Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 08/48/01. The contractual start date was in January 2009. The draft report began editorial review in August 2009 and was accepted for publication in November 2009. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
None
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Myocardial infarction
A myocardial infarction (MI) occurs when an area of the myocardium is exposed to prolonged ischaemia, usually as a result of a failure of the blood supply from one or more coronary arteries; the affected myocardium necroses and heals leaving a non-contractile scar. Depending upon the size of the infarcted area, heart function can be impaired leading to varying degrees of contractile function known as ‘systolic dysfunction’. 1 For many years, the commonest criteria used to diagnose MI were those of the World Health Organization (WHO), which required the presence of any two of the following three criteria: ischaemic symptoms, electrocardiographic changes and elevated creatine kinase-MB (CK-MB) level. 1 In 2000, the American College of Cardiology and the European Society of Cardiology redefined MI. 1 The new definition combined typical changes in biochemical markers, preferably the cardiac-specific troponins T or I (which show a rise and gradual fall), or CK-MB (which shows a more rapid rise and fall), with ischaemic symptoms, electrocardiographic (ECG) changes and/or coronary intervention. 1,2 Applying the new criteria to patients presenting with suspected cardiac chest pain leads to a 26% increase in the number of events diagnosed as MI (11% increase in the number of patients diagnosed with MI); approximately 66% of the additional MI would previously have been diagnosed as unstable angina. 3 This increase in the frequency of diagnosis of MI gives an indication of the impact of changes in diagnostic criteria on clinical practice.
Postmyocardial infarction heart failure
A large MI stimulates adaptations in cardiac structure and function (usually those of the left ventricle) known as ventricular remodelling in an attempt to compensate for reduced overall cardiac performance. Ventricular remodelling occurs rapidly immediately postMI, and more slowly thereafter. 4 The initial stage of ventricular remodelling is the thinning of the wall of the ventricle in the area of the infarct, and the dilatation of the ventricular chamber. This is followed by hypertrophy and fibrosis including lengthening of the non-infarcted part of the myocardium. 4 Initially ventricular remodelling preserves stroke volume and pump function of the left ventricle, however, these changes become maladaptive over time. The hormone aldosterone facilitates ventricular remodelling by promoting the development of myocardial fibrosis, leading to the progression of heart failure (HF) and its symptoms. 5 Left ventricular systolic dysfunction (LVSD) is the most common cause of postMI HF. 4 Other causes of HF after MI are papillary muscle dysfunction and mitral regurgitation or arrhythmias (for example atrial fibrillation), or rarer complications such as ventricular rupture or formation of ventricular septal defects; HF may also develop after an MI in the absence of any of these problems. 6 However, over time these adaptations can become counterproductive with dilatation and hypertrophy of the left ventricle eventually leading to impaired cardiac function. This reduced cardiac output leads to symptoms of breathlessness and fatigue, the syndrome known as HF. 4
The risk of developing HF postMI varies with the site, severity and type of MI, and the presence of comorbid conditions. Predictors of HF/LVSD after an MI have been reported as higher blood pressure,4,7,8 previous HF,4,7,8 left bundle branch block,7 anterior MI,4,7,8 diabetes,4,8 higher heart rate,4,7, older age,4,7–9 and larger infarcts (indicated by peak creatine phosphokinase level). 4,10 Patients who develop HF after an MI have been reported as having an increased risk of cardiovascular (CV) events including recurrent acute MI, stroke, atrioventricular block, ventricular arrhythmias cardiac rupture, and unexpected cardiac arrest8 and death. 8,11–13 Patients with impaired left ventricular ejection fraction (LVEF) have been shown to have worse outcomes than those with preserved LVEF. 14,15
Heart failure classification
There are two clinical subgroups of HF patients; either a reduction in the efficiency of the left ventricle to relax and fill with blood (diastolic HF), or to contract and pump blood (systolic HF), resulting in the heart becoming unable to meet the demands of the body. 16 Patients with ventricular dysfunction, whether diastolic or systolic, exhibit the same signs and symptoms; shortness of breath (dyspnoea) particularly when lying down (orthopnoea), fatigue and oedema. Cardiac remodelling occurs with both diastolic and systolic HF. Patients with diastolic HF develop concentric hypertrophy, with a normal or reduced heart cavity size, an increase in wall thickness, and a high mass : cavity ratio, end-systolic and diastolic volumes are generally normal and LVEF is usually normal or elevated. 16,17 In contrast, patients with systolic HF have an increase in cavity size, decreased or unchanged wall thickness, normal or reduced mass : cavity ratio, elevated systolic and diastolic volumes, and a lower LVEF. 16,17 Left ventricular (LV) dilatation always occurs with systolic dysfunction; however, with diastolic dysfunction, LV dilatation only occurs when additional injury is sustained such as an MI. 16 The differential diagnosis of diastolic and systolic HF can require the measurement of LVEF once the diagnosis of the presence of HF has been made; LVEF ≤ 45% is considered to be indicative of LVSD, indicating impaired contractile function, and a diagnosis of systolic HF can be made. 16
Coronary heart disease, hypertension, diabetes, increasing age and obesity are all risk factors for both diastolic and systolic HF. 16,17 Hypertension is a more common risk factor with diastolic HF because it increases LV afterload, which results in delayed relaxation time, elevated LV filling pressure and reduced end-diastolic volume. 16,17
The most commonly used systems for classifying patients with HF are the New York Heart Association (NYHA) and Killip classification systems; the classification system of the American College of Cardiology/American Heart Association (ACC/AHA) is also used. The NYHA classification has four classes based upon physical function and symptoms and the ACC/AHA provides definitions of different stages of HF. These two systems are compared in Table 1. 18 The Killip classification is used to stratify postMI patients into risk groups. 19
-
Killip class I: No clinical signs of HF.
-
Killip class II: Rales or crackles in the lungs, an S3 gallop (an additional heart sound), and elevated jugular venous pressure.
-
Killip class III: Acute pulmonary oedema.
-
Killip class IV: Cardiogenic shock.
| ACC/AHA stage | NYHA functional class | ||
|---|---|---|---|
| Stage | Description | Class | Description |
| A | Patients at high-risk of developing HF because of the presence of conditions that are strongly associated with the development of HF. Such patients have no identified structural or functional abnormalities of the pericardium, myocardium or cardiac valves and have never shown signs or symptoms of HF | No comparable functional class | |
| B | Patients who have developed structural heart disease that is strongly associated with the development of HF but who have never shown signs or symptoms of HF | I (Mild) | No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation or dyspnoea |
| C | Patients who have current or prior symptoms of HF associated with underlying structural heart disease | II (Mild) | Slight limitation of physical activity. Comfortable at rest, but ordinary physical activity results in fatigue, palpitation or dyspnoea |
| III (Moderate) | Marked limitation of physical activity. Comfortable at rest, but less than ordinary activity causes fatigue, palpitation or dyspnoea | ||
| D | Patients with advanced structural heart disease and marked symptoms of HF at rest despite maximal medical therapy and who require specialised interventions | IV (Severe) | Unable to carry out any physical activity without discomfort. Symptoms of cardiac insufficiency at rest. If any physical activity is undertaken, discomfort is increased |
Burden on the NHS
The incidence of postMI HF is increasing in the UK because of the shifting age distribution of the population and increased survival after acute MI. 4,20 Approximately 707,000 people in the UK aged 45 years and older are thought to have HF (393,000 men; 314,000 women), with prevalence increasing steeply with age (1% under 65 years; 6–7% 75 to 84 years; 12–22% 85 years and older). 9 Approximately 40% of patients that experience an acute MI suffer HF. 20 The number of people with postMI HF in the UK for the year 2000 was estimated to be between 130,000 and 202,000, with associated annual costs to the NHS in the region of £125M to £181M. 21
Management of disease
The main drugs used to treat patients with HF are angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, beta-blockers and aldosterone antagonists (mineralocorticoid receptor antagonists). This review will focus on the effectiveness of aldosterone antagonists, which include spironolactone, eplerenone, and the active metabolite of spironolactone, canrenone, and its salt, potassium canrenoate.
Spironolactone
Spironolactone (Aldactone, Pharmacia Ltd, Sandwich, Kent, UK; alternative names include Novo-Spiroton, Spiractin, Spirotone, Verospiron and Berlactone) is licensed for use for HF in the UK. It inhibits the effect of aldosterone by competing for intracellular aldosterone receptors in the collecting ducts in the kidney, increasing water and sodium excretion and decreasing the excretion of potassium. Spironolactone is available alone or combined with hydroflumethiazide (Aldactide, Pharmacia Ltd), hydrochlorothiazide (Aldactazide, Pfizer Ltd) or furosemide (Lasilactone, Sanofi-Aventis). Spironolactone is indicated in patients with moderate to severe HF (NYHA class III and IV) caused by LVSD. 22,23
According to the Summary of Product Characteristics (SPC), the advised dose of spironolactone (Aldactone) in adults with congested cardiac failure is 100 mg/day, gradually increased up to 400 mg/day if required, with a maintenance dose of 25–200 mg/day once oedema is controlled. 24 However, high doses of spironolactone are rarely used, with most patients receiving between 12.5 and 50 mg/day. The SPC states that adverse drug reactions associated with spironolactone include electrolyte disturbances, hyperkalaemia, leukopenia, thrombocytopenia, malaise, gastrointestinal disturbances and drowsiness, rashes and abnormal hepatic and renal function. As a result of the effect on androgen receptors and other steroid receptors, gynaecomastia, testicular atrophy, sexual dysfunction and menstrual irregularities may occur. Being a mineralocorticoid antagonist, spironolactone may reduce the effectiveness of antidepressant drugs in the treatment of major depression, presumably by interfering with normalisation of the hypothalamic–pituitary–adrenal axis in patients receiving antidepressant therapy.
Eplerenone
Eplerenone (Ispra; Pfizer Ltd) is a selective aldosterone antagonist used as an adjunct in the management of chronic HF. It is specifically indicated for the reduction of risk of CV death in patients with HF and LV dysfunction within 3–14 days of an acute MI, in combination with standard therapies and as treatment for hypertension.
Eplerenone is similar to spironolactone, but has a greater affinity for the mineralocorticoid receptor and as a result is thought to have fewer side effects, in particular gynaecomastia. According to the SPC, the usual starting dose of eplerenone is 25 mg once daily, increasing to 50 mg/day after approximately 4 weeks. 24 The SPC state that common adverse drug reactions associated with eplerenone include hyperkalaemia, hypotension, dizziness, altered renal function and increased creatinine concentration.
Canrenone
Canrenone is the active metabolite of both spironolactone and potassium canrenoate. Neither canrenone nor potassium canrenoate are licensed as human medicines in either Europe or the USA so they will not be considered in this assessment. Available data on canrenone and potassium canrenoate may be used where appropriate to complement the evidence base for spironolactone and eplerenone.
Costs
Based on British Heart Foundation statistics, the annual cost of HF to the NHS was £628.6M in 2000,25 and £250M when the cost of inpatient hospitalisations was excluded. Given that these costings were based on assuming a prevalence of HF of 707,000 in the UK,26 this equates to an annual cost of HF of £354 per person. Eplerenone 25 mg/day for the first 4 weeks, and 50 mg thereafter, results in an annual cost of £557 per year that the patient continues to receive the drug. For 100 mg per day of spironolactone, the annual cost is £46 for the duration of treatment.
Available evidence
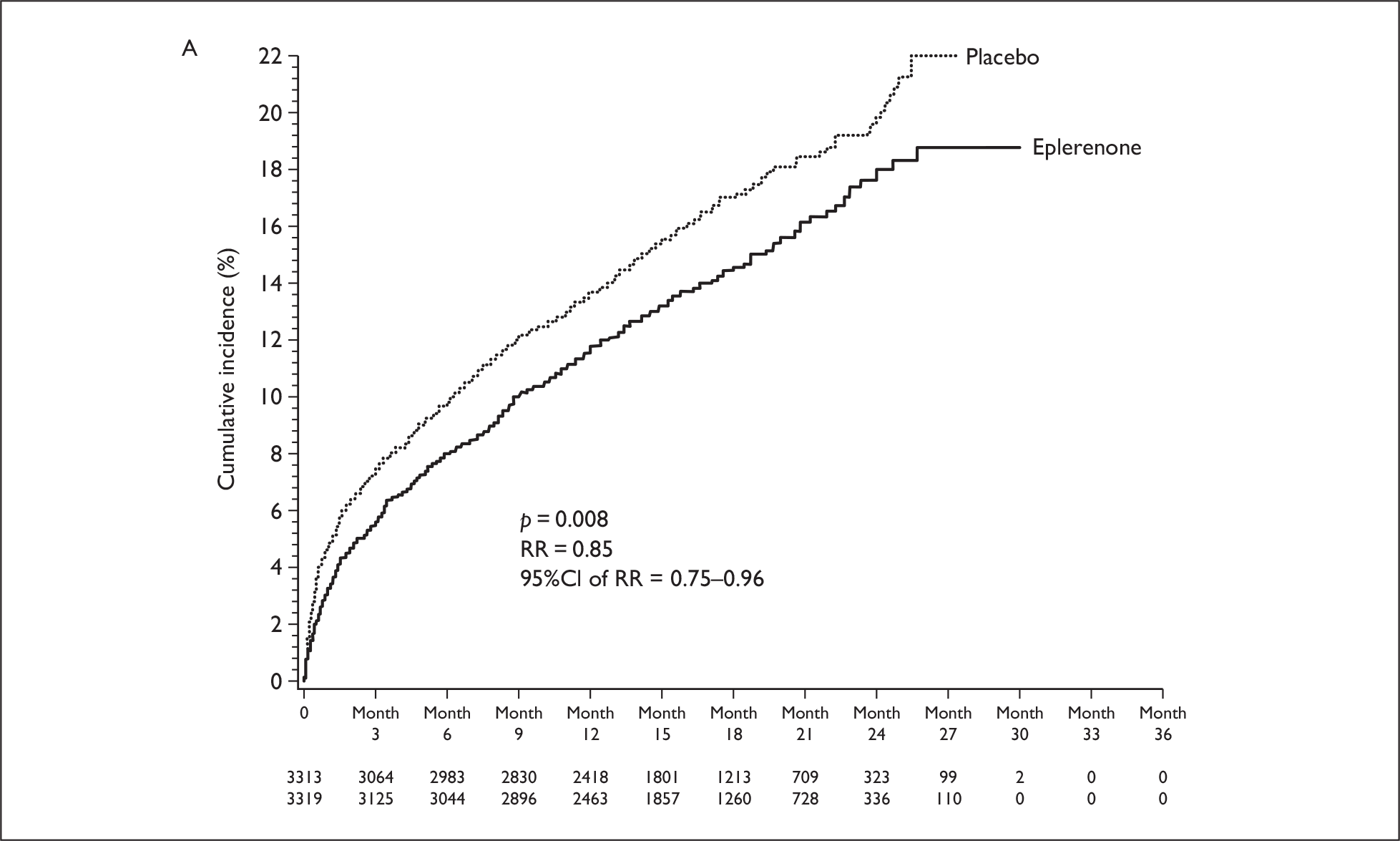
There are two aldosterone antagonists licensed for HF in the UK: spironolactone for chronic HF and eplerenone for postMI HF. There are two large good-quality randomised control trials (RCTs) of aldosterone antagonists in patients with HF and LV dysfunction; The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) and the Randomised Aldactone Evaluation Study (RALES). Only EPHESUS specifically examined the effectiveness in patients with LVSD following an MI. 27 This was a multicentre, randomised, double-blind, parallel group comparison, administering 25–50 mg/day of eplerenone to patients with ischaemic HF and an LVEF 40% or less. 27 Eplerenone, in addition to ACE inhibitors and beta-blockers, was associated with a reduced risk of the two primary end points: death from any cause [relative risk (RR) 0.85, 95% confidence interval (CI): 0.75 to 0.96, p = 0.008] and the combination of death from CV causes or hospitalisation for CV events (RR 0.87, 95% CI: 0.79 to 0.95, p = 0.002). In addition, evidence from two studies was identified that examined the cost-effectiveness of eplerenone based on the effectiveness data from EPHESUS. 28,29 Both studies demonstrated that eplerenone appears to be cost-effective in patients early after MI with LV dysfunction.
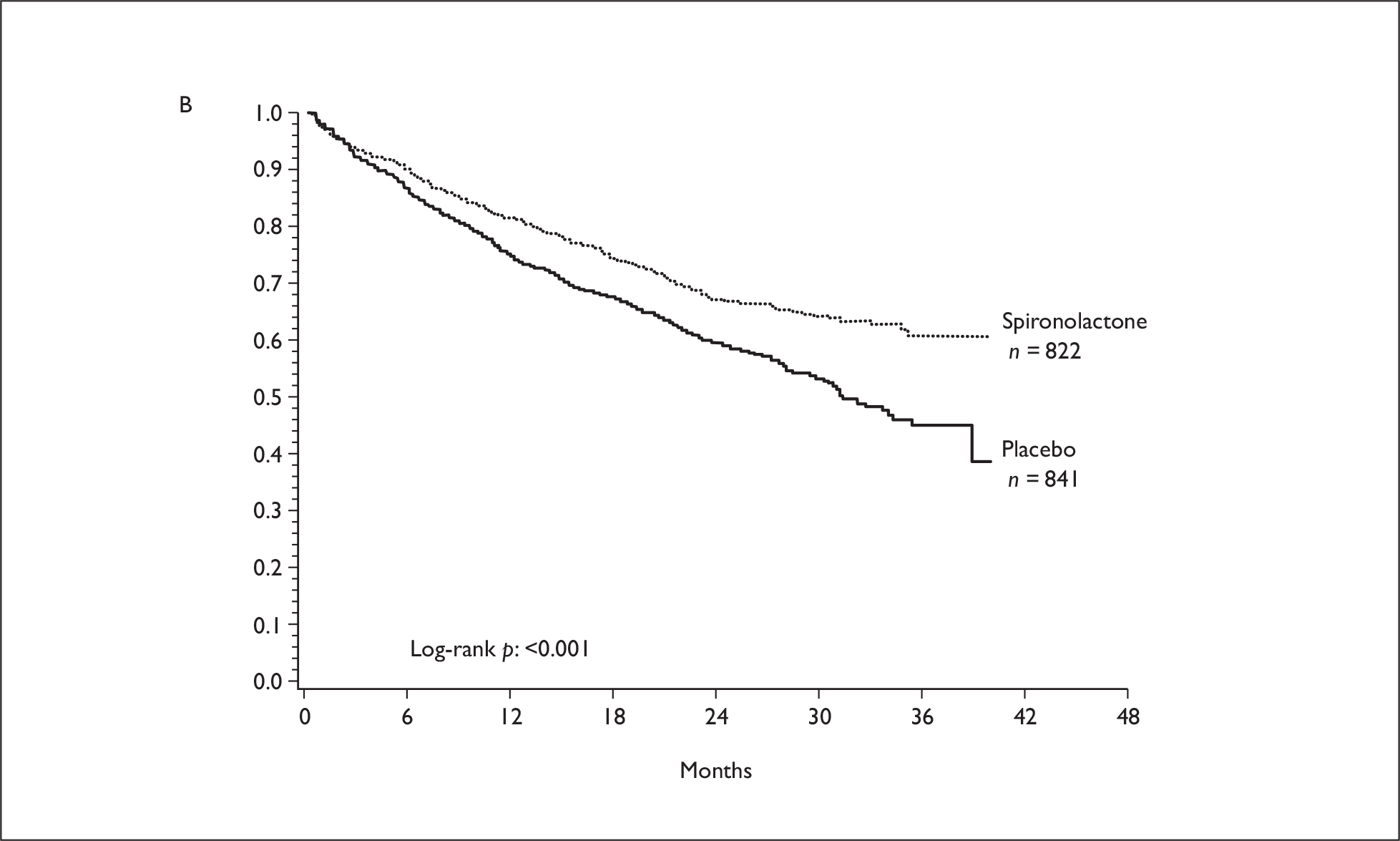
There is evidence that spironolactone administered with an ACE inhibitor can prevent postMI remodelling more effectively than an ACE inhibitor alone. 30 The use of spironolactone in addition to standard therapy, has been demonstrated in RALES to significantly reduce the risk of both morbidity and death among patients with severe HF. 31 Spironolactone was administered in doses of 25–50 mg/day to patients with HF and an LVEF of 35% or less; approximately 54% of patients in the study had ischaemic HF. The trial found that the addition of spironolactone to an ACE inhibitor and loop diuretic significantly reduced mortality in patients with severe HF (RR 0.70, 95% CI: 0.60 to 0.82, p < 0.001). 31 Several studies have also evaluated the cost-effectiveness of spironolactone in the overall population, demonstrating that spironolactone appears to be highly cost-effective or may even dominate standard care, being potentially both cheaper and more effective.
Canrenone has been studied in the Antiremodeling Effect of Aldosterone receptors blockade with canrenone In mild Chronic Heart Failure (AREA IN-CHF) trial. This is a multicentre, randomised, double-blind, parallel group comparison, administering 25 to 50 mg/day of canrenone to patients with NYHA class II HF and an LVEF < 45%. 32 Of the 467 patients recruited into the trial, 52% had ischaemic HF. There was no difference between canrenone and placebo in terms of CV death; however, there was a significant reduction in the composite outcome of cardiac death or hospitalisation for cardiac causes; 7.9% with canrenone and 15.1% with placebo (p = 0.02). 33
A recent pharmacoeconomic review of eplerenone concluded that future clinical and economic comparison of eplerenone and spironolactone would be of particular interest, particularly in view of the lower drug acquisition costs for spironolactone than for eplerenone. 34 However, this review reached the conclusion that a comparison of these two drugs in patients who have had an acute MI and who have symptoms and/or signs of HF and LV dysfunction is not currently feasible because of the limited data on spironolactone, specifically in patients after an MI, and the lack of clinical data directly comparing the two drugs.
Current guidance
The current National Institute for Health and Clinical Excellence (NICE) guidelines (CG5) for chronic HF states: all patients with HF due to LVSD should be considered for treatment with an ACE inhibitor, which should be introduced prior to beta-blockade, and titrated upwards at short intervals until the optimal tolerated or target dose is achieved; diuretics should be routinely used for the relief of congestive symptoms and fluid retention in patients, titrated up and down as required following the initiation of subsequent HF therapies; beta-blockers licensed for use in HF should be initiated after diuretic and ACE inhibitor therapy, regardless of whether or not symptoms persist; and if symptoms remain moderate to severe despite optimal therapy, patients should be prescribed spironolactone, 12.5–50 mg/day. 22 Eplerenone is currently only recommended in patients whose HF develops following an MI;34 the most recent NICE guidelines for HF state that eplerenone is not licensed for use in the treatment of HF in the UK. 22 Canrenone is not licensed in the UK.
The NICE guidelines for secondary prevention in primary and secondary care of patients following an MI (CG 48, May 2007), recognised that from an economic perspective, the correct comparison should be between eplerenone and spironolactone. 35 Although spironolactone was reported to be widely used in postMI patients, in the absence of direct effectiveness evidence in this patient group the guideline recommended treatment with eplerenone for patients who have had an acute MI and who have symptoms and/or signs of HF and LV dysfunction.
Based on the results of EPHESUS, the Scottish Intercollegiate Guidelines Network guidelines for acute coronary syndromes indicate that patients with clinical MI complicated by LV dysfunction (LVEF 40% or less) where there are clinical signs of HF should take eplerenone therapy in addition to standard therapy. 36 Eplerenone was approved by Food and Drug Administration (FDA) for treatment of hypertension in 2002, and for patients who have congested cardiac failure after an MI in 2004.
Chapter 2 Definition of decision problem
Decision problem
Eplerenone is recommended by NICE in postMI HF and has been shown to reduce mortality in these patients. The pharmacologically similar aldosterone antagonist spironolactone has lower acquisition costs and potentially increased or equivalent efficacy compared with eplerenone in patients with severe HF. However, paucity of evidence for efficacy of spironolactone specifically in postMI HF precludes recommendation of this drug at present.
To investigate the potential interchangeability of spironolactone and eplerenone in practice, and to assess whether a trial comparing these drugs is feasible, a systematic review of the clinical evidence is required. The evidence used to assess clinical effectiveness will be obtained from RCTs of spironolactone, eplerenone or canrenone in addition to optimal medical management for postMI HF, compared with placebo plus optimal medical management or optimal medical management alone. The population being studied is adults with LVSD (LVEF ≤ 45%) and clinical signs of HF following an MI. All-cause or CV mortality, all-cause or CV hospitalisation rates, composite outcomes of mortality and hospitalisation rates, CV events, change in NYHA classification, drug-related adverse effects (specifically gynaecomastia and hyperkalaemia or clinical events resulting from hyperkalaemia) and health-related quality of life (HRQoL) will be considered the main outcomes. A subgroup of interest is those with severe LVSD (LVEF < 30%). For evaluation of adverse effects any population that were treated with an aldosterone antagonist will be included.
Overall aims and objectives of the assessment
The primary objective is to evaluate the relative clinical effectiveness and cost-effectiveness of spironolactone and eplerenone in patients with HF following MI, and to explore the possibility of conducting an indirect comparison of spironolactone and eplerenone in postMI HF. A range of alternative approaches will be considered and will be used as the basis for considering issues related to the relative cost-effectiveness of spironolactone and epleronone. A second objective is to use value-of-information (VOI) analyses to determine the need for further research, to identify the research questions critical to decision-making, and to help inform the design of future studies and to consider implementation issues.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing clinical effectiveness
Search strategy
Resources searched
The following resources were searched to identify information relating to the use of spironolactone, eplerenone, canrenone and canrenoate potassium for postMI HF or HF. A variety of search strategies were used that included relevant free-text terms and subject headings (see Appendix 1). No language restrictions were applied.
To identify clinical trials of spironolactone, eplerenone, canrenone or potassium canrenoate for postMI HF, a search strategy was designed to be run on MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials (CENTRAL) with no date restrictions. This was supplemented by a second search strategy to locate clinical trials of the named drugs for HF (as distinct from postMI HF) that may have had an ischaemic subgroup. A date limit of 1995 was applied to this search, to retrieve studies that were more comparable with recent trials and current clinical practice.
Bibliographies of all relevant reviews, guidelines and included studies were scrutinised for further relevant studies.
Databases searched for systematic reviews and guidelines
-
Cochrane Database of Systematic Reviews – www.interscience.wiley.com/cgi-in/mrwhome/106568753/HOME
-
Database of Abstracts of Reviews of Effects – www.interscience.wiley.com/cgi-bin/mrwhome/106568753/HOME
-
Heath Technology Assessment (HTA) Database – www.interscience.wiley.com/cgi-bin/mrwhome/106568753/HOME
-
National Library for Health (Guidelines Finder) – www.library.nhs.uk/guidelinesFinder/
-
United States (US) National Guidelines Clearing House – www.guideline.gov/
Databases searched for clinical trials
-
MEDLINE (OvidSP) – http://gateway.ovid.com/athens
-
EMBASE (OvidSP) – http://gateway.ovid.com/athens
-
CENTRAL – www.interscience.wiley.com/cgi-bin/mrwhome/106568753/HOME
-
Clinical Trials.gov – www.clinicaltrials.gov/
-
MetaRegister of Controlled Trials – www.controlled-trials.com/
Databases and resources searched for adverse effects information
-
MEDLINE (OvidSP) – http://gateway.ovid.com/athens
-
EMBASE (OvidSP) – http://gateway.ovid.com/athens
-
Toxicology Literature Online (TOXLINE) – http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?TOXLINE
-
Dynamed – www.ebscohost.com/dynamed/
-
Drugs@FDA – www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/
-
European Medicines Agency (EMEA) – www.emea.europa.eu/
-
American Hospital Formulary Service (AHFS) Drug Information. Bethesda, MD: American Society of Hospital Pharmacists, 2008
-
Dukes MNG, Aronson JK, (editors) Meylers side effects of drugs. 14th edition. Amsterdam: Elsevier; 2000
Study selection
Titles and abstracts were screened independently by two reviewers using endnote X1; disagreements were resolved by consensus, or if consensus could not be reached, a copy of the full paper was retrieved. Two reviewers independently screened full papers according to the criteria detailed below using eppi-reviewer, version 3.0, software (Evidence for Policy and Practice Information; EPPI). Discrepancies were resolved by discussion, or by referral to a third reviewer if necessary. Studies in any language were eligible for inclusion; non-English language papers were screened by one reviewer with a native speaker. Details of included studies are provided in Appendix 2 and a list of excluded studies and reasons for exclusion is provided in Appendix 3.
Inclusion and exclusion criteria
Study designs
For the assessment of clinical effectiveness, RCTs of any size were included if conducted in a postMI HF population. Trials of general HF patients that included a subgroup of patients whose HF was preceded at some point by an ischaemic event such as an MI, were considered further if they had at least 100 ischaemic participants per arm and the authors provided subgroup data when contacted. All trials had to have at least 6 months of follow-up to be included in the assessment of clinical effectiveness. For the assessment of adverse events, summary data from recognised reference sources [Martindale complete drug reference,33 Meyler’s side effects of drugs,37 AHFS drug information,38,39 FDA medical reviews40 and label information,41 and the British National Formulary (BNF)42] and RCTs or observational studies in any population that recruited more than 100 participants, were sought.
Interventions and comparators
Randomised controlled trials of spironolactone or eplerenone in addition to optimal medical management for postMI HF, compared with placebo plus optimal medical management or optimal medical management alone, were included in the review of clinical effectiveness. RCTs that evaluated the effectiveness of canrenone or potassium canrenoate were also sought to provide supplementary information on efficacy.
Population
Studies of adults with LVSD (LVEF ≤ 45%), in addition to clinical signs of HF, following an MI were included. A subgroup of interest was those with severe LVSD (LVEF < 30%). For the evaluation of adverse effects the criteria were broadened to include general HF populations that were treated with an aldosterone antagonist. The criteria were not extended further to include any patient treated with an aldosterone antagonist because other conditions for which these drugs are used require much higher doses so the results would not be generalisable to the population that is of interest in this review. NICE guidelines recommend 12.5–50 mg/day of spironolactone for symptomatic HF due to LVSD,43 and a starting dose of 25 mg/day of eplerenone, rising to 50 mg/day after 4 weeks, for the treatment of postMI HF. 35 When used for the treatment of conditions such as hepatic cirrhosis with ascites and oedema, malignant ascites, nephritic syndrome and primary aldosteronism, the doses prescribed tend to range between 100 and 400 mg/day, making their adverse event profile not comparable to those of the drugs when used in populations with HF. 44 There is some evidence for the efficacy of low doses of 12.5 to 50 mg/day in patients with resistant hypertension;45–47 however, this population was not considered sufficiently comparable to that being studied in this review.
Outcomes
The outcomes of interest were:
-
all-cause or CV mortality
-
all-cause or CV hospitalisation rates
-
composite outcomes of mortality and hospitalisation rates
-
CV events
-
change in NYHA classification
-
drug-related adverse effects (specifically gynaecomastia and hyperkalaemia or clinical events resulting from hyperkalaemia) and HRQoL.
Data extraction
Data were extracted by one reviewer and checked by a second reviewer, using a standardised data extraction form for effectiveness data, and extracting as reported in the studies for adverse events data. Discrepancies were resolved by discussion. Non-English language studies were extracted by one reviewer with a native speaker. Authors were contacted to obtain additional information on the baseline characteristics and outcome measures for ischaemic (postMI) subgroups from trials of general HF populations, where subgroup data were not reported in the published papers. Data from multiple publications of the same study were extracted as one study. The data extracted included: study characteristics (e.g. author, year, number of participants, countries and study centres, and duration of follow-up), intervention (type of aldosterone antagonist, dose and dosing regimen), comparator (placebo or optimal medical management), population characteristics (e.g. NYHA class included, proportion of population that were ischaemic, time from MI, LVEF, age, gender, using a standardised data extraction form), and data for the outcomes of interest as described above.
Quality assessment
The methodological quality of the included RCTs was assessed in terms of randomisation, allocation concealment, blinding, reporting of withdrawals, completeness of follow-up and the use of a power calculation and an intention-to-treat (ITT) analysis. Review-specific criteria included the requirement of baseline characteristics of the ischaemic population, the duration of follow-up and whether the population recruited was representative of the postMI HF population seen in clinical practice. Observational studies of adverse events data were assessed in terms of the use of a control group and the comparability of these groups at baseline, the method of data collection, the potential for selection bias and generalisability of the patient spectrum, length of follow-up, attrition and the appropriateness of the analysis. Full results of the quality assessment are presented in Appendix 4. One reviewer initially assessed study quality and this was checked by a second reviewer. Disagreements were resolved by discussion.
Data analysis
As a result of the clinical heterogeneity observed between studies, the results are presented in a narrative synthesis. The synthesis will explore the exhangeability between the drugs on a pharmacological basis, and the trials in relation to the population recruited. Although the focus of the review is the postMI population, only eplerenone has been investigated directly in this population. Results of subgroups of patient with chronic HF who have a history of cardiac ischaemia will be investigated in an attempt to provide some indication as to how spironolactone may perform in the postMI population.
Results of review of clinical effectiveness
Quantity and quality of research available
Effectiveness
The searches for studies of clinical effectiveness identified 1616 papers; 37 were considered potentially relevant and were retrieved as full papers for assessment. Five trials (across 10 publications) met the inclusion criteria; two papers provided adverse events data only. The flow of studies is shown in Figure 1. Three trials evaluated spironolactone,31,48,49 one evaluated eplerenone27 and one evaluated canrenone. 50 Of the five trials, three were conducted specifically in postMI HF patients,27,48,49 and two in a general HF population with a subgroup of patients in whom the cause of HF was ischaemic. 31,50 The authors of the two trials in the general HF population were contacted for data for the ischaemic subgroup; additional data were denied by the manufacturer of the drug for one trial,31 and were provided by the triallists for the second. 50
FIGURE 1.
Flow of studies through the review obtained from the searches for clinical effectiveness studies.
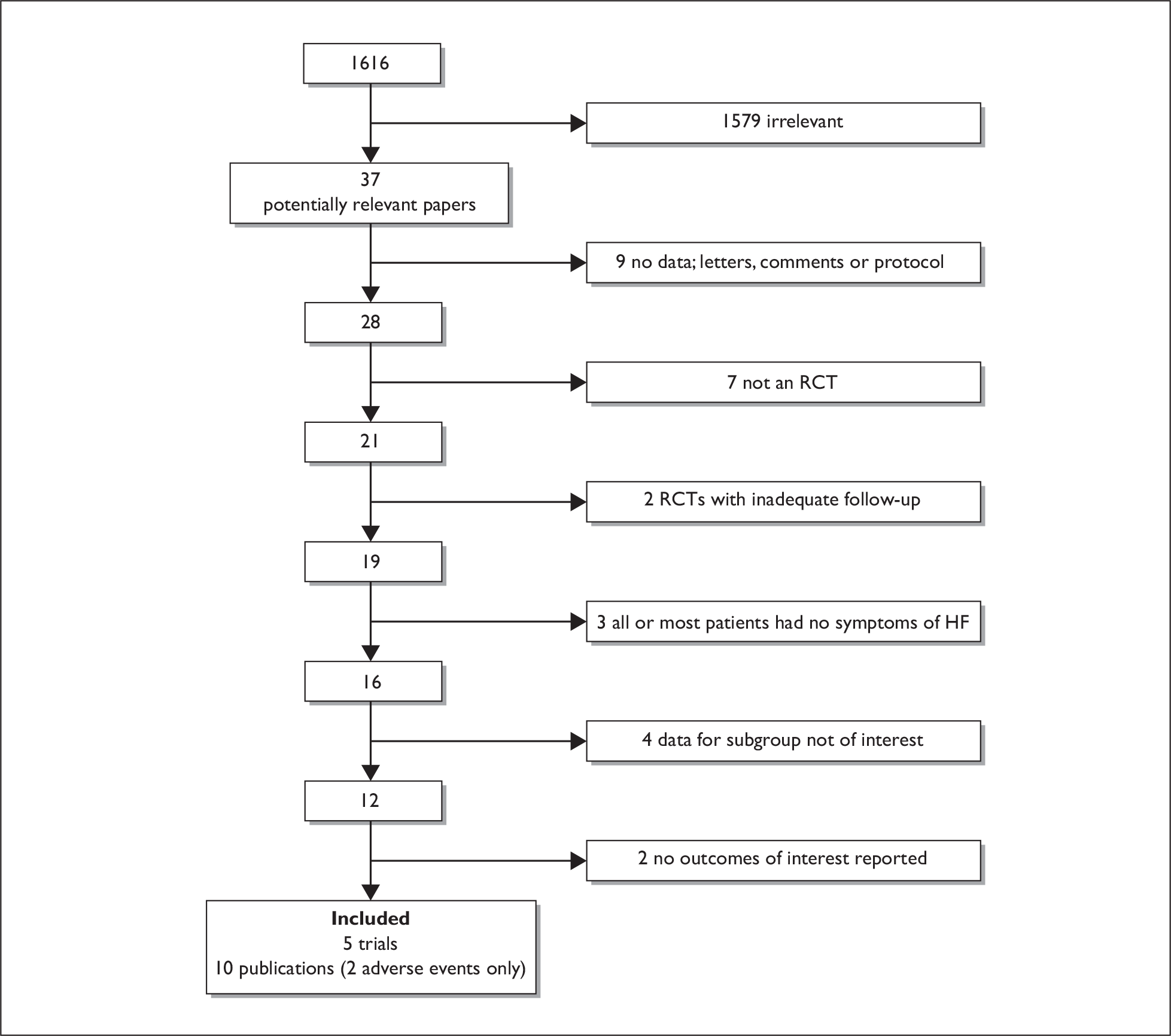
All five studies were described as randomised trials; however, none provided details of the randomisation method. Three trials reported allocation concealment, double blinding (although no details as to who this referred to were provided), and the use of a power calculation. 27,31,50 All five studies had groups that were comparable at baseline, clearly described eligibility criteria, had at least 12 months of follow-up, and used an ITT analysis. Of the three larger trials,27,31,50 only one had at least 90% follow-up. 50 Two of the studies were very small, poorly reported and were of poor quality; one was published in Polish48 and the other in Chinese. 49 Results of the quality assessment are given in Table 2, with guidelines for scoring each criterion provided in Appendix 4.
| RALES | EPHESUS | AREA-IN-CHF | Ruta (2006) | Tu (2003) | |
|---|---|---|---|---|---|
| Number randomised reported | Y | Y | Y | Y | Y |
| Randomisation method used reported | U | U | U | U | U |
| Allocation concealment implemented | Y | Y | Y | N | U |
| Groups comparability at baseline | Y | Y | Y | Y | Y |
| Study reported as double blind | Y | Y | Y | N | N |
| Patient blinded specifically stated | U | U | U | U | U |
| Outcome assessors blinded specifically stated | U | U | U | U | U |
| Care givers blinded specifically stated | U | U | U | U | U |
| Power calculation used | Y | Y | Y | N | N |
| Eligibility criteria clearly described | Y | Y | Y | Y | Y |
| Baseline characteristics of ischaemic population provided | N | Y | N | Y | Y |
| At least 12 months of follow-up | Y | Y | Y | Y | Y |
| Representative sample recruited | N | Y | N | N | U |
| ITT analysis used | Y | Y | Y | Y | Y |
| Losses to follow described | Y | Y | Y | Y | N |
| At least 90% follow-up | N | N | Y | Y | Y |
Adverse events
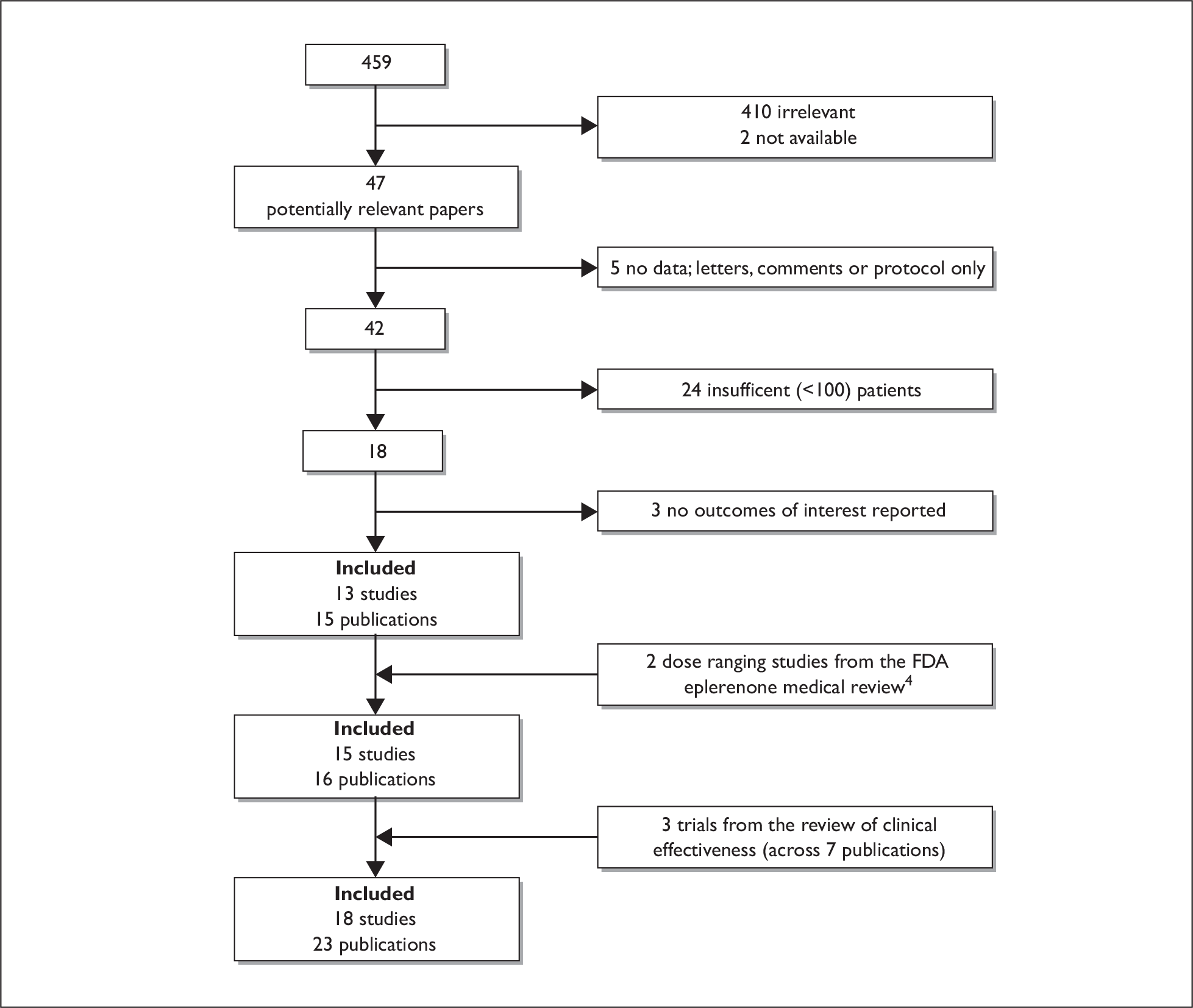
Initially, information relating to adverse events was sought from reference sources such as Martindale,33 Meyler’s,37 AHFS,38,39 FDA medical reviews and label information,40,41 and the BNF. 42 Little information was provided in these sources (see section Adverse events, p. 21), and therefore additional searches were conducted to identify studies reporting adverse events using the inclusion criteria stated previously (see Inclusion and exclusion criteria). From these additional searches, 459 papers were identified. Of these, 49 were considered potentially relevant for full paper review. Two papers were unobtainable. 55,56 Of the 47 papers screened, 15 met the inclusion criteria; one was only available as an abstract,57 and two papers were results from the same consecutive sample. 58,59 Two dose-ranging studies described only in the FDA eplerenone medical review were also included. 40 With three studies identified from the search for studies of clinical effectiveness, 18 studies (across 23 publications) were finally included in the review of adverse events. The flow of studies is shown in Figure 2. Results of the quality assessment for studies included only for adverse events are given in Table 3 and Table 4, with guidelines for scoring each criterion provided in Appendix 4.
| Dose ranging: 01140 | Dose ranging: Japanese40 | |
|---|---|---|
| Number randomised reported | Y | Y |
| Randomisation method used reported | U | U |
| Allocation concealment implemented | Y | Y |
| Groups comparability at baseline | U | U |
| Study reported as double blind | Y | Y |
| Patient blinded specifically stated | U | U |
| Outcome assessors blinded specifically stated | U | U |
| Care givers blinded specifically stated | U | U |
| Power calculation used | U | U |
| Eligibility criteria clearly described | Y | Y |
| Baseline characteristics of ischaemic population provided | N | N |
| At least 12 months follow-up | N | N |
| Representative sample recruited | U | N |
| ITT analysis used | Y | Y |
| Losses to follow described | N | N |
| At least 90% follow-up | N | N |
| Reference numbera: | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 60 | 61 | 62 | 63 | 64 | 65 | 57 | 66 | 67 | 58, 59, 68 | 69 | 70 | 71 | |
| Control group recruited? | N | N | N | N | N | N | Y | N | N | N | N | Nb | N |
| Was the data obtained prospectively? | N | Y | N | Y | N | Y | Y | N | Y | Y | N | Y | N |
| Consecutive patients? | U | Y | U | U | U | Y | Y | Y | U | Y | U | Y | U |
| Inclusion criteria clearly reported | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Study size explained | N | N | N | N | Y | Y | N | N | N | N | N | N | N |
| Similar baseline characteristics? | NA | NA | NA | NA | NA | NA | U | NA | NA | NA | NA | NA | NA |
| Conducted in patients with post-MI HF? | N | N | N | N | N | N | N | N | N | N | N | N | N |
| Confounders identified and accounted for? | Y | Y | N | N | N | Y | U | Y | Y | Y | Y | Y | Y |
| Losses to follow-up accounted for? | NA | NA | Y | NA | NA | NA | U | Y | Y | Y | Y | Y | Y |
| All recruited participants included in the final analysis? | Y | Y | N | Y | Y | Y | U | N | Y | Y | N | Y | Y |
| Outcomes measured at least 6 months after initiation of treatment? | U | U | N | Y | U | U | Y | Y | Y | Y | Y | Y | U |
| Adverse events were a primary outcome? | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Attrition rate less than 90%? | NA | NA | U | Y | NA | NA | U | U | Y | Y | U | Y | Y |
FIGURE 2.
Flow of studies through the review obtained from the searches for adverse events data.
Result of the review of clinical effectiveness
Effectiveness
Five RCTs were included in the assessment of the clinical effectiveness of aldosterone antagonists; brief study details are provided in Table 5, and full study details are given in Appendix 2. All five trials reported all-cause mortality and four reported either cardiac or CV mortality in patients with ischaemic HF (Table 6). Only one study reported increased all-cause mortality in patients receiving spironolactone; this was the smallest study, poorly reported and published in Polish. 48 Of the studies that reported a reduced mortality with treatment, the reduction in all-cause mortality ranged from 15% to 86%, and CV mortality ranged from 17% to 84% (Table 6). The largest reductions in mortality were reported in the second smallest study, which was published in Chinese and also of poor quality. 49
| RALES 31 (March 1995 to December 1996) (in English) | EPHESUS27,52 (December 1999 to 2001) (in English) | AREA IN-CHF50 (September 2002 to July 2005) (in English) | Ruta (2006)48 (December 2000–2) (in Polish) | Tu (2003)49 (2000–1) (in Chinese) | ||
|---|---|---|---|---|---|---|
| General HF population | Ischaemic HF population | |||||
| Population | 1663 patients | 6642 patients | 467 patients | 241 patients | 47 patients | 85 patients |
| Spironolactone (n = 822) | Eplerenone (n = 3319) | Canrenone (n = 231) | Canrenone (n = 118) | Spironolactone (n = 22) | Spironolactone (n = 43) | |
| Placebo (n = 841) | Placebo (n = 3313) | Placebo (n = 236) | Placebo (n = 123) | No spironolactone (n = 25) | Controls (n = 42) | |
| Drug regimen | Spironolactone 25 mg/day; maximum 50 mg/day | Eplerenone 25 mg/day; maximum 50 mg/day | Canrenone 25 mg/day; 50 mg/day after first month | Spironolactone 25 to 50 mg/day | Spironolactone 20 mg/day | |
| Matching placebo | Matching placebo | Matching placebo | No spironolactone | Placebo | ||
| Dose decreased or discontinued if hyperkalaemia or serious hyperkalaemia occurred | Dose decreased or discontinued if hyperkalaemia occurred | Dose not increased if hyperkalaemia occurred, or serum creatinine > 2.5 mg/dl | ||||
| LVEF inclusion criteria | 35% or less | 40% or less | 45% or less in 6 months prior to enrolment | 45% or less | 30% or less | All patients had symptomatic LVSD |
| NYHA Heart Failure Classification | I: 1864 | NR | ||||
| II: 0.5% | II: 3279 | II: 100% | II: 28% | |||
| III: 70.5% | III: 1049 | III:72% | ||||
| IV: 29% | IV: 103 | IV: 0% | ||||
| 337 not classified | ||||||
| Other medication | Beta-blockers: 10.5% | Beta-blockers: 75% | Beta-blockers: 79% | Beta-blockers: 80% | Beta-blockers: 85% | Beta-blockers: 100% |
| ACE inhibitors: 94.5% | ACE inhibitors/ARBs: 86.5% | ACE inhibitors: 80% | ACE inhibitors: 81% | ACE inhibitors: 60% | ACE inhibitors: 100% | |
| Digoxin: 73.5% | Digoxin: NR | Digoxin: 26% | Digoxin:18% | Digoxin: 30% | Digoxin: ‘usually’ prescribed | |
| Length of follow-up | 24 months (median 103 weeksa) | Mean 16 months (range 0–33 monthsa) | 12 months | 24 months | 12 months | |
| Withdrawals | 414 discontinued; 214 spironolactone, 200 placebo | 1021 discontinued; 528 eplerenone, 493 placebo | 38 patients disqualified; inadequate consent | 7 patients in each arm at 12 months | 2 patients discontinued spironolactone due to gynaecomastia | None reported |
| Treatment; comparison; timepoint/follow-up | LVEF; mean (SD) | All-cause mortality | Cardiovascular mortality | |||||
|---|---|---|---|---|---|---|---|---|
| n/N | RR (95% CI) | Change in risk with treatment | n/N | RR (95% CI) | Change in risk with treatment | |||
| RALES31 | Spironolactone, 25–50 mg/day | 25.6 (6.7) | 171a/454 | 0.77 (0.66 to 0.89)b | 28% reduction | NR | NR | NR |
| Matching placebo | 25.2 (6.8) | 222a/453 | ||||||
| Median 103 weeksa | ||||||||
| EPHESUS: all patients52 | 33 (6) | 478/3319 | 0.84 (0.73 to 0.96)b | 15% reduction | 407/3319 | 0.82 (0.71 to 0.94)b | 17% reduction | |
| Matching placebo | 33 (6) | 554/3313 | Kaplan–Meier estimate: 0.85 (0.75 to 0.96) | 483/3313 | Kaplan–Meier estimate: 0.83 (0.72 to 0.94) | |||
| Mean 16 months; range 0–33 monthsa | ||||||||
| EPHESUS: poor LVEF27 | Eplerenone, 25–50 mg/day | 26 (4) | 205/1048 | 0.81 (0.69 to 0.96)b | 21% reduction | 177/1048 | 0.79 (0.66 to 0.94)b | 23% reduction |
| Matching Placebo | 26 (5) | 254/1058 | Kaplan–Meier estimate: 0.79 (NR) | 226/1058 | Kaplan–Meier estimate: 0.77 (NR) | |||
| Mean 16 months; range 0–33 monthsa | ||||||||
| AREA IN-CHF50 | Canrenone, 25 mg/day (50mg after first month) | 39.1 (8) | 4/118 | 0.42 (0.13 to 1.29)b | 58% reduction | 4/118 | 0.69 (0.20 to 2.40)b | 31% reduction |
| Matching placebo | 38.3 (8.5) | 10/123 | 6/123 | |||||
| 12 months | ||||||||
| Ruta (2006)48 | Spironolactone, 25–50mg/day | 23 (5) | 11/22 | 1.56 (0.77 to 3.17)b | 50% increase | NR | NR | NR |
| No spironolactone | 26 (4) | 8/25 | ||||||
| 4 months | ||||||||
| Tu (2003)49 | Spironolactone, 25 mg/day | NR | 1/43 | 0.14 (0.02 to 1.09)b | 86% reduction | 1/43 | 0.16 (0.02 to 1.30)b | 84% reduction |
| Placebo (unclear if matching) | 7/42 | 6/42 | ||||||
| 12 months | ||||||||
Exchangeability between RALES, EPHESUS and AREA IN-CHF
If a reliable indirect comparison is to be made between eplerenone, spironolactone and canrenone the trials need to be comparable, both in terms of the intervention evaluated and the population recruited. Given the small size and poorer quality of the trials by Ruta et al. 48 and Tu and Chen,49 clinical effectiveness can only be reliably assessed using the three main trials: EPHESUS (eplerenone),27,52 RALES (spironolactone),31 and AREA IN-CHF (canrenone). 50 The exchangeability between the the drugs and the three trials are discussed in the following sections.
Pharmacology of spironolactone (and canrenone) and eplerenone
Increased plasma aldosterone is associated with adverse CV effects in HF. Originally, this was thought to be because of its impact on sodium and water retention and potassium excretion, by binding to the mineralocorticoid receptor in epithelial tissues, such as the kidney. 72,73 Subsequent research has shown aldosterone to also act on non-epithelial tissues, such as the heart, brain and vasculature, and therefore to have a role in the pathophysiology of CV disease beyond ion transport,72,73 and to contribute to a number of non-renal mechanisms that progress disease such as thrombogenesis, cardiac fibrosis and cardiac remodelling. 72 The role of aldosterone in the pathophysiology of CV disease is still not fully understood. 72,73
Spironolactone (7α-acetylthio-3-oxo-17 α-pregn-4-ene-21,17β-carbolactone; C24H32O4S; molecular weight 416.58) (Figure 3) is a competitive non-selective antagonist of the aldosterone receptor. The term non-selective is used because of spironolactone’s moderate affinity to other steroid receptors, including the progesterone and androgen receptors. 72–74 This affinity for these steroid receptors results in progestational and antiandrogenic side effects, such as gynaecomastia, disruptions to the menstrual cycle and impotence. 33,37,38,75,76 The peak plasma concentration of spironolactone is reached approximately 2.6 hours after oral administration. 24 Spironolactone is quickly metabolised by the liver into a number of metabolites. 74 The two main active metabolites of spironolactone are canrenoate and canrenone, which have elimination half-lives of approximately 13.8–16.5 hours. 41,72,73 These metabolites are mainly excreted in urine, but also in bile. 76
FIGURE 3.
Molecular structure of eplerenone and spironolactone.
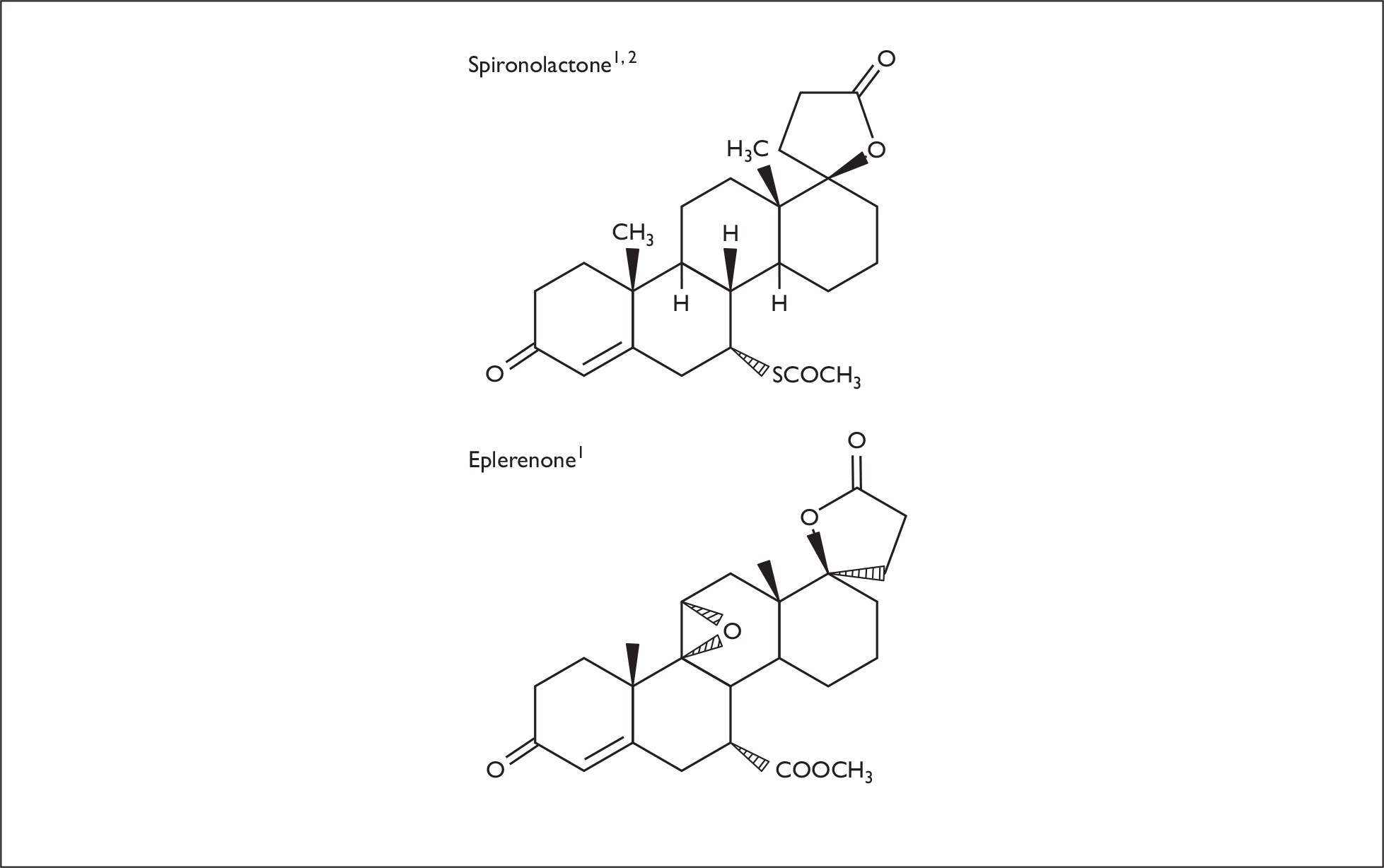
Eplerenone [pregn-4-ene-7,21-dicarboxylic acid, 9,11-epoxy-17-hydroxy-3-oxo-,γ-lactone, methyl ester, (7α,11α,17α); C24H30O6; molecular weight 414.50] is a derivative of spironolactone, and is therefore also a competitive antagonist of the aldosterone receptor; the 17α-thioacetyl group of spironolactone is replaced with a carbomethoxy group (Figure 3). 72,75,77 Eplerenone has a very low affinity for steroid receptors in vitro; however, it has an increased selectivity for the mineralocorticoid receptor, resulting in a comparable bioavailablity with spironolactone in vivo. 72,73,75 With a low affinity for other steroid receptors, eplerenone has fewer progestational and antiandrogenic side effects than spironolactone. 72,74,75,77,78 The mean peak plasma concentration of eplerenone is reached approximately 1.5 hours after oral administration. 41,77 Eplerenone is metabolised to inactive metabolites, primarily to the 6β-OH metabolite SC71597, predominantly by cytochrome P450 3A4 metabolism (CYP3A4). Most of the metabolites are excreted in urine and faeces (67% and 32%, respectively); less than 5% of eplerenone is excreted unchanged. 41,79–81 The elimination half-life of eplerenone is shorter than those of the active metabolites of spironolactone (4–6 hours versus 13.8–16.5 hours). 41,73 As a result, exposure to eplerenone after administration is less prolonged than with spironolactone metabolites and therefore the risk of hyperkalaemia is potentially reduced with eplerenone. 74
A study investigating the binding specificity and the ability to compete for binding sites using mineralocorticoid receptor–glucocorticoid receptor chimeras, showed that the amino acids 804–874, thought to affect the overall shape of the binding pocket of the mineralocorticoid receptor, are essential for the binding of eplerenone, spironolactone and aldosterone. 82 The study showed that the binding determinants of eplerenone and spironolactone were very similar, attributed to their very similar structure – a lactone ring at the C17 position and similar C7 side chains. 82 This demonstrates the similarity in structure and mode of action between these drugs, and therefore their pharmacological exchangeability.
Comparability of the populations in RALES, EPHESUS and AREA IN-CHF
There are several important ways in which the HF populations in these three trials differ from each other, and from current clinical practice.
Beta-blocker usage
Following an MI, beta-blockers decrease myocardial oxygen demand by decreasing heart rate and blood pressure, and increasing coronary artery flow by prolonging diastole. 83,84 They also decrease ventricular arrhythmias by blocking the activation of the sympathetic nervous system. 83 When beta-blockers were first used in HF patients, they were prescribed at full dose, and became contraindicated because of negative inotropic properties (weakening myocardial contractility). 85 Large RCTs conducted in the 1990s of bisoprolol, metoprolol and carvedilol demonstrated reductions in mortality and morbidity in patients with chronic HF with the use of beta-blockers when slow individualised upward titration was applied. 85 This altered method of administration resulted in beta-blockers no longer being considered contraindicated in patients with chronic HF, but an essential component of the medical management of these patients. 85 The number of prescriptions for beta-blockers has risen steeply in the UK, from 14,282,000 in 1991 to 26,810,000 in 2007 in England alone. 86 Even in the relatively short time between RALES and EPHESUS, beta-blocker prescriptions increased in England from 14,375,000 in 1996 to 20,439,000 in 2001. 86
Over the years, beta-blockers have been developed, and their pharmacological properties have changed:83,87
-
first-generation (propanolol; timolol): non-selective antagonists, with equal affinity for beta-1 and beta-2 receptors
-
second-generation (atenolol; metoprolol; bisoprolol): beta-1 selective
-
third-generation (carvedilol): non-selective activity against alpha-1, beta-1, and beta-2 receptors.
Propanolol was the first beta-blocker licensed in the UK, for angina in 1965 and then hypertension in 1969. 88 Atenolol became available in 1976,88 metoprolol in 1997,24 and carvedilol in 2001. 89 The later, thrid-generation beta-blockers have vasodilatory effects,85 and are more effective at lowering aortic pressure than conventional beta-blockers. 87 Carvedilol also has antioxidant,90,91 antiarrhythmic,91,92 and anti-inflammatory91 properties. Currently the most commonly used beta-blockers for HF are metoprolol, bisoprolol and carvedilol. 85
Prior to the standard use of thrombolytics, ACE inhibitors and aspirin, beta-blockers produced a 20–30% reduction in mortality in postMI patients with LVSD; however, it was unclear whether this benefit would be sustained after the introduction of these treatments. 83,93 Retrospective analyses showed continued significant reductions in mortality and progression to severe HF in patients receiving beta-blockers alongside captopril94 or ramipril95 compared with those not receiving beta-blockers. A more recent prospective study showed carvedilol to reduce all-cause mortality by 23%, CV mortality by 25% and non-fatal MI by 41%; of the 1959 patients included, 98% were on ACE inhibitors, 86% were on aspirin and 45% received reperfusion treatment. 96 From these studies it can be seen that despite the introduction of thrombolytics, ACE inhibitors and aspirin into the standard treatment for postMI HF, beta-blockers continue to have a significant benefit in terms of mortality and morbidity and their use is now part of standard management postMI.
As a result, the use of beta-blockers in only 10.5% of the population in RALES, compared with 75% in EPHESUS and 80% in AREA IN-CHF, makes the exchangeability of the populations in these three trials questionable. In addition, it is likely that the type of beta-blocker would vary between RALES and the more recent trials EPHESUS and AREA IN-CHF. With RALES being conducted in 1995–6, patients recruited would not generally have been prescribed beta-blockers, and those that were, would not have been receiving third-generation agents. By the time EPHESUS was conducted in 1999–2001, third-generation beta-blockers would have been available and may have been prescribed to some patients. The use of third-generation beta-blockers was likely to have been common in the AREA IN-CHF trial. The use of third-generation beta-blockers would afford the patients receiving these drugs additional benefits over patients receiving first- or second-generation beta-blockers.
It is also likely that the type of patients prescribed beta-blockers will differ between RALES and the other two trials. Although higher-risk patients gain most from beta-blocker therapy,97 it is unlikely that the 10.5% of patients in RALES who received beta-blockers are those with more severe illness because at the time of the trial, beta-blockers tended to be used on patients with milder disease. In EPHESUS and AREA IN-CHF, beta-blocker use probably encompasses a wide range of disease severity; to confirm this, access to patient-level data would be required. There is also evidence that some patients are more likely to receive treatment with beta-blockers than others, over and above the severity of illness. One study showed that from 7106 patients with non-ST-elevation MI eligible for beta-blocker treatment, early beta-blocker therapy (within 24 hours of admission) was initiated in 76%; patients prescribed a beta-blocker were more commonly younger, and had a previous MI, hypertension or hyperlipidemia. 97 In RALES, patients likely to do well, young men with mild HF, were more likely to receive beta-blockers.
The time between MI and recruitment into the trial
The patients in EPHESUS were randomised between 3 and 14 days postMI. In RALES, patients had HF for at least 6 weeks, and in AREA IN-CHF for at least 3 months. Therefore, the ischaemic patients in RALES and AREA IN-CHF were further ahead in their postMI recovery and were a more stable chronic HF population compared with the EPHESUS population. Evidence shows that the risk of death and CV events is greatest in the first 24 to 48 hours after an MI. This declines over the subsequent 3 to 5 days, with survival curves becoming relatively flat after this period. 98 This would imply that the patients enrolled in EPHESUS would have an increased baseline risk of death and CV events early in the trial because of their recent history of MI, compared with the ischaemic subgroups of RALES and AREA IN-CHF.
The primary mode of action of aldosterone antagonists may differ between acute and chronic HF populations. Therefore, differences between the trials in terms of response may be attributed to the population being treated, rather than the drug administered. Reanalysis of the data from EPHESUS and RALES were reported in the FDA eplerenone medical review. 40 It was noted that approximately 66% of the difference in deaths between eplerenone and placebo occurred within the first 30 days with little continued divergence of the survival curves during the remainder of the trial [Figure 4(A)]. The reductions in deaths in patients taking eplerenone were considered to be primarily the result of a reduction in sudden death, with reductions in deaths due to recurrent MI and HF also prominent. In RALES, the survival curves did not start to diverge until approximately 3 months, with the divergence continuing over the course of the trial [Figure 4(B)]. The major contributors to the reduction in deaths with spironolactone were considered to be reductions in sudden death and the progression of HF.
The diagnosis of MI
In 2000, the European Society of Cardiology and the American College of Cardiology produced a consensus document that changed the way in which MI is diagnosed. A study comparing the patient diagnoses using the old and new systems showed that the new definition increased the number of events diagnosed as MI by approximately 26% (an 11% increase in the number of patients diagnosed with MI); two-thirds of the additional events diagnosed as MI having previously been diagnosed as unstable angina. 3 Therefore EPHESUS and AREA IN-CHF may have a number of patients diagnosed with MI that would not have received that diagnosis in RALES.
The severity of LVSD
The inclusion criteria relating to LVEF varied across the three trials. In RALES, patients had to have an LVEF of 35% or less, compared with 40% or less in EPHESUS, and 45% or less in AREA IN-CHF. A subgroup of patients with severe LSVD in EPHESUS with LVEF < 35% has been investigated. 52 Baseline LVEF was approximately 25% in RALES, 33% in EPHESUS and 38% for the ischaemic group from AREA IN-CHF; the range of LVEF in the ischaemic group from AREA IN-CHF was 16–58%, therefore some patients were recruited who did not have LVSD despite the inclusion criteria.
Other comparability issues
The proportion of patients on ACE inhibitors is similar in both trials; however, the proportion prescribed a diuretic (100% in RALES, 60.5% in EPHESUS and 63% in AREA IN-CHF) and aspirin (36.5% in RALES, 88.5% in EPHESUS and 71% in AREA IN-CHF) varied greatly. Digoxin is indicated for chronic HF; the proportion of patients prescribed digoxin was high in RALES (73.5%), and low in the AREA IN-CHF trial (18%) despite patients having HF for at least 3 months (digoxin use not reported for EPHESUS).
There has been a substantial increase in the use of percutaneous coronary interventions over recent years, making AREA IN-CHF the most likely to reflect current practice. 99
Some outcomes of interest in this review were described differently across the trials. Deaths and hospitalisations were reported as cardiac in RALES and AREA IN-CHF, whereas in EPHESUS they were cardiovascular, which is a broader term including non-cardiac and CV outcomes (such as stroke). As a consequence, the results for these outcomes are not comparable.
The mix of NYHA classifications of the populations varied across the three trials. In RALES most patients were NYHA class III, with the majority of the remaining patients class IV (only 0.5% were class II). In EPHESUS most patients were NYHA class II, with few patients in class IV; 28% were NYHA class I. In AREA IN-CHF, all patients were NYHA class II. The usefulness of this classification system when applied to hopsitalised patients is questionable, as much of the assessment is based on the ability to undertake physical activity and the exacerbation of symptoms, which are less easily assessed in hospitalised patients.
Summary
Although the drugs being evaluated could be considered pharmacologically exchangeable, the populations in which they have been studied are not. In terms of beta-blocker and percutaneous coronary intervention usage, RALES is not comparable to either EPHESUS or AREA IN-CHF, and RALES and EPHESUS are not comparable to current practice. The patients recruited into EPHESUS are early postMI, whereas in RALES and AREA IN-CHF the patients had their ishaemic event at least 6 weeks and 3 months before randomisation, respectively. The patients recruited into RALES were a more chronic HF population than in the other two trials, with HF newly developing in patients in EPHESUS who had only just experienced an MI. EPHESUS and AREA IN-CHF had patients with less severe HF compared with RALES in terms of LVSD and NYHA class. Most patients in RALES were NYHA class III, whereas in EPHESUS most patients were NYHA class II, with few patients in class IV and 28% in class I, and in AREA IN-CHF, all patients were NYHA class II. In addition, not only were there differences in the rate of beta-blocker usage, which was much higher in EPHESUS and AREA IN-CHF compared with RALES, there were likely to be differences in the types of beta-blockers prescribed, with an increase in the more effective third-generation beta-blockers over time.
Adverse events
Adverse events related to spironolactone, canrenone and eplerenone as reported in reference sources (Martindale,33 Meyler’s,37 AHFS,38,39 FDA medical reviews40 and label information,41 and the BNF42) are shown in Table 7. Where information was provided, this was based either on old studies which generally recruited few patients, or case reports. To further investigate the rates of adverse events associated with spironolactone, canrenone and eplerenone, RCTs or observational studies with at least 100 patients treated with one of these drugs were retrieved. Of the nine studies cited in the reference sources, eight were excluded from further consideration as individual studies: three were rejected for being too small to provide reliable rates of adverse events with 30,100 46,101 and 54102 patients, and five others were case reports. 103–107 Therefore a total of 18 studies were identified that met these criteria, one cited in a reference source above,61 three RCTs from the clinical effectiveness section, and 14 studies identified through additional searches (see Figure 2 for the flow of studies in this section). Brief details of the studies that were included are given in Table 8, with further study details provided in Appendix 2.
| Source | Adverse events | |
|---|---|---|
| Spironolactone/canrenone | Eplerenone | |
| Martindale33 |
Rates of hyperkalaemia from a US drug surveillance programme are reported as 8.6% overall, 42.1% in patients with uraemia and 2.8% in patients without uraemia61 Gynaecomastia was reported as occurring in 62%100 to 100%101 of men Gynaecomastia was reported to be reduced in patients with liver cirrhosis101 or eliminated in a patient with hyperaldosteronism103 when prescribed potassium canrenoate |
Not reported |
| Meyler’s37 |
Caused gynaecomastia, reduced libido or erectile disfunction in 4–30% of men Rates of gynaecomastia were reduced in patients with hepatic cirrhosis, from 42% to 20%, with the use of potassium canrenoate |
Not reported |
| AHFS38,39 | A list of potential adverse events was provided, but the rates of these adverse events were not reported | |
| FDA medical reviews/label information40,41 | Not reported |
The pooled estimate for the rate of gynaecomastia from hypertension trials was approximately 0.5% for all trials and 0.7% for trials of at least 6 months duration The proportion of patients in the hypertension trials with a serum potassium over 5.5 mmol/l was 1% or less with doses up to 200 mg/day (674 patients), but rose to 8.7% (104 patients) with a dose of 400 mg/day |
| BNF42 | A list of potential adverse events was provided, but the rates of these adverse events were not reported | |
| Study ID | Study design | Population | Drug regimens |
|---|---|---|---|
| Dose ranging study 011: FDA medical review40 |
Double-blind RCT 1997–8 12-week treatment duration; 16-week follow-up |
321 patients with HF LVEF: 40% or less 56 patients lost to follow-up |
Eplerenone: 25 mg two or four times/day 50 mg four times/day 50 mg four times/day for 1 week then increased to 100 mg four times/day for 11 weeks Spironolactone: 25 mg four times/day Placebo Doses of eplerenone doubled in last 4 weeks; spironolactone remained unchanged |
| Japanese dose ranging study: FDA medical review 4 |
Double-blind RCT 2000–2 12-week follow-up |
161 patients LVEF: 40% or less 26 patients lost to follow-up |
Eplerenone: 25 mg four times/day 50 mg four times/day 100 mg four times/day Placebo |
| Lawson (1982)60 |
Surveillance programme Dates not reported |
783 HF patients taking spironolactone | Spironolactone: dose not reported |
| Greenblatt (1973)61 |
Surveillance programme From 1966; end date not reported |
788 patients treated for fluid retention | Spironolactone: up to 150 mg/day |
| Shah (2006)62 |
Record examination 1999–2004 |
840 patients with congested cardiac failure; 556 patients had laboratory tests results | Spironolactone: dose not reported |
| Smith (1980)63 |
Case series 48-week prospective study |
115 patients with congested cardiac failure with oedema | Spironolactone: 100 mg/day |
| Nassiacos (2005)57 |
Retrospective case series 1995–2003 Abstract only |
124 consecutive paitents admitted with heart failure treated with antialdosterone therapy | Canrenone: mean 37 (± 19.9) mg/day |
| Witham (2004)66 |
Retrospective case series 2001–2 |
226 patients with chronic HF and objective evidence of LVSD | Spironolactone: dose not reported |
| Sligl (2004)67 |
Prospective cohort 1989–2001 |
136 patients with confirmed HF | Spironolactone: mean 24 mg/day |
| Svensson (2003/4)58, 59, 68 |
Prospective case series 1999–2001 |
125 consecutive patients with congested cardiac failure and an LVEF < 45% | Spironolactone: full dose information not reported; 48 patients treated with 50 mg/day at some point |
| Anton (2003)69 |
Retrospective cohort 2000–1 |
110 patients prescribed spironolactone and an ACE inhibitor for whom clinical data could be obtained | Spironolactone: full dose information not reported; 24 patients received >25 mg/day |
| Tamirisa (2004)70 |
Case–control study 1998–2002 |
926 patients with heart failure and a documented LVEF < 35%; 67 cases: discontinued treatment and 134 controls continued treatment | Spironolactone: dose not reported |
| Williams (2006)71 |
Patient record review 1996–2003 |
762 patients prescribed spironolactone | Spironolactone: mean 38.4 (± 1.49) mg/day |
| Hauben (2007)64 |
Rate of spironolactone-associated hyperkalaemia per 1000 reports per year; 1970–2005 Cases identified in USFDA Adverse Event Reporting System (AERS) |
||
| Juurlink (2004)65 |
Population-based time-series; 1994–2001 Rate of spironolactone prescriptions and hyperkalaemia in ambulatory patients Computerised prescription records of the Ontario Drug Benefit Program |
||
The results for hyperkalaemia, gynaecomastia and discontinuations because of adverse events are provided in Tables 9 to 14; other adverse events reported in the studies are provided in Appendix 2. From these tables it can be seen that data relating to adverse events were sparsely reported.
| Study | Definition (mmol/l) | Eplerenone | Spironolactone | Canrenone | Placebo |
|---|---|---|---|---|---|
| EPHESUS; all patients27,40,108 | > 5.5 | 508a/3319 (15.6%) | – | – | 363a/3313 (11.2%) |
| EPHESUS; first 30 days of study109 | 23/3319 (0.69%) | – | – | 15/3313 (0.45%) | |
| RALES40,31 | > 5.5 | – | 83b/822 (10%) | – | 30a/841 (4%) |
| AREA IN-CHF; all patients50 | > 5.5 | – | – | 23/231 (10%) | 8/236 (3.5%) |
| AREA IN-CHF – ischaemic | > 5.5 | – | – | 10/118 (8.5%) | 4/123 (3%) |
| Dose ranging study 01140 | NR | 19/214b (9%a); 25–100 mg/day | 6/46b (13%a) | – | 2/57b (4%a) |
| Greenblatt (1973)61 | NR | – | 68/788 (8.6%) | – | – |
| Shah (2006)62 | ≥ 5.5 | – | 83/556 (15%) | – | – |
| Svensson (2003)58,59,68 | ≥ 5.5 | – | 21/125 (17%) | – | – |
| Anton (2003)69 | ≥ 5.5 | – | 42/110 (38.2%) | – | – |
| Williams (2006)71 | > 5.0 | – | 40/762 (5.3%) | – | – |
| Study | Eplerenone | Spironolactone | Canrenone | Placebo |
|---|---|---|---|---|
| EPHESUS; all patients27,40 | 180a/3319 (5.5%) | – | – | 126a/3313 (3.9%) |
| EPHESUS; patients with poor LVEF 52 | 62b/1048 (5.9%) | – | – | 37b/1058 (3.5%) |
| RALES31 | – | 14/822 (1.7%) | – | 10/841 (1.2%) |
| AREA IN-CHF; all patients50 | – | – | 3/231 (1.3%b) | 2/236 (0.4%b) |
| AREA IN-CHF; ischaemic patients | – | – | 1/118 (0.9%) | 1/123 (0.8%) |
| Lawson (1982)60 | – | 57/783 (7.3%) | – | – |
| Shah (2006)62 | – | 33/556 (6%) | – | – |
| Svensson (2003)58,59,68 | – | 12/125 (9.6%) | – | – |
| Anton (2003)69 | – | 26/110 (23.6%) | – | – |
| Tamirisa (2004)70 | – | 15/926 (1.6%) | – | – |
| Study | Eplerenone | Spironolactone | Canrenone | Placebo |
|---|---|---|---|---|
| EPHESUS; all patients27,40,108 | 22a/3319 (0.7%) | – | – | 10a/3313 (0.3%) |
| RALES31 | – | 9/822 (1.1%) | – | 3/841 (0.4%) |
| AREA IN-CHF; all patients50 | – | – | 13/231 (5.6%b) | 3/236 (1.3%b) |
| AREA IN-CHF; ischaemic patients | – | – | 9/118 (7.6%) | 1/123 (0.8%) |
| Japanese dose ranging study40 | 4a/114 (3.5%b); 25–100 mg/day | – | – | 1/38a (2.6%b) |
| Nassiacos (2005)57 | – | – | 8/124 (6.5%) | – |
| Witham (2004)66 | – | 15/141 (10.6%) | – | – |
| Tamirisa (2004)70 | – | 33/926 (3.6%) | – | – |
| Study | Eplerenone | Spironolactone | Canrenone | Placebo |
|---|---|---|---|---|
| EPHESUS; all patients27,40 | 12a/3319 (0.5%) | – | – | 14a/3313 (0.6%) |
| RALES31 | – | 55/822 (9%) | – | 8/841 (1%) |
| AREA IN-CHF; ischaemic patients | – | – | 0/118 | 0/123 |
| Dose ranging study 01140 | 2a/214b (0.9%b); 25–100 mg/day | 1a/46b (2%b) | – | 0/57b |
| Greenblatt (1973)61 | – | 2/788 (0.25%) | – | – |
| Smith (1980)63 | – | 3/115 (2.6%) | – | |
| Witham (2004)66 | – | 5/141 (3.5%) | – | – |
| Williams (2006)71 | – | 14/762 (1.8%) | – | – |
| Study | Eplerenone | Spironolactone | Canrenone | Placebo |
|---|---|---|---|---|
| RALES31,40 | – | 8a/822 (1.0%) | – | 3a/841 (0.4%) |
| AREA IN-CHF; ischaemic patients | – | – | 0/118 | 0/123 |
| Japanese dose ranging study40 | 0/114a; 25–100 mg/day | – | – | 0/38a |
| Smith (1980)63 | – | 1/115 (0.8%) | – | – |
| Nassiacos (2005)57 | – | – | 2/124 (1.6%) | – |
| Sligl (2004)67 | – | 6/114 (5%) | – | – |
| Study | Eplerenone | Spironolactone | Canrenone | Placebo | |
|---|---|---|---|---|---|
| EPHESUS; all patients27,40 | 163a/3319 (4.9%) | – | – | 155a/3313 (4.7%) | |
| EPHESUS; first 30 days of study109 | 134/3319 (4.0%) | – | – | 139/3313 (4.2%) | |
| EPHESUS; open label extension110 | Drug-related | 75/1425 (5.3%) | – | – | – |
| Not drug-related | 190/1425 (13.3%) | – | – | – | |
| RALES31 | – | 62/822 (8%) | – | 40/841 (5%) | |
| AREA IN-CHF; all patients50 | – | – | 27b/231 (11.7%) | 15b/236 (6.5%) | |
| AREA IN-CHF; ischaemic patients | – | – | 12/118 (10.2%) | 2/123 (1.6%) | |
| Japanese dose ranging study40 |
25 mg: 6/37 (16%)a 50 mg: 7/39 (18%)a 100 mg: 3/38 (8%)a |
– | – | 3/38 (8%)a | |
| Smith (1980)63 | – | 11/115 (9.5%) | – | ||
| Sligl (2004)67 | – | 29/114 (25%) | – | – | |
| Anton (2003)69 | – | 44/93 (47.3%) | – | – | |
Hyperkalaemia
Hyperkalaemia was better reported than other adverse events (Tables 9 and 10). With spironolactone, the rates of hyperkalaemia ranged from 8.6% to 38.2%, and serious hyperkalaemia from 1.7% to 23.6%. Most of the studies evaluating spironolactone did not provide full details of the dose used; it seems that older studies used doses up to 150 mg/day, whereas more recent studies tended to use up to 50 mg/day. Where dose was reported, there did not seem to be a direct dose–response relationship with hyperkalaemia. Fewer studies of eplerenone and canrenone reported the rate of hyperkalaemia and serious hyperkalaemia, but those that did, reported rates within the range reported for spironolactone. Insufficient evidence was available to reliably assess the rate of discontinuation as a result of hyperkalaemia; where data were available the rates for the three drugs seemed to overlap, suggesting that they may be similar (Table 11). Two studies reported that hyperkalaemia was primarily associated with the use of potassium chloride supplements,60,61 with one of these also highlighting the link with renal insufficiency. 61 Hyperkalaemia was considered to have contributed to the deaths of two patients in one study. 61 Reanalysis of the data from EPHESUS and RALES in the FDA eplerenone medical review showed that both eplerenone and spironolactone had greater effectiveness when baseline serum potassium was lower, which was considered most likely to be the result of the reduced risk of arrhythmias. 40
Gynaecomastia
Gynaecomastia is a concern when prescribing drugs that bind to androgen and other steroid receptors, and may reduce compliance. Table 12 shows the rate of gynaecomastia with spironolactone ranging between 0.25% and 9%, with no obvious dose-related response. Data were lacking on the rates of gynaecomastia in eplerenone and canrenone, but where reported, the rates were consistently lower than those of spironolactone, being less than 1% for both drugs (Table 12). This difference between spironolactone and the other two drugs may be reflected in the data for discontinuations because of gynaecomastia; however, data were scarce and the difference was less apparent (Table 13). When considering the discontinuations for any adverse event, the rates varied greatly, with overlap in the rates reported across the three drugs (Table 14). These data suggest that the rate of overall adverse events and hyperkalaemia may be similar across the three drugs, but the rate of gynaecomastia seems to be higher with spironolactone.
Time to adverse events
The rate of adverse events is not the only aspect of interest. The time between commencing treatment and the occurrence of an adverse event is also important. Of the 23 publications reporting adverse events, five provided some information on the time to event (Table 15). 40,60,66,108,109 From Table 15, it can be seen that a large proportion of adverse events occur soon after initiation of treatment. However, late occurring events are not uncommon, as demonstrated by the time to death and hyperkalaemia in EPHESUS. The relationship between study duration and the rate of gynaecomastia in RALES and EPHESUS was discussed in the FDA eplerenone medical review. 40 The median follow-up was reported as 736 days (interquartile range 473–920 days) in RALES and 495 days (interquartile range 366–629 days) in EPHESUS. 40 The median time to the development of gynaecomastia was 677 days (interquartile range 400–867 days) in RALES and 491 days (interquartile range 372–674 days) in EPHESUS. 40 These figures show that gynaecomastia continues to develop beyond the duration of the EPHESUS trial, and that the rates of gynaecomastia reported in EPHESUS may be underestimated.
| Study | Time to adverse event |
|---|---|
| EPHESUS27,108,109 | Eplerenone:
|
| Dose ranging sudy 01140 |
Eplerenone: Spironolactone: |
| Lawson (1982)60 | Spironolactone:
|
| Witham (2004)66 | Spironolactone:
|
Two studies investigated the impact of the publication of RALES on the rate of prescriptions of spironolactone and the rate of spironolactone-associated hyperkalaemia; both studies reported increased rates of prescribing, and reporting of hyperkalaemia, after the publication of RALES. 64,65
Summary
The evidence relating to adverse events associated with aldosterone antagonists is sparcely reported. Where evidence is available it appears that the overall rate adverse events and the rate of hyperkalaemia may be similar across the three drugs, but the rate of gynaecomastia seems to be higher with spironolactone. These data also indicate that although a large proportion of adverse events occur soon after the initiation of treatment, late events do occur, and the lack of long-term data may underestimate the rate of serious adverse events such as hyperkalaemia and gynaecomastia.
Discussion of the clinical evaluation
The original purpose of this review was to systematically evaluate the evidence in order to assess the exchangeability of the aldosterone antagonists spironolactone and eplerenone in patients with postMI HF. Additionally, we aimed to determine whether further research comparing these two drugs in this indication is warranted and potentially cost-effective.
Strengths and limitations
This review employed systematic methods to identify relevant studies, both published and unpublished, and was conducted in a transparent, unbiased and reproducible manner. The searches yielded two non-English trials of spironolactone in postMI populations that had not been previously reviewed, and the assistance of translators was obtained so that the evidence in these studies could be included. In addition, trials of the active metabolite of spironolactone, canrenone, were included to increase the understanding of the action and clinical outcomes associated with this class of drugs, and to further strengthen our evidence base.
To fully evaluate the evidence base for aldosterone antagonists in postMI HF the exchangeability of data between trials was investigated, both in terms of the pharmacology of the drugs and in terms of the populations recruited into the trials, which enabled appropriate decisions regarding synthesis and interpretation of the available studies.
The evidence base was dominated by two large trials, RALES and EPHESUS. Given the age of the trials, neither was considered to be representative of current clinical practice for HF. As this review aimed to inform current practice, this was a major limitation. The AREA IN-CHF trial that evaluated canrenone, being more recent, had the potential of better reflecting current practice, and provided additional information on aldosterone antagonists; however, this drug is not licensed for use in the UK.
Key findings
Efficacy
Searches yielded five RCTs; three evaluated spironolactone,31,48,49 one eplerenone27 and one canrenone. 50 Of the three larger, better-quality trials, one investigated spironolactone (RALES),31 one eplerenone (EPHESUS)27 and one canrenone (AREA IN-CHF). 50 Only one of these trials specifically examined postMI HF (EPHESUS). 27 AREA IN-CHF50 and RALES31 recruited HF patients and some data were available for the ischaemic subgroup. The two remaining spironolactone trials conducted by Tu and Chen (2003)49 and Ruta et al. (2006)48 were small, poorly detailed and seemingly of poor methodological quality.
The relative risk of all-cause mortality was reported in EPHESUS for eplerenone, in RALES for spironolactone and in AREA IN-CHF for canrenone; this was highest in EPHESUS and lowest in AREA IN-CHF. The relative risk of CV mortality was reported only in the AREA IN-CHF trial and EPHESUS, and was lower in the AREA IN-CHF trial. The results of the two small spironolactone trials did not reflect those of these larger, better-quality studies. One reported a far greater reduction in all-cause and CV mortality,49 and the other an increase in all-cause mortality with spironolactone. 48 It is unclear what the three larger trials tell us about the relative efficacy of spironolactone, eplerenone and canrenone in postMI HF, and the relevance of this to current clinical practice. To further investigate this, their exchangeability was explored.
Exchangeability
The structural similarity of spironolactone and eplerenone suggests that these drugs may be interchangeable in terms of efficacy, although eplerenone is likely to have fewer progestational and antiandrogenic adverse effects because of its greater selectivity for the mineralocorticoid receptor and lower affinity for steroid receptors. There were, however, a number of issues that severely limited the exchangeability of the RALES, EPHESUS and AREA IN-CHF trials.
During the years between the conduct of the trials, the drug treatment for HF progressed, in particular, beta-blocker use. Beta-blockers are used in contemporary postMI management because they have been demonstrated to confer significant improvements in all-cause mortality, including sudden death and non-fatal reinfarction. 84 The proportion of patients that used beta-blockers varied from 10.5% in RALES to 75–80% in EPHESUS and AREA IN-CHF. This increase in usage is likely to have conferred increased survival benefits in the newer trials, independent of aldosterone antagonist use. This has been demonstrated in two large trials published since 2000: the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) study showed a 23% risk reduction in all-cause mortality and reductions in CV mortality and non-fatal reinfarction,111 and the Metoprolol CR/XL Randomized Intervention Trial in Heart Failure (MERIT-HF) showed similar significant reductions in patients with severe LVSD postMI. 112 Compounding this effect is the advancement in beta-blocker development, with the more recent trials benefiting from the availability of third-generation beta-blockers that provide vasodilatory effects independent of beta-blockade. 87
Another major difference is the time since MI, which would be expected to impact on mortality and HF progression. The Valsartan in Acute Myocardial Infarction Trial (VALIANT) found that the risk of sudden death or cardiac arrest was highest in the first 30 days after MI in patients with LVSD and/or HF. 113 In this study the rate of sudden death or cardiac arrest with resuscitation was more than six times as high in the first month as after 1 year. Therefore, it can be assumed that the patients in EPHESUS who were within 14 days of their MI were at a greater risk of death than those in RALES and the AREA IN-CHF trial who had been diagnosed with HF for at least 6 weeks and 3 months, respectively. The survival curves (Figure 4) demonstrate the difference in response of the two populations to spironolactone and eplerenone.
Further factors identified as reducing the comparability between RALES, EPHESUS and the AREA IN-CHF trial were the differences in baseline LVEF, and the possible inclusion of a different population as a result of introduction of new criteria for diagnosis of MI in 2000. A lower LVEF has been associated with an increase in rates of both pump failure death and sudden cardiac death;16 an inverse relationship between mortality risk and LVEF has been demonstrated in a study of 1906 patients with chronic HF. 114 Similar patterns of increasing mortality with increasing NYHA class have also been demonstrated. 16,114 The use of the more sensitive measures of troponins T or I as part of the new diagnostic criteria of MI resulted in the identification of smaller infarcts and any amount of myocardial damage (CK-MB used in the old criteria can remain normal with minimal cardiac damage). 1 It is unclear, however, whether this change would have impacted on the populations recruited into the three main efficacy trials. There is a correlation between severity, location, and size of an infarct and LVEF,115 and therefore, EPHESUS and AREA IN-CHF may have included patients with less severe MIs than those recruited into RALES. However, it is possible that the additional patients diagnosed using the criteria would have been those with smaller infarcts, and therefore those less likely to develop LVEF, and may not have met the inclusion criteria for EPHESUS and AREA IN-CHF. In addition, even if the recruitment into trials was affected, a study comparing outcomes of patients diagnosed using the old and new criteria found that the 6-month prognosis was similar in both groups. 3
Other differences between trials that affected their exchangeability were apparent, such as concomitant medication (other than beta-blockers). Diuretic use was considerably more common, and aspirin use was less common in RALES than in EPHESUS and AREA IN-CHF. As diuretics are titrated up according to severity of symptoms, this disparity may have been indicative of the increased severity of symptoms in RALES. According to current NICE guidance, aspirin should be routinely prescribed in patients with HF and atherosclerotic arterial disease22 and has been shown in several trials to decrease the risk of all-cause mortality and reinfarction in postMI patients. 116 It is therefore possible that the low rates of aspirin use in RALES negatively influenced mortality in these patients. The high rate of dignoxin use in RALES compared to AREA IN-CHF suggests that patients in RALES had more severe HF than those in the AREA IN-CHF trial. However, this is not reflected in the inclusion criteria of the two trials, where the time since onset of HF was 6 weeks in RALES and 3 months in AREA IN-CHF. This may reflect prescribing differences between the UK and Italy, rather than the severity of the HF population.
Safety
In addition to their relative efficacy, the relative safety of eplerenone, spironolactone and canrenone was investigated. Little information was provided in reference sources regarding adverse events (see Results of the review of clinical effectiveness; Adverse events). Data were obtained from 13 observational studies, two dose ranging studies, RALES, EPHESUS and the AREA IN-CHF trial. The observational studies were generally of poor quality, being small and with most having no control group; and were not conducted in patients with postMI HF. Consequently, there was a lack of data on the adverse events associated with the aldosterone antagonists.
The rates of hyperkalaemia varied widely for eplerenone, spironolactone and canrenone but were generally higher than those reported for placebo. Data were insufficient to assess discontinuation because of hyperkalaemia. Although the pharmacological differences between aldosterone antagonists suggest that the risk of hyperkalaemia may be less with eplerenone than spironolactone, the adverse event data reviewed did not support this. The data showed no obvious dose–response relationship in the occurrence of hyperkalaemia. This is not altogether unexpected because data from hypertension trials have shown doses up to 200 mg/day as used in the studies in this review had similar, low rates of hyperkalaemia, with the rate increasing only at doses above 200 mg/day. 40
The rates of gynaecomastia were generally higher with spironolactone, consistent with the lower affinity of eplerenone for androgen and progesterone receptors compared with spironolactone. This could result in improved compliance with eplerenone compared with spironolactone, which could have implications for clinical effectiveness and cost-effectiveness. However, data relating to the discontinuation of treatment because of adverse events were poorly reported, and were insufficient to determine a relationship. As with hyperkalaemia, the data showed no obvious dose–response relationship in the occurrence of gynaecomastia; however, this result was not expected. The FDA medical review highlighted the possibility that the higher rates of gynaecomastia in RALES could be a result of either the more effective doses used, or the longer duration of the trial; gynaecomastia is likely to become apparent with higher doses and longer durations of treatment. 40 Therefore the lack of a dose–response for this outcome may be primarily the result of the lack of data.
Time to adverse event data was also sparse and few useful data were obtained. The FDA medical review highlighted the possibility that the higher rates of gynaecomastia seen with spironolactone in RALES could be a result of the longer duration of the trial, as gynaecomastia is likely to become apparent with longer durations of treatment. 40
Given the paucity of good-quality evidence and the diversity of dosages and populations from which data were derived, it was not possible to draw reliable conclusions.
Conclusions
The only good-quality trial evidence for aldosterone inhibitors in the postMI HF population comes from a trial of eplerenone (EPHESUS) and spironolactone has been studied in HF in RALES. The lack of exchangeability of these trials with respect to study populations, beta-blocker use and other issues such as concurrent medication, means that a simple indirect comparison between these drugs using these trials could not produce clinically meaningful results. As a consequence, to evaluate the efficacy of spironolactone in postMI HF patients, a contemporary trial comparing eplerenone and spironolactone directly appears warranted. However, whether this would be worthwhile from a cost-effectiveness and clinical standpoint is unknown.
To further explore the relative efficacy and cost-effectiveness of eplerenone and spironolactone in postMI HF, and to determine the potential cost-effectiveness of a future trial, a decision model will be developed.
Chapter 4 Systematic review of existing cost-effectiveness evidence
Methods
A broad range of studies were considered for inclusion in the assessment of cost-effectiveness, including economic evaluations conducted alongside trials, modelling studies and analyses of administrative databases. Only full economic evaluations that compared two or more options and considered both costs and consequences (including cost-effectiveness, cost–utility or cost–benefit analyses) were included.
The following databases were searched for economic evaluations of spironolactone, eplerenone, canrenone and canrenoate potassium for postMI HF or HF:
-
NHS Economic Evaluations Database – www.crd.york.ac.uk/crdweb/.
-
Health Economic Evaluation Database – www.interscience.wiley.com/cgi-bin/mrwhome/114130635/HOME.
-
IDEAS – http://ideas.repec.org/.
Full details of the main search strategy for this review are presented in Appendix 1.
Two reviewers independently assessed all obtained titles and abstracts for inclusion. Any discrepancies were resolved by discussion and consultation with a third reviewer. All studies meeting the inclusion criteria were summarised and used as the basis for identifying major structural issues, assumptions and key drivers of cost-effectiveness. The quality of the cost-effectiveness studies was assessed according to a checklist updated from that developed by Drummond and Jefferson. 117 This information is summarised within the text of the report, alongside a detailed critique of the study and its relevance to the UK NHS.
Results
The systematic literature search identified 31 references, of which six studies met the inclusion criteria for the cost-effectiveness review. 29,118–122 None of these studies attempted to undertake a direct comparison of the cost-effectiveness of spironolactone versus eplerenone. Two of the studies reported the cost-effectiveness of spironolactone versus standard care in patients with severe chronic HF and the remainder evaluated the cost-effectiveness of eplerenone versus standard care in patients with HF after acute MI (AMI). All of the epleronone studies29,119,121,122 were based on the same general approach and methods originally reported in the cost-effectiveness study by Weintraub et al. 29 with country-specific estimates applied to particular inputs (e.g. resource use and unit costs). Given the significant overlap between these country-specific cost-effectiveness studies and the original study by Weintraub et al. ,29 only the latter is considered in detail within this review. A useful summary of the wider set of cost-effectiveness studies for eplererone has been previously published. 33
The following sections provide a detailed critique of the cost-effectiveness evidence from the three main studies included29,118,120 and an assessment of the quality and relevance of the data from the perspective of the UK NHS. A quality assessment checklist for each study is provided in Appendix 4.
Review of Tilson et al. (2003). Cost-effectiveness of spironolactone in patients with severe heart failure
Overview
The study by Tilson et al. 120 was designed to establish the cost-effectiveness of spironolactone when added to standard therapy in patients with severe chronic HF in an Irish health-care setting. The two treatment strategies were: spironolactone in combination with standard therapy and standard therapy alone. Standard therapy could include a loop diuretic, an ACE inhibitor, digoxin, nitrate, a beta-blocker, or a combination of these. The study started with the hypothesis that the use of spironolactone in addition to standard therapy may be cost-effective because it reduces mortality and hospital admission rates. The main outcomes assessed were the probabilities of death and hospitalisation under the alternative treatment strategies.
The study was based on a deterministic, Markov decision-analytic model with three health states defined as: severe HF; severe HF with hospitalisation; and death. The model was evaluated over a 10-year time horizon employing annual cycle lengths. The study population comprised a hypothetical cohort of patients with similar characteristics to patients in the RALES trial. On average, patients were 65 years old with severe HF (defined as NYHA class III and IV with LVEF ≤ 35%). The perspective of the study was not explicitly reported, but it could be inferred to have been that of the hospital.
Summary of effectiveness data
The probabilities of death (0.26) and hospitalisation (0.38) for patients on standard therapy alone were obtained from a cohort of patients attending an Irish teaching hospital over a 12-month period. The differences in the probabilities of death and hospitalisation for patients treated with spironolactone plus standard therapy compared with standard therapy alone were obtained from RALES. 31 The corresponding probabilities were 0.18 and 0.25 for death and hospitalisation, respectively. After the mean follow-up period of RALES of 2 years, the probabilities of mortality and hospitalisation among the patients on spironolactone were assumed to revert to those of patients receiving standard therapy alone over the subsequent 8 years of the model.
Summary of resource utilisation and cost data
Costs associated with treatment (drug acquisition costs of spironolactone), hospitalisation for the treatment of severe HF and outpatient clinic visits were considered. Drug costs were obtained from the Irish Monthly Index of Medical Specialities and the cost of spironolactone was based on the mean dose prescribed in RALES (25 mg/day). The cost of hospitalisation (€3019) and outpatient visits (€83 per patient per visit) were based on costs within the Irish teaching hospital centre. 123 The costs were calculated in Irish pounds in 2000, converted to euros and inflated to 2000–1 (5.6%) and 2001–2 (4.9%) prices using the annual consumer price index. Costs were discounted at an annual rate of 5%.
Summary of cost-effectiveness data
The measure of benefit used in the cost-effectiveness study was life-years gained (LYG). An annual discount rate of 1.5% was applied to outcomes. The addition of spironolactone to standard therapy for the management of chronic severe HF was estimated to be more effective, in terms of LYG, but more costly than standard therapy alone. The incremental cost-effectiveness ratio (ICER) was €466 per LYG for treatment with spironolactone. An ICER of €20,000 per LYG was considered by the authors to be cost-effective in this setting. The favourable cost-effectiveness results are largely driven by the relatively low drug acquisition costs for spironolactone combined with significant clinical benefits in terms of associated reductions in subsequent hospitalisations and additional survival benefits.
A series of one-way and two-way sensitivity analyses were performed on the cost of hospitalisation, the number of outpatient visits, and the probabilities of death and hospitalisation. When the cost of hospitalisation was reduced to €1060 the ICER increased to €728 per LYG. When the cost of hospitalisation was increased to €9319 the ICER was dominated, indicating that spironolactone was both more effective and less costly than standard therapy. Increasing the number of outpatient visits required with spironolactone treatment compared with standard therapy, from one extra visit to four extra visits, increased the ICER to €1136 per LYG. Varying the probability of hospitalisation from 0.21 to 0.29 and the probability of death from 0.16 to 0.21 resulted in an ICER ranging from €309 to €624 per LYG. All the sensitivity analyses demonstrated an ICER in the range of €75 to €1136 per LYG, indicating that the addition of spironolactone to standard therapy for patients with severe chronic HF appeared both highly cost-effective and robust to alternative costing assumptions.
Commentary
The study employs a simple decision model to represent the main outcomes of death and hospitalisation for patients with severe HF receiving spironolactone. No details are reported on whether a systematic approach was employed to inform the effectiveness data and other inputs into the model. Similarly there is limited discussion on the validity of the assumptions employed within the model itself.
The source of effectiveness estimates come from two separate studies with follow-up periods of 1 and 2 years. The data reported in these studies are subsequently extrapolated over a 10-year time horizon assuming that the mortality and hospitalisation rates applied to standard care remain constant over time and that after 2 years the event rates for spironolactone revert to those of standard care. Neither of these assumptions is discussed in detail and no attempt is made to validate them. Furthermore, no justification is provided for employing a 10-year time horizon, and this appears to be a relatively arbitrary choice that may not fully capture the LYG arising from the mortality differences modelled within the initial 2-year period. Although a sensitivity analysis is performed to assess the probabilities of hospitalisation and mortality in patients treated with spironolactone within the first 2 years, no assessment was made of the potential impact of employing alternative assumptions over a longer time horizon.
The perspective of the analysis was not stated and it is unclear whether all appropriate cost components were included. Resource use and costs were not reported separately, which limits the generalisability of the data to other settings. The only costs considered where hospitalisation, drug costs and number of outpatient visit costs. Any additional costs which could differ between the two treatment arms were not discussed.
From a UK NHS perspective, the study has a number of additional limitations: the baseline event rates applied to standard care and the resource utilisation and costs are specific to Ireland, and as such, may not be relevant to the UK; and the effectiveness of spironolactone was measured in terms of changes in life expectancy rather than quality-adjusted life-years (QALYs), which limits the comparison of the achieved health benefits with other interventions in the UK NHS.
Review of Glick et al. (2002). Economic evaluation of the randomised aldactone evaluation study (RALES): treatment of patients with severe heart failure
Overview
The study by Glick et al. 118 uses individual patient-level data from RALES to assess the cost-effectiveness of spironolactone plus standard treatment versus standard treatment alone for patients with severe HF. Standard therapy consisted of an ACE inhibitor and a loop diuretic, with or without digoxin. The primary outcome from the trial was all-cause mortality and CV hospitalisations were a secondary outcome. These were combined in cost-effectiveness analysis to estimate QALYs. The time horizon applied in the cost-effectiveness analysis was restricted to the follow-up period from RALES (approximately 35 months).
The study adopted a previously published Markov decision-analytic model, which was developed to assess functional status through NYHA class and quality-adjusted survival over the lifetime of patients. 124 At each follow-up visit in RALES, NYHA class was evaluated. This was used to estimate the proportion of time that study participants were in each of the four NYHA classes during each month. Quality-adjusted survival was assessed by multiplying the number of years spent in the NYHA class states by a set of quality-adjustment factors. The factors were derived from the responses of 1601 participants in a study of LV dysfunction124 to a visual analogue scale. The Ladder of Life questionnaire was used to rate the health of patients in the four NYHA classes as a fraction of healthy life. The study population comprised a hypothetical cohort of patients with similar characteristics to patients in RALES. Patients were on average 65 years old with severe HF (defined as NYHA class III and IV with LVEF ≤ 35%). The study reports a truncated societal perspective that was limited to the evaluation of direct medical costs. The exclusion of productivity costs suggests that the perspective was more akin to that of a health-care provider/payer.
Summary of effectiveness data
The effectiveness data were based on the first 35 months of observation in RALES. The average survival time during this follow-up for patients who received spironolactone was 2.28 years. This was 0.22 years longer than the average survival time of 2.07 years among patients who received standard therapy. Spironolactone therapy also led to improved functional status evaluated by NYHA class. Of the 0.22-year increase in survival, 0.05 years was spent in NYHA class I and 0.13 years was spent in class II.
The primary health outcome used in the analysis was QALYs. The quality-adjustment factors were 0.71, 0.61, 0.52 and 0.47 for NYHA classes I to IV, respectively. During the 35 months of follow-up, patients who received spironolactone experienced 1.27 QALYs, whereas those who received standard care in the same period experienced 1.14 QALYs. This corresponds to a gain of 0.13 QALYs associated with spironolactone therapy.
Summary of resource utilisation and cost data
The costs of spironolactone, non-fatal and fatal hospitalisations, ambulatory care and deaths outside the hospital were included in the estimates of total costs. Costs were reported in US dollars for the year 1999 and were discounted at an annual rate of 3%.
Resource utilisation was based on data collected in RALES. Treatment-specific monthly probabilities of all-cause non-fatal hospitalisations were also derived from RALES. Total hospitalisations during the 35 months of follow-up were estimated by multiplying the monthly probabilities by the survival time in a month. Given that RALES was a multicentre trial with participants enrolled from 16 countries, cost estimates for hospitalisation were derived from a number of separate countries. Daily costs of hospitalisation were derived from Belgium, Brazil, France, Spain and the UK (these five countries enrolled 70% of the trial participants). The per-patient total hospital costs in these countries were estimated by multiplying days in the hospital (by reason for admission) by the admission-specific daily cost estimates. Hospitalisation costs for the developing countries of Mexico, South Africa and Venezuela were estimated by multiplying days in hospital by the daily cost estimates of Brazil. For the other eight countries (Australia, Canada, Germany, Japan, the Netherlands, New Zealand, Switzerland and the USA), the days in hospital were multiplied by the admission-specific average cost estimates from Belgium, France, Spain and the UK.
The cost associated with death outside the hospital was estimated to be $1000, which included the costs of an ambulance, emergency services and emergency department care. A year of ambulatory care was estimated to be $436. The cost of spironolactone treatment was based on the US average wholesale price of $48.30 per 100 25-mg tablets.
Summary of cost-effectiveness data
One thousand bootstrap replications of total costs and QALYs for patients receiving spironolactone or standard therapy during the first 35 months of RALES were estimated. The mean total costs associated with spironolactone treatment were $8762, whereas the mean total costs for standard care were $9475, giving an average cost saving of $713 with spironolactone treatment. Given that spironolactone therapy lengthens quality-adjusted survival time by an average of 0.13 years, spironolactone was reported to dominate standard care (i.e. spironolactone resulted in both lower costs and higher QALYs). A total of 80.4% of the bootstrap replications fell in the dominant, south-east quadrant of the cost-effectiveness plane. The other 19.6% of replications fell in the north-east quadrant, where spironolactone therapy increased costs and QALYs. The 95% CI for incremental costs and QALYs indicated that spironolactone therapy either dominated placebo (lower limit) or had a cost per QALY ratio as high as $6650 (upper limit). Based on the results of the bootstrap, the probability that spironolactone has a cost-effectiveness ratio below $20,000 per QALY is 1.
A series of univariate sensitivity analyses were performed based on assumptions used in the model. Five variables were considered: (1) survival benefit (varied by ± 33%), (2) costs of spironolactone (varied by ± 50%), (3) daily costs of hospitalisation (varied by ± 50%), (4) ambulatory care costs (varied by ± 50%), and (5) discount rate (0% and 7% on both costs and outcomes). In all cases, the average incremental cost and QALYs indicated that spironolactone dominated standard therapy. The maximum upper limit of the 95% CI was $9050. An additional sensitivity analysis was undertaken to address the impact that gynaecomastia may have on quality of life. This was achieved by assuming that (1) gynecomastia had an additional quality-adjustment factor of – 0.10, and (2) patients who developed this side effect experienced it for the 35 months of follow-up. The impact on QALYs was a reduction of 0.02 associated with spironolactone. The upper limit of the ICER was $8050 per QALY gained.
A best-case and worst-case scenario was reported by combining the variables that led to the most optimistic results for spironolactone and the variables that led to the most pessimistic results. In the best-case scenario, spironolactone dominated standard therapy with an upper 95% CI of $2400. In the worst-case analysis, spironolactone again dominated standard therapy but with a higher upper 95% CI of $20,300 per QALY gained.
Commentary
The study appears comprehensive and well conducted. The authors report that the patient population relates to severe HF and therefore the results should not be extrapolated to patients with less severe heart disease. It should also be noted that the study is based on a time horizon comparable to the follow-up period reported in RALES. Consequently no attempt is made to quantify any additional benefit or costs accruing beyond the 35 months of follow-up. However, given the dominance results reported within the current study, a longer time horizon is unlikely to alter the study conclusions. From a UK NHS perspective, the study has a number of potential limitations because many of the data employed are not specific to a UK setting. For example, resource utilisation for hospitalisations and their associated costs were derived from 16 countries participating in RALES and the generalisability and transferability of the data to a UK setting is a major issue.
Review of Weintraub et al. (2005). Cost-effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure
Overview
The study by Weintraub et al. 29 was designed to evaluate the cost-effectiveness of eplerenone compared with placebo in patients with LVSD and HF after AMI. The study uses data from EPHESUS where patients were randomised to eplerenone or placebo and followed up for a mean time of 16 months. The two treatment strategies included standard optimal therapy, which could consist of ACE inhibitors or angiotensin receptor blockers, diuretics, beta-blockers, statin therapy and coronary reperfusion. The two primary outcomes from the trial were time to death from any cause and time to death caused by CV causes or first hospitalisation for a CV event. Secondary outcomes included death from CV causes and death from any cause or any hospitalisation. These outcomes were included in the cost-effectiveness analysis to estimate LYG and QALYs over a lifetime time horizon.
Lifetime costs and life-years for the two treatment strategies were derived from combining in-trial estimates of event rates and resource utilisation with estimates of lost life expectancy associated with in-trial deaths derived from three separate observational sources. The primary analysis was based on estimates of lifetime cost-effectiveness in terms of cost per LYG. Results of a sensitivity analysis reporting cost-effectiveness using cost per QALY gained were also presented. Costs were expressed in $US using 2001 as a base year (with the exception of the cost of eplerenone which was not marketed until 2004) and both costs and outcomes were discounted using an annual rate of 3%. A societal perspective is stated, although the evaluation of costs was restricted to direct medical care costs only and the actual perspective appears closer to that of a health-care provider/payer.
Summary of effectiveness data
Mortality estimates were derived directly from EPHESUS. The average survival at 1 year was 88.2% for the eplerenone group and 86.4% for the placebo group. These mortality estimates were used as the basis for estimating LYG and QALYs associated with the alternative treatments. Estimates of lost life expectancy associated with the in-trial deaths were derived from three sources: the Framingham Heart Study,125 the Saskatchewan Health database126 and the Worcester Heart Attack Registry. 127 Three sources were used because there was not a single source that was considered to be ideal for the purposes of extrapolation. These sources were used to derive long-term estimates of the hazard of mortality according to particular patient characteristics. For patients who died within the trial, life-years lost were obtained by subtracting the in-trial survival times from the estimated age-specific and sex-specific life expectancy estimates. Patients were considered to have zero life-years lost if they survived the trial period. Average life-years lost for each of the treatment groups were calculated across all patients who survived or died in each arm of the trial.
Quality-adjusted survival was considered as part of a sensitivity analysis. This approach used quality of life data collected within EPHESUS using a generic instrument, European Quality of Life-5 Dimensions (EQ-5D) from a subset of patients from English-speaking countries (1792 patients at baseline, 1530 patients at 6 months and 1123 patients at 12 months). QALYs were then estimated by multiplying survival rates at particular time points by the corresponding utility values derived from EQ-5D. The utility values with eplerenone and placebo were 0.637 and 0.638 at baseline, 0.764 and 0.763 at 6 months and 0.802 and 0.7709 at 1 year, respectively. None of these differences was reported to be statistically significant. In the absence of data on quality of life after 12 months, the utility values reported at 12 months were carried forward.
Summary of resource utilisation and cost data
The study considered the direct medical-care costs associated with hospitalisations, outpatient procedures and drugs incurred during the follow-up period for EPHESUS. The additional health-care costs attributed to life-years gained by treatments were estimated as part of a sensitivity analysis.
