Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 08/35/01. The protocol was agreed in January 2009. The assessment report began editorial review in November 2009 and was accepted for publication in June 2010. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2011 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
2011 Queen’s Printer and Controller of HMSO
Chapter 1 Background
Description of health problem
Breast cancer and axillary metastases
Breast cancer is the most common type of cancer in women, with 38,048 new cases registered in women in England1 and 2457 in Wales2 in 2007. The usual site of spread outside the breast in newly diagnosed cases is to the lymph nodes in the axilla (underarm). The presence of axillary metastases and the extent of their spread are important prognostic factors for staging disease and planning treatment, whilst removal of any spread is essential to prevent recurrence and wider metastatic spread.
Aetiology, pathology and prognosis
Aetiology
A range of risk factors for breast cancer have been identified, including genetic, hormonal and lifestyle factors. 3 It has been estimated that 12% of women with breast cancer have one affected family member and 1% have two or more affected. 4 Genetic predisposition is mediated by high-penetrance genes such as breast cancer susceptibility gene 1 (BRCA1) and breast cancer susceptibility gene 2 (BRCA2) (responsible for around 80%–90% of hereditary cancers), and low-penetrance genes which confer increased and decreased risk. 3
Environmental and lifestyle factors as well as genetic factors influence breast cancer risk. Asian migrants to the West have increased levels of risk compared with the indigenous population, while Asian-Americans born in the West have incidence rates approximating the US average. 5 Lifestyle and environmental factors thought to increase risk include hormonal factors such as taking the oral contraceptive pill6 or hormone replacement therapy,7 higher age at menopause, early age at menarche, late age at first birth and not giving birth. A recent systematic review suggests night work increases risk. 8 Factors which decrease risk include higher folate intake, higher number of pregnancies, breast feeding and younger age at first birth. 3 Obesity increases risk of breast cancer in post-menopausal women. 9,10 The picture is less clear for pre-menopausal women in whom the risk may be lower, but prognosis poorer. 11 Obesity may affect oestradiol or insulin levels, which is thought to account in part for the increased incidence. Obesity is also correlated to increased severity at presentation, which may be related to lower screening attendance for obese women. 12 Physical activity in adolescence and young adulthood confers a decreased risk of breast cancer,13 which may be mediated hormonally. The protective effect is less evident in pre-menopausal cancers than post-menopausal. 14
Other factors that confer risk include certain high- and low-penetrance genes, increasing age, height, dense breast tissue, alcohol consumption and exposure to ionising radiation in childhood. Among other protective factors are certain low-penetrance genes, fruit and vegetable consumption, chemopreventative agents and use of non-steroidal anti-inflammatory drugs. 3 For men, genetic predisposition, exposure to radiation (especially at a young age), obesity and high levels of oestrogen due to other conditions all affect risk. 15
Pathology
Breast cancer starts with genetic changes in a single cell or small group of cells in the epithelia of the ducts or the lobules of the breast. The genetic change allows cells to reproduce uncontrollably, creating a tumour. Tumours that have not yet spread to surrounding tissue are known as ‘carcinoma in situ’ and may be ductal carcinoma in situ (DCIS) or lobular carcinoma in situ (LCIS). Once spread to surrounding tissue begins, a tumour is known as ‘invasive’. More rapid growth and spread occurs once a blood supply is secured. Cancer spreads via the lymphatic system or the bloodstream. Lymphatic spread is usually first to the axillary lymph nodes. Spread via the bloodstream can lead to distant metastases in the bone or viscera, which are considered incurable.
The presence or absence of axillary metastases is a key indicator of stage of disease and prognosis, and adjuvant therapy is, in part, planned based on their presence and extent. 16 They are caused when a single cell or small numbers of cells detach from the main tumour, travel via the lymphatic system and establish themselves in the tissue of the lymph nodes. Axillary metastases occur in approximately 41%17 of cases and prognosis is better where there is no axillary spread. Where metastases are present, surgical removal of axillary lymph nodes is indicated in order to prevent further spread and ensure local disease control.
Prognosis
Overall, breast cancer 5-year, age-standardised survival rates are around 80%. 18 Survival varies with age (Table 1) and stage of disease (Table 2).
| Age (years) | ||||||
|---|---|---|---|---|---|---|
| 15–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80–99 | |
| 5-year survival rate (%) | 81 | 86 | 89 | 87 | 78 | 64 |
| Stage of disease | ||||
|---|---|---|---|---|
| I | II | III | IV | |
| 5-year survival (%) | 88 | 69 | 43 | 12 |
Other factors can affect prognosis. Clinicians may use tools such as the Nottingham Prognostic Index,20 which takes into account grade as well as size and spread, or Adjuvant Online,21 which uses patient data such as age, tumour size, nodal involvement, hormonal receptor status and histological grade to predict disease course and treatment options. Good prognosis is associated with small tumour size, node-negative status and younger age, oestrogen receptor-positive and progesterone receptor-positive status. HER2 (human epidermal growth factor receptor 2) over-expression is associated with poor prognosis.
Epidemiology and incidence
Incidence varies most with gender. Women are far more likely to get breast cancer than men. For both men and women, incidence also varies with age (Table 3). Approximately 81% of cases occur in women aged 50 years and over. 22
| Population | Country | Age (years) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | 50–54 | 55–59 | 60–64 | 65–69 | 70–74 | 75–79 | 80–84 | 85+ | Total | ||
| Women | Wales | 2 | 21 | 64 | 123 | 186 | 256 | 286 | 324 | 328 | 254 | 201 | 199 | 213 | 2457 | ||
| England | 4 | 18 | 138 | 414 | 1178 | 2390 | 3239 | 4047 | 4347 | 5077 | 4637 | 3243 | 3406 | 2788 | 3122 | 38,048 | |
| Men | Wales | 1 | 1 | 1 | 1 | 2 | 0 | 2 | 1 | 0 | 9 | ||||||
| England | 1 | 2 | 4 | 5 | 14 | 25 | 29 | 23 | 36 | 45 | 34 | 25 | 243 | ||||
| Total | 4 | 18 | 141 | 435 | 1244 | 2517 | 3431 | 4318 | 4659 | 5431 | 4990 | 3533 | 3654 | 3022 | 3360 | 40,757 | |
Incidence varies with ethnicity. Asian, Chinese and Black ethnic groups and those with mixed heritage have a lower incidence than the white ethnic group in England. The rate ratios are 0.65, 0.75, 0.49 and 0.58, respectively, when compared with the white group. 23 Incidence is generally > 80 in 100,000 in developed regions compared with < 30 per 100,000 in developing regions of the world. 24 Similarly, within the developed world, incidence varies with socioeconomic status. In both England25 and Wales26 those who are classed as most deprived have a lower incidence of breast cancer. However, there is some evidence to suggest that the trend for mortality is reversed, with better survival for those from more affluent areas. It is unclear why this is, but may be due to lower levels of screening compliance, worse overall general health status and lower levels of treatment due to limited access to health care27 and poorer compliance with treatment regimens.
Significance in terms of ill-health (burden of disease)
Breast cancer is a significant cause of death in England and Wales, ranking among the five leading causes when age-standardised rates are compared. It is currently the second biggest cause of cancer death in women after lung cancer, with an age-standardised mortality rate of 26 per 100,000 women. In 2008 this constituted 10,716 deaths for women in England and Wales. 28 Breast cancer deaths have been steadily falling since a peak in 1988. 29 The fall in mortality may be due to screening (earlier detection and more successful treatment), improvements in social awareness, diagnostic techniques and treatment options such as tamoxifen, chemotherapy and more recently trastuzumab. 30,31 As screening programmes detect more cancers at an earlier stage and women live longer after diagnosis, the long-term morbidity associated with treatment, such as lymphoedema, is becoming more significant.
Measurement of disease
Breast cancer has few obvious symptoms and can easily go undetected for many years. Among the more noticeable symptoms is a palpable lump in the breast, a change in breast shape and skin appearance or changes to the nipple such as inversion, a rash or discharge. Women are encouraged to be breast aware, and to seek medical advice if they notice anything unusual. 32 Screening was introduced in the UK in 1988. 33 Currently, women between the ages of 50 and 70 years are routinely invited to attend. Screening is thought to have reduced breast cancer deaths in the 55–69 years age category by an estimated 6.4% in addition to the effects of tamoxifen, chemotherapy and earlier presentation outside of screening. 30 Screening increases the proportion of tumours detected in the early, more curable stages.
A suspicious breast mass may be identified through screening, or via presentation to a general practitioner. The breast mass and axillary areas are investigated clinically through palpation and mammography or ultrasound for younger women, and the status of the tumour confirmed by histology of biopsied tissue. Staging of the disease depends on tumour size, the number of involved lymph nodes and the presence or absence of distant metastases. Tumour size and axillary metastases can be estimated by clinical examination and imaging techniques, but definitive status is achieved through surgery. Those with small tumours and no axillary metastases have the best prognosis, while those with distant metastases are considered incurable.
Current service provision
Current methods for staging of breast cancer
Three main factors are used to stage breast cancer. These are tumour size, metastases to the regional lymph nodes and distant metastases. The tumour/node/metastases (TNM) staging system was developed and is maintained by the American Joint Committee on Cancer and the Union for International Cancer Control. 34,35 T stage is classified according to size of the tumour and degree of local infiltration; N stage is classified according to the number and location of metastases to the lymph nodes in the axilla, between the ribs (internal mammary nodes) and above or below the collarbone (supraclavicular and infraclavicular nodes); and M stage is classified by the presence of metastases beyond the breast and regional lymph nodes (Table 4). The overall TNM stage of the cancer is defined as in Table 5.
| Stage | Description |
|---|---|
| T: tumour stage | |
| Tx | Primary tumour cannot be assessed |
| T0 | No evidence of primary tumour |
| Tis | Carcinoma in situ |
| T1 | Tumour ≤ 2 cm across |
| T2 | Tumour 2–5 cm across |
| T3 | Tumour > 5 cm across |
| T4 | Tumour of any size with direct extension to skin or chest wall, or inflammatory breast cancer |
| N: lymph node stage | |
| Nx | Nodal stage cannot be assessed |
| N0 | No metastases to any ipsilateral lymph nodes |
| N1 | Metastases to 1–3 axillary nodes or axillary nodes that are mobile |
| N2 | Metastases to 4–9 axillary nodes, or axillary nodes that are fixed to one another or other structures, or clinically apparent metastases to internal mammary nodes |
| N3 | Metastasis to nodes above or below collarbone (supraclavicular/infraclavicular), or to both axillary and internal mammary nodes, or to 10 + axillary nodes |
| M: metastasis stage | |
| Mx | Presence of metastases cannot be assessed |
| M0 | No distant metastases |
| M1 | Distant metastases |
| Stage | T | N | M |
|---|---|---|---|
| 0 (DCIS/LCIS) | Tis | N0 | M0 |
| I | T1 | N0 | M0 |
| IIA | T0–1 | N1 | M0 |
| T2 | N0 | M0 | |
| IIB | T2 | N1 | M0 |
| T3 | N0 | M0 | |
| IIIA | T0–2 | N2 | M0 |
| T3 | N1–2 | M0 | |
| IIIB | T4 | N0–2 | M0 |
| IIIC | T(any) | N3 | M0 |
| IV | T(any) | N(any) | M1 |
Early breast cancer is generally defined as cancer which has not spread beyond the breast or the ipsilateral axillary lymph nodes and is confined to stages I, II or IIIA. 36–38
Current methods for assessment of axillary metastases
Axillary lymph nodes are the most common site of spread outside the breast (occurring in approximately 41% of cases)17 and removal of lymph nodes affected by tumour is crucial to prevent recurrence. Assessment of whether cancer has spread to the lymph nodes is also important for staging, assessing prognosis and selection of adjuvant therapy. Clinical examination via palpation has a sensitivity of approximately 46% based on pooled data from a number of studies (in other words, of those patients with metastases, 46% can be detected via clinical examination). 41–46 Therefore, of all patients presenting with breast cancer, approximately 19% (41% × 46%) have axillary metastases that can be detected via clinical examination, while 22% (41% × 54%) have occult axillary metastases.
The following steps (see also Figure 1) for assessing the axilla are recommended in the 2009 National Institute for Health and Clinical Excellence (NICE) breast cancer guideline. 17 A clinical examination and an ultrasound scan are carried out. If these examinations suggest nodal metastases on the basis of size or abnormal morphology, ultrasound-guided needle biopsy [either fine-needle aspiration cytology (FNAC) or core biopsy] of abnormal nodes is undertaken, which detects 45% of axillary node metastases. 47 For patients shown by needle biopsy to have nodal metastases, standard management is surgery to remove all the lymph nodes in the axilla, known as axillary lymph node dissection (ALND) or axillary clearance.
FIGURE 1.
Diagnostic pathway for axillary metastases as recommended in the 2009 NICE breast cancer guidelines. 17 *Either fine-needle aspiration cytology or core biopsy.
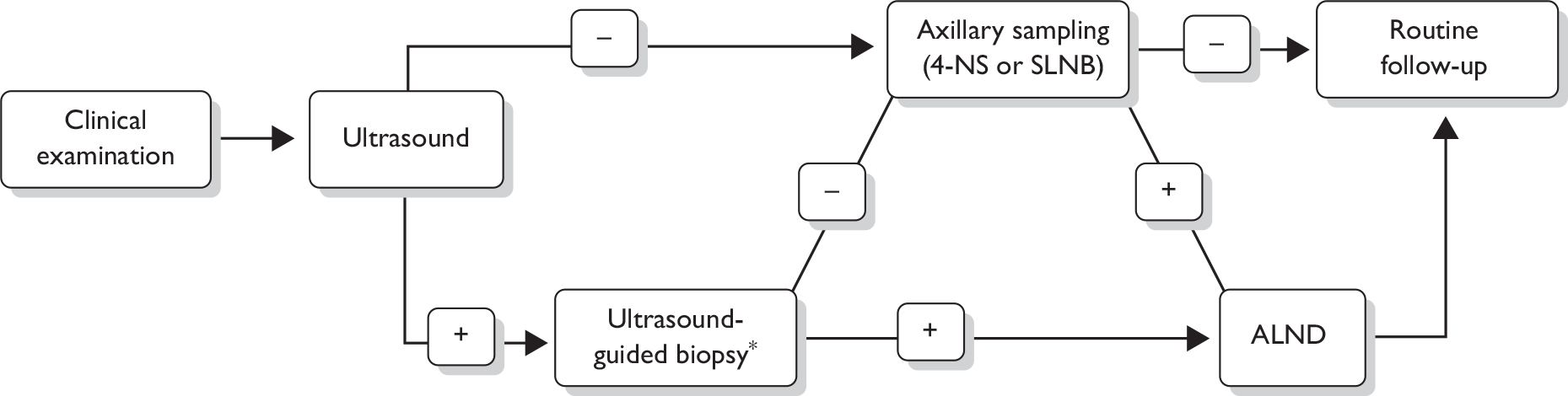
As well as being the treatment for those with positive nodes, ALND has been considered the ‘gold standard’ procedure for staging the axilla. Typically, 10–15 lymph nodes are removed and at least one section from each is assessed via haematoxylin and eosin (H&E) staining. ALND is very accurate in establishing the presence of axillary disease and has the therapeutic advantage of being associated with a high long-term local disease control rate. However, ALND is associated with significant complications, including a 21% incidence of arm lymphoedema,48–50 a 22% incidence of seromas48,51 and a 14% infection rate. 48,52 In addition, insertion of a surgical drain during surgery is commonplace (79%) and usually necessitates prolongation of hospital stay. 52
For patients with no evidence of lymph node involvement on ultrasound, or for whom the ultrasound-guided biopsy is negative, surgery to remove a sample of axillary lymph nodes is recommended, as opposed to full axillary clearance. 17 There are two axillary sampling techniques in current practice: sentinel lymph node biopsy (SLNB) and 4-node sampling (4-NS). SLNB is a procedure to identify and remove the sentinel lymph node, which is the first axillary lymph node to which lymphatic fluid drains from the breast, and therefore the most likely to be affected by metastases. The sentinel node may be identified via blue dye, radioactive isotope or a combination. Sentinel lymph nodes may be examined via H&E staining, and/or immunohistochemistry for epithelial or cytokeratin markers. 4-NS involves a more random surgical removal of a minimum of four lymph nodes (sometimes more) from the lower axilla, and may involve use of blue dye to aid in identification of nodal tissue in the axilla. The false-negative (FN) rate of SLNB and 4-NS have been estimated at between 0% and 10%. 17 As stated in the NICE guideline, data on 4-NS are limited and there are currently insufficient data to compare the diagnostic accuracy of SLNB and 4-NS. 17
Sentinel lymph node biopsy and 4-NS involve shorter surgical procedures than ALND, and are associated with lower incidence of surgical complications and long-term adverse effects than ALND. Lymphoedema incidence falls from 21%48–50 for ALND to 7%53 for SLNB, seroma incidence falls from 22%48,51 to 7%48,51, surgical drain requirement from 79% to 2%52,54 and infection incidence from 14%48,52 to 2%. 48,52,54
Patients in whom axillary lymph node metastases are identified via SLNB or 4-NS are advised to undergo ALND as a subsequent procedure to remove all axillary lymph nodes. 17 Some units are investigating the use of intraoperative cytology for assessment of axillary lymph nodes, whereby patients undergo axillary sampling (e.g. via SLNB) and the dissected lymph nodes are assessed while the surgical procedure is still ongoing. If any metastases are identified in the sampled nodes then the procedure is converted to a full ALND, bypassing the requirement for a second surgical procedure. However, it is currently unclear whether immediate or delayed ALND differ significantly in terms of adverse effects. Since intraoperative cytology is not currently used as standard, it is not included in this assessment.
Cost of current methods for assessment of axillary metastases
The costs of clinical examination, ultrasound, and ultrasound-guided biopsy are £86, £53 and £147, respectively55,56 ALND when carried out as a stand-alone procedure costs £2448. 56 All costs have been adjusted to 2007 prices.
Sentinel lymph node biopsy and 4-NS are normally performed at the same time as the main breast cancer surgery while ALND may be performed at the same time as the breast surgery or as a stand-alone surgical procedure. No UK costs are identified from previous studies regarding the combined costs of SLNB, 4-NS and ALND when performed at the same time as the breast surgery. However, it is widely accepted that SLNB has a significantly higher cost than 4-NS.
Relevant national guidelines
The 2009 NICE guideline Early and locally advanced breast cancer: diagnosis and treatment includes recommendations on methods for assessment of the axilla. 17
Variation in services and uncertainty about best practice
Until recently, ALND was the gold standard technique for assessing axillary lymph node status. A 2006 audit of 271 UK breast surgeons, asked how they would manage a woman with small, clinically node-negative breast cancer, reported that 27% performed ALND, 21% used 4-NS and 52% used SLNB. 57 The 2009 NICE guideline recommends that axillary sampling techniques (SLNB or 4-NS) should be used instead of ALND for patients with clinically node-negative disease, stating that SLNB is the preferred technique. 17 However, there are insufficient data to allow comparison of SLNB and 4-NS, either in terms of diagnostic accuracy or complication rate. 17 Therefore, there is likely to be variation in practice within the UK in the use of ALND, SLNB or 4-NS to assess axillary status.
Description of technology under assessment
Summary of diagnostic tests under assessment (index tests)
This review assesses two imaging techniques: positron emission tomography (PET) and magnetic resonance imaging (MRI).
Positron emission tomography
Positron emission tomography is a nuclear medicine imaging technique that produces a three-dimensional image or map of functional processes in the body. The system detects pairs of gamma rays emitted indirectly by a positron-emitting radioactive tracer, which is introduced into the body attached to a biologically active molecule. Images of tracer concentration in three-dimensional space within the body are then reconstructed by computer analysis. A tracer commonly used for PET scanning is 18F-FDG, which is a glucose analogue (2-fluoro-2-deoxy-d-glucose; FDG) attached to the radioactive isotope fluorine-18. When FDG is injected into a patient, a PET scanner can form images of the distribution of FDG within the body. This provides a picture of the glucose uptake, i.e. metabolic activity, in different areas of the body. As cancer cells frequently have a higher glucose requirement and uptake than many normal body cells, areas of cancerous activity can be detected. 58,59
The definition of increased uptake on a PET scan may be based on the reader’s qualitative visual impression, or more formally by using indices such as the standardised uptake value (SUV) (tissue radioactivity concentration divided by the total injected dose, normalised to body size). 58
Positron emission tomography/computed tomography
In recent practice, PET scans are commonly undertaken as PET/CT scans, in which a computed tomography (CT) scan is taken alongside the PET scan. Modern PET scanners often perform a reduced-dose CT scan and a PET scan on the patient during the same session, using the same machine. CT scans use a large series of two-dimensional X-rays to generate a three-dimensional image of body structures. This modality combination allows concurrent visualisation of both the anatomy of tissues and their metabolic activity. 58–60 This review includes both studies of PET only and studies of PET/CT.
Cautions and contraindications for positron emission tomography and computed tomography scanning
In terms of safety, PET and CT scanning are non-invasive, but do involve exposure to ionising radiation. The effective radiation dose from a CT scan is approximately 10 millisieverts (mSv), which is about the same as the average person receives from background radiation in 3–4 years. 61 Patients with small children may be advised to limit proximity to them for several hours following the completion of a PET scan. CT scanning is not generally recommended for pregnant women unless it is essential, owing to the potential risk to the baby. Nursing mothers are recommended to wait for 24 hours after contrast material injection before resuming breast-feeding. Serious allergic reaction to contrast materials containing iodine is rare. 61
Magnetic resonance imaging
Magnetic resonance imaging provides detailed images of the body in any plane. MRI provides much greater contrast between the different soft tissues of the body than does CT. MRI scanning uses a powerful magnetic field to align the nuclear magnetisation of (usually) hydrogen atoms in water in the body. Radiofrequency fields are used to systematically alter the alignment of this magnetisation, causing the hydrogen nuclei to produce a rotating magnetic field detectable by the scanner. This signal can be manipulated by additional, time-varying magnetic field gradients to introduce spatial information that is used to construct two- or three-dimensional images of the area of interest. Different imaging ‘sequences’ are used in which the relative timings of these and other sequence components are altered to produce various contrasts between different tissue types, states and pathologies. This is often referred to as defining the degree of proton density, T1-, T2- and T2*-weighting that is present in the resultant set of images.
An MRI scan may provide information on whether a lesion is suspicious for metastasis, based on criteria such as size, morphology and enhancement characteristics following administration of a contrast agent. 62,63
Variations in method of magnetic resonance imaging
Magnetic resonance imaging techniques may vary in terms of factors such as the field strength, image sequence parameters and type of coil used. Also, a contrast agent is often administered via intravenous injection during the MR procedure. Images obtained post contrast are usually compared with those obtained pre contrast in order to determine where the contrast agent has accrued. Gadolinium-based agents form one class of contrast agent. Another class of contrast agent is ultrasmall super-paramagnetic iron oxide (USPIO), also known as ferumoxtran-10. Dynamic-contrast-enhanced MRI (DCE-MRI) uses repeated imaging to track the entrance of contrast agents into tissue over time. The pattern of uptake over time can be used to assess whether or not cancerous tissue is present.
Magnetic resonance (MR) spectroscopy is used to measure the levels of different metabolites (other than water) in body tissues. Studies of in vitro and in vivo proton MR spectroscopy of the breast have shown that high levels of choline-containing compounds may indicate metastatic lesions. 64
Cautions and contraindications for magnetic resonance imaging scanning
Magnetic resonance imaging scanning is non-invasive, but use of contrast agents may need to be reviewed for patients with reduced renal function. Different chelating agents may be used to minimise the associated risk. These compounds can have differential effects on resultant image contrast. Allergic reactions to contrast agents have been observed and these agents may be contraindicated in patients with a strong allergic disposition. MRI is contraindicated in people with pacemakers, some artificial heart valves, electronically or magnetically-activated implants and some types of metallic implants and foreign bodies. Full safety guidelines for MRI are available from the Medicines and Healthcare products Regulatory Agency. 65
Identification of important subgroups
Subgroup analyses were undertaken according to the different methods of PET and MRI, the reference standard test used, and the clinical characteristics of included patients (see Chapter 2, Decision Problem for a full list). Sensitivity analyses were also undertaken according to study quality criteria.
Current usage in the NHS
In recent years, imaging techniques such as PET and MRI have been increasingly used for diagnosis and staging in various types of cancer. However, at present they are not routinely used for staging the axilla in breast cancer.
Chapter 2 Definition of the decision problem
Decision problem
Population and relevant subgroups
Population
The relevant population consists of patients newly diagnosed with early-stage invasive primary breast cancer.
In this review, early-stage breast cancer is defined as TNM stage I, II or IIIA. The included studies recruited patients before full staging investigations had been undertaken, as would be the case in practice, and therefore tended to include patients with a range of cancer stages. Where sufficient data were reported in the primary study, patients with advanced, metastatic or recurrent cancer were excluded from the analysis. Patients with carcinoma in situ (DCIS or LCIS) were also excluded where possible, as these patients do not generally undergo diagnostic axillary surgery. Where separate data were not presented, studies were included if at least 80% of the study population had early-stage, newly diagnosed breast cancer (a sensitivity analysis was undertaken including early-stage patients only). Only patients with histologically confirmed breast cancer were included in this analysis.
Since there are several studies of PET in this setting with large sample sizes, PET studies with < 20 analysable patients were excluded. MRI studies of all sizes were included as there are few with a large sample size.
Subgroups
Subgroup analyses were undertaken according to the following variables:
-
PET alone or PET/CT
-
MRI using different contrast agents and methods
-
criteria for defining a node as metastatic
-
reference standard test used
-
whether the included patients were all early stage and newly diagnosed
-
size of axillary metastases and nodal stage
-
prevalence of patients with axillary metastases within the study
-
clinical axillary nodal status (positive or negative)
-
study quality.
Diagnostic tests under assessment (index tests)
The index tests assessed in this review are:
-
PET, including:
-
– ET alone
-
– PET/CT
-
-
MRI, including:
-
– gadolinium-enhanced MRI
-
– dynamic gadolinium-enhanced MRI
-
– USPIO-enhanced MRI
-
– MR spectroscopy.
-
Reference standard tests (comparator tests)
The most relevant reference standard tests were considered to be ALND, SLNB and 4-NS. ALND is the ‘gold standard’ method of staging the axilla. SLNB and 4-NS were also thought to be acceptable comparators, since these techniques are now recommended by NICE and are not associated with a survival detriment in the long term. 17 Studies using other reference standard tests, or where the reference standard is not clear, were included, but a sensitivity analysis excluding these studies was also undertaken.
Outcomes
Relevant outcomes include:
-
sensitivity and specificity [or data required to calculate these, i.e. numbers of true-positive (TP), false-negative (FN), true-negative (TN) and false-positive (FP) results]
-
adverse effects and withdrawals
-
health-related quality of life
-
cost-effectiveness and cost–utility.
Studies were only included if they report the numbers of TP, FN, TN and FP results for PET or MRI scanning in comparison with a reference standard test. These values can be used to calculate measures of diagnostic accuracy such as sensitivity and specificity.
Patients are classified by the index test (the diagnostic test being investigated) as either positive (axillary metastases) or negative (no axillary metastases). The reference standard (an established diagnostic test) is also undertaken to identify patients’ true health status. The reference standard is assumed to have 100% sensitivity and specificity; however, subgroup analyses are undertaken to test the effect of using different reference standards. Patients fall into one of four groups. Where the index test is positive, patients may be TP where both tests agree that they have metastases, or FP where the index test indicates that they have metastases but the reference standard does not. Where the index test is negative, patients may be TN where both tests agree they are metastasis free, or FN where the index test incorrectly classifies them as metastasis free. This can be represented in a 2 × 2 table (Table 6). In the clinical setting, FPs can result in patients receiving unnecessary treatment, while FNs can result in people not receiving treatment they require. Sensitivity indicates the effectiveness of the index test in correctly identifying metastases (TPs divided by all persons with metastases). Specificity indicates the effectiveness of the index test in correctly classifying people as metastasis free (TNs divided by all persons without metastases). Sensitivity and specificity can be calculated as simple percentages, as shown in Table 6. In practice, diagnostic tests often have a high sensitivity at the expense of a low specificity and vice versa. Ideally, a test would have both high sensitivity and high specificity.
| Index test result | Reference standard-positive | Reference standard-negative |
|---|---|---|
| Index test positive | TP | FP |
| Index test negative | FN | TN |
| Sensitivity = [TP/(TP + FN)] × 100 | Specificity = [TN/(TN + FP)] × 100 |
Study design
Studies of a cohort design (prospective or retrospective) were included. Studies that were both prospective and consecutive were examined in a separate sensitivity analysis as part of the assessment of study quality. Case–control studies (where the test is evaluated in a group of patients already known to have the outcome and a separate group of patients without the outcome) were excluded; however, no studies of this type were identified within this review.
Overall aims and objectives of assessment
The aim of this assessment was to assess the diagnostic accuracy, cost-effectiveness, and effect on patient outcomes of PET and MRI in the evaluation of axillary lymph node metastases in patients with newly diagnosed, early-stage invasive primary breast cancer. PET and MRI are assessed, firstly, as a replacement for SLNB or 4-NS and, secondly, as an additional test prior to SLNB or 4-NS.
The objectives of the assessment are:
-
To conduct a systematic review of the published evidence on the diagnostic accuracy and cost-effectiveness of PET and MRI for the assessment of axillary lymph node metastases in newly diagnosed, early-stage breast cancer.
-
To develop a decision model to investigate the benefits, harms, and cost-effectiveness of PET and MRI, either as a replacement for surgical assessment of the axilla or as an additional test in the diagnostic pathway for assessing the axilla. Outcomes from the model will be expressed in terms of net health benefit and cost per quality-adjusted life-year (QALY).
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing effectiveness
A systematic review was undertaken according to the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 67,68
Identification of studies
Search strategy
The search strategy comprised the following elements:
-
searching of electronic databases
-
scrutiny of bibliographies of retrieved papers and previous reviews
-
contact with experts in the field.
The MEDLINE search strategy is included in Appendix 1, and comprised medical subject heading (MeSH) terms and free-text terms as follows: terms for breast cancer, terms for PET and MRI, terms for the axilla or lymph nodes and terms to identify diagnostic studies. No restrictions were used according to language or date of publication. Searches were undertaken in April 2009.
Databases
The following databases were searched:
-
MEDLINE
-
MEDLINE In-Process & Other Non-Indexed Citations
-
EMBASE
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
-
Cochrane Library including Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (NHS EED) and HTA databases
-
Science Citation Index (via Web of Science)
-
BIOSIS Previews
-
National Research Register archive (www.nrr.nhs.uk; searched until 2007)
-
UK NIHR Clinical Research Network (www.ukcrn.org.uk; searched post-2007)
-
ClinicalTrials.gov (www.clinicaltrials.gov)
-
current controlled trials (www.controlled-trials.com)
-
American Society of Clinical Oncology abstracts (conference proceedings)
-
European Society for Medical Oncology abstracts (conference proceedings).
Titles and abstracts were screened for inclusion by two reviewers. Full text relevant papers were screened against the inclusion criteria by two reviewers and any disagreements resolved by consensus.
Inclusion and exclusion criteria
Inclusion criteria
Studies were included if they assessed the diagnostic accuracy of PET or MRI for use in the assessment of axillary metastases in patients newly diagnosed with early-stage invasive primary breast cancer. Studies were only included if they reported the numbers of TP, FN, TN and FP results for PET or MRI scanning in comparison with a reference standard test.
Exclusion criteria
As there are several studies of PET in this setting with large sample sizes, PET studies with < 20 analysable patients were excluded, since they were thought to add little to the overall estimates of accuracy. MRI studies of all sizes were included as there are few with a large sample size. Studies were also excluded if > 20% of the study population had breast cancer that was non-early stage, non-newly diagnosed or DCIS. Animal models, pre-clinical and biological studies, narrative reviews, editorials, opinion papers and non-English-language papers were excluded. Case–control studies were excluded (although no studies of this type were identified within this review).
Data extraction strategy
Data were extracted by one reviewer using a standardised data extraction form and checked by a second reviewer. Discrepancies were resolved by discussion.
Critical appraisal strategy
Study quality was considered with the aid of the quality assessment of diagnostic accuracy studies (QUADAS) checklist. 69 Quality assessment was performed by one reviewer and checked by a second. The quality assessment criteria were scored as detailed in Appendix 2. Three items from the published checklist were not used within our assessment, as follows. The ‘description of selection criteria’ item was omitted as this was thought to be covered by the ‘representative patient spectrum’ item, where studies with insufficient description of the selection criteria scored ‘unclear’. The ‘partial verification bias’ item was omitted because, in almost all studies included in this review, all patients received a reference standard. The ‘incorporation bias’ item was also omitted because, in this review, the reference standard was always independent of the index test (it was noted by Whiting et al. 69 that the above two items are not relevant in every review).
Methods of data synthesis
Sensitivity and specificity are presented for each study. Meta-analysis was undertaken to calculate a mean sensitivity and specificity across studies. Sensitivity and specificity are linked, so that changing the threshold at which a test is considered positive will tend to increase the sensitivity but decrease the specificity, or vice versa. Therefore, sensitivity and specificity were meta-analysed using a bivariate random effects method within stata (StataCorp©, College Station, TX, USA). This approach assumes a bivariate normal distribution for the logits of sensitivity and specificity, which allows the correlation between them to be accounted for in the meta-regression model; covariates may be used to adjust the (marginal) logits of both sensitivity and specificity. 70,71 Where significant heterogeneity was observed, the random effects method was used in order to account for variation both within and between studies. Forest plots and receiver operating characteristic (ROC) plots were generated within review manager (version 5.0, Cochrane Collaboration©, Copenhagen, Denmark). 72 To explore possible sources of bias, all study quality variables were added as covariates in univariate regression models for sensitivity and specificity for PET and MRI, in order to test whether any variables had a significant effect (p < 0.10) on sensitivity or specificity.
Results
Quantity and quality of research available
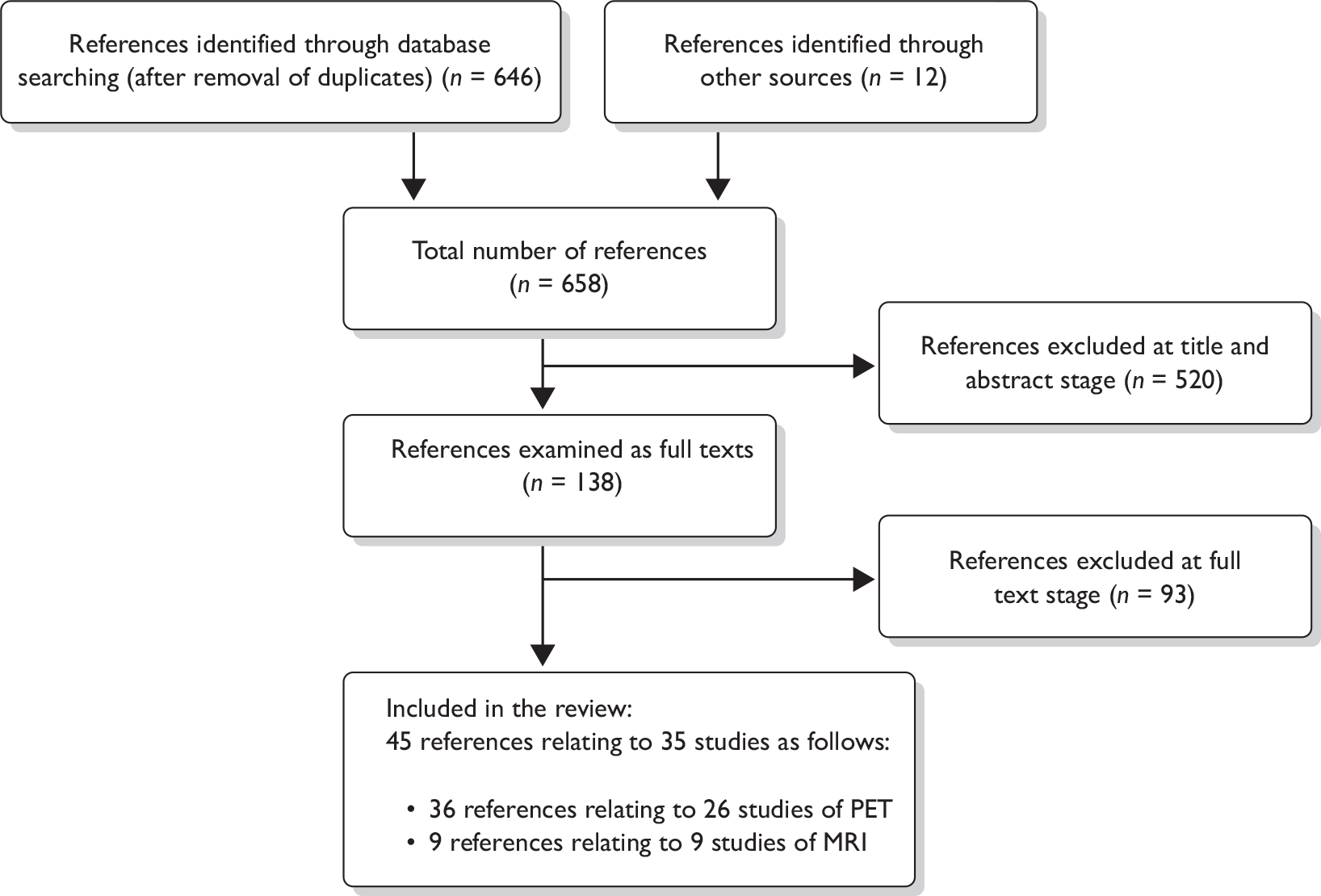
The search identified 658 citations (646 from the literature search and 12 from other sources such as relevant reviews; Figure 2). Of these, 520 were excluded at the title/abstract stage and 138 were obtained for examination of the full text. Ninety-three citations were excluded at the full text stage (Appendix 3). In total, 45 citations relating to 35 studies were included in the review: 26 studies of PET and nine studies of MRI.
FIGURE 2.
Preferred reporting items for systematic reviews and meta-analyses flow chart of included and excluded studies.
Study characteristics
Positron emission tomography studies
The characteristics of the 26 included studies of PET for assessment of the axilla are described in Table 7. There were seven studies assessing PET/CT73–79 and 19 studies assessing PET alone. 44,80–97
| Study | Country | Index test | Reference standard | Prospective/retrospective? | Consecutive? | n met criteria? | n analysed | Mean age (range) (years) | Gender | Cancer stage | Clinical nodal status | Prevalence of axillary metastases (%) | Confirmation of breast cancer | Other inclusion and exclusion criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies of PET/CT | ||||||||||||||
| Chae 200973 | South Korea | PET/CT | 100% ALND (plus SLNB) | Retrospective | NR | 108 | 108 | 49 (27–75) | NR |
T1 = 71% T2 = 27% T3 = 2% N0 = 69% N1 = 17% N2 = 9% N3 = 5% |
100% negative; micrometastases/ITCs excluded | 31 | CNB or FNAC | NR |
| Heusner 200974 | Germany | PET/CT | ALND and/or SLNB | Retrospective | Consecutive | 54 | 54 | 56 (28–78) | Female |
T1 = 44% T2 = 56% N0 = 59% N1 = 26% N2 = 7% N3 = 7% |
Positive and negative (% NR) | 41 | Histopathology | NR |
| Kim 200975 | South Korea | PET/CT | ALND and/or SLNB (plus non-SLNB) | Prospective | Consecutive | 137 | 137 | 51 (27–85) | 99% female | T1/T2 = 100%, no DCIS | NR | 26 | Biopsy | Exclusion: diabetes, neoadjuvant chemotherapy, excisional biopsy |
| Taira 200976 | Japan | PET/CT | ALND (if PET or SLNB positive) and/or SLNB | Retrospective | NR | 92 axilla | 92 axilla | 55 (21–82)a | Female |
T1 = 68% T2 = 29% T3 = 2%, no DCIS |
100% negative | 29 | Six EB; remainder NR | Exclusion: prior chemotherapy, hormone therapy, radiotherapy |
| Fuster 200877 | Spain | PET/CT | 100% ALND | Prospective | Consecutive | 60 | 52 | 57 ± 13 | NR |
T1 = 0% T2 = 21% T3 = 68% T4 = 11% (all primary tumour > 3 cm) |
97% negative, 3% positive | 38 | CNB | Exclusion: IBC, breast surgery, chemotherapy or radiotherapy, pregnant, diabetes, age < 18 years |
| Ueda 200878 | Japan | PET/CT | ALND and/or SLNB | Prospective | Unclear (states ‘series’) | 183 | 183 | 57 (32–81) | NR |
Tis = 5% T1 = 50% T2 = 37% T3 = 8% |
Positive and negative (% NR) | 32 | CNB | Exclusion: distant metastases, systemic therapy, excisional biopsy, diabetes, pregnancy |
| Veronesi 200779 | Italy | PET/CT | ALND (if PET or SLNB positive) and/or SLNB | Prospective | Consecutive | 236 | 236 | 49 (24–79)a | 99.6% female |
T1 = 58% T2 = 37% T3 = 6%, no DCIS N0 = 56% N1 = 32% N2 = 8% N3 = 4% |
100% negative | 44 | FNAC, CNB or EB | Exclusion: neoadjuvant chemotherapy |
| Studies of PET only | ||||||||||||||
|
Cermik 2008,80 Kumar 2006102 |
USA | PET only | ALND and/or SLNB | Prospective | Consecutive | 188 axilla | 188 axilla | 51 (24–80) | Female (confirmed by author) |
N0 = 61% N1 = 31% N2 = 8% |
Positive and negative (% NR) | 39 | CNB 67.5%, EB 32.5% | NR |
|
Gil-Rendo 2006,81 Zornoza 2004103 |
Spain | PET only | ALND (first n = 150 and next n = 125 if PET/SLNB-positive) and/or SLNB | Prospective | Unclear (states ‘series’) | 275 | 275 | 51 (24–87) | Female |
Stage I–II = 100% T1 = 49% extensive DCIS = 4% neoadjuvant chemotherapy = 2% |
100% negative | 52 | CNB | Exclusion: stage III/IV, biopsy or surgery to breast or axilla, uncontrolled diabetes |
| Weir 200582 | Canada | PET only | ALND and/or SLNB | Retrospective | NR | 40 | 40 | 52 (30–88)a | Female | Stage not reported. Newly diagnosed | NR | 45 | Histology (no further detail) | Exclusion: non-newly diagnosed, axillary surgery, chemotherapy |
|
Agresti 2004,83 Agresti 2001104 |
Italy | PET only | ALND (if PET or SLNB-positive) and/or SLNB | NR | NR | 71 | 71 | 55 (24–78) | NR |
T1 = 86% T2 = 14% |
100% negative | 44 | NR | Exclusion: primary tumour > 2.5 cm |
| Fehr 200484 | Switzer-land | PET only | 100% ALND (plus SLNB) | NR | NR | 24 | 24 | 56 ± 10.8 | NR |
T1 = 14 (58%) T2 = 9 (38%) T3 = 1 (4%) |
100% negative | 42 | FNAC | Exclusion: tumour > 3 cm, IBC, multifocality, pregnancy, lactation, diabetes, radiotherapy, breast/axilla surgery |
| Inoue 2004,85 Yutani 2000,106 Yutani 1999107 | Japan | PET only | All confirmed by histology, ‘almost all’ ALND (via author) | Retrospective (via author) | NR | 81 | 81 | 53 (32–78)a | Female |
T1 = 41% T2 = 46% T3 = 14% N0 = 23% N1–N2 = 77% |
23% negative, 77% positive | 43 | Histology after surgery (via author) | Exclusion: distant metastases |
|
Lovrics 2004,86 Lovrics 2002105 |
Canada | PET only | ALND and/or SLNB | Prospective | Consecutive | 115 | 90 | 56 (SD 11) | Female |
T1 = 81% T2 = 18% T3 = 1% |
Positive and negative (% NR) | 28 | Histology: 14 EB, NR for remainder | Exclusion: stage III/IV, multiple, multicentric, IBC, male, prior ALND, pregnancy, uncontrolled diabetes |
| Wahl 200444 | USA | PET only | 100% ALND (some SLNB also) | Prospective | Consecutive (via author) | 330 axilla | 308 axilla | 52 (27–82) | Female |
T1 = 65% T2 = 28% T3 = 2% Tx = 1% missing = 4% |
92% negative, 8% positive | 35 | NR | Exclusion: non-invasive, distant metastases, diabetes, infections, serious organ dysfunction, neoadjuvant chemotherapy, SLNB only |
| Barranger 200387 | France | PET only | 100% ALND (plus SLNB) | Prospective | Consecutive | 32 | 32 | 58 (29–77) | NR |
T0 = 28% T1 = 56% T2 = 16% |
100% negative | 47 | FNAC or CNB | Exclusion: neoadjuvant chemotherapy, pregnancy, diabetes |
| Guller 200288 | Switzer-land | PET only | ALND and/or SLNB | Prospective | Consecutive | 31 | 31 | 65 (47–88) | Female |
T1 = 61% T2 = 39% |
100% negative | 45 | Histopathology (no further detail) | Exclusion: pregnancy, diabetes, inability to lie still in PET scanner |
| Nakamoto 200289 | USA | PET only | 100% ALND | Prospective | NR | 30 | 30 | 51 (28–78) | 94% female |
T1 = 67% T2 = 37% T3 = 7% |
NR | 43 | Histology (no further detail) | NR |
| Rieber 200290 | Germany | PET only | Histology (no further details) | NR | NR | 43 | 40 | 53 (27–84) | NR |
DCIS = 3% Tmic = 3% T1 = 21% T2 = 49% T3 = 10% T4 = 15% |
NR | 50 | Histology (no further detail) | NR |
| van der Hoeven 200291 | Nether-lands | PET only | ALND and/or SLNB (depending on tumour size) | Prospective | Unclear | 70 | 70 | 58 (SD 13) | NR |
T0 = 6% T1 = 53% T2 = 26% T3 = 6% T4 = 4% Unknown = 6% |
71% negative, 29% positive |
46 | EB 17%, FNAC 83% | Exclusion: diabetes |
|
Greco 2001,92 Crippa 199898 Bombardieri 1998,99 Crippa 1997100 |
Italy | PET only | 100% ALND | Prospective | Consecutive | 167 | 167 | 54 (28–84) | NR |
T1 = 59% T2 = 41% |
77% negative, 23% positive |
43 | Histology (no further detail) | Exclusion: primary tumour > 5 cm, abnormal blood glucose |
| Noh 199893 | South Korea | PET only | Histology (no further details) | NR | NR | 31 axilla | 27 axilla | NR | NR |
Stage not reported DCIS = 12%; not clear if included in analysis |
70% negative, 30% positive |
56 |
Histology (no further detail), 1 FNAC |
NR |
| Smith 199894 | UK | PET only | ALND 90%; FNAC 10% (large/locally advanced) | NR | NR | 38 | 38 | 67 (26–89) | Female |
T1 = 21% T2 = 55% T3 = 24% |
70% negative, 30% positive |
42 | FNAC | Exclusion: age < 18 years, pregnant, diabetes, unable to lie still on PET scanner |
| Adler 1997,95 Adler 1996101 | USA | PET only | 100% ALND | Part of prospective study | NR | 54 axilla | 52 axilla | 36–79 | NR |
Benign = 2% T1 = 60% T2 = 33% T3 = 6% |
NR | 38 | NR | Exclusion: primary tumour < 0.5 cm, < level II ALND, < 10 nodes dissected, age < 30 years, prior ALND, neoadjuvant chemotherapy |
| Avril 199696 | Germany | PET only | ALND 90%; clinical examination 10% (locally advanced) | NR | NR | 41 | 41 | 50 (18–74) | Female |
T1 = 44% T2 + = 56% Locally advanced = 10% Distant or non-axillary metastases ≥ 12% |
Positive and negative (% NR) | 59 | Histology (no further detail) after surgery | Exclusion: age < 18 years, pregnancy, diabetes |
| Utech 199697 | USA/Germany | PET only | ALND 44%; modified radical mastectomy 56% | Prospective | NR | 124 | 124 | 59 (32–94) | NR |
T1 = 67% T2 = 29% T3 = 4% N0 = 64% N1 = 35% N2 = 2% |
92% negative, 8% positive | 35 | CNB 42%, EB 54%, partial mastectomy 4% | NR |
Eight PET studies used ALND as the reference standard for all patients,44,73,77,84,87,89,92,95,98–101 12 studies used a mixture of ALND and SLNB,74–76,78–83,86,88,91,102–105 and six studies did not specify the reference standard or used a method other than ALND/SLNB for some of the patients. Nine studies recruited patients both prospectively and consecutively. 85,90,93,94,96,97,106,107 The number of analysed patients (relevant to this review) ranged from 24 to 308. Fourteen studies presented data such that the patients analysed in this review were entirely early stage (stage I, II or IIIA), newly diagnosed and non-DCIS,44,75,76,80,83–89,92,94,97 while the remaining 12 studies comprised up to 20% of patients who did not meet these criteria. 73,74,77–79,81,82,90,91,93,95,96 Eight studies consisted entirely of patients who were clinically node negative,73,76,79,81,83,84,87,88 13 studies included a mixture of node-negative and node-positive patients,44,74,77,78,80,85,86,91–94,96,97 and in five studies nodal status was not reported. 75,82,89,90,95 The mean age of the included patients ranged from 49 to 67 years (mean across studies was 56 years), and the majority of patients were female. The prevalence of axillary metastases (as measured via the reference standard) ranged from 26% to 59%, the average across studies being 41%. Further details of the methods of PET scanning and the reference standard are described in Appendix 4.
