Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 05/53/01. The assessment report began editorial review in July 2007 and was accepted for publication in November 2008. See the HTA programme website (www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical and cost-effectiveness of docetaxel for the adjuvant treatment of early node-positive breast cancer based upon the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The manufacturer’s scope restricts the intervention to docetaxel in combination with doxorubicin and cyclophosphamide (TAC), and the comparator to anthracycline-based chemotherapy. Based on the BCIRG 001 trial, the submitted evidence shows that TAC is associated with superior disease-free and overall survival at 5 years compared with the anthracycline-based regimen FAC. The absolute risk reduction in patients treated with TAC compared with those treated with FAC was 7% for disease-free survival and 6% for overall survival. However, TAC was associated with significantly greater toxicity than FAC. There is also evidence that docetaxel, in an unlicensed sequential regimen FEC100-T, is associated with superior diseasefree and overall survival at 5 years compared with FEC100. An economic model was developed by the manufacturer based on the BCIRG 001 trial. This generated central estimates of the cost per life-year gained and cost per quality-adjusted lifeyear (QALY) gained of TAC compared with FAC of £7900 and £9800 respectively. The manufacturer’s submission predicts a cost-effectiveness of £15,000–£20,000 per QALY gained for TAC compared with E-CMF (epirubicin in sequential therapy with cyclophosphamide, methotrexate, and fluorouracil), and estimates the cost-effectiveness of FEC100-T to be £8200 per QALY compared with FEC100. Taking into account a number of issues identified by the ERG this may generate higher estimates of cost-effectiveness, but these are unlikely to exceed £35,000 per QALY gained. Importantly, FAC is not commonly used in clinical practice in the UK and, therefore, the submitted evidence does not indicate whether TAC is superior to the anthracycline-based regimens that are in common use (FEC or E-CMF). The indirect comparisons presented suggest that the economic case for TAC in comparison to current UK practice may not be proven. The manufacturer’s submission failed to record evidence of three serious adverse events in patients receiving docetaxel with doxorubicin or to mention the concern of the European Medicines Agency regarding TAC’s long-term adverse events. The guidance issued by NICE in June 2006 as a result of the STA states that docetaxel, when given concurrently with doxorubicin and cyclophosphamide (the TAC regimen), is recommended as an option for the adjuvant treatment of women with early nodepositive breast cancer.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of docetaxel for the adjuvant treatment of early node-positive breast cancer. 2
Description of the underlying health problem
Breast cancer is the most common cancer among women in England and Wales. Around one in nine women will be diagnosed with breast cancer at some time in their lives. In 2002, 37,134 new cases of breast cancer were diagnosed in women in England and Wales. 3 The risk of breast cancer increases with age; over 80% of cases occur in women aged over 50. 3
In breast cancer, prognosis is related to a number of factors, including the extent of disease progression identified at diagnosis or initial surgery.
Scope of the decision problem
The scope of the manufacturer’s submission was limited to docetaxel in combination with doxorubicin and cyclophosphamide (TAC) for the adjuvant treatment of women diagnosed with operable node-positive breast cancer (i.e. the relevant licensed application), compared with anthracycline-based chemotherapy. It thus excludes women with high-risk node-negative cancers. Such women, who are at intermediate risk of recurrence, would, in clinical practice, be considered for adjuvant chemotherapy. The scope also excludes docetaxel used in sequential therapy (i.e. following or preceding several cycles of other cytotoxic drugs), although current clinical opinion appears to favour such regimens rather than combination regimens such as TAC. The anthracycline-based regimens in common use in the UK at the time of the assessment were FEC and E-CMF. The limitation of the comparators to anthracycline-based regimens excludes paclitaxel, another taxane, which, like docetaxel, is licensed for use in the UK as adjuvant therapy for operable node-positive breast cancer in sequential therapy following treatment with doxorubicin and cyclophosphamide.
Methods
The ERG report comprised a critical review of the evidence for the clinical and cost-effectiveness of the technology, based upon the manufacturer’s/sponsor’s initial submission to NICE and subsequent clarification of issues raised by the ERG early in the STA process. A narrative critique of the submitted evidence was presented. The economic model submitted by the manufacturer was analysed to investigate the impact of different assumptions regarding potential indirect comparisons with UK comparator therapies.
Results
Summary of submitted clinical evidence
There is evidence from a randomised controlled trial (RCT) that, compared with the anthracycline-based regimen FAC, TAC is associated with superior disease-free and overall survival at 5 years (HR 0.72, 95% CI 0.59–0.88, p = 0.001 versus HR 0.70, 95% CI 0.53–0.91, p = 0.008). 4 The absolute risk reduction at 5 years in patients treated with TAC compared with those treated with FAC was 7% for disease-free survival and 6% for overall survival, and the number of patients who had to be treated with TAC rather than FAC for one additional patient to benefit was 14 for disease-free survival and 17 for overall survival. However, TAC was associated with significantly greater toxicity than FAC.
There is also RCT evidence that a sequential regimen, FEC100-T, in which docetaxel is used after the anthracycline-based regimen FEC100, is associated with superior disease-free and overall survival at 5 years (adjusted HR 0.83, 95% CI 0.69–0.99, p = 0.041 versus HR 0.77, 95% CI 0.59–1.00, p = 0.05) compared with FEC100. 5 The estimated absolute risk reduction at 5 years in patients treated with FEC100-T compared with those treated with FEC100 was 5.1% for disease-free survival and 4.0% for overall survival, and the number of patients who had to be treated with FEC100-T rather than FEC100 for one additional patient to benefit was 20 for disease-free survival and 25 for overall survival.
Summary of submitted cost-effectiveness evidence
An economic model was developed by the manufacturer, based primarily on the single trial BCIRG 001. 4 This submission model generates central estimates of the cost per life-year gained and cost per quality-adjusted life-year (QALY) gained of TAC compared with FAC of £7900 and £9800 respectively.
The manufacturer’s submission predicts a cost-effectiveness of £15,000–£20,000 per QALY gained for TAC compared with E-CMF (epirubicin in sequential therapy with cyclophosphamide, methotrexate, and fluorouracil). This estimate was based upon an indirect comparison of absolute disease-free survival rates.
Based upon the RCT of FEC100-T compared with FEC100, the manufacturer’s submission estimates the cost-effectiveness of FEC100-T to be £8200 (£3500–£56,000) per QALY compared with FEC100. Only four of the six potentially relevant studies reported overall survival and/or disease-free survival (Table 1).
| Study Intervention/comparator | Population | Median follow-up | Number of patients | Outcome | Intervention | Comparator | Hazard ratio (95% CI) | Absolute risk reduction |
|---|---|---|---|---|---|---|---|---|
| BCIRG 001, six cycles of TAC, six cycles of FAC | Node-positive | 55 months | 1491 | Recurrence of breast cancer within 5 years | 19% | 26% | 0.72 (0.59-0.88), p = 0.001 | 7% |
| PACS 01, three cycles of FEC100 followed by three cycles of docetaxel, six cycles of FEC100 | Node-positive | 60 months | 1999 | Recurrence of breast cancer within 5 years | 22% | 27% | 0.83 (0.69-0.99), p = 0.041 | 5% |
| ECOG 2197, four cycles of AT, four cycles of AC | Node-positive and high-risk node negative | 53 months | 2952 | 4-year disease-free survival | 87% | 87% | 1.08 (0.89-1.31), p = 0.43 | 0% |
| 4-year overall survival | 93% | 93% | 1.09 (0.84-1.43), p = 0.48 | 0% | ||||
| USO 9735, four cycles of TC, four cycles of AC | Node-positive and high-risk node-negative | 66 months | 1016 | Estimated 5-year disease-free survival | 86% | 80% | 0.67 (0.50-0.94), p = 0.015 | 6% |
| Estimated 5-year overall survival | 90% | 87% | 0.76, p = 0.131 | 3% |
Commentary on the robustness of submitted evidence
The ERG identified four other potentially relevant studies that do not meet the inclusion criteria in full, which were missed by the manufacturer in their literature search. These are the ECOG 2197,6 GEICAM 9805,7 USO 97358 and RAPP 019 studies.
The submitted clinical evidence depends primarily on an interim analysis from one trial, BCIRG 001, which uses docetaxel in its licensed regimen (TAC). 4 This is a large study carried out in a population that appears to be representative of the population for whom adjuvant docetaxel is licensed and who are expected to be eligible to receive it. However, there is no evidence that the study outcome assessors were blinded to treatment allocation, although the US Food and Drug Administration (FDA) recommends such blinding when disease-free survival is measured and considers it necessary to minimise bias in the assessment of drug toxicity. FAC, the anthracycline-based regimen used as the comparator in the trial, is not in common use in the UK, where FEC and E-CMF predominate. The submitted evidence does not therefore indicate whether TAC is superior to the anthracycline-based regimens that are in common use.
No evidence of systematic bias has been found in the primary economic analysis of TAC compared with FAC presented within the manufacturer’s submission. It is the ERG’s opinion that a revised model taking into account a number of modelling issues identified by the ERG may generate higher estimates of cost-effectiveness (Table 2), but it is unlikely that these estimates would exceed £35,000 per QALY gained. The manufacturer’s submission presents a probabilistic sensitivity analysis of uncertainty in the economic estimates; the certainty in the cost-effectiveness estimates is overestimated.
Conclusions
Docetaxel has been licensed for use in combination with doxorubicin and cyclophosphamide (TAC) for the adjuvant treatment of women diagnosed with operable node-positive breast cancer. Evidence from a large RCT demonstrates that TAC is superior to the anthracycline-based FAC regimen in terms of disease-free and overall survival at 5 years. However, the same evidence suggests that TAC is associated with significantly greater toxicity than FAC. Importantly, FAC is not commonly used in clinical practice in the UK. The most common adjuvant chemotherapy regimens currently in use in the UK are FEC, using an epirubicin dose of 75 mg/m2 or greater, or E-CMF. FAC has not been demonstrated to be superior to these anthracycline regimens.
The manufacturer’s submission to NICE failed to record the premature termination of the French RAPP 01 trial following three fatal or life-threatening adverse events in patients receiving docetaxel with doxorubicin. Furthermore, the submission does not mention the concern of the European Medicines Agency (EMEA) regarding TAC’s long-term adverse events, as a result of which intensive monitoring for cardiotoxicity, secondary leukaemia and serious gastrointestinal toxicity is ongoing.
There also exists RCT evidence that docetaxel, in an unlicensed sequential regimen FEC100-T, is associated with superior disease-free and overall survival at 5 years compared with FEC100.
The health economic model submitted by Sanofi-aventis estimates that TAC has a cost-effectiveness in the order of £10,000 per QALY gained compared with FAC. Indirect comparisons presented within this review (Figure 1) suggest that the economic case for TAC in comparison to current UK practice is not proven. As part of the unlicensed FEC100-T regimen, the manufacturer’s submission estimates that the cost-effectiveness for docetaxel is in the order of £10,000 per QALY gained compared with FEC100, a comparator that is currently used in the UK.
FIGURE 1.
Indirect comparisons. CMF, cyclophosphamide, methotrexate and fluorouracil; E-CMF, epirubicin with cyclophosphamide, methotrexate and fluorouracil; FAC, fluorouracil, doxorubicin and cyclophosphamide; TAC, docetaxel with doxorubicin and cyclophosphamide.
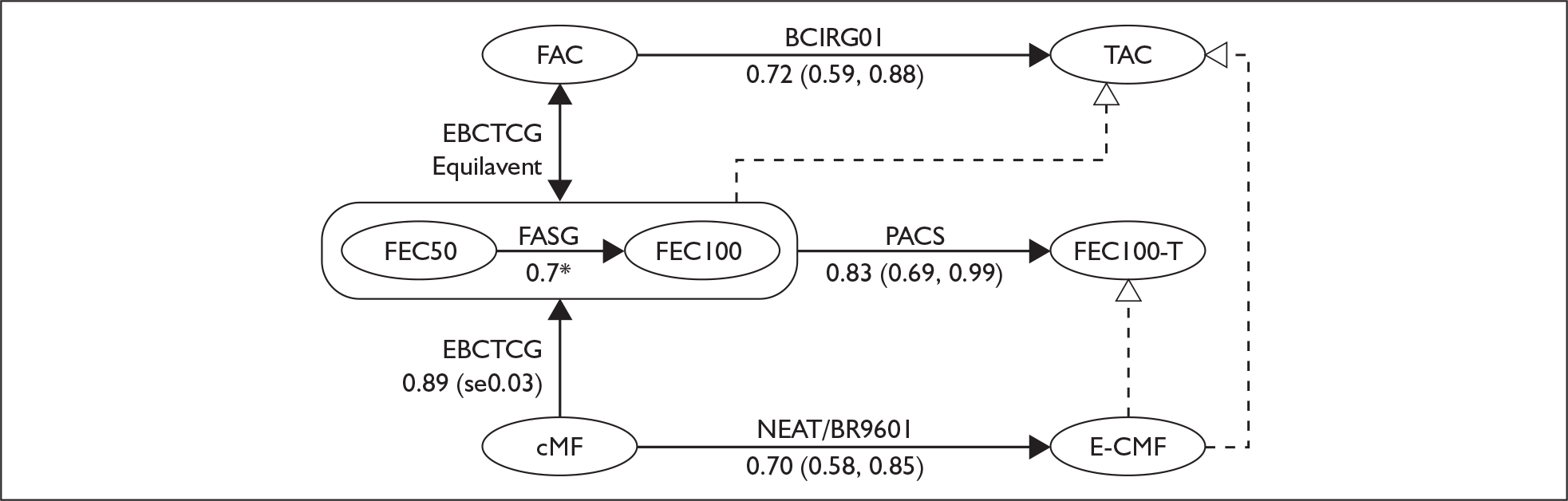
The relevance of the cost-effectiveness estimates put forward in the manufacturer’s submission depends on subjective judgments regarding the likely superiority of TAC over FEC75–100 or E-CMF (Table 3).
| ICER threshold (£/QALY) | % Responders in E-CMF arm | Average relative monthly hazard of relapse over 5 years |
|---|---|---|
| £10,000 | 87.9 | 0.75 |
| £20,000 | 92.3 | 0.84 |
| £30,000 | 93.8 | 0.88 |
| £40,000 | 94.7 | 0.90 |
| £50,000 | 95.2 | 0.91 |
| £60,000 | 95.5 | 0.92 |
| £70,000 | 95.8 | 0.93 |
| £100,000 | 96.2 | 0.95 |
| E-CMF indirect estimate | 0.92 |
Summary of NICE guidance issued as a result of the STA
The guidance issued by NICE in June 2006 states that:
Docetaxel, when given concurrently with doxorubicin and cyclophosphamide (the TAC regimen) as per its licensed indication, is recommended as an option for the adjuvant treatment of women with early node-positive breast cancer.
Acknowledgements
The authors would like to thank: Professor R Coleman, Head of Clinical Oncology, Cancer Research Centre, Weston Park Hospital, Sheffield; Dr J Brown, Lecturer in Clinical Oncology, Weston Park Hospital, Sheffield; Dr David Dodwell, Consultant Oncologist, Cookridge Hospital, Leeds; Dr Gregory Wilson, Consultant Oncologist, the Christie Hospital, Manchester; and Mr P Golightly, Director of Trent Medicines Information Service, Leicester, who all acted as clinical advisors in this project. The authors would also like to thank Gill Rooney, Project Administrator, for her help with formatting the document, setting up meetings and liaising with the clinical advisors.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Key references
- National institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.
- Chilcott J, Lloyd Jones M, Wilkinson A. Docetaxel for the Adjuvant Treatment of Early Node-Positive Breast Cancer: A Single Technology Appraisal 2006. www.nice.org.uk/Guidance/TA109.
- Cancer Research UK . UK Breast Cancer Incidence Statistics 2006. info.cancerresearchuk.org/cancerstats/types/breast/incidence/.
- Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005;352:2302-13.
- Roche H, Fumoleau P, Spielman M, Canon J-L, Delozier T, Kerbrat P, et al. 6 Cycles of FEC 100 Followed by 3 Cycles of Docetaxel for Node-Positive Breast Cancer Patients: Analysis at 5 Years of the Adjuvant PACS 01 Trial n.d.
- Goldstein L, O’Neill A, Sparano J, Perez E, Shulman L, Martino S, et al. E2197: phase III AT (doxorubicin/docetaxel) vs. AC (doxorubicin/cyclophosphamide) in the adjuvant treatment of node positive and high risk node negative breast cancer. Proc Am Soc Clin Oncol 2005.
- Martin M, Lluch A, Segui MA, Anton A, Ruiz A, Ramos M, et al. Prophylactic growth factor (GF) support with adjuvant docetaxel, doxorubicin, and cyclophosphamide (TAC) for node-negative breast cancer (BC): an interim safety analysis of the GEICAM 9805 study. Proc Am Soc Clin Oncol 2004.
- Jones SE, Savin MA, Holmes FA, O’Shaughnessy JA, Blum JL, Vukelja SJ, et al. Final Analysis: TC (docetaxel Cyclophosphamide, 4 Cycles) Has a Superior Disease-Free Survival Compared to Standard AC (doxorubicin Cyclophosphamide) in 1016 Women With Early Stage Breast Cancer n.d.
- Brain EG, Bachelot T, Serin D, Kirscher S, Graic Y, Eymard JC, et al. Life-threatening sepsis associated with adjuvant doxorubicin plus docetaxel for intermediate-risk breast cancer. JAMA 2005;293:2367-71.