Initial and subsequent hospitalisations from case-report forms from patients in EPHESUS were assigned to a diagnosis-related group, as used in the Medicare programme in the USA, by an investigator blinded to treatment group. Costs for each diagnosis-related group were estimated from average Medicare reimbursement rates and professional costs were estimated by percentage share by diagnosis-related group. Outpatient procedures were coded by a blinded investigator and costs were assigned based on the Medicare fee schedule. The costs of medications were based on the Red Book average wholesale price.
The additional health-care costs attributed to additional years of life saved by the treatments were estimated by calculating the cost in each arm of the trial for each year of follow-up, and carrying forward the average cost estimate from years 2 and 3 of the trial in subsequent years considered in the extrapolation.
Summary of cost-effectiveness data
Bootstrap methods were employed to quantify the uncertainty surrounding the estimates of cost-effectiveness expressed in terms of the probability that each intervention was cost-effective at a threshold of $50,000 per LYG.
The ICER per LYG for eplerenone compared with placebo was reported to be $13,718 (Framingham), $21,876 (Saskatchewan) and $10,402 (Worcester). The corresponding probabilities that eplerenone was cost-effective at a $50,000 per LYG threshold were 0.967, 0.938 and 0.988. These ICER estimates increased to $21,072 (Framingham), $30,349 (Saskatchewan) and $17,374 (Worcester) when the costs resulting from additional LYG were added.
The ICER per QALY gained for eplerenone compared with placebo were higher than the estimates based on LYG because quality of life was assumed to be < 1. The ICER per QALY gained, excluding the costs incurred during additional LYG, were $20,579 (Framingham), $32,405 (Saskatchewan) and $15,330 (Worcester). These ICER estimates increased to $29,469 (Framingham), $43,301 (Saskatchewan) and $23,724 (Worcester) when the costs resulting from additional LYG were added.
A range of subgroups were also considered based on age (> 65 or < 65 years), gender, presence or absence of diabetes and presence or absence of a previous AMI. The ICER for these subgroups ranged from $10,000 to $21,000 per LYG, with the exception of patients with diabetes for whom the ICER was $42,160.
Commentary
In general the study appears comprehensive and well conducted. The authors attempted to appropriately quantify the longer-term survival gains (and quality-adjusted estimates) attributed to differences in the in-trial estimates of mortality between eplerenone and placebo reported in EPHESUS. Uncertainty in the extrapolation of LYG because of in-trial mortality was also explored using a range of alternative sources. In addition, the additional costs that may be incurred because of longer survival were also considered. Finally, variation in the cost-effectiveness estimates was explored using subgroup analyses. Importantly, the ICER results remained relatively robust to a range of alternative assumptions and approaches.
Although the study is of high-quality, from a UK NHS perspective, the study has a number of potential limitations. First, estimates of the in-trial mortality and the longer-term projections of survival gains are derived from trial and US data sources and may not be generalisable to current practice in the UK. Second, estimates of quality of life and the costs assigned in the additional LYG, over the longer-term time horizon, are based on the assumption that both of these elements (and any difference between the groups) remain constant in both arms over a lifetime time horizon and no attempt is made to consider alternative assumptions for either of these elements. Finally, it should be noted that the extrapolation approach employed only captures the impact of differences based on in-hospital mortality on the overall estimates of LYG. Consequently, the potential prognostic benefits that may arise as the result of differences in non-fatal events (e.g. recurrent AMI, deterioration in HF) observed during the trial period are not considered within the estimates of LYG.
Discussion
The review of existing economic evidence identified three main published studies evaluating the cost-effectiveness of aldosterone antagonists as an adjunctive therapy to standard care, for either severe chronic HF or for patients with HF after AMI. None of these studies attempted to undertake a direct comparison of the cost-effectiveness of spironolactone versus eplerenone in either of these populations. Two of the three studies considered were based on the results from RALES and both were conducted before results from EPHESUS were available. 118,120 Consequently, the exclusion of eplerenone can be justified on the basis of data availability at the time the studies were conducted. The third study by Weintraub et al. 29 evaluated the cost-effectiveness of eplerenone compared with standard care alone based on the results from EPHESUS. Although spironolactone was acknowledged by the authors as a potentially relevant comparator, the lack of any direct evidence comparing eplerenone to spironolactone led them to conclude that any comparison would be speculative. The methods and approaches reported by Weintraub et al. 29 have since been used within a series of related publications reporting country-specific estimates of cost-effectiveness for eplerenone compared with standard care derived from applying country-specific sources of resource utilisation and costs. These individual studies were not considered within this review given the significant overlap with the earlier publication by Weintraub et al. 29 and the absence of a UK-specific analysis.
Despite the different approaches and assumptions employed, all of the studies consistently reported that the addition of an aldosterone antagonist to standard care for patients with either severe chronic HF or for patients with postMI HF appeared to be highly cost-effective compared with standard care alone. In addition, the results in each of the studies appeared relatively robust to a range of alternative assumptions or inputs. However, despite the consistent findings emerging from these studies, a number of important uncertainties remain surrounding the potential generalisability of these results to the UK NHS. Furthermore, the lack of existing evidence on the potential cost-effectiveness of eplerenone compared with spironolactone presents an important limitation to current decision-making.
Two of the three studies were based directly on the results of RALES and EPHESUS. 29,118 While the use of RCT data as the main source of evidence provides a high level of internal validity, the external validity and generalisability of these findings to clinical practice remains uncertain. The study by Tilson et al. 120 attempted to evaluate the potential impact of spironolactone within the context of current clinical practice by combining evidence on the relative effectiveness of spironolactone from RALES with data on the absolute event rates associated with standard care reported in an Irish setting. However, data for standard care were derived from a single acute teaching hospital in Ireland and the assumptions employed to extrapolate this data from a 1-year time horizon to a 10-year time horizon were not considered to have been sufficiently justified. Issues concerning the generalisability of the clinical data applied within existing studies to an NHS setting, combined with the application of non-UK sources to key resource utilisation and cost assumptions, meant that none of the three studies reported results that were considered to be directly applicable to the UK NHS.
Although one of the studies118 provided an evaluation of cost-effectiveness over a similar time horizon to the follow-up period reported in RALES, the extrapolation of the within-trial results to a longer time horizon was central to the other two studies. The extrapolation was considered necessary to appropriately quantify the estimate of LYG due to the mortality differences from the within-trial results. When the primary outcomes of interest for cost-effectiveness are expressed in terms of life-years or QALYs gained, the consequences of dying (i.e. the subsequent loss in life-years and QALYs) during the trial period can only be appropriately quantified by considering the remaining life expectancy for a patient who survives until the end of the trial follow-up period. Inevitably this requires the extrapolation of survival estimates over a longer time horizon and preferably over a lifetime time horizon if differences in LYG are to be fully captured. For this extrapolation, both of the studies used observational data sources relevant to the specific settings considered within each study. However, the extrapolation approach employed by Tilson et al. 120 was considered to be subject to a number of potential limitations and associated uncertainties arising from employing an arbitrary 10-year time horizon and using relatively short-term mortality data (12 months) from a single acute hospital as the basis for informing the longer-term mortality rates. In contrast, the approach employed by Weintraub et al. 29 used several large observational datasets with long-term follow-up and used these datasets to estimate the remaining life expectancy over a lifetime time horizon.
A potentially important limitation of the extrapolation approaches employed by both Tilson et al. 120 and Weintraub et al. 29 is that neither study attempted to project longer-term mortality differences because of non-fatal events arising during the trial period within the calculations used to estimate LYG and QALYs. Hence, the cost-effectiveness results reported in both studies may be considered potentially conservative estimates. Although the inclusion of this element is unlikely to alter the cost-effectiveness conclusions, it may have an important effect on the uncertainty surrounding the cost-effectiveness results, which could have important implications when it comes to estimating the costs of decision uncertainty using VOI approaches.
Although the extrapolation approach employed by Weintraub et al. 29 incorporates estimates of the additional costs and quality of life resulting from the additional life-years saved, it is important to note that this approach does not explicitly model the longer term prognosis of a patient in terms of the likelihood of their experiencing further non-fatal events over their remaining lifetime. Instead, the approach employed assumes that the differences in the average costs and quality of life reported for each treatment, at between 2- to 3-years postrandomisation within EPHESUS, will remain at this level over the longer term. However, if either the incidence of non-fatal events or the severity of HF increases, then the approach employed by Weintraub et al. 29 will potentially underestimate the costs and overestimate the quality of life assigned to the additional life-years saved, and the resulting cost-effectiveness estimates may not be reliable.
A final limitation of the existing cost-effectiveness evidence is that none of the studies have considered the potential cost-effectiveness of alternative treatment durations with aldosterone antagonists. Although different approaches and assumptions were applied within the studies for the purposes of extrapolating the effectiveness data, all of the studies assumed that treatment with either spironolactone or eplerenone would not be continued beyond the follow-up period of the trials.
Although several potential limitations have been identified concerning the current cost-effectiveness evidence for aldosterone antagonists, there are two particular issues that are likely to represent major challenges to ensuring current NHS decision-making: (1) the lack of prior cost-effectiveness evidence for either eplerenone or spironolactone from a UK NHS perspective and (2) the absence of existing studies comparing the cost-effectiveness of eplerenone and spironolactone directly. Although epleronone and spironolactone are clearly relevant comparators for the treatment of postMI HF from an economic perspective, there are real concerns about the feasibility of such a comparison because of the limited data on spironolactone in this specific indication, and the lack of clinical data directly comparing the two drugs. 29,33,35
The next chapter presents the methods and results of a new decision analytic model that has been specifically developed to address both of these limitations more formally. Central to this model is the need to provide estimates that are more relevant to the UK NHS and the feasibility of undertaking a direct comparison between the alternative aldosterone antagonists.
Chapter 5 The York Economic Assessment
Overview
The review of cost-effectiveness studies in Chapter 4 identified a number of potential limitations of previously published studies in relation to the cost-effectiveness of aldosterone antagonists in the UK. In particular, the lack of previous studies evaluating the cost-effectiveness of both eplerenone and spironolactone, and the absence of UK studies reporting the cost-effectiveness of aldosterone antagonists for the management of postMI HF more generally, represent important evidence gaps for decision-making in the NHS. A new decision analytic model was therefore developed to more formally assess and compare the cost-effectiveness of spironolactone and eplerenone in patients with postMI HF in the NHS.
In developing and populating the decision model there were three issues that were considered central to the approaches and methods employed:
-
the need to extend the evidence ‘network’ considered in Chapter 3 to address the issues identified regarding the exchangeability of the trials and populations to facilitate an indirect comparison of eplerenone and spironolactone
-
the requirement to extrapolate outcomes beyond the time horizon of the main RCTs to ensure that differences in LYG and QALYs were appropriately quantified
-
the need to ensure that the data inputs and assumptions were relevant to informing current NHS practice.
The decision analytic model provides a framework for combining data from the wider evidence ‘network’, assumptions concerning the longer-term impact of the use of eplerenone and spironolactone on mortality and quality of life, and other inputs reflecting current NHS practice, to evaluate the cost-effectiveness of the alternative therapies. The model was developed using Microsoft excel and the evidence synthesis was undertaken using winbugs.
The model considers the long-term prognosis of postMI HF patients to capture the long-term costs and consequences associated with the natural history of these patients in the absence of aldosterone antagonists. The evidence synthesis approach considers the effect of using either spironolactone or eplerenone in addition to standard care. The model is made up of two parts: a short-term element, which relates to a period of 3 months after a patient presents with an AMI, and a long-term element, which extrapolates a patient’s lifetime costs and outcomes conditional on surviving the first 3 months. The model evaluates costs from the perspective of the NHS and Personal Social Services, expressed in UK £ sterling at a 2008–9 price base. Outcomes in the model are expressed in terms of QALYs. Both costs and outcomes are discounted using a 3.5% annual discount rate, in line with current NICE guidelines. 128 All stages of the work were informed by discussion with our clinical advisors, who provided feedback on specific aspects of the analysis such as the model structure, data inputs and assumptions.
The model is probabilistic in that input parameters are entered into the model as probability distributions to reflect uncertainty in the mean estimates. 129,130 Monte Carlo simulation is used to propagate uncertainty in input parameters through the model in such a way that the results of the analysis can also be presented with their associated uncertainty. The probabilistic analysis also provides a formal approach to quantifying the consequences associated with the uncertainty surrounding the model results and can be used to identify priorities for future research.
The following sections outline the decision problem, the structure of the model, and an overview of the key assumptions and data used to populate the model.
Treatment strategies and population
The decision problem addressed by the model relates to the cost-effectiveness of spironolactone and eplerenone for the treatment of patients with postMI HF compared with standard care without aldosterone antagonists. The model considers this in the context of patients presenting with an AMI and at the start of their HF development. The base-case population is based on a hypothetical cohort of patients with similar baseline characteristics of the population who entered EPHESUS under the assumption that this trial population is representative of postMI HF patients. The decision model evaluates three treatment strategies:
-
treatment with spironolactone as an adjunct to standard therapy
-
treatment with eplerenone as an adjunct to standard therapy
-
treatment with standard therapy alone.
In projecting to the lifetime of patients, assumptions concerning the duration of treatment and the duration of the effect of treatment need to be made. In the base-case analysis, treatment with spironolactone and eplerenone was assumed to be 2 years, consistent with the follow-up of the main RCTs. However, a range of additional scenarios were also explored to examine the robustness of alternative assumptions including the impact of different treatment durations.
Model structure
The model is made up of two components: a short-term element, which characterises a period of 3 months, and a long-term element, which considers the costs and outcomes over the remaining lifetime of a patient conditional on surviving the first 3 months. The period represented by the short-term model captures the initial acute postMI period when the impact of treatment on the rates of CV death or hospitalisations for CV events may differ depending on the time since MI. A 3-month period was chosen following the advice of clinicians who would define the acute postMI period as 6–12 weeks. Separating the acute and longer-term periods also provides additional flexibility to allow different data and assumptions to be employed within these periods. This was considered particularly important for the purposes of extrapolation and ensuring that the data were also generalisable to the NHS.
Short-term model
The short-term model is structured as a Markov model131 as shown in Figure 5. Monthly cycles were used to reflect the events that occur in each of the first 3 months of the acute postMI period. The primary events of interest were all-cause mortality and hospitalisations for CV causes. Four mutually exclusive health states are defined: (1) index hospitalisation, (2) non-fatal CV event, (3) CV death, and (4) non-CV death (not shown on figure). Patients enter the index hospitalisation state at the point at which they have an AMI and remain in this state until they experience either a CV event (fatal or non-fatal) or non-CV death. These outcomes also represent states in the long-term model. The probabilities of the different end points during the acute postMI period are used to estimate the proportion of patients starting in the health states in the long-term model.
FIGURE 5.
Structure of the short-term model.
Long-term model
Any assessment of the cost-effectiveness of aldosterone antagonists must allow for the long-term cost and outcome implications of treatment. This ‘extrapolation’ is needed for two reasons. First, many patients who are treated for postMI HF will continue to consume health-service resources for the remainder of their life, and the effectiveness of aldosterone antagonists in the short-term may influence these costs. Second, to compare the cost-effectiveness of aldosterone antagonists with other uses of health-service resources (inside and outside cardiology), it is necessary to express the benefits of the drugs in terms of a generic measure of health gain that can be compared across treatment areas. The most frequently used generic measure for this purpose is the QALY. To provide a realistic estimate of the QALY impact of aldosterone antagonists, the long-term implications for survival and HRQoL need to be modelled.
The long-term model (Figure 6) is structured as a Markov model with yearly cycles used to model the progression of postMI HF over the longer term. Five health states are defined: (1) postMI HF (i.e. no additional CV events have incurred since the index hospitalisation), (2) 1st year postCV event, (3) subsequent years postCV event, (4) CV death, and (5) non-CV death (not shown on figure). Depending on progress through the short-term model, patients enter the model in the postMI HF state, the 1st year postCV event state, or the death states (CV or non-CV). The proportion of patients starting in each of these states will differ between the treatment strategies depending on their relative effectiveness during the acute period.
FIGURE 6.
Structure of the long-term model.
Patients who do not experience any events in the short term enter the long-term model in the postMI HF state. Individuals remain in this state until they experience a further event either CV in nature (e.g. AMI, progression of HF, stroke, ventricular arrhythmia, or CV death) or non-CV death. Patients who experience a fatal event exit the model. Patients who experience a non-fatal CV event, enter the ‘1st year postCV event’ state for 1 year. Once that year has elapsed they can experience another non-fatal CV event and re-enter the 1st year postCV event state for another year; have no additional events; or die and exit the model. Those patients who do not experience additional events within the Markov cycle move to the subsequent years postCV event state, where they remain until they experience another event (nonfatal CV event, CV death, or non-CV death).
The period after a non-fatal CV event is split into ‘1st year postevent’ and ‘subsequent years postevent’ to reflect the elevated risk of a subsequent event in the initial year following an event. Additional tunnel states (not shown in the figure) were also incorporated into the model to keep track of the number of rehospitalisations for recurrent CV events. Tunnel states are arranged so that they overcome the ‘memoryless’ feature of Markov models regarding where the patient has come from or the timing of the transition. It was necessary to record the number of rehospitalisations for CV events to correctly assign unit costs and quality of life adjustments to the period of these events (see Resource use and unit costs). A maximum of three recurrent CV events (four events in total) since the initial hospitalisation for MI was allowed because the utility estimates applied in the model relate to three or more rehospitalisations. Although the likelihood of having five or more CV events is not fully explored the difference in the results would be expected to be negligible.
From any of the states in the model where individuals are alive, there is a competing risk of a non-CV death.
Model inputs
A full list of parameter inputs applied in the model is reported in Appendix 5. Each of the main parameter groups is discussed in detail in the following sections.
Baseline events rates
The RCTs undertaken to evaluate the clinical effectiveness of eplerenone and spironolactone were mainly or wholly undertaken outside the UK. In many respects, treatment patterns and resource use in the UK can be expected to differ from those in the centres involved in the trials. One implication of this UK-specific practice is that the baseline event rates observed in the control group of the trials are unlikely to provide reliable estimates for UK practice. In addition, the limited follow-up reported in both EPHESUS and RALES and the generalisability of RALES to a postMI HF population, raise additional issues regarding their suitability for the purposes of long-term extrapolation.
Long-term survival from EPHESUS and RALES was estimated by fitting parametric curves to the published empirical Kaplan–Meier survival data to predict the survival of individuals beyond the published follow-up. The trials provide survival curves for all-cause mortality up to a period of 27 months in EPHESUS and 33 months in RALES. A parametric Weibull distribution was fitted to the data to predict survival over a patient’s lifetime, as shown in Figure 7. The difference in survival between EPHESUS and RALES is largely the result of the differences in the trials regarding the time since AMI and the severity of HF. The EPHESUS population is immediately postMI (up to 14 days) and at the start of HF development (approximately 80% NYHA class I or II), whereas the RALES population is further into the progression of HF (NYHA class III or IV), and members of the population with ischaemic HF were at least 6 weeks postMI. It is also worth noting that the longer-term extrapolation of EPHESUS appears to underestimate mortality in the long term with over 50% of patients still predicted to be alive after 20 years.
FIGURE 7.
Extrapolated survival curves based on EPHESUS and RALES.
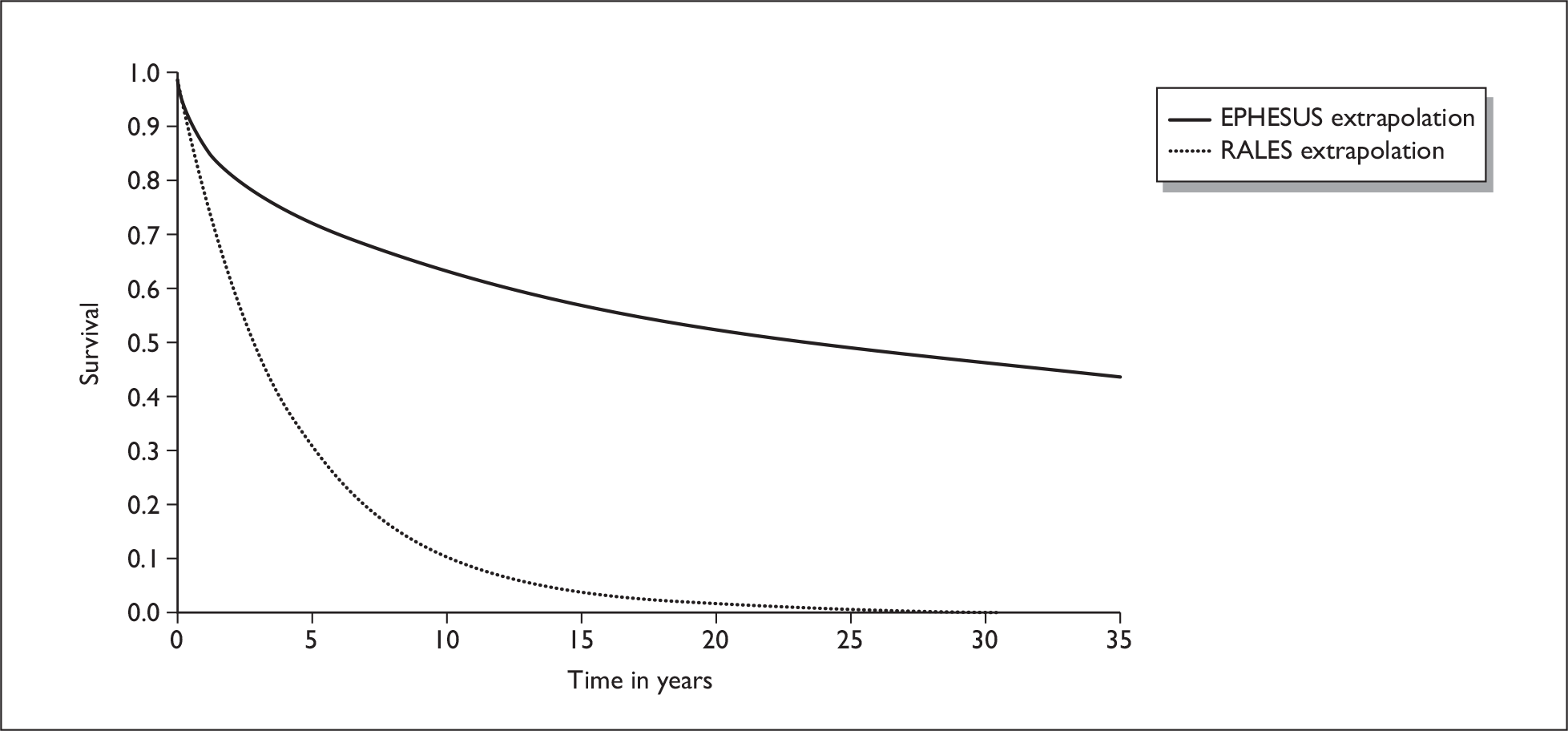
Given the concerns noted, the use of baseline data from EPHESUS or RALES was not considered appropriate for informing the long-term extrapolation. Instead, an alternative source of baseline event rates, specific for UK practice, was obtained from the linked Scottish Morbidity Record (SMR), which records all hospitalisations and subsequent deaths in Scotland. 132 These data are collated by the Information and Statistics Division, Scotland. Record linkage, using probability matching (with an accuracy of approximately interquartile range 98%), permits analysis of data at the level of the individual patient as well as the episode of care.
For this study, data from the SMR was obtained on all individuals with a ‘first’ discharge from hospital, between 1993 and 2003, with a principal diagnosis of HF [International Classification of Diseases (ICD) 9 425.4, 425.5, 425.9, 428.0, 428.1, 428.9, 402, ICD10 I50, I42.0, I42.6, I42.7, I42.9]. 133 A ‘first’ discharge was defined as one with an HF code in a primary diagnostic position, with no previous hospitalisation for HF (in any diagnostic position) for a minimum of 5 years before the index admission. For these individuals with a ‘first’ discharge for HF, data were also available on subsequent major CV events (MI, stroke, angina and other HF events) as well as deaths from CV and non-CV causes, until 31 December 2005.
Data on comorbid diagnoses (coded as a secondary diagnosis during the index hospitalisation or as the principal diagnosis during a previous hospitalisation within 5 years of the index hospitalisation) were also included. Data on MI as a comorbid diagnosis was used as a means of obtaining estimates for a postMI cohort. However, since the timing of the previous AMI was not recorded in the SMR data, it was considered that the SMR data might not adequately capture the initial risk of the acute episode. Given this concern, it was considered more appropriate to use the SMR data as part of the longer-term extrapolation and to use data from the control group of EPHESUS to characterise the initial acute period of 3 months.
A subset of the SMR data was used representing 39,307 individuals out of the overall dataset of 71,848. This subset of individuals has recently been used to estimate the progression of HF in patients who survived the initial episode and had stable HF. 134 Of the sample of 39,307 individuals, 23,150 (59%) had a primary non-fatal CV event, and 7509 (19%) had a fatal CV event as the primary (first) event. For those with non-fatal primary events, 6233 went on to have a subsequent non-fatal event, and 13,662 died of a subsequent CV event. The extrapolation is therefore based on a large and robust dataset. Figure 8 provides a comparison of the extrapolated survival curve from the SMR data compared with those estimates from EPHESUS and RALES.
FIGURE 8.
Extrapolated survival curves from Scottish Morbidity Register data.
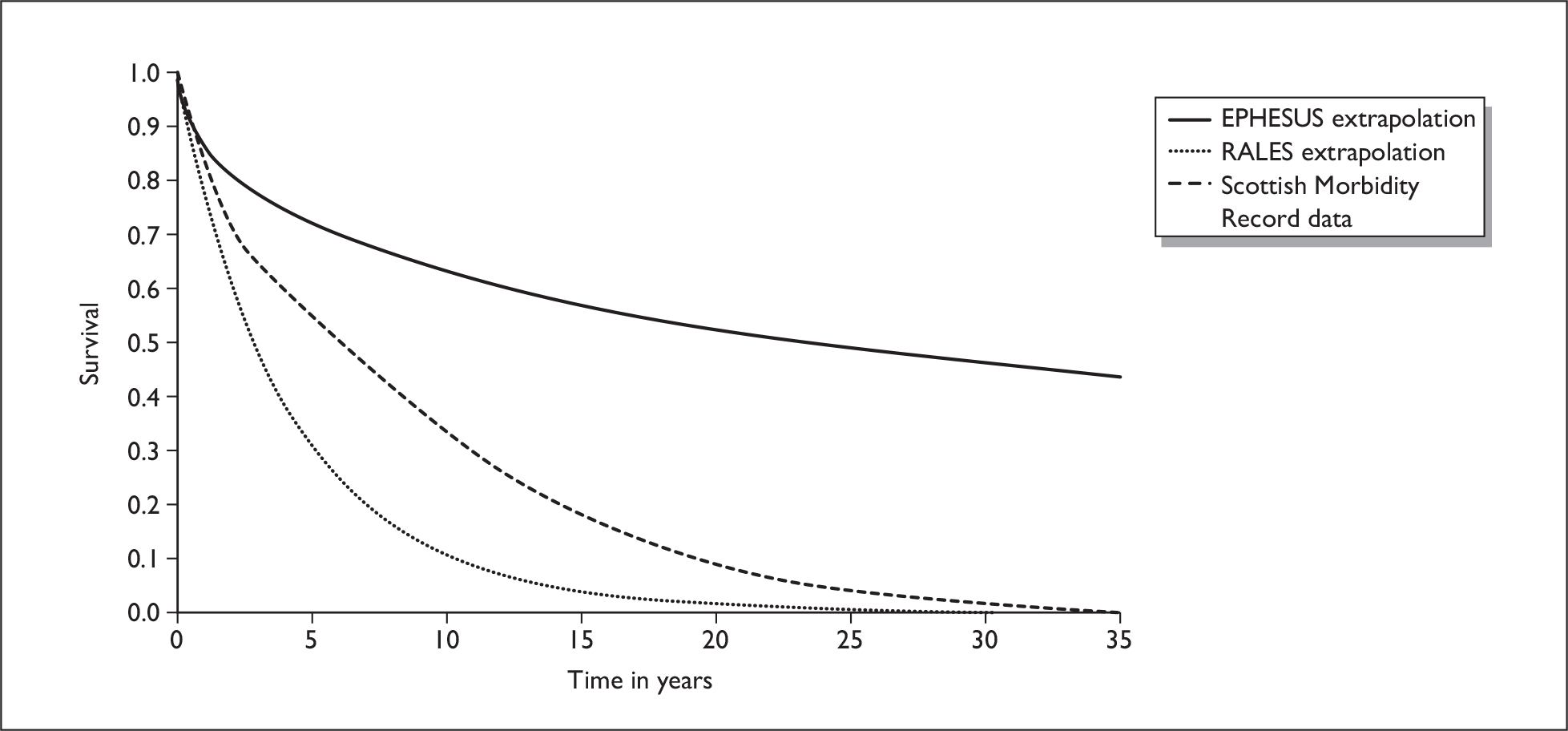
Short-term model
Baseline event rates for mortality and hospitalisation for the first 3 months observed in the control group of EPHESUS were used in the short-term model to reflect the initial acute period. The two primary end points considered were time to death from any cause, and time to death from CV causes or first hospitalisation for a CV event including HF, recurrent AMI, stroke or ventricular arrhythmia. Data were extracted from the published Kaplan–Meier curve of the rate of death from any cause to provide estimates of the monthly probabilities for all-cause mortality. A parametric Weibull distribution was fitted to the empirical Kaplan–Meier data using simple regression analysis by transforming the survivor function to a linear function with time. 135 The resulting mean estimates for the ancillary gamma and lambda parameters of the Weibull curve were 0.46 and 0.05, respectively. These parameters were used to estimate monthly transition probabilities for all-cause mortality. The resulting estimates for months 1, 2 and 3 were 4.73%, 1.81% and 1.36%, respectively.
In the absence of a single Kaplan–Meier curve reporting the rate of first hospitalisation for a CV event (only a composite curve was reported), baseline hospitalisation rates were derived from data reported on the proportion of patients hospitalised over the follow-up period. Over a mean follow-up period of 16 months, 649 patients out of 3313 were hospitalised for a CV event. This equates to a mean risk of a first hospitalisation for CV events of 1.35% per month. A beta distribution was used to characterise the uncertainty in the mean estimate of hospitalisation for a CV event.
Long-term model
Baseline transition probabilities used in the long-term Markov model (as indicated by arrows in Figure 6) were estimated using the SMR data previously described, with the exception of the risk of non-CV death, which was taken from UK population lifetables. The SMR data were used to identify individuals at the start of their HF development and to identify subsequent major CV events for these individuals over time, to derive survival equations or logit models, whereby time-to-event or death could be predicted based on a set of baseline characteristics. Each of the arrows in Figure 6 is labelled with an equation number. These equations estimate the relationship between the event and the individual’s characteristics (based on the SMR data) to facilitate simulation of the fatal and non-fatal events that a cohort of patients is expected to experience over the long-term.
The first two equations (Equations 1 and 2 in Figure 6) predict the primary outcomes of a fatal CV event and a non-fatal CV event, respectively. The estimated equation follows a standard parametric time-to-event survival analysis. The risk is predicted based on a set of baseline characteristics of age, gender, prior medical history (comordities of diabetes, atrial fibrillation, hypertension, angina, stroke and MI) and year of admission/diagnosis. Equations 3 and 4 relate to the probability that, having had a non-fatal CV event, there will be a subsequent event (non-fatal and fatal, respectively) within 12 months of the first event. This is estimated as a standard logistic regression with an indicator for the individuals who went on to have a second event within the year. The logistic regressions control for the same baseline variables as in Equations 1 and 2. Equations 5 and 6 estimate the risk of a subsequent CV event (non-fatal and fatal, respectively) from the first event. A standard Weibull time-to-event analysis was employed using the time from the first event. The risk is predicted using similar covariates to those in Equations 1 and 2, except that given the information on the primary event, it is possible to update the prior history variables of stroke, angina and MI. All subsequent events are assumed to follow a similar trajectory as the primary event.
Although the SMR data were used to derive the equations described above, the predicted risk of the events in the long-term model is based on the baseline characteristics and subsequent events of the EPHESUS population, given that this population is representative of the population to be addressed in the decision problem. The full set of equations applied in the decision model is reported in Appendix 6.
Risk of non-cardiovascular mortality
The risk of dying from CV causes is described above using risk equations, but there is a competing risk of non-CV mortality. The age-dependent risk of other-cause mortality was estimated using UK age-specific and sex-specific mortality rates. 136 These rates were adjusted to exclude those deaths pertaining to CV mortality using a cause elimination approach. This involved eliminating deaths caused by CV disease (ICD10 code) from the UK lifetables according to standard methods used by the Office for National Statistics. 137
Relative treatment effect
In the absence of any direct head-to-head trials comparing aldosterone antagonists simultaneously in postMI HF, indirect evidence is required to establish the effectiveness of the interventions. The effect of spironolactone or eplerenone, when added to standard care, has been evaluated in RCTs with standard therapy as a control. The review reported in Chapter 3 identified a number of issues with regard to exchangeability of the trial evidence; heterogeneity between the trials with respect to different study populations (postMI HF versus HF more generally), timing of MI, beta-blocker usage and severity of HF, which made any formal ‘pooling’ of the individual trials inappropriate. A recent systematic review by Ezekowitz and McAlister138 that included studies of aldosterone antagonists in postMI without clinical HF as well as those in postMI HF and general HF, reinforces the concerns expressed in Chapter 3 that any pooling across the different populations (i.e. postMI HF and HF) would not be appropriate because of heterogeneity. Although the Ezekowitz and McAlister review138 has limitations in that many of the included trials were small, of poor quality and reported few (in some cases zero) events, it does show that pooling across studies within these populations may be less problematic given a lack of significant heterogeneity within the populations. In the context of the decision model the combined weight of evidence from all relevant trials and comparators is necessary to provide a useful aid to decision-making. To facilitate an indirect comparison of eplerenone and spironolactone, there was a need to extend the evidence ‘network’ considered in Chapter 3 to overcome the issues regarding exchangeability of trials and populations. The review by Ezekowitz and McAlister138 was important in this respect for two reasons.
-
It provided additional RCT evidence reporting on the use of aldosterone antagonists (including canrenoate) in different populations. Although many of these studies were small, reported few events, were of unknown quality, and did not meet the inclusion criteria for the review of clinical effectiveness in Chapter 3, they provided evidence on the use of aldosterone antagonists for the treatment of MI and HF.
-
It showed that there was no evidence of statistical heterogeneity within the different study populations despite differences that exist between the populations.
The additional evidence reported by Ezekowitz and McAlister138 was used to complement the RCT evidence reported in Chapter 3 to obtain a wider network of evidence on the use of aldosterone antagonists in different populations (including those beyond the scope of the decision problem). This wider network of evidence was used to facilitate an indirect comparison of eplerenone and spironolactone by examining the relationships that exist between the different treatments and study populations. A summary of the network of evidence for all-cause mortality is reported in Table 16.
| Study | Treatment | Population | Mean follow-up (months) | Treatment | Standard care | Trial-specific estimates of RR | |||
|---|---|---|---|---|---|---|---|---|---|
| Dead | Total | Dead | Total | Relative risk | 95% CI | ||||
| Agostoni (2005)140 | Spironolactone | General HF | 6 | 0 | 15 | 0 | 15 | – | – |
| Akbulut (2003)141 | Spironolactone | General HF | 3 | 0 | 35 | 0 | 70 | – | – |
| Berry (2007)142 | Spironolactone | General HF | 3 | 0 | 20 | 0 | 20 | – | – |
| Chan (2007)143 | Spironolactone | General HF | 12 | 0 | 23 | 0 | 25 | – | – |
| Cicoira (2002)144 | Spironolactone | General HF | 12 | 3 | 54 | 4 | 52 | 0.722 | [0.170 to 3.072] |
| Gao (2007)145 | Spironolactone | General HF | 6 | 0 | 58 | 0 | 58 | – | – |
| Pitt (1996)146 | Spironolactone | General HF | 3 | 0 | 174 | 0 | 40 | – | – |
| Pitt (1999)31 | Spironolactone | General HF | 24 | 284 | 822 | 386 | 841 | 0.753 | [0.668 to 0.848] |
| Rodriguez (1997)147 | Spironolactone | PostMI HF | 6 | 1 | 23 | 2 | 24 | 0.522 | [0.051 to 5.370] |
| Ruta (2006)48 | Spironolactone | PostMI HF | 11 | 22 | 8 | 25 | 1.562 | [0.770 to 3.171] | |
| Tsutamoto (2001)148 | Spironolactone | General HF | 4 | 0 | 20 | 0 | 17 | – | – |
| Tu (2003)49 | Spironolactone | PostMI HF | 1 | 43 | 7 | 42 | 0.140 | [0.018 to 1.086] | |
| Pitt (2003)27 | Eplerenone | PostMI HF | 16 | 478 | 3319 | 554 | 3313 | 0.861 | [0.770 to 0.964] |
| Study402 (2004)138 | Eplerenone | General HF | 3 | 1 | 114 | 1 | 38 | 0.333 | [0.021 to 5.201] |
| Boccanelli (2007)54 | Canrenoate | General HF | 12 | 6 | 231 | 12 | 236 | 0.511 | [0.195 to 1.338] |
| Di Pasquale (2005)149 | Canrenoate | PostMI HF | 6 | 22 | 341 | 32 | 346 | 0.698 | [0.414 to 1.175] |
| Modena (2001)150 | Canrenoate | PostMI HF | 12 | 0 | 24 | 2 | 22 | 0.184 | [0.009 to 3.634] |
A Bayesian evidence synthesis approach was employed which draws on the relationships that exist between treatments and populations while preserving differences that exist across populations. The approach essentially assumes that there is a correlation between the relative effectiveness of a treatment (spironolactone, eplerenone or canrenoate) in one study population (e.g. postMI HF) and another population (e.g. HF more generally). A metaregression analysis was used to examine the relationship between the treatments and the size of the treatment effect in the two populations (postMI HF and general HF) identified in the network of trials reported in Table 16. Distinct indicator variables were incorporated in the regression for the two populations (e.g. postMI HF assumes a value of 1, while general HF assumes a value of 0) and treatments to allow an estimate of the increment in relative risk for a particular treatment in a particular study population.
Formally, the regression takes the form:
where MI assumes the value 1 for postMI HF and 0 for general HF, EPL and CAN assume the value 1 for treatment with eplerenone and canrenoate, respectively, or 0 for treatment with spironolactone. The coefficient β0 refers to the reference category, which estimates the log of the relative risk for treatment with spironolactone in general HF. The coefficient β1 estimates the increment in relative risk for postMI HF relative to general HF. Similarly, the coefficients β2 and β3 estimate the increment in relative risk for treatment with eplerenone and canrenoate, respectively, relative to spironolactone. Details of the winbugs code are reported in Appendix 7. A fixed effects model was used in the analysis. A random effects model was investigated but resulted in problems with convergence because of the small amount of data available across the different populations and treatments.
The metaregression approach allows treatment specific estimates to be modelled in each population by drawing strength from the network of evidence, and assuming that all treatments share a similar reduction/improvement in efficacy in one population compared with another. The main advantage of this approach is that it provides predictions on efficacy for the treatments in the populations where there are limited or no data. For example, there are limited data to estimate the effect of spironolactone in postMI HF, or eplerenone in general HF, therefore informed predictions can be made by observing the relationships that exist between the treatments and between the populations. The inclusion of the canrenoate trials is essential to this synthesis because they provide sizeable studies in both populations.
The primary outcome reported across the trials is mortality. The relative risks with 95% credibility intervals (CrI) for spironolactone and eplerenone compared with standard care are shown in Table 17 for postMI HF and general HF. The estimates for the treatments in the populations where the evidence is more certain, i.e. eplerenone in postMI HF and spironolactone in general HF, are consistent with EPHESUS and RALES, respectively. The predictions for the treatments in the populations where there is a paucity of evidence (spironolactone in postMI HF and eplerenone in general HF) are more uncertain with wide 95% CrI.
| Mean | Median | 95% CrI | |
|---|---|---|---|
| Relative risk for postMI HF | |||
| Spironolactone | 1.020 | 0.988 | [0.575 to 1.652] |
| Eplerenone | 0.861 | 0.860 | [0.767 to 0.964] |
| Relative risk for general HF | |||
| Spironolactone | 0.753 | 0.752 | [0.666 to 0.843] |
| Eplerenone | 0.683 | 0.656 | [0.377 to 1.152] |
The estimates for postMI HF are used within the model because this is the population of interest to the decision problem. The results, on average, suggest that eplerenone is associated with a significant reduction in mortality compared with spironolactone; however, there is high uncertainty around the estimate for spironolactone (95% CrI 0.575 to 1.652). The simulated output (10,000 Markov Chain Monte Carlo simulations implemented in winbugs139) is used directly in the model to maintain correlation between the estimates for the separate treatment strategies.
A secondary outcome of hospitalisations for CV events was not reported consistently across trials. Therefore meta-analytic approaches could not be used to derive a relative effect measure for this outcome. The relative risk of hospitalisation for CV events for eplerenone compared with standard care was informed by EPHESUS. Over a mean follow-up time of 16 months, the relative risk was 0.91 (95% CI 0.81 to 1.01). Within the model, this relative risk was incorporated as a log-normal distribution to allow for uncertainty in the parameter. The relative risk was assumed to remain the same over the individual’s lifetime. No information was available to inform the effect of spironolactone on the risk of hospitalisation for CV causes in postMI HF, therefore an assumption was required to estimate this measure. The model assumes that the increment in relative risk observed for mortality between eplerenone and spironolactone can be applied to hospitalisations. This relative increment was then applied to the relative risk of hospitalisations for eplerenone to obtain an estimate of the risk of hospitalisations for spironolactone.
The relative treatment effect measure for mortality and hospitalisations for CV causes is applied to the baseline event rates (see Baseline events rates) to obtain absolute event rates for the different treatment comparators. In separate scenarios the robustness of the results are explored by changing the network of evidence available to inform the evidence synthesis. Three scenarios are considered: (1) exclusion of HF trials reporting follow-up durations of < 3 months; (2) excluding canrenone studies; and (3) excluding lowest quality trials.
Resource use and unit costs
Costs were incorporated into the Markov model by attaching a unit cost to each non-fatal CV event (AMI, HF, stroke, ventricular arrhythmia) which occurs over time. The 1st year postCV event state in the model represents three tunnel states, which record the number of rehospitalisations for CV events. Once a patient enters one of these states, it is assumed that there is a proportional risk that the event which occurred was AMI, HF, stroke or ventricular arrhythmia. The proportion of patients experiencing each of these events was informed by EPHESUS: 31.6% AMI, 53.9% HF, 7% stroke and 7.5% ventricular arrhythmia. The unit costs attached to each event were based on the National Schedule of Reference Costs 2007–08. 151 The reference costs are the average costs to the NHS of providing a defined service/resource in a given financial year. The costs are categorised into particular groups (Health Resource Groups; HRGs) according to episodes that are clinically coherent and consume similar resources. Table 18 presents the non-elective inpatient HRG costs for the CV events of interest in the model. The costs for each event are based on a weighted average of short-stay and long-stay visits, as well as the numbers of patients that incur complications. An annual background cost of HF relating to the routine management of HF, excluding event costs, was also applied for the length of time that a patient is alive within the model. This cost was based on British Heart Foundation statistics, which estimate that the annual cost of HF to the NHS was £628.6M in 2000. 25 Excluding inpatient hospitalisations, the annual cost is £250M. Assuming a prevalence of HF of 707,000 in the UK. 26 This equates to an annual background cost of HF of £354 per person (2000 prices). The price was uprated to the current year to give an annual cost of £462 per person per year alive.
| HRG code | HRG label – CV event | FCEs (n) | National average unit cost | Lower quartile unit cost | Upper quartile unit cost | |
|---|---|---|---|---|---|---|
| AA22Z | Non-transient stroke or cerebrovascular accident, nervous system infections or encephalopathy | Short stay | 33,213 | £351 | £246 | £405 |
| Long stay | 90,071 | £2718 | £2019 | £3124 | ||
| Strokea | £2081 | £1541 | £2391 | |||
| EB03H | Heart failure or Shock with CC | Short stay | 7674 | £356 | £248 | £406 |
| Long stay | 24,194 | £2589 | £1949 | £2976 | ||
| EB03I | Heart failure or Shock without CC | Short stay | 18,302 | £340 | £248 | £388 |
| Long stay | 37,513 | £1685 | £1259 | £1935 | ||
| Heart failurea | £1538 | £1150 | £1765 | |||
| EB07H | Arrhythmia or Conduction disorders with CC | Short stay | 7376 | £354 | £245 | £408 |
| Long stay | 13,899 | £1814 | £1320 | £2107 | ||
| EB07I | Arrhythmia or Conduction disorders without CC | Short stay | 58,022 | £339 | £248 | £393 |
| Long stay | 42,747 | £1041 | £778 | £1204 | ||
| Ventricular arrhythmiaa | £754 | £555 | £873 | |||
| EB10Z | Actual or suspected myocardial infarction | Short stay | 35,669 | £381 | £251 | £439 |
| Long stay | 71,478 | £1523 | £1126 | £1757 | ||
| Myocardial infarctiona | £1143 | £835 | £1318 |
The cost of spironolactone and eplerenone were obtained from the BNF. 42 The dosage of eplerenone was assumed to be 25 mg/day for the first 4 weeks and 50 mg thereafter. A 28-tab pack of 50-mg strength costs £42.72. This resulted in an annual cost of £557 incurred for each year that the patient continues to receive the drug. The dosage of spironolactone was assumed to be 100 mg/day. A 28-tab pack of 100-mg strength of generic spironolactone costs £3.55. This resulted in an annual cost of £46 for the duration of treatment. All patients, regardless of the treatment strategy were assumed to receive standard therapy.
Quality of life
In order to estimate QALYs, it is necessary to quality-adjust the period of time for which the average patient is alive within the model using an appropriate utility or preference score. Ideally, utility data are required to differentiate between the health status of patients in the different states of the Markov model using a generic measure of health such as the EQ-5D. 152 A recent study by Gohler et al. 153 addresses the limitations of currently available data with respect to providing utility estimates that can be linked to common proxy health states in HF. The study uses primary data from EPHESUS to estimate utility values for patients with HF according to NYHA classification and number of CV rehospitalisations. This study is therefore directly applicable to the current model. The 1st year postCV event state in the model represents three tunnel states, which record the number of rehospitalisations for CV events. A utility decrement, based on the Gohler et al. study,153 is applied to each of these rehospitalisations (Table 19) and a gamma distribution is used to characterise uncertainty in the mean values. The underlying baseline utility for a 64-year-old patient with postMI HF was also taken from Gohler et al. 153 (Table 19). To reflect the decreasing utility of patients as they age through the model, age and sex adjusted norms for the UK154 were adjusted downwards by approximately 5% to reflect the existence of HF. The ‘adjustment’ factor was estimated by comparing the baseline utility of Gohler et al. 153 to the average utility of a 64-year-old person in the UK, derived from a nationally representative UK sample using EQ-5D. 154
| Health status | Estimate (standard error) |
|---|---|
| Baseline | 0.759 (0.040) |
| 0 rehospitalisations | Reference group |
| 1 rehospitalisations | – 0.024 (0.007) |
| 2 rehospitalisations | – 0.031 (0.009) |
| 3 rehospitalisations | – 0.055 (0.001) |
Analytic methods
Cost-effectiveness
The model was developed in excel and is run probabilistically for 10,000 iterations incorporating all the estimates and assumptions described previously. Mean costs and QALYs for spironolactone, eplerenone and standard care alone are presented and the cost-effectiveness of each is compared using conventional decision rules, estimating ICERs as appropriate. 155 The ICER examines the additional costs that one strategy incurs over another and compares this with the additional benefits. When more than two interventions are being compared the ICERs are calculated using the following process:
-
The strategies are ranked in terms of cost (least expensive to most costly).
-
If a strategy is more expensive and less effective than any previous strategy, then this strategy is said to be dominated and is excluded from the calculation of the ICERs.
-
The ICERs are calculated for each successive alternative, from the cheapest to the most costly. If the ICER for a given strategy is higher than that of any more effective strategy, then this strategy is ruled out on the basis of extended dominance.
-
Finally, the ICERs are recalculated excluding any strategies that are ruled out by principles of dominance or extended dominance.
Uncertainty in the estimates of cost-effectiveness of the alternative strategies is reflected using cost-effectiveness acceptability curves (CEACs). 156 These show the probability that each strategy is more cost-effective than the others using alternative values for the maximum value that the health service is willing to pay for an additional QALY in these patients.
Value of information
Value of information analysis is used to quantify the cost associated with the decision uncertainty related to the use of aldosterone antagonists for postMI HF. This approach provides an explicit and quantitative method to value the consequences of decision uncertainty based on existing evidence. 157,158 The analysis can be used as the basis to inform future research priorities and can assist in establishing the potential value of a future head-to-head trial of spironolactone and epelerenone.
Using VOI approaches, the expected cost of uncertainty surrounding the adoption decision can be determined by the expected value of perfect information (EVPI). 159 The expected costs of decision uncertainty represent the consequences in terms of costs incurred and lost benefits that would have occurred should it later transpire that the optimal decision on cost-effectiveness grounds was not correct. The VOI analysis therefore involves establishing the difference between the expected value of a decision made on the basis of existing evidence and, following the arrival of further information and the subsequent resolution of uncertainty in the estimates, the expected value of a decision made on the basis of new evidence. The EVPI provides an estimate of the value of completely resolving all existing uncertainty, through the provision of perfect information, and provides a measure of the maximum return to further research. Consequently EVPI represents an upper bound to the amount a decision-maker should be willing to pay for additional evidence to support the current decision based on expected cost-effectiveness estimates. That is, if the EVPI exceeds the expected costs of additional research then it is potentially cost-effective to acquire more information by undertaking this research. 159
The overall EVPI surrounding a health-care policy decision depends upon the number of times that the decision is faced over the lifetime of the technology. This is termed the population level EVPI and is estimated by scaling up the individual (per-patient) EVPI estimates according to the number of people that would be affected by the information over the anticipated lifetime of the technology. Formally this can be expressed as:
where I is the incidence in the period, t is the period, T is the total number of periods for which information from research would be useful and r is the discount rate.
Based on published statistics it has been estimated that postMI HF accounts for approximately 20% of all HF cases. 21 The British Heart Foundation estimates that the prevalence of HF in the UK is 707,000 (393,000 men, 314,000 women) and the annual incidence of HF is estimated to be 68,000 cases (38,000 men, 30,000 women). 26 Together these sources suggest a prevalence of about 141,400 and an annual incidence of around 13,600 for postMI HF. Assuming a 10-year time horizon for the lifetime of the technologies being considered, this gives an effective population of 258,465 (prevalent population plus incident population per annum discounted at 3.5% over the lifetime of the technology) for the population EVPI calculations.
In addition to determining the EVPI surrounding the decision as a whole, VOI approaches can also be used for particular elements of the decision to direct and focus research towards the areas where the elimination of uncertainty has the most value. The partial EVPI can be calculated for individual or subsets of parameters.
On the basis of EVPI and partial EVPI calculations, the potential value of a future trial or other potential research designs can be evaluated. The VOI which could be acquired by conducting further research depends crucially on the number of future patients who could benefit from it and the time horizon over which the information would be useful. It has recently been shown that selecting a value for the time horizon is essentially a proxy for a more complex and uncertain process of future changes to the decision problem which impact on the EVPI. 160 One future change that can be anticipated in the short-term is the potential impact of the arrival of a generic version of epelerenone. The potential impact on EVPI estimates of this change was therefore an important consideration.
Scenarios
Cost-effectiveness and EVPI results are presented for a base-case analysis and a number of separate scenarios. All scenarios consider a cohort of postMI HF patients (mean age 65 years) over a time horizon of 40 years. The base-case analysis assumes that treatment with aldosterone antagonists will be given for a maximum of 2 years to ensure consistency with the available trial evidence for these treatments. The relative treatment effects for mortality and non-fatal events, derived from results of the evidence synthesis for spironolactone and eplerenone compared with standard care, are applied to the baseline risk prediction equations for the first 2 years only. After 2 years all patients share the same set of common risks from each health state in the decision model, regardless of their initial treatment assignment. However, since each strategy in the model will result in a different proportion of patients who reside in each of the separate health states of the model at 2 years, this means that the model will continue to account for the longer-term prognostic benefit associated with a reduction in non-fatal events incurred during the initial 2 year treatment period.
In addition to the base-case analysis a range of separate scenarios are explored to examine the robustness of these results to a range of alternative assumptions and parameter inputs. These include:
Scenario 1: Lifetime treatment duration with aldosterone antagonists.
This scenario considers the potential impact of continuing treatment beyond the 2-year period assumed in the base-case analysis. In this scenario it is assumed that treatment with aldosterone antagonists is continued for the remainder of a patient’s lifetime and the treatment effects observed during the follow-up of the trials are applied to the baseline event risks over the lifetime of the patient.
In each of the subsequent scenarios, the cost-effectiveness and VOI results are presented separately assuming either a 2-year or lifetime treatment with aldosterone antagonists.
Scenario 2: Calibration of the baseline event risks.
This scenario considers the potential impact on the results of adjusting the baseline event risks estimated from the SMR data to reflect potential changes in standard care that may have occurred since these data were collected and also to consider the generalisability of the SMR cohort to the population recruited in EPHESUS.
Scenario 3: Investigating the impact of the introduction of generic eplerenone.
Given the imminent arrival of a generic version of eplerenone, it is important to consider the potential implications of this anticipated future change. Alternative scenarios are considered where the price of eplerenone is reduced by 25–75% of the branded version.
Scenario 4: Network of evidence.
Given the methodological uncertainty surrounding the evidence synthesis approaches applied in the main analyses, a range of alternative approaches are considered and the robustness of the results is explored. These scenarios consider the potential impact of excluding particular studies from the evidence network. Three scenarios are considered: (1) exclusion of HF trials reporting follow-up durations of < 3 months; (2) excluding canrenone studies; and (3) excluding lowest quality trials.
Scenario 5: Assuming equivalent efficacy.
This scenario considers an alternative approach to populating the effectiveness inputs reflecting the concerns that may be expressed regarding the robustness of the underlying assumptions of the evidence synthesis model. In this scenario a class effect is assumed for the aldosterone antagonists (i.e. the relative effect on mortality and non-fatal events of eplerenone and spironolactone compared with standard care is assumed to be the same) and that the main difference relates to the potential incidence of side effects. Separate analyses are therefore undertaken altering either compliance or quality of life to reflect plausible differences in the side effect profiles of the alternative aldosterone antagonists.
Cost-effectiveness and EVPI results
The results are presented for the base-case anaysis and for each of the separate scenarios. In each case, mean lifetime costs and QALYs are estimated for each strategy and ICERs are presented where appropriate. The probability that each intervention is cost-effective is also reported for three select values of the cost-effectiveness threshold representing a maximum willingness to pay between £10,000 and £30,000 per QALY. The value of the uncertainty surrounding a decision based on the expected ICER estimates is expressed using both individual (per-patient) estimates of EVPI and population EVPI assuming a 10-year time horizon.
Results of the base-case analysis (2-year treatment duration)
Table 20 reports the cost-effectiveness results for the base-case scenario incorporating a maximum 2-year treatment duration for both spironolactone and eplerenone. In terms of cost-effectiveness, spironolactone appears less effective and more costly than standard care, and is therefore dominated by standard care. Standard care appears the least costly strategy and eplerenone is estimated to be both more effective and more costly than either spironolactone or standard care. Compared with standard care, eplerenone increases mean total costs by approximately £1120 per patient but results in an additional gain of 0.25 QALYs; the ICER for eplerenone compared to standard care alone is £4457 per additional QALY gained. At a threshold of £10,000 per QALY the probability that eplerenone is cost-effective is 0.61. At higher thresholds of £20,000 and £30,000 per QALY the probabilities that eplerenone is cost-effective increase to 0.66 and 0.68, respectively.
| Strategy | QALY | Cost | ICER | Probability cost-effectivea | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5972 | £4129 | N/A | 0.013 | 0.004 | 0.002 |
| Spironolactone | 4.5551 | £4191 | D | 0.375 | 0.338 | 0.322 |
| Eplerenone | 4.8486 | £5249 | £4457 | 0.612 | 0.658 | 0.676 |
Interestingly, although spironolactone is dominated by standard care, the probability that spironolactone is cost-effective is 0.38 at a cost-effectiveness threshold of £10,000 per QALY. In other words, across the 10,000 simulations applied in the model spironolactone appeared the most cost-effective intervention in approximately 38% of these simulations at this particular threshold. Hence, while spironolactone appears dominated based on the expected (mean) values of costs and QALYs, there appears a high-degree of uncertainty surrounding the overall decision based on cost-effectiveness considerations.
The degree of decision uncertainty is illustrated more clearly in Figure 9, which presents the CEACs for each strategy. At low values of the threshold of cost-effectiveness, standard care has the highest probability of being cost-effective. However, as the threshold increases the probability that eplerenone and spironolactone are cost-effective begin to increase. At cost-effectiveness thresholds above £10,000 per QALY the probability that standard care is cost-effective becomes almost negligible and the uncertainty surrounding cost-effectiveness is almost entirely between eplerenone and spironolactone.
FIGURE 9.