Magnetic resonance imaging studies
The characteristics of the nine41,42,64,108–113 included studies of MRI for assessment of the axilla are described in Table 8. There were five studies of USPIO-enhanced MRI,108–112 two studies of dynamic gadolinium-enhanced MRI,41,113 one study of (non-dynamic) gadolinium-enhanced MRI,42 and one study of in vivo proton MR spectroscopy. 64 Several of the studies reported more than one set of results on diagnostic accuracy, according to different criteria for defining whether axillary metastases were present (e.g. based on USPIO or gadolinium uptake, size, morphology, or combinations of these criteria).
| Study | Country | Index test | Reference standard | Prospective/retrospective? | Consecutive? | N met criteria? | N analysed | Mean age (range) (years) | Gender | Cancer stage | Clinical nodal status | Prevalence of axillary metastases (%) | Confirmation of breast cancer | Other inclusion and exclusion criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kimura 2010108 | Japan | USPIO-enhanced | ALND and/or SLNB | Prospective | Consecutive | 10 | 10 | 66 (35 to 79) | Female | 100% clinically T2 N0 M0 (stage IIA) | 100% negative | 20 | Pathology (no further detail) | Exclusion: strong allergic disposition, liver dysfunction |
| Harada 2007109 | Japan | USPIO-enhanced | 100% ALND | Prospective | Consecutive | 33 | 33 | 58 (36–77) | 97% female |
Stage II = 73% Stage IIIA = 24% Stage IIIB = 3% |
NR | 70 | Pathology (no further detail) | Exclusion: stage I, strong allergic disposition, liver dysfunction |
| Memarsadeghi 2006110 | Austria | USPIO-enhanced | 100% ALND | Prospective | Consecutive | 24 | 22 | 60 (40–79) | Female |
T1 = 59% T2 = 41% |
NR | 27 | CNB | Exclusion: contraindication to MRI, allergy to dextran or iron salts, chemotherapy or radiotherapy, no ALND, pregnancy, lactation, unable to cooperate, other trial, under care of guardian |
| Stadnik 2006111 | Belgium | USPIO-enhanced | 100% ALND | Prospective | NR | 10 | 10 | 56 (41–74) | Female | Stage not reported. Included pts scheduled for mastectomy | NR | 50 | NR | Exclusion: not scheduled for mastectomy, contraindication for MRI, strong allergic disposition to gadolinium, dextrans or iron salts, unable to obtain PET (for technical or accessibility reasons) |
| Michel 2002112 | Switzerland | USPIO-enhanced | 100% ALND | Prospective | Consecutive | 18 | 18 | 53 (22–76) | Female |
T1 = 56% T2 = 39% T4 = 6% |
NR | 61 | Cytology 95%, histology 5% | Exclusion: strong allergic disposition, contraindication to MRI |
| Murray 2002113 | UK | Dynamic gadolinium-enhanced | 100% ALND | NR | NR | 47 | 47 | 63 (50–87) | Female | T1/T2 = 100% | NR | 21 | Histology (no further detail) | Exclusion: primary tumour < 0.5 cm or > 3.1 cm. |
| Kvistad 200041 | Norway | Dynamic gadolinium-enhanced | 100% ALND | NR | NR | 67 | 65 | 59 (38–79) | NR |
T1 = 58% T2 = 31% T3/T4 = 11% (neoadjuvant chemotherapy) |
Positive and negative (% NR) | 37 | Histology or FNAC | NR |
| Mumtaz 199742 | UK | Gadolinium-enhanced | 100% ALND | NR | NR | 92 axilla | 75 axilla | 49 (29–80)a | NR |
T1 = 11% T2 = 72% T3 = 3% T4 = 3% Tx = 11% DCIS = 4% |
NR | 53 | FNAC 90%, CNB 10% (if equivocal) | NR |
| Yeung 200264 | Hong Kong | MR spectroscopy | 100% ALND | Prospective | Consecutive | 32 | 27 | 53 (26–82) | NR | Stage not reported | 52% negative 48% positive | 63 | CNB | Exclusion: receiving chemotherapy |
Eight MRI studies used ALND as the reference standard for all patients,41,42,64,109–113 while one study used a mixture of ALND and SLNB. 108 Five studies recruited patients both prospectively and consecutively. 64,108–110,112 The number of analysed patients (relevant to this review) ranged from 10 to 75. Three studies presented data such that the patients analysed in this review were entirely early stage (stage I, II or IIIA), newly diagnosed and non-DCIS,108,110,113 while the remaining six studies comprised up to 20% of patients who did not meet these criteria. 41,42,64,109,111,112 One study consisted entirely of patients who were clinically node negative,108 two studies included a mixture of node-negative and node-positive patients,41,64 and in six studies nodal status was not reported. 42,109–113 The mean age of the included patients ranged from 53 to 66 years (mean across studies was 59 years), and the majority of patients were female. The prevalence of axillary metastases (as measured via the reference standard) ranged from 20% to 70%, the average across studies being 45%. Further details of the methods of MRI scanning and the reference standard are described in Appendix 4.
Study quality
Figures 3 and 4 provide an overview of the methodological quality of the 35 included studies. 41,42,44,64,73–97,108–113 Of the PET studies, quality items that scored poorly overall were representative patient spectrum, availability of relevant clinical information, handling/reporting of uninterpretable results and interpretation of the reference standard with blinding to index test results. Patient spectrum scored negatively either because the study included up to 20% participants who were not early stage or newly diagnosed (12 studies)73,74,77–79,81,82,90,91,93,95,96 or because the patients were not recruited both prospectively and consecutively (two studies). 76,85 It was unclear if participants were recruited prospectively and consecutively in 12 cases. 73,78,81,83,84,89,91,93–97 The index test was interpreted blind to reference standard results in most studies. The index test was often interpreted blind to other clinical data in addition to the reference standard, possibly to ensure a robust evaluation of PET; however, as this is likely to differ from expected clinical practice, studies where this occurred were scored negatively for the ‘relevant clinical information’ item. Uninterpretable results were dealt with well in only eight studies,75,77,79,80,86–88,92 and were not discussed in 16 cases. 73,74,76,78,81–85,87,89–91,93,94,96 Similarly, blinding of the reference standard results was under-reported, with 20 studies scoring ‘unclear’. 73–75,77,78,80–85,87–89,92–96 It is difficult to know what impact these have on study quality and transferability to real life practice. The reference standard was adequate in nearly all cases, with only three studies failing to give sufficient detail,85,90,93 and two not performing ALND on those patients with large or locally advanced disease. 94,96 The delay between reference standard and index test was acceptable in 14 studies44,76,78,79,83–88,91,92,94,95 and not reported in 12. 73–75,77,80–82,87,89,90,93,96 Where patients were given a different reference standard depending on the index test results (six studies),75,76,78,79,81,83 this was either ALND or SLNB. SLNB is thought to have slightly lower accuracy so differential verification bias may occur in these studies. The index test was usually very well described, while the reference standard was often only partially described, probably due to the widespread routine use of these techniques. Most studies did not have any withdrawals to explain, so this item scored well overall.
FIGURE 3.
Methodological quality: summary across all studies. (a) PET studies; (b) MRI studies.
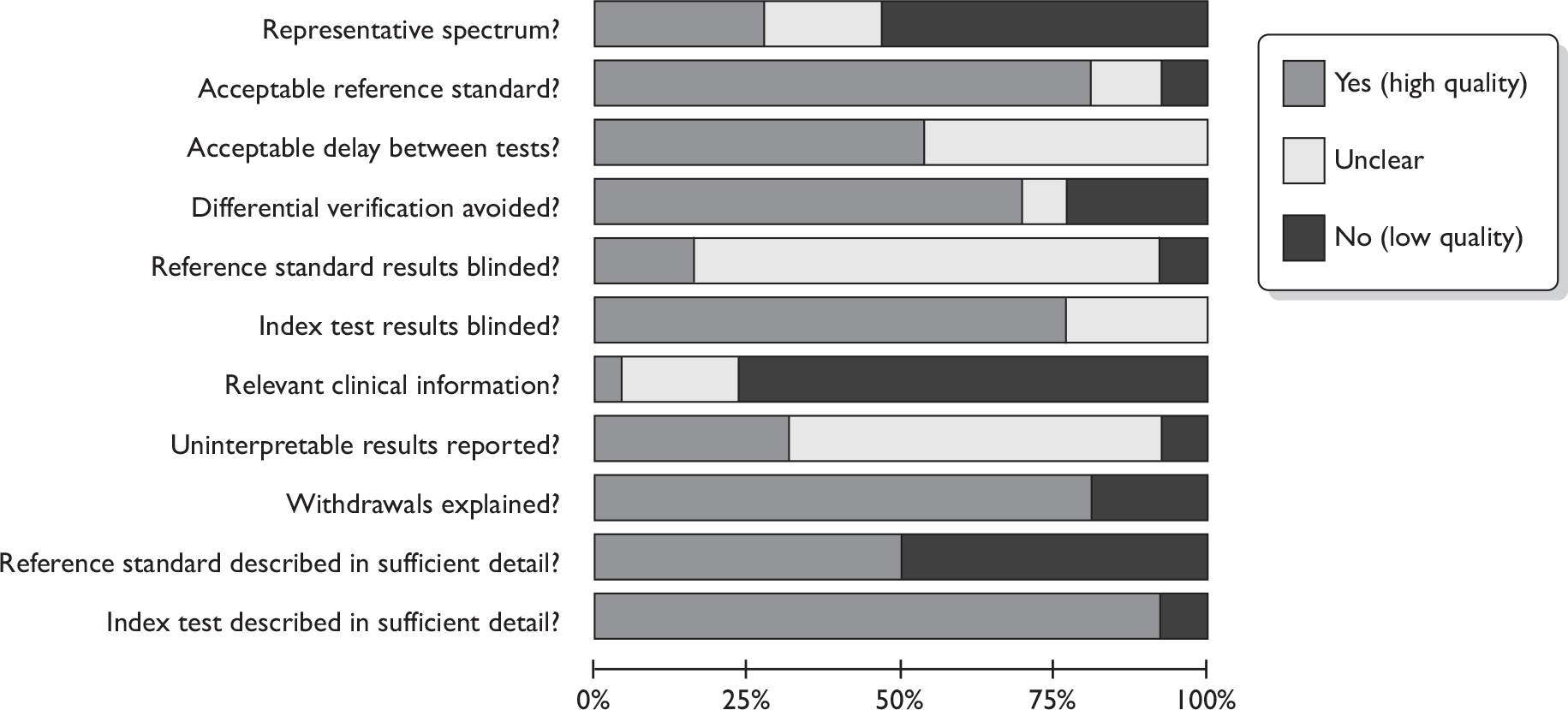
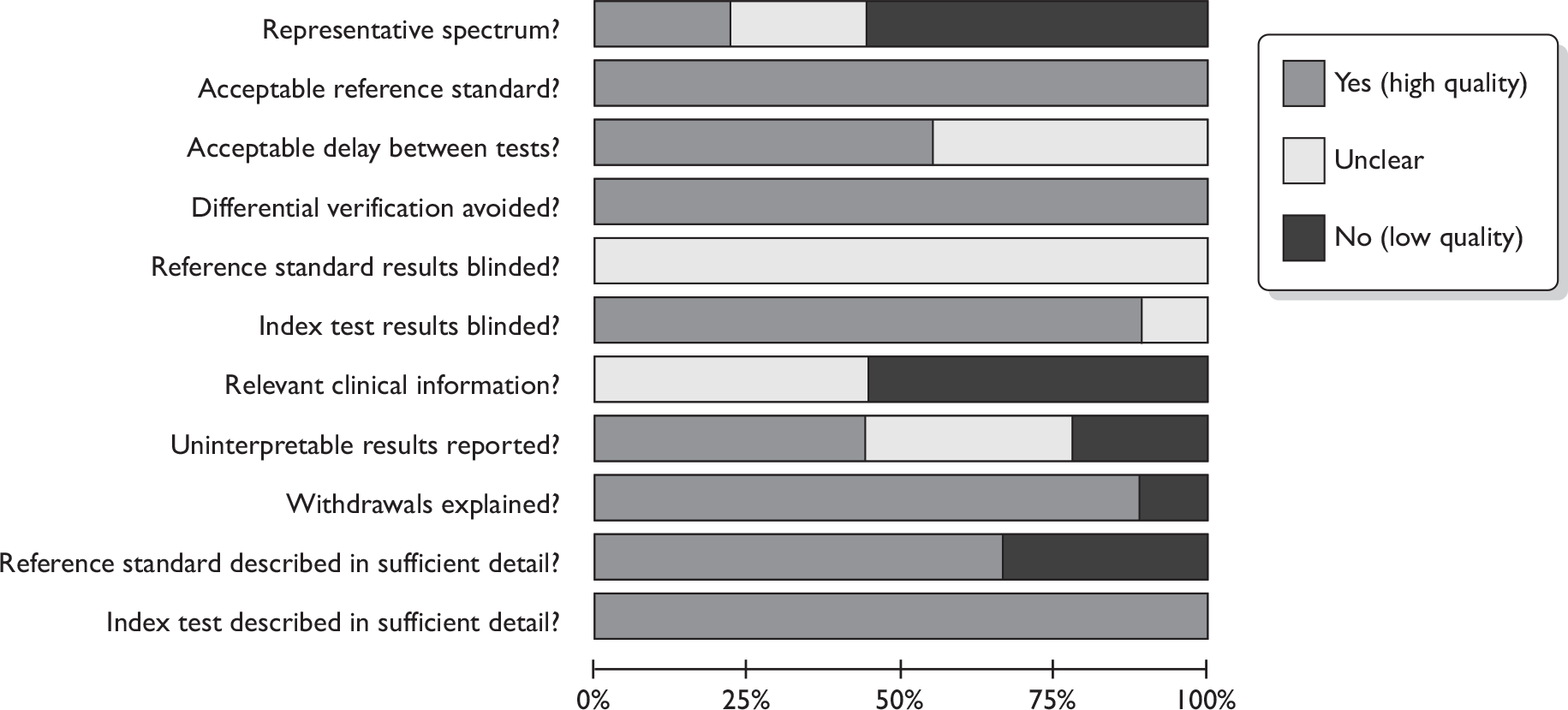
FIGURE 4.
Methodological quality: summary for each individual study. (a) PET studies; (b) MRI studies.
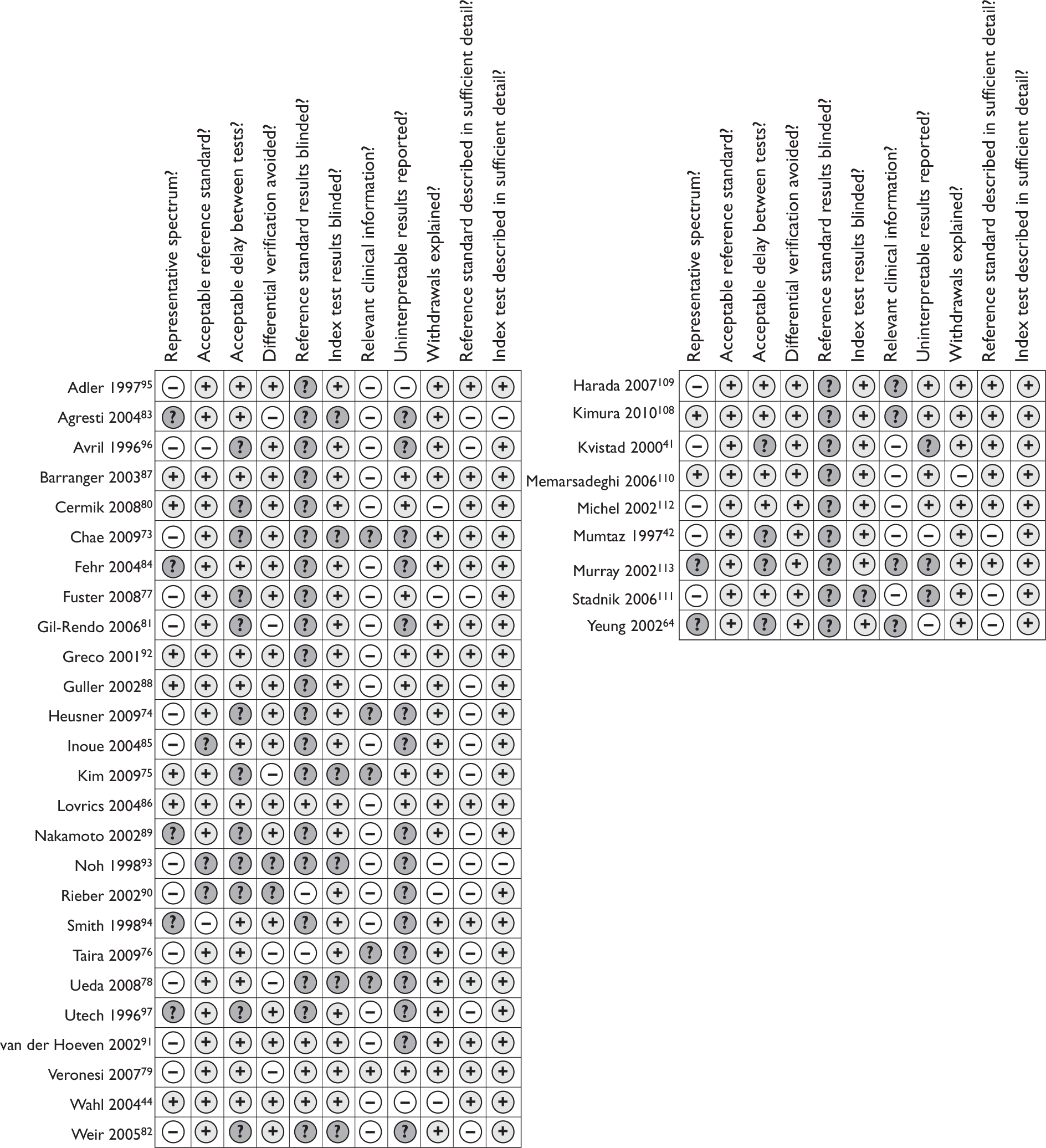
All nine MRI studies used an acceptable reference standard and avoided differential verification bias. 41,42,64,108–113 All described the index test in detail, probably due to the novel nature of the techniques, and usually described the reference standard well. Withdrawals were explained or did not occur in eight cases. 41,42,64,108,109,111–113 However, as with PET studies, the spectrum of patients was mixed, with over half not recruiting only early stage and newly diagnosed, or not recruiting prospectively or consecutively. Relevant clinical information was not available to the image interpreter in five cases41,42,110,111,112 and not reported in the other four,64,108,109,113 and uninterpretable results were dealt with well by only four studies. 108–110,112 None of the studies reported whether the reference standard results were interpreted blindly.
Assessment of diagnostic accuracy
Positron emission tomography studies: diagnostic accuracy results
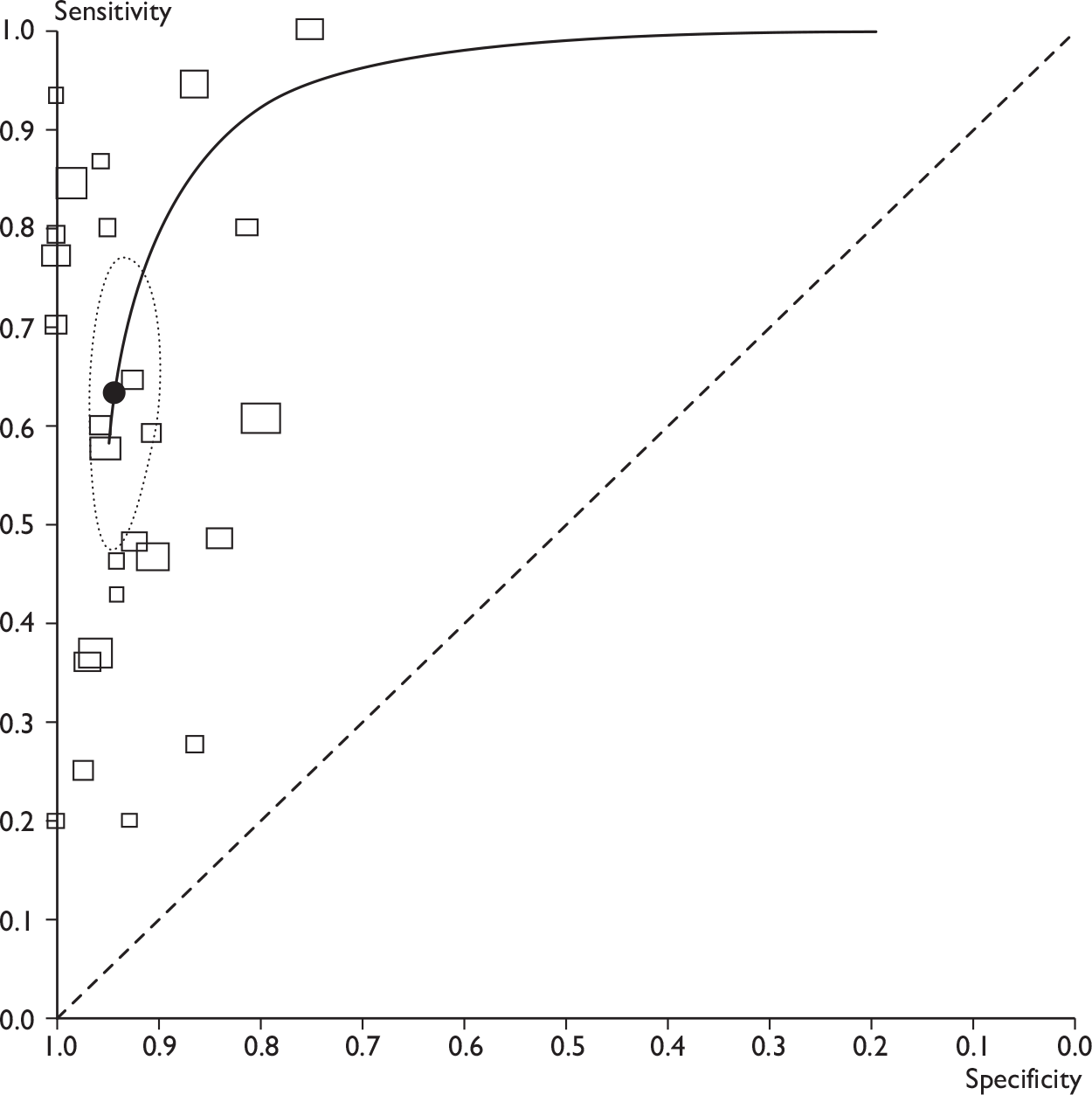
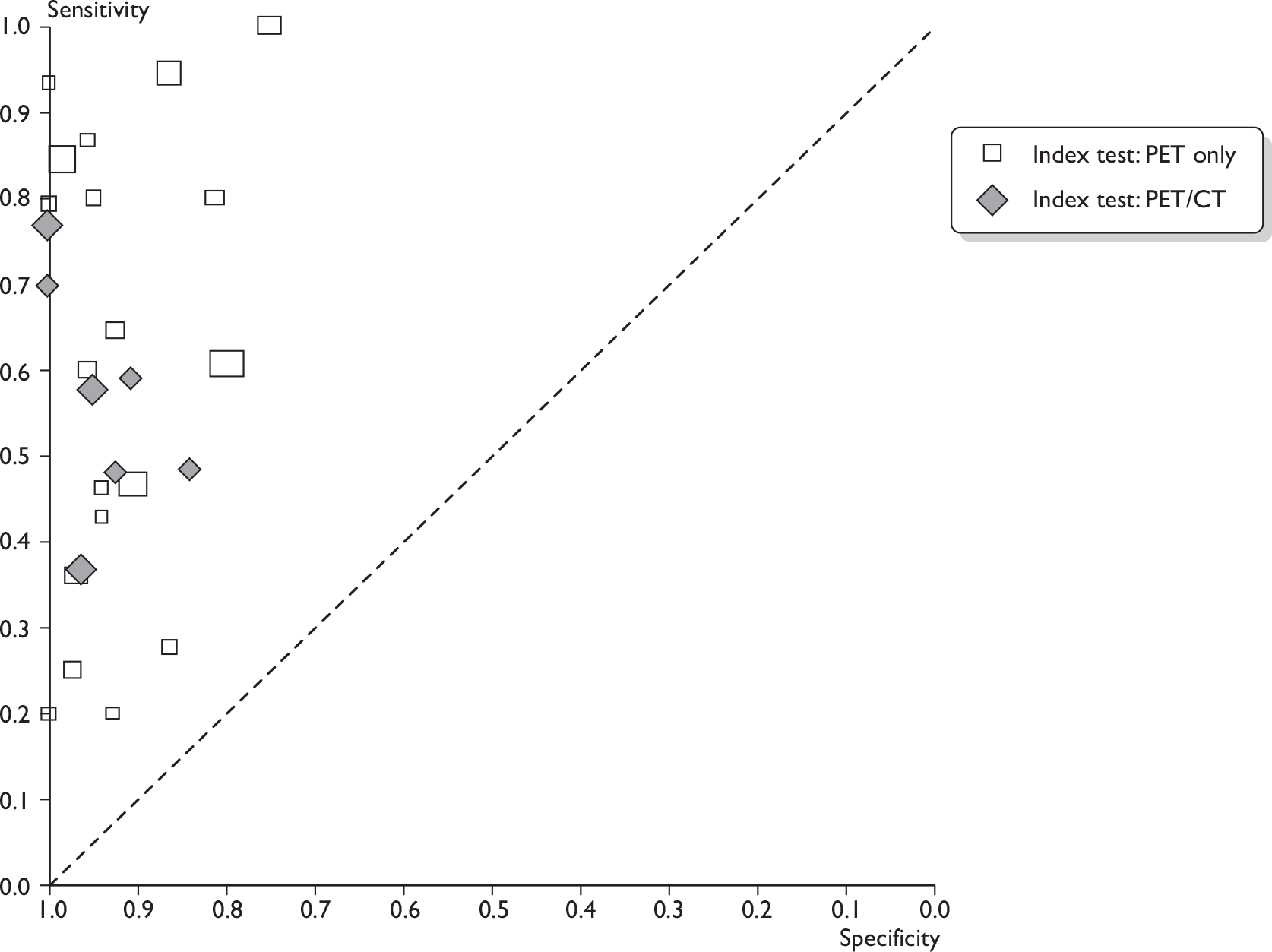
Summary findings
Across 26 studies of PET or PET/CT (n = 2591 patients),44,73–97 the mean sensitivity was 63% [95% confidence interval (CI) 52% to 74%; range 20%–100%] and the mean specificity was 94% (95% CI 91% to 96%; range 75%–100%) (Table 9 and Figures 5 and 6). For the seven studies of PET/CT (n = 862),73–79 the mean sensitivity was 56% (95% CI 44% to 67%) and the mean specificity was 96% (95% CI 90% to 99%). For the 19 studies of PET only (n = 1729),44,80–97 the mean sensitivity was 66% (95% CI 50% to 79%) and the mean specificity was 93% (95% CI 89% to 96%). Therefore PET/CT gave a slightly lower mean sensitivity than PET only. The reason for this is not clear, as a concurrent CT scan is generally thought to enhance the accuracy of PET. As sensitivity varied widely between studies, this finding may have been due to chance. It may also have been due to differences in populations or study methods; for example, PET/CT studies used ALND/SLNB for all patients, whereas some PET-only studies used another reference standard for some patients, which may have led to overestimates of sensitivity (see Effect of reference standard). Also, three of seven PET/CT studies73,76,79 (compared with 5,83,84,87,88 of 19 PET-only studies) were restricted to clinically node-negative patients, which may have decreased the overall sensitivity estimate for the PET/CT studies (see Effect of clinical nodal status).
| Diagnostic test | No. of studies | No. of patients | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|
| All PET studies | ||||
| All PET studies44,73–97 | 26 | 2591 | 63 (52 to 74) | 94 (91 to 96) |
| PET studies with or without CT | ||||
| PET/CT73–79 | 7 | 862 | 56 (44 to 67) | 96 (90 to 99) |
| PET (no CT)44,80–97 | 19 | 1729 | 66 (50 to 79) | 93 (89 to 96) |
FIGURE 5.
Forest plot of all PET studies. Brackets show 95% CIs. The figure shows the sensitivity and specificity for each study (squares) and 95% CIs (horizontal lines).
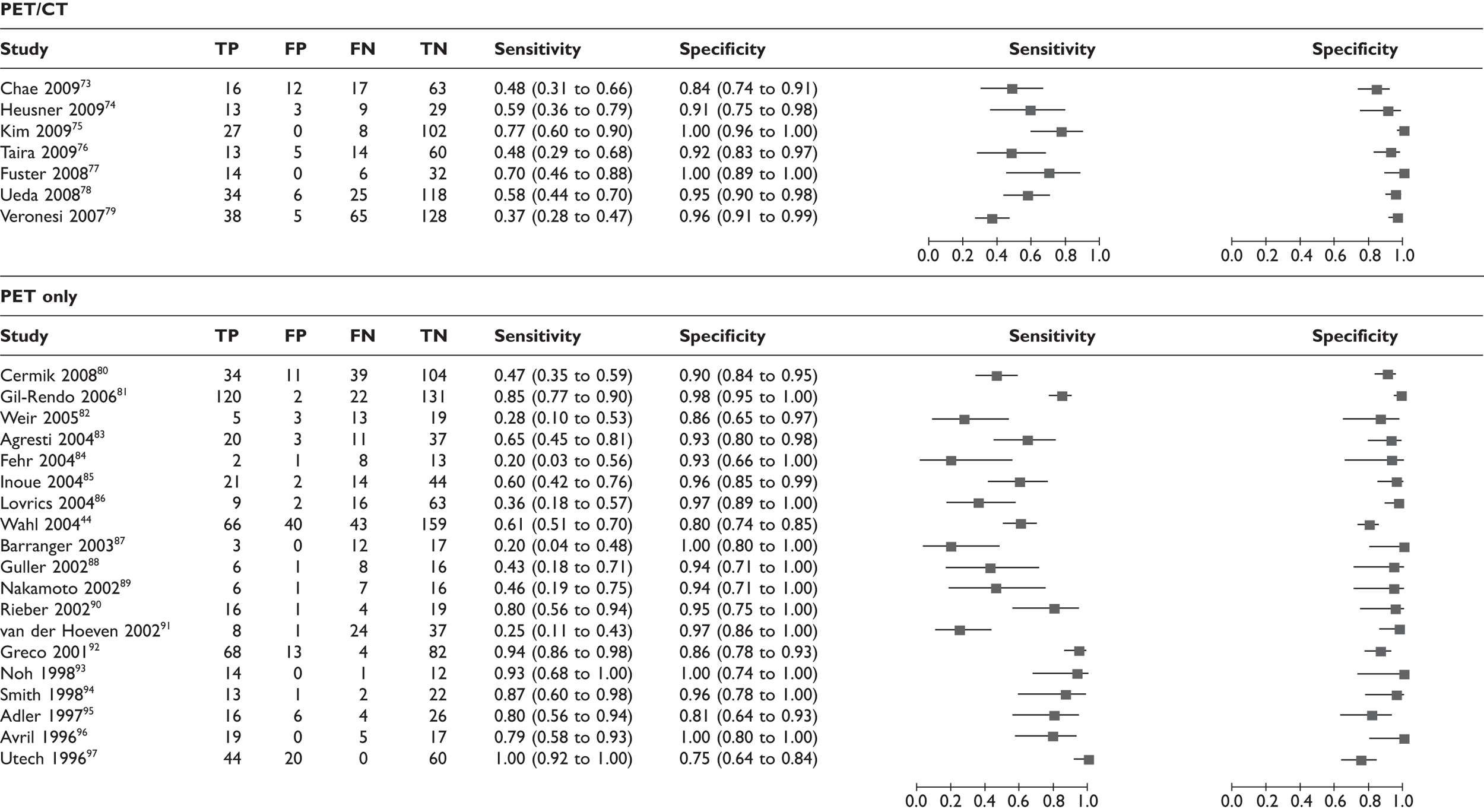
FIGURE 6.
Receiver operating characteristic plots for PET. (a) All PET studies, showing ROC curve (solid line), mean sensitivity/specificity (black spot) and 95% confidence region (dashed ellipse); (b) studies of PET only (rectangles) and PET/CT (diamonds). Rectangles/diamonds indicate sensitivity and specificity for individual studies (size proportional to sample sizes).
Effect of date of publication
Although the majority of studies do not show a clear trend in results according to date of publication, the earliest six studies (published between 1996 and 2001)92–97 do appear to report higher sensitivities than later studies (see Figure 5). This may reflect differences in methodology; for example, four93,94,96,97 of six of these early studies did not report using ALND or SLNB for all patients, which may have led to overestimates of sensitivity. Also, none of these early studies restricted inclusion by clinical nodal status, whereas a subset of the later studies included clinically node-negative patients only, which may have decreased the overall sensitivity estimate for the later studies.
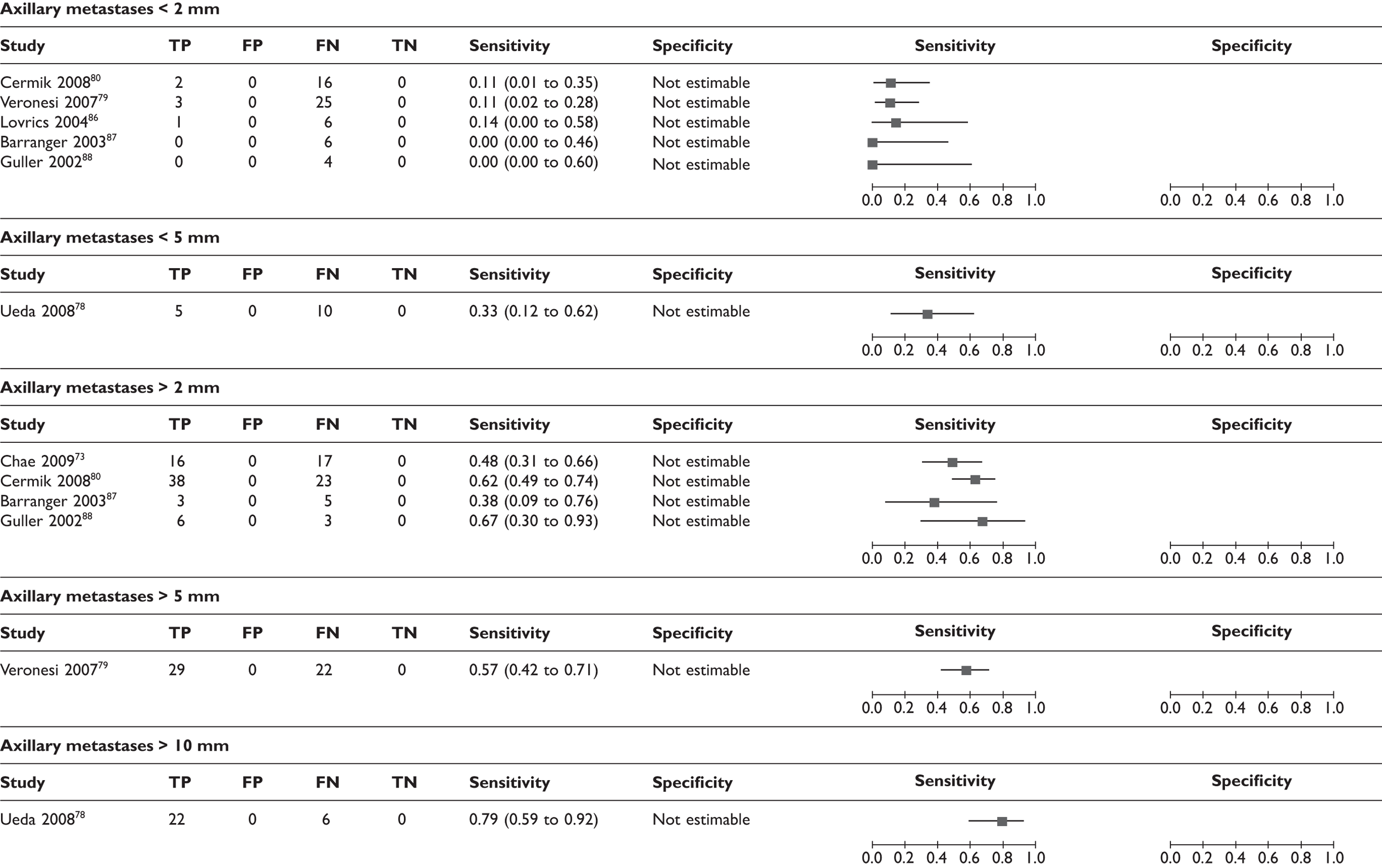
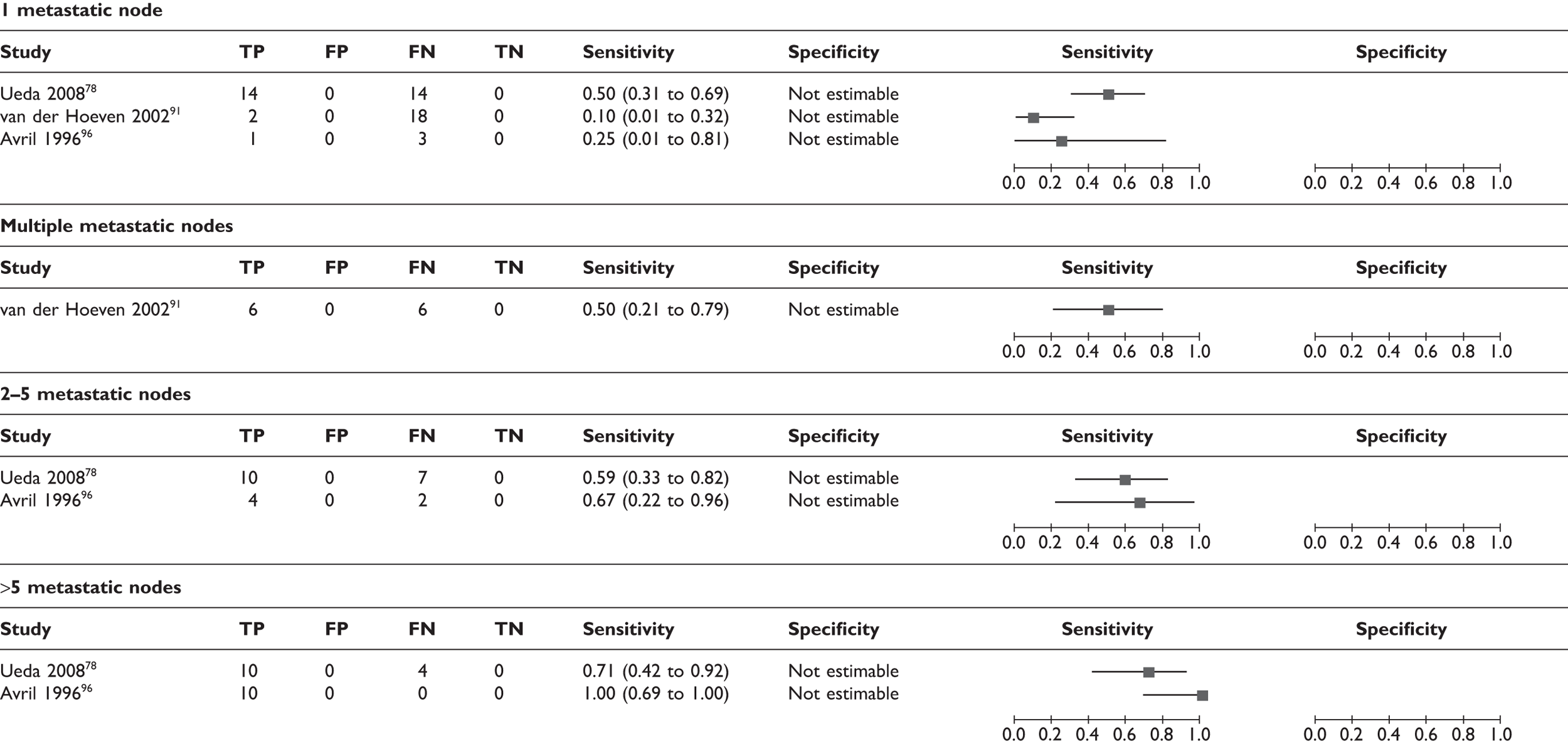
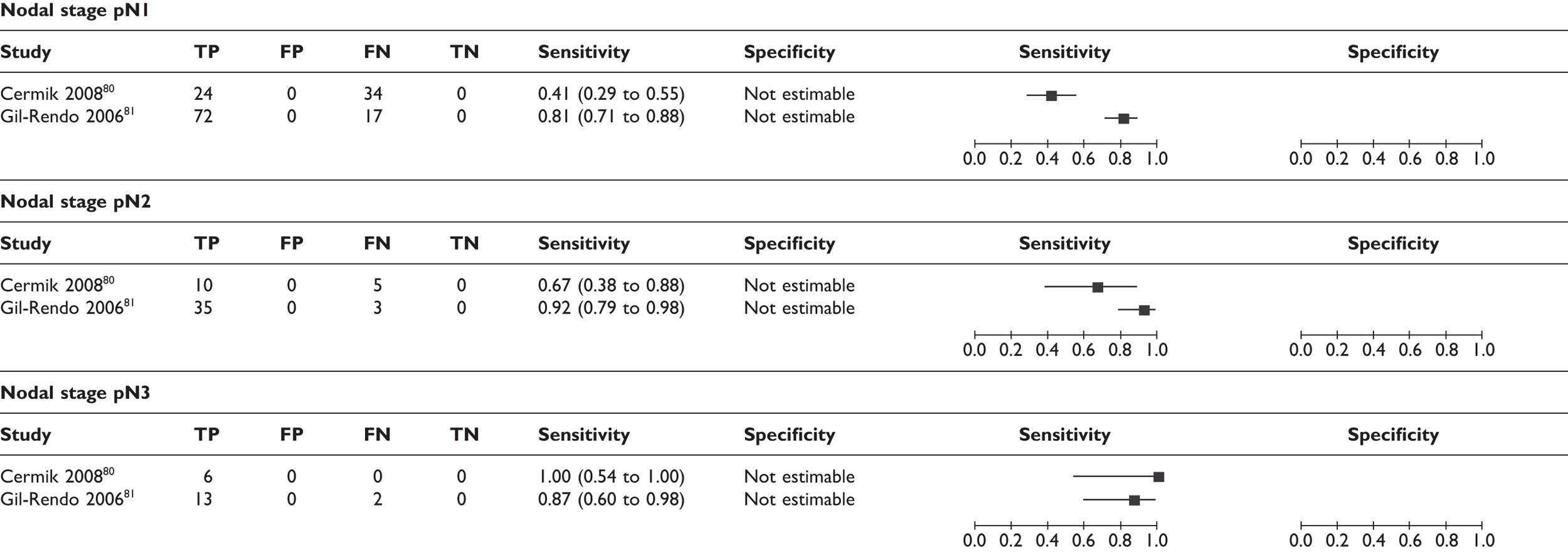
Effect of size and number of axillary metastases
A few studies analysed the sensitivity according to the size and number of axillary metastases. Thresholds for size were not consistent across studies and the number of analysed patients was small. However, there was a trend for lower sensitivities where metastatic lymph nodes were smaller or fewer in number (Table 10; Figure 7). Axillary micrometastases (≤ 2 mm) were associated with a mean sensitivity of 11% (95% CI 5% to 22%) based on data from five studies (n = 63 patients),79,80,86–88 while macrometastases (> 2 mm) were associated with a mean sensitivity of 57% (95% CI 47% to 66%) based on data from four studies (n = 111 patients). 73,80,87,88 In addition, some studies reported the mean size and number of axillary metastases in TP patients (i.e. those detected by PET) and FN patients (i.e. those which PET failed to detect). As shown in Table 11, the cases which PET failed to detect tended to have smaller and fewer axillary metastases, although there was variation between studies. The smallest metastatic nodes detected by PET measured 3 mm,87,88 while PET failed to detect some nodes measuring > 15 mm86,94 (including one case of 25 mm in one study94).
| Subgroup | No. of studies | No. of patients | Sensitivity, % (95% CI) | Specificitya, % (95% CI) |
|---|---|---|---|---|
| Size of axillary metastases | ||||
| ≤ 2 mm79,80,86–88 | 5 | 63 | 11 (5 to 22) | Not calculable |
| < 5 mm78 | 1 | 15 | 33 (15 to 59) | Not calculable |
| > 2 mm73,80,87,88 | 4 | 111 | 57 (47 to 66) | Not calculable |
| > 5 mm79 | 1 | 51 | 57 (43 to 70) | Not calculable |
| > 10 mm78 | 1 | 28 | 79 (60 to 90) | Not calculable |
| Number of axillary metastases | ||||
| 1 metastatic node78,91,96 | 3 | 52 | 27 (7 to 63) | Not calculable |
| Multiple metastatic nodes91 | 1 | 12 | 50 (21 to 79) | Not calculable |
| 2–5 metastatic nodes78,96 | 2 | 23 | 61 (40 to 78) | Not calculable |
| > 5 metastatic nodes78,96 | 2 | 24 | 77 (53 to 91) | Not calculable |
| Nodal stage | ||||
| pN180,81 | 2 | 147 | 63 (24 to 91) | Not calculable |
| pN280,81 | 2 | 53 | 83 (51 to 96) | Not calculable |
| pN380,81 | 2 | 21 | 88 (66 to 97) | Not calculable |
| Study | TP cases (PET detected) | FN cases (PET failed to detect) | ||||
|---|---|---|---|---|---|---|
| No. of patients in analysis | Mean | Range | No. of patients in analysis | Mean | Range | |
| Size of largest metastatic node per patient (mm) | ||||||
| Heusner 200974 | 13 | 9.0 | 4–22 | 9 | 3.0 | 0.8–6 |
| Kim 200975 | 8 | 2.6 | 1–7 | |||
| Weir 200582 | 13 | All ≤ 10 | ||||
| Fehr 200484 | 2 | 11.0 | 10–12 | 8 | 7.5 | Micro–15 |
| Lovrics 200486 | 9 | 22.0 | 16 | 13.0 | NR (> 15 in 7 cases) | |
| Wahl 200444 | 66 | 15.6 | 43 | 11.5 | ||
| Barranger 200387 | 3 | 12.0 | 3–20 | 12 | 4.2 | 0.1–10 |
| Guller 200288 | 6 | 13.5 | 3–30 | 8 | 2.9 | ITCs–13 |
| Smith 199894 | 19 | 8–NR | 2 | 18.5a | 12–25 | |
| Number of metastatic nodes | ||||||
| Heusner 200974 | 13 | 4.3 | 1–11 | 9 | 1.8 | 1–4 |
| Kim 200975 | 8 | 1.4 | 1–3 | |||
| Fuster 200877 | 14 | 5.2 | 1–19 | 6 | 1.8 | 1–3 |
| Lovrics 200486 | 9 | 5.8 | 16 | 2.1 | NR (≥ 3 in 5 cases) | |
| Wahl 200444 | 66 | 5.0 | 43 | 2.7 | ||
| Greco 200192 | 4 | 1.3 | 1–2 | |||
| Avril 199696 | 5 | 1–4 | ||||
FIGURE 7.
Forest plot of sensitivity of PET according to (a) size of axillary metastases; (b) number of axillary metastases; and (c) nodal stage. Brackets show 95% CIs. The figure shows the sensitivity and specificity for each study (squares) and 95% CIs (horizontal lines).
Effect of clinical nodal status
Studies in which all patients were clinically node negative, which generally referred to non-palpable axillary nodes, had a trend towards lower sensitivity and similar specificity when compared with studies including both clinically node-negative and node-positive patients (Table 12). This may reflect the fact that clinically negative axillary metastases are likely to be smaller and more difficult to detect via PET. This analysis was limited by the fact that, even in studies with a mixed population, the majority of patients were clinically node negative (see Table 7). One study reported separate results according to clinical nodal status: among clinically node-negative patients the sensitivity was 39/42 (93%) and the specificity 76/87 (87%), while among clinically node-positive patients the sensitivity was 29/30 (97%) and the specificity 6/8 (75%). 92 The mix of clinically node-positive and node-negative patients within the included studies was thought to be representative of clinical practice.
| Subgroup | No. of studies | No. of patients | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|
| Clinical nodal status | ||||
| All clinically negative73,76,79,81,83,84,87,88 | 8 | 869 | 48 (32 to 64) | 94 (90 to 97) |
| Mix of positive and negative44,74,77,78,80,85,86,91–94,96,97 | 13 | 1423 | 72 (54 to 85) | 93 (88 to 96) |
| Nodal status not reported75,82,89,90,95 | 5 | 299 | 65 (44 to 81) | 95 (82 to 99) |
| Patient sample | ||||
| All patients early stage and newly diagnosed44,75,76,80,83–89,92,94,97 | 14 | 1413 | 63 (44 to 79) | 94 (89 to 96) |
| Not all patients early stage and newly diagnosed73,74,77–79,81,82,90,91,93,95,96 | 12 | 1178 | 63 (49 to 76) | 95 (91 to 98) |
| T stage (size) of breast tumour | ||||
| T1 (≤ 2 cm)74,79,81,84,88,89,92,94,96,97 | 10 | 451 | 53 (34 to 72) | 87 (82 to 91) |
| T2 (> 2 cm, ≤ 5 cm)74,77,79,81,84,88,89,92,94 | 9 | 343 | 67 (40 to 86) | 86 (78 to 92) |
| T2 or above (> 2 cm)96,97 | 2 | 82 | 96 (84 to 99) | 82 (13 to 99) |
| T3 (> 5 cm)74,77,79,84,89,94 | 6 | 41 | 65 (44 to 82) | 88 (58 to 98) |
| T4 (tumour spread/fixed to skin/chest wall, or IBC)94 | 1 | 10 | 100 (54 to 100) | 100 (40 to 100) |
| Reference standard | ||||
| 100% ALND44,73,77,84,87,89,92,95 | 8 | 773 | 59 (36 to 78) | 90 (80 to 95) |
| ALND and/or SLNB74–76,78–83,86,88,91 | 12 | 1467 | 52 (40 to 63) | 95 (93 to 97) |
| Not all ALND/SLNB, or not reported85,90,93,94,96,97 | 6 | 351 | 88 (68 to 96) | 94 (85 to 98) |
| Prevalence of axillary metastases | ||||
| < 40%44,73,75–78,80,86,95,97 | 10 | 1334 | 66 (49 to 80) | 92 (85 to 96) |
| 40%–49%74,79,82–85,87–89,91,92,94 | 12 | 874 | 51 (34 to 68) | 94 (91 to 96) |
| ≥ 50%81,90,93,96 | 4 | 383 | 84 (78 to 88) | 98 (94 to 99) |
Effect of patient sample
The mean sensitivity and specificity were not affected by whether all analysed patients were early stage (stage I, II or IIIA), newly diagnosed and non-DCIS; this may be explained by the fact that all included studies had a majority of patients who were early stage, newly diagnosed and non-DCIS (see Table 12).
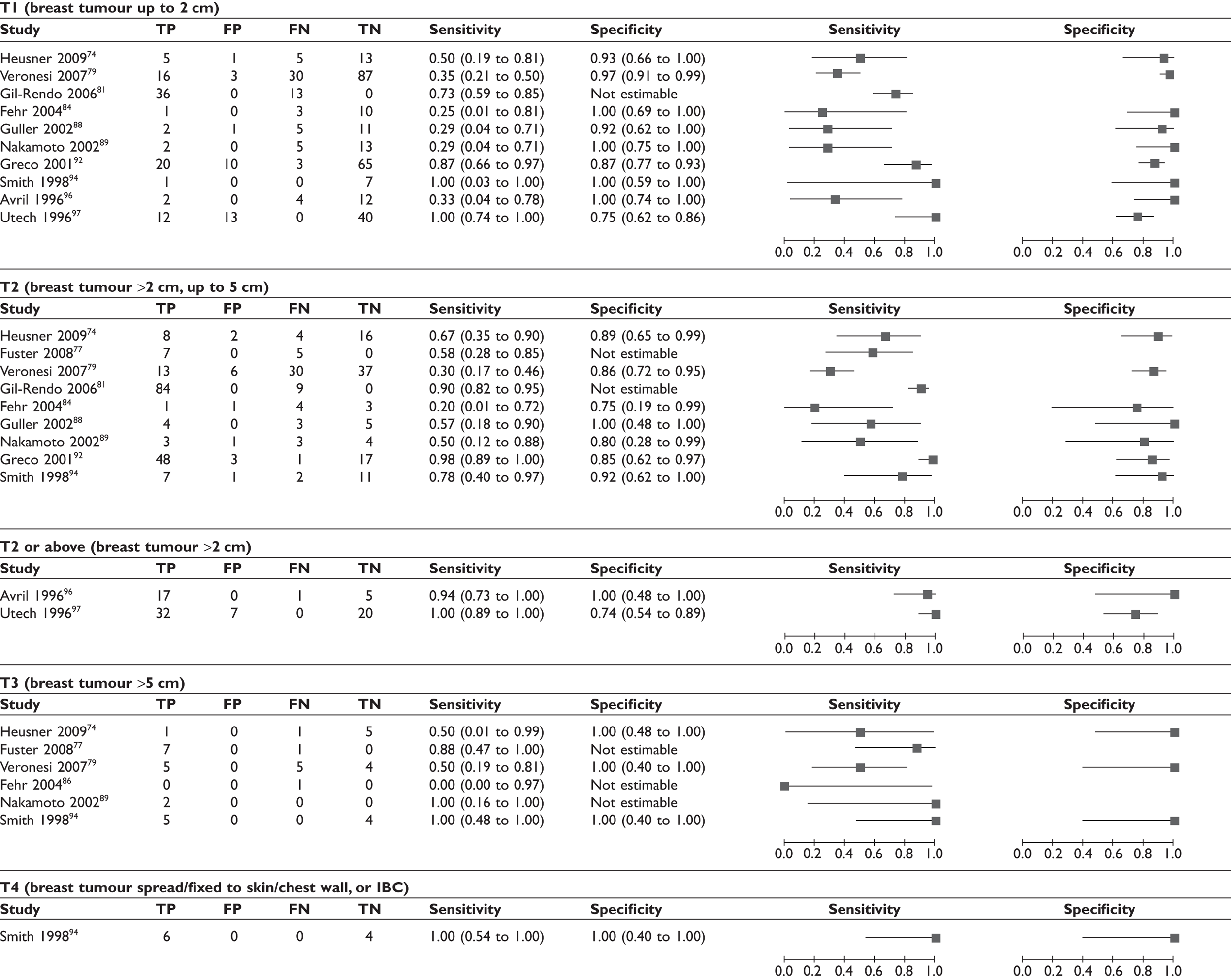
Effect of T stage (size) of the breast tumour
Some studies reported sensitivity and specificity according to the T stage (size) of the primary breast tumour (see Table 12; Figure 8). The pattern was difficult to interpret due to the wide variation in sensitivity and specificity between studies and small patient numbers per subgroup. Data from some of the individual studies suggest a trend for lower sensitivity in patients with smaller breast tumours (e.g. between T1 and T274,81,92 and between T2 and T379,94), although the pattern for the meta-analysed data is less clear.
FIGURE 8.
Forest plot of sensitivity of PET according to T stage (size) of breast tumour. Brackets show 95% CIs. The figure shows the sensitivity and specificity for each study (squares) and 95% CIs (horizontal lines). IBC, inflammatory breast cancer.
Effect of reference standard
In terms of reference standard, studies in which all patients received ALND had similar mean sensitivity and specificity values to studies in which some patients received ALND and some SLNB. Studies in which the reference standard was not stated (or some patients did not receive ALND or SLNB) had a higher sensitivity, possibly due to the poorer-quality reference standard in these studies (see Table 12).
Effect of prevalence of axillary metastases
There was no clear correlation between prevalence of axillary metastases and sensitivity or specificity (see Table 12).
Sensitivity analysis for study quality
Eleven quality items from the QUADAS checklist69 were used to assess study quality (see Appendix 2). For PET, study quality variables which had a significant effect (p < 0.10) on either sensitivity or specificity were as follows. Studies with a representative patient spectrum (all early stage, newly diagnosed and non-DCIS, with prospective and consecutive recruitment) had a higher sensitivity than those in which the patient spectrum was unclear (p = 0.001). Studies that used the same reference standard regardless of index test results (differential verification avoided) had a lower sensitivity than studies in which differential verification occurred or those in which this was unclear (p < 0.001). Studies in which the index test was interpreted blind to reference standard results had a higher sensitivity than studies in which this was unclear (p < 0.001). Studies in which the reference standard was interpreted blind to index test results had a lower sensitivity than studies in which this was unclear, but a higher sensitivity than studies where this was unblinded (although there were only two studies76,90 known to be unblinded) (p < 0.001). Studies in which uninterpretable test results did not occur, or occurred and were included in the analysis, had a lower sensitivity than studies in which this was unclear (p < 0.001). No study quality variables had a significant effect on the specificity of PET. These results show a mixed pattern. Studies which score ‘unclear’ on reporting of quality variables may be expected to be of poorer quality, but this could theoretically lead to either underestimates or overestimates of diagnostic accuracy.
Magnetic resonance imaging studies: diagnostic accuracy results
Summary findings
Of the nine studies evaluating MRI (n = 307 patients),41,42,64,108–113 several reported more than one set of results on diagnostic accuracy, according to different criteria for defining whether axillary metastases were present. Results are first presented across all studies using the highest reported sensitivity and specificity for each study (Table 13 and Figures 9 and 10). Results are then presented according to each criterion for positivity (Table 14 and Figures 11 and 12).
| Diagnostic test | No. of studies | No. of patients | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|
| All MRI studies | ||||
| All MRI studies41,42,64,108–113 | 9 | 307 | 90 (78 to 96) | 90 (75 to 96) |
| MRI studies by type of MRI | ||||
| USPIO-enhanced MRI108–112 | 5 | 93 | 98 (61 to 100) | 96 (72 to 100) |
| Gadolinium-enhanced MRI41,42,113 | 3 | 187 | 88 (78 to 94) | 73 (63 to 81) |
| MR spectroscopy64 | 1 | 27 | 65 (38 to 86) | 100 (69 to 100) |
| Criteria for positivity | No. of studies | No. of patients | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|
| USPIO-based criteria | ||||
| USPIO uptake108–111 | 4 | 75 | 98 (63 to 100) | 94 (69 to 99) |
| USPIO uptake, size > 10 mm, round shape112 (not clear if ‘and’ or ‘or’) | 1 | 18 | 82 (48 to 98) | 100 (59 to 100) |
| Gadolinium-based criteria | ||||
| Gd uptake, size > 5 mm42 (not clear if ‘and’ or ‘or’) | 1 | 75 | 90 (76 to 97) | 83 (66 to 93) |
| Dynamic Gd signal intensity increase41,113 | 2 | 112 | 86 (68 to 94) | 59 (45 to 72) |
| Dynamic Gd + positive washout41 | 1 | 65 | 71 (49 to 87) | 90 (77 to 97) |
| Dynamic Gd + size > 4 sq-mm113 | 1 | 47 | 100 (69 to 100) | 54 (37 to 71) |
| Dynamic Gd + size > 5 mm + abnormal morphology41 | 1 | 65 | 63 (41 to 81) | 93 (80 to 98) |
| MR spectroscopy | ||||
| MR spectroscopy64 | 1 | 27 | 65 (38 to 86) | 100 (69 to 100) |
| Size and/or morphological criteria | ||||
| Size > 4 sq-mm113 | 1 | 47 | 100 (69 to 100) | 19 (8 to 35) |
| Size > 5 mm109 | 1 | 33 | 100 (85 to 100) | 10 (0 to 45) |
| Size > 10 mm109 | 1 | 33 | 43 (23 to 66) | 80 (44 to 97) |
| Abnormal morphology109 | 1 | 33 | 96 (78 to 100) | 20 (3 to 56) |
| Size > 5 mm + abnormal morphology109 | 1 | 65 | 63 (41 to 81) | 80 (65 to 91) |
| Size > 10 mm and/or round shape110 | 1 | 22 | 83 (36 to 100) | 31 (11 to 59) |
FIGURE 9.
Forest plot of all MRI studies. Brackets show 95% CIs. The figure shows the sensitivity and specificity for each study (squares) and 95% CIs (horizontal lines). Where studies report results using more than one set of criteria for positivity, these analyses use data corresponding to the criteria with the highest reported estimates of diagnostic accuracy per study. The criteria used for each study are shown on the plot.
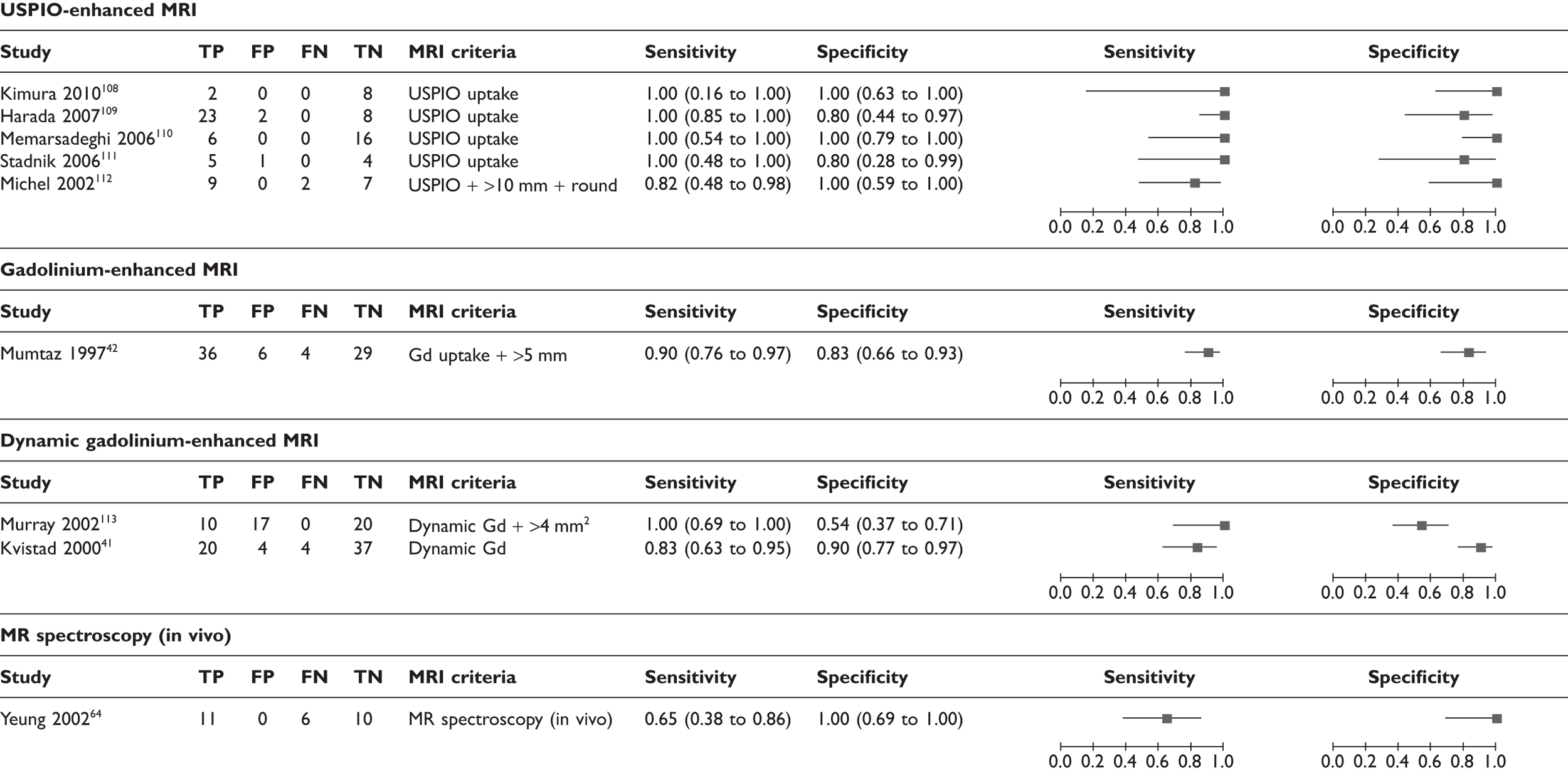
FIGURE 10.
Receiver operating characteristic plots for MRI. (a) All MRI studies, showing ROC curve (solid line), mean sensitivity/specificity (black spot) and 95% confidence region (dashed ellipse); (b) all MRI studies, showing type of MRI. Shapes indicate sensitivity and specificity for individual studies (size proportional to sample sizes, legend shows type of MRI). Studies appear only once, using the highest reported sensitivity and specificity per study.
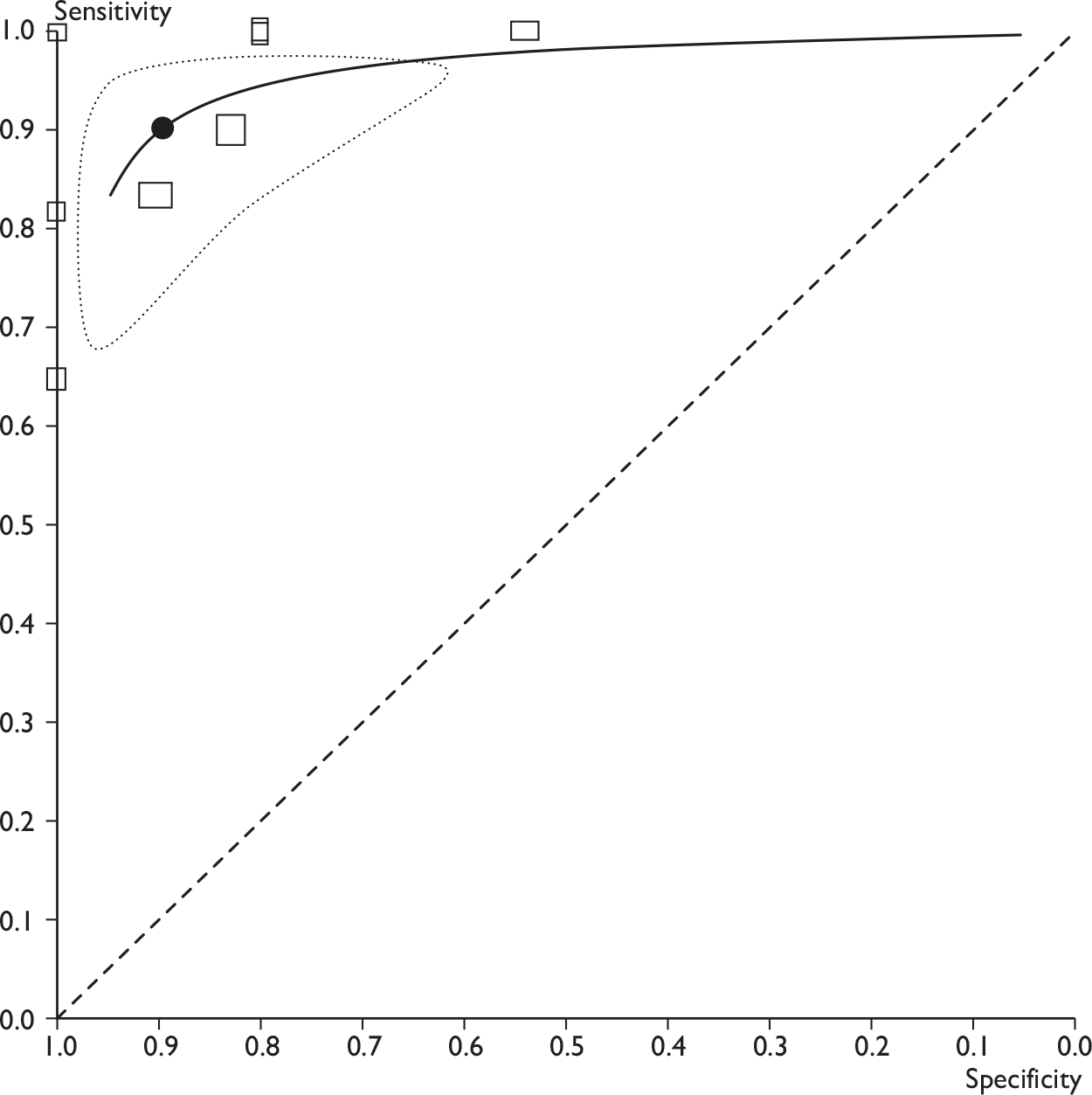
FIGURE 11.
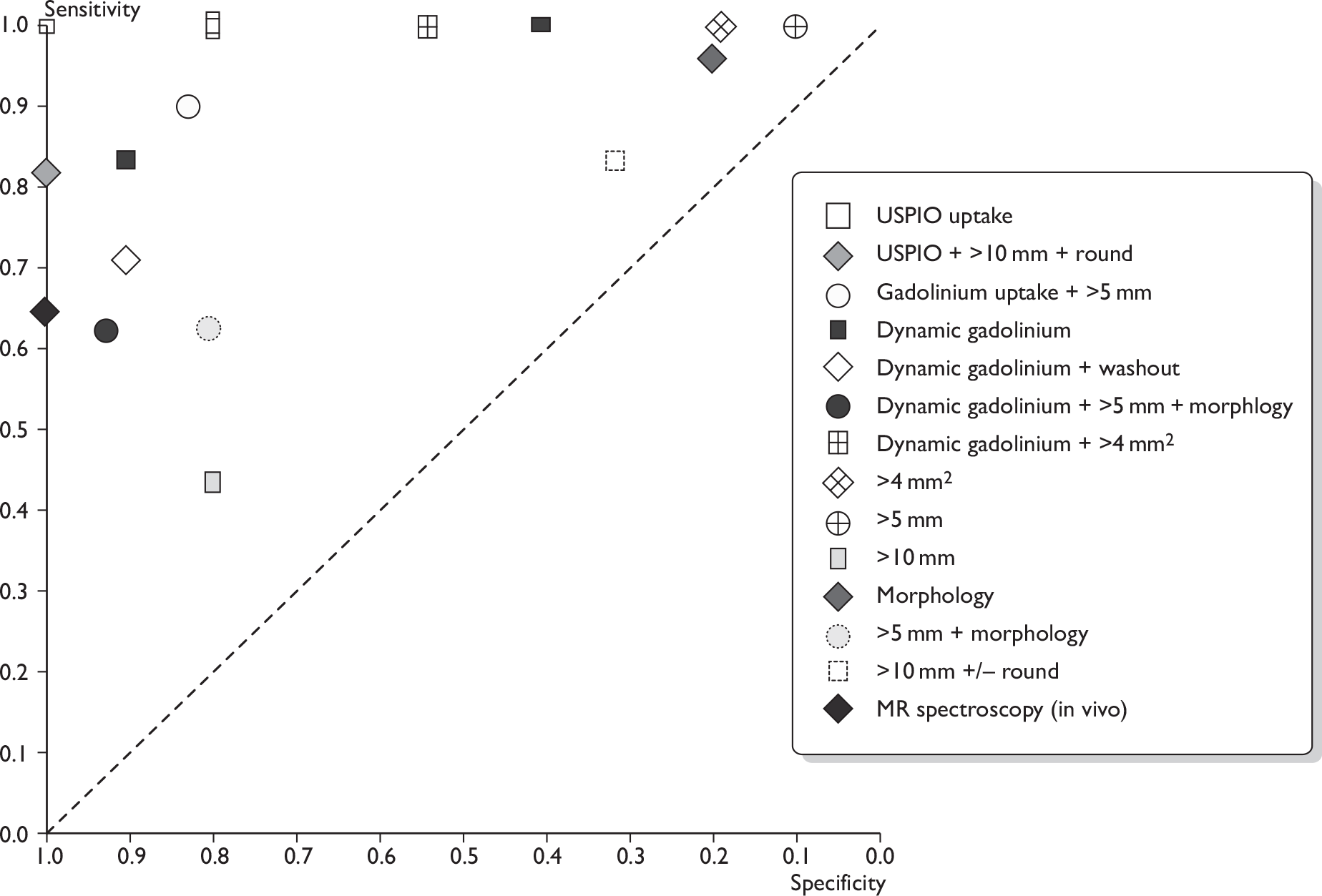
Forest plots of MRI studies (using various criteria for defining a node as metastatic; many studies appear more than once). (a) Based on ultrasmall super-paramagnetic iron oxide uptake (and size/morphology); (b) based on gadolinium uptake (and size/morphology); (c) Based on size and/or morphology; (d) based on MR spectroscopy. Brackets show 95% CIs. The figure shows the sensitivity and specificity for each study (squares) and 95% CIs (horizontal lines).
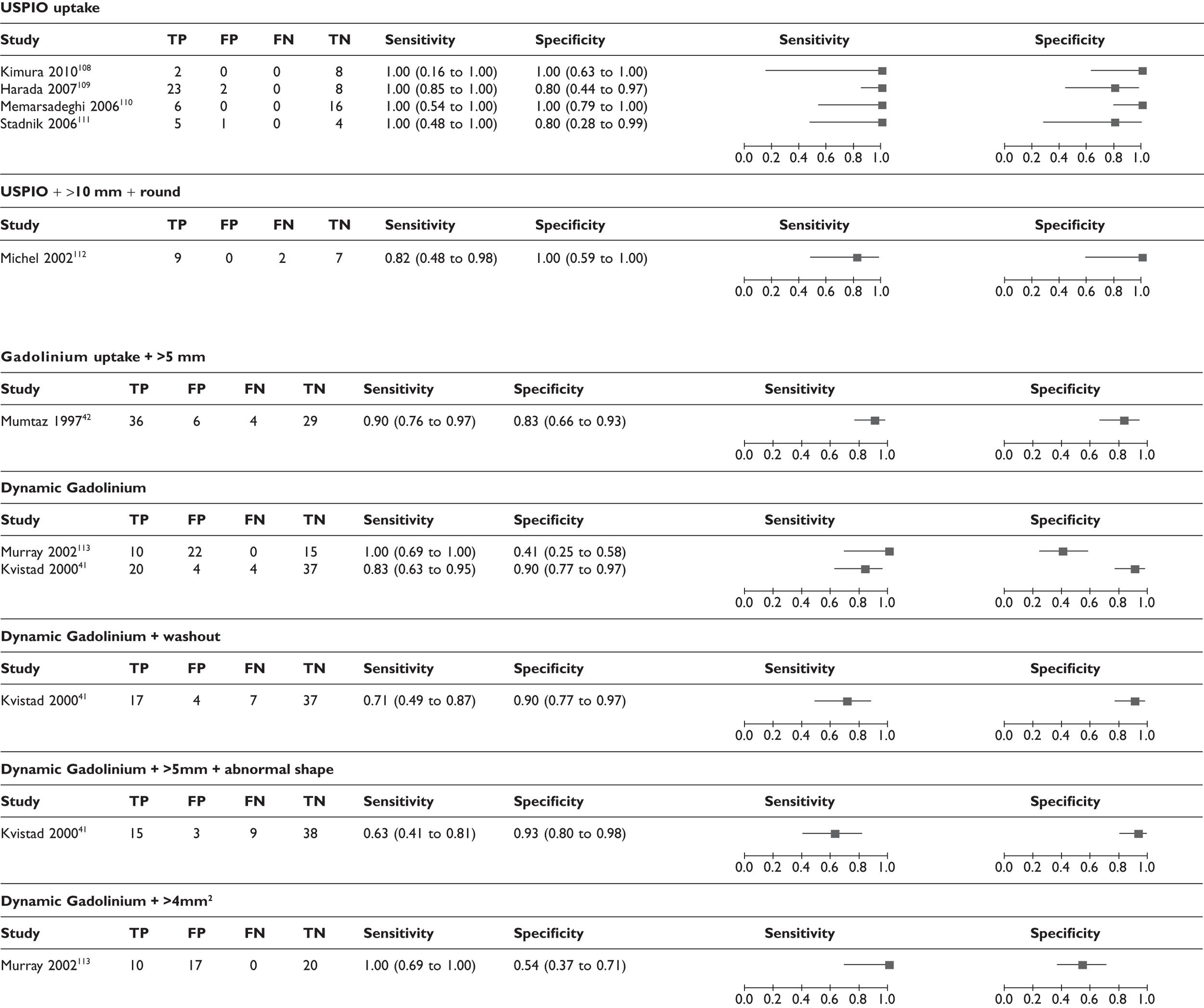
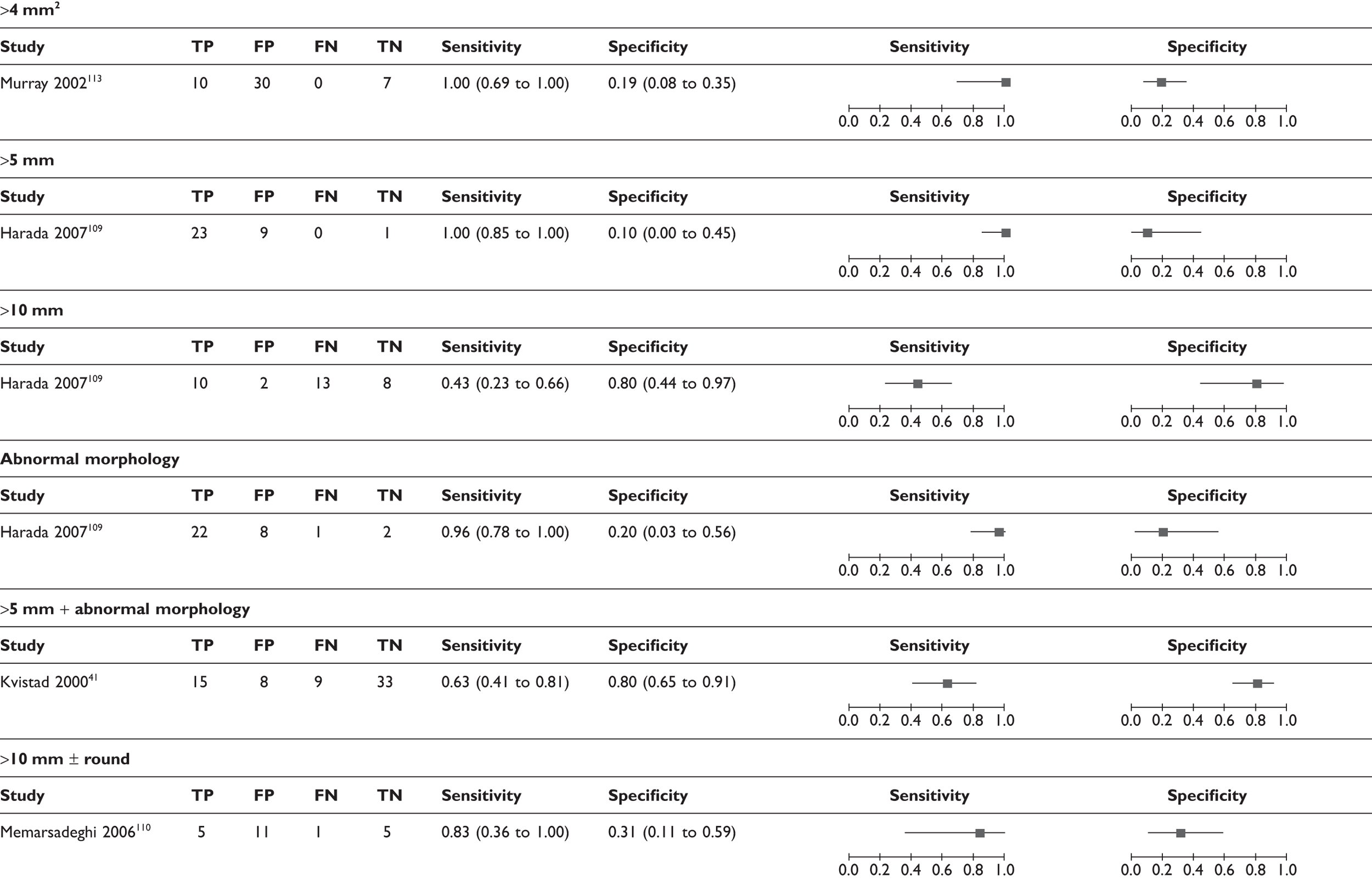
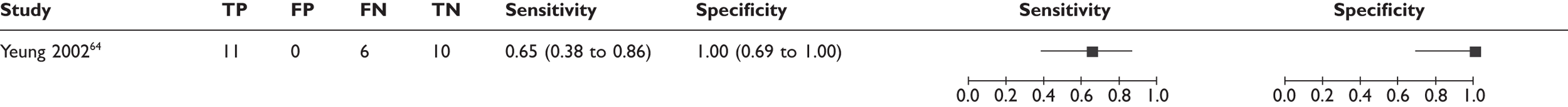
FIGURE 12.
Receiver operating characteristic plot for MRI (using various criteria for defining a node as metastatic). Shapes indicate sensitivity and specificity for individual studies (size proportional to sample sizes, legend shows criteria used for defining a node as metastatic). Many studies appear more than once.
Across all nine MRI studies, using the highest sensitivity and specificity for each study, the mean sensitivity was 90% (95% CI 78% to 96%; range 65%–100%) and the mean specificity was 90% (95% CI 75% to 96%; range 54%–100%) (see Table 13 and Figures 9 and 10). According to the type of MRI, the mean estimates of sensitivity and specificity were as follows (see Table 13). Across five studies of USPIO-enhanced MRI (n = 93),108–112 the mean sensitivity was 98% (95% CI 61% to 100%)and specificity 96% (95% CI 72% to 100%). Across three studies of gadolinium-enhanced MRI (n = 187),41,42,113 the mean sensitivity was 88% (95% CI 78% to 94%) and specificity 73% (95% CI 63% to 81%). In the single study of in vivo proton MR spectroscopy (n = 27),64 the sensitivity was 65% (95% CI 38% to 86%) and the specificity 100% (95% CI 69% to 100%). Therefore, USPIO-enhanced MRI showed a trend towards higher sensitivity and specificity than gadolinium-enhanced MRI.
In addition, the diagnostic accuracy data were analysed according to the criteria for defining whether axillary metastases were present (e.g. based on USPIO or gadolinium uptake, size, morphology, or combinations of these criteria) (see Table 14 and Figures 11 and 12). Within this analysis, some studies appear more than once. The exact combinations of criteria were often not consistent across studies. The use of contrast uptake pattern as the main criterion for defining a node as metastatic appeared to give better combined sensitivity and specificity than size and morphology, although many studies used criteria based on both uptake and size/morphology, and the methods of interpreting uptake patterns varied within and between studies.
Effect of size and number of axillary metastases
No MRI studies presented data allowing calculation of sensitivity according to size and number of axillary metastases.
Effect of clinical nodal status
It was difficult to assess the effect of clinical nodal status as six42,109–113 of nine studies did not report these data (Table 15).
| Subgroup | No. of studies | No. of patients | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|---|---|
| Reference standard | ||||
| 100% ALND41,42,64,109–113 | 8 | 297 | 91 (78 to 97) | 88 (73 to 95) |
| ALND and/or SLNB108 | 1 | 10 | 100 (16 to 100) | 100 (63 to 100) |
| Not all ALND/SLNB or not reported | 0 | 0 | N/A | N/A |
| Cancer stage | ||||
| All patients early stage and newly diagnosed108,110,113 | 3 | 79 | 90 (63 to 98) | 61 (46 to 74) |
| Not all patients early stage and newly diagnosed41,42,64,109,111,112 | 6 | 228 | 83 (74 to 89) | 86 (78 to 92) |
| Clinical nodal status | ||||
| All clinically negative108 | 1 | 10 | 100 (16 to 100) | 100 (63 to 100) |
| Mix of positive and negative41,64 | 2 | 92 | 75 (69 to 79) | 91 (86 to 94) |
| Nodal status not reported42,109–113 | 6 | 205 | 94 (83 to 98) | 85 (63 to 95) |
| Prevalence of axillary metastases | ||||
| < 40%41,108,110,113 | 4 | 144 | 86 (71 to 94) | 71 (59 to 81) |
| 40%–49% | 0 | 0 | N/A | N/A |
| ≥ 50%42,64,109,111,112 | 5 | 163 | 94 (89 to 97) | 84 (73 to 91) |
Effect of patient sample
Studies in which all analysed patients were early stage (stage I, II or IIIA), newly diagnosed and non-DCIS had a trend towards a higher sensitivity, and a significantly lower specificity, than studies in which not all patients were early stage, newly diagnosed and non-DCIS; however, there was wide variation in results between studies (see Table 15).
Effect of T stage (size) of the breast tumour
There were insufficient consistently reported data to assess the relationship between individual size or stage categories for the primary tumour, and the sensitivity and specificity of MRI in detecting axillary metastases.
Effect of reference standard
It was not possible to assess the effect of reference standard as, in eight41,42,64,109–113 of nine studies, all patients received ALND (see Table 15).
Effect of prevalence of axillary metastases
There was no clear correlation between prevalence of axillary metastases and sensitivity or specificity (see Table 15).
Sensitivity analysis for study quality
Eleven quality items from the QUADAS checklist69 were used to assess study quality (see Appendix 2). For MRI, no study quality variables had a significant effect on sensitivity or specificity, although this analysis was limited by the small number of studies and the fact that there was little variation in scoring between studies for some quality variables.
Withdrawal rates, adverse events and contraindications
Positron emission tomography studies
In 2073–76,78–85,87–89,91,92,94,96,97 of the 26 PET studies, no withdrawals were reported (however, studies that were not prospective and consecutive may have included only patients with complete data for both tests). The remaining six studies reported that between 4% and 22% of patients withdrew. 44,77,86,90,93,95 Reasons for withdrawal included no ALND, anxiety and inconvenience, unavailability of PET scanner, and unusable PET scans (Table 16). No adverse events were reported for any of the 26 PET studies. In addition, many PET studies excluded patients who were pregnant or had diabetes mellitus.
| Study | Index test | No. met criteria? | Withdrawals (%) | Reasons (%) | Adverse effects |
|---|---|---|---|---|---|
| Chae 200973 | PET/CT | 108 | None reported | None reported | |
| Heusner 200974 | PET/CT | 54 | None reported | None reported | |
| Kim 200975 | PET/CT | 137 | None reported | None reported | |
| Taira 200976 | PET/CT | 92 axilla | None reported | None reported | |
| Fuster 200877 | PET/CT | 60 | 8/60 (13) | Eight (13) no ALND (reason not reported) | None reported |
| Ueda 200878 | PET/CT | 183 | None reported | None reported | |
| Veronesi 200779 | PET/CT | 236 | None reported | None reported | |
| Cermik 200880 | PET only | 188 axilla | None reported | None reported | |
| Gil-Rendo 200681 | PET only | 275 | None reported | None reported | |
| Weir 200582 | PET only | 40 | None reported | None reported | |
| Agresti 200483 | PET only | 71 | None reported | None reported | |
| Fehr 200484 | PET only | 24 | None reported | None reported | |
| Inoue 200485 | PET only | 81 | None reported | None reported | |
| Lovrics 200486 | PET only | 115 | 25/115 (22) | Seventeen (15) withdrew (anxiety and inconvenience), six (5) no PET (machine unavailable), two (2) no PET (anxiety) | None reported |
| Wahl 200444 | PET only | 330 axilla | 22/330 (7) | Five (1.5) no surgery (reason not reported), 15 (5) SLNB, only two (0.6) unusable PET scans | None reported |
| Barranger 200387 | PET only | 32 | None reported | None reported | |
| Guller 200288 | PET only | 31 | None reported | None reported | |
| Nakamoto 200289 | PET only | 30 | None reported | None reported | |
| Rieber 200290 | PET only | 43 | 3/43 (7) | Three (7) reason not reported | None reported |
| van der Hoeven 200291 | PET only | 70 | None reported | None reported | |
| Greco 200192 | PET only | 167 | None reported | None reported | |
| Noh 199893 | PET only | 31 axilla | 4/31 (13) | Four (13) reason not reported | None reported |
| Smith 199894 | PET only | 38 | None reported | None reported | |
| Adler 199795 | PET only | 54 axilla | 2/54 (4) | Two (4) PET scans uninterpretable due to high FDG accumulation in myocardium | None reported |
| Avril 199696 | PET only | 41 | None reported | None reported | |
| Utech 199697 | PET only | 124 | None reported | None reported |
Magnetic resonance imaging studies
In five108,109,111–113 of nine MRI studies, no withdrawals were reported (however, studies that were not prospective and consecutive may have included only patients with complete data for both tests). The remaining four studies reported that between 3% and 18% of patients withdrew. 41,42,64,110 Reasons for withdrawal included no ALND, inadequate MRI data, and claustrophobia or poor health (Table 17). No serious adverse effects were reported in any of the MRI studies. Mild-to-moderate adverse effects included mild rash following USPIO administration (recovered without treatment or following antihistamine treatment) and inability to complete the MRI scan due to claustrophobia or back pain as a result of holding the same position for some time (see Table 17). In addition, many of the studies excluded patients with contraindications to MRI, such as strong allergic disposition, allergy to contrast agents or liver dysfunction (see Table 8).
| Study | Index test | No. met criteria? | Withdrawals (%) | Reasons (%) | Adverse effects |
|---|---|---|---|---|---|
| Kimura 2010108 | USPIO-enhanced | 10 | None reported | No serious adverse effects. Mild rash on four limbs of one patient after USPIO; recovered without further treatment | |
| Harada 2007109 | USPIO-enhanced | 33 | None reported | No serious adverse effects. 2/33 (6%) patients had minor adverse event of mild rash; one (3%) required oral antihistamine and one (3%) recovered without further treatment | |
| Memarsadeghi 2006110 | USPIO-enhanced | 24 | 2/24 (8) | 2/24 (8) no ALND (reason not reported) | No discomfort or adverse reactions were observed (0/22, 0%) |
| Stadnik 2006111 | USPIO-enhanced | 10 | None reported | None reported | |
| Michel 2002112 | USPIO-enhanced | 18 | None reported | No serious adverse effects. Of 22 examinations in 20 patients: mild adverse effects in 3/22 (14%); moderate adverse effect in 1/22 (5%); and antihistamines administered. Symptoms: rash (n = 2), pruritis (n = 2), abdominal and or lumbar pain (n = 1), chest pain (n = 3), orthostatic reaction (n = 1). MRI of axilla performed in all 20 patients, but three wished to terminate scan before MRI of breast due to claustrophobia or back pain as a result of holding same position | |
| Murray 2002113 | Dynamic gadolinium-enhanced | 47 | None reported | None reported | |
| Kvistad 200041 | Dynamic gadolinium-enhanced | 67 | 2/67 (3) | One (1.5) no ALND due to old age, one (1.5) died before surgery | None reported |
| Mumtaz 199742 | Gadolinium-enhanced | 92 axilla | 17/92 (18) | Six (7) image obscured by cardiac flow or not in field of view, eight (9) inadequate MR data and three (3) no ALND data | None reported |
| Yeung 200264 | MR spectroscopy | 32 | 5/32 (16) | One (3) no MR spectroscopy due to machine breakage, three (9) no MR spectroscopy due to claustrophobia or poor general health, and one (3) refused surgery | 3/39 (8%) could not complete procedure due to claustrophobia or poor general health |
Discussion for clinical effectiveness
Diagnostic accuracy of positron emission tomography
Across all 26 studies (n = 2591 patients)44,73–97 evaluating PET or PET/CT for assessment of axillary metastases, the mean sensitivity was 63% (95% CI 52% to 74%; range 20%–100%) and the mean specificity was 94% (95% CI 91% to 96%; range 75%–100%). For the seven studies (n = 862)73–79 evaluating PET/CT, the mean sensitivity was 56% (95% CI 44% to 67%) and the mean specificity was 96% (95% CI 90% to 99%). For the 19 studies (n = 1729)44,80–97 evaluating PET only, the mean sensitivity was 66% (95% CI 50% to 79%) and the mean specificity was 93% (95% CI 89% to 96%). PET performed less well in terms of identifying small metastases; micrometastases (≤ 2 mm) were associated with a mean sensitivity of 11% (95% CI 5% to 22%) based on data from five studies (n = 63),79,80,86–88 while macrometastases (> 2 mm) were associated with a mean sensitivity of 57% (95% CI 47% to 66%) based on data from four studies (n = 111). 73,80,87,88 The smallest metastatic nodes reported as being detected by PET measured 3 mm,87,88 while PET failed to detect some nodes measuring > 15 mm. 86,94 Current PET cameras are thought to achieve spatial resolutions of 4–7 mm (around 4–5 mm in the centre of the field of view). 115 PET studies in which all patients were clinically node negative showed a trend towards lower sensitivity compared with studies that included both clinically node-negative and node-positive patients, which may reflect the fact that clinically-negative axillary metastases are likely to be smaller.
Diagnostic accuracy of magnetic resonance imaging
The review identified nine studies (n = 307 patients) evaluating MRI. 41,42,64,108,–113 Several MRI studies reported more than one set of diagnostic accuracy results, according to different criteria for defining whether axillary metastases were present. Based on the highest reported sensitivity and specificity per study, the mean sensitivity across all nine MRI studies was 90% (95% CI 78% to 96%; range 65%–100%) and the mean specificity was 90% (95% CI 75% to 96%; range 54%–100%). Across the five studies (n = 93 patients) evaluating USPIO-enhanced MRI,108–112 the mean sensitivity was 98% (95% CI 61% to 100%) and specificity 96% (95% CI 72% to 100%). Across three studies of gadolinium-enhanced MRI (n = 187),41,42,113 the mean sensitivity was 88% (95% CI 78% to 94%) and specificity 73% (95% CI 63% to 81%). In the single study of in vivo proton MR spectroscopy (n = 27),64 the sensitivity was 65% (95% CI 38% to 86%) and specificity 100% (95% CI 69% to 100%). USPIO-enhanced MRI showed a trend towards higher sensitivity and specificity than gadolinium-enhanced MRI. However, studies of USPIO-enhanced MRI are currently small and in the experimental stages. The use of contrast uptake pattern as the main criterion for defining a node as metastatic appeared to give better combined sensitivity and specificity than size and morphology, although some studies used criteria based on both uptake and size/morphology. No data were presented according to size of metastases for the MRI studies. However, current MRI methods are thought to achieve a resolution of approximately 1 mm using modern scanners and based on the methods used in the included papers.
Adverse effects and contraindications
Both PET and MRI appeared to be relatively safe in this setting, with no adverse effects reported for any of the 26 PET studies,44,73–97 and no serious adverse effects reported in any of the MRI studies. Mild-to-moderate adverse effects of MRI included mild rash following USPIO administration and inability to complete the MRI scan due to claustrophobia or back pain. Previous studies of USPIO have suggested an adverse event rate > 2%; most events are minor and include lumbar pain, rash, transient decrease in blood pressure, and arrhythmia. 116–118 The mechanism of lumbar pain is unknown, but this is also observed with other particulate agents, and usually disappears when the infusion is stopped or its speed is reduced. 116 Cautions and contraindications exist for both PET (pregnancy) and MRI (allergy to contrast agents, renal or liver dysfunction, pacemakers and other metallic implants). Several PET studies excluded patients who had diabetes mellitus (or high serum glucose levels). In addition, some patients are unable to complete an MRI scan due to claustrophobia. These factors may limit the applicability of PET and MRI for some patients.
Internal and external validity
The sensitivity and specificity of both PET and MRI varied widely between studies, so the pooled accuracy data should be interpreted with caution. The included MRI studies were relatively small and there was variation between and within studies in terms of the MRI method used (MRI or MR spectroscopy; contrast agent; field strength; image sequence parameters; type of coil) and the criteria for defining a node as positive.
Study quality was assessed using the QUADAS checklist69 and was mixed. In the majority of studies, the reference standard was adequate (ALND or SLNB). Studies using ALND reported similar sensitivity and specificity to studies using a combination of ALND and SLNB, while studies in which not all patients had ALND or SLNB (or in which the reference standard was not stated) had a higher mean sensitivity, which may represent an overestimate. Differential verification bias was avoided in most studies, but in six PET studies75,76,78,79,81,83 patients received a different reference standard (ALND or SLNB) depending on the PET results. Nineteen studies were not considered to have a representative patient spectrum,73,74,76–79,81,82–85,89–91,93–97 either because recruitment was not prospective and consecutive or because some of the included patients were non-early stage, non-newly diagnosed or had DCIS. Most studies reported few or no withdrawals. The index test was interpreted blind to reference standard results in most studies. Interpretation of the reference standard with blinding to index test results and handling of uninterpretable results were often not reported. Index test details were very well reported, while reference standard details were sometimes incomplete, probably due to the widespread routine use of these techniques. For PET, two quality variables (representative patient spectrum and index test interpreted blind to reference standard results) were associated with higher sensitivity in studies scoring ‘yes’ than in studies scoring ‘unclear’, while three quality variables (differential verification avoided, reference standard interpreted blind to index test and uninterpretable test results did not occur or occurred and were included in the analysis) were associated with lower sensitivity in studies scoring ‘yes’ than in studies scoring ‘unclear’. For MRI, no study quality variables had a significant effect on sensitivity or specificity, although this analysis was limited by the small number of studies. These results show a mixed pattern. Studies that score ‘unclear’ on reporting of quality variables may be expected to be of poorer quality, but this could theoretically lead to either underestimates or overestimates of diagnostic accuracy.
The external validity of the included studies relates to the extent to which the populations and methods are generalisable to clinical practice in the UK. The average age of the patients in the included studies was 56 years, while the average age at diagnosis using published data was 64 years. 1,2 This may reflect slightly younger patients being recruited into clinical studies or being candidates for ALND. However, this is unlikely to substantially affect the accuracy estimates of PET and MRI. The majority of patients in the included studies had early-stage, newly diagnosed breast cancer; patients with DCIS were excluded where possible as these patients would not usually undergo surgical assessment of the axilla in the UK (with the exception of patients with extensive DCIS). Within PET studies, sensitivity and specificity were not affected by whether or not all analysed patients were early stage, newly diagnosed and non-DCIS; for MRI, sensitivity was slightly higher and specificity slightly lower where all patients were early stage, newly diagnosed and non-DCIS. The mean prevalence of axillary metastases across all studies was 42% (range 20%–70%); there was no clear relationship with sensitivity and specificity.
Comparison of positron emission tomography and magnetic resonance imaging with current techniques for assessing the axilla
Positron emission tomography and MRI have lower sensitivity and specificity than SLNB and 4-NS, but are associated with fewer adverse effects. The potential use of PET or MRI, either as a replacement for SLNB or 4-NS or as an additional test prior to SLNB or 4-NS, was evaluated using a decision model. The model was used to assess both the cost-effectiveness and clinical acceptability of each potential diagnostic strategy, via an estimation of the number of correct and incorrect diagnoses, costs, and impact on QALYs due to cancer recurrence and adverse effects associated with each strategy. The results of this analysis are described further within the cost-effectiveness section of this report (see Chapter 4).
Ultrasound is currently recommended prior to surgical assessment of the axilla for all patients with early-stage breast cancer. 17 In order to be useful, any additional imaging technique would need a higher sensitivity and/or specificity than ultrasound. A systematic review estimated the average sensitivity of ultrasound at 69%–71% in all patients and 44%–61% in patients with non-palpable axillary nodes, and the specificity at 75%–86% in all patients and 77%–92% in patients with non-palpable axillary nodes. 47 In terms of sensitivity, PET appears similar to the above estimates for ultrasound, while MRI appears slightly higher. As ultrasound is not currently considered sensitive enough to completely replace SLNB or 4-NS, it is unlikely that PET could fulfil this role either. In terms of specificity, PET and USPIO-enhanced MRI appear slightly higher than ultrasound.
Conclusions for clinical effectiveness
The studies in this review demonstrate a significantly higher sensitivity for MRI than for PET, with USPIO-enhanced MRI providing the highest sensitivity. However, since none of the included studies directly compared PET and MRI, caution should be taken when comparing these estimates. Sensitivity of PET is reduced for smaller metastases. Specificity was similar for PET and MRI. However, this analysis is limited by the small number and size of MRI studies, and the wide variations between and within studies in terms of the MRI method used and the criteria for defining a node as positive. The sensitivity and specificity of both PET and MRI vary widely between studies, which is likely to reflect differences in imaging methods and interpretation. Therefore, caution should be taken when interpreting these results, particularly for MRI.
Positron emission tomography and MRI have lower sensitivity and specificity than the current surgical diagnostic techniques of SLNB and 4-NS, but are associated with fewer adverse events. The potential use of PET or MRI, either as a replacement for SLNB or 4-NS or as an additional test prior to SLNB or 4-NS, was evaluated using a decision model (described further within Assessment of cost-effectiveness). As the sensitivity of PET is only moderate and is similar to that of ultrasound, it appears unlikely that PET could entirely replace SLNB or 4-NS in the assessment of axillary metastases.
As data on MRI are currently in the experimental stage, further large, well-conducted studies using up-to-date MRI methods are required to obtain more accurate data on the sensitivity and specificity of MRI in the assessment of axillary metastases. Further studies of USPIO-enhanced MRI would be valuable in order to gain more robust data on sensitivity and specificity, adverse effects and which are the best criteria for defining a node as metastatic.
Chapter 4 Assessment of cost-effectiveness
This section of the assessment focuses on the health economics of enhanced imaging techniques in the assessment of axillary lymph node metastases in comparison with standard diagnostic techniques. It includes a brief review of existing economic evaluations of the relevant imaging techniques in the assessment of axillary lymph node metastases and a detailed explanation of the methodologies and results of the economic model.
Review of existing cost-effectiveness evidence presents the results of the review of economic literature. The modelling approach adopted for this study is discussed in Independent economic assessment – methods, with the results of the analysis being presented in Independent economic assessment – results.
Review of existing cost-effectiveness evidence
The primary objective of this review was to identify and evaluate studies exploring the cost-effectiveness of enhanced imaging techniques in the assessment of axillary lymph node metastases. The secondary objective was to evaluate methodologies used to inform our own economic evaluation.
The literature was searched using the strategy described in Chapter 3, Methods for reviewing effectiveness and Appendix 1. Published economic evaluations of PET or MRI in the assessment of axillary lymph node metastases in breast cancer were included in the review. The search identified 245 citations. Of these, 242 were excluded at the title/abstract stage and one was excluded at the full-text stage. In total, two studies were included in the review: one of PET119 and one of MRI. 120
One published economic evaluation of PET in the assessment of axillary lymph node metastases in breast cancer was identified. 119 The model studied breast cancer in general, rather than early-stage breast cancer in particular. A decision tree model was built which did not include the lifetime of the patient or breast cancer recurrence. Furthermore, patient utilities and QALYs were not used in the model. The second paper compared the cost-effectiveness of MR lymphangiography-based strategies with that of SLNB in the axillary staging of early breast cancer. 120 However, the model did not consider the short- and long-term adverse events (e.g. lymphoedema) that are associated with SLNB. The diagnostic pathway modelled did not represent the typical pathway in the UK, where ultrasound and ultrasound-guided biopsy are also used. The disease pathway in the model did not include the locoregional relapse and subsequent remission states which are important health states for breast cancer patients. All costs of the study were based in the USA and are unlikely to represent the costs in the UK, given the significant difference in the organisation and funding of health services between the two countries. The literature review confirmed the need for new published economic evaluations in this area.
Independent economic assessment – methods
Objective
The aim of the model was to evaluate the effects on patient outcomes and cost-effectiveness of enhanced imaging techniques (MRI and PET) compared with standard techniques in the assessment of axillary lymph node metastases in women with early-stage breast cancer. Two axillary sampling techniques, 4-NS and SLNB, are used currently in the UK. It is beyond the remit of this assessment to compare 4-NS and SLNB. The enhanced imaging techniques (PET and MRI) are therefore compared with the two baseline sampling techniques separately.
Diagnostic methods
The diagnostic methods that were evaluated in the model (MRI, PET, 4-NS and SLNB) were discussed in detail in Chapter 1, Current methods for assessment of axillary metastases and Chapter 1, Description of technology under assessment.
Structure of the model
A probabilistic discrete-event simulation model has been developed in simul8 (SIMUL8®, Boston, MA, USA) to explore the costs and health outcomes associated with the assessment of axillary lymph node metastases and the treatment of women with early breast cancer.
Discrete-event simulation concerns the modelling of a system as it evolves over time by a representation in which the state variables change instantaneously at separate and countable points in time. 121 In the context of health-care modelling, patients are individually represented in a discrete-event simulation model, and normally have associated attributes indicating their distinctive demographical information and diagnostic and/or disease history.
The model differs from classical Markov state transition models, in which the state transition probabilities of patients are evaluated during fixed time intervals and patients may remain in one state after each evaluation. In discrete-event simulation, the time spent in each health state is sampled from a distribution when an individual patient enters this state. If one state can transit to multiple states, then the timing to each subsequent state is sampled and compared, and the patient will transit to the state that has the shortest transition delay.
The discrete-event simulation model consists of two main parts: the diagnostic pathway, which represents current and alternative diagnostic strategies (including PET and MRI), and the treatment pathway, which represents the disease progression among various health states and the management of patients with early breast cancer after the diagnosis of axillary lymph node metastases. A hypothetical cohort of 5000 early breast cancer patients was modelled. Each individual patient follows a specific diagnostic path and will obtain one of the four diagnostic results: TN, FP, TP or FN. Short- and long-term adverse events associated with sampling diagnostic techniques (4-NS and SLNB) and the associated cost and utility implications are also determined for each patient. The diagnostic results will influence the time spent in each of the subsequent health states, which is sampled from exponential distributions based on yearly transition probabilities. The starting age of patients was 56 years, which is based on the clinical effectiveness review within this assessment. The model was run for the remaining lifetime of patients.
Resource use and utilities are mainly taken from published literature. Input parameters are assigned probability distributions to reflect their imprecision and Monte Carlo techniques are performed to reflect this uncertainty in the results. Results are presented in terms of net health benefit and cost per incremental QALY gained.
Diagnostic pathway
The status of axillary lymph node metastases was assessed for all early breast cancer patients. Current methods of assessment consist of clinical examination followed by ultrasound. 17 If the result of ultrasound is positive, an ultrasound-guided fine-needle or core-needle biopsy will be conducted. If axillary metastases are identified via the biopsy (TP or FP), then the patients will be managed as node-positive patients (i.e. patients who have axillary lymph node metastases) and ALND will be performed, normally at the same time as the main breast cancer surgery is carried out.
For those women whose axillary metastases have not been identified by ultrasound or biopsy (i.e. either ultrasound or biopsy result is negative), current management involves the surgical removal of only some of the axillary lymph nodes (axillary sampling via either 4-NS or SLNB) for histological examination. Axillary sampling is normally performed at the same time as the main breast cancer surgery. Depending on the results of axillary sampling, patients will be managed either as node-negative patients (i.e. patients who do not have axillary lymph node metastases), who will receive no further investigation at that time, or as node-positive patients, who will undergo ALND to remove all axillary lymph nodes. Figure 13 illustrates the current standard diagnostic pathway.
FIGURE 13.
Standard and alternative imaging replacement diagnostic pathway in the School of Health and Related Research (ScHARR) model.
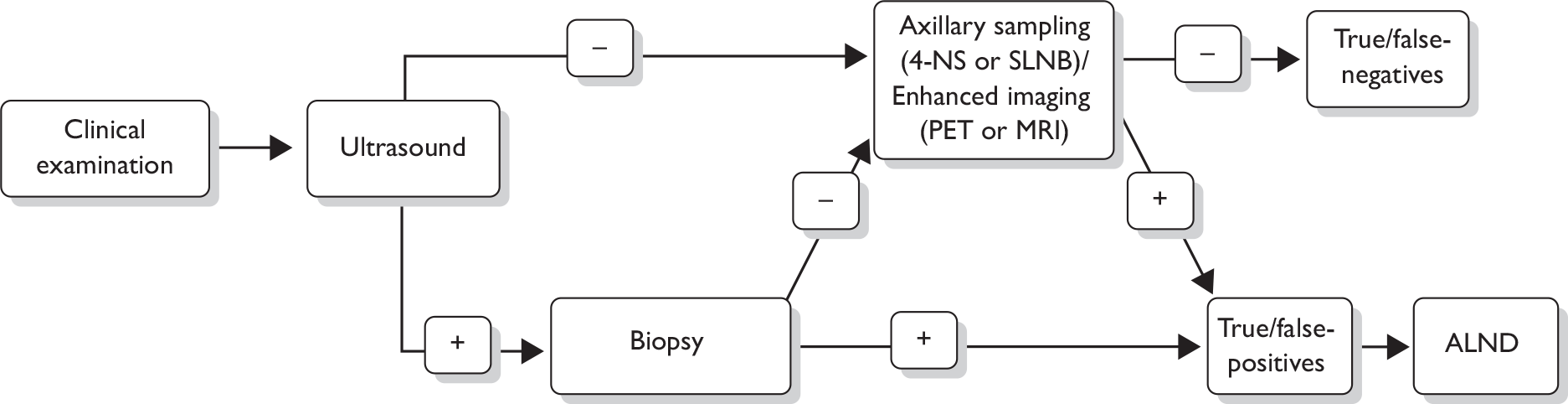
Apart from the two baseline 4-NS and SLNB strategies, six alternative strategies that involve either MRI or PET were evaluated. Two alternative strategies are to replace the axillary sampling methods with either MRI or PET (scenarios 1 and 2). Another four alternative strategies are to add MRI or PET before 4-NS (scenarios 3 and 4), and to add MRI or PET before SLNB (scenarios 5 and 6) in the diagnostic pathway. In theory, a biopsy (fine-needle or core-needle biopsy) could be undertaken in the event of a positive MRI or PET result in the alternative diagnostic pathways. However, MRI-guided or PET-guided biopsy is not currently available in most centres in the UK and no clinical studies were identified to provide data on this. Therefore, these techniques were not included in our assessment. The eight diagnostic strategies were as follows:
-
baseline 1: 4-NS
-
baseline 2: SLNB
-
scenario 1: replace sampling with MRI
-
scenario 2: replace sampling with PET
-
scenario 3: add MRI before 4-NS
-
scenario 4: add PET before 4-NS
-
scenario 5: add MRI before SLNB
-
scenario 6: add PET before SLNB.
The cost-effectiveness of the alternative MRI or PET replacement strategies was evaluated using the diagnostic pathway illustrated in Figure 13, in which MRI or PET replaces the current axillary sampling procedures. In order to evaluate the other four alternative strategies, the standard pathway was modified to create an alternative diagnostic pathway (Figure 14). In the alternative pathway, enhanced imaging techniques will be carried out for patients who have negative ultrasound or biopsy results. If the results of the imaging techniques are positive, no axillary sampling is performed and the patients are regarded as node positive and go on to receive ALND. If the results of imaging techniques are negative, further axillary sampling (4-NS or SLNB) is still performed as in the standard pathway.
FIGURE 14.
Alternative imaging addition diagnostic pathway in the School of Health and Related Research (ScHARR) model.
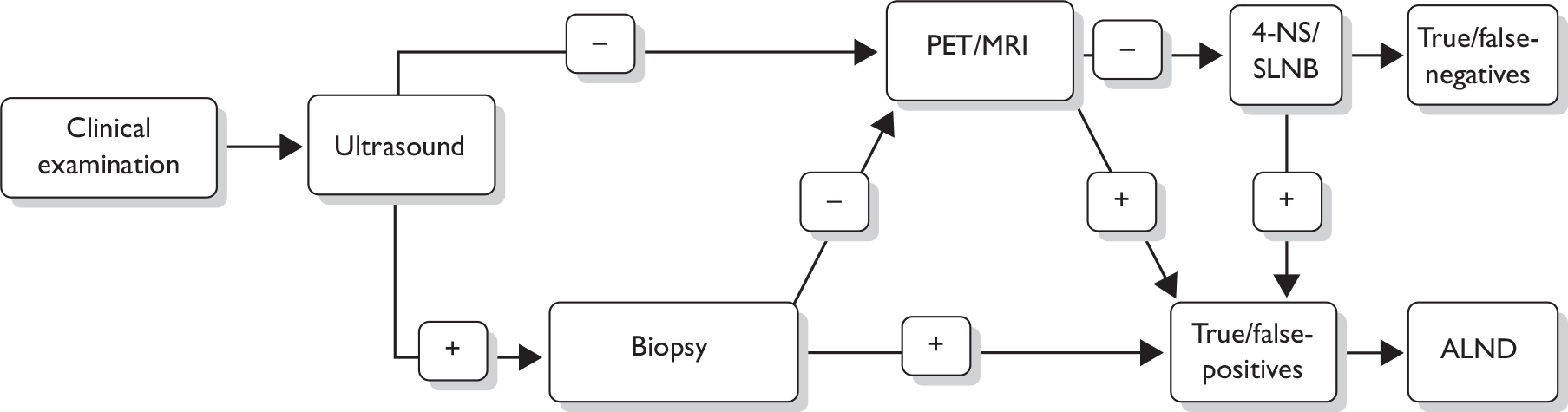
Disease pathway
The diagnostic results will affect the choice of adjuvant therapies and the probability of locoregional relapse and developing metastatic diseases (e.g. patients with FN diagnoses, who have metastatic nodes which are not detected and removed, are more likely to suffer from relapse). Certain diagnostic techniques are associated with long-term adverse events, such as lymphoedema, which may have lifetime cost and utility implications. Therefore, the disease pathway of patients with early breast cancer is also modelled.
All patients with early breast cancer receive adjuvant therapy after the main breast cancer surgery. Node-positive patients normally receive chemotherapy followed by hormonal therapy (where appropriate) while node-negative patients normally receive only hormonal therapy (where appropriate). 17 The aim of the adjuvant therapies is to reduce the risk of cancer recurrences. Following the adjuvant therapy, patients may enter into a disease-free survival state of post-adjuvant therapy and may potentially stay in this state for the rest of their lives (i.e. cured). Some patients may, however, experience locoregional relapse during or after the therapy. Patients who are in the post-adjuvant therapy state may experience locoregional or metastatic relapse. Patients experiencing locoregional relapse receive further treatment (e.g. surgical removal of lymph nodes, chemotherapy, radiotherapy, hormonal therapy). The patients may then enter a further remission period without evidence of cancer until death or further relapse to metastatic disease. Metastatic/distant relapse is not considered curable. Patients experiencing a metastatic relapse receive active palliative treatment to control symptoms and improve quality of life, a period of supportive care and ultimately a period of intensive end-of-life care for the last few days/weeks of life. Patients may also die owing to other causes in any health state.
In the UK, women with breast cancer receive follow-up examinations for a number of years following their treatment. The aim of this follow-up is to detect any recurrences earlier and therefore the frequency and effectiveness of follow-up may affect the overall effectiveness of the diagnostic strategies assessed in this study. In theory, axillary metastases in patients misdiagnosed as FN may be identified by either follow-up or self-presentation. However, in practice it is difficult for a clinician to determine whether axillary metastases identified by follow-up or self-presentation are actually due to previous misdiagnosis or due to recurrence. Because the issue of follow-up is outside the scope of the assessment and not enough published evidence is available on the effectiveness of follow-up (especially for patients with FN results), the model did not explicitly represent follow-up.
Health states
There are 10 health states within the disease pathway part of the model:
-
adjuvant therapy – node (true) negative patients
-
adjuvant therapy – node (false) positive patients
-
adjuvant therapy – node (true) positive patients
-
adjuvant therapy – node (false) negative patients
-
post-adjuvant therapy
-
locoregional relapse
-
remission (post-locoregional relapse)
-
metastatic relapse
-
death from breast cancer
-
death from other causes.
The disease pathway is shown in Figure 15. Each individual patient starts from one of the four adjuvant therapy states, depending on the previous diagnostic result.
FIGURE 15.
Treatment pathways in the School of Health and Related Research (ScHARR) model.
Model state transitions
The following health-state transitions are possible in the model:
-
adjuvant therapy – patients can move to:
-
post-adjuvant therapy
-
locoregional relapse
-
death from other causes
-
-
post-adjuvant therapy – patients can move to:
-
locoregional relapse
-
metastatic relapse
-
death from other causes
-
-
locoregional relapse – patients can move to:
-
remission
-
metastatic relapse
-
death from other causes
-
-
remission – patients can move to:
-
metastatic relapse
-
death from other causes
-
-
metastatic relapse – patients can move to:
-
death from breast cancer
-
death from other causes
-
-
death from breast cancer – absorbing state
-
death from other causes – absorbing state.
Model assumptions
The model employs a number of simplifying assumptions, which are detailed below.
-
The sensitivity and specificity of biopsy, axillary sampling (4-NS and SLNB), and imaging techniques (MRI and PET) are independent of preceding diagnostic results.
-
The sensitivity and specificity of MRI is based on all identified studies, with no distinction made between different types of MRI (see Chapter 3, Quantity and quality of research available). This assumption is tested in the sensitivity analysis.
-
Seroma, surgical drain and infection are the short-term adverse events associated with diagnostic techniques (4-NS, SLNB and ALND) considered by the model.
-
Lymphoedema is the only long-term adverse event considered. Lymphoedema is classified as either mild/moderate or severe.
-
Studies have reported adverse events associated with SLNB, whereas no studies were identified which quantify the short-term adverse events associated with 4-NS or compare the probability of adverse events between SLNB and 4-NS. Therefore, the probability of adverse events is assumed to be equal for 4-NS and SLNB.
-
Short-term adverse events increase the costs, but do not affect quality of life.
-
Long-term adverse events (i.e. lymphoedema) affect both costs and quality of life for the rest of the patient’s life.
-
During the adjuvant therapy period, node-positive patients receive chemotherapy plus hormonal therapy (where appropriate) and node-negative patients receive hormonal therapy (where appropriate).
-
Patients receive adjuvant therapy for a fixed 5-year period. Node-positive patients receive chemotherapy for half a year, followed by hormonal therapy for 4.5 years. Node-negative patients receive hormonal therapy for 5 years. This is the maximum time patients may stay in this state. Patients may, owing to model dynamics, spend < 5 years in the adjuvant therapy state if the sampled time to locoregional relapse or death from other causes is < 5 years.
-
Following locoregional relapse patients cannot experience further locoregional relapse; they can only experience metastatic relapse.
-
Death rates for non-breast cancer causes are based on UK mortality statistics and applied across all health states. These are not adjusted to exclude breast cancer mortality, and so may overestimate the risk of dying due to non-breast cancer causes.
Model inputs: accuracy and costs of diagnostic techniques
The model inputs of accuracy and costs of diagnostic methods were summarised in Table 18. The sensitivity and specificity of clinical examination and biopsy were based on previous published studies. 41,42,47,94 The sensitivity and specificity of ultrasound for clinically negative patients were calculated based on either the size criterion (60.9% and 77.3%, respectively) or morphology criterion (43.9% and 92.4%, respectively) from a systematic review by Alvarez et al. 47 We used the averages to represent overall sensitivity and specificity assuming both size and morphology are used to assess axillary lymph node metastases. The sensitivity and specificity of ultrasound for clinically-positive patients were estimated based on expert opinion, as no data were identified from published studies.
| Diagnostic technique | Parameter | Value | Distribution | Source |
|---|---|---|---|---|
| Clinical examination | Sensitivity (%) | 45.9 | Beta | Mumtaz et al. 1997;42 Smith et al. 1998;94 Kvistad et al. 200041 |
| Specificity (%) | 78.1 | |||
| Costs (£) | 86 | Normal | NHS Reference Costs 2008 56 | |
| Ultrasound | Sensitivity for clinically positive (%) | 90.0 | Expert opinion | |
| Specificity for clinically positive (%) | 70.0 | |||
| Sensitivity for clinically negative (%) | 52.4 | Beta | Alvarez et al. 200647 | |
| Specificity for clinically negative (%) | 84.9 | |||
| Costs (£) | 53 | Normal | NHS Reference Costs 2008 56 | |
| Biopsy | Sensitivity (%) | 45.4 | Beta | Alvarez et al. 200647 |
| Specificity (%) | 99.6 | |||
| Costs (£) | 147 | Normal | NHS Reference Costs 200355 (adjusted to 2007 prices) | |
| 4-NS | Sensitivity (%) | 94.5 | Beta | Sato et al. 2001;124 Tanaka et al. 2006123 |
| Specificity (%) | 100.0 | |||
| Costs (breast surgery and 4-NS) (£) | 2099 | Assumption | ||
| SLNB | Sensitivity (%) | 93.0 | Beta | Kim et al. 2006125 |
| Specificity (%) | 100.0 | |||
| Costs (breast surgery and SLNB) (£) | 2728 | Pandharipande et al. 2008120 | ||
| MRI | Sensitivity (%) | 90.3 | Beta | Systematic review by this assessment |
| Specificity (%) | 89.7 | |||
| Costs (£) | 232 | Normal | NHS Reference Costs 200856 | |
| PET | Sensitivity (%) | 63.4 | Beta | Systematic review within this assessment |
| Specificity (%) | 94.2 | |||
| Costs (£) | 978 | Jacklin et al. 200266 (adjusted to 2007 prices) | ||
| ALND | Costs of ALND alone (£) | 2448 | Normal | NHS Reference Costs 2008 56 |
| Costs of ALND and breast surgery (£) | 3186 | Pandharipande et al. 2008120 | ||
| Costs of breast surgery (£) | 1908 | NHS Reference Costs 2008 56 | ||
The sensitivity of 4-NS was based on two identified studies with data from 335 patients. 122,123 The sensitivity of SLNB was based on a systematic review and meta-analysis of 69 studies undertaken by Kim et al. 125 with data from over 8000 patients. All studies were included in the NICE guideline. 17 The mean sensitivity of 4-NS is slightly higher than that of SLNB (94.5% vs 93%) according to literature reviewed within the NICE guideline. However, it is important to note that the sensitivity of SLNB is based on a significantly larger sample size and should be more robust. The specificities of 4-NS and SLNB are set at 100% as, by definition, there should be no FP cases when histological methods are used.
The costs of clinical examination, ultrasound, biopsy, MRI and ALND were obtained from NHS reference costs. 55,56 Apart from scanning the axilla, MRI is currently also used in the pre-surgical evaluation of breast tumours for some patients. However, the procedure is performed in a small proportion of women with breast cancer (e.g. lobular cancers and patients having neoadjuvant chemotherapy) and it is likely that axillary and breast MRI scans would be undertaken separately, as pre-contrast scans would be required for both procedures. Therefore marginal cost savings of axillary MRI scans due to breast MRI scans are not considered and we assumed the full NHS reference costs for MRI in the model.
The costs of PET were calculated based on a published UK study. 66 The costs of breast surgery were calculated based on NHS costs of total mastectomy surgery [Healthcare Resource Group (HRG) codes are JA07A, JA07B and JA07C] and intermediate breast surgery (HRG codes are JA09A and JA09B),56 assuming that two-thirds of patients receive intermediate breast surgery and one-third of patients receive mastectomy.
Sentinel lymph node biopsy and 4-NS are assumed to be carried out at the same time as the breast surgery. ALND is assumed to be carried out at the same time as the breast surgery, if no sampling is performed beforehand (e.g. patients are diagnosed as node positive by biopsy). No UK costs of SLNB, 4-NS and ALND (at the same time as breast surgery) were identified from published studies. One study from the USA was identified which reported the costs of breast surgery alone, SLNB together with breast surgery and ALND together with breast surgery. 20 The relative ratios between breast surgery and SLNB together with breast surgery, and between breast surgery and ALND together with breast surgery, can be calculated. These ratios were used to adjust the UK costs of breast surgery to obtain the costs of SLNB and ALND together with breast surgery. The procedure of 4-NS together with breast surgery was assumed to increase the costs of breast surgery alone by 10%.
Model inputs: probability and costs of short-term adverse events
The model inputs of probability and costs of short-term adverse events were summarised in Table 19. The probabilities of developing short-term adverse events due to SLNB and ALND were estimated based on published studies. No studies were identified that quantify the short-term adverse events associated with 4-NS. Based on expert opinion, it is assumed that 4-NS is associated with the same probabilities of developing short-term adverse events as SLNB. Sensitivity analysis was carried out to test the assumption that patients are more likely to develop adverse events for 4-NS than SLNB. The costs of short-term adverse events were based on a study in the US, as no UK costs were identified.
| Short-term adverse events | Parameter | Value | Distribution | Source |
|---|---|---|---|---|
| Seroma | Probability (4-NS) | 0.072 | Beta | Expert opinion – assumed same as SLNB |
| Probability (SLNB) | 0.072 (95% CI 0.065 to 0.08) | Beta | Blanchard et al. 2003;48 Purushotham et al. 2005;51 Wilke et al. 200654 | |
| Probability (ALND) | 0.221 (95% CI 0.174 to 0.278) | Beta | Blanchard et al. 2003;48 Purushotham et al. 200551 | |
| Costs (£) | 292 | Jeruss et al. 2006127 (adjusted to 2007 prices) | ||
| Surgical drains | Probability (4-NS) | 0.02 | Beta | Expert opinion – assumed same as SLNB |
| Probability (SLNB) | 0.02 (95% CI 0.016 to 0.025) | Beta | Mansel et al. 2006;128 Wilke et al. 200654 | |
| Probability (ALND) | 0.792 (95% CI 0.753 to 0.827) | Beta | Mansel et al. 2006128 | |
| Costs (£) | 292 | Jeruss et al. 2006127 (adjusted to 2007 prices) | ||
| Infection | Probability (4-NS) | 0.021 | Beta | Expert opinion – assumed same as SLNB |
| Probability (SLNB) | 0.021 (95% CI 0.018 to 0.026) | Beta | Blanchard et al. 2003;48 Mansel et al. 2006;128 Wilke et al. 200654 | |
| Probability (ALND) | 0.142 (95% CI 0.115 to 0.1730) | Beta | Blanchard et al. 2003;48 Mansel et al. 2006128 | |
| Costs (£) | 292 | Jeruss et al. 2006127 (adjusted to 2007 prices) |
Model inputs: costs and utilities of health states
The model inputs of costs and utilities of health states are summarised in Table 20. Patients with node-negative results (TN and FN) are assumed to receive hormonal therapy for 5 years (where appropriate). For patients with FP results, the TN nodal status will be picked up by the following ALND. Therefore, patients with FP diagnoses also receive hormonal therapy. It is assumed that 81% of patients are oestrogen receptor positive, which means that they will respond to hormonal therapy. 126 Among these patients, it is assumed that 90% receive aromatase inhibitors [anastrozole (Armidex®, AstraZeneca), exemestane (Aromasin®, Pfizer) or letrozole (Femara®, Novartis)] and 10% receive tamoxifen (Nolvadex, Istubal, Valodex). It is also assumed that each patient has one clinic visit and one mammogram per year in the adjuvant therapy state. The annual cost of aromatase inhibitors, tamoxifen and mammography is estimated to be £1002 based on a published study. 129
| Health state | Parameter | Value | Distribution | Source |
|---|---|---|---|---|
| Adjuvant therapy (TN and FN, FP) | Annual costs (£) | 1002 | Ward et al. 2007128 (adjusted to 2007 prices) | |
| Utility | 0.82 | Beta | Tengs and Wallace 2000129 | |
| Adjuvant therapy (TP) | Costs of chemotherapy for 6 months (£) | 8788 | Ward et al. 2007128 (adjusted to 2007 prices) | |
| Annual costs after chemotherapy (£) | 1002 | |||
| Utility | 0.74 | Beta | Tengs and Wallace 2000129 | |
| Post-adjuvant therapy | Annual costs (£) | 0 | Assumption | |
| Utility | 0.94 | Beta | Tengs and Wallace 2000129 | |
| Locoregional relapse | Annual costs (£) | 4737 | Ward et al. 2007128 (adjusted to 2007 prices) | |
| Utility | 0.70 | Beta | Tengs and Wallace 2000129 | |
| Remission | Annual costs for the first 5 years (£) | 1002 | Ward et al. 2007128 (adjusted to 2007 prices) | |
| Annual costs after 5 years (£) | 0 | |||
| Utility | 0.85 | Beta | Tengs and Wallace 2000129 | |
| Metastatic relapse | Annual costs (£) | 10,196 | Ward et al. 2007128 (adjusted to 2007 prices) | |
| Utility | 0.40 | Beta | Tengs and Wallace 2000129 | |
| Death | Costs (£) | 3321 | Ward et al. 2007128 (adjusted to 2007 prices) | |
| Utility | 0 | By definition |
Patients with TP results are assumed to receive chemotherapy for 6 months (£8788) followed by hormonal therapy for 4.5 (£1002 per year). It is assumed docetaxel (Taxotere®, Sanofi-aventis)-based chemotherapy is used. 17 The costs of chemotherapy were calculated based on a published study. 129
Annual costs for the post-adjuvant therapy state are assumed to be £0. Annual costs for the locoregional and metastatic relapse states and the death state were calculated based on a published study and are £4737, £10,196 and £3321, respectively. 129 For patients in the remission state after locoregional relapse, the annual costs for the first 5 years were assumed to be the same as for patients in the adjuvant therapy state receiving hormonal therapy (£1002). The costs are assumed to be £0 after 5 years in the remission state.
The source of utility data used in the model is from Tengs and Wallace,130 which is a systematic review of health-related quality of life estimates from publicly available source documents. Given that the health-related quality of life in the general population decreases with age, it is important to take this into account in the model. General population utility estimates from Ara and Brazier131 were applied using a regression analysis of utility versus age. The age-related utility is calculated by the following formula (Equation 1):
where A = –0.0001728, B = –0.000034 and C = 0.9584588.
The utilities for all health states are multiplied by this age-related utility value for each year of the model.
Model inputs: transition probabilities between health states
Transition probabilities between health states are summarised in Table 21. When a patient starts adjuvant therapy, the expected life expectancy of the patient is determined by the life table. 132 The patient will die from other causes once the expected life expectancy is met. The transition probabilities from the adjuvant therapy state to the locoregional recurrence state depend on the diagnostic results for lymph node metastases (i.e. TN, FP, TP and FN). Node-negative patients, including TN and FP, will have lower transition probabilities and node-positive patients, including TP and FN, will have higher transition probabilities. In particular, patients with FN results will have the highest transition probability because they have been denied ALND and chemotherapy, needed to reduce the risk of recurrence. The annual transition probabilities for locoregional recurrence were based on a study by Orr et al. ,133 which provided the estimates of annual transition probabilities of recurrence in patients with negative results (0.03), TP results (0.09) and FN results (0.14). The study assumed that patients with TP results receive chemotherapy and patients with FN results do not receive chemotherapy (and only receive hormonal therapy). The same assumption is used in this assessment.
| Parameter | Value | Source |
|---|---|---|
| Annual probability of locoregional relapse (TN and FP) | 0.03 | Orr et al. 1999133 |
| Annual probability of locoregional relapse (TP) | 0.09 | |
| Annual probability of locoregional relapse (FN) | 0.14 | |
| Annual probability of metastatic relapse (TN and FP) | 0.0023 | Pandharipande et al. 2008120 |
| Annual probability of metastatic relapse (TP) | 0.0052 | |
| Annual probability of metastatic relapse (FN) | 0.0094 | |
| Annual probability of metastatic relapse from locoregional relapse | 0.18 | Kamby and Sengelov 1997134 |
| Annual probability of metastatic relapse from remission | 0.13 | |
| Annual probability of death from locoregional relapse | 0.30 | Orr et al. 1999133 |
| Annual probability of death from metastatic relapse | 0.37 | Ward et al. 2007129 |
When a patient enters the adjuvant therapy state, the model uses exponential distributions to sample the time to locoregional relapse according to the above annual transition probabilities. The sampled time to locoregional relapse will then be compared with the time to death (according to the life table) and the 5-year maximum period for adjuvant therapy. Depending on which event happens first (locoregional relapse, death due to other causes or finishing the adjuvant therapy), the patient will transit to the corresponding state after the time delay. The methodology used to determine which state the patient transits to is the same for other health states.
When patients enter the post-adjuvant therapy state, they may experience locoregional or metastatic relapse, or they may die from other causes. The annual transition probabilities of locoregional relapse and the time to death from other causes are assumed to be the same as under the adjuvant therapy state. The transition probabilities to metastatic relapse are 0.0023 for node-negative patients (TN and FP), 0.0052 for TP patients, and 0.0094 for FN patients. 120
If patients enter the locoregional relapse state, they may enter a subsequent remission state, may have metastatic relapse, or may die from other causes. It is assumed that patients can stay in the locoregional relapse state for a maximum of 1 year. The annual probability of developing metastatic cancer in the first year of locoregional relapse is 0.18,134 which is much higher compared with disease-free survival states (i.e. adjuvant therapy and post-therapy states). Orr et al. 133 suggested that the annual probability of death for patients with locoregional relapse is 0.30. This includes death due to both breast cancer and other causes. The model does not distinguish between death from breast cancer and death from other causes once a patient enters the locoregional relapse state. The maximum lifetime of a patient is still bounded by the life expectancy of the patient (i.e. death due to other causes).
If patients enter the remission state, they may still experience metastatic relapse before death. The average annual probability of developing metastatic cancer from the remission state is 0.13134 and the probability of death (for all reasons) is assumed to be the same as in the locoregional relapse state, which is 0.30.
Metastatic cancer is assumed not to be curable and the annual probability of death from metastatic relapse is 0.37. 129
Model inputs: probability, costs and utilities of long-term adverse events
The model inputs of probability and costs of long-term adverse events (i.e. lymphoedema) are summarised in Table 22. The probabilities of developing lymphoedema due to SLNB and ALND were estimated based on published studies. 48–50,53 The probability of having lymphoedema due to 4-NS was assumed to be the same as SLNB, as no data were identified for 4-NS.
| Long-term adverse events | Parameter | Value | Distribution | Source |
|---|---|---|---|---|
| Lymphoedema | Probability (4-NS) | 0.068 | Beta | Expert opinion – assumed same as SLNB |
| Probability (SLNB) | 0.068 (95% CI 0.062 to 0.074) | Beta | Liu et al. 200953 | |
| Probability (ALND) | 0.214 (95% CI 0.18 to 0.252) | Beta | Blanchard et al. 2003;48 Crane-Okada et al. 2008;49 McLaughlin et al. 200850 | |
| Proportion of mild/moderate lymphoedema (%) | 66.3 | Mak et al. 2009135 | ||
| Proportion of severe lymphoedema (%) | 33.7 | |||
| Additional costs of mild/moderate lymphoedema (£) | 66.50 | Expert opinions from the Sheffield Lymphoedema Service | ||
| Additional costs of severe lymphoedema (£) | 1180.00 | |||
| Assumed utility decrement due to mild/moderate lymphoedema (%) | 9.9 | Mak et al. 2009135 | ||
| Assumed utility decrement due to severe lymphoedema (%) | 12.3 |
Lymphoedema was classified as either mild/moderate or severe. A literature search was undertaken, but no studies reporting utility for patients with lymphoedema were identified. The proportion of patients within each category and the utility decrements of each category were therefore estimated from a published study reporting quality of life using the FACT-B + 4 (Functional Assessment of Cancer Therapy for Breast Cancer, adding a four-item arm subscale) quality-of-life instrument. 135 The study reported the data regarding the quality of life of breast cancer patients who suffer from different degrees of lymphoedema, using the FACT-B + 4 quality-of-life instrument. The utility decrements were estimated based on these quality-of-life data, therefore the decrements do not represent the true utility decrements due to lymphoedema. Sensitivity analyses were carried out to explore the impact on the cost-effectiveness results caused by changing the estimated utility decrements. The annual additional costs due to lymphoedema were based on expert opinions from the Sheffield Lymphoedema Service.
The utility decrement represents the reduced quality of life for patients with lymphoedema.
Discounting
The economic analysis assumes that both costs and QALYs are discounted at 3.5% per annum, in line with current recommendations from Her Majesty’s Treasury. 136
Univariate sensitivity analysis
In order to explore the impact on the cost-effectiveness results of changes to individual parameters and assumptions, a number of sensitivity analyses were performed.
Sensitivity and specificity of magnetic resonance imaging and positron emission tomography
The analysis is limited by the small number and size of MRI studies, and the wide variations between and within studies in terms of the MRI method used. A sensitivity analysis was carried out to decrease the mean sensitivity of MRI to the lower CI (from 90% to 78%) and maintain the mean specificity of MRI. Another sensitivity analysis was carried out to decrease the mean specificity of MRI to the lower CI (from 90% to 75%) and maintain the mean sensitivity of MRI.
In order to test the sensitivity of model results to increased MRI accuracy, a sensitivity analysis was carried out to increase both the sensitivity and specificity of MRI to the levels for USPIO-enhanced MRI. USPIO-enhanced MRI is a subtype of MRI that appears to have higher sensitivity and specificity (98% and 96%, respectively). In this sensitivity analysis the cost of MRI was assumed to increase by £100 to take account of the additional cost of the contrast agent used in USPIO-enhanced MRI.
Regarding PET, a sensitivity analysis was carried out to increase the sensitivity of PET to the higher CI (from 63% to 74%) and maintain the mean specificity.
Utility decrements and additional costs for lymphoedema
The main advantage of imaging techniques is to reduce short- and long-term adverse events including lymphoedema. Therefore, the utility decrements and additional costs for lymphoedema will impact on the cost-effectiveness of imaging techniques compared with sampling methods. Data on the long-term costs and utility impact of lymphoedema are, however, limited. Two sensitivity analyses were carried out to increase/decrease the utility decrements for lymphoedema by 50%. Another two sensitivity analyses were performed to increase/decrease the additional costs due to lymphoedema by 20%.
Probabilities of relapse for false-negative patients
Two sensitivity analyses were carried out to increase/decrease the probabilities of locoregional relapse for patients with FN diagnoses by 20%. Due to lower sensitivity, imaging techniques, especially PET, produce more FN cases than 4-NS and SLNB. The probabilities of relapse for patients with FN diagnoses were changed so that the impact on model results can be assessed.
Costs of sampling methods
High-quality UK cost data for 4-NS and SLNB procedures have not been identified. The costs used in the model were derived from non-UK studies. Sensitivity analyses were carried out to increase/decrease the costs of 4-NS and SLNB by 20%.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis (PSA) was undertaken to demonstrate the impact of uncertainty in the key model parameters and to generate information on the likelihood that each of the diagnostic strategies is optimal.
The sensitivity and specificity of a diagnostic test are generally correlated. To maintain this correlation, the sensitivities of each diagnostic method are sampled from a beta distribution, while the specificities of the test are derived based on the sampled sensitivity. The sensitivity and specificity are linked by the prevalence and the overall accuracy of the test, which are assumed to be constants. Therefore, when the sensitivity is sampled, the specificity can be calculated deterministically.
Equation 2 is the formula for calculating the test accuracy. After rearranging Equation 2, the model applies Equation 3 to derive test specificity from sensitivity. The overall accuracy of each diagnostic method is presented in Table 23, where the prevalence of early breast cancer among breast cancer patients is assumed to be fixed at 41.2%. The beta distributions representing the sensitivity of each diagnostic method are based on trial data from previous literature and the systematic review within this assessment (MRI and PET).
| Diagnostic method | Overall accuracy (%) |
|---|---|
| Clinical examination | 64.8 |
| Ultrasound for clinically negative | 78.2 |
| Ultrasound for clinically positive | 71.5 |
| Biopsy | 88.7 |
| 4-NS | 97.7 |
| SLNB | 97.1 |
| MRI | 89.9 |
| PET | 81.5 |
| ALND | 100.0 |
Health utilities and the probabilities of developing short- and long-term adverse events were modelled using beta distributions. Costs were sampled from normal distributions where standard errors can be calculated. Transition probabilities among health states and other costs were sampled from uniform distributions bounded by a 10% increase or decrease in the mean value.
The PSA was carried out by allowing the key model input parameters to vary according to the uncertainty specified in their probability distributions, with 500 sets of random numbers used to generate 500 parameter configurations, which produce 500 sets of model outputs. All model results were based on the PSA model outputs.
To demonstrate that 500 replications is enough to obtain accurate model outputs, the cumulative mean total costs and total QALYs of the baseline 4-NS diagnostic strategy based on 2000 replications were calculated. A significance level of 5% was used to construct the CI around the cumulative means. The analysis suggests that the CI was sufficiently narrow and the cumulative mean stabilises after 500 replications are performed.
Independent economic assessment – results
This section details the results of the health economic model. The cost-effectiveness results of imaging techniques are presented as marginal estimates when compared against the standard sampling methods of 4-NS and SLNB. All results are presented in terms of net benefits and incremental cost per QALY gained.
Base-case estimates of cost-effectiveness
The base-case estimates given below are the mean estimates from the 500 runs of the PSA. For each strategy, results are presented in terms of the diagnostic results, the total number of diagnostic and surgical procedures performed, the total costs and QALYs, the net benefit and incremental cost-effectiveness.
Diagnostic results
The proportions of patients whose lymph node diagnostic results are TN, FP, TP and FN are presented in Table 24 and Figure 16.
| Diagnostic strategy | TN (%) | FP (%) | TP (%) | FN (%) | Total (%) |
|---|---|---|---|---|---|
| Baseline 1: 4-NS | 58.6 | 0.2 | 40.1 | 1.1 | 100.0 |
| Baseline 2: SLNB | 58.6 | 0.2 | 39.9 | 1.3 | 100.0 |
| Scenario 1: replace sampling with MRI | 52.5 | 6.3 | 39.3 | 1.9 | 100.0 |
| Scenario 2: replace sampling with PET | 55.2 | 3.6 | 34.0 | 7.2 | 100.0 |
| Scenario 3: add MRI before 4-NS | 52.5 | 6.3 | 41.1 | 0.1 | 100.0 |
| Scenario 4: add PET before 4-NS | 55.2 | 3.6 | 40.8 | 0.4 | 100.0 |
| Scenario 5: add MRI before SLNB | 52.5 | 6.3 | 41.1 | 0.1 | 100.0 |
| Scenario 6: add PET before SLNB | 55.2 | 3.6 | 40.7 | 0.5 | 100.0 |
FIGURE 16.
Proportion of people with FP and FN diagnostic results under each strategy.
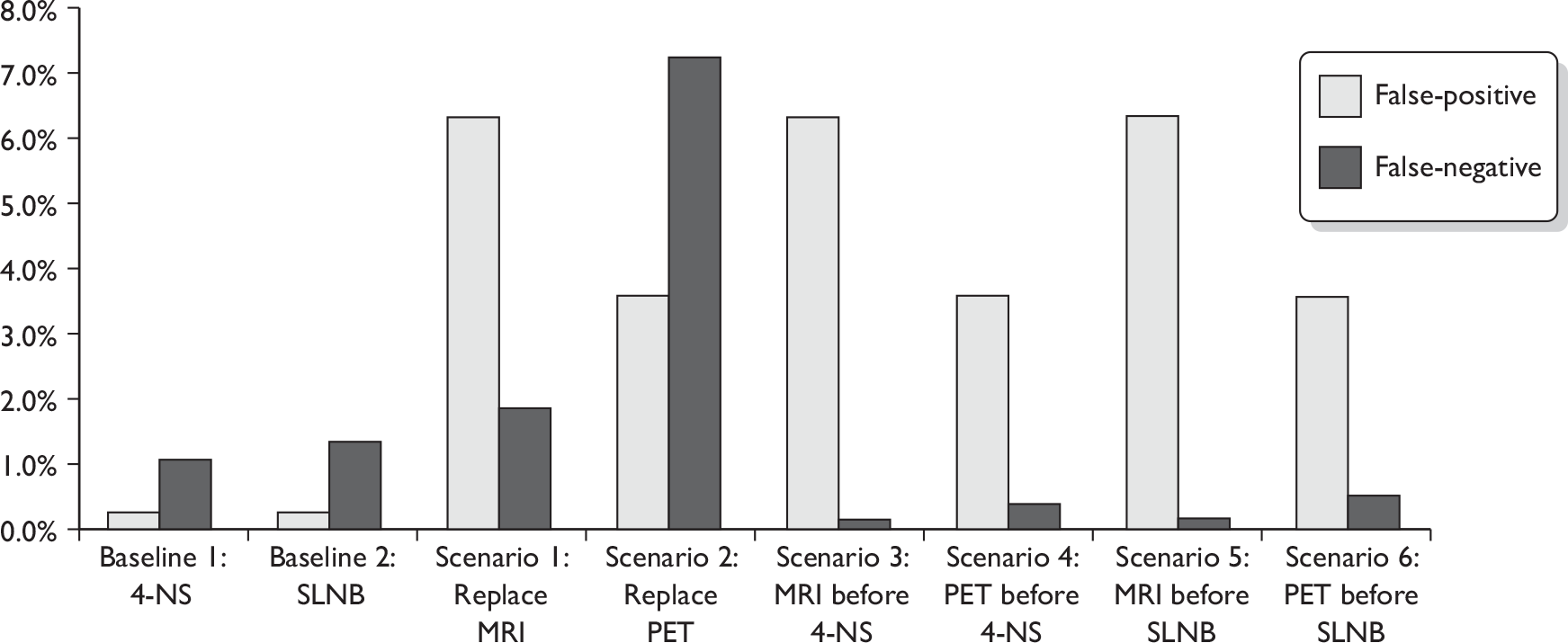
Key findings from the diagnostic results
Replacing sampling
-
The number of FP cases increases significantly when sampling is replaced with imaging techniques (from 0.2% to 6.3% and 3.6% for MRI and PET, respectively). The main reason is that imaging techniques have lower specificity (89.7% and 94.2% for MRI and PET, respectively).
-
Patients with FP diagnoses will receive ALND, as a stand-alone procedure (if detected by 4-NS or SLNB where the breast surgery is performed at the same time) or at the same time as the breast surgery (if detected by imaging techniques), which they actually do not need. The surgery is associated with short- and long-term adverse events which will both increase costs and affect quality of life of the patients. Since ALND is carried out for patients with FP diagnoses, the negative nodal status will be confirmed and the patients will be managed as node-negative patients afterwards.
-
The number of FN cases increases slightly when the sampling methods are replaced with MRI. FN cases increase significantly when the sampling methods are replaced with PET (from around 1% to 7.2%) due to the low sensitivity of PET (63.4%).
-
For patients with FN diagnoses, as no ALND will be performed, the true nodal status will remain unknown. The FN patients will miss the ALND which they actually need, resulting in a higher risk of locoregional and distant relapse which have significant costs and quality of life implications.
-
As MRI has a lower specificity and a higher sensitivity than PET, the strategy that involves MRI will have more FP cases and fewer FN cases than strategies involving PET. However, the evidence on the accuracy of MRI is less robust, given that there are fewer studies on MRI than PET. The systematic review in the assessment also demonstrated that the sensitivity and specificity of both PET and MRI vary significantly between studies.
-
The two strategies of replacing axillary sampling with imaging techniques produce higher levels of FP cases and (particularly for PET) FN cases. These strategies may be considered unacceptable on clinical grounds, even if they are found to be more cost-effective.
Adding imaging techniques before sampling
-
When the imaging techniques are placed before the sampling methods (scenarios 3–6), the number of FP cases is increased from baseline to the same extent as in the corresponding replacement strategies (as expected according to the diagnostic pathway).
-
The benefit of putting imaging techniques before sampling methods is that they will identify a proportion of TP patients (depending on the sensitivity of the tests) who will undergo the ALND straight away (at the same time as the main breast surgery), rather than undergoing both sampling (at the same time as the breast surgery) and a separate ALND procedure. This will reduce costs and possibly morbidity associated with having two sequential operations.
-
As expected, the number of FN cases is reduced when imaging techniques are placed before sampling due to the use of two sequential diagnostic tests. When MRI is placed before the sampling methods, the proportion of FN cases drops to about 0.1%.
-
Strategies that place imaging techniques before sampling methods produce fewer FN cases. However, they also produce significantly more FP cases, especially in the case of PET. This may be considered unacceptable on clinical grounds.
Resource use for diagnosis and surgical procedures
Table 25 presents the number of main diagnostic tests and surgical procedures carried out under each diagnostic strategy based on 5000 patients. Breast surgery is performed as a stand-alone procedure only if the negative nodal status is obtained by an imaging technique and no sampling methods are used (in scenario 1 or 2).
| Diagnostic strategy | MRI | PET | 4-NS (with breast surgery) | SLNB (with breast surgery) | ALND (stand-alone) | ALND (with breast surgery) | ALND – subtotal | Breast surgery (stand-alone) |
|---|---|---|---|---|---|---|---|---|
| Baseline 1: 4-NS | N/A | N/A | 3913 | N/A | 930 | 1087 | 2017 | 0 |
| Baseline 2: SLNB | N/A | N/A | N/A | 3913 | 916 | 1087 | 2003 | 0 |
| Scenario 1: replace sampling with MRI | 3913 | N/A | N/A | N/A | 0 | 2283 | 2283 | 2717 |
| Scenario 2: replace sampling with PET | N/A | 3913 | N/A | N/A | 0 | 1877 | 1877 | 3123 |
| Scenario 3: add MRI before 4-NS | 3913 | N/A | 2716 | N/A | 88 | 2284 | 2372 | 0 |
| Scenario 4: add PET before 4-NS | N/A | 3914 | 3123 | N/A | 341 | 1877 | 2219 | 0 |
| Scenario 5: add MRI before SLNB | 3914 | N/A | N/A | 2716 | 87 | 2284 | 2371 | 0 |
| Scenario 6: add PET before SLNB | N/A | 3914 | N/A | 3123 | 336 | 1877 | 2213 | 0 |
The key findings from the resource use results
Replacing sampling
When sampling methods are replaced with imaging techniques, all ALND procedures will be performed at the same time as the breast surgery as node-positive patients are diagnosed by either biopsy or non-invasive imaging techniques. It is also possible that breast surgery is performed as a stand-alone procedure if negative nodal status is obtained by the imaging techniques.
The same numbers of imaging techniques are carried out in scenarios 1 and 2, in which sampling methods are replaced with MRI and PET. There are more ALND procedures in scenario 1 than in scenario 2 (2283 vs 1877) as MRI is associated with more TP and FP cases than PET (see Table 24) due to its higher sensitivity and lower specificity. For the same reason, there are fewer stand-alone breast surgeries in scenario 1 than in scenario 2 (2717 vs 3123).
Adding imaging techniques before sampling
When imaging techniques are introduced before the sampling methods, ALND will be performed as a stand-alone procedure if positive nodal status is obtained from sampling procedures. ALND will be carried out at the same time as the breast surgery if positive nodal status is obtained from either imaging techniques or biopsy. As in the two baseline scenarios, it is not possible to have a stand-alone breast surgery when the imaging techniques are placed before the sampling methods.
As the imaging techniques are performed before the sampling methods, the numbers of imaging techniques performed are the same as the replacement scenarios. Compared with the two baseline scenarios, the numbers of sampling procedures carried out are reduced (from 3913 to 2716 if MRI is placed before sampling and to 3123 if PET is placed before sampling). The reduction is more evident when MRI is placed before sampling than PET, as MRI is associated with more positive cases (both true and false), which do not require further sampling methods.
Adverse events
The numbers of short- and long-term adverse events associated with each diagnostic strategy are presented in Table 26 and Figure 17. The numbers of short- and long-term adverse events are proportional to the number of sampling and ALND procedures undertaken (see Table 25). The model assumes that the probabilities of short-term adverse events are the same for both 4-NS and SLNB, while ALND is associated with much higher probabilities of developing adverse events.
| Diagnostic strategy | Short-term adverse events | Long-term adverse events (lymphoedema) |
|---|---|---|
| Baseline 1: 4-NS | 2781 | 633 |
| Baseline 2: SLNB | 2764 | 631 |
| Scenario 1: replace sampling with MRI | 2642 | 487 |
| Scenario 2: replace sampling with PET | 2172 | 401 |
| Scenario 3: add MRI before 4-NS | 3055 | 684 |
| Scenario 4: add PET before 4-NS | 2925 | 662 |
| Scenario 5: add MRI before SLNB | 3055 | 684 |
| Scenario 6: add PET before SLNB | 2919 | 661 |
FIGURE 17.
Adverse event cases associated with each diagnostic strategy.
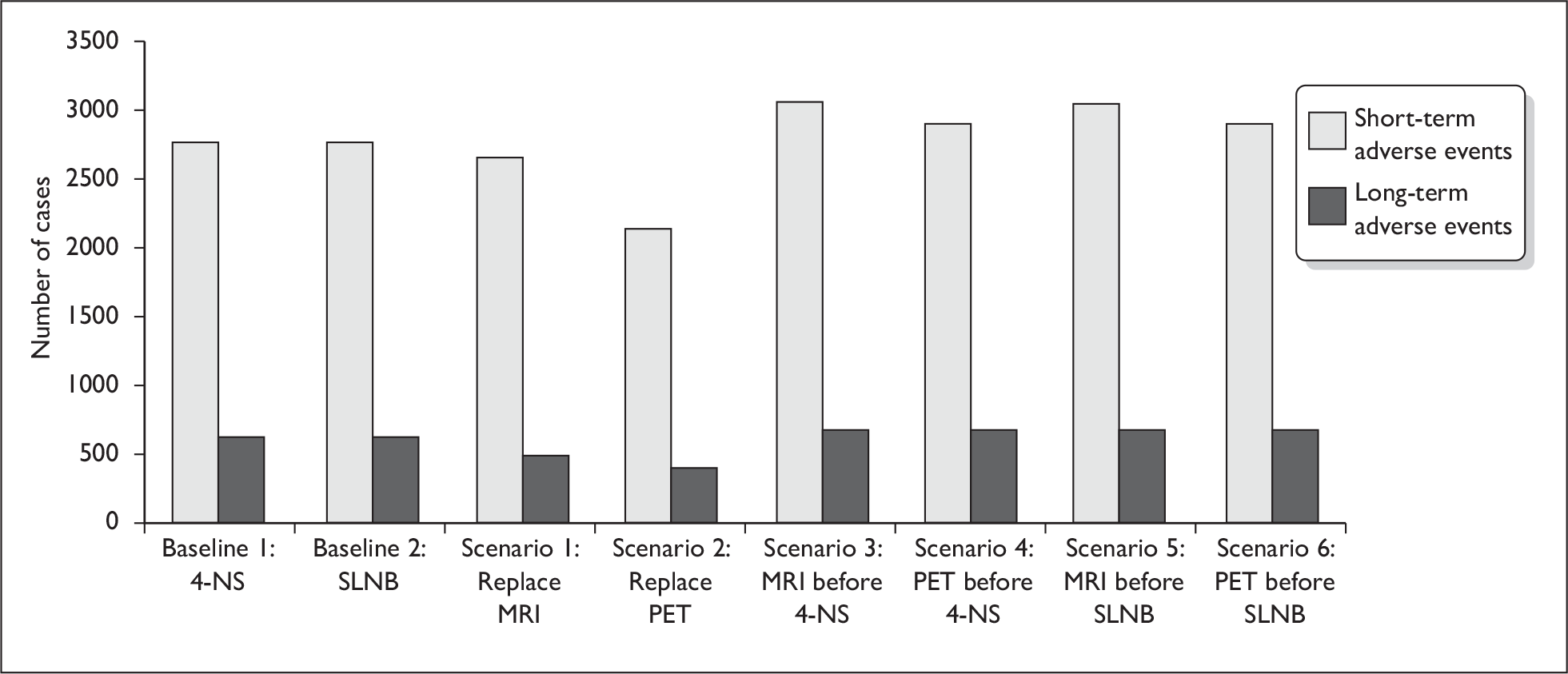
The key findings from the adverse event results
Replacing sampling
The two replacement strategies (scenarios 1 and 2) have both fewer short- and long-term adverse events than the two baseline strategies, despite more ALND procedures being undertaken. This is due to the number of sampling procedures avoided (see Table 25). The PET replacement strategy has fewer adverse events than the MRI replacement strategy as fewer ALND procedures are performed.
Adding imaging techniques before sampling
In general, there are more short- and long-term adverse events when imaging techniques are placed before sampling methods (scenarios 3–6). Under these scenarios, some sampling procedures are avoided (if positive nodal status is obtained from imaging techniques). However, more patients will undergo ALND (either stand-alone or with breast surgery) in these scenarios as the number of patients with both TP and FP diagnoses will increase (see Table 24). The model suggests that the adverse events associated with increased ALND procedures outnumber the adverse events associated with avoided sampling procedures (ALND is associated with significantly higher probability of developing adverse events than sampling methods).
Survival results
The 5-year survival rates for patients with different diagnostic results are presented in Figure 18. Patients with axillary lymph node metastases (both TP and FN) have lower survival rates than patients with negative nodal status. In particular, patients with FN results are associated with the lowest survival rate, because they do not receive ALND or chemotherapy that may reduce the chance of recurrence.
FIGURE 18.
The 5-year survival rates for patients with different diagnostic results.
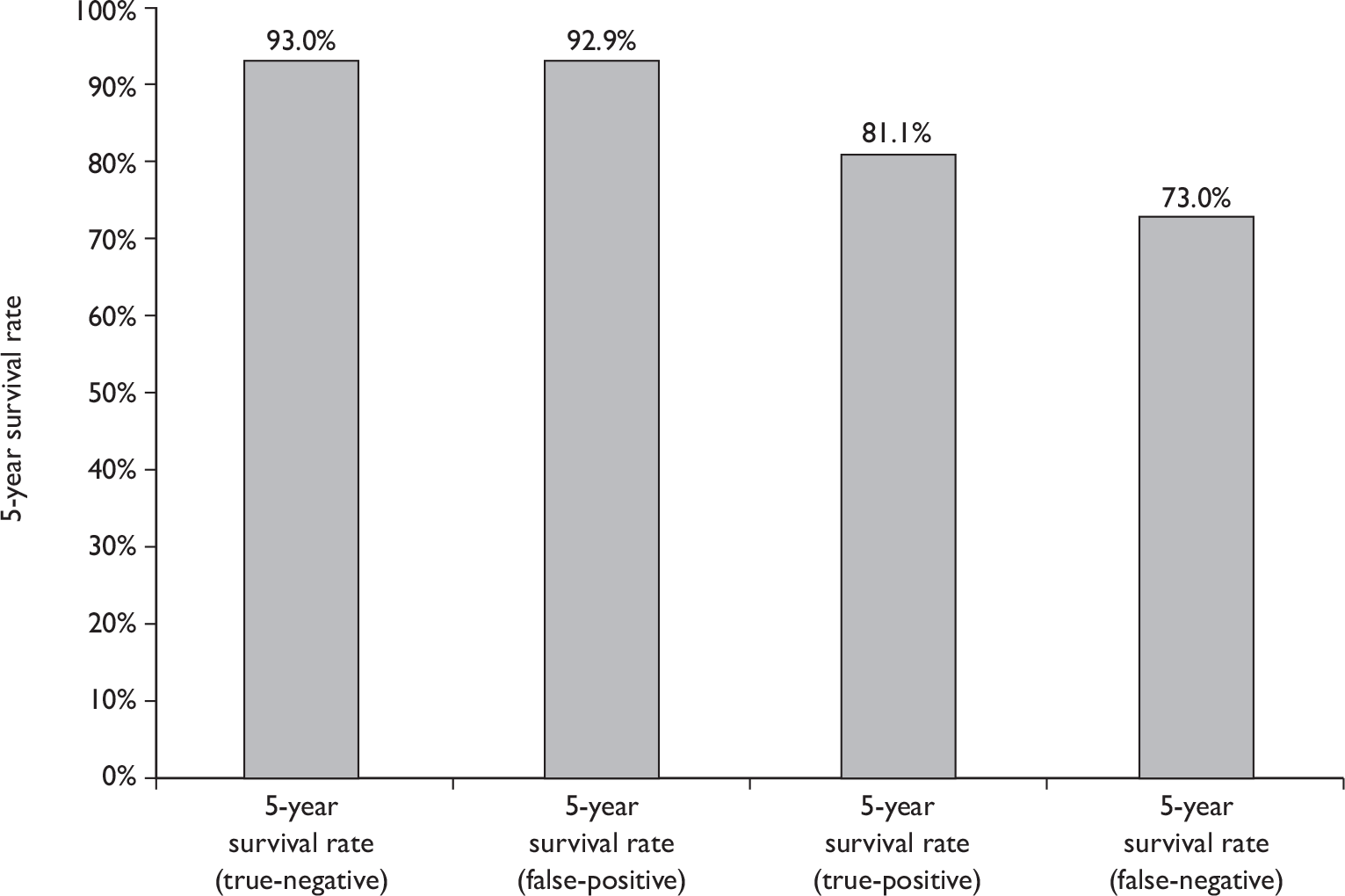
The 5-year survival rates of all patients with early breast cancer and the absolute number of deaths per 10,000 patients presenting with early breast cancer are presented in Table 27 for each tested diagnostic strategy. The overall survival rate of each strategy is dependent on the proportion of patients with each diagnostic result (see Table 24) and the individual survival rate for patients with different diagnostic results (see Figure 18). As patients with FN results have the lowest survival rate, diagnostic strategies that are associated with more FN results have lower overall survival rate (e.g. PET replacement strategy). The absolute differences in overall survival rates among tested diagnostic strategies are relatively small (range from 87.52% to 88.1%). This is because the absolute numbers of patients with FN results only account for a small proportion of patients (range from 0.1% to 7.2% as shown in Table 24). The absolute difference in the number of deaths during the first 5 years per 10,000 early-stage breast cancer patients is also presented in Table 27. This shows the change in absolute mortality for each tested diagnostic strategy, using the 4-NS baseline as the reference strategy.
| Diagnostic strategy | 5-year survival rate (all patients) (%) | Number of deaths during first 5 years (per 10,000 patients) | Absolute difference in number of deaths during first 5 years (per 10,000 patients) |
|---|---|---|---|
| Baseline 1: 4-NS | 88.03 | 1197 | 0 |
| Baseline 2: SLNB | 88.00 | 1200 | 3 |
| Scenario 1: replace MRI | 87.96 | 1204 | 7 |
| Scenario 2: replace PET | 87.52 | 1248 | 51 |
| Scenario 3: MRI before 4-NS | 88.10 | 1190 | –7 |
| Scenario 4: PET before 4-NS | 88.08 | 1192 | –5 |
| Scenario 5: MRI before SLNB | 88.10 | 1190 | –7 |
| Scenario 6: PET before SLNB | 88.07 | 1193 | –4 |
Cost-effectiveness results (net benefit analysis)
Positron emission tomography and MRI are assumed to be associated with no short- and long-term adverse events. However, due to the lower accuracy of the imaging techniques, more FP and FN cases will be produced, which will lead to increased costs, worse quality of life due to adverse events, and in some cases higher probability of recurrence and subsequent death from breast cancer. Economic modelling provides a systematic way to understand and quantify the complex trade-offs between the advantages and disadvantages of the imaging techniques, so that the overall cost-effectiveness of alternative strategies can be determined.
Net benefit is the increase in effectiveness (Δ E), multiplied by the amount the decision-maker is willing to pay per unit of increased effectiveness (RT), less the increase in cost (Δ C). The formula for calculating net benefit is:
A strategy is most cost-effective if it has the highest positive net benefit. Thresholds of willingness to pay per QALY of £10,000, £20,000 and £30,000 were used to calculate the net benefit of each strategy.
Two baseline strategies (4-NS and SLNB) are considered. Both 4-NS and SLNB are currently used in the UK. It is not within the scope of this assessment to compare these sampling methods. Tables 28 and 29 summarise the net benefits of each strategy using either 4-NS or SLNB as the baseline strategy. Note that scenarios 1 and 2 appeared in both tables because they are comparable to both 4-NS and SLNB strategies. The total costs and QALYs of each diagnostic strategy are plotted in Figures 19 and 20.
| Costs associated with diagnostic tests or short-term adverse events (£) | Costs associated with health states or long-term adverse events (£) | Total costs (£) | Total QALYs | Net benefit (£10,000 per QALY) | Net benefit (£20,000 per QALY) | Net benefit (£30,000 per QALY) | |
|---|---|---|---|---|---|---|---|
| Baseline 1: 4-NS | 3157 | 16,571 | 19,728 | 8.122 | Baseline | ||
| Scenario 1: replace sampling with MRI | 3030 | 16,295 | 19,325 | 8.174 | 919 | 1435 | 1952 |
| Scenario 2: replace sampling with PET | 3484 | 15,835 | 19,319 | 8.126 | 450 | 490 | 531 |
| Scenario 3: add MRI before 4-NS | 3204 | 16,655 | 19,859 | 8.125 | –104 | –78 | –51 |
| Scenario 4: add PET before 4-NS | 3818 | 16,623 | 20,440 | 8.126 | –681 | –649 | –618 |
| Costs associated with diagnostic tests or short-term adverse events (£) | Costs associated with health states or long-term adverse events (£) | Total costs (£) | Total QALYs | Net benefit (£10,000 per QALY) | Net benefit (£20,000 per QALY) | Net benefit (£30,000 per QALY) | |
|---|---|---|---|---|---|---|---|
| Baseline 2: SLNB | 3642 | 16,547 | 20,189 | 8.119 | Baseline | ||
| Scenario 1: replace sampling with MRI | 3030 | 16,295 | 19,325 | 8.174 | 1412 | 1959 | 2507 |
| Scenario 2: replace sampling with PET | 3484 | 15,835 | 19,319 | 8.126 | 942 | 1014 | 1085 |
| Scenario 5: add MRI before SLNB | 3546 | 16,655 | 20,201 | 8.124 | 35 | 82 | 129 |
| Scenario 6: add PET before SLNB | 4208 | 16,614 | 20,822 | 8.125 | –577 | –520 | –464 |
FIGURE 19.
Total costs and QALYs of diagnostic strategies using 4-node sampling as baseline.
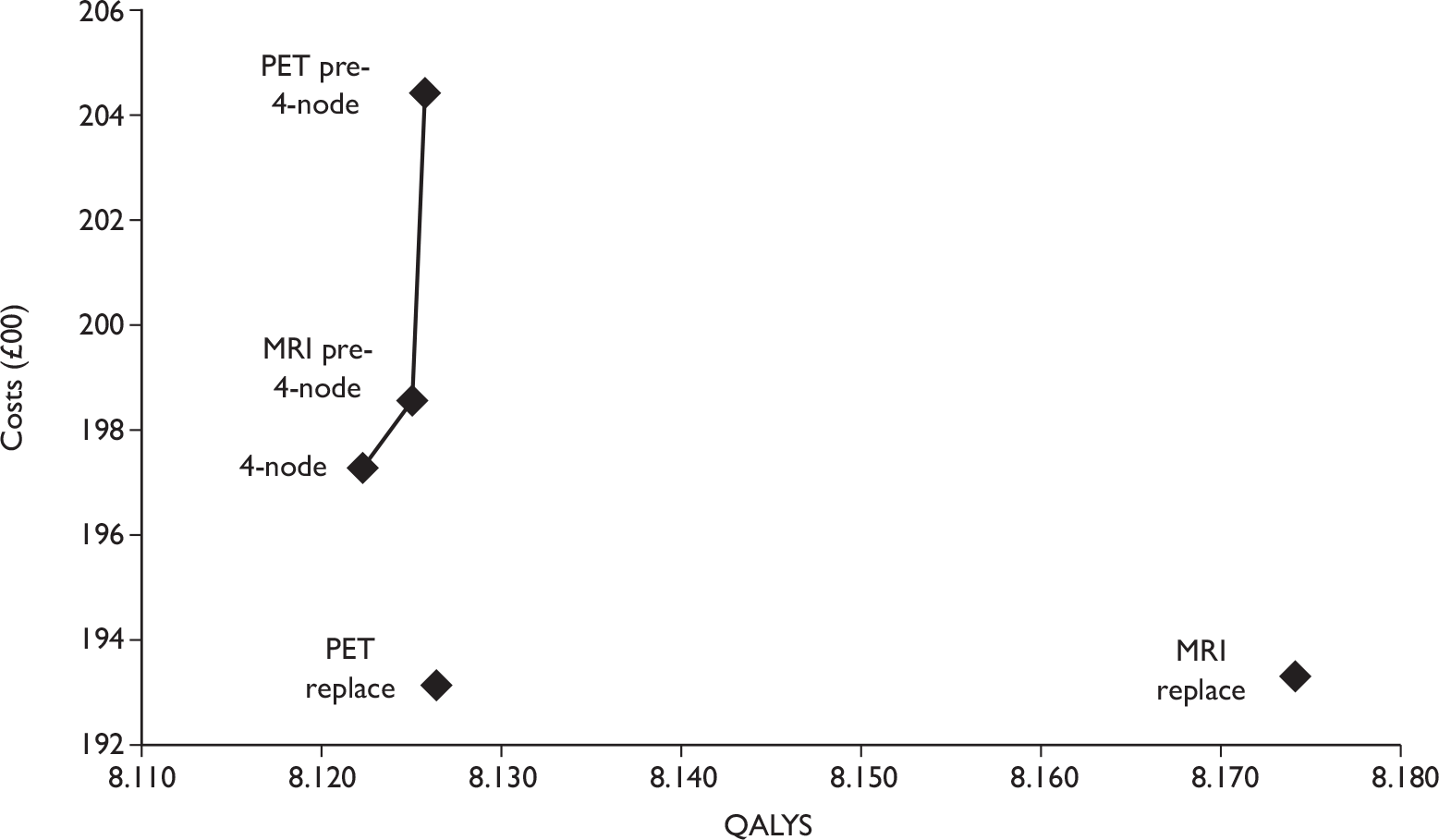
FIGURE 20.
Total costs and QALYs of diagnostic strategies using sentinel lymph node biopsy as baseline.
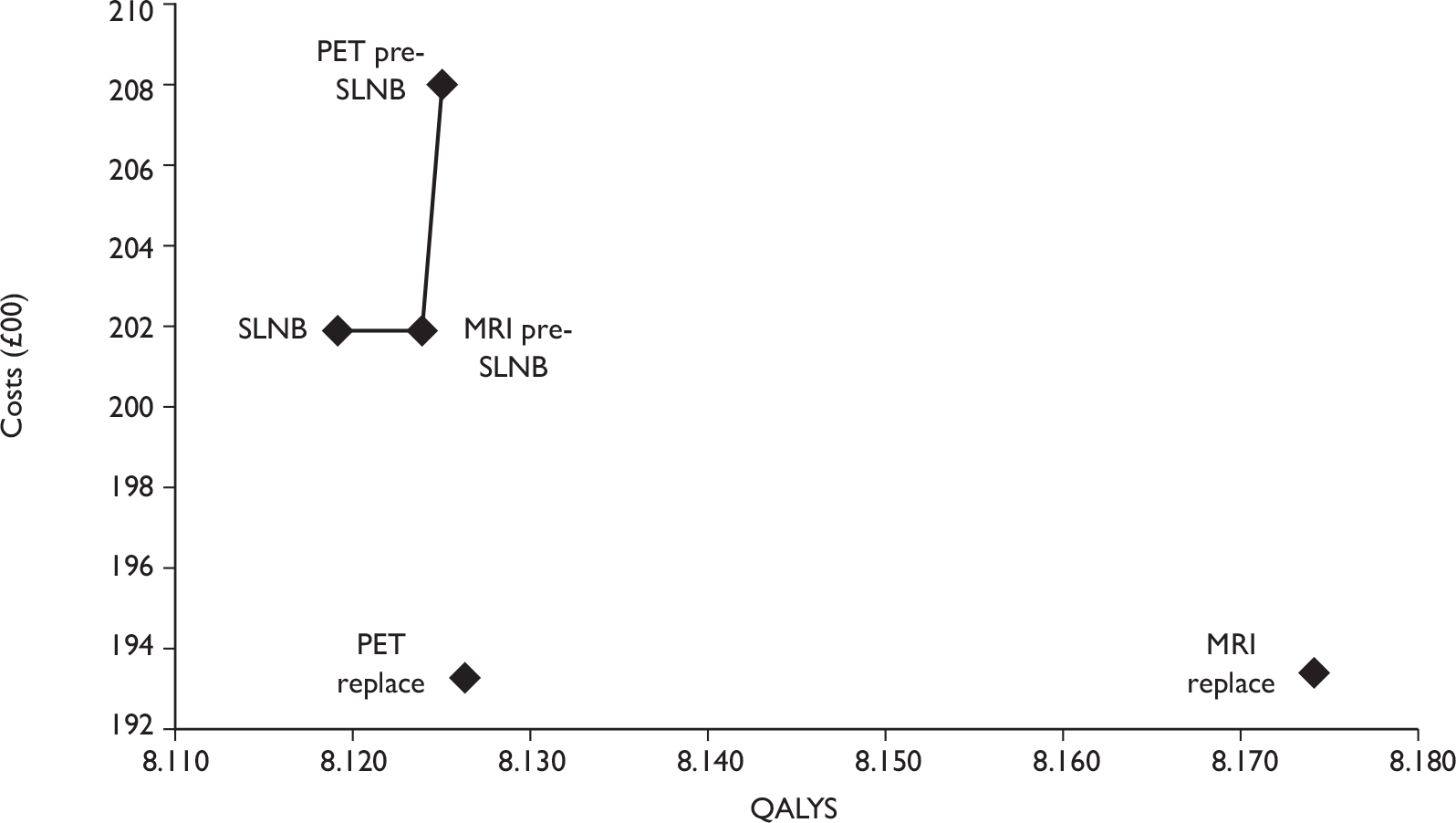
The horizontal axis of Figures 19 and 20 represents the total QALYs accrued by each diagnostic strategy and the vertical axis represents the total costs associated with each strategy. The lines in the figures denote the cost-effective frontiers if PET and MRI replacement strategies are excluded based on clinical grounds.
The total and breakdown costs (costs associated with diagnostic tests or short-term adverse events and costs associated with health states or long-term adverse events) of each strategy are illustrated in Figure 21.
FIGURE 21.
Costs associated with each diagnostic strategy.
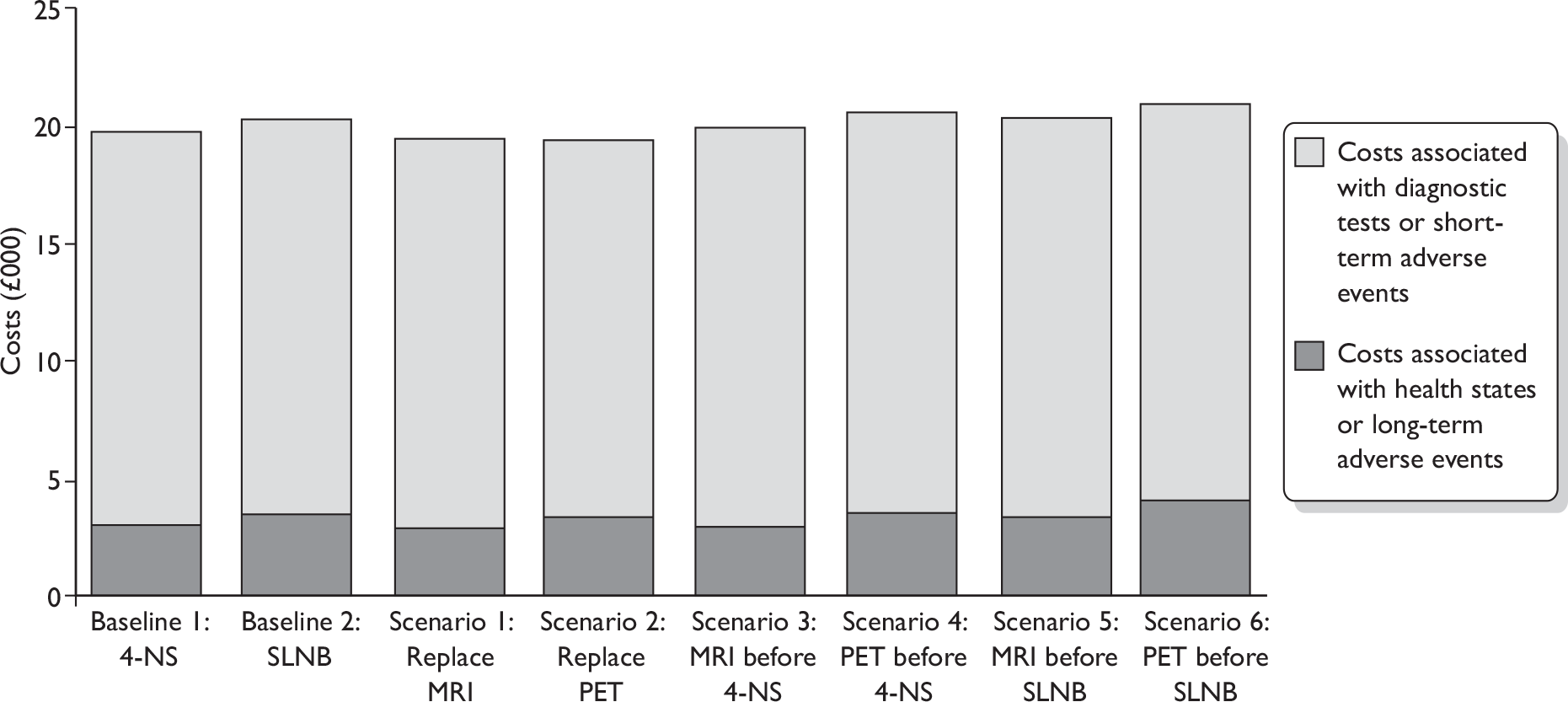
Key findings from the net benefit results
Replacing sampling
-
When 4-NS is used as the baseline (see Table 28 and Figure 19), the most cost-effective strategy is to replace sampling with MRI (scenario 1), which has the highest net benefits under all willingness-to-pay thresholds tested. The next most cost-effective strategy is to replace sampling with PET (scenario 2). The baseline 4-NS strategy is dominated by both scenario 1 and scenario 2, as they have lower total costs and higher total QALYs.
-
When SLNB is used as the baseline (see Table 29 and Figure 20), the most cost-effective strategy is still to replace sampling with MRI (scenario 1), which has the highest net benefits under all willingness-to-pay thresholds tested. The next most cost-effective strategy is also to replace sampling with PET (scenario 2). The baseline SLNB strategy is dominated by both scenarios 1 and 2, as they have lower total costs and higher total QALYs.
-
MRI has reasonably good sensitivity and specificity and lower cost than PET, 4-NS and SLNB. Compared with the baseline 4-NS and SLNB strategies, the disadvantages of the MRI replacement strategy are that it is associated with more FP cases (increased from 0.2% to 6.3%) resulting in many node-negative patients undergoing unnecessary ALND, and more FN cases (increased from around 1.0% to 1.9%) who are left at higher risk of cancer recurrence. The advantage of the MRI replacement strategy, compared with the two baseline strategies, is that many node-positive patients will be correctly diagnosed by MRI and undergo ALND (at the same time as the breast surgery) rather than undergoing two surgical procedures (4-NS or SLNB followed by ALND). For TN patients, the advantage of the MRI replacement strategy is that these patients will be correctly diagnosed without the need for a sampling procedure. The sampling procedures are associated with increased costs and risk of short- and long-term adverse events. The model suggests that the advantages of the MRI replacement strategy outweigh the disadvantages in relation to benefits as expressed by QALYs.
-
The advantages and disadvantages of MRI compared with sampling methods also applies to PET. Compared with the baseline strategies, the model also suggests that the advantages of PET outweigh the disadvantages for both costs and QALYs. Compared with the MRI replacement strategy, the PET replacement strategy has similar total costs but significantly lower total QALYs. This is because PET is associated with more FN cases due to lower sensitivity and patients with FN diagnoses are more likely to experience locoregional and metastatic relapse.
Adding magnetic resonance imaging or positron emission tomography before sampling
The MRI and PET replacement strategies may be deemed unacceptable on clinical grounds due to higher numbers of patients with FN and FP diagnoses. When the replacement strategies are excluded, the baseline 4-NS and SLNB strategies were only compared with the strategies of adding MRI or PET before sampling methods.
-
The most cost-effective strategy is to retain the baseline 4-NS strategy (when 4-NS is used as the baseline) or to place MRI before SLNB (when SLNB is used as the baseline).
-
The advantages of the strategies of adding MRI or PET before sampling methods are that there are fewer FN cases (reduced from around 1.0% to 0.1% for MRI) due to the use of two sequential tests, and fewer sampling procedures performed (because sampling methods are avoided if MRI or PET results are positive). The disadvantages of these strategies are that there are more FP cases because the specificities of MRI and PET are lower than those of SLNB and 4-NS (FPs increase from 0.2% to 6.3% for MRI prior to SLNB, which is the same as for the MRI replacement strategy). Overall, the cost-effectiveness results suggest that there are both higher costs and higher QALYs associated with strategies of adding MRI or PET before sampling methods compared with the baseline 4-NS and SLNB strategies.
-
In terms of cost-effectiveness, the model results suggest that adding MRI prior to SLNB is cost-effective, whereas adding MRI prior to 4-NS is not. This is because the addition of MRI means that fewer sampling procedures are required, and the cost saving associated with this is greater for SLNB than for 4-NS because SLNB is more costly than 4-NS.
-
The absolute differences in QALYs among the baseline 4-NS and SLNB strategies and the strategies of adding MRI or PET before sampling are very small. When the MRI and PET replacement strategies are excluded, the total QALYs range from 8.122 to 8.126 when 4-NS is used as the baseline and from 8.119 to 8.125 when SLNB is used as the baseline. This implies that there is no significant absolute improvement in QALYs when MRI or PET are added before 4-NS or SLNB. For example, although the model results suggest the strategy of adding MRI before SLNB is cost-effective, the change from the baseline SLNB strategy to the alternative strategy of MRI before SLNB only increases the QALYs by 0.005. The main reason that the alternative strategy is cost-effective is that there is an even smaller increase in total costs (relative to the QALY increase).
Cost-effectiveness results (incremental analysis)
The incremental cost-effectiveness analyses were performed assuming that MRI and PET replacement strategies are deemed unacceptable on clinical grounds. Tables 30 and 31 show the incremental cost-effectiveness analyses where 4-NS and SLNB are used as the baseline strategy.
| Total cost (£) | Total QALYs | Incremental cost (£) | Incremental QALY | ICER (£) | |
|---|---|---|---|---|---|
| Baseline 1: 4-NS | 19,728 | 8.122 | Baseline | ||
| Scenario 3: add MRI before 4-NS | 19,859 | 8.125 | 131 | 0.003 | 48,986 |
| Scenario 4: add PET before 4-NS | 20,440 | 8.126 | 581 | 0.000 | 1,200,212 |
| Total cost (£) | Total QALYs | Incremental cost (£) | Incremental QALY | ICER (£) | |
|---|---|---|---|---|---|
| Baseline 2: SLNB | 20,189 | 8.119 | Baseline | ||
| Scenario 5: add MRI before SLNB | 20,201 | 8.124 | 11 | 0.005 | 2451 |
| Scenario 6: add PET before SLNB | 20,822 | 8.125 | 622 | 0.001 | 647,415 |
The cost-effectiveness plane for the MRI before 4-NS strategy versus the baseline 4-NS strategy is presented in Figure 22. The cost-effectiveness plane for the MRI before SLNB strategy versus the baseline SLNB strategy is presented in Figure 23.
FIGURE 22.
Cost-effectiveness plane for MRI before 4-node sampling (4-NS) strategy versus baseline 4-NS strategy.
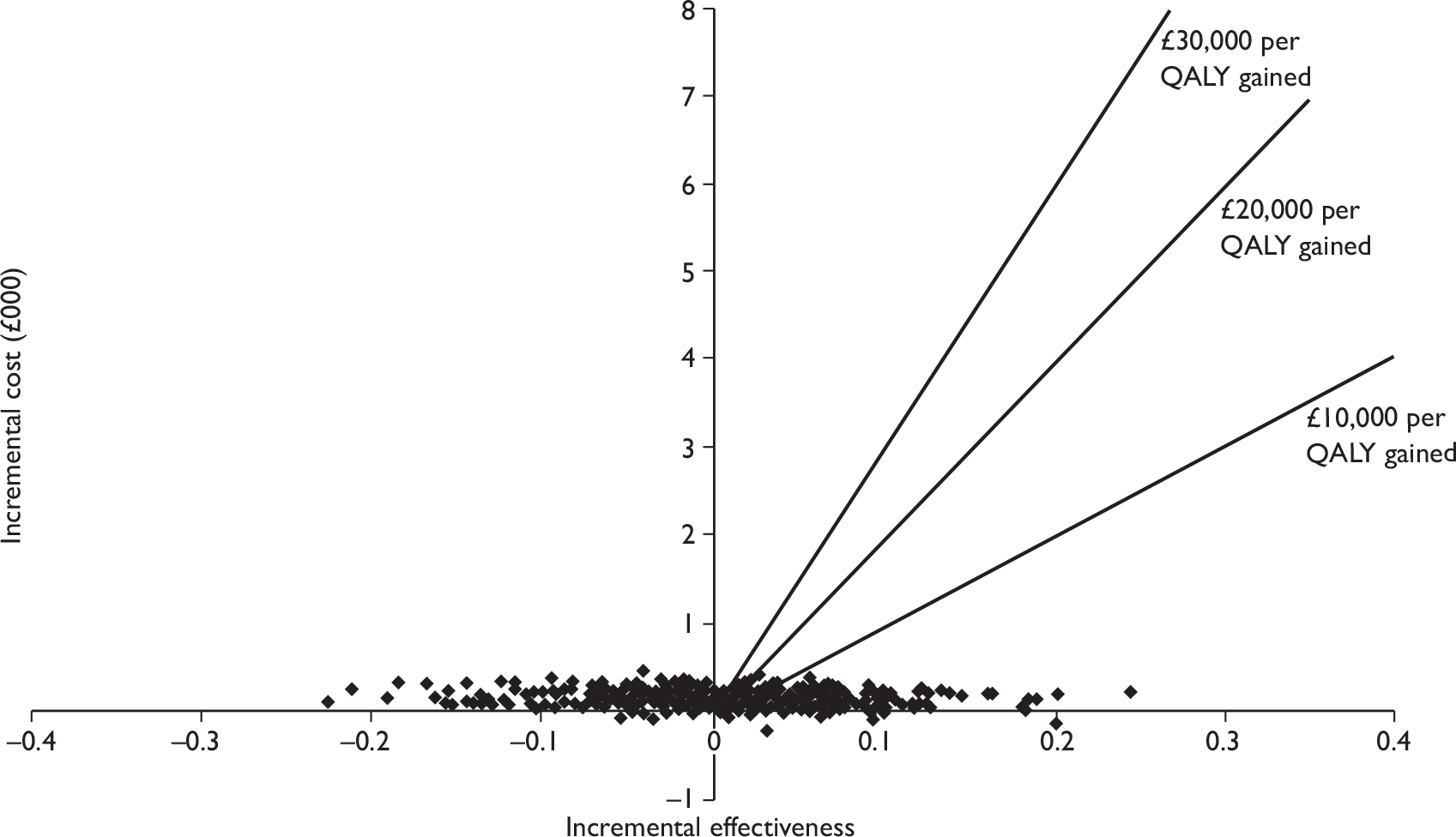
FIGURE 23.
Cost-effectiveness plane for MRI before SLNB strategy versus baseline SLNB strategy.
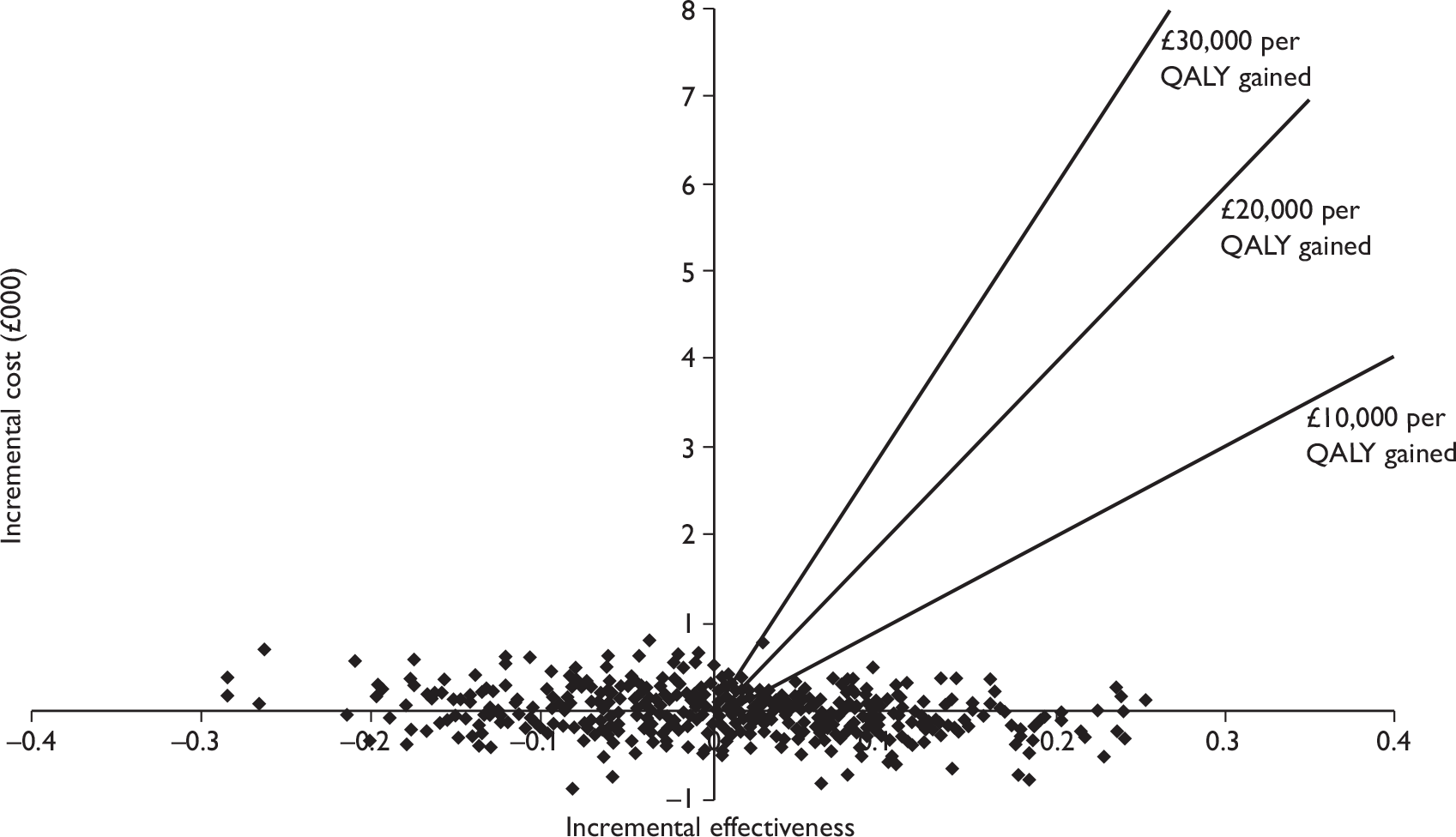
Both Figures 22 and 23 demonstrate that MRI before sampling strategy may lead to either an increase or decrease in QALYs compared with the baseline strategy. The advantage of this strategy in terms of QALYs is therefore uncertain. Figure 22 shows that the MRI before 4-NS typically generates higher total costs than the 4-NS strategy. Figure 23 shows that MRI before SLNB strategy may lead to either an increase or a decrease in total costs compared with the SLNB strategy. The benefits offered by these strategies are not clear-cut.
Univariate sensitivity analysis
Sensitivity and specificity of magnetic resonance imaging
When the sensitivity of MRI is reduced from 90% to 78% (the lower CI) while maintaining the mean specificity of 90%, the strategy of MRI replacement is still the most cost-effective strategy when either 4-NS or SLNB is used as baseline. If the MRI and PET replacement strategies are rejected on clinical grounds, then the conclusions differ from the baseline results. When SLNB is used as the baseline, the most cost-effective strategy is to retain the baseline SLNB strategy when a willingness-to-pay threshold of £20,000 per QALY is used. This differs from the baseline results, where the most cost-effective strategy is the addition of MRI before SLNB.
When the specificity of MRI is reduced from 90% to 75% (the lower CI), while maintaining the mean sensitivity of 90%, the PET replacement strategy becomes the most cost-effective strategy when a willingness-to-pay threshold of £20,000 per QALY is used. This conclusion differs from the baseline results where the MRI replacement strategy is most cost-effective. If the MRI and PET replacement strategies are rejected on clinical grounds, then the strategy of adding MRI before SLNB is dominated by the baseline SLNB strategy. This means it is cost-effective to retain the baseline SLNB strategy.
When the sensitivity and specificity of MRI are increased to the levels of USPIO-enhanced MRI (from 90% to 98% and from 90% to 96%, respectively), the MRI replacement strategy remains the most cost-effective strategy. If the MRI and PET replacement strategies are rejected based on clinical grounds, then the most cost-effective strategy is to add MRI before 4-NS (when 4-NS is used as baseline) and to add MRI before SLNB (when SLNB is used as baseline).
The sensitivity analyses demonstrate that the sensitivity and specificity of MRI have a significant impact on the cost-effectiveness results.
Sensitivity of positron emission tomography
When the sensitivity of PET is increased from 63% to 74% (the upper CI), while maintaining the mean specificity of 94%, the baseline cost-effectiveness results do not change. The total QALYs of the three strategies that involve PET, including the PET replacement strategy and strategies to add PET before 4-NS and SLNB, were all increased. However, the increase is not significant enough to alter the conclusions relating to cost-effectiveness. The sensitivity analysis demonstrates that the sensitivity of PET does not appear to have a significant impact on cost-effectiveness results.
Utility decrement for lymphoedema
When the assumed utility decrement for lymphoedema is reduced by 50% (i.e. from 9.9% to 5.0% for mild/moderate lymphoedema and from 12.3% to 6.2% for severe lymphoedema), the MRI replacement strategy is still the most cost-effective strategy. However, the total QALYs for the PET replacement strategy, which is the second most effective strategy, becomes the smallest among all diagnostic strategies modelled. The PET replacement strategy is associated with the fewest cases of lymphoedema (see Table 26) and is therefore most affected. The cost-effectiveness results remain unchanged if the MRI and PET replacement strategies are rejected on clinical grounds.
When the assumed utility decrement for lymphoedema is increased by 50% (i.e. from 9.9% to 14.9% for mild/moderate lymphoedema and from 12.3% to 18.5% for severe lymphoedema), the baseline cost-effectiveness results do not change. Although the total QALYs for all strategies are reduced due to a higher utility decrement for lymphoedema, the sensitivity analysis shows that the decrease is smallest for the PET replacement strategy, as this strategy has the smallest number of lymphoedema cases. However, the MRI replacement strategy is still the most cost-effective.
The sensitivity analyses demonstrate that the PET replacement strategy is most affected by the change of utility decrements for lymphoedema. However, the overall cost-effectiveness results do not appear to be significantly affected by this parameter.
Additional costs of lymphoedema
When the annual additional cost of lymphoedema is decreased by 20% (i.e. from £66.50 to £53.20 for mild/moderate lymphoedema and from £1180 to £944 for severe lymphoedema) or increased by 20% (i.e. from £66.50 to £79.80 for mild/moderate lymphoedema and from £1180 to £1416 for severe lymphoedema), the baseline cost-effectiveness results do not change. The sensitivity analysis demonstrates that the additional cost of lymphoedema does not appear to have a significant impact on cost-effectiveness results.
Probability of relapse for false-negative patients
When the probability of relapse for patients with FN diagnoses is reduced by 20% (i.e. from 0.140 to 0.112 for locoregional relapse and from 0.0094 to 0.0075 for metastatic relapse), the MRI and PET replacement strategies remain the most and second most effective strategies. However, if the MRI or PET replacement strategies are rejected on clinical grounds, then the most cost-effective strategy is either the baseline 4-NS strategy or the SLNB strategy (depending on which baseline is used) which dominate the strategies of adding MRI before 4-NS and SLNB, respectively.
When the probability of relapse for patients with FN diagnoses is increased by 20% (i.e. from 0.14 to 0.17 for locoregional relapse and from 0.0094 to 0.0113 for metastatic relapse), baseline cost-effectiveness results do not change. The total QALYs for all strategies are reduced due to the increased probability of relapse. The sensitivity analysis shows that the decrease in QALYs is most significant for the PET replacement strategy. The total QALYs for this strategy, which dominates the baseline 4-NS and SLNB strategies in the baseline results, becomes the lowest among all strategies. The PET replacement strategy is affected the most because it is associated with the largest number of patients with FN diagnoses.
The sensitivity analysis shows that cost-effectiveness results are affected by the change in probability of relapse for FN patients. Among all strategies, the PET replacement strategy is most affected.
Cost of 4-node sampling
When the cost of 4-NS is decreased by 20% (i.e. from £2099 to £1679) or increased by 20% (i.e. from £2099 to £2518), the baseline cost-effectiveness results do not change. The sensitivity analyses demonstrate that the cost of 4-NS does not appear to have a significant impact on cost-effectiveness results.
Cost of sentinel lymph node biopsy
When the cost of SLNB is decreased by 20% (i.e. from £2728 to £2182), the MRI replacement strategy remains the most cost-effective strategy. However, if the MRI and PET replacement strategies are rejected on clinical grounds and SLNB is used as the baseline, then the most cost-effective strategy is to retain the baseline SLNB strategy when a willingness-to-pay threshold of £20,000 is used. This differs from the baseline results where the most cost-effective strategy is the addition of MRI before SLNB. When the cost of SLNB is increased by 20% (i.e. from £2728 to £3274), the baseline cost-effectiveness results do not change.
The sensitivity analyses demonstrate that the cost of SLNB influences the cost-effectiveness results, when the cost of SLNB is decreased.
Discussion of cost-effectiveness and modelling results
Diagnostic results
The baseline (4-NS and SLNB) strategies produce the smallest number of FP cases among all strategies. The number of FN cases produced by the two baseline strategies is also very small (1.1% for 4-NS and 1.3% for SLNB). In the MRI replacement strategy, the number of FP cases is increased significantly from 0.2% to 6.3% and the number of FN cases is increased to a lesser extent, from around 1.0% to 1.9%. In the PET replacement strategy the numbers of both FP and FN cases are increased significantly, from 0.2% to 3.6% for FP cases and from around 1.0% to 7.2% for FN cases. Overall, the PET replacement strategy produces the largest number of FP/FN cases among all the diagnostic strategies tested.
If MRI or PET is placed before sampling methods, the number of FN cases is reduced from around 1.0% to 0.1% if MRI is placed before sampling and to around 0.5% if PET is placed before sampling. The number of FP cases remains the same as for the MRI and PET replacement strategies.
The baseline strategies produce the lowest number of FP cases. The strategies of adding MRI before 4-NS and SLNB produce the lowest number of FN cases. Overall, the baseline strategies produce the smallest number of combined FP and FN cases. The MRI and PET replacement strategies may be considered unacceptable on clinical grounds, as they both generate more FP and FN cases than current standard practice. The strategies of adding MRI or PET before sampling also produces more FP cases, although the number of FN cases is reduced.
Number of procedures
Under the baseline sampling strategies a proportion of patients will need two separate surgical procedures: a sampling procedure (4-NS or SLNB) and subsequent ALND. In the replacement strategies, no sampling procedures are needed and the ALND procedures are carried out for TP and FP cases. Compared with the baseline strategies, the total number of ALND procedures carried out is increased for the MRI replacement strategy (due to more FP cases) and decreased for the PET replacement strategy (due to less TP cases).
If MRI or PET is placed before the sampling methods, the numbers of sampling procedures carried out are reduced compared with the baseline 4-NS and SLNB strategies because both TP and FP cases detected by MRI or PET will receive ALND surgery without sampling procedures. The reduction is more evident when MRI rather than PET is placed before sampling, as MRI is associated with more positive cases (both true and false). However, due to the increase in FP cases, the strategies of adding MRI or PET before sampling are associated with more ALND procedures than the baseline 4-NS and SLNB strategies.
Adverse events
The number of short- and long-term adverse events is proportional to the number of 4-NS, SLNB and ALND surgical procedures carried out. Adverse events are more frequent for ALND than 4-NS and SLNB. Among all diagnostic strategies modelled, the PET replacement strategy is associated with the lowest number of adverse events, followed by the MRI replacement strategy. The PET replacement strategy is associated with the smallest number of ALND procedures. The strategies of adding MRI or PET before sampling produce more adverse events than the baseline 4-NS and SLNB strategies, because more ALND procedures are carried out.
Survival rates
Patients with axillary lymph node metastases have lower survival rates (both TP and FN) than patients with negative nodal status (both TN and FP). Patients with FN results have the lowest survival rates because they do not receive ALND or chemotherapy that may reduce the risk of recurrence.
Compared with the two baseline strategies, the overall survival rates of early-stage breast cancer patients are lower for the MRI or PET replacement strategies and higher for the strategies where MRI or PET is placed before 4-NS and SLNB. The absolute differences in overall survival rate among tested diagnostic strategies are relatively small, as the absolute number of patients with FN results only account for a small proportion of all patients.
Cost-effectiveness analyses – baseline results
The PET and MRI strategies are compared with two baseline techniques – SLNB and 4-NS – which are both used currently in the UK. It is beyond the remit of this assessment to compare 4-NS and SLNB.
The MRI replacement strategy is the most cost-effective strategy and dominates the two baseline strategies. The higher QALYs of the MRI replacement strategy are driven by fewer cases of lymphoedema, which has a lifelong impact on quality of life. The PET replacement strategy is the next most cost-effective strategy and also dominates the two baseline strategies. Compared with the MRI replacement strategy, the PET replacement strategy has significantly lower QALYs, which is driven by more FN cases who are more likely to experience locoregional and metastatic relapse.
The cost-effectiveness results demonstrate that, on the population level, it is beneficial to replace invasive sampling methods with the non-invasive imaging techniques of MRI or PET. If the MRI or PET replacement strategies are used, a small proportion of patients will be wrongly diagnosed as FP or FN, which will impact on life-years gained and quality of life for these patients. However, the majority of patients will be correctly diagnosed by MRI or PET, without the need for sampling procedures. This will improve their quality of life owing to avoidance of short- and long-term adverse events such as lymphoedema. The model results suggest that the health benefits gained by the majority of patients outweigh the negative impact on reduced survival and lower quality of life of a small proportion of patients. The imaging replacement strategies, especially for MRI, also cost less than the baseline 4-NS and SLNB strategies. Overall, the analysis predicts that it is cost-effective to replace 4-NS or SLNB with MRI or PET.
Despite the cost-effectiveness results, the MRI and PET replacement strategies may be considered unacceptable on clinical grounds, due to higher numbers of FP and FN cases. If this is the case, then the most cost-effective strategy is the 4-NS strategy (if 4-NS is used as the baseline) or the addition of MRI before SLNB (if SLNB is used as the baseline). However, these results are less robust than the results for the replacement strategies. The differences in costs and QALYs between strategies are small and therefore the results are sensitive to changes in the input parameters. More robust evidence is needed on the costs of 4-NS and SLNB and the costs of MRI and PET. In addition more robust evidence on the sensitivity and specificity of 4-NS is also needed.
The strategies of placing MRI or PET before sampling may also be rejected on the clinical grounds that they are associated with more FP cases. In order to have a similar level of FP cases to the sampling methods, the specificity of MRI and PET needs to be improved to be close to 100% which, by definition, is the specificity of 4-NS and SLNB. For the MRI or PET replacement strategies, in order to have similar levels of FP and FN cases to the sampling methods, both the sensitivity and specificity have to be improved to the levels of 4-NS and SLNB. The most promising technique is the USPIO-enhanced MRI, which has a mean sensitivity of 98% and specificity of 96%. These figures are, however, based on a limited number of small studies. Further studies are needed to confirm the robustness of these figures.
In general, based on the estimates of sensitivity and specificity of MRI and PET in this assessment, the analysis predicts that it is cost-effective to replace 4-NS or SLNB with MRI or PET which will accrue more QALYs and cost less at the population level. Within the two replacement strategies, it is more cost-effective to replace sampling with MRI than PET.
Cost-effectiveness analyses – sensitivity analysis results
The sensitivity and specificity of MRI have a significant impact on the cost-effectiveness results, and change the results in terms of the most cost-effective strategy. Further evidence on the sensitivity and specificity of MRI is therefore needed.
The utility decrement for lymphoedema has a significant impact on the cost-effectiveness of the PET replacement strategy, as the strategy is associated with the smallest number of lymphoedema cases. If the assumed utility decrement for lymphoedema is reduced, the PET replacement strategy no longer dominates the baseline 4-NS and SLNB strategies. Further studies on the costs and quality of life of lymphoedema are needed.
The probabilities of relapse for FN patients also have a significant impact on the cost-effectiveness results, particularly for the PET replacement strategy. The PET replacement strategy no longer dominates the baseline 4-NS and SLNB strategies when the probabilities of relapse for FN patients are increased.
The change in cost of SLNB has an impact on the cost-effectiveness of the strategy of adding MRI before SLNB. When the cost of SLNB is decreased, the strategy of adding MRI before SLNB becomes less cost-effective than the baseline SLNB strategy.
Sensitivity analyses also suggest that cost-effectiveness results appear to be robust when the model inputs of sensitivity of PET, the additional costs of lymphoedema and the costs of 4-NS are changed.
In general, the MRI replacement strategy remains the most cost-effective strategy and dominates the baseline 4-NS and SLNB strategies in most of the sensitivity analyses undertaken. The PET replacement strategy is not as robust as the MRI replacement strategy, as its cost-effectiveness is significantly affected by the utility decrement for lymphoedema and the probability of relapse for FN patients. When the imaging replacement strategies are excluded, the cost-effectiveness of adding MRI before 4-NS or SLNB is affected by the change of model inputs of MRI sensitivity and specificity, the probabilities of relapse for FN patients and the cost of SLNB.
Limitations of the analysis
The main limitations of the cost-effectiveness analyses include:
-
Evidence on the sensitivity and specificity of 4-NS is limited and is less robust than evidence on SLNB. The adverse event rates of 4-NS are assumed to be the same as rates for SLNB, but this may underestimate the adverse event rates of 4-NS.
-
The sensitivity and specificity of MRI and PET vary significantly across different studies. The evidence for MRI is less robust than the evidence for PET, given that it based on a limited number of small studies.
-
The sensitivity and specificity of MRI and PET are assumed to be independent of previous tests.
-
The quality of evidence on the cost of lymphoedema and the impact of lymphoedema on quality of life is poor.
-
The quality of evidence on the costs of short-term adverse events is poor.
-
More robust costing information for the baseline sampling procedures and for MRI and PET are also needed for the UK setting.
Chapter 5 Assessment of factors relevant to the NHS and other parties
Positron emission tomography and MRI are used in the management of many cancers, for staging and for assessing response to treatment. In the context of breast cancer, PET scanning at the time of diagnosis may have other advantages in addition to detection of axillary metastases. For example, whole-body PET scans may detect undiagnosed distant metastases or synchronous tumours. 137,138
Availability of PET and MRI scanning facilities may be limited. If PET or MRI were recommended as part of the routine screening pathway for all patients with early breast cancer, this would mean a large increase in the number of PET or MRI procedures required. This issue would require careful consideration if PET or MRI were to be used in this setting. Also, a requirement for additional diagnostic techniques may add to the delay between diagnosis and treatment.
As there are contraindications associated with both PET and MRI, and some patients are unable to complete an MRI scan owing to claustrophobia, a subset of patients may be unable to undergo these techniques and may require alternative investigations.
Chapter 6 Discussion
Statement of principal findings
Summary of clinical results
Diagnostic accuracy of positron emission tomography
Across all 26 studies (n = 2591 patients) evaluating PET or PET/CT for assessment of axillary metastases, the mean sensitivity was 63% (95% CI 52% to 74%; range 20%–100%) and the mean specificity was 94% (95% CI 91% to 96%; range 75%–100%). For the seven studies (n = 862) evaluating PET/CT,73–79 the mean sensitivity was 56% (95% CI 44% to 67%) and the mean specificity was 96% (95% CI 90% to 99%). For the 19 studies (n = 1729) evaluating PET only,44,80–97 the mean sensitivity was 66% (95% CI 50% to 79%) and the mean specificity was 93% (95% CI 89% to 96%). Therefore PET/CT gave a slightly lower mean sensitivity than PET only; this may have been due to chance variation or to differences in the populations or study methods.
Positron emission tomography performed less well in terms of identifying small metastases; micrometastases (≤ 2 mm) were associated with a mean sensitivity of 11% (95% CI 5% to 22%) based on data from five studies (n = 63),79–80,86–88 while macrometastases (> 2 mm) were associated with a mean sensitivity of 57% (95% CI 47% to 66%) based on data from four studies (n = 111). 73,80,87,88 The smallest metastatic nodes detected by PET measured 3 mm,87,88 while PET failed to detect some nodes measuring > 15 mm. 86,94 Current PET cameras are thought to achieve spatial resolutions of 4–7 mm (around 4–5 mm in the centre of the field of view). 115 PET studies in which all patients were clinically node negative showed a trend towards lower sensitivity compared with studies which included both clinically node-negative and node-positive patients, which may reflect the fact that clinically negative axillary metastases are likely to be smaller. This mix of node-positive and node-negative patients is likely to reflect clinical practice. Studies using ALND reported similar sensitivity and specificity to studies using a combination of ALND and SLNB, while studies in which not all patients had ALND or SLNB (or in which the reference standard was not stated) had a higher mean sensitivity which may represent an overestimate.
Diagnostic accuracy of magnetic resonance imaging
The review identified nine studies (n = 307 patients) evaluating MRI. 41,42,64,108–113 Several MRI studies reported more than one set of diagnostic accuracy results, according to different criteria for defining whether axillary metastases were present. Based on the highest reported sensitivity and specificity per study, the mean sensitivity across all nine MRI studies was 90% (95% CI 78% to 96%; range 65%–100%) and the mean specificity was 90% (95% CI 75% to 96%; range 54%–100%). Across the five studies (n = 93 patients) evaluating USPIO-enhanced MRI,108–112 the mean sensitivity was 98% (95% CI 61% to 100%) and specificity 96% (95% CI 72% to 100%). Across three studies of gadolinium-enhanced MRI (n = 187),41,42,113 the mean sensitivity was 88% (95% CI 78% to 94%) and specificity 73% (95% CI 63% to 81%). In the single study of in vivo proton MR spectroscopy (n = 27),64 the sensitivity was 65% (95% CI 38% to 86%) and specificity 100% (95% CI 69% to 100%). Therefore USPIO-enhanced MRI showed a trend towards higher sensitivity and specificity than gadolinium-enhanced MRI. No data were presented according to the size of metastases for the MRI studies. However, current MRI methods are thought to achieve a resolution of approximately 1 mm using modern scanners and based on the methods used in the included papers.
Adverse effects and contraindications
Both PET and MRI appeared to be relatively safe in this setting, with no adverse effects reported for PET, and only mild rash, claustrophobia and back pain reported as adverse effects of MRI. There are some contraindications to both PET (pregnancy) and MRI (allergy to contrast agents, renal or liver dysfunction, pacemakers and other metallic implants). In addition, claustrophobia may prevent scanning in some patients. These factors may limit the applicability of PET and MRI for some patients.
Comparison between positron emission tomography and magnetic resonance imaging
The mean sensitivity of MRI for the studies included here was significantly higher than for PET. However, as none of the included studies directly compared PET and MRI, caution should be taken when comparing these estimates. In particular, USPIO-enhanced MRI showed high sensitivity and specificity in these preliminary studies. However, these results should be interpreted with caution, as the included MRI studies were relatively small and there was variation between and within studies in terms of the MRI method used, the criteria for defining a node as positive, and the sensitivity and specificity results for individual studies. The included studies for both PET and MRI were heterogeneous in their results. This may reflect differences in conduct and interpretation of the index test and reference standard between studies, not all of which will have been captured in the study reports. Therefore, the mean sensitivity and specificity data should be interpreted with caution.
Summary of cost-effectiveness and benefits versus risks for each strategy
The decision model evaluated the benefits, harms and cost-effectiveness of PET and MRI, either as a replacement for SLNB or 4-NS or as an additional test prior to SLNB or 4-NS. Comparison of SLNB with 4-NS was not part of the remit of this assessment, but both techniques are in current use. Therefore, two baseline strategies (SLNB and 4-NS) were modelled and strategies involving SLNB were assessed separately to strategies involving 4-NS.
The results of the decision modelling suggest that the most cost-effective strategy is to replace sampling (SLNB or 4-NS) with MRI. This strategy dominates the baseline SLNB and 4-NS strategies, generating higher QALYs and lower costs. Axillary sampling (SLNB and 4-NS) is avoided for all patients, leading to fewer adverse effects, especially lymphoedema, which has an assumed lifelong impact on quality of life. However, MRI has lower sensitivity than SLNB and 4-NS (leading to more FNs) and a lower specificity (leading to more FPs). Patients with a FN diagnosis will not receive ALND or adjuvant therapy, leading to a higher risk of cancer recurrence. Patients with a FP diagnosis will receive ALND unnecessarily, with the accompanying increased risk of adverse effects. On the population level, the model results suggest that the MRI replacement strategy costs less, and also the health benefits gained by the majority of patients outweigh the negative impact on survival and quality of life of a small proportion of patients. This strategy may, however, be rejected on clinical grounds, owing to the increase in FP and FN cases compared with current practice.
If the replacement strategies are rejected on clinical grounds, the most cost-effective strategy is predicted to be the baseline 4-NS strategy (compared with 4-NS as the baseline) or the use of MRI prior to SLNB (compared with SLNB as the baseline). For strategies that place MRI or PET before sampling, patients with TP PET or MRI results receive immediate ALND without the need to carry out a separate SLNB or 4-NS procedure. However, the need for a second surgical procedure may also be averted through the use of intraoperative cytology, whereby the axillary sampling procedure may be converted immediately to full ALND for patients with positive nodes. Intraoperative cytology is currently under investigation at a number of units. 139 The number of FN cases is also reduced due to the use of two sequential tests. The disadvantage is that, due to the lower specificity of PET and MRI compared with SLNB and 4-NS, there are still more patients with FP results who receive ALND unnecessarily, with the accompanying increased risk of adverse effects. The total QALYs generated by the strategies of adding MRI prior to 4-NS or SLNB are very similar to those in the baseline strategies and these results are not considered to be robust, based on the quality of the data available.
The model results indicate that, as would be expected, patients with axillary lymph node metastases (either TP or FN) have lower survival rates than patients with negative nodal status. Patients with FN results have the lowest survival rates because they do not receive ALND or chemotherapy that may reduce the risk of recurrence. Compared with the two baseline strategies, the overall survival rates are lower for the MRI or PET replacement strategies (due to an increase in FNs) and higher for the strategies where MRI or PET are placed before 4-NS and SLNB (due to a decrease in FNs). However, the absolute differences in overall survival rate among tested diagnostic strategies are relatively small, as the absolute number of patients with FN results only account for a small proportion of all patients.
Sensitivity analysis suggests that the MRI replacement strategy remains the most cost-effective strategy in the majority of the one-way sensitivity analyses undertaken. The sensitivity analyses indicate that the cost-effectiveness of the PET replacement strategy is significantly affected by the assumption relating to utility decrements for lymphoedema and the probability of relapse for FN patients. The quality of data on long-term utility decrements generated by lymphoedema is particularly poor. If the PET and MRI replacement strategies are excluded, the cost-effectiveness of the strategies of adding MRI prior to 4-NS or SLNB is affected by the assumed MRI sensitivity and specificity, the probability of relapse for FN patients, and the costs of SLNB. The results relating to addition of MRI prior to 4-NS or SLNB are not considered to be robust.
Strengths and limitations of the assessment
This assessment provides a comprehensive evaluation of PET and MRI for the assessment of axillary metastases. Mean values for sensitivity and specificity have been calculated using the bivariate random effects approach, which accounts for variation within and between studies as well as taking into account the correlation between sensitivity and specificity. 70,71
Uncertainties
The included studies for both PET and MRI were heterogeneous in their results, implying that their sensitivities and specificities may vary in practice according to the methods used. For MRI there are fewer studies with smaller numbers of patients, and there are wide variations between studies in terms of the MRI methods used and the criteria for defining a node as positive. Therefore, caution should be taken when interpreting these results, particularly for MRI. The sensitivity and specificity data for 4-NS are not as robust as for SLNB due to the fewer studies identified and the smaller patient sample sizes within studies.
There are a number of key uncertainties and limitations relating to the cost-effectiveness modelling analyses. The evidence for MRI is based on a limited number of small studies. The sensitivity and specificity of PET and MRI are assumed to be independent of previous tests. There are insufficient high-quality data to estimate the costs of SLNB, 4-NS and ALND procedures, the costs of short-term adverse events and the impact of lymphoedema on patient utility. Results for the replacement strategies are considered to be more robust than for strategies in which MRI or PET are added in before sampling.
Other relevant factors
As there are contraindications associated with both PET and MRI, and some patients are unable to complete an MRI scan due to claustrophobia, a subset of patients may be unable to undergo these techniques and may require alternative investigations. Additional diagnostic techniques may add to the delay between diagnosis and treatment. Conversely, PET and MRI may have additional advantages; for example, whole-body PET scanning may detect undiagnosed distant metastases or synchronous tumours. Availability of PET and MRI scanning facilities would need to be considered if PET or MRI were recommended as part of the routine screening pathway for all patients with early breast cancer.
Chapter 7 Conclusions
Implications for service provision
The studies in this review demonstrate a significantly higher sensitivity for MRI than for PET, with USPIO-enhanced MRI providing the highest sensitivity. However, as none of the included studies directly compared PET and MRI, caution should be taken when comparing these estimates. Sensitivity of PET is reduced for smaller metastases. Specificity was similar for PET and MRI. However, this analysis is limited by the small number and size of MRI studies, and the wide variations between and within studies in terms of the MRI method used and the criteria for defining a node as positive. The sensitivity and specificity of both PET and MRI vary widely between studies, which is likely to reflect differences in imaging methods and interpretation. Therefore, caution should be taken when interpreting these results, particularly for MRI.
All patients currently receive ultrasound prior to other investigations; the sensitivity of PET appears similar to that of ultrasound while the sensitivity of MRI appears slightly higher. As ultrasound is not currently considered sensitive enough to completely replace SLNB or 4-NS, it is unlikely that PET could fulfil this role either. Specificity of PET and USPIO-enhanced MRI appear slightly higher than for ultrasound.
Positron emission tomography and MRI have lower sensitivity and specificity than the current surgical diagnostic techniques of SLNB and 4-NS, but are associated with fewer adverse events. Decision modelling suggests that MRI has the potential to offer an alternative to current sampling techniques. The current analysis suggests that the most cost-effective strategy may be MRI rather than SLNB or 4-NS, reducing costs and increasing QALYs due to fewer adverse events for the majority of patients. More robust data on the sensitivity and specificity of MRI techniques, particularly USPIO-enhanced MRI, are required, along with more accurate UK costs for the diagnostic and sampling procedures and high-quality utility data on the impact of lymphoedema. If the replacement strategy is considered clinically unacceptable due to higher numbers of FN cases (leading to higher risk of recurrence) and FP cases (leading to unnecessary ALND), then the most cost-effective strategy appears to be to retain the baseline 4-NS strategy (if 4-NS is used as the baseline) or to use MRI prior to SLNB (if SLNB is used as the baseline). However, the results relating to addition of MRI prior to 4-NS or SLNB are not considered to be robust, based on the quality of the input parameters available and further work is required to provide more reliable estimates.
Suggested research priorities
If the use of MRI is deemed clinically acceptable (either to replace SLNB or 4-NS or as an additional test prior to SLNB or 4-NS), then further large, well-conducted studies using up-to-date MRI methods are required to obtain more accurate data on the sensitivity and specificity of MRI in this setting. Further studies of USPIO-enhanced MRI would be valuable in order to gain more robust data on sensitivity and specificity, adverse effects and which are the best criteria for defining a node as metastatic. In addition, further data on the long-term impacts of differing severities of lymphoedema on cost and patient utility would be valuable. More robust UK cost data are needed for 4-NS and SLNB, as well as the cost of MRI and PET techniques. It would also be useful to identify the dependencies of different diagnostic methods in the pathway; in particular, whether and to what extent the accuracy of MRI or PET depends on the diagnostic results of ultrasound and ultrasound-guided biopsy. One potential use of MRI or PET might be to triage patients with a positive result to immediate ALND, avoiding a prior SLNB or 4-NS procedure. However, the need for two sequential surgical procedures may also be averted through the use of intraoperative cytology. Therefore, further studies on this technique would be of relevance when assessing the potential benefits of PET or MRI in this setting. A major limitation of imaging techniques such as PET and MRI is their limited ability to detect small metastases. It is therefore important that future research into diagnostic techniques should assess accuracy in detecting metastases of different sizes, including micrometastases. It may also be useful for future studies to report diagnostic accuracy according to subgroups of patients with different sizes and stages of primary breast tumour, in order to inform management decisions for these different patient groups.
Acknowledgements
Contributions of authors
Katy Cooper coordinated the review; Diana Papaioannou undertook the literature searches; Katy Cooper and Sue Harnan extracted data from papers, undertook data analyses and wrote the clinical review; Patrick Fitzgerald provided statistical support and undertook the meta-analyses; Yang Meng and Sue Ward undertook the health economic review and economic modelling and wrote the cost-effectiveness assessment; and, Lynda Wyld, Christine Ingram, Iain Wilkinson and Eleanor Lorenz provided clinical advice.
About ScHARR
The School of Health and Related Research (ScHARR) is one of the nine departments that comprise the Faculty of Medicine, Dentistry and Health at the University of Sheffield. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of the public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical and cost-effectiveness of healthcare interventions for the National Institute for Health Research (NIHR) Health Technology Assessment Programme on behalf of a range of policy-makers, including the National Institute for Health and Clinical Excellence. ScHARR-TAG is part of a wider collaboration of six units from other regions. The other units are: Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen Health Technology Assessment Group (Aberdeen HTA Group), University of Aberdeen; Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; Peninsular Technology Assessment Group (PenTAG), University of Exeter; NHS Centre for Reviews and Dissemination, University of York; and West Midlands Health Technology Assessment Collaboration (WMHTAC), University of Birmingham.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Office for National Statistics . Cancer Registration Statistics England 2007: MB1 No 38 n.d. www.statistics.gov.uk/StatBase/Product.asp?vlnk=7720%26Pos=1%26ColRank=1%26Rank=272 (accessed 29 November 2010).
- Welsh Cancer Intelligence & Surveillance Unit (WCISU) . Cancer Incidence in Wales 2003–2007 n.d. www.wales.nhs.uk/sites3/Documents/242/Cancer%20Incidence%20in%20Wales%202003–2007.pdf (accessed 23 January 2009).
- Dumitrescu RG, Cotarla I. Understanding breast cancer risk – where do we stand in 2005?. J Cell Mol Med 2005;9:208-21.
- Collaborative Group on Hormonal Factors in Breast Cancer . Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58 209 women with breast cancer and 101 986 women without the disease. Lancet 2001;358:1389-99.
- Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst 1993;85:1819-27.
- Kahlenborn C, Modugno F, Potter DM, Severs WB. Oral contraceptive use as a risk factor for premenopausal breast cancer: a meta-analysis. Mayo Clin Proc 2006;81:1290-302.
- Beral V. Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52, 705 women with breast cancer and 108, 411 women without breast cancer. Lancet 1997;350:1047-59.
- Megdal SP, Kroenke CH, Laden F, Pukkala E, Schernhammer ES. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer 2005;41:2023-32.
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78.
- Loi S, Milne RL, Friedlander ML, McCredie MRE, Giles GG, Hopper JL, et al. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev 2005;14:1686-91.
- Kerlikowske K, Walker R, Miglioretti DL, Desai A, Ballard-Barbash R, Buist DSM, et al. Obesity, mammography use and accuracy, and advanced breast cancer risk. J Natl Cancer Inst 2008;100:1724-33.
- Lagerros YT, Hsieh SF, Hsieh CC. Physical activity in adolescence and young adulthood and breast cancer risk: a quantitative review. Eur J Cancer Prev 2004;13:5-12.
- Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, et al. Physical activity and breast cancer: a systematic review. Epidemiology 2007;18:137-57.
- Cancer Research UK. Breast Cancer in Men n.d. www.cancerhelp.org.uk/help/default.asp?page=5075 (accessed 1 May 2009).
- Fisher B, Bauer M, Wickerham DL, Redmond CK, Fisher ER, Cruz AB, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983;52:1551-7.
- National Institute for Health and Clinical Excellence (NICE), National Collaborating Centre for Cancer . Early and Locally Advanced Breast Cancer: Diagnosis and Treatment 2009.
- Office for National Statistics . Survival Rates in England, Patients Diagnosed 2001–2006 Followed up to 2007 2007. www.statistics.gov.uk/StatBase/Product.asp?vlnk=14007.
- West Midlands Cancer Intelligence Unit . 0–10 Year Relative Survival for Cases of Breast Cancer by Stage Diagnosed in the West Midlands 1985–1989 Followed up to the End of 1999, As at January 2002 2009.
- Galea MH, Blamey RW, Elston CE, Ellis IO. The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 1992;22:207-19.
- Adjuvant! Online . Adjuvant! Online. Decision Making Tools for Health Care Professionals n.d. www.adjuvantonline.com/index.jsp (accessed 29 November 2010).
- Cancer Research UK . Cancer Statistics: Breast Cancer UK n.d. http://publications.cancerresearchuk.org/WebRoot/crukstoredb/CRUK_PDFs/CSBREA09.pdf (accessed 1 May 2009).
- Cancer Research UK, National Cancer Intelligence Network . Cancer Incidence and Survival by Major Ethnic Group, England, 2002–2006 n.d. http://publications.cancerresearchuk.org/WebRoot/crukstoredb/CRUK_PDFs/CR-UKNCIN_ETHNIC.pdf (accessed 1 June 2009).
- Parkin DM, Fernandez LM. Use of statistics to assess the global burden of breast cancer. Breast J 2006;12:S70-S80.
- National Cancer Intelligence Network . Cancer Incidence by Deprivation England, 1995–2004 n.d. www.ncin.org.uk/publications/reports/default.apsx.
- Welsh Cancer Intelligence & Surveillance Unit . Cancer Incidence, Mortality and Survival by Deprivation in Wales n.d. www.wales.nhs.uk/sites3/Documents/242/Deprivation%20in%20Wales%201993–2007.pdf (accessed 1 July 2009).
- Cancer Research UK . Cancer Survival and Deprivation Statistics n.d. http://info.cancerresearchuk.org/cancerstats/survival/survivaldeprivation/ (accessed 4 April 2005).
- Office for National Statistics . Death Registrations in England and Wales, 2008, Causes n.d. www.statistics.gov.uk/pdfdir/dthreg0809.pdf (accessed 26 August 2009).
- South East England Public Health Observatory, Oxford University . Breast Cancer in England 1996 to 2004. Mortality Trends n.d. www.uhce.ox.ac.uk/epidembase/temp_epi/trends_mortality.php.
- Blanks RG, Moss SM, McGahan CE, Quinn MJ, Babb PJ. Effect of NHS breast screening programme on mortality from breast cancer in England and Wales, 1990–8: comparison of observed with predicted mortality. BMJ 2000;321:665-9.
- Kobayashi S. What caused the decline in breast cancer mortality in the United Kingdom?. Breast Cancer 2004;11:156-9.
- NHS Cancer screening programme . Breast Awareness n.d. www.cancerscreening.nhs.uk/breastscreen/breastawareness.html (accessed 29 November 2010).
- NHS Cancer screening programme . Breast Cancer Screening n.d. www.cancerscreening.nhs.uk/breastscreen/index.html (accessed 29 November 2010).
- International Union Against Cancer . TNM Classification of Malignant Tumours 1997.
- Singletary SE, Connolly JL. Breast cancer staging: working with the sixth edition of the AJCC Cancer Staging Manual. CA Cancer J Clin 2006;56:37-4.
- Cancer Research UK . TNM Breast Cancer Staging n.d. www.cancerhelp.org.uk/help/default.asp?page = 3316 (accessed 29 November 2010).
- Agency for Healthcare Research and Quality (AHRQ) . Surgery Choices for Women With Early-Stage Breast Cancer n.d. www.ahrq.gov/consumer/brcanchoice.htm (accessed 29 November 2010).
- US National Cancer Institute (NCI) . Surgery Choices for Women With Early-Stage Breast Cancer 2009. www.cancer.gov/cancertopics/breast-cancer-surgery-choices.
- American Cancer Society . How Is Breast Cancer Staged? n.d. www.cancer.org/docroot/CRI/content/CRI_2_4_3X_How_is_breast_cancer_staged_5.asp (accessed 29 November 2010).
- Cancer Research UK . Number Stages of Breast Cancer n.d. www.cancerhelp.org.uk/help/default.asp?page=3315 (accessed 29 November 2010).
- Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjosne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol 2000;10:1464-71.
- Mumtaz H, Hall-Craggs MA, Davidson T, Walmsley K, Thurell W, Kissin MW, et al. Staging of symptomatic primary breast cancer with MR imaging. AJR 1997;169:417-24.
- Smith IC, Welch AE, Chilcott F, Heys SD, Sharp P, Eremin O. Gamma emission imaging in the management of breast disorders. Eur J Surg Oncol 1998;24:320-9.
- Wahl RL, Siegel BA, Coleman RE, Gatsonis CG. PET Study Group . Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol 2004;22:277-85.
- Wallace IW, Champton HR. Axillary nodes in breast cancer. Lancet 1972;1:217-18.
- Fisher B, Wolmark N, Bauer M, Redmond C, Gebhardt M. The accuracy of clinical nodal staging and of limited axillary dissection as a determinant of histologic nodal status in carcinoma of the breast. Surg Gynecol Obstet 1981;152:765-72.
- Alvarez S, Anorbe E, Alcorta P, Lopez F, Alonso I, Cortes J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol 2006;186:1342-8.
- Blanchard DK, Donohue JH, Reynolds C, Grant CS. Relapse and morbidity in patients undergoing sentinel lymph node biopsy alone or with axillary dissection for breast cancer. Arch Surg 2003;138:482-7.
- Crane-Okada R, Wascher RA, Elashoff D, Giuliano AE. Long-term morbidity of sentinel node biopsy versus complete axillary dissection for unilateral breast cancer. Ann Surg Oncol 2008;15:1996-2005.
- McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol 2008;26:5213-19.
- Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol 2005;23:4312-21.
- Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-60.
- Liu CQ, Guo Y, Shi JY, Sheng Y. Late morbidity associated with a tumour-negative sentinel lymph node biopsy in primary breast cancer patients: a systematic review. Eur J Cancer 2009;45:1560-8.
- Wilke LG, McCall LM, Posther KE, Whitworth PW, Reintgen DS, Leitch AM, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol 2006;13:491-500.
- Department of Health . NHS Reference Costs 2003 2004.
- Department of Health . NHS Reference Costs 2008 2009.
- Mansfield L, Sosa I, Dionello R, Subramanian A, Devalia H, Mokbel K. Current management of the axilla in patients with clinically node-negative breast cancer: a nationwide survey of United Kingdom breast surgeons. Int Semin Surg Oncol 2007;4.
- Facey K, Bradbury I, Laking G, Payne E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol Assess 2007;11.
- Cherry SR. The 2006 Henry N. Wagner lecture: of mice and men (and positrons)--advances in PET imaging technology. J Nucl Med 2006;47:1735-45.
- Messa C, Bettinardi V, Picchio M, Pelosi E, Landoni C, Gianolli L, et al. PET/CT in diagnostic oncology. Q J Nucl Med Mol Imaging 2004;48:66-75.
- Radiology Info n.d. www.radiologyinfo.org/en/info.cfm?pg=abdominct (accessed 29 November 2010).
- Erguvan-Dogan B, Whitman GJ, Kushwaha AC, Phelps MJ, Dempsey PJ. BI-RADS-MRI: a primer. AJR Am J Roentgenol 2006;187:W152-W160.
- Lehman CD, Schnall MD. Imaging in breast cancer: magnetic resonance imaging. Breast Cancer Res 2005;7:215-19.
- Yeung DK, Yang WT, Tse GM. Breast cancer: in vivo proton MR spectroscopy in the characterization of histopathologic subtypes and preliminary observations in axillary node metastases. Radiology 2002;225:190-7.
- Medicines and Healthcare products Regulatory Agency (MHRA) . Safety Guidelines for Magnetic Resonance Imaging Equipment in Clinical Use: Device Bulletin n.d. www.mhra.gov.uk/Publications/Safetyguidance/DeviceBulletins/CON2033018 (accessed 29 November 2010).
- Jacklin PB, Barrington SF, Roxburgh JC, Jackson G, Sariklis D, West PA, et al. Cost-effectiveness of preoperative positron emission tomography in ischemic heart disease. Ann Thorac Surg 2002;73:1403-9.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9.
- PRISMA Statement Website n.d. www.prisma-statement.org (accessed 29 November 2010).
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90.
- Leeflang MM, bets-Ossenkopp YJ, Visser CE, Scholten RJ, Hooft L, Bijlmer HA, et al. Galactomannan detection for invasive aspergillosis in immunocompromized patients. Cochrane Database Syst Rev 2008;4.
- Review Manager (revman). Copenhagen, The Nordic Cochrane Centre, The Cochrane Collaboration; 2008.
- Chae BJ, Bae JS, Kang BJ, Kim SH, Jung SS, Song BJ. Positron emission tomography-computed tomography in the detection of axillary lymph node metastasis in patients with early stage breast cancer. Jpn J Clin Oncol 2009;39:284-9.
- Heusner TA, Kuemmel S, Hahn S, Koeninger A, Otterbach F, Hamami ME, et al. Diagnostic value of full-dose FDG PET/CT for axillary lymph node staging in breast cancer patients. Eur J Nucl Med Mol Imaging 2009;36:1543-50.
- Kim J, Lee J, Chang E, Kim S, Suh K, Sul J, et al. Selective sentinel node plus additional non-sentinel node biopsy based on an FDG-PET/CT scan in early breast cancer patients: single institutional experience. World J Surg 2009;33:943-9.
- Taira N, Ohsumi S, Takabatake D, Hara F, Takashima S, Aogi K, et al. Determination of indication for sentinel lymph node biopsy in clinical node-negative breast cancer using preoperative 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging. Jpn J Clin Oncol 2009;39:16-21.
- Fuster D, Duch J, Paredes P, Velasco M, Munoz M, Santamaria G, et al. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol 2008;26:4746-51.
- Ueda S, Tsuda H, Asakawa H, Omata J, Fukatsu K, Kondo N, et al. Utility of 18F-fluoro-deoxyglucose emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer 2008;8.
- Veronesi U, De CC, Galimberti VE, Fernandez JR, Rotmensz N, Viale G, et al. A comparative study on the value of FDG-PET and sentinel node biopsy to identify occult axillary metastases. Ann Oncol 2007;18:473-8.
- Cermik TF, Mavi A, Basu S, Alavi A. Impact of FDG PET on the preoperative staging of newly diagnosed breast cancer. Eur J Nucl Med Mol Imaging 2008;35:475-83.
- Gil-Rendo A, Zornoza G, Garcia-Velloso MJ, Regueira FM, Beorlegui C, Cervera M. Fluorodeoxyglucose positron emission tomography with sentinel lymph node biopsy for evaluation of axillary involvement in breast cancer. Br J Surg 2006;93:707-12.
- Weir L, Worsley D, Bernstein V. The value of FDG positron emission tomography in the management of patients with breast cancer. Breast J 2005;11:204-9.
- Agresti R, Crippa F, Gerali A, Maccauro M, Giovanazzi R, Bombardieri E, et al. Lymph node metastases detection by FDG-PET and sentinel node biopsy in breast cancer patients: comparison of these different approaches. EJC Supplements 2004;2.
- Fehr MK, Hornung R, Varga Z, Burger D, Hess T, Haller U, et al. Axillary staging using positron emission tomography in breast cancer patients qualifying for sentinel lymph node biopsy. Breast J 2004;10:89-93.
- Inoue T, Yutani K, Taguchi T, Tamaki Y, Shiba E, Noguchi S. Preoperative evaluation of prognosis in breast cancer patients by [(18)F]2-Deoxy-2-fluoro-d-glucose-positron emission tomography. J Cancer Res Clin Oncol 2004;130:273-8.
- Lovrics PJ, Chen V, Coates G, Cornacchi SD, Goldsmith CH, Law C, et al. A prospective evaluation of positron emission tomography scanning, sentinel lymph node biopsy, and standard axillary dissection for axillary staging in patients with early stage breast cancer. Ann Surg Oncol 2004;11:846-53.
- Barranger E, Grahek D, Antoine M, Montravers F, Talbot JN, Uzan S. Evaluation of fluorodeoxyglucose positron emission tomography in the detection of axillary lymph node metastases in patients with early-stage breast cancer. Ann Surg Oncol 2003;10:622-7.
- Guller U, Nitzsche EU, Schirp U, Viehl CT, Torhorst J, Moch H, et al. Selective axillary surgery in breast cancer patients based on positron emission tomography with 18F-fluoro-2-deoxy-d-glucose: not yet!. Breast Cancer Res Treat 2002;71:171-3.
- Nakamoto Y, Chang AE, Zasadny KR, Wahl RL, Nakamoto Y, Chang AE, et al. Comparison of attenuation-corrected and non-corrected FDG-PET images for axillary nodal staging in newly diagnosed breast cancer. Mol Imaging Biol 2002;4:161-9.
- Rieber A, Schirrmeister H, Gabelmann A, Nuessle K, Reske S, Kreienberg R, et al. Pre-operative staging of invasive breast cancer with MR mammography and/or PET: boon or bunk?. Br J Radiol 2002;75:789-98.
- van der Hoeven JJ, Hoekstra OS, Comans EF, Pijpers R, Boom RP, van Geldere D, et al. Determinants of diagnostic performance of [F-18]fluorodeoxyglucose positron emission tomography for axillary staging in breast cancer. Ann Surg 2002;236:619-24.
- Greco M, Crippa F, Agresti R, Seregni E, Gerali A, Giovanazzi R, et al. Axillary lymph node staging in breast cancer by 2-fluoro-2-deoxy-d-glucose-positron emission tomography: clinical evaluation and alternative management. J Natl Cancer Inst 2001;93:630-5.
- Noh DY, Yun IJ, Kim JS, Kang HS, Lee DS, Chung JK, et al. Diagnostic value of positron emission tomography for detecting breast cancer. World J Surg 1998;22:223-8.
- Smith IC, Ogston KN, Whitford P, Smith FW, Sharp P, Norton M, et al. Staging of the axilla in breast cancer – accurate in vivo assessment using positron emission tomography with 2-(fluorine-18)-fluoro-2-deoxy-d-glucose. Ann Surg 1998;228:220-7.
- Adler LP, Faulhaber PF, Schnur KC, Al-Kasi NL, Shenk RR. Axillary lymph node metastases: screening with [F-18]2-deoxy-2-fluoro-d-glucose (FDG) PET. Radiology 1997;203:323-7.
- Avril N, Dose J, Janicke F, Ziegler S, Romer W, Weber W, et al. Assessment of axillary lymph node involvement in breast cancer patients with positron emission tomography using radiolabeled 2-(fluorine-18)-fluoro-2-deoxy-d-glucose. J Natl Cancer Inst 1996;88:1204-9.
- Utech CI, Young CS, Winter PF. Prospective evaluation of fluorine-18 fluorodeoxyclucose positron emission tomography in breast cancer for staging of the axilla related to surgery and immunocytochemistry. Eur J Nucl Med 1996;23:1588-93.
- Crippa F, Agresti R, Seregni E, Greco M, Pascali C, Bogni A, et al. Prospective evaluation of fluorine-18-FDG PET in presurgical staging of the axilla in breast cancer. J Nucl Med 1998;39:4-8.
- Bombardieri E, Crippa F, Agresti R, Leutner M, Delledonne V, Greco M, et al. FDG-PET in the assessment of axillary metastases in breast cancer. Clinical application and economic implications. J Nucl Med 1998;39.
- Crippa F, Agresti R, Donne VD, Pascali C, Bogni A, Chiesa C, et al. The contribution of positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) in the preoperative detection of axillary metastases of breast cancer: the experience of the National Cancer Institute of Milan. Tumori 1997;83:542-3.
- Adler LP, Schnur KC, Faulhaber PF, Shenk RR. High sensitivity interpretation PET of the axilla: screening test for axillary lymph node metastases in patients with breast cancer. Radiology 1996;201.
- Kumar R, Zhuang H, Schnall M, Conant E, Damia S, Weinstein S, et al. FDG PET positive lymph nodes are highly predictive of metastasis in breast cancer. Nucl Med Commun 2006;27:231-6.
- Zornoza G, Garcia-Velloso MJ, Sola J, Regueira FM, Pina L, Beorlegui C. F-18-FDG PET complemented with sentinel lymph node biopsy in the detection of axillary involvement in breast cancer. Eur J Surg Oncol 2004;30:15-9.
- Agresti R, Crippa F, Gerali A, Maccauro M, Giovanazzi R, Guida V, et al. Lymph node metastases detection by FDG-PET and sentinel node biopsy in breast cancer patients: comparison of these different approaches. Eur J Cancer 2001;37.
- Lovrics PJ, Chen V, Coates G, Cornacchi SD, Goldsmith C, Law C, et al. A prospective comparison of positron emission tomography scanning, sentinel lymph node biopsy and axillary dissection in staging patients with early stage breast cancer. Breast Cancer Res Treat 2002;76.
- Yutani K, Shiba E, Kusuoka H, Tatsumi M, Uehara T, Taguchi T, et al. Comparison of FDG-PET with MIBI-SPECT in the detection of breast cancer and axillary lymph node metastasis. J Comput Assist Tomogr 2000;24:274-80.
- Yutani K, Tatsumi M, Shiba E, Kusuoka H, Nishimura T. Comparison of dual-head coincidence gamma camera FDG imaging with FDG PET in detection of breast cancer and axillary lymph node metastasis. J Nucl Med 1999;40:1003-8.
- Kimura K, Tanigawa N, Matsuki M, Nohara T, Iwamoto M, Sumiyoshi K, et al. High-resolution MR lymphography using ultrasmall superparamagnetic iron oxide (USPIO) in the evaluation of axillary lymph nodes in patients with early stage breast cancer: preliminary results. Breast Cancer 2010;17:241-6.
- Harada T, Tanigawa N, Matsuki M, Nohara T, Narabayashi I. Evaluation of lymph node metastases of breast cancer using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging. Eur J Radiol 2007;63:401-7.
- Memarsadeghi M, Riedl CC, Kaneider A, Galid A, Rudas M, Matzek W, et al. Axillary lymph node metastases in patients with breast carcinomas: assessment with nonenhanced versus uspio-enhanced MR imaging. Radiology 2006;241:367-77.
- Stadnik TW, Everaert H, Makkat S, Sacre R, Lamote J, Bourgain C. Breast imaging. Preoperative breast cancer staging: comparison of USPIO-enhanced MR imaging and 18F-fluorodeoxyglucose (FDC) positron emission tomography (PET) imaging for axillary lymph node staging – initial findings. Eur Radiol 2006;16:2153-60.
- Michel SC, Keller TM, Frohlich JM, Fink D, Caduff R, Seifert B, et al. Preoperative breast cancer staging: MR imaging of the axilla with ultrasmall superparamagnetic iron oxide enhancement. Radiology 2002;225:527-36.
- Murray AD, Staff RT, Redpath TW, Gilbert FJ, Ah-See AK, Brookes JA, et al. Dynamic contrast enhanced MRI of the axilla in women with breast cancer: comparison with pathology of excised nodes. Br J Radiol 2002;75:220-8.
- Lind PG. Radioimmunoscintigraphy with Tc-99m labeled monoclonal antibody 170H.82 in suspected primary, recurrent, or metastatic breast cancer. Clin Nucl Med 1997;22:30-4.
- Pichler BJ, Wehrl HF, Judenhofer MS. Latest advances in molecular imaging instrumentation. J Nucl Med 2008;49:5S-23S.
- Bellin MF, Thomsen HS, Webb JAW. Contrast media: safety issues and ESUR guidelines. Berlin: Springer-Verlag; 2009.
- Bellin MF, Roy C, Kinkel K, Thoumas D, Zaim S, Vanel D, et al. Lymph node metastases: safety and effectiveness of MR imaging with ultrasmall superparamagnetic iron oxide particles--initial clinical experience. Radiology 1998;207:799-808.
- Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med 2003;348:2491-9.
- Sloka JS, Hollett PD, Mathews M. Cost-effectiveness of positron emission tomography in breast cancer. Mol Imaging Biol 2005;7:351-60.
- Pandharipande PV, Harisinghani MG, Ozanne EM, Specht MC, Hur C, Lee JM, et al. Staging MR lymphangiography of the axilla for early breast cancer: cost-effectiveness analysis. AJR Am J Roentgenol 2008;191:1308-19.
- Law AM. Simulation modeling and analysis. New York, NY: McGraw Hill; 2007.
- Sato T, Yamaguchi K, Morita Y, Noikura T, Sugihara K, Matsune S. Lymphoscintigraphy for interpretation of changes of cervical lymph node function in patients with oral malignant tumors: comparison of Tc-99m-Re and Tc-99m-HSA-D. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:525-37.
- Tanaka K, Yamamoto D, Kanematsu S, Okugawa H, Kamiyama Y. A four node axillary sampling trial on breast cancer patients. Breast 2006;15:203-9.
- Sato K, Tamaki K, Takeuchi H, Tsuda H, Kosuda S, Kusano S, et al. Management of the axilla in breast cancer: a comparative study between sentinel lymph node biopsy and four-node sampling procedure. Jpn J Clin Oncol 2001;31:318-21.
- Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer 2006;106:4-16.
- Hind D, Ward S, De Nigris E, Simpson E, Carroll C, Wyld L. Hormonal therapies for early breast cancer: systematic review and economic evaluation. Health Technol Assess 2007;11.
- Jeruss JS, Hunt KK, Xing Y, Krishnamurthy S, Meric-Bernstam F, Cantor SB, et al. Is intraoperative touch imprint cytology of sentinel lymph nodes in patients with breast cancer cost effective?. Cancer 2006;107:2328-36.
- Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst 2006;98:599-60.
- Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A. Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation. Health Technol Assess 2007;11.
- Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care 2000;38:583-637.
- Ara R, Brazier J. Populating an economic mode with health state utility values: moving towards better practice. Value Health 2010;13:S09-18.
- Office for National Statistics . Interim Life Tables, United Kingdom, 1980–82 to 2005–07 2008.
- Orr RK, Col NF, Kuntz KM. A cost-effectiveness analysis of axillary node dissection in postmenopausal women with estrogen receptor-positive breast cancer and clinically negative axillary nodes. Surgery 1999;126:568-76.
- Kamby C, Sengelov L. Pattern of dissemination and survival following isolated locoregional recurrence of breast cancer. A prospective study with more than 10 years of follow up. Breast Cancer Res Treat 1997;45:181-92.
- Mak SS, Mo KF, Suen JJ, Chan SL, Ma WL, Yeo W. Lymphedema and quality of life in Chinese women after treatment for breast cancer. Eur J Oncol Nurs 2009;13:110-15.
- Her Majesty’s Treasury . The Green Book: Appraisal and Evaluation in Central Government 2003.
- Escalona S, Blasco JA, Reza MM, Andradas E, Gomez N. A systematic review of FDG-PET in breast cancer. Med Oncol 2010;27:114-29.
- Samson D, Redding FC, Aronson N. FDG positron emission tomography for evaluating breast cancer. Chicago, IL: Blue Cross and Blue Shield Association; 2001.
- Olson JA, McCall LM, Beitsch P, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. J Clin Oncol 2008;26:3530-5.
- Bland KI. Re: axillary staging using positron emission tomography in breast cancer patients qualifying for sentinel lymph node biopsy. Breast J 2004;10:83-4.
- Guller U, Nitzsche E, Moch H, Zuber M. Is positron emission tomography an accurate non-invasive alternative to sentinel lymph node biopsy in breast cancer patients?. J Natl Cancer Inst 2003;95:1040-3.
- Wahl RS. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging of breast cancer with PET study group. Womens Oncol Rev 2004;4:161-2.
- Bahri S, Chen JH, Yu HJ, Kuzucan A, Nalcioglu O, Su MY. Can dynamic contrast-enhanced MRI (DCE-MRI) predict tumor recurrence and lymph node status in patients with breast cancer?. Ann Oncol 2008;19:822-4.
- Hsiang DJ, Yamamoto M, Mehta RS, Su MY, Baick CH, Lane KT, et al. Predicting nodal status using dynamic contrast-enhanced magnetic resonance imaging in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy with and without sequential trastuzumab. Arch Surg 2007;142:855-61.
- Zytoon AA. Clinical relevance of the standardized uptake value (Suv) in staging breast cancer with FDG-PET/CT. Dokkyo J Med Sci 2007;34:193-204.
- Adler LP, Crowe JP, al-Kaisi NK, Sunshine JL. Evaluation of breast masses and axillary lymph nodes with [F-18] 2-deoxy-2-fluoro-d-glucose PET. Radiology 1993;187:743-50.
- Bassa P, Kim EE, Inoue T, Wong FC, Korkmaz M, Yang DJ, et al. Evaluation of preoperative chemotherapy using PET with fluorine-18-fluorodeoxyglucose in breast cancer. J Nucl Med 1996;37:931-8.
- Beer AJ, Niemeyer M, Carlsen J, Sarbia M, Nahrig J, Watzlowik P, et al. Patterns of alphavbeta3 expression in primary and metastatic human breast cancer as shown by 18F-Galacto-RGD PET. J Nucl Med 2008;49:255-9.
- Bombardieri E, Crippa F, Maffioli L, Chiti A, Castellani MR, Greco M, et al. Axillary lymph node metastases detection with nuclear medicine approaches in patients with newly diagnosed breast cancer: can positron emission tomography (PET) with 18F-FDG be considered as the best method?. Int J Oncol 1996;8:693-9.
- Burcombe RJ, Makris A, Pittam M, Lowe J, Emmott J, Wong WL. Evaluation of good clinical response to neoadjuvant chemotherapy in primary breast cancer using [18F]-fluorodeoxyglucose positron emission tomography. Eur J Cancer 2002;38:375-9.
- Crowe JP, Adler LP, Shenk RR, Sunshine J. Positron emission tomography and breast masses: comparison with clinical, mammographic, and pathological findings. Ann Surg Oncol 1994;1:132-40.
- Danforth DN, Aloj L, Carrasquillo JA, Bacharach SL, Chow C, Zujewski J, et al. The role of F-18-FDG-PET in the local/regional evaluation of women with breast cancer. Breast Cancer Res Treat 2002;75:135-46.
- Harisinghani MG, Saksena MA, Hahn PF, King B, Kim J, Torabi MT, et al. Ferumoxtran-10-enhanced MR lymphangiography: does contrast-enhanced imaging alone suffice for accurate lymph node characterization?. AJR Am J Roentgenol 2006;186:144-8.
- Harisinghani MG, Weissleder R. Sensitive, noninvasive detection of lymph node metastases. PLoS Med 2004;1:202-9.
- Hoh CK, Hawkins RA, Glaspy JA, Dahlbom M, Tse NY, Hoffman EJ, et al. Cancer-detection with whole-body Pet using 2-[F-18]Fluoro-2-Deoxy-d-Glucose. J Comput Assist Tomogr 1993;17:582-9.
- Kelemen PR, Lowe V, Phillips N. Positron emission tomography and sentinel lymph node dissection in breast cancer. Clin Breast Cancer 2002;3:73-7.
- Luciani A, Pigneur F, Ghozali F, Dao TH, Cunin P, Meyblum E, et al. Ex vivo MRI of axillary lymph nodes in breast cancer. Eur J Radiol 2009;69:59-66.
- Noh DY, Yun IJ, Kang HS, Kim YC, Kim JS, Chung JK, et al. Detection of cancer in augmented breasts by positron emission tomography. Eur J Surg 1999;165:847-51.
- Palmedo H, Bender H, Grunwald F, Mallmann P, Zamora P, Krebs D, et al. Comparison of fluorine-18 fluorodeoxyglucose positron emission tomography and technetium-99m methoxyisobutylisonitrile scintimammography in the detection of breast tumours. Eur J Nucl Med 1997;24:1138-45.
- Scheidhauer K, Scharl A, Pietrzyk U, Wagner R, Gohring UJ, Schomacker K, et al. Qualitative [18F]FDG positron emission tomography in primary breast cancer: clinical relevance and practicability. Eur J Nucl Med 1996;23:618-23.
- Smyczek-Gargya B, Fersis N, Dittmann H, Vogel U, Reischl G, Machulla HJ, et al. PET with [18F]fluorothymidine for imaging of primary breast cancer: a pilot study. Eur J Nucl Med Mol Imaging 2004;31:720-4.
- Sun X-CL. Comparative analysis of multi-slice spiral CT and positron emission tomography-CT in evaluation of axillary lymph nodes in breast cancer patients. Zhonghua Fang She Xian Yi Xue Za Zhi 2008;42:68-72.
- Tse NY, Hoh CK, Hawkins RA, Zinner MJ, Dahlbom M, Choi Y, et al. The application of positron emission tomographic imaging with fluorodeoxyglucose to the evaluation of breast disease. Ann Surg 1992;216:27-34.
- Vansant J, Balfore J, Monticciolo D, Votaw D, Carlson G, Garcia E, et al. (F-18)-FDG PET qualitative and quantitative assessment of breast cancer. J Nucl Med 1996;37.
- Yang J-HN. Comparison of intraoperative frozen section analysis of sentinel node with preoperative positron emission tomography in the diagnosis of axillary lymph node status in breast cancer patients. Jpn J Clin Oncol 2001;31:1-6.
- Black D, Specht M, Lee JM, Dominguez F, Gadd M, Hughes K, et al. Detecting occult malignancy in prophylactic mastectomy: preoperative MRI versus sentinel lymph node biopsy. Ann Surg Oncol 2007;14:2477-84.
- Bollet MA, Thibault F, Bouillon K, Meunier M, Sigal-Zafrani B, Savignoni A, et al. Role of dynamic magnetic resonance imaging in the evaluation of tumor response to preoperative concurrent radiochemotherapy for large breast cancers: a prospective phase II study. Int J Radiat Oncol Biol Phys 2007;69:13-8.
- Dose J, Bleckmann C, Bachmann S, Bohuslavizki KH, Berger J, Jenicke L, et al. Comparison of fluorodeoxyglucose positron emission tomography and “conventional diagnostic procedures” for the detection of distant metastases in breast cancer patients. Nucl Med Commun 2002;23:857-64.
- Gil-Rendo A, Martinez-Regueira F, Zornoza G, Garcia-Velloso MJ, Beorlegui C, Rodriguez-Spiteri N, et al. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg 2009;96:166-70.
- Kim S-JK. Predictive value of [18F]FDG PET for pathological response of breast cancer to neo-adjuvant chemotherapy. Ann Oncol 2004;15:1352-7.
- Kubota K, Ogawa Y, Nishioka A, Murata Y, Itoh S, Hamada N, et al. Radiological imaging features of invasive micropapillary carcinoma of the breast and axillary lymph nodes. Oncol Rep 2008;20:1143-7.
- Kumar R, Chauhan A, Zhuang HM, Chandra P, Schnall M, Alavi A. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat 2006;98:267-74.
- Perman WH, Heiberg EV, Herrmann VM. Half-Fourier, three-dimensional technique for dynamic contrast-enhanced MR imaging of both breasts and axillae: initial characterization of breast lesions. Radiology 1996;200:263-9.
- Alberini JL, Lerebours F, Wartski M, Fourme E, Le SE, Gontier E, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) imaging in the staging and prognosis of inflammatory breast cancer. Cancer 2009;115:5038-47.
- Bathen TF, Jensen LR, Sitter B, Fjosne HE, Halgunset J, Axelson DE, et al. MR-determined metabolic phenotype of breast cancer in prediction of lymphatic spread, grade, and hormone status. Breast Cancer Res Treat 2007;104:181-9.
- Bleckmann C, Dose J, Bohuslavizki KH, Buchert R, Klutmann S, Mester J, et al. Effect of attenuation correction on lesion detectability in FDG PET of breast cancer. J Nucl Med 1999;40:2021-4.
- Buchmann I, Riedmuller K, Hoffner S, Mack U, Aulmann S, Haberkorn U. Comparison of 99mtechnetium-pertechnetate and 123iodide SPECT with FDG-PET in patients suspicious for breast cancer. Cancer Biother Radiopharm 2007;22:779-89.
- Carkaci S, Macapinlac HA, Cristofanilli M, Mawlawi O, Rohren E, Gonzalez Angulo AM, et al. Retrospective study of 18F-FDG PET/CT in the diagnosis of inflammatory breast cancer: preliminary data. J Nucl Med 2009;50:231-8.
- Chen JH, Baek HM, Nalcioglu O, Su MY. Estrogen receptor and breast MR imaging features: a correlation study. J Magn Reson Imaging 2008;27:825-33.
- Chung A, Liou D, Karlan S, Waxman A, Fujimoto K, Hagiike M, et al. Preoperative FDG-PET for axillary metastases in patients with breast cancer. Arch Surg 2006;141:783-8.
- Dizendorf EV, Baumert BG, von Schulthess GK, Lutolf UM, Steinert HC. Impact of whole-body 18F-FDG PET on staging and managing patients for radiation therapy. J Nucl Med 2003;44:24-9.
- Groheux D, Moretti JL, Baillet G, Espie M, Giacchetti S, Hindie E, et al. Effect of (18)F-FDG PET/CT imaging in patients with clinical Stage II and III breast cancer. Int J Radiat Oncol Biol Phys 2008;71:695-704.
- Heusner TA, Kuemmel S, Umutlu L, Koeninger A, Freudenberg LS, Hauth EA, et al. Breast cancer staging in a single session: whole-body PET/CT mammography. J Nucl Med 2008;49:1215-22.
- Matsushima S, Sasaki F, Yamaura H, Iwata H, Ohsaki H, Era S, et al. Equivalent cross-relaxation rate imaging for sentinel lymph node biopsy in breast carcinoma. Magn Reson Med 2005;54:1300-4.
- Matsushima S, Nishiofuku H, Iwata H, Era S, Inaba Y, Kinosada Y. Equivalent cross-relaxation rate imaging of axillary lymph nodes in breast cancer. J Magn Reson Imaging 2008;27:1278-83.
- Mavi A, Cermik TF, Urhan M, Zhuang H, Alavi A. Dual time point imaging method may increase the sensitivity and the specificity of FDG PET for detecting axillary metastasis from primary breast cancer. Eur J Nucl Med Mol Imaging 2006;33.
- Mortellaro VE, Marshall J, Singer L, Hochwald SN, Chang M, Copeland EM, et al. Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging 2009;30:309-12.
- Mussurakis S, Buckley DL, Horsman A. Dynamic MR imaging of invasive breast cancer: correlation with tumour grade and other histological factors. Br J Radiol 1997;70:446-51.
- Nieweg OE, Kim EE, Wong WH, Broussard WF, Singletary SE, Hortobagyi GN, et al. Positron emission tomography with fluorine-18-deoxyglucose in the detection and staging of breast cancer. Cancer 1993;71:3920-5.
- Rotaru N, Luciani A, Rotaru N, Luciani A. Magnetic resonance imaging of the breast: potential for lesion characterization. J BUON 2004;9:77-82.
- Schirrmeister H, Kuhn T, Guhlmann A, Santjohanser C, Horster T, Nussle K, et al. Fluorine-18 2-deoxy-2-fluoro-d-glucose PET in the preoperative staging of breast cancer: comparison with the standard staging procedures. Eur J Nucl Med 2001;28:351-8.
- Schirrmeister HH, Kuehn T, Buck AC, Santjohanser C, Reske SN. FDG-PET in preoperative assessment of newly diagnosed breast cancer. Breast Cancer Res Treat 2000;64.
- Song B, Kim M, Lee J, Seo Y, Park W, Oh S, et al. Evaluation of PET-CT for axillary lymph node staging in patients with early stage breast cancer. EJC Supplements 2006;4.
- Stets C, Brandt S, Wallis F, Buchmann J, Gilbert FJ, Heywang-Kobrunner SH. Axillary lymph node metastases: a statistical analysis of various parameters in MRI with USPIO. J Magn Reson Imaging 2002;16:60-8.
- Torrenga HL. Re: Axillary lymph node staging in breast cancer by 2-fluoro-2-deoxy-D-glucose-positron emission tomography: clinical evaluation and alternative management. J Natl Cancer Inst 2001;93:1659-61.
- Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol 2008;38:250-8.
- Yang SK, Cho N, Moon WK. The role of PET/CT for evaluating breast cancer. Korean J Radiol 2007;8:429-37.
- Bock E, Bock C, Belli P, Campioni P, Manfredi R, Pastore G. Role of diagnostic imaging of the breast in patients treated with postsurgical radiotherapy or presurgical radiotherapy or chemotherapy. Radiologia Medica 1998;95:38-43.
- Chen SH, Ma QH, Li ZJ, Hu CW, Chen Sh, Ma Qh, et al. Diagnosis of breast diseases by mammography in combination with MRI. Zhongguo Yiliao Qixie Zazhi 2007;31:216-18.
- Dose-Schwarz J, Mahner S, Schirrmacher S, Jenicke L, Muller V, Habermann CR, et al. Detection of metastases in breast cancer patients: comparison of FDG PET with chest X-ray, bone scintigraphy and ultrasound of the abdomen. Nuclear-Medizin 2008;47:97-103.
- Enya M, Goto H, Nandate Y, Kiryu T, Kanematsu M, Hoshi H. Usefulness of dynamic MR mammography for diagnosis of axillary lymph node status in breast cancer patient. Nippon Igaku Hoshasen Gakkai Zasshi 2000;60:764-70.
- Koga S, Nakano S, Honma Y, Ogasawara N. FDG-PET (positron emission tomography) in the detection of primary breast cancer and lymph node involvement. Nippon Rinsho 2007;65:379-84.
- Nakayama S, Kakizaki D, Kaise H, Kusama M, Ishikawa A, Amino M, et al. Three-dimentional volumetric interpolated breath-hold magnetic resonance imaging for the diagnosis of breast tumors. Nippon Rinsho 2004;62:790-8.
- Romer W, Avril N, Schwaiger M. Possible uses of positron emission tomography in breast carcinoma. Acta Medica Austriaca 1997;24:60-2.
- Tiutin L, Fadeev N, Ryzhkova D, Kostenikiv N, Arzumanov A, Savello V, et al. Clinical experience with positron emission tomography with 2-fluorine, 18F-2-deoxy-d-glucose used for the diagnosis of malignant breast neoplasms. Vestn Rentgenol Radio 2001;6:14-8.
- Zhao TT, Li JG, Li YM. Performance of 18F-FDG PET/CT in the detection of primary breast cancer and staging of the regional lymph nodes. Chung-Hua Chung Liu Tsa Chih 2007;29:206-9.
- al-Yasi AR, Carroll MJ, Ellison D, Granowska M, Mather SJ, Wells CA, et al. Axillary node status in breast cancer patients prior to surgery by imaging with Tc-99m humanised anti-PEM monoclonal antibody, hHMFG1. Br J Cancer 2002;86:870-8.
- Altinyollar H, Dingil G, Berberoglu U. Detection of infraclavicular lymph node metastases using ultrasonography in breast cancer. J Surg Oncol 2005;92:299-303.
- Altomare V, Guerriero G, Carino R, Battista C, Primavera A, Altomare A, et al. Axillary lymph node echo-guided fine-needle aspiration cytology enables breast cancer patients to avoid a sentinel lymph node biopsy. Preliminary experience and a review of the literature. Surg Today 2007;37:735-9.
- Holle LH, Trampert L, Lung-Kurt S, Villena-Heinsen CE, Puschel W, Schmidt S, et al. Investigations of breast tumors with fluorine-18-fluorodeoxyglucose and SPECT. J Nucl Med 1996;37:615-22.
- Lumachi FT. Accuracy of ultrasonography and 99m Tc-sestamibi scintimammography for assessing axillary lymph node status in breast cancer patients. A prospective study. Eur J Surg Oncol 2006;32:933-6.
- Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK, Drucker MJ, Livingston RB. Monitoring the response of patients with locally advanced breast carcinoma to neoadjuvant chemotherapy using [technetium 99m]-sestamibi scintimammography. Cancer 1999;85:2410-23.
- Mansi L, Rambaldi PF, Procaccini E, DiGregorio F, Laprovitera A, Pecori B, et al. Scintimammography with technetium-99m tetrofosmin in the diagnosis of breast cancer and lymph node metastases. Eur J Nucl Med 1996;23:932-9.
- Myslivecek M, Koranda P, Kaminek M, Husak V, Hartlova M, Duskova M, et al. Technetium-99m-MIBI scintimammography by planar and SPECT imaging in the diagnosis of breast carcinoma and axillary lymph node involvement. Nucl Med Rev 2004;7:151-5.
- Paredes P, Vidal-Sicart S, Zanon G, Pahisa J, Fernandez PL, Velasco M, et al. Clinical relevance of sentinel lymph nodes in the internal mammary chain in breast cancer patients. Eur J Nucl Med Mol Imaging 2005;32:1283-7.
- Schillaci O, Scopinaro F, Danieli R, Tavolaro R, Cannas P, Picardi V, et al. Technetium-99m sestamibi imaging in the detection of axillary lymph node involvement in patients with breast cancer. Anticancer Res 1997;17:1607-10.
- Shien T, kashi-Tanaka S, Yoshida M, Hojo T, Iwamoto E, Miyakawa K, et al. Evaluation of axillary status in patients with breast cancer using thin-section CT. Int J Clin Oncol 2008;13:314-19.
- Spanu A, Chessa F, Sanna D, Cottu P, Manca A, Nuvoli S, et al. Breast cancer axillary lymph node metastasis detection by a high-resolution dedicated breast camera: a comparative study with SPECT and pinhole SPECT. Cancer Biother Radiopharm 2007;22:799-811.
- Spanu A, Dettori G, Chessa F, Porcu A, Cottu P, Solinas P, et al. Tc-99m-tetrofosmin pinhole-SPECT (P-SPECT) and radioguided sentinel node (SN) biopsy and in breast cancer axillary lymph node staging. Cancer Biother Radiopharm 2001;16:501-13.
- Spanu A, Dettori G, Chiaramida P, Cottu P, Falchi A, Porcu A, et al. The role of Tc-99m-Tetrofosmin Pinhole-SPECT in breast cancer axillary lymph node staging. Cancer Biother Radiopharm 2000;15:81-9.
- Spanu A, Tanda F, Dettori G, Manca A, Chessa F, Porcu A, et al. The role of Tc-99m-tetrofosmin pinhole-SPECT in breast cancer non palpable axillary lymph node metastases detection. Q J Nucl Med 2003;47:116-28.
- Sperber FW. Preoperative clinical, mammographic and sonographic assessment of neoadjuvant chemotherapy response in breast cancer. Isr Med Assoc J 2006;8:342-6.
- Taillefer R, Robidoux A, Turpin S, Lambert R, Cantin J, Leveille J. Metastatic axillary lymph node technetium-99m-MIBI imaging in primary breast cancer. J Nucl Med 1998;39:459-64.
- Taillefer R, Robidoux A, Lambert R, Turpin S, Laperriere J. Technetium-99M-sestamibi prone scintimammography to detect primary breast-cancer and axillary lymph-node involvement. J Nucl Med 1995;36:1758-65.
- Dose J, Avril N, Graeff H, Jaenicke F. Positron emission tomography for diagnosis of breast tumors. Onkologie 1997;20:190-5.
- Prati R, Minami CA, Gornbein JA, Debruhl N, Chung D, Chang HR, et al. Accuracy of clinical evaluation of locally advanced breast cancer in patients receiving neoadjuvant chemotherapy. Cancer 2009;115:1194-202.
- Smith IC, Welch AE, Hutcheon AW, Miller ID, Payne S, Chilcott F, et al. Positron emission tomography using [F-18]-fluorodeoxy-d-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 2000;18:1676-88.
- Bradley AJ, Carrington BM, Hammond CL, Swindell R, Magee B. Accuracy of axillary MR imaging in treated breast cancer for distinguishing between recurrent tumour and treatment effects: does intravenous Gd-DTPA enhancement help in cases of diagnostic dilemma?. Clin Radiol 2000;55:921-8.
- Lin W-YT. Fluorine-18 FDG-PET in detecting local recurrence and distant metastases in breast cancer – Taiwanese experiences. Cancer Invest 2002;20:725-9.
- Mustafa S, Marshall C, Griffiths PA, Chuthapisith S, Wheatley DC, Eremin JM, et al. 18F-FDG dual-headed gamma camera PET in detection of axillary nodal disease in patients with large or locally advanced breast cancer: possible alternative staging of axilla. Oncol Rep 2007;18:1545-9.
- Ohta M, Tokuda Y, Saitoh Y, Suzuki Y, Okumura A, Kubota M, et al. Comparative efficacy of positron emission tomography and ultrasonography in preoperative evaluation of axillary lymph node metastases in breast cancer. Breast Cancer 2000;7:99-103.
- Rostom AY, Powe J, Kandil A, Ezzat A, Bakheet S, el-Khwsky F, et al. Positron emission tomography in breast cancer: a clinicopathological correlation of results. Br J Radiol 1999;72:1064-8.
Appendix 1 Literature search strategies
The numbers in the brackets are the number of citations identified by each search term.
MEDLINE search strategy for clinical effectiveness studies
-
exp Breast Neoplasms/ (160,544)
-
exp Neoplasms/ (1,998,053)
-
exp Carcinoma/ (378,056)
-
exp Adenocarcinoma/ (215,523)
-
4 or 3 or 2 (1,998,053)
-
exp Breast/ (25,097)
-
6 and 5 (13,438)
-
((breast$or mamma$) adj5 (cancer$or carcin$or tumour$or tumor$or neoplasm$or malignan$)).tw. (162,727)
-
1 or 7 or 8 (203,102)
-
Positron-Emission Tomography/ (11,502)
-
positron emission tomography.tw. (20,247)
-
PET.tw. (29,636)
-
exp Magnetic Resonance Imaging/ (198,356)
-
magnetic resonance.tw. (129,805)
-
mri.tw. (79,788)
-
or/10–15 (290,335)
-
9 and 16 (4802)
-
exp “Sensitivity and Specificity”/ (276,863)
-
sensitivity.tw. (353,675)
-
specificity.tw. (227,637)
-
((pre-test or pretest) adj probability).tw. (755)
-
post-test probability.tw. (217)
-
predictive value$.tw. (43,259)
-
likelihood ratio$.tw. (4749)
-
or/18–24 (702,339)
-
25 and 17 (1471)
-
Axilla/ (7809)
-
axilla$.tw. (19,578)
-
exp lymphatic system/or exp lymph nodes/ (197,948)
-
Lymphatic Metastasis/ (52,995)
-
lymphatic system/or exp lymphatic vessels/or exp lymphoid tissue/ (197,847)
-
lymph$.tw. (548,454)
-
or/27–32 (691,123)
-
33 and 26 (299)
MEDLINE search strategy for cost-effectiveness studies
-
exp Breast Neoplasms/ (160,809)
-
exp Neoplasms/ (2,000,086)
-
exp Carcinoma/ (378,481)
-
exp Adenocarcinoma/ (215,800)
-
4 or 3 or 2 (2,000,086)
-
exp Breast/ (25,139)
-
6 and 5 (13,472)
-
((breast$or mamma$) adj5 (cancer$or carcin$or tumour$or tumor$or neoplasm$or malignan$)).tw. (163,002)
-
1 or 7 or 8 (203,430)
-
Positron-Emission Tomography/ (11,569)
-
positron emission tomography.tw. (20,284)
-
PET.tw. (29,688)
-
exp Magnetic Resonance Imaging/ (198,724)
-
magnetic resonance.tw. (129,992)
-
mri.tw. (79,951)
-
or/10–15 (290,851)
-
9 and 16 (4816)
-
Axilla/ (7823)
-
axilla$.tw. (19,613)
-
exp lymphatic system/or exp lymph nodes/ (198,061)
-
Lymphatic Metastasis/ (53,075)
-
lymphatic system/or exp lymphatic vessels/or exp lymphoid tissue/ (197,960)
-
lymph$.tw. (548,945)
-
or/18–23 (691,694)
-
24 and 17 (855)
-
Economics/ (25,278)
-
“costs and cost analysis”/ (36,971)
-
Cost allocation/ (1848)
-
Cost–benefit analysis/ (44,801)
-
Cost control/ (17,850)
-
cost savings/ (6180)
-
Cost of illness/ (11,331)
-
Cost sharing/ (1482)
-
“deductibles and coinsurance”/ (1202)
-
Health care costs/ (17,597)
-
Direct service costs/ (860)
-
Drug costs/ (8947)
-
Employer health costs/ (998)
-
Hospital costs/ (5768)
-
Health expenditures/ (10,378)
-
Capital expenditures/ (1841)
-
Value of life/ (5003)
-
exp economics, hospital/ (15,808)
-
exp economics, medical/ (11,638)
-
Economics, nursing/ (3779)
-
Economics, pharmaceutical/ (2001)
-
exp “fees and charges”/ (23,905)
-
exp budgets/ (10,044)
-
(low adj cost).mp. (11,582)
-
(high adj cost).mp. (5361)
-
(health?care adj cost$).mp. (1899)
-
(fiscal or funding or financial or finance).tw. (49,856)
-
(cost adj estimate$).mp. (918)
-
(cost adj variable).mp. (23)
-
(unit adj cost$).mp. (941)
-
(economic$or pharmacoeconomic$or price$or pricing).tw. (108,763)
-
or/26–56 (327,682)
-
25 and 57 (21)
Appendix 2 Quality assessment
Quality assessment of diagnostic accuracy studies (QUADAS)69 and details of criteria for scoring studies.
| 1. Was the spectrum of patients representative of the patients who will receive the test in practice? | |
|---|---|
| Yes | All patients were early stage, newly diagnosed and non-DCIS, and patients were recruited both prospectively and consecutively |
| No | Not all patients were early stage/newly diagnosed, or some had DCIS, or patients were studied retrospectively or non-consecutively |
| Unclear | Insufficient details given about stage or recruitment methods to make a judgement about whether the patient spectrum would be scored ‘yes’ |
| 2. Is the reference standard likely to correctly classify the target condition? | |
| Yes | All patients received either ALND or SLNB |
| No | Some or all patients received any other reference standard, including FNAC, or no reference standard |
| Unclear | Reference standard is not stated |
| 3. Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | |
| Yes | Reference standard was performed within 3 months of the index test |
| No | Reference standard was performed more than 3 months after the index test |
| Unclear | The time between reference standard and index test is not stated |
| 4. Did patients receive the same reference standard regardless of the index test result? | |
| Yes | Selection of reference standard was not determined by the index text result |
| No | Selection of reference standard was determined by the index test result |
| Unclear | It is not clear whether selection of reference standard was determined by the index test result |
| 5. Was the execution of the index test described in sufficient detail to permit replication of the test? | |
| 6. Was the execution of the reference standard described in sufficient detail to permit replication of the test? | |
| Yes | Sufficient details of the test execution are reported |
| No | Sufficient details are not reported |
| Unclear | Not applicable |
| 7. Were the index test results interpreted without knowledge of the results of the reference standard? | |
| 8. Were the reference standard results interpreted without knowledge of the results of the index test? | |
| Yes | The index test was interpreted without knowledge of the results of the reference standard or vice versa. If the test was clearly interpreted before the results of the other test were available then this was scored as ‘yes’ |
| No | The person interpreting the index test was aware of the results of the reference standard or vice versa |
| Unclear | No information is provided regarding whether tests were interpreted blindly |
| 9. Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | |
| Yes | PET studies included a CT scan; reference standard interpreted using usual methods; and clinical data relating to the primary tumour (examination, ultrasound, biopsy results) were available to the interpreting radiologist |
| No | PET studies did NOT include a CT scan; and/or reference standard was NOT interpreted using usual methods; and/or clinical data relating to the primary tumour (examination, ultrasound, biopsy results) were NOT available to the interpreting radiologist |
| Unclear | Insufficient data reported regarding the text methods and availability of clinical data |
| 10. Were uninterpretable/intermediate test results reported? | |
| Yes | |
| No | |
| Unclear | |
| 11. Were withdrawals from the study explained? | |
| Yes | All patients recruited to the study were accounted for |
| No | There appear to be patients who were recruited into the study who are not accounted for |
| Unclear | It is not clear whether any withdrawals occurred |
Appendix 3 Summary of excluded studies
| Study | Number | Reason for exclusion |
| Bland 2004,140 Guller 2003,141 Wahl 2004142 | 3 | Comment, not original research |
| Bahri 2008,143 Hsiang 2007,144 Zytoon 2007145 | 3 | Imaging of breast not axilla |
| Adler 1993,146 Bassa 1996,147 Beer 2008,148 Bombardieri 1996,149 Burcombe 2002,150 Crowe 1994,151 Danforth 2002,152 Harisinghani 2006,153 Harisinghani 2004,154 Hoh 1993,155 Kelemen 2002,156 Luciani 2009,157 Noh 1999,158 Palmedo 1997,159 Scheidhauer 1996,160 Smyczek-Gargya 2004,161 Sun 2008,162 Tse 1992,163 Vansant 1996,164 Yang 2001165 | 20 | n < 20, PET |
| Black 2007,166 Bollet 2007,167 Dose 2002,168 Gil-Rendo 2009,169 Kim 2004,170 Kubota 2008,171 Kumar 2006,172 Perman 1996173 | 8 | No data on axilla |
| Alberini 2009,174 Bathen 2007,175 Bleckmann 1999,176 Buchmann 2007,177 Carkaci 2009,178 Chen 2008,179 Chung 2006,180 Dizendorf 2003,181 Groheux 2008,182 Heusner 2008,183 Matsushima 2005,184 Matsushima 2008,185 Mavi 2006,186 Mortellaro 2009,187 Mussurakis 1997,188 Nieweg 1993,189 Rotaru 2004,190 Schirrmeister 2001,191 Schirrmeister 2000,192 Song 2006,193 Stets 2002,194 Torrenga 2001,195 Ueda 2008,196 Yang 2007197 | 24 | No relevant data |
| Bock 1998,198 Chen 2007,199 Dose-Schwarz 2008,200 Enya 2000,201 Koga 2007,202 Nakayama 2004,203 Romer 1997,204 Tiutin 2001,205 Zhao 2007206 | 9 | Non-English language |
| Al-Yasi 2002,207 Altinyollar 2005,208 Altomare 2007,209 Holle 1996,210 Lumachi 2006,211 Mankoff 1999,212 Mansi 1996,213 Myslivecek 2004,214 Paredes 2005,215 Schillaci 1997,216 Shien 2008,217 Spanu 2007,218 Spanu 2001,219 Spanu 2000,220 Spanu 2003,221 Sperber 2006,222 Taillefer 1998,223 Taillefer 1995224 | 18 | Not PET or MRI |
| Dose 1997225 | 1 | Review |
| Prati 2009,226 Smith 2000,227 Bradley 2000,228 Lin 2002,229 Mustafa 2007,230 Ohta 2000,231 Rostom 1999232 | 7 | > 20% not correct population (not early stage, had neoadjuvant chemotherapy or not newly diagnosed) |
Appendix 4 Details of index test and reference standard
Positron emission tomography studies
| Study | PET or PET/CT | PET details | Reference standard (summary) | Reference standard (detail) |
|---|---|---|---|---|
| Chae 200973 | PET/CT | Fasted for at least 6 hours. Serum glucose levels < 120 mg/dl. 5 mCi FDG injected, 1 hour later whole body scan and abdominal tomoscintigraphy performed in supine position, thoracic tomoscintigraphy in prone position with arms extended. SUVmax measurement was corrected for body weight. Scans interpreted quantitatively using an SUV cut-off ≥ 0.64. Not reported who interpreted scans | 100% ALND (all SLNB in addition; ALND results used in analysis) |
SLNB: each half-sentinel node sectioned at 3 mm intervals; each section analysed by four levels of 150 µm and four parallel sections. One level used for H&E staining. Immunohistochemical staining was NOT performed routinely. ALND: following SLNB, level I and II dissection performed |
| Heusner 200974 | PET/CT | Fasted 6 hours before intravenous injection of 271 ± 35 MBq FDG (210–360 MBq). Blood glucose < 150 mg/dl. 1500 ml water-based oral contrast agent applied for bowel marking. Whole body PET imaging 1 hour after FDG injection. CT acquired before PET with 100 mA/s at 130 kV; 140 ml of an iodinated contrast material administered by injection. All images reconstructed with 5 mm slice thickness and 2.4 mm increment. PET emission time adapted to the patient’s body weight: < 65 kg = 4 minutes per bed position, 65–85 kg = 5 minutes per bed position, > 85 kg = 6 minutes per bed position Image reconstruction with two iterations and eight subsets. Data filtered and corrected for scatter. Experienced radiologist and nuclear medicine physician analysed PET/CT data and diagnosis was made by consensus. Scans evaluated in three orthogonal planes (axial, coronal and sagittal). Visual analysis considered positive if abnormally high uptake corresponding with a lymph node | ALND and/or SLNB |
SLNB for those with cT1 or cT2 and no clinical suspicion of lymph node metastases. ALND for everyone else and those with positive SLNB. SLNB: intraoperative frozen section, remove perinodal fatty tissue, section into 2 mm slices. Only most suspicious slice snap frozen and one superficial cryostat section examined histologically. Standard fixation overnight and paraffin embedded; H&E staining at three serial 500 µm levels, reviewed by two gynaecological pathologists. ALND: no details reported |
| Kim 200975 | PET/CT | Fasted for 6 hours, serum glucose ≤ 150 mg/dl, whole-body scan. CT scan (non-contrast) 1 hour after injection of 7.4 MBq/kg (0.2 mCi/kg) of 18-FDG for attenuation correction. Transaxial, coronal, sagittal planes. Interpreted by two experienced nuclear medicine physicians. Visual assessment of nodes; considered positive if more uptake than background. SUVmax measurement corrected for body weight | ALND and/or SLNB (SLNB included additional non-SLNB) | SLNB + ADD: injection of blue dye followed by gentle breast massage for 3 minutes. 5 minutes after injection, incision made over lower axilla to remove sentinel nodes and additional non-SLNB enlarged at lower axilla. 2 mm slices examined in frozen section intraoperatively, H&E staining also performed on paraffin-embedded permanent sections. ALND for those with positive PET/CT scan or positive intraoperative sample on SLNB or ADD. No details reported |
| Taira 200976 | PET/CT | Four-hour fast, 3 MBq/kg body weight 18-F-FDG injected, scan performed 60 minutes later. Whole-body scan in supine position with elevation of arms. Analysed by two expert radiologists. Visual analysis; positive if more uptake than background. Quantitative SUVmax measurement if abnormal uptake | ALND and/or SLNB (ALND if PET or SLNB positive or due to patient preference) |
SLNB: blue dye method. 3 ml injected, massaged 5 minutes, then excised. Rapid pathological diagnosis with H&E. ALND performed if SLNB positive. SLNB post-operative histology: fixed with formalin and embedded in paraffin. 200 µm whole cross section, with H&E staining and immunostaining with anti-cytokeratin antibody. If positive, secondary ALND performed. Non-NO sentinel nodes 1–2 sections prepared from each lymph node, H&E staining |
| Fuster 200877 | PET/CT | Fast 4 hours prior. Blood glucose required to be < 7 mmol/l. 740 MBq FDG injected, scan 1 hour later. No oral or i.v. contrast used. Whole-body PET. Reconstructed with and without CT data for attenuation correction. Interpreted separately by two nuclear medicine physicians. Visual analysis and SUV measurement | 100% ALND | None reported |
| Ueda 200878 | PET/CT | Fast 4 hours prior. Blood glucose required to be ≤ 120 mg/dl. 3.7 Mbq/kg FDG injected, scan 1 hour later. Transmission scan with CT for attenuation correction and anatomical imaging for 90 seconds. i.v. contrast not administered for CT. Gaussian filter, back projection image obtained, spacial resolution 6–7 mm cranio-caudal, 6.3–7.1 mm right–left, 6.3 and 7.1 mm in anterior-posterior directions. Two experienced radiologists interpreted scans. PET results determined via visual assessment (abnormal axillary uptake) and also via various SUV cut-offs. Results using visual assessment presented here | ALND and/or SLNB |
ALND for those positive at ultrasound or due to patient preference. SLNB for those negative at ultrasound, with full ALND if intraoperative histology positive SLNB histology: 2 mm thick slices, haematoxylin and eosin stain, two pathologists examined |
| Veronesi 200779 | PET/CT | Fast 6 hours prior. Blood glucose required to be < 140 mg/dl. 5.3 MBq/kg FDG injected, three glasses of water, empty bladder. Scan 45 minutes later. Supine position, arms over head. CT scan before PET, attenuation-corrected images reconstructed in transaxial, coronal and sagittal planes. Interpreted independently by three experienced nuclear medicine physicians. Initial qualitative assessment of images, quantitative analysis of those equivocal, probably abnormal or abnormal. Any focal uptake greater than normal considered, definitely abnormal if SUV (normalised to body weight) > 1.2 | ALND and/or SLNB (ALND if PET or SLNB positive) |
SLNB only if PET/CT negative: SN dissected along major axis if > 5 mm, uncut if < 5 mm, then sectioned at 50/100 µm intervals. H&E stain. Suspicious sections: untreated portion of section stained with cytokeratins. Micrometastases ≤ 2 mm diameter. ALND if SLNB or PET/CT positive. Level I, II and III cleared – SNs removed during ALND were treated differently, sectioned 100–500 µm apart, stained with H&E only |
| Cermik 200880 | PET only | Fast 4 hours prior. Serum glucose levels required to be < 140 mg/dl. Intravenous FDG (5.2 MBq/kg), PET scan 1 hour later. Whole-body PET scans. Transmission images interleaved between multiple emission scans. Images interpreted with and without attenuation correction. Interpreted by single experienced nuclear medicine physician. Visual analysis and SUV measurement | ALND and/or SLNB |
Kumar 2006:102 radiotracer and blue dye injected (subareolar) to identify sentinel lymph nodes. SLNs dissected. Dissected lymph nodes formalin fixed and paraffin embedded. SLNs evaluated by H&E and by immunohistochemistry (cytokeratin antibody). Non-sentinel nodes evaluated by H&E Cermik 2008:80 no details reported |
| Gil-Rendo 200681 | PET only | Fast 6 hours prior. Blood glucose required to be < 120 mg/dl. 370 MBq FDG injected, scan 40–60 minutes later. Whole-body emission-transmission scan. Reconstructed with and without attenuation correction. From Zornoza paper:103 supine position, arms separated from side by roller. Four-to-five overlapping emission-transmission images obtained in 2D acquisition mode. Two experienced nuclear medicine physicians interpreted scans. Visual analysis; considered positive if axillary uptake higher than surrounding tissue. SUVmax also measured | ALND and/or SLNB (first n = 150: ALND; second n = 125: ALND if PET or SLNB positive; SLNB otherwise) |
Lymphatic mapping using blue dye. SLNs studied intraoperatively (sections stained with H&E) and all patients with SLN metastases received completion ALND. If SLN negative on initial analysis, further sections analysed via immunocytochemistry with anti-cytokeratin antibodies. ALND: level I and II. Nodes sectioned and stained with H&E. Note: nodes undergoing SLNB and immunocytochemistry are sectioned multiple times compared to ALND nodes therefore may have detected more micrometastases |
| Weir 200582 | PET only | Six-hour fast. 5.5 MBq/Kg of FDG given intravenously on contralateral arm, 40–60 minutes before scan. Three-to-six bed position interleaved emission and transmission scans. Images reconstructed using iterative methods. Images interpreted by ‘experienced nuclear medicine physicians’ | ALND and/or SLNB | ALND level I and II |
| Agresti 200483 | PET only | No details reported | ALND and/or SLNB (ALND if PET or SLNB positive) | SLNB: radioguided using lymphoscintigraphy. ALND/SLNB evaluated on sections |
| Fehr 200484 | PET only | Four-hour fast. 600–700 MBq of FDG given intravenously, 40–50 minutes before scan. Whole body scan in supine position plus scan of breasts and axilla in prone position. Images reconstructed using standard back-projections and with segmented attenuation correction. Images interpreted by two nuclear medicine physicians. Considered positive if axilla showed more uptake than surrounding tissue | 100% ALND (all SLNB in addition) |
SLNB: radioguided using lymphoscintigraphy (technetium 99 m colloidal albumin) and blue dye. SLNs formalin fixed, paraffin embedded, half the sections stained with H&E and half via immunohistochemistry using pan-cytokeratin staining. No frozen sections. ALND: level I and II. Formalin fixed, paraffin embedded, H&E stained (and immunohistochemistry using pan-cytokeratin staining for cases of invasive lobular carcinoma) |
| Inoue 200485 | PET only | Fast 4 hours prior. Blood glucose required to be < 150 mg/dl. 300–510 MBq FDG injected, scan 1 hour later. Reconstructed with and without attenuation correction using filtered back-projection and Butterworth filter. From Yutani 2000:107 emission then transmission scans. Images interpreted by two experienced radiologists. Considered positive if focal FDG uptake present in axilla (SUV also measured for primary tumour) | All confirmed by histology, ‘almost all’ ALND (information from author) | No details reported |
| Lovrics 200486 | PET only | Patients fasted for at least 4 hours and had two glasses of water 30–60 minutes before scan. If plasma glucose levels were > 10 mmol/l, given insulin at nuclear physician’s discretion. Intravenous FDG (5 mCi) in contralateral hand, PET scan 45 minutes later. Whole-body PET scans, arms over head. Forty-seven slices per bed position (mean of five positions), overlap of 5 cm, resolution 6 mm, axial field of view 15 cm. No attenuation correction. Visual and semi-quantitative analysis. Interpreted by single nuclear physician | ALND and/or SLNB |
ALND: morning of surgery or afternoon of day before surgery, 1.0 mCi technetium 99 sulfur colloid injected peritumorally/at excision site (in cases of excision biopsy?) Lymphoscintigraphy not routinely performed. Intraoperatively vital blue dye injected peritumorally, Sentinel node identified with hand-held gamma probe. Histology: level I and II and routine histology: sentinal nodes > 0.5 cm sectioned transversely, < 0.5 cm bisected. The H&E SLNB as above. Where routine histology negative, micrometastasis histology performed: Cam 5.2 (anti-cytokeratin) stain and serial sections at 50 µm |
| Wahl 200444 | PET only | Four-hour fast, checked fasting blood sugar. Injected with 17 ± 3 mCi of FDG in opposite arm or foot if bilateral disease, 40 minutes before scanning. Nearly all cases, arms above head for entire scanning period. Emission and transmission scans at two levels. Images reconstructed by filtered back-projection using a Hann filter. Quality control committee assessed image quality. Three experienced nuclear medicine physicians independently interpreted results. Visual analysis; probably or definitely abnormal PET was considered positive. Also SUV measurement for any abnormal images | 100% ALND (some SLNB in addition) | At least level II. Nodes fixed in formalin, embedded in paraffin and stained with H&E. Depending on size, each lymph node sectioned into two or three parts, and one or more sections prepared from each part |
| Barranger 200387 | PET only | Fast 6 hours prior. Blood glucose required to be < 7 mmol/l. 4 MBq/kg FDG injected, scan 1 hour later. Whole-body scan and abdominal tomoscintigraphy in supine position, and thoracic tomoscintigraphy in prone position with arms extended, using mammoscintigraphy table. Reconstructed by iterative algorithm without attenuation correction. Two experienced nuclear medicine physicians interpreted scans. Visual analysis; consensus regarding presence or absence of abnormal uptake in axilla (and degree of uptake) | 100% ALND (all SLNB in addition) |
SLNB: (all with blue dye, 28 also with lymphoscintigraphy). 3 mm sections, H&E staining and immunohistochemistry with anti-cytokeratin antibody cocktail. ALND: level I and II. Sections stained with H&E |
| Guller 200288 | PET only | Fast ≥ 12 hours prior. 5 MBq/kg FDG injected, scan 90 minutes later. Whole-body scan. Prone position. Not reported who analysed. Considered positive if detectable lymph node metastases present | ALND and/or SLNB | SLN identified by combination of blue dye and radioisotope (99 m-Tc-colloid). Histopathologic analysis of SLN (H&E staining, immunohistochemistry with CK22 and Lu-5, step sections of lymph nodes) |
| Nakamoto 200289 | PET only | Fast ≥ 4 hours prior. 370 MBq FDG injected. Arms at side of body during scan. Transmission scans obtained using 68-Ge rod source for purpose of attenuation correction. Dynamic images at breast level, then static images of axillary area, obtained shortly after injection of FDG. Images reconstructed using filtered back projection algorithm and Hanning filter. Images generated with and without attenuation correction. Two experienced nuclear medicine physicians independently reviewed images. Visual analysis. Considered positive if uptake was probably or definitely abnormal on consensus between analysts. Attenuation-corrected and non-attenuation-corrected images analysed separately. SUV also measured | 100% ALND | No details reported |
| Rieber 200290 | PET only | Eight-hour fast. Average 370 MBq FDG injected. Scan carried out 45–60 minutes after injection. Prone position, elevated arms. Emission scans at three bed positions. No attenuation correction. Clearly visible, focally increased tracer uptake = positive scan. One experienced observer | Histology (no further details) | Unclear – simply states histology |
| van der Hoeven 200291 | PET only | Six-hour fast, serum glucose measured. 370 MBq FDG in contralateral arm 60 minutes before scan. Emission scans of chest (two-dimensional mode, two bed positions) with patient in supine position. Images reconstructed with filtered back-projection using a Hanning filter, resulting in spatial resolution of ~7 mm full-width at half maximum. Three experienced clinicians interpreted images. Final diagnosis was taken as the result agreed upon by at least two of the three. Considered positive if PET scan showed at least moderately enhanced uptake | ALND and/or SLNB (patients were stratified for ALND or SLNB largely depending on tumour size) | ALND nodes sliced at one or two levels (< 1 cm and > 1 cm, respectively) and stained with CAM5.2 before examination SLNB used combined blue dye/radiocolloid approach. Sentinel nodes were sliced at four-to-six other levels if the first slice was negative. H&E and CAM5.2 staining. (CAM5.2 is a immunohistochemical stain) |
| Greco 200192 | PET only | Fast ≥ 5 hours prior. Required to have normal fasting blood glucose levels. 400 MBq (11 mCi) FDG injected. Most patients scanned in supine position with arms raised. Two transmission scans in bed position before FDG injection for attenuation correction. Two static emission scans 45–60 minutes after FDG injection. Images attenuation-corrected with filtered back-projection using a Hanning filter. Three nuclear medicine physicians interpreted. Visual analysis; considered positive if axillary uptake higher than surrounding tissue | 100% ALND | ALND (three levels). Lymph nodes formalin fixed, paraffin embedded, sectioned, stained with H&E |
| Noh 199893 | PET only | Injection of 370 MBq (10 mCi) FDG. Scan 60 minutes later. Whole-body emission scan and regional transmission scan for attenuation correction. SUV measured. No details of who analysed or what was considered positive | Histology (no further details) | No details reported |
| Smith 199894 | PET only | Six-hour fast, injection of 185 MBq of FDG, contralateral arm, scan 40 minutes later. Prone position, arms by side. Imaging at two bed positions. Two experienced independent observers who reached consensus. Visual analysis; considered positive if abnormal uptake in axilla | 45/50 (90%) ALND; 5/50 (10%) FNAC (large/locally advanced) | 4-NS or level III ALND nodes embedded in paraffin blocks, sections of each node cut at two-to-four levels, examined by a single pathologist using standard preparation and histochemical techniques |
| Adler 199795 | PET only | Four-hour fast, injection of 740 MBq (20 mCi) FDG (note high dose of FDG). Supine position, arms at sides. One- or two-level transmission scans of breast and axilla before FDG injection. One- or two-level emission scans 1 hour after FDG injection. Reconstructed using filtered back projection with Hann filters. Interpreted by two independent observers, discrepancies resolved by consensus. Visual analysis for increased FDG uptake compared with background. Within this review, considered positive if probably or definitely abnormal, i.e. score of 4–5 on 1–5 scale | 100% ALND | ALND: at least level II. Formalin fixed, paraffin embedded, single 3 µm planes, stained with H&E |
| Avril 199696 | PET only | Four-hour fast, injection of 270–390 MBq FDG, scan 60–80 minutes later. Prone position, arms at sides. Whole-body PET scanner. Emission scans, then transmission scans for attenuation correction. Attenuation-corrected images reconstructed using filtered back projection with Hanning filter. Normalised for dose of FDG and body weight. Interpreted by two nuclear medicine physicians, consensus reached. Visual analysis; considered positive if focally increased FDG uptake in axilla | 37/41 (90%) ALND; 4/41 (10%, locally advanced) no reference standard; assumed positive due to clinical evidence | ALND (levels I and II and possibly III) |
| Utech 199697 | PET only | At least 4 hours fast, serum glucose monitored. 10 mCi FDG given intravenously 1 hour before emission scan. Whole-body scan, four bed positions. Transmission scan before FDG injection. Emission scan 1 hour after FDG injection. Images reconstructed via filtered back projection. Three experienced nuclear radiologists reviewed scan, and final reading by experienced nuclear medicine physician. Visual analysis; considered positive if discrete focal uptake in axilla greater than background | 55/124 (44%) ALND; 69/124 (56%) modified radical mastectomy | ALND level not reported |
Magnetic resonance imaging studies
| Study | Type of MRI | MRI details | Reference standard (summary) | Reference standard (detail) |
|---|---|---|---|---|
| Kimura 200998 | USPIO-enhanced | USPIO administered intravenously 24-hours prior to MRI through 5 µm microfilter (2.6 mg/kg iron diluted in 100 ml of 0.9% saline solution) by drip infusion for 90 minutes. MRI on 1.5T MRI scanner. 3-inch surface coil placed over SLN area identified by RI lymphoscintigraphic images. Arms elevated, prone position. T1W axial and coronal MR images and T2*W axial and coronal MR images obtained. Other technical details given. Images evaluated by one radiologist and one surgeon; reached consensus. Nodes classified as (A) low signal intensity on T2*W images, (B1) partial high signal intensity on T2*W, isointense to the fatty tissue on T1W images, (B2) partial high signal intensity on T2*W images, not identified as fat on T1W images and (C) high signal intensity on T2*W images. Types C and B2 were classified as metastatic, therefore criteria for positivity was USPIO uptake | ALND and/or SLNB |
SLNB: blue dye and radioactive tracer-guided procedure in all patients. SLNs bisected or sectioned into 2 mm slices and embedded. H&E staining used. Immunohistochemical-keratin staining if H&E negative. ALND: if SLNB positive. Details on level not given. Non SLN H&E only |
| Harada 200799 | USPIO-enhanced | Images both pre-contrast and 24–36 hours post-administration of USPIO, which was given as infusion over 90 minutes (2.6 mg/kg iron diluted in 100 ml 0.9% saline). Transverse and coronal T1-weighted and T2-weighted images, patients in supine position, cardiac surface coil used. (Other technical details of MRI given.) Analysed independently by one radiologist and one surgeon; consensus via discussion. Various alternative criteria were used to define a positive result, as follows. The main analysis (giving the highest sensitivity and specificity) used: (a) contrast uptake on USPIO-enhanced images (homogenous USPIO intake = benign; heterogeneous or no uptake = metastatic); (b) comparison of contrast uptake on pre-contrast and USPIO-enhanced images (homogenous USPIO intake = benign; heterogeneous or no uptake = metastatic); (c) size of short axis > 5 mm (pre-contrast images); (d) size of short axis > 10 mm (pre-contrast images); (e) abnormal morphology (replacement of central fatty hilum and/or irregular margins) (pre-contrast images) | 100% ALND | ALND (levels I and II): nodes formalin fixed, sectioned, stained with H&E and studied under microscope |
| Memarsadeghi 2006100 | USPIO-enhanced | Images both pre-contrast, and 24–36 hours post-administration of USPIO, which was given as infusion (2.6 mg/kg iron diluted in 100 ml saline at rate of 4 ml/minute). T1-weighted images (in transverse plane) and T2-weighted images (in transverse and sagittal planes). Patients in supine position, arms by sides and then elevated (sagittal plane arms elevated only), surface coil used. (Other technical details of MRI given.) Pre-contrast and USPIO-enhanced images interpreted independently by two experienced radiologists (consensus via discussion, third radiologist if consensus could not be reached). Criteria for positive node: (a) for non-enhanced images, (i) size of short axis > 10 mm, and/or (ii) shape round rather than oval (ratio of long-to-short axis < 1.5); and (b) for USPIO-enhanced images, (i) visual analysis comparing contrast uptake on enhanced and non-enhanced images (homogenous decrease in uptake on enhanced vs non-enhanced image = benign; no decrease or partial decrease in uptake or uniformly high uptake on enhanced image = metastatic) and/or (ii) quantitative analysis showing signal-to-noise ratio decreased by < 30% on enhanced vs non-enhanced image | 100% ALND | ALND (levels I and II): histopathological analysis, regarded as metastatic if tumour cells present at light microscopy |
| Stadnik 2006101 | USPIO-enhanced (also used gadolinium for one of the MRI sequences) | USPIO on slow-drip infusion for 24–36 hours prior to imaging (2.6 mg/kg iron diluted in 100 ml 0.9% saline), 3 ml/minute. 1.5T magnet, surface coil on axilla, patient supine. Sequences in transverse plane (section thickness 3 mm for all): T2-weighted 2D spin-echo images; T1-weighted 3D gradient echo; T2*-weighted 2D gradient echo. After injection of gadolinium (0.1 mmol/kg Gd), T1-weighted 3D gradient echo with spectral fat saturation. Images analysed in consensus by two experienced radiologists. Criteria for positivity based on pattern of nodal enhancement after USPIO administration (considered benign if normal morphology with uniform or central signal drop; considered metastatic if normal morphology or focal or global volume increase, with or without partial signal drop). Stated that USPIO-enhanced T1 gradient echo after gadolinium injection and fat saturation was a useful method | 100% ALND | Nodes considered positive if tumour cells present at light microscopy |
| Michel 2002102 | USPIO-enhanced | USPIO on slow drip infusion for 24–36 hours prior to imaging of axilla (2.6 mg/kg iron body weight diluted in 100 ml 0.9% saline soln). 2 ml/minute for first 10 minutes, to test tolerance, then 4 ml/minute. Infusion for 30 minutes overall. Technical details of MRI given. MRI of the primary breast tumour also performed using two contrast agents (USPIO and 0.1 mmol/kg Gd), but only USPIO used for imaging axilla. Criteria for metastatic node: size (short-axis diameter > 10 mm), shape (round rather than oval, i.e. ratio of long-to-short axes < 1.5), USPIO uptake (heterogeneous uptake or lack of uptake = malignant; homogenous uptake = benign; rim enhancement could be classified as malignant if on round node with other imaging properties). Not clear whether nodes were considered positive if they met just one of these criteria or some/all these criteria. Images interpreted by radiologist and an experienced resident | 100% ALND | Block resection of levels I and II of the ipsilateral side, though later mentions level III clearance in results section. Nodes positive if cancer cells positive at light microscopy, independent of results of immunohistochemical staining |
| Murray 2002103 | Dynamic gadolinium-enhanced | Contrast agent: Gd injected at 0.1 mmol/kg. Anatomical imaging prior to Gd enhanced imaging at oblique sagittal plane to optimise axillary imaging. More detail about how to interpret images given. Uses technique of comparing node uptake to uptake in adjacent fat tissue. Single experienced analyst interpreted images. Criteria for metastatic node: (a) size (cross-sectional area > 4 mm2) AND signal intensity change > 21% (compared with adjacent fat tissue); (b) signal intensity change only; and (c) size only | 100% ALND | Level II, bisection of smaller nodes, 2 mm macrosection of lager nodes, H&E staining, then embedded in paraffin, and 5 µm slices mounted on slides. In women with primary lobular carcinoma, immunocytochemical staining for cytokeratin with CAM5.2 also performed |
| Kvistad 200041 | Dynamic gadolinium-enhanced | Prone position, coil covering breast and axilla. Pre-contrast axial T1-weighted spin-echo images obtained. Then, injection of 0.1 mmol/kg gadodiamide followed by 20 ml isotonic saline. Following contrast injection, six T1-weighted dynamic contrast-enhanced 3D images acquired. Both pre-contrast images and dynamic contrast-enhanced images analysed independently by two radiologists (discrepancies resolved by consensus). Time-vs-signal intensity curves obtained. Various alternative criteria were used to define a positive result, as follows. The main analysis (giving the highest sensitivity and specificity) used: (a) positive if signal intensity increased by > 100% during the first post-contrast image (after contrast injection) compared with the pre-contrast image; (b) decrease in signal intensity between second and sixth post-contrast images (known as ‘positive wash-out sign’ and previously shown to be sign of malignancy); (c) size of short axis > 5 mm; and (d) abnormal morphology (replacement of central fatty hilus and/or irregular margins) | 100% ALND | ALND (at least levels I and II): nodes paraffin embedded, sectioned and stained with H&E |
| Mumtaz 199742 | Gadolinium-enhanced | Enhancement with Gd 0.1 mmol/kg. Extensive technical details given. Criteria for metastatic node: size > 5 mm, higher than soft-tissue intensity and enhancement with Gd. Not clear whether nodes were considered positive if they met just one of these criteria or some/all these criteria. Assessed by single radiologist | 100% ALND | None given |
| Yeung 200264 | In vivo proton MR spectroscopy | MRI performed first to identify enhancing lesions, using Gd 0.2 mmol/kg body weight. Then areas of interest (including the largest visible axillary lymph node) imaged by in vivo proton MR spectroscopy. Point-resolved spectroscopic (PRESS) sequence used. Three water-suppressed spectra acquired for each volume of interest. Spectra analysed for choline level; signal-to-noise ratio ≥ 2 indicated that choline present | 100% ALND | Few technical details given |
Glossary
- 4-node sampling (4-NS)
- An alternative staging technique to sentinel lymph node biopsy. A minimum of four lymph nodes (sometimes more) are surgically removed and examined histologically to determine the presence of axillary metastases.
- Axillary lymph nodes
- Located in the armpit area, they receive lymph fluid from the arm, breast and ipsilateral upper torso.
- Axillary lymph node dissection (ALND)
- Surgical removal of most or all axillary lymph nodes, to level I, II or III. Removed tissue is histologically examined to determine axillary spread of breast cancer.
- Axillary sampling
- Within this assessment, axillary sampling refers to sentinel lymph node biopsy or 4-node sampling.
- Computed tomography (CT)
- CT scans use a series of two-dimensional X-rays to generate a three-dimensional image of body structures. In modern positron emission tomography scanning, CT scans are often used alongside the positron emission tomography scan to allow concurrent visualisation of the anatomy of tissues and their metabolic activity.
- False-negative (FN)
- A patient with a condition who is wrongly diagnosed as not having it.
- False-positive (FP)
- A patient without a condition who is wrongly diagnosed as having it.
- Lymph nodes
- Small glands that are part of the lymphatic system. White blood cells in the lymph nodes attack bacteria and viruses as they pass through the node.
- Magnetic resonance imaging (MRI)
- MRI uses a magnetic field to provide a net alignment of hydrogen nuclei, predominantly in water molecules. These nuclei resonate when a non-ionising radiofrequency field is applied and give out a signal when it is turned off. An image is generated by encoding this signal using noisy switched magnetic field gradients. Nuclei in different tissues and pathology yield different image characteristics. In contrast-enhanced MRI, intravenously administered agents such as gadolinium chelates and USPIO (ultrasmall super-paramagnetic iron oxide) are used to provide localised image enhancement.
- Positron emission tomography (PET)
- PET uses a positron-emitting radionucleotide tracer to create a three-dimensional image of the functional processes of the body.
- Sensitivity
- The effectiveness of a diagnostic test in correctly identifying persons with a condition (true-positives divided by all persons with the condition).
- Sentinel lymph node biopsy (SLNB)
- A surgical diagnostic technique used to determine metastatic spread in primary breast cancer. The sentinel node (the first draining node from the breast) is identified using a blue dye and/or radiotracer, and is dissected out and sent for histological examination. SLNB is a less invasive surgical procedure than axillary lymph node dissection as fewer nodes are removed.
- Specificity
- The effectiveness of a diagnostic test in correctly diagnosing as negative persons who do not have a condition (true-negatives divided by all persons without the condition).
- True-negative (TN)
- A patient without a condition who is correctly diagnosed as not having it.
- True-positive (TP)
- A patient with a condition who is correctly diagnosed as having it.
List of abbreviations
- 4-NS
- 4-node sampling
- ALND
- axillary lymph node dissection
- CI
- confidence interval
- CT
- computed tomography
- DCE-MRI
- dynamic contrast-enhanced magnetic resonance imaging
- DCIS
- ductal carcinoma in situ
- FDG
- 2-fluoro-2-deoxy-d-glucose
- FN
- false-negative
- FNAC
- fine-needle aspiration cytology
- FP
- false-positive
- H&E
- haematoxylin and eosin
- LCIS
- lobular carcinoma in situ
- MeSH
- medical subject heading
- MR
- magnetic resonance
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Clinical Excellence
- PET
- positron emission tomography
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QUADAS
- Quality Assessment of Diagnostic Accuracy Studies
- ROC
- receiver operating characteristic
- SLNB
- sentinel lymph node biopsy
- SUV
- standardised uptake value
- TN
- true-negative
- TNM stage
- tumour, node, metastases stage
- TP
- true-positive
- USPIO
- ultrasmall super-paramagnetic iron oxide
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Service User Representative
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Service User Representative
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Service User Representative
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Service User Representative
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Service User Representative
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Service User Representative
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Service User Representative
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Service User Representative
-
Mr David P Britt, Service User Representative
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Service User Representative
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Service User Representative
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Service User Representative
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Service User Representative
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Service User Representative
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington