Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 06/27/01 from route sheet. The protocol was agreed in November 2006. The assessment report began editorial review in November 2007 and was accepted for publication in March 2008. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NCCHTA, Alpha House, Enterprise Road, Southampton Science Park, Chilworth, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Prostate cancer is one of the leading causes of cancer death among men worldwide. 1 It is considered to be the most common malignant disease in Western Europe and North America. 2 Despite these alarming statistics, prostate cancer frequently grows slowly and does not always cause a problem. 3 The difficulty for clinicians is in deciding which men have fast-growing cancers that need essential treatment and which have slow-growing cancers that will never trouble them. There is still a lack of understanding of the markers for prostate cancer’s presence and progression; this understanding is important to avoid unnecessary treatment, predict disease course, signal the extent of cancer, and develop more effective treatment and implement definitive guidelines. 4 The focus of this systematic review will be on novel markers (i.e. newer markers) and their added benefit over existing classical markers, and an evaluation of models that combine markers.
Aetiology
The specific causes of prostate cancer remain unknown. Hsing and Chokkalingam5 provided a comprehensive review of prostate cancer epidemiology. They reported that there are several risk factors that can increase the chances of developing prostate cancer, related to age, genetics and family history. They further reported that putative risk factors include obesity, hormones, smoking, dietary factors, physical inactivity, occupation, vasectomy, genetic susceptibility and sexual factors; however, there is a lack of good-quality evidence concerning the role of these factors.
Incidence and prevalence
The age-adjusted prostate cancer incidence rates vary considerably throughout the world. 6 In the US during 2005 it was estimated that there were 230,000 new cases of prostate cancer and 30,000 deaths due to prostate cancer. 7 Based on statistics produced by the Office for National Statistics from registrations of cancer diagnosed in 1993–1996 in England and Wales, the lifetime risk of being diagnosed with prostate cancer is 1 in 13. 8 More recent statistics concerning the incidence rates of prostate cancer in the UK during 2002 are reported in Table 1.
| England | Wales | Scotland | Northern Ireland | UK | |
|---|---|---|---|---|---|
| Cases | |||||
| Males | 27,174 | 1766 | 2335 | 648 | 31,923 |
| Crude rate per 100,000 | |||||
| Males | 113.0 | 125.4 | 96.0 | 78.3 | 111.2 |
| Age-standardised rate (European) per 100,000 | |||||
| Males | 92.6 | 93.4 | 80.1 | 78.7 | 91.3 |
| 95%CI | 91.5–93.7 | 89.0–97.7 | 76.9–83.4 | 72.7–84.8 | 90.3–92.3 |
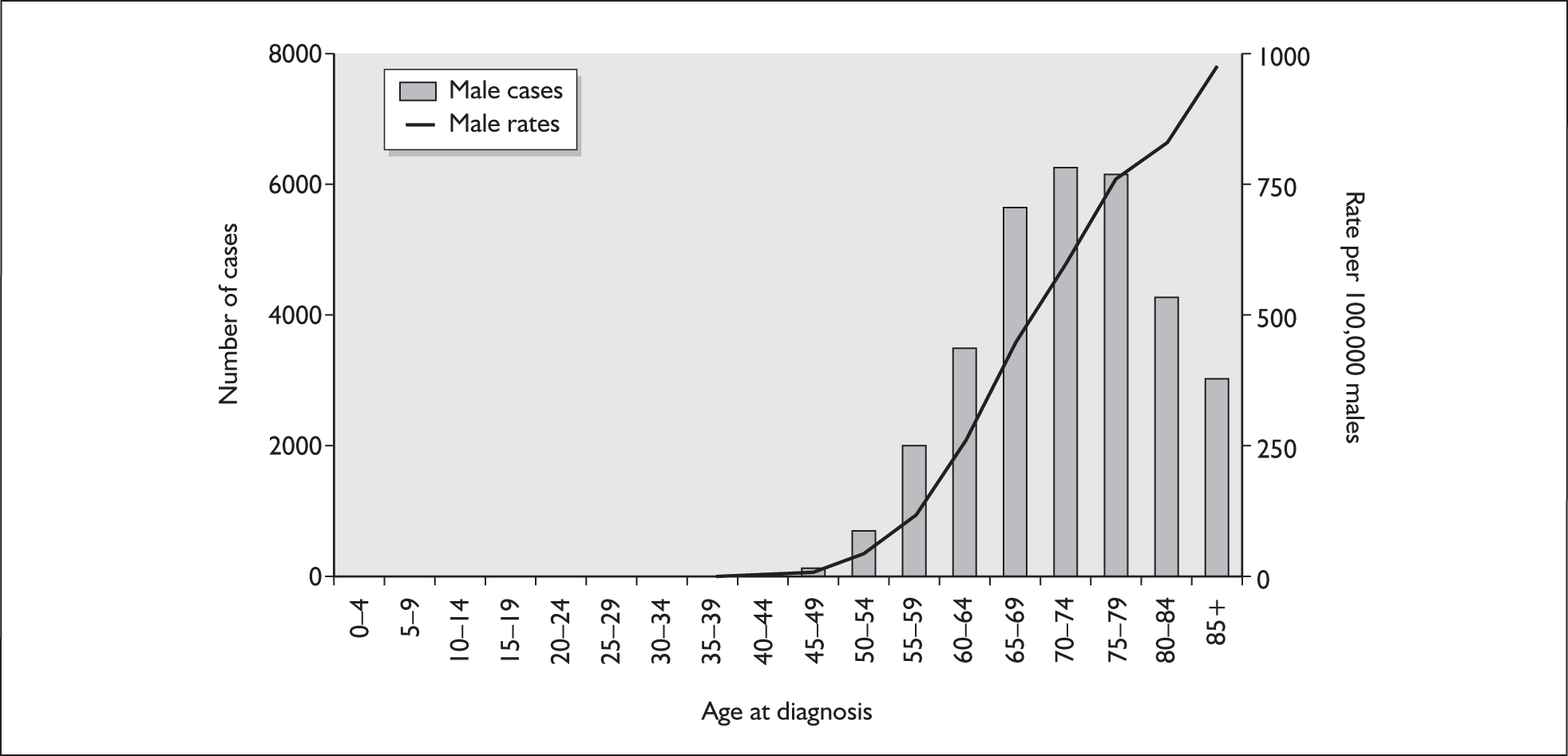
The risk of developing prostate cancer is strongly related to age: very few cases are registered in men under 50 years of age and more than 60% of cases occur in men over 70 years. The largest number of cases were diagnosed in the 70–74 and 75–79 age groups. Figure 1 reports the age-specific incidence rates of male prostate cancer in the UK during 2002.
FIGURE 1.
Numbers of new cases and age-specific incidence rates of male prostate cancer in the UK during 2002. From UK Prostate Cancer Mortality Statistics,9 with permission from Cancer Research UK.
Definitions of prognosis
Srigley et al. 10 present a discussion of prognostic and predictive factors in prostate cancer. Prognosis refers to the ability to distinguish clinically important variation and reliably forecast the course, progression, pattern and end of disease. 11 This ability to forecast the outcome of a disease is an important aspect of medical practice, which presents a challenge given the heterogeneity of cancer at a clinical, biomolecular, morphological and outcome level. 10 Prognostic factors might account for some of the heterogeneity that is associated with the expected outcome and course of the disease, relating more to probability of a cure or prolonged survival. 10 Prognostic markers are those that are associated with prognosis, independent of the treatment received. They are prognostic of the natural outcome of disease before an intervention is applied or regardless of it. Prognostic factors should, however, be considered in the context of a treatment and therapeutic intervention and for a specific end point of interest (e.g. local control, survival or organ preservation). 10 This is because the treatment can change the prognosis in addition to the end point relevant to it.
It is important to recognise that ‘predictive’ and ‘prognostic’ are often used interchangeably in the medical and research literature. Prediction is frequently used in the context of tumour reduction following specific intervention, whereas factors that influence the response are referred to as predictive factors, in contrast to prognostic factors. A predictive marker is one that predicts the outcome of a treatment, thus allowing the identification of those who will benefit from particular therapies, whereas a prognostic factor is a marker for disease severity and outcome that is independent of treatment.
Impact of the health problem
Prostate cancer is reported to be a primary reason for consultation with a general practitioner (GP) amongst men with cancer. In an earlier review of prostate cancer12 information on the burden of the disease on health services was reported. In 1994 the cost to the NHS in terms of consultations with GPs was over £2 million, whereas the cost of prescribing for prostate cancer was £24 million and hospital inpatient costs were around £19 million.
Current service provision
Management of disease
At present it is not NHS policy to screen for prostate cancer. There is uncertainty about the benefits of screening for prostate cancer. In a recent systematic review there was no support found for a reduction in prostate cancer deaths as a result of screening, but only two poor-quality studies [one randomised controlled trial (RCT), one quasi-RCT] met the inclusion criteria. 13 Some attribute the decline in prostate cancer mortality over recent years to screening, but improvements in treatment may also have had an effect. There are several large-scale trials that are currently investigating the effectiveness of screening [e.g. Prostate, Lung, Colorectal and Ovary (PCLO) trial, European Randomised Study of Screening for Prostate Cancer (ERSPC), UK Prostate Testing for Cancer and Treatment (ProtecT) trial]. Several other systematic reviews have argued against screening until more information is available on the natural history of the disease and the optimum treatment of organ-confined disease. 12,14 In contrast, there has been a large amount of published literature about the risks of screening and resultant treatments. 15
Clear guidelines have been developed for managing patients who present, usually to a GP, with lower urinary tract symptoms (LUTS). 15 The Prostate Cancer Specialty Working Group (PCSWG) recommends that patients presenting with LUTS have a digital rectal examination (DRE) by someone who performs these on a regular basis. 15 For this examination the doctor uses his/her finger to feel for prostate enlargement and surface irregularities via the rectum. The drawbacks of this test are that it is unable to detect tumours in the anterior and medial lobes of the prostate, and it appears to be of limited value in detecting early localised cancer. Because not all tumours are palpable a GP can be alerted to the presence of such a tumour by an elevated prostate-specific antigen (PSA) level. It is accepted therefore that a GP would want to make use of such a diagnostic tool for patients with significant symptoms. For radiological staging purposes magnetic resonance imaging (MRI) is thought to give the most accurate and complete assessment of local disease and spread. 15 When this is not available other methods of radiological staging are required: transrectal ultrasound (TRUS) is often used as an aid to biopsy, computerised tomography (CT) is used to detect spread to the lymph nodes, and radionuclide bone scans may detect metastases.
Before the start of treatment, confirmation of a diagnosis of prostate cancer is required via histological examination of prostate tissue from biopsy samples. This examination provides information on the grade of the tumour, which is an important prognostic indicator.
Current service cost
An earlier Health Technology Assessment (HTA) review17 of new and emerging treatments for early localised prostate cancer claimed that, given the lack of evidence of clinical effectiveness and the variation in estimated treatment costs presented in the economic analysis, it was not considered appropriate to estimate the overall cost of the technologies to the NHS in England and Wales. The evidence presented by Hummel et al. 16 considered technologies only in terms of clinical effectiveness and cost-effectiveness and did not consider matters relating to implementation. An evaluation of implementation other than clinical effectiveness and cost-effectiveness has been outlined in the NHS guidance on urological cancers issued by the National Institute for Health and Clinical Excellence (NICE). 17 The guidance states that centres should aim to provide conformal radiotherapy and that radical surgery should be undertaken only by teams performing at least 50 such procedures per year. Patients for whom radical treatment may be appropriate should have the opportunity for a joint meeting with urologist, oncologist and specialist nurse.
Description of technology under assessment
A group of prognostic factors known as markers or biomarkers has received considerable interest from clinical trials. These markers can be found in blood, urine or tissue samples, and histological specimens. Few markers have achieved widespread clinical utility and there is an increasing need to develop and identify markers that provide more clinical information and allow risk-based individual therapy. 4 There is a growing need to identify new prognostic markers in prostate cancer to avoid excessive or inappropriate treatment of patients. Furthermore, they may be helpful in identifying patients with poor outcomes who would be candidates for trials of adjuvant treatment. No novel markers have been uniformly recommended for routine application in prostate cancer since the advent of PSA over 20 years ago, despite the plethora of studies of prognostic factors. In the following sections we will differentiate the large number of markers into classical markers (the more commonly used markers) and novel markers (those markers that are of potential benefit).
Classical markers
The most commonly used classical markers are PSA, cancer stage (or extent of the cancer within and beyond the prostate) and histopathological evaluation from diagnostic biopsy, including Gleason grade (a classification system based on the appearance of the cancer tissue in a biopsy specimen). PSA has had the greatest impact on the management and evaluation of prostate cancer. Gleason grade and tumour stage have been recognised as essential descriptors of prostate cancer for over 50 years in prediction and treatment evaluation. 10 These classical biomarkers are used singly and combined in models to predict biochemical (PSA) recurrence (signifying disease progression) and mortality.
PSA
The most well-known prognostic marker that has been used to assess prognosis (as well as detection of early disease) is PSA. PSA is a 30- to 33-kDa protease belonging to the kallikrein family, which is made up of 15 serine proteases encoded by a cluster of genes on chromosome 19q3. 18 The earliest reported investigations of tissue-specific antigens in the human prostate were conducted by Ablin and colleagues in 1970. 19 Further investigations resulted in the discovery of prostatic antigens in seminal plasma. 20,21 Sensabaugh and Crim22 went on to characterise and isolate PSA from human seminal plasma during investigations into potential markers to aid detection of rape crimes. Wang and colleagues23 purified and isolated an antigen from prostate tissue that was considered to be prostate specific in nature. A large number of men are being diagnosed with early-stage prostate cancer as a result of the increasing use of PSA testing. 24
Stage
In the TNM system, the extent of primary tumour (T category), regional lymph node involvement (N category) and distant metastasis (M category) are determined. The TNM system for classifying the anatomic extent of disease in cancer has been in existence for more than 50 years. 25 Over time the TNM classification has evolved to accommodate new knowledge from the growth in medical research to improve its prognostic ability and keep pace with the demands of clinical practice. 26 The TNM system was last updated in 2002. 27 The latest version of the TNM staging system is used to stage prostate cancer (Table 2). 28 Two main changes have been made to the new TNM classification system compared with the older versions: (1) subdivision of T2 disease into three clinical substages and (2) the recommendation that the Gleason scoring system is used for grading.
| Primary tumour, clinical (T) | ||||
|---|---|---|---|---|
| TX | Primary tumour cannot be assessed | |||
| T0 | No evidence of primary tumour | |||
| T1 | Clinically unapparent tumour not palpable or visible by imaging | |||
| T1a | Tumour incidental histological finding in less than or equal to 5% of tissue resected | |||
| T1b | Tumour incidental histological finding in greater than 5% of tissue resected | |||
| T1c | Tumour identified by needle biopsy (because of elevated PSA level); tumours found in one or both lobes by needle biopsy but not palpable or reliably visible by imaging | |||
| T2 | Tumour confined within prostate | |||
| T2a | Tumour involving less than or equal to half a lobe | |||
| T2b | Tumour involving more than half a lobe but not more than one lobe | |||
| T2c | Tumour involving both lobes | |||
| T3 | Tumour extending through the prostatic capsule; no invasion into the prostatic apex or into, but not beyond, the prostatic capsule | |||
| T3a | Extracapsular extension (unilateral or bilateral) | |||
| T3b | Tumour invading seminal vesicle(s) | |||
| T4 | Tumour fixed to or invading adjacent structures other than seminal vesicles (e.g. bladder neck, external sphincter, rectum, levator muscles, pelvic wall) | |||
| Primary tumour, pathological (pT) | ||||
| pT2 | Organ-confined | |||
| pT2a | Tumour involves half of one lobe, but not both lobes | |||
| pT2b | Tumour involves more than half of one lobe, but not both lobes | |||
| pT2c | Tumour involves both lobes | |||
| pT3 | Extraprostatic extension | |||
| pT3a | Extraprostatic extension | |||
| pT3b | Seminal vesicle invasion | |||
| pT4 | Invasion of bladder, rectum | |||
| Regional lymph nodes (N) | ||||
| NX | Regional lymph nodes (cannot be assessed) | |||
| N0 | No regional lymph node metastasis | |||
| N1 | Metastasis in regional lymph node or nodes | |||
| Distant metastasis (M) | ||||
| PM1c | More than one site of metastasis present | |||
| MX | Distant metastasis cannot be assessed | |||
| M0 | No distant metastasis | |||
| M1 | Distant metastasis | |||
| M1a | Non-regional lymph node(s) | |||
| M1b | Bone(s) | |||
| M1c | Other site(s) | |||
| Stage grouping | ||||
| Stage I | T1a | NO | MO | G1 (Gleason score 2–4) |
| Stage II | T1a | NO | MO | G2–4 (Gleason score 5–10) |
| T1b | NO | MO | Any G | |
| T1c | NO | MO | Any G | |
| T1 | NO | MO | Any G | |
| T2 | NO | MO | Any G | |
| Stage III | T3 | NO | MO | Any G |
| Stage IV | T4 | NO | MO | Any G |
| Any T | N1 | MO | Any G | |
| Any T | Any N | M1 | Any G | |
The clinical stage is based on information obtained before surgery to remove the tumour. The pathological stage provides additional information from the examination of the tumour microscopically. Pathological staging provides a more direct examination of the tumour and its spread, whereas clinical staging can be limited as the information is obtained by making an indirect assessment of the tumour whilst it is still in the patient. In Europe the TNM staging system is most commonly used. In stage T1 the tumour is located within the prostate gland only and is too small to be felt on DRE. In stage T2 the tumour is still located only within the prostate but it can be felt on DRE. In stage T3 the tumour has spread from the prostate into the immediate surrounding tissue. The seminal vesicles may be included. In stage T4 the tumour is still within the pelvic region but may have spread to other areas, i.e. metastatic disease may be present. Both T3 and T4 are often referred to as locally advanced disease. However, it should be noted that, for the purposes of this review, despite being interested only in early localised prostate cancer, we shall still evaluate stages T1, T2 and T3 with no lymph node involvement or metastases.
Although the TNM system stages are universally used, a similar system called the Jewett–Whitmore system is sometimes used in the US (Table 3). This has more specific alphanumeric subcategories. The Jewett–Whitmore system classifies prostate cancer first into stages A, B, C or D. Stages A and B are considered curable, whereas stages C and D are treatable. A number is given to describe a condition within each stage.
It is important to recognise that patients may move stages over the course of disease progression. Upstaging or downstaging has been found following treatment and also stage classification can depend on the imaging procedure used. 30
| Stage A | Very early and without symptoms; cancer cells confined to the prostate |
| A1 | Well-differentiated and slightly abnormal cancer cells |
| A2 | Moderately or poorly differentiated and abnormal cancer cells in several locations within the prostate |
| Stage B | Confined to the prostate, but palpable (detectable by digital rectal examination) and/or detectable by elevated PSA |
| B0 | Confined to the prostate, non-palpable; PSA elevated |
| B1 | Single cancerous nodule in one lobe of the prostate |
| B2 | Extensive, involvement in one or both prostate lobes |
| Stage C | Cancer cells found outside the prostate capsule (membrane covering the prostate); spread confined to surrounding tissues and/or seminal vesicles |
| C1 | Extends outside the prostate capsule |
| C2 | Bladder or urethral obstruction |
| Stage D | Metastasis (spread) to regional lymph nodes or to distant bones, organs (e.g. liver, lungs) and/or other tissues |
| D0 | Metastatic, clinically localised and showing elevated blood PAP levels |
| D1 | Regional lymph nodes involved |
| D2 | Distant lymph nodes, bones or organs involved |
| D3: | Metastatic disease after treatment |
Gleason
The most commonly used scheme for reporting histological grade is the Gleason score. Within this scheme there are five possible tissue patterns with 1 being well differentiated (good prognosis) and 5 being poorly differentiated (poor prognosis). The two most frequent patterns are added together to give a score. Albertsen31 reported that over the last 20 years there has been a significant shift in the use of the Gleason scoring system: tumours scored as Gleason 2–5 a decade ago are more likely to be scored as Gleason 6 tumours today. Men with high-grade prostate cancers (Gleason scores 7–10) appear to be at greater risk of disease progression and death if managed expectantly, whereas for men with low-grade prostate cancers (Gleason scores 6 or less) the outcome is unclear.
Surgical margins
A positive margin of resection means that the tumour extends to the inked surface of the prostate specimen removed by the surgeon. 32 Although this definition is useful it presents difficulties in terms of its practical application as the prostate is surrounded by many structures that limit its the radical removal. There appear to be two main causes of positive margins: (1) non-iatrogenic and (2) transection of intraprostatic tumour (capsular incision). 32 The incidence of positive margins following radical prostatectomy (RP) has significantly decreased over the last decade. 33–35 Although this may be partly the result of improvements in surgical techniques, it is likely that the majority of the decrease is due to stage migration and careful patient selection. 32 It has been reported that patients with positive margins have an increased risk of progression compared with patients with negative margins. 33,36 These studies by Epstein and colleagues found that the probability of being progression free at 5 years following RP ranged from approximately 81% to 83% for margin-negative disease and from 58% to 64% for margin-positive disease.
Novel markers
It has become increasingly apparent that the incidence of prostate cancer has increased significantly over the last 10–15 years and that this is largely due to increasing use of opportunistic screening or case finding and the use of PSA testing in serum. 37 The use of such an approach tends to result in prostate cancer being detected 5–10 years before it gives rise to any symptoms and approximately 17 years before causing death. 37 This has resulted in a large number of patients being diagnosed inappropriately. It remains clear, therefore, that researchers need to provide methods that will enable those patients who need to be treated to be identified while avoiding diagnosing patients who will not benefit, and to develop new prognostic markers that can predict those patients that need to be diagnosed and those that do not. However, one must also recognise that the incidence of prostate cancer is often also linked to an increase in mortality because of the cause of death being erroneously ascribed to prostate cancer once a patient has been diagnosed with it. It has been claimed that this is another reason why there has been an increase in prostate cancer mortality. 38
Several reviews of novel markers have been published. 4,10,37,39 These reviews have detailed a large number of potential prognostic markers. Several subcategories of novel markers have been proposed. Grizzle39 reported that markers which are used in the characterisation of disease processes fall into three major categories: (1) histopathological biomarkers (e.g. stage, Gleason score); (2) demographic biomarkers (e.g. age, race, sex); and (3) molecular biomarkers (e.g. E-cadherin, p53, p27Kip-1). In using biomarkers to characterise disease processes, the three types of biomarker may be used in combination.
Recent advances in molecular biology have identified a large number of novel biomarkers that might have prognostic significance. PSA kinetics [e.g. PSA doubling time (PSADT)] is becoming increasingly well established. 40 Morphology-based approaches, especially Gleason scoring, have enabled clinicians to evaluate prognostic information, especially when combined with other clinical parameters of T stage and PSA. 41–47 However, the prognostic value of the Gleason score is limited by the fact that the vast majority of prostate cancer patients present with moderately differentiated tumours (e.g. Gleason score of 6) in the PSA era, limiting the prognostic utility of morphological features. Since the introduction of microarrays there has been considerable interest in using whole-genome expression profiling to gain insight into a particular cancer and to identify key genetic mediators. 48
Screening for prostate cancer aims to advance the time of diagnosis (lead time) and detect cancers that would not have been found without screening (overdetection). Draisma49 estimated the mean lead times and rates of overdetection associated with different PSA screening programs using the simulation program MISCAN (microsimulation screening analysis). The rate of overdetection was expressed in different ways (e.g. detection of non-lethal cancer). The estimated mean lead times and rates of overdetection were significantly associated with age at the time of screening. At age 55 years the estimated mean lead time was 12.3 years and the overdetection rate was 27%, whereas at age 75 years these were 6 years and 56% respectively.
Clinical evaluation of markers
It is important to consider how one might validate the clinical usefulness of any marker. Tricoli et al. 4 suggested that it was necessary to establish what the end point will be, which will in turn determine the study population to be investigated. The appropriate statistical design of the study will require information on the prevalence and strengths of the association of marker expression with the outcomes being examined. These factors will help determine the specificity and sensitivity of the marker. Other considerations relate to a possible control population and suitable sample collection, preparation and assay method.
Despite the large amount of published research concerning the prognostic value of markers for prostate cancer, the number of clinically useful novel markers that have emerged appears to be very small. Quite often, an initial report of a particular marker suggests that it has great potential, but further research yields different conclusions or even contradicts the initial promising results. A discussion of these problems is presented in a commentary by McShane et al. 50 These authors highlight the variety of reasons that have been proposed to explain these inconsistencies: (1) methodological differences; (2) poor study design; (3) assays that are not standardised or lack reproducibility; (4) inappropriate or misleading statistical analyses which are often based on sample sizes that are too small to draw meaningful conclusions from; and (5) quantity, quality and preservation method of the specimens. McShane and colleagues further comment on the use of retrospective studies, as patient populations are often biased towards patients with available tumour specimens.
Other explanations have been proposed in terms of common statistical problems across differences studies (e.g. underpowered studies, subset analyses, optimistic effect size reporting and significance levels, consideration of multiple testing, and cut-point optimisation). 51,52
Several consensus conferences and initiatives have examined prognostic markers in prostate cancer, including two College of American Pathologists (CAP) conferences (1994 and 1999), a World Health Organization (WHO) conference (1999) and the International Union Against Cancer (IUCC) prognostic factor project committee. In 1995 an international consultation meeting on prostatic intraepithelial neoplasia and pathological staging of prostate cancer was held. Several new and evolving markers were assessed and classified according to the following four categories: (1) well supported for widespread application; (2) supported for further investigation; (3) insufficient data to make a decision; and (4) of no value. From this work some of the evolving biomarkers that were considered to be of potential importance were markers of apoptosis (Bcl-2); microvessel density; PSA isoforms; prostate-specific membrane antigen; androgen receptor mutation; neuroendocrine cell status; E-cadherin; interphase cytogenetics; and tumour suppressor genes such as p53. 53 Following this, a large amount of other consensus work has been achieved in this field of prognostic factors in prostate cancer. Classical markers including stage, Gleason score, preoperative serum PSA and even post-radical prostatectomy margin status have come to be regarded as independent predictors of patient outcome. The developments of prognostic indices and nomograms have allowed these classical markers to be combined and now they are regularly used in the clinical management of patients. What remains unclear is which of the novel and promising factors that are emerging from the extensive research are going to be appropriate for future clinical use. Most of these novel markers require considerably more analysis and assessment in the context of multifactor prognostic indices. 38 There is a growing need for consensus in the field of prognostic factors and for an analysis of the new and emerging prognostic factors through a more rigorous evidence-based approach and to help develop guidelines. 54
Bostwick and Foster55 reported on recommended predictive factors in prostate cancer following two international consensus conferences held in 1999. Both conferences recommended several predictive factors for routine use based on evidence from multiple published trials: TNM stage, histological grade using the Gleason system, serum PSA concentration and surgical margin status. Furthermore, the WHO conference recommended the use of WHO nuclear grade, location of cancer within the prostate and pathological effects of treatment. Other promising factors included histopathological and genetic markers. Bostwick and Foster concluded that standards are needed for analysis and quantifying methods of tissue analysis, particularly for immunohistochemical studies and genotypic studies.
Issues related to handling of prostatectomy specimens were recently discussed in a review. 33 In relation to biomarkers, differences were raised amongst studies in relation to methodology, preparation, analysis and measurement. There appears to be subjectivity in the interpretation of some test results, and where one decides the cut-off between negative and positive can be subjective (i.e. using image analysis or the human eye). All of these factors can produce potentially conflicting results concerning the prognostic value of a biomarker for prostate cancer.
Prognostic models
Prognostic models combine individual prognostic markers to predict patient outcomes. They may be used to inform patient treatment, counsel patients and inform future research. The most common methods for developing prognostic models are Cox regression, recursive partitioning and artificial neural networks (ANN).
The most commonly used form of Cox regression is the proportional hazards model, which makes two important implicit assumptions. First, it assumes that the hazard ratios (HRs) are constant over time and, second, it assumes that there is a log-linear relationship between the explanatory (independent) variables and the hazard function. The model does not make any assumptions regarding the underlying survival distribution. The proportionality assumption (constant HRs) should be tested for each variable included in the model. One simple method is to check that the Kaplan–Meier survival curves are parallel, but this is not practical for continuous variables or categorical variables with many levels. Another method is to introduce into the model interactions of independent variables and survival time to determine if they are significant. Another form of the model is the parametric Cox model in which it is assumed that the underlying hazard follows a mathematical distribution, commonly the Weibull, lognormal or gamma distribution.
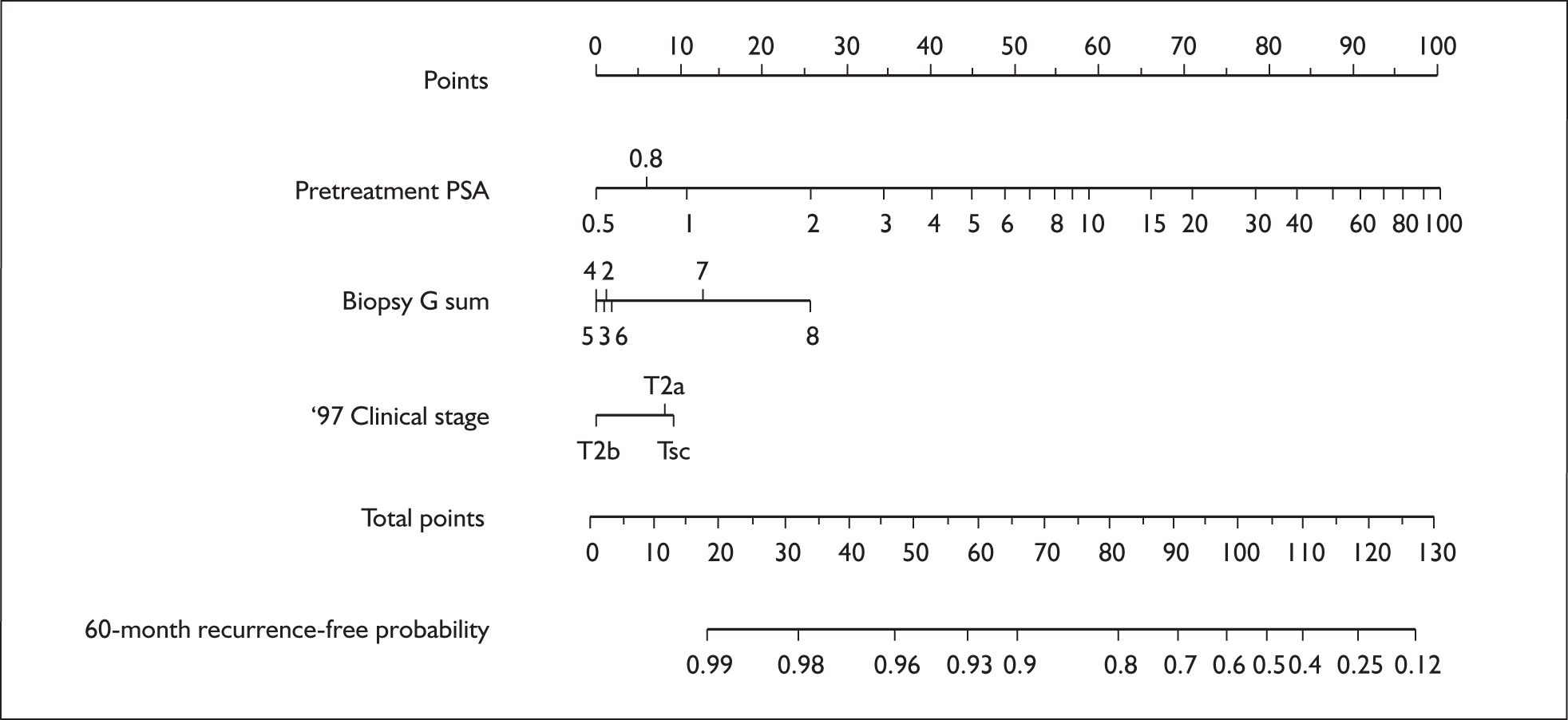
Survival predictions derived from Cox regression models are typically presented in tables showing survival for different risk groups, or graphically. Graphical representations are commonly used in prostate cancer and are referred to as nomograms. Chun et al. 56 define the term nomogram as applying ‘to a specific functional representation that graphically displays prediction models based on traditional statistical methods such as multivariable logistic regression analysis to predict a binary outcome or Cox regression analysis to predict a prognostic outcome’. An example is shown in Figure 2.
The number of points for each prognostic marker matching the patient value is found by drawing a vertical line to the points scale at the top of the diagram. The points are summed for all prognostic variables and estimated survival is read from the corresponding value of the total points scale.
In recursive partitioning the data are split using the variable and cut-point to give the greatest separation on the prognostic outcome. This procedure is applied to the data repeatedly until the criteria for stopping are met. This method is also sometimes referred to as classification trees.
ANN are one of several artificial intelligence techniques that use machine learning to examine relationships between variables. Their advantage compared with algebraic modelling is that they can more easily capture complex interactions, so in theory they should provide more accurate models. These methods are computing intensive and critics point to the lack of transparency in the models. A review of 28 studies by Sargent,58 which compared ANN with regression models, was inconclusive as to which method was better, reporting that the development of both was required to achieve the desired performance. ANN and other artificial intelligence methods have been used for prognostic modelling in prostate cancer. 59,60
There have been many prognostic models developed for use in prostate cancer, for many different purposes, including predicting positive biopsy and pathological stage, as well as outcomes following prostatectomy, radiotherapy and brachytherapy. Many of these are listed in Ross et al. 61 The Memorial Sloan-Kettering Center in the United States has been particularly active in recent years in developing nomograms for different patient groups (pretreatment, and at surgery) and for different treatments (radiotherapy, brachytherapy and prostatectomy). 57,62–70 These models are now freely available via the internet for clinician and patient use. 71
Study end points
Survival
Few studies report survival outcomes, mainly because patients diagnosed with low-stage localised prostate cancer typically survive for several years and in fact many will die of other causes. This demonstrates the importance of an adequate length of follow-up, although even then the number of events may be small. Those studies that do report survival outcomes vary in their definitions of survival.
The most reliable outcome in prostate cancer is all-cause mortality, but as most patients with prostate cancer do not die of the disease it is not a sensitive measure and is also highly dependent on the age distribution of the study population.
Prostate cancer survival is a more sensitive measure of prostate cancer outcome than all-cause mortality; however, a potential problem with prostate cancer survival as an outcome is ensuring that cause of death has been accurately determined. 72,73
Clinical failure
Clinical failure may refer to local disease recurrence, the development of metastatic disease, or both. For patients who do not have radical treatment for prostate cancer there is no definition of biochemical failure, and disease progression is usually measured in terms of those developing symptomatic or metastatic disease. There are variations between studies in the frequency of follow-up and methods for identifying and confirming disease recurrence that may affect this outcome measure. Clinical failure may be biased if prognostic factors influence the frequency of follow-up.
Biochemical failure
As prostate cancer is a slowly progressive disease and has many competing causes of death, the development of biochemical failure may not necessarily be associated with prostate cancer mortality or clinical failure. There has been a surge of interest in attempting to identify a definition of biochemical failure after RP or radiation therapy that is both sensitive and specific in predicting subsequent clinically significant failure. Although the principle of using biochemical failure is a useful one, in practice it has proved difficult to determine an appropriate definition of what constitutes failure. For example, there is a difference in PSA behaviour following different treatment modalities. In principle, PSA levels fall to zero after a few weeks’ washout period following prostatectomy. Subsequent re-emergence of detectable PSA is interpreted as disease recurrence. However, radiotherapy does not necessarily destroy the entire prostate and it may take several months for PSA levels to reach the lowest point or ‘nadir’. Other treatments such as brachytherapy are also now available and each has a differing effect on subsequent PSA behaviour.
Following a consensus conference in 1996 the American Society for Therapeutic Radiology and Oncology (ASTRO) established a definition of biochemical failure following radiotherapy. 74 The definition was three consecutive rises in PSA after a nadir, with the date of failure defined as a point half-way between the nadir date and the first rise, or any rise great enough to provoke initiation of salvage therapy. It was also recommended that a minimum period of follow-up of 2 years after therapy was required. Problems subsequently emerged with this definition, including the non-comparability of survival estimates based on different follow-up periods, as the backdating in the definition biases the survival estimates, the bias being worse the shorter the follow-up: results change dramatically if follow-up is only 3 years compared with 6 years. Another criticism of the 1996 definition of biochemical failure was that there had been no attempt to link it to clinical outcomes. To resolve these issues a second ASTRO consensus conference was held in 2005. A new definition of biochemical failure following radiotherapy, to be known as the ‘Phoenix definition’, was agreed: an increase of 2 ng/ml or more above the nadir PSA (lowest PSA attained following treatment). Data presented at the conference suggest that this definition yields a sensitivity and specificity of 66% and 77% for predicting clinical failure at 10 years. Patients who undergo salvage therapies without meeting the PSA failure definition should also be counted as failures at the time of positive biopsy or salvage treatment, whichever is first. A further recommendation of the conference was that control rates should be quoted at a time 2 years before the median follow-up to avoid the artefacts that may result from a short follow-up, including the backdating issue of the first ASTRO definition and the more favourable short-term outcomes that result from using the new Phoenix definition of PSA failure compared with the original ASTRO definition. However, it was emphasised that these definitions of PSA failure do not address the issue of cure rates, for which more data and longer follow-up are needed. As the new Phoenix definition was only published in 2006 it is unlikely that it will be used in many of the studies included in this review.
Cookson et al. 75 recently reviewed the variability in published definitions of biochemical recurrence and provided recommendations for a standard definition in patients treated with RP. Their review followed the American Urological Association (AUA) Prostate Guideline Update Panel being given the task of updating the guidelines for clinically localised prostate cancer. It became clear to the AUA that there were a substantial number of definitions being used to describe biochemical recurrence. Cookson and colleagues found 13,800 citations between 1991 and 2004 that included the terms prostate cancer and prostatic neoplasm, with 436 articles dealing with the clinical T1–T2N0M0 prostate definition of biochemical recurrence. Of these, 145 articles contained 53 different definitions of biochemical recurrence for those treated with RP. The most common definition after RP was a PSA of > 0.2 ng/ml or a slight variation of this. For radiation therapy, 208 articles were found reporting 99 varying definitions of biochemical failure. The most common definition for radiation failure was the ASTRO definition, three consecutive rises in PSA after a nadir. Overall, 166 different definitions of biochemical failure were found. The review shows the high degree of variability that is being used in the definition of biochemical recurrence following treatment for localised prostate cancer. These differences in definition can have a considerable effect on failure rates, as illustrated in a study by Amling et al. 76 For thresholds of 0.2 ng/ml and 0.5 ng/ml, biochemical survival was 62% and 78%, respectively, at 5 years. The authors concluded that strict definitions for biochemical recurrence are necessary to identify men at risk for disease progression and to allow reliable comparisons among patients treated similarly.
Following RP, the AUA recommends defining biochemical recurrence as an initial serum PSA of ≥ 0.2 ng/ml or more, with a second confirmatory PSA level of > 0.2 ng/ml. The panel recommended the use of the ASTRO criteria for patients treated with radiation therapy but recognised that these criteria will soon be updated. 75
Description of new and emerging technologies
Biomarkers
It is apparent that improved diagnostic and prognostic markers are needed to discriminate between men with curable prostate cancer, those with clinically irrelevant prostate cancer and those with life-threatening prostate cancer. Several clinical trials are currently attempting to investigate this.
The ProtecT study is currently evaluating the effectiveness, cost-effectiveness and acceptability to men with localised prostate cancer of active monitoring (monitoring with regular check-ups), RP and radical radiotherapy (the study does not include brachytherapy). The ProtecT study is an RCT investigating general health, quality of life, prostate cancer development, treatment outcome, length of life and cost implications. Several papers have been published from the ProtecT trial. For example, Mills et al. 77 reported the differences found at baseline between the sociodemographic status and psychological status of those randomised and those self-selecting treatment; there were no psychological differences at short-term follow-up. The study is still recruiting patients and follow-up will continue for 10–15 years. As there is a growing awareness of the importance of examining long-term overall survival when evaluating the clinical effectiveness of a trial, periods of 5, 10 and 15 years following treatment are being analysed. However, as in many other studies the trial will also measure short- and medium-term outcomes such as disease progression. Often, because of the short duration of many studies and the consequent lack of long-term follow-up, disease progression is the only reported outcome. Disease progression is thought to give some indication of the likelihood of longer-term survival. There are, however, differing definitions of disease progression. Biochemical no evidence of disease rates are often reported at varying times post treatment. This measure relates to levels of serum PSA and/or rising levels of PSA. A rising PSA level can predate other signs of progression. There is controversy, however, about the use and interpretation of serial changes in PSA values for assessing outcomes and determining prognosis. 78 It is useful, therefore, to have details about the rates of disease progression as defined in clinical terms, that is, evidence of recurrence of disease collected via patient history, DRE, radiography, scans, biopsies, etc. Because new and emerging prognostic marker studies have shorter follow-up periods than studies concerning the more classical markers, disease progression, either biochemical or clinical, is the most commonly measured outcome. For many of the potential novel markers it will be many years before overall survival can be reported.
The P-Mark trial aims to improve prognostic and diagnostic prostate cancer markers by the evaluation and identification of novel markers in addition to the validation of recently developed markers. The novel serum and urine markers will be identified and evaluated for their clinical importance using mass spectrometry tools and antibody-based immunoassays. Those markers that prove their clinical value during the evaluation will be validated on a sample set derived from two European screening studies. 79
With recent advances in functional genomics and proteomics there has been a growing research interest in investigating whether more molecular-based prognostic factors could be utilised to assay original needle biopsy specimens to allow the tailoring of the primary treatment to individual prostate cancer patients. 80–83 As targeted therapy in oncology becomes increasingly powerful there is a significant interest in finding prognostic markers in prostate cancer that could be used as targets for novel biotherapies. Many molecular- and genetic-based biomarkers have been discovered over the last two decades and they are summarised in review articles (see Abate-Shen and Shen84).
Treatments
As well as considering the potential novel markers being developed, one must also recognise that there are a number of new and developing therapies that aim to treat early localised cancer effectively in terms of survival, are minimally invasive and aim to reduce complications. 16 It remains unclear what is the most effective treatment for patients with localised prostate cancer.
At present we do not know enough about the outcomes of the many different forms of treatments for prostate cancer to guarantee that men are receiving the most appropriate treatment. Several trials are currently investigating the effectiveness of various treatments for prostate cancer to form consensus over which treatment is most appropriate. The Prostate Cancer Research International: Active Surveillance (PRIAS) trial is a prospective, observational study that aims to validate the treatment option of active surveillance in men with localised, well-differentiated prostate cancer in an attempt to limit overtreatment (Roemeling et al. 85). A number of factors are being studied: (1) PSA velocity (PSAV); (2) the pathological findings in radical prostatectomy specimens; and (3) the effect of expectancy on quality of life. Other trials include the ProStart trial (Principal Investigator Dr Chris Parker; CR-UK Feasibility Studies Committee funding), which is also comparing active surveillance with radical intervention options in localised prostate cancer. Clearly there is a need for further research to assess whether treatment preferences impact upon the processes and outcomes of RCTs.
Many patients with early localised disease have a good prognosis without treatment but because of the difficulties in identifying this group of patients the majority will require radical local treatment. Bill-Axelson et al. 86 found a significant advantage of RP over watchful waiting in patients with localised (T1, T2), well- to moderately differentiated cancers, but the absolute risk reduction in all-cause mortality was relatively small. There were also benefits in terms of other end points such as less local progression and distant metastases but, nevertheless, after 10 years the majority of patients on watchful waiting had not developed distant metastases or died of prostate cancer. The study was not powered for subgroup analysis. The trial also included few screen-detected patients (5.2%) and compared surgery with watchful waiting rather than active monitoring, the latter allowing for radical treatment at a later time if there are indications that the disease is aggressive. Thus, the question remains for most men diagnosed with localised prostate cancer whether they will benefit from radical treatment. Prognostic markers may help to determine which cancers are indolent and therefore do not require treatment.
Chapter 2 Definition of the decision problem
Decision problem
Patients diagnosed with localised prostate cancer face the difficult decision of whether to opt for radical treatment or not. Even without radical treatment, patients are much more likely to die of other causes. 87 Nevertheless, some will progress to metastatic disease, which has serious consequences for quality of life and which ultimately leads to death. In 2005, prostate cancer was the cause of 10,000 deaths in the UK, comprising around 13% of male deaths from cancer. 9
Radical treatment for prostate cancer has adverse effects including erectile dysfunction (80%)88 and urinary leakage (49%)88 following surgery, which may also severely compromise quality of life. Furthermore, the benefits of immediate radical therapy over a strategy of active monitoring of the disease are unknown. To our knowledge the results of only one RCT of treatment have been published. 86 This trial compared surgery with watchful waiting, the traditional form of disease monitoring, and the patient sample pre-dated PSA screening. The latter is important as there is evidence that since the advent of PSA screening tumours are diagnosed with smaller volumes, with lower grades and at a younger age. 89 Thus, although the trial did report improved survival, prostate cancer survival and freedom from metastatic disease after surgery compared with watchful waiting, there are still questions as to the benefit of immediate radical treatment for most patients. Following radical treatment, results are also very heterogeneous and the question also arises as to whether some patients may benefit from adjuvant treatment.
Ideally, a marker, or a combination of markers, would allow slow-growing, non-aggressive tumours to be accurately differentiated from those that will rapidly develop into metastatic disease, hence the interest in prognostic markers and models in prostate cancer. There is a considerable volume of literature on both prognostic markers and models in prostate cancer. Yet the last new marker to be widely adopted is PSA, which first emerged in the 1970s. 19,23 There is clearly a need to review what has been achieved to date to inform future research in this area. Although previous reviews have been undertaken for prognostic markers and prognostic models, to our knowledge there has been none undertaken for all markers using a systematic review methodology.
However, it must be noted that patient outcomes are not only dependent on an individual’s disease characteristics but also on the treatment received and possibly interactions between the two. Most research on prognostic markers is undertaken in cohort studies, usually with all patients treated in the same way. A marker that is found to be associated with an outcome in such circumstances can be said to be a predictive marker, that is, useful in predicting patient outcome given that treatment. Clinical understanding of the potential interactions between treatment and marker and/or studies with different treatment modes are required to determine if the marker is truly prognostic.
Once an effective prognostic marker or model has been identified the question remains as to the optimum treatment for each prognostic group. Only RCTs can ensure the avoidance of bias in answering this question. Thus, there are many steps in the research process that are needed to inform the decision problem of which patients with localised prostate cancer will benefit from radical treatment. This review forms one step in that process.
Overall aims and objectives of assessment
The current systematic review aims to provide an evidence-based perspective on the prognostic value of novel markers. Through systematic, explicit and rigorous methods of identifying, critically appraising and synthesising evidence, systematic reviews are considered a useful and appropriate means of identifying and combining existing evidence. 90,91 Some systematic reviews are able to conduct a meta-analysis of the data pooled across studies. This synthesis of the data across several studies attempts to overcome limitations of small samples or scope in individual studies. However, the combining of relevant data to produce results that are more precise than those from individual studies is not always possible because of the differences in characteristics (e.g. population, intervention, comparator and outcomes) between studies.
The focus of this review is on novel markers (as opposed to classical markers) and prognostic models. These terms were defined as follows:
-
Classical markers that are currently in widespread use were defined as PSA, biopsy or pathological Gleason grade (score), and clinical or pathological stage. For patients who had surgery, positive margins were also considered to be a classical marker.
-
Novel markers were defined as all disease-specific markers other than those previously defined as classical markers (clinical or pathological stage, total Gleason score, single PSA measurement, surgical margins) but excluding epidemiological markers or measures of co-morbidity.
-
A prognostic model was defined as a model developed using statistical methodology to combine two or more factors to predict a relevant prostate cancer outcome.
The objective of this review is to identify the best prognostic model(s) that include(s) the three classical markers and to see if any models incorporating novel markers are better than these. Additionally, novel markers will be reviewed and their potential for incorporation into a prognostic model assessed. This will allow the need to be determined for further research to develop prognostic models for early localised prostate cancer patients.
To achieve these objectives two systematic reviews of prognostic models for patients with early localised prostate cancer will be undertaken. A separate review of novel prognostic markers will allow their potential for inclusion in a prognostic model to be assessed.
Chapter 3 Assessment of prognostic markers and models
Methods for reviewing prognostic markers and models
Search strategies
The search aimed to identify all references relating to novel markers and prognostic models. An iterative procedure was used, with input from clinical advisors and a previous HTA review. Copies of the search strategies used in the major databases are included in Appendix 1. The main searches were conducted in March and April 2007.
Searches were performed in MEDLINE, EMBASE, the Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CCTR), the Database of Abstracts of Reviews of Effects (DARE), the Science Citation Index, the NHS Economic Evaluation Database (NHS EED), the Health Technology Assessment Database (NHS HTA), the Current Index to Nursing and Allied Health Literature (CINAHL), the Current Controlled Trials Meta-Register and the National Research Register.
In addition, the reference lists of relevant articles were checked and various health services research-related resources were consulted via the internet. These included HTA organisations, guideline-producing bodies and generic research and trials registers.
Search restrictions
No study- or publication-type restrictions were applied, but the search was restricted to publications from 1970 onwards in the English language. The decision not to include publications before 1970 was considered appropriate as the classical marker PSA was not discovered until 1970. 19
Inclusion and exclusion criteria
The review of the evidence for prognostic markers and models was undertaken systematically following the general principles recommended in the QUOROM statement. Few or no RCTs were expected, so all study designs were accepted. The inclusion and exclusion criteria were generic to the whole review with the exception of the following specific criteria for the two main parts of the review.
Review of novel markers
To be included the article had to report a primary prognostic study of (a) novel marker(s). Novel markers were defined as all disease-specific markers other than those previously defined as classical markers (clinical or pathological stage, total Gleason score, single pretreatment PSA measurement, surgical margins) but excluding epidemiological markers or measures of co-morbidity.
Review of prognostic models
To be included the article had to report a primary study or validation of a prognostic model. A prognostic model is defined as a model developed using statistical methodology to combine two or more factors to predict a relevant prostate cancer outcome. It should be noted that, although the statistical methods used to test the novel prognostic markers and to develop prognostic models are the same, to be classified as a review of a model the study needed to present predicted outcomes for different prognostic groups based on a multivariate analysis. Model articles that included novel markers were also included in the novel marker review.
Generic inclusion criteria
Population
Males with a diagnosis of early localised prostate cancer (i.e. clinical or pathological stage TI/T2/T3N0M0 or Jewett–Whitmore system stages A, B, C) before treatment (radical or not) or at the time of radical treatment (prognostic markers taken before or at treatment). Studies were included if at least 80% of the study sample were in the target patient group.
Study end points
All reported measures of the prognostic value of individual or combinations of markers that predict the following outcomes:
-
overall survival
-
disease-specific survival
-
disease-free survival
-
biochemical (PSA) recurrence
-
biochemical (PSA) freedom from recurrence
-
clinical recurrence.
Generic exclusion criteria
-
Study populations with more than 20% not in the target study group (i.e. not TI/T2/T3N0M0) unless results for target study group are reported separately.
-
Studies that do not report the statistical differences between prognostic groups.
-
Studies that do not report when in the treatment course the biomarkers were measured (before, during, after) or what principal treatments (e.g. prostatectomy, radiotherapy) patients received.
-
Non-English language papers.
-
Studies that are reported only in abstract form.
-
Reviews of primary studies – not included in the analysis but retained for discussion.
-
Studies with fewer than 200 patients in the target group (i.e. T1/T2/T3N0M0).
-
Studies with less than 5 years’ mean or median follow-up (included if either greater than 5 years).
Rationale for the exclusion of small studies and those with a follow-up period of less than 5 years
Exclusion of studies with fewer than 200 patients in the target group (T1/T2/T3N0M0)
Given the large volume of literature that the scoping literature searches indicated would be identified, we needed a simple method that would enable us to quickly identify the higher quality studies. Studies with a low number of outcome events (death or clinical/biochemical recurrence) tend to yield statistically weak analyses. It is recommended that analyses should have at least ten events per variable (EPV), if not 20,92 and so, with at least three (or four if pathological variables are included) classical variables that should be included in any multivariate analysis, as well as any novel markers, the very minimum number of events is 40–50. However, the number of events is often not reported and the reporting of the number of EPV is even more rare. The EPV can sometimes be estimated if sufficient information is presented, but this is often difficult to locate in an article. It was therefore decided that it was not practical to use number of events or EPV as a study inclusion criterion. Instead, a minimum number of patients used in the analysis was specified as an inclusion criterion for the review. This allowed small studies to be sifted out relatively quickly. The minimum was set at 200 based on an approximate calculation of the number of outcome events expected with a median follow-up of 5 years. This was carried out as follows. The outcome with the highest event rate is biochemical recurrence. Approximately 30% of patients suffer biochemical recurrence at 5 years following radiotherapy, with a similar proportion following surgery, dependent on the definition of biochemical recurrence. 76,93 Approximately 10% of treated patients with localised prostate cancer will die within 5 years86 and we allowed a further 10% loss to follow-up. Thus, after 5 years in a cohort of 100 patients, 24 events {30 × [1.0–(0.1 + 0.1)]} might be expected. As a minimum of 40–50 events are required, a cohort of 200 was specified as an inclusion criterion. Note that other prostate cancer outcomes have much lower event rates and therefore need much larger cohorts to achieve 40–50 events. For the outcomes of local progression and prostate cancer death with cumulative incidence rates of 8.1% and 2.3% respectively,86 similar calculations to that shown above suggest that cohort sizes of at least 600 and 2000 respectively are required to obtain the same number of events.
Length of follow-up
Patients diagnosed with localised prostate cancer usually live for several years with their disease and are more likely to die of other causes. For those who have radical treatment, approximately 8.1% and 19.2% will have experienced local recurrence at 5 and 10 years respectively. Prostate cancer mortality at the same time intervals is 2.3% and 9.6% respectively. 86 Clearly, studies with a follow-up of only a few months will identify only a small proportion of those who will eventually experience disease recurrence and almost none of those who will die of prostate cancer. In a study of radiotherapy94 24% of recurrences were recorded after 5 years of follow-up (median 6 years’ follow-up, maximum 11). This study quotes results from a study of prostatectomy95 showing that the proportion is similar following this mode of treatment: 27% of all recurrences occurred after 5 years in a series with a median follow-up of 8.8 years. They argued in favour of a follow-up period of at least 5 years following radiation therapy. In an editorial comment concerning a review of prognostic models used in prostate cancer61 it was noted that PSA recurrence in the reviewed nomograms was reported at between 2 and 6 years, ‘which is too short to be definitive’.
Another issue in determining the length of follow-up that is adequate for prognostic studies is the phenomenon of PSA ‘bounce’, which may occur following radiotherapy treatment. This is a temporary rise in PSA level, which with a short follow-up period may appear to be a failure. The American Society of Clinical Oncology (ASCO) recommends a minimum follow-up period of 2 years following radiotherapy. 74
On the basis of the above discussion one might argue that the prognostic studies should have a follow-up of several years. However, there must be a balance between a sufficiently long follow-up, so that a significant proportion of those destined to suffer disease progression have done so, and the relevance of studies conducted several years previously when screening, diagnosis and treatments will have been different.
Scanning the literature indicated that using a minimum follow-up period as an inclusion criterion for the review would not be useful, as most studies do not report this statistic. Those that do report a measure of the follow-up period usually give a mean or median. Similarly, relying on the timing of the reported outcome (e.g. 5-year progression-free survival) was also unsatisfactory for two reasons. First, not all studies report the outcome in this way and, second, for those that do, it was clear that in some studies median follow-up represented only a fraction of the time to the reported outcome, suggesting a low level of events at this time and therefore potentially unreliable results.
It was decided pragmatically to apply a mean or median follow-up of 5 years as an inclusion criterion. Clearly the two measures are not the same as the distribution of follow-up time is often skewed, but as many studies report only one measure this was a practical method of eliminating studies with the shortest follow-up times.
All articles produced by the searches were entered into a Reference Manager database. All identified titles were screened by at least one of three reviewers (PS, SH, ES). If there was any doubt as to the relevance of the article to the review the article was included at this stage. All abstracts were read by at least two reviewers and consensus obtained. The reviewers held regular meetings to discuss the review process and the assessment of the literature.
Data abstraction strategy
A data extraction form was developed based on that used by Williams et al. 92 for prognostic models in breast cancer. The data abstraction tool includes study design, the study population, details of univariate and multivariate analyses and the results of those analyses. The model data extraction form included the same items as well as more details of the analysis and details of any validation. The forms are shown in Appendix 2. All data from included studies were extracted by two reviewers and any disagreements were resolved by discussion.
Assessing methodological quality
There are no widely agreed quality criteria for assessing prognostic studies. 96 In determining how to approach quality assessment in this review of prognostic markers and models we identified some recent (all published after 2000) systematic reviews of prognostic studies to see how the issue had been addressed. These included two reviews for stroke,97,98 one for liver transplantation99 and three for different forms of cancer. 92,100,101 With the exception of one study100 all assessed study quality and two of the five calculated an overall quality score. The value of an overall quality score, which mixes different issues, has been questioned. 92 Common themes in the assessments were internal, external and statistical validity.
In our search to identify an instrument that we could use or adapt for this review we discovered a study by Hayden et al. 102 that appraised how authors of reviews of prognostic studies had assessed study quality. This study also made recommendations of the domains that should be considered and the questions that might contribute to the assessment of each domain. The domains proposed by Hayden and colleagues to assess potential biases in prognostic studies were:
-
study population
-
study attrition
-
prognostic factor measurement
-
outcome measurement
-
confounding measurement and account
-
analysis.
Within each of these categories questions are proposed by Hayden and colleagues to help assess the extent of possible biases. These questions were adapted to make them relevant to the disease area and the types of studies available in this review, and also to clarify what each of the questions meant in the context of the study. As with any study, pragmatic decisions needed to be made on the value of collecting data. With more than a handful of studies to assess there was a certain prioritisation of the elements that it was believed would contribute most to differentiating between the quality of the studies included. The approach taken in this review to assessing each of the domains listed above will be discussed in turn. The resulting quality assessment tool is shown in Appendix 3.
Study population
It was clear from the outset that the studies were not reporting on entirely homogeneous populations. Rather than defining some theoretical ideal population and then determining how actual study populations would be biased to representations of that ideal, it was decided that the most important factor was that studies reported sufficient information on the principal factors known to affect patient prognosis so that it would be clear to which population the results were applicable.
The key factors known to affect patient outcome, and which were considered essential to report for the population studied, were treatment, recruitment dates and the established prognostic markers of PSA, clinical or pathological stage, biopsy or pathological Gleason grade, and surgical margins (where relevant). A TNM stage of T1–T3N0M0 or stage A–C on the Jewett–Whitmore system was an inclusion criterion so that, as a minimum, all studies included in the review reported clinical or pathological stage.
Treatment
It was noted whether the principal treatment (usually surgery, radiotherapy or watchful waiting) and also the proportion of patients who had had adjuvant or neoadjuvant treatment were recorded. Note that in none of the studies were patients randomised to treatment and it is likely that there are differences between populations selected for the different treatment modes.
Recruitment dates
Many factors that affect prognosis may change with time. A particular example in prostate cancer is the introduction of PSA testing, which has considerably changed the population of patients newly diagnosed with prostate cancer, who on average have lower-stage cancers than those diagnosed before the introduction of PSA testing. 103 Biopsy methods and surgical techniques have also evolved. The staging classifications used in the TNM system have also undergone several minor changes. It is therefore important to know over what period of time the patients were recruited. The more recent studies are likely to be most relevant to new patients.
Baseline characteristics
It is important to describe the study population with regard to known prognostic factors. In particular, there were differences between studies in terms of the stages of the cancers included and whether postoperatively those who had had positive surgical margins were included or not. The availability of PSA measurements was also an indication, together with the recruitment dates, of whether the patient population may have been initially identified through PSA screening.
The reporting of diagnostic methods and ‘time zero’ were not recorded. For both issues the differences in populations arising through variations in these factors were considered to be small in comparison to those resulting from the advent of PSA screening, which has resulted in younger patients being diagnosed with lower-stage cancers. Furthermore, time zero, where stated, is generally defined as the start of treatment. In the traditional model of care the decision of whether to have radical treatment or not is made close to the time of diagnosis. It is only more recently that a different model of care has emerged, in which a patient is monitored and is possibly offered radical treatment at a later date, and this model is still unusual. Thus, generally, it is unlikely that there will be large discrepancies between the approaches to the definition of time zero.
Study attrition
It was apparent that the majority of studies were going to be retrospective and so the assessment of attrition had to be relevant to this type of study.For these studies, loss to follow-up was not the only issue to consider; the selection of cases was also important, on the basis of either complete follow-up data or complete baseline data. The question regarding baseline information was awarded a ‘yes’ if the total number of patients from which the study population was selected was given, together with reasons for patient exclusion. If some of this information was given, the question was ranked ‘partly’. Similarly, with loss to follow-up, a ‘yes’ was given only if either the number or the percentage lost to follow-up was reported or if the number of patients at risk was recorded at least one time point after time zero.
Biases due to such selection are difficult to assess from a publication. Ideally, the authors discussed what biases such selection may have introduced and we recorded whether they had done so.
Prognostic factor measurement
For a prognostic marker to be useful its measurement must be consistent. This means that there must be a well-defined and reproducible method of extraction and measurement. Some markers may be affected by how they are stored before measurement and so it is important to know that studies have considered this issue. We looked for a description of the measurement of the prognostic markers, with a particular emphasis on the novel markers. A full description of measurement methods was considered less important for the classical markers, for which methods are more established, although for PSA measurements there are different assays in use. Hayden and colleagues102 also consider the issue of how continuous variables are treated in the analysis in this section and we followed suit. In summary, categorising continuous variables leads to the loss of statistical power, and data-dependent categorisation leads to overoptimism. In the latter case, studies were graded ‘no’ on this issue. If the data were categorised, but using well-established groups such as are often used for PSA, the study was graded as ‘partly’ satisfying this question.
Outcome measurement
The most reliable outcome in prostate cancer is all-cause mortality but as most patients with prostate cancer do not die of the disease it is not a sensitive measure and is also highly dependent on the age distribution of the study population. The potential problem with prostate cancer survival as an outcome is ensuring that cause of death has been accurately determined. 72,73
Because of the long average survival time of prostate cancer patients most studies in fact use freedom from biochemical (PSA) recurrence as the outcome measure. As discussed in Chapter 1 (see section Biochemical failure), with PSA being a continuous measure the problem is the definition of PSA recurrence. There are, however, consensus recommendations for the definition of PSA recurrence following surgery and radiotherapy, and we recorded whether these had been used. Two definitions were allowed following radiotherapy as the original 1996 recommendation was changed in 2005.
It was also recorded whether a unique definition of PSA recurrence was used: it is important that the outcome is defined consistently so that the predicted outcomes are unambiguous.
Length of follow-up was not included in the quality assessment as this was an inclusion criterion for the review.
Confounding measurements
The most important confounders were considered to be the classical markers. In this section it was noted whether a multivariate analysis was reported that included all appropriate classical markers (dependent on whether the model was pretreatment or at surgery). At pretreatment the markers should include clinical stage, PSA and biopsy or pathological Gleason score. At treatment (only relevant for surgery) the markers should include clinical or pathological stage, pretreatment PSA, biopsy or pathological Gleason score and positive or negative surgical margins.
Treatment was another potential confounder but in the majority of studies all patients had the same principal treatment (usually surgery). Ideally, if some patients have had adjuvant or neoadjuvant treatment this should be included as a confounding variable, as should age if the end point is all-cause mortality. A recent review104 concluded that age is not a prognostic factor for prostate cancer outcome.
Analysis
In addition to an adequate description of the analysis, to determine whether there were sufficient data to assess the quality of the study the reporting that a univariate analysis had been undertaken was considered essential; this resulted in a ‘yes’ score and was used as an indication that the authors had undertaken a systematic analysis of their data.
The question regarding model building was relevant only to the multivariate models. Although there is some controversy regarding the optimum method of developing multivariate regression models all reasonable approaches were accepted (forward and backward removal of variables, all plausible variables), as long as variables were not introduced that were not included in the univariate analysis.
For a model to be considered adequate it had to include a time-to-survival analysis such as the Cox regression and have no other major inadequacies. Ideally, a multivariate analysis with novel and established markers was sought. Thus, if only a log-rank test of difference between survival curves was used (a univariate analysis) instead of multivariate regression analysis the maximum score was ‘partly’. Division of patients into groups and testing of survival differences using a t-test were considered inadequate.
In total, there were 23 questions. Each question was scored as yes (y), no (n), partly clear (p), unsure (?) or not applicable (na). There was also an overall question on the conclusion for each domain.
The quality of each study was assessed by at least two of the three members of the research team (PS, SH, ES). There is an element of subjectivity in quality assessment, as well as a need for attention to detail as reporting methods and formats vary widely, so disagreement between the two reviewers was common. Regular discussion meetings were arranged to resolve uncertainty between the two members who had completed the assessment. The third team member attended the meetings when agreement could not be reached. A statistician (TY) provided additional support for the interpretation of the statistical models and validation of the quality assessment scores assigned by the two reviewers. It was always possible to reach a consensus among the team members.
It is important to recognise that, as with all forms of systematic review, our review may be influenced by publication bias. By this we mean that the findings from the individual studies that have been published might be different from the findings of individual studies that have not been published. The exclusion of smaller studies may have reduced the possibility of publication bias, but with the literature comprising retrospective case series the possibility of publication bias remains considerable. Furthermore, with several possible outcome measures available there is scope for selective outcome reporting.
Data synthesis
Studies were assessed for the suitability of pooling results with regard to populations, outcomes and study type. Because of the lack of sufficient similarity regarding these components, meta-analyses were not undertaken and the results are presented in a tabulated format with a narrative synthesis of the results.
Chapter 4 Results of searches
Number of studies identified
A flow chart describing the process of identifying relevant literature can be found in Figure 3. Following the removal of duplicates our searches identified 12,963 potentially relevant articles. A total of 8934 articles that did not meet our inclusion criteria were removed at title sift, leaving a total of 4029 articles to be screened at the abstract sifting stage. It should be noted that 795 articles were excluded because they had no abstract. Of these, 28 articles were concerned with prognostic novel markers and five with prognostic models. Note that three articles were included in both the novel markers and the prognostic models sections.
FIGURE 3.
Summary of study selection and exclusion. *795 articles were excluded because they had no abstract. **Three articles were included in both the novel markers and prognostic models sections.
Number of studies excluded
A list of the 365 articles that were excluded at full paper sift with reasons for exclusion is provided in Appendix 4.
Chapter 5 Results for systematic review of novel prognostic markers
This chapter aims to evaluate the additional prognostic value of novel markers over the prognostic value of markers in current widespread use (classical markers) in prostate cancer.
The heterogeneous nature of the studies precluded the use of meta-analysis. One of the main sources of heterogeneity was in the measures of outcome, with all-cause mortality, prostate cancer mortality and clinical and biochemical recurrence all being used, and the definition of the last two also varying. The heterogeneity of the definitions used in the literature for biochemical recurrence and the effect that it can have on outcomes has been previously highlighted (see Chapter 1, Biochemical failure). Other important differences between studies were the covariates included in multivariate analysis and marker measurement methods and cut-points used to define prognostic groups. In general, the patient groups were fairly homogeneous with almost all patients clinically T1–T2N0M0, but there were some exceptions, and in some older studies patients were diagnosed from transurethral resection of the prostate (TURP) specimens rather than via the PSA screening/biopsy route, which is current practice. Although most patients had surgery as their principal treatment, in some studies radiotherapy was used and adjuvant treatment was treated differently in the various studies. Some studies excluded those who had had adjuvant treatment (risking bias in their study population) whereas others included these patients (with or without adjuvant treatment as a covariate in analysis); many did not report this item. Finally, as well as the heterogeneity in study design and analysis methods, the poor reporting of models and particularly the lack of HRs sometimes made meta-analysis impossible.
The evidence for each marker, taking into account the direction of evidence and the strengths and weaknesses of studies, is discussed in a narrative format. Note that, although the primary aim is to evaluate the additional prognostic value of the novel markers over the classical markers, to assess this requires the novel markers to have been tested in a multivariate model that included all the classical markers. As many novel markers were not tested in such models, the multivariate results with different covariates are not comparable. Also, in some instances only univariate results were reported. For this reason the univariate results are also presented. It must be noted, however, that these results demonstrate only the prognostic value of the marker independently and do not show whether the marker would add prognostic information to those already in current use.
There was only a small number of studies, or sometimes only a single study, for each marker. It was not possible to examine the potential issues of publication bias or selective outcome reporting. The exclusion of smaller studies may have reduced the possibility of publication bias, but with the literature comprising retrospective case series the possibility of publication bias remains considerable. Furthermore, with several possible outcome measures available there is scope for selective outcome reporting. It is possible for many markers that a single unpublished study could alter the conclusions considerably, and this should be taken into consideration in interpreting the results.
Novel marker categories identified
A total of 17 novel marker categories was identified from the 28 studies included in this section. A list of these novel marker categories is presented in Table 4. Of these 28 studies, three105–107 also appear in Chapter 6 as they also present prognostic models.
| Novel marker category | Studies |
|---|---|
| β-Catenin expression: < 10% vs ≥ 10% nuclei | Horvath, 2005108 |
| Acid phosphatase level | Anscher, 1991;109 Han, 2001;110 Perez, 1989;111 Roach, 1999;112 Zagars, 1993113 |
| Androgen receptor: CAG repeats | Nam, 2000;114 Powell, 2005115 |
| Creatinine | Merseburger, 2001;116 Zagars, 1987117 |
| CYP3A4 genotypes | Powell, 2004118 |
| DNA ploidy | Blute, 2001;105 Lieber, 1995;106 Siddiqui, 2006119 |
| Germline genetic variation in the vitamin D receptor | Williams, 2004120 |
| Non-classical use of Gleason measurements (three prognostic submarker categories): | Egevad, 2002;121 Gonzalgo, 2006;122 Tollefson, 2006;123 Vis, 2007;124 Vollmer, 2001107 |
| (a) Gleason pattern in Gleason score 7 (4 + 3 vs 3 + 4) | |
| (b) Amount of high-grade cancer | |
| (c) Modified Gleason score | |
| Ki67 LI | Zellweger, 2003125 |
| Bcl-2 | Zellweger, 2003125 |
| p53 | Zellweger, 2003125 |
| Syndecan-1 | Zellweger, 2003125 |
| CD10 | Zellweger, 2003125 |
| Proportion cancer: | Antunes, 2005;126 Egevad, 2002;121 Potters, 2005;127 Selek, 2003;128 Vis, 2007;124 Vollmer, 2001107 |
| (a) Percentage positive biopsy cores | |
| (b) Percentage cancer in surgical specimen | |
| PSA kinetics | D’Amico, 2004;129 Sengupta, 2005130 |
| Stat5 activation status | Li, 2005131 |
| Tumour size: | Blute, 2001;105 Lieber, 1995;106 Salomon, 2003;132 Sengupta, 2005;130 Vis, 2007124 |
| (a) Maximum tumour dimension | |
| (b) Tumour volume |
Descriptions of studies
We first present a short discussion of the overall quality assessment of the included studies. We then focus on the identified prognostic maker categories and evaluate the evidence for each of the markers.
Quality assessment tables of included studies
Each article was assessed according to the six subheadings (study population, study attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, analysis). An overall quality score was not assigned to each article. Rather, the quality assessment tool was used to help identify factors that needed to be taken into account when interpreting the results of the study. The key items are discussed in each of the marker sections.
Table 5 provides a summary of the 23 questions for the six subheadings (A–F).
| Marker category/study | Subheadings and questions (Q) of quality assessmenta,b | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||||||||||||||||||
| Q1 | Q 2 | Q 3 | Q 4 | Q 5 | Q 6 | Q 7 | Q 8 | Q 9 | Q 10 | Q 11 | Q 12 | Q 13 | Q 14 | Q 15 | Q 16 | Q 17 | Q 18 | Q 19 | Q 20 | Q 21 | Q 22 | Q 23 | |
| β-Catenin expression | |||||||||||||||||||||||
| Horvath, 2005108 | p | p | p | y | n | n | ? | y | y | n | p | y | n | na | y | p | y | y | y | y | y | y | y |
| Acid phosphatase level | |||||||||||||||||||||||
| Anscher, 1991109 | y | p | p | y | n | n | ? | p | n | p | p | y | na | na | y | y | p | y | y | y | y | n | p |
| Han, 2001110 | y | y | y | y | y | n | p | y | n | y | p | y | y | na | n | y | y | p | y | y | n | ? | p |
| Perez, 1989111 | y | p | p | p | y | n | p | n | n | p | n | y | na | na | na | y | p | n | y | y | n | ? | ? |
| Roach, 1999112 | y | p | p | y | n | n | ? | n | n | p | n | y | na | na | na | y | p | y | y | y | y | y | y |
| Zagars, 1993113 | y | p | p | y | n | n | ? | y | n | p | y | y | na | na | na | y | p | y | y | y | p | p | y |
| Androgen receptor: CAG repeats | |||||||||||||||||||||||
| Nam, 2000114 | y | y | y | y | y | n | p | y | n | p | p | y | y | na | y | y | y | y | y | y | y | y | y |
| Powell, 2005115 | y | y | y | y | n | n | ? | y | n | p | p | n | n | na | y | p | y | p | y | y | y | y | p |
| Creatinine | |||||||||||||||||||||||
| Merseburger, 2001116 | y | y | y | n | n | n | ? | p | n | y | p | y | y | na | na | y | p | p | ? | y | y | ? | p |
| Zagars, 1987117 | y | p | p | y | y | n | p | p | n | p | p | y | na | na | na | y | n | p | na | n | y | na | p |
| CYP3A4 genotypes | |||||||||||||||||||||||
| Powell, 2004118 | y | y | y | y | n | n | ? | y | n | ? | p | y | n | na | y | p | y | p | y | y | n | ? | p |
| DNA ploidy | |||||||||||||||||||||||
| Blute, 2001105 | y | y | y | y | p | n | ? | p | y | y | y | y | n | na | y | p | p | y | y | y | y | y | y |
| Lieber, 1995106 | y | p | p | y | y | n | p | y | y | p | p | p | na | na | na | p | p | y | y | y | y | y | y |
| Siddiqui, 2006119 | y | y | y | p | y | n | p | p | p | p | p | y | na | na | y | y | p | y | y | y | n | ? | y |
| Germline genetic variation in the vitamin D receptor | |||||||||||||||||||||||
| Williams, 2004120 | y | y | y | y | n | n | ? | y | n | y | y | n | ? | na | ? | ? | y | p | y | y | n | ? | p |
| Non-classical use of Gleason measurements | |||||||||||||||||||||||
| Egevad, 2002121 | y | p | p | n | y | n | p | y | n | p | p | y | na | na | na | y | n | y | y | y | y | y | y |
| Gonzalgo, 2006122 | y | y | y | y | y | n | p | y | n | na | y | y | y | na | n | y | n | p | na | p | n | na | ? |
| Tollefson, 2006123 | y | y | y | y | y | n | p | y | n | ? | p | y | n | na | y | p | ? | p | ? | y | n | ? | ? |
| Vis, 2007124 | y | y | y | p | y | p | ? | p | n | ? | p | y | n | na | y | p | n | y | y | y | y | p | p |
| Vollmer, 2001107 | p | y | p | n | na | n | ? | n | n | ? | n | y | na | na | y | p | n | n | ? | y | y | y | p |
| Ki67 LI, Bcl-2, p53, syndecan-1, CD10 | |||||||||||||||||||||||
| Zellweger, 2003125 | y | p | p | y | p | n | ? | y | n | p | p | p | ? | na | ? | n | n | p | y | y | p | p | p |
| Percentage positive biopsy cores | |||||||||||||||||||||||
| Antunes, 2005126 | y | y | y | p | n | n | ? | y | n | p | p | y | n | na | y | y | y | p | y | y | y | y | y |
| Egevad, 2002121 | y | p | p | n | y | n | p | y | n | p | p | y | na | na | na | y | n | y | y | y | y | y | y |
| Potters, 2005127 | y | y | y | n | n | n | ? | n | n | ? | n | y | na | n | y | p | y | p | y | y | n | ? | p |
| Selek, 2003128 | y | y | y | y | y | n | p | y | n | p | p | y | na | y | y | y | p | p | y | y | y | y | y |
| Vis, 2007124 | y | y | y | p | y | p | ? | p | n | ? | p | y | n | na | y | p | n | y | y | y | y | p | p |
| Vollmer, 2001107 | p | y | p | n | na | n | ? | n | n | ? | n | y | na | na | y | p | n | n | ? | y | y | y | p |
| PSA kinetics | |||||||||||||||||||||||
| D’Amico, 2004129 | y | y | y | y | y | n | p | y | n | n | p | y | y | na | y | y | y | y | y | y | y | p | y |
| Sengupta, 2005130 | y | y | y | y | y | p | y | y | n | n | p | y | n | na | y | p | p | y | y | y | y | p | y |
| Stat5 activation status | |||||||||||||||||||||||
| Li, 2005131 | y | p | p | y | p | p | p | y | n | p | p | n | n | na | ? | p | p | y | y | y | n | ? | p |
| Tumour size | |||||||||||||||||||||||
| Blute, 2001105 | y | y | y | y | p | n | ? | p | y | y | y | y | n | na | y | p | p | y | y | y | y | y | y |
| Lieber, 1995106 | y | p | p | y | y | n | p | y | y | p | p | p | na | na | na | p | p | y | y | y | y | y | y |
| Salomon, 2003132 | y | y | y | n | n | n | ? | y | n | p | p | y | y | na | p | y | p | p | y | n | y | y | p |
| Sengupta, 2005130 | y | y | y | y | y | p | y | y | n | n | p | y | n | na | y | p | p | y | y | y | y | p | y |
| Vis, 2007124 | y | y | y | p | y | p | ? | p | n | ? | p | y | n | na | y | p | n | y | y | y | y | p | p |
| Total ratingsc | |||||||||||||||||||||||
| Yes (y) | 26 | 18 | 17 | 19 | 13 | 0 | 1 | 18 | 3 | 4 | 4 | 23 | 6 | 1 | 15 | 15 | 9 | 13 | 23 | 25 | 17 | 11 | 12 |
| Partly (p) | 2 | 10 | 11 | 4 | 3 | 3 | 12 | 6 | 1 | 15 | 20 | 2 | 0 | 0 | 1 | 11 | 12 | 13 | 0 | 1 | 2 | 5 | 13 |
| No (n) | 0 | 0 | 0 | 5 | 11 | 25 | 0 | 4 | 24 | 3 | 4 | 3 | 9 | 1 | 2 | 1 | 6 | 2 | 0 | 2 | 9 | 1 | 3 |
| Unsure (?) | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 0 | 0 | 5 | 0 | 0 | 2 | 0 | 3 | 1 | 1 | 0 | 3 | 0 | 0 | 9 | 0 |
| Not applicable (na) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 11 | 26 | 7 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 |
Description of quality
Study population
All of the studies adequately reported (n = 26) or partly reported (n = 2) the inclusion and exclusion criteria (including treatment, start/finish date for recruitment). The baseline study sample (i.e. individuals entering the study) was adequately described (n = 18) or partly described (n = 10) for key characteristics (age, PSA, clinical and/or pathological stage, biopsy and/or pathological Gleason grade, surgical margins) among the included papers. Overall, the study populations of the 28 included studies were considered to sufficiently represent the population of interest on key characteristics to limit potential bias to results in 17 studies and to partly limit potential bias in 11 studies. The quality of reporting of the study population was in most cases adequate and no study failed to report information concerning the study population.
Study attrition
The majority of studies reported (n = 19) or partly reported (n = 4) the exclusions due to missing data at baseline, but several studies did not (n = 5). In comparison with the missing data at baseline, fewer studies reported (n = 13) or partly reported (n = 3) the exclusions due to missing data at follow-up. A large number of studies (n = 11) did not provide any details about the exclusions due to missing data at follow-up, and this was not considered an appropriate quality assessment for one study. None of the studies gave a clear statement of the possible effects on the results of missing data; the majority of studies (n = 25) failed to provide this information and it was partly reported in a few studies (n = 3). Overall, in evaluating the study quality in terms of whether the loss to follow-up was associated with key characteristics (i.e. differences between key characteristics and outcomes in participants who completed the study and those who did not), sufficient to limit potential bias, only one study was considered adequate, 12 studies were partly satisfactory and 15 studies were unclear. In conclusion, the quality of the reporting of study attritionwas poor and many studies failed to adequately provide details about exclusions due to missing data at baseline and follow-up.
Prognostic factor measurement
A clear definition of the prognostic factors measured was provided (e.g. extraction method, measurement described) in the majority of studies (n = 18); six studies partly reported this information and four studies did not provide a clear definition of the prognostic factors measured. There was poor reporting of the material storage method used (n = 24), with only a small number of studies clearly (n = 3) or partly (n = 1) reporting this. The reporting of continuous variables or appropriate (i.e. not data dependent) cut-points was found in four studies and partly found in 15 studies. A few studies (n = 3) did not provide suitable information, in five studies it was unclear and in one it was not considered an appropriate quality assessment. Overall, the prognostic factors of interest were adequately measured in the majority of included studies to sufficiently limit potential bias in four studies and partly limit potential bias in 20 studies. Four studies did not adequately measure the prognostic factors. The section has clearly demonstrated that there was a lack of adequate reporting of the material storage methods used in a large proportion of the identified studies.
Outcome measurement
The majority of studies provided a clear (n = 23) or partly clear (n = 2) definition of the outcome. Only a small number of studies (n = 3) failed to adequately provide this information. Out of those studies that had an outcome of PSA recurrence (n = 15), there was no reporting of the internationally agreed definition of PSA recurrence (e.g. PSA > 0.2 ng/ml after prostatectomy) in nine, with only a small number of studies (n = 6) adequately meeting this quality assessment criteria. This was not considered an appropriate quality assessment for a large proportion of the included studies (n = 11) and for one study it was unsure (n = 2). In those studies that had an outcome of PSA recurrence, there was good reporting in one study and poor reporting in another of the internationally agreed definition of PSA recurrence [i.e. a rise by 2 ng/ml or more above the nadir PSA (2005) or three consecutive PSA rises above nadir (1997) after radiotherapy]. This was not considered an appropriate quality assessment for a large proportion of the included studies (n = 26). In those studies that had a biochemical outcome (PSA), a unique definition of failure was adequately used in 15 and partly used in one; two studies did not use a unique definition of failure and for three studies it was unsure. This was not considered an appropriate quality assessment for a proportion of the included studies (n = 7). Overall, the outcome of interest was considered to be adequately measured in study participants to sufficiently limit potential bias in 15 studies and partly in 11 studies. Only one study did not adequately satisfy this overall quality criterion and for another study it was unsure.
Confounding measurement and account
In quality assessing whether the statistical model included all classical markers (PSA, stage and grade, surgical margins if applicable) in an attempt to determine whether the important potential confounders are appropriately accounted for, sufficiently limiting potential bias with respect to the prognostic factor of interest, nine studies adequately met and 12 partly met the criteria. A further six studies did not include all of the classical markers and in one study it was unclear. There was good reporting of the possible confounding measures and how they were accounted for.
Analysis
In quality assessing the analysis of the included studies there were sufficient data presented to assess the adequacy of the analysis in 13 studies and to partly assess the adequacy of the analysis in another 13 studies. There were, however, two studies that failed to provide sufficient data to assess the adequacy of the analysis. The strategy for statistical analysis building (i.e. inclusion of variables) was considered appropriate and based on a conceptual framework or statistical analysis for the majority of studies (n = 23). There was some uncertainty in three of the studies and this was not considered an appropriate quality assessment in two studies. For a large proportion of the included studies the selected statistical analysis was considered adequate (n = 25) or partly adequate (n = 1) for the design of the study. For a few studies the selected statistical analysis was not considered adequate (n = 2). The number of events or EPV was adequately reported (n = 17) or partly reported (n = 2) in the majority of included studies. However, a large proportion failed to provide this information (n = 9). In terms of the actual number of EPV being reported, several studies adequately reported (n = 11) or partly reported (n = 5) this information; however, one study did not report this information, in nine studies it was unclear, and in two it was not considered an appropriate quality assessment. Overall, in considering whether the statistical analysis was appropriate for the design of the study, limiting the potential for the presentation of invalid results, 12 studies were considered appropriate, 13 were considered partly appropriate and only three studies were considered not appropriate.
Summary of overall quality assessment
This section has shown that the quality of the novel marker studies varied in terms of study population, study attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, and analysis.
Evaluation of prognostic markers identified
Because of the wealth of literature in this section we will first provide a summary of the key characteristics of the 28 included studies concerned with novel prognostic markers (Table 6).
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Median age (years) | 10 | 65.30 | 1.54 |
| Mean age (years) | 16 | 64.17 | 3.47 |
| Median follow-up (months) | 18 | 75.63 | 15.63 |
| Mean follow-up (months) | 9 | 70.06 | 9.93 |
| Mean length of study (years) | 27 | 11.67 | 6.08 |
| Clinically organ confined (%) | 27 | 81.64 | 31.22 |
| Clinically non-organ confined (%) | 27 | 18.29 | 31.22 |
| Pathologically organ confined (%) | 15 | 65.16 | 16.90 |
| Pathologically non-organ confined (%) | 15 | 34.03 | 17.35 |
| PSA level taken from median (ng/ml) | 9 | 7.19 | 1.75 |
| PSA level taken from mean (ng/ml) | 6 | 8.43 | 4.43 |
| Positive surgical margins (%) | 14 | 29.71 | 15.85 |
| Positive lymph nodes (%) | 14 | 4.89 | 3.89 |
The large majority of included studies used retrospective data; however, three studies112,129,132 appeared to use prospective data. The sample sizes ranged from 200 to 5509 men. The treatments used across the studies varied: RP alone (n = 19); radiotherapy alone (n = 5); either RP or TURP (n = 2); TURP alone (n = 1); and brachytherapy (n = 1). As the minimum mean or median follow-up period for inclusion in the study was 5 years, all studies adequately met this criterion; however, six studies did not provide a mean or median follow-up statistic, rather they stated that a minimum follow-up of 5 years was an inclusion criterion for their study or they provided only the range or minimum number of years of follow-up. Other more specific details concerning the study population (clinically organ confined, clinically non-organ confined, pathologically organ confined, pathologically non-organ confined, PSA level taken from median, PSA level taken from mean, positive surgical margins, positive lymph nodes) are provided in Table 6. It is important to note that not all studies reported this information.
Each study will now be discussed in relation to its respective novel prognostic marker category. Full data abstraction tables of the included studies for all novel prognostic markers are provided in Appendices 5 and 6.
β-Catenin expression
One study108 evaluated the prognostic value of preoperative serum β-catenin in men with localised prostate cancer.
Brief description of the prognostic marker
β-Catenin is an intracellular protein that is involved in intercellular adhesion at the cellular membrane and cell signalling in the nucleus. It has been implicated in prostate carcinogenesis primarily through modulation of androgen receptor activity. The loss of expression of membrane β-catenin has been associated with progression from benign to malignant prostate pathology. 133 The definition of the marker and its distribution in the population studied are shown in Table 7.
| Study | Definition | Population distribution |
|---|---|---|
| Horvath, 2005108 | β-Catenin is a ubiquitously expressed intracellular protein that has roles in both intercellular adhesion at the cellular membrane and cell signalling in the nucleus | Number of cases with β-catenin score < 10%: 83 (36%); number of cases with β-catenin score ≥ 10%: 149 (64%) |
| Detected using a mouse monoclonal antibody | ||
| Patients who had < 10% of cells expressing β-catenin in the nucleus were compared with those who had ≥ 10% of malignant cells demonstrating β-catenin expression |
Brief description of the objectives of the individual study identified
The primary aim of the identified study was to assess β-catenin as a prognostic marker in patients with localised prostate cancer treated with RP. Horvath et al. 108 chose to investigate β-catenin expression as it is thought to have a significant role as a signal transduction molecule in both in vitro and in vivo models of prostate cancer. They attempted to define the pattern of β-catenin protein expression in the nuclei of normal, hyperplastic and malignant human prostate tissue to evaluate whether differences in expression in patients with cancer were related to disease progression. The basic study design characteristics are summarised in Table 8.
| Study | n | Primary aim to assess prognostic marker | Treatment |
|---|---|---|---|
| Horvath, 2005108 | 232 | Yes | Radical prostatectomy |
Quality of the individual study identified
Although the statistical analysis in this study is appropriate and the multivariate model includes the recognised classical markers, a weakness of the study is that the cut-point for differentiating between high and low β-catenin levels was determined within the data. This means that the results are likely to be overoptimistic as the β-catenin variable has been optimised to the data. At a value of 10 EPV the model just meets the minimum criterion in the quality assessment. However, with most of the variables entered into the model as dichotomous rather than continuous variables, an EPV of 10 is low and may lead to unreliable results. The overall concluding questions for each of the six subheadings are presented in Table 9.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Horvath, 2005108 | p | ? | p | p | y | y |
Summary of the baseline characteristics of the sample
Horvath and colleagues used a sample of 232 participants who had had RP, 22% of whom also had some form of adjuvant therapy (hormone therapy, radiotherapy or orchidectomy). Participants all had clinically localised cancers and were pathological T1/T2 (47%) or T3/T4 (53%). The Gleason scores and PSA distributions appeared to be within the usual range. Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual study identified
Table 10 presents a summary of the main statistical findings from the single study included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Horvath, 2005108 | Univariate | Not applicable | Survival from biochemical relapse (PSA 0.4 ng/ml or greater over 3 months or local recurrence on DRE confirmed by biopsy or subsequent rise in PSA) | Estimated from survival curve; 5-year survival: β-catenin < 10%: 60%; ≥ 10% 78% | Cox proportional hazards; β-catenin < 10% with reference ≥ 10%: HR 1.9 (95% CI 1.2–3.0) | 0.008 (log-rank test from survival curve, p = 0.007) |
| Multivariate | Clinical PSA, pathological stage, Gleason score, surgical margins (also seminal vesicle involvement, adjuvant treatment) | Survival from biochemical relapse (PSA 0.4 ng/ml or greater over 3 months or local recurrence on DRE confirmed by biopsy or subsequent rise in PSA) | Not applicable | Cox proportional hazards; β-catenin < 10% with reference ≥ 10%: HR 1.4 (95% CI 0.8–2.3) | 0.2 |
In a Cox univariate analysis β-catenin was found to be significantly prognostic for biochemical recurrence (p = 0.008. However, in a Cox multivariate analysis including the classical markers it was not (HR 1.4, 95% CI 0.8–2.3, p = 0.2).
Overall conclusions based on the results and quality of the findings
The results of this study indicate that, although β-catenin may be prognostic for biochemical recurrence following RP, its association with the existing widely used PSA marker means that it would not provide additional prognostic information. In addition, the quality issues raised above mean that the results are inconclusive.
Acid phosphatase
Five studies109–113 were identified that were concerned with the prognostic value of preoperative serum acid phosphatase (ACP) in men with localised prostate cancer following radical RP or other treatment methods.
Brief description of the prognostic marker
Prostatic acid phosphatase (PAP) is an enzyme produced by the prostate. Serum ACP was used as a marker for prostate cancer before the 1980s. 134 However, with the development of assays for PSA, the use of ACP has diminished. The measurement methods, definitions and distributions of the marker in the populations studied are compared in Table 11.
| Study | Definition | Population distribution |
|---|---|---|
| Anscher, 1991109 | Elevated preoperative ACP defined as > 5.4 IU/l | Normal (≤ 5.4 IU/l) = 212; elevated (> 5.4 IU/l) = 47 |
| Han, 2001110 | ACP level was measured using an enzymatic assay with sodium thymolphthalein monophosphatase as a substrate (Roy assay), which is more specific for prostatic ACP. Normal range in this assay for men without prostatic disease is between 0 and 0.8 U/l | < 0.4 = 996 (59.2%); 0.4–0.5 = 573 (34.1%); > 0.5 = 112 (6.7%); total = 1681 (100%) |
| Perez, 1989111 | Not stated | Normal = 241 (73.5%); abnormal = 87 (26.5%) |
| Roach, 1999112 | Not stated | Serum acid phosphatase: not elevated = 1107 (71%); elevated = 389 (25%); unknown = 61 (4%) |
| Zagars, 1993113 | Serum PAP level was determined in 838 cases (96%) with either the Bessie-Lowrie (103 cases) or Roy (735 cases) method. Only results obtained from the Roy method presented. Upper limit for normal range was 0.8 U/l | Normal PAP = 682 (92.8%); elevated PAP = 53 (7.2%) |
Note that the proportion of patients in the elevated PAP groups, however defined, is relatively small, varying from 6.7%110 to 25%. 112 With the exception of Han et al. ,110 all studies used a binary measure for ACP, sometimes resulting in a relatively small number of patients in the elevated group (e.g. n = 47109), and probably a small number of outcome events, making the results of the analyses less reliable.
Brief description of the objectives of the individual studies identified
Only three of the studies109–111 had a primary aim of assessing ACP as a prognostic marker. The aims of these studies were to: (1) identify those patients at most risk for local failure;109 (2) investigate the prognostic value of preoperative serum ACP in men with localised prostate cancer following radical retropubic prostatectomy;110 and (3) identify prognostic factors for prostate cancer treated by external beam radiation. 111 Of the other studies, one112 was concerned with long-term survival in patients treated with radiotherapy and one,113 although concerned with prognostic factors in prostate cancer, did not specifically investigate ACP. The basic study design characteristics are summarised in Table 12.
Quality of the individual studies identified
The five studies varied in quality. The overall concluding questions for each of the six subheadings are presented in Table 13. The study considered to be of the highest quality for this novel prognostic marker was conducted by Han et al. 110 This was the most recent study involving ACP. Most of the other studies,109,111,113 being older, do not report PSA measurements and do not have this measurement available to enter as a covariate in multivariate models. Some also omit grade109,113 or stage. 111 The only study to report a multivariate analysis including all classical markers was that of Han et al. 110 Some of the models also have a low number of events, for example that of Anscher et al. 109 has only six. Perez et al. 111 did not state the number of events but with a patient sample of 328 and 12 variables in their model the EPV is likely to be low.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Anscher, 1991109 | p | ? | p | y | p | p |
| Han, 2001110 | y | p | p | y | y | p |
| Perez, 1989111 | p | p | n | y | p | ? |
| Roach, 1999112 | p | ? | n | y | p | y |
| Zagars, 1993113 | p | ? | y | y | p | y |
Summary of the baseline characteristics of the sample
In only two studies109,110 did most patients (> 95%) have clinically organ-confined disease. In these two studies patients were treated with surgery. The other studies111–113 are all atypical of the majority of studies in this review in that most of the patients did not have organ-confined tumours; in one study all patients had extraprostatic disease. 111 Two studies111,112 report relatively high proportions of patients with high-grade tumours (31% and 28% respectively), whereas one113 does not report grade. In all three studies with high proportions of patients with non-organ-confined disease, patients were treated with radiotherapy. Additional summary characteristics are provided in Appendix 7.
Brief description of the results from individual studies identified
Table 14 presents a summary of the main statistical findings from the five studies included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Events | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|---|
| Anscher, 1991109 | Univariate | Not applicable | Local relapse rate (local failure confirmed by biopsy, with or without distant metastases) | Elevated ACP (> 5.4 IU/l): 12/47 (26%); normal ACP (≤ 5.4 IU/l): 30/212 (14%) | Not reported | HR not reported | 0.06 |
| Multivariate | Clinical stage, poor differentiation, surgical margins (also age, type of biopsy, hormonal therapy given, seminal vesicles involved) | Local relapse rate (local failure confirmed by biopsy, with or without distant metastases), median follow-up 66 months | Elevated ACP (> 5.4 IU/l): 12/47 (26%); normal ACP (≤ 5.4 IU/l): 30/212 (14%) | Not applicable | HR not reported | 0.0273 | |
| Univariate | Not applicable | Distant metastases | Not reported | Not reported | HR not reported | Not significant | |
| Multivariate | Clinical stage, poor differentiation, surgical margins (also age, type of biopsy, hormonal therapy given, seminal vesicles involved) | Distant metastases | Not reported | Not applicable | HR not reported | Not significant | |
| Han, 2001110 | Univariate | Not applicable | Biochemical (PSA) recurrence (PSA > 0.2 ng/ml) | Not reported | 5-year survival: ACP < 0.4 U/l: 87% (from n = 996); ACP 0.4–0.5 U/l: 79% (from n = 573); ACP > 0.5 U/l: 63% (from n = 112) | HR not reported | Not reported |
| 10-year survival: ACP < 0.4 U/l: 77%; ACP 0.4–0.5 U/l: 65%; ACP > 0.5 U/l: 44%. | |||||||
| Multivariate | Clinical PSA, stage, Gleason (also age) | Biochemical (PSA) recurrence (PSA > 0.2 ng/ml) | Not reported | Not applicable | Normalised HR (per 1 standard deviation change in predictor variable): 1.22 (SE 0.03) | < 0.001 | |
| Perez, 1989111 | Univariate | Not applicable | Overall survival (events – death from any cause) | Not reported | 5-year survival: ACP normal: 64% (from n = 241); ACP abnormal: 64% (from n = 87) | Not reported | Not reported |
| Univariate | Not applicable | Disease-free survival (events – any tumour progression, local or distant) | Not reported | 5-year survival: ACP normal: 52% (from n = 241); ACP abnormal: 45% (from n = 87) | Not reported | Not reported | |
| Multivariate | Histological grade (well, moderate, poor) (also age, race, positive or negative lymphadenectomy, type of biopsy, hormonal status, dose of irradiation) | Overall survival (events – death from any cause) | Not reported | Not applicable | Not reported | 0.76 | |
| Multivariate | Clinical histological grade (well, moderate, poor) (also age, race, positive or negative lymphadenectomy, type of biopsy, hormonal status, dose of irradiation) | Disease-free survival (events – any tumour progression, local or distant) | Not reported | Not applicable | Not reported | 0.23 | |
| Roach, 1999112 | Univariate | Not applicable | Overall survival (events – death from any cause) | Not reported | Not reported | ACP elevated vs not elevated: risk ratio 1.277 | 0.004 |
| Univariate | Not applicable | Survival from prostate cancer death (events – prostate cancer death only) | Not reported | Not reported | ACP elevated vs not elevated: risk ratio 1.717 | 0.0001 | |
| Multivariate | Clinical stage + nodal status, pathological; Gleason grade (also race, age) | Overall survival (events – death from any cause) | Not reported | Not applicable | ACP elevated vs not elevated: risk ratio not reported | Not significant | |
| Multivariate | Clinical stage + nodal status, pathological; Gleason grade (also race, age) | Survival from prostate cancer death (events – prostate cancer death only) | Not reported | Not applicable | ACP elevated vs not elevated: risk ratio 1.294 | 0.037 | |
| Zagars, 1993113 | Univariate | Not applicable | Survival from local recurrence | Total 142 | 5-year survival: PAP normal: 88% (from n = 682); PAP elevated: 86% (from n = 53) | Not reported | 0.442 (log-rank) |
| 10-year survival: PAP normal: 76%; PAP elevated: 74% | |||||||
| Univariate | Not applicable | Survival from metastatic failure | Total 263 | 5-year survival: PAP normal: 78% (from n = 682); PAP elevated: 47% (from n = 53) | Not reported | < 0.001 (log-rank) | |
| 10-year survival: PAP normal: 66%; PAP elevated: 37% | |||||||
| Univariate | Not applicable | Disease-free survival (events – first relapse, whether it is local, nodal or metastatic) | Total 348 | 5-year survival: PAP normal: 70% (from n = 682); PAP elevated: 41% (from n = 53) | Not reported | < 0.001 (log-rank) | |
| 10-year survival: PAP normal: 51%; PAP elevated: 22% | |||||||
| Univariate | Not applicable | Overall survival (events – death from any cause) | Not reported | 5-year survival: PAP normal: 80% (from n = 682); PAP elevated: 70% (from n = 53) | Not reported | 0.059 (log-rank) | |
| 10-year survival: PAP normal: 51%; PAP elevated: 49% | |||||||
| Multivariate | Pathological stage, pathological MD Anderson grade (age, TURP vs no TURP in stage C) | Survival from local recurrence | Total 142 | Not applicable | Not reported | Not significant | |
| Multivariate | Pathological stage, pathological MD Anderson grade (age, TURP vs no TURP in stage C) | Survival from metastatic failure | Total 263 | Not applicable | Not reported | < 0.0016 | |
| Multivariate | Pathological stage, pathological MD Anderson grade (age, TURP vs no TURP in stage C) | Disease-free survival (events – first relapse, whether it is local, nodal or metastatic) | Total 348 | Not applicable | Not reported | 0.005 | |
| Multivariate | Pathological stage, pathological MD Anderson grade (age, TURP vs no TURP in stage C) | Overall survival (events – death from any cause) | Not reported | Not applicable | Not reported | Not significant |
Most of the univariate analyses on ACP level as a prognostic marker found it to be significantly associated with outcome (local recurrence,109 survival from metastatic failure and disease-free survival112,113), and some found it to be highly so (prostate cancer survival, p = 0.0001;112 survival from metastatic failure and disease-free survival, both p < 0.001113). All of these last three analyses have a large number of outcome events. In three univariate analyses, ACP failed to reach significance at the 95% confidence level (metastases,109 local recurrence and any death113). These analyses include patients treated both with RP and with radiotherapy.
None of the multivariate analyses for which the outcome was survival from all causes of death showed ACP to be a statistically significant marker of outcome,111–113 but, as many patients will die from causes other than prostate cancer, the outcome is not highly sensitive to prostate cancer-specific markers. In the study by Zagars et al. ,113 ACP was also not found to be significant in the multivariate analysis with an outcome of local recurrence.
In the other multivariate analyses with prostate cancer-specific outcome events – biochemical recurrence110 or local or distant failure109,111,113 or prostate cancer death112 – ACP was shown to be a significant prognostic marker in all with the exception of that of Perez et al. 111 (p = 0.23). This analysis may be statistically weak. Although the EPV is not reported the number of patients (n = 328) and the number of variables in the model (n = 12) suggest that it may be low. This may also be a problem with one of the studies that found a positive result109 (EPV = 6), and although the EPV is large in the study by Zagars et al. 113 the number of events in the elevated ACP group is likely to be very small as only 43 of 357 cases were in this category. It should also be noted that only one of these studies included all of the classical markers in the model110 and so the prognostic value of ACP in addition to that of the classical markers has only been demonstrated in one study. In this study ACP was found to be a highly significant marker (p < 0.001) for biochemical recurrence in patients who had RP.
Overall conclusions based on the results and quality of the findings
The studies for this marker are particularly heterogeneous, with two109,110 of the five studies based on patients with organ-confined tumours and the rest with all, or the majority of, patients with non-organ-confined tumours. In the former studies patients were treated with surgery, whereas in the latter patients were treated with radiotherapy. However, the results do not appear to be dependent on these factors. In the multivariate analyses four of five analyses that had prostate cancer-specific outcomes found ACP to be a statistically significant marker. However, only one of these analyses110 included all of the classical markers in the multivariate model. Although the number of events for this analysis was not stated, the large sample size and the fact that ACP was entered in the model as a continuous variable suggest that the study was statistically well powered. Thus, although the direction of evidence from several studies suggests that ACP is prognostic of prostate cancer outcomes, there is only one study that shows that it is prognostic independently of the established markers.
Androgen receptor: CAG repeats
Two studies114,115 were concerned with androgen receptor CAG repeats.
Brief description of the prognostic marker
Androgen function is mediated by the androgen receptor, which is a ligand-dependent steroid hormone transactivation factor located on the X chromosome. 115 Nam et al. 114 hypothesised that CAG repeats may be associated with prognosis as it has been shown in other studies that men with ≤ 18 CAG repeats have an increased risk for developing prostate cancer compared with men with a longer CAG sequence and also have a 2.1-fold increased risk for developing advanced-stage or high-grade prostate cancer. 135 The measurement methods, definitions and distributions of the marker in the populations studied are compared in Table 15.
| Study | Definition | Population distribution |
|---|---|---|
| Nam, 2000114 | Examined as both a continuous and a categorical variable. The number of CAG repeats was categorised dichotomously as: (1) ≤ 18 repeats; and (2) > 18 repeats | ≤ 18 repeats: n = 39 (12.3%); > 18 repeats:n = 279 (87.7%) |
| Powell, 2005115 | The number of repeats in the exon 1 CAG microsatellite of the androgen receptor gene was determined using polymerase chain reaction analysis. Stratification of CAG results was made: (1) ≤ 18 repeats; (2) 19–22 repeats; and (3) ≥ 22 repeats. Also, to enable a comparison to be made with the study by Nam114 the authors also used: (1) ≤ 18 CAG repeats; and (2) > 18 repeats | Not stated |
Note that the proportion of patients with ≤ 18 CAG repeats in the study by Nam et al. 114 is relatively small (n = 39). In the study by Powell et al. 115 the distribution of the marker according to the groups used in the analysis is not stated, but if the three groups are of similar size this should not be a problem as there are 711 patients in total.
Brief description of the objectives of the individual studies identified
Both studies had the primary aim of assessing the prognostic marker. Nam et al. 114 examined the significance of the CAG repeat polymorphism of the androgen receptor gene for predicting biochemical progression among patients treated by RP for clinically localised prostate cancer. The hypothesis was that a high level of androgen receptor activity associated with short CAG repeats may be important in prostate cancer progression. Powell et al. 115 also examined the impact of the number of CAG repeats in the androgen receptor on disease progression (not defined) among men with prostate carcinoma following prostatectomy. The basic study design characteristics are summarised in Table 16.
Quality of the individual studies identified
A summary of the quality assessment for the studies is shown in Table 17. Both studies were of reasonable quality. However, in the study by Nam et al. 114 there are only a small number of patients with ≤ 18 CAG repeats. This weakens their analysis and is a particular issue in the model in which CAG repeats is used as a binary variable. In the study by Powell et al. 115 it is not clear exactly what the end point is: biochemical recurrence or biochemical or clinical recurrence.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Nam, 2000114 | y | p | p | y | y | y |
| Powell, 2005115 | y | ? | p | p | y | p |
Summary of the baseline characteristics of the sample
The patient populations appear similar with all of the patients having clinically localised cancers, just over 40% of patients having pathologically organ-confined tumours, and around 14% having high-grade tumours (Gleason score 8–10), although for Powell et al. 115 the Gleason score is pathological rather than clinical. In both studies patients were treated with RP. Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual studies identified
Table 18 presents a summary of the main statistical findings from the two studies included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| aNam, 2000114 | Multivariate | Clinical PSA, Gleason grade, stage | Biochemical recurrence-free survival (PSA ≥ 0.2 ng/ml on two consecutive measurements at least 3 months apart; date of recurrence was time of initial increase) | Not applicable | Adjusted relative risk for ≤ 18 repeats (with reference > 18 repeats) = 0.93 (95% CI 0.5–1.8) | Categorical: p = 0.83; continuous variable: p = 0.79 |
| When analysed as a continuous variable, relative risk = 1.01 (95% CI 0.9–1.1) | ||||||
| Powell, 2005115 | Multivariate | Clinical PSA, Gleason grade, stage (also race and age) | Biochemical recurrence-free survival (PSA level > 0.4 ng/ml, which persisted for more than one reading) | Not applicable | HR of recurrence > 18 CAG repeats (with reference ≤ 18 repeats) = 1.52 (95% CI 1.03–2.23) | > 18 CAG repeats (with reference ≤ 18 repeats): p = 0.03; one-category increase: p = 0.32 |
| HR for a one-category increase in CAG repeats (≤ 18 repeats; 19–22 repeats; and ≥ 22 repeats) = 1.11 (95% CI 0.90–1.38) |
In the univariate analysis, Nam et al. 114 did not find the number of CAG repeats to be prognostic for biochemical recurrence-free survival (p = 0.80). Both studies present multivariate analyses. Both include the classical markers of PSA, Gleason grade and stage. Both studies also present two analyses, with the number of CAG repeats entered into the models in a different form. Nam et al. 114 entered CAG repeats as a dichotomous variable and as a continuous variable. In neither analysis was it a significant predictor of outcome. Powell et al. 115 used the same two categories as Nam et al. 114 for CAG repeats but with the opposite category entered as the baseline. Thus, the direction of the risk reduction is actually the same as for Nam et al. :114 those with ≤ 18 CAG repeats are at lower risk for disease recurrence and this result was statistically significant at the 95% confidence level (p = 0.03). The fact that this result was significant, whereas that for Nam et al. 114 was not, may be due to the larger sample size. The results of the other analysis by Powell et al. ,115 which examined the increase in risk for each category of CAG repeats (≤ 18, 19 22 and ≥ 22), were not significant (p = 0.32). This analysis may be considered less reliable as it treats three categories of the CAG repeat variable as a continuous variable in the analysis.
Overall conclusions based on the results and quality of the findings
Although otherwise of reasonable quality, the results of the study by Nam et al. 114 might be considered less reliable because of the small number of patients with short CAG repeats (≤ 18 CAG repeats). In the study by Powell et al. 115 with a larger patient sample, and possibly a larger proportion in the group with ≤ 18 repeats, an analysis with the number of CAG repeats entered as a binary variable did show a significant association between this marker and disease progression. Another analysis by Powell et al. in which the marker was entered in a different format did not show a significant association but this may be less reliable. The results are inconclusive as to whether the number of CAG repeats is prognostic of prostate cancer outcome.
Creatinine
Two studies116,117 were concerned with assessing serum creatinine as a putative marker for prognosis in localised prostate cancer.
Brief description of the prognostic marker
Creatinine is a by-product of muscle metabolism. It is widely used to measure kidney function. It was hypothesised by Merseburger116 that in localised disease creatinine could be associated with good prognosis as a high proportion of low-volume cancers are in enlarged glands, which may be associated with renal insufficiency and creatinine elevation. The definitions and distributions of the marker in the populations studied are shown in Table 19.
| Study | Definition | Population distribution |
|---|---|---|
| Merseburger, 2001116 | Creatinine is a metabolic by-product of muscle metabolism. Levels were determined within 6 months before surgery. Creatinine was entered into the statistical model as a continuous variable and was also stratified into 0.7–1.0 mg/dl, 1.1–1.3 mg/dl and 1.4–2.3 mg/dl creatinine | 0.7–1.0 mg/dl: n = 87; 1.1–1.3 mg/dl: n = 280; 1.4–2.3 mg/dl: n = 42 |
| Range 0.1–2.3 mg/dl (mean and median 1.1 mg/dl) | ||
| Zagars, 1987117 | Creatinine level divided into ≤ 1.5 mg/dl, > 1.5 mg/dl | Creatinine: ≤ 1.5 mg/dl: n = 455; > 1.5 mg/dl: n = 28 |
Note that in both studies the proportion of patients with a high level of creatinine (> 1.3 mg/dl,116 > 1.5 mg/dl117) is relatively small. This is an issue, particularly in the analyses carried out by Zagars et al. 117 and in a univariate analysis by Merseburger et al. ,116 in which patients are grouped according to their level of creatinine, with only a very small number of patients in the elevated creatinine group.
Brief description of the objectives of the individual studies identified
Only the study by Merseburger116 had a primary aim of assessing this prognostic marker. Merseburger116 investigated the ability of creatinine to predict PSA recurrence using Cox regression analysis. Zagars et al. 117 studied outcomes for patients with stage C cancer. The basic study design characteristics are summarised in Table 20.
Quality of the individual studies identified
The two included studies varied in quality (Table 21). Zagars et al. 117 did not conduct a multivariate analysis but rather compared survival curves for patients with normal and elevated creatinine. There were only 28 patients in the elevated creatinine group and so the number of events is likely to be very low. Merseburger116 did undertake multivariate analysis that included several covariates including Gleason grade, PSA and stage. It did not, however, include surgical margins. The multivariate model was not fully presented and it is not entirely clear exactly which covariates were included in the model; therefore, although there are a reasonable number of outcome events (n = 130) the EPV may be below 10.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Merseburger, 2001116 | y | ? | p | y | p | p |
| Zagars, 1987117 | p | p | p | y | n | p |
Summary of the baseline characteristics of the sample
The clinical stage of the participants was very different in the two studies. Merseburger116 used a sample that was almost entirely clinically organ confined, whereas the participants in the Zagars et al. 117 study were all stage C or non-organ confined. We were unable to compare the participants according to Gleason score or PSA level as these were not reported by Zagars et al. 117 The patients in the Merseburger116 study were treated with surgery where those in the Zagars et al. 117 study were treated with radiotherapy. Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual studies identified
Table 22 presents a summary of the main statistical findings from the two studies included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Merseburger, 2001116 | Univariate | Clinical Gleason grade, PSA, stage (also age, weight, prostate weight, history of prostatism, treatment of BPH) | Biochemical recurrence (two successive PSA measurements > 0.2 ng/ml) | Unclear: stratified into 0.7–1.0 mg/dl, 1.1–1.3 mg/dl and 1.4–2.3 mg/dl creatinine; survival curve indicates just under 80% for all three groups | Log-rank, stratified into 0.7–1.0 mg/dl, 1.1–1.3 mg/dl and 1.4–2.3 mg/dl creatinine | 0.845 |
| Multivariate | Clinical Gleason grade, PSA, stage (also age, weight, prostate weight, history of prostatism, treatment of BPH) | Biochemical recurrence (two successive PSA measurements > 0.2 ng/ml) | Not applicable | Analysed as continuous variable by Cox regression | Not significant | |
| Zagars, 1987117 | Univariate | Not applicable | Overall survival (events – death from any cause) | 5-year survival: creatinine ≤ 1.5 ng/ml: 75% (from n = 455); creatinine > 1.5 ng/ml: 67% (from n = 28) | Not reported | 0.32 |
| 10-year survival: creatinine ≤ 1.5 ng/ml: 45%; creatinine > 1.5 ng/ml: 39% | ||||||
| Univariate | Not applicable | Disease-free survival (events – any relapse – censored at death) | 5-year survival: creatinine ≤ 1.5 ng/ml: 61% (from n = 455); creatinine > 1.5 ng/ml: 44% (from n = 28) | Not reported | 0.05 | |
| 10-year survival: creatinine ≤ 1.5 ng/ml: 47%; creatinine > 1.5 ng/ml: 30% |
Zagars et al. 117 conducted three univariate analyses using the log-rank statistic to compare survival curves with three different end points: all deaths, any disease relapse and local control. As previously discussed there were only a small number of patients in the elevated creatinine group (n = 28) and so the results may be unreliable. Of these three analyses only one, that with any disease relapse as the outcome measure, showed a statistically significant association between elevated creatinine and outcome (p = 0.05).
Merseburger116 also reported a log-rank analysis to compare survival by creatinine stratified into three groups. The curves were not statistically significantly different (p = 0.845). Again, there were only a small number of patients in the elevated creatinine group (n = 42). In the multivariate analysis with creatinine entered into the analysis as a continuous variable with several other covariates including PSA, Gleason grade and stage, Merseburger116 found no significant effect of creatinine on PSA recurrence (p-value not stated). The analysis may be statistically weak with a low EPV.
Overall conclusions based on the results and quality of the findings
These two studies were carried out on different patient groups (organ confined and non-organ confined) and patients had different treatments. The results of neither study indicate that creatinine is a useful prognostic marker for prostate cancer. However, the results cannot be considered conclusive as both studies had statistical weaknesses.
CYP3A4 genotypes
One study118 was concerned with the impact of CYP3A4 on the risk of biochemical recurrence after prostatectomy.
Brief description of the prognostic marker
Cytochrome P450 3A4 (CYP3A4) is a member of the cytochrome P450 supergene group. It is thought to be involved in the oxidative deactivation of testosterone to biologically less active metabolites. Testosterone is a major contributor to prostate cancer progression. A germline genetic variant in the 5′ regulatory region of the CYP3A4 gene (A to G transition) on chromosome 7 has been reported and named as CYP3A4*1B (otherwise known in the literature as –392A > G and CYP3A4-V). This CYP3A4 genetic variant was the prognostic factor of consideration in this section. The definition and distribution of the marker in the population studied are shown in Table 23.
| Study | Definition | Population distribution |
|---|---|---|
| Powell, 2004118 |
Germline genetic variant in the 5′ regulatory region of the CYP3A4 gene (A to G transition) on chromosome 7 |
The distribution of AA alleles [92% white men (WM), 17% African American men (AAM)], AG alleles (7% WM, 39% AAM) and GG alleles (1% WM, 43% AAM) was associated with race (p = 0.00002) |
| Used two methods to genotype the individual DNA samples: (1) Ampliflour single nucleotide polymorphism genotyping system; and (2) a second assay primer extension using high-performance liquid chromatography | The progression-free survival for all men of all races was: AA alleles, n = 446; AG alleles, n = 153; and GG alleles, n = 138 | |
| DNA was isolated using the QIAamp Tissue Kit using a modification of the procedure recommended by the manufacturer |
Brief description of the objectives of the individual study identified
The primary aim of this study was to assess CYP3A4 genotypes as prognostic markers. The study examined the survival of men with localised prostate cancer who had undergone RP to evaluate whether CYP3A4*1B was associated with disease progression and whether it was independently prognostic of outcome. The basic study design characteristics are summarised in Table 24.
| Study | n | Primary aim to assess prognostic marker | Treatment |
|---|---|---|---|
| Powell, 2004118 | 737 | Yes | Radical prostatectomy |
Quality of the individual study identified
An important quality item that needs to be considered in the interpretation of the study results is that the number of EPV is unknown. In common with many studies there was poor reporting of the effects of missing data on the results, the authors did not use the internationally agreed definitions of PSA recurrence after prostatectomy and the methods of storage of materials were not reported. Generally the study was of adequate quality. The overall concluding questions to each of the six subheadings are presented in Table 25.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Powell, 2004118 | y | ? | p | p | y | p |
Summary of the baseline characteristics of the sample
Powell and colleagues used a sample of 737 participants in the analysis, all treated with RP. Participants were all clinical stages T1/T2. Pathologically, 50% of the white men (WM) and 37% of the African American men (AAM) had organ-confined tumours. More of the AAM than the WM had high-grade (Gleason score 8–10) tumours (17% and 13% respectively) and fewer had low (≤ 10 ng/ml) preoperative PSA levels (WM 67%; AAM 57%). Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual study identified
The association between CYP3A4 genotypes and biochemical progression was examined using a multivariate Cox proportional hazards model that included the classical prognostic markers. Although a model including both WM and AAM is presented, the authors argue that the strong association between CYP3A4 genotype and race means that race-stratified models should be used to avoid co-linearity. These are also presented. Table 26 presents a summary of the main statistical findings from this study.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Powell, 2004118 | Multivariate | Not applicable | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | 5-year survival: AA alleles: 76%; AG alleles: 65%; GG alleles: 58% | Not reported | Not reported |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; all men AG (reference AA): HR 1.45 (1.03–2.04) |
0.03 | |
| Multivariate | Not applicable | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; all men GG (reference AA): HR 1.58 (1.12–2.23) |
0.01 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; all men Copies of G allele (0, 1, 2): HR 1.27 (1.08–1.50) |
0.0049 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; all men AG plus GG (reference AA): HR 1.51 (1.14–2.00) |
0.004 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; all men GG (reference AA plus AG): HR 1.41 (1.02–1.96) |
0.04 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; WM men AG (reference AA): HR 2.1 (0.95–4.64) |
0.068 | |
| Multivariate | Not applicable | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; WM men GG (reference AA): HR 3.29 (0.45–24.36) |
0.24 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; WM men Copies of G allele (0, 1, 2): HR 1.98 (1.06–3.70) |
0.033 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; WM men AG plus GG (reference AA): HR 2.2 (1.04–4.65) |
0.04 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; WM men GG (reference AA plus AG): HR 3.07 (0.42–22.61) |
0.27 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; AAM men AG (reference AA): HR 0.87 (0.49–1.54) |
0.64 | |
| Multivariate | Not applicable | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; AAM men GG (reference AA): HR 0.96 (0.55–1.68) |
0.88 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; AAM men Copies of G allele (0, 1, 2): HR 1.004 (0.77–1.32) |
0.97 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; AAM men AG plus GG (reference AA): HR 0.92 (0.54–1.55) |
0.75 | |
| Multivariate | Clinical PSA, pathological stage, Gleason (also age) | Survival from progression (events – first recurrence; censored at last follow-up if no recurrence) | Not applicable |
Cox proportional hazards; AAM men GG (reference AA plus AG): HR 1.06 (0.72–1.55) |
0.78 |
Powell et al. 118 report several analyses that look at the effect of the G alleles in different ways. The analyses including all men showed a significant association between the CYP3A4*1B genotype and progression-free survival, with the most statistically significant result obtained with the number of copies of G allele (p = 0.0049). The presentation of race-stratified results is justified by the author by the strong association found between the AA, AG and GG alleles and race (p = 0.00002). They suggest that the G allele was not associated with biochemical progression-free survival in AAM. In WM some of the associations were of marginal significance at the 95% confidence level: the number of copies of the G allele in a dose model (p = 0.03) and the comparison of men with the AA genotype versus men with AG and GG (p = 0.04).
Overall conclusions based on the results and quality of the findings
This single study presents some evidence in support of CYP3A4 genotype as a prognostic marker in localised prostate cancer. The CYP3A4 variant was shown to be significantly more prevalent among AAM but was not prognostic in this group.
DNA ploidy
Three studies105,106,119 were included concerning the prognostic value of DNA ploidy in localised prostate cancer. It should also be noted that two other studies136,137 included DNA ploidy in their analyses and met the review inclusion criteria. However, it appeared highly likely that the study by Amling et al. 136 was based on a subset of the same data as that used by Siddiqui et al. 119 and Blute et al. ,105 and the study by Montgomery et al. 137 was based on similar data to that of Lieber et al. ,106 and so they were omitted from the review. All of the excluded studies were older than the included studies and they contained fewer data, were of poorer quality in general and did not add any additional prognostic information to that reported by the later studies. Although it is also likely that the data used by Blute et al. 105 (Mayo Clinic January 1990–December 1993) were a subset of that used by Siddiqui et al. 119 (Mayo Clinic 1987–1995), they were retained as there were some differences in the analyses.
Brief description of the prognostic marker
DNA ploidy is a test to measure the DNA content within tumour cells. The definitions and distributions of the marker in the populations studied are shown in Table 27.
| Study | Definition | Population distribution |
|---|---|---|
| Blute, 2001105 | Classified as diploid, tetraploid and aneuploid using a technique developed by Winkler et al.138 | Diploid: 1935 (77%); tetraploid: 451 (18%); aneuploid: 132 (5%) |
| Lieber, 1995106 | Authors state that they assigned tumours as DNA diploid, tetraploid and aneuploid in a uniform manner as described in previous publications. Used DNA ploidy analysis techniques developed by Hedley et al.170 Tumours that had > 13% of nuclei in the 2G or 4C peak were DNA tetraploid. Tumours with a clearly abnormal third peak that was neither 2C or 4C were considered DNA aneuploid | Diploid: 283; tetraploid: 181; aneuploid: 30 |
| Siddiqui, 2006119 | DNA ploidy was assessed by flow cytometry.139 Classified as diploid, tetraploid and aneuploid | Diploid: 3720 (71.6%); tetraploid: 1141 (22%); aneuploid: 332 (6.4%) |
Brief description of the objectives of the individual studies identified
The study by Lieber and colleagues106 had the primary objective of investigating whether measurement of DNA ploidy provided additional unique prognostic information beyond the customary parameters of tumour stage and grade for patients with prostate cancer. Blute and colleagues105 were interested in predicting biochemical failure following prostatectomy, and the main aim of the study by Siddiqui and colleagues119 was to assess whether age at treatment was a predictor of survival following prostatectomy. The basic study design characteristics are summarised in Table 28.
Quality of the individual studies identified
The principal limitation of all of these studies is that an absolute measure of PSA is not included in any of the multivariate models, thus limiting the conclusions that can be reached regarding the prognostic value of DNA ploidy in the presence of established markers. The Lieber et al. study106 pre-dates routine PSA measurement, but it is not clear why it was omitted from the models of Blute et al. 105 and Siddiqui et al. 119 The Blute et al. 105 model does, however, include a measure of PSA doubling. Two of the studies105,119 have a very large number of participants and therefore should give good statistical power, although the number of outcome events is not reported by Siddiqui et al. 119 The Lieber et al. study106 is smaller than the other two studies but reports an adequate number of events, and, in a rare example of good practice, also reports the number of patients and events in each marker category. Thus, we know that 283, 181 and 30 patients had diploid, tetraploid and aneuploid tumours respectively, with 60, 90 and 24 respectively experiencing disease progression.
A major drawback of the Siddiqui et al. study is that it is not clear in what form ploidy is entered into the statistical analysis (i.e. diploid/non-diploid), which means that the results are difficult to interpret. The overall concluding questions to each of the six subheadings are presented in Table 29.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Blute, 2001105 | p | p | p | p | p | y |
| Lieber, 1995106 | y | p | p | y | p | y |
| Siddiqui, 2006119 | y | ? | y | p | p | y |
Summary of the baseline characteristics of the sample
In all three studies patients had been treated with RP. However, the clinical stage of the patients in the Lieber et al. study106 was more advanced, with only 52% having organ-confined tumours compared with around 90% for those in the Blute et al. 105 and Siddiqui et al. 119 studies. The proportion of patients with pathologically high-grade cancers was not dissimilar across the studies, ranging from 4%105 to 9%. 106 Additional summary characteristics are provided in Appendix 7.
Brief description of the results from individual studies identified
Table 30 presents a summary of the main statistical findings from the three studies included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Events | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|---|
| Blute, 2001105 | Univariate | Not applicable | Survival from progression (events – local recurrence or systemic progression or biochemical recurrence defined as PSA 0.4 ng/ml or greater) | Not reported | 5-year survival: diploid 81% (SE 0.9); tetraploid 67% (SE 2.3); aneuploid 60% (SE4.4) | Not reported | < 0.001 |
| Multivariate | Pathological Gleason grade, PSA doubling, surgical margins (factors used to define pathological stage including seminal vesicle involvement and extraprostatic extension, adjuvant hormonal or radiation therapy) | Survival from progression (events – local recurrence or systemic progression or biochemical recurrence defined as PSA 0.4 ng/ml or greater) | Not reported | Not applicable | Estimated risk ratio: tetraploid vs diploid: 1.24 (95% CI 1.00–1.53); aneuploid vs diploid: 1.43 (95% CI 1.03–2.00) | Tetraploid vs diploid: p = 0.05; aneuploid vs diploid: p = 0.04 | |
| Lieber, 1997106 | Univariate | Not applicable | Survival from progression [events – disease progression based on clinical examination (not routine PSA measurements), censoring at last follow-up for patients who had not had progression or who had died] | Diploid 60; tetraploid 90; aneuploid 24 | 10-year survival: diploid 82%; tetraploid 49%; aneuploid 24% | HR: tetraploid with reference diploid: 3.025 (95% CI 2.178–4.200); aneuploid with reference diploid: 7.102 (95% CI 4.394–11.497); log-rank χ2 for ploidy: 91.75 | < 0.0001 (log-rank) |
| Univariate | Not applicable | Survival from death from prostate cancer, ‘cause-specific survival’ (events – death from prostate cancer only, censoring at last follow-up for patients who had not had progression or who had died) | Diploid 20; tetraploid 38; aneuploid 15 | 10-year survival: diploid 93%; tetraploid 79%; aneuploid 61% | HR: tetraploid with reference diploid: 3.192 (95% CI 1.856–5.489); aneuploid with reference diploid: 8.690 (95% CI 4.427–17.06); log-rank χ2 for ploidy: 51.20 | < 0.0001 (log-rank) | |
| Univariate | Not applicable | Overall survival (events – death from any cause, censoring at last follow-up for patients who had not had progression or who had died) | Diploid 92; tetraploid 71; aneuploid 16 | 10-year survival: diploid 73%; tetraploid 68%; aneuploid 59% | HR: tetraploid with reference diploid: 1.320 (95% CI 0.968–1.801); aneuploid with reference diploid: 2.094 (95% CI 1.227–3.572); log-rank χ2 for ploidy: 8.79 | 0.0124 (log-rank) | |
| Multivariate | Pathological Gleason grade, stage | Survival from progression [events – disease progression based on clinical examination (not routine PSA measurements), censoring at last follow-up for patients who had not had progression or who had died] | Not reported | Not applicable | Stepwise Cox regression, ploidy, relative hazard: 2.59 | < 0.0001 | |
| Multivariate | Pathological Gleason grade, stage | Survival from death from prostate cancer, ‘cause-specific survival’ (events – death from prostate cancer only, censoring at last follow-up for patients who had not had progression or who had died) | Not reported | Not applicable | Stepwise Cox regression, ploidy, relative hazard: 2.49 | 0.0011 | |
| Multivariate | Pathological Gleason grade, stage | Overall survival (events – death from any cause, censoring at last follow-up for patients who had not had progression or who had died) | Not reported | Not applicable | Stepwise Cox regression, ploidy, relative hazard: 1.18 | 0.2925 | |
| Siddiqui, 2006119 | Univariate | Not applicable | Systemic progression risk (events – demonstrable metastatic disease on radionuclide bone scintigraphy or plain radiography, or pathological evidence of failure as on lymph node biopsy) | Not reported | Not reported | Relative risk, tumour DNA ploidy: 2.63 (95% CI 2.16–3.20) | < 0.0001 |
| Univariate | Not applicable | Risk of death from prostate cancer (events – death from prostate cancer) | Not reported | Not reported | Relative risk, tumour DNA ploidy: 3.20 (95% CI 2.46–4.16) | < 0.0001 | |
| Multivariate | Pathological stage and Gleason score, surgical margins, categorised age (also lymph node involvement, adjuvant hormonal therapy, adjuvant radiation therapy) | Systemic progression risk (events – demonstrable metastatic disease on radionuclide bone scintigraphy or plain radiography, or pathological evidence of failure as on lymph node biopsy) | Not reported | Not applicable | Cox proportional hazard regression, relative risk, tumour DNA ploidy (risk of diploid with reference non-diploid?): 1.72 (95% CI 1.39–2.13) | < 0.0001 | |
| Multivariate | Pathological stage and Gleason score, surgical margins, categorised age (also lymph node involvement, adjuvant hormonal therapy, adjuvant radiation therapy) | Risk of death from prostate cancer (events – death from prostate cancer) | Not reported | Not applicable | Relative risk, tumour DNA ploidy: 1.92 (95% CI 1.44–2.55) | < 0.0001 |
In the univariate analyses of Blute et al. 105 and Lieber et al. 106 tetraploid and aneuploid tumours are compared with diploid tumours, and Blute et al. also carry out this comparison in multivariate analysis. In the multivariate analysis Lieber et al. enter a binary ploidy variable (non-diploid versus diploid). In the Siddiqui et al. 119 study only one ploidy variable is entered into the analyses and this is not defined. Lieber et al. and Siddiqui et al. both examine ploidy as a prognostic marker for survival from clinical progression (although not necessarily similarly defined) and prostate cancer death, whereas the end point for the Blute et al. study is biochemical or clinical (local or distant) progression. Lieber et al. also use crude survival as an end point.
All studies present univariate analyses and for all studies and all outcomes ploidy was found to be a significant predictor, in many analyses highly so (see Table 30).
In the multivariate analyses two studies106,119 found ploidy to be highly significantly prognostic for clinical progression and prostate cancer death (p-value ranged from 0.0011 to < 0.0001). The Lieber et al. 106 model included grade and stage, and the Siddiqui et al. 119 model grade and pathological variables including stage T3. Neither study included PSA. An analysis by Lieber et al. 106 did not find ploidy to be prognostic for all-cause death, but this outcome is less sensitive to prostate cancer markers than the others.
Blute et al. 105 found ploidy to be significantly prognostic for biochemical or clinical recurrence, but marginally so at the 95% confidence level (tetraploid versus diploid, p = 0.05, anueploid versus diploid, p = 0.04). This analysis included similar covariates to that of Siddiqui et al. 119 but with the addition of PSA doubling.
Overall conclusions based on the results and quality of the findings
Although two studies106,119 found DNA ploidy to be highly significantly prognostic for prostate cancer outcomes, another105 found it to be only marginally significant. The fact that the data used in the study by Blute and colleagues105 are probably included in the analysis of Siddiqui et al. 119 makes this more puzzling. All three studies are large and so are more likely to be statistically reliable than many other studies included in this review.
The most obvious differences between the analyses of Blute et al. 105 and Siddiqui et al. 119 are that Siddiqui et al. had no measure of PSA in their analysis and used clinical outcomes only whereas Blute et al. included a measure of PSA (PSA doubling) and used an outcome of biochemical or clinical progression. Vollmer et al. 107 suggest that pathological variables may be better at predicting clinical outcomes, whereas PSA is a better predictor of biochemical recurrence. This might explain the results. Neither analysis includes the usual absolute measure of preoperative PSA, although these data are presented in the baseline statistics and therefore must be available in the data set. The relationship between DNA ploidy and clinical and biochemical outcomes with and without PSA as a covariate could be explored in this data set (Siddiqui et al. 119 and/or Blute et al. 105 if not the same) and might resolve the contradictions apparent from the current analyses.
Germline genetic variation in the vitamin D receptor
One study by Williams et al. 120 was concerned with the impact of germline genetic variation in the vitamin D receptor on the risk of recurrence after prostatectomy.
Brief description of the prognostic marker
Vitamin D binds to the vitamin D receptor in the prostate and forms a complex with other factors such as retinoid X receptors. It is believed that this complex binds to vitamin D response elements on DNA and regulates the transcription of a number of genes involved in cell growth, differentiation and metastasis. Prostate cancer mortality rates appear to increase significantly with decreased ultraviolet radiation exposure, which decreases vitamin synthesis in the skin. This has led to the hypothesis that those men with a vitamin D deficiency might be at increased risk of prostate cancer. The definition and distribution of the marker in the population studied are shown in Table 31.
| Study | Definition | Population distribution |
|---|---|---|
| Williams, 2004120 | Vitamin D binds to the vitamin D receptor (VDR) in the prostate and forms a complex with other factors such as retinoid X receptors. The primary effects of vitamin D on the prostate are mediated through its receptor. DNA was isolated from fixed tissues by a modified procedure using the QIAamp Tissue Kit. Genotyping was performed using a 5-nuclease (TaqMan) assay in an ABI7700 Sequence Detector for VDR BsmI and TaqI genotypes | VDR BsmI genotypes for WM were: Bb, n = 164 (38%); Bb, n = 195 (46%); BB, n = 69 (16%) |
| VDR BsmI genotypes for AAM were: Bb, n = 168 (54%); Bb, n = 107 (35%); BB, n = 35 (11%) |
Brief description of the objectives of the individual study identified
Williams et al. 120 aimed to analyse the associations between germline genetic variation in the vitamin D receptor with clinical and pathological factors at the time of prostate cancer diagnosis and progression after RP. The basic study design characteristics are summarised in Table 32.
| Study | n | Primary aim to assess prognostic marker | Treatment |
|---|---|---|---|
| Williams, 2004120 | 738 | Yes | Radical prostatectomy |
Quality of the individual study identified
In general this is a good quality study but there are some issues that need to be considered when interpreting the results. First, the end point, disease recurrence, is not defined. It is not even clear if a consistent definition was used. Also, the number of events is not stated. It is possible that there is a low EPV rate, particularly in the second analysis, which is conducted on white men only with separate models for organ-confined and locally advanced tumours. The patient samples in these two models were 213 and 215 respectively. The overall concluding questions to each of the six subheadings are presented in Table 33.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Williams, 2004120 | y | ? | y | ? | y | p |
Summary of the baseline characteristics of the sample
Williams et al. 120 used a sample of 738 participants in the analysis (428 WM and 310 AAM), all of whom were treated with RP. Participants were all clinical stages T1/T2. More of the AAM than the WM had high-grade (Gleason score 8–10) tumours (16.5% and 12.7% respectively) and more also had pathologically non-confined tumours (WM: 50.2%, n = 213; AAM: 62.6%, n = 215) and high (≥ 20 ng/ml) preoperative PSA levels (WM 10.3%; AAM 22.9%). Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual study identified
The association between Bsm1 genotypes and progression was examined using a multivariate Cox proportional hazards model. The model was stratified by race to avoid multicolinearity effects between race and genotype, as the two were associated. Table 34 presents a summary of the main statistical findings from this study.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Williams, 2004120 | Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | Cox proportional hazards: WM, number of B alleles (0, 1, 2): HR 0.80 (95% CI 0.59–1.08) | 0.14 |
| Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | Cox proportional hazards: AAM, number of B alleles (0, 1, 2): HR 0.98 (95% CI 0.73–1.31) | 0.89 | |
| Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | bb vs Bb (WM): 0.85 (95% CI 0.55–1.33) | 0.47 | |
| Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | bb vs Bb (AAM): 0.74 (95% CI 0.48–1.15) | 0.18 | |
| Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | bb vs BB (WM): 0.60 (95% CI 0.31–1.18) | 0.14 | |
| Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | bb vs BB (AAM): 1.25 (95% CI 0.69–2.30) | 0.46 | |
| Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | bb vs Bb plus BB (WM): 0.78 (95% CI 0.51–1.19) | 0.25 | |
| Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | bb vs Bb plus BB (AAM): 0.85 (95% CI 0.57–1.25) | 0.40 | |
| Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | bb plus Bb vs BB (WM): 0.66 (95% CI 0.35–1.24) | 0.19 | |
| Multivariate | Clinical PSA, Gleason, pathological stage (also age) | Survival from progression (events – first recurrence; censoring at last follow-up) | Not applicable | bb plus Bb vs BB (AAM): 1.40 (95% CI 0.78–2.51) | 0.27 |
In neither model were Bsm1 genotypes significant predictors of progression; however, they were classified [according to the number of copies of the B allele (allele dose); the individual genotypes included in the same model (genotype specific); comparing bb with Bb plus BB (dominant effect of B); and comparing bb plus Bb with BB (recessive effect of B)].
A graphical analysis had suggested a differential effect of Bsm1 by pathological stage. In a further exploratory analysis a Cox regression model on WM was stratified by organ-confined status. In this analysis Bsm1 status showed high HRs for WM with organ-confined tumours, although they were not significant. For men with locally advanced tumours, the B allele was associated with a lower recurrence risk, with the HRs of marginal significance at the 95% confidence level.
It was reported that similar results were obtained for the Taq1 genotype but none of the analyses were shown.
Overall conclusions based on the results and quality of the findings
The primary analysis indicated that vitamin D receptor gene polymorphisms are not prognostic in prostate cancer. A secondary analysis on WM stratified by pathological organ-confined status did yield statistically significant associations between the Bsm1 genotype classifications and progression, with the B allele having an opposite effect in the two groups, but the statistical power of the analysis may have been weak. The authors claim that the complexity of the biological effects of vitamin D in experimental studies supports the possibility of complex clinical effects. The plausibility of such effects would need to be considered before pursuing vitamin D receptor gene polymorphisms as a prognostic marker in prostate cancer.
Non-classical use of Gleason measurements (divided into three submarker categories)
Conventionally, a patient is assigned a Gleason score, a measure of tumour differentiation, based on the sum of the scores for the primary and secondary most dominant patterns observed in the prostate specimen (either biopsy or surgical). Five included studies were interested in examining whether further prognostic information could be derived from different measures of Gleason grade: Egevad et al. ,121 Gonzalgo et al. ,122 Tollefson et al. ,123 Vis et al. 124 and Vollmer et al. 107
Brief description of the prognostic marker
Two studies122,123 examined whether the primary Gleason grade could differentiate between the prognostic outcomes of patients with a Gleason score of 7, a patient group that has particularly heterogeneous outcomes, i.e. whether there was a difference between patients whose Gleason pattern was 4 + 3 and those whose pattern was 3 + 4. These studies are shown in Table 35.
| Study | Definition | Population distribution |
|---|---|---|
| Gonzalgo, 2006122 | Classified prostatectomy (pathological) Gleason score 7 patients as Gleason pattern 3 + 4 or 4 + 3 on biopsy and created four categories for comparison: group A (clinical 3 + 4, pathological ≤ 3 + 4); group B (clinical 3 + 4, pathological ≥ 4 + 3); group C (clinical 4 + 3, pathological ≤ 3 + 4); group D (clinical 4 + 3, pathological ≥ 4 + 3) | Group A: 191 (59.7%); group B: 61 (19.1%); group C: 32 (10.0%); group D: 36 (11.3%) |
| Tollefson, 2006123 | Classified biopsy Gleason score 7 patients as Gleason pattern 3 + 4 or 4 + 3 | Pattern 3 + 4: 1256 patients; pattern 4 + 3: 432 patients |
Three studies107,121,124 examined whether some measure of the amount of high-grade cancer was prognostic of outcomes. The measures included percentage of tumour grade 4 or 5,121,124 length of high-grade tumour124 and the presence or not of grade 5 cancer in the primary and secondary prostatectomy specimens. 107 Samples were taken from TURP, biopsy and prostatectomy specimens. Details, as far as provided by the study authors, of the different definitions and measurement methods of these different measures of high-grade cancer are shown in Table 36.
| Study | Definition | Population distribution |
|---|---|---|
| Egevad, 2002121 | Percentage of tumour Gleason grade 4/5. Slides from TURP had cancerous areas outlined in ink and the percentage of tumour Gleason grade 4/5 by area was estimated as focal (≤ 5%) and at 10% intervals (0%, 1–5%, 6–10%, 11–20%, 21–30%, etc.). The variable was analysed as continuous data at 10% increments | Percentage grade 4/5 = 0%: n = 104; percentage grade 4/5 = up to 5%: n = 40; percentage grade 4/5 = 10–50%: n = 40; percentage grade 4/5 = 51–100%: n = 121 |
| Vis, 2007124 | Length of high-grade cancer (Gleason grade 4/5) (mm) from each biopsy core: continuous variable in analysis? Percentage of high-grade cancer (Gleason grade 4/5) from biopsy specimen (percentage of cancer with high-grade components) from prostatectomy specimen: continuous variable in analysis? | Median length of high-grade cancer = 0 mm (range 0.00–42.0 mm) |
| 0 mm: n = 1201 (71.5%); > 0–3 mm: n = 137 (13.2%); 3–10 mm: n = 129 (10.3%); > 10 mm: n = 114 (5.0%) | ||
| Median percentage of high-grade cancer = 0% (range = 0–100%) | ||
| Vollmer, 2001107 | Presence of primary/secondary grade 5 versus absence (prostatectomy specimen) | Not reported |
Egevad et al. 121 also calculated a modified Gleason score, which was the sum of the dominant (primary) and worst Gleason grades.
Brief description of the objectives of the individual studies identified
Four of the studies121–124 had a primary aim of assessing the prognostic value of different methods of measurement or scoring of Gleason grade assessments of tumour differentiation.
Two studies122,123 examined whether the primary Gleason grade could differentiate between the prognostic outcomes of patients with Gleason score 7, a patient group that has particularly heterogeneous outcomes, i.e. whether there was a difference between patients whose Gleason pattern was 4 + 3 and those whose pattern was 3 + 4. Note that Gonzalgo et al. 122 selected a population who were all biopsy Gleason score 7, whereas Tollefson et al. 123 selected a population who were all pathologically Gleason score 7. Egevad et al. 121 also included an analysis of Gleason pattern in Gleason score 7 patients but as this analysis had fewer than 200 participants it did not meet the inclusion criteria for Gleason score 7.
Both Egevad et al. 121 and Vis et al. 124 had the aim of examining the amount of high-grade cancer as a prognostic factor, whereas Vollmer et al. 107 was interested in the relative importance of anatomic and PSA factors for prostate cancer outcomes.
The basic study design characteristics are summarised in Table 37.
| Study | n | Primary aim prognostic marker | Treatment |
|---|---|---|---|
| Egevad, 2002121 | 305 | Yes | TURP |
| Gonzalgo, 2006122 | 320 | Yes | Radical prostatectomy |
| Tollefson, 2006123 | 1688 | Yes | Radical prostatectomy |
| Vis, 2007124 | 281 | Yes | Radical prostatectomy |
| Vollmer, 2001107 | 203 | No | Radical prostatectomy |
Quality of the individual studies identified
Perhaps because the focus of most of these studies was on different measures of Gleason grade, only one study123 reports a multivariate analysis including ‘known risk factors’ as well as the novel Gleason measure, although the former are not specified. The statistical analysis in two of the studies122,123 is also poorly reported and therefore difficult to assess. The number of events or EPV is low in some studies. Both the Vis and Vollmer studies have adequate EPV in their final models according to our criteria but that is only because they have removed most variables. The initial models that were used to select variables for the final model will have had low EPV and therefore may not have been reliable. In the analysis by Gonzalgo et al. 122 the number of events is not stated, but there are relatively small numbers of patients in two of the four groups (C: n = 32; D: n = 36) and so there are likely few events for these patients on which to base the analysis. The EPV is adequate in the study by Egevad et al. 121 and although the number of events is not stated by Tollefson et al. 123 the large sample size suggests that it is also adequate. The overall concluding questions to each of the six subheadings are presented in Table 38.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Egevad, 2002121 | p | p | p | y | n | y |
| Gonzalgo, 2006122 | y | p | y | y | n | ? |
| Tollefson, 2006123 | y | p | p | p | ? | ? |
| Vis, 2007124 | y | ? | p | p | n | p |
| Vollmer, 2001107 | p | ? | n | p | n | p |
Summary of the baseline characteristics of the sample
With the exception of Egevad et al. 121 the patients in all of the studies had more than 90% organ-confined tumours. The study population in Egevad et al. 121 was different from the others, with prostate cancer diagnosed at TURP because of obstructive symptoms. In total, 83% of these patients had organ-confined tumours. These patients also had a high proportion of high-grade cancers (31% pathologically Gleason score 8–10). The Gonzalgo et al. 122 and Tollefson et al. 123 studies included only patients with Gleason score 7. The patients in all studies, with the exception of those in the Egevad et al. 121 study who had deferred treatment following TURP, were treated with RP.
Brief description of the results from the individual studies identified
Table 39 presents a summary of the main statistical findings from the two studies on Gleason patterns 3 + 4 and 4 + 3 included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Gonzalgo, 2006122 | Univariate | Not applicable | Biochemical recurrence (PSA 0.2 ng/ml or greater) (measured in terms of likelihood of undetectable PSA level) |
Estimated from survival curve at 5 years. Scored on scale 0–1, probability of undetectable PSA (higher score indicates better prognosis) Group A significantly better prognosis than group B (p = 0.002) and group D (p < 0.001); group C significantly better prognosis than group D (p = 0.03) |
Log-rank test for comparison of survival curves |
Group A (clinical 3 + 4 not upgraded at prostatectomy), p = 0.89; group B (clinical 3 + 4 upgraded at prostatectomy), p = 0.74; group C (clinical 4 + 3 downgraded), p = 0.86; group D (clinical 4 + 3 not downgraded), p = 0.55 Non-significant between groups A and C (p < 0.17), groups B and D (p = 0.07) and groups B and C (p = 0.47) All four curves χ2 = 28.80 (p < 0.0001) |
| Tollefson, 2006123 | Univariate (analysis method not specified) | Not applicable | Biochemical failure (events – single serum PSA of > 0.4 ng/ml) | 10-year survival: Gleason 3 + 4: 52%; Gleason 4 + 3: 62% | Not reported | < 0.0001 |
| Univariate (analysis method not specified) | Not applicable | Systemic recurrence (events – positive bone scan or other lesion identifying metastatic prostate cancer) | 10-year survival: Gleason 3 + 4: 8%; Gleason 4 + 3: 15% | Not reported | < 0.0001 | |
| Univariate (analysis method not specified) | Not applicable | Cancer-specific survival (events – death from prostate cancer) | 10-year survival: Gleason 3 + 4: 97%; Gleason 4 + 3: 93% | Not reported | 0.013 | |
| Multivariate | Clinical PSA, stage, margin status (also seminal vesicle involvement, DNA ploidy)? | Biochemical failure (events – single serum PSA of > 0.4 ng/ml) | Not applicable | Not reported | < 0.0001 | |
| Multivariate | Clinical PSA, stage, margin status (also seminal vesicle involvement, DNA ploidy)? | Systemic recurrence (events – positive bone scan or other lesion identifying metastatic prostate cancer) | Not applicable | Not reported | 0.002 | |
| Multivariate | Clinical PSA, stage, margin status (also seminal vesicle involvement, DNA ploidy)? | Cancer-specific survival (events – death from prostate cancer) | Not applicable | Not reported | 0.029 |
Primary Gleason pattern in Gleason score 7 patients
In the study by Gonzalgo et al. 122 patients (all biopsy Gleason score 7) were divided into four groups according to whether they were Gleason pattern 3 + 4 or 4 + 3 at biopsy and after prostatectomy. The prognosis of these four groups in terms of freedom from biochemical recurrence was compared using a log-rank test to test the significance of differences between pairs of the four survival curves, and also using an overall test of the four curves. Survival at 5 years ranged from 89% for group A to 55% for group D. Not all of the pairs of curves were significantly different from each other (see Table 39), but groups A and B (both biopsy Gleason pattern 3 + 4) had significantly different outcomes (p = 0.002) as did groups C and D (both biopsy Gleason pattern 4 + 3) (p = 0.03). The latter analysis may be unreliable because of the small numbers of patients in groups B and C. The overall log-rank statistic for all curves was significant (p < 0.0001). A comparison between all those with clinical Gleason pattern 3 + 4 and those with pattern 4 + 3 was not made.
In a univariate analysis Tollefson et al. 123 found significant differences in prognosis between patients with biopsy Gleason pattern 3 + 4 and those with Gleason pattern 4 + 3 with outcomes of biochemical recurrence-free survival (p < 0.0001), systemic recurrence-free survival (p < 0.002) and cancer-specific survival (p = 0.013). In a multivariate analysis ‘correcting for known risk factors’, primary Gleason score was an independent significant predictor of biochemical failure (p < 0.0001), systemic recurrence (p = 0.002) and cancer-specific survival (p = 0.029). The lower p-values for the relationship between primary Gleason score and outcome in both univariate and multivariate analyses when the outcome was survival rather than disease recurrence (even biochemical or systemic) may be due to the lower number of events for the survival outcome compared with the recurrence outcomes, rather than any difference in the strength of the relationship. The number of events is not reported in the study but, after 10 years, although around 95% of patients have survived prostate cancer death, only around 50% are biochemical progression free. Table 40 presents a summary of the main statistical findings from the three studies included in this section on the amount of high-grade cancer.
| Study | Statistical analysis | Classical markers in model | End point | Events | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|---|
| Egevad, 2002121 (percentage Gleason grade 4/5) | Univariate | Not applicable | Survival from death from prostate cancer, ‘disease-specific survival’ (events – death from prostate cancer) | At mean follow-up of 7.3 years for censored patients, 5.9 uncensored | Not reported | Cox analysis, percentage Gleason grade 4/5 (from TURP) (continuous data at 10% increments): χ2 = 92.3 | < 0.001 |
| Percentage grade 4/5 = 0%: 8% died of prostate cancer (of n = 104); percentage grade 4/5 = up to 5%: 28% died (of n = 40); percentage grade 4/5 = 10–50%: 38% died (of n = 40); percentage grade 4/5 = 51–100%: 65% died (of n = 121) | |||||||
| Multivariate | Pathological Gleason score | Survival from death from prostate cancer, ‘disease-specific survival’ (events – death from prostate cancer) | Not applicable | Not applicable | Multivariate Cox analysis, percentage Gleason grade 4/5 (from TURP) (continuous data at 10% increments): χ2 = 9.5 | 0.002 | |
| Vis, 2007124 (percentage high-grade tumour involvement) | Univariate | Not applicable | Biochemical recurrence (PSA ≥ 0.1 ng/ml) | Not reported | Not reported | Cox regression analysis, percentage high-grade tumour involvement (biopsy cores): HR 1.029 | < 0.001 |
| Multivariate | Surgical margins (also invasion of adjacent organs) | Biochemical recurrence (PSA ≥ 0.1 ng/ml) | Not reported | Not applicable | Cox regression analysis, percentage high-grade tumour involvement (biopsy cores): HR 1.023 | < 0.001 | |
| Vis, 2007124 (proportion of high-grade cancer) | Multivariate | Not stated | Biochemical recurrence (PSA ≥ 0.1 ng/ml) | Not reported | Not applicable | Cox multiple regression, proportion of high-grade cancer | 0.001 |
| Vis, 2007124 [length (mm) of high-grade cancer] | Univariate | Not applicable | Biochemical recurrence (PSA ≥ 0.1 ng/ml) | Not reported | Estimation from survival curve, 5-year survival: length of high-grade cancer (Gleason 4/5): 0 mm 92%; 0–3 mm 90%; 3–10 mm 72%; > 10 mm 50% | Cox proportional hazards model, length (mm) of high-grade cancer (biopsy cores): HR 1.079 | < 0.001 |
| Univariate | Not applicable | Clinical progression (local progression and/or distant metastases) | Not reported | Extrapolating from survival curve, 5-year survival: length of high-grade cancer (Gleason 4/5): 0 mm 99%; 0–3 mm 98%; 3–10 mm 88%; > 10 mm 78% | Length (mm) of high-grade cancer (biopsy cores): HR 1.074 | 0.004 | |
| Multivariate | PSA (also length of tumour in mm) | Biochemical recurrence (PSA ≥ 0.1 ng/ml) | Not reported | Not applicable | Length (mm) of high-grade cancer (biopsy cores): HR 1.033 | 0.006 | |
| Multivariate | None as all removed, therefore as univariate | Clinical progression (local progression and/or distant metastases) | Not reported | Not applicable | Length (mm) of high-grade cancer (biopsy cores): HR 1.074 | 0.004 | |
| Vollmer, 2001107 (Gleason grade 5 in primary or secondary) | Multivariate | None (also percentage cancer) | Time to death from prostate cancer [censored if died without elevated (> 0.5 ng/ml) postoperative PSA level] | Not reported | Not applicable | Presence of either primary or secondary Gleason grade 5 from prostatectomy specimen (with reference absence of Gleason grade 5): Cox model analysis coefficient 1.17 (SE 0.450) | 0.0096 |
Amount of high-grade tumour
In univariate analysis both Egevad et al. 121 and Vis et al. 124 found the percentage of high-grade tumour to be significantly prognostic for prostate cancer death (p < 0.001) and biochemical progression (p < 0.001) respectively. Using multivariate analysis Egevad et al. 121 examined the performance of the percentage of high-grade tumour in a model with Gleason score but no other covariates, in which it was significant (p = 0.002). Vis et al. 124 found the percentage of high-grade tumour to be significantly prognostic for biochemical progression (p < 0.001) in a multivariate model that included PSA. Gleason score was removed from the model because of non-significance.
Vis et al. 124 also tested a variable of length of high-grade cancer from the biopsy core. In univariate analysis it was significant for the outcomes of survival from biochemical and clinical progression. In multivariate analysis it was significant for biochemical survival with PSA as the only covariate, but for the outcome of clinical recurrence all of the other covariates were removed from the model using a stepwise process and so the result reported is the same as that for the univariate analysis.
Vollmer et al. 107 found the presence of Gleason grade 5 in either the primary or secondary prostatectomy specimen to be significantly prognostic for prostate cancer death (p = 0.0096) in a multivariate model with no classical markers but with percentage of tumour in the prostate.
Modified Gleason score
Egevad et al. 121 also found a modified Gleason score [sum of the dominant (primary) and worst Gleason grades] to be prognostic of prostate cancer death in univariate analysis (p < 0.001) and in a multivariate model with Gleason score (p < 0.001).
Overall conclusions based on the results and quality of the findings
Two studies122,123 showed that primary Gleason grade in Gleason score 7 patients was prognostic, although Gonzalgo et al. 122 report only a univariate analysis. In the multivariate analysis reported by Tollefson et al. 123 primary Gleason grade was prognostic for biochemical failure (p < 0.0001), systemic recurrence (p = 0.002) and cancer-specific survival (p = 0.029). This study was likely to have been adequately powered but poor reporting of the analysis makes it difficult to assess. The results needed to be confirmed.
Gleason pattern has already been used by Han et al. 140 in a prognostic model, which is discussed in Chapter 6. If further prognostic information could be derived from what is routinely collected data this would clearly be advantageous.
Two studies121,124 found the percentage of high-grade tumour to be prognostic for prostate cancer death and biochemical progression respectively, and in both it appeared to outperform Gleason score. In neither study was percentage of high-grade tumour tested in a multivariate model with all of the established markers and so its additional prognostic value is not established. Vis et al. 124 also found length of high-grade cancer to be prognostic in univariate and multivariate analysis, but most covariates were removed from the analysis and so its performance in the presence of the classical markers is not shown. Vollmer et al. 107 found the presence of Gleason grade 5 to be significantly prognostic for prostate cancer death (p = 0.0096), but this marker also was not tested in a multivariate model with classical markers. Thus, although measured differently, all measures of amount of high-grade cancer were found to be prognostic, but none was tested in models including all of the established markers.
One study121 found a modified Gleason score [sum of the dominant (primary) and worst Gleason grades] to be prognostic of prostate cancer death.
All of the studies in this section report a variety of novel Gleason measures to be significantly prognostic of various prostate cancer outcomes. However, only one study123 was (probably) tested in models including all of the established markers and the quality of the studies was generally worse than average. The positive results, combined with the relative ease with which some of these measures could be applied as the data are currently collected, suggest that more rigorous studies would be worth undertaking.
Ki67 LI, Bcl-2, p53, syndecan-1 and CD10
One study by Zellweger et al. 125 was concerned with the prognostic significance of the four novel markers Ki67 LI, Bcl-2, p53, syndecan-1 and CD10.
Brief description of the prognostic markers
Tissue microarrays are emerging as powerful tools to rapidly analyse the clinical significance of new molecular markers in human tumours. Ki67 LI (labelling index) is a nuclear antigen that is present throughout the cell cycle but not at rest (GO phase) or in the early G1 phase. 141 Antibodies to the p53 protein bind both normal (wild type) and mutant forms. 141 The Bcl-2 oncoprotein inhibits apoptosis, such that its overexpression leads to increased cell growth. 141 Syndecan-1 (also known as CD138, CD138 antigen, SDC, SYND1, syndecan-1 precursor) is a multifunctional transmembrane heparan sulfate proteoglycan that is present on many cell types and which mediates growth factor binding. 142
The definitions and distributions of the markers in the population studied are shown in Table 41.
| Study | Definition | Population distribution |
|---|---|---|
| Zellweger, 2003125 | The expression of Ki67, Bcl-2, p53, CD10 (neutral endopeptidase) and syndecan-1 (CD138) was analysed by immunohistochemistry. For Ki67, immunostaining was visually scored and stratified into two groups (< 10% and ≥ 10%). The intensity of the immunostaining for p53, Bcl-2 and syndecan-1 was visually scored and stratified into four groups (negative, weak, moderate and strong). Overexpression was defined as at least moderate staining intensity in > 10% of the tumour cells | High Ki67 LI expression (≥ 10%) was found in 14.5% of 515 specimens. Cytoplasmic Bcl-2 overexpression was present in 13.7% of 493 specimens. p53 overexpression was found in 3.9% of 534 specimens. Syndecan-1 overexpression was present in 36.7% of 501 specimens. CD10 overexpression was present in 22.5% of 510 specimens |
Brief description of the objectives of the individual study identified
The study examined the expression of the molecular markers Ki67, Bcl-2, p53, syndecan-1 and CD10 for prognostic significance. The basic study design characteristics are summarised in Table 42.
| Study | n | Primary aim to assess prognostic marker | Treatment |
|---|---|---|---|
| Zellweger, 2003125 | 551 | Yes | Radical prostatectomy or TURP |
Quality of the individual study identified
The study does poorly on many quality assessment criteria. One important issue is recognised by the authors, that is the heterogeneity of the study cohort. Participants were accrued over a considerable period of time between 1971 and 1996. This means that there were different staging, treatment and follow-up methods. There is also heterogeneity in how disease progression was defined, with it being defined clinically in some patients and biochemically (by PSA) in others. Furthermore, the definition of PSA failure is not given and may have been variable.
The statistical analysis may also be weak as there are relatively small numbers of patients in each of the ‘high-risk’ marker categories and thus the number of events in these groups is likely to be small (Table 43). With the exception of pathological grade, classical markers were not included in the model and therefore the prognostic significance of these markers over those in current use is not demonstrated.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Zellweger, 2003125 | p | ? | p | n | n | p |
Summary of the baseline characteristics of the sample
The study involved 551 participants who had been treated with RP or TURP. All participants were organ confined at clinical stage. At pathological stage there were still a greater number of organ-confined (71.9%) compared with non-organ-confined participants (18.5%), with a small number of participants having missing data (9.6%). Only Gleason grade (as opposed to Gleason score) was reported because of the small size of the specimens. PSA levels were not reported. The failure to measure and report this information limits the comparison of this study with other prognostic studies involving other types of markers. Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual study identified
Table 44 presents a summary of the main statistical findings from the single study included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Zellweger, 2003125 | Univariate | Not applicable | Time to progression – two definitions according to dates: before 1992 clinical progression (bone scans/chest radiography/digital rectal examination); after 1992 defined by increasing PSA (no definition of level of increase reported) | From survival curve: Ki67 LI high 70%, low 85%; Bcl-2 negative 85%, positive 72%; p53 negative 82%, positive 82%; syndecan-1 negative 84%, positive 78%; CD10 negative 81%, positive 78% | Log-rank | Ki67: p < 0.01; Bcl-2: p < 0.05; p53: p = 0.38; syndecan-1: p < 0.02; CD10: p = 0.22 |
| Univariate | Not applicable | Overall survival (not defined) | From survival curve: Ki67 LI high 72%, low 86%; Bcl-2 negative 94%, positive 88%; p53 negative 90%, positive 71%; syndecan-1 negative 90%, positive 79%; CD10 negative 85%, positive 85% | Log-rank | Ki67: p < 0.05; Bcl-2: p = 0.28; p53: p < 0.05; syndecan-1: p = 0.07; CD10: p = 0.87 | |
| Univariate | Not applicable | Tumour-specific survival (not defined) | From survival curve: Ki67 LI high 90%, low 98%; Bcl-2 negative 96%, positive 96%; p53 negative 97%, positive 87%; syndecan-1 negative 99%, positive 92%; CD10 negative 95%, positive 95% | Log-rank | Ki67: p < 0.01; Bcl-2: p = 0.79; p53: p < 0.05; syndecan-1: p < 0.01; CD10: p = 0.68 | |
| Multivariate | Gleason grade | Time to progression – two definitions according to dates: before 1992 clinical progression (bone scans/chest radiography/digital rectal examination); after 1992 defined by increasing PSA (no definition of level of increase reported) | Not applicable | Cox proportional hazards (stepwise, included if significant in univariate analysis) | Ki67 LI: 0.178; Bcl-2: 0.816; syndecan-1: 0.147; p53 not included as not significant in univariate analysis | |
| Multivariate | Gleason grade | Overall survival (not defined) | Not applicable | Cox proportional hazards (stepwise, included if significant in univariate analysis) | Ki67 LI: 0.071; p53: 0.84; Bcl-2 and syndecan-1 not included as not significant in univariate analysis | |
| Multivariate | Gleason grade | Tumour-specific survival (not defined) | Not applicable | Cox proportional hazards (stepwise, included if significant in univariate analysis) | Ki67 LI: 0.023; p53: 0.542; syndecan-1: 0.051; Bcl-2 not included as not significant in univariate analysis |
Zellweger et al. 125 reports the p-values of the markers (Ki67, Bcl-2, p53, syndecan-1 and CD10) in three different Cox regression models, each with a different end point: progression, overall survival and tumour-specific survival. Markers were only introduced into the multivariate model if they were found to be statistically significant predictors of that outcome in univariate analysis. Gleason grade was the only classical marker entered into the statistical model. Marker Ki67 LI (p = 0.023) was the only marker found to be statistically significant for all end points in univariate analysis. It remained significant in multivariate analysis for the end points of overall survival and tumour-specific survival, but with Gleason score as the only classical marker in the model. CD10 was not significant in any of the univariate analyses and thus was not tested in the multivariate models.
Bcl-2 and p53 were not significant in any of the multivariate analyses. The marker syndecan-1 was of marginal significance for tumour-specific survival (p = 0.051).
It should be noted that Zellweger et al. 125 reported many significant associations between the markers and this may have affected their individual performances in the multivariate models.
Overall conclusions based on the results and quality of the findings
The weaknesses of this study make the results inconclusive. Of the markers studied Ki67 LI appeared to be the most strongly associated with the study end points and in particular tumour-specific survival (p = 0.023). p53 was of marginal significance for this end point (p = 0.051).
Proportion of cancer
Six studies107,121,124,126–128 were concerned with the prognostic significance of the proportion of cancer in the specimen.
Brief description of the prognostic marker
These studies all used some measure of the proportion of the prostate affected by cancer as a prognostic marker. Four studies124,126–128 achieved this by counting the number of biopsy cores containing cancer, usually expressing this as a proportion of cores affected. Two studies107,121 used a measure of the percentage of the prostate involved with cancer, estimated from the surgical specimens; however, the Egevad et al. 121 study used TURP specimens whereas in the Vollmer et al. 107 study patients had RP. The definitions and the marker distributions in the different studies are shown in Table 45.
| Study | Definition | Population distribution |
|---|---|---|
| Antunes, 2005126 | Percentage positive biopsy cores (PPBC). A total of 6–18 cores were taken under TRUS guidance. PPBC was defined as the ratio of positive cores to total cores | < 25, n = 164 (30.7%); 25.1–50, n = 242 (45.3%); 50.1–75, n = 76 (14.2%); 75.1–100, n = 52 (9.7%) |
| Egevad, 2002121 | Percentage cancer. The slides from TURP were reviewed and the cancer outlined in ink. The percentage of the total specimen area involved with tumour was estimated at 10% intervals | Not stated |
| Potters, 2005127 | PPBC | <50%, n = 808 (55.8%); ≥ 50%, n = 641 (44.2%) |
| Selek, 2003128 | PPBC. Only patients with systematic biopsies were considered. In total, 74% had sextant biopsies, 8% had < 6 and 18% had > 6. PPBC was defined as the number of cores that contained prostate cancer of any length divided by the total number of cores sampled | < 50%, n = 266 (77.1%); ≥ 50%, n = 79 (32.9%) |
| Vis, 2007124 | Number of positive tumour biopsy cores. All patients had sextant biopsies | 1, n = 101 (35.9%); 2, n = 82 (29.2%); 3, n = 49 (17.4%); 4–6, n = 49 (17.4%) |
| Vollmer, 2001107 | Percentage cancer. Defined as the percentage of prostate tissue with tumour in the RP specimen. Measurement method not specified | Median = 15%; range = 0.1–89.0% |
Brief description of the objectives of the individual studies identified
It is important to note that only two of the studies126,128 had a primary aim of assessing positive biopsy cores as a prognostic marker. Antunes et al. 126 evaluated the prognostic value of the percentage of positive biopsy cores (PPBC) in determining the pathological features and biochemical outcome of patients with prostate cancer treated by R.P. Selek et al. 128 aimed to determine the utility of the PPBC in predicting PSA outcome after external beam radiotherapy alone. Potters et al. 127 assessed the outcomes of men undergoing prostate brachytherapy and evaluated factors that could impact on disease-specific survival. Vis et al. 124 and Egevad et al. 121 investigated the predictive value of the amount of high-grade cancer (Gleason growth patterns 4/5) in the biopsy following RP and TURP, respectively. Vollmer et al. 107 compared anatomic and PSA factors as prognostic markers.
Quality of the individual studies identified
One of the key failings amongst these studies is the omission of classical markers in the reported multivariate models,107,121,124,128 usually because of stepwise removal of variables rather than lack of data. The statistical power of some of the studies107,124,128 in terms of EPV may also be weak, although in the case of Selek et al. 128 and Vis et al. 124 the assessment criterion of an EPV of at least 10 in the final model was met. The study by Antunes et al. 126 avoids both of these issues and is overall probably the best quality study for this marker. In the four studies that had an end point of biochemical recurrence124,126–128 only one used a recognised definition;128 the definition therefore varied across the studies, although at least all of the studies were internally consistent. The overall concluding questions to each of the six subheadings are presented in Table 46.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Antunes, 2005126 | y | ? | p | y | y | y |
| Egevad, 2002121 | p | p | p | y | n | y |
| Potters, 2005127 | y | ? | n | p | y | p |
| Selek, 2003128 | y | p | p | y | p | y |
| Vis, 2007124 | y | ? | p | p | n | p |
| Vollmer, 2001107 | p | ? | n | p | n | p |
Two studies107,128 failed to present sufficient data to assess the adequacy of the analysis.
Summary of the baseline characteristics of the sample
Three of the studies107,124,126 used RP treatment. Potters et al. 127 used brachytherapy (some in combination with radiotherapy), Selek et al. 128 used radiotherapy alone and Egevad et al. 121 used TURP. The studies varied in population size ranging from 203 to 1449 (Table 47). The largest study was conducted by Potters et al. 127 and the smallest by Vollmer et al. 107
| Study | n | Primary aim prognostic marker | Treatment |
|---|---|---|---|
| Antunes, 2005126 | 534 | Yes | Radical prostatectomy |
| Egevad, 2002121 | 305 | Yes | TURP |
| Potters, 2005127 | 1449 | No | Brachytherapy (some in combination with radiotherapy) |
| Selek, 2003128 | 345 | Yes | Radiotherapy |
| Vis, 2007124 | 281 | Yes | Radical prostatectomy |
| Vollmer, 2001107 | 203 | Yes | Radical prostatectomy |
In evaluating the results of the six studies it is important to consider the differences in sample characteristics (e.g. stage, Gleason score and PSA distributions). The clinical stage of the participants was provided in all six studies. More than 98% of the samples in five of the studies were organ-confined cancers at clinical stage. The exception was the study of Egevad et al.,121 in which 17% of cancers were non-organ confined and whose participants also had a high proportion of high-grade cancers (35% Gleason score 8–10). This study pre-dates PSA screening and the patients had their tumours detected on TURP carried out for obstructive symptoms. The distributions of Gleason and PSA scores (where reported) were similar across the other studies. Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual studies identified
Table 48 presents a summary of the main statistical findings from the six studies included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Antunes, 2005126 (percentage positive biopsy cores) | Univariate | Not applicable | Survival from biochemical recurrence (PSA ≥ 0.4 ng/ml) | Estimated from survival curve, 5-year survival, percentage positive biopsy curves: < 25: 85%; 25.1–50: 76%; 50.1–75: 72%; 75.1–100: 43% | Cox regression, percentage positive biopsy cores (continuous variable): HR 5.13 (95% CI 2.86–9.21) | < 0.001 |
| Multivariate | Clinical stage, PSA, Gleason score | Survival from biochemical recurrence (PSA ≥ 0.4 ng/ml) | Not applicable | Cox regression, percentage positive biopsy cores (continuous variable): HR 3.46 (95% CI 1.89–6.33) | < 0.001 | |
| Potters, 2005127(percentage positive biopsy cores) | Multivariate | Clinical PSA, Gleason score, stage (also percentage D90, hormone addition, external beam radiotherapy addition) | Survival from biochemical recurrence (ASTRO–Kattan definition) | Not applicable | Cox proportional hazards model, percentage positive biopsy cores (< 50% compared with ≥ 50%): Exp(B) 1.492 (95% CI 1.024–2.173) | 0.037 |
| Selek, 2003128 (percentage positive biopsy cores) | Univariate proportional hazards | Not applicable | Survival from biochemical recurrence (events from ASTRO definition) | Not reported | Proportional hazards model, percentage positive biopsy cores (analysed as continuous variable) | 0.0053 |
| Univariate log-rank | Not applicable | Survival from biochemical recurrence (events from ASTRO definition) | Not reported | Log-rank, percentage positive biopsy cores (< 50% compared with ≥ 50%) | 0.0077 | |
| Multivariate | Clinical PSA, Gleason score | Survival from biochemical recurrence (events from ASTRO definition) | Not applicable | Percentage positive biopsy cores (analysed as continuous variable): HR 1.001 | 0.13 | |
| Multivariate | Clinical PSA, Gleason score | Survival from biochemical recurrence (ASTRO definition) | Not applicable | Cox regression analysis, percentage positive biopsy cores (≥ 50% compared with < 50%): HR 1.40 | 0.22 | |
| Vis, 2007124 (number of positive biopsy cores) | Univariate | Not applicable | Biochemical recurrence (PSA ≥ 0.1 ng/ml) | Not reported | Cox proportional hazards model, number of positive tumour biopsy cores (continuous variable): HR 1.439 | 0.001 |
| Univariate | Not applicable | Clinical progression (local progression and/or distant metastases) | Not reported | Cox proportional hazards model, number of positive tumour biopsy cores: HR 1.513 | 0.025 | |
| Multivariate | Clinical stage, Gleason score, PSA (also length of tumour and length of high-grade cancer in mm) | Biochemical recurrence (PSA ≥ 0.1 ng/ml) | Not applicable | Cox proportional hazards model, number of positive tumour biopsy cores: HR not reported | Not significant | |
| Multivariate | Clinical stage, Gleason score, PSA (also length of tumour and length of high-grade cancer in mm) | Clinical progression (local progression and/or distant metastases) | Not applicable | Cox proportional hazards model, number of positive tumour biopsy cores: HR not reported | Not significant |
All of the studies provided a Cox multivariate analysis of the data. As shown in Table 48 all studies used an end point of biochemical recurrence but the definition varied between studies, and in the Selek et al. 128 study patients were treated with radiotherapy and so PSA behaviour following treatment is different from that in the other studies. Vis et al. 124 also used an outcome of clinical progression. Table 48 shows the different clinical and pathological classical markers entered into the statistical models across the four studies: all included the classical markers in their models with the exception of the Selek analysis, which does not include stage.
All of the studies that reported a univariate analysis124,126,128 found PPBC to be prognostic. However, only two studies126,127 showed PPBC to be prognostic in multivariate analysis, both for PSA survival. Of these, one126 has a large EPV ratio (30) suggesting a statistically strong analysis and the other,127 although it is not stated, is likely to be more than adequate because of the sample size (n = 1449). The studies of Antunes et al. 126 and Potters et al. 127 both also include all of the classical prognostic markers, suggesting that the proportion of positive biopsy cores may add prognostic value to that of the established markers.
The multivariate results of three analyses in two studies124,128 indicate that PPBC is not prognostic. The study end points were biochemical progression and clinical progression. The number of events in both of these studies may have been low, making the analyses less reliable. The analyses of Selek et al. 128 and Vis et al. 124 met the quality criterion of an EPV of at least 10, but for Selek et al. 128 it was only 13 and not all continuous variables were treated as continuous, thus weakening the analysis. Vis et al. 124 achieved adequate EPV in their final models by eliminating most variables. However, there were only 39 events in total and so the EPV for the full models (when the number of positive cores would have been eliminated for non-significance) would have been low.
Table 49 presents a summary of the results of the studies concerning the percentage of cancer in the specimen.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Egevad, 2002121 (percentage of cancer in TURP specimen) | Univariate | Not applicable | Survival from death from prostate cancer, ‘disease-specific survival’ (events – death from prostate cancer) | Not reported | Cox analysis, percentage cancer (continuous data at 10% increments): χ2 = 73.5 | < 0.001 |
| Multivariate | Pathological Gleason score (also percentage Gleason grade 4/5) | Survival from death from prostate cancer, ‘disease-specific survival’ (events – death from prostate cancer) | Not applicable | Multivariate Cox analysis, percentage cancer (continuous data at 10% increments): χ2 = 10.6 | 0.011 | |
| Vollmer, 2001107 (percentage cancer in prostatectomy specimen) | Multivariate | Gleason grade 5 | Time to death from prostate cancer [censored if died without elevated (> 0.5 ng/ml) postoperative PSA level] | Not applicable | Cox model analysis, percentage carcinoma (continuous variable): coefficient 0.029 (SE 0.009), HR 1.03 | 0.0014 |
Percentage of cancer in the surgical specimen
Both of the studies provided a Cox multivariate analysis of the data but with very limited covariates, which did not include PSA or stage. Both used prostate cancer survival as their outcome measure. Note that the estimates of percentage of cancer are derived differently, with it being estimated from the TURP specimen in Egevad et al. 121 and from the prostatectomy specimen in Vollmer et al. 107 The patient sample in Egevad et al. 121 also had slightly more advanced disease, as described in the section on the baseline characteristics of the sample.
Both studies found the percentage of cancer in the surgical specimen to be prognostic for prostate cancer death, but in neither multivariate analysis was PSA or stage included. Given the range of values for this variable quoted by Vollmer et al. 107 (0.1–89%), it has prognostic potential but needs to be tested in a model with the classical variables. The results from the current evidence must be considered inconclusive.
Overall conclusions based on the results and quality of the findings
Percentage of positive biopsy cores
The results of the four studies are mixed, with two of the studies126,127 suggesting that the proportion of cancer in a biopsy specimen is prognostic in the presence of the classical variables and three analyses from the other two studies124,128 suggesting that it is not. However, the two studies that found a positive result were statistically stronger than the others in terms of having a large ratio of events to the number of variables in the analyses; these two analyses also included all of the established classical markers in the final analysis. This suggests that the proportion of cancer in a biopsy specimen may have additional prognostic value for biochemical recurrence over the established markers. However, the evidence is currently limited.
Percentage of cancer in the surgical specimen
Two studies107,121 found the percentage of cancer in a surgical specimen to be prognostic for prostate cancer death, but in neither multivariate analysis was PSA or stage included. Given the range of values for this variable quoted by Vollmer et al. 107 (0.1–89%), it has prognostic potential but needs to be tested in a model with the classical variables. The results from the current evidence must be considered inconclusive.
Prostate-specific antigen kinetics
Two studies129,130 were concerned with the prognostic significance of the novel markers PSAV or PSADT.
Brief description of the prognostic markers
Both studies used linear regression to calculate the rate of rise in the PSA level (PSAV) in the year before diagnosis129 or 2 years before treatment130 using all available values. PSADT is the time that it takes for the PSA value to double; this was calculated by Sengupta et al. 130 using log-linear regression. The definitions and the marker distributions are shown in Table 50.
| Study | Definition | Population distribution |
|---|---|---|
| D’Amico, 2004129 | PSAV was defined as the rate of rise in the PSA level. PSA measurements were made at intervals of 6–12 months. PSAV during the year before diagnosis was considered as a categorical variable. In the 2 years before RP multiple PSA values (mean 3.05, range 2–14) were taken at least 90 days apart. Note that in models with clinical variables PSAV at diagnosis was used, whereas in models with pathological variables PSAV on prostatectomy was used. However, the numbers in the two groups are the same for both measures and so it is not evident that they are actually different | End point recurrence – PSAV at diagnosis: ≤ 2.0 ng/ml/year, n = 816; > 2.0 ng/ml/year, n = 247 |
| End points prostate cancer death and any death – PSAV at diagnosis or at prostatectomy: ≤ 2.0 ng/ml/year, n = 833; > 2.0 ng/ml/year, n = 262 | ||
| Sengupta, 2005130 | A cut-off value of 3.4 ng/ml/year was chosen for PSAV. For PSADT a value of 18 months was chosen | PSADT < 18 months, n = 506 (22.1%); PSADT ≥ 18 months, n = 1784 |
| PSAV > 3.4 ng/ml/year, n = 460 (20.1%); PSAV ≤ 3.4 ng/ml/year, n = 1830 |
Brief description of the objectives of the individual studies identified
Both of the included studies had a primary aim of assessing PSA kinetics as a prognostic marker. D’Amico et al. 129 evaluated whether the rate of rise in the PSA level (i.e. PSAV) during the year before diagnosis could predict PSA recurrence, prostate cancer mortality and all-cause mortality. Sengupta et al. 130 also used three separate end points for different analyses: PSA recurrence, clinical recurrence and prostate cancer mortality. In both studies two models are presented for each end point, the first using only clinical variables and the second including pathological variables. Sengupta et al. 130 assessed preoperative PSADT as a predictor of outcome following RP.
Quality of the individual studies identified
Both studies are large and of good quality. However, they both determined the cut-point for differentiating between high and low PSAV within their respective data sets. The same applies to the doubling time (18 months) used by Sengupta et al. 130 This means that the results are likely to be over-optimistic as the PSAV and PSADT variables have been optimised to the data. The overall concluding questions to each of the six subheadings are presented in Table 51.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| D’Amico, 2004129 | y | p | p | y | y | y |
| Sengupta, 2005130 | y | y | p | p | p | y |
Summary of the baseline characteristics of the sample
The two studies both had over 1000 participants, with almost all (> 95%) having clinically organ-confined tumours. In the largest study Sengupta et al. 130 evaluated 2290 men who were treated with RP for prostate cancer between 1990 and 1999, with multiple preoperative PSA measurements available. In the study by D’Amico et al. 129 patients were also treated by RP (Table 52).
| Study | n | Primary aim prognostic marker | Treatment |
|---|---|---|---|
| D’Amico, 2004129 | 1095 | Yes | Radical prostatectomy |
| Sengupta, 2005130 | 2290 | Yes | Radical prostatectomy |
The distributions of Gleason and PSA scores (where reported) were similar across studies. Although different cut-points were used in the two studies for PSAV, the proportions in the high-velocity groups were similar at 20.1% and 23.9% respectively. Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual studies identified
Table 53 presents a summary of the main statistical findings from the two studies included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Events | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|---|
| D’Amico, 2004129 (PSAV at diagnosis/also at prostatectomy) | Univariate | Not applicable | Recurrence (two consecutive PSA > 0.2 ng/ml) | PSAV ≤ 2.0 ng/ml/year 247; PSAV > 2.0 ng/ml/year 119 | Not reported | Cox regression, PSAV at diagnosis: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 1.6 (95% CI 1.3–2.1) | < 0.001 |
| Univariate | Not applicable | Death from prostate cancer | PSAV ≤ 2.0 ng/ml/year 3; PSAV > 2.0 ng/ml/year 24 | Not reported | Cox regression, PSAV at diagnosis: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 20.4 (95% CI 6.2–67.9) | < 0.001 | |
| Univariate | Not applicable | Death from any cause | PSAV ≤ 2.0 ng/ml/year 45; PSAV > 2.0 ng/ml/year 39 | Not reported | Cox regression, PSAV at diagnosis: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 2.6 (95% CI 1.6–4.1) | < 0.001 | |
| D’Amico, 2004129 (PSAV at prostatectomy) | Univariate | Not applicable | Death from prostate cancer | PSAV ≤ 2.0 ng/ml/year 3; PSAV > 2.0 ng/ml/year 24 | Cox regression, PSAV at prostatectomy: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 20.4 (95% CI 6.2–67.9) | < 0.001 | |
| Univariate | Not applicable | Death from any cause | PSAV ≤ 2.0 ng/ml/year 45; PSAV > 2.0 ng/ml/year 39 | Cox regression, PSAV at prostatectomy: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 2.2 (95% CI 1.4–3.4) | < 0.001 | ||
| D’Amico, 2004129 (PSAV at diagnosis) | Multivariate | Clinical PSA, Gleason score | Recurrence (two consecutive PSA > 0.2 ng/ml) | PSAV ≤ 2.0 ng/ml/year 247; PSAV > 2.0 ng/ml/year 119 | Not applicable | Cox regression, PSAV at diagnosis: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 1.5 (95% CI 1.1–1.9) | 0.003 |
| Multivariate | Clinical PSA, Gleason score | Death from prostate cancer | PSAV ≤ 2.0 ng/ml/year 3; PSAV > 2.0 ng/ml/year 24 | Not applicable | Cox regression, PSAV at diagnosis: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 9.8 (95% CI 2.8–34.3) | < 0.001 | |
| Multivariate | Clinical PSA, Gleason score | Death from any cause | PSAV ≤ 2.0 ng/ml/year 45; PSAV > 2.0 ng/ml/year 39 | Not applicable | Cox regression, PSAV at diagnosis: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 1.9 (95% CI 1.2–3.2) | 0.01 | |
| D’Amico, 2004129 (PSAV at prostatectomy) | Multivariate | Pathological Gleason score, surgical margins (also nodal status) | Death from prostate cancer | PSAV ≤ 2.0 ng/ml/year 3; PSAV > 2.0 ng/ml/year 24 | Not applicable | Cox regression, PSAV at prostatectomy: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 12.8 (95% CI 3.7–43.7) | < 0.001 |
| Multivariate | Pathological Gleason score, surgical margins (also nodal status) | Death from any cause | PSAV ≤ 2.0 ng/ml/year 45; PSAV > 2.0 ng/ml/year 39 | Not applicable | Cox regression, PSAV at prostatectomy: PSAV > 2.0 ng/ml/year (reference PSAV ≤ 2.0 ng/ml/year): RR 1.8 (95% CI 1.1–2.8) | 0.01 | |
| Sengupta, 2005130 (PSADT) | Univariate | Not applicable | Survival from biochemical progression (PSA ≥ 0.4 ng/ml; patients without progression censored at time of last PSA determination) | Not reported | Preoperative PSADT < 18 months 74%; PSADT ≥ 18 months 84% | Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR 1.58 (95% CI 1.32–1.89) | < 0.0001 |
| Univariate | Not applicable | Survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa) | Not reported | Preoperative PSADT < 18 months 92%; PSADT ≥ 18 months 96% | Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR 2.53 (95% CI 1.83–3.48) | < 0.0001 | |
| Univariate | Not applicable | Survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) | Not reported | Preoperative PSADT < 18 months 96%; PSADT ≥ 18 months 99% | Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR 6.22 (95% CI 3.33–11.61) | < 0.0001 | |
| Sengupta, 2005130 (PSAV) | Univariate | Not applicable | Survival from biochemical progression (PSA ≥ 0.4 ng/ml; patients without progression censored at time of last PSA determination) | Not reported | Preoperative PSAV > 3.4 ng/ml/year 66%; preoperative PSAV ≤ 3.4 ng/ml/year 86% | Cox proportional hazards, preoperative PSAV > 3.4 ng/ml/year (reference preoperative PSAV ≤ 3.4 ng/ml/year or less): HR 2.28 (95% CI 1.92–2.71) | < 0.0001 |
| Univariate | Not applicable | Survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa) | Not reported | Preoperative PSAV > 3.4 ng/ml/year 96%; preoperative PSAV ≤ 3.4 ng/ml/year 90% | Cox proportional hazards, preoperative PSAV > 3.4 ng/ml/year (reference preoperative PSAV ≤ 3.4 ng/ml/year or less): HR 2.53 (95% CI 1.83–3.50) | < 0.0001 | |
| Univariate | Not applicable | Survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) | Not reported | Preoperative PSAV > 3.4 ng/ml/year 98%; preoperative PSAV ≤ 3.4 ng/ml/year 96% | Cox proportional hazards, preoperative PSAV > 3.4 ng/ml/year (reference preoperative PSAV ≤ 3.4 ng/ml/year or less): HR 6.54 (95% CI 3.51–12.19) | < 0.0001 | |
| Multivariate | Clinical PSA, stage, Gleason (also treatment year) (PSADT removed from model) | Survival from biochemical progression (PSA ≥ 0.4 ng/ml; patients without progression censored at time of last PSA determination) | Not reported | Not applicable | Stepwise Cox proportional hazards, preoperative PSAV > 3.4 ng/ml/year (reference preoperative PSAV ≤ 3.4 ng/ml/year or less): HR 1.49 (95% CI 1.17–1.90) | PSAV: p = 0.001 (PSADT not included, not significant) | |
| Sengupta, 2005130 (PSADT) | Multivariate | Clinical stage, Gleason (PSAV removed from model) | Survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa) | Not reported | Not applicable | Stepwise Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR 1.83 (95% CI 1.24–2.72) | PSADT: p = 0.003 (PSAV not included, not significant) |
| Sengupta, 2005130 (PSADT and PSAV) | Multivariate | Clinical Gleason (also treatment year) (PSAV removed from model) | Survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) | Not reported | Not applicable | Stepwise Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR 2.30 (95% CI 1.77–2.98) | PSADT: p < 0.0001 (PSAV not included, not significant) |
| Sengupta, 2005130 (PSAV) | Multivariate | Clinical PSA, pathological stage, Gleason, surgical margins (also treatment year, seminal vesicle involvement, lymph node involvement, adjuvant therapy) (PSADT removed from model) | Survival from biochemical progression (PSA ≥ 0.4 ng/ml; patients without progression censored at time of last PSA determination) | Not reported | Not applicable | Stepwise Cox proportional hazards, preoperative PSAV > 3.4 ng/ml/year (reference preoperative PSAV ≤ 3.4 ng/ml/year): HR 1.30 (95% CI 1.06–1.58) | PSAV: p = 0.011 (PSADT not included, not significant) |
| Sengupta, 2005130 (PSADT) | Multivariate | Pathological Gleason, surgical margins (also treatment year, seminal vesicle involvement, adjuvant therapy, estimated cancer volume) (PSAV removed from model) | Survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa) | Not reported | Not applicable | Stepwise Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR 1.80 (95% CI 1.26–2.57) | PSADT: p = 0.001 (PSAV not included, not significant) |
| Multivariate | Pathological Gleason, surgical margins (also treatment year, seminal vesicle involvement, estimated cancer volume) (PSAV removed from model) | Survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) | Not reported | Not applicable | Stepwise Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR 3.92 (95% CI 1.95–7.85) | PSADT: p = 0.0001 (PSAV not included, not significant) |
Both studies report a Cox multivariate analysis of the data. Table 53 shows the different clinical and pathological classical markers entered into the statistical models across the studies, together with the results of each analysis.
Sengupta et al. 130 calculated PSADT by log-linear regression and PSAV by linear regression. Each of these parameters was used in preoperative and postoperative multivariate models for the end points of biochemical and clinical progression, and cancer death, but only one remained in each model. PSAV appeared to be a better predictor of biochemical progression, and PSADT of clinical progression and death. Of all the predicted outcomes the association with cancer death appeared to be the strongest. In the clinical model the HR for death from prostate cancer was 6.18 (95% CI 2.75–13.88, p < 0.0001) in men with a PSADT of less than 18 months versus men with a PSADT of 18 months or more; similarly, the HR was 3.92 (95% CI 1.95–7.85, p = 0.0001) in the pathological model.
D’Amico et al. 129 also reports a particularly strong association between PSAV and prostate cancer death in both clinical and pathological models. In the clinical model the HR for death from prostate cancer was 9.8 (95% CI 2.8–34.3, p < 0.001) in men with an annual PSAV of more than 2 ng/ml versus an annual PSAV of 2 ng/ml or less; similarly, the HR was 12.8 (95% CI 3.7–43.7, p < 0.001) in the pathological model.
Overall conclusions based on the results and quality of the findings
Both of these large, good-quality studies report compelling results showing an association between PSA kinetics and prostate cancer outcomes, and in particular cause-specific mortality. This result remained significant in the presence of other clinical and pathological variables. However, with both studies using data-dependent cut-points to define high and low PSAV the results will be over-optimistic. Whereas D’Amico et al. 129 derived an optimum cut-point of 2.0 ng/ml/year, Sengupta et al. 130 found 3.4 ng/ml/year gave the best results. Use of the other cut-points in the two data sets would give more realistic estimates of how this prognostic marker would perform in practice. A review of monitoring protocols for men with localised prostate cancer143 showed that in some research protocols PSAV and PSADT were already used, in conjunction with other factors, to identify disease progression that might require radical treatment. Note that in the UK regular measurements of PSA are not routinely available before diagnosis as was the case in these two studies, as regular PSA screening is not normal practice.
Sengupta et al. 130 concluded that, although PSADT may perform more accurately and strongly in multivariate analysis than PSAV, PSAV is simpler to derive and therefore more easily used in clinical practice.
Stat5 activation status
One study131 was concerned with the prognostic significance of the novel marker Stat5 activation status.
Brief description of the prognostic marker
Signal transducer and activator of transcription-5 (Stat5) is a signalling protein that is activated by prolactin in normal and malignant prostates. The definition of the marker and its distribution in the population studied are shown in Table 54.
| Study | Definition | Population distribution |
|---|---|---|
| Li, 2005131 | Signal transducer and activator of transcription-5 (Stat5) is a signalling protein that is activated by prolactin in normal and malignant prostates. Individual prostate tumour samples were scored (MTN and HL) for active and nuclear Stat5 levels on a scale from 0 to 1, where 0 was undetectable and 1 represented positive immunostaining | Stat5 activation status: negative, n = 141 (25.7%); positive, n = 216 (39.4%); unknown, n = 191 (34.9%) |
Brief description of the objectives of the individual study identified
The study aimed to investigate whether activation of Stat5 in prostate cancer was linked to clinical outcome with disease recurrence as an end point. The basic study design characteristics are summarised in Table 55.
| Study | n | Primary aim to assess prognostic marker | Treatment |
|---|---|---|---|
| Li, 2005131 | 357 | Yes | Radical prostatectomy or TURP |
Quality of the individual study identified
In general this was a good quality study. Unusually it was very specific as to the events that were included as the end points, but the number of events was not stated and so the EPV is unknown. In interpreting the results the omission of PSA from the multivariate analysis must be considered. As with many prognostic studies in this systematic review the study did not provide details about the storage of materials, although it was clear that the study was based on archival specimens. The overall concluding questions to each of the six subheadings are presented in Table 56.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Li, 2005131 | p | p | p | p | p | p |
Summary of the baseline characteristics of the sample
The study involved 357 participants who had been treated with RP or TURP. At pathological stage there were still a greater number of organ-confined (79.5%) than non-organ-confined participants (19.7%), with a small number of participants having missing data (0.7%). The Gleason scores ranged between 2 and 5 but PSA levels were not reported. The failure to measure and report this information limits the ability to compare this study with other prognostic studies involving other types of markers. Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual study identified
Li et al. 131 provided a multivariate analysis of the data. Non-significant factors were removed from the multivariate model. The end point was progression-free survival, with clinical recurrence, PSA recurrence and prostate cancer deaths all treated as events. The HRs and p-values are shown for the univariate analyses and for the variables kept in the multivariate model. Univariate analysis showed that Stat5 activation was associated with early disease recurrence (p = 0.04). However, in multivariate analysis Stat5 activation status only reached borderline significance in its association with progression-free survival (HR 1.63; 95% CI 0.99–2.69; p = 0.057) in a model that included Gleason grade and stage but not PSA. The effect size (HR = 1.6) was similar to that for grade (HR = 2.0) and stage (HR = 2.0). A subgroup analysis of patients with intermediate Gleason grade prostate cancers (3 and 4; 325 of the total patient sample of 357) showed similar results. Table 57 presents a summary of the main statistical findings from this study.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Li, 2005131 | Univariate | Not applicable | Survival from progression [events – clinical (bone scan, chest radiography, digital rectal examination) and increase in PSA125] | Estimated from survival curve, 5-year survival: positive for active Stat5 80%; negative for active Stat5 88% | Cox proportional hazards, Stat5 positive with reference negative: regression coefficient 0.4884 (SE 0.256) | 0.0399 |
| Multivariate | Pathological stage, Gleason grade (also perineural invasion, seminal vesicle infiltration) | Survival from progression [events – clinical (bone scan, chest radiography, digital rectal examination) and increase in PSA125] | Not applicable | Cox proportional hazards, Stat5 positive with reference negative: HR 1.630 (95% CI 0.99–2.69) | 0.0565 |
Overall conclusions based on the results and quality of the findings
Although the current study was found to be adequate in terms of key quality factors considered to be important when evaluating prognostic studies, there were shortcomings that make the result inconclusive: the absence of PSA from the analysis and the uncertain (possibly inadequate) number of EPV needed to give a statistically reliable result. To establish whether Stat5 really adds prognostic value to the established markers it needs to be tested in a study that addresses these issues. The authors claim that the predictive value of active Stat5 in prostate cancers of intermediate and low histological grades might be improved by an analysis of other prognostic markers in conjunction with active Stat5 (e.g. Ki67, p53, Bcl-2, syndecan-1125). This hypothesis needs to be tested.
Tumour size
Five studies105,106,124,130,132 were concerned with the prognostic significance of tumour size.
Brief description of the prognostic marker
Two principal approaches have been used to estimate tumour size: tumour volume and maximum tumour dimension. The estimate used in each study together with the measurement methods and values are shown in Table 58.
| Study | Definition | Population distribution |
|---|---|---|
| Blute, 2001105 | Maximum tumour dimension (mm). Measurement method not specified (pathological) | < 1.5 mm, n = 369 (15%); 1.5–2.4, n = 706 (28%); 2.5–3.0, n = 292 (12%); 3.0+, n = 805 (32%); missing 14% |
| Lieber, 1995106 | Tumour volume (cm3) ‘crudely estimated by three-dimensional measurements of cut specimens. Serial sectioning and mapping were not performed’ (pathological) | ≤ 1 cm3, n = 228 (47.5%); > 1 cm3 n = 252 (52.5%) |
| Salomon, 2003132 | Tumour volume (cc = cm3) estimated from the area of each slide, with all volume calculations multiplied by a factor of 1.5 to take into account differences between fresh and processed specimens. More detail in paper (pathological) | Mean = 1.35 ± 1.5; range = 0.01–8.1 |
| Sengupta, 2005130 | Maximum tumour dimension and tumour volume ‘estimated based on measured tumour dimensions using an elliptical formula’ (pathological) | Not stated |
| Vis, 2007124 | Length of tumour (mm) (biopsy specimen) | Median = 7.2; range = 0.4–51.0 |
It is not clear whether any of the measures are the same, but the values for tumour volume reported by Lieber et al. 106 and Salomon et al. 132 appear consistent with each other. Note that the measure of tumour dimension used by Vis et al. 124 is clearly different to those used by Blute et al. 105 and Sengupta et al. ,130 being from biopsy cores rather than from the pathological specimen.
Brief description of the objectives of the individual studies identified
Only one of the studies had a primary objective of assessing the prognostic significance of tumour size. 132 Salomon et al. 132 aimed to evaluate the association between Gleason score, stage and status of surgical margins and tumour volume in prostate cancer progression after RP. Three studies had the objective of investigating other novel markers,106,124,130 and one developed a prognostic model. 105
Quality of the individual studies identified
The overall concluding questions to each of the six subheadings are presented in Table 59.
| Study | Study population | Study attrition | Prognostic factor measurement | Outcome measurement | Confounding measurement and account | Analysis |
|---|---|---|---|---|---|---|
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results | Loss to follow-up is not associated with key characteristics | Prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | Outcome of interest is adequately measured in study participants to sufficiently limit potential bias | Model includes all classical markers | Statistical analysis is appropriate for the study design, limiting potential for the presentation of invalid results | |
| Blute, 2001105 | y | ? | y | p | p | y |
| Lieber, 1995106 | p | p | p | p | p | y |
| Salomon, 2003132 | y | ? | p | y | p | p |
| Sengupta, 2005130 | y | y | p | p | p | y |
| Vis, 2007124 | y | ? | p | p | n | p |
The principal weakness present in all of these studies is that the classical markers were not present or kept in all analyses and so the additional prognostic value of tumour size in the presence of known markers is not clear. In particular, several analyses omitted PSA, a classical marker that may be associated with tumour volume. The only study that had the assessment of tumour size as its main objective132 did not use a time to failure analysis (Cox regression) and so the statistical analysis is weak.
Summary of the baseline characteristics of the sample
The five studies included a wide range of samples sizes, from 281124 to 2290. 130 All five studies were based on patients who had received RP treatment (Table 60).
| Study | n | Primary aim prognostic marker | Treatment |
|---|---|---|---|
| Blute, 2001105 | 2000 | No | Radical prostatectomy |
| Lieber, 1995106 | 494 | Yes | Radical prostatectomy |
| Salomon, 2003132 | 357 | Yes | Radical prostatectomy |
| Sengupta, 2005130 | 2290 | Yes | Radical prostatectomy |
| Vis, 2007124 | 281 | Yes | Radical prostatectomy |
In evaluating the results of the five studies it is important to consider the differences in sample characteristics (e.g. stage, Gleason score and PSA distributions). The clinical stage of the participants was provided in four of the five studies (not that of Lieber et al. 106). More than 90% of the samples in the four studies were made up of organ-confined participants at clinical stage. Lieber et al. 106 had 18% of patients who were found pathologically to have positive regional lymph nodes, which is high compared with the other studies in this group. The distributions of Gleason and PSA scores (where reported) were similar across studies. Additional summary characteristics are provided in Appendix 7.
Brief description of the results from the individual studies identified
Tables 61 and 62 present a summary of the main statistical findings from the five studies included in this section.
| Study | Statistical analysis | Classical markers in model | End point | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|
| Blute, 2001105 (maximum tumour dimension) | Univariate | Not applicable | Biochemical progression-free survival (events – local recurrence or systemic progression or biochemical recurrence defined as PSA ≥ 0.4 ng/ml) | 5-year survival, maximum tumour dimension: < 1.5 mm 86% (SE 1.9); 1.5–2.4 mm 82% (SE 1.5); 2.5–3.0 mm 79% (SE 2.5); ≥ 3.0 mm 68% (SE 1.7) | Not reported | < 0.001 |
| Sengupta, 2005130 (maximum tumour dimension) | Univariate | Not applicable | Survival from biochemical progression (PSA ≥ 0.4 ng/ml; patients without progression censored at time of last PSA determination) | Not reported | Cox proportional hazards model, maximum cancer dimension: HR 1.19 (95% CI 1.15–1.23) | < 0.0001 |
| Univariate | Not applicable | Survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa) | Not reported | Cox proportional hazards model, maximum cancer dimension: HR 1.24 (1.17–1.30) | < 0.0001 | |
| Univariate | Not applicable | Survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) | Not reported | Cox proportional hazards model, maximum cancer dimension: HR 1.28 (1.18–1.39) | < 0.0001 | |
| Multivariate | PSA, clinical stage, biopsy Gleason, pathological stage, pathological Gleason, surgical margin (also age, treatment year, PSADT, PSAV, cancer volume, seminal vesicle, lymph nodes, adjuvant therapy) | All above outcomes: survival from biochemical progression; survival from clinical progression; survival from death from prostate cancer | Not applicable | Not reported | Not significant (removed by forward selection if p > 0.10) | |
| Vis, 2007124 [length (mm) of tumour] | Univariate | Not applicable | Biochemical recurrence (PSA ≥ 0.1 ng/ml) | Not reported | Cox proportional hazards model, length (mm) of tumour (as continuous variable): HR 1.055 | < 0.001 |
| Univariate | Not applicable | Clinical progression (local progression and/or distant metastases) | Not reported | Cox proportional hazards model, length (mm) of tumour (as continuous variable): HR 1.037 | 0.098 | |
| Multivariate | PSA (also length of high-grade cancer in mm) | Biochemical recurrence (PSA ≥ 0.1 ng/ml) | Not applicable | Length (mm) of tumour : HR 1.012 | 0.04 | |
| Multivariate | Clinical stage, Gleason score, PSA (also number of positive biopsy cores and length of high-grade cancer in mm) | Clinical progression (local progression and/or distant metastases) | Not applicable | Length (mm) of tumour : not reported | Not reported but not significant |
| Study | Statistical analysis | Classical markers in model | End point | Events | Survival | Outcome measure | p-value |
|---|---|---|---|---|---|---|---|
| Lieber, 1997106 (tumour volume) | Univariate | Not applicable | Survival from clinical progression [events – disease progression based on clinical examination (not routine PSA measurements); censoring at last follow-up for patients who had not had progression or who had died] | Tumour volume ≤ 1 cm3, 64; tumour volume > 1 cm3, 106 | Not reported | HR for tumour volume > 1 cm3 (with reference tumour volume ≤ 1 cm3) 1.691 (95% CI 1.239–1.486); χ2 = 11.24 | Log-rank 0.0008 |
| Univariate | Not applicable | Survival from death from prostate cancer, ‘cause-specific survival’ (events – death from prostate cancer only; censoring at last follow-up for patients who had not had progression or who had died) | Tumour volume ≤ 1 cm3, 23; tumour volume > 1 cm3, 48 | Not reported | HR for tumour volume > 1 cm3 (with reference tumour volume ≤ 1 cm3) 1.891 (95% CI 1.150–3.111); χ2 = 6.52 | Log-rank 0.0107 | |
| Univariate | Not applicable | Overall survival (events – death from any cause; censoring at last follow-up for patients who had not had progression or who had died) | Tumour volume ≤ 1 cm3, 77; tumour volume > 1 cm3, 96 | Not reported | HR for tumour volume > 1 cm3 (with reference tumour volume ≤ 1 cm3) 1.1.0 (95% CI 0.821–1.497); χ2 = 0.45 | Log-rank 0.5026 | |
| Multivariate | Gleason score, pathological stage (also ploidy, adjuvant therapy) | All outcomes: survival from clinical progression; survival from death from prostate cancer; overall survival | Not reported | Not applicable | Not reported | Not significant (removed from model in stepwise process) | |
| Salomon, 2003132 (tumour volume) | Univariate | Not applicable | Survival from biochemical recurrence (events single PSA level > 0.2 ng/ml) | Not reported | Not reported | Tumour volume (Fisher test) | 0.009 |
| Multivariate | Pathological stage, Gleason score, surgical margins | Survival from biochemical recurrence (events single PSA level > 0.2 ng/ml) | Not reported | Not applicable | Tumour volume (unclear, but possibly analysed as continuous): OR 1.09 (95% CI 0.90–1.31) | 0.35 | |
| Vis, 2007124 [tumour volume (ml)] | Univariate | Not applicable | Biochemical recurrence (PSA ≥ 0.1 ng/ml after RP) | Not reported | Not reported | Cox regression model, tumour volume (ml): HR 1.401 | < 0.001 |
| Multivariate | RP Gleason score, surgical margins (also extraprostatic extension, invasion of adjacent organs) | Biochemical recurrence (PSA ≥ 0.1 ng/ml after RP) | Not reported | Not applicable | Cox regression model, tumour volume (ml): not reported | Not reported but not significant | |
| Sengupta, 2005130 (estimated cancer volume) | Univariate | Not applicable | Survival from biochemical progression (PSA ≥ 0.4 ng/ml; patients without progression censored at time of last PSA determination) | Not reported | Not reported | Cox proportional hazards model, estimated cancer volume: HR 1.05 (95% CI 1.04–1.06) | < 0.0001 |
| Univariate | Not applicable | Survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa) | Not reported | Not reported | Cox proportional hazards model, estimated cancer volume: HR 1.06 (95% CI 1.04–1.07) | < 0.0001 | |
| Univariate | Not applicable | Survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) | Not reported | Not reported | Cox proportional hazards model, estimated cancer volume: HR 1.07 (95% CI 1.06–1.09) | < 0.0001 | |
| Mulitvariate | PSA, pathological stage, Gleason score, surgical margins (also treatment year, preoperative PSAV, seminal vesicle involvement, lymph node involvement, adjuvant therapy) | Survival from biochemical progression (PSA ≥ 0.4 ng/ml; patients without progression censored at time of last PSA determination) | Not reported | Not applicable | Not reported | Not significant (removed in stepwise process if p > 0.10) | |
| Mulitvariate | Gleason score, surgical margins (also treatment year, preoperative PSDAT, preoperative PSAV, seminal vesicle involvement, adjuvant therapy) | Survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa) | Not reported | Not applicable | Stepwise analysis: HR 1.03 (95% CI 1.01–1.05) | 0.0008 | |
| Mulitvariate | Gleason score, surgical margins (also treatment year, preoperative PSDAT, preoperative PSAV, seminal vesicle involvement) | Survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) | Not reported | Not applicable | Stepwise analysis: HR 1.05 (95% CI 1.02–1.08) | 0.003 |
Maximum tumour dimension
Two studies105,130 report analyses of maximum tumour dimension with PSA recurrence, clinical recurrence and prostate cancer death all used as outcomes in different analyses. In both studies maximum tumour dimension was found to be significant in univariate analysis but not in multivariate analysis. With biochemical progression as the outcome, Vis et al. 124 found length of tumour in biopsy cores significant in univariate and multivariate analysis (p = 0.04), but the multivariate analysis included only one of the classical markers, PSA. With the outcome of clinical progression, length of tumour in biopsy cores was not significant in univariate or multivariate analysis.
Tumour volume
Four studies106,124,130,132 report several analyses of this marker with different end points: PSA recurrence, clinical recurrence, prostate cancer death and all deaths. In univariate analyses, except that with all deaths as the outcome,106 tumour volume was reported to be significant. In multivariate analysis it was not found to be significant in the studies of Lieber et al. 106, Salomon et al. 132 or Vis et al. 124 Sengupta et al. 130 did not find it to be significant in an analysis with biochemical recurrence as the end point but did find it to be a significant predictor of clinical progression (p = 0.0008) and prostate cancer death (p = 0.003). It may be of note that PSA and stage were included in the first analysis but were not in the last two analyses (i.e. tumour volume was only significant in the absence of PSA and stage in the model). The association between tumour volume and PSA may account for the results of Sengupta et al. 130
Overall conclusions based on the results and quality of the findings
All of these studies have weaknesses that make their individual results inconclusive with respect to the significance of tumour size as a prognostic indicator; however, the direction of evidence suggests that maximum tumour dimension, length of tumour in the biopsy core and tumour volume are not independent prognostic parameters after other routinely assessed variables are accounted for. Tumour volume was only found to be significant in multivariate models that did not include PSA or stage. 130
Conclusions
This chapter has provided the first comprehensive systematic review of all potential novel prognostic markers for patients with early localised prostate cancer. It also included a quality assessment of all studies. In total, 28 relevant novel marker articles met the inclusion criteria, reporting 17 novel marker categories. Previous reviews have listed tens of potential markers (e.g. Tricoli et al. 4). The inclusion criteria used in this review, particularly the restriction of the sample size to 200 or more and the requirement for a mean or median follow-up of at least 5 years, led to many papers being rejected. This suggests that much of the research on novel markers is based on sample sizes that are likely to be too small to yield statistically reliable results, and of insufficient follow-up to provide reliable indicators of long-term outcomes. Despite having to meet the inclusion criteria used in this review, many of the included studies were found to be lacking statistical power in terms of having insufficient events for the number of variables in the multivariate models.
The considerable variability in the results reported within the prognostic marker categories and the lack of studies for some categories has made it difficult to provide clear conclusions as to which markers might offer the most potential as prognostic parameters for localised prostate cancer. The large heterogeneity and poor standard of reporting/quality meant that it was not possible to quantitatively synthesise the results. We have paid particular attention in this chapter to the quality of studies. Key quality issues that commonly affected the potential to draw conclusions from these studies were the lack of classical markers in the statistical models and insufficient EPV. Other common issues were the failure to indicate reasons for drop out, the failure to adequately describe the storage of material and specific aspects of analysis and reporting. In general, the description of the study population was reported to a higher quality standard than the other quality criteria. We believe that our systematic review has provided an important insight into the complexities of developing a suitable quality tool for assessing the quality of studies.
There is insufficient evidence at present to judge the clinical utility of most prognostic markers highlighted in this chapter. However, the review has gone some way to identifying those markers that have possible prognostic importance. The clinical interpretation of these findings is difficult because of the differences in quality and the inconsistency of reporting across the literature. Note that in none of the novel marker studies was it considered whether a marker was prognostic or predictive. Given that in the majority of studies patients all had the same principal treatment this was not possible to assess.
In Table 63 each of the markers has been placed into one of three categories dependent on the direction and strength of the evidence for each in terms of adding prognostic value to the established markers: (i) promising; (ii) not promising; (iii) inconclusive. Note that the classifications are indicative only: the evidence for most markers is poor, and publication bias and selective reporting of outcomes may have affected the results. The text after the classification summarises the nature of the evidence; however, the evidence reported in the main body of this section must also be considered. Those markers that did not appear to be prognostic according to the studies included in this review were placed in the ‘not promising’ category. However, many of these studies have weaknesses or are simply too small to give reliable results. Those placed in the category of ‘promising’ were supported by at least one good quality multivariate study or several weaker studies with consistent results or when the stronger of several studies consistently showed a positive result. The rest of the markers, those for which the studies gave contradictory results or for which there was very little evidence (e.g. only one univariate analysis) on which to base a conclusion, were placed in the ‘inconclusive’ category.
| Study | Relevant articles (first author, year of publication) | Assessment of future application |
|---|---|---|
| β-catenin expression: < 10% vs ≥ 10% nuclei | Horvath, 2005108 | Not promising |
| Association between PSA and β-catenin found. If this is confirmed β-catenin is unlikely to add prognostic value to existing markers. Significant predictor in univariate analysis, but not in multivariate analysis, for biochemical recurrence in a single study of low power | ||
| Acid phosphatase level | Anscher, 1991;109 Han, 2001;110 Perez, 1989;111 Roach, 1999;112 Zagars, 1993113 | Promising |
| One study110 of reasonable quality and likely statistically well powered included all of the classical markers in the multivariate model and found the marker to be highly significant. The other studies were weaker and did not include PSA in analysis, but most analyses with prostate-specific outcomes found this marker to be significantly prognostic | ||
| Androgen receptor: CAG repeats | Nam, 2000;114 Powell, 2005115 | Inconclusive |
| One study114 did not find the marker to be significant in univariate or multivariate analysis but this study must be considered unreliable because of the small number of patients with short CAG repeats (≤ 18 CAG repeats). Powell et al.115 with a larger patient sample did show a significant association between this marker and disease progression in one analysis | ||
| Creatinine | Merseburger, 2001;116 Zagars, 1987117 | Not promising |
| The results of neither study indicate that creatinine is a useful prognostic marker for prostate cancer; however, the results cannot be considered conclusive as both studies had statistical weaknesses | ||
| CYP3A4 genotypes | Powell, 2004118 | Inconclusive |
| A single study found CYP3A4 genotypes to be significantly prognostic. May be race/genotype interactions | ||
| DNA ploidy | Blute, 2001;105 Lieber, 1995;106 Siddiqui, 2006119 | Inconclusive |
| Contradictory results from large studies, two of which may share some data. None of the studies include an absolute measure of preoperative PSA, although it appears to be available in some of the data. The relationship between DNA ploidy and clinical and biochemical outcomes with and without PSA as a covariate could be explored in the data of Siddiqui et al.119 and/or Blute et al.105 (if not the same) and this might resolve the contradictions apparent from the current analyses | ||
| Germline genetic variation in the vitamin D receptor | Williams, 2004120 | Not promising |
| The primary analysis indicated that vitamin D receptor gene polymorphisms are not prognostic in prostate cancer but some (possibly statistically weak) subgroup analyses gave some significant results, with the B allele having an opposite effect in different groups. The authors claim that the complexity of the biological effects of vitamin D in experimental studies supports the possibility of complex clinical effects. The plausibility of such effects would need to be considered before pursuing vitamin D receptor gene polymorphisms as a prognostic marker in prostate cancer | ||
| Non-classical use of Gleason measurements: (a) Gleason pattern in Gleason score 7 (4 + 3 vs 3 + 4); (b) amount of high-grade cancer; (c) modified Gleason score | Egevad, 2002;121 Gonzalgo, 2006;122 Tollefson, 2006;123 Vollmer, 2001107 | (a) Promising |
| But on the basis of only one poorly reported multivariate analysis that was likely adequately powered. Would be simple to implement as uses data already collected | ||
| (b) Promising | ||
| On the basis of three studies using three different measures, none of which included all of the classical markers | ||
| (c) Inconclusive | ||
| A single study121 found a modified Gleason score to be prognostic of prostate cancer death but the marker was not tested in a multivariate model with classical markers | ||
| Ki67 LI, Bcl-2, p53, syndecan-1, CD10 | Zellweger, 2003125 | Inconclusive |
| The weaknesses of the study make the results inconclusive. Ki67 LI appeared to be the most strongly associated with the study end points and in particular tumour-specific survival (p = 0.023) | ||
| Proportion cancer: (a) percentage positive biopsy cores; (b) percentage of cancer in surgical specimen | Antunes, 2005;126 Egevad, 2002;121 Potters, 2005;127 Selek, 2003;128 Vis, 2007;124 Vollmer, 2001107 | (a) Promising |
| The results of these studies are mixed, but the two studies that showed positive results had greater statistical power than the others, and also included the classical markers in multivariate analysis126,127 | ||
| (b) Inconclusive | ||
| Two studies found the marker significantly prognostic, but neither included PSA or stage in their models | ||
| PSA kinetics | D’Amico, 2004;129 Potters, 2005;127 Sengupta, 2005130 | Promising |
| Two large, good-quality studies reported a strong association between PSA kinetics and prostate cancer outcomes, the result remaining significant in the presence of classical markers. However, both studies used (different) data-dependent cut-points to define high and low PSAV and so the results will be over-optimistic. Use of the other cut-point in the two data sets would give more realistic estimates of how this prognostic marker would perform in practice | ||
| Stat5 activation status | Li, 2005131 | Inconclusive |
| A single study with some limitations found Stat5 to be marginally significant for disease progression | ||
| Tumour size: (a) maximum tumour dimension; (b) tumour volume | Blute, 2001;105 Egevad, 2002;121 Lieber, 1995;106 Salomon, 2003;132 Vis, 2007124 | (a) Not promising |
| Pathological tumour dimension not significant in two studies with multivariate analyses. Length of cancer from biopsy core marginally significant in only one of three analyses | ||
| (b) Not promising | ||
| Only significant in one of several multivariate analyses, and this did not include PSA or stage as a covariate |
To summarise, the markers fall into the following categories:
-
Promising:
-
acid phosphatase level
-
Gleason pattern in Gleason score 7 (4 + 3 versus 3 + 4) (non-classical use of Gleason measurements)
-
amount of high-grade cancer (non-classical use of Gleason measurements)
-
PSA kinetics (PSAV/PSADT)
-
percentage positive biopsy cores (proportion of cancer).
-
-
Not promising:
-
β-catenin expression
-
creatinine
-
germline genetic variation in the vitamin D receptor
-
maximum tumour dimension (tumour size)
-
tumour volume (tumour size).
-
-
Inconclusive:
-
percentage cancer in surgical specimen (proportion of cancer)
-
androgen receptor: CAG repeats
-
DNA ploidy
-
CYP3A4 genotypes
-
modified Gleason score (non-classical use of Gleason measurements)
-
Ki67 LI
-
Bcl-2
-
p53
-
syndecan-1
-
CD10
-
Stat5 activation status.
-
The evidence for all markers is weak, with the exception of that for PSAV for which there are two large, good-quality studies. However, even in this case the results are likely to be over-optimistic because of methodological weaknesses and in particular the use of multiple testing to determine the optimum cut-point for high- and low-risk groups. 144 It is clear that large studies are needed with adequate follow-up. Particular attention needs to be paid to ensuring sufficient outcome events in minority prognostic groups. To combine data from different centres there must be agreement on study outcomes, and in particular disease recurrence. A bank of stored prostate material together with long-term follow-up data would allow the rapid evaluation of new markers as they become available. Almost none of the studies makes reference to patient consent. Clearly this should be addressed if such archive material and data are put to this use.
Chapter 6 Results for systematic review of prognostic models
In this chapter some general features of prognostic models will be presented, followed by the results of the review. The prognostic models identified by the literature search that met our inclusion criteria will be discussed in terms of the study objectives, study design, study quality, presentation of models and model performance.
General issues in prognostic modelling
It is generally agreed in the literature that, when creating a prognostic model, the aim is to produce a model that makes sense clinically as well as statistically. Altman and Royston145 suggest that it is more important to focus on a prognostic model that makes clinical sense – one in which the variables included in the model are known predictors of survival – and that ‘a clinically validated model is likely to be more useful than a statistically validated model’.
The literature on prognostic models also seems to agree that external validity is much more important than internal validity, as the whole idea of producing a prognostic model is that it can be used on other cohorts of patients to predict their prognosis. 146,147 However, a model should not be assessed based on one criterion alone, for example the C-statistic for discrimination, but should be assessed based upon general performance across a set of clinical, internal performance and external performance criteria.
Internal validation
Internal validity should consider the following questions:
-
Are the data of an acceptable quality (e.g. attrition, etc.)?
-
Does the model make sense clinically and statistically?
-
Has the EPV criterion been met?
Calibration – the predictive probability of the model is measured by comparing observed and predicted values and should be neither too low nor too high.
Discrimination relates to the ranking of severity and can be measured in a number of ways [the relative ranking of risk/severity groups should be ordered, C-statistic, PSEP (Prognostic Separation Index)]. The C-statistic gives a general overview of the discrimination of the model by estimating the probability of all possible pairs of results in which one patient dies and the second patient lives; a discrimination of 0.5 shows no discrimination and a value of 1.0 shows perfect discrimination. The C-statistic should be presented with 95% confidence intervals so that the model reviewer can assess the uncertainty around the estimate; if the CI spans 0.5 this suggests that the model is not discriminating. Similarly, the PSEP statistic, which measures the distance between the probability of prognosis in the most severe group and the least severe group, can be used; the distance should account for the overall degree of severity in the population (a homogeneous population will show little spread). It should be noted that Altman and Royston stress that discrimination should not be the sole criterion used for assessing the usefulness of a prognostic model.
A number of articles suggest that authors of prognostic models should use techniques such as bootstrapping to allow for the problem of overfitting a model (predictions are more precise when validated internally). 145–149 Another possible validation technique is jack-knifing. Although not described as such, it appears that one study used this technique to estimate model performance. 150 Few authors acknowledge or adjust for model overfitting.
External validation
Prognostic models are usually derived to be used in populations other than the data set from which they are being derived. Therefore, external validation is probably the most important step in validating a model, yet it is the step that is the least checked. In terms of external validation the article by Justice et al. 146 presents a comprehensive hierarchy of levels of external validation and this is a good starting point when assessing the external validity of a model. Robust prognostic models should be shown to have predictive accuracy in external data sets that differ historically, geographically and methodologically (in the way the data is collected, e.g. PSA assay technique used), and should be validated across multiple sites, and different risk groups and disease severities.
Model uncertainty
Any estimates that are reported in the models, whether they are regression coefficients, probabilities or nomograms, are based on point estimates and as such they are subject to statistical uncertainty. Therefore, the authors of such models should report a measure of this uncertainty so that future users can account for this in their prognostic estimates and in any decisions that might be made or any information that might be given to patients about future treatments and likely outcomes.
Review of prognostic models in prostate cancer
Only five papers reporting eight models met the inclusion criteria, all of which developed new models. The study by Cowen et al. 150 also included a validation of two other prognostic models, but as neither of these models met the study inclusion criteria the validation part of the study was not included in this review. Although the original objectives were set out in terms of reviewing separately the models with classical markers only and those including novel markers, in view of the small number of models identified they will be discussed together. Only two models do not include any novel markers,105,150 and one of those included several demographic and co-morbidity variables. 150 Han et al. 140 included Gleason pattern in their two models, Lieber et al. 106 tumour ploidy, and Vollmer et al. 107 percentage carcinoma and the presence of high-grade tumour (Gleason 5) in the prostatectomy specimen.
It should be noted that, although the statistical models used to test the novel prognostic markers and to develop prognostic models are the same, to be classified as a model the study needed to present predicted outcomes for different prognostic groups based on a multivariate analysis. Model papers that included novel markers were also included in the novel marker review.
The principal characteristics of the studies are shown in Table 64. Two of the models used prognostic markers that are only available before treatment, whereas the others included some pathological markers. All models were developed on patient groups that had had radical surgery (prostatectomy) except that of Cowen et al. ,150 which included patients who had had different modes of treatment. The end points for the analyses included crude mortality, prostate cancer mortality, clinical recurrence and biochemical (PSA) recurrence. The inclusion criteria for the review meant that all of the included models were based on data that had a mean or median follow-up of at least 5 years. For two studies, follow-up was considerably greater, with Cowen et al. 150 reporting a minimum of 13 years and Lieber et al. 106 a minimum of 10 years.
| Model (study) | Pre or post treatment | Analysis methods | Outcome measure | Novel markers | Prediction form | Measure of performance | Comments |
|---|---|---|---|---|---|---|---|
| Cowen, 2005150 | Pre | A multivariate Cox proportional hazards model with restricted cubic spline to allow for non-linear relationships was used. Missing data values were estimated by imputation. The accuracy of the nomogram was tested using a subset of the population used to develop the model that had complete data | Crude survival at 5, 10 and 15 years | Nomogram | C-statistic = 0.73 | Includes demographic and disease variables | |
| Han, 2003140 | Pre | Several multivariate Cox models were fitted to the data from which the proportional hazards model was chosen in preference to parametric models by comparing the model predictions to the actual outcomes. From the chosen model the nomograms were constructed from the biochemical recurrence-free survival probability with corresponding 95% confidence intervals, adjusting for the latest year in which surgical data were available (1999) | Survival from PSA recurrence at 3, 5, 7 and 10 years | Gleason 3 + 4, 4 + 3 | Table | None | Includes year of surgery as a variable |
| Post | Survival from PSA recurrence at 3, 5, 7 and 10 years | Gleason 3 + 4, 4 + 3 | Table | None | Includes year of surgery as a variable | ||
| Blute, 2001105 | Post | Several multivariate Cox regression models were developed. The final model was selected to balance predictive power (as measured by the C-statistic) and parsimony. To develop the scoring algorithm the model was refitted with PSA as a categorical variable and the coefficients rounded | Survival from PSA recurrence | Formula for risk score | C-statistic = 0.72 | Novel markers (DNA ploidy, maximum tumour dimension) included in the initial model but not in the final model as they did not improve model performance as measured by the C-statistic | |
| Lieber, 1995106 | Post | The regression coefficients from multivariate Cox models were used to calculate HRs and predicted survival probabilities for hypothetical patients with different combinations of variable values. The most favourable prognostic group was assigned an HR of 1 | Survival from clinical recurrence | Tumour ploidy (diploid/not) | Table | None | Other markers included at univariate but not significant in multivariate model were tumour volume and Mayo nuclear grade |
| Pre-PSA era | |||||||
| Post | Prostate cancer survival | Tumour ploidy (diploid/not) | Table | None | |||
| Post | Crude survival | Tumour ploidy (diploid/not) | Table | None | |||
| Vollmer, 2001107 | Post | A hazard score was developed from the results of a Cox regression analysis. Patients were divided into two groups based on scores of less than or more than 1.5 (reason not given), and the differences in survival between the two groups illustrated graphically | Prostate cancer survival | Percentage carcinoma in RP specimen, Gleason 5 (binary variable) | Formula | None | Other variables not significant in multivariate analysis |
Study objectives
In all but one of the studies106 the development of some sort of prognostic tool is a stated objective, but the rationale for doing this is not always clear. In the studies by Vollmer et al. 107 and Lieber et al. 106 no reasons were given and it appears to have been carried out as a means of illustrating the results of the Cox regression model.
Han et al. 140 stated that, as a significant proportion of men who have a prostatectomy for clinically localised prostate cancer experience PSA elevation during long-term follow-up, it is important that patients and treating physicians know the probability of recurrence following surgery, based on preoperative and/or postoperative parameters, when making treatment decisions. The issue of the model results only being applicable to patients who have already made these decisions is not discussed. Patients who had had adjuvant therapy were excluded from the analysis, but these are likely to represent a different population from the patients who were not so treated, unless treatment was given at random. It is not clear whether reference is being made to radical or adjuvant treatment decisions. Clearly, their model that includes parameters known only following surgery is of no use to a patient before surgery, for which these parameters are unknown. However, as Han et al. excluded all patients who had had adjuvant or neoadjuvant treatment from their analysis, for patients who have chosen surgery it does show whether their expected survival is good without further treatment, which may help in the decision as to whether further treatment may be beneficial, but only if the efficacy of that treatment is known. The preoperative model shows patients’ expected survival with parameters known to the patient and his physician before surgery, but only given surgery. Only randomised trials of radical treatment powered to analyse the effectiveness of treatment in patients with different disease parameters can answer the question as to whether the patient’s prognosis will be improved or not with radical treatment.
Blute et al. 105 argue that ‘although few clinical failures will occur within 10 years after RP for organ-confined disease, early assessment of risks of biochemical failure allows identification of patients at highest risk for testing the efficacy of adjuvant therapy, establishing intervals of surveillance and, most importantly, counselling’. They further state that ‘early stratification of high-risk patients will facilitate timing and entry into adjuvant therapy trials or lessen the need for strict surveillance’. Thus they make no claim that their model will in itself assist patients in making decisions regarding their treatment.
The stated objective of Cowen et al. 150 was to develop a prediction rule for deriving estimates of life expectancy in men with clinically localised prostate cancer. Furthermore, they stated that such a tool is needed to implement the common recommendation to consider life expectancy when determining how to manage a man presenting with localised prostate cancer. The prognostic tool developed shows the estimated probability of survival for a patient given various diseases, treatment, and demographic and co-morbid characteristics. However, it seems that what a patient and his clinician really want to know is, given various treatment choices for prostate cancer, is the patient more likely to die from other causes before suffering serious consequences from his prostate cancer.
Study design
All of the studies were apparently retrospective. The use of retrospective data may affect studies in two related ways: poor data quality and the potential for bias arising from the possible need to exclude otherwise eligible patients on factors such as data availability, which may be non-random.
The first of these issues was recognised by Cowen et al. 150 who state: ‘We cannot assume that all of our subjects received the same intensity of staging or followed a particular treatment protocol. . . we did not record subsequent treatments given, and so cannot quantify the potential relationship that they may have had with survival.’ One study tried to partially address such issues by uniform analysis of archival material,106 an approach only possible for some variables and dependent on the availability of material. Another reviewed charts to confirm the original diagnosis of clinically localised tumour. 150
In terms of potential bias from the exclusion of patients, this is difficult to assess as in only two studies were the numbers excluded and reasons for exclusion given. 105,140 In the study by Blute et al. 105 missing data is given as one of the reasons for exclusion. However, in the study by Lieber et al. 106 the availability of data is an inclusion criterion. Cowen et al. 150 and Han et al. 140 appear to include patients with missing data, as both stated the proportion of patients for whom each variable was available, but only Cowen et al. described how the missing data was dealt with (imputation). Han et al. may have excluded cases with missing data from the multivariate analysis. Imputation can be a valuable technique to avoid the possible biases that may result from omitting patients with missing data; it also requires assumptions to be made with respect to the nature of the missing data. In the Cowen et al. study one key variable, PSA, was missing in 67% of cases, a weakness that the authors recognise may have affected the results. Other reasons for omitting patients were unknown treatment150 or adjuvant/neoadjuvant treatment. 140
With the exception of Cowen et al. , none of the studies discusses how omitted patients or loss to follow-up may have affected the results. Clearly the use of retrospective data has implications for data completeness and quality, an issue that does not appear to have been considered in most studies.
A key issue in these studies is whether they are adequately powered for the analyses undertaken, meaning that there are sufficient outcome events (such as deaths) per explanatory variable in the analysis (EPV). None of the studies makes any comment on this and so it is unclear whether the issue was considered, although sufficient data were presented in all studies to allow estimation of the EPV.
Only one study mentions patient consent for access to their records. 105 It remains unclear whether the majority of these studies have been undertaken without such consent.
Study quality
The results of the study quality assessment are summarised in Table 65. None of the studies fully addressed all of the potential issues assessed. The issue that all studies failed to consider properly was study attrition, but treatment of confounding variables was also poor. The different elements of the quality assessment will be discussed in more detail in the following sections.
| Study | Subheadings and questions (Q) of quality assessment | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||||||||||||||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Q15 | Q16 | Q17 | Q18 | Q19 | Q20 | Q21 | Q22 | Q23 | |
| Blute, 2001105 | y | y | y | y | p | n | ? | p | y | y | y | y | n | na | y | p | p | y | y | y | y | y | y |
| Cowen, 2006150 | y | p | p | y | n | p | p | y | n | y | p | y | na | na | na | y | p | p | y | y | y | y | y |
| Han, 2003140 | y | y | y | p | n | n | ? | y | n | p | p | y | y | na | y | y | p | p | y | y | y | y | y |
| Lieber, 1997106 | y | p | p | y | y | n | p | y | y | p | p | p | na | na | na | p | p | y | y | y | y | y | y |
| Vollmer, 2001107 | p | y | p | n | na | n | ? | n | n | ? | n | y | na | na | y | p | n | n | ? | y | y | y | p |
Study populations
All of the studies made clear statements about the patients included and the dates that marked the start and finish of patient recruitment, with the exception of Vollmer et al. 107 These were the principal criteria for the quality assessment. Only two reported on the setting, one reported zero time (Lieber et al. 106) and none mentioned diagnostic methods.
Specification of the principal treatment was a condition for inclusion in the review. All models applied to patients treated with RP except that of Cowen et al. ,150 in which patients had a mixture of prostatectomy, radiotherapy and ‘other treatment’, the last being principally watchful waiting. Two studies did not specify if any patients had had adjuvant or neoadjuvant treatment,107,150 and Han et al. 140 excluded such patients from their analysis. The patient cohort of Blute et al. 105 comprised 15% who had had adjuvant therapy, a group that they considered excluding ‘but thought it would have resulted in a lower risk cohort that would not be reflective of our practice’. Instead they included adjuvant therapy as a covariate in their models. A total of 17% of the patients in the Lieber et al. 106 cohort had had ‘early endocrine therapy’, but as this factor was not statistically significant it was not included in the final model.
The studies in general gave good descriptions of the key characteristics, as demonstrated in Tables 66, 67 and 68, which show the study populations by stage, Gleason grade and PSA respectively. As far as it is possible to tell from the different statistics reported for these factors it appears that the study populations are broadly similar.
| Study | Staging system | Clinical/pathological stage | Stage | Missing | |||
|---|---|---|---|---|---|---|---|
| T1 (or Jewett–Whitmore A) | T2 (or Jewett–Whitmore B) | T3 (or Jewett–Whitmore C) | T4 or N,M > 0 (or Jewett–Whitmore D) | ||||
| Cowen, 2005150 | TNM and Jewett–Whitmore | Clinical | 100% | ||||
| Han, 2003140 | TNM | Clinical | 100% | ||||
| Blute, 2001105 | TNM | Clinical | 90% | 10% | < 1% | ||
| Lieber, 1995106 | Jewett–Whitmore | Pathological | 52% | 30% | 18% D1 | ||
| Vollmer, 2001107 | TNM | Clinical | 100% | ||||
| Study | Clinical/pathological Gleason | Gleason score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Cowen, 2005150 | Clinical | 22.0 | 43.3 | 24.3 | 10.4 | |||||
| 67.6 | ||||||||||
| Han, 2003140 | Clinical | 0 | 12 | 49 | 33 | 6 | ||||
| 94 | ||||||||||
| Blute, 2001105 | Pathological | 11 | 42 | 17 | 25 | 4 | ||||
| 84 | ||||||||||
| Lieber, 1995106 | Pathological | 14.4 | 76.7 | 8.8 | ||||||
| Vollmer, 2001107 | Pathological | R | Median | R | ||||||
| Study | Recruitment years | PSA (ng/ml) | Missing | |||
|---|---|---|---|---|---|---|
| < 4 | 4.1–10 | 10.1–20 | > 20 | |||
| Cowen, 2005150 | 1987–89 | Mean 18.8, SD 77.6 | 66.8% | |||
| aHan, 2003140 | 1982–99 | 24% | 55% | 17% | 4% | 10.5% |
| aBlute, 2001105 | 1990–93 | 18% | 46% | 22% | 14% | |
| Lieber, 1995106 | 1967–81 | 100% | ||||
| Vollmer, 2001107 | Not specified | R = 0.2 | Median 8.8 | R = 283 | ||
The stage distribution of the Lieber et al. 106 study population is not comparable with that of the other studies as only pathological stage was reported. Many patients have their tumours upstaged on surgery. Of the studies that reported pathological stage as well as clinical stage, Han et al. 140 reported that 50% of study patients had pathologically non-organ-confined tumours and 5% had positive lymph nodes; Vollmer et al. 107 and Blute et al. 105 reported 43% and 13% extracapsular tumours respectively. This demonstrates the differences that may be found between clinical and pathological staging, but there also appear to be differences in the accuracy of clinical staging, although study exclusion criteria (for example Blute et al. excluded patients with pathologically positive lymph nodes) may be the reason for this.
The Gleason distributions of Cowen et al. 150 and Han et al. 140 are not strictly comparable with those of the other studies as many patients’ Gleason scores are upgraded when pathological specimens are available. This may explain the relatively high proportion of patients with low-grade cancers in the Cowen study. Low Gleason scores (2–4) are usually no longer assigned to biopsy specimens, which may explain their absence in the study of Han et al. Of the studies that report pathological Gleason scores the populations appear similar on this factor.
The Lieber et al. 106 study is based on a pre-PSA era cohort of patients, and the very high proportion of missing PSA values in the Cowen et al. 150 study may be for the same reason. The distributions in the other studies appear comparable, with the median PSA in the 4.1–10 ng/ml range.
Study attrition
Study attrition included both the omission of patients because of the lack of baseline variables and loss to follow-up. Although most studies stated the total population from which the study sample was drawn, together with reasons for exclusions, none reported the extent of loss to follow-up. However, Lieber et al. 106 showed the number at risk for the three different outcome measures used in their models for all three factors in the models at 10 years. Two studies105,140 reported how loss to follow-up was dealt with in the analyses. None discussed the biases that may have been introduced from the loss of patients from the analyses, although Cowen et al. 150 did discuss the potential effect of a high proportion of missing PSA data on their results.
Prognostic factor measurement
Most studies gave some information regarding the measurement of some of the prognostic markers used. Both of the studies that included the novel ploidy marker described its measurement;105,106 however, only two studies reported the PSA assay that was used,140,150 although there are several. Material storage was only described in two studies, i.e. those in which ploidy was measured105,106 There was no evidence of data-dependent cut-points being used for any continuous variables in the studies, but in two of the five studies continuous variables were categorised106,140 and in a further study it was not clear what was done. 107
Outcome measurement
The end points used in the studies, together with some of their properties, are shown in Table 69. Four different end points for the outcome measurement (all deaths, prostate cancer deaths, clinical recurrence and biochemical recurrence) were used in the eight models. Of these, only all-cause death was unambiguously defined. 106,150 Lieber et al. 106 and Vollmer et al. 107 report models with prostate cancer death as the end point, but they do not report how attribution of cause of death was made. The Lieber study also uses clinical recurrence as a model end point, but, although reporting tests that were given to patients to establish recurrence, the frequency of follow-up is not stated. This outcome is now used more rarely and has generally been superseded by PSA recurrence, which was used by Han et al. 140 and Blute et al. 105 Both used a unique definition of PSA recurrence, but only the study of Han et al. used the consensus definition of 0.2 ng/ml. In none of the three studies in which recurrence was an outcome105,106,140 was it clear whether deaths were treated as events or censored.
| Study | Deaths | Clinical recurrence | Biochemical (PSA) recurrence | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Prostate cancer | Unclear | Outcome | Defined | Outcome | Consensus definition | Unique definition | Deaths as events or censored | |
| Cowen, 2005150 | y | na | na | na | na | na | na | na | na |
| Han, 2003140 | na | na | na | na | na | y | y | y | ? |
| Blute, 2001105 | na | na | na | na | na | y | n | y | ? |
| Lieber, 1995106 | y | y | na | y | p | na | na | na | na |
| Vollmer, 2001107 | na | y | na | na | na | na | na | na | na |
Confounding measurement
Confounding measurement, considered principally as the inclusion of the classical markers in the models, was also dealt with poorly in the studies. Only two models included all confounders in their analysis,140,150 and in one instance this was not a deliberate choice but the result of all of the established markers remaining significant in the stepwise variable selection process. 140 In the Cowen et al. 150 study all potential covariates were kept in the model but most patients had missing data on a key confounding variable, PSA, and so the study could not be awarded a ‘yes’ for this category. None of the other studies forced known confounders into their analysis, although omitting them can result in a misleading model. The inclusion of the classical markers in the prognostic models is shown in Table 70. Note that the inclusion of other factors is also relevant in particular circumstances, such as age for an end point of all-cause mortality and treatment when this varied (see Table 61).
| Study | Pre or post treatment | PSA | Gleason grade | Stage (or organ-confined status) | Surgical margins |
|---|---|---|---|---|---|
| Cowen, 2005150 | Pre | y | y | y (as binary variable) | na |
| Han, 2003140 | Pre | y | y | y | na |
| Post | y | y | (y) | n | |
| Blute, 2001105 | Post | y | y | n | y |
| Lieber, 1995106 | Post (three models) | n | y | y (pathological) | n |
| Vollmer, 2001107 | Post | n | y (as binary variable) | n | n |
Statistical analysis
All of the models included in the review were developed using a multivariate Cox proportional hazards regression. None of the studies reports testing the proportionality assumption, although Han et al. 140 tried parametric (Weibull, lognormal and gamma) Cox models. They selected the proportional hazards model on the basis of a comparison of actual and predicted survival curves (calibration) for four risk groups.
All of the models used were considered to be methodologically adequate and all had at least 10 EPV in the multivariate model.
In general the statistical methods used were well reported, although presentation of the univariate results was not universal. Univariate analysis was reported to have been carried out in three studies,105,106,140 was presented in two,105,106 but was only used in one140 to select variables to enter into the multivariate model. There was further heterogeneity in the methods used to select variables for the final models presented. Three studies106,107,140 appear to have used a stepwise process, either forwards106 or backwards. 140 The method used by Vollmer et al. 107 was not specified. Cowen et al. 150 state that the variables for their model were chosen on a ‘conceptual basis’. Blute et al. 105 start with ‘established predictors’ in their model and then add and remove variables to determine the effect on the predictive power of the model, as judged by the C-statistic. When model predictive power was similar despite the inclusion or exclusion of variables, these variables were removed from the model. These variable selection processes, as well as the lack of availability of data, resulted in well-established markers [Gleason score, PSA, stage (or organ-confined status) and surgical margins (when relevant)] being omitted from all but two of the eight final models, as discussed above.
Presentation of the model results
For prognostic models to be usable the results must be presented in such a way that the predicted outcome or risk group can be easily calculated for an individual patient. In two studies,106,140 reporting five models, the model predictions are presented in tables, showing survival probabilities according to patient disease characteristics. For example, the Han et al. 140 pretreatment model shows the estimated biochemical recurrence-free survival probability at 5 years to be 96% for a patient with clinical stage T2a disease, biopsy Gleason score 6 and PSA measurement between 4.1 and 10 ng/ml. These tables are easy to use but they become more unwieldy the more variables there are in the model. Han et al. present three tables for their pretreatment model, with 60 different risk groups. Some of the groups have large confidence intervals around the results. Taking another example from the Han et al. pretreatment model the estimated biochemical recurrence-free survival probability for a patient with clinical stage T2b/c disease, biopsy Gleason score 8–10 and PSA greater than 20 ng/ml is 51%, with a 95% confidence interval ranging from 7% to 84%. To develop such tables continuous variables have to be categorised, reducing the power of the model. The practical value of reporting results for such a large number of groups must be open to question. However, in table form it is easy to present the confidence intervals around the predicted probabilities, which both Han et al. 140 and Lieber et al. 106 do, and so the uncertainty around the predictions is transparent.
Two approaches that overcome some of the disadvantages discussed above are the creation of a reduced number of risk groups and the presentation of the results in nomogram form. Examples of both of these methods were found in the reviewed studies.
Blute et al. 105 state that it was ‘our goal to have a scoring algorithm that was easy to calculate’. To achieve this they adapted their initial model, converting PSA from a continuous to a categorical variable, and rounded the model coefficients. They report that the changes had a negligible effect on model performance, measured by the C-statistic. Thus, the index, or Gleason, PSA, seminal vesicle and margin (GPSM) score, was calculated as:
GPSM = Gleason grade + 1 (PSA 4–10), + 2 (PSA 10.1–20), + 3 (PSA > 20), + 2 (seminal vesicle positive), + 2 (margin positive), – 4 (adjuvant hormonal treatment), – 2 (only adjuvant radiation treatment)
This formula resulted in scores between 1 and 16. Each value of the score was considered as a different risk group, although at both extremes of the scale, with low patient numbers, the scores were concatenated (scores 1–4 and 13–16). The most common score was 6, which had a 5-year progression-free survival probability of 91% (SE 3.0) in the test data set. In comparison, the group with the highest scores (GPSM = 13–16) had an estimated survival probability of only 30% (SE 10.2).
Cowen et al. 150 presented their model results in the form of a nomogram. The advantage of this form of model presentation is that it allows continuous variables to be kept as such and, as with an index, can easily accommodate several variables, although this makes calculation of the final score more time consuming. A disadvantage of this form of presentation is that the confidence limits cannot be easily presented, as is the case with the Cowen et al. model. Both of these problems could potentially be overcome through the use of computer models, which are now available via the internet, such as those provided by the Memorial Sloan-Kettering Center in the US. 71 However, these do not provide any information on the uncertainty around the survival estimates provided. Note that none of the studies on which the Sloan-Kettering Center computer prediction tools are based that were identified by our searches met the inclusion criteria for this review.
Performance of the prognostic models
Only two models reported any measure of model performance,105,150 and both used the concordance index or C-statistic to do this. For both models the result was similar, with Cowen et al. 150 and Blute et al. 105 reporting C-statistics of 0.73 and 0.72 respectively. Neither study reported a confidence limit around the statistic and so it is not certain that they are significantly different from 0.5, which is what is achieved by chance. The C-statistics from the two studies are not comparable for two reasons. First, the models do very different things. In the Cowen model clinical prostate cancer and demographic and co-morbidity variables are used to predict survival from all-cause mortality, whereas the Blute model uses clinical and pathological prostate cancer variables to predict survival from PSA recurrence. Second, the statistic was calculated differently in the two studies. Whereas Blute et al. split their data set to provide separate modelling and validation cohorts, Cowen et al. validated their model by systematically omitting each case from model building and then predicting the outcome for the omitted patient. Both of these methods of internal validation are discussed by Altman and Royston in an overview of prognostic model validation,145 who suggest that the method used by Cowen et al. is preferable to splitting the data set. Neither study reports an external validation in an independent data set, which is required to demonstrate the generalisability of a model.
Conclusions
This review included only five studies, reporting eight prognostic models, although there are many more models reported in the literature. In this review, as papers were only assessed as to whether they concerned novel prognostic markers or prognostic models after determining whether they met the inclusion criteria, it is not possible to state the reasons for the rejection of papers reporting prognostic models. However, during the sifting process it was clear that many models that otherwise met our inclusion criteria were rejected because they included a mean or median follow-up of less than 5 years.
Typically models predict survival at 5 years, with some also predicting survival at 10 years. As discussed in Chapter 1, long-term outcomes are very important in this disease, with disease recurrence being common after 5 years. The reliability of many models in the literature in predicting long-term outcomes must be questionable when the median follow-up is less than 5 years.
In general, the quality of the prognostic model studies, as assessed by our criteria, was good and overall better than the quality of the studies on prognostic markers. Nevertheless, there were two issues that were poorly dealt with in most or all of the prognostic model studies: inclusion of established markers and consideration of the possible biases from study attrition. An issue not considered in the quality assessment, but of primary importance, is the lack of external validation of any of the models, which have therefore not been demonstrated to be reliable outside of the original data.
Only two models reported in two different studies140,150 included all of the established markers in their model, and in one instance this was not a deliberate choice but the result of all of the established markers remaining significant in the stepwise variable selection process. 140 According to Williams et al. ,92 ‘recognised prognostic factors are generally not be subjected to the selection process. If they are excluded because by chance they do not reach a specified level of significance in that particular study, the resulting model can be misleading.’ They go on to note that collapsing variables into binary categories makes such exclusions more likely.
There were few reports of study attrition and so one might assume that little thought has been given to biases due to the exclusion of patients, missing data or loss to follow-up. If any of these are not random the data may not be representative of the population of interest. Only one study106 reported the number of patients at risk after time zero, in this case at 10 years.
So is it possible to choose one model as being better than any of the others? Given the heterogeneity of the models, particularly in terms of the outcomes predicted and whether they include clinical variables only or also pathological variables, the models cannot be considered comparable. Furthermore, only two studies reported a measure of model performance and in neither of these cases was the statistic calculated in an external data set, which is essential for validation. Only two models did not include a novel marker. It was not possible to conclude whether the inclusion of novel markers improved the performance of the prognostic models.
However, as the discussion of prognostic models at the beginning of this chapter highlighted, even in appropriate circumstances it is not a straightforward question to answer as a model should not be assessed based on one criterion alone, for example the C-statistic for discrimination, but should be assessed based upon general performance across a set of clinical, internal performance and external performance criteria.
An associated issue to validation is that of the generalisability of models. All of the models included in this review were developed in the US. How applicable are their results to the UK population with prostate cancer? Graefen151 set out to answer a similar question by validating in a German population a prognostic model developed in the US. The model, by Partin, was used to predict pathological features such as organ confinement and lymph node involvement from clinical variables. Using the area under the receiver operating characteristic (ROC) curve as the measure of performance, Graefen found that the model performed well in the German data, and in fact that the accuracy was better than that achieved in a validation cohort from the US.
Whether validated or not it, is clear that the predictions for some groups of patients in particular have considerable uncertainty, as demonstrated by the wide confidence limits. It is essential that users of these models are aware of the uncertainty around the model predictions. The presentation of models in nomogram form does not allow this. Tabular presentation of prediction models is unwieldy but does allow confidence limits to be presented alongside the survival estimates. Computer models potentially offer a solution, but one such model that is available on the internet71 does not provide any estimate of uncertainty.
Future model development
This review has highlighted some issues in the development and reporting of prognostic models for early prostate cancer. Future model developers should particularly consider the following:
-
validation of the models with independent (external) data
-
the reporting of the uncertainty around model predictions
-
the inclusion of classical markers in multivariate models, whether statistically significant or not
-
the adequacy of the data for predicting long-term outcomes (and the reporting of numbers at risk at the different time points for survival predictions)
-
the size of the data set that is to be used to develop the model, particularly ensuring adequate representation of less common prognostic groups.
Chapter 7 Discussion
Statement of principal findings
Novel prognostic markers
A total of 21 novel markers were identified from the 28 studies that met the inclusion criteria for this section.
The considerable variability in the results reported within the prognostic marker categories, the poor quality of studies and the lack of studies for some categories have made it difficult to provide clear conclusions as to which markers might offer the most potential as prognostic parameters for localised prostate cancer. These reasons also meant that it was not possible to quantitatively synthesise the results. Key quality issues that commonly affected the potential to draw conclusions on the novel markers were the lack of classical markers in the statistical models and insufficient EPV.
Nevertheless, on the available evidence the 21 prognostic markers were placed into one of three categories dependent on the direction and strength of the evidence for each in terms of adding prognostic value to the established markers: (1) promising; (2) not promising; and (3) inconclusive:
-
Promising:
-
acid phosphatase level
-
Gleason pattern in Gleason score 7 (4 + 3 versus 3 + 4) (non-classical use of Gleason measurements)
-
amount of high-grade cancer (non-classical use of Gleason measurements)
-
PSA kinetics (PSAV/PSADT)
-
percentage positive biopsy cores (proportion cancer).
-
-
Not promising:
-
β-catenin expression
-
creatinine
-
germline genetic variation in the vitamin D receptor
-
maximum tumour dimension (tumour size)
-
tumour volume (tumour size).
-
-
Inconclusive:
-
percentage cancer in surgical specimen (proportion cancer)
-
androgen receptor: CAG repeats
-
DNA ploidy
-
CYP3A4 genotypes
-
modified Gleason score (non-classical use of Gleason measurements)
-
Ki67 LI
-
Bcl-2
-
p53
-
syndecan-1
-
CD10
-
Stat5 activation status.
-
The marker with the strongest evidence for its prognostic significance, and which also has relatively large HRs, is PSAV.
Prognostic models
In the review of prognostic models only five articles reporting eight models met the inclusion criteria, all of which developed new models. In general, the quality of the prognostic model studies, as assessed by our criteria, was adequate and overall better than the quality of the studies on prognostic markers. Nevertheless, there were two issues that were poorly dealt with in most or all of the prognostic model studies: inclusion of established markers and consideration of the possible biases from study attrition.
Given the heterogeneity of the models, particularly in terms of the outcomes predicted and whether they included clinical variables only or also pathological variables, the models cannot be considered comparable. Only two models did not include a novel marker, and one of these included several demographic and co-morbidity variables to predict all-cause mortality. Only two models reported a measure of model performance, the C-statistic, and for neither was it calculated in an external data set. It was not possible to assess whether the models that included novel markers performed better than those without. In addition, with regard to the need for external model validation, a key recommendation is that the uncertainty around model predictions should be reported.
Strengths and limitations
Literature search
A comprehensive literature search was undertaken in eight electronic bibliographic databases using terms to capture both novel prognostic markers and prognostic models. The searches identified 12,963 potentially relevant articles. Only one of three reviewers screened titles but if there was any doubt as to the relevance of an article to the review the article was included at this stage, so although a few articles may have been erroneously rejected at this stage the effect is expected to be very limited. A total of 8934 articles not meeting our inclusion criteria were removed at title sift, leaving a total of 4029 abstracts to be screened. All abstracts were read by at least two reviewers and consensus obtained. It should be noted that 795 articles were excluded because they had no abstract and foreign language articles were also excluded.
Inclusion and exclusion criteria
Given the large volume of literature that the scoping literature searches indicated would be identified, we needed a simple method that would enable us to quickly identify the studies most likely to yield good-quality evidence. Clinical consideration of the often slow course of the disease indicated that studies should have a mean or median follow-up of at least 5 years. For this length of follow-up it was estimated that, for the most commonly occurring outcome, PSA recurrence, a sample size of at least 200 was required to yield sufficient events for statistical analysis.
In principle, a criterion based on the number of events or EPV would have been preferable, but studies report the number of patients more commonly than the number of events. If we had used a criterion based on the number of events or EPV we would have excluded nine studies that were included in this review, some of which had large sample sizes and which probably do have an adequate number of events. More sensitive criteria could be designed based on a combination of the number of events (or when these data are missing on an estimate based on patient numbers), outcome variable and length of follow-up. This would require considerably more resources to screen papers for inclusion in the review than the simple threshold based on patient numbers that we used and would not have been possible to implement for this review.
Despite the inclusion criteria used in this review some of the included studies were nevertheless found to be lacking statistical power in terms of having insufficient events for the number of variables in the multivariate models.
The inclusion criterion requiring a follow-up period of a mean or median of 5 years was based on clinical considerations. In reviewing the articles it was evident that most studies used a Cox proportional hazards model, which assumes that the HR is constant over time. The assumption is reported to have been tested in six studies, with only one study112 reporting that it did not hold (for Gleason scores, for which the risk ratios decreased with extended follow-up). If the proportional hazards assumption holds it suggests that some studies with a follow-up of less than 5 years may have made a useful contribution to the literature on prognostic studies if their sample sizes were sufficiently large to generate enough events. However, there would be more uncertainty over the results. This would particularly affect the confidence limits around the predictions of the prognostic models.
The inclusion criteria of a sample size of 200 and a median or mean follow-up of 5 years are likely to be the reason why other markers and models have not been included in this review. This review aimed to systematically assess the best-quality evidence rather than be exhaustive. Several non-systematic reviews have identified many other novel prognostic biomarkers. 4,10,141,152 These include prostate-specific membrane antigen (PSMA), MIB-1, Bax, interleukin 6 (IL-6) soluble receptors, transforming growth factor (TGF)-β1, prostate cancer antigen 3 (PCA3), TMPRSS2-Erg, circulating tumour cells, DDA3, caveolin-1, estrogen receptor, cyclin D1 and E-cadherin. The fact that these markers are not included in this review does not mean that they are not promising, rather that the published studies reporting them at the time of our searches did not meet the review inclusion criteria and that more high-quality research will be required to assess their value. Two recent systematic reviews, both led by Harnden, studied the prognostic significance of tertiary Gleason grade in pathological samples and perineural invasion in biopsy samples respectively. 153,154 As with this review, the poor quality of the studies and the heterogeneity between them limited the strength of the conclusions that could be drawn, but for both markers the authors concluded on the basis of the evidence available that the markers were promising.
The exclusion criteria also meant that some of the models which are familiar to clinicians, such as those developed at the Memorial Sloan-Kettering Center, have not been included in this review. Although some report outcomes at 10 years, such as the preoperative and postoperative nomograms of Stephenson et al. , the median patient follow-up is less than 5 years and in the model of Stephenson et al. it is only 25 months. 63,64
Quality assessment
A study by Hayden et al. 102 that appraised how authors of reviews of prognostic studies had assessed study quality proposed a list of questions that could be used to assess biases in six domains: study population, attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, and analysis. This provided an excellent template from which to develop a quality assessment instrument specific to the needs of this review. An overall quality score was not assigned to each paper; rather the quality assessment tool was used to help identify factors that needed to be taken into account when interpreting the results of the study. Key quality issues that commonly affected the potential to draw conclusions on the novel markers were the lack of classical markers in the statistical models and insufficient EPV.
Analysis and interpretation
Study heterogeneity
The heterogeneity between studies precluded the use of meta-analysis. One of the main sources of heterogeneity was in the measures of outcome, with all-cause mortality, prostate cancer mortality, and clinical and biochemical recurrence all being used, with the definition of the last two also varying. Other important differences between studies were the covariates included in the multivariate analyses and the marker measurement methods and cut-points used to define prognostic groups. As well as the heterogeneity in study design and analysis methods, the poor reporting of models and particularly the lack of HRs sometimes made meta-analysis impossible. Methods are available to estimate HRs from other results presented, but this would have been possible in a limited number of cases and would not have affected the possibility of undertaking meta-analysis because of the other sources of heterogeneity. Similarly, if more articles had been included in this review it is very unlikely to have affected the ability to have undertaken meta-analyses.
The heterogeneity between studies, poor quality of studies and the limited number of studies for each marker also mean that the classification of markers into ‘promising’ and ‘not promising’ groups can be considered indicative only, based on the generally weak evidence available. Other reviews of prognostic markers and models, not only in cancer, have also commented on the generally poor quality of studies in this field92,97,99,100 and the issues have been more generally discussed in the literature. 96,155,156
There is increasing interest in meta-analysis using pooled individual patient data from different studies. 156–158 This method allows differences in statistical models, and particularly differences in the treatment of covariates and marker cut-points in reported studies, to be standardised in a single analysis (assuming covariate data are available) and reduces the potential for misleading results. 158 However, not all differences between studies can be retrospectively overcome through uniform analysis. Some of these differences are common to all prognostic marker studies, such as the different (or unspecified) definitions and measurement methods of novel markers. For prostate cancer studies a particular issue is the variation in definition of PSA failure, as failure may result in different patient treatment and so different failure thresholds cannot be applied retrospectively.
Publication and reporting bias
There was only a small number of studies, or sometimes only a single study, for each marker. It was not possible to examine the potential issues of publication bias or selective outcome reporting. The exclusion of smaller studies may have reduced the possibility of publication bias, but with the literature comprising retrospective case series the possibility of publication bias remains considerable. Furthermore, with several possible outcome measures available there is scope for selective outcome reporting. Kyzas et al. 159 evaluated publication bias and variation in outcome definitions in the literature on prognostic factors for head and neck squamous cell cancer. Their analysis showed that these biases may inflate the apparent importance of prognostic markers. This must be considered in the interpretation of the results of this review. It is possible for many markers that a single unpublished study could have altered the conclusions considerably.
Prognostic or predictive marker?
In none of the novel marker studies was it considered whether a marker was prognostic or predictive. Given that in the majority of studies patients all had the same principal treatment this was not possible to assess. Before a marker is adopted it needs to be considered whether it is truly prognostic or whether it may be predictive, i.e. whether there is an interaction with any particular treatment.
Economic evaluation
This study did not include an economic evaluation of the use of novel markers. The clinical and financial consequences of the use of prognostic markers will be known only if research is carried out to show which prognostic groups are likely to benefit from radical treatment. Currently most men who are otherwise healthy have radical treatment. The consequences of introducing a novel prognostic marker will depend on whether some men opt not to have such treatment as a result of the test and how their disease subsequently progresses compared with men of the same prognostic status who do have treatment. The advantage of immediate radical treatment compared with active monitoring is not yet fully understood for the prognostic groups defined by the classical markers in current use.
Uncertainties
The main sources of uncertainty for the results of the novel prognostic marker review were the small number of studies and the poor quality of those studies, which made it difficult to reach firm conclusions on the prognostic value of the novel markers.
For the review of prognostic models the lack of external validation of any of the models and lack of a well-established measure of performance, together with the heterogeneity of the models, made it impossible to compare the performances of the different models as prognostic tools.
Other factors that affected both reviews were the heterogeneity in marker measurement methods and categorisation; outcome heterogeneity and in particular the many variations in the definition of disease progression; the different approaches to including covariates in the models; and the varied reporting of the models and their results. Furthermore, reporting of these items was poor and so it was often unclear in studies exactly how markers or outcomes were defined, how many patients were used in different analyses and what covariates were entered in multivariate models.
Other relevant factors
Costs and implementation
As the evidence presented in this systematic review considers prognostic markers only in terms of their prognostic value, we are not able to make conclusions about the costs or matters relating to implementation.
Chapter 8 Conclusions
Implications for service provision
Novel markers
In common with many other reviews of prognostic markers this review has highlighted the poor quality of studies and the heterogeneity between studies, which makes the results of much of this research inconclusive. As a result it is not possible to make any immediate recommendations for service provision.
However, one marker, PSAV (or doubling time), did stand out, not only in terms of the strength of the evidence supporting its prognostic value but also in terms of the relatively high HRs. The studies included in this review measured PSAV before diagnosis. This information is not generally available in the UK as most men do not have regular PSA screening. However, there is great interest in PSAV post diagnosis as a monitoring tool for active surveillance. It appears that in some centres it is already being used for this purpose, although there is no consensus on how it should be used and in particular what threshold should indicate the need for radical treatment.
Models
This review highlights the small proportion of models reported in the literature that are based on patient cohorts with a mean or median follow-up of at least 5 years. Users of models need to be aware that long-term predictions may be unreliable. We note that our inclusion criteria, for pragmatic reasons, were somewhat arbitrary. It is possible that some large cohorts with a follow-up of less than 5 years that were excluded from this review may have had as many patients at risk at 5 years as some smaller studies with a longer follow-up that were included. When using any form of prediction tool model users should look at the confidence intervals around the survival estimates. None of the models in this review were externally validated. Confidence intervals would be expected to be greater in external data.
Users should also be aware that prognostic models have been developed using cohort data. These models cannot be used to predict whether a patient’s survival probabilities are better with one or other treatment as they have not been developed on randomised data and apparent differences in survival may be due to selection biases that are not necessarily controlled for with the model covariates.
Implications for future research
The only way to determine the optimum treatment for different prognostic groups whilst ensuring lack of bias in treatment estimates is to conduct randomised controlled trials. However, it is not practicable or even desirable to test all potential prognostic markers in this way. Much more could be achieved to identify the most promising prognostic markers with cohort studies if the research was conducted in an organised and scientific manner. Many of the current studies appear ad hoc and poorly designed. Specific recommendations are as follows:
-
Data could be collected prospectively for later retrospective studies. If this is combined with storage of biopsy and pathological material new markers could be rapidly assessed using existing long-term follow-up data. The methods of collecting and storing marker materials need careful consideration to ensure consistency of results. This review has shown that marker storage is poorly reported in the majority of studies. Patient consent is also rarely reported.
-
Centres need to work collaboratively so that larger patient cohorts are available for analysis. Many of the current studies are statistically underpowered. It should be noted that one such initiative is already being established. The P-Mark project (validation of recently developed diagnostic and prognostic markers and identification of novel markers for prostate cancer using European databases) is establishing a serum and urine repository with matching patient data. 79
-
If data are to be combined from different centres common definitions of PSA and clinical disease recurrence should be agreed on so that outcomes are not ambiguous. Ideally these would be agreed across all research centres to assist the synthesis of evidence. The consensus recommendations of what constitutes PSA failure following RP and radiotherapy go some way towards this (if followed), but the treatment of clinical progression and the censoring (or not) of death also vary between studies. Marker measurement methods and marker cut-points also need to be agreed. These recommendations should be considered in the context of the advances in prospective meta-analysis techniques. 160–163
-
The analysis and reporting of prognostic marker studies must be improved. Readers are referred to other sources in the literature for guidelines on the designing, reporting, conduct and analysis of prognostic studies. 51,52,92,160,162–168 Some of the key failings that were highlighted by this review include:
-
– poor reporting of marker measurement methods, exact definitions of outcome (recurrence, etc.), number of outcome events, models and their results
-
– handling of continuous variables, which were often categorised (with the categories sometimes treated as continuous variables, which is not recommended); variables should be kept continuous when possible and, when categorised, the cut-points should not be determined within the data144
-
– the failure to report a multivariate model that includes all of the established markers
-
– the failure to assess the statistical power of the analysis, with particular attention paid to the number of events in each group for categorical variables
-
– the failure to clearly report the number of outcome events and what variables were included in the multivariate analysis (particularly those removed through stepwise processes).
-
The issues considered in our quality assessment, which was based on a review of potential sources of bias in prognostic studies, are those that need to be considered when designing prognostic studies. 102 The main categories identified by Hayden et al. 102 for sources of bias are study population, study attrition, prognostic factor measurement, outcome measurement, confounding measurement and account, and analysis methods. Within each of these Hayden proposes items that may need to be examined. A summary of these is listed below to illustrate the many issues that must be considered by those undertaking prognostic studies.
Study participation
Does the study sample represent the population of interest, considering adequate description of key characteristics including recruitment methods, period and place of recruitment, inclusion and exclusion criteria, zero time description and adequate participation of eligible individuals?
Study attrition
Do the study data adequately represent the sample, considering response rates, attempts to collect data from participants who dropped out of the study, characteristics of ‘dropouts’, reasons for loss to follow-up reported, and differences between dropouts and participants who completed the study?
Prognostic factor measurement
Are the prognostic factors of interest adequately measured, considering the presentation of clear definitions of markers (including measurement methods), the treatment of continuous variables in the analysis (avoiding use of data-dependent cut-points), the reliability of marker measurements, the consistency of measurements and the proportion of participants with complete data for prognostic factors?
Outcome measurement
Is the outcome of interest adequately measured, considering whether a clear definition is provided (including duration of follow-up), the possibility of misclassification and the consistency of measurement?
Confounding measurement and account
Are important potential confounders accounted for, considering the completeness of reporting of their definitions and values, the reliability and consistency of their measurement, and whether they are accounted for in the study design and analysis?
Analysis
Is the statistical design appropriate for the study, considering the adequacy of the reporting to make an assessment, the strategy for model building, the appropriateness of the model for the study design and full (no selective) reporting of results?
Similar issues are highlighted in REMARK,50 developed in response to a recommendation of the National Cancer Institute – European Organisation for Research and Treatment of Cancer (NCI-EORTC) First International Meeting on Cancer Diagnostics, in which the inadequacies of prognostic studies and their reporting had been highlighted.
Future reviews will be able to undertake meta-analyses of prognostic studies in this field only if there is greater standardisation across studies, particularly in the definitions of outcomes and in marker measurement methods. Use of pooled individual patient data from different studies allows differences in statistical models, and particularly differences in the treatment of covariates and marker cut-points in reported studies, to be standardised in a single analysis (assuming covariate data are available). However, as biochemical failure may result in different patient treatment, different failure thresholds cannot be retrospectively applied.
The key message of this section is well summarised by McShane et al. :155
The tumor marker research community must come to the same realization that clinical trialists came to decades ago. If sound scientific principles of careful study design, adequate study size, scrupulous data collection and documentation, and appropriate analysis strategies are not adhered to, the field will flounder. Culture changes will be required. Stable and adequate funding will be required to have necessary personnel and infrastructure to collect, annotate, and maintain valuable specimen collections essential for high-quality retrospective studies. More importantly, the necessity of large, definitive prospective studies or prospectively planned meta-analyses for tumor marker research must be recognized.
Acknowledgements
Freddie Hamdy (Consultant in Urology, Royal Hallamshire Hospital, Sheffield), John Staffurth (Clinical Senior Lecturer/Honorary Consultant, Department of Clinical Oncology, Velindre Hospital, Cardiff) and Noel Clarke (Consultant Urologist, Christie Hospital and Salford Royal Hospital, Manchester) provided specialist clinical advice during the study. Chris Parker (Clinical Senior Lecturer, Institute of Cancer Research, Surrey), Richard Riley (Research Fellow, Centre for Medical Statistics and Health Evaluation, University of Liverpool), James Michael Olu N’Dow (Professor of Urology, Academic Urology Department, University of Aberdeen) and Howard Kynaston (Professor of Urology, Department of Urology, University Hospital of Wales, Cardiff) provided valuable comments and support during the internal peer reviewing stage of the study.
Gill Rooney and Andrea Shippam, Project Administrators, ScHARR, organised the retrieval of papers and helped in preparing and formatting the report.
The authors wish to thank all of the above.
Contribution of authors
Paul Sutcliffe, Research Fellow, and Silvia Hummel, Senior Operational Research Analyst, coordinated the review.
Paul Sutcliffe, Silvia Hummel, Angie Rees (Systematic Reviews Information Officer) and Anna Wilkinson (Systematic Reviews Information Officer) developed the search strategy and undertook searches. Paul Sutcliffe, Silvia Hummel and Emma Simpson (Research Fellow) screened the search results. Paul Sutcliffe, Emma Simpson and Silvia Hummel screened retrieved articles against the inclusion criteria. Silvia Hummel and Paul Sutcliffe developed the critical appraisal tool and appraised the quality of papers. Emma Simpson, Silvia Hummel and Paul Sutcliffe abstracted data from papers. Statistical support was provided by Tracey Young (Lecturer in Medical Statistics). Silvia Hummel, Emma Simpson and Paul Sutcliffe analysed the data. Paul Sutcliffe and Silvia Hummel wrote the background chapter. Silvia Hummel, Paul Sutcliffe and Emma Simpson wrote the chapters on novel prognostic markers. Silvia Hummel and Tracey Young wrote the chapter on prognostic models. Paul Sutcliffe and Silvia Hummel wrote the discussion chapter.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA Programme or the Department of Health.
References
- Zhang C, Li HR, Fan JB, Wang-Rodriguez J, Downs T, Fu XD, et al. Profiling alternatively spliced mRNA isoforms for prostate cancer classification. BMC Bioinformatics 2006;7.
- Gronau E, Goppelt M, Harzmann R, Weckermann D. Prostate cancer relapse after therapy with curative intention: a diagnostic and therapeutic dilemma. Onkologie 2005;28:361-6.
- Harris R. Screening for prostate cancer: an update of the evidence for the US Preventive Services Task Force. Ann Intern Med 2002;137:917-29.
- Tricoli JV, Schoenfeldt M, Conley BA. Detection of prostate cancer and predicting progression: current and future diagnostic markers. Clin Cancer Res 2004;10:3943-53.
- Hsing AW, Chokkalingam AP. Prostate cancer epidemiology. Front Biosci 2006;11:1388-413.
- Parkin DM, Whelan J, Ferlay L, Teppo L, Thomas DB. Cancer incidence in five continents. Volume VIII. Lyon: IARC; 2002.
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin 2005;55:10-3.
- Office for National Statistics . Registrations of cancer diagnosed in 1993–1996, England and Wales. Health Stat Quart 1999;4:59-70.
- Cancer Research UK . UK Prostate Cancer Mortality Statistics n.d. http://info.cancerresearchuk.org/cancerstats/types/prostate/mortality/2007 (accessed 11 October 2007).
- Srigley JR, Amin M, Boccon-Gibod L, Egevad L, Epstein JI, Humphrey PA, et al. Prognostic and predictive factors in prostate cancer: historical perspectives and recent international consensus initiatives. Scand J Urol Nephrol Suppl 2005;216:8-19.
- Bailey JA. Concise dictionary of medical-legal terms. New York: Parthanon Publishing Group; 1998.
- Chamberlain J, Melia J, Moss S, Brown J. The diagnosis, management, treatment and costs of prostate cancer in England and Wales. Health Technol Assess 1997;1.
- Ilic D, O’Connor D, Green S, Wilt T. Screening for prostate cancer: a Cochrane systematic review. Cancer Causes Control 2007;18:279-85.
- Middleton RG, Thompson IM, Austenfeld MS, Cooner WH, Correa RJ, Gibbons RP, et al. Prostate cancer clinical guidelines panel summary report on the management of clinically localized prostate cancer. J Urol 1995;154:2144-8.
- Prostate Cancer Speciality Working Group . Clinical Information Network Guidelines on the Management of Prostate Cancer 1999.
- Hummel S, Paisley S, Morgan A, Currie E. Clincial and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review. Health Technol Assess 2003;7.
- NICE . Improving Outcomes in Urological Cancers 2002. http://guidance.nice.org.uk/csguc/guidance/pdf/English.
- Diamandis EP, Yousef GM, Clements J, Ashworth LK, Yoshida S, Egelrud T, et al. New nomenclature for the human tissue kallikrein gene family. Clin Chem 2000;46:1855-8.
- Ablin RJ, Bronson RT, Soanes WA. Tissue- and species-specific antigens of normal human prostatic tissue. J Immunol 1970;104:1329-39.
- Hara M, Koyanagi Y, Inoue T. Some physiochemical characteristics of seminoprotein, an antigenic component specific for human seminal plasma. Nippon Hoigaku Zasshi 1971;25:322-4.
- Li TS, Beling CG. Isolation and characterisation of two specific antigens of human seminal plasma. Fertil Steril 1973;24:134-44.
- Sensabaugh GF, Crim D. Isolation and characterisation of a semen-specific protein from human seminal plasma: a potential new market for semen idenitification. J Forensic Sci 1978;23:106-15.
- Wang MC, Valenzuela LA, Murphy GP. Purification of a human prostate specific antigen. Invest Urol (Berlin) 1979;17:159-63.
- Hughes C, Murphy A, Martin C, Sheils O, O’Leary J. Molecular pathology of prostate cancer. J Clin Pathol 2005;58:673-84.
- Gospodarowicz MK, Miller D, Groome PA, Greene FL, Logan PA, Sobin LH. The process for continuous improvement of the TNM classification. Cancer 2003;100:1-5.
- Balch C, Soong CM, Gershenwald JE. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 2001;19:3622-34.
- Sobin LH, Wittekind CL. TNM classification of malignant tumors. New York: John Wiley; 2002.
- Brierley J. The evolving TNM cancer staging system: an essential component of cancer care. CMAJ 2006.
- Jewett HJ. The present status of radical prostetectomy for stages A and B prostatic cancer. Urol Clin N Am 1975;2:105-24.
- Jackson AS, Parker CC, Norman AR, Padhani AR, Huddart RA, Horwich A, et al. Tumour staging using magnetic resonance imaging in clinically localised prostate cancer: relationship to biochemical outcome after neo-adjuvant androgen deprivation and radical radiotherapy. Clin Oncol 2005;17:167-71.
- Albertsen PC. PSA and the conservative treatment of early prostate cancer. Arch Ital Urol Androl 2006;78:152-3.
- Epstein JI. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol n.d.;39:34-63.
- Epstein JI, Pizov G, Walsh PC, Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer 1993;71:3582-93.
- Ohori M, Wheeler TM, Kattan MW, Goto Y, Scardino PT. Prognostic significance of positive surgical margins in radical prostatectomy specimens. J Urol 1995;154:1818-24.
- Trapasso JG, deKernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol 1994;152:1821-5.
- Epstein JI. Prediction of progression following radical prostatectomy: a multivariate analysis of 721 men with long-term follow-up. Am J Surg Pathol 1996;20:286-92.
- Stenman UH, Abrahamsson PA, Aus G, Lilja H, Bangma C, Hamdy FC, et al. Prognostic value of serum markers for prostate cancer. Scand J Urol Nephrol 2005;216:64-81.
- Etzioni R, Penson DF, Legler JM, di Tommaso D, Boer R, Gann PH, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from US prostate cancer incidence trends. J Natl Cancer Inst 2002;94:981-90.
- Grizzle W. Biomarkers in prostate cancer. Stanford, CA: American Association for Cancer Research; 2005.
- Barranco C. Preoperative PSA kinetics predict prostate cancer outcomes. Nat Clin Pract Urol 2006;3:64-5.
- Polascik TJ, Pearson JD, Partin AW. Multivariate models as predictors of pathological stage using Gleason score, clinical stage, and serum prostate-specific antigen. Semin Urol Oncol 1998;16:160-71.
- Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA 1997;277:1445-51.
- Partin AW, Yoo J, Carter HB, Pearson JD, Chan DW, Epstein JI, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol 1993;150:110-14.
- Partin AW, Steinberg GD, Pitcock RV, Wu L, Piantadosi S, Coffey DS, et al. Use of nuclear morphometry, Gleason histologic scoring, clinical stage, and age to predict disease-free survival among patients with prostate cancer. Cancer 1992;70:161-8.
- Hammond ME, Fitzgibbons PL, Compton CC, Grignon DJ, Page DL, Fielding LP, et al. College of American Pathologists Conference XXXV: solid tumor prognostic factors – which, how and so what? Summary document and recommendations for implementation. Cancer Committee and Conference Participants. Arch Pathol Lab Med 2000;124:958-65.
- Ross JS, Sheehan CE, Fisher HA, Kauffman RA, Dolen EM, Kallakury BV, et al. Prognostic markers in prostate cancer. Exp Rev Mol Diagn 2002;2:129-42.
- Ross JS, Sheehan CE, Dolen EM, Kallakury BV, Ross JS, Sheehan CE, et al. Morphologic and molecular prognostic markers in prostate cancer. Adv Anat Pathol 2002;9:115-28.
- Ergun A, Lawrence CA, Kohanski A, Brennan TA, Collins JJ. A network biology approach to prostate cancer. Mol Syst Biol 2007;3.
- Draisma, G . Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst 2003;95:868-78.
- McShane LM, Altman DA, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst 2005;97:1180-4.
- Riley RD, Abrams KR, Sutton AJ, Lambert PC, Jones DR, Heney D. Reporting of prognostic markers: current problems and development of guidelines for evidence-based practice in the future. Br J Cancer 2003;88:1191-8.
- Altman D, Lyman GH. Methodological challenges in the evaluation of prognostic factors in breast cancer. Breast Cancer Res Treat 1998;52:289-303.
- von Eschenbach AC, Brawer M, di Sant’ Agnese PA, Humphrey P, Mahran HE, Murphy G, et al. Exploration of new pathologic factors in prostate cancer in terms of potential for pronostic significance and future applications. Cancer 1996;78:372-5.
- Marchevsky AM, Wick MR. Evidence-based medicine, medical decision analysis, and pathology. Hum Pathol 2004;35:1179-88.
- Bostwick DG, Foster CS. Predictive factors in prostate cancer: current concepts from the 1999 College of American Pathologists Conference on Solid Tumor Prognostic Factors and the 1999 World Health Organization Second International Consultation on Prostate Cancer. Semin Urol Oncol 1999;17:222-72.
- Chun FK, Karakiewicz PI, Briganti A, Gallina A, Kattan MW, Montorsi F, et al. Prostate cancer nomograms: an update. Eur Urol 2006;50:914-26.
- Kattan MW, Potters L, Blasko JC, Beyer DC, Fearn P, Cavanagh W, et al. Pretreatment nomogram for predicting freedom from recurrence after permanent prostate brachytherapy in prostate cancer. Urology 2001;58:393-9.
- Sargent DJ. Comparison of artificial neural networks with other statistical approaches: results from medical data sets. Cancer 2001;91:1636-42.
- Abbod MF, Catto JW, Linkens DA, Hamdy FC. Application of artificial intelligence to the management of urological cancer. J Urol 2007;178:1150-6.
- Babaian RJ, Zhang Z. Computer-assisted diagnostics: application to prostate cancer. Mol Urol 2001;5:175-80.
- Ross PL, Scardino PT, Kattan MW, Ross PL, Scardino PT, Kattan MW. A catalog of prostate cancer nomograms. J Urol 2001;165:1562-8.
- Kattan MW, Shariat SF, Andrews B, Zhu K, Canto E, Matsumoto K, et al. The addition of interleukin-6 soluble receptor and transforming growth factor beta1 improves a preoperative nomogram for predicting biochemical progression in patients with clinically localized prostate cancer. J Clin Oncol 2003;21:3573-9.
- Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, Fearn PA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst 2006;98:715-17.
- Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Dotan ZA, DiBlasio CJ, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol 2005;23:7005-12.
- Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol 1999;17:1499-507.
- Kattan MW, Zelefsky MJ, Kupelian PA, Scardino PT, Fuks Z, Leibel SA. Pretreatment nomogram for predicting the outcome of three-dimensional conformal radiotherapy in prostate cancer. J Clin Oncol 2000;18:3352-9.
- Kattan MW, Zelefsky MJ, Kupelian PA, Cho D, Scardino PT, Fuks Z, et al. Pretreatment nomogram that predicts 5-year probability of metastasis following three-dimensional conformal radiation therapy for localized prostate cancer. J Clin Oncol 2003;21:4568-71.
- Kattan MW. A nomogram which predicts 7-year metastasis-free survival following 3D conformal radiation therapy. J Urol 2002;167.
- Kattan MW. A nomogram which uses postoperative factors to predict PSA progression after radical prostatectomy for clinically localized prostate cancer. J Urol 1998;159.
- Stephenson AJ, Smith A, Kattan MW, Satagopan J, Reuter VE, Scardino PT, et al. Integration of gene expression profiling and clinical variables to predict prostate carcinoma recurrence after radical prostatectomy. Cancer 2005;104:290-8.
- Memorial Sloan-Kettering Center prostate cancer nomograms n.d. http://info.cancerresearchuk.org/cancerstats/types/prostate/mortality/2007.
- Hoffman RM, Stone SN, Hunt WC, Key CR, Gilliland FD. Effects of misattribution in assigning cause of death on prostate cancer mortality rates. Ann Epidemiol 2003;13:450-4.
- Feuer EJ, Merrill RM, Hankey BF. Cancer surveillance series: interpreting trends in prostate cancer. II. Cause of death misclassification and the recent rise and fall in prostate cancer mortality. J Natl Cancer Inst 1999;91:1025-32.
- Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG–ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965-74.
- Cookson MS, Aus G, Burnett AL, Canby-Hagino ED, D’Amico AV, Dmochowski RR, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 2007;177:540-5.
- Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point?. J Urol 2001;165:1146-51.
- Mills N, Metcalfe C, Ronsmans C, Davis M, Lane JA, Sterne JAC, et al. A comparison of socio-demographic and psychological factors between patients consenting to randomisation and those selecting treatment (the ProtecT study). Contemp Clin Trials 2006;27:413-19.
- Stattin P, Damber JE, Karlberg L, Nordgren H, Bergh A, Stattin P, et al. Bcl-2 immunoreactivity in prostate tumorigenesis in relation to prostatic intraepithelial neoplasia, grade, hormonal status, metastatic growth and survival. Urol Res 1996;24:257-64.
- van Gils MP, Stenman UH, Schalken JA, Schroder FH, Luider TM, Lilja H, et al. Innovations in serum and urine markers in prostate cancer current European research in the P-Mark project. Eur Urol 2005;48:1031-41.
- Bubendorf L. High-throughput microarray technologies: from genomics to clinics. Eur Urol 2001;40:231-8.
- Bok RA, Small EJ. Bloodborne biomolecular markers in prostate cancer development and progression. Nat Rev Cancer 2002;2:918-26.
- Falcone A, Antonuzzo A, Danesi R, Allegrini G, Monica L, Pfanner E, et al. Suramin in combination with weekly epirubicin for patients with advanced hormone-refractory prostate carcinoma. Cancer 1999;86:470-6.
- Alers JC, Rochat J, Krijtenburg PJ, Hop WC, Kranse R, Rosenberg C, et al. Identification of genetic markers for prostatic cancer progression. Lab Invest 2000;80:931-42.
- Abate-Shen C, Shen M. Molecular genetics of prostate cancer. Genes Dev 2000;14:2410-34.
- Roemeling S, Schroder FH, Bangma CH. Guideline and study for the expectant management of localized prostate cancer with curative intent. Rotterdam: Department of Urology, Erasmus MC, University Medical Center; 2006.
- Bill-Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Scandinavian Prostate Cancer Group . Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2005;352:1977-84.
- Johansson J, Andren O, Andersson S, Dickman PW, Holmberg L, Magnuson A, et al. Natural history of early, localized prostate cancer. JAMA 2004;291:2713-9.
- Steineck G, Helgesen F, Adolfsson J, Dickman PW, Johansson JE, Johan Norlen B, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med 2002;347:790-6.
- D’Amico AV, Chen MH, Oh-Ung J, Renshaw AA, Cote K, Loffredo M, et al. Changing prostate-specific antigen outcome after surgery or radiotherapy for localized prostate cancer during the prostate-specific antigen era. Int J Radiat Oncol Biol Phys 2002;54:436-41.
- Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Fujian S. Methods for meta-analysis in medical research. Chichester: John Willey & Sons; 2000.
- Egger M, Davey Smith G, Altman DG. Systematic reviews in health care: meta-analsysis in context. London: BMJ Books; 2001.
- Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al. Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy. Health Technol Assess 2006;10.
- Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, Sokol GH, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: receommendations of the RTOG–ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2007;66:1274-5.
- Critz FA, Levinson K. 10-year disease-free survival rates after simultaneous irradiation for prostate cancer with a focus on calculation methodology. J Urol 2004;172:2232-8.
- Ward JF, Blute ML, Slezak J, Bergstralh EJ, Zincke H. The long-term clinical impact of biochemical recurrence of prostate cancer 5 or more years after radical prostatectomy. J Urol 2003;170:1872-6.
- Altman D. Systematic reviews in health care: systematic reviews of evaluations of prognostic variables. BMJ 2001;323:224-8.
- Counsell C, Dennis M. Systematic review of prognostic models in patients with acute stroke. Cerebrovasc Dis 2001;12:159-70.
- Meijer R, Ihnenfeldt DS, de Groot IJM, van Limbeek J, Vermeulen M, de Haan RJ. Prognostic factors for ambulation and activities of daily living in the subacute phase after stroke. A systematic review of the literature. Clin Rehabil 2003;17:119-29.
- Jacob M, Lewsey JD, Sharpin C, Gimson A, Rela M, van der Meulen JHP. Systematic review and validation of prognostic models in liver transplantation. Liver Transpl 2005;11:814-25.
- Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al. A systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma. Health Technol Assess 2003;7.
- Martin B, Paesmans M, Mascaux C, Berghmans T, Lothaire P, Meert AP, et al. P. Ki-67 expression and patients survival in lung cancer: systematic review of the literature with meta-analysis. Br J Cancer 2004;91:2018-25.
- Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427-37.
- Stemey TA, Caldwell MC, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years?. J Urol 2004;172:1297-301.
- Parker CC, Gospodarowicz M, Warde P. Does age influence the behaviour of localized prostate cancer?. BJU Int 2001;87:629-37.
- Blute ML, Bergstralh EJ, Iocca A, Scherer B, Zincke H. Use of Gleason score, prostate specific antigen, seminal vesicle and margin status to predict biochemical failure after radical prostatectomy. J Urol 2001;165:119-25.
- Lieber MM, Murtaugh P, Farrow GM, Myers RP, Blute M. DNA ploidy and surgically treated prostate cancer: important independent association with prognosis for patients with prostate carcinoma treated by radical prostatectomy. Cancer 1995;75:1935-43.
- Vollmer RT, Humphrey PA, Vollmer RT, Humphrey PA. The relative importance of anatomic and PSA factors to outcomes after radical prostatectomy for prostate cancer. Am J Clin Pathol 2001;116:864-70.
- Horvath LG, Henshall SM, Lee CS, Kench JG, Golovsky D, Brenner PC, et al. Lower levels of nuclear beta-catenin predict for a poorer prognosis in localized prostate cancer. Int J Cancer 2005;113:415-22.
- Anscher MS, Prosnitz LR. Multivariate analysis of factors predicting local relapse after radical prostatectomy – possible indications for postoperative radiotherapy. Int J Radiat Oncol Biol Phys 1991;21:941-7.
- Han M, Piantadosi S, Zahurak ML, Sokoll LJ, Chan DW, Epstein JI, et al. Serum acid phosphatase level and biochemical recurrence following radical prostatectomy for men with clinically localized prostate cancer. Urology 2001;57:707-11.
- Perez CA, Garcia D, Simpson JR, Zivnuska F, Lockett MA. Factors influencing outcome of definitive radiotherapy for localized carcinoma of the prostate. Radiother Oncol 1989;16:1-21.
- Roach M, Lu J, Pilepich MV, Asbell SO, Mohiuddin M, Terry R, et al. Long-term survival after radiotherapy alone: radiation therapy oncology group prostate cancer trials. J Urol 1999;161:864-8.
- Zagars GK, von Eschenbach AC, Ayala AG. Prognostic factors in prostate cancer. Analysis of 874 patients treated with radiation therapy. Cancer 1993;72:1709-25.
- Nam RK, Elhaji Y, Krahn MD, Hakimi J, Ho M, Chu W, et al. Significance of the CAG repeat polymorphism of the androgen receptor gene in prostate cancer progression. J Urol 2000;164:567-72.
- Powell IJ, Land SJ, Dey J, Heilbrun LK, Hughes MB, Sakr W, et al. The impact of CAG repeats in exon 1 of the androgen receptor on disease progression after prostatectomy. Cancer 2005;103:528-37.
- Merseburger AS. Use of serum creatinine to predict pathologic stage and recurrence among radical prostatectomy patients. Urology 2001;58:729-34.
- Zagars GK, von Eschenbach AC, Johnson DE, Oswald MJ. Stage C adenocarcinoma of the prostate. An analysis of 551 patients treated with external beam radiation. Cancer 1987;60:1489-99.
- Powell IJ, Zhou J, Sun Y, Sakr WA, Patel NP, Heilbrun LK, et al. CYP3A4 genetic variant and disease-free survival among white and black men after radical prostatectomy. J Urol 2004;172:1848-52.
- Siddiqui SA, Sengupta S, Slezak JM, Bergstralh EJ, Leibovich BC, Myers RP, et al. Impact of patient age at treatment on outcome following radical retropubic prostatectomy for prostate cancer. J Urol 2006;175:952-7.
- Williams H, Powell IJ, Land SJ, Sakr WA, Hughes MR, Patel NP, et al. Vitamin D receptor gene polymorphisms and disease free survival after radical prostatectomy. Prostate 2004;61:267-75.
- Egevad L, Granfors T, Karlberg L, Bergh A, Stattin P. Percent Gleason grade 4/5 as prognostic factor in prostate cancer diagnosed at transurethral resection. J Urol 2002;168:509-13.
- Gonzalgo ML, Bastian PJ, Mangold LA, Trock BJ, Epstein JI, Walsh PC, et al. Relationship between primary Gleason pattern on needle biopsy and clinicopathologic outcomes among men with Gleason score 7 adenocarcinoma of the prostate. Urology 2006;67:115-19.
- Tollefson MK, Leibovich BC, Slezak JM, Zincke H, Blute ML. Long-term prognostic significance of primary Gleason pattern in patients with Gleason score 7 prostate cancer: impact on prostate cancer specific survival. J Urol 2006;175:547-51.
- Vis AN, Roemeling S, Kranse R, Schroder FH, van der Kwast TH. Should we replace the Gleason score with the amount of high-grade prostate cancer?. Eur Urol 2007;51:931-9.
- Zellweger T, Ninck C, Mirlacher M, Annefeld M, Glass AG, Gasser TC, et al. Tissue microarray analysis reveals prognostic significance of syndecan-1 expression in prostate cancer. Prostate 2003;55:20-9.
- Antunes AA, Srougi M, Dall’Oglio MF, Crippa A, Campagnari JC, Leite KR. The percentage of positive biopsy cores as a predictor of disease recurrence in patients with prostate cancer treated with radical prostatectomy. BJU Int 2005;96:1258-63.
- Potters L, Morgenstern C, Calugaru E, Fearn P, Jassal A, Presser J, et al. 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol 2005;173:1562-6.
- Selek U, Lee A, Levy L, Kuban DA. Utility of the percentage of positive prostate biopsies in predicting PSA outcome after radiotherapy for patients with clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2003;57:963-7.
- D’Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med 2004;351:125-35.
- Sengupta S, Myers RP, Slezak JM, Bergstralh EJ, Zincke H, Blute ML. Preoperative prostate specific antigen doubling time and velocity are strong and independent predictors of outcomes following radical prostatectomy. J Urol 2005;174:2191-6.
- Li H, Zhang Y, Glass A, Zellweger T, Gehan E, Bubendorf L, et al. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res 2005;11:5863-8.
- Salomon L, Levrel O, Anastasiadis AG, Irani J, de la Taille A, Saint F, et al. Prognostic significance of tumor volume after radical prostatectomy: a multivariate analysis of pathological prognostic factors. Eur Urol 2003;43:39-44.
- Morita N, Uemura H, Tsumatani K, Cho M, Hirao Y, Okajima E, et al. E-cadherin and alpha-, beta- and gamma-catenin expression in prostate cancers: correlation with tumour invasion. Br J Cancer 1999;79:1879-83.
- Lowe FC, Trauzzi SJ. Prostatic acid phoshatase in 1993. Its limited clinical utility. Urol Clin N Am 1993;20:589-95.
- Giovannucci E. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci USA 1997;94:3320-3.
- Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol 2000;164:101-5.
- Montgomery BT, Nativ O, Blute ML, Farrow GM, Myers RP, Zincke H, et al. Stage B prostate adenocarcinoma. Flow cytometric nuclear DNA ploidy analysis. Arch Surg 1990;125:327-31.
- Winkler HZ, Rainwater LM, Myers RP, Farrow GM, Therneau TM, Zincke H, et al. Stage D1 prostatic adenocarcinoma: significance of nuclear DNA ploidy patterns studied by flow cytometry. Mayo Clin Proc 1988;63:103-12.
- So MJ, Cheville JC, Katzmann JA, Riehle DL, Lohse CM, Pankratz VS, et al. Factors that influence the measurement of prostate cancer DNA ploidy and proliferation in paraffin embedded tissue evaluated by flow cytometry. Mod Pathol 2001;14:906-12.
- Han M, Partin AW, Zahurak M, Piantadosi S, Epstein JI, Walsh PC. Biochemical (prostate specific antigen) recurrence probability following radical prostatectomy for clinically localized prostate cancer. J Urol 2003;169:517-23.
- Epstein JI, Amin M, Boccon-Gibod L, Egevad L, Humphrey PA, Mikuz G, et al. Prognostic factors and reporting of prostate carcinoma in radical prostatectomy and pelvic lymphadenectomy specimens. Scand J Urol Nephrol 2005;216:34-63.
- Anttonen A, Leppä S, Heikkilä P, Grenman R, Joensuu H. Effect of treatment of larynx and hypopharynx carcinomas on serum syndecan-1 concentrations. J Cancer Res Clin Oncol 2006;7:451-7.
- Martin RM, Gunnell D, Hamdy F, Neal D, Lane A, Donovan J. Continuing controversy over monitoring men with localized prostate cancer: a systematic review of programs in the prostate specific antigen era. J Urol 2006;176:439-49.
- Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using ‘optimal’ cutpoints in the evaluation of prognositc factors. J Natl Cancer Inst 1994;86:829-35.
- Altman DG, Royston P. What do we mean by validating a prognostic model?. Stat Med 2000;19:453-73.
- Justice AC, Covinsky KE, Berlin JA. Assessing the generalisability of prognostic information. Ann Intern Med 1999;130:515-24.
- Vergouwe Y, Steyerberg EW, Eijkemans MJC, Habbema JDF. Validity of prognostic models: when is a model clinically useful?. Semin Oncol 2002;20:96-107.
- Braitman LE, Davidoff F. Predicting clinical states in individual patients. Ann Intern Med 1996;125:406-12.
- Harrell FE, Lee KL, Mark DB. Multivariate prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361-87.
- Cowen ME, Halasyamani LK, Kattan MW. Predicting life expectancy in men with clinically localized prostate cancer. J Urol 2006;175:99-103.
- Graefen M. Can nomograms derived in the US be applied to German patients? A study about the validation of preoperative nomograms predicting the risk of recurrence after radical prostatectomy. Urologe A 2003;42:685-92.
- Dunsmuir WD, Gillett CE, Meyer LC, Young MP, Corbishley C, Eeles RA, et al. Molecular markers for predicting prostate cancer stage and survival. BJU Int 2000;86:869-78.
- Harnden P, Shelley MD, Coles B, Staffurth J, Mason MD. Should the Gleason grading system for prostate cancer be modified to account for high-grade tertiary components? A systematic review and meta-analysis. Lancet Oncol 2007;8:411-19.
- Harnden P, Shelley MD, Clements H, Coles B, Tyndale-Biscoe RS, Naylor B, et al. The prognostic significance of perineural invasion in prostatic cancer biopsies: a systematic review. Cancer 2007;109:13-24.
- McShane LM, Altman DG, Sauerbrei W. Identification of clinically useful cancer prognostic factors: what are we missing?. J Natl Cancer Inst 2005;97:1023-5.
- Altman DG, Egger M, Davy Smith G, Altman DG. Systematic reviews of health care: meta-analysis in context. London: BMJ Books; 2001.
- Hutchon DJR. Publishing raw data and real time statistical analysis on e-journals. Br Med J 2001;322.
- Stewart LA, Palmar MKB. Meta-analysis of the literature or of individual patient data: is there a difference?. Lancet 1993;341:418-22.
- Kyzas PA, Loizou KT, Ioannidis JPA. Selective reporting biases in cancer prognostic factor studies. J Natl Cancer Inst 2005;97:1043-55.
- Sauerbrei W, Hollander N, Riley RD, Altman DG. Evidence based assessment and application of prognostic markers: the long way from single studies to meta-analysis. Commun Stat Theor Methods 2006;35:1333-42.
- Altman DG, Trivella M, Pezzella F, Harris AL, Pastorino U, Auget J-L, et al. Advances in statistical methods for the health sciences. Basel: Birkhauser; 2007.
- Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med 2006;25:127-41.
- Riley RD, Ridley G, William K, Altman DG, Hayden J, de Vet HC. Prognosis research: towards evidence-based results and a Cochrane methods group. J Clin Epidemiol 2007;60:863-5.
- Holländer N, Sauerbrei W, Auget J-L, Balakrishna L, Mesbah N, Molenberghs. Advances in statistical methods for the health sciences. Basel: Birkhauser; 2007.
- Simon R, Altman D. Statistical aspects of prognostic factor studies in onclology. Br J Cancer 1994;69:979-85.
- Burton A, Altman DG. Missing covariate data within cancer prognostic studies: a review of current reporting and proposed guidelines. Br J Cancer 2004;91:4-8.
- Altman DG, De Stavola BL, Love SB, Stepiewska KA. Review of survival analyses published in cancer journals. Br J Cancer 1995;72:511-18.
- Riley RD, Abrams KR, Lambert PC, Sutton AJ, Altman DG, Auget J-L, et al. Advances in statistical methods for the health sciences. Basel: Birkhauser; 2007.
- Antunes AA, Dall’Oglio MF, Sant’Anna AC, Paranhos M, Leite KR, Srougi M. Prognostic value of the percentage of positive fragments in biopsies from patients with localized prostate cancer. Int Braz J Urol 2005;31:34-41.
- Hedley DW. DNA flow cytometry and breast cancer. Breast Cancer Res Treat 1993;28:51-3.
Appendix 1 Literature search strategies
Searches were conducted in March and April 2007 on studies published between January 1970 and March/April 2007.
MEDLINE
-
prostatic neoplasms/
-
(prostat$adj5 (cancer$or carcin$or tumor$or tumour$or neoplasm$)).tw.
-
((carcinoma or neoplasia or neoplasm$or adenocarcinoma or cancer$or tumor$or tumour$or malignan$) adj3 prostat$).tw.
-
or 2 or 3
-
prognostic methods.mp.
-
predictive factors.mp.
-
(prognos$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(predict$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(neural network$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
survival rate/
-
exp prognosis/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$).ti,ab.
-
disease free survival/
-
mortality/
-
recurrence/
-
neural networks computer/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$).ti,ab.
-
exp models statistical/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$).ti,ab.
-
algorithms/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$).ti,ab.
-
(algorithm$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
exp survival analysis/
-
nomogram$.mp.
-
((marker$or biomarker$) adj10 (prognos$or predict$)).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
or/5–21
-
letter.pt.
-
comment.pt.
-
(animal or cell line$or vitro or invitro or rat or rats or mouse or mice).ti,ab.
-
or/23–25
-
(4 and 22) not 26
Current Index to Nursing and Allied Health Literature (CINAHL)
-
Prostatic Neoplasms/
-
(prostat$adj5 (cancer$or carcin$or tumor$or tumour$or neoplasm$)).tw.
-
((carcinoma or neoplasia or neoplasm$or adenocarcinoma or cancer$or tumor$or tumour$or malignan$) adj3 prostat$).tw.
-
or 2 or 3
-
prognostic methods.mp.
-
predictive factors.mp.
-
(prognos$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(predict$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(neural network$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
survival rate.tw.
-
exp prognosis/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$).ti,ab.
-
disease free survival.tw.
-
mortality/
-
recurrence/
-
neural networks computer/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$).ti,ab.
-
exp models statistical/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$).ti,ab.
-
algorithms/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$).ti,ab.
-
(algorithm$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
exp survival analysis/
-
nomogram$.mp.
-
((marker$or biomarker$) adj10 (prognos$or predict$)).mp. [mp=title, subject heading word, abstract, instrumentation]
-
or/5–21
-
letter.pt.
-
(animal or cell line$or vitro or invitro or rat or rats or mouse or mice).ti,ab.
-
(4 and 22) not (23 or 24)
BIOSIS
-
(prostat$adj5 (cancer$or carcin$or tumor$or tumour$or neoplasm$)).tw.
-
((carcinoma or neoplasia or neoplasm$or adenocarcinoma or cancer$or tumor$or tumour$or malignan$) adj3 prostat$).tw.
-
1 or 2
-
prognostic methods.ti,ab.
-
predictive factors.ti,ab.
-
(prognos$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(predict$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(neural network$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
survival rate.ti,ab.
-
(prognosis and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
disease free survival.ti,ab.
-
mortality.ti,ab.
-
recurrence.ti,ab.
-
(neural networks computer and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(models statistical and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(algorithm$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
survival analysis.ti,ab.
-
nomogram$.ti,ab.
-
((marker$or biomarker$) adj10 (prognos$or predict$)).ti,ab.
-
or/4–19
-
letter.pt.
-
(animal or cell line$or vitro or invitro or rat or rats or mouse or mice).ti,ab.
-
(20 and 3) not (21 or 22)
-
(prostat$adj5 (cancer$or carcin$or tumor$or tumour$or neoplasm$)).tw.
-
((carcinoma or neoplasia or neoplasm$or adenocarcinoma or cancer$or tumor$or tumour$or malignan$) adj3 prostat$).tw.
-
24 or 25
-
prognostic methods.ti,ab.
-
predictive factors.ti,ab.
-
(prognos$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(predict$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(neural network$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
survival.ds.
-
(prognosis and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
mortality.ds.
-
recurrence$.ds.
-
recurrent.ds.
-
(neural networks computer and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(models statistical and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
(algorithm$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or psa failure$or biochemical failure$)).ti,ab.
-
survival analysis.ti,ab.
-
nomogram$.ti,ab.
-
((marker$or biomarker$) adj10 (prognos$or predict$)).ti,ab.
-
letter.pt.
-
(animal or cell line$or vitro or invitro or rat or rats or mouse or mice).ti,ab.
-
26 and (or/27–42)
-
45 not (43 or 44)
EMBASE
-
prostatic neoplasms/
-
(prostat$adj5 (cancer$or carcin$or tumor$or tumour$or neoplasm$)).tw.
-
((carcinoma or neoplasia or neoplasm$or adencarcinoma or cancer$or tumor$or tumour$or malignan$) adj3 prostat$).tw.
-
1 or 2 or 3
-
prognostic methods.mp.
-
predictive factors.mp.
-
(prognos$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or pda failure$or biochemical failure$)).ti,ab.
-
(predict$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or pda failure$or biochemical failure$)).ti,ab.
-
(neural network$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or pda failure$or biochemical failure$)).ti,ab.
-
survival rate/
-
exp prognosis/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or pda failure$or biochemical failure$).ti,ab.
-
disease free survival/
-
mortality/
-
Recurrent Disease/
-
Artificial Neural Networks/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or pda failure$or biochemical failure$).ti,ab.
-
Statistical Model/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or pda failure$or biochemical failure$).ti,ab.
-
algorithms/and (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or pda failure$or biochemical failure$).ti,ab.
-
(algorithm$adj10 (relapse$or recurrence$or survival$or death$or mortality or progress$or disease free or pda failure$or biochemical failure$)).ti,ab.
-
survival analysis.ti,ab.
-
nomogram/
-
nomogram$.ti,ab.
-
((marker$or biomarker$) adj10 (prognos$or predict$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
or/5–22
-
23 and 4
-
letter.pt.
-
editorial.pt.
-
24 not (25 or 26)
Web of Science
-
#1 TS=(prostat*) SAME TS=(cancer* or neoplasm* or neoplasia or tumor* or tumour* or carcin* or adenocarcinoma* or malignan*)
-
#2 TS=(prognostic methods or predictive factors)
-
#3 TS=(prognos*) SAME TS=(relapse* or recurrence* or survival* or death* or mortality* or progress* or disease free or psa failure or biochemical failure)
-
#4 TS=(predict*) SAME TS=(relapse* or recurrence* or survival* or death* or mortality* or progress* or disease free or psa failure or biochemical failure)
-
#5 TS=(neural network*) SAME TS=(relapse* or recurrence* or survival* or death* or mortality* or progress* or disease free or psa failure or biochemical failure)
-
#6 TS=disease free survival
-
#7 TS=(algorithm*) SAME TS=(cancer* or neoplasm* or neoplasia or tumor* or tumour* or carcin* or adenocarcinoma* or malignan*)
-
#8 TS=(statistical model*) SAME TS=(cancer* or neoplasm* or neoplasia or tumor* or tumour* or carcin* or adenocarcinoma* or malignan*)
-
#9 TS=nomogram*
-
#10 TS=(marker* or biomarker*) SAME TS=(prognos* or predict*)
-
#11 #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2
-
#12 #11 AND #1
Cochrane Library
-
#1 MeSH descriptor Prostatic Neoplasms explode all trees
-
#2 prostat* (cancer or neoplams* or carcin* or tumour* or tumor* or malignan* or neoplasia or adenocarcinoma*)
-
#3 (#1 OR #2)
-
#4 (prognos* or predict*)
-
#5 disease free survival
-
#6 survival rate*
-
#7 recurren*
-
#8 neural network*
-
#9 statistical model*
-
#10 algorithm*
-
#11 survial analysis
-
#12 nomogram*
-
#13 marker* or biomarker
-
#14 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13)
-
#15 #14 AND #3)
This search strategy was repeated on the National Research Register and a modified version was used on the meta-register of Current Controlled Trials.
Appendix 2 Data abstraction tables
Prostate novel prognostic markers data extraction
Article ID
First author Year Ref ID
Reviewer
Article category
| Pretreatment only = 1 | At treatment (may also include pretreatment variables) = 2 |
Principal treatment
| 0 = NS (exclude) | 1 = Watchful waiting/active monitoring | 2 = Surgery |
| 3 = Radiotherapy | 4 = Conformal radiotherapy | 5 = Brachytherapy |
| 6 = Other/mixed |
Study design
| Cohort = 1 | Comparative study = 2 | Other = 3 |
| Retrospective = 1 | Prospective = 2 |
| Sample size (indicate sample size dependent on category of study: model development, validation or both) | Initial | In analysis |
| Developing model | ||
| Validating model |
| Length of follow-up: | Median = | Mean = |
| Results reported at X years, X = |
| Study participation | |||||
|---|---|---|---|---|---|
| Are there any inclusion/exclusion criteria specified? | |||||
| Detail: | |||||
| Age (any reported values): | Value | ||||
| Median: | |||||
| Mean: | |||||
| Range: | |||||
| Distribution, specify (only if mean or median not available): | |||||
| Clinical stage (T) | Clinical, n (%) | Pathological, n (%) | |||
| Organ confined (T1, T2 or A, B): | |||||
| Non-organ confined (T3 or C): | |||||
| Missing: | |||||
| Gleason (list groups reported) | Biopsy, n (%) | Pathological, n (%) | |||
| 2 | 2 | ||||
| 3 | 3 | ||||
| 4 | 4 | ||||
| 5 | 5 | ||||
| 6 | 6 | ||||
| 7 | 7 | ||||
| 8 | 8 | ||||
| 9 | 9 | ||||
| 10 | 10 | ||||
| Missing | |||||
| PSA (any reported values): | Value | ||||
| Median: | |||||
| Mean: | |||||
| Range: | |||||
| Distribution specify: | |||||
| Missing | |||||
| Recruitment dates: | Start (YYYY) | End (YYYY) | |||
| Adjuvant/neoadjuvant treatment: | |||||
| 0 = none | 1 = all | 2 = some | 3 = NS | ||
| Post surgical: | |||||
| Positive surgical margins, % | Lymph node involvement, % | ||||
Novel marker definitions (where applicable)
| Marker | Definition |
|---|---|
Univariate analysis
Analysis 1 methods:
| End point (tick all that apply): | |||
|---|---|---|---|
| Expressed as: | Survival = 1 | Failure (e.g. death, recurrence) = 2 | |
| Events: | All death = 1 | Prostate cancer death = 2 | Death – unclear = 3 |
| Biochemical (PSA) recurrence = 4 | Clinical recurrence = 5 | ||
| Marker | Measure (e.g. HR, actuarial survival) | Resulta | CI | p-value |
|---|---|---|---|---|
Multivariate analysis
| Model used: | 0 = None | 1 = Cox | 2 = Logistic | 3 = Weibull | 4 = Artificial neural network |
| 5 = Multinomial logistic | 6 = Other, please specify | 7 = Not specified | |||
| Classical markers included? 0 = Not specified | 1 = None | 2 = Yes, at least one (see below) | |||
| Marker | Clinical | Pathological |
|---|---|---|
| PSA | ||
| Gleason grade | ||
| Stage (or organ confined) | ||
| Surgical margins | ||
| Number of factors (prognostic markers) in final model? | ||
| 0 = Not specified | ||
Number of factors (prognostic markers) in final model?
0 = Not specified
Results
| Analysis 1 methods: | |||
| End point (tick all that apply): | All death = 1 | Prostate cancer death = 2 | Death – unclear = 3 |
| Biochemical (PSA) recurrence = 4 | Clinical recurrence = 5 | ||
| Marker | Measure (e.g. HR, actuarial survival) | Resulta | CI | p-value |
|---|---|---|---|---|
Conclusions
Novel marker and model studies data extraction continuation sheet no.
Univariate results
| End point (tick all that apply): | |||
|---|---|---|---|
| Expressed as: | Survival = 1 | Failure (e.g. death, recurrence) = 2 | |
| Events: | All death = 1 | Prostate cancer death = 2 | Death – unclear = 3 |
| Biochemical (PSA) recurrence = 4 | |||
| Marker | Measure (e.g. HR, actuarial survival) | Resulta | CI | p-value |
|---|---|---|---|---|
Univariate analysis number: Methods:
| End point (tick all that apply): | |||||
|---|---|---|---|---|---|
| Expressed as: | Survival = 1 | Failure (e.g. death, recurrence) = 2 | |||
| Events: | All death = 1 | Prostate cancer death = 2 | Death – unclear = 3 | Biochemical (PSA) recurrence = 4 | Clinical recurrence = 5 |
| Marker | Measure (e.g. HR, actuarial survival) | Resulta | CI | p-value |
|---|---|---|---|---|
Prostate novel prognostic markers data extraction continuation sheet no.
Multivariate results
Multivariate analysis number:
| Model used: | 0 = None | 1 = Cox | 2 = Logistic | 3 = Weibull | 4 = Artificial neural network |
| 5 = Multinomial logistic | 6 = Other, please specify | 7 = Not specified | |||
| Classical markers included? 0 = Not specified | 1 = None | 2 = Yes, at least one (see below) | |||
| Marker | Clinical | Pathological |
|---|---|---|
| PSA | ||
| Gleason grade | ||
| Stage (or organ confined) | ||
| Surgical margins | ||
| Number of factors (prognostic markers) in final model? | ||
| 0 = Not specified | ||
Number of factors (prognostic markers) in final model?
0 = Not specified
Results
Analysis methods:
| End point (tick all that apply): | ||
|---|---|---|
| All death = 1 | Prostate cancer death = 2 | |
| Death – unclear = 3 | Biochemical (PSA) recurrence = 4 | Clinical recurrence = 5 |
| Marker | Measure (e.g. HR, actuarial survival) | Resulta | CI | p-value |
|---|---|---|---|---|
Appendix 3 Quality assessment
| Potential bias | Items to be considered for assessment of potential opportunity for bias | Yes | Partly | No | Unsure | NA |
|---|---|---|---|---|---|---|
| Study population | Inclusion and exclusion criteria are adequately described (including treatment, start/finish date recruitment) | |||||
| Baseline study sample (i.e. individuals entering the study) is adequately described for key characteristics: age, PSA, clinical and/or pathological stage, biopsy and/or pathological Gleason grade, surgical margins (where relevant) | ||||||
| Study sample represents population of interest on key characteristics, sufficient to limit potential bias to results (note inherent bias from treatment selection) | ||||||
| Study attrition | Statement as to exclusions due to missing data: | |||||
| baseline variables | ||||||
| loss to follow-up | ||||||
| Statement as to the possible effect on the results from missing data | ||||||
| Loss to follow-up is not associated with key characteristics (i.e. there are no important differences between key characteristics and outcomes in participants who completed the study and those who did not), sufficient to limit potential bias | ||||||
| Prognostic factor measurement | Clear definitions of the prognostic factors measured are provided (e.g. extraction method, measurement described) | |||||
| Material storage is described | ||||||
| Continuous variables are reported or appropriate (i.e. not data dependent) cut-points are used | ||||||
| The prognostic factor(s) of interest is(are) adequately measured in study participants to sufficiently limit potential bias | ||||||
| Outcome measurement | Is the outcome (e.g. survival, PSA survival) clearly defined? (Any death? Prostate cancer death? Clinical recurrence?) | |||||
| If the study has an outcome of PSA recurrence have the internationally agreed definitions of PSA recurrence been used: | ||||||
| PSA > 0.2 ng/ml after prostatectomy | ||||||
| following radiotherapy, a rise by 2 ng/ml or more above the nadir PSA (2005) or three consecutive PSA rises above the nadir (1997) | ||||||
| If there is a biochemical outcome (PSA), is a unique definition of failure used? | ||||||
| The outcome of interest is adequately measured in study participants to sufficiently limit potential bias | ||||||
| Confounding measurement and account | Does the model include all classical markers (PSA, stage and grade, surgical margins if applicable)? (i.e. the important potential confounders are appropriately accounted for, sufficiently limiting potential bias with respect to the prognostic factor of interest) | |||||
| Analysis | There is sufficient presentation of data to assess the adequacy of the analysis | |||||
| The strategy for model building (i.e. inclusion of variables) is appropriate and is based on a conceptual framework or model | ||||||
| The selected model is adequate for the design of the study | ||||||
| The number of events or events per variable is reported | ||||||
| Events per variable (minimum 10; 20 more robust) | ||||||
| The statistical analysis is appropriate for the study design, limiting the potential for the presentation of invalid results |
First author: Year: ID: Reviewer:
Overall opinion of study quality:
Appendix 4 References excluded at full sifting and reasons for exclusion
A total of 365 articles were excluded at full paper sift. A summary of the reasons for exclusion is shown in Table 72. For each article the name of the first author, year of publication, journal and reason for exclusion are reported in Table 73. Note that in both tables only one reason for exclusion is shown. Many articles were excluded on several criteria.
| Reason for exclusion | n |
|---|---|
| Commentary | 1 |
| n < 200 at 5 years’ follow-up | 1 |
| No appropriate outcome | 1 |
| Nodal status not identified | 1 |
| Risk groups are not based on statistical model | 1 |
| Treatment evaluation study | 1 |
| Animal study | 2 |
| Follow-up 2–5 years in radiation-treated group | 2 |
| Gleason score only with no novel markers | 2 |
| Mx patients | 2 |
| n < 200 | 2 |
| Not a full paper | 2 |
| Not a pretreatment PSADT | 2 |
| Not the correct type of marker | 2 |
| Predicts what will find at surgery | 2 |
| PSADT after surgery | 2 |
| Secondary study | 2 |
| Unclear number of T4 patients | 2 |
| Validation of excluded models | 2 |
| Wrong outcomes | 2 |
| Wrong patient group | 3 |
| Not a primary study | 3 |
| Review | 3 |
| Foreign language article | 4 |
| Not prognosis | 4 |
| Nx patients | 4 |
| Early data from trial | 4 |
| Screening article | 6 |
| Predicts stage | 7 |
| Follow-up below 2 years | 15 |
| > 20% metastases | 20 |
| n < 200 in relevant analysis group | 22 |
| No follow-up data | 22 |
| No novel marker and no model | 28 |
| Follow-up 2–5 years | 186 |
| Total | 365 |
| First author, year of publication | Journal | Reason for exclusion |
|---|---|---|
| Aaltomaa, 1999 | British Journal of Cancer | > 20% metastases |
| Aaltomaa, 1999 | Prostate | > 20% metastases |
| Aaltomaa, 1999 | Prostate | > 20% metastases |
| Aaltomaa, 2001 | European Urology | > 20% metastases |
| Aaltomaa, 2006 | Anticancer Research | n < 200 |
| Adami, 1986 | Scandinavian Journal of Urology and Nephrology | No novel marker and no model |
| Albertsen, 2001 | Journal of Urology | Not the correct type of marker |
| Alcantara, 2007 | Cancer | Follow-up 2–5 years |
| Aleman, 2003 | Urology | Wrong outcomes |
| Algaba, 2005 | European Urology | No follow-up data |
| Ali, 2007 | International Journal of Cancer | n < 200 in relevant analysis group |
| Amling, 1998 | Mayo Clinic Proceedings | No follow-up data |
| Amling, 2000 | Journal of Urology | Early data from trial |
| Andrén, 2006 | Journal of Urology | Nx patients |
| Antenor, 2005 | Journal of Urology | Follow-up 2–5 years |
| Antunes, 2005 | International Brazilian Journal of Urology | Early data from trial |
| Aref, 1998 | British Journal of Radiology | Follow-up 2–5 years |
| Augustin, 2003 | Prostate | Follow-up 2–5 years |
| Augustin, 2003 | Urology | Follow-up 2–5 years |
| Ayala, 2003 | Clinical Cancer Research | Follow-up 2–5 years |
| Ayala, 2003 | Cancer Research | Follow-up 2–5 years |
| Ayala, 2004 | Clinical Cancer Research | Follow-up 2–5 years |
| Babaian, 2005 | Nature Clinical Practice Urology | Not a full paper |
| Badalament, 1996 | Journal of Urology | n < 200 in relevant analysis group |
| Banerjee, 2000 | Cancer | Follow-up 2–5 years |
| Bastian, 2006 | Cancer | No follow-up data |
| Bauer, 1998 | Urology | Follow-up 2–5 years |
| Bauer, 1998 | Military Medicine | Predicts what will find at surgery |
| Bauer, 1998 | Journal of Urology | Follow-up 2–5 years |
| Beard, 2004 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Bettuzzi, 2003 | Cancer Research | n < 200 in relevant analysis group |
| Beyer, 1997 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Bianco, 2002 | Urologic Oncology | Follow-up 2–5 years |
| Bianco, 2003 | Journal of Urology | Validation of excluded models |
| Bianco, 2003 | Clinical Prostate Cancer | Follow-up 2–5 years |
| Bloom, 2004 | Urology | Follow-up 2–5 years |
| Blute, 1989 | Journal of Urology | n < 200 in relevant analysis group |
| Blute, 2000 | Journal of Urology | No follow-up data |
| Borre, 1998 | Prostate Cancer and Prostatic Diseases | > 20% metastases |
| Borre, 1998 | British Journal of Cancer | > 20% metastases |
| Borre, 2000 | Journal of Urology | > 20% metastases |
| Borre, 2000 | Clinical Cancer Research | > 20% metastases |
| Bostwick, 1993 | Urology | Secondary study |
| Bostwick, 1996 | Journal of Urology | No follow-up data |
| Brassell, 2005 | Urology | Follow-up 2–5 years |
| Brenner, 2005 | Journal of Clinical Oncology | Screening paper |
| Briganti, 2006 | BJU International | Predicts stage |
| Buskirk, 2006 | Journal of Urology | Wrong patient group |
| Calvert, 2003 | British Journal of Cancer | Not a primary study |
| Cappello, 2003 | Anticancer Research | n < 200 in relevant analysis group |
| Carvalhal, 2000 | Cancer | Follow-up below 2 years |
| Catalona, 1994 | Journal of Urology | Follow-up 2–5 years |
| Catalona, 1998 | Journal of Urology | Follow-up 2–5 years |
| Catton, 2002 | Canadian Journal of Urology | Follow-up 2–5 years |
| Cheng, 2005 | Journal of Clinical Oncology | Follow-up below 2 years |
| Chism, 2004 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Chun, 2006 | European Urology | Review |
| Chun, 2006 | World Journal of Urology | Follow-up 2–5 years |
| Chun, 2006 | BJU International | No follow-up data |
| Chun, 2007 | European Journal of Cancer | Follow-up 2–5 years |
| Chun, 2007 | European Urology | Follow-up 2–5 years |
| Chun, 2007 | European Urology | Follow-up 2–5 years |
| Coetzee, 1997 | Journal of Urology | Follow-up below 2 years |
| Cooperberg, 2005 | Journal of Urology | Follow-up 2–5 years |
| Crippa, 2006 | International Brazilian Journal of Urology | Predicts stage |
| Critz, 2004 | Journal of Urology | Nx patients |
| Dahm, 2000 | World Journal of Urology | Follow-up 2–5 years |
| Dall’Oglio, 2005 | International Brazilian Journal of Urology | No novel marker and no model |
| D’Amico, 1994 | International Journal of Radiation Oncology, Biology, Physics | Not prognosis |
| D’Amico, 1995 | Journal of Urology | Follow-up 2–5 years |
| D’Amico, 1996 | Journal of Clinical Oncology | Follow-up 2–5 years |
| D’Amico, 1996 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| D’Amico, 1997 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| D’Amico, 1998 | Journal of Urology | Follow-up 2–5 years |
| D’Amico, 1998 | Urology | Follow-up 2–5 years |
| D’Amico, 1998 | Cancer | Follow-up 2–5 years |
| D’Amico, 1998 | Cancer | Follow-up 2–5 years |
| D’Amico, 1999 | Journal of Clinical Oncology | Follow-up 2–5 years |
| D’Amico, 1999 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| D’Amico, 2000 | Molecular Urology | Follow-up 2–5 years |
| D’Amico, 2000 | Urology | Follow-up 2–5 years |
| D’Amico, 2000 | Journal of Urology | Follow-up 2–5 years |
| D’Amico, 2000 | Cancer | Follow-up 2–5 years |
| D’Amico, 2000 | Cancer | Follow-up 2–5 years |
| D’Amico, 2001 | Journal of Urology | Follow-up 2–5 years |
| D’Amico, 2001 | Journal of Urology | Follow-up 2–5 years |
| D’Amico, 2001 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| D’Amico, 2001 | Urology | Follow-up 2–5 years |
| D’Amico, 2002 | Journal of Urology | Follow-up 2–5 years |
| D’Amico, 2002 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| D’Amico, 2002 | Cancer | Follow-up 2–5 years |
| D’Amico, 2003 | Journal of Urology | Follow-up 2–5 years |
| D’Amico, 2003 | Journal of National Cancer Institute | Follow-up 2–5 years |
| D’Amico, 2004 | Journal of Clinical Oncology | Follow-up 2–5 years |
| D’Amico, 2004 | Journal of Urology | Not a pretreatment PSADT |
| D’Amico, 2005 | JAMA | Follow-up 2–5 years |
| D’Amico, 2005 | Journal of Clinical Oncology | Follow-up 2–5 years |
| D’Amico, 2006 | Journal of Urology | Follow-up 2–5 years in radiation-treated group |
| Darson, 1997 | Urology | No follow-up data |
| De%%La%%Taille, 2000 | European Urology | Follow-up 2–5 years |
| Demsar, 1999 | Studies in Health Technology and Informatics | No follow-up data |
| Dillioglugil, 1997 | Urology | Follow-up 2–5 years |
| Douglas, 1997 | Cancer | n < 200 in relevant analysis group |
| Draisma, 2006 | International Journal of Cancer | Screening paper |
| Eastham, 1999 | Urology | No follow-up data |
| Egawa, 2001 | Japanese Journal of Clinical Oncology | n < 200 in relevant analysis group |
| Egawa, 2004 | Prostate Cancer and Prostatic Diseases | No novel marker and no model |
| Egevad, 2002 | BJU International | No novel marker and no model |
| Eggener, 2005 | Journal of Urology | Follow-up below 2 years |
| Eichelberger, 2005 | Modern Pathology | Follow-up 2–5 years |
| Epstein, 1988 | Journal of Urology | n < 200 in relevant analysis group |
| Epstein, 1996 | American Journal of Surgical Pathology | No novel marker and no model |
| Fang, 2001 | Urology | Follow-up 2–5 years |
| Fatih, 2005 | Archivos Españoles de%%Urologia | Follow-up below 2 years |
| Feigenberg, 2004 | International Journal of Radiation Oncology, Biology, Physics | No novel marker and no model |
| Ferrari, 2004 | Urology | No novel marker and no model |
| Finne, 2002 | European Urology | Screening paper |
| Fitzsimons, 2006 | Journal of Urology | Follow-up 2–5 years |
| Fowler, 2000 | Journal of Urology | No novel marker and no model |
| Freedland, 2002 | Urology | Follow-up 2–5 years |
| Freedland, 2003 | Journal of Urology | Follow-up 2–5 years |
| Freedland, 2003 | Journal of Urology | Follow-up 2–5 years |
| Freedland, 2003 | Journal of Urology | Follow-up 2–5 years |
| Freedland, 2003 | Journal of Urology | Follow-up 2–5 years |
| Freedland, 2003 | Journal of Urology | Follow-up 2–5 years |
| Freedland, 2003 | Urology | Follow-up 2–5 years |
| Freedland, 2003 | Prostate Cancer and Prostatic Diseases | Gleason score only with no novel markers |
| Freedland, 2003 | Cancer | Follow-up 2–5 years |
| Freedland, 2004 | Cancer | Follow-up 2–5 years |
| Freedland, 2004 | Cancer | No novel marker and no model |
| Freedland, 2004 | Cancer | Follow-up 2–5 years |
| Freedland, 2004 | Journal of Urology | Follow-up 2–5 years |
| Freedland, 2005 | JAMA | PSADT after surgery |
| Freedland, 2005 | Journal of Urology | Follow-up 2–5 years |
| Gettman, 1999 | Adult Urology | Mx patients |
| Giovannucci, 1997 | Proceedings of the National Academy of Sciences of the United States of America | Not prognosis |
| Glinsky, 2004 | Journal of Clinical Investigation | Animal study |
| Gonzalez, 2004 | Urology | No novel marker and no model |
| Graefen, 1999 | Journal für Urologie und Urogynäkologie | Foreign language paper |
| Graefen, 2002 | Urologic Oncology | Follow-up 2–5 years |
| Graefen, 2002 | Journal of Clinical Oncology | Follow-up 2–5 years |
| Graefen, 2002 | Journal of Urology | Follow-up 2–5 years |
| Graefen, 2002 | Journal of Clinical Oncology | Follow-up 2–5 years |
| Graefen, 2003 | Urologe A | Foreign language paper |
| Graefen, 2003 | European Urology | Predicts stage |
| Graefen, 2004 | Journal of Urology | Follow-up 2–5 years |
| Greene, 2006 | Journal of Urology | Follow-up below 2 years |
| Grossfeld, 2000 | Journal of Urology | Follow-up below 2 years |
| Grossfeld, 2002 | Journal of Urology | Follow-up 2–5 years |
| Grubb, 2006 | Nature Clinical Practice Urology | Commentary |
| Han, 2000 | Urology | n < 200 at 5 years’ follow-up |
| Hattab, 2006 | Journal of Urology | Follow-up 2–5 years |
| Haukaas, 2006 | BJU International | No novel marker and no model |
| Hayes, 2006 | Cancer Epidemiology, Biomarkers and Prevention | Unclear number of T4 patients |
| Henshall, 2001 | Clinical Cancer Research | n < 200 in relevant analysis group |
| Herman, 2000 | American Journal of Surgical Pathology | Follow-up 2–5 years |
| Herman, 2001 | American Journal of Surgical Pathology | Follow-up 2–5 years |
| Horwitz, 2006 | Cancer | No novel marker and no model |
| Imai, 1990 | Japanese Journal of Cancer Research | Follow-up 2–5 years |
| Jani, 2005 | Urology | Follow-up 2–5 years |
| Johansson, 1992 | Cancer | Nodal status not identified |
| Johansson, 1997 | JAMA | > 20% metastases |
| Johnstone, 2003 | International Journal of Radiation Oncology, Biology, Physics | No novel marker and no model |
| Jones, 2005 | BJU International | Follow-up 2–5 years |
| Jones, 2006 | BJU International | Follow-up below 2 years |
| Joseph, 2004 | BJU International | Follow-up 2–5 years |
| Kahl, 2006 | Cancer Research | Follow-up below 2 years |
| Kaminski, 2002 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Karakiewicz, 2005 | Urology | Follow-up 2–5 years |
| Kattan, 1998 | Journal of the National Cancer Institute | Follow-up 2–5 years |
| Kattan, 2000 | Journal of Clinical Oncology | Follow-up 2–5 years |
| Kattan, 2001 | Urology | No follow-up data |
| Kattan, 2003 | Journal of Clinical Oncology | Follow-up 2–5 years |
| Kattan, 2003 | Journal of Clinical Oncology | Follow-up 2–5 years |
| Kattan, 2003 | Journal of Urology | Follow-up 2–5 years |
| Kausik, 2002 | Cancer | Follow-up 2–5 years |
| Kestin, 2004 | International Journal of Radiation Oncology, Biology, Physics | No appropriate outcome |
| Khan, 2003 | Urology | Risk groups are not based on statistical model |
| Khan, 2005 | Prostate Cancer and Prostatic Diseases | Follow-up 2–5 years |
| Khoddami, 2004 | BJU International | Follow-up below 2 years |
| Klotz, 2006 | European Urology Supplements | Review |
| Kreisberg, 2004 | Cancer Research | n < 200 in relevant analysis group |
| Kuban, 1995 | International Journal of Radiation Oncology, Biology, Physics | No novel marker and no model |
| Kuban, 2003 | International Journal of Radiation Oncology, Biology, Physics | No novel marker and no model |
| Kupelian, 1997 | Cancer Journal from Scientific American | Follow-up 2–5 years |
| Kupelian, 1997 | Journal of Clinical Oncology | Follow-up 2–5 years |
| Kupelian, 1997 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Kurek, 1999 | Prostate Cancer and Prostatic Diseases | Not a primary study |
| Lam, 2006 | BJU International | Follow-up 2–5 years |
| Latil, 2003 | Clinical Cancer Research | Follow-up 2–5 years |
| Latini, 2006 | Cancer | Follow-up 2–5 years |
| Lee, 2002 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Leibovici, 2005 | Cancer | Wrong patient group |
| Lerner, 1996 | Journal of Urology | Follow-up 2–5 years |
| Li, 2003 | Anticancer Research | No follow-up data |
| Li, 2004 | American Journal of Surgical Pathology | Follow-up 2–5 years |
| Li, 2006 | Urologic Oncology | n < 200 in relevant analysis group |
| Li, 2006 | Journal of Urology | Follow-up 2–5 years |
| Lieberfarb, 2002 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Lind, 2005 | Prostate | n < 200 in relevant analysis group |
| Lipponen, 1996 | Anticancer Research | > 20% metastases |
| Lipponen, 1997 | Prostate | > 20% metastases |
| Lipponen, 2000 | European Urology | > 20% metastases |
| Lowe, 1988 | Journal of Urology | n < 200 in relevant analysis group |
| McAleer, 2005 | Urologic Oncology | Follow-up 2–5 years |
| McAlhany, 2004 | Prostate | Follow-up 2–5 years |
| McIntire, 1988 | American Journal of Clinical Pathology | n < 200 in relevant analysis group |
| McNeal, 1996 | American Journal of Surgical Pathology | No follow-up data |
| Makarov, 2002 | Journal of Urology | Predicts stage |
| Man, 2003 | Journal of Urology | No novel marker and no model |
| Massengill, 2003 | Journal of Urology | Follow-up 2–5 years |
| May, 2001 | BJU International | No novel marker and no model |
| Merrick, 1985 | British Journal of Urology | Treatment evaluation study |
| Merrick, 2005 | Urology | No novel marker and no model |
| Merrill, 2002 | Cancer Causes and Control | No follow-up data |
| Mitchell, 2005 | Journal of Urology | Follow-up 2–5 years |
| Miyake, 2005 | Acta Urologica Japonica | Follow-up 2–5 years |
| Molitierno, 2006 | Urologia Internationalis | Follow-up 2–5 years |
| Montgomery, 1990 | Archives of Surgery | Early data from trial |
| Moul, 1998 | Journal of Urology | Follow-up 2–5 years |
| Moul, 1999 | European Urology | n < 200 in relevant analysis group |
| Moul, 2001 | Journal of Urology | No novel marker and no model |
| Myers, 1983 | Prostate | No novel marker and no model |
| Nelson, 2003 | Urologic Oncology | Follow-up 2–5 years |
| Ng, 2004 | Journal of Urology | Follow-up below 2 years |
| Nguyen, 2004 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Nickers, 2006 | Radiotherapy and Oncology | Follow-up below 2 years |
| Nielsen, 2006 | Journal of Urology | No novel marker and no model |
| Noguchi, 2000 | Urologia Internationalis | n < 200 in relevant analysis group |
| Noguchi, 2003 | Journal of Urology | Follow-up 2–5 years |
| Norlen, 1991 | Acta Oncologica | No novel marker and no model |
| Norrish, 1999 | BJU International | No novel marker and no model |
| Oakley-Girvan, 2003 | American Journal of Public Health | No novel marker and no model |
| Ogawa, 2006 | Anticancer Research | No novel marker and no model |
| Ohori, 1993 | American Journal of Surgical Pathology | No follow-up data |
| Ohori, 1999 | Journal of Urology | Follow-up 2–5 years |
| Optenberg, 1995 | JAMA | No novel marker and no model |
| Orvieto, 2006 | BJU International | No novel marker and no model |
| Osman, 2004 | Clinical Cancer Research | Follow-up 2–5 years |
| Parker, 2004 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Partin, 1993 | Journal of Urology | Predicts stage |
| Partin, 1995 | Urology | Follow-up 2–5 years |
| Pollack, 2004 | Journal of Clinical Oncology | Unclear number of T4 patients |
| Paulson, 2002 | Critical Reviews in Oncology Hematology | Follow-up 2–5 years |
| Perlman, 2000 | Genome Biology | Not a primary study |
| Pettus, 2004 | Journal of Urology | Follow-up 2–5 years |
| Pienta, 1995 | Urology | No novel marker and no model |
| Pilepich, 1980 | Journal of Urology | No reporting of statistical differences |
| Pinover, 1996 | Cancer | Follow-up 2–5 years |
| Pisansky, 1997 | Cancer | Follow-up 2–5 years |
| Pisansky, 2002 | Cancer | Not prognosis |
| Polednak, 2003 | Ethnicity and Disease | No follow-up data |
| Pootrakul, 2006 | Clinical Cancer Research | n < 200 in relevant analysis group |
| Porter, 2006 | Journal of Urology | No novel marker and no model |
| Potter, 1999 | Urology | n < 200 in relevant analysis group |
| Potters, 2002 | Prostate Cancer and Prostatic Diseases | Follow-up 2–5 years |
| Pound, 1997 | Urologic Clinics of North America | No report of statistical differences between groups |
| Pousette, 1999 | Scandinavian Journal of Clinical and Laboratory Investigation Supplement | n < 200 in relevant analysis group |
| Powell, 2002 | Urology | No novel marker and no model |
| Powell, 2004 | Journal of Urology | No novel marker and no model |
| Presti, 1998 | Urology | Follow-up 2–5 years |
| Prtilo, 2005 | Journal of Urology | > 20% metastases |
| Quan, 2006 | Urology | Follow-up 2–5 years |
| Quinn, 2001 | Journal of Clinical Oncology | Follow-up 2–5 years |
| Rabbani, 1998 | Molecular Urology | No follow-up data |
| Ramos, 2004 | Journal of Urology | Follow-up 2–5 years |
| Rasiah, 2006 | Cancer Epidemiology, Biomarkers and Prevention | n < 200 in relevant analysis group |
| Renshaw, 1999 | American Journal of Clinical Pathology | Follow-up 2–5 years |
| Rhodes, 2003 | Journal of the National Cancer Institute | Follow-up 2–5 years |
| Ricciardelli, 1997 | Clinical Cancer Research | Follow-up 2–5 years |
| Ricciardelli, 1998 | Clinical Cancer Research | Follow-up 2–5 years |
| Risbridger, 2004 | Journal of Urology | n < 200 in relevant analysis group |
| Roach, 2000 | Seminars in Urologic Oncology | Follow-up 2–5 years |
| Roach, 2000 | International Journal of Radiation Oncology, Biology, Physics | Nx and N1 patients |
| Roach, 2003 | Journal of Urology | No novel marker and no model |
| Roach, 2003 | Urology | No novel marker and no model |
| Roach, 2006 | Journal of Urology | No follow-up data |
| Robbins, 2000 | American Journal of Epidemiology | No novel marker and no model |
| Roberts, 2001 | Urology | Follow-up 2–5 years |
| Rodriguez, 2001 | Cancer Epidemiology, Biomarkers and Prevention | No novel marker and no model |
| Roehl, 2004 | Journal of Urology | No novel marker and no model |
| Rosser, 2003 | Journal of Urology | No novel marker and no model |
| Rosser, 2004 | Journal of the National Medical Association | Follow-up 2–5 years |
| Rossi, 2004 | Urology | No novel marker and no model |
| Rubin, 2005 | Cancer Epidemiology, Biomarkers and Prevention | Follow-up 2–5 years |
| Saito, 2006 | Acta Urologica Japonica | Foreign language paper |
| Salomon, 2003 | Urologia Internationalis | Follow-up 2–5 years |
| Sandblom, 2000 | Urology | > 20% metastases |
| Schafer, 2006 | Journal of Urology | Unknown number of lymph nodes reported |
| Schellhammer, 1993 | Urology | < 5 years follow-up in analysis group |
| Secin, 2006 | Cancer | No novel marker and no model |
| Seligson, 2005 | Nature | Follow-up below 2 years |
| Severi, 2006 | Cancer Epidemiology, Biomarkers and Prevention | No novel marker and no model |
| Shariat, 2004 | Journal of Clinical Oncology | No follow-up data |
| Shariat, 2004 | Journal of Urology | Follow-up 2–5 years |
| Shariat, 2006 | European Urology | Follow-up 2–5 years |
| Shuford, 2004 | Journal of Urology | Follow-up 2–5 years |
| Singh, 2002 | Cancer Cell | Follow-up below 2 years |
| Smedley, 1983 | British Journal of Urology | No novel marker and no model |
| Smith, 1991 | Urologic Clinics of North America | n < 200 in relevant analysis group |
| Smith, 1992 | Cancer | n < 200 in relevant analysis group |
| Snow, 2002 | Journal of Urology | No follow-up data |
| Sofer, 2002 | Journal of Urology | n < 200 in relevant analysis group |
| Soloway, 2005 | Cancer | Review |
| Stamey, 1999 | Journal of the American Medical Association | Follow-up 2–5 years |
| Stephenson, 2006 | Journal of the National Cancer Institute | Follow-up 2–5 years |
| Steuber, 2006 | Cancer | Follow-up 2–5 years |
| Steuber, 2006 | International Journal of Cancer | Follow-up 2–5 years |
| Steuber, 2007 | Clinical Chemistry | Follow-up 2–5 years |
| Steyerberg, 2007 | Journal of Urology | No follow-up data |
| Stokes, 2000 | International Journal of Radiation Oncology, Biology, Physics | No novel marker and no model |
| Sumiya, 1990 | European Journal of Cancer | n < 200 in relevant analysis group |
| Suzuki, 2002 | European Urology | No novel marker and no model |
| Swindle, 2005 | Journal of Urology | No novel marker and no model |
| Tahir, 2006 | Clinical Cancer Research | Follow-up 2–5 years |
| Takahashi, 2002 | Prostate | Not prognosis |
| Tarman, 2000 | Urology | Follow-up 2–5 years |
| Taylor, 2005 | Journal of Clinical Oncology | Follow-up 2–5 years |
| Tewari, 2004 | Journal of Urology | Nodal status unclear |
| Tewari, 2005 | BJU International | No novel marker and no model |
| Tewari, 2005 | BJU International | No novel marker and no model |
| Thompson, 2005 | Journal of the American Medical Association | No novel marker and no model |
| Thompson, 2006 | Urology | n < 200 in relevant analysis group |
| Thrasher, 1994 | Cancer | n < 200 in relevant analysis group |
| Tiguert, 1998 | Prostate | No novel marker and no model |
| Tombal, 2002 | Urology | Follow-up 2–5 years |
| Tribukait, 1993 | European Urology | No novel marker and no model |
| Tsai, 2006 | Cancer | Follow-up 2–5 years |
| Underwood, 2004 | Urologic Oncology | Follow-up 2–5 years |
| van den Ouden, 1997 | British Journal of Urology | Follow-up 2–5 years |
| van den Ouden, 1998 | Urologia Internationalis | Follow-up 2–5 years |
| van den Ouden, 2005 | European Urology | Follow-up 2–5 years |
| Vesalainen, 1994 | European Journal of Cancer | > 20% metastases |
| Vesalainen, 1994 | British Journal of Cancer | > 20% metastases |
| Vesalainen, 1995 | Anticancer Research | n < 200 in relevant analysis group |
| Vesalainen, 1995 | Acta Oncologica | > 20% metastases |
| Vesalainen, 1995 | Prostate | > 20% metastases |
| Vira, 2005 | Urology | Follow-up 2–5 years |
| Vis, 2006 | European Urology | No novel marker and no model |
| Vollmer, 1999 | Clinical Cancer Research | No follow-up data |
| Weight, 2006 | International Journal of Radiation Oncology, Biology, Physics | Follow-up 2–5 years |
| Went, 2006 | British Journal of Cancer | Not prognosis |
| Wheeler, 1998 | Human Pathology | Follow-up 2–5 years |
| Wilcox, 1998 | Human Pathology | Follow-up 2–5 years |
| Williams, 2004 | International Journal of Radiation Oncology, Biology, Physics | No novel marker and no model |
| Williams, 2004 | International Journal of Radiation Oncology, Biology, Physics | Nx patients |
| Williams, 2006 | International Journal of Radiation Oncology, Biology, Physics | No follow-up data |
| Winkler, 2004 | BJU International | No follow-up data |
| Wise, 2002 | Urology | Follow-up 2–5 years |
| Wu, 2004 | Journal of Urology | Follow-up 2–5 years |
| Yang, 2002 | Clinical Cancer Research | Follow-up 2–5 years |
| Yang, 2004 | Cancer Research | Follow-up 2–5 years |
| Yeole, 2001 | Indian Journal of Cancer | > 20% metastases |
| Young, 2000 | Seminars Urologic Oncology | Follow-up 2–5 years |
| Yu, 2006 | Urology | No follow-up data |
| Zagars, 1994 | Journal of Urology | Follow-up 2–5 years |
| Zagars, 1995 | International Journal of Radiation Oncology, Biology, Physics | Nx patients pre-PSA group and follow-up 2–5 years for post-PSA group |
| Zagars, 1995 | International Journal of Radiation Oncology, Biology, Physics | Nx patients |
| Zetterberg, 1991 | Acta Oncologica | n < 200 in relevant analysis group |
| Zhang, 2004 | Cancer | No novel marker and no model |
| Zhang, 2006 | Journal of Urology | Follow-up 2–5 years |
| Ziada, 2001 | Cancer | Follow-up 2–5 years |
| Zincke, 1981 | Cancer | Follow-up 2–5 years |
| Zincke, 1994 | Journal of Clinical Oncology | No novel marker and no model |
Appendix 5 Included studies for novel prognostic markers
Novel prognostic markers
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Horvath, 2005108 Australia International Journal of Cancer |
Aim: to determine whether differences in the pattern of β-catenin protein expression were associated with disease progression and prognosis Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery (78%), hormone, radiotherapy and orchidectomy Study design: cohort retrospective study Sample size: initial, 732 patients; in analysis, 232 specimens Inclusion criteria: clinically localised prostate cancer patients No neoadjuvant hormonal therapy Start and finish dates: NS |
Age: median, NS; mean, 63 years; range, 44–76 years; distribution, NS Stage (T): clinical: organ confined, 232 (100%); non-organ confined, 0 (0%); missing, 0 (0%); pathological: organ confined, 111 (47%); non-organ confined, 121 (53%); missing, 0 (0%) |
Gleason: biopsy: NS; pathological: range, 4–10; median = 6 PSA (ng/ml) (pathological): median, 10.1; mean, NS; range, 1–182; distribution, NS Adjuvant or neoadjuvant treatment: none Positive surgical margins: 122 (53%) Lymph node involvement: 5 (2.2%) Length of follow-up: median, 78 months; mean, NS; range, 1–160 months Results reported at x years: NS |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Anscher, 1991109 USA International Journal of Radiation Oncology, Biology, Physics |
Aim: to identify those patients at most risk for local failure Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NA; in analysis, 273 patients Inclusion criteria: underwent radical surgery for newly diagnosed adenocarcinoma of the prostate No adjuvant postoperative irradiation Start and finish dates: 1970 and 1983 |
Age: median, 64 years; mean, NS; range, 40–80 years; distribution, NA Stage (T): clinical: organ confined, 261 (95.6%); non-organ confined, 12 (4.4%); missing, 0 (0%); pathological: organ confined, 156 (57%); non-organ confined, 127 (43%); missing, 0 (0%) |
Gleason: biopsy: NS; pathological: grade 2–4 = 201 (73.6%), grade 8–10 = 72 (26.4%) PSA (ng/ml): median, NS; mean, NS; range, NS; distribution, NS Adjuvant or neoadjuvant treatment: none Positive surgical margins: 102 (37%) Lymph node involvement: 4 (1%) Length of follow-up: median, 66 months; mean, 73 months; range, 1–183 months Results reported at x years: NS |
|
Han, 2001110 USA Urology |
Aim: to investigate the prognostic value of preoperative serum ACP in predicting prognosis for men with localised prostate cancer following radical retropubic prostatectomy Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NS; in analysis, 1681 clinically localised men Inclusion criteria: clinically localised prostate cancer; underwent pelvic lymphadenectomy/RP Start and finish dates: 1982–1998 |
Age: median, NS; mean, 58.4 years (SD = 6.6 years); range, 33–76 years; distribution, NS Stage (T): clinical: organ confined, 1633 (97.14%); non-organ confined, 47 (2.8%); Tx, 1 (0.06%); missing, NS; pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: Gleason 2–4 = 83 (5%), Gleason 5 = 276 (16.7%), Gleason 6 = 926 (56.1%), Gleason 7 = 295 (17.9%), Gleason 8–9 = 72 (4.3%); pathological: Gleason 2–4 = 42 (2.5%), Gleason 5 = 243 (14.5%), Gleason 6 = 693 (41.2%), Gleason 7 = 565 (33.6%), Gleason 8–9 = 138 (8.2%) PSA (ng/ml): median, NS; mean, NS; range, NS; distribution: 0–4 = 426 (27.9%), 4.1–10 = 735 (48.1%), 10.1–20 = 283 (18.5%), > 20 = 84 (5.5%) Adjuvant or neoadjuvant treatment: none Positive surgical margins: NS Lymph node involvement: 89 (5.3%) (this refers to the number with seminal vesicle involvement, negative lymph nodes) Length of follow-up: median, NS; mean, 6.3 years; range, 1–17 years Results reported at x years: NS |
|
Perez, 1989111 USA Radiotherapy and Oncology |
Aim: to assess the impact of a variety of prognostic factors on the outcome of radiation therapy in localised carcinoma of the prostate Was primary aim of paper to assess prognostic marker(s)? Partiallya Pre/at treatment category: at treatment Principal treatment: radiotherapy Study design: cohort retrospective study Sample size: initial, 577; in analysis, 328 (only grade C) Inclusion criteria: patients with histologically confirmed carcinoma of the prostate localised to the pelvis Start and finish dates: 1967 and 1983 |
Age: median, NS; mean, NS; range, NS; distribution: ≤ 60 years = 92 patients; > 60 years = 236 patients Stage (T): clinical: organ confined, 0 (0%); non-organ confined, 328 (100%); missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: NS; pathological: well = 90 (27.4%), moderate = 131 (39.9%), poor or undifferentiated = 102 (31.1%), ungraded = 5 (0.02%) PSA (ng/ml): median, NS; mean, NS; range, NS; distribution, NS Adjuvant or neoadjuvant treatment: some Positive surgical margins: NS Lymph node involvement: 15 patients Length of follow-up: median, 6.5 years; mean, NS; range, NS Results reported at x years: 5 years |
|
Roach, 1999112 USA Journal of Urology |
Aim: to assess the relative importance of the several pretreatment characteristics in predicting death from prostate cancer in patients treated with curative intent with external beam radiotherapy alone Was primary aim of paper to assess prognostic marker(s)? No Pre/at treatment category: at treatment Principal treatment: radiotherapy Study design: cohort retrospective study; there is uncertainty whether the study used prospectively collected data Sample size: initial, 1557; in analysis, 1459 Inclusion criteria: no hormonal therapy; initial treatment and follow-up data were available; all entered a prospective phase III trial Start and finish dates: 1975 and 1992 |
Age: median, NS; mean, NS; range, NS; distribution: < 56 years = 66 (4%), 56–65 years = 421 (27%), 66–75 years = 845 (54%), > 75 years = 225 (14%) Stage (T): clinical: organ confined, 631 (41%); non-organ confined, 926 (59%); missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: Gleason 2–5 = 208 (13%), Gleason 6–7 = 825 (53%), Gleason 8–10 = 426 (27%), missing = 98 (6%); pathological: NS PSA (ng/ml): median, 22.3 (RTOG 85–31), 33.8 (RTOG 86–10); mean, NS; range, 1.22–560 (RTOG 85–31), 1.9–264.6 (RTOG 86–10); distribution: data were only available from RTOG 85–31 and RTOG 86–10. A total of 237 (16%) patients provided data Adjuvant or neoadjuvant treatment: none Positive surgical margins: NS Lymph node involvement: 152 (10%) Length of follow-up: median, NS; mean, NS; range, > 6 years Results reported at x years: NS |
|
Zagars, 1993113 USA Cancer (See also preliminary findings in Zagars, 1987,117 USA, Cancer) |
Aim: to delineate independently significant prognostic factors for prostate cancer treated by external beam radiation Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: radiotherapy and surgery Study design: cohort retrospective study Sample size: initial, 874; in analysis, 735 Inclusion criteria: patients who had received radiation and were grade A2–C; no patient had received hormone treatment Start and finish dates: 1966 and 1988 |
Age: median, 68 years; mean, 65 years; range, 41–81 years; distribution, NA Stage (T): clinical: organ confined, 272 (31%); non-organ confined, 602 (69%); missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy, NA; pathological, NA PSA (ng/ml): median, NA; mean, NA; range, NA; distribution, NA Adjuvant or neoadjuvant treatment: none Positive surgical margins: NA Lymph node involvement: NA Length of follow-up: median, 68 months; mean, 86 months; range, NS Results reported at x years: 5, 10, 15 years |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Nam, 2000114 USA Journal of Urology |
Aim: to examine the significance of the CAG repeat polymorphism of the androgen receptor gene for predicting prostate cancer progression Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, 318; in analysis, 318 Inclusion criteria: patients treated by RP for clinically localised prostate cancer; only patients without evidence of metastases or residual disease; no pelvic lymph nodes; no previous primary malignancy or organ transplantation; the cancer had to be sufficiently large to grade; had to be a resident of Ontario Start and finish dates: 1987 and 1994 |
Age: median, NS; mean, 62.9 years (at diagnosis), 69.6 years (at current); range, 45–74 years (at diagnosis) 54–83 years (at current); distribution, NS Stage (T): clinical: organ confined, 43.4%; non-organ confined, 56.6%; missing, NA; pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: Gleason 2–6 = 35.2%, Gleason 7 = 51.3%, Gleason 8–10 = 13.5%; pathological: NS PSA (ng/ml): median, NS; mean, 11.2; range, NS; distribution: < 4 = 27.4%, 4.1–10 = 38.4%, 10.1–20 = 22.6%, > 20 = 11.6% Adjuvant or neoadjuvant treatment: none Positive surgical margins: NS Lymph node involvement: NS Length of follow-up: median, NS; mean, 61.8 months; range, 2.1–135.9 months Results reported at x years: NS |
|
Powell, 2005115 USA Cancer |
Aim: to examine the impact of the number of CAG repeats in exon 1 of the androgen receptor on disease progression among men with prostate carcinoma after prostatectomy Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, 413 American white men (WM) and 298 African American men (AAM); in analysis, 711 Inclusion criteria: patients receiving RP; all patients were from the USA; no salvage prostatectomy or missing clinical data; patients for whom PSA levels did not decline to < 0.4 ng/ml or who had neoadjuvant therapy were excluded Start and finish dates: 1991 and 1996 |
Age: median, NS; mean, NS; range, NS; distribution: ≤ 65 years: 262 WM, 159 AAM; > 65 years: 151 WM, 139 AAM Stage (T): clinical: organ confined, 711 (100%); non-organ confined, 0 (0%); missing, 0 (0%); pathological: organ confined, 318 (45%); non-organ confined, 393 (55%); missing, 0 (0%) |
Gleason: biopsy, NS; pathological: Gleason < 7 = 251 (35%), Gleason 7 = 359 (50%), Gleason > 7 = 99 (14%) PSA (ng/ml): median, NS; mean, NS; range, NS; distribution: preoperative PSA ≤ 10 = 451 (63%), preoperative PSA 10–20 = 162 (23%), preoperative PSA > 20 = 108 (15%) Adjuvant or neoadjuvant treatment: none Positive surgical margins: 160 (23%) Lymph node involvement:47(7%) Length of follow-up: median, NS; mean, NS; range, 5–10 years Results reported at x years: NS |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Merseburger, 2001116 USA Urology |
Aim: to assess serum creatinine as a putative marker for staging/prognosis in localised prostate cancer Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NA; in analysis, 409 Inclusion criteria: patients who underwent RP; serum creatinine measured within 6 months pre surgery; pathological disease stage was known Start and finish dates: 1990 and 1996 |
Age: median, 63 years; mean, 63.1 years; range, NS; distribution, NA Stage (T): clinical: organ confined, 403 (99%); non-organ confined, 4 (0.7%); missing, 2 (0.3%); pathological: organ confined, 402 (98.3%); non-organ confined, 7 (1.7%); missing, 0 (0%) |
Gleason: biopsy: NS; pathological: Gleason 2–4 = 21.1%, Gleason 5–7 = 50.5%, Gleason 8–10 = 28.4% PSA (ng/ml): median, 6.9; mean, 9.9; range, NS; distribution: 0–4 = 95 (24.2%), 4.1–10 = 179 (45.4%), 10.1–20 = 90 (22.8%), 20.1+ = 30 (7.6%) (14 unknown) Adjuvant or neoadjuvant treatment: NS Positive surgical margins: 0 Lymph node involvement: 0 Length of follow-up: median, NS; mean, 60.6 months; range, NS Results reported at x years: NS |
|
Zagars, 1987117 USA Cancer |
Aim: to identify the prognostic factors likely to necessitate modifications of radiation dose–volume factors Was primary aim of paper to assess prognostic marker(s)? No Pre/at treatment category: at treatment Principal treatment: radiotherapy Study design: cohort retrospective study Sample size: initial, NA; in analysis, 551 Inclusion criteria: clinical stage C prostatic adenocarcinoma; external beam radiation patients Start and finish dates: 1965 and 1982 |
Age: median, 65 years; mean, 64 years; range, 47–78 years; distribution, NA Stage (T): clinical: organ confined, 0 (0%); non-organ confined, 551 (100%); missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: NS; pathological: NS PSA (ng/ml): median, NS; mean, NS; range, NS; distribution, NS Adjuvant or neoadjuvant treatment: some Positive surgical margins: NS Lymph node involvement: NS Length of follow-up: median, 6.5 years; mean, 7 years; range, 16–201 months Results reported at x years: NS |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Powell, 2004118 USA Journal of Urology |
Aim: to investigate whether CYP3A4*1B is associated with disease progression and whether it is an independent predictor of outcome Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, 428 white men (WM) and 309 African American men (AAM); in analysis, 737 Inclusion criteria: > 5 years follow-up; clinically localised prostate cancer; no salvage prostatectomy; no adjuvant therapy; had Gleason score measures Start and finish dates: 1991 and 1996 |
Age: median, NS; mean, NS; range, NS; distribution: ≤ 65 years: 268 WM, 168 AAM; > 65 years: 160 WM, 141 AAM Stage (T): clinical: organ confined, 737 (100%); non-organ confined, 0 (0%); missing, 0 (0%); pathological: organ confined, 327 (44%); non-organ confined, 410 (56%); missing, 0 (0%) |
Gleason: biopsy: NS; pathological: Gleason < 7 = 262 (36%), Gleason 7 = 367 (50%), Gleason > 7 = 106 (14%) PSA (ng/ml): median, NS; mean, NS; range, NS; distribution: preoperative PSA ≤ 10 = 462 (63%), preoperative PSA 10–20 = 160 (22%), preoperative PSA > 20 = 115 (16%) Adjuvant or neoadjuvant treatment: none Positive surgical margins: 156 (21%) Lymph node involvement: 49 (7%) Length of follow-up: median, NS; mean, NS; range, 5–10 years Results reported at x years: NS |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Blute, 2001105 USA Journal of Urology |
Aim: to determine the importance of clinical and pathological variables for predicting biochemical progression in patients after surgery for specimen-confined prostate cancer; to develop a simple scoring algorithm for biochemical progression in node-negative cases with testing of the algorithm performance on an independent group Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial: 3188 patients with pT2N0 or pT3N0 disease with complete records (for PSA, Gleason and ploidy) treated between 1990 and 1993; in analysis: 2000 in analysis, 518 validation Inclusion criteria: no preoperative therapy; no positive nodes; agreed to records being accessed Start and finish dates: 1990 and 1993 |
Age: median, NS; mean, 63 years; range, NS; distribution: < 63 years, 717 (29%); 63–68 years, 900 (36%), 69+ years, 901 (36%) Stage (T): clinical: organ confined, 2258 (90%); non-organ confined, 255 (10%); missing, 5 (< 1%); pathological: organ confined, 1555 (87%); non-organ confined, 963 (13%); missing, 0 (0%) |
Gleason: biopsy: NS; pathological: Gleason 2–4 = 286 (11%), Gleason 5 = 1060 (42%), Gleason 6 = 440 (17%), Gleason 7 = 635 (25%), Gleason 8–10 = 97 (4%) PSA (ng/ml): median, NS; mean, NS; range, NS; distribution: ≤ 4.0, 18%; 4.1–10.0, 46%; 10.1–20.0, 22%; > 20.0, 14% Adjuvant or neoadjuvant treatment: 398 (15% adjuvant) Positive surgical margins: 978 (39%) Lymph node involvement: 0 (0%) Length of follow-up: median, NS; mean, 5.6 years; range, NS Results reported at x years: NS |
|
Lieber, 1995106 USA Cancer (See also overlapping findings in Montgomery, 1990137) |
Aim: to determine if DNA ploidy measurement provides additional unique prognostic information beyond the customary parameters of tumour stage and histological grade for patients with prostate adenocarcinoma; to summarise prognostic risk in tables using the above variables Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Participants: treated with RP at Mayo clinic Sample size: initial, 635; in analysis, 494 (78%) Inclusion criteria: patients whose DNA ploidy was measurable Start and finish dates: 1967 and 1981 |
Age: median, NS; mean, NS; range, NS; distribution, NS Stage (T): clinical: organ confined, 258 (52%); non-organ confined, 236 (48%) (note 18% of total had DI); missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: NS; pathological: Gleason 2–4 = 70 (14.4%); Gleason 5–7 = 373 (76.7%); Gleason 8–10 = 43 (8.8%) PSA (ng/ml): median, NS; mean, NS; range, NS; distribution, NS Adjuvant or neoadjuvant treatment: NS Positive surgical margins: NS Lymph node involvement: NS Length of follow-up: median, NS; mean, NS; range, minimum 10 years Results reported at x years: 10 years |
|
Siddiqui, 2006119 USA Journal of Urology (See also overlapping findings in Amling, 2000136) |
Aim: to assess whether age at treatment was a predictor of post-RP survival Was primary aim of paper to assess prognostic marker(s)? No Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NA; in analysis, 5509 Inclusion criteria: patients treated with RP for prostate cancer; no neoadjuvant therapy before surgery Start and finish dates: 1987 and 1995 |
Age: median, 66 years; mean, NS; range, NS; distribution: < 55 to > 70 years Stage (T): clinical: organ confined, 4907 (89%); non-organ confined, 602 (11%); missing, 0 (0%); pathological: organ confined, 3215 (58.6%); non-organ confined, 2276 (41.4%); missing, 0 (0%) |
Gleason: biopsy: Gleason 2–4 = 529 (17.9%), Gleason 5 = 974 (32.9%), Gleason 6 = 634 (21.4%), Gleason 7 = 668 (22.6%), Gleason 8–10 = 156 (5.3%); pathological: Gleason 2–4 = 435 (8.4%), Gleason 5 = 1788 (34.3%), Gleason 6 = 1107 (21.3%), Gleason 7 = 1526 (29.3%), Gleason 8–10 = 353 (6.8%) PSA (ng/ml): median, 7.8; mean, NS; range, 4.9–13.9; distribution, NS Adjuvant or neoadjuvant treatment: some Positive surgical margins: 2135 (38.8%) Lymph node involvement: NS Length of follow-up: median, 10.6 years; mean, NS; range, 8.7–12.4 years Results reported at x years: NS |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Williams, 2004120 USA Prostate |
Aim: to investigate whether germline genetic variation in the vitamin D receptor impacts on progression of prostate cancer after RP Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, 792; in analysis, 428 white men (WM) and 310 African American men (AAM) Inclusion criteria: RP; only patients residing in the USA; no patient had received salvage surgery or neoadjuvant therapy; patients had complete data for Gleason/preoperative PSA/tissue blocks; patients had postoperative PSA < 0.4 ng/ml Start and finish dates:1991 and 1996 |
Age: median, NS; mean, NS; range, NS; distribution: ≤ 65 years: WM 160/428 (37.4%), AAM 141/310 (45.5%); > 65 years: WM 268/428 (62.6%), AAM 169/310 (54.5%) Stage (T): clinical: organ confined, WM 428, AAM 310 (100%); non-organ confined, 0 (0%); missing, 0 (0%); pathological: organ confined, WM 213/428 (49.7%), AAM 116/310 (37.4%); non-organ confined, WM 215/428 (50.2%), AAM 194/310 (62.6%); missing, 0 (0%) |
Gleason: biopsy: Gleason 2–6 = WM 159/428 (37.1%), AAM 102/310 (32.9%); Gleason 7 = WM 213/428 (49.8%), AAM 157/310 (50.6%); Gleason 8–10 = WM 54/428 (12.6%), AAM 51/310 (16.5%); pathological: NS PSA (ng/ml): median, NS; mean, NS; range, NS; distribution: preoperative PSA ≤ 10 = WM 287/428 (67.1%), AAM 176/310 (56.8%); PSA 10–20 = WM 97/428 (22.7%), AAM 63/310 (20.3%); PSA 20+ = WM 44/428 (10.4%), AM 71/310 (22.8%) Adjuvant or neoadjuvant treatment: none Positive surgical margins: WM 74/428 (17.3%), AAM 82/310 (26.5%), total = 156 (21%) Lymph node involvement: WM 31/428 (7.2%), AAM 18/310 (5.8%), total = 49 (9.1%) Length of follow-up: median, NS; mean, NS; range, 60–120 months Results reported at x years: NA |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Egevad, 2002121 Sweden Journal of Urology |
Aim: to investigate the value of percentage Gleason grade 4/5 as a predictor of long-term outcome in men with prostate cancer diagnosed at transurethral resection who received deferred treatment Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NA; in analysis, 305 Inclusion criteria: patients diagnosed at transurethral resection; no hormonal treatment/radiotherapy before transurethral prostate resection Start and finish dates: 1975 and 1990 |
Age: median, NS; mean, 74 years; range, 52–95 years; distribution, NA Stage (T): clinical: organ confined, 252 (82.6%); non-organ confined, 53 (17.3%); missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: grade 4 = 13 (4%), grade 5 = 54 (18%), grade 6 = 89 (29%), grade 7 = 55 (18%), grade 8 = 37 (12%), grade 9 = 39 (13%), grade 10 = 18 (6%); pathological: NS PSA (ng/ml): median, NS; mean, NS; range, NS; distribution, NS Adjuvant or neoadjuvant treatment: none Positive surgical margins: NS Lymph node involvement: NS Length of follow-up: median, 7.3 years (censored), 5.9 years (uncensored); mean, NS; range, 0–22 years (censored and uncensored) Results reported at x years: NS |
|
Gonzalgo, 2006122 USA Urology |
Aim: to examine the relationship between needle biopsy primary grade, prostatectomy grade and post-prostatectomy biochemical recurrence among men with Gleason score 7 disease Was primary aim of paper to assess prognostic marker(s)? No Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NS; in analysis, 320 men with Gleason score 7 tumours on prostate biopsy Inclusion criteria: no patient had received neoadjuvant or adjuvant hormonal therapy or radiotherapy; men with Gleason score 7 tumours on prostate biopsy; treated with RP Start and finish dates: 1991 and 2001 |
Age: median, NS; mean, 59 years ± 5.9 years; range, NS; distribution, NS Stage (T): clinical: organ confined, 213 (98%); non-organ confined, 7 (2%); missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: group 3 + 4 = 7, 252 (79%); group 4 + 3 = 7, 68 (21%); pathological: NS PSA (ng/ml): median, 7.1; mean, NS; range, 0.1–38; distribution, NS Adjuvant or neoadjuvant treatment: none Positive surgical margins: 28 (9%) Lymph node involvement: 25 (8%) Length of follow-up: median, 5 years; mean, NS; range, 1–13 years Results reported at x years: NS |
|
Tollefson, 2006123 USA Journal of Urology |
Aim: to determine the long-term clinical significance of primary Gleason pattern in patients with Gleason score 7 prostate cancer Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NA; in analysis, 1688 Inclusion criteria: Gleason 7 tumour pathological; no hormonal/radiation therapy Start and finish dates: 1987 and 2000 |
Age: median, 66 years; mean, 64.8 ± 6.69 years; range, 43–82 years; distribution: 3 + 4 group: median = 65 years, mean = 64.5 ± 6.78 years, range = 43–82 years; 4 + 3 group: median = 67 years; mean = 65.5 ± 6.39 years; range = 47–80 years Stage (T): clinical: organ confined, 1544 (91.5%); non-organ confined, 139 (8.2%); missing, 5 (0.3%); pathological: organ confined, 999 (59.2%); non-organ confined, 689 (40.8%); missing, 0 (0%) |
Gleason: biopsy: Gleason 2–5 = 232 (13.7%), Gleason 6 = 431 (25.5%), Gleason 7 = 552 (32.7%), Gleason 8+ = 66 (3.9%), missing = 407 (24.1%); pathological: Gleason 7 = 1688 (100%) PSA (ng/ml): median, 7.8; mean, 0 (0%); range, 0.5–219; distribution, quartile 1, 3 = 5.5, 12.3 ng/ml Adjuvant or neoadjuvant treatment: none Positive surgical margins: 612 (36.3%) Lymph node involvement: NS Length of follow-up: median, 6.9 years; mean, NS; range, NS Results reported at x years: 10 years |
|
Vis, 2007124 The Netherlands European Urology |
Aim: to investigate the predictive value of the amount of high-grade cancer (Gleason growth patterns 4/5) in the biopsy for PSA and clinical relapse after RP Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NA; in analysis, 281 Inclusion criteria: underwent RP; all had pelvic lymph node dissection before RP; no hormonal treatment or transurethral resection before operation Start and finish dates: 1994 and 1999 |
Age: median, NS; mean, 64 years; range, 55–73 years; distribution, NS Stage (T): clinical: organ confined, 277 (98.6%); non-organ confined, 4 (1.4%); missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: Gleason 2–6 = 203 (72.2%), Gleason 7 = 66 (23.5%), Gleason 8–10 = 12 (4.3%); pathological: NS PSA (ng/ml): median, 5.2; mean, NS; range, 0.8–29.5; distribution, NS Adjuvant or neoadjuvant treatment: none Positive surgical margins: NS Lymph node involvement: NS Length of follow-up: median, 81 months; mean, NS; range, 5–120 months Results reported at x years: NS |
|
Vollmer, 2001107 USA American Journal of Clinical Pathology |
Aim: to explore the relationship between PSA-derived and pathology-derived prognostic information and different outcomes for prostate cancer; to derive an algorithm to determine risk category immediately after surgery (note only one of two models meets inclusion criteria) Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, 216; in analysis, 203 Inclusion criteria: evaluation of prostate specimen by dedicated uropathologist; long-term follow-up Start and finish dates: NS |
Age: median, 67 years; mean, NS; range, 44–83 years; distribution, NS Stage (T): clinical: organ confined, 216 (100%); non-organ confined, 0 (0%); missing, 0 (0%); pathological: organ confined, 124 (57.4%); non-organ confined, 92 (42.6%); missing, 0 (%) |
Gleason: biopsy: NS; pathological: median, 7; range, 3–9 PSA (ng/ml): median, 8.8; mean, NS; range, 0.2–283.0; distribution, NS Adjuvant or neoadjuvant treatment: NS Positive surgical margins: 127 (58.8%) Lymph node involvement: NS Length of follow-up: median, 70 months; mean, > 6 years; range, < 1–148 months Results reported at x years: NS |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Zellweger, 2003125 Switzerland Prostate |
Aim: to test Gleason grading and the expression of the molecular markers Ki67, Bcl-2, p53 and syndecan-1 in relation to prognostic significance Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort-retrospective study Sample size: initial, NA; in analysis, specimens were from 551 patients with prostate cancer and long-term follow-up information on progression Inclusion criteria: clinically localised prostate cancer; RP or TURP; no chemotherapy; complete follow-up data; no patients with tumours; no distant metastases before TURP Start and finish dates: 1971 and 1996 |
Age: median, 63.6 years; mean, NS; range, 45–92 years; distribution, NS Stage (T): clinical: organ confined, 551 (100%); non-organ confined, NA; missing, NA; pathological: organ confined, 396 (71.9%); non-organ confined, 102 (18.5%); missing, 53 (9.6%) |
Gleason: biopsy: NS; pathological: NS PSA (ng/ml): median, NS; mean, NS; range, NS; distribution, NS Adjuvant or neoadjuvant treatment: 101/498 (20.3%) Positive surgical margins: NS Lymph node involvement: 14/428 (3.3%) Length of follow-up: median, 5.3 years; mean, NS; range, 0.5–20 years Results reported at x years: NS |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Antunes, 2005126 Brazil International Brazilian Journal of Urology (See also preliminary findings in Antunes, 2005169) |
Aim: to analyse the prognostic value of the percentage of positive biopsy cores (PPBC) in determining the pathological features and biochemical outcomes of patients with prostate cancer treated by RP Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NA; in analysis, 534 Inclusion criteria: patients with clinically localised prostate cancer; RP; sufficient clinical data; patients receiving treatment from same pathologist and surgeon Start and finish dates: 1991 and 2000 |
Age: median, NS; mean, 63 years; range, 40–83 years; distribution, NS Stage (T): clinical: organ confined, 532 (99.6%); non-organ confined, 2 (0.4%); missing, 0 (0%); pathological: organ confined, 401 (75.1%); non-organ confined, 133 (24.9%); missing, 0 (0%) |
Gleason: biopsy: grade 2–6 = 423 (79.2%), grade 7 = 76 (14.2%), grade 8–10 = 35 (6.6%); pathological: grade 2–6 = 335 (62.7%), grade 7 = 105 (19.7%), grade 8–10 = 94 (17.6%) PSA (ng/ml): median, NS; mean, 10.5; range, 0.3–63.5; distribution, NA Adjuvant or neoadjuvant treatment: NS Positive surgical margins: NS Lymph node involvement: none Length of follow-up: median, 58.3 months; mean, 60.5 months; range, 1.2–130.5 months Results reported at x years: NA |
|
Potters, 2005127 USA Journal of Urology |
Aim: to assess the outcomes of men undergoing prostate brachytherapy and to evaluate factors that could impact on disease-specific survival Was primary aim of paper to assess prognostic marker(s)? No Pre/at treatment category: at treatment Principal treatment: radiotherapy and brachytherapy Study design: cohort retrospective study Sample size: initial, NA; in analysis, 1449 Inclusion criteria: men treated with permanent prostate brachytherapy; clinically localised prostate cancer; biopsy-proven adenocarcinoma; all patients underwent transrectal ultrasound to assess prostate size Start and finish dates: 1992 and 2000 |
Age: median, NS; mean, 68.05 years; range, 43.5–84.4 years; distribution, NS Stage (T): clinical: organ confined, 1449 (100%); non-organ confined, NA; missing, NA; pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: Gleason 2–6 = 965 (66.6%), Gleason 7 = 412 (28.4%), Gleason 8–10 = 72 (5%); pathological: NS PSA (ng/ml): median, NS; mean, 7.2 (follow-up), 10.1 (pretreatment); range, NS; distribution, NS Adjuvant or neoadjuvant treatment: NS Positive surgical margins: NS Lymph node involvement: NS Length of follow-up: median, 82 months; mean, NS; range, NS Results reported at x years: NS |
|
Selek, 2003128 USA International Journal of Radiation Oncology, Biology, Physics |
Aim: to determine the utility of the percentage of positive prostate biopsies (PPPB) in predicting PSA outcome after external beam radiotherapy alone Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: radiotherapy Study design: cohort retrospective study Sample size: initial, 750; in analysis, 345 Inclusion criteria: stage T1 and T2 patients treated by external beam radiotherapy alone Start and finish dates: 1987 and 1998 |
Age: median, NS; mean, NS; range, NS; distribution: < 65 years = 86 (24.9%), 65–69 years = 104 (30.2%), ≥ 70 years = 145 (44.9%) Stage (T): clinical: organ confined, 345 (100%); non-organ confined, 0 (0%); missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: Gleason 2–6 = 200 (58%), Gleason 7 = 112 (32.4%), Gleason 8–10 = 33 (9.6%); pathological: NS PSA (ng/ml): median, NS; mean, NS; range, NS; distribution: ≤ 10 = 240 (69.6%), 10.1–20 = 92 (26.6%), > 20 = 13 (3.8%) Adjuvant or neoadjuvant treatment: none Positive surgical margins: NS Lymph node involvement: NS Length of follow-up: median, 80 months; mean, NS; range, 4–158 months Results reported at x years: NS |
|
Vis, 2007124 The Netherlands European Urology |
See details in Table 81 | See details in Table 81 | See details in Table 81 |
|
Vollmer, 2001107 USA American Journal of Clinical Pathology |
See details in Table 81 | See details in Table 81 | See details in Table 81 |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
D’Amico, 2004129 USA New England Journal of Medicine |
Aim: to evaluate whether men at risk for death from prostate cancer after RP can be identified using information available at diagnosis; to assess whether the rate of rise in the PSA level – the PSAV – during the year before diagnosis, the PSA level at diagnosis, the Gleason score and the clinical tumour stage could predict the time to death from prostate cancer and death from any cause after RP Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study carried out on prospectively collected data Sample size: initial, NA; in analysis, 1095 men with localised prostate cancer Inclusion criteria: localised prostate cancer (T1, T2); treated with RP; no lymph node metastases; no men with a single measurement of PSA postoperatively; no men receiving adjuvant radiotherapy Start and finish dates: 1989 and 2002 |
Age: median, 65.4 years; mean, NS; range, 43.3–83.5 years; distribution, NA Stage (T): clinical: organ confined, 1095 (100%); non-organ confined, NS; missing, 0 (0%); pathological: organ confined, NS; non-organ confined, NS; missing, NS |
Gleason: biopsy: grade 2–7 = 916 (84%), grade 7 = 133 (12%), grade 8–10 = 46 (4%); pathological: NS PSA (ng/ml): median, 4.3; mean, NS; range, 0.3–58.2; distribution, 95% have PSA level of 10 ng/ml or less Adjuvant or neoadjuvant treatment: none Positive surgical margins: 237 (22%) Lymph node involvement: 2 (11%) Length of follow-up: median, 5.1 years; mean, NS; range, 0.5–13.1 years Results reported at x years: 7 years |
|
Sengupta, 2005130 USA Journal of Urology |
Aim: to assess preoperative PSADT and PSAV as predictors of outcome following RP Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, NA; in analysis: 2290 Inclusion criteria: treated with RP for prostate cancer; no neoadjuvant treatment Start and finish dates: 1990 and 1999 |
Age: median, NS; mean, 64.8 years (SD = 6.8 years); range, 40–83 years; distribution, NS Stage (T): clinical: organ confined, 2198 (95.9%); non-organ confined, 70 (3.1%); missing, 22 (1%); pathological: organ confined, 1794 (78.3%); non-organ confined, 481 (21%); missing, 15 (0.7%) |
Gleason: biopsy: Gleason 2–5 = 588 (30.8%), Gleason 6 = 870 (45.5%), Gleason 7 = 362 (18.9%), Gleason 8–10 = 92 (4.8%); pathological: Gleason 2–5 = 624 (27.4%), Gleason 6 = 952 (41.9%), Gleason 7 = 589 (25.9%), Gleason 8–10 = 109 (4.8%) PSA (ng/ml): median, 6.7; mean, NS; range, 4.7–9.9; distribution, NS Adjuvant treatment: some; neoadjuvant treatment: none Positive surgical margins: 757 (33.1%) Lymph node involvement: NS Length of follow-up: median, 7.1 years; mean, NS; range, 0.1–14.5 years Results reported at x years: NS |
| Study | Method | Study participation | Study participation |
|---|---|---|---|
|
Li, 2005131 USA Clinical Cancer Research |
Aim: to investigate whether activation of Stat5 in prostate cancer is linked to clinical outcome with disease recurrence as end point Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study Sample size: initial, 548 patients treated for clinically localised prostate cancer; in analysis, 357 paraffin-embedded prostate cancer specimens Inclusion criteria: clinically localised prostate cancer Start and finish dates: 1971 and 1996 |
Age: median, 65 years; mean, 64.61 years (SD = 0.3 years); range, 45–88 years; distribution, NS Stage (T): clinical: organ confined, NA; non-organ confined, NA; missing, NA; pathological: organ confined, 436 (79.5%); non-organ confined, 108 (19.7%); missing, 4 (0.7%) |
Gleason: biopsy: Gleason 2 = 26 (4.7%), Gleason 3 = 333 (60.8%), Gleason 4 = 171 (31.2%), Gleason 5 = 18 (3.3%); pathological:NS PSA (ng/ml): median, NS; mean, NS; range, NS; distribution, NS Adjuvant or neoadjuvant treatment: NS Positive surgical margins: NS Lymph node involvement: NS Length of follow-up: median, 6.01 years (overall survival follow-up); mean, NS; range, 0.93–28.36 years Results reported at x years: NS |
| Study | Method | Study participation | Study participation (continued) |
|---|---|---|---|
| Blute, 2001105 | See details in earlier Table 79 | See details in Table 79 | See details in earlier Table 79 |
| USA | |||
| Journal of Urology | |||
| Lieber, 1997106 | See details in earlier Table 79 | See details in earlier Table 79 | See details in earlier Table 79 |
| USA | |||
| Cancer | |||
|
Salomon, 2003132 France European Urology |
Aim: to investigate the association between Gleason score, stage and status of surgical margins with tumour volume in prostate cancer progression after RP Was primary aim of paper to assess prognostic marker(s)? Yes Pre/at treatment category: at treatment Principal treatment: surgery Study design: cohort retrospective study although unclear whether prospective data used Sample size: initial, 200 consecutive RP specimens; in analysis: 200 Inclusion criteria: surgery; preoperative physical; PSA levels reported; biopsy; no neoadjuvant hormonal treatment or adjuvant radiotherapy Start and finish dates: 1992 and 1998 |
Age: median, NS; mean, 65 years ± 5.6 years; range, 46.9–75.7 years; distribution, NS Stage (T): clinical: organ confined, 200 (100%); non-organ confined, 0 (0%); missing, 0 (0%); pathological: organ confined, 149 (74.5%); non-organ confined, 51 (25.5%); missing, 0 (0%) |
Gleason: biopsy: Gleason 2–4 = 34 (17%), Gleason 5–6 = 126 (63%), Gleason 7–10 = 40 (20%); pathological: Gleason 2–4 = 4 (2%), Gleason 5–6 = 122 (61%), Gleason 7–10 = 74 (37%) PSA (ng/ml): median, NS; mean, 11.8 ± 10.9; range, 1.3–82; distribution, NS Adjuvant or neoadjuvant treatment: none Positive surgical margins: 48 (24%) Lymph node involvement: NS Length of follow-up: median, NS; mean, 63.6 months; range, NS Results reported at x years: 5 years |
|
Sengupta, 2005130 USA Journal of Urology |
See details in earlier Table 84 | See details in earlier Table 84 | See details in earlier Table 84 |
|
Vis, 2007124 The Netherlands European Urology |
See details in earlier Table 81 | See details in earlier Table 81 | See details in earlier Table 81 |
Appendix 6 Included studies for novel prognostic markers: analysis methods, results and conclusions
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Horvath, 2005108 Australia International Journal of Cancer |
Univariate analysis Marker(s): β-catenin expression Analysis methods: Cox proportional hazards: < 10% with reference ≥ 10% End point: survival from biochemical relapse (PSA 0.4 ng/ml or greater over 3 months or local recurrence on digital rectal examination confirmed by biopsy or subsequent rise in PSA) Multivariate analysis Marker(s): β-catenin expression (< 10% vs ≥ 10% nuclei) Analysis methods: disease-specific survival was measured from the date of RP to relapse or the date of last follow-up. Kaplan–Meier and log-rank analyses evaluating disease relapse were performed on the raw nuclear β-catenin scores in a stepwise fashion (i.e. using a cut-off of 5%, then 10% up to 95%). Further survival analysis was performed using univariate and multivariate Cox proportional hazards model for β-catenin status End point: survival from biochemical relapse (PSA 0.4 ng/ml or greater over 3 months or local recurrence on digital rectal examination confirmed by biopsy or subsequent rise in PSA) Model used: multivariate Cox proportional hazards model Classical clinical markers included: PSA Classical pathological markers included: stage; Gleason score; surgical margins Factors (prognostic markers) in final model? Clinical PSA, pathological stage, Gleason score, surgical margins, seminal vesicle involvement, adjuvant treatment |
Univariate analysis Measure: HR Result: 1.9; 95% CI: 1.2–3.0; p-value: 0.008 (log-rank from survival curve p = 0.007) Survival: extrapolated from survival curve: 5-year survival for β-catenin < 10% = 60%, β-catenin ≥ 10% = 78% Multivariate analysis Measure: HR Result: 1.4; 95% CI: 0.8–2.3; p-value: 0.2 |
Lower levels of nuclear β-catenin expression are found in malignant than in benign prostate tissue. In addition, lower nuclear β-catenin expression is associated with a poorer prognosis in localised prostate cancer, in particular in the low-risk subgroup of patients with preoperative PSA levels < 10 ng/ml. Thus, the level of nuclear β-catenin expression may be of clinical utility as a preoperative prognostic marker in low-risk localised prostate cancer. Although β-catenin may be prognostic for biochemical recurrence following RP, its association with the existing widely used PSA marker means that it would not provide additional prognostic information. There are several quality issues related to this study that make the results inconclusive |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Anscher, 1991109 USA International Journal of Radiation Oncology, Biology, Physics |
Univariate analysis Marker(s): elevated preoperative acid phosphatase (EPAP) End point: (a) local relapse rate (local failure confirmed by biopsy, with or without distant metastases); (b) distant metastases Multivariate analysis Marker(s): EPAP Analysis method: multivariate analysis was used to measure the influence of the following variables on the development of local relapse and distant metastases: age, type of biopsy (TURP vs needle), use of adjuvant hormonal therapy, histological grade and clinical stage, histological involvement of the seminal vesicles or positive surgical margins, and EPAP. Variables were combined in a stepwise fashion to determine the combination that proved powerful in distinguishing groups End point: (a) local relapse rate (local failure confirmed by biopsy, with or without distant metastases), median follow-up 66 months; (b) distant metastases Model used: multivariate Cox proportional hazards model Classical clinical markers included: clinical stage Classical pathological markers included: surgical margins Factors (prognostic markers) in final model? Clinical stage, surgical margins, age, type of biopsy, hormonal therapy given, poorly differentiated, seminal vesicles involved |
Univariate analysis (a) Measure: HR Events: elevated ACP (> 5.4 IU/l) 12/47 (26%); normal ACP (≤ 5.4 IU/l) 30/212 (14%) Result: HR not reported; CI not reported; p-value: 0.06 (b) Measure: HR Result: HR not reported, not significant; CI not reported; p-value: not reported Multivariate analysis (a) Measure: local relapse Events: elevated ACP (> 5.4 IU/l) 12/47 (26%); normal ACP (≤ 5.4 IU/l) 30/212 (14%) Result: EPAP was a significant predictor of local relapse; CI not reported; p-value: 0.0273 (b) Measure: HR Result: HR not reported, not significant; CI not reported; p-value: not reported |
The presence of an EPAP, poorly differentiated histology and/or positive surgical margins identified patients at high risk for local relapse following radical surgery for prostate cancer |
|
Han, 2001110 USA Urology |
Univariate analysis No univariate analysis Multivariate analysis Marker(s): acid phosphatase level Analysis methods: multivariate logistic regression model was constructed using the preoperative variables to determine whether preoperative ACP levels represented an independent predictor of pathological stage End point: biochemical (PSA) recurrence (PSA > 0.2 ng/ml) Model used: multivariate logistic regression model Classical clinical markers included: PSA, Gleason grade, stage Classical pathological markers included: none Factors (prognostic markers) in final model? Clinical PSA, clinical Gleason grade, clinical stage, age |
Univariate analysis No univariate analysis Multivariate analysis Measure: normalised HR (HR per 1 standard deviation change in predictor variable) Survival: 5-year survival: ACP < 0.4 U/l 87% (from n = 996), ACP 0.4–0.5 U/l 79% (from n = 573), ACP > 0.5 U/l 63% (from n = 112); 10-year survival: ACP < 0.4 U/l 77%, ACP 0.4–0.5 U/l 65%, ACP > 0.5 U/l 44% Result: 1.22 (SE 0.03); CI not reported; p-value: < 0.001 |
Stratification of men according to their preoperative ACP levels was predictive of patient outcome after RP. Proportional hazards modelling using preoperative variables demonstrated that the serum ACP level is an independent predictor of tumour recurrence following RP |
|
Perez, 1989111 USA Radiotherapy and Oncology |
Univariate analysis No univariate analysis Multivariate analysis Marker(s): acid prostatic phosphatase level Analysis methods: all survivals and survival functions utilise the actuarial life table and test statistics provided by generalised Wilcoxon (Breslow), generalised salvage (Mantel–Cox) and Tarone–Ware. Trend analysis was performed using the Tarone method. The Mantel–Cox method was used to test for potential significant factors for survival End point: (a) overall survival (events – death from any cause); (b) disease-free survival (events – any tumour progression, local or distant) Model used: unclear – possible Mantel–Cox Classical clinical markers included: none Classical pathological markers included: clinical histological grade (well, moderate, poor) Factors (prognostic markers) in final model? Clinical histological grade (well, moderate, poor), age, race, positive or negative lymphadenectomy, type of biopsy, hormonal status, dose of irradiation |
Univariate analysis No univariate analysis Multivariate analysis (a) Measure: 5-year survival Result: ACP normal 64% (from n = 241); ACP abnormal 64% (from n = 87); CI not reported; p-value: 0.76 (b) Measure: 5-year survival Result: ACP normal 52% (from n = 241); ACP abnormal 45% (from n = 87); CI not reported; p-value: 0.23 |
This study looked at some patients with stage B carcinoma, but n < 200 so these data were not included; data on ACP was presented separately for stage B and stage C (i.e. not combined for stages B and C). A broader utilisation of the PSA assay will eventually replace the plasma acid phosphatase in assessing prognosis after therapy |
|
Roach, 1999112 USA Journal of Urology |
Univariate analysis Marker(s): serum acid phosphatase End point: (a) overall survival (events – death from any cause); (b) survival from prostate cancer death (events – prostate cancer death only) Multivariate analysis Marker(s): serum acid phosphatase Analysis methods: Cox proportional hazard models were used to assess the impact of risk factors on overall survival and disease-specific survival. Actuarial estimates of overall survival and disease-free survival were performed using Kaplan–Meier methods End point: (a) Overall survival (events – death from any cause); (b) survival from prostate cancer death (events – prostate cancer death only) Model used: Cox proportional hazard models Classical clinical markers included: stage Classical pathological markers included: Gleason grade Factors (prognostic markers) in final model? Clinical stage, nodal status, pathological Gleason grade, race, age |
Univariate analysis (a) Measure: ACP elevated vs not elevated: risk ratio Result: 1.277; CI not reported; p-value: 0.004 (b) Measure: ACP elevated vs not elevated: risk ratio Result: 1.717; CI not reported; p-value: 0.0001 Multivariate analysis (a) Measure: ACP elevated vs not elevated: risk ratio not reported Result: not significant; CI not reported; p-value: not reported (b) Measure: ACP elevated vs not elevated: risk ratio Result: 1.294; CI not reported; p-value: 0.037 |
Tumour grade was the single most important predictor of death, whereas stage was less important. No conclusions about the prognostic use of serum acid phosphatase were presented in the discussion |
|
Zagars, 1993113 USA Cancer (See also preliminary findings in Zagars, 1987,117 USA, Cancer) |
Univariate analysis Marker(s): elevated prostatic acid phosphatase (PAP) End point: (a) disease-free survival (events – first relapse, whether local, nodal or metastatic); (b) overall survival (events – death from any cause) Multivariate analysis Marker(s): elevated PAP Analysis methods: multiple covariate actuarial analysis was performed with the proportional hazards model and log-linear relative hazard function of Cox End point: (a) disease-free survival (events – first relapse, whether local, nodal or metastatic); (b) overall survival (events – death from any cause) Model used: Cox proportional hazards model Classical clinical markers included: NS Classical pathological markers included: stage Factors (prognostic markers) in final model? Pathological stage (pathological MD Anderson grade, age, TURP vs no TURP in stage C); analysis 1 method = 11 factors, analysis 2 method = 9 factors |
Univariate analysis (a) Measure: survival normal vs elevated PAP Result: 5-year survival: PAP normal 70% (from n = 682), PAP elevated 41% (from n = 53); 10-year survival: PAP normal 51%, PAP elevated 22%; CI: not reported; p-value: < 0.001 (b) Measure: survival normal vs elevated PAP Result: 5-year survival: PAP normal 80% (from n = 682), PAP elevated 70% (from n = 53); 10-year survival: PAP normal 51%, PAP elevated 49%; CI: not reported; p-value: 0.059 Multivariate analysis (a) Measure: survival normal vs elevated PAP Result: not reported; CI: not reported; p-value: 0.005 (b) Measure: survival normal vs Elevated PAP Result: not significant; CI: not reported; p-value: not reported |
Elevated PAP correlated with metastasis not local control |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Nam, 2000114 USA Journal of Urology |
Univariate analysis Reported in paper Multivariate analysis Marker(s): androgen receptor Analysis methods: effect of the number of CAG repeats of the androgen receptor gene in predicting disease recurrence was examined by multivariate Cox proportional hazard modelling End point: biochemical recurrence-free survival (PSA greater than or equal to 0.2 ng/ml on two consecutive measurements at least 3 months apart; date of recurrence was time of initial increase) Model used: multivariate Cox proportional hazard modelling Classical clinical markers included: PSA, Gleason grade, stage Classical pathological markers included: none Factors (prognostic markers) in final model? Clinical PSA, Gleason grade, stage |
Univariate analysis Reported in paper Multivariate analysis Measure: adjusted relative risk for ≤ 18 repeats (with reference > 18 repeats) Result: 0.93 (when analysed as a continuous variable: RR = 1.01); CI: 0.5–1.8 (when analysed as a continuous variable: CI = 0.9–1.1); p-value: 0.83 (when analysed as a continuous variable: p = 0.79) |
The length of the CAG repeat polymorphism of the androgen receptor gene may be important in predicting prostate cancer recurrence among patients who are otherwise at low risk for recurrence after RP |
|
Powell, 2005115 USA Cancer |
Univariate analysis Marker(s): number of CAG repeats End point: biochemical recurrence-free survival Multivariate analysis Marker(s): number of CAG repeats Analysis methods: Kendall τ b correlation coefficients were used to assess associations between CAG repeats and clinical variables. When analyses required stratification of CAG results, results were grouped by ≤ 18 repeats and > 18 repeats. Non-parametric Kaplan–Meier survival function estimates for progression-free survival distributions after RP were obtained. Finally, Cox proportional hazard regression models were used to determine the impact of CAG repeats on disease-free survival End point: biochemical recurrence-free survival (PSA level> 0.4 ng/ml that persisted for more than one reading) Model used: Cox proportional hazard regression models Classical clinical markers included: PSA Classical pathological markers included: Gleason grade, stage Factors (prognostic markers) in final model? Clinical PSA, Gleason grade, stage, race and age |
Univariate analysis Measure: (a) HR of recurrence > 18 CAG repeats (with reference ≤ 18 repeats); (b) HR for a one-category increase in CAG repeats (≤ 18 repeats; 19–22 repeats; and ≥ 22 repeats) Result: (a) 1.09, (b) 1.00; 95% CI: (a) 0.6–2.1, (b) 0.9–1.1; p-value: (a) 0.80, (b) 0.94 Multivariate analysis Measure: (a) HR of recurrence > 18 CAG repeats (with reference ≤ 18 repeats); (b) HR for a one-category increase in CAG repeats (≤ 18 repeats; 19–22 repeats; and ≥ 22 repeats) Result: (a) 1.52, (b) 1.11; 95% CI: (a) 1.03–2.23, (b) 0.90–1.38; p-value: (a) 0.03, (b) 0.32 |
Overall, men with prostate carcinoma who had > 18 CAG repeats had an estimated 52% increased risk of disease recurrence. The increased risk could be attributed to men who were at high risk of recurrence |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Merseburger, 2001116 USA Urology |
Univariate analysis Marker(s): pretreatment serum creatinine End point: biochemical recurrence (two successive PSA measurements > 0.2 ng/ml) Multivariate analysis Marker(s): pretreatment serum creatinine Analysis methods: multivariable logistic regression analysis assessed the clinical usefulness of creatinine as a predictor of disease recurrence End point: biochemical recurrence (two successive PSA measurements > 0.2 ng/ml) Model used: multivariable logistic regression analysis Classical clinical markers included: unclear Classical pathological markers included: unclear Factors (prognostic markers) in final model? Unclear – clinical Gleason grade, PSA, stage, age, weight, prostate weight, history of prostatism, treatment of benign prostatic hyperplasia |
Univariate analysis Measure: log-rank, stratified into creatinine 0.7–1.0, 1.1–1.3, 1.4–2.3 Result: unclear – survival curve indicates just under 80% for all three groups; CI not reported; log-rank p-value: 0.845 Multivariate analysis Measure: recurrence-free survival Result: no significant differences between creatinine groups (analysed as continuous variable by Cox regression); CI not reported; log-rank p-value not reported |
Creatinine did not provide independent information for predicting pathological stage or disease recurrence in patients with early prostate cancer |
|
Zagars, 1987117 USA Cancer |
Univariate analysis Marker(s): creatinine Analysis methods: tests to determine whether the significance between actuarial curves (local control, disease-free survival) was achieved with log-rank statistic End point: (a) overall survival (events – death from any cause); (b) disease-free survival (events – any relapse; censored at death) Multivariate analysis Not reported |
Univariate analysis (a) Measure: survival Result: 5-year survival: creatinine ≤ 1.5 ng/ml = 75% (from n = 455), creatinine > 1.5 ng/ml = 67% (from n = 28); 10-year survival: creatinine ≤ 1.5 ng/ml = 45%, creatinine > 1.5 ng/ml = 39%; CI not reported; p-value: 0.32 (b) Measure: survival Result: 5-year survival: creatinine ≤ 1.5 ng/ml = 61% (from n = 455), creatinine > 1.5 ng/ml = 44% (from n = 28); 10-year survival: creatinine ≤ 1.5 ng/ml = 47%, creatinine > 1.5 ng/ml = 30%; CI not reported; p-value: 0.05 Multivariate analysis Not reported |
No specific conclusions made related to creatinine as a prognostic marker |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Powell, 2004118 USA Journal of Urology |
Univariate analysis Not reported Multivariate analysis Marker(s): CYP3A4 genetic variant Analysis methods: Cox proportional hazards regression models were used to examine the impact of polymorphisms on progression-free survival, controlling for other established prognostic factors. HRs were estimated classifying genotypes according to the number of copies of the G allele (allele dose), individually for AG and GG genotypes (genotype specific), comparing AA with AG + GG (dominant effect of G), and comparing AA + GG with GG (recessive effect of G) End point: all men: (a) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (b) survival from progression (events –first recurrence; censored at last follow-up if no recurrence); (c) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (d) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (e) survival from progression (events – first recurrence; censored at last follow-up if no recurrence) End point: white men (WM): (a) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (b) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (c) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (d) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (e) survival from progression (events – first recurrence; censored at last follow-up if no recurrence) End point: African American men (AAM): (a) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (b) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (c) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (d) survival from progression (events – first recurrence; censored at last follow-up if no recurrence); (e) survival from progression (events – first recurrence; censored at last follow-up if no recurrence) Model used: Cox proportional hazards regression models Classical clinical markers included: PSA, Gleason grade Classical pathological markers included: pathological stage Factors (prognostic markers) in final model? Clinical PSA, pathological stage, Gleason grade, age |
Univariate analysis Not reported Multivariate analysis All men: (a) Measure: AG (reference AA): HR Result: 1.45; CI: 1.03–2.04; p-value: 0.03 (b) Measure: GG (reference AA): HR Result: 1.58; CI: 1.12–2.23; p-value: 0.01 (c) Measure: copies of G allele (0, 1, 2): HR Result: 1.27; CI: 1.08–1.5; p-value: 0.0049 (d) Measure: AA (reference AG + GG): HR Result: 1.51; CI: 1.14–2.00; p-value: 0.004 (e) Measure: GG (reference AA + AG): HR Result: 1.41; CI: 1.02–1.96; p-value: 0.04 White men: (a) Measure: AG (reference AA): HR Result: 2.1; CI: 0.95–4.64; p-value: 0.068 (b) Measure: GG (reference AA): HR Result: 3.29; CI: 0.45–24.36; p-value: 0.24 (c) Measure: copies of G allele (0, 1, 2): HR Result: 1.98; CI: 1.06–3.70; p-value: 0.033 (d) Measure: AA (reference AG + GG): HR Result: 2.2; CI: 1.04–4.65; p-value: 0.04 (e) Measure: GG (reference AA + AG): HR Result: 3.07; CI: 0.42–22.61; p-value: 0.27 African American men: (a) Measure: AG (reference AA): HR Result: 0.87; CI: 0.49–1.54; p-value: 0.64 (b) Measure: GG (reference AA): HR Result: 0.96; CI: 0.55–1.68; p-value: 0.88 (c) Measure: copies of G allele (0, 1, 2): HR Result: 1.004; CI: 0.77–1.32; p-value: 0.97 (d) Measure: AA (reference AG + GG): HR Result: 0.92; CI: 0.54–1.55; p-value: 0.75 (e) Measure: GG (reference AA + AG): HR Result: 1.06; CI: 0.72–1.55; p-value: 0.78 |
The CYP3A4 genotype studied was not associated with pathological features of prostate cancer for men of either race. Unstratified analyses of men of both races and stratified analyses of WM demonstrated poorer progression-free survival after prostatectomy for those with the G allele, but the G allele did not predict progression-free survival among AAM |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Blute, 2001105 USA Journal of Urology |
Univariate analysis Marker(s): DNA ploidy End point: survival from progression (events – local recurrence or systemic progression or biochemical recurrence defined as PSA 0.4 ng/ml or greater) Multivariate analysis Maximum tumour dimension (mm) was not used in the multivariate analysis. Reasons for this exclusion are unclear Marker(s): DNA ploidy Analysis methods: Cox proportional hazards End point: survival from progression (events – local recurrence or systemic progression or biochemical recurrence defined as PSA 0.4 ng/ml or greater) Model used: Cox regression analyses Classical clinical markers included: PSA Classical pathological markers included: Gleason grade, surgical margins Factors (prognostic markers) in final model? Pathological Gleason grade, PSA doubling, surgical margins; factors used to define pathological stage including seminal vesicle involvement and extraprostatic extension, adjuvant hormonal or radiation therapy |
Univariate analysis Measure: 5-year survival: diploid 81% (SE 0.9), tetraploid 67% (SE 2.3), aneuploid 60% (SE 4.4) p-value: < 0.001 Multivariate analysis Measure: DNA ploidy, tetraploid vs diploid: relative risk Result: 1.24; CI: 1.00–1.53; p-value: 0.05 Measure: DNA ploidy, aneuploid vs diploid: estimated risk ratio Result: 1.43; CI: 1.03–2.00; p-value: 0.04 |
In a multivariate model to predict progression-free survival with several factors DNA ploidy was much less important than the other factors. Excluding extraprostatic extension and ploidy resulted in a model with nearly identical predictive power. When maximum tumour dimension was added to the final model it did not improve the model performance as judge by the concordance statistic. No conclusions were made by the authors regarding the prognostic significance of maximum tumour dimension |
|
Lieber, 1995106 USA Cancer (See also overlapping findings in Montgomery, 1990137) |
Univariate analysis Marker(s): DNA ploidy End point: (a) survival from progression (events – disease progression based on clinical examination, not routine PSA measurements; censoring at last follow-up for patients who had not had progression or who had died); (b) survival from death from prostate cancer, ‘cause-specific survival’ (events – death from prostate cancer only; censoring at last follow-up for patients who had not had progression or who had died); (c) overall survival (events – death from any cause; censoring at last follow-up for patients who had not had progression or who had died) Multivariate analysis Marker(s): DNA ploidy Analysis methods: Cox proportional hazards with stepwise variable selection on all variables except ploidy (forwards/backwards not specified) End point: (a) clinical progression; (b) cause-specific death; (c) all death Model used: Cox proportional hazards Classical clinical markers included: none Classical pathological markers included: Gleason grade, stage (Jewett–Whitmore) Factors (prognostic markers) in final model? Pathological Gleason grade, stage |
Univariate analysis (a) Measure: HR 10-year survival: diploid 82%; tetraploid 49%; aneuploid 24% Events: diploid 60; tetraploid 90; aneuploid 24 Result: tetraploid with reference diploid = 3.025 (95% CI 2.178–4.200); aneuploid with reference diploid = 7.102 (4.394–11.497); log-rank χ2 for ploidy = 91.75; p-value: < 0.0001 (log-rank) (b) Measure: HR 10-year survival: diploid 93%; tetraploid 79%; aneuploid 61% Events: diploid 20; tetraploid 38; aneuploid 15 Result: tetraploid with reference diploid = 3.192 (95% CI 1.856–5.489); aneuploid with reference diploid = 8.690 (95% CI 4.427–17.06); log-rank χ2 for ploidy = 51.20; p-value: < 0.0001 (log-rank) (c) Measure: HR 10-year survival: diploid 73%; tetraploid 68%; aneuploid 59% Events: diploid 92; tetraploid 71; aneuploid 16 Result: tetraploid with reference diploid = 1.320 (95% CI 0.968–1.801); aneuploid with reference diploid = 2.094 (95% CI 1.227–3.572); log-rank χ2 for ploidy 8.79; p-value: 0.0124 (log-rank) Multivariate analysis Measure: ploidy coefficient (SE), HR Result: (a): 0.950 (0.171), 2.59; CI not reported; p-value: < 0.0001 (b): 0.914 (0.280), 2.49; CI not reported; p-value: 0.0011 (c): 0.166 (0.157), 1.18l; CI not reported; p-value: 0.2925 |
Tumour volume was statistically significant in the univariate analyses but not in the multivariate analyses. It was noted that the tumour volume was estimated by three-dimensional measurements of cut specimens. PSA was not available. In the multivariate analyses ploidy was a significant predictor of clinical progression and cause-specific survival but not of all-cause mortality. In the latter model only Gleason grade was significant |
|
Siddiqui, 2006119 USA Journal of Urology (See also overlapping findings in Amling, 2000136) |
Univariate analysis Marker(s): tumour DNA ploidy End point: (a) systemic progression risk (events – demonstrable metastatic disease on radionuclide bone scintigraphy or plain radiography, or pathological evidence of failure as on lymph node biopsy); (b) risk of death from prostate cancer (events – death from prostate cancer) Multivariate analysis Marker(s): tumour DNA ploidy: diploid; tetraploid; aneuploid Analysis methods: overall survival and progression-free survival was estimated using the Kaplan–Meier method. Association of age at treatment and other clinical pathological features with prostate cancer progression and death were assessed using Cox proportional hazard regression models End point: (a) systemic progression risk (events – demonstrable metastatic disease on radionuclide bone scintigraphy or plain radiography, or pathological evidence of failure as on lymph node biopsy); (b) risk of death from prostate cancer (events – death from prostate cancer) Model used: Cox proportional hazard regression models Classical clinical markers included: none Classical pathological markers included: Gleason grade, stage, surgical margins Factors (prognostic markers) in final model? Pathological stage and Gleason score, surgical margins, categorised age, lymph node involvement, adjuvant hormonal therapy, adjuvant radiation therapy |
Univariate analysis (a) Measure: relative risk Result: 2.63; CI: 2.16–3.20; p-value: < 0.0001 (b) Measure: relative risk Result: 3.20; CI: 2.46–4.16; p-value: < 0.0001 Multivariate analysis (a) Measure: Cox proportional hazard regression: relative risk, tumour DNA ploidy (risk of diploid with reference non-diploid) Result: 1.72; CI: 1.39–2.13; p-value: < 0.0001 (b) Measure: Cox proportional hazard regression: relative risk, tumour DNA ploidy Result: 1.92; CI: 1.44–2.55; p-value: < 0.0001 |
No conclusions about tumour DNA ploidy prognostic factors are made |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Williams, 2004120 USA Prostate |
Univariate analysis No univariate analysis Multivariate analysis Marker(s): BsmI polymorphism Analysis methods: Cox proportional regression analysis models were used to examine the impact of the polymorphisms on progression-free survival, controlling for effects of other established prognostic factors. Using the BsmI polymorphism, genotypes were classified in several ways: according to the number of copies of the B allele (allele dose); the individual genotypes included in the same model (genotype specific); comparing bb with Bb + BB (dominant effect of B); comparing bb + Bb with BB (recessive effect of B) End point: survival from progression (events – first recurrence; censoring at last follow-up). This is split into white men (WM) and African American Men (AAM) Model used: multivariable Cox proportional hazard regression model Classical clinical markers included: PSA, Gleason grade Classical pathological markers included: stage Factors (prognostic markers) in final model? Clinical PSA, Gleasongrade, pathological stage and age |
Univariate analysis No univariate analysis Multivariate analysis White men: (a) Measure: number of B alleles (0, 1, 2), progression-free survival: HR Result: 0.80; CI: 0.59–1.08; p-value: 0.14 (b) Measure: Bb vs Bb, progression-free survival: HR Result: 0.85; CI: 0.55–1.33; p-value: 0.47 (c) Measure: Bb vs BB, progression-free survival: HR Result: 0.60; CI: 0.31–1.18; p-value: 0.14 (d) Measure: bb vs (Bb + BB), progression-free survival: HR Result: 0.78; CI: 0.51–1.19; p-value: 0.25 (e) Measure: (bb + Bb) vs BB, progression-free survival: HR Result: 0.66; CI: 0.35–1.24; p-value: 0.19 African American men: (a) Measure: number of B alleles (0, 1, 2), progression-free survival: HR Result: 0.98; CI: 0.73–1.31; p-value: 0.89 (b) Measure: Bb vs Bb, progression-free survival: HR Result: 0.74; CI: 0.48–1.15; p-value: 0.18 (c) Measure: Bb vs BB, progression-free survival: HR Result: 1.25; CI: 0.69–2.30; p-value: 0.46 (d) Measure: bb vs (Bb + BB), progression-free survival: HR Result: 0.85; CI: 0.57–1.25; p-value: 0.40 (e) Measure: (bb + Bb) vs BB, progression-free survival: HR Result: 1.40; CI: 0.78–2.51; p-value: 0.27 |
Overall, vitamin D receptor polymorphisms did not predict pathological features of prostate cancer but they may impact on risk of recurrence among men in certain risk groups Although the B allele was protective for WM with locally advanced disease, it tended to be associated with a poorer prognosis among men with organ-confined disease. However, the adverse effect of the B allele among men with organ-confined disease was not statistically significant |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Egevad, 2002121 Sweden Journal of Urology |
Univariate analysis Marker(s): percentage Gleason grade 4/5 End point: survival from death from prostate cancer, ‘disease-specific survival’ (events – death from prostate cancer) Multivariate analysis Marker(s): percentage Gleason grade 4/5 Analysis methods: survival was analysed by Kaplan–Meier plots using log-rank comparisons of groups. The Cox proportional hazards model was used to compare prognostic parameters End point: (a) survival from death from prostate cancer, ‘disease-specific survival’ (events – death from prostate cancer); (b) survival from death from prostate cancer, ‘disease-specific survival’ (events – death from prostate cancer) Model used: Cox proportional hazards Classical clinical markers included: none Classical pathological markers included: Gleason score Factors (prognostic markers) in final model? (a) pathological Gleason score; (b) pathological Gleason score (also percentage cancer) |
Univariate analysis (a) Measure: percentage Gleason grade 4/5 (continuous data at 10% increments) Events (at mean follow-up 7.3 years for censored patients, 5.9 for uncensored): percentage grade 4/5 = 0%, 8% died of prostate cancer (of n = 104); percentage grade 4/5 = up to 5%, 28% (of n = 40); percentage grade 4/5 = 10–50%, 38% (of n = 40); percentage grade 4/5 = 51–100%, 65% (of n = 121) Result: χ2 = 92.3; CI not applicable; p-value: < 0.001 Multivariate analysis (a) Measure: percentage Gleason grade 4/5 (continuous data at 10% increments) Events (at mean follow-up 7.3 years for censored patients, 5.9 for uncensored): percentage grade 4/5 =0%, 8% died of prostate cancer (of n = 104); percentage grade 4/5 = up to 5%, 28% (of n = 40); percentage grade 4/5 = 10–50%, 38% (of n = 40); percentage grade 4/5 = 51–100%, 65% (of n = 121) Result: χ2 = 9.5; CI not applicable; p-value: 0.002 (b) Measure: percentage Gleason grade 4/5 (continuous data at 10% increments) Events: see above Result: χ2 = 4.7; CI not applicable; p-value: 0.030 |
The strong prognostic value of percentage Gleason grade 4/5 was confirmed. Percentage Gleason grade 4/5 was superior to conventional Gleason score as a predictor of biochemical failure (PSA relapse). In the univariate Cox models, percentage Gleason grade 4/5, Gleason score, Gleason score categories, modified Gleason score and percentage cancer were significant predictors of disease-specific survival |
|
Gonzalgo, 2006122 USA Urology |
Univariate analysis Marker(s): Gleason score 7: biopsy 3 + 4, prostatectomy ≤ 3 + 4; biopsy 3 + 4, prostatectomy ≥ 4 + 3; biopsy 4 + 3, prostatectomy ≤ 3 + 4; biopsy 4 + 3, prostatectomy ≥ 4 + 3 End point: biochemical recurrence (PSA ≥ 0.2 ng/ml) (measured in terms of likelihood of undetectable PSA level) Multivariate analysis No multivariate analysis |
Univariate analysis Measure: log-rank test for comparison of survival curves; chi-squared test Survival: estimated from survival curve; scored on scale 0–1, likelihood of undetectable PSA (higher score indicates better prognosis). Group A (clinical 3 + 4 not upgraded at prostatectomy), p = 0.89; group B (clinical 3 + 4 upgraded at prostatectomy), p = 0.74; group C (clinical 4 + 3 downgraded), p = 0.86; group D (clinical 4 + 3 not downgraded), p = 0.55 Result: log-rank test for comparison of all four survival curves, χ2 = 28.80 (p < 0.0001); CI not applicable; p-value: < 0.0001 Additional results: group A significantly better prognosis than group B (p = 0.002) and group D (p < 0.001); group C significantly better prognosis than group D (p = 0.03); non-significant between groups A and C (p < 0.17), groups B and D (p = 0.07), groups B and C (p = 0.47) |
Approximately 47% of men with a diagnosis of Gleason pattern 4 + 3 on needle biopsy are downgraded at RP and have biochemical PSA recurrence-free outcomes similar to those of patients originally diagnosed with Gleason pattern 3 + 4 adenocarcinoma |
|
Tollefson, 2006123 USA Journal of Urology |
Univariate analysis Marker(s): Gleason pattern: 3 + 4/4 + 3 Analysis methods: not specified End point: (a) biochemical recurrence-free survival (events – single serum PSA of > 0.4 ng/ml); (b) systemic recurrence (events – positive bone scan or other lesion identifying metastatic prostate cancer); (c) cancer-specific survival (events – death from prostate cancer) Multivariate analysis Marker(s): Gleason pattern: 3 + 4/4 + 3 Analysis methods: NS End point: (a) biochemical recurrence-free survival (events – single serum PSA of > 0.4 ng/ml); (b) systemic recurrence (events – positive bone scan or other lesion identifying metastatic prostate cancer); (c) cancer-specific survival (events – death from prostate cancer) Model used: not reported Classical clinical markers included: clinical PSA, stage Classical pathological markers included: none Factors (prognostic markers) in final model? Unclear: clinical PSA, stage, margin status, seminal vesicle involvement, DNA ploidy |
Univariate analysis (a) Measure: survival Result: 10-year survival: Gleason 3 + 4, 48%; Gleason 4 + 3, 38%; CI not reported; p-value: < 0.001 (b) Measure: survival Result: 10-year survival: Gleason 3 + 4, 8%; Gleason 4 + 3, 15%; CI not reported; p-value: < 0.001 (c) Measure: survival Result: 10-year survival: Gleason 3 + 4, 97%; Gleason 4 + 3, 93%; CI not reported; p-value: < 0.001 Multivariate analysis (a) Measure: biochemical progression: survival Result: 10-year survival: Gleason 3 + 4, 48%; Gleason 4 + 3, 38%; CI not reported; p-value: < 0.0001 (b) Measure: systemic recurrence: survival Result: 10-year survival: Gleason 3 + 4, 8%; Gleason 4 + 3, 15%; CI not reported; p-value: 0.002 (c) Measure: cancer-specific death: survival Result: 10-year survival: Gleason 3 + 4, 97%; Gleason 4 + 3, 93%; CI not reported; p-value: 0.013 |
Patients with Gleason score 4 + 3 prostate cancer have more aggressive disease and experience higher rates of biochemical failure, systemic recurrence and cancer-specific death. The study firmly established pathological primary Gleason pattern as an independent predictor of survival in patients with Gleason score 7 prostate cancer. Primary Gleason pattern is independently associated with biochemical recurrence, systemic recurrence and cancer-specific survival |
|
Vis, 2007124 The Netherlands European Urology |
Univariate analysis Marker(s): length (mm) of high-grade cancer End point: (a) biochemical recurrence (PSA ≥ 0.1 ng/ml); (b) clinical progression (local progression and/or distant metastases); (c) biochemical recurrence (PSA ≥ 0.1 ng/ml) Analysis method: Cox proportional hazards model Multivariate analysis Marker(s): length (mm) of high-grade cancer End point: (a) biochemical recurrence (PSA ≥ 0.1 ng/ml); (b) biochemical recurrence (PSA ≥ 0.1 ng/ml); (c) clinical progression (local progression and/or distant metastases); (d) biochemical recurrence (PSA ≥ 0.1 ng/ml) Analysis methods: Cox proportional regression analysis was used to assess the relationship between preoperative and postoperative variables and PSA relapse (≥ 0.1 ng/ml, ≥ 1.0 ng/ml) or clinical relapse after RP. Subsequent analyses were also performed when Gleason score 7 cancers were divided into 3 + 4 and 4 + 3 categories. To identify independent prognostic factors, backwards stepwise Cox regression analysis was performed by removing variables from the model that were not significant at the univariate level. Forwards stepwise elimination was performed to verify that the same parameters remained of prognostic significance in the final models Model used: Cox proportional regression analysis Classical clinical markers included: PSA, Gleason grade, stage Classical pathological markers included: Gleason grade, stage, surgical margins Factors (prognostic markers) in final model? For end point (a), PSA and length of tumour in mm; for end point (b), not stated; for endpoint (c), none as all removed, therefore as univariate; for endpoint (d), surgical margins (also invasion of adjacent organs) |
Univariate analysis (a) Measure: HR Result: 1.079 Survival (estimated from survival curve): 5-year survival, length of high-grade cancer (Gleason 4/5): 0 mm 92%, 0–3 mm 90%, 3–10 mm 72%, > 10 mm 50%; CI not reported; p-value: < 0.001 (b) Measure: HR Result: 1.074 Survival (extrapolating from survival curve): 5-year survival, length of high-grade cancer (Gleason 4/5): 0 mm 99%, 0–3 mm 98%, 3–10 mm 88%, > 10 mm 78%; CI not reported; p-value: < 0.004 (c) Measure: HR Result: 1.029; CI not reported; p-value: < 0.001 Multivariate analysis (a) Measure: length (mm) of high-grade cancer: HR Result: 1.033; CI not reported; p-value: 0.006 (b) Measure: Cox multiple regression, proportion of high-grade cancer (note not length) Result: NS; CI not reported; p-value: 0.001 (c) Measure: length (mm) of high-grade cancer: HR Result: 1.074; CI not reported; p-value: 0.004 (d) Measure: Cox regression analysis, percentage high-grade tumour volume: HR Result: 1.023; CI not reported; p-value: < 0.001 |
Amount of high-grade cancer in diagnostic biopsy proved to be an independent and stronger prognostic factor for relapse after RP than Gleason score |
|
Vollmer, 2001107 USA American Journal of Clinical Pathology |
Univariate analysis Not reported Multivariate analysis Marker(s): Gleason grade 5 present or not Analysis methods: Cox proportional hazards, with removal of insignificant variables (method not specified) End point: time to death from prostate cancer [censored if died without elevated (> 0.5 ng/ml) postoperative PSA level] Model used: Cox proportional hazards Classical clinical markers included: none Classical pathological markers included: none Factors (prognostic markers) in final model? None |
Univariate analysis Not reported Multivariate analysis Measure: Gleason grade 5: coefficient [presence of either primary or secondary Gleason grade 5 (with reference absence of Gleason grade 5) Cox model analysis] Result: coefficient = 1.17 (SE = 0. 450); CI not reported; p-value: 0.0096 |
‘selecting a PSA end point favours models with PSA-related prognostic factors. Using time to death as the end point, on the other hand, seems to favour anatomic factors.’ The presence of Gleason grade 5 was significantly related to survival, regardless of how much was present |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Zellweger, 2003125 Switzerland Prostate |
Univariate analysis Marker(s): Ki67 LI Analysis methods: log-rank End point: (a) time to progression – two definitions according to dates, before 1992 clinical progression (bone scans/chest radiography/digital rectal examination), after 1992 defined by increasing PSA (no definition of level of increase reported); (b) overall survival (not defined); (c) tumour-specific survival (not defined) Multivariate analysis Marker(s): Ki67 LI Analysis methods: Cox proportional hazards model (stepwise, included if significant in univariate analysis) End point: (a) time to progression – two definitions according to dates, before 1992 clinical progression (bone scans/chest radiography/digital rectal examination), after 1992 defined by increasing PSA (no definition of level of increase reported); (b) overall survival (not defined); (c) tumour-specific survival (not defined) Model used: Cox proportional hazards model Classical clinical markers included: Gleason grade Classical pathological markers included: none Factors (prognostic markers) in final model? Gleason grade |
Univariate analysis (a) Measure: log-rank Result: from survival curve: Ki67 LI high, 70%; Ki67 LI low, 85%; CI not reported; p-value: < 0.01 (b) Measure: log-rank Result: from survival curve: Ki67 LI high, 72%; Ki67 LI low, 86%; CI not reported; p-value: < 0.05 (c) Measure: log-rank Result: from survival curve: Ki67 LI high, 90%; Ki67 LI low, 98%; CI not reported; p-value: < 0.01 Multivariate analysis (a) Measure: Cox proportional hazards Result: not reported; CI not reported; p-value: 0.178 (b) Measure: Cox proportional hazards Result: not reported; CI not reported; p-value: 0.071 (c) Measure: Cox proportional hazards Result: not reported; CI not reported; p-value: 0.023 |
The results confirm a dominant prognostic significance of Gleason grading and Ki67 LI in prostate cancer and a less pronounced role of Bcl-2 and p53. Syndecan-1 was identified as a new prognostic factor. Also the evidence supports androgen-dependent regulation of CD10 expression |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Zellweger, 2003125 Switzerland Prostate |
Univariate analysis Marker(s): Bcl-2 Analysis methods: log-rank End point: (a) time to progression – two definitions according to dates, before 1992 clinical progression (bone scans/chest radiography/digital rectal examination), after 1992 defined by increasing PSA (no definition of level of increase reported; (b) overall survival (not defined); (c) tumour-specific survival (not defined) Multivariate analysis Marker(s): Bcl-2 Analysis methods: Cox proportional hazards model (stepwise, included if significant in univariate analysis) End point: (a) Time to progression – two definitions according to dates, before 1992 clinical progression (bone scans/chest radiography/digital rectal examination), after 1992 defined by increasing PSA (no definition of level of increase reported) Model used: Cox proportional hazards model Classical clinical markers included: Gleason grade Classical pathological markers included: none Factors (prognostic markers) in final model? Gleason grade |
Univariate analysis (a) Measure: log-rank Result: from survival curve: Bcl-2 negative 85%, Bcl-2 positive 72%; CI not reported; p-value: < 0.05 (b) Measure: log-rank Result: from survival curve: Bcl-2 negative 94%, Bcl-2 positive 88%; CI not reported; p-value: 0.28 (c) Measure: log-rank Result: from survival curve: Bcl-2 negative 96%, Bcl-2 positive 96%; CI not reported; p-value: 0.79 Multivariate analysis (a) Measure: Cox proportional hazards Result: not reported; CI not reported; p-value: 0.816 |
See Table 95 |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Zellweger, 2003125 Switzerland Prostate |
Univariate analysis Marker(s): p53 Analysis methods: log-rank End point: (a) time to progression – two definitions according to dates, before 1992 clinical progression (bone scans/chest radiography/digital rectal examination), after 1992 defined by increasing PSA (no definition of level of increase reported); (b) overall survival (not defined); (c) tumour-specific survival (not defined) Multivariate analysis Marker(s): p53 Analysis methods: Cox proportional hazards model (stepwise, included if significant in univariate analysis) End point: (a) overall survival (not defined); (b) tumour-specific survival (not defined) Model used: Cox proportional hazards model Classical clinical markers included: Gleason grade Classical pathological markers included: none Factors (prognostic markers) in final model? Gleason grade |
Univariate analysis (a) Measure: log-rank Result: from survival curve: p53 negative 82%, p53 positive 82%; CI not reported; p-value: 0.38 (b) Measure: log-rank Result: from survival curve: p53 negative 90%, p53 positive 71%; CI: not reported; p-value: < 0.05 (c) Measure: log-rank Result: from survival curve: Ki67 LI high 97%; Ki67 LI low 87%; CI not reported; p-value: < 0.05 Multivariate analysis (a) Measure: Cox proportional hazards Result: not reported; CI not reported; p-value: 0.84 (b) Measure: Cox proportional hazards Result: not reported; CI not reported; p-value: 0.542 |
See Table 95 |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Zellweger, 2003125 Switzerland Prostate |
Univariate analysis Marker(s): syndecan-1 Analysis methods: log-rank End point: (a) Time to progression – two definitions according to dates, before 1992 clinical progression (bone scans/chest radiography/digital rectal examination), after 1992 defined by increasing PSA (no definition of level of increase reported); (b) overall survival (not defined); (c) tumour-specific survival (not defined) Multivariate analysis Marker(s): syndecan-1 Analysis methods: Cox proportional hazards model (stepwise, included if significant in univariate analysis) End point: (a) Time to progression – two definitions according to dates, before 1992 clinical progression (bone scans/chest radiography/digital rectal examination), after 1992 defined by increasing PSA (no definition of level of increase reported); (b) tumour-specific survival (not defined) Model used: Cox proportional hazards model Classical clinical markers included: Gleason grade Classical pathological markers included: none Factors (prognostic markers) in final model? Gleason grade |
Univariate analysis (a) Measure: log-rank Result: from survival curve: syndecan-1 negative 84%, syndecan-1 positive 78%; CI not reported; p-value: < 0.02 (b) Measure: log-rank Result: from survival curve: syndecan-1 negative 90%, syndecan-1 positive 79%; CI not reported; p-value: 0.07 (c) Measure: log-rank Result: from survival curve: syndecan-1 negative 99%, syndecan-1 positive 92%; CI not reported; p-value: < 0.01 Multivariate analysis (a) Measure: Cox proportional hazards Result: not reported; CI not reported; p-value: 0.147 (b) Measure: Cox proportional hazards Result: not reported; CI not reported; p-value: 0.051 |
See Table 95 |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Zellweger, 2003125 Switzerland Prostate |
Univariate analysis Marker(s): CD10 Analysis methods: log-rank End point: (a) time to progression – two definitions according to dates, before 1992 clinical progression (bone scans/chest radiography/digital rectal examination), after 1992 defined by increasing PSA (no definition of level of increase reported); (b) overall survival (not defined); (c) tumour-specific survival (not defined) Multivariate analysis Not reported |
Univariate analysis (a) Measure: log-rank Result: from survival curve: CD10 negative 81%, CD10 positive 78%; CI not reported; p-value: 0.22 (b) Measure: log-rank Result: from survival curve: CD10 negative 85%, CD10 positive 85%; CI not reported; p-value: 0.87 (c) Measure: log-rank Result: from survival curve: CD10 negative 95%, CD10 positive 95%; CI not reported; p-value: 0.68 Multivariate analysis Not reported |
See Table 95 |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Antunes, 2005126 Brazil International Brazilian Journal of Urology (See also preliminary findings in Antunes, 2005169) |
Univariate analysis Marker(s): percentage of positive biopsy cores (PPBC) Analysis methods: the survival analysis considered biochemical recurrence as the main end point using a Cox regression model. In all tests the level of significance was set at p < 0.05 End point: survival from biochemical recurrence (PSA ≥ 0.4 ng/ml) Multivariate analysis Marker(s): PPBC Analysis methods: the survival analysis considered biochemical recurrence as the main end point using a Cox regression model. In all tests the level of significance was set at p < 0.05 End point: survival from biochemical recurrence (PSA ≥ 0.4 ng/ml) Model used: Cox regression model Classical clinical markers included: stage, PSA, Gleason score Classical pathological markers included: NA Factors (prognostic markers) in final model? Clinical stage, PSA, Gleason score |
Univariate analysis Measure: Cox regression: percentage positive biopsy cores (continuous variable) Result: 3.46; extrapolated from survival curve , 5-year survival: PPBC: under 25 85%, 25.1–50 76%, 50.1–75 72%, 75.1–100 43%; CI: 1.89–6.33; p-value: < 0.001 Multivariate analysis Measure: Cox regression: PPBC (continuous variable) Result: 3.46; CI: 1.89–6.33; p-value: < 0.001 |
Confirmed the clinical utility of the PPBC in determining the pathological features and biochemical outcomes of patients with prostate cancer treated with RP, and established thresholds for use in patients in the three risk groups. Also PPBC was related to the biochemical outcome with thresholds of 75%, 25% and 50% in the low-, intermediate- and high-risk groups respectively |
|
Egevad, 2002121 Sweden Journal of Urology |
Univariate analysis Marker(s): percentage of prostate showing tumour in transurethral section specimen Analysis methods: Cox analysis model End point: survival from death from prostate cancer, ‘disease-specific survival’ (events – death from prostate cancer) Multivariate analysis Marker(s): percentage of prostate showing tumour in transurethral section specimen Analysis methods: Cox multivariate analysis model End point: (a) survival from death from prostate cancer, ‘disease-specific survival’ (events – death from prostate cancer) Model used: Classical clinical markers included: none Classical pathological markers included: Gleason score Factors (prognostic markers) in final model? Pathological Gleason score, percentage Gleason grade 4/5 |
Univariate analysis Measure: percentage cancer in transurethral specimen (continuous data at 10% increments): chi-squared test Result: 73.5; CI not applicable; p-value: < 0.001 Multivariate analysis Measure: (a) multivariate Cox analysis; percentage cancer in transurethral specimen (continuous data at 10% increments): chi-squared test Result: 10.6; CI not applicable; p-value: 0.011 |
Confirmed the clinical utility of the PPBC in determining the pathological features and biochemical outcome of patients with prostate cancer treated with RP, and established thresholds for use in patients in the three risk groups. Also PPBC was related to the biochemical outcome with thresholds of 75%, 25% and 50% in the low-, intermediate- and high-risk groups, respectively |
|
Potters, 2005127 USA Journal of Urology |
Univariate analysis No univariate analysis Multivariate analysis Marker(s): positive biopsy core Analysis methods: multivariate analyses were performed by the Cox proportional square hazards model testing. Kaplan–Meier curves were constructed to demonstrate survival distributions End point: survival from biochemical recurrence (ASTRO–Kattan definition) Model used: Cox proportional square hazards model Classical clinical markers included: PSA, Gleason grade, stage Classical pathological markers included: none Factors (prognostic markers) in final model? Clinical PSA, Gleason score, stage, percentage D90, hormone addition, external beam radiotherapy addition |
Univariate analysis No univariate analysis Multivariate analysis Measure: Cox proportional hazards: percentage positive biopsy cores < 50% compared with those ≥ 50% Result: 1.492; CI: 1.024–2.173; p-value: 0.037 |
PPB offers acceptable 12-year BFR in patients who present with clinically localised prostate cancer. Implant dosimetry continues as an important predictor for BFR, while the addition of adjuvant therapies such as hormones and external radiation is insignificant |
|
Selek, 2003128 USA International Journal of Radiation Oncology, Biology, Physics |
Univariate analysis Marker(s): positive biopsy core Analysis methods: Cox proportional hazards model for (a); univariate log-rank for (b) End point: (a) survival from biochemical recurrence (events from ASTRO definition); (b) survival from biochemical recurrence (events from ASTRO definition) Multivariate analysis Marker(s): percentage of positive prostate biopsies (PPPB) Analysis methods: Cox regression analysis was performed evaluating the ability of pretreatment serum PSA level, PPPBs, clinical stage, and biopsy Gleason score to predict the time to post-external beam radiotherapy (EBRT) PSA failure End point: (a) survival from biochemical recurrence (ASTRO definition); (b) survival from biochemical recurrence (ASTRO definition) Model used: Cox regression multivariate analysis Classical clinical markers included: PSA, Gleason score Classical pathological markers included: none Factors (prognostic markers) in final model? Clinical PSA, Gleason score |
Univariate analysis (a) Measure: proportional hazards: percentage positive biopsy cores (analysed as continuous variable) Result: not reported; CI not reported; p-value: 0.0053 (b) Measure: log-rank: percentage positive biopsy cores < 50% compared with those ≥ 50% Result: not reported; CI not reported; p-value: 0.0077 Multivariate analysis (a) Measure: percentage positive biopsy cores (analysed as continuous variable): HR Result: 1.001; CI not reported; p-value: 0.13 (b) Measure: Cox regression analysis: percentage positive biopsy cores ≥ 50% compared with those < 50%: HR Result: 1.40; CI not reported; p-value: 0.22 |
PPPB was a predictor of post-EBRT PSA outcome in clinically localised prostate cancer but in this cohort it did not provide additional information beyond the traditional risk stratification schema |
|
Vis, 2007124 The Netherlands European Urology |
Univariate analysis Marker(s): number of positive tumour biopsy cores Analysis methods: Cox proportional hazards model End point: (a) biochemical recurrence (PSA ≥ 0.1 ng/ml); (b) clinical progression (local progression and/or distant metastases) Multivariate analysis Marker(s): biopsy cores Analysis methods: Cox proportional regression analysis was used to assess the relationship between preoperative and postoperative variables and PSA relapse (≥ 0.1 ng/ml, ≥ 1.0 ng/ml) or clinical relapse after RP. Subsequent analyses were also performed when Gleason score 7 cancers were divided into 3 + 4 and 4 + 3 categories. To identify independent prognostic factors, backwards stepwise Cox regression analysis was performed by removing variables from the model that were not significant at univariate level. Forwards stepwise elimination was performed to verify that the same parameters remained of prognostic significance in the final models End point: (a) biochemical recurrence (PSA ≥ 0.1 ng/ml); (b) clinical progression (local progression and/or distant metastases) Model used: Cox proportional regression analysis Classical clinical markers included: PSA, Gleason grade, stage Classical pathological markers included: Gleason grade, stage, surgical margins Factors (prognostic markers) in final model? preoperative = 6; postoperative = 6 |
Univariate analysis (a) Measure: number of positive tumour biopsy cores (continuous variable): HR Result: 1.439; CI not reported; p-value: 0.001 (b) Measure: number of positive tumour biopsy cores (continuous variable): HR Result: 1.513; CI not reported; p-value: 0.025 Multivariate analysis (a) Measure: number of positive tumour biopsy cores (continuous variable): HR Result: non-significant; CI not reported; p-value not reported (b) Measure: number of positive tumour biopsy cores (continuous variable): HR Result: non-significant; CI not reported; p-value not reported |
In biopsy and RP specimens of surgically treated prostate cancer, the amount of high-grade cancer is superior to the Gleason grading system in predicting patient outcome. Amount of high-grade cancer in diagnostic biopsy proved to be an independent and stronger prognostic factor for relapse after RP than Gleason score |
|
Vollmer, 2001107 USA American Journal of Clinical Pathology |
Univariate analysis Not reported Multivariate analysis Marker(s): percentage of the prostate showing tumour in the RP specimen Analysis methods: Cox proportional hazards, with removal of insignificant variables (method not specified) End point: time to death from prostate cancer [censored if died without elevated (> 0.5 ng/ml) postoperative PSA level] Model used: Cox proportional hazards Classical clinical markers included: clinical PSA, grade Classical pathological markers included: stage Factors (prognostic markers) in final model? Gleason 5 |
Univariate analysis Not reported Multivariate analysis Measure: percentage carcinoma (continuous variable) Result: 0.029 (SE = 0.009); HR: 1.03; CI not reported; p-value: 0.0014 |
‘selecting a PSA end point favours models with PSA-related prognostic factors. Using time to death as the end point, on the other hand, seems to favour anatomic factors.’ ‘The importance of percentage carcinoma for death but not for biochemical failure probably relates to how some have found tumour volume to be prognostic, while others have not.’ |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
D’Amico, 2004129 USA New England Journal of Medicine |
Univariate analysis Marker(s): PSAV Analysis methods: Cox regression on PSAV at diagnosis, PSAV > 2 ng/ml/year (reference PSAV ≤ 2 ng/ml/year), see end points (a), (b) and (c); Cox regression on PSAV at prostatectomy, PSAV > 2 ng/ml/year (reference PSAV ≤ 2 ng/ml/year), see end points (d) and (e) End point: (a) recurrence (two consecutive PSA > 0.2 ng/ml); (b) death from prostate cancer; (c) death from any cause; (d) death from prostate cancer; (e) death from any cause Multivariate analysis Marker(s): PSAV: (1) PSAV ≤ 2 ng; (2) PSAV > 2 ng; (3) PSAV on prostate ≤ 2 ng; (4) PSAV on prostate > 2 ng Analysis methods: used PSA measurement closest in time before diagnosis and all previous PSA values that had been obtained within 1 year before diagnosis. Linear regression analysis was used to calculate the PSAV during the year before diagnosis. Cox regression on PSAV at diagnosis, PSAV > 2 ng/ml/year (reference PSAV ≤ 2 ng/ml/year), see end points (a), (b) and (c); Cox regression on PSAV at prostatectomy, PSAV > 2 ng/ml/year (reference PSAV ≤ 2 ng/ml/year), see end points (d) and (e) End point: (a) recurrence (two consecutive PSA > 0.2 ng/ml); (b) death from prostate cancer; (c) death from any cause; (d) death from prostate cancer; (e) death from any cause Model used: Cox regression analysis Classical clinical markers included: PSA, Gleason grade, stage Classical pathological markers included: Gleason grade, stage, surgical margins Factors (prognostic markers) in final model? For end points (a), (b) and (c): clinical PSA, Gleason, stage. For end points (d) and (e): pathological Gleason, stage, surgical margins, nodal status |
Univariate analysis (a) Measure: relative risk Events: PSAV ≤ 2ng/ml/year, 247; PSAV > 2 ng/ml/year, 119 Result: 1.6; CI: 1.3–2.1; p-value: < 0.001 (b) Measure: relative risk Events: PSAV ≤ 2 ng/ml/year, 3; PSAV > 2 ng/ml/year, 24 Result: 20.4; CI: 6.2–67.9; p-value: < 0.001 (c) Measure: relative risk Events: PSAV ≤ 2 ng/ml/year, 45; PSAV > 2 ng/ml/year, 39 Result: 2.6; CI: 1.6–4.1; p-value: < 0.001 (d) Measure: relative risk Events: PSAV ≤ 2 ng/ml/year, 3; PSAV > 2 ng/ml/year, 24 Result: 20.4; CI: 6.2–67.9; p-value: < 0.001 (e) Measure: relative risk Events: PSAV ≤ 2 ng/ml/year, 45; PSAV > 2 ng/ml/year, 39 Result: 2.2; CI: 1.4–3.4; p-value: < 0.001 Multivariate analysis (a) Measure: PSAV ≤ 2 ng vs PSAV > 2 ng: HR Events: PSAV ≤ 2ng/ml/year, 247; PSAV > 2 ng/ml/year, 119 Result: 1.5; CI: 1.1–1.9; p-value: 0.003 (b) Measure: PSAV ≤ 2 ng vs PSAV > 2 ng: HR Events: PSAV ≤ 2 ng/ml/year, 3; PSAV > 2 ng/ml/year, 24 Result: 9.8; CI: 2.8–34.3; p-value: < 0.001 (c) Measure: PSAV ≤ 2 ng vs PSAV > 2 ng: HR Events: PSAV ≤ 2 ng/ml/year, 45; PSAV > 2 ng/ml/year, 39 Result: 1.9; CI: 1.2–3.2; p-value: < 0.01 (d) Measure: PSAV on prostate ≤ 2 ng vs PSAV > 2 ng: HR Events: PSAV ≤ 2 ng/ml/year, 3; PSAV > 2 ng/ml/year, 24 Result: 12.8; CI: 3.7–43.7; p-value: < 0.001 (e) Measure: PSAV on prostate ≤ 2 ng vs PSAV > 2 ng: HR Events: PSAV ≤ 2 ng/ml/year, 45; PSAV > 2 ng/ml/year, 39 Result: 1.8; CI: 1.1–2.8; p-value: 0.01 |
Men whose PSA level increases by > 2 ng/ml during the year before the diagnosis of prostate cancer may have a relatively high risk of death from prostate cancer or death from any cause despite undergoing RP; however, the CIs are large |
|
Sengupta, 2005130 USA Journal of Urology |
Univariate analysis Marker(s): PSADT, see end points (a), (b) and (c); PSAV, see end points (d), (e) and (f) Analysis methods: preoperative and postoperative prognostic factors were assessed using Cox proportional hazards models End point: (a) survival from biochemical progression (PSA 0.4 ng/ml or greater; patients without progression censored at time of last PSA determination); (b) survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa); (c) survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa); (d) survival from biochemical progression (PSA 0.4 ng/ml or greater; patients without progression censored at time of last PSA determination); (e) survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa); (f) survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) Multivariate analysis Marker(s): PSADT; PSAV Analysis methods: preoperative and postoperative prognostic factors were assessed using Cox proportional hazards models End point: biochemical progression; clinical progression; prostate cancer death Model used: Cox proportional hazards models Classical clinical markers included: PSA, Gleason grade, stage Classical pathological markers included: Gleason grade, stage, surgical margins Factors (prognostic markers) in final model? Six multivariate preoperative factors; 11 multivariate postoperative factors |
Univariate analysis (a) Measure: Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR Events: preoperative PSADT < 18 months, 74%; PSADT ≥ 18 months, 84% Result: 1.58; CI: 1.32–1.89; p-value: < 0.0001 (b) Measure: Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR Events: preoperative PSADT < 18 months, 92%; PSADT ≥ 18 months, 96% Result: 2.53; CI: 1.83–3.48; p-value: < 0.0001 (c) Measure: Cox proportional hazards, preoperative PSADT < 18 months (reference PSADT ≥ 18 months): HR Events: preoperative PSADT < 18 months, 96%; PSADT ≥ 18 months, 99% Result: 2.53; CI: 1.83–3.48; p-value: < 0.0001 (d) Measure: Cox proportional hazards, preoperative PSAV > 3.4 ng/ml/year (reference preoperative PSAV ≤ 3.4 ng/ml/year): HR Events: preoperative PSAV > 3.4 ng/ml/year, 66%; preoperative PSAV ≤ 3.4 ng/ml/year, 86% Result: 2.28; CI: 1.92–2.71; p-value: < 0.0001 (e) Measure: Cox proportional hazards, preoperative PSAV > 3.4 ng/ml/year (reference preoperative PSAV ≤ 3.4 ng/ml/year): HR Result: 2.53; CI: 1.83–3.50; p-value: < 0.0001 (f) Measure: Cox proportional hazards, preoperative PSAV > 3.4 ng/ml/year (reference preoperative PSAV ≤ 3.4 ng/ml/year): HR Events: preoperative PSAV > 3.4 ng/ml/year, 98%; preoperative PSAV ≤ 3.4 ng/ml/year, 96% Result: 6.54; CI: 3.51–12.19; p-value: < 0.0001Multivariate analysis Measure: preoperative PSAV > 3.4 ng/ml/year with preoperative factors predictive of biochemical recurrence: HR Result: 1.49; CI: 1.17–1.90; p-value: 0.001 Measure: preoperative PSADT < 18 months with preoperative factors predictive of clinical recurrence: HR Result: 1.83; CI: 1.24–2.72; p-value: 0.003 Measure: preoperative PSADT < 18 months with preoperative factors predictive of prostate cancer death: HR Result: 6.18; CI: 2.75–13.88; p-value: < 0.0001 Measure: preoperative PSAV > 3.4 ng/ml/year with postoperative factors predictive of biochemical recurrence: HR Result: 1.30; CI: 1.06–1.58; p-value: 0.011 Measure: preoperative PSADT < 18 months with postoperative factors predictive of clinical recurrence: HR Result: 1.80; CI: 1.26–2.57; p-value: 0.001 Measure: preoperative PSADT < 18 months with postoperative factors predictive of prostate cancer death: HR Result: 3.92; CI: 1.95–7.85; p-value: 0.0001 |
Preoperative PSA kinetics appear to be useful for predicting post-RP outcomes. Although PSADT may be biologically more accurate and stronger on multivariate analysis, PSAV is clinically easier to use and a good approximation in the short term. Preoperative PSADT and PSAV are associated with clinical and pathological indicators of prostate cancer aggressiveness but they are independent predictors of cancer progression and death |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Li, 2005131 USA Clinical Cancer Research |
Univariate analysis Marker(s): Stat5 activation status (positive for active Stat5 vs negative for active Stat5) Analysis methods: Cox regression models were separately fit to progression-free survival data End point: survival from progression [events clinical (bone scan, chest radiography, digital rectal examination) and by increase in PSA (as referenced in Zellweger et al. 125) Multivariate analysis Marker(s): Stat5 activation status (positive for active Stat5 vs negative for active Stat5) Analysis methods: multivariate Cox regression models were separately fit to progression-free survival data End point: survival from progression [events clinical (bone scan, chest radiography, digital rectal examination) and by increase in PSA (as referenced in Zellweger et al. 125) Model used: multivariate Cox regression models Classical clinical markers included: none Classical pathological markers included: Gleason grade, stage Factors (prognostic markers) in final model? Pathological stage, Gleason grade, perineural invasion, seminal vesicle infiltration |
Univariate analysis Measure: regression coefficient Result: 0.4884 (SE 0.256); extrapolated from survival curve, 5-year survival: positive for active Stat5 80%, negative for active Stat5 88%; CI not applicable; p-value: 0.0399 Multivariate analysis Measure: Cox proportional hazards, Stat5 positive with reference negative: HR Result: 1.630; CI: 0.99–2.69; p-value: 0.0565 |
Active Stat5 distinguished prostate cancer patients whose disease was likely to progress earlier. Active Stat5 may be a useful marker for selection of more individualised treatment |
| Study | Analysis methods | Results | Conclusions |
|---|---|---|---|
|
Blute, 2001105 USA Journal of Urology |
Univariate analysis Marker(s): maximum tumour dimension (mm) was not used in a multivariate analysis. Reasons for this exclusion are unclear Analysis methods: Cox proportional hazards End point: biochemical progression-free survival (events – local recurrence or systemic progression or biochemical recurrence defined as PSA ≥ 0.4 ng/ml) Model used: Cox regression analyses |
Univariate analysis Measure: survival Result: 5-year survival; maximum tumour dimension: < 1.5 mm 86% (SE = 1.9), 1.5–2.4 mm 82% (SE = 1.5), 2.5–3.0 mm 79% (SE = 2.5), ≥ 3.0 mm 68% (SE = 1.7); CI not applicable; p-value: 0.001 Multivariate analysis Not reported |
No conclusions are made regarding the prognostic significance of maximum tumour dimension |
|
Lieber, 1995106 USA Cancer |
Univariate analysis Marker(s): tumour volume cm3 (> 1 compared to ≤ 1) Analysis methods: Cox proportional hazards and log-rank test of differences between survival curves End point: (a) survival from progression [events – disease progression based on clinical examination (not routine PSA measurements; censoring at last follow-up for patients who had not had progression or died)]; (b) survival from death from prostate cancer, ‘cause-specific survival’ (events – death from prostate cancer only; censoring at last follow-up for patients who had not had progression or who had died); (c) overall survival (events – death from any cause; censoring at last follow-up for patients who had not had progression or who had died) Multivariate analysis Not reported |
Univariate analysis (a) Measure: HR for tumour volume > 1 cm3 (with reference tumour volume ≤ 1 cm3) Events: tumour volume ≤ 1 cm3 64; tumour volume > 1 cm3 106 Result: HR: 1.691; χ2 = 11.24; CI: 1.239–1.486; p-value: log-rank = 0.0008 (b) Measure: HR for tumour volume > 1 cm3 (with reference tumour volume ≤ 1 cm3) Events: tumour volume ≤ 1 cm3 23; tumour volume > 1 cm3 48 Result: HR: 1.891; χ2 = 6.52; CI: 1.150–3.111; p-value: log-rank = 0.0107 (c) Measure: HR for tumour volume > 1 cm3 (with reference tumour volume ≤ 1 cm3) Events: tumour volume ≤ 1 cm3 77; tumour volume > 1 cm3 96 Result: HR: 1.10; χ2 = 0.45; CI: 0.821–1.497; p-value: log-rank = 0.5026 Multivariate analysis Not reported |
Tumour volume was statistically significant in two of the univariate analyses: those with clinical progression and cause-specific survival as end points. It was noted that the tumour volume was estimated by three-dimensional measurements of cut specimens. PSA was not available |
|
Salomon, 2003132 France European Urology |
Univariate analysis Marker(s): tumour volume End point: survival from biochemical recurrence (events – single PSA level > 0.2 ng/ml) Multivariate analysis Marker(s): tumour volume Analysis methods: multivariate analysis using stepwise logistic regression was performed to identify parameters with additional prognostic value End point: survival from biochemical recurrence (events – single PSA level > 0.2 ng/ml) Model used: multivariate stepwise logistic regression Classical clinical markers included: none Classical pathological markers included: Gleason score, stage, surgical margins Factors (prognostic markers) in final model? Pathological stage, Gleason score, surgical margins |
Univariate analysis Measure: tumour volume (Fisher’s test) Result: not reported; CI not applicable; p-value: 0.009 Multivariate analysis Measure: odds ratio (note: it was unclear but possibly analysed as continuous variable) Result: 1.09; CI: 0.9–1.31; p-value: 0.35 |
Gleason score and pathological stage are independent factors that predict prostate cancer progression after RP. When these parameters are known, tumour volume does not provide additional information |
|
Sengupta, 2005130 USA Journal of Urology |
Univariate analysis Marker(s): maximum cancer dimension [for end points (a), (b) and (c)]; estimated cancer volume [for end points (d), (e) and (f)] Analysis methods: Cox proportional hazards End points: (a) survival from biochemical progression (PSA 0.4 ng/ml or greater; patients without progression censored at time of last PSA determination); (b) survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa); (c) survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes); (d) survival from biochemical progression (PSA 0.4 ng/ml or greater; patients without progression censored at time of last PSA determination); (e) survival from clinical progression (demonstrable disease on radionuclide bone scintigraphy or histological examination of biopsy material from enlarged lymph nodes or the prostatic fossa); (f) survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) Multivariate analysis Marker(s): maximum cancer dimension [for end point (a)]; estimated cancer volume [for end points (b), (c)] Analysis methods: stepwise analysis End point: (a) all above outcomes: survival from biochemical progression; survival from clinical progression; survival from death from prostate cancer; (b) survival from clinical progression (PSA 0.4 ng/ml or greater; patients without progression censored at time of last PSA determination); (c) survival from death from prostate cancer (events – death from prostate cancer; censored at last follow-up if alive or died of other causes) Model used: multivariate stepwise logistic regression Classical clinical markers included: Gleason score, PSA Classical pathological markers included: pathological stage, surgical margins Factors (prognostic markers) in final model? Pathological stage, Gleason score, surgical margins, treatment year, preoperative PSA, preoperative PSADT, preoperative PSAV, seminal vesicle involvement, lymph node involvement, adjuvant therapy |
Univariate analysis (a) Measure: HR Result: 1.19; CI: 1.15–1.23; p-value: < 0.0001 (b) Measure: HR Result: 1.24; CI: 1.17–1.30; p-value: < 0.0001 (c) Measure: HR Result: 1.28; CI: 1.18–1.39; p-value: < 0.0001 (d) Measure: HR Result: 1.05; CI: 1.04–1.06; p-value: < 0.0001 (e) Measure: HR Result: 1.06; CI: 1.04–1.07; p-value: < 0.0001 (f) Measure: HR Result: 1.07; CI: 1.06–1.09; p-value: < 0.0001 Multivariate analysis (a) Measure: HR Result: not significant (removed by forward selection if p > 0.10); CI not reported; p-value: not reported (b) Measure: HR Result: 1.03; CI: 1.01–1.05; p-value: 0.0008 (c) Measure: HR Result: 1.05; CI: 1.02–1.08; p-value: 0.003 |
The study reported analyses of tumour volume (as continuous measure) and maximum tumour dimension (as continuous measure) with different end points: PSA recurrence, clinical recurrence, prostate cancer death and all deaths. All analyses of tumour volume were significant on univariate analysis. The study did not find this marker to be a significant predictor in an analysis with biochemical recurrence as the end point but did find it a significant predictor of clinical progression and prostate cancer death. It should be noted that PSA was not included in the multivariate analysis |
|
Vis, 2007124 The Netherlands European Urology |
Univariate analysis Marker(s): length (mm) of tumour (as continuous variable) [end points (a) and (b)]; tumour volume [end point (c)] Analysis methods: Cox proportional hazards End points: (a) biochemical recurrence (PSA ≥ 0.1 ng/ml); (b) clinical progression (local progression and/or distant metastases); (c) biochemical recurrence (PSA ≥ 0.1 ng/ml after RP) Multivariate analysis Marker(s): length (mm) of tumour (as continuous variable) [end points (a) and (b)]; tumour volume [end point (c)] End point: (a) biochemical recurrence (PSA ≥ 0.1 ng/ml); (b) clinical progression (local progression and/or distant metastases); (c) biochemical recurrence (PSA ≥ 0.1 ng/ml after RP) Model used: Cox proportional hazards model Classical clinical markers included: stage, Gleason score, PSA Classical pathological markers included: none Factors (prognostic markers) in final model? Clinical stage, Gleason score, PSA, number of positive biopsy cores |
Univariate analysis (a) Measure: length (mm) of tumour: HR Result: 1.055; CI not reported; p-value: 0.001 (b) Measure: length (mm) of tumour: HR Result: 1.037; CI not reported; p-value: 0.098 (c) Measure: tunour volume: HR Result: 1.401; CI not reported; p-value: < 0.001 Multivariate analysis (a) Measure: length (mm) of tumour: HR Result: 1.012; CI not reported; p-value: 0.04 (b) Measure: length (mm) of tumour: HR Result: not significant; CI not reported; p-value not reported (c) Measure: tumour volume: HR Result: not significant; CI not reported; p-value not reportedMultivariate analysis (a) Measure: length (mm) of tumour: HR Result: 1.012; CI not reported; p-value: 0.04 (b) Measure: length (mm) of tumour: HR Result: not significant; CI not reported; p-value not reported (c) Measure: tumour volume: HR Result: not significant; CI not reported; p-value not reported |
Amount of high-grade cancer in diagnostic biopsy proved to be a independent and stronger prognostic factor for relapse after RP than Gleason score |
Appendix 7 Sample characteristics of included novel marker studies
Summary of included novel marker studies (n = 28)
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 28 | 921.18 | 1076.90 |
| Median age (years) | 10 | 65.30 | 1.54 |
| Mean age (years) | 16 | 64.17 | 3.47 |
| Median follow-up (months) | 18 | 75.63 | 15.63 |
| Mean follow-up (months) | 9 | 70.06 | 9.93 |
| Mean length of study (years) | 27 | 11.67 | 6.08 |
| Clinically organ confined (%) | 27 | 81.64 | 31.22 |
| Clinically non-organ confined (%) | 27 | 18.29 | 31.22 |
| Pathologically organ confined (%) | 15 | 65.16 | 16.90 |
| Pathologically non-organ confined (%) | 15 | 34.03 | 17.35 |
| PSA level taken from median (ng/ml) | 9 | 7.19 | 1.75 |
| PSA level taken from mean (ng/ml) | 6 | 8.43 | 4.43 |
| Positive surgical margins (%) | 14 | 29.71 | 15.85 |
| Positive lymph nodes (%) | 14 | 4.89 | 3.89 |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 1 | 232.00 | NS |
| Median age (years) | 0 | NS | NS |
| Mean age (years) | 1 | 63.00 | NS |
| Median follow-up (months) | 1 | 78.00 | NS |
| Mean follow-up (months) | 0 | NS | NS |
| Mean length of study (years) | 0 | NS | NS |
| Clinically organ confined (%) | 1 | 100.00 | NS |
| Clinically non-organ confined (%) | 1 | 0.00 | NS |
| Pathologically organ confined (%) | 1 | 47.00 | NS |
| Pathologically non-organ confined (%) | 1 | 53.00 | NS |
| PSA level taken from median (ng/ml) | 1 | 10.10 | NS |
| PSA level taken from mean PSA (ng/ml) | 0 | NS | NS |
| Positive surgical margins (%) | 1 | 53.00 | NS |
| Positive lymph nodes (%) | 1 | 2.20 | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 5 | 895.20 | 646.12 |
| Median age (years) | 2 | 66.00 | 2.83 |
| Mean age (years) | 2 | 61.70 | 4.67 |
| Median follow-up (months) | 3 | 66.33 | 1.53 |
| Mean follow-up (months) | 3 | 78.00 | 7.00 |
| Mean length of study (years) | 5 | 16.80 | 3.27 |
| Clinically organ confined (%) | 5 | 52.95 | 42.43 |
| Clinically non-organ confined (%) | 5 | 47.05 | 42.43 |
| Pathologically organ confined (%) | 1 | 57.00 | NS |
| Pathologically non-organ confined (%) | 1 | 43.00 | NS |
| PSA level taken from median (ng/ml) | 0 | NS | NS |
| PSA level taken from mean PSA (ng/ml) | 0 | NS | NS |
| Positive surgical margins (%) | 1 | 37.00 | NS |
| Positive lymph nodes (%) | 4 | 5.23 | 3.70 |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 2 | 514.50 | 277.89 |
| Median age (years) | 0 | NS | NS |
| Mean age (years) | 1 | 62.90 | NS |
| Median follow-up (months) | 0 | NS | NS |
| Mean follow-up (months) | 1 | 61.80 | NS |
| Mean length of study (years) | 2 | 6.00 | 1.41 |
| Clinically organ confined (%) | 2 | 71.70 | 40.02 |
| Clinically non-organ confined (%) | 2 | 28.30 | 40.02 |
| Pathologically organ confined (%) | 1 | 45.00 | NS |
| Pathologically non-organ confined (%) | 1 | 55.00 | NS |
| PSA level taken from median (ng/ml) | 0 | NS | NS |
| PSA level taken from mean PSA (ng/ml) | 1 | 11.20 | NS |
| Positive surgical margins (%) | 1 | 23.00 | NS |
| Positive lymph nodes (%) | 1 | 7.00 | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 2 | 480.00 | 100.41 |
| Median age (years) | 2 | 64.00 | 1.41 |
| Mean age (years) | 2 | 63.55 | 0.64 |
| Median follow-up (months) | 1 | 77.00 | NS |
| Mean follow-up (months) | 2 | 72.30 | 16.55 |
| Mean length of study (years) | 2 | 11.50 | 7.78 |
| Clinically organ confined (%) | 2 | 49.50 | 70.00 |
| Clinically non-organ confined (%) | 2 | 50.35 | 70.22 |
| Pathologically organ confined (%) | 1 | 98.30 | NS |
| Pathologically non-organ confined (%) | 1 | 1.70 | NS |
| PSA level taken from median (ng/ml) | 1 | 6.90 | NS |
| PSA level taken from mean PSA (ng/ml) | 1 | 9.90 | NS |
| Positive surgical margins (%) | 1 | 0 | NS |
| Positive lymph nodes (%) | 1 | 0 | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 1 | 737.00 | NS |
| Median age (years) | 0 | NS | NS |
| Mean age (years) | 0 | NS | NS |
| Median follow-up (months) | 0 | NS | NS |
| Mean follow-up (months) | 0 | NS | NS |
| Mean length of study (years) | 1 | 5.00 | NS |
| Clinically organ confined (%) | 1 | 100.00 | NS |
| Clinically non-organ confined (%) | 1 | 0.00 | NS |
| Pathologically organ confined (%) | 1 | 44.00 | NS |
| Pathologically non-organ confined (%) | 1 | 56.00 | NS |
| PSA level taken from median (ng/ml) | 0 | NS | NS |
| PSA level taken from mean PSA (ng/ml) | 0 | NS | NS |
| Positive surgical margins (%) | 1 | 21.00 | NS |
| Positive lymph nodes (%) | 1 | 7.00 | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 3 | 2667.67 | 2573.30 |
| Median age (years) | 1 | 66.00 | NS |
| Mean age (years) | 1 | 63.00 | NS |
| Median follow-up (months) | 1 | 126.00 | NS |
| Mean follow-up (months) | 1 | 66.00 | NS |
| Mean length of study (years) | 3 | 8.33 | 5.51 |
| Clinically organ confined (%) | 3 | 77.00 | 21.66 |
| Clinically non-organ confined (%) | 3 | 23.00 | 21.66 |
| Pathologically organ confined (%) | 2 | 72.00 | 20.08 |
| Pathologically non-organ confined (%) | 2 | 27.20 | 20.08 |
| PSA level taken from median (ng/ml) | 1 | 7.80 | NS |
| PSA level taken from mean PSA (ng/ml) | 0 | NS | NS |
| Positive surgical margins (%) | 2 | 38.90 | 0.14 |
| Positive lymph nodes (%) | 1 | 0.00 | 0.00 |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 1 | 738.00 | NS |
| Median age (years) | 0 | NS | NS |
| Mean age (years) | 0 | NS | NS |
| Median follow-up (months) | 0 | NS | NS |
| Mean follow-up (months) | 0 | NS | NS |
| Mean length of study (years) | 1 | 5.00 | NS |
| Clinically organ confined (%) | 1 | 100.00 | NS |
| Clinically non-organ confined (%) | 1 | 0.00 | NS |
| Pathologically organ confined (%) | 1 | 44.58 | NS |
| Pathologically non-organ confined (%) | 1 | 54.52 | NS |
| PSA level taken from median (ng/ml) | 0 | NS | NS |
| PSA level taken from mean PSA (ng/ml) | 0 | NS | NS |
| Positive surgical margins (%) | 1 | 21.00 | NS |
| Positive lymph nodes (%) | 1 | 9.10 | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 5 | 559.40 | 632.51 |
| Median age (years) | 2 | 66.50 | 0.71 |
| Mean age (years) | 4 | 65.45 | 6.25 |
| Median follow-up (months) | 5 | 76.00 | 11.02 |
| Mean follow-up (months) | 0 | NS | NS |
| Mean length of study (years) | 5 | 11.00 | 3.81 |
| Clinically organ confined (%) | 5 | 94.14 | 7.23 |
| Clinically non-organ confined (%) | 5 | 5.78 | 7.17 |
| Pathologically organ confined (%) | 2 | 58.30 | 1.27 |
| Pathologically non-organ confined (%) | 2 | 41.70 | 1.27 |
| PSA level taken from median (ng/ml) | 4 | 7.23 | 1.52 |
| PSA level taken from mean PSA (ng/ml) | 1 | 0.00 | NS |
| Positive surgical margins (%) | 3 | 34.70 | 24.94 |
| Positive lymph nodes (%) | 1 | 8.00 | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 1 | 551.00 | NS |
| Median age (years) | 1 | 63.60 | NS |
| Mean age (years) | 0 | NS | NS |
| Median follow-up (months) | 1 | 63.00 | NS |
| Mean follow-up (months) | 0 | NS | NS |
| Mean length of study (years) | 1 | 25.00 | NS |
| Clinically organ confined (%) | 1 | 100.00 | NS |
| Clinically non-organ confined (%) | 1 | 0.00 | NS |
| Pathologically organ confined (%) | 1 | 71.90 | NS |
| Pathologically non-organ confined (%) | 1 | 18.50 | NS |
| PSA level taken from median (ng/ml) | 0 | NS | NS |
| PSA level taken from mean PSA (ng/ml) | 0 | NS | NS |
| Positive surgical margins (%) | 0 | NS | NS |
| Positive lymph nodes (%) | 1 | 3.30 | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 6 | 519.50 | 468.55 |
| Median age (years) | 1 | 67.00 | NS |
| Mean age (years) | 4 | 67.26 | 4.99 |
| Median follow-up (months) | 6 | 76.55 | 10.66 |
| Mean follow-up (months) | 1 | 60.50 | NS |
| Mean length of study (years) | 6 | 10.00 | 3.46 |
| Clinically organ confined (%) | 6 | 96.80 | 6.98 |
| Clinically non-organ confined (%) | 6 | 3.18 | 6.94 |
| Pathologically organ confined (%) | 2 | 66.25 | 12.52 |
| Pathologically non-organ confined (%) | 2 | 33.75 | 12.52 |
| PSA level taken from median (ng/ml) | 2 | 7.00 | 2.55 |
| PSA level taken from mean PSA (ng/ml) | 2 | 8.85 | 2.33 |
| Positive surgical margins (%) | 1 | 58.80 | NS |
| Positive lymph nodes (%) | 1 | 0.00 | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 2 | 1692.50 | 8.44.99 |
| Median age (years) | 1 | 65.40 | NS |
| Mean age (years) | 1 | 64.80 | NS |
| Median follow-up (months) | 2 | 72.55 | 17.61 |
| Mean follow-up (months) | 0 | NS | NS |
| Mean length of study (years) | 2 | 11.00 | 2.83 |
| Clinically organ confined (%) | 2 | 97.95 | 2.90 |
| Clinically non-organ confined (%) | 2 | 1.55 | 2.19 |
| Pathologically organ confined (%) | 1 | 78.30 | NS |
| Pathologically non-organ confined (%) | 1 | 21.00 | NS |
| PSA level taken from median (ng/ml) | 2 | 5.50 | 1.70 |
| PSA level taken from mean PSA (ng/ml) | 0 | NA | NS |
| Positive surgical margins (%) | 2 | 27.55 | 7.85 |
| Positive lymph nodes (%) | 1 | 11.00 | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 1 | 357.00 | NS |
| Median age (years) | 1 | 65.00 | NS |
| Mean age (years) | 1 | 64.61 | NS |
| Median follow-up (months) | 1 | 73.00 | NS |
| Mean follow-up (months) | 0 | NS | NS |
| Mean length of study (years) | 1 | 25.00 | NS |
| Clinically organ confined (%) | 0 | NS | NS |
| Clinically non-organ confined (%) | 0 | NS | NS |
| Pathologically organ confined (%) | 1 | 79.50 | NS |
| Pathologically non-organ confined (%) | 1 | 19.70 | NS |
| PSA level taken from median (ng/ml) | 0 | NS | NS |
| PSA level taken from mean PSA (ng/ml) | 0 | NS | NS |
| Positive surgical margins (%) | 0 | NS | NS |
| Positive lymph nodes (%) | 0 | NS | NS |
| Characteristics | n | Mean | SD |
|---|---|---|---|
| Sample size in analysis | 5 | 1053.00 | 1007.85 |
| Median age (years) | 0 | NS | NS |
| Mean age (years) | 4 | 64.20 | 0.91 |
| Median follow-up (months) | 2 | 83.00 | 2.83 |
| Mean follow-up (months) | 2 | 64.80 | 1.70 |
| Mean length of study (years) | 5 | 7.40 | 4.28 |
| Clinically organ confined (%) | 5 | 87.30 | 20.10 |
| Clinically non-organ confined (%) | 5 | 12.50 | 20.21 |
| Pathologically organ confined (%) | 3 | 79.93 | 6.41 |
| Pathologically non-organ confined (%) | 3 | 19.83 | 6.33 |
| PSA level taken from median (ng/ml) | 2 | 5.95 | 1.06 |
| PSA level taken from mean PSA (ng/ml) | 1 | 11.80 | NS |
| Positive surgical margins (%) | 3 | 32.03 | 7.56 |
| Positive lymph nodes (%) | 1 | 0.00 | 0.00 |
Glossary
- Biochemical
- Involves chemical processes in living organisms.
- Biomarker
- Specific biochemical in the body that might help to measure the progress of disease or the effectiveness of treatment.
- Biopsy
- Sampling of tissue from a specific area of the body (e.g. the prostate) to check for abnormalities such as cancer.
- Brachytherapy
- Form of radiation therapy involving radioactive seeds that are implanted within the prostate, which then emit radiation to help destroy the cancer.
- Cancer
- Growth of abnormal cells in the body in an uncontrolled manner.
- Downstaging
- Lowering the clinical stage of prostate cancer before attempted curative treatment (e.g. from stage T3a to stage T2b).
- Early localised prostate cancer
- In the current report this is defined as clinical or pathological stage TI/T2/T3N0M0, or Jewett–Whitmore system stages A, B and C.
- Epidemiology
- Study of the causes, distribution and control of disease in populations.
- Etiology
- Study of factors involved in the development of a disease.
- External beam radiation therapy
- Radiation delivered by a machine directed at the area to be radiated.
- Frozen section
- Technique involving the removal and freezing of tissue, which is cut into thin slices and stained for microscopic examination.
- Gleason grade
- Method of classifying prostate cancer tissue for degree of loss of normal glandular architecture; a grade from 1 to 5 is assigned, with high numbers indicating poor differentiation and therefore more aggressive cancer.
- Gleason score
- Two Gleason grade numbers are added together to produce the Gleason score (e.g. Gleason score of 4 + 3 = 7 means that Gleason grade 4 is the most commonly found type of cell and Gleason grade 3 is the second most commonly found, producing a total Gleason score of 7).
- Grade
- Describes the degree of severity of a cancer.
- Heterogeneous (heterogeneity)
- Composed of a diverse mixture of different kinds or subgroups.
- Hormone therapy
- Use of hormones, hormone analogues and specific surgical techniques to treat a disease.
- Prognosis
- Potential clinical outlook or chance of recovery based on the status and likely course of the disease.
- Progression
- Continuing growth of a cancer.
- Prostate
- Gland surrounding the urethra, located immediately below the bladder in males.
- Prostatectomy
- Surgical procedure to remove part or all of the prostate gland.
- Prostate-specific antigen
- Protein secreted by epithelial cells of the prostate gland; it has been used to identify potential problems in the prostate gland.
- Prostate-specific antigen doubling time
- Calculation of the time taken for the prostate-specific antigen value to double using at least three values separated by at least 3 months each.
- Prostate-specific antigen velocity
- Calculation of the rate of increase in prostate-specific antigen levels in succeeding prostate-specific antigen tests.
- Radiation therapy
- Use of X-rays and other types of radiation to destroy malignant tissue and cells.
- Radical prostatectomy
- Surgical procedure to remove the entire prostate gland and seminal vesicles.
- Recurrence
- Reappearance of disease.
- Risk
- Probability or chance that a specific event will or will not happen.
- Stage
- Term used to define the size and physical extent of a cancer.
- Staging
- Process of determining the extent of disease in a patient from all available information. The two staging methods are the Whitmore-Jewett staging classification and the more detailed TNM classification.
- Transurethral resection of the prostate
- Surgical procedure to remove tissue obstructing the urethra.
List of abbreviations
- ACP
- acid phosphatase
- AAM
- African American men
- ASCO
- American Society of Clinical Oncology
- ASTRO
- American Society for Therapeutic Radiology and Oncology
- AUA
- American Urological Association
- BDF(s)
- biochemical disease-free (survival)
- BP
- biochemical progression
- BPH
- benign prostatic hyperplasia
- CAP
- College of American Pathologists
- CCTR
- Cochrane Central Register of Controlled Trials
- CDSR
- Cochrane Database of Systematic Reviews
- CI
- confidence interval
- CINAHL
- Current Index to Nursing and Allied Health Literature
- CP
- clinical progression
- CT
- computerised tomography
- DRE
- digital rectal examination
- EBRT
- external beam radiation therapy
- EPV
- events per variable
- ERSPC
- European Randomised Study of Screening for Prostate Cancer
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- iPSA
- initial prostate-specific antigen
- IMRT
- intensity-modulated conformal radiotherapy
- IUCC
- International Union Against Cancer
- LUTS
- lower urinary tract symptoms
- MRI
- magnetic resonance imaging
- NA
- not applicable
- NHS EED
- NHS Economic Evaluation Database
- NHT
- neoadjuvant hormonal therapy
- NS
- not stated
- OR
- odds ratio
- PAP
- prostatic acid phosphatase
- PCD
- prostate cancer death
- PCLO
- Prostate, Lung, Colorectal, and Ovary Trial
- PCSWG
- Prostate Cancer Specialty Working Group
- PFS
- progression-free survival
- Preop
- preoperative
- ProtecT
- Prostate Testing for Cancer and Treatment
- PSA
- prostate-specific antigen
- PSAV
- prostate-specific antigen velocity
- PSADT
- prostate-specific antigen doubling time
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QUOROM
- Quality of Reporting of Meta-analyses
- RCT
- randomised controlled trial
- RP
- radical prostatectomy
- RR
- relative risk
- RTOG
- Radiation Therapy and Oncology Group
- SCIM-RT
- short-course intensity-modulated radiotherapy
- SE
- standard error
- SG
- standard gamble
- SRT
- standard radiotherapy
- Stat5
- signal transducer and activator of transcription-5
- TNM
- size of the primary tumour, extent of lymph node involvement, presence or absence of metastases
- TRUS
- transrectal ultrasound sonography
- TURP
- transurethral resection of the prostate
- WM
- white men
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
Health Technology Assessment Programme
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NCCHTA
-
Dr Andrew Cook, Consultant Advisor, NCCHTA
-
Dr Peter Davidson, Director of Science Support, NCCHTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NCCHTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NCCHTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NCCHTA
HTA Commissioning Board
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programmes, Research and Development Directorate, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington