Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 05/56/01. The assessment report began editorial review in July 2007 and was accepted for publication in December 2008. See the HTA programme website (www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of bortezomib for the treatment of multiple myeloma patients at first relapse and beyond, in accordance with the licensed indication, based upon the evidence submission from Ortho Biotech to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The outcomes stated in the manufacturer’s definition of the decision problem were time to disease progression, response rate, survival and quality of life. The literature searches for clinical and cost-effectiveness studies were adequate and the one randomised controlled trial (RCT) included was of reasonable quality. Results from the RCT suggest that bortezomib increases survival and time to disease progression compared with high-dose dexamethasone (HDD) in multiple myeloma patients who have had a relapse after one to three treatments. Cost-effectiveness analysis based on the same trial and an observational study was reasonable and gave an estimated cost per life-year gained of £30,750, which ranged from £27,957 to £36,747 on sensitivity analysis. An attempt was made to replicate the results of the manufacturer’s model and to compare the results to the Kaplan–Meier survival curve presented in the manufacturer’s submission. In addition, a one-way sensitivity analysis and a probabilistic sensitivity analysis were undertaken, as well as additional scenario analyses. Based on these analyses the ERG suggests that the cost-effectiveness results presented in the manufacturer’s submission may underestimate the cost per life-year gained for bortezomib therapy (versus high-dose dexamethasone) when potential UK practice and scenarios are considered. The guidance issued by NICE in June 2006 as a result of the STA states that bortezomib monotherapy for the treatment of relapsed multiple myeloma is clinically effective compared with HDD but has not been shown to be cost-effective and is not recommended for the treatment of progressive multiple myeloma in patients who have received at least one previous therapy and who have undergone, or are unsuitable for, bone marrow transplantation.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of bortezomib for the treatment of multiple myeloma patients.
Description of the underlying health problem
Multiple myeloma is a haematological cancer that progresses rapidly and is incurable. As well as reducing life expectancy it causes significant morbidity with painful symptoms including lytic bone lesions. These lead to pathological fractures of the long bones and vertebral collapse. Patients may also suffer renal failure, anaemia and neutropenia leading to infections. In the UK the median age at diagnosis is 65 years, with 1-year survival rates of approximately 60% and 5-year survival rates of approximately 25%. 2,3 Multiple myeloma is more common in men than women3,4 and the incidence rate among Afro-Caribbean populations is higher than for Caucasians of European descent. 5
Scope of the ERG report
The ERG critically evaluated the evidence submission from Ortho Biotech for the use of bortezomib monotherapy for the treatment of multiple myeloma patients at first relapse and beyond, in accordance with the licensed indication. 6 Bortezomib is a proteasome inhibitor and works by disrupting normal intracellular protein regulation, leading to programmed cell death (apoptosis).
Bortezomib was licensed for the treatment of people with relapsed and refractory multiple myeloma in 2004. The marketing authorisation was extended in April 2005 to allow use as a monotherapy for the treatment of progressive multiple myeloma in patients who have received at least one previous therapy (at first relapse) and who have already undergone (or who are unsuitable for) bone marrow transplantation.
The outcomes stated in the manufacturer’s definition of the decision problem were time to disease progression, response rate, survival and quality of life.
Methods
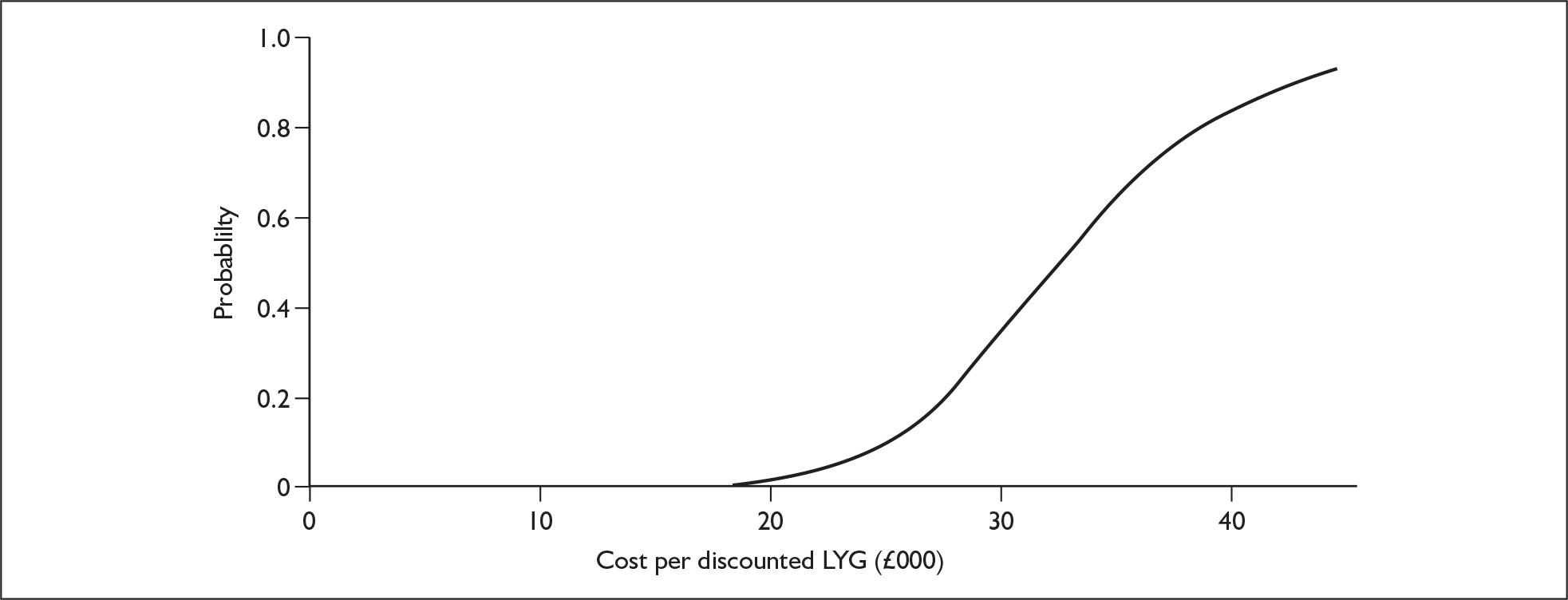
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process. The ERG checked the literature searches and applied the NICE critical appraisal checklist to the included studies. In addition, the ERG attempted to replicate the results of the manufacturer’s model and also compared the model’s results to the Kaplan–Meier survival curve presented in the manufacturer’s submission (Figure 1). A one-way sensitivity analysis and a probabilistic sensitivity analysis (Figure 2) were undertaken by the ERG, as well as additional scenario analyses.
FIGURE 1.
Patient survival for high-dose dexamethasone (HDD) and bortezomib for the APEX trial and model results.
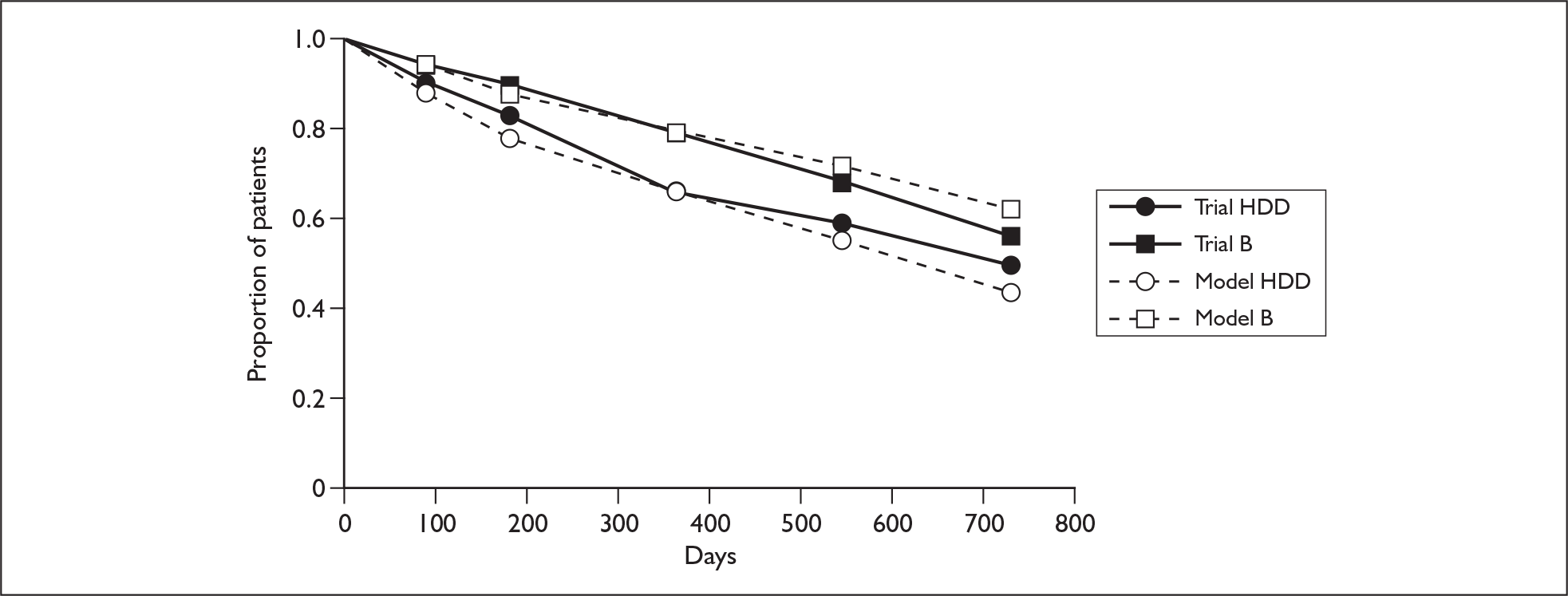
FIGURE 2.
Cost-effectiveness acceptability curve from the evidence review group probabilistic sensitivity analyses. LYG, life-years gained.
Results
Summary of submitted clinical evidence
The manufacturer based the submission on one randomised controlled trial (RCT) comparing bortezomib with high-dose dexamethasone (HDD) in multiple myeloma patients who have had a relapse after one to three treatments. Results of the RCT suggest that bortezomib increases survival and time to disease progression compared with high-dose dexamethasone in these patients.
Summary of submitted cost-effectiveness evidence
The manufacturer submitted a cost-effectiveness analysis that used a decision-analytic model (quasi-Markov) to estimate the treatment effect with bortezomib compared with high-dose dexamethasone. The model used clinical effectiveness data from the RCT supplemented with data from an observational study. Primary analysis presented an estimated cost per life-year gained of £30,750. Cost per life-year gained ranged from £27,957 to £36,747 from sensitivity analyses.
Commentary on the robustness of submitted evidence
The literature searches for clinical and cost-effectiveness studies were adequate and all available evidence was included. The RCT was of reasonable quality when assessed according to NICE internal validity criteria. However, the reporting of the trial lacked detail and clarity making interpretation of clinical effectiveness results difficult. Furthermore, the included RCT does not reflect current UK clinical practice, calling into question its external validity. However, the lack of standardisation in the clinical management of relapsed myeloma suggests that the impact of this on the generalisability of the economic model in terms of patient group and comparator may be minimal.
The manufacturer’s approach taken to model disease progression and cost-effectiveness in this patient group seemed reasonable. However, the manufacturer’s submission did not originally present quality of life issues in the economic model, although an additional analysis on cost per QALY was subsequently submitted.
The ERG considered that the economic model in the manufacturer’s submission may overestimate the treatment effect from the trial for a UK setting (Figure 1). Furthermore, sensitivity analyses undertaken in the economic evaluation were considered to be limited. Using what the ERG considered to be appropriate ranges for the one-way sensitivity analysis (Table 1), the most influential variables were the time to (disease) progression (TTP) hazard ratio and the cost of bortezomib. A sensitivity analysis was run in which each of the hazard ratios [TTP and overall survival (OS)] were varied in the same direction at the same time (low and high scenarios) and the cost-effectiveness ratios ranged from £23,287 to £46,814. A sensitivity analysis in which the cost of bortezomib was varied by ±50% gave a cost-effectiveness ratio ranging from £18,311 to £43,850.
| Variable | Base case | Inputs | Cost-effectiveness ratios | Range | ||
|---|---|---|---|---|---|---|
| Left | Right | Left | Right | |||
| Hazard ratio – TTP | 0.56 | 0.44 | 0.69 | £25,339 | £39,141 | £13,802 |
| Cost of bortezomib per course | £21,035 | £15,776 | £26,294 | £24,365 | £37,136 | £12,770 |
| Duration of treatment effect (years) | 3 | 4 | 2 | £27,957 | £36,747 | £8790 |
| Cost of other care – bortezomib preprogression | £470 | £352 | £588 | £28,266 | £33,892 | £5627 |
| Hazard ratio – OS (year 1) | 0.42 | 0.30 | 0.60 | £29,317 | £33,175 | £3858 |
| Cost of other care – pre- and postprogression | £470 | £352 | £588 | £29,682 | £32,476 | £2795 |
| Cost of HDD per course | £82 | £103 | £62 | £30,725 | £30,774 | £50 |
The ERG also ran an additional scenario analysis, which was a combination of the three scenarios run in the original submission (limiting the number of cycles of treatment from eight to three; assuming 40% of patients were treated at first relapse, with the remaining 60% at second relapse and beyond; and using a combination of bortezomib and HDD as treatment). The results of the ERG scenario are summarised in Table 2.
| Patient group | Cost per life-year gained |
|---|---|
| All patients treated at first relapse | £27,334 |
| 80% of patients treated at first, 20% at second relapse | £30,219 |
| 60% of patients treated at first, 30% at second, 10% at third relapse | £35,783 |
| 40% of patients treated at first, 40% at second, 20% at third relapse | £44,602 |
The ERG probabilistic sensitivity analysis used the 95% confidence intervals for the hazard ratios and has estimated a range of ±25% for the costs. A cost of £470 has been used for the ‘other care costs’. The baseline scenario is shown in Figure 2 with more appropriate ranges for the probabilistic sensitivity analysis. The results of the probabilistic sensitivity analysis show that the fifth percentile is £22,693 and the 95th percentile is £46,751 (cost per life-year gained). A probabilistic sensitivity analysis in which the cost of bortezomib varies by ±50% had a fifth percentile of £20,364 and 95th percentile of £49,876.
The ERG identified that adverse events had not been included in the manufacturer’s model, either in terms of loss of quality of life or increased resource use.
Conclusions
The ERG suggests that the cost-effectiveness results presented in the manufacturer’s submission may underestimate the cost per life-year gained for bortezomib therapy (versus HDD) when potential UK practice and scenarios are considered.
There is no standard treatment for relapsed multiple myeloma patients, which makes assessing the effectiveness and cost-effectiveness of new treatments problematic in terms of the individuality of treatment protocols and which comparators to use. It would be useful for future trials to reflect current practice but this may be difficult as it is a quickly developing area in which clinicians are eager to have new treatments options for patients who do not easily fit into stereotypical groups.
Summary of NICE guidance issued as a result of the STA
The following guidance was issued by NICE in October 2007:
-
1.1 Bortezomib monotherapy is recommended as an option for the treatment of progressive multiple myeloma in people who are at first relapse having received one prior therapy and who have undergone, or are unsuitable for, bone marrow transplantation, under the following circumstances:
-
– the response to bortezomib is measured using serum M protein after a maximum of four cycles of treatment, and treatment is continued only in people who have a complete or partial response (that is, reduction in serum M protein of 50% or more or, where serum M protein is not measurable, an appropriate alternative biochemical measure of response) and
-
– the manufacturer rebates the full cost of bortezomib for people who, after a maximum of four cycles of treatment, have less than a partial response (as defined above).
-
-
1.2 People currently receiving bortezomib monotherapy who do not meet the criteria in paragraph 1.1 should have the option to continue therapy until they and their clinicians consider it appropriate to stop.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process n.d. www.nice.org.uk/page.aspx?o=STAprocessguide (accessed 19 September 2006).
- Coleman MP, Rachet B, Woods LM, Mitry E, Riga M, Cooper N, et al. Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. Br J Cancer 2004;90:1367-73.
- Cancer Research UK . Multiple Myeloma Statistics for the UK n.d. http://info.cancerresearchuk.org/cancerstats/types/multiplemyeloma/?a=5441.
- Zappasodi P, Corso A, Klersy C, Pica G, Mangiacavalli S, Varettoni M, et al. Changes in multiple myeloma epidemiology in the last thirty years: a single centre experience. Eur J Cancer 2006;42:396-402.
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, et al. SEER Cancer Statistics Review, 1975–2002. Bethesda, MD: National Cancer Institute; n.d.
- Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al. Bortezomib for the Treatment of Multiple Myeloma Patients 2006.