Cost-effectiveness acceptability curves (base-case).
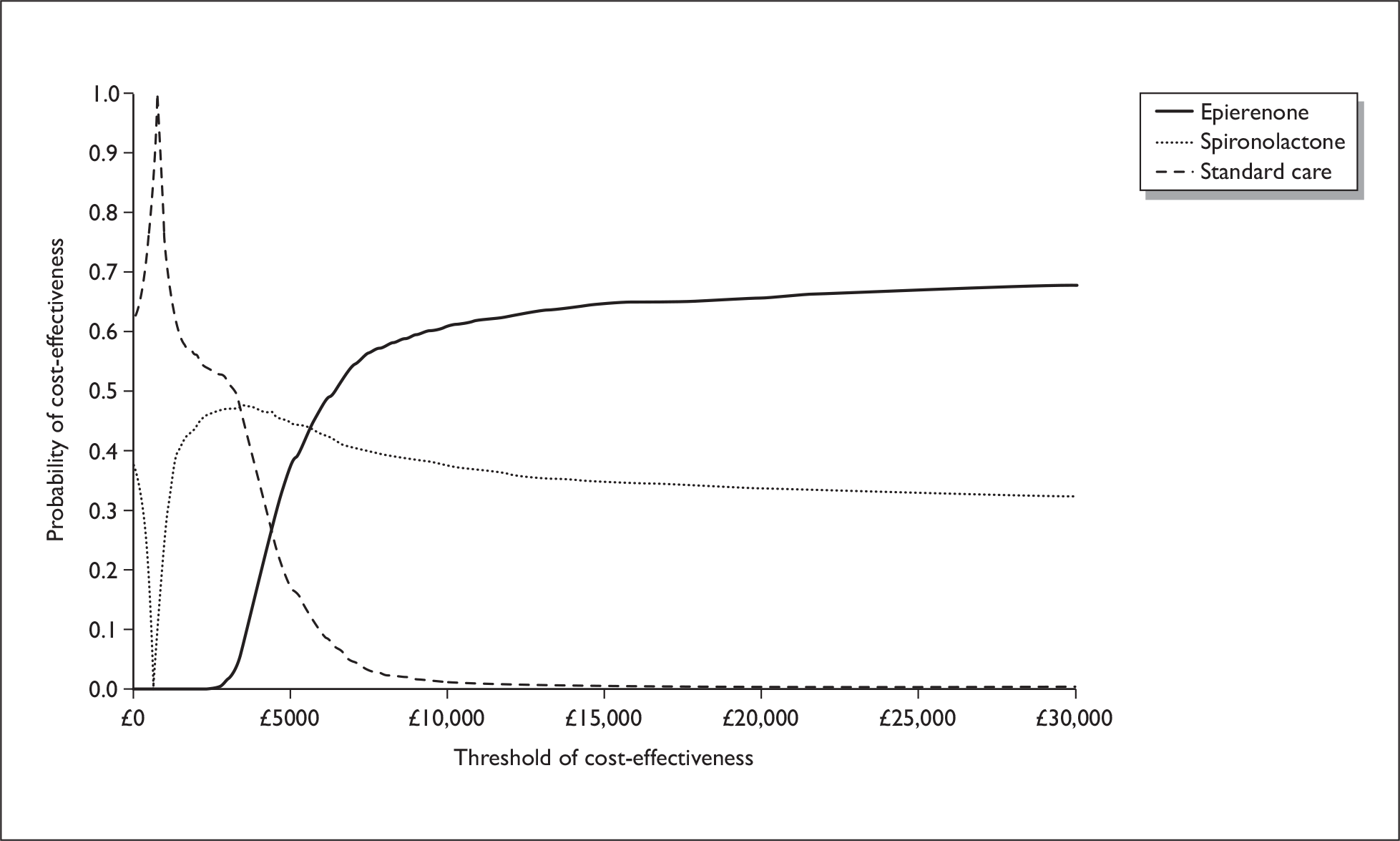
The uncertainty surrounding cost-effectiveness translates into a high cost of decision uncertainty demonstrated in the EVPI results presented in Table 21. This table presents both the individual (per-patient) EVPI estimates and the total population EVPI estimates based on a 10-year time horizon. Estimates of total EVPI ranged from between £277M to £696M across the different range of thresholds. The estimates demonstrate that there appears significant value in undertaking additional research to reduce the existing decision uncertainty.
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £1073 | £277,255,219 |
| £20,000 | £1876 | £484,859,920 |
| £30,000 | £2694 | £696,178,334 |
Results of the scenarios
Scenario 1: Lifetime treatment duration
The base-case analysis assumes that treatment with eplerenone and spironolactone will be discontinued after 2 years. This assumption was employed to be consistent with the average duration of follow-up reported in EPHESUS. In the absence of longer-term follow-up data, the effectiveness of continuing to treat patients with aldosterone antagonists over a longer time horizon remains highly uncertain. However, clearly the optimal duration of treatment with either spironolactone or eplerenone is an important issue. For chronic diseases, such as postMI HF, patients may continue to face an elevated risk of major clinical events for the remainder of their lifetime. The potential cost-effectiveness of maintaining treatment with aldosterone antagonists over the longer-term should therefore be explored. Thus, a separate scenario was undertaken to evaluate the potential cost-effectiveness of continuing treatment with aldosterone antagonists for the remainder of a patient’s lifetime. In this scenario the relative treatment effects applied to fatal and non-fatal events for spironolactone and eplerenone compared with standard care within the initial 2-year period were also applied to subsequent years.
Table 22 presents the cost-effectiveness results for the scenario assuming lifetime treatment with aldosterone antagonists. In common with the base-case scenario, the relevant ICER calculation was based on a comparison of eplerenone versus standard care. The ICER for lifetime treatment was £7893 per QALY compared with £4457 per QALY based on a treatment duration of 2 years. Hence, although the mean ICER of eplerenone compared with standard care becomes less favourable over a lifetime duration, the ICER estimate still remains well below conventional cost-effectiveness thresholds considered to represent value for money in the NHS.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5981 | £4130 | N/A | 0.116 | 0.009 | 0.003 |
| Spironolactone | 4.6196 | £4446 | ED | 0.441 | 0.366 | 0.346 |
| Eplerenone | 5.1108 | £8177 | £7893 | 0.443 | 0.625 | 0.651 |
In contrast to the base-case scenario, spironolactone was not dominated in the lifetime treatment duration scenario by standard care. Instead spironolactone was now extendedly dominated by eplerenone. The different findings between these scenarios may appear potentially counterintuitive because the same relative effect is being applied to spironolactone in both the base-case and lifetime treatment scenarios. However, the results clearly demonstrate the non-linear relationship between the model inputs and outputs. That is, although the mean of the relative risk estimates assigned to spironolactone are slightly higher than one (i.e. indicating that, on average, spironolactone is less effective than routine care) there is considerable uncertainty surrounding this estimate. As the model is non-linear, the simulations in which spironolactone is estimated to be more effective than standard care has a greater subsequent impact on the mean outputs (i.e. mean costs and QALYs) of the model in the lifetime treatment scenario than those in which spironolactone is estimated to be less effective. Hence, while the mean relative risk of spironolactone compared with standard care exceeds the value one, the expected values for QALYs derived from averaging across the entire 10,000 simulation are higher for spironolactone because of the non-linear relationship that exists in the model.
Although the lifetime treatment duration assumption and the non-linear relationship between the model inputs and outputs do not alter the choice of strategy on cost-effectiveness grounds (i.e. eplerenone appears to be the most cost-effective strategy in both scenarios), these factors do appear to have marked effect on the decision uncertainty and the costs of this uncertainty. At a threshold of £10,000 per QALY, the probability that eplerenone is cost-effective is only marginally higher than the estimate for spironolactone. Although the probability that eplerenone is cost-effective increases at higher thresholds, the results are less certain than in the base-case scenario. Figure 10 reports the CEACs assuming a lifetime duration which demonstrates higher uncertainty across the range of cost-effectiveness thresholds considered.
FIGURE 10.
Cost-effectiveness acceptability curves (lifetime treatment duration).
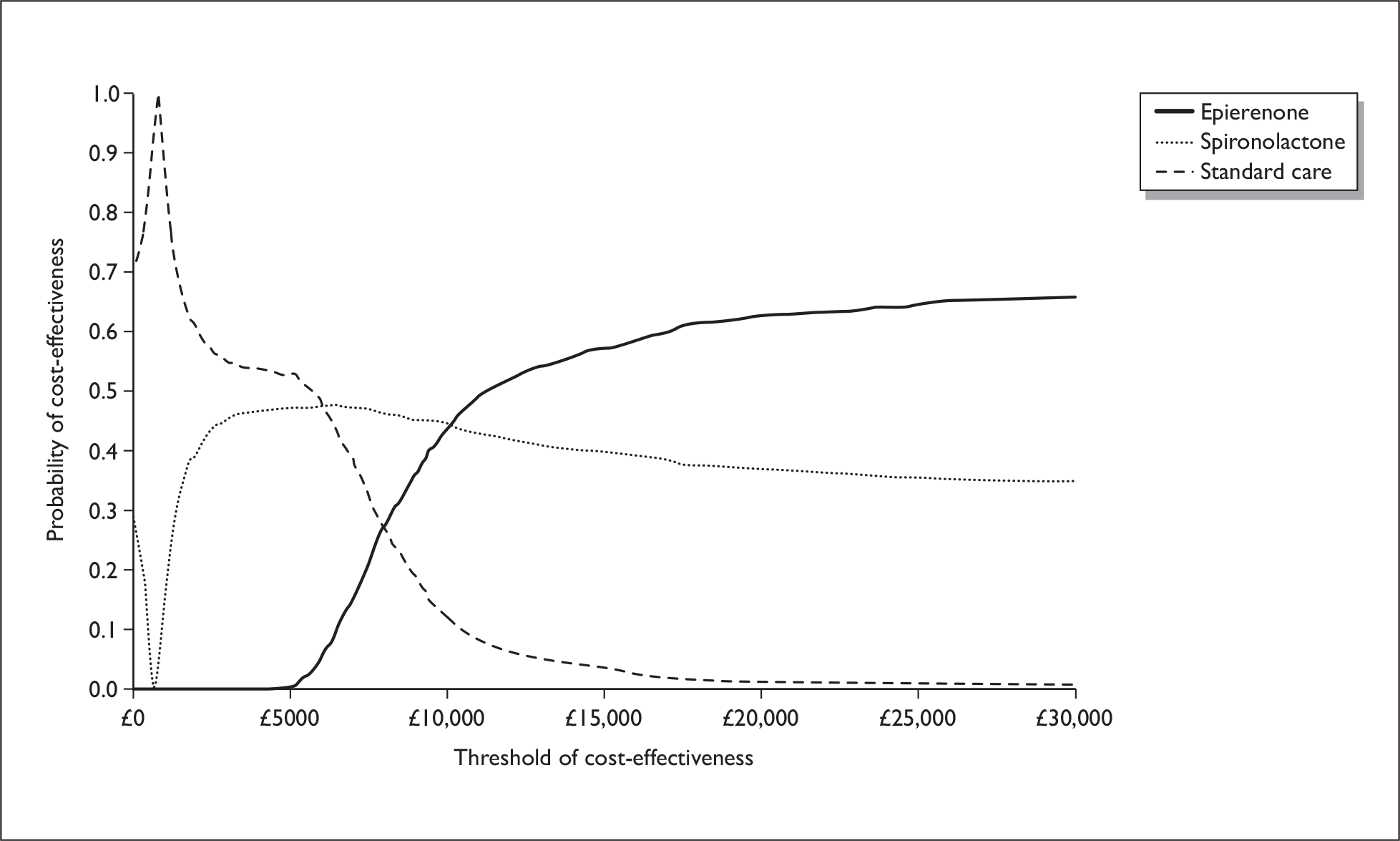
The higher decision uncertainty in the lifetime treatment duration is reflected in the higher EVPI estimates for this scenario reported in Table 23. These estimates are between two and three times higher than the comparable estimates reported for a 2-year treatment duration. The estimates of total EVPI range between £820M and £1748M. The estimates reinforce the conclusions based on the base-case scenario that there appears to be significant value to the NHS in undertaking additional research to reduce the existing decision uncertainty.
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £3172 | £819,942,905 |
| £20,000 | £4893 | £1,264,550,867 |
| £30,000 | £6764 | £1,748,146,894 |
Scenario 2: External calibration
Both the base-case and lifetime treatment scenarios are based on the risk prediction equations derived from the SMR data used to estimate the baseline event rates assigned to standard care after the initial acute episode. Although this approach was considered to provide a number of advantages for informing NHS decision-making, compared with using the event rates reported in the control arm from EPHESUS, there remain a number of potential uncertainties with this approach. First, although covariate adjustment based on the year of the cohort provided an approach to ensure that the risk predictions reflected the event rates for the most recent cohort possible (year 2002), important changes may have occurred within standard care since this time. Second, the identification of patients in the SMR dataset required patients to be hospitalised for their HF. Although information on the underlying severity of HF was not reported in the SMR dataset, it is plausible that this cohort may have more severe HF than the patients recruited within EPHESUS. To examine the robustness of the results to these sources of uncertainty, an attempt was made to calibrate and subsequently adjust the risk prediction equations applied to mortality using external sources.
The approach to calibration and subsequent adjustment of the mortality rates assigned to standard care was based on data from the Seattle Heart Failure Model. 161 This model, developed at the University of Washington, can be used to estimate survival rates up to 5 years after the diagnosis of HF. The risk calculator derived from the model can also be used to estimate survival rates according to particular patient characteristics and current medical management. Five-year mortality rates were therefore estimated from the Seattle model for a cohort of patients matched according to the baseline characteristics reported in EPHESUS and assumed to be receiving optimal medical management (excluding aldosterone antagonists). The 5-year mortality rates were approximately 30% lower than those derived from the SMR risk prediction equations. An adjustment was therefore applied to the SMR mortality prediction equations to provide comparable 5-year predictions.
The cost-effectiveness and EVPI results, based on the adjustments applied to the SMR mortality predictions are reported in Table 24 and Table 25, assuming a maximum treatment duration of 2 years. Although the adjusted estimates had an important effect on the absolute QALY estimates, both the ICER and EVPI estimates appeared robust to the adjustment applied. The ICER of eplerenone compared with standard care was £5010 per QALY compared with £4457 per QALY reported in the base-case scenario, which used the SMR risk prediction equations directly. The EVPI results did not appear significantly altered by the adjustment.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 5.5422 | £4977 | N/A | 0.02 | 0.003 | 0.002 |
| Spironolactone | 5.5037 | £5046 | D | 0.388 | 0.344 | 0.328 |
| Eplerenone | 5.7720 | £6128 | £5010 | 0.592 | 0.653 | 0.67 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £1029 | £266,061,414 |
| £20,000 | £1754 | £453,336,787 |
| £30,000 | £2497 | £645,260,716 |
Table 26 and Table 27 report the cost-effectiveness and EVPI results assuming a lifetime treatment duration. Again both sets of results appeared robust to the adjustment applied, resulting in similar conclusions regardless of whether the baseline mortality rates were adjusted or not.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 5.5410 | £4975 | N/A | 0.262 | 0.026 | 0.008 |
| Spironolactone | 5.5356 | £5344 | D | 0.461 | 0.39 | 0.359 |
| Eplerenone | 6.0230 | £9733 | £9869 | 0.277 | 0.584 | 0.633 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £3498 | £904,155,971 |
| £20,000 | £4845 | £1,252,334,517 |
| £30,000 | £6501 | £1,680,350,316 |
Scenario 3: Generic eplerenone
Although not currently available in the UK NHS, the arrival of a generic version of eplerenone appears imminent. The potential lower price of a generic version may have important implications for the cost-effectiveness and VOI estimates. In the absence of a current price, the price of generic eplerenone is subject to uncertainty. In general, generic prices are highly dependent on the policy environment162 and competition. Impact factors may include the following:163,164
-
average revenue per brand name extended unit
-
number of extended units sold before patent loss
-
age of market in terms of time the brand-name product was sold
-
time since the patent expired
-
average revenue per generic extended unit.
Given that generic competition can be expected for eplerenone, three alternative scenarios were considered: (1) a price of 25% of the branded version, corresponding with the ratio of the average price of generic to branded drugs in the UK, (2) a price of 50% of the branded version, and (3) a price of 75% of the branded version.
The cost-effectiveness and EVPI results based on a 25% reduction are reported in Tables 28–31 for a 2-year treatment duration and lifetime treatment duration. Applying a 25% price reduction did not alter the ordering of the strategies for the ICER calculations and spironolactone was either dominated (2-year treatment duration) or extendedly dominated (lifetime treatment duration). The ICER of eplerenone compared with standard care alone was £3257 per QALY (2-year treatment duration) and £6080 per QALY (lifetime treatment duration). The corresponding ICERs based on the current branded price were £4457 per QALY and £7893 per QALY. As expected, the reduction in price improved the cost-effectiveness estimates for eplerenone in both scenarios, reinforcing the conclusion that eplerenone appears the most cost-effective strategy. The improved ICER estimates also resulted in a reduction in the uncertainty surrounding the decision itself and the subsequent EVPI estimates. However, the reduction in uncertainty appeared relatively marginal with significant uncertainty still apparent between eplerenone and spironolactone. The EVPI estimates ranged from £254M to £677M and from £699M to £1658M, across the cost-effectiveness thresholds considered assuming a 2-year and lifetime treatment duration, respectively.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.6011 | £4131 | N/A | 0.008 | 0.001 | 0.001 |
| Spironolactone | 4.5592 | £4193 | D | 0.355 | 0.325 | 0.32 |
| Eplerenone | 4.8525 | £5018 | £3527 | 0.637 | 0.674 | 0.679 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £984 | £254,207,448 |
| £20,000 | £1798 | £464,655,674 |
| £30,000 | £2619 | £676,984,002 |
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5968 | £4131 | N/A | 0.043 | 0.004 | 0.002 |
| Spironolactone | 4.6177 | £4445 | ED | 0.405 | 0.348 | 0.332 |
| Eplerenone | 5.1103 | £7253 | £6080 | 0.552 | 0.648 | 0.666 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £2706 | £699,486,288 |
| £20,000 | £4529 | £1,170,572,893 |
| £30,000 | £6416 | £1,658,182,211 |
The cost-effectiveness and EVPI results based on a 50% reduction are reported in Tables 32–35 for a 2-year treatment duration and lifetime treatment duration. Applying a higher price reduction resulted in further improvements in the ICER for eplerenone compared with standard care, although significant decision uncertainty still remained.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5975 | £4129 | N/A | 0.002 | 0.001 | 0.001 |
| Spironolactone | 4.5547 | £4192 | D | 0.335 | 0.321 | 0.318 |
| Eplerenone | 4.8484 | £4782 | £2602 | 0.663 | 0.678 | 0.681 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £897 | £231,968,238 |
| £20,000 | £1716 | £443,555,913 |
| £30,000 | £2537 | £655,828,534 |
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5924 | £4130 | N/A | 0.015 | 0.005 | 0.002 |
| Spironolactone | 4.6133 | £4444 | ED | 0.361 | 0.33 | 0.321 |
| Eplerenone | 5.1051 | £6329 | £4289 | 0.624 | 0.665 | 0.677 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £2324 | £600,798,024 |
| £20,000 | £4204 | £1,086,657,391 |
| £30,000 | £6107 | £1,578,465,962 |
Given that there could be substantial generic competition for eplerenone, the cost-effectiveness and EVPI results based on a 75% reduction are reported in Tables 36–39 for a 2-year treatment duration and lifetime treatment duration. Again, the higher price reduction resulted in improvements in the ICER for eplerenone compared with standard care but significant decision uncertainty remains. A change in the cost of eplerenone has only a small effect on EVPI relative to a change in the effectiveness parameters.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.6021 | £4131 | N/A | 0.000 | 0.000 | 0.000 |
| Spironolactone | 4.5598 | £4193 | D | 0.318 | 0.315 | 0.312 |
| Eplerenone | 4.8535 | £4550 | £1666 | 0.682 | 0.685 | 0.688 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £820 | £211,998,091 |
| £20,000 | £1643 | £424,732,168 |
| £30,000 | £2467 | £637,557,938 |
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5976 | £4129 | N/A | 0.004 | 0.001 | 0.001 |
| Spironolactone | 4.6186 | £4444 | ED | 0.325 | 0.318 | 0.316 |
| Eplerenone | 5.1107 | £5405 | £2487 | 0.671 | 0.681 | 0.683 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £1998 | £516,398,468 |
| £20,000 | £3910 | £1,010,644,127 |
| £30,000 | £5825 | £1,505,674,962 |
Scenario 4: Network of evidence
Given the methodological uncertainty surrounding the evidence synthesis approaches applied in the main analyses, a range of alternative approaches were considered and the robustness of the results was explored. In particular, the evidence synthesis undertaken to inform the decision model incorporated a range of trials that were not considered within the main clinical effectiveness review. Although it was considered necessary within the modelling framework to link to a wider evidence network to address the problems noted in the clinical effectiveness review of making indirect comparisons based on RALES and EPHESUS, it should be recognised that this approach incorporated a number of additional studies that were beyond the specific patient population of interest, and had not been quality assessed or did not meet the inclusion criteria for the clinical review in Chapter 3. As a result, it is important to consider the robustness of the evidence synthesis to the inclusion of these additional studies.
The first scenario considered was the exclusion of HF trials reporting follow-up durations of < 3 months. As noted in Chapter 3, the survival curves in RALES did not start to diverge until approximately 3 months, with the divergence continuing over the course of the trial. Consequently, the inclusion of HF studies with short-term follow-up could potentially introduce a source of potential bias into the wider evidence network used to inform the decision model.
Tables 40–43 report the cost-effectiveness and EVPI results, excluding these trials, for a 2-year treatment duration and lifetime treatment duration. The results demonstrate that the results appeared robust to exclusion of HF trials reporting follow-up durations of < 3 months. Estimates of the ICER of eplerenone compared with routine care were very close to those obtained by including these studies. However, spironolactone was no longer dominated for the lifetime treatment duration scenario. In this scenario the ICER for spironolactone was £3684 per QALY compared with standard care. As spironolactone was not excluded from the ICER calculations, the ICER for eplerenone was now compared with spironolactone. The ICER for eplerenone was £8962 per QALY compared with spironolactone, demonstrating that eplerenone remained the most cost-effective strategy based on conventional cost-effectiveness thresholds. These results demonstrate the robustness of the main synthesis and provide an indication that the results from the shorter-term studies were not inconsistent with the other studies included in the wider network of evidence.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.6027 | £4132 | N/A | 0.019 | 0.004 | 0.002 |
| Spironolactone | 4.6008 | £4220 | D | 0.419 | 0.384 | 0.367 |
| Eplerenone | 4.8517 | £5250 | £4489 | 0.562 | 0.612 | 0.631 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £1216 | £314,208,489 |
| £20,000 | £2148 | £555,297,529 |
| £30,000 | £3095 | £799,840,794 |
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5990 | £4129 | N/A | 0.122 | 0.011 | 0.005 |
| Spironolactone | 4.6979 | £4493 | £3684 | 0.471 | 0.413 | 0.392 |
| Eplerenone | 5.1076 | £8165 | £8962 | 0.407 | 0.576 | 0.603 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £3548 | £917,064,170 |
| £20,000 | £5574 | £1,440,561,037 |
| £30,000 | £7753 | £2,003,813,110 |
Although the exclusion of these trials did not alter the cost-effectiveness conclusions, there was marginally higher uncertainty surrounding this decision. The higher uncertainty translated into higher EVPI estimates. This provides an indication of the value derived from linking to the wider evidence network in the overall approach in terms of reducing the overall uncertainty surrounding the decision of interest.
Another important issue is the inclusion of canrenone studies in the overall network of evidence used to inform the cost-effectiveness analysis. As noted in Chapter 1 (see Canrenone), neither canrenone nor potassium canrenoate are licensed as human medicines in the UK. However, the wider network of evidence incorporated trial evidence for canrenone to strengthen the overall evidence base considered for the comparison of spironolactone and eplerenone. Clearly it is possible that the relationships assumed between the different drugs and the populations being considered may not be appropriate for each of the separate drugs. The inclusion of canrenone studies could introduce another source of potential bias into the synthesis results so the impact of excluding these studies was considered.
Tables 44–47 report the cost-effectiveness and EVPI results excluding the canrenone trials for a 2-year treatment duration and lifetime treatment duration. The impact of excluding these trials was similar to that of excluding the shorter-term trials in HF. That is, while the ICER results appeared robust to the exclusion of this evidence, the decision uncertainty increased accordingly. In addition to demonstrating the robustness of the main synthesis, the analysis provides further evidence that the canrenone studies did not appear to be inconsistent with the wider set of studies included in the overall network and that their inclusion was important in terms of reducing the overall uncertainty.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5947 | £4130 | N/A | 0.016 | 0.005 | 0.004 |
| Spironolactone | 4.5340 | £4177 | D | 0.374 | 0.343 | 0.324 |
| Eplerenone | 4.8445 | £5249 | £4480 | 0.61 | 0.652 | 0.672 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £1212 | £313,203,503 |
| £20,000 | £2162 | £558,730,255 |
| £30,000 | £3124 | £807,399,276 |
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.6000 | £4131 | N/A | 0.113 | 0.018 | 0.011 |
| Spironolactone | 4.6091 | £4431 | ED | 0.429 | 0.367 | 0.351 |
| Eplerenone | 5.1096 | £8171 | £7928 | 0.458 | 0.615 | 0.638 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £3522 | £910,410,749 |
| £20,000 | £5613 | £1,450,803,067 |
| £30,000 | £7853 | £2,029,740,960 |
The final scenario considered, restricted the evidence network to the four trials that were considered to be of highest overall quality as determined by the clinical effectiveness review. 27,31,54,149 Tables 48–51 report the cost-effectiveness and EVPI results including only these four trials for a 2-year treatment duration and lifetime treatment duration. In contrast to the previous analyses, spironolactone was no longer ruled out either by dominance or by extended dominance in either the 2-year or the lifetime scenarios. Assuming a 2-year treatment duration, the ICER of spironolactone compared with standard care was £532 per QALY and the ICER of eplerenone compared with spironolactone was £1949 per QALY. Assuming lifetime treatment duration, the ICER of spironolactone compared with standard care was £43 per QALY and the ICER of eplerenone compared with spironolactone was £4798 per QALY. Hence, although spironolactone was not ruled out of the ICER calculations, the ICER of eplerenone compared with spironolactone demonstrated that eplerenone remained the most cost-effective strategy based on current cost-effectiveness thresholds.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5986 | £4131 | N/A | 0.02 | 0.008 | 0.006 |
| Spironolactone | 4.1520 | £3893 | £532 | 0.384 | 0.366 | 0.363 |
| Eplerenone | 4.8472 | £5249 | £1949 | 0.596 | 0.626 | 0.631 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £1868 | £482,896,840 |
| £20,000 | £3511 | £907,496,642 |
| £30,000 | £5163 | £1,334,413,488 |
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5958 | £4128 | N/A | 0.132 | 0.019 | 0.007 |
| Spironolactone | 4.2601 | £4114 | £43 | 0.411 | 0.381 | 0.368 |
| Eplerenone | 5.1038 | £8162 | £4798 | 0.457 | 0.6 | 0.625 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £5326 | £1,376,652,538 |
| £20,000 | £9344 | £2,415,044,069 |
| £30,000 | £13,487 | £3,485,922,590 |
While restricting the evidence network to the four highest quality studies did not alter the cost-effectiveness conclusions, decision uncertainty again increased as a result of drawing on a more restricted set of trials. Consequently the EVPI results increased markedly compared with those estimated using the wider evidence network.
Scenario 5: Assuming equivalent efficacy
The final scenario considered an alternative approach to populating the effectiveness inputs reflecting the concerns that may be expressed regarding the robustness of the underlying assumptions of the evidence synthesis model. In this scenario a class effect was assumed for the aldosterone antagonists and it was assumed that the only differences considered related to the incidence of side effects. The class effect assumes that the relative effect on mortality and non-fatal events of eplerenone and spironolactone compared with standard care are the same. In other words, the relative efficacy (and the uncertainty surrounding it) of eplerenone compared with standard care is assumed for spironolactone. Therefore, the only difference between the two drugs is differences related to the incidence of adverse effects.
It was previously reported in Chapter 3 that the evidence relating to adverse events associated with aldosterone antagonists was sparsely reported, and where evidence was available it appeared that the overall rate of adverse events and the rate of hyperkalaemia may be similar across the drugs, but the rate of gynaecomastia could be higher with spironolactone. These data also indicate that although a large proportion of adverse events occur soon after the initiation of treatment, late events do occur, and the lack of long-term data may underestimate the rate of serious adverse events.
In the absence of robust data on the incidence of side effects, this scenario focused on the potential impact of incorporating higher rates of gynaecomastia for spironolactone. Two alternative scenarios were considered: (1) gynaecomastia would reduce overall compliance with spironolactone (i.e. patients would discontinue treatment if they had gynaecomastia) and (2) gynaecomastia would not alter compliance but would result in a decrement in overall quality of life for the entire duration of treatment with spironolactone. In both scenarios it was assumed that the incidence of gynaecomastia would be 10% for patients receiving spironolactone and 0% in the other strategies. The incidence of gynaecomastia ranged from 0.25% to 9% in Table 12. However, the evidence relating to adverse events associated with aldosterone antagonists was sparsely reported, and in the absence of robust data, 10% was considered a conservative estimate.
Tables 52–55 report the cost-effectiveness and EVPI results, assuming a 10% discontinuation rate each year in the spironolactone strategy for a 2-year treatment duration and lifetime treatment duration. As anticipated, assuming a class effect and considering potential differences in side effects only had a marked effect on the overall cost-effectiveness and EVPI results. In both the 2-year and lifetime duration scenarios, the ICER of eplerenone increased above conventional thresholds of cost-effectiveness (£44,134 per QALY and £70,509 per QALY for 2-year and lifetime durations, respectively). Despite incorporating an additional risk of discontinuation for spironolactone, this strategy appears the most cost-effective treatment with an ICER of £1046 per QALY (2-year treatment duration) and £1280 per QALY (lifetime treatment duration) compared with standard care alone.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5994 | £4128 | N/A | 0.001 | 0.001 | 0.001 |
| Spironolactone | 4.8309 | £4370 | £1046 | 0.999 | 0.999 | 0.944 |
| Eplerenone | 4.8507 | £5248 | £44,134 | 0 | 0 | 0.055 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £1 | £176,276 |
| £20,000 | £1 | £330,064 |
| £30,000 | £7 | £1,813,176 |
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5975 | £4129 | N/A | 0 | 0 | 0 |
| Spironolactone | 5.0624 | £4724 | £1280 | 1 | 1 | 1 |
| Eplerenone | 5.1113 | £8173 | £70,509 | 0 | 0 | 0 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £1 | £311,307 |
| £20,000 | £2 | £536,661 |
| £30,000 | £3 | £767,520 |
Employing this particular set of assumptions has an equally marked effect on the EVPI estimates and the conclusions that could be drawn concerning the potential value of a future head-to-head trial. The results indicate that the probability that spironolactone is the most cost-effective strategy is almost one across the different cost-effectiveness thresholds. This results in estimates of the total EVPI of between £176,000 and £1.8M across the different treatment durations and thresholds, suggesting that a further trial would not appear to provide value for money.
Tables 56–59 report the cost-effectiveness and EVPI results assuming 10% of patients receiving spironolactone incur a decrement in quality of life because of adverse events, for 2 years of treatment or lifetime duration of treatment. A decrement in utlitity of 0.1 was applied in the model in this scenario consistent with the approach reported by Glick et al. 118 The results and conclusions were similar to those where a lower compliance was assumed.
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5968 | £4130 | N/A | 0.002 | 0.001 | 0.001 |
| Spironolactone | 4.8338 | £4393 | £1110 | 0.998 | 0.999 | 0.999 |
| Eplerenone | 4.8481 | £5250 | £59,996 | 0 | 0 | 0 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £1 | £259,191 |
| £20,000 | £2 | £482,336 |
| £30,000 | £3 | £707,994 |
| Strategy | QALY | Cost | ICER | Probability cost-effective | ||
|---|---|---|---|---|---|---|
| @£10,000 | @£20,000 | @£30,000 | ||||
| Standard care | 4.5912 | £4129 | N/A | 0.005 | 0.004 | 0.004 |
| Spironolactone | 5.0398 | £4790 | £1473 | 0.995 | 0.996 | 0.996 |
| Eplerenone | 5.1035 | £8173 | £53,063 | 0 | 0 | 0 |
| Cost-effectiveness threshold | Individual EVPI | Population EVPI |
|---|---|---|
| £10,000 | £4 | £1,157,492 |
| £20,000 | £7 | £1,860,689 |
| £30,000 | £10 | £2,599,258 |
Partial EVPI
Although estimates of the total EVPI provide a useful global estimate of the uncertainty surrounding the adoption decision, this estimate does not provide an indication of where further research would be of most value. Partial EVPI can be used to consider particular elements of the decision problem to direct and focus research towards the specific areas where the elimination of uncertainty has the most value. The partial EVPI can be calculated for individual parameters or subsets of parameters. This can be particularly relevant to the design of any future research because subsets of parameters can be grouped according to related areas and may also be used to separate parameters for which a randomised design is necessary and those where this may not be essential (e.g. effectiveness parameters are likely to need a randomised design to minimise bias; however, issues of bias are likely to be less critical for obtaining epidemiological or utility data and observational design may be more appropriate). Given the computational time required to perform these calculations, partial EVPI was undertaken using the base-case scenario and lifetime treatment duration scenarios only. However, the results are likely to be generalisable to the other scenarios considered.
Parameters in the model were separated into a number of distinct areas:
-
effectiveness – baseline event and relative risk estimates applied in the synthesis model; separate results are presented for mortality and hospitalisation
-
epidemiology – baseline events and long-term prognosis parameters only
-
quality of life – quality of life inputs applied in the model.
The areas reflect distinct types of research which could be conducted using different research designs. For example, decision uncertainty concerning the effectiveness parameters might be most appropriately informed by undertaking an additional RCT comparing eplerenone and spironolactone given the importance of minimising potential biases. However, uncertainty in epidemiology and quality of life would not necessarily require a randomised design and additional data could be obtained using less costly observational designs.
Figure 11 and Figure 12 report the partial EVPI estimates for a 2-year and lifetime treatment duration at a cost-effectiveness threshold of £20,000 per QALY, respectively. In this example, the partial EVPI associated with the effectiveness parameters accounts for the majority of decision uncertainty surrounding the current cost-effectiveness. Within the effectiveness parameters, the relative risks associated with mortality appear to be the main source of uncertainty in the analyses. The partial EVPI estimate clearly demonstrates the importance of the uncertainty surrounding the treatment effect of aldosterone antagonists on mortality compared with the other parameters considered in the decision model.
FIGURE 11.
Partial EVPI estimates for 2-year treatment duration.
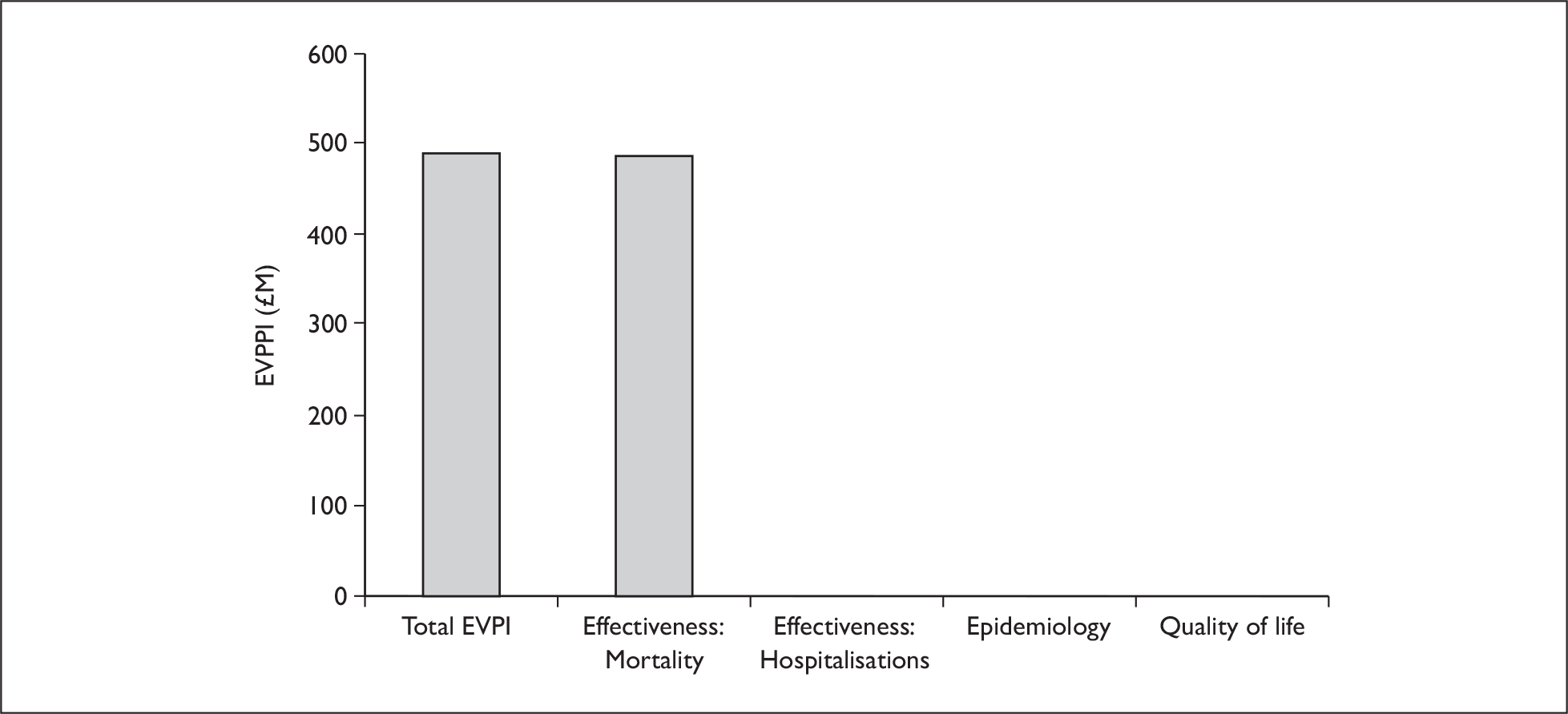
FIGURE 12.
Partial EVPI estimates for lifetime treatment duration.
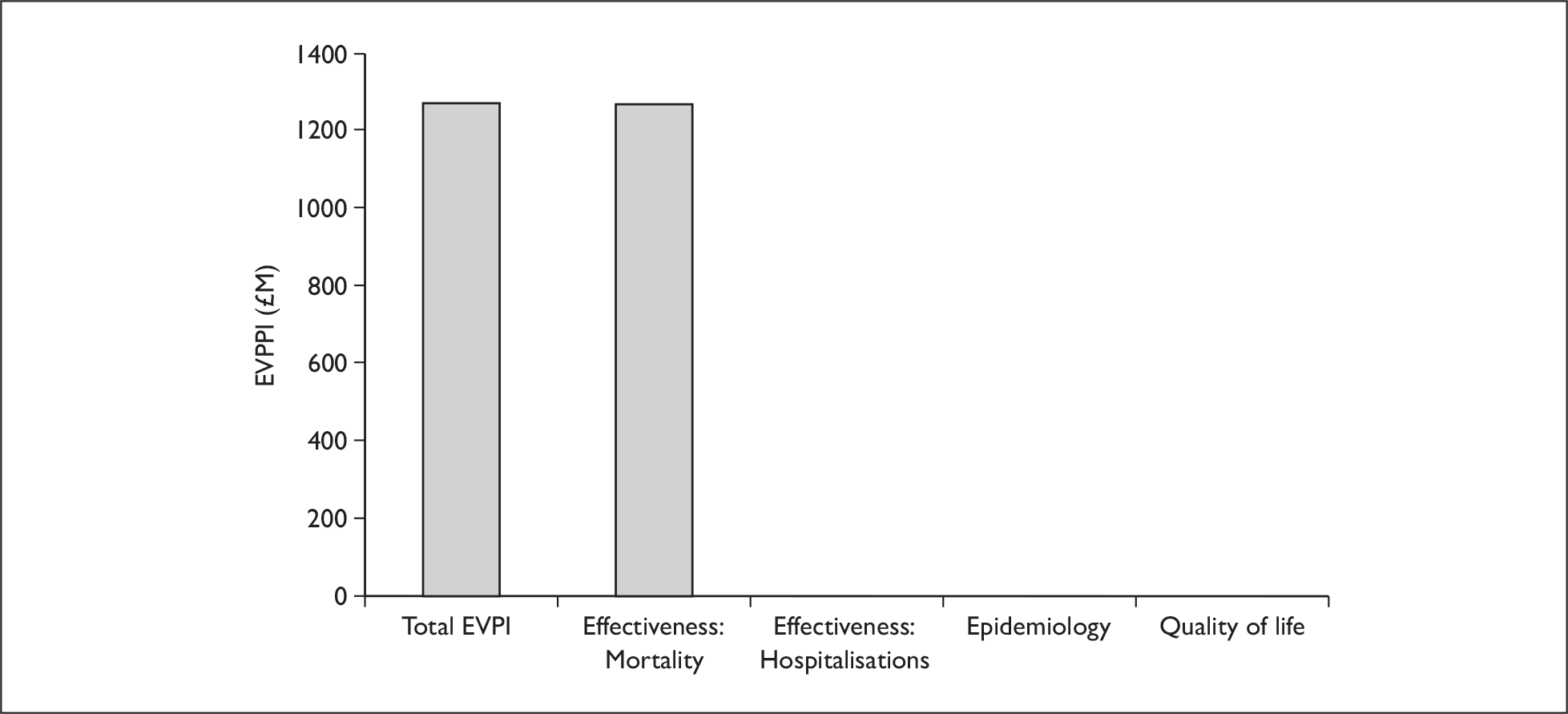
Discussion of findings from the York model
A new decision analytic model was developed to estimate the cost-effectiveness of spironolactone and eplerenone, in addition to standard care, for the management of postMI HF. Results from this model were presented for a base-case analysis assuming 2-year treatment duration and for a lifetime treatment duration. A range of scenarios were considered to explore the robustness of the cost-effectiveness results to alternative parameter inputs and assumptions.
A Bayesian meta-regression approach was used to model the relationship between the relative effectiveness of spironolactone and eplerenone in the separate populations (postMI HF and HF). This incorporated evidence from a wider ‘network’ of trials to those considered in the main clinical effectiveness review. This approach was taken because of the difficulties in basing an indirect comparison on the results of the trials of postMI/ischaemic patients with reduced LVEF and clinical signs of HF that met the inclusion criteria for the review of clinical effectiveness. Trials were incorporated that recruited three distinct populations: postMI with reduced LVEF but without clinical signs of HF; postMI with reduced LVEF and clinical signs of HF; and general HF due to causes other than MI. By drawing on a wider evidence network, more information was used to derive estimates of the efficacy of each treatment in the separate populations and also provided estimates to inform an indirect comparison of eplerenone and spironolactone in the postMI HF population. The results indicated that for postMI HF, eplerenone is associated with a significant reduction in mortality compared with standard care alone (RR = 0.86, 95% CrI 0.77 to 0.96). However, there appeared greater uncertainty around the estimate for spironolactone, which was not significantly different from standard care alone (RR = 1.02, 95% CrI 0.58 to 1.66).
The results of the York model are not directly comparable to those of the three published models because none of the previous models have attempted to undertake a direct comparison of the cost-effectiveness of spironolactone versus eplerenone. The previous models have only considered the cost-effectiveness of spironolactone or eplerenone versus standard care. The results from the previous models suggest that an aldosterone antagonist (spironolactone or eplerenone) relative to standard care is cost-effective. This is consistent with the results of the York model but the York model addresses the relative cost-effectiveness of spironolactone to eplerenone.
Key findings from cost-effectiveness analysis
When the results of the evidence synthesis were used to inform the relative effectiveness estimates in the model, eplerenone consistently emerged as the most cost-effective strategy for the management of postMI HF. For 2-year treatment duration, the ICER of eplerenone compared to standard care was £4457 per QALY. This increased to £7893 per QALY assuming that treatment with eplerenone was continued over a patient’s lifetime. In both of these scenarios spironolactone was either dominated or extendedly dominated by eplerenone or standard care. The results were robust to a range of alternative assumptions including applying alternative baseline event rates and incorporating potential price reductions for a generic version of eplerenone. In each of the scenarios based on the evidence synthesis results, the ICER of eplerenone was consistently under the £20,000–£30,000 per QALY threshold of cost-effectiveness conventionally used to establish value for money in the NHS.
In the absence of direct head-to-head evidence and the inevitable uncertainties in the meta-regression analysis, an alternative scenario was also considered assuming a ‘class effect’ for the aldosterone antagonists in terms of major clinical events but allowing for potential differences in their side effect profiles. This scenario had an important effect on the cost-effectiveness results with spironolactone now appearing the most cost-effective strategy. Although eplerenone was not ruled out by either dominance or extended dominance, the ICER of eplerenone compared with spironolactone exceeded the £20,000–30,000 threshold.
Key findings from the value of information analysis
Bayesian VOI analysis was undertaken to determine the expected costs of decision uncertainty predicted by the model and the maximum value that can be placed on additional research aimed at reducing this uncertainty. The estimates of EVPI provide an upper boundary for the value of additional research and provide a necessary hurdle for determining the potential efficiency of further primary research. This analysis can therefore be used as the basis to inform policy decisions relating to future research priorities and study design issues in this area. The population EVPI estimates suggest that further research is likely to be of significant value. The results indicate a considerable range in the population EVPI estimates, between £820M (base-case) and £1265M (lifetime treatment duration scenario). This uncertainty was driven by the relative treatment effects of mortality between eplerenone and spironolactone, indicating that a future head-to-head RCT of these two treatments in a postMI HF population would be likely to be highly valuable. This conclusion remained robust to a range of alternative assumptions including the potential price reduction of a generic version of eplerenone. However, the results of the scenario analyses also demonstrated that the EVPI results appear highly sensitive to whether the cost-effectiveness analysis was based on the results of the Bayesian meta-regression or a class-effect was assumed. In the latter case the EVPI results indicate that undertaking further primary research would be unlikely to represent value for money.
Strengths and limitations of the assessment
Strengths
The decision model was developed to address a number of important evidence gaps identified concerning the use of aldosterone antagonists for the management of post-MI HF; most notably the lack of previous studies directly comparing the potential cost-effectiveness of alternative licensed aldosterone antagonists (eplerenone and spironolactone) and the absence of cost-effectiveness studies undertaken from the perspective of the NHS. The Bayesian synthesis incorporated evidence from a wider evidence network of aldosterone trials to address the problems noted with basing an indirect comparison on the results of the postMI HF trials alone. The main strength of this approach is that it provides more robust predictions on efficacy for the treatments in the populations where existing data are limited by ‘borrowing strength’ from the relationships that exist between the treatments and between the separate populations. A further strength is that the robustness of the results to the inclusion/exclusion of particular studies (e.g. studies considered to be of a lower quality) was explored and provided evidence that appeared to support consistency in the wider set of studies included in the Bayesian synthesis with those considered in the main clinical effectiveness review. Finally, a range of scenarios were explored to examine the robustness of the cost-effectiveness and EVPI results to a series of alternative assumptions.
Limitations
The cost-effectiveness and EVPI results are subject to several important limitations. The strength of conclusions that can be drawn based on the current set of cost-effectiveness results clearly depends on the validity of the Bayesian evidence synthesis approach employed. There remain a number of difficulties in making a reliable comparison between spironolactone and eplerenone based on evidence from either the main RCTs (RALES, EPHESUS) or from combining these with the results of the additional studies included in the Bayesian synthesis. The synthesis approach employed requires the trials to be exchangeable within, although not across, the HF and postMI HF populations. Given the relatively low number of studies and the small sample sizes for many of the studies considered, the statistical absence of heterogeneity within the separate populations may not provide sufficient evidence to support the assumption employed. Furthermore, it should be emphasised that the existing RCT evidence for spironolactone in a postMI HF population is extremely limited compared with that for eplerenone. While this is reflected in the wider confidence intervals for the treatment effect estimate applied to spironolactone, the lack of robust evidence specifically in the postMI HF population represents a major limitation in terms of informing current service provision in the NHS.
It should also be recognised that data on other key inputs into the model were less well reported in the RCTs. In particular, data on non-fatal events requiring hospitalisation were only reported in a few of the RCTs, which meant that it was not possible to undertake a robust synthesis of these data directly. Instead it was assumed that the relative difference between eplerenone and spironolactone estimated for all-cause mortality would also be similar for non-fatal events. There was also a lack of data on the adverse events associated with the aldosterone antagonists. Given the paucity of good quality evidence and the diversity of dosages and populations from which data were derived, it was not considered possible to draw reliable conclusions.
There also exist limitations with respect to the strength of existing evidence regarding the optimal duration of treatment with aldosterone antagonists. The base-case analysis assumed a maximum duration of treatment of 2 years consistent with the mean follow-up reported for EPHESUS. An alternative scenario was also considered which demonstrated that longer-term treatment could be potentially cost-effective. However, it should be noted that there is limited evidence on the longer-term effectiveness of aldosterone antagonists and hence the results of this scenario (based on assuming that the relative effect will remain constant over the longer term) should be seen as exploratory.
The use of long-term observational evidence from the SMR data addressed potential concerns over the generalisability of the baseline event rates reported in the trials to UK practice. However, the SMR data are subject to several potential limitations. First, the long-term follow-up available in the SMR data represents both a relative strength and a potential limitation because the data may no longer adequately reflect current NHS practice. Second, the population included from the SMR data is unlikely to precisely match the postMI HF population considered in the EPHESUS study. That is, details of the timing of previous MI were not reported in the SMR data and similarly no details were reported in relation to the severity of the HF. Consequently there exist a number of potential sources of uncertainty related to the use of these data as a source of baseline data within the cost-effectiveness analysis. However, it should also be noted that approaches were taken to explore the impact of these sources of uncertainty by using data from EPHESUS for the acute period and attempting to recalibrate the baseline mortality data to more closely reflect the baseline characteristics of the EPHESUS population and to incorporate changes in current practice. Importantly, the cost-effectiveness and EVPI results appeared robust to these alterations.
Finally, although the EVPI results demonstrated significant potential value in undertaking further research in the form of a head-to-head RCT, the EVPI results present an upper bound to further research and hence do not provide both a necessary and sufficient condition, even if the cost of a trial fell below this amount. This is because a trial will resolve only a proportion of the uncertainty and as such, the amount of uncertainty that is likely to be resolved would have to be assessed against the cost of the trial to ensure that any further research was considered an efficient use of resources. Additional work would therefore need to be undertaken to establish the efficiency and optimal design of a future RCT.
Uncertainties
There remains a large degree of uncertainty surrounding the relative effectiveness of spironolactone and eplerenone in a postMI HF population. This uncertainty relates both to major clinical events as well as to the incidence of side effects. This uncertainty also extends to the optimal duration of treatment with aldosterone antagonists. Although these issues were considered in the cost-effectiveness analysis, they can only be reliably addressed by further research. The most appropriate study design would appear to be an RCT directly comparing eplerenone and spironolactone.
Chapter 6 Conclusions
Conclusions from review of clinical effectiveness
The only good quality trial evidence for aldosterone inhibitors in the postMI HF population comes from a trial of eplerenone (EPHESUS) whereas spironolactone has been studied in HF in RALES. The lack of exchangeability of these trials with respect to study populations, beta-blocker use and other issues such as concurrent medication, means that a simple indirect comparison between these drugs using these trials could not produce clinically meaningful results. To evaluate the efficacy of spironolactone in postMI HF patients a contemporary trial directly comparing eplerenone and spironolactone appears warranted. However, whether this is would be worthwhile from a cost-effectiveness and clinical standpoint is unknown.
Conclusions from economic evaluation
When the results of the Bayesian synthesis were applied within the economic model, eplerenone appeared to be the most cost-effective strategy for the management of postMI HF. The cost-effectiveness results were remarkably robust to a range of alternative assumptions and parameter inputs and the ICER of eplerenone was consistently under the threshold of cost-effectiveness conventionally used to establish value for money in the NHS. The only scenario considered that resulted in a different conclusion regarding cost-effectiveness was when the results from the evidence synthesis were ignored and instead a class effect was assumed for both of the aldosterone antagonists.
When the results from the Bayesian evidence synthesis were used, the EVPI results consistently demonstrated potential value to the NHS in undertaking additional research to reduce the existing decision uncertainty. Decision uncertainty and the population EVPI estimates were primarily caused by the level of uncertainty surrounding the relative treatment effects of mortality between eplerenone and spironolactone. However, in common with the cost-effectiveness conclusions, when a class effect was assumed, different conclusions were reached and further primary research would appear unlikely to represent value for money to the NHS.
Despite the challenges and difficulties that emerged in attempting to undertake a formal comparison of the effectiveness and cost-effectiveness of spironolactone and eplerenone, an important finding has consistently emerged. That is, the use of an aldosterone antagonist more generally appears to be a highly cost-effective strategy for the management of postMI HF patients in the UK NHS.
Implications for service provision
Current guidelines from NICE and the Scottish Intercollegiate Guidelines Network recommend treatment with eplerenone in addition to standard therapy for patients who have had an AMI and who have symptoms and/or signs of HF and LV dysfunction. 35,36 Although neither of these guidelines formally evaluated the relative cost-effectiveness of eplerenone compared with spironolactone, the results from our study provide additional evidence to further support the recommendations from NICE and the Scottish Intercollegiate Guidelines Network at the current time. Using the best available evidence, eplerenone appears both more effective and cost-effective than either spironolactone or standard care alone for patients with postMI HF. While spironolactone appeared potentially cost-effective when a class-effect was assumed, the limited data that exist for spironolactone in a postMI HF population means that the existence of potentially important differences in the efficacy of spironolactone and eplerenone cannot be ruled out based on current evidence.
Recommendations for research
Ideally, an adequately powered, well-conducted RCT that directly compares spironolactone and eplerenone would be required to provide more robust evidence on the optimal management of postMI HF patients. Our results indicate that differences in mortality appear to be the major source of current uncertainty and hence the design and follow-up should reflect this. However, given that there is a lack of evidence for either drug in terms of hospitalisations, additional data on non-fatal events requiring hospitalisation and side effects would also be important outcomes. Although a formal assessment of the costs and benefits of a future RCT were not considered, the estimates of EVPI appear sufficiently high to conclude that a head-to-head RCT is likely to provide value for money. However, should a future RCT be considered, then before formal commissioning, a more formal assessment of the costs and benefits of alternative designs (i.e. sample size, length of follow-up) should be conducted using the cost-effectiveness model presented here, to ensure that this is done efficiently and to assess the feasibility of conducting such a trial.
Acknowledgements
The authors would like to thank Pardeep Jhund, BHF Glasgow Cardiovascular Research Centre, for assistance with the SMR data, and John Cleland, Azam Torabi and Kevin Goode, Department of Cardiology, Hull Royal Infirmary, University of Hull, for their help and advice provided at the start of the project. We would also like to thank Marek Grzes and Zhi Quan for their help with translations. The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA Programme. Any errors are the responsibility of the authors.
Contribution of authors
Claire McKenna was responsible for the review of cost-effectiveness evidence and the overall development of the economic model. Jane Burch was the reviewer responsible for study selection, data extraction, validity assessment, data analysis and writing the report. Sara Suekarran assisted in study selection, data extraction, validity assessment, data analysis and writing the report. Simon Walker assisted in the review of cost-effectiveness evidence and development of the economic model. Ameet Bakhai provided clinical input for all stages of the project and assisted in writing the report. Klaus Witte provided clinical input for all stages of the project and assisted in writing the report. Melissa Harden devised the search strategy, carried out the literature searches and wrote the search methodology sections of the report. Kath Wright helped to devise the search strategy, conduct the literature searches and assisted in the writing of the search methodology sections of the report. Nerys Woolacott provided input at all stages of the review, and took overall responsibility for the clinical effectiveness sections of the report. Paula Lorgelly undertook the analysis of the Scottish Morbidity Record (SMR) data and contributed to the development of the economic model. Liz Fenwick undertook the analysis of the SMR data and contributed to the development of the economic model. Stephen Palmer provided input at all stages of the review, and took overall responsibility for the cost-effectiveness sections of the report.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- European Society of Cardiology/American College of Cardiology Committee . Myocardial infarction redefined: a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502-13.
- Roger VL. Epidemiology of myocardial infarction. Med Clin North Am 2007;91:537-52.
- Trevelyan J, Needham EW, Smith SC, Mattu RK. Impact of the recommendations for the redefinition of myocardial infarction on diagnosis and prognosis in an unselected United Kingdom cohort with suspected cardiac chest pain. Am J Cardiol 2004;93:817-21.
- Cowie MR, Lacey L, Tabberer M. Heart failure after myocardial infarction: a neglected problem?. Br J Cardiol 2005;12:205-8.
- Miller AB. Aldosterone antagonism in heart failure. Vasc Health Risk Manag 2007;3:605-9.
