Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 06/51/01. The assessment report began editorial review in October 2008 and was accepted for publication in March 2009. See the HTA programme web site for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group report into the clinical effectiveness and cost-effectiveness of alteplase for the treatment of acute ischaemic stroke, in accordance with the licensed indication, based upon the evidence submission from the manufacturer to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The submitted clinical evidence included several randomised controlled trials indicating that, in highly selected patients, alteplase administered at a licensed dose within 3 hours of the onset of acute ischaemic stroke is associated with a statistically significant reduction in the risk of death or dependency at 3 months compared with placebo, despite a significantly increased risk of symptomatic intracranial haemorrhage within the first 7–10 days. Data from the National Institute of Neurological Disorders and Stroke (NINDS) trial suggest that the benefit of treatment is sustained at 6 and 12 months. However, data from observational studies suggest that few patients with acute ischaemic stroke will be eligible for alteplase therapy under the terms of the current licensing agreement. In particular, many patients will be excluded by virtue of their age, and many more by the restriction of therapy to patients in whom treatment can be initiated within 3 hours of symptom onset. The manufacturer’s submission included a state transition model evaluating the impact of treatment with alteplase within 3 hours of onset of stroke symptoms compared to standard treatment reporting that, in the base-case analysis, alteplase was both less costly and more effective than standard treatment. This increased to a maximum of approximately £4000 upon one-way sensitivity analysis of the parameters. The probabilistic sensitivity analysis presented within the submission suggests that the probability that alteplase has a cost-effectiveness ratio greater than £20,000 per quality-adjusted life-year (QALY) gained is close to 1 (0.99). The results of the short-term model demonstrate that alteplase is cost-effective over a 12-month period, with an incremental cost-effectiveness ratio of £14,026 per QALY gained. This increased to a maximum of £50,000 upon one-way sensitivity analysis of the parameters. At 12 months, the probabilistic sensitivity analysis presented within the submission suggests that the probability that alteplase has a cost-effectiveness ratio greater than £20,000 per QALY gained is approximately 0.7. The guidance issued by NICE in April 2007 as a result of the STA states that alteplase is recommended for the treatment of acute ischaemic stroke only when used by physicians trained and experienced in the management of acute stroke and in centres with the required facilities.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG); an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of alteplase for the treatment of acute ischaemic stroke. 2
Description of the underlying health problem
‘Stroke’ is a term used to refer to the clinical syndrome that results from the interruption of the blood supply to an area of the brain. Approximately 85% of all strokes occur when the blood supply to the brain is blocked, either by a blood clot or by narrowing of the blood vessels: such strokes are termed ischaemic strokes. 3 Most other strokes occur when a blood vessel in or around the brain ruptures: these are termed haemorrhagic strokes. 3
In England, stroke is one of the top three causes of death. 3 It is also the leading cause of adult disability;4 at least 300,000 people in England live with moderate to severe disabilities as a result of stroke. 3
Alteplase is an enzyme that causes blood clots to dissolve. It is therefore of potential value in ischaemic stroke because it may enable the restoration of the blood supply to the affected area of the brain. However, it is also associated with a risk of intracerebral haemorrhage. Moreover, because it dissolves blood clots, its use in haemorrhagic stroke is potentially fatal or disabling. Alteplase is not licensed for use in patients older than 80 years.
Scope of the evidence review group report
The principal research question relates to the clinical effectiveness and cost-effectiveness of alteplase for the treatment of acute ischaemic stroke. The manufacturer’s scope restricts the intervention to intravenous alteplase given to adults with ischaemic stroke within 3 hours of symptom onset, in a secondary care setting, under the guidance of experienced stroke and neuro-imaging specialists, and after prior exclusion of intracranial haemorrhage. The scope restricts the comparator to placebo or standard medical and supportive management without thrombolysis. This is because no thrombolytic treatment other than alteplase is licensed in the UK for use in acute ischaemic stroke, and other stroke treatment or prevention therapies that function in different ways would not be relevant comparators.
The single most clinically relevant and important outcome measure is the proportion of patients suffering death or dependency (reported as a score of 3–6 inclusive on the modified Rankin scale). This captures in one measure alteplase’s impact on both the proportion of patients making a good functional recovery and the proportion suffering asymptomatic intracranial haemorrhage (SICH), an outcome associated with death or increased disability. Other relevant outcomes include survival; neurological deficit; mental health (including anxiety and depression); adverse effects of treatment (including bleeding events); and health-related quality of life. Economic outcomes include cost per quality-adjusted life-year (QALY) gained.
Methods
The ERG report comprised a critical review of the evidence for the clinical evidence and cost-effectiveness of the technology based upon the manufacturer’s submission to NICE as part of the STA process. In addition, in an attempt to ensure that no relevant randomised controlled trials were overlooked, the ERG reran in MEDLINE both the manufacturer’s search strategy and the search strategy previously used in the Cochrane review of thrombolysis for acute stroke. 5 This established that, while the manufacturer’s MEDLINE search strategy identified the key publication relating to each of the included trials, it did not identify the important reanalysis of the National Institute of Neurological Disorders and Stroke (NINDS) study,6 two supplementary analyses that the submission identified as relevant,7,8 or the Cochrane review5 on which the submission drew heavily.
The manufacturer’s submission also drew on evidence from a number of observational studies. It is not clear how these were identified. The submission implied that the same search strategies were used to identify both randomised controlled trials and studies investigating or evaluating service delivery or provision of technology. However, as the manufacturer’s EMBASE and MEDLINE search strategies both contained a term limiting the search to clinical trials, neither would have reliably identified observational studies. Supplementary data provided by the manufacturer stated that a systematic search was undertaken for observational studies, but did not provide a relevant search strategy and, within the time available, the ERG was not able to conduct supplementary searches to ensure that relevant observational studies were not missed. The manufacturer’s exclusion criteria arbitrarily excluded observational studies that were small (< 100 patients) or added nothing to the conclusions that could be drawn from the larger studies. No indication was given as to the number of studies that were excluded for these reasons. Inclusion of those studies that were excluded because they did not contain a new message would have enabled estimation of the strength of evidence for the messages contained in the included studies.
The manufacturer did not undertake independent meta-analyses, but referred to those undertaken for the Cochrane review (which were calculated as odds ratios using the Peto fixed-effects method),5 and the pooled analysis of the Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke (ATLANTIS) A and B, European
Cooperative Acute Stroke Study (ECASS) II, and NINDS 1 and 2 trials8 (which again used the odds ratio). The ERG therefore carried out meta-analyses to explore the effects of excluding a study (ECASS I) that used an unlicensed dose of alteplase and of presenting the results as relative risks, as required by NICE, rather than as Peto odds ratios.
The ERG had concerns about some of the methods used by the manufacturer in the cost-effectiveness modelling. This included the use of odds ratios in the model instead of relative risks, and the length of the model cycle time. The manufacturers were asked to justify the use of these methods and were requested to perform additional analyses using methods considered by the ERG to be more appropriate. In all cases the manufacturers complied with these requests. The additional analyses showed no meaningful differences in either the direction or the magnitude of the results compared with the original work.
Results
Summary of submitted clinical evidence
Evidence from randomised controlled trials indicates that, in highly selected patients, alteplase administered at a licensed dose within 3 hours of the onset of acute ischaemic stroke is associated with a statistically significant reduction in the risk of death or dependency at 3 months compared with placebo [relative risk (RR) 0.82, 95% confidence interval (CI) 0.72 to 0.93, absolute risk reduction 11%; Figure 1], despite a significantly increased risk of SICH within the first 7–10 days [RR 4.24, 95% confidence interval (CI) 1.52 to 11.83, absolute risk increase 6%]. Data from the NINDS trial, the only study which presented data relating to a time point later than 3 months from stroke onset, suggest that the benefit of treatment is sustained at 6 and 12 months.
FIGURE 1.
All patients treated within 3 hours: death or dependency at 3 months. ATLANTIS, Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke; CI, confidence interval; ECASS, European Cooperative Acute Stroke Study; NINDS, National Institute of Neurological Disorders and Stroke; RR, relative risk.
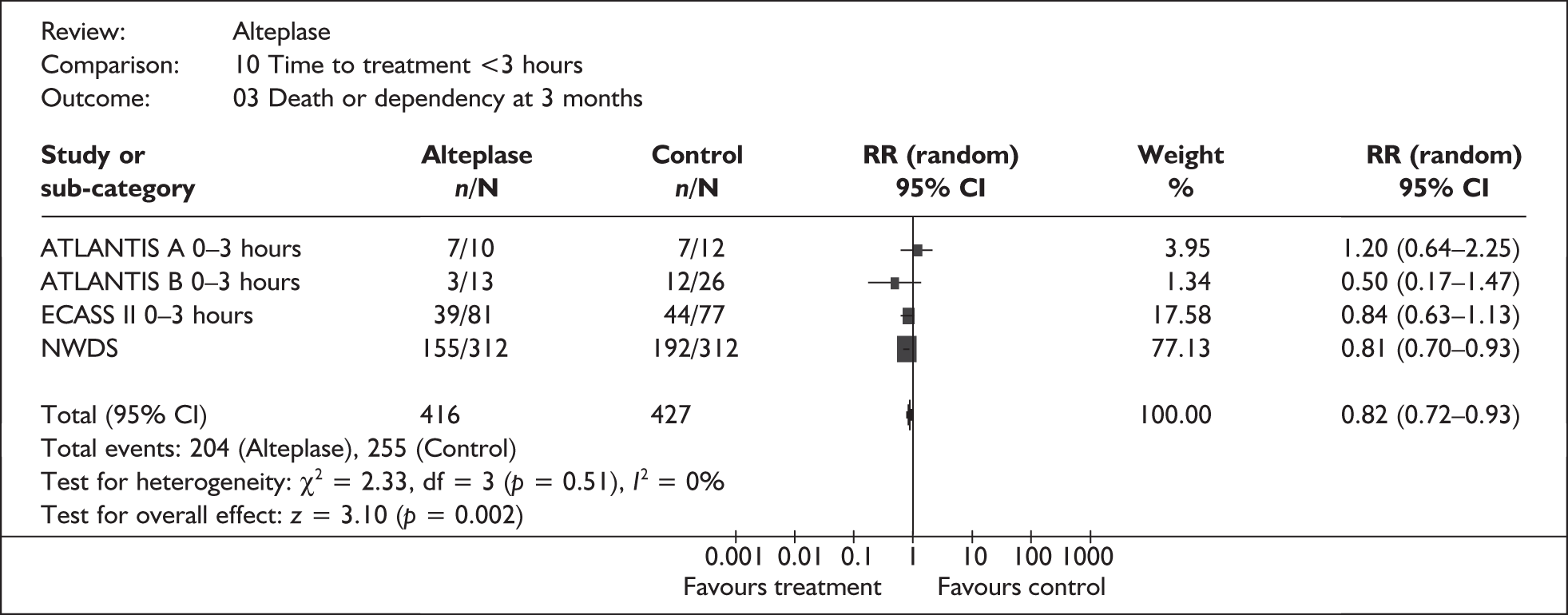
However, data from observational studies suggest that few patients with acute ischaemic stroke will be eligible for alteplase therapy under the terms of the current licensing agreement. In particular, many patients will be excluded by virtue of their age, and many more by the restriction of therapy to patients in whom treatment can be initiated within 3 hours of symptom onset. In principle, it may be possible to increase the proportion of patients who both reach hospital and are assessed for alteplase therapy within 3 hours, but to do so would require substantial investment in public education, and possibly also service reconfiguration. Moreover, the risk of major protocol violations in the administration of alteplase should be noted. In two comprehensive independent community-based studies, the Cleveland9 and Connecticut10 studies (of which only the former was cited in the manufacturer’s submission), such violations, most of which appeared to have been accidental,10 affected 67% of patients receiving alteplase in Connecticut and 50% in the Cleveland area.
Summary of submitted cost-effectiveness evidence
A state transition model was used to evaluate the impact of treatment with alteplase within 3 hours of onset of stroke symptoms compared to standard treatment. The time horizon for this long-term model was 40 years. In addition, a short-term (12-month follow-up) model is included. The model is based on work published as part of the Health Technology Appraisal (HTA) of thrombolytic therapy by Sandercock et al. 11
The main data source for the model is a Cochrane review meta-analysis of the NINDS,12 ECASS I,13 ECASS II,14 ATLANTIS A,15 ATLANTIS B16 and Haley et al. 17 studies. Outcomes from this meta-analysis are extrapolated over a time horizon of 40 years in order to assess the long-term benefits and costs of alteplase. The model takes into account the increased rate of haemorrhage seen in alteplase-treated patients.
The health states used within the model and the costs and utilities associated with each health state are considered to be appropriate for the required analysis.
The Boehringer Ingelheim model estimated that, in the base-case analysis, alteplase was both less costly and more effective than standard treatment. This increased to a maximum of approximately £4000 upon one-way sensitivity analysis of the parameters.
The probabilistic sensitivity analysis presented within the submission suggests that the probability that alteplase has a cost-effectiveness ratio greater than £20,000 per QALY gained is close to 1 (0.99).
The results of the short-term model demonstrate that alteplase is cost-effective over a 12-month period, with an incremental cost-effectiveness ratio (ICER) of £14,026 per QALY gained. This increased to a maximum of £50,000 upon one-way sensitivity analysis of the parameters.
At 12 months, the probabilistic sensitivity analysis presented within the submission suggests that the probability that alteplase has a cost-effectiveness ratio greater than £20,000 per QALY gained is approximately 0.7.
Commentary on the robustness of submitted evidence
The evidence for the clinical effectiveness of alteplase when used within the 3-hour licensed window for the treatment of acute ischaemic stroke is not robust and, as noted in a recent Cochrane review,5 should be treated with extreme caution. It is based on a total of only 416 patients who received the current licensed dose of alteplase within the 3-hour time window (see Figure 1). Moreover, 312 of these patients were enrolled in one trial, the NINDS trial, in which a substantial imbalance in baseline stroke severity, a key prognostic factor, favoured alteplase. 11 An additional analysis undertaken by the Cochrane reviewers suggested that the imbalance probably caused the effect of alteplase on death and dependency to be overestimated by around 3%. 5 However, a subsequent independent analysis of the NINDS data considered that there was no evidence that the imbalance in the distribution of baseline NIHSS (National Institute for Health Stroke Scale) scores had either a statistically or a clinically significant effect on the trial results. 6 The randomised trials were not stratified by any potential prognostic factor other than time to treatment, and therefore any post hoc analyses designed to explore the extent to which different groups might benefit from therapy can only be regarded as hypothesis generating. Nonetheless, it is interesting to note that a pooled analysis of data from the ATLANTIS A and B, ECASS II, and NINDS trials18 appeared to indicate that alteplase therapy was of significant benefit in women, but not in men (Table 1).
| Alteplase | Placebo | p-value (alteplase vs placebo) | |
|---|---|---|---|
| Men | 38.5% | 36.7% | 0.52 |
| Women | 40.5% | 30.3% | < 0.001 |
| p-value (men vs women) | 0.50 | 0.03 |
The model structure is appropriate and allows sensitivity analysis to be carried out easily. Given a 40-year time horizon, one-way sensitivity analysis suggests that variations in the majority of the parameters do not have a large effect upon the ICER. Alteplase dominates (i.e. costs less and is more effective than) standard treatment; potential parameter variations are unlikely to increase the ICER beyond the currently accepted threshold values. 19
The results at 12 months, when the full lifetime costs associated with disability due to stroke and the QALY gain associated with increased survival are not captured, indicate that alteplase is still cost-effective. No weaknesses in the model structurewere identified that would alter the results significantly. However, the model rests on evidence for the clinical effectiveness of alteplase administered with 3 hours of symptom onset which, as noted above, is not robust. Moreover, although the risks and benefits of alteplase are unknown beyond 12 months, the manufacturer’s health economic model has used a lifetime horizon of 40 years. In addition, the economic evaluation relies heavily on the results of the NINDS trial in which, as noted above, a substantial imbalance in baseline stroke severity favoured alteplase. Thus, the results of the cost-effectiveness analysis should be treated with extreme caution.
One important issue which is not explicitly taken into account in the economic modelling is the possible impact of trying to increase the number of patients who could be treated within the 3-hour window. This could have a significant cost impact to the NHS in terms of both the need to educate the public on the importance of early treatment and potential substantial service reconfiguration.
Conclusions
The evidence from randomised controlled trials suggests that, in highly selected patients, alteplase administered within 3 hours of the onset of acute ischaemic stroke is associated with a statistically significant reduction in the risk of death or dependency at 3 months compared with placebo, despite the statistically significant increase in the risk of early SICH. However, this evidence should be treated with extreme caution as it is based on a total of only 416 patients who received the current licensed dose of alteplase, and 312 of these patients were included in a trial in which a substantial imbalance in baseline stroke severity, a key prognostic factor, favoured alteplase.
Observational studies suggest that few patients with ischaemic stroke will be eligible for alteplase therapy under the terms of the current licensing agreement. In particular, many patients will be excluded because they are older than 80 years, and many more will be excluded because treatment cannot be initiated within 3 hours of symptom onset. Any increase in the number of patients in whom treatment can be initiated within 3 hours is likely to require substantial efforts in terms of public education and service reconfiguration.
The critical appraisal of the Boehringer Ingelheim model undertaken by the ERG suggests that alteplase can result in long-term cost savings and is more effective than standard treatment.
In the short-term, when the full lifetime costs associated with disability due to stroke and the QALY gain associated with increased survival are not captured, alteplase was still shown to be cost-effective compared to standard treatment.
Summary of NICE guidance issued as a result of the STA
At the time of writing, the final appraisal determination document issued by NICE in April 2007 states that:
Alteplase is recommended for the treatment of acute ischaemic stroke when used by physicians trained and experienced in the management of acute stroke. It should only be administered in centres with facilities that enable it to be used in full accordance with its marketing authorisation.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Institute for Health and Clinical Excellence (NICE) . Guide to the Single Technology (STA) Process. 2008. www.nice.org.uk/media/8DE/74/ (accessed 25 August 2008).
- Lloyd Jones M, Holmes M. Alteplase for the Treatment of Acute Ischaemic Stroke: A Single Technology Appraisal 2007.
- National Audit Office . Reducing Brain Damage: Faster Access to Better Stroke Care 2005.
- Bath PMW, Lees KR. ABC of arterial and venous disease. Acute stroke. BMJ 2000;320:920-3.
- Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2003.
- Ingall TJ, O’Fallon WM, Asplund K, Goldfrank LR, Hertzberg VS, Louis TA, et al. Findings from the reanalysis of the NINDS tissue plasminogen activator for acute ischemic stroke treatment trial. Stroke 2004;35:2418-24.
- Broderick JP, Lu M, Kothari R, Levine SR, Lyden PD, Haley EC, et al. Finding the most powerful measures of the effectiveness of tissue plasminogen activator in the NINDS tPA stroke trial. Stroke 2000;31:2335-41.
- Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768-74.
- Katzan IL, Furlan AJ, Lloyd LE, Frank JI, Harper DL, Hinchey JA, et al. Use of tissue-type plasminogen activator for acute ischemic stroke: the Cleveland area experience. JAMA 2000;283:1151-8.
- Bravata DM, Kim N, Concato J, Krumholz HM, Brass LM. Thrombolysis for acute stroke in routine clinical practice. Arch Intern Med 2002;162:1994-2001.
- Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al. A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS. Health Technol Assess 2002;6.
- The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581-7.
- Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995;274:1017-25.
- Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998;352:1245-51.
- Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Thrombolytic therapy in acute ischemic stroke study investigators. Stroke 2000;31:811-16.
- Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999;282:2019-26.
- Haley EC, Brott TG, Sheppard GL, Barsan W, Broderick J, Marler JR, et al. Pilot randomized trial of tissue plasminogen activator in acute ischemic stroke. The TPA Bridging Study Group. Stroke 1993;24:1000-4.
- Kent DM, Price LL, Ringleb P, Hill MD, Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke 2005;36:62-5.
- National Institute for Health and Clinical Excellence . Guide to Methods of Technology Appraisal 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 30 September 2008).