the intervention group did, though, close to recruitment early due to reaching their recruitment target, which could have an impact in a definitive cRCT. Even though there are limited PICUs in the UK, the sample size simulations indicated that there are sufficient available clusters to adequately power a definitive cRCT on patient-centred clinical outcomes, including healthcare-acquired infection and days alive and free from ventilation.
Potential modifications
Periods (e.g. ecology, transition, intervention) need to be long enough to embed the new processes. Contracting procedures for sites need to require enrolment of patients for the full period of the cRCT.
Role of patient and public involvement
Patients and family representatives were involved in the design of the study, including one ex-patient being a coinvestigator of the study and a member of the TMG. The involvement of PPI representatives shaped the study design and materials as described previously.
Screening, recruitment and consent
Screening and recruitment processes worked efficiently, with the vast majority of potential eligible patients being enrolled across the ecology periods and Periods One and Two. Potentially eligible patients may have been missed due to the subjective estimate of whether the patient was likely to be extubated within the next 48 hours. When comparing against the potentially eligible patients within the participating sites, the patients enrolled were representative in terms of age and ethnicity as well as admission diagnosis, with no evidence of issues with inclusion of any patient cohorts.
Sites implemented the consent model inconsistently, especially early in the pilot cRCT, with a number of parents not being approached for additional samples or data collection. Parents did not object to the consent process, as long as additional samples were only taken with consent. This was shown, as consent for data collection was good, but there was a number of refusals for collection of additional samples.
Potential modifications
Inclusion criteria need to include all ventilated children, with an aim to reduce the burdensome repeat screening at sites. This would also better match how SDD would be implemented in practice.
Delivery of the selective decontamination of the digestive tract intervention
Adherence to the SDD intervention was high, with SDD commencing in a timely manner in the majority of children. The time-critical nature of the study intervention made delivery challenging and has resource implications. Most of the staff found SDD administration acceptable or very acceptable; however, views on SDD were polarised and some clinicians were sharply for or against the intervention. The paste was frequently described as unpleasant in taste and thick. The volume of paste for babies compared to older children was the same, but in practice only the amount needed to smear inside the mouth was needed but some staff may have tried to use the full amount based on pharmacy information leaflets. Some were previously concerned the feeding tubes would become blocked, although this was not reported at any sites during the intervention period.
There appeared to be subtle differences in how staff described the nature of the intervention at sites administering SDD, which may have led to parental misconceptions.
Potential modifications
A definitive cRCT would need to ensure parental communication is clear about the design of the study and the experimental nature of SDD in an infection control regime. Information and training to engage those staff who were unsure or opposed to SDD will be vital to ensure equipoise in the intervention. The time-critical nature of the intervention will need to be considered in the funding model for a definitive cRCT in order to ensure site staff felt able to deliver workload.
The dosing of the paste for babies should be reviewed to generate more specific guidance and a more paediatric-focused package size prepared.
Outcome measures
With regard to the ecological outcomes, the consent rate was very low for the collection of additional samples. Collection of additional samples was challenging clinically for various reasons, such as the perceived burden on the patient and staff capacity issues, particularly during night shifts, which meant that swabs became less of a priority or forgotten. Parents who declined consent for additional samples also mentioned the child having gone through a lot and they did not want to add further distress. When consent was obtained, sample collection was very high. In addition, specific antimicrobial resistance testing at sites was inconsistent.
With regard to potential clinical outcomes, consent for data collection was high, and the completeness, either from collection directly from sites or through PICANet reports, was very high.
Survival and health complications/AEs were ranked most highly by parents. Duration of organ support and recovery/re-admission were also considered as most important outcomes by caregivers. Some parents highlighted that VAP rates should be included.
Potential modifications
The design of a definitive trial must include consideration of how the ecological impact of SDD can be examined. In the recently concluded adult SDD trial,7 microbiology outcomes utilised samples routinely collected during the PICU stay. If more specific profiling of the resistance to the SDD components was considered integral to the assessment of this intervention, a definitive cRCT should consider adaptation to site ecology monitoring, either to minimise additional swabs or samples or to integrate monitoring into existing clinically indicated infection control practices to allow more complete data collection. This would need careful discussion with the microbiology departments of participating sites. Site selection should be dependent on agreement of the local laboratories to process samples and allowance made to allocate appropriate resource to the microbiology departments to support this.
The potential primary outcomes for a definitive RCT should include a measure of organ recovery and the development of complications during their PICU stay.
Strengths and limitations
Strengths
The study was supported by the PCCS-SG, which provided peer review and support for the pilot trial and mixed-methods study design, as well as the dissemination of results. Further advice on study design and conduct was made possible through collaboration with clinicians involved with the adult SDD trial and ensured the trial design was optimised. Furthermore, the study intervention was made possible through agreements to contract the supplier to the adult SDD trial and use their good manufacturing practice (GMP) compliant formulation.
From among caregivers, we spoke to parents from children admitted to six additional PICUs as well as the six pilot PICUs, and their views were consistent with support for the proposed definitive clinical trial, which mirrors the views of PICU clinicians, with modifications to study design and outcomes as recommended below.
Data collection was facilitated by a national clinical audit to minimise staffing burden at sites and to inform the wider UK generalisability of the findings. This was important in the context of site working capacity and the burden of other concurrent studies.
Our mixed-methods approach means that our findings provide both qualitative insight into parents’ and staff experiences, as well as the wider perspectives of parents and PICU staff captured by the surveys conducted.
The original aim was to conduct two focus groups (one intervention and one control). We extended this work to ensure all sites had the opportunity to take part and conducted six focus groups with 25 staff. The results of the mixed-methods study therefore reflect the views and experiences of a more representative sample of those who took part in the pilot trial than our original design had intended.
All parents who took part in our study experienced recruitment to the pilot trial, which means our findings are less hypothetical than those of feasibility studies that do not incorporate a pilot trial and are likely to be similar to decisions made by parents in the proposed trial.
Limitations
The COVID-19 pandemic had an impact in delivery of the pilot cRCT. Delays in local approvals and competition of resources for non-COVID-19-related studies led to pressure on research teams, which may have impacted consent and sample collection.
No bereaved parents took part in the mixed-methods study as only one child died during the pilot cRCT. In addition, potentially due to these staffing pressures, despite using different strategies to assist recruitment of wider PICU staff and extending the data collection period of the survey, the response was limited. Due to the COVID-19 restrictions, site training had to be undertaken remotely, and this may have limited the number of staff who could be reached. Although the talks were made available online, a future definitive trial would be best supported by on-site training, especially in sites allocated to administer SDD.