- Cleland JGF, Torabi A, Khan NK. Epidemiology and management of heart failure and left ventricular systolic dysfunction in the aftermath of a myocardial infarction. Heart 2005;91:ii7-ii13.
- Velazquez EJ, Francis GS, Armstrong PW, Aylward PE, Diaz R, O’Connor CM, et al. An international perspective on heart failure and left ventricular systolic dysfunction complicating myocardial infarction: the VALIANT registry. Eur Heart J 2004;25:1911-19.
- Wu AH, Parsons L, Every NR, Bates ER. Hospital outcomes in patients presenting with congestive heart failure complicating acute myocardial infarction: a report from the Second National Registry of Myocardial Infarction (NRMI-2). J Am Coll Cardiol 2002;40:1389-94.
- British Heart Foundation Statistics database . Morbidity 2008. www.heartstats.org (accessed September 2008).
- Mattichak SJ, Harjai KJ, Dutcher JR, Boura JA, Stone G, Cox D, et al. Left ventricular remodeling and systolic deterioration in acute myocardial infarction: findings from the Stent-PAMI Study. J Interv Cardiol 2005;18:255-60.
- Weir RA, McMurray JJ, Velazquez EJ. Epidemiology of heart failure and left ventricular systolic dysfunction after acute myocardial infarction: prevalence, clinical characteristics, and prognostic importance. Am J Cardiol 2006;97:13F-25F.
- Hernandez AF, Velazquez EJ, Solomon SD, Kilaru R, Diaz R, O’Connor CM, et al. Left ventricular assessment in myocardial infarction: the VALIANT registry. Arch Intern Med 2005;165:2162-9.
- Torabi A, Cleland JG, Khan NK, Loh PH, Clark AL, Alamgir F, et al. The timing of development and subsequent clinical course of heart failure after a myocardial infarction. Eur Heart J 2008;29:859-70.
- Hellermann JP, Jacobsen SJ, Redfield MM, Reeder GS, Weston SA, Roger VL. Heart failure after myocardial infarction: clinical presentation and survival. Eur J Heart Fail 2005;7:119-25.
- Macin SM, Perna ER, Augier N, Cialzeta J, Farias EF, Fontana M, et al. Clinical characteristics and long-term outcome in patients with heart failure complicating acute myocardial infarction. Rev Esp Cardiol 2005;58:789-96.
- Chatterjee K, Massie B. Systolic and diastolic heart failure: differences and similarities. J Card Fail 2007;13:569-76.
- Gary R, Davis L. Diastolic heart failure. Heart Lung 2008;37:405-16.
- Brookes L. Incorrect Classification of Patients by the AHA ACC Stages of Heart Failure. Medscape Conference Coverage, Based on Selected Sessions at The: Heart Failure Society of America 8th Annual Scientific Meeting 2004. www.medscape.com/viewarticle/490041 (accessed 15 January 2009).
- Killip T, Kimball JT. Treatment of myocardial infarction in a coronary care unit. A two year experience with 250 patients. Am J Cardiol 1967;20:457-64.
- Jessani S, Lip GYH. Death or heart failure post acute myocardial infarction? The role of cardiac biomarkers. J Card Fail 2006;12:641-3.
- Lacey L, Tabberer M. Economic burden of post-acute myocardial infarction in the United Kingdom. Eur J Heart Fail 2005;7:677-83.
- National Collaborating Centre for Chronic Conditions . Chronic Heart Failure. National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care 2003.
- Scottish Intercollegiate Guidelines Network . Management of Chronic Heart Failure: A National Clinical Guideline 2007.
- Electronic Medicines Compendium. London: Datapharm Communications Ltd; 2008.
- British Heart Foundation statistics website . Total Cost of Heart Failure to the NHS 2009. www.heartstats.org/datapage.asp?id=817 (accessed 1 May 2009).
- British Heart Foundation statistics website . Morbidity. 2009. www.heartstats.org/topic.asp?id=18 (accessed 1 May 2009).
- Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-21.
- Scottish Medicines Consortium . Eplerenone 25mg and 50mg Tablets (Ispra). Pfizer Ltd. Resubmission 2005. www.scottishmedicines.org.uk/smc/files/eplerenone_Inspra_RESUBMISSION(136–04).pdf (accessed September 2008).
- Weintraub WS, Zhang Z, Mahoney EM, Kolm P, Spertus JA, Caro J, et al. Cost-effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure. Circulation 2005;111:1106-13.
- Hayashi M, Tsutamoto T, Wada A, Tsutsui T, Ishii C, Ohno K, et al. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post-infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation 2003;107:2559-65.
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709-17.
- Cleland JGF, Coletta AP, Abdellah AT, Witte KK, Hobson N, Clark AL. Clinical trials update from Heart Rhythm 2007 and Heart Failure 2007: CARISMA, PREPARE, DAVID II, SAVE-PACE, PROTECT and AREA-IN-CHF. Eur J Heart Fail 2007;9:850-3.
- Martindale: the complete drug reference. London: Pharmaceutical Press; 2007.
- Croom K, Plosker G. Eplerenone: a pharmacoeconomic review of its use in post-myocardial infarction heart failure. Pharmacoeconomics 2005;23:1057-72.
- Cooper A, Skinner J, Nherera L, Feder G, Ritchie G, Kathoria M, et al. Clinical guidelines and evidence review for post myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction. London: National Collaborating Centre for Primary Care and Royal College of General Practitioners; 2007.
- Scottish Intercollegiate Guidelines Network . Acute Coronary Syndrome: A National Clinical Guideline 2007.
- McInnes GT, Aronson JK, Dukes MNG. Meyler’s Side effects of drugs. Amsterdam: Elsevier Science; 2000.
- McEvoy GK. AHFS drug information. Bethesda: American Society of Health-System Pharmacists, Inc.; 2008.
- McEvoy GK. AHFS drug information. Bethesda: American Society of Health-system Pharmacists, Inc.; 2008.
- Marciniak TA. U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Clinical Review for SNDA 21–437 Inspra Tablets (Parts 1–4) 2003. www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21–437s002_Inspra.cfm (accessed 28 January 2009).
- US Food and Drug Administration Centre for Drug Evaluation and Research . Label Information INSPRA (eplerenone) Tablets 2008. www.accessdata.fda.gov/drugsatfda_docs/label/2008/021437s006lbl.pdf (accessed 20 January 2009).
- British National Formulary (BNF). London: BMA and RPS; 2009.
- National Institue of Clinical Effectiveness . Management of Chronic Heart Failure in Adults in Primary and Secondary Care. Clinical Guideline 5. 2003. www.nice.org.uk/nicemedia/pdf/CG5NICEguideline.pdf (accessed September 2008).
- Pharmacia Limited . Aldactone 25mg, 50mg and 100mg Tablets 2007. http://emc.medicines.org.uk/ (accessed 13 November 2008).
- Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, et al. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens 2004;18:139-85.
- British Hypertension Society . Drug Classes: Other Diuretics 2008. www.bhsoc.org/pdfs/therapeutics/Other%20Diuretics.pdf (accessed 16 March 2009).
- National Institute of Clinical Effectiveness . Management of Hypertension in Adults in Primary Care. NICE Clinical Guideline 34 2006. www.nice.org.uk/nicemedia/pdf/CG034NICEguideline.pdf (accessed September 2008).
- Ruta J, Ptaszyski P, Maciejewski M, Chizyski K, Goch JH. Effect of spironolactone on mortality in patients with severe left ventricular dysfunction after acute myocardial infarction. Przegl Lek 2006;63:1249-51.
- Tu H, Chen HB. The efficacy and safety of spironolactone in the treatment of chronic heart failure after acute myocardial infarction. Hebei Med 2003;9:908-10.
- Boccanelli A, Mureddu GF, Cacciatore G, Clemenza F, Di Lenarda A, Gavazzi A, et al. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (AREA IN-CHF study): final results. Eur J Heart Fail 2009;11:68-76.
- Pitt B, Ahmed A, Love TE, Krum H, Nicolau J, Cardoso JS, et al. History of hypertension and eplerenone in patients with acute myocardial infarction complicated by heart failure. Hypertension 2008;52:271-8.
- Pitt B, Gheorghiade M, Zannad F, Anderson JL, van Veldhuisen DJ, Parkhomenko A, et al. Evaluation of eplerenone in the subgroup of EPHESUS patients with baseline left ventricular ejection fraction ≤ 30%. Eur J Heart Fail 2006;8:295-301.
- Cacciatore G, Boccanelli A, Mureddu G, Maggioni A, Latini R, Masson S, et al. The AREA IN-CHF trial (antiremodeling effect of aldosterone receptors blockade with canrenone in mild chronic heart failure): rationale and design. Ital Heart J 2005;6:66S-74S.
- Boccanelli A, Cacciatore G, Mureddu GF, de Simone G, Clemenza F, De Maria R, et al. Baseline characteristics of patients recruited in the AREA IN-CHF study (Antiremodelling Effect of Aldosterone Receptors Blockade with Canrenone in Mild Chronic Heart Failure). J Cardiovasc Med 2007;8:683-91.
- Casado-Escribano PP, Gargallo-Garcia E, Conthe P. Follow-up of patients with heart insufficiency in anti-aldosterone therapy in clinical practice. Med Clin Monograf 2005;6:33-8.
- Passino C, Ry SD, Severino S, Gabutti A, Prontera C, Clerico A, et al. C-type natriuretic peptide expression in patients with chronic heart failure: effects of aerobic training. Eur J Cardiovasc Prev Rehabil 2008;15:168-72.
- Nassiacos D, Meloni S. Tolerability and efficacy of aldosterone inhibition with canrenone in heart failure: the real-world experience of an outpatient heart failure clinic. Ital Heart J 2005;6:51S-65S.
- Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. Hyperkalaemia and impaired renal function in patients taking spironolactone for congestive heart failure: retrospective study. BMJ 2003;327:1141-2.
- Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure?. J Card Fail 2004;10:297-303.
- Lawson DH, O’Connor PC, Jick H. Drug attributed alterations in potassium handling in congestive cardiac failure. Eur J Clin Pharmacol 1982;23:21-5.
- Greenblatt DJ, Koch-Weser J. Adverse reactions to spironolactone. a report from the Boston Collaborative Drug Surveillance Program. JAMA 1973;225:40-3.
- Shah KB, Gottlieb SS. Laboratory monitoring for spironolactone in congestive heart failure. Cardiol Rev 2006;23:35-7.
- Smith AGE. Spironolactone in the long-term management of patients with congestive heart failure. Curr Med Res Opin 1980;7:131-6.
- Hauben M, Reich L, Gerrits CM, Madigan D. Detection of spironolactone-associated hyperkalaemia following the Randomized Aldactone Evaluation Study (RALES). Drug Saf 2007;30:1143-9.
- Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, et al. Rates of hyperkalemia after publication of the randomized aldactone evaluation study. N Engl J Med 2004;351:543-51.
- Witham MD, Gillespie ND, Struthers AD. Tolerability of spironolactone in patients with chronic heart failure: a cautionary message. Br J Clin Pharmacol 2004;58:554-7.
- Sligl W, McAlister FA, Ezekowitz J, Armstrong PW. Usefulness of spironolactone in a specialized heart failure clinic. Am J Cardiol 2004;94:443-7.
- Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. Hyperkalemia and impaired renal function in patients taking spironolactone for congestive heart failure. A retrospective analysis of 125 consecutive cases. Ugeskr Laeger 2004;166:3201-3.
- Anton C, Cox AR, Watson RDS, Ferner RE. The safety of spironolactone treatment in patients with heart failure. J Clin Pharm Ther 2003;28:285-7.
- Tamirisa KP, Aaronson KD, Koelling TM. Spironolactone-induced renal insufficiency and hyperkalemia in patients with heart failure. Am Heart J 2004;148:971-8.
- Williams EM, Katholi RE, Karambelas MR. Use and side-effect profile of spironolactone in a private cardiologist’s practice. Clin Cardiol 2006;29:149-53.
- Delyani JA. Mineralocorticoid receptor antagonists: the evolution of utility and pharmacology. Kidney Int 2000;57:1408-11.
- Struthers A, Krum H, Williams GH. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin Cardiol 2008;31:153-8.
- Sica DA. Pharmacokinetics and pharmacodynamics of mineralocorticoid blocking agents and their effects on potassium homeostasis. Heart Fail Rev 2005;10:23-9.
- Garthwaite SM, McMahon EG. The evolution of aldosterone antagonists. Mol Cell Endocrinol 2004;217:27-31.
- Dynamed Editorial Team . Spironolactone for Heart Failure 2008. http://dynaweb.ebscohost.com/Detail.aspx?id=233224%26sid=8a5af014–22bb-4fa6–826f-f327310661b3@sessionmgr9 (accessed 2 September 2008).
- Brown NJ. Eplerenone: cardiovascular protection. Circulation 2003;107:2512-18.
- Reyes AJ, Leary WP, Crippa G, Maranhao MF, Hernandez-Hernandez R. The aldosterone antagonist and facultative diuretic eplerenone: a critical review. Eur J Intern Med 2005;16:3-11.
- Dynamed Editorial Team . Eplerenone 2008. http://dynaweb.ebscohost.com/Detail.aspx?id=232890%26sid=8a5af014–22bb-4fa6–826f-f327310661b3@sessionmgr9.
- Cook CS, Berry LM, Bible RH, Hribar JD, Hajdu E, Liu NW. Pharmacokinetics and metabolism of [14C]eplerenone after oral administration to humans. Drug Metab Dispos 2003;31:1448-55.
- Ravis WR, Reid S, Sica DA, Tolbert DS. Pharmacokinetics of eplerenone after single and multiple dosing in subjects with and without renal impairment. J Clin Pharmacol 2005;45:810-21.
- Rogerson FM, Yao Y, Smith BJ, Fuller PJ. Differences in the determinants of eplerenone, spironolactone and aldosterone binding to the mineralocorticoid receptor. Clin Exp Pharmacol Physiol 2004;31:704-9.
- Thattassery E, Gheorghiade M. Beta blocker therapy after acute myocardial infarction in patients with heart failure and systolic dysfunction. Heart Fail Rev 2004;9:107-13.
- Borrello F, Beahan M, Klein L, Gheorghiade M. Reappraisal of beta-blocker therapy in the acute and chronic post-myocardial infarction period. Rev Cardiovasc Med 2003;4:S13-24.
- Azuma J, Nonen S. Chronic heart failure: beta-blockers and pharmacogenetics. Eur J Clin Pharmacol 2009;65:3-17.
- British Heart Foundation . British Heart Foundation Statistics Website. n.d. www.heartstats.org (accessed 12 January 2009).
- Pedersen ME, Cockcroft JR. The latest generation of beta-blockers: new pharmacologic properties. Curr Hypertens Rep 2006;8:279-86.
- Bryan J. Inderal: a forerunner of a radically new generation of products. Pharm J 2007;279:538-9.
- Midland Therapeutic Review and Advisory Committee . Carvedilol (Eucardic®) for the Treatment of Chronic Heart Failure 2004. www.keele.ac.uk/schools/pharm/MTRAC/ProductInfo/verdicts/C/CARVEDILOL1.PDF (accessed 22 December 2008).
- Dulin B, Abraham WT. Pharmacology of carvedilol. Am J Cardiol 2004;93:3B-6B.
- Kopecky SL. Effect of beta blockers, particularly carvedilol, on reducing the risk of events after acute myocardial infarction. Am J Cardiol 2006;98:1115-19.
- McMurray J, Kober L, Robertson M, Dargie H, Colucci W, Lopez-Sendon J, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol 2005;45:525-30.
- Gheorghiade M, Goldstein S. Beta-blockers in the post-myocardial infarction patient. Circulation 2002;106:394-8.
- Vantrimpont P, Rouleau JL, Wun CC, Ciampi A, Klein M, Sussex B, et al. Additive beneficial effects of beta-blockers to angiotensin-converting enzyme inhibitors in the Survival and Ventricular Enlargement (SAVE) Study. SAVE Investigators. J Am Coll Cardiol 1997;29:229-36.
- Spargias KS, Hall AS, Greenwood DC, Ball SG. Beta blocker treatment and other prognostic variables in patients with clinical evidence of heart failure after acute myocardial infarction: evidence from the AIRE study. Heart 1999;81:25-32.
- Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385-90.
- Emery M, Lopez-Sendon J, Steg PG, Anderson FA, Jr, Dabbous OH, Scheuble A, et al. Patterns of use and potential impact of early beta-blocker therapy in non-ST-elevation myocardial infarction with and without heart failure: the Global Registry of Acute Coronary Events. Am Heart J 2006;152:1015-21.
- Newby LK, Hasselblad V, Armstrong PW, Van de Werf F, Mark DB, White HD, et al. Time-based risk assessment after myocardial infarction. Implications for timing of discharge and applications to medical decision-making. Eur Heart J 2003;24:182-9.
- British Cardiovascular Intervention society (BCIS) . Audit Returns of Interventional Procedures 2007. 2008. www.bcis.org.uk/resources/documents/BCIS%20Audit%20web.pdf (accessed September 2008).
- Huffman DH, Kampmann JP, Hignite CE, Azarnoff DL. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther 1978;24:465-73.
- Bellati G, Ideo G. Gynaecomastia after spironolactone and potassium canrenoate. Lancet 1986;1.
- Hughes BR, Cunliffe WJ. Tolerance of spironolactone. Br J Dermatol 1988;118:687-91.
- Dupont A. Disappearance of spironolactone-induced gynaecomastia during treatment with potassium canrenoate. Lancet 1985;2.
- O’Reilly PH, Upsdell SM, Brough RJ. Life-threatening hyperkalaemia after bladder decompression for high pressure chronic retention. Lancet 1987;2.
- Greenlaw C. Spironolactone induced gynecomastia: a case report. Drug Intell Clin Pharm 1977;11:70-3.
- Udezue EO, Harrold BP. Hyperkalaemic paralysis due to spironolactone. Postgrad Med J 1980;56:254-5.
- Pongpaew C, Songkhla RN, Kozam RL. Hyperkalemic cardiac arrhythmia secondary to spironolactone. Chest 1973;63:1023-5.
- Pitt B, Bakris G, Ruilope LM, DiCarlo L, Mukherjee R. EPHESUS investigators . Serum potassium and clinical outcomes in the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS). Circulation 2008;118:1643-50.
- Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol 2005;46:425-31.
- Pfizer Inc . Clinical Protocol for an Open-Label Extension Study Evaluating the Safety of Eplerenone in Patients With Heart Failure. Protocol no.: EPLA-0501–076 A6141015. n.d. http://pdf.clinicalstudyresults.org/documents/company-study_4388_0.pdf (accessed 20 September 2008).
- Macdonald PS, Keogh AM, Aboyoun CL, Lund M, Amor R, McCaffrey DJ. Tolerability and efficacy of carvedilol in patients with New York Heart Association class IV heart failure. J Am Coll Cardiol 1999;33:924-31.
- Janosi A, Ghali JK, Herlitz J, Czuriga I, Klibaner M, Wikstrand J, et al. Metoprolol CR/XL in postmyocardial infarction patients with chronic heart failure: experiences from MERIT-HF. Am Heart J 2003;146:721-8.
- Solomon SD, Zelenkofske S, McMurray JJV, Finn PV, Velazquez E, Ertl G, et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N Engl J Med 2005;352:2581-8.
- Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, et al. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 2000;102:203-10.
- Sciagra R, Imperiale A, Antoniucci D, Migliorini A, Parodi G, Comis G, et al. Relationship of infarct size and severity versus left ventricular ejection fraction and volumes obtained from 99mTc-sestamibi gated single-photon emission computed tomography in patients treated with primary percutaneous coronary intervention. Eur J Nucl Med Mol Imaging 2004;31:969-74.
- Amin A. Improving the management of patients after myocardial infarction, from admission to discharge. Clin Ther 2006;28:1509-39.
- Drummond MF, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83.
- Glick HA, Orzol SM, Tooley JF, Remme WJ, Sasayama S, Pitt B. Economic evaluation of the randomized aldactone evaluation study (RALES): treatment of patients with severe heart failure. Cardiovasc Drugs Ther 2002;16:53-9.
- Kvasz M, Zhang Z, Lenz C, Mundhenke M, Weintraub WS. Cost-effectiveness evaluation of eplerenone in patients with heart failure following AMI in Germany [abstract PCV13]. Value Health 2005;8.
- Tilson L, McGowan B, Ryan M, Barry M. Cost-effectiveness of spironolactone in patients with severe heart failure. Ir J Med Sci 2003;172:70-2.
- Van Genugten MLL, Weintraub WS, Zhang Z, Voors AA. Cost-effectiveness of eplerenone plus standard treatment compared with standard treatment in patients with myocardial infarction complicated by left ventricular systolic dysfunction and heart failure in the Netherlands. Neth Heart J 2005;13:393-400.
- Weintraub WS, Zhang Z, Kvasz M, Willke R. Cost-effectiveness of aldosterone blockade with eplerenone in patients with heart failure after acute myocardial infarction: results from EPHESUS applied to four European countries [abstract P-2299]. Eur Heart J 2005;26.
- McGowan B. The Clinical and Economic Aspects of the Present Management of Heart Failure in an Irish Teaching Hospital 2001.
- Glick H, Cook J, Kinosian B, Pitt B, Bourassa MG, Pouleur H, et al. Costs and effects of enalapril therapy in patients with symptomatic heart failure: an economic analysis of the Studies of Left Ventricular Dysfunction (SOLVD) Treatment Trial. J Card Fail 1995;1:371-80.
- Peeters A, Mamun AA, Willekens F, Bonneux L. A cardiovascular life history: a life course analysis of the original Framingham Heart Study cohort. Eur Heart J 2002;23:458-66.
- Downey W, Beck P, McNutt M, Stang MR, Osei W, Nichol J, et al. Pharmacoepidemiology. Chichester, UK: Wiley; 2000.
- Goldberg RJ, Yarzebski J, Lessard D, Gore JM. A two-decades (1975 to 1995) long experience in the incidence, in-hospital and long-term case-fatality rates of acute myocardial infarction: a community-wide perspective. J Am Coll Cardiol 1999;33:1533-9.
- National Institute for Clinical Excellence . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 22 May 2009).
- Briggs AH, Goeree R, Blackhouse G, O’Brien BJ. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making 2002;22:290-308.
- Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation: a practical approach. Med Decis Making 1985;5:157-77.
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13:397-409.
- Kendrick S, Clarke J. The Scottish Record Linkage System. Health Bull (Edinb) 1993;51:72-9.
- MacIntyre K, Capewell S, Stewart S, Chalmers JW, Boyd J, Finlayson A, et al. Evidence of improving prognosis in heart failure: trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation 2000;102:1126-31.
- Fenwick E, Lorgelly PK, Jhund PS, Gandhi S, Briggs AH. Designing a Disease Model for Heart Failure to Examine the Cost-Effectiveness of Statins n.d.
- Tappenden P, Chilcott J, Ward S, Eggington S, Hind D, Hummel S. Methodological issues in the economic analysis of cancer treatments. Eur J Cancer 2006;42:2867-75.
- GAD . Life Tables. London: Government Actuary’s Department; 2007 n.d. www.gad.gov.uk/Demography%20Data/Life%20Tables/index.html (accessed 1 May 2009).
- Mortality statistics. Deaths registered in 2007. Newport, South Wales: Office for National Statistics; n.d.
- Ezekowitz JA, McAlister FA. Aldosterone blockade and left ventricular dysfunction: a systematic review of randomized clinical trials. Eur Heart J 2009;30:469-77.
- Spiegelhalter D, Thomas A, Best N, Lunn D. WINBUGS user manual: version 1.4. Cambridge: MRC Biostatistics Unit; 2001.
- Agostoni P, Magini A, Andreini D, Contini M, Apostolo A, Bussotti M, et al. Spironolactone improves lung diffusion in chronic heart failure. Eur Heart J 2005;26:159-64.
- Akbulut M, Ozbay Y, Ilkay E, Karaca I, Arslan N. Effects of spironolactone and metoprolol on QT dispersion in heart failure. Jpn Heart J 2003;44:681-92.
- Berry C, Murphy N, De Vito G, Galloway S, Seed A, Fisher C, et al. Effects of aldosterone receptor blockade in patients with mild-moderate heart failure taking a beta-blocker. Eur J Heart Fail 2007;9:429-34.
- Chan AKY, Sanderson JE, Wang T, Lam W, Yip G, Wang M, et al. Aldosterone receptor antagonism induces reverse remodeling when added to angiotensin receptor blockade in chronic heart failure. J Am Coll Cardiol 2007;50:591-6.
- Cicoira M, Zanolla L, Rossi A, Golia G, Franceschini L, Brighetti G, et al. Long-term, dose-dependent effects of spironolactone on left ventricular function and exercise tolerance in patients with chronic heart failure. J Am Coll Cardiol 2002;40:304-10.
- Gao X, Peng L, Adhikari CM, Lin J, Zuo Z. Spironolactone reduced arrhythmia and maintained magnesium homeostasis in patients with congestive heart failure. J Card Fail 2007;13:170-7.
- The RALES. Investigators. Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol 1996;78:902-7.
- Rodriguez JA, Godoy I, Castro P, Quintana JC, Chavez E, Yovanovich J, et al. Randomized controlled trial on the effects of ramipril and spironolactone on ventricular remodeling after an acute myocardial infarction. Rev Med Chil 1997;125:643-52.
- Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol 2001;37:1228-33.
- Di Pasquale P, Cannizzaro S, Scalzo S, Parrinello G, Fasullo S, Giambanco F, et al. Effects of canrenoate plus angiotensin-converting enzyme inhibitors versus angiotensin-converting enzyme inhibitors alone on systolic and diastolic function in patients with acute anterior myocardial infarction. Am Heart J 2005;150.
- Modena MG, Aveta P, Menozzi A, Rossi R. Aldosterone inhibition limits collagen synthesis and progressive left ventricular enlargement after anterior myocardial infarction. Am Heart J 2001;141:41-6.
- Department of Health . National Schedule of Reference Costs 2007–08. 2009. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_098945 (accessed 8 May 2009).
- Kind P, Spilker B. Quality of life and pharmacoeconomics in clinical trials. Philadelphia, PA Lippincott-Raven; 1996.
- Gohler A, Geisler BP, Manne JM, Kosiborod M, Zhang Z, Weintraub WS, et al. Utility estimates for decision-analytic modeling in chronic heart failure-health states based on New York Heart Association classes and number of rehospitalizations. Value Health 2009;12:185-7.
- Kind P, Hardman G, Macran S. UK population norms for EQ-5D. York: Centre for Health Economics, University of York; 1999.
- Johannesson M, Weinstein MC. On the decision rules of cost-effectiveness analysis. J Health Econ 1993;12:459-67.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87.
- Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S. A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme. Health Technol Assess 2004;8:1-103.
- Claxton KP, Sculpher MJ. Using value of information analysis to prioritise health research: some lessons from recent UK experience. Pharmacoeconomics 2006;24:1055-68.
- Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of health care technologies. J Health Econ 1999;18:341-64.
- Philips Z, Claxton K, Palmer S. The half-life of truth: what are appropriate time horizons for research decisions?. Med Decis Making 2008;28:287-99.
- Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation 2006;113:1424-33.
- Garattini L, Tediosi F. A comparative analysis of generics markets in five European countries. Health Policy 2000;51:149-62.
- Frank R, Salkever D. Generic entry and the pricing of pharmaceuticals. J Econ Manag Strategy 1997;6:75-90.
- Magazzini L, Pammolli F, Riccaboni M. Dynamic competition in pharmaceuticals. Patent expiry, generic penetration, and industry structure. Eur J Health Econ 2004;5:175-82.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 2008. www.cochrane-handbook.org.
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of randomized trials published world-wide – the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. Emerg Themes Epidemiol 2008;5.
- Banas J, White H, Parkhomenko A, Patni R, Mukherjee R, Zannad F. Favorable outcomes with 25 mg of eplerenone in patients with post-AMI heart failure: results from the EPHESUS trial. J Card Fail 2005;11.
- Barr CS, Lang CC, Hanson J, Arnott M, Kennedy N, Struthers AD. Effects of adding spironolactone to an angiotensin-converting enzyme inhibitor in chronic congestive heart failure secondary to coronary artery disease. Am J Cardiol 1995;76:1259-65.
- Rousseau MF, Gurne O, Duprez D, Van Mieghem W, Robert A, Ahn S, et al. Beneficial neurohormonal profile of spironolactone in severe congestive heart failure: results from the RALES neurohormonal substudy. J Am Coll Cardiol 2002;40:1596-601.
- Rumsfeld JS, Jones PG, Whooley MA, Sullivan MD, Pitt B, Weintraub WS, et al. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. Am Heart J 2005;150:961-7.
- Tsutamoto T. Mineralocorticoid receptor antagonist spironolactone improves left ventricular remodeling in patients with congestive heart failure and acute myocardial infarction. Nippon Yakurigaku Zasshi 2004;124:90-100.
- Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 2000;102:2700-6.
- O’Keefe JH, Abuissa H, Pitt B. Eplerenone improves prognosis in postmyocardial infarction diabetic patients with heart failure: results from EPHESUS. Diabetes Obes Metab 2008;10:492-7.
- Kohya T, Tsuzuki N, Kaji T, Tomita F, Ono T, Nakamura M, et al. Effects of the aldosterone antagonist spironolactone on ventricular arrhythmias and serum electrolyte levels in congestive heart failure. Clin Drug Investig 1995;10:264-75.
- Berry C, McMurray JJ. Serious adverse events experienced by patients with chronic heart failure taking spironolactone. Heart 2001;85.
- Konopa J, Bullo B, Rutkowski B. Life threatening drug-induced hyperkalaemia: case report. Pol Arch Med Wewn 2006;115:238-42.
- Butler JV, McAvoy H, McEnroy D, Mulkerrin EC. Spironolactone therapy in older patients – the impact of renal dysfunction. Arch Gerontol Geriatr 2002;35:45-9.
- Masoudi FA, Gross CP, Wang Y, Rathore SS, Havranek EP, Foody JM, et al. Adoption of spironolactone therapy for older patients with heart failure and left ventricular systolic dysfunction in the United States, 1998–2001. Circulation 2005;112:39-47.
- Nurnberger J, Daul A, Philipp T. A patient with severe hyperkalaemia – an emergency after RALES. Dtsch Med Wochenschr 2005;130:2008-11.
- Cox AR. Hyperkalaemia associated with use of spironolactone in heart failure. Pharmacy in Practice 2001;11:187-9.
- Debugne G, Schoevaerdts D, Botelho A, Mwenge B, Spinewine A, Vanpee D, et al. Spironolactone in the treatment of heart failure among elderly patients: The evidences and the reality. Louv Med 2006;125:23-9.
- Dinsdale C, Wani M, Steward J, O’Mahony MS. Tolerability of spironolactone as adjunctive treatment for heart failure in patients over 75 years of age. Age Ageing 2005;34:395-8.
- Raebel MA, McClure DL, Chan KA, Simon SR, Feldstein AC, Lafata JE, et al. Laboratory evaluation of potassium and creatinine among ambulatory patients prescribed spironolactone: are we monitoring for hyperkalemia?. Ann Pharmacother 2007;41:193-200.
- Saudan P, Mach F, Perneger T, Schnetzler B, Stoermann C, Fumeaux Z, et al. Safety of low-dose spironolactone administration in chronic haemodialysis patients. Nephrol Dial Transplant 2003;18:2359-63.
- Skvortsov AA, Mareev VY, Chelmakina SM, Baklanova NA, Belenkov IN. Efficacy and safety of long-term application of spironolactone in patients with moderate and severe chronic heart failure receiving optimal therapy. Kardiologiia 2007;47:12-23.
- Teive HAG, Munhoz RP, Werneck LC. Worsening of motor symptoms and gynecomastia during spironolactone treatment in a patient with Parkinson’s disease and congestive heart failure. Mov Disord 2007;22:1678-9.
- Hussain S, Dreyfus DE, Marcus RJ, Biederman RWW, McGill RL. Is spironolactone safe for dialysis patients?. Nephrol Dial Transplant 2003;18:2364-8.
- Karagiannis A, Tziomalos K, Papageorgiou A, Kakafika AI, Pagourelias ED, Anagnostis P, et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert Opin Pharmacother 2008;9:509-15.
- Vanpee D, Swine CH. Elderly heart failure patients with drug-induced serious hyperkalemia. Aging Clin Exp Res 2000;12:315-19.
- Kauffmann R, Orozco R, Venegas JC. Severe hyperkalemia associated to the use of losartan and spironolactone: case report. Rev Med Chil 2005;133:947-52.
- Wrenger E, Muller R, Moesenthin M, Welte T, Frolich JC, Neumann KH. Lesson of the week: interaction of spironolactone with ACE inhibitors or angiotensin receptor blockers: analysis of 44 cases. BMJ 2003;327:147-9.
- Ko DT, Juurlink DN, Mamdani MM, You JJ, Wang JT, Donovan LR, et al. Appropriateness of spironolactone prescribing in heart failure patients: a population-based study. J Card Fail 2006;12:205-10.
Appendix 1 Literature search strategies
Clinical effectiveness
Search strategies to identify systematic reviews and guidelines of spironolactone and eplerenone for postMI HF or HF.
The Cochrane Library
www.interscience.wiley.com/cgi-bin/mrwhome/106568753/HOME
-
Search date: 3 September 2008.
-
Records retrieved:
-
– 4 records were retrieved from the Cochrane Database of Systematic Reviews
-
– 14 records were retrieved from the NHS Economic Evaluation Database (NHS EED)
-
– 11 records were retrieved from the Cochrane Central Register of Controlled Trials (CENTRAL)
-
– no records were retrieved from the Database of Abstracts of Reviews of Effects and the Health Technology Assessment Database (HTA).
-
#1 MeSH descriptor Heart Failure explode all trees
#2 MeSH descriptor Myocardial Infarction explode all trees
#3 (#1 AND #2)
#4 ‘post acute myocardial infarction heart failure’
#5 ‘post-acute myocardial infarction heart failure’
#6 ‘post-AMI heart failure’
#7 ‘post AMI heart failure’
#8 ‘post-AMI HF’
#9 ‘post AMI HF’
#10 (#3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Spironolactone, this term only
#12 MeSH descriptor Aldosterone Antagonists explode all trees
#13 eplerenone
#14 aldactone
#15 inspra
#16 (#11 OR #12 OR #13 OR #14 OR #15)
#17 (#10 AND #16)
#18 (#10 AND #16)
National Library for Health (Guidelines Finder)
www.library.nhs.uk/guidelinesFinder/
-
Search date: 11 September 2008.
-
Searched for ‘eplerenone’ or ‘spironolactone’.
-
No guidelines identified.
US National Guidelines Clearing House
-
Search date: 11 September 2008.
-
Searched for ‘spironolactone’ Publication Date(s): 2008, 2007, 2006, 2005, 2004.
-
Twenty-two guidelines identified.
Search strategies to identify completed and ongoing clinical trials of spironolactone, eplerenone, canrenone and canrenoate potassium for postMI HF or HF
MEDLINE
(OvidSP) http://gateway.ovid.com/athens
Both MEDLINE search strategies below incorporated the sensitivity maximising version of the Cochrane Highly Sensitive Search Strategy for randomised trials in MEDLINE. 165
MEDLINE search strategy to retrieve clinical trials of spironolactone, eplerenone, canrenone and canrenoate potassium for postMI HF
-
Date range: 1950 to November Week 2 2008.
-
Search date: 25 November 2008.
The following search strategy retrieved 185 records:
-
exp Heart Failure/(63163)
-
‘heart failure$’.ti,ab. (69378)
-
3‘HF’.ti,ab. (10060)
-
‘CHF’.ti,ab. (7471)
-
‘cardiac failure$’.ti,ab. (8325)
-
‘coronary failure$’.ti,ab. (130)
-
‘myocardial failure$’.ti,ab. (650)
-
‘heart decompensation’.ti,ab. (90)
-
‘cardia$decompensation’.ti,ab. (829)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (108267)
-
exp Myocardial Infarction/(123075)
-
‘heart attack$’.ti,ab. (2700)
-
‘heart infarct$’.ti,ab. (712)
-
‘cardiac infarct$’.ti,ab. (608)
-
‘cardial infarct$’.ti,ab. (14)
-
‘myocardial infarct$’.ti,ab. (109338)
-
‘myocardium infarct$’.ti,ab. (129)
-
‘MI’.ti,ab. (17744)
-
‘AMI’.ti,ab. (9154)
-
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 (164235)
-
10 and 20 (15453)
-
‘post AMI HF’.ti,ab. (1)
-
‘postmyocardial infarct$’.ti,ab. (700)
-
‘postmyocardium infarct$’.ti,ab. (1)
-
‘post MI’.ti,ab. (1261)
-
21 or 22 or 23 or 24 or 25 (16909)
-
exp Aldosterone Antagonists/(6212)
-
‘aldosterone antagonist$’.ti,ab. (734)
-
27 or 28 (6445)
-
Spironolactone/(4764)
-
spironolactone.ti,ab,rn. (5868)
-
52–01–7.rn. (4764)
-
aldactone.ti,ab,rn. (269)
-
(novo-spiroton or novospiroton or ‘novo spiroton’).ti,ab,rn. (0)
-
spiractin.ti,ab,rn. (3)
-
spirotone.ti,ab,rn. (0)
-
verospiron.ti,ab,rn. (15)
-
berlactone.ti,ab,rn. (0)
-
30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 (5927)
-
eplerenone.ti,ab,rn. (452)
-
inspra.ti,ab,rn. (22)
-
40 or 41 (452)
-
Canrenone/(194)
-
canrenone.ti,ab,rn. (274)
-
976–71–6.rn. (194)
-
contaren.ti,ab,rn. (0)
-
luvion.ti,ab,rn. (0)
-
aldadiene.ti,ab,rn. (17)
-
phanurane.ti,ab,rn. (1)
-
43 or 44 or 45 or 46 or 47 or 48 or 49 (290)
-
Canrenoate Potassium/(252)
-
(canrenoate adj potassium).ti,ab,rn. (76)
-
2181–04–6.rn. (252)
-
canrenol.ti,ab,rn. (0)
-
soldactone.ti,ab,rn. (11)
-
’Kalium-Can’.ti,ab,rn. (0)
-
diurek.ti,ab,rn. (0)
-
Kanrenol.ti,ab,rn. (0)
-
luvion.ti,ab,rn. (0)
-
venactone.ti,ab,rn. (0)
-
spiroctan.ti,ab,rn. (0)
-
51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 (260)
-
29 or 39 or 42 or 50 or 62 (7491)
-
randomized controlled trial.pt. (268895)
-
controlled clinical trial.pt. (80708)
-
randomized.ab. (176810)
-
placebo.ab. (111127)
-
drug therapy.fs. (1316446)
-
randomly.ab. (128361)
-
trial.ab. (184184)
-
groups.ab. (888547)
-
64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 (2382102)
-
(animals not (humans and animals)).sh. (3307879)
-
72 not 73 (2019865)
-
26 and 63 and 74 (172)
-
RALES.ti,ab. (660)
-
EPHESUS.ti,ab. (95)
-
‘AREA IN CHF’.ti,ab. (5)
-
77 or 76 or 78 (728)
-
63 or 79 (8084)
-
26 and 74 and 80 (185)
MEDLINE search strategy to retrieve clinical trials of spironolactone, eplerenone, canrenone, canrenoate potassium for HF
-
Date range: 1995 to November Week 2 2008.
-
Search date: 25 November 2008.
The following search strategy retrieved 898 records:
-
exp Heart Failure/(63163)
-
‘heart failure$’.ti,ab. (69378)
-
‘HF’.ti,ab. (10060)
-
‘CHF’.ti,ab. (7471)
-
‘cardiac failure$’.ti,ab. (8325)
-
‘coronary failure$’.ti,ab. (130)
-
‘myocardial failure$’.ti,ab. (650)
-
‘heart decompensation’.ti,ab. (90)
-
‘cardia$decompensation’.ti,ab. (829)
-
or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (108267)
-
exp Aldosterone Antagonists/(6212)
-
‘aldosterone antagonist$’.ti,ab. (734)
-
11 or 12 (6445)
-
Spironolactone/(4764)
-
spironolactone.ti,ab,rn. (5868)
-
52–01–7.rn. (4764)
-
aldactone.ti,ab,rn. (269)
-
(novo-spiroton or novospiroton or ‘novo spiroton’).ti,ab,rn. (0)
-
spiractin.ti,ab,rn. (3)
-
spirotone.ti,ab,rn. (0)
-
verospiron.ti,ab,rn. (15)
-
berlactone.ti,ab,rn. (0)
-
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 (5927)
-
eplerenone.ti,ab,rn. (452)
-
inspra.ti,ab,rn. (22)
-
24 or 25 (452)
-
Canrenone/(194)
-
canrenone.ti,ab,rn. (274)
-
976–71–6.rn. (194)
-
contaren.ti,ab,rn. (0)
-
luvion.ti,ab,rn. (0)
-
aldadiene.ti,ab,rn. (17)
-
phanurane.ti,ab,rn. (1)
-
27 or 28 or 29 or 30 or 31 or 32 or 33 (290)
-
Canrenoate Potassium/(252)
-
(canrenoate adj potassium).ti,ab,rn. (76)
-
2181–04–6.rn. (252)
-
canrenol.ti,ab,rn. (0)
-
soldactone.ti,ab,rn. (11)
-
’Kalium-Can’.ti,ab,rn. (0)
-
diurek.ti,ab,rn. (0)
-
Kanrenol.ti,ab,rn. (0)
-
luvion.ti,ab,rn. (0)
-
venactone.ti,ab,rn. (0)
-
spiroctan.ti,ab,rn. (0)
-
35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 (260)
-
13 or 23 or 26 or 34 or 46 (7491)
-
randomized controlled trial.pt. (268895)
-
controlled clinical trial.pt. (80708)
-
randomized.ab. (176810)
-
placebo.ab. (111127)
-
drug therapy.fs. (1316446)
-
randomly.ab. (128361)
-
trial.ab. (184184)
-
groups.ab. (888547)
-
48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 (2382102)
-
(animals not (humans and animals)).sh. (3307879)
-
56 not 57 (2019865)
-
10 and 47 and 58 (1089)
-
RALES.ti,ab. (660)
-
EPHESUS.ti,ab. (95)
-
‘AREA IN CHF’.ti,ab. (5)
-
60 or 61 or 62 (728)
-
47 or 63 (8084)
-
10 and 58 and 64 (1136)
-
limit 65 to yr=’1995 – 2008’ (898)
EMBASE
(OvidSP) http://gateway.ovid.com/athens
Both EMBASE search strategies below incorporated a search filter to identify randomised trials based on the filter developed by Lebfebvre et al. 166
EMBASE search strategy to retrieve clinical trials of spironolactone, eplerenone, canrenone and canrenoate potassium for postMI HF
-
Date range: 1980 to November Week 47 2008.
-
Search date: 25 November 2008.
The following search strategy retrieved 195 records:
-
exp Heart Failure/(117131)
-
‘heart failure$’.ti,ab. (62220)
-
‘HF’.ti,ab. (10000)
-
‘CHF’.ti,ab. (7192)
-
‘cardiac failure$’.ti,ab. (6679)
-
‘coronary failure$’.ti,ab. (30)
-
‘myocardial failure$’.ti,ab. (497)
-
‘heart decompensation’.ti,ab. (40)
-
‘cardia$decompensation’.ti,ab. (499)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (139605)
-
exp Heart Infarction/(116134)
-
‘heart attack$’.ti,ab. (1941)
-
‘heart infarct$’.ti,ab. (777)
-
‘cardiac infarct$’.ti,ab. (302)
-
‘cardial infarct$’.ti,ab. (8)
-
‘myocardial infarct$’.ti,ab. (83484)
-
‘myocardium infarct$’.ti,ab. (99)
-
‘MI’.ti,ab. (16777)
-
‘AMI’.ti,ab. (8413)
-
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 (140223)
-
10 and 20 (22964)
-
‘post AMI HF’.ti,ab. (2)
-
‘postmyocardial infarct$’.ti,ab. (552)
-
‘postmyocardium infarct$’.ti,ab. (0)
-
‘post MI’.ti,ab. (1282)
-
21 or 22 or 23 or 24 or 25 (24195)
-
exp Aldosterone Antagonists/(15156)
-
‘aldosterone antagonist$’.ti,ab. (589)
-
27 or 28 (15219)
-
Spironolactone/(13115)
-
spironolactone.ti,ab,rn. (2878)
-
52–01–7.rn. (13115)
-
aldactone.ti,ab,rn. (137)
-
(novo-spiroton or novospiroton or ‘novo spiroton’).ti,ab,rn. (0)
-
spiractin.ti,ab,rn. (0)
-
spirotone.ti,ab,rn. (0)
-
verospiron.ti,ab,rn. (7)
-
berlactone.ti,ab,rn. (0)
-
30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 (13353)
-
Eplerenone/(1301)
-
eplerenone.ti,ab,rn. (399)
-
inspra.ti,ab,rn. (20)
-
40 or 41 or 42 (1317)
-
Canrenone/(399)
-
canrenone.ti,ab,rn. (180)
-
976–71–6.rn. (399)
-
contaren.ti,ab,rn. (0)
-
luvion.ti,ab,rn. (0)
-
aldadiene.ti,ab,rn. (0)
-
phanurane.ti,ab,rn. (2)
-
44 or 45 or 46 or 47 or 48 or 49 or 50 (432)
-
Canrenoate Potassium/(443)
-
(canrenoate adj potassium).ti,ab,rn. (28)
-
2181–04–6.rn. (443)
-
canrenol.ti,ab,rn. (0)
-
soldactone.ti,ab,rn. (4)
-
’Kalium-Can’.ti,ab,rn. (0)
-
diurek.ti,ab,rn. (0)
-
Kanrenol.ti,ab,rn. (0)
-
luvion.ti,ab,rn. (0)
-
venactone.ti,ab,rn. (1)
-
spiroctan.ti,ab,rn. (0)
-
52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 (446)
-
29 or 39 or 43 or 51 or 63 (15441)
-
random$.ti,ab. (383957)
-
factorial$.ti,ab. (7997)
-
crossover$.ti,ab. (26959)
-
cross-over$.ti,ab. (12168)
-
placebo$.ti,ab. (107934)
-
(doubl$adj blind$).ti,ab. (83454)
-
(singl$adj blind$).ti,ab. (7323)
-
assign$.ti,ab. (106212)
-
allocat$.ti,ab. (33593)
-
volunteer$.ti,ab. (97507)
-
Crossover Procedure/(20719)
-
double blind procedure/(70553)
-
Randomized Controlled Trial/(163066)
-
single blind procedure/(7817)
-
65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 (645400)
-
26 and 64 and 79 (189)
-
RALES.ti,ab. (581)
-
EPHESUS.ti,ab. (91)
-
‘AREA IN CHF’.ti,ab. (4)
-
81 or 82 or 83 (639)
-
64 or 84 (15918)
-
26 and 79 and 85 (195)
-
from 86 keep 1–195 (195)
EMBASE search strategy to retrieve clinical trials of spironolactone, eplerenone, canrenone and canrenoate potassium for HF
-
Date range: 1995 to November Week 47 2008.
-
Search date: 25 November 2008.
The following search strategy retrieved 805 records:
-
exp Heart Failure/(117131)
-
‘heart failure$’.ti,ab. (62220)
-
‘HF’.ti,ab. (10000)
-
‘CHF’.ti,ab. (7192)
-
‘cardiac failure$’.ti,ab. (6679)
-
‘coronary failure$’.ti,ab. (30)
-
‘myocardial failure$’.ti,ab. (497)
-
‘heart decompensation’.ti,ab. (40)
-
‘cardia$decompensation’.ti,ab. (499)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 (139605)
-
exp Aldosterone Antagonists/(15156)
-
‘aldosterone antagonist$’.ti,ab. (589)
-
11 or 12 (15219)
-
Spironolactone/(13115)
-
spironolactone.ti,ab,rn. (2878)
-
52–01–7.rn. (13115)
-
aldactone.ti,ab,rn. (137)
-
(novo-spiroton or novospiroton or ‘novo spiroton’).ti,ab,rn. (0)
-
spiractin.ti,ab,rn. (0)
-
spirotone.ti,ab,rn. (0)
-
verospiron.ti,ab,rn. (7)
-
berlactone.ti,ab,rn. (0)
-
14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 (13353)
-
Eplerenone/(1301)
-
eplerenone.ti,ab,rn. (399)
-
inspra.ti,ab,rn. (20)
-
24 or 25 or 26 (1317)
-
Canrenone/(399)
-
canrenone.ti,ab,rn. (180)
-
976–71–6.rn. (399)
-
contaren.ti,ab,rn. (0)
-
luvion.ti,ab,rn. (0)
-
aldadiene.ti,ab,rn. (0)
-
phanurane.ti,ab,rn. (2)
-
28 or 29 or 30 or 31 or 32 or 33 or 34 (432)
-
Canrenoate Potassium/(443)
-
(canrenoate adj potassium).ti,ab,rn. (28)
-
2181–04–6.rn. (443)
-
canrenol.ti,ab,rn. (0)
-
soldactone.ti,ab,rn. (4)
-
’Kalium-Can’.ti,ab,rn. (0)
-
diurek.ti,ab,rn. (0)
-
Kanrenol.ti,ab,rn. (0)
-
luvion.ti,ab,rn. (0)
-
venactone.ti,ab,rn. (1)
-
spiroctan.ti,ab,rn. (0)
-
36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 (446)
-
13 or 23 or 27 or 35 or 47 (15441)
-
random$.ti,ab. (383957)
-
factorial$.ti,ab. (7997)
-
crossover$.ti,ab. (26959)
-
cross-over$.ti,ab. (12168)
-
placebo$.ti,ab. (107934)
-
(doubl$adj blind$).ti,ab. (83454)
-
(singl$adj blind$).ti,ab. (7323)
-
assign$.ti,ab. (106212)
-
allocat$.ti,ab. (33593)
-
volunteer$.ti,ab. (97507)
-
Crossover Procedure/(20719)
-
double blind procedure/(70553)
-
Randomized Controlled Trial/(163066)
-
single blind procedure/(7817)
-
49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 (645400)
-
10 and 48 and 63 (838)
-
RALES.ti,ab. (581)
-
EPHESUS.ti,ab. (91)
-
‘AREA IN CHF’.ti,ab. (4)
-
65 or 66 or 67 (639)
-
48 or 68 (15918)
-
10 and 63 and 69 (852)
-
limit 70 to yr=’1995 – 2008’ (805)
Cochrane Central Register of Controlled Trials (CENTRAL)
www.interscience.wiley.com/cgi-bin/mrwhome/106568753/HOME
CENTRAL search strategy to retrieve clinical trials of spironolactone, eplerenone, canrenone and canrenoate potassium for postMI HF
-
2008, Issue 4.
-
Search date: 25 November 2008.
The following search strategy retrieved 18 records:
#1 MeSH descriptor Heart Failure explode all trees
#2 ’heart failure*’:ti,ab
#3 ’HF’:ti,ab
#4 ‘CHF’:ti,ab
#5 ‘cardiac failure*’:ti,ab
#6 ‘coronary failure*’:ti,ab
#7 ‘myocardial failure*’:ti,ab
#8 ‘heart decompensation’:ti,ab
#9 ‘cardia* decompensation’:ti,ab
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Myocardial Infarction explode all trees
#12 ‘heart attack*’:ti,ab
#13 ‘heart infarct*’:ti,ab
#14 ‘cardiac infarct*’:ti,ab
#15 ‘cardial infarct*’:ti,ab
#16 ’myocardial infarct*’:ti,ab
#17 ’myocardium infarct*’:ti,ab
#18 ’MI’:ti,ab
#19 ‘AMI’:ti,ab
#20 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19)
#21 (#10 AND #20)
#22 ‘post AMI HF’:ti,ab
#23 ‘postmyocardial infarct*’:ti,ab
#24 ‘postmyocardium infarct*’:ti,ab
#25 ‘post MI’:ti,ab
#26 (#21 OR #22 OR #23 OR #24 OR #25)
#27 MeSH descriptor Aldosterone Antagonists explode all trees
#28 ’aldosterone antagonist*’:ti,ab
#29 (#27 OR #28)
#30 MeSH descriptor Spironolactone, this term only
#31 spironolactone:ti,ab
#32 52–01–7:ab
#33 aldactone:ti,ab
#34 (novo-spiroton or novospiroton or ‘novo spiroton’):ti,ab
#35 spiractin:ti,ab
#36 spirotone:ti,ab
#37 verospiron:ti,ab
#38 berlactone:ti,ab
#39 (#30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38)
#40 eplerenone:ti,ab
#41 inspra:ti,ab
#42 (#40 OR #41)
#43 MeSH descriptor Canrenone, this term only
#44 canrenone:ti,ab
#45 976–71–6:ab
#46 contaren:ti,ab
#47 luvion:ti,ab
#48 aldadiene:ti,ab
#49 phanurane:ti,ab
#50 (#43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49)
#51 MeSH descriptor Canrenoate Potassium, this term only
#52 (canrenoate NEAR potassium):ti,ab
#53 canrenol:ti,ab
#54 soldactone:ti,ab
#55 ’Kalium-Can’:ti,ab
#56 diurek:ti,ab
#57 Kanrenol:ti,ab
#58 luvion:ti,ab
#59 venactone:ti,ab
#60 spiroctan:ti,ab
#61 2181–04–6:ab
#62 (#51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61)
#63 (#29 OR #39 OR #42 OR #50 OR #62)
#64 RALES:ti,ab
#65 EPHESUS:ti,ab
#66 ‘AREA IN CHF’:ti,ab
#67 (#64 OR #65 OR #66)
#68 (#63 OR #67)
#69 (#26 AND #68)
CENTRAL search strategy to retrieve clinical trials of spironolactone, eplerenone, canrenone and canrenoate potassium for heart failure
-
Date range: 1995 to 2008, Issue 4.
-
Search date: 25 November 2008.
The following search strategy retrieved 100 records:
#1 MeSH descriptor Heart Failure explode all trees
#2 ‘heart failure*’:ti,ab
#3 ‘HF’:ti,ab
#4 ‘CHF’:ti,ab
#5 ‘cardiac failure*’:ti,ab
#6 ‘coronary failure*’:ti,ab
#7 ‘myocardial failure*’:ti,ab
#8 ‘heart decompensation’:ti,ab
#9 ‘cardia* decompensation’:ti,ab
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Aldosterone Antagonists explode all trees
#12 ‘aldosterone antagonist*’:ti,ab
#13 (#11 OR #12)
#14 MeSH descriptor Spironolactone, this term only
#15 spironolactone:ti,ab
#16 52–01–7:ab
#17 aldactone:ti,ab
#18 (novo-spiroton or novospiroton or ‘novo spiroton’):ti,ab
#19 spiractin:ti,ab
#20 spirotone:ti,ab
#21 verospiron:ti,ab
#22 berlactone:ti,ab
#23 (#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22)
#24 eplerenone:ti,ab
#25 inspra:ti,ab
#26 (#24 OR #25)
#27 MeSH descriptor Canrenone, this term only
#28 canrenone:ti,ab
#29 976–71–6:ab
#30 contaren:ti,ab
#31 luvion:ti,ab
#32 aldadiene:ti,ab
#33 phanurane:ti,ab
#34 (#27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33)
#35 MeSH descriptor Canrenoate Potassium, this term only
#36 (canrenoate NEAR potassium):ti,ab
#37 canrenol:ti,ab
#38 soldactone:ti,ab
#39 ‘Kalium-Can’:ti,ab
#40 diurek:ti,ab
#41 Kanrenol:ti,ab
#42 luvion:ti,ab
#43 venactone:ti,ab
#44 spiroctan:ti,ab
#45 2181–04–6:ab
#46 (#35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45)
#47 (#13 OR #23 OR #26 OR #34 OR #46)
#48 RALES:ti,ab
#49 EPHESUS:ti,ab
#50 ‘AREA IN CHF’:ti,ab
#51 (#48 OR #49 OR #50)
#52 (#47 OR #51)
#53 (#10 AND #52), from 1995 to 2008
Clinical Trials.gov
-
Search date: 4 September 2008.
-
Series of short searches undertaken:
-
– spironolactone and heart failure –19 records retrieved
-
– spironolactione and heart attack – two records retrieved
-
– eplerenone and heart failure – eight records retrieved
-
– eplerenone and heart attack – two records retrieved.
-
MetaRegister of Controlled Trials
-
Search date: 5 September 2008.
-
Series of short searches undertaken:
-
– spironolactone and ‘heart attack’ – three records retrieved
-
– spironolactone and ‘heart failure’ – 65 records retrieved
-
– eplerenone and ‘heart failure’ – 22 records retrieved
-
– eplerenone and ‘heart attack’ – two records retrieved.
-
Search strategies to identify adverse effects information for spironolactone and eplerenone
MEDLINE
(OvidSP) http://gateway.ovid.com/athens
-
Date range: 1950 to November Week 3 2008.
-
Search date: 11 December 2008.
The following search strategy retrieved 167 records:
-
Gynecomastia/(2696)
-
gyn?ecomast$.ti,ab. (2696)
-
(breast adj3 (enlarg$or larg$or engorg$or swell$or disten$or growth)).ti,ab. (4374)
-
1 or 2 or 3 (7844)
-
Hyperkalemia/(4124)
-
(hyperkal?em$or hyperkali?em$or hyper-kal?em$or hyper-kali?em$).ti,ab. (4864)
-
(hyperpotass?emi$or hyper-potass?emi$).ti,ab. (136)
-
5 or 6 or 7 (6688)
-
4 or 8 (14510)
-
exp Heart Failure/(63303)
-
‘heart failure$’.ti,ab. (69532)
-
‘HF’.ti,ab. (10094)
-
‘CHF’.ti,ab. (7484)
-
‘cardiac failure$’.ti,ab. (8338)
-
‘coronary failure$’.ti,ab. (131)
-
‘myocardial failure$’.ti,ab. (650)
-
‘heart decompensation’.ti,ab. (90)
-
‘cardia$decompensation’.ti,ab. (829)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (108476)
-
Spironolactone/(4771)
-
spironolactone.ti,ab,rn. (5879)
-
52–01–7.rn. (4771)
-
aldactone.ti,ab,rn. (269)
-
(novo-spiroton or novospiroton or ‘novo spiroton’).ti,ab,rn. (0)
-
spiractin.ti,ab,rn. (3)
-
spirotone.ti,ab,rn. (0)
-
verospiron.ti,ab,rn. (15)
-
berlactone.ti,ab,rn. (0)
-
20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 (5938)
-
eplerenone.ti,ab,rn. (455)
-
inspra.ti,ab,rn. (22)
-
30 or 31 (455)
-
29 or 32 (6001)
-
9 and 19 and 33 (167)
EMBASE
(OvidSP) http://gateway.ovid.com/athens
-
Date range: 1980 to week 49 2008.
-
Search date: 11 December 2008.
The following search strategy retrieved 679 records:
-
Gynecomastia/(4383)
-
gyn?ecomast$.ti,ab. (1976)
-
(breast adj3 (enlarg$or larg$or engorg$or swell$or disten$or growth)).ti,ab. (3811)
-
1 or 2 or 3 (8465)
-
Hyperkalemia/(6913)
-
(hyperkal?em$or hyperkali?em$or hyper-kal?em$or hyper-kali?em$).ti,ab. (3849)
-
(hyperpotass?emi$or hyper-potass?emi$).ti,ab. (51)
-
5 or 6 or 7 (8125)
-
4 or 8 (16299)
-
exp Heart Failure/(117544)
-
‘heart failure$’.ti,ab. (62401)
-
‘HF’.ti,ab. (10037)
-
‘CHF’.ti,ab. (7209)
-
‘cardiac failure$’.ti,ab. (6686)
-
‘coronary failure$’.ti,ab. (31)
-
‘myocardial failure$’.ti,ab. (497)
-
‘heart decompensation’.ti,ab. (40)
-
‘cardia$decompensation’.ti,ab. (501)
-
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (140059)
-
Spironolactone/(13167)
-
spironolactone.ti,ab,rn. (2882)
-
52–01–7.rn. (13167)
-
aldactone.ti,ab,rn. (137)
-
(novo-spiroton or novospiroton or ‘novo spiroton’).ti,ab,rn. (0)
-
spiractin.ti,ab,rn. (0)
-
spirotone.ti,ab,rn. (0)
-
verospiron.ti,ab,rn. (7)
-
berlactone.ti,ab,rn. (0)
-
20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 (13405)
-
Eplerenone/(1317)
-
eplerenone.ti,ab,rn. (405)
-
inspra.ti,ab,rn. (20)
-
30 or 31 or 32 (1334)
-
29 or 33 (13823)
-
9 and 19 and 34 (679)
Toxicology Literature Online (TOXLINE)
http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?TOXLINE
-
Date range: 1965 to 15 December 2008.
-
Date searched: 15 December 2008.
The following search retrieved 88 records:
#1 [mh] (gynecomastia)
#2 (gynecomast* OR gyneacomast*)
#3 (‘breast enlarg*’ OR ‘breast larg$’ OR ‘breast engorg*’ OR ‘breast swell*’ OR ‘breast disten*’ OR ‘breast growth’)
#4 (#1 OR #2 OR #3)
#5 [mh] (hyperkalemia)
#6 (hyperkalaem* OR hyperkalem* OR hyperkaliaem* OR hyperkaliem* OR hyper kalaem* OR hyper kalem* OR hyper kaliaem* OR hyper kaliem*)
#7 (hyperpotassaemi* OR hyperpotassemi* OR hyper potassaemi* OR hyper potassemi*)
#8 (#5 OR #6 OR #7)
#9 ‘heart failure’ [mh]
#10 (‘heart failure*’ OR ‘hf’ OR ‘chf’ OR ‘cardiac failure*’ OR ‘coronary failure*’ OR (myocardial OR myocardium) failure* ‘heart decompensation’ OR ‘cardia* decompensation’)
#11 (#9 OR #10)
#12 [mh] (spironolactone OR aldactone OR verospirone OR verospiron OR uractone OR ‘spironolactone a’ OR spirone OR spiridon OR spiresis OR ‘aldactone a’)
#13 (spironolactone OR aldactone OR verospirone OR verospiron OR uractone OR ‘spironolactone a’ OR spirone OR spiridon OR spiresis OR ‘aldactone a’ OR 52–01–7 [rn])
#14 (#12 OR #13)
#15 [mh] (eplerenone OR inspra OR epoxymexrenone)
#16 (eplerenone OR inspra OR epoxymexrenone OR 107724–20–9 [rn])
#17 (#15 OR #16)
#18 (#17 OR #14)
#19 (#4 AND #8 AND #11 AND #18)
#20 (#11 AND #18)
#21 (#4 OR #8)
#22 (#21 AND #11 AND #18)
Dynamed
-
Date of search: 11 December 2008.
-
The search terms used to search Dynamed were:
-
– spironolactone
-
– eplerenone
-
– Inspra.
-
-
Three relevant Dynamed topics were retrieved: spironolactone, spironolactone for heart failure and eplerenone.
Drugs@FDA
www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/
-
Date of search: 9 December 2008.
-
Search terms used to search drugs@FDA were:
-
– spironolactone
-
– eplerenone
-
– Inspra.
-
-
Information for each of the drugs was retrieved.
European Medicines Agency (EMEA)
-
Date of search: 28 January 2009.
-
European Public Assessment Reports (EPAR) were searched for on the EMEA website using the following search terms:
-
– spironolactone
-
– eplerenone
-
– Inspra.
-
-
No EPARs were found for these drugs.
AHFS Drug Information
AHFS Drug Information. Bethesda, MD: American Society of Hospital Pharmacists; 2008.
The above reference text was searched for entries on spironolactone, eplerenone and Inspra. Information on spironolactone and eplerenone only were retrieved.
Meyler’s side effects of drugs
Dukes MNG, Aronson JK, (editors) Meylers side effects of drugs. 14th edn. Amsterdam: Elsevier; 2000.
The above reference text was searched for entries on spironolactone, eplerenone and Inspra. Information on spironolactone only was retrieved.
Cost-effectiveness
NHS Economic Evaluations Database (NHS EED)
via Centre for Reviews and Dissemination website: www.crd.york.ac.uk/crdweb/
-
Search date: 6 January 2009.
The following search strategy retrieved 15 records from NHS EED:
-
MeSH Aldosterone Antagonists
-
aldosterone NEAR antagonist*
-
MeSH Spironolactone
-
spironolactone
-
aldactone
-
novo-spiroton OR novospiroton OR ‘novo spiroton’
-
spiractin
-
spirotone
-
verospiron
-
berlactone
-
eplerenone
-
inspra
-
canrenone
-
contaren
-
luvion
-
aldadiene
-
phanurane
-
MeSH Canrenone
-
MeSH Canrenoate Potassium
-
‘canrenoate potassium’
-
‘potassium canrenoate’
-
canrenol
-
soldactone
-
‘Kalium-Can’
-
diurek
-
kanrenol
-
luvion
-
venactone
-
spiroctan
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29
Health Economic Evaluation Database
www.interscience.wiley.com/cgi-bin/mrwhome/114130635/HOME
-
Search date: 6 January 2009.
The following search strategy retrieved 20 records:
-
AX = Spironolactone
-
AX = aldactone
-
AX = (novo-spiroton OR novospiroton OR ‘novo spiroton’)
-
AX = spiractin
-
AX = spirotone
-
AX = verospiron
-
AX = berlactone
-
AX = Eplerenone
-
AX = inspra
-
AX = Canrenone
-
AX = contaren
-
AX = luvion
-
AX = aldadiene
-
AX = phanurane
-
AX = (‘Canrenoate Potassium’ OR ‘Potassium Canrenoate’)
-
AX = canrenol
-
AX = soldactone
-
AX = ‘Kalium-Can’
-
AX = diurek
-
AX = Kanrenol
-
AX = luvion
-
AX = venactone
-
AX = spiroctan
-
CS = (1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23)
-
AX = (aldosterone AND antagonist*)
-
CS = (24 OR 25)
IDEAS
-
Search date: 6 January 2009.
The following search strategy retrieved five records:
spironolactone | aldactone | novospiroton | spiractin | spirotone | verospiron | berlactone | eplerenone | inspra | ‘aldosterone antagonist*’ | canrenone | contaren | luvion | aldadiene | phanurane | ‘canrenoate potassium’ | ‘potassium canrenoate’ | canrenol | soldactone | ‘kalium-can’ | diurek | kanrenol | luvion | venactone | spiroctan
Appendix 2 Details of included studies
Clinical effectiveness
| RALES31,40 General HF population | EPHESUS27,40,52 Ischaemic HF population | AREA IN-CHF50 | |
|---|---|---|---|
| General HF population | Ischaemic HF population | ||
| Study design | |||
| 1663 patients; 822 spironolactone, 841 placebo | 6642 patients; 3319 eplerenone, 3313 placebo | 467 patients; 231 canrenone, 236 placebo | 241 patients; 118 canrenone, 123 placebo |
| Follow-up: 24 months (median 103 weeksa) | Follow-up: mean 16 months (range 0 to 33 monthsa) | Follow-up: 12 months | |
| Conducted March 1995 to December 1996 | Conducted December 1999 to 2001 | Conducted September 2002 to July 2005 | |
| 195 study centres; 15 countries | 647 study centres; 37 countries | 46 cardiology centres in Italy | |
| Drug regimen | |||
| Spironolactone 25 mg daily increasing to a maximum of 50 mg daily as required | Eplerenone 25 mg daily increasing to a maximum of 50 mg daily as required | Canrenone 25 mg daily increasing to 50 mg daily after first month of treatment | |
| Matching placebo | Matching placebo | Matching placebo | |
| Drug dose could be decreased to 25 mg every other day if hyperkalaemia occurred. Medication could be withheld if serious hyperkalaemia (6.0 mmol/l or over) occurred or a serum creatinine of more than 4.0 mg/dl | Drug dose decreased or discontinued if serum potassium exceeded 5.5 mmol/l | Drug dose not increased if serum potassium exceeded 5.0 mmol/l or serum creatinine exceeded 2.5 mg/dl | |
| Population | |||
| NYHA class III or IV HF at enrolment; IV in previous 6 months | PostMI HF | NYHA class II HF | NYHA class II HF |
| 54% ischaemic HF | 100% ischaemic HF | 52% ischaemic, 49% postMI HF | 100% ischaemic HF |
| Mean time from MI: NR | Mean time from MI: 7.3 days | Mean time from MI: NR | Mean time from MI: at least 3 months since MI |
| Range time from MI: HF for at least 6 weeks | Range time from MI: 3–14 days | Time from MI: HF for over 12 months in 75% | |
| LVEF 35% or less | LVEF 40% or less | LVEF 45% or less within 6 months prior to enrolment | LVEF 45% or less |
| Diabetics included despite no symptoms of HF | |||
| Approximate baseline characteristics | |||
| Mean age 65 years (range 21–91 years) | Mean age 64 years | Mean age 62.5 years | Mean age 62.5 years |
| 86.5% white | 90% white | Ethnicity: NR | Ethnicity: NR |
| 73% male | 70% male | 84% male | 93% male |
| BP: 122.5/75 | BP: 120/70 | BP: 128/80 | BP: NR |
| First MI: 73% | |||
| LVEF 25% | LVEF: 33% | LVEF: 40% (IQR 33 to 45) | LVEF: 38% (range 16 to 58) |
| Serum potassium: NR | Serum potassium: 4.3 mmol/dl | Serum potassium: 4.4 mmol/dl | Serum potassium: 4.4 mmol/dl |
| Serum creatinine: NR | Serum creatinine: 1.1 mg/dl | Serum creatinine: 1.02 mg/dl | Serum creatinine: 1.1 mg/dl |
| Creatinine clearance: NR | Creatinine clearance: 78.5 ml/min | Creatinine clearance: 79 ml/min | Creatinine clearance: NR |
| NYHA classification: | NYHA classificationa: | NYHA classification: | NYHA classification: |
| II: 0.5%; III: 70.5%; IV: 29% | I: 1864; II: 3279; III: 1049; IV: 103; 337 not classified | II: 100% | II: 100% |
| Killip classification: | Killip classificationa: | Killip classification: | Killip classification: |
| NR | 1: 15%; 2: 65%; 3: 17%; 4: 3% | NR | NR |
| Concomitant medications | |||
| ACE inhibitors: 94.5% | ACE inhibitors/ARBs: 86.5% | ACE inhibitors: 80% | ACE inhibitors: 81% |
| ARBs: NR | ARBs: NR separately | ARBs: 18% | ARBs: 16% |
| Thiazides: 5% | |||
| Beta-blockers: 10.5% | Beta-blockers: 75% | Beta-blockers: 79% | Beta-blockers: 80% |
| Diuretics generally: 100% | Diuretics generally: 60.5% | Diuretics: 63% | |
| Loop diuretics specifically: 100% | Loop diuretics specifically: NR | Loop diuretics specifically: 60% | |
| Aspirin: 36.5% | Aspirin: 88.5% | Aspirin: 48% | Aspirin: 71% |
| Statins: NR | Statins: 47% | Statins: 45% | Statins: 68% |
| Digoxin: 73.5% | Digoxin: NR | Digoxin: 26% | Digoxin: 18% |
| Nitrates: NR | Nitrates: NR | Nitrates: 26.5% | Nitrates: 39% |
| Amiodarone: NR | Amiodarone: NR | Amiodarone: 18% | Amiodarone: NR |
| Dihydropyridines: NR | Dihydropyridines: NR | Dihydropyridines: 7% | Dihydropyridines: NR |
| Calcium channel blockers: NR | Calcium channel blockers: NR | Calcium channel blockers: 7.5% | Calcium channel blockers: NR |
| Medical history | |||
| Acute MI: NR | Acute MI: 27% | Acute MI: 49% | Acute MI: 92% |
| Diabetes: NR | Diabetes: 32% | Diabetes: 20% | Diabetes: 24.5% |
| HF: NR | HF: 14.5% | HF: 47% | HF: 38.5% |
| Hypertension: NR | Hypertension: 60.5% | Hypertension: 45% | Hypertension: 44% |
| LBBB: NR | LBBB: NR | LBBB: 28.5% | LBBB: NR |
| Exclusions | |||
| Use of potassium sparing diuretics | Use of potassium sparing diuretics | Use of potassium sparing diuretics | |
| Serum creatinine 2.5 mg/dl or over | Serum creatinine 2.5 mg/dl or over | Serum creatinine 2.5 mg/dl or over | |
| Serum potassium 5 mmol/l or over | Serum potassium 5 mmol/l or over | Serum potassium 5 mmol/l or over | |
|
Heart transplant Primary operable valvular heart disease Congenital heart disease Unstable angina Primary hepatic failure Active cancer Primary life threatening disease other than HF |
IV inotropic agents 3 months prior to study History or resuscitation, VF, or tachycardia unless due to MI in last 24 hours Use of lithium salts, tumor necrosis factor antagonists, or investigational drugs 3 months prior to study |
||
| Outcomes of interest reported | |||
|
Mortality – all-cause, cardiac Cardiac hospitalisation Cardiac death/hospitalisation Change in NYHA classification Serious hyperkalaemia (6.0 mmol/l or over) |
Mortality at 1 year Mortality – all-cause, cardiovascular, sudden CV, acute MI, HF Cardiovascular hospitalisation Death/hospitalisation – all-cause, cardiac Serious hyperkalaemia (6.0 mmol/l or over) |
Mortality – cardiac Cardiac hospitalisation Cardiac death/hospitalisation Change in NYHA classification Hyperkalaemia (5.5mEq/l) Ventricular function outcomes also reported |
Mortality – all-cause, cardiac Hospitalisation – all-cause, cardiac Death/hospitalisation – all-cause, cardiac Hyperkalaemia (5.5mEq/l) Serious hyperkalaemia (6.0 mEq/l) Gynaecomastia Dropouts overall and due to any adverse event, gynaecomastia, hyperkalaemia Change in serum creatinine |
| Withdrawals | |||
| 414 discontinued; 214 spironolactone, 200 placebo | 1021 discontinued; 528 eplerenone, 493 placebo | 38 patients disqualified due to inadequacy of informed consent | 7 patients in each arm at 12 months |
Results for the general ischaemic HF population
| EPHESUS | AREA IN-CHF |
|---|---|
|
RRs are Kaplan–Meier estimates Mortality at 1 year: Ep 11.8%; Pl 13.6% (66% of the deaths were in the first 30 daysa) Mortality – all-cause: Ep 478 (14.4%); Pl 554 (16.7%); RR 0.85 (95% CI: 0.75 to 0.96) Mortality – all-cause within first 30 days: Ep 107; Pl 158; RR 0.68a Mortality – all-cause after 30 days: Ep 371; Pl 396; RR 0.92a Mortality – CV: Ep 407 (12.3%); Pl 483 (14.6%); RR 0.83 (95% CI: 0.72 to 0.94) Death – sudden CV: Ep 162 (4.9%); Pl 201 (6.1%); RR 0.79 (95% CI: 0.64 to 0.97) Death – acute MI: Ep 78 (2.4%); Pl 94 (2.8%); RR 0.82 (95% CI: 0.61 to 1.10) Death – HF: Ep 104 (3.1%); Pl 127 (3.8%); RR 0.80 (95% CI: 0.62 to 1.04) Death – stroke: Ep 26 (0.8%); Pl 28 (0.8%); RR 0.91 (95% CI: 0.53 to 1.55) Hospitalisation – CV: Ep 606 (18.3%); Pl 649 (19.6%); RR 0.91 (95% CI: 0.81 to 1.01) Hospitalisation – all-cause: Ep 1493 (45.0%); Pl 1526 (46.1%); RR 0.95 (95% CI: 0.89 to 1.02) Hospitalisation – acute MI: Ep 224 (6.7%); Pl 229 (6.9%); RR 0.97 (95% CI: 0.80 to 1.16) Hospitalisation – HF: Ep 345 (10.4%); Pl 391 (11.8%); RR 0.85 (95% CI: 0.74 to 0.99) Hospitalisation – stroke: Ep 70 (2.1%); Pl 51 (1.5%); RR 1.34 (95% CI: 0.94 to 1.93) Hospitalisation – ventricular arrhythmia: Ep 52 (1.6%); Pl 54 (1.6%); RR 0.95 (95% CI: 0.65 to 1.39) Mortality/hospitalisation – cardiac: Ep 885 (26.7%); Pl 993 (30.0%); RR 0.87 (95% CI: 0.79 to 0.95) Mortality/hospitalisation – all-cause: Ep 1730 (52.1%); Pl 1829 (55.2%); RR 0.92 (95% CI: 0.86 to 0.98) Recurrent MI (fatal or non-fatal): Ep 8.8%; Pl 9.4%a Change in NYHA classificationa: Worsened: Ep 779 (24.7%); Pl 902 (28.7%) No change: Ep 1582 (50.2%); Pl 1527 (48.6%) Improved: Ep 790 (25.1%); Pl 715 (22.7%) |
Mortality – all-cause: Can 4 (0%); Pl 10 (0%); RR 0.00 (95% CI: 0.00 to 0.00) Mortality – cardiac: Can 4 (0%); Pl 6 (0%); RR 0.00 (95% CI: 0.00 to 0.00) Hospitalisation – all-cause: Can 22 (0%); Pl 30 (0%); RR 0.00 (95% CI: 0.00 to 0.00) Hospitalisation – cardiac: Can 9 (0%); Pl 20 (0%); RR 0.00 (95% CI: 0.00 to 0.00) Mortality/hospitalisation – all-cause: Can 26 (0%); Pl 35 (0%); RR 0.00 (95% CI: 0.00 to 0.00) Mortality/hospitalisation – cardiac: Can 13 (0%); Pl 24 (0%); RR 0.00 (95% CI: 0.00 to 0.00) Relative mean (SE) change in serum creatinine: Can 0.047 (0.015); Pl 0.018 (0.015) Absolute mean (SE) change in serum creatinine: Can 0.046 (0.017); Pl 0.010 (0.017) |
Results for the ischaemic HF population with poor LVEF
| RALES | EPHESUS |
|---|---|
|
Mortality – all-cause: Sp 171/454a (37.6%); Pl 222/453a (49.0%) RR 0.72 (95% CI: 0.58 to 0.88; extrapolated from graph) RR 0.77 (95% CI: 0.66 to 0.89)b |
RRs are Kaplan–Meier estimates Mortality – all-cause: Ep 205 (19.6%); Pl 254 (24.0%); RR 0.79 (95% CI: NR) Mortality – CV: Ep 177 (16.9%); Pl 226 (21.4%); RR 0.77 (95% CI: NR) Death – sudden cardiac: Ep 71 (6.8%); Pl 103 (9.7%); RR 0.67 (95% CI: NR) Death – HF: Ep 49 (4.7%); Pl 59 (5.6%); RR 0.81 (95% CI: NR) Hospitalisation – HF: Ep 152 (14.5%); Pl 181 (17.1%); RR 0.80 (95% CI: NR) Mortality/hospitalisation – CV: Ep 359 (34.3%); Pl 433 (40.9%); RR 0.79 (95% CI: NR) Mortality/hospitalisation – HF: Ep 176 (16.8%); Pl 221 (20.9%); RR 0.75 (95% CI: NR) |
| Ruta et al. (2006)48 | Tu (2003)49 |
|---|---|
| Study design | |
| Published in Polish | Published in Chinese |
| 47 patients; 22 spironolactone, 25 no spironolactone | 85 patients; 43 spironolactone, 42 controls |
| Follow-up 24 months | Follow-up 12 months |
| Conducted December 2000 to 2002 | Conducted 2000 to 2001 |
| Conducted in specialist cardiology units; number NR | Setting: NR |
| Drug regimen | |
| Spironolactone 25–50 mg daily | Spironolactone 20 mg daily |
| No placebo | Placebo – unclear if matching |
| Population | |
| PostMI with LEVF 30% or less | PostMI with clinical signs of HF; LVEF: NR |
| 100% ischaemic HF | 100% ischaemic HF |
| Time from MI: immediately postMI | Time from MI: appears recruitment on admission for acute MI |
| Approximate baseline characteristics for entire population | |
| Mean age: 62.5 years (range 38 to 79 years) | Mean age: 67.9 years |
| Ethnicity: NR | Ethnicity: NR |
| 81% male | 65% male |
| BP: NR | BP: NR |
| LVEF: 24.5% | LVEF: NR |
| Anterior MI: 32% | Anterior MI: NR |
| Mean time from MI: NR | Mean time from MI: NR |
| Range time from MI: recruited immediately post-MI | Range time from MI: NR |
| Serum potassium: NR | Serum potassium: NR |
| Serum creatinine: NR | Serum creatinine: NR |
| Creatinine clearance: NR | Creatinine clearance: NR |
| NYHA classification: II: 28%; III: 72%; IV: 0% | NYHA classification: NR |
| Concomitant medications | |
| ACE inhibitors: 60% | ACE inhibitors: 100% |
| ARBs: NR | ARBs: NR |
| Beta-blockers: 85% | Beta-blockers: 100% |
| Diuretics generally: 79% | Diuretics generally: 100% |
| Aspirin: 100% | Aspirin: NR |
| Statins: 53% | Statins: NR |
| Digoxin: 30% | Digoxin: ‘usually’ prescribed |
| Amiodarone: 19% | Amiodarone: NR |
| Medical history | |
| Previous MI: 74% | Previous MI: NR |
| Diabetes: 23% | Diabetes: NR |
| HF: NR | HF: NR |
| Hypertension: 47% | Hypertension: NR |
| LBBB: NR | LBBB: NR |
| Stated: no differences at baseline in previous stroke or diabetes | |
| Exclusions | |
| Patients referred for revascularisation |
Serum creatinine 177 µmol/l (2 mg/dl) or over Serum potassium 5 mmol/l or over |
| Outcomes reported | |
|
All-cause mortality Gynaecomastia |
Mortality – all-cause and cardiovascular Rehospitalisation for HF Hyperkalaemia Gynaecomastia |
| Withdrawals | |
| Two patients stopped taking spironolactone due to gynaecomastia | None reported |
| Effectiveness results for ischaemic patients with poor LVEF | |
| Mortality – all-cause: Sp 11/22; no Sp 8/25 |
Mortality – all-cause: Sp 1/43; Pl 7/42 Mortality – CV: Sp 1/43; Pl 6/42 Mortality – haemorrhagic stroke: Sp 0/43; Pl 1/42 Rehospitalisation: Sp 4/43; Pl 11/42 |
Adverse events
| Study ID | Study design | Population | Drug regimens | Results | ||
|---|---|---|---|---|---|---|
| Dose ranging study 01140 |
Double-blind RCT 1997–8 12-week treatment duration; 16-week follow-up 57 sites, six countries; US, Poland, France, Germany, Belgium, the Netherlands |
321 patients with HF; mean age 61 (range 31 to 87) years LVEF: 40% or less NYHA: II: 39% III: 59% IV: 2% 56 patients lost to follow-up |
Eplerenone: 25 mg two or four times daily 50 mg four times daily 50 mg four times daily for 1 week then increased to 100 mg four times daily for 11 weeks Spironolactone: 25 mg four times daily Placebo Doses of eplerenone doubled in last 4 weeks; spironolactone remained unchanged |
Withdrawal due to adverse event: Ep 27 (12%); Sp 6 (13%); Pl 3 (5%) Serious adverse event: Ep 30 (14%); Sp 6 (13%); Pl 4 (7%) Hyperkalaemia: Ep 9%; Sp 13%; Pl 4% Gynaecomastia: Ep 2; Sp 1; Pl 0 |
||
| Japanese dose ranging study40 |
Double-blind RCT 2000–2 12-week follow-up 36 sites |
161 patients; mean age 64 (range 29 to 88) years LVEF: 40% or less NYHA: II: 63% III: NR IV: NR 26 patients lost to follow-up |
Eplerenone: 25 mg four times daily 50 mg four times daily 100 mg four times daily Placebo |
Adverse event: 25 mg 25/37 (68%) 50 mg 28/39 (72%) 100 mg 26/38 (68%) Placebo 29/38 (76%) Serious adverse event: 25 mg 2/37 (5%) 50 mg 4/39 (10%) 100 mg 2/38 (5%) Placebo 2/38 (5%) Discontinued due to adverse event: 25 mg 6/37 (16%) 50 mg 7/39 (18%) 100 mg 3/38 (8%) Placebo 3/38 (8%) Withdrawal due to specific adverse event: Hyperkalaemia: Ep 4, Pl 1 Hypokalaemia: Ep 1, Pl 1 Gynaecomastia: Ep 0, Pl 0 |
||
| Lawson (1982)60 |
Surveillance programme From/to: NR US, Canada, NZ, UK, Italy, Germany, and Israel |
3879 HF patients; 783 taking spironolactone | Spironolactone: dose not reported | Hyperkalaemia (6.0 mmol/l or over): 57/783 (7.3%) | ||
| Greenblatt (1973)61 |
Surveillance programme From 1966, to NR US, Canada, NZ and Israel |
788 patients Treated for fluid retention due to liver cirrhosis (366), congested cardiac failure (313) or neoplastic disease (25), hypertension (25); 59 patients for other less frequent indications that were not specified |
Spironolactone: 150 mg/day or less |
Of 788 patients: 148 died while in hospital (19%) 164 had adverse event (20.8%): 68 hyperkalaemia (not defined; 8.6%) 3.4% dehydration 2.4% hyponatraemia 18 gastrointestinal disturbances (2.3%) 16 neurological disturbances (2%) Of 164 with an adverse event: 1.2% gynaecomastia (translates two patients, 0.25% overall population) |
||
| Shah (2006)62 |
Record examination 1999–2004 |
840 patients with congested cardiac failure; 556 patients had laboratory tests results | Spironolactone: dose not reported |
Hyperkalaemia (5.5 mmol/l or greater): 83/556 (15%) Serious hyperkalaemia (6.0 mmol/l or greater): 33/556 (6%) Renal failure (creatinine levels over 2.5 mg/dl): 51/556 (9%) |
||
| Smith (1980)63 |
Case series 48 weeks prospective study |
115 patients admitted with congested cardiac failure with oedema | Spironolactone: 100 mg daily | No. reporting | No. dropping out | |
| Limb pain/cramps | 11 | 1 | ||||
| Vertigo/dizziness | 11 | 2 | ||||
| Tiredness/drowsiness | 5 | 2 | ||||
| Nausea | 5 | 4 | ||||
| Gynaecomastia/sore nipples | 3 | 1 | ||||
| Gastrointestinal upset | 3 | |||||
| Depression | 3 | |||||
| Dry mouth | 3 | 2 | ||||
| Palpitations | 2 | |||||
| Indigestion | 2 | 1 | ||||
| Headache | 2 | |||||
| Soles of feet hot | 1 | |||||
| Chest pains | 1 | |||||
| Increased micturition | 2 | |||||
| Fibrillation | 1 | 1 | ||||
| Discomfort | 1 | 1 | ||||
| Loss of appetite | 1 | 1 | ||||
| Vaginal bleeding | 1 | 1 | ||||
| Others (reported once) | 14 | |||||
| Total | 36 | 11 | ||||
| Nassiacos (2005)57 |
Retrospective case series 1995–2003 Abstract only |
157 consecutive patients admitted with HF 124 patients received antialdosterone therapy and 33 patients did not 71 patients had ischaemic aetiology, and 86 did not |
Canrenone: mean 37 (± 19.9) mg/day |
Serum potassium levels significantly increased in patients receiving canrenone and those with ischaemic aetiology compared with those who did not receive canrenone (p < 0.01) and without ischaemic aetiology (p < 0.01), respectively Canrenone discontinuation: Hyperkalaemia: 8/124 (6.5%) Gynaecomastia: 2/124 (1.6%) Urticaria: 1/124 (0.8%) Nausea: 1/124 (0.8%) |
||
| Witham (2004)66 |
Retrospective case series 2001–2 |
226 patients with chronic HF and objective evidence of LVSD | Spironolactone: dose not reported | Hyperkalaemia | 15/141 (10.6%) | |
| Raised creatinine | 16/141 (11.0%) | |||||
| Hyperkalaemia or raised creatinine | 30/141 (21.3%) | |||||
| Hypotension | 7/141 (5.0%) | |||||
| Breast pain | 5/141 (3.5%) | |||||
| Gynaecomastia | 5/141 (3.5%) | |||||
| Hyponatraemia | 3/141 (2.1%) | |||||
| Nausea and vomiting | 3/141 (2.1%) | |||||
| Headache | 1/141 (0.7%) | |||||
| Cramps | 1/141 (0.7%) | |||||
| Impotence | 1/141 (0.7%) | |||||
| Lightheadedness | 1/141 (0.7%) | |||||
| Tinnitus | 1/141 (0.7%) | |||||
| Stomach pain | 1/141 (0.7%) | |||||
| Sligl (2004)67 |
Prospective cohort 1989–2001 |
136 patients with confirmed HF 114 patients treated since 1998 and focus of adverse event data 57% had ischaemic aetiology |
Spironolactone: mean 24 mg/day | Discontinuations due to: | ||
| Any adverse event | 29/114 (25%) | |||||
| Hyperkalaemia and/or raised creatinine | 10/114 (9%) | |||||
| Dehydration or hyponatraemia | 7/114 (6%) | |||||
| Gynaecomastia | 6/114 (5%) | |||||
| Decreased sympoms | 3/114 (3%) | |||||
| Unpsecified reasons | 3/114 (3%) | |||||
| Svensson (2003)58,59 |
Prospective case series 1999–2001 |
125 consecutive patients with congested cardiac failure and an LVEF < 45% | Spironolactone: full dose information not reported; 48 patients were reported to have been treated with 50 mg/day at some point |
Increased serum creatinine concentration by: 20%: 69/125 (55%) 50%: 30/125 (24%) 100%: 11/125 (9%) Serum creatinine concentration exceeded: 130 µmol/l: 72/125 (58%) > 220 µmol/l: 23/72 with levels over 130 µmol/l Serum potassium concentration: > 5.0 mmolll: 45/125 (36%) > 5.5 mmol/l: 21/125 (17%) > 6 mmol/l: 12/125 (9.6%) Mean increase in serum potassium: from 4.2 (SD ± 0.3) mmol/l at baseline to 5.0 (SD ± 0.4) |
||
| Anton (2003)69 |
Retrospective cohort 2000–1 |
110 patients prescribed spironolactone and an ACE inhibitor for whom clinical data could be obtained | Spironolactone: full dose information not reported; 24 patients were reported to have received a dose > 25 mg/day |
Hyperkalaemia: 42/110 (38.2%) Severe hyperkalaemia: 26/110 (23.6%) Discontinued treatment: 44/93 (47.3%) |
||
| Tamirisa (2004)70 |
Case–control study 1998–2002 |
926 patients with heart failure and a documented LVEF < 35% 67 cases: discontinued treatment due to hyperkalaemia or renal failure 134 controls (randomly selected from possible 834): did not discontinue spironolactone; treated for at least 6 months |
Spironolactone: dose not reported |
Discontinued due to: Hyperkalaemia: 33/926 (3.6%) Renal failure: 34/926 (3.7%) Serum potassium > 6 mmol/l: 15/926 (1.6%) Serum creatinine > 4.0 mg.dl: 3/926 (0.3%) |
||
| Williams (2006)71 |
Patient record review 1996–2003 |
762 patients prescribed spironolactone for a range of disorders | Spironolactone: mean 38.4 (± 1.49) mg/day |
Any adverse event: 81/762 (10.6%) Hyperkalaemia: 40/762 (5.3%) Gynaecomastia: 14/762 (1.8%) Gastritis: 15/762 (2%) Other (decreased libido, hair loss, hypotension, metallic taste, withdrawal after trial period): 12/762 (1.6%) |
||
| Hauben (2007)64 |
Calculated the reporting rate of spironolactone-associated hyperkalaemia per 1000 reports per year 1970–2005 Cases identified in US FDA AE Reporting System (AERS) |
The rate per 1000 reports was consistently < 0.1 (and always < 0.3) between 1970 and 2000, and always over 0.3 between 2000 and 2005. Change attributed to an increase in reporting after the publication of RALES | ||||
| Juurlink (2004)65 |
Population-based time-series; 1994–2001 Rate of prescriptions of spironolactone and rate of hyperkalaemia in ambulatory patients, before and after publication of RALES Computerised prescription records of the Ontario Drug Benefit Program |
Patients 66 years or older treated with an ACE inhibitor after hospitalisation for HF Prescription rates for spironolactone: 1994 34 per 1000, 1999 30 per 1000, 2001 149 per 1000 Hospital admission for hyperkalaemia (not defined) rates: 1994 2.4 per 1000, 1999 4.0 per 1000, 2001 11.0 per 1000 Within-hospital hyperkalaemia-associated death: 1994 0.3 per 1000, 1999 0.7 per 1000, 2001 2.0 per 1000 Patients 66 years or older treated with an ACE inhibitor with or without and admission for HF: Prescription rates for spironolactone: 1994 12 per 1000, 2001 32 per 1000 Hospital admission for hyperkalaemia (not defined) rates: 1994 0.9 per 1000, 1999 1.2 per 1000, 2001 2.8 per 1000 Within-hospital hyperkalaemia-associated death: 1994 0.1 per 1000, 1999 0.17 per 1000, 2001 0.39 per 1000 |
||||
Appendix 3 Excluded studies with rationale
Effectiveness
| aBanas (2005)167 | bRodriguez (1997)147 |
| cBarr (1995)168 | dRousseau (2002)169 |
| bDi Pasquale (2005)149 | aRumsfeld (2005)170 |
| cHayashi (2003)30 | dTsutamoto (2004)171 |
| bModena (2001)150 | aZannad (2000)172 |
| aO’Keefe (2008)173 |
Adverse events
| aBellati (1986)101 | aKohya (1995)174 |
| aBerry (2001)175 | aKonopa (2006)176 |
| aButler (2002)177 | bMasoudi (2005)178 |
| cCasado-Escribano (2005)55 | aNurnberger (2005)179 |
| aCox (2001)180 | aO’Reilly (1987)104 |
| aDebugne (2006)181 | cPassino (2008)56 |
| aDinsdale (2005)182 | aPongpaew (1973)107 |
| aDupont (1985)103 | bRaebel (2007)183 |
| aGreenlaw (1977)105 | aSaudan (2003)184 |
| aHuffman (1986)100 | aSkvortsov (2007)185 |
| aHughes (1988)102 | aTeive (2007)186 |
| aHussain (2003)187 | aUdezue (1980)106 |
| aKaragiannis (2008)188 | aVanpee (2000)189 |
| aKauffmann (2005)190 | aWrenger (2003)191 |
| bKo (2006)192 |
Appendix 4 Quality assessment guidelines
Clinical-effectiveness
Randomised controlled trials
| Was the number of participants randomised stated? |
|---|
|
Yes: Number of people randomised to each arm of the trial was reported No: Only the total number of participants was reported or only the number that actually received each treatment was reported |
| Was the method of randomisation appropriate? |
|
Yes: Computer-generated random numbers or the use of random number tables No: Any other method of randomisation Unclear: The study stated that randomisation occurred, but did not report the method |
| Was allocation concealment adequate? |
|
Yes: Any robust method that would not allow the patient status to influence the allocation of treatment No: Other methods of allocation concealment Unclear: Either allocation was concealed but the method was not reported, or the concealment of allocation was not reported |
| Were the treatment groups comparable at baseline? |
|
Yes: There were no significant differences between the participants of the treatment arms at baseline No: There were significant differences between the participants of the treatment arms at baseline Unclear: Baseline characteristics were not reported |
| Was the study reported as being at least double blind? |
|
Yes: The study was reported as being double or triple blind No: The study did not report whether it was double blind or not |
| Were patients blinded? |
|
Yes: It was explicitly stated that patients were blinded to treatment or the methods described implied that patients were blinded No: It was explicitly stated that patients were not blinded to treatment Unclear: No specific information regarding the blinding of patients was reported |
| Were outcome assessors blinded? |
|
Yes: It was explicitly stated that outcome assessors were blinded to treatment or the methods described implied that outcome assessors were blinded No: It was explicitly stated that outcome assessors were not blinded to treatment Unclear: No specific information regarding the blinding of outcome assessors was reported |
| Were care givers blinded? |
|
Yes: It was explicitly stated that care givers were blinded to treatment or the methods described implied that care givers were blinded No: It was explicitly stated that care givers were not blinded to treatment Unclear: No specific information regarding the blinding of care givers was reported |
| Power calculation used? |
|
Yes: Power calculation was used No: Power calculation was not used, or its use was not reported |
| Selection/eligibility criteria reported? |
|
Yes: Selection/eligibility criteria were reported No: Selection/eligibility criteria were not adequately reported |
| Baseline characteristics of ischaemic group provided? |
|
Yes: Adequate baseline characteristics of the ischaemic group were provided No: Baseline characteristics of the ischaemic group were not provided or were inadequate |
| At least 12 months follow-up? |
|
Yes: Patients were followed up for 12 months or longer No: Patients were followed up for less than 12 months |
| Representative sample recruited? |
|
Yes: The study sample was representative of the study population in clinical practice No: The study sample was not truly representative of the study population (e.g. age range for a given group was too narrow) Unclear: Criteria were not adequately reported |
| ITT analysis used? |
|
Yes: An ITT analysis was presented No: An ITT analysis was not used |
| Losses to follow-up reported/explained? |
|
Yes: Losses to follow-up were reported/explained No: Losses to follow-up were not reported/explained |
| Were at least 90% of those randomised followed up? |
|
Yes: At least 90% were followed up at the final time point reported No: < 90% were followed up at the final time point reported Unclear: Loss to follow-up was not reported |
Observational studies
| Was there a control group? |
|---|
|
Yes: There were at least two arms to the study No: The study included a single group |
| Were data obtained prospectively? |
|
Yes: The data were obtained prospectively No:The study was retrospective in design Unclear:The study design was not clearly reported |
| Were participants selected in an unbiased way? |
|
Yes: A consecutive sample was used No: There was evidence of selective sampling Unclear: Patient selection methods were not clearly described |
| Inclusion criteria clearly reported? |
|
Yes: Inclusion criteria were clearly reported No: Inclusion criteria were not clearly reported |
| Study size explained? |
|
Yes: Sample size calculation for prospective studies or explanation for dates selected for retrospective studies provided No: No sample size calculation or explanation for dates selected |
| Were the baseline characterstics of participants similar? |
|
Yes: No significant differences between the participants of the treatment arms at baseline No: Significant differences between the participants of the treatment arms at baseline Unclear: Baseline characteristics were not reported NA: There was no control group |
| Was the study conducted in the population of interest (postMI HF)? |
|
Yes: Participants were matched or stratified according to confounders, restricted, or multivariate analysis used No: Confounders were not adequately investigated or reported |
| Have losses to follow-up been accounted for? |
|
Yes: Losses to follow-up have been investigated and reported No: Losses to follow-up have not been reported or accounted for NA: There were no losses to follow-up or the study was retrospective |
| Were all participants recruited included in the analysis? |
|
Yes: All participants were included in the final analysis No: Some participants were excluded from the final analysis |
| Outcomes measured up to at least 6 months after initiation of treatment? |
|
Yes: Outcomes were measured at 6 months or longer No: Outcomes were measured at less than 6 months Unclear: The duration of follow-up was not reported |
| Was the detection of adverse events a primary goal of the study? |
|
Yes: The detection of adverse events was the primary outcome of the study No: The detection of adverse events was a secondary outcome of the study |
| Were at least 90% of those recruited followed up? |
|
Yes: At least 90% were followed up at the final time point reported No: < 90% were followed up at the final time point reported Unclear: Loss to follow-up was not reported or the study was retrospective |
Cost-effectiveness
All items are graded as either ✓ yes (item adequately addressed); ✗ no (item not adequately addressed); ? unclear or not enough information; N/A, not applicable; or NS, not stated.
Tilson et al. (2003)120 Cost-effectiveness of spironolactone in patients with severe heart failure
| Study question | Grade | Comments |
|---|---|---|
| 1. Costs and effects examined | ✓ | |
| 2. Alternatives compared | ✓ | |
| 3. The viewpoint(s)/perspective of the analysis is clearly stated (e.g. NHS, society) | ✗ | The perspective was not explicitly reported. It appears to have been that of the hospital |
| Selection of alternatives | ||
| 4. All relevant alternatives are compared (including do-nothing if applicable) | ✓ | The study was undertaken prior to the availability of results from EPHESUS and hence restricting the comparison to spironolactone and usual care was appropriate based on data available at the time of the cost-effectiveness analysis |
| 5. The alternatives being compared are clearly described (who did what, to whom, where and how often) | ✓ | The addition of spironolactone to standard therapy is compared with standard therapy alone |
| 6. The rationale for choosing the alternative programmes or interventions compared is stated | ✓ | A justification was given for the comparator used. It represented current practice in the authors’ setting |
| Form of evaluation | ||
| 7. The choice of form of economic evaluation is justified in relation to the questions addressed | ✓ | |
| 8. If a cost-minimisation design is chosen, have equivalent outcomes been adequately demonstrated? | N/A | |
| Effectiveness data | ||
| 9. The source(s) of effectiveness estimates used are stated (e.g. single study, selection of studies, systematic review, expert opinion) | ✓ | Very little detail is reported. A systematic review of the literature does not appear to have been performed. The source of effectiveness estimates is two studies with follow-up periods of 1 and 2 years |
| 10. Effectiveness data from RCT or review of RCTs | ✓ | The main effectiveness data are derived from one major RCT |
| 11. Potential biases identified (especially if data not from RCTs) | ✗ | No attempt to identify sources of possible bias was made |
| 12. Details of the method of synthesis or meta-analysis of estimates are given (if based on an overview of a number of effectiveness studies) | N/A | No formal synthesis undertaken |
| Costs | ||
| 13. All the important and relevant resource use included | ? | Resource use and costs were not reported separately. Only costs associated with treatment, hospitalisation and outpatient clinic visits were considered |
| 14. All the important and relevant resource use measured accurately (with methodology) | ? | No information is provided on how resource use was measured |
| 15. Appropriate unit costs estimated (with methodology) | ✓ | |
| 16. Unit costs reported separately from resource use data | ✗ | |
| 17. Productivity costs treated separately from other costs | N/A | |
| 18. The year and country to which unit costs apply is stated with appropriate adjustments for inflation and/or currency conversion | ✓ | 2000–2 Irish € |
| Benefit measurement and valuation | ||
| 19. The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | Benefits were measured in terms of life years gained. Quality-adjusted life-years gained was not considered because of the lack of available data |
| 20. Methods to value health states and other benefits are stated | ✗ | No health states were valued |
| 21. Details of the individuals from whom valuations were obtained are given | N/A | |
| Decision modelling | ||
| 22. Details of any decision model used are given (e.g. decision tree, Markov model) | ✓ | |
| 23. The choice of model used and the key input parameters on which it is based are adequately detailed and justified | ✓ | |
| 24. All model outputs described adequately | ? | The number of life-years gained (LYG) in each of the two treatment arms is not reported. Only the incremental number of LYG is reported. Similarly, the total cost associated with any of the two treatment arms is not reported separately, only the incremental cost is reported |
| Discounting | ||
| 25. Discount rate used for both costs and benefits | ✓ | Costs were discounted at 5% per annum and life expectancy at 1.5% per annum |
| 26. Do discount rates accord with NHS guidance? | ✗ | NHS guidance currently recommends 3.5% per year for costs and benefits |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ✗ | |
| 27. Details of statistical tests and confidence intervals are given for stochastic data | N/A | |
| 28. Uncertainty around cost-effectiveness expressed [e.g. confidence interval around incremental cost-effectiveness ratio (ICER), cost-effectiveness acceptability curves] | N/A | |
| 29. Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | N/A | |
| Stochastic analysis of decision models | ||
| 30. Are all appropriate input parameters included with uncertainty? | ✓ | Only in one-way and two-way sensitivity analyses |
| 31. Is second-order uncertainty (uncertainty in means) included rather than first-order (uncertainty between patients)? | ✓ | |
| 32. Are the probability distributions adequately detailed and appropriate? | ✗ | No distributions are used |
| 33. Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unizt costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | ✓ | Limited sensitivity analysis |
| Deterministic analysis | ||
| 34. The approach to sensitivity analysis is given (e.g. univariate, threshold analysis etc.) | ✓ | One-way and two-way sensitivity analysis |
| 35. The choice of variables for sensitivity analysis is justified | ✓ | |
| 36. The ranges over which the variables are varied are stated | ✓ | |
| Presentation of results | ||
| 37. Incremental analysis is reported using appropriate decision rules | ✓ | |
| 38. Major outcomes are presented in a disaggregated as well as aggregated form | ✗ | Only incremental results are reported |
| 39. Applicable to the NHS setting | ? | Based in the Irish health-care setting. Unclear how valid the long-term extrapolation assumptions are and also issues about whether resource use and costs are generalisable to a UK setting |
Glick et al. (2002)118 Economic evaluation of the randomised aldactone evaluation study (RALES): treatment of patients with severe heart failure
| Study question | Grade | Comments |
|---|---|---|
| 1. Costs and effects examined | ✓ | |
| 2. Alternatives compared | ✓ | The study was undertaken before the availability of results from EPHESUS |
| 3. The viewpoint(s)/perspective of the analysis is clearly stated (e.g. NHS, society) | ✓ | It is stated that the analyses took a truncated societal perspective that was limited to the evaluation of direct medical costs |
| Selection of alternatives | ||
| 4. All relevant alternatives are compared (including do-nothing if applicable) | ✓ | The study was undertaken before the availability of results from EPHESUS, restricting the comparison to spironolactone and usual care was appropriate based on data available at the time of the cost-effectiveness analysis |
| 5. The alternatives being compared are clearly described (who did what, to whom, where and how often) | ✓ | Spironolactone is compared with placebo. Both treatments are in addition to standard therapy |
| 6. The rationale for choosing the alternative programmes or interventions compared is stated | ✓ | |
| Form of evaluation | ||
| 7. The choice of form of economic evaluation is justified in relation to the questions addressed | ✓ | |
| 8. If a cost-minimisation design is chosen, have equivalent outcomes been adequately demonstrated? | N/A | |
| Effectiveness data | ||
| 9. The source(s) of effectiveness estimates used are stated (e.g. single study, selection of studies, systematic review, expert opinion) | ✓ | The source of effectiveness is a single study |
| 10. Effectiveness data from RCT or review of RCTs | ✓ | Effectiveness data are derived from one major RCT |
| 11. Potential biases identified (especially if data not from RCTs) | ✗ | No discussion on potential biases made |
| 12. Details of the method of synthesis or meta-analysis of estimates are given (if based on an overview of a number of effectiveness studies) | N/A | No formal synthesis undertaken |
| Costs | ||
| 13. All the important and relevant resource use included | ✓ | |
| 14. All the important and relevant resource use measured accurately (with methodology) | ✓ | |
| 15. Appropriate unit costs estimated (with methodology) | ✓ | |
| 16. Unit costs reported separately from resource use data | ✗ | |
| 17. Productivity costs treated separately from other costs | N/A | |
| 18. The year and country to which unit costs apply is stated with appropriate adjustments for inflation and/or currency conversion | ✓ | 1999 US$. Costs are derived from 16 countries based on resource use within a multicentre trial |
| Benefit measurement and valuation | ||
| 19. The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | |
| 20. Methods to value health states and other benefits are stated | ✗ | No health states were valued |
| 21. Details of the individuals from whom valuations were obtained are given | N/A | |
| Decision modelling | ||
| 22. Details of any decision model used are given (e.g. decision tree, Markov model) | ✓ | The author refers to a previously published decision analytic model |
| 23. The choice of model used and the key input parameters on which it is based are adequately detailed and justified | ✓ | |
| 24. All model outputs described adequately | ✓ | |
| Discounting | ||
| 25. Discount rate used for both costs and benefits | ✓ | Costs and benefits were discounted at 3% per year in accordance with US guidance |
| 26. Do discount rates accord with NHS guidance? | ✗ | NHS guidance recommends 3.5% per year for costs and benefits |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ✓ | Bootstrapping is used to reflect uncertainty in the analysis. Limited details are given |
| 27. Details of statistical tests and confidence intervals are given for stochastic data | ✓ | No details of statistical tests given. Confidence intervals are presented |
| 28. Uncertainty around cost-effectiveness expressed [e.g. confidence interval around incremental cost-effectiveness ratio (ICER), cost-effectiveness acceptability curves] | ✓ | |
| 29. Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | ✓ | Univariate sensitivity analysis is performed |
| Stochastic analysis of decision models | ||
| 30. Are all appropriate input parameters included with uncertainty? | ✓ | Only in a sensitivity analysis |
| 31. Is second-order uncertainty (uncertainty in means) included rather than first-order (uncertainty between patients)? | ✓ | |
| 32. Are the probability distributions adequately detailed and appropriate? | ✗ | No distributions are used |
| 33. Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | ✓ | |
| Deterministic analysis | ||
| 34. The approach to sensitivity analysis is given (e.g. univariate, threshold analysis etc.) | ✓ | Univariate sensitivity analysis |
| 35. The choice of variables for sensitivity analysis is justified | ✓ | |
| 36. The ranges over which the variables are varied are stated | ✓ | |
| Presentation of results | ||
| 37. Incremental analysis is reported using appropriate decision rules | ✓ | |
| 38. Major outcomes are presented in a disaggregated as well as aggregated form | ✓ | |
| 39. Applicable to the NHS setting | ? | Unclear how generalisable the results are to a UK setting given potential differences between UK practice and the population considered in the RALES study and the generalisability of resource use and costs estimated as part of a multinational study to the UK |
Weintraub et al. (2005)29 Cost-effectiveness of eplerenone compared with placebo in patients with myocardial infarction complicated by left ventricular dysfunction and heart failure
| Study question | Grade | Comments |
| 1. Costs and effects examined | ✓ | |
| 2. Alternatives compared | ✓ | |
| 3. The viewpoint(s)/perspective of the analysis is clearly stated (e.g. NHS, society) | ✓ | A societal perspective is stated, although it is made clear that the evaluation of costs is restricted to direct medical care costs only |
| Selection of alternatives | ||
|---|---|---|
| 4. All relevant alternatives are compared (including do-nothing if applicable) | ? | Spironolactone is not explicitly considered as a comparator in the analysis although it is discussed as a potentially relevant comparator in the study limitations sections. However, the authors consider that any attempt to make a comparison of the two aldosterone blockers would be speculative |
| 5. The alternatives being compared are clearly described (who did what, to whom, where and how often) | ✓ | Eplerenone is compared with placebo. Both treatments are in addition to standard therapy |
| 6. The rationale for choosing the alternative programmes or interventions compared is stated | ✓ | |
| Form of evaluation | ||
| 7. The choice of form of economic evaluation is justified in relation to the questions addressed | ✓ | |
| 8. If a cost-minimisation design is chosen, have equivalent outcomes been adequately demonstrated? | N/A | |
| Effectiveness data | ||
| 9. The source(s) of effectiveness estimates used are stated (e.g. single study, selection of studies, systematic review, expert opinion) | ✓ | The source of effectiveness is a single RCT. The in-trial differences in mortality are extrapolated to estimates of life-years gained using three separate observational datasets |
| 10. Effectiveness data from RCT or review of RCTs | ✓/? | Effectiveness data are derived from one major RCT. The estimates of life-years gained are derived from observational sources |
| 11. Potential biases identified (especially if data not from RCTs) | × | No discussion on potential biases made |
| 12. Details of the method of synthesis or meta-analysis of estimates are given (if based on an overview of a number of effectiveness studies) | N/A | No formal synthesis undertaken |
| Costs | ||
| 13. All the important and relevant resource use included | ✓ | |
| 14. All the important and relevant resource use measured accurately (with methodology) | ✓ | |
| 15. Appropriate unit costs estimated (with methodology) | ✓ | |
| 16. Unit costs reported separately from resource use data | × | |
| 17. Productivity costs treated separately from other costs | N/A | |
| 18. The year and country to which unit costs apply is stated with appropriate adjustments for inflation and/or currency conversion | ✓ | 2001 US$ |
| Benefit measurement and valuation | ||
| 19. The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | The primary analysis was based on estimates of life-years gained |
| 20. Methods to value health states and other benefits are stated | ✓ | A sensitivity analysis presented results based on QALYs. Estimates were based on patient-level data on EQ-5D from a sample of patients from the EPHESUS study. Differences in utility scores between the interventions at 12 months were projected over a lifetime |
| 21. Details of the individuals from whom valuations were obtained are given | ✓ | |
| Decision modelling | ||
| 22. Details of any decision model used are given (e.g. decision tree, Markov model) | N/A | The analysis is based on an extrapolation of survival used to project life-years lost due to in-hospital mortality. These estimates are based on estimates obtained from survival functions derived from three observational sources as opposed to using a formal decision model framework |
| 23. The choice of model used and the key input parameters on which it is based are adequately detailed and justified | N/A | |
| 24. All model outputs described adequately | N/A | |
| Discounting | ||
| 25. Discount rate used for both costs and benefits | ✓ | Costs and benefits were discounted at 3% per year in accordance with US guidance |
| 26. Do discount rates accord with NHS guidance? | × | NHS guidance recommends 3.5% per year for costs and benefits |
| Allowance for uncertainty | ||
| Stochastic analysis of patient-level data | ✓ | Bootstrapping is used to reflect uncertainty in the sample mean ICER estimates |
| 27. Details of statistical tests and confidence intervals are given for stochastic data | ✓ | |
| 28. Uncertainty around cost-effectiveness expressed [e.g. confidence interval around incremental cost-effectiveness ratio (ICER), cost-effectiveness acceptability curves] | ✓ | |
| 29. Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | ✓ | The impact of alternative assumptions is explored using separate scenarios |
| Stochastic analysis of decision models | ||
| 30. Are all appropriate input parameters included with uncertainty? | ? | Bootstrapping is used to reflect uncertainty in the ICER estimates based on sampling uncertainty. It is unclear whether uncertainty surrounding the estimates of life-years lost derived from the observational sources have been included |
| 31. Is second-order uncertainty (uncertainty in means) included rather than first-order (uncertainty between patients)? | ✓ | |
| 32. Are the probability distributions adequately detailed and appropriate? | N/A | |
| 33. Sensitivity analysis used to assess uncertainty in non-stochastic variables (e.g. unit costs, discount rates) and analytic decisions (e.g. methods to handle missing data) | ✓ | |
| Deterministic analysis | ||
| 34. The approach to sensitivity analysis is given (e.g. univariate, threshold analysis etc.) | ✓ | Univariate sensitivity analysis |
| 35. The choice of variables for sensitivity analysis is justified | ✓ | |
| 36. The ranges over which the variables are varied are stated | ✓ | |
| Presentation of results | ||
| 37. Incremental analysis is reported using appropriate decision rules | ✓ | |
| 38. Major outcomes are presented in a disaggregated as well as aggregated form | ✓ | |
| 39. Applicable to the NHS setting | ? | Unclear how generalisable the results are to a UK setting given potential differences between UK practice and the population considered in the EPHESUS study and the generalisability of resource use and costs estimated from US sources |
Appendix 5 Input parameters for the decision model
| Parameter | Mean (95% CI) | Distribution | Description | Source |
| Baseline patient characteristics | ||||
|---|---|---|---|---|
| Age (years) | 65 | Fixed | Average age of patients at start of treatment | EPHESUS27 |
| Gender | 70% male | Fixed | Proportion of patients that are male | EPHESUS27 |
| Baseline event rates in short-term model | ||||
| All-cause mortality | 4.73%, 1.81%, 1.36% in months 1–3, respectively | Weibull (γ = 0.4614, λ = 0.0484) | Monthly probability of all-cause mortality | EPHESUS27 |
| CV hospitalisation | 1.35% per month | Beta | Monthly probability of first CV hospitalisation | EPHESUS27 |
| Baseline event rates in long-term model | ||||
| Time to fatal CV event | Weibull (γ = 0.5194, λ = 0.0076) | Time to a fatal CV event | SMR132 | |
| Time to non-fatal CV event | Weibull (γ = 0.6427, λ = 0.0149) | Time to a non-fatal CV event | ||
| Non-fatal CV event < 1 year | 0.1423 | Exponential | Probability of non-fatal subsequent event within 1 year of first event | |
| Fatal CV event < 1 year | 0.1891 | Exponential | Probability of fatal subsequent event within 1 year of first event | |
| Time to non-fatal CV event > 1 year | Weibull (γ = 1.6183, λ = 0.0000) | Time to non-fatal subsequent event (> 1 year) | ||
| Time to fatal CV event > 1 year | Weibull (γ = 1.6090, λ = 0.0000) | Time to non-fatal subsequent event (> 1 year) | ||
| Relative treatment effect (RR) a | ||||
| All-cause mortality | Relative risk for all-cause mortality | |||
| - Spironolactone | 1.020 (0.575 to 1.652)b | Posterior of synthesis | Spironolactone vs standard care | |
| - Eplerenone | 0.861 (0.767 to 0.964)b | Posterior of synthesis | Eplerenone vs standard care | |
| CV hospitalisation | Relative risk for CV hospitalisation | |||
| - Spironolactone | 1.002 (0.997 to 1.008) | Lognormal | Spironolactone vs standard care | |
| - Eplerenone | 0.910 (0.810 to 1.010) | Lognormal | Eplerenone vs standard care | |
| Proportion experiencing CV events | ||||
| Acute MI | 0.316 | Dirichlet | Proportion of CV events attributed to: AMI | EPHESUS27 |
| Heart failure | 0.539 | Dirichlet | Heart failure | |
| Stroke | 0.070 | Dirichlet | Stroke | |
| Ventricular arrhythmia | 0.075 | Dirichlet | Ventricular arrhythmia | |
| Cost of CV events | ||||
| Acute MI | £1143 | Fixed | Cost of acute MI | NHS Reference costs151 |
| Heart failure | £1538 | Fixed | Cost of heart failure | |
| Stroke | £2081 | Fixed | Cost of stroke | |
| Ventricular arrhythmia | £754 | Fixed | Cost of ventricular arrhythmia | |
| Drug costs | ||||
| Spironolactone | £3.86/month | Fixed | Monthly cost of spironolactone | BNF42 |
| Eplerenone | £46.41/month | Fixed | Monthly cost of eplerenone | BNF42 |
| Utilities | ||||
| Baseline | 0.759 (SE 0.040) | Beta | Average utility at baseline | Gohler et al (2009)153 |
| 0 rehospitalisations | Reference group | – | ||
| 1 rehospitalisation | –0.024 (SE 0.007) | Gamma | Utility decrement for 1 rehospitalisation | |
| 2 rehospitalisations | –0.031 (SE 0.009) | Gamma | Utility decrement for 2 rehospitalisations | |
| 3 rehospitalisations | –0.055 (SE 0.001) | Gamma | Utility decrement for 3 rehospitalisations | |
| Annual discount rate (%) | ||||
| On costs | 3.5% | Fixed | Cost discount rate | NICE128 |
| On QALYs | 3.5% | Fixed | Outcome discount rate | NICE128 |
Appendix 6 Risk prediction equations
Description of all covariates used in the risk prediction equations
| Covariate (short form) | Description |
|
age6570 age7075 age7580 age8085 age8590 age90plus sex year93 year94 year95 year96 year97 year98 year99 year00 year01 year02 priordiab priorcvd prioraf priorhyp priorang priorami newstroke newang newmi |
Age group 65–70 years Age group 70–75 years Age group 75–80 years Age group 80–85 years Age group 85–90 years Age group 90+ years Sex Year of admission/diagnosis 1993 Year of admission/diagnosis 1994 Year of admission/diagnosis 1995 Year of admission/diagnosis 1996 Year of admission/diagnosis 1997 Year of admission/diagnosis 1998 Year of admission/diagnosis 1999 Year of admission/diagnosis 2000 Year of admission/diagnosis 2001 Year of admission/diagnosis 2002 Prior medical history of diabetes Prior medical history of cardiovascular disease Prior medical history of atrial fibrillation Prior medical history of hypertension Prior medical history of angina Prior medical history of myocardial infarction Updated prior medical history of stroke Updated prior medical history of angina Updated prior medical history of myocardial infarction |
Equation 1 – Weibull survival model for risk of fatal primary CV event
| Covariate | Coefficient | Standard error | p-value |
|
age6570 age7075 age7580 age8085 age8590 age90plus sex year93 year94 year95 year96 year97 year98 year99 year00 year01 year02 priordiab priorcvd prioraf priorhyp priorang priorami constant ln(gamma) |
0.1142104 0.3103986 0.498815 0.7343063 1.043028 1.377505 0.0915804 0.2736026 0.2378531 0.2172512 0.2066953 0.1212795 0.1856557 0.1143468 0.0808038 0.099048 0.0160397 0.0488248 0.4771201 0.0012858 –0.170741 –0.0565406 0.2548452 –5.716321 –0.6550324 |
0.0617953 0.0574377 0.055914 0.0554494 0.0563111 0.0596971 0.0242287 0.0632319 0.0635091 0.0641984 0.0650187 0.0658669 0.0660883 0.0675563 0.0686749 0.0700405 0.0726802 0.0362625 0.0339001 0.0274591 0.0353889 0.0378009 0.0315411 0.0827735 0.0096661 |
0.065 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.000 0.001 0.001 0.066 0.005 0.091 0.239 0.157 0.825 0.178 0.000 0.963 0.000 0.135 0.000 0.000 0.000 |
Equation 2 – Weibull survival model for risk of non-fatal primary CV event
| Covariate | Coefficient | Standard error | p-value |
|
age6570 age7075 age7580 age8085 age8590 age90plus sex year93 year94 year95 year96 year97 year98 year99 year00 year01 year02 priordiab priorcvd prioraf priorhyp priorang priorami constant ln(gamma) |
0.0239187 0.1012257 0.1615768 0.2233147 0.2241962 0.1694864 0.0647581 0.0353563 0.0770093 0.0610026 0.0819559 0.0019114 0.0343983 0.0659367 0.0437398 0.0750242 0.0441458 0.2938997 0.2794145 0.0438589 0.0690824 0.3483781 0.2144575 –4.949428 –0.442019 |
0.0276758 0.0261908 0.0259465 0.0265048 0.0288188 0.0357627 0.0136374 0.0354168 0.0351939 0.035684 0.0358969 0.0363661 0.0366146 0.0367755 0.0373659 0.0379737 0.0390768 0.0180644 0.0208758 0.0155184 0.0177999 0.0181344 0.0174307 0.0446597 0.0053258 |
0.387 0.000 0.000 0.000 0.000 0.000 0.000 0.318 0.029 0.087 0.022 0.958 0.347 0.073 0.242 0.048 0.259 0.000 0.000 0.005 0.000 0.000 0.000 0.000 0.000 |
Equation 3 – Logistic regression model for the odds of a non-fatal subsequent CV event within 12 months of first event
| Covariate | Coefficient | Standard error | p-value |
|
age6570 age7075 age7580 age8085 age8590 age90plus sex year93 year94 year95 year96 year97 year98 year99 year00 year01 year02 priordiab newstroke prioraf priorhyp newang newmi constant |
0.1125991 0.0169313 0.0200039 0.0517668 –0.0209989 –0.3127574 –0.0025111 –0.0792919 0.067295 0.0571968 –0.0452664 0.0172286 –0.0903866 –0.041936 –0.0067343 –0.0629235 0.002814 0.2500783 0.4110265 –0.0139245 0.1892357 0.7842072 0.4374084 –2.271575 |
0.0760088 0.0730381 0.0728453 0.0743678 0.0827711 0.1130157 0.0386127 0.1001969 0.097925 0.0992959 0.1006584 0.100902 0.102685 0.1028882 0.103809 0.1062944 0.108462 0.0476905 0.0437008 0.0443889 0.0471495 0.0401597 0.041228 0.1085895 |
0.139 0.817 0.784 0.486 0.800 0.006 0.948 0.429 0.492 0.565 0.653 0.864 0.379 0.684 0.948 0.554 0.979 0.000 0.000 0.754 0.000 0.000 0.000 0.000 |
Equation 4 – Logistic regression model for the odds of a fatal subsequent CV event within 12 months of first event
| Covariate | Coefficient | Standard error | p-value |
|
age6570 age7075 age7580 age8085 age8590 age90plus sex year93 year94 year95 year96 year97 year98 year99 year00 year01 year02 priordiab newstroke prioraf priorhyp newang newmi constant |
0.2900759 0.5852352 0.7031345 0.7722055 1.016184 1.218653 0.0451671 0.5586984 0.4586728 0.4504893 0.4348143 0.4071992 0.3977228 0.2819047 0.2483304 0.2299843 0.214318 0.0490557 0.2661526 –0.0427512 –0.135624 –0.5791964 0.3554937 –2.056677 |
0.0739599 0.0687861 0.0679141 0.0687101 0.072307 0.0842087 0.0312922 0.0866315 0.0865509 0.0876677 0.0882558 0.0893249 0.0901089 0.0912563 0.0928475 0.0946006 0.097208 0.0424563 0.0358641 0.0359094 0.0423044 0.0393138 0.0352831 0.1001297 |
0.000 0.000 0.000 0.000 0.000 0.000 0.149 0.000 0.000 0.000 0.000 0.000 0.000 0.002 0.007 0.015 0.027 0.248 0.000 0.234 0.001 0.000 0.000 0.000 |
Equation 5 – Weibull survival model for risk of non-fatal subsequent CV event (> 12 months)
| Covariate | Coefficient | Standard error | p-value |
|
age6570 age7075 age7580 age8085 age8590 age90plus sex year93 year94 year95 year96 year97 year98 year99 year00 year01 year02 priordiab newstroke prioraf priorhyp newang newmi constant ln(gamma) |
0.104049 0.3243485 0.4655076 0.5094514 0.5153933 0.1912532 0.005879 –0.2432886 –0.1966869 –0.3115933 –0.2727395 –0.2379322 –0.0834489 –0.1272264 –0.0962688 –0.1381184 –0.002711 0.2234116 0.3521316 0.0518128 0.0924553 0.4253138 0.2584302 –13.49448 0.4813593 |
0.0721596 0.0681069 0.0688277 0.0719766 0.085695 0.1333063 0.0394383 0.1415689 0.1405879 0.1427461 0.1425461 0.1440017 0.1422557 0.1438267 0.147216 0.1525536 0.1606974 0.0522731 0.047867 0.0452664 0.0491622 0.04093 0.0439301 0.2091183 0.0136849 |
0.149 0.000 0.000 0.000 0.000 0.151 0.882 0.086 0.162 0.029 0.056 0.098 0.557 0.376 0.513 0.365 0.987 0.000 0.000 0.252 0.06 0.000 0.000 0.000 0.000 |
Equation 6 – Weibull survival model for risk of fatal subsequent CV event (> 12 months)
| Covariate | Coefficient | Standard error | p-value |
|
age6570 age7075 age7580 age8085 age8590 age90plus sex year93 year94 year95 year96 year97 year98 year99 year00 year01 year02 priordiab newstroke prioraf priorhyp newang newmi constant ln(gamma) |
0.2279961 0.5016003 0.7102732 0.8584889 1.066231 1.289183 0.214799 0.3733868 0.3173388 0.2987303 0.2575228 0.3579099 0.3220833 0.127605 0.2806149 0.2614406 0.0238035 0.2965929 0.1302423 0.092758 –0.0811671 –0.3020755 0.1480251 –13.94932 0.475612 |
0.078882 0.0740511 0.073719 0.0754133 0.0826222 0.0997929 0.0387423 0.1694596 0.1693354 0.170376 0.1709435 0.1716071 0.1718932 0.1752378 0.1766743 0.1807961 0.1990109 0.053845 0.0488128 0.0438206 0.0537953 0.0472979 0.0458127 0.2279898 0.0131998 |
0.004 0.000 0.000 0.000 0.000 0.000 0.000 0.028 0.061 0.08 0.132 0.037 0.061 0.467 0.112 0.148 0.905 0.000 0.008 0.034 0.131 0.000 0.001 0.000 0.000 |
Appendix 7 winbugs code
Below is the winbugs code used to synthesise the RCT evidence.
The robustness of the model was assessed by examining the sensitivity of the model results to different prior distributions and initial values, and by examining the convergence diagnostics for evidence of when the simulation appears to have stabilised.
A burn-in period of 100,000 simulations was used. This was followed by a further 100,000 simulations. Convergence of the model was assessed using the sample trace plots and the Gelman–Rubin convergence diagnostic implemented in winbugs (Figure 13 and Figure 14).
FIGURE 13.
Gelman and Rubin diagnostic test for two chains (different initial values) where convergence looked reasonable.
FIGURE 14.
Sample traces of chains where convergence looked reasonable.
The sensitivity of the model results to changing the prior distribution on the treatment effect parameters was assessed by changing from a normal distribution to a uniform distribution over the range from negative to positive infinity (Table 60).
| Relative risk for all-cause mortality in postMI HF (95% CrI) | ||
|---|---|---|
| Spironolactone | Eplerenone | |
| Normal prior | 1.020 (0.575 to 1.652) | 0.861 (0.767 to 0.964) |
| Uniform prior | 1.022 (0.569 to 1.673) | 0.861 (0.767 to 0.963) |
List of abbreviations
- ACC
- American College of Cardiology
- ACE
- angiotensin-converting enzyme
- AHFS
- American Hospital Formulary Service
- AHA
- American Heart Association
- AMI
- acute myocardial infarction
- ARB
- angiotensin receptor blocker
- AREA IN-CHF
- Antiremodeling Effect of Aldosterone receptors blockade with canrenone In mild Chronic Heart Failure
- BNF
- British National Formulary
- BP
- blood pressure
- CAPRICORN
- Carvediliol Post-infaret Survival Control in Left Ventricular Dysfunction
- CC
- complications and/or comorbidities
- CEAC
- cost-effectiveness acceptability curve
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CK-MB
- creatine kinase-MB
- CrI
- credibility intervals
- CV
- cardiovascular
- D
- dominated
- ECG
- electrocardiograph
- ED
- extendedly dominated
- EMEA
- European Medicines Agency
- Ep
- eplerenone
- EPHESUS
- Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study
- EQ-5D
- European Quality of Life-5 Dimensions
- EVPI
- expected value of perfect information
- FCE
- finished consultant episodes
- FDA
- Food and Drug Administration
- HF
- heart failure
- HRG
- Health Resource Group
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICD
- International Classification of Diseases
- ICER
- incremental cost-effectiveness ratio
- ITT
- intention to treat
- LV
- left ventricular
- LBBB
- left bundle branch block
- LVEF
- left ventricular ejection fraction
- LVSD
- left ventricular systolic dysfunction
- LYG
- life-years gained
- MERIT-HF
- Metoprolol CR/XL Randomized Intervention Trial in Heart Failure
- MeSH
- Medical subject headings in the MEDLINE thesaurus
- MI
- myocardial infarction
- N/A
- not applicable
- NA
- not available
- NICE
- National Institute for Health and Clinical Excellence
- NHS EED
- NHS Economic Evaluation Database
- NR
- not reported
- NYHA
- New York Heart Association
- Pl
- placebo
- QALY
- quality-adjusted life-year
- RALES
- Randomised Aldactone Evaluation Study
- RCT
- randomised controlled trial
- RR
- relative risk
- SD
- standard deviation
- SE
- standard error
- SMR
- Scottish Morbidity Record
- Sp
- spironolactone
- SPC
- Summary of Product Characteristics
- TOXLINE
- Toxicology literature online
- VALIANT
- Valsartan in Acute Myocardial Infarction
- VOI
- value of information
- WHO
- World Health Organization
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment reports published to date
-
Home parenteral nutrition: a systematic review.
By Richards DM, Deeks JJ, Sheldon TA, Shaffer JL.
-
Diagnosis, management and screening of early localised prostate cancer.
A review by Selley S, Donovan J, Faulkner A, Coast J, Gillatt D.
-
The diagnosis, management, treatment and costs of prostate cancer in England and Wales.
A review by Chamberlain J, Melia J, Moss S, Brown J.
-
Screening for fragile X syndrome.
A review by Murray J, Cuckle H, Taylor G, Hewison J.
-
A review of near patient testing in primary care.
By Hobbs FDR, Delaney BC, Fitzmaurice DA, Wilson S, Hyde CJ, Thorpe GH, et al.
-
Systematic review of outpatient services for chronic pain control.
By McQuay HJ, Moore RA, Eccleston C, Morley S, de C Williams AC.
-
Neonatal screening for inborn errors of metabolism: cost, yield and outcome.
A review by Pollitt RJ, Green A, McCabe CJ, Booth A, Cooper NJ, Leonard JV, et al.
-
Preschool vision screening.
A review by Snowdon SK, Stewart-Brown SL.
-
Implications of socio-cultural contexts for the ethics of clinical trials.
A review by Ashcroft RE, Chadwick DW, Clark SRL, Edwards RHT, Frith L, Hutton JL.
-
A critical review of the role of neonatal hearing screening in the detection of congenital hearing impairment.
By Davis A, Bamford J, Wilson I, Ramkalawan T, Forshaw M, Wright S.
-
Newborn screening for inborn errors of metabolism: a systematic review.
By Seymour CA, Thomason MJ, Chalmers RA, Addison GM, Bain MD, Cockburn F, et al.
-
Routine preoperative testing: a systematic review of the evidence.
By Munro J, Booth A, Nicholl J.
-
Systematic review of the effectiveness of laxatives in the elderly.
By Petticrew M, Watt I, Sheldon T.
-
When and how to assess fast-changing technologies: a comparative study of medical applications of four generic technologies.
A review by Mowatt G, Bower DJ, Brebner JA, Cairns JA, Grant AM, McKee L.
-
Antenatal screening for Down’s syndrome.
A review by Wald NJ, Kennard A, Hackshaw A, McGuire A.
-
Screening for ovarian cancer: a systematic review.
By Bell R, Petticrew M, Luengo S, Sheldon TA.
-
Consensus development methods, and their use in clinical guideline development.
A review by Murphy MK, Black NA, Lamping DL, McKee CM, Sanderson CFB, Askham J, et al.
-
A cost–utility analysis of interferon beta for multiple sclerosis.
By Parkin D, McNamee P, Jacoby A, Miller P, Thomas S, Bates D.
-
Effectiveness and efficiency of methods of dialysis therapy for end-stage renal disease: systematic reviews.
By MacLeod A, Grant A, Donaldson C, Khan I, Campbell M, Daly C, et al.
-
Effectiveness of hip prostheses in primary total hip replacement: a critical review of evidence and an economic model.
By Faulkner A, Kennedy LG, Baxter K, Donovan J, Wilkinson M, Bevan G.
-
Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomised controlled trials.
By Song F, Glenny AM.
-
Bone marrow and peripheral blood stem cell transplantation for malignancy.
A review by Johnson PWM, Simnett SJ, Sweetenham JW, Morgan GJ, Stewart LA.
-
Screening for speech and language delay: a systematic review of the literature.
By Law J, Boyle J, Harris F, Harkness A, Nye C.
-
Resource allocation for chronic stable angina: a systematic review of effectiveness, costs and cost-effectiveness of alternative interventions.
By Sculpher MJ, Petticrew M, Kelland JL, Elliott RA, Holdright DR, Buxton MJ.
-
Detection, adherence and control of hypertension for the prevention of stroke: a systematic review.
By Ebrahim S.
-
Postoperative analgesia and vomiting, with special reference to day-case surgery: a systematic review.
By McQuay HJ, Moore RA.
-
Choosing between randomised and nonrandomised studies: a systematic review.
By Britton A, McKee M, Black N, McPherson K, Sanderson C, Bain C.
-
Evaluating patient-based outcome measures for use in clinical trials.
A review by Fitzpatrick R, Davey C, Buxton MJ, Jones DR.
-
Ethical issues in the design and conduct of randomised controlled trials.
A review by Edwards SJL, Lilford RJ, Braunholtz DA, Jackson JC, Hewison J, Thornton J.
-
Qualitative research methods in health technology assessment: a review of the literature.
By Murphy E, Dingwall R, Greatbatch D, Parker S, Watson P.
-
The costs and benefits of paramedic skills in pre-hospital trauma care.
By Nicholl J, Hughes S, Dixon S, Turner J, Yates D.
-
Systematic review of endoscopic ultrasound in gastro-oesophageal cancer.
By Harris KM, Kelly S, Berry E, Hutton J, Roderick P, Cullingworth J, et al.
-
Systematic reviews of trials and other studies.
By Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F.
-
Primary total hip replacement surgery: a systematic review of outcomes and modelling of cost-effectiveness associated with different prostheses.
A review by Fitzpatrick R, Shortall E, Sculpher M, Murray D, Morris R, Lodge M, et al.
-
Informed decision making: an annotated bibliography and systematic review.
By Bekker H, Thornton JG, Airey CM, Connelly JB, Hewison J, Robinson MB, et al.
-
Handling uncertainty when performing economic evaluation of healthcare interventions.
A review by Briggs AH, Gray AM.
-
The role of expectancies in the placebo effect and their use in the delivery of health care: a systematic review.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Thomas H.
-
A randomised controlled trial of different approaches to universal antenatal HIV testing: uptake and acceptability. Annex: Antenatal HIV testing – assessment of a routine voluntary approach.
By Simpson WM, Johnstone FD, Boyd FM, Goldberg DJ, Hart GJ, Gormley SM, et al.
-
Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review.
By Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ.
-
Assessing the costs of healthcare technologies in clinical trials.
A review by Johnston K, Buxton MJ, Jones DR, Fitzpatrick R.
-
Cooperatives and their primary care emergency centres: organisation and impact.
By Hallam L, Henthorne K.
-
Screening for cystic fibrosis.
A review by Murray J, Cuckle H, Taylor G, Littlewood J, Hewison J.
-
A review of the use of health status measures in economic evaluation.
By Brazier J, Deverill M, Green C, Harper R, Booth A.
-
Methods for the analysis of quality-of-life and survival data in health technology assessment.
A review by Billingham LJ, Abrams KR, Jones DR.
-
Antenatal and neonatal haemoglobinopathy screening in the UK: review and economic analysis.
By Zeuner D, Ades AE, Karnon J, Brown J, Dezateux C, Anionwu EN.
-
Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses.
A review by Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al.
-
‘Early warning systems’ for identifying new healthcare technologies.
By Robert G, Stevens A, Gabbay J.
-
A systematic review of the role of human papillomavirus testing within a cervical screening programme.
By Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al.
-
Near patient testing in diabetes clinics: appraising the costs and outcomes.
By Grieve R, Beech R, Vincent J, Mazurkiewicz J.
-
Positron emission tomography: establishing priorities for health technology assessment.
A review by Robert G, Milne R.
-
The debridement of chronic wounds: a systematic review.
By Bradley M, Cullum N, Sheldon T.
-
Systematic reviews of wound care management: (2) Dressings and topical agents used in the healing of chronic wounds.
By Bradley M, Cullum N, Nelson EA, Petticrew M, Sheldon T, Torgerson D.
-
A systematic literature review of spiral and electron beam computed tomography: with particular reference to clinical applications in hepatic lesions, pulmonary embolus and coronary artery disease.
By Berry E, Kelly S, Hutton J, Harris KM, Roderick P, Boyce JC, et al.
-
What role for statins? A review and economic model.
By Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al.
-
Factors that limit the quality, number and progress of randomised controlled trials.
A review by Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al.
-
Antimicrobial prophylaxis in total hip replacement: a systematic review.
By Glenny AM, Song F.
-
Health promoting schools and health promotion in schools: two systematic reviews.
By Lister-Sharp D, Chapman S, Stewart-Brown S, Sowden A.
-
Economic evaluation of a primary care-based education programme for patients with osteoarthritis of the knee.
A review by Lord J, Victor C, Littlejohns P, Ross FM, Axford JS.
-
The estimation of marginal time preference in a UK-wide sample (TEMPUS) project.
A review by Cairns JA, van der Pol MM.
-
Geriatric rehabilitation following fractures in older people: a systematic review.
By Cameron I, Crotty M, Currie C, Finnegan T, Gillespie L, Gillespie W, et al.
-
Screening for sickle cell disease and thalassaemia: a systematic review with supplementary research.
By Davies SC, Cronin E, Gill M, Greengross P, Hickman M, Normand C.
-
Community provision of hearing aids and related audiology services.
A review by Reeves DJ, Alborz A, Hickson FS, Bamford JM.
-
False-negative results in screening programmes: systematic review of impact and implications.
By Petticrew MP, Sowden AJ, Lister-Sharp D, Wright K.
-
Costs and benefits of community postnatal support workers: a randomised controlled trial.
By Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A.
-
Implantable contraceptives (subdermal implants and hormonally impregnated intrauterine systems) versus other forms of reversible contraceptives: two systematic reviews to assess relative effectiveness, acceptability, tolerability and cost-effectiveness.
By French RS, Cowan FM, Mansour DJA, Morris S, Procter T, Hughes D, et al.
-
An introduction to statistical methods for health technology assessment.
A review by White SJ, Ashby D, Brown PJ.
-
Disease-modifying drugs for multiple sclerosis: a rapid and systematic review.
By Clegg A, Bryant J, Milne R.
-
Publication and related biases.
A review by Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ.
-
Cost and outcome implications of the organisation of vascular services.
By Michaels J, Brazier J, Palfreyman S, Shackley P, Slack R.
-
Monitoring blood glucose control in diabetes mellitus: a systematic review.
By Coster S, Gulliford MC, Seed PT, Powrie JK, Swaminathan R.
-
The effectiveness of domiciliary health visiting: a systematic review of international studies and a selective review of the British literature.
By Elkan R, Kendrick D, Hewitt M, Robinson JJA, Tolley K, Blair M, et al.
-
The determinants of screening uptake and interventions for increasing uptake: a systematic review.
By Jepson R, Clegg A, Forbes C, Lewis R, Sowden A, Kleijnen J.
-
The effectiveness and cost-effectiveness of prophylactic removal of wisdom teeth.
A rapid review by Song F, O’Meara S, Wilson P, Golder S, Kleijnen J.
-
Ultrasound screening in pregnancy: a systematic review of the clinical effectiveness, cost-effectiveness and women’s views.
By Bricker L, Garcia J, Henderson J, Mugford M, Neilson J, Roberts T, et al.
-
A rapid and systematic review of the effectiveness and cost-effectiveness of the taxanes used in the treatment of advanced breast and ovarian cancer.
By Lister-Sharp D, McDonagh MS, Khan KS, Kleijnen J.
-
Liquid-based cytology in cervical screening: a rapid and systematic review.
By Payne N, Chilcott J, McGoogan E.
-
Randomised controlled trial of non-directive counselling, cognitive–behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care.
By King M, Sibbald B, Ward E, Bower P, Lloyd M, Gabbay M, et al.
-
Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?
By Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J.
-
Systematic reviews of wound care management: (3) antimicrobial agents for chronic wounds; (4) diabetic foot ulceration.
By O’Meara S, Cullum N, Majid M, Sheldon T.
-
Using routine data to complement and enhance the results of randomised controlled trials.
By Lewsey JD, Leyland AH, Murray GD, Boddy FA.
-
Coronary artery stents in the treatment of ischaemic heart disease: a rapid and systematic review.
By Meads C, Cummins C, Jolly K, Stevens A, Burls A, Hyde C.
-
Outcome measures for adult critical care: a systematic review.
By Hayes JA, Black NA, Jenkinson C, Young JD, Rowan KM, Daly K, et al.
-
A systematic review to evaluate the effectiveness of interventions to promote the initiation of breastfeeding.
By Fairbank L, O’Meara S, Renfrew MJ, Woolridge M, Sowden AJ, Lister-Sharp D.
-
Implantable cardioverter defibrillators: arrhythmias. A rapid and systematic review.
By Parkes J, Bryant J, Milne R.
-
Treatments for fatigue in multiple sclerosis: a rapid and systematic review.
By Brañas P, Jordan R, Fry-Smith A, Burls A, Hyde C.
-
Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial.
By Baxter-Jones ADG, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al.
-
Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis.
By Marks D, Wonderling D, Thorogood M, Lambert H, Humphries SE, Neil HAW.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists in the medical management of unstable angina.
By McDonagh MS, Bachmann LM, Golder S, Kleijnen J, ter Riet G.
-
A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma.
By Turner J, Nicholl J, Webber L, Cox H, Dixon S, Yates D.
-
Intrathecal pumps for giving opioids in chronic pain: a systematic review.
By Williams JE, Louw G, Towlerton G.
-
Combination therapy (interferon alfa and ribavirin) in the treatment of chronic hepatitis C: a rapid and systematic review.
By Shepherd J, Waugh N, Hewitson P.
-
A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies.
By MacLehose RR, Reeves BC, Harvey IM, Sheldon TA, Russell IT, Black AMS.
-
Intravascular ultrasound-guided interventions in coronary artery disease: a systematic literature review, with decision-analytic modelling, of outcomes and cost-effectiveness.
By Berry E, Kelly S, Hutton J, Lindsay HSJ, Blaxill JM, Evans JA, et al.
-
A randomised controlled trial to evaluate the effectiveness and cost-effectiveness of counselling patients with chronic depression.
By Simpson S, Corney R, Fitzgerald P, Beecham J.
-
Systematic review of treatments for atopic eczema.
By Hoare C, Li Wan Po A, Williams H.
-
Bayesian methods in health technology assessment: a review.
By Spiegelhalter DJ, Myles JP, Jones DR, Abrams KR.
-
The management of dyspepsia: a systematic review.
By Delaney B, Moayyedi P, Deeks J, Innes M, Soo S, Barton P, et al.
-
A systematic review of treatments for severe psoriasis.
By Griffiths CEM, Clark CM, Chalmers RJG, Li Wan Po A, Williams HC.
-
Clinical and cost-effectiveness of donepezil, rivastigmine and galantamine for Alzheimer’s disease: a rapid and systematic review.
By Clegg A, Bryant J, Nicholson T, McIntyre L, De Broe S, Gerard K, et al.
-
The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review.
By Stewart A, Sandercock J, Bryan S, Hyde C, Barton PM, Fry-Smith A, et al.
-
Equity and the economic evaluation of healthcare.
By Sassi F, Archard L, Le Grand J.
-
Quality-of-life measures in chronic diseases of childhood.
By Eiser C, Morse R.
-
Eliciting public preferences for healthcare: a systematic review of techniques.
By Ryan M, Scott DA, Reeves C, Bate A, van Teijlingen ER, Russell EM, et al.
-
General health status measures for people with cognitive impairment: learning disability and acquired brain injury.
By Riemsma RP, Forbes CA, Glanville JM, Eastwood AJ, Kleijnen J.
-
An assessment of screening strategies for fragile X syndrome in the UK.
By Pembrey ME, Barnicoat AJ, Carmichael B, Bobrow M, Turner G.
-
Issues in methodological research: perspectives from researchers and commissioners.
By Lilford RJ, Richardson A, Stevens A, Fitzpatrick R, Edwards S, Rock F, et al.
-
Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy.
By Cullum N, Nelson EA, Flemming K, Sheldon T.
-
Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: a systematic review.
By Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al.
-
Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review.
By Jobanputra P, Parry D, Fry-Smith A, Burls A.
-
Statistical assessment of the learning curves of health technologies.
By Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT.
-
The effectiveness and cost-effectiveness of temozolomide for the treatment of recurrent malignant glioma: a rapid and systematic review.
By Dinnes J, Cave C, Huang S, Major K, Milne R.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of debriding agents in treating surgical wounds healing by secondary intention.
By Lewis R, Whiting P, ter Riet G, O’Meara S, Glanville J.
-
Home treatment for mental health problems: a systematic review.
By Burns T, Knapp M, Catty J, Healey A, Henderson J, Watt H, et al.
-
How to develop cost-conscious guidelines.
By Eccles M, Mason J.
-
The role of specialist nurses in multiple sclerosis: a rapid and systematic review.
By De Broe S, Christopher F, Waugh N.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of orlistat in the management of obesity.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The clinical effectiveness and cost-effectiveness of pioglitazone for type 2 diabetes mellitus: a rapid and systematic review.
By Chilcott J, Wight J, Lloyd Jones M, Tappenden P.
-
Extended scope of nursing practice: a multicentre randomised controlled trial of appropriately trained nurses and preregistration house officers in preoperative assessment in elective general surgery.
By Kinley H, Czoski-Murray C, George S, McCabe C, Primrose J, Reilly C, et al.
-
Systematic reviews of the effectiveness of day care for people with severe mental disorders: (1) Acute day hospital versus admission; (2) Vocational rehabilitation; (3) Day hospital versus outpatient care.
By Marshall M, Crowther R, Almaraz- Serrano A, Creed F, Sledge W, Kluiter H, et al.
-
The measurement and monitoring of surgical adverse events.
By Bruce J, Russell EM, Mollison J, Krukowski ZH.
-
Action research: a systematic review and guidance for assessment.
By Waterman H, Tillen D, Dickson R, de Koning K.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer.
By Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, et al.
-
A rapid and systematic review of the evidence for the clinical effectiveness and cost-effectiveness of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer.
By Lloyd Jones M, Hummel S, Bansback N, Orr B, Seymour M.
-
Comparison of the effectiveness of inhaler devices in asthma and chronic obstructive airways disease: a systematic review of the literature.
By Brocklebank D, Ram F, Wright J, Barry P, Cates C, Davies L, et al.
-
The cost-effectiveness of magnetic resonance imaging for investigation of the knee joint.
By Bryan S, Weatherburn G, Bungay H, Hatrick C, Salas C, Parry D, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.
By Forbes C, Shirran L, Bagnall A-M, Duffy S, ter Riet G.
-
Superseded by a report published in a later volume.
-
The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial.
By Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M.
-
Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients.
By McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.
By Clegg A, Scott DA, Sidhu M, Hewitson P, Waugh N.
-
Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives.
By Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G.
-
Depot antipsychotic medication in the treatment of patients with schizophrenia: (1) Meta-review; (2) Patient and nurse attitudes.
By David AS, Adams C.
-
A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression.
By Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al.
-
Cost analysis of child health surveillance.
By Sanderson D, Wright D, Acton C, Duree D.
-
A study of the methods used to select review criteria for clinical audit.
By Hearnshaw H, Harker R, Cheater F, Baker R, Grimshaw G.
-
Fludarabine as second-line therapy for B cell chronic lymphocytic leukaemia: a technology assessment.
By Hyde C, Wake B, Bryan S, Barton P, Fry-Smith A, Davenport C, et al.
-
Rituximab as third-line treatment for refractory or recurrent Stage III or IV follicular non-Hodgkin’s lymphoma: a systematic review and economic evaluation.
By Wake B, Hyde C, Bryan S, Barton P, Song F, Fry-Smith A, et al.
-
A systematic review of discharge arrangements for older people.
By Parker SG, Peet SM, McPherson A, Cannaby AM, Baker R, Wilson A, et al.
-
The clinical effectiveness and cost-effectiveness of inhaler devices used in the routine management of chronic asthma in older children: a systematic review and economic evaluation.
By Peters J, Stevenson M, Beverley C, Lim J, Smith S.
-
The clinical effectiveness and cost-effectiveness of sibutramine in the management of obesity: a technology assessment.
By O’Meara S, Riemsma R, Shirran L, Mather L, ter Riet G.
-
The cost-effectiveness of magnetic resonance angiography for carotid artery stenosis and peripheral vascular disease: a systematic review.
By Berry E, Kelly S, Westwood ME, Davies LM, Gough MJ, Bamford JM, et al.
-
Promoting physical activity in South Asian Muslim women through ‘exercise on prescription’.
By Carroll B, Ali N, Azam N.
-
Zanamivir for the treatment of influenza in adults: a systematic review and economic evaluation.
By Burls A, Clark W, Stewart T, Preston C, Bryan S, Jefferson T, et al.
-
A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models.
By Richards RG, Sampson FC, Beard SM, Tappenden P.
-
Screening for gestational diabetes: a systematic review and economic evaluation.
By Scott DA, Loveman E, McIntyre L, Waugh N.
-
The clinical effectiveness and cost-effectiveness of surgery for people with morbid obesity: a systematic review and economic evaluation.
By Clegg AJ, Colquitt J, Sidhu MK, Royle P, Loveman E, Walker A.
-
The clinical effectiveness of trastuzumab for breast cancer: a systematic review.
By Lewis R, Bagnall A-M, Forbes C, Shirran E, Duffy S, Kleijnen J, et al.
-
The clinical effectiveness and cost-effectiveness of vinorelbine for breast cancer: a systematic review and economic evaluation.
By Lewis R, Bagnall A-M, King S, Woolacott N, Forbes C, Shirran L, et al.
-
A systematic review of the effectiveness and cost-effectiveness of metal-on-metal hip resurfacing arthroplasty for treatment of hip disease.
By Vale L, Wyness L, McCormack K, McKenzie L, Brazzelli M, Stearns SC.
-
The clinical effectiveness and cost-effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation.
By Woolacott NF, Jones L, Forbes CA, Mather LC, Sowden AJ, Song FJ, et al.
-
A systematic review of effectiveness and economic evaluation of new drug treatments for juvenile idiopathic arthritis: etanercept.
By Cummins C, Connock M, Fry-Smith A, Burls A.
-
Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation.
By Bryant J, Cave C, Mihaylova B, Chase D, McIntyre L, Gerard K, et al.
-
Clinical effectiveness and cost-effectiveness of growth hormone in adults in relation to impact on quality of life: a systematic review and economic evaluation.
By Bryant J, Loveman E, Chase D, Mihaylova B, Cave C, Gerard K, et al.
-
Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial.
By Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freementle N, Vail A.
-
The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation.
By Jobanputra P, Barton P, Bryan S, Burls A.
-
A systematic review and economic evaluation of computerised cognitive behaviour therapy for depression and anxiety.
By Kaltenthaler E, Shackley P, Stevens K, Beverley C, Parry G, Chilcott J.
-
A systematic review and economic evaluation of pegylated liposomal doxorubicin hydrochloride for ovarian cancer.
By Forbes C, Wilby J, Richardson G, Sculpher M, Mather L, Riemsma R.
-
A systematic review of the effectiveness of interventions based on a stages-of-change approach to promote individual behaviour change.
By Riemsma RP, Pattenden J, Bridle C, Sowden AJ, Mather L, Watt IS, et al.
-
A systematic review update of the clinical effectiveness and cost-effectiveness of glycoprotein IIb/IIIa antagonists.
By Robinson M, Ginnelly L, Sculpher M, Jones L, Riemsma R, Palmer S, et al.
-
A systematic review of the effectiveness, cost-effectiveness and barriers to implementation of thrombolytic and neuroprotective therapy for acute ischaemic stroke in the NHS.
By Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, et al.
-
A randomised controlled crossover trial of nurse practitioner versus doctor-led outpatient care in a bronchiectasis clinic.
By Caine N, Sharples LD, Hollingworth W, French J, Keogan M, Exley A, et al.
-
Clinical effectiveness and cost – consequences of selective serotonin reuptake inhibitors in the treatment of sex offenders.
By Adi Y, Ashcroft D, Browne K, Beech A, Fry-Smith A, Hyde C.
-
Treatment of established osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Brazier JE, Stevenson M, Calvert NW, Lloyd Jones M.
-
Which anaesthetic agents are cost-effective in day surgery? Literature review, national survey of practice and randomised controlled trial.
By Elliott RA Payne K, Moore JK, Davies LM, Harper NJN, St Leger AS, et al.
-
Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice.
By Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al.
-
The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature.
By Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al.
-
The effectiveness and cost-effectiveness of imatinib in chronic myeloid leukaemia: a systematic review.
By Garside R, Round A, Dalziel K, Stein K, Royle R.
-
A comparative study of hypertonic saline, daily and alternate-day rhDNase in children with cystic fibrosis.
By Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al.
-
A systematic review of the costs and effectiveness of different models of paediatric home care.
By Parker G, Bhakta P, Lovett CA, Paisley S, Olsen R, Turner D, et al.
-
How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study.
By Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure.
By Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al.
-
Systematic review and economic evaluation of the effectiveness of infliximab for the treatment of Crohn’s disease.
By Clark W, Raftery J, Barton P, Song F, Fry-Smith A, Burls A.
-
A review of the clinical effectiveness and cost-effectiveness of routine anti-D prophylaxis for pregnant women who are rhesus negative.
By Chilcott J, Lloyd Jones M, Wight J, Forman K, Wray J, Beverley C, et al.
-
Systematic review and evaluation of the use of tumour markers in paediatric oncology: Ewing’s sarcoma and neuroblastoma.
By Riley RD, Burchill SA, Abrams KR, Heney D, Lambert PC, Jones DR, et al.
-
The cost-effectiveness of screening for Helicobacter pylori to reduce mortality and morbidity from gastric cancer and peptic ulcer disease: a discrete-event simulation model.
By Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Bhandari P, et al.
-
The clinical effectiveness and cost-effectiveness of routine dental checks: a systematic review and economic evaluation.
By Davenport C, Elley K, Salas C, Taylor-Weetman CL, Fry-Smith A, Bryan S, et al.
-
A multicentre randomised controlled trial assessing the costs and benefits of using structured information and analysis of women’s preferences in the management of menorrhagia.
By Kennedy ADM, Sculpher MJ, Coulter A, Dwyer N, Rees M, Horsley S, et al.
-
Clinical effectiveness and cost–utility of photodynamic therapy for wet age-related macular degeneration: a systematic review and economic evaluation.
By Meads C, Salas C, Roberts T, Moore D, Fry-Smith A, Hyde C.
-
Evaluation of molecular tests for prenatal diagnosis of chromosome abnormalities.
By Grimshaw GM, Szczepura A, Hultén M, MacDonald F, Nevin NC, Sutton F, et al.
-
First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS).
By Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM.
-
The effectiveness and cost-effectiveness of ultrasound locating devices for central venous access: a systematic review and economic evaluation.
By Calvert N, Hind D, McWilliams RG, Thomas SM, Beverley C, Davidson A.
-
A systematic review of atypical antipsychotics in schizophrenia.
By Bagnall A-M, Jones L, Lewis R, Ginnelly L, Glanville J, Torgerson D, et al.
-
Prostate Testing for Cancer and Treatment (ProtecT) feasibility study.
By Donovan J, Hamdy F, Neal D, Peters T, Oliver S, Brindle L, et al.
-
Early thrombolysis for the treatment of acute myocardial infarction: a systematic review and economic evaluation.
By Boland A, Dundar Y, Bagust A, Haycox A, Hill R, Mujica Mota R, et al.
-
Screening for fragile X syndrome: a literature review and modelling.
By Song FJ, Barton P, Sleightholme V, Yao GL, Fry-Smith A.
-
Systematic review of endoscopic sinus surgery for nasal polyps.
By Dalziel K, Stein K, Round A, Garside R, Royle P.
-
Towards efficient guidelines: how to monitor guideline use in primary care.
By Hutchinson A, McIntosh A, Cox S, Gilbert C.
-
Effectiveness and cost-effectiveness of acute hospital-based spinal cord injuries services: systematic review.
By Bagnall A-M, Jones L, Richardson G, Duffy S, Riemsma R.
-
Prioritisation of health technology assessment. The PATHS model: methods and case studies.
By Townsend J, Buxton M, Harper G.
-
Systematic review of the clinical effectiveness and cost-effectiveness of tension-free vaginal tape for treatment of urinary stress incontinence.
By Cody J, Wyness L, Wallace S, Glazener C, Kilonzo M, Stearns S, et al.
-
The clinical and cost-effectiveness of patient education models for diabetes: a systematic review and economic evaluation.
By Loveman E, Cave C, Green C, Royle P, Dunn N, Waugh N.
-
The role of modelling in prioritising and planning clinical trials.
By Chilcott J, Brennan A, Booth A, Karnon J, Tappenden P.
-
Cost–benefit evaluation of routine influenza immunisation in people 65–74 years of age.
By Allsup S, Gosney M, Haycox A, Regan M.
-
The clinical and cost-effectiveness of pulsatile machine perfusion versus cold storage of kidneys for transplantation retrieved from heart-beating and non-heart-beating donors.
By Wight J, Chilcott J, Holmes M, Brewer N.
-
Can randomised trials rely on existing electronic data? A feasibility study to explore the value of routine data in health technology assessment.
By Williams JG, Cheung WY, Cohen DR, Hutchings HA, Longo MF, Russell IT.
-
Evaluating non-randomised intervention studies.
By Deeks JJ, Dinnes J, D’Amico R, Sowden AJ, Sakarovitch C, Song F, et al.
-
A randomised controlled trial to assess the impact of a package comprising a patient-orientated, evidence-based self- help guidebook and patient-centred consultations on disease management and satisfaction in inflammatory bowel disease.
By Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al.
-
The effectiveness of diagnostic tests for the assessment of shoulder pain due to soft tissue disorders: a systematic review.
By Dinnes J, Loveman E, McIntyre L, Waugh N.
-
The value of digital imaging in diabetic retinopathy.
By Sharp PF, Olson J, Strachan F, Hipwell J, Ludbrook A, O’Donnell M, et al.
-
Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy.
By Law M, Wald N, Morris J.
-
Clinical and cost-effectiveness of capecitabine and tegafur with uracil for the treatment of metastatic colorectal cancer: systematic review and economic evaluation.
By Ward S, Kaltenthaler E, Cowan J, Brewer N.
-
Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review.
By Hummel S, Paisley S, Morgan A, Currie E, Brewer N.
-
Literature searching for clinical and cost-effectiveness studies used in health technology assessment reports carried out for the National Institute for Clinical Excellence appraisal system.
By Royle P, Waugh N.
-
Systematic review and economic decision modelling for the prevention and treatment of influenza A and B.
By Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K.
-
A randomised controlled trial to evaluate the clinical and cost-effectiveness of Hickman line insertions in adult cancer patients by nurses.
By Boland A, Haycox A, Bagust A, Fitzsimmons L.
-
Redesigning postnatal care: a randomised controlled trial of protocol-based midwifery-led care focused on individual women’s physical and psychological health needs.
By MacArthur C, Winter HR, Bick DE, Lilford RJ, Lancashire RJ, Knowles H, et al.
-
Estimating implied rates of discount in healthcare decision-making.
By West RR, McNabb R, Thompson AGH, Sheldon TA, Grimley Evans J.
-
Systematic review of isolation policies in the hospital management of methicillin-resistant Staphylococcus aureus: a review of the literature with epidemiological and economic modelling.
By Cooper BS, Stone SP, Kibbler CC, Cookson BD, Roberts JA, Medley GF, et al.
-
Treatments for spasticity and pain in multiple sclerosis: a systematic review.
By Beard S, Hunn A, Wight J.
-
The inclusion of reports of randomised trials published in languages other than English in systematic reviews.
By Moher D, Pham B, Lawson ML, Klassen TP.
-
The impact of screening on future health-promoting behaviours and health beliefs: a systematic review.
By Bankhead CR, Brett J, Bukach C, Webster P, Stewart-Brown S, Munafo M, et al.
-
What is the best imaging strategy for acute stroke?
By Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PAG, Dennis MS, et al.
-
Systematic review and modelling of the investigation of acute and chronic chest pain presenting in primary care.
By Mant J, McManus RJ, Oakes RAL, Delaney BC, Barton PM, Deeks JJ, et al.
-
The effectiveness and cost-effectiveness of microwave and thermal balloon endometrial ablation for heavy menstrual bleeding: a systematic review and economic modelling.
By Garside R, Stein K, Wyatt K, Round A, Price A.
-
A systematic review of the role of bisphosphonates in metastatic disease.
By Ross JR, Saunders Y, Edmonds PM, Patel S, Wonderling D, Normand C, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda®) for locally advanced and/or metastatic breast cancer.
By Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R.
-
Effectiveness and efficiency of guideline dissemination and implementation strategies.
By Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al.
-
Clinical effectiveness and costs of the Sugarbaker procedure for the treatment of pseudomyxoma peritonei.
By Bryant J, Clegg AJ, Sidhu MK, Brodin H, Royle P, Davidson P.
-
Psychological treatment for insomnia in the regulation of long-term hypnotic drug use.
By Morgan K, Dixon S, Mathers N, Thompson J, Tomeny M.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: development of a patient-based measure of outcome.
By Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ.
-
A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography.
By Kaltenthaler E, Bravo Vergel Y, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al.
-
The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis.
By Barton P, Jobanputra P, Wilson J, Bryan S, Burls A.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.
By Pandor A, Eastham J, Beverley C, Chilcott J, Paisley S.
-
Clinical effectiveness and cost-effectiveness of pioglitazone and rosiglitazone in the treatment of type 2 diabetes: a systematic review and economic evaluation.
By Czoski-Murray C, Warren E, Chilcott J, Beverley C, Psyllaki MA, Cowan J.
-
Routine examination of the newborn: the EMREN study. Evaluation of an extension of the midwife role including a randomised controlled trial of appropriately trained midwives and paediatric senior house officers.
By Townsend J, Wolke D, Hayes J, Davé S, Rogers C, Bloomfield L, et al.
-
Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach.
By Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al.
-
A multi-centre randomised controlled trial of minimally invasive direct coronary bypass grafting versus percutaneous transluminal coronary angioplasty with stenting for proximal stenosis of the left anterior descending coronary artery.
By Reeves BC, Angelini GD, Bryan AJ, Taylor FC, Cripps T, Spyt TJ, et al.
-
Does early magnetic resonance imaging influence management or improve outcome in patients referred to secondary care with low back pain? A pragmatic randomised controlled trial.
By Gilbert FJ, Grant AM, Gillan MGC, Vale L, Scott NW, Campbell MK, et al.
-
The clinical and cost-effectiveness of anakinra for the treatment of rheumatoid arthritis in adults: a systematic review and economic analysis.
By Clark W, Jobanputra P, Barton P, Burls A.
-
A rapid and systematic review and economic evaluation of the clinical and cost-effectiveness of newer drugs for treatment of mania associated with bipolar affective disorder.
By Bridle C, Palmer S, Bagnall A-M, Darba J, Duffy S, Sculpher M, et al.
-
Liquid-based cytology in cervical screening: an updated rapid and systematic review and economic analysis.
By Karnon J, Peters J, Platt J, Chilcott J, McGoogan E, Brewer N.
-
Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement.
By Avenell A, Broom J, Brown TJ, Poobalan A, Aucott L, Stearns SC, et al.
-
Autoantibody testing in children with newly diagnosed type 1 diabetes mellitus.
By Dretzke J, Cummins C, Sandercock J, Fry-Smith A, Barrett T, Burls A.
-
Clinical effectiveness and cost-effectiveness of prehospital intravenous fluids in trauma patients.
By Dretzke J, Sandercock J, Bayliss S, Burls A.
-
Newer hypnotic drugs for the short-term management of insomnia: a systematic review and economic evaluation.
By Dündar Y, Boland A, Strobl J, Dodd S, Haycox A, Bagust A, et al.
-
Development and validation of methods for assessing the quality of diagnostic accuracy studies.
By Whiting P, Rutjes AWS, Dinnes J, Reitsma JB, Bossuyt PMM, Kleijnen J.
-
EVALUATE hysterectomy trial: a multicentre randomised trial comparing abdominal, vaginal and laparoscopic methods of hysterectomy.
By Garry R, Fountain J, Brown J, Manca A, Mason S, Sculpher M, et al.
-
Methods for expected value of information analysis in complex health economic models: developments on the health economics of interferon-β and glatiramer acetate for multiple sclerosis.
By Tappenden P, Chilcott JB, Eggington S, Oakley J, McCabe C.
-
Effectiveness and cost-effectiveness of imatinib for first-line treatment of chronic myeloid leukaemia in chronic phase: a systematic review and economic analysis.
By Dalziel K, Round A, Stein K, Garside R, Price A.
-
VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers.
By Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, on behalf of the VenUS Team.
-
Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of myocardial perfusion scintigraphy for the diagnosis and management of angina and myocardial infarction.
By Mowatt G, Vale L, Brazzelli M, Hernandez R, Murray A, Scott N, et al.
-
A pilot study on the use of decision theory and value of information analysis as part of the NHS Health Technology Assessment programme.
By Claxton K, Ginnelly L, Sculpher M, Philips Z, Palmer S.
-
The Social Support and Family Health Study: a randomised controlled trial and economic evaluation of two alternative forms of postnatal support for mothers living in disadvantaged inner-city areas.
By Wiggins M, Oakley A, Roberts I, Turner H, Rajan L, Austerberry H, et al.
-
Psychosocial aspects of genetic screening of pregnant women and newborns: a systematic review.
By Green JM, Hewison J, Bekker HL, Bryant, Cuckle HS.
-
Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status.
By Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B.
-
Coronary artery stents: a rapid systematic review and economic evaluation.
By Hill R, Bagust A, Bakhai A, Dickson R, Dündar Y, Haycox A, et al.
-
Review of guidelines for good practice in decision-analytic modelling in health technology assessment.
By Philips Z, Ginnelly L, Sculpher M, Claxton K, Golder S, Riemsma R, et al.
-
Rituximab (MabThera®) for aggressive non-Hodgkin’s lymphoma: systematic review and economic evaluation.
By Knight C, Hind D, Brewer N, Abbott V.
-
Clinical effectiveness and cost-effectiveness of clopidogrel and modified-release dipyridamole in the secondary prevention of occlusive vascular events: a systematic review and economic evaluation.
By Jones L, Griffin S, Palmer S, Main C, Orton V, Sculpher M, et al.
-
Pegylated interferon α-2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J.
-
Clopidogrel used in combination with aspirin compared with aspirin alone in the treatment of non-ST-segment- elevation acute coronary syndromes: a systematic review and economic evaluation.
By Main C, Palmer S, Griffin S, Jones L, Orton V, Sculpher M, et al.
-
Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under-represented groups.
By Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al.
-
Involving South Asian patients in clinical trials.
By Hussain-Gambles M, Leese B, Atkin K, Brown J, Mason S, Tovey P.
-
Clinical and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes.
By Colquitt JL, Green C, Sidhu MK, Hartwell D, Waugh N.
-
Identification and assessment of ongoing trials in health technology assessment reviews.
By Song FJ, Fry-Smith A, Davenport C, Bayliss S, Adi Y, Wilson JS, et al.
-
Systematic review and economic evaluation of a long-acting insulin analogue, insulin glargine
By Warren E, Weatherley-Jones E, Chilcott J, Beverley C.
-
Supplementation of a home-based exercise programme with a class-based programme for people with osteoarthritis of the knees: a randomised controlled trial and health economic analysis.
By McCarthy CJ, Mills PM, Pullen R, Richardson G, Hawkins N, Roberts CR, et al.
-
Clinical and cost-effectiveness of once-daily versus more frequent use of same potency topical corticosteroids for atopic eczema: a systematic review and economic evaluation.
By Green C, Colquitt JL, Kirby J, Davidson P, Payne E.
-
Acupuncture of chronic headache disorders in primary care: randomised controlled trial and economic analysis.
By Vickers AJ, Rees RW, Zollman CE, McCarney R, Smith CM, Ellis N, et al.
-
Generalisability in economic evaluation studies in healthcare: a review and case studies.
By Sculpher MJ, Pang FS, Manca A, Drummond MF, Golder S, Urdahl H, et al.
-
Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations.
By Wallace P, Barber J, Clayton W, Currell R, Fleming K, Garner P, et al.
-
Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne.
By Ozolins M, Eady EA, Avery A, Cunliffe WJ, O’Neill C, Simpson NB, et al.
-
Do the findings of case series studies vary significantly according to methodological characteristics?
By Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L.
-
Improving the referral process for familial breast cancer genetic counselling: findings of three randomised controlled trials of two interventions.
By Wilson BJ, Torrance N, Mollison J, Wordsworth S, Gray JR, Haites NE, et al.
-
Randomised evaluation of alternative electrosurgical modalities to treat bladder outflow obstruction in men with benign prostatic hyperplasia.
By Fowler C, McAllister W, Plail R, Karim O, Yang Q.
-
A pragmatic randomised controlled trial of the cost-effectiveness of palliative therapies for patients with inoperable oesophageal cancer.
By Shenfine J, McNamee P, Steen N, Bond J, Griffin SM.
-
Impact of computer-aided detection prompts on the sensitivity and specificity of screening mammography.
By Taylor P, Champness J, Given- Wilson R, Johnston K, Potts H.
-
Issues in data monitoring and interim analysis of trials.
By Grant AM, Altman DG, Babiker AB, Campbell MK, Clemens FJ, Darbyshire JH, et al.
-
Lay public’s understanding of equipoise and randomisation in randomised controlled trials.
By Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ, et al.
-
Clinical and cost-effectiveness of electroconvulsive therapy for depressive illness, schizophrenia, catatonia and mania: systematic reviews and economic modelling studies.
By Greenhalgh J, Knight C, Hind D, Beverley C, Walters S.
-
Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology.
By Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al.
-
Clinical effectiveness and cost-effectiveness of drotrecogin alfa (activated) (Xigris®) for the treatment of severe sepsis in adults: a systematic review and economic evaluation.
By Green C, Dinnes J, Takeda A, Shepherd J, Hartwell D, Cave C, et al.
-
A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy.
By Dinnes J, Deeks J, Kirby J, Roderick P.
-
Cervical screening programmes: can automation help? Evidence from systematic reviews, an economic analysis and a simulation modelling exercise applied to the UK.
By Willis BH, Barton P, Pearmain P, Bryan S, Hyde C.
-
Laparoscopic surgery for inguinal hernia repair: systematic review of effectiveness and economic evaluation.
By McCormack K, Wake B, Perez J, Fraser C, Cook J, McIntosh E, et al.
-
Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation.
By Wilby J, Kainth A, Hawkins N, Epstein D, McIntosh H, McDaid C, et al.
-
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.
By Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, Moore M, et al.
-
Clinical effectiveness and cost-effectiveness of immediate angioplasty for acute myocardial infarction: systematic review and economic evaluation.
By Hartwell D, Colquitt J, Loveman E, Clegg AJ, Brodin H, Waugh N, et al.
-
A randomised controlled comparison of alternative strategies in stroke care.
By Kalra L, Evans A, Perez I, Knapp M, Swift C, Donaldson N.
-
The investigation and analysis of critical incidents and adverse events in healthcare.
By Woloshynowych M, Rogers S, Taylor-Adams S, Vincent C.
-
Potential use of routine databases in health technology assessment.
By Raftery J, Roderick P, Stevens A.
-
Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study.
By Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al.
-
A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis.
By Stevenson M, Lloyd Jones M, De Nigris E, Brewer N, Davis S, Oakley J.
-
A systematic review to examine the impact of psycho-educational interventions on health outcomes and costs in adults and children with difficult asthma.
By Smith JR, Mugford M, Holland R, Candy B, Noble MJ, Harrison BDW, et al.
-
An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales.
By Roderick P, Nicholson T, Armitage A, Mehta R, Mullee M, Gerard K, et al.
-
Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation.
By Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al.
-
Indirect comparisons of competing interventions.
By Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al.
-
Cost-effectiveness of alternative strategies for the initial medical management of non-ST elevation acute coronary syndrome: systematic review and decision-analytical modelling.
By Robinson M, Palmer S, Sculpher M, Philips Z, Ginnelly L, Bowens A, et al.
-
Outcomes of electrically stimulated gracilis neosphincter surgery.
By Tillin T, Chambers M, Feldman R.
-
The effectiveness and cost-effectiveness of pimecrolimus and tacrolimus for atopic eczema: a systematic review and economic evaluation.
By Garside R, Stein K, Castelnuovo E, Pitt M, Ashcroft D, Dimmock P, et al.
-
Systematic review on urine albumin testing for early detection of diabetic complications.
By Newman DJ, Mattock MB, Dawnay ABS, Kerry S, McGuire A, Yaqoob M, et al.
-
Randomised controlled trial of the cost-effectiveness of water-based therapy for lower limb osteoarthritis.
By Cochrane T, Davey RC, Matthes Edwards SM.
-
Longer term clinical and economic benefits of offering acupuncture care to patients with chronic low back pain.
By Thomas KJ, MacPherson H, Ratcliffe J, Thorpe L, Brazier J, Campbell M, et al.
-
Cost-effectiveness and safety of epidural steroids in the management of sciatica.
By Price C, Arden N, Coglan L, Rogers P.
-
The British Rheumatoid Outcome Study Group (BROSG) randomised controlled trial to compare the effectiveness and cost-effectiveness of aggressive versus symptomatic therapy in established rheumatoid arthritis.
By Symmons D, Tricker K, Roberts C, Davies L, Dawes P, Scott DL.
-
Conceptual framework and systematic review of the effects of participants’ and professionals’ preferences in randomised controlled trials.
By King M, Nazareth I, Lampe F, Bower P, Chandler M, Morou M, et al.
-
The clinical and cost-effectiveness of implantable cardioverter defibrillators: a systematic review.
By Bryant J, Brodin H, Loveman E, Payne E, Clegg A.
-
A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study.
By Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, Pickering R, et al.
-
The causes and effects of socio-demographic exclusions from clinical trials.
By Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al.
-
Is hydrotherapy cost-effective? A randomised controlled trial of combined hydrotherapy programmes compared with physiotherapy land techniques in children with juvenile idiopathic arthritis.
By Epps H, Ginnelly L, Utley M, Southwood T, Gallivan S, Sculpher M, et al.
-
A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study.
By Hobbs FDR, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, et al.
-
Displaced intracapsular hip fractures in fit, older people: a randomised comparison of reduction and fixation, bipolar hemiarthroplasty and total hip arthroplasty.
By Keating JF, Grant A, Masson M, Scott NW, Forbes JF.
-
Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland.
By Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al.
-
The effectiveness and cost-effectiveness of dual-chamber pacemakers compared with single-chamber pacemakers for bradycardia due to atrioventricular block or sick sinus syndrome: systematic review and economic evaluation.
By Castelnuovo E, Stein K, Pitt M, Garside R, Payne E.
-
Newborn screening for congenital heart defects: a systematic review and cost-effectiveness analysis.
By Knowles R, Griebsch I, Dezateux C, Brown J, Bull C, Wren C.
-
The clinical and cost-effectiveness of left ventricular assist devices for end-stage heart failure: a systematic review and economic evaluation.
By Clegg AJ, Scott DA, Loveman E, Colquitt J, Hutchinson J, Royle P, et al.
-
The effectiveness of the Heidelberg Retina Tomograph and laser diagnostic glaucoma scanning system (GDx) in detecting and monitoring glaucoma.
By Kwartz AJ, Henson DB, Harper RA, Spencer AF, McLeod D.
-
Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation.
By Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, et al.
-
Systematic review of effectiveness of different treatments for childhood retinoblastoma.
By McDaid C, Hartley S, Bagnall A-M, Ritchie G, Light K, Riemsma R.
-
Towards evidence-based guidelines for the prevention of venous thromboembolism: systematic reviews of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis.
By Roderick P, Ferris G, Wilson K, Halls H, Jackson D, Collins R, et al.
-
The effectiveness and cost-effectiveness of parent training/education programmes for the treatment of conduct disorder, including oppositional defiant disorder, in children.
By Dretzke J, Frew E, Davenport C, Barlow J, Stewart-Brown S, Sandercock J, et al.
-
The clinical and cost-effectiveness of donepezil, rivastigmine, galantamine and memantine for Alzheimer’s disease.
By Loveman E, Green C, Kirby J, Takeda A, Picot J, Payne E, et al.
-
FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke.
By Dennis M, Lewis S, Cranswick G, Forbes J.
-
The clinical effectiveness and cost-effectiveness of computed tomography screening for lung cancer: systematic reviews.
By Black C, Bagust A, Boland A, Walker S, McLeod C, De Verteuil R, et al.
-
A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery.
By Whiting P, Gupta R, Burch J, Mujica Mota RE, Wright K, Marson A, et al.
-
Comparison of conference abstracts and presentations with full-text articles in the health technology assessments of rapidly evolving technologies.
By Dundar Y, Dodd S, Dickson R, Walley T, Haycox A, Williamson PR.
-
Systematic review and evaluation of methods of assessing urinary incontinence.
By Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al.
-
The clinical effectiveness and cost-effectiveness of newer drugs for children with epilepsy. A systematic review.
By Connock M, Frew E, Evans B-W, Bryan S, Cummins C, Fry-Smith A, et al.
-
Surveillance of Barrett’s oesophagus: exploring the uncertainty through systematic review, expert workshop and economic modelling.
By Garside R, Pitt M, Somerville M, Stein K, Price A, Gilbert N.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.
By Main C, Bojke L, Griffin S, Norman G, Barbieri M, Mather L, et al.
-
Evaluation of molecular techniques in prediction and diagnosis of cytomegalovirus disease in immunocompromised patients.
By Szczepura A, Westmoreland D, Vinogradova Y, Fox J, Clark M.
-
Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis. The Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study.
By Wu O, Robertson L, Twaddle S, Lowe GDO, Clark P, Greaves M, et al.
-
A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers.
By Nelson EA, O’Meara S, Craig D, Iglesias C, Golder S, Dalton J, et al.
-
Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial).
By Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al.
-
The cost-effectiveness of screening for oral cancer in primary care.
By Speight PM, Palmer S, Moles DR, Downer MC, Smith DH, Henriksson M, et al.
-
Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis.
By Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S, et al.
-
Systematic review of the effectiveness and cost-effectiveness of HealOzone® for the treatment of occlusal pit/fissure caries and root caries.
By Brazzelli M, McKenzie L, Fielding S, Fraser C, Clarkson J, Kilonzo M, et al.
-
Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment.
By Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, et al.
-
Diagnostic tests and algorithms used in the investigation of haematuria: systematic reviews and economic evaluation.
By Rodgers M, Nixon J, Hempel S, Aho T, Kelly J, Neal D, et al.
-
Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: randomised controlled trial.
By Kennedy TM, Chalder T, McCrone P, Darnley S, Knapp M, Jones RH, et al.
-
A systematic review of the clinical effectiveness and cost-effectiveness of enzyme replacement therapies for Fabry’s disease and mucopolysaccharidosis type 1.
By Connock M, Juarez-Garcia A, Frew E, Mans A, Dretzke J, Fry-Smith A, et al.
-
Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation.
By Wright M, Grieve R, Roberts J, Main J, Thomas HC, on behalf of the UK Mild Hepatitis C Trial Investigators.
-
Pressure relieving support surfaces: a randomised evaluation.
By Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al.
-
A systematic review and economic model of the effectiveness and cost-effectiveness of methylphenidate, dexamfetamine and atomoxetine for the treatment of attention deficit hyperactivity disorder in children and adolescents.
By King S, Griffin S, Hodges Z, Weatherly H, Asseburg C, Richardson G, et al.
-
The clinical effectiveness and cost-effectiveness of enzyme replacement therapy for Gaucher’s disease: a systematic review.
By Connock M, Burls A, Frew E, Fry-Smith A, Juarez-Garcia A, McCabe C, et al.
-
Effectiveness and cost-effectiveness of salicylic acid and cryotherapy for cutaneous warts. An economic decision model.
By Thomas KS, Keogh-Brown MR, Chalmers JR, Fordham RJ, Holland RC, Armstrong SJ, et al.
-
A systematic literature review of the effectiveness of non-pharmacological interventions to prevent wandering in dementia and evaluation of the ethical implications and acceptability of their use.
By Robinson L, Hutchings D, Corner L, Beyer F, Dickinson H, Vanoli A, et al.
-
A review of the evidence on the effects and costs of implantable cardioverter defibrillator therapy in different patient groups, and modelling of cost-effectiveness and cost–utility for these groups in a UK context.
By Buxton M, Caine N, Chase D, Connelly D, Grace A, Jackson C, et al.
-
Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation.
By Shepherd J, Jones J, Takeda A, Davidson P, Price A.
-
An evaluation of the clinical and cost-effectiveness of pulmonary artery catheters in patient management in intensive care: a systematic review and a randomised controlled trial.
By Harvey S, Stevens K, Harrison D, Young D, Brampton W, McCabe C, et al.
-
Accurate, practical and cost-effective assessment of carotid stenosis in the UK.
By Wardlaw JM, Chappell FM, Stevenson M, De Nigris E, Thomas S, Gillard J, et al.
-
Etanercept and infliximab for the treatment of psoriatic arthritis: a systematic review and economic evaluation.
By Woolacott N, Bravo Vergel Y, Hawkins N, Kainth A, Khadjesari Z, Misso K, et al.
-
The cost-effectiveness of testing for hepatitis C in former injecting drug users.
By Castelnuovo E, Thompson-Coon J, Pitt M, Cramp M, Siebert U, Price A, et al.
-
Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation.
By Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, et al.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.
By Williams C, Brunskill S, Altman D, Briggs A, Campbell H, Clarke M, et al.
-
Psychological therapies including dialectical behaviour therapy for borderline personality disorder: a systematic review and preliminary economic evaluation.
By Brazier J, Tumur I, Holmes M, Ferriter M, Parry G, Dent-Brown K, et al.
-
Clinical effectiveness and cost-effectiveness of tests for the diagnosis and investigation of urinary tract infection in children: a systematic review and economic model.
By Whiting P, Westwood M, Bojke L, Palmer S, Richardson G, Cooper J, et al.
-
Cognitive behavioural therapy in chronic fatigue syndrome: a randomised controlled trial of an outpatient group programme.
By O’Dowd H, Gladwell P, Rogers CA, Hollinghurst S, Gregory A.
-
A comparison of the cost-effectiveness of five strategies for the prevention of nonsteroidal anti-inflammatory drug-induced gastrointestinal toxicity: a systematic review with economic modelling.
By Brown TJ, Hooper L, Elliott RA, Payne K, Webb R, Roberts C, et al.
-
The effectiveness and cost-effectiveness of computed tomography screening for coronary artery disease: systematic review.
By Waugh N, Black C, Walker S, McIntyre L, Cummins E, Hillis G.
-
What are the clinical outcome and cost-effectiveness of endoscopy undertaken by nurses when compared with doctors? A Multi-Institution Nurse Endoscopy Trial (MINuET).
By Williams J, Russell I, Durai D, Cheung W-Y, Farrin A, Bloor K, et al.
-
The clinical and cost-effectiveness of oxaliplatin and capecitabine for the adjuvant treatment of colon cancer: systematic review and economic evaluation.
By Pandor A, Eggington S, Paisley S, Tappenden P, Sutcliffe P.
-
A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness.
By Chen Y-F, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al.
-
Telemedicine in dermatology: a randomised controlled trial.
By Bowns IR, Collins K, Walters SJ, McDonagh AJG.
-
Cost-effectiveness of cell salvage and alternative methods of minimising perioperative allogeneic blood transfusion: a systematic review and economic model.
By Davies L, Brown TJ, Haynes S, Payne K, Elliott RA, McCollum C.
-
Clinical effectiveness and cost-effectiveness of laparoscopic surgery for colorectal cancer: systematic reviews and economic evaluation.
By Murray A, Lourenco T, de Verteuil R, Hernandez R, Fraser C, McKinley A, et al.
-
Etanercept and efalizumab for the treatment of psoriasis: a systematic review.
By Woolacott N, Hawkins N, Mason A, Kainth A, Khadjesari Z, Bravo Vergel Y, et al.
-
Systematic reviews of clinical decision tools for acute abdominal pain.
By Liu JLY, Wyatt JC, Deeks JJ, Clamp S, Keen J, Verde P, et al.
-
Evaluation of the ventricular assist device programme in the UK.
By Sharples L, Buxton M, Caine N, Cafferty F, Demiris N, Dyer M, et al.
-
A systematic review and economic model of the clinical and cost-effectiveness of immunosuppressive therapy for renal transplantation in children.
By Yao G, Albon E, Adi Y, Milford D, Bayliss S, Ready A, et al.
-
Amniocentesis results: investigation of anxiety. The ARIA trial.
By Hewison J, Nixon J, Fountain J, Cocks K, Jones C, Mason G, et al.
-
Pemetrexed disodium for the treatment of malignant pleural mesothelioma: a systematic review and economic evaluation.
By Dundar Y, Bagust A, Dickson R, Dodd S, Green J, Haycox A, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of docetaxel in combination with prednisone or prednisolone for the treatment of hormone-refractory metastatic prostate cancer.
By Collins R, Fenwick E, Trowman R, Perard R, Norman G, Light K, et al.
-
A systematic review of rapid diagnostic tests for the detection of tuberculosis infection.
By Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al.
-
The clinical effectiveness and cost-effectiveness of strontium ranelate for the prevention of osteoporotic fragility fractures in postmenopausal women.
By Stevenson M, Davis S, Lloyd-Jones M, Beverley C.
-
A systematic review of quantitative and qualitative research on the role and effectiveness of written information available to patients about individual medicines.
By Raynor DK, Blenkinsopp A, Knapp P, Grime J, Nicolson DJ, Pollock K, et al.
-
Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: a systematic review and economic evaluation.
By Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al.
-
Glucocorticoid-induced osteoporosis: a systematic review and cost–utility analysis.
By Kanis JA, Stevenson M, McCloskey EV, Davis S, Lloyd-Jones M.
-
Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection.
By Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al.
-
Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation.
By Connock M, Juarez-Garcia A, Jowett S, Frew E, Liu Z, Taylor RJ, et al.
-
Exercise Evaluation Randomised Trial (EXERT): a randomised trial comparing GP referral for leisure centre-based exercise, community-based walking and advice only.
By Isaacs AJ, Critchley JA, See Tai S, Buckingham K, Westley D, Harridge SDR, et al.
-
Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation.
By Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N.
-
Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer.
By Tappenden P, Jones R, Paisley S, Carroll C.
-
A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment.
By Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al.
-
A systematic review and economic evaluation of statins for the prevention of coronary events.
By Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al.
-
A systematic review of the effectiveness and cost-effectiveness of different models of community-based respite care for frail older people and their carers.
By Mason A, Weatherly H, Spilsbury K, Arksey H, Golder S, Adamson J, et al.
-
Additional therapy for young children with spastic cerebral palsy: a randomised controlled trial.
By Weindling AM, Cunningham CC, Glenn SM, Edwards RT, Reeves DJ.
-
Screening for type 2 diabetes: literature review and economic modelling.
By Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, et al.
-
The effectiveness and cost-effectiveness of cinacalcet for secondary hyperparathyroidism in end-stage renal disease patients on dialysis: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Mealing S, Roome C, Snaith A, et al.
-
The clinical effectiveness and cost-effectiveness of gemcitabine for metastatic breast cancer: a systematic review and economic evaluation.
By Takeda AL, Jones J, Loveman E, Tan SC, Clegg AJ.
-
A systematic review of duplex ultrasound, magnetic resonance angiography and computed tomography angiography for the diagnosis and assessment of symptomatic, lower limb peripheral arterial disease.
By Collins R, Cranny G, Burch J, Aguiar-Ibáñez R, Craig D, Wright K, et al.
-
The clinical effectiveness and cost-effectiveness of treatments for children with idiopathic steroid-resistant nephrotic syndrome: a systematic review.
By Colquitt JL, Kirby J, Green C, Cooper K, Trompeter RS.
-
A systematic review of the routine monitoring of growth in children of primary school age to identify growth-related conditions.
By Fayter D, Nixon J, Hartley S, Rithalia A, Butler G, Rudolf M, et al.
-
Systematic review of the effectiveness of preventing and treating Staphylococcus aureus carriage in reducing peritoneal catheter-related infections.
By McCormack K, Rabindranath K, Kilonzo M, Vale L, Fraser C, McIntyre L, et al.
-
The clinical effectiveness and cost of repetitive transcranial magnetic stimulation versus electroconvulsive therapy in severe depression: a multicentre pragmatic randomised controlled trial and economic analysis.
By McLoughlin DM, Mogg A, Eranti S, Pluck G, Purvis R, Edwards D, et al.
-
A randomised controlled trial and economic evaluation of direct versus indirect and individual versus group modes of speech and language therapy for children with primary language impairment.
By Boyle J, McCartney E, Forbes J, O’Hare A.
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.
By Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L.
-
Cardioprotection against the toxic effects of anthracyclines given to children with cancer: a systematic review.
By Bryant J, Picot J, Levitt G, Sullivan I, Baxter L, Clegg A.
-
Adalimumab, etanercept and infliximab for the treatment of ankylosing spondylitis: a systematic review and economic evaluation.
By McLeod C, Bagust A, Boland A, Dagenais P, Dickson R, Dundar Y, et al.
-
Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost-effectiveness and expected value of information analyses.
By Colbourn T, Asseburg C, Bojke L, Philips Z, Claxton K, Ades AE, et al.
-
Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review.
By Garrison KR, Donell S, Ryder J, Shemilt I, Mugford M, Harvey I, et al.
-
A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial.
By Prescott RJ, Kunkler IH, Williams LJ, King CC, Jack W, van der Pol M, et al.
-
Current practice, accuracy, effectiveness and cost-effectiveness of the school entry hearing screen.
By Bamford J, Fortnum H, Bristow K, Smith J, Vamvakas G, Davies L, et al.
-
The clinical effectiveness and cost-effectiveness of inhaled insulin in diabetes mellitus: a systematic review and economic evaluation.
By Black C, Cummins E, Royle P, Philip S, Waugh N.
-
Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.
By Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al.
-
The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Homebased compared with hospital-based cardiac rehabilitation in a multi-ethnic population: cost-effectiveness and patient adherence.
By Jolly K, Taylor R, Lip GYH, Greenfield S, Raftery J, Mant J, et al.
-
A systematic review of the clinical, public health and cost-effectiveness of rapid diagnostic tests for the detection and identification of bacterial intestinal pathogens in faeces and food.
By Abubakar I, Irvine L, Aldus CF, Wyatt GM, Fordham R, Schelenz S, et al.
-
A randomised controlled trial examining the longer-term outcomes of standard versus new antiepileptic drugs. The SANAD trial.
By Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al.
-
Clinical effectiveness and cost-effectiveness of different models of managing long-term oral anti-coagulation therapy: a systematic review and economic modelling.
By Connock M, Stevens C, Fry-Smith A, Jowett S, Fitzmaurice D, Moore D, et al.
-
A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder.
By Soares-Weiser K, Bravo Vergel Y, Beynon S, Dunn G, Barbieri M, Duffy S, et al.
-
Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation.
By Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A.
-
The clinical effectiveness and cost-effectiveness of screening for open angle glaucoma: a systematic review and economic evaluation.
By Burr JM, Mowatt G, Hernández R, Siddiqui MAR, Cook J, Lourenco T, et al.
-
Acceptability, benefit and costs of early screening for hearing disability: a study of potential screening tests and models.
By Davis A, Smith P, Ferguson M, Stephens D, Gianopoulos I.
-
Contamination in trials of educational interventions.
By Keogh-Brown MR, Bachmann MO, Shepstone L, Hewitt C, Howe A, Ramsay CR, et al.
-
Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers.
By Facey K, Bradbury I, Laking G, Payne E.
-
The effectiveness and cost-effectiveness of carmustine implants and temozolomide for the treatment of newly diagnosed high-grade glioma: a systematic review and economic evaluation.
By Garside R, Pitt M, Anderson R, Rogers G, Dyer M, Mealing S, et al.
-
Drug-eluting stents: a systematic review and economic evaluation.
By Hill RA, Boland A, Dickson R, Dündar Y, Haycox A, McLeod C, et al.
-
The clinical effectiveness and cost-effectiveness of cardiac resynchronisation (biventricular pacing) for heart failure: systematic review and economic model.
By Fox M, Mealing S, Anderson R, Dean J, Stein K, Price A, et al.
-
Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study.
By Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al.
-
Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial.
By Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al.
-
Evaluation of diagnostic tests when there is no gold standard. A review of methods.
By Rutjes AWS, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PMM.
-
Systematic reviews of the clinical effectiveness and cost-effectiveness of proton pump inhibitors in acute upper gastrointestinal bleeding.
By Leontiadis GI, Sreedharan A, Dorward S, Barton P, Delaney B, Howden CW, et al.
-
A review and critique of modelling in prioritising and designing screening programmes.
By Karnon J, Goyder E, Tappenden P, McPhie S, Towers I, Brazier J, et al.
-
An assessment of the impact of the NHS Health Technology Assessment Programme.
By Hanney S, Buxton M, Green C, Coulson D, Raftery J.
-
A systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery.
By Cranny G, Elliott R, Weatherly H, Chambers D, Hawkins N, Myers L, et al.
-
‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis.
By Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D.
-
A systematic review of the effectiveness of strategies for reducing fracture risk in children with juvenile idiopathic arthritis with additional data on long-term risk of fracture and cost of disease management.
By Thornton J, Ashcroft D, O’Neill T, Elliott R, Adams J, Roberts C, et al.
-
Does befriending by trained lay workers improve psychological well-being and quality of life for carers of people with dementia, and at what cost? A randomised controlled trial.
By Charlesworth G, Shepstone L, Wilson E, Thalanany M, Mugford M, Poland F.
-
A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study.
By Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al.
-
Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Meads CA, Cnossen JS, Meher S, Juarez-Garcia A, ter Riet G, Duley L, et al.
-
The use of economic evaluations in NHS decision-making: a review and empirical investigation.
By Williams I, McIver S, Moore D, Bryan S.
-
Stapled haemorrhoidectomy (haemorrhoidopexy) for the treatment of haemorrhoids: a systematic review and economic evaluation.
By Burch J, Epstein D, Baba-Akbari A, Weatherly H, Fox D, Golder S, et al.
-
The clinical effectiveness of diabetes education models for Type 2 diabetes: a systematic review.
By Loveman E, Frampton GK, Clegg AJ.
-
Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study.
By Raftery J, Bryant J, Powell J, Kerr C, Hawker S.
-
Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation.
By Chen Y-F, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, et al.
-
The clinical effectiveness and cost-effectiveness of central venous catheters treated with anti-infective agents in preventing bloodstream infections: a systematic review and economic evaluation.
By Hockenhull JC, Dwan K, Boland A, Smith G, Bagust A, Dundar Y, et al.
-
Stepped treatment of older adults on laxatives. The STOOL trial.
By Mihaylov S, Stark C, McColl E, Steen N, Vanoli A, Rubin G, et al.
-
A randomised controlled trial of cognitive behaviour therapy in adolescents with major depression treated by selective serotonin reuptake inhibitors. The ADAPT trial.
By Goodyer IM, Dubicka B, Wilkinson P, Kelvin R, Roberts C, Byford S, et al.
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.
By Hind D, Tappenden P, Tumur I, Eggington E, Sutcliffe P, Ryan A.
-
Ranibizumab and pegaptanib for the treatment of age-related macular degeneration: a systematic review and economic evaluation.
By Colquitt JL, Jones J, Tan SC, Takeda A, Clegg AJ, Price A.
-
Systematic review of the clinical effectiveness and cost-effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of coronary artery disease.
By Mowatt G, Cummins E, Waugh N, Walker S, Cook J, Jia X, et al.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.
By Albon E, Tsourapas A, Frew E, Davenport C, Oyebode F, Bayliss S, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in adults and children aged 12 years and over.
By Shepherd J, Rogers G, Anderson R, Main C, Thompson-Coon J, Hartwell D, et al.
-
Systematic review and economic analysis of the comparative effectiveness of different inhaled corticosteroids and their usage with long-acting beta2 agonists for the treatment of chronic asthma in children under the age of 12 years.
By Main C, Shepherd J, Anderson R, Rogers G, Thompson-Coon J, Liu Z, et al.
-
Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation.
By Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, et al.
-
Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study.
By Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al.
-
A prospective randomised comparison of minor surgery in primary and secondary care. The MiSTIC trial.
By George S, Pockney P, Primrose J, Smith H, Little P, Kinley H, et al.
-
A review and critical appraisal of measures of therapist–patient interactions in mental health settings.
By Cahill J, Barkham M, Hardy G, Gilbody S, Richards D, Bower P, et al.
-
The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation.
By Carlton J, Karnon J, Czoski-Murray C, Smith KJ, Marr J.
-
A systematic review of the clinical effectiveness and cost-effectiveness and economic modelling of minimal incision total hip replacement approaches in the management of arthritic disease of the hip.
By de Verteuil R, Imamura M, Zhu S, Glazener C, Fraser C, Munro N, et al.
-
A preliminary model-based assessment of the cost–utility of a screening programme for early age-related macular degeneration.
By Karnon J, Czoski-Murray C, Smith K, Brand C, Chakravarthy U, Davis S, et al.
-
Intravenous magnesium sulphate and sotalol for prevention of atrial fibrillation after coronary artery bypass surgery: a systematic review and economic evaluation.
By Shepherd J, Jones J, Frampton GK, Tanajewski L, Turner D, Price A.
-
Absorbent products for urinary/faecal incontinence: a comparative evaluation of key product categories.
By Fader M, Cottenden A, Getliffe K, Gage H, Clarke-O’Neill S, Jamieson K, et al.
-
A systematic review of repetitive functional task practice with modelling of resource use, costs and effectiveness.
By French B, Leathley M, Sutton C, McAdam J, Thomas L, Forster A, et al.
-
The effectiveness and cost-effectivness of minimal access surgery amongst people with gastro-oesophageal reflux disease – a UK collaborative study. The reflux trial.
By Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al.
-
Time to full publication of studies of anti-cancer medicines for breast cancer and the potential for publication bias: a short systematic review.
By Takeda A, Loveman E, Harris P, Hartwell D, Welch K.
-
Performance of screening tests for child physical abuse in accident and emergency departments.
By Woodman J, Pitt M, Wentz R, Taylor B, Hodes D, Gilbert RE.
-
Curative catheter ablation in atrial fibrillation and typical atrial flutter: systematic review and economic evaluation.
By Rodgers M, McKenna C, Palmer S, Chambers D, Van Hout S, Golder S, et al.
-
Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement.
By Lourenco T, Armstrong N, N’Dow J, Nabi G, Deverill M, Pickard R, et al.
-
Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation.
By Wang D, Cummins C, Bayliss S, Sandercock J, Burls A.
-
Deferasirox for the treatment of iron overload associated with regular blood transfusions (transfusional haemosiderosis) in patients suffering with chronic anaemia: a systematic review and economic evaluation.
By McLeod C, Fleeman N, Kirkham J, Bagust A, Boland A, Chu P, et al.
-
Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis.
By Simpson EL, Stevenson MD, Rawdin A, Papaioannou D.
-
Surgical procedures and non-surgical devices for the management of non-apnoeic snoring: a systematic review of clinical effects and associated treatment costs.
By Main C, Liu Z, Welch K, Weiner G, Quentin Jones S, Stein K.
-
Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea–hypopnoea syndrome: a systematic review and economic analysis.
By McDaid C, Griffin S, Weatherly H, Durée K, van der Burgt M, van Hout S, Akers J, et al.
-
Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review.
By Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al.
-
The harmful health effects of recreational ecstasy: a systematic review of observational evidence.
By Rogers G, Elston J, Garside R, Roome C, Taylor R, Younger P, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of oesophageal Doppler monitoring in critically ill and high-risk surgical patients.
By Mowatt G, Houston G, Hernández R, de Verteuil R, Fraser C, Cuthbertson B, et al.
-
The use of surrogate outcomes in model-based cost-effectiveness analyses: a survey of UK Health Technology Assessment reports.
By Taylor RS, Elston J.
-
Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) – a randomised controlled trial.
By Potter J, Mistri A, Brodie F, Chernova J, Wilson E, Jagger C, et al.
-
Routine antenatal anti-D prophylaxis for RhD-negative women: a systematic review and economic evaluation.
By Pilgrim H, Lloyd-Jones M, Rees A.
-
Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation.
By Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, et al.
-
Improving the evaluation of therapeutic interventions in multiple sclerosis: the role of new psychometric methods.
By Hobart J, Cano S.
-
Treatment of severe ankle sprain: a pragmatic randomised controlled trial comparing the clinical effectiveness and cost-effectiveness of three types of mechanical ankle support with tubular bandage. The CAST trial.
By Cooke MW, Marsh JL, Clark M, Nakash R, Jarvis RM, Hutton JL, et al. , on behalf of the CAST trial group.
-
Non-occupational postexposure prophylaxis for HIV: a systematic review.
By Bryant J, Baxter L, Hird S.
-
Blood glucose self-monitoring in type 2 diabetes: a randomised controlled trial.
By Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al.
-
How far does screening women for domestic (partner) violence in different health-care settings meet criteria for a screening programme? Systematic reviews of nine UK National Screening Committee criteria.
By Feder G, Ramsay J, Dunne D, Rose M, Arsene C, Norman R, et al.
-
Spinal cord stimulation for chronic pain of neuropathic or ischaemic origin: systematic review and economic evaluation.
By Simpson, EL, Duenas A, Holmes MW, Papaioannou D, Chilcott J.
-
The role of magnetic resonance imaging in the identification of suspected acoustic neuroma: a systematic review of clinical and cost-effectiveness and natural history.
By Fortnum H, O’Neill C, Taylor R, Lenthall R, Nikolopoulos T, Lightfoot G, et al.
-
Dipsticks and diagnostic algorithms in urinary tract infection: development and validation, randomised trial, economic analysis, observational cohort and qualitative study.
By Little P, Turner S, Rumsby K, Warner G, Moore M, Lowes JA, et al.
-
Systematic review of respite care in the frail elderly.
By Shaw C, McNamara R, Abrams K, Cannings-John R, Hood K, Longo M, et al.
-
Neuroleptics in the treatment of aggressive challenging behaviour for people with intellectual disabilities: a randomised controlled trial (NACHBID).
By Tyrer P, Oliver-Africano P, Romeo R, Knapp M, Dickens S, Bouras N, et al.
-
Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study.
By Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, Peveler R, et al.
-
Diagnostic strategies using DNA testing for hereditary haemochromatosis in at-risk populations: a systematic review and economic evaluation.
By Bryant J, Cooper K, Picot J, Clegg A, Roderick P, Rosenberg W, et al.
-
Enhanced external counterpulsation for the treatment of stable angina and heart failure: a systematic review and economic analysis.
By McKenna C, McDaid C, Suekarran S, Hawkins N, Claxton K, Light K, et al.
-
Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE).
By Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al.
-
A systematic review of presumed consent systems for deceased organ donation.
By Rithalia A, McDaid C, Suekarran S, Norman G, Myers L, Sowden A.
-
Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial.
By Hay AD, Redmond NM, Costelloe C, Montgomery AA, Fletcher M, Hollinghurst S, et al.
-
A randomised controlled trial to compare minimally invasive glucose monitoring devices with conventional monitoring in the management of insulin-treated diabetes mellitus (MITRE).
By Newman SP, Cooke D, Casbard A, Walker S, Meredith S, Nunn A, et al.
-
Sensitivity analysis in economic evaluation: an audit of NICE current practice and a review of its use and value in decision-making.
By Andronis L, Barton P, Bryan S.
-
Trastuzumab for the treatment of primary breast cancer in HER2-positive women: a single technology appraisal.
By Ward S, Pilgrim H, Hind D.
-
Docetaxel for the adjuvant treatment of early node-positive breast cancer: a single technology appraisal.
By Chilcott J, Lloyd Jones M, Wilkinson A.
-
The use of paclitaxel in the management of early stage breast cancer.
By Griffin S, Dunn G, Palmer S, Macfarlane K, Brent S, Dyker A, et al.
-
Rituximab for the first-line treatment of stage III/IV follicular non-Hodgkin’s lymphoma.
By Dundar Y, Bagust A, Hounsome J, McLeod C, Boland A, Davis H, et al.
-
Bortezomib for the treatment of multiple myeloma patients.
By Green C, Bryant J, Takeda A, Cooper K, Clegg A, Smith A, et al.
-
Fludarabine phosphate for the firstline treatment of chronic lymphocytic leukaemia.
By Walker S, Palmer S, Erhorn S, Brent S, Dyker A, Ferrie L, et al.
-
Erlotinib for the treatment of relapsed non-small cell lung cancer.
By McLeod C, Bagust A, Boland A, Hockenhull J, Dundar Y, Proudlove C, et al.
-
Cetuximab plus radiotherapy for the treatment of locally advanced squamous cell carcinoma of the head and neck.
By Griffin S, Walker S, Sculpher M, White S, Erhorn S, Brent S, et al.
-
Infliximab for the treatment of adults with psoriasis.
By Loveman E, Turner D, Hartwell D, Cooper K, Clegg A
-
Psychological interventions for postnatal depression: cluster randomised trial and economic evaluation. The PoNDER trial.
By Morrell CJ, Warner R, Slade P, Dixon S, Walters S, Paley G, et al.
-
The effect of different treatment durations of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes: a systematic review and value of information analysis.
By Rogowski R, Burch J, Palmer S, Craigs C, Golder S, Woolacott N.
-
Systematic review and individual patient data meta-analysis of diagnosis of heart failure, with modelling of implications of different diagnostic strategies in primary care.
By Mant J, Doust J, Roalfe A, Barton P, Cowie MR, Glasziou P, et al.
-
A multicentre randomised controlled trial of the use of continuous positive airway pressure and non-invasive positive pressure ventilation in the early treatment of patients presenting to the emergency department with severe acute cardiogenic pulmonary oedema: the 3CPO trial.
By Gray AJ, Goodacre S, Newby DE, Masson MA, Sampson F, Dixon S, et al. , on behalf of the 3CPO study investigators.
-
Early high-dose lipid-lowering therapy to avoid cardiac events: a systematic review and economic evaluation.
By Ara R, Pandor A, Stevens J, Rees A, Rafia R.
-
Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation.
By Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al.
-
Methods to identify postnatal depression in primary care: an integrated evidence synthesis and value of information analysis.
By Hewitt CE, Gilbody SM, Brealey S, Paulden M, Palmer S, Mann R, et al.
-
A double-blind randomised placebo-controlled trial of topical intranasal corticosteroids in 4- to 11-year-old children with persistent bilateral otitis media with effusion in primary care.
By Williamson I, Benge S, Barton S, Petrou S, Letley L, Fasey N, et al.
-
The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model.
By Bond M, Pitt M, Akoh J, Moxham T, Hoyle M, Anderson R.
-
Rehabilitation of older patients: day hospital compared with rehabilitation at home. A randomised controlled trial.
By Parker SG, Oliver P, Pennington M, Bond J, Jagger C, Enderby PM, et al.
-
Breastfeeding promotion for infants in neonatal units: a systematic review and economic analysis.
By Renfrew MJ, Craig D, Dyson L, McCormick F, Rice S, King SE, et al.
-
The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation.
By Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, et al.
-
Rapid testing for group B streptococcus during labour: a test accuracy study with evaluation of acceptability and cost-effectiveness.
By Daniels J, Gray J, Pattison H, Roberts T, Edwards E, Milner P, et al.
-
Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling.
By Honest H, Forbes CA, Durée KH, Norman G, Duffy SB, Tsourapas A, et al.
-
The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model.
By Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al.
-
Gemcitabine for the treatment of metastatic breast cancer.
By Jones J, Takeda A, Tan SC, Cooper K, Loveman E, Clegg A.
-
Varenicline in the management of smoking cessation: a single technology appraisal.
By Hind D, Tappenden P, Peters J, Kenjegalieva K.
-
Alteplase for the treatment of acute ischaemic stroke: a single technology appraisal.
By Lloyd Jones M, Holmes M.
-
Rituximab for the treatment of rheumatoid arthritis.
By Bagust A, Boland A, Hockenhull J, Fleeman N, Greenhalgh J, Dundar Y, et al.
-
Omalizumab for the treatment of severe persistent allergic asthma.
By Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al.
-
Rituximab for the treatment of relapsed or refractory stage III or IV follicular non-Hodgkin’s lymphoma.
By Boland A, Bagust A, Hockenhull J, Davis H, Chu P, Dickson R.
-
Adalimumab for the treatment of psoriasis.
By Turner D, Picot J, Cooper K, Loveman E.
-
Dabigatran etexilate for the prevention of venous thromboembolism in patients undergoing elective hip and knee surgery: a single technology appraisal.
By Holmes M, C Carroll C, Papaioannou D.
-
Romiplostim for the treatment of chronic immune or idiopathic thrombocytopenic purpura: a single technology appraisal.
By Mowatt G, Boachie C, Crowther M, Fraser C, Hernández R, Jia X, et al.
-
Sunitinib for the treatment of gastrointestinal stromal tumours: a critique of the submission from Pfizer.
By Bond M, Hoyle M, Moxham T, Napier M, Anderson R.
-
Vitamin K to prevent fractures in older women: systematic review and economic evaluation.
By Stevenson M, Lloyd-Jones M, Papaioannou D.
-
The effects of biofeedback for the treatment of essential hypertension: a systematic review.
By Greenhalgh J, Dickson R, Dundar Y.
-
A randomised controlled trial of the use of aciclovir and/or prednisolone for the early treatment of Bell’s palsy: the BELLS study.
By Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, et al.
-
Lapatinib for the treatment of HER2-overexpressing breast cancer.
By Jones J, Takeda A, Picot J, von Keyserlingk C, Clegg A.
-
Infliximab for the treatment of ulcerative colitis.
By Hyde C, Bryan S, Juarez-Garcia A, Andronis L, Fry-Smith A.
-
Rimonabant for the treatment of overweight and obese people.
By Burch J, McKenna C, Palmer S, Norman G, Glanville J, Sculpher M, et al.
-
Telbivudine for the treatment of chronic hepatitis B infection.
By Hartwell D, Jones J, Harris P, Cooper K.
-
Entecavir for the treatment of chronic hepatitis B infection.
By Shepherd J, Gospodarevskaya E, Frampton G, Cooper, K.
-
Febuxostat for the treatment of hyperuricaemia in people with gout: a single technology appraisal.
By Stevenson M, Pandor A.
-
Rivaroxaban for the prevention of venous thromboembolism: a single technology appraisal.
By Stevenson M, Scope A, Holmes M, Rees A, Kaltenthaler E.
-
Cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck.
By Greenhalgh J, Bagust A, Boland A, Fleeman N, McLeod C, Dundar Y, et al.
-
Mifamurtide for the treatment of osteosarcoma: a single technology appraisal.
By Pandor A, Fitzgerald P, Stevenson M, Papaioannou D.
-
Ustekinumab for the treatment of moderate to severe psoriasis.
By Gospodarevskaya E, Picot J, Cooper K, Loveman E, Takeda A.
-
Endovascular stents for abdominal aortic aneurysms: a systematic review and economic model.
By Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al.
-
Clinical and cost-effectiveness of epoprostenol, iloprost, bosentan, sitaxentan and sildenafil for pulmonary arterial hypertension within their licensed indications: a systematic review and economic evaluation.
By Chen Y-F, Jowett S, Barton P, Malottki K, Hyde C, Gibbs JSR, et al.
-
Cessation of attention deficit hyperactivity disorder drugs in the young (CADDY) – a pharmacoepidemiological and qualitative study.
By Wong ICK, Asherson P, Bilbow A, Clifford S, Coghill D, R DeSoysa R, et al.
-
ARTISTIC: a randomised trial of human papillomavirus (HPV) testing in primary cervical screening.
By Kitchener HC, Almonte M, Gilham C, Dowie R, Stoykova B, Sargent A, et al.
-
The clinical effectiveness of glucosamine and chondroitin supplements in slowing or arresting progression of osteoarthritis of the knee: a systematic review and economic evaluation.
By Black C, Clar C, Henderson R, MacEachern C, McNamee P, Quayyum Z, et al.
-
Randomised preference trial of medical versus surgical termination of pregnancy less than 14 weeks’ gestation (TOPS).
By Robson SC, Kelly T, Howel D, Deverill M, Hewison J, Lie MLS, et al.
-
Randomised controlled trial of the use of three dressing preparations in the management of chronic ulceration of the foot in diabetes.
By Jeffcoate WJ, Price PE, Phillips CJ, Game FL, Mudge E, Davies S, et al.
-
VenUS II: a randomised controlled trial of larval therapy in the management of leg ulcers.
By Dumville JC, Worthy G, Soares MO, Bland JM, Cullum N, Dowson C, et al.
-
A prospective randomised controlled trial and economic modelling of antimicrobial silver dressings versus non-adherent control dressings for venous leg ulcers: the VULCAN trial
By Michaels JA, Campbell WB, King BM, MacIntyre J, Palfreyman SJ, Shackley P, et al.
-
Communication of carrier status information following universal newborn screening for sickle cell disorders and cystic fibrosis: qualitative study of experience and practice.
By Kai J, Ulph F, Cullinan T, Qureshi N.
-
Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation.
By Burch J, Paulden M, Conti S, Stock C, Corbett M, Welton NJ, et al.
-
Development of a toolkit and glossary to aid in the adaptation of health technology assessment (HTA) reports for use in different contexts.
By Chase D, Rosten C, Turner S, Hicks N, Milne R.
-
Colour vision testing for diabetic retinopathy: a systematic review of diagnostic accuracy and economic evaluation.
By Rodgers M, Hodges R, Hawkins J, Hollingworth W, Duffy S, McKibbin M, et al.
-
Systematic review of the effectiveness and cost-effectiveness of weight management schemes for the under fives: a short report.
By Bond M, Wyatt K, Lloyd J, Welch K, Taylor R.
-
Are adverse effects incorporated in economic models? An initial review of current practice.
By Craig D, McDaid C, Fonseca T, Stock C, Duffy S, Woolacott N.
-
Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE).
By Turnbull LW, Brown SR, Olivier C, Harvey I, Brown J, Drew P, et al.
-
Bevacizumab, sorafenib tosylate, sunitinib and temsirolimus for renal cell carcinoma: a systematic review and economic evaluation.
By Thompson Coon J, Hoyle M, Green C, Liu Z, Welch K, Moxham T, et al.
-
The clinical effectiveness and cost-effectiveness of testing for cytochrome P450 polymorphisms in patients with schizophrenia treated with antipsychotics: a systematic review and economic evaluation.
By Fleeman N, McLeod C, Bagust A, Beale S, Boland A, Dundar Y, et al.
-
Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer.
By Mowatt G, Zhu S, Kilonzo M, Boachie C, Fraser C, Griffiths TRL, et al.
-
Effectiveness and cost-effectiveness of arthroscopic lavage in the treatment of osteoarthritis of the knee: a mixed methods study of the feasibility of conducting a surgical placebo-controlled trial (the KORAL study).
By Campbell MK, Skea ZC, Sutherland AG, Cuthbertson BH, Entwistle VA, McDonald AM, et al.
-
A randomised 2 × 2 trial of community versus hospital pulmonary rehabilitation for chronic obstructive pulmonary disease followed by telephone or conventional follow-up.
By Waterhouse JC, Walters SJ, Oluboyede Y, Lawson RA.
-
The effectiveness and cost-effectiveness of behavioural interventions for the prevention of sexually transmitted infections in young people aged 13–19: a systematic review and economic evaluation.
By Shepherd J, Kavanagh J, Picot J, Cooper K, Harden A, Barnett-Page E, et al.
-
Dissemination and publication of research findings: an updated review of related biases.
By Song F, Parekh S, Hooper L, Loke YK, Ryder J, Sutton AJ, et al.
-
The effectiveness and cost-effectiveness of biomarkers for the prioritisation of patients awaiting coronary revascularisation: a systematic review and decision model.
By Hemingway H, Henriksson M, Chen R, Damant J, Fitzpatrick N, Abrams K, et al.
-
Comparison of case note review methods for evaluating quality and safety in health care.
By Hutchinson A, Coster JE, Cooper KL, McIntosh A, Walters SJ, Bath PA, et al.
-
Clinical effectiveness and cost-effectiveness of continuous subcutaneous insulin infusion for diabetes: systematic review and economic evaluation.
By Cummins E, Royle P, Snaith A, Greene A, Robertson L, McIntyre L, et al.
-
Self-monitoring of blood glucose in type 2 diabetes: systematic review.
By Clar C, Barnard K, Cummins E, Royle P, Waugh N.
-
North of England and Scotland Study of Tonsillectomy and Adeno-tonsillectomy in Children (NESSTAC): a pragmatic randomised controlled trial with a parallel non-randomised preference study.
By Lock C, Wilson J, Steen N, Eccles M, Mason H, Carrie S, et al.
-
Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial.
By Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FGR, Gillespie I, et al.
-
A randomised controlled multicentre trial of treatments for adolescent anorexia nervosa including assessment of cost-effectiveness and patient acceptability – the TOuCAN trial.
By Gowers SG, Clark AF, Roberts C, Byford S, Barrett B, Griffiths A, et al.
-
Randomised controlled trials for policy interventions: a review of reviews and meta-regression.
By Oliver S, Bagnall AM, Thomas J, Shepherd J, Sowden A, White I, et al.
-
Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery: a systematic review.
By McDaid C, Maund E, Rice S, Wright K, Jenkins B, Woolacott N.
-
A systematic review of outcome measures used in forensic mental health research with consensus panel opinion.
By Fitzpatrick R, Chambers J, Burns T, Doll H, Fazel S, Jenkinson C, et al.
-
The clinical effectiveness and cost-effectiveness of topotecan for small cell lung cancer: a systematic review and economic evaluation.
By Loveman E, Jones J, Hartwell D, Bird A, Harris P, Welch K, et al.
-
Antenatal screening for haemoglobinopathies in primary care: a cohort study and cluster randomised trial to inform a simulation model. The Screening for Haemoglobinopathies in First Trimester (SHIFT) trial.
By Dormandy E, Bryan S, Gulliford MC, Roberts T, Ades T, Calnan M, et al.
-
Early referral strategies for management of people with markers of renal disease: a systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis.
By Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, et al.
-
A randomised controlled trial of cognitive behaviour therapy and motivational interviewing for people with Type 1 diabetes mellitus with persistent sub-optimal glycaemic control: A Diabetes and Psychological Therapies (ADaPT) study.
By Ismail K, Maissi E, Thomas S, Chalder T, Schmidt U, Bartlett J, et al.
-
A randomised controlled equivalence trial to determine the effectiveness and cost–utility of manual chest physiotherapy techniques in the management of exacerbations of chronic obstructive pulmonary disease (MATREX).
By Cross J, Elender F, Barton G, Clark A, Shepstone L, Blyth A, et al.
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
Prioritisation Strategy Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Dr Bob Coates, Consultant Advisor, NETSCC, HTA
-
Dr Andrew Cook, Consultant Advisor, NETSCC, HTA
-
Dr Peter Davidson, Director of Science Support, NETSCC, HTA
-
Professor Robin E Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Professor Paul Glasziou, Professor of Evidence-Based Medicine, University of Oxford
-
Dr Nick Hicks, Director of NHS Support, NETSCC, HTA
-
Dr Edmund Jessop, Medical Adviser, National Specialist, National Commissioning Group (NCG), Department of Health, London
-
Ms Lynn Kerridge, Chief Executive Officer, NETSCC and NETSCC, HTA
-
Dr Ruairidh Milne, Director of Strategy and Development, NETSCC
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Ms Pamela Young, Specialist Programme Manager, NETSCC, HTA
HTA Commissioning Board
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Director, Medical Care Research Unit, University of Sheffield
-
Senior Lecturer in General Practice, Department of Primary Health Care, University of Oxford
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics, Queen Mary, University of London
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Nicky Cullum, Director of Centre for Evidence-Based Nursing, University of York
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, University of Sheffield
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford?
-
Professor Stuart Logan, Director of Health & Social Care Research, The Peninsula Medical School, Universities of Exeter and Plymouth
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, Univeristy of Oxford
-
Professor Ian Roberts, Professor of Epidemiology & Public Health, London School of Hygiene and Tropical Medicine
-
Professor Mark Sculpher, Professor of Health Economics, University of York
-
Professor Helen Smith, Professor of Primary Care, University of Brighton
-
Professor Kate Thomas, Professor of Complementary & Alternative Medicine Research, University of Leeds
-
Professor David John Torgerson, Director of York Trials Unit, University of York
-
Professor Hywel Williams, Professor of Dermato-Epidemiology, University of Nottingham
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Professor of Evidence-Based Medicine, University of Oxford
-
Consultant Paediatrician and Honorary Senior Lecturer, Great Ormond Street Hospital, London
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, Imaging Science and Biomedical Engineering, Cancer & Imaging Sciences, University of Manchester
-
Mr A S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital
-
Dr Dianne Baralle, Consultant & Senior Lecturer in Clinical Genetics, Human Genetics Division & Wessex Clinical Genetics Service, Southampton, University of Southampton
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Service User Representative
-
Professor Anthony Robert Kendrick, Professor of Primary Medical Care, University of Southampton
-
Dr Susanne M Ludgate, Director, Medical Devices Agency, London
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee
-
Dr David Mathew Service User Representative
-
Dr Michael Millar, Lead Consultant in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mr Stephen Pilling, Director, Centre for Outcomes, Research & Effectiveness, University College London
-
Mrs Una Rennard, Service User Representative
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Ultrasound Department, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr W Stuart A Smellie, Consultant, Bishop Auckland General Hospital
-
Professor Lindsay Wilson Turnbull, Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Dr Alan J Williams, Consultant in General Medicine, Department of Thoracic Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Neuroscience and Mental Health Board
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Disease Prevention Panel
-
Medical Adviser, National Specialist Commissioning Advisory Group (NSCAG), Department of Health
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook Clinical Programmes Director, Bazian Ltd, London
-
Dr Elizabeth Fellow-Smith, Medical Director, West London Mental Health Trust, Middlesex
-
Dr Colin Greaves Senior Research Fellow, Peninsular Medical School (Primary Care)
-
Dr John Jackson, General Practitioner, Parkway Medical Centre, Newcastle upon Tyne
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Chris McCall, General Practitioner, The Hadleigh Practice, Corfe Mullen, Dorset
-
Miss Nicky Mullany, Service User Representative
-
Dr Julie Mytton, Locum Consultant in Public Health Medicine, Bristol Primary Care Trust
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Service User Representative
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Ms Kay Pattison NHS R&D Programme/DH, Leeds
-
Dr Caroline Stone, Programme Manager, Medical Research Council
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust, Bristol
-
Reader in Wound Healing and Director of Research, University of Leeds, Leeds
-
Professor Bipin Bhakta Charterhouse Professor in Rehabilitation Medicine, University of Leeds, Leeds
-
Mrs Penny Calder Service User Representative
-
Professor Paul Carding, Professor of Voice Pathology, Newcastle Hospital NHS Trust, Newcastle
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry, London
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol, Bristol
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Professor Christopher Griffiths, Professor of Primary Care, Barts and the London School of Medicine and Dentistry, London
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester, Manchester
-
Dr Peter Martin, Consultant Neurologist, Addenbrooke’s Hospital, Cambridge
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham, Nottingham
-
Dr Kate Radford, Division of Rehabilitation and Ageing, School of Community Health Sciences. University of Nottingham, Nottingham
-
Mr Jim Reece, Service User Representative
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton, Southampton
-
Dr Pippa Tyrrell, Stroke Medicine, Senior Lecturer/Consultant Stroke Physician, Salford Royal Foundation Hospitals’ Trust, Salford
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford, Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University, Cardiff
-
Dr Phillip Leech, Principal Medical Officer for Primary Care, Department of Health , London
-
Ms Kay Pattison, Section Head R&D, DH, Leeds
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Dr Ursula Wells PRP, DH, London
Interventional Procedures Panel
-
Consultant Surgeon & Honorary Clinical Lecturer, University of Sheffield
-
Mr David P Britt, Service User Representative, Cheshire
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Mr Michael Thomas, Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Mrs Isabel Boyer, Service User Representative, London
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Unit Manager, Pharmacoepidemiology Research Unit, VRMM, Medicines & Healthcare Products Regulatory Agency
-
Mrs Nicola Carey, Senior Research Fellow, School of Health and Social Care, The University of Reading
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Professor Robin Ferner, Consultant Physician and Director, West Midlands Centre for Adverse Drug Reactions, City Hospital NHS Trust, Birmingham
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr Bill Gutteridge, Medical Adviser, London Strategic Health Authority
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Yoon K Loke, Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Dr Martin Shelly, General Practitioner, Leeds, and Associate Director, NHS Clinical Governance Support Team, Leicester
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Mr David Symes, Service User Representative
-
Ms Kay Pattison, Section Head, NHS R&D Programme, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Dr Ursula Wells, Principal Research Officer, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Service User Representative, Gloucestershire
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician & Cardiologist, County Durham & Darlington Foundation Trust
-
Mr John Needham, Service User, Buckingmashire
-
Ms Mary Nettle, Mental Health User Consultant, Gloucestershire
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Howard Ring, Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry & Warwickshire Partnership Trust
-
Dr Alastair Sutcliffe, Senior Lecturer, University College London
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Ms Kay Pattison, Section Head, R&D, DH, Leeds
-
Dr Morven Roberts, Clinical Trials Manager, MRC, London
-
Professor Tom Walley, HTA Programme Director, Liverpool
-
Dr Ursula Wells, Policy Research Programme, DH, London
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation for Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Dr Katherine Darton, Information Unit, MIND – The Mental Health Charity, London
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Mr George Levvy, Chief Executive, Motor Neurone Disease Association, Northampton
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Rajan Madhok, Medical Director and Director of Public Health, Directorate of Clinical Strategy & Public Health, North & East Yorkshire & Northern Lincolnshire Health Authority, York
-
Professor Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, National Co-ordinator, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Susan Schonfield, Consultant in Public Health, Hillingdon Primary Care Trust, Middlesex
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington