Notes
Article history
The research reported in this article of the journal supplement was commissioned and funded by the HTA programme on behalf of NICE as project number 07/91/01. The assessment report began editorial review in January 2009 and was accepted for publication in June 2009. See the HTA programme website for further project information (www.hta.ac.uk). This summary of the ERG report was compiled after the Appraisal Committee’s review. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2010 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2010 Queen’s Printer and Controller of HMSO
This paper presents a summary of the evidence review group (ERG) report into the clinical effectiveness and cost-effectiveness of cetuximab for the first-line treatment of metastatic colorectal cancer (mCRC), in accordance with the licensed indication, based upon the manufacturer’s submission to the National Institute for Health and Clinical Excellence (NICE) as part of the single technology appraisal process. The ERG project ran between 22 January 2008 and 4 November 2008. The clinical evidence came from two unpublished randomised controlled trials (RCTs) of cetuximab plus chemotherapy versus chemotherapy alone in the first-line treatment of mCRC. A third RCT submitted later compared cetuximab with irinotecan in combination with 5-fluorouracil (5-FU) and folinic acid (FA) and cetuximab with oxaliplatin in combination with 5-FU and FA in patients with mCRC with liver metastases only. No published economic evaluations of cetuximab for first-line chemotherapy in mCRC were identified in the submission. A de novo model examined the cost-effectiveness of cetuximab in patients with mCRC that was epidermal growth factor receptor positive, k-ras wild type and with liver metastases. The main source of clinical effectiveness evidence came from the first two RCTs which provided follow up information for 1–2 years. Secondary information was used to estimate survival for a further 22 years. The model focused on the patients for whom the treatment had been licensed. This limited the applicability of the model to the NHS setting in which patients would be a mixture of k-ras wild type and mutations and also a mixture of patients with liver metastases and other metastases. The difference in progress-free survival for the two trials was between 0.5 to 1.2 months over a 7–10 month period. Eight months’ treatment with cetuximab, given as an initial loading dose and then weekly until progression, would cost around £22,932 for an average man and £18,427 for an average woman. It is uncertain whether this constitutes good value for money. The guidance issued by NICE on 25 September 2008 stated that cetuximab was not recommended for the first-line treatment of mCRC and people currently receiving cetuximab for the first-line treatment of mCRC should have the option to continue treatment until they and their clinicians consider it appropriate to stop.
Introduction
The National Institute for Health and Clinical Excellence (NICE) is an independent organisation within the NHS that is responsible for providing national guidance on the treatment and care of people using the NHS in England and Wales. One of the responsibilities of NICE is to provide guidance to the NHS on the use of selected new and established health technologies, based on an appraisal of those technologies.
NICE’s single technology appraisal (STA) process is specifically designed for the appraisal of a single product, device or other technology, with a single indication, where most of the relevant evidence lies with one manufacturer or sponsor. 1 Typically, it is used for new pharmaceutical products close to launch. The principal evidence for an STA is derived from a submission by the manufacturer/sponsor of the technology. In addition a report reviewing the evidence submission is submitted by the evidence review group (ERG); an external organisation independent of NICE. This paper presents a summary of the ERG report for the STA entitled ‘Cetuximab for the first-line treatment of metastatic colorectal cancer (mCRC)’. 2
Description of the underlying health problem
Colorectal cancer is a malignant neoplasm arising from the lining (mucosa) of the large intestine (colon and rectum). Colorectal cancer is the third most common cancer in the UK, with approximately 30,000 new cases registered in England and Wales in 2002. This represents 12% of all new cancer cases in women and 14% of all new cancer cases in men. In people between the ages of 45 and 49 years, the incidence is 20 per 100,000. Amongst those over 75 years of age, the incidence is over 300 per 100,000 for men and 200 per 100,000 per year for women. The median age of patients at diagnosis is over 70 years.
In mCRC the tumour has spread beyond the confines of the locoregional lymph nodes to other parts of the body. This is described as stage IV of the American Joint Committee on Cancer tumour node metastases system, or stage D of Dukes’ classification. Estimates of people presenting with mCRC range from 20% to 55% of new cases. In addition, out of patients who have undergone surgery for early stage colorectal cancer with apparently complete excision, approximately 50% will eventually develop advanced disease and distant metastases (typically presenting within 2 years of initial diagnosis). The 5-year survival rate for metastatic colorectal disease is 12%.
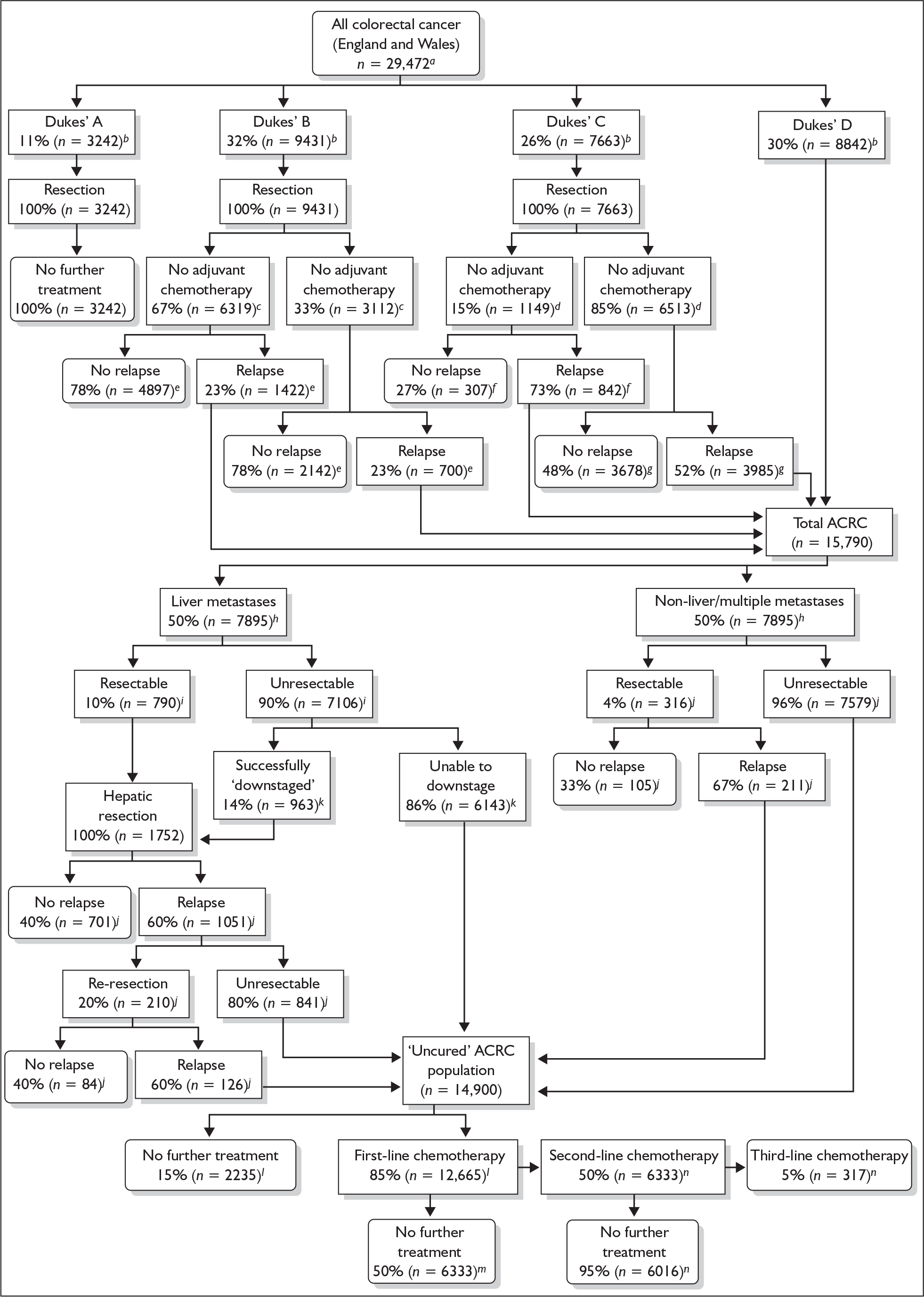
The management of mCRC is mainly palliative and involves a combination of specialist treatments (such as palliative surgery, chemotherapy and radiation), symptom control and psychosocial support. However, approximately 20% of patients with mCRC present with potentially resectable liver metastases. In addition, estimates suggest that for between 10% and 50% of patients, chemotherapy may render unresectable liver metastases operable. The resection of metastases can result in longer term survival for a proportion of patients. Flow of patients and approximate percentages can be seen in Figure 1, reproduced with permission from a recent HTA report. 3
FIGURE 1.
Treatment algorithm for people with colorectal cancer in England and Wales a, Office for National Statistics,4 Welsh Cancer Intelligence and Surveillance Unit;5 b, South West Cancer Intelligence Service;13 c, Seymour M, Leeds Teaching Hospitals NHS Trust, personal communication: between 33% and 60% of people with Dukes’ B cancer receive adjuvant chemotherapy (this study assumed the lower estimate); d, Seymour M, personal communication: more than 85% receive adjuvant chemotherapy; e, Seymour M, personal communication: 20–25% of patients with Dukes’ B will relapse; f, estimated 40% relative risk increase of relapse for surgery alone versus chemotherapy, from pooled multicentre trial.39 Relative risk increase applied to 5-year disease-free survival estimates from X-ACT trial;40 g, 5-year disease-free survival estimates from X-ACT trial;40 h, Maughan T, Velindre Hospital, Cardiff, personal communication; i, data from case series41 suggest up to 20% may be resectable, although this is an aggressive stance; a maximum of 15% of patients are suitable; Maughan T, personal communication; j, Poston G, Royal Liverpool University Hospital, personal communication; k, data from case series;41 l, Seymour M, personal communication: 85–90% of advanced patients receive chemotherapy;42 m, preliminary data from FOCUS trial;42 n, Glynne Jones R, Watford and Barnet General Hospitals, London, personal communication: only 3–5% patients would receive third-line therapy. [Note: the numbers in the text above refer to references in Hind et al.,1 boxes with subscript letters c and d have error where the 33% and the 85% boxes (right-hand side of each pair) should read adjuvant chemotherapy whereas the left-hand side boxes should read no adjuvant chemotherapy (Steven N, University of Birmingham, July 2008, personal communication).]
Current guidance from NICE recommends oxaliplatin in combination with 5-fluorouracil (5-FU) and folinic acid (FA) (FOLFOX) and irinotecan in combination with 5-FU/FA (FOLFIRI) as first-line treatment options (technology appraisal 93). The oral analogues of 5-FU capecitabine and tegafur with uracil are also recommended as treatment options (technology appraisal 61). Bevacizumab as a first-line treatment and cetuximab as a treatment following the failure of an irinotecan-including chemotherapy regimen are not recommended as treatment options (technology appraisal 118).
Scope of the evidence review group report
The purpose of the ERG report is to comment on the validity of the manufacturer’s submission on the technology of interest. The scope for this submission and hence the scope for the ERG report was:
To appraise the clinical and cost effectiveness of cetuximab within its licensed indication for the first line treatment of metastatic colorectal cancer. 4
The relevant Commitee for Medicinal Products for Human Use (CHMP) positive opinion for cetuximab (Erbitux) was:
Erbitux is indicated for the treatment of patients with epidermal growth factor receptor (EGFR) expressing k-ras (k-ras is the gene that encodes for KRAS, a protein that acts in cellular proliferation and transformation) wild-type mCRC:
-
in combination with chemotherapy
-
as a single agent in patients who have failed oxaliplatin- and irinotecan-based therapy and who are intolerant to irinotecan.
Methods
The ERG report comprised a critical review of the evidence for the clinical evidence and cost-effectiveness of cetuximab based upon the manufacturer’s submission to NICE as part of the STA process. Specific steps undertaken by the ERG included:
-
discussion of the nature of the problem with a clinical expert
-
reanalysis of the nature of the clinical question
-
rerunning searches indicated to have been carried out to inform the manufacturer’s submission
-
extending searches, particularly for ongoing trials
-
formal critical appraisal of the systematic review of clinical effectiveness and cost-effectiveness data underpinning the manufacturer’s submission
-
reappraisal and checking of effectiveness and safety data from unpublished trial reports
-
formal critical appraisal of the de novo economic model
-
checking the consistency of the effectiveness estimates emerging from the trials with the parameters used in the economic model
-
rerunning the model for a 5-year time horizon instead of the original 23-year time horizon as submitted by the manufacturer
-
evaluating the evidence regarding clinical sensitivity and specificity of k-ras and EGFR tests
-
evaluating a best-case scenario analysis of the model submitted as a response to clarifications
-
evaluating a separate budget impact model submitted by the manufacturer
-
evaluation of additional material from a third randomised controlled trial (RCT) and a reworked economic model between the first and second appraisal committee meetings.
The work was carried out between 22 January 2008 and 4 November 2008 (report submitted 22 July 2008). Members of the ERG team attended and advised the meetings of the NICE Appraisal Committee in which this guidance was discussed, on 3 September 2008 and 4 November 2008.
Results
Summary of submitted clinical evidence
The originally submitted clinical effectiveness consists of two unpublished RCTs of cetuximab plus chemotherapy versus chemotherapy alone in the first-line treatment of mCRC. The CRYSTAL trial enrolled 1217 patients with EGFR expressing mCRC, and the combination chemotherapy was FOLFIRI. The OPUS trial enrolled 337 patients with previously untreated EGFR expressing mCRC that was not resectable with curative intent, and the chemotherapy used was FOLFOX4. Full follow-up was for [commercial-in-confidence data removed] months in the CRYSTAL trial when [commercial-in-confidence data removed] in both arms of the trial either had died or was lost to follow up. Full follow-up in the CRYSTAL trial, k-ras wild-type subgroup was given at 16 months, and there were six patients remaining in the intervention arm and three in the control arm. For the OPUS trial, full trial results for progression-free survival and overall survival were not found in the submission or the trial report, so are not presented here. For the OPUS trial k-ras subgroup, the equivalent numbers at 12-month follow-up were four patients in the intervention arm and two in the control arm. The difference in median progression-free survival in the CRYSTAL trial, for the k-ras wild-type subgroup, was 1.2 months (9.9 months versus 8.7 months) and for the OPUS trial k-ras wild-type subgroup was 0.5 months (7.7 months versus 7.2 months). Survival curves for these two trials are presented in Figure 2. A third RCT, the CELIM trial, was submitted for assessment between the first and second appraisal committee meetings. 5 This compared cetuximab with FOLFIRI and cetuximab with FOLFOX in 111 patients with mCRC with liver metastases only. Interim results only were presented.
FIGURE 2.
Survival curves for the CRYSTAL and OPUS trials
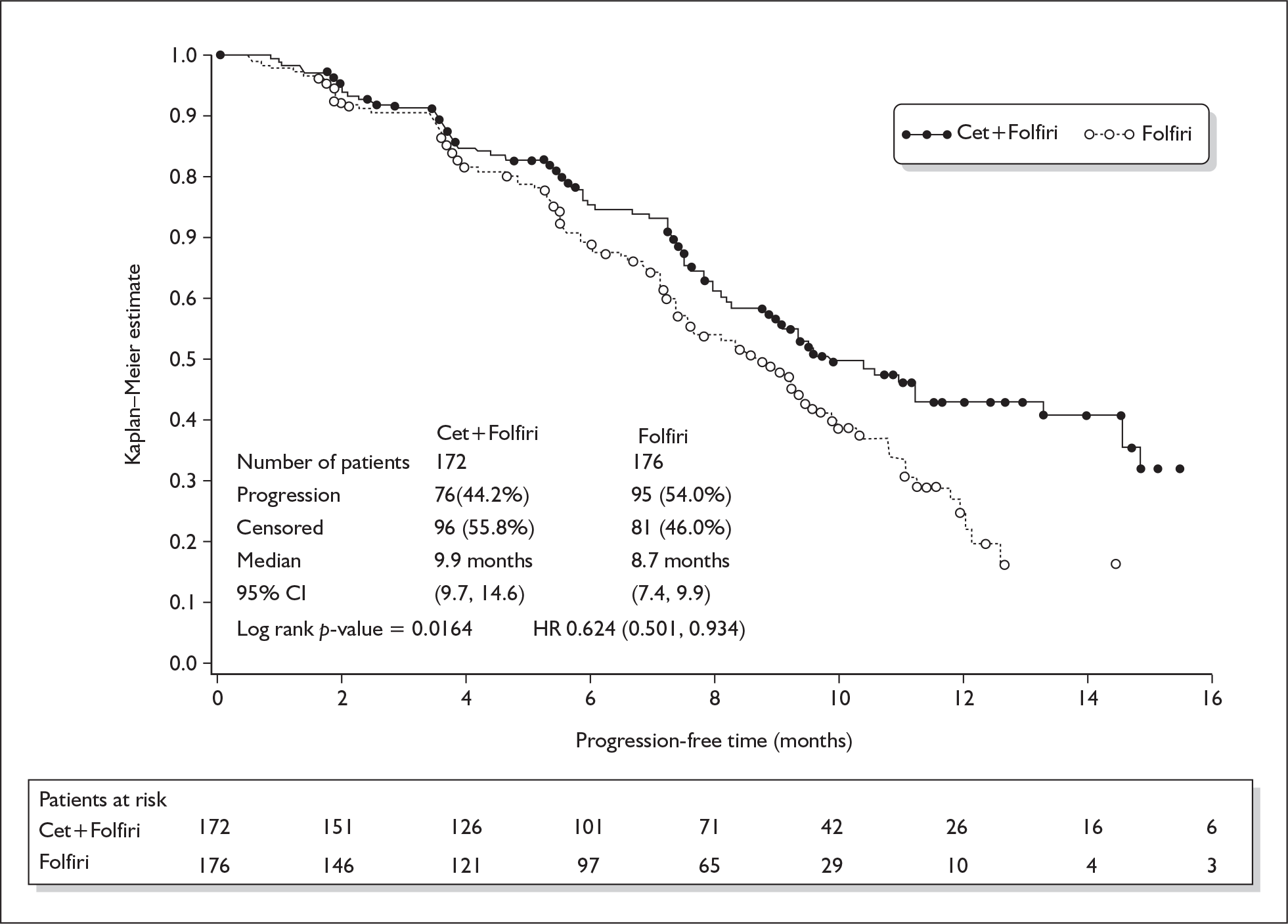
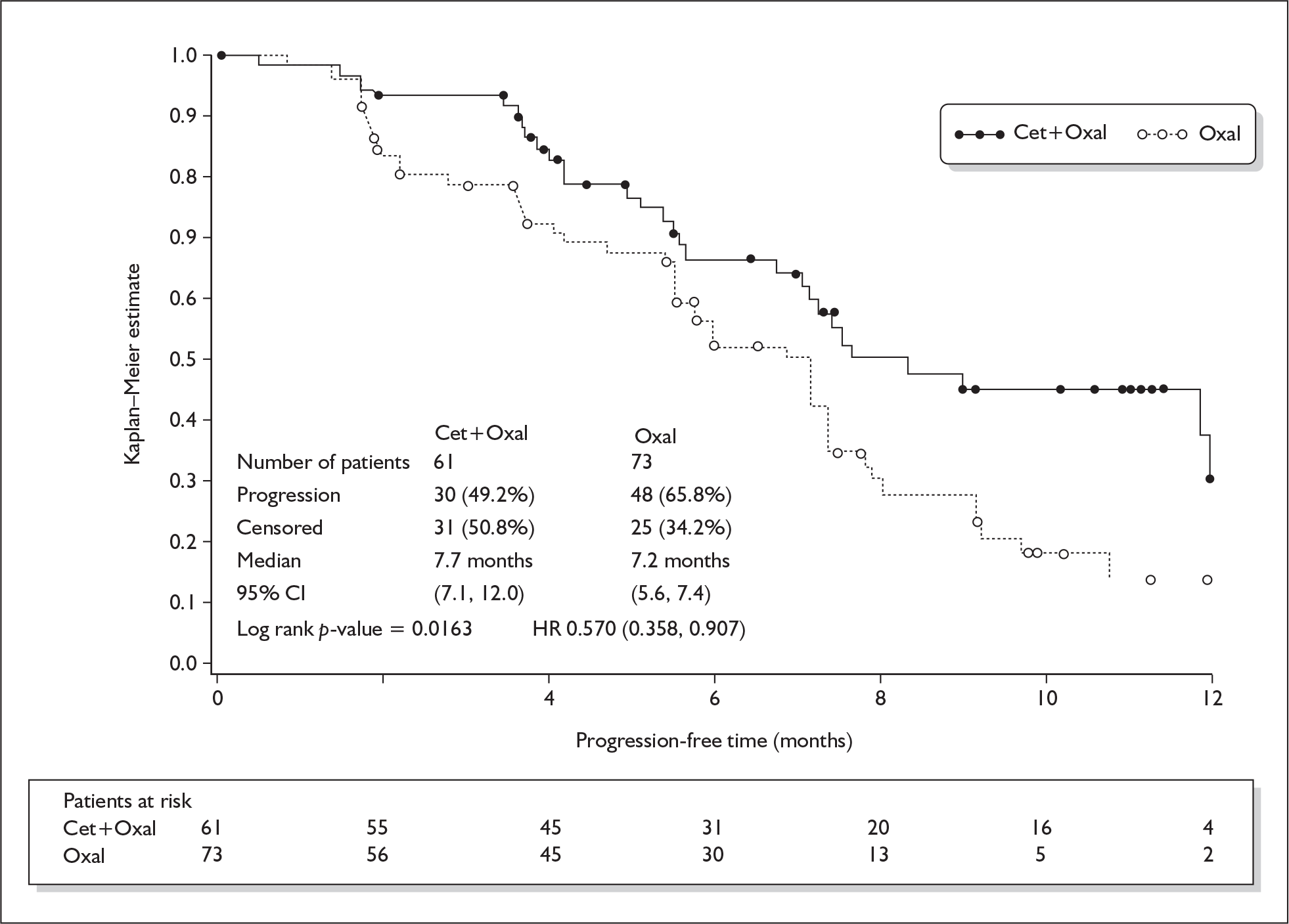
Table 1 shows a comparison of the NICE scope, the CHMP positive opinion, the submission and the two originally submitted trials.
| NICE scope | CHMP | Submission | Trials | |
|---|---|---|---|---|
| Patients | Untreated mCRC, first-line palliative | EGFR expressing k-ras wild type mCRC | Untreated EGFR expressing k-ras wild type mCRC | Previously untreated mCRC |
| Metastases | Untreated, any location | Any location | In model – metastases only in liver | Untreated, non-resectable |
| Intervention | Cetuximab with chemotherapy | In combination with chemotherapy | Cetuximab plus FOLFIRI or FOLFOX | Cetuximab + FOLFIRI (CRYSTAL), cetuximab + FOLFOX (OPUS) |
| Comparators | Oxaloplatin-including regimens, irinotecan including regimens, 5-FU/FA (including oral analogues, capecitabine and tegafur with uracil) | FOLFIRI or FOLFOX only | FOLFIRI (CRYSTAL), FOLFOX (OPUS) |
Summary of submitted cost-effectiveness evidence
No published economic evaluations of cetuximab for first-line chemotherapy in mCRC were identified in the submission, but additional searches by the ERG suggested that six cost-effectiveness papers may have been of relevance. A de novo model examined the cost-effectiveness of cetuximab in patients with mCRC that was EGFR positive, k-ras wild type and with liver metastases. The model was a time dependent state transition (Markov) model with a cycle length of 1 week and a 23-year time horizon (1200 cycles). The main source of evidence came from the two RCTs (CRYSTAL and OPUS) and used progression-free survival and mortality results. Other sources of cost and clinical model inputs were included such as Eastern Co-operative Oncology Group performance status, results of second and third-line treatment, costs of k-ras (but not EGFR) tests and costs of hospitalisation. Sensitivity analyses (scenario, one-way and probabilistic) were performed and reported. A reworked economic model using inputs from the CELIM trial5 and the GERCOR trial6 was submitted between the first and second appraisal committee meetings.
Commentary on the robustness of submitted evidence
The strength of the submitted clinical effectiveness was because it was based on two RCTs rather than a single RCT or non-RCT evidence, and then a further two trials were introduced. The original trials were sufficiently large and follow-up was sufficiently long {[commercial-in-confidence data removed] months for CRYSTAL and 12 months for OPUS} to establish median survival times and obtain statistically significant results. However, only the k-ras wild-type subgroup of results was presented for clinical effectiveness. It was acknowledged in the submission that they were post hoc tests carried out for licensing purposes. In addition, a subgroup of liver metastases from the k-ras wild-type subgroup was also presented and it was these subgroup results that were used for the economic model. For the CRYSTAL and OPUS trials, respectively, the full intention-to-treat populations, k-ras wild-type subgroups and liver metastases subgroups of subgroups were 1198, 348 and 67, and 336, 134 and 38.
The CELIM and GERCOR trials were introduced at the committee stage. For the CELIM trial, the inclusion criteria stated that patients were to have non-resectable liver metastases at baseline yet randomisation was stratified by whether the liver metastases were technically resectable or not, and evaluation of resectability occurred 4 months after randomisation. This seemed contradictory. The GERCOR trial compared using FOLFIRI first the FOLFOX to FOLFOX first the FOLFIRI in mCRC.
The Markov model was appropriate for the decision problem. Having two RCTs (CRYSTAL and OPUS) as the main source of clinical effectiveness evidence was a strength in that the two trials’ results were similar even though the comparator arms used different chemotherapy regimens (FOLFIRI and FOLFOX). Most of the other sources of cost and clinical model inputs were appropriate within the context of the model. Extensive and appropriate sensitivity analyses were conducted.
The model did not wholly address the problem as stated in the scope issued by NICE. Instead the model focused on the patients for whom the treatment had been licensed. This limited the applicability of the model to the NHS setting in which patients would be a mixture of k-ras wild type and mutations and also a mixture of patients with liver metastases and other metastases. Although the manufacturer cannot be held responsible for the differences between the licensed population and the population as set out in the NICE scope, strictly speaking the model did not answer the specified decision problem.
The model structure did not include provision for the identification of k-ras wild-type patients. In order to evaluate the cost-effectiveness of a treatment it is important to know the outcomes for all patients. In this case, the model assumed that all patients who were suitable for treatment were identified and treated (those who were k-ras wild type). It also assumed that no patients who were not suitable for treatment (those who were not k-ras wild type) were treated. No evidence is provided to support this key assumption. Given the importance of estimating the outcomes for those treated incorrectly (either not receiving treatment when they should receive it, or being incorrectly given treatment) in reaching a conclusion on the cost-effectiveness of the treatment, this omission from the model should be considered a serious flaw in the model design.
The reworked model had the same structure as the original, but it was difficult to determine how accurate the clinical effectiveness inputs from the CELIM and GERCOR trials were, given that neither were RCTs of cetuximab versus placebo.
Conclusions
The NICE scope did not specify the k-ras subgroup of patients so it is uncertain as to how the clinical effectiveness results presented matched the population specified in the decision problem. It is also uncertain as to how accurate k-ras testing is in clinical practice.
The effectiveness estimates for the economic model were based on 105 patients (67 CRYSTAL, 38 OPUS). It is uncertain how accurate these effectiveness estimates are, given that they were derived from small post hoc subgroup analyses of trial results.
The lifetime time horizon selected for the model was the appropriate approach to take in a decision analysis such as the one submitted by the manufacturer. However, the average age of patients developing colorectal cancer and being treated in the NHS was in the region of 10 years greater than the average age assumed in the economic model. This limited the applicability of the results to the NHS. The manufacturer’s revised submission included a model that ran for 10 years to examine the impact that this would have on the results. Information from the CRYSTAL and OPUS trials was only available for a period of just over 1 year. Secondary information was used to estimate survival over a further 22-year period. This increased greatly the uncertainty in the results although this increased uncertainty was not discussed in sufficient detail.
The difference in median progression-free survival in the CRYSTAL trial, for the k-ras wild-type subgroup, was 1.2 months (9.9 months versus 8.7 months) and for the OPUS trial was 0.5 months (7.7 months versus 7.2 months). Eight months’ treatment with cetuximab, given as an initial loading dose and then weekly until progression, would cost around £22,932 for an average man and £18,427 for an average woman. It is uncertain whether this constitutes good value for money.
Summary of NICE guidance issued as a result of the STA
At the time of writing, the Appraisal Consultation Document issued by NICE on 25 September 2008 states that:
-
Cetuximab is not recommended for the first-line treatment of metastatic colorectal cancer
-
People currently receiving cetuximab for the first-line treatment of metastatic colorectal cancer should have the option to continue treatment until they and their clinicians consider it appropriate to stop.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
Key references
- National Institute for Health and Clinical Excellence . Guide to the Single Technology Appraisal (STA) Process. n.d. www.nice.org.uk/nicemedia/pdf/STA_Process_Guide.pdf (accessed September 2006).
- Meads C, Round J, Tubeuf S, Moore D, Pennant M, Bayliss S, et al. Cetuximab for the first-line treatment of metastatic colorectal cancer. WMHTAC, University of Birmingham; 2008.
- Hind D, Tappenden P, Tumut I, Eggington S, Sutcliffe P, Ryan A. The use of irenotecan, oxaloplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation. Health Technol Assess 2008;12.
- NICE . Cetuximab for the First Line Treatment of Metastatic Colorectal Cancer n.d. www.nice.org.uk/guidance/index.jsp?action=byID%26o=11918 (accessed 10 November 2008).
- Folprecht G, Gruenberger T, Hartmann JT, Lordick F, Stoehlmacher J, Bechstein W, et al. Randomised Multicenter Study of Cetuximab Plus FOLFOX or Cetuximab Plus FOLFIRI in Neoadjuvant Treatment of Non-Resectable Colorectal Liver Metastases (CELIM-Study) n.d.
- Tournigand C, Andre T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomised GERCOR study. J Clin Oncol 2004;22:229-37.