Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 05/51/01. The assessment report began editorial review in July 2007 and was accepted for publication in November 2008. See the HTA programme website (www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the the clinical and cost-effectiveness of trastuzumab for the treatment of primary breast cancer in human epidermal growth factor 2 (HER2)-positive women based upon a review of the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The manufacturer’s scope restricts the intervention to intravenous trastuzumab given for 1 year after surgery and after the completion of standard adjuvant chemotherapy, and the comparator to standard therapy without trastuzumab. The clinical rationale for the duration of treatment in the scope is open to question and leads to the exclsuion of one potentially relevant trial. The submitted evidence reports that the 3-weekly regimen of trastuzumab produced a relative reduction in all-cause mortality of 24–33%. Meta-analysis of all available studies based on 12 months of trastuzumab showed that there was a statistically significant 30% relative improvement in overall survival using the 3-weekly regimen. A study looking at weekly cycles of trastuzumab, excluded in the manufacturer’s submission, produced a relative reduction in all-cause mortality of 59%, which was not statistically significant. All included studies showed a statistically significant difference in the risk of recurrence or death from any cause (disease-free survival), favouring trastuzumab. There was a statistically significant increase in the relative risk of a serious adverse event in women treated with 3-weekly cycles of trastuzumab, with no excess toxicity in the study evaluating weekly cycles. Estimates of cost-effectiveness provided by the manufacturer were based on data from the HERA trial using the 3-weekly regimen of trastuzumab. The economic model was a state-transition model that compared the lifetime impact of adding 1 year of trastuzumab therapy to standard care with standard care alone. The initial cost-effectiveness estimate was £5687 per additional quality-adjusted life-year (QALY) gained, rising to a maximum of £8689 upon one-way sensitivity analysis. The base-case estimate of cost-effectiveness was subsequently revised by the manufacturer, resulting in an estimated incremental cost per additional QALY gained of £2387. A number of assumptions behind the manufacturer’s model may be optimistic and could mean that the incremental costs per QALY gained were underestimated. Additional analysis carried out by the evidence review group concluded that the incremental cost-effectiveness ratio (ICER) is expected to be around £20,000 to £30,000. The addition of potential long-term cardiac events could push the ICER above £30,000, although there is no long-term evidence to date surrounding this issue. In addition, the small study excluded from the manufacturer’s submission raises the possibility of an equally effective but shorter regimen, incurring lower cost and toxicity and with greater patient convenience. The guidance issued by NICE in June 2006 as a result of the STA states that trastuzumab, given at 3-week intervals for 1 year or until disease recurrence, is recommended as a treatment option for women with early-stage HER2-positive breast cancer following surgery, chemotherapy and radiotherapy.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of trastuzumab for the treatment of primary breast cancer in HER2-positive women. 2
Description of the underlying health problem
HER2-positive breast cancer is a breast cancer that tests positive for human epidermal growth factor receptor 2 (HER2). This protein promotes cancer cell growth. Cancer cells produce an excess of HER2 as a result of gene mutation in about one-third of cases of breast cancer. HER2-positive breast cancers are more aggressive than other types of breast cancer and are less responsive to hormone treatment.
Scope of the ERG report
No scoping exercise was undertaken by NICE for this STA. The scope as defined by Roche (the manufacturer of trastuzumab), restricts the intervention to intravenous trastuzumab given for 1 year after surgery and after the completion of standard adjuvant chemotherapy. It restricts the comparator to standard therapy without trastuzumab, which by implication is NICE’s recommended four to eight cycles of anthracycline-containing chemotherapy postsurgery and 5 years of hormonal therapy. The primary outcome is defined as disease-free survival (cancer recurrence or death from any cause); secondary outcomes include overall survival, breast cancer recurrence and cardiotoxicity. Economic outcomes include cost per life-year gained (LYG) and cost per quality-adjusted life-year (QALY) gained.
Methods
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process. In addition, the ERG carried out a meta-analysis of trials to derive a more precise estimate of treatment effect in terms of overall survival, disease-free survival, distant recurrence and cardiac toxicity. The ERG also critically evaluated the role of a study excluded in the manufacturer’s submission (FinHER study3) in decision-making. Sensitivity analysis was also carried out to evaluate the robustness of the manufacturer’s model, as well as the impact of the ERG’s revised base-case assumptions.
Results
Summary of submitted clinical evidence
Five relevant phase III trials were identified by systematic review: HERA (n = 3387),4 BCIRG-006 (n = 2148),5 NCCTG N9831 (n = 1615),6 NSABP B-31 (n = 1736),6 and FinHER (n = 229). 3 The published evidence reports that 18 × 3-weekly cycles of trastuzumab produced a relative reduction in the hazard of all-cause mortality from 24% [hazard ratio (HR) 0.76, 95% CI 0.47–1.23; absolute risk reduction 0.5%) at a median follow-up of 1 year in the HERA trial to 33% (HR 0.67, 95% CI 0.48–0.93; absolute risk reduction 1.8%) at a median follow-up of 2 years in the combined B-31 and N9831 analysis. When all studies with available data were meta-analysed there was a 30% relative improvement in overall survival and this was statistically significant at the 5% level (HR 0.70, 95% CI 0.53–0.92, p = 0.010). The excluded study,4 which looked at nine weekly cycles of trastuzumab, given concurrently with three cycles of docetaxel or eight cycles of vinorelbine, produced a relative reduction in the hazard of all-cause mortality of 59% (HR 0.41, 95% CI 0.16–1.08; absolute risk reduction 6.9%) at a median follow-up of 3 years (the longest follow-up available for any trastuzumab schedule). This study had a small sample size and was not statistically significant at the 5% level.
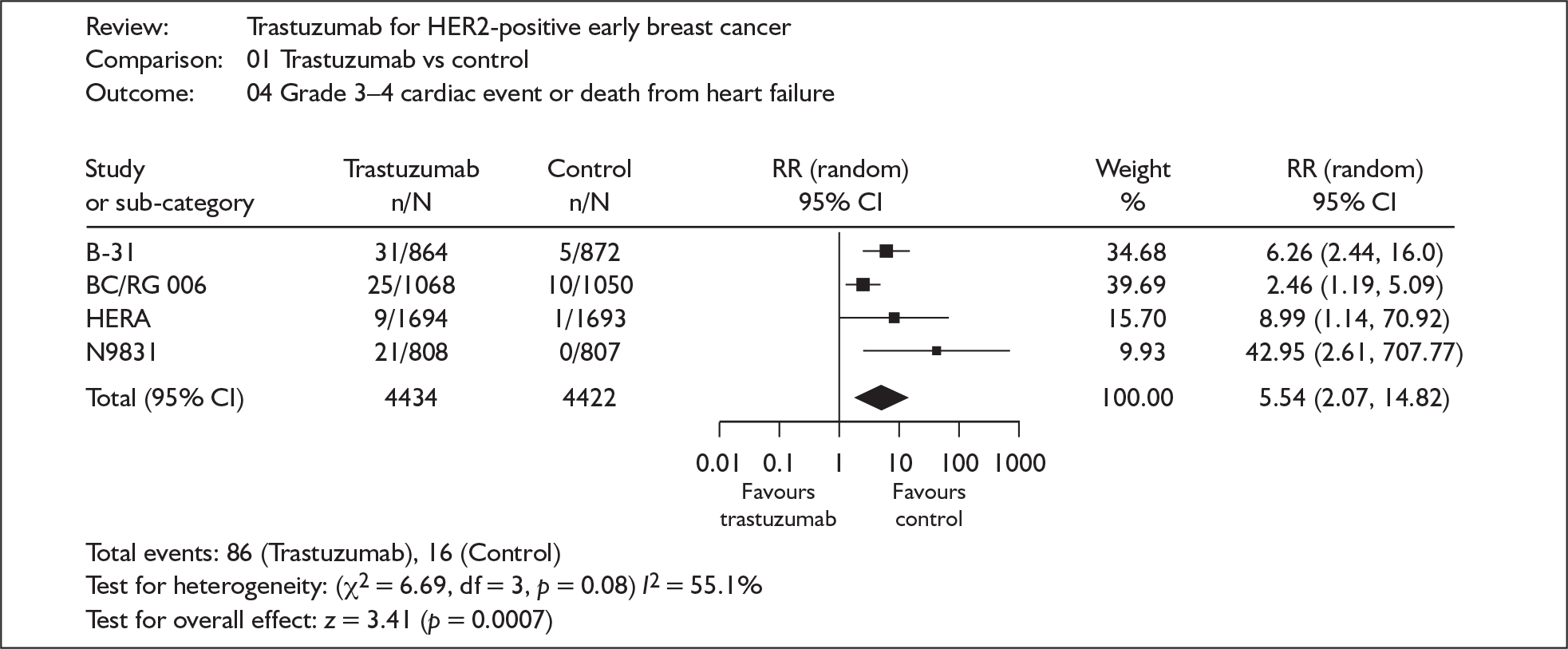
All included studies, at whatever schedule or length of follow-up, showed a statistically significant difference in the risk of recurrence or death from any cause (disease-free survival), favouring trastuzumab. The combined HR for 18 × 3-weekly cycles was 0.50 (95% CI 0.44–0.57, p < 0.00001). In the study evaluating nine weekly cycles the HR was 0.42 (95% CI 0.21–0.83, p = 0.01). There was a statistically significant (almost sixfold) increase in the relative risk (5.54, 95% CI 2.07–14.82, p = 0.0007) of a serious life-threatening or fatal cardiac event in women treated with 18 three-weekly cycles of trastuzumab, although this represents an absolute risk increase of just 1.6% (Figure 1). In the FinHER study evaluating nine weekly cycles there was no excess toxicity. 3
FIGURE 1.
Cardiac toxicity.
Summary of submitted cost-effectiveness evidence
Roche have developed a state-transition cohort model to compare the lifetime impact of 1 year of adjuvant trastuzumab therapy with no trastuzumab following standard chemotherapy. The main data source for the model is the Herceptin Adjuvant (HERA) trial, an international, multicentre, randomised trial on women with HER2-positive primary breast cancer, with a median of 1 year of follow-up. Outcomes from the HERA trial are extrapolated over a lifetime horizon to assess the long-term benefits and costs of trastuzumab. The model takes into account cardiac toxicity but does not consider other adverse events. The health states used within the model are considered to be appropriate for the required analysis. The cost of trastuzumab has been underestimated in the Roche submission, along with the cost of monitoring for cardiac toxicity. The costs and utilities associated with each health state were based upon studies carried out by the MEDTAP (Medical Technology Assessment and Policy) research centre specifically for the model. These costs appear high relative to other recent breast cancer models. 8,9
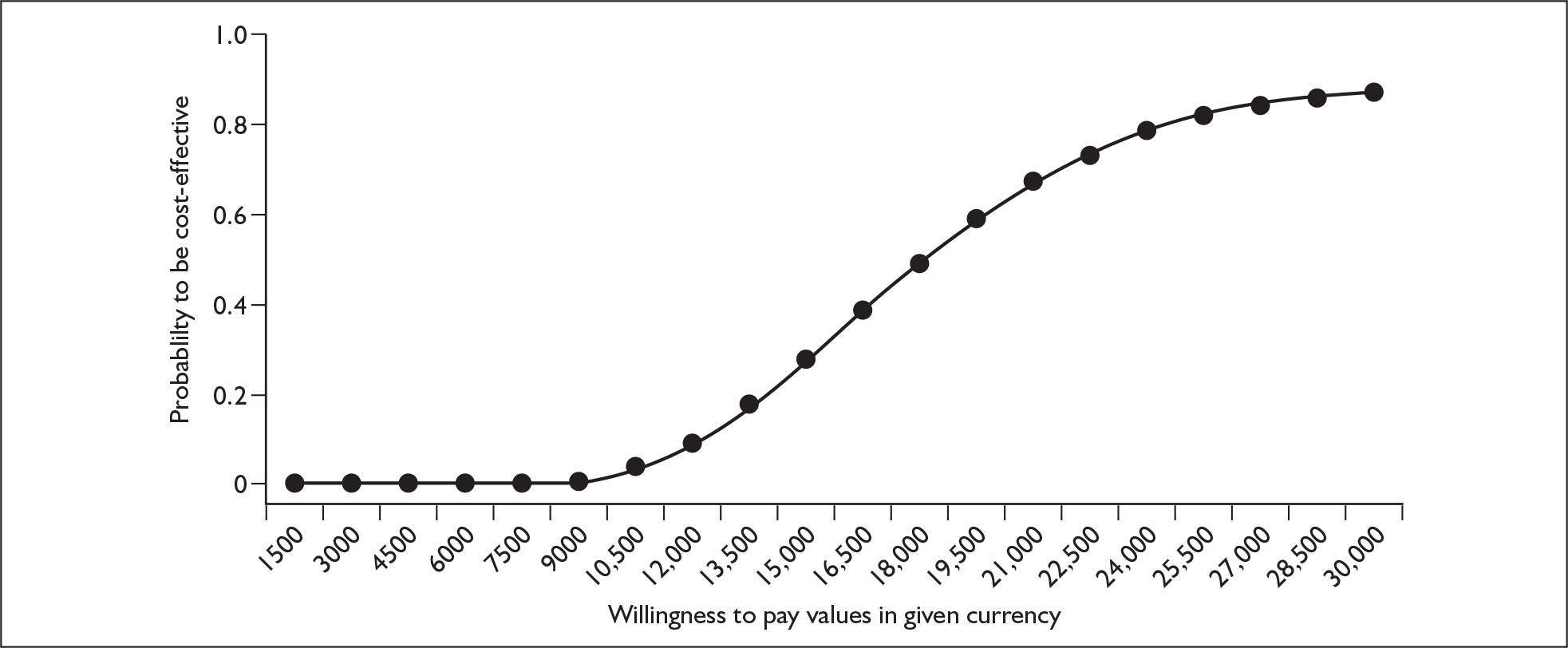
The Roche model estimated that the base-case incremental cost-effectiveness ratio (ICER) of chemotherapy plus trastuzumab versus chemotherapy is £5687 per QALY gained, rising to a maximum of £8689 upon one-way sensitivity analysis of the parameters. However, in the view of the ERG several of the baseline costs were underestimated and some of the upper or lower parameter values tested within the sensitivity analysis were not sufficiently extreme. In addition, there was no sensitivity analysis around the extrapolation of rate of recurrence in the comparator arm and limited sensitivity analysis around the relative risk of recurrence for trastuzumab. With respect to the probabilistic sensitivity analysis the description of uncertainty surrounding the mean values of many of the model parameters was considered to be insufficient or incomplete. However, following responses from Roche to queries raised by the ERG in a letter dated 8 March 2006 a revised base case of £2387 was presented by Roche (section 6 of ERG report2). This included a correction to an error in the original model, which reduced the ICER. Based on further sensitivity analysis carried out by the ERG (e.g. Figure 2) the ERG conclude that the ICER presented by Roche is too low. The combined effect of the uncertainties has the potential to increase the central estimate of the ICER to around £20,000–£30,000 (Figure 3). The addition of potential long-term cardiac events could push the ICER above £30,000, although the ICER is not expected to rise above £35,000–£50,000.
FIGURE 2.
Sensitivity analysis around rate of recurrence over time in comparator arm.
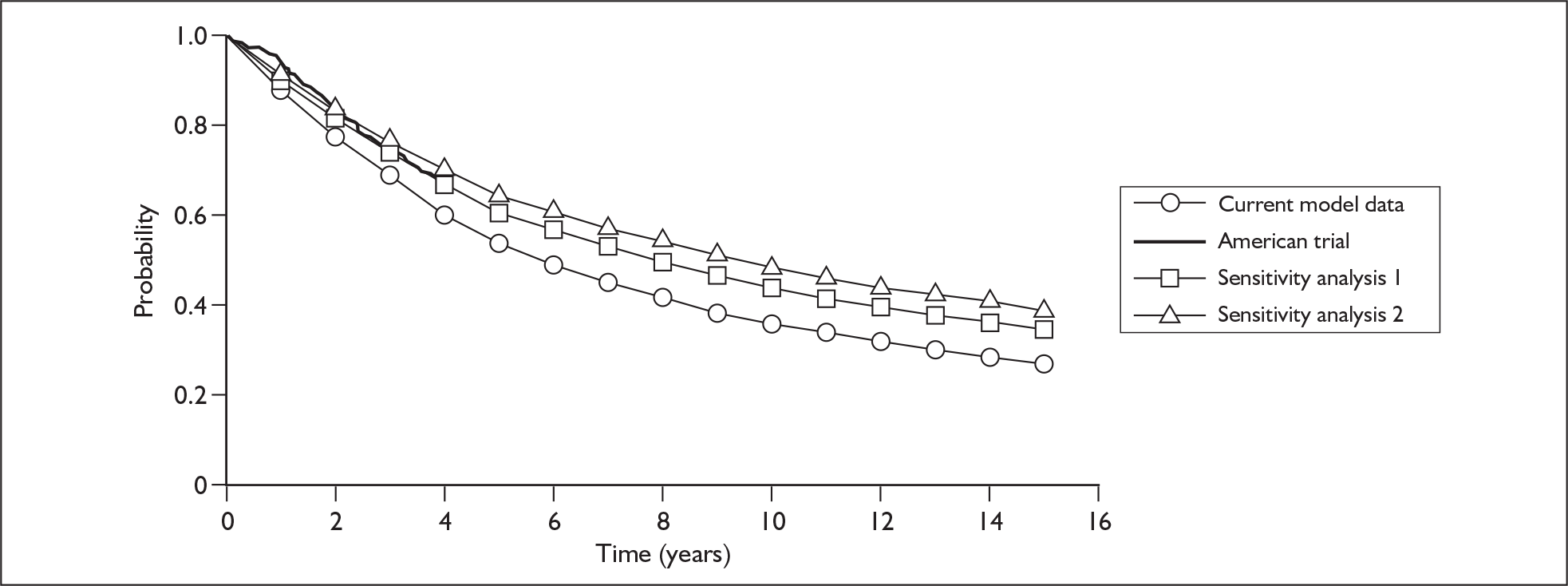
FIGURE 3.
Evidence review group’s base case – cost-effectiveness acceptability curve. QALY, quality-adjusted life-year.
Commentary on the robustness of submitted evidence
The model structure is appropriate and allows sensitivity analysis to be carried out easily. One-way sensitivity analysis suggests that variations in the majority of the parameters do not have a large effect upon the ICER. The baseline ICER is relatively modest, such that potential parameter variations are unlikely to increase the ICER beyond the currently accepted threshold values. However, no sensitivity analysis has been undertaken to explore the impact of uncertainty surrounding the comparator arm on the ICER. Little sensitivity analysis has been carried out around the long-term benefits of trastuzumab. Confidence intervals of some of the parameters do not adequately describe the uncertainty. For instance, the upper values of the cost of trastuzumab and cardiac monitoring were considered to be unrealistic.
There are a number of major areas of uncertainty. Disease-free and overall survival may differ from the comparator arm in the model, depending on the chemotherapy regimens being used in the UK. The benefits of trastuzumab regarding rates of recurrence are unknown beyond 3–4 years. There is little evidence to date on the effects of trastuzumab upon overall survival. There is no evidence on the effects of trastuzumab upon long-term cardiac dysfunction.
Conclusions
The following issues have the potential to impact on the cost-effectiveness results: the uncertainty surrounding the long-term benefits of trastuzumab in terms of reduction in the risk of recurrence; the extent to which reductions in the rate of recurrence will translate into benefits in overall survival; the extent to which patients in both the comparator arm and the trastuzumab arm are likely to receive trastuzumab in the metastatic setting; and the uncertainty generated by long-term extrapolation of the comparator arm. The combined effect of these uncertainties has the potential to increase the ICER from below £5000 to around £20,000–£30,000. The addition of potential long-term cardiac events could push the ICER above £30,000 although there is no long-term evidence to date surrounding this issue.
There are also a number of other important issues that are not explicitly taken into account in the economic modelling. A small study (the FinHER trial,3 n = 229), excluded from the manufacturer’s submission, raises the possibility of an equally effective but shorter regimen, incurring lower cost and toxicity and with greater patient convenience. Finally, there are a number of capacity issues for the NHS: HER2 testing, the preparation and administration of trastuzumab and cardiac monitoring will all require the augmentation of currently available facilities.
Summary of NICE guidance issued as a result of the STA
The guidance issued by NICE in June 2006 states that:
Trastuzumab, given at 3-week intervals for 1 year or until disease recurrence (whichever is the shorter period), is recommended as a treatment option for women with early-stage HER2-positive breast cancer following surgery, chemotherapy (neoadjuvant or adjuvant) and radiotherapy (if applicable).
Cardiac function should be assessed prior to the commencement of therapy and trastuzumab treatment should not be offered to women who have a left ventricular ejection fraction (LVEF) of 55% or less, or who have any of the following:
-
a history of documented congestive heart failure
-
high-risk uncontrolled arrhythmias
-
angina pectoris requiring medication
-
clinically significant valvular disease
-
evidence of transmural infarction on electrocardiograph
-
poorly controlled hypertension.
Cardiac functional assessments should be repeated every 3 months during trastuzumab treatment. If the LVEF drops by 10 percentage (ejection) points or more from from baseline and to below 50% then trastuzumab treatment should be suspended. A decision to resume trastuzumab therapy should be based on a further cardiac assessment and a fully informed discussion of the risks and benefits between the individual patient and their clinician.
Acknowledgements
The authors wish to thank Rob Coleman (Professor of Medical Oncology, University of Sheffield), Michael Dixon (Consultant Surgeon and Senior Lecturer, Edinburgh Breast Unit), Frances Lambert (Macmillan Breast Care Nurse Specialist), David Thomson (Lead Pharmacist, British Oncology Pharmacists Association), Andy Hanby (Professor of Breast Pathology, Leeds Teaching Hospital NHS Trust), Catherine Dickens (Consultant Cardiologist, Leeds Teaching Hospital NHS Trust), Laurence O’Toole (Consultant Cardiologist, Sheffield Teaching Hospitals NHS Trust) and Tim Perren (Consultant Oncologist, Leeds Teaching Hospitals NHS Trust) who provided advice during the project.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.
- Ward S, Pilgrim H, Hind D. Trastuzumab for the Treatment of Primary Breast Cancer in HER2-Positive Women: A Single Technology Appraisal 2006.
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, et al. FinHer Study I, Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med 2006;354:809-20.
- Piccant-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsh A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER-2 positive breast cancer. N Engl J Med 2005;353:1657-72.
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Pawlicki M, et al. Phase III Trial Comparing AC-T With AC-TH and With TCH in the Adjuvant Treatment of HER2 Positive Early Breast Cancer Patients: First Interim Efficacy Analysis n.d.
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84.
- Karnon J, Delea T, Johnston S, Smith R, Brandman J. Cost effectiveness of extended adjuvant letrozole in postmenopausal women after adjuvant tamoxifen therapy. Pharmacoeconomics 2006;24:237-50.
- Remak E, Brazil L. Cost of managing women presenting with stage IV breast cancer in the United Kingdom. Br J Cancer 2004;91:77-83.