Notes
Article history
This themed issue of the Health Technology Assessment journal series contains a collection of research commissioned by the NIHR as part of the Department of Health’s (DH) response to the H1N1 swine flu pandemic. The NIHR through the NIHR Evaluation Trials and Studies Coordinating Centre (NETSCC) commissioned a number of research projects looking into the treatment and management of H1N1 influenza. NETSCC managed the pandemic flu research over a very short timescale in two ways. Firstly, it responded to urgent national research priority areas identified by the Scientific Advisory Group in Emergencies (SAGE). Secondly, a call for research proposals to inform policy and patient care in the current influenza pandemic was issued in June 2009. All research proposals went through a process of academic peer review by clinicians and methodologists as well as being reviewed by a specially convened NIHR Flu Commissioning Board.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
Chapter 1 Introduction
In the last century, there were three pandemics (global epidemics) of influenza (1918–19, 1957–58, 1968–69), with these resulting in considerable morbidity and mortality; the numbers of deaths in these pandemics have been estimated at 20–40 million, 1 million and 1 million, respectively. The lack of herd immunity to the novel influenza viruses implicated (i.e. H1N1, H2N2 and H3N2) is believed to have been a key factor contributing to these very high numbers of deaths. 1 The influenza A subtype: H1N1 virus, which emerged in Mexico in March 2009, was subsequently declared a pandemic by the World Health Organization in June 2009. 2
In the light of data that incentivised immunisation programmes delivered in primary health-care settings being shown to be acceptable (as evidenced by high uptake rates) and effective in reducing morbidity and mortality,3 and evidence that seasonal influenza vaccination has been shown to reduce the risk of hospitalisation and death from pneumonia or influenza by 27% and 48% respectively,4 the Chief Medical Officer (CMO) for England and the Department of Health instituted a targeted vaccination programme. 5 This was mirrored in Scotland by the CMO (Scotland) and Scottish Government. Production of influenza A (H1N1)v vaccinations began soon after outbreaks in the USA and Europe, with two vaccines being adopted for the UK national immunisation programme: Pandemrix (GlaxoSmithKline), which requires one dose, and Celvapan (Baxter Healthcare), which requires two doses at least 3 weeks apart. The vaccination process in the UK began on 22 October 2009 and was initially offered to frontline health-care workers and pregnant women; those with underlying health conditions that may predispose (and in particular people with respiratory disease) (Table 1) who were at increased risk of serious illness or death from influenza-like illness were also targeted in this first phase. In December 2009, phase II of the immunisation programme targeted children aged between 6 months and 5 years to receive the vaccination.
| Disease group | Medical codes |
|---|---|
| Chronic respiratory disease (including asthma) | Read codes: H33 and below, H3.., H31.., H32.., H34.., H35.., H36.., H37.., H38.., H3y.., H3z.., C370., H40.., H41.., H42.., H43.., H44.., H45.., H46.., H47y0, H48.. H4y.., H4z.., H5410., H55.., H563., H57.., H583., H591. H592., H593., Hyu3., Hyu40, Hyu41, Hyu5. |
| Chronic heart disease | Read codes: G3 and below, G58 and below, G21 and below, G220. G222., G55 and below, G5yy9, G5yyA, G23 and below, G41 and below, G1 and below, P5 and below, P60 and below, P61 and below, P62 and below, P63 and below, P64 and below, P65 and below, P66 and below, P67 and below, P68 and below, P6W and below, P6X and below, P6y.., P6y0., P6y1., P6y2., P6y3 and below, P6y63, P6y64, P6y6z, P6yy and below, P6z and below, 33BA. |
| Chronic kidney disease | Read codes: 1z12., 1z13., 1z13. 1z15, 1z16., 1z1B., 1z1C., 1z1D., 1z1E., 1z1F., 1Z1G., 1z1H., 1Z1J. 1Z1K., 1z1L., K01 and below, K02 and below, K0A3 and below, K05 and below, K0D.., 7B00 and below, 7B012, 7B015, 7B063, 8L50., SP083, TB001, ZV420 |
| Chronic liver disease | Read codes: J6…, J61 and below, J62y., J62z., J6353, J6354, J6355, J6356, J63B., PB61 and below, PB63 and below, PB6y1 |
| Chronic neurological disease | G51.., G610., G611., G612., G613., G614., G615., G616., G618., G61X., G61X0, G61X1, G61z.., G63y0, G63y1, G64.., G640. G6400, G641., G6410, G64z., G64z0, G64z1, G64z2, G64z3, G64z4, G66 and below, G6760., G6W.. |
| Immunosuppression | Read codes: PK01., 14N7., 7840 and below, D4154, D4156, 2J30., Drugs: alkylating drugs cytotoxic antibiotics antimetabolites vinca alkaloids and etoposide, other cytotoxix drugs, antagonists, cytotoxic immunosuppressants other immunosuppressants, other antineoplastic agents, leflunomide |
| Diabetes | Drugs: short-acting insulin preparations, medium/long-acting insulins sulphonylureas, biguanides repaglinide Rosiglitazone pioglitazone nateglinide short with intermediate-acting insulins |
| Pregnancy | Read code: 62… |
| Influenza hospitalisation | ICD10: J10,J100,J101,J108,J11,J110,J111,J118 |
| Pneumonia hospitalisation | ICD10: J12,J13,J14,J15,J16,J17,J18 |
| COPD hospitalisation | ICD10:J40,J41,J42,J43,J44,J45,J46,J47,J80,J81,J82,J83,J84,J60,J61,J62,J63,J64,J65,J66,J67,J68,J69,J70, |
| CRH | ICD10:I05,I06,I07,I08,I09,I10,I11,I12,I13,I15,I20,I21,I22,I23,I24,I25,I26,I27,I28,I30,I31,I32,I33,I34,I35,I36,I37,I38,I39,I40,I41,I42,I43,I44,I45,I46,I47,I48,I49,I50,I51,I52,I60,I61,I62,I63,I64,I65,I66,I67,I68,I69 |
| Trauma (including bone fracture), appendicitis or hernia hospitalisation | ICD10: S, T K35,K36,K37,K38,K40,K41,K42,K43,K44,K45,K46 |
Observational studies can be used to estimate the effectiveness of health-care interventions in situations where it is unethical and/or not feasible to mount more rigorous experimental studies, as is the case in the context of the 2009 HIN1 pandemic. 6 Building on related pilot work,3 we sought to determine influenza A (H1N1)v vaccine uptake and effectiveness for 2009 in the Scottish population using a sentinel surveillance network of general practices, the Practice Team Information (PTI) network.
Chapter 2 Methods
Overview of methods
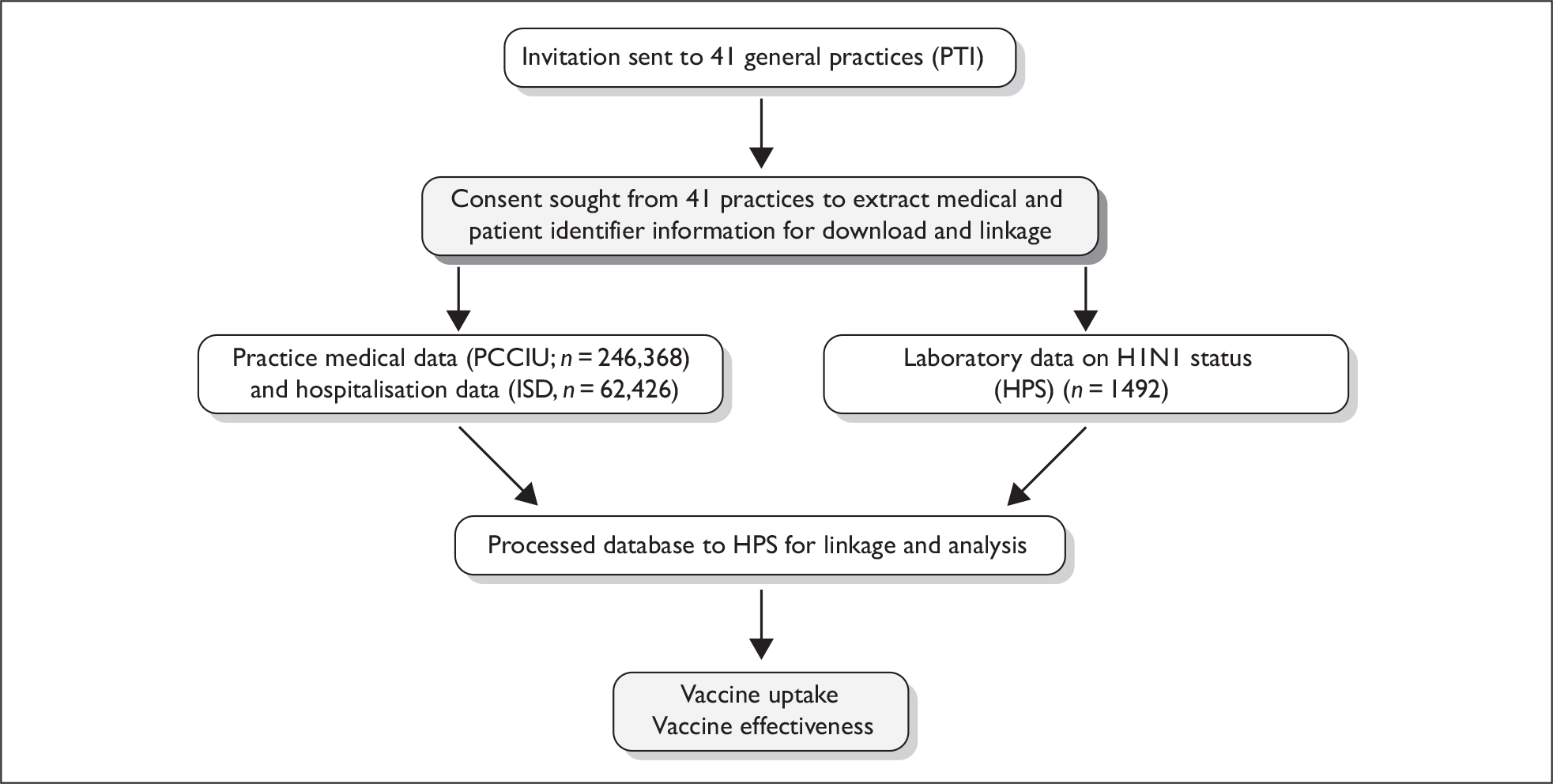
The impact of the Scottish 2009 pandemic H1N1 vaccination programme was evaluated using a retrospective cohort design to study vaccination effectiveness (VE). This was achieved by ascertaining the uptake of the influenza A (H1N1)v vaccine by the relevant at-risk populations, i.e. patients with relevant comorbidities and pregnant women, and assessing the reduction in the expected incidence of influenza-related serious morbidity. Figure 1 gives an overview of the study design and the data sources used.
FIGURE 1.
Flow diagram for the VIPER study. HPS, Health Protection Scotland; ISD, Information Services Division; PCCIU, Primary Care Clinical Informatics Unit; PTI, Practice Team Information (network).
Setting
The PTI network of 41 general practices covers a 5% broadly representative sample of the Scottish population (n = 246,368). These practices receive an annual financial incentive to record practice data electronically. 7 Data from practices within Scotland have shown to be of high quality and useful for epidemiological research. 8 The completeness of capture of contacts and accuracy of clinical event coding in primary care (using Read codes) has been found to be above 91%. 9 Using the unique Community Health Index (CHI) number, general practice patient-level data were extracted and linked to the Scottish Morbidity Record (SMR) catalogue, which has information on all inpatient hospitalisations within Scotland [as well as information on death certification linked from the General Register Office for Scotland (GROS)]. 10 Hospital data are reliable from 1981, with completeness and accuracy rates exceeding 90%. 11 We also used the Health Protection Scotland (HPS) data set, which consists of all laboratory-confirmed cases of influenza A (H1N1)v from the general practices. We determined key characteristics of each identified patient in the cohort: sex, age (0–4, 5–14, 15–44, 45–64, 65–74 and 75+ years), socioeconomic status (Carstairs deprivation category scores12 expressed as deciles: 1 = most affluent and 10 = most deprived, and quintiles: 1 = most affluent and 5 = most deprived), clinical at-risk groups (i.e. chronic respiratory disease, chronic heart disease, chronic kidney disease, chronic liver disease, chronic neurological disease, immunosuppression and diabetes) and pregnancy (at start date of H1N1 vaccination). The practices were also asked to collect information on health-care worker status and immunisation (in addition to information routinely recorded as part of usual clinical care).
Interventions
The study involved a quantitative evaluation of the aspect of the H1N1 vaccination programme implemented through general practice during 2009. General practices were given financial incentives to record and code additional data electronically, over and above that routinely recorded for clinical care or as part of the PTI project including: H1N1 vaccination status, age, deprivation status, pregnancy, and, where it was feasible, health-worker status.
Outcome measures
During 2009, swabbing was undertaken to test patients for influenza A (H1N1)v as part of a sentinel swabbing scheme. This was carried out by the practices on a convenience sampling basis, with each practice being encouraged to submit around 10 samples per week from patients presenting with influenza-like illness. To calculate effectiveness, patient swab data were linked to the patient data (from primary and secondary care) using the CHI number.
We assessed the vaccination uptake in the relevant populations, i.e. children (< 5 years), pregnant women, health-care workers and patients with at-risk comorbidities, recorded by general practice prior to 25 December 2009, and influenza, pneumonia, chronic obstructive pulmonary disease (COPD) and cardiovascular-related hospitalisations (CRHs) (both individually and as composite outcomes, for emergency admissions and any admission reason),13 and, for comparison, hospitalisation for other serious morbidity (e.g. trauma, appendicitis and hernia) in vaccinated and unvaccinated patients (Table 1). For patients who remained unvaccinated and who had not been hospitalised, the risk period of interest was 65 days, i.e. from 21 October 2009 (date of first vaccination in the data set) to 24 December 2009 (date of last recorded hospital admission and the study census end point – the vaccination programme continued beyond this time). For those who had been vaccinated, the risk period of interest began 7 days after the vaccination date. [Conventionally, the seasonal influenza vaccine is thought to require 14 days to establish a protective effect; however, there is evidence from ongoing studies (involving HPS and the Health Protection Agency, Colindale) that 7 days is probably sufficient for the influenza A (H1N1)v vaccine.] Hospital admissions before the date of vaccination among those vaccinated were ignored. For those with an admission, the risk period ended on admission. This type of analysis was required so as to ensure that hospitalisations before vaccination could not be attributed to a vaccine effect.
Statistical methods
Odds ratios (adjusted for age, sex and deprivation) were calculated for differences in vaccine uptake rates between groups of patients. For VE using information from linked virological swab data, a logistic regression model was fitted, adjusting for the effects of gender, age, deprivation and being in an at-risk morbidity group. Some of these patients did not receive the influenza A (H1N1)v vaccine, some received the vaccine (but after they were tested) and some received the vaccine before they were tested. We therefore measured VE first by comparing swabs taken after vaccination with swabs taken before vaccination for all vaccinated individuals, and second, by comparing swabs taken after vaccination among those vaccinated with swabs taken among those never vaccinated. A delay of 7 days after vaccination was used to establish a protective effect of the influenza A (H1N1)v vaccine. Confidence intervals for the rate ratio (RR) and tests of the differences between two rates were carried out using the ‘MIDP method’ in the ‘RR’ function and rate2by2.test function respectively, using the ‘epitools’ package in r. 14 For small samples, confidence intervals for the RR were estimated using the excel workbook. 15
Illness RRs are the ratio of the rate of first admission to hospital in the vaccinated compared with the rate of first admission to hospital among those who did not receive the vaccine. This is a direct measure of VE. The unadjusted estimate of VE = (1 – RR) × 100. Adjusted RRs of VE for prevention of first hospitalisation were derived from Poisson regression models, adjusting for gender, age, deprivation and clinical risk group. An adjustment to the standard error of the estimated effect to account for clustering of patients within practices was carried out using the ‘survey’ package in r. Statistical analysis was carried out using r version 2.9.0.
Summary of changes to the project protocol
We were unable in this interim analysis to calculate rates of mortality in vaccinated and unvaccinated patients, as only confirmed deaths prior to 30 September 2009 were available. A definitive analysis with a repeat linkage to SMR and HPS records will be required later in 2010.
We planned to use the ‘Farrington’ method, as detailed in our original project protocol, as we were confident of at least obtaining practice-level vaccination uptake data. 6 We were fortunate, however, to be able to obtain individual patient-level data and so could use the more robust cohort method as described in the statistical methods section above.
Chapter 3 Results
We recruited all 41 PTI practices with a combined list size of 246,368 patients. As only one practice software system, gpass, was used for the study, the general practitioner (GP) practices in this study are not representative of the spatial distribution of the population of Scotland. There was an under-representation of practices from the north-east of Scotland, in particular Orkney, Grampian and Tayside areas (where practices tend to use other GP software systems). There was also a preponderance of practices in west and central Scotland, which have higher levels of socioeconomic deprivation (Table 2).
| Total patients (% within category) | No. with at least one at-risk comorbidity group, n (%, 95% CI) | Vaccine uptake for not-at-risk comorbidity group, n (%, 95% CI) | Vaccine uptake for at-risk comorbidity group, n (%, 95% CI) | |
|---|---|---|---|---|
| Sex | ||||
| Female | 124,177 (50.4) | 30,400 (24.5, 24.2 to 24.7) | 4823 (5.1, 5.0 to 5.3) | 11,557 (38.0, 37.5 to 38.6) |
| Male | 122,193 (49.6) | 29,321 (24.0, 23.8 to 24.2) | 2356 (2.5, 2.4 to 2.6) | 10,840 (37.0, 36.4 to 37.5) |
| Age group (years) | ||||
| 0–4 | 13,245 (5.4) | 434 (3.3, 3.0 to 3.6) | 384 (3.0, 2.7 to 3.3) | 207 (47.7, 43.0 to 52.4) |
| 5–34 | 25,932 (10.5) | 3951 (15.2, 14.8 to 15.7) | 288 (1.3, 1.2 to 1.5) | 1166 (29.5, 28.1 to 31.0) |
| 35–49 | 103,888 (42.2) | 17,666 (17.0, 16.8 to 17.2) | 2674 (3.1, 3.0 to 3.2) | 4006 (22.7, 22.1 to 23.3) |
| 50–64 | 64,823 (26.3) | 16,101 (24.8, 24.5 to 25.2) | 2571 (5.3, 5.1 to 5.5) | 7933 (49.3, 48.5 to 50.0) |
| 65–74 | 20,625 (8.4) | 10,089 (48.9, 48.2 to 49.6) | 767 (7.3, 6.8 to 7.8) | 4946 (49.0, 48.0 to 50.6) |
| ≥ 75 | 17,855 (7.2) | 6375 (64.3, 63.6 to 65.0) | 495 (7.8, 7.1 to 8.4) | 4139 (36.1, 35.2 to 36.9) |
| Deprivation decile | ||||
| 1 | 15,538 (6.3) | 3413 (22.0, 21.3 to 22.6) | 511 (4.2, 3.9 to 4.6) | 1549 (45.4, 43.7 to 47.1) |
| 2 | 11,594 (4.7) | 2277 (19.6, 18.9 to 20.4) | 248 (2.7, 2.4 to 3) | 694 (30.5, 28.6 to 32.4) |
| 3 | 12,818 (5.2) | 3194 (24.9, 24.2 to 25.7) | 572 (5.9, 5.5 to 6.4) | 1381 (43.2, 41.5 to 45) |
| 4 | 11,693 (4.7) | 2511 (21.5, 20.7 to 22.2) | 222 (2.4, 2.1 to 2.8) | 842 (33.5, 31.7 to 35.4) |
| 5 | 38,742 (15.7) | 8996 (23.2, 22.8 to 23.6) | 1060 (3.6, 3.4 to 3.8) | 3302 (36.7, 35.7 to 37.7) |
| 6 | 28,558 (11.6) | 7281 (25.5, 25 to 26) | 963 (4.5, 4.3 to 4.8) | 2704 (37.1, 36 to 38.3) |
| 7 | 43,324 (17.6) | 11,318 (26.1, 25.7 to 26.5) | 1299 (4.1, 3.8 to 4.3) | 4310 (38.1, 37.2 to 39) |
| 8 | 18,111 (7.4) | 4430 (24.5, 23.8 to 25.1) | 438 (3.2, 2.9 to 3.5) | 1358 (30.7, 29.3 to 32) |
| 9 | 41,831 (17.0) | 10,459 (25, 24.6 to 25.4) | 1288 (4.1, 3.9 to 4.3) | 3980 (38.1, 37.1 to 39) |
| 10 | 24,159 (9.8) | 5842 (24.2, 23.6 to 24.7) | 578 (3.2, 2.9 to 3.4) | 2277 (39.0, 37.7 to 40.2) |
| Pregnant women | 2203 (4.3) | 360 (16.3, 14.9 to 17.9) | 575 (31.2, 29.1 to 33.4) | 151 (41.9, 37.0 to 47.1) |
| Health-care worker | 1314 (0.8) | 347 (26.4, 24.0 to 28.8) | 229 (23.6, 21.0 to 26.4) | 118 (34.0, 29.0 to 39.0) |
Overall, 24.2% individuals (n = 59,721) were deemed to be in the at-risk category on the basis of existing illnesses; 4.3% (n = 2203) of 15- to 44-year-old women (n = 51,404) were found to be pregnant and 0.8% (n = 1314) of people of working age (18–64 years inclusive, n = 159,873) registered with the practices worked for the NHS (Table 2).
Vaccine uptake
Influenza A (H1N1)v vaccine uptake estimates for the whole population as obtained at 25 December 2009 were 12.0% (95% CI 11.9 to 12.1) (Table 2). These uptake estimates reflect the early stage of the H1N1v vaccination programme, which continued into 2010. For those patients in an at-risk comorbidity group, the uptake rate was 37.5% (95% CI 37.1 to 37.9). Men and younger people (outwith the youngest age group 0–4 years) were less likely to take up the vaccine than women, infants and older adults (Table 3). Uptake rates among pregnant women and health-care workers can be found in Table 2. More male [odds ratio (OR) 2.67, 95% CI 1.44 to 4.96] and older (45- to 64-years-olds; 1.67, 95% CI 0.87 to 3.19) health-care workers were vaccinated than female and younger (16- to 44-year-old) health-care workers.
| OR | 95% CI | |
|---|---|---|
| Gender | ||
| Females | 1.00 | |
| Males | 0.96 | 0.92 to 0.99 |
| Age group (years) | ||
| 0–4 | 1.00 | |
| 5–14 | 0.46 | 0.35 to 0.60 |
| 15–44 | 0.32 | 0.24 to 0.43 |
| 45–64 | 1.06 | 0.76 to 1.47 |
| 65–74 | 1.05 | 0.71 to 1.54 |
| ≥ 75 | 0.61 | 0.42 to 0.90 |
| Socioeconomic statusa | ||
| Quintile 1 | 1.00 | |
| Quintile 2 | 0.93 | 0.55 to 1.58 |
| Quintile 3 | 0.86 | 0.47 to 1.59 |
| Quintile 4 | 0.84 | 0.56 to 1.27 |
| Quintile 5 | 0.92 | 0.56 to 1.52 |
Virology
Among the 1492 patients swabbed, 467 were positive for H1N1, giving an influenza A (H1N1)v positive rate of 31.3% (95% CI 29.0 to 33.7). Out of the 1492 patients, 1301 (87.2%; 95% CI 85.5 to 88.9) were never vaccinated, 160 (10.7%; 95% CI 9.2 to 12.3) were swabbed before being vaccinated, and 31 (2.1%; 95% CI 1.4 to 2.8) were tested after vaccination. The ORs in Table 4 show that during the study period those who were in a clinical risk group had an 82.0% (95% CI 37.0 to 141.0) increase in the odds of being positive for H1N1 compared with those with no clinical risk group. Those in age groups of less than 45 years of age were more likely to test positive for H1N1.
| Description | Adjusted OR | 95% CI |
|---|---|---|
| At-risk comorbidity | ||
| No | 1.00 | |
| Yes | 1.82 | 1.37 to 2.41 |
| Gender | ||
| Female | 1.00 | |
| Male | 1.00 | 0.79 to 1.27 |
| Age group (years) | ||
| 0–4 | 1.00 | |
| 5–14 | 3.87 | 2.65 to 5.72 |
| 15–44 | 1.48 | 1.05 to 2.10 |
| 45–64 | 0.95 | 0.95 to 1.46 |
| 65–74 | 0.21 | 0.05 to 0.61 |
| ≥ 75 | 0.08 | 0.00 to 0.37 |
| Deprivation quintile | ||
| 1 | 1.00 | |
| 2 | 0.91 | 0.54 to 1.52 |
| 3 | 1.06 | 0.67 to 1.69 |
| 4 | 0.92 | 0.58 to 1.48 |
| 5 | 0.91 | 0.58 to 1.44 |
Only one vaccinated patient swabbed after their vaccination tested positive for influenza A (H1N1)v (Table 5). For patients not vaccinated during the study period more patients within a clinical at-risk comorbidity group tested positive for H1N1 than those outwith the clinical at-risk groups (p < 0.01).
| Description | Swab test result for H1N1 | |
|---|---|---|
| Total tested (n) | Positive, n (%, 95% CI) | |
| No clinical risk group | ||
| No vaccination | 996 | 323 (32.4, 29.6 to 35.4) |
| Vaccinated: swabbed after vaccination | 11 | 0 (0.0, 0.0 to 25.9) |
| Vaccinated: swabbed before vaccination | 31 | 0 (0.0, 0.0 to 11.0) |
| At least one clinical risk group | ||
| No vaccination | 305 | 126 (41.3, 35.6 to 46.9) |
| Vaccinated: swabbed after vaccination | 20 | 1 (5.0, 0.3 to 23.6) |
| Vaccinated: swabbed before vaccination | 129 | 17 (13.2, 8.4 to 20.1) |
Comparing swabs taken after vaccination with swabs taken before vaccination for all vaccinated individuals, there was a VE of 70.0% (95% CI –58.0 to 98.0). By comparing swabs taken after vaccination among those vaccinated with swabs taken among those never vaccinated, the VE was found to be 95.0% (95% CI 76.0 to 100.0). The former vaccine effect is estimated with much lower precision, as it is based upon fewer cases.
Influenza A (H1N1)v vaccination effectiveness
During the study period there were 2739 admissions to hospital in our cohort, of which 1241 were emergency admissions. All emergency hospitalisations for influenza and pneumonia occurred in patients who did not receive the vaccine (Table 6). Patients with an at-risk comorbidity were 12 times more likely to be hospitalised than not-at-risk patients for the composite outcome: influenza, pneumonia, COPD and CRH (0.43 versus 5.18 per 100,000 person-days). Patients who were at risk and vaccinated were less likely than their unvaccinated counterparts to be admitted into hospital for the composite outcome. Vaccinated patients were more likely to be admitted to hospital for trauma.
| Hospitalisation | Vaccination status | Hospitalisation events | |||||
|---|---|---|---|---|---|---|---|
| All patients | Not in at-risk comorbidity group | At-risk comorbidity group | |||||
| Rate per 100,000 person-days (n) | Crude risk ratio (95% CI) | Rate per 100,000 person-days (n) | Crude risk ratio (95% CI) | Rate per 100,000 person-days (n) | Crude risk ratio (95% CI) | ||
| Influenza | No | 0.10 (14) | 1.00 | 0.05 (6) | 1.00 | 0.32 (8) | 1.00 |
| Yes | 0.00 (0) | 0.00 (0.00 to 7.10) | 0.00 (0) | 0.00 (0.00 to 49.23) | 0.00 (0) | 0.00 (0.00 to 3.12) | |
| Pneumonia | No | 0.25 (34) | 1.00 | 0.13 (14) | 1.00 | 0.81 (20) | 1.00 |
| Yes | 0.00 (0) | 0.00 (0.00 to 2.92) | 0.00 (0) | 0.00 (0.00 to 21.10) | 0.00 (0) | 0.00 (0.00 to 1.25) | |
| COPD | No | 0.39 (53) | 1.00 | 0.03 (3) | 1.00 | 2.03 (50) | 1.00 |
| Yes | 0.96 (5) | 2.51 (0.86 to 5.71) | 0.00 (0) | 0.00 (0.00 to 99.47) | 1.32 (5) | 0.67 (0.23 to 1.53) | |
| CRH | No | 0.65 (88) | 1.00 | 0.23 (25) | 1.00 | 2.56 (63) | 1.00 |
| Yes | 0.57 (3) | 0.93 (0.22 to 2.47) | 0.70 (1) | 3.50 (0.15 to 16.36) | 0.53 (2) | 0.22 (0.03 to 0.71) | |
| Influenza and pneumonia | No | 0.36 (48) | 1.00 | 0.18 (20) | 1.00 | 1.14 (28) | 1.00 |
| Yes | 0.00 (0) | 0.00 (0.00 to 2.07) | 0.00 (0) | 0.00 (0.00 to 14.77) | 0.00 (0) | 0.00 (0.00 to 0.89) | |
| Influenza, pneumonia and COPD | No | 0.75 (101) | 1.00 | 0.21 (23) | 1.00 | 3.17 (78) | 1.00 |
| Yes | 0.96 (5) | 1.32 (0.46 to 2.92) | 0.00 (0) | 0.00 (0.00 to 12.84) | 1.32 (5) | 0.43 (0.15 to 0.96) | |
| Influenza, pneumonia, COPD and CRH | No | 1.39 (187) | 1.00 | 0.43 (47) | 1.00 | 5.69 (140) | 1.00 |
| Yes | 1.53 (8) | 1.13 (0.51 to 2.14) | 0.70 (1) | 1.87 (0.08 to 8.40) | 1.85 (7) | 0.33 (0.14 to 0.66) | |
| Trauma-associated emergency admissiona | No | 1.60 (230) | 1.00 | 1.40 (163) | 1.00 | 2.52 (67) | 1.00 |
| Yes | 5.18 (30) | 3.40 (2.27 to 4.88) | 2.09 (4) | 1.96 (0.59 to 4.63) | 6.36 (26) | 2.54 (1.59 to 3.95) | |
| Any emergency admission | No | 8.99 (1209) | 1.00 | 6.42 (707) | 1.00 | 20.56 (502) | 1.00 |
| Yes | 6.14 (32) | 0.69 (0.47 to 0.96) | 5.58 (8) | 0.89 (0.40 to 1.66) | 6.35 (24) | 0.31 (0.20 to 0.46) | |
Statistically significant findings consistent with protection in recipients of influenza A (H1N1)v vaccine were evident in the adjusted VE seen for emergency admissions with a CRH alone, or in preventing an emergency admission for any one or more of influenza and pneumonia plus COPD and CRH (for all patients) (Table 7).
| Description | Unadjusted vaccine effectiveness, % (95% CI) | Adjusted vaccine effectiveness, % (95% CI)a |
|---|---|---|
| Influenza | 100.00 (∞ to 100.00) | 100.00 (∞ to 100.00) |
| Pneumonia | 100.00 (∞ to 100.00) | 100.00 (∞ to 100.00) |
| COPD | –144.01 (–526.65 to 4.98) | 40.61 (–57.91 to 77.66) |
| CRH | 11.84 (–167.58 to 70.79) | 71.11 (11.26 to 90.59) |
| Influenza and pneumonia | 100.00 (∞ to 100.00) | 100.00 (∞ to 100.00) |
| Influenza, pneumonia and COPD | –28.02 (–223.79 to 49.38) | 59.46 (–5.79 to 84.46) |
| Influenza, pneumonia, COPD and CRH | –10.61 (–169.34 to 54.41) | 64.69 (12.04 to 85.82) |
Chapter 4 Discussion
During the immediate period after the introduction of the influenza A (H1N1)v vaccination, more than one-third of patients registered in primary care with PTI practices and deemed to have an at-risk comorbidity were vaccinated for influenza A (H1N1)v. However, men and younger patients (outwith the 0–4 years age group) were less likely to be vaccinated. Our interim results suggest that during the study period, the vaccine seems to have been particularly effective for people with an at-risk comorbidity. This is reassuring as they were also more likely to be tested positive for having influenza A (H1N1)v (when compared with those not at risk) and to be at much higher rates of hospitalisation for severe complication of influenza. Of the patients tested using swabs after their vaccination, only one tested positive for influenza A (H1N1)v.
Our findings indicate that our estimated influenza A (H1N1)v VE is at least comparable to the seasonal influenza vaccine in preventing hospitalisation admission for: influenza and/or pneumonia (27%),16 influenza-like illness (27%) in all patients,4 acute respiratory disease and cardiovascular disease (97%) in high-risk patients,17 and medically attended acute respiratory illness in children (18% in those aged 18 months to 18 years). 18 As expected, overall uptake rates of vaccine reported in this study were similar to those reported by HPS in similar practices. 19 However, rates of vaccine uptake for health-care workers in occupational settings were lower than those reported by HPS, which relied on occupational health services reporting (as opposed to information captured by the practices). This under-reporting probably reflects a partial success in practices recording of occupational status on patients records, and therefore any inference from the occupational data should be treated with caution. Our findings that females and, outwith the youngest age group (0–4 years), older patients were more likely to be vaccinated is similar to other studies looking at uptake of vaccines by different groups. 20 This is likely to be due to greater levels of perceived susceptibility to, and perceived severity of, influenza A (H1N1)v and a greater belief in the effectiveness of recommended behaviours to protect against the disease. There is also evidence that greater levels of state anxiety and greater trust in authorities are associated with increased vaccine uptake. Our finding that the vaccine seemed to be particularly effective in patients with an at-risk comorbidity endorses the targeted approach advised by the Joint Committee on Vaccination and Immunisation and adopted by the CMOs for England, Northern Ireland, Scotland and Wales and their respective Government Health Departments/Directorates. 5
There are benefits as well as drawbacks to the evaluation of an influenza vaccine campaign based on just a single postimplementation season. Retrospective ascertainment of vaccination status is less dependable, of course, than prospective clarification, but the use of GP records is more reliable than self-reporting methods,21 as is the electronic recording of uptake rates in the sample PTI population. Also, the relatively small size of the Scottish population makes it feasible to centrally collate almost all cases of H1N1 disease, allowing for completeness of reporting. Observational studies can be used to assess the effects of health-care interventions without influencing the care that is provided or the patients who receive it;6 therefore, when used in the assessment of vaccination programmes they have high external validity and broad generalisability. However, non-randomised studies, such as the current evaluation, are limited by the extent to which there may be dissimilarities between vaccinated and non-vaccinated persons, in both their likelihood of receiving vaccination and in their subsequent care and follow-up. Our findings that at-risk patients who received the vaccine were more likely to be admitted for a trauma-associated emergency (and were possibly more likely to be frail, thus leading to negative confounding) than those who did not may mean that there is some underestimate of VE. A further assessment of the possible impact of any bias caused by the preferential receipt of vaccine by relatively healthy individuals will need to be checked, however, outside the influenza season. 22 The results from the single outcome of emergency admission for cardiovascular-related illness or the use of the composite outcome: influenza, pneumococcal disease, COPD and cardiovascular disease should also be treated with some caution as it may have led to a healthy vaccine effect. For instance, within the high-risk group those at lower risk of heart disease or those who do not smoke (and hence are less likely to have COPD) may be more likely to be vaccinated. However, it is of note that other authors have considered the role of influenza in the generation of cardiac-related conditions and debated its contribution to the excess mortality observed each winter during the annual influenza season. 23–25 The lack of a recorded vaccination amongst health-care workers (which was probably carried out in occupational settings) may have biased the estimation of VE towards zero. The fact that PTI is based on a small sample of practices in Scotland means that the data collected may be subject to fluctuations as a result of any factors that have an impact locally, such as changes to the way that PTI practices manage their services. 9 However, apart from a reduction in precision, it is unlikely that the small sample size, or other associated factors, will have a substantial impact on the overall estimates of VE. A convenience sampling approach was used to collect data on patient H1N1 virological status rather than a more systematic sampling approach. This was adopted in recognition that the implementation of a surveillance programme could not affect routine clinical practice. Conventionally, the seasonal influenza vaccine is thought to require 14 days to establish a protective effect; however, there is evidence from ongoing studies (involving HPS and the Health Protection Agency, Colindale), that 7 days is probably sufficient for the influenza A (H1N1)v vaccine. A sensitivity analysis using the 14-day cut-off period was carried out with similar estimates of vaccine effect being found (results not presented).
Chapter 5 Implications for practice
Policy-makers and clinicians should be encouraged that the VE estimates obtained are comparable to those found for seasonal influenza, which should strengthen the evidence base for health-care practitioners involved in distributing vaccine in other countries.
Influenza A (H1N1)v immunisation in primary health-care settings is both effective and widely acceptable as evidenced by high uptake rates, and should continue to be the mainstay of disease prevention for at-risk patients.
Chapter 6 Research recommendations
Study time constraints resulting from the unexpected ‘slow-burn’ nature of the second wave of influenza A (H1N1)v and the later than expected roll-out of the vaccine meant that this analysis was limited to studying only the short-term effectiveness of the influenza A (H1N1)v vaccine. A further analysis encompassing the whole influenza season is required to cover more days of vaccination exposure, which will increase precision (with resulting narrower confidence intervals). Also, for pregnant women and under-5-year-olds, a further study using a greater time period of exposure is required to calculate and present meaningful results. We were also unable, in this study, to estimate whether the vaccine was effective in reducing mortality (as only mortality data for the prevaccination period were available). A future study that repeats this data linkage and allows the calculation of longer-term VE (in reducing both morbidity and mortality) should be undertaken later in 2010.
Chapter 7 Conclusions
Evidence from swabs submitted from patients in the cohort presenting with influenza-like illness in general practice suggests that the introduction of influenza A (H1N1)v vaccine in Scotland during 2009 was associated with a high degree of protection against influenza A (H1N1)v. In addition, receipt of influenza A (H1N1)v vaccine was associated with a reduction in both admission for cardiac-related conditions and for the combined category of influenza, pneumonia, COPD and cardiac conditions. Policy-makers ought to be encouraged that the VE estimates obtained are comparable to those found for seasonal influenza, and this possibly reflects the suitability of primary care as a means of delivery. Additionally, as the first large-scale demonstration of effectiveness in a UK population, the results should strengthen the evidence base for health-care practitioners involved in distributing vaccine in other countries. Influenza A (H1N1)v immunisation in primary health-care settings is both effective and widely acceptable, as evidenced by high uptake rates, and should continue to be the mainstay of disease prevention for at-risk patients. Whether the reduced incidence of severe complications of influenza or death will persist will be apparent only when data from later in 2010 are analysed.
Acknowledgements
The authors would like to thank Dr Martin Donaghy, Dr Oliver Blatchford, Arlene Reynolds, Heather Murdoch, Eisin McDonald, Alison Potts, Ruth Thomson, Cathy Hunter, Lynne Otterson and Stuart Yule at HPS, the staff at all of the general practices and virus laboratories that contributed data to the study, and also Katie Wilde, Fiona Chaloner, Dr Margaret Watson, Carol Mackie, Alasdair Coutts, Alastair Soutar and Cristina de Cario at PCCIU, and Carole Morris and Bradley Kirby at ISD. We would also like to thank the Independent Steering Committee, which comprised Dr Neil Kelly and Dr Robert Milne.
Contribution of authors
Dr Colin Simpson (Chief Scientist Office, Health Services and Health of the Public, Postdoctoral Fellow, Epidemiology) was principal investigator and led the writing of this report. Professor Lewis Ritchie and Professor Aziz Sheikh (Professors of Primary Care) helped design the study and commented on drafts of the paper. Professor Chris Robertson (Professor of Statistics) and Dr Jim McMenamin (Consultant Epidemiologist) helped to design the study, carry out the analyses and write the paper.
Disclaimers
The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the Department of Health.
References
- Nicholls H. Pandemic influenza: the inside story. PLoS Biol 2006;4.
- Chan M. World now at start of 2009 influenza pandemic. Geneva: World Health Organization; 2009.
- Mooney JD, Weir A, McMenamin J, Ritchie LD, Macfarlane TV, Simpson CR, et al. The impact of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis 2008;8.
- Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med 2007;14:1373-81.
- Donaldson LJ, Rutter PD, Ellis BM, Greaves FEC, Mytton OT, Pebody RG, et al. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ 2009;339.
- Smith S, Sinclair D, Raine R, Reeves B, Smith S, Sinclair D, et al. Health care evaluation. London: Open University Press; 2005.
- Whitelaw FG, Nevin SL, Milne RM, Taylor RJ, Taylor MW, Watt AH. Completeness and accuracy of morbidity and repeat prescribing records held on general practice computers in Scotland. Br J Gen Pract 1996;46:181-6.
- Ekins-Daukes S, Helms PJ, Taylor MW, Simpson CR, McLay JS. Utility of routinely acquired primary care data for epidemiology and pharmacoepidemiology. Brit J Clin Pharmacol 2005;59:684-90.
- Information Services Division . General Practice – Practice Team Information 2008. www.isdscotland.org/pti (accessed November 2009).
- General Register Office for Scotland . GRO Statistics Library (population Estimates) n.d. www.gro-scotland.gov.uk/statistics/publications-and-data/populationestimates/index.html (accessed July 2005).
- Harley K, Jones C. Quality of Scottish Morbidity Record (SMR) data. Health Bull 1996;54:410-17.
- Carstairs V, Morris R. Deprivation and health in Scotland. Aberdeen: Aberdeen University Press; 1991.
- Fleming DM, Elliot AJ. Estimating the risk population in relation to influenza vaccination policy. Vaccine 2006;24:4378-85.
- Graham PL, Mengersen K, Morton AP. Confidence limits for the ratio of two rates based on likelihood scores: non-iterative method. Stat Med 2003;22:2071-83.
- Newcombe R. Confidence Intervals for Proportions and Related Quantities n.d. www.cardiff.ac.uk/medic/aboutus/departments/primarycareandpublichealth/ourresearch/resources/index.html (accessed January 2010).
- Jefferson T, Rivetti D, Rivetti A, Rudin M, Di Pietrantonj C, Demicheli V. Efficacy and effectiveness of influenza vaccines in elderly people: a systematic review. Lancet 2005;366:1165-74.
- Hak E, Buskens E, Nichol KL, Verheij TJM. Do recommended high-risk adults (18–64 years) benefit from a first influenza vaccination?. Vaccine 2006;24:2799-802.
- Holloran ME, Longini IM, Gaglani MJ, Piedra PA, Chu H, Herschler GB, et al. Estimating efficacy of trivalent, cold-adapted, influenza virus vaccine (CAIV-T) against influenza A (H1N1) and B using surveillance cultures. Am J Epidemiol 2003;158:305-11.
- Health Protection Scotland . Weekly Influenza Situation Report 2009. www.documents.hps.scot.nhs.uk/respiratory/swine-influenza/situation-reports/weekly-influenza-sitrep-2009–12–24.pdf (accessed January 2010).
- Bish A, Michie S. Demographic and attitudinal determinants of protective behaviours during a pandemic: a review. Br J Health Psych 2010. 10.1348/135910710X485826.
- Mangtani P, Shah A, Roberts JA. Validation of influenza and pneumococcal vaccine status in adults based on self-report. Epidemiol Infect 2007;135:139-43.
- Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol 2006;35:337-44.
- Madjid M, Aboshady I, Awan I, Litovsky S, Ward Casscells S. Influenza and cardiovascular disease: is there a causal relationship?. Tex Heart Inst J 2004;31:4-13.
- Mamas MA, Fraser D, Neyses L. Cardiovascular manifestations associated with influenza virus infection. Int J Cardiol 2008;130:304-9.
- Warren-Gash C, Smeeth L, Hayward A. Cardiovascular manifestations associated with influenza virus infection. Lancet Infect Dis 2009;9:601-10.
List of abbreviations
- CHI
- Community Health Index
- CI
- confidence interval
- CMO
- Chief Medical Officer
- COPD
- chronic obstructive pulmonary disease
- CRH
- cardiovascular-related hospitalisation
- GP
- general practitioner
- GROS
- General Register Office for Scotland
- HPS
- Health Protection Scotland
- ISD
- Information Services Division
- OR
- odds ratio
- PCCIU
- Primary Care Clinical Informatics Unit
- PTI
- Practice Team Information
- RR
- rate ratio
- SMR
- Scottish Morbidity Record
- VE
- vaccination effectiveness
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.