Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 02/09/04. The contractual start date was in November 2003. The draft report began editorial review in September 2007 and was accepted for publication in March 2009. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Disease condition
Bell’s palsy is an acute unilateral paralysis of the facial nerve first described by the Scottish surgeon Sir Charles Bell (1774–1842). 1 Its cause is unknown but animal studies have suggested the possibility that reactivation of herpes viruses may be responsible for demyelination. 2,3 It affects 11–40 people per 100,000 in the population per annum, most commonly in the age group 30–45. 4 The condition presents disproportionately among pregnant women and people who have diabetes, influenza, a cold or some other upper respiratory ailment. On average every year a general practitioner will see one or two patients who have developed the condition. A recent UK study using the General Practice Research Database (GPRD) showed that 36% of patients were treated with oral steroids and 19% were referred to hospital. 5 Although most patients recover well, 30% have a poor recovery with continuing facial disfigurement, psychological difficulties and sometimes facial pain (though the presence and course of pain is unclear from current knowledge). 6 In the absence of an established aetiology, treatment continues to be based upon the established pathophysiology: swelling and entrapment of the nerve.
Two recent Cochrane reviews concerning the treatment of Bell’s palsy have examined the effectiveness of oral prednisolone and aciclovir. 7,8 These found that insufficient data exist to conclude that either or both therapies are effective. Many of the studies included in the reviews either failed to randomise patients or, when correctly randomised, were erroneously interpreted in a favourable light. 9,10 In addition, high-dose steroid therapy has numerous potential side effects including peptic ulceration, hypertension and confusional states. Antiviral therapy is expensive and should be reserved for circumstances where definite benefits are likely to be obtained. Current recommendations suggest that aciclovir needs to be started within 48 hours, though more recent studies of viral replication in patients with Bell’s palsy suggest that this might be extended. 11
Provenance of the Scottish Bell’s Palsy Study
Given this lack of evidence the UK National Institute for Health Research (NIHR) Health Technology Assessment programme commissioned an independent academic group to conduct a randomised clinical trial to determine whether prednisolone or aciclovir, used separately or in combination and used early in the course of Bell’s palsy, improved the chances of recovery at 3 and 9 months.
With this defined as the primary research question, the protocol for the Scottish Bell’s Palsy Study was developed and submitted and is provided as Appendix 1. A small number of variations to the protocol are summarised where they arise.
Governance
We established three committees for the oversight of the study – a Trial Steering Committee (TSC: constitution and personnel listed in Appendix 2); a Data Monitoring and Ethics Committee (DMEC: constitution and personnel listed in Appendix 3); and a Management Committee for the day-to-day monitoring of the progress of the trial, comprising the nine Principal Investigators, Trial Co-ordinator and three Researchers (personnel listed in Appendix 4).
The lead host organisation and the sponsor of the study was the University of Dundee.
Associate host organisations (trial centres) were the Universities of Glasgow, Edinburgh and Aberdeen.
Approvals
In common with many researchers and triallists setting up studies during 2003–4 raising questions in the clinical arena and requiring the participation of patient groups, we found the processes for obtaining approvals demanding and time-consuming in a way that they had not been previously. With other research teams we were invited to summarise our experience for an investigative commission headed by Professor Adrian Grant on behalf of Scotland’s Chief Scientist Office (CSO), and did so as outlined in Appendix 5. We recognise that our experience was not untypical of that of other researchers at the time, but we include our report to the CSO and this account of it because of the depth of feeling that was then commonly reported by researchers.
Ethical approval for the study was provided by the lead research ethics committee, Multicentre Research Ethics Committee (MREC) Scotland (Edinburgh) reference MREC 03/0/74, and by Local Research Ethics Committees (LRECs) where patients were referred to local sites, or where the study recruited patients. Research and Development (R&D) approval was provided by local R&D offices likewise. The Clinical Trials Authority to use prednisolone, aciclovir and lactose placebo was provided by the Medicines and Healthcare Products Regulatory Agency (MHRA), references MF8000/13139 and 13140 respectively. The study was registered with Current Controlled Trials reference ISRCTN 71548196 under the title Bell’s palsy: Early acicLovir and/or prednisoLone in Scotland (‘BELLS’) and from 07/08/2007 its registered status is ‘Completed’.
Study calendar
The study calendar is shown in Table 1. The total duration of the study was 44 months of which 25 months were dedicated to patient recruitment.
| Dates | Duration | Activity |
|---|---|---|
| Nov 2003–May 2004 | 7 months | Approvals, staff recruitment and training |
| Jun 2004–Jun 2006 | 25 months | Recruitment of patients |
| Jul 2006–Mar 2007 | 9 months | Follow-up of patients |
| Apr 2007–Jun 2007 | 3 months | Analysis of results |
The calendar represents an amendment to the original timetable, being an 8-month extension to the study overall, which had comprised 3 months for approvals, 18 months for patient recruitment, 9 months for patient follow-up and 6 months for analysis (i.e. 36 months altogether).
Submission of a paper describing the results of the study to an appropriate journal, and of a draft final report to the funder were scheduled to take place as soon as possible following completion of the analysis of results.
Chapter 2 Methods
Referrers
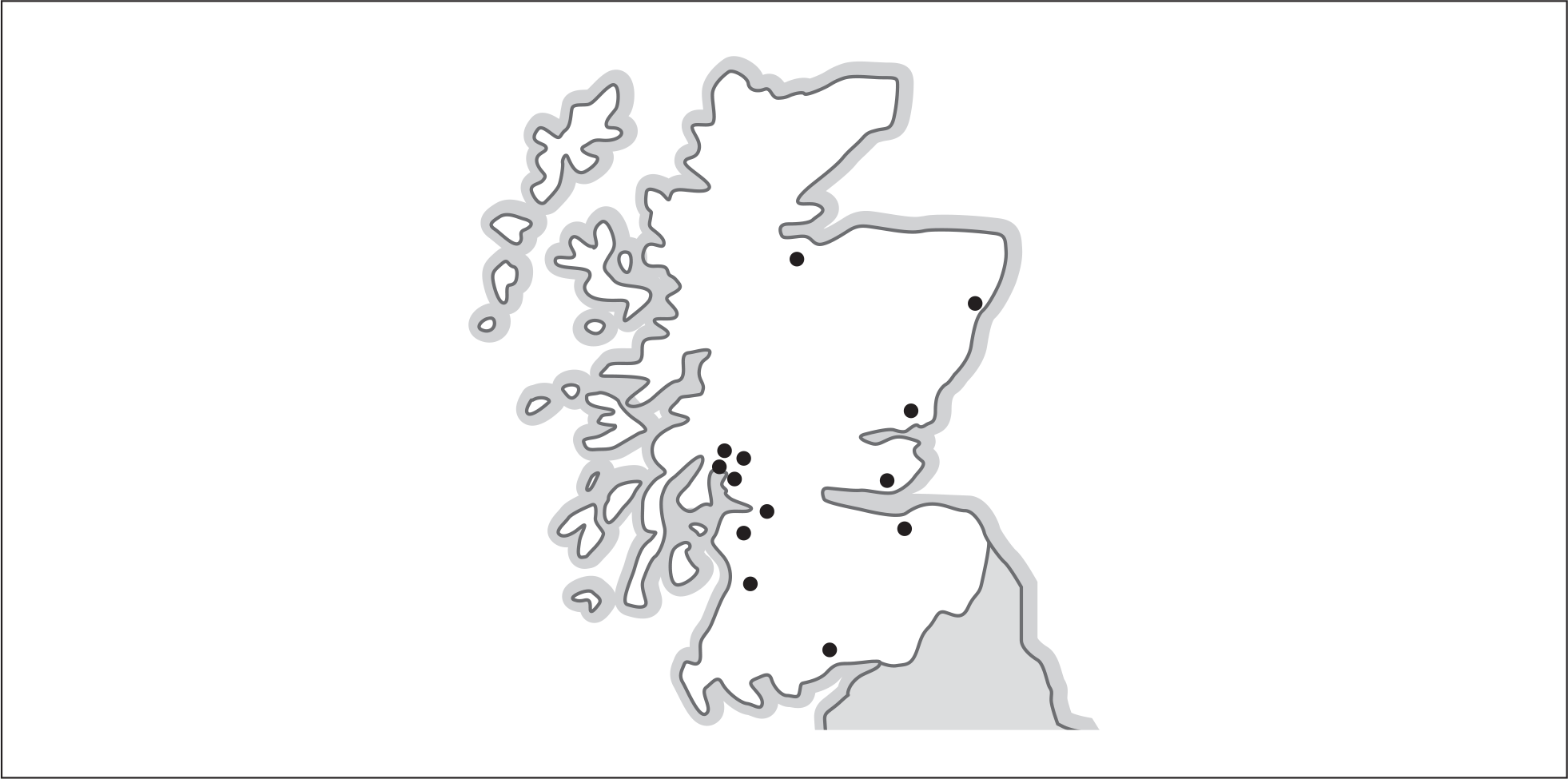
Patients identified in primary medical and dental care or accident and emergency (A&E) departments and those who approached NHS24 (a 24-hour medical advice line in Scotland similar to NHS Direct in England and Wales, which also co-ordinates all general practice out-of-hours consultations) with an appropriate description of symptoms, were asked to attend 1 of 17 hospital sites where trial arrangements were in place. The geographical coverage of the BELLS study is shown in Figure 1. The contributing sites are listed in Table 2.
FIGURE 1.
Map of hospital sites contributing to the BELLS study.
| Raigmore Hospital, Inverness |
| Aberdeen Royal Infirmary |
| Ninewells Hospital, Dundee (two sites: ward and clinic) |
| Perth Royal Infirmary |
| Victoria Hospital, Kirkcaldy |
| Royal Infirmary, Edinburgh |
| Western General Hospital, Edinburgh |
| St John’s Hospital, Livingston |
| Borders General Hospital, Melrose |
| Crosshouse Hospital, Kilmarnock |
| Royal Alexandra Hospital, Paisley (two sites: ward and annex) |
| Monklands Hospital, Airdrie |
| Gartnavel General Hospital, Glasgow |
| Victoria Infirmary, Glasgow |
| Stobhill Hospital, Glasgow |
| Glasgow Royal Infirmary |
| Southern General Hospital, Glasgow |
We recognised that not all of these patients would be notified to the study or recruited to it. It was important therefore to pilot notification of the condition prior to running the study to determine if general practitioners considered the condition to be of sufficiently significant importance to become involved in a trial, and what proportion of patients would be recruited.
In order to test this we piloted a notification process in one region of Scotland. With the co-operation of the local research networks in Tayside and Fife we asked general practitioners to notify us of all patients presenting with Bell’s palsy over a period of 1 month. As a result of this exercise we determined that we would be able to recruit one-third of those presenting within 48 hours of diagnosis. Of those, we assumed that two-thirds would remain in the trial for review at 9 months. In order therefore to recruit and retain the 480 patients necessary to detect a 12% difference in treatment effect from Scottish recruitment, we needed to recruit continuously for 25 months.
As it was unlikely that individual general practitioners or A&E doctors would be involved more than once in the trial it was essential that their role should be clearly delineated and relatively simple to carry out, and that instruction should be available relatively easily. The involvement of recruiting doctors was restricted to diagnosis followed by determination of the patients’ interest in participating, exclusion of ineligible patients, and a telephone referral to the on-call otolaryngology specialist. The trial process actually constituted a reduction in clinical workload for most general pratitioners, who would normally undertake follow-up of patients without input from hospital colleagues. 12 The trial also offered immediate access to specialist assessment, which would not be provided under normal care. Both of these attributes were found to be very attractive to general practitioners and patients during the planning phase of the trial.
Doctors need to be reminded regularly of an ongoing trial of a condition that occurs relatively sporadically. 13 In addition, there is a high turnover among staff in A&E departments and training grades in general practice and otolaryngology. A variety of strategies publicising the trial were set in motion.
Mailshots
The responsibility for keeping doctors informed about the on-going trial was taken on by SPPIRe14 (Scottish Professionals and Practices Interested in Research, since renamed SPCRN, the Scottish Primary Care Research Network, to fit the SPCRN model). All general practitioners in the four participating regions of Scotland were sent a mailshot outlining the trial and explaining how to take part. We emphasised the importance of the condition and the simplicity of involvement. The mailshots were in colour and designed to be attractive; further, based on evidence from the literature, we highlighted the benefits to patients15 and remuneration to general practitioners16 for taking part, and letters were signed by well-known local general practitioner ‘champions’. 17 Separate mailshots went out to non-principal doctors and registrars.
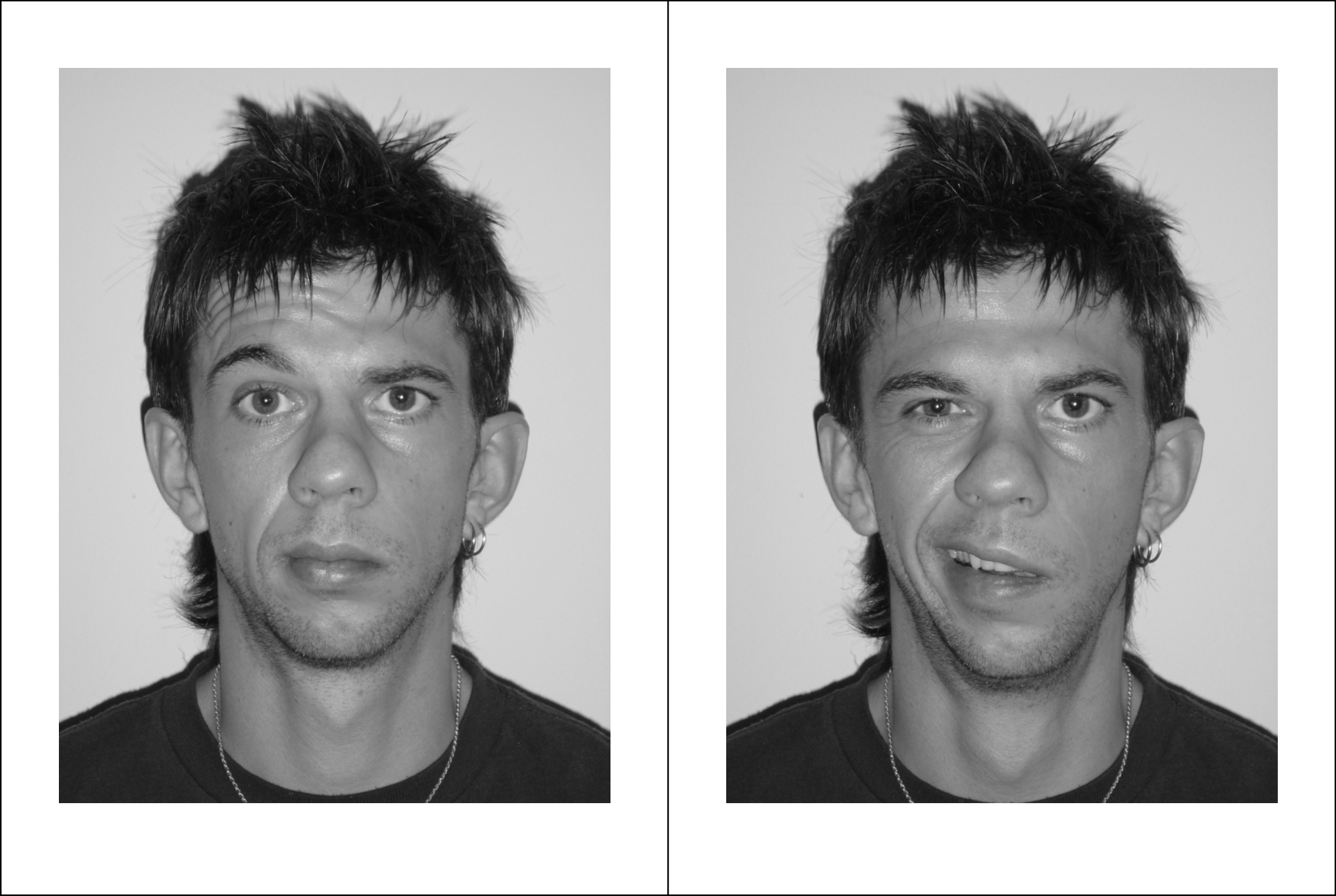
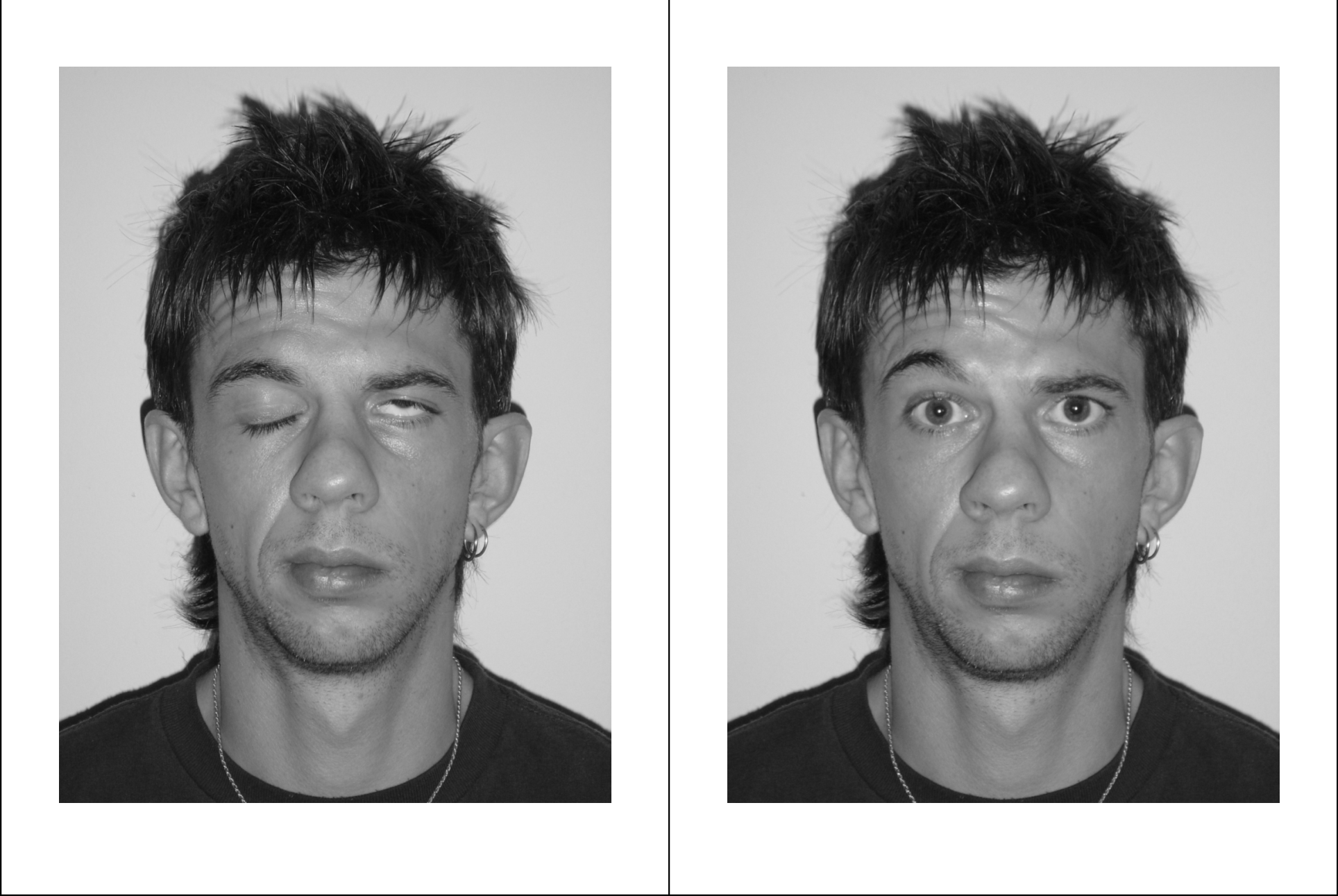
The trial was also highlighted in Local Medical Committee briefings to general practitioners throughout the country. We estimate that each quarterly mailshot took about a day of researcher time in each of the four participating regions. A&E departments were kept informed by literature and posters from the centre; similarly, general practice co-operatives were informed through literature and posters distributed with SPPIRe’s help, while NHS24 was in direct contact with the study centre. We found that the most attention-grabbing poster was one showing photographs of a patient at onset (see Figure 2). Every mailshot included the project’s web address and telephone contact details.
FIGURE 2.
Posed portrait photographs (at rest, smiling, eyes tight shut, eyebrows raised). Note: This patient was graded HB5 by the panel of assessors.


Project website
The project website18 had a simple web address, was clear and easy to navigate, with instructions on how to take part in the trial, and was regularly updated at its Stop Press page19 with information on the progress of the trial. The site was easily found with simple Google terms.
Media
In order to heighten and maintain the profile of the study we contacted professional magazines, national press and radio. We were fortunate that a medical graduate and former sufferer who regularly works in a variety of media, Graeme Garden, offered to speak to media colleagues on our behalf to provide his insight into the condition. (See Appendix 6 for Graeme’s story.)
In all we had one professional magazine article,20 several newspaper articles,21,22 a radio programme23 and a BBC Health website24 dealing with the topic during recruitment.
We took advantage of two articles in the British Medical Journal about Bell’s palsy25,26 and the resultant correspondence of 48 rapid responses (with potentially problematic influences on referral of patients into the study) to respond with details of the study. All of these activities may have helped to keep the study in the eye of our target group for recruitment. Such activities did take several days in terms of planning, writing and interviews but could reasonably be fitted in around the general work of the project.
Educational meetings
We took every opportunity to raise awareness and build the profile of the study including conference presentations and workshops. However, these exercises connected with relatively few recruiting general practitioners and emergency room staff, and it is hard to know what impact, if any, they had on recruitment.
Regular feedback on the trial
In the quarterly mailings to general practitioners organised by the SPPIRe nodes we took the opportunity both to let them know that the study was still ongoing and the current recruiting status.
Remuneration
Following negotiation with primary care R&D departments, general practices were offered £51 per patient for recruiting patients into the trial and for any ongoing explanation and care that might be required. This fee was intended additionally to cover the situation where in rare cases a patient preferred to use their general practitioner surgery rather than their home for the researcher’s visits.
Recruiters
Heads of ear, nose and throat (ENT) departments at 20 hospitals in Scotland that could contribute to the trial as recruiting sites were approached, and 17 agreed to join. It was never anticipated that the trial would recruit in the island regions; however, both potential centres in NHS Forth Valley (Stirling Royal Infirmary, Falkirk & District Royal Infirmary) and also that for NHS Dumfries & Galloway (Dumfries & Galloway Royal Infirmary) declined to join, reducing the national coverage to an estimated 88% of the Scottish population. In all cases, staffing difficulties were cited as the reason for non-participation. The three main Scottish dental institutions (Dundee Dental Hospital, Glasgow Dental Hospital and Edinburgh Dental Institute) were also approached but rather than act as recruiting sites agreed to refer potential patients to the nearest site. The 17 sites (with additional premises at Ninewells Hospital and Royal Alexandra Hospital) finally organised and provisioned with study medications and stationery were the ENT wards and clinics listed in Table 2. The head of each contributing clinic or department was formally designated Local Principal Investigator with site-specific approvals from their LREC and R&D division; these are listed in Appendix 7.
Each trial site was supplied with stationery (site folders; patient information sheets, consent forms and case record forms – see Appendices referred to later); instructions for the process of randomisation; and patient packs comprising the trial medications in appropriate storage locations (e.g. drugs cupboards).
Prior to the start of recruitment all sites were briefed by the Local Principal Investigator or a member of the BELLS team or both. The briefing was provided as part of the standard educational programme and potential recruiters were taken step-by-step through the processes of recruitment as follows.
Recruitment step 1: Patient awareness
Staff were requested to explain the condition to the patient and the options for treatment, and to bring to the patient’s attention the ongoing Scottish Bell’s Palsy Study. Potential patients were presented with the site-specific study Patient Information Sheet, an example of which is provided in Appendix 8.
Recruitment step 2: Data collection
All patients and recruiting staff provided a completed site-specific patient case record form, irrespective of final recruitment status. An example is provided in Appendix 9.
Recruitment step 3: Consent
For eligible patients consenting to join the study, the BELLS consent form was then completed. The patient consent form was health region specific. An example is provided in Appendix 10.
Recruitment step 4: Randomisation to treatment
The patient was then randomised to treatment according to the following schedule, and utilising the services of the Health Services Research Unit (HSRU) randomisation facility at the University of Aberdeen. The randomisation scheme was site-specific. An example is provided in Appendix 11.
Recruitment step 5: Initiation of treatment
Finally, staff were instructed to request the patient to commence treatment immediately by taking the first dose. Staff at the recruiting sites changed twice a year: briefing of new staff was handled by the local principal investigator, who supplied briefing notes, including expanded instructions on what to do if any aspect of the randomisation process differed from that expected (see Appendix 12).
Remuneration
Departments were paid £50 for each recruitment irrespective of the patient’s completion status, paid from study funds.
Feedback
Local principal investigators were advised monthly by email of the current status of the study (recruitment figures, retention figures, adherence to target). The Management Committee were updated weekly by email: see Appendix 13 for a typical example.
Patients
Patients were referred to participating sites after presentation at GP surgeries, A&E, NHS24 or (rarely) their dentist. After the diagnosis of Bell’s palsy was confirmed then inclusion and exclusion criteria were examined. See Table 3 for a full listing of inclusion and exclusion criteria.
| Inclusion criteria |
|---|
| Adults (16 or older) |
| Unilateral facial nerve weakness of no identifiable cause confirmed as Bell’s palsy |
| Seen within 72 hours of the onset of weakness |
| Exclusion criteria |
| Pregnancy |
| Uncontrolled diabetes (HbA1c > 8%) |
| Peptic ulcer disease |
| Suppurative otitis media |
| Herpes zoster |
| Multiple sclerosis |
| Sarcoidosis and other rarer conditions |
| Inability to give informed consent |
| Breast-feeding |
| Patients with systemic infection |
Interventions
Patients satisfying the criteria for entry into the Scottish Bell’s Palsy Study and who were willing to join it, and who had provided signed witnessed consent, were immediately randomised into the trial as follows. In order to accommodate the intended 2 × 2 factorial design (see Table 4) patients were randomised to prednisolone/placebo and to aciclovir/placebo as follows, with all processes necessary to achieve balance attended to by the randomisation unit at HSRU.
| Treatment | Prednisolone P | Placebo P′ |
|---|---|---|
| Aciclovir A | aciclovir–prednisolone AP | aciclovir–placebo AO |
| Placebo A′ | placebo–prednisolone OP | placebo–placebo OO |
In Table 4 we use the shorthand abbreviations that will be used throughout this report to distinguish between the treatment groups, specifically:
| aciclovir–prednisolone | AP |
| aciclovir–placebo | AO |
| placebo–prednisolone | OP |
| placebo–placebo | OO |
and
| aciclovir | A | AP + AO |
| no aciclovir | A′ | OP + OO |
| prednisolone | P | AP + OP |
| no prednisolone | P′ | AO + OO |
Medications were prescribed according to the doses described in Table 5.
| Prednisolone | 2 × 25 mg/day = 50 mg/day for 10 days, starting immediately |
| Placebo equivalent | Indistinguishable capsules (red) |
| Aciclovir | 5 × 400 mg/day = 2000 mg/day for 10 days, starting immediately |
| Placebo equivalent | Indistinguishable capsules (green) |
The four treatment combinations were provided in packs labelled Treatment 1, 2, 3, 4 with all participants masked (referrers, recruiters, patients and researchers, and, later, assessors). The bottles, labelling and capsules are shown in Figure 3.
FIGURE 3.
(a) Study medications: bottles (ten days’ treatment). (b) Study medications: capsules (one day’s dose).
Patients were requested to commence the first dose on site, even if there was sufficient time only to complete a half-day’s dose.
Identification of coded treatments
The identification of treatments was established by code break in the presence of the Chief Investigator, Trial Statistician and Trial Co-ordinator on 20 March 2007 by agreement with the TSC and DMEC, after the last patient was followed up and all primary and secondary outcomes obtained.
For clarity in the sequel, the identification of treatments is provided in Table 6.
| Treatment (code) | Treatment (actual) | Treatment (shorthand) |
|---|---|---|
| 1 | placebo–prednisolone | OP |
| 2 | placebo–placebo | OO |
| 3 | aciclovir–prednisolone | AP |
| 4 | aciclovir–placebo | AO |
Researchers
The geographical coverage of the study was split into four regions, each staffed by one researcher as shown in Table 7.
| Region | Coverage | Proportion |
|---|---|---|
| North | NHS Highland, NHS Grampian | 16.4% |
| East and South-East | NHS Tayside, NHS Fife | 16.6% |
| South | NHS Lothian, NHS Lothian West NHS Borders, NHS Dumfries and Galloway | 17.5% |
| West | NHS Argyll & Clyde, NHS Forth Valley, NHS Greater Glasgow, NHS Ayrshire & Arran, NHS Lanarkshire | 49.5% |
After interview and appointment researchers were briefed to practise as outlined in Appendix 14, in order to achieve as uniform an approach as possible. During the first patient visit (as well as completing study instruments; see later) the researcher completed Form C (checklist, see Appendix 15) and Form B (patient details, see Appendix 16). In particular, Form C contains the check item that the treatment dispensed to the patient was that allocated.
Patients were assessed during a home visit 3–5 days after randomisation, i.e. up to 8 days after onset of symptoms; again after 3 months; and finally at a third assessment after 9 months, if they were still unrecovered (House–Brackmann grade II–VI) at 3 months.
The preceding paragraph encapsulates two variations to protocol made on clinical grounds within weeks of the commencement of patient randomisation. These were:
Definition of ‘complete recovery’
Our definition of recovery stated in the trial protocol is attainment of House–Brackmann grade I or II (HBI or HBII).
Within a few weeks of commencing 3-month assessment visits to BELLS patients, i.e. after November 2004, it was evident to investigators and from the patients’ own accounts of their progress towards recovery that patients looked, and felt, fully recovered only when a status of HBI was attained.
Researchers were instructed to make the final follow-up visit at 9 months only to patients graded HBII or higher. At a meeting of the trial DMEC on 24 August 2005 and following a scheduled analysis of 3-month data only (independent of and blind to investigators) the Chair of the Committee drew to the attention of principal investigators the discrepancy between the definition of complete recovery (HBI) and the definition stated in the protocol (HBI or II). At the next meeting of Principal Investigators on 2 December 2005 under Item 2(ii) it was noted as follows:
-
the opinion of the meeting was that HBI at V2 was the definition of ‘recovery..’ and that further data collection on recovered patients is irrelevant;
-
the comments from DMEC that the definition of recovery (HBI) differed from that in the protocol (HBI-II); the feeling of the meeting was that if this was a real issue then it could be covered by discussion in the final report [to the funder].
We did not alter the protocol or seek an amendment to it to address this difference, but we determined to continue to use the definition of HBI as ‘completely recovered’ to impose our strategy for 9-month visits, and finally in our pre-specified primary analysis for the funder’s final report.
A supplementary analysis based around HBI-II for recovery (‘good’ not ‘complete’) is included in this final report.
Baseline visits
At the same time as it was determined to distinguish between ‘good’ and ‘complete’ recovery (HBI-II and HBI respectively) and for the same reason (clinical response to patient feedback) it was decided to extend the time lag between notification of a new randomisation and the baseline assessment visit from ‘as soon as possible’ to ‘3–5 days’. Earlier, patients recruited to the trial had reported with regret that researchers were visiting ‘too soon’ and that their symptoms – specifically, poor appearance and pain – worsened after that visit had been made. In order to capture the patient experience adequately, researchers were instructed to negotiate the timing of the baseline visit with the greater flexibility indicated.
Assessors
The fifth and final group of participants were the assessors, whose role was to assess the patients’ recovery status (House–Brackmann grading I to VI) on the basis of the posed photographs taken by the researcher during patient visits. These were experts in their field (one otolaryngologist, one neurosurgeon, one plastic surgeon) and were blinded to the patient treatment allocation, and to the timing of the visit (onset, 3 months or 9 months) throughout the assessment period. A typical set of the four required posed photographs is shown as Figure 2. (This patient’s signed consent for publication is available on file.)
Assessors provided independent gradings for each patient visit, based on the four posed portrait photographs.
Patients graded ‘well’ at 3 months (House–Brackmann grade I) did not receive a 9-month visit.
The identities of the members of the panel of assessors are provided in Appendix 17.
In order to achieve a clear definition of the attainment of recovery we wanted to make the assumption that in Bell’s palsy no patient’s condition worsened from one visit to the next. In fact there were just four cases where this assumption failed (0.8%; three patients were graded I–II–I, i.e. ‘well–ill–well’ at the respective assessment visits, and one was graded III–I–II, i.e. ‘ill–well–ill’). Given the small number of such cases and the comparatively greater variation in individual gradings by experts, we elected in all four cases to allow the minority judgement to over-rule the majority or median judgement, the patients being regraded II–II–I and III–II–II respectively, and thus we achieved a trajectory that, for the purposes of an exploration of recovery after onset of Bell’s palsy, is satisfactorily defined.
Objectives
The trial objectives are listed in Table 8.
| 1. | To describe the resolution of neurological deficit and cosmetic, psychological and functional recovery in each of four groups of patients: those treated with prednisolone, aciclovir, both, or neither |
| 2. | To determine which group of patients has the greatest reduction in neurological disability scores on the House–Brackmann grading system at 3 and 9 months after randomisation |
| 3. | To compare self-reported health status (including assessments of pain) at 3 and 9 months after randomisation |
| 4. | To compare the incremental cost per neurological deficit resolved (case cured) and incremental cost per QALY in the study groups |
Primary outcomes
House–Brackmann grade
Our primary disease measurement was the commonly used and easily administered House–Brackmann scale for facial paralysis, where a score of I is ‘normal’ (or ‘recovered’, in the language of the trial) and scores II–VI reflect increasing dysfunction from ‘minor asymmetry e.g. when smiling’ (graded II) to ‘no perceptible movement’ (graded VI). The complete scale is provided in Table 9.
| Grade | Definition |
|---|---|
| I | Normal symmetrical function in all areas |
| II | Slight weakness noticeable only on close inspection. Complete eye closure with minimal effort. Slight asymmetry of smile with maximal effort. Synkinesis barely noticeable; contracture or spasm absent |
| III | Obvious weakness, but not disfiguring. May not be able to lift eyebrow. Complete eye closure; strong but asymmetrical mouth movement with maximal effort. Obvious, but not disfiguring synkinesis, mass movement or spasm |
| IV | Obvious disfiguring weakness. Inability to lift brow. Incomplete eye closure and asymmetry of mouth with maximal effort. Severe synkinesis, mass movement, spasm |
| V | Motion barely perceptible. Incomplete eye closure; slight movement of corner of mouth. Synkinesis, contracture and spasm usually absent |
| VI | No movement; loss of tone; no synkinesis, contracture, or spasm |
Patients were assessed during a home visit 3–5 days after randomisation, i.e. up to 8 days after onset of symptoms; again after 3 months; and finally at a third assessment after 9 months, if they were still unrecovered (House–Brackmann II–VI) at 3 months. Judgements were made by expert review of four posed portrait photographs taken during the assessment visit (at rest, smiling, eyebrows raised, eyes tight shut). Three clinicians (one otolaryngologist, one neurologist, one plastic surgeon) independently reviewed the posed photographs and recorded a grading I–VI. If there was disagreement by more than one point on the scale, a revised opinion was requested from all three assessors. The majority or median judgement was taken as providing the patient’s health status on the day of the visit.
Patients, referrers, recruiters, research visitors and assessors were all blinded to treatment throughout the duration of the study.
Secondary outcomes
There are three secondary outcomes for patients on the BELLS study, measured once at each visit.
Health Utilities Index Mark 3 (HUI3)
The Health Utilities Index Mark 3 (HUI3)27 is a multi-attribute health status classification system providing an aggregated score on eight variables, i.e. vision, hearing, speech, ambulation, dexterity, emotion, cognition and pain. It is designed for use in clinical practice and research, health policy evaluations, and general population surveys. It is constructed for self-administration by persons 14 years of age and older, and for administration by a trained interviewer in person or by telephone. It takes about 5–10 minutes to administer. A copy of the form for the collection of HUI3 data is provided in Appendix 18. Scoring is achieved through the HUI3 Multi-Attribute Utility Function on the Dead-Healthy Scale28 as shown in Table 10.
| Vision x1 b1 | Hearing x2 b2 | Speech x3 b3 | Ambulation x4 b4 | Dexterity x5 b5 | Emotion x6 b6 | Cognition x7 b7 | Pain x8 b8 |
|---|---|---|---|---|---|---|---|
| 1 1.00 | 1 1.00 | 1 1.00 | 1 1.00 | 1 1.00 | 1 1.00 | 1 1.00 | 1 1.00 |
| 2 0.98 | 2 0.95 | 2 0.94 | 2 0.93 | 2 0.95 | 2 0.95 | 2 0.92 | 2 0.96 |
| 3 0.89 | 3 0.89 | 3 0.89 | 3 0.86 | 3 0.88 | 3 0.85 | 3 0.95 | 3 0.90 |
| 4 0.84 | 4 0.80 | 4 0.81 | 4 0.73 | 4 0.76 | 4 0.64 | 4 0.83 | 4 0.77 |
| 5 0.75 | 5 0.74 | 5 0.68 | 5 0.65 | 5 0.65 | 5 0.46 | 5 0.60 | 5 0.55 |
| 6 0.61 | 6 0.61 | 6 0.58 | 6 0.56 | 6 0.42 |
Here xn is the attribute level and bn is the attribute utility score. Then a patient’s HUI3 score on the Dead-Healthy scale is defined by the formula on the Dead–Perfect Health scale:
where u is the utility of a chronic health state on a utility scale where ‘dead’ has a utility of 0.00 and ‘healthy’ has a utility of 1.00. The range of the score is – 0.371 to + 1.000.
Brief Pain Inventory (BPI)
The Brief Pain Inventory (BPI) is based on a measure known as the Wisconsin Brief Pain Questionnaire29 and was developed by the Pain Research Group to provide information on the intensity of pain (the sensory dimension) as well as the degree to which pain interferes with function (the reactive dimension). The BPI also asks questions about pain relief, pain quality, and the patient’s perception of the cause of pain. It uses numerical rating scales of 0 to 10 for item ratings because of their simplicity, lack of ambiguity and because they seemed the best to use for cross-linguistic pain measurement. A copy of the form for data collection is provided in Appendix 19.
The pain score is obtained by adding together the scores provided by the patient’s responses to Questions 2 to 12. The range of scores for any individual patient visit is thus 0 to 110. The higher the score, the greater the impact of pain on the patient’s daily living.
Derriford Appearance Scale (DAS59)
The Derriford Appearance Scales (DAS24 and DAS59)30 are psychological measures of concern about appearance, developed and validated in the UK for use in clinical and research settings (e.g. in plastic surgery, oncology and psychology). They have excellent validity and reliability, and have been independently recommended as a measure of choice. A copy of the DAS59 form is provided in Appendix 20. We obtained permission for use of the form from the copyright owners, who generously waived copyright charges after the first 250 copies were obtained.
Scoring of the DAS59
The score for the DAS59 is obtained by summing different components as follows:
-
items on page 1 do not contribute;
-
throughout the scale ‘N/A’ scores 0;
-
items 52, 54, 55, 56, 57 have their score values reversed (1 becomes 5, 2 becomes 4, 3 is unchanged, 4 becomes 2, 5 becomes 1).
The DAS59 generates six measures of psychological distress and dysfunction as well as a measure of physical distress and dysfunction (items 25 and 26). Full-scale and factorial sub-scale scores are obtained by adding the scores of individual items as shown in Table 11.
| Factor | Label | Items |
|---|---|---|
| Factor 1 | General self-consciousness of appearance (GSC) | 1, 8, 10, 12, 15, 17, 27, 28, 30, 31, 34, 35, 36, 38, 41, 42, 58 |
| Factor 2 | Social self-consciousness of appearance (SSC) | 2, 3, 5, 6, 7, 13, 14, 16, 18, 19, 20, 21, 22, 29, 32, 33, 39, 40, 47, 50 |
| Factor 3 | Sexual and bodily self-consciousness of appearance (SBSC) | 4, 9, 23, 24, 37, 43, 45, 46, 49 |
| Factor 4 | Negative self-concept (NSC) | 52, 54, 55, 56, 57 |
| Factor 5 | Facial self-consciousness of appearance (FSC) | 11, 44, 48, 51 |
The range of DAS59 scores is 8 to 262. The higher the score, the greater the patient’s level of distress and dysfunction.
Methods used to enhance the quality of measurements
All participants were briefed similarly (including patients, through the medium of the Patient Information Sheet and discussion with the referring and recruiting clinicians). General practitioners were provided with instructions for referral on the study website. Referrers followed identical procedures as far as it was possible to contrive this. Researchers used the same model of camera (Sony DSC–P12), with the same settings, and requested the same poses of their patients; follow-up procedures (phone calls, visits) were managed identically. Assessors calibrated their measurements through an initial training period with discussion (assessment of 10 sample portrait sets, with a discussion of differences, followed by assessment of a further 20 sample portrait sets); assessors were at all times made aware of any ‘large’ discrepancies in their grading of patient recovery (i.e. any difference in grading exceeding one grade point) and in all such cases the portrait sets were reassessed.
At the centre there was double entry of data and all discrepancies were identified, discussed and corrected.
Two statisticians independently pursued separate analyses of the data.
Unblinding took place in the presence of the Chief Investigator, one principal investigator and the study co-ordinator, who independently interpreted the decoding key and agreed that interpretation.
Chapter 3 Study design
Sample size
The relevant Cochrane reviews suggested potential effect sizes from 4% to 17%. A difference in complete recovery of 10–12% or more was considered to be clinically meaningful. Randomising 240 patients per active treatment (e.g. aciclovir or not; a total of 480 patients) was calculated to provide 80% power to detect a difference of the order of 12% at the 5% level. Since the study design is factorial the attained power is the same for each pair-wise comparison of treatments (assuming no interaction between treatments and groups). Assuming an incidence rate of 24 per 100,000 per annum based on population access and age range we would have anticipated 2235 cases to have occurred in the study catchment area during the recruitment period. We therefore aimed to refer approximately one-third of all cases of Bell’s palsy arising in Scotland during the study period, and after excluding ineligible cases, to recruit approximately one-quarter of all cases.
Thus the number of patients required to achieve the intended design is 240 per treatment arm, as shown in Table 12 (in brackets after the treatment code).
| Treatment | Prednisolone | Placebo | Total |
|---|---|---|---|
| Aciclovir | AP (120) | AO (120) | 240 |
| Placebo | OP (120) | OO (120) | 240 |
| Total | 240 | 240 | 480 |
We aimed, therefore, to assess 720 patients for eligibility (approximately one-third of all cases in Scotland over the 25-month recruitment period) and to randomise 540 of those to treatment (three-quarters: in other words, to randomise one-quarter of all cases in Scotland) in order to achieve 480 completed patients (about nine-tenths of those randomised; this retention rate represents a very high proportion but one that was realised in fact).
Explanation of any interim analyses and stopping rules
No formal interim analysis was scheduled or requested; however, as part of a quality control exercise an analysis of completed data was carried out in August 2005 at the request of the Chair of the study DMEC, and managed by the host institution. A statistician co-opted to the committee to analyse the partial data remained blinded to the treatment key, having been made aware only of the contrasts by Tayside Pharmaceuticals, the drug manufacturers. At the conclusion of this analysis the team was requested to continue recruitment and follow-up; no other illumination of progress was provided.
Randomisation
Allocation to treatment
The randomisation processes were designed and managed by the HSRU at the University of Aberdeen. After witnessing signed informed consent from an intending patient, the recruiting clinician telephoned a 24-hour automated contact at HSRU. Telephone key-presses advised HSRU of the site, and the HSRU randomisation service responded with a unique patient ID carrying a code for the recruiting trial site and patient accession number, and finally with a decision about the treatment allocation. The allocated treatment was taken from local storage and administration of the allocated medication commenced immediately.
Pharmaceutical stock control
Stock control was managed through constant monitoring of the treatment supplies at each of the treatment sites. When supplies were noted to be reducing, stock was ordered directly from Tayside Pharmaceuticals, who attended to the draw from stock and delivery to the sites. Stocks were replenished by instruction to Tayside Pharmaceuticals, requesting 40 or 80 patient packs (10 or 20 packs of each of the four treatments) at a time. Quality control, batch control and labelling were all attended to by staff at Tayside Pharmaceuticals.
Sequence generation
Randomisation was achieved using a permuted block randomisation technique with block sizes of four or eight, and no stratification. The sequence remained entirely concealed until the intervention was assigned.
Blinding (masking)
Patients, referrers, recruiters, research visitors and assessors were all masked to treatment throughout the duration of the study.
How the success of blinding was evaluated
No formal checks of the quality of blinding were implemented. However, no attempts were made individually or collectively, formally or informally, to match treatment to side effects, or to speed of recovery, or to extent of recovery. Where masking might have been threatened (e.g. consideration of the three deaths that occurred during follow-up, or during the quality-driven analysis) the opinion of independent experts was sought and study staff remained entirely insulated from the process. Occasionally patients hazarded a guess (as in ‘I’m sure I’m on placebo’); a small number of patients claimed to ‘know’ the treatment allocated, through misinterpretation of the labelling on the patient packs. Three members of the team (the Chief Investigator, the Trial Statistician and the Trial Co-ordinator) confirmed the decode envelope to be sealed at the end of study, and it was opened only when it became necessary to do so in order for the treatments to be identified, the analysis having taken place masked. The primary statistical analysis was completed before decoding took place.
Statistical methods
All analyses were based on intention-to-treat and specific comparisons were pre-specified in the protocol.
The primary outcome measure of complete recovery (House–Brackmann I) at 3 and 9 months was compared initially between those who did and did not receive prednisolone using a two-sided Fisher’s exact test. This was repeated for aciclovir. We tested the data for any interaction between the groups prior to these tests.
Pre-specified secondary analyses compared HUI3, DAS59 and BPI scores. Then, our analysis was adjusted for all baseline characteristics measured: age, gender, interval between onset and receiving treatment, and scores on House–Brackmann, HUI3, DAS59 and BPI.
The results were also assessed for sensitivity to drop-out, assuming missing at random. A propensity score for drop-out at 9 months (Yes/No) was estimated using logistic regression, and a further analysis carried out weighting the results by the reciprocal of the probability of remaining in the study.
If there is a significant interaction the overall efficiency of the design is maintained as long as the two drugs do not act antagonistically to cancel each other out, which was considered unlikely in this case. In the presence of an interaction it is still possible to assess each drug separately, albeit with reduced power (72% instead of 80%) for the effect size (12%) or alternatively to detect effect sizes greater than 15% with the same power. Randomisation does not always result in perfect balance of all factors that may affect the primary outcome and it is important to adjust even for minor differences. The differences between odds ratios (ORs), and adjusted odds ratios were not substantial; nevertheless the odds ratios were lowered on adjustment, showing that crude unadjusted results would have given an over-optimistic impression of effect size.
Economic evaluation was an integral part of the trial. Furthermore, a series of subgroup analyses were also considered. The rationale, methods and results of these analyses are presented in Chapters 7 and 8 respectively.
Subgroup analyses
Post hoc analyses were performed of (1) the effectiveness of prednisolone and its dependence on the time of commencement of administration of treatment after onset of symptoms, and (2) the effectiveness of prednisolone and its dependence on the severity of symptoms at onset. We also examined (3) the inter- and intra-assessor reliability of the primary outcome measurement.
Chapter 4 Results
Participant flow
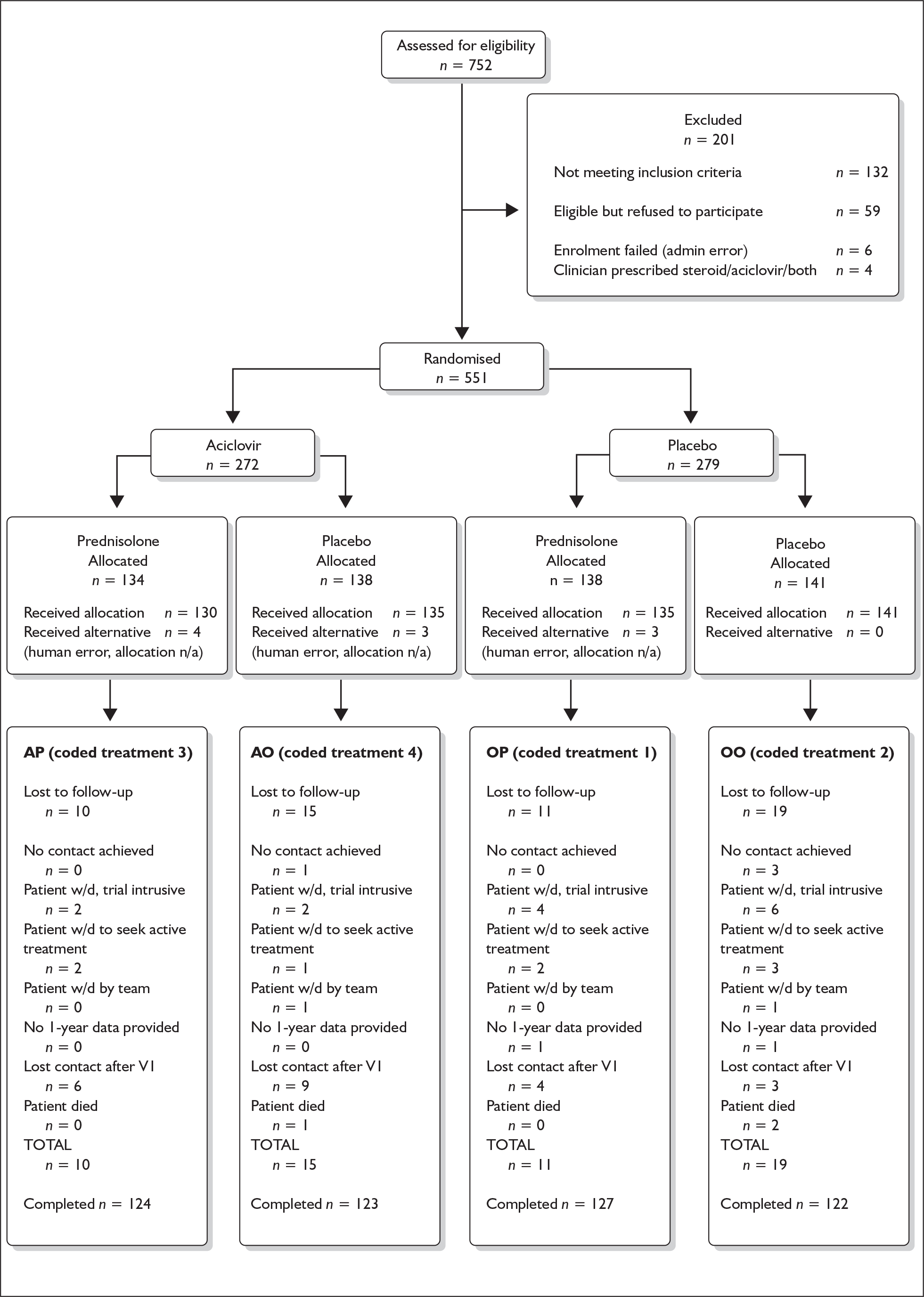
The flow of participants through each stage of the study is shown in Figure 4. Specifically, this shows for each treatment group the numbers of participants randomly assigned, how many received the intended treatment, how many completed the study protocol, and how many were analysed for the primary outcome (‘Completed’).
FIGURE 4.
CONSORT Framework diagram, BELLS study. AO, aciclovir–placebo group; AP, aciclovir–prednisolone group; OO, placebo–placebo group; OP, placebo–prednisolone group; n/a, not available.
Protocol deviations from study as planned, together with reasons
In two cases patients were recruited to the study but later it was decided that the diagnosis of Bell’s palsy was probably mistaken.
In one case a patient with diabetes was recruited to the study. It is not known how this occurred.
In a small number of cases, it became apparent during the researchers’ follow-up visits that the delay between onset of symptoms and the commencement of treatment probably exceeded 72 hours. It is unlikely that this aspect of the consent process was neglected: in fact, patients’ definition of the onset of symptoms, and consequently of the time of onset of symptoms, was in a few cases very vague indeed.
It is our belief that signed consent was always obtained at the time of recruitment; however, the signed consent form along with the patient case record form was occasionally returned to hospital notes rather than being retained in the site folder. In these cases strenuous efforts, usually successful, were always made to locate and retrieve the signed consent form.
In nine cases, patients received a treatment different to that allocated, and in one case a patient was sent away with no treatment at all. Although stocks were monitored and maintained, occasionally they were not available for issue at the site (simply, they were temporarily mislaid). In these cases an alternative was offered and the alternative noted.
In one case, a patient successfully recruited to the study and randomised to treatment was not offered the allocated treatment: instead a 7-day regime of prednisolone and aciclovir in combination was prescribed. It is not known how this breakdown between briefing and practice occurred.
Under intention to treat (ITT) all these patients were followed up and their data retained in the analysis of results.
Recruitment
The study ran during 2003–7 and included 25 months’ recruitment and 9 months’ patient follow-up. At an estimated 30 cases/100,000/year (estimates vary from 11 to 40 cases/100,000/year31) in the Scottish population (5.04 million, of whom 4.10 million are aged over 16, the minimum age for recruitment to this clinical trial) and with an estimated geographical trial catchment area of 88%, we sought to refer one-third of all cases (approximately 720) and to recruit three-quarters of those (approximately 540) to achieve our target of 480 completed patients (see Table 12).
In the event we assessed 752 patients for eligibility and randomised 551 of those to treatment, of whom 496 patients completed follow-up, as shown in Table 13.
| Treatment | Prednisolone | Placebo | Total |
|---|---|---|---|
| Aciclovir | AP (124) | AO (123) | A (247) |
| Placebo | OP (127) | OO (122) | A′ (249) |
| Total | P (251) | P′ (245) | (496) |
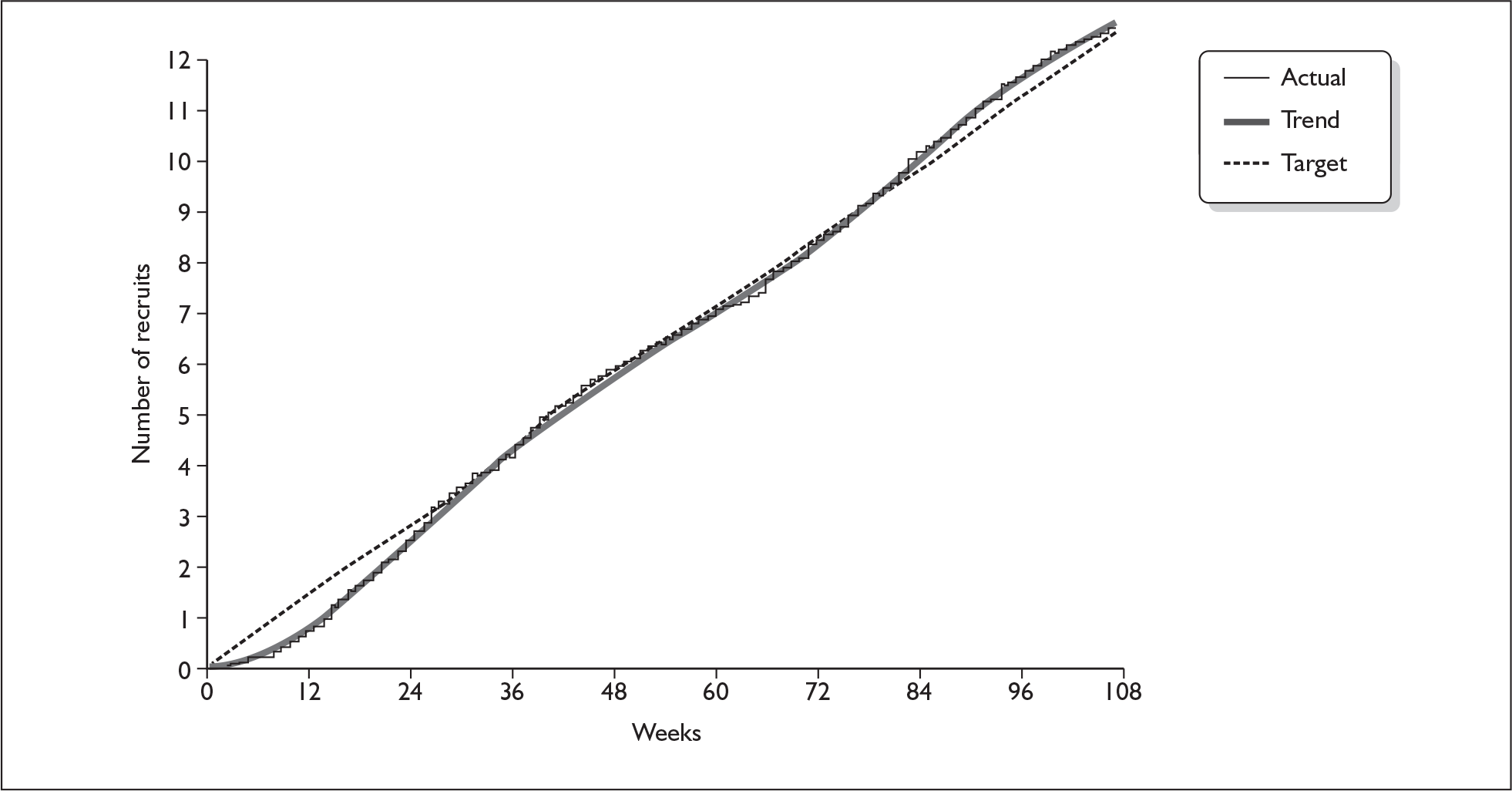
The pattern in weekly recruitment and overall retention to target over the 108 weeks of recruitment to the BELLS study are shown in Figures 5 and 6. Summary totals are shown in Table 13.
FIGURE 5.
Weekly recruitment to the BELLS study.
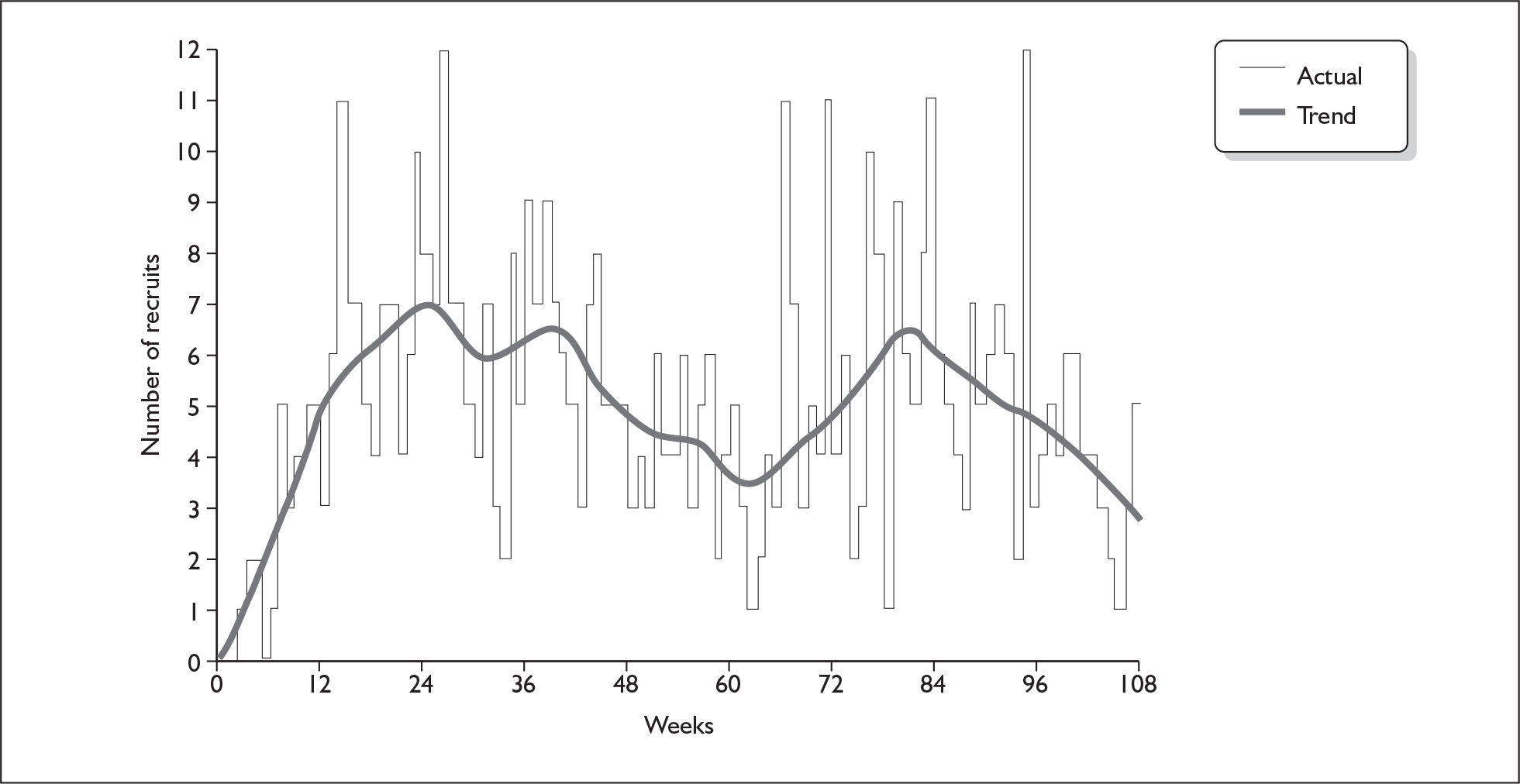
FIGURE 6.
Retention to target on the BELLS study.
Despite a very flexible and convenient system for patient appointments and assessment, not all patients were assessed at all the required time points: altogether there were 19 missed appointments (of course, there were many more missed appointments for the 55 patients of the 551 randomised into the trial who did not complete follow-up, chronic missed appointments being the most common reason for loss to follow-up). The number of attained appointments for the collection of data on the BELLS Study is shown in Table 14.
| Patient health status as defined by House–Brackmann grade | |||
|---|---|---|---|
| Visit 1 (soon after onset) | Visit 2 (∼ 3 months after onset) | Visit 3 (∼ 9 months after onset) | Frequency |
| Missed | Missed | Well | 2 |
| Missed | Missed | Ill | 0 |
| Missed | Well | 8 | |
| Missed | Ill | Well | 0 |
| Missed | Ill | Ill | 1 |
| Well | Missed | Well | 1 |
| Ill | Missed | Well | 4 |
| Ill | Missed | Ill | 1 |
| Well | Well | 30 | |
| Ill | Well | 319 | |
| Ill | Ill | Well | 73 |
| Ill | Ill | Ill | 57 |
| Total | 496 | ||
We noted a small number of cases (31) where the patient was graded well (HBI) at the baseline visit. In fact this is not clinically very remarkable. One of several characteristics of Bell’s palsy is that it is capable of very rapid recovery, and there were many anecdotal reports amongst patients visited soon after onset commenting that they felt ‘better already’ and those visited at 3 months who reported that the condition ‘cleared up soon after you came to see me the first time’.
Eight patients received their first visit after completing the 10-day course of treatment; and three patients received their final assessment a year after onset. The reasons for this were always related to the difficulty of following up a mobile population and arranging a visit that was convenient to the patient.
Baseline data
Table 15 shows the baseline characteristics of groups. The patients were balanced for gender, the mean age was 44 years, and the degree of initial facial paralysis was moderate to severe. Most patients (53%) initiated treatment within 24 hours of onset of symptoms, 32% within the 24–48-hour period, and 15% from 48–72 hours.
| Prednisolone P (n = 251) | No prednisolone P′ (n = 245) | Aciclovir A (n = 247) | No aciclovir A′ (n = 249) | All (n = 496) | |
|---|---|---|---|---|---|
| Demography | |||||
| Male | 53.8 (135) | 48.2 (118) | 48.2 (119) | 53.8 (134) | 51.0 (253) |
| Female | 46.2 (116) | 51.8 (127) | 51.8 (128) | 46.2 (115) | 49.0 (243) |
| Age | 43.2 (16.2) | 44.9 (16.6) | 45.0 (16.6) | 43.0 (16.1) | 44.0 (16.4) |
| Primary oucome | |||||
| House–Brackmann gradea | 3.5 (1.2) | 3.8 (1.3) | 3.6 (1.3) | 3.7 (1.2) | 3.6 (1.3) |
| Secondary outcome | |||||
| HUI3b | 0.796 (0.225) | 0.775 (0.206) | 0.792 (0.209) | 0.779 (0.223) | 0.786 (0.216) |
| DAS59b | 71 (37) | 75 (41) | 72 (39) | 74 (38) | 73 (39) |
| BPIc | 10 (18) | 16 (21) | 12 (18) | 14 (21) | 13 (20) |
| Time to commencement of treatment | |||||
| Within 24 h | 47.8 (120) | 60.0 (147) | 55.5 (137) | 52.2 (130) | 53.8 (267) |
| 24–48 h | 37.8 (95) | 26.1 (64) | 30.4 (75) | 33.7 (84) | 32.1 (159) |
| 48–72 h | 10.0 (25) | 7.3 (18) | 10.1 (25) | 7.2 (18) | 8.7 (43) |
| Unknown but < 72 h | 4.4 (11) | 6.5 (16) | 4.0 (10) | 6.8 (17) | 5.4 (27) |
Outcomes and estimation
Any patient graded ‘well’ (i.e. House–Brackmann I) at 3 months was deemed ‘completed’, and no visit was made to the patient at 9 months. Altogether 496 patients (90% of all those recruited) completed follow-up.
Primary outcome
Following the classical procedure for a factorial design, we first independently assessed outcome in the marginal treatment groups prednisolone versus no prednisolone (P vs P′; i.e. AP + OP vs AO + OO) and aciclovir versus no aciclovir (A vs A′; i.e. AP + AO vs OP + OO).
Table 16 presents the unadjusted and adjusted outcome data on patients who completed the study. Altogether 357 patients had recovered by 3 months and did not require a further visit. Of the remainder, 80 had recovered at 9 months, leaving 59 with a residual facial nerve deficit.
| Treatment % (n) | No treatment % (n) | OR (95% CI) | p value | |
|---|---|---|---|---|
| Prednisolone | ||||
| HB I at 3 months | 83.0% (205/247) | 63.6% (152/239) |
2.79 (1.82 to 4.35) (u) 2.44 (1.55 to 3.84) (a) |
< 0.001 (u) < 0.001 (a) |
| HB I at 9 months | 94.4% (237/251) | 81.6% (200/245) |
3.81 (2.01 to 7.56) (u) 3.32 (1.72 to 6.44) (a) |
< 0.001 (u) < 0.001 (a) |
| Aciclovir | ||||
| HB I at 3 months | 71.2% (173/243) | 75.7% (184/243) |
0.79 (0.53 to1.21) (u) 0.86 (0.55 to 1.34) (a) |
0.304 (u) 0.504 (a) |
| HB I at 9 months | 85.4% (211/247) | 90.8% (226/249) |
0.60 (0.34 to 1.07) (u) 0.61 (0.33 to 1.11) (a) |
0.072 (u) 0.105 (a) |
The analysis in this section was pre-specified and all analyses were based on intention-to-treat.
The effect of adjustment was to attenuate odds ratios in the case of the prednisolone comparison, and thus adjustment acted conservatively. Unadjusted odds ratios (column 4) were respectively 2.79 and 3.81 (prednisolone at 3 months and 9 months); and 0.79 and 0.60 (aciclovir at 3 months and 9 months).
There were significant differences in complete recovery at 3 months between the prednisolone comparison groups (83.0% for prednisolone, 63.6% for no prednisolone, a difference of + 19.4%; 95% confidence interval (CI): + 11.7% to + 27.1%, p < 0.001); but no significant difference between the aciclovir comparison groups (71.2% for aciclovir and 75.7% for no aciclovir, a difference of – 4.5% (95% CI: – 12.4% to + 3.3%, p = 0.30, adjusted 0.50). Nine-month assessments of patients at House–Brackmann grade I were: 94.4% for prednisolone compared with 81.6% for no prednisolone, a difference of + 12.8% (95% CI: + 7.2% to + 18.4%, p < 0.001); and 85.4% for aciclovir and 90.8% for no aciclovir, a difference of – 5.3% (95% CI: – 1.0% to + 0.3%, p = 0.07, adjusted 0.10).
Although neither the results at 3 months nor 9 months were statistically significant, we noted that recovery rates were higher in the no-aciclovir group than in the aciclovir group.
Next we pursued the corresponding four-arm analysis, i.e. comparison of the four delivered treatments (AP, AO, OP, OO). The trial was not powered for this, but primarily it provided an opportunity to assess any interaction between the active therapies (aciclovir and prednisolone) and secondly it provided a very convenient and interpretable comparison for clinicians and researchers (and for this reason was also adopted in the health costs analyses in Chapter 7).
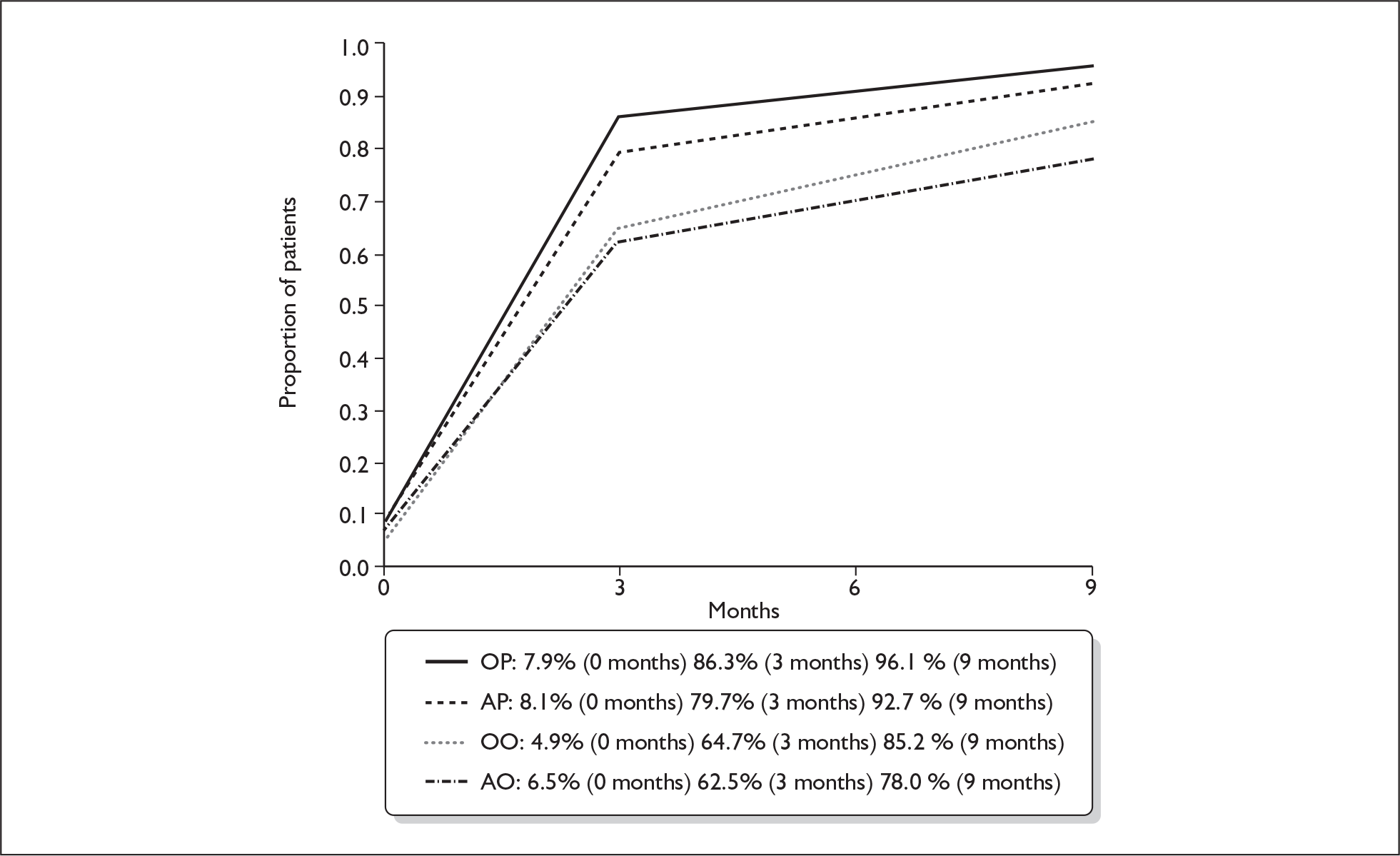
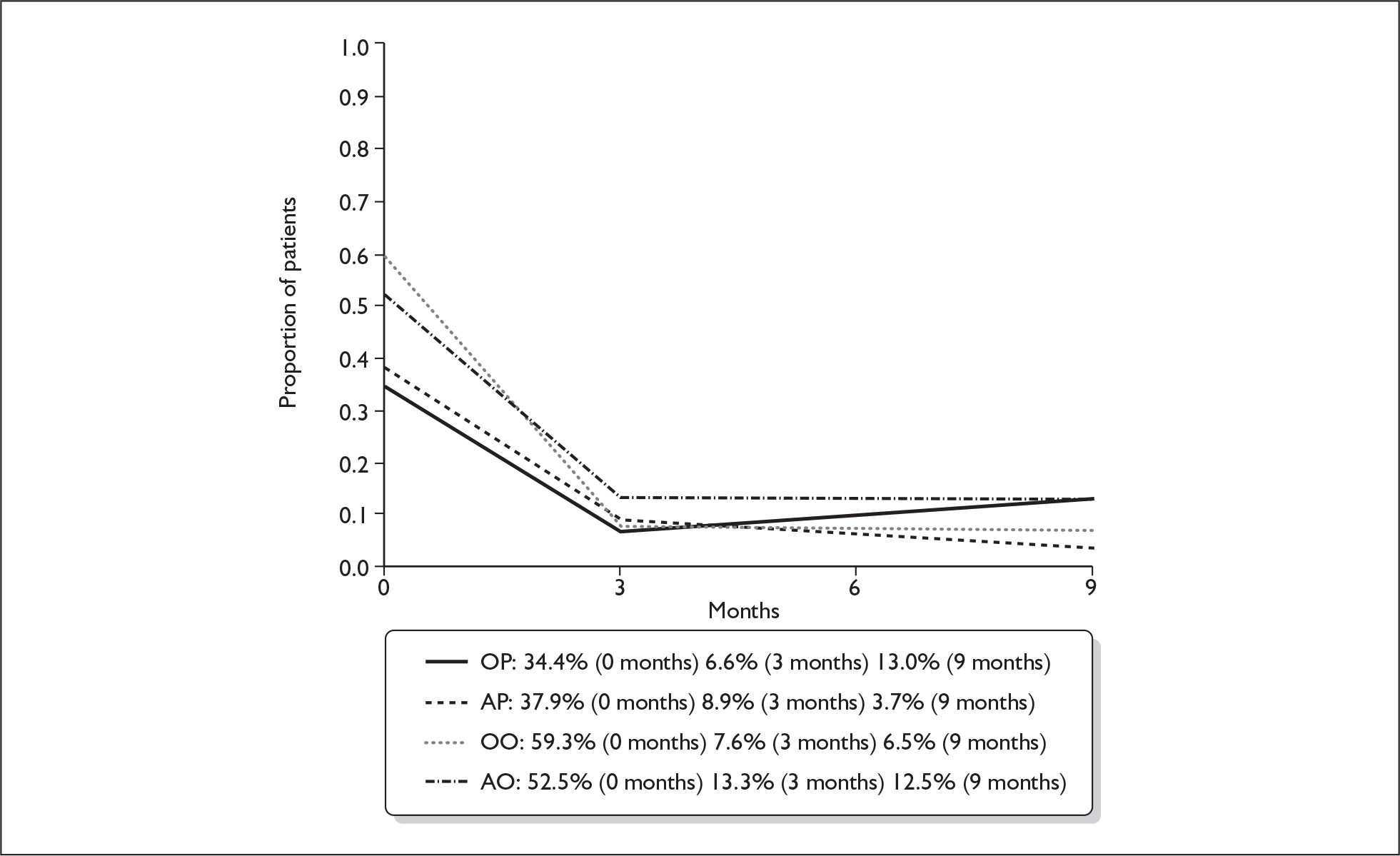
Table 17 and Figure 7 demonstrate the proportion of patients assessed as making a full recovery, i.e. having normal facial function (House–Brackmann I) at baseline, 3 and 9 months in the four treatment subgroups.
| Treatment | 0 months | 3 months | 9 months |
|---|---|---|---|
| OP | 10/127 = 7.9% | 107/124 = 86.3% | 122/127 = 96.1% |
| AP | 10/124 = 8.1% | 98/123 = 79.7% | 115/124 = 92.7% |
| OO | 6/122 = 4.9% | 77/119 = 64.7% | 104/122 = 85.2% |
| AO | 8/123 = 6.5% | 75/120 = 62.5% | 96/123 = 78.0% |
FIGURE 7.
Proportion of patients making a full recovery (House–Brackmann I) at three and 9 months. AO, aciclovir–placebo group; AP, aciclovir–prednisolone group; OO, placebo–placebo group; OP, placebo–prednisolone group.
There was no significant aciclovir–prednisolone interaction at 3 months or at 9 months (p = 0.32, p = 0.72 respectively).
We found a marginally significant aciclovir effect when added to placebo (AO vs OO, p = 0.078) and when added to prednisolone (AP vs OP, p = 0.074); however, there was consistency in the effect previously noted: aciclovir added to prednisolone tended to decrease the recovery rate, and also the recovery rate in those receiving aciclovir was lower than in the group receiving double-placebo.
We noted as before a highly significant prednisolone effect (p < 0.001).
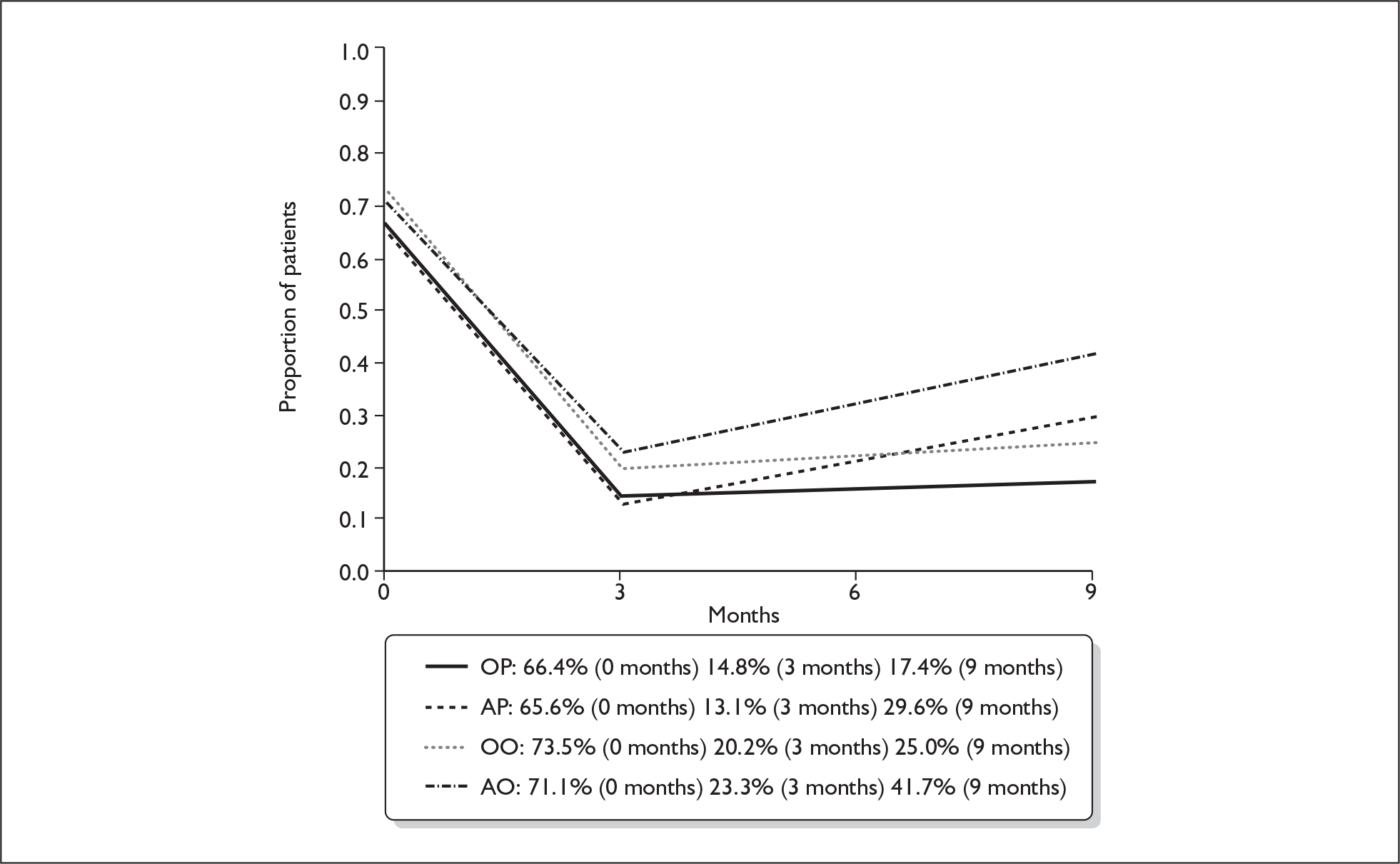
We repeated the foregoing analysis of primary outcome for patients making a ‘good’ recovery, i.e. House–Brackmann grade I or II, with the results shown in Table 18.
| Treatment | None | OR (95% CI) | p value | |
|---|---|---|---|---|
| Unadjusted % (n)a | Unadjusted % (n)a | |||
| Prednisolone | ||||
| HB I/II at 3 months | 93.9 (232/247) | 77.8 (186/239) | 4.41 (2.35 to 8.19) | <0.001 |
| HB I/II at 9 months | 97.2 (244/251) | 94.7 (232/245) | 1.95 (0.74 to 5.41) | 0.176 |
| Aciclovir | ||||
| HB I/II at 3 months | 84.0 (204/243) | 88.0 (214/243) | 0.71 (0.41 to 1.19) | 0.239 |
| HB I/II at 9 months | 95.1 (235/247) | 96.8 (241/249) | 0.65 (0.26 to 1.71) | 0.372 |
The proportions making a good recovery (House–Brackmann I or II) at 3 months and 9 months in the four treatment subgroups shown are in Figure 8.
FIGURE 8.
Proportion of patients making a good recovery (House–Brackmann I or II) at 3 and 9 months. AO, aciclovir–placebo group; AP, aciclovir–prednisolone group; OO, placebo–placebo group; OP, placebo–prednisolone group.
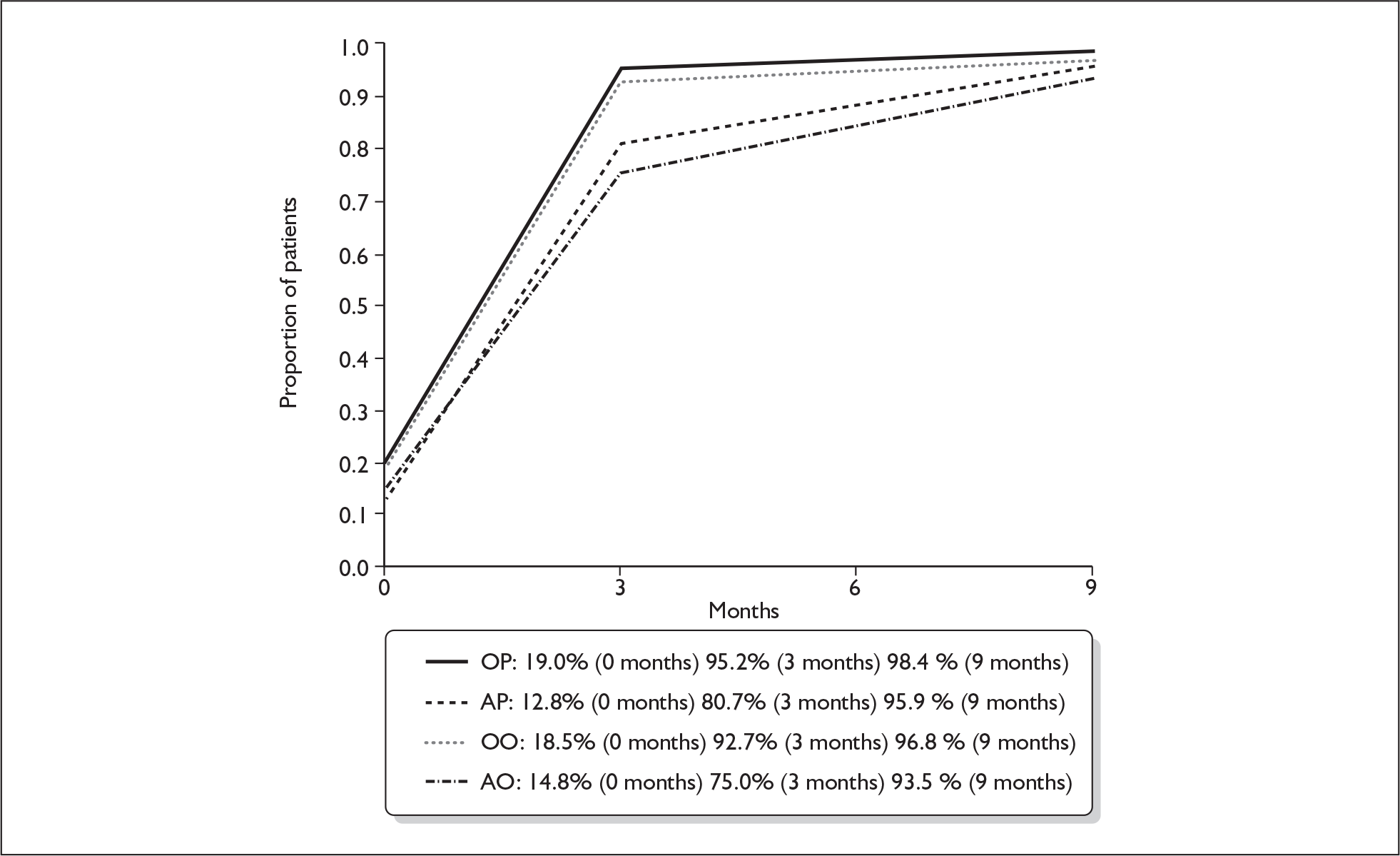
There was no significant aciclovir–prednisolone interaction at 3 months or at 9 months (p = 0.78, p = 0.87 respectively). There were significant differences in complete recovery at 3 months between the prednisolone comparison groups (93.9% for prednisolone, 77.8% for no prednisolone, a difference of + 16.1% (95% CI + 10.1% to + 22.2%, p < 0.001); but otherwise there were no significant differences to be identified between the treatment comparison groups at 3 months or at 9 months.
Secondary outcomes
All the analyses in this section were pre-specified.
Reduction in neurological disability scores
We explored differences in House–Brackmann score at baseline and 9 months, and examined which treatment combination led to the greatest reduction.
We first looked at treatment differences A versus A′ and P versus P′. The mean reduction was greater for those not receiving aciclovir (2.54) than for those receiving aciclovir (2.44) but the difference was not significant (p = 0.345). Similarly, and surprisingly in the context of other results, the mean reduction was greater for those not receiving prednisolone (2.59) than for those receiving prednisolone (2.39) but the difference was not significant (p = 0.074).
We then looked at the extent of reduction for the four different therapies: the mean reduction from greatest to least was 2.64 (OO), 2.54 (AO), 2.45 (OP) and 2.33 (AP); however, there is no evidence that any treatment combination achieved a greater reduction than any other.
Measurement of pain
We first distinguished between patients who reported themselves as ‘in pain, attributed to Bell’s palsy’ and those reporting no pain attributable to their diagnosis. Figure 9 shows the proportion of patients describing themselves as in pain at onset, after 3 months and after 9 months.
FIGURE 9.
Proportion of patients describing themselves as ‘in pain’ at onset, after 3 months and after 9 months. AO, aciclovir–placebo group; AP, aciclovir–prednisolone group; OO, placebo–placebo group; OP, placebo–prednisolone group.
In all four treatment groups there was significantly reduced incidence of pain after 3 months, with no significant additional reduction therafter.
We next assessed patients according to their score on the BPI. Table 19 gives the number of patients assessed, the mean score and standard deviation (SD) for the BPI in each of the treatment groups at onset, after 3 months and after 9 months. The lower number of 9-month visits in groups OP and AP confirms the more rapid recovery of patients randomised to receive prednisolone.
| Treatment group | 0 months | 3 months | 9 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| OP | 125 | 10.9 | 19.6 | 122 | 1.2 | 5.4 | 23 | 1.6 | 4.1 |
| AP | 124 | 9.9 | 16.7 | 123 | 1.8 | 7.2 | 27 | 1.2 | 6.0 |
| OO | 118 | 17.6 | 22.8 | 118 | 2.2 | 9.3 | 46 | 1.8 | 6.9 |
| AO | 122 | 13.8 | 1.4 | 120 | 1.8 | 6.8 | 48 | 1.9 | 5.7 |
In all cases the high coefficient of variation is explained by the high proportion of patients scoring 0 (‘no pain’) during their visit assessment.
The lower BPI scores at the baseline visit in patients who had started oral steroids confirms their rapid effectiveness in reducing the swelling associated with the underlying inflammatory pathophysiology.
Measurement of self-assessed appearance
First we assessed patients according to whether they reported themselves to be dissatisfied or otherwise concerned with their appearance (Part I of the DAS59). Figure 10 gives the proportion of patients expressing themselves as ‘dissatisfied with appearance’ at onset, after 3 months and after 9 months. Patients randomised to prednisolone were least bothered by their appearance at 3 months, and those most bothered were those who received aciclovir. Those on prednisolone alone (OP) are significantly less bothered by their appearance at 9 months.
FIGURE 10.
Proportion of patients dissatisfied with appearance. AO, aciclovir–placebo group; AP, aciclovir–prednisolone group; OO, placebo–placebo group; OP, placebo–prednisolone group.
The DAS is a measuring instrument with wide applicability and (unlike the BPI) the patients were not instructed to restrict their concerns about appearance to symptoms and consequences of Bell’s palsy. Thus, there were references to (e.g.) anxieties about weight and hairline (men) and weight, size and the signs of ageing (women). We attribute the apparent increase in anxiety at the 9-month visits, in all treatment groups, to an increasing willingness to engage with these other issues, once the immediate and pressing issues of Bell’s palsy became diminished with the passage of time.
We next assessed patients according to their score on the DAS59 (Part II). Table 20 gives the number of patients assessed, the mean score and standard deviation for the DAS59 in each of the treatment groups at onset, after 3 months and after 9 months.
| Treatment group | 0 months | 3 months | 9 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| OP | 125 | 72.0 | 36.5 | 121 | 42.8 | 32.1 | 24 | 30.8 | 28.8 |
| AP | 121 | 69.8 | 36.8 | 122 | 42.0 | 32.3 | 27 | 48.2 | 39.1 |
| OO | 116 | 75.7 | 39.7 | 117 | 39.9 | 28.2 | 46 | 49.7 | 38.1 |
| AO | 121 | 74.6 | 41.5 | 120 | 46.5 | 37.3 | 47 | 50.0 | 32.3 |
The reductions noted in all treatment groups after the first 3 months were all statistically significant (p < 0.001 in all cases); the prednisolone-only group (OP) appears unique in that the reduction continues to the 9-month assessment whereas in the three other treatment groups the mean score rises. However, all the perceived differences are non-significant (OP: p = 0.091; AP: p = 0.387; OO: p = 0.074; AO: p = 0.573).
Measurement of self-assessed health utility
Table 21 gives the number of patients assessed, the mean score and standard deviation for the HUI3 in each of the treatment groups at onset, after 3 months and after 9 months.
| Treatment group | 0 months | 3 months | 9 months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| OP | 125 | 0.80 | 0.24 | 121 | 0.92 | 0.16 | 22 | 0.83 | 0.25 |
| AP | 124 | 0.80 | 0.21 | 121 | 0.90 | 0.18 | 27 | 0.85 | 0.26 |
| OO | 114 | 0.76 | 0.20 | 117 | 0.91 | 0.12 | 46 | 0.90 | 0.15 |
| AO | 120 | 0.79 | 0.21 | 119 | 0.90 | 0.13 | 47 | 0.86 | 0.17 |
The quality of life measured using HUI3 at 9 months was significantly higher for patients who did not receive prednisolone than for those who did (p = 0.04). Given that the secondary measures were obtained only in patients who had not recovered at 3 months and given the problem of multiple testing, this result should be interpreted with caution.
Ancillary analyses
We performed one additional analysis of time to recovery against treatment group, fitting a non-parametric Kaplan–Meier survival model for the time to recovery. We found a highly significant beneficial prednisolone effect (p < 0.001 for OP vs OO, p < 0.001 for AP vs AO) and a marginally significant aciclovir effect (p = 0.079 for AO vs OO, p = 0.081 for AP vs OP) with the suggestion that its addition to treatment may slow or impede recovery; and there was negligible aciclovir–prednisolone interaction (p = 0.536).
Key outcome measures for each treatment group are shown in Table 22.
| Treatment | Lower quartile time to full recovery (days) | Median time to full recovery (days) | Upper quartile time to full recovery (days) | Mean time to full recovery (days) |
|---|---|---|---|---|
| OP | 20 | 45 | 77 | 67 |
| AP | 24 | 54 | 87 | 85 |
| OO | 34 | 71 | 174 | 126 |
| AO | 40 | 79 | 240 | 150 |
Adverse events and side effects
Adverse events (deaths)
Three patient deaths were reported to study personnel during follow-up. Such are our processes for patient contact and the maintenance of links with general practices that we are satisfied this is a complete accounting. All deaths were explored as rapidly as possible with relevant hospitals and practices. All were deemed non-treatment-related and there were no requests for or discussions about individual decoding of study treatment allocations. The patient-specific de-identified data are provided in Appendix 21.
Adverse events (other)
Initially the study team advised DMEC of all reports from patients of side effects, albeit standard and well-known. After four reports the Committee chairman indicated that these reports were not necessary. Thereafter symptoms were noted in patient study notes. Any patient reporting anxiety with troubling or persistent symptoms was immediately directed to their general practitioner.
Side effects
A log was maintained of any reports from patients of side effects experienced during the treatment period, these reports being solicited at mid-treatment and end-of-treatment telephone calls, and again discussed at the 3-month assessment visit. The results are provided in Table 23.
| OP | AP | OO | AO | Total | |
|---|---|---|---|---|---|
| Dizziness | 5 | 4 | 4 | 5 | 18 |
| Dyspepsia | 2 | 4 | 3 | 1 | 10 |
| Nausea | 1 | 2 | 3 | 3 | 9 |
| Constipation | 3 | 2 | 1 | 0 | 6 |
| Hunger | 1 | 1 | 0 | 2 | 4 |
| Vomiting | 0 | 2 | 1 | 0 | 3 |
| Insomnia | 1 | 1 | 1 | 0 | 3 |
| Night sweats | 2 | 1 | 0 | 0 | 3 |
| Rash | 0 | 1 | 0 | 2 | 3 |
| Hot flushes | 1 | 1 | 0 | 0 | 2 |
| Depression | 0 | 0 | 0 | 1 | 1 |
| Thirst | 0 | 0 | 1 | 0 | 1 |
| Anorexia | 0 | 1 | 0 | 0 | 1 |
| Diarrhoea | 0 | 0 | 0 | 1 | 1 |
| Drowsiness | 0 | 0 | 1 | 0 | 1 |
| Pruritus | 0 | 1 | 0 | 0 | 1 |
| Combinations of minor symptomsa | 8 | 4 | 3 | 3 | 18 |
| Subtotal | 24 | 25 | 18 | 18 | 85 |
| Death | 0 | 0 | 2 | 1 | 3 |
| Total | 24 | 25 | 20 | 19 | 88 |
Chapter 5 Economic evaluation of treatments
Introduction
Health-care resources are always scarce. The cost of a particular treatment can be seen not as its monetary value but as the foregone benefits [i.e. years of life, better quality of life (QoL)] of an alternative treatment that we cannot provide when we decide to use these scarce resources in a particular way. This is the notion of opportunity cost – the central concept of economics – that is used to help identify how we can get the maximum benefit from the limited resources available (i.e. obtain an efficient allocation of resources). Economic evaluation provides guidance on how best to use resources as it is a systematic analysis comparing the resources used (costs) and benefits of alternative courses of action. 32 An economic evaluation, in this context, would involve assessing the relative costs and benefits associated with the alternative treatments, included in this clinical trial for the treatment of Bell’s palsy. 33
How an economic evaluation brings together information on costs and effects is illustrated in Figure 11. The vertical axis represents the difference in costs between an experimental (e.g. an ‘active treatment’ for Bell’s palsy) and a control treatment (e.g. a ‘placebo’). The difference in cost will reflect the difference in the value of the resources used to provide treatment (e.g. medications) as well as the resource consequences of treatment (e.g. the costs of the use of health services during the follow-up period). The horizontal axis represents differences in effectiveness between the two approaches, which might be measured in clinical terms, e.g. the reduction in House–Brackmann grading score, or other measures such as quality-adjusted life-years (QALYs). The latter combines estimates of both QoL with estimates of length of life. The wider the definition of effectiveness used, usually, the more likely it is to measure outcomes of importance to individuals.
FIGURE 11.
Relationship between the difference in costs and effects between a new (experimental) intervention and a standard (control) intervention.
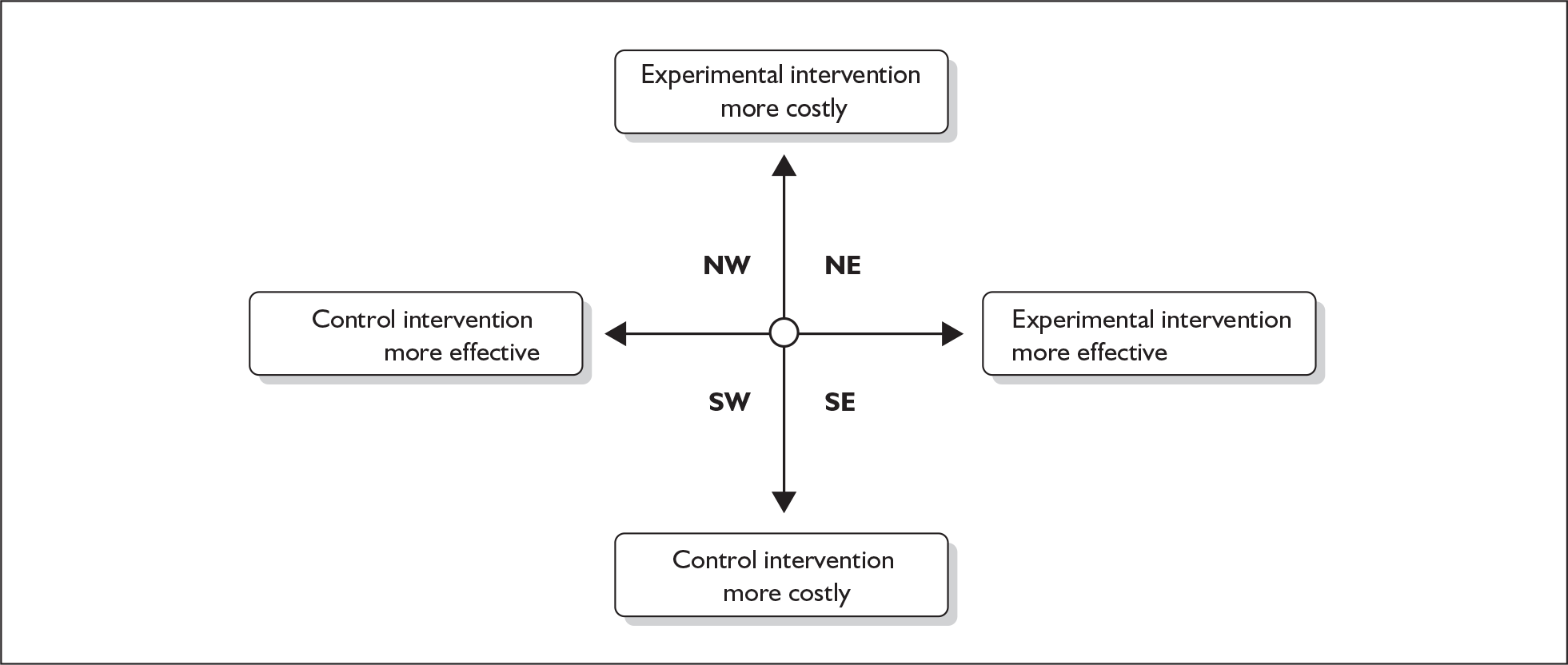
In the north-west (NW) and south-east (SE) quadrants of Figure 11 a clear decision about which treatment should be preferred is provided because one or the other treatment ‘dominates’. In the NW quadrant the experimental treatment is more costly and provides less benefit and therefore the control treatment is more efficient (is dominant). In the SE quadrant the opposite situation occurs and the experimental treatment is more efficient (is dominant) as it is less costly and provides more benefit. The circle in the centre of the figure represents the possibility that no meaningful differences in costs or benefits exist between the treatments and for practical purposes the two interventions are equally efficient. In the two remaining areas of the figure, the north-east (NE) and south-west (SW) quadrants, a judgement is required as to whether the more effective treatment is worth the extra cost. To aid these judgements, information can be provided in terms of an incremental cost-effectiveness ratio (ICER). This is the difference in mean costs between treatment and the control groups divided by the difference in mean effectiveness between treatment and control groups. The higher the ICER for the comparison of one intervention with another, then the less likely it is that this intervention will be considered efficient.
Aim
The purpose of this section is to assess the cost-effectiveness of early administration of prednisolone and or aciclovir compared with placebo for treatment of Bell’s palsy in an adult population in the UK.
Three separate analyses are presented, which correspond to the factorial design of the trial:
-
prednisolone versus no prednisolone (P vs P′)
-
aciclovir versus no aciclovir (A vs A′)
-
AP versus OP versus AO versus OO.
As described below the methods used to make these three comparisons are similar. The study was designed as a 2 × 2 factorial design. The first two analyses can be conducted as there is no evidence of any interaction between prednisolone and aciclovir. Arguably, for an economic evaluation the more useful comparison is the four-arm comparison. However, as the study was not powered to compare the four treatments the results of any economic evaluation are subject to a further lack of precision.
Methods
The trial, as is commonly the case, was not powered to detect difference in the cost-effectiveness of the different treatments. Thus, a modelling approach was adopted as a way of gaining precision in the cost-effectiveness estimates.
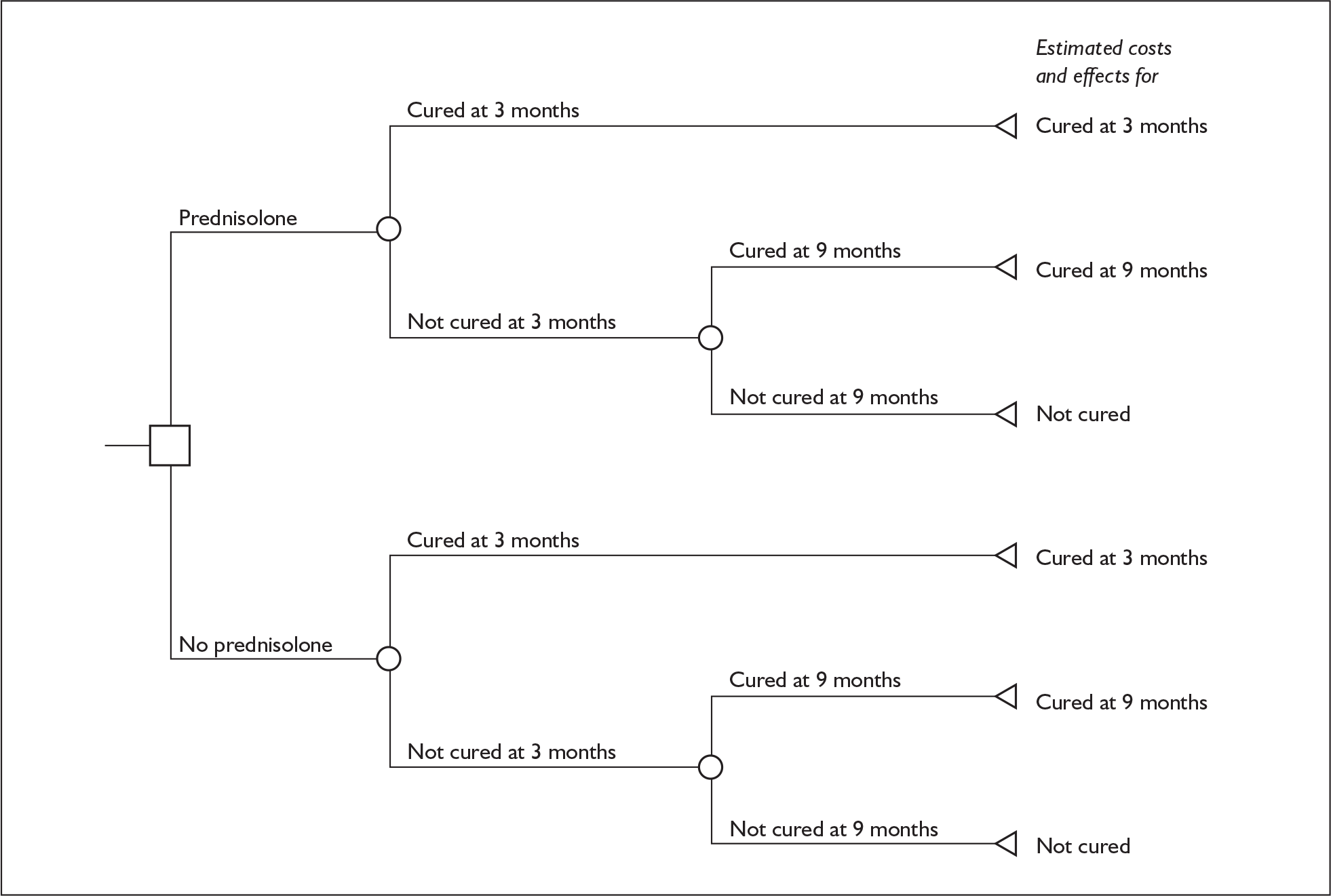
Model structure of the two-arm models
Decision tree models were constructed to compare the cost-effectiveness of prednisolone against no prednisolone and aciclovir against no aciclovir. Figure 12 shows the structure of the model for prednisolone, and a tree with a similar structure was used for the aciclovir cost-effectiveness analysis. Within these models it is assumed that the different trial interventions affect the probability of being cured or not cured. The consequences of being cured or not cured are assumed to be independent of the initial therapy to which an individual was allocated. The definition of ‘cured’ follows that already used in the analysis of treatment differences: namely, an individual is classified as being cured if he or she has a value for the House–Brackmann grading system equal to 1.
FIGURE 12.
Bell’s palsy decision tree model: prednisolone vs no prednisolone.
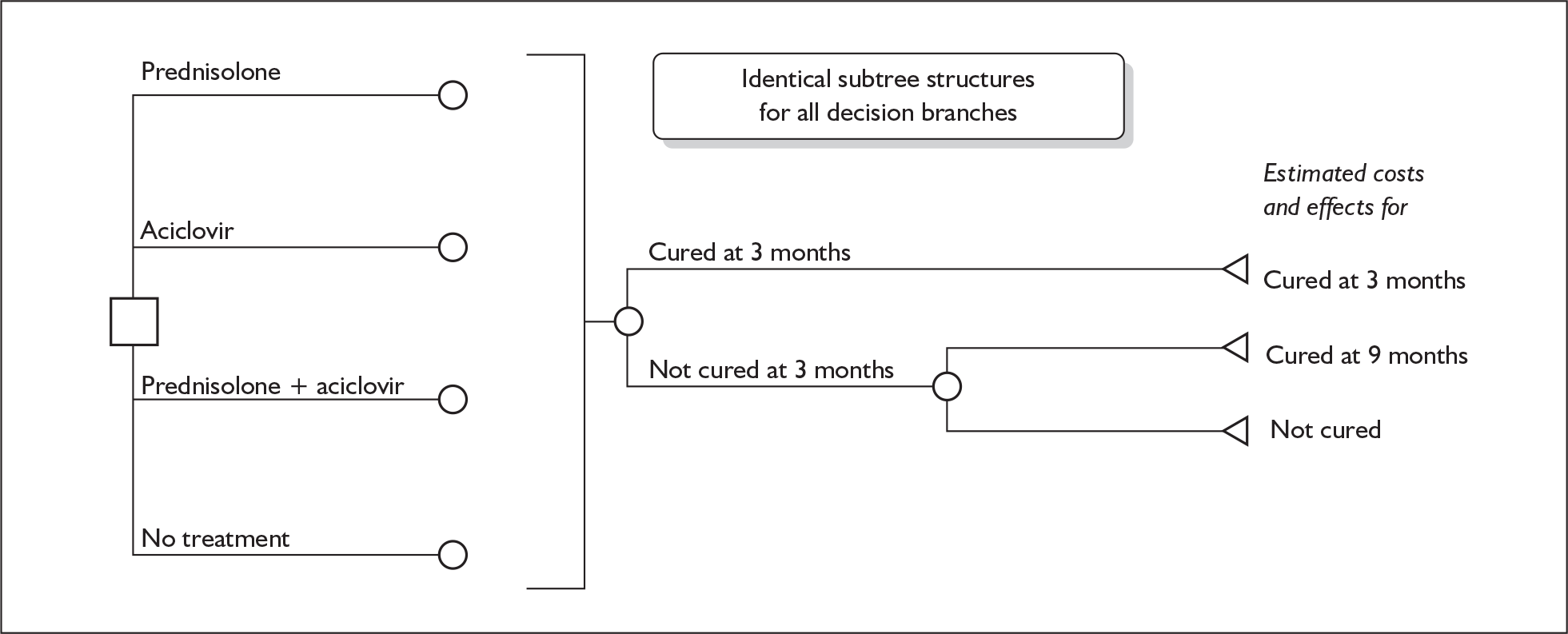
Model structure of the four-arm model
A further decision tree model was developed for the third comparison performed (see Figure 13). This decision tree has four decision branches, which reflect the four groups provided by the 2 × 2 factorial trial design. Again it has been assumed that the costs of the consequences of being cured or not cured are independent of the initial treatment a person was allocated to.
FIGURE 13.
Decision tree model for early treatment for Bell’s palsy: prednisolone alone vs aciclovir alone vs prednisolone + aciclovir vs no treatment (placebo).
Parameter estimates used in the model
Parameter estimates on probabilities, costs and effectiveness, required to populate the model, were developed mainly from trial data. These data related to the risk of being cured or not cured at different time points, health services resource use, and costs and health state utilities.
Probability of cure and not cure
Two-arm model
Tables 24 and 25 show the proportion of subjects cured and not cured at 3 and 9 months. These proportions were used as the probability of being cured and not cured at 3 and 9 months within the models. Normal probability distributions were attached to the difference of mean values between groups to allow for parameter uncertainty.
| Prednisolone | No prednisolone | Difference (95% CIs) | PD assumed for difference | |
|---|---|---|---|---|
| Probability of being cured at 3 months | 0.83 | 0.64 | 0.19 (0.12 to 0.27) | Normal |
| Probability of being cured at 9 months given not cured at 3 months | 0.49 | 0.61 |
| Aciclovir | No aciclovir | Difference (95% CIs) | PD assumed for difference | |
|---|---|---|---|---|
| Probability of being cured at 3 months | 0.71 | 0.76 | – 0.05 (– 0.12 to 0.03) | Normal |
| Probability of being cured at 9 months given not cured at 3 months | 0.49 | 0.61 |
Four-arm model
Table 26 shows the same data but reported to the four groups of the 2 × 2 factorial trial.
| Prednisolone alone | Aciclovir alone | Aciclovir + prednisolone | Placebo alone | |
|---|---|---|---|---|
| Probability of being cured at 3 months (SE) | 0.84 (0.03) | 0.60 (0.04) | 0.78 (0.04) | 0.65 (0.04) |
| Probability of being cured at 9 months given not cured at 3 months (SE) | 0.71 (0.11) | 0.44 (0.07) | 0.68 (0.09) | 0.57 (0.08) |
| Probability distributions | Normal | Normal | Normal | Normal |
Health-care resource use and costs
The costs estimates used in the model were based on the cost of the initial treatments, and follow-up costs. Follow-up costs included the use of resources in primary and secondary care, the unit costs of these resources, and the subsequent use of other medications.
Treatment costs
The doses and length of treatment for trial medications were defined by the trial protocol. The unit costs were obtained from the British National Formulary (BNF)34 (see Table 27). These data were applicable to both the two-arm and four-arm models.
| Drug | Dose | Cost | Note | BNF web pagea |
|---|---|---|---|---|
| Prednisolone | 50 mg/day× 10 days | 4.32 |
Prednisolone Tablets, 25 mg, 56-tab pack = £12.09 |
http://www.bnf.org |
| Aciclovir | 2000 mg/day× 10days | 6.57 |
Aciclovir Tablets, 400 mg, 56-tab pack = £7.31; 800 mg, 35-tab pack = £9.22 |
http://www.bnf.org |
Follow-up costs
Primary and secondary care resource use
Health-care resources used were collected from primary care case notes on any contacts made with health services or resources used by trial participants. This analysis was based on a 15% sample of study participants who completed the trial (n = 74). In order to maximise efficiency of researcher time in visiting practices to collect data, only practices who had referred two or more subjects into the study were visited. Data collected on primary care resource use included visits or phone calls to: a general practitioner, practice nurse, district nurse, community therapy services, and health visitor. Data collection on the use of secondary care services included: hospital inpatient and day-case admissions to general medicine and general surgery. Finally, hospital outpatient resource use included contacts with A&E, acute services, dermatology, ENT, gastroenterology, mental health, neurology, occupational therapist, ophthalmology, orthopaedics, physiotherapy, radiology, speech therapist, urology and general health-care assistants. All data collection was masked as to the allocation group.
Tables 28–32 present selected summary statistics for the main resource use categories, by trial arm and also whether someone was cured or not cured. It is these latter estimates of resource use for those cured or not cured that were used within all three models.
| Concept | No prednisolone | Prednisolone | ||||
|---|---|---|---|---|---|---|
| Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | |
| n | 42 | 42 | 40 | 32 | 33 | 31 |
| Mean (SD) | 3.07 (4.47) | 0.07 (0.34) | 0.88 (1.84) | 1.78 (2.25) | 0.12 (0.33) | 0.65 (1.05) |
| Median [IQR] | 2 [0–4] | 0 [0–0] | 0 [0–1] | 1 [0–2.5] | 0 [0–0] | 0 [0–1] |
| Concept | No aciclovir | Aciclovir | ||||
|---|---|---|---|---|---|---|
| Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | |
| n | 48 | 48 | 46 | 26 | 27 | 25 |
| Mean (SD) | 2.25 (2.67) | 0.08 (0.28) | 0.74 (1.36) | 3 (5.15) | 0.11 (0.42) | 0.84 (1.86) |
| Median [IQR] | 2 [0–3] | 0 [0–0] | 0 [0–1] | 1 [0–3] | 0 [0–0] | 0 [0–0] |
| Concept | Prednisolone only | Aciclovir only | ||||
|---|---|---|---|---|---|---|
| Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | |
| n | 22 | 22 | 21 | 16 | 16 | 15 |
| Mean (SD) | 1.77 (1.88) | 0.14 (0.35) | 0.62 (0.8) | 3.75 (6.09) | 0.13 (0.5) | 0.93 (2.12) |
| Median [IQR] | 1 [0–3] | 0 [0–0] | 0 [0–1] | 2 [0.5–4.5] | 0 [0–0] | 0 [0–1] |
| Concept | Prednisolone and aciclovir | Placebo only | ||||
|---|---|---|---|---|---|---|
| Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | |
| n | 10 | 11 | 10 | 25 | 25 | 25 |
| Mean (SD) | 1.8 (3.05) | 0.09 (0.3) | 0.7 (1.49) | 2.76 (3.19) | 0.04 (0.2) | 0.84 (1.7) |
| Median [IQR] | 1 [0–2] | 0 [0–0] | 0 [0–0] | 2 [1–4] | 0 [0–0] | 0 [0–1] |
| Concept | Cured at 3 months | Cured at 9 months | Not cured | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | Primary care (contacts) | Hospital (inpatient days and day cases) | Hospital outpatient (visits) | |
| n | 52 | 53 | 51 | 11 | 11 | 10 | 9 | 9 | 9 |
| Mean (SD) | 2.15 (3.9) | 0.11 (0.38) | 0.49 (1.17) | 3.82 (3.68) | 0.09 (0.3) | 1.8 (2.62) | 3.22 (2.73) | 0 (0) | 1.22 (1.56) |
| Median [IQR] | 1 [0–2] | 0 [0–0] | 0 [0–1] | 3 [1–5] | 0 [0–0] | 0.5 [0–3] | 3 [2–3] | 0 [0–0] | 1 [0–1] |
Primary and secondary care resource use for the two-arm comparisons drawn in the trial
Primary and secondary care resource use for the four arms of the trial
Unit costs of primary and secondary care services
Unit costs for hospital-based services (inpatient days, day cases and outpatient visits) were obtained from Information Services Department (ISD) for Scotland. 35 Day cases and inpatient unit costs were calculated using total gross cost and deducting the overheads allocated to the particular cost category (e.g. total allocated cost per case within the ISD tables). Furthermore, total direct costs per attendance were used for outpatient visits. 35 Unit costs for primary care-based services were obtained from Unit costs of health and social care36 (see Table 33). These unit costs were applicable to both the two-arm and four-arm models.
| Service/ward | Unit | Cost per unit £2005/06 | Note |
|---|---|---|---|
| Hospital costs | |||
| General Medicine | Day case | 309 | ISD35 Table R042 |
| General Medicine | Day | 1061 | ISD35 Table R040 |
| General Surgery | Day case | 506 | ISD35 Table R042 |
| General Surgery | Day | 1671 | ISD35 Table R040 |
| General Practice | Day case | 243 | ISD35 Table R042 |
| Hospital outpatient costs | |||
| A&E | Visit | 64 | ISD35 Table R044 |
| Acute Services | Visit | 238 | ISD35 Table R044 |
| Dermatology | Visit | 66 | ISD35 Table R044 |
| ENT | Visit | 74 | ISD35 Table R044 |
| Gastroenterology | Visit | 142 | ISD35 Table R044 |
| Mental Health | Visit | 98 | ISD35 Table R044 |
| Neurology | Visit | 131 | ISD35 Table R044 |
| Occupational Therapist | Visit | 34 | ISD35 Table R046 |
| Ophthalmology | Visit | 52 | ISD35 Table R044 |
| Orthopaedics | Visit | 79 | ISD35 Table R044 |
| Physiotherapy | Visit | 19 | ISD35 Table R046 |
| Radiology | Visit | 32 | ISD35 Table R046 |
| Speech Therapist | Visit | 49 | ISD35 Table R046 |
| Urology | Visit | 65 | ISD35 Table R044 |
| Health-Care Assistant | Visit | 24 | ISD35 Table R045 |
| Primary care costs | |||
| General Practice | Visit | 23 | PSSRU36 Table 9.8b General practitioner, p. 143 |
| General Practice | Telephone consultation | 25 | PSSRU36 Table 9.8b General practitioner, p. 143 |
| Practice Nurse | Visit | 8 | PSSRU36 Table 9.6 Nurse (GP practice), p. 140 |
| Practice Nurse | Telephone consultation | 8 | PSSRU36 Table 9.6 Nurse (GP practice), p. 140 |
| District Nurse | Visit | 18 | PSSRU36 Table 9.1 Community nurse (includes district nursing sister, district nurse), p. 135 |
| Community Therapy Services | Visit | 14 | Assumed same as above Therapists Services36 |
| Health Visitor | Visit | 31 | PSSRU36 Table 9.3 Health visitor, p.137 |
Unit costs of health and social care
Use and cost of medications
This category relates to the use of any subsequent therapy to manage symptoms. The use of other medications was identified from patient primary care case notes. Unit costs were again obtained from the BNF website in May 2007 (see Table 34). Due to the wide variety of medications identified only summary cost details have been reported in terms of the mean costs for those cured and not cured. These data were used to inform both the two- and four-arm models.
| n | Mean (£) | SD (£) | Range (£) | Interquartile range (£) | |||
|---|---|---|---|---|---|---|---|
| Min. | Max. | p25 | p75 | ||||
| Cured (at 3 months) | 53 | 29.13 | 59.56 | 0 | 365.91 | 0 | 34.32 |
| Not cured (at 3months) | 20 | 113.17 | 274.66 | 0 | 1205.02 | 0 | 48.06 |
Determination of mean cost estimates
Using data described in this section (Health-care resource use and costs), estimates of the total mean costs for those cured and not cured were determined (see Table 35). To obtain these cost estimates a simple ordinary least squared (OLS) regression was fitted to the data obtained from the 74 people for whom data collection was possible. The total mean values used within the three models were £210 and £315, for cured and not cured at 3 months, respectively. To these costs the cost of initial medication was added (see Table 27).
| Dependent variable total costa | |||
|---|---|---|---|
| Coefficient | SE | 95% CI | |
| Constant | 210 | 58.39 | 93.28 to 326.32 |
| Not cured at 3 months | 105 | 112.08 | – 118.59 to 328.73 |
Normal distributions were added to the total cost of being cured and not cured. The total cost of not cured was bounded at zero within the probabilistic sensitivity analysis.
Estimation of utilities
Data were collected on HUI3 at baseline, 3 months and, if trial participants were not cured at 3 months, they were assessed also at 9 months. Two analyses of covariance adjusting for baseline HUI3 scores were used to obtain utility weights for participants who were cured and not cured at 3 and 9 months. Table 36 shows the results of these analyses. These data were used to estimate the utility scores for those cured at 3 months, those cured at 9 months and those not cured. In order to reflect the statistical imprecision surrounding these estimates when used in the model, normal distributions were attached to the mean values based upon the results of a regression analysis. These data were used in both the two-arm and the four-arm models. Table 36 also reports data for HUI3 at baseline for all participants from the trial analyses.
| Dependent variable: HUI3 at 3 months | |||
|---|---|---|---|
| Number of obs = 487 | |||
| Coefficient | SE | 95% CI | |
| Constant | 0.6146 | 0.0235 | 0.5684 to 0.6609 |
| Cured | 0.0574 | 0.0132 | 0.0314 to 0.0834 |
| Dependent variable: HUI3 at 9 months | |||
| Number of obs = 137 | |||
| Coefficient | SE | 95% CI | |
| Constant | 0.5265 | 0.0495 | 0.4287 to 0.6243 |
| Cured | – 0.0019 | 0.0293 | – 0.0599 to 0.0561 |
| Utility weights (mean values) | |||
| Cured at 3 months | Cured at 9 months | Not cured | |
| 0.9947 | 0.9900 | 0.9919 | |
| Baseline characteristics: HUI3 data all participants | |||
| Mean = 0.786 | SD = 0.216 | ||
With the information in Table 36 utility weights were calculated using the area under the curve method. For instance, for a participant cured at 3 months the QALY weight used in the model would be:
Substituting values into this expression gives:
A similar approach was used to estimate QALYs for those cured at 9 months and QALYs for those not cured.
Base case analysis
Base case analyses were conducted for the two randomised controlled comparisons included in the trial: prednisolone versus no prednisolone and aciclovir versus no aciclovir. A further analysis comparing all four randomised arms was also conducted although, as noted above, the trial was not adequately powered even for its primary outcome for this comparison. For all analyses cumulative mean costs were estimated for the 9-month follow-up period of the trial. All costs were expressed in 2006/07 pounds Sterling. Effectiveness was measured in terms of number of cases cured (e.g. House–Brackmann score = 1), and mean QALYs for the 9-month time horizon. As the time horizon for the analyses was less than a year, neither cost nor effectiveness outcomes were discounted. ICERs were calculated; these measure the extra cost needed for gaining a further unit of effectiveness.
Deterministic and probabilistic sensitivity analyses were conducted. The latter involved attaching probability distributions to the model parameters and conducting Monte Carlo simulations. One thousand iterations were obtained for each Monte Carlo simulation conducted. Therefore, for both base case analyses, the analysis comparing the four arms of the trial and for each of the sensitivity analyses, one thousand mean cost and mean effects pairs were obtained. From these, incremental cost and incremental effectiveness could be obtained and/or cost-effectiveness acceptability curves (CEAC) developed using the net benefit approach. The net benefit approach is entirely equivalent to the standard rule in terms of ICER; however, when applying this approach to Monte Carlo simulation data, it has the advantage of unambiguously sorting out the acceptability of an individual simulation trial on the cost-effectiveness plane. This is not the case with the ICER approach, where simulations of the same sign but within opposite quadrants could be mixed up. 37
Point estimates are shown for deterministic results, whereas scatter plots and cost-effectiveness acceptability curves are used for the main probabilistic analyses. These curves plot the probability of each strategy being the optimal decision against a range of values for society’s willingness to pay for an extra unit of effectiveness. For the probabilistic analysis results are also reported on the likelihood of an intervention being considered cost-effective for society’s willingness to pay at threshold values of £10,000, £20,000, £30,000 and £50,000.
Central to the assessment of cost-effectiveness is the value that society would put on gaining an additional QALY. For instance, the National Institute for Health and Clinical Excellence (NICE) states that ‘Below a most plausible ICER of £20,000 per QALY, judgements about the acceptability of a technology as an effective use of NHS resources are based primarily on the cost-effectiveness estimate. Above a most plausible ICER of £20,000 per QALY, judgements about the acceptability of the technology as an effective use of NHS resources are more likely to make more explicit reference to factors including:
-
the degree of uncertainty surrounding the calculation of ICERs;
-
the innovative nature of the technology;
-
the particular features of the condition and population receiving the technology; and
-
where appropriate, the wider societal costs and benefits.
Above an ICER of £30,000 per QALY, the case for supporting the technology on these factors has to be increasingly strong.’38 (p. 33). In the absence of a more definitive statement this report focuses on a willingness-to-pay of £30,000 for a QALY.
Sensitivity analysis
Although probabilistic analyses were performed that reflect the statistical imprecision in model parameters, there are other forms of imprecision that need to be explored using sensitivity analysis. These sensitivity analyses related to changes in key parameters used in the model, for example unit cost values, or to changes in model assumptions relating to the derivation of cost and the definition of cure. With respect to cost, it is well known that often cost data are skewed to the right. In other words, there are usually a few trial participants for whom costs are extremely high. A sensitivity analysis was conducted taking these potential outliers out of the analysis.
Potential drivers in these models are the probability of being cured or not cured at 3 months; therefore, threshold analysis was also used to explore the effect of the probability of being cured or not cured on the model results. In addition, subgroup analyses by age and gender were also performed. Finally, structural uncertainty was explored by assuming an exponential regression analysis for total costs instead of the original ordinary least-squared regression.
Results
Two-arm models
Prednisolone vs no prednisolone model
Cost-effectiveness analysis
When the proportion of cases cured is used as the measure of effectiveness, prednisolone has a lower mean cost and is more effective than the no prednisolone alternative, i.e. it is the SE quadrant of Figure 11 (see Table 37). Thus, prednisolone dominates the ‘no prednisolone intervention.
Cost–utility analysis
Table 38 shows cost-effectiveness deterministic and probabilistic results when QALYs are used as the effectiveness. This table shows the likelihood of a particular treatment to be considered cost-effective for alternative values of willingness to pay for an extra QALY. As Table 38 shows, the results of the cost–utility analysis are similar to those from the cost-effectiveness analysis.
| Treatment | Cost (£) | QALYs | ICER | Probability that intervention is cost-effective for different threshold values for society’s willingness to pay for a QALY | |||
|---|---|---|---|---|---|---|---|
| 10,000 | 20,000 | 30,000 | 50,000 | ||||
| Prednisolone | 231.98 | 0.718 | 79.3% | 77.5% | 77.0% | 76.0% | |
| No prednisolone | 248.05 | 0.717 | Dominated | 20.7% | 22.5% | 23.0% | 24.0% |
The results of the probabilistic analysis indicate that prednisolone is likely to be considered a cost-effective treatment at all values for society’s willingness to pay for a QALY. A further illustration of these results is provided by the scatterplot (Figure 14) and the cost-effectiveness acceptability curves (Figure 15).
FIGURE 14.
Incremental cost-effectiveness scatterplot. Prednisolone vs no prednisolone model.
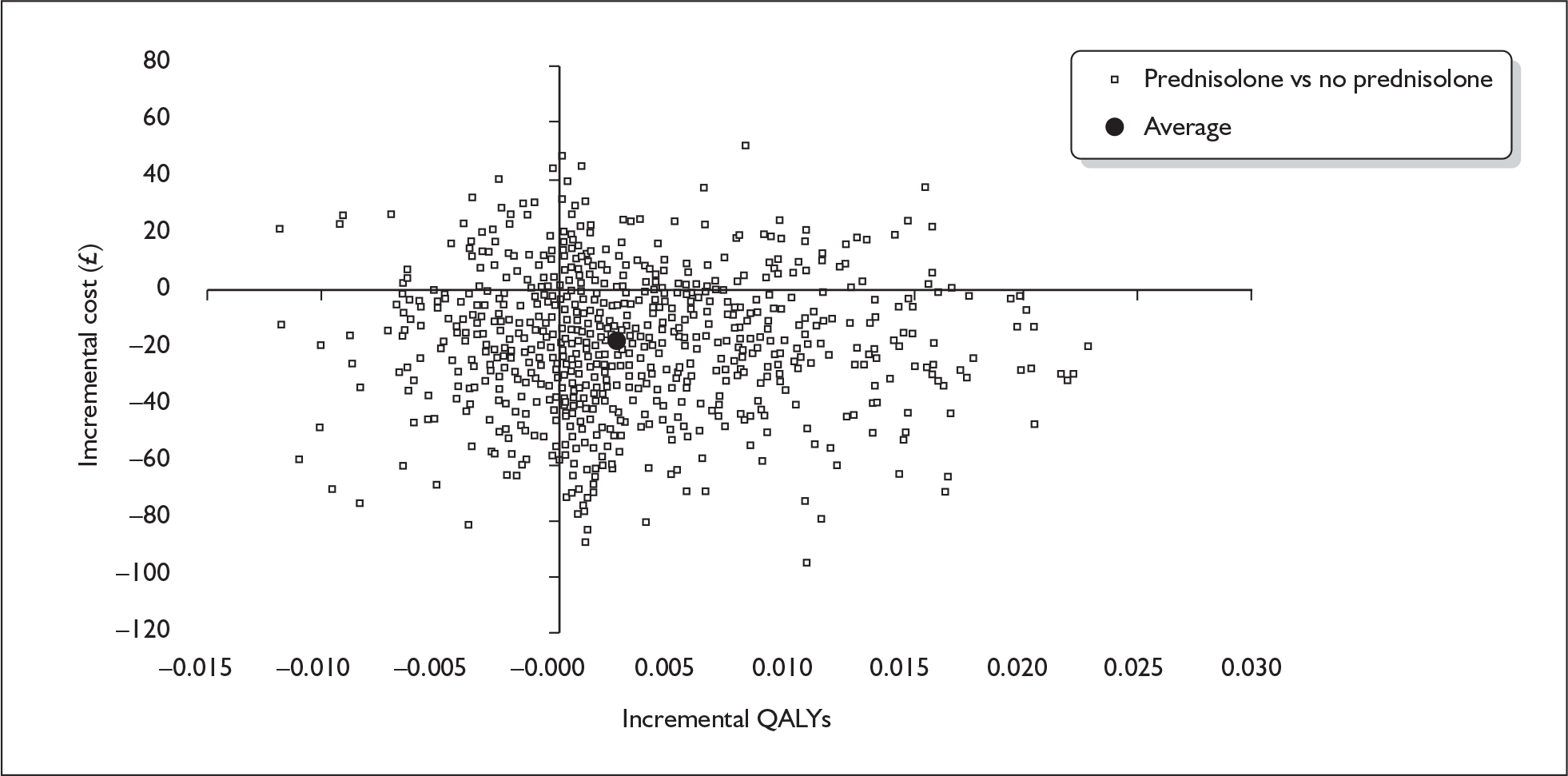
FIGURE 15.
Cost-effectiveness acceptability curves. Prednisolone vs no prednisolone model.
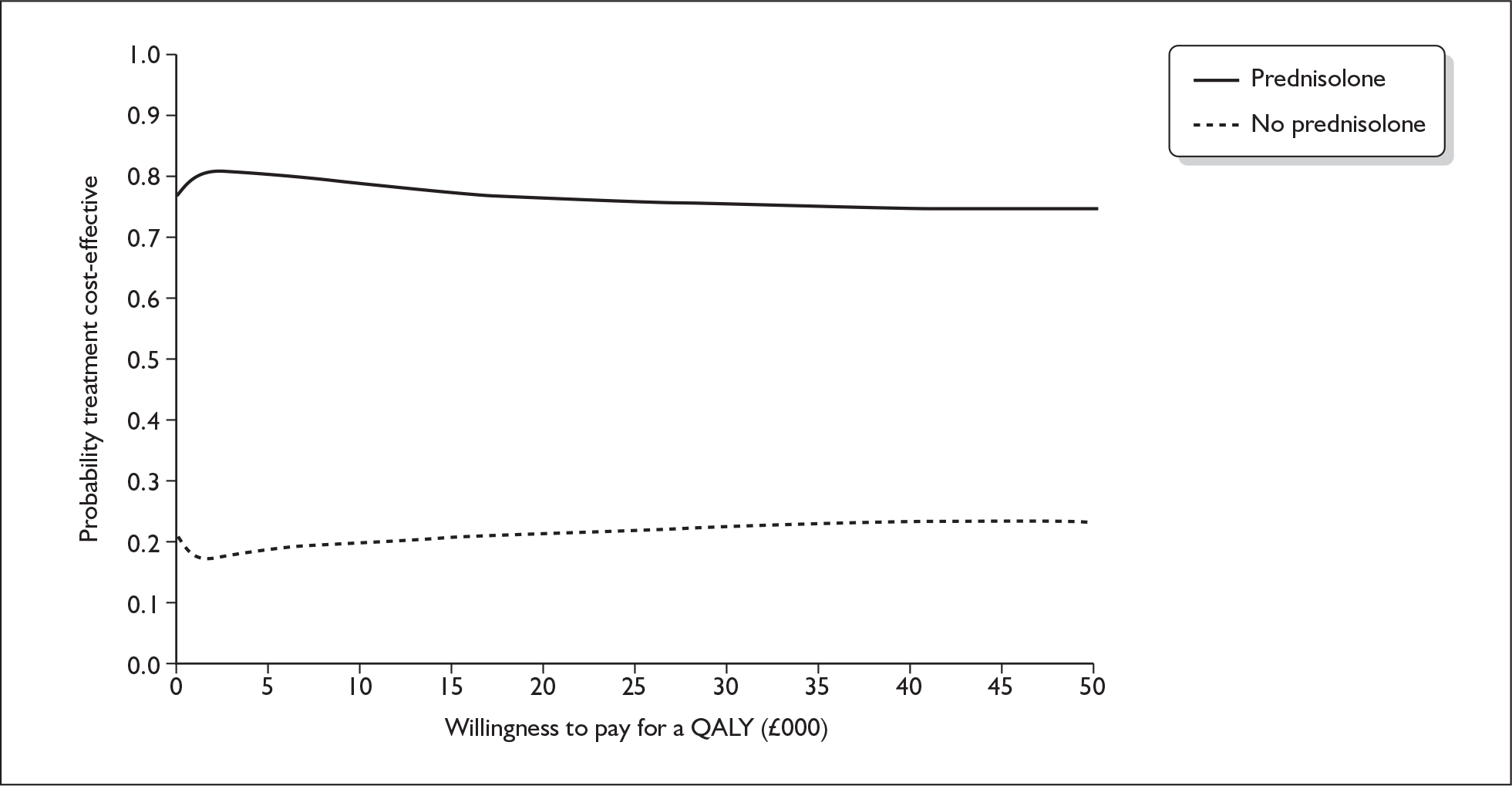
Figure 14 shows the scatterplot of the difference in cost and effects pairs for the comparison of prednisolone with no prednisolone from the Monte Carlo simulation (represented by the clear squares on the figure). A high proportion of the dots are allocated within the SE quadrant. This means that, for those cases, prednisolone produced more QALYs and was less costly than no prednisolone, and therefore prednisolone is cost-effective for these iterations. The opposite argument applies to those cases that fall within the NW quadrant (for these iterations the no prednisolone option is cost-effective). Finally, for those iterations that fall within the NE and SW quadrants the decision for or against prednisolone will depend on the threshold value of WTP for an extra QALY.
Figure 15 shows the cost-effectiveness acceptability curves derived using the cost-effectiveness data. These curves show how likely a particular option would be considered cost-effective for alternative values for society’s willingness to pay for an extra QALY. As this figure shows there is approximately an 80% chance that prednisolone will be considered cost-effective compared with no prednisolone for values for a cost per QALY that society might consider worthwhile.
Aciclovir vs no aciclovir model
Cost-effectiveness analysis
Table 39 shows the incremental cost per case cured for the comparison of aciclovir with no aciclovir. The no aciclovir alternative has on average lower costs and a higher proportion of individuals recovered. Therefore, on average no aciclovir dominates aciclovir treatment.
| Treatment | Cost (£) | Cured casesa at 9 months (%) | ICER |
|---|---|---|---|
| No aciclovir | 235.33 | 90.8% | |
| Aciclovir | 246.63 | 85.4% | Dominated |
Cost–utility analysis
In terms of incremental cost per QALY, the results suggest that no aciclovir is on average more effective and less costly that aciclovir, and probabilistic analysis reinforces this finding (see Table 40).
| Treatment | Cost (£) | QALYs | ICER | Probability that intervention is cost-effective for different threshold values for society’s willingness to pay for a QALY | |||
|---|---|---|---|---|---|---|---|
| 10,000 | 20,000 | 30,000 | 50,000 | ||||
| No aciclovir | 235.33 | 0.718 | 91.1% | 85.1% | 82.2% | 79.0% | |
| Aciclovir | 246.63 | 0.717 | Dominated | 8.9% | 14.9% | 17.8% | 21.0% |
The scatterplot (Figure 16) of the incremental cost and QALY pairs from the Monte Carlo simulation shows that the majority of the iterations lie within the NW quadrant (e.g. aciclovir more costly and less effective than no aciclovir). The cost-effectiveness acceptability curves (Figure 17) show that aciclovir was unlikely to be considered cost-effective at values compared with no aciclovir.
FIGURE 16.
Incremental cost-effectiveness scatterplot. Aciclovir vs no aciclovir model.
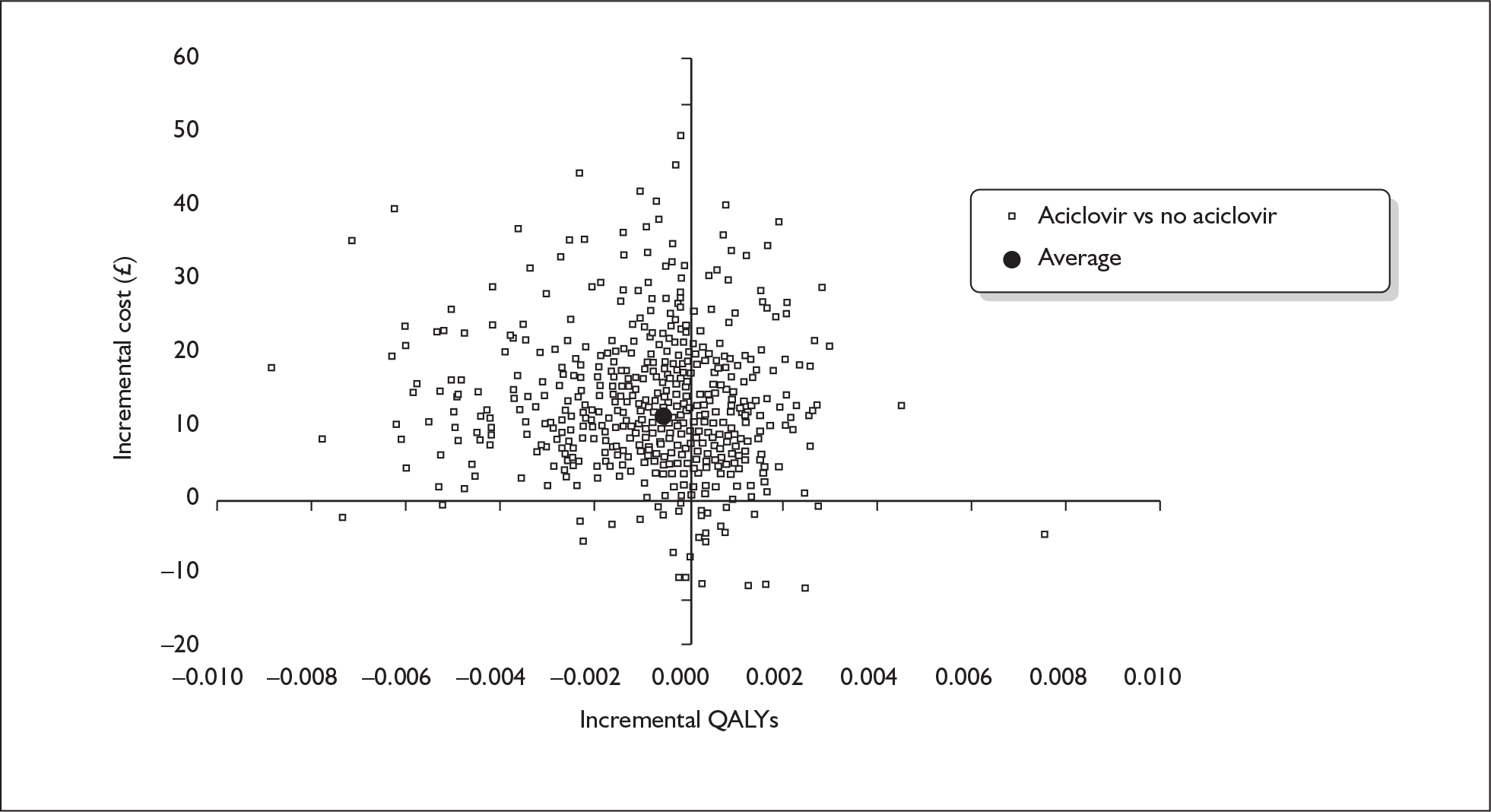
FIGURE 17.
Cost-effectiveness acceptability curves. Aciclovir vs no aciclovir model.
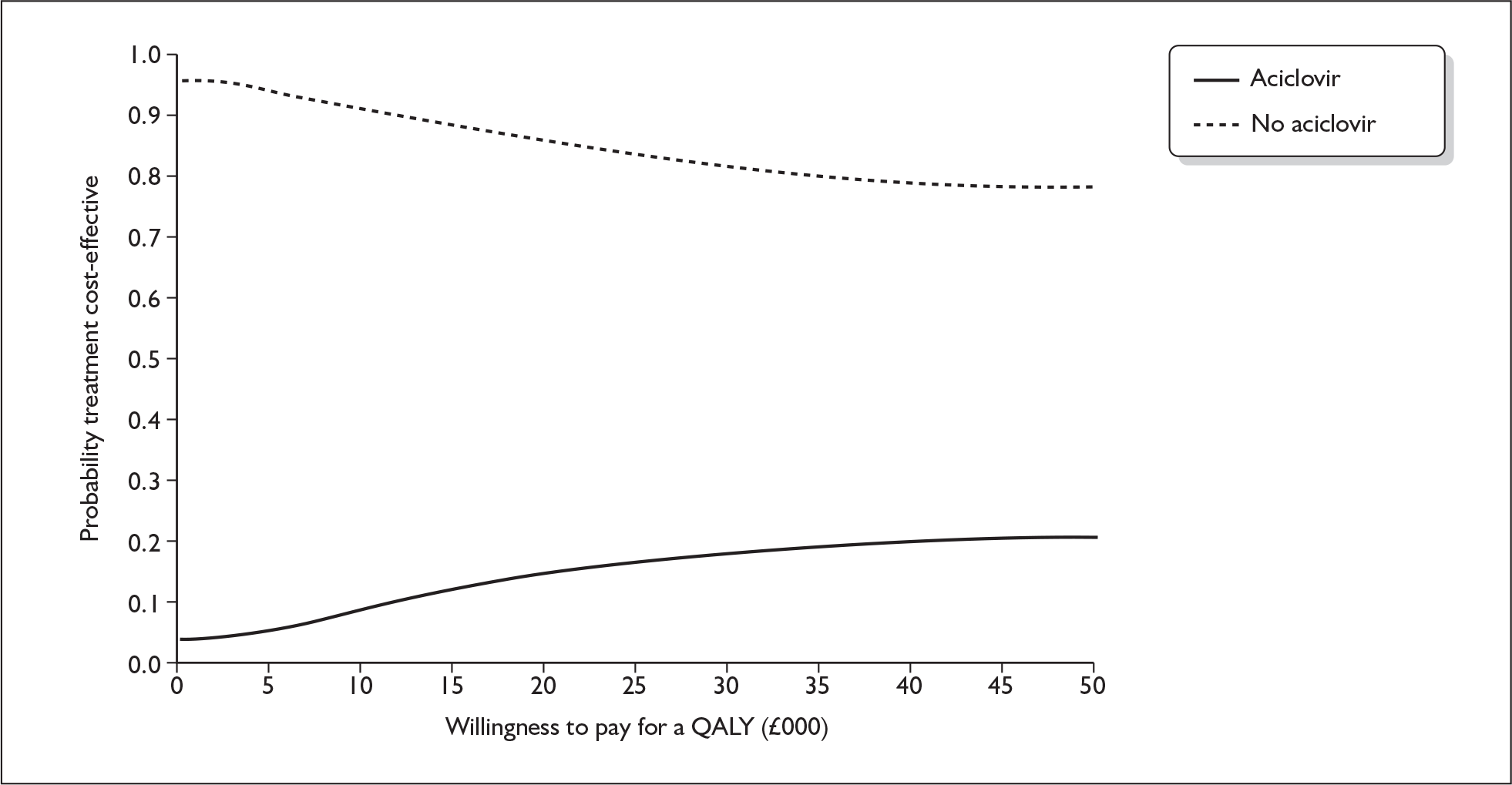
Comparison of all four randomised groups in the four-arm model
Cost-effectiveness analysis
Table 41 shows the deterministic cost-effectiveness analysis results. On average prednisolone only is the least costly and most effective of the four alternative interventions out of the prednisolone-only, aciclovir-only, aciclovir and prednisolone, and placebo-only arms model, i.e. it dominates the other interventions.
Cost–utility analysis
Prednisolone only is on average less costly and produces more QALYs than any of the other treatments (see Table 42). Furthermore, it has approximately an 80% chance of being considered cost-effective compared with the other treatments. This is illustrated by the cost-effectiveness acceptability curves (Figure 18), which indicate that collectively the other interventions have only a 20% chance of being considered cost-effective.
| Treatment | Cost (£) | QALYs | ICER | Probability that intervention is cost-effective for different threshold values for society’s willingness to pay for a QALY | |||
|---|---|---|---|---|---|---|---|
| 10,000 | 20,000 | 30,000 | 50,000 | ||||
| Prednisolone | 230.62 | 0.719 | 79.1% | 77.4% | 76.9% | 75.9% | |
| Aciclovir and prednisolone | 244.02 | 0.718 | Dominated | 0.0% | 0.0% | 0.0% | 0.1% |
| No treatment | 246.47 | 0.717 | Dominated | 12.5% | 9.5% | 7.2% | 5.2% |
| Aciclovir | 258.93 | 0.716 | Dominated | 8.4% | 13.1% | 15.9% | 18.8% |
FIGURE 18.
Cost-effectiveness acceptability curves. Four-arms model.
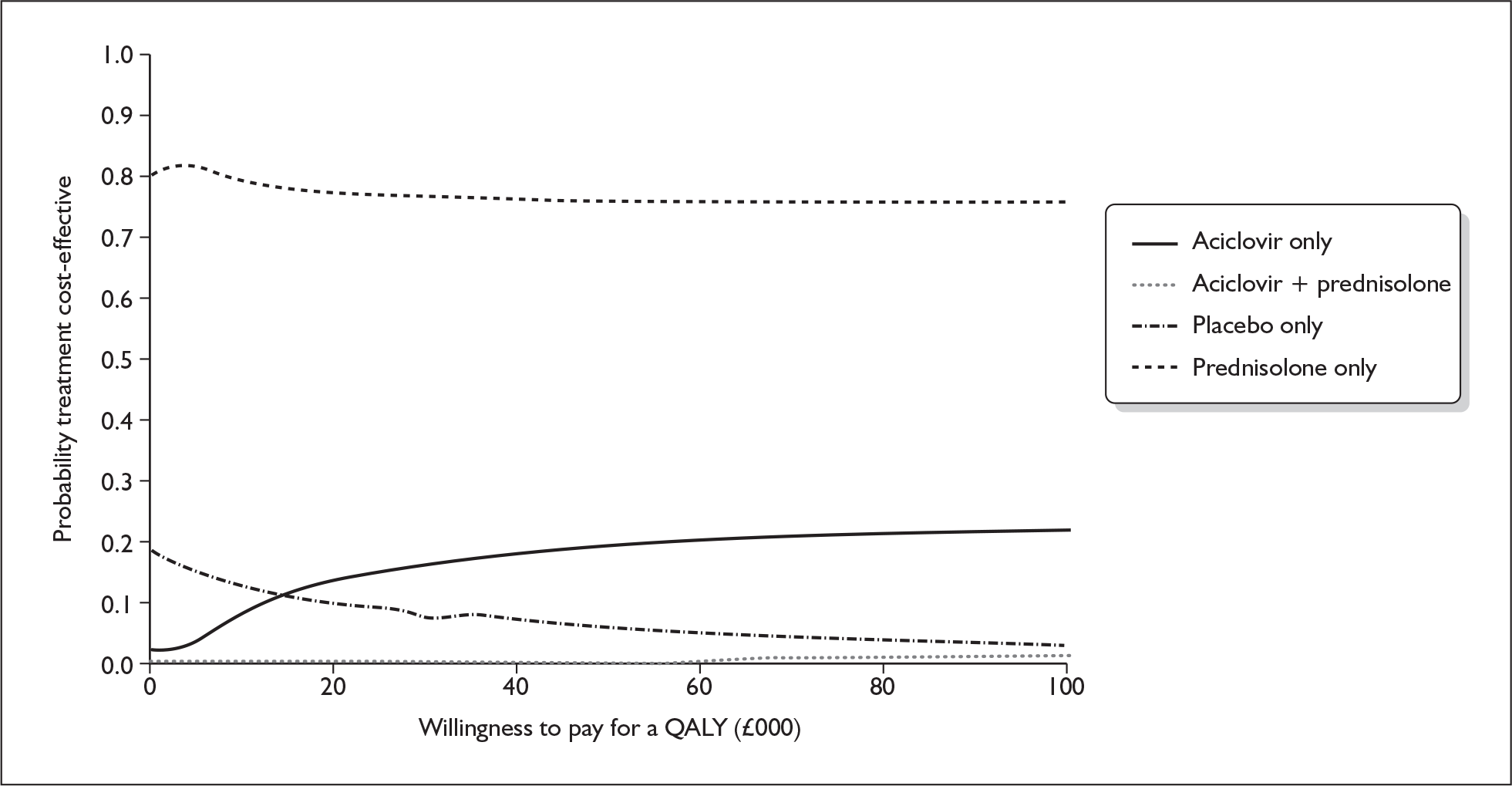
Sensitivity analysis
Sensitivity analyses on costs
Sensitivity analysis was conducted by taking out of the analysis the five highest total costs participants as outliers. Average total strategy costs were reduced by around £100. However, none of the cost-effectiveness or cost–utility analyses results changed.
Further scenario sensitivity analyses were conducted. Unit costs for hospital-based resource use, outpatient visits, primary care visits, or medications were cut by 50% or doubled. Results were not sensitive to any of these changes.
As is well known, cost data might not follow a normal distribution. In this case, cost data presented a few zero observations and were skewed to the right as there were a minority of trial participants for whom costs were extremely high. Therefore, an exponential regression was fitted to obtain total cost for cured and not cured participants. Deterministic and probabilistic results were not sensitive to this change in the way average total costs for cured and not cured participants were calculated.
Probability of being cured at 3 months
One-way sensitivity analyses were conducted on the difference in the probability of being cured at 3 months. The 95% CI upper and lower limits were used for this (Tables 24 and 25). Cost-effectiveness or cost–utility analysis results were not sensitive to these changes for the prednisolone versus no prednisolone model. If the difference in the probability of being cured at 3 months between prednisolone and no prednisolone arms was around 2%, well below the lower 95% CI limit, the ICER would be of about £21,000 per additional QALY.
However, results were sensitive to this sensitivity analysis within the aciclovir versus no aciclovir model. Specifically, when the difference in the probability of being cured at 3 months between the aciclovir arm and no aciclovir arm was 0.033 (the upper limit of the 95% CI), the ICER was £9576. Further threshold analyses were conducted and at ICERs of about £20,000 and £30,000 were obtained for 2% and 1.5% differences in the absolute probability of cure, respectively. Nonetheless, when this difference was around – 0.32% the no aciclovir arm would dominate the aciclovir arm. All of these difference values are within the 95% CI results reported in this chapter (see Table 25).
Age group and gender
Regression analyses for total cost and for utility weights show age group variables as well as gender as statistically non-significant. In other words, there was no evidence that total costs would differ by age group or between males and females. Similarly, there was no evidence of a difference in utility weights between male or female participants cured at 3 months, males and females cured at 9 months, or males and females not cured. Given these data no estimates of incremental cost per QALY were estimated for different age groups or by gender.
After completion of our analyses we were made aware of a late reference to a Japanese study finding in favour of treatment with aciclovir. 39 In the language of the BELLS Study, researchers found the overall rate of patient recovery among those treated with AP (96.5%) was significantly better (p < 0.05) than the rate among those treated with OP (89.7%). We examined these findings in detail. This study was smaller than ours, treated patients in tertiary referral centres, and the outcome assessors were not masked to treatment allocation. We concluded that their results should be treated with caution.
Chapter 6 Subgroup analyses
Outcome dependent on the time delay to commencement of treatment
Where the time delay was known, we compared the recovery rate at 3 months and at 9 months, only for those patients treated with prednisolone with the recovery rate for those not treated with prednisolone, according to whether the time delay between onset and the commencement of treatment was less than 24 hours, less than 48 hours or less than 72 hours. The recovery rates are shown in Table 43.
| Delay | 0–24 hours | 24–48 hours | 48–72 hours | |
|---|---|---|---|---|
| P | 90/115 (0.78) | 81/93 (0.87) | 20/23 (0.87) | 191/231 (0.83) |
| P′ | 84/142 (0.59) | 45/63 (0.71) | 13/17 (0.76) | 142/222 (0.64) |
| 174/257 (0.68) | 126/156 (0.81) | 33/40 (0.83) | 333/453 (0.74) |
| Delay | 0–24 hours | 24–48 hours | 48–72 hours | |
|---|---|---|---|---|
| P | 111/117 (0.9487) | 90/94 (0.9574) | 23/23 (1) | 224/234 (0.9573) |
| P′ | 118/146 (0.8082) | 55/63 (0.8730) | 15/17 (0.8824) | 188/226 (0.8319) |
| 229/263 (0.8707) | 145/157 (0.9236) | 38/40 (0.9500) | 412/460 (0.8957) |
In all cases there is the suggestion that treatment commenced within 24 hours leads to lower recovery rates than treatment commenced within 24–72 hours. Collapsing both tables to a 2 × 2 factorial format permits a comparison of treatment (prednisolone with non-prednisolone) and time to commencement of treatment (a delay of less than 24 hours compared with a delay of 24–72 hours).
At 3 months treatment with prednisolone is more effective than treatment without prednisolone (82.7% recovery vs 64.0%; OR = 2.69; 95% CI: 1.71 to 4.20; p < 0.001); comparison of the recovery rates at 3 months suggests that treatment delayed by at least 24 hours is useful (67.7% recovery for those treated within 24 hours vs 73.5% for those treated later; OR = 0.49; 95% CI: 0.31 to 0.78; p = 0.002). At 9 months, the recovery rate after treatment with prednisolone is significantly higher than the recovery rate without prednisolone treatment (95.7% vs 83.2%; OR = 4.53; 95% CI: 2.14 to 9.17; p < 0.001); the effect of a 24-hour delay before commencing treatment is still apparent (87.1% if treatment commences early, 89.6% otherwise; OR = 0.52; 95% CI: 0.26 to 0.99; p = 0.046).
We can currently neither identify nor suggest a convincing rationale for delaying treatment for 24 hours, in the face of all the evidence (or conventional wisdom) that early commencement of treatment is an important adjunct to successful resolution of Bell’s palsy.
Neither at 3 months nor at 9 months is any interaction between treatment and commencement of administration evident (p = 0.73 and p = 0.97).
Outcome dependent on severity at onset
We looked at 9-month recovery rates for those patients treated with prednisolone and for those not treated with prednisolone, and explored the extent to which the severity of the episode of Bell’s palsy as measured at onset dictated recovery. There were 34 patients graded I at onset who were also graded I at 3 months and not visited at 9 months. We characterised those graded II to IV at onset as ‘moderate’, and those graded V to VI at onset as ‘severe’. The recovery rates are shown in Table 45.
| Severity at onset | P | P′ | |
|---|---|---|---|
| Moderate (HB II–IV) | 169/176 (0.9602) | 122/145 (0.8414) | 291/321 (0.9065) |
| Severe (HB V–VI) | 42/49 (0.8571) | 59/80 (0.7375) | 101/129 (0.7829) |
| 211/225 (0.9378) | 181/225 (0.8044) | 392/450 (0.8711) |
The 9-month recovery rate is substantially higher in both treatment groups when the severity at onset is only moderate; also the 9-month recovery rate is higher in the prednisolone subgroups, irrespective of severity. The 9-month recovery rate in the prednisolone group is 93.8% compared with 80.4% in the non-prednisolone group (OR 3.66; 95% CI: 1.92 to 7.28; p < 0.001); the 9-month recovery rate in the moderately affected group is 90.7% compared with 78.3% in the severely affected group (OR 2.69; 95% CI: 1.49 to 4.82; p < 0.001). The interaction is non-significant (p = 0.182).
Assessors’ concordance
The three assessors considered altogether 1172 patient photo sets in order to provide our primary outcome measure. When all three assessors agreed to within one grade, the median or majority grade (the decision is equivalent) was the one awarded for that patient visit. Otherwise, all three assessors were invited to provide a new assessment, when the median grade was the one awarded. Throughout, all the assessors were masked to the treatment and the time of the visit (i.e. whether the portrait records derived from the onset visit, or the 3-month or 9-month visit). The extent of agreement is summarised in Table 46.
| Pairing | Unanimous | Agreement ± 1 | Reassessment | Cohen’s kappa |
|---|---|---|---|---|
| J1 with J2 | 750 | 388 | 34 | 0.53 |
| J1 with J3 | 722 | 395 | 55 | 0.50 |
| J2 with J3 | 769 | 355 | 48 | 0.54 |
So Judge 1 was in agreement with Judge 2 in 97% of cases, with Judge 3 in 95% of cases, and Judge 2 with Judge 3 in 96% of cases. The kappa values awarded are all ‘moderate’. 40
We also tested the assessors for consistency by sending them 92 repeat photo sets for assessment without additional explanation. The agreements were as shown in Table 47.
| Assessor | Same grading | Same grading ± 1 | Difference ≥ 2 | Cohen’s kappa |
|---|---|---|---|---|
| J1 | 80 | 12 | 0 | 0.83 |
| J2 | 71 | 21 | 0 | 0.70 |
| J3 | 71 | 21 | 0 | 0.71 |
All repeat gradings were within one grade of the original decision. In the terminology of Landis and Koch, the consistency of Judge 1’s assessments is ‘almost perfect’ and that of both Judge 2 and Judge 3 is ‘substantial’.
Chapter 7 Discussion
This is the largest randomised controlled trial of the effectiveness of treatment for Bell’s palsy in the world literature. We have confirmed the generally favourable outcome for untreated Bell’s palsy, with 63% of patients recovered at 3 months, increasing to 85% after 9 months. Early treatment (within 72 hours of onset of weakness) with prednisolone increased these rates to 81% and 94% respectively. Aciclovir produced no benefit over placebo and there was no benefit from its addition to prednisolone.
The trial was not powered to detect a difference in cost-effectiveness. However, the results of the economic evaluation suggest that the use of prednisolone is likely to be considered cost-effective. Aciclovir, in contrast, appears to be on average no more effective but more costly than no treatment or treatment with prednisolone. Thus, it is unlikely to be considered to be cost-effective. The time horizon of the model was only 9 months. Therefore, an implicit assumption of the model is that there are no further benefits and cost savings from the use of prednisolone after the end of the time horizon. Given the difference in cure rates that existed at 9 months it is possible that should the time horizon be extended treatment of Bell’s palsy with prednisolone would be associated with further gains in QoL. Furthermore, it is likely that those who did not receive prednisolone would make more use of health services, thus increasing their cost relative to those who received prednisolone.
Strengths and limitations of the study
This study, which was independently funded by the HTA, has several advantages compared to earlier trials, which have lacked power and produced inconsistent results. We have recruited double the number of patients that were included in the Cochrane systematic reviews. We recruited the majority of patients from primary care, thus reducing the selection bias inherent in hospital-based studies. The high acceptance of randomisation and low drop-out rate during the study suggest that these results are likely to be generalisable to other settings with similar populations. We used drugs that are relatively inexpensive and readily available worldwide. The diagnosis and absence of exclusion factors was confirmed by experienced, trained otorhinolaryngologists. The randomisation procedure and allocation to treatment was managed by an experienced, dedicated, independent trials unit. The assessment of outcomes using validated study tools was undertaken by observers who were masked to treatment allocation. The factorial design has permitted separate assessment of the effectiveness of both treatments as well as serving to exclude the possibility of a beneficial or antagonistic interaction.
We used the House–Brackmann scale to grade lower motor neurone facial nerve function because it reliably assigns patients to a recovered status. The scale has been criticised for not being sufficiently sensitive to change, and for having grades that are sometimes difficult to assign because patients may have contrasting degrees of function in different parts of their face. Alternative scales such as Sydney or Sunnybrook are available, but are more demanding to use for frontline clinicians. We used posed, static images rather than moving video clips as we believe these were adequate for our purpose. However, researchers noticed both asymmetry and slowness in blinking in patients, and that this was one of the last symptoms to disappear. Patients’ enunciation was occasionally compromised and in a few cases the disability was very severe. Neither of these is evident from static poses, and both would be readily identifiable even in short video clips. The cameras issued to researchers were inexpensive and (even in 2004) possessed the capability to record and save many minutes of moderately high quality video material. This capability was not used. We visited patients three times over 9 months, but would have preferred to visit more frequently in the early course of the illness to capture more detail about the recovery process and to obtain serology. We did not collect 9-month data on the subgroup whose facial nerve function had fully recovered at 3 months because patients with Bell’s palsy who improve do not subsequently deteriorate. We considered that we were likely to be underpowered to detect significant differences in the secondary end points with this design.
The economic analysis used a modelling framework to estimate relative efficiency. This approach has the advantage of making the best use of the limited data available but it made the assumption that the main determinant of relative efficiency is whether or not the Bell’s palsy was cured or not. If a standard trial-based cost-effectiveness analysis had been conducted with the available data it is likely that on average broadly similar results would have been achieved but the results would have lacked precision. Furthermore, the lack of data on costs and the decision not to follow up those deemed cured at 3 months would have necessitated similar assumptions being made in order to handle the missing cost and utilities data.
The data on costs used within the model came from a sample of only 74 of the trial participants – only a small proportion (15%) of the total trial sample. This led to a reduction in the precision of the estimates. Despite this limitation these data appear representative of the whole sample and the reasons for non-response were unconnected to the therapy the participant received or their outcomes. With respect to the estimation of QALYs measurements of health state utilities were censored for those trial participants who were judged to be cured at the 3-month follow-up. Therefore, an assumption was made within the modelling exercise that was tantamount to imputing utility data using the ‘last value carried forward’ method. Ordinarily this approach, while simple, is normally considered to be a poor method of imputation. 41,42 However, in this situation it may not be wholly unrealistic as these trial participants were judged to be cured at the time of censoring. Nevertheless, it assumes that there is no possible further improvement in health status for these people nor is there any possibility of relapse. This latter situation is clinically implausible unless there is a new episode of Bell’s palsy. The results of the economic evaluation would have been strengthened by further data on both costs and health state utilities.
Within the model the results are driven by the probability of being cured at 3 months and to a lesser extent, the probability of being cured at 9 months. Both probabilistic and deterministic sensitivity analyses were conducted. The probabilistic sensitivity analysis focused on the statistical imprecision surrounding the model parameters using parameter distributions that were plausible and based upon the available data. Further deterministic sensitivity analysis was conducted to address uncertainty in the model structure or uncertainty surrounding model parameters that were obtained from outwith the randomised controlled trial (RCT). The results of these sensitivity analyses indicate that conclusions are only sensitive to assumptions on the probability of being cured for the aciclovir versus no aciclovir model.
Overall, based on the data available it appears that treatment of Bell’s palsy with prednisolone is likely to be considered cost-effective while treatment with aciclovir is highly unlikely to be considered cost-effective. Given the limited data available on costs and utilities, further data would be useful to confirm findings. Similarly, although it is unlikely to change the conclusion, further data on costs and outcomes in the longer term (i.e. for a follow-up greater than 9 months) would also serve to confirm the findings of the study.
Implications for practice
Of the two most common treatments for Bell’s palsy the results of this study indicate that prednisolone may improve outcomes and potentially lower net NHS costs than other treatments (including doing nothing). Should the NHS subsequently recommend the adoption of prednisolone for the firstline treatment of people presenting with the symptoms of Bell’s palsy the following factors should be considered: contraindications to prednisolone, severity at onset and time of commencement of treatment.
Chapter 8 Conclusion
In conclusion, we have provided robust evidence that the early use of oral prednisolone in Bell’s palsy is an effective treatment. The mechanism of action is uncertain but may be due to modulation of the immune response to the causative agent or by direct reduction of oedema around the facial nerve within the facial canal. Treatment with unesterified aciclovir at these doses as used in other trials, either alone or with steroids, had no effect on outcome, and this drug should not be used in Bell’s palsy unless evidence becomes available that better absorbed formulations are effective. We still do not know how best to treat patients who present later than 72 hours, and this should stimulate early and rapid assessment of all patients with suspected Bell’s palsy. Most patients with Bell’s palsy recover fully without any treatment but this study has demonstrated that the number needed to treat for prednisolone to achieve one additional complete recovery is six at 3 months and eight at 9 months.Inevitably, therefore, for some clinicians and their patients, offering no treatment will remain an appropriate strategy, but we can now have an informed discussion with our patients regarding the use of steroids, based on data that were lacking hitherto.
Chapter 9 Opportunities for further research
The opportunities for further research identified during the course of this study are described briefly below.
Primary research
Important questions unanswered by our study design include:
-
the extent of recovery within the first four weeks on steroid therapy
-
any benefit in using steroids if started after 72 hours
-
the optimally effective dose of prednisolone in the 25–100-mg range that has been used in other studies
-
the hazards, if any, pertaining to short courses of high-dose steroid
-
the contribution of serological studies to the underlying cause of Bell’s palsy
-
confirmation whether higher tissue concentrations of antiviral agents – e.g. higher doses or esterified forms – may have benefits we were unable to detect
-
whether static poses accurately reflect the extent of functional disability compared to moving images or direct observation by an experienced clinician.
Evidence synthesis
We have agreed to undertake an update of the Antivirals in Bell’s Palsy (Cochrane review) and this was submitted in February 2009.
Acknowledgements
We would like to take this opportunity to thank all the patients who have helped us by agreeing to participate in this national clinical trial; our patient David Bell for permission to use his photographs on our website and in presentations and publications; all the general practitioners, A&E staff, NHS24 nurses and others who helped by referring patients to the hospital sites; all the ENT consultants and senior house officers in the hospital wards and clinics who took the time to explain the study to patients, randomise them to treatment and issue the treatment capsules; to the researchers who visited the patients; to the expert panel who provided the House–Brackmann grading assessments and hence our primary outcome measure; to the Scottish School of Primary Care for their role in assisting the processes of referral of patients to the trial sites; to Tayside Pharmaceuticals at Dundee for their dedicated attention to detail in manufacturing the capsules, bottling, labelling and distributing them; to the randomisation unit at HSRU Aberdeen for the efficiency of their systems in allocating the treatments to patients; and to Health Boards, R&D Departments and Medical Research Ethics Committees throughout Scotland who provided facilities and the ethical foundation for the delivery of this study.
The Health Services Research Unit and the Health Economics Research Unit are both core funded by the Chief Scientist Office of the Scottish Government Health Directorates. This study is sponsored by the University of Dundee.
Trial Steering Committee
Professor Chris van Weel, University of Nijmegen (Chair); Dr Ian Williamson, University of Southampton; Professor Sally Wyke, University of Stirling; Mrs Caryl Hamilton (Patient representative).
Data Monitoring and Ethics Committee
Professor Marion Campbell, University of Aberdeen (Chair); Dr Carl Counsell, University of Aberdeen; Mr Rodney Mountain, University of Dundee; co-opted member: Dr Simon Ogston, University of Dundee (Statistician).
Local Principal Investigators
Mr Kim Ah-See, Aberdeen Royal Infirmary; Mr Natarajan Balaji, Monklands Hospital, Airdrie; Mr Hasan Beg, Victoria Hospital, Kirkcaldy; Mr Quentin Gardiner, Perth Royal Infirmary; Mr Musheer Hussain, Ninewells Hospital, Dundee; Mr Alastair Kerr, Western General Hospital and Royal Infirmary, Edinburgh; Mr John Marshall, Southern General Hospital, Glasgow; Mr William McKerrow, Raigmore Hospital, Inverness; Ms Mary Shanks, Crosshouse Hospital, Kilmarnock; Mr David Simpson, Stobhill Hospital, Glasgow; Mr Guy Vernham, St John’s Hospital, Livingston; Miss Aileen White, Royal Alexandra Hospital, Paisley.
Scottish School of Primary Care (SSPC)
Dr Lucy McCloughan (SPPIRe); Marie Pitkethly, Kim Stringer (East of Scotland); Susan Campbell, Amanda Cardy (North of Scotland); Dr Colette Fulton (South East of Scotland); Colin Cowan, June McGill (West of Scotland); Dr Karen Bell (NHS Ayrshire and Arran); Dr Brian Rae (NHS North Glasgow).
Contribution of authors
Additional to their roles on the BELLS Trial Management Committee or as advisers to it (R Hernández, K Stewart) and to their specific contributions to the production of this report and the approval of this version, the contributions of authors are as follows: FM Sullivan (trial design, links to primary care in Scotland, overall oversight as Chief Investigator); IRC Swan (trial design, links to ENT in Scotland, assessment of primary outcome); PT Donnan (trial design, statistics); JM Morrison (trial design, primary care, detection and assessment of psychological distress); BH Smith (trial design, primary care, assessment of pain); B McKinstry (primary care, recruitment methodology); RJ Davenport (neurosciences, assessment of primary outcome); LD Vale, R Hernández (health economics); JE Clarkson (trial design, links to dentistry in Scotland); K Stewart (plastic surgery, assessment of primary outcome); V Hammersley, S Hayavi, A McAteer, D Gray (patient contact, data collection); F Daly (trial coordination, statistics, data collection).
Publications
Sullivan F, Daly F. The Scottish Bell’s Palsy Study. Scottish Primary Care 2006;47:10.
Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al. Early treatment with prednisolone or aciclovir in Bell’s palsy. N Engl J Med 2007;357:16,1598–1607.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Petruzelli GJ, Hirsch BE. Bell’s Palsy. Postgrad Med 1991;90:115-27.
- Sugita T, Murakami S, Yanagihara N, Fujiwara Y, Hirata Y, Kurata T. Facial nerve paralysis induced by herpes simplex virus in mice: an animal model of acute and transient facial paralysis. Ann Otol Rhinol Laryngol 1995;104:574-81.
- Morgan M, Moffat M, Ritchie L, Collacott I, Brown T. Is Bell’s palsy a reactivation of varicella Zoster virus?. J Infect 1995;30:29-36.
- Bateman DE. Facial Palsy. Br J Hosp Med 1992;47:430-1.
- Rowlands S, Hooper R, Hughes R, Burney P. The epidemiology and treatment of Bell’s palsy in the UK. Eur J Neurol 2002;91:63-7.
- Morgenlander JC, Massey EW. Bell’s Palsy: ensuring the best possible outcome. Postgrad Med 1990;88:157-62.
- Salinas RA, Alvarez G, Alvarez MI, Ferreira J. The Cochrane Library. Oxford: Update Software; 2002.
- Sipe J, Dunn L. Acyclovir for Bell’s palsy (idiopathic facial paralysis) (Cochrane Review). The Cochrane Library 2002.
- May M, Wette R, Hardin WB. The use of steroids in Bell’s palsy: a prospective controlled study. Laryngoscope 1976;86:1111-2.
- Wolf SM, Wagner JH, Davidson S, Forsythe A. Treatment of Bell’s palsy with prednisone: a prospective randomised study. Neurology 1978;28:158-61.
- Abiko Y, Ikeda M, Hondo R. Secretion and dynamics of herpes simplex virus in tears and saliva of patients with Bell’s Palsy. Otol Neurotol 2002;23:779-83.
- Rowlands S, Hooper R, Hughes R, Burney P. The epidemiology and treatment of Bell’s palsy in the UK. Eur J Neurol 2002;91:63-7.
- Foy R, Duggan A, Delaney B, Wilson S, Lewin-van den Broek N, lassen A, et al. How evidence based are recruitment strategies to randomized controlled trials in primary care? Experience from seven studies. Fam Pract 2003;20:83-92.
- Scottish Primary Care Network Research (SPCRN) n.d. http://www.sspc.ac.uk/spcrn/.
- Prout H, Butler C, Kinnersley P, Robling M, Hood K, Tudor-Jones R. A qualitative evaluation of implementing a randomized controlled trial in general practice. Fam Pract 2003;20:675-81.
- Deehan A, Templeton L, Taylor C, Drummond C, Strang J. The effect of cash and other financial inducements on the response rate of general practitioners in a national postal survey. Br J Gen Pract 1997;47:87-90.
- Soumerai SB, McLaughlin TJ, Gurwitz JH, Guadagndi E, Hauptman PJ, Borbas C, et al. Effect of local medical opinion leaders on quality of care for acute myocardial infarction. A randomized controlled trial. JAMA 1998;279:1358-63.
- Scottish Bell’s Palsy Study n.d. http://www.dundee.ac.uk/bells/ (accessed 22 August 2007).
- Scottish Bell’s Palsy Study. Stop Press; n.d.
- Sullivan F, Daly F. The Scottish Bell’s Palsy Study. Scottish Primary Care 2006;47.
- Puttick H. Research looks to ease misery for Bell’s palsy sufferers. The Herald 2004.
- Cunningham J. I had never heard of Bell’s palsy but it sounded quite scary. The Herald 2005.
- Wave 102: Interview With Fergus Daly 2005.
- Elliott J. ‘I Had to Eat My Food through a straw’ n.d. http://news.bbc.co.uk/1/hi/health/4703481.stm (accessed 22 August 2007).
- Holland NJ, Weiner GM. Clinical review, Recent developments in Bell’s palsy. BMJ 2004;329:553-7. 10.1136/bmj.329.7465.553 (accessed 22 August 2007).
- Piercy J. Primary care: 10-minute consultation: Bell’s palsy. BMJ 2005;330. 10.1136/bmj.330.7504.1374 (accessed 22 August 2007).
- Furlong WJ, Feeny DH, Torrance GW, Barr RD. The Health Utilities Index (HUI3) system for assessing health-related quality of life in clinical studies. Ann Med 2001;33:375-84.
- Furlong W, Feeny D, Torrance GW, Goldsmith C, DePauw S, Boyle M, et al. Multiplicative Multi-Attribute Utility Finction for the Health Utilities Index Mark 3 (HUI3) System: A Technical Report. Working Paper No. 98-11 n.d.
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain 2004;20:309-18.
- Harris DL, Carr AT. The Derriford Appearance Scale (DAS59): a new psychometric scale for the evaluation of patients with disfigurements and aesthetic problems of appearance. Br J Plast Surg 2001;54:216-22.
- De Diago-Sastre JI, Prim-Espada MP, Fernandez-Garcia F. The epidemiology of Bell’s palsy. Rev Neurol 2005;41:287-90.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. New York: Oxford University Press; 2005.
- Glick H, Polsky D, Schulman K, Drummond M, McGuire A. Economic evaluation in health care: merging theory with practice. Oxford: Oxford University Press; 2001.
- British Medical Association/Royal Pharmaceutical Society of Great Britain . British National Formulary n.d. http://www.bnf.org/bnf.
- Information Services Department for Scotland; NHS National Services Scotland n.d. www.isdscotland.org.
- Personal Social Services Research Unit (PSSRU) . Unit Costs of Health and Social Care 2006 n.d. http://www.pssru.ac.uk/pdf/uc/uc2006/uc2006.pdf (accessed May 2007).
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
- National Institute for Clinical Excellence . Guide to the Methods of Technology Appraisal 2004.
- Hato N, Yamada H, Kohno H, Matsumoto S, Honda N, Gyo K, et al. Valacyclovir and prednisolone treatment for Bell’s Palsy: a multicenter, randomized, placebo-controlled study. Otol Neurotol 2007;28:408-13.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74.
- White IR, Wood A, Royston P. Editorial: Multiple imputation in practice. Stat Methods Med Res 2007;16:195-7.
- Kenward MG, Carpenter J. Multiple imputation: current perspectives. Stat Methods Med Res 2007;16:199-218.
Appendix 1 BELLS protocol
Project title
Bell’s palsy: Early acicLovir and/or prednisoLone in Scotland – ‘BELLS’: a multicentre factorial trial of the early administration of steroids and/or antivirals for Bell’s palsy
1 Principal Investigators
Professor Frank Sullivan, NHS Tayside Professor of R&D in General Practice and Primary Care, Tayside Centre for General Practice, University of Dundee
Professor Jillian Morrison, Professor of General Practice, Department of General Practice, University of Glasgow
Mr Iain Swan, Senior Lecturer in Otolaryngology, Department of Medicine, University of Glasgow
Professor John Cairns, Reader in Health Economics, Health Economics Research Unit, University of Aberdeen
Dr Peter Donnan, Senior Lecturer in Medical Statistics, Tayside Centre for General Practice, University of Dundee
Dr Blair H Smith, Senior Lecturer in General Practice, Department of General Practice and Primary Care, University of Aberdeen
Dr Brian McKinstry, Medical Director, Lothian and Borders Primary Care Research Network, Ashgrove Health Centre, Blackburn, West Lothian
Dr Richard Davenport, Consultant Neurologist, Western General Hospital, Edinburgh
Dr Janet Clarkson, Senior Lecturer in Dental Primary Care, Dental Health Services Research Unit, University of Dundee
2 Planned investigation
(a) Research objectives
-
To describe the resolution of neurological deficit and cosmetic, psychological and functional recovery in each of four groups of patients: those treated with prednisolone, aciclovir, both or neither.
-
To determine which group of patients have the greatest reduction in neurological disability scores on the House–Brackmann grading system at 3 and 9 months after randomisation.
-
To compare self-reported health status (including assessments of pain) at 3 and 9 months after randomisation.
-
To compare the incremental cost per neurological deficit resolved and incremental cost per QALY in the study groups.
(b) Existing research
Bell’s palsy is an acute unilateral paralysis of the facial nerve first described by the Scottish surgeon Sir Charles Bell (1774–1842). 1 Its cause is unknown but animal studies have suggested the possibility that reactivation of herpes viruses may be responsible for demyelination. 2,3 It affects 25–35 people per 100,000 in the population per annum, most commonly in the age group 30–45. 4 The condition presents disproportionately amongst pregnant women and people who have diabetes, influenza, a cold, or some other upper respiratory ailment. On average every year a general practitioner will see one or two patients who have developed the condition. A recent UK study using the general practice research database (GPRD) showed that 36% of patients were treated with oral steroids and 19% were referred to hospital. 5 Although most recover well, 30% of patients have a poor recovery with continuing facial disfigurement, psychological difficulties and sometimes facial pain (though the presence and course of pain is unclear from current knowledge). 6 In the absence of an established aetiology, treatment continues to be based upon the established pathophysiology: swelling and entrapment of the nerve.
Two recent Cochrane reviews concerning the treatment of Bell’s palsy have examined the effectiveness of oral prednisolone and aciclovir. 7,8 These found that insufficient data exist to conclude that either or both therapies are effective. Many of the studies included in the reviews either failed to randomise patients or, when correctly randomised, were erroneously interpreted in a favourable light. 9,10 In addition, high-dose steroid therapy has numerous potential side effects including peptic ulceration, hypertension and confusional states. Antiviral therapy is expensive and should be reserved for circumstances where definite benefits are likely to be obtained. Current recommendations suggest that aciclovir needs to be started within 48 hours, though more recent studies of viral replication in patients with Bell’s palsy suggest that this might be extended. 11
(c) Research methods
Design
A randomised 2 × 2 factorial design to assess whether prednisolone and/or aciclovir commenced within the first 72 hours after onset of Bell’s palsy results in the same level of disability and pain after 9 months as treatment with placebo. 12
Recruitment
In order to establish whether aciclovir or steroids are an effective therapy, initial treatment needs to be given in the first 72 hours after the onset of symptoms.
Because Bell’s palsy is a comparatively rare condition only a co-ordinated approach across a large population will provide sufficient numbers to allow a satisfactory power to be achieved in this study. Our pilot work with the Scottish research networks led to almost 100% participation rates. We intend to approach every medical and dental practice in Scotland and to enhance recruitment through existing networks of influence, which will encourage referral of a higher proportion of cases than would be expected from more anonymised exhortation. The Scottish School of Primary Care is able to co-ordinate recruitment through several networks, which overlap in their membership:
-
general medical practices already associated with existing primary care research networks, n = 273
-
networks of practices already involved in undergraduate and postgraduate teaching, n = 482
-
general medical practice out of hours co-operatives, n = 89
-
NHS24, which will cover most of Scotland by the time the study commences (we have arranged to amend their referral scripts and train their nurses)
-
the Medical Research Council General Practice Research Framework (MRC GPRF), n = 90
-
Scottish Dental Practice Based Research Network dental practices, n = 270
-
A&E departments across Scotland.
We will also advertise the study with articles in the free, weekly medical press. Clinicians throughout Scotland will be reminded on a quarterly basis to recruit into the study any patients who present with Bell’s palsy. As soon as a suitable patient presents to the GMP, GDP, A&E or Out of Hours Co-op clinician within 72 hours of onset, they will telephone the nearest ENT unit and arrange with the ENT surgeon on call for the patient to be seen immediately. When the patient is seen by the ENT surgeon the criteria for study entry will be confirmed, consent will be obtained and the randomisation centre will be contacted. All consultant ENT surgeons in Scotland have been contacted and with one exception have agreed to take part in the study. We will ensure that randomisation is secure by telephone randomisation, which will be centrally controlled by the Health Services Research Unit (HSRU) in Aberdeen. 13 When an eligible patient has given consent to participation, the doctor will telephone the computerised randomisation service, via a hot-key, and receive instructions about which numbered pack is to be supplied. The on-call ENT Registrar will immediately supply the medication from the supplies available in the unit.
The HSRU computer will notify the study co-ordinator of the patient’s study details by an immediate email. This mechanism has been extensively used in multicentre trials before. The co-ordinator will arrange for the nearest research assistant to visit within the next three days to complete the baseline assessments and arrange follow-up.
(d) Planned interventions
Patients will be randomised to receive two identical preparations for 10 days simultaneously, creating four patient groups: (1) prednisolone (50 mg per day) and placebo; (2) aciclovir (2000 mg per day) and placebo; (3) prednisolone and aciclovir; and (4) placebo and placebo. Each patient will be supplied with two bottles of medication (marked by a code).
(e) Planned inclusion and exclusion criteria
Inclusion criteria:
-
Adults (16 or older) with unilateral facial nerve weakness of no identifiable cause seen within 72 hours of the onset of weakness.
Exclusion criteria:
-
Pregnancy
-
Uncontrolled diabetes (HbA1c > 8%)
-
Peptic ulcer disease
-
Suppurative otitis media
-
Herpes zoster
-
Multiple sclerosis
-
Sarcoidosis and other rarer conditions
-
Inability to give informed consent
and two further exclusions identified during the processes of MREC application
-
Breast-feeding
-
Patients with systemic infection.
(f) Ethical arrangements
Risks and anticipated benefits
-
No adverse events have been reported for the interventions in this study when administered in similar settings. There is a theoretical risk of adverse events from the prednisolone, which should be greatly reduced by adherence to the exclusion criteria above.
-
The potential benefits include faster and/or more complete resolution of neurological deficit and cosmetic, psychological and functional recovery.
Informed consent
-
Will be obtained before entry to the study.
Actions where informed consent is not possible
-
Unless the person is able to provide informed consent they will not enter the study.
Proposed time period for retention of documentation
-
At least 20 years in electronic format in which all study documentation will be retained. (‘Personal Information in Medical Research’, MRC 2000, 2003; also ‘MRC Population Data Archiving and Access Project, Consultants’ Report, Draft 2’, MRC 2002.)
(g) Sample size
This has been calculated using the primary endpoint of incomplete recovery of facial motor function (House–Brackmann grade III or greater) 9 months after randomisation.
Design
2 × 2 factorial randomised controlled trial with the following treatments:
-
Aciclovir (A)
-
Steroids (B)
-
Aciclovir + Steroids (AB)
-
Placebo (O)
Note that this assumes A and B have independent effects and do not interact.
The literature is sparse about likely effect sizes. One systematic review suggests a relative risk (RR) (of incomplete recovery) = 0.86 (95% CI: 0.47 to 1.59) or 22% on steroids compared with 26% in the control group. Other results are few and contradictory; Adour et al. 14 suggest 24% on steroids compared with 7.5% on aciclovir plus steroids, while De Diego et al. 15 suggest 6.4% on steroids and 22% on aciclovir alone. Hence the literature gives effect sizes from 4% to 17%. We regard a difference in incomplete recovery of 10% or more to be clinically meaningful and so, in Table 1 below, sample sizes are given for differences in percentage with incomplete recovery from 10% to 15%.
| Trt 1 | Trt 2 | Difference | Relative risk | n per group | Total |
|---|---|---|---|---|---|
| % incomplete recovery | % incomplete recovery | ||||
| 22% | 32% | 10% | 0.69 | 328 | 656 |
| 22% | 34% | 12% | 0.65 | 235 | 470 |
| 22% | 37% | 15% | 0.59 | 157 | 314 |
If we simultaneously randomise approximately 240 patients per treatment (a total of 480) this would allow detection of differences of the order of 12%. Since the study design is factorial the power is the same for each pair-wise comparison of treatments (assuming no interaction). Note that we will also treat the House–Brackmann scale as ordinal as well as binary, which serves to increase power.
The ability to detect an interaction if it exists is an attractive feature of the factorial design. If there is a significant interaction the overall efficiency of the design is maintained as long as the two drugs do not act antagonistically to cancel each other out, which is unlikely. With an interaction it is still possible to assess each drug separately, albeit with reduced power (72% instead of 80%) for the effect size (12%) or alternatively to detect effect sizes > 15% with the same power.
For clarity of numbers: we will seek to recruit 720 patients commencing treatment within 72 hours after onset, of whom 480 have commenced treatment within 48 hours after onset.
The incidence of Bell’s palsy in Scottish adults is 33 per 100,000 per year, and with an eligible population of 4.3 million for Scotland the expected number of new cases per year in Scotland would be 1419 after one year, and 2129 over 18 months. 16 We have piloted a notification process for early cases in four primary care research networks covering 1.4 million patients in Scotland. During one month of observation 74 cases of Bell’s palsy were seen and 35 of these within 48 hours. Based on this, we believe we are able to recruit approximately one-third of those who develop Bell’s palsy within 48 hours (710) and 50% by 72 hours, and that 70% (a conservative estimate) will attend for review at 9 months (see Figure 19). From the work of Adour and others we believe that at least 50% of patients will have fully recovered by 3 months, so a smaller number will require to be visited at 9 months17 (see Figure 20). We will require 18 months of a recruitment period to achieve 480 completed examinations at 9 months. If we collaborate with another co-ordinating centre of an equivalent size, this could be reduced to 9 months.
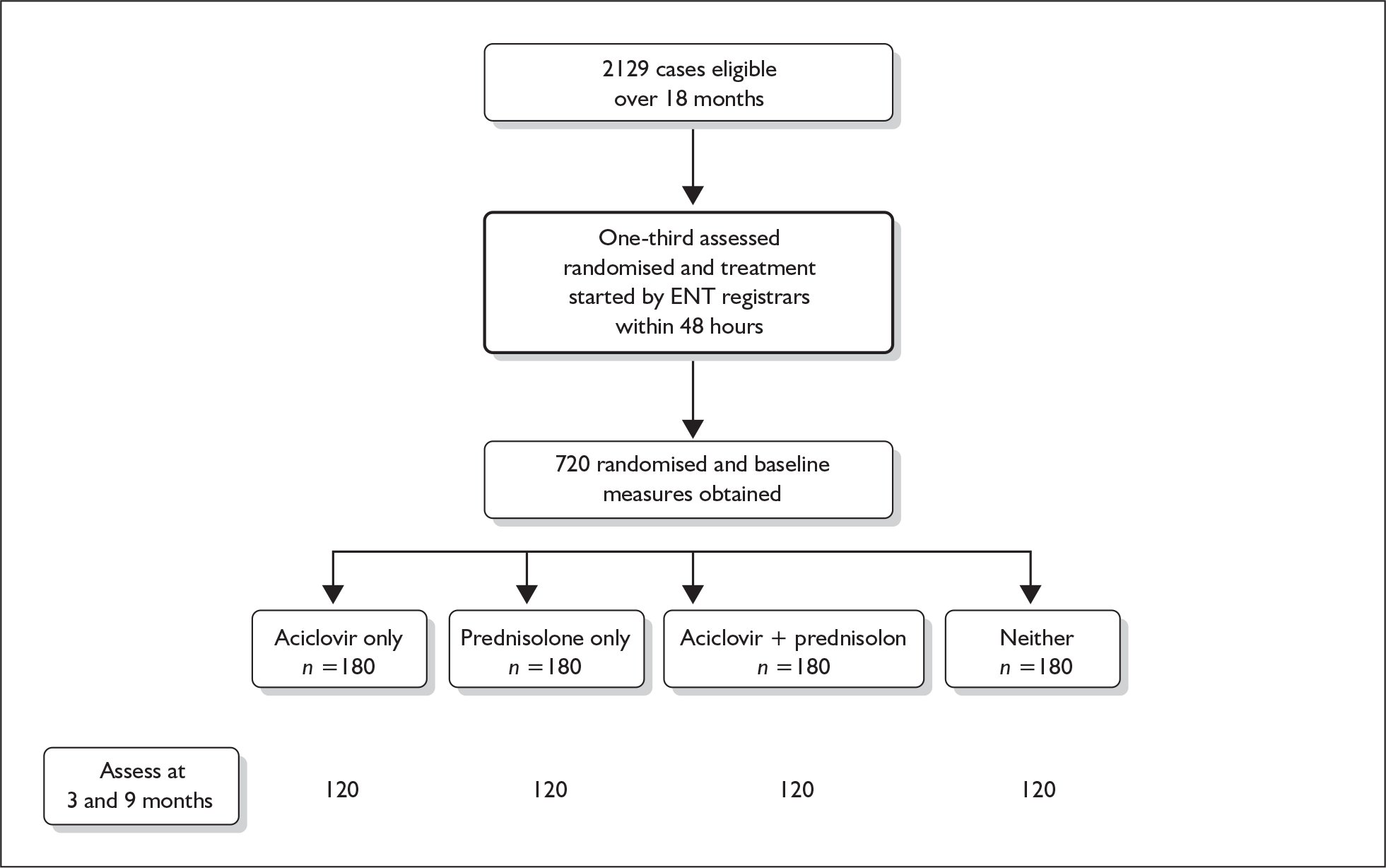
For the analyses, results will be stratified and analysed according to whether treatment started within 48 hours.
(h) Statistical analyses
Reporting will adhere to revised CONSORT criteria. 18
The baseline characteristics in each treatment group will be described. The following will be used to adjust the results: severity at initial presentation, age and gender. The main outcome of incomplete recovery (House–Brackmann grade III or higher) will be compared between treatment groups using chi-squared tests and also tests for linear trends as the nerve function scale is ordinal. We will use logistic regression to adjust for the prespecified confounding factors above. All analyses will be based on an intention-to-treat principle. Similar methods will be used for the ordinal scale for pain. The mean or median HUI score will be compared using t tests or Mann–Whitney tests, depending on the distributions found. Two-sided tests will be implemented throughout, using spss for data analysis.
Generally, subgroup analyses should be avoided in randomised controlled trials, or at least specified before data collection. It is intended to carry out formal tests of interaction between treatment and severity of House–Brackmann scale to assess whether treatment effects are greater in those most severely affected. The results of such analyses will be treated with caution and as hypothesis-generating. 19
(i) Outcome measures
Following the email alert from HSRU to the research co-ordinator, patients will then be seen within three days by the research assistants, at their GP surgery or at home, for more detailed assessment. At this first post-randomisation visit the degree of facial nerve denervation will be recorded by a digital camera and the other study instruments administered. Patients will be reassessed by questionnaire and digital camera at 3 and 9 months post-randomisation. We will capture facial appearances in digital images in standard positions (at rest, forced smile, bared teeth and buried eyelashes). These will be assessed blindly by a panel of three experts in otorhinolaryngology, neurology and plastic surgery.
The key outcome measure to be used in research objectives 1, 2 and 4 is the House–Brackmann grading system for facial nerve function shown below. 20 It has been validated against electrophysiological studies.
| Grade | Definition |
|---|---|
| I | Normal symmetrical function in all areas |
| II | Slight weakness noticeable only on close inspection. Complete eye closure with minimal effort. Slight asymmetry of smile with maximal effort. Synkinesis barely noticeable; contracture, or spasm absent |
| III | Obvious weakness, but not disfiguring. May not be able to lift eyebrow. Complete eye closure and strong but asymmetrical mouth movement with maximal effort. Obvious, but not disfiguring synkinesis, mass movement or spasm |
| IV | Obvious disfiguring weakness. Inability to lift brow. Incomplete eye closure and asymmetry of mouth with maximal effort. Severe synkinesis, mass movement, spasm |
| V | Motion barely perceptible. Incomplete eye closure, slight movement corner mouth. Synkinesis, contracture, and spasm usually absent |
| VI | No movement, loss of tone, no synkinesis, contracture, or spasm |
The Health Utilities Index version 3 (HUI3) will be used to assess research objectives 1 and 3. The HUI3 represents a global assessment of overall health status. It has eight dimensions: vision; hearing; speech; ambulation; dexterity; emotion; cognition and pain. For details see www.fhs.mcmaster.ca/hug/. It is designed for use in clinical practice and research, health policy evaluations, and general population surveys. It is constructed for self-administration by persons 14 years of age and older, and for administration by a trained interviewer in person or by telephone. It takes about 5 to 10 minutes to administer.
Pain measures will be used to achieve research objective 3.
The presence and duration of facial pain will be determined by a simple set of screening questions, based on a validated chronic pain case definition questionnaire. 21 The global severity of any pain will be assessed using a visual analogue scale, and the Chronic Pain Grade, a simple validated measure of intensity and pain-related disability, providing classification into four hierarchical grades of severity (Grade I low intensity–low disability; Grade IV high disability–severely limiting). 22,23
| Measure | Baseline | 3 months | 9 months |
|---|---|---|---|
| House–Brackmann | 720 | 576 | 138 |
| Health Utilities Index | 720 | 576 | 504 |
| Chronic Pain Grade | 720 | 576 | 504 |
| Costs | 720 | 576 | 504 |
(j) Economic evaluation
The cost-effectiveness of early treatment can be evaluated by calculating the incremental cost per additional neurological deficit resolved. However, it is not clear what incremental cost per additional deficit resolved decision-makers should consider good or poor value for money. The results of the trial will be rendered more informative by also translating the resolution of a neurological deficit into a quality-adjusted life-year equivalent by estimating the duration of the effect and the impact on the patient’s quality of life. This will be achieved by using the Health Utilities Index to measure quality of life at baseline, 3 months and 9 months. 24 Unresolved deficits will have resource implications for the NHS and thus it is appropriate to subtract any savings that result from successful treatment from the cost of that treatment. These savings will be estimated by comparing the use of health-care resources by those with resolved and unresolved deficits.
We will obtain permission from participating patients to review their primary care records and extract data on treatments in practice and elsewhere. Other variables to be collected are patient demographic data, blood pressure, current drug therapy and concomitant medical conditions. The numbers of patients undergoing tarsorrhaphy or attending outpatient clinics for corneal ulceration will also be collected from patient records. Scottish Morbidity Register (SMR1) data on outpatient attendances will also be obtained. 25
No electrophysiological studies are proposed because these are normally unavailable to GPs within the timeframe of therapy initiation and because of uncertainty as to what useful information they add to the clinical decision, which is usually based on clinical examination and an assessment of the patient’s psychological state.
(k) Independent supervision
In accordance with the HTA’s research governance framework we will form an independent Trial Steering Committee (TSC) under the chairmanship of Professor Chris van Weel (Catholic University, Nijmegen) and a Data Monitoring and Ethics Committee (DMEC) under the chairmanship of Dr Marion Campbell (HSRU, University of Aberdeen). We are continuing our efforts to identify a suitable patient representative through health councils and the Cochrane collaboration.
3 Project timetable and milestones
| Month | Activity |
|---|---|
| – 3 | MREC Submission |
| Inform practices via local research, teaching and training networks, the MRC GP Research Framework, two direct mailshots to every medical and dental practice and out of hours co-operatives in Scotland. Train NHS24 nurses and ENT registrars. General advertisement via medical press | |
| 1 | Project-specific training of research team to use study instruments |
| Pilot study instruments and recruitment process | |
| 2 | Begin patient recruitment. Monthly reminders to health professionals. Data entry by research secretary |
| 5 | Begin 3-month assessments |
| 6 | Provide first interim report and prepare conference submissions |
| 11 | Begin 9-month assessments |
| 12 | Provide second interim report |
| 18 | Complete patient recruitment. Provide third interim report |
| 21 | Complete 3-month assessments |
| 24 | Provide fourth interim report |
| 27 | Complete 9-month assessments. Final analysis and writing up |
| 31 | Provide final report and submit papers |
4 Expertise
The research team comprises multidisciplinary expertise in health economics, medical statistics, neurology, otorhinolaryngology, general medical and dental practice. Members of the team will work within the framework of the Scottish School of Primary Care (SSPC), which co-ordinates primary care research throughout Scotland.
Professor Sullivan, the lead investigator, has experience of participating in six RCTs and of leading one RCT. He has 15 years of experience of other types of research in primary care. As the clinical director of one of the research networks, TayRen, and head of department in one of the participating universities, he will be able positively to influence recruitment in the East of Scotland.
Professor Morrison contributes expertise in the detection and assessment of psychological distress in primary care. She also has experience of participating in and leading RCTs in primary care. She will be able to influence the recruitment of study subjects by general practitioners in teaching and research networks in the West of Scotland.
Dr Smith is a primary care career scientist with expertise in the epidemiology of pain in the community. As a respected researcher in the north-east of Scotland he will be able to influence the recruitment of study subjects by general practitioners in teaching and research networks in Grampian and Highland.
Dr McKinstry has experience of leading two RCTs in telephone triage and hypertension, and is taking part in another. He has 15 years’ experience of other types of research in primary care. He is a working general practitioner and Medical Director of Lothian and Borders Primary Care Research Network and can therefore facilitate recruitment in this area.
Mr Swan is one of two postgraduate training directors in ENT in Scotland. He is able to train and support all Scottish ENT registrars likely to see patients eligible for study. He has already secured agreement from the clinical directors of 12/14 Scottish ENT units to participate.
Dr Donnan is a senior medical statistician who has advised on the study methodology and will remain in daily contact with the principal investigator and research fellow as the study proceeds.
Professor Cairns is a senior health economist with longstanding interests in community interventions.
Dr Davenport is a neurologist who has provided advice on the study methodology and will be a member of the panel of photographic reviewers providing independent assessment of the key outcome variable.
Dr Clarkson is the director of the Scottish Dental Practices Research Network. She will be able to increase the recruitment of study subjects by general dental practitioners in teaching and research networks throughout Scotland.
5 Trial process
The trial may be separated into eight stages as follows:
-
– 1. Approvals, advertisement, awareness-raising
This stage covers trial administration [MREC approvals, appropriate LREC approvals, Doctors’ and Dentisits’ Exemption (DDX) certification, etc.] and advice of the existence of the trial to all relevant persons with a potential role, including GPs, A&E departments, NHS24, dentists (stage 1) and ENT consultants, registrars and nurses (stage 2).
Stages 0 to 6 are taken from the patient’s point of view and are as follows:
-
0. Onset (or symptoms noticed)
-
1. Seek advice (visit GP, dentist or A&E; or contact NHS24)
These experts need to be sufficiently aware of the trial and its design to refer the patient onward to:
-
2. Visit the nearest ENT acute receiving clinic
of which there are 14 identified so far; of these, 13 have agreed to collaborate. Here diagnosis is confirmed and, if appropriate, consent, randomisation and the commencement of treatment follow. It is here that the first written record is taken from the patient. The Trial Co-ordinator is advised of the new recruitment, and the appropriate local Research Associate is informed.
It is crucial to the design of the trial that the time delay from onset to first administration of treatment should not exceed 72 hours.
On receipt of notification from the Co-ordinator, the local RA arranges
-
3. Baseline visit by local RA to the patient
to take place at the patient’s home or GP surgery (if preferred) within 3 days (72 hours) of the ENT visit. At this point photographs of the patient’s condition are taken and further details are recorded. This is followed two or three days later by
-
4. A telephone contact
to enquire about the patient’s condition, to check adherence to the treatment and their general progress on the trial. The two final stages are
-
5. 3-month visit
at which follow-up photographs and further details are taken. The final stage of patient involvement is
-
6. 9-month visit
if deemed necessary.
It is anticipated that recruitment will commence on 1 February 2004 and continue for 18 months up to 31 July 2005. The last recruit’s 9-month visit will therefore take place on or about 30 April 2006.That 27-month period is also the approximate period of employment of the local RAs. The trial is scheduled to end 6 months later on 31 October 2006.
6 Justification of support requested
Study Co-ordinator
A single, full-time postdoctoral health services researcher is required to co-ordinate all the components of the study, visit each of the centres and arrange meetings of the study team. This post will be full-time for the duration of the study and the postholder will be experienced in multi-centre health services research. They will be supported in this role by the Scottish Clinical Trials co-ordinators group based at HSRU in Aberdeen They will also be responsible for visiting the study subjects in their own region of Scotland, i.e. Tayside, Fife and Forth Valley.
Centre Co-ordinators
In the three other centres, Glasgow, Edinburgh and Aberdeen, the baseline, 3-month and 9-month visits will be conducted by centre co-ordinators working respectively full-time (covering all West of Scotland NHS boards), half-time (covering Edinburgh, Lothian and Borders) and half-time (covering Grampian and Highland).
Secretary
A half-time research secretary will be responsible for correspondence with patients and practitioners, data entry, typing reports and minutes of meetings, and general office administration.
Statistician
For a total of one day per month throughout the study and 3 months at the end of the study a postdoctoral statistician will be required to assist with analysis of the data and assist the principal investigators in completing the report to the HTA and preparation of papers for publication.
Health economist
For 3 months at the end of the study a postdoctoral health economist will be required to assist with the analysis of the economic data and assist the principal investigators in completing the report to the HTA and preparation of papers for publication.
Randomisation service
The Health Services Research Unit in Aberdeen has experience of 24-hour factorial randomisation for clinical trials. This will be accessed by an 0800 number from the ENT department where the patient is first seen, and data on entry of study subjects will be sent every day to the study co-ordinator with monthly summary statistics provided.
Office equipment
-
One laptop PC for the study co-ordinator to use on site visits and conferences as well as daily trial management (e.g. Dell C640–256Mb, CD, 20 GB).
-
Printer: e.g. HP LaserJet 1200.
-
Other equipment will be supplied by the study sites.
Postage + stationery
-
3000 initial requests to participate to all medical and dental practices as well as A&E units and out of hours co-operatives. Production of laminated sheets to go in every participating site’s consulting areas.
-
1500 reminders to participants. Monthly reminders to practices and hospitals.
Cameras
For baseline assessment and follow-up of patients at 3 and 9 months the research co-ordinators in each centre will need a camera.
-
Four Canon A40 8Mb USB cameras for onward transmission of digital images to the three assessors.
Travel to patients’ homes and conference expenses
-
This has been costed on the assumption of a return car journey of 10 miles either of the patient to the practice or the co-ordinator to the patient’s home.
7 References
- Petruzelli GJ, Hirsch BE. Bell’s Palsy. Postgrad Med 1991;90:115-27.
- Sugita T, Murakami S, Yanagihara N, Fujiwara Y, Hirata Y, Kurata T. Facial nerve paralysis induced by herpes simplex virus in mice: an animal model of acute and transient facial paralysis. Ann Otol Rhinol Laryngol 1995;104:574-81.
- Morgan M, Moffat M, Ritchie L, Collacott I, Brown T. Is Bell’s palsy a reactivation of varicella Zoster virus?. J Infect 1995;30:29-36.
- Bateman DE. Facial Palsy. Br J Hosp Med 1992;47:430-1.
- Rowlands S, Hooper R, Hughes R, Burney P. The epidemiology and treatment of Bell’s palsy in the UK. Eur J Neurol 2002;91:63-7.
- Morgenlander JC, Massey EW. Bell’s Palsy: ensuring the best possible outcome. Postgrad Med 1990;88:157-62.
- Salinas RA, Alvarez G, Alvarez MI, Ferreira J. The Cochrane Library. Oxford: Update Software; 2002.
- Sipe J, Dunn L. The Cochrane Library. Oxford: Update Software; 2002.
- May M, Wette R, Hardin WB. The use of steroids in Bell’s palsy: a prospective controlled study. Laryngoscope 1976;86:1111-2.
- Wolf SM, Wagner JH, Davidson S, Forsythe A. Treatment of Bell’s palsy with prednisone: a prospective randomised study. Neurology 1978;28:158-61.
- Abiko Y, Ikeda M, Hondo R. Secretion and dynamics of herpes simplex virus in tears and saliva of patients with Bell’s Palsy. Otol Neurotol 2002;23:779-83.
- Stampfer MJ, Buring JE, Willett W, Rosner B, Eberlein K, Hennekens CH. The 2x2 factorial design: its application to a randomised trial of aspirin and carotene in US physicians. Stat Med 1985;4:111-6.
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses?. Lancet 1998;352:609-13.
- Adour KK, Ruboyianes JM, Von Doeersten PG, Byl FM, Trent CS, Queensberry CP, et al. Bell’s palsy treatment with acyclovir and prednisone compared with prednisone alone: a double-blind, randomised, controlled trial. Ann Otol Rhinol Laryngol 1996;105:371-8.
- De Diego JI, Prim MP, De Sarria MJ, Madero R, Gavilan J. Idiopathic facial paralysis: A randomised, prospective and controlled study using single-dose prednisone versus acyclovir three times daily. Laryngoscope 1998;108:573-5.
- Simpson C. Personal communication. Annual Incidence of Bell’s Palsy (Read Code F130) in Scottish Continuous Morbidity Recording Practices n.d.
- Ekstran T. Bell’s palsy: prognostic accuracy of case history, sialometry and taste impairment. Clin Otolaryngol 1979;4:183-96.
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663-94.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey-Smith G. Subgroup analyses in randomized controlled trials: quantifying the risks of false-positives and false-negatives. Health Technol Assess 2001;5.
- House JW, Brackmann DE. Facial nerve grading system. Otolaryng Head Neck 1985;93:146-7.
- Purves AM, Penny KI, Munro C, Smith BH, Grimshaw J, Wilson B, et al. Defining chronic pain for epidemiological research – assessing a subjective definition. The Pain Clinic 1998;10:139-47.
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain 1992;50:133-49.
- Smith BH, Penny KI, Purves AM, Munro C, Smith WC, Grimshaw J, et al. The Chronic Pain Grade questionnaire: acceptability and validity in postal research. Pain 1997;71:141-7.
- Feeny D, Furlong W, Boyle M, Torrance GW. Multi-attribute health status classification systems. Health Utilities Index. Pharmacoeconomics 1995;7:490-502.
- Information and Statistics Division . ISD Scotland Guide: an A-Z of the Work of the Information &Amp; Statistics Division 1996.
Appendix 2 Trial Steering Committee
A Trial Steering Committee (TSC) should be set up with the following terms of reference:
Terms of reference
-
To monitor and supervise the progress of the trial towards its interim and overall objectives.
-
To review at regular intervals relevant information from other sources (e.g. other related trials).
-
To consider the recommendations of the Data Monitoring and Ethics Committee (DMEC).
-
To advise on publicity and the presentation of all aspects of the trial.
Membership of TSC
The membership should be limited and include an independent Chairman (not involved directly with the trial other than as a member of the TSC), two or more other independent expert members and the Principal Investigator. Where possible the membership should include a lay/consumer representative. The trial co-ordinator, trial statistician, etc., should attend meetings as appropriate. Observers from the Host Institution may be invited to all meetings.
Guidance notes
Meetings
Before the trial starts, the PI should organise a meeting of the TSC to finalise the protocol. The TSC should then meet at least annually, although there may be periods when more frequent meetings are necessary. Meetings should be organised by the PI. Papers for the meeting should be circulated in advance. An accurate minute should be prepared by the PI and agreed by all the members.
Trial steering and management
The role of the TSC is to provide overall supervision of the trial. In particular, the TSC should concentrate on the progress of the trial, adherence to the protocol, patient safety and consideration of new information. Day-to-day management of the trial is the responsibility of the PI. The PI may wish to set up a separate Trial Management Group to assist with this function.
Good clinical practice
The TSC should endeavour to ensure that the trial is conducted at all times to the standards set out in the MRC Guidelines for Good Clinical Practice (GCP).
Patient safety
In all the deliberations of the TSC the rights, safety and well-being of the trial participants are the most important considerations. The TSC should ensure that freely given informed consent is obtained from each trial participant. The TSC should advise the investigators on the completeness and suitability of the patient information provided.
Progress of the trial
It is the role of the TSC to monitor the progress of the trial and to maximise the chances of completing the trial within the agreed time scale. At the first TSC meeting, targets for recruitment, data collection, compliance, etc., should be agreed with the PI. Based on these targets, the TSC should agree a set of data that should be presented at each meeting (see template).
The PI is required to submit an annual report to HTA. This report should be endorsed by the TSC, should stand alone, and contain sufficient information to enable HTA to assess the progress of the trial without the need to refer back to the original application. The annual report should inform HTA of any new information that has a bearing on safety or ethical acceptability of the trial or any significant complaints arising, with a justification of the decisions taken.
The DMEC should be asked to advise the TSC, and may be required to provide information on the availability of data collected to date (from this and other studies) and advice on the likelihood that continuation of the trial will allow detection of an important effect. This should be done using methods that do not unblind the trial.
Adherence to protocol
The full protocol should be presented and agreed at the first TSC meeting. Any subsequent changes to the protocol must be approved by the TSC, LREC/MREC (and by HTA).
Data Monitoring and Ethics Committee
At its first meeting, the TSC should establish a Data Monitoring and Ethics Committee (DMEC) that meets regularly to review the data and results of any interim analyses.
Members of the DMEC should be independent of both the trial and TSC.
Consideration of new information
The TSC should consider new information relevant to the trial including reports from the DMEC. It is the responsibility of the PI, the Chairman and other independent members to bring results from other studies that may have a direct bearing on future conduct of the trial to the attention of the TSC.
On consideration of this information the TSC should recommend appropriate action, such as changes to the protocol, additional patient information, or stopping the trial. The rights, safety and well-being of the trial participants should be the most important consideration.
It is the responsibility of the PI to notify the TSC, DMEC and relevant regulatory authority (if applicable) immediately of any unexpected serious adverse events occurring during the course of the trial.
Template for Trial Steering Committee agendas and reports
The TSC should meet at least once a year and compose an annual report.
The table below outlines the information that should be provided by the PI at each meeting. This template should be used as a basis for the agenda of TSC meetings and a template for the annual report. These headings may not be appropriate at every stage of an individual trial, or for all trials.
Trial Steering Committee
Professor Chris van Weel, University of Nijmegen
Dr Ian Williamson, University of Southampton
Professor Sally Wyke, University of Stirling
Mrs Caryl Hamilton (Patient representative)
Professor Frank Sullivan, University of Dundee
Dr Peter Donnan, University of Dundee
In attendance:
Dr Fergus Daly, University of Dundee.
| Target (date set) | Achieved (date) | |
|---|---|---|
| Sample size sought | ||
| Date recruitment started | ||
| Proposed date for end of recruitment | ||
| Actual recruitment rate vs target rate (by month/quarter) | ||
| Acceptance rate, as a proportion (i) of those invited to participate | ||
| (ii) of all eligible participants, if known | ||
| Quarterly/monthly forecasts of recruitment for the planned remainder of the trial | ||
| Losses to follow-up (i) as a proportion of those entered | ||
| (ii) per month/quarter | ||
| Number still being followed up successfully and number who have completed follow-up | ||
| Completeness of data collected | ||
| Any available results (pooled) | ||
| Any organisational problems | ||
| Issues specific to the trial (as specified by the TSC) |
Appendix 3 Data Monitoring and Ethics Committee (DMEC)
The Data Monitoring and Ethics Committee (DMEC) is established to safeguard the interests of patients participating in randomised controlled trials.
The terms of reference and membership are based on the Medical Research Council Guidelines for Good Clinical Practice In Clinical Trials (1998).
The DMEC is the only body involved in the trial that has access to the unblinded comparative data. The role of committee members is to monitor these data and make recommendations to the Trial Steering Committee (TSC) if there are any ethical or safety reasons why the trial should not be continued.
The Chair of the TSC should be made aware of all communication between DMEC and the Principal Investigator (PI).
The membership of the DMEC will incorporate a pool of statisticians and clinicians/epidemiologists, consisting of at least three members to represent clinical, statistical and clinical trial expertise.
All members of the group should be independent of the trial they are monitoring. The frequency with which the DMEC subgroup meets will be dependent on the needs of the individual trial. The PI should submit a detailed plan for the interim analysis before the trial commences. The plan must satisfy members of the DMEC group.
Communication between the PI and the DMEC Chair is encouraged but should not bypass the TSC Chair. The PI and the Chair of the TSC will agree with their DMEC group Chair a timely mechanism for reporting to the DMEC group. With the help of the trial statistician, the PI must provide blinded data, in strict confidence, to the DMEC group as frequently as the members of the subgroup request. Serious adverse events must be reported to the lead clinician of the DMEC group and chairperson of the relevant research ethics committee immediately. If appropriate, the Medicines Control Agency must also be informed of all serious adverse events. The template for reporting interim data should be used by all the PIs.
The DMEC group will discuss the data on adverse events and, if appropriate, efficacy data, either in a meeting or by teleconference. If necessary, they may request further data from the PI and trial statistician. In the light of interim data, and other evidence from relevant studies (including updated overviews of the relevant randomised controlled trials), the DMEC group will inform the TSC if, in their view, the trial should proceed or be terminated. They may also advise the TSC on modification of the protocol.
Unless cessation of the protocol is recommended by the DMEC, the TSC and collaborators and administrative staff will remain ignorant of the results of the interim analysis of efficacy and toxicity. Collaborators and all others associated with the study may write to the DMEC, to draw attention to any concerns they may have about the possibility of harm arising from the treatment under study, or about any other matters that may be relevant.
Terms of reference
-
To set up and maintain direct communication with the PI and Chair of the TSC. The Chair of the TSC should be made aware of all communication between the PI and DMEC group.
-
To receive a copy of the trial protocol and plans for interim analysis prior to commencement of the trial, or, in the case of the first wave of trials, as early as possible.
-
To receive reports (as per template) during the trial at intervals agreed with the TSC and PI. It would be expected that these would be 6-monthly in the first year, and no less frequent than 12-monthly after that.
-
If interim analysis of the trial data is not planned in the protocol the subgroup should determine whether interim analysis should be undertaken.
-
To consider data from interim analyses, unblinded if considered appropriate, plus any additional safety issues for the trial and relevant information from the template and other sources.
-
In the light of points 3, 4 and 5, and ensuring that ethical considerations are of prime importance, to report to the TSC and recommend on the continuation of the trial.
Output and reporting by DMEC group
Format of first meeting
-
The first meeting of the group will generally be an open meeting with the trial investigators (PI and trial statistician). The output of that meeting will include agreement on the relevant material for that particular trial that needs to be reported subsequently within the template.
-
The report of the trial statistician to the DMEC group will be seen only by the group members. Each group meeting should be summarised in the form of brief minutes. These minutes and the report of the trial statistician will be circulated only to the DMEC group members.
-
A very brief summary of the recommendations of each subgroup meeting should be sent to the Chairman of the TSC.
Data Monitoring and Ethics Committee
Professor Marion Campbell, University of Aberdeen
Dr Carl Counsell, University of Aberdeen
Mr Rodney Mountain, University of Dundee
Dr Simon Ogston (Statistician), University of Dundee
DATA MONITORING AND ETHICS COMMITTEE REPORT TEMPLATE 1
Trial Summary and Analysis Plan for Pre-trial Submission
-
1. Title:
-
2. Grant No.:
-
3. Principal Investigator:
-
4. Introduction to the trial
-
4.1 Background in brief:
-
-
5. Methods in brief
-
5.1 Design of the trial
-
5.2 Details of interventions
-
5.3 Outcome measures
-
Primary:
-
Secondary:
-
-
5.4 Eligibility criteria
-
Inclusion criteria:
-
Exclusion criteria:
-
-
5.5 Sample size and analysis
-
-
6. Baseline characteristics that will be analysed for internal and external validity
-
Internal validity
-
(Comparability between the treatment groups)
-
-
External validity
-
(Comparability between trial participants and non-participants)
-
(Comparability between high and low recruiting centres)
-
-
All serious adverse events, as defined below, must be reported to the lead clinician of the DMEC monitoring subgroup and chairperson of the ethics committee as soon as possible.
Any untoward medical occurrence that at any dose:
-
Results in death
-
Is life threatening
-
Requires inpatient hospitalisation or prolongation of existing hospitalisation
-
Results in persistent or significant disability/incapacity
-
Is a congenital anomaly/birth defect
DATA MONITORING AND ETHICS COMMITTEE REPORT TEMPLATE 2
DMEC Report Date:
Report Number:
-
1. Title:
-
2. Trial progress
-
2.1 Trial recruitment
-
2.1.1. Plan of recruitment
-
Start date of recruitment =
-
End date of recruitment =
-
Recruitment period =
-
Expected average monthly recruitment =
-
Recruiting centres =
-
-
2.1.2 Recruitment to date
-
Recruitment period to date =
-
Total recruitment to date =
-
Observed average monthly recruitment =
-
Recruitment stratified by centre =
-
Expected recruitment period (based on current recruitment rate) =
-
End date of recruitment (based on expected recruitment patterns) =
Please insert a graph showing the planned and observed recruitment rates
-
-
2.1.3. Recruitment based on eligibility
-
Inclusion/exclusion
-
Number ineligible
-
Non-consent
-
Protocol violation
-
-
-
2.2 Internal validity
-
Comparability of selected baseline characteristics between the treatment groups
-
-
2.3 External validity
-
Selected baseline characteristics of trial participants and non-participants
-
Selected baseline characteristics of subjects in high and low recruiting centres
-
-
2.4 Protocol compliance
-
Number of patients withdrawn from treatment but continued being followed up
-
Number of patients who have been lost to follow-up
-
Number of patients with missing follow-up data
-
-
2.5 Frequency of primary events (if applicable)
-
-
3. Did you submit any data for interim analysis of efficacy?
-
Yes/No
-
-
4. Analysis of safety data.
-
These are to be presented overall and by group:
-
4.1 Serious adverse events
These are defined as any untoward medical occurrence that at any dose:
-
Results in death
-
Is life threatening
-
Requires inpatient hospitalisation or prolongation of existing hospitalisation
-
Results in persistent or significant disability/incapacity
-
Is a congenital anomaly/birth defect
To be summarised here but reported immediately to the Chairs of the DMEC subgroup and ethics committee and if appropriate to the Medicines Control Agency
-
-
4.2 Other adverse events
-
4.3 Abnormal laboratory tests (if applicable)
-
-
5. List any new publications on the safety and efficacy of the trial medications and provide copies.
-
6. List any new national or international guidelines on the treatment of the disease being studied and provide copies.
Appendix 4 BELLS Study Management Committee
Frank M Sullivan PhD, Scottish School of Primary Care, University of Dundee
Iain RC Swan MD, Department of Otolaryngology, University of Glasgow
Peter T Donnan PhD, Community Health Sciences, University of Dundee
Jillian M Morrison PhD, Division of Community Based Sciences, University of Glasgow
Blair H Smith MD, Department of General Practice and Primary Care, University of Aberdeen
Brian McKinstry MD, Community Health Sciences, University of Edinburgh
Richard J Davenport DM, Department of Clinical Neurosciences, University of Edinburgh
Luke D Vale PhD, Health Economics Research Unit, University of Aberdeen
Janet E Clarkson PhD, Dental Health Services Research Unit, University of Dundee
Victoria Hammersley BSc, Community Health Sciences, University of Edinburgh
Sima Hayavi PhD, Division of Community Based Sciences, University of Glasgow
Anne McAteer MSc, Department of General Practice and Primary Care, University of Aberdeen
Fergus Daly PhD, Community Health Sciences, University of Dundee
Appendix 5 Timetable of approvals: Copy of report to Chief Scientist Office
Scottish Bell’s Palsy Study The ‘BELLS’ study
HTA 02/09/04
The application to HTA was naturally preceded by an interval of some 3 months for negotiation and detailed discussion, the identification of a research team, the refinement of the study protocol, and for approaches to be made to, and provisional agreements reached with, local investigators at 18 hospital sites throughout Scotland. This relatively short timescale is illustrative of the enthusiasm and energy that the project was generating in the scientific and medical community.
Notwithstanding substantial changes in the MREC approvals process that were taking place during this period, there was altogether an interval of five months between the date of the successful application to HTA and the date of final MREC approval. This is normal and acceptable, taking account of the 6-week period for advertisement and appointment of staff, which could only reasonably commence after provisional MREC approval had been gained.
The project was originally planned to start on 1 October 2003, but the University of Dundee and HTA agreed a revised start date of 1 November 2003.
Approvals from local committees
1. Legal, indemnity, sponsorship
Study activity in Tayside and Fife was covered by the appointment of the study co-ordinator at the University of Dundee. Three universities were required to sign Letters of Agreement before staff could be appointed to progress the study in Grampian and Highlands (University of Aberdeen), Lothian and Borders (University of Edinburgh) and Glasgow and the West of Scotland (University of Glasgow).
Each of these institutions understandably needed to scrutinise the terms of the Agreement and also to explore all issues of indemnity given the nature of the project and particular its aspects of patient care and contact. It is notable that notwithstanding the award of the DDX, one university raised questions about the safety of steroid medication. There were some considerable delays induced by the lack of clarity surrounding the identity of the project sponsor, and this role was finally accepted by the University of Dundee in late April 2004 (ref. 2002PS27). Only then could the process of staff appointments commence. The third of three research assistants finally commenced employment on 5 July 2004.
2. NHS contracts
The four research assistants (study co-ordinator and three others) are all non-clinical and required NHS contracts to cover their dual roles within hospitals (issue, handling and return of drug stocks, secure handling of patient data and documentation) and with patients (up to three home visits, incorporating posed portrait photographs for expert clinical assessment, completion of questionnaires and gathering of additional data).
Each RA required a contract to cover activity within their allocated region and within others’, in order to achieve a proper coverage during holiday and other absences (e.g. maternity leave).
At the time of writing not all these contracts had been issued, although we were assured that merely having applied for them provides a sufficient foundation for the Research Associates to commence their work.
3. Local ethical approvals
Our applications for local ethics approvals commenced on 18 December 2003 with 13 applications despatched to regional committees. Later it emerged that some Glasgow hospitals have their own committees and Greater Glasgow has its own Primary Care ethics committee, and another five applications were despatched up to March 2004.
We had been led to believe, and it was the intention of the new MREC structure, that the process of local approvals for a multi-centre trial would be substantially eased, but in practice this was not the case. The new procedures would only come into place in May 2004, and the study team found themselves caught between the new MREC application form and old procedures in the regions.
For instance, one ethics committee requested their own version of the previously approved Patient Information Sheet for the study. In practice this is a requirement that could have been met without great effort (and the labour of its construction might have been less than that expended during a discussion of the principles involved) but the formal requirements at this time were very blurred for all concerned.
4. Local R&D approvals
At the same time (18 December 2003) we made simultaneous applications to 19 primary and acute care R&D departments, with another three up to March 2004. The first approval came through before the new year, but three difficulties became apparent in February.
First, the study team wish to reimburse the efforts of general practitioners in referring patients into the study. Early discussion with the HTA had confirmed the principle, but HTA insisted that the funding for this should come from Support for Science. At this stage neither the mechanisms for making and paying claims, nor the amount to be reimbursed had been agreed. It became apparent that not all R&D departments had applied for Support for Science and therefore not all had funding available to make reimbursements, which had been agreed at £51 per referral. This situation is now resolved, though not without some administrative difficulties and some feeling on both sides that advantage had been taken.
Second, in order to smooth the process of claim, it was required to identify research-active professionals additional to the team of nine principal investigators and 17 local principal investigators, and two additional local principal investigators were added to the list of team members. Notwithstanding the professional wisdom and experience added to the team by their appointment, this is essentially a device invented for no better reason than to allow an administrative process to take place.
Third, as a consequence of the requirement for uniformity in appearance of the study medications, the study budget has been used for the purchase of all treatments, active and placebo. In fact the study is funded only for the costs of placebo, according to the reasonable principle that primary care organisations should bear the cost of active treatment. These costs needed to be recovered from the primary care organisations by the study, but only through an administrative mechanism that has yet to be invented.
5. Pharmacies
By an oversight, the new local approvals form designed by COREC then lacked an entry allowing for the approval of pharmacies to contribute expertise to the running of a study, and the team found itself in the position of having to approach Chief Pharmacists for their approval after R&D approval to mount the study locally had already been gained. In many cases the individuals were aware of the BELLS Study through their position on local ethics committees or local R&D panels, but in all cases it was necessary for us to adopt the role of supplicant in our approach to pharmacies. Altogether 17 separate applications have been made.
In one case the pharmacy has required the issue of a steroid card with the patient pack, but also kindly agreed to the printing and administration of the card themselves.
Commencement of recruitment
Recruitment to the study finally commenced on 4 June 2004, with two of four researchers in post, 15 months after the invitation to tender, 11 months after our successful application to the HTA, 10 months after the first application to MREC and allowing altogether 5 months for the processes of local approvals.
We would state again the need for the project as clearly articulated in successive Cochrane reviews and by professionals; we would draw attention to the prevailing and undiminished enthusiasm and energy for the project from the scientists and researchers involved in the project; and finally and crucially we would reaffirm the fact that its design, its approach to patient care and its ethical framework have never been questioned on any other than trivial grounds that were easily and immediately addressed.
The calendar of the application process and approvals from central committees was as follows:
| Invitation to tender (HTA) | March 2003 |
| Application from University of Dundee to HTA | June 2003 |
| Date of award letter from HTA | 27 June 2003 |
| First application to MREC | 14 August 2003* |
| Provisional approval from MREC | 26 August 2003 |
*This date, 14 August 2003, may be regarded as the start of the approvals process.
| Appointment of Study Co-ordinator | 16 October 2003 |
| Application to MHRA for DDX | 16 October 2003 |
| Official start date for BELLS | 1 November 2003 |
| DDX received (MF8000/13139–141) | 12 November 2003 |
| Revised application to MREC | 19 November 2003 |
| MREC approval received (MREC/03/0/74) | 26 November 2003 |
The delays may be summarised as shown in Table 48.
| Item | Number of applications | Time taken |
|---|---|---|
| MREC approval | 1 + revision | 15 weeks |
| MHRA (DDX certificate) | 1 | 4 weeks |
| University agreements | 4 | 4 months |
| University sponsorship | 2 months after identification of this item as an issue | |
| NHS contracts | 4 × 4 | Variable – some came back by return of post |
| Local ethics approvals | 18 | 5 months. By the time the last two applications were made the form and associated requirements had altered |
| R&D approvals | 22 | 5 months. Some took less than 3 weeks (including Christmas closure) |
| Pharmacies | 17 | Altogether about 6 weeks, with most taking 2 weeks on average |
Appendix 6 How cruel to call it Bell’s palsy: by Graeme Garden

On December 1st 2002, while driving south along the M6, I discovered that I couldn’t whistle. I don’t know why I wanted to whistle; perhaps it was because I was on the way home after a particularly busy week. The previous Sunday evening we had recorded two editions of ‘I’m Sorry I Haven’t a Clue’ in front of a live audience at Blackpool’s Grand Theatre. Next morning I drove up to Edinburgh for a lunchtime meeting with a group of writers, with whom I spent the week outlining 13 episodes for a children’s drama series called ‘The Shoebox Zoo’, then back on Saturday to visit my mother in Preston, before driving home again on the Sunday, which was when I must have felt the need to whistle, and discovered it was impossible.
At home that evening the need to whistle didn’t arise, but I did notice that my mouth felt odd on the left hand side; not numb exactly, or puffy, but sort of weak and loose and… odd. I began to suspect what was going on, and so it didn’t come as an enormous surprise the next morning when I woke to find the left side of my face completely paralysed. I revealed the condition to my wife, Emma, as gently as I could; having caught sight of myself in the mirror it appeared that my face in repose took on an expression of shock or despair, and if I tried to smile, what came across was a most unsettling (and uncharacteristic) leer. She coped with this apparition pretty well, but was concerned about the cause. As a non-practising qualified doctor I knew a little about Bell’s palsy, at least enough to diagnose myself and rule out the more frightening possibilities such as a stroke. A visit to my GP confirmed the diagnosis, and I was duly prescribed a short, sharp course of prednisolone and aciclovir (no famciclovir being immediately available). After that, all being well, I could look forward to a lop-sided month or two, followed by a complete recovery. Meanwhile, I had to phone my agent.
The call to my agent, also named Emma, was a matter of some urgency, as the following morning, Tuesday, I was due to report to the set of ‘Holby City’ to record two episodes playing the role of a cardio-thoracic surgeon. It was only fair to let the producer know that, although I was fit enough to work, 50% of my face was simply not up to the job. It was too late to recast the part, so I said I was prepared to give it a go, and with luck we’d get away with it, but if the results were unacceptable then a rethink would be required. There then followed a series of phone calls between my agent Emma, my wife Emma, and the producer – yet another Emma – about the state of my face, how bad it really looked, and whether viewers might think I was ill or had suffered a stroke, or was drunk, or just playing the fool. We decided to go for it, so it was off to Elstree for three days’ recording.
The production staff and the cast were very understanding and supportive. My performance as Mr Loftwood was perhaps a little more muted than normal, but I think we got away with it. It was helped in the Operating Theatre scenes by the fact that I wore a surgical mask, and in the other scenes they managed to favour my good side to the camera, although there were moments when Mr Hyde was rather more in evidence than Dr Jekyll.
On the Saturday at the end of the first week of affliction we had a family dinner party to celebrate our son Tom’s 18th birthday. I sat at the head of the table, and towards the end of the meal noticed that everybody on one side of the table was in great high spirits, while those on the other side seemed rather dour and gloomy. My wife pointed out that those sitting on my right could see me smiling and animated, while those on the left saw only the paralysed, grim and unresponsive side of my face, which put a bit of a damper on their mood. Even when people understand the problem, they still can’t help reacting to the message they perceive the Bell’s-palsied face to be sending out, however unintentionally, and it is also very tedious having to keep explaining to people why you look the way you do. The other peculiar sensation was of the affected side of the head having its own personality, being cold and unresponsive, unlike the ‘normal’ side, which at times felt somehow out of control. If I smiled or laughed, it was as if the left side was unamused, and saying ‘get a grip of yourself!’ while the right side contorted itself uncontrollably into half a grin or giggle: a strange and disconcerting experience. Eating and drinking could also be troublesome, with a spurt of tea or gravy suddenly scooting out of the affected corner of the mouth. I could understand why people suffering from this embarrassing condition often like to hide themselves away and avoid social contact wherever possible. Unfortunately my diary was not prepared to allow me this luxury. Next Monday, December 9th, I was to record another two editions of ‘I’m Sorry I Haven’t a Clue’, this time at Sadlers Wells in London.
The audience of a thousand or so responded to my opening contributions rather coolly. Perhaps they were overcome with sympathy, or concern, or confusion, or fear, or perhaps were simply not minded to mock the afflicted. It therefore seemed a good idea to explain to them about Bell’s palsy, and how it affected the facial expression, and indeed speech. The loose lips find it difficult to pronounce Bs and Ps – which makes it especially cruel of the medical profession to call it Bell’s palsy. When someone asks you what’s wrong, you tend to reply ‘It’s Whbhell’s Whphalsy!’
That Thursday saw me at a school prize-giving, and once again I had to explain the condition to the assembled sixth-formers. It also seemed prudent to let them know that, in view of my lopsided leer and one winking eye, Social Services had been informed! In the days that followed there was a radio pilot in which I played a character who was supposed to be pretty leery anyway, so that was all right, and then a signing session for Dr Who fans to promote an audio episode I’d been in. The ‘Whovians’ who flocked to get the signatures and buy the merchandise may just have thought I was in some clever prosthetic make-up, and on that occasion I didn’t feel the need to do any explaining at all. After that, a few meetings, brief visits to the odd party, and Christmas. By the end of January I was counting wrinkles again, and by February my face was back to as near normal as it ever was.
I am very well aware that I got off lightly. The condition ran its course according to the textbook, and the prompt medication may well have helped. However, I am also aware that some people recover without treatment, while others are treated but have problems lasting many months or years. One doctor wrote to me saying that she had suffered for 12 months, then fell head first down a flight of stairs in New Zealand, and on regaining consciousness found she was cured. She does not recommend this course of treatment. What causes the condition is also currently unclear, although it does seem to be associated with the herpes simplex virus. Then again, we can’t rule out the old tale that sitting in a draught brings it on; my own week of driving long distances and sitting in a draughty Edinburgh hotel bedroom working on a laptop might be seen to point in that direction. One thing I did learn from my own, comparatively brief, experience of the palsy was that, once they understand what the problem is, people are much more supportive and sympathetic than you might suppose.
Appendix 7 Local Principal Investigators
Mr Kim Ah-See, Aberdeen Royal Infirmary
Mr Natarajan Balaji, Monklands Hospital, Airdrie
Mr Hasan Beg, Victoria Hospital, Kirkcaldy
Mr Quentin Gardiner, Perth Royal Infirmar
Mr Musheer Hussain, Ninewells Hospital, Dundee
Mr Alastair Kerr, Western General Hospital and Royal Infirmary, Edinburgh
Mr John Marshall, Southern General Hospital, Glasgow
Mr William McKerrow, Raigmore Hospital, Inverness
Ms Mary Shanks, Crosshouse Hospital, Kilmarnock
Mr David Simpson, Stobhill Hospital, Glasgow
Mr Guy Vernham, St John’s Hospital, Livingston
Miss Aileen White, Royal Alexandra Hospital, Paisley
Appendix 8 Scottish Bell’s Palsy Study: ‘BELLS’: Patient Information Sheet www.dundee.ac.uk/bells/
What is Bell’s palsy and how is it treated?
A doctor has told you that you may have a condition known as Bell’s palsy. The most common symptoms are weakness or paralysis on one side of your face, with others that may include dizziness, difficulty with blinking, problems tasting and increased sensitivity to sound. The cause of Bell’s palsy is not known, but it may be due to local swelling or a viral infection of the facial nerve on one side of your face. There is good recovery in the majority of cases. Unfortunately there is no medical treatment that is known for certain to improve how quickly people recover, and most patients with Bell’s palsy in the UK are currently given no medication.
It has been suggested that some medicines might improve patients’ recovery, and the two most commonly used by doctors are prednisolone (a steroid) and aciclovir (a medicine for treating viral infections). These medicines are in common use and licensed for other conditions, but not for use in treating Bell’s palsy, because it is not known which, if either, is better at aiding recovery, or if giving no medication at all is just as good.
-
The NHS has asked us to find out whether either of these medicines, separately or together, is most helpful in achieving a good recovery from Bell’s palsy.
To do this, we need your help. The Scottish Bell’s Palsy Study (‘BELLS’) is being conducted throughout Scotland and has been approved as an ethical study by the Scottish Multicentre Research Ethics Committee and by all the regional Research Ethics Committees.
We would like to invite you to join the Study. Please take time to read the following information carefully and discuss it with others if you wish. Ask if there is anything that is not clear, or if you would like more information.
-
The purpose of the study is to try to find out which of two treatments, a steroid (prednisolone) and an antiviral medicine (aciclovir), or neither, or both, is most effective in achieving a good recovery from Bell’s palsy.
-
You have been invited to join the study because you have recently been diagnosed with Bell’s palsy. It is fairly common (about 1 person in 60 during their lifetime) and there will be up to 500 fellow sufferers included in the study.
-
It is up to you to decide whether to join. If you decide not to (or if after joining the study you change your mind) your decision will not affect your future care. You don’t have to say why, and the doctor will just treat you as they normally would if the study was not taking place.
What will happen if I decide I am interested in taking part in the study?
If you decide you do want to take part in the study (and if you are suitable: not everybody will be) then this is what will happen to you.
-
You will be given two bottles of tablets by a doctor to take away with you, providing a 10-day course of treatment, starting immediately. One bottle will contain prednisolone (the steroid) or a ‘placebo’ (a harmless substance that looks exactly the same as the medicine, but has no effect). The second bottle will contain aciclovir (the medicine for treating viral infections) or another placebo.
So the four possible combinations of tablets are:
| Steroid and Antiviral | Steroid and Placebo | Placebo and Antiviral | Placebo and Placebo |
There is an equal chance that you will be given any of these combinations. Since nobody knows which of these possibilities is better than any other, you are not advantaged or disadvantaged by the choice of treatment allocated to you.
-
The doctor has checked that there is no reason why you cannot take either of these medicines.
The choice of treatment is made by a computer, and neither you nor the doctor will know which tablets you have been given. We will only find this out after all the results of the study are collected. If for medical reasons we need to find out before the end of the study what medication you are taking, we can do this. You can carry on your life completely as normal during the treatment and thereafter. There will be no added difficulty or complications from taking part in the study other than remembering to take your medicine, and some assessments that would comprise part of normal care anyway. You can continue to take exercise, drive, and eat and drink as you normally do.
You may take any additional medicines you may require such as pain relief, headache remedies, indigestion pills and so on: however, you should avoid any other steroid or antiviral preparations.
How will recovery be measured?
The severity of your condition, and how fast and how well you recover, will be assessed three times: once as soon as possible during the 10-day treatment period, again after 3 months, and finally after 9 months. A researcher will visit you at home or you can meet them at your GP’s surgery, if you prefer. If you have to travel from home for the assessments, then your travelling expenses will be reimbursed.
The assessment will consist of questionnaires to record your symptoms, and a series of photographs, taken to provide a record of how the condition has affected the appearance and control of your face. The photographs and all other information collected that is personal to you will be stored securely and will only be made available to those connected with the study in an official capacity. If at any time you decide to withdraw from the study, it will be necessary to retain your data for safety and monitoring purposes, but it will be maintained as carefully and with the same security as that of other patients who complete the study. You will not be identified in any report.
When do I need to decide?
-
We think that if these medicines are effective, then they have to be taken early, preferably within 48 hours of you first noticing your condition and definitely within 72 hours.
-
So, please let us know very soon if you are happy to join the study so that treatment can commence. Preferably, tell the doctor that gave you this information sheet now.
Will there be side effects?
Almost all medicines lead to occasional side effects, as well as helping the condition for which they were given. These ‘side effects’ are usually unwanted, but they are well-known.
For the medicines used in this study the side effects may include sickness, headache, dizziness, sleepiness or rash.
So, what are we saying?
We cannot guarantee you a benefit by taking part in the study, but given current medical knowledge, you are not being disadvantaged by joining the study, whichever choice of treatment is allocated to you. Other people in the future will be helped when we know which treatment is best. Thank you very much for helping us to learn more about an effective and appropriate treatment for patients with Bell’s palsy.
Finally…
If at any time you would like additional advice or consultation whilst you are on the study, then this can be obtained from < contact name and details >. The results of this study will be published in medical journals. An internal report will be written on completion of the study in 2006 and will be distributed to all taking part in it. Individuals will not be identified in any report.
Appendix 9 Form A
Appendix 10 Consent form
Appendix 11 Randomisation Service User Guide
Appendix 12 Recruiters’ notes
You probably already know that your hospital is one of 17 sites in Scotland that is contributing to a NHS-funded national study, aimed at establishing once and for all the effectiveness or otherwise of prednisolone/aciclovir separately or in combination as treatments for Bell’s palsy.
The stationery for the study
-
patient information sheet (for issue)
-
patient case record form (‘Form A’, for completion and filing)
-
consent form (for signature and filing)
is already provided at your site, as are the medications, labelled
-
Treatment 1 or 2 or 3 or 4
There is also a
-
laminated telephone dialling instruction sheet
explaining the procedures for registration and randomisation to treatment. If a patient with suspected Bell’s palsy presents at your clinic (usually though not necessarily as a referral from their GP) then please explain that this important national study is in progress, and
-
complete Form A (confirmation of diagnosis, inclusions/exclusions)
-
if patient is eligible and interested, issue Patient Information Sheet
-
if appropriate, get signatures on Consent Form
then
-
complete registration and randomisation to treatment
by
-
dialling HSRU at 0800 000000 and following instructions
Two things might go wrong:
-
If for some reason the allocated treatment pack is not available to you, please do not make a 2nd call to HSRU; simply allocate at random from the treatments available to you and note your decision on Form A.
-
If the call fails altogether, then again please simply allocate at random from the treatments available to you and note your decision on Form A.
In either case and as soon as you can, please telephone the study co-ordinator on
-
01382 000000 (W) 0771 000 000 (M) 01738 000000 (H)
and say what has happened.
Finally, please
-
issue designated treatment and commence dose immediately
Appendix 13 Weekly recruitment update (example)
Dear PIs, RAs:
-
BELLS: weekly recruitment update
-
Week number 81 (16.12.2005 – 23.12.2005)
of 108 recruitment weeks. The current figures for the BELLS study are always to be found at http://www.dundee.ac.uk/bells/index_files/stoppress.htm.
Part 1 Recruitment
The cumulative regional figures showing last week’s recruits are
| Grampian and Highland | + 2 | 76 |
| Tayside and Fife | + 0 | 67 |
| Lothian and Borders | + 1 | 73 |
| Glasgow and the West | + 3 | 198 |
| Total | + 6 | 414 |
of which M : F = 209 : 205.
FIGURE 1.
Weekly recruitment figures showing underlying recruitment rate.
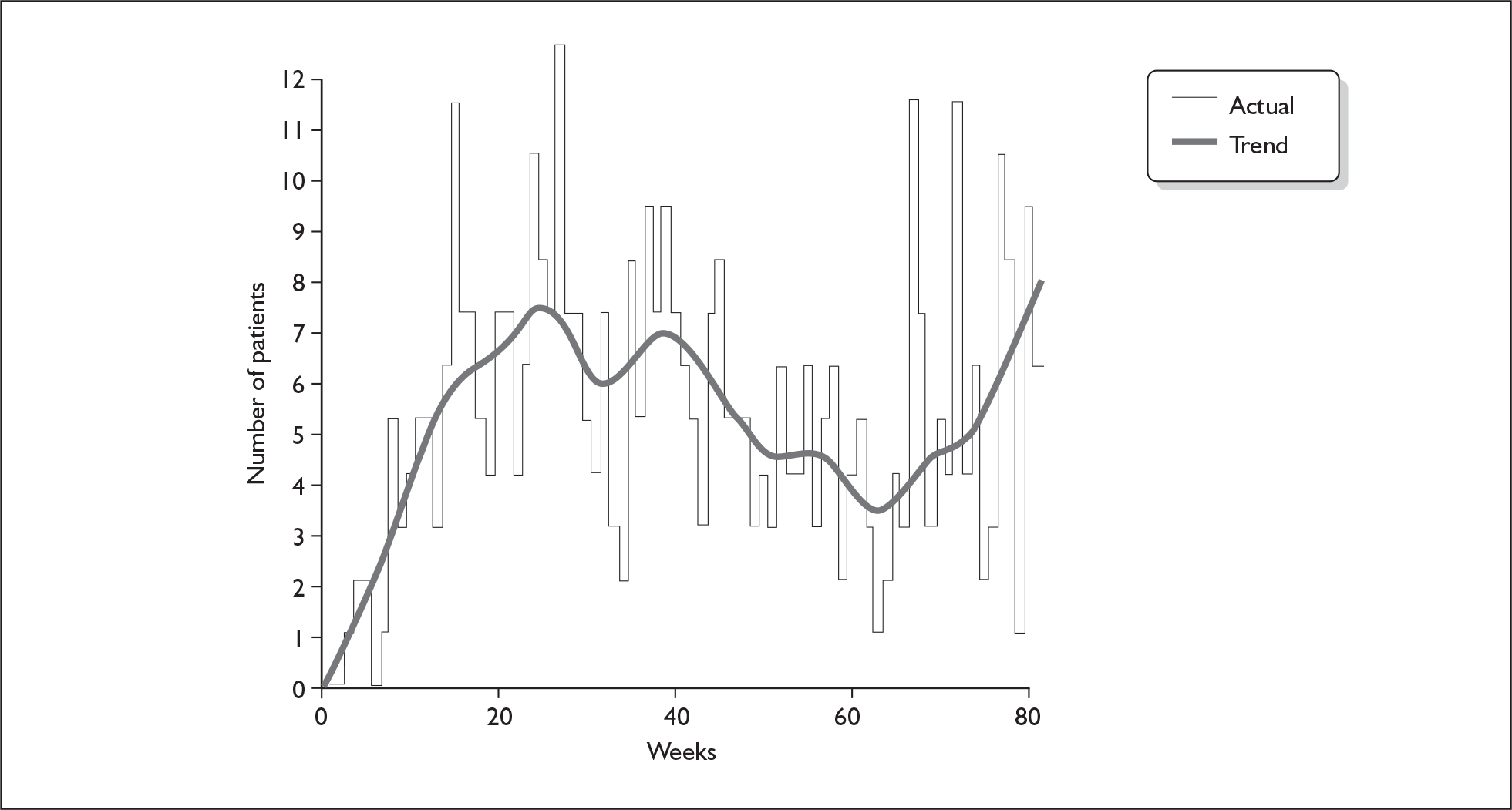
Part 2 Retention and adherence to target
There were 0 patients lost to follow-up this week. Since the start of the project 23 patients have been LTF, so there are 391 patients ‘live’ on the study. The study is currently
-
1 patient below target/1 day behind schedule.
Seasonal comparison: average recruitment over the last month is 6.1 pts/week; cf. the same period last year: 8.0 pts/week.
The next figure shows current retention and our final target.
FIGURE 2.
Retention and target.
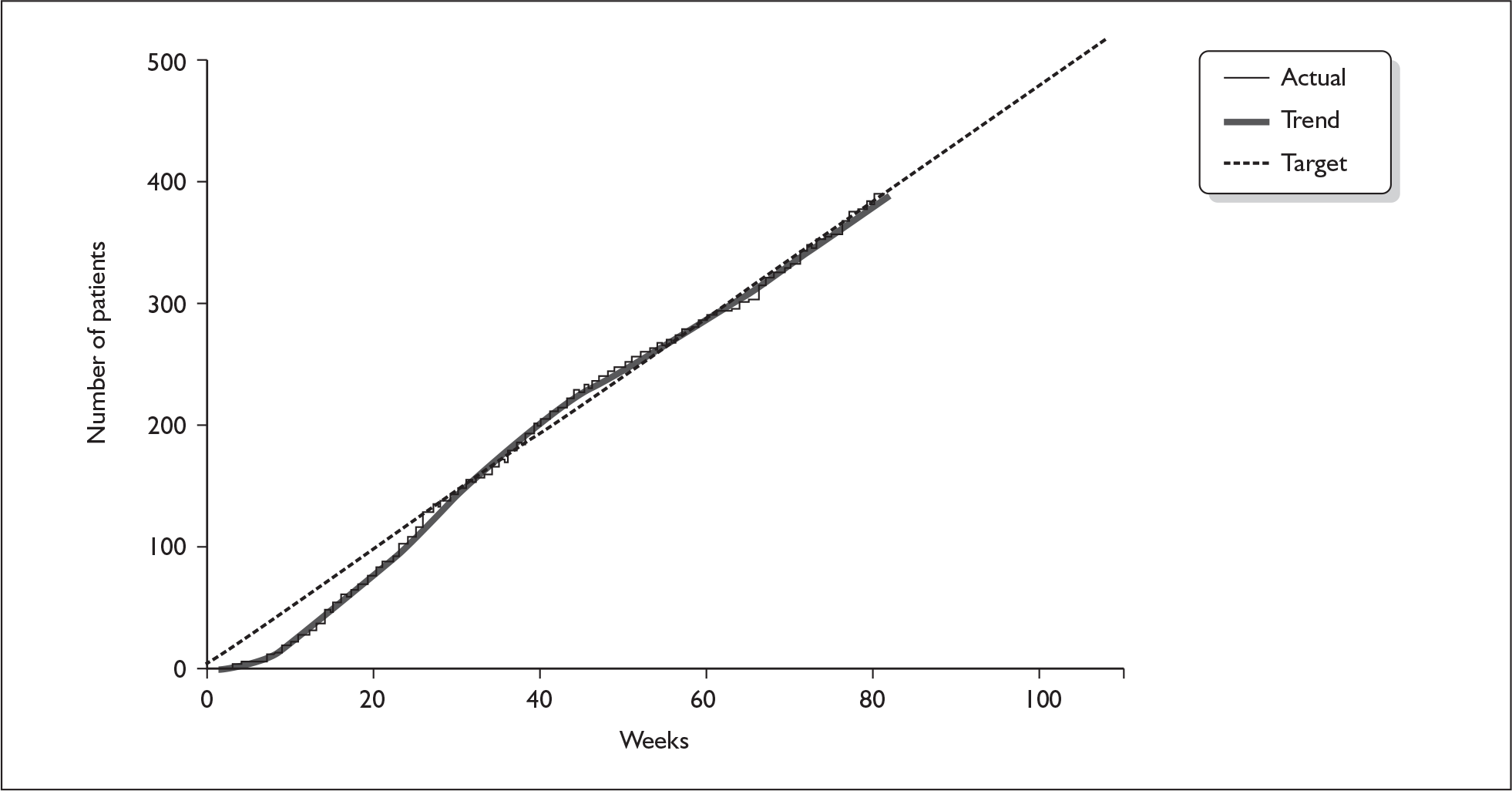
Part 3 Patients missed
There were 2 missed cases advised to us this week (one herpes zoster; in the other case a locum GP started steroid treatment and told the patient to go home and phone me. Which she did, to some mutual confusion). The total number of missed patients (incl. 87 found at ENT to have exclusions) is 155.
Part 4 Snapshot
Activity this week: 6 recruits, 6 V1s, 2 V2s, 0 V3s, 0 LTF.
Part 5 Status of the study
| Recruited, awaiting V1 | 3 |
| V1 made, awaiting V2 | 70 |
| V2 made, awaiting V2HB | 90 |
| V2HB known, V3 not necessary | 150 |
| V2HB known, awaiting V3 | 0 |
| V3 made, awaiting V3HB | 42 |
| V3HB known | 27 |
| Not easily classified* | 9 |
| Subtotal | 391 |
| LTF | 23 |
| Total | 414 |
* At any one time there is a small number of patients whose status is not easily classified, mainly through a failure to agree appointments or through other communication difficulties.
The number of completed patients is 150 + 27 = 177.
Fergus
Dr Fergus Daly
Coordinator, Scottish Bell’s Palsy Study
Tayside Centre for General Practice
University of Dundee Kirsty Semple Way
Dundee
DD2 4BF, Scotland
tel 01382 000000 (direct) 000000 (secretary) 000000 (reception)
fax 01382 000000
tel 01738 000000 (home) 07715 000000 (mobile)
email f.daly@tcgp.dundee.ac.uk
project email bells@tcgp.dundee.ac.uk
project web http://www.dundee.ac.uk/bells/
Appendix 14 Briefing notes for researchers
What happens after a new randomisation
When the doctor telephoned the HSRU computer in Aberdeen at the time of a patient’s hospital visit in order to register the patient and to be told what treatment to offer, a number of other events were set in train behind the scenes. The Aberdeen computer immediately sent an automatic email to the study co-ordinator in Dundee, copied to all the other three RAs. This email includes an electronic recording of the patient’s personal details (name, address and contact telephone number) as reported by the doctor during the telephone call.
If the arrival took place at one of ‘your’ hospitals, then, you will be alerted to this fact either by reading the email or, if the co-ordinator sees the message before you do, he will contact you by phone to alert you to the new arrival. Once you know that a new arrival is ‘yours’ please
-
advise the other RAs by email that you have picked up responsibility for the call
-
contact the patient and arrange your first visit
At this first visit you will need to
-
complete Form B
-
get the patient to complete three questionnaires
-
take 4 posed photographs
together with other minor tasks listed at Form C, which is your checklist.
Taking the photographs
Please use the green background supplied. It is convenient and aids uniformity in the poses if the patient is seated in front of it. (One idea that seems to work is sticking the background to the back of a door and placing a chair appropriately.) The tripod will aid steadiness and clarity of the images. Please set the camera as follows. Settings 1 to 5 can be set beforehand and will remain set thereafter. Unfortunately setting 6 needs to be attended to at each use.
-
Resolution: 1280 × 960
-
Macro (the ‘flower’ setting): No
-
Redeye reduction: Yes
-
Flash: Automatic (camera decides)
-
Date/time: No
and
-
Zoom: suggest 3.0, but local conditions may vary
If there is a source of daylight (e.g. a window) try to place the patient so that they are facing the source and you have your back to it. Then the photograph should be taken as though for a passport (portrait not landscape, some clearance around the face). You will find that you are quite close to the subject but they should not find this too oppressive.
The required poses are
-
at rest (eyes open, no expression)
-
smiling
-
eyes tight shut, clenched
-
raised eyebrows
Note: all of poses 2–4 are highly exaggerated, ‘forced’. (Quite tiring if you try it yourself.)
Later, please download the images to your computer for onward communication to TCGP. Please label the .jpg files as shown in the following example, showing the study title, patient ID, date, visit number and pose:
-
BELLS ID2708033 20040518 V2 Pose3.jpg
If you are uncertain/unhappy with any of the poses then it is OK to send more than one, but we should try not to send more than two. In such a case please call the pose numbers Pose3a and Pose3b.
Appendix 15 Form C
Appendix 16 Form B
Appendix 17 House–Brackmann Assessors
Mr Iain Swan, Glasgow Royal Infirmary
Dr Richard Davenport, Western General Hospital, Edinburgh
Mr Ken Stewart, St John’s Hospital, Livingston
Appendix 18 Health Utilities Index Mark 3
Appendix 19 Brief Pain Inventory
Appendix 20 Derriford Appearance Scale (DAS59)
Appendix 21 Patient deaths on the BELLS study
Patient reference 2613014
| Date of Consent to BELLS study | 27.03.2005 |
| Site | Monklands Hospital Airdrie |
| Allocated treatment | Trt 2 (not decoded) |
| Planned treatment period | 27.03.2005 – 5.04.2005 |
| Date of Death | 04.2005 |
| Age | 78 |
| Cause of Death | 1A bronchial pneumonia |
| 1B stroke | |
| 2 liver metastases, primary unknown | |
| Compliance | This patient never commenced BELLS medications |
Patient reference 2617009
| Date of Consent to BELLS study | 17.12.2004 |
| Site | Glasgow Royal Infirmary |
| Allocated treatment | Trt 4 (not decoded) |
| Planned treatment period | 17.12.2004 – 26.12.2004 |
| Date of Death | 04.2005 |
| Age | 53 |
| Cause of Death | Sudden Death (believed MI) |
| Compliance | 9/10 days prednisolone/placebo, 9/10 days aciclovir/placebo |
Patient reference 2613030
| Date of Consent to BELLS study | 14/01/2006 |
| Site | Monklands Hospital Airdrie |
| Allocated treatment | Trt 2 (not decoded) |
| Planned treatment interval | 14/01/2006 – 24/01/2006 |
| Date of Death | 04/2006 |
| Cause of Death | Ischaemic heart disease |
| Coronary atheroma | |
| Hypertensive heart disease | |
| Compliance | Not known (containers not returned) assumed complete |
The treatments were decoded following the end of patient follow-up in March 2007.
Trt 2 (two of the three events) is double-placebo and Trt 4 is aciclovir with placebo.
Appendix 22 CONSORT Checklist of items to include when reporting a randomised trial
| SECTION/topic | Item | Description | Page |
|---|---|---|---|
| TITLE & ABSTRACT | 1 | How participants were allocated to interventions (e.g., ‘random allocation’, ‘randomised’, or ‘randomly assigned’) | i,iii |
|
INTRODUCTION Background |
2 | Scientific background and explanation of rationale | 1,2 |
|
METHODS Participants |
3 | Eligibility criteria for participants and the settings and locations where the data were collected | 5,6 |
| Interventions | 4 | Precise details of the interventions intended for each group and how and when they were actually administered | 6, 7 |
| Objectives | 5 | Specific objectives and hypotheses | 9 |
| Outcomes | 6 | Clearly defined primary and secondary outcome measures and, when applicable, any methods used to enhance the quality of measurements (e.g., multiple observations, training of assessors) | 9–11 |
| Sample size | 7 | How sample size was determined and, when applicable, explanation of any interim analyses and stopping rules | 13 |
| Randomisation – Sequence generation | 8 | Method used to generate the random allocation sequence, including details of any restrictions (e.g., blocking, stratification) | 13 |
| Randomisation – Allocation concealment | 9 | Method used to implement the random allocation sequence (e.g., numbered containers or central telephone), clarifying whether the sequence was concealed until interventions were assigned | 13, 14 |
| Randomisation – Implementation | 10 | Who generated the allocation sequence, who enrolled participants, and who assigned participants to their groups | 13, 14 |
| Blinding (masking) | 11 | Whether or not participants, those administering the interventions, and those assessing the outcomes were blinded to group assignment. When relevant, how the success of blinding was evaluated | 14 |
| Statistical methods | 12 | Statistical methods used to compare groups for primary outcome(s); Methods for additional analyses, such as subgroup analyses and adjusted analyses |
13, 14, 43–45 |
|
RESULTS Participant flow |
13 | Flow of participants through each stage (a diagram is strongly recommended). Specifically, for each group report the numbers of participants randomly assigned, receiving intended treatment, completing the study protocol, and analysed for the primary outcome. Describe protocol deviations from study as planned, together with reasons | 16 |
| Recruitment | 14 | Dates defining the periods of recruitment and follow-up | 15, 17, 18 |
| Baseline data | 15 | Baseline demographic and clinical characteristics of each group | 18 |
| Numbers analysed | 16 | Number of participants (denominator) in each group included in each analysis and whether the analysis was by ‘intention-to-treat’. State the results in absolute numbers when feasible (e.g., 10/20, not 50%) | 17 |
| Outcomes and estimation | 17 | For each primary and secondary outcome, a summary of results for each group, and the estimated effect size and its precision (e.g., 95% confidence interval) | 18–21 |
| Ancillary analyses | 18 | Address multiplicity by reporting any other analyses performed, including subgroup analyses and adjusted analyses, indicating those prespecified and those exploratory | 25 |
| Adverse events | 19 | All important adverse events or side effects in each intervention group | 25,26 |
|
DISCUSSION Interpretation |
20 | Interpretation of the results, taking into account study hypotheses, sources of potential bias or imprecision and the dangers associated with multiplicity of analyses and outcomes | 47, 48 |
| Generalisability | 21 | Generalisability (external validity) of the trial findings | 47, 48 |
| Overall evidence | 22 | General interpretation of the results in the context of current evidence | 48, 49 |
List of abbreviations
- AP
- aciclovir–prednisolone group
- AO
- aciclovir–placebo group
- OP
- placebo–prednisolone group
- OO
- placebo–placebo group
- A
- aciclovir group (i.e. AP + AO)
- A′
- no-aciclovir group (i.e. OP + OO)
- P
- prednisolone group (i.e. AP + OP)
- P′
- no-prednisolone group (i.e. AO + OO)
- HB
- House–Brackmann
Prior to decoding, the four treatments were labelled Trt 1, Trt 2, Trt 3, Trt 4, and after decoding these were revealed to be:
- OP Trt 1
- (prednisolone + placebo)
- AP Trt 3
- (aciclovir + prednisolone)
- OO Trt 2
- (placebo + placebo)
- AO Trt 4
- (aciclovir + placebo)
- A&E
- accident and emergency
- BNF
- British National Formulary
- BPI
- Brief Pain Inventory
- CEAC
- cost-effectiveness acceptability curves
- CI
- confidence interval
- CSO
- Chief Scientist Office (of the Scottish Government)
- DAS59
- Derriford Appearance Scale
- DDX
- Doctors’ and Dentists’ Exemption
- DMEC
- Data Monitoring and Ethics Committee
- ENT
- ear, nose and throat
- GPRD
- General Practice Research Database
- HSRU
- Health Services Research Unit
- HUI3
- Health Utilities Index Mark 3
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ISD
- Information Services Department
- LREC
- Local Research Ethics Committee
- MREC
- Multicentre Research Ethics Committee
- NNT
- number needed to treat
- OR
- odds ratio
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- SD
- standard deviation
- SE
- standard error
- SPCRN
- Scottish Primary Care Research Network
- SPPIRe
- Scottish Professionals and Practices Interested in Research
- TSC
- Trial Steering Committee
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Reimsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson, EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and costeffectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebocontrolled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
-
Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis.
By Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al.
-
The clinical effectiveness and costeffectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.
By Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al.
-
Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness.
By Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al.
-
Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al.
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.
By Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al.
-
Gemcitabine for the treatment of metastatic breast cancer.
By Jones J, Takeda A, Tan SC, Cooper K, Loveman E, Clegg A.
-
Varenicline in the management of smoking cessation: a single technology appraisal.
By Hind D, Tappenden P, Peters J, Kenjegalieva K.
-
Alteplase for the treatment of acute ischaemic stroke: a single technology appraisal.
By Lloyd Jones M, Holmes M.
-
Rituximab for the treatment of rheumatoid arthritis.
By Bagust A, Boland A, Hockenhull J, Fleeman N, Greenhalgh J, Dundar Y, et al.
-
Omalizumab for the treatment of severe persistent allergic asthma.
By Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al.
-
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma.
By Boland A, Bagust A, Hockenhull J, Davis H, Chu P, Dickson R.
-
Adalimumab for the treatment of psoriasis.
By Turner D, Picot J, Cooper K, Loveman E.
-
Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a single technology appraisal.
By Holmes M, C Carroll C, Papaioannou D.
-
Romiplostim for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a single technology appraisal.
By Mowatt G, Boachie C, Crowther M, Fraser C, Hernández R, Jia X, et al.
-
Sunitinib for the treatment of gastrointestinal stromal tumours: a critique of the submission from Pfizer.
By Bond M, Hoyle M, Moxham T, Napier M, Anderson R.
-
Vitamin K to prevent fractures in older women: systematic review and economic evaluation.
By Stevenson M, Lloyd-Jones M, Papaioannou D.
-
The effects of biofeedback for the treatment of essential hypertension: a systematic review.
By Greenhalgh J, Dickson R, Dundar Y.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of Science Support, NETSCC, HTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies & Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Ms Jane Bates, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Professor Glyn Elwyn, Primary Medical Care Research Group, Swansea Clinical School, University of Wales
-
Dr Ron Gray, Consultant Clinical Epidemiologist, Department of Public Health, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, University of Sheffield
-
Dr Jennifer J Kurinczuk, Consultant Clinical Epidemiologist, National Perinatal Epidemiology Unit, Oxford
-
Dr Susanne M Ludgate, Medical Director, Medicines & Healthcare Products Regulatory Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, Joint Director, National Collaborating Centre for Mental Health, University College London
-
Mrs Una Rennard, Service User Representative
-
Dr Phil Shackley, Senior Lecturer in Health Economics, School of Population and Health Sciences, University of Newcastle upon Tyne
-
Dr W Stuart A Smellie, Consultant in Chemical Pathology, Bishop Auckland General Hospital
-
Dr Nicholas Summerton, Consultant Clinical and Public Health Advisor, NICE
-
Ms Dawn Talbot, Service User Representative
-
Dr Graham Taylor, Scientific Advisor, Regional DNA Laboratory, St James’s University Hospital, Leeds
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Pharmaceuticals Panel
-
Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor in Child Health, University of Nottingham
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Mrs Barbara Greggains, Service User Representative
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Professor Jonathan Ledermann, Professor of Medical Oncology and Director of the Cancer Research UK and University College London Cancer Trials Centre
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Dr Lesley Wise, Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Therapeutic Procedures Panel
-
Consultant Physician, North Bristol NHS Trust
-
Professor of Psychiatry, Division of Health in the Community, University of Warwick, Coventry
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School, Coventry
-
Ms Maree Barnett, Acting Branch Head of Vascular Programme, Department of Health
-
Mrs Val Carlill, Service User Representative
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Mr Mark Emberton, Senior Lecturer in Oncological Urology, Institute of Urology, University College Hospital, London
-
Professor Steve Goodacre, Professor of Emergency Medicine, University of Sheffield
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and The London School of Medicine and Dentistry
-
Mr Paul Hilton, Consultant Gynaecologist and Urogynaecologist, Royal Victoria Infirmary, Newcastle upon Tyne
-
Professor Nicholas James, Professor of Clinical Oncology, University of Birmingham, and Consultant in Clinical Oncology, Queen Elizabeth Hospital
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Kate Radford, Senior Lecturer (Research), Clinical Practice Research Unit, University of Central Lancashire, Preston
-
Mr Jim Reece Service User Representative
-
Dr Karen Roberts, Nurse Consultant, Dunston Hill Hospital Cottages
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
-
Professor Tom Walley, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist, National Commissioning Group (NCG), London
-
Director, NHS Sustainable Development Unit, Cambridge
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Professor Mike Kelly, Director, Centre for Public Health Excellence, NICE, London
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Ms Jeanett Martin, Director of Nursing, BarnDoc Limited, Lewisham Primary Care Trust
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Miss Nicky Mullany, Service User Representative
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Ken Stein, Senior Clinical Lecturer in Public Health, University of Exeter
-
Dr Kieran Sweeney, Honorary Clinical Senior Lecturer, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Professor Margaret Thorogood, Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Caroline Stone, Programme Manager, Medical Research Council
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington