Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 07/14/01. The assessment report began editorial review in October 2008 and was accepted for publication in May 2009. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Declared competing interests of authors
Mark Sculpher has been a consultant to Sanofi-Aventis and Roche, but in areas unrelated to obesity and/or the products covered in this single technology appraisal. The remaining authors of this report have no conflicts of interest.
Permissions
Copyright statement
© 2009 Queen’s Printer and Controller of HMSO. This monograph may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2009 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical and cost-effectiveness of rimonabant for the treatment of obese or overweight patients based upon a review of the manufacturer’s submission to the National Centre for Health and Clinical Excellence (NICE) as part of the single technology appraisal (STA) process. The submission’s main evidence came from four randomised controlled trials. Rimonabant resulted in a significantly greater benefit than placebo for all primary weight loss outcomes. At 1 year, rimonabant had a statistically significant beneficial effect on systolic blood pressure, high-density lipoprotein cholesterol, triglycerides and fasting plasma glucose in diabetics and non-diabetics, and glycosylated haemoglobin in diabetics. Improvements were maintained over 2 years with rimonabant; withdrawal of rimonabant at 1 year resulted in a reduction in weight loss until there was no difference from placebo at 2 years. Psychiatric adverse events were experienced by 26% and 14% of rimonabant and placebo patients respectively; figures for symptoms of depression were 9% and 5% respectively. Pairwise comparisons of orlistat, sibutramine and rimonabant showed beneficial effects of rimonabant over orlistat and sibutramine for weight loss outcomes; however, response hurdles imposed on orlistat or sibutramine in clinical practice may not have been applied in the orlistat and sibutramine trials. The manufacturer’s Markov cohort model evaluated rimonabant versus orlistat, sibutramine and diet and exercise alone for three base-case populations. The incremental cost-effectiveness ratio (ICER) of rimonabant varied from £10,534–£13,236 per quality-adjusted life-year (QALY) versus diet and exercise, to £8977–£12,138 per QALY versus orlistat, to £1463–£3908 per QALY versus sibutramine. In subgroup analysis there was a wider variation in the ICER estimates although none exceeded £20,000 per QALY. The ICER of rimonabant remained under £20,000 per QALY in reanalyses by the manufacturer and the ERG, with the results sensitive to the source of health-related quality of life (HRQoL) benefits in the model. Four treatment strategies were modelled in comparisons of rimonabant versus diet and exercise alone and orlistat and sibutramine in which rimonabant was continued only in patients achieving 5% weight loss at 3, 6, 9 or 12 months. In pairwise comparisons rimonabant remained below a threshold of £30,000 per QALY in 70% of the comparisons reported. The results were most sensitive to the decrement applied to depression and the costs of screening for depression. In conclusion, areas of uncertainty remain in relation to the clinical effectiveness and cost-effectiveness of rimonabant, for example lack of evidence on long-term outcomes and the effect of rimonabant on cardiovascular events, developing diabetes and mortality, and lack of data on the HRQoL benefits associated with rimonabant. The lack of response hurdles applied to sibutramine and orlistat means that the comparator strategies were not considered by the ERG to reflect their respective product licenses or current NHS use. The NICE guidance issued as a result of the STA states that rimonabant is recommended as an adjunct to diet and exercise for adults who are obese or overweight and who have had an inadequate response to, are intolerant of or are contraindicated to orlistat and sibutramine.
Introduction
The National Institute for Health Research Health Technology Assessment (NIHR HTA) programme supports the National Institute for Health and Clinical Excellence (NICE) by funding independent academic input to NICE technology appraisal activities. NICE is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, for which most of the relevant evidence lies with one manufacturer or sponsor (Sanofi-Aventis). 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology to NICE. In addition, a report reviewing the evidence submission is submitted by the evidence review group (ERG), an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA of rimonabant for the treatment of overweight and obese patients,2 which was submitted on 5 October 2007 to NICE, with a subsequent submission of a commentary on 24 January 2008.
In October 2008, the European Medicines Agency (EMEA), based on new evidence that became available from postmarketing surveillance studies following the NICE appraisal of rimonabant, concluded that the balance of risks and benefits no longer supported the use of rimonabant and the drug was withdrawn from use. It should therefore be noted that this report is based only on evidence available to NICE at the time of its appraisal of rimonabant and does not include any further evidence that informed the EMEA’s decision on withdrawal.
Description of the underlying health problem
Obesity is a chronic condition which is associated with a number of conditions such as type 2 diabetes that have a significant impact on morbidity and quality of life and reduce life expectancy. There are currently several options for the treatment of overweight and obese patients, including lifestyle changes, drug treatments and bariatric surgery. According to NICE guidelines, the initial treatments of choice for overweight and obese patients are multicomponent interventions that include behavioural change strategies to promote physical activity and improve eating habits.
Three drugs are currently used in practice to treat obesity: orlistat (Xenical®, Roche), sibutramine (Reductil®, Abbott) and rimonabant (Accomplia®). Orlistat is a specific and long-acting inhibitor of the enzyme lipase, which results in the inability to hydrolyse dietary fat in the form of triglycerides into absorbable free fatty acids and monoglycerides, therefore preventing fat absorption. The net price per 84-cap pack is £33.58, with an approximate annual cost of £438. Sibutramine produces secondary and primary amine metabolites that inhibit noradrenaline, serotonin and dopamine reuptake, which in turn suppresses appetite by producing a feeling of satiety. The net price per 28-cap pack of 10 mg is £36.90. The net price per 28-cap pack of 15 mg is £43.65. The approximate annual cost is £481 for 10 mg and £569 for 15 mg. Rimonabant is a selective CB1 cannabinoid receptor antagonist and acts by decreasing appetite. The net price per 28-tab pack is £44.00, with an approximate annual cost of £574.
The manufacturer’s submission to NICE stated that, since the introduction of rimonabant until the end of June 2007, approximately 32,500 patients have been prescribed rimonabant in England and Wales, accounting for 16.4% of prescription initiations for obesity treatments during that period. Patients with comorbidities accounted for a large majority of rimonabant prescriptions.
Concerns have been raised relating to the licensing of rimonabant, both in the UK and in the USA. In January 2007, the Scottish Medicines Consortium decided that the economic case for prescribing rimonabant had not been demonstrated and therefore did not recommend its use within NHS Scotland as an adjunct to diet and exercise for the treatment of obese or overweight patients. The US Food and Drugs Administration (FDA) also did not recommend a license for rimonabant in the USA because of the risk of psychiatric adverse events, particularly the incidence of suicidality and suicidal ideation. The safety profile of rimonabant was reviewed by the EMEA and its use in patients with ongoing major depressive illness and/or ongoing antidepressive treatment is now precluded.
Scope of the ERG report
The ERG report presented a critical evaluation of the manufacturer’s submission (Sanofi-Aventis), which evaluated the evidence for the clinical effectiveness, safety, tolerability and cost-effectiveness of rimonabant in its licensed indication as an adjunct to diet and exercise, relative to other licensed antiobesity drugs (orlistat and sibutramine) and diet and exercise alone.
Methods
The ERG report comprised a critical review of the evidence for the clinical effectiveness and cost-effectiveness of the technology based upon the manufacturer’s/sponsor’s submission to NICE as part of the STA process. In addition, the ERG:
-
Generated tables from data provided in the body of the original and clarification submissions, and the appendices of the original submission, in order to present a clear summary of the relative and absolute weight effects of rimonabant at 1 year.
-
Repeated the meta-analyses for the primary weight loss outcomes [except for body mass index (BMI) as insufficient data were provided] including all four RIO trials (Rimonabant In Obesity).
-
Compared the results for orlistat and sibutramine included in the submission with those presented in the NICE guidelines3 because of concerns about how representative of the general literature the trials of orlistat and sibutramine included in the submission were.
-
Conducted additional analyses to provide further insight into the potential impact on the cost-effectiveness estimates of key issues and uncertainties identified during the structured critique of the manufacturer’s submission.
-
Conducted additional analyses to clarify the relative importance of the independent effect of BMI on utilities compared with the impact of the other risk factors on cardiovascular disease (CVD) and diabetes event rates in the incremental cost-effectiveness ratio (ICER) estimates.
Results
Summary of submitted clinical evidence
Effectiveness of rimonabant
The evaluation of the efficacy of rimonabant focused primarily on the results of four Sanofi-Aventis-sponsored randomised control trials (RCTs): (RIO-Europe4, RIO-North America5, RIO-Diabetes6 and RIO-Lipids7). Two further trials were cited but did not contribute to the main meta-analyses [SERENADE (Study Evaluating Rimonabant Efficacy in drug-NAive DiabEtic patients) and REBA (Riminobant Eating Behaviour Assessment study)]. Data from two unpublished studies were used to inform the analysis of adverse effects (EFC5745 and ACT3801).
Rimonabant resulted in a significantly greater benefit than placebo in terms of all primary weight loss outcomes:
-
change in weight (kg): non-diabetics: weighted mean difference (WMD) –4.91 [95% confidence interval (CI) –5.35 to –4.48]; diabetics: WMD–3.90 (95% CI –4.57 to –3.23)
-
proportion of patients losing 5% body weight: non-diabetics: relative risk (RR) 2.61 (95% CI 2.32 to 2.95); diabetics: RR 3.41 (95% CI 2.58 to 4.50)
-
proportion of patients losing 10% body weight: non-diabetics: RR 3.48 (95% CI 2.84 to 4.27); diabetics: RR 8.07 (95% CI 3.37 to 17.46)
-
change in waist circumference (cm): nondiabetics: WMD –4.01 (95% CI –4.50 to –3.53); diabetics: WMD –3.30 (95% CI –4.17 to –2.43)
-
BMI (kg/m2): non-diabetics: WMD –1.76 (95% CI –1.92 to –1.60); diabetics: WMD –3.90 (95% CI –4.57 to –3.23); for any baseline BMI, the average weight loss beyond that which can be achieved with diet and exercise over a 1-year period is around 5 kg, with a fall in BMI of 1.7 kg/m2.
The ERG generated pooled estimates using data from all four of the RIO trials. The results for the change in weight and the proportion who achieved 5% weight loss are shown in Figures 1 and 2 respectively. These analyses show that the a priori decision by the manufacturer to pool data for diabetics and non-diabetics separately was justified statistically as well as clinically. However, although the mean weight loss and placebosubtracted reduction in BMI in the RIO-Diabetes trial were slightly lower than in the other RIO trials, the other primary outcomes did not indicate any materially different treatment effect in this population.
FIGURE 1.
Meta-analyses for change in weight (kg) from baseline to 1 year (intention to treat data) (ERG generated). CI, confidence interval; SD, standard deviation; WMD, weighted mean difference.
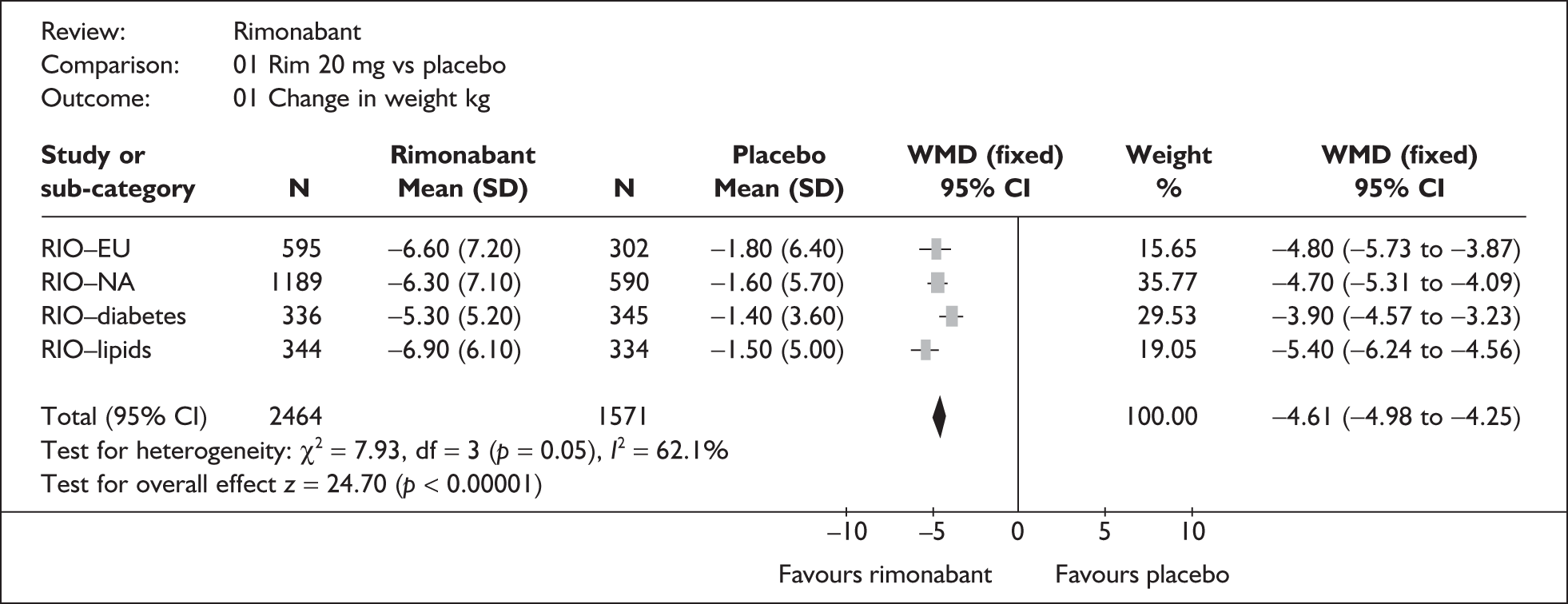
FIGURE 2.
Proportion of patients achieving 5% weight loss. CI, confidence interval; RR, relative risk.
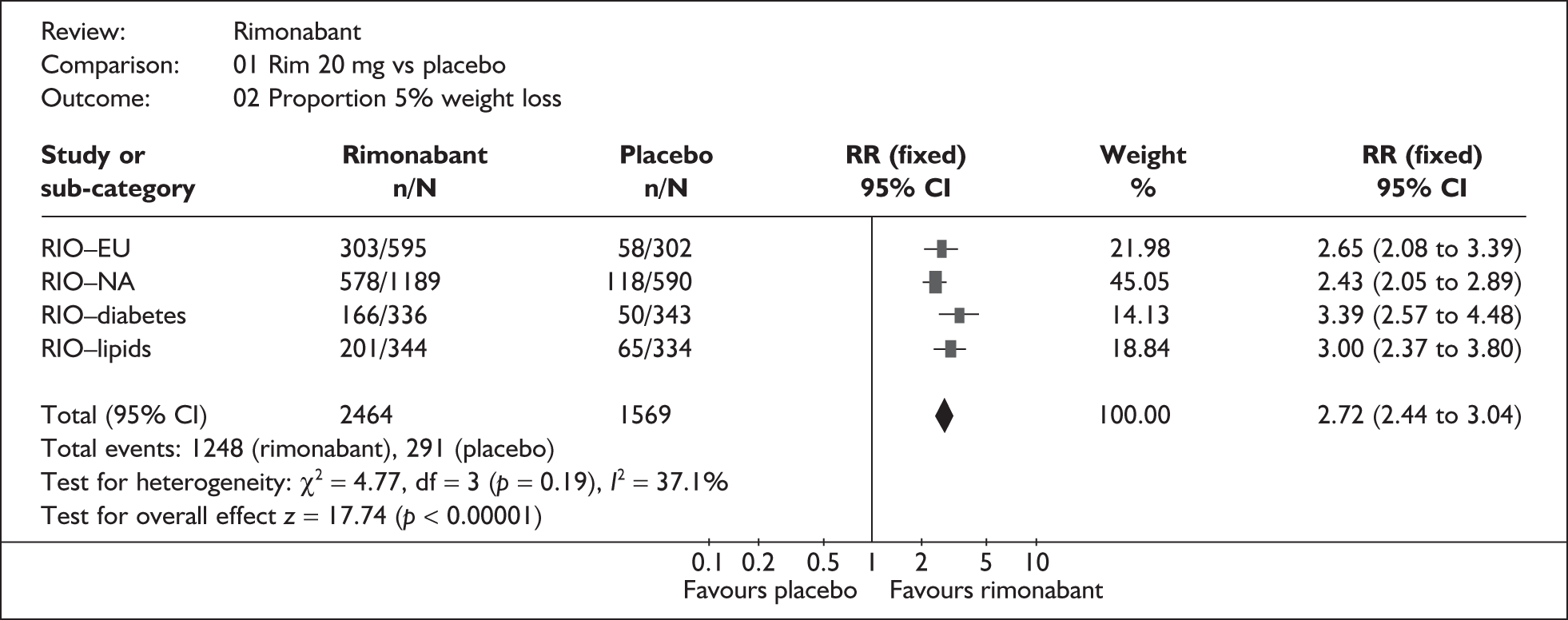
Two of the RIO trials (RIO-North America, RIOLipids) reported significantly greater reductions in body weight in patients achieving at least 5% weight loss with rimonabant than with placebo. None of the trials reported significantly greater reductions in body weight in patients achieving at least 10% weight loss with rimonabant, or in waist circumference in patients achieving at least 5% or 10% weight loss with rimonabant compared with placebo.
At 1 year, rimonabant had a statistically significant beneficial effect on systolic blood pressure, high-density lipoprotein cholesterol, triglycerides and fasting plasma glucose in both diabetic and non-diabetic patients, and glycosylated haemoglobin
(HbA1c) in diabetic patients. Weight loss and improvements in associated cardiovascular and diabetes risk factors were maintained over 2 years when rimonabant was continued; however, the relative benefit over placebo was lower in year 2. Following withdrawal of rimonabant treatment at 1 year, there was a gradual reduction in the rate of weight loss until there was no difference from placebo at 2 years.
In total, 13 adverse events were identified by the manufacturer as being associated with rimonabant at a rate of ≥ 2%, and at a rate of ≥ 1% greater than placebo (Table 1). Some form of psychiatric adverse event was experienced by 26% of patients receiving 20 mg rimonabant across the four RIO trials, compared with 14% of patients receiving placebo. Symptoms of depression were reported in 9% of patients taking 20 mg rimonabant compared with 5% of patients taking placebo. These rates were broken down further, with the most commonly reported psychiatric adverse events as stated in the FDA briefing shown in Table 2. 8
| Year 1 | ||
|---|---|---|
| Rimonabant (n = 2742) | Placebo (n = 2474) | |
| Any event | 86.3 | 81.4 |
| Nausea | 13.6 | 4.7 |
| Diarrhoea | 7.7 | 5.8 |
| Vomiting | 4.7 | 2.3 |
| Dizziness | 7.3 | 4.1 |
| Anxiety | 5.9 | 2.1 |
| Insomnia | 5.8 | 3.4 |
| Mood alterations with depressive symptoms | 4.7 | 2.8 |
| Depressive disorders | 3.9 | 1.7 |
| Influenza | 10.3 | 9.1 |
| Asthenia/fatigue | 6.1 | 4.4 |
| Gastroenteritis | 4.5 | 3.5 |
| Contusion | 3.1 | 1.1 |
| Hot flush | 2 | 0.8 |
| 20 mg rimonabant | Placebo | |
|---|---|---|
| Any psychiatric adverse event | 569 (26.2) | 226 (14.1) |
| Anxiety | 131 (6.02) | 40 (2.50) |
| Insomnia | 118 (5.42) | 53 (3.31) |
| Depressed mood | 83 (3.81) | 45 (2.81) |
| Depression | 74 (3.40) | 23 (1.44) |
| Irritability | 1.93% | 0.56% |
| Stress | 38 (1.75) | 28 (1.75) |
| Nervousness | 31 (1.42) | 5 (0.31) |
| Depressive symptoms | 23 (1.06) | 12 (0.75) |
| Sleep disorder | 21 (0.97) | 7 (0.44) |
| Nightmare | 21 (0.97) | 3 (0.19) |
Two separate instruments were used to evaluate the effect of rimonabant on health-related quality of life (HRQoL). One was the obesity-specific Impact of Weight on Quality of Life-Lite (IWQOL-Lite) and the other the generic Medical Outcomes Study Short Form-36 (SF-36). Rimonabant provided benefits in some areas of HRQoL, particularly physical functioning, but was associated with a significant deterioration in mental health.
On request, the manufacturer provided analyses of responder and non-responder data for 3, 6, 9 and 12 months. These analyses were based on patients with complete weight measurements for months 3, 6 and 9. Data at 12 months were based on an intention-to-treat (ITT) analysis using last observation carried forward (LOCF). For rimonabant-treated patients, responders lost more weight than non-responders. Comparison of the 12-month response data based on the LOCF and completer analysis indicates the use of the completer analysis is likely to result in higher response rates than the LOCF approach (e.g. 49.3% using LOCF compared with 64.4% using a completer analysis for one of the two populations considered, 49.4% versus 56% for the other).
In addition, the manufacturer provided an assessment of the diagnostic value of predicting a 1-year response at earlier time points by calculating the sensitivity and specificity of these time points. At 3 months sensitivity was 0.57 and specificity 0.89; sensitivity increased at 6 and 9 months (0.85 and 0.91 respectively) and specificity remained high (0.80 and 0.81 respectively).
Comparison of rimonabant with orlistat and sibutramine
In the absence of head-to-head trials, the manufacturer provided tabulated comparisons between the placebo-subtracted results for orlistat, sibutramine and rimonabant. On request, pairwise comparisons between rimonabant and sibutramine and orlistat were provided for the primary outcomes. These pairwise comparisons showed a significant increase in the number of patients achieving 5% weight loss with rimonabant compared with sibutramine in the non-diabetic population. In addition, rimonabant compared favourably with orlistat in terms of body weight (non-diabetics, diabetics and dyslipidaemics); waist circumference (non-diabetics and dyslipidaemics); change in BMI (non-diabetics); patients who achieved 5% weight loss (non-diabetics and diabetics); and patients who achieved 10% weight loss (non-diabetics and diabetics). There was no comparison of adverse events or HRQoL between rimonabant and orlistat or sibutramine.
Summary of submitted cost-effectiveness evidence
Only one previously published study reporting on the cost-effectiveness of rimonabant was identified. This study evaluated the cost-effectiveness of rimonabant compared with diet and exercise alone. No published studies were identified that had compared rimonabant with other licensed antiobesity drugs.
The manufacturer’s submission was based on a de novo economic evaluation of rimonabant compared with orlistat, sibutramine and diet and exercise alone. Separate models were presented based on a Markov cohort model and a patient-level approach using discrete event simulation. The main submission focused on the Markov cohort model.
The Markov model evaluated the following treatment comparisons: (1) lifetime rimonabant plus diet and exercise versus lifetime diet and exercise alone; (2) lifetime rimonabant plus diet and exercise versus lifetime orlistat plus diet and exercise; and (3) 1-year rimonabant plus diet and exercise versus 1-year sibutramine plus diet and exercise. The results of the economic evaluation were presented for three base-case populations: (1) overweight or obese patients with treated type 2 diabetes (diabetic group); (2) overweight or obese patients with dyslipidaemia, not treated with a statin and without type 2 diabetes (dyslipidaemic group); and (3) obese patients with or without comorbidities (obese group). A number of additional subgroups were considered as part of the sensitivity analysis.
In the absence of direct head-to-head RCT data for the alternative strategies, indirect approaches were employed to assess the relative effectiveness of each treatment strategy in terms of its impact on a number of established risk factors for CVD and diabetes. A series of published risk equations was used to translate changes in these risk factors to a reduced risk of CVD and, in patients without diabetes, to a reduced risk of developing diabetes. The effect of the treatments on BMI was also assumed independently to influence HRQoL beyond that attributed to the effect on CVD and diabetes risks. These approaches were used as the basis for estimating quality-adjusted life-years (QALYs) over a lifetime time horizon. Costs were based on the drug acquisition and monitoring costs, adverse events and the costs of CVD and diabetes. Costs and QALYs were compared and ICERs of rimonabant estimated when appropriate. The robustness of the results was assessed using deterministic and probabilistic sensitivity analyses.
Across the base-case populations, the ICER of rimonabant varied from £10,534 to £13,236 per QALY versus diet and exercise, from £8977 to £12,138 per QALY versus orlistat and from £1463 to £3908 per QALY versus sibutramine. In the additional subgroups considered there was a wider variation in the ICER estimates; however, none of the individual pairwise ICERs for rimonabant exceeded £20,000 per QALY in any of the subgroups. The ICER estimates across the majority of the sensitivity analyses were broadly consistent with the base-case results.
The ERG considered that the original submission contained a number of important uncertainties and issues which potentially compromised the validity of the model results. A number of these issues were addressed by the manufacturer as part of their response to the ERG’s points for clarification. The ERG identified a number of remaining issues related to the manufacturer’s response and several of these were subsequently addressed with additional analyses conducted by the ERG. The ICER of rimonabant remained relatively robust throughout the reanalyses by the manufacturer and the ERG (< £20,000 per QALY), although the results did appear to be sensitive to the source of HRQoL benefits assumed in the model, with markedly less favourable ICER estimates using data from the RIO trials. However, the ERG considered that several important caveats and uncertainties remained.
On request, the manufacturer provided comparisons of rimonabant versus diet and exercise alone and orlistat and sibutramine; in each analysis, four treatment strategies were modelled in which treatment with rimonabant was continued only in patients achieving 5% weight loss at 3, 6, 9 or 12 months. Further modifications to the model included: discontinuation of treatment when a patient returned to their original weight while on treatment; a disutility for depressive adverse events associated with rimonabant; inclusion of costs of screening/monitoring for depression for patients treated with rimonabant; and long-term deterioration of efficacy of all treatments after 1 year.
Compared with diet and exercise alone, the response hurdles for rimonabant of between 6 and 9 months were demonstrated to be more cost-effective than a response hurdle of 3 months in the analyses of overweight or obese patients with diabetes and obese patients with or without risk factors. When compared with orlistat and sibutramine, the ICER of rimonabant employing a 6-month response hurdle was £30,743 per QALY compared with a 3-month response hurdle for sibutramine for overweight or obese patients with diabetes, and £23,644 per QALY for obese patients with or without risk factors.
Pairwise comparisons were presented, showing the upper and lower ICERs for rimonabant versus the three comparators. Rimonabant was reported to remain below a threshold of £30,000 per QALY in 70% of the pairwise comparisons reported. The results appeared most sensitive to the decrement applied to depression and the costs of screening for depression.
Commentary on the robustness of submitted evidence
Strengths
The manufacturer’s submission presented a clear overview of the four major trials (RIO trials4–7) conducted with rimonabant in overweight or obese patients with data for up to 2 years. The submission also included a comparison with the appropriate comparators orlistat and sibutramine.
The manufacturer used appropriate criteria to assess the quality of the RIO trials, although the ERG noted some discrepancies between the assessments provided in the submission and the information available in published trial reports. The ERG assumes that the manufacturer had access to the full trial reports.
The manufacturer’s submission was considered to comprise the most relevant source of cost-effectiveness evidence relating to the use of rimonabant. The ERG identified a number of strengths in the manufacturer’s cost-effectiveness analysis. The overall model structure, approaches to estimating long-term costs and outcomes (expressed using QALYs), the time horizon employed and the approach to handling parameter uncertainty were all consistent with the NICE reference case for cost-effectiveness analysis. The ERG also noted that the manufacturer had compared rimonabant against other licensed antiobesity drugs as well as against diet and exercise alone. A broad range of sensitivity analyses was also undertaken to explore alternative assumptions. Variation in the cost-effectiveness estimates for rimonabant was considered in a number of different patient subgroups. The ERG also felt that the validation approaches employed by the company (including presenting the results of a separate discrete event simulation) were a relative strength of the submission. Finally, the ERG felt that the manufacturer had attempted to address a number of areas of uncertainty identified by the ERG in their response to the points for clarification.
In general, the ERG felt that the revised submission provided by the manufacturer had adequately addressed the main clarification points raised. The ERG noted that several of the assumptions employed by the manufacturer to address these points were conservative towards rimonabant; however, a more limited range of subgroups was considered in the resubmission – data on overweight and obese patients with risk factors other than diabetes were omitted.
Weaknesses
The four included trials may not be generalisable to the UK population, both in terms of baseline BMI and the differences in lifestyle, diet and attitudes towards alcohol consumption and exercise between the UK and the USA and other European countries. Furthermore, the diabetic patients included in the manufacturer’s submission did not include insulin-dependant diabetics and so may not be generalisable to the broader diabetic population.
The comparison of the effects of rimonabant with those of orlistat and sibutramine on weight loss outcomes is uncertain given the differences in diet and exercise that might have been employed across the different trials. There was no comparison of 2-year data between rimonabant and orlistat. There are differences in the licensing of rimonabant compared with that of orlistat and sibutramine; orlistat and sibutramine are subject to response ‘hurdles’ in practice that may not be applied in trials and therefore any additional benefit of rimonabant over orlistat or sibutramine may be overestimated and may not be apparent in normal clinical practice.
Overall, the ERG found the presentation of the data unclear, particularly that for orlistat and sibutramine. The ERG has concerns over how representative of the general literature the trials of orlistat and sibutramine in the submission are, and how objectively the data have been used.
The ERG identified a number of potential weaknesses in the manufacturer’s cost-effectiveness analysis. The most significant was considered to be the lack of response hurdles applied to sibutramine and orlistat, such that the comparator strategies were not considered by the ERG to reflect their respective product licenses or current NHS use. Although this issue was partially addressed by the manufacturer in the response to the ERG points for clarification, the ERG did not consider that this aspect had been robustly considered by the manufacturer and hence it represents a major limitation. The revised submission by the manufacturer addressed this issue further. However, there remained potential inconsistency in the approaches used to estimate the response rates for the alternative time points representing continuation hurdles for rimonabant; at 3, 6, and 9 months completer data were used and at 12 months LOCF was used. Although the ERG recognises that the manufacturer presented a more consistent approach as part of their clarification, the ERG considers that the full ITT LOCF would represent a more conservative approach and that the current analyses may overstate the response rates at 3, 6 and 9 months. In addition, there was a lack of conditional response data for sibutramine and orlistat (the change in individual risk factors for responders and non-responders) resulting in the use of different approaches to estimate the cost-effectiveness of rimonabant versus diet and exercise alone (patient-level data from the RIO trials) and versus orlistat and sibutramine [applying the average change in risk factors reported for the active treatments (regardless of response status) to responders, and the average change in risk factors for diet and exercise to non-responders].
The ERG also considered the manufacturer’s approach to evaluating HRQoL benefits to be subject to a number of important uncertainties. The ERG considered that the manufacturer’s reliance on external utility estimates, as opposed to the HRQoL data reported in the RIO trials, was a potential weakness. Indeed, the HRQoL benefits associated with rimonabant remain highly uncertain and need more detailed investigation by the manufacturer.
Conclusions
Key issues
The adequacy of the cost-effectiveness modelling and assumptions regarding strategies utilising response hurdles for rimonabant and comparator treatments is a key concern. Also, the use of external evidence on the HRQoL impact of BMI independent of longer-term clinical events rather than estimates from the trials, and the choice of this external evidence, are key issues.
The lack of evidence on the effect of rimonabant on ‘hard’ end points, such as CVD, diabetes and mortality, is a major limitation. Data are also lacking on the effectiveness and safety of rimonabant beyond 2 years. In addition, the appropriateness of incorporating the link between BMI reductions and a lower risk of diabetes and CVD and the choice of evidence to inform this link are questionable.
There are concerns over the psychiatric morbidity associated with rimonabant and, given the lack of long-term data, the cumulative data on less common side-effects are uncertain. The generalisability to the UK overweight and obese population is uncertain, particularly in the broader diabetic population as there are no data on the effectiveness or safety of rimonabant in insulindependant diabetics.
Areas of uncertainty
Areas of uncertainty remain in relation to the clinical effectiveness and safety of rimonabant. A major area where data are lacking relates to the long-term outcomes, with no effectiveness or safety data presented for rimonabant beyond 2 years and limited data available beyond 1 year. Also, the manufacturer has identified no direct evidence for the effect of rimonabant on hard clinical end points, such as cardiovascular events, developing diabetes and mortality. The manufacturer states that results from an ongoing trial, CRESCENDO (Comprehensive Rimonabant Evaluation Study of Cardiovascular Endpoints and Outcomes), which is evaluating the effect of rimonabant on cardiovascular morbidity and mortality, are expected to be available in 2011.
Given the lack of head-to-head comparisons between rimonabant and orlistat or sibutramine with all three drugs given as per license, it is unclear whether the pairwise comparisons between rimonabant and orlistat and sibutramine, presented in the clarification submission, will reflect that seen in clinical practice; response hurdles imposed on orlistat or sibutramine in clinical practice may not have been applied in the orlistat and sibutramine trials.
With respect to cost-effectiveness, a number of issues and uncertainties were addressed by the manufacturer in their response to the ERG’s points for clarification. Some remaining issues relating to the manufacturer’s response were subsequently addressed with additional analyses conducted by the ERG and a revised submission by the manufacturer. However, some caveats and uncertainties remain with respect to the modelling of the comparator technologies and the HRQoL benefits associated with rimonabant.
Summary of NICE guidance issued as a result of the STA
The guidance issued by NICE in March 2008 states that:
Rimonabant, within its licensed indications, is recommended as an adjunct to diet and exercise for adults who are obese or overweight and who have had an inadequate response to, are intolerant of or are contraindicated to orlistat and sibutramine.
Rimonabant treatment should be continued beyond 6 months only if the person has lost at least 5% of their initial body weight since starting rimonabant treatment.
Rimonabant treatment should be discontinued if a person returns to their original weight while on rimonabant treatment.
Rimonabant treatment should not be continued for longer than 2 years without a formal clinical assessment and discussion of the individual risks and benefits with the person receiving treatment.
Disclaimers
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology (STA) Process 2006. www.nice.org.uk/page.aspx?o=STAprocessguide.
- McKenna C, Palmer S, Burch J, Norman G, Glanville J, Sculpher M, et al. Rimonabant for the treatment of overweight and obese patients. NICE; 2007.
- National Institute for Health and Clinical Excellence . CG43 Obesity: Full Guideline 2006. www.nice.org.uk/CG043fullguideline (accessed 26 September 2007).
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. RIO-Europe Study Group . Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 2005;365:1389-97.
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. RIO-North America Study Group . Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 2006;295:761-75.
- Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 2006;368:1660-72.
- Despres JP, Golay A, Sjöström L. Rimonabant in Obesity-Lipids Study Group . Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005;353:2121-34.
- Food and Drug Administration . Rimonabant Briefing Document n.d. www.fda.gov/ohrms/dockets/AC/07/briefing/2007–4306b1-fda-backgrounder.pdf (accessed 27 September 2007).