Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 06/17/01. The assessment report began editorial review in July 2007 and was accepted for publication in December 2008. See the HTA programme website (www.hta.ac.uk) for further project information. This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical and cost-effectiveness of fludarabine phosphate or fludarabine plus cyclophosphamide for the first-line treatment of chronic lymphocytic leukaemia, based upon the evidence submission from Schering Health Care (SHC) to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The submission was of good quality with no major errors or omissions in the clinical evidence. Two published studies and seven abstracts were included in the company submission, which showed improvements in overall response and progression-free survival (PFS) and a higher complete response rate in the fludarabinecontaining arms; however, until the complete data are made available for evaluation these results must be interpreted with caution. The manufacturer’s decision-analytic Markov model to estimate the cost-effectiveness of treatment with fludarabine monotherapy, fludarabine plus cyclophosphamide and chlorambucil was considered to be the most relevant source for informing this STA; it was appropriate for the decision problem and the data sources used to inform the model were appropriate from a UK NHS perspective. The incremental cost-effectiveness ratio of fludarabine plus cyclophosphamide compared with chlorambucil from the revised model presented in the manufacturer’s addendum was £3244 per additional quality-adjusted life-year. The results were robust to a range of subgroup and sensitivity analyses. Additional sensitivity and survival analyses were carried by the ERG to investigate possible bias in the results. This brought into question the validity of the assumptions underpinning the extrapolation of data over a lifetime time horizon and showed that the ICER estimates submitted by the manufacturer were not calculated correctly and uncertainty surrounding the decision problems was not expressed fully. Based on these analyses the ERG suggests that further evidence is needed to enable an accurate assessment to be made of the clinical and cost-effectiveness of fludarabine as first-line treatment for chronic lymphocytic leukaemia. The guidance issued by NICE in December 2006 as a result of the STA states that fludarabine monotherapy, within its licensed indication, is not recommended for the first-line treatment of chronic lymphocytic leukaemia; no recommendations have been made with respect to fludarabine plus cyclophosphamide combination therapy because the current marketing authorisation does not specifically provide a recommendation that fludarabine should be used concurrently with other drugs for the treatment of chronic lymphocytic leukaemia.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of fludarabine phosphate for the first-line treatment of chronic lymphocytic leukaemia. 2
Description of the underlying health problem
Chronic lymphocytic leukaemia (CLL) is defined as a slow progressive form of leukaemia characterised by an increased number of lymphocytes,3 mostly of small or medium size, with clumped nuclear material (chromatin), an indistinct or absent nucleoli and little cytoplasm. 4 The other type of lymphocyte commonly observed in approximately 15% of patients is a prolymphocyte, which appears large with a prominent nucleolus. 4,5 The general symptoms of CLL are tiredness, night sweats, weight loss, anaemia and associated symptoms, and increased susceptibility to infection. 4 The lymphocytes may also accumulate in the lymph nodes and spleen resulting in lymphadenopathy, splenomegaly and other abdominal masses. 4,5 Frequently the condition is identified by chance during a routine blood test in the absence of specific symptoms or physical signs. At the point of diagnosis CLL is usually widespread and with some degree of bone marrow involvement. With the exception of blood and marrow transplantation, the condition is inherently incurable with treatment emphasis on maintaining an acceptable state of health and inducing remission when required. 5
B-cell CLL is reported to be the most common leukaemia, representing approximately 25% of all cases of leukaemia. 6 In England and Wales in 2003 there were 6198 cases of leukaemia;7,8 assuming that 25% of these are B-cell CLL means that there were approximately 1550 new cases of B-cell CLL diagnosed in 2003. This indicates a crude incidence in this population of approximately 3 per 100,000 population per year;7–9 however, this belies the demographics of its incidence. CLL is rare below the age of 30 years with 20–30% of patients presenting under the age of 55 years. 4 The peak incidence is between 60 and 80 years, with the incidence increasing up to almost 50 per 100,000 population per year after the age of 70 years. 6 It is male dominant, occurring with a male–female ratio of 2:1. 4,10
Scope of the ERG report
The report critically evaluates the evidence submission from Schering Health Care (SHC) on the clinical and cost-effectiveness of fludarabine phosphate (Fludara®) or fludarabine plus cyclophosphamide for the first-line treatment of chronic lymphocytic leukaemia.
Methods
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process.
The ERG undertook additional work to examine the potential robustness of the base-case results to several of the assumptions made in the manufacturer’s model and also to identify possible sources of bias. This work was performed on the revised model presented in the manufacturer’s addendum and was separated into three main areas: (1) additional one-way sensitivity analyses to examine the robustness of the base-case incremental cost-effectiveness ratio (ICER) to alternative assumptions related to the response rate for retreatment and the duration of this response; (2) a more appropriate presentation of the probabilistic sensitivity analysis results from the submission; and (3) formal survival analysis of the individual patient data from the CLL4 trial to explore the appropriateness of assuming constant transition probabilities to extrapolate over a lifetime time horizon. These were selective analyses and the revised economic analyses were undertaken to examine the robustness of the manufacturer’s own model to alternative assumptions. These analyses were thus subject to potential limitations regarding the structural assumptions, the general approach used to estimate transition probabilities and issues related to the modelling of second-line treatments. The results should, therefore, be taken as indicative of the potential impact on the cost-effectiveness estimates.
Results
Summary of submitted clinical evidence
Two published studies11,12 and seven abstracts were included in the company submission. Fludarabine or fludarabine plus cyclophosphamide were compared with chlorambucil (Chl) in five studies12–16 and two studies11,17 compared fludarabine with fludarabine plus cyclophosphamide. Only one study compared all three regimens. 13,18
All studies, with one exception,16 showed an improvement in overall response (OR) in those patients receiving fludarabine compared with those receiving Chl. 11–15,17 In all but one15 of the studies comparing fludarabine or fludarabine plus cyclophosphamide with Chl there was a higher complete response (CR) rate for the fludarabine-containing arms. 12–14,16 Although progression-free survival (PFS) was stated as a primary outcome measure in five studies,11–14,17 this outcome was fully reported in only three. 11,12,17 In one study comparing differences in median PFS between the fludarabine and Chl regimens there was a significantly longer duration of response in the fludarabine arm. 12 Two studies demonstrated a significantly longer duration of response with the fludarabine plus cyclophosphamide combination compared with fludarabine alone. 11,17 At present, the follow-up periods of the studies included in the submission are too short to demonstrate any significant improvement in overall survival (OS). Therefore, fully matured survival data are necessary to ascertain whether any improvement in PFS translates into an increase in OS. Three studies included quality of life (QoL) analyses; however, only limited data from the CLL4 study are presented. 18 In this study QoL was the same for each treatment group at baseline and at 12 months and correlated with the quality of response. It is anticipated that the results of further QoL analyses are likely to become available within the next year. Because five of the studies included in the submission are not fully published and report only preliminary results in abstract form there are insufficient data presented to fully assess the validity of these studies. 13–16 Although the unpublished CLL4 study13 is supplemented with additional patient-level data18 provided by the manufacturer to support the health economic analyses, these supplemental data are not in the public domain and therefore cannot be verified externally. Until these studies are fully published and the complete data made available for evaluation, these results must be interpreted with caution.
Summary of submitted cost-effectiveness evidence
Two papers were identified in both the manufacturer’s submission and the ERG searches that reported on the cost-effectiveness of fludarabine monotherapy compared with Chl in the management of CLL in previously untreated patients. 19,20 Neither of the studies was considered particularly relevant because of the limited clinical and economic evidence on which the studies were based (mainly because of the limited evidence available at the time that these studies were undertaken) and the restricted range of comparators considered. Neither of these studies considered the cost-effectiveness of fludarabine combined with cyclophosphamide as a first-line treatment for CLL. Consequently, the submission by the manufacturer was considered to comprise the most relevant evidence for the purposes of this STA.
The manufacturer’s submission included a de novo decision-analytic Markov model to estimate the cost-effectiveness of treatment with (1) fludarabine monotherapy, (2) fludarabine in combination with cyclophosphamide and (3) Chl. The model used individual patient data from the CLL4 trial to model transition probabilities related to first-line treatment with these therapies. The costs of first-line treatment were derived from an audit of UK patients from the CLL4 trial. The model was based on a lifetime time horizon and included the costs and consequences of further treatments required after first-line treatment had failed. Data on the costs and effects of further treatment (including retreatment and second-line and salvage therapies) were derived from a combination of secondary sources and assumptions by the manufacturer. Results were presented in terms of the incremental cost per quality-adjusted life-year (QALY) gained, with QoL estimates informed by a separate systematic review. In the original submission by the manufacturer, the incremental cost-effectiveness (ICER) of fludarabine in combination with cyclophosphamide compared with Chl was £2602 per additional QALY. Fludarabine in combination with cyclophosphamide was reported to dominate fludarabine (i.e. was less costly and more effective). These results were based on an approach which assumed that median (as opposed to mean) survival was equal in all treatments. An addendum was submitted by the manufacturer, which presented similar results based on an approach that equalised mean survival. This latter approach was considered by the ERG to be a more appropriate assumption. The results presented in the addendum increased the ICER of fludarabine in combination with cyclophosphamide compared with Chl to £3244 per additional QALY. Fludarabine plus cyclophosphamide continued to dominate fludarabine. The results of the subgroup analysis presented by age and Binet stage did not substantially alter these results. Similarly, the results were reported to be robust to a wide range of sensitivity analyses undertaken by the manufacturer. The results were most sensitive to the time horizon of the model, such that fludarabine plus cyclophosphamide did not appear cost-effective at a time horizon of 5 years.
Commentary on the robustness of submitted evidence
Strengths
The ERG felt that the SHC submission was generally of good quality. There were no major errors or omissions in the clinical evidence. The majority of the data quoted within the submission was a fair and accurate representation of the original reference data. The ERG noted the limitations of existing cost-effectiveness studies in this area and considered the economic model submitted by the manufacturer to be the most relevant source for the purpose of informing this STA. The economic model structure (including the comparators) was considered appropriate for the decision problem, and the data sources used to inform the model were deemed appropriate from a UK NHS perspective. A range of subgroups was considered and uncertainty in parameter estimates was addressed using probabilistic approaches.
Weaknesses
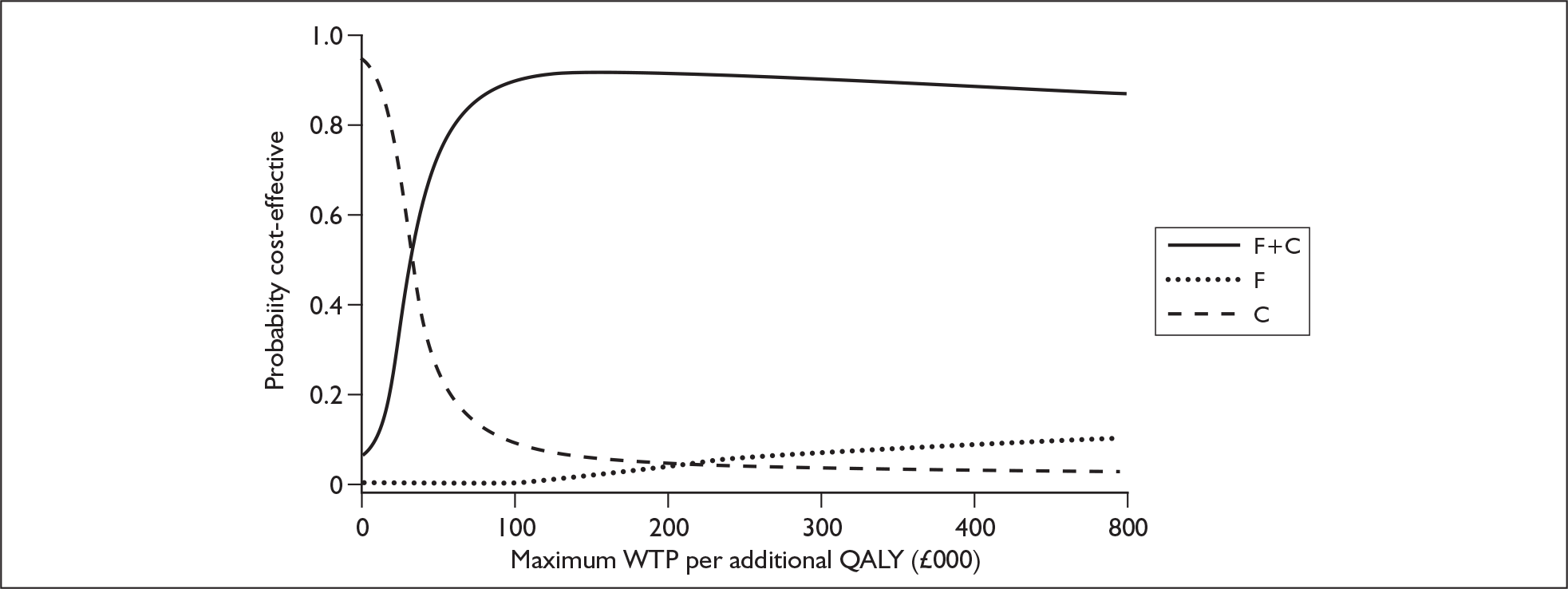
The majority of the reference data presented in the submission was not fully published and was only available in abstract form. Therefore, the ERG felt that, until these studies are fully published and the complete data made available for evaluation, these results must be interpreted with caution. The ERG identified a number of potential sources of weakness in the manufacturer’s economic submission. In particular, a number of issues were identified that may have introduced possible bias into the results. Most of these issues appeared to act in favour of the fludarabine plus cyclophosphamide regimen such that it is likely that the manufacturer’s results are overly optimistic towards this regimen. The robustness of the manufacturer’s results to some of these issues was explored in additional work undertaken by the ERG. The cost-effectiveness of fludarabine in combination with cyclophosphamide appeared relatively robust to wide variation in several of the key assumptions made by the manufacturer. The ERG was concerned with the approach that the manufacturer used to estimate a number of key probabilities derived from the CLL4 trial data. Because of the structure of the model it was not possible to fully explore the potential robustness of the manufacturer’s results to alternative assumptions. However, work undertaken by the ERG brought into question the validity of the assumptions underpinning the extrapolation of data over a lifetime time horizon. In addition, the ERG noted that the ICER estimates submitted by the manufacturer were not calculated correctly and uncertainty surrounding the decision problems was not expressed fully. The revised ICER results are presented in Table 1, with the associated cost-effectiveness acceptability curves given in Figure 1.
| Comparator | Mean costs | Mean QALYs | ICER (compared with Chl) | Probability cost-effective at willingness to pay | ||
|---|---|---|---|---|---|---|
| £20,000 | £30,000 | £40,000 | ||||
| Chi | £11,836 | 5.48 | – | 0.047 | 0.032 | 0.028 |
| F | £17,840 | 5.70 | Dominated by FC | 0.04 | 0.067 | 0.09 |
| FC | £13,291 | 6.13 | £3213 | 0.913 | 0.901 | 0.882 |
FIGURE 1.
Cost-effectiveness acceptability curves – revised by the ERG. QALY, quality-adjusted life-year; WTP, willingness to pay.
Areas of uncertainty
The fludarabine summary of product characteristics (SPC) does not mention the use of fludarabine in combination with other chemotherapeutic agents. The dose for oral therapy in combination with cyclophosphamide does not appear to be a licensed dose and is not mentioned in the SPC. The SPC for cyclophosphamide states that it is frequently used in combination chemotherapy regimens involving other cytotoxic drugs and that it is recommended that the calculated dose be reduced at the discretion of the clinician when it is given in combination with other antineoplastic agents or radiotherapy and in patients with bone marrow suppression. However, the ERG feels that the efficacy of the fludarabine plus cyclophosphamide regimen is still under investigation and that the recommendations outlined in the British Committee for Standards in Haematology (BCSH) guidelines are expected to be revised following the outcomes of the CLL4 study. Therefore, the ERG sought clarification on this matter from the manufacturer. The manufacturer believes that the proposed regimen falls within the current licenses and states that they are not, therefore, considering an extension to the fludarabine license. The dosing 11 regimen for the fludarabine plus cyclophosphamide combination was agreed by expert clinicians within the MRC/LRF UK-CLL group. However, independent expert advice given to the ERG confirms that the fludarabine plus cyclophosphamide regimen is increasingly used for the first-line treatment of CLL and that the dosing regimen chosen also reflects current practice.
Conclusions
To enable an accurate assessment to be made of the clinical and cost-effectiveness of fludarabine as first-line treatment for chronic lymphocytic leukaemia there is a need for further evidence to clarify areas of uncertainty.
Summary of NICE guidance issued as a result of the STA
The guidance issued by NICE in December 2006 states that:
Fludarabine monotherapy, within its licensed indication, is not recommended for the first-line treatment of chronic lymphocytic leukaemia. No recommendations have been made with respect to fludarabine plus cyclophosphamide combination therapy because the current marketing authorisation does not specifically provide a recommendation that fludarabine should be used concurrently with other drugs for the treatment of chronic lymphocytic leukaemia.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.
- Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al. Fludarabine Phosphate for the First-Line Treatment of Chronic Lymphocytic Leukaemia 2006.
- Centre for Cancer Education, University of Newcastle upon Tyne . CancerWEB Online Medical Dictionary n.d. http://cancerweb.ncl.uk/cgi-bin/omd (accessed 24 May 2006).
- Oscier D, Fegan C, Hillmen P, Illidge T, Johnson S, Maguire P, et al. Guidelines on the diagnosis and management of chronic lymphocytic leukaemia. Br J Haematol 2004;125:294-317.
- Warell DA, Cox TM, Firth JD. Oxford textbook of medicine. Oxford: Oxford university Press; 2003.
- Hallek M, Stahel RA, Greil R. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of chronic lymphocytic leukemia. Ann Oncol 2005;16:i50-1.
- Welsh Cancer Intelligence and Surveillance Unit . Cancer Incidence 2003 2005.
- Office for National Statistics . Cancer Registrations in England 2003 2005.
- Office for National Statistics . Components of Population Change (constituent Countries of the United Kingdom) 2005.
- Shanafelt TD, Call TG. Current approach to diagnosis and management of chronic lymphocytic leukemia. Mayo Clin Proc 2004;79:388-98.
- Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecker C, et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006;107:885-91.
- Rai K, Peterson B, Appelbaum F, Kolitz J, Elias L, Shepherd L, et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 2000;343:1750-7.
- Catovsky D, Richrads S, Hillmen P. Early results from LRF CLL4: a UK multicentre randomised trial. ASH Annual Meeting Abstracts. Blood 2005;106.
- Eichhorst BF, Strömberg M, Jönsson V, Gill D, Hammerström J, Wallivik J, et al. Comparison of the efficacy and toxicity of fludarabine (F) in firstline therapy of younger versus elderly patients (Pts) with advanced chronic lymphocytic leukemia (CLL): results of a meta-analysis of two phase III trials of the German CLL study group (GCLLSG). ASH Annual Meeting Abstracts. Blood 2005;106.
- Karlsson K, Busch R, Wendtner CN, Hallek M, . Cladribine (CdA) or fludarabine (F) or highdose intermittent chlorambucil (Chl) as first-line treatment of symptomatic chronic lymphocytic leukemia? First interim analysis of data from the international randomized phase III trial. ASH Annual Meeting Abstracts. Blood 2004;104.
- Spriano M, Chiurazzi F, Liso V, Mazza P, Molica S, Gobbi M, et al. Multicentre prospective randomized trial of fludarabine versus chlorambucil and prednisone in previously untreated patients with active B-cell CLL: final report. Haematol Cell Ther 2000;42.
- Flinn W, Kumm E, Grever MR, Neuberg D, Dewald GW, Bennet JM, et al. Fludarabine and cyclophosphamide produces a higher complete response rate and more durable remissions than fludarabine in patients with previously untreated CLL: Intergroup Trial E2997. ASH Annual Meeting Abstracts. Blood 2004;104.
- Schering Health Care Ltd . Fludarabine Phosphate for the First-Line Treatment of Chronic Lymphcytic Leukaemia 2006.
- Best L. Fludarabine in the treatment of chronic lymphocytic leukaemia. Bristol: South and West Regional Health Authority; 1995.
- Hancock S, Wake B, Hyde C. Fludarabine as first line therapy for chronic lymphocytic leukaemia. Birmingham: West Midlands Health Technology Assessment Collaboration; 2002.