The time frame of the pilot RCT meant that sites had to switch through different phases of the study quickly, which may have impacted the ability to ensure all staff were fully trained. A larger, definitive trial would be undertaken over a longer period, allowing more time for training and uptake of study-specific training.
The oral paste that was part of the trial intervention was problematic. Some of this may be resolved by communicating that the dose can be adapted to body size so that only the amount needed to smear the inside of the oral cavity is needed. Some sites reported that using all the paste in smaller patients was challenging and led to gagging, although there was no AE reported in terms of clinical risk. However, this is possible to address through improved communication and study drug information.
Recruitment in one of the intervention sites was stopped when the target of patient enrolments was reached. This had an impact on the overall recruitment to the intervention as seasonal variation meant the other sites had lower numbers of eligible patients admitted in the remainder of the intervention period. A definitive trial will need to adapt staff training and unit agreements to ensure participation for the full duration of the study.
As a small pilot study, there was not time or resource to include the cost of translation of materials used to communicate with families and this may impact the inclusivity of the study to all ethnicities. A definitive trial should therefore explicitly include the ability to translate study documents and communication of the study to caregivers, given the nature of the study and the unit-wide consent process.
Summaries of key research recommendations
The design of the pilot cRCT was found to be acceptable to both staff and parents in the mixed-methods study. We recommend that a future definitive PICnIC RCT should use a largely similar design of cluster randomisation with the same eligibility criteria, control and intervention arms, but consideration should be given to the following: SDD paste formulation and dosing regimen, ecology monitoring and consent processes, ensuring consistency and appropriate rigour in collecting swabs to monitor ecology in enrolled patients without overburdening the PICU clinical staff.
Implications for health care/practice
As a feasibility study, this project has no direct implications for health care or practice.
Equality, diversity and inclusion statement
Through its cluster design, clinicians were able to ensure that the study was inclusive to all children who would be eligible. Our data suggest that our study population reflects the national population of children admitted to PICU as reflected in the PICANet annual report. The contracted nature of the pilot trial and mixed-methods study meant translated material was not available for those for whom English was not a language they understood or were able to communicate in. A future definitive study should ensure translation of materials is incorporated into the study design.
Acknowledgements
We are grateful to the NIHR (HTA) programme for commissioning this study, and to the NIHR Biomedical Research Centres at Cambridge University Hospitals NHS Foundation Trust, Birmingham Children’s Hospital, Southampton Children’s Hospital, St George’s University Hospital London, John Radcliffe Hospital Oxford and Bristol Royal Hospital for Children for supporting the study at their sites.
The sponsor (Cambridge University and Cambridge University Hospitals NHS Foundation Trust) and the clinical trials unit (ICNARC) provided tremendous support to overcome logistical and study process hurdles to deliver this feasibility study in a timely manner.
The study would not have been possible with the consent and participation of children admitted to the PICUs involved in the study and their caregivers, along with the PICU, microbiology and pharmacy staff who undertook the study in a backdrop of NHS recovery from the COVID-19 pandemic and all the restrictions and clinical pressures that imposed.
Outside of the PICnIC study sites, clinicians working in PICUs across the UK and their allied team members regularly reviewed and advised on study development and progress as part of the Paediatric Critical Care Society Study Group.
Information governance
The University of Cambridge is committed to handling all personal information in line with the UK Data Protection Act (2018) and the General Data Protection Regulation (EU GDPR) 2016/679. Under Data Protection legislation, ICNARC is the Data Processor, the University of Cambridge is the Data Controller and we process personal data in accordance with their instructions. You can find out more about how we handle personal data, including how to exercise your individual rights and the contact details for the University of Cambridge Data Protection Officer (www.information-compliance.admin.cam.ac.uk/contact-us; data.protection@admin.cam.ac.uk)
Research staff at participating sites
We acknowledge that there have been many other individuals who contributed within the participating sites. It is impossible to thank everyone personally; however, we would like to thank the following research staff:
Dr Charlotte Fulham, Melanie James and Kirsten Beadon from John Radcliffe Hospital; Cat Postlethwaite, Jenny Pond and Antonia Hargadon-Lowe from University Hospital Southampton; Jane Cassidy, Ceri Robbins and Phil Milner from Birmingham Hospital; Dr Buvana Dwarakanathan and Ms Joana Gomes De Queiroz from St George’s Hospital; Dr Nazima Pathan, Esther Daubney and Deborah White from Addenbrooke’s Hospital; and Peter Davis, Laura Dodge and Francesca Moody from Bristol Royal Hospital for Children.
Contribution of authors
Alanna Brown (https://orcid.org/0000-0002-0104-3251) (Project Manager) set up and managed the studies, contributed to the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Paloma Ferrando-Vivas (https://orcid.org/0000-0002-2163-645X) (Statistician) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Mariana Popa (https://orcid.org/0009-0006-9237-4094) (Research Associate) carried out the mixed-methods work package of the study, contributed to acquisition, analysis and interpretation of the data, and drafted and critically revised the manuscript.
Gema Milla de la Fuente (https://orcid.org/0000-0002-2134-3462) (Project Manager) managed the studies, contributed to the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
John Pappachan (https://orcid.org/0000-0002-3559-0595) (Professor of Paediatric Intensive Care) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Brian H Cuthbertson (https://orcid.org/0000-0003-4174-9424) (Professor of Critical Care) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Laura Drikite (https://orcid.org/0000-0002-5194-4189) (Project Manager) managed the studies, contributed to the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Richard Feltbower (https://orcid.org/0000-0002-1728-9408) (PICANet) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Theodore Gouliouris (https://orcid.org/0000-0002-2011-1205) (Microbiologist) contributed to the microbiology design of the trial, the acquisition, analysis and interpretation of microbiological data, and drafted and critically reviewed the manuscript.
Isobel Sale (Lay member of the coinvestigator team) contributed to the study design, reviewing study documentation and helping to draft the study from the perspective of a former PICU patient.
Robert Shulman (Pharmacist) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Lyvonne N Tume (https://orcid.org/0000-0002-2547-8209) (Professor of Paediatric Intensive Care Nursing) contributed to the design of the trial and the acquisition, analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
John Myburgh (https://orcid.org/0000-0003-4088-7016) (Professor of Critical Care) contributed to the design of the trial and the protocol and on the design and agreements for the acquisition of the SDD formulation used in the PICnIC study. He also contributed to the analysis and interpretation of the data and drafted and critically reviewed the manuscript.
Kerry Woolfall (https://orcid.org/0000-0002-5726-5304) (Reader in Health Research Methodology) conceived, designed and led the mixed-methods work package of the study, contributed to acquisition, analysis and interpretation of the data, and drafted and critically revised the manuscript.
David A Harrison (https://orcid.org/0000-0002-9002-9098) (Head Statistician) contributed to the design of the study, the statistical analysis and interpretation of the data, and drafted and critically reviewed the manuscript.
Paul R Mouncey (https://orcid.org/0000-0002-8510-8517) (CTU Head of Research) designed the trial, contributed to acquisition, analysis and interpretation of the data, and drafted and critically revised the manuscript.
Kathryn Rowan (https://orcid.org/0000-0001-8217-5602) (CTU Director) designed the trial, contributed to acquisition, analysis and interpretation of the data, and drafted and critically revised the manuscript.
Nazima Pathan (https://orcid.org/0000-0001-7656-9453) (Associate Professor of Paediatric Intensive Care) was the chief investigator. With the coinvestigators, she conceived and designed the study and had overall responsibility for study conduct and progress. She contributed to the acquisition and analysis of data and drafted and critically revised the manuscript.
Trial Management Group
Nazima Pathan, Lyvonne N Tume, Paul Mouncey, David A Harrison, Kathryn Rowan, Richard Feltbower, Theodore Gouliouris, Kerry Woolfall, John Pappachan, Robert Shulman, Brian Cuthbertson, Meredith Malloy, John Myburgh, Paloma Ferrando-Vivas
Trial Steering Committee
Simon Nadel, Sarah Seaton, Louise Rose, Penny Revitt, Nazima Pathan
Data Monitoring and Ethics Committee
Padmanabhan Ramnarayan, Patrick Davies, Jennifer Bell
Ethics statement
The study was approved by the West Midlands – Black Country Research Ethics Committee (20/WM/0061) and received a favourable opinion on 3 November 2020, with approval granted by the HRA on 20 November 2020.
Data-sharing statement
All data requests should be submitted to the corresponding author for consideration. Access to all anonymised data may be granted following review by the data monitoring committee and completion of a data-sharing agreement.
Publications
Pathan N, Woolfall K, Popa M, de la Fuente GM, Ferrando-Vivas P, Brown A, et al. Selective digestive tract decontamination to prevent healthcare associated infections in critically ill children: the PICNIC multicentre randomised pilot clinical trial. Sci Rep 2023 Dec 7;13(1):21668. https://doi.org/10.1038/s41598-023-46232-7
Disclaimers
This manuscript presents independent research funded by the National Institute for Health and Care Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, the HTA programme or the Department of Health and Social Care.
References
- Patrick SW, Kawai AT, Kleinman K, Jin R, Vaz L, Gay C, et al. Health care-associated infections among critically ill children in the US, 2007–2012. Pediatrics 2014;134:705-12.
- Field-Ridley A, Dharmar M, Steinhorn D, McDonald C, Marcin JP. ICU-acquired weakness is associated with differences in clinical outcomes in critically ill children*. Pediatr Crit Care Med 2016;17:53-7. https://doi.org/10.1097/PCC.0000000000000538.
- Choong K, Al-Harbi S, Siu K, Wong K, Cheng J, Baird B, et al. Functional recovery following critical illness in children: The ‘wee-Cover’ pilot study. Pediatr Crit Care Med 2015;16:310-8.
- Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. Am J Respir Crit Care Med 1999;159:1249-56.
- Bekaert M, Timsit JF, Vansteelandt S, Depuydt P, Vésin A, Garrouste-Orgeas M, et al. Outcomerea Study Group . Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med 2011;184:1133-9.
- de Smet AMGA, Kluytmans JAJW, Cooper BS, Mascini EM, Benus RFJ, van der Werf TS, et al. Decontamination of the digestive tract and oropharynx in ICU patients. N Engl J Med 2009;360:20-31. https://doi.org/10.1056/NEJMoa0800394.
- Group TSI for the A and NZICSCT . Effect of selective decontamination of the digestive tract on hospital mortality in critically ill patients receiving mechanical ventilation: a randomized clinical trial. JAMA 2022;328:1911-21. https://doi.org/10.1001/jama.2022.17927.
- Hammond NE, Myburgh J, Seppelt I, Garside T, Vlok R, Mahendran S, et al. Association between selective decontamination of the digestive tract and in-hospital mortality in intensive care unit patients receiving mechanical ventilation: a systematic review and meta-analysis. JAMA 2022;328:1922-34. https://doi.org/10.1001/jama.2022.19709.
- Francis JJ, Duncan EM, Prior ME, MacLennan GS, Dombrowski SU, Bellingan G, et al. Selective decontamination of the digestive tract in critically ill patients treated in intensive care units: a mixed-methods feasibility study (the SuDDICU study). Health Technol Assess (Rockv) 2014;18:1-170.
- Canter RR, Harvey SE, Harrison DA, Campbell MK, Rowan KM, Cuthbertson BH. Selective Decontamination of the Digestive tract in critically ill patients treated in Intensive Care Unit (SuDDICU) Investigators . Observational study of current use of selective decontamination of the digestive tract in UK Critical Care units. B J Anaesth 2014;113:610-7. https://doi.org/10.1093/bja/aeu108.
- Daneman N, Sarwar S, Fowler RA, Cuthbertson BH. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013;13:737-8.
- Liberati A, D’Amico R, Pifferi S, Torri V, Brazzi L, Parmelli E. Antibiotic prophylaxis to reduce respiratory tract infections and mortality in adults receiving intensive care. Cochrane Database Syst Rev 2009;2009.
- Oostdijk EAN, De Smet AMGA, Blok HEM, Thieme Groen ES, Van Asselt GJ, Benus RFJ, et al. Ecological effects of selective decontamination on resistant gram-negative bacterial colonization. Am J Respir Crit Care Med 2010;181:452-7.
- Plantinga NL, Bonten MJM. Selective decontamination and antibiotic resistance in ICUs. Critical Care 2015;19.
- Price RJ, Cuthbertson BH. SuDDICU Collaboration . Selective decontamination of the digestive tract and oropharynx: after 30 years of debate is the definitive answer in sight?. Curr Opin Crit Care 2016;22:161-6.
- Petros A, Silvestri L, Booth R, Taylor N, Van Saene H. Selective decontamination of the digestive tract in critically ill children: systematic review and meta-analysis. Pediatr Crit Care Med 2013;14:89-97.
- Woolfall K, Frith L, Dawson A, Gamble C, Lyttle M, Young B. Fifteen-minute consultation: an evidence-based approach to research without prior consent (deferred consent) in neonatal and paediatric critical care trials. Archiv Dis Child: Educ Pract 2016;101:49-53.
- Straney L, Clements A, Parslow RC, Pearson G, Shann F, Alexander J, et al. ANZICS Paediatric Study Group and the Paediatric Intensive Care Audit Network . Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatr Crit Care Med 2013;14:673-81. https://doi.org/10.1097/PCC.0b013e31829760cf.
- Health Services Research Unit . Cluster Sample Size Calculator: User Manual 1999. www.abdn.ac.uk/hsru/documents/calculationmanual.pdf (accessed 15 August 2023).
- Moher D, Schulz KF, Altman D, Group for the C. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA 2001;285:1987-91. https://doi.org/10.1001/jama.285.15.1987.
- Campbell MK, Piaggio G, Elbourne DR, Altman DG. CONSORT Group . Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345.
- Deja E, Peters MJ, Khan I, Mouncey PR, Agbeko R, Fenn B, et al. Establishing and augmenting views on the acceptability of a paediatric critical care randomised controlled trial (the FEVER trial): a mixed methods study. BMJ Open 2021;11.
- Deja E, Roper L, Tume LN, Dorling J, Gale C, Arch B, et al. Can they stomach it? Parent and practitioner acceptability of a trial comparing gastric residual volume measurement versus no gastric residual volume in UK NNU and PICUs: a feasibility study. Pilot Feasibility Stud 2021;7.
- Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res 2016;26:1753-60.
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77-101.
- Glaser BG. The constant comparative method of qualitative analysis. Soc Probl 1965;12:436-45.
- Morse JM, Barrett M, Mayan M, Olson K, Spiers J. Verification strategies for establishing reliability and validity in qualitative research. Int J Qual Methods 2002;1:13-22.
Appendix 1 Selective decontamination of the digestive tract formulation
-
1. Selective decontamination of the digestive tract paste
| Material (BD identifier #) | Material description |
|---|---|
| 305851 | BD Syringe Oral/Enteral 1 mL |
| 302435 | BD Oral/Enteral Tip Cap |
-
2. Selective decontamination of the digestive tract powder for suspension
| Material | Material description |
|---|---|
| Pharmacy Lite/EZ-75 | Graduated Amber plastic bottle 240 mL |
| Pharmacy Lite/EZ-CR | Threaded child-resistant cap 33 mL |
| Cormack/220231 | Press-in bottle adapter 33 mL |
| Tri seal/33 mm PS22 WAD (Die 1–250) | Pressure seal liner PS22 |
Formulation
-
1. Selective decontamination of the digestive tract paste
| Ingredient | Specification | Purpose | Concentration (per gram) |
|---|---|---|---|
| Colistin sulphate | BP | Active | 20 mg |
| Nystatin | Eur. Ph. 8th Ed. | Active | 0.25 MU |
| Tobramycin sulphate | USP | Active | 20 mg |
| Mineral oil light | USP | Excipient | 50 mg |
| Methocel E4M premium | USP | Excipient | 177 mg |
| Petrolatum white | USP | Excipient | a686 mg |
-
2. Selective decontamination of the digestive tract powder for suspension
| Ingredient | Specification | Purpose | Concentration after reconstitution (unit/ml) |
|---|---|---|---|
| Colistin sulphate | BP | Active | 10 mg |
| Nystatin | Eur. Ph. 8th Ed. | Active | 0.20 MU |
| Tobramycin sulphate | USP | Active | 8 mg |
| Syrspend SF pH4 | USP | Excipient | 45.5 mg |
| Citric acid monohydrate | BP | Excipient | 2.86 mg |
| Potassium sorbate | USP | Preservative | 2.0 mg |
Appendix 2 Topic guide for parents’ interviews
Topic guide for interviewing parents – PICnIC
Please note: Italic text indicates instruction for researcher and will not be read to participant .
Introduction call: My name is Mariana and I am a researcher from the University of Liverpool. I am carrying out a study that is looking at the feasibility of conducting a full PICnIC trial. I don’t know if you remember, but you completed a consent form when your child was in hospital and was approached about the PICnIC feasibility study? On that form you stated that you would like to take part in an interview, which is why I am ringing. (Obtain consent for interview and arrange time.)
Start of interview: I have a few questions about the PICnIC recruitment and consent process.
IF ECOLOGY WEEK RECRUITMENT CHECK THAT PIS FOR INTERVENTION HAS BEEN READ AND THEN TALK THROUGH THE KEY ASPECTS OF THE STUDY (AIMS, RANDOMISATION, INTERVENTION AND CONTROL, OPT-OUT CONSENT), CHECK UNDERSTANDING.
Obtain consent:
Bereaved: I have some idea about your circumstances, if there is anything that you find difficult to talk about please don’t feel that you have to, or you want to stop the interview at any point, then let me know. Is that ok?
The reason that you were invited to take part in this interview study is because if we only ask those parents who have children who have recovered from their hospital admission the information we gather won’t be complete. The findings will be biased. We know even less about what it is like for parents who have been bereaved to have discussions with doctors and nurses about the trial and what it was like to be involved in the trial. Doctors and nurses want to understand what it is like for parents in this situation and whether they should approach them about the trial. Does that make sense? Do you have any questions before I start?
I will start with some questions about you if that’s ok and then I will ask you about your experience of being approached about the PICnIC study. Is that ok?
| Section 1: Demographic information
Do you mind if I start by asking a few questions about you and your child, for administrative purposes. |
||
| 1.1 | Where do you live/What is the first part of your postcode? | |
| 1.2 | Would you describe yourself as being: employed or unemployed? – (If employed, what is your profession?) | |
| 1.3 | How old is your child? Boy or girl if not mentioned already? | |
| 1.4 | What would you describe as being your first language and ethnicity? | |
| 1.5 | So just to get a background of what happened: what first prompted you to seek medical help when your child became unwell – how did they end up in intensive care? (Prompt: explore what happened, how they were admitted to hospital) | |
| 1.6 | Did the doctor give your child a diagnosis (e.g. did they tell you what had caused the illness?) If unclear: Does your child suffer from an ongoing health condition? |
|
| 1.7 | How long ago was this? Request month and date. How long were you in hospital for? How is [child name] now? Has he/she recovered from his/her hospital visit? |
|
| Section 2: The PICnIC consent process – Baseline knowledge | ||
| 2.1 | Would you mind if I start by getting an overall picture of what happened when you first heard about the PICnIC study … could you tell me a bit about that? Explore any knowledge about the trial before approach. Explore where parents were when they first heard the trial mentioned. If bereaved and received information by post, go to section 7. |
|
| 2.2 | Did you see any leaflets or posters about the study? If yes, where were the posters (Prompt: on the wall in PICU) If Yes, was this before or after the nurse approached you about the study (Explore initial reactions if before). Were you pointed towards the posters or leaflets at all? Could you tell me what you thought about the leaflets/posters? |
|
| 2.3 a | How were you introduced to the study? Was it a doctor or nurse who spoke to you about the trial? Did one of the nursing staff looking after your child introduce you to the research nurse or doctor? Who do you think should approach the parents about a trial? Prompt: Do you think it should be a doctor or nurse involved in a child’s care who approaches parents about a trial?a Do you think it should be someone separate from the care team?a Could you tell me why you think this? Did you have an opportunity to discuss your child’s condition with a member of the clinical team? Explore preferences for this, or just research staff. |
|
| 2.4 | Did the research nurse/doctor check with you that it was a good time to talk about research? If so, when was this? Do you think that this was the best time? If not, when would have been the best time? Explore whether parents were surprised to be asked about research at that point in time. How could this be improved? |
|
| 2.5 | Could you tell me what they explained about the PICnIC study? This is a question I ask all parents and it’s not a test, but just so we can gauge whether the trial is being explained clearly enough. Could you tell me what the PICnIC study was looking at? Describe your understanding of what the PICnIC study is aiming to do? |
|
| 2.6 | Very unwell children are at higher risk of getting an infection while in hospital. Many of these infections are caused by bacteria already in the body such as those in the mouth or stomach. When children are very ill the level of naturally occurring ‘good bacteria’ is reduced, meaning ‘bad bacteria’ can lead to serious infections. | |
| The PICnIC study will compare current infection control procedures (such as strict hand washing) with a treatment called selective digestive decontamination (SDD). SDD works by stopping bacteria growing in the digestive tract that could cause infections. This will allow researchers to decide which treatment is better at preventing infections and improving outcomes for children. | ||
| In order to fairly compare both treatments, we have randomly assigned six participating PICUs to two groups. One group will deliver SDD in addition to usual infection control procedures and one group will continue to deliver infection control procedures as usual. PERIOD TWO ONLY: Do you know if you child was in a unit that was receiving the SDD intervention or where they in the comparison group (no SDD intervention)? |
||
Depending on trial arm:
|
||
| Depending on response:
Do you know what the SDD medication is? If not explain that it is a mix of non-absorbable antibiotic and antifungal medication. When children are very ill the level of naturally occurring ‘good bacteria’ is reduced, meaning ‘bad bacteria’ can lead to serious infections. SDD works by stopping bacteria growing in the digestive tract that could cause infections. A pea-sized amount of paste is given around the mouth and a tiny bit of liquid through his feeding tube. Explore if this changes their views on the study at all? Also explore whether they think this is important information/should be clarified? (What SDD is) |
||
| The intervention will be started in all eligible patients as it will form part of the standard infection control strategy in the participating PICU, but this will not have been the standard before the trial. Does that change your view of the study? Do you think it’s important to clarify that it is standard procedure only during the trial? Clarify that this is a pilot/feasibility study rather than the full trial (if they are unclear). Is there anything about how PICnIC was explained to you that could have been handled a bit differently? | ||
| 2.7 | Did they go through any of the potential risks or benefits of your child taking part in the trial at that point? If yes, how did they describe these? Was there anything that you found: (a) unclear? (b) surprising? Is there anything else that sticks out in your mind about the discussion? |
|
| 2.8 | Could you tell me about any written information you were given by a nurse or doctor about the study? Explore whether they were given the information leaflet – short version and/or the full patient information sheet.
Prompts: Did you read the information leaflet/sheet? (Prompt: If they read the short information PIS leaflet or the full information PIS) What did you think about the information leaflet/sheet? Was there anything that you found: (a) unclear? (b) surprising? Could the information leaflet be improved in any way? (Prompt: If so, how?) |
|
| Section 3: Randomisation | ||
| 3.1 | I mentioned earlier that hospital PICUs taking part in the PICnIC study were split into two groups by a process called cluster randomisation. So, half of the units are giving SDD, and half are not. That means that children in one intensive care unit (AD/BI/SG) all are given SDD and all children in another unit (Bristol) do not, they follow usual care procedures. We would then compare the study findings between whole units rather than by individual child.
Could you tell me if this process was explained to you? Explore if parents think this is important to know? How was this process explained? Were you aware that there were two possibilities in terms treatment? Was there anything about this process that you were unclear about? CONTROL GROUP: How do you think you would feel if you were informed that the intensive care unit your child was in was taking part in PICnIC and all children were given SDD? Explore any questions, any concerns, would you provide permission to use data already collected? INTERVENTION GROUP: How would you feel if you were in a unit who were NOT given SDD (control group)? Explore any questions, any concerns, would you provide permission to use data already collected? |
|
| 3.2 | How acceptable do you feel it is to give SDD to half the babies who take part in this study? | |
| 3.3 | How acceptable do you think it is to not give SDD to half the babies in this study? | |
| SECTION 4: OPT-OUT CONSENT AND SAMPLE COLLECTION | ||
| 4.1 | Explain: Babies are included in the PICnIC study if they are in a PICU that is taking part in this feasibility study. All babies are included, and parents are informed about the study through posters, leaflets, and a discussion with the research team. Parents can ‘opt out’ of their babies’ involvement in the study at any point. Information collected up until the point that parents ‘opt out’ are included in the study. This is called ‘opt-out consent’. What do you think about the use of opt-out consent in the PICnIC study (e.g. all babies are included, and parents can withdraw)? | |
| 4.2 | How did the nurse/doctor explain opt-out consent to you? Was it explained clearly? If not, provide explanation. Check understanding. | |
| 4.3 | What did you think when you found out that your child had already been entered into the study before you were approached by the doctor or nurse about your consent? Were you surprised at all? If YES, could you tell me a bit about that? |
|
| 4.4 | Did you have any concerns about this method? If so, could you tell me a little bit more about these? If not, what were your reasons for this? Did you raise these concerns with a practitioner? If so, were they addressed/alleviated and how? If not, why? IF NOT IN THE INTERVENTION GROUP: How would you have felt if your baby had been in one of the units giving the SDD intervention without prior consent? Explore any concerns |
|
| 4.5 | Explain: All babies in PICnIC have samples taken for the study, regardless of whether or not they received the SDD intervention. This included:
If doctors were concerned about specific infections, they may have also taken urine samples, Respiratory samples (collection of sputum) and Swabs of any wounds your child may have. Some of these samples are part of routine care and consent was not sought before they were taken. Some samples are additional samples for the study and your consent was sought before they were taken. Had any of these samples been taken before you were aware of the PICnIC study, e.g. samples that already taken as part of routine care? (Explore how they knew, e.g. was this explained, or did you see the samples being taken?) |
|
| 4.6 | Did you provide consent for the collection of additional samples in the PICnIC study (e.g. samples that were not part of routine care in that unit)? Do you have any concerns about the collection of samples approach in the PICnIC study? |
|
| 4.7 | Do you think it is acceptable to collect routine care samples from babies who take part in the PICnIC study without prior consent from parents? | |
| Section 5: OUTCOMES | ||
| 5.1 | As we have discussed, we want to find out if a study looking at the use of selective gut decontamination (SDD) in PICU is feasible, acceptable and what the trial should look like.
In the full trial we will collect information on Outcomes (the list that I sent to you prior to interview). For example, the PICnIC trial may explore whether SDD helps to reduce the number of hospital-acquired infections or incidence of ventilator-acquired pneumonia. These are called outcome measures. We collect data on outcomes to see if a trial medication is effective or not. However, these outcomes have come from research papers and don’t really give us much information on how children or families feel, or what is important to them. It is important that we include outcome measures that matter to children and their families. Thinking about your experience of your child being admitted to the PICU, what would you hope the study would do to help your child? (Prompt: what effect would the treatment have to be useful/what would you be looking for as an indicator that your child was getting better?) |
|
| 5.2 | Refer to outcomes list. What do you think about the outcome measures (re-cap measures in the list provided) Is there another outcome measure that you think is important to families which we should be collecting information about in the PICnIC Study? |
|
| 5.3 | Recap on outcomes measured and ask them to put in order of importance for example So far you have mentioned x outcomes, X, Y and Z. which would you say is the most important for this study? Second most important for this study? |
|
| SECTION 6: DECISION-MAKING | ||
| 6.1 | Opted in/out – if not known ask which | |
| Opt-outs | ||
| 6.2 | Was it difficult to say no? | |
| 6.3 | Would you mind telling me your reason for saying no to your child’s involvement in the study? | |
| 6.4 | Did you worry about how the doctor or nurse would respond? How did they respond? | |
| Opt-ins | ||
| 6.5 | In making the decision about your child’s continued participation in PICnIC, what sort of things went through your mind? | |
| 6.6 | Some parents have said that it’s difficult to take in all the information about a research study when their child is ill. Could you tell me about what it is like to have this information given to you and for you to think about it at this difficult time. |
|
| 6.7 | When do you think is the best time to approach parents about the PICnIC study? | |
| 6.8 | How long did you get to think about whether you wanted your child’s information to be used in the PICnIC study? Do you think this was long enough/How long do you think people should be given to think about taking part in a trial like PICnIC? Could you describe the possible benefits you expected your child to gain from taking part in PICnIC? Did this influence your decision in any way? (Prompt: Did taking part in research provide some distraction/foster a sense of control? If so, how? If not, did it increase stress levels?) |
|
| 6.9 | Did you see or envisage any possible risks to your child in participating? Did you have any concerns about your child’s involvement in the PICnIC study? If so, what were these concerns? Did you directly raise these concerns with a nurse or doctor? If so, were these concerns addressed/alleviated during this conversation? What was most helpful about these conversations? |
|
| 6.10 | In making your decision, did you think about how the research may benefit other children in the future? | |
| 6.11 | Apart from the doctor or nurse, did you discuss it with anyone else? [Can you tell me a bit about that?] | |
| 6.12 | Did you ever feel under pressure in making your mind up? [If yes: where did that pressure come from?] | |
| 6.13 | Did you know the doctor or nurse who approached you/interacted with them before? In your opinion, did your relationship with the doctor or nurse who asked you to take part in PICnIC influence your decision? | |
| 6.14 | How was the nurse/doctor in dealing with you that day? Prompts: how was their manner, how did they come across, were they empathetic, explained things clearly – not too leading |
|
| 6.15 | In making your decision how important was their manner/their level of expertise/professionalism, such as what they said and how they said it? | |
| Did you have the opportunity to ask any other questions about the study? Have you thought of any questions you would have liked to have asked that you didn’t ask at the time? | ||
| 6.16 | Now that a little time has passed, how do you feel about the decision you made? | |
| SECTION 7: Seeking child assent (NOT BEREAVED PARENTS OR PARENTS OF CHILDREN UNDER 5 GO TO SECTION 8) | ||
| 7.1 | Did the nurse or doctor explain the PICnIC trial to your child and give them an information sheet to seek their permission to take part? IF YES: (a) Could you tell me a bit more about that? (b) Do you think they understood the information they were given? (c) Did they give their permission to take part? (d) Did they ask any questions? |
|
| 7.2 | Did the nurse/doctor give you an information sheet to help you discuss the trial with your child when you got home? IF yes: (a) Did you discuss the PICnIC trial with your child? [Could you tell me about that?] (b) Could you tell me your reasons for discussing/not discussing the trial with them? IF TRIAL DISCUSSED WITH (a) Was the information sheet useful in helping you discuss the study with your child? (b) how do you think the discussion went? EXPLORE (could you tell me a bit more about that) (c) Is there anything that could have helped you discuss the trial with you child? (d) Did your child ask any questions about the study? (e) Did they want to take part? (f) Did they raise any concerns? (g) If your child had not wanted to take part in PICnIC would their opinion have influenced your decision about the study? [Could you tell me a bit more about that] |
|
| 7.3 | What do you think about involving children more in making the decision about the use of their information in a trial? | |
| 7.4 | What do you think about children being involved in the trial discussion between doctors and parents as part of a family approach to consent? Or do you think it should discussion should be kept separate | |
| SECTION 8: IMPROVING THE TRIAL AND RESEARCH DISCUSSION IN THE FUTURE | ||
| 8.1 | Before the PICnIC trial, have you ever been approached to consent for your child to participate in medical research? (If yes) [If more than one go through the trial prior to PICnIC] Could you tell me a bit more about it? Did you provide consent for your child to take part in the research? Could you tell me a bit about what informed your decision (not) to take part? Could you tell me anything about being approached about this research that has stuck in your mind? |
|
| SECTION 9: CONCLUDING | ||
| 9.1 | Overall, how acceptable do you think it is to conduct a future full PICnIC trial across the UK? | |
| 9.2 | Is there anything else that you think is important to mention about the proposed clinical trial? | |
| 9.3 | Would you like to be sent a copy of the study findings when they are published? | yes |
| 9.4 | Would you like to be contacted in the future about any further research connected to this study? | yes |
| SECTION 10: BEREAVED PARENTS ONLY | ||
| 10.1 | We don’t have much information about what parents who have lost a child think about how parents should be asked for consent. Even though some of these issues have been mentioned earlier, I’d like to check through these questions again and make sure I have your full answers, if it’s ok? (Use judgement to see whether further probing here is appropriate) | |
| 10.2 | (IF RECEIVED LETTER IN POST) How long after leaving hospital did you receive the letter about the PICnIC trial?
|
|
| 10.3 | What advice would you give doctors and nurses on how to go about approaching bereaved parents to discuss PICnIC before they leave hospital? | |
| 10.4 | Do you think bereaved parents should be informed about their child’s involvement in the PICnIC study? When do you think is the best time for doctors and nurses to approach bereaved parents for deferred consent? Who approached you to discuss PICnIC? (Prompt: Was it a doctor or nurse? Did you know them?) Do you think that this was the most appropriate person to approach you? If yes/no, what were your reasons for this? If not, who do you believe would have been the best person to approach you? |
|
| 10.5 | How do you think this should be done? Explore response and:
If letter, do you think the letter should be ‘opt out’ where parents didn’t have to do anything if they wanted their child’s data to be in the trial (they are automatically enrolled like all other children in the trial)? In person, do you think this should be done face to face or via a telephone call? Out of all the options we have discussed which would you recommend? In PICnIC we send parents a letter 4 weeks after they leave hospital to explain how their child was included in the trial and how their child’s data will be included in the trial unless they contact the hospital to ‘opt out’. There is a number for parents to contact the doctor if they have any questions. What do you think about this approach? Do you think it is OK to send a second letter in case of no response (e.g. in case the first letter was not opened by parents?). Explore 8-week timeframe. Explore any concerns. |
|
| 10.6 | If the doctor or nurse had approached a parent about a trial before their child had passed away and left them to consider the information, do you think it is ok for the doctor or nurse to then contact the parent for their decision after their child has died? (Prompt, letter home or face to face?) | |
| 10.7 | Some nurses have suggested involving a bereavement counsellor when contacting parents. What do you think about this? Did you take up the opportunity to speak to a bereavement counsellor? |
|
Appendix 3 Topic guide for practitioners
PICnIC qualitative study clinician focus group topic guide
(Researcher introduction) The proposed PICnIC trial will aim to determine the effectiveness of SDD compared to standard infection control in reducing HCAIs. As part of the trial feasibility study the aim of this focus group is to explore your views on the acceptability of the proposed PICnIC trial. During the next hour, we will go through different aspects of the trial, including its aims and our suggested approach to recruitment and consent. We would also appreciate your views on the type of training you think would be needed to prepare for recruitment and consent to PICnIC. This is one of four focus groups we are conducting as part of the qualitative element of the PICnIC feasibility study. Your views will help us explore the feasibility of conducting the definitive trial.
In addition to talking to yourselves I have been interviewing parents about their experience of being part of the pilot study. The main aim of the focus group is to find out how clinicians think the full PICnIC trial should look like and to identify what has worked well and perhaps what hasn’t with the recruitment to the pilot.
We will combine your feedback with parent feedback to design the trial moving forward.
Introduce voting system and consent process.
We’ll start by asking you to introduce yourselves, but this is only for our own notes about who is here today.
Section 1: your role
Can we go around the table and introduce ourselves?
Ice breaker question using handset (e.g. if you could have a superpower what would it be? Options: e.g. invisibility/flying/super strength. X-ray vision).
Please tell us what your role is at this hospital: Junior doctor/senior doctor/junior nurse senior nurse
Are you involved in the clinical care of children? Yes/No
How much, if any, experience you have in recruiting to paediatric clinical trials?
Direct to those who have recruitment experience: Have any of these trials involved opt-out consent?
Could you please tell me how you have been involved in PICnIC (e.g. your role, such as recruitment or delivering the intervention).
Section 2: current practice, SDD and randomisation
Could you tell me about the processes that were in place to reduce infection risk in your PICU before involvement in PICnIC? Was the SDD intervention being used in your unit before PICnIC?
(Control group: I am aware you don’t have an experience of this) What are your views on giving SDD to children admitted to PICU? Explore: would you have any concerns?
Please explain your answer. (Everyone said … why is that. Different – Some of you said … but others said …. Can you explain your answers?)
Do you have any concerns about antimicrobial resistance by children having too many antibiotics?
How did you feel about your patients being randomised to *Intervention/Control group*? How would you have felt if your PICU was randomised to the other trial arm?
Could you tell me how acceptable you feel it is to give SDD to only half of the babies/children in this study?
Similarly, how acceptable do you feel it is to not give SDD to half the babies in the study?
Section 3: recruitment, consent and samples
SDD has been shown to influence the rate of hospital-acquired infections in all patients within a unit, not just those receiving it. As such, the intervention will be delivered to all eligible patients in participating PICUs as a standard of care for the period of the study, in addition to their standard infection control policies; individual patient consent will not be requested for their child’s involvement. Parents can opt out of continued involvement.
Thinking about your experience of recruitment in PICnIC, how do you gauge when is best to approach parents about the study? Explore: do you speak to the clinical care team? Have you ever asked the clinical team to introduce you to the parents?
-
At what time have you usually approached parents? (Explore minimum and maximum time frames)
-
Have any parents had prior knowledge of PICnIC before you have approached them? For example, after seeing posters or leaflets? (Check if posters are up in PICU)
-
Could you tell me how you explained the PICnIC trial to parents?
-
Mention of SDD arm – becoming standard practice during trial.
-
-
Risk – study drug information sheet supplied?
-
In general, how have you found having those discussions with parents?
-
Do you explicitly inform them that their child was given a trial intervention without their prior consent … How have they reacted to finding out that their child has already been entered into a clinical trial intervention/have been given the SDD intervention without their prior consent? Do you explicitly inform them that this is the case and that data collected before they are approached will be kept? Explore.
-
Prompt: parents under the impression that they would have gotten it anyway – explore – is this true?
-
-
Have any parents been angry/upset, etc. – what happened?
-
Do you ever check parents’ understanding of the trial?
-
Is there anything that you find particularly helpful when consenting parents to PICnIC?
-
What type of questions do they ask?
-
Is there anything that you find particularly difficult when consenting parents to PICnIC?
-
Do you explain the reasons why PICnIC uses an opt-out approach for the intervention? Explore reasons and experiences.
-
Do you explain the concept of cluster randomisation? Explore reasons and experiences.
How acceptable is the use of opt-out consent in PICnIC? Explore any particular concerns.
All babies in PICnIC have samples taken for the study, regardless of whether they received the SDD intervention. This included:
-
Nasopharyngeal (swab from nose and throat)
-
Stool samples or rectal swabs
If doctors were concerned about specific infections, they may also take urine samples, respiratory samples (collection of sputum) and swabs of any wounds the child may have.
Some of these samples are part of routine care and consent will not be before they are taken. Some samples are additional samples for the study and the parents’ consent was sought before they were taken.
Do you think it is acceptable to collect routine care samples from babies who take part in the PICnIC study without prior consent from parents? Yes/No
Have parents expressed any concerns about the collection of samples?
Explore view in focus group.
Section 4: screening and inclusion/exclusion criteria
Have any of you been involved in the screening process?
If yes, please talk me though how you have found screening.
Are you satisfied with the inclusion and exclusion criteria? Any suggestions for improvement?
Section 5: training and protocol adherence
Do you feel the training adequately prepared you to take part in the pilot trial? Yes/No
What aspects, if any, of the training did you find useful?
What aspects, if any, of the training do you think needed development?
Is there anything that the study team can put into place to improve the training process if we proceed to the full trial?
Do you think there will be challenges in adhering to the trial protocol? Yes/No
Explore perceived problems and any potential solutions.
Coenrolment
Do you think that recruitment to other studies has impacted on the pilot? Prompt: prioritising other studies.
Section 6: general views on the pilot and proposed clinical trial – challenges and ways to overcome them
Do you have any concerns about the proposed PICnIC trial? Yes/No
What, if any, barriers would there be to conducting a full PICnIC trial? What practical or logistical challenges have you encountered while recruiting parents to the pilot phase of the PICnIC study in your unit?
What do you think would help facilitate recruitment to the full PICnIC trial?
Section 7: concluding questions/remarks.
Given everything that you’ve discussed so far today and your experience of taking part in the pilot study, overall, do you think the full PICnIC trial is practically possible to conduct?
Lastly, how acceptable is the proposed PICnIC trial to conduct? Very acceptable/acceptable/neutral/not acceptable/very unacceptable
Before we finish, is there anything else that you would like to say (about the PICnIC pilot/full trial)?
Thank you for your time today.
Appendix 4 Outcomes for parent interviews
OUTCOMES
THIS INFORMATION WILL BE EXPLAINED TO YOU FULLY DURING THE INTERVIEW:
-
An outcome measure refers to ‘what’ should be measured in a research study to find out whether a treatment is effective (whether the treatment helps to make children better).
-
Studies often have a number of outcome measures to determine whether a treatment is effective – some are measured during a child’s stay in hospital, while others are measured either at the end of their hospital stay or when they have left hospital.
-
Researchers or doctors often suggest what outcomes should be measured in a research study. However, they do not always fully understand what it’s like either to be a sick child or to be the parent/guardian of a sick child. That is why it’s important we ask parents/guardians what outcomes they think a research study should measure to determine whether a treatment is effective.
-
For the PICnIC study, we have reviewed lots of previous research studies on very ill children, including those that had a severe infection.
-
Below is a list of outcomes that might be useful to measure. During the telephone interview, we will ask you what you think about the outcome measures on this list.
-
It’s not a test! We just want to make sure we include outcomes that are important to parents and children.
Outcomes that are measured during a child’s stay in hospital
-
Number of, duration spent, and type of treatment for your child’s vital organs (including mechanical support).
-
Health complication/AE.
-
The number of health complications/AEs that occurred as part of your child’s illness (or from a treatment).
-
Infections acquired in the ICU – drill down to VAP/line sepsis/UTI/other.
-
The number of specific treatments your child received (e.g. number during course of stay).
-
Tolerance for the intervention (e.g. how children tolerate the SDD when given).
-
Length of PICU and hospital stay.
-
Looking and behaving like normal self – that is overall feeling of return to health or normality including a reduction in the magnitude of swelling and skin discolouration. Improvements in child temperament included references to behaviour and mood, including increased alertness and decreased irritability.
-
-
Survival at PICU and hospital discharge.
-
Whether your child survived to a certain time point (usually time point at months/years) or to a specific event (e.g. hospital discharge).
-
The total period of time (usually period of months/years) that your child survived for.
-
Outcomes that are measured at the end of care or after a child has left hospital
-
Survival.
-
Whether your child survived to a certain time point (usually time point at months/years) or to a specific event.
-
The total period of time (usually period of months/years) that your child survived for.
-
-
Current health.
-
Your child’s health status in terms of development, functioning and/or life quality at a certain time point (usually time point at months/years).
-
-
Re-admission to hospital.
-
The number of times your child was re-admitted to hospital for the same/different illness within a certain time period (usually period of months/years).
-
List of abbreviations
- AE
- adverse event
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- cRCT
- cluster-randomised controlled trial
- CRF
- case report form
- CTU
- Clinical Trials Unit
- eCRF
- electronic case report form
- HCAI
- healthcare-associated infection
- ICC
- intracluster correlation coefficient
- ICNARC
- Intensive Care National Audit and Research Centre
- ICU
- intensive care unit
- IQR
- interquartile range
- PCCS-SG
- Paediatric Critical Care Society Study Group
- PICANet
- Paediatric Intensive Care Audit Network
- PICnIC
- Infection Control in Paediatric Intensive Care
- PICU
- paediatric intensive care unit
- PI
- principal investigator
- PIS
- participant information sheet
- PPI
- patient and public involvement
- RWPC
- research without prior consent
- SAE
- serious adverse event
- SD
- standard deviation
- SDD
- selective decontamination of the digestive tract
- SMG
- Study Management Group
- TMG
- Trial Management Group
- VAP
- ventilator-associated pneumonia
