Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA Programme as project number 05/08/01. The contractual start date was in May 2007. The draft report began editorial review in December 2007 and was accepted for publication in October 2008. As the funder, by devising a commissioning brief, the HTA Programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NCCHTA, Alpha House, Enterprise Road, Southampton Science Park, Chilworth, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
Chapter 1 Background and main questions
Introduction
Advances in technology within health care should lead us to continually question the most effective methods for investigation, diagnosis, intervention and rehabilitation. Recent advances in imaging techniques raise questions of clinical effectiveness and cost-effectiveness in many areas of health care. This report aims to address some of these questions in the identification of acoustic neuroma. Specifically:
-
What is the place of magnetic resonance (MR) imaging in investigating patients with unilateral hearing loss and/or tinnitus for suspected acoustic neuroma?
-
What is the cost-effectiveness of MR imaging compared with other diagnostic strategies in these patients?
-
What is known about the natural history of acoustic neuroma?
The objectives of the study were to:
-
evaluate the clinical effectiveness and cost-effectiveness of a range of diagnostic strategies for investigating patients with unilateral hearing loss and/or tinnitus with a view to confirming or eliminating a diagnosis of acoustic neuroma
-
describe the natural history of acoustic neuroma
-
synthesise the findings from these two elements of the study to formulate guidelines for clinical practice and proposals for future primary research priorities.
It is important to note that this report does not address issues of treatment options nor outcomes.
Design of the report
This introductory chapter sets the background to the report by summarising the clinical context of acoustic neuroma, providing a historical overview of our knowledge and investigation of the condition, and discussing the issues raised by technological advances. Each of the above questions is then addressed by a systematic review of the evidence in a separate chapter, and each of these chapters begins with an introduction summarising the key background information specifically relevant to that area of study.
Chapter 2 comprises two subsections: a comparison of the use of auditory brainstem responses (ABR) with the use of MR imaging in investigation and a comparison of the use of differing MR imaging protocols in investigation. Chapter 3 comprises two subsections: the costs of different investigative strategies and the modelling of different investigative/diagnostic scenarios. Chapter 4 addresses three areas of natural history in three subsections: epidemiology, including incidence and prevalence, presenting symptoms and growth.
Methods (Chapter 2) and Chapter 3 present detailed reviews of the included papers together with summaries of the evidence. This allows exploration of the methodology of each paper and permits interpretation by the reader.
Chapter 2 (Introduction) and Chapter 4 tabulate the data from papers included in the review and then draw conclusions in summary, thus providing an overview from studies for which individual methodological variation is less crucial for interpretation. All papers included in the review have undergone quality assessment (see Appendix 3).
Finally, Chapter 5 draws conclusions from each of the previous chapters and makes recommendations for further primary research in this area
Acoustic neuroma
Anatomy
Acoustic neuromas are benign, slow-growing tumours which arise from cells in the sheaths that surround the VIIIth cranial nerve (the vestibulocochlear nerve), which carries information from the organs of hearing and balance to their relay stations in the mid-brain.
The vestibulocochlear nerve measures about 2 cm from its root entry zone at the brainstem to the peripheral end organ, and runs within the bony internal auditory canal (IAC) accompanied by the cochlear and facial nerves. A transition zone (called the Obersteiner–Redlich zone) exists on the nerve where the covering myelin changes from a central to a peripheral type, and it is here that the tumours are thought to originate. Schwann cells proliferate, causing compression of adjacent axons, resulting in the formation of a schwannoma in the vestibular (balance) portion of the nerve. Thus, anatomically and pathologically, acoustic neuromas are more correctly called vestibular schwannomas. However, to make this report accessible to all, including many who may only be aware of the more common usage of acoustic neuroma, we have used this term throughout this report.
The vestibular portion of the nerve has a superior and inferior division and the origin of an acoustic neuroma can now be identified by MR imaging (superior division,1 inferior division2). The proportion of tumours arising from each of the two divisions has previously been reported to be 50:50. 3,4 More recently, Jacob and colleagues5 reported a series of 359 patients from Ohio, USA, with unilateral acoustic neuroma undergoing surgical removal. Results from patients in a ‘watch, wait and rescan’ regime were not reported. It was found that the inferior vestibular nerve (IVN) was the nerve of origin in 84 of 359 cases (23.3%), whereas the superior vestibular nerve (SVN) was the nerve of origin in 36 patients (10%); in 239 of 359 cases (66.6%) the nerve of origin was not identified.
Prevalence and incidence
In the early 1990s acoustic neuroma was shown to represent 6% of all intracranial tumours, with approximately 10 cases per million population being diagnosed annually in the developed world. 6 Similarly, the incidence of acoustic neuromas – the number of newly diagnosed cases per year – was reported to be 13 cases per million population per year by Moffat and colleagues. 7 More recently, Tos and colleagues8 have shown that incidence figures in Denmark have increased over the last 20 years from 7.8 to 17.4 cases per million population per year. The size of tumours diagnosed has decreased from a median of 35 mm in 1979 to 10 mm in 2001 but the median age at diagnosis has remained unchanged at 55 years. 9 Thus, these data are probably a reflection of better diagnostic methods rather than a true increase in neuroma incidence. A review and synthesis of the relevant literature is presented in Chapter 4 and provides evidence of the changing natural history that will contribute to decisions on future strategies for identification.
Age at onset
Patients with unilateral acoustic neuromas most commonly present in their 40s and 50s but the reason for this late onset remains unknown. Research suggests that the primary event in the causation of all acoustic neuromas may be dysfunction of the neurofibromatosis type 2 (NF2) gene on chromosome 22, the product of which is a suppressor protein (merlin) that primarily regulates cell division. Without this protein uncontrolled Schwann cell proliferation takes place. 10 This should not be confused with the inherited condition of NF2, which is a distinct disease involving bilateral tumours and with an onset much earlier in life. This report addresses issues concerned with the identification of unilateral acoustic neuroma and therefore does not consider cases of NF2 as the pathology, natural history and management are distinctly different from those of the much more common solitary acoustic neuroma.
Because the average age at presentation is typically in the 40s or 50s, patients could expect, on average, to have 15–20 years of working life remaining. Delays in diagnosis could result in some loss of productivity and thus this should be borne in mind when considering alternative diagnostic strategies. 11
Clinical presentation
Not all acoustic neuromas grow. For those that do it is generally considered that they do so through a number of stages that relate to their clinical presentation. Although substantial variability exists in clinical manifestations of the tumour, particular symptoms are more common with particular stages as adjacent structures are compressed:
-
Preclinical stage. An acoustic neuroma of any size may be present but may not cause symptoms or the symptoms may be present but disregarded by the patient. Such tumours may, for instance, be diagnosed when patients are referred for an assessment of their hearing, for example following routine health checks or following referral for a hearing aid. Some may also be diagnosed incidentally as a result of having a brain scan for reasons unrelated to hearing.
-
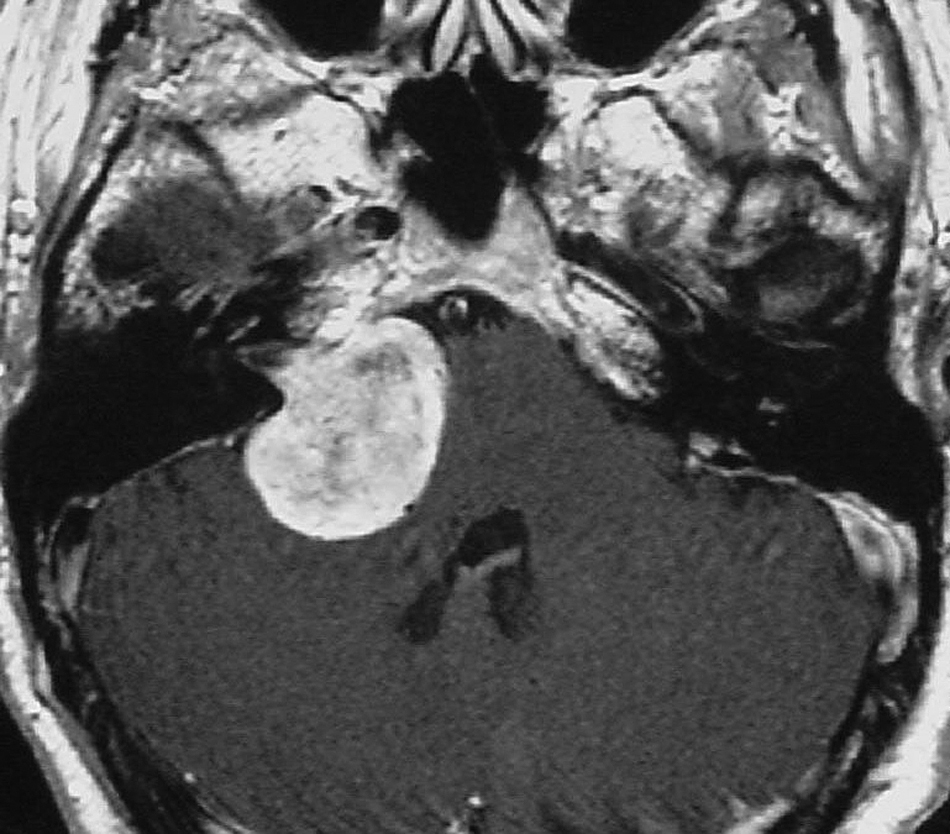
Intracanalicular stage (Figure 1). The tumour is small and confined within the IAC. Symptoms are due to compression of the vestibulocochlear nerve and may be auditory or vestibular, such as asymmetric sensorineural hearing impairment (ASHI), which may be sudden and/or gradually progressive, tinnitus and/or vertigo.
-
Cisternal stage (Figure 2). The tumour extends beyond the limited rigid confines of the IAC into the basal cisterns of the cerebellopontine angle (CPA). Progression to this stage may result in further compression of the vestibulocochlear nerve leading to a worsening of audiovestibular symptoms, while localised dural irritation may cause headache. Patients may also develop disequilibrium and/or other cranial nerves may become involved (e.g. the trigeminal nerve, resulting in a disturbance of facial sensation).
-
Brainstem compressive stage (Figure 3). This is characterised by the additional symptoms and signs of brainstem compression such as headache, visual disturbance, numbness or ataxia.
FIGURE 1.
MR scan of acoustic neuroma at the intracanalicular stage.
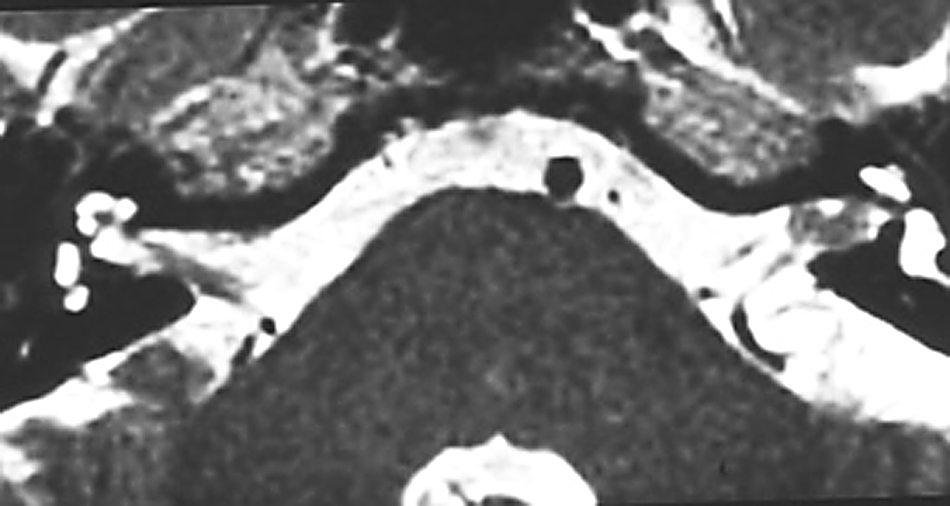
FIGURE 2.
MR scan of acoustic neuroma at the cisternal stage.
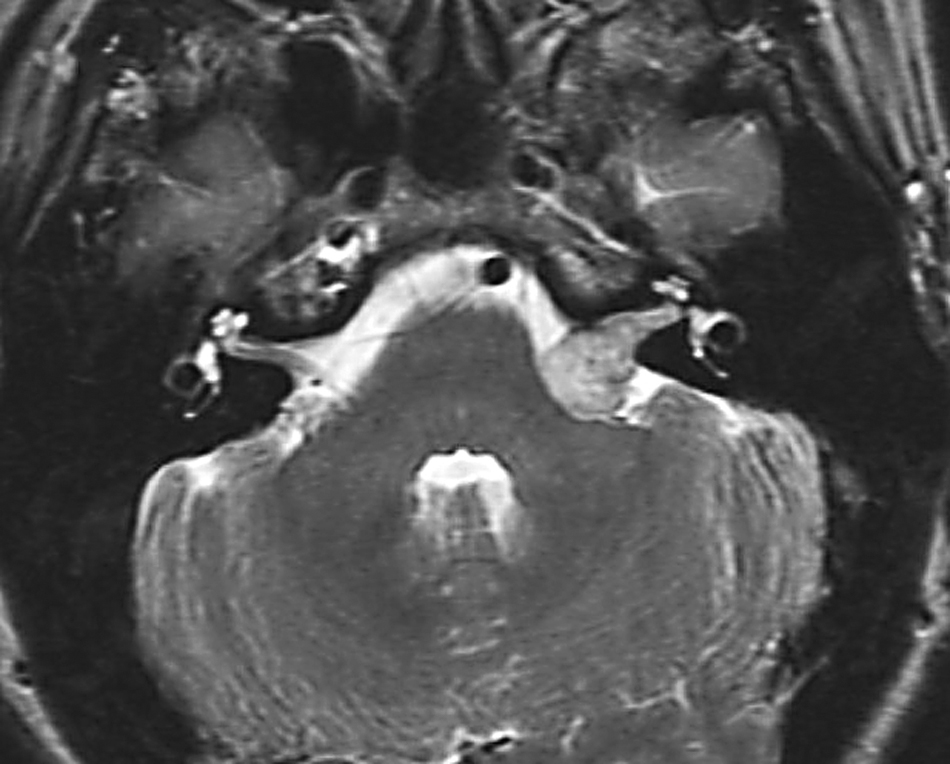
FIGURE 3.
MR scan of acoustic neuroma at the brainstem compressive stage.
The number of patients with asymmetrical hearing symptoms who attend ear, nose and throat (ENT) clinics and are eventually diagnosed with acoustic neuroma ranges from 1% to 7.5%12–14 dependent on the criteria thresholds set. The National Study of Hearing15 showed that 2.9% of the population has an asymmetry greater or equal to 15 dB across 0.5–4.0 kHz. For the high frequencies, 4, 6 and 8 kHz, this prevalence increases to 10.4%. When the better ear has hearing thresholds better than 25 dB, the prevalence values are 5.2% and 10.9% respectively. 15 Thus, the burden of patients in whom the exclusion of an acoustic neuroma is indicated is significant upon individual departments of otolaryngology and upon the health economy.
Many patients with acoustic neuroma experience hearing impairment, tinnitus and imbalance. There are a number of less common symptoms including facial numbness, headaches and otalgia. In some cases there are markedly unusual patterns of symptoms at presentation, and some asymptomatic patients have an acoustic neuroma diagnosed whilst undergoing radiological investigation for unrelated symptoms. Chapter 4 (see Symptoms) summarises the literature on presenting symptoms.
Growth
When growth occurs, acoustic neuromas are typically slow growing. A recent systematic review16 of 26 studies (published up to 2002) including 1340 patients reported a combined mean growth rate of 29–46% dependent on study design, and a mean annual growth rate of 1.2 mm/year. Not all tumours grow. The range in the individual studies reported by Yoshimoto16 was 9–84% (mean growth rate not reported). The mean tumour regression rate is reported as 8%.
The capacity to image even the smallest tumours has introduced management dilemmas. It is known, for instance, that many patients live undisturbed by their tumours, ultimately dying with them but not because of them. Other tumours, however, progress to cause life-threatening neurological symptoms. Therapeutic intervention, by surgery or radiation treatment, is associated with significant morbidity and consequences for quality of life. 17,18 Distinguishing those patients whose tumours pose a threat from those whose tumours may safely be left without intervention remains one of the main therapeutic questions confronting this field.
An update of the evidence on patterns and predictors of growth, including recent studies using modern technology for measurement, is presented in Chapter 4 (see Growth).
Diagnostic strategies
Taking into account the evidence on growth, early diagnosis offers the patient a range of management options and may significantly reduce morbidity. It is therefore important to identify patients who are at risk and use appropriate investigations to identify any tumours early.
The priority should be to confirm that there is no abnormality (rather than to detect abnormality). False positives can be followed up, but false negatives may be lost to follow-up and present late with progressive disease. Early diagnosis is also a priority as treatment of smaller lesions has lower risk. Small lesions may not be detected by ABR and very small lesions may be overlooked by MR imaging, even with the use of gadolinium-enhanced T1-weighted (GdT1W) sequences.
A variety of diagnostic tools and protocols exist for the detection of acoustic neuroma but there are no current guidelines on which is the most clinically effective and cost-effective to use. Clinical effectiveness guidelines for the investigation and management of acoustic neuroma were commissioned by the Clinical Practice Advisory Group of the British Association of Otorhinolaryngologists – Head and Neck Surgeons in 2000. A working party produced a report in 2002. 19 The one recommendation concerned with investigation stated that MR imaging represented the method of choice for identifying acoustic neuroma in patients presenting with unilateral or asymmetric auditory symptoms, but this was not based on a complete and systematic review of the evidence.
There remains no agreement as to which screening protocols are most appropriate for the population presenting with symptoms that might indicate the presence of an acoustic neuroma. The clinical investigations available historically and reported in the literature comprise audiological assessments including pure tone and speech audiometry, electrophysiological tests and diagnostic imaging [either computerised tomography (CT) scanning or MR imaging]. Each of these strategies will be briefly introduced here and then addressed with a review of the evidence in Chapter 2.
The effectiveness of a diagnostic test can be considered in terms of the sensitivity and specificity of the test. 20 The sensitivity of a test refers to the proportion of patients with the target disorder who have a positive test result. The specificity refers to the proportion of patients who do not have the target disorder and who have negative or normal test results. These concepts require a gold standard test (GdT1W MR imaging in the case of acoustic neuroma) and so make the assumption that the gold standard has 100% sensitivity and specificity. In the case of MR imaging and acoustic neuroma this cannot be entirely true: although the sensitivity approximates 100%, there will be some microscopic tumours that are not detectable. Further, some false-positive diagnoses may occur, so that the specificity approximates 100% but will not achieve that figure.
Audiological tests
Patients typically present with one or more audiovestibular symptoms that result in referral to an otolaryngology clinic. If a history and physical examination suggest the possibility of an acoustic neuroma, an audiological examination will be requested. This will generally consist of pure tone and speech recognition audiometry, although different criteria are used by different departments in defining what constitutes an abnormal result warranting further investigation. Historically these tests proved to be of limited value, as they often gave a mixed picture11 and did not in fact diagnose acoustic neuroma; rather the likelihood of this pathology was increased in the clinician’s mind. More recent work has indicated that the mixed picture was the result of the acoustic neuroma potentially causing both cochlear hearing impairment because of ischaemia or biochemical degradation21 and retrocochlear hearing impairment by nerve compression. Recent work has indicated that measures of audiological handicap may have utility in the management of patients with acoustic neuroma. 22 A brief summary of the role of audiological investigation in the identification of acoustic neuroma is presented in Chapter 2 (see Comparison of the use of auditory brainstem response with the use of magnetic resonance imaging).
Electrophysiological tests
Electrodiagnostic recordings of the ABR enable the investigation of the functioning of the peripheral auditory nerve and the auditory brainstem pathways. 23,24 For ABR (or other physiological tests such as caloric testing, speech audiometry, pure tone audiometry) to indicate a retrocochlear lesion that might be tumour, the tumour has to exert some effect (most commonly thought to be physical pressure) on the neurological structures involved. An acoustic neuroma usually has the effect of slowing the speed of propagation of the action potentials as they progress through the ascending auditory pathway or causing dys-synchrony of the potentials. These two mechanisms lead to a delayed latency response or an abolished response respectively. However, it is important to remember that an abolished response can be the result of a severe/profound cochlear impairment as well as the result of an acoustic neuroma. The ABR has been studied extensively over the last 20 years with respect to its diagnostic capability, it’s relationship to other diagnostic tests, its effect on the management of patients,25,26 including comparative roles of ABR and other diagnostic investigations,12,27,28 it’s limitations in the identification of small tumours,29,30 and potential techniques to enhance it’s diagnostic power. 31,32 ABR can also provide additional information such as the degree of brainstem compression by measurement of the ABR on the non-tumour side. 11,33 This may be monitored postoperatively as an index of recovery.
Chapter 2 (see Comparison of the use of ABR with the use of MR imaging) summarises the published data to evaluate the role of ABR with respect to other diagnostic tests in the screening and diagnosis of patients with suspected acoustic neuroma. When evaluating the role of ABR compared with other technologies such as MR imaging, it should not be overlooked that there are some patients for whom MR imaging is not appropriate and for whom some alternative strategy is needed to cater for them. ABR or CT may represent an option for the majority of such patients. 34–36
Imaging tests
MR imaging detects tumours on the basis of abnormal anatomy and is a structural investigation, whereas ABR relies on altered physiology and is a functional investigation.
Imaging has evolved from diagnostic tests that involved clinical risk (CT with cisternography), through non-invasive, low spatial resolution axial examinations (CT plus contrast), to high-resolution MR imaging using three-dimensional T2W or gradient echo sequences and possibly T1W sequences post contrast. Current 1.5- or 3.0-Tesla (T) clinical MR imaging scanners offer high-resolution three-dimensional volume sequences that enable effective screening of the majority of patients without a requirement for T1W sequences post gadolinium (reducing cost). Most units will reserve postcontrast sequences for those cases in which the diagnosis of acoustic neuroma cannot be confidently excluded by a three-dimensional T2W or gradient echo sequence. 37,38
MR imaging with gadolinium (GdT1W) is considered by many as the gold standard diagnostic test for acoustic neuroma,39–44 and is used to evaluate positive findings following the MR screening examination. There are patients for whom MR imaging is inappropriate, such as claustrophobic patients and those with implanted metal. In these patients CT scanning is the alternative imaging test.
One important issue is that of supply and demand. The prevalence of ‘incidental acoustic neuroma’ has been estimated at 2/10,000. 45,46 The incidence of patients presenting to a general ENT clinic with symptoms that might indicate a CPA lesion has been reported as nearly 20%. 47 Even among those exhibiting symptoms of asymmetrical hearing impairment or unilateral tinnitus who have been referred for investigation, as few as 1% might have acoustic neuromas. 13,48,49 Thus, the large number of patients ‘eligible’ for investigation generates waiting list issues in many NHS MR imaging units, and the low incidence coupled with a high relative cost of GdT1W MR imaging (see Chapter 3) have contributed to reservations regarding its use as a first-line (albeit definitive) basis for diagnosis.
Debate therefore surrounds the most efficient MR imaging protocol to use in screening. Most NHS units are selecting sequence protocols that balance scanner/radiology time with waiting list pressures. In some centres excessive waiting list times for MR imaging are influencing clinical decisions and referral patterns, and selected patients are undergoing CT examinations to exclude CPA space-occupying lesions as the first (and possibly only) investigation, particularly in elderly subjects in whom diagnosis of intracanalicular lesions is not a clinical priority. CT cannot exclude small tumours within the IAC but, given the low prevalence of the condition and the reduced cost, this approach may be cost-effective, providing the majority of patients do not go on to MR imaging or to develop tumours.
Other resource savings may be made if medical staff do not directly supervise the screening process. Suitable patients can be ‘batched’, allowing large numbers to be scanned in a given MR imaging session. 50 However, this approach may not be suitable for older generation or lower field (< 1 T) scanners in which spatial resolution is insufficient to clearly define the individual nerves. It may prove necessary to obtain additional contrast-enhanced T1W images for a small percentage of patients with equivocal findings on T2W images or when patient movement leads to an inability to resolve the individual components of the nerve complex. For scans that are medically unsupervised, this requires patients to be recalled. It should be recognised that radiologists reporting these scans will have varying degrees of training and familiarity with imaging this region, and some may have a preference for and greater confidence with reporting contrast-enhanced T1W images.
Chapter 2 (see Comparison of the role of different protocols for MR imaging) summarises the recent evidence on the effectiveness of different MR imaging protocols in the investigation of patients presenting with symptoms that might indicate the presence of an acoustic neuroma.
Cost-effectiveness
Many studies of the various diagnostic tests and protocols existing for the detection of acoustic neuroma have been published. These studies have shown that the level of cost-effectiveness is not simply a question of the technology deployed but also the manner in which it is deployed. Factors that might be considered to influence cost-effectiveness include the nature of the technology, the characteristics of the patients referred for diagnosis, the test protocol and the skill of those using the technology.
A review of the literature, addressing the issues highlighted above, is presented in Chapter 3 (see Cost-effectiveness review); Chapter 3 (see Cost-effectiveness model) also explores these issues further using economic modelling.
Chapter 2 Clinical effectiveness of imaging and non-imaging strategies in the investigation of acoustic neuroma
Introduction
This chapter addresses two specific research questions:
-
In patients with ASHI and/or tinnitus what is the diagnostic accuracy of MR imaging compared with other tests?
-
In patients with ASHI and/or tinnitus what is the diagnostic accuracy of non-contrast-enhanced compared with contrast-enhanced magnetic resonance sequences?
To do so it considers the technologies currently used as the basis for investigative strategies, and reports the findings from a systematic review of the evidence.
First, it considers electrophysiological testing represented by ABR compared with MR imaging in terms of diagnostic accuracy and, second, it reviews the evidence for different protocols based on MR imaging. Although CT may be used as an alternative imaging investigation when MR imaging is contraindicated or unavailable, it falls outside the scope of this review and is not considered further.
Methods
Search strategy
The systematic review was designed to identify evidence that compared the diagnostic accuracy of MR imaging with all relevant comparators and to assess the diagnostic accuracy of different MR imaging strategies. It was not designed to identify studies that compared any two tests that did not include MR imaging.
The search terms used and databases searched to identify articles for possible inclusion in the clinical effectiveness review are listed in Appendix 1.
The flow chart in Figure 11 (Appendix 2) details the number of references found and the exclusion of irrelevant references at each stage of the review process. The initial search yielded 13,887 titles, with 12,732 remaining after exclusion of duplicates. All titles were reviewed by at least two members of the research team and a further 12,366 references were excluded as not relevant to the review. The remaining 366 abstracts together with five titles from the natural history theme that were identified as being relevant to clinical effectiveness were sought and 336 were reviewed by the content experts for the theme (RL, GL, SM); 167 were considered to be not relevant and two additional conference abstracts were identified, resulting in 171 full papers sought. Nine full papers were in a language inaccessible to the research team and a further two were found to be either a duplicate or not relevant, resulting in 160 full papers finally reviewed. A further 104 papers were excluded at this stage for the reasons given in Figure 11, Appendix 2, and one paper was identified as relevant to clinical effectiveness from the natural history search, resulting in 57 papers being retained. Finally, 25 further papers were excluded at the data extraction stage because of relevant data being insufficiently clear for extraction or analysis, leaving a total of 32 papers in the review reporting data from 27 studies. The search was updated to cover the period October 2006 to August 2008. No additional papers were identified that contributed data to the review.
Quality assessment
Papers were assessed for quality using the QUADAS (quality assessment of diagnostic accuracy studies) tool. Details of the questions used in the assessment and the score for each paper can be found in Tables 26 and 27 (Appendix 3).
Inclusion criteria
Papers were included if they met the following criteria:
-
presented data only on adults over 16 years of age (or included only a few patients under 16 years of age)
-
provided a case definition of at least one of the following: unilateral sensorineural hearing impairment (SNHI), ASHI, unilateral tinnitus
-
compared an investigative test with MR imaging
-
reported sensitivity/specificity of the diagnostic test or provided data from which these could be calculated
-
contained adequate data for extraction.
In addition, papers were excluded if they met the following criteria:
-
patients with NF2 could not be excluded
-
published before 1990.
Data extraction
The following data were extracted from each paper:
-
author(s) and year of publication
-
the country in which the study was undertaken
-
the study design – retrospective, prospective
-
dates of the study
-
source of patients
-
number of patients, age and sex distribution
-
number followed up
-
comparator for MR imaging
-
diagnostic cut-off for MR imaging
-
diagnostic cut-off for comparator
-
additional notes on text protocol
-
number of patients presenting with audiological symptoms including unilateral or asymmetric hearing impairment, sudden hearing impairment, tinnitus, vestibular/balance symptoms, other symptoms
-
symptom duration
-
location of acoustic neuroma
-
size of acoustic neuroma at diagnosis/entry to the study (mean, median, range, etc.)
-
data to populate 2 × 2 table, number present or absent on MR imaging, number present or absent on comparator test
-
sensitivity (if quoted or calculable)
-
specificity (if quoted or calculable)
-
any important additional information or comments.
Analyses
Data concerning the reference standard and test accuracy measures (true positives, true negatives, false positives and false negatives) were initially extracted into a spreadsheet. When possible we sought results reported according to the denominator of the number of patients; however, some studies only reported their test results using the number of ears tested as the denominator, so these data were used instead.
Results are presented and analysed separately for studies according to the reference test [i.e. ABR or different MR imaging protocols – fast spin echo (FSE) or gradient echo (GE) studies]. Sensitivities and specificities across studies are shown as Forest plots and statistical heterogeneity assessed using both the χ2 and I2 statistics. 51 To take into account the low power of the χ2 test the cut-offs of p < 0.1 and I2 ≤ 50% were used to indicate that the studies were considered sufficiently homogenous to proceed with pooling to derive an overall summary estimate of the test accuracy. Pooled sensitivities and specificities were calculated according to the Der Simonian–Laird random effects model. 51 Analyses were conducted using the Meta-DiSc software. 52
Comparison of the use of auditory brainstem response with the use of magnetic resonance imaging
Introduction
The ABR has been employed extensively in the past to assist with the differential diagnosis of cochlear and retrocochlear pathology, and specifically the identification of acoustic neuroma. This is demonstrated by the plethora of papers published in the 1980s and 1990s that examine the performance of the ABR.
Diagnostic criteria of the ABR waveform
The various peaks and troughs of the ABR waveform originate from the auditory nerve and progressively higher nuclei and tracts within the brainstem as reviewed by Moller. 23 Using a click stimulus results in a sequence of components labelled as waves I–VII, where waves I, III and V are the most robust and are employed as the main diagnostic components of the response. The origins of the components of the click-evoked ABR are as follows (Figure 4):
-
wave I – the distal auditory nerve close to the cochlea
-
wave II – the proximal auditory nerve as it approaches the cochlear nucleus
-
wave III – the cochlear nucleus
-
wave IV – the superior olivary complex
-
wave V – the lateral lemniscus
-
waves VI and VII – the inferior colliculus.
FIGURE 4.
Generators of the auditory brainstem response (ABR) and the ABR waves. (a) A schematic drawing of the presumed generators of the ABR. BIC, brachium of the inferior colliculus; CIC, Commisure of the inferior colliculus; CN, cochlear nucleus; CP, Commisure of the Probst; GC, Gudden’s commisure; IC, inferior colliculus; LL, lateral lemniscus; MGB, medial geniculate body; NLL, nucleus of the lateral lemniscus; RE, in reference to; SG, spiral ganglion; SOC, superior olivary complex; TB, trapezoid body. (b) ABR showing presumed anatomic correlations of major peaks. Note that one anatomic structure may give rise to more than one ABR wave and, conversely, more than one anatomic structure may contribute to a single ABR wave. From James W. Hall. New Handbook For Auditory Evoked Responses, 1/e. Published by Allyn and Bacon/Merrill Education, Boston, MA. Copyright © 2007 by Pearson Education. Reprinted by permission of the publisher.
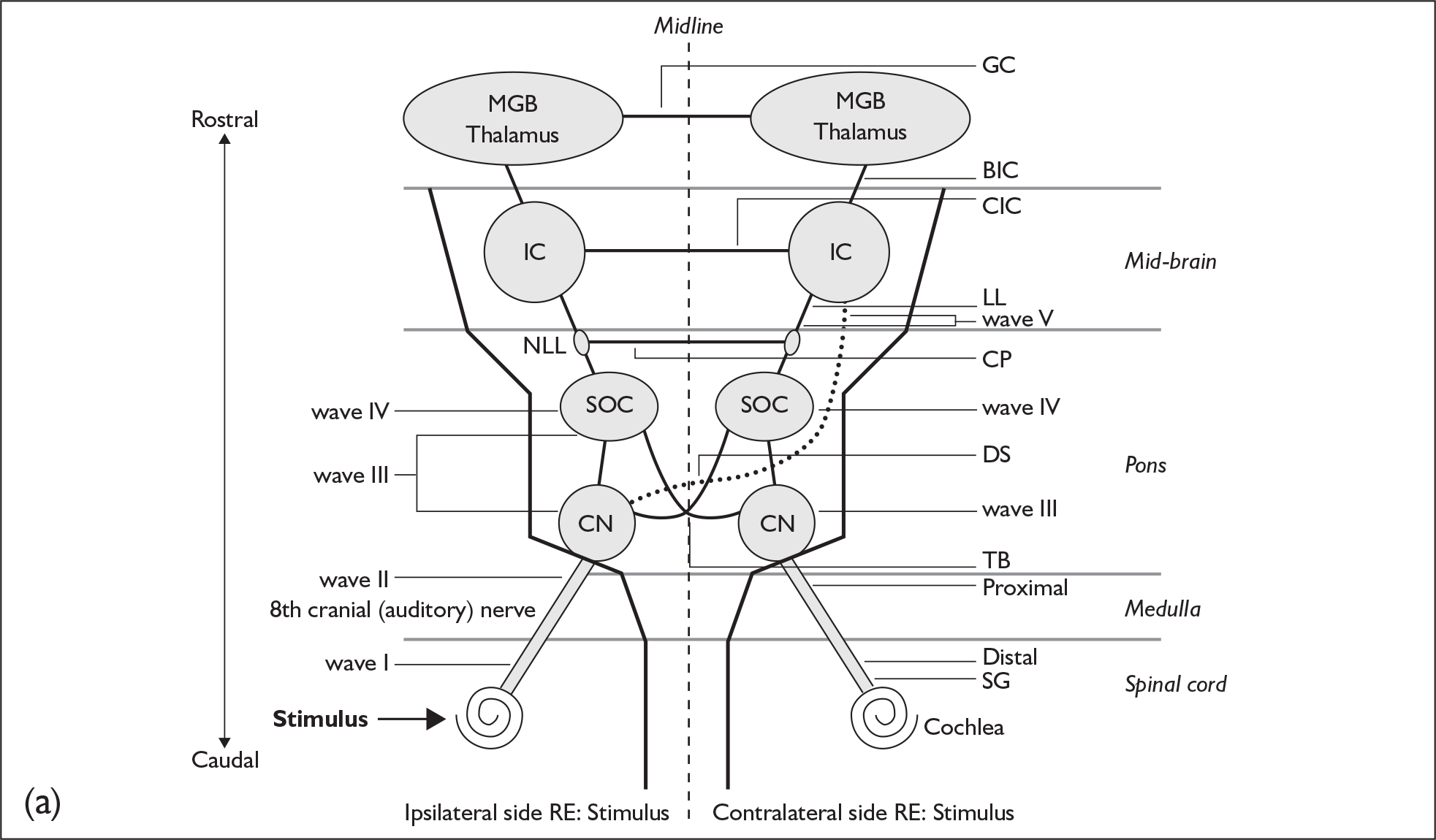
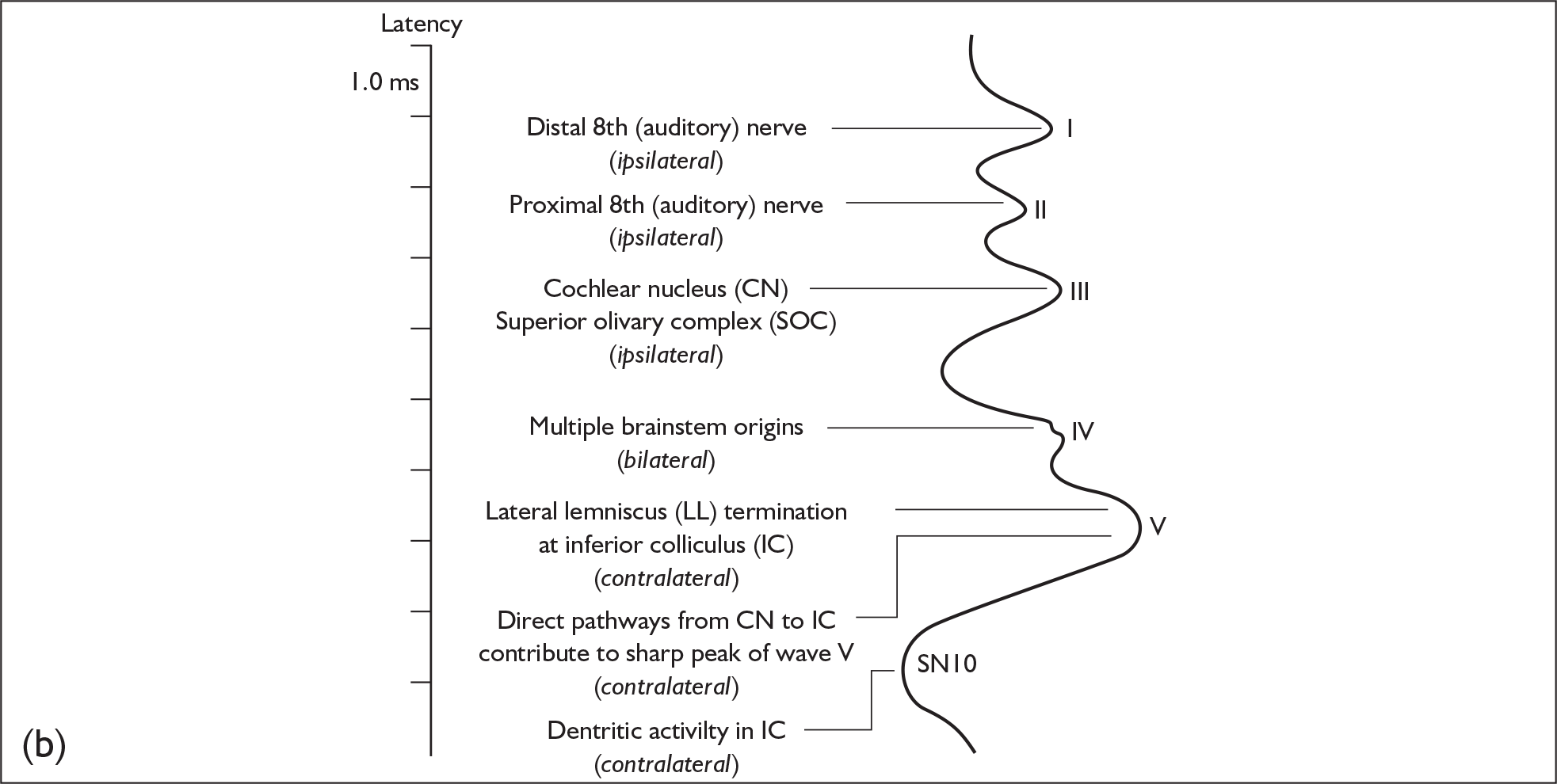
The location of acoustic neuroma usually involves regions of the auditory nerve beyond the generator site for wave I. The normality of components originating more proximally than wave I is therefore compromised in cases of acoustic neuroma. For this reason the waves I–III and waves I–V interpeak intervals are the main diagnostic indicators of normality. The wave V component is the single most robust component of the ABR. This has resulted in the waves I–V component being the gold standard measurement and the one referred to most extensively in the literature. In some recordings the identification of wave I can be problematic because of the level of hearing impairment, and in these cases some reports refer to the absolute wave V latency. Correction of wave V latency with respect to hearing impairment can be applied. 54 As acoustic neuroma are predominantly unilateral, the interaural differences in the waves I–V latency interval and absolute wave V latency are important, as the ‘normal’ side can be used as a control for the suspect side. A summary of the important diagnostic criteria is as follows:
-
monaural waves I–III and waves I–V interpeak latency intervals
-
interaural difference of the waves I–V interpeak interval
-
absolute latency of wave V with correction for hearing impairment
-
interaural difference of wave V latencies (ILDV or IT5), corrected for hearing impairment.
In addition to the above criteria the total absence of a recognisable ABR is sometimes seen in acoustic neuroma. However, the ABR may also be absent in cases of severe/profound cochlear hearing impairment (especially at high frequencies) and so the audiometric status of the patient must be known to interpret the clinical significance of an absent ABR. If the patient’s hearing threshold is greater than 75 dBHL (decibel hearing level) at 4 kHz, for example, an absent ABR is non-diagnostic; as milder hearing thresholds are considered, an absent ABR becomes increasingly suggestive of neuropathy.
It is important to note that the characteristics of the ABR are also sometimes affected by a range of different pathologies (e.g. multiple sclerosis, other space-occupying tumours of the brainstem) in addition to acoustic neuroma. Interpretation of the waveform must take this into account in more complex cases.
Interpretive strategy
It is common practice to define a test’s performance in terms of sensitivity, the rate of true positives identified, and specificity, the rate of true negatives identified. However, these two measures do not carry equal weight, as the consequences of a false-negative result (a tumour that is present but that is missed) are more serious than those of a false-positive result (identification of a tumour leading to further testing, with no tumour being present).
Although the performance of individual ABR measurements may be known, patients are often judged using a combination of ABR measurements that are imperfectly correlated. There have been very few attempts to construct and evaluate efficient ABR protocols using a number of measurements, and the true specificity of most ABR protocols is likely to be somewhat worse than clinicians expect. 55
Study groups
Reports on the performance of the ABR in identification of acoustic neuroma cases are almost exclusively retrospective studies. Patients within a study group will have had ABR investigation previously and will have subsequently received confirmation of the presence of an acoustic neuroma or not using imaging techniques and/or during surgery. In earlier ABR reports the imaging confirmation will be based on CT scanning or early developments of MR imaging technology and procedures. This should be considered when interpreting the results on the diagnostic performance of the ABR presented by the papers included in this review.
Results
Sixteen papers met the inclusion criteria for the review of the use of ABR in the investigation of acoustic neuroma.
Table 1 lists these studies together with the demographic details of the participants. The majority of studies were retrospective reviews of patients with proven acoustic neuroma and therefore only allowed quantification of true-positive and false-negative rates and sensitivity.
| Study | Country | Date of study | n | Age of participants (years) |
|---|---|---|---|---|
| Weiss, 199056 | USA | 1983–8 | 750 | NR |
| Levine, 199157 | USA | 1986–9 | 27 | By size: ≥ 10 mm: 47.6 ± 10.4; < 10 mm + ABR: 47.6 ± 13.3; < 10 mm – ABR: 47.0 ± 9.5 |
| Wilson et al., 199258 | USA | NR | 51 | NR |
| Selesnick and Jackler, 199359 | USA | 1986–90 | 126 but only 35 had ABR | 50 (n = 126). By size: < 10 mm: 54; 10–30 mm: 51; > 30 mm: 43 |
| Gordon, 199560 | USA | NR | 105 | NR |
| Chandrasekhar et al., 199561 | USA | 1988–93 | 197 (4 = NF2) | 48 (SD 12.5) (13–78) |
| Zappia et al., 199762 | USA | 1988–96 | 111 | 47 (18–68) |
| Godey et al., 199863 | France | 1989–95 | 89 | NR |
| El-Kashlan et al., 200029 | USA | 1988–97 | 25 | NR |
| Haapaniemi et al., 200064 | Finland | 1992–7 | 41 | 55 |
| Robinette et al., 200065 | USA | NR | 75 | NR |
| Marangos, 200166 | Germany | 1986 to NR | 261 | NR |
| Schmidt et al., 200130 | USA | NR | 58 | 52 |
| Rupa et al., 200367 | India | NR | 90 | 15–66 |
| Skinner et al., 200368 | Italy | 1988–2001 | 426 | 48.6 (15–79) |
| Cueva, 200469 | USA | NR | 312 | 53.9 (18–87) |
Tables 2 and 3 summarise the diagnostic performance of ABR testing in the investigation of patients suspected of having an acoustic neuroma. We have included the available details of the hearing status of subjects, as severe hearing impairment has a profound effect on the ability of the ABR to produce clinically useful results. The inconsistency of this issue across studies makes the comparison of reported ABR sensitivities problematic.
| Study | n | Study population | Tumour size | Audiometric details |
|---|---|---|---|---|
| Weiss, 199056 | 750 | Patients referred for ABR testing | NR | Impairments > 75 dB excluded because no ABR expected |
| Levine, 199157 | 27 | Series of patients with AN excluding those with other CPA tumours, previously treated AN or incomplete documentation | < 10 mm: n = 8 (29.6%), mean 7.3 mm ± 1.4; ≥ 10 mm: n = 19 (70.4%), mean 18.2 mm ± 10.2 | No mention of hearing impairment being used in the interpretation of ABR. Absent ABR may have been a positive result even if loss was marked |
| Normal ABR: n = 3, mean 4.0 mm ± 1.0 mm | ||||
| Wilson et al., 199258 | 51 | Consecutive series of patients with surgically confirmed AN | Normal ABR: 3–13 mm. | 11 of 51 insufficient hearing (not defined) to obtain ABR |
| Selesnick et al., 199359 | 35 | Patients newly diagnosed with AN with ABR results and sufficient clinical documentation | Mean 21 mm (range 3–55 mm); < 10 mm (mean 8 mm); 10–30 mm (mean 21 mm); > 30 mm (mean 41 mm) | No discussion of impairment related to ABR. Absent ABR considered abnormal regardless of loss |
| Gordon, 199560 | 105 | Random patients with confirmed AN | ≤ 9 mm (including intracanalicular): n = 13 (12.4%); 10–15 mm: n = 45 (42.9%); 16–20 mm: n = 29 (27.6%); 21–24 mm: n = 6 (5.7%); ≥ 25 mm: n = 12 (11.4%) | 100/105 had SRT < 50 dB. Absent ABR taken as a positive result |
| Chandrasekhar et al., 199561 | 197 | Patients with confirmed AN with both MR imaging and ABR results | Mean: interaural difference (IT5) normal: 11.5 mm; IT5 not normal or no response: 18.9 mm; waveform normal: 11.3 mm; waveform abnormal: 23.1 mm; not recorded: 19.1 mm | Mean PTA (three frequencies, not stated): 30 dB (SD 20) (range 0 – no response) |
| Zappia et al., 199762 | 111 | Patients with surgically confirmed AN with MR imaging and ABR test results | Mean: 16.5 mm (range 4–50 mm) | ABR absent (positive result) in 36/111 (32%). Relation to hearing impairment not stated |
| Godey et al., 199863 | 89 | Patients undergoing surgery for AN with ABR results | Extracanalicular: with normal ABR: 15 mm (maximum 18 mm); with abnormal ABR: 26 mm | Further 13 patients in whom ABR not measured if impairment > 75 dB (n = 11) or ABR unreadable (n = 2) |
| El-Kashlan et al., 200029 | 25 | Patients with surgically confirmed AN of < 10 mm (25/252 assessed) | Mean: 8.2 mm ± 2.3 mm (range 3–10 mm) | PTA (0.5, 1, 2 kHz): 22.9 dB ± 12.7; SRT: 19.2 dB ± 12.8: SDS: 75.4% ± 22.3. Absent ABR taken as a positive result |
| Haapaniemi et al., 200064 | 41 | Patients with MR imaging-confirmed AN | Anteroposterior: n = 41, 14.4 mm (range 3–34 mm); mediolateral: n = 41, 16.3 mm (range 4–38mm ); intracanalicular: n = 9 (22.0%), 4–13 mm; extracanalicular: n = 32 (78.0%), 9–38 mm | PTA (0.5, 1 2 kHz): 42dB ± 23; SRT: 42dB ± 24; SDS: 68% ± 31. Marked loss with absent ABR interpreted as positive result |
| Robinette et al., 200065 | 75 | Patients with surgically confirmed AN and group matched for hearing impairment without AN | ≤ 10 mm: n = 22 (29.3%); 11–20 mm: n = 30 (40%); > 20 mm: n = 23 (30.7%) | Asymmetry defined as interaural difference ≥ 15 dB at 0.5, 1, 2 and 3 kHz |
| Marangos, 200166 | 261 | Patients with CPA tumours confirmed to be unilateral AN by CT or MR imaging | ≤ 15 mm: n = 84 (32.3%); 16–25 mm: n = 75 (28.7%); 26–39 mm: n = 61 (23.4%); ≥ 40mm: n = 41 (15.7%) | ABR not expected if loss > 70 dB at > 1 kHz |
| Schmidt et al., 200130 | 58 | Patients with confirmed AN who had both MR imaging and ABR data | ≤ 10mm: n = 12 (20.7%); 11–15 mm: n = 17 (29.3%); > 15 mm: n = 29 (50.0%) | Absent ABR is positive result regardless of hearing impairment |
| Normal ABR: n = 6, 7 mm (4–13 mm); abnormal ABR: n = 52, 20.8 mm (4–70 mm); all: 19.4 mm (4–70 mm) | ||||
| Rupa et al., 200367 | 90 | Patients with asymmetric audiovestibular symptoms (> 15 dB at ≥ two frequencies between 0.125 and 8 kHz) | NR (stated to be commonly > 20 mm) | No response on ABR (n = 18) excluded from analyses |
| Skinner et al., 200368 | 426 | Retrospective review of ABR results in 13-year series of radiologically confirmed AN | Of 91 (21.4%) with ‘good’ ABR morphology (waves I–V present): intracanalicular: n = 24 (26.4%); extracanalicular extension ≤ 10 mm: n = 27 (29.7%); > 10 mm: n = 40 (44.0%) | 242 (56.8%) resulted in no recordable ABR (because of either impairment or pathology); 93 (21.8%) had ‘poor’ ABR morphology (wave V present); 91 (21.4%) had ‘good’ ABR morphology (waves I–V present) |
| Normal ABR based on IT5: intracanalicular, n = 6; extracanalicular extension ≤ 12 mm, n = 8 | ||||
| Cueva, 200469 | 312 | Patients with ASHI excluding those with contraindications for MR imaging, clear aetiology for hearing impairment, NF2 or hearing levels > 70 dB between 2 and 4 kHz | Normal ABR: n = 7, 9.71 mm ± 4.27 (5–16 mm); abnormal ABR: n = 17, 14.23 mm ± 6.21 mm (5–26 mm) | Asymmetry defined as ≥ 15 dB in two or more frequencies or asymmetry of ≥ 15% in SDS |
| Study | Detected with comparator | Detected with ABR | Sensitivity and other reported measures | ABR criteria for abnormality |
|---|---|---|---|---|
| Weiss, 199056 | NA | 26 abnormal ABR, 4 = AN | PPV: 15.4% | Waves I–V IPL ≥ 4.45 ms; wave V interaural latency ≥ 0.4 ms; wave V absolute latency ≥ 6.3 ms if 4 kHz threshold was ≤ 70 dBHL, for every 10-dB shift in threshold above 40 dBHL, 0.1 ms was subtracted from wave V latency |
| Levine, 199157 | MR imaging: 27 | 24 | 88.9%. By size: < 10 mm: 62.5%; ≥ 10 mm: 100% | Based upon standard criteria |
| Wilson et al., 199258 | IC: 15; EC: 25 | IC: 10; EC: 24 | 85%. By location: IC: 66.7%; EC: 96% | At 80 dB interaural absolute latency difference of wave V ≥ 0.4 ms; interaural I–V ≥ 0.4 ms; interwave I–V interval ≥ 4.4 ms; poor morphology in spite of adequate hearing |
| Selesnick and Jackler, 199359 | 35 | 33 | 94%. By size: < 10 mm: 82%; 10–30 mm: 100%; > 30 mm: 100% | Increased interaural waves I–V latency (IT5); lack of discernible waveforms |
| Gordon, 199560 | Surgery: 105 | 92 | 87.6%. By size: ≤ 9 mm: 69.2%; 10–15 mm: 90.8%; 16–20 mm: 86.2%; 21–24 mm: 100%; ≥ 25 mm: 100% | Interaural waves I–V latency difference > 0.2 ms; absolute wave V latency abnormally prolonged; abnormal or absent waveform morphology |
| Chandrasekhar et al., 199561 | MR imaging: 197 | IT5 ≤ 0.2 ms: 15; > 0.2 ms: 130; no response: 52 | IT5 92.4%; waveform morphology 81.6%. By size: < 11 mm: 83.1%; 11–20 mm: 97.4%; 21–30 mm: 93.5%; > 30 mm: 100% | Interaural difference > 0.2 ms; no response; abnormal waveforms |
| Waveform morphology: normal: 14; abnormal ipsilaterally: 54; abnormal bilaterally: 8; not recorded: 121 | ||||
| Zappia et al., 199762 | Surgery: 111 | 103 (36 = absent) | ABR abnormal (ILDV > 0.2 ms) or absent: 95.5%. By size: ≤ 10 mm: 89.2%; 11–20 mm: 97.8%; ≥ 20 mm: 100% | Abnormal: ILDV > 0.2 ms; absent or abnormal waveform morphology |
| ABR waveform morphology abnormal or absent: 93.0%. By size: ≤ 10 mm: 62.2%; 11–20 mm: 71.7%; ≥ 20 mm: 89.3% | ||||
| Godey et al., 199863 | 89 | 82 | 92.1%. By location: IC: 77.8%; EC: 93.8%. By abnormality criteria: IPL I–III: 77.5%; IPL I–IV: 84.3%; ILDV: 88.8%; ID I–V: 91.0% | Absence of response, wave I only; I–III IPL > 2.5 ms; I–V IPL > 4.4 ms; interaural latency difference wave V (ILDV) > 0.2 ms; interaural difference of I–V IPL (ID I–V) > 0.2 ms |
| El-Kashlan et al., 200029 | MR imaging: 25 | 23 | 92% | IPL: I–III > 2.3 ms, III–V > 2.1 ms, I–V > 4.40 ms; ILDV > 0.40 ms; absolute latency of wave V > 7.75 ms; ILDV > 0.60 ms for 75- to 95-dBHL 1000-Hz tone pips; complete absence of identifiable waves in presence of adequate PTA or absence of waves beyond wave I |
| Haapaniemi et al., 200064 | MR imaging: 41; 38 had ABR | 37 | 97.4% | Interaural absolute latency difference of wave V (ILDV) ≥ 0.4 ms; I–V IPL ≥ 4.4 ms; interaural difference of I–V IPL (ID I–V) ≥ 0.4 ms; tracings showed poor or absent wave morphology |
| Robinette et al., 200065 | Surgery: 75 subjects, 75 matched control subjects | 69 true positives; 66 true negatives in control group | 92%. By size: ≤ 10 mm: 82%; 11–20 mm: 93%; > 20 mm: 100% | NR |
| Specificity: 88% (66/75) | ||||
| Marangos, 200166 | CT/MR imaging: 261 | Normal: 50; abnormal: 88; no response: 123 | Excluding no response: 88/138 = 63.7%. By size: ≤ 15 mm: 74.5%; 16–25 mm: 30.9%; 26–39 mm: 93.5%; ≥ 40 mm: 100% | IPL I–V > 4.4 ms; interaural wave V difference > 0.3 ms; interaural IPL I–V difference > 0.2 ms |
| Including no response as positive: 211/261 = 80.8%. By size: ≤ 15 mm: 58.3%; 16–25 mm: 82.7%; 26–39 mm: 96.7%; ≥ 40 mm: 100% | ||||
| Schmidt et al., 200130 | 58 | 52 | 89.7%. By size: 4–10 mm: 58.3%; 11–15 mm:94%; > 15 mm: 100% | Interaural difference in waves I–V delay (IPD 1–V) > 0.2 ms; interaural difference in absolute wave V latency (IT5) > 0.2 ms, when wave I could not be identified in one or both ears and when Brackmann correction factor applied for ears for hearing impairment > 50 dB at 4000 Hz; no identifiable waves |
| Rupa et al., 200367 | MR imaging: 6/90 (4/72 excluding ABR no response) | 30 retrocochlear pathology (4 = AN); 18 no response (2 = AN) | 100% (4/4) | Interpeak intervals: I–III ≥ 2.5 ms, III–V ≥ 2.3 ms or I–V ≥ 4.4 ms; interaural latency difference of ≥ 0.3 ms; poor waveform morphology; absent response despite normal or only mildly elevated audiometric thresholds |
| Specificity: 61.8% (42/68) | ||||
| PPV: 13.3%; NPV: 100% | ||||
| Skinner et al., 200368 | Radiology: 426 | 77 based on ILDV | 84.6% | IPL: I–III > 2.3 ms, III–V > 2.1 ms, I–V > 4.40 ms; ILDV > 0.2 ms; absolute latency of wave V > 7.75 ms; ILDV > 0.60 ms for 75- to 95-dBHL 1000-Hz tone pips; complete absence of identifiable waves in presence of adequate PTA or absence of waves beyond wave I |
| 53 based on III–V interpeak latency | 58.2% | |||
| 89% ILDV for 61 with normal hearing (≤ 25 dB PTA, ≥ 80% SDS) | ||||
| Cueva, 200469 | MR imaging: 24 | 17 | 70.8% | IT5 interpeak latency > 0.2 ms; absolute wave V latencies abnormal; absent or distorted waveform morphology |
| Specificity: 72.9% (210/288) | ||||
| False-positive rate: 82/1% (78/95); false-negative rate: 29.2% (7/24) | ||||
| PPV: 17.9% (17/95); NPV: 96.8% (210/217) |
ABR criteria are reported as the values used and the combination of criteria applied influence both the sensitivity and the specificity of the test. This diversity across studies reflects clinical practice. ABR test performance is therefore likely to differ from one centre to another. Operator skill in interpreting ABR results is another important factor but not one that could be accessed in this review. One could speculate that the level of operator skill in centres publishing major studies might be somewhat higher than average.
Pooled synthesis
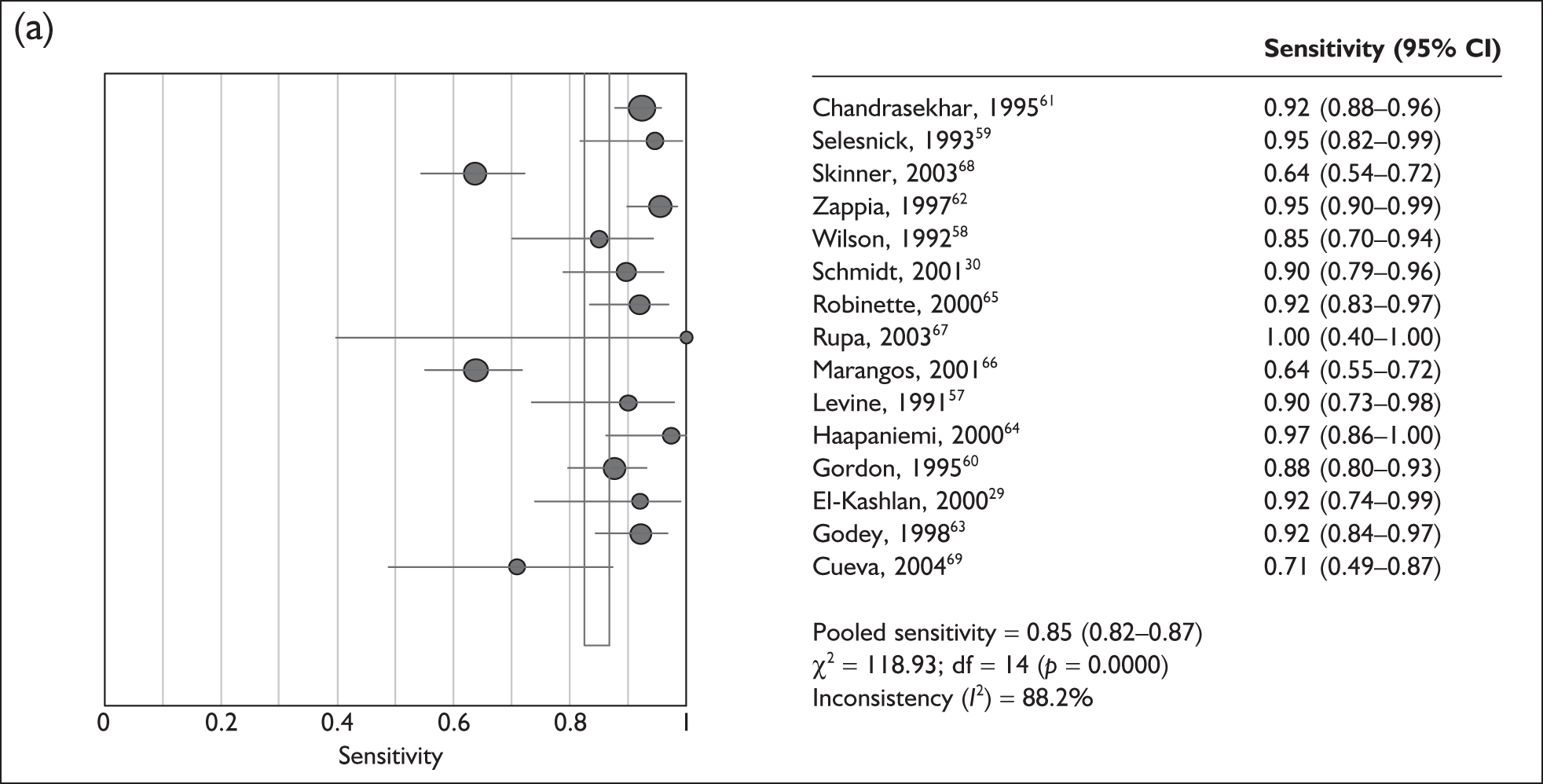
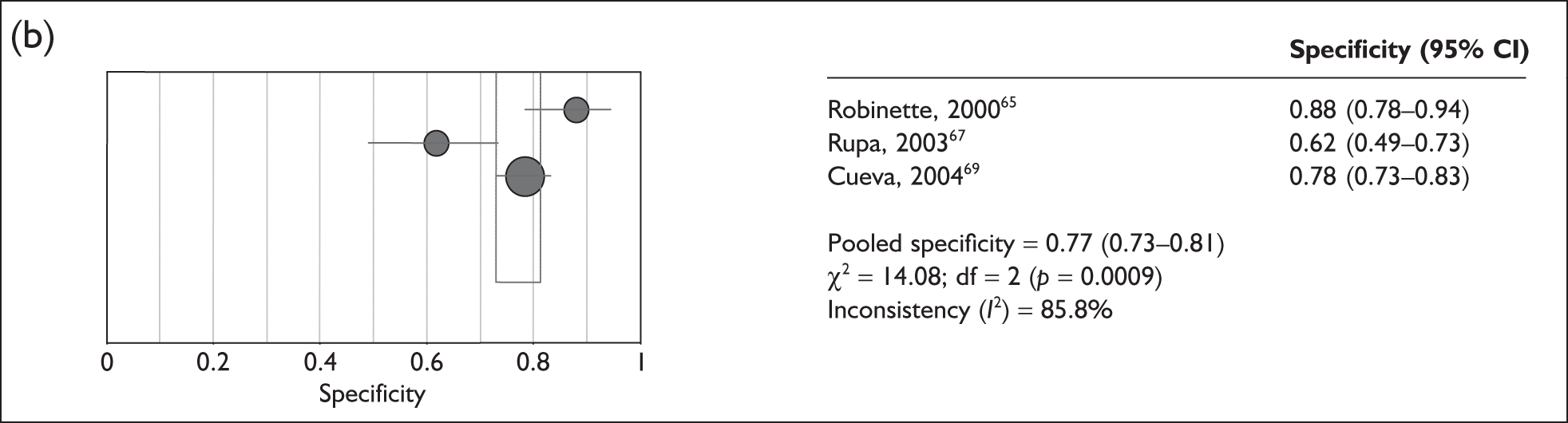
The 15 studies that assessed the sensitivity of ABR against MR imaging were highly heterogeneous (p < 0.0001, I2 = 88%), ranging from 64% (95% CI 54–72%)66,68 to 100% (95% CI 40–100%)67 (Figure 5a). Only three studies reported the specificity of ABR versus MR imaging and these were also highly heterogeneous (p < 0.001, I2 = 86%), ranging from 62% (95% CI 49–73%)67 to 88% (95% CI 78–94%)65 (Figure 5b).
FIGURE 5.
(a) Pooled sensitivity for 15 studies assessing the use of auditory brainstem response (ABR) versus the use of MR imaging. The size of the symbol is in proportion to the size and weight of each individual study. (b) Pooled specificity for 15 studies assessing the use of ABR versus the use of MR imaging. The size of the symbol is in proportion to the size and weight of each individual study. df, degrees of freedom.
Exploration of heterogeneity
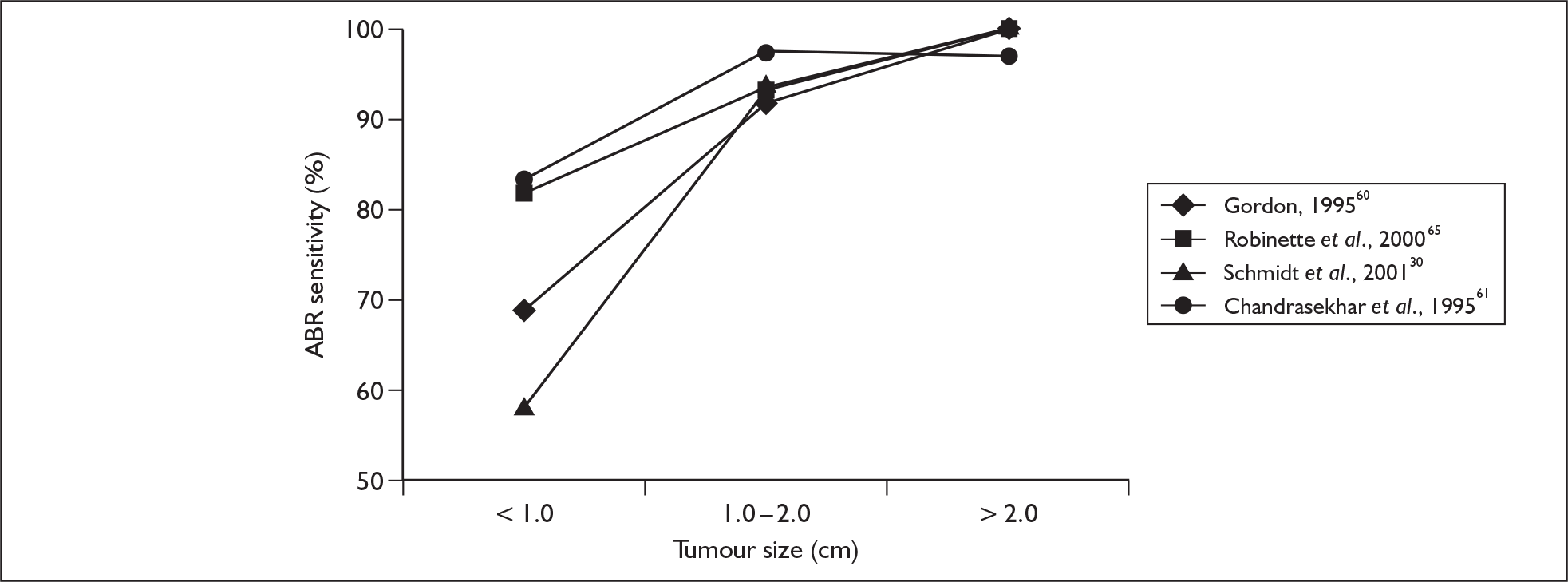
A major potential driver of the heterogeneity in the diagnostic accuracy of ABR is likely to be the size of the acoustic neuroma. A number of studies reported their true-positive and false-negative results separately, according to the size of the acoustic neuroma. When we stratified the sensitivity results according to the categories of acoustic neuroma size, heterogeneity was substantially reduced and the following trend in pooled sensitivity results was obtained: acoustic neuroma size ≤ 1.0 cm: 79% (95% CI 72–85%), heterogeneity p-value = 0.98, I2 = 32%; acoustic neuroma size 1.0–2.0 cm: 95% (95% CI 91–97%), heterogeneity p-value = 0.46, I2 = 0%; acoustic neuroma size > 2.0 cm: 98% (95–99%), heterogeneity p-value = 0.347, I2 = 10%. Sensitivity results of the individual studies are plotted by mean tumour size categories in Figure 6.
FIGURE 6.
Auditory brainstem response (ABR) sensitivity versus tumour size derived from four studies.
Discussion
The effects of hearing impairment on the ABR
The recording of an ABR waveform whose clarity is sufficient to allow measurements of peak latencies requires that a well-synchronised action potential volley be induced in the auditory nerve. This is lost at low-stimulus intensities or in cases of elevated high-frequency hearing thresholds (regardless of site of lesion). In our review of the ABR literature we noted that some studies excluded those cases in which a clear (quantifiable) ABR was not seen, whereas other studies included them. Still other studies excluded cases in which the subjects’ hearing impairment exceeded a defined value, in the expectation that a measurable ABR would be unlikely. Most studies that evaluated the effect of hearing impairment on ABR included a statement which suggested that the authors considered that a quantifiable waveform was not expected if subjects’ hearing thresholds exceeded 70 or 75 dBHL at 4 kHz (or an average of two or three frequencies in the mid- to high-frequency range).
The effects of tumour size on the ABR
A review of the literature shows that the sensitivity of the ABR is dependent on the size of the tumour. High levels of ABR sensitivity are reported for tumours in excess of 1 cm, and several studies57,60,65 report values of 100%. However, in some cases this is achieved by classing no ABR as a positive finding, which results in relatively low levels of specificity. There is a significant reduction in ABR sensitivity for smaller tumours (< 1 cm). Values range from as low as 58%30 up to 92%. 29 In the work by El-Kashlan and colleagues29 this fairly high level of sensitivity again results from classing no ABR as a positive finding.
Because several studies provided ABR sensitivity data for tumours of less than 1 cm, between 1 and 2 cm and more than 2 cm, we were able to establish indicative sensitivities of 79%, 95% and 98% respectively. Thus, ABR provides a clinically acceptable sensitivity for medium to larger tumours but is not able to reliably identify small (often intracanalicular) tumours. One observation made58,61 was that the presence of a normal ABR waveform (indicating good functional integrity of the nerve) was a good prognostic indicator for hearing preservation, given an appropriate surgical approach.
Role of the ABR in screening for acoustic neuroma
In the 1980s and 1990s the ABR became popular as a cost-effective initial screen for patients considered at risk of an acoustic neuroma, its performance being judged in the light of the size of tumours then being detected radiologically (typically over 1 cm). However, the advent of gadolinium-enhanced MR imaging in the late 1980s and the subsequent development of non-contrast MR imaging has allowed much smaller tumours to be detected, leading to improved surgical outcome and hearing preservation. 70
The current aim of screening for acoustic neuroma is to detect tumours at an early stage when they are relatively small. This is a challenge for the ABR in view of the relatively low sensitivities reported for small tumours. The performance of the ABR for larger tumours is clinically more acceptable, but it is expected that identification of acoustic neuroma will be achieved before this stage. Application of ABR as a screening tool is therefore limited for the general population of patients when compared with the MR scan, particularly as the mean size at diagnosis is now 10 mm (see Chapter 4, Growth).
There are some situations in which MR imaging might not be possible or might be contraindicated. Alternative strategies, including ABR, may have a role to play in the following scenarios:
-
in patients who are unable to routinely undertake an MR scan because of claustrophobia (2.8–4.0%);34,35 in such cases the use of sedation or a general anaesthesia, or an open MR imaging system or CT, could be considered as an alternative imaging strategy
-
when physical limitations limit access to the scanner, such as severe obesity (1 in 1139 and 2 in 913);34,36 imaging on an open MR scanner or CT may be possible in these patients
-
in patients with metallic implants, pacemakers, etc. (2 in 1139)34
-
when access to an MR scan is limited because of time constraints and waiting times.
Modifications of the ABR
One research group has developed a modified ABR method that seeks to make the ABR sensitive to small (< 1 cm) acoustic neuroma. 71 The so-called ‘stacked ABR’ claims to allow the involvement of nerve fibres of all frequencies (rather than just the high-frequency fibres upon which the standard ABR relies), thus making it more sensitive to selective effects of even small acoustic neuroma. However, this method requires specialist equipment, demands a high level of operator skill and takes substantially longer than the standard ABR. Initial reports of the sensitivity of the stacked ABR for small tumours have been encouraging but further and independent studies are needed before it becomes clear whether it is a viable clinical tool worthy of routine clinical implementation.
With a similar aim, Bush and colleagues72 have explored the use of another index to improve the detection rates in smaller acoustic neuroma. Their method evaluates the difference between the behavioural and electrophysiological (ABR) thresholds, which they show to be abnormally large in acoustic neuroma. In a small series (seven acoustic neuroma cases, four of which had tumour diameters of 5 mm or less) the technique gave a positive result. Further studies will determine whether this method delivers the promise suggested by this study.
Role of other techniques in screening for acoustic neuroma
In the past, audiovestibular investigations have been employed to assist with the identification of acoustic neuroma. These tests have included stapedius reflex threshold, threshold tone decay, speech audiometry, alternate binaural loudness balance, otoacoustic emissions (OAE) and caloric testing. In early papers the value of these tests has been investigated with respect to ABR, CT and early MR imaging technology. A prospective study by Ferguson and colleagues12 showed that audiovestibular tests had sensitivities in the range of 45–85%, with caloric testing highest at 85% and speech discrimination lowest at 45%. Specificities were in the range of 66–90%, with alternate binaural loudness balance at 90% and caloric testing at 66%. The conclusion from this study was that the performance of audiovestibular tests as screening tools was limited when compared with the ABR. However, Haapaniemi and colleagues64 confirmed earlier reports that there is a positive relationship between the size of an acoustic neuroma and a decrement of caloric function.
Evoked OAE can be employed to assess outer hair cell activity. In some acoustic neuroma cases cochlear hair cell function can be preserved such that an OAE will be present, in contrast to conductive and cochlear losses in which a hearing impairment of greater than 30 dB will result in an absent emission. However, Quaranta and colleagues73 showed that only 18 of 47 patients (38%) with acoustic neuroma had an OAE present, which demonstrates the limitation of the technique as a screening tool. In cases with absent OAE the hair cells will have been compromised by the tumour.
Prasher and colleagues21 reported absent transient-evoked otoacoustic emissions (TEOAE) in 19 of 26 patients with acoustic neuroma (73%); in all patients in whom TEOAE was absent, a hearing impairment of 40 dBHL or greater was present, and this was assumed to be cochlear in origin. Telischi and colleagues74 undertook distortion product otoacoustic emission (DPOAE) measurements in 44 patients with unilateral acoustic neuroma. On the basis of the presence or absence of the DPOAE, 26 (59%) tumour ears were classified as having a cochlear loss, 13 (30%) as retrocochlear (DPOAE recorded in the presence of a hearing impairment > 40 dB) and five (11%) as mixed. Ferber-Viart and colleagues75 attempted TEOAE recordings in 168 ears with acoustic neuroma, and in 79% were not able to demonstrate good cochlear function, thus indicating a cochlear dysfunction in addition to the tumour. Ferguson and colleagues76 were unable to evoke TEOAE in 78 patients of a series of 100 with unilateral acoustic neuroma. The various mechanisms by which cochlear dysfunction may be involved in tinnitus generation are described in Chapter 4.
Comparison of the role of different protocols for magnetic resonance imaging
Introduction
Magnetic resonance imaging provides images of the brain and skull without the use of ionising radiation. When a patient is placed into an MR scanner all of the protons in the body align in the same direction due to a powerful background magnetic field. Radiofrequency pulses are then emitted by the scanner, which cause the protons to resonate and deflect coherently to various degrees. As the protons return to alignment with the background magnetic field, they lose spin coherence (dephase) and release a signal that is detected and then processed into a medical image.
The main factors that influence the amount of signal released by tissue are the proton density and the longitudinal (T1) and transverse (T2) relaxation time constants. The T1 relaxation time is a property of a material and describes the profile of the distribution of energy back to the surrounding tissues. The T2 relaxation time is also a material property and characterises the loss of coherent signal as energy is passed between adjacent protons and their spins desynchronise. The T2 star (T2*) describes the loss of energy in a non-uniform magnetic field; although similar to the dephasing T2 process, the T2* is not a property of the sampled material but is dependent on the characteristics of the MR imaging system.
Gadolinium is a paramagnetic material and is used as a contrast agent in MR imaging. It has the effect of shortening the T1 time of a tissue resulting in increased signal on T1W images. An enhancing object, such as an acoustic neuroma, ‘stands out’ against the background lower signal of cerebrospinal fluid (CSF) and brain tissue.
The data acquired during an MR imaging sequence are presented as an anatomical image, or ‘slice’, in one of three orientations: axial, coronal or sagittal. Data acquired as a three-dimensional volume can be reviewed on a workstation and evaluated from any slice orientation using multiplanar reformat (mpr) software.
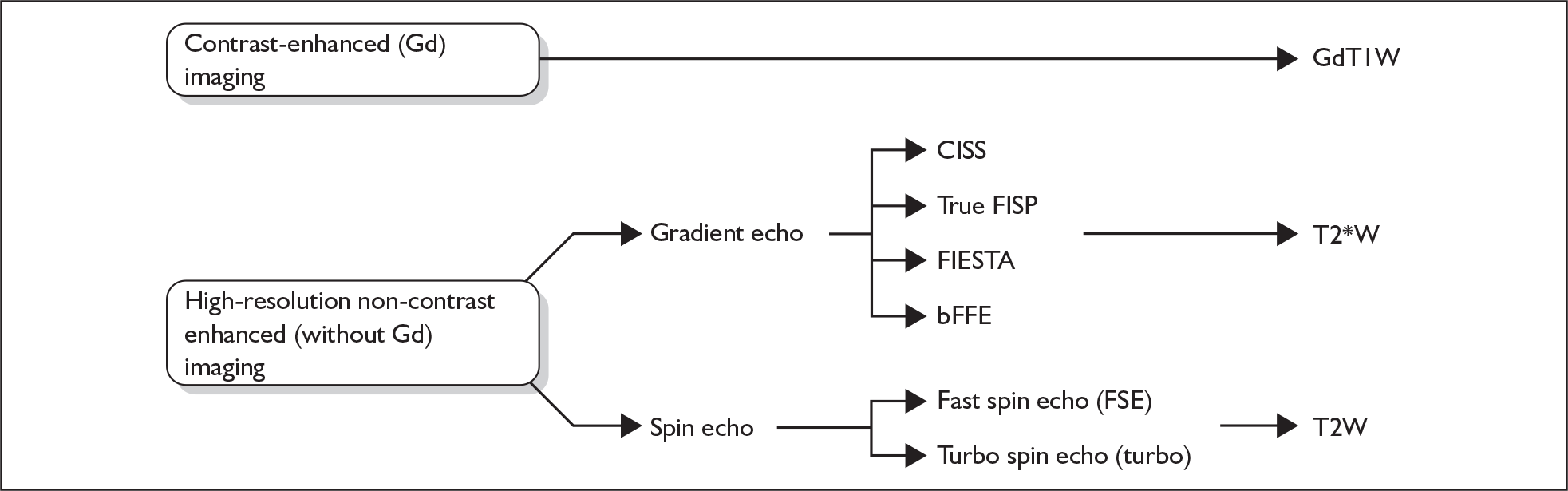
The images are weighted to distinguish tissues by their differing T1, T2 or T2* properties, so that, for example, CSF will appear bright and cranial nerves will appear dark on T2W or T2*W images. There are many acronyms for the different sequences (see List of abbreviations), summarised in Figure 7. In the following text, gadolinium-enhanced T1W sequences will be referred to as GdT1W images; T2W sequences and T2*W sequences will be referred to as T2W, T2*W or high-resolution imaging without gadolinium.
FIGURE 7.
Currently used MR imaging protocols. bFFE, balanced fast field echo; CISS, constructive interference in the steady state; FIESTA, fast imaging employing steady-state acquisition; true FISP, true free induction with steady-state precession; Gd, gadolinium; GdT1W, gadolinium-enhanced T1-weighted imaging; T2W, T2 weighted; T2*W, T2 star weighted.
Over the last two decades, advances in computer processing speeds, data storage capacity and materials technology have been applied in medical MR imaging, resulting in the development of MR imaging equipment and scanning sequences that have delivered incremental improvements in image quality, acquisition and processing times.
The advances have been associated with the following benefits:
-
shorter image acquisition times (patient comfort, faster turnover, shorter waiting lists)
-
faster/in-time data processing (images available immediately, reduced post processing time)
-
higher spatial/contrast resolution (improved image quality, enhanced diagnostic confidence, improved accuracy, reduced requirement for GdT1W images, reduced recall rate, reduced scan time and cost)
-
reduced gadolinium usage (reduced radiologist time commitment and costs; the use of high-resolution three-dimensional T2W or T2*W sequences that do not require gadolinium allows services to arrange unsupervised out of hours/weekend scanning in batched lists, with improved efficiency).
In addition to advances in MR technology there has been a significant expansion in the number of MR scanners in England and Wales, supported by the New Opportunities Fund cancer initiative. Government initiatives have aimed to improve access to MR imaging (wave 1 and 2 outsourcing, 18-week wait). 77
The infrastructural improvements described have helped address limited access to MR technology and the cost of MR service delivery. In light of the technological and infrastructural changes over the last decade, the role of investigation protocols incorporating other screening techniques that have previously been justified on the basis of limited clinical access to, and efficacy and cost of, MR imaging need to be re-evaluated.
Shortly after the clinical introduction of MR imaging, the GdT1W MR sequence was agreed to be the ‘gold standard’ diagnostic test for the detection of acoustic neuroma when compared with the established axial imaging technology, CT. 70,78
As MR technology evolved, GdT1W images were compared with a variety of sequences without gadolinium. Early studies evaluating high-resolution three-dimensional GE techniques for the acquisition of both T1W and T2W images were limited by magnetic susceptibility artefacts that reduced spatial resolution and decreased the signal-to-noise ratio. The IAC is especially prone to this artefact because of the close proximity of soft tissue structures, bone and air. 79
An early paper in 199380 describing the constructive interference in the steady state sequence (CISS; a three-dimensional GE technique that nulls the effect of CSF motion, thereby improving nerve/CSF discrimination) on a 1-T magnet using a standard head coil showed very promising results in a small selected population. The individual nerve branches in the IAC could be identified in 90–95% of cases and the cochlea and vestibule were well demonstrated, with the vestibular aqueduct visible in 75% of cases. Disease extension into the vestibule was documented. The paper did not contain extractable data on sensitivity and specificity and false-positive and false-negative rates.
In practice, two-dimensional T2W FSE or turbo spin echo (TSE) sequences had the advantage of reduced sensitivity to susceptibility artefacts and developed an early lead in the literature, demonstrating improved visualisation of normal and pathological processes involving the IAC and CPA. The scan acquisition time was reduced compared with conventional spin-echo sequences. 81,82
This section reviews the literature on the comparison of high-resolution imaging without gadolinium (T2W or T2*W) with gadolinium-enhanced imaging (GdT1W).
Results
Eleven papers were considered in the review of different protocols of investigation using MR imaging. All of the included studies were diagnostic comparative studies comparing a non-contrast-enhanced MR sequence with the accepted gold standard GdT1W sequence. They are summarised in Table 4.
| Study | Dates of study | Index testa,b | Country | Study design | Number of participants | Sex | Age (years), mean (range) |
|---|---|---|---|---|---|---|---|
| Allen et al., 199682 | NR | 2D FSE | USA | Retrospective case-matched cohort | 50 | NR | 49 (20–83) |
| Stuckey et al., 199683 | NR | 3D CISS | Australia | Prospective cohort of consecutive patients with clinical features necessitating evaluation of CPA | 125 | M: 49.6%, F: 50.4% | 50 (19–80) |
| Soulié et al., 199784 | NR | 2D FSE | France | Prospective cohort of patients referred for suspected retrocochlear pathology due to SNHI and/or vertigo | 110 | M: 64.5% F: 35.5% | 55 (22–85) |
| Hermans et al., 199785 | NR | 3D CISS | Belgium | Retrospective cohort referred for exclusion of AN | 83 | NR | NR |
| Naganawa, 199886 | 1996–7 | 3D FSE | Japan | Prospective cohort of consecutive patients referred for evaluation of ear symptoms | 205 | M: 53.2% F: 46.8% | 48.4 ± 16.4 (12–79) |
| Held et al., 199987 | 1995–7 | 3D CISS | Germany | Retrospective cohort of patients with AN | 20 (3 = NF2) | M: 45% F: 55% | 50 (12–80); 54 excluding NF2 |
| Marx et al., 199988 | NR | 2D FSE | USA | Retrospective case-matched cohort of patients suspected to have AN | 25 | M: 44% F: 56% | NR |
| Schmalbrock et al., 199989 | NR | 3D GE | USA | Prospective cohort | 21 | M: 47.6% F: 52.4% | 53.2 (10–88); 3/21 < 16, 11/21 < 65 |
| Zealley et al., 200044 | NR but 2-year period | 2D FSE | UK | Prospective cohort | 1233 | NR | 50.6 (14–81) |
| Annesley-Williams, 200138 | 1996–8 | 2- and 3D FSE | UK | Prospective consecutive cohort referred for exclusion of AN | 513 (2D = 340, 3D = 173) | ‘Almost equal’ | 55 (15–98) |
| Ben Salem, 200190 | 1995–8 | 2D GE | France | Retrospective cohort referred for exclusion of AN | 190 | NR | 52 (13–79) |
Assessment of the value and quality of the studies is dependent upon the methodology used. The results of the formal quality assessment are detailed in Table 27 (Appendix 3). Table 5 summarises the methodology of the observation protocol for each of the 11 studies.
| Study | Index test | Number of observers | Number of observations (ears × observers × observations) | Independence of observers | Blind to results of other tests |
|---|---|---|---|---|---|
| Allen et al., 199682 | 2D FSE | Four | 400 | Y | Y |
| Stuckey et al., 199683 | 3D CISS | Two | 250 | Y | Y |
| Soulié et al., 199784 | 2D FSE | Two | 110 | NR | N |
| Hermans et al., 199785 | 3D CISS | Two | 664 | Y | Y |
| Naganawa, 199886 | 3D FSE | Two | 820 | Y | Y |
| Held et al., 199987 | 3D CISS | Three | All had AN | N | N |
| Marx et al., 199988 | 2D FSE | One | 50 | Y | N |
| Schmalbrock et al., 199989 | 3D GE | One | 42 | Y | N |
| Zealley et al., 200044 | 2D FSE | Two in each of three centres | 2466 | Y | N |
| Annesley-Williams, 200138 | 2- and 3D FSE | Three | 1026 | Y | NR |
| Ben Salem, 200190 | 2D GE | Two | 380 | Y | Y |
Values of sensitivity and specificity for the various high-resolution imaging without gadolinium protocols compared with gadolinium-enhanced imaging were systematically recalculated for the data presented when possible and are presented in Table 6. Further detail is provided in 2 × 2 tables in Appendix 5.
| Study | Index test | Number of AN found | Size | Sensitivity (%) | Specificity (%) | False negatives | Details of false negatives | False positives | Details of false positives |
|---|---|---|---|---|---|---|---|---|---|
| Allen et al., 199682 | 2D FSE | 25 | Cases × range: 1 × 0–5 mm; 3 × 6–10 mm; 14 × > 10 mm | 98 quoted | 99.7 quoted | 2/100 | 2 and 3 mm in small IAC | 1/300 | Result of crowded nerve roots in IAC |
| Stuckey et al., 199683a | 3D CISS | 18 | Cases × range: 11 × < 10 mm; 14 × 10–36 mm | Obs 1: 100, obs 2: 94.4, overall: 97.2 | Obs 1: 98.1, obs 2: 93.5, overall: 95.8 | Obs 1: 0/18, obs 2: 1/18, overall: 1/36 | Obs 1: 2/107, obs 2: 7/107, overall: 9/214 | Related to clustering of nerve roots in IAC or narrow IACs | |
| Soulié et al., 199784 | 2D FSE | 25 | Cases × location, mean: 1 × labyrinth; 5 × IAC, 5 mm; 3 × porus, 11 mm; 9 × CPA, 19 mm | 100 quoted | 93 quoted | 0/24 | 6/86 | ||
| Hermans et al., 199785 | 3D CISS | 18 | Cases × range: 1 × < 2 mm; 4 × 2–5 mm; 6 × 5–10 mm; 7 × 10–20 mm; 1 × > 20 mm | 89–94 quoted | 94–97 quoted | 5/72 | Intralabyrinthine overlooked by one observer; smallest IAC lesion missed twice by both observers | 18/592 | |
| Naganawa, 199886 | 3D FSE | 19 | Cases × range:b 5 × < 5 mm; 7 × 5–10 mm; 4 × 11–20 mm; 1 × > 20 mm | 100 quoted and calculated | 99.5 quoted, obs 1: 99.7, obs 2: 99.5 calculated overall | 0/19 | 2/391 | NRc | |
| Held et al., 199987 | 3D CISS | 20d | Size, range: 10, 4–15 mm | 100 | All had AN | 0/20 | All had AN | ||
| Marx et al., 199988 | 2D FSE | 11 | Volume: 0.06–3.00 cm3 | 100 | 100 | 0/11 | 0/39 | 0 | |
| Schmalbrock et al., 199989 | 3D GE | 27 | Location, number, size: IAC, 10, 3–12 mm; CPA, 23, 6–58 mm | 100 | All had AN | 0/27 | All had AN | ||
| 6 = bilateral | |||||||||
| Zealley et al., 200044 | 2D FSE | 33 | Range: 4–22 mm | 97 calculated | 95.3 calculated | 2/66 | 114/2400 | ||
| Annesley-Williams, 200138 | 2- and 3D FSE | 23 | Range: 2.5–42 mm | See 2 × 2 tables | See 2 × 2 tables | See 2 × 2 tables | 5 = intralabyrinthine | See 2 × 2 tables | |
| Ben Salem, 200190 | 2D GE | 19 | 100 | 96 | 0/19 | 15/361 |
Pooled synthesis
Although the sensitivities were found to be relatively homogeneous (T2W: p = 0.34, I2 = 11%; T2*W: p = 0.19, I2 = 40%), specificities were highly heterogeneous (T2W: p < 0.0001, I2 = 89%). The pooled test sensitivity for the T2W reference was 98% (95% CI 94–99%) and for the T2*W reference it was 96% (95% CI 86–99%). The specificity of the T2W studies ranged from 90% (85–94%)38 to 100% (91–100%). 88 For the T2*W studies specificity ranged from 86% (75–93%)85 to 99% (98–100%)86 (Figures 8 and 9).
FIGURE 8.
Pooled sensitivity and specificity for T2-weighted reference studies. The sizes of the symbols are in proportion to the size and weight of each individual study. df, degrees of freedom.
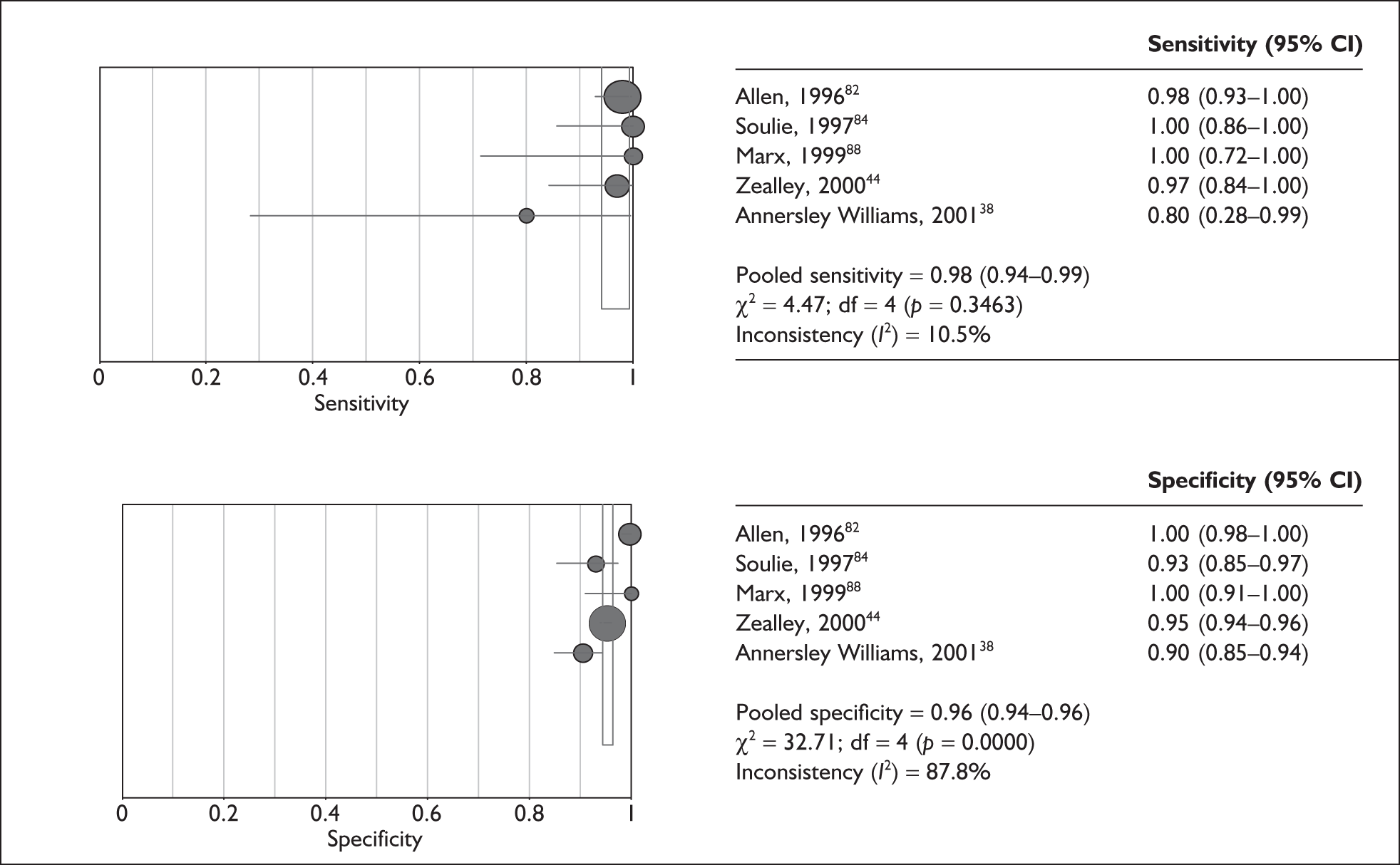
FIGURE 9.
Pooled sensitivity and specificity for T2-star-weighted reference studies. The sizes of the symbols are in proportion to the size and weight of each individual study. df, degrees of freedom.
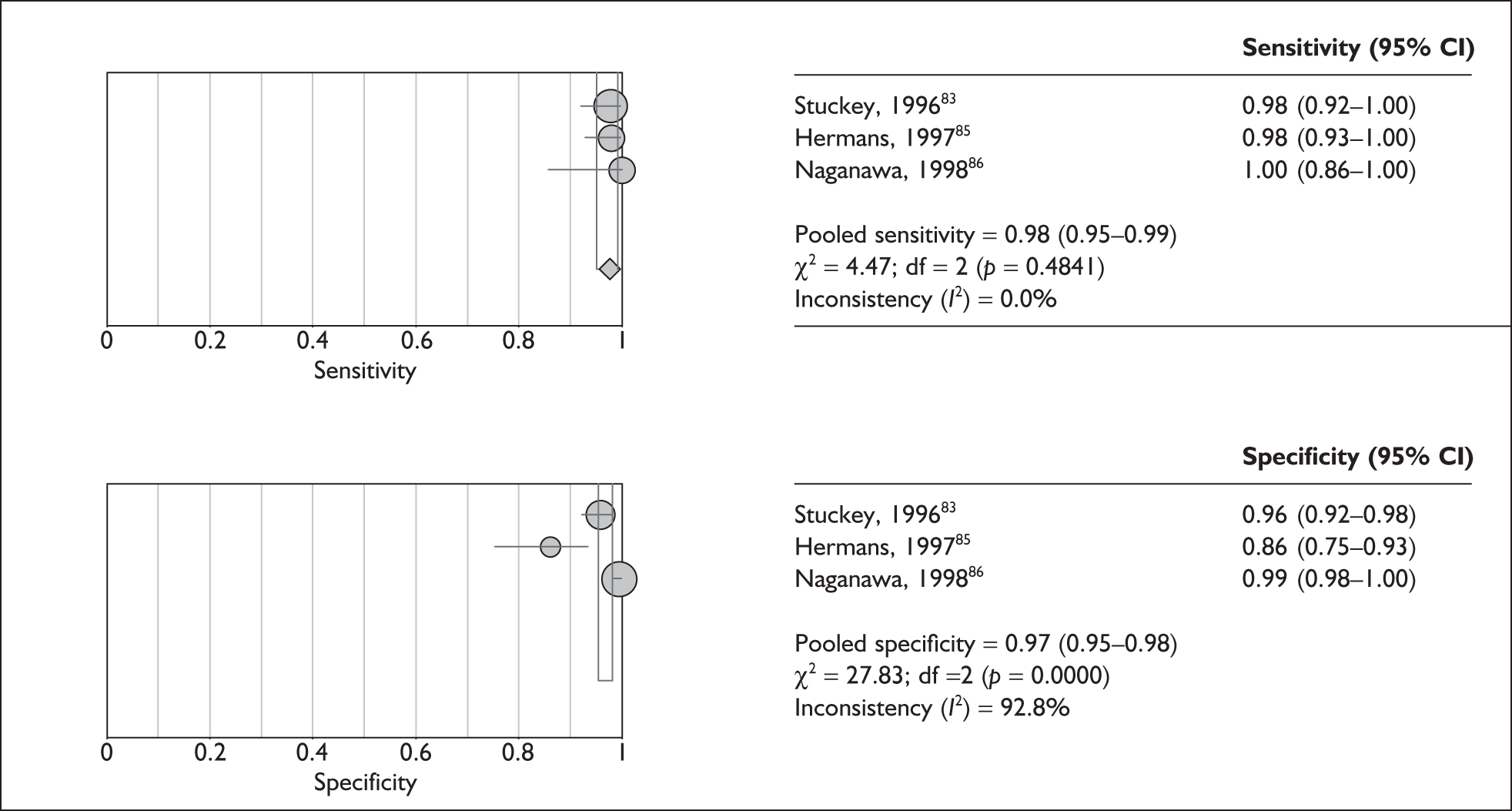
Compared with the gold standard of GdT1W MR imaging, the high-resolution non-enhanced T2W and T2*W sequences appear to have good test accuracy as assessed by both sensitivity and specificity. The level of test specificity was found to be heterogeneous across the included studies in this review.
In such analyses the pooled effect may change if poorer quality studies are excluded. The number of studies included in these analyses was not large enough, nor did the studies differ sufficiently in quality (see Appendix 3) to make such a direction of analyses useful.
Evolution of MR technology subsequent to the evidence reviewed
During the 1990s progressive improvement of two-dimensional and then three-dimensional T2W or T2*W sequences acquired without Gd allowed researchers to question the requirement for GdT1W imaging in all cases, on the basis of improved image quality, diagnostic accuracy and time/cost savings. 91,92
Reports subsequent to 1998 have compared T2W with T2*W sequences for specific attributes that improve acquisition time, acoustic neuroma detection or characterisation. These papers were not included in the analysis on the basis that the sequences were not specifically evaluated for performance of acoustic neuroma detection by comparison with a GdT1W sequence. Selected references have been included as part of a short narrative review to document advances in imaging technology that have been applied to clinical practice subsequent to the data evaluated in the systematic review.
The time advantage of using an ultra-long echo train length (ETL) and half-Fourier three-dimensional FSE to acquire images with high spatial resolution in less than 3 minutes was reported by Naganawa in 1998. 86 The advantage of high-resolution T2W imaging in identifying the path of the VIIth nerve in relation to acoustic neuroma compared with the limited spatial and contrast resolution of GdT1W imaging was illustrated by Sartorelli-Schefer et al. 93 The improvement of image quality, due to reduced CSF flow artefact and shorter imaging time, by the use of a driven equilibrium radio frequency reset pulse (DRIVE) in adjunct with a T2W three-dimensional TSE sequence was reported by Ciftci et al. in 2004. 94
T2*W sequences, including balanced fast field echo (bFFE), fast imaging employing steady-state acquisition (FIESTA), or true free induction with steady-state precession (true FISP), have also become standard internal auditory meatus (IAM) sequences in clinical practice. These sequences use very short repetition times and balanced gradients to generate steady-state free precession. They use signal from free induction decays, spin echoes and stimulated echoes to generate images with a high signal-to-noise ratio.
The contrast in T2*W images is dependent on the T2/T1 ratio and CSF, which has long T2 and T1 relaxation times, returns a high signal intensity and outlines normal structures, which return a lower signal. Balanced gradients also result in flow compensation and reduce image degradation by CSF flow. 95
Some groups prefer TSE (T2W) to GE (T2*W) sequences because of their reduced degradation by magnetic susceptibility artefact. However, magnetic susceptibility is an inherent tissue parameter that cannot be completely avoided and occurs with both sequence types. Three-dimensional TSE sequences with a long ETL have very short acquisition times but are susceptible to blurring, depending on ETL and k-space trajectory. 96
The sequences used in current practice allow reliable acquisition of three-dimensional T2W or T2*W data sets that can be interrogated from any orientation using a submillimetre slice thickness on a workstation immediately after the study. Sequence selection is based on the equipment available and operator preference.
Discussion
Allen et al., 199682
This study selected pathologically proven acoustic neuroma cases thereby facilitating validation of the gold standard reference test (GdT1W) but introducing a selection bias compared with a screening population.
Four neuroradiologists were blind to the clinical data and GdT1W results and used an observer confidence rating (1, ‘definitely not present’, to 5, ‘definitely present’), with scores 1–3 representing a negative test and 4 and 5 a positive test.
There was good agreement (no statistical difference using k-coefficients) between FSE and GdT1W MR imaging, but case selection could have facilitated the excellent performance of the index test. The authors’ conclusion that two-dimensional FSE was a cost-effective alternative to GdT1W MR imaging should be treated with caution.
The findings of two false-negative index test results and one false-positive result were not regarded as significant compared with the large number of observations in the study. The authors did not develop a discussion of the implications of indeterminate results, or the influence of the diagnostic threshold on sensitivity/specificity, but instead commented that, at the time of publication, the slice thickness of acquisitions had reduced from 3.0 to 0.8 mm, making a significant miss even less likely.
The authors commented that clinicians at their institution had abandoned impedance testing and brainstem-evoked responses in favour of limited-cost high-resolution MR imaging if an audiogram suggested the possibility of acoustic neuroma. They also commented that a negative MR test result would result in transfer back to the primary care provider rather than clinic follow-up. This documents a significant practice shift away from the routine use of the ‘gold standard’ test, based on case experience at the centre and the existing literature quoted in the paper.
Stuckey et al., 199683
This study emphasised the potential benefit of a high-resolution CISS sequence in which supplementary GdT1W imaging would be required only if the CISS imaging was suboptimal, equivocal or positive, or if an abnormality on whole brain T2 imaging required further characterisation.
The study included a recognised selection bias due to variable application of CT and non-imaging tests to the same cohort, with patients often referred for MR imaging to clarify existing findings. The authors acknowledged that this bias resulted in the high incidence (14%) of acoustic neuroma in this screening population.
A normal appearance was defined by the ability to identify normal-sized VIIth and VIIIth nerves clearly throughout their course, with no evidence of a mass lesion. The two independent, blinded observers graded the images as optimal, suboptimal but adequate, or inadequate and interpreted the images as normal, indeterminate or abnormal, with the latter two categories constituting a positive result. Each interpretation was rated with a subjective confidence score of low, moderate or high.
False-positive observations were caused by nerve clustering and/or a small IAC. Differences in observer performance (not confirmed statistically) were possibly related to experience and non-availability of MPR facilities. The authors commented that a high false-positive rate would have increased the necessity for GdT1W imaging and costs.
The single false-negative interpretation was the result of ‘satisfaction of search’ (identifying an obvious lesion in the opposite CPA) and the missed lesion having a homogeneous, relatively high signal intensity on the CISS sequences, resulting in reduced conspicuity compared with CSF in the IAC.
Although the study did not evaluate the role of GdT1W screening, the authors commented that omitting these sequences could result in missing lesions with no mass effect such as nerve enhancement associated with inflammatory lesions of the labyrinth or cranial nerves, or leptomeningeal sarcoidosis, metastasis or lymphoma. They commented that the incidence of such significant pathologies was very low and that the implications of missing more benign pathologies such as labyrinthitis were minimal.
The authors concluded that three-dimensional CISS was a sensitive test that could reasonably be used alone if gadolinium was contraindicated. They advised further evaluation before advocating the sequence as a stand-alone screening test.
Soulié et al., 199784
Normality was defined as a high CSF signal in the IAC and nerves visible; uncertain cases, in which the nerves could not be clearly identified, were classified as positives.
False-positive results were the result of recognised limitations of the technique (narrow IACs and CSF flow artefacts) and the authors proposed that these would be addressed by the future introduction of three-dimensional FSE scanning with submillimetre slice thicknesses.
The authors emphasised that the criteria set for normality of high-resolution T2 images were strongly predictive of normality on the GdT1W images and could rule out acoustic neuroma in 73% of their patients. The data were supportive of this and provided further support for non-contrast high-resolution screening although the methodology was flawed.
The authors recognised selection bias in their population, excluding patients with pathologies that would obligate GdT1W sequences (history of malignancy, human immunodeficiency virus, cranial neuropathy or middle ear disease); however, the prevalence of these conditions is low in the screening population and this bias was likely to be less significant than the methodological biases also acknowledged by the authors. For these reasons they did not advocate abandoning the GdT1W sequence for screening patients but proposed that this should be considered given the time/cost savings that might be involved.
Hermans et al., 199785
The independent, blinded observers looked for abnormality, defined as nodular low signal relative to CSF, and scored the degree of confidence on a five-point scale. They also documented any cause of uncertainty related to artefact or a technical limitation of the examination.
Test specificity values quoted were calculated on the basis of the number of ears examined. If recalculated on the basis of the number of patients, test specificity falls from 97% to 86% (see 2 × 2 tables in Appendix 5)
The authors commented that identification of the nerves in the IAC was not a prerequisite in the study and that making it so would have reduced the specificities obtained. This interpretive bias partly compensated for the fact that volume averaging, which was recorded as the most frequent cause of uncertainty, could have been reduced if the observers had been able to review MPR on a workstation. This facility, which might have improved specificity, was not available.
The authors explored the consequences of varying the diagnostic threshold for the test. When the operating point was set at a level felt to be equivalent to the operating point for screening, the quoted sensitivities and specificities were achieved. At this point the screening service would miss tumours in 2/18 cases and recall 9/65 negative cases for reimaging unnecessarily. At the more lenient operating point (including ‘probably negative’ cases), 0.5/18 cases would be missed (the intralabyrinthine tumour was scored ‘definitely negative’ on one occasion) and 34.5% of the patients would require GdT1W imaging.
The authors recognised the potential measurement bias in the study and commented that the significance of missing the smallest IAC tumours would depend on the natural history of that tumour and the clinical consequences of the miss. They did not discuss the cost implications of reduced test specificity (increased GdT1W usage), but they did discuss potential causes and the implications of a false-positive reference test (none were noted in the study though).
Naganawa, 199886
The high-resolution technique resulted in very high-quality imaging in the hands of these investigators, with a false-negative rate of zero. The improved spatial resolution also permitted identification of labyrinthine pathologies that could explain the presentation in 13 ears (eight patients, 4%).
Although the quality of imaging was high in this study, the acquisition time was long (11 minutes) and the technique was not available/relevant to the screening population at the time. The potential of high spatial resolution imaging to minimise false-negative test results was an important observation and the authors did recognise that false negatives may arise from a larger study population.
The false-positive rate was low but the significance of false positives was not considered, the authors commenting that they would be verified by GdT1W imaging.
Held et al., 199987
The authors’ conclusions that CISS provided better nerve attribution and improved evaluation of labyrinthine involvement in a screening population may be subject to selection and measurement biases.
The authors recognised this limitation by commenting that all cases in their institution would continue to undergo both three-dimensional CISS and three-dimensional GdT1W imaging routinely. They also acknowledged the time-penalty implications of workstation review of three-dimensional T2*W and three-dimensional GdT1W datasets, which was not a time-effective approach compared with conventional two-dimensional GdT1W imaging at the time.
Marx et al., 199988
The single independent but unblinded reviewer defined the results as positive, indeterminate or negative, but evaluation of the image quality of the index and reference test was subjective. The sensitivity and specificity of the index test were each 100%, there were no indeterminate readings and the two-dimensional FSE images were felt to be as good as the reference images in all cases. These results and the authors’ conclusion that FSE MR imaging was an excellent choice as a screening test for acoustic neuroma should be viewed with caution, given the lack of detail on subject selection and lesion measurement.
The authors intended to demonstrate the accuracy of two-dimensional FSE imaging using ‘readily available hardware’, and, although the results do not justify the conclusions, the intention recognises that a screening test has to be deliverable at a community rather than at a tertiary level. The authors emphasised that the sequence could be acquired at low cost without purchase of specialised hardware.
The authors also commented that FSE provided better evaluation of acoustic neuroma in the lateral IAC (although they did not evaluate this point) and that, ultimately, dropping GdT1W sequences would eliminate the confusion generated by a false-positive reference test.
Schmalbrock et al., 199989
The patients were prospectively selected from a cohort undergoing surveillance imaging or postoperative follow-up. Selection of pathologically proven acoustic neuroma cases facilitated validation of the gold standard reference test (GdT1W) but introduced a selection bias compared with a screening population, as did inclusion of eight patients with NF2.
The researchers were interested in clear demonstration of the outline of nerves in CSF, in attribution of a lesion to a nerve of origin and in accurately defining a lesion outline so that growth could be characterised. These objectives recognised the requirements for ‘watch and wait policies’ (for over 65-year-olds in their practice) and for accurate follow-up post surgery or radiosurgery.
The images were evaluated by a single unblinded radiologist. Grading scales were used to evaluate tumour conspicuity and contrast. Tumour volume measurements were calculated ‘offline’ on a workstation by a neuroradiologist using manual tracing and computed summation. The volumes on the three-dimensional GE sequence were assessed first to avoid biasing by the reference test. This was time-consuming and would not have been applicable to the screening population as a cost-effective technique.
Very small lesions were identified (0.05 cm3) and the cochlear, vestibule and semicircular canal were all clearly demonstrated on the three-dimensional GE sequence but not on the three-dimensional GdT1W sequence. The number of small lesions in the study provided supportive evidence of the applicability of the index test to the screening population.
The index test was shown to be highly sensitive and specific, even in small (< 5 mm) acoustic neuroma. The three-dimensional GE sequence was better at defining tumour outline with CSF (but not brain) and better at identifying the related nerves. Cystic tumour components were harder to distinguish from CSF on the index test, but cystic, necrotic or haemorrhagic components were better defined by the index than the reference test.
The authors emphasised the importance of the very high spatial resolution required for a screening test if subtle nerve pathologies were to be detected without the use of GdT1W imaging, and commented that achieving a slice section thickness comparable to, or less than, the width of a cranial nerve would allow this to be achieved in practice.
Evidence from the study did support the use of both the index and the reference test sequences to provide optimal evaluation of acoustic neuroma post treatment.
Zealley et al., 200044
A criterion for normality in this study was clear visualisation of the VIIth and VIIIth nerves, but further specific criteria for abnormality were not defined.
The authors discussed different screening strategies including FSE alone, GdT1W alone, FSE with GdT1W in selected patients and FSE plus GdT1W imaging in all patients. Their preferred strategy was the last, on the basis that the strengths/weaknesses of both sequences were complimentary and the approach would not require supervised image review or delayed recall of patients.
The authors’ conclusions were strongly influenced by the relatively high percentage of tumours (44% overall) that were not allocated to the ‘definitely tumour’ group in their study. They did not explore the implications of varying the diagnostic threshold of the test on test performance or of individual operator performance on test results.
The results presented in Appendix 5 were extracted from the data presented in the paper. If the diagnostic threshold is set at a level appropriate to a population screening test (including ‘uncertain’, ‘probable acoustic neuroma’ and ‘definite acoustic neuroma’ categories as a positive result), test sensitivity is 97%, specificity is 95% and there would be 32 true-positive, one false-negative, 57 false-positive and 1143 true-negative results in the population of 1233 patients screened.
If an ‘FSE alone’ policy had been followed using the suggested ‘screening threshold’, 1144 patients would have not required GdT1W imaging with one false-negative result. In addition to the 32 proven cases, only 57 would have required GdT1W imaging to confirm their false-positive status. The published results would appear to support an ‘FSE alone’ policy with this selected diagnostic threshold.
If the threshold is moved to include ‘acoustic neuroma probably not present’ as well, test sensitivity becomes 100% but specificity falls to 61% and there are 473 false-positive results with 506 patients requiring GdT1W imaging to characterise the tumour or confirm the false-positive status. This represents a significant cost to exclude one false-negative result in the population.
The range in the percentage of FSE scans correctly allocated to ‘definitely normal’ (44–77%), and the moderate correlation for interobserver score allocation, was an illustration in practice of the potential implications of variation in image quality, operator experience and confidence. Variable image quality and operator experience could increase or reduce the number of patients in the uncertain categories and therefore the performance of the screening test.
Two cases of labyrinthine enhancement (2/1233) were detected in the screening population. These were not characterised or discussed.
Annesley-Williams, 200138
This study excluded patients from the results analysis when the reference test was graded inadequate. This resulted in 52 (15%) of the two-dimensional cases and 16 (9.25%) of the three-dimensional cases being excluded. The author stated that they would have recalled these cases for GdT1W imaging and so, in the context of evaluating the FSE sequence as a screening test, these should have been regarded as false-positive results. As a consequence, the sensitivity and specificity calculable for the two-dimensional and three-dimensional FSE sequences in Appendix 5 differ from those quoted in the original paper.
The author rightly comments that the two-dimensional FSE sequence was accurate in detecting acoustic neuroma in the IAC (20/20 detected), providing image quality was adequate. However, the recalculated false-negative and false-positive rates for the two-dimensional FSE sequence illustrate the limitations of this sequence in practice at the time, with the inability to detect lesions as filling defects in the labyrinth and a relatively higher number of inadequate scans because of the inability to define nerve roots in the IAC on 3-mm sections.
Despite the biases described, the results suggest that the performance of the index test in practice improved after the introduction of the three-dimensional FSE sequence, with no false-negative results reported for tumour, and a reduced indeterminate scan rate. The author commented that they had expected a more significant reduction in inadequate rates using the three-dimensional TSE sequence with 1-mm slices; however, they suspected that the scan time of 8 minutes was associated with increased movement artefact.
The author advocated confirmation of all pathological cases with GdT1W imaging to avoid surgery on false-positive cases and discussed the role of GdT1W imaging in detecting subtle intralabyrinthine tumours and other inflammatory lesions. They speculated that, with improved acquisition times, the image quality of three-dimensional FSE might improve to a level at which it was possible to detect intralabyrinthine schwannoma. They also highlighted the potential value of detecting such lesions reliably with GdT1W imaging, facilitating appropriate management of patients with possible NF2.
Ben Salem, 200190
Two radiologists were blinded but reviewed the images jointly, first the index and then the reference test. Only five of the 19 acoustic neuroma were 5 mm or smaller, but the smallest (2.5 mm) was detected. Sensitivity and specificity were excellent and there were no false-negative and only 15 false-positive readings.
The author acknowledged a selection bias in their population resulting in the high incidence (10%) of acoustic neuroma.
Quality of the included papers
Appendix 3 details the results of the application of the QUADAS tool to the selected studies. The following discussion gives a narrative summary of these conclusions.
All of the selected studies set a clear research question and were either prospective or retrospective case–control studies; 6/12 studies included a spectrum of patients that was comparable with a screening population. 38,44,84–86,90
None of the selected papers specified inclusion and exclusion criteria for patients and only four described the subjects’ symptoms explicitly. 84–87 The papers in which the study population was most comparable with the screening population included consecutive series of patients with appropriate symptoms. 38,44,84–86,90
All of the papers compared an index sequence with the reference test, GdT1W imaging, and applied both the index and the reference tests to all cases, without a delay between acquisitions. Although all patients underwent GdT1W imaging, three series employed three-dimensional T1W sequences with MPR with submillimetre slice thickness86,87,89 rather than two-dimensional T1W sequences with 3-mm axial slices. This is unlikely to have influenced the sensitivity of the index test with regard to lesion detection (but does raise the theoretical possibility of a false-negative result for the lower resolution reference test).
Acquisition of the index test was independent of the reference test in all cases, and all studies described the execution of both index and reference tests in sufficient detail for other investigators to replicate them. The index test was interpreted without knowledge of the results of the reference standard in eight out of 12 studies. 38,44,81–83,85,86,88 The reference test was interpreted without knowledge of the results of the index test in five out of 12 studies. 81,82,85,86,88
Three out of 12 of the selected papers did not define the parameters that determined a positive or negative index test result. 81,82,86 This is unlikely to have biased the results of the studies given that the investigators knew what they were looking for.
None of the selected papers provided sufficient data to evaluate test performance for detection and characterisation of acoustic neuroma by patient age, sex or specific subsite location (such as IAC, porus acousticus or CPA). Although this does not undermine the performance of the index test as a screening tool, it impairs the extraction of natural history data from these cohorts.
The method used to evaluate tumour size or volume was documented in only two out of 12 of the selected papers. 38,89 Again, this did not influence lesion detection but illustrates the potential for measurement bias within the population. Significant intra- or interobserver variation at follow-up has profound implications for the evaluation of natural history and clinical effectiveness, as the proportion of patients under serial observation is substantial and increasing.
Indeterminate test results or observer confidence ratings were reported in five out of 12 of the selected series. 38,44,82,83,85 There were no withdrawals from any of the studies, other than omission of the indeterminate results from the analysis of test sensitivity and specificity in one paper;38 these results have been incorporated with the original data for analysis in this review.
Although the evidence evaluated in this review was acquired using ‘old technology’ and might be regarded as out of date, re-evaluation has provided additional supportive evidence for screening using non-contrast sequences, on the basis of data derived from the clinical use of a relatively low-resolution two-dimensional T2W sequence, which was initially reported with a cautious recommendation to use both non-contrast and GdT1W sequences routinely. 44
Factors such as case selection, equipment and image quality, variability in the number and experience of observers, variation in the diagnostic threshold and counting ears evaluated rather than cases, all have the potential to affect the calculation of sensitivity and specificity. It was not possible to control for these factors.
The evidence in this systematic review was therefore extracted from a heterogeneous population with variation in demographic features and investigator methodology over a time frame in which technology evolved and clinical practice changed. Allowing for this, the following specific themes may be drawn from the evidence evaluated in this chapter and from related papers that were reviewed informally as part of a narrative process during the project.
Detection of small acoustic neuromas
None of the papers evaluated in the evidence review had a significant population of patients with small acoustic neuroma. As such, the evidence extracted from the papers that compared a new index sequence with the reference standard (GdT1W) can only robustly support a statement that two-dimensional or three-dimensional T2W or T2*W sequences can reliably detect acoustic neuroma larger than 5 mm without the requirement for GdT1W imaging. However, technological advances in MR have improved the spatial resolution of current three-dimensional T2W or T2*W sequences to a degree that illustrates that this statement is not applicable to current practice, in which high-resolution sequences are used routinely to screen for acoustic neuroma of all sizes.
The evidence evaluated in this review was published between 1995 and 2001. To our knowledge, no studies comparing a T2W or T2*W sequence with a reference GdT1W sequence have been published since. We believe that this reflects the wide acceptance within the diagnostic community that good-quality, high-resolution T2W or T2*W sequences permit exclusion of acoustic neuroma of any size with sufficiently high diagnostic confidence to abandon routine GdT1W imaging. Practice change reflecting this belief was documented in 199682 and discussed in editorials shortly afterwards. 91,92
The refinement of MR technology over time has been a continual process that has resulted in either improved image quality or preserved image quality with shorter imaging times (reducing patient motion). Evidence drawn from a brief narrative review of publications since 2001 is supportive of the assertion that current three-dimensional T2W or T2*W sequences allow confident, reliable identification of the VIIIth and VIIth nerves within the CPA and IAM. In the context of population screening, the criteria that define normality would exclude acoustic neuroma.
This issue of size of tumour impacts on the quality assessment using the QUADAS tool. Only two points are lost in this assessment if there is significant selection bias in the study population that could be responsible for the apparently good performance of a test evaluated using excellent methodology in all other regards (e.g. selecting patients with moderate-sized tumours unlikely to be missed by any radiologist using any MR sequence).
Definition of normality
The majority of papers in the review defined criteria for a normal or abnormal study. The definition of a normal, high-resolution, non-contrast study should include clear visualisation of normal VIIIth and VIIth nerves within the CPA and IAC, along with clear identification of normal cochlear and labyrinthine structures. If a high-resolution study is confidently normal, GdT1W imaging is unlikely to improve diagnostic confidence. 38,44,83–85
Reported advantages of high-resolution T2W or T2*W imaging without contrast
-
Exquisite anatomy: high-resolution T2W or T2*W images provide an accurate anatomical representation of the CPA and temporal bone and enable detection of the normal nerves, or acoustic neuroma, without requiring GdT1W sequences. 81–86
-
Identification of VIIIth and VIIth nerve relationships: high-resolution T2W or T2*W images allow lesion attribution to specific nerve branches. 81,87,89 This can inform the surgical approach and may have prognostic implications, as tumours arising from the superior division of the vestibulocochlear nerve have a higher rate of hearing preservation. Spatial relationships between acoustic neuroma and the VIIth nerve can be determined preoperatively. 93
-
Improved evaluation of inner ear structures: high-resolution T2W or T2*W images permit detection of inner ear extension87 and evaluation of inner ear pathology and dysplasias. 86,97
Reported disadvantages of high-resolution T2W and T2*W imaging without contrast
-
Multiplanar reformats (postprocessing time) are required to confidently exclude subtle pathology at the porus acousticus or in the IAC. 86 Advances in computer workstation technology now permit rapid evaluation of thin-section MPRs, in any orientation, immediately following image acquisition.
-
The incidence of small tumours in the study populations reported is relatively low, with approximately 30% of the reported acoustic neuroma being smaller than 5 mm. Very small tumours may be missed if there is nerve root clumping,44,82 and if there is nerve root clumping or image degradation by artefact, the examination should be reported as indeterminate (requiring recall for GdT1W imaging).
-
High-resolution three-dimensional T2W or T2*W sequences will identify structures that are outlined by, or contain, fluid, for example, CSF or perilymphatic fluid. They will not clearly identify inflammatory or other processes within the temporal bone or brain that may also be responsible for the patient’s symptoms. Other sequences are required for this. 38,81,86
-
High-resolution three-dimensional T2W or T2*W sequences will not necessarily characterise a filling defect that may also be a normal variant, vessel loop, exostosis, lipoma, cavernoma, meningioma or metastasis, although more complex presentations are more likely with some of these pathologies. 38,87
-
High-resolution three-dimensional T2W or T2*W sequences will not detect microscopic acoustic neuroma that do not alter the contour of the VIIIth or VIIth nerves, or the anatomy of the cochlea or labyrinth. GdT1W imaging might detect such subtle pathology but cannot distinguish acoustic neuroma from other inflammatory pathologies (the specificity of GdT1W imaging would be low, the false-negative rate for acoustic neuroma would be high).
Image quality and radiologist experience
Image quality and diagnostic ability are paramount for the clinical effectiveness and cost-effectiveness of a non-contrast screening test.
False positives or negatives may occur as a result of poor image quality and limited radiologist experience or confidence. 38,83 Optimal image quality can be ensured by using modern equipment with good quality assurance and experienced radiographers. Normality should be defined (as above) and indeterminate scans should be either reviewed (second opinion) or recalled for GdT1W imaging.
Radiologists with a detailed knowledge of temporal bone anatomy and neuroanatomy, including anatomical variations and pathology, will reduce the false-negative rate and the requirement for GdT1W imaging for ‘uncertain’ cases. 44,83 The QUADAS assessment tool does not account for the experience of radiologists interpreting images.
Variation in observer confidence and sensitivity/specificity was illustrated by two studies,83,85 and there were moderate interobserver agreement scores in another. 44
The potential impact of radiologist experience can be illustrated by the effect of varying the diagnostic threshold for a positive test result on the false-positive result rate in the study by Zealley and colleagues;44 the false-positive rate increases from 57 to 473 patients when the threshold includes ‘acoustic neuroma probably not present’ cases. In this scenario an additional 416 patients would require GdT1W imaging to prevent one false-negative examination result. 44
Timing of radiology review
If a review is undertaken during the first attendance, an immediate decision regarding the requirement for GdT1W may be made, resulting in no need for recall or a second appointment and a reduced time to diagnosis. However, the radiology cost of supervision of all IAM scans is prohibitive given the low prevalence of acoustic neuroma in the screening population.
Thus, delaying the radiology review and undertaking unsupervised examinations may allow efficient high patient volume and out of hours scanning. The following factors will enhance the efficacy of this strategy: experienced radiographers, optimal scan quality, an experienced radiologist (low recall rate for GdT1W) and a relatively low prevalence of acoustic neuroma in the population.
GdT1W imaging as the gold standard test
GdT1W imaging has evolved from being the gold standard diagnostic test to the gold standard reference test for further evaluation of indeterminate images or characterisation of defined pathology. As very few false-negative cases have been reported for GdT1W imaging,98,99 its role as the gold standard reference test for lesion confirmation has remained unchallenged.
However, as lesion size reduces, the specificity and ultimately the sensitivity of the gold standard declines. 98 Although the sensitivity of GdT1W imaging approaches 100%, the specificity for acoustic neuroma is lower. The reported differential diagnosis for enhancing lesions in the IAC or CPA includes meningioma, metastasis, lymphoma, labyrinthitis, sarcoid and haemangioma. 100 False-positive results for GdT1W MR imaging have been widely reported. 81,82,101–103
Three-dimensional GdT1W sequences have improved the signal-to-noise ratio compared with a two-dimensional T1W sequence, with the equivalent slice thickness, and the MPR facility allows evaluation in any slice orientation, resulting in improved comparability with the three-dimensional T2W or T2*W sequence. 87
The relatively large (3 mm) section thickness of conventional two-dimensional GdT1W images and the low signal of non-enhancing structures (nerves/CSF) limit its accuracy for the measurement of tumour volume in small tumours, the identification of cystic components (particularly within the IAC) and the attribution of a lesion to a specific nerve. 89 Areas of non-enhancement of acoustic neuroma within the IAC may impact on outcome by misleading the choice of surgical approach, for example selecting a retrosigmoid approach to acoustic neuroma involving the lateral IAC. 104
In practice, GdT1W images are rarely interpreted in isolation as they are usually acquired to clarify findings on the screening three-dimensional T2W or T2*W sequence. The described limitations are of limited relevance in the context of screening but are more significant for follow-up of known acoustic neuroma.
Philosophy of cost-effective limited screening versus a more complete examination to detect all possible causes of hearing impairment
The quoted rate of normal scans in acoustic neuroma screening populations is 86–88%. 34,97 The abnormal rate of 12–14% includes a heterogeneous definition of abnormalities and inclusion/exclusion of pathologies. In addition to detection of acoustic neuroma or other soft tissue lesions involving the VIIIth or VIIth nerves, high-resolution three-dimensional T2W or T2*W imaging enables detection of arachnoid or epidermoid cysts and vessel loops within the CPA or IAC and evaluation of the inner ear for malformations and tumours.
Pathologies that may be missed by omitting the GdT1W sequence include small acoustic neuroma, intralabyrinthine acoustic neuroma, labyrinthitis, metastasis, and inflammatory dural and leptomeningeal lesions such as sarcoidosis. Pathologies that may be misinterpreted on three-dimensional T2W or T2*W imaging alone also include vessel loop, arachnoid cyst and lipoma. 38,82,83,85
Arguments for omitting GdT1W imaging from a screening examination include the fact that the incidence of the more aggressive pathologies in the screening population is low and that their presentation with isolated symptoms would be unusual. The benefit of identifying benign processes such as labyrinthitis is limited. 38,82,83,85
A number of abnormalities potentially related to hearing impairment or clinically significant but incidental to the patient’s presentation may be missed if a T2W evaluation of the brain is omitted from the screening evaluation. The ‘incidental abnormality rate’ detected in larger screening cohorts depends on what observers regard as pathological and also on the philosophy of the screening process (cost-minimal focused on acoustic neuroma detection versus inclusive aimed at detecting CNS problems as well). The incidence of unexpected pathology in the healthy population has been documented in a study from Rotterdam105 in which the authors reported the identification of 212 asymptomatic brain lesions in a population of 2000 (10.6%).
The average age of acoustic neuroma screening populations is around 50 years. Small vessel white matter lesions have been detected in up to 20% of such patients. 106 White matter lesions are a marker for cardiovascular risk factors, increased stroke risk and risk for intracerebral haemorrhage in patients placed on anticoagulants. 107 Such patients may benefit from investigation and treatment of previously undetected cardiovascular risk factors (e.g. a potential beneficial ‘side effect’ of the scan).
The prevalence of serious incidental pathologies requiring referral to neurology or neurosurgery was (10/1139) 0.9%34 to (11/644) 1.7%. 106 An approximation to a 1% prevalence would seem reasonable and would correlate with the findings in the Rotterdam study. 105 In our view, this is sufficiently high to justify inclusion of whole brain imaging with the screening IAM scan (which is current practice in the UK).
Contraindications to MR imaging
Absolute contraindications to MR imaging include the presence of a cardiac pacemaker, an intraocular metallic foreign body and some neurosurgical aneurysm clips. In such cases, an alternative imaging strategy is required.
Other factors that may limit the success of an MR examination include claustrophobia and severe obesity (increasing in the UK population). In this case, options include sedation, access to open MR units, CT or alternative non-imaging strategies.
Conclusions
In current clinical practice, MR imaging is the first-line investigation for the identification of suspected acoustic neuroma in appropriately selected patients. The GdT1W sequence remains the gold standard sequence for evaluating an indeterminate test result and for characterising any suspected pathology.
Non-contrast, high-resolution three-dimensional T2W or T2*W sequences enable accurate evaluation of the VIIIth and VIIth cranial nerves within the CPA and IAC as well as evaluation of the cochlea and labyrinth. When these structures are clearly and confidently identified, inclusion of GdT1W sequences is unlikely to contribute information that would alter patient management in the screening population.
The quality of the imaging and the experience of the reporting radiologist are key factors determining the efficacy of a non-contrast screening strategy. Poor image quality or lack of diagnostic confidence or ability will result in increased patient recall rates and use of GdT1W imaging at additional cost or, worse, increased false-negative rates and the cost burden of late re-presentation with an advanced acoustic neuroma.
Chapter 3 Cost-effectiveness
Introduction
The central purpose of an economic evaluation in the context of this report is to compare the relative value of alternative diagnostic algorithms and in so doing to provide information that can aid decision-makers in addressing resource allocation questions. This chapter reviews evidence from the published literature in which the relative value of alternative algorithms has been compared, or from which these can be inferred (see Cost-effectiveness review) and develops a model considering available published evidence in the context of current UK practice (see Cost-effectiveness model).
Two principle types of study are reviewed: cost-minimisation analyses and cost-effectiveness analyses. In cost-minimisation analysis it is assumed that alternative ways of achieving a particular outcome are equally effective. In this context it could mean, for example, that two strategies for investigating acoustic neuroma are equally likely to detect a neuroma of a given size – if one is present – everything else besides cost being equal. Under this condition, only costs need vary for us to establish which strategy represents the more efficient use of resources.
In a cost-effectiveness analysis alternative ways of achieving a particular outcome can differ both in terms of how effective they are and in terms of how costly they are. In this context it could mean, for example, that one strategy for investigating acoustic neuroma is more likely to detect a neuroma of a given size – if one is present – than another strategy. Equally, it could mean that two strategies differed both in terms of effectiveness and cost. To ascertain which strategy is the more efficient would entail a comparison of costs and effects across the alternatives available. That which delivers the greatest effect per pound spent represents the most efficient use of resources.
As the stage at which a diagnosis is made has implications for treatment and long-term outcomes, the chapter briefly reviews treatment options before proceeding to review the literature relating to diagnosis.
Management options
The most common management options for acoustic neuroma are surgery, radiosurgery, radiotherapy108 and interval scanning (‘watchful waiting’ or ‘wait and scan’). Although the risks associated with each may be relatively small, the impact can be dramatic. In the case of surgery these may include facial paralysis and (in very rare cases) death. 57,109,110 In the case of radiosurgery weakness of the facial nerve can result. 108 These risks, coupled with the slow growth of some tumours, can make cautious monitoring a more attractive management strategy, especially in the case of particular groups such as older patients or the medically infirm in whom the risks of intervention relative to potential benefits may be considered high. Outcomes and treatment costs vary with tumour size, a fact that can have a bearing on the cost-effectiveness of alternative diagnostic strategies. That confusion exists regarding the reporting of tumour size across studies is worth noting111 because of the complications it creates for assessing outcomes.
Diagnosis of acoustic neuroma typically involves a physical examination during which a patient history is taken. Audiological tests, which might include pure tone audiometry and speech discrimination, are conducted. It is at this stage that choices emerge in how best to investigate instances of suspected neuroma. Auditory brainstem response, CT, non-contrast-enhanced MR imaging (non-contrast-enhanced MR imaging here can refer to any of the two-dimensional or three-dimensional T2W or T2*W sequences) and GdT1W MR imaging have all been used in a variety of contexts in the investigation of acoustic neuroma. GdT1W MR imaging is considered the gold standard for tumour detection112 (see Chapter 2). Debate, however, exists in the literature as to the value of GdT1W MR imaging relative to other tests given its expense, as well as how the various tests might best be combined in a diagnostic algorithm. 69
Methods
To examine the cost-effectiveness of GdT1W MR in the diagnosis of acoustic neuroma, we undertook a systematic review of the cost-effectiveness of alternative protocols for the identification of suspected acoustic neuroma. Subsequent to a review of the literature, a decision tree was constructed based on expert opinion and then populated with data from the literature and/or expert opinion. The costs of pursuing two alternative diagnostic strategies (contrast-enhanced imaging for all patients in whom pathology is suspected after audiometry versus a strategy in which non-contrast-enhanced MR imaging is used as an intermediate screen) was estimated using this decision model, with a series of sensitivity analyses used to test the robustness of its conclusions. The model and its results are discussed later in this chapter. The cost-effectiveness review section focuses on the review of the literature.
Search strategy
The search terms used and databases searched to identify articles for inclusion are listed in Appendix 1.
The flow chart in Figure 12 (Appendix 2) details the number of references found and the exclusion of irrelevant references at each stage of the review process. The initial search yielded 2754 titles, which were then reviewed by three of the project team (HF, CM, HJ) for relevance. Duplicates (n = 249) were deleted and a further 1755 titles deleted based on relevance. The remaining 750 titles were further sifted by four team members (CO’N, HF, CM, HJ). A total of 85 papers were identified as being potentially relevant based on their titles and recency of publication. One title could not be located and one was found to be a duplicate, leaving 83 in total. These 54 full papers and 29 abstracts were then reviewed by one economist (CO’N). From a review of the bibliographies of the papers three additional papers were located and from among the abstracts obtained one additional full paper was located. In total, 58 papers were reviewed. These included original articles, editorials, reviews and published correspondence between authors. The publications covered the time period from 1977 to 2005, although the majority of the papers were from the mid-1990s onwards. The review was deliberately kept wide to gain as good an appreciation as possible of the issues that impinge on the cost-effectiveness of alternative diagnostic strategies. At this stage it included papers on the long-term consequences of living with an acoustic neuroma or of living with the consequences following treatment. For the systematic review of cost-effectiveness it was decided to include only original articles that compared both the costs and the effectiveness of alternative diagnostic strategies or from which this information could be inferred. This reduced the number of papers to 13. One paper81 was adjudged to report substantially the same data as another82 and was removed from the review, leaving 12 papers. The search was updated to cover the period from October 2006 to August 2008. No additional papers were identified for inclusion in the review.
Quality assessment
The quality of each paper was assessed by two of the authors (CO’N and HF) using the CASP (Critical Appraisal Skills Programme) guidelines on economic appraisals adapted from the checklist of Drummond and colleagues. 113 The assessment of quality is detailed in Appendix 3 together with details of the specific interpretation attached to these criteria.
Data extraction
The following data were extracted from each paper:
-
author(s) and year
-
the test/tests/protocol that were studied
-
the country in which the study was undertaken
-
the study design (retrospective, prospective) and numbers studied
-
data sources used
-
results
-
sensitivity analyses
-
conclusions
-
any additional comments.
Data from the studies included were summarised and critiqued by a single economist (CO’N) to identify common results, variations and weaknesses between studies. No formal attempt was made to synthesise quantitatively the data from the identified studies, although an informal comparison of results from the selected studies is summarised in the discussion section of the cost-effectiveness review. A quantitative analysis was not thought to be appropriate given the differences between the studies in terms of the application of the technologies assessed (e.g. in relation to the definition of ASHI), the patient groups used (some studies were retrospective, others prospective), the publication years (publications spanned 13 years) and the countries in which the studies were conducted (USA, UK, India and Canada, between which substantial differences in unit costs exist).
Cost-effectiveness review
Results
Table 7 summarises the characteristics of the twelve included studies.
| Study | Country | Sample size | Design |
|---|---|---|---|
| Welling et al., 1990114 | USA | 70 | An estimation of costs from a retrospective review of the process leading to the diagnosis of AN |
| Robinette et al., 200065 | USA | 75 | Hypothetical costs associated with identification of AN estimated from a retrospective review of patients’ medical records |
| Robson et al., 199343 | UK | 99 | Prospective study to audit the cost-effectiveness and accuracy of audiovestibular investigations compared with MR imaging |
| Saeed et al., 199542 | UK | 139 | Determination of costs of detecting AN by examination of case notes |
| Ravi and Wells, 199641 | UK | 100 | Examination of costs of confirming or refuting a diagnosis of AN from a retrospective review of clinical records of all patients presenting with suspected symptoms of AN |
| Cheng et al., 2003115 | Canada | 270 | Retrospective chart review of patient records and cost comparison of ABR vs MR screening of AN |
| Carrier and Arriaga, 199713 | USA | 485 | Retrospective review of a focused MR imaging sequence for patients with asymmetric sensorineural hearing impairment and its cost-effectiveness |
| Rupa et al., 200367 | India | 90 | Prospective study to determine the cost-effectiveness of ABR as a screening test in the diagnosis of AN in patients presenting with asymmetric audiovestibular symptoms |
| Daniels et al., 1998116 | USA | 58 | Retrospective cost comparison of routine workup and screening of ANs with cost of fast spin echo MR imaging |
| Allen et al., 199682 | USA | 50 | Blinded review and comparison of unenhanced fast spin echo images with enhanced T1W conventional spin echo images from 25 patients with AN and 25 control patients |
| Marx et al., 199988 | USA | 25 | Prospective study comparing accuracy of fast spin echo MR imaging with gadolinium-enhanced MR imaging in the diagnosis of AN |
| Tan, 1999117 | Singapore | 123 | Prospective study of patients presenting with symptoms of sensorineural hearing impairment, and undergoing MR imaging for the diagnosis of AN |
The studies can be divided into two broad groups: those that compared protocols involving ABR and GdT1W MR imaging in differing combinations (n = 8), and those that compared protocols involving non-contrast-enhanced MR imaging and GdT1W MR imaging (n = 4). It is extremely difficult to classify the papers definitively as cost-minimisation or cost-effectiveness analyses based on the information contained in them. Most focus on costs, dealing with detection rates discursively. In broad terms, 11 have been classified as cost-minimisation analyses (although in a number of cases the authors state that equality of outcomes across protocols does not exist, but nevertheless they do not quantify ‘effects’ and they may perhaps be better thought of as cost–consequence studies) and one as a cost-effectiveness analysis. Just three studies were based on UK data, the remainder spanning the USA, Canada, Singapore and India. A summary of the key findings across the various studies is provided in Table 8. The next section includes a brief review and critique of each paper.
| Study | Country | Sample size | Cost of GdT1W MR imaging | Cost of ABR | Study type | Superior strategy in terms of efficiency |
|---|---|---|---|---|---|---|
| GdT1W MR imaging studies | ||||||
| Welling et al., 1990114 | USA | 70 | US$1235 | US$220 | CMA | ABR/GdT1W MR imaging > traditionala |
| Robinette et al., 200065 | USA | 75 | US$1500 | US$300 | CEA | ABR/GdT1W MR imaging > GdT1W MR imaging |
| Robson et al., 199343 | UK | 99 | £130 | Unknown | CMA | GdT1W MR imaging > traditionala |
| Saeed et al., 199542 | UK | 139 | £165 | Unknown | CMA | GdT1W MR imaging > traditionala |
| Ravi and Wells, 199641 | UK | 100 | £137 | Unknown | CMA | GdT1W MR imaging > traditionala |
| Cheng et al., 2003115 | Canada | 270 | C$260 | C$44.60 | CMA | Traditionala > GdT1W MR imaging |
| Carrier and Arriaga, 199713 | USA | 485 | US$300–500 | US$300 | CMA | GdT1W MR imaging > traditionala |
| Rupa et al., 200367 | India | 90 | US$200 | US$13.33 | CMA | ABR/GdT1W MR imaging > GdT1W MR imaging |
| Non-contrast-enhanced MR imaging studies | ||||||
| Daniels et al., 1998116 | USA | 58 | GdT1W MR imaging US$1200; non-contrast-enhanced MR imaging US$415 | US$245 | CMA | Non-contrast-enhanced MR imaging > traditionala |
| Allen et al., 199682 | USA | 50 | GdT1W MR imaging US$1200; non-contrast-enhanced MR imaging US$400 | N/A | CMA | Non-contrast-enhanced MR imaging > GdT1W MR imaging |
| Marx et al., 199988 | USA | 25 | Unknown | N/A | CMA | Non-contrast-enhanced MR imaging > GdT1W MR imaging |
| Tan, 1999117 | Singapore | 123 | GdT1W MR imaging S$950; non-contrast-enhanced MR imaging S$400 | N/A | CMA | Non-contrast-enhanced MR imaging > GdT1W MR imaging |
Discussion
Staged ABR and GdT1W MR imaging protocols
The study by Welling and colleagues114 compared the cost of a ‘traditional’ diagnostic protocol [including electronystagmography (ENG), CT and unenhanced MR imaging as well as ABR and GdT1W MR imaging in the algorithm] with the cost of one that deployed GdT1W MR imaging or ABR after audiometry was used to assess the likelihood that a neuroma may be present. Strength of suspicion was determined using an assessment of the patient’s history, a physical examination and audiometry results (pure tone air and bone conduction levels and speech discrimination). Only those patients considered to have a low risk of neuroma (less than 5%) were referred to ABR. Those at intermediate or high risk (5% or above as assessed by reviewer) were referred for GdT1W MR imaging immediately after audiometry. (If an individual’s ABR results were abnormal among the low-risk group, a subsequent referral for GdT1W MR imaging was advocated.)
Unit costs were estimated from a survey of four independent institutions conducted in 1989. (The average cost of ABR was reported as US$220 and of GdT1W MR imaging as US$1235.) The average cost of the traditional diagnostic work-up (in which ENG and CT, etc. might feature) was based on a retrospective review of 70 patient records and application to these of unit costs. The average cost of the traditional protocol was estimated at US$2568 (this figure excluded office visits and treatment). By comparison, the estimated average cost of the alternative diagnostic strategy (ABR and/or GdT1W MR imaging immediately after audiometry based on an assessment of risk) was US$1150. (Assuming a dollar to sterling conversion of £1 = US$2, the cost of the traditional protocol in 1989 prices would be £1284 and that of the alternative strategy would be £575.)
The paper only considers other costs related to treatment of the neuroma, long-term sequelae and litigation, and offers some estimates in relation to the first two from a range of sources. The average cost of hospitalisation for excision of an acoustic neuroma was reported as US$9400 when there are no complications and US$39,950 when major complications arise. (Surgery was only attempted on a subgroup of patients, the team opting to manage seven through observation rather than intervention. Six of those operated on, however, experienced major complications.) When a delay in diagnosis results in profound unilateral SNHI, an impairment equivalent to 6% of annual salary – US$18,000 over the average remaining working life – was hypothesised as an estimate of the cost of lost production (35% lost income being suggested for bilateral deafness).
A number of problems arise with this study. First, the 70 patients in the study were individuals known to have tumours. Given this, although the detection rates across protocols could be treated as being equal and thus the study amounts effectively to a cost-minimisation analysis, whether this group would be representative of those to whom the protocols would be applied in practice is debatable. Individuals exhibiting symptoms who are not imaged may well experience a different diagnostic process (and cost) to that of those encountered in this group. From this study, for example, we do not know how many false negatives might have arisen in the ABR examinations of those judged ‘low risk’ among an unselected group. In consequence, we do not know the cost associated with false negatives in this protocol. Similarly, we do not know what the compliance might be for those assigned to recall monitoring within such a protocol, or again what costs might arise from this in terms of missed tumours. Although a sensitivity analysis could potentially have gone some way to addressing these issues, none was undertaken in the study.
A second, although relatively minor, issue concerns the discussion of the treatment and costs of long-term sequelae. This study is almost unique in making reference to such costs, but is somewhat naïve in its treatment of them. Considerable caution must therefore be exercised in interpretation of this discussion. For example, no discounting of future lifetime earnings is undertaken in the estimate of future lost earnings. Again, although this is an issue that could perhaps have been dealt with in a sensitivity analysis, that none was undertaken means that the result must be viewed cautiously. Although the study makes no claim to have definitively established the cost-effectiveness of a risk-assessed protocol over a traditional approach, the inference that this is the case is made.
The study by Robinette and colleagues65 essentially follows the same approach as that of Welling and colleagues114 and is again US based. The authors take data relating to 75 patients (all of whom had been surgically confirmed as having tumours) and assign the patients to the Welling and colleagues’114 groupings of high, intermediate and low risk. (As the symptoms presented in case notes did not always fit the Welling and colleagues’114 criteria, some interpretation was used on occasion.) Based on the prevalence of tumours among individuals exhibiting the symptoms described by Welling and colleagues114 (prevalence rates as reported in Buach and colleagues118), the number of individuals needed to generate the number of tumours observed was calculated. The costs of screening this number with an GdT1W MR imaging protocol as the first test after audiometry and of using a protocol that incorporates a risk-assessed screen in which ABR is included (in line with Welling and colleagues114) are calculated. The sensitivity for ABR and GdT1W MR imaging from Buach and colleagues118 is then used to determine the expected number of false negatives in each risk grouping, and, finally, the additional cost required to detect these by adopting an GdT1W MR imaging first test protocol is calculated. The study design can be described as a cost-effectiveness analysis in as much as costs are related to the number of expected missed tumours under the two protocols.
Unit costs are presented as US$300 for ABR and US$1500 for GdT1W MR imaging. The source for these is not identified, although from the context in which they are presented they are likely to reflect billable charges levied at the study site. The cost-effectiveness of the risk-assessed diagnostic algorithm over the GdT1W MR imaging first test algorithm is demonstrated by reference to the cost that would be incurred were one required to detect the tumours that would otherwise be missed. This cost is shown to rise across the risk groups, the low-risk group being higher than the intermediate-risk group and the intermediate-risk group being higher than the high-risk group. Thus, to find the one tumour missed in the high-risk group, it is estimated that a protocol deploying GdT1W MR imaging immediately after audiometry would cost an additional US$30,900. To find the four missed tumours in the intermediate risk group would cost an estimated additional US$858,000 (or US$214,500 per tumour), and to find the one missed tumour in the low-risk group would cost an estimated US$1.6 million.
The study is not compromised by selection bias to the same extent as that of Welling and colleagues. 114 Although a selected sample is used – all individuals were known to have tumours – the number of persons one would expect to screen in order to generate the number of tumours observed was derived from a study by Bauch and colleagues118 in which both tumour and non-tumour patients were present. In this respect, the data are more likely to be representative of patients to whom tests would be applied in practice. However, the use of prevalence data from another study as well as one set of unit costs does build assumptions into the analysis upon which the study’s results and conclusions are predicated. Varying these assumptions, as the authors state (and provide some guidance on), would alter the study results. A formal sensitivity analysis is, however, not undertaken nor are dollar estimates of costs per missed tumour across groups given under different assumptions.
These caveats noted (and broadly acknowledged by the authors), the study nevertheless extends the conclusion of Welling and colleagues114 that a diagnostic strategy based on risk assessment in which ABR has a role is not only likely to be more cost-effective than one based on a more protracted ad hoc battery of tests but also more cost-effective than one that deploys GdT1W MR imaging as a first-line test after audiometry.
Robson and colleagues,43 Saeed and colleagues42 and Ravi and Wells41 report the results of studies undertaken in the UK. In each, comparisons are made between protocols involving specific GdT1W MR imaging tests immediately after audiometry and traditional diagnostic protocols involving a range of intervening tests. Each is undertaken broadly within what might be considered as being cost-minimisation analyses.
Robson and colleagues43 report what is effectively a prospective study of 99 patients. The authors state that all 99 patients were referred for GdT1W MR imaging before the results of their audiovestibular examinations were known. The results of these tests should not therefore have influenced the decision to refer patients for GdT1W MR imaging and thus the study can be thought of as being prospective. Although it is conceivable that an elevated suspicion of neuroma must have been present for GdT1W MR imaging to have been requested, that other tests were requested first suggests that any elevated risk cannot have been very high. Unit costs are based on charges levied on private patients at a single UK hospital (Radcliffe Infirmary, Oxford) in 1992. The cost of directing 99 patients to imaging immediately after audiometry is estimated at £12,900, the unit cost of GdT1W MR imaging being reported at £130. The cost of the existing protocol that included CT and ABR (as indicated by need) before imaging is estimated at £12,545 (these costs exclude GdT1W MR imaging and any repeat CT scans that may in reality have been undertaken). The imaging protocol involves patients being processed in batches (thus obviating the need to adjust equipment, and saving on scan time) as well as the use of a reduced amount of gadolinium. The elimination of the need for repeat CT and/or ABR or GdT1W MR imaging when equivocal results from CT and ABR remain equivocal, or of missed false negatives when GdT1W MR imaging is not used to exclude a tumour, would likely erase the small cost difference between the two protocols. As with the other papers reviewed thus far, however, no sensitivity analysis is undertaken to illustrate this.
The prospective study design in which all patients presenting with symptoms giving rise to a suspicion of acoustic neuroma are included in the study is one of the paper’s strengths, although the absence of a sensitivity analysis means that the conclusions must be treated with some caution. In as much as only costs are formally compared, it can be thought of as a cost-minimisation analysis. GdT1W MR charges reflect in part the reduced scan time (20 minutes compared with the standard of approximately 60 minutes) as well as the reduced dose of gadolinium. Using an exchange rate of £1 = US$2 and tripling the scan time involved, the dollar equivalent would be US$780. As can be seen, this is somewhat less than the costs reported by Welling and colleagues114 and Robinette and colleagues. 65 The reduced dose of gadolinium may in part explain the difference as may the different geographical settings for the studies; however, the possibility that unit costs are not calculated on the same basis remains.
With these caveats in mind the study does seem to provide prima facie evidence that GdT1W MR imaging immediately after audiometry for patients in whom an acoustic neuroma is suspected is more cost-effective than a strategy in which GdT1W MR imaging is deployed only after further intervening tests. Although this would appear to contradict the findings of the study by Robinette and colleagues,65 the different unit costs and the different protocol of tests used between the two studies must be borne in mind. To what extent a direct comparison is possible is debatable.
In the study by Saeed and colleagues,42 a traditional protocol (involving CT as well as ENG, ABR and GdT1W MR imaging) is again compared with a protocol in which GdT1W MR imaging is conducted immediately after audiometry has failed to remove suspicion of a neuroma. The cost of processing patients who would undergo GdT1W MR imaging was estimated using details of tests involved in the traditional protocol. Unit costs relate to the authors’ own department (Manchester, UK), although how exactly these have been arrived at is unclear. The unit costs are similar to those of Robson and colleagues43 for imaging (£165) and, as with Robson and colleagues,43 were based on a protocol that involved processing patients in batches (requiring a 30-minute scan time) as well as use of a reduced dose of gadolinium. (Adjusting these for a 60-minute scan time and converting to dollars at an exchange rate of £1 = US$2, the GdT1W MR unit cost would equate to US$660.) The cost per patient of the traditional diagnostic process is estimated at £188.22, and the cost of an GdT1W MR imaging first protocol is estimated at £180.05. Cases in which tumours may have been missed because of equivocal CT or ABR results are presented suggesting that the two protocols would not be equally effective. As with Robinette and colleagues,65 costs per tumour missed are not calculated. The costs of operating the two protocols are very similar. The similarity of the costs forms the basis for the study’s conclusion and thus it can be thought of as a cost-minimisation analysis. Given the similarity of costs under the two protocols and the likely incomplete nature of costs under the traditional protocol, the dominance of the GdT1W MR imaging first protocol over the traditional diagnostic strategy is implicit.
The relative cost-effectiveness of the protocol in which imaging is undertaken more widely and earlier in part reflects the effect of eliminating equivocal intervening tests. If these results were from an unselected group of patients (as was the case in Robson and colleagues43) there is also a strong likelihood that the traditional protocol would miss some tumours because of the lower sensitivity of the tests deployed (i.e. like is not compared with like in terms of effectiveness). The corollary of this is that if this was an unselected group of patients there would be good reason to believe that the cost advantage reported for a GdT1W MR imaging first test protocol after audiometry would be greater than is indicated here. However, it is not entirely clear that this is an unselected group of patients. It is not stated what prompted the GdT1W MR imaging request for this group, merely that ‘139 requests were made as part of the investigative protocol for patients with unilateral or asymmetrical sensorineural hearing impairment’. In other words, it is possible, as with Welling and colleagues,114 that the request for imaging was informed by the results of other tests besides audiometry.
If we assume that this is not the case, then the study is similar to that of Robson and colleagues43 and provides further evidence that a strategy in which GdT1W MR imaging is deployed immediately after audiometry is more cost-effective than one in which it is deployed only after other intervening tests. If we do not make this assumption then the result must be treated with somewhat more care. A straight to GdT1W MR strategy may see many referred for GdT1W MR imaging who could be (and implicitly were) eliminated from further investigation by cheaper tests. That a GdT1W MR image was requested for all in this group may indicate that it was in fact needed to positively diagnose or eliminate acoustic neuroma as a cause of the symptoms exhibited. In this situation, savings attributed to the exclusion of intermediate diagnostic steps would then merely reflect that, for a group that could be defined ex post (i.e. once its participants have been the tested we know its value), by definition such tests were of no value. As with the other studies, no formal sensitivity analysis is undertaken, again indicating the need for some care to be exercised in drawing conclusions from the study.
In Ravi and Wells,41 similar issues arise. These authors compare the cost of a traditional protocol, in which CT as well as ENG and GdT1W MR imaging are used to investigate suspected acoustic neuroma, with that of a GdT1W MR image immediately after audiometry protocol. Case notes on all tests conducted on 100 patients are examined to identify those in whom a GdT1W MR image was requested to eliminate an acoustic neuroma. The unit costs for GdT1W MR imaging are taken from one of the study’s centres, although these are demonstrated to be similar to those at the other study sites (£110, £137 and £158, £137 being that used in the study) and are similar to those of Robson and colleagues. 43 When, following a pure tone audiogram, patients were referred to GdT1W MR scanning as their next investigation, the cost per patient is estimated at £220.72 (if results were communicated by the GP) or £258.72 (if results were communicated via an outpatient visit). The £220.72 comprises £137 for a GdT1W MR image, £75 for an ENT consultation and £8.72 for pure tone audiometry. By comparison, when other tests besides a pure tone audiogram were undertaken before GdT1W MR imaging was conducted, the cost per patient is markedly higher – £417.31 for those who underwent a pure tone audiogram, ‘special’ audiological tests and ENG as well as GdT1W MR imaging. (The precise GdT1W MR test is not described, although the costs suggest it is similar to that in the studies by Robson and colleagues43 and Saeed and colleagues. 42) As with Saeed and colleagues,42 the analysis of cost-effectiveness in terms of cost per tumour missed or detected is not pursued, and the study may be thought of in terms of a cost-minimisation analysis. That a protocol in which GdT1W MR imaging is deployed as a first test costs less than a protocol involving intervening steps is sufficient to demonstrate its dominance.
As with Saeed and colleagues,42 the interpretation of results here depends on the interpretation of recruitment to the study. If the 100 participants whose notes were examined reflect a selected sample – individuals for whom other tests after pure tone audiometry were equivocal – they cannot be viewed as being equivalent to the general population of individuals for whom audiometry has failed to rule out a tumour (among a group comprising the latter, other tests may not have been as equivocal and imaging may have been pursued). Were this the case, any savings attributed to the exclusion of intermediate diagnostic steps would merely reflect that, for a group defined ex post (i.e. for when having conducted the tests we know they were of no value), savings could be derived by the elimination of intermediate steps. If, on the other hand, the sample represents an unselected group (everyone for whom pure tone audiometry has failed to exclude a tumour) then, as with Robson and colleagues,43 the study lends weight to the argument that GdT1W MR imaging immediately after audiometry is a more cost-effective diagnostic strategy for acoustic neuroma than one that involves additional steps. From the text, one cannot be certain of which of these is the case. The fact that it is not stated explicitly that this is an unselected group adds to the suspicion that this was not the case. As with the other studies, no formal sensitivity analysis is undertaken and again caution is warranted in regard to the study’s results.
In Cheng and colleagues,115 the cost of a protocol that involves GdT1W MR investigation after audiometry is compared with that associated with a traditional protocol. Included in the battery of tests deployed in the traditional protocol are ABR, CT and ENG as well as GdT1W MR imaging. Data on the tests performed on 270 patients who presented consecutively at a Canadian hospital and in whom an acoustic neuroma was suspected were extracted from their notes. Unit costs for the tests used were derived from the Ontario Health Insurance Plan for 1998 and combined with data on test use to produce an estimate of total costs for all patients. The unit cost of an uncontrasted MR image is reported as C$260 and that of an ABR as C$44.60 (broadly equivalent to US$244 and US$42 respectively). This was then averaged across the 270 patients to produce an estimate for the traditional protocol of C$191.19 (US$180). The average cost for the 270 patients to receive audiometry (pure tone, speech discrimination and acoustic reflex tests) followed by GdT1W MR imaging without other intervening tests was estimated at C$310.30 (US$292).
In as much as the study deals with an unselected group of patients, comparing the actual costs of administering one protocol with the estimated costs of another (effectively a cost-minimisation analysis), it does not suffer from selection bias as was the case in some of the other studies. However, against this, under the traditional protocol the number of false negatives from ABR and other tests are unknown. (‘Most patients with normal ABRs were assumed to have no retrocochlear pathology. No further testing was performed on such patients’.) As the number of tumours missed is unknown, like is not being compared with like in terms of effectiveness across the two protocols. It follows that the costs associated with an unknown number of potentially missed tumours are implicitly ignored in the study. Although the study could be classified as a cost-minimisation analysis, the validity of the assumption that the two diagnostic strategies have the same outcomes is difficult to sustain (an unknown amount of costs associated with missed tumours has been omitted from the traditional protocol). As no formal sensitivity analysis is undertaken, the impact on the findings of relaxation of the implicit assumptions regarding missed tumours or of varying the costs associated with the conduct of any of the diagnostic tests is unknown. [The figures quoted in the paper are somewhat confusing. The authors quote a unit cost of GdT1W MR imaging of C$310 but use a cost of C$260 in their calculations, the unit cost they quote for non-contrast enhanced MR imaging. The total cost of processing the 270 patients would be C$83,700; adding to this audiometry costs (C$13,581) the total for a GdT1W MR imaging protocol would be C$97,281 and not the C$83,781 quoted in the paper.]
In Carrier and Arriaga,13 a protocol that involves imaging immediately after audiometry is compared with a protocol that permits further investigation before imaging based on ABR – similar to the study by Welling and colleagues114 but without formal risk assessment. As with Robson and colleagues,43 a specific imaging strategy in which a reduced dose of gadolinium is administered is followed, as well as a reduction in the number of images printed. Although the authors make no reference to batching patients, the total imaging time including set-up and administration of gadolinium is reported at between 20 and 25 minutes, similar to the times reported by Robson and colleagues43 and Saeed and colleagues42 when batching does take place.
The images of 485 patients who presented with symptoms of ASHI or unilateral non-pulsate tinnitus were reviewed and a diagnosis arrived at. The cost of the ‘straight to imaging’ protocol is estimated based on direct progression to imaging after audiometry. This is estimated at US$300–500 per scan, the lower cost reflecting the lower scan time as well as use of a reduced amount of gadolinium. The cost of an ABR is quoted at US$200–300 per test. In what amounts to a cost-minimisation analysis the authors conclude that, given the lower sensitivity of ABR compared with GdT1W MR imaging and the associated costs of additional confirmatory tests that would likely be involved if ABR was deployed, as well as the costs of potentially missed tumours, the direct to imaging protocol is more cost-effective than traditional algorithms.
As with the other papers reviewed, however, a number of issues arise in relation to this study. First, it is not clear if the sample used here has been selected – it is one for which images exist, and others may have been filtered out before reaching this stage. It seems unlikely that imaging would have been requested unnecessarily and thus it is likely that it is a selected sample. This need not effect the conclusion of the study as the costs of the diagnostic tests are not actually calculated based on the use of tests in the sample. Rather, the relative cost-effectiveness of the direct to GdT1W MR imaging strategy hinges on the relative unit costs and assumptions regarding repeat examinations and missed tumours. Although the unit cost reported for an ABR (US$300) is in line with the costs reported by Robinette and colleagues65 and Welling and colleagues,114 it is at variance with those reported in the UK studies as well as in other US studies. Thus, the unit cost reported for an ABR by Cheng and colleagues,115 for example, is US$44.60 (£22.30); in Robson and colleagues43 a unit cost of £55 (approximately US$110) is reported and in Saeed and colleagues42 there is a cost of £23 (approximately US$46). As with the other studies, a sensitivity analysis could have assessed the impact of uncertainties regarding these costs (as well as those regarding the sensitivity of ABR and the cost of missed tumours) but none was undertaken. If the unit costs are considered credible the study does provide evidence supporting the contention that a direct to GdT1W MR imaging protocol is likely to be more cost-effective than one that entails intervening tests.
Finally, the prospective study by Rupa and colleagues67 examined 90 patients, comparing a protocol using ABR and GdT1W MR imaging in the investigation of suspected acoustic neuroma with a protocol using GdT1W MR imaging only after audiometry. The study was conducted in India with unit costs for GdT1W MR imaging and ABR based on those operating at the hospital in which the study was conducted (although it is not stated, one assumes that these reflect charges levied on users). In total, 18 patients were unsuitable for ABR in the study. Among the remainder, all received ABR and GdT1W MR imaging. ABR had 100% sensitivity among those tested (as confirmed by GdT1W MR imaging results) and cost one-fifteenth of GdT1W MR imaging (US$13.33 versus US$200). If all 90 patients were to receive GdT1W MR imaging after audiometry, diagnostic costs would have been US$18,000. If only the 18 unsuited to ABR and the 30 whose ABR results suggested retrocochlear pathology were to receive GdT1W MR imaging, all others receiving only ABR, total costs would have been US$10,800. This indicates that a diagnostic algorithm incorporating ABR as an initial screen followed by GdT1W MR imaging is more cost-effective than one deploying GdT1W MR imaging immediately. This study thus apparently contradicts that of Carrier and Arriaga13 as well as the UK studies but is in line with those of Welling and colleagues114 and Robinette and colleagues. 65
At face value, this study offers perhaps the most convincing evidence of the various studies examined thus far that a diagnostic algorithm incorporating GdT1W MR imaging after initial screening with ABR is more cost-effective than one with GdT1W MR imaging after audiometry. The patient group is unselected and information on false negatives under the different search strategies is known (there were none). On closer examination, however, the patient group studied is found to be atypical of that likely to be encountered in most Western hospitals. Of the six tumours detected among this group, four were in excess of 1.5 cm in size and two were 3 cm in size. These are somewhat larger than is typically the case in other studies (see Chapter 4, Growth). This clearly explains the high sensitivity of ABR in this study (see Chapter 2, Comparison of the use of auditory brainstem response with the use of magnetic resonance imaging). Moreover, although the unit cost of GdT1W MR imaging is broadly comparable to that reported in other studies, the unit cost of ABR does seem somewhat cheap. Although the study does appear valid – even in the absence of a sensitivity analysis – whether the results are applicable to a UK setting is highly questionable.
GdT1W MR imaging versus non-contrast-enhanced MR imaging
The study by Daniels and colleagues116 compares a diagnostic algorithm that deploys non-contrast-enhanced MR imaging as a first test after audiometry with a diagnostic strategy in which ABR is used as a screen followed by GdT1W MR imaging. Unit costs are based on the charges levied at the study institution and are US$245 for ABR, US$1200 for GdT1W MR imaging and US$415 for non-contrast-enhanced MR imaging. The patient group studied is selected, comprising only those who have experienced sudden SNHI. The sample includes 58 individuals, and the use of various tests is based on a retrospective review of patient notes regarding ABR testability and results. Those who were not ABR testable were assigned the cost of GdT1W MR imaging (this being the diagnostic test they would have been referred to) and those who were ABR testable were assigned the cost of an ABR and the cost of GdT1W MR imaging if their ABR results were abnormal (for those with normal ABR results five cycles of audiometry follow-up were assigned).
Total costs for the ABR/GdT1W MR imaging protocol for the 58 patients were estimated as US$52,175. Using non-contrast-enhanced MR imaging as the sole test after audiometry, total costs were estimated as $24,070, a difference of $485 per patient evaluated. If we assume that the two protocols are equally effective in the diagnosis of tumours this would indicate that the non-contrast-enhanced MR diagnostic protocol is more cost-effective than the traditional protocol. If, more realistically, we assume that the traditional protocol is less sensitive than non-contrast-enhanced MR imaging (because of the role of accorded ABR), more false negatives would be associated with the traditional protocol and costs associated with complications and long-term sequelae would also be higher. Interpreting this as a cost-minimisation study it seems reasonable to assume that, for this patient group, the extent to which non-contrast-enhanced MR imaging is more cost-effective than the traditional protocol is underestimated. The extent of any overestimation though is impossible to assess.
An attempt at a sensitivity analysis is undertaken in this study. In the sensitivity analysis the percentages of patients that one would expect to be ABR non-testable and, of those who are ABR testable, that one would expect to exhibit abnormal ABR results are estimated for a hypothetical cohort of 100 patients. Rates are based on a meta-analysis of the literature for patients with sudden SNHI. Under these revised assumptions the non-contrast-enhanced MR imaging protocol was estimated to have a slightly lower saving of US$431 per patient associated with it relative to the ABR/GdT1W MR imaging protocol.
A key issue with this study, however, (echoing the criticisms levelled at a number of the other studies already examined) is that the findings relate to a selected (and in this case very specific) patient group – namely those who have experienced sudden SNHI. This group represents only a small proportion of those who experience acoustic neuroma – reported as 5–15% in this study based on the literature (see Chapter 4, Symptoms). How representative the detection rates and costs associated with the different diagnostic strategies applied to this group are for the other 85–95% is unclear (the authors make no claims in regard to this broader group).
A second issue with the paper (as with various others) is the absence of a formal sensitivity analysis. Although the unit costs deployed, for example, are in line with those contained in several of the studies already examined, as has already been noted, considerable variation in costs exists in the literature and the costs reported here are somewhat more expensive than those reported by other studies. A sensitivity analysis would have let the authors identify the range over which costs could vary without effecting their conclusions. Similarly, based on the literature, the paper reports the sensitivity of non-contrast-enhanced MR imaging as 100%. This is by no means universally agreed (as discussed below). The authors could have used sensitivity analyses to explore the range of sensitivities for non-contrast-enhanced MR imaging and ABR for which one protocol was more cost-effective than another in this patient group; however, no such analysis was undertaken.
In the study by Allen and colleagues,82 a patient group with more general symptoms was examined with non-contrast-enhanced MR imaging and conventional GdT1W MR imaging to determine the relative cost-effectiveness of the two imaging approaches. A group of 25 patients with GdT1W MR imaging-confirmed diagnoses of acoustic neuroma and 25 control subjects were evaluated in blinded readings of non-contrast-enhanced MR images and GdT1W MR images by four neuroradiologists. GdT1W MR imaging was assumed to have 100% sensitivity and non-contrast-enhanced MR imaging was demonstrated in the study to have 98% sensitivity. Unit costs were based on billable charges; for GdT1W MR imaging the unit cost was US$1200 and for the non-contrast-enhanced MR examination the unit cost was US$400. The authors assert that there is no statistical difference between the two tests in terms of the results obtained but that non-contrast-enhanced MR imaging is US$800 cheaper per test than GdT1W MR imaging and therefore more cost-effective. The non-contrast-enhanced MR imaging protocol indeed can be made even cheaper by removing unnecessary ABR and impedance testing from the GdT1W MR imaging protocol and made more effective by deploying a more refined search approach (taking thinner sliced images), which is now possible. In effect, the study amounts to a cost-minimisation analysis in which non-contrast-enhanced MR imaging is clearly less costly.
However, the false-negative rates reported in the study indicate that GdT1W MR imaging and non-contrast-enhanced MR imaging are not equally effective despite their treatment as such by the authors in this study. It follows that a cost-minimisation analysis is not appropriate here. The cost of missed tumours should have been factored into the analysis. Although the low false-negative rates may make it unlikely that this would have effected the result (non-contrast-enhanced MR imaging would have remained the more cost-effective strategy), no sensitivity analyses were undertaken that could perhaps have addressed this issue. A further issue commented on by Jackler91 is whether the high sensitivity achieved at this centre could reasonably be expected to be replicated elsewhere. Other centres are unlikely to have the experience and expertise in the use of the non-contrast-enhanced MR technology that exists at this site. Other centres may not achieve the high sensitivity achieved here and may experience higher false-negative rates (and costs associated with these). Thus, the study’s findings must be treated with some caution, especially given the small sample size involved.
The study by Marx and colleagues,88 although not claiming to examine cost-effectiveness, nevertheless contains information from which an assessment of cost-effectiveness can be made. The study compared the sensitivity of non-contrast-enhanced MR imaging with GdT1W MR imaging prospectively in 25 patients whose history, physical examination and audiometry results gave rise to a suspicion of acoustic neuroma. Patients were investigated with both protocols; all tumours detected with GdT1W MR imaging were detected with non-contrast-enhanced MR imaging giving 100% sensitivity. The cost of non-contrast-enhanced MR imaging is reported as being one-third of that for GdT1W MR imaging at this institution, although no actual figures are given. The lower cost is attributed to a shorter scan time, absence of contrasting and a reduced number of printed images.
The study’s strength is its prospective design of an unselected group of patients. That the study group exhibited many smaller tumours – as small as 4 mm – adds weight to the contention that non-contrast-enhanced MR imaging is as sensitive as GdT1W MR imaging (the average size of tumours in the group was 1 cm), although the fact that this is a small sample studied by an experienced team and that these are results obtained in a trial, in which greater care is deployed than might be under normal circumstances, may all have affected the outcome. The advantages of GdT1W MR imaging in detecting other pathology are, as the authors point out, also perhaps worth considering. The authors are cautious in recommending non-contrast-enhanced MR imaging over GdT1W MR imaging commenting on the need to ensure that the skills of the team using the technology are adequate. In as much as this was not a cost-effectiveness analysis the study cannot really be criticised for failing to conduct a sensitivity analysis, although one would certainly have been useful. With these caveats in mind the study does provide evidence that a diagnostic algorithm deploying non-contrast-enhanced MR imaging after audiometry may be more cost-effective than one relying on GdT1W MR imaging.
Finally, as with Marx and colleagues,88 the study by Tan117 does not purport to be a cost-effectiveness analysis but it contains information from which inferences regarding cost-effectiveness may be drawn. The study relates to 123 individuals who presented with audiovestibular symptoms indicative of acoustic neuroma. As non-contrast-enhanced MR tests that were negative for acoustic neuroma were not confirmed by GdT1W MR imaging, the sensitivity of non-contrast-enhanced MR imaging cannot be assessed in this study. The authors, however, refer to the literature, including the study by Allen and colleagues,82 to assert that non-contrast-enhanced MR imaging may miss between 6% and 8% of smaller tumours. The cost of GdT1W MR imaging (S$950 or US$627) is based on billable charges at this institution; this compares with a cost of S$400 (US$264) for non-contrast-enhanced MR imaging. The lower charge is attributed to a shorter scan time as a result of not using contrast. The authors are conservative in their conclusions, suggesting that non-contrast-enhanced MR imaging be used for those who cannot afford GdT1W MR imaging, for those whose presenting symptoms indicate a low probability of acoustic neuroma (e.g. vertigo and/or tinnitus without SNHI or when the history suggests that any tumour present is likely to be large) and in the case of elderly and/or medically infirm patients in whom no real harm may result from small tumours missed. GdT1W MR imaging by inference is still seen as the gold standard and the test that should be used when a strict budget or other information does not contraindicate it.
Conclusions
Table 8 provides a summary of the key information taken from the various papers. Compared with traditional protocols that deploy what have essentially become redundant tests such as CT and ENG, strategies that deploy GdT1W MR imaging immediately or in conjunction with ABR appear to be more cost-effective. A consensus on this would appear to exist based on the work of Welling and colleagues,114 Robson and colleagues,43 Saeed and colleagues,42 Ravi and Wells,41 and Carrier and Arriaga. 13 Cheng and colleagues115 appear to be the one dissenting voice, but care is warranted in making these comparisons given the differences in patient groups, the construction of costs, the performance of tests and the criteria used to define ‘abnormal’. The quality of all of these studies is questionable. Exposure to selection bias, the failure to include important elements of cost or assess efficacy convincingly and the failure to include meaningful sensitivity analyses or assess incremental cost-effectiveness, as well as differences in patient groups and the timing of the studies and the fact that a number of the studies are now almost 20 years old mean that the results from each must be treated with some care and comparisons between them made with extreme caution. That few of the studies made any reference to any costs borne by the patients and none made any reference to costs associated with travel related to repeat visits is also notable, especially as these may have important implications for compliance with recall in a wait and watch strategy. The impact of rapid developments in imaging technology, cost and patient expectations,119 as well as emerging evidence in respect of quality of life after surgery,120 must also be borne in mind [emerging evidence regarding the relationship between gadolinium and nephrogenic systemic fibrosis (NSF)/nephrogenic fibrosing dermopathy (NFD) in patients with renal failure suggests that additional screening costs may also be required in relation to its use;121 among patients with renal failure this is a further complicating factor in assessing cost-effectiveness]. Although the evidence base reported in the literature on cost-effectiveness may lag behind technical developments and emerging evidence, such developments should not be ignored.
The two studies that compared ABR/GdT1W MR imaging protocols with a direct to GdT1W MR imaging protocol after audiometry – Robinette and colleagues65 and Rupa and colleagues67 – both concluded that interposing an intervening screen was more cost-effective than going directly to GdT1W MR imaging. These studies were among the strongest of those reviewed in terms of study design although, the study by Rupa and colleagues67 related to a relatively small sample size and a group of tumours that were somewhat larger than one might typically expect. That sensitivity analyses did not cover variations in test costs – although such variations clearly exist in the literature – is an issue.
The three studies that compared non-contrast-enhanced MR imaging with GdT1W MR imaging – Allen and colleagues,82 Marx and colleagues88 and Tan117 – each found non-contrast-enhanced MR imaging to be a more cost-effective test for acoustic neuroma than GdT1W MR imaging. However, as expressed by several of the authors themselves, and as noted by Jackler,91 caution is warranted in the interpretation of these results. In particular, whether other centres would experience the high sensitivity of non-contrast-enhanced MR imaging exhibited at these centres is debatable. It must also be borne in mind that these studies are now either approaching or over 10 years old.
The one remaining study, that by Daniels and colleagues,116 compares non-contrast-enhanced MR imaging with a protocol deploying ABR and GdT1W MR imaging in the search strategy. This study found that non-contrast-enhanced MR imaging is more cost-effective than the ABR/GdT1W MR approach. In all four of these studies the absence of a formal sensitivity analysis is problematic.
Cost-effectiveness model
Introduction
None of the acoustic neuroma diagnostic algorithms reviewed in the previous sections was considered by the clinical experts in this project to closely resemble current practice in the NHS. ABR does not currently have a role in the investigation of acoustic neuroma in the UK except in cases in which imaging cannot be used. The cost-effectiveness of a screening strategy for acoustic neuroma that included ABR was therefore not assessed.
Among the other imaging algorithms several were somewhat dated, reporting sensitivity rates that are at odds with those currently attainable with the technology. Others, again because they are now quite dated or because of location, assumed much lower access to imaging technology (which in turn had implications for the stage at which tumours were detected) than is currently the case in the UK. For example, the model of Welling and colleagues114 is now almost 20 years old. In the intervening period not only have equipment, techniques and costs changed but so too has our knowledge (e.g. the relatively low sensitivity of ABR in the detection of small tumours has become apparent). Similarly, although more recent, the study by Rupa and colleagues67 was carried out in an area where access to scanning equipment is much lower than in the UK (it is notable that this study67 reports an average tumour size of 1.73 cm, with one-third of the tumours detected in the study being 3 cm in size).
Rather than deploy models that were thought not to reflect current practice in the UK to assess cost-effectiveness, it was decided to construct a diagnostic protocol based on the best available published evidence following consultation among the authors of this report. The model that resulted from our deliberations is presented in the form of a decision tree in Figure 10 and assesses the cost-effectiveness of a screening strategy in which imaging begins with non-contrast MR imaging (non-contrast MR imaging covering any of the two-dimensional or three-dimensional T2W or T2*W sequences with differing acronyms) compared with that of a screening strategy in which imaging begins with contrast GdT1W MR imaging.
Methodology of the model
In our decision model an NHS perspective is taken. A cost-minimisation approach is adopted on the assumption that both algorithms are equally effective in the detection of acoustic neuromas (see Figures 8 and 9). A decision tree is used to describe the algorithm and point estimates of costs based on the parameters of the model under a range of assumptions derived.
In the model, all patients receive a physical examination, a patient history is taken and audiological tests including pure tone and speech discrimination are conducted. When an acoustic neuroma is suspected based on the results of these examinations, patients are referred initially to non-contrast MR imaging. The small percentage of patients for whom MR imaging is unsuitable because of claustrophobia, obesity and/or implanted metal (perhaps 5%) are not included in the imaging protocol as such patients would not be scanned; these patients would be referred instead to alternatives such as ABR or CT scanning. This group would be equally unsuited to contrast and non-contrast imaging and would affect both protocols equally, their effects on costs cancelling each other out.
Six possible outcomes emerge from the non-contrast MR imaging. An individual may be:
-
correctly diagnosed as having no pathology (true negative)
-
incorrectly diagnosed as having no pathology (false negative)
-
incorrectly diagnosed as having an acoustic neuroma (false positive)
-
diagnosed as having serious incidental pathology
-
diagnosed as having incidental (non-serious) pathology
-
diagnosed as having or suspected of having an acoustic neuroma (subset are true positives).
Among true negatives the patients can be either followed up or not followed up. Follow-up (in line with Welling and colleagues. 114) is assumed to consist of 5 years of audiometry tests (one test in each successive year) after which no further follow-up or investigation take place, the diagnostic protocol being considered to be complete.
False negatives can also be followed up or not. Follow-up is as detailed above. Those not followed up are assumed in the base case not to present further costs; that is, no complications or adverse effects are assumed to arise from their misdiagnosis. This is at odds with the assumptions made in several of the papers reviewed, but is considered to more accurately reflect current knowledge. Having screened patients with audiometry and a physical examination as well as non-contrast MR imaging it is unlikely that anything but the smallest of tumours would be missed. The lack, or slow rate, of growth of tumours122 would support the view that no further costs are incurred. The impact of relaxing this assumption on cost-effectiveness (assuming that a cost is associated with false negatives that are not followed up) is examined in a series of one-way sensitivity analyses (discussed below). Among all negative results, the diagnostic algorithm is considered complete at this stage.
Non-contrast MR results indicative of pathology (abnormal results) can be true or false positives. Among the true positives (in which an abnormality of some type is seen to exist), non-contrast MR results may clearly identify the pathology to be other than an acoustic neuroma (e.g. meningioma). Among this ‘other’ true pathology group, the pathology may be identified as serious and require further investigation, or may be identified as not presenting an issue and deemed not to warrant further investigation. In respect of both types of ‘other’ pathology, however, acoustic neuroma is assumed to be definitively ruled out and no further investigation related to diagnosis of neuroma is required. The identification of ‘other’ pathology in other words completes the diagnostic protocol for these groups in respect of acoustic neuroma. Whether further scans are required or not, no further costs in respect of neuroma are involved.
Among those whose non-contrast MR results indicate evidence of a neuroma, further scanning sequences may be requested or it may be decided, based on evidence available from the non-contrast MR imaging, that no further scans are required. For those in whom a further scan is indicated, the scan used will be contrast-enhanced GdT1W MR imaging. The costs of further scanning are included in the diagnostic protocol. When no further scanning is required, no further diagnostic costs are assumed to arise. At this stage (having decided to have further scans or that no further scans are required) the diagnostic protocol is assumed to be complete.
Finally, for the false positives emerging from non-contrast MR imaging we assume that all are referred to contrast-enhanced GdT1W MR imaging to definitively rule out pathology. The performance of the contrast-enhanced GdT1W MR imaging is again assumed to terminate the diagnostic protocol for this group, with them emerging with a definitive diagnosis excluding neuroma.
The probabilities associated with movement along different routes of the diagnostic pathway were based, when possible, on data taken from the literature. When such data were not available, consensus opinion from among the authors was used. Probabilities are shown in Figure 10. By way of example, between 12% and 14% of patients are assumed to emerge from non-contrast MR imaging with an indication of pathology. These figures are based on data from Daniels and colleagues97 and Dawes and colleagues34 respectively. Considering the 14% figure, less than 1% (we assumed conservatively 0.9%) are false positives, 1% have serious incidental pathology, between 8% and 10% (8.32%) have incidental pathology of minor significance and between 3% and 5% (3.78%) are diagnosed with acoustic neuroma (total 14%). The percentage of patients with suspected neuroma (3–5%) was again taken from Dawes and colleagues34 and Daniels and colleagues97. These publications describe large screening populations. The percentages reported, however, depend on the pretest selection for MR imaging in the respective populations. Some UK operators may regard this incidence as high, based perhaps on less discriminating selection criteria for screening in their populations; however, the estimates are considered reasonable by the authors.
Costs associated with contrast MR imaging (£233) and non-contrast MR imaging (£151) were taken from correspondence with ENT departments in the UK undertaken by the group; audiometry costs (£35) were taken from a review of reported charges. When follow-up involving audiometry is required, the protocol assumes that the individual concerned receives one examination each year for 5 years subsequent to the initial investigation. A discount rate of 3.5% was applied to costs to ascertain their present value. When additional scans beyond non-contrast MR imaging are required, these are assumed to take place within the same calendar year, no discounting therefore being necessary.
Results
Using these percentages and costs, the expected cost of processing 100 hypothetical patients through the protocol (excluding initial audiometry and examination, which all patients receive) is estimated at £16,121.26 (or £161.12 per patient). This compares with a cost, were a patient to proceed immediately after audiometry and examination to contrast MR imaging (based on suspicion of pathology), of £233 per patient or £23,300 for 100 patients. If 3–5% of those investigated ultimately are found to have an acoustic neuroma, and the total cost of these initial tests for 100 patients is £16,121, this contributes £3224–5374 to the cost per case detected. Clearly, under the assumptions detailed, a protocol that involves non-contrast MR imaging as an initial screen before deployment of contrast MR imaging is more cost-effective in the diagnosis of acoustic neuroma than one that deploys contrast MR imaging directly.
In Table 9, the expected costs for which the assumptions used in the model have been adjusted are reported. In scenario 1, the base case model identified above is presented together with the net additional cost associated with pursuing a strategy of contrast imaging all patients instead. As can be seen, a ‘contrast all’ strategy would cost an additional £71.79 per patient.
| Scenario | Non-contrast screening strategy | Costs (£) | ||
|---|---|---|---|---|
| Non-contrast-enhanced MR imaging | GdT1W MR imaging | Difference | ||
| 1 | Base case (see Figure 10) | 161.21 | 233.00 | 71.79 |
| 2 | As in base case, but percentage of normal results on non-contrast imaging is 88%a | 160.88 | 233.00 | 72.12 |
| 3 | As in scenario 1, but percentage of non-contrast followed up is 10% rather than 5% | 168.01 | 233.00 | 64.99 |
| 4 | As in scenario 2, but percentage followed up is 10% rather than 5% | 167.83 | 233.00 | 65.17 |
| 5 | As in scenario 1, but 1% of false negatives not followed up suffer profound unilateral sensorineural hearing impairment as a resultb | 162.71 | 233.00 | 70.29 |
| 6 | As in scenario 1, but 10% of false negatives not followed up suffer profound unilateral sensorineural hearing impairment as a resultb | 176.16 | 233.00 | 56.84 |
| 7 | As in scenario 4, but 10% of false negatives not followed up suffer profound unilateral sensorineural hearing impairmentb | 183.13 | 233.00 | 49.87 |
| 8 | As in scenario 4, but 10% of false negatives not followed up suffer profound bilateral sensorineural hearing impairmentc | 251.79 | 233.00 | –18.79 |
In scenario 2, a model which assumes that 88% of those scanned with non-contrast MR imaging are normal (12% abnormal) and that all other assumptions are as in the base case is presented. As can be seen, the slightly lower hit rate for the detection of pathology results in savings associated with subsequent MR investigations on selected groups. This improves the cost-effectiveness of the non-contrast imaging screening strategy relative to imaging all patients with contrast MR. The net saving per patient of a non-contrast screen is £72.12.
In scenario 3, a detection rate of 86% for pathology from non-contrast MR imaging is again assumed, but rather than only 5% of those in whom no pathology is suspected being followed up (95% not followed up), 10% are now followed up (90% not). The additional follow-up effort is seen to reduce the cost-effectiveness of the non-contrast MR screening strategy relative to a ‘contrast all’ MR imaging strategy. This is as one would expect given that non-contrast MR imaging now has additional follow-up tests associated with it. In scenario 4, similar results are found when the percentage of patients in whom pathology is suspected after non-contrast MR imaging is 88% rather than 86%, all other assumptions remaining unchanged. Under the base case assumptions, varying the percentage of normal to abnormal results within reasonable ranges seems unlikely to affect the conclusion that a non-contrast MR screening strategy is more cost-effective than contrast imaging all patients.
If we assume that 1% of false negatives develop unilateral SNHI associated with a failure to detect neuroma, costs associated with a non-contrast MR screening strategy will increase relative to a ‘contrast all’ image approach. Assuming a loss of productivity equal to 6% of earnings for anyone whose neuroma is missed (in line with Welling and colleagues114), the cost-effectiveness of the non-contrast screening strategy is seen to fall (scenario 5 relative to scenario 1). (Unilateral and later bilateral hearing loss are used here as examples of costs that could arise from the failure to detect false negatives. As noted in the previous chapter, other adverse events can occur including, in rare and extreme cases, death.) Nevertheless, the non-contrast screening strategy remains relatively more cost-effective than a ‘contrast all’ image strategy. This is in part because the strategy continues to be highly sensitive (giving rise to very few false negatives) and in part because only a small percentage of these false negatives develop significant complications as a result of having been missed. Scenarios 6 and 7, in which 10% rather than 1% of false negatives are assumed to develop profound hearing impairment (the percentages of abnormal results and of those followed up varying between the two scenarios), continue to show non-contrast MR imaging as being more cost-effective than contrast MR imaging for all patients, although the relative cost-effectiveness, as one would expect, is reduced. Of the scenarios presented, it is not until we assume that 10% of false negatives develop very significant problems (bilateral profound SNHI, scenario 8) that a direct to contrast imaging protocol is seen to be more cost-effective than the non-contrast MR imaging screening protocol. This extreme case is thought to be unrealistic given the observations in the literature.
Conclusions
A comparison of the models presented here indicates the cost-effectiveness of a non-contrast MR screen before contrast MR imaging relative to the cost-effectiveness of a direct to contrast MR imaging strategy for all patients in the investigation of acoustic neuromas. This analysis is supported by the series of one-way sensitivity analyses in which case detection rates, costs associated with false negatives and the percentage of patients with negative non-contrast MR imaging results followed up are varied within plausible ranges.
Based on current UK practice, we have not compared the cost-effectiveness of an initial non-contrast MR screening strategy with one that includes ABR.
It is our assessment that a diagnostic algorithm that deploys non-contrast MR as an initial imaging screen in the investigation of acoustic neuroma is more cost-effective than available comparators. This finding is in agreement with those of Allen and colleagues82 and Marx and colleagues. 88 It is again worth stating, however, that both technology and costs are evolving rapidly and therefore cost-effectiveness is likely to change.
Chapter 4 Natural history of acoustic neuroma
Introduction
This chapter addresses the question ‘What is known about the natural history of acoustic neuroma?’ in relation to the underlying principal objective of the study, which was to investigate the place of MR imaging in the investigation of patients with symptoms that might indicate the presence of an acoustic neuroma.
The key element of the natural history is therefore knowledge of the extent of the problem, including:
-
the incidence and prevalence of acoustic neuroma
-
the characteristics of patients presenting for investigation
-
the characteristics of patients diagnosed with acoustic neuroma
-
the clinical characteristics of acoustic neuroma growth.
This chapter reports the findings from a systematic review examining these areas under three headings: epidemiology, symptoms and growth.
Methods
Search strategy
The search terms used and databases searched to identify articles for possible inclusion in the natural history review are listed in Appendix 1.
The flow chart in Figure 13 (Appendix 2) details the number of references found and the exclusion of irrelevant references at each stage of the review process. The initial search yielded 3330 titles, which was reduced to 2455 after exclusion of duplicates. All titles were reviewed by at least two members of the research team and a further 2050 references were excluded as not being relevant to the review. The remaining 405 titles were reviewed by the content experts for the theme (TN, GO’D, DB) and abstracts sought for 209. In addition, seven further abstracts were identified from reference lists and 35 from the proceedings of an international conference and the searches in the clinical effectiveness theme. Of the 251 abstracts sought, five could not be retrieved, leaving 246 for review, of which 58 were considered to be not relevant. Seventeen full papers were in a language inaccessible to the research team, resulting in 171 full papers finally reviewed. A further 70 papers were excluded at the data extraction stage for the reasons given in Figure 13, resulting in 101 papers reporting 89 studies remaining in the review. The search was updated to cover the period from October 2006 to August 2008. Six additional papers were identified that contributed data to the review as well as one update of a previously included paper (see Figure 14, Appendix 2).
Quality assessment
Papers were assessed for quality using the CASP guidelines. Details of the questions used in the assessment and the score for each paper can be found in (Tables 29–32, Appendix 3). The four systematic reviews included in the section on growth were assessed separately.
Data extraction
The following data were extracted from each paper:
-
author(s) and year of publication
-
country in which the study was undertaken
-
study design – retrospective, prospective
-
dates of the study
-
number of patients, age and sex distribution
-
length of follow-up
-
incidence (if quoted or extractable)
-
prevalence (if quoted or extractable)
-
number of patients presenting with audiological symptoms including unilateral or asymmetric hearing impairment, sudden hearing impairment, tinnitus, vestibular/balance symptoms, other symptoms
-
number of patients identified from further investigation to have audiovestibular impairments, tinnitus or other impairments
-
location of acoustic neuroma
-
size of acoustic neuroma at diagnosis/entry to the study (mean, median, range, etc.)
-
method of measurement of acoustic neuroma
-
method of measuring growth (e.g. change in diameter, volume)
-
definition of growth
-
numbers and percentages of tumours that grew, were found to be stable or regressed
-
growth rates
-
predictors of growth
-
numbers receiving intervention after a conservative management approach
-
loss to follow-up
-
any important additional information or comments.
Inclusion criteria
Papers were included if they met the following criteria:
-
presented data only on adults over 16 years of age (or included only a few patients under 16 years of age)
-
provided a case definition of at least one of the following: unilateral SNHI, ASHI, unilateral tinnitus
-
contained adequate data for extraction.
In addition, papers were excluded if they met the following criteria:
-
patients with NF2 could not be excluded
-
published before 1990
-
presenting data from patients included in the study only before 1990.
Epidemiology
Introduction
Acoustic neuromas are uncommon in the general population but they are nonetheless responsible for about 6% of all intracranial tumours and about 80% of CPA tumours. Screening for acoustic neuromas accounts for about 20% of the activity of ENT departments in district hospitals47 (this percentage may well have increased over the years since this study was undertaken) and approximately 3% of all referrals to a diagnostic imaging department in a large acute teaching hospital are for scanning of the internal auditory meatus (Lenthall, Nottingham, 2007, personal communication).
Risk factors
Public concern that the use of mobile telephones might increase the risk of brain tumours because of microwave exposure led to a range of epidemiological studies designed to estimate any possible injurious effects, especially of prolonged use. The proximity of the acoustic nerve to the handset and hence to the radiation field might be expected to place the nerve at particular risk. There is no agreement between studies but it would appear that, at least in the short term (i.e. less than 10 years of mobile phone use), the risk is likely to be small; beyond that period the risk seems to be elevated but it is not sufficiently quantified at present. 123
Occupational noise exposure has also been evaluated as a possible risk factor for acoustic neuroma. Preston-Martin and colleagues124 compared 86 acoustic neuroma patients with 86 control subjects and reported an odds ratio of 2.2 for noise exposure, which increased to 13.2 for noise exposure of more than 20 years. The most recent Swedish study125 examined occupational noise exposure data for 599 cases of acoustic neuroma and 101,756 control subjects, and reported no increased risk of acoustic neuroma even after allowing for a long latency period.
Using material from a population-based tumour registry (National Cancer Registry 1961–79), Haas and colleagues126 evaluated the influence of pregnancy at the time of diagnosis. Observed cases for malignancy and meningioma were the same as for a control population, except for acoustic tumours, the latter presenting more frequently during pregnancy. Although interesting, this finding has not been independently replicated.
Incidence and prevalence
Various attempts have been made to evaluate the incidence and prevalence of acoustic neuroma in the general population. Original attempts were based on hospital autopsy studies. These studies represented a valuable source of tumour material, but their usefulness is restricted by limited availability. Hardy and Crowe127 examined the available autopsy material at the Johns Hopkins Hospital in Baltimore, representing material from 1928 to 1941, and found six tumours in the 250 temporal bone pairs (2.4%). Leonard and Talbot,128 in a later series of similar size from the same institution, revealed acoustic neuromas in 0.8% of subjects. In the Hamburg series based on the Wittmaack Collection,129 1720 temporal bones were collected between 1906 and 1945; 30 tumours were identified, many of them (n = 22) being large tumours. In the Harvard temporal bone collection,130 in 893 temporal bones from 517 individuals, five tumours were diagnosed. The authors considered that ‘the finding of acoustic neuromas in 0.9% of individuals in this series indicates the high incidence of this tumour in the general population’. However, to suggest that findings in a temporal bone collection reflect the incidence in the general population is not tenable given the highly selected provenance and small sample size of the material in such collections. Interestingly, they also commented that ‘the location and size of these tumours indicate that clinical diagnosis would have been difficult or impossible by any method of study’, reflecting the rather crude diagnostic techniques available at that time.
In 1984, a Finnish study131 reported an analysis of 298 temporal bones from 168 cases (129 paired specimens). No occult neuromas were discovered in the material, although one or two cases were expected based on previous autopsy studies. The authors found only one large CPA acoustic neuroma in a patient who died after a neurosurgical operation. A further autopsy study from Copenhagen132 based on a collection of 150 temporal bones revealed eight (2.4%) acoustic tumours.
Tos and colleagues132 have used the above data to infer tumour incidence in the general population (i.e. the number of new cases of acoustic neuroma in 1 year), which resulted in conservative estimates of acoustic neuroma of 8000 tumours per million population, which they recognised as totally unrealistic. Although autopsy studies might at best give an idea of prevalence (i.e. the total number of cases of acoustic neuroma in the population at a given time), they are unlikely to provide data on incidence. Some authors have also confused the terms ‘incidence’ (i.e. the number of new cases of a disease during a given time interval, usually 1 year) and ‘prevalence’ (defined as the total number of cases of a disease in the population at a given time), which has caused confusion in the literature.
The advent of non-invasive diagnostic intracranial imaging by means of CT and MR imaging has afforded another means of assessing the prevalence in the general population. Thus, more recently, epidemiological studies (e.g. from tumour registries and population-based studies), clinical studies based on referral to specialist units and databases of confirmed cases, and exploration of MR imaging findings in large numbers of individuals have been used to estimate tumour incidence and prevalence. We present data from such studies that met the inclusion criteria for this review.
Results
Table 10 presents an overall summary of the papers included in the review of epidemiology.
| Study | Study population and design | Country | Number of patients | Study dates |
|---|---|---|---|---|
| Propp et al., 2006133 | National registry data: (a) CBTRUS 1995–9; (b) LACCSP 1995–8 | USA | (a) 1424; (b) 256 | (a) 1995–9; (b) 1975–98 |
| Evans et al., 2005134 | Review of incidental cases and cancer registry | UK | 419 | 1990–9 |
| Stangerup et al., 2004;9 Tos et al., 2004;132 Howitz et al., 2000;135 Tos et al., 19998 | AN registry data | Denmark | 1976–83: 278; 1983–90: 337; 1990–5: 355; 1995–2001: 542; 1976–2001: 1446 | 1976–2001 |
| Prospective | ||||
| Tali et al., 1993136 | Consecutive patients referred for suspected AN | USA | 411 | 1986–92 |
| Retrospective | ||||
| Lin et al., 200545 | Case review of ANs | USA | 688 | 1980–99 |
| Frohlich et al., 1993137 | Patients with surgically or radiologically confirmed AN | Canada | 69 (2 = bilateral) | 1987–91 |
| Retrospective | ||||
| Moffat et al., 1995138 | Patients with confirmed AN | UK | 321 | 1981–93 |
| Retrospective database review | ||||
| Moffat et al., 2004139 | Patients with confirmed AN | UK | 626 | 1994–2002 |
| Retrospective database review | ||||
| Seedat et al., 2002140 | Patients diagnosed with AN | South Africa | 115 | 2000 |
| Retrospective telephone survey | ||||
| Kwan et al., 2004141 | Patients with sensorineural/mixed hearing impairment | Hong Kong | 1821: 132 = +ve, 54 = AN | 1999–2001 |
| Retrospective | ||||
| Dawes et al., 200034 | Asymmetric hearing loss > 20 dB at two adjacent frequencies or < 20 dB plus neurological signs. Unilateral tinnitus, Meniere’s triad, sudden hearing loss | UK | 1077 | 1994–7 |
| Retrospective | ||||
| Urben et al., 1999142 | Patients with ASHI ≥ 10 dB at two frequencies or ≥ 15 dB at one frequency | USA | 325 with ASHI, 193 had diagnostic studies | 1990–4 |
| Retrospective | ||||
| Verret et al., 2006143 | Patients with isolated ASHI > 15 dB at one frequency or > 10 dB at two frequencies | USA | 146 | 2002–3 |
| Retrospective case review | ||||
| Daniels et al., 200097 | Patients with isolated unilateral or asymmetric bilateral SNHI | USA | 1070 | 1994–8 |
| Retrospective case review | ||||
| Carrier and Arriaga, 199713 | Patients with ASHI or unilateral non-pulsatile tinnitus | USA | 485 | 1994–5 |
| Retrospective case review | ||||
| Dawes and Basiouny, 199935 | Patients with unilateral tinnitus only | UK | 174 | 1994–7 |
| Retrospective case review | ||||
| Sheppard et al., 199636 | Patients with asymmetric audiovisual symptoms | UK | 913 (892 scanned) | 1990–3 |
| Prospective screening | ||||
| Saunders et al., 1995144 | Patient documented SHI | USA | 836 | 1989–93 |
| Retrospective case review | ||||
| Schick et al., 2001145 | Consecutive patients with SHI, unilateral tinnitus and/or vestibular disorders | Germany | 354 | 1994–9 |
| Retrospective evaluation | ||||
| Kosugi et al., 2004146 | Patients with SHI (unilateral > 30 dB) | Brazil | 49 | 2001–3 |
| Prospective | ||||
| Fitzgerald and Mark, 1998147 | Patients with SHI | USA | 78 | 1989–95 |
| Retrospective case review | ||||
| Lin et al., 200545 | Patients undergoing MR imaging (excluded MR imaging of IAC) | USA | 46414 | 1995–2003 |
| Database review | ||||
| Anderson et al., 2000148 | Patients undergoing MR imaging investigation | USA | 24246 | 1993–7 |
| Retrospective |
Data from registries
Table 11 details three sets of studies on registry data. Propp and colleagues133 analysed the epidemiology of acoustic neuroma using combined data from central brain tumour registries in the USA (1975–99). The overall incidence of primary nerve sheath tumours increased significantly over the study period, as did the incidence of acoustic neuroma, whereas the incidence of benign schwannomas at other sites decreased or remained static. The annual incidence of acoustic neuroma was found to be between six and eight per million person-years. In their data, acoustic neuroma accounted for 57% of benign intracranial schwannoma but further scrutiny of data from Los Angeles county confirmed that acoustic neuroma accounted for 90% of intracranial nerve sheath tumours. Acoustic neuroma were found to occur with equal incidence in men and women, but the non-white population had a significantly lower incidence than the white population. The authors felt that better diagnostic techniques, especially for the symptomatic elderly, contributed to the apparent increased incidence. They also felt that more accurate coding, avoidance of misclassification and more complete active case ascertainment would provide a much more accurate picture. Adding to the difficulty is that many cases are now ‘diagnosed’ on imaging without having histological confirmation (as biopsy alone would require major surgery), thus the accuracy of the diagnosis in these cases is open to question; in addition, these histologically unconfirmed cases may not be reported to a tumour registry.
| Study | Study population | Age (years) | Sex | Number of patients | Study date | Incidence per 100,000 person-years (95% CI) | Comments |
|---|---|---|---|---|---|---|---|
| Propp et al., 2006133 | National registry data: (a) CBTRUS 1995–9; (b) LACCSP 1995–8 | NR | NR | (a) 1424; (b) 256 | (a) 1995–9; (b) 1975–98 | (a) 0.55 (0.52–0.58); (b) 0.82 (0.71–0.92) | Incidence increased significantly with time: (a) 14% per year, (b) 6% but no data on size for interpretation |
| 0–19 years: (a) 0, (b) 0; 20–44 years: (a) 0.4, (b) 0.5; 45–64 years: (a) 1.2, (b) 2.0; > 65 years: (a) 1.0, (b) 1.5 | |||||||
| M: (a) 0.56 (0.52–0.60), (b) 0.83 (0.68–0.99); F: (a) 0.55 (0.51–0.58), (b) 0.80 (0.66–0.94) | |||||||
| White: (a) 0.58 (0.55–0.61), (b) 0.89 (0.77–1.01); non-white: (a) 0.23 (0.18–0.28), (b) 0.51 (0.36–0.67) | |||||||
| Evans et al., 2005134 | Review of incidental cases and cancer registry | Mean 54.9 (15.7–91.9) | M 48.4%; F 51.6% | 419 | 1990–9 | 1990–9: 1.04; 1995–9: 1.27 | Age < 60 years F/M = 1.02:1; age ≥ 60 years F/M = 1.14:1. Highest incidence at 60–69 years: 113/419 |
| Stangerup et al., 2004;9 Tos et al., 2004;132 Howitz et al., 2000;135 Tos et al., 19998 |
AN registry data Prospective |
Median 55 (15–84) | M 48.3%; F 51.7% | 1976–83: 278; 1983–90: 337; 1990–5: 355; 1995–2001: 542; 1976–2001: 1446 | 1976–2001 | 1976–83: 0.78; 1983–90: 0.94; 1990–5: 1.24; 1996–2001: 1.74 | Size of diagnosed tumours decreased over the time periods but median age at diagnosis did not change. Increase in number of intrameatal tumours as number of MR scanners increased |
In 2005, Evans and colleagues134 reported incidence data from the North West of England, accrued from the major neurosurgical centres and cross-referenced with the regional tumour registry (data from 1990 to 1999). From a sample of 419 sporadic acoustic neuroma and 64 NF2-related acoustic neuroma, they estimated the incidence of acoustic neuroma as 1.04 per 100,000 population per year in the first 5 years of the study period, increasing to 1.4 per 100,000 population in the last 5 years. They also pointed out that more acoustic neuroma than previously considered were the result of NF2, due to a greater awareness of the mosaic forms of this disease. An epidemiological study of the incidence of brain tumours in Devon and Cornwall confirmed that the incidence of cranial nerve tumours (unspecified) was approximately 2.3 per 100,000 person-years, with the peak in incidence between the fifth and sixth decades, the precise incidence of acoustic neuromas not being stated. 149
The leading studies on epidemiology have come from Denmark (population 5.2 million), in large part because of the establishment of a comprehensive database for these tumours in 1975. In this central register, data are entered from all neurological and otological clinics in the country on all confirmed tumours. This has resulted in sequential publications9,122,132,150, covering the time span from 1975 to 2001, offering the most comprehensive data yet available on the epidemiology of these tumours in a stable population of meaningful size.
The incidence of these tumours has changed over the years since data collection commenced. 8,9,122 There has been a dramatic decline in the incidence of giant tumours (> 40 mm extracanalicular extension), from 28% initially down to 1%, with a commensurate increase in tumours with an extracanalicular extension of 11–40 mm; the incidence of small tumours (between 1 and 10 mm extracanalicular extension) has remained steady at 20%. The mean size of the extrameatal portion of the tumour has dropped from 28 to 16 mm. These improvements are largely attributable to the advent of MR imaging and increased accessibility to the technology over time. In particular, the non-invasive nature of MR imaging has resulted in the evaluation of elderly patients who might not otherwise have been fit to undergo more invasive assessments. Also, there has been a reduction in the time taken for patients to seek advice (‘patient delay’) and physician time to respond (‘doctor delay’) because of greater awareness and public education. Patient organisations (acoustic neuroma support groups, tinnitus groups, etc.) have also played a very positive role in creating a much more informed and demanding public.
Clinical studies based on referral to specialist units and databases of confirmed cases
Table 12 summarises the studies that have reported incidence data for populations of patients confirmed to have acoustic neuromas, and Table 13 summarises the prevalence data from papers that examined populations of patients presenting with various audiovestibular symptoms suggesting the possibility of acoustic neuroma.
| Study | Study population | Number of patients | Study date | Age (years) | Sex | Incidence per 100,000 per year | Comments | Tumour size |
|---|---|---|---|---|---|---|---|---|
| Frohlich et al., 1993137 | Patients with surgically or radiologically confirmed AN | 69 (2 = bilateral) | 1987–91 | NR | M 51%; F 49% | 1.27; M: 1.31, F: 1.24 | Incidence peak for females at 60–69 years (4.1/100,000) then decline; incidence peak for males at 50–59 years (3.3/100,000) then plateau. | ≤ 1 cm = 10 (14.1%); 1.1–2.5 cm = 40 (56.3%); 2.6–4.0 cm = 17 (23.9%); > 4 cm = 4 (5.6%) |
| Moffat et al., 1995138 | Patients with confirmed AN | 321 | 1981–93 | 52.1 (SD 12.3) | M 47.4%; F 52.6% | 1981–91: 2.02 | 1982–2002: 1.8–3.0 cm | |
| Moffat et al., 2004139 | Patients with confirmed AN | 626 | 1994–2002 | 54.8 (SD 13.7) | M 49.2%; F 50.8% | 2002: 0.83; 1981–2002: 1.36 | ||
| Seedat et al., 2002140 | Patients diagnosed with AN | 115 | 2000 | NR | M 52%; F 48% | 0.3 overall; racially white: 1.76, racially black: 0.01 (based on n = 95) | Significant racial differences in incidence – much more rare in blacks. In telephone survey, some patients may have been missed or counted twice |
| Study | Study population | Age (years), mean (range) | Sex | Number of patients | Study date | Prevalence | Comments |
|---|---|---|---|---|---|---|---|
| Tali et al., 1993136 | Consecutive patients referred for suspected AN | NR | NR | 411 | 1986–92 | 19.5% (80/411) | |
| Kwan et al., 2004141 | Patients with sensorineural/mixed hearing impairment | NR | NR | 1821: 132 = +ve, 54 = AN | 1999–2001 | 3% (54/1821) | Tumour size: < 1 cm = 22 (40.7%); 1.0–1.5 cm = 15 (27.8%); > 1.5 cm = 17 (31.5%) |
| Dawes et al., 200034 | Asymmetric hearing loss > 20 dB at two adjacent frequencies or < 20 dB plus neurological signs. Unilateral tinnitus, Meniere’s triad, sudden hearing loss | NR | NR | 1077 | 1994–7 | 3.2% (34/1077 | |
| Urben et al., 1999142 | Patients with ASHI ≥ 10 dB at two frequencies or ≥ 15 dB at one frequency | 51 ± 13.2 | M: 66%; F: 34% | 325; 193 had diagnostic studies | 1990–4 | 2.1% (4/193) | 4 ANs found (1 = NF2); AN 29–68 years: 2 M, 2 F |
| Verret et al., 2006143 | Patients with isolated ASHI > 15 dB at one frequency or > 10 dB at two frequencies | 55 (20–84) | M: 38.4%; F: 61.4% | 146 | 2002–3 | 0/146 | |
| Daniels et al., 200097 | Patients with isolated unilateral or asymmetric bilateral SNHI | > 18 | NR | 1070 | 1994–8 | 5.2% (56/1070) | |
| Carrier and Arriaga, 199713 | Patients with ASHI or unilateral non-pulsatile tinnitus | NR | NR | 485 | 1994–5 | 1.44% (7/485) | |
| Dawes and Basiouny, 199935 | Patients with unilateral tinnitus only | 50 (23–76) | M: 46.0%; F: 54.0% | 174 | 1994–7 | 0.57% (1/174) | |
| Sheppard et al., 199636 | Patients with asymmetric audiovisual symptoms | From 10–19 to 80–89 | M: 48.5%; F: 51.5% | 913 (892 scanned) | 1990–3 | 4.26% (38/892) | 21/913 (2.3%) referred could not have MR because of claustrophobia (17), pregnancy (2) or patient too large to enter scanner (2); AN: 16 M, 22 F |
| Saunders et al., 1995144 | Patient-documented SHI | NR | NR | 836 | 1989–93 | 1.5% (13/836) | |
| Schick et al., 2001145 | Consecutive patients with SHI, unilateral tinnitus and/or vestibular disorders | 49 (8–86) | M: 49.2%; F: 50.8% | 354 | 1994–9 | 1.41% (5/354) | |
| Kosugi et al., 2004146 | Patients with SHI (unilateral > 30 dB) | 45.4 (15–91) | M: 47%; F: 53% | 49 | 2001–3 | 6.1% (3/49) | Age: 42, 43 and 55 years |
| Fitzgerald and Mark, 1998147 | Patients with SHI | 45 (13–79) | M: 51.3%; F: 48.7% | 78 | 1989–95 | 3.8% (3/78) |
In Manitoba, Canada, Frohlich and colleagues137 reported an overall incidence of acoustic neuroma of 1.27 cases per 100,000 per year, with differences reported in the incidence and peak age between men and women; however, numbers in this study (n = 69) were small.
Moffat and colleagues,138 evaluating the population in the greater Cambridge area in the UK, estimated an overall annual incidence for 1981–91 of 2.02 per 100,000 per year, which was an exceptionally high incidence for this time period (before routine MR scanning). In 2002,139 an incidence of 0.83 was reported and the authors estimate the overall incidence to be 1.36 per 100,000 per year accounting for changes in the catchment population. Mean tumour size at presentation in the period 1982–2002 (1.8–3.0 cm) showed a slight trend towards an overall decrease, which was paradoxically accompanied by an increase in the numbers of large tumours (e.g. those > 45 mm).
The incidence of acoustic neuroma in the South African population has been reported by Seedat and colleagues140 as being approximately 0.3 per 100,000 population per year; interestingly, racial differences were apparent, with the incidence among the white population being significantly higher than that among the black population. Whether this represents a true racial difference in the susceptibility to develop these tumours or whether it reflects social and economic factors is uncertain; for instance, of the 65 MR imaging units in South Africa, 63 are in the private sector, resulting in restricted access for the poorer (black) population.
Szyfter and colleagues151 undertook a study of the epidemiology of acoustic neuroma in the Polish population based on questionnaires sent to seven neurosurgical and three ENT centres to determine the numbers undergoing surgery in an indicative year (1997–8). In total, 72 patients underwent surgery, equating to an annual surgical incidence of 1.9 acoustic neuroma per million population; however, the true incidence (as distinct from the surgical incidence) of these tumours in the general Polish population could not be assessed.
The prevalence of acoustic neuroma reported in Table 13 for symptomatic populations varies from 0% to 6.1%. The design of each study was different, and the populations studied were heterogeneous in terms of age, sex and presenting symptoms, and firm conclusions cannot be drawn.
MR findings in studies of large numbers of individuals
Table 14 summarises the data from MR studies of large numbers of patients who were being scanned for reasons other than to exclude an acoustic tumour. These have been used to estimate the prevalence of acoustic tumours in the general population. Anderson and colleagues148 retrospectively evaluated 24,246 brain MR studies (19,405 with contrast and 4841 without contrast) and found seven unsuspected cases of acoustic neuroma per 10,000 brain MR imaging studies. They concluded that the true prevalence of acoustic neuroma is likely to be greater ‘than the 1.0 per 100,000 population per year previously reported’, but their data (on prevalence) cannot be used to evaluate the incidence of these tumours. Besides, it is uncertain how representative MR imaging data of this kind is of the general population. Lin and colleagues45 studied 46,414 brain scans on the MR database at the University of California in San Francisco, and eight tumours were discovered incidentally (the scans being undertaken for reasons other than to investigate the presence of an acoustic neuroma). The figures suggested that acoustic tumours may be present in at least 0.02% of the population; however, it is difficult to extrapolate prevalence data in a hospital-based population to the general population.
| Study | Study population | Age (years), mean (range) | Sex | Number of patients | Study date | Prevalence | Size |
|---|---|---|---|---|---|---|---|
| Anderson et al., 2000148 | Patients undergoing MR investigation; excluded patients with suspected AN (i.e. symptomatic patients referred for MR imaging to rule out AN) | 56 (26–74) in 17 patients with AN | M: 52.9%; F: 47.1% | 24,246 | 1993–7 | 17/24,246; 7 per 10,000 scans (0.07%) | < 1 cm = 8 (47.1%); 1–2 cm = 6 (35.3%); > 2 cm = 3 (17.6%); largest = 2.9 cm |
| Lin et al., 200545 | Patients undergoing MR imaging (excluded MR imaging of internal auditory canal) | 58 (20–83) | NR; AN: 6 M, 2 F | 46,414 | 1995–2003 | 505/46,414 (1.1%); AN 8/46,414 (0.02%) with no audiovisual symptoms |
Discussion
The Danish data have posed some unresolved questions. If indeed the true incidence of these tumours approaches 2 per 100,000 population, and the numbers actually presenting for treatment are considerably smaller, what has happened to those patients (nearly 2500 over a 39-year period) who, in the authors’ estimation, may have evaded diagnosis?152 It is likely that in the vast majority of these patients the tumours never grew and the patients lived and died with their tumours but were never sufficiently troubled by them in life to seek medical help or, if they sought medical help, no action was taken. Furthermore, once identified, which patients should be offered treatment, treatment that can often negatively impact on quality of life? What is also puzzling in the Danish data is that, given greater access to MR scanning, one might have thought that the mean age at diagnosis would have decreased significantly over time; however, this was not the case, with the median age at diagnosis remaining the same (55 years) over the 26 years of the study. This may, at least in part, be due to the scanning of elderly patients who would not have previously been subjected to investigation.
Symptoms
Introduction
In this section, which considers the symptoms of acoustic neuroma, there is an explicit and important distinction between symptoms with which the person presents to seek medical advice and those additional symptoms that patients may only volunteer on direct questioning or that are identified by further investigation. When possible, this distinction is made in presentation of the data. When this distinction is not clear it renders analysis of patterns of presentation of patients with acoustic neuroma problematic.
An additional point is that variation between the symptoms reported in series of acoustic neuroma by authors may reflect differences in the patient populations, such as size of tumour, distribution, length of history and perhaps patient age or gender, and also differences in health-care systems and technologies.
The advent of MR imaging made it possible to diagnose smaller tumours than were previously possible59 and to make more reliable differential diagnoses between acoustic neuroma and other CPA lesions and represented a step change in the ability to accurately diagnose acoustic neuroma. 59 However, such technologies did not become available to all health economies at the same time and, indeed, access remains limited outside the developed world.
Each of these factors contributes to the variation in the reports of symptoms of acoustic neuroma in the literature and so obscures analyses. It should also be noted that there is an innate tautology regarding papers that report symptoms of acoustic neuroma in that the reported data can only relate to those patients in whom a diagnosis was achieved. There is a small amount of literature describing asymptomatic patients, but this is not of high quality.
Schucknecht153 describes three potential mechanisms for the origin of the prevalent auditory/vestibular symptoms experienced by patients with acoustic neuroma:
-
the destruction of cochlear and vestibular nerve fibres by pressure atrophy or invasion
-
ischaemia, causing atrophy of the neurosensory elements within the cochlea and the vestibular labyrinth by compromising blood flow in the labyrinthine artery that runs through the IAC
-
biochemical degradation of the cochlea and the vestibular labyrinth.
The evidence for each of these mechanisms is considered below.
A review of pathophysiology associated with nerve compression was undertaken by Sunderland. 154 It was noted that a nerve is particularly at risk of compression injury when ‘it passes through, or is contained within, a compartment with unyielding walls’, which is the case for the cochlear, vestibular and facial nerves within the bony cylinder of the IAC. Because of the slow growth of the acoustic neuroma, the compression exerted by the growth within the IAC is chronic rather than acute. Sunderland154 noted that theories of mechanical damage to nerves in this situation have been superseded by models of ischaemic damage. The evidence suggests that mechanical changes occur only at higher pressures than are found in chronic compression conditions (such as carpal tunnel syndrome). Additionally, the fact that some chronic compression conditions (again such as carpal tunnel syndrome) respond rapidly and significantly to surgical decompression mitigates against the possibility of permanent structural/mechanical nerve damage. Axon155 has reviewed the pathophysiological effects of the compressive action of acoustic neuroma upon the facial nerve in the IAC and noted the controversies between mechanisms of mechanical damage and ischaemia. It is similarly not possible to be definitive about the effects of compression upon the cochlear and vestibular nerves. Sunderland154 noted that an ischaemic component will be present in every nerve compression injury as it is not possible to compress nerve fibres without involvement of the intraneural blood vessels. Thus, models of mechanical and ischaemic nerve injury may be complementary rather than mutually exclusive.
Evidence for ischaemic and biochemical injury to the vestibular labyrinth in acoustic neuroma has been reported by Jahnke and Neuman,156 who studied specimens taken from nine patients during translabyrinthine surgery. Examination with electron microscopy demonstrated significant degenerative changes that were thought to be the result of prolonged protein intoxication of the labyrinth (via increased perilymph protein concentrations) and compression of labyrinthine blood vessels by the tumour. Similar mechanisms were suggested for cochlear dysfunction in such cases. O’Connor and colleagues157 had earlier identified high protein levels in the perilymph of patients with acoustic neuroma but not in a patient group with meningioma in the IAC, and suggested that this may be a mechanism specific to acoustic neuroma.
Results
Table 15 summarises the studies included in the evaluation of the evidence on symptoms of acoustic neuroma.
| Study | Date of study | Study design and population | Country | Number of patients | Age (years), mean (SD) (range) | Sex |
|---|---|---|---|---|---|---|
| Fucci et al., 1999158 | 1988–96 | Chart review of patients with AN managed conservatively | USA | 119 | 65 (SD 10) (37–84) | M: 47.9%; F: 52.1% |
| Tschudi et al., 2000159 | 1989–94 | Chart review of patients with AN managed conservatively | Switzerland | 74 | 52.6 (19–78) | M: 59.5%; F: 40.5% |
| Kentala and Pyykko, 2001160 | NR | Patients with AN identified from review of questionnaires completed at diagnosis by patients with vertigo | Finland | 122; 2 = NF2 | 47 (13–73) | M: 39.3%; F: 60.6% |
| Sauvaget et al., 2005161 | 2000–2 | Consecutive surgical series of patients with unilateral AN | France | 139 | n = 28 with sudden hearing impairment: 44.4 (20–74) | n = 28 with sudden hearing impairment: M:57.1%; F: 42.9% |
| Wandong et al., 2005162 | 1996–2001 | Retrospective cohort of patients with cystic AN | China | 22 | 46 (14–69) | M: 54.5%; F: 45.5% |
| Matthies and Samii, 1997163 | 1978–93 | Patients of one surgeon with surgically confirmed AN | Germany | 1000; 38 = bilateral | 46.3 (11–87) | M: 45.7%; F: 54.3% |
| van Leeuwen et al., 1995164 | 1980–92 | Case review of patients with surgically confirmed AN | the Netherlands | 164 | 49.2 (17–79) | M: 46.3%; F: 53.7% |
| Selesnick et al., 1993;59 Selesnick and Jackler, 1993182 | 1986–90 | Case review of patients newly diagnosed with AN excluding those with insufficient documentation | USA | 126; 3 = NF2 | 50 | NR |
| Ogawa et al., 1991;165 Ogawa et al., 1991166 | 1976–89 | Case review of patients with surgically proven AN | Japan | 132 | 46.6 (18–69) | M: 50.8%; F: 49.2% |
| Sai, 1990167 | 1976–89 | Case review of patients confirmed with AN | Japan | 84 | NR | NR |
| Diensthuber et al., 2006168 | 1996–2002 | Case review of patients with surgically confirmed sporadic unilateral AN | Germany | 118 | 53.9 (SD 12.9) (19–77) | M: 56.8%; F: 43.2% |
| Haapaniemi et al., 200064 | 1992–7 | Database review of patients with AN confirmed by MR imaging | Finland | 41 | 55 | M: 58.5%; F: 41.5% |
| Aslan et al., 1997169 | 1987–95 | Patients with surgically confirmed AN | Italy | 192 | NR | NR |
| Berrettini et al., 1996170 | 1990–4 | Case review of consecutive patients with unilateral AN | Italy | 42 | 50.8 (26–77) | M: 40.5%; F: 59.5% |
| Are et al., 1995171 | 1988–93 | Case review of patients with surgically confirmed AN | Ireland | 58 | NR | NR |
| Moffat et al., 1993172 | 1990–1 | Case review of patients with surgically and histologically confirmed AN | UK | 38 | 50 | M:44.7%; F: 55.3% |
| Leonetti, 1995173 | 1988–93 | Case review of patients with AN | USA | 204 | 56.4 (16–82) | M:43.6%; F: 56.4% |
| Magdziarz et al., 2000174 | 1980–97 | Case review of patients with surgically proven AN | USA | 369 | 49.9 (10–86) | NR |
| Moffat et al., 1994175 | NR | Case review of patients with surgically proven unilateral sporadic AN | UK | 284 | NR | NR |
| Saleh et al., 1996176 | 1987–93 | Case review of patients with AN | Italy | 128; 1 = NF2 | 47.8 (17–79) | M: 45.3%; F: 54.7% |
| Tos et al., 19998 | 1976–95 | Database of patients with AN having translabyrinthine surgery | Denmark | 703 | NR | NR |
| Lustig et al., 1998177 | 1983–96 | Case review of patients with AN | USA | 546; 6 = NF2 | NR | NR |
| Frohlich and Sutherland, 1993137 | 1987–91 | Case review of patients with surgically or radiologically confirmed AN | Canada | 69 | NR | M: 50.7%; F: 49.3% |
| Moffat et al., 2004139 | 1981–93 | Database review of patients with AN | UK | 321 | 52.1 (SD 12.3). | M: 47.4%; F: 52.6% |
| Moffat et al., 2004139 | 1994–2002 | Database review of patients with AN | UK | 626 | 54.8 (SD 13.7) | M: 49.2%; F: 50.8% |
| Artz et al., 2008178 | 1994–2006 | Prospective series of patients with sporadic unilateral AN diagnosed from symptoms, AV data and MR images | the Netherlands | 234 | 57 (SD 11.7) (6–82) | M: 50.9%; F: 49.1% |
| Day et al., 2008179 | ‘Past decade’ | Database review of patients with AN | Taiwan | 44 | 50 (20–80) | M: 50%; F: 50% |
| Baguley et al., 2006180 | 1986–2002 | Retrospective case-note review of patients with unilateral sporadic AN diagnosed with ABR screening, CT scans and, latterly, MR imaging | UK | 941 | 54.3 (SD 13.3) | M: 52%; F: 48% |
| Mackle et al., 2007181 | 1993–2006 | Database review of patients attending AN clinic with radiological diagnosis of tumour in CPA or IAM | Ireland | 398 | NR | M: 58.3%; F: 41.7% |
Hearing impairment
Table 16 summarises the data from the included papers on the hearing impairment at presentation and the audiological findings on investigation.
| Study | Unilateral/asymmetric hearing impairment, n (%) | Progressive hearing impairment, n (%) | Sudden hearing impairment N (%) | Other audiometric data |
|---|---|---|---|---|
| Fucci et al., 1999158 | 113 (95%) unilateral ‘presenting symptoms’ | NR | NR | |
| Tschudi et al., 2000159 | NR | 50 (67.6%); only initial symptom, n = 17 | 20 (27.0%); only initial symptom, n = 5 | Mean PTA on diagnosis 49.4 dB; n = 8 totally deaf, n = 3 with no symptoms |
| Kentala and Pyykko, 2001160 | 98 (80.3%) initial symptom (elicited by questionnaire); only initial symptom, n = 32; 115 (94.3%) on investigation | NR | NR | Initial symptoms: hearing impairment alone 32 (25%); hearing impairment and tinnitus 44 (43%); hearing impairment and tinnitus and vertigo 22 (17%) |
| Sauvaget et al., 2005161 | NR | NR | 28 (20.1%) (revealing symptoms found in medical history) | |
| Matthies and Samii, 1997163 | NR | NR | NR | 95% (of 841) reported some hearing disturbance; 16% total deafness on exploration of history |
| van Leeuwen et al., 1995164 | 73% as main presenting symptom; 93% on investigation (95% asymmetric) | NR | 3% presenting and on investigation | Mean interaural difference 57.5 dB |
| Selesnick et al., 1993;59 Selesnick and Jackler, 1993182 | 85% subjective hearing impairment (67% = earliest symptom) (77% < 1 cm; 88% 1–3 cm; 95% > 3 cm) | 26% overall (20% < 1 cm, 27% 1–3 cm, 33% > 3 cm) | SRT average 46 dB; SDS average 53%; n = 2 (1.6%) with no symptoms (tumour size < 10 mm) | |
| Ogawa et al., 1991;165 Ogawa et al., 1991166 | 125 (94.7%) at initial visit | 29 (22.0%) | n = 10 normal hearing on investigation (< 25 dB from 500 Hz to 4kHz and interaural differences < 15 dB at 125 Hz to 8 kHz) | |
| Sai, 1990167 | NR | NR | 12 (14.3%) initial symptom | |
| Diensthuber et al., 2006168 | 68.4% as initial symptom; 89.4% ‘in the longer term’ | NR | 23.6% as initial symptom; 26.3% ‘in the longer term’ | |
| Haapaniemi et al., 200064 | Unclear | NR | NR | Mean PTA 42 ± 23 dB; mean SRT 42 ± 24 dB; mean SDS 68 ± 31% on investigation |
| Aslan et al., 1997169 | NR | NR | 8 (4.2%) as initial symptom; 14 (7.3%) on investigation | |
| Berrettini et al., 1996170 | 95% on investigation | NR | NR | Mean hearing impairment by tumour size: < 10 mm: 34 dBHL; 10–30 mm: 53 dBHL; > 30 mm: 53 dBHL |
| Are et al., 1995171 | 56 (96.6%) as initial symptom | NR | 1 (1.7%) as initial symptom | |
| Moffat et al., 1993172 | 26 (68.4%) as initial presenting symptom | NR | NR | |
| Leonetti, 1995173 | 190 (93%) as initial symptom; 178 (87.3%) > 20 dB at 0.5, 1.0 and 2.0 kHz on investigation | NR | NR | 38 (19%) had no measurable hearing on investigation; n = 2 had no symptoms |
| Magdziarz et al., 2000174 | 348 (94.3%) as initial symptom; 359 (97.3%) on investigation | 301 (81.6%) as initial symptom | 38 (10.3%) | 10 (2.7%) normal hearing on investigation (< 20 dB from 500 Hz to 2 kHz, interaural differences < 10 dB at each frequency and SDS > 90%) |
| Moffat et al., 1994175 | 171 (60.2%) as principal presenting symptom; 236 (83.1%) on investigation | 29 (10.2%) as initial symptom; 34 (12.0%) on investigation | Normal hearing: 12 (4.2%) on investigation | |
| Saleh et al., 1996176 | 62 (48.4%) as initial symptom | NR | NR | Normal hearing: 16 (12.5%) on investigation (PTA < 25 dB at 0.25, 0.5, 1.0, 2.0 and 4.0 kHz and SDS ≥ 80%) |
| Tos et al., 1998152 | 402 (57%) as first symptom | 47 (6.7%) as first symptom | ||
| Lustig et al., 1998177 | 517 (94.7%) | NR | NR | Normal hearing or symmetrical hearing impairment: 29 (5.3%) (interaural difference < 15 dB at one frequency or < 10 dB at two frequencies) |
| Frohlich and Sutherland, 1993137 | Unilateral: 59 (85.5%); bilateral asymmetric: 8 (11.6%) | NR | 4 (5.8%) | |
| Moffat et al., 2004139 | 58% principal presenting symptom | 10% principal presenting symptom | ||
| Moffat et al., 2004139 | 59% principal presenting symptom | 8% principal presenting symptom | ||
| Artz et al., 2008178 | Hearing loss reported at diagnosis: 216 (92.3%) | NR | 13.2% | Mean PTA on diagnosis: 38.9 dB (SD 25.9); n = 6 totally deaf |
| Day et al., 2008179 | NR | NR | NR | 4 (9%) normal hearing on investigation (< 25 dBHL from 250–8000 Hz); 12 (27%) total deafness |
| Baguley et al., 2006180 | NR | 575 (61%) presenting symptom | 77 (8%) presenting symptom | |
| Mackle et al., 2007181 | 97% on investigation | 346 (86.9%) | 17 (4.3%) |
Unilateral sensorineural hearing impairment
There is a consensus in the literature that the majority of patients who come to be diagnosed with an acoustic neuroma had a principal presenting symptom of a progressive unilateral SNHI. The incidence of SNHI in acoustic neuroma has been reported as over 90%;160,163,164,173 the incidence has been stable over time, specifically comparing presenting symptoms for diagnoses in 1981–93 and 1994–2002. 139 Authors have considered whether tumour size is related to the extent of hearing impairment, but this has not been robustly demonstrated. 164,179 Tumour morphology has been categorised and some associations with the extent of SNHI have been reported152,172 in that, when laterally and medially arising lesions are compared, the extent of SNHI is greater in the laterally arising tumours. 172
Sudden hearing impairment
There are a number of other symptoms that can be associated with the diagnosis of acoustic neuroma. Sudden SNHI is a surprisingly prevalent condition, affecting perhaps 7500 individuals in the UK each year. 183 The proportion of acoustic neuroma patients who experience a sudden hearing impairment and who have this as their principal presenting symptom is variable, ranging from 1.7%171 to 27.0%. 159 This may represent variance in the extent to which sudden hearing impairment is seen as requiring urgent otological consutation. 184 Interestingly, of those patients with sudden SNHI, it has been reported that approximately 2% are diagnosed with an acoustic neuroma when investigated radiologically. 144,161
Normal hearing
There are reports in the literature of patients being diagnosed with acoustic neuroma but having normal hearing. Analysis is confounded by variation in the definition of normal hearing, some choosing 20 dBHL174 and others 25 dBHL as the cut-off. 165 Some authors consider normal hearing as being symmetrical, hence allowing for some age-appropriate hearing impairment. 182 Unsurprisingly, there is marked variability in reports of the incidence of normal hearing in acoustic neuroma patients, ranging from 5.0%177 to 12.5%. 176 In papers in which normal hearing is strictly defined, the incidence is lower. 181
Table 17 summarises the data on tinnitus and vestibular symptoms at presentation and/or as determined on further investigation.
| Study | Time of recording data as noted in publication | Tinnitus, n (%) | Vestibular symptoms, n (%) |
|---|---|---|---|
| Fucci et al., 1999158 | Presenting symptom | 77 (65%) | Dizziness: 55 (46%) |
| Tschudi et al., 2000159 | Main presenting symptom at time of diagnosis | 37 (50%) | Dizziness: 11 (14.9%) |
| Only initial symptom | 6 | Dizziness: 2 | |
| Kentala and Pyykko, 2001160 | Initial symptom elicited by questionnaire | 75 (61.5%) | Vertigo: 34 (27.9%) |
| Symptoms at any time elicited by questionnaire | 101 (82.8%) | Vertigo: 60 (49.2%) | |
| Only initial symptom | 9 | Vertigo: 12 | |
| Wandong et al., 2005162 | Signs and symptoms at presentation | 15 (68.2%) | Vertigo: 1 (4.5%) |
| Matthies and Samii, 1997163 | On exploration of history | 63.3% (of 841) | Vestibular disturbance: 61.1% (of 841); vertigo: 34.4%; dizziness: 27.5%; unsteadiness: 40.3% |
| van Leeuwen et al., 1995164 | Presenting (main) symptom | 13% | Vertigo/unsteadiness: 7% |
| On further investigation | 57% | Vertigo/unsteadiness: 29% | |
| Selesnick et al., 1993;59 Selesnick and Jackler, 1993182 | Initial symptom | 36% (seldom sole initial symptom) | Vertigo: 7%; disequilibrium: 9% |
| On investigation | 56%; 53% tumour size < 1 cm, 59% tumour size 1–3 cm, 52% tumour size > 3 cm (measured as maximal diameter of CPA component parallel to petrous face) | Vertigo: 19%; 27% tumour size < 1 cm, 19% tumour size 1–3 cm, 10% tumour size > 3 cm | |
| Disequilibrium: 48%; 37% tumour size < 1 cm, 47% tumour size 1–3 cm, 71% tumour size > 3 cm | |||
| Ogawa et al., 1991;165 Ogawa et al., 1991166 | Symptom at initial visit | 113 (85.6%) | Vertigo: 26 (19.7%); dizziness: 62 (47.0%) |
| Diensthuber et al., 2006168 | Initial symptom | 44.7% | Unsteadiness/vertigo 26.3% |
| In the longer term | 70.1% | Unsteadiness/vertigo: 49.1% | |
| Berrettini et al., 1996170 | On questioning at time of diagnosis | 52.3%; 40% tumour size < 10 mm, 42.8% tumour size 10–30 mm, 68.7% tumour size > 30 mm (measured as greatest absolute diameter on MR scan, including intracanalicular portion when involved) | Disequilibrium: 52.3%; 40% tumour size < 10 mm, 28.6% tumour size 10–30 mm, 87.5% tumour size > 30 mm: |
| Rotary vertigo: 19%; 20% tumour size < 10 mm, 19% tumour size 10–30 mm, 12.5% tumour size > 30 mm | |||
| Are et al., 1995171 | Volunteered by patients | 43% | Imbalance: 44% |
| Moffat et al., 1993172 | Initial presenting symptom | 5 (13.2%) | Vertigo: 3 (7.9%) |
| On examination | Vestibular gait disturbance: 30 (78.9%) | ||
| Leonetti, 1995173 | Presenting symptom | 124 (60.8%) | Imbalance: 73 (35.8%) |
| Magdziarz et al., 2000174 | Reported by patients | 290 (78.6%) | Dysequilibrium: 144 (39.0%); vertigo: 77 (20.9%) |
| Moffat et al., 1994175 | Principle presenting symptom | 23 (8.09%) | Imbalance: 29 (10.2%) |
| On investigation | Calorics: reduced 48 (16.9%), absent 141 (49.3%), normal 31 (10.9%), not known 64 (22.5%) | ||
| Saleh et al., 1996176 | Initial complaint | 46 (35.9%) | Dysequilibrium: 14 (10.9%); vertigo: 13 (10.2%) |
| Tos et al., 1998152 | First symptom | 87 (12.4%) | Vertigo: 99 (14.1%) |
| Frohlich and Sutherland, 1993137 | Presenting symptom | 42 (60.9%) | Vertigo/dizziness: 20.3% |
| Moffat et al., 2004139 | Principle presenting symptom | 12% | Imbalance: 10%; vertigo: 0 |
| Moffat et al., 2004139 | Principle presenting symptom | 13% | Imbalance: 10%; vertigo: 1% |
| Artz et al., 2008178 | Reported at diagnosis | 151 (64.5%) | Unsteadiness/vertigo: 103 (44.0%) |
| Day et al., 2008179 | Clinical symptom | 38 (86%) | Vertigo: 18 (41%); dizziness: 20 (45%) |
| Baguley et al., 2006180 | Presenting symptom | 114 (12%) | Imbalance: 95 (11%) |
| Mackle et al., 2007181 | Principle complaint | 20 (5%) | Dysequilibrium: 109 (27%) |
| Presenting symptom | 53 (13.3%) constant; 163 (41%) intermittent | Rotatory vertigo: 13 (3.3%) |
Tinnitus
Several studies152,164,175 have reported that the incidence of tinnitus as a principal presenting symptom of acoustic neuroma ranges from 8% to 13%. As stated above, this is at odds with the prevalence of tinnitus in this patient group, which has been reported to be between 60% and 83%,59,158,160,163 and this has led to a proposal that tinnitus associated with a acoustic neuroma may evoke less distress than a clinician would predict. 22,183
Further exploration of the data of Moffat and colleagues138 by Baguley et al. 180 reviewed the characteristics of 941 patients with a radiological diagnosis of unilateral sporadic acoustic neuroma. Of these, 717 experienced tinnitus (76%) and in 114 (12%) tinnitus was the principle presenting symptom. Statistically significant associations were found between tinnitus presence/absence and tumor size (p = 0.012) (although these were non-linear) and type of hearing impairment (progressive, sudden, fluctuant, nil), with a tendency for patients without hearing impairment to be less likely to experience tinnitus. Statistically significant associations were identified between classification of tinnitus severity and age at diagnosis (p < 0.001) (greater age being associated with greater tinnitus severity), abnormal findings on caloric testing (p = 0.01) (abnormal calorics being associated with greater tinnitus severity) and tinnitus as a principal presenting symptom (p < 0.001) (this being associated with greater tinnitus severity). The common sense expectation that those patients who indicate tinnitus to be their principle presenting symptom experience more severe tinnitus than those who do not was supported.
Imbalance
A further proportion of patients describe symptoms of imbalance at presentation, which have been reported to include rotary vertigo, unsteadiness and imbalance. There is a lack of consistency in how these terms are applied in the literature regarding patients with acoustic neuroma, and this is a significant impediment to interpretation. There is evidence that vestibular symptoms in acoustic neuroma tend to be mild22,160 and involve non-specific dysequilibrium. 170,174,182 A small proportion of patients, between 10%176 and 19%170 of the patient population with acoustic neuroma, are described as experiencing rotary vertigo. Relatively few patients with acoustic neuroma present with a primary complaint of symptoms of imbalance, reported as between 7% and 26% when the principle presenting symptom is robustly defined. 139,164,168,172,175
Table 18 summarises the data on all other symptoms at presentation or as determined by further investigation. These data illustrate the relative frequency of trigeminal (Vth) nerve involvement, a nerve that supplies sensation to the face and eyes. As an acoustic neuroma expands superiorly, the sensory fibres of this nerve are particularly vulnerable to compression by the tumour. Hence, impairment of facial sensation (loss of sensation, paraesthesia, etc.) or loss of sensation on the cornea are typically seen in larger tumours. A small number of patients may even present with excruciating facial pain, evoking a misdiagnosis of trigeminal neuralgia. The facial nerve, although more intimately related to the tumour, is less frequently involved clinically, presumably because of the greater resilience of motor nerve fibres to the effects of compression. Indeed, the presence of a facial paralysis in a patient presenting with what appears to be an acoustic neuroma should lead to the consideration of other petrous apex pathology (e.g. non-acoustic tumours such as cholesteatoma). The evidence also demonstrates that the facial nerve may appear to have normal function to an examining clinician but may, on investigation, exhibit signs of weakness.
| Study | Headache, n (%) | Facial (VIIth) nerve problems, n (%) | Other cranial nerve symptoms, n (%) | Other symptoms, n (%) | Time of presentation |
|---|---|---|---|---|---|
| Fucci et al., 1999158 | 2 (2%) | NR | NR | Presenting symptom | |
| Kentala and Pyykko, 2001160 | 21 (17.2%) [1] | Facial sensitivity disturbance: 30 (24.6%) [2] | Lightheadedness: 22 (18.0%); movement difficulties outside, vertigo: 39 (32.0%); anxiety: 15 (12.3%) [1] | [1] Elicited by questionnaire; [2] on investigation | |
| Wandong et al., 2005162 | 19 (86.4%) | Facial nerve palsy: 6 (27.3%) | Impaired facial sensation: 18 (81.8%) | At presentation | |
| Matthies and Samii, 1997163 | 12.2% (mostly occipital) | Facial paresis: 5.2% | Trigeminal nerve symptoms: 9%; nerve VI (double vision): 1.8%, | Nausea and vomiting, visual disturbances and swallowing disturbances: 1–3% | On exploration of history |
| van Leeuwen et al., 1995164 | 0% [1]; 24% [2] | 1% [1]; 22% [2]; facial paresis: 5% [2] | Otalgia: 12%; epilepsy: 4%; ataxia/tremor: 33%; eye symptoms: 7%; nausea/vomiting: 3%; dysarthria: 3%; all [2] | [1] Presenting symptom; [2] on investigation | |
| Selesnick et al., 1993;59 Selesnick and Jackler, 1993182 | 2% [1]; 19% [2] (0% tumour size < 1 cm, 20% tumour size 1–3 cm, 43% tumour size > 3 cm) | Facial nerve dysfunction: 2% [1]; 10% [2] (7% tumour size < 1 cm, 11% tumour size 1–3 cm, 10% tumour size > 3 cm) | Trigeminal nerve dysfunction: 3% [1]; 20% [2] (0% tumour size < 1 cm, 20% tumour size 1–3 cm, 48% tumour size > 3 cm) | Diplopia: 3% [2] (1% tumour size < 1 cm, 1% tumour size 1–3 cm, 14% tumour size > 3 cm) | [1] Initial symptom; [2] on investigation |
| Ogawa et al., 1991;165 Ogawa et al., 1991166 | NR | Facial paralysis: 8 (6.1%) | Trigeminal paralysis: 36 (27.3%) | Plugged sensation of the ear: 50 (37.9%) | Symptoms at initial visit |
| Berrettini et al., 1996170 | 20%; 43.7% of tumours > 3 cm | 39% (60% tumour size < 1 cm, 47.6% tumour size 1–3 cm, 18.7% tumour size > 3 cm) | Trigeminal hypesthesia–paresthesia: 17% (4.8% tumour size 1–3 cm, 37.5% tumour size > 3 cm) | On questioning on diagnosis | |
| Are et al., 1995171 | Weakness: 10% | On investigation | |||
| Moffat et al., 1993172 | NR | Decreased facial sensation: 5 (13.2%) [2] | Numbness: 2 (5.3%) [1] | Otalgia: 2 (5.3%) [1] | [1] Initial presenting symptom; [2] on investigation |
| Leonetti, 1995173 | 10 (4.9%) [1] | Twitching: 9 (4.4%); paralysis: 7 (3.4%); both [2] | Facial numbness: 7 (3.4%) [2] | Aural fullness: 48 (40.8%) [1] | [1] Initial symptom; [2] on investigation |
| Magdziarz et al., 2000174 | 51(13.8%) | Weakness: 25 (6.8%) | Numb mouth/tongue: 1 (0.3%); facial numbness: 40 (10.8%); facial pain: 8 (2.2%); tingling ear/face: 1 (0.3%) | Aural fullness: 102 (27.6%); ataxia: 8 (2.2%); seizure: 2 (0.5%); arm/hand discomfort: 1 (0.3%); poor attention span: 1 (0.3%); nausea: 1 (0.3%) | |
| Moffat et al., 1994175 | 5 (1.8%) | Visual symptoms: 3 (1.05%); facial numbness: 12 (4.2%); 63 (22.2%) on investigation | Otalgia: 2 (0.7%); other (unspecified): 10 (1.2%) | Principal presenting symptom | |
| Saleh et al., 1996176 | 1 (0.8%) | Dysfunction: 4 (3.1%) | Trigeminal neuralgia: 1 (0.8%); trigeminal symptoms: 4 (3.1%); facial numbness: 3 (2.3%) | Diplopia: 1 (0.8%) | Initial symptom |
| Tos et al., 1998152 | 9 (1.3%) | Paresis: 8 (1.1%) | Trigeminal symptoms: 29 (4.1%) | Cerebellar symptoms: 20 (2.8%); epilepsy: 1 (0.1%) | First symptom |
| Frohlich and Sutherland, 1993137 | 18 (26.1%) [1] | Facial paresthesia: 16 (23.2%) [1] | Facial pain: 5 (7.2%) [1] | Ataxia/unsteady gait: 26 (37.7%) [1], 24 (34.8%) [2]; visual impairment: 9 (13%); retroauricular fullness: 9 (13%); otalgia: 1 (1.4%); diplopia: 3 (4.4%); difficulty swallowing: 2 (2.9%), all [1] | [1] Presenting symptom; [2] on examination |
| Moffat et al., 2004139 | 1% | Weakness: 0% | Visual disturbance: 1%; facial numbness: 5% | Otalgia: 1% | Principal presenting symptom |
| Moffat et al., 2004139 | 1% | Weakness: 1% | Visual disturbance: 1%; facial numbness: 3% | Otalgia: 1% | Principal presenting symptom |
| Artz et al., 2008178 | 8 (3.4%) | NR | NR | Otalgia: 10 (4.3%); aural fullness: 27 (11.5%) | Reported at diagnosis |
| Day et al., 2008179 | 14 (32%) | Facial palsy: 3 (7%) | Ataxia: 13 (30%); aural fullness: 10 (23%) | Unclear |
Symptoms and tumour characteristics
The association between tumour characteristics and symptom profile has been considered. The influence of tumour size has been analysed and the observation made that small tumours (< 1 cm diameter) may be associated with fewer symptoms;185 however, the most rigorous analysis failed to demonstrate any association between size and symptoms. 164
Tumour morphology has also been considered. Medially arising tumours have been demonstrated to be associated with a higher incidence of symptoms deriving from cerebellar, trigeminal nerve and brainstem involvement,152 such as ataxia, disdiadokinesis and decreased facial and corneal sensation. Conversely, an association between medial acoustic neuroma and normal hearing has been reported. 170,172
Studies have failed to demonstrate a consistent correlation between the pattern or degree of hearing impairment and any aspect of tumour morphology. Many studies demonstrate a worsening of hearing threshold over time159,186–195 but relatively few are able to report a statistically significant correlation with growth. 179,186,190–192
Diagnosis of asymptomatic tumours
Table 14 summarises the data from two studies reporting on large series of MR scans. Anderson and colleagues148 retrospectively evaluated 24,246 brain MR imaging studies (19,405 with contrast and 4841 without contrast) and found 17 incidental acoustic neuromas or approximately seven per 10,000 routine brain MR studies being undertaken for reasons other than the exclusion of an acoustic neuroma. A further study at the University of California in San Francisco45 analysed the MR imaging database of 46,414 patients who had brain scans with no documented audiovestibular symptoms, and revealed eight patients with incidental acoustic neuromas. Three of these patients were found to have audiovestibular symptoms on enquiry after diagnosis; three patients had asymmetry on audiometry at 4 kHz, with otherwise normal audiometry for age in the remaining patients. Tumour size ranged from 3 to 28 mm.
Duration of symptoms and delay to diagnosis
An interesting consideration is that of the duration of symptoms. This is a complex area as some of the symptoms of an acoustic neuroma are insidious and may not be given due attention by the patient or GP. Van Leeuwen and colleagues184 studied a series of 164 patients who underwent surgical removal of a unilateral acoustic neuroma in Nijmegen, the Netherlands, between 1980 and 1992. The mean delay from initial symptom onset to seeing a specialist was 35.7 months [standard deviation (SD) 62.2, range 0–468 months]; such delay could be due to patient reluctance to seek advice or to the GP not referring on for specialist consultation. A further mean delay of 15.2 months (SD 36.2, range 0–242 months) was evident from specialist input to diagnosis. Although there is an obvious caveat in that the period studied predates the modern era of straightforward access to MR imaging, this work does demonstrate the complexity of the situation regarding the duration of symptoms and the delay before definitive diagnosis. Moffat and colleagues139 report the length of history in a cohort of acoustic neuroma patients undergoing surgery in the years 1981–3 as 42 months (SD 51) and from 1994 to 2004 as 45 months (SD 108.64). These figures are not statistically significantly different (p = 0.4726, Mann–Whitney U-test) and therefore access to MR imaging may not have influenced this issue.
Within the literature, the complexity of the issue of length of symptoms and diagnostic delay has not been adequately addressed and, although some papers report a duration of symptoms,59,152,163,164 the lack of rigour hampers systematic analysis. Specifically, a possible association between size of tumour and duration of symptoms has either been excluded164 or been suggested and not statistically verified. 59,163
Discussion
The data in Tables 16–18 are illustrative of the dichotomy between the prevalence of some symptoms determined after further investigation, examination and questioning, and the number of patients who report that symptom as their principal complaint.
van Leeuwen and colleagues164 noted that such a disparity between the presence of a symptom and the incidence of that symptom as the primary presenting complaint of an acoustic neuroma may reflect either a lack of concern or distress associated with that symptom by the patient or a reluctance to refer a patient for that symptom on the part of a patient’s primary care physician.
This consideration of the symptoms of acoustic neuroma is pertinent to the subject of this review. It is apparent that the majority of patients diagnosed with acoustic neuroma present with insidious symptoms of unilateral hearing impairment, tinnitus and/or vertigo, which may not have been prioritised by the patient or the GP.
A somewhat different perspective was taken by Humphriss and colleagues,22 who considered the audiovestibular handicap in a series of 145 patients scheduled for surgical excision of a unilateral acoustic neuroma. Using standard questionnaire instruments for the determination of hearing, tinnitus and dizziness handicap, 68% had significant hearing handicap, 30% had significant tinnitus handicap and 75% had significant dizziness handicap. In total, 88% of patients had some handicap in at least one domain and 23% had some handicap in all three domains. A total of 7% of patients had severe handicap in all three domains. There was no significant association between tumour size and any of the questionnaire scores. It is apparent that the audiovestibular symptoms experienced by patients diagnosed with acoustic neuroma are associated with a significant burden.
The data in Table 18 regarding the non-audiovestibular symptoms that a patient with acoustic neuroma may experience or indeed present with are of some clinical interest. The implication is that even when symptoms that a patient describes are non-audiovestibular but indicate potential cranial nerve involvement the possibility of acoustic neuroma should be included in the differential diagnosis. 196
Growth
Introduction
Acoustic neuromas usually arise in the IAM and fill it before emerging and growing into the cranial cavity. Thus, an acoustic neuroma has an intracranial component of a certain size in addition to the intrameatal component. The advent of MR scanning ushered in a new era for acoustic neuroma diagnosis, permitting identification of tumours of no more than a few millimetres in diameter. However, the capacity to so readily image even the smallest tumours has introduced management dilemmas. It is known, for instance, that many patients live undisturbed by their tumours, ultimately dying with them but not because of them. Other tumours, however, progress to cause life-threatening neurological symptoms. Therapeutic intervention, by surgery or radiation treatment, is associated with significant morbidity and consequences for quality of life. 17,18 Thus, distinguishing those patients whose tumours pose a threat from those whose tumours may safely be left without intervention is the key to current acoustic neuroma management. In many centres evidence of tumour growth has become the defining criterion for intervention, especially for small or medium-sized tumours that have not caused any neurological symptoms or signs. Therefore, the current issues in this field concern methods of measuring these tumours, definitions of growth and estimation of actual growth.
MR imaging is now widely accepted as the gold standard for assessing acoustic neuroma size and is considered to be superior to all previous diagnostic imaging methods. 197 However, to accumulate numbers and increase follow-up, numerous studies have used CT scanning either as the only method of measuring or in combination with MR or even cisternography. 187,198–200 This makes comparisons difficult and introduces a significant bias in any conclusions derived from such studies. On the other hand, such studies are important as they include patients with very long follow-up, a key issue when exploring natural history and conservative management.
Results
Table 19 summarises the studies reviewed in this section reporting data on the characteristics of growth of acoustic neuromas.
| Study | Country | Number of participantsa | Age of participants (years), mean (range) | Sex |
|---|---|---|---|---|
| Jorgensen and Pedersen, 1994198 | Denmark | 78 (3 NF2) | For the 18 unilaterals followed, 55 (16–74) | Overall M: 47%; F: 53%. Of 18 followed: 8 M, 10 F |
| Bozorg Grayeli et al., 2005186 | France | 111 | 59 (19–87) | M: 44%; F: 56% |
| Flint et al., 2005187 | Australia | 100 | 61 (31–86) | M: 46%; F: 54% |
| Piazza et al., 2003201 | Italy | 44 | 70 (65–77) | M: 48%; F: 52% |
| Glasscock et al., 1997202 | USA | 48 | 75 (70–90) | M: 37.5%; F: 62.5% |
| Strasnick et al., 1994203 | USA | 51 | 68 (40–90) | M: 35%; F: 65% |
| Bederson et al., 1991204 | Switzerland | 70 | 57 ± 1.4 (26–79) | M: 44%; F: 56% |
| Valvassori et al., 1991200 | USA | 50 | 16–81 | M: 40%; F: 60% |
| Herwadker et al., 2005205 | UK | 63 | Median 62 (36–88) | M: 52%; F: 48% |
| Caye-Thomasen et al., 2003206 | Denmark | 15 | 60 (37–79) | |
| Mohyuddin et al., 2003207 | UK | 50 | 64.1 ± 12.8 | M: 52%; F: 48% |
| Niemczyk et al., 2002208 | France | 17 | NR | NR |
| Vokurka et al., 2002209 | UK | 63 | Median 62 (36–88) | M: 52%; F: 48% |
| Stipkovits et al., 2001197 | the Netherlands | 44 | 58 (29–76) | M: 66%; F:34% |
| Hoistad et al., 2001210 | USA | 102 | 64 (25–89) | M: 43%; F: 57% |
| Nutik and Babb, 2001211 | USA | 433 | NR | M: 52%; F: 48% |
| Sakamoto et al., 2001212 | Japan | 31 | 57.1 (9–81) | NR |
| Massick et al., 2000213 | USA | 21 | 63.3 (15–84) | M: 48%; F: 52% |
| O’Reilly et al., 2000214 | UK | 44 | 64.3 (30–85) | M: 30%; F: 70% |
| Shin et al., 2000215 | France | 97 | 63 (29–89) | M: 35%; F: 65% |
| Modugno et al., 1999189 | Italy | 47 | 60 (26–84) | M: 55%; F: 45% |
| Niemczyk et al., 1999216 | France | 15 | 59.5 | NR |
| Yamamoto et al., 1998217 | Japan | 16 (4 lost to follow-up) | 58 (38–87) | M: 50%; F: 50% |
| Levo et al., 1997218 | Finland | 31 (7 NF2) | For 24 in unilateral group, median age 63.1 (31.6–74.6) | M: 26%; F: 74% |
| Deen et al., 1996219 | USA | 68 | 67.1 (35–80) | M: 46%; F: 54% |
| Wiet et al., 1995220 | USA | 53 | 66 (25–89) | M: 47%; F: 53% |
| Martin et al., 1994190 | France | 39 | NR | NR |
| Rosenberg et al., 1993221 | USA | 23 | 73 (65–86) | NR |
| Ogawa et al., 1991199 | Japan | 36 (13 NF2) | Mean 40.7 (15–73) (for all patients, not just unilateral AN) | M: 50%; F: 50% |
| Al Sanosi et al., 2006222 | Australia | 205 | 60.84 (26–89) | M: 44%; F: 56% |
| Shin et al., 2003223 | France | 123 | 64 (29–90) | M: 39%; F: 61% |
| Moller et al., 2003224 | Norway | 239 | NR | NR |
| Kishore et al., 2003225 | UK | 100 | 59 (39–80) | NR |
| Ramsden et al., 2003226 | UK | 244 (54 NF2) | NR | NR |
| Ferri et al., 2008227 | Italy | 123 | 61.1 (25–84) | M: 50%; F: 50% |
| Quaranta et al., 2003228 | UK | 129 | 62 (29–86) | M: 52%; F: 48% |
| Battaglia et al., 2006229 | USA | 164 | 71 (35–94) | |
| Battaglia et al., 2006229 | USAb | n = 5 studies | NR | NR |
| Stangerup et al., 2006;122 Charabi et al., 2000230 (also Charabi et al., 1995;188 Thomsen et al., 2000;231 Caye-Thomasen et al., 2006232) | Denmark | 552 | 59 (15–83) | M: 52%; F: 48% |
| Hajioff et al., 2008191 (also Raut et al., 2004;233 Walsh et al., 2000;193 Walsh et al., 2000;195 Walsh et al., 2000234) | Canada | 72 | 61 (36–78) | M: 44%; F: 56% |
| Mirz et al., 2000235 (also Mirz et al., 1999236) | Denmark | 64 | 55 (23–75) | M: 48%; F: 52% |
| Rosenberg, 2000;237 also included in reviews | USA | 80 | At diagnosis: 69.7 (35.9–84.2). At symptoms: 61.9 (23–83.2) | M: 55%; F: 45% |
| Rosenberg, 2000;237 also included in reviews | USA | 49 | At diagnosis: 66 (31–84.6). At symptoms: 60 (29.7–84.5) | M: 29%; F: 71% |
| Smouha et al., 2005194 | USA | 64 | ||
| Quaranta et al., 2007192 | UK | 70 | 60 (29–81) | M: 48.6%; F: 51.4% |
| Fucci et al., 1999158 | USA | 119 | 65 (37–84) | M: 47.9%; F: 52.1% |
| Tschudi et al., 2000159 | Switzerland | 84 | 52.6 (19–78) | M: 59.5%; F: 40.5% |
| Artz et al., 2008178 | the Netherlands | 234 | 57 (16–82) | M: 49.1%; F: 50.9% |
| Solares and Panizza, 2008238 | USA | 110 | 62.4 (32–91) | M: 59%; F: 41% |
| Systematic reviews | ||||
| Yoshimoto, 200516 | Japan | 1340 (12–127 per study) | 62 (52–75) | |
| Smouha et al., 2005194 | USA | 1345 (13–123) | 62 | |
| Yamakami et al., 2003239 | Japanb | 894 in 13 studies | 65 (12–94) | |
| Selesnick and Johnson, 1998240 | USA | 571 (16 NF2), so 555 with unilateral AN; 13 studies | 64 (56–75) (n = 555) | |
Measurement
An important issue in measuring acoustic neuromas is how their size is measured or calculated even within the same imaging technique. The most common procedure is to measure the maximum diameter of the tumour; however, this entails gross simplification, because tumours are three-dimensional structures and often irregular in shape. Moreover, a change in the diameter implies an exponential change in the volume of the lesion. For example, when the diameter has doubled, the volume has increased eight times, assuming that the shape of the tumour has not changed. 197 Rosenberg237 compared rigorous computer analysis of size and growth rate with the radiologists’ usual measurements of maximal diameters. 237 The computer analysis used a new method of measurement: equivalent diameter as the diameter of a perfect circle that corresponds to the measured tumour area (largest area in both axial and coronal plane). Rosenberg237 concluded that the measurement of the maximal tumour diameter on MR imaging is still a reliable method for following acoustic neuroma growth and that there is no need to perform a rigorous analysis of tumour size to determine whether the tumour is growing significantly.
The confusion and possible bias in measuring acoustic neuroma size is confounded when we take into account that, in addition to the maximal diameter method, numerous other descriptions of measurement have been reported by different centres. These are summarised in Table 20.
| Study | Country | Measurement |
|---|---|---|
| Jorgensen and Pedersen, 1994198 | Denmark | Greatest extrameatal diameter (measured by CT only) |
| Nutik and Babb, 2001211 | USA | Greatest extrameatal diameter (measured by CT or MR imaging) |
| Flint et al., 2005187 | Australia | |
| Bozorg Grayeli et al., 2005186 | France | Greatest diameter including intracanalicular portion measured by MR imaging |
| Modugno et al., 1999189 | Italy | |
| Al Sanosi et al., 2006222 | Australia | |
| Quaranta et al., 2003228 | UK | |
| Ferri et al., 2008227 | Italy | Greatest diameter including intracanalicular portion in the three axes of projection measured by MR imaging |
| Ramsden et al., 2003226 | UK | Lateral extent in the IAM to medial pole |
| Glasscock et al., 1997202 | USA | Greatest mediolateral extent within CPA |
| Strasnick et al., 1994203 | USA | Mean of greatest anteroposterior and mediolateral tumour extent |
| Bederson et al., 1991204 | Switzerland | |
| Deen et al., 1996219 | USA | |
| Shin et al., 2003223 | France | |
| Mirz et al., 2000235 | Denmark | Maximal anteroposterior dimension along the pyramid |
| Raut et al., 2004233 | Canada | The axis of the canal from the fundus to the porus |
| Massick et al., 2000213 | USA | Maximal diameter and tumour volume |
| Quaranta et al., 2007192 | UK | Measurement along longest axis including intrameatal portion |
| Niemczyk et al., 1999216 | France | Tumour volume measured using visible tumour slices; values of all slices saved and shape of tumour slices was contoured |
| Yamamoto et al., 1998217 | Japan | Three diameters on MR imaging, two determined on axial slice including maximum diameter and other on coronal slice measured in centimetres. Tumour volume calculated by multiplication of three diameters by 0.52 |
| Ogawa et al., 1991199 | Japan | Tumour size calculated using (long axis × short axis) to the power ½ |
| Rosenberg, 2000237 | USA | Equivalent diameter as the diameter of a perfect circle that corresponds to the measured tumour area (largest area in both axial and coronal plane) |
| Tschudi et al., 2000159 | Switzerland | Anteroposterior and mediolateral diameters |
| Wiet et al., 1995220 | USA | Greatest axial dimension along longitudinal plane of IAC (including intrameatal portion) |
| Valvassori and Shannon, 1991200 | USA | No definition of measurement method |
| Levo et al., 1997218 | Finland | |
| Kishore et al., 2003225 | UK | |
| Artz et al., 2008178 | the Netherlands | Maximum diameter along length of IAC (IAC tumours); maximum diameter parallel to petrous ridge or IAC in the axial plane (CPA tumours) |
| Solares and Panizza, 2008238 | USA | Greatest extracanalicular dimension by MR imaging |
To limit the variance, the American Academy of Otolaryngology – Head and Neck Surgery – recommended that size should be measured by taking the square root of the product of the two standardised diameters in the axial plane. Although this method was introduced in 1995, very few centres have been using it. In addition, the procedure has its own drawbacks. One is that the superior and inferior extents of the tumour as well as the volume are not taken into account. Another is that this procedure is intended to classify tumours in such a manner as to allow comparison of the results of surgery, not to establish changes in the size over time. 197
Apart from the numerous different formulae used to measure size and/or volume, there is also evidence that the reliability of measuring acoustic neuromas is far from satisfactory. Marshall and colleagues241 assessed the inter- and intraobserver reliability of measuring acoustic neuromas, including in their study the method proposed by the American Academy of Otolaryngology, and concluded that, in routine clinical practice, differences in tumour size of the order of 2 mm cannot be reliably measured, even by the same radiologist. On the other hand, in another study,197 when the maximal surface of the tumour in the axial plane and SD were calculated – SD was taken as the indicator of growth or shrinkage – interobserver agreement was found to be very high and clinical judgements agreed with surface axial computer measurements. However, this study had several weaknesses including a small number of patients (n = 44) and the fact that interobserver agreement was dependent on the threshold set (e.g. if growth is defined as a large difference of 3 mm, interobserver agreement becomes very high, and vice versa). Reported growth of acoustic tumours should therefore be interpreted with caution, especially if this is the criterion for recommending treatment.
Definition of growth
It might be thought that any growth, even that which is minimal, should be considered as such; however, the reality is quite different as terms such as ‘important growth’, ‘definite growth’, ‘probable growth’, ‘minimal growth’, ‘slow or rapid growth’ and various other definitions have been used in the literature. Table 21 summarises the various definitions that have been used to report growth. For example, an increase of 1 mm in size may be considered as growth in one study198 and ‘stable’ in another. 186 In addition, regression has been separately reported in some papers,186,198,210 whereas in others221 stability and regression count as one. Finally, mathematical formulae, both simple and complex, have been used to assess growth and growth rates.
| Definition of growth | Study |
|---|---|
| Increase > 0.5 mm/year | Jorgensen and Pedersen, 1994198 |
| Increase > 1 mm/year (American Academy guidelines followed in some cases) | Hajioff et al., 2008;191 Walsh et al., 2000;193 Mirz et al., 2000;235 Strasnick et al., 1994;203 Sakamoto et al., 2001;212 Kishore et al., 2003225 |
| Increase > 1 mm along the same axis | Hoistad et al., 2001;210 Artz et al., 2008178 |
| Increase > 1 mm in either tangential or perpendicular planes | O’Reilly et al., 2000214 |
| Increase > 2 mm/year | Bozorg Grayeli et al., 2005;186 Glasscock et al., 1997202 |
| Increase ≥ 2 mm | Ferri et al., 2008227 |
| Increase in diameter > 2 mm | Solares and Panizza, 2008238 |
| Increase > 2 mm along the same axis | Quaranta et al., 2003228 |
| Percentage increase in maximum tumour diameter | Nutik and Babb, 2001211 |
| Percentage increase in volume | Niemczyk et al., 1999216 |
| Increase in tumour volume > 10% | Massick et al., 2000213 |
| Increase in tumour volume > 20% | Yamamoto et al., 1998217 |
| Change in tumour volume | Shin et al., 2000215 |
| Minimal tumour growth as < 20% of original size, 20–50% moderate growth, > 50% marked growth | Valvassori and Shannon, 1991200 |
| Slowly growing if < 2 mm increase, rapidly growing if > 2 mm increase | Al Sanosi et al., 2006222 |
| Internal auditory canal tumours: growth to extrameatal dimensions. Extrameatal tumours: largest diameter change > 2 mm | Stangerup et al., 2006122 |
| Clinical growth index = maximal tumour diameter at second scan divided by time of onset of first symptom to time of second scan | Mohyuddin et al., 2003207 |
| Definite growth: increase > 3 times estimated measurement error. Probable growth: increase 1–3 times measurement error | Herwadker et al., 2005205 |
| Tumour doubling time | Yamamoto et al., 1998217 |
| Formulae used to calculate tumour increasing size and tumour volume doubling | Ogawa et al., 1991199 |
| Standard deviation used to indicate growth or shrinkage | Stipkovits et al., 2001197 |
Radiosurgical tumour control
In addition to the problems related to measuring tumour size and growth, comparisons between conservative management and radiosurgery have introduced new challenges. Radiosurgeons also have various definitions of tumour control, i.e. non-growth. A widely accepted radiosurgical definition of tumour control is that of Flickinger and colleagues242 who described tumour control as a less than 1-mm increase in tumour diameter in any two directions or 2 mm in one direction. Another widely accepted definition of tumour control is freedom from surgical resection or lack of surgical intervention. In a study that compared conservative management in patients from a single centre with radiosurgery patients from the literature,229 using Flickinger’s242 definition of control, the control rate in their population of 71 patients after a mean of 3.1 years was 87%, which was only 6% worse than Flickinger’s242 results after a 24-month median follow-up period with 313 patients, and 1% better than that reported by Iwai and colleagues243, the radiosurgical study with one of the longest median follow-up periods (60 months).
In another report,223 data from a study of conservative management were compared with data from three studies of radiosurgery. The results revealed that the risk of growth was statistically higher in the conservative management group, whereas stability was comparable. Regarding other factors, the risks of losing useful hearing, developing trigeminal nerve dysfunction and/or developing hydrocephalus requiring shunt placement were statistically higher after radiosurgery. Ultimately, however, without standardised ways of measuring tumour growth and reporting the results, a fair comparison of radiosurgical results with the natural history of acoustic neuromas is not possible. 229
Growth pattern during conservative management
Studies that have compared the various methods of assessing growth have revealed that reports of growth depend on the measurement method used and the respective differences may be huge. In one study that compared different measurement methods, tumour volume doubling time assessed by a Bayesian partial volume tissue segmentation method demonstrated tumour growth in 80% of cases that appeared to show no growth when measured by manual segmentation techniques. 207,209 In another study, volume calculation was carried out manually and compared with a computerised method; the difference was statistically significant and in some cases as much as double or triple the volume. 215
Although the literature reports different methods of measuring and comparing growth, any review may only be a rough estimate of what actually happens in ‘real life’. Table 22 summarises the results of the papers included in this review that reported the growth pattern of acoustic neuroma during conservative management, that is, ‘wait and rescan’. Besides the absolute growth, an estimate of the growth rate of these tumours will contribute to the decision of when, if at all, to intervene. A very slowly growing tumour may have a completely different management in comparison to a rapidly growing tumour, which may result in neurological complications in the short term. Of course, the task of assessing growth rate has the same weaknesses mentioned previously. In addition, the various studies on growth rate have used different criteria or methods of reporting. For example, some studies report the growth rate of tumours that were increasing in size, whereas other studies report the growth rate of all tumours. Finally, some studies introduce the term ‘tumour doubling time’, which is another type of growth rate. Table 22 summarises the outcomes of papers reporting on acoustic neuroma growth rate.
| Study | Number followed up | Length of follow-up, mean (range) | No growth (%) | Growth (%) | Regression (%) | Growth rate (mm/year), mean (range) |
|---|---|---|---|---|---|---|
| (1) Growth rate applies to all tumours | ||||||
| Bederson et al., 1991204 | 70 | 26 ± 2 months (6–84 months) | 41.4 | 52.9 | 5.7 | 1.6 ± 0.4 in first year; 1.9 ± 1.0 in second year |
| Ogawa et al., 1991199 | 23 | 51.3 months (6–127 months) | NR | NR | NR | 4.8 (SD 2.4) |
| Jorgensen and Pedersen, 1994198 | 18 | 4.5 years (1–7 years) | 27.8 | 66.7 | 5.6 | 1.61 |
| Strasnick et al., 1994203 | 50 | 2.3 years (6–74 months) | 32 | 68 | 0 | 1.1 (0–11.0) |
| Glasscock et al., 1997202 | 34 | 28.5 months (5–108 months) | 45 | 55 | 0 | 2.9 (0–19) |
| Levo et al., 1997218 | 24 | 2 years (1–6 years) | 58 | Not clear | Not clear | 0.35 |
| Fucci et al., 1999158 | 119 | 2.5 years (5 months–8 years) | 66 | 30 | 3 | 1.2 (SD 3.1) |
| Rosenberg, 2000237 | 80 | 4.4 years (0.01–17.2 years) | 34.6 | 57.8 | 7.7 | 0.91 (–3.9 to 10.34) |
| Mirz et al., 2000235 | 64 | 3.6 years (5 months to 14.8 years) | 55 | 23 | 22 | 0.4 (SD 1.52) |
| Shin et al., 2000215 | 87 | 31 months (4 months to 10 years) | 36 | 53 | 11 | 1.5 (–13 to18) |
| Hajioff et al., 2008191 | 72 | 121 months (median) (89–271 months) | 38 | 40 | 22 | 1.0 (median) (–0.53 to 7.84) |
| Sakamoto et al., 2001212 | 31 | 33 months (6–92 months) | 45 | 45 | 10 | 2.4 (–2 to 17). Solid type: 2.1; cystic type: 3.7 |
| Caye-Thomasen et al., 2003206 | 15 | 19 months (6–41 months) | NR | NR | NR | 0.62 cm3/year (0.086–2.609) |
| Quaranta et al., 2003228 | 122 | 41.5 months (5–136 months) | 53 | 40 | 7 | 1.09 (–6.32 to 10) |
| Raut et al., 2004233 | Extrameatal: 43 | 80 months (52–234 months) | 30 | 50 | 20 | 1.3 (–0.84 to 9.65). |
| IAC: 18 | 78 | 6 | 17 | 0 (–0.36 to 0.45) | ||
| Bozorg Grayeli et al., 2005186 | 111 | 33 months (6–111months) | 47 | 47 | 6 | 1.1 (SE 0.21) |
| Herwadker et al., 2005205 | 50 | 17.4 months (5–22 months) | 0 (unusual method) | 42% definite, 38% probable | 20% probable involution | Median 109 mm3/year (–380 to 765) |
| Battaglia et al., 2006229 | 111 | 38 months (1–13 years) | 45 | 50 | 5 | 0.7 (SE 1.4) |
| (2) Growth rate applies to growing tumours only | ||||||
| Jorgensen and Pedersen, 1994198 | 18 | 4.5 years (1–7 years) | 27.8 | 66.7 | 5.6 | 2.42 (0.28–4.8) |
| Wiet et al., 1995220 | 53 | 25.8 months (5–99 months) | 60 | 40 | 0 | 4.2 (0.6–16.4) |
| Yamamoto et al., 1998217 | 12 | 18 months (3–46 months) | 9 | 73 | 18 | Mean tumour doubling time 5 years (6 months–29 years) |
| Fucci et al., 1999158 | 119 | 2.5 years (5 months–8 years) | 66 | 30 | 3 | 3.8 (SD 4.6) |
| Modugno et al., 1999189 | 47 | 36 months (6–91 months) | 64 | 36 | 0 | 4 (1–12) |
| Mirz et al., 2000235 | 64 | 3.6 years (5 months to 14.8 years) | 55 | 23 | 22 | 2.3 |
| Tschudi et al., 2000159 | 74 | 35 months (12–108 months) | 58 | 31 | 11 | 2.2 (0.3–7.7); mean for first year 2.7 |
| Hoistad et al., 2001210 | 102 | 28.5 months (6 months to 10 years) | 53 | 44 | 3 | 2.1 |
| Nutik and Babb, 2001211 | 75 | 4.1 years (SD 2.8 years) | 59 | 41 | 0 | 3.1 (SD 2.8) |
| Ferri et al., 2008227 | 123 | 57.4 months (6–182 months) | 60 | 35 | 5 | 1.2 |
| Flint et al., 2005187 | 100 | 25.5 months (5–150 months) | 62 | 36 | 2 | 2.7 |
| Stangerup et al., 2006122 | Extrameatal: 322 | 3.6 years (1–15 years) | 70 | 29 | 1 | 4.9 in first year |
| IAC: 230 | 3.6 years (1–15 years) | 83 | 17 | 0 | 10.3 | |
| (3) Application of growth rate to tumour state is not reported or not clear | ||||||
| Martin et al., 1994190 | 37 | 5.5 years (6 months–14 years) | 65 | 30 | 5 | NR |
| Deen et al., 1996219 | 68 | 3.4 years (6 months–12 years) | 71 | 29 | 0 | Overall growth rate not clear |
| Niemczyk et al., 1999216 | 15 | 6.3 months | 53 (may include regression) | 47 | 0 | Mean volume gain 14.4% |
| Massick et al., 2000213 | 21 | 3.8 years (2–5 years) | 24 | 66 | 10 | NR |
| O’Reilly et al., 2000214 | 43 | 30.5 months (12–120 months) | 70 | 30 | 0 | NR |
| Stipkovits et al., 2001197 | 44 | 3.5 years (12–74 months) | 75 | 18 | 7 | NR |
| Shin et al., 2003223 | 123 | 33 months (5–132 months) | 64 | 25 | 11 | NR |
| Moller et al., 2003224 | 82 | Minimum 3 years | 57 | 43 | 0 | NR |
| Kishore et al., 2003225 | 100 | 38 months (12 months to 12 years) | 71 | 29 | 0 | NR |
| Al Sanosi et al., 2006222 | 197 | 40.8 months (12–180 months) | 69 | 28 | 3 | NR |
| Quaranta et al., 2007192 | 70 | 33.3 months (7–111 months) | 60 | 40 | 0 | NR |
| Solares and Panizza, 2008238 | 110 | 31.4 months (6–156 months) | 70.6 (5-year no growth rate) | 21 | 10 | NR |
| (4) Growth rate has another definition | ||||||
| Rosenberg et al., 1993221 | 16 | 4.3 years (0.7–9.2 years) | 44 (may include regression) | 56 | 0 | 0.6 |
To facilitate comparisons within the table we have ordered the studies as follows: (1) growth rate applies to all tumours, (2) growth rate applies only to tumours that grow, (3) application of growth rate to tumour state is not reported or is not clear or (4) growth rate has another definition.
In a meta-analysis comprising 1244 patients in 19 studies, 51% of tumours showed no growth, 43% of tumours grew and 6% showed regression. 194 In another meta-analysis of 555 patients in 13 studies, 54% showed growth (range 14–74%) and 26% had rapid growth of more than 2 mm/year (range 0–47%). 240 In a systematic review of 1340 patients, 46% showed growth (95% CI 43–48%, range 15–85%) and 8% regression (95% CI 6–10%). 16 In a small group of four prospective studies, 29% of tumours showed growth (95% CI 21–37%). 16 In another systematic review of 879 patients, 51% of tumours grew and 4% showed regression. 239 An additional problem is that there are various patterns of growth, and a tumour that shows growth may stop doing so and vice versa. In a study215 that assessed these patterns, tumours were classified into five categories: continuous growth (15%), negative growth (5%), growth followed by shrinkage (40%), negative growth followed by growth (20%) and no variation in tumour size (20%). In another study,228 five patterns of growth were also found: continuous growth (25%), stability/growth (16%), stability (50%), negative growth (7%) and growth/stability (3%). Finally, a third study237 reported six patterns: no growth (35%), growth only (21%), no growth to growth (13%), growth to stability (13%), growth to regression (10%) and regression (8%).
Growth rate
In one meta-analysis180 of 793 patients in 13 studies, the mean growth rate was 1.9 mm/year (range 0–10 mm/year) and in another meta-analysis240 of 508 patients in 13 studies, the mean growth rate was 1.8 mm/year (range 0.5–3.2 mm/year). In a systematic review of 964 patients, the mean growth rate was 1.2 mm/year (range 0.4–2.9 mm/year). 16 In another systematic review of 879 patients, the mean growth rate was 1.8 mm/year (range 0.3–30 mm/year) (including those with regression the mean growth rate was –3.9 to 30.0 mm/year). 239 It seems that the mean growth rate for all tumours varies between 1 and 2 mm/year. Including only tumours that grow, the growth rate varies between 2 and 4 mm/year. However, there are cases with significant regression or exceptional growth that may exceed 18 mm/year.
Time frame of growth
Irrespective of growth rate, the time scale in which this growth may occur is very important. For example, if growing in a specific time period (e.g. within 1 or 2 years from diagnosis) predicts later tumour behaviour, this would influence management and follow-up intervals. Therefore, some studies have analysed the time frame of growth (Table 23).
| Growth pattern | Study |
|---|---|
| Growth always evident in the first year | Moller et al., 2003;224 O’Reilly et al., 2000214 |
| Growth in first year highly predictive of overall growth | Bederson et al., 1991;204 Tschudi et al., 2000;159 Quaranta et al., 2003228 |
| 80% of tumours that grew did so in the first year | Flint et al., 2005187 |
| 59% of tumours that grew did so in the first year | Modugno et al., 1999189 |
| Patients with higher growth rate at 1 year statistically more likely to have surgery | Deen et al., 1996219 |
| Tumours with significant growth did so within an average of 2 years of follow-up | Glasscock et al., 1997202 |
| 97% of tumours that grew did so in the first follow-up interval (but this could be as much as 5 years) | Nutik and Babb, 2001211 |
| 45% of those growing did so in the first year, but 23% grew after 3 years of stability and 0% grew after 6 or more years of observation | Ferri et al., 2008227 |
| Within the first year 62% of those growing did grow and growth occurs only in the first 5 years after diagnosis | Stangerup et al., 2006122 |
| 42% of those that grew remained stable for > 1 year followed by continuous growth | Massick et al., 2000213 |
| 65% of tumours grew within the first 4 years after diagnosis, but 14% started to grow after 60 months of stability | Quaranta et al., 2003228 |
| Tumour volume doubling time may be within 6 months; therefore, follow-up intervals should be short initially | Yamamoto et al., 1998217 |
| No significant difference in tumour growth rates using 6-month, 12-month or > 1-year intervals | Tschudi et al., 2000159 |
Many of these studies suggest that the first year after diagnosis is crucial for determining the pattern of tumour growth, but it is clear from several other reports that this may not be the case for some patients. For example, a patient with a 4-mm extension in the CPA did not have any growth for 6 years and then the tumour started to grow requiring radiosurgery. 219 Another case showed an exceptional growth in just 7 months from diagnosis (17 mm),221 whereas another small tumour was stable for 6 years and then showed a continuous growth for the next 5 years. 237
Predictors of growth
Determinants or predictors of growth would be very helpful in management planning and patient counselling. Therefore, many studies have attempted to identify such factors and apply them to clinical practice (Table 24).
| Predictors of growth | Study |
|---|---|
| No correlation of growth with age, initial size, initial symptoms, duration of symptoms, laterality, gender | Bozorg Grayeli et al., 2005;186 Flint et al., 2005;187 Bederson et al., 1991;204 Herwadker et al., 2005;205 Hoistad et al., 2001;210 Nutik and Babb, 2001;211 Massick et al., 2000;213 Modugno et al., 1999;189 Niemczyk et al., 1999;216 Wiet et al., 1995;220 Moller et al., 2003;224 Stangerup et al., 2006;122 Raut et al., 2004;233 Rosenberg, 2000;237 Walsh et al., 2000193 |
| In tumours with confirmed growth there is a significant correlation between volume and growth rate and between clinical stage and growth rate | Niemczyk et al., 2002208 |
| No patient with a tumour < 15 mm had rapid growth or neurological symptoms | Rosenberg et al., 1993221 |
| The more rapid the tumour growth, the younger the age or the smaller the initial tumour size | Ogawa et al., 1991199 |
| Tumours smaller at presentation tend to grow less frequently and slower | Kishore et al., 2003225 |
| The smaller the initial size, the less likely statistically the tumour is to grow | Ramsden et al., 2003226 |
| No robust predictors of growth apart from presentation (typical vs atypical grew less) and duration of symptoms (inverse correlation with growth) | Quaranta et al., 2003228 |
| Growth correlated with tinnitus as an initial symptom and inversely correlated with intracanalicular tumours and duration of symptoms of more than 10 years – no other predictors | Ferri et al., 2008227 |
| Two groups: (1) high risk for growth: (a) those with an extrameatal component and short duration of hearing loss and at least one of the other two predictors (unsteadiness/vertigo or no sudden hearing loss), (b) those with intrameatal localisation and all three other predictors; (2) low risk for growth: (a) those with an extrameatal component and no other predictor, (b) those with an intrameatal localisation and at most one other predictor | Artz et al., 2008178 |
| Significant difference between intracanalicular and stage I tumours versus stage II tumours regarding growth | Battaglia et al., 2006229 |
| Significant difference between growth rates of CPA and IAC tumours (IAC significantly slower growth) | Hajioff et al., 2008191 |
| Size of tumours in regression group was statistically larger than in other groups. Statistically significant inverse correlation between growth and size at presentation | Mirz et al., 2000235 |
| The only significant factor in predicting growth was size at presentation, with tumours > 20 mm having a higher chance of growing; however, mean initial size of tumours that regressed was larger than that of other tumours | Fucci et al., 1999158 |
| Intracanalicular tumours less likely to grow compared with stage II tumours | Solares and Panizza, 2008238 |
It seems that most studies fail to identify predictors of growth. Some studies have found large initial size to be a determinant of later growth although the opposite has also been reported. In a systematic review of 1340 patients16 no correlation was found between tumour growth frequency and patient age at diagnosis or follow-up duration; prospective design and serial MR imaging were associated with lower tumour growth frequency and larger tumours were associated with a lower risk of enlargement.
A meta-analysis of 10 studies and 620 patients did not find any predictive factors for tumour growth, whereas in four studies including 255 patients positive growth at 1 year was found to be predictive of future growth. 194 In another meta-analysis of 555 patients (13 studies) no statistically significant differences were found regarding mean age and mean initial size between growth and non-growth groups. 240
Failure of conservative management mainly due to tumour growth
Conservative management or the ‘wait and see’ de facto policy fails when treatment is decided. However, this does not necessarily mean that the tumour has grown because patients may change their minds and have treatment for other reasons such as personal preference, symptoms, etc. Table 25 summarises the ‘failure’ rates of conservative management taken from the literature.
| Study | Number followed up | Length of follow-up, mean (range) | Failure rate (%) | n | Reasons for failure |
|---|---|---|---|---|---|
| Bozorg Grayeli et al., 2005186 | 111 | 33 months (6–111 months) | 16a | 18 | Growth (18) |
| Flint et al., 2005187 | 100 | 25.5 months (5–150 months) | 11b | 11 | Growth (6), growth and symptoms (4), vertigo (1) |
| Glasscock et al., 1997202 | 34 | 28.5 months (5–108 months) | 24b | 8 | Accelerated growth (8) |
| Strasnick et al., 1994203 | 51 | 2.3 years (6–74 months) | 24c | 12 | Growth (12) |
| Bederson et al., 1991204 | 70 | 26±2 months (6–84 months) | 13b | 9 | Growth (7), not stated (2) |
| Sakamoto et al., 2001212 | 31 | 33 months (6–92 months) | 48d | 15 | Growth or worse hearing (15) |
| Tschudi et al., 2000159 | 74 | 35 months (12–108 months) | 12b | 9 | Symptoms (7), growth (2) |
| Hoistad et al., 2001210 | 102 | 28.5 months (6–120 months) | 33c | 34 | Growth (34), no information on other reasons |
| Yamamoto et al., 1998217 | 12 | 18 months (3–46 months) | 42d | 5 | Growth and/or symptoms (5) |
| Quaranta et al., 2007192 | 70 | 33.3 months (7–111 months) | 39d | 27 | Growth, symptoms or patient choice (detail not reported) |
| Fucci et al., 1999158 | 119 | 2.5 years (5 months to 8 years) | 19d | 23 | Growth (15), and/or symptoms (17), and/or patient choice (2) |
| Hajioff et al., 2008191 | 72 | 121 months (89–271 months) | 35d | 25 | Not stated |
| Deen et al., 1996219 | 68 | 3.4 years (6 months to 12 years) | 15d | 10 | Growth and symptoms (6), growth (4) |
| Shin et al., 2000215 | 87 | 31 months (4 months to 10 years) | 12d | 12 | Growth (12) |
| Al Sanosi et al., 2006222 | 197 | 40.8 months (12–180 months) | 8d | 15 | Growth (9) and/or symptoms (9), patient choice (1) |
| Rosenberg, 2000237 | 80 | 4.4 years (0.01–17.2 years) | 7d | 6 | Growth and symptoms (4), not stated (2) |
| Solares and Panizza, 2008238 | 110 | 31.4 months (6–156 months) | 18.7 (5-year intervention rate) | 12 | Not stated |
In a meta-analysis of 15 studies and 1000 patients,194 20% of patients required treatment. In another meta-analysis of 13 studies and 1000 patients,240 26% of patients required treatment (range in these studies 0–50%). Finally, in a systematic review of 1340 patients,16 18% (95% CI 16–21%) required treatment. It seems that approximately one in every four or five cases fails conservative management; however, the range is very wide (0–50%), suggesting that there are many factors influencing the percentage of patients who finally have treatment, such as length of follow-up, selection of patients, counselling, etc.
Discussion
Numerous studies have explored the natural history and growth of acoustic neuromas; however, most of them have one or more serious weaknesses:
-
retrospective design
-
number of patients lost to follow-up (often not even mentioned)
-
selection of patients according to various criteria (not always clear)
-
multiple publications from the same centres over time
-
relatively short follow-up times
-
failure to distinguish NF2 patients
-
failure to distinguish between CT and MR measurements
-
current clinical measures of tumour size and growth have serious weaknesses and limitations
-
variable definitions of growth
-
variable definitions of growth rate
-
treatment decisions based on subjective reasons and symptomatology as well as on growth.
Any analysis of the literature regarding acoustic neuroma tumour size and possible associations with symptoms, signs or diagnostic test efficacy suffers from the great number of different methods used to determine and report tumour size. Different radiological techniques have been utilised, and many different methods to report size are evident, including diameter of both intra- and extracanalicular components, maximum extracanalicular diameter and tumour volume (this last method often making the assumption that tumour morphology approximates a sphere). Further, work has indicated marked interobserver and test–retest variability in estimates of acoustic neuroma size. 241,244
An attempt to address the issue of the great variability in the methods for the reporting of acoustic neuroma tumour size was made by a group of surgeons who formulated a consensus statement. 111 The following proposals were made:
-
an explicit distinction should be made between the intra- and extrameatal components
-
linear planimetric measurements (in millimetres) should be reported rather than volumetric measures
-
the intrameatal component should be measured along the length of the IAC, and the width measured perpendicular to that plane
-
the largest extrameatal diameter should be reported.
Further, the following tumour classification framework was proposed:
-
grade 1 – small: 1–10 mm extrameatal
-
grade 2 – medium: 11–20 mm
-
grade 3 – moderately large: 21–30 mm
-
grade 4 – large: 31–40 mm
-
grade 5 – giant: > 40 mm.
For tumours confined to the IAC, the term intrameatal tumour was proposed.
We can conclude from the evidence reviewed that:
-
At least 50% of acoustic neuromas do not grow, at least for some years after diagnosis.
-
No reliable predictors of growth have been identified. Some studies have found large initial size to be a determinant of later growth although the opposite has also been reported.
-
The mean growth rate for all tumours varies between 1 and 2 mm/year and, for only those that grow, between 2 and 4 mm/year; however, there are cases with significant regression or exceptional growth that may exceed 18 mm/year.
-
Regression is a small but real possibility (around 5%).
-
There are various patterns of growth and a tumour that shows growth may stop doing so and one that does not grow initially may begin to do so.
-
The first year after diagnosis may be crucial for determining the pattern of tumour growth; however, this is not always the case, and a tumour may be stable for many years before showing continuous growth.
Chapter 5 Conclusions and recommendations
Conclusions
The majority of the evidence reviewed in all three themes was generally poorly reported and there is therefore an inherent risk of bias. This is reflected to a degree in the quality assessment but not completely as the problems often concerned a lack of detail of reporting rather than poor methods per se. Absence of evidence is not evidence of poor quality. The conclusions drawn should be considered in this light.
Auditory brainstem response
Given the recent improvement in resolution and the reduction in cost of MR imaging, ABR can no longer be considered appropriate as the primary test used to screen for an acoustic neuroma. Although it is relatively inexpensive and offers acceptable sensitivity for medium to larger tumours, its ability to reliably indicate tumours of less than 1 cm is poor. ABR also fails to provide clinically useful results in patients with severe to profound hearing impairment (typically a hearing threshold greater than 70 dBHL at 4 kHz). Despite these considerable disadvantages, ABR might be considered to be the test of choice for identifying acoustic neuroma in the majority of patients unable to undergo routine MR imaging. The use of sedation and a general anaesthetic for claustrophobic patients and an open magnet for obese patients may ultimately be required in some cases.
Magnetic resonance imaging
In current clinical practice, MR imaging is the first-line investigation for the identification of suspected acoustic neuroma in appropriately selected patients. The GdT1W sequence remains the gold standard sequence for evaluating cases in which the screening sequence is indeterminate and for characterising any suspected pathology.
Non-contrast high-resolution three-dimensional T2W or T2*W sequences enable accurate evaluation of the VIIIth and VIIth cranial nerves within the CPA and IAC as well as evaluation of the cochlea and labyrinth. When these structures are clearly and confidently identified, inclusion of GdT1W sequences is unlikely to contribute information that would alter patient management in the screening population.
The quality of the imaging chain and the experience of the reporting radiologist are key factors determining the efficacy of a non-contrast screening strategy. Poor image quality, or lack of diagnostic confidence or ability, will result in increased patient recall rates and use of GdT1W imaging at additional cost or, worse, increased false-negative rates and the cost burden of late re-presentation with an advanced acoustic neuroma.
CT
CT is considered by some clinicians to be the test of choice if MR imaging is contraindicated. We are unable to comment further as this review did not extend to an examination of the relative performance of CT and ABR.
Cost-effectiveness
Compared with ‘traditional’ protocols that deploy what have become essentially redundant tests such as CT and ENG, strategies that deploy GdT1W MR imaging immediately or in conjunction with ABR appear to be more cost-effective.
The applicability of previous studies reporting cost and cost-effectiveness data is limited given their age and the fact that many were undertaken outside the UK. Based on a cost-effectiveness model developed to reflect UK practice, it was concluded that a diagnostic algorithm that deploys non-contrast MR imaging as an initial imaging screen in the investigation of acoustic neuroma is less costly than and likely to be as effective as available contrast MR imaging.
Epidemiology
Epidemiological studies have reported a significant increase in the incidence of acoustic neuroma over the past 30 years. In 1976, the incidence was approximately five tumours per million population per year whereas in 2001 the incidence had reached just under 20 tumours per million population per year. Much of this increase in incidence is due to the advent of better non-invasive diagnostic techniques, especially MR scanning. This has also resulted in individuals being investigated for acoustic neuroma who in the past were considered unsuitable (e.g. the elderly, those with significant comorbidity, etc.). The incidence of giant tumours has dropped, whereas that of small and medium-sized tumours has increased. Overall, the median age at diagnosis has not changed (around 55 years).
There are no regional or national tumour registries in the UK for acoustic neuroma. Adding to the challenge of data collection is that many of these tumours are ‘diagnosed’ on imaging alone without histological confirmation and may escape entry into any database. Thus, trends in incidence are difficult to capture and one is heavily reliant on data from tertiary centres, which is often unrepresentative of what is happening in the general population.
Symptoms
The typical presentation of acoustic neuroma is with symptoms of progressive unilateral hearing impairment and associated tinnitus and imbalance. These should be clear ‘red flags’ for investigation and this would usefully be enshrined in clinical protocols. It should also be borne in mind that atypical presentation with facial pain, otalgia or facial numbness occurs, and the clinician’s acumen should bear this possibility in mind.
Although the biology of the tumours is well understood, the pathophysiological mechanisms by which patients become symptomatic are not, and much of the relevant literature is inferential rather than based on experimental evidence.
Growth
Most of the studies that have explored the natural history and growth of acoustic neuromas have one or more serious weaknesses. It is therefore very difficult to draw any conclusions or make any comparisons between studies. However, the evidence is that the pattern and rate of growth and the predictors of growth are highly variable. Acoustic neuromas may grow, not grow or get smaller, the rate of change may be fast, slow or delayed, and no reliable predictors of growth have been identified, i.e. there is no useful information in the reviewed literature.
Recommendations
Future research
-
The evidence highlights the need for primary longitudinal studies to address several unanswered questions. The studies reviewed were generally of poor quality in terms of the detail of reporting of the methodology as well as the consistency of reporting, and it is recommended that studies be undertaken to provide evidence of the true incidence and natural history of acoustic neuroma. To ensure that the findings are timely, apply to current practice and have sufficient numbers of subjects to draw robust conclusions, such studies should be collaborative and multicentre.
-
A national audit should explore the true prevalence of unilateral auditory symptoms and their relation to acoustic neuroma.
-
This review did not address issues of treatment strategies or outcomes, and useful knowledge would be gathered and disseminated by a systematic review of the evidence around these issues.
-
Further research is required to provide evidence to more fully understand the pathophysiological mechanisms by which patients become symptomatic.
-
The evidence reviewed was not current and it is recommended that studies of current practice be undertaken. Developments in technology have reduced the costs of imaging and increased the resolution achievable.
Imaging strategies
In determining the most appropriate approach to imaging, consideration should be given to:
-
the demographics of the referral population
-
the clinical and audiological criteria for ASHI or tinnitus
-
ensuring that referring clinicians are aware of the referral criteria
-
the definitions of the pathologies to be detected
-
the definitions of the criteria for a normal or abnormal study
-
the experience of the radiologist
-
variations to the management pathways for specific pathologies, including variations dependent on age, lesion location, lesion size and comorbidities (NF2)
-
evaluation of the whole brain to enable detection of related unexpected or coincidental pathologies
-
standardisation and specification of the measurement method used to document acoustic neuroma size, to minimise inter- and intra-observer variation at follow-up.
Costs
-
The range of national costs should be available for contrast- and non-contrast-enhanced MR imaging and for ABR measurement, taking into account differing service provision in different sites.
Natural history
-
A national tumour registry should be established for acoustic neuroma in the UK.
-
There is an urgent need for a consensus method of measuring tumours and evaluating growth, taking into account their three dimensions.
Acknowledgements
We thank the members of our advisory committee for their contribution: Dr Tim Coleman (Associate Professor and Reader in Primary Care and practising general practitioner), Dr Ralph Holme [Biomedical Research Manager for the Royal National Institute for Deaf People (RNID)] and Jackey Weightman [representing the British Acoustic Neuroma Association (BANA)].
We thank Lisa Hayman Tansley (MRC Institute of Hearing Research) for help with accessing journals and Diana Papaiouannou and Anna Wilkinson (School of Health and Related Research, Sheffield) for conducting the searches.
Contribution of authors
Heather Fortnum (Associate Professor and Reader in Hearing Research, systematic reviews and epidemiology) led the research team. Ciaran O’Neill (Professor, Health Economics) conducted the review of the cost-effectiveness literature and the modelling analyses. Rod Taylor (Associate Professor in Health Services Research, systematic review of diagnostic strategies) led the review of diagnostic strategies and conducted the synthesis analyses. Rob Lenthall, (Neuroradiologist, MR imaging) conducted the review of the MR literature and contributed to the review of diagnostic strategies. Thomas Nikolopolous (Otorhinolaryngologist, systematic reviews and ENT) conducted the review of the growth literature and contributed to the review of natural history. Guy Lightfoot (Consultant Clinical Scientist, Audiology) conducted the review of the ABR literature and contributed to the review of diagnostic strategies. Gerry O’Donoghue (Otorhinolaryngologist, ENT) conducted the review of the epidemiological literature and contributed to the review of natural history. Steve Mason (Consultant Clinical Scientist, electrophysiological measurement) conducted the review of the ABR literature and contributed to the review of diagnostic strategies. David Baguley (Consultant Clinical Scientist, Audiology) conducted the review of the symptomatology literature and contributed to the review of natural history. Helen Jones (Research Fellow, hearing research) contributed to all aspects of the review. Caroline Mulvaney (Research Fellow, systematic reviews) contributed to all aspects of the review and co-ordinated the editing of the final report. All authors contributed to writing and editing.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA Programme or the Department of Health.
References
- Ludman CN, O’Donoghue GM. Magnetic resonance imaging demonstrating the origin of a vestibular schwannoma to be the superior division of the vestibular nerve. Am J Otol 2000;21:135-6.
- Baguley DM, Jones SE, Moffat DA. A small vestibular schwannoma arising from the inferior vestibular nerve. J Laryngol Otol 2003;117:498-500.
- Clemis JD, Ballad WJ, Baggot PJ, Lyon ST. Relative frequency of inferior vestibular schwannoma. Arch Otolaryngol Head Neck Surg 1986;112:190-4.
- Jackler R, Jackler RK, Brackmann D. Neurotology. St Louis, MO: Mosby; 1994.
- Jacob A, Robinson LL, Bortman JS, Yu L, Dodson EE, Welling DB. Nerve of origin, tumor size, hearing preservation, and facial nerve outcomes in 359 vestibular schwannoma resections at a tertiary care academic center. Laryngoscope 2007;117:2087-92.
- National Institutes of Health . Acoustic neuroma. NIH Consensus Statement 1991;9:1-24.
- Moffat D, Baguley D, Hardy D, Tsui Y. Contralateral auditory brainstem response abnormalities in acoustic neuroma. J Laryngol Otol 1989;103:835-8.
- Tos M, Charabi S, Thomsen J. Incidence of vestibular schwannomas. Laryngoscope 1999;109:736-40.
- Stangerup S-E, Tos M, Caye-Thomasen P, Tos T, Klokker M, Thomsen J. Increasing annual incidence of vestibular schwannoma and age at diagnosis. J Laryngol Otol 2004;118:622-7.
- Scherer S, Gutmann D. Expression of the neurofibromatosis 2 tumor suppressor gene product, merlin, in Schwann cells. J Neurosci Res 1996;46:595-60.
- Moffat D, Hardy D, Baguley D. Strategy and benefits of acoustic neuroma searching. J Laryngol Otol 1989;103:51-9.
- Ferguson MA, Smith PA, Lutman ME, Mason SM, Coles RR, Gibbin KP. Efficiency of tests used to screen for cerebello-pontine angle tumours: a prospective study. Br J Audiol 1996;30:159-76.
- Carrier D, Arriaga M. Cost-effective evaluation of asymmetric sensorineural hearing loss with focused magnetic resonance imaging. Otolaryngol Head Neck Surg 1997;116:567-74.
- Hollingworth W, Mell M, Dixon A, Antoun N, Moffat D, Todd C. Measuring the effects of medical imaging in patients with possible cerebellopontine angle lesions: a four-center study. Acad Radiol 1998;5:s306-9.
- Davis A. Hearing in adults. London: John Wiley; 1995.
- Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. J Neurosurg 2005;103:59-63.
- Roland PS, Eston D. Stereotactic radiosurgery of acoustic tumours. Otolaryngol Clin N Am 2002;35:343-55.
- Nikolopoulos TP, Johnson I, O’Donoghue GM. Quality of life after acoustic neuroma surgery. Laryngoscope 1998;108:1382-5.
- British Association of Otorhinolaryngologists – Head and Neck Surgeons . Clinical Effectiveness Guidelines Acoustic Neuroma (vestibular Schwanomma) 2002.
- Sackett D, Straus S, Richardson W, Rosenburg W, Haynes R. Evidence based medicine: how to practice and teach EBM. London: Churchill Livingstone; 2000.
- Prasher D, Tun T, Brookes G, Luxon L. Mechanisms of hearing loss in acoustic neuroma: an otoacoustic emission study. Acta Otolaryngol 1995;115:375-81.
- Humphriss R, Baguley D, Axon P, Moffat D. Change in hearing handicap following translabyrinthine vestibular schwannoma excision. Otol Neurotol 2004;25:371-8.
- Moller AR. Neural mechanisms of BAEP. Electroencephalogr Clin Neurophysiol Suppl 1999;49:27-35.
- Hood L. Clinical applications of the auditory brainstem response. San Diego, CA: Singular Publishing; 1998.
- Brackmann D. Current status of ABR audiometry in acoustic neuroma diagnosis. Arch Otolaryngol Head Neck Surg 1999;125.
- Doyle K. Is there still a role for auditory brainstem response audiometry in the diagnosis of acoustic neuroma. Arch Otolaryngol Head Neck Surg 1999;125:232-4.
- Ruckenstein M, Cueva R, Morrison D, Press G. A prospective study of ABR and MRI in the screening for vestibular schwannomas. Am J Otol 1996;17:317-20.
- Wilson D, Talbot J, Mills L. A critical appraisal of the role of auditory brainstem response and magnetic resonance imaging in acoustic neuroma diagnosis. Am J Otol 1997;18:673-81.
- El-Kashlan H, Eisenmann D, Kileny P. Auditory brain stem response in small acoustic neuromas. Ear Hear 2000;21:257-62.
- Schmidt RJ, Sataloff RT, Newman J, Spiegel JR, Myers DL. The sensitivity of auditory brainstem response testing for the diagnosis of acoustic neuromas. Arch Otolaryngol Head Neck Surg 2001;127:19-22.
- Lightfoot G. ABR screening for acoustic neuromata; the role of rate induced latency shift measurements. Br J Audiol 1992;26:217-27.
- Don M, Masuda A, Nelson R, Brackmann D. Successful detection of small acoustic tumors using the stacked derived-band auditory brainstem response amplitude. Am J Otol 1997;18:608-21.
- Musiek F, Kibbe K. Auditory brain stem response wave IV–V abnormalities from the ear opposite large cerebellopontine lesions. Am J Otol 1986;7:253-7.
- Dawes PJD, Mehta D, Arullendran P. Screening for vestibular schwannoma: magnetic resonance imaging findings and management. J Laryngol Otol 2000;114:584-8.
- Dawes PJD, Basiouny HE. Outcome of using magnetic resonance imaging as an initial screen to exclude vestibular schwannoma in patients presenting with unilateral tinnitus. J Laryngol Otol 1999;113:818-22.
- Sheppard IJ, Milford CAM, Anslow P. MRI in the detection of acoustic neuromas – a suggested protocol for screening. Clin Otolaryngol 1996;21:301-4.
- Curtin H. Rule out eighth nerve tumour: contrast enhanced T1-weighted or high resolution T2-weighted MR?. Am J Neuroradiol 1997;18:1834-8.
- Annesley-Williams DJ. Magnetic resonance imaging in the investigation of sensorineural hearing loss: is contrast enhancement still necessary?. J Laryngol Otol 2001;115:14-21.
- Stack J, Ramsden R, Anton N, Lye R, Sheppard I, Jenkins J. Magnetic resonance imaging of acoustic neuromas: the role of gadolinium – DPTA. Br J Radiol 1988;61:800-5.
- Mark AS, Seltzer S, Harnsberger HR. Sensorineural hearing loss: more than meets the eye?. Am J Neuroradiol 1993;14:37-45.
- Ravi K, Wells S. A cost effective screening protocol for vestibular schwannoma in the late 90s. J Laryngol Otol 1996;110:1129-32.
- Saeed S, Woolford T, Ramsden R, Lye R. Magnetic resonance imaging: a cost-effective first line investigation in the detection of vestibular schwannomas. Br J Neurosurg 1995;9:497-503.
- Robson A, Leighton S, Anslow P, Milford C. MRI as a single screening procedure for acoustic neuroma: a cost effective protocol. J R Soc Med 1993;86:455-7.
- Zealley IA, Cooper RC, Clifford KM, Campbell RS, Potterton AJ, Zammit-Maempel I, et al. MRI screening for acoustic neuroma: a comparison of fast spin echo and contrast enhanced imaging in 1233 patients [see comment]. Br J Radiol 2000;73:242-7.
- Lin D, Hegarty JL, Fischbein NJ, Jackler RK. The prevalence of ‘incidental’ acoustic neuroma. Arch Otoloryngol Head Neck Surg 2005;131:241-4.
- Jeyakumar A, Seth R, Brickman TM, Dutcher P. The prevalence and clinical course of patients with ‘incidental’ acoustic neuromas. Acta Otolaryngol 2007;127:1051-7.
- Harcourt J, Vijaya-Sekaran S, Loney E, Lennox P. The incidence of symptoms consistent with cerebellopontine angle lesions in a general ENT out-patient clinic. J Laryngol Otol 1999;113:518-22.
- Bonneville F, Savatovsky J, Chiras J. Imaging of cerebellopontine angle lesions: an update. Part 1: enhancing extra-axial lesions. Eur Radiol 2007;17:2472-82.
- Chisholm EJ, Savy L, Geyer M, Choa D. Magnetic resonance imaging scans for vestibulocochlear nerve tumours: what is actually found?. J Laryngol Otol 2006;120:1019-22.
- Dubrulle F, Delomez J, Kiaei A, Berger P, Vincent C, Vaneecloo F-M, et al. Mass screening for retrocochlear disorders: low-field-strength (0.2-T) versus high-field-strength (1.5-T) MR imaging. Am J Neuroradiol 2002;23:918-23.
- Deeks J. Systematic reviews of evaluations for diagnostic and screening tests. Br Med J 2001;323:157-62.
- Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Method 2006;6.
- Hall JW. New handbook of auditory evoked potentials. Boston, MA: Pearson Education; 2007.
- Selters WA, Brackmann DE. Acoustic tumor detection with brain stem electric response audiometry. Arch Otolaryngol 1977;103:181-7.
- Montaguti M, Bergonzoni C, Zanetti MA, Ceroni AR. Comparative evaluation of ABR abnormalities in patients with and without neurinoma of VIII cranial nerve. Acta Otorhinolaryngol Ital 2007;27:68-72.
- Weiss MH, Kisiel DL, Bhatia P. Predictive value of brainstem evoked response in the diagnosis of acoustic neuroma. Otolaryngol Head Neck Surg 1990;103:583-5.
- Levine SC, Antonelli PJ, Le CT, Haines SJ. Relative value of diagnostic tests for small acoustic neuromas. Am J Otol 1991;12:341-6.
- Wilson DF, Hodgson RS, Gustafson MF, Hogue S, Mills L. The sensitivity of auditory brainstem response testing in small acoustic neuromas [see comment]. Laryngoscope 1992;102:961-4.
- Selesnick SH, Jackler RK, Pitts LH. The changing clinical presentation of acoustic tumors in the MRI era. Laryngoscope 1993;103:431-6.
- Gordon MA, Cohen NL. Efficacy of auditory brainstem response as a screening test for small acoustic neuromas. Am J Otol 1995;16:136-9.
- Chandrasekhar SS, Brackmann DE, Devgan KK. Utility of auditory brainstem response audiometry in diagnosis of acoustic neuromas [see comment]. Am J Otol 1995;16:63-7.
- Zappia JJ, O’Connor CA, Wiet RJ, Dinces EA. Rethinking the use of auditory brainstem response in acoustic neuroma screening. Laryngoscope 1997;107:1388-92.
- Godey B, Morandi X, Beust L, Brassier G, Bourdiniere J. Sensitivity of auditory brainstem response in acoustic neuroma screening. Acta Otolaryngol 1998;118:501-4.
- Haapaniemi JJ, Laurikainen ET, Johansson R, Rinne T, Varpula M. Audiovestibular findings and location of an acoustic neuroma. Eur Arch Otorhinolaryngol 2000;257:237-41.
- Robinette MS, Bauch CD, Olsen WO, Cevette MJ. Auditory brainstem response and magnetic resonance imaging for acoustic neuromas: costs by prevalence. Arch Otolaryngol Head Neck Surg 2000;126:963-6.
- Marangos N. Brainstem response in cerebellopontine angle tumors. Otol Neurotol 2001;22:95-9.
- Rupa V, Job A, George M, Rajshekhar V. Cost-effective initial screening for vestibular schwannoma: auditory brainstem response or magnetic resonance imaging?. Otolaryngol Head Neck Surg 2003;128:823-8.
- Skinner LJ, Piccirillo E, Agarwal M, Falcioni M, Sanna M. A Retrospective Correlation of Auditory Brainstem Response and Magnetic Resonance Imaging for Acoustic Neuromas Vestibular Scwannomas n.d.:84-5.
- Cueva RA. Auditory brainstem response versus magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope 2004;114:1686-92.
- Gentry L, Jacoby C, Turski P, Houston L, Strother C, Sackett J. Cerebellopontine angle petro-mastoid mass lesions: comparative study of diagnosis with MR imaging and CT. Radiology 1987;162:513-20.
- Don M, Kwong B, Tanaka C, Brackmann D, Nelson R. The stacked ABR: a sensitive and specific screening tool for detecting small acoustic tumors. Audiol Neurootol 2005;10:274-90.
- Bush ML, Jones RO, Shinn JB. Auditory brainstem response threshold differences in patients with vestibular schwannoma: a new diagnostic index. Ear Nose Throat J 2008;87:458-62.
- Quaranta A, Scaringi A, Quaranta N. Auditory Brainstem Responses, Otoacoustic Emissions and Efferent Acoustic Reflexes in Ears With Vestibular Schwannoma n.d.:91-2.
- Telischi F, Roth J, Stanger B, Lonsbury-Martin B, Balkany T. Patterns of evoked otoacoustic emissions associated with acoustic neuromas. Laryngoscope 1995;105:675-82.
- Ferber-Viart C, Colleaux B, Laoust L, Dubreuil C, Duclaux R. Is the presence of transient evoked otoacoustic emissions in ears with acoustic neuroma significant?. Laryngoscope 1998;18:605-9.
- Ferguson M, O’Donoghue G, Owen V. Contralateral suppression of transient evoked otoacoustic emissions in patients with cerebello-pontine angle tumor. Ear Hear 2001;22:173-81.
- Department of Health . Tackling Hospital Waiting: The 28 Week Patient Pathway. An Implementation Framework 2006.
- Haughton VM, Rimm A, Czervionke L, Breger R, Fisher M, Papke R, et al. Sensitivity of Gd-DTPA enhanced MR imaging of benign extraaxial tumours. Radiology 1988;166:829-33.
- Schmalbrock P, Brogan M, Chakeres D, Hacker V, Ying K, Clymer B. Optimization of submillimeter resolution MR imaging methods for the inner ear. J Magn Reson Imaging 1993;3:451-9.
- Casselman J, Kuhweide R, Deimling M, Ampe W, Dehaene I, Meeus L. Constructive interference in the steady state: 3DFT MR imaging of the inner ear and cerebellopontine angle. Am J Neuroradiol 1993;14:47-5.
- Shelton C, Harnsberger HR, Allen R, King B. Fast spin echo magnetic resonance imaging: clinical application in screening for acoustic neuroma. Otolaryngol Head Neck Surg 1996;114:71-6.
- Allen RW, Harnsberger HR, Shelton C, King B, Bell DA, Miller R, et al. Low-cost high-resolution fast spin-echo MR of acoustic schwannoma: an alternative to enhanced conventional spin-echo MR? [see comment]. Am J Neuroradiol 1996;17:1205-10.
- Stuckey SL, Harris AJ, Mannolini SM. Detection of acoustic schwannoma: use of constructive interference in the steady state three-dimensional MR [see comment]. Am J Neuroradiol 1996;17:1219-25.
- Soulié D, Cordoliani YS, Vignaud J, Cosnard G. MR imaging of acoustic neuroma with high resolution fast spin echo T2-weighted sequence. Eur J Radiol 1997;24:61-5.
- Hermans R, Van der Goten GA, De Foer B, Baert AL. MRI screening for acoustic neuroma without gadolinium: value of 3DFT-CISS sequence. Neuroradiology 1997;39:593-8.
- Naganawa S, Ito J, Fukatsu H, Ishigaki T, Nakashima T, Ichinose N, et al. MR imaging of the inner ear: comparison of a three-dimensional fast spin-echo sequence with use of a dedicated quadrature-surface coil with a gadolinium-enhanced spoiled gradient-recalled sequence. Radiology 1998;208:679-85.
- Held P, Fellner C, Seitz J, Graf S, Fellner F, Strutz J. The value of T2(*)-weighted MR images for the diagnosis of acoustic neuromas. Eur J Radiol 1999;30:237-44.
- Marx SV, Langman AW, Crane RC. Accuracy of fast spin echo magnetic resonance imaging in the diagnosis of vestibular schwannoma. Am J Otol 1999;20:211-16.
- Schmalbrock P, Chakeres DW, Monroe JW, Saraswat A, Miles BA, Welling DB. Assessment of internal auditory canal tumors: a comparison of contrast-enhanced T1-weighted and steady-state T2-weighted gradient-echo MR imaging. Am J Neuroradiol 1999;20:1207-13.
- Ben Salem D. MRI screening of vestibular schwannomas without gadolinium: usefulness of the turbo gradient spin echo T2-weighted pulse sequence. J Neuroradiol 2001;28:97-102.
- Jackler RK. Cost-effective screening for acoustic neuroma with unenhanced MR: a clinician’s perspective. Am J Neuroradiol 1996;17:1226-8.
- Curtin HD. Rule out eighth nerve tumor: contrast-enhanced T1-weighted or high-resolution T2-weighted MR?. Am J Neuroradiol 1997;18:1834-8.
- Sartorelli-Schefer S, Kollias S, Valavanis A. Spatial relationship between vestibular schwannoma and facial nerve on three-dimensional T2-weighted fast spin-echo MR images. Am J Neuroradiol 2000;21:810-16.
- Ciftci E, Anik Y, Arslan A, Akansel G, Sarisoy T, Demirci A. Driven equilibrium (DRIVE) MR imaging of the cranial nerves V–VIII: comparison with the T2-weighted 3D TSE sequence. Eur J Radiol 2004;51:234-40.
- Tsuchiya K, Aoki C, Hachiya J. Evaluation of MR cisternography of the cerebellopontine angle using a balanced fast-field-echo sequence: preliminary findings. Eur Radiol 2004;14:239-42.
- Held P, Fründ R, Seitz J. Comparison of a T2* w. 3D CISS and a T2 w. 3D turbo spin echo sequence for the anatomical study of facial and vestibulocochlear nerves. J Neuroradiol 2000;27:173-8.
- Daniels RL, Swallow C, Shelton C, Davidson HC, Krejci CS, Harnsberger HR. Causes of unilateral sensorineural hearing loss screened by high-resolution fast spin echo magnetic resonance imaging: review of 1,070 consecutive cases. Am J Otol 2000;21:173-80.
- Kernohan M, Blackmore K, Johnson I, Zammit-Maempel I. Magnetic resonance imaging: is a single scan ever enough for the diagnosis of acoustic neuroma. J Laryngol Otol 2006;120:1061-3.
- Montague M, Kishore A, Hadley D, O’Reilly B. MR findings in intralabyrinthine schwannomas. Clin Radiol 2002;57:355-8.
- Lhuillier F, Doyon D, Halimi P, Sigal R, Sterkers J. Magnetic resonance imaging of acoustic neuromas: pitfalls and differential diagnosis. Neuroradiology 1992;34:144-9.
- Donnelly MJ, Cass A, Ryan L. False positive diagnosis of small intracanalicular vestibular schwannomas. J Laryngol Otol 1994;108:986-8.
- Han M, Jabour B, Andrews J, Canalis R, Chen F, Anzai Y, et al. Non-neoplastic enhancing lesions mimicking intracanalicular acoustic neuroma on gadolinium-enhanced MR images. Radiology 1991;179:795-6.
- Maeta M, Saito R, Nameki H. False-positive magnetic resonance image in the diagnosis of small acoustic neuroma. J Laryngol Otol 2001;115:842-4.
- Selesnick SH, Rebol J, Heier LA, Wise JB, Gutin PH, Lavyne MH. Internal auditory canal involvement of acoustic neuromas: surgical correlates to magnetic resonance imaging findings. Otol Neurotol 2001;22:912-16.
- Vernooij M, Ikram M, Tanghe H, Vincent A, Hofman A, Krestin G, et al. Incidental findings on brain MRI in the general population. N Engl J Med 2007;357:1821-8.
- Mirza S, Malik TH, Ahmed A, Willatt DJ, Hughes DG. Incidental findings on magnetic resonance imaging screening for cerebellopontine angle tumours. J Laryngol Otol 2000;114:750-4.
- Ovbiagele B, Saver J. Cerebral white matter hyperintensities on MRI: current concepts and therapeutic implications. Cerebrovasc Dis 2006;22:83-90.
- Rutherford S, King A. Vestibular schwannoma management: what is the ‘best’ option?. Br J Neurosurg 2005;19:309-16.
- Coca Pelaz A, Gomez JR, Llorente JL, Rodrigo JP, Nunez F, Sevilla MA, et al. Complications and sequelae in acoustic neuroma surgery. Acta Otorrinolaringol Esp 2007;58:470-5.
- Cardoso A, Fernandes Y, Ramina R, Borges G. Acoustic neuroma (vestibular schwannoma): surgical results on 240 patients operated on dorsal decubitus position. Arq Neuropsiquiatr 2007;65:605-9.
- Kanzaki J, Tos M, Sanna M, Moffat D, Monsell E, Berliner K. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol 2003;24:642-9.
- Dawes PJD, Jeannon JP. Audit of regional screening guidelines for vestibular schwannoma. J Laryngol Otol 1998;112:860-4.
- Drummond M, Stoddart G, Torrance G. Methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 1987.
- Welling D, Glasscock M, Woods C, Jackson C. Acoustic neuroma: a cost-effective approach. Otolaryngol Head Neck Surg 1990;103:364-70.
- Cheng G, Smith R, Tan AKW. Cost comparison of auditory brainstem response versus magnetic resonance imaging screening of acoustic neuroma. J Otolaryngol 2003;32:394-9.
- Daniels R, Shelton C, Harnsberger H. Ultra high resolution nonenhanced fast spin echo magnetic resonance imaging: cost-effective screening for acoustic neuroma in patients with sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 1998;119:364-9.
- Tan TY. Non-contrast high resolution fast spin echo magnetic resonance imaging of acoustic schwannoma. Singapore Med J 1999;40:27-31.
- Bauch CD, Olsen WO, Pool AF. ABR indices: sensitivity, specificity and tumour size. Am J Audiol 1996;5:97-104.
- Chen D. Acoustic neuroma in a private neurotology practice: trends in demographics and practice patterns. Laryngoscope 2007;117:2003-12.
- Alfonso C, Lassaletta L, Sarria J, Gavilan J. Quality of life following vestibular schwannoma surgery. Acta Otorrhinolaringol 2007;58:61-5.
- Stratta P, Canavese C, Aime S. Gadolinium-enhanced magnetic resonance imaging, renal failure and nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy. Curr Med Chem 2008;15:1229-35.
- Stangerup S-E, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol 2006;27:547-52.
- Schüz J, Jacobsen R, Olsen JH, Boice JD, McLaughlin JK, Johansen C. Cellular telephone use and cancer risk: update of a nationwide Danish cohort. J Natl Cancer Inst 2006;98:1707-13.
- Preston-Martin S, Thomas D, Wright W, Henderson B. Noise trauma in the aetiology of acoustic neuromas in men in Los Angeles County, 1978–1985. Br J Cancer 1989;59:783-6.
- Edwards C, Schwartzbaum J, Nise G, Forssen U, Ahlbom A, Lonn S, et al. Occupational noise exposure and risk of acoustic neuroma. Am J Epidemiol 2007;116:1252-8.
- Haas J, Janisch W, Staneczek W. Newly diagnosed primary intracranial neoplasms in pregnant women: a population-based assessment. J Neurol Neurosurg Psychiatr 1986;49:874-80.
- Hardy M, Crowe SJ. Early asymptomatic acoustic tumor. Arch Surg 1936;32:292-301.
- Leonard JR, Talbot ML. Asymptomatic acoustic neurilemoma. Arch Otolaryngol 1970;91:117-24.
- Eckermeier L, Pirsig W, Mueller D. Histopathology of 30 non-operated acoustic schwannomas. Arch Otorhinolaryngol 1979;222:1-9.
- Stewart TJ, Liland J, Schuknecht HF. Occult schwannomas of the vestibular nerve. Arch Otolaryngol 1975;101:91-5.
- Karjalainen S, Nuutinen J, Neittaanmaki H, Naukkarinen A, Asikainen R. The incidence of acoustic neuroma in autopsy material. Arch Otorhinolaryngol 1984;240:91-3.
- Tos M, Stangerup S-E, Caye-Thomasen P, Tos T, Thomsen J. What is the real incidence of vestibular schwannoma?. Arch Otolaryngol Head Neck Surg 2004;130:216-20.
- Propp JM, McCarthy BJ, Davis FG, Preston-Martin S. Descriptive epidemiology of vestibular schwannomas. Neurooncology 2006;8:1-11.
- Evans DGR, Moran A, King A, Saeed S, Gurusinghe N, Ramsden R. Incidence of vestibular schwannoma and neurofibromatosis 2 in the North West of England over a 10-year period: higher incidence than previously thought. Otol Neurotol 2005;26:93-7.
- Howitz MF, Johansen C, Tos M, Charabi S, Olsen JH. Incidence of vestibular schwannoma in Denmark, 1977–1995. Am J Otol 2000;21:690-4.
- Tali ET, Yuh WTC, Nguyen HD, Feng G, Koci TM, Jinkins JR, et al. Cystic acoustic schwannomas: MR characteristics. Am J Neuroradiol 1993;14:1241-7.
- Frohlich A, Sutherland G. Epidemiology and clinical features of vestibular schwannoma in Manitoba, Canada. Can J Neurol Sci 1993;20:126-30.
- Moffat D, Hardy D, Irving R, Viani L, Beynon G, Baguley D. Referral patterns in vestibular schwannomas. Clin Otolaryngol 1995;20:80-3.
- Moffat DA, Jones SEM, Mahendran S, Humphriss R, Baguley DM. Referral patterns in vestibular schwannomas – 10 years on. Clin Otolaryngol 2004;29:515-17.
- Seedat R, Clasen A, Mol D. Incidence and management of acoustic neuromas in South Africa. Otol Neurotol 2002;23:996-8.
- Kwan TL, Tang KW, Pak KKT, Cheung JYL. Screening for vestibular schwannoma by magnetic resonance imaging: analysis of 1821 patients. Hong Kong Med J 2004;10:38-43.
- Urben SL, Benninger MS, Gibbens ND. Asymmetric sensorineural hearing loss in a community-based population. Otolaryngol Head Neck Surg 1999;120:808-14.
- Verret DJ, Adelson RT, Defatta RJ. Asymmetric sensorineural hearing loss evaluation with T2 FSE-MRI in a public hospital. Acta Otolaryngol 2006;126:705-7.
- Saunders JE, Luxford WM, Devgan KK, Fetterman BL. Sudden hearing loss in acoustic neuroma patients. Otolaryngol Head Neck Surg 1995;113:23-31.
- Schick B, Brors D, Koch O, Schafers M, Kahle G. Magnetic resonance imaging in patients with sudden hearing loss, tinnitus and vertigo. Otol Neurotol 2001;22:808-12.
- Kosugi EM, Tangerina RP, Dib GC, Ramos HVL, Penido NO. Vestibular schwannoma presenting as sudden hearing loss. Rev Bras Otorrinolaringol 2004;70:795-9.
- Fitzgerald DC, Mark AS. Sudden hearing loss: frequency of abnormal findings on contrast-enhanced MR studies. Am J Neuroradiol 1998;19:1433-6.
- Anderson TD, Loevner LA, Bigelow DC, Mirza N. Prevalence of unsuspected acoustic neuroma found by magnetic resonance imaging. Otolaryngol Head Neck Surg 2000;122:643-6.
- Poberskin L, Chadduck J. Incidence of brain tumours in two English counties – a population based study. J Neurol Neurosurg Psychiatry 2000;69:464-71.
- Tos M, Thomsen J. Epidemiology of acoustic neuromas. J Laryngol Otol 1984;98:685-92.
- Szyfter W, Kopec T. Epidemiology of acoustic neuromas in Poland. Otolaryngol Pol 2001;55:533-8.
- Tos M, Charabi S, Thomsen J. Clinical experience with vestibular schwannomas: epidemiology, symptomatology, diagnosis, and surgical results. Eur Arch Otorhinolaryngol 1998;255:1-6.
- Schuknecht H. Pathology of the ear. Philadelphia, PA: Lea and Febiger; 1993.
- Sunderland S. Nerve injuries and their repair: a critical appraisal. Edinburgh: Churchill Livingstone; 1991.
- Axon P. An Electrophysiological Study of Facial Nerve Injury Caused by Vestibular Schwannomata and Their Surgical Management 1999.
- Jahnke K, Neuman T, Tos M, Thomsen J. Acoustic neuroma. Amsterdam: Kugler; 1992.
- O’Connor A, France M, Morrison A. Perilymph total protein levels associated with cerebellopontine angle lesion. Am J Otol 1981;2:193-5.
- Fucci MJ, Buchman CA, Brackmann DE, Berliner KI. Acoustic tumor growth: implications for treatment choices. Am J Otol 1999;20:495-9.
- Tschudi DC, Linder TE, Fisch U. Conservative management of unilateral acoustic neuromas. Am J Otol 2000;21:722-8.
- Kentala E, Pyykko I. Clinical picture of vestibular schwannoma. Auris Nasus Larynx 2001;28:15-22.
- Sauvaget E, Kici S, Kania R, Herman P, Tran Ba Huy P. Sudden sensorineural hearing loss as a revealing symptom of vestibular schwannoma. Acta Otolaryngol 2005;125:592-5.
- Wandong S, Meng L, Xingang L, Yuguang L, Shugan Z, Lei W, et al. Cystic acoustic neuroma. J Clin Neurosci 2005;12:253-5.
- Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery 1997;40:1-10.
- van Leeuwen JPPM, Cremers CWRJ, Thewissen NPMW, Harhangi BS, Meijer E. Acoustic neuroma: correlation among tumor size, symptoms, and patient age. Laryngoscope 1995;105:701-7.
- Ogawa K, Kanzaki J, Ogawa S, Tsuchihashi N, Yamamoto M. Acoustic neuromas with normal hearing. Acta Otolaryngol 1991;487:144-9.
- Ogawa K, Kanzaki J, Ogawa S, Tsuchihashi N, Inoue Y. Acoustic neuromas presenting as sudden hearing loss. Acta Otolaryngol Suppl 1991;487:138-43.
- Sai M. Acoustic neuroma presenting as sudden hearing loss. Practica Otologica Kyoto 1990;83.
- Diensthuber M, Lenarz T, Stover T. Determination of the clinical growth index in unilateral vestibular schwannoma. Skull Base 2006;16:31-8.
- Aslan A, de Donato G, Balyan FR, Falcioni M, Russo A, Taibah A, et al. Clinical observations on coexistence of sudden hearing loss and vestibular schwannoma. Otolaryngol Head Neck Surg 1997;117:580-2.
- Berrettini S, Ravecca F, Sellari-Franceschini S, Bruschini P, Casani A, Padolecchia R. Acoustic neuroma: correlations between morphology and otoneurological manifestations. J Neurol Sci 1996;144:24-33.
- Are CK, Eljamel MS, McConachie N, Viani L, Rawluk D. Acoustic neuromas: a review of 58 patients and correlation of tumour-size to the outcome. Ir Med J 1995;88:104-5.
- Moffat DA, Golledge J, Baguley DM, Hardy DG. Clinical correlates of acoustic neuroma morphology. J Laryngol Otol 1993;107:290-4.
- Leonetti JP. The diagnosis and management of acoustic neuromas: contemporary practice guidelines. Compr Ther 1995;21:68-73.
- Magdziarz DD, Wiet RJ, Dinces EA, Adamiec LC. Normal audiologic presentations in patients with acoustic neuroma: an evaluation using strict audiologic parameters. Otolaryngol Head Neck Surg 2000;122:157-62.
- Moffat DA, Baguley DM, von Blumenthal H, Irving RM, Hardy DG. Sudden deafness in vestibular schwannoma. J Laryngol Otol 1994;108:116-19.
- Saleh EA, Aristegui M, Naguib MB, Cokesser Y, Landolfi M, Sanna M. Normal hearing in acoustic neuroma patients: a critical evaluation. Am J Otol 1996;17:127-32.
- Lustig LR, Rifkin S, Jackler RK, Pitts LH. Acoustic neuromas presenting with normal or symmetrical hearing: factors associated with diagnosis and outcome. Am J Otol 1998;19:212-18.
- Artz J, Timmer F, Mulder J, Cremers C, Graamans K. Predictors of future growth of sporadic vestibular schwannomas obtained by history and radiologic assessment of the tumor. Eur Arch Otorhinolaryngol n.d.
- Day A-S, Wang C-T, Chen C-N, Young Y-H. Correlating the cochleovestibular deficits with tumor size of acoustic neuroma. Acta Otolaryngol 2008;128:756-60.
- Baguley D, Humphriss R, Axon P, Moffat D. The clinical characteristics of tinnitus in patients with vestibular schwannoma. Skull Base 2006;16:49-58.
- Mackle T, Rawluk D, Walsh R. Atypical clinical presentations of vestibular schwannomas. Otol Neurotol 2007;28:526-8.
- Selesnick SH, Jackler RK. Atypical hearing loss in acoustic neuroma patients. Laryngoscope 1993;103:437-41.
- Baguley D, Bird J, Humphriss R, Prevost A. The evidence base for the application of contralateral bone anchored hearing aids in acquired unilateral sensorineural hearing loss in adults. Clin Otolaryngol 2006;31:6-14.
- van Leeuwen J, Harhangi B, Thewissen N, Thijssen H, Cremers C. Delays in the diagnosis of acoustic neuromas. Am J Otol 1996;17:321-5.
- Frohlich AM, Sutherland GR. Epidemiology and clinical features of vestibular schwannoma in Manitoba, Canada. Can J Neurol Sci 1993;20:126-30.
- Bozorg Grayeli A, Kalamarides M, Ferrary E, Bouccara D, Elgharem H, Rey A, et al. Conservative management versus surgery for small vestibular schwannomas. Acta Otolaryngol 2005;125:1063-8.
- Flint D, Fagan P, Panarese A. Conservative management of sporadic unilateral acoustic neuromas. J Laryngol Otol 2005;119:424-8.
- Charabi S, Thomsen J, Mantoni M, Charabi B, Jorgensen B, Borgesen SE, et al. Acoustic neuroma (vestibular schwannoma): growth and surgical and nonsurgical consequences of the wait-and-see policy. Otolaryngol Head Neck Surg 1995;113:5-14.
- Modugno GC, Pirodda A, Ferri GG, Fioravanti A, Calbucci F, Pezzi A, et al. Small acoustic neuromas: monitoring the growth rate by MRI. Acta Neurochir 1999;141:1063-7.
- Martin CH, Fraysse B, Chelikh L, Cadene MV. Acoustic neuroma natural growth in older patients. J Fr Otorhinolaryngol 1994;43:392-7.
- Hajioff D, Raut VV, Walsh RM, Bath AP, Bance ML, Guha A, et al. Conservative management of vestibular schwannomas: third review of a 10-year prospoective study. Clin Otolaryngol 2008;33:255-64.
- Quaranta N, Baguley DM, Moffat DA. Change in hearing and tinnitus in conservatively managed vestibular schwannomas. Skull Base 2007;17:223-8.
- Walsh RM, Bath AP, Bance ML, Keller A, Rutka JA. Consequences to hearing during the conservative management of vestibular schwannomas. Laryngoscope 2000;110:250-5.
- Smouha EE, Yoo M, Mohr K, Davis RP. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope 2005;115:450-4.
- Walsh RM, Bath AP, Bance ML, Keller A, Tator CH, Rutka JA. The natural history of untreated vestibular schwannomas. Is there a role for conservative management?. Rev Laryngol Otol Rhinol 2000;121:21-6.
- Shaan M, Vassalli L, Landolfi M, Taibah A, Russo A, Sanna M. Atypical presentation of acoustic neuroma. Otolaryngol Head Neck Surg 1993;109:865-70.
- Stipkovits EM, Graamans K, Vasbinder GBC, van Dijk JE, Beek FJA. Assessment of vestibular schwannoma growth: application of a new measuring protocol to the results of a longitudinal study. Ann Otol Rhinol Laryngol 2001;110:326-30.
- Jorgensen BG, Pedersen CB. Acoustic neuroma: follow-up of 78 patients. Clin Otolaryngol 1994;19:478-84.
- Ogawa K, Kanzaki J, Ogawa S, Yamamoto M, Ikeda S, Shiobara R. The growth rate of acoustic neuromas. Acta Otolaryngol Suppl 1991;487:157-63.
- Valvassori GE, Shannon M. Natural history of acoustic neuromas. Skull Base Surg 1991;1:165-7.
- Piazza F, Frisina A, Gandolfi A, Quaranta N, Zini C. Management of acoustic neuromas in the elderly: retrospective study. Ear Nose Throat J 2003;82:374-8.
- Glasscock ME, Pappas DG, Manolidis S, von Doersten PG, Jackson CG, Storper IS. Management of acoustic neuroma in the elderly population. Am J Otol 1997;18:236-42.
- Strasnick B, Glasscock ME, Haynes D, McMenomey SO, Minor LB. The natural history of untreated acoustic neuromas. Laryngoscope 1994;104:1115-19.
- Bederson JB, von Ammon K, Wichmann WW, Yasargil MG. Conservative treatment of patients with acoustic tumors. Neurosurgery 1991;28:646-51.
- Herwadker A, Vokurka EA, Evans DGR, Ramsden RT, Jackson A. Size and growth rate of sporadic vestibular schwannoma: predictive value of information available at presentation. Otol Neurotol 2005;26:86-92.
- Caye-Thomasen P, Baandrup L, Jacobsen GK, Thomsen J, Stangerup S-E. Immunohistochemical demonstration of vascular endothelial growth factor in vestibular schwannomas correlates to tumor growth rate. Laryngoscope 2003;113:2129-34.
- Mohyuddin A, Vokurka EA, Evans DGR, Ramsden RT, Jackson A. Is clinical growth index a reliable predictor of tumour growth in vestibular schwannomas?. Clin Otolaryngol 2003;28:85-90.
- Niemczyk K, Dubrulle F, Vaneecloo F-M, Lejeune J-P, Lemaitre L, Bruzgielewicz A, et al. Clinical implications of acoustic neuromas growth rate in volumetric study. Ann Otolaryngol Chir Cervicofac 2002;119:259-63.
- Vokurka EA, Herwadker A, Thacker NA, Ramsden RT, Jackson A. Using Bayesian tissue classification to improve the accuracy of vestibular schwannoma volume and growth measurement. Am J Neuroradiol 2002;23:459-67.
- Hoistad DL, Melnik G, Mamikoglu B, Battista R, O’Connor CA, Wiet RJ. Update on conservative management of acoustic neuroma. Otol Neurotol 2001;22:682-5.
- Nutik SL, Babb MJ. Determinants of tumor size and growth in vestibular schwannomas. J Neurosurg 2001;94:922-6.
- Sakamoto T, Fukuda S, Inuyama Y. Hearing loss and growth rate of acoustic neuromas in follow-up observation policy. Auris Nasus Larynx 2001;28:S23-7.
- Massick DD, Welling DB, Dodson EE, Scholfield M, Nagaraja HN, Schmalbrock P, et al. Tumor growth and audiometric change in vestibular schwannomas managed conservatively. Laryngoscope 2000;110:1843-9.
- O’Reilly B, Murray CD, Hadley DM. The conservative management of acoustic neuroma: a review of forty-four patients with magnetic resonance imaging. Clin Otolaryngol 2000;25:93-7.
- Shin YJ, Fraysse B, Cognard C, Gafsi I, Charlet J-P, Berges C, et al. Effectiveness of conservative management of acoustic neuromas. Am J Otol 2000;21:857-62.
- Niemczyk K, Vaneecloo F-M, Lemaitre L, Lejeune J-P, Skarzynski H, Dubrulle F, et al. The growth of acoustic neuromas in volumetric radiologic assessment. Am J Otol 1999;20:244-8.
- Yamamoto M, Hagiwara S, Ide M, Jimbo M, Arai Y, Ono Y. Conservative management of acoustic neurinomas: prospective study of long-term changes in tumor volume and auditory function. Minim Invasive Neurosurg 1998;41:86-92.
- Levo H, Pyykko I, Blomstedt G. Non-surgical treatment of vestibular schwannoma patients. Acta Otolaryngol Suppl 1997;529:56-8.
- Deen HG, Ebersold MJ, Harner SG, Beatty CW, Marion MS, Wharen RE, et al. Conservative management of acoustic neuroma: an outcome study. Neurosurgery 1996;39:260-6.
- Wiet RJ, Zappia JJ, Hecht CS, O’Connor CA. Conservative management of patients with small acoustic tumors. Laryngoscope 1995;105:795-800.
- Rosenberg SI, Silverstein H, Gordon MA, Flanzer JM, Willcox TO, Silverstein J. A comparison of growth rates of acoustic neuromas: nonsurgical patients vs. subtotal resection. Otolaryngol Head Neck Surg 1993;109:482-7.
- Al Sanosi A, Fagan PA, Biggs NDW. Conservative management of acoustic neuroma. Skull Base 2006;16:95-100.
- Shin YJ, Lapeyre-Mestre M, Gafsi I, Cognard C, Deguine O, Tremoulet M, et al. Neurotological complications after radiosurgery versus conservative management in acoustic neuromas: a systematic review-based study. Acta Otolaryngol 2003;123:59-64.
- Moller P, Myrseth E, Pedersen P-H, Lund-Johannessen M, Krakenes J, Moen G. Small vestibular schwannoma: results with observation, surgery and gamma-knife n.d.:39-40.
- Kishore A, Handoura L, O’Reilly BF, Crowther J. Outcome of 100 Vestibular Schwannomas Managed Conservatively: A 10 Year Follow up Study n.d.
- Ramsden RT, Suryanarayanan R, Orabi A, Saeed SR, King A, Evans DGR, et al. Wait-Rescan As an Option for the Management of a Vestibular Schwannoma: A Retrospective Review of 267 Cases over a Period of 15 Years n.d.:60-1.
- Ferri GG, Modugno GC, Pirodda A, Fioravanti A, Calbucci F, Ceroni A. Conservative management of vestibular schwannomas: an effective strategy. Laryngoscope 2008;118:951-7.
- Quaranta N, Baguley DM, Axon PR, Moffat DA. Conservative Management of Vestibular Schwannomas n.d.:256-7.
- Battaglia A, Mastrodimos B, Cueva R. Comparison of growth patterns of acoustic neuromas with and without radiosurgery. Otol Neurotol 2006;27:705-12.
- Charabi S, Tos M, Thomsen J, Charabi B, Mantoni M. Vestibular schwannoma growth – long-term results. Acta Otolaryngol Suppl 2000;543:7-10.
- Thomsen J, Charabi S, Tos M, Mantoni M, Charabi B. Intracanalicular vestibular schwannoma – therapeutic options. Acta Otolaryngol Suppl 2000;543:38-40.
- Caye-Thomasen P, Hansen S, Dethloff T, Stangerup S-E, Thomsen J. Sublocalization and volumetric growth pattern of intracanalicular vestibular schwannomas. Laryngoscope 2006;116:1131-5.
- Raut VV, Walsh RM, Bath AP, Bance ML, Guha A, Tator CH, et al. Conservative management of vestibular schwannomas – second review of a prospective longitudinal study. Clin Otolaryngol 2004;29:505-14.
- Walsh RM, Bath AP, Bance ML, Keller A, Tator CH, Rutka JA. The role of conservative management of vestibular schwannomas. Clin Otolaryngol 2000;25:28-39.
- Mirz F, Pedersen CB, Fiirgaard B, Lundorf E. Incidence and growth pattern of vestibular schwannomas in a Danish county, 1977–98. Acta Otolaryngol Suppl 2000;543:30-3.
- Mirz F, Jorgensen BG, Fiirgaard B, Lundorf E, Pedersen CB. Investigations into the natural history of vestibular schwannomas. Clin Otolaryngol 1999;24:13-8.
- Rosenberg SI. Natural history of acoustic neuromas. Laryngoscope 2000;110:497-508.
- Solares C, Panizza B. Vestibular schwannoma: an understanding of growth should influence management decisions. Otol Neurotol 2008;29:829-34.
- Yamakami I, Uchino Y, Kobayashi E, Yamaura A. Conservative management, gamma-knife radiosurgery, and microsurgery for acoustic neurinomas: a systematic review of outcome and risk of three therapeutic options. Neurol Res 2003;25:682-90.
- Selesnick SH, Johnson G. Radiologic surveillance of acoustic neuromas. Am J Otol 1998;19:846-9.
- Marshall AH, Owen VMF, Nikolopoulos TP, O’Donoghue GM. Acoustic schwannomas: awareness of radiologic error will reduce unnecessary treatment. Otol Neurotol 2005;26:512-15.
- Flickinger J, Kondziolka D, Niranjan JC, Voynov G, Maitz A, Lunsford L. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys 2004;60:225-30.
- Iwai Y, Yamanaka K, Shiotani M, Uyama T. Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery 2003;52:282-7.
- Cross J, Baguley D, Antoun N, Moffat D, Prevost A. Reproducibility of volume measurements of vestibular schwannomas – a preliminary study. Clin Otolaryngol 2006;31:123-9.
Appendix 1 Search strategies used in the systematic review
Search strategies
A comprehensive literature search was conducted in October 2006 to identify relevant literature pertaining to acoustic neuroma. Three major searches were undertaken, which were designed to retrieve:
-
papers describing the natural history of acoustic neuroma
-
papers on the effectiveness of different techniques for diagnosing acoustic neuroma
-
papers on the costs associated with acoustic neuroma.
The following electronic bibliographic databases were searched:
-
Allied and Complementary Medicine (AMED) via Ovid Online, 1982–
-
BIOSIS previews (Biological Abstracts) via Webspirs, 1986–
-
British Nursing Index (BNI) via Dialog, 1994–
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) via Ovid Online, 1982–
-
Cochrane Database of Systematic Reviews (CDSR), 1991–
-
Cochrane Central Register of Controlled Trials (CENTRAL), 1991–
-
EMBASE via Ovid Online, 1980–
-
HEED via OHE
-
MEDLINE via Ovid Online, 1966–
-
MEDLINE In-Process & Other Non-Indexed Citations via Ovid Online
-
NHS Database of Abstracts of Reviews of Effects (DARE), 1994–
-
NHS Economic Evaluations Database (NHS EED), 1995–
-
NHS Health Technology Assessment (HTA) Database, 1998–
-
PsycINFO via Dialog, 1806–
-
Science Citation Index (SCI) via WOK, 1900–
-
Social Sciences Citation Index (SSCI) via WOK, 1956–
Attempts were also made to identify ‘grey’ literature by searching appropriate databases (e.g. King’s Fund via Dialog, 1979–; DH-Data via Dialog, 1983–) and current research registers (e.g. National Research Register).
The main searches were limited to literature from 1980 to 2006. A further simplified updating search (October 2006–August 2008) was conducted before the final submission of the report. This search used only the terms acoustic neuroma and vestibular schwannoma and searched MEDLINE, EMBASE, PubMed and HEED (see Figure 14, Appendix 2).
With the larger and more sophisticated databases (e.g. MEDLINE, CINAHL, EMBASE) search filters were utilised to retrieve relevant studies. A natural history filter was added on to the searches to retrieve papers on the natural history of acoustic neuroma. A diagnostic filter was used to retrieve studies on the effectiveness of the different diagnostic techniques for acoustic neuroma. An economics filter was used to retrieve papers on the costs associated with acoustic neuroma.
The full search strategies for each database are provided below.
AMED
-
acoustic neuroma$.mp.
-
acoustic lesion$.mp.
-
acoustic neurinoma$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve tumour$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma$.mp.
-
intracranial tumor$.mp.
-
intracranial tumour$.mp.
-
intracranial lesion$.mp.
-
cerebellopontine angle lesion$.mp.
-
cerebellopontine angle tumor$.mp.
-
cerebellopontine angle tumour$.mp.
-
or/1–13
Filters were not used on the AMED database.
BIOSIS
Natural history
-
acoustic neuroma$.mp.
-
acoustic lesion$.mp.
-
acoustic neurinoma$.mp.
-
vestibular nerve tumour$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
intracranial tumor$.mp.
-
intracranial tumour$.mp.
-
intracranial lesion$.mp.
-
cerebellopontine angle tumour$.mp.
-
cerebellopontine angle tumor$.mp.
-
cerebellopontine angle lesion$.mp.
-
or/1–13
-
natural history.mp.
-
incidence.mp. or Epidemiology/
-
epidemiology.mp.
-
prevalence.mp.
-
or/15–18
-
14 and 19
The terms describing acoustic neuroma 1–13 were combined with the natural history filters, terms 15–18.
Clinical effectiveness and cost-effectiveness
-
acoustic neuroma$.mp.
-
acoustic lesion$.mp.
-
acoustic neurinoma$.mp.
-
vestibular nerve tumour$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
intracranial tumor$.mp.
-
intracranial tumour$.mp.
-
intracranial lesion$.mp.
-
cerebellopontine angle tumour$.mp.
-
cerebellopontine angle tumor$.mp.
-
cerebellopontine angle lesion$.mp.
-
or/1–13
-
magnetic resonance imaging.mp.
-
mri.mp.
-
nmr.mp.
-
antoni classification.mp.
-
computed tomography.mp.
-
CAT.mp.
-
electrocochleography.mp.
-
EcochG.mp.
-
auditory steady state response.mp.
-
ASSR.mp.
-
electric response audiometry.mp.
-
ERA.mp.
-
auditory brainstem response.mp.
-
ABR.mp.
-
brainstem evoked response.mp.
-
BSER.mp.
-
BAER.mp.
-
otoacoustic emission$.mp.
-
(OAE or OAE DP OAE).mp.
-
stapedial reflex.mp.
-
tone decay reflex.mp.
-
vestibular function.mp.
-
(caloric test$or calorics).mp.
-
or/15–37
-
14 and 38
The acoustic neuroma terms 1–13 were combined with terms for the different diagnostic techniques 15–37.
British Nursing Index
-
ACOUSTIC ADJ NEUROMA$
-
ACOUSTIC ADJ NEURINOMA$
-
ACOUSTIC ADJ TUMOR$
-
ACOUSTIC ADJ TUMOUR$
-
ACOUSTIC ADJ LESION$
-
VESTIBULAR ADJ NERVE ADJ TUMOR$
-
VESTIBULAR ADJ NERVE ADJ TUMOUR$
-
VESTIBULAR ADJ NERVE ADJ LESION$
-
VESTIBULAR ADJ SCHWANNOMA$
-
INTRACRANIAL ADJ TUMOUR$
-
INTRACRANIAL ADJ TUMOR$
-
INTRACRANIAL ADJ LESION$
-
CEREBELLOPONTINE ADJ ANGLE ADJ LESION$
-
CEREBELLOPONTINE ADJ ANGLE ADJ TUMOR$
-
CEREBELLOPONTINE ADJ ANGLE ADJ TUMOUR$
-
CPA
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16
Filters were not used on the BNI database.
CRD
acoustic AND neuroma* OR acoustic AND lesion* OR acoustic AND neurinoma* OR vestibular AND nerve AND tumor* OR vestibular AND nerve AND tumour* OR vestibular AND nerve AND lesion* OR vestibular AND schwannoma* OR intracranial AND tumor* OR intracranial AND tumour* OR intracranial AND lesion* OR cerebellopontine AND angle AND lesion* OR cerebellopontine AND angle AND tumor* OR cerebellopontine AND angle AND tumour*
Filters were not used on the CRD databases.
CINAHL
Natural history
-
exp Neuroma, Acoustic/
-
acoustic neuroma$.mp.
-
acoustic lesion$.mp.
-
vestibular nerve tumour$.mp.
-
acoustic neurinoma$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
(intracranial tumour$or intracranial tumor$).mp. [mp=title, subject heading word, abstract, instrumentation]
-
intracranial lesion$.mp.
-
cerebellopontine angle lesion$.mp.
-
cerebellopontine angle tumour$.mp.
-
cerebellopontine angle tumor$.mp.
-
or/1–13
-
natural history.mp.
-
epidemiology.mp. or exp EPIDEMIOLOGY/
-
incidence.mp. or exp INCIDENCE/
-
prevalence.mp. or exp PREVALENCE/
-
or/15–18
-
14 and 19
The terms describing acoustic neuroma 1–13 were combined with the natural history filters, terms 15–18.
Clinical effectiveness
With diagnostic filter
-
exp Neuroma, Acoustic/
-
acoustic neuroma$.mp.
-
acoustic lesion$.mp.
-
vestibular nerve tumour$.mp.
-
acoustic neurinoma$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
(intracranial tumour$or intracranial tumor$).mp. [mp=title, subject heading word, abstract, instrumentation]
-
intracranial lesion$.mp.
-
cerebellopontine angle lesion$.mp.
-
cerebellopontine angle tumour$.mp.
-
cerebellopontine angle tumor$.mp.
-
or/1–13
-
exp Magnetic Resonance Imaging/
-
magnetic resonance imag$.mp.
-
mri.mp.
-
nmr.mp.
-
antoni classification.mp.
-
exp Tomography, X-Ray Computed/or computed tomography.mp.
-
CAT.mp.
-
electrocochleography.mp. or exp Audiometry, Evoked Response/
-
EcochG.mp.
-
auditory state response.mp.
-
ASSR.mp.
-
electric response audiometry.mp.
-
auditory brainstem response.mp. or exp Evoked Potentials, Auditory, Brainstem/
-
AMR.mp.
-
brainstem evoked response.mp.
-
BSER.mp.
-
BAER.mp.
-
otoacoustic emission$.mp.
-
(OAE or OAE DP OAE).mp.
-
stapedial reflex.mp.
-
tone decay reflex.mp.
-
vestibular function.mp.
-
exp Vestibular Function Tests/
-
(caloric$or caloric test$).mp. [mp=title, subject heading word, abstract, instrumentation]
-
or/15–38
-
exp “Sensitivity and Specificity”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
or/40–46
-
14 and 39 and 47
The terms describing acoustic neuroma 1–13 were combined with the terms describing the different diagnostic techniques filters, terms 15–38, and the diagnostic search filters, terms 40–46.
Without diagnostic filter
-
exp Neuroma, Acoustic/
-
acoustic neuroma$.mp.
-
acoustic lesion$.mp.
-
vestibular nerve tumour$.mp.
-
acoustic neurinoma$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
(intracranial tumour$or intracranial tumor$).mp. [mp=title, subject heading word, abstract, instrumentation]
-
intracranial lesion$.mp.
-
cerebellopontine angle lesion$.mp.
-
cerebellopontine angle tumour$.mp.
-
cerebellopontine angle tumor$.mp.
-
or/1–13
-
exp Magnetic Resonance Imaging/
-
magnetic resonance imag$.mp.
-
mri.mp.
-
nmr.mp.
-
antoni classification.mp.
-
exp Tomography, X-Ray Computed/or computed tomography.mp.
-
CAT.mp.
-
electrocochleography.mp. or exp Audiometry, Evoked Response/
-
EcochG.mp.
-
auditory state response.mp.
-
ASSR.mp.
-
electric response audiometry.mp.
-
auditory brainstem response.mp. or exp Evoked Potentials, Auditory, Brainstem/
-
AMR.mp.
-
brainstem evoked response.mp.
-
BSER.mp.
-
BAER.mp.
-
otoacoustic emission$.mp.
-
(OAE or OAE DP OAE).mp.
-
stapedial reflex.mp.
-
tone decay reflex.mp.
-
vestibular function.mp.
-
exp Vestibular Function Tests/
-
(caloric$or caloric test$).mp. [mp=title, subject heading word, abstract, instrumentation]
-
or/15–38
-
14 and 39
The search was also run without the diagnostic filter. The additional references retrieved (without the diagnostic filter) were then reviewed after the references retrieved with the diagnostic filter to ensure that no important studies were missed.
Cost-effectiveness
-
exp Neuroma, Acoustic/
-
acoustic neuroma$.mp.
-
acoustic lesion$.mp.
-
vestibular nerve tumour$.mp.
-
acoustic neurinoma$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
(intracranial tumour$or intracranial tumor$).mp. [mp=title, subject heading word, abstract, instrumentation]
-
intracranial lesion$.mp.
-
cerebellopontine angle lesion$.mp.
-
cerebellopontine angle tumour$.mp.
-
cerebellopontine angle tumor$.mp.
-
or/1–13
-
exp economics/
-
exp “financial management”/
-
exp “financial support”/
-
exp “financing organized”/
-
exp “business”/
-
or/16–19
-
20 not 15
-
Health resource allocation.sh.
-
Health resource utilization.sh.
-
22 or 23
-
21 or 24
-
(cost or costs or economic$or pharmacoeconomic$or price$or pricing$).tw.
-
25 or 26
-
Editorial.pt.
-
Letter.pt.
-
News.pt.
-
or/28–30
-
27 not 31
-
“Animal studies”/
-
32 not 33
-
Cochrane library.so.
-
Anonymous.au.
-
34 not (35 or 36)
-
14 and 37
The terms describing acoustic neuroma 1–13 were combined with the economics filters, terms 15–37.
The Cochrane Library including Cochrane Reviews, CENTRAL, DARE, HTA and NHS EED
Natural history
-
MeSH descriptor Neuroma, Acoustic explode all trees
-
acoustic neuroma*
-
acoustic neurinoma*
-
acoustic tumor*
-
acoustic tumour*
-
acoustic lesion*
-
vestibular nerve tumor*
-
vestibular nerve tumour*
-
vestibular nerve lesion*
-
vestibular schwannoma
-
(intracranial tumour*):ti or (intracranial tumour*):ab or (intracranial tumor*):ab or (intracranial tumor*):ti
-
(intracranial lesion*):ab or (intracranial lesion*):ti
-
(cerebellopontine angle lesion*):ti or (cerebellopontine angle lesion*):ab
-
(cerebellopontine angle tumor*):ti or (cerebellopontine angle tumor*):ab or (cerebellopontine angle tumour*):ab or (cerebellopontine angle tumour*):ti
-
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14)
-
MeSH descriptor Natural History explode all trees
-
(natural history):ti,ab,kw
-
MeSH descriptor Prevalence explode all trees
-
(prevalence):ti,ab,kw
-
MeSH descriptor Incidence explode all trees
-
(incidence):ti,ab,kw
-
MeSH descriptor Epidemiology explode all trees
-
(epidemiology):ti,ab,kw
-
(#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23)
-
(#15 AND #24)
The terms describing acoustic neuroma 1–14 were combined with the natural history filters, terms 16–23.
Clinical effectiveness
-
MeSH descriptor Neuroma, Acoustic explode all trees
-
(acoustic neuroma*)
-
acoustic neurinoma*
-
acoustic tumor*
-
acoustic tumour*
-
acoustic lesion*
-
vestibular nerve tumor*
-
vestibular nerve tumour*
-
vestibular nerve lesion*
-
vestibular schwannoma
-
(intracranial tumour*):ti or (intracranial tumour*):ab or (intracranial tumor*):ab or (intracranial tumor*):ti
-
(intracranial lesion*):ab or (intracranial lesion*):ti
-
(cerebellopontine angle lesion*):ti or (cerebellopontine angle lesion*):ab
-
(cerebellopontine angle tumor*):ti or (cerebellopontine angle tumor*):ab or (cerebellopontine angle tumour*):ab or (cerebellopontine angle tumour*):ti
-
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14)
-
MeSH descriptor Magnetic Resonance Imaging explode all trees
-
(magnetic resonance imag*):ab or (magnetic resonance imag*):ti
-
(mri):ti or (mri):ab
-
(nmr):ti or (nmr):ab
-
(antoni classification):ab or (antoni classification):ti
-
(computed tomography):ab or (computed tomography):ti
-
(cat):ab or (cat):ti
-
(electrocochleography):ab or (electrocochleography):ti
-
(ecochg):ti or (ecochg):ab
-
(auditory steady state response*):ti or (auditory steady state response*):ab
-
(assr):ab or (assr):ti
-
(electric response audiometry):ab or (electric response audiometry):ti
-
(era):ab or (era):ti
-
(auditory brainstem response*):ab or (auditory brainstem response*):ti
-
(abr):ab or (abr):ti
-
(brainstem evoked response*):ab or (brainstem evoked response*):ti
-
(bser):ab or (bser):ti or (baer):ab or (baer):ti
-
(otoacoustic emission*):ab or (otoacoustic emission*):ti
-
(oae):ab or (oae):ti
-
(eoae):ab or (eoae):ti
-
(dpoae):ab or (dpoae):ti
-
(stapedial reflex*):ab or (stapedial reflex*):ti
-
(tone delay reflex*):ab or (tone delay reflex*):ti
-
MeSH descriptor Vestibular Function Tests explode all trees
-
(vestibular function*):ab or (vestibular function*):ti
-
(calorics):ab or (calorics):ti
-
(#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41)
-
(#15 AND #42)
The terms describing acoustic neuroma 1–14 were combined with the terms describing the different diagnostic techniques 16–41.
Cost-effectiveness
-
MeSH descriptor Neuroma, Acoustic explode all trees
-
(acoustic neuroma*)
-
acoustic neurinoma*
-
acoustic tumor*
-
acoustic tumour*
-
acoustic lesion*
-
vestibular nerve tumor*
-
vestibular nerve tumour*
-
vestibular nerve lesion*
-
vestibular schwannoma
-
(intracranial tumour*):ti or (intracranial tumour*):ab or (intracranial tumor*):ab or (intracranial tumor*):ti
-
(intracranial lesion*):ab or (intracranial lesion*):ti
-
(cerebellopontine angle lesion*):ti or (cerebellopontine angle lesion*):ab
-
(cerebellopontine angle tumor*):ti or (cerebellopontine angle tumor*):ab or (cerebellopontine angle tumour*):ab or (cerebellopontine angle tumour*):ti
-
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14)
The NHS EED database in the Cochrane Library was searched for cost-effectiveness papers using the acoustic neuroma, terms 1–14.
DH-Data
-
ACOUSTIC ADJ NEUROMA$
-
ACOUSTIC ADJ NEURINOMA$
-
ACOUSTIC ADJ TUMOR$
-
ACOUSTIC ADJ TUMOUR$
-
ACOUSTIC ADJ LESION$
-
VESTIBULAR ADJ NERVE ADJ TUMOR$
-
VESTIBULAR ADJ NERVE ADJ TUMOUR$
-
VESTIBULAR ADJ NERVE ADJ LESION$
-
VESTIBULAR ADJ SCHWANNOMA$
-
INTRACRANIAL ADJ TUMOUR$
-
INTRACRANIAL ADJ TUMOR$
-
INTRACRANIAL ADJ LESION$
-
CEREBELLOPONTINE ADJ ANGLE ADJ LESION$
-
CEREBELLOPONTINE ADJ ANGLE ADJ TUMOR$
-
CEREBELLOPONTINE ADJ ANGLE ADJ TUMOUR$
-
CPA
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16
Filters were not used on the DH-Data database.
EMBASE
Natural history
-
exp Acoustic Neurinoma/
-
acoustic neuroma$.mp.
-
(acoustic and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
(vestibular nerve and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
vestibular schwannoma.mp.
-
exp Intracranial Tumor/
-
(intracranial and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
cerebellopontine angle lesion$.mp.
-
(cerebellopontine angle and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
acoustic lesion$.mp.
-
vestibular nerve lesion$.mp.
-
intracranial lesion$.mp.
-
CPA.mp.
-
or/1–13
-
natural history.mp. or exp History/
-
incidence.mp. or exp INCIDENCE/
-
prevalence.mp. or exp PREVALENCE/
-
exp EPIDEMIOLOGY/or epidemiology.mp.
-
or/15–18
-
14 and 19
The terms describing acoustic neuroma 1–13 were combined with the natural history filters, terms 15–18.
Clinical effectiveness
With diagnostic filter
-
exp Acoustic Neurinoma/
-
acoustic neuroma$.mp.
-
(acoustic and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
(vestibular nerve and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
vestibular schwannoma.mp.
-
exp Intracranial Tumor/
-
(intracranial and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
cerebellopontine angle lesion$.mp.
-
(cerebellopontine angle and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
acoustic lesion$.mp.
-
vestibular nerve lesion$.mp.
-
intracranial lesion$.mp.
-
CPA.mp.
-
magnetic resonance imaging.mp. or exp Nuclear Magnetic Resonance Imaging/
-
magnetic resonance image$.mp.
-
mri.mp. or Nuclear Magnetic Resonance Imaging/
-
nmr.mp.
-
antoni classification.mp.
-
computed tomography.mp. or exp Computer Assisted Tomography/
-
CAT.mp.
-
electrocochleography.mp. or exp ELECTROCOCHLEOGRAPHY/
-
ecochG.mp.
-
auditory steady state response/or auditory steady state response$.mp.
-
ASSR.mp.
-
electric response audiometry.mp.
-
ERA.mp. or exp Evoked Response Audiometry/
-
auditory brainstem response.mp.
-
ABR.mp.
-
brainstem evoked response.mp. or exp Evoked Brain Stem Response/
-
BSER.mp.
-
BAER.mp.
-
otoacoustic emissions.mp. or exp Otoacoustic Emission/
-
OAE.mp.
-
stapedial reflex.mp. or exp Acoustic Reflex/
-
tone decay reflex.mp.
-
exp Vestibular Function/
-
calorics.mp. or CALORIC VESTIBULAR TEST/
-
calorics.mp. or exp CALORIC VESTIBULAR TEST/
-
or/14–38
-
or/1–13
-
39 and 40
-
exp “SENSITIVITY AND SPECIFICITY”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
*Diagnostic Accuracy/
-
or/42–49
-
41 and 50
The terms describing acoustic neuroma 1–13 were combined with the terms describing the different diagnostic techniques filters, terms 14–38, and the diagnostic search filters, terms 42–49.
Without diagnostic filter
-
exp Acoustic Neurinoma/
-
acoustic neuroma$.mp.
-
(acoustic and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
(vestibular nerve and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
vestibular schwannoma.mp.
-
exp Intracranial Tumor/
-
(intracranial and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
cerebellopontine angle lesion$.mp.
-
(cerebellopontine angle and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
acoustic lesion$.mp.
-
vestibular nerve lesion$.mp.
-
intracranial lesion$.mp.
-
CPA.mp.
-
magnetic resonance imaging.mp. or exp Nuclear Magnetic Resonance Imaging/
-
magnetic resonance image$.mp.
-
mri.mp. or Nuclear Magnetic Resonance Imaging/
-
nmr.mp.
-
antoni classification.mp.
-
computed tomography.mp. or exp Computer Assisted Tomography/
-
CAT.mp.
-
electrocochleography.mp. or exp ELECTROCOCHLEOGRAPHY/
-
ecochG.mp.
-
auditory steady state response/or auditory steady state response$.mp.
-
ASSR.mp.
-
electric response audiometry.mp.
-
ERA.mp. or exp Evoked Response Audiometry/
-
auditory brainstem response.mp.
-
ABR.mp.
-
brainstem evoked response.mp. or exp Evoked Brain Stem Response/
-
BSER.mp.
-
BAER.mp.
-
otoacoustic emissions.mp. or exp Otoacoustic Emission/
-
OAE.mp.
-
stapedial reflex.mp. or exp Acoustic Reflex/
-
tone decay reflex.mp.
-
exp Vestibular Function/
-
calorics.mp. or CALORIC VESTIBULAR TEST/
-
calorics.mp. or exp CALORIC VESTIBULAR TEST/
-
or/14–38
-
or/1–13
-
39 and 40
The search was also run without the diagnostic filter. The additional references retrieved (without the diagnostic filter) were then reviewed after the references retrieved with the diagnostic filter to ensure that no important studies were missed.
Cost-effectiveness
-
exp Acoustic Neurinoma/
-
acoustic neuroma$.mp.
-
(acoustic and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
(vestibular nerve and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
vestibular schwannoma.mp.
-
exp Intracranial Tumor/
-
(intracranial and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
cerebellopontine angle lesion$.mp.
-
(cerebellopontine angle and (tumour$or tumor$)).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
-
acoustic lesion$.mp.
-
vestibular nerve lesion$.mp.
-
intracranial lesion$.mp.
-
CPA.mp.
-
exp SOCIOECONOMICS/
-
exp “Cost Benefit Analysis”/
-
exp “Cost Effectiveness Analysis”/
-
exp “Cost of Illness”/
-
exp “Cost Control”/
-
exp Economic Aspect/
-
exp Financial Management/
-
exp “Health Care Cost”/
-
exp Health Care Financing/
-
exp Health Economics/
-
exp “Hospital Cost”/
-
(financial or fiscal or finance or funding).tw.
-
exp “Cost Minimization Analysis”/
-
(cost adj estimate$).mp.
-
(cost adj variable$).mp.
-
(unit adj cost$).mp.
-
or/14–29
-
or/1–13
-
30 and 31
The terms describing acoustic neuroma 1–13 were combined with the economics filters, terms14–29.
HEED
Cost-effectiveness
ALL DATA (acoustic neuroma OR acoustic neurinoma OR acoustic tumor OR acoustic tumour OR vestibular nerve tumor OR vestibular nerve tumour OR vestibular schwannoma OR intracranial tumor OR intracranial tumour OR cerebellopontine angle lesion OR cerebellopontine tumor OR cerebellopontine tumour OR acoustic lesion OR vestibular nerve lesion OR intracranial lesion)
HEED is a database of economic evaluation so it was searched only for cost-effectiveness papers.
King’s Fund
-
ACOUSTIC ADJ NEUROMA$
-
ACOUSTIC ADJ NEURINOMA$
-
ACOUSTIC ADJ TUMOR$
-
ACOUSTIC ADJ TUMOUR$
-
ACOUSTIC ADJ LESION$
-
VESTIBULAR ADJ NERVE ADJ TUMOR$
-
VESTIBULAR ADJ NERVE ADJ TUMOUR$
-
VESTIBULAR ADJ NERVE ADJ LESION$
-
VESTIBULAR ADJ SCHWANNOMA$
-
INTRACRANIAL ADJ TUMOUR$
-
INTRACRANIAL ADJ TUMOR$
-
INTRACRANIAL ADJ LESION$
-
CEREBELLOPONTINE ADJ ANGLE ADJ LESION$
-
CEREBELLOPONTINE ADJ ANGLE ADJ TUMOR$
-
CEREBELLOPONTINE ADJ ANGLE ADJ TUMOUR$
-
CPA
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16
Filters were not used on the King’s Fund database.
MEDLINE and MEDLINE In-Process
Natural history
-
acoustic neuroma$.mp. or exp Neuroma, Acoustic/
-
acoustic neurinoma$.mp.
-
acoustic lesion$.mp.
-
vestibular nerve tumour$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
(intracranial tumor$or intracranial tumour$).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
intracranial lesion$.mp.
-
cerebellopontine angle lesion$.mp.
-
cerebellopontine angle tumor$.mp.
-
cerebellopontine angle tumour$.mp.
-
or/1–12
-
natural history.mp. or exp Natural History/
-
incidence.mp. or exp Incidence/
-
prevalence.mp. or exp Prevalence/
-
exp Epidemiology/or epidemiology.mp.
-
or/14–17
-
13 and 18
The terms describing acoustic neuroma 1–12 were combined with the natural history filters, terms 14–17.
Clinical effectiveness
With diagnosis filter
-
acoustic neuroma$.mp. or exp Neuroma, Acoustic/
-
acoustic neurinoma$.mp.
-
acoustic lesion$.mp.
-
vestibular nerve tumour$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
(intracranial tumor$or intracranial tumour$).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
intracranial lesion$.mp.
-
cerebellopontine angle lesion$.mp.
-
cerebellopontine angle tumor$.mp.
-
cerebellopontine angle tumour$.mp.
-
or/1–12
-
exp magnetic resonance imaging/or magnetic resonance imag$.mp.
-
MRI.mp.
-
NMR.mp.
-
antoni classification.mp.
-
exp tomography, X-ray computed/or computed tomography.mp.
-
CAT.mp.
-
electrocochleography.mp. or exp Audiometry, evoked response/
-
EcochG.mp.
-
auditory steady state response.mp.
-
ASSR.mp.
-
electric response audiometry.mp.
-
ERA.mp.
-
auditory brainstem response.mp. or exp evoked potentials, auditory, brain stem/
-
ABR.mp.
-
brainstem evoked response.mp.
-
BSER.mp.
-
BAER.mp.
-
otoacoustic emission$.mp.
-
(OAE or OAE DP OAE).mp.
-
stapedial reflex.mp.
-
tone decay reflex.mp.
-
vestibular function.mp.
-
exp caloric tests/or calorics.mp.
-
or/14–36
-
exp “Sensitivity and Specificity”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
or/38–44
-
13 and 37 and 45
The terms describing acoustic neuroma 1–12 were combined with the terms describing the different diagnostic techniques filters, terms 14–36, and the diagnostic search filters, terms 39–45.
Without diagnostic filter
-
acoustic neuroma$.mp. or exp Neuroma, Acoustic/
-
acoustic neurinoma$.mp.
-
acoustic lesion$.mp.
-
vestibular nerve tumour$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
(intracranial tumor$or intracranial tumour$).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
intracranial lesion$.mp.
-
cerebellopontine angle lesion$.mp.
-
cerebellopontine angle tumor$.mp.
-
cerebellopontine angle tumour$.mp.
-
or/1–12
-
exp magnetic resonance imaging/or magnetic resonance imag$.mp.
-
MRI.mp.
-
NMR.mp.
-
antoni classification.mp.
-
exp tomography, X-ray computed/or computed tomography.mp.
-
CAT.mp.
-
electrocochleography.mp. or exp Audiometry, evoked response/
-
EcochG.mp.
-
auditory steady state response.mp.
-
ASSR.mp.
-
electric response audiometry.mp.
-
ERA.mp.
-
auditory brainstem response.mp. or exp evoked potentials, auditory, brain stem/
-
ABR.mp.
-
brainstem evoked response.mp.
-
BSER.mp.
-
BAER.mp.
-
otoacoustic emission$.mp.
-
(OAE or OAE DP OAE).mp.
-
stapedial reflex.mp.
-
tone decay reflex.mp.
-
vestibular function.mp.
-
exp caloric tests/or calorics.mp.
-
or/14–36
-
13 and 37
The search was also run without the diagnostic filter. The additional references retrieved (without the diagnostic filter) were then reviewed after the references retrieved with the diagnostic filter to ensure that no important studies were missed.
Cost-effectiveness
-
acoustic neuroma$.mp. or exp Neuroma, Acoustic/
-
acoustic neurinoma$.mp.
-
acoustic lesion$.mp.
-
vestibular nerve tumour$.mp.
-
vestibular nerve tumor$.mp.
-
vestibular nerve lesion$.mp.
-
vestibular schwannoma.mp.
-
(intracranial tumor$or intracranial tumour$).mp. [mp=title, original title, abstract, name of substance word, subject heading word]
-
intracranial lesion$.mp.
-
cerebellopontine angle lesion$.mp.
-
cerebellopontine angle tumor$.mp.
-
cerebellopontine angle tumour$.mp.
-
or/1–12
-
Economics/
-
exp “Costs and Cost Analysis”/
-
economic value of life/
-
exp economics hospital/
-
exp economics medical/
-
economics nursing/
-
exp models economic/
-
Economics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp budgets/
-
ec.fs.
-
(cost or costs or costed or costly or costing$).tw.
-
(economic$or pharmacoecomomic$or price$or pricing$).tw.
-
quality adjusted life years/
-
(qaly or qaly$).af.
-
or/14–28
-
13 and 29
The terms describing acoustic neuroma 1–12 were combined with the economics filters, terms 13–28.
PsycINFO
Natural history
-
acoustic neuroma*
-
acoustic neurinoma*
-
acoustic tumor*
-
acoustic tumour*
-
vestibular nerve tumor*
-
vestibular nerve tumour*
-
vestibular schwannoma*
-
intracranial tumour*
-
intracranial tumor*
-
cerebellopontine angle lesion*
-
cerebellopontine angle tumor*
-
cerebellopontine angle tumour*
-
cpa
-
intracranial lesion*
-
vestibular nerve lesion*
-
acoustic lesion*
-
(cerebellopontine angle lesion*) or (intracranial tumor*) or (intracranial tumour*) or (vestibular schwannoma*) or (vestibular nerve tumour*) or (vestibular nerve tumor*) or (acoustic tumour*) or (acoustic tumor*) or (acoustic neurinoma*) or (acoustic neuroma*) or (acoustic lesion*) or (vestibular nerve lesion*) or (intracranial lesion*) or (cpa) or (cerebellopontine angle tumour*) or (cerebellopontine angle tumor*)
-
NATURAL-HISTORY
-
NATURAL-HISTORY-OF-DISEASE
-
natural history
-
(natural history) or (NATURAL-HISTORY-OF-DISEASE) or (NATURAL-HISTORY)
-
PREVALENCE
-
INCIDENCE
-
“Epidemiology-” in MJ,MN
-
epidemiology
-
(“Epidemiology-” in MJ,MN) or (INCIDENCE) or (PREVALENCE) or ((natural history) or (NATURAL-HISTORY-OF-DISEASE) or (NATURAL-HISTORY)) or (natural history) or (NATURAL-HISTORY-OF-DISEASE) or (NATURAL-HISTORY) or (epidemiology)
-
((“Epidemiology-” in MJ,MN) or (INCIDENCE) or (PREVALENCE) or ((natural history) or (NATURAL-HISTORY-OF-DISEASE) or (NATURAL-HISTORY)) or (natural history) or (NATURAL-HISTORY-OF-DISEASE) or (NATURAL-HISTORY) or (epidemiology)) and ((cerebellopontine angle lesion*) or (intracranial tumor*) or (intracranial tumour*) or (vestibular schwannoma*) or (vestibular nerve tumour*) or (vestibular nerve tumor*) or (acoustic tumour*) or (acoustic tumor*) or (acoustic neurinoma*) or (acoustic neuroma*) or (acoustic lesion*) or (vestibular nerve lesion*) or (intracranial lesion*) or (cpa) or (cerebellopontine angle tumour*) or (cerebellopontine angle tumor*))
The terms describing acoustic neuroma 1–16 were combined with the natural history filters, terms 18–25.
Clinical effectiveness and cost-effectiveness
-
acoustic neuroma*
-
acoustic neurinoma*
-
acoustic tumor*
-
acoustic tumour*
-
vestibular nerve tumor*
-
vestibular nerve tumour*
-
vestibular schwannoma*
-
intracranial tumour*
-
intracranial tumor*
-
cerebellopontine angle lesion*
-
cerebellopontine angle tumor*
-
cerebellopontine angle tumour*
-
cpa
-
intracranial lesion*
-
vestibular nerve lesion*
-
acoustic lesion*
-
(cerebellopontine angle lesion*) or (intracranial tumor*) or (intracranial tumour*) or (vestibular schwannoma*) or (vestibular nerve tumour*) or (vestibular nerve tumor*) or (acoustic tumour*) or (acoustic tumor*) or (acoustic neurinoma*) or (acoustic neuroma*) or (acoustic lesion*) or (vestibular nerve lesion*) or (intracranial lesion*) or (cpa) or (cerebellopontine angle tumour*) or (cerebellopontine angle tumor*)
A diagnostic and cost-effectiveness filter was not used.
Research Findings Register (ReFeR)
acoustic neuroma* OR acoustic lesion* OR acoustic neurinoma* OR vestibular nerve tumor* OR vestibular nerve tumour* OR vestibular nerve lesion* OR vestibular schwannoma* OR intracranial tumor* OR intracranial tumour* OR intracranial lesion* OR cerebellopontine angle lesion* OR cerebellopontine angle tumor* OR cerebellopontine angle tumour*
Filters were not used on ReFeR.
Science and Social Science Citation Indexes
Natural history
-
TI=(acoustic neuroma* or acoustic neurinoma* or AN or acoustic tumour* or acoustic tumor* or vestibular nerve tumour* or vestibular nerve tumor* or vestibular schwannoma or VS)
-
TI=(intracranial tumour* or intracranial tumor* or cerebellopontine angle lesion* or cerebellopontine angle tumour* or cerebellopontine angle tumor* or CPA)
-
1 or 2
-
TI=(natural history or prevalence or incidence or epidemiology)
-
3 and 4
The acoustic neuroma terms 1 and 2 were combined with the natural history term 4.
Clincial effectiveness and cost-effectiveness
-
TI=(magnetic resonance imag* or MRI or NMR or Antoni classification of computed tomography or CAT or electrocochleography or EcochG or auditory steady state response* or ASSR or electric response audiometry or ERA or auditory brainstem response* or ABR or brainstem evoked response* or BSER or BAER or otoacoustic emission* or OAE or DP OAE or OAE DP OAE or stapedial reflex or tone decay reflex or vestibular function or calorics)
-
TI=(acoustic neuroma* or acoustic neurinoma* or AN or acoustic tumour* or acoustic tumor* or vestibular nerve tumour* or vestibular nerve tumor* or vestibular schwannoma or VS)
-
TI=(intracranial tumour* or intracranial tumor* or cerebellopontine angle lesion* or cerebellopontine angle tumour* or cerebellopontine angle tumor* or CPA)
-
2 or 3
-
1 and 4
The acoustic neuroma terms 2 and 3 were combined with the diagnostic technique term 1.
TRIP
(acoustic neuroma OR acoustic neurinoma OR acoustic tumor OR acoustic tumour OR vestibular nerve tumor OR vestibular nerve tumour OR vestibular schwannoma OR intracranial tumor OR intracranial tumour OR cerebellopontine angle lesion OR cerebellopontine tumor OR cerebellopontine tumour OR acoustic lesion OR vestibular nerve lesion OR intracranial lesion) Title and text
Filters were not used on TRIP.
Appendix 2 Process of inclusion of selected studies
Figure 11.
Flow chart detailing the process of identification and selection of relevant papers: clinical effectiveness. AN, acoustic neuroma; CPA, cerebellopontine angle; VS, vestibular schwannoma.
Figure 12.
Flow chart detailing the process of identification and selection of relevant papers: cost-effectiveness.
Figure 13.
Flow chart detailing the process of identification and selection of relevant papers: natural history. AN, acoustic neuroma; CPA, cerebellopontine angle; VS, vestibular schwannoma.
Figure 14.
Flow chart detailing the process of identification and selection of relevant papers in the additional search covering October 2006–August 2008 in all three areas of the review.
Appendix 3 Quality assessment of included studies
For those studies for which there was one paper plus comments or letters we extracted data from the paper only and we have therefore only assessed the quality of the paper.
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Scorea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chandrasekhar et al., 199561 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | No | Unclear | Unclear | Yes | No | No | 8 |
| Cueva, 200469 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | 11 |
| El-Kashlan et al., 200029 | Yes | Yes | Yes | Unclear | No | Yes | Yes | Yes | No | Unclear | Unclear | Yes | Yes | Yes | 9 |
| Godey et al., 199863 | No | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | No | Unclear | Unclear | Unclear | Yes | Yes | 8 |
| Gordon, 199560 | No | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No | Yes | Unclear | Yes | Yes | 9 |
| Haapaniemi et al., 200064 | Unclear | No | Yes | Unclear | Yes | Yes | Yes | Yes | No | Unclear | Unclear | Unclear | Yes | Yes | 7 |
| Levine, 199157 | Yes | Yes | Yes | Unclear | Yes | No | Yes | No | No | Unclear | Unclear | Unclear | Yes | Yes | 7 |
| Marangos, 200166 | No | No | Yes | Unclear | Yes | No | Yes | Yes | No | Unclear | Unclear | No | Yes | Yes | 6 |
| Robinette et al., 200065 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | No | No | Unclear | Unclear | Yes | Yes | Yes | 9 |
| Rupa et al., 200367 | Yes | No | Yes | Unclear | Yes | Yes | Yes | Yes | No | Unclear | Unclear | Unclear | Yes | Yes | 8 |
| Schmidt et al., 200130 | Yes | No | Yes | Unclear | Yes | Yes | Yes | No | No | Unclear | Unclear | Yes | Yes | Yes | 8 |
| Weiss, 199056 | No | Yes | No | Unclear | Yes | Unclear | Yes | Yes | No | Yes | No | Unclear | Yes | Yes | 7 |
| Wilson et al., 199258 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | No | Unclear | No | Unclear | Yes | Yes | 9 |
| Zappia et al., 199762 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | No | No | Unclear | Unclear | Yes | Yes | Yes | 9 |
| Skinner et al., 200368 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | No | No | Unclear | No | Unclear | Yes | Yes | 8 |
| Selesnick and Jackler, 1993182 | No | Yes | Unclear | Unclear | Yes | No | Yes | No | No | Unclear | Unclear | Unclear | Yes | Yes | 5 |
| Totalsb | 10 | 12 | 14 | 1 | 15 | 12 | 16 | 8 | 0 | 2 | 2 | 6 | 15 | 15 |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Q11 | Q12 | Q13 | Q14 | Scorea |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allen et al., 199682 | Unclear | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 12 |
| Ben Salem, 200190 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | NA |
| Held et al., 199987 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes | 11 |
| Hermans et al., 199785 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 12 |
| Marx et al., 199988 | Unclear | No | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 11 |
| Naganawa, 199886 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 13 |
| Annesley-Williams, 200138 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | No | Unclear | Yes | Yes | 11 |
| Schmalbrock et al., 199989 | Unclear | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Unclear | Yes | Yes | 9 |
| Soulié et al., 199784 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Unclear | 10 |
| Stuckey et al., 199683 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | 11 |
| Zealley et al., 200044 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 12 |
| Totalsb | 7 | 4 | 10 | 6 | 10 | 10 | 10 | 10 | 10 | 7 | 4 | 5 | 10 | 9 |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Scorea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Welling et al., 1990114 | No | Yes | No | No | No | Yes | No | Yes | Yes | No | 4 |
| Robinette et al., 200065 | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | No | 6 |
| Robson et al., 199343 | Yes | Yes | No | No | No | Yes | No | No | Yes | Yes | 5 |
| Saeed et al., 199542 | Yes | No | Yes | No | No | Yes | No | No | Yes | No | 4 |
| Ravi and Wells, 199641 | Yes | Yes | No | No | No | Yes | No | No | No | No | 3 |
| Cheng et al., 2003115 | Yes | Yes | No | No | No | No | No | No | No | No | 2 |
| Carrier and Arriaga, 199713 | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | 7 |
| Rupa et al., 200367 | Yes | Yes | Yes | No | No | Yes | No | No | No | No | 4 |
| Daniels et al., 200097 | No | Yes | No | Yes | No | Yes | No | Yes | Yes | Yes | 6 |
| Allen et al., 199682 | Yes | Yes | Yes | No | No | Yes | No | No | No | No | 4 |
| Marx et al., 199988 | Yes | Yes | Yes | No | No | Yes | No | No | No | No | 4 |
| Tan, 1999117 | Yes | No | Yes | No | No | Yes | No | Yes | No | Yes | 5 |
| Totalsb | 10 | 10 | 7 | 1 | 0 | 11 | 1 | 4 | 6 | 4 |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Scorea |
|---|---|---|---|---|---|---|---|
| Propp et al., 2006133 | Yes | Yes | Yes | Yes | No | Yes | 5 |
| Evans et al., 2005134 | Yes | Yes | Yes | Yes | No | No | 4 |
| Kwan et al., 2004141 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Kosugi et al., 2004146 | Yes | Yes | Yes | No | Yes | No | 4 |
| Seedat et al., 2002140 | Yes | Yes | Yes | No | No | No | 3 |
| Dawes et al., 200034 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Anderson et al., 2000148 | Yes | Yes | Yes | Unclear | Yes | No | 4 |
| Urben et al., 1999142 | Yes | Yes | Yes | Yes | No | No | 4 |
| Saunders et al., 1995144 | Yes | Yes | Yes | Unclear | No | Yes | 4 |
| Tali et al., 1993136 | Yes | Yes | Yes | Unclear | Yes | No | 4 |
| Frohlich and Sutherland, 1993185 | Yes | Yes | Yes | Unclear | No | Yes | 4 |
| Fitzgerald and Mark, 1998147 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Verret et al., 2006143 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Daniels et al., 200097 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Carrier and Arriaga, 199713 | Yes | Yes | Yes | Unclear | Yes | No | 4 |
| Dawes and Basiouny, 199935 | Yes | Unclear | Yes | Unclear | Yes | No | 3 |
| Sheppard et al., 199636 | Yes | Yes | Yes | Yes | Yes | No | 5 |
| Lin et al., 200545b | Yes | No | Yes | No | Yes | No | 3 |
| Lin et al., 200545c | Yes | Yes | Yes | Yes | No | Yes | 5 |
| Tos et al., 2004132 | Yes | Yes | Yes | Yes | No | No | 4 |
| Moffat et al., 2004139 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Totalsd | 21 | 19 | 21 | 12 | 13 | 5 |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Scorea |
|---|---|---|---|---|---|---|---|
| Kentala and Pyykko, 2001160 | Yes | Yes | Yes | No | No | Yes | 4 |
| Sauvaget et al., 2005161 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Wandong et al., 2005162 | Yes | Yes | Yes | Yes | No | Yes | 5 |
| Lustig et al., 1998177 | Yes | Yes | Yes | No | Yes | Yes | 5 |
| Matthies and Samii, 1997163 | Yes | Yes | Yes | No | No | Yes | 4 |
| van Leeuwen et al., 1995164 | Yes | Yes | Yes | No | Yes | Yes | 5 |
| Frohlich and Sutherland, 1993185 | Yes | Yes | Yes | Unclear | No | Yes | 4 |
| Selesnick et al., 199359 | Yes | Yes | Yes | Yes | No | Yes | 5 |
| Selesnick and Jackler, 1993182 | Yes | Yes | Yes | Yes | No | Yes | 5 |
| Ogawa et al., 1991165 | Yes | Yes | Yes | Unclear | Yes | Yes | 5 |
| Ogawa et al., 1991166 | Yes | Yes | Yes | Unclear | Yes | Yes | 5 |
| Sai, 1990167 | Yes | Yes | Yes | Unclear | No | Yes | 4 |
| Diensthuber et al., 2006168 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Haapaniemi et al., 200064 | Yes | Yes | Yes | Unclear | Yes | Yes | 5 |
| Tschudi et al., 2000159 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Magdziarz et al., 2000174 | Yes | Yes | Yes | Yes | No | Yes | 5 |
| Moffat et al., 1994175 | Yes | Yes | Yes | Unclear | No | Yes | 4 |
| Saleh et al., 199617 | Yes | Yes | No | Yes | No | Yes | 4 |
| Fucci et al., 1999158 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Aslan et al., 1997169 | Yes | Yes | No | Yes | No | Yes | 4 |
| Berrettini et al., 1996170 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Are et al., 1995171 | Yes | Yes | Yes | Unclear | No | Yes | 4 |
| Moffat et al., 1993172 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Leonetti, 1995173 | No | Yes | Yes | Unclear | Yes | Yes | 4 |
| Tos et al., 1998152 | Yes | Yes | Yes | Yes | No | Yes | 5 |
| Artz et al., 2008178 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Day et al., 2008179 | Yes | Yes | Yes | Yes | Yes | Yes | 6 |
| Mackle et al., 2007181 | Yes | Yes | Yes | Yes | Unclear | Yes | 5 |
| Baguley et al., 2006180 | Yes | Yes | Yes | Yes | In part | Yes | 5–6 |
| Totalsb | 28 | 29 | 27 | 17 | 13–14 | 29 |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Scorea |
|---|---|---|---|---|---|---|---|---|---|
| Jorgensen and Pedersen, 1994198 | Yes | Yes | No | Yes | No | Yes | Yes | Yes | 6 |
| Bozorg Grayeli et al., 2005186 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Flint et al., 2005187 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | 6 |
| Piazza et al., 2003201 | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 6 |
| Glasscock et al., 1997202 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 7 |
| Strasnick et al., 1994203 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 7 |
| Bederson et al., 1991204 | Yes | Yes | Yes | Unclear | No | Yes | Yes | Yes | 6 |
| Valvassori and Shannon, 1991200 | Yes | Yes | Yes | Unclear | No | No | Yes | No | 4 |
| Herwadker et al., 2005205 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Caye-Thomasen et al., 2003206 | Yes | Yes | No | Unclear | Yes | Yes | Yes | No | 5 |
| Mohyuddin et al., 2003207 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Niemczyk et al., 2002208 | Yes | Yes | No | No | Yes | No | Yes | No | 4 |
| Vokurka et al., 2002209 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | 7 |
| Stipkovits et al., 2001197 | Yes | Yes | Yes | Unclear | Yes | No | Yes | Yes | 6 |
| Hoistad et al., 2001210 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 7 |
| Nutik and Babb, 2001211 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 7 |
| Sakamoto et al., 2001212 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Massick et al., 2000213 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 7 |
| O’Reilly et al., 2000214 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Shin et al., 2000215 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Tschudi et al., 2000159 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Fucci et al., 1999158 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Modugno et al., 1999189 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 7 |
| Niemczyk et al., 1999216 | Yes | Yes | No | Yes | Yes | No | Yes | No | 5 |
| Yamamoto et al., 1998217 | Yes | Yes | No | Yes | Yes | Yes | No | No | 5 |
| Levo et al., 1997218 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Deen et al., 1996219 | Yes | Yes | Yes | Unsure | No | No | Yes | Yes | 5 |
| Wiet et al., 1995220 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 7 |
| Martin et al., 1994190 | Yes | Yes | Yes | No | No | No | Yes | Yes | 5 |
| Rosenberg et al., 1993221 | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 6 |
| Ogawa et al., 1991199 | Yes | Yes | Yes | Unclear | No | Yes | Yes | Yes | 6 |
| Al Sanosi et al., 2006222 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 7 |
| Shin et al., 2003223 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | 6 |
| Moller et al., 2003224 | Yes | Yes | Yes | Unclear | No | No | Yes | Yes | 5 |
| Kishore et al., 2003225 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 7 |
| Ramsden et al., 2003226 | Yes | Yes | Yes | Unclear | No | No | Yes | Yes | 5 |
| Ferri et al., 2003227 | Yes | Yes | Yes | Unclear | Yes | No | Yes | Yes | 6 |
| Quaranta et al., 2003228 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Battaglia et al., 2006229 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| Stangerup et al., 2006122 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | 6 |
| Raut et al., 2004233 | Yes | Yes | Yes | Unclear | No | Yes | Yes | Yes | 6 |
| Mirz et al., 2000235 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | 7 |
| Rosenberg, 2000237 | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 6 |
| Smouha et al., 2005194 | Yes | Unclear | Yes | Unclear | Unclear | No | Yes | No | 3 |
| Quaranta et al., 2007192 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 7 |
| Solares and Panizza, 2008238 | Yes | Yes | Yes | Yes | Yes | No | No | Yes | 6 |
| Artz et al., 2008178 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | 7 |
| Hajioff et al., 2008191 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | 7 |
| Totalsb | 48 | 47 | 42 | 28 | 28 | 33 | 47 | 41 |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Scorea |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Smouha et al., 2005194 | Yes | Yes | No | No | Unclear | No | No | No | Yes | No | 3 |
| Yoshimoto, 200516 | Yes | Yes | No | No | Yes | Yes | Yes | Unclear | Yes | Unclear | 6 |
| Yamakami et al., 2003239 | Yes | Yes | No | No | No | Yes | Yes | No | Yes | Unclear | 5 |
| Selesnick and Johnson, 1998240 | Yes | Yes | No | No | Unclear | Yes | Unclear | Unclear | Yes | Unclear | 4 |
| Totalsb | 4 | 4 | 0 | 0 | 1 | 3 | 2 | 0 | 4 | 0 |
Appendix 4 Excluded studies and reasons for exclusion
Studies excluded from the clinical effectiveness theme
Bance ML, Hyde ML, Malizia K. Decision and cost analysis in acoustic neuroma diagnosis. J Otolaryngol 1994;23:109. [No clinical data.]
Bauch CD, Olsen WO, Pool AF. ABR indices: sensitivity, specificity, and tumor size. Am J Audiol 1996;5:97. [No comparative MR imaging data.]
Brackmann DE. The role of auditory brain stem response and magnetic resonance imaging in acoustic neuroma diagnosis: a critical appraisal – invited comments. Am J Otol 1997;18:682. [No comparative MR imaging data.]
Burkey JM, Rizer FM, Schuring AG, Fucci MJ, Lippy WH. Acoustic reflexes, auditory brainstem response, and MRI in the evaluation of acoustic neuromas. Laryngoscope 1996;106:839–41. [No comparative MR imaging data.]
Carrier DA, Arriaga M. Cost-effective evaluation of asymmetric sensorineural hearing loss with focused magnetic resonance imaging. Otolaryngol Head Neck Surg 1997;116:567. [No comparative MR imaging data.]
Charabi S, Lassen NA, Thomsen J, Tos M, Rossen K, Jacobsen GK. Thallium chloride 201Tl combined with single photon emission computed tomography (SPECT) in the evaluation of vestibular schwannoma growth. Acta Otolaryngol 1997;117:35. [No detection of acoustic neuroma.]
Charabi S, Klinken L, Mantoni M, Tos M, Thomsen J. Histology and neuro-imaging in cystic acoustic neuromas. Acta Otolaryngol 1993;113:519. [No comparative MR imaging data.]
Charabi S, Thomsen JC, Tos M, Charabi S, Thomsen JC, Tos M. [False-positive diagnostic findings in acoustic neurinomas]. [Danish]. Ugeskrift for Laeger 1991;154:19. [No comparative MR imaging data.]
Cross JJ, Baguley DM, Antoun NM, Moffat DA, Prevost AT. Reproducibility of volume measurements of vestibular schwannomas – a preliminary study. Clin Otolaryngol 2006;31:123. [No clinical data.]
Curtin HD. Rule out eighth nerve tumor: contrast-enhanced T1-weighted or high-resolution T2-weighted MR? Am J Neuroradiol 1997;18:1834. [No clinical data.]
Curtin HD, Hirsch WL, Jr. Imaging of acoustic neuromas. Otolaryngol Clin N Am 1992;25:553. [No clinical data.]
Czepko R, Libionka W, Urbanik A, Adamek D, Uhl H, Kwinta B, et al. Evaluation of the accuracy with which radiologists define intracranial tumor histology on CT and MRI. Pol J Radiol 2006;71:13. [Foreign language: Polish. No usable data in the abstract.]
Dal PG, Mascalchi M, Fonda C, Olmastroni M, Pandolfo C, Vannucchi P, et al. Gadolinium DTPA in the NMR study of acoustic neurinoma. Riv Neuroradiol 1992;5:161. [Foreign language: Italian. No usable data in the abstract.]
D’Ambrosio F, Benfari G. [Magnetic resonance with GD-DTPA in acoustic neuroma: personal experience] [Italian]. Acta Otorhinolaryngol Ital 1993;13:21. [Foreign language: Italian. No usable data in the abstract.]
Daniels RL, Shelton C, Harnsberger HR. Ultra high resolution nonenhanced fast spin echo magnetic resonance imaging: cost-effective screening for acoustic neuroma in patients with sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 1998;119:364. [No clinical data.]
Daniels RL, Swallow C, Shelton C, Davidson HC, Krejci CS, Harnsberger HR. Causes of unilateral sensorineural hearing loss screened by high- resolution fast spin echo magnetic resonance imaging: review of 1,070 consecutive cases. Am J Otol 2000;21:173. [No comparator to MR imaging.]
Dawes PJ, Basiouny H. Outcome of using magnetic resonance imaging as an initial screen to exclude vestibular schwannoma in patients presenting with unilateral tinnitus. J Laryngol Otol 1999;113:818. [No comparator to MR imaging.]
Dawes PJ, Jeannon JP. Audit of regional screening guidelines for vestibular schwannoma. J Laryngol Otol 1998;112:860. [No comparator to MR imaging.]
Dawes PJD. Vestibular schwannoma screening: closing the audit loop. J Laryngol Otol 2001;115:719. [No comparator to MR imaging.]
Dawes PJD, Mehta D, Arullendran P. Screening for vestibular schwannoma: magnetic resonance imaging findings and management. J Laryngol Otol 2000;114:584. [No comparator to MR imaging.]
Desaulty A, Lansiaux V, Moreau L, Vandorpe C, Desaulty A, Lansiaux V, et al. [Retrocochlear deafness, acoustic neurinoma and early evoked auditory potentials – apropos of a series of 113 patients] [French]. Acta Otorhinolaryngol Belg 1992;46:77. [No usable clinical data.]
Diensthuber M, Lenarz T, Stover T. Determination of the clinical growth index in unilateral vestibular schwannoma. Skull Base 2006;16:31. [No comparator to MR imaging.]
Dobie RA. Hearing threshold differences and risk of acoustic tumor [comment]. Otolaryngol Head Neck Surg 1992;107:493. [No usable clinical data.]
Doherty JK, Friedman RA. Controversies in building a management algorithm for vestibular schwannomas. Curr Opin Otolaryngol Head Neck Surg 2006;14:305. [No clinical data.]
Don M, Kwong B, Tanaka C, Brackmann D, Nelson R. The stacked ABR: a sensitive and specific screening tool for detecting small acoustic tumors. Audiol Neurootol 2005;10:274. [Unproven new technique.]
Don M. Auditory brainstem response testing in acoustic neuroma diagnosis. Curr Opin Otolaryngol Head Neck Surg 2002;10:376. [No comparator to MR imaging.]
Don M, Masuda A, Nelson R, Brackmann D. Successful detection of small acoustic tumors using the stacked derived-band auditory brain stem response amplitude. Am J Otol 1997;18:608. [No clinical data.]
Dornhoffer JL, Helms J, Hoehmann DH. Presentation and diagnosis of small acoustic tumors. Otolaryngol Head Neck Surg 1994;111:232. [No comparative MR imaging data.]
Dort JC. Screening for cerebellopontine angle tumours: conventional MRI vs T2 fast spin echo MRI. Can J Neurol Sci 2001;28:47–50. [Data incomplete.]
Doyle KJ. Is there still a role for auditory brainstem response audiometry in the diagnosis of acoustic neuroma? Arch Otolaryngol Head Neck Surg 1999;125:232. [No clinical data.]
El-Gammal T. MR techniques for the internal auditory canal [comment]. Am J Neuroradiol 1997;18:1393. [No acoustic neuromas.]
Esteve-Fraysse MJ, Vincent M, Rugui MG, Bounaix MJ, Fraysse B. [Value of auditory evoked potentials and electrocochleography in the diagnosis of small acoustic neuromas. Our experience on 131 cases] [French]. Ann Otolaryngol Chir Cervicofac 1993;110:203. [No comparative MR imaging data.]
Fiirgaard B, Pedersen CB, Lundorf E. [Size of acoustic neurinomas assessed by CT and magnetic resonance scanning] [Danish]. Ugeskrift for Laeger 1995;157:6855. [Foreign language: Danish. No usable data in the abstract.]
Fisher EW, Parikh AA, Harcourt JP, Wright A. The burden of screening for acoustic neuroma: asymmetric otological symptoms in the ENT clinic. Clin Otolaryngol Allied Sci 1994;19:19. [No clinical data.]
Fitzgerald DC, Mark AS. Sudden hearing loss: frequency of abnormal findings on contrast-enhanced MR studies. Am J Neuroradiol 1998;19:1433. [No comparator to MR imaging.]
Gilain L, Bouccara D, Jacquier I, Achouche J, Casteran JM, Freyss G, et al. Diagnostic strategy of acoustic neuroma. Evaluation of efficacy of auditory evoked potentials. Apropos of a series of 50 neuroma cases] [French]. Ann Otolaryngol Chir Cervicofac 1991;108:257. [No clinical data.]
Gosepath K, Maurer J, Mann W. Detection of intracellular acoustic neuromas by auditory evoked brainstem audiometry and other otoneurologic testmethods. Laryngo-Rhino-Otologie 1995;74:728. [Foreign language: German. No usable data in the abstract.]
Grabel JC, Zappulla RA, Ryder J, Wang WJ, Malis LI. Brain-stem auditory evoked responses in 56 patients with acoustic neurinoma. J Neurosurg 1991;74:749. [Unclear how many acoustic neuromas were confirmed by MR imaging or CT.]
Gstoettner W, Neuwirth-Riedl K, Swoboda H, Mostbeck W, Burian M. Specificity of auditory brainstem response audiometry criteria in acoustic neuroma screening as a function of deviations of reference values in patients with cochlear hearing loss. Eur Arch Otorhinolaryngol 1992;249:253. [No comparative MR imaging data.]
Hampton SM, Adler J, Atlas MD. Evaluating the role of magnetic resonance imaging scans in the surgical management of acoustic neuromas. Laryngoscope 2000;110:1194. [No comparator to MR imaging.]
Harner SG. Contemporary algorithm for acoustic neuroma: assessment and management. Curr Opin Otolaryngol Head Neck Surg 2001;9:290. [No clinical data.]
Hayashi M, Kubo O, Sato H, Taira T, Tajika Y, Izawa M, et al. Correlation between MR image characteristics and histological features of acoustic schwannoma. Noshuyo Byori 1996;13:139. [No comparator to MR imaging.]
Herwadker A, Vokurka EA, Evans DGR, Ramsden RT, Jackson A. Size and growth rate of sporadic vestibular schwannoma: predictive value of information available at presentation. Otol Neurotol 2005;26:86. [No comparator to MR imaging.]
Hohmann D, Dornhoffer JL. Clinical presentation and diagnosis of small acoustic neuromas. Laryngorhinootologie 1994;73:320. [Foreign language: German. No usable data in the abstract.]
Hoth S. [Changes in early auditory evoked potentials in acoustic neuroma] [German]. HNO 1991;39:343. [No comparative MR imaging data.]
Itasaka Y, Ishikawa K, Wong WH, Shibata Y. Application of vestibular evoked myogenic potential test in patients with acoustic neuroma. Equilib Res 2004;63:353. [No comparative MR imaging data.]
Jackler RK. Cost-effective screening for acoustic neuroma with unenhanced MR: a clinician’s perspective [comment]. Am J Neuroradiol 1996;17:1226. [No clinical data.]
Jeng CM, Huang JS, Lee WY, Wang YC, Kung CH, Lau MK. Magnetic resonance imaging of acoustic schwannomas. J Formosan Med Assoc 1995;94:487. [No comparator to MR imaging.]
Johansson R, Magnusson M, Fransson PA, Karlberg M. Discrimination of patients with acoustic neuroma and peripheral vestibular lesions with human posture dynamics. Acta Otolaryngol Suppl 1995;520:27. [No acoustic neuromas.]
Kanzaki J, Ogawa K, Ogawa S, Yamamoto M, Ikeda S, Uchi T. Audiological findings in acoustic neuroma. Acta Otolaryngol Suppl 1991:487:125–32. [No comparative MR imaging data.]
Katsura M, Oosato Y, Dotsu M, Yoshida H, Kumagami H, Takahashi H. Usefulness of magnetic resonance imaging (MRI) for patients with unilateral tinnitus. Practica Otologica Kyoto 2004;97:481. [No comparator to MR imaging.]
Kent DL, Haynor DR, Longstreth WT, Jr, Larson EB. The clinical efficacy of magnetic resonance imaging in neuroimaging. Ann Intern Med 1994;120:856. [No acoustic neuromas.]
Kosugi EM, Tangerina RP, Dib GC, Ramos HVL, Penido NO. Vestibular schwannoma presenting as sudden hearing loss. Rev Bras Otorrinolaringol 2004;70:795–9. [No comparator to MR imaging.]
Kovacsovics B, Davidsson L, Harder H, Magnuson B, Ledin T. MRI screening of the cerebellopontine angle and inner ear with fast spin-echo T2 technique. Arch Ital Biol 2000;138:87. [Foreign language: Italian. No usable data in the abstract.]
Kubo T, Sakashita T, Kusuki M, Kyunai K, Ueno K, Hikawa C, et al. Evaluation of radiological examination for sensorineural hearing loss. Acta Otolaryngol Suppl 2000;542:34–8. [No comparator to MR imaging.]
Kwan TL, Tang KW, Pak KK, Cheung JY, Cheung JYL. Screening for vestibular schwannoma by magnetic resonance imaging: analysis of 1821 patients. Hong Kong Med J 2004;10:38. [No comparator to MR imaging.]
Lajtman Z, Bartolek D, Manestar D, Krpan D, Kovacic J, Vincelj J. Auditory brain stem responses in patients with pontocerebellar angle tumors and severe sensorineural hearing loss. Neurol Croatica 2002;51:3. [No comparative MR imaging data.]
Lalwani AK. Preoperative differentiation between meningioma of the cerebellopontine angle and acoustic neuroma using MRI. Otolaryngol Head Neck Surg 1993;109:88–95. [No comparator to MR imaging.]
Lenarz T. The role of auditory brain stem response and magnetic resonance imaging in acoustic neuroma diagnosis: a critical appraisal – invited comments. Am J Otol 1997;18:683. [No comparator to MR imaging.]
Levine SC, Muckle RP, Anderson JH. Evaluation of patients with acoustic neuroma with dynamic posturography. Otolaryngol Head Neck Surg 1993;109:392. [No usable clinical data.]
Lhuillier FM, Doyon DL, Halimi PM, Sigal RC, Sterkers JM. Magnetic resonance imaging of acoustic neuromas: pitfalls and differential diagnosis. Neuroradiology 1992;34:144. [No comparator to MR imaging.]
Lightfoot GR. ABR screening for acoustic neuromata: the role of rate-induced latency shift measurements. Br J Audiol 1992;26:217. [No comparative MR imaging data.]
Litt AW, Mirsky P, Berson BD, Kricheff II. Screening protocol for MR imaging of the internal auditory canal. J Comput Assist Tomogr 1991;15:930. [Data too old.]
Long SA, Arriaga M, Nelson RA. Acoustic neuroma volume: MRI-based calculations and clinical implications. Laryngoscope 1993;103:1093. [No comparator to MR imaging.]
Lustig LR, Rifkin S, Jackler RK, Pitts LH. Acoustic neuromas presenting with normal or symmetrical hearing: factors associated with diagnosis and outcome. Am J Otol 1998;19:212. [No comparative MR imaging data.]
Magdziarz DD, Wiet RJ, Dinces EA, Adamiec LC. Normal audiologic presentations in patients with acoustic neuroma: an evaluation using strict audiologic parameters. Otolaryngol Head Neck Surg 2000;122:157. [No comparator to MR imaging.]
Mangham C. Hearing threshold difference between ears and risk of acoustic tumor. Otolaryngol Head Neck Surg 1991;105:814–7. [No usable clinical data.]
Mangham CA. Expert opinion on the diagnosis of acoustic tumors. Otolaryngol Head Neck Surg 1997;117:622. [No clinical data.]
Martinez RJ. Contribution of MR with gadolinium to the assessment of acoustic neurinoma. Radiologia (Madrid) 1991;33. [Foreign language: Spanish. No usable data in the abstract.]
Massick DD, Welling DB, Dodson EE, Scholfield M, Nagaraja HN, Schmalbrock P, et al. Tumor growth and audiometric change in vestibular schwannomas managed conservatively. Laryngoscope 2000;110:1843. [No comparator to MR imaging.]
Matsumoto K, Niitsu M, Yoshioka H, Tanaka Y, Anno I, Kuramoto K, et al. MRI of acoustic neurinoma. Jap J Clin Radiol 1994;39:451. [Foreign language: Japanese. No usable data in the abstract.]
Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery 1997;40:1–9. [No comparator to MR imaging.]
Maurer J, Beck A, Mann Mintert WR. Changes of amplitude of otoacoustic emissions under contralateral noise in normal hearing persons and in patients with unilateral acoustic neuroma. Laryngorhinootologie 1992;71:69. [No comparative MR imaging data.]
Mausolf A, Laubert A. [Neuro-otologic criteria in the diagnosis of tumor-induced hearing disorders. Studies of 300 patients with acoustic neuroma] [German]. HNO 1990;38:50. [No comparative MR imaging data.]
Mirza S. Incidental findings on magnetic resonance imaging screening for cerebellopontine angle tumours. J Laryngol Otol 2000;114:750–4. [No usable clinical data.]
Monsell EM, Cody DD, Spickler E, Windham JP. Segmentation of acoustic neuromas with magnetic resonance imaging and Eigen image filtering. Am J Otol 1997;18:602. [No usable clinical data.]
Moretz WH, Jr, Orchik DJ, Shea JJ, Jr, Emmett JR. Low-frequency harmonic acceleration in the evaluation of patients with intracanalicular and cerebellopontine angle tumors. Otolaryngol Head Neck Surg 1986;95:324. [No comparative MR imaging data.]
Mulkens TH, Parizel PM, De Schepper AM, van de Heyning PH, Forton GE, Martin JJ, et al. MRI of acoustic schwannoma: a retrospective study of 89 tumours. Rofo 1993;158:362. [Foreign language: German. No usable data in the abstract.]
Murofushi T, Matsuzaki M, Mizuno M. Vestibular evoked myogenic potentials in patients with acoustic neuromas. Arch Otolaryngol Head Neck Surg 1998;124:509. [No comparative MR imaging data.]
Murphy MR, Selesnick SH. Cost-effective diagnosis of acoustic neuromas: a philosophical, macroeconomic, and technological decision. Otolaryngol Head Neck Surg 2002;127:253. [No clinical data.]
Musiek FE, McCormick CA, Hurley RM. Hit and false-alarm rates of selected ABR indices in differentiating cochlear disorders from acoustic tumors. Am J Audiol 1996;5:90. [No comparative MR imaging data.]
Naganawa S, Itoh T, Fukatsu H, Ishigaki T, Nakashima T, Kassai Y, et al. Three-dimensional fast spin-echo MR of the inner ear: ultra-long echo train length and half-Fourier technique. Am J Neuroradiol 1998;19:739. [No usable clinical data.]
Nakamura T, Naganawa S, Fukatsu H, Sakurai Y, Aoki I, Ninomiya A, et al. Contrast enhancement of the cochlear aqueduct in MR imaging: its frequency and clinical significance. Neuroradiology 2003;45:626. [No usable clinical data.]
Neary WJ, Newton VE, Laoide-Kemp SN, Ramsden RT, Hillier VF, Kan SW. A clinical, genetic and audiological study of patients and families with unilateral vestibular schwannomas. II. Audiological findings in 93 patients with unilateral vestibular schwannomas. J Laryngol Otol 1996;110:1120. [No usable clinical data.]
Negrevergne M, Ribeiro S, Moraes CLO, Maunsell R, Morata GC, Darrouzet V. Videonystagmography and vibratory test in the diagnosis bilan of vestibular schwannoma. Report of 100 cases. Rev Laryngol Otol Rhinol 2003;124:91. [No comparative MR imaging data.]
Nguyen HD, Simonson TM, Fisher DJ, Mayr NA, Tali ET, Gao F, et al. MR evaluation of acoustic schwannoma with fractional contrast doses. J Comput Assist Tomogr 1995;19:23. [No usable clinical data.]
Nishizawa S, Yokoyama T, Uemura K. Magnetic resonance cisternography using the fast spin echo method for the evaluation of vestibular schwannoma. Neurol Med Chir 1999;39:282. [No comparator to MR imaging.]
Obholzer RJ, Harcourt JP. Magnetic resonance imaging screening for vestibular schwannoma: analysis of published protocols. J Laryngol Otol 2004;118:329. [No usable clinical data.]
O’Connell ME. The combined role of ABR, vestibular function and blink reflex tests in the detection of VIII nerve and brainstem lesions. Aust J Otolaryngol 1992;1:23. [No usable clinical data.]
Odabasi AO, Telischi FF, Gomez-Marin O, Stagner B, Martin G. Effect of acoustic tumor extension into the internal auditory canal on distortion-product otoacoustic emissions. Ann Otol Rhinol Laryngol 2002;111:912. [No comparative MR imaging data.]
Oeken J. Detection of cochlear function in patients with acoustic neuromas by means of distortion-product otoacoustic emissions. HNO 1996;44:677. [No comparative MR imaging data.]
Okubo T, Yoshioka N, Hayashi N, Abe O, Masumoto T, Sasaki T, et al. Contrast-enhanced magnetic resonance imaging of the cranial nerves in patients with acoustic schwannoma: correlation with surgical findings. Acta Otolaryngol Suppl 2000;542:13–17. [No usable clinical data.]
O’Leary DP, Davis LL, Maceri DR. Vestibular autorotation test asymmetry analysis of acoustic neuromas. Otolaryngol Head Neck Surg 1991;104:103. [No comparative MR imaging data.]
Park CH, Kim SM, Zhang J, McEwan JR, Intenzo C. Tc-99m MIBI brain SPECT of an acoustic schwannoma. Clin Nucl Med 1994;19:152. [No usable clinical data.]
Patko T, Vidal PP, Vibert NH, Huy PT, de-Waele C. Vestibular evoked myogenic potentials in patients suffering from a unilateral acoustic neuroma: a study of 170 patients. Clin Neurophysiol 2003;114:1344–50. [No usable clinical data.]
Plaza G, Lopez LJ, Aparicio JM, Herraiz C, Mate MA, Toledano A, et al. [Magnetic resonance: first choice test in the screening of internal auditory canal and cerebellopontine angle tumors] [Spanish]. Acta Otorrinolaringol Esp 2001;52:651. [No comparator to MR imaging.]
Portier F, Herman P, Lot G, Tran Ba Huy P. [Contribution and cost-effectiveness of ABR and MRI in acoustic neuroma screening. Retrospective study of 151 cases] [French]. Ann Otolaryngol Chir Cervicofac 2002;119:67. [No useable clinical data.]
Prosser S, Arslan E, Turrini M, Rosignoli M. Cochlear and neural dysfunction in acoustic neuroma: can they be separately revealed by auditory brain-stem wave V latency? Scand Audiol 1992;21:195. [No comparative MR imaging data.]
Quaranta A, Zini C, Gandolfi A, Piazza F, De Thomasis G, Frisina A, et al. [Acoustic neuroma: clinical-functional finding, results and surgical complication] [Italian]. Acta Otorhinolaryngol Ital 2001;21:1. [No comparative MR imaging data.]
Rostkowska-Nadolska B, Sterkers JM, Pospiech L. [Auditory evoked potentials from brain stem determined in acoustic nerve neurinomas] [Polish]. Neurol Neurochir Pol 1999;33:43. [Foreign language: Polish. No usable data in the abstract.]
Saeed S, Woolford T, Ramsden R, Lye R. Magnetic resonance imaging: a cost-effective first line investigation in the detection of vestibular schwannomas. Br J Neurosurg 1995;9:497–503. [No usable clinical data.]
Sakamoto H, Nakai Y, Matsuda M, Ohashi Y, Tsuyuguchi N, Kawabe J, et al. Positron emission tomographic imaging of acoustic neuromas. Acta Otolaryngol Suppl 2000;542:18. [No detection of acoustic neuroma.]
Saleh EA, Aristegui M, Naguib MB, Cokesser Y, Landolfi M, Sanna M. Normal hearing in acoustic neuroma patients: a critical evaluation. Am J Otol 1996;17:127. [Unclear how many acoustic neuromas were confirmed by MR imaging or CT.]
Saunders JE, Luxford WM, Devgan KK, Fetterman BL. Sudden hearing loss in acoustic neuroma patients. Otolaryngol Head Neck Surg 1995;113:23–31. [No usable clinical data.]
Scherler M, Bohmer A. The value of clinical examination methods in diagnosis of acoustic neuroma. HNO 1995;43:487–91. [Duplicate.]
Schick B, Brors D, Koch O, Schafers M, Kahle G. Magnetic resonance imaging in patients with sudden hearing loss, tinnitus and vertigo. Otol Neurotol 2001;22:808. [No usable clinical data.]
Schlauch RS, Levine S, Li Y, Haines S. Evaluating hearing threshold differences between ears as a screen for acoustic neuroma. J Speech Hear Res 1995;38:1168–75. [No comparative MR imaging data.]
Selesnick SH, Rebol J, Heier LA, Wise JB, Gutin PH, Lavyne MH. Internal auditory canal involvement of acoustic neuromas: surgical correlates to magnetic resonance imaging findings. Otol Neurotol 2001;22:912. [Not about detection of acoustic neuroma.]
Shelton C. Fast spin echo magnetic resonance imaging: clinical application in screening for acoustic neuroma. Otolaryngol Head Neck Surg 1996;114:71. [Duplicate.]
Slattery WH, III, Fisher LM, Yoon G, Sorensen G, Lev M. Magnetic resonance imaging scanner reliability for measuring changes in vestibular schwannoma size. Otol Neurotol 2003;24:666. [No acoustic neuromas.]
Smilauer T, Kluh J, Zverina E, Betka J. Contribution of BERA to the diagnosis of neurinomas of the acoustic nerve. Otorinolaryngol Foniatr 2001;50:99. [No comparative MR imaging data.]
Smith IM, Turnbull LW, Sellar RJ, Murray JA, Best JJ. A modified screening protocol for the diagnosis of acoustic neuromas. Clin Otolaryngol Allied Sci 1990;15:167. [No comparative MR imaging data.]
Soda T. Auditory brainstem responses and the diagnosis of acoustic and cerebellopontine angle tumors. Otologia Fukuoka 2002;30. [No comparative MR imaging data.]
Soto SC, Garcia JJ, Farina Conde JL, Tedin Garcia SL. [New protocol for the early diagnosis of acoustic schwannoma in the outpatient clinic] [Spanish]. Acta Otorrinolaringol Esp 1995;46:353. [Foreign language: Spanish. No usable data in the abstract.]
Swan IR, Swan IRC, Gatehouse S. Costs of investigative protocols for cerebellopontine angle lesions in Scotland. Health Bull 1991;49:329. [No comparative MR imaging data.]
Swan IRC, Gatehouse S. Clinical and financial audit of diagnostic protocols for lesions of the cerebellopontine angle. Br Med J 1991;302:701. [No comparative MR imaging data.]
Takeichi N, Sakamoto T, Fukuda S, Inuyama Y. Vestibular evoked myogenic potential (VEMP) in patients with acoustic neuromas. Auris Nasus Larynx 2001;28:S39. [No comparative MR imaging data.]
Tan TY. Non-contrast high resolution fast spin echo magnetic resonance imaging of acoustic schwannoma. Singapore Med J 1999;40:27. [Not all patients had gold standard.]
Tanaka H. Usefulness of auditory brainstem responses at high stimulus rates in the diagnosis of acoustic neuroma. ORL (Basel) 1996;58:224–8. [No comparative MR imaging data.]
Telischi FF, Lo WW, Arriaga MA, De la Cruz A. Lateral extent of internal auditory canal involvement by acoustic neuromas: a surgical-radiologic correlation. Am J Otol 1993;14:446. [No comparator to MR imaging.]
Tsuchiya K, Aoki C, Hachiya J. Evaluation of MR cisternography of the cerebellopontine angle using a balanced fast-field-echo sequence: preliminary findings. Eur Radiol 2004;14:239. [No gold standard.]
Urben SL, Benninger MS, Gibbens ND. Asymmetric sensorineural hearing loss in a community-based population. Otolaryngol Head Neck Surg 1999;120:809. [No comparator to MR imaging.]
Verret DJ, Adelson RT, Defatta RJ. Asymmetric sensorineural hearing loss evaluation with T2 FSE-MRI in a public hospital. Acta Otolaryngol 2006;126:705. [No acoustic neuromas.]
Veysel PA, Akan H, Yazici B, Ozturk A, Belet U, Koyuncu M. The accuracy of computed tomography in internal acoustic canal tumors. Ondokuz Mayis Universitesi Tip Dergisi 2001;18:265. [Foreign language: Turkish. No usable data in the abstract.]
Wareing MJ, Baguley D, Moffat DA. Investigations for vestibular schwannoma. CME Bull Otorhinolaryngol Head Neck Surg 2002;6:12. [No clinical data.]
Weber PC. Appropriateness of magnetic resonance imaging in sudden sensorineural hearing loss. Otolaryngol Head Neck Surg 1997;116:153–6. [No comparative MR imaging data.]
Wilson DF. A critical appraisal of the role of auditory brain stem response and magnetic resonance imaging in acoustic neuroma diagnosis. Am J Otol 1997;18:673–85. [No clinical data.]
Wu X, Wu H, Li Z, Lu C. [The study of MRI examination of acoustic neuromas] [Chinese]. Lin Chuang Erh Pi Yen Hou Ko Tsa Chih J Clin Otorhinolaryngol 2002;16:340. [Foreign language: Chinese. No usable data in the abstract.]
Zymon-Zagorska A, Kwiek S, Baron J, Gibinska J, Bazowski P, Pieta M, et al. Efficacy of CT in diagnostics of cerebellopontine angle region. Pol J Radiol 2004;69:76. [No comparative MR imaging data.]
Studies excluded from the costs theme
Annesley-Williams DJ. Magnetic resonance imaging in the investigation of sensorineural hearing loss: is contrast enhancement still necessary? J Laryngol Otol 2001;115:14–21. [Does not contain cost data.]
Arriaga M, Gorum M, Kennedy A. Clinical pathways in acoustic tumor management. Laryngoscope 1997;107:602–6. [Does not relate to diagnosis.]
Avruch L. The real cost of MRI in Canada [comment]. CMAJ 1993;149:1772–3. [Does not contain any useable data.]
Brackmann D. Current status of ABR audiometry in acoustic neuroma diagnosis. Arch Otolaryngol Head Neck Surg 1999;125:235. [Does not contain any useable data.]
Brackmann DE. The role of auditory brain stem response and magnetic resonance imaging in acoustic neuroma diagnosis: a critical appraisal – invited comments. Am J Otol 1997;18:682. [Does not contain any useable data.]
Byrne E. MRI as a single screening-procedure for acoustic neuroma. J R Soc Med 1994;87:307. [Does not contain any useable data.]
Chandrasekhar SS, Brackmann DE, Devgan KK. Auditory brainstem response audiometry and acoustic neuromas – reply. Am J Otol 1997;18:128. [Does not contain any useable data.]
Cueva RA. Auditory brainstem response versus magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope 2004;114:1686. [Does not contain cost data.]
Dawes PJD, Jeannon JP. Audit of regional screening guidelines for vestibular schwannoma. J Laryngol Otol 1998;112:860–4. [Does not contain cost data.]
Dawes PJD, Mehta D, Arullendran P. Screening for vestibular schwannoma: magnetic resonance imaging findings and management. J Laryngol Otol 2000;114:584–8. [Does not contain cost data.]
Dawes PJD. Vestibular schwannoma screening: closing the audit loop. J Laryngol Otol 2001;115:719–22. [Does not contain cost data.]
Dobie RA. The role of auditory brain stem response and magnetic resonance imaging in acoustic neuroma diagnosis: a critical appraisal – invited comments. Am J Otol 1997;18:684–5. [Does not contain cost data.]
Don M, Kwong B, Tanaka C, Brackmann D, Nelson R. The stacked ABR: a sensitive and specific screening tool for detecting small acoustic tumors. Audiol Neurootol 2005;10:274. [Does not contain cost data.]
Don M. The detection of small acoustic tumors: the stacked derived-band ABR procedure – reply. Am J Otol 2000;21:149–51. [Does not contain any useable data.]
Donnelly MJ, Cass A, Ryan L. False positive diagnosis of small intracanalicular vestibular schwannomas. J Laryngol Otol 1994;108:986–8. [Does not contain cost data.]
Doyle K. Is there still a role for auditory brainstem response audiometry in the diagnosis of acoustic neuroma. Arch Otolaryngol Head Neck Surg 1999;125:232–4. [Does not contain any useable data.]
El-Kashlan H, Eisenmann D, Kileny P. Auditory brain stem response in small acoustic neuromas. Ear Hear 2000;21:257–62. [Does not contain cost data.]
Erdem A. False-positive findings on magnetic resonance imaging mimicking vestibular schwannoma: case illustration. J Neurosurg 2000;92:733. [Does not contain cost data.]
Fitzgerald DC, Mark AS, Fitzgerald DC, Mark AS. Cost-effective screening for acoustic neuroma [comment]. Otolaryngol Head Neck Surg 1999;121:846. [Does not contain any useable data.]
Gillespie JE. MRI screening for acoustic neuroma [comment]. Br J Radiol 2000;73:1129. [Does not contain any useable data.]
Godey B, Morandi X, Beust L, Brassier G, Bourdiniere J. Sensitivity of auditory brainstem response in acoustic neuroma screening. Acta Otolaryngol 1998;118:501. [Does not contain cost data.]
Hermans R, Van der Goten G, De Foer B, Baert A. MRI screening for acoustic neuroma without gadolinium: value of 3DFT-CISS sequence. Neuroradiology 1997;39:593. [Does not contain cost data.]
Jackler RK. Cost-effective screening for acoustic neuroma with unenhanced MR: a clinician’s perspective. Am J Neuroradiol 1996;17:1226–8. [Does not contain any useable data.]
Kevanishvili Z. The detection of small acoustic tumors: the stacked derived-band ABR procedure. Am J Otol 2000;21:148–9. [Does not contain any useable data.]
Konrad HR. There is a role for ABR audiometry in the diagnosis of acoustic neuroma. Arch Otolaryngol Head Neck Surg 1999;125:234–5. [Does not contain any useable data.]
Lenarz T. The role of auditory brain stem response and magnetic resonance imaging in acoustic neuroma diagnosis: a critical appraisal – invited comments. Am J Otol 1997;18:683–4. [Does not contain any useable data.]
Maeta M, Saito R, Nameki H. False-positive magnetic resonance image in the diagnosis of small acoustic neuroma. J Laryngol Otol 2001;115:842–4. [Does not contain cost data.]
Mangham CA, Mangham CA. Acoustic neuroma: cost-effective approach [comment]. Otolaryngol Head Neck Surg 1992;106:315–16. [Does not contain any useable data.]
Mangham CA. Expert opinion on the diagnosis of acoustic tumors. Otolaryngol Head Neck Surg 1997;117:622–7. [Does not contain any useable data.]
Moffat DA. Early diagnosis and surgical management of acoustic neuroma: is it cost effective? J R Soc Med 1989;2:329–32. [Does not contain cost data.]
Moffat D, Hardy D, Baguley D. Strategy and benefits of acoustic neuroma searching. J Laryngol Otol 1989;103:51–9. [Does not contain cost data.]
Murphy MR, Selesnick SH. Cost-effective diagnosis of acoustic neuromas: a philosophical, macroeconomic, and technological decision. Otolaryngol Head Neck Surg 2002;127:253–9. [Does not contain any useable data.]
Nishizawa S. Magnetic resonance cisternography using the fast spin echo method for the evaluation of vestibular schwannoma. Neurol Med Chir 1999;39:282–6. [Does not contain cost data.]
O’Donoghue GM. The role of auditory brain stem response and magnetic resonance imaging in acoustic neuroma diagnosis: a critical appraisal – invited comments. Am J Otol 1997;18:682–3. [Does not contain cost data.]
Parker S, Davis K. Limitations of computed tomography in the investigation of acoustic neuromas. Ann Otol Rhinol Laryngol 1977;86:436–40. [Does not include MR imaging data.]
Raber E, Dort J, Sevick R, Winkelaar R. Asymmetric hearing loss: toward a cost-effective diagnosis. J Otolaryngol 1997;26:88–91. [Does not contain cost data.]
Schmidt RJ, Sataloff RT, Newman J, Spiegel JR, Myers DL. The sensitivity of auditory brainstem response testing for the diagnosis of acoustic neuromas. Arch Otolaryngol Head Neck Surg 2001;127:19–22. [Does not contain cost data.]
Schwartz MS, Slattery WH, Oppenheimer M, Fisher LM, Schwartz MS, Slattery WH, et al. CSNS Young Neurosurgeon Award: acoustic neuroma surgical cost and outcome by hospital volume. Clin Neurosurg 2005;52:359–65. [Does not relate to diagnosis.]
Shelton C. Fast spin echo magnetic resonance imaging: clinical application in screening for acoustic neuroma. Otolaryngol Head Neck Surg 1996;114:71. [Duplicate.]
Slattery WH, Schwartz MS, Fisher LM, Oppenheimer M, Slattery W, Schwartz M, et al. Acoustic neuroma surgical cost and outcome by hospital volume in California. Otolaryngol Head Neck Surg 2004;130:726–35. [Does not relate to diagnosis.]
Telian SA. Management of the small acoustic neuroma: a decision analysis. Am J Otol 1994;15:358–64. [Does not relate to diagnosis.]
Wilson D, Talbot J, Mills L. A critical appraisal of the role of auditory brainstem response and magnetic resonance imaging in acoustic neuroma diagnosis. Am J Otol 1997;18:673–81. [Does not contain cost data.]
Zappia JJ, Oconnor CA, Wiet RJ, Dinces EA. Rethinking the use of auditory brainstem response in acoustic neuroma screening. Laryngoscope 1997;107:1388. [Does not contain cost data.]
Zealley IA, Cooper RC, Clifford KM, Campbell RS, Potterton AJ, Zammit-Maempel I, et al. MRI screening for acoustic neuroma: a comparison of fast spin echo and contrast enhanced imaging in 1233 patients [see comment]. Br J Radiol 2000;73:242–7. [Does not contain cost data.]
Zealley IA, Cooper RC, Clifford KM, Campbell RS, Potterton AJ, Zammit-Maempel I, et al. MRI screening for acoustic neuroma – author’s reply. Br J Radiol 2000;73:1129. [Does not contain cost data.]
Studies excluded from the natural history theme
Anand VT, Kerr AG, Byrnes DP, Smyth GDL. Non-surgical management of acoustic neuromas. Clin Otolaryngol 1992;17:406–10. [No relevant data.]
Arriaga MA, Gorum M, Kennedy A. Clinical pathways in acoustic tumor management. Laryngoscope 1997;107:602–6. [Management pathway data only.]
Arriaga MA, Major MC, Long S, Nelson R. Clinical correlates of acoustic neuroma volume. Am J Otol 1993;14:465–8. [Surgical data only.]
Baguley DM. Tinnitus and vestibular schwannoma. Semin Hearing 2001;22:77–87. [Review paper, no original data.]
Belal A, Rahman NA, Hashem N. Acoustic tumors in developing countries. Am J Otol 1984;5:360–4. [Paper pre-1990.]
Benech F, Perez R, Maria Fontanella M, Morra B, Albera R, Ducati A. Cystic versus solid vestibular schwannomas: a series of 80 grade III–IV patients. Neurosurg Rev 2005;28:209–13. [No relevant data.]
Berrettini S, Ravecca F, Russo F, Bruschini P, Sellari-Franceschini S. Some uncharacteristic clinical signs and symptoms of acoustic neuroma. J Otolaryngol 1997;26:97–103. [Audio/vestibular test data only.]
Caldera SRM, Pearson CR. Risk management of asymmetrical hearing impairment in an armed forces population. J Laryngol Otol 2000;114:345–9. [No relevant data.]
Chaimoff M, Nageris BI, Sulkes J, Spitzer T, Kalmanowitz M. Sudden hearing loss as a presenting symptom of acoustic neuroma. Am J Otolaryngol 1999;20:157–60. [No relevant data.]
Chandler CL, Ramsden RT. Acoustic schwannoma. Br J Hosp Med 1993;49:335–43. [Review paper, no original data.]
Charabi S, Engel P, Charabi B, Jacobsen GK, Overgaard J, Thomsen J, et al. Growth of vestibular schwannomas: in situ model employing the monoclonal antibody Ki-67 and DNA flow cytometry. Am J Otol 1996;17:301–6. [Histology only.]
Charabi S, Thomsen J, Tos M, Charabi B, Mantoni M, Borgesen SE. Acoustic neuroma/vestibular schwannoma growth: past, present and future. Acta Otolaryngol 1998;118:327–32. [Data repeated elsewhere.]
Clark WC, Moretz WH, Acker JD, Gardner LG, Eggers F, Robertson JH. Nonsurgical management of small and intracanalicular acoustic tumors. Neurosurgery 1985;16:801–3. [Paper pre-1990.]
Duvoisin B, Fernandes J, Doyon D, Denys A, Sterkers J-M, Bobin S. Magnetic resonance findings in 92 acoustic neuromas. Eur J Radiol 1991;13:96–102. [Data pre-1990.]
Eldridge R, Parry DM. Summary of the vestibular schwannoma consensus development conference, National Institutes of Health, Bethesda, Maryland, December 11–13, 1991. Otolaryngol Clin N Am 1992;25:729–35. [Review paper, no original data.]
Ferber-Viart C, Colleaux B, Laoust L, Dubreuil C, Duclaux R. Is the presence of transient evoked otoacoustic emissions in ears with acoustic neuroma significant? Laryngoscope 1998;108:605–9. [Audio/vestibular test data only.]
Fisher EW, Parikh AA, Harcourt JP, Wright A. The burden of screening for acoustic neuroma: asymmetric otological symptoms in the ENT clinic. Clin Otolaryngol 1994;19:19–21. [No relevant data.]
Forton GEJ, Cremers CWRJ, Offeciers FE. Acoustic neuroma ingrowth in the cochlear nerve: does it influence the clinical presentation? Ann Otol Rhinol Laryngol 2004;113:582–6. [No relevant data.]
Gardner G, Moretz WH, Robertson JH, Clark C, Shea JJ. Nonsurgical management of small and intracanalicular acoustic tumors. Otolaryngol Head Neck Surg 1986;94:328–33. [Paper pre-1990.]
Gomez-Brouchet A. Vestibular schwannomas: correlations between magnetic resonance imaging and histopathologic appearance. Otol Neurotol 2001;22:79–86. [Mainly histopathological.]
Greene S, David F, McCarthy B, Kupelian V, Surawicz T. What is the risk of vestibular schwannoma in the US population? Am J Epidemiol 2000;151:S30. [Data repeated elsewhere.]
Hahn A, Fundova P, Schneider D. Audiovestibular findings prior to and after acoustic neuroma surgery. Int Tinnitus J 2000;6:67–9. [Audio/vestibular test data only.]
Harcourt JP, Vijaya-Sekaran S, Loney E, Lennox P. The incidence of symptoms consistent with cerebellopontine angle lesions in a general ENT out-patient clinic. J Laryngol Otol 1999;113:518–22. [No relevant data.]
Hashimoto S, Furukawa K, Sasaki T. The natural history of vestibular schwannomas. Proceedings of the Fourth International Conference on Vestibular Schwannoma and other CPA Lesions. Cambridge, UK, 13–17 July 2003. p. 136. [No relevant data.]
Kale SU, Morgan DW, Cruickshank G. Wait and rescan policy for patients with acoustic neuroma. Proceedings of the Fourth International Conference on Vestibular Schwannoma and other CPA Lesions. Cambridge, UK, 13–17 July 2003. pp. 237–8. [No relevant data.]
Kanzaki J. Present state of early neurotological diagnosis of acoustic neuroma. Otorhinolaryngology 1986;48:193–8. [Paper pre-1990.]
Kanzaki J, Ogawa K, Ogawa S, Yamamoto M, Ikeda S, O-Uchi T. Audiological findings in acoustic neuroma. Acta Otolaryngol Suppl 1991;487:125–32. [Audio/vestibular test data only.]
Karjalainen S, Nuutinen J, Neittaanmaki H, Naukkarinen A, Asikainen R. The incidence of acoustic neuroma in autopsy material. Arch Otorhinolaryngol 1984;240:91–3. [Paper pre-1990.]
Laasonen EM, Troupp H. Volume growth rate of acoustic neurinomas. Neuroradiology 1986;28:203–7. [Paper pre-1990.]
Lanser MJ, Sussman SA, Frazer K. Epidemiology, pathogenesis, and genetics of acoustic tumors. Otolaryngol Clin N Am 1992;25:499–520. [Review paper, no original data.]
MacAndie C, Crowther JA. Quality of life in patients with vestibular schwannomas managed conservatively. Clin Otolaryngol 2004;29:215–18. [Quality of life only.]
Matthies C, Samii M, Krebs S. Management of vestibular schwannomas (acoustic neuromas): radiological features in 202 cases – their value for diagnosis and their predictive importance. Neurosurgery 1997;40:469–82. [No relevant data.]
McLeod B, Upfold L. The incidence of surgery for acoustic neuroma in Australia, 1993/1994 to 2003/2004. Aust J Otolaryngol 2005;8:5–8. [Surgical data only.]
Meyer TA, Canty PA, Wilkinson EP, Hansen MR, Rubinstein JT, Gantz BJ. Small acoustic neuromas: surgical outcomes versus observation or radiation. Otol Neurotol 2006;27:380–92. [No relevant data.]
Mirza S, Malik TH, Ahmed A, Willatt DJ, Hughes DG. Incidental findings on magnetic resonance imaging screening for cerebellopontine angle tumours. J Laryngol Otol 2000;114:750–4. [Not detection of acoustic neuroma.]
Nageris BI, Popovtzer A. Acoustic neuroma in patients with completely resolved sudden hearing loss. Ann Otol Rhinol Laryngol 2003;112:395–7. [Audio/vestibular test data only.]
Neary WJ, Newton VE, Laoide-Kemp SN, Ramsden RT, Hillier VF, Kan SW. A clinical, genetic and audiological study of patients and families with unilateral vestibular schwannomas. II. Audiological findings in 93 patients with unilateral vestibular schwannomas. J Laryngol Otol 1996;110:1120–8. [Audio/vestibular test data only.]
Nedzelski JM, Canter RJ, Kassel EE, Rowed DW, Tator CH. Is no treatment good treatment in the management of acoustic neuromas in the elderly? Laryngoscope 1986;96:825–9. [Paper pre-1990.]
Nedzelski JM, Schessel DA, Pfleiderer A, Kassel EE, Rowed DW. Conservative management of acoustic neuromas. Otolaryngol Clin N Am 1992;25:691–705. [No relevant data (mainly old data).]
Nestor JJ, Korol HW, Nutik SL, Smith R. The incidence of acoustic neuromas. Arch Otolaryngol Head Neck Surg 1988;114:680. [Paper pre-1990.]
Noguchi Y, Komatsuzaki A, Nishida H. Cochlear microphonic potentials in patients with vestibular schwannomas. Otorhinolaryngology 1998;60:283–90. [Audio/vestibular test data only.]
Noguchi Y, Komatsuzaki A, Nishida H. Electrocochleographic study in patients with vestibular schwannomas and U-shaped audiograms. Audiology 2000;39:19–23. [Audio/vestibular test data only.]
Norman M, Thornton ARD, Phillips AJ, Slaven A. Otoacoustic emissions recorded at high rates in patients with confirmed acoustic neuromas. Am J Otol 1996;17:763–72. [Audio/vestibular test data only.]
O’Connell ME. The combined role of ABR, vestibular function and blink reflex tests in the detection of VIII nerve and brainstem lesions. Aust J Otolaryngol 1992;1:23–9. [Audio/vestibular test data only.]
Okada Y, Takahashi M, Saito A, Kanzaki J. Electronystagmographic findings in 147 patients with acoustic neuroma. Acta Otolaryngol Suppl 1991;487:150–6. [Old data only (≤ 1990).]
O’Reilly BF, Kishore A, Crowther JA, Smith C. Correlation of growth factor receptor expression with clinical growth in vestibular schwannomas. Otol Neurotol 2004;25:791–6. [Histology only.]
O-Uchi T, Kanzaki J, Ogata A, Inoue T, Mashino H, Yoshihara S, et al. Pathophysiology of hearing impairment in acoustic neuroma with profound deafness: analysis by evoked otoacoustic emission and promontory stimulation test. Acta Otolaryngol Suppl 1994;514:95–100. [Audio/vestibular test data only.]
Piazza F, Frisina A, Gandolfi A, Quaranta N, Zini C. Management of acoustic neuromas in the elderly: retrospective study. Ear Nose Throat J 2003;82:374–8. [Surgical management.]
Ramamurthi B. The continuing challenge of acoustic neurinomas (1949–1993). Br J Neurosurg 1995;9:361–6. [No relevant data.]
Sarazin L, Jolivet O, Doyon D. Computerized evaluation of tumor volume with MRI. Applications to the surveillance of acoustic neurinoma. J Radiol 1993;74:455–60. [No relevant data.]
Schneider J. Tumors of the central nervous system in biopsy and autopsy material. 7th communication: neurinomas and neurofibromatoses with central nervous system involvement. Zentrallblatt fuer Allgemeine Pathologie und Pathologische Anatomie 1983;127:5–6. [Paper pre-1990.]
Selesnick SH, Deora M, Droitman MB, Heier LA. Incidental discovery of acoustic neuromas. Otolaryngol Head Neck Surg 1999;120:815–18. [No relevant data.]
Silverstein H, McDaniel A, Norrell H, Wazen J. Conservative management of acoustic neuroma in the elderly patient. Laryngoscope 1985;95:766–70. [Paper pre-1990.]
Simpson RHW, Sparrow OC, Duffield MS. Cerebellopontine angle tumours in black South Africans – how rare are acoustic schwannomas? S Afr Med J 1990;78:11–14. [Old data only (≤ 1990).]
Sterkers J-M, Desgeorges M, Sterkers O, Grob R. Acoustic neuromas in the elderly patient. Rev Laryngol Otol Rhinol 1989;110:55–7. [Paper pre-1990.]
Stipkovits EM, Graamans K, Van Dijk JE. Vestibular schwannoma: negative growth and audiovestibular features. Eur Arch Otorhinolaryngol 2001;258:467–71. [No relevant data.]
Stipkovits EM, van Dijk JE, Graamans K. Electronystagmographic changes in patients with unilateral vestibular schwannomas in relation to tumor progression and central compensation. Eur Arch Otorhinolaryngol 1999;256:173–6. [Audio/vestibular test data only.]
Thomason JE-M, Smyth V, Murdoch BE. Acoustic neuroma and non-tumour retrocochlear patients: audiological features. Scand Audiol 1993;22:19–23. [Audio/vestibular test data only.]
Thomsen J, Tos M. Acoustic neuromas. Diagnostic delay, growth rate and possible non-surgical treatment. Acta Otolaryngol Suppl 1988;452:26–33. [Paper pre-1990.]
Thomsen J, Tos M. Acoustic neuroma: clinical aspects, audiovestibular assessment, diagnostic delay, and growth rate. Am J Otolaryngol 1990;11:12–19. [Old data only (≤ 1990).]
Tos M, Charabi S, Thomsen J. Increase of diagnosed vestibular schwannoma in Denmark. Acta Otolaryngol Suppl 1997;529:53–5. [Data repeated elsewhere.]
Tos M, Thomsen J. Epidemiology of acoustic neuromas. J Laryngol Otol 1984;98:685–92. [Paper pre-1990.]
Tos M, Thomsen J. Management of acoustic neuromas. A review. Acta Otolaryngol 1991;111:616–32. [Review paper, no original data.]
Tos M, Thomsen J, Charabi S. Incidence of acoustic neuromas. Ear Nose Throat J 1992;71:391–3. [Old data only (≤ 1990).]
Valvassori GE, Guzman M. Growth rate of acoustic neuromas. Am J Otol 1989;10:174–6. [Paper pre-1990.]
van Dijk JE. Acoustic neuroma: deterioration of speech discrimination related to thresholds in pure-tone audiometry. Acta Otolaryngol 2000;120:627–32. [Audio/vestibular test data only.]
Vokurka EA, Herwadker A, Thacker NA, Ramsden RT, Jackson A. Using Bayesian tissue classification to improve the accuracy of vestibular schwannoma volume and growth measurement. Am J Neuroradiol 2002;23:459–67. [No relevant data.]
Warrick P, Bance M, Rutka J. The risk of hearing loss in nongrowing, conservatively managed acoustic neuromas. Am J Otol 1999;20:758–62. [Audio/vestibular test data only.]
Yanagihara N, Asai M. Sudden hearing loss induced by acoustic neuroma: significance of small tumors. Laryngoscope 1993;103:308–11. [Data incomplete.]
Yasumoto Y, Sato K, Sakata E. A retrospective clinical analysis of acoustic neurinomas: hearing disturbances and postoperative hearing preservation. Jap J Neurosurg 1995;4:376–82. [Surgical data only.]
Studies excluded from the additional search covering October 2006–August 2008 in all areas of interest
Arts HA, Telian SA, El-Kashlan H, Thompson BG. Hearing preservation and facial nerve outcomes in vestibular schwannoma surgery: results using the middle cranial fossa approach. Otol Neurotol 2006;27:234–41. [No relevant data.]
Bird PA, MacFarlane MR. Management of unilateral vestibular schwannoma/acoustic neuroma. N Z Med J 2007;120:1265. [No data.]
Bonneville F, Savatovsky, J, Chiras J. Imaging of cerebeloopontine angle lesions: an update. Part 2: intra-axial lesions, skull base lesions that may invade the CPA region, and non-enhancing extra-axial lesions. Eur Radiol 2007;17:2908–20. [No relevant data.]
Bozorg Grayeli AB, Fond C, Kalamarides M, Bouccara D, Cazals-Hatem D, Cyna-Gorse F, et al. Diagnosis and management of intracochlear schwannomas. Otol Neurotol 2007;28:951–7. [No relevant data.]
Bozorg Grayeli A, Refass A, Smail M, Elgarem H, Kalamarides M, Bouccara D, et al. Diagnostic value of auditory brainstem responses in cerebellopontine angle tumours. Acta Otolaryngol 2008;128:1096–1100. [No relevant data.]
Caye-Thomasen P, Dethloff T, Hansen S, Stangerup SE, Thomsen J. Hearing in patients with intracanalicular vestibular schwannomas. Audiol Neurotol 2007;12:1–12. [No new data.]
Edwards CG, Schwartzbaum JA, Lönn S, Ahlbom A, Feychting M. Exposure to loud noise and risk of acoustic neuroma. Am J Epidemiol 2005;163:327–33. [No relevant data.]
Forssén UM, Lönn S, Ahlbom A, Savitz DA, Feychting M. Occupational magnetic field exposure and the risk of acoustic neuroma. Am J Ind Med 2006;49:112–18. [No relevant data.]
Humphriss RL, Baguley DM, Axon PR, Moffat DA. Preoperative audiovestibular handicap in patients with vestibular schwannoma. Skull base: an interdiscplinary approach. Skull Base 2006;16:193–9. [No relevant data.]
Luppino FS, Grooters E, de Bruïne FT, Zwinderman AH, van der May AGL. Volumetrical measurements in vestibular schwannoma, the influence of slice thickness and patient’s repositioning. Otol Neurotol 2006;27:962–8. [No relevant data.]
Mahrous AK, Kalepu R. Positive findings on MRI in patients with assymmetrical SNHL. Eur Arch Otorhinolaryngol 2008;265:1471–5. [No relevant data.]
Myrseth E, Pedersen P-H, Møller P, Lund-Johansen M. Treatment of vestibular schwannomas. Why, when and how? Acta Neurochir (Wien) 2007;149:647–60. [No relevant data.]
Nouraei SAR, Huys QJM, Chatrath P, Powles J, Harcourt JP. Screening patients with sensorineural hearing loss for vestibular schwannoma using a Bayesian classifier. Clin Otolaryngol 2007;32:248–54. [No comparator to MR imaging.]
Penido N de O, Tangerina RP, Kosugi EM, de Abreu CEC, Vasco MB. Vestibular schwannoma: spontaneous tumor involution. Rev Bras Otorrinolaringol 2007;73:867–71. [No relevant data.]
Prasad J, Cousins VC. Asymmetrical hearing loss. Aust Fam Physician 2008;37:312–20. [No data.]
Roche PH, Robitail S, Régis J. Two- and three dimensional measures of vestibular schwannomas and posterior fossa – implications for the treatment. Acta Neurochir (Wien) 2007;149:267–73. [No relevant data.]
Sahu RN, Behari S, Agarwal VK, Giri PJ, Jain VK. Taste dysfunction in vestibular schwannomas. Neurol India 2008;56:42–6. [No relevant data.]
Snelling JD, Krywawych M, Majithia A, Harcourt JP. The compliance, true positive and false negatives of the Charing Cross protocol for magnetic resonance imaging screening for cerebellopontine angle lesions. J Laryngol Otol 2008;122:255–8. [No comparator to MR imaging.]
Stangerup S-E, Caye-Thomasen P, Thomasen MT. Change in hearing during ‘wait and scan’ management of patients with vestibular schwannoma. J Laryngol Otol 2008;122:673–81. [No new data.]
Tringal S, Dubreuil C, Zaouche S, Feber-Viart C. Are stage IV schwannomas preoperatively different from other stages? Otol Neurotol 2007;29:46–9. [No relevant data.]
Appendix 5 2 × 2 Results tables
Tables for selected auditory brainstem response (ABR) versus magnetic resonance (MR) imaging papers
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 182 | 0 | 182 |
| Negative | 15 | 0 | 15 |
| Total | 197 | 0 | 197 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 35 | 0 | 35 |
| Negative | 2 | 0 | 2 |
| Total | 37 | 0 | 37 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 77 | 0 | 77 |
| Negative | 44 | 0 | 44 |
| Total | 121 | 0 | 121 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 106 | 0 | 106 |
| Negative | 5 | 0 | 5 |
| Total | 111 | 0 | 111 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 34 | 0 | 34 |
| Negative | 6 | 0 | 6 |
| Total | 40 | 0 | 40 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 52 | 0 | 52 |
| Negative | 6 | 0 | 6 |
| Total | 58 | 0 | 58 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 69 | 9 | 78 |
| Negative | 6 | 66 | 72 |
| Total | 75 | 75 | 150 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 4 | 26 | 30 |
| Negative | 0 | 42 | 42 |
| Total | 4 | 68 | 72 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 88 | 0 | 88 |
| Negative | 50 | 0 | 50 |
| Total | 138 | 0 | 138 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 27 | 0 | 27 |
| Negative | 3 | 0 | 3 |
| Total | 30 | 0 | 30 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 37 | 0 | 37 |
| Negative | 1 | 0 | 1 |
| Total | 38 | 0 | 38 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 92 | 0 | 92 |
| Negative | 13 | 0 | 13 |
| Total | 105 | 0 | 105 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 23 | 0 | 23 |
| Negative | 2 | 0 | 2 |
| Total | 25 | 0 | 25 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 82 | 0 | 82 |
| Negative | 7 | 0 | 7 |
| Total | 89 | 0 | 89 |
| Index test: ABR | Reference test: MR imaging | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 17 | 58 | 75 |
| Negative | 7 | 210 | 217 |
| Total | 24 | 268 | 292 |
Tables for selected MR imaging versus MR imaging papers
| Index test: 2D FSE | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 98 | 1 | 99 |
| Negative | 2 | 299 | 301 |
| Total | 100 | 300 | 400 |
| Index test: 3D CISS | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 35 | 9 | 44 |
| Negative | 1 | 205 | 206 |
| Total | 36 | 214 | 250 |
| Index test: 2D FSE | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 24 (1 NF2) | 6 | 30 |
| Negative | 0 | 80 | 80 |
| Total | 24 | 86 | 110 |
| Index test: 3D CISS | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 16 | 9 | 25 |
| Negative | 2 | 56 | 58 |
| Total | 18 | 65 | 83 |
| Index test: 3D CISS | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 67 | 18 | 85 |
| Negative | 5 | 574 | 579 |
| Total | 72 | 592 | 664 |
| Index test: 3D FSE | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 19 | 2 | 21 |
| Negative | 0 | 389 | 389 |
| Total | 19 | 391 | 410 |
Held et al., 199887
No 2 × 2 table.
All patients known to have acoustic neuroma. Study compared characterisation of tumour with different sequences rather than detection.
| Index test: 2D FSE | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 11 (1 NF2) | 0 | 11 |
| Negative | 0 | 39 | 39 |
| Total | 11 | 39 | 50 |
Schmalbrock et al., 199989
No 2 × 2 table.
All patients known to have acoustic neuroma. Study compared characterisation of tumour with different sequences rather than detection.
| Index test: 2D FSE | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 64 | 114 | 178 |
| Negative | 2 | 2286 | 2288 |
| Total | 66 | 2400 | 2466 |
| Index test: 2D FSE | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 66 | 946 | 1012 |
| Negative | 0 | 1454 | 1454 |
| Total | 66 | 2400 | 2466 |
| Index test: 2D FSE | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 24 | 53 | 77 |
| Negative | 8 | 255 | 263 |
| Total | 32 | 308 | 340 |
| Index test: 3D FSE | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 4 | 16 | 20 |
| Negative | 1 | 152 | 153 |
| Total | 5 | 168 | 173 |
| Index test: 2D TGSE | Reference test: GdT1W | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 19 | 15 | 34 |
| Negative | 0 | 346 | 346 |
| Total | 19 | 361 | 380 |
List of abbreviations
- ABR
- auditory brainstem response
- AN
- acoustic neuroma
- ASHI
- asymmetric sensorineural hearing impairment
- bFFE
- balanced fast field echo
- CASP
- Critical Appraisal Skills Programme
- CI
- confidence interval
- CISS
- constructive interference in the steady state
- CPA
- cerebellopontine angle
- CSF
- cerebrospinal fluid
- CT
- computerised tomography
- dBHL
- decibel hearing level
- DPOAE
- distortion product otoacoustic emissions
- ENG
- electronystagmography
- ENT
- ear, nose and throat
- ETL
- echo train length
- FIESTA
- fast imaging employing steady-state acquisition
- FSE
- fast spin echo (MR imaging)
- GdT1W
- gadolinium-enhanced T1 weighted
- GE
- gradient echo
- IAC
- internal auditory canal
- IAM
- internal auditory meatus
- ILDV
- interaural difference of wave V
- IT5
- interaural difference of wave V
- MPR
- multiplanar reformats
- MR
- magnetic resonance
- MRI
- magnetic resonance imaging
- NF2
- neurofibromatosis type 2
- OAE
- otoacoustic emissions
- QUADAS
- quality assessment of diagnostic accuracy studies
- SHI
- sudden hearing impairment
- SNHI
- sensorineural hearing impairment
- T1W
- T1 weighted
- T2W
- T2 weighted
- T2*W
- T2 star weighted
- TEOAE
- transient otoacoustic emissions
- true FISP
- true free induction with steady-state precession
- TSE
- turbo spin echo
- VS
- vestibular schwannoma
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS) or it has been used only once or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson, EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
Health Technology Assessment Programme
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NCCHTA
-
Dr Andrew Cook, Consultant Advisor, NCCHTA
-
Dr Peter Davidson, Director of Science Support, NCCHTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NCCHTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NCCHTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NCCHTA
HTA Commissioning Board
-
Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programmes, Research and Development Directorate, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA Programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington