Notes
Article history
The research reported in this issue of the journal was commissioned by the HTA programme as project number 06/29/02. The contractual start date was in July 2009. The draft report began editorial review in December 2009 and was accepted for publication in September 2010. As the funder, by devising a commissioning brief, the HTA programme specified the research question and study design. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the referees for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© 2011 Queen’s Printer and Controller of HMSO. This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE) (http://www.publicationethics.org/). This journal may be freely reproduced for the purposes of private research and study and may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NETSCC, Health Technology Assessment, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK. 2011Queen’s Printer and Controller of HMSO
Chapter 1 Aim of the report
The original Health Technology Assessment (HTA) report on this topic was Wang D, Cummins C, Bayliss S, Sandercock J, Burls A. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assess 2008;12(36). 1
This update report develops the economic model from the first report by exploring cost-effectiveness in different subgroups of children with RSV infection.
Chapter 2 Background
As the original report has full details of the condition, current treatment options and information about palivizumab (Synagis®, MedImmune), only brief details will be given here.
Description of health problem
Respiratory syncytial virus is a seasonal infectious disease, with epidemics occurring annually from October to March in the UK. It is a very common infection in young children, with up to half of all infants becoming infected by the age of 1 year. 1 A proportion of children with RSV are seriously affected by the virus and may need to be hospitalised owing to life-threatening complications such as bronchiolitis (inflammation of the smaller airways of the lung) and pneumonia. Children who are at high risk of hospitalisation for these reasons include premature infants, children with chronic lung disease due to abnormal development of the lungs or cystic fibrosis, children who were born with certain types of heart problems and children who have limited resistance to disease because of a weakened immune system. 2 Many of these high-risk infants may need to be hospitalised and some may require admission to an intensive care unit. 3
Detection of RSV in children with lower respiratory tract infections is by direct immunofluorescence assay, enzyme immunoassay or a positive viral culture for RSV from nasopharyngeal secretions.
Current service provision
Beyond supportive care (such as mechanical assistance with breathing, intravenous fluids and oxygen), the only treatment available for severe RSV infection causing bronchiolitis is ribavirin (Virazole®, ICN Pharmaceuticals). 4 This is an antiviral treatment available orally and by inhalation. It is licensed for inhaled administration for severe bronchiolitis caused by RSV infection in infants, especially when they have other serious conditions, such as when they are immunocompromised. However, ‘there is no evidence that ribavirin produces clinically relevant benefit in RSV bronchiolitis’. 4 Its use requires hospitalisation, which increases the risk of spreading the infection, and it is costly and has a number of unwanted side effects. 4
Attempts to develop a vaccine to prevent RSV infection have so far been unsuccessful. Strategies to prevent infection are therefore of considerable interest.
Description of technology under assessment
Palivizumab has a proprietary name of Synagis®. It is a monoclonal antibody and is indicated for the prevention of serious lower respiratory tract disease requiring hospitalisation caused by RSV in children at high risk for RSV disease. 4 It is used in the following high-risk groups:
-
children < 6 months with haemodynamically significant left to right shunt, congenital heart disease (CHD) or pulmonary hypertension
-
children < 2 years with chronic lung disease requiring oxygen at home (or who have been on prolonged oxygen treatment)
-
children < 2 years with severe congenital immunodeficiency
-
children born at 35 weeks of gestation or less and < 6 months of age at the onset of the RSV season and considered to be at high risk of RSV hospitalisation. 4
Common side effects of palivizumab include injection site reactions, nervousness and fever. Less common side effects include diarrhoea and vomiting, constipation, haemorrhage, rhinitis, respiratory problems, pain, drowsiness, asthenia, hyperkinesia, leucopenia and rash. 4
The recommended dose of palivizumab is 15 mg per kg body weight, injected intramuscularly, given once a month during anticipated periods of RSV risk in the community. Where possible, the first dose should be administered prior to commencement of the RSV season. Subsequent doses should be administered monthly throughout the RSV season. To reduce the risk of rehospitalisation, it is recommended that children receiving palivizumab who are hospitalised with RSV continue to receive monthly doses of palivizumab for the duration of the RSV season. 4
For children undergoing cardiac bypass, it is recommended that a 15 mg/kg injection of palivizumab be administered as soon as the child is stable after surgery to ensure adequate palivizumab serum levels. Subsequent doses should resume monthly through the remainder of the RSV season for children that continue to be at high risk of RSV disease.
The cost of palivizumab (Synagis®) is £360.00 for a 50-mg vial and £663.11 for a 100-mg vial. 4 If a baby at 6 months weighs 7.5 kg the cost of one dose of palivizumab is £1023.11 if vial wastage is assumed, plus cost of administration.
Chapter 3 Definition of the decision problem
This report investigated cost-effectiveness only. We are unaware of any other work investigating the cost-effectiveness of palivizumab by different subgroups, particularly where based on a systematic review.
The original report1 found the following cost-effectiveness results:
-
In pre-term children without chronic lung disease (CLD), the base-case estimate of cost-effectiveness was £475,600/quality-adjusted life-year (QALY). When this was varied by a range of mortality rate estimates the results varied between £24,100/QALY and £3M/QALY.
-
In children with CLD the base-case estimate of cost-effectiveness was £66,900/QALY. When this was varied by a range of mortality rate estimates the results varied between £51,000/QALY and £85,000/QALY.
-
In children with CHD, the base-case estimate of cost-effectiveness was £83,200/QALY. When this was varied by whether the children had cyanotic or acyanotic CHD, the results varied between £49,100/QALY and £159,400/QALY. When this was varied by age of the child and hospitalisation rates the results varied between £63,300/QALY and £457,900/QALY.
These results are listed in Table 1.
| Category | Base estimate (£/QALY) | Sensitivity analyses (£) |
|---|---|---|
| Children without CLD | 475,600 | By mortality rate: 24,100–3,905,500 |
| Children with CLD | 66,900 | By mortality rate: 51,000–85,000 |
| Children with CHD | 83,200 | By cyanotic vs not: 49,100–159,400 |
| By age and hospitalisation rate: 63,300–457,900 |
There was also further work on the subgroup of CLD children and children with siblings in day care, which provided Table 2.
| Birth age (months) | GA (weeks) | |||||
|---|---|---|---|---|---|---|
| ≤ 24 | 24–26 | 26–28 | 28–30 | 30–32 | 32–34 | |
| < 3 | 9000 | 10,000 | 12,000 | 15,000 | 19,000 | 25,000 |
| 3–6 | 13,000 | 15,000 | 19,000 | 24,000 | 33,000 | 42,000 |
| 6–9 | 19,000 | 24,000 | 33,000 | 42,000 | 59,000 | 75,000 |
| 9–12 | 33,000 | 45,000 | 58,000 | 76,000 | 105,000 | 141,000 |
| 12–15 | 59,000 | 83,000 | 105,000 | 140,000 | 212,000 | 284,000 |
| 15–18 | 105,000 | 141,000 | 214,000 | 286,000 | 430,000 | 430,000 |
| 18–21 | 213,000 | 285,000 | 42,000 | 429,000 | 863,000 | 866,000 |
| 21–24 | 430,000 | 431,000 | 867,00 | 870,000 | 859,000 | ∞000 |
Note that these were point estimates of cost-effectiveness only. There was no information on credible intervals, for example, for the 9000 in the upper left box, i.e. whether it might vary between £8500 and £9500 or between £1000 and £17,000.
The original decision problem for the current report was in two parts.
-
The population is infants and young children at high risk of hospitalisation, morbidity or death due to RSV infection, including children < 2 years of age, and with haemodynamically significant CHD. This group was stratified by age to find out whether administration is cost-effective for any age group.
-
Using the whole data set, further analyses of other potential risk groups were investigated in healthy children or children with acyanotic or cyanotic CHD or any form of significant CLD:
-
gestational age (GA)
-
male gender
-
siblings at school (SAS)
-
multiple births (MBs)
-
exposure to passive smoke (SE)
-
overcrowding (OC) in the family home
-
parental education (PE)
-
age < 6 weeks at the start of the RSV season
-
lack of, or minimal, breast feeding
-
family history of atopy.
-
The subgroups above were suggested by members of the RSV subcommittee from the UK Joint Committee on Vaccination and Immunisation. The last three listed were not included in the final model owing to lack of good-quality information from included studies.
Chapter 4 Assessment of clinical effectiveness
Methods for reviewing effectiveness
Although this report is on the cost-effectiveness of subgroups of children with potential risk factors for hospitalisation with RSV, the process for finding relevant studies for use in the model is very similar to that used for a systematic review of clinical effectiveness. Therefore, these methods will be described in this section.
Identification of studies
The original searches for this review topic for the previous HTA report were carried out in March 2007, following preliminary scoping in 2006. 1 No date or language limits were applied.
To find appropriate prognostic studies for this report, three main strategies were used:
-
conducting new searches for prognostic and hospitalisation studies covering 1950–2009, making extensive use of searching of reference lists from recently published studies
-
rerunning of the original report searches in August 2009, to cover the interim period 2007–9, for clinical effectiveness and cost-effectiveness research as detailed below
-
sifting through the database of all references from the original report to find any relevant studies that may have been missed.
Prognosis and hospitalisation studies
The following sources were searched for relevant studies:
-
Ovid MEDLINE(R) 1950 to July Week 4 2009
-
the original HTA review database of all references
-
reference lists of relevant studies.
The reason for running the specific prognosis searches on MEDLINE only was because there was a large number of hits, but very few studies of relevance. Therefore, extensive use was made of searching reference lists of relevant studies instead.
Clinical effectiveness review
The following sources were searched for systematic reviews and primary studies:
-
Bibliographic databases: Cochrane Library (John Wiley & Sons, Inc. internet version) [Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE) and HTA], 2009 Issue 3, MEDLINE (Ovid) 1950 to July Week 4 2009, MEDLINE In-Process and other Non-Indexed Citations (Ovid) 3 August 2009, EMBASE (Ovid) 1980–2009 Week 31, Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO Host) 1982 to 4 August 2009 and Science Citation Index (Web of Knowledge) at 4 August 2009.
-
Research registries of ongoing trials including Current Controlled Trials metaRegister, Clinical Trials.gov and the National Institute for Health Research Clinical Research Network Portfolio.
-
Relevant internet sources.
Searches were limited by date to the period 2007–9 and there were no language restrictions.
Cost-effectiveness review and modelling
Studies on costs, quality of life, cost-effectiveness and modelling were identified from the following sources:
-
Bibliographic databases: MEDLINE (Ovid) 1950 to July Week 5 2009, EMBASE (Ovid) 1980 to 2009 Week 32, Cochrane Library (John Wiley & Sons, Inc. internet version) [NHS Economic Evaluation Database (EED) and DARE] 2009 Issue 3.
-
Relevant internet sites.
Searches were limited by date to the period 2007–9 and there were no language restrictions. All relevant references were inserted into a new reference manager database.
Inclusion and exclusion criteria
The inclusion criteria for this report are listed in Table 3.
| Population | Infants or children aged up to 5 years, with at least some having confirmed RSV infection, can be term or premature or mixed, healthy or can have CHD or CLD (any definition) |
| Intervention(s) | – |
| Comparator(s) | – |
| Outcomes | Reporting age specific hospitalisation rates |
| Reporting ORs and CIs for any of the listed subgroupsa | |
| Study design | Prospective or retrospective cohort, case–control, cross-sectional |
Inclusion decisions were made by one reviewer and checked by the modeller. Any disagreements were resolved through discussion.
Data abstraction strategy
Data abstraction was done straight into data tables by one reviewer and checked by the modeller. Any discrepancies were resolved through discussion.
Critical appraisal strategy
Quality assessment was by assessment of four relevant factors derived from the Critical Appraisal Skills Programme checklists for randomised controlled trials (RCTs), cohort and case–control studies (Appendix 3).
Data analysis and evidence synthesis
The data analysis and evidence synthesis process consists of the following steps:
(1) The outcomes of the risk factors, including types of population (pre-term infants and children without CLD/CHD or children with CLD or CHD), GA, birth age (AGE), SAS, gender [BOY, SEX(male)], MB, SE, OC, and PE of high school or less (≤ 12 years) were analysed, updated and combined (when it was possible). All values of parameters based on whole weeks, for example 34.5 weeks gestational age was rounded to 35 weeks. Meta-analysis was carried out with the stata program (version 10; StataCorp LP, College Station, TX, USA) using log(odds ratio, OR) and standard error [se(logOR)] for each study and drawing the plot using the meta, rather than metan function because for some studies, only OR and 95% confidence intervals (CIs) were available. Fixed and random effects models were used according to the level of heterogeneity. Heterogeneity was assessed with the Q statistic.
(2) It was assumed that the effect of the risk factors follows an addition rule in the log scale. The outcomes of different combinations of risk factors were derived by:
where x is an indicator variable for study population, with 0 for children without CLD, CLD for children with CLD, and CHD for children with CHD. SAS, BOY, MB, SE, OC and PE are indicator variables for the presence/absence of the risk factors of siblings at school, gender, MB, smoke exposure, OC and PE of high school or less. Note that the lnORs for GA and AGE are included as negative terms because our model uses increasing risk with lower values compared with the reference (OR > 1), whereas some of the ORs in the papers were reported the other way around (OR < 1).
Establishment of cost-effectiveness
The cost-effectiveness threshold used in this report is a willingness to pay of £30,000 per QALY. This is predefined by the National Institute for Health and Clinical Evidence (NICE) as their normal higher threshold for cost-effectiveness. 5
Results
Quantity and quality of research available
There were 13 studies included in total (14 papers) and 82 excluded articles for which the full paper was ordered (Figure 1). The excluded studies that were closest to being included are listed in Appendix 2 with their reasons for exclusion. The original report chose studies that were ‘of most relevance to the current UK context’ so similar studies were used in the update. Therefore, studies from Taiwan and Mexico were excluded. Otherwise, the excluded studies could not have been used as they were too small (total n = 18) or carried out in a very specific population with different hospitalisation characteristics (Down’s syndrome) or because no subgroup results were given or not with the right metrics, they were replications of included studies or were reviews.
FIGURE 1.
Flow diagram showing identification of studies.
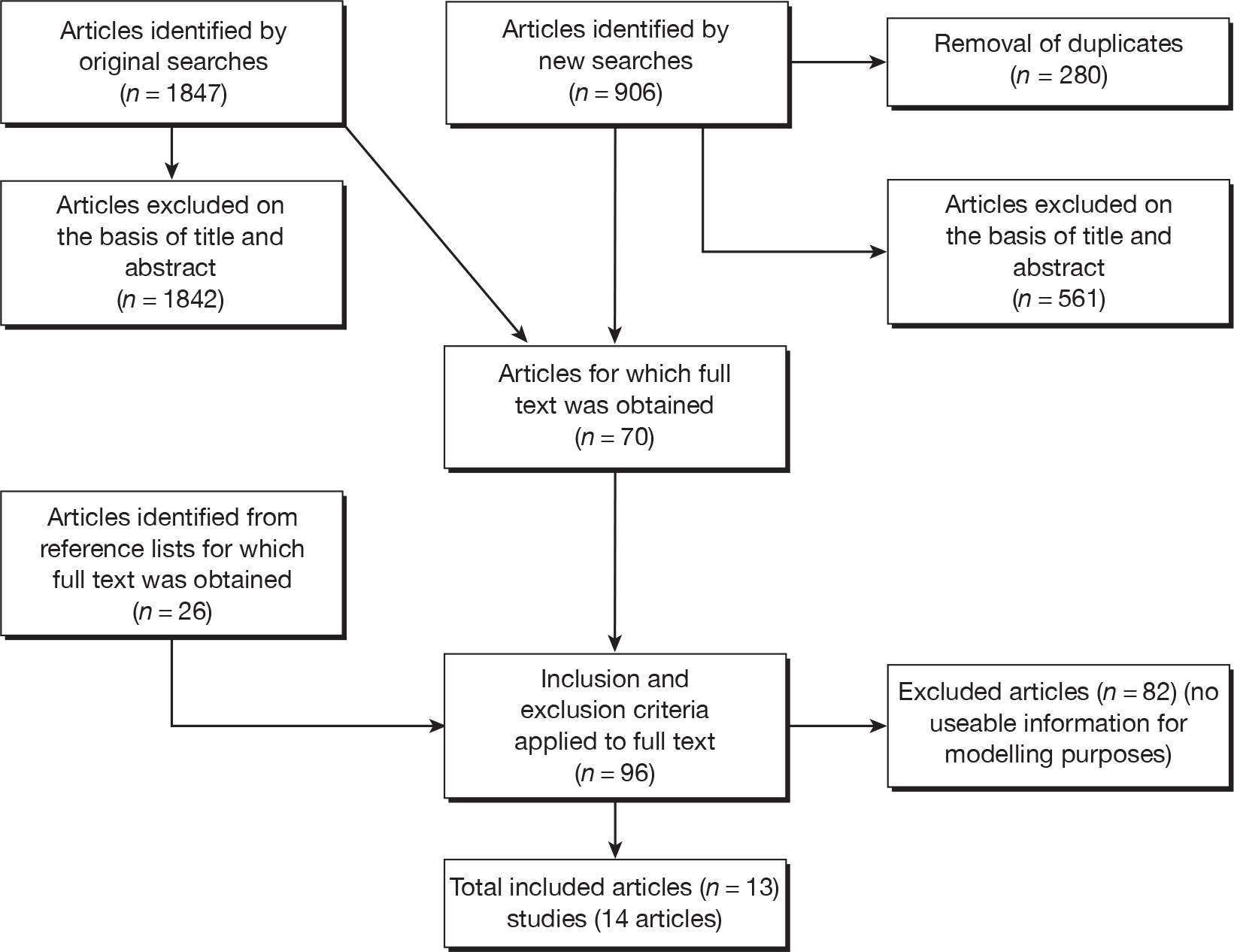
Some of the included studies, provided data for both questions 1 and 2. In the original HTA report, one study6 provided an estimate of monthly hospitalisation rate in young children with no CLD. It is unclear whether any of the children in this study had CHD. For the update, although five studies were found reporting hospitalisation for RSV by different ages,6–10 only Rietveld et al. 6 reported monthly hospitalisation rates by age so this was used in the model. In the original HTA report, two studies6,11 were used to estimate subgroup risks of hospitalisation by GA, for CLD and whether there were SAS (see Table 14 in original report1). In this update, 13 studies were used, including Carbonell-Estrany et al. 11 and Rietveld et al. 6 The baseline characteristics of the included studies are shown in Table 4. The results for individual subgroups used are shown in Table 5. Quality assessment of the included studies is in Appendix 3.
| Study name, date, country | Type | Parameters measured | Number of children in study | Number of children admitted to hospital | Premature/term/mixed | CHD/CLD/mixed | Comment |
|---|---|---|---|---|---|---|---|
| Carbonell-Estrany 2000 Spain11 | Prospective cohort | GA, CHD, CLD, SAS, SE | 584 | 118 | All premature younger than 33 weeks | Mixed | |
| Carbonell-Estrany 2001 Spain14 | Prospective cohort | GA, CHD, CLD, SAS, MB, SE | 999 | 207 | All premature younger than 33 weeks | Mixed | None given palivizumab |
| Eriksson 2002 Sweden15 | Cohort | SAS | 48,715 (total population) | 1503 | Mixed | Mixed | Unclear whether prospective or retrospective |
| Figueras-Aloy 2004 Spain16,17 | Prospective case–control | SAS, SE, OC, PE | 557 | 186 cases and 371 controls | All premature (33–35 weeks) | Mixed | Study design odd |
| Figueras-Aloy 2008 Spain18 | Prospective cohort | SAS, SE, OC | 5441 | 202 | All premature (32–35 weeks) | Mixed | |
| Frogel 2008 USA19 | Prospective registry | CHD | 19,474 | 2532 | Mixed | Mixed | All given palivizumab |
| Grimwood 2008 NZ20 | Prospective cohort | GA, gender, MB | 11,270 (total eligible population) | 141 | Mixed | Mixed | |
| Kristensen 2009 Denmark9 | Case–control | Gender | 626 | 313 cases, 313 controls | Mixed | CHD only | |
| Law 2004 Canada21 | Prospective cohort | Gender, SAS, SE, OC | 1862 | 1862 | All premature (33–35 weeks) | Mixed | |
| Liese 2003 Germany25 | Prospective cohort | Gender, CLD | 717 | 76 | All premature (35 weeks or less) | Mixed | None given palivizumab |
| Nielsen 2003 Denmark23 | Retrospective case–control | GA, SAS | 7327 | 1252 cases, 6075 controls | Mixed | Mixed | |
| Rietveld 2006 Netherlands6 | Retrospective cohort | GA, gender | 140,661 | 2469 | Mixed | Mixed | |
| Rossi 2007 Italy24 | Case–control | GA, gender, SE | 440 | 145 | Mixed | Mixed |
| Study | Risk factors | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | GA | Gender (male/female) | CHD | CLD | SAS | MB | SE | OC | PE of high school or less (≤ 12 years) | |
| Number of studies with this outcome | 1 | 1 | 6 | 3 | 2 | 6 | 1 | 5 | 3 | 1 |
| Carbonell-Estrany 200011 | – | Combined with Carbonell-Estrany 200114 | – | OR = 1.42 (0.57, 3.51) (estimated from raw numbers) | OR = 3.1 (1.22, 7.91) (multivariate logistic regression) | Combined with Carbonell-Estrany 200114 | – | Combined with Carbonell-Estrany 200114 | – | – |
| Carbonell-Estrany 200114 | – | OR = 0.87 (0.77, 0.97) (multivariate logistic regression) | – | OR = 1.07 (0.41, 2.79) (estimated from raw numbers) | – | OR = 1.64 (1.05, 2.55) (multivariate logistic regression) | – | OR = 1.63 (1.05, 2.56) (multivariate logistic regression) | – | – |
|
Eriksson 200215 |
– | – | – | – | – | Previously healthy OR 2.42 (2.08, 2.81) | – | – | – | – |
| Figueras-Aloy 200416,17 | – | – | – | – | – | OR = 2.40 (1.61, 3.57) (bivariate analysis) | – | OR = 0.95 (1.01, 2.18) (bivariate analysis) | OR = 1.79 (1.18, 2.72) (bivariate analysis) | OR = 1.48 (0.98, 2.23) (bivariate analysis) |
| Figueras-Aloy 200818 | – | – | – | – | – | OR = 1.96 (1.47, 2.60) (bivariate analysis) | – | OR = 1.59 (1.12, 2.26) (bivariate analysis) | OR 1.37 (0.85, 2.20) (bivariate analysis) | – |
| Frogel 200819 | – | – | – | OR = 1.55 (1.04, 2.31) (CIs estimated) | – | – | – | – | – | – |
| Grimwood 200820 | – | – | Crude RR = 1.30 (0.93, 1.82) | – | – | – | Crude RR = 1.57 (0.83, 2.96) | – | – | – |
| Kristensen 20099 | – | – | Crude OR = 1.10 (0.80, 1.50) | – | – | – | – | – | – | – |
| Law 200421 | – | – | OR = 1.91 (1.10, 3.31) (logistic regression) | – | – | OR = 2.76 (1.51, 5.03) (logistic regression) | – | OR = 1.71 (0.97, 3.00) (logistic regression) | OR = 1.69 (0.93, 3.10) (logistic regression) | – |
| Liese 200325 | – | – | OR = 8.7 (2.6, 29.1) (multivariate logistic regression) | – | OR = 3.9 (1.4, 11.2) (multivariate logistic regression) | – | – | – | – | – |
| Nielsen 200323 | – | – | – | – | – | OR = 1.10 (0.92, 1.35) (multivariate logistic regression) | – | – | – | – |
| Rietveld 20066 | OR = 0.8 (0.8, 0.8) (univariable regression analysis) | – | OR = 1.4 (1.3, 1.5) (univariate regression analysis) | – | – | – | – | – | – | – |
| Rossi 200724 | – | – | OR = 0.98 (0.66, 1.47) [calculated from female OR 1.02 (0.68, 1.52)] | – | – | – | – | OR = 0.81 (0.54, 1.21) (bivariate analysis) | – | – |
Assessment of inputs to model
In the original HTA report, two RCTs12,13 were used for establishing the relative risk of hospitalisation in children given palivizumab compared with those without. No additional RCTs of palivizumab were found for this update.
There were a number of issues associated with the risk factor inputs to the model. Most of the studies were small and not powered to investigate subgroups, so had wide CIs. The quality of reporting was not always adequate, so it was difficult to determine whether the results were a fair representation or due to biases. Also, there was some difficulty with establishing correct comparators. The required comparator was children hospitalised with RSV who did not have the attribute. For some factors this was straightforward, such as males versus females hospitalised with RSV. Other studies compared, for example, hospitalised males with non-hospitalised males with RSV infection. The results for studies could not be used unless the required comparator was available. Another issue was that some of the studies presented only regression results adjusted for confounding factors whereas other presented raw data. We have used unadjusted results by preference where available. If they were not available, this is shown in Table 5. The definitions of CLD, CHD and other risk factors were not reported in most included studies so may have varied between studies.
Chapter 5 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
No systematic review of cost-effectiveness studies was appropriate for this report as there are no models available investigating the listed subgroups.
Independent economic assessment
Methods
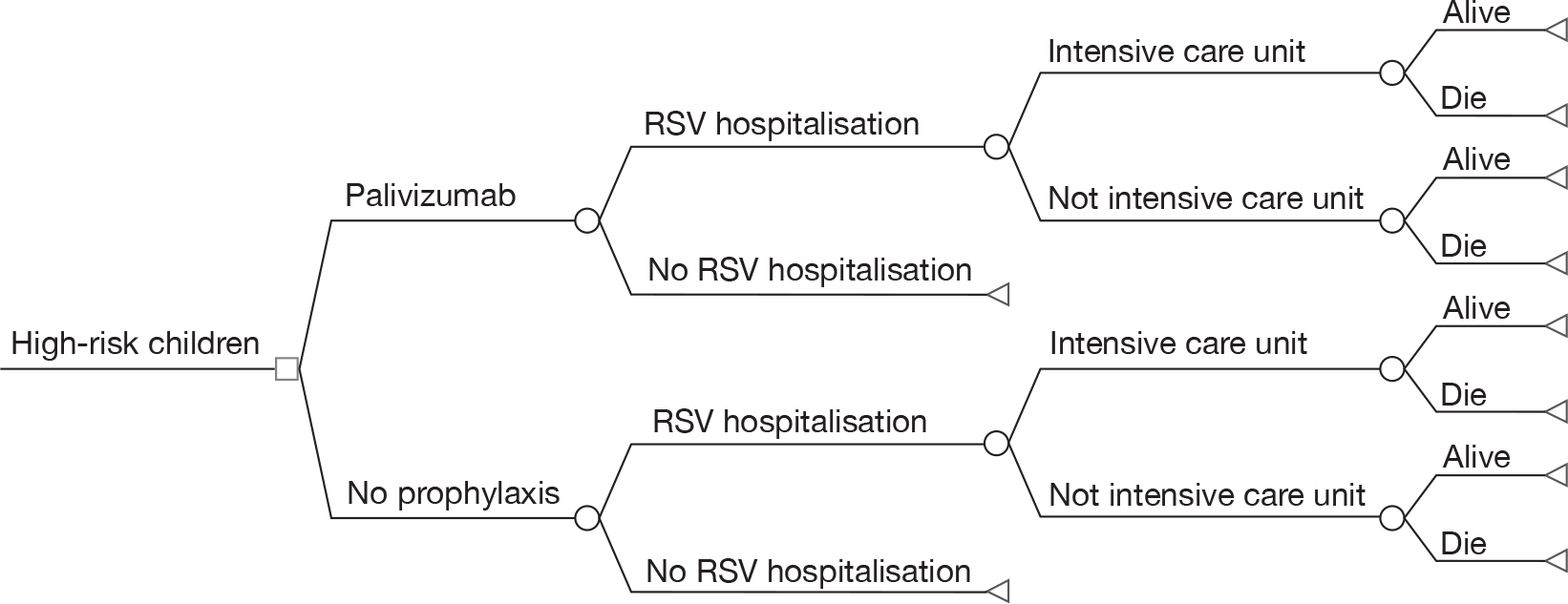
To estimate the cost-effectiveness of immunoprophylaxis of RSV using palivizumab for different subgroups of children who are at high risk of serious morbidity from RSV infection, the base-case decision tree model developed in the original HTA journal publication1 is used. The model structure is shown in Figure 2. All costs are presented in 2006 UK pounds sterling (£). Both costs and benefits are discounted at 3.5%. A time horizon of lifetime is used to take into account the impact of palivizumab on long-term morbidity and mortality from RSV infection. As a large number of subgroup analyses were involved, only the NHS perspective is adopted in this report. The detailed description of the model can be found in the HTA journal publication. 1
FIGURE 2.
Model structure for palivizumab versus no prophylaxis.
As the best summary estimate for policy-makers is currently considered to be the average ICER from the probabilistic sensitivity analysis (PSA), the results of cost-effectiveness subgroup analysis in this report are expressed as the mean ICER from PSA, where models are run for 5000 simulations for each of the combined risk factors.
Cost-effectiveness for different risk groups
Because the parameters required by the economic model have been updated since the search was made for the original HTA journal publication,1 and more risk factors have been added, the cost-effectiveness for different risk groups has been re-analysed/extended for children without CLD/CHD, or children with CLD or CHD. All results for such subgroup analyses presented in this report overwrite the subgroup analysis results carried out in the original HTA journal publication. 1 In this report, comprehensive subgroup analyses were carried out in four categories:
-
children without CLD/CHD
-
children with CLD
-
children with acyanotic CHD
-
children with cyanotic CHD.
In each category, the cost-effectiveness for 64 different combinations of risk factors was derived and is presented. Each combination of risk factors contained 63 subgroups, cross-tabulated by GA and AGE. In total, 256 combinations of risk factors (corresponding to 16,128 subgroups) were analysed.
Results
Risk factors
The studies listed in Table 5 were identified and used to derive the risk factors.
Hospitalisation at different ages
Only the study by Rietveld et al. 6 reported OR per month, which is required by the model. Therefore, an OR of 0.8 (95% CI 0.8 to 0.8) was used to estimate the risk of hospitalisation by age in the model.
Gestational age
Only the study by Carbonell-Estrany et al. 14 reported OR per week, which is required by the model. Therefore, an OR of 0.85 (95% CI 0.77 to 0.97) was used to estimate the risk of hospitalisation by GA in the model.
Gender
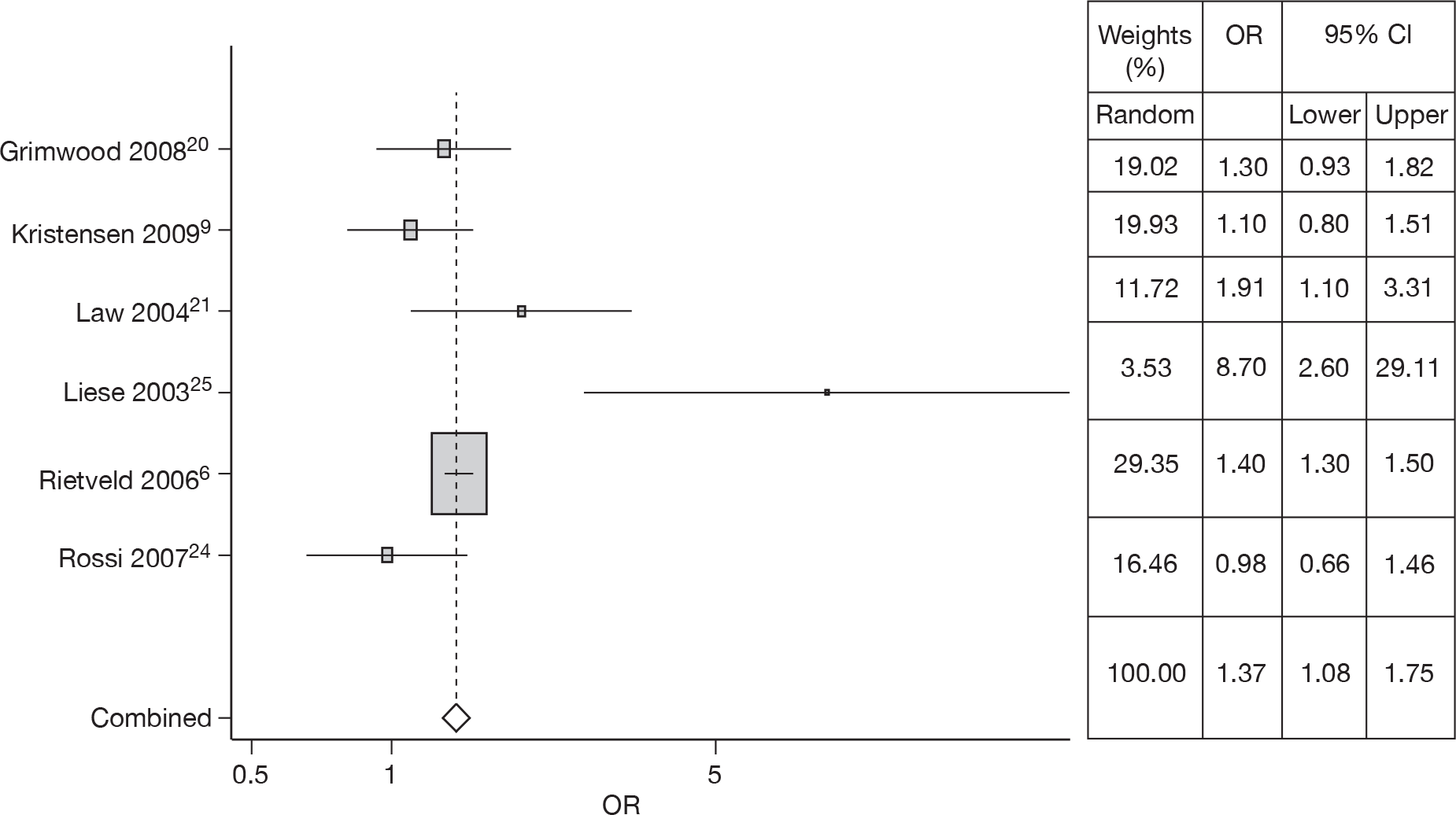
Six studies6,9,20,21,24,25 estimated the risk of gender in RSV hospitalisation; a meta-analysis was carried out, heterogeneity was observed (Q = 15.35, p = 0.009), and thus the OR of 1.37 (95% CI 1.08 to 1.75) from the random effects model was used in the model. The forest plot is shown in Figure 3.
FIGURE 3.
Combined OR for gender (male), random effects model.
Congenital heart disease
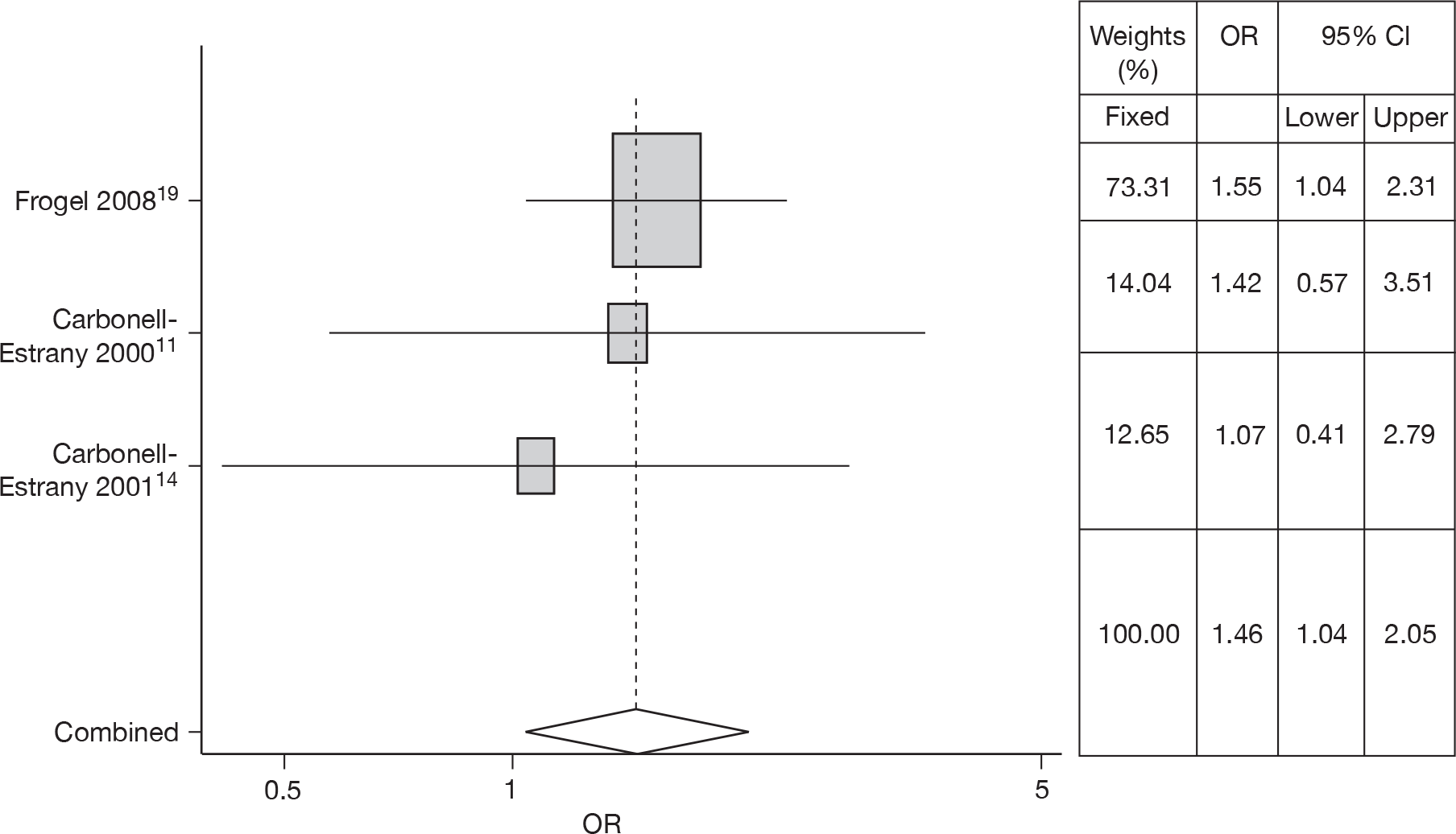
No studies were found that gave ORs and CIs for CHD. Three studies11,14,19 estimated the risk of CHD in RSV hospitalisation and provided sufficient results with which to estimate ORs and CIs. There was no heterogeneity (Q = 0.489, p = 0.783) so a fixed effects model was used. The meta-analysis gave an OR of 1.46 (95% CI 1.04 to 2.05). The forest plot is shown in Figure 4.
FIGURE 4.
Combined OR for CHD, fixed effects model.
Chronic lung disease
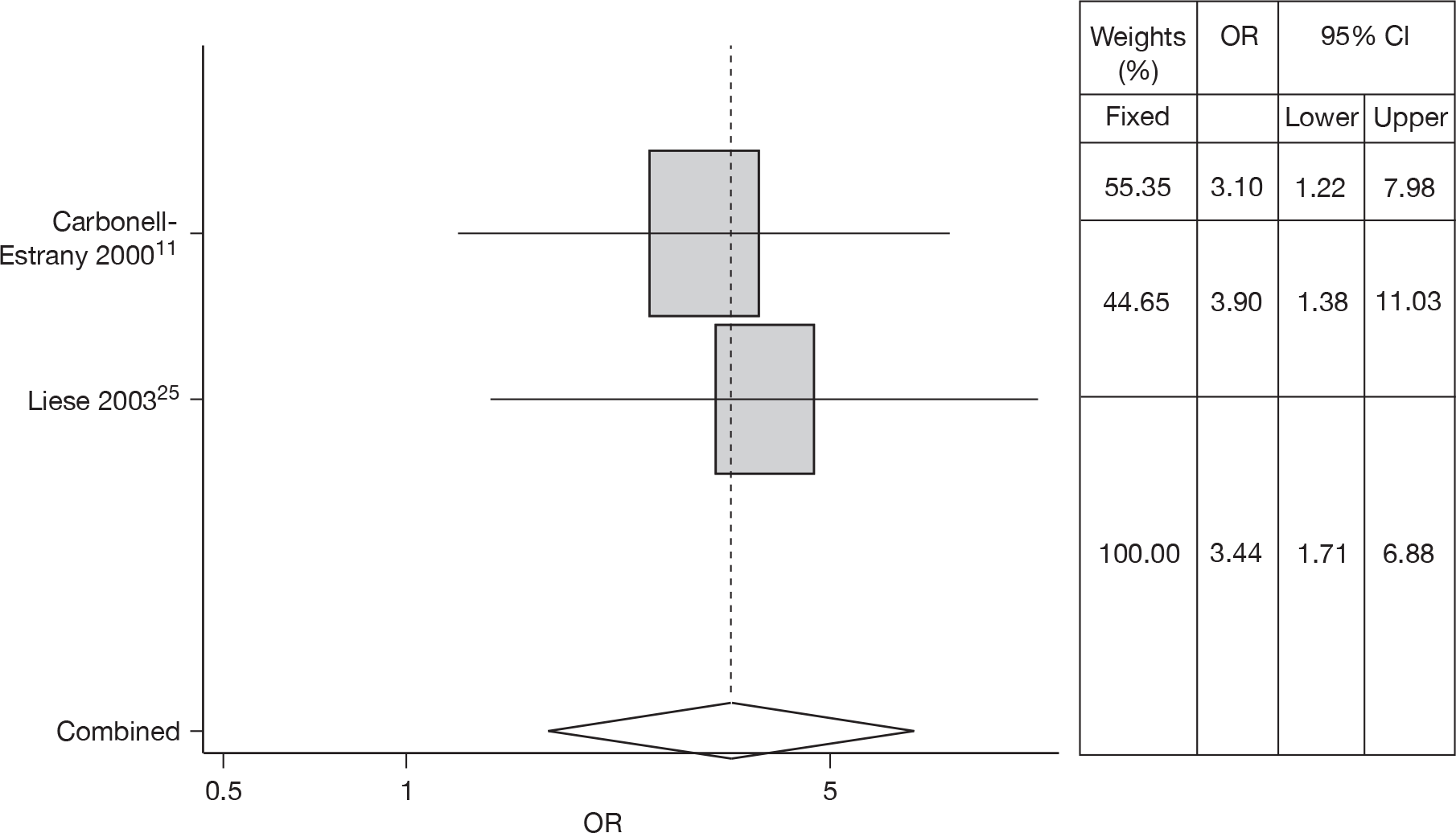
Two studies14,25 estimated the risk of CLD in RSV hospitalisation; a meta-analysis gave an OR of 3.44 (95% CI 1.71 to 6.88). There was no heterogeneity (Q = 0.104, p = 0.748) so a fixed effects model was used. The forest plot is shown in Figure 5.
FIGURE 5.
Combined OR for CLD, fixed effects model.
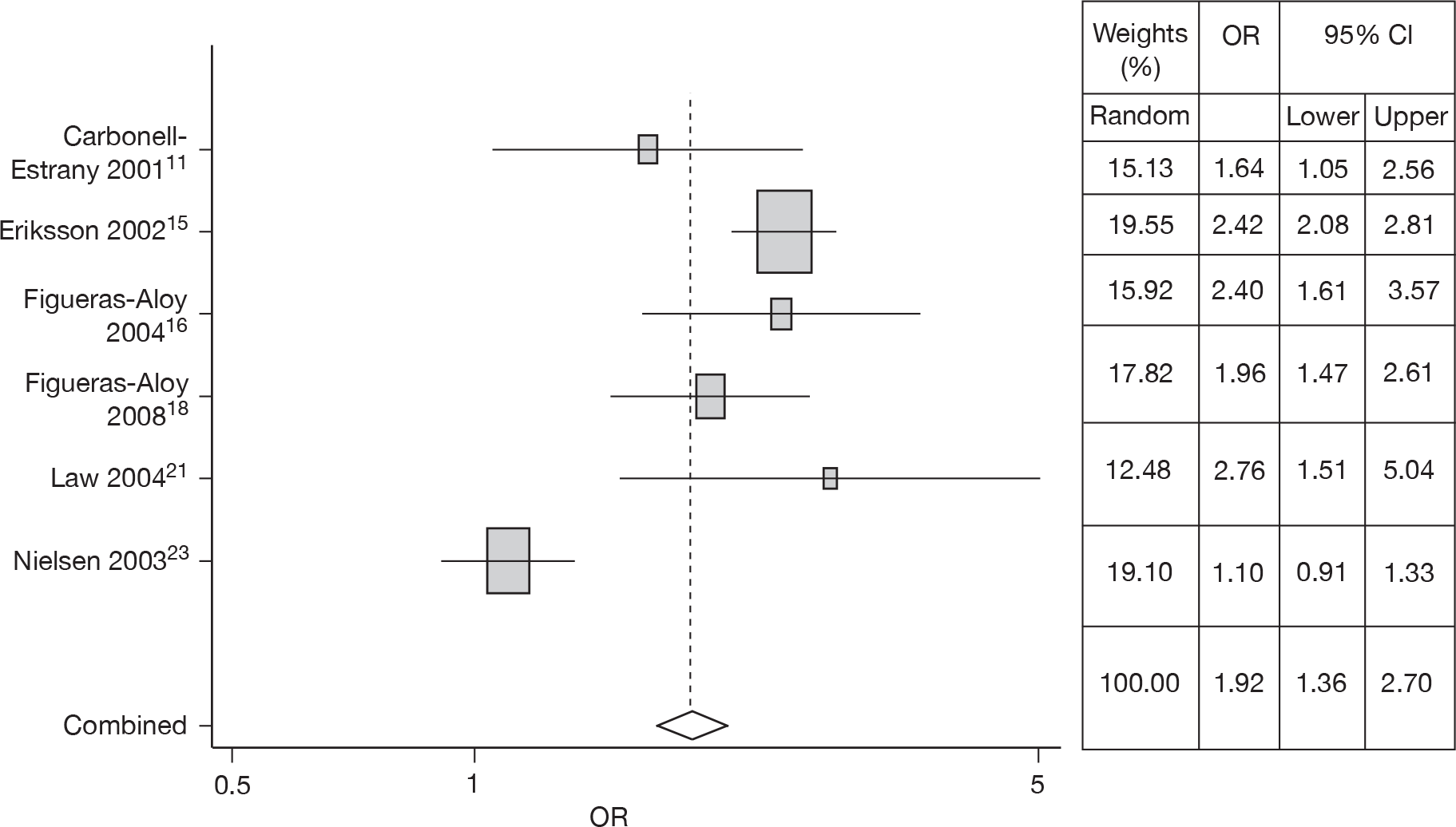
Siblings at school (SAS)
Six studies14–18,21,23 estimated the risk of SAS. Meta-analysis gave an OR of 1.92 (95% CI 1.36 to 2.70). The forest plot is shown in Figure 6. Heterogeneity was observed (Q = 44.26, p < 0.001).
FIGURE 6.
Combined OR for siblings at school, random effects model.
Multiple births
One study, by Grimwood et al.,20 reported OR of MBs for RSV hospitalisation. Therefore, an OR of 1.57 (95% CI 0.83 to 2.96) was used to estimate the risk of MBs in the model.
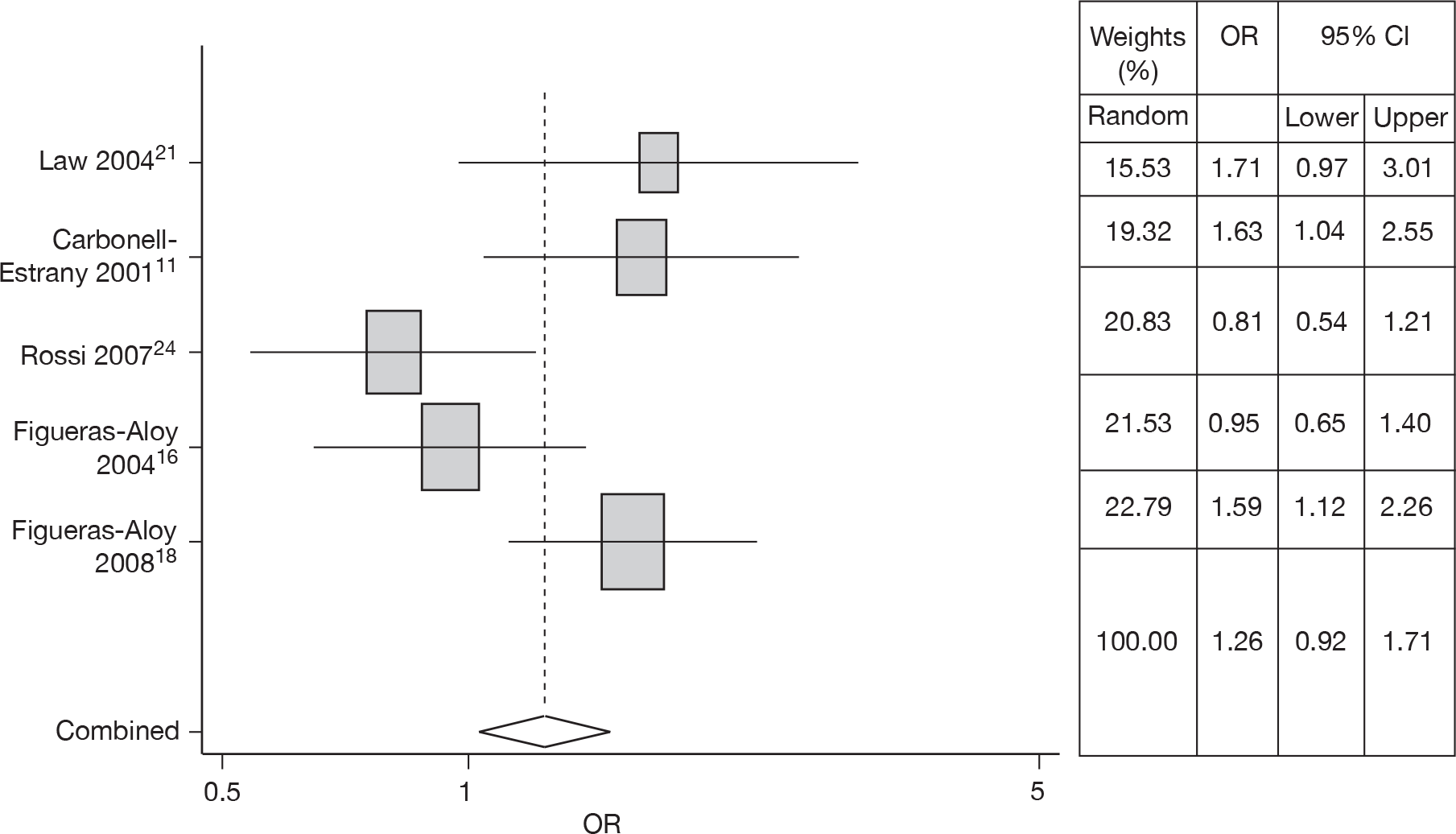
Smoking exposure
Five studies14,16–18,21,24 estimated the risk of SE in RSV hospitalisation. Meta-analysis gave an OR of 1.26 (95% CI 0.92 to 1.71). The forest plot is shown in Figure 7. Heterogeneity was observed (Q = 10.74, p = 0.03).
FIGURE 7.
Combined OR for SE, random effects model.
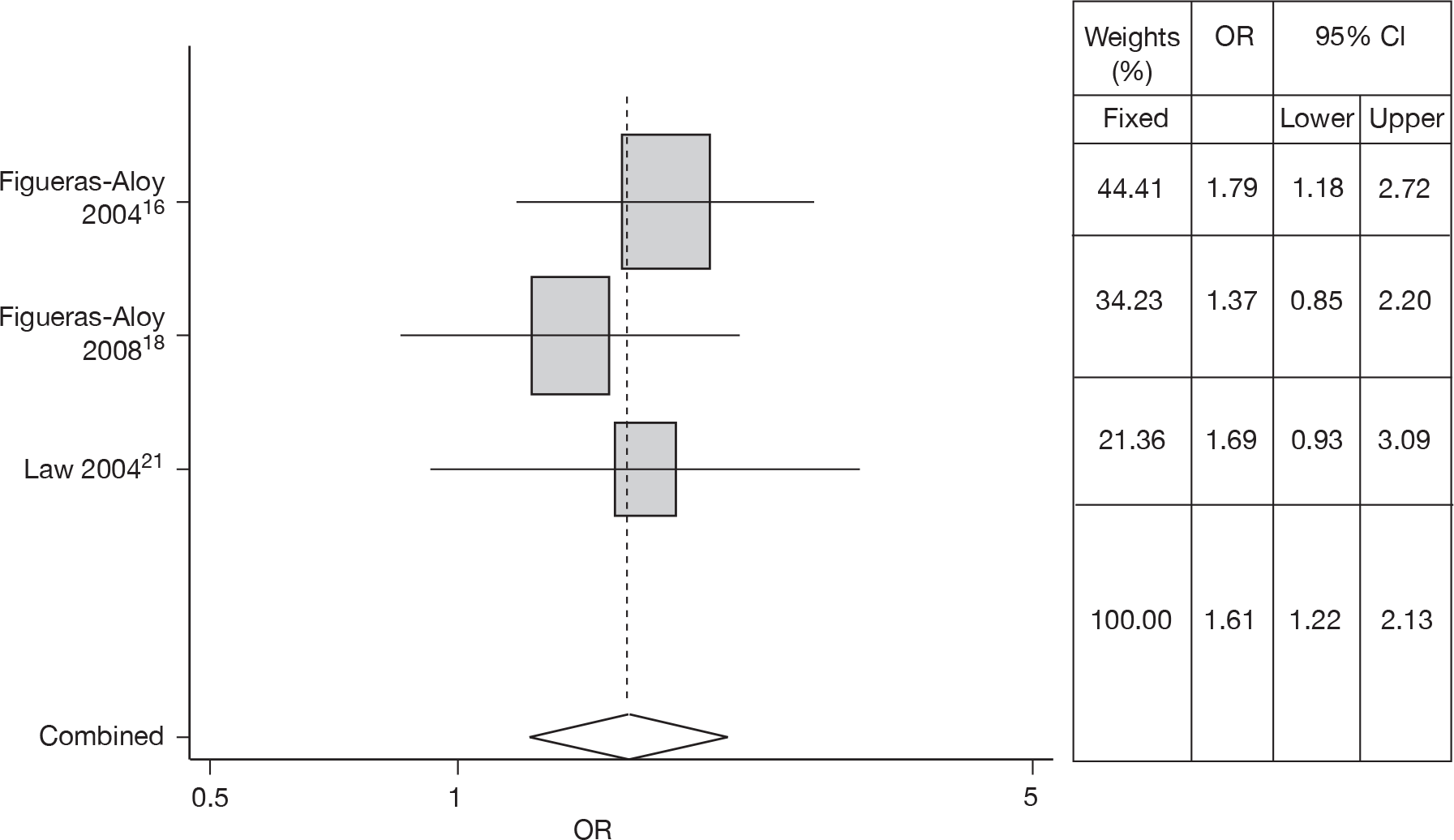
Overcrowding
Three studies16–18,21 estimated the risk of OC in RSV hospitalisation. There was no heterogeneity (Q = 0.715, p = 0.699) so a fixed effects model was used. Meta-analysis gave an OR of 1.61 (95% CI 1.22 to 2.13). The forest plot is shown in Figure 8.
FIGURE 8.
Combined OR for OC, fixed effects model.
Low parental education
The study by Figueras-Aloy et al. 16,17 reported OR of low PE in RSV hospitalisation. Therefore, an OR of 1.48 (95% CI 0.98 to 2.23) was used to estimate the risk of low PE in the model.
Other risk factors
Several studies were identified for the risk factors of age < 6 weeks at the start of the RSV season, lack of or minimal breastfeeding and family history of atopy. Some studies showed association between the risk factors and RSV hospitalisation; others did not. To avoid introducing unreliable parameters into the models, which might reduce the accuracy and precision of the model estimates to an unacceptable degree, we did not include the risk factors of age < 6 weeks at the start of the RSV season, lack of or minimal breastfeeding and family history of atopy in the model. Table 6 lists all parameters of the considered risk factors that were used in the subgroup analysis.
| Risk factors, OR (95% CI) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| AGE | GA | SEX (male) | CHD | CLD | SAS | MB | SE | OC | PE (≤ 12 years) |
| 0.80 (0.80 to 0.80) | 0.85 (0.77 to 0.97) | 1.37 (1.08 to 1.75) | 1.46 (1.04 to 2.05) | 3.44 (1.71 to 6.88) | 1.91 (1.36 to 2.70) | 1.57 (0.83 to 2.96) | 1.26 (0.92 to 1.71) | 1.61 (1.22 to 2.13) | 1.48 (0.98 to 2.23) |
Costs and outcomes
The costs considered in the model included medical costs, administration costs and hospitalisation costs. The detailed calculation of these costs can be found in the original HTA journal publication. 1 The costs and outcomes for children without CLD, children with CLD, children with acyanotic CHD and children with cyanotic CHD in the base-case model are listed in Tables 7–10, respectively. Note that we used a viral sharing scheme in the model, as described in the previous journal publication. 1 For all children, five doses were given. The assumption on vial use was that, among children with or without CLD, 38.7% used a 50-mg vial and 91.3% used a 100-mg vial. For children with CHD, 39.6% used a 50-mg vial, 100.0% used a 100-mg vial, and 3.8% used 200 mg (2 × 100-mg vials). These assumptions were made based on (1) 15 mg/kg weight and (2) the average weight reported in the trials.
| Parameters | Palivizumab | No prophylaxis | Cost difference | Outcome difference |
|---|---|---|---|---|
| Costs (£) | ||||
| Drug | 3437 | |||
| Drug administration (GP) | 21 | |||
| Drug administration (nurse) | 39 | |||
| Hospital | 67 | 301 | ||
| Total cost (NHS) | 3564 | 301 | 3263 | |
| Outcomes | ||||
| Discounting QALYs | 26.5163 | 26.5092 | 0.0072 | |
| Parameters | Palivizumab | No prophylaxis | Cost difference | Outcome difference |
|---|---|---|---|---|
| Costs (£) | ||||
| Drug | 3437 | |||
| Drug administration (GP) | 21 | |||
| Drug administration (nurse) | 39 | |||
| Hospital | 293 | 475 | ||
| Total cost (NHS) | 3790 | 475 | 3315 | |
| Outcomes | ||||
| Discounting QALYs | 26.4346 | 26.3826 | 0.0520 | |
| Parameters | Palivizumab | No prophylaxis | Cost difference | Outcome difference |
|---|---|---|---|---|
| Costs (£) | ||||
| Drug | 3714 | |||
| Drug administration (GP) | 21 | |||
| Drug administration (nurse) | 39 | |||
| Hospital | 359 | 647 | ||
| Total cost (NHS) | 4132 | 847 | 3285 | |
| Outcomes | ||||
| Discounting QALYs | 26.4187 | 26.3518 | 0.0670 | |
| Parameters | Palivizumab | No prophylaxis | Cost difference | Outcome difference |
|---|---|---|---|---|
| Costs (£) | ||||
| Drug | 3714 | |||
| Drug administration (GP) | 21 | |||
| Drug administration (nurse) | 39 | |||
| Hospital | 402 | 567 | ||
| Total cost (NHS) | 4176 | 567 | 3609 | |
| Outcomes | ||||
| Discounting QALYs | 26.4128 | 26.3902 | 0.0226 | |
Utilities
The study by Greenough et al. 26 assessed the health-related quality of life (HRQoL) for pre-term children at the age of 5 years using the Health Utilities Index (HUI). The HUI described a family of genetic health status and HRQoL measures. Parents were sent the HUI2/3 and asked to complete the 15 questions to reflect their child’s health over the previous 4 weeks. The HUI2 measured seven attributes of health status describing 24,000 unique health states, while HUI3 described 972,000 unique health states. The HUI2 was originally developed for paediatric application and clinical evaluation studies, whereas HUI3 was developed for use in adults and population surveys. The median HUI2 multiattribute utility function was 0.88 (range 0.16–1.00) in the RSV-proven children, while the median HUI2 multiattribute utility function was 0.95 (range 0.03–1.00) in the non-RSV children. The median HUI3 multiattribute scores were 0.93 (range –0.05–1.00) for RSV-proven children and 0.97 (range –0.32–1.00) for non-RSV children. These utility values are used in the model for children with or without CLD and are listed in Table 11. As mentioned above, the utility estimate was made by asking parents (rather than children themselves) to complete the questions to reflect their child’s health. This might introduce a bias in the utility estimate. However, because the utility estimates for children with RSV hospitalisation and without RSV hospitalisation were evaluated in the same way (i.e. parents completed the questionnaire), the effect of utility estimate made by parents for a child on the overall results was likely to be small and conclusions unaltered. Utility data for children and adults with CHD are lacking. The economic evaluation study by Yount et al. 27 extrapolated data from congestive heart failure to the CHD population and used a utility of 0.71 for children with CHD. The same utility values for children with CHD as those for children with or without CLD were used here.
Parameter values and their distributions
The parameter values and their distributions used in the subgroup analysis are shown in Tables 12–15 for children without CLD/CHD, children with CLD, children with acyanotic CHD and children with cyanotic CHD, respectively.
| Parameter | Expected value | α | β |
|---|---|---|---|
| Probability of RSV hospitalisation (no prophylaxis) | 0.081 | 344.384 | 3934.08 |
| Mortality rate of RSV hospitalisation | 0.0043 | 17.221 | 3982.226 |
| Utility of RSV hospitalisation | 0.880 | 702.101 | 95.770 |
| Utility of non-RSV hospitalisation | 0.950 | 976.417 | 51.397 |
| Probability of ICU | 0.107 | 26.270 | 219.218 |
| Parameter | Expected value | a | b |
|---|---|---|---|
| Dose of palivizumab | 5 | 4 | 6 |
| Period of morbidity due to RSV | 5 | 2 | 8 |
| Parameter | Mean | SD2 |
|---|---|---|
| Log relative risk of RSV hospitalisation | –1.5404 | 0.0771 |
| Length of ICU stay | 1.370 | 0.259 |
| Length of general ward stay | 6.470 | 0.644 |
| Life expectancy | 77.800 | 11.830 |
| Parameter | Expected value | α | β |
|---|---|---|---|
| Probability of RSV hospitalisation (no prophylaxis) | 0.128 | 573.974 | 3900.294 |
| Utility of RSV hospitalisation | 0.880 | 702.101 | 95.770 |
| Utility of non-RSV hospitalisation | 0.950 | 976.417 | 51.397 |
| Probability of ICU | 0.107 | 26.270 | 219.218 |
| Parameter | Expected value | a | b |
|---|---|---|---|
| Dose of palivizumab | 5 | 4 | 6 |
| Period of morbidity due to RSV | 5 | 2 | 8 |
| Mortality rate of RSV hospitalisation | 0.040 | 0.030 | 0.050 |
| Parameter | Mean | SD2 |
|---|---|---|
| Log relative risk of RSV hospitalisation | –0.4826 | 0.0253 |
| Length of ICU stay | 1.370 | 0.259 |
| Length of general ward stay | 6.470 | 0.644 |
| Life expectancy | 77.800 | 11.830 |
| Parameter | Expected value | α | β |
|---|---|---|---|
| Probability of RSV hospitalisation (no prophylaxis) | 0.097 | 21.895 | 203.830 |
| Mortality rate of RSV hospitalisation | 0.0372 | 8.012 | 207.920 |
| Utility of RSV hospitalisation | 0.880 | 702.101 | 95.770 |
| Utility of non-RSV hospitalisation | 0.950 | 976.417 | 51.397 |
| Probability of ICU | 0.387 | 123.685 | 195.916 |
| Parameter | Expected value | a | b |
|---|---|---|---|
| Dose of palivizumab | 5 | 4 | 6 |
| Period of morbidity due to RSV | 5 | 2 | 8 |
| Parameter | Mean | SD2 |
|---|---|---|
| Log relative risk of RSV hospitalisation | –0.859 | 0.088 |
| Length of ICU stay | 6.140 | 1.009 |
| Length of general ward stay | 6.250 | 0.635 |
| Life expectancy | 77.110 | 11.830 |
| Parameter | Expected value | α | β |
|---|---|---|---|
| Probability of RSV hospitalisation (no prophylaxis) | 0.097 | 21.895 | 203.830 |
| Mortality rate of RSV hospitalisation | 0.0372 | 8.012 | 207.920 |
| Utility of RSV hospitalisation | 0.880 | 702.101 | 95.770 |
| Utility of non-RSV hospitalisation | 0.950 | 976.417 | 51.397 |
| Probability of ICU | 0.387 | 123.685 | 195.916 |
| Parameter | Expected value | a | b |
|---|---|---|---|
| Dose of palivizumab | 5 | 4 | 6 |
| Period of morbidity due to RSV | 5 | 2 | 8 |
| Parameter | Mean | SD2 |
|---|---|---|
| Log relative risk of RSV hospitalisation | –0.340 | 0.084 |
| Length of ICU stay | 6.140 | 1.009 |
| Length of general ward stay | 6.250 | 0.635 |
| Life expectancy | 77.110 | 11.830 |
Results of cost-effectiveness subgroup analysis
Detailed numerical results of the outcomes of cost-effectiveness for children without CLD/CHD, children with CLD, children with acyanotic CHD and children with cyanotic CHD alone, plus other risk factors are given below. Detailed numerical results are listed in Tables 16–19.
| AGE (months) | GA (weeks) | ||||||
|---|---|---|---|---|---|---|---|
| ≤ 24 | > 24–26 | > 26–28 | > 28–30 | > 30–32 | > 32–34 | ≥ 35 | |
| Risk factors GA, AGE | |||||||
| < 1.5 | 78 | 104 | 140 | 192 | 264 | 365 | 831 |
| 1.5–3 | 104 | 142 | 196 | 267 | 370 | 497 | 1147 |
| 3–6 | 200 | 276 | 370 | 515 | 708 | 965 | 2234 |
| 6–9 | 383 | 520 | 728 | 984 | 1372 | 1872 | 4379 |
| 9–12 | 752 | 1001 | 1371 | 1959 | 2640 | 3684 | 8420 |
| 12–15 | 1443 | 1956 | 2725 | 3852 | 5234 | 7164 | 16,437 |
| 15–18 | 2777 | 3900 | 5298 | 7326 | 10,248 | 14,121 | 32,663 |
| 18–21 | 5395 | 7497 | 10,309 | 14,173 | 19,697 | 27,134 | 62,539 |
| 21–24 | 10,578 | 14,665 | 20,117 | 28,330 | 38,777 | 54,436 | 124,424 |
| Risk factors GA, AGE, plus PE ≤ 12 years | |||||||
| < 1.5 | 56 | 73 | 98 | 132 | 180 | 244 | 559 |
| 1.5–3 | 74 | 95 | 133 | 182 | 250 | 345 | 776 |
| 3–6 | 137 | 184 | 251 | 348 | 478 | 661 | 1505 |
| 6–9 | 258 | 355 | 488 | 662 | 920 | 1280 | 2980 |
| 9–12 | 501 | 694 | 951 | 1296 | 1813 | 2517 | 5777 |
| 12–15 | 964 | 1316 | 1848 | 2571 | 3503 | 4866 | 11,093 |
| 15–18 | 1867 | 2592 | 3572 | 4957 | 6815 | 9442 | 21,764 |
| 18–21 | 3678 | 5095 | 7096 | 9735 | 13,485 | 18,498 | 42,614 |
| 21–24 | 7127 | 9827 | 13,615 | 18,944 | 26,094 | 36,267 | 83,156 |
| Risk factors GA, AGE, plus OC | |||||||
| < 1.5 | 51 | 66 | 91 | 122 | 166 | 230 | 517 |
| 1.5–3 | 67 | 91 | 122 | 167 | 229 | 314 | 721 |
| 3–6 | 126 | 171 | 236 | 325 | 448 | 599 | 1425 |
| 6–9 | 235 | 329 | 453 | 619 | 836 | 1181 | 2737 |
| 9–12 | 463 | 640 | 859 | 1199 | 1660 | 2268 | 5354 |
| 12–15 | 893 | 1228 | 1665 | 2350 | 3267 | 4443 | 10,262 |
| 15–18 | 1739 | 2386 | 3280 | 4578 | 6289 | 8627 | 20,090 |
| 18–21 | 3342 | 4641 | 6406 | 8924 | 12,401 | 17,062 | 39,345 |
| 21–24 | 6518 | 9135 | 12,736 | 17,382 | 23,827 | 33,731 | 77,069 |
| Risk factors GA, AGE, plus SE | |||||||
| < 1.5 | 63 | 84 | 112 | 156 | 208 | 289 | 653 |
| 1.5–3 | 86 | 115 | 159 | 214 | 295 | 402 | 918 |
| 3–6 | 157 | 216 | 299 | 408 | 555 | 778 | 1760 |
| 6–9 | 304 | 423 | 571 | 793 | 1101 | 1504 | 3489 |
| 9–12 | 589 | 792 | 1103 | 1539 | 2119 | 3039 | 6752 |
| 12–15 | 1122 | 1559 | 2159 | 2922 | 4098 | 5809 | 13,137 |
| 15–18 | 2182 | 3052 | 4336 | 5844 | 8164 | 11,009 | 26,309 |
| 18–21 | 4358 | 5978 | 8224 | 11,469 | 15,958 | 22,160 | 50,304 |
| 21–24 | 8498 | 11,730 | 16,319 | 22,063 | 30,773 | 42,314 | 96,398 |
| Risk factors GA, AGE, plus MB | |||||||
| < 1.5 | 52 | 70 | 92 | 124 | 171 | 232 | 524 |
| 1.5–3 | 70 | 93 | 128 | 170 | 235 | 318 | 735 |
| 3–6 | 130 | 174 | 238 | 327 | 453 | 632 | 1431 |
| 6–9 | 244 | 340 | 465 | 630 | 888 | 1215 | 2752 |
| 9–12 | 480 | 669 | 901 | 1225 | 1664 | 2361 | 5532 |
| 12–15 | 903 | 1244 | 1744 | 2390 | 3288 | 4594 | 10,542 |
| 15–18 | 1771 | 2466 | 3364 | 4637 | 6456 | 8811 | 20,352 |
| 18–21 | 3444 | 4758 | 6588 | 9101 | 12,582 | 17,622 | 40,237 |
| 21–24 | 6632 | 9313 | 12,932 | 18,053 | 24,955 | 34,215 | 77,992 |
| Risk factors GA, AGE, plus SEX (male) | |||||||
| < 1.5 | 58 | 78 | 108 | 142 | 196 | 265 | 603 |
| 1.5–3 | 79 | 105 | 141 | 196 | 266 | 369 | 845 |
| 3–6 | 147 | 200 | 276 | 373 | 515 | 730 | 1643 |
| 6–9 | 275 | 381 | 531 | 722 | 1013 | 1385 | 3191 |
| 9–12 | 540 | 733 | 1008 | 1408 | 1956 | 2657 | 6296 |
| 12–15 | 1055 | 1460 | 1986 | 2765 | 3820 | 5312 | 12,220 |
| 15–18 | 2020 | 2779 | 3865 | 5379 | 7550 | 10,085 | 23,610 |
| 18–21 | 3866 | 5411 | 7545 | 10,589 | 14,230 | 20,087 | 46,417 |
| 21–24 | 7678 | 10,694 | 14,926 | 20,588 | 28,282 | 39,169 | 90,652 |
| Risk factors GA, AGE, plus SAS | |||||||
| < 1.5 | 43 | 59 | 78 | 103 | 140 | 192 | 441 |
| 1.5–3 | 59 | 77 | 105 | 140 | 190 | 266 | 604 |
| 3–6 | 106 | 146 | 199 | 272 | 374 | 522 | 1161 |
| 6–9 | 203 | 279 | 385 | 517 | 718 | 1003 | 2260 |
| 9–12 | 384 | 543 | 721 | 1003 | 1400 | 1943 | 4406 |
| 12–15 | 741 | 1043 | 1402 | 1955 | 2744 | 3771 | 8780 |
| 15–18 | 1450 | 2010 | 2780 | 3851 | 5340 | 7369 | 17,162 |
| 18–21 | 2860 | 3854 | 5524 | 7566 | 10,420 | 14,534 | 33,362 |
| 21–24 | 5589 | 7604 | 10,581 | 15,009 | 20,458 | 28,088 | 64,781 |
| Risk factors GA, AGE, plus OC, PE ≤ 12 years | |||||||
| < 1.5 | 36 | 47 | 64 | 85 | 114 | 156 | 359 |
| 1.5–3 | 48 | 63 | 84 | 115 | 155 | 216 | 490 |
| 3–6 | 87 | 119 | 160 | 219 | 294 | 412 | 940 |
| 6–9 | 162 | 223 | 308 | 425 | 578 | 796 | 1832 |
| 9–12 | 314 | 430 | 580 | 818 | 1126 | 1593 | 3587 |
| 12–15 | 594 | 837 | 1131 | 1586 | 2184 | 3008 | 7037 |
| 15–18 | 1156 | 1637 | 2226 | 3073 | 4273 | 5828 | 13,540 |
| 18–21 | 2272 | 3168 | 4360 | 6039 | 8353 | 11,509 | 25,969 |
| 21–24 | 4491 | 6125 | 8542 | 11,747 | 16,214 | 22,474 | 51,712 |
| Risk factors GA, AGE, plus SE, PE ≤ 12 years | |||||||
| < 1.5 | 45 | 59 | 79 | 105 | 143 | 199 | 441 |
| 1.5–3 | 60 | 81 | 107 | 145 | 201 | 269 | 618 |
| 3–6 | 109 | 148 | 202 | 280 | 379 | 520 | 1185 |
| 6–9 | 206 | 282 | 386 | 544 | 739 | 1020 | 2365 |
| 9–12 | 403 | 556 | 750 | 1039 | 1450 | 1970 | 4574 |
| 12–15 | 771 | 1070 | 1440 | 2030 | 2782 | 3887 | 9029 |
| 15–18 | 1515 | 2079 | 2854 | 3915 | 5505 | 7612 | 17,155 |
| 18–21 | 2916 | 3996 | 5466 | 7663 | 10,623 | 14,445 | 34,348 |
| 21–24 | 5780 | 7825 | 10,819 | 15,063 | 20,897 | 28,700 | 65,220 |
| Risk factors GA, AGE, plus SE, OC | |||||||
| < 1.5 | 42 | 55 | 72 | 98 | 136 | 183 | 405 |
| 1.5–3 | 55 | 73 | 99 | 137 | 184 | 251 | 570 |
| 3–6 | 102 | 135 | 187 | 254 | 347 | 479 | 1104 |
| 6–9 | 190 | 262 | 360 | 500 | 681 | 937 | 2157 |
| 9–12 | 364 | 501 | 689 | 976 | 1304 | 1831 | 4227 |
| 12–15 | 704 | 980 | 1350 | 1893 | 2588 | 3585 | 8148 |
| 15–18 | 1354 | 1855 | 2642 | 3608 | 4976 | 6915 | 15,597 |
| 18–21 | 2678 | 3724 | 5148 | 7085 | 9738 | 13,903 | 31,177 |
| 21–24 | 5171 | 7265 | 9987 | 13,794 | 19,171 | 26,365 | 60,631 |
| Risk factors GA, AGE, plus MB, PE ≤ 12 years | |||||||
| < 1.5 | 37 | 49 | 67 | 86 | 118 | 160 | 359 |
| 1.5–3 | 50 | 66 | 87 | 119 | 161 | 225 | 498 |
| 3–6 | 89 | 120 | 166 | 226 | 307 | 421 | 969 |
| 6–9 | 170 | 230 | 315 | 427 | 593 | 820 | 1874 |
| 9–12 | 318 | 445 | 596 | 830 | 1152 | 1581 | 3684 |
| 12–15 | 621 | 856 | 1188 | 1626 | 2223 | 3087 | 7116 |
| 15–18 | 1195 | 1679 | 2277 | 3146 | 4310 | 5956 | 14,119 |
| 18–21 | 2351 | 3210 | 4448 | 6147 | 8450 | 11,975 | 27,003 |
| 21–24 | 4580 | 6348 | 8819 | 12,047 | 16,565 | 23,112 | 53,447 |
| Risk factors GA, AGE, plus SEX (male) | |||||||
| < 1.5 | 34 | 45 | 60 | 81 | 107 | 146 | 335 |
| 1.5–3 | 46 | 61 | 80 | 110 | 148 | 204 | 457 |
| 3–6 | 82 | 114 | 152 | 210 | 283 | 386 | 903 |
| 6–9 | 154 | 211 | 294 | 400 | 554 | 759 | |
| 9–12 | 302 | 411 | 572 | 768 | 1065 | 1495 | 3394 |
| 12–15 | 562 | 787 | 1084 | 1501 | 2058 | 2873 | 6467 |
| 15–18 | 1098 | 1521 | 2122 | 2899 | 4094 | 5520 | 13,045 |
| 18–21 | 2154 | 2994 | 4092 | 5813 | 7916 | 10,848 | 24,742 |
| 21–24 | 4220 | 5823 | 7972 | 10,967 | 15,332 | 21,137 | 48,664 |
| Risk factors GA, AGE, plus MB, SE | |||||||
| < 1.5 | 42 | 56 | 75 | 99 | 137 | 187 | 430 |
| 1.5–3 | 57 | 76 | 101 | 139 | 191 | 254 | 582 |
| 3–6 | 104 | 142 | 192 | 264 | 363 | 499 | 1129 |
| 6–9 | 195 | 270 | 368 | 504 | 691 | 957 | 2185 |
| 9–12 | 374 | 506 | 717 | 968 | 1365 | 1880 | 4268 |
| 12–15 | 715 | 1007 | 1359 | 1891 | 2695 | 3672 | 8349 |
| 15–18 | 1394 | 1953 | 2658 | 3695 | 5188 | 6983 | 16,374 |
| 18–21 | 2763 | 3789 | 5290 | 7250 | 10,058 | 13,796 | 31,814 |
| 21–24 | 5390 | 7452 | 10,122 | 13,944 | 19,885 | 26,950 | 62,317 |
| Risk factors GA, AGE, plus SEX (male), PE ≤ 12 years | |||||||
| < 1.5 | 41 | 54 | 75 | 100 | 135 | 182 | 414 |
| 1.5–3 | 55 | 74 | 99 | 139 | 180 | 253 | 565 |
| 3–6 | 101 | 136 | 187 | 258 | 353 | 485 | 1100 |
| 6–9 | 193 | 257 | 364 | 493 | 690 | 931 | 2128 |
| 9–12 | 368 | 508 | 687 | 952 | 1309 | 1833 | 4152 |
| 12–15 | 695 | 980 | 1318 | 1878 | 2578 | 3555 | 8313 |
| 15–18 | 1414 | 1880 | 2636 | 3656 | 5059 | 6907 | 16,050 |
| 18–21 | 2689 | 3697 | 5172 | 7067 | 9830 | 13,465 | 30,882 |
| 21–24 | 5180 | 7231 | 10,027 | 13,745 | 19,082 | 26,551 | 61,279 |
| Risk factors GA, AGE, plus SEX (male), OC | |||||||
| < 1.5 | 38 | 51 | 69 | 89 | 124 | 169 | 387 |
| 1.5–3 | 52 | 69 | 91 | 128 | 168 | 234 | 526 |
| 3–6 | 94 | 127 | 174 | 238 | 328 | 442 | 1022 |
| 6–9 | 177 | 238 | 331 | 452 | 630 | 865 | 2008 |
| 9–12 | 337 | 459 | 638 | 880 | 1214 | 1683 | 3891 |
| 12–15 | 658 | 884 | 1227 | 1721 | 2338 | 3275 | 7535 |
| 15–18 | 1273 | 1725 | 2373 | 3333 | 4615 | 6382 | 14,877 |
| 18–21 | 2453 | 3421 | 4724 | 6485 | 9111 | 12,432 | 28,831 |
| 21–24 | 4830 | 6626 | 9260 | 12,808 | 17,678 | 24,225 | 55,848 |
| Risk factors GA, AGE, plus SEX (male), SE | |||||||
| < 1.5 | 48 | 63 | 85 | 113 | 156 | 213 | 493 |
| 1.5–3 | 64 | 87 | 115 | 157 | 214 | 296 | 664 |
| 3–6 | 118 | 162 | 218 | 301 | 418 | 572 | 1310 |
| 6–9 | 219 | 306 | 418 | 576 | 802 | 1103 | 2475 |
| 9–12 | 430 | 592 | 806 | 1142 | 1554 | 2121 | 4943 |
| 12–15 | 824 | 1144 | 1588 | 2181 | 3011 | 4135 | 9836 |
| 15–18 | 1609 | 2248 | 3063 | 4281 | 5811 | 8140 | 18,858 |
| 18–21 | 3134 | 4346 | 5971 | 8206 | 11,640 | 16,117 | 37,082 |
| 21–24 | 6109 | 8547 | 11,685 | 15,968 | 22,380 | 31,581 | 72,274 |
| Risk factors GA, AGE, plus SEX (male), MB | |||||||
| < 1.5 | 40 | 53 | 70 | 94 | 128 | 169 | 394 |
| 1.5–3 | 53 | 70 | 94 | 130 | 173 | 239 | 540 |
| 3–6 | 96 | 132 | 177 | 244 | 330 | 459 | 1058 |
| 6–9 | 178 | 244 | 336 | 462 | 635 | 889 | 2034 |
| 9–12 | 346 | 480 | 654 | 917 | 1243 | 1745 | 3965 |
| 1288 | 668 | 923 | 1288 | 1756 | 2452 | 3342 | 7740 |
| 24253377 | 1312 | 1780 | 2425 | 3377 | 4700 | 6507 | 15,079 |
| 18–21 | 2535 | 3478 | 4896 | 6624 | 9375 | 12,838 | 28,966 |
| 21–24 | 4918 | 6710 | 9439 | 12,819 | 17,920 | 24,539 | 58,181 |
| Risk factors GA, AGE, plus SAS, PE ≤ 12 years | |||||||
| < 1.5 | 31 | 42 | 54 | 71 | 98 | 132 | 299 |
| 1.5–3 | 42 | 55 | 72 | 99 | 134 | 186 | 417 |
| 3–6 | 75 | 99 | 134 | 187 | 257 | 348 | 807 |
| 6–9 | 140 | 187 | 259 | 353 | 493 | 675 | 1567 |
| 9–12 | 266 | 364 | 507 | 681 | 930 | 1318 | 2952 |
| 12–15 | 515 | 707 | 980 | 1328 | 1863 | 2534 | 5880 |
| 15–18 | 1005 | 1383 | 1876 | 2596 | 3575 | 4911 | 11,278 |
| 18–21 | 1940 | 2672 | 3694 | 5099 | 6985 | 9669 | 22,195 |
| 21–24 | 3780 | 5103 | 7159 | 9934 | 13,633 | 19,106 | 43,645 |
| Risk factors GA, AGE, plus SAS, OC | |||||||
| < 1.5 | 30 | 38 | 51 | 67 | 90 | 122 | 278 |
| 1.5–3 | 39 | 51 | 68 | 93 | 124 | 169 | 383 |
| 3–6 | 69 | 94 | 127 | 172 | 235 | 318 | 732 |
| 6–9 | 130 | 177 | 241 | 328 | 456 | 615 | 1416 |
| 9–12 | 243 | 334 | 465 | 644 | 888 | 1219 | 2786 |
| 12–15 | 468 | 647 | 888 | 1242 | 1697 | 2360 | 5470 |
| 15–18 | 916 | 1255 | 1718 | 2408 | 3341 | 4672 | 10,756 |
| 18–21 | 1755 | 2452 | 3403 | 4744 | 6472 | 8970 | 20,696 |
| 21–24 | 3431 | 4838 | 6650 | 9248 | 12,663 | 17,337 | 40,181 |
| Risk factors GA, AGE, plus SAS, SE | |||||||
| < 1.5 | 37 | 47 | 64 | 84 | 112 | 156 | 346 |
| 1.5–3 | 47 | 63 | 86 | 116 | 157 | 213 | 487 |
| 3–6 | 86 | 118 | 156 | 219 | 299 | 413 | 932 |
| 6–9 | 160 | 219 | 303 | 416 | 562 | 775 | 1817 |
| 9–12 | 314 | 425 | 581 | 813 | 1110 | 1513 | 3501 |
| 12–15 | 597 | 824 | 1143 | 1586 | 2155 | 3025 | 6803 |
| 15–18 | 1157 | 1597 | 2217 | 3064 | 4276 | 5912 | 13,425 |
| 18–21 | 2258 | 3106 | 4312 | 5888 | 8244 | 11,361 | 25,945 |
| 21–24 | 4446 | 6111 | 8373 | 11,525 | 15,943 | 22,374 | 51,355 |
| Risk factors GA, AGE, plus SAS, MB | |||||||
| < 1.5 | 31 | 39 | 52 | 70 | 91 | 126 | 285 |
| 1.5–3 | 40 | 52 | 70 | 94 | 127 | 172 | 392 |
| 3–6 | 70 | 94 | 129 | 177 | 239 | 329 | 752 |
| 6–9 | 133 | 181 | 245 | 328 | 461 | 641 | 1432 |
| 9–12 | 247 | 344 | 466 | 649 | 884 | 1256 | 2872 |
| 12–15 | 484 | 674 | 905 | 1255 | 1760 | 2465 | 5557 |
| 15–18 | 929 | 1295 | 1807 | 2455 | 3441 | 4738 | 10,835 |
| 18–21 | 1829 | 2491 | 3423 | 4774 | 6653 | 9145 | 21,118 |
| 21–24 | 3476 | 4874 | 6780 | 9360 | 12,957 | 18,070 | 41,265 |
| Risk factors GA, AGE, plus SAS, SEX (male) | |||||||
| < 1.5 | 34 | 44 | 58 | 77 | 106 | 139 | 320 |
| 1.5–3 | 44 | 59 | 79 | 107 | 143 | 198 | 449 |
| 3–6 | 81 | 108 | 145 | 202 | 269 | 382 | 863 |
| 6–9 | 150 | 206 | 281 | 394 | 525 | 742 | 1701 |
| 9–12 | 286 | 387 | 541 | 747 | 1036 | 1408 | 3235 |
| 12–15 | 545 | 751 | 1025 | 1437 | 2009 | 2767 | 6340 |
| 15–18 | 1084 | 1475 | 2062 | 2806 | 3869 | 5379 | 12,487 |
| 18–21 | 2100 | 2894 | 3972 | 5453 | 7795 | 10,586 | 24,415 |
| 21–24 | 4002 | 5534 | 7770 | 10,797 | 14,993 | 20,601 | 47,849 |
| Risk factors GA, AGE, plus SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 31 | 39 | 52 | 69 | 93 | 125 | 284 |
| 1.5–3 | 39 | 52 | 70 | 94 | 128 | 174 | 393 |
| 3–6 | 70 | 93 | 128 | 177 | 237 | 330 | 758 |
| 6–9 | 133 | 180 | 239 | 335 | 462 | 640 | 1434 |
| 9–12 | 246 | 345 | 467 | 656 | 880 | 1229 | 2880 |
| 12–15 | 481 | 658 | 915 | 1265 | 1738 | 2408 | 5583 |
| 15–18 | 929 | 1299 | 1798 | 2458 | 3409 | 4717 | 10,838 |
| 18–21 | 1802 | 2512 | 3469 | 4737 | 6633 | 9317 | 21,432 |
| 21–24 | 3589 | 4827 | 6770 | 9373 | 13,139 | 17,483 | 40,239 |
| Risk factors GA, AGE, plus MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 26 | 33 | 42 | 56 | 76 | 103 | 231 |
| 1.5–3 | 33 | 44 | 58 | 76 | 102 | 138 | 313 |
| 3–6 | 59 | 78 | 103 | 143 | 196 | 273 | 611 |
| 6–9 | 106 | 142 | 194 | 269 | 376 | 511 | 1167 |
| 9–12 | 204 | 273 | 383 | 533 | 731 | 992 | 277 |
| 12–15 | 385 | 531 | 736 | 1023 | 1377 | 1926 | 4510 |
| 15–18 | 746 | 1017 | 1439 | 1964 | 2713 | 3761 | 8654 |
| 18–21 | 1426 | 1981 | 2740 | 3841 | 5333 | 7224 | 16,866 |
| 21–24 | 2807 | 3887 | 5449 | 7661 | 10,376 | 14,381 | 33,105 |
| Risk factors GA, AGE, plus MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 31 | 40 | 53 | 71 | 93 | 128 | 288 |
| 1.5–3 | 40 | 54 | 72 | 96 | 132 | 180 | 405 |
| 3–6 | 74 | 98 | 131 | 177 | 247 | 339 | 769 |
| 6–9 | 135 | 180 | 255 | 347 | 469 | 638 | 1513 |
| 9–12 | 256 | 356 | 483 | 658 | 925 | 1281 | 2948 |
| 12–15 | 500 | 676 | 946 | 1280 | 1809 | 2465 | 5728 |
| 15–18 | 977 | 1298 | 1844 | 2574 | 3510 | 4900 | 11,091 |
| 18–21 | 1872 | 2581 | 3488 | 4937 | 6906 | 9318 | 21,707 |
| 21–24 | 3643 | 5075 | 6763 | 9540 | 13,498 | 18,451 | 42,669 |
| Risk factors GA, AGE, plus MB, SE, OC | |||||||
| < 1.5 | 29 | 37 | 49 | 65 | 88 | 118 | 269 |
| 1.5–3 | 38 | 50 | 67 | 88 | 118 | 162 | 371 |
| 3–6 | 67 | 90 | 121 | 165 | 224 | 314 | 710 |
| 6–9 | 123 | 170 | 231 | 317 | 435 | 601 | 1369 |
| 9–12 | 238 | 319 | 442 | 611 | 860 | 1154 | 2704 |
| 12–15 | 455 | 620 | 859 | 1186 | 1687 | 2250 | 5171 |
| 15–18 | 892 | 1198 | 1680 | 2318 | 3231 | 4446 | 9963 |
| 18–21 | 1688 | 2328 | 3283 | 4525 | 6293 | 8580 | 19,504 |
| 21–24 | 3305 | 4619 | 6294 | 8818 | 12,275 | 16,936 | 39,178 |
| Risk factors GA, AGE, plus MB, PE ≤ 12 years | |||||||
| < 1.5 | 29 | 37 | 48 | 64 | 86 | 116 | 263 |
| 1.5–3 | 37 | 49 | 65 | 87 | 118 | 157 | 363 |
| 3–6 | 67 | 86 | 119 | 163 | 222 | 299 | 686 |
| 6–9 | 123 | 161 | 224 | 313 | 423 | 578 | 1349 |
| 9–12 | 230 | 314 | 434 | 599 | 814 | 1117 | 2609 |
| 12–15 | 445 | 604 | 849 | 1151 | 1591 | 2223 | 5075 |
| 15–18 | 853 | 1180 | 1650 | 2223 | 3131 | 4307 | 9963 |
| 18–21 | 1652 | 2271 | 3199 | 4474 | 6077 | 8427 | 19,593 |
| 21–24 | 3286 | 4485 | 6197 | 8450 | 11,968 | 16,420 | 37,390 |
| Risk factors GA, AGE, plus SEX (male), SE, PE ≤ 12 years | |||||||
| < 1.5 | 35 | 45 | 60 | 79 | 106 | 149 | 334 |
| 1.5–3 | 46 | 60 | 80 | 108 | 146 | 200 | 459 |
| 3–6 | 82 | 110 | 148 | 205 | 283 | 383 | 880 |
| 6–9 | 154 | 211 | 287 | 391 | 548 | 739 | 1689 |
| 9–12 | 292 | 406 | 561 | 765 | 1048 | 1442 | 3322 |
| 12–15 | 559 | 762 | 1070 | 1485 | 2037 | 2814 | 6484 |
| 15–18 | 1087 | 1498 | 2098 | 2834 | 4021 | 5589 | 12,750 |
| 18–21 | 2107 | 2952 | 4095 | 5638 | 7897 | 10,884 | 25,124 |
| 21–24 | 4104 | 5757 | 7922 | 11,014 | 15,059 | 21,098 | 47,913 |
| Risk factors GA, AGE, plus SEX (male), SE, OC | |||||||
| < 1.5 | 32 | 42 | 55 | 74 | 99 | 133 | 306 |
| 1.5–3 | 42 | 55 | 75 | 100 | 137 | 185 | 418 |
| 3–6 | 75 | 101 | 139 | 189 | 260 | 354 | 814 |
| 6–9 | 140 | 193 | 256 | 359 | 494 | 687 | 1587 |
| 9–12 | 272 | 371 | 508 | 697 | 954 | 1343 | 3114 |
| 12–15 | 512 | 712 | 975 | 1371 | 1873 | 2585 | 5870 |
| 15–18 | 1009 | 1397 | 1909 | 2644 | 3660 | 5019 | 11,692 |
| 18–21 | 1967 | 2735 | 3798 | 5170 | 6976 | 9963 | 22,879 |
| 21–24 | 3829 | 5302 | 7238 | 10,015 | 13,969 | 19,709 | 44,533 |
| Risk factors GA, AGE, plus SEX (male), MB, PE ≤ 12 years | |||||||
| < 1.5 | 29 | 37 | 50 | 67 | 87 | 121 | 268 |
| 1.5–3 | 37 | 50 | 66 | 88 | 121 | 163 | 370 |
| 3–6 | 66 | 90 | 123 | 167 | 225 | 314 | 715 |
| 6–9 | 126 | 169 | 234 | 311 | 434 | 602 | 1387 |
| 9–12 | 238 | 321 | 444 | 609 | 852 | 1166 | 2682 |
| 12–15 | 452 | 630 | 846 | 1170 | 1650 | 2274 | 5174 |
| 15–18 | 866 | 1220 | 1669 | 2325 | 3170 | 4366 | 10,032 |
| 18–21 | 1708 | 2346 | 3230 | 4423 | 6214 | 8668 | 19,694 |
| 21–24 | 3238 | 4716 | 6497 | 9007 | 12,334 | 16,731 | 39,106 |
| Risk factors GA, AGE, plus SEX (male), MB, OC | |||||||
| < 1.5 | 27 | 35 | 46 | 61 | 82 | 110 | 245 |
| 1.5–3 | 36 | 47 | 62 | 82 | 111 | 151 | 339 |
| 3–6 | 62 | 82 | 111 | 152 | 208 | 289 | 653 |
| 6–9 | 115 | 155 | 215 | 292 | 403 | 549 | 1278 |
| 9–12 | 215 | 297 | 406 | 562 | 785 | 1072 | 2503 |
| 12–15 | 425 | 574 | 781 | 1084 | 1500 | 2051 | 4884 |
| 15–18 | 821 | 1121 | 1539 | 2116 | 2975 | 4082 | 9362 |
| 18–21 | 1568 | 2169 | 3025 | 4136 | 5700 | 7909 | 18,155 |
| 21–24 | 3047 | 4197 | 5814 | 8233 | 11,202 | 15,502 | 35,680 |
| Risk factors GA, AGE, plus SEX (male), MB, SE | |||||||
| < 1.5 | 33 | 44 | 56 | 76 | 102 | 135 | 314 |
| 1.5–3 | 43 | 57 | 75 | 103 | 139 | 194 | 420 |
| 3–6 | 78 | 104 | 142 | 195 | 266 | 366 | 848 |
| 6–9 | 146 | 197 | 268 | 372 | 507 | 703 | 1625 |
| 9–12 | 276 | 379 | 522 | 724 | 991 | 1375 | 3143 |
| 12–15 | 529 | 728 | 997 | 1396 | 1921 | 2625 | 6295 |
| 15–18 | 1036 | 1429 | 2001 | 2710 | 3731 | 5139 | 11,884 |
| 18–21 | 2003 | 2798 | 3767 | 5257 | 7387 | 10,204 | 23,456 |
| 21–24 | 3914 | 5529 | 7424 | 10,402 | 14,207 | 19,872 | 45,432 |
| Risk factors GA, AGE, plus SAS, OC, PE ≤ 12 years | |||||||
| < 1.5 | 23 | 28 | 37 | 48 | 63 | 85 | 193 |
| 1.5–3 | 29 | 36 | 48 | 64 | 85 | 117 | 261 |
| 3–6 | 48 | 64 | 86 | 118 | 161 | 219 | 498 |
| 6–9 | 90 | 121 | 165 | 226 | 310 | 419 | 982 |
| 9–12 | 167 | 231 | 314 | 429 | 599 | 823 | 1905 |
| 12–15 | 317 | 440 | 602 | 840 | 1146 | 1607 | 3685 |
| 15–18 | 618 | 848 | 1168 | 1634 | 2266 | 3110 | 7278 |
| 18–21 | 1207 | 1642 | 2267 | 3141 | 4401 | 6038 | 14,051 |
| 21–24 | 2311 | 3244 | 4457 | 6136 | 8545 | 11,783 | 27,242 |
| Risk factors GA, AGE, plus SAS, SE, PE ≤ 12 years | |||||||
| < 1.5 | 27 | 34 | 44 | 59 | 81 | 107 | 237 |
| 1.5–3 | 34 | 45 | 60 | 79 | 106 | 148 | 333 |
| 3–6 | 60 | 82 | 108 | 151 | 200 | 275 | 646 |
| 6–9 | 111 | 152 | 208 | 287 | 392 | 534 | 1231 |
| 9–12 | 210 | 286 | 397 | 547 | 754 | 1040 | 2382 |
| 12–15 | 404 | 554 | 771 | 1081 | 1486 | 2042 | 4668 |
| 15–18 | 738 | 1079 | 1516 | 2084 | 2815 | 3931 | 9118 |
| 18–21 | 1515 | 2128 | 2916 | 4081 | 5543 | 7711 | 17,652 |
| 21–24 | 2971 | 4119 | 5627 | 7857 | 10,901 | 15,265 | 34,420 |
| Risk factors GA, AGE, plus SAS, SE, OC | |||||||
| < 1.5 | 24 | 32 | 42 | 54 | 73 | 97 | 222 |
| 1.5–3 | 33 | 42 | 55 | 74 | 100 | 137 | 302 |
| 3–6 | 56 | 75 | 101 | 136 | 187 | 259 | 584 |
| 6–9 | 103 | 140 | 192 | 259 | 363 | 499 | 1119 |
| 9–12 | 195 | 264 | 370 | 512 | 698 | 976 | 2198 |
| 12–15 | 373 | 516 | 697 | 974 | 1349 | 1884 | 4309 |
| 15–18 | 727 | 989 | 1372 | 1909 | 2614 | 3592 | 8402 |
| 18–21 | 1390 | 1935 | 2674 | 3684 | 5206 | 7186 | 16,160 |
| 21–24 | 2754 | 3752 | 5158 | 7461 | 10,121 | 13,943 | 32,491 |
| Risk factors GA, AGE, plus SAS, MB, PE ≤ 12 years | |||||||
| < 1.5 | 22 | 29 | 37 | 49 | 66 | 88 | 193 |
| 1.5–3 | 29 | 38 | 50 | 66 | 88 | 118 | 265 |
| 3–6 | 50 | 68 | 90 | 121 | 164 | 229 | 512 |
| 6–9 | 93 | 122 | 170 | 232 | 315 | 430 | 982 |
| 9–12 | 171 | 232 | 319 | 452 | 604 | 839 | 1939 |
| 12–15 | 330 | 453 | 615 | 860 | 1180 | 1625 | 3686 |
| 15–18 | 634 | 871 | 1215 | 1689 | 2300 | 3167 | 7254 |
| 18–21 | 1205 | 1691 | 2342 | 3273 | 4534 | 6311 | 14,293 |
| 21–24 | 2393 | 3295 | 4554 | 6263 | 8688 | 12,136 | 27,931 |
| Risk factors GA, AGE, plus SAS, MB, OC | |||||||
| < 1.5 | 21 | 27 | 36 | 45 | 61 | 81 | 180 |
| 1.5–3 | 27 | 35 | 46 | 60 | 83 | 111 | 246 |
| 3–6 | 46 | 62 | 83 | 114 | 151 | 204 | 483 |
| 6–9 | 84 | 113 | 155 | 215 | 289 | 404 | 930 |
| 9–12 | 157 | 214 | 298 | 406 | 568 | 763 | 1751 |
| 12–15 | 303 | 416 | 565 | 788 | 1069 | 1495 | 3418 |
| 15–18 | 582 | 811 | 1096 | 1530 | 2071 | 2917 | 6762 |
| 18–21 | 1132 | 1529 | 2193 | 3037 | 4137 | 5598 | 13,239 |
| 21–24 | 2169 | 3030 | 4142 | 5823 | 8082 | 10,955 | 26.042 |
| Risk factors GA, AGE, plus SAS, MB, SE | |||||||
| < 1.5 | 25 | 33 | 43 | 57 | 75 | 103 | 230 |
| 1.5–3 | 33 | 43 | 56 | 76 | 100 | 137 | 314 |
| 3–6 | 58 | 79 | 105 | 142 | 192 | 268 | 606 |
| 6–9 | 105 | 145 | 195 | 269 | 365 | 511 | 1169 |
| 9–12 | 199 | 271 | 376 | 518 | 710 | 977 | 2258 |
| 12–15 | 386 | 541 | 730 | 992 | 1383 | 1927 | 4389 |
| 15–18 | 740 | 1009 | 1426 | 1911 | 2696 | 3743 | 8593 |
| 18–21 | 1451 | 1994 | 2750 | 3847 | 5216 | 7156 | 16,888 |
| 21–24 | 2798 | 3892 | 5488 | 7261 | 10,192 | 14,140 | 32,426 |
| Risk factors GA, AGE, plus SAS, SEX (male), PE ≤ 12 years | |||||||
| < 1.5 | 25 | 32 | 42 | 54 | 74 | 100 | 224 |
| 1.5–3 | 32 | 42 | 55 | 74 | 99 | 132 | 301 |
| 3–6 | 57 | 75 | 101 | 136 | 185 | 257 | 596 |
| 6–9 | 103 | 141 | 191 | 262 | 355 | 498 | 1126 |
| 9–12 | 195 | 271 | 370 | 503 | 702 | 956 | 2210 |
| 12–15 | 374 | 514 | 708 | 970 | 1350 | 1844 | 4324 |
| 15–18 | 732 | 987 | 1370 | 1902 | 2603 | 3682 | 8301 |
| 18–21 | 1407 | 1906 | 2648 | 3688 | 5138 | 7050 | 16,452 |
| 21–24 | 2751 | 3810 | 5272 | 7270 | 9915 | 13,822 | 31,581 |
| Risk factors GA, AGE, plus SAS, SEX (males), OC | |||||||
| < 1.5 | 24 | 30 | 40 | 51 | 69 | 93 | 203 |
| 1.5–3 | 30 | 39 | 51 | 69 | 92 | 125 | 281 |
| 3–6 | 52 | 70 | 95 | 127 | 172 | 237 | 536 |
| 6–9 | 94 | 130 | 173 | 242 | 337 | 459 | 1054 |
| 9–12 | 178 | 245 | 335 | 474 | 642 | 874 | 2045 |
| 12–15 | 346 | 467 | 646 | 901 | 1255 | 1703 | 3893 |
| 15–18 | 665 | 910 | 1258 | 1730 | 2421 | 3389 | 7747 |
| 18–21 | 1286 | 1790 | 2462 | 3373 | 4679 | 6525 | 15,110 |
| 21–24 | 2520 | 3509 | 4793 | 6757 | 9241 | 12,680 | 30,006 |
| Risk factors GA, AGE, plus SAS, SEX (male), SE | |||||||
| < 1.5 | 28 | 37 | 47 | 63 | 87 | 114 | 256 |
| 1.5–3 | 37 | 49 | 65 | 86 | 116 | 160 | 361 |
| 3–6 | 64 | 88 | 119 | 164 | 220 | 297 | 695 |
| 6–9 | 120 | 165 | 223 | 301 | 432 | 573 | 1359 |
| 9–12 | 225 | 311 | 434 | 601 | 829 | 1120 | 2574 |
| 12–15 | 446 | 607 | 830 | 1146 | 1580 | 2225 | 5085 |
| 15–18 | 858 | 1170 | 1611 | 2240 | 3070 | 4269 | 9910 |
| 18–21 | 1672 | 2278 | 3180 | 4422 | 6096 | 8457 | 19,408 |
| 21–24 | 3229 | 4456 | 6110 | 8522 | 11,754 | 16,276 | 37,137 |
| Risk factors GA, AGE, plus SAS, SEX (male), MB | |||||||
| < 1.5 | 24 | 31 | 40 | 52 | 70 | 93 | 209 |
| 1.5–3 | 31 | 39 | 53 | 69 | 95 | 127 | 286 |
| 3–6 | 53 | 73 | 97 | 131 | 177 | 245 | 555 |
| 6–9 | 99 | 134 | 181 | 250 | 341 | 468 | 1070 |
| 9–12 | 185 | 257 | 347 | 479 | 660 | 902 | 2082 |
| 12–15 | 347 | 484 | 671 | 922 | 1277 | 1751 | 4027 |
| 15–18 | 664 | 955 | 1303 | 1774 | 2462 | 3435 | 8003 |
| 18–21 | 1318 | 1832 | 2551 | 3541 | 4861 | 6684 | 15,371 |
| 21–24 | 2592 | 3581 | 4870 | 6750 | 9317 | 13,004 | 30,146 |
| Risk factors GA, AGE, plus MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 22 | 27 | 36 | 47 | 62 | 81 | 185 |
| 1.5–3 | 28 | 35 | 47 | 62 | 83 | 112 | 255 |
| 3–6 | 48 | 62 | 85 | 115 | 156 | 214 | 483 |
| 6–9 | 86 | 116 | 159 | 215 | 296 | 411 | 931 |
| 9–12 | 162 | 222 | 304 | 413 | 578 | 797 | 1827 |
| 12–15 | 308 | 424 | 578 | 799 | 1116 | 1538 | 3554 |
| 15–18 | 605 | 814 | 1125 | 1559 | 2157 | 2958 | 6899 |
| 18–21 | 1154 | 1579 | 2214 | 3056 | 4212 | 5750 | 12,407 |
| 21–24 | 2265 | 3120 | 4271 | 5971 | 8331 | 11,439 | 26,311 |
| Risk factors GA, AGE, plus SEX (male), SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 24 | 30 | 40 | 52 | 71 | 92 | 210 |
| 1.5–3 | 31 | 40 | 53 | 70 | 95 | 129 | 286 |
| 3–6 | 53 | 71 | 95 | 128 | 180 | 240 | 561 |
| 6–9 | 98 | 131 | 184 | 249 | 342 | 463 | 1072 |
| 9–12 | 182 | 250 | 347 | 467 | 652 | 915 | 2046 |
| 12–15 | 353 | 484 | 661 | 930 | 1291 | 1735 | 4064 |
| 15–18 | 675 | 929 | 1283 | 1776 | 2476 | 3479 | 7796 |
| 18–21 | 1330 | 1843 | 2489 | 3478 | 4850 | 6733 | 15,208 |
| 21–24 | 2585 | 3594 | 4975 | 6714 | 9429 | 12,999 | 30,536 |
| Risk factors GA, AGE, plus SEX (male), MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 21 | 26 | 33 | 43 | 57 | 76 | 168 |
| 1.5–3 | 26 | 34 | 43 | 57 | 77 | 104 | 236 |
| 3–6 | 44 | 59 | 79 | 107 | 143 | 196 | 449 |
| 6–9 | 80 | 111 | 144 | 196 | 275 | 370 | 870 |
| 9–12 | 151 | 205 | 275 | 387 | 531 | 733 | 1684 |
| 12–15 | 285 | 389 | 534 | 755 | 1030 | 1401 | 3228 |
| 15–18 | 550 | 760 | 1032 | 1421 | 1982 | 2736 | 6398 |
| 18–21 | 1075 | 1460 | 2035 | 2815 | 3928 | 5386 | 12,215 |
| 21–24 | 2085 | 2853 | 3940 | 5482 | 7623 | 10,677 | 24,025 |
| Risk factors GA, AGE, plus SEX (male), MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 25 | 31 | 40 | 53 | 70 | 96 | 214 |
| 1.5–3 | 32 | 41 | 54 | 72 | 96 | 129 | 291 |
| 3–6 | 56 | 74 | 99 | 133 | 184 | 251 | 577 |
| 6–9 | 100 | 133 | 187 | 251 | 344 | 478 | 1099 |
| 9–12 | 188 | 255 | 353 | 490 | 668 | 926 | 2123 |
| 12–15 | 357 | 506 | 679 | 939 | 1302 | 1783 | 4146 |
| 15–18 | 696 | 964 | 1343 | 1837 | 2546 | 3501 | 8038 |
| 18–21 | 1370 | 1862 | 2589 | 3588 | 5050 | 6957 | 15,604 |
| 21–24 | 2652 | 3593 | 5023 | 7069 | 9748 | 13,752 | 31,161 |
| Risk factors GA, AGE, plus SEX (male), MB, SE, OC | |||||||
| < 1.5 | 23 | 29 | 38 | 49 | 66 | 87 | 197 |
| 1.5–3 | 29 | 38 | 50 | 65 | 90 | 119 | 273 |
| 3–6 | 51 | 67 | 89 | 124 | 166 | 233 | 522 |
| 6–9 | 93 | 126 | 170 | 232 | 321 | 434 | 996 |
| 9–12 | 171 | 235 | 329 | 452 | 618 | 854 | 1962 |
| 12–15 | 330 | 466 | 629 | 871 | 1203 | 1662 | 3723 |
| 15–18 | 645 | 891 | 1208 | 1716 | 2324 | 3226 | 7410 |
| 18–21 | 1264 | 1687 | 2399 | 3360 | 4604 | 6348 | 14,575 |
| 21–24 | 2463 | 3379 | 4651 | 6349 | 8925 | 12,290 | 28,297 |
| Risk factors GA, AGE, plus SAS, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 19 | 24 | 31 | 40 | 51 | 69 | 153 |
| 1.5–3 | 24 | 30 | 40 | 52 | 69 | 92 | 209 |
| 3–6 | 40 | 52 | 69 | 94 | 127 | 178 | 407 |
| 6–9 | 72 | 97 | 132 | 178 | 247 | 336 | 768 |
| 9–12 | 132 | 182 | 246 | 342 | 470 | 653 | 1496 |
| 12–15 | 258 | 344 | 481 | 660 | 945 | 1261 | 2903 |
| 15–18 | 491 | 670 | 940 | 1298 | 1771 | 2461 | 5644 |
| 18–21 | 941 | 1327 | 1842 | 2494 | 3458 | 4767 | 10,946 |
| 21–24 | 1872 | 2541 | 3556 | 4937 | 6707 | 9275 | 21,672 |
| Risk factors GA, AGE, plus SAS, MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 17 | 20 | 26 | 33 | 43 | 56 | 122 |
| 1.5–3 | 20 | 26 | 34 | 44 | 57 | 77 | 171 |
| 3–6 | 34 | 45 | 58 | 78 | 103 | 145 | 321 |
| 6–9 | 59 | 80 | 107 | 145 | 202 | 272 | 622 |
| 9–12 | 111 | 146 | 202 | 276 | 376 | 529 | 1197 |
| 12–15 | 203 | 285 | 383 | 528 | 735 | 1010 | 2365 |
| 15–18 | 401 | 543 | 736 | 1033 | 1425 | 1947 | 4600 |
| 18–21 | 776 | 1037 | 1446 | 2022 | 2829 | 3848 | 8930 |
| 21–24 | 1492 | 2056 | 2861 | 3865 | 5526 | 7581 | 17,138 |
| Risk factors GA, AGE, plus SAS, MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 20 | 24 | 30 | 40 | 52 | 70 | 155 |
| 1.5–3 | 24 | 31 | 40 | 54 | 71 | 97 | 214 |
| 3–6 | 41 | 55 | 73 | 97 | 134 | 176 | 407 |
| 6–9 | 74 | 98 | 135 | 182 | 254 | 347 | 806 |
| 9–12 | 139 | 192 | 250 | 355 | 480 | 655 | 1531 |
| 12–15 | 265 | 356 | 494 | 672 | 928 | 1311 | 2912 |
| 15–18 | 501 | 703 | 960 | 1307 | 1831 | 2534 | 5765 |
| 18–21 | 973 | 1351 | 1869 | 2566 | 3546 | 4961 | 11,259 |
| 21–24 | 1899 | 2651 | 3662 | 5084 | 6958 | 9548 | 21,768 |
| Risk factors GA, AGE, plus SAS, MB, SE, OC | |||||||
| < 1.5 | 18 | 23 | 29 | 38 | 49 | 67 | 142 |
| 1.5–3 | 23 | 29 | 38 | 49 | 65 | 89 | 200 |
| 3–6 | 39 | 50 | 67 | 91 | 121 | 165 | 381 |
| 6–9 | 69 | 92 | 126 | 168 | 233 | 315 | 728 |
| 9–12 | 127 | 173 | 236 | 320 | 451 | 604 | 1401 |
| 12–15 | 242 | 333 | 448 | 635 | 867 | 1181 | 2730 |
| 15–18 | 455 | 620 | 877 | 1231 | 1668 | 2336 | 5277 |
| 18–21 | 890 | 1245 | 1718 | 2388 | 3261 | 4494 | 10,331 |
| 21–24 | 1754 | 2393 | 3381 | 4593 | 6396 | 8785 | 20,319 |
| Risk factors GA, AGE, plus SAS, SEX (male), OC, PE ≤ 12 years | |||||||
| < 1.5 | 18 | 22 | 29 | 37 | 49 | 64 | 141 |
| 1.5–3 | 22 | 29 | 38 | 48 | 65 | 86 | 193 |
| 3–6 | 37 | 50 | 66 | 88 | 118 | 164 | 365 |
| 6–9 | 67 | 89 | 121 | 165 | 226 | 310 | 697 |
| 9–12 | 125 | 170 | 229 | 315 | 432 | 604 | 1372 |
| 12–15 | 235 | 325 | 440 | 601 | 831 | 1145 | 2667 |
| 15–18 | 461 | 634 | 861 | 1180 | 1640 | 2281 | 5195 |
| 18–21 | 870 | 1200 | 1681 | 2292 | 3215 | 4423 | 10,219 |
| 21–24 | 1725 | 2354 | 3255 | 4451 | 6332 | 8597 | 19,660 |
| Risk factors GA, AGE, plus SAS, SEX (male), SE, PE ≤ 12 years | |||||||
| < 1.5 | 21 | 27 | 35 | 45 | 61 | 78 | 179 |
| 1.5–3 | 27 | 34 | 45 | 60 | 83 | 109 | 250 |
| 3–6 | 46 | 61 | 82 | 111 | 150 | 205 | 465 |
| 6–9 | 83 | 114 | 151 | 207 | 287 | 395 | 904 |
| 9–12 | 158 | 212 | 290 | 396 | 554 | 758 | 1726 |
| 12–15 | 299 | 405 | 560 | 777 | 1054 | 1509 | 3447 |
| 15–18 | 581 | 790 | 1095 | 1508 | 2087 | 2871 | 6634 |
| 18–21 | 1117 | 1547 | 2122 | 2938 | 4093 | 5575 | 13,017 |
| 21–24 | 2167 | 2992 | 4178 | 5736 | 7778 | 10,929 | 25,255 |
| Risk factors GA, AGE, plus SAS, SEX (male), SE, OC | |||||||
| < 1.5 | 20 | 25 | 32 | 42 | 55 | 74 | 169 |
| 1.5–3 | 25 | 32 | 42 | 56 | 74 | 101 | 225 |
| 3–6 | 44 | 57 | 76 | 105 | 139 | 189 | 428 |
| 6–9 | 78 | 104 | 142 | 193 | 264 | 366 | 824 |
| 9–12 | 144 | 197 | 270 | 372 | 521 | 711 | 1630 |
| 12–15 | 272 | 378 | 517 | 720 | 993 | 1355 | 3197 |
| 15–18 | 529 | 728 | 1011 | 1386 | 1924 | 2661 | 6110 |
| 18–21 | 1027 | 1416 | 1966 | 2729 | 3771 | 5178 | 12,123 |
| 21–24 | 2005 | 2777 | 3828 | 5238 | 7312 | 10,027 | 23,334 |
| Risk factors GA, AGE, plus SAS, SEX (male), MB, PE ≤ 12 years | |||||||
| < 1.5 | 19 | 23 | 29 | 37 | 49 | 67 | 145 |
| 1.5–3 | 23 | 30 | 38 | 51 | 66 | 87 | 198 |
| 3–6 | 38 | 51 | 69 | 91 | 124 | 168 | 384 |
| 6–9 | 69 | 92 | 125 | 170 | 231 | 316 | 723 |
| 9–12 | 127 | 172 | 232 | 324 | 449 | 617 | 1434 |
| 12–15 | 241 | 333 | 452 | 618 | 848 | 1203 | 2773 |
| 15–18 | 464 | 641 | 860 | 1214 | 1692 | 2314 | 5384 |
| 18–21 | 906 | 1255 | 1709 | 2326 | 3247 | 4416 | 10,527 |
| 21–24 | 1769 | 2355 | 3311 | 4628 | 6303 | 8847 | 20,325 |
| Risk factors GA, AGE, plus SAS, SEX (male), MB, OC | |||||||
| < 1.5 | 18 | 21 | 26 | 35 | 46 | 61 | 134 |
| 1.5–3 | 21 | 28 | 36 | 46 | 60 | 82 | 184 |
| 3–6 | 36 | 47 | 65 | 85 | 113 | 154 | 352 |
| 6–9 | 63 | 86 | 115 | 159 | 215 | 289 | 678 |
| 9–12 | 119 | 159 | 213 | 301 | 408 | 567 | 1292 |
| 12–15 | 220 | 305 | 412 | 578 | 787 | 1088 | 2539 |
| 15–18 | 425 | 584 | 814 | 1135 | 1545 | 2101 | 4924 |
| 18–21 | 828 | 1146 | 1582 | 2174 | 3031 | 4162 | 9501 |
| 21–24 | 1650 | 2224 | 3082 | 4261 | 5892 | 8054 | 18,615 |
| Risk factors GA, AGE, plus SAS, SEX (male), MB, SE | |||||||
| < 1.5 | 21 | 26 | 32 | 42 | 57 | 75 | 169 |
| 1.5–3 | 25 | 33 | 43 | 57 | 76 | 103 | 228 |
| 3–6 | 44 | 58 | 78 | 105 | 142 | 193 | 440 |
| 6–9 | 80 | 105 | 145 | 199 | 271 | 379 | 851 |
| 9–12 | 146 | 201 | 277 | 381 | 514 | 717 | 1642 |
| 12–15 | 278 | 386 | 528 | 735 | 1007 | 1381 | 3254 |
| 15–18 | 550 | 759 | 1019 | 1419 | 1950 | 2720 | 6312 |
| 18–21 | 1053 | 1460 | 1998 | 2747 | 3839 | 5276 | 12,332 |
| 21–24 | 2078 | 2805 | 3936 | 5443 | 7349 | 10,448 | 23,544 |
| Risk factors GA, AGE, plus SAS, SEX (male), MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 17 | 22 | 28 | 36 | 46 | 61 | 137 |
| 1.5–3 | 22 | 28 | 37 | 47 | 63 | 84 | 189 |
| 3–6 | 36 | 48 | 65 | 85 | 114 | 156 | 358 |
| 6–9 | 66 | 86 | 121 | 159 | 215 | 298 | 681 |
| 9–12 | 121 | 163 | 223 | 303 | 416 | 581 | 1342 |
| 12–15 | 224 | 316 | 432 | 600 | 811 | 1140 | 2566 |
| 15–18 | 437 | 596 | 830 | 1152 | 1591 | 2184 | 5038 |
| 18–21 | 841 | 1178 | 1628 | 2216 | 3071 | 4265 | 9908 |
| 21–24 | 1604 | 2240 | 3119 | 4277 | 6013 | 8471 | 19,239 |
| Risk factors GA, AGE, plus SAS, MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 14 | 18 | 22 | 27 | 35 | 47 | 102 |
| 1.5–3 | 18 | 23 | 27 | 36 | 47 | 63 | 137 |
| 3–6 | 28 | 36 | 47 | 64 | 84 | 114 | 257 |
| 6–9 | 48 | 63 | 86 | 115 | 157 | 218 | 502 |
| 9–12 | 88 | 118 | 163 | 221 | 304 | 419 | 965 |
| 12–15 | 167 | 226 | 310 | 422 | 591 | 810 | 1850 |
| 15–18 | 318 | 434 | 600 | 824 | 1138 | 1556 | 3655 |
| 18–21 | 611 | 854 | 1147 | 1595 | 2188 | 3103 | 7051 |
| 21–24 | 1149 | 1645 | 2219 | 3077 | 4303 | 5989 | 13,786 |
| Risk factors GA, AGE, plus SAS, SEX (male), SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 16 | 20 | 23 | 31 | 39 | 52 | 115 |
| 1.5–3 | 20 | 24 | 32 | 41 | 53 | 71 | 156 |
| 3–6 | 31 | 40 | 53 | 72 | 97 | 130 | 295 |
| 6–9 | 54 | 73 | 99 | 133 | 184 | 250 | 559 |
| 9–12 | 101 | 133 | 182 | 251 | 347 | 475 | 1101 |
| 12–15 | 186 | 258 | 349 | 488 | 684 | 908 | 2143 |
| 15–18 | 360 | 494 | 684 | 942 | 1316 | 1830 | 4176 |
| 18–21 | 687 | 974 | 1347 | 1821 | 2587 | 3512 | 8175 |
| 21–24 | 1346 | 1865 | 2572 | 3583 | 5014 | 6840 | 16,027 |
| Risk factors GA, AGE, plus SAS, SEX (male), MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 14 | 17 | 20 | 26 | 34 | 44 | 93 |
| 1.5–3 | 17 | 21 | 26 | 33 | 44 | 56 | 128 |
| 3–6 | 26 | 34 | 45 | 60 | 79 | 106 | 243 |
| 6–9 | 45 | 60 | 82 | 110 | 148 | 198 | 456 |
| 9–12 | 82 | 111 | 151 | 205 | 277 | 389 | 887 |
| 12–15 | 156 | 210 | 286 | 388 | 560 | 745 | 1686 |
| 15–18 | 295 | 402 | 546 | 746 | 1043 | 1435 | 3278 |
| 18–21 | 555 | 777 | 1056 | 1488 | 2059 | 2851 | 6481 |
| 21–24 | 1084 | 1517 | 2028 | 2868 | 3976 | 5459 | 12,490 |
| Risk factors GA, AGE, plus SAS, SEX (male), MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 16 | 19 | 25 | 31 | 41 | 53 | 121 |
| 1.5–3 | 20 | 24 | 32 | 40 | 54 | 72 | 158 |
| 3–6 | 32 | 41 | 55 | 74 | 101 | 136 | 303 |
| 6–9 | 55 | 75 | 100 | 135 | 187 | 254 | 585 |
| 9–12 | 103 | 140 | 189 | 258 | 355 | 489 | 1126 |
| 12–15 | 193 | 265 | 369 | 493 | 682 | 948 | 2208 |
| 15–18 | 369 | 510 | 704 | 973 | 1347 | 1852 | 4226 |
| 18–21 | 716 | 915 | 1360 | 1870 | 2605 | 3580 | 8346 |
| 21–24 | 1405 | 1902 | 2607 | 3675 | 5085 | 6967 | 16,232 |
| Risk factors GA, AGE, plus SAS, SEX (male), MB, SE, OC | |||||||
| < 1.5 | 15 | 18 | 23 | 29 | 38 | 50 | 110 |
| 1.5–3 | 19 | 23 | 30 | 38 | 50 | 66 | 147 |
| 3–6 | 30 | 38 | 51 | 69 | 92 | 124 | 279 |
| 6–9 | 52 | 69 | 92 | 126 | 174 | 234 | 525 |
| 9–12 | 94 | 129 | 178 | 235 | 330 | 448 | 1034 |
| 12–15 | 177 | 243 | 331 | 459 | 632 | 873 | 2007 |
| 15–18 | 351 | 468 | 645 | 888 | 1217 | 1681 | 3914 |
| 18–21 | 653 | 902 | 1247 | 1729 | 2391 | 3338 | 7697 |
| 21–24 | 1274 | 1769 | 2422 | 3360 | 4703 | 6482 | 14,873 |
| Risk factors GA, AGE, plus SAS, SEX (male), MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 12 | 15 | 18 | 22 | 28 | 35 | 75 |
| 1.5–3 | 15 | 18 | 23 | 27 | 36 | 47 | 103 |
| 3–6 | 22 | 28 | 36 | 48 | 64 | 85 | 192 |
| 6–9 | 38 | 49 | 64 | 87 | 116 | 162 | 365 |
| 9–12 | 66 | 90 | 120 | 164 | 223 | 302 | 704 |
| 12–15 | 123 | 167 | 227 | 315 | 429 | 595 | 1362 |
| 15–18 | 231 | 316 | 437 | 605 | 816 | 1146 | 2662 |
| 18–21 | 445 | 616 | 855 | 1178 | 1596 | 2275 | 5118 |
| 21–24 | 874 | 1200 | 1672 | 2295 | 3168 | 4320 | 10,097 |
| AGE (months) | GA (weeks) | ||||||
|---|---|---|---|---|---|---|---|
| ≤ 24 | > 24–26 | > 26–28 | > 28–30 | > 30–32 | > 32–34 | ≥ 35 | |
| Risk factors GA, AGE, CLD | |||||||
| < 1.5 | 10 | 11 | 14 | 18 | 22 | 29 | 59 |
| 1.5–3 | 12 | 14 | 17 | 22 | 29 | 38 | 78 |
| 3–6 | 18 | 23 | 29 | 38 | 49 | 66 | 147 |
| 6–9 | 29 | 40 | 51 | 71 | 94 | 128 | 289 |
| 9–12 | 53 | 71 | 96 | 129 | 178 | 246 | 560 |
| 12–15 | 97 | 135 | 182 | 253 | 346 | 467 | 1079 |
| 15–18 | 187 | 257 | 353 | 485 | 668 | 919 | 2095 |
| 18–21 | 359 | 492 | 672 | 916 | 1295 | 1793 | 4107 |
| 21–24 | 693 | 951 | 1292 | 1791 | 2504 | 3456 | 7893 |
| Risk factors GA, AGE, CLD plus PE ≤ 12 years | |||||||
| < 1.5 | 8 | 10 | 11 | 14 | 17 | 21 | 42 |
| 1.5–3 | 9 | 11 | 13 | 17 | 22 | 27 | 56 |
| 3–6 | 14 | 17 | 21 | 27 | 36 | 47 | 103 |
| 6–9 | 22 | 27 | 37 | 48 | 65 | 90 | 193 |
| 9–12 | 38 | 49 | 66 | 92 | 125 | 167 | 382 |
| 12–15 | 66 | 92 | 122 | 172 | 233 | 325 | 723 |
| 15–18 | 128 | 174 | 242 | 326 | 450 | 616 | 1423 |
| 18–21 | 238 | 336 | 468 | 628 | 873 | 1209 | 2849 |
| 21–24 | 469 | 638 | 879 | 1225 | 1722 | 2335 | 5373 |
| Risk factors GA, AGE, CLD, plus OC | |||||||
| < 1.5 | 8 | 9 | 11 | 13 | 16 | 20 | 39 |
| 1.5–3 | 9 | 11 | 13 | 16 | 20 | 26 | 53 |
| 3–6 | 13 | 16 | 20 | 25 | 33 | 44 | 97 |
| 6–9 | 20 | 26 | 35 | 45 | 61 | 83 | 185 |
| 9–12 | 35 | 46 | 62 | 83 | 114 | 155 | 340 |
| 12–15 | 63 | 85 | 113 | 158 | 215 | 294 | 687 |
| 15–18 | 115 | 159 | 222 | 300 | 419 | 571 | 1305 |
| 18–21 | 224 | 306 | 418 | 583 | 814 | 1119 | 2543 |
| 21–24 | 434 | 590 | 816 | 1113 | 1572 | 2146 | 4994 |
| Risk factors GA, AGE, CLD, plus SE | |||||||
| < 1.5 | 9 | 10 | 12 | 14 | 18 | 22 | 44 |
| 1.5–3 | 10 | 12 | 14 | 18 | 22 | 29 | 60 |
| 3–6 | 14 | 18 | 23 | 29 | 39 | 52 | 114 |
| 6–9 | 23 | 30 | 40 | 52 | 70 | 95 | 212 |
| 9–12 | 39 | 53 | 71 | 96 | 129 | 182 | 408 |
| 12–15 | 71 | 97 | 133 | 186 | 250 | 345 | 785 |
| 15–18 | 135 | 187 | 258 | 350 | 488 | 674 | 1546 |
| 18–21 | 264 | 360 | 485 | 678 | 919 | 1295 | 2933 |
| 21–24 | 515 | 687 | 964 | 1314 | 1852 | 2543 | 5875 |
| Risk factors GA, AGE, plus SAS | |||||||
| < 1.5 | 8 | 9 | 10 | 12 | 14 | 17 | 34 |
| 1.5–3 | 9 | 10 | 12 | 14 | 18 | 22 | 45 |
| 3–6 | 12 | 14 | 18 | 22 | 29 | 38 | 83 |
| 6–9 | 18 | 23 | 30 | 39 | 51 | 70 | 156 |
| 9–12 | 30 | 39 | 53 | 71 | 96 | 129 | 293 |
| 12–15 | 53 | 73 | 98 | 133 | 183 | 248 | 571 |
| 15–18 | 100 | 134 | 186 | 255 | 357 | 487 | 1092 |
| 18–21 | 189 | 266 | 363 | 480 | 688 | 922 | 2158 |
| 21–24 | 359 | 497 | 708 | 966 | 1296 | 1819 | 4211 |
| Risk factors GA, AGE, CLD plus OC, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 8 | 9 | 10 | 12 | 15 | 28 |
| 1.5–3 | 8 | 9 | 10 | 12 | 15 | 19 | 38 |
| 3–6 | 10 | 13 | 15 | 20 | 25 | 32 | 66 |
| 6–9 | 16 | 19 | 25 | 32 | 42 | 57 | 125 |
| 9–12 | 25 | 32 | 44 | 59 | 78 | 104 | 243 |
| 12–15 | 45 | 59 | 81 | 107 | 145 | 199 | 469 |
| 15–18 | 81 | 109 | 151 | 207 | 286 | 390 | 892 |
| 18–21 | 154 | 209 | 293 | 397 | 547 | 747 | 1735 |
| 21–24 | 295 | 405 | 559 | 757 | 1046 | 1476 | 3308 |
| Risk factors GA, AGE, CLD plus SE, PE ≤ 12 years | |||||||
| < 1.5 | 8 | 9 | 10 | 12 | 14 | 18 | 34 |
| 1.5–3 | 9 | 10 | 12 | 14 | 18 | 23 | 46 |
| 3–6 | 12 | 15 | 18 | 23 | 29 | 39 | 83 |
| 6–9 | 18 | 23 | 30 | 40 | 53 | 71 | 158 |
| 9–12 | 30 | 40 | 54 | 72 | 98 | 134 | 304 |
| 12–15 | 55 | 72 | 100 | 134 | 188 | 260 | 582 |
| 15–18 | 102 | 138 | 186 | 257 | 360 | 500 | 1138 |
| 18–21 | 197 | 264 | 367 | 508 | 690 | 951 | 2204 |
| 21–24 | 370 | 513 | 698 | 994 | 1368 | 1863 | 4259 |
| Risk factors GA, AGE, CLD plus SE, OC | |||||||
| < 1.5 | 7 | 8 | 10 | 11 | 14 | 17 | 32 |
| 1.5–3 | 8 | 10 | 11 | 14 | 17 | 21 | 43 |
| 3–6 | 12 | 14 | 17 | 21 | 28 | 36 | 80 |
| 6–9 | 17 | 22 | 28 | 37 | 49 | 66 | 145 |
| 9–12 | 29 | 38 | 49 | 68 | 91 | 126 | 275 |
| 12–15 | 50 | 70 | 92 | 125 | 170 | 233 | 531 |
| 15–18 | 94 | 128 | 175 | 241 | 330 | 464 | 1039 |
| 18–21 | 175 | 246 | 334 | 460 | 647 | 867 | 2047 |
| 21–24 | 341 | 471 | 645 | 907 | 1244 | 1720 | 3939 |
| Risk factors GA, AGE, CLD plus MW, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 8 | 9 | 10 | 12 | 15 | 29 |
| 1.5–3 | 8 | 9 | 10 | 12 | 15 | 19 | 38 |
| 3–6 | 11 | 13 | 15 | 19 | 25 | 32 | 68 |
| 6–9 | 16 | 20 | 26 | 33 | 43 | 58 | 126 |
| 9–12 | 26 | 33 | 44 | 59 | 78 | 109 | 244 |
| 12–15 | 45 | 60 | 81 | 110 | 151 | 208 | 472 |
| 15–18 | 85 | 112 | 155 | 213 | 286 | 396 | 907 |
| 18–21 | 158 | 213 | 295 | 406 | 556 | 759 | 1767 |
| 21–24 | 302 | 412 | 567 | 791 | 1093 | 1529 | 3483 |
| Risk factors GA, AGE, CLD plus MB, OC | |||||||
| < 1.5 | 7 | 8 | 9 | 10 | 12 | 15 | 27 |
| 1.5–3 | 8 | 9 | 10 | 12 | 15 | 18 | 36 |
| 3–6 | 10 | 12 | 15 | 18 | 24 | 31 | 63 |
| 6–9 | 15 | 19 | 24 | 30 | 40 | 53 | 117 |
| 9–12 | 24 | 31 | 41 | 55 | 75 | 102 | 223 |
| 12–15 | 42 | 55 | 75 | 102 | 140 | 188 | 428 |
| 15–18 | 76 | 102 | 141 | 192 | 266 | 364 | 824 |
| 18–21 | 143 | 198 | 273 | 372 | 516 | 710 | 1595 |
| 21–24 | 280 | 375 | 524 | 734 | 1006 | 1379 | 3218 |
| Risk factors GA, AGE, CLD plus MB, SE | |||||||
| < 1.5 | 8 | 8 | 10 | 12 | 14 | 17 | 34 |
| 1.5–3 | 9 | 10 | 11 | 14 | 17 | 21 | 43 |
| 3–6 | 12 | 14 | 17 | 22 | 28 | 37 | 80 |
| 6–9 | 17 | 22 | 29 | 38 | 50 | 66 | 149 |
| 9–12 | 29 | 39 | 51 | 69 | 92 | 126 | 285 |
| 12–15 | 53 | 70 | 95 | 130 | 176 | 239 | 553 |
| 15–18 | 96 | 131 | 177 | 252 | 340 | 461 | 1089 |
| 18–21 | 184 | 249 | 342 | 478 | 660 | 906 | 2066 |
| 21–24 | 355 | 486 | 667 | 911 | 1305 | 1779 | 4020 |
| Risk factors GA, AGE, CLD plus SEX (male), PE ≤ 12 years | |||||||
| < 1.5 | 8 | 8 | 10 | 11 | 14 | 17 | 31 |
| 1.5–3 | 8 | 10 | 11 | 14 | 17 | 21 | 43 |
| 3–6 | 11 | 14 | 17 | 22 | 28 | 36 | 77 |
| 6–9 | 17 | 22 | 28 | 36 | 50 | 65 | 147 |
| 9–12 | 28 | 37 | 49 | 67 | 92 | 123 | 282 |
| 12–15 | 51 | 68 | 92 | 127 | 171 | 238 | 526 |
| 15–18 | 95 | 129 | 176 | 238 | 331 | 459 | 1042 |
| 18–21 | 179 | 249 | 331 | 470 | 643 | 896 | 2025 |
| 21–24 | 348 | 468 | 651 | 917 | 1213 | 1707 | 3951 |
| Risk factors GA, AGE, CLD plus MB, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 8 | 9 | 11 | 13 | 16 | 30 |
| 1.5–3 | 8 | 9 | 11 | 13 | 16 | 20 | 40 |
| 3–6 | 11 | 13 | 16 | 21 | 26 | 34 | 72 |
| 6–9 | 16 | 21 | 27 | 34 | 45 | 60 | 133 |
| 9–12 | 26 | 35 | 47 | 62 | 84 | 112 | 257 |
| 12–15 | 47 | 63 | 85 | 115 | 158 | 217 | 494 |
| 15–18 | 86 | 119 | 158 | 225 | 309 | 422 | 947 |
| 18–21 | 164 | 223 | 306 | 419 | 592 | 815 | 1889 |
| 21–24 | 317 | 428 | 604 | 827 | 1143 | 1601 | 3597 |
| Risk factors GA, AGE, CLD plus SEX (male) | |||||||
| < 1.5 | 8 | 9 | 10 | 12 | 15 | 19 | 37 |
| 1.5–3 | 9 | 10 | 12 | 15 | 19 | 24 | 49 |
| 3–6 | 13 | 15 | 20 | 24 | 31 | 43 | 89 |
| 6–9 | 19 | 25 | 32 | 42 | 56 | 76 | 168 |
| 9–12 | 33 | 42 | 58 | 77 | 104 | 140 | 323 |
| 12–15 | 59 | 79 | 107 | 146 | 201 | 276 | 624 |
| 15–18 | 110 | 150 | 207 | 281 | 383 | 537 | 1234 |
| 18–21 | 210 | 289 | 392 | 544 | 756 | 1047 | 2336 |
| 21–24 | 407 | 550 | 761 | 1047 | 1461 | 2053 | 4746 |
| Risk factors GA, AGE, CLD plus SEX (male), MB | |||||||
| < 1.5 | 7 | 8 | 9 | 11 | 13 | 16 | 31 |
| 1.5–3 | 8 | 9 | 11 | 13 | 16 | 21 | 41 |
| 3–6 | 11 | 13 | 16 | 21 | 26 | 35 | 73 |
| 6–9 | 16 | 21 | 27 | 35 | 46 | 62 | 138 |
| 9–12 | 27 | 36 | 46 | 63 | 86 | 117 | 260 |
| 12–15 | 48 | 66 | 87 | 116 | 162 | 222 | 502 |
| 15–18 | 90 | 124 | 168 | 229 | 312 | 432 | 977 |
| 18–21 | 167 | 232 | 325 | 446 | 603 | 837 | 1911 |
| 21–24 | 320 | 451 | 628 | 836 | 1183 | 1648 | 3721 |
| Risk factors GA, AGE, CLD plus SAS, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 8 | 9 | 9 | 11 | 14 | 25 |
| 1.5–3 | 8 | 8 | 10 | 11 | 13 | 17 | 32 |
| 3–6 | 10 | 12 | 14 | 17 | 22 | 28 | 56 |
| 6–9 | 14 | 17 | 22 | 28 | 37 | 49 | 104 |
| 9–12 | 22 | 28 | 37 | 50 | 66 | 90 | 203 |
| 12–15 | 39 | 51 | 67 | 91 | 124 | 174 | 383 |
| 15–18 | 69 | 95 | 128 | 175 | 233 | 325 | 753 |
| 18–21 | 126 | 178 | 241 | 336 | 459 | 639 | 153 |
| 21–24 | 247 | 334 | 468 | 652 | 885 | 1230 | 2808 |
| Risk factors GA, AGE, CLD plus SAS, OC | |||||||
| < 1.5 | 7 | 7 | 8 | 9 | 11 | 14 | 25 |
| 1.5–3 | 8 | 8 | 10 | 11 | 13 | 17 | 32 |
| 3–6 | 10 | 12 | 14 | 17 | 22 | 28 | 56 |
| 6–9 | 14 | 17 | 22 | 28 | 37 | 49 | 104 |
| 9–12 | 22 | 28 | 37 | 50 | 66 | 90 | 203 |
| 12–15 | 39 | 51 | 67 | 91 | 124 | 174 | 383 |
| 15–18 | 69 | 95 | 128 | 175 | 233 | 325 | 753 |
| 18–21 | 126 | 178 | 241 | 336 | 459 | 639 | 1453 |
| 21–24 | 247 | 334 | 468 | 652 | 885 | 1230 | 2808 |
| Risk factors GA, AGE, CLD plus SAS, OC | |||||||
| < 1.5 | 7 | 7 | 8 | 9 | 11 | 13 | 23 |
| 1.5–3 | 7 | 8 | 9 | 11 | 13 | 16 | 30 |
| 3–6 | 9 | 11 | 13 | 16 | 20 | 26 | 54 |
| 6–9 | 13 | 17 | 20 | 26 | 34 | 45 | 98 |
| 9–12 | 21 | 27 | 34 | 46 | 62 | 84 | 185 |
| 12–15 | 36 | 47 | 63 | 85 | 115 | 158 | 355 |
| 15–18 | 64 | 89 | 118 | 163 | 220 | 298 | 690 |
| 18–21 | 119 | 160 | 225 | 305 | 431 | 582 | 1355 |
| 21–24 | 227 | 318 | 434 | 588 | 837 | 1132 | 2608 |
| Risk factors GA, AGE, CLD plus SAS, SE | |||||||
| < 1.5 | 7 | 8 | 9 | 10 | 12 | 15 | 27 |
| 1.5–3 | 8 | 9 | 11 | 12 | 15 | 18 | 37 |
| 3–6 | 10 | 13 | 15 | 19 | 24 | 32 | 66 |
| 6–9 | 15 | 19 | 25 | 33 | 42 | 56 | 124 |
| 9–12 | 25 | 32 | 43 | 58 | 76 | 106 | 237 |
| 12–15 | 44 | 57 | 78 | 108 | 147 | 199 | 454 |
| 15–18 | 80 | 111 | 151 | 205 | 282 | 385 | 875 |
| 18–21 | 148 | 211 | 287 | 387 | 549 | 748 | 1730 |
| 21–24 | 291 | 403 | 555 | 758 | 1053 | 1431 | 3308 |
| Risk factors GA, AGE, CLD plus SAS, MB | |||||||
| < 1.5 | 7 | 8 | 9 | 9 | 11 | 13 | 24 |
| 1.5–3 | 8 | 8 | 9 | 11 | 13 | 16 | 31 |
| 3–6 | 9 | 11 | 13 | 16 | 21 | 26 | 55 |
| 6–9 | 14 | 16 | 21 | 26 | 35 | 46 | 100 |
| 9–12 | 22 | 27 | 36 | 46 | 63 | 86 | 193 |
| 12–15 | 36 | 48 | 64 | 87 | 120 | 159 | 367 |
| 15–18 | 65 | 88 | 121 | 164 | 225 | 306 | 711 |
| 18–21 | 123 | 167 | 227 | 318 | 429 | 598 | 1355 |
| 21–24 | 237 | 326 | 444 | 618 | 845 | 1164 | 2657 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male) | |||||||
| < 1.5 | 7 | 8 | 9 | 10 | 12 | 14 | 26 |
| 1.5–3 | 8 | 9 | 10 | 12 | 14 | 18 | 34 |
| 3–6 | 10 | 12 | 14 | 18 | 23 | 30 | 61 |
| 6–9 | 15 | 18 | 24 | 30 | 40 | 52 | 114 |
| 9–12 | 23 | 30 | 40 | 53 | 72 | 97 | 218 |
| 12–15 | 41 | 55 | 75 | 99 | 138 | 184 | 411 |
| 15–18 | 74 | 100 | 137 | 188 | 256 | 356 | 813 |
| 18–21 | 141 | 191 | 257 | 366 | 499 | 674 | 1603 |
| 21–24 | 266 | 368 | 507 | 704 | 941 | 1317 | 3038 |
| Risk factors GA, AGE, CLD plus SE, OC , PE ≤ 12 years | |||||||
| < 1.5 | 7 | 7 | 8 | 9 | 11 | 13 | 24 |
| 1.5–3 | 8 | 8 | 9 | 11 | 13 | 16 | 31 |
| 3–6 | 9 | 11 | 13 | 17 | 20 | 26 | 55 |
| 6–9 | 13 | 17 | 21 | 27 | 36 | 47 | 99 |
| 9–12 | 21 | 27 | 35 | 46 | 64 | 85 | 189 |
| 12–15 | 36 | 48 | 64 | 86 | 121 | 161 | 363 |
| 15–18 | 66 | 88 | 119 | 164 | 223 | 315 | 705 |
| 18–21 | 122 | 167 | 232 | 318 | 441 | 613 | 1387 |
| 21–24 | 238 | 319 | 446 | 617 | 844 | 1166 | 2656 |
| Risk factors GA, AGE, CLD plus MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 7 | 7 | 8 | 10 | 12 | 20 |
| 1.5–3 | 7 | 8 | 9 | 9 | 12 | 14 | 25 |
| 3–6 | 9 | 10 | 12 | 14 | 18 | 22 | 45 |
| 6–9 | 12 | 14 | 18 | 23 | 30 | 38 | 84 |
| 9–12 | 18 | 23 | 29 | 38 | 52 | 69 | 154 |
| 12–15 | 29 | 40 | 52 | 72 | 96 | 131 | 297 |
| 15–18 | 55 | 73 | 97 | 131 | 184 | 242 | 578 |
| 18–21 | 101 | 135 | 187 | 252 | 349 | 479 | 1105 |
| 21–24 | 188 | 258 | 352 | 492 | 678 | 932 | 2139 |
| Risk factors GA, AGE, CLD plus MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 8 | 8 | 9 | 11 | 13 | 24 |
| 1.5–3 | 8 | 8 | 9 | 11 | 13 | 16 | 31 |
| 3–6 | 10 | 11 | 14 | 17 | 21 | 27 | 55 |
| 6–9 | 14 | 17 | 20 | 27 | 35 | 46 | 103 |
| 9–12 | 22 | 27 | 36 | 50 | 63 | 89 | 193 |
| 12–15 | 36 | 48 | 65 | 89 | 122 | 164 | 372 |
| 15–18 | 67 | 90 | 124 | 167 | 228 | 319 | 723 |
| 18–21 | 126 | 168 | 237 | 318 | 444 | 625 | 1416 |
| 21–24 | 238 | 325 | 445 | 624 | 872 | 1188 | 2804 |
| Risk factors GA, AGE, CLD plus MW, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 7 | 8 | 9 | 11 | 13 | 23 |
| 1.5–3 | 7 | 8 | 9 | 11 | 12 | 15 | 30 |
| 3–6 | 9 | 11 | 13 | 16 | 20 | 25 | 51 |
| 6–9 | 13 | 16 | 20 | 25 | 33 | 45 | 96 |
| 9–12 | 21 | 27 | 33 | 45 | 60 | 80 | 178 |
| 12–15 | 34 | 46 | 60 | 84 | 111 | 152 | 343 |
| 15–18 | 62 | 83 | 113 | 155 | 215 | 289 | 650 |
| 18–21 | 114 | 159 | 215 | 299 | 405 | 564 | 1291 |
| 21–24 | 221 | 304 | 416 | 569 | 805 | 1099 | 2534 |
| Risk factors GA, AGE, CLD plus SEX (male), OC, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 7 | 8 | 9 | 11 | 13 | 22 |
| 1.5–3 | 7 | 8 | 9 | 11 | 12 | 15 | 29 |
| 3–6 | 9 | 11 | 13 | 15 | 19 | 25 | 51 |
| 6–9 | 13 | 16 | 20 | 25 | 33 | 43 | 93 |
| 9–12 | 20 | 26 | 33 | 43 | 58 | 79 | 172 |
| 12–15 | 34 | 44 | 59 | 80 | 108 | 149 | 341 |
| 15–18 | 60 | 82 | 109 | 152 | 206 | 285 | 646 |
| 18–21 | 111 | 154 | 213 | 289 | 396 | 548 | 1272 |
| 21–24 | 220 | 296 | 408 | 560 | 773 | 1085 | 2451 |
| Risk factors GA, AGE, CLD plus SEX (male), SE, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 8 | 9 | 10 | 12 | 14 | 27 |
| 1.5–3 | 8 | 9 | 10 | 12 | 14 | 18 | 35 |
| 3–6 | 10 | 12 | 15 | 18 | 22 | 30 | 62 |
| 6–9 | 15 | 19 | 23 | 31 | 40 | 54 | 117 |
| 9–12 | 24 | 31 | 41 | 55 | 75 | 99 | 218 |
| 12–15 | 42 | 55 | 74 | 101 | 137 | 190 | 428 |
| 15–18 | 74 | 103 | 142 | 195 | 268 | 362 | 841 |
| 18–21 | 144 | 195 | 264 | 364 | 510 | 700 | 1630 |
| 21–24 | 272 | 380 | 515 | 720 | 1011 | 1371 | 3172 |
| Risk factors GA, AGE, CLD plus SEX (male), SE, OC | |||||||
| < 1.5 | 7 | 7 | 8 | 10 | 11 | 14 | 25 |
| 1.5–3 | 7 | 8 | 10 | 11 | 14 | 16 | 32 |
| 3–6 | 10 | 12 | 14 | 17 | 22 | 28 | 58 |
| 6–9 | 14 | 17 | 22 | 28 | 37 | 49 | 109 |
| 9–12 | 22 | 29 | 38 | 51 | 67 | 91 | 206 |
| 12–15 | 37 | 52 | 68 | 92 | 126 | 175 | 396 |
| 15–18 | 71 | 94 | 130 | 173 | 246 | 334 | 761 |
| 18–21 | 131 | 180 | 249 | 340 | 470 | 642 | 1490 |
| 21–24 | 253 | 345 | 477 | 658 | 916 | 1256 | 2903 |
| Risk factors GA, AGE, CLD plus SEX (male) MB, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 7 | 8 | 9 | 11 | 13 | 23 |
| 1.5–3 | 7 | 8 | 9 | 11 | 13 | 15 | 29 |
| 3–6 | 9 | 11 | 13 | 16 | 19 | 25 | 52 |
| 6–9 | 13 | 16 | 20 | 26 | 33 | 44 | 95 |
| 9–12 | 20 | 26 | 34 | 45 | 61 | 82 | 180 |
| 12–15 | 34 | 45 | 59 | 83 | 112 | 154 | 344 |
| 15–18 | 62 | 86 | 114 | 152 | 214 | 292 | 679 |
| 18–21 | 117 | 157 | 214 | 304 | 414 | 560 | 1288 |
| 21–24 | 220 | 307 | 412 | 570 | 774 | 1074 | 2500 |
| Risk factors GA, AGE, CLD plus SEX (male), MB, OC | |||||||
| < 1.5 | 7 | 7 | 8 | 9 | 10 | 12 | 21 |
| 1.5–3 | 7 | 8 | 9 | 10 | 12 | 15 | 27 |
| 3–6 | 9 | 11 | 13 | 15 | 19 | 24 | 48 |
| 6–9 | 12 | 15 | 19 | 24 | 31 | 41 | 89 |
| 9–12 | 19 | 25 | 31 | 42 | 55 | 74 | 168 |
| 12–15 | 32 | 42 | 57 | 76 | 100 | 140 | 320 |
| 15–18 | 57 | 78 | 104 | 146 | 193 | 269 | 616 |
| 18–21 | 106 | 145 | 199 | 275 | 376 | 520 | 1165 |
| 21–24 | 202 | 274 | 384 | 532 | 747 | 1019 | 2307 |
| Risk factors GA, AGE, CLD plus SEX (male), MB, SE | |||||||
| < 1.5 | 7 | 8 | 8 | 10 | 11 | 14 | 26 |
| 1.5–3 | 8 | 9 | 10 | 12 | 14 | 17 | 33 |
| 3–6 | 10 | 11 | 14 | 17 | 22 | 28 | 59 |
| 6–9 | 15 | 18 | 22 | 29 | 38 | 50 | 111 |
| 9–12 | 23 | 30 | 38 | 52 | 69 | 94 | 209 |
| 12–15 | 39 | 52 | 71 | 94 | 131 | 176 | 408 |
| 15–18 | 71 | 97 | 133 | 177 | 247 | 337 | 767 |
| 18–21 | 135 | 187 | 255 | 349 | 475 | 651 | 1480 |
| 21–24 | 260 | 356 | 493 | 682 | 952 | 1323 | 2998 |
| Risk factors GA, AGE, CLD plus SAS, OC, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 7 | 7 | 8 | 9 | 10 | 18 |
| 1.5–3 | 7 | 7 | 8 | 9 | 10 | 12 | 22 |
| 3–6 | 8 | 9 | 11 | 13 | 15 | 19 | 38 |
| 6–9 | 11 | 12 | 15 | 19 | 25 | 32 | 68 |
| 9–12 | 16 | 20 | 25 | 33 | 43 | 59 | 127 |
| 12–15 | 25 | 33 | 45 | 58 | 79 | 107 | 238 |
| 15–18 | 45 | 61 | 80 | 110 | 150 | 207 | 485 |
| 18–21 | 82 | 111 | 153 | 210 | 288 | 405 | 915 |
| 21–24 | 156 | 217 | 290 | 404 | 551 | 775 | 1795 |
| Risk factors GA, AGE, CLD plus SAS, SE, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 7 | 8 | 9 | 10 | 12 | 21 |
| 1.5–3 | 7 | 8 | 9 | 10 | 12 | 14 | 27 |
| 3–6 | 9 | 10 | 12 | 14 | 18 | 23 | 47 |
| 6–9 | 12 | 15 | 18 | 23 | 30 | 41 | 85 |
| 9–12 | 19 | 24 | 31 | 40 | 55 | 73 | 159 |
| 12–15 | 31 | 41 | 56 | 73 | 101 | 137 | 311 |
| 15–18 | 55 | 75 | 102 | 138 | 191 | 260 | 595 |
| 18–21 | 104 | 141 | 191 | 267 | 368 | 510 | 1153 |
| 21–24 | 197 | 270 | 370 | 522 | 710 | 987 | 2294 |
| Risk factors GA, AGE, CLD plus SAS, Se, OC | |||||||
| < 1.5 | 6 | 7 | 7 | 8 | 10 | 11 | 20 |
| 1.5–3 | 7 | 8 | 8 | 10 | 11 | 14 | 25 |
| 3–6 | 8 | 10 | 11 | 14 | 17 | 21 | 44 |
| 6–9 | 12 | 14 | 17 | 22 | 28 | 37 | 80 |
| 9–12 | 17 | 22 | 28 | 37 | 50 | 67 | 150 |
| 12–15 | 29 | 39 | 52 | 69 | 94 | 126 | 287 |
| 15–18 | 52 | 70 | 94 | 128 | 175 | 238 | 547 |
| 18–21 | 96 | 129 | 181 | 241 | 342 | 462 | 1079 |
| 21–24 | 181 | 256 | 341 | 469 | 648 | 900 | 2098 |
| Risk factors GA, AGE, CLD plus SAS, MB, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 7 | 7 | 8 | 9 | 11 | 18 |
| 1.5–3 | 7 | 7 | 8 | 9 | 11 | 13 | 23 |
| 3–6 | 8 | 9 | 11 | 13 | 15 | 20 | 39 |
| 6–9 | 11 | 13 | 16 | 20 | 25 | 33 | 70 |
| 9–12 | 16 | 20 | 26 | 34 | 45 | 59 | 130 |
| 12–15 | 26 | 34 | 46 | 61 | 82 | 108 | 250 |
| 15–18 | 46 | 61 | 83 | 113 | 154 | 213 | 483 |
| 18–21 | 85 | 114 | 155 | 216 | 303 | 401 | 933 |
| 21–24 | 159 | 215 | 295 | 415 | 580 | 798 | 1806 |
| Risk factors GA, AGE, CLD plus SAS, MB, OC | |||||||
| < 1.5 | 6 | 7 | 7 | 8 | 9 | 10 | 17 |
| 1.5–3 | 7 | 7 | 8 | 9 | 10 | 12 | 21 |
| 3–6 | 8 | 9 | 10 | 12 | 15 | 19 | 37 |
| 6–9 | 10 | 12 | 15 | 19 | 23 | 31 | 66 |
| 9–12 | 15 | 19 | 24 | 32 | 41 | 55 | 120 |
| 12–15 | 24 | 32 | 42 | 56 | 74 | 102 | 229 |
| 15–18 | 43 | 57 | 77 | 105 | 144 | 193 | 440 |
| 18–21 | 78 | 107 | 145 | 197 | 273 | 371 | 839 |
| 21–24 | 147 | 207 | 273 | 377 | 520 | 721 | 1689 |
| Risk factors GA, AGE, CLD plus SAS, MB, SE | |||||||
| < 1.5 | 6 | 7 | 8 | 8 | 10 | 11 | 20 |
| 1.5–3 | 7 | 8 | 9 | 10 | 11 | 14 | 26 |
| 3–6 | 9 | 10 | 12 | 14 | 17 | 22 | 46 |
| 6–9 | 12 | 14 | 18 | 22 | 29 | 38 | 81 |
| 9–12 | 17 | 22 | 29 | 39 | 51 | 69 | 153 |
| 12–15 | 30 | 39 | 52 | 70 | 95 | 130 | 292 |
| 15–18 | 53 | 71 | 97 | 131 | 183 | 252 | 570 |
| 18–21 | 98 | 132 | 183 | 254 | 351 | 486 | 1089 |
| 21–24 | 188 | 252 | 352 | 487 | 671 | 927 | 2126 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), PE ≤ 12 years | |||||||
| < 1.5 | 7 | 7 | 8 | 8 | 10 | 11 | 19 |
| 1.5–3 | 7 | 8 | 9 | 10 | 11 | 14 | 25 |
| 3–6 | 8 | 10 | 11 | 14 | 17 | 22 | 44 |
| 6–9 | 12 | 14 | 17 | 22 | 28 | 37 | 80 |
| 9–12 | 18 | 22 | 29 | 38 | 50 | 67 | 149 |
| 12–15 | 30 | 38 | 51 | 68 | 93 | 128 | 287 |
| 15–18 | 52 | 70 | 94 | 128 | 178 | 240 | 541 |
| 18–21 | 93 | 131 | 180 | 246 | 341 | 462 | 1076 |
| 21–24 | 182 | 255 | 344 | 478 | 665 | 917 | 2053 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), OC | |||||||
| < 1.5 | 6 | 7 | 7 | 8 | 9 | 11 | 18 |
| 1.5–3 | 7 | 7 | 8 | 9 | 11 | 13 | 24 |
| 3–6 | 8 | 9 | 11 | 13 | 16 | 20 | 41 |
| 6–9 | 11 | 13 | 16 | 20 | 26 | 34 | 74 |
| 9–12 | 17 | 20 | 27 | 35 | 46 | 62 | 137 |
| 12–15 | 27 | 36 | 46 | 64 | 87 | 117 | 265 |
| 15–18 | 49 | 64 | 86 | 118 | 163 | 220 | 500 |
| 18–21 | 88 | 122 | 165 | 227 | 313 | 434 | 956 |
| 21–24 | 169 | 231 | 317 | 443 | 604 | 838 | 1880 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), SE | |||||||
| < 1.5 | 7 | 7 | 8 | 9 | 11 | 12 | 22 |
| 1.5–3 | 7 | 8 | 9 | 10 | 12 | 15 | 28 |
| 3–6 | 9 | 11 | 13 | 15 | 19 | 24 | 50 |
| 6–9 | 13 | 16 | 19 | 25 | 33 | 43 | 92 |
| 9–12 | 19 | 25 | 33 | 44 | 59 | 77 | 173 |
| 12–15 | 33 | 44 | 58 | 79 | 106 | 143 | 333 |
| 15–18 | 61 | 80 | 110 | 149 | 208 | 282 | 639 |
| 18–21 | 113 | 150 | 208 | 287 | 395 | 547 | 1256 |
| 21–24 | 214 | 294 | 408 | 555 | 756 | 1066 | 2437 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), MB | |||||||
| < 1.5 | 7 | 7 | 7 | 8 | 10 | 11 | 19 |
| 1.5–3 | 7 | 8 | 8 | 9 | 11 | 13 | 25 |
| 3–6 | 8 | 9 | 11 | 13 | 16 | 20 | 41 |
| 6–9 | 11 | 13 | 17 | 21 | 27 | 35 | 75 |
| 9–12 | 17 | 21 | 28 | 36 | 47 | 63 | 142 |
| 12–15 | 28 | 36 | 48 | 65 | 88 | 119 | 271 |
| 15–18 | 49 | 65 | 89 | 123 | 170 | 229 | 525 |
| 18–21 | 91 | 127 | 170 | 234 | 320 | 435 | 1000 |
| 21–24 | 172 | 238 | 327 | 439 | 627 | 850 | 1982 |
| Risk factors GA, AGE, CLD plus MB, SE, OC6, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 7 | 7 | 8 | 9 | 10 | 17 |
| 1.5–3 | 7 | 7 | 8 | 9 | 10 | 12 | 22 |
| 3–6 | 8 | 9 | 10 | 12 | 15 | 19 | 37 |
| 6–9 | 11 | 12 | 15 | 19 | 24 | 31 | 66 |
| 9–12 | 15 | 20 | 25 | 32 | 41 | 57 | 122 |
| 12–15 | 24 | 33 | 44 | 58 | 78 | 103 | 236 |
| 15–18 | 44 | 58 | 78 | 106 | 147 | 200 | 453 |
| 18–21 | 81 | 106 | 148 | 201 | 278 | 384 | 864 |
| 21–24 | 149 | 213 | 282 | 386 | 530 | 747 | 1687 |
| Risk factors GA, AGE, CLD plus SEX (male), SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 7 | 7 | 8 | 9 | 11 | 19 |
| 1.5–3 | 7 | 7 | 8 | 10 | 11 | 13 | 24 |
| 3–6 | 8 | 10 | 11 | 13 | 16 | 20 | 42 |
| 6–9 | 11 | 14 | 17 | 21 | 27 | 35 | 76 |
| 9–12 | 17 | 22 | 27 | 36 | 47 | 64 | 141 |
| 12–15 | 28 | 36 | 49 | 65 | 87 | 120 | 271 |
| 15–18 | 49 | 65 | 89 | 119 | 166 | 228 | 511 |
| 18–21 | 91 | 124 | 170 | 228 | 319 | 444 | 1006 |
| 21–24 | 171 | 230 | 330 | 451 | 617 | 847 | 1945 |
| Risk factors GA, AGE, CLD plus SEX (male),MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 6 | 7 | 8 | 8 | 10 | 16 |
| 1.5–3 | 6 | 7 | 8 | 9 | 10 | 12 | 21 |
| 3–6 | 8 | 9 | 10 | 12 | 14 | 18 | 34 |
| 6–9 | 10 | 12 | 14 | 18 | 22 | 29 | 63 |
| 9–12 | 15 | 18 | 23 | 30 | 38 | 52 | 112 |
| 12–15 | 23 | 30 | 40 | 52 | 72 | 96 | 214 |
| 15–18 | 41 | 54 | 73 | 97 | 134 | 182 | 412 |
| 18–21 | 73 | 100 | 136 | 184 | 258 | 352 | 811 |
| 21–24 | 137 | 191 | 266 | 358 | 499 | 697 | 1583 |
| Risk factors GA, AGE, CLD plus SEX (male), MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 7 | 7 | 8 | 8 | 10 | 11 | 19 |
| 1.5–3 | 7 | 7 | 8 | 9 | 11 | 13 | 24 |
| 3–6 | 9 | 9 | 11 | 13 | 16 | 21 | 43 |
| 6–9 | 11 | 14 | 17 | 21 | 27 | 35 | 77 |
| 9–12 | 17 | 22 | 28 | 36 | 49 | 65 | 141 |
| 12–15 | 28 | 37 | 50 | 66 | 90 | 122 | 277 |
| 15–18 | 50 | 67 | 91 | 123 | 167 | 232 | 532 |
| 18–21 | 93 | 126 | 170 | 237 | 335 | 450 | 1025 |
| 21–24 | 174 | 243 | 328 | 450 | 628 | 871 | 2011 |
| Risk factors GA, AGE, CLD plus SEX (male), MB, SE, OC | |||||||
| < 1.5 | 6 | 7 | 7 | 8 | 9 | 11 | 18 |
| 1.5–3 | 7 | 7 | 8 | 9 | 11 | 12 | 23 |
| 3–6 | 8 | 9 | 11 | 13 | 16 | 20 | 40 |
| 6–9 | 11 | 13 | 16 | 20 | 26 | 34 | 71 |
| 9–12 | 16 | 21 | 26 | 34 | 46 | 60 | 133 |
| 12–15 | 26 | 34 | 45 | 61 | 83 | 114 | 256 |
| 15–18 | 47 | 62 | 84 | 115 | 155 | 217 | 483 |
| 18–21 | 84 | 118 | 157 | 221 | 297 | 410 | 954 |
| 21–24 | 163 | 225 | 302 | 434 | 589 | 810 | 1841 |
| Risk factors GA, AGE, CLD plus SAS, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 7 | 7 | 7 | 8 | 9 | 15 |
| 1.5–3 | 6 | 7 | 7 | 8 | 10 | 11 | 19 |
| 3–6 | 8 | 8 | 9 | 11 | 13 | 16 | 31 |
| 6–9 | 9 | 11 | 13 | 17 | 21 | 27 | 56 |
| 9–12 | 14 | 17 | 21 | 27 | 36 | 47 | 103 |
| 12–15 | 22 | 28 | 36 | 47 | 65 | 87 | 191 |
| 15–18 | 37 | 49 | 65 | 89 | 121 | 164 | 373 |
| 18–21 | 67 | 89 | 123 | 169 | 226 | 314 | 720 |
| 21–24 | 125 | 168 | 236 | 321 | 438 | 615 | 1409 |
| Risk factors GA, AGE, CLD plus SAS, MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 6 | 7 | 7 | 8 | 8 | 13 |
| 1.5–3 | 6 | 7 | 7 | 8 | 9 | 10 | 17 |
| 3–6 | 7 | 8 | 9 | 10 | 12 | 14 | 26 |
| 6–9 | 9 | 10 | 12 | 14 | 18 | 23 | 45 |
| 9–12 | 12 | 14 | 18 | 23 | 30 | 39 | 83 |
| 12–15 | 19 | 23 | 30 | 40 | 53 | 70 | 160 |
| 15–18 | 31 | 41 | 54 | 72 | 98 | 132 | 303 |
| 18–21 | 54 | 74 | 100 | 135 | 187 | 255 | 572 |
| 21–24 | 102 | 139 | 187 | 262 | 361 | 487 | 1120 |
| Risk factors GA, AGE, CLD plus SAS, MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 7 | 7 | 7 | 8 | 9 | 16 |
| 1.5–3 | 6 | 7 | 7 | 8 | 9 | 11 | 19 |
| 3–6 | 8 | 8 | 9 | 11 | 13 | 17 | 32 |
| 6–9 | 10 | 11 | 14 | 17 | 21 | 28 | 57 |
| 9–12 | 14 | 17 | 22 | 28 | 36 | 48 | 104 |
| 12–15 | 22 | 28 | 37 | 49 | 64 | 88 | 198 |
| 15–18 | 37 | 50 | 66 | 91 | 123 | 167 | 385 |
| 18–21 | 68 | 93 | 125 | 169 | 238 | 324 | 738 |
| 21–24 | 129 | 172 | 237 | 328 | 452 | 624 | 1457 |
| Risk factors GA, AGE, CLD plus SAS, MB, SE, OC | |||||||
| < 1.5 | 6 | 6 | 7 | 7 | 8 | 9 | 15 |
| 1.5–3 | 6 | 7 | 7 | 8 | 9 | 11 | 18 |
| 3–6 | 7 | 8 | 9 | 11 | 13 | 15 | 30 |
| 6–9 | 9 | 10 | 13 | 16 | 20 | 25 | 54 |
| 9–12 | 13 | 16 | 20 | 26 | 33 | 45 | 96 |
| 12–15 | 21 | 26 | 34 | 46 | 61 | 80 | 184 |
| 15–18 | 34 | 47 | 62 | 85 | 112 | 154 | 352 |
| 18–21 | 62 | 85 | 117 | 159 | 218 | 299 | 676 |
| 21–24 | 118 | 160 | 220 | 307 | 407 | 583 | 1317 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), OC, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 7 | 7 | 7 | 8 | 9 | 14 |
| 1.5–3 | 6 | 7 | 7 | 8 | 9 | 11 | 18 |
| 3–6 | 7 | 8 | 9 | 11 | 13 | 15 | 29 |
| 6–9 | 9 | 10 | 13 | 15 | 20 | 25 | 52 |
| 9–12 | 13 | 16 | 20 | 25 | 33 | 43 | 95 |
| 12–15 | 20 | 26 | 34 | 46 | 60 | 81 | 177 |
| 15–18 | 33 | 45 | 61 | 80 | 113 | 152 | 339 |
| 18–21 | 64 | 85 | 113 | 154 | 212 | 290 | 665 |
| 21–24 | 116 | 157 | 218 | 295 | 400 | 568 | 1271 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), SE, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 7 | 7 | 7 | 9 | 10 | 17 |
| 1.5–3 | 7 | 7 | 8 | 9 | 10 | 12 | 22 |
| 3–6 | 8 | 9 | 10 | 12 | 15 | 18 | 36 |
| 6–9 | 11 | 12 | 15 | 19 | 23 | 31 | 66 |
| 9–12 | 15 | 19 | 24 | 31 | 42 | 54 | 118 |
| 12–15 | 24 | 31 | 42 | 55 | 74 | 102 | 226 |
| 15–18 | 43 | 57 | 75 | 104 | 140 | 195 | 436 |
| 18–21 | 77 | 103 | 144 | 198 | 270 | 371 | 839 |
| 21–24 | 146 | 202 | 278 | 382 | 520 | 715 | 1663 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), SE, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 6 | 7 | 7 | 8 | 10 | 16 |
| 1.5–3 | 7 | 7 | 8 | 8 | 9 | 11 | 20 |
| 3–6 | 8 | 9 | 10 | 11 | 14 | 17 | 33 |
| 6–9 | 10 | 12 | 14 | 17 | 22 | 29 | 60 |
| 9–12 | 14 | 17 | 23 | 28 | 38 | 51 | 110 |
| 12–15 | 23 | 29 | 39 | 52 | 68 | 93 | 211 |
| 15–18 | 39 | 51 | 70 | 94 | 130 | 179 | 410 |
| 18–21 | 72 | 97 | 132 | 178 | 254 | 342 | 774 |
| 21–24 | 137 | 186 | 252 | 346 | 489 | 661 | 1522 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), MB, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 6 | 7 | 8 | 8 | 9 | 14 |
| 1.5–3 | 6 | 7 | 7 | 8 | 9 | 10 | 18 |
| 3–6 | 7 | 8 | 9 | 11 | 13 | 16 | 30 |
| 6–9 | 9 | 11 | 13 | 16 | 20 | 25 | 52 |
| 9–12 | 13 | 16 | 20 | 25 | 35 | 45 | 97 |
| 12–15 | 20 | 26 | 34 | 46 | 61 | 83 | 183 |
| 15–18 | 35 | 46 | 63 | 86 | 114 | 156 | 354 |
| 18–21 | 64 | 85 | 116 | 157 | 216 | 291 | 695 |
| 21–24 | 116 | 159 | 223 | 304 | 421 | 578 | 1327 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), MB, OC | |||||||
| < 1.5 | 6 | 6 | 7 | 7 | 8 | 9 | 14 |
| 1.5–3 | 6 | 7 | 7 | 8 | 9 | 10 | 17 |
| 3–6 | 7 | 8 | 9 | 10 | 12 | 15 | 28 |
| 6–9 | 9 | 11 | 12 | 15 | 19 | 24 | 49 |
| 9–12 | 12 | 16 | 19 | 24 | 31 | 42 | 90 |
| 12–15 | 19 | 24 | 32 | 42 | 56 | 76 | 167 |
| 15–18 | 33 | 43 | 57 | 77 | 104 | 143 | 328 |
| 18–21 | 59 | 79 | 106 | 148 | 202 | 271 | 628 |
| 21–24 | 110 | 148 | 206 | 274 | 387 | 530 | 1240 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), MB, SE | |||||||
| < 1.5 | 6 | 7 | 7 | 7 | 8 | 10 | 16 |
| 1.5–3 | 6 | 7 | 8 | 9 | 10 | 12 | 21 |
| 3–6 | 8 | 9 | 10 | 12 | 14 | 18 | 34 |
| 6–9 | 10 | 12 | 14 | 18 | 23 | 29 | 61 |
| 9–12 | 14 | 18 | 23 | 29 | 39 | 51 | 111 |
| 12–15 | 23 | 30 | 39 | 52 | 70 | 96 | 213 |
| 15–18 | 41 | 54 | 72 | 98 | 134 | 183 | 409 |
| 18–21 | 74 | 99 | 134 | 182 | 249 | 345 | 796 |
| 21–24 | 138 | 190 | 260 | 355 | 486 | 679 | 1542 |
| Risk factors GA, AGE, CLD plus SEX (male), MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 6 | 7 | 7 | 8 | 9 | 14 |
| 1.5–3 | 6 | 7 | 7 | 8 | 9 | 10 | 18 |
| 3–6 | 7 | 8 | 9 | 10 | 12 | 15 | 28 |
| 6–9 | 9 | 10 | 12 | 15 | 19 | 24 | 50 |
| 9–12 | 13 | 15 | 19 | 25 | 32 | 43 | 90 |
| 12–15 | 19 | 25 | 33 | 43 | 57 | 77 | 172 |
| 15–18 | 33 | 44 | 59 | 78 | 107 | 146 | 333 |
| 18–21 | 60 | 80 | 110 | 150 | 205 | 287 | 635 |
| 21–24 | 112 | 154 | 209 | 282 | 391 | 549 | 1234 |
| Risk factors GA, AGE, CLD plus SAS, MB, SE, OC | |||||||
| < 1.5 | 6 | 6 | 6 | 7 | 7 | 8 | 12 |
| 1.5–3 | 6 | 6 | 7 | 7 | 8 | 9 | 14 |
| 3–6 | 7 | 7 | 8 | 9 | 10 | 12 | 22 |
| 6–9 | 8 | 9 | 10 | 13 | 15 | 19 | 38 |
| 9–12 | 11 | 12 | 15 | 19 | 24 | 31 | 69 |
| 12–15 | 15 | 20 | 25 | 32 | 43 | 57 | 126 |
| 15–18 | 25 | 34 | 43 | 58 | 80 | 107 | 242 |
| 18–21 | 45 | 59 | 80 | 109 | 147 | 204 | 465 |
| 21–24 | 81 | 110 | 152 | 208 | 285 | 389 | 893 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 6 | 6 | 7 | 8 | 8 | 13 |
| 1.5–3 | 6 | 6 | 7 | 7 | 8 | 9 | 15 |
| 3–6 | 7 | 8 | 8 | 10 | 11 | 14 | 24 |
| 6–9 | 9 | 9 | 11 | 14 | 17 | 21 | 43 |
| 9–12 | 12 | 14 | 16 | 21 | 27 | 36 | 76 |
| 12–15 | 17 | 22 | 28 | 36 | 49 | 65 | 141 |
| 15–18 | 28 | 36 | 49 | 67 | 89 | 121 | 273 |
| 18–21 | 50 | 66 | 92 | 123 | 172 | 234 | 523 |
| 21–24 | 92 | 125 | 175 | 237 | 327 | 446 | 1016 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 6 | 6 | 7 | 7 | 8 | 11 |
| 1.5–3 | 6 | 6 | 7 | 7 | 8 | 9 | 13 |
| 3–6 | 7 | 7 | 8 | 9 | 10 | 12 | 21 |
| 6–9 | 8 | 9 | 10 | 12 | 15 | 18 | 35 |
| 9–12 | 10 | 12 | 15 | 18 | 24 | 30 | 64 |
| 12–15 | 15 | 18 | 24 | 30 | 40 | 52 | 115 |
| 15–18 | 24 | 30 | 41 | 55 | 73 | 99 | 222 |
| 18–21 | 41 | 55 | 74 | 101 | 139 | 189 | 420 |
| 21–24 | 75 | 102 | 139 | 190 | 265 | 360 | 823 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 6 | 6 | 7 | 7 | 7 | 8 | 13 |
| 1.5–3 | 6 | 6 | 7 | 7 | 8 | 10 | 15 |
| 3–6 | 7 | 8 | 8 | 10 | 11 | 13 | 25 |
| 6–9 | 8 | 10 | 11 | 13 | 17 | 21 | 43 |
| 9–12 | 12 | 14 | 17 | 22 | 28 | 37 | 77 |
| 12–15 | 17 | 22 | 29 | 37 | 50 | 67 | 146 |
| 15–18 | 30 | 38 | 50 | 68 | 94 | 124 | 282 |
| 18–21 | 50 | 69 | 95 | 130 | 172 | 239 | 538 |
| 21–24 | 94 | 129 | 174 | 241 | 336 | 453 | 1015 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), MB, SE, OC | |||||||
| < 1.5 | 6 | 6 | 6 | 7 | 7 | 8 | 12 |
| 1.5–3 | 6 | 6 | 7 | 7 | 8 | 9 | 15 |
| 3–6 | 7 | 7 | 8 | 9 | 11 | 13 | 23 |
| 6–9 | 8 | 9 | 11 | 13 | 16 | 20 | 40 |
| 9–12 | 11 | 13 | 16 | 21 | 26 | 35 | 73 |
| 12–15 | 16 | 21 | 27 | 35 | 46 | 62 | 135 |
| 15–18 | 27 | 35 | 46 | 62 | 84 | 115 | 256 |
| 18–21 | 48 | 65 | 86 | 116 | 159 | 220 | 490 |
| 21–24 | 87 | 120 | 162 | 220 | 308 | 423 | 951 |
| Risk factors GA, AGE, CLD plus SAS, SEX (male), MB, SE, OC | |||||||
| < 1.5 | 6 | 6 | 6 | 6 | 7 | 7 | 10 |
| 1.5–3 | 6 | 6 | 6 | 7 | 7 | 8 | 12 |
| 3–6 | 6 | 7 | 7 | 8 | 9 | 11 | 18 |
| 6–9 | 7 | 8 | 9 | 11 | 12 | 15 | 29 |
| 9–12 | 9 | 11 | 13 | 15 | 19 | 25 | 50 |
| 12–15 | 13 | 16 | 20 | 26 | 33 | 43 | 93 |
| 15–18 | 20 | 26 | 34 | 45 | 59 | 79 | 176 |
| 18–21 | 33 | 45 | 61 | 81 | 109 | 149 | 340 |
| 21–24 | 61 | 82 | 110 | 156 | 212 | 289 | 653 |
| AGE (months) | GA (weeks) | ||||||
|---|---|---|---|---|---|---|---|
| ≤ 24 | > 24–26 | > 26–28 | > 28–30 | > 30–32 | > 32–34 | ≥ 35 | |
| Risk factors GA, AGE, CHD (acyanotic) | |||||||
| < 1.5 | 8 | 11 | 16 | 22 | 31 | 43 | 100 |
| 1.5–3 | 11 | 17 | 22 | 31 | 42 | 59 | 138 |
| 3–6 | 23 | 32 | 44 | 62 | 83 | 114 | 266 |
| 6–9 | 45 | 61 | 86 | 119 | 162 | 224 | 523 |
| 9–12 | 90 | 120 | 166 | 235 | 323 | 443 | 996 |
| 12–15 | 167 | 234 | 324 | 443 | 611 | 854 | 2021 |
| 15–18 | 333 | 465 | 642 | 874 | 1221 | 1613 | 3960 |
| 18–21 | 641 | 900 | 1258 | 1717 | 2397 | 3266 | 7739 |
| 21–24 | 1253 | 1761 | 2439 | 3434 | 4593 | 6473 | 14,545 |
| Risk factors GA, AGE, CHD (acyanotic) plus PE ≤ 12 years | |||||||
| < 1.5 | 5 | 8 | 10 | 15 | 20 | 28 | 67 |
| 1.5–3 | 7 | 11 | 15 | 21 | 29 | 40 | 93 |
| 3–6 | 15 | 22 | 30 | 41 | 56 | 78 | 180 |
| 6–9 | 30 | 42 | 56 | 79 | 110 | 154 | 347 |
| 9–12 | 58 | 80 | 113 | 157 | 217 | 302 | 706 |
| 12–15 | 116 | 158 | 221 | 299 | 417 | 584 | 1330 |
| 15–18 | 225 | 309 | 421 | 596 | 829 | 1114 | 2585 |
| 18–21 | 436 | 606 | 860 | 1144 | 1650 | 2216 | 5185 |
| 21–24 | 835 | 1179 | 1651 | 2282 | 3149 | 4443 | 9755 |
| Risk factors GA, AGE, CHD (acyanotic) plus OC | |||||||
| < 1.5 | 5 | 7 | 10 | 13 | 18 | 27 | 60 |
| 1.5–3 | 7 | 10 | 14 | 19 | 26 | 37 | 87 |
| 3–6 | 14 | 19 | 26 | 37 | 52 | 72 | 168 |
| 6–9 | 27 | 39 | 52 | 72 | 102 | 140 | 323 |
| 9–12 | 55 | 75 | 105 | 141 | 199 | 277 | 627 |
| 12–15 | 103 | 140 | 202 | 282 | 399 | 544 | 1261 |
| 15–18 | 201 | 287 | 395 | 556 | 756 | 1040 | 2388 |
| 18–21 | 399 | 556 | 765 | 1053 | 1467 | 2064 | 4782 |
| 21–24 | 757 | 1100 | 1499 | 2065 | 2800 | 4031 | 9320 |
| Risk factors GA, AGE, CHD (acyanotic) plus SE | |||||||
| < 1.5 | 6 | 9 | 12 | 17 | 24 | 32 | 77 |
| 1.5–3 | 9 | 13 | 17 | 24 | 34 | 47 | 110 |
| 3–6 | 18 | 25 | 35 | 48 | 65 | 92 | 210 |
| 6–9 | 35 | 49 | 68 | 95 | 131 | 179 | 420 |
| 9–12 | 68 | 95 | 133 | 183 | 255 | 347 | 801 |
| 12–15 | 136 | 183 | 254 | 356 | 495 | 698 | 1571 |
| 15–18 | 260 | 360 | 504 | 681 | 960 | 1306 | 3078 |
| 18–21 | 520 | 713 | 974 | 1369 | 1883 | 2606 | 6022 |
| 21–24 | 1012 | 1393 | 1908 | 2640 | 3679 | 5232 | 11,957 |
| Risk factors GA, AGE, CHD (acyanotic) plus MB | |||||||
| < 1.5 | 5 | 7 | 10 | 14 | 19 | 27 | 61 |
| 1.5–3 | 7 | 10 | 14 | 199 | 28 | 37 | 87 |
| 3–6 | 14 | 20 | 27 | 38 | 54 | 75 | 171 |
| 6–9 | 28 | 39 | 54 | 75 | 108 | 143 | 331 |
| 9–12 | 55 | 77 | 107 | 149 | 201 | 277 | 652 |
| 12–15 | 109 | 149 | 209 | 291 | 397 | 545 | 1230 |
| 15–18 | 212 | 291 | 400 | 558 | 780 | 1083 | 2455 |
| 18–21 | 405 | 566 | 780 | 1103 | 1502 | 2119 | 4816 |
| 21–24 | 792 | 1102 | 1525 | 2163 | 2955 | 4009 | 9519 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male) | |||||||
| < 1.5 | 6 | 8 | 11 | 16 | 22 | 31 | 72 |
| 1.5–3 | 8 | 12 | 16 | 23 | 31 | 43 | 99 |
| 3–6 | 16 | 23 | 32 | 43 | 61 | 83 | 195 |
| 6–9 | 32 | 46 | 63 | 85 | 121 | 164 | 375 |
| 9–12 | 63 | 87 | 119 | 170 | 234 | 325 | 748 |
| 12–15 | 125 | 174 | 238 | 330 | 452 | 617 | 1464 |
| 15–18 | 240 | 342 | 461 | 665 | 894 | 1209 | 2892 |
| 18–21 | 468 | 639 | 911 | 1267 | 1744 | 2414 | 5556 |
| 21–24 | 913 | 1282 | 1802 | 2421 | 3376 | 4844 | 11,101 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS | |||||||
| < 1.5 | 4 | 6 | 8 | 11 | 15 | 22 | 52 |
| 1.5–3 | 6 | 8 | 11 | 16 | 22 | 31 | 72 |
| 3–6 | 12 | 16 | 22 | 32 | 43 | 61 | 140 |
| 6–9 | 23 | 31 | 46 | 62 | 85 | 117 | 273 |
| 9–12 | 45 | 62 | 87 | 121 | 167 | 232 | 522 |
| 12–15 | 87 | 124 | 170 | 241 | 327 | 458 | 1019 |
| 15–18 | 177 | 238 | 338 | 462 | 640 | 877 | 2007 |
| 18–21 | 340 | 467 | 646 | 900 | 1254 | 1718 | 4020 |
| 21–24 | 661 | 926 | 1266 | 1740 | 2432 | 3350 | 7724 |
| Risk factors GA, AGE, CHD (acyanotic) plus OC, PE ≤ 12 years | |||||||
| < 1.5 | 3 | 4 | 6 | 9 | 13 | 18 | 41 |
| 1.5–3 | 5 | 6 | 9 | 13 | 17 | 24 | 60 |
| 3–6 | 9 | 13 | 18 | 25 | 34 | 48 | 113 |
| 6–9 | 18 | 26 | 36 | 48 | 68 | 97 | 220 |
| 9–12 | 35 | 51 | 69 | 95 | 137 | 189 | 429 |
| 12–15 | 70 | 97 | 137 | 191 | 259 | 357 | 837 |
| 15–18 | 141 | 193 | 267 | 371 | 504 | 715 | 1614 |
| 18–21 | 271 | 386 | 521 | 722 | 1040 | 1395 | 3145 |
| 21–24 | 540 | 749 | 1010 | 1384 | 1951 | 2688 | 6046 |
| Risk factors GA, AGE, CHD (acyanotic) plus SE, PE ≤ 12 years | |||||||
| < 1.5 | 4 | 6 | 8 | 12 | 16 | 22 | 52 |
| 1.5–3 | 6 | 8 | 12 | 17 | 22 | 31 | 74 |
| 3–6 | 12 | 17 | 23 | 32 | 45 | 62 | 140 |
| 6–9 | 23 | 32 | 46 | 64 | 87 | 120 | 281 |
| 9–12 | 46 | 65 | 88 | 124 | 166 | 236 | 537 |
| 12–15 | 90 | 125 | 174 | 243 | 340 | 461 | 1053 |
| 15–18 | 181 | 244 | 337 | 481 | 655 | 890 | 2135 |
| 18–21 | 344 | 489 | 673 | 929 | 1284 | 1717 | 4056 |
| 21–24 | 675 | 943 | 1306 | 1754 | 2515 | 3487 | 7991 |
| Risk factors GA, AGE, CHD (acyanotic) plus SE, OC | |||||||
| < 1.5 | 4 | 5 | 7 | 11 | 15 | 29 | 48 |
| 1.5–3 | 5 | 8 | 11 | 15 | 21 | 29 | 68 |
| 3–6 | 11 | 15 | 21 | 30 | 42 | 56 | 128 |
| 6–9 | 22 | 30 | 42 | 59 | 77 | 109 | 258 |
| 9–12 | 43 | 58 | 81 | 113 | 155 | 222 | 501 |
| 12–15 | 82 | 114 | 160 | 220 | 309 | 424 | 975 |
| 15–18 | 166 | 221 | 313 | 428 | 587 | 836 | 1884 |
| 18–21 | 315 | 441 | 623 | 864 | 1184 | 1632 | 3679 |
| 21–24 | 631 | 873 | 1202 | 1667 | 2248 | 3121 | 7383 |
| Risk factors GA, AGE, CHD (acyanotic) plus MB, PE ≤ 12 years | |||||||
| < 1.5 | 3 | 6 | 7 | 10 | 13 | 18 | 43 |
| 1.5–3 | 5 | 7 | 9 | 13 | 18 | 25 | 57 |
| 3–6 | 10 | 13 | 19 | 26 | 36 | 50 | 115 |
| 6–9 | 19 | 26 | 36 | 50 | 72 | 95 | 231 |
| 9–12 | 37 | 52 | 71 | 98 | 138 | 193 | 434 |
| 12–15 | 77 | 101 | 138 | 195 | 263 | 365 | 838 |
| 15–18 | 143 | 198 | 271 | 376 | 527 | 714 | 1709 |
| 18–21 | 280 | 383 | 538 | 747 | 1012 | 1380 | 3240 |
| 21–24 | 547 | 750 | 1042 | 1449 | 1992 | 2752 | 6356 |
| Risk factors GA, AGE, CHD (acyanotic) plus MB, OC | |||||||
| < 1.5 | 3 | 4 | 6 | 8 | 12 | 16 | 39 |
| 1.5–3 | 4 | 6 | 8 | 12 | 17 | 23 | 54 |
| 3–6 | 9 | 12 | 17 | 24 | 32 | 46 | 106 |
| 6–9 | 17 | 23 | 34 | 47 | 67 | 90 | 208 |
| 9–12 | 33 | 47 | 66 | 90 | 124 | 174 | 404 |
| 12–15 | 66 | 92 | 130 | 176 | 254 | 342 | 778 |
| 15–18 | 133 | 180 | 253 | 355 | 483 | 674 | 1548 |
| 18–21 | 256 | 349 | 491 | 684 | 961 | 1302 | 3069 |
| 21–24 | 510 | 690 | 971 | 1336 | 1850 | 2459 | 5880 |
| Risk factors GA, AGE, CHD (acyanotic) plus MB, SE | |||||||
| < 1.5 | 4 | 5 | 8 | 11 | 14 | 22 | 50 |
| 1.5–3 | 6 | 8 | 11 | 16 | 21 | 29 | 68 |
| 3–6 | 11 | 15 | 22 | 31 | 42 | 58 | 135 |
| 6–9 | 22 | 32 | 42 | 59 | 84 | 114 | 263 |
| 9–12 | 45 | 60 | 85 | 118 | 163 | 224 | 524 |
| 12–15 | 84 | 118 | 165 | 226 | 313 | 430 | 1012 |
| 15–18 | 161 | 238 | 325 | 443 | 612 | 855 | 2017 |
| 18–21 | 319 | 442 | 623 | 857 | 1209 | 1682 | 3870 |
| 21–24 | 644 | 876 | 1212 | 1697 | 2375 | 3208 | 7465 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), PE ≤ 12 years | |||||||
| < 1.5 | 4 | 6 | 8 | 11 | 15 | 21 | 49 |
| 1.5–3 | 5 | 8 | 11 | 15 | 21 | 29 | 67 |
| 3–6 | 11 | 15 | 21 | 29 | 41 | 56 | 133 |
| 6–9 | 22 | 30 | 42 | 57 | 83 | 111 | 257 |
| 9–12 | 42 | 58 | 81 | 112 | 156 | 223 | 511 |
| 12–15 | 85 | 113 | 157 | 224 | 307 | 427 | 979 |
| 15–18 | 161 | 229 | 320 | 440 | 611 | 825 | 1890 |
| 18–21 | 320 | 440 | 624 | 855 | 1170 | 1626 | 3774 |
| 21–24 | 612 | 864 | 1212 | 1646 | 2282 | 3154 | 7333 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), OC | |||||||
| < 1.5 | 3 | 5 | 7 | 10 | 13 | 18 | 45 |
| 1.5–3 | 5 | 7 | 10 | 14 | 20 | 27 | 63 |
| 3–6 | 10 | 14 | 20 | 28 | 36 | 53 | 122 |
| 6–9 | 20 | 28 | 38 | 53 | 72 | 100 | 242 |
| 9–12 | 39 | 54 | 75 | 103 | 143 | 196 | 468 |
| 12–15 | 76 | 105 | 143 | 204 | 285 | 387 | 908 |
| 15–18 | 153 | 211 | 281 | 390 | 540 | 773 | 1770 |
| 18–21 | 298 | 400 | 561 | 774 | 1053 | 1463 | 3419 |
| 21–24 | 576 | 793 | 1102 | 1515 | 2157 | 2886 | 6873 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), SE | |||||||
| < 1.5 | 5 | 6 | 9 | 12 | 18 | 24 | 59 |
| 1.5–3 | 6 | 9 | 13 | 18 | 24 | 34 | 78 |
| 3–6 | 13 | 18 | 25 | 34 | 48 | 67 | 155 |
| 6–9 | 26 | 35 | 50 | 69 | 96 | 130 | 306 |
| 9–12 | 52 | 69 | 97 | 135 | 184 | 261 | 589 |
| 12–15 | 98 | 136 | 190 | 262 | 367 | 493 | 1139 |
| 15–18 | 193 | 268 | 363 | 519 | 693 | 959 | 2292 |
| 18–21 | 374 | 523 | 715 | 1016 | 1390 | 1933 | 4372 |
| 21–24 | 738 | 1032 | 1424 | 1943 | 2710 | 3654 | 8508 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), MB | |||||||
| < 1.5 | 3 | 5 | 7 | 10 | 14 | 19 | 45 |
| 1.5–3 | 5 | 7 | 10 | 14 | 19 | 27 | 65 |
| 3–6 | 11 | 14 | 19 | 28 | 38 | 54 | 127 |
| 6–9 | 21 | 28 | 40 | 55 | 75 | 107 | 245 |
| 9–12 | 40 | 56 | 77 | 106 | 146 | 205 | 473 |
| 12–15 | 77 | 110 | 149 | 211 | 291 | 395 | 925 |
| 15–18 | 155 | 214 | 292 | 410 | 572 | 806 | 1802 |
| 18–21 | 303 | 412 | 572 | 818 | 1131 | 1536 | 3521 |
| 21–24 | 578 | 821 | 1122 | 1545 | 2144 | 2953 | 6868 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, PE ≤ 12 years | |||||||
| < 1.5 | 3 | 4 | 5 | 7 | 11 | 15 | 35 |
| 1.5–3 | 4 | 5 | 7 | 10 | 15 | 21 | 48 |
| 3–6 | 8 | 11 | 15 | 22 | 29 | 40 | 96 |
| 6–9 | 15 | 21 | 30 | 43 | 59 | 77 | 184 |
| 9–12 | 30 | 42 | 59 | 80 | 114 | 161 | 364 |
| 12–15 | 60 | 82 | 115 | 158 | 219 | 302 | 700 |
| 15–18 | 118 | 163 | 220 | 309 | 439 | 610 | 1360 |
| 18–21 | 227 | 320 | 442 | 617 | 847 | 1143 | 2734 |
| 21–24 | 461 | 624 | 866 | 1183 | 1632 | 2318 | 5268 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, OC | |||||||
| < 1.5 | 2 | 3 | 5 | 7 | 9 | 13 | 32 |
| 1.5–3 | 3 | 5 | 7 | 10 | 14 | 19 | 44 |
| 3–6 | 7 | 10 | 14 | 20 | 27 | 38 | 87 |
| 6–9 | 14 | 19 | 28 | 39 | 53 | 72 | 170 |
| 9–12 | 27 | 39 | 53 | 75 | 105 | 144 | 333 |
| 12–15 | 55 | 76 | 104 | 144 | 203 | 286 | 656 |
| 15–18 | 105 | 148 | 205 | 282 | 395 | 548 | 1284 |
| 18–21 | 209 | 293 | 401 | 557 | 772 | 1056 | 2458 |
| 21–24 | 411 | 563 | 801 | 1107 | 1516 | 2122 | 4798 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SE | |||||||
| < 1.5 | 3 | 4 | 6 | 9 | 13 | 18 | 42 |
| 1.5–3 | 5 | 7 | 9 | 12 | 18 | 25 | 58 |
| 3–6 | 9 | 13 | 18 | 24 | 36 | 48 | 115 |
| 6–9 | 18 | 24 | 35 | 48 | 67 | 95 | 218 |
| 9–12 | 35 | 50 | 70 | 97 | 130 | 185 | 424 |
| 12–15 | 69 | 97 | 132 | 182 | 258 | 373 | 810 |
| 15–18 | 139 | 193 | 266 | 368 | 504 | 689 | 1650 |
| 18–21 | 269 | 371 | 527 | 708 | 994 | 1390 | 3105 |
| 21–24 | 539 | 733 | 1006 | 1415 | 1945 | 2675 | 6071 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, MB | |||||||
| < 1.5 | 3 | 3 | 5 | 7 | 10 | 14 | 33 |
| 1.5–3 | 3 | 5 | 7 | 10 | 14 | 20 | 46 |
| 3–6 | 8 | 10 | 14 | 20 | 27 | 38 | 89 |
| 6–9 | 15 | 20 | 29 | 40 | 53 | 76 | 172 |
| 9–12 | 28 | 40 | 55 | 77 | 106 | 147 | 330 |
| 12–15 | 56 | 77 | 110 | 152 | 212 | 287 | 667 |
| 15–18 | 112 | 153 | 211 | 292 | 409 | 551 | 1273 |
| 18–21 | 215 | 306 | 416 | 588 | 795 | 1098 | 2511 |
| 21–24 | 421 | 582 | 814 | 1117 | 1555 | 2142 | 4890 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male) | |||||||
| < 1.5 | 3 | 4 | 6 | 8 | 12 | 16 | 38 |
| 1.5–3 | 4 | 6 | 8 | 12 | 16 | 22 | 52 |
| 3–6 | 9 | 11 | 16 | 23 | 32 | 45 | 102 |
| 6–9 | 17 | 23 | 32 | 45 | 62 | 86 | 204 |
| 9–12 | 32 | 45 | 65 | 89 | 120 | 166 | 385 |
| 12–15 | 64 | 89 | 124 | 172 | 239 | 328 | 769 |
| 15–18 | 126 | 174 | 238 | 335 | 464 | 640 | 1483 |
| 18–21 | 248 | 340 | 475 | 662 | 915 | 1244 | 2869 |
| 21–24 | 478 | 671 | 934 | 1278 | 1794 | 2444 | 5595 |
| Risk factors GA, AGE, CHD (acyanotic) plus SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 2 | 4 | 5 | 7 | 10 | 14 | 33 |
| 1.5–3 | 3 | 5 | 7 | 10 | 14 | 20 | 46 |
| 3–6 | 7 | 10 | 14 | 19 | 28 | 38 | 88 |
| 6–9 | 14 | 20 | 28 | 39 | 55 | 77 | 172 |
| 9–12 | 29 | 40 | 56 | 75 | 103 | 146 | 338 |
| 12–15 | 57 | 78 | 108 | 148 | 205 | 289 | 660 |
| 15–18 | 110 | 152 | 211 | 296 | 405 | 572 | 1284 |
| 18–21 | 211 | 298 | 419 | 564 | 784 | 1110 | 2488 |
| 21–24 | 417 | 584 | 806 | 1124 | 1558 | 2118 | 4737 |
| Risk factors GA, AGE, CHD (acyanotic) plus MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 2 | 3 | 4 | 6 | 8 | 11 | 27 |
| 1.5–3 | 3 | 4 | 6 | 8 | 11 | 15 | 37 |
| 3–6 | 6 | 8 | 11 | 16 | 22 | 31 | 71 |
| 6–9 | 12 | 17 | 23 | 31 | 43 | 61 | 139 |
| 9–12 | 23 | 32 | 44 | 61 | 85 | 118 | 269 |
| 12–15 | 46 | 63 | 84 | 121 | 171 | 227 | 535 |
| 15–18 | 88 | 124 | 166 | 239 | 326 | 455 | 1006 |
| 18–21 | 173 | 245 | 325 | 463 | 434 | 875 | 1983 |
| 21–24 | 337 | 467 | 649 | 908 | 1232 | 1729 | 3970 |
| Risk factors GA, AGE, CHD (acyanotic) plus MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 3 | 4 | 5 | 7 | 10 | 14 | 34 |
| 1.5–3 | 4 | 5 | 7 | 10 | 14 | 20 | 47 |
| 3–6 | 7 | 10 | 15 | 20 | 29 | 40 | 90 |
| 6–9 | 15 | 20 | 29 | 39 | 56 | 76 | 182 |
| 9–12 | 30 | 41 | 57 | 79 | 109 | 149 | 351 |
| 12–15 | 59 | 80 | 112 | 157 | 217 | 298 | 675 |
| 15–18 | 113 | 159 | 218 | 297 | 413 | 574 | 1301 |
| 18–21 | 216 | 307 | 417 | 587 | 836 | 1146 | 2604 |
| 21–24 | 428 | 610 | 838 | 1126 | 1579 | 2224 | 4982 |
| Risk factors GA, AGE, CHD (acyanotic) plus MB, SE, OC | |||||||
| < 1.5 | 2 | 3 | 5 | 7 | 9 | 13 | 31 |
| 1.5–3 | 3 | 5 | 7 | 9 | 13 | 18 | 43 |
| 3–6 | 7 | 10 | 13 | 19 | 25 | 36 | 84 |
| 6–9 | 13 | 19 | 26 | 37 | 51 | 70 | 160 |
| 9–12 | 27 | 38 | 53 | 72 | 99 | 137 | 321 |
| 12–15 | 54 | 73 | 102 | 143 | 199 | 274 | 626 |
| 15–18 | 103 | 144 | 195 | 280 | 379 | 542 | 1223 |
| 18–21 | 197 | 282 | 395 | 539 | 736 | 1066 | 2349 |
| 21–24 | 395 | 575 | 742 | 1029 | 1466 | 2008 | 4664 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), OC, PE ≤ 12 years | |||||||
| < 1.5 | 2 | 3 | 5 | 7 | 9 | 13 | 30 |
| 1.5–3 | 3 | 5 | 6 | 9 | 13 | 18 | 44 |
| 3–6 | 7 | 9 | 13 | 18 | 25 | 35 | 83 |
| 6–9 | 14 | 18 | 25 | 36 | 49 | 68 | 162 |
| 9–12 | 27 | 35 | 51 | 72 | 97 | 134 | 322 |
| 12–15 | 50 | 73 | 101 | 141 | 191 | 271 | 606 |
| 15–18 | 103 | 137 | 194 | 267 | 372 | 519 | 1175 |
| 18–21 | 198 | 283 | 388 | 523 | 728 | 998 | 2351 |
| 21–24 | 385 | 534 | 745 | 1054 | 1443 | 2031 | 4479 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), SE, PE ≤ 12 years | |||||||
| < 1.5 | 3 | 4 | 6 | 8 | 12 | 16 | 39 |
| 1.5–3 | 4 | 66 | 9 | 12 | 16 | 23 | 55 |
| 3–6 | 8 | 12 | 16 | 24 | 33 | 45 | 103 |
| 6–9 | 17 | 24 | 32 | 46 | 65 | 91 | 199 |
| 9–12 | 33 | 48 | 64 | 91 | 124 | 172 | 400 |
| 12–15 | 66 | 92 | 128 | 177 | 242 | 346 | 777 |
| 15–18 | 132 | 180 | 253 | 352 | 474 | 665 | 1554 |
| 18–21 | 257 | 355 | 493 | 669 | 918 | 1308 | 3000 |
| 21–24 | 504 | 691 | 970 | 1303 | 1817 | 2502 | 5618 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), SE, OC | |||||||
| < 1.5 | 3 | 4 | 5 | 8 | 11 | 15 | 35 |
| 1.5–3 | 4 | 5 | 8 | 11 | 15 | 21 | 51 |
| 3–6 | 8 | 11 | 16 | 21 | 31 | 41 | 98 |
| 6–9 | 16 | 22 | 29 | 42 | 60 | 80 | 183 |
| 9–12 | 32 | 43 | 60 | 83 | 113 | 157 | 369 |
| 12–15 | 62 | 83 | 116 | 157 | 231 | 312 | 717 |
| 15–18 | 118 | 168 | 226 | 322 | 449 | 610 | 1372 |
| 18–21 | 233 | 316 | 440 | 611 | 847 | 1164 | 2745 |
| 21–24 | 460 | 619 | 874 | 1198 | 1656 | 2321 | 5407 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), MB | |||||||
| < 1.5 | 2 | 3 | 5 | 7 | 9 | 13 | 31 |
| 1.5–3 | 3 | 5 | 7 | 9 | 14 | 18 | 43 |
| 3–6 | 7 | 10 | 13 | 18 | 27 | 36 | 83 |
| 6–9 | 13 | 19 | 27 | 36 | 51 | 73 | 165 |
| 9–12 | 27 | 38 | 52 | 74 | 99 | 136 | 321 |
| 12–15 | 53 | 74 | 102 | 142 | 199 | 270 | 635 |
| 15–18 | 100 | 145 | 199 | 269 | 384 | 547 | 1244 |
| 18–21 | 208 | 282 | 386 | 536 | 736 | 1056 | 2384 |
| 21–24 | 390 | 549 | 764 | 1038 | 1443 | 2054 | 4710 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), MB, OC | |||||||
| < 1.5 | 2 | 3 | 4 | 6 | 9 | 12 | 28 |
| 1.5–3 | 3 | 4 | 6 | 8 | 12 | 17 | 38 |
| 3–6 | 6 | 9 | 12 | 17 | 23 | 34 | 77 |
| 6–9 | 12 | 17 | 24 | 34 | 46 | 65 | 151 |
| 9–12 | 25 | 34 | 48 | 68 | 91 | 129 | 296 |
| 12–15 | 49 | 67 | 92 | 130 | 177 | 248 | 570 |
| 15–18 | 94 | 136 | 179 | 255 | 353 | 494 | 1121 |
| 18–21 | 188 | 265 | 355 | 502 | 696 | 957 | 2124 |
| 21–24 | 368 | 508 | 692 | 973 | 1327 | 1843 | 4328 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), MB, SE | |||||||
| < 1.5 | 3 | 4 | 6 | 8 | 11 | 15 | 37 |
| 1.5–3 | 4 | 6 | 8 | 11 | 16 | 22 | 51 |
| 3–6 | 8 | 11 | 16 | 21 | 31 | 42 | 100 |
| 6–9 | 16 | 22 | 30 | 44 | 59 | 83 | 193 |
| 9–12 | 31 | 44 | 61 | 85 | 120 | 161 | 382 |
| 12–15 | 63 | 85 | 118 | 165 | 234 | 328 | 724 |
| 15–18 | 121 | 167 | 237 | 326 | 467 | 622 | 1467 |
| 18–21 | 242 | 328 | 460 | 637 | 873 | 1203 | 2844 |
| 21–24 | 471 | 644 | 909 | 1262 | 1701 | 2410 | 5600 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, OC, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 2 | 3 | 5 | 7 | 9 | 22 |
| 1.5–3 | 2 | 3 | 5 | 7 | 9 | 13 | 31 |
| 3–6 | 5 | 7 | 9 | 13 | 18 | 25 | 59 |
| 6–9 | 9 | 13 | 18 | 26 | 36 | 49 | 114 |
| 9–12 | 19 | 27 | 36 | 51 | 70 | 96 | 230 |
| 12–15 | 38 | 50 | 69 | 100 | 135 | 190 | 439 |
| 15–18 | 73 | 100 | 137 | 195 | 264 | 365 | 843 |
| 18–21 | 145 | 197 | 271 | 382 | 519 | 720 | 1629 |
| 21–24 | 268 | 380 | 537 | 737 | 1027 | 1396 | 3291 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SE, PE ≤ 12 years | |||||||
| < 1.5 | 2 | 3 | 4 | 6 | 8 | 12 | 27 |
| 1.5–3 | 3 | 4 | 6 | 8 | 11 | 17 | 39 |
| 3–6 | 6 | 8 | 12 | 17 | 23 | 33 | 77 |
| 6–9 | 12 | 17 | 24 | 33 | 47 | 64 | 146 |
| 9–12 | 24 | 34 | 46 | 63 | 90 | 120 | 286 |
| 12–15 | 48 | 67 | 92 | 128 | 173 | 246 | 551 |
| 95 | 95 | 128 | 180 | 242 | 346 | 474 | 1088 |
| 18–21 | 182 | 256 | 345 | 489 | 678 | 919 | 2089 |
| 21–24 | 360 | 480 | 680 | 930 | 1320 | 1846 | 4187 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SE, OC | |||||||
| < 1.5 | 2 | 3 | 4 | 5 | 7 | 11 | 25 |
| 1.5–3 | 3 | 4 | 5 | 8 | 11 | 15 | 36 |
| 3–6 | 5 | 8 | 11 | 15 | 21 | 30 | 69 |
| 6–9 | 11 | 16 | 22 | 30 | 43 | 59 | 138 |
| 9–12 | 23 | 30 | 42 | 61 | 81 | 116 | 261 |
| 12–15 | 44 | 60 | 84 | 118 | 161 | 229 | 504 |
| 15–18 | 85 | 122 | 164 | 227 | 312 | 450 | 1002 |
| 18–21 | 168 | 231 | 326 | 441 | 607 | 835 | 1950 |
| 21–24 | 337 | 456 | 623 | 878 | 1232 | 1633 | 3805 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, MB, PE ≤ 12 years | |||||||
| < 1.5 | 2 | 2 | 3 | 5 | 6 | 9 | 22 |
| 1.5–3 | 2 | 3 | 5 | 6 | 10 | 13 | 31 |
| 3–6 | 5 | 7 | 10 | 13 | 19 | 25 | 61 |
| 6–9 | 10 | 13 | 19 | 27 | 36 | 50 | 117 |
| 9–12 | 19 | 26 | 37 | 51 | 69 | 99 | 229 |
| 12–15 | 39 | 52 | 73 | 103 | 140 | 199 | 446 |
| 15–18 | 73 | 102 | 149 | 194 | 272 | 385 | 889 |
| 18–21 | 149 | 204 | 276 | 400 | 535 | 734 | 1692 |
| 21–24 | 278 | 399 | 548 | 752 | 1039 | 1420 | 3357 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, MB, OC | |||||||
| < 1.5 | 1 | 2 | 3 | 4 | 6 | 9 | 21 |
| 1.5–3 | 2 | 3 | 4 | 6 | 8 | 12 | 28 |
| 3–6 | 4 | 6 | 9 | 12 | 17 | 23 | 56 |
| 6–9 | 9 | 13 | 17 | 24 | 33 | 47 | 101 |
| 9–12 | 18 | 24 | 35 | 48 | 67 | 94 | 210 |
| 12–15 | 35 | 49 | 68 | 96 | 130 | 186 | 401 |
| 15–18 | 70 | 93 | 130 | 182 | 255 | 350 | 807 |
| 18–21 | 136 | 184 | 256 | 358 | 486 | 674 | 1554 |
| 21–24 | 263 | 362 | 496 | 695 | 974 | 1358 | 3128 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, MB, SE | |||||||
| < 1.5 | 2 | 3 | 4 | 5 | 8 | 11 | 25 |
| 1.5–3 | 3 | 4 | 6 | 8 | 11 | 15 | 36 |
| 3–6 | 6 | 8 | 11 | 15 | 22 | 31 | 72 |
| 6–9 | 12 | 16 | 22 | 30 | 43 | 60 | 142 |
| 9–12 | 22 | 32 | 44 | 61 | 84 | 118 | 275 |
| 12–15 | 44 | 63 | 85 | 17 | 165 | 227 | 531 |
| 15–18 | 90 | 124 | 169 | 236 | 317 | 441 | 1026 |
| 18–21 | 173 | 242 | 337 | 454 | 631 | 866 | 2042 |
| 21–24 | 338 | 452 | 637 | 891 | 1221 | 1725 | 3934 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), PE ≤ 12 years | |||||||
| < 1.5 | 2 | 2 | 4 | 5 | 7 | 11 | 25 |
| 1.5–3 | 3 | 4 | 5 | 7 | 11 | 15 | 37 |
| 3–6 | 6 | 8 | 11 | 16 | 21 | 29 | 79 |
| 6–9 | 11 | 16 | 22 | 30 | 42 | 57 | 139 |
| 9–12 | 22 | 31 | 43 | 60 | 83 | 110 | 272 |
| 12–15 | 45 | 61 | 81 | 118 | 161 | 222 | 510 |
| 15–18 | 87 | 118 | 164 | 226 | 311 | 441 | 1002 |
| 18–21 | 168 | 229 | 317 | 438 | 615 | 853 | 1989 |
| 21–24 | 323 | 450 | 632 | 866 | 1209 | 1648 | 3892 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), OC | |||||||
| < 1.5 | 2 | 3 | 4 | 5 | 7 | 10 | 23 |
| 1.5–3 | 2 | 3 | 5 | 7 | 10 | 14 | 33 |
| 3–6 | 5 | 7 | 10 | 14 | 20 | 28 | 64 |
| 6–9 | 10 | 15 | 20 | 27 | 39 | 54 | 127 |
| 9–12 | 21 | 29 | 39 | 57 | 75 | 105 | 247 |
| 12–15 | 40 | 55 | 76 | 109 | 149 | 205 | 472 |
| 15–18 | 79 | 109 | 154 | 206 | 293 | 395 | 929 |
| 18–21 | 155 | 210 | 289 | 406 | 559 | 782 | 1862 |
| 21–24 | 304 | 420 | 578 | 769 | 1105 | 1481 | 3536 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), SE | |||||||
| < 1.5 | 2 | 3 | 4 | 7 | 9 | 12 | 29 |
| 1.5–3 | 3 | 4 | 6 | 9 | 13 | 18 | 42 |
| 3–6 | 6 | 9 | 13 | 18 | 24 | 35 | 81 |
| 6–9 | 13 | 18 | 25 | 36 | 50 | 68 | 158 |
| 9–12 | 26 | 37 | 49 | 70 | 96 | 133 | 311 |
| 12–15 | 53 | 70 | 100 | 139 | 192 | 253 | 589 |
| 15–18 | 101 | 139 | 190 | 269 | 377 | 509 | 1199 |
| 18–21 | 194 | 270 | 366 | 510 | 712 | 988 | 2298 |
| 21–24 | 380 | 533 | 723 | 991 | 1442 | 1977 | 4472 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), MB | |||||||
| < 1.5 | 2 | 2 | 4 | 5 | 7 | 10 | 25 |
| 1.5–3 | 3 | 3 | 5 | 7 | 10 | 14 | 34 |
| 3–6 | 5 | 7 | 10 | 14 | 20 | 28 | 67 |
| 6–9 | 10 | 15 | 20 | 29 | 39 | 55 | 127 |
| 9–12 | 21 | 29 | 40 | 56 | 74 | 108 | 251 |
| 12–15 | 40 | 56 | 80 | 108 | 156 | 205 | 483 |
| 15–18 | 80 | 110 | 115 | 215 | 295 | 415 | 961 |
| 18–21 | 158 | 218 | 305 | 425 | 577 | 794 | 1902 |
| 21–24 | 305 | 424 | 598 | 809 | 1135 | 1549 | 3633 |
| Risk factors GA, AGE, CHD (acyanotic) plus MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 2 | 3 | 4 | 6 | 8 | 21 |
| 1.5–3 | 2 | 3 | 4 | 6 | 9 | 12 | 30 |
| 3–6 | 5 | 6 | 9 | 12 | 18 | 24 | 58 |
| 6–9 | 9 | 13 | 18 | 25 | 35 | 48 | 112 |
| 9–12 | 18 | 26 | 35 | 49 | 67 | 92 | 219 |
| 12–15 | 36 | 50 | 70 | 94 | 132 | 187 | 421 |
| 15–18 | 71 | 96 | 136 | 184 | 268 | 350 | 828 |
| 18–21 | 137 | 188 | 262 | 363 | 505 | 707 | 1620 |
| 21–24 | 277 | 375 | 507 | 705 | 975 | 1359 | 3118 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 2 | 2 | 4 | 5 | 7 | 10 | 24 |
| 1.5–3 | 2 | 4 | 5 | 7 | 10 | 14 | 34 |
| 3–6 | 5 | 7 | 10 | 14 | 20 | 27 | 64 |
| 6–9 | 11 | 15 | 21 | 28 | 40 | 56 | 128 |
| 9–12 | 20 | 29 | 41 | 55 | 77 | 107 | 243 |
| 12–15 | 42 | 57 | 80 | 109 | 152 | 210 | 477 |
| 15–18 | 80 | 111 | 155 | 216 | 298 | 407 | 925 |
| 18–21 | 159 | 217 | 303 | 413 | 572 | 801 | 1834 |
| 21–24 | 311 | 435 | 600 | 836 | 1114 | 1528 | 3471 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), MB, OC | |||||||
| < 1.5 | 1 | 2 | 3 | 4 | 5 | 8 | 19 |
| 1.5–3 | 2 | 3 | 4 | 6 | 8 | 11 | 27 |
| 3–6 | 4 | 6 | 8 | 12 | 16 | 23 | 52 |
| 6–9 | 8 | 12 | 16 | 23 | 31 | 44 | 104 |
| 9–12 | 17 | 24 | 32 | 45 | 62 | 87 | 199 |
| 12–15 | 33 | 46 | 63 | 87 | 120 | 172 | 377 |
| 15–18 | 64 | 87 | 122 | 173 | 237 | 331 | 759 |
| 18–21 | 125 | 175 | 247 | 344 | 450 | 657 | 1470 |
| 21–24 | 249 | 339 | 482 | 654 | 918 | 1259 | 2929 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 2 | 2 | 4 | 5 | 7 | 10 | 25 |
| 1.5–3 | 3 | 4 | 5 | 7 | 10 | 15 | 35 |
| 3–6 | 5 | 7 | 11 | 15 | 21 | 28 | 67 |
| 6–9 | 11 | 16 | 21 | 29 | 40 | 56 | 128 |
| 9–12 | 21 | 30 | 42 | 59 | 77 | 110 | 252 |
| 12–15 | 41 | 59 | 82 | 114 | 158 | 219 | 492 |
| 15–18 | 82 | 116 | 160 | 221 | 308 | 413 | 982 |
| 18–21 | 164 | 220 | 306 | 422 | 586 | 825 | 1878 |
| 21–24 | 321 | 438 | 596 | 836 | 1166 | 1612 | 3700 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), MB, SE, OC | |||||||
| < 1.5 | 2 | 2 | 3 | 5 | 7 | 9 | 23 |
| 1.5–3 | 2 | 3 | 5 | 6 | 9 | 13 | 32 |
| 3–6 | 5 | 7 | 10 | 14 | 19 | 27 | 61 |
| 6–9 | 10 | 14 | 19 | 27 | 39 | 51 | 120 |
| 9–12 | 20 | 28 | 38 | 53 | 73 | 100 | 236 |
| 12–15 | 39 | 54 | 75 | 102 | 141 | 197 | 450 |
| 15–18 | 75 | 104 | 148 | 205 | 276 | 384 | 868 |
| 18–21 | 150 | 202 | 287 | 394 | 542 | 762 | 1769 |
| 21–24 | 285 | 404 | 561 | 746 | 1075 | 1468 | 3389 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 2 | 2 | 3 | 5 | 7 | 17 |
| 1.5–3 | 2 | 2 | 4 | 5 | 7 | 10 | 24 |
| 3–6 | 3 | 5 | 7 | 10 | 14 | 20 | 47 |
| 6–9 | 8 | 10 | 15 | 20 | 28 | 40 | 93 |
| 9–12 | 15 | 22 | 29 | 40 | 55 | 76 | 180 |
| 12–15 | 30 | 40 | 58 | 79 | 110 | 151 | 348 |
| 15–18 | 58 | 79 | 110 | 150 | 213 | 301 | 671 |
| 18–21 | 113 | 157 | 220 | 303 | 414 | 564 | 307 |
| 21–24 | 223 | 308 | 426 | 585 | 819 | 1127 | 2510 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 1 | 2 | 3 | 4 | 6 | 14 |
| 1.5–3 | 1 | 2 | 3 | 4 | 5 | 8 | 20 |
| 3–6 | 3 | 4 | 6 | 8 | 11 | 16 | 37 |
| 6–9 | 6 | 9 | 12 | 16 | 23 | 32 | 75 |
| 9–12 | 12 | 17 | 23 | 32 | 45 | 62 | 143 |
| 12–15 | 23 | 32 | 45 | 62 | 88 | 121 | 282 |
| 15–18 | 46 | 64 | 87 | 123 | 172 | 232 | 543 |
| 18–21 | 91 | 126 | 172 | 241 | 334 | 472 | 1095 |
| 21–24 | 177 | 246 | 328 | 482 | 659 | 902 | 2070 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 2 | 2 | 4 | 5 | 7 | 18 |
| 1.5–3 | 2 | 3 | 4 | 5 | 7 | 10 | 24 |
| 3–6 | 4 | 5 | 8 | 10 | 15 | 20 | 47 |
| 6–9 | 8 | 11 | 15 | 21 | 29 | 40 | 93 |
| 9–12 | 15 | 21 | 29 | 41 | 55 | 79 | 183 |
| 12–15 | 30 | 41 | 59 | 83 | 113 | 155 | 364 |
| 15–18 | 58 | 82 | 116 | 156 | 214 | 299 | 689 |
| 18–21 | 114 | 157 | 219 | 311 | 438 | 589 | 1320 |
| 21–24 | 227 | 317 | 437 | 596 | 830 | 1141 | 2636 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, MB, SE, OC | |||||||
| < 1.5 | 1 | 1 | 2 | 3 | 5 | 7 | 17 |
| 1.5–3 | 1 | 2 | 3 | 5 | 7 | 10 | 23 |
| 3–6 | 3 | 5 | 7 | 9 | 14 | 19 | 45 |
| 6–9 | 7 | 10 | 14 | 20 | 26 | 37 | 90 |
| 9–12 | 14 | 19 | 27 | 37 | 50 | 73 | 168 |
| 12–15 | 28 | 39 | 53 | 74 | 101 | 141 | 334 |
| 15–18 | 54 | 75 | 102 | 143 | 196 | 276 | 646 |
| 18–21 | 104 | 146 | 211 | 279 | 388 | 524 | 1233 |
| 21–24 | 208 | 280 | 396 | 548 | 747 | 1057 | 2484 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), OC, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 1 | 2 | 3 | 5 | 7 | 16 |
| 1.5–3 | 1 | 2 | 3 | 5 | 7 | 9 | 22 |
| 3–6 | 3 | 5 | 7 | 9 | 13 | 18 | 44 |
| 6–9 | 7 | 9 | 14 | 19 | 25 | 35 | 85 |
| 9–12 | 13 | 19 | 27 | 37 | 51 | 71 | 160 |
| 12–15 | 27 | 38 | 51 | 72 | 101 | 139 | 315 |
| 15–18 | 54 | 76 | 105 | 142 | 200 | 269 | 632 |
| 18–21 | 105 | 144 | 200 | 278 | 389 | 529 | 1213 |
| 21–24 | 203 | 276 | 386 | 542 | 743 | 1041 | 2364 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), SE, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 2 | 3 | 4 | 6 | 8 | 20 |
| 1.5–3 | 2 | 3 | 4 | 6 | 8 | 12 | 28 |
| 3–6 | 4 | 6 | 9 | 12 | 16 | 23 | 54 |
| 6–9 | 9 | 13 | 17 | 24 | 33 | 46 | 107 |
| 9–12 | 17 | 24 | 35 | 47 | 65 | 88 | 205 |
| 12–15 | 34 | 46 | 67 | 90 | 126 | 176 | 408 |
| 15–18 | 68 | 95 | 128 | 182 | 251 | 338 | 805 |
| 18–21 | 136 | 184 | 253 | 354 | 479 | 666 | 1539 |
| 21–24 | 261 | 359 | 497 | 699 | 951 | 1321 | 3064 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), SE, OC | |||||||
| < 1.5 | 1 | 2 | 3 | 4 | 5 | 8 | 19 |
| 1.5–3 | 2 | 3 | 4 | 6 | 8 | 11 | 27 |
| 3–6 | 4 | 6 | 8 | 11 | 16 | 21 | 51 |
| 6–9 | 8 | 12 | 16 | 22 | 31 | 44 | 99 |
| 9–12 | 16 | 23 | 31 | 44 | 59 | 83 | 190 |
| 12–15 | 33 | 44 | 62 | 85 | 120 | 162 | 392 |
| 15–18 | 63 | 88 | 120 | 165 | 226 | 327 | 732 |
| 18–21 | 121 | 169 | 231 | 329 | 450 | 612 | 1404 |
| 21–24 | 238 | 326 | 467 | 642 | 869 | 1209 | 2851 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), MB, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 2 | 2 | 3 | 5 | 7 | 16 |
| 1.5–3 | 1 | 2 | 3 | 5 | 7 | 9 | 23 |
| 3–6 | 4 | 5 | 7 | 10 | 13 | 19 | 45 |
| 6–9 | 7 | 10 | 14 | 19 | 27 | 36 | 85 |
| 9–12 | 14 | 20 | 27 | 38 | 53 | 72 | 164 |
| 12–15 | 27 | 38 | 53 | 74 | 104 | 141 | 322 |
| 15–18 | 55 | 76 | 107 | 143 | 198 | 281 | 636 |
| 18–21 | 107 | 146 | 206 | 277 | 392 | 545 | 1255 |
| 21–24 | 212 | 284 | 400 | 548 | 760 | 1040 | 2351 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), MB, OC | |||||||
| < 1.5 | 1 | 1 | 2 | 3 | 4 | 6 | 14 |
| 1.5–3 | 1 | 2 | 3 | 4 | 6 | 8 | 21 |
| 3–6 | 3 | 4 | 6 | 9 | 12 | 17 | 41 |
| 6–9 | 6 | 9 | 13 | 18 | 25 | 34 | 80 |
| 9–12 | 13 | 18 | 25 | 34 | 49 | 67 | 160 |
| 12–15 | 26 | 36 | 50 | 68 | 95 | 130 | 299 |
| 15–18 | 49 | 70 | 95 | 134 | 183 | 245 | 585 |
| 18–21 | 99 | 136 | 188 | 257 | 351 | 504 | 1131 |
| 21–24 | 192 | 266 | 367 | 493 | 711 | 971 | 2216 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), MB, SE | |||||||
| < 1.5 | 1 | 2 | 3 | 4 | 6 | 8 | 19 |
| 1.5–3 | 2 | 3 | 4 | 5 | 8 | 11 | 26 |
| 3–6 | 4 | 6 | 8 | 12 | 16 | 22 | 52 |
| 6–9 | 8 | 11 | 16 | 23 | 31 | 44 | 100 |
| 9–12 | 17 | 23 | 32 | 45 | 62 | 85 | 195 |
| 12–15 | 33 | 45 | 62 | 85 | 122 | 167 | 394 |
| 15–18 | 64 | 87 | 120 | 172 | 242 | 335 | 735 |
| 18–21 | 129 | 172 | 241 | 330 | 467 | 639 | 1468 |
| 21–24 | 244 | 344 | 459 | 649 | 915 | 1241 | 2926 |
| Risk factors GA, AGE, CHD (acyanotic) plus SEX (male), MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 1 | 2 | 3 | 4 | 6 | 16 |
| 1.5–3 | 2 | 2 | 3 | 4 | 6 | 9 | 22 |
| 3–6 | 3 | 4 | 7 | 9 | 13 | 17 | 41 |
| 6–9 | 7 | 10 | 13 | 18 | 24 | 34 | 80 |
| 9–12 | 13 | 18 | 26 | 35 | 50 | 67 | 155 |
| 12–15 | 25 | 35 | 51 | 69 | 95 | 139 | 307 |
| 15–18 | 52 | 72 | 98 | 138 | 189 | 265 | 615 |
| 18–21 | 99 | 136 | 194 | 269 | 369 | 504 | 1159 |
| 21–24 | 197 | 266 | 378 | 535 | 705 | 1001 | 2293 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 1 | 1 | 2 | 3 | 4 | 11 |
| 1.5–3 | 1 | 1 | 2 | 3 | 4 | 6 | 16 |
| 3–6 | 2 | 3 | 4 | 6 | 9 | 12 | 29 |
| 6–9 | 5 | 7 | 9 | 13 | 18 | 25 | 58 |
| 9–12 | 9 | 13 | 19 | 26 | 35 | 49 | 116 |
| 12–15 | 19 | 26 | 37 | 49 | 70 | 98 | 221 |
| 15–18 | 37 | 51 | 69 | 97 | 136 | 190 | 433 |
| 18–21 | 72 | 100 | 140 | 189 | 269 | 368 | 847 |
| 21–24 | 143 | 192 | 271 | 378 | 505 | 711 | 1633 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 1 | 2 | 2 | 3 | 5 | 12 |
| 1.5–3 | 1 | 2 | 2 | 4 | 5 | 7 | 18 |
| 3–6 | 2 | 4 | 5 | 7 | 10 | 14 | 35 |
| 6–9 | 5 | 7 | 10 | 15 | 21 | 29 | 67 |
| 9–12 | 11 | 15 | 20 | 29 | 40 | 57 | 133 |
| 12–15 | 21 | 30 | 40 | 58 | 77 | 110 | 253 |
| 15–18 | 43 | 58 | 80 | 109 | 153 | 216 | 499 |
| 18–21 | 84 | 115 | 163 | 219 | 304 | 424 | 966 |
| 21–24 | 161 | 220 | 302 | 427 | 592 | 836 | 1851 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 1 | 1 | 2 | 3 | 4 | 11 |
| 1.5–3 | 1 | 1 | 2 | 3 | 5 | 6 | 14 |
| 3–6 | 2 | 3 | 4 | 6 | 8 | 11 | 28 |
| 6–9 | 4 | 6 | 8 | 11 | 17 | 23 | 53 |
| 9–12 | 8 | 12 | 17 | 23 | 32 | 45 | 106 |
| 12–15 | 17 | 23 | 32 | 47 | 63 | 88 | 203 |
| 15–18 | 34 | 47 | 66 | 90 | 125 | 171 | 393 |
| 18–21 | 66 | 91 | 125 | 173 | 242 | 343 | 785 |
| 21–24 | 130 | 179 | 244 | 344 | 478 | 646 | 1504 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 1 | 1 | 2 | 3 | 4 | 5 | 13 |
| 1.5–3 | 1 | 2 | 2 | 4 | 6 | 8 | 18 |
| 3–6 | 3 | 4 | 5 | 7 | 11 | 15 | 35 |
| 6–9 | 6 | 8 | 11 | 15 | 22 | 30 | 67 |
| 9–12 | 11 | 16 | 22 | 29 | 42 | 57 | 137 |
| 12–15 | 22 | 30 | 43 | 58 | 81 | 114 | 259 |
| 15–18 | 43 | 60 | 82 | 117 | 154 | 218 | 501 |
| 18–21 | 85 | 117 | 162 | 227 | 313 | 429 | 975 |
| 21–24 | 162 | 233 | 314 | 436 | 613 | 843 | 1961 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), MB, SE, OC | |||||||
| < 1.5 | 1 | 1 | 2 | 2 | 3 | 5 | 12 |
| 1.5–3 | 1 | 2 | 2 | 3 | 5 | 7 | 17 |
| 3–6 | 2 | 3 | 5 | 7 | 10 | 14 | 32 |
| 6–9 | 5 | 7 | 10 | 14 | 19 | 27 | 64 |
| 9–12 | 10 | 14 | 20 | 28 | 37 | 52 | 122 |
| 12–15 | 20 | 28 | 38 | 53 | 75 | 100 | 233 |
| 15–18 | 39 | 55 | 77 | 106 | 147 | 200 | 469 |
| 18–21 | 78 | 106 | 149 | 205 | 286 | 395 | 897 |
| 21–24 | 153 | 212 | 285 | 406 | 556 | 780 | 1741 |
| Risk factors GA, AGE, CHD (acyanotic) plus SAS, SEX (male), MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 0 | 1 | 1 | 1 | 2 | 3 | 8 |
| 1.5–3 | 1 | 1 | 1 | 2 | 3 | 4 | 11 |
| 3–6 | 1 | 2 | 3 | 5 | 7 | 9 | 23 |
| 6–9 | 3 | 5 | 7 | 9 | 13 | 18 | 42 |
| 9–12 | 7 | 9 | 3 | 18 | 25 | 36 | 83 |
| 12–15 | 14 | 18 | 26 | 35 | 51 | 70 | 159 |
| 15–18 | 26 | 36 | 50 | 70 | 101 | 136 | 310 |
| 18–21 | 52 | 71 | 98 | 138 | 193 | 269 | 608 |
| 21–24 | 100 | 141 | 197 | 272 | 390 | 525 | 1204 |
| AGE (months) | GA (weeks) | ||||||
|---|---|---|---|---|---|---|---|
| ≤ 24 | > 24–26 | > 26–28 | > 28–30 | > 30–32 | > 32–34 | ≥ 35 | |
| Risk factors GA, AGE, CHD (cyanotic) | |||||||
| < 1.5 | 26 | 33 | 41 | 56 | 81 | 100 | 230 |
| 1.5–3 | 31 | 47 | 56 | 76 | 101 | 134 | 307 |
| 3–6 | 59 | 78 | 109 | 147 | 184 | 254 | 596 |
| 6–9 | 106 | 140 | 195 | 271 | 357 | 490 | 1127 |
| 9–12 | 200 | 259 | 368 | 526 | 710 | 946 | 2068 |
| 12–15 | 367 | 502 | 710 | 964 | 1319 | 1850 | 4364 |
| 15–18 | 761 | 1043 | 1421 | 1898 | 2627 | 3417 | 8903 |
| 18–21 | 1398 | 1959 | 2808 | 3711 | 5088 | 7090 | 17,071 |
| 21–24 | 2723 | 3848 | 5431 | 7525 | 9736 | 13,547 | 30,203 |
| Risk factors GA, AGE, CHD (cyanotic) plus PE ≤ 12 years | |||||||
| < 1.5 | 21 | 26 | 30 | 40 | 51 | 66 | 155 |
| 1.5–3 | 24 | 31 | 44 | 54 | 71 | 95 | 217 |
| 3–6 | 41 | 59 | 76 | 97 | 131 | 177 | 398 |
| 6–9 | 73 | 100 | 127 | 173 | 245 | 354 | 771 |
| 9–12 | 133 | 173 | 255 | 352 | 481 | 658 | 1596 |
| 12–15 | 263 | 354 | 489 | 643 | 932 | 1275 | 2855 |
| 15–18 | 498 | 683 | 901 | 1309 | 1830 | 2408 | 5658 |
| 18–21 | 935 | 1275 | 1854 | 2450 | 3674 | 4641 | 11,031 |
| 21–24 | 1757 | 2544 | 3685 | 4894 | 7176 | 9817 | 20,326 |
| Risk factors GA, AGE, CHD (cyanotic) plus OC | |||||||
| < 1.5 | 20 | 22 | 30 | 37 | 46 | 68 | 133 |
| 1.5–3 | 23 | 28 | 38 | 52 | 65 | 90 | 196 |
| 3–6 | 39 | 49 | 61 | 91 | 120 | 163 | 387 |
| 6–9 | 68 | 94 | 125 | 159 | 230 | 311 | 674 |
| 9–12 | 124 | 173 | 235 | 303 | 436 | 607 | 1325 |
| 12–15 | 232 | 293 | 447 | 618 | 915 | 1199 | 2811 |
| 15–18 | 434 | 632 | 853 | 1247 | 1677 | 2277 | 5062 |
| 18–21 | 876 | 1202 | 1677 | 2192 | 3098 | 4609 | 10,263 |
| 21–24 | 1598 | 2338 | 3245 | 4437 | 5856 | 8621 | 20,345 |
| Risk factors GA, AGE, CHD (cyanotic) plus SE | |||||||
| < 1.5 | 21 | 30 | 33 | 43 | 59 | 77 | 177 |
| 1.5–3 | 28 | 35 | 45 | 63 | 82 | 115 | 252 |
| 3–6 | 47 | 63 | 86 | 112 | 152 | 209 | 470 |
| 6–9 | 86 | 114 | 150 | 215 | 299 | 393 | 942 |
| 9–12 | 153 | 210 | 294 | 416 | 557 | 752 | 1763 |
| 12–15 | 299 | 384 | 568 | 781 | 1061 | 1574 | 3458 |
| 15–18 | 570 | 772 | 1105 | 1436 | 2107 | 2761 | 6438 |
| 18–21 | 1154 | 1520 | 2129 | 2980 | 3954 | 5694 | 13,149 |
| 21–24 | 2206 | 2992 | 4117 | 5756 | 8126 | 11,306 | 26,446 |
| Risk factors GA, AGE, CHD (cyanotic) plus MB | |||||||
| < 1.5 | 19 | 24 | 30 | 39 | 51 | 67 | 139 |
| 1.5–3 | 25 | 29 | 39 | 47 | 68 | 89 | 190 |
| 3–6 | 39 | 53 | 67 | 89 | 130 | 174 | 371 |
| 6–9 | 69 | 90 | 127 | 170 | 248 | 312 | 746 |
| 9–12 | 128 | 178 | 238 | 330 | 443 | 595 | 1403 |
| 12–15 | 248 | 328 | 479 | 643 | 863 | 1208 | 2646 |
| 15–18 | 475 | 643 | 847 | 1227 | 1721 | 2363 | 5415 |
| 18–21 | 877 | 1244 | 1660 | 2514 | 3198 | 4587 | 10,222 |
| 21–24 | 1715 | 2427 | 3390 | 4690 | 6249 | 8577 | 21,675 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS | |||||||
| < 1.5 | 18 | 20 | 27 | 33 | 41 | 59 | 126 |
| 1.5–3 | 20 | 25 | 31 | 45 | 55 | 74 | 167 |
| 3–6 | 36 | 42 | 56 | 77 | 100 | 137 | 319 |
| 6–9 | 58 | 74 | 110 | 144 | 194 | 256 | 601 |
| 9–12 | 104 | 139 | 191 | 268 | 368 | 508 | 1097 |
| 12–15 | 198 | 278 | 384 | 539 | 716 | 1026 | 2227 |
| 15–18 | 396 | 517 | 749 | 982 | 1404 | 1910 | 4497 |
| 18–21 | 777 | 1003 | 1399 | 1976 | 2728 | 3728 | 8763 |
| 21–24 | 1390 | 2021 | 2724 | 3657 | 5346 | 7206 | 16,436 |
| Risk factors GA, AGE, CHD (cyanotic) plus OC, PE ≤ 12 years | |||||||
| < 1.5 | 15 | 18 | 21 | 27 | 36 | 47 | 97 |
| 1.5–3 | 19 | 23 | 27 | 36 | 45 | 62 | 142 |
| 3–6 | 27 | 36 | 47 | 63 | 81 | 110 | 256 |
| 6–9 | 46 | 64 | 86 | 112 | 152 | 224 | 480 |
| 9–12 | 81 | 119 | 165 | 215 | 312 | 424 | 942 |
| 12–15 | 158 | 212 | 304 | 430 | 563 | 810 | 1820 |
| 15–18 | 305 | 430 | 589 | 815 | 1065 | 1614 | 3392 |
| 18–21 | 594 | 881 | 1121 | 1558 | 2221 | 3024 | 6798 |
| 21–24 | 1200 | 1667 | 2128 | 2985 | 4368 | 5784 | 12,620 |
| Risk factors GA, AGE, CHD (cyanotic) plus SE, PE ≤ 12 years | |||||||
| < 1.5 | 18 | 20 | 24 | 35 | 42 | 55 | 119 |
| 1.5–3 | 21 | 26 | 33 | 47 | 55 | 75 | 164 |
| 3–6 | 35 | 45 | 57 | 77 | 106 | 142 | 305 |
| 6–9 | 59 | 76 | 111 | 152 | 194 | 278 | 606 |
| 9–12 | 110 | 149 | 198 | 276 | 366 | 533 | 1144 |
| 12–15 | 201 | 281 | 373 | 520 | 746 | 1003 | 2256 |
| 15–18 | 399 | 532 | 732 | 1093 | 1437 | 1882 | 4671 |
| 18–21 | 750 | 1074 | 1505 | 2048 | 2845 | 3581 | 8777 |
| 21–24 | 1444 | 1998 | 2865 | 3810 | 5434 | 7674 | 17,116 |
| Risk factors GA, AGE, CHD (cyanotic) plus SE, OC | |||||||
| < 1.5 | 17 | 20 | 24 | 30 | 40 | 53 | 107 |
| 1.5–3 | 19 | 25 | 32 | 40 | 56 | 72 | 156 |
| 3–6 | 35 | 42 | 53 | 74 | 101 | 125 | 272 |
| 6–9 | 55 | 74 | 100 | 143 | 168 | 237 | 564 |
| 9–12 | 98 | 136 | 182 | 245 | 341 | 483 | 1121 |
| 12–15 | 181 | 249 | 354 | 497 | 687 | 923 | 2120 |
| 15–18 | 382 | 471 | 685 | 947 | 1270 | 1849 | 4004 |
| 18–21 | 673 | 966 | 1396 | 1894 | 2604 | 3592 | 7812 |
| 21–24 | 1418 | 1893 | 2678 | 3617 | 4828 | 6614 | 16,790 |
| Risk factors GA, AGE, CHD (cyanotic) plus MB, PE ≤ 12 years | |||||||
| < 1.5 | 15 | 18 | 23 | 31 | 36 | 47 | 105 |
| 1.5–3 | 18 | 25 | 28 | 37 | 48 | 63 | 126 |
| 3–6 | 31 | 37 | 49 | 66 | 86 | 113 | 256 |
| 6–9 | 49 | 62 | 88 | 114 | 167 | 209 | 510 |
| 9–12 | 90 | 122 | 157 | 221 | 311 | 427 | 919 |
| 12–15 | 186 | 222 | 304 | 427 | 589 | 801 | 1758 |
| 15–18 | 321 | 435 | 581 | 818 | 1129 | 1515 | 3845 |
| 18–21 | 617 | 826 | 1137 | 1650 | 2203 | 2884 | 7095 |
| 21–24 | 1193 | 1615 | 2296 | 3131 | 4372 | 6066 | 13,923 |
| Risk factors GA, AGE, CHD (cyanotic) plus MB, OC | |||||||
| < 1.5 | 14 | 18 | 21 | 28 | 34 | 42 | 93 |
| 1.5–3 | 18 | 22 | 26 | 37 | 46 | 59 | 124 |
| 3–6 | 29 | 35 | 45 | 59 | 77 | 110 | 245 |
| 6–9 | 46 | 59 | 80 | 109 | 155 | 211 | 468 |
| 9–12 | 78 | 109 | 153 | 199 | 272 | 389 | 897 |
| 12–15 | 148 | 209 | 284 | 383 | 554 | 737 | 1707 |
| 15–18 | 291 | 395 | 542 | 768 | 1081 | 1427 | 3404 |
| 18–21 | 559 | 745 | 1111 | 1478 | 2098 | 2836 | 6706 |
| 21–24 | 1134 | 1448 | 2085 | 2935 | 3996 | 5194 | 13,168 |
| Risk factors GA, AGE, CHD (cyanotic) plus MB, SE | |||||||
| < 1.5 | 16 | 20 | 25 | 33 | 40 | 54 | 120 |
| 1.5–3 | 21 | 24 | 32 | 44 | 54 | 72 | 152 |
| 3–6 | 34 | 41 | 55 | 77 | 99 | 134 | 296 |
| 6–9 | 55 | 79 | 98 | 140 | 196 | 267 | 570 |
| 9–12 | 110 | 135 | 199 | 267 | 367 | 502 | 1203 |
| 12–15 | 189 | 268 | 379 | 498 | 679 | 919 | 2210 |
| 15–18 | 346 | 534 | 713 | 966 | 1362 | 1868 | 4544 |
| 18–21 | 686 | 937 | 1357 | 1906 | 2724 | 3750 | 8474 |
| 21–24 | 1426 | 1915 | 2604 | 3713 | 5213 | 6872 | 16,151 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), PE ≤ 12 years | |||||||
| < 1.5 | 17 | 22 | 26 | 33 | 40 | 53 | 113 |
| 1.5–3 | 19 | 26 | 33 | 41 | 54 | 74 | 151 |
| 3–6 | 33 | 43 | 56 | 70 | 97 | 129 | 293 |
| 6–9 | 54 | 71 | 100 | 126 | 192 | 246 | 581 |
| 9–12 | 96 | 131 | 178 | 245 | 348 | 502 | 1153 |
| 12–15 | 196 | 249 | 335 | 502 | 667 | 972 | 2144 |
| 15–18 | 353 | 507 | 735 | 983 | 1339 | 1760 | 4015 |
| 18–21 | 687 | 939 | 1394 | 1897 | 2551 | 3563 | 8578 |
| 21–24 | 1337 | 1918 | 2732 | 3549 | 4851 | 6713 | 16,047 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), OC | |||||||
| < 1.5 | 15 | 19 | 24 | 29 | 38 | 47 | 107 |
| 1.5–3 | 17 | 23 | 29 | 38 | 54 | 69 | 144 |
| 3–6 | 29 | 36 | 52 | 70 | 84 | 126 | 280 |
| 6–9 | 50 | 70 | 91 | 125 | 165 | 218 | 539 |
| 9–12 | 91 | 124 | 173 | 219 | 315 | 425 | 1014 |
| 12–15 | 173 | 228 | 317 | 441 | 636 | 857 | 1991 |
| 15–18 | 348 | 459 | 603 | 846 | 1145 | 1697 | 3723 |
| 18–21 | 656 | 839 | 1213 | 1643 | 2293 | 3251 | 7245 |
| 21–24 | 1249 | 1690 | 2405 | 3212 | 4752 | 6284 | 15,765 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), SE | |||||||
| < 1.5 | 19 | 22 | 30 | 33 | 48 | 59 | 136 |
| 1.5–3 | 23 | 28 | 35 | 47 | 63 | 80 | 171 |
| 3–6 | 36 | 47 | 64 | 81 | 112 | 154 | 332 |
| 6–9 | 66 | 85 | 116 | 161 | 221 | 291 | 679 |
| 9–12 | 126 | 166 | 222 | 311 | 410 | 597 | 1265 |
| 12–15 | 224 | 292 | 418 | 559 | 810 | 1073 | 2494 |
| 15–18 | 425 | 595 | 776 | 1112 | 1473 | 2050 | 4951 |
| 18–21 | 812 | 1209 | 1585 | 2239 | 3072 | 4218 | 9531 |
| 21–24 | 1629 | 2317 | 3100 | 4214 | 5744 | 7792 | 18,295 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), MB | |||||||
| < 1.5 | 16 | 20 | 23 | 30 | 39 | 52 | 104 |
| 1.5–3 | 20 | 23 | 30 | 41 | 51 | 70 | 155 |
| 3–6 | 33 | 41 | 50 | 70 | 88 | 127 | 285 |
| 6–9 | 55 | 68 | 94 | 127 | 172 | 240 | 535 |
| 9–12 | 92 | 130 | 177 | 233 | 325 | 453 | 1020 |
| 12–15 | 172 | 246 | 325 | 470 | 652 | 842 | 2069 |
| 15–18 | 348 | 476 | 624 | 876 | 1245 | 1831 | 4104 |
| 18–21 | 659 | 897 | 1252 | 1749 | 2496 | 3383 | 7590 |
| 21–24 | 1204 | 1786 | 2396 | 3416 | 4648 | 6262 | 14,784 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, PE ≤ 12 years | |||||||
| < 1.5 | 14 | 17 | 19 | 24 | 32 | 41 | 82 |
| 1.5–3 | 17 | 19 | 25 | 31 | 41 | 55 | 110 |
| 3–6 | 25 | 33 | 44 | 57 | 70 | 95 | 215 |
| 6–9 | 40 | 53 | 77 | 104 | 136 | 169 | 408 |
| 9–12 | 71 | 100 | 132 | 181 | 252 | 356 | 800 |
| 12–15 | 137 | 190 | 261 | 339 | 494 | 654 | 1491 |
| 15–18 | 266 | 369 | 479 | 680 | 957 | 1377 | 2894 |
| 18–21 | 508 | 695 | 966 | 1322 | 1818 | 2486 | 6169 |
| 21–24 | 1032 | 1384 | 1867 | 2555 | 3564 | 5031 | 11,480 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, OC | |||||||
| < 1.5 | 14 | 16 | 19 | 25 | 29 | 38 | 76 |
| 1.5–3 | 16 | 19 | 24 | 32 | 38 | 51 | 106 |
| 3–6 | 25 | 32 | 41 | 52 | 68 | 93 | 200 |
| 6–9 | 42 | 49 | 70 | 95 | 119 | 166 | 377 |
| 9–12 | 66 | 96 | 121 | 174 | 236 | 321 | 715 |
| 12–15 | 128 | 168 | 234 | 311 | 444 | 674 | 1485 |
| 15–18 | 240 | 327 | 467 | 615 | 848 | 1188 | 2767 |
| 18–21 | 451 | 662 | 873 | 1193 | 1699 | 2287 | 5270 |
| 21–24 | 876 | 1207 | 1760 | 2370 | 3261 | 4576 | 10,201 |
| Risk factors GA, AGE, CHD (cyanotic) SAS, SE | |||||||
| < 1.5 | 15 | 18 | 22 | 28 | 37 | 47 | 99 |
| 1.5–3 | 19 | 23 | 26 | 35 | 48 | 64 | 135 |
| 3–6 | 27 | 38 | 51 | 62 | 93 | 113 | 258 |
| 6–9 | 44 | 60 | 83 | 112 | 150 | 218 | 484 |
| 9–12 | 84 | 120 | 161 | 225 | 300 | 414 | 945 |
| 12–15 | 153 | 221 | 289 | 395 | 569 | 842 | 1765 |
| 15–18 | 313 | 421 | 602 | 799 | 1063 | 1469 | 3640 |
| 18–21 | 593 | 814 | 1168 | 1536 | 2155 | 3133 | 6701 |
| 21–24 | 1161 | 1601 | 2275 | 3156 | 4220 | 5783 | 12,350 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, MB | |||||||
| < 1.5 | 14 | 16 | 19 | 23 | 29 | 38 | 81 |
| 1.5–3 | 16 | 20 | 24 | 30 | 38 | 54 | 111 |
| 3–6 | 26 | 29 | 40 | 54 | 67 | 88 | 201 |
| 6–9 | 42 | 52 | 69 | 100 | 122 | 174 | 383 |
| 9–12 | 68 | 95 | 128 | 177 | 239 | 331 | 692 |
| 12–15 | 130 | 172 | 247 | 340 | 468 | 618 | 1436 |
| 15–18 | 252 | 341 | 470 | 636 | 906 | 1190 | 2739 |
| 18–21 | 464 | 683 | 922 | 1266 | 1715 | 2383 | 5275 |
| 21–24 | 926 | 1299 | 1754 | 2399 | 3395 | 4589 | 10,165 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male) | |||||||
| < 1.5 | 16 | 18 | 20 | 27 | 36 | 43 | 88 |
| 1.5–3 | 18 | 21 | 28 | 35 | 43 | 56 | 118 |
| 3–6 | 27 | 32 | 42 | 57 | 80 | 106 | 227 |
| 6–9 | 46 | 62 | 77 | 108 | 138 | 194 | 458 |
| 9–12 | 75 | 108 | 155 | 204 | 263 | 361 | 875 |
| 12–15 | 148 | 199 | 273 | 381 | 517 | 719 | 1686 |
| 15–18 | 274 | 383 | 520 | 747 | 1001 | 1391 | 3240 |
| 18–21 | 531 | 728 | 1026 | 1433 | 1981 | 2685 | 5983 |
| 21–24 | 1052 | 1445 | 1997 | 2791 | 3942 | 5135 | 11,757 |
| Risk factors GA, AGE, CHD (cyanotic) plus SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 13 | 16 | 19 | 24 | 29 | 41 | 82 |
| 1.5–3 | 16 | 19 | 24 | 29 | 36 | 53 | 108 |
| 3–6 | 24 | 32 | 39 | 48 | 68 | 91 | 193 |
| 6–9 | 40 | 50 | 70 | 92 | 127 | 178 | 377 |
| 9–12 | 69 | 96 | 132 | 163 | 226 | 322 | 746 |
| 12–15 | 135 | 173 | 244 | 323 | 440 | 624 | 1442 |
| 15–18 | 240 | 345 | 472 | 654 | 881 | 1249 | 2813 |
| 18–21 | 447 | 672 | 950 | 1240 | 1693 | 2376 | 5344 |
| 21–24 | 890 | 1303 | 1702 | 2418 | 3458 | 4533 | 9882 |
| Risk factors GA, AGE, CHD (cyanotic) plus MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 13 | 16 | 18 | 21 | 25 | 33 | 66 |
| 1.5–3 | 14 | 17 | 21 | 26 | 31 | 42 | 87 |
| 3–6 | 21 | 27 | 33 | 43 | 56 | 75 | 161 |
| 6–9 | 35 | 45 | 56 | 78 | 99 | 147 | 310 |
| 9–12 | 60 | 80 | 99 | 136 | 198 | 269 | 582 |
| 12–15 | 110 | 146 | 183 | 276 | 386 | 483 | 1154 |
| 15–18 | 204 | 274 | 359 | 533 | 718 | 1012 | 2119 |
| 18–21 | 387 | 546 | 695 | 1007 | 1405 | 1832 | 4092 |
| 21–24 | 732 | 1025 | 1434 | 2043 | 2681 | 3693 | 8584 |
| Risk factors GA, AGE, CHD (cyanotic) plus MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 14 | 16 | 20 | 25 | 31 | 39 | 80 |
| 1.5–3 | 16 | 20 | 25 | 30 | 39 | 51 | 109 |
| 3–6 | 23 | 29 | 41 | 49 | 72 | 95 | 200 |
| 6–9 | 42 | 52 | 74 | 91 | 128 | 167 | 407 |
| 9–12 | 76 | 95 | 127 | 184 | 235 | 329 | 785 |
| 12–15 | 139 | 187 | 250 | 365 | 493 | 662 | 1434 |
| 15–18 | 246 | 353 | 477 | 645 | 889 | 1255 | 2733 |
| 18–21 | 463 | 673 | 909 | 1302 | 1898 | 2459 | 5703 |
| 21–24 | 948 | 1347 | 1868 | 2307 | 3357 | 4926 | 10,837 |
| Risk factors GA, AGE, CHD (cyanotic) plus MB, SE, OC | |||||||
| < 1.5 | 14 | 15 | 19 | 22 | 29 | 36 | 74 |
| 1.5–3 | 15 | 19 | 22 | 28 | 38 | 48 | 99 |
| 3–6 | 25 | 29 | 37 | 50 | 62 | 85 | 188 |
| 6–9 | 37 | 52 | 64 | 91 | 121 | 154 | 352 |
| 9–12 | 66 | 93 | 125 | 172 | 233 | 303 | 695 |
| 12–15 | 132 | 169 | 235 | 321 | 448 | 590 | 1335 |
| 15–18 | 226 | 319 | 421 | 623 | 819 | 1257 | 2608 |
| 18–21 | 427 | 603 | 870 | 1185 | 1597 | 2334 | 4933 |
| 21–24 | 864 | 1303 | 1619 | 2197 | 3228 | 4348 | 10,038 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), OC, PE ≤ 12 years | |||||||
| < 1.5 | 12 | 15 | 19 | 24 | 27 | 35 | 71 |
| 1.5–3 | 16 | 20 | 21 | 27 | 37 | 47 | 104 |
| 3–6 | 23 | 29 | 36 | 49 | 62 | 83 | 194 |
| 6–9 | 37 | 47 | 60 | 85 | 111 | 153 | 348 |
| 9–12 | 68 | 81 | 117 | 166 | 214 | 301 | 733 |
| 12–15 | 116 | 170 | 242 | 314 | 433 | 595 | 1361 |
| 15–18 | 236 | 299 | 425 | 613 | 796 | 1122 | 2550 |
| 18–21 | 440 | 643 | 878 | 1096 | 1580 | 2114 | 5151 |
| 21–24 | 844 | 1163 | 1612 | 2356 | 3199 | 4683 | 9667 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), SE, PE ≤ 12 years | |||||||
| < 1.5 | 14 | 18 | 21 | 26 | 36 | 44 | 93 |
| 1.5–3 | 17 | 20 | 28 | 34 | 45 | 62 | 129 |
| 3–6 | 26 | 35 | 43 | 60 | 79 | 106 | 233 |
| 6–9 | 45 | 61 | 76 | 107 | 149 | 210 | 422 |
| 9–12 | 80 | 113 | 147 | 203 | 281 | 376 | 870 |
| 12–15 | 150 | 206 | 286 | 399 | 518 | 772 | 1750 |
| 15–18 | 310 | 412 | 558 | 778 | 1017 | 1468 | 3502 |
| 18–21 | 563 | 802 | 1082 | 1449 | 1946 | 2878 | 6474 |
| 21–24 | 1104 | 1487 | 2214 | 2787 | 3918 | 5379 | 11,857 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), SE, OC | |||||||
| < 1.5 | 15 | 18 | 20 | 25 | 32 | 41 | 84 |
| 1.5–3 | 18 | 20 | 24 | 31 | 43 | 56 | 120 |
| 3–6 | 24 | 31 | 44 | 55 | 75 | 100 | 221 |
| 6–9 | 44 | 58 | 69 | 100 | 138 | 176 | 388 |
| 9–12 | 80 | 102 | 142 | 186 | 250 | 354 | 790 |
| 12–15 | 137 | 187 | 257 | 339 | 522 | 682 | 1529 |
| 15–18 | 257 | 388 | 495 | 726 | 1007 | 1299 | 2951 |
| 18–21 | 510 | 698 | 952 | 1333 | 1887 | 2468 | 6024 |
| 21–24 | 1017 | 1366 | 1894 | 2516 | 3569 | 5097 | 11,692 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), MB, PE ≤ 12 years | |||||||
| < 1.5 | 14 | 15 | 19 | 22 | 30 | 36 | 74 |
| 1.5–3 | 15 | 19 | 24 | 28 | 40 | 50 | 98 |
| 3–6 | 23 | 29 | 37 | 46 | 70 | 88 | 186 |
| 6–9 | 36 | 49 | 68 | 86 | 119 | 170 | 371 |
| 9–12 | 68 | 94 | 122 | 179 | 222 | 300 | 719 |
| 12–15 | 124 | 172 | 234 | 314 | 445 | 574 | 1421 |
| 15–18 | 217 | 323 | 444 | 592 | 846 | 1246 | 2777 |
| 18–21 | 471 | 618 | 828 | 1164 | 1547 | 2329 | 5150 |
| 21–24 | 846 | 1224 | 1667 | 2189 | 3207 | 4407 | 10,484 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), MB, OC | |||||||
| < 1.5 | 14 | 15 | 18 | 20 | 28 | 33 | 69 |
| 1.5–3 | 13 | 18 | 22 | 27 | 34 | 47 | 89 |
| 3–6 | 22 | 28 | 35 | 45 | 57 | 81 | 172 |
| 6–9 | 33 | 45 | 62 | 86 | 105 | 150 | 330 |
| 9–12 | 64 | 81 | 113 | 159 | 203 | 301 | 658 |
| 12–15 | 118 | 155 | 203 | 283 | 379 | 565 | 1246 |
| 15–18 | 205 | 306 | 386 | 563 | 745 | 1070 | 2334 |
| 18–21 | 417 | 585 | 783 | 1139 | 1489 | 2069 | 4455 |
| 21–24 | 816 | 1100 | 1514 | 2145 | 2829 | 3946 | 9494 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), MB, SE | |||||||
| < 1.5 | 15 | 18 | 22 | 25 | 33 | 41 | 91 |
| 1.5–3 | 17 | 21 | 25 | 32 | 42 | 56 | 121 |
| 3–6 | 26 | 32 | 44 | 52 | 75 | 97 | 229 |
| 6–9 | 43 | 54 | 74 | 106 | 131 | 195 | 427 |
| 9–12 | 75 | 102 | 139 | 199 | 265 | 333 | 838 |
| 12–15 | 147 | 190 | 259 | 361 | 526 | 729 | 1473 |
| 15–18 | 269 | 375 | 517 | 719 | 1046 | 1372 | 3306 |
| 18–21 | 534 | 702 | 1024 | 1403 | 1900 | 2579 | 6244 |
| 21–24 | 1017 | 1436 | 2073 | 2746 | 3633 | 5329 | 11,816 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, OC, PE ≤ 12 years | |||||||
| < 1.5 | 12 | 14 | 15 | 18 | 24 | 30 | 54 |
| 1.5–3 | 13 | 14 | 19 | 23 | 29 | 36 | 82 |
| 3–6 | 18 | 22 | 29 | 36 | 48 | 62 | 137 |
| 6–9 | 27 | 36 | 47 | 64 | 87 | 113 | 249 |
| 9–12 | 50 | 67 | 83 | 123 | 163 | 214 | 520 |
| 12–15 | 92 | 117 | 153 | 231 | 291 | 407 | 978 |
| 15–18 | 167 | 227 | 295 | 445 | 578 | 775 | 1777 |
| 18–21 | 321 | 434 | 587 | 854 | 1165 | 1560 | 3466 |
| 21–24 | 571 | 834 | 1188 | 1615 | 2242 | 3040 | 7129 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SE, PE ≤ 12 years | |||||||
| < 1.5 | 13 | 14 | 16 | 20 | 25 | 34 | 66 |
| 1.5–3 | 15 | 18 | 21 | 27 | 32 | 46 | 95 |
| 3–6 | 22 | 25 | 34 | 44 | 56 | 79 | 177 |
| 6–9 | 36 | 44 | 61 | 79 | 116 | 149 | 321 |
| 9–12 | 62 | 81 | 110 | 149 | 203 | 267 | 614 |
| 12–15 | 111 | 159 | 203 | 290 | 373 | 547 | 1210 |
| 15–18 | 225 | 282 | 407 | 526 | 796 | 1063 | 2391 |
| 18–21 | 400 | 575 | 751 | 1076 | 1511 | 1976 | 4476 |
| 21–24 | 794 | 1006 | 1482 | 2001 | 2843 | 4040 | 9330 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SE, OC | |||||||
| < 1.5 | 13 | 15 | 17 | 20 | 24 | 33 | 59 |
| 1.5–3 | 15 | 17 | 20 | 25 | 34 | 41 | 88 |
| 3–6 | 20 | 25 | 32 | 42 | 54 | 74 | 162 |
| 6–9 | 34 | 44 | 55 | 74 | 102 | 135 | 307 |
| 9–12 | 60 | 73 | 99 | 141 | 180 | 265 | 564 |
| 12–15 | 104 | 135 | 191 | 267 | 359 | 529 | 1085 |
| 15–18 | 191 | 285 | 350 | 495 | 680 | 1029 | 2114 |
| 18–21 | 382 | 518 | 743 | 960 | 1324 | 1828 | 4125 |
| 21–24 | 773 | 1036 | 1363 | 1927 | 2787 | 3494 | 8013 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, MB, PE ≤ 12 years | |||||||
| < 1.5 | 12 | 13 | 16 | 19 | 22 | 28 | 59 |
| 1.5–3 | 15 | 16 | 20 | 22 | 31 | 36 | 75 |
| 3–6 | 19 | 23 | 30 | 36 | 50 | 63 | 145 |
| 6–9 | 30 | 35 | 52 | 67 | 85 | 117 | 260 |
| 9–12 | 49 | 65 | 89 | 118 | 151 | 222 | 500 |
| 12–15 | 96 | 120 | 166 | 246 | 312 | 457 | 991 |
| 15–18 | 166 | 226 | 335 | 409 | 610 | 873 | 1964 |
| 18–21 | 347 | 453 | 607 | 897 | 1176 | 1564 | 3644 |
| 21–24 | 592 | 882 | 1218 | 1650 | 2191 | 3018 | 7202 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, MB, OC | |||||||
| < 1.5 | 11 | 15 | 15 | 18 | 21 | 28 | 55 |
| 1.5–3 | 12 | 15 | 17 | 23 | 27 | 37 | 67 |
| 3–6 | 18 | 22 | 28 | 35 | 46 | 58 | 128 |
| 6–9 | 29 | 36 | 45 | 60 | 79 | 115 | 240 |
| 9–12 | 48 | 62 | 85 | 115 | 159 | 217 | 450 |
| 12–15 | 82 | 118 | 157 | 224 | 285 | 446 | 828 |
| 15–18 | 165 | 204 | 281 | 403 | 564 | 770 | 1773 |
| 18–21 | 304 | 421 | 556 | 802 | 1072 | 1448 | 3289 |
| 21–24 | 588 | 774 | 1073 | 1503 | 2109 | 3073 | 6786 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, MB, SE | |||||||
| < 1.5 | 13 | 13 | 18 | 20 | 26 | 33 | 61 |
| 1.5–3 | 14 | 16 | 21 | 26 | 33 | 43 | 86 |
| 3–6 | 21 | 26 | 32 | 40 | 56 | 75 | 170 |
| 6–9 | 36 | 46 | 58 | 71 | 103 | 137 | 319 |
| 9–12 | 57 | 77 | 102 | 140 | 190 | 265 | 597 |
| 12–15 | 107 | 148 | 192 | 259 | 365 | 503 | 1172 |
| 15–18 | 207 | 268 | 375 | 519 | 681 | 969 | 2286 |
| 18–21 | 395 | 527 | 779 | 961 | 1349 | 1897 | 4519 |
| 21–24 | 744 | 951 | 1344 | 1901 | 2614 | 3681 | 8691 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), PE ≤ 12 years | |||||||
| < 1.5 | 12 | 13 | 17 | 21 | 24 | 32 | 61 |
| 1.5–3 | 15 | 17 | 20 | 24 | 31 | 41 | 91 |
| 3–6 | 21 | 25 | 32 | 42 | 53 | 70 | 159 |
| 6–9 | 32 | 41 | 54 | 73 | 101 | 130 | 328 |
| 9–12 | 57 | 76 | 106 | 137 | 186 | 240 | 621 |
| 12–15 | 105 | 141 | 180 | 274 | 362 | 482 | 1085 |
| 15–18 | 204 | 268 | 362 | 487 | 682 | 966 | 2170 |
| 18–21 | 363 | 515 | 690 | 945 | 1364 | 1843 | 4431 |
| 21–24 | 701 | 978 | 1394 | 1883 | 2710 | 3631 | 8513 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), OC | |||||||
| < 1.5 | 11 | 15 | 17 | 20 | 25 | 31 | 57 |
| 1.5–3 | 14 | 16 | 20 | 22 | 31 | 38 | 81 |
| 3–6 | 20 | 26 | 29 | 40 | 51 | 70 | 142 |
| 6–9 | 30 | 40 | 52 | 67 | 92 | 127 | 293 |
| 9–12 | 60 | 68 | 92 | 134 | 168 | 246 | 540 |
| 12–15 | 95 | 127 | 168 | 264 | 330 | 449 | 1025 |
| 15–18 | 177 | 239 | 356 | 438 | 657 | 847 | 2008 |
| 18–21 | 353 | 468 | 611 | 887 | 1172 | 1732 | 4139 |
| 21–24 | 667 | 915 | 1252 | 1619 | 2378 | 3084 | 7768 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), SE | |||||||
| < 1.5 | 13 | 14 | 17 | 23 | 28 | 35 | 71 |
| 1.5–3 | 15 | 18 | 22 | 28 | 35 | 48 | 102 |
| 3–6 | 21 | 27 | 37 | 49 | 61 | 83 | 180 |
| 6–9 | 38 | 49 | 62 | 90 | 115 | 157 | 342 |
| 9–12 | 63 | 91 | 110 | 160 | 215 | 298 | 716 |
| 12–15 | 125 | 154 | 232 | 309 | 431 | 542 | 1277 |
| 15–18 | 234 | 307 | 423 | 601 | 823 | 1101 | 2620 |
| 18–21 | 440 | 593 | 778 | 1101 | 1533 | 2179 | 4977 |
| 21–24 | 821 | 1146 | 1602 | 2157 | 3241 | 4375 | 9666 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), SE | |||||||
| < 1.5 | 13 | 13 | 16 | 19 | 23 | 31 | 66 |
| 1.5–3 | 15 | 15 | 20 | 25 | 28 | 39 | 81 |
| 3–6 | 18 | 24 | 29 | 39 | 52 | 67 | 152 |
| 6–9 | 31 | 40 | 53 | 71 | 95 | 126 | 278 |
| 9–12 | 55 | 72 | 93 | 128 | 163 | 242 | 545 |
| 12–15 | 93 | 130 | 183 | 234 | 361 | 443 | 1029 |
| 15–18 | 192 | 250 | 347 | 471 | 623 | 910 | 2196 |
| 18–21 | 339 | 494 | 666 | 966 | 1244 | 1682 | 4328 |
| 21–24 | 666 | 923 | 1289 | 1736 | 2471 | 3350 | 7827 |
| Risk factors GA, AGE, CHD (cyanotic) plus MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 11 | 12 | 16 | 18 | 22 | 25 | 54 |
| 1.5–3 | 12 | 16 | 18 | 23 | 28 | 35 | 78 |
| 3–6 | 18 | 21 | 27 | 35 | 48 | 62 | 135 |
| 6–9 | 29 | 36 | 48 | 61 | 84 | 115 | 256 |
| 9–12 | 46 | 65 | 82 | 115 | 154 | 200 | 490 |
| 12–15 | 87 | 121 | 157 | 212 | 303 | 425 | 923 |
| 15–18 | 160 | 217 | 312 | 399 | 593 | 770 | 1829 |
| 18–21 | 305 | 409 | 566 | 812 | 1107 | 1535 | 3573 |
| 21–24 | 617 | 831 | 1082 | 1525 | 2086 | 2986 | 6642 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), SE, OC | |||||||
| < 1.5 | 12 | 14 | 17 | 19 | 26 | 30 | 60 |
| 1.5–3 | 14 | 17 | 19 | 23 | 29 | 39 | 85 |
| 3–6 | 19 | 25 | 31 | 40 | 53 | 65 | 144 |
| 6–9 | 32 | 42 | 52 | 70 | 96 | 129 | 281 |
| 9–12 | 49 | 71 | 97 | 127 | 176 | 240 | 525 |
| 12–15 | 101 | 130 | 183 | 249 | 337 | 453 | 1034 |
| 15–18 | 184 | 247 | 350 | 477 | 635 | 872 | 1986 |
| 18–21 | 355 | 469 | 671 | 896 | 1207 | 1738 | 3958 |
| 21–24 | 671 | 961 | 1308 | 1872 | 2399 | 3290 | 7119 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 11 | 12 | 14 | 18 | 19 | 25 | 48 |
| 14 | 13 | 14 | 15 | 21 | 25 | 32 | 64 |
| 3–6 | 17 | 22 | 26 | 33 | 43 | 59 | 117 |
| 6–9 | 28 | 34 | 41 | 61 | 76 | 102 | 237 |
| 9–12 | 44 | 62 | 79 | 108 | 144 | 198 | 440 |
| 12–15 | 79 | 103 | 142 | 195 | 263 | 385 | 827 |
| 15–18 | 147 | 192 | 272 | 384 | 532 | 716 | 1714 |
| 18–21 | 272 | 394 | 554 | 770 | 987 | 1487 | 3173 |
| 21–24 | 547 | 711 | 1089 | 1432 | 2004 | 2644 | 6330 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 12 | 13 | 16 | 20 | 24 | 31 | 63 |
| 1.5–3 | 14 | 16 | 20 | 25 | 32 | 42 | 86 |
| 3–6 | 20 | 24 | 32 | 41 | 54 | 71 | 158 |
| 6–9 | 34 | 44 | 55 | 75 | 93 | 128 | 286 |
| 9–12 | 55 | 73 | 100 | 141 | 172 | 245 | 550 |
| 12–15 | 95 | 136 | 186 | 261 | 349 | 496 | 1085 |
| 15–18 | 180 | 257 | 360 | 489 | 657 | 882 | 2104 |
| 18–21 | 379 | 479 | 652 | 925 | 1309 | 1804 | 4022 |
| 21–24 | 717 | 935 | 1279 | 1816 | 2555 | 3528 | 7756 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), MB, SE, OC | |||||||
| < 1.5 | 13 | 14 | 15 | 18 | 23 | 30 | 63 |
| 1.5–3 | 14 | 15 | 19 | 22 | 29 | 37 | 77 |
| 3–6 | 19 | 24 | 29 | 38 | 48 | 66 | 137 |
| 6–9 | 30 | 37 | 50 | 66 | 94 | 121 | 265 |
| 9–12 | 50 | 70 | 91 | 126 | 164 | 232 | 541 |
| 93 | 93 | 126 | 177 | 229 | 308 | 437 | 1019 |
| 15–18 | 174 | 238 | 338 | 446 | 604 | 819 | 1844 |
| 18–21 | 343 | 444 | 608 | 870 | 1176 | 1644 | 3900 |
| 21–24 | 609 | 891 | 1211 | 1547 | 2347 | 3143 | 7349 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 11 | 12 | 15 | 16 | 19 | 23 | 46 |
| 1.5–3 | 11 | 14 | 16 | 20 | 24 | 32 | 60 |
| 3–6 | 15 | 20 | 25 | 30 | 38 | 49 | 112 |
| 6–9 | 25 | 31 | 41 | 50 | 72 | 94 | 211 |
| 9–12 | 40 | 59 | 72 | 97 | 127 | 171 | 399 |
| 12–15 | 76 | 92 | 131 | 178 | 247 | 333 | 751 |
| 15–18 | 136 | 178 | 244 | 324 | 475 | 673 | 1426 |
| 18–21 | 250 | 344 | 489 | 691 | 911 | 1215 | 2787 |
| 21–24 | 486 | 670 | 928 | 1241 | 1819 | 2490 | 5209 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 10 | 11 | 12 | 14 | 19 | 20 | 37 |
| 1.5–3 | 11 | 12 | 14 | 18 | 21 | 26 | 51 |
| 3–6 | 15 | 17 | 21 | 23 | 33 | 43 | 87 |
| 6–9 | 21 | 27 | 33 | 43 | 60 | 78 | 175 |
| 9–12 | 35 | 47 | 59 | 76 | 102 | 140 | 323 |
| 12–15 | 60 | 79 | 105 | 144 | 204 | 275 | 614 |
| 15–18 | 111 | 154 | 193 | 271 | 389 | 504 | 1185 |
| 18–21 | 206 | 281 | 369 | 535 | 723 | 1109 | 2399 |
| 21–24 | 386 | 550 | 699 | 1067 | 1411 | 2004 | 4500 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 11 | 12 | 14 | 17 | 18 | 23 | 47 |
| 1.5–3 | 11 | 14 | 18 | 20 | 25 | 31 | 58 |
| 3–6 | 17 | 21 | 25 | 30 | 44 | 51 | 111 |
| 6–9 | 26 | 32 | 41 | 54 | 69 | 95 | 205 |
| 9–12 | 40 | 54 | 71 | 98 | 121 | 178 | 401 |
| 12–15 | 77 | 97 | 143 | 189 | 249 | 346 | 798 |
| 15–18 | 135 | 186 | 256 | 341 | 451 | 639 | 1490 |
| 18–21 | 248 | 335 | 478 | 685 | 951 | 1300 | 2810 |
| 21–24 | 495 | 723 | 965 | 1299 | 1766 | 2496 | 5636 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, MB, SE, OC | |||||||
| < 1.5 | 11 | 11 | 14 | 16 | 18 | 23 | 44 |
| 1.5–3 | 11 | 13 | 16 | 18 | 22 | 30 | 59 |
| 3–6 | 16 | 20 | 22 | 27 | 39 | 47 | 106 |
| 6–9 | 23 | 31 | 39 | 53 | 64 | 89 | 210 |
| 9–12 | 40 | 48 | 68 | 86 | 113 | 170 | 377 |
| 12–15 | 71 | 94 | 125 | 169 | 222 | 316 | 764 |
| 15–18 | 124 | 174 | 222 | 314 | 429 | 604 | 1435 |
| 18–21 | 229 | 330 | 479 | 595 | 825 | 1151 | 2775 |
| 21–24 | 459 | 608 | 871 | 1174 | 1592 | 2291 | 5415 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), OC, PE ≤ 12 years | |||||||
| < 1.5 | 11 | 11 | 13 | 15 | 19 | 24 | 42 |
| 1.5–3 | 12 | 13 | 16 | 18 | 23 | 29 | 58 |
| 3–6 | 15 | 18 | 23 | 29 | 37 | 47 | 107 |
| 6–9 | 24 | 29 | 38 | 52 | 63 | 84 | 200 |
| 9–12 | 38 | 48 | 67 | 91 | 117 | 160 | 345 |
| 12–15 | 69 | 91 | 115 | 164 | 232 | 307 | 675 |
| 15–18 | 127 | 179 | 237 | 316 | 467 | 596 | 1348 |
| 18–21 | 242 | 313 | 438 | 598 | 869 | 1171 | 2569 |
| 21–24 | 453 | 606 | 831 | 1173 | 1649 | 2286 | 5067 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), SE, PE ≤ 12 years | |||||||
| < 1.5 | 11 | 12 | 15 | 18 | 21 | 26 | 52 |
| 1.5–3 | 13 | 15 | 17 | 22 | 26 | 35 | 66 |
| 3–6 | 17 | 22 | 27 | 35 | 41 | 57 | 122 |
| 6–9 | 27 | 36 | 45 | 59 | 80 | 106 | 237 |
| 9–12 | 45 | 61 | 87 | 110 | 144 | 194 | 440 |
| 12–15 | 82 | 106 | 154 | 206 | 269 | 382 | 869 |
| 15–18 | 156 | 215 | 288 | 426 | 564 | 716 | 1801 |
| 18–21 | 315 | 406 | 569 | 768 | 1027 | 1409 | 3254 |
| 21–24 | 562 | 784 | 1064 | 1502 | 2085 | 2929 | 6774 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), SE, OC | |||||||
| < 1.5 | 11 | 13 | 14 | 16 | 20 | 26 | 50 |
| 1.5–3 | 12 | 14 | 18 | 21 | 25 | 32 | 73 |
| 3–6 | 18 | 21 | 25 | 32 | 44 | 55 | 120 |
| 6–9 | 27 | 34 | 41 | 55 | 77 | 103 | 213 |
| 9–12 | 42 | 59 | 78 | 106 | 134 | 184 | 422 |
| 12–15 | 79 | 102 | 144 | 191 | 272 | 365 | 904 |
| 15–18 | 151 | 198 | 276 | 374 | 491 | 713 | 1573 |
| 18–21 | 269 | 369 | 509 | 731 | 971 | 1327 | 3051 |
| 21–24 | 521 | 688 | 1042 | 1409 | 1948 | 2579 | 6383 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), MB, PE ≤ 12 years | |||||||
| < 1.5 | 10 | 12 | 12 | 15 | 19 | 23 | 44 |
| 1.5–3 | 11 | 14 | 15 | 19 | 23 | 27 | 59 |
| 3–6 | 17 | 19 | 23 | 31 | 37 | 49 | 106 |
| 6–9 | 23 | 29 | 39 | 48 | 64 | 83 | 190 |
| 9–12 | 38 | 51 | 69 | 94 | 127 | 169 | 356 |
| 12–15 | 65 | 89 | 120 | 173 | 236 | 315 | 722 |
| 15–18 | 128 | 174 | 255 | 309 | 432 | 601 | 1401 |
| 18–21 | 241 | 332 | 454 | 606 | 879 | 1187 | 2737 |
| 21–24 | 468 | 629 | 865 | 1202 | 1628 | 2194 | 4996 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), MB, OC | |||||||
| < 1.5 | 11 | 12 | 13 | 15 | 19 | 23 | 39 |
| 1.5–3 | 11 | 14 | 16 | 17 | 22 | 26 | 55 |
| 3–6 | 16 | 18 | 22 | 26 | 34 | 46 | 95 |
| 6–9 | 22 | 27 | 37 | 46 | 64 | 82 | 180 |
| 9–12 | 35 | 45 | 64 | 80 | 117 | 159 | 357 |
| 12–15 | 64 | 88 | 121 | 164 | 210 | 291 | 661 |
| 15–18 | 114 | 165 | 210 | 296 | 398 | 521 | 1236 |
| 18–21 | 222 | 303 | 412 | 551 | 757 | 1159 | 2482 |
| 21–24 | 437 | 579 | 778 | 1064 | 1570 | 2100 | 4715 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), MB, SE | |||||||
| < 1.5 | 11 | 12 | 15 | 17 | 22 | 26 | 51 |
| 1.5–3 | 13 | 14 | 16 | 19 | 27 | 32 | 65 |
| 3–6 | 17 | 22 | 26 | 35 | 42 | 55 | 121 |
| 6–9 | 26 | 33 | 44 | 60 | 74 | 106 | 218 |
| 9–12 | 45 | 57 | 80 | 108 | 142 | 189 | 426 |
| 12–15 | 81 | 106 | 142 | 188 | 279 | 373 | 907 |
| 15–18 | 147 | 191 | 275 | 387 | 544 | 726 | 1605 |
| 18–21 | 293 | 373 | 546 | 729 | 1038 | 1366 | 3240 |
| 21–24 | 538 | 791 | 1013 | 1381 | 2014 | 2696 | 6283 |
| Risk factors GA, AGE, CHD (cyanotic) plus SEX (male), MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 10 | 12 | 13 | 17 | 18 | 22 | 43 |
| 1.5–3 | 12 | 13 | 16 | 17 | 21 | 28 | 56 |
| 3–6 | 15 | 18 | 23 | 30 | 36 | 45 | 98 |
| 6–9 | 24 | 31 | 37 | 48 | 59 | 87 | 182 |
| 9–12 | 37 | 49 | 67 | 81 | 117 | 154 | 341 |
| 12–15 | 62 | 84 | 119 | 153 | 210 | 332 | 676 |
| 15–18 | 121 | 164 | 218 | 318 | 422 | 582 | 1361 |
| 18–21 | 222 | 290 | 440 | 594 | 833 | 1077 | 2506 |
| 21–24 | 425 | 585 | 838 | 1176 | 1482 | 2155 | 4984 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 10 | 11 | 11 | 13 | 16 | 18 | 33 |
| 1.5–3 | 10 | 12 | 13 | 15 | 18 | 22 | 43 |
| 3–6 | 12 | 15 | 17 | 22 | 28 | 36 | 72 |
| 6–9 | 19 | 24 | 28 | 37 | 46 | 65 | 137 |
| 9–12 | 27 | 36 | 50 | 64 | 86 | 114 | 258 |
| 12–15 | 51 | 61 | 88 | 116 | 163 | 227 | 502 |
| 15–18 | 91 | 121 | 154 | 212 | 297 | 428 | 947 |
| 18–21 | 160 | 219 | 316 | 426 | 616 | 830 | 1891 |
| 21–24 | 315 | 430 | 603 | 843 | 1087 | 1505 | 3452 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 10 | 10 | 11 | 14 | 16 | 18 | 35 |
| 1.5–3 | 11 | 12 | 14 | 17 | 20 | 24 | 49 |
| 3–6 | 13 | 16 | 20 | 23 | 30 | 40 | 84 |
| 6–9 | 19 | 24 | 31 | 41 | 54 | 72 | 152 |
| 9–12 | 31 | 43 | 53 | 74 | 95 | 136 | 310 |
| 12–15 | 54 | 73 | 93 | 132 | 171 | 243 | 555 |
| 15–18 | 108 | 133 | 181 | 236 | 328 | 485 | 1059 |
| 18–21 | 199 | 257 | 380 | 469 | 675 | 895 | 2141 |
| 21–24 | 350 | 496 | 680 | 932 | 1284 | 1815 | 3976 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), MB, OC, PE ≤ 12 years | |||||||
| < 1.5 | 10 | 10 | 12 | 14 | 15 | 18 | 32 |
| 1.5–3 | 10 | 11 | 14 | 15 | 17 | 21 | 39 |
| 3–6 | 13 | 14 | 18 | 21 | 27 | 33 | 69 |
| 6–9 | 17 | 22 | 26 | 33 | 47 | 56 | 120 |
| 9–12 | 26 | 35 | 44 | 58 | 78 | 106 | 249 |
| 12–15 | 46 | 60 | 77 | 111 | 141 | 198 | 439 |
| 15–18 | 86 | 111 | 155 | 210 | 282 | 374 | 853 |
| 18–21 | 152 | 206 | 276 | 369 | 541 | 756 | 1737 |
| 21–24 | 297 | 394 | 541 | 760 | 1052 | 1432 | 3231 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), MB, SE, PE ≤ 12 years | |||||||
| < 1.5 | 10 | 11 | 14 | 15 | 17 | 19 | 35 |
| 1.5–3 | 11 | 12 | 13 | 16 | 22 | 25 | 48 |
| 3–6 | 15 | 16 | 21 | 25 | 33 | 41 | 82 |
| 6–9 | 21 | 25 | 32 | 41 | 56 | 76 | 151 |
| 9–12 | 33 | 42 | 59 | 70 | 101 | 131 | 307 |
| 12–15 | 55 | 76 | 100 | 137 | 179 | 260 | 560 |
| 15–18 | 97 | 136 | 183 | 268 | 341 | 467 | 1082 |
| 18–21 | 195 | 260 | 359 | 499 | 684 | 923 | 2101 |
| 21–24 | 354 | 521 | 701 | 934 | 1367 | 1894 | 4298 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), MB, SE, OC | |||||||
| < 1.5 | 10 | 10 | 11 | 13 | 15 | 18 | 35 |
| 1.5–3 | 10 | 12 | 15 | 15 | 20 | 24 | 46 |
| 3–6 | 14 | 16 | 18 | 24 | 29 | 38 | 73 |
| 6–9 | 19 | 25 | 30 | 39 | 51 | 65 | 145 |
| 9–12 | 29 | 39 | 51 | 68 | 87 | 122 | 277 |
| 12–15 | 54 | 67 | 91 | 124 | 174 | 222 | 499 |
| 15–18 | 93 | 130 | 175 | 240 | 335 | 429 | 1042 |
| 18–21 | 175 | 235 | 320 | 465 | 634 | 855 | 1962 |
| 21–24 | 348 | 469 | 610 | 898 | 1225 | 1737 | 3634 |
| Risk factors GA, AGE, CHD (cyanotic) plus SAS, SEX (male), MB, SE, OC, PE ≤ 12 years | |||||||
| < 1.5 | 9 | 9 | 10 | 11 | 14 | 15 | 26 |
| 1.5–3 | 11 | 11 | 11 | 13 | 15 | 18 | 33 |
| 3–6 | 12 | 13 | 16 | 18 | 23 | 29 | 58 |
| 6–9 | 16 | 18 | 22 | 27 | 36 | 48 | 98 |
| 9–12 | 24 | 27 | 36 | 48 | 61 | 87 | 189 |
| 12–15 | 38 | 46 | 66 | 82 | 118 | 163 | 353 |
| 15–18 | 63 | 86 | 115 | 157 | 233 | 295 | 649 |
| 18–21 | 119 | 163 | 218 | 296 | 440 | 589 | 1342 |
| 21–24 | 223 | 308 | 425 | 603 | 876 | 1191 | 2654 |
Chapter 6 Summary of key results
The assessment group used the decision tree model developed in the original HTA journal publication1 to assess the cost-effectiveness of prophylaxis with palivizumab, compared with no prophylaxis, for subgroups of pre-term infants and young children with different risk factors. This report covers four categories (children without CLD or CHD, with CLD, with acyanotic CHD and with cyanotic CHD), a total of 256 different combinations of risk factors, corresponding to 16,128 subgroups. Cost-effectiveness is defined as the ICER being less than or equal to the UK conventional cost-effectiveness threshold (a willingness-to-pay threshold of £30,000/QALY).
Compared with no prophylaxis, prophylaxis with palivizumab for children without CLD/CHD:
-
is not cost-effective for any GA and AGE if there are no more than one of the other risk factors that were considered in this report
-
is cost-effective for children < 6 weeks old at the start of the RSV season who had at least two of the other risk factors that were considered in this report and were born at 24 weeks GA or less
-
is cost-effective for children < 6 weeks old at the start of the RSV season who had at least three of the other risk factors that were considered in this report and were born at 26 weeks GA or less
-
is cost-effective for children < 3 months old at the start of the RSV season who had at least four of the other risk factors that were considered in this report and were born at 28 weeks GA or less
-
is cost-effective for children < 6 months old at the start of the RSV season who had at least five of the other risk factors that were considered in this report and were born at 26 weeks GA or less.
Compared with no prophylaxis, prophylaxis with palivizumab for pre-term infants with CLD:
-
is cost-effective for children < 9 months old at the start of the RSV season who had no other risk factors and were born at 24 weeks GA or less, for children < 6 months old at the start of the RSV season who had no other risk factors and were born at 28 weeks GA or less, for children < 3 months old at the start of the RSV season who had no other risk factors and were born at 32 weeks GA or less, and for children < 6 weeks old at the start of the RSV season who had no other risk factors and were born at 34 weeks GA or less.
-
is cost-effective for children < 9 months old at the start of the RSV season who had at least one of the other risk factors that were considered in this report and were born at 26 weeks GA or less
-
is cost-effective for children < 9 months old at the start of the RSV season who had at least two of the other risk factors that were considered in this report and were born at 28 weeks GA or less
-
is cost-effective for children < 12 months old at the start of the RSV season who had at least three of the other risk factors that were considered in this report and were born at 26 weeks GA or less
-
is cost-effective for children < 15 months old at the start of the RSV season who had at least four of the other risk factors that were considered in this report and were born at 24 weeks GA or less
-
is cost-effective for children < 18 months old at the start of the RSV season who had at least five of the other risk factors that were considered in this report and were born at 24 weeks GA or less.
Compared with no prophylaxis, prophylaxis with palivizumab for children with acyanotic CHD:
-
is cost-effective for children < 6 months old at the start of the RSV season who had no other risk factors and were born at 24 weeks GA or less, for children < 3 months old at the start of the RSV season who had no other risk factors and were born at 28 weeks GA or less, and for infants under 6 weeks old at the start of the RSV season who had no other risk factors and were born at 30 weeks GA or less.
-
is cost-effective for children < 6 months old at the start of the RSV season who had at least one of the other risk factors that were considered in this report and were born at 26 weeks GA or less
-
is cost-effective for children < 9 months old at the start of the RSV season who had at least two of the other risk factors that were considered in this report and were born at 24 weeks GA or less
-
is cost-effective for children < 12 months old at the start of the RSV season who had at least three of the other risk factors that were considered in this report and were born at 24 weeks GA or less
-
is cost-effective for children < 9 months old at the start of the RSV season who had at least four of the other risk factors that were considered in this report and were born at 30 weeks GA or less
-
is cost-effective for children < 15 months old at the start of the RSV season who had at least five of the other risk factors and were born at 24 weeks GA or less.
Compared with no prophylaxis, prophylaxis with palivizumab for children with cyanotic CHD:
-
is cost-effective for children < 6 weeks old at the start of the RSV season who had no other risk factors and were born at 24 weeks GA or less
-
is cost-effective for children < 3 months old at the start of the RSV season who had at least one of the other risk factors that were considered in this report and were born at 24 weeks GA or less
-
is cost-effective for children < 6 months old at the start of the RSV season who had at least two of the other risk factors that were considered in this report and were born at 24 weeks GA or less
-
is cost-effective for children < 9 months old at the start of the RSV season who had at least four of the other risk factors and were born at 24 weeks GA or less
-
is cost-effective for children < 12 months old at the start of the RSV season who had at least five of the other risk factors and were born at 24 weeks GA or less.
Credible intervals, cost-effectiveness planes and cost-effectiveness acceptability curves
Credible intervals were derived for one subset of the analysis for illustrative purposes only. The table chosen was acyanotic CHD children who have additional risk factors of GA, AGE and PE ≤ 12 years because this had three results around £20,000 per QALY within it. The mean values of the ICERs and their 95% credible intervals are listed in Table 20. The results showed that for a point estimate of ICERs around £20,000/QALY, the upper 95% credible intervals may far exceeds the UK conventional cost-effective threshold (of £30,000/QALY), and that a point estimate of ICERs has to be < £8000/QALY if its upper 95% credible intervals falls within the UK conventional cost-effective threshold. However, we have some comments on interpretation of these further credible interval analysis results.
| AGE (months) | GA (weeks) | ||||||
|---|---|---|---|---|---|---|---|
| ≤ 24 | > 24–26 | > 26–28 | > 28–30 | > 30–32 | > 32–34 | ≥ 35 | |
| < 1.5 | 5000 (0 to 27,000) | 8 (2000 to 33,000) | 10,000 (3000 to 40,000) | 15,000 (5000 to 56,000) | 20,000 (5000 to 56,000) | 28,000 (8000 to 66,000) | 67, 000 (31,000 to 246,000) |
| 1.5–3 | 7000 (2000 to 33,000) | 11,000 (3000 to 41,000) | 15,000 (6000 to 54,000) | 21,000 (9000 to 78,000( | 29,000 (11,000 to 112,000) | 40,000 (18,000 to 132,000) | 93,000 (42,000 to 309,000) |
| 3–6 | 15,000 (5000 to 57,000) | 22,000 (9000 to 75,000) | 30,000 (13,000 to 108,000) | 41,000 (18,000 to 136,000) | 56,000 (26,000 to 177,000) | 78,000 (35,000 to 252,000) | 180,000 (78,000 to 636,000) |
| 6–9 | 30,000 (12,000 to 95,000) | 42,000 (18,000 to 141,000) | 56,000 (24,000 to 179,000) | 79,000 (33,000 to 249,000) | 110,000 (50,000 to 323,000) | 154,000 (69,000 to 494,000) | 347,000 (161,000 to 1,151,000) |
| 9–12 | 58,000 (27,000 to 188,000) | 80,000 (37,000 to 254,000) | 113,000 (51,000 to 379,000) | 157,000 (73,000 to 523,000) | 217,000 (103,000 to 747,000) | 302,000 (138,000 to 1,060,000) | 706,000 (318,000 to 2,323,000) |
| 12–15 | 116,000 (51,000 to 360,000) | 158,000 (71,000 to 547,000) | 221,000 (106,000 to 724,000) | 299,000 (139,000 to 893,000) | 417,000 (185,000 to 1,406,000) | 584,000 (286,000 to 1,871,000) | 1,330,000 (628,000 to 4,069,000) |
| 15–18 | 225,000 (102,000 to 717,000) | 309,000 (145,000 to 962,000) | 421,000 (203,000 to1,286,000) | 596,000 (273,000 to 1,906,000) | 829,000 (389,000 to 2,411,000) | 1,115,000 (499,000 to 3,673,000) | 2,586,000 (1,223,000 to 9,295,000) |
| 18–21 | 436,000 (203,000 to 1,571,000) | 607,000 (298,000 to 1,955,00) | 860,000 (373,000 to 2,960,000) | 1,144,000 (529,000 to 3,820,000) | 1,650,000 (775,000 to 4,974,000) | 2,216,000 (1,091,000 to 6,390,000) | 5,185,000 (2,468,000 to 15,218,000) |
| 21–24 | 835,000 (379,000 to 2,773,000) | 1,179,000 (544,000 to 3,433,000) | 1,651,000 (802,000 to 5,112,000) | 2,282,000 (1,090,000 to 6,820,000) | 3,149,000 (1,412,000 to 9,553,000) | 4,443,000 (2,112,000 to 12,917,000) | 9,755,000 (4,440,000 to 36,592,000) |
-
The National Institute for Health and Clinical Excellence (NICE) usually considers the mean values of ICERs when making decisions,5 i.e. the point estimates of the ICERs presented in the results section of this report.
-
As shown in Table 20, the 95% credible intervals of the point estimate of £20,000/QALY is £8000/QALY to £66,000/QALY. The wide credible intervals can be explained by the fact that ICER does not follow a normal distribution (see Figure 9 showing that the distribution has a long tail to the right side).
-
– In fact, for this example, the probability of an ICER < £30,000/QALY is 0.74. The probability of ICER < £51,000/QALY is 0.95.
-
FIGURE 9.
Distribution of the ICER with a mean value of £20,000/QALY.
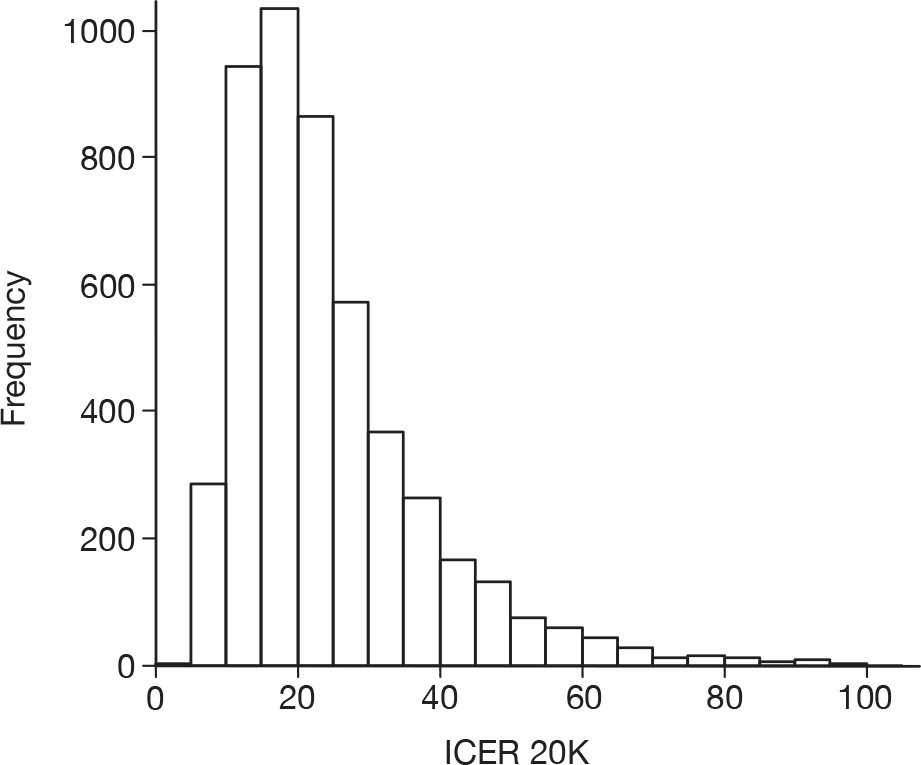
The incremental cost-effectiveness plane and the cost-effectiveness acceptability curve (CEAC) for prophylaxis with palivizumab compared with no prophylaxis for the three cases highlighted in Table 20 are shown in Figures 10–15. These results show that, compared with no prophylaxis, palivizumab has a probability of 74%, 73% and 72% of having an ICER below £30,000/QALY for the three cases, respectively.
FIGURE 10.
Incremental cost-effectiveness plane for prophylaxis with palivizumab compared with no prophylaxis for children < 6 weeks old at the start of the RSV season who had acyanotic CHD and were born at 32 weeks gestational age or less, and whose parental education is < 12 years.
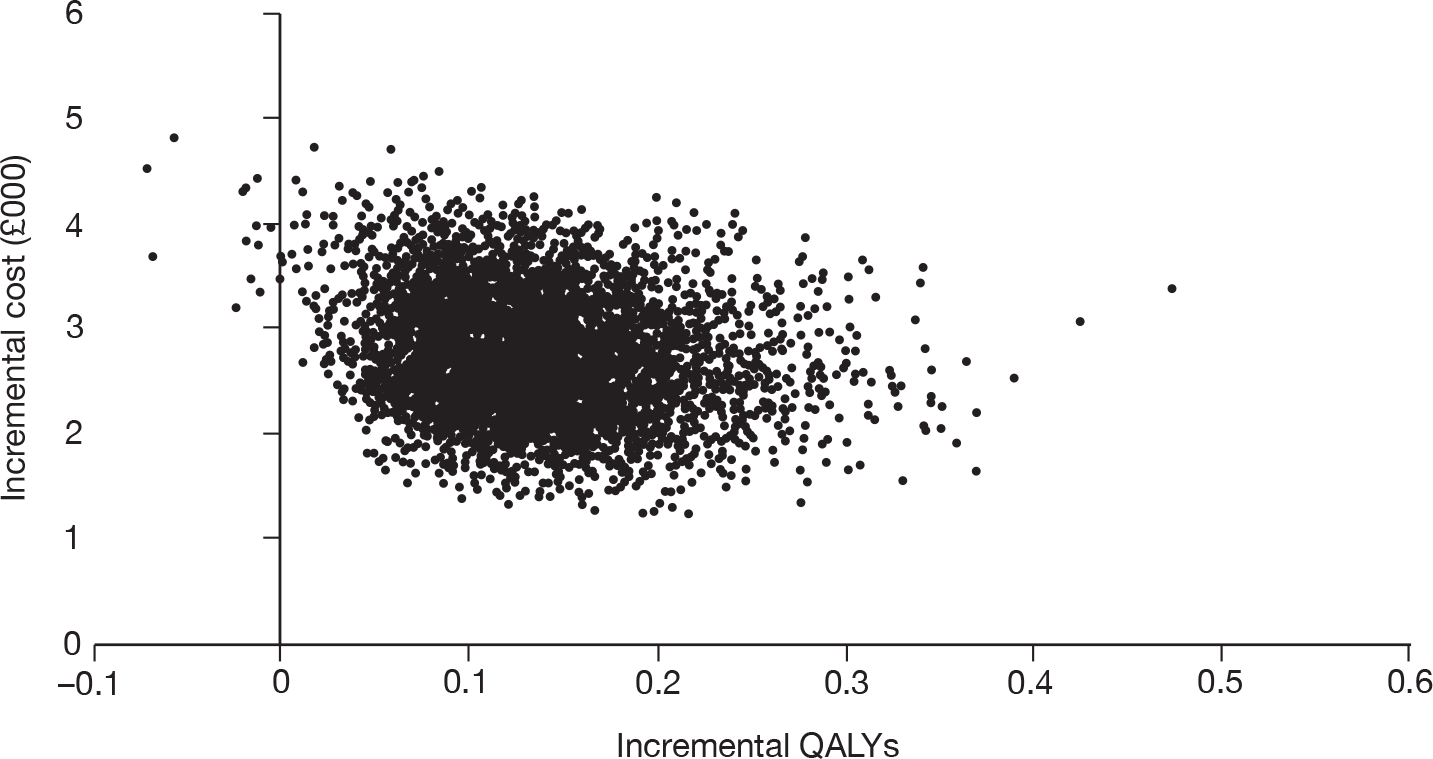
FIGURE 11.
Cost-effectiveness acceptability curve for prophylaxis with palivizumab compared with no prophylaxis for children < 6 weeks old at the start of the RSV season who had acyanotic CHD and were born at 32 weeks gestational age or less, and whose parental education is < 12 years.
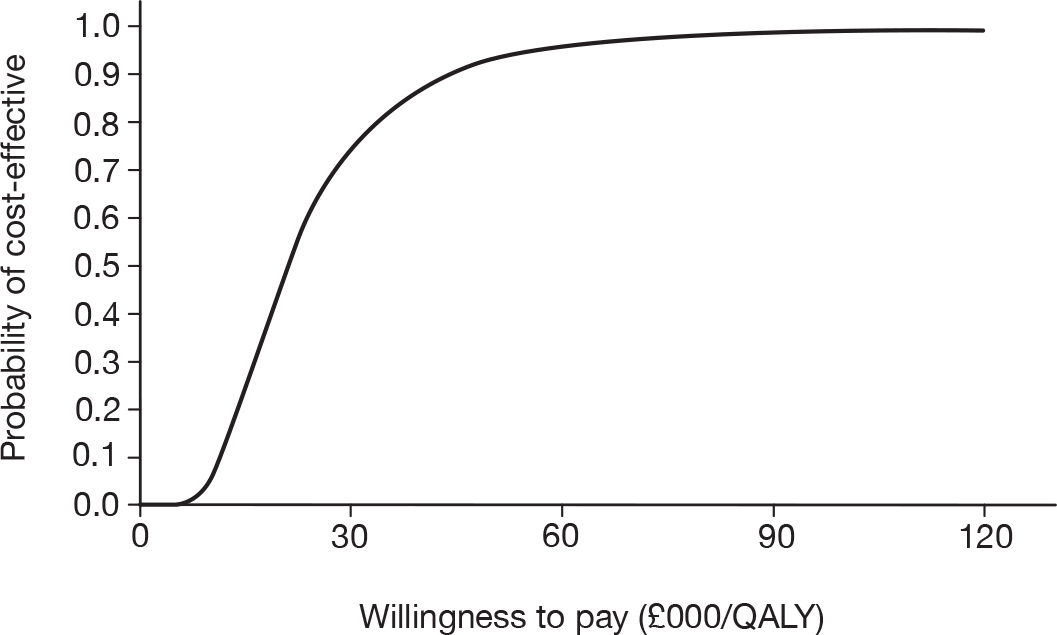
FIGURE 12.
Incremental cost-effectiveness plane for prophylaxis with palivizumab compared with no prophylaxis for children < 3 months old at the start of the RSV season who had acyanotic CHD and were born at 30 weeks gestational age or less, and whose parental education is < 12 years.
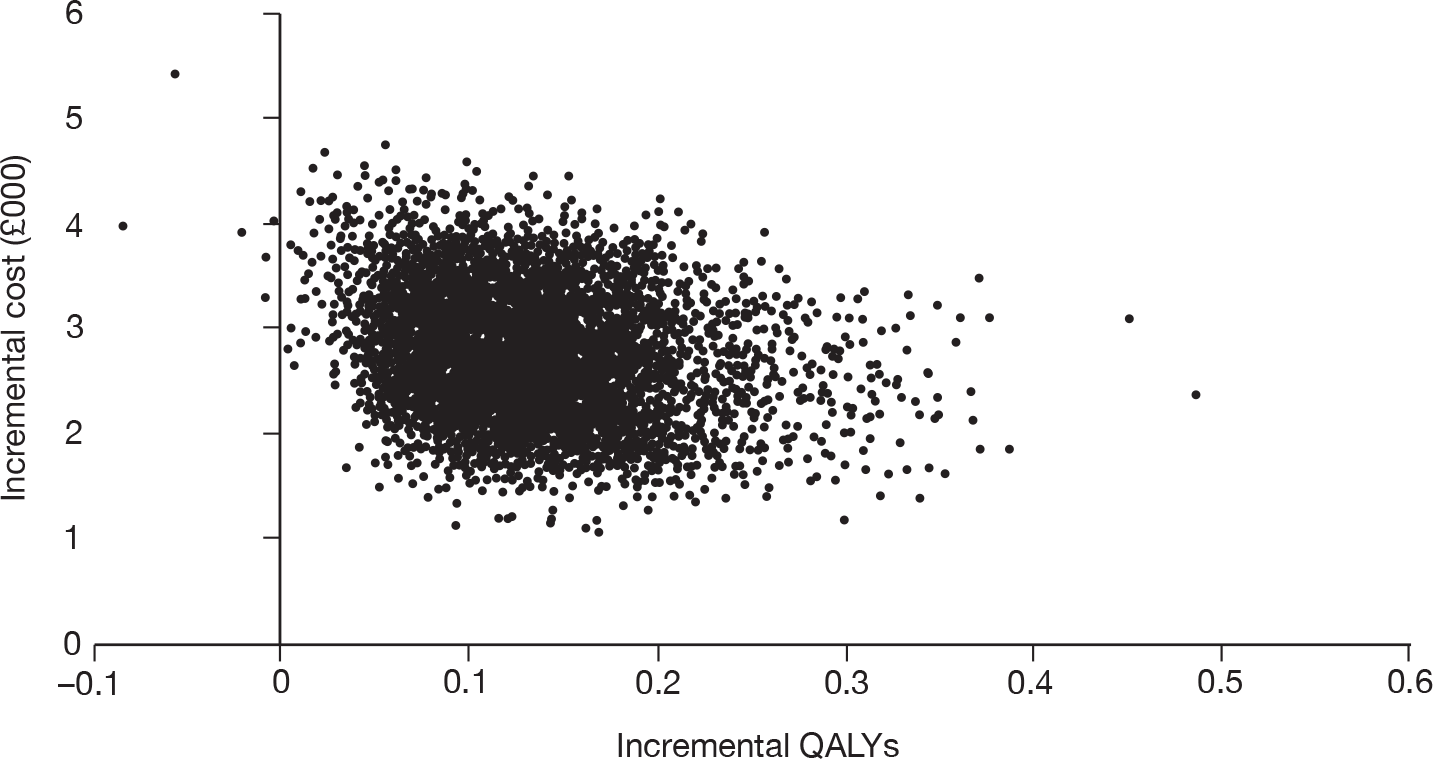
FIGURE 13.
Cost-effectiveness acceptability curve for prophylaxis with palivizumab compared with no prophylaxis for children < 3 months old at the start of the RSV season who had acyanotic CHD and were born at 30 weeks gestational age or less, and whose parental education is < 12 years.
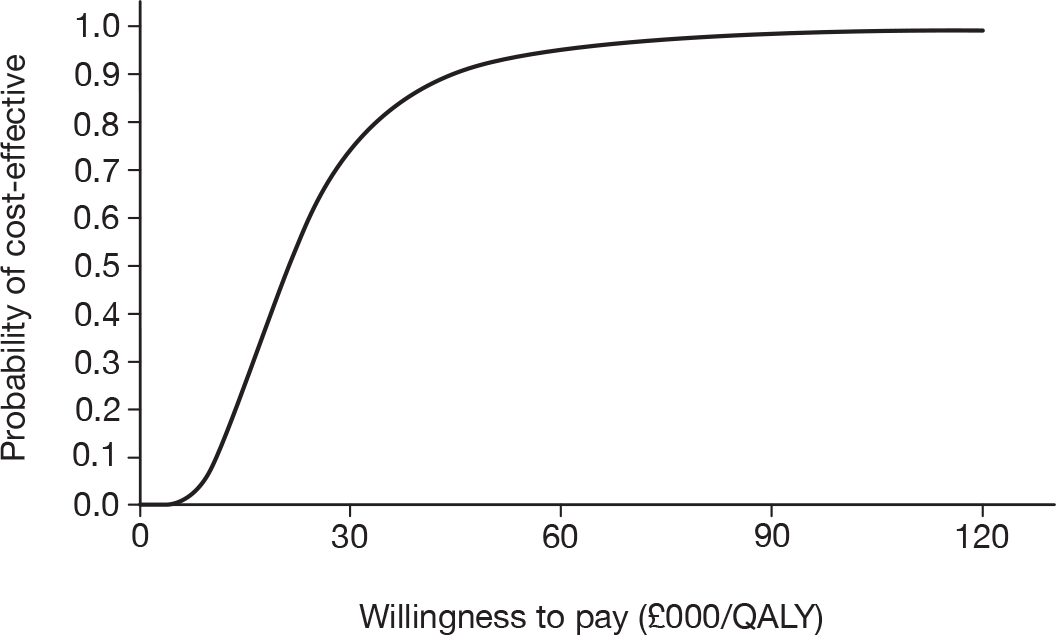
FIGURE 14.
Incremental cost-effectiveness plane for prophylaxis with palivizumab compared with no prophylaxis for children < 6 months old at the start of the RSV season who had acyanotic CHD and were born at 26 weeks gestational age or less, and whose parental education is < 12 years.
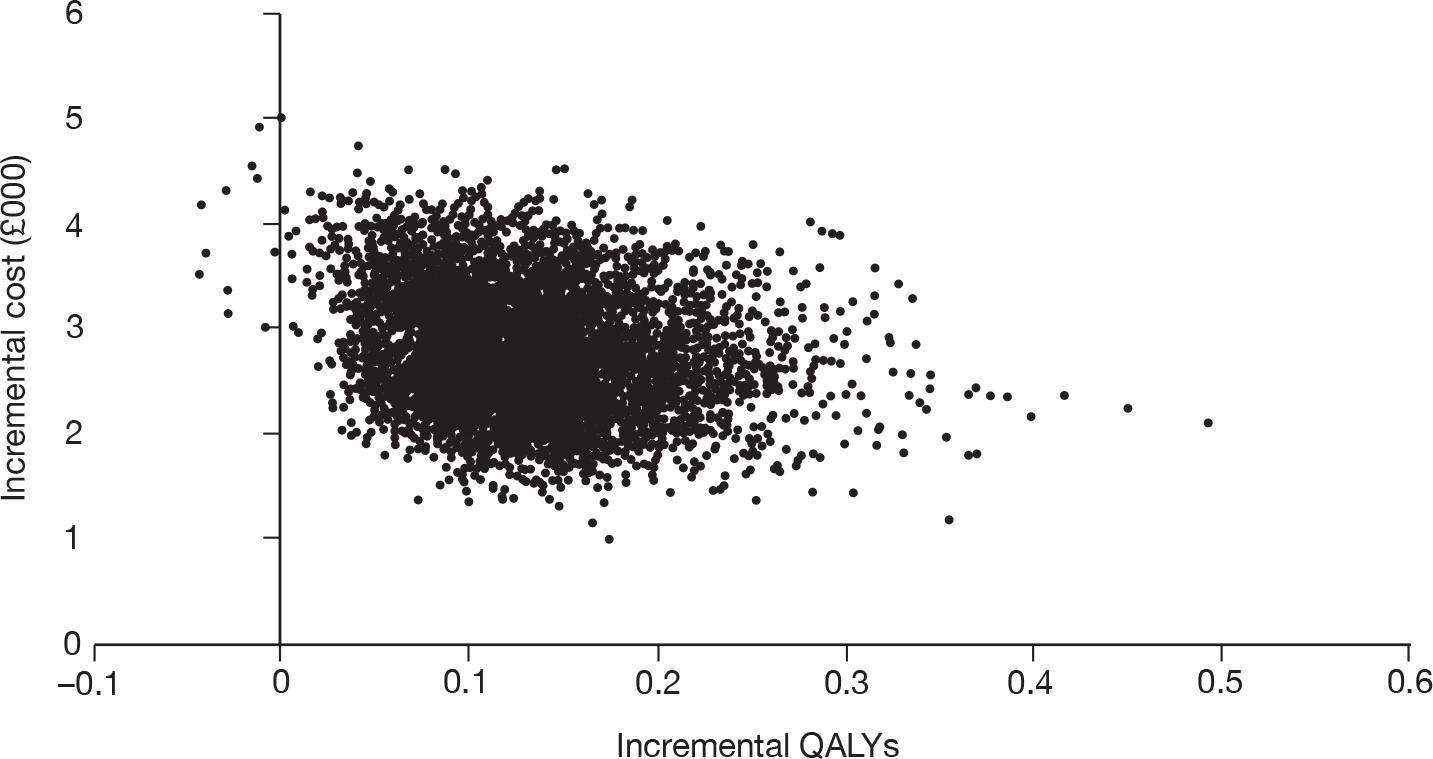
FIGURE 15.
Cost-effectiveness acceptability curve for prophylaxis with palivizumab compared with no prophylaxis for children < 6 months old at the start of the RSV season who had acyanotic CHD and were born at 26 weeks gestational age or less, and whose parental education is < 12 years.
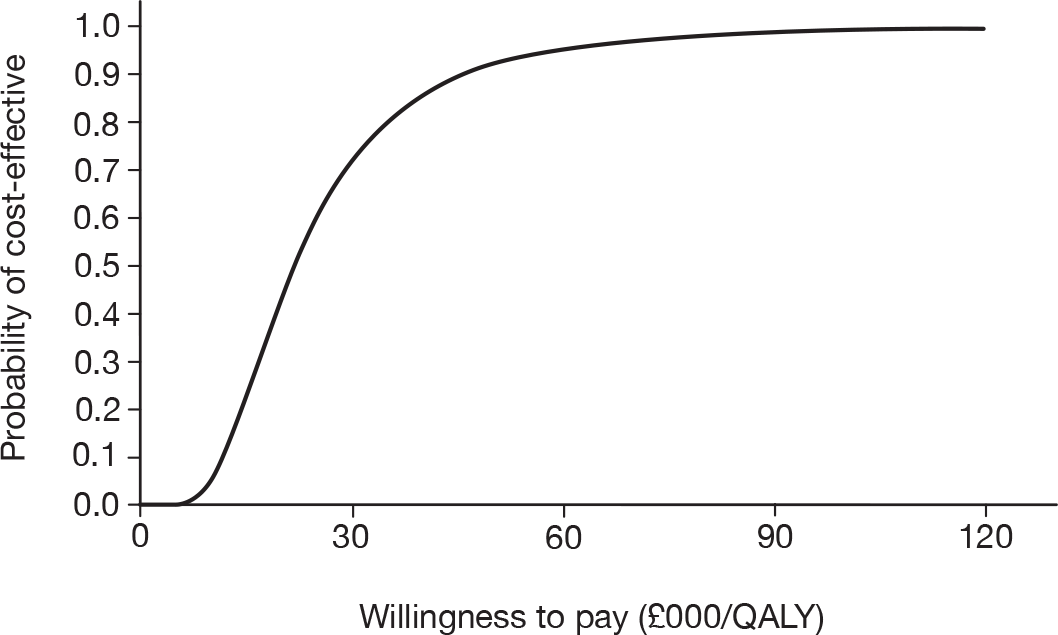
Chapter 7 Discussion
Statement of principal findings
For pre-term infants and young children without CLD/CHD, prophylaxis with palivizumab compared with no prophylaxis is not cost-effective for any GA and AGE subgroup if children had no more than one other risk factor. However, from the cost-effective spectra, we did find cost-effective GA and AGE subgroups for children without CLD/CHD who had at least two other risk factors that were considered in this report. For example, prophylaxis with palivizumab compared with no prophylaxis was found to be cost-effective for children < 6 weeks old at the start of the RSV season who had no CLD/CHD, but have at least two of SAS, male, MB, SE, OC or PE of high school or less (≤ 12 years) and were born at 24 weeks GA or less. Furthermore, the cost-effective subgroups would not include children who had no CLD/CHD and were > 9 months old at the start of the RSV season or had a GA of > 32 weeks.
The cost-effective spectra were also derived for children with CLD, children with acyanotic CHD and children with cyanotic CHD. Unlike in the case of children without CLD/CHD, the cost-effective subgroups were found for GA and AGE subgroups for children with CLD, children with acyanotic CHD, and children with cyanotic CHD, who had no SAS, were male, MB, SE, OC or PE of high school or less (≤ 12 years). These include children < 6 months old at the start of the RSV season who had CLD and were born at 28 weeks GA or less, children < 6 months old at the start of the RSV season who had acyanotic CHD and were born at 24 weeks GA or less, and children < 6 weeks old at the start of the RSV season who had cyanotic CHD and were born at 24 weeks GA or less. However, the cost-effective subgroups would not include children who had CLD and were > 21 months old at the start of the RSV season, children who had cyanotic CHD and were > 12 months old at the start of the RSV season, and children who had acyanotic CHD and were > 21 months old at the start of the RSV season.
Strengths and limitations of the assessment
The strengths of the assessment include the following aspects:
-
This report presents a comprehensive subgroup analysis for cost-effectiveness of prophylaxis with palivizumab compared with no prophylaxis. It covers four categories of children (pre-term infants and young children without CLD/CHD, with CLD, with acyanotic CHD and with cyanotic CHD), 10 risk factors of RSV hospitalisation, and a total of 16,128 subgroups.
-
Meta-analysis was applied to deriving RSV hospitalisation outcomes for gender, CHD, CLD, SAS, SE, and OC.
The assessment includes the following limitations.
-
There was only one study contributing to the risk factors of AGE, GA, MB and PE of high school or less (≤ 12 years), which means that these estimates may not be as reliable as when more than one study is available. Therefore, one should be careful when interpreting the cost-effectiveness for children with different combination of risk factors, especially for the cases that involve risk factors that were derived from only one study.
-
Many of the included studies were relatively small and their quality was frequently poor, so it was difficult to know the accuracy of the inputs into the modelling. Therefore several of the meta-analyses had relatively high heterogeneity. The implication of this is that there will be a relatively high degree of uncertainty around the point estimates of cost-effectiveness. To illustrate this, an example estimate of CIs for point estimates was made.
-
The definitions of risk factors were not available for most studies and may have varied between studies. This may have been contributing to the heterogeneity seen.
-
The diagnosis of RSV was made by different methods in different included studies and unfortunately details were lacking in some of the included studies. This variation in methods will have introduced heterogeneity into the results.
-
An additive rule was used to assess the impact of different risk factors. However, it is acknowledged that some of the risk factors will interact with each other to some extent, but this interaction will vary in magnitude and direction by risk factor. Univariate estimates of risk factor OR were used by preference as most studies did not report multivariate estimates. The implications of having interacting risk factors are unknown as they could potentially positively or negatively interact. This interaction is likely to reduce confidence in the point estimates of cost-effectiveness.
-
Credible intervals of ICERs were derived for a small subset for illustrative purposes only. It should be noted that, on average, using more risk factors in the estimates will give wider CIs.
-
Risk factors of lack of or minimal breastfeeding and family history of atopy were not included in the model due to lack of consistent information from primary studies, giving large amounts of heterogeneity in meta-analyses.
-
Assessment of quality of life was derived from parental estimates so may not be accurate. It is not possible to derive accurate preference-based quality of life estimates from infants and young children.
Other relevant factors
There are several factors that may have impact on the evaluation of cost-effectiveness of prophylaxis with palivizumab compared with no prophylaxis. Firstly, other risk factors, such as lack of or minimal breastfeeding and family history of atopy, may further affect the probability that children will be hospitalised for RSV and this will influence the results of ICERs. However, inconsistent results for lack of or minimal breastfeeding and family history of atopy were observed. Without further work to identify relevant studies systematically and consider whether pooling of estimates would be appropriate, there is the risk of reducing the accuracy and precision of the model estimates to an unacceptable degree. Therefore, the risk factors of lack of or minimal breastfeeding and family history of atopy were not included in the model. However, the presence of the two additional risk factors may not play a very important role in making the clinical decision on whether to offer palivizumab prophylaxis to a particular baby as the model considered 10 risk factors.
Secondly, residual confounding is likely to influence the estimate of risk in the included observational studies. Where potential risk factors are associated with each other, the choice of factors entered into the model will influence the factors included in the final model.
Thirdly, vial wastage is an important problem with palivizumab. The drug is packaged in two vial sizes only and cannot be stored once opened. Infants and young children vary in weight so there will be an unknown amount of wastage. A vial sharing scheme was included in the model, using average weights reported in the trials.
Finally, it should be remembered that factors identified as important in one society will not necessarily have the same impact in other settings, for example, the impact of race and rural residence may be different in Northern Europe from Southern USA.
Chapter 8 Conclusions
Implications for service provision
Prophylaxis with palivizumab does not represent good value based on the current UK ICER threshold of £30,000/QALY when used unselectively in pre-term infants and children without CLD/CHD or children with CLD or CHD. This subgroup analysis does show that prophylaxis with palivizumab may be cost-effective for some subgroups, which have been identified in this report. In summary, the cost-effective subgroups for children who had no CLD/CHD have to contain at least two of the other risk factors examined here apart from GA and AGE. The cost-effective subgroups for children who had CLD/CHD do not necessarily have any other of the modelled risk factors apart from GA and AGE.
Suggested research priorities
Future research should be directed towards the following:
-
to conduct much larger, better powered and better reported studies to derive better estimates of risk factor effect sizes
-
to update the effect sizes of the risk factors used in the current model, especially age, GA, MB and PE as the values of these parameters were derived from only one study
-
to systematically identify the effect size of other risk factors, such as lack of or minimal breastfeeding and family history of atopy and enter them into the model to estimate the effect of additional risk factors on the cost-effectiveness
-
to derive credible intervals for the 16,128 point estimates of cost-effectiveness of prophylaxis with palivizumab compared with no prophylaxis.
Acknowledgements
Linda Briscoe and Anne Massey for their administrative assistance with this project.
Olalekan Uthman – Preliminary inclusion and exclusion of papers.
David Moore for assistance with management of the project.
Contributions of authors
Dechao Wang – Data extraction, data analysis and synthesis, economic modelling, writing report.
Sue Bayliss, – Prepared and ran search strategies, wrote preliminary searching methods section.
Catherine Meads – Inclusion and exclusion of papers, data extraction and data checking, writing report, management and overview of project.
Disclaimers
The views expressed in this publication are those of the authors and not necessarily those of the HTA programme or the Department of Health.
References
- Wang D, Cummins C, Bayliss S, Sandercock J, Burls A. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assess 2008;12.
- Fjaerli HO, Farstad T, Bratlid D. Hospitalisations for respiratory syncytial virus bronchiolitis in Akershus, Norway, 1993–2000: a population-based retrospective study. BMC Pediatrics 2004;4:1-7.
- Cabalka AK. Physiologic risk factors for respiratory viral infections and immunoprophylaxis for respiratory syncytial virus in young children with congenital heart disease. Pediatr Infect Dis J 2004;23:S41-S45.
- British Medical Association and Royal Pharmaceutical Society of Great Britain . British National Formulary. 2010.
- Anon. Guide to the methods of technology appraisal. London: National Institute for Health and Clinical Excellence; 2009.
- Rietveld E, Vergouwe Y, Steyerberg EW, Huysman MW, de Groot R, Moll HA, et al. Hospitalization for respiratory syncytial virus infection in young children: development of a clinical prediction rule. Pediatr Infect Dis J 2006;25:201-7.
- Duppenthaler A, Ammann RA, Gorgievski-Hrisoho M, Pfammatter JP, Aebi C. Low incidence of respiratory syncytial virus hospitalisations in haemodynamically significant congenital heart disease. Arch Dis Child 2004;89:961-5.
- Feltes TF, Sondheimer HM. Palivizumab and the prevention of respiratory syncytial virus illness in pediatric patients with congenital heart disease. Expert Opin Biol Ther 2007;7:1471-80.
- Kristensen K, Stensballe LG, Bjerre JV, Roth D, Fisker N, Kongstad T, et al. Risk factors for RSV hospitalisation in children with heart disease. Arch Dis Child 2009;94:785-9.
- Simoes EA, Sondheimer HM, Top FH, Jr, Meissner HC, Welliver RC, Kramer AA, et al. Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus disease in infants and children with congenital heart disease. The Cardiac Study Group. J Pediatr 1998;133:492-9.
- Carbonell-Estrany X, Quero J, Bustos G, Cotero A, Domenech E, Figueras-Aloy J, et al. Rehospitalization because of respiratory syncytial virus infection in premature infants younger than 33 weeks of gestation: a prospective study. IRIS Study Group. Pediatr Infect Dis J 2000;19:592-7.
- Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003;143:532-40.
- The IMpact-RSV Study Group . Palivisumab, a humanised respiratory syncytial virus monoclonal antibody, reduces hospitalisation from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998;102:531-7.
- Carbonell-Estrany X, Quero J. IRIS Study Group . Hospitalization rates for respiratory syncytial virus infection in premature infants born during two consecutive seasons. Pediatr Infect Dis J 2001;20:874-9.
- Eriksson M, Bennet R, Rotzen-Ostlund M, von Sydow M, Wirgart BZ. Population-based rates of severe respiratory syncytial virus infection in children with and without risk factors, and outcome in a tertiary care setting. Acta Paediatr 2002;91:593-8.
- Figueras-Aloy J, Carbonell-Estrany X, Quero J. IRIS Study Group . Case-control study of the risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born at a gestational age of 33–35 weeks in Spain. Pediatr Infect Dis J 2004;23:815-20.
- Simoes EA, Carbonell-Estrany X, Fullarton JR, Liese JG, Figueras-Aloy J, Doering G, et al. A predictive model for respiratory syncytial virus (RSV) hospitalisation of premature infants born at 33–35 weeks of gestational age, based on data from the Spanish FLIP Study. Respir Res 2008;9.
- Figueras-Aloy J, Carbonell-Estrany X, Quero-Jimenez J, Fernandez-Colomer B, Guzman-Cabanas J, Echaniz-Urcelay I, et al. FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J 2008;27:788-93.
- Frogel M, Nerwen C, Cohen A, VanVeldhuisen P, Harrington M, Boron M. Prevention of hospitalization due to respiratory syncytial virus: results from the Palivizumab Outcomes Registry. J Perinatol 2008;28:511-17.
- Grimwood K, Cohet C, Rich FJ, Cheng S, Wood C, Redshaw N, et al. Risk factors for respiratory syncytial virus bronchiolitis hospital admission in New Zealand. Epidemiol Infect 2008;136:1333-41.
- Law BJ, Langley JM, Allen U, Paes B, Lee DS, Mitchell I, et al. The Pediatric Investigators Collaborative Network on infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J 2004;23:806-14.
- Liese J. Comment on the Austrian consensus paper on prophylaxis of RSV infection with palivizumab and post-RSV respiratory tract disease. Monatsschrift Fur Kinderheilkunde 2008;156.
- Nielsen HE, Siersma V, Andersen S, Gahrn-Hansen B, Mordhorst CH, Norgaard-Pedersen B, et al. Respiratory syncytial virus infection--risk factors for hospital admission: a case-control study. Acta Paediatr 2003;92:1314-21.
- Rossi GA, Medici MC, Arcangeletti MC, Lanari M, Merolla R, Paparatti UD, et al. Risk factors for severe RSV-induced lower respiratory tract infection over four consecutive epidemics. Eur J Pediatr 2007;166:1267-72.
- Liese JG, Grill E, Fischer B, Roeckl-Wiedmann I, Carr D, Belohradsky BH. Incidence and risk factors of respiratory syncytial virus-related hospitalizations in premature infants in Germany. Eur J Pediatr 2003;162:230-6.
- Greenough A, Alexander J, Burgess S, Bytham J, Chetcuti PA, Hagan J, et al. Health care utilisation of prematurely born, preschool children related to hospitalisation for RSV infection. Arch Dis Child 2004;89:673-8.
- Yount LE, Mahle WT, Yount LE, Mahle WT. Economic analysis of palivizumab in infants with congenital heart disease. Pediatrics 2004;114:1606-11.
- Nuijten MJC, Wittenberg W, Lebmeier M. Cost effectiveness of palivizumab for respiratory syncytial virus prophylaxis in high-risk children: A UK analysis. Pharmacoeconomics 2007;25:55-71.
Appendix 1 Literature search strategies
Clinical effectiveness
Database: Cochrane Library (Wiley) 2009 Issue 3
-
#1 respiratory next syncytial
-
#2 rsv
-
#3 bronchiolitis
-
#4 MeSH descriptor Bronchiolitis, Viral, this term only
-
#5 MeSH descriptor Respiratory Syncytial Virus, Human, this term only
-
#6 (#1 OR #2 OR #3 OR #4 OR #5)
-
#7 immunoprophylaxis
-
#8 monoclonal next _ntibody*
-
#9 MeSH descriptor Antibodies, Monoclonal explode all trees
-
#10 palivizumab
-
#11 synagis
-
#12 (#7 OR #8 OR #9 OR #10 OR #11)
-
#13 (#6 AND #12)
-
#14 <nothing>, from 2007 to 2009
-
#15 (#13 AND #14)
Database: Ovid MEDLINE® In-Process & Other Non-Indexed Citations 3 August 2009
-
respiratory syncytial virus.mp.
-
bronchiolitis.mp. or exp Bronchiolitis, Viral/
-
palivizumab.mp.
-
monoclonal antibod$.mp.
-
synagis.mp.
-
exp Immunotherapy/or immunoprophylaxis.mp.
-
or/1-2
-
or/3-6
-
7 and 8
-
limit 9 to yr=“2007 – 2009”
Database: Ovid MEDLINE® 1950 to week 4 July 2009
-
exp Respiratory Syncytial Virus, Human/or rsv.mp.
-
respiratory syncytial virus.mp.
-
bronchiolitis.mp. or exp Bronchiolitis, Viral/
-
or/1-3
-
palivizumab.mp.
-
monoclonal _ntibody$.mp.
-
exp Antibodies, Monoclonal/
-
synagis.mp.
-
exp Immunotherapy/or immunoprophylaxis.mp.
-
or/5-9
-
4 and 10
-
(systematic adj review$).tw.
-
(data adj synthesis).tw.
-
(published adj studies).ab.
-
(data adj extraction).ab.
-
meta-analysis/
-
meta-analysis.ti.
-
comment.pt.
-
letter.pt.
-
editorial.pt.
-
animal/
-
human/
-
not (21 and 22)
-
11 not (18 or 19 or 20 or 23)
-
or/12-17
-
24 and 25
-
limit 26 to yr=“2007 – 2009”
Database: Ovid MEDLINE® 1950 to week 4 July 2009
-
exp Respiratory Syncytial Virus, Human/or rsv.mp.
-
respiratory syncytial virus.mp.
-
bronchiolitis.mp. or exp Bronchiolitis, Viral/
-
or/1-3
-
palivizumab.mp.
-
monoclonal _ntibody$.mp.
-
exp Antibodies, Monoclonal/
-
synagis.mp.
-
exp Immunotherapy/or immunoprophylaxis.mp.
-
or/5-9
-
4 and 10
-
randomized controlled trial.pt.
-
controlled clinical trial.pt.
-
randomized controlled trials.sh.
-
random allocation.sh.
-
double blind method.sh.
-
single-blind method.sh.
-
or/12-17
-
(animals not human).sh.
-
18 not 19
-
clinical trial.pt.
-
(clin$adj25 trial$).ti,ab.
-
((singl$or doubl$or trebl$or tripl$) adj25 (blind$or mask$)).ti,ab.
-
placebos.sh.
-
placebo$.ti,ab.
-
random$.ti,ab.
-
research design.sh.
-
or/21-27
-
28 not 19
-
29 not 20
-
comparative study.sh.
-
exp evaluation studies/
-
follow up studies.sh.
-
prospective studies.sh.
-
(control$or _ntibody_ve$or volunteer$).ti,ab.
-
or/31-35
-
not 19
-
20 or 30 or 38
-
11 and 39
-
limit 40 to yr=“2007 – 2009”
Database: EMBASE 1980 to week 31 2009
-
exp Respiratory Syncytial Pneumovirus/or rsv.mp. or exp Bronchiolitis/
-
bronchiolitis.mp.
-
respiratory syncytial virus.mp.
-
or/1-3
-
palivizumab.mp. or exp PALIVIZUMAB/
-
exp Monoclonal Antibody/or monoclonal _ntibody$.mp.
-
synagis.mp.
-
immunoprophylaxis.mp. or exp IMMUNOPROPHYLAXIS/
-
or/5-8
-
4 and 9
-
“meta-analysis”/
-
metaanalys$.ti,ab.
-
meta-analys$.ti,ab.
-
meta analys$.ti,ab.
-
cochrane.ti,ab,de.
-
(review$or overview$).ti,ab.
-
(synthes$adj3 (literature$or research$or study or studies or data)).mp.
-
pooled analy$.ti,ab.
-
(systematic$adj2 review$).ti,ab.
-
or/11-19
-
10 and 20
-
19 or 11
-
10 and 22
-
limit 23 to yr=“2007 – 2009”
Database: EMBASE 1980 to week 31 2009
-
exp Respiratory Syncytial Pneumovirus/or rsv.mp. or exp Bronchiolitis/
-
bronchiolitis.mp.
-
respiratory syncytial virus.mp.
-
or/1-3
-
palivizumab.mp. or exp PALIVIZUMAB/
-
exp Monoclonal Antibody/or monoclonal _ntibody$.mp.
-
synagis.mp.
-
immunoprophylaxis.mp. or exp IMMUNOPROPHYLAXIS/
-
or/5-8
-
4 and 9
-
randomized controlled trial/
-
exp clinical trial/
-
exp controlled study/
-
or/11-12
-
10 and 14
-
limit 15 to yr=“2007 – 2009”
Database: CINAHL (EBSCO Host) 1982 to 4 August 2009
Terms used: RSV or respiratory syncytial virus or bronchiolitis or palivizumab or synagis or immunoprophylaxis or monoclonal _ntibody* random* or trial*
Database: Science Citation Index (Web of Science) 1900 to 4 August 2009
Terms used: RSV or respiratory syncytial virus or bronchiolitis or palivizumab or synagis or immunoprophylaxis or monoclonal _ntibody* or random* or trial*
Cost-effectiveness/modelling
Database: Ovid MEDLINE® 1950 to week 5 July 2009
-
exp Respiratory Syncytial Virus, Human/or rsv.mp.
-
respiratory syncytial virus.mp.
-
bronchiolitis.mp. or exp Bronchiolitis, Viral/
-
or/1-3
-
palivizumab.mp.
-
monoclonal _ntibody$.mp.
-
exp Antibodies, Monoclonal/
-
synagis.mp.
-
exp Immunotherapy/or immunoprophylaxis.mp.
-
or/5-9
-
4 and 10
-
economics/
-
exp “costs and cost analysis”/
-
cost of illness/
-
exp health care costs/
-
economic value of life/
-
exp economics medical/
-
exp economics hospital/
-
economics pharmaceutical/
-
exp “fees and charges”/
-
(econom$or cost or costs or costly or costing or price or pricing or pharmacoeconomic$).tw.
-
(expenditure$not energy).tw.
-
(value adj1 money).tw.
-
budget$.tw.
-
or/12-24
-
11 and 25
-
limit 26 to yr=“2007 –Current”
Database: Ovid MEDLINE® 1950 to week 5 July 2009
-
exp Respiratory Syncytial Virus, Human/or rsv.mp.
-
respiratory syncytial virus.mp.
-
bronchiolitis.mp. or exp Bronchiolitis, Viral/
-
or/1-3
-
palivizumab.mp.
-
monoclonal _ntibody$.mp.)
-
exp Antibodies, Monoclonal/
-
synagis.mp.
-
exp Immunotherapy/or immunoprophylaxis.mp.
-
or/5-9
-
4 and 10
-
decision support techniques/
-
markov.mp.
-
exp models economic/
-
decision analysis.mp.
-
cost benefit analysis/
-
or/12-16
-
11 and 17
-
4 and 17
-
18 or 19
-
limit 20 to yr=“2007 –Current”
Database: Ovid MEDLINE® 1950 to week 5 July 2009
-
exp Respiratory Syncytial Virus, Human/or rsv.mp.
-
respiratory syncytial virus.mp.
-
bronchiolitis.mp. or exp Bronchiolitis, Viral/
-
or/1-3
-
palivizumab.mp.
-
monoclonal _ntibody$.mp.
-
exp Antibodies, Monoclonal/
-
synagis.mp.
-
exp Immunotherapy/or immunoprophylaxis.mp.
-
or/5-9
-
4 and 10
-
quality of life/
-
life style/
-
health status/
-
health status indicators/
-
or/12-15
-
4 and 16
-
limit 17 to yr=“2007 –Current”
Database: EMBASE 1980 to week 32 2009
-
exp Respiratory Syncytial Pneumovirus/or rsv.mp. or exp Bronchiolitis/
-
bronchiolitis.mp.
-
respiratory syncytial virus.mp.
-
or/1-3
-
palivizumab.mp. or exp PALIVIZUMAB/
-
exp Monoclonal Antibody/or monoclonal _ntibody$.mp.
-
synagis.mp.
-
immunoprophylaxis.mp. or exp IMMUNOPROPHYLAXIS/
-
or/5-8
-
4 and 9
-
cost benefit analysis/
-
cost effectiveness analysis/
-
cost minimization analysis/
-
cost utility analysis/
-
economic evaluation/
-
(cost or costs or costed or costly or costing).tw.
-
(economic$or pharmacoeconomic$or price$or pricing).tw.
-
(technology adj assessment$).tw.
-
or/11-18
-
10 and 19
-
limit 20 to yr=“2007 –Current”
Database: EMBASE 1980 to week 32 2009
-
exp Respiratory Syncytial Pneumovirus/or rsv.mp. or exp Bronchiolitis/
-
bronchiolitis.mp.
-
respiratory syncytial virus.mp.
-
or/1-3
-
quality of life.mp. or exp “Quality of Life”/
-
health status.mp. or exp Health Status/
-
or/5-6
-
4 and 7
-
lung transplant$.mp.
-
8 not 9
-
limit 10 to yr=“2007 –Current”
Database: EMBASE 1980 to week 32 2009
-
exp Respiratory Syncytial Pneumovirus/or rsv.mp. or exp Bronchiolitis/
-
bronchiolitis.mp.
-
respiratory syncytial virus.mp.
-
or/1-3
-
palivizumab.mp. or exp PALIVIZUMAB/
-
exp Monoclonal Antibody/or monoclonal _ntibody$.mp.
-
synagis.mp.
-
immunoprophylaxis.mp. or exp IMMUNOPROPHYLAXIS/
-
or/5-8
-
4 and 9
-
decision support technique$.mp.
-
exp statistical model/or markov model$.mp.
-
exp “cost effectiveness analysis”/or economic model$.mp.
-
decision analysis.mp.
-
or/11-14
-
10 and 15
-
limit 16 to yr=“2007 –Current”
Additional searches:
Prognosis
Database: Ovid MEDLINE® 1950 to week 4 July 2009
-
exp Respiratory Syncytial Virus, Human/or rsv.mp.
-
respiratory syncytial virus.mp.
-
bronchiolitis.mp. or exp Bronchiolitis, Viral/
-
or/1-3
-
prognosis.mp. or exp Prognosis/
-
outcome$.mp.
-
risk$.mp. or exp Risk Factors/
-
hospitali?ation.mp.
-
exp Follow-Up Studies/or follow-up.mp.
-
complication$.mp.
-
exp Cohort Studies/or cohort$.mp.
-
or/5-10
-
4 and 12
-
11 and 13
-
limit 14 to yr=“2007 – 2009”
Hospitalisation
Database: Ovid MEDLINE® 1950 to week 4 July 2009
-
exp Respiratory Syncytial Virus, Human/or rsv.mp.
-
respiratory syncytial virus.mp.
-
bronchiolitis.mp. or exp Bronchiolitis, Viral/
-
or/1-3
-
hospitali?ation.mp.
-
4 and 5
-
limit 6 to yr=“2007 – 2009”
Appendix 2 Table of excluded studies with rationale
Below are listed studies that were nearly included, with reasons for exclusion. The remaining studies were excluded because they did not report any of the listed subgroups.
| Study | Reason for exclusion |
|---|---|
| Aujard Y, Fauroux B. Risk factors for severe respiratory syncytial virus infection in infants. Respir Med 2002;96:S9–S14 | Review |
| Bloemers B, van Furth M, Weijerman ME, Gemke RJ, Broers CJ, van den Ende, et al. Down syndrome: a novel risk factor for respiratory syncytial virus bronchiolitis – a prospective birth-cohort study. Pediatrics 2007;120(4):1076–81 | All children with Down’s syndrome |
| Boyce TG, Mellen BG, Mitchel EF, Wright PF, Griffin MR. Rates of hospitalisation for respiratory syncytial virus infection among children in Medicaid. J Pediatr 2000;137:865–70 | Reported incidence rate ratios and could not convert to ORs |
| Breese Hall C, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360(6):588–98 | No CIs given for ORs in outcomes where ORs and CIs available from other studies |
| Cabalka AK. Physiologic risk factors for respiratory viral infections and immunoprophylaxis for respiratory syncytial virus in young children with congenital heart disease. Pediatr Infect Dis J 2004;23(1):S41–S5 | Review |
| Carbonell-Estrany X, Figueras-Aloy J, IRIpVRS Study Group. Identifying risk factors for severe respiratory syncytial virus among infants born after 33 through 35 completed weeks of gestation. Pediatr Infect Dis J 2004;23(11):S193–S201 | PICNIC and FLIP studies already included |
| Carbonell-Estrany X, Bont L, Doerling G, Gouyon J-B, Lanari M. Clinical relevance of prevention of respiratory syncytial virus lower respiratory tract infection in pre-term infants born between 33 and 35 weeks gestational age. Eur J Clin Microbiol Infect Dis 2008;27:891–9 | Review |
| Clark SJ, Beresford MW, Subhedar NV, Shaw NJ. Respiratory syncytial virus infection in high risk infants and the potential impact of prophylaxis in a United Kingdom cohort. Arch Dis Child 2000;83:313–6 | No subgroup results |
| Doering G, Guselnleitner W, Belohradsky BH, Burdach S, Resch B, Liese JG. The risk of respiratory syncytial virus-related hospitalisation in preterm infants of 29 to 35 weeks gestational age. Pediatr Infect Dis J 2006;25(12):1188–91 | Duplicates Liese et al.25 study |
| Duppenthaler A, Ammann RA, Gorgievski-Hrisoho M, Pfammatter JP, Aebi C. Low incidence of respiratory syncytial virus hospitalisations in haemodynamically significant congenital heart disease. Arch Dis Child 2003;89:961–5 | No suitable subgroup results |
| Everard ML. The relationship between respiratory syncytial virus infection and the development of wheezing and asthma in children. Curr Opin Allergy Clin Immunol 2006;6(1):56–61 | Review |
| Fjaerli HO, Farstad T, Bratlid D. Hospitalisations for respiratory syncytial virus bronchiolitis in Akerhus, Norway, 1993–2000: a population-based retrospective study. BMC Pediatr 2004;4(25):1–7 | No suitable subgroup results |
| Greenough A, Alexander J, Boit P, Boorman J, Burgess S, Burke A, et al. School-age outcome of hospitalisation with respiratory syncytial virus infection of prematurely born infants. Thorax 2009;64:490–5 | No suitable subgroup results |
| Grimaldi M, Cornet B, Milou C, Gouyon JB. Etude prospective regionale d’une epidemie de bronchiolites a virus respiratoire syncytial (VRS). Arch Pediatri 2002;9:572–80 | Relevant subgroups not given |
| Groothuis JR, Gutierrez KM, Lauer BA. Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics 1988;82:199–203 | Study too small to use (total n = 18 for risk factors) |
| Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM, et al. Hospitalisation for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol 2005;16:386–92 | No subgroups given |
| Holberg CJ, Wright AL, Martinez FD, Ray CG, Taussing LM, Lebowitz MD. Risk factors for respiratory syncytial virus-associated lower respiratory illnesses in first year of life. Am J Epidemiol 1991;133(11):1135–51 | RSV hospitalisation related risk factors not given |
| Kneyber MC, Steyerberg EW, de Groot R, Moll HA. Long term effects of respiratory syncytial virus (RSV) bronchiolitis in infants and young children: a quantitative review. Acta Paediatr 2000;89:654–60 | Early meta-analysis |
| Lee JT, Chang LY, Wang LC, Kao CL, Shao PL, Lu CY, et al. Epidemiology of respiratory syncytial virus infection in northern Taiwan, 2001–2005 – seasonality, clinical characteristics and disease burden. J Microbiol Immunol Infect 2007;40:293–301 | Not generalisable to UK population |
| Medrano C, Garcia-Guereta L, Grueso J, Insa B, Ballesteros F, Casaldaliga J, et al. Respiratory infection in congenital heart disease. Hospitalisations in young children in Spain during 2004 and 2005: the CIVIC Epidemiological Study. Cardiol Young 2007;17:360–71 | No suitable subgroup results given |
| Navas L, Wang E, de Carvalho V, Robinson J. Improved outcome of respiratory syncytial virus infection in a high risk hospitalised population of Canadian children. Pediatrics 1992;121(3):347–54 | No suitable subgroup results given |
| Noyola DE, Zuviri-Gonzalez A, Casto-Garcia JA, Ochoa-Zavala JR. Impact of respiratory syncytial virus on hospital admissions in children younger than 3 years of age. Infection 2007;54:180–4 | Study from Mexico and results not UK generalisable |
| Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, Quero J, Iris Study Group. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalisations in premature infants. Pediatr Infect Dis J 2003;22(9):823–7 | Partial duplication of Carbonell-Estrany et al. 200011 and 200114 |
| Prevent Study Group. Reduction in respiratory syncytial virus hospitalisation among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 1997;99:93–9 | No suitable subgroups given |
| Resch B. Palivizumab for the prophylaxis of respiratory syncytial virus infection. Ped Health 2008;2(3):265–78 | Review of other included studies |
| Simoes EA, Sondheimer HM, Top FH, Meissner C, Welliver RC, Kramer AA, et al. Respiratory syncytial virus immune globulin for prophylaxis against respiratory syncytial virus disease in infants and children with congenital heart disease. J Pediatr 1998;133(4):492–9 | No suitable subgroups given |
| Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr 2003;143:S118–S126 | Semi-systematic review |
| Wang EE, Law BJ, Stephens D, the PICNIC Study Group. Pediatric investigators collaborative network in infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalised with respiratory syncytial viral lower respiratory tract infection. J Pediatr 1995;126(2):212–9 | PICNIC study already included (Law) |
| Wang EE, Law BJ, Boucher FD, the PICNIC Study Group. Pediatric investigators collaborative network in infections in Canada (PICNIC) study of admission and management variation in patients hospitalised with respiratory syncytial viral lower respiratory tract infection. J Pediatr 1996;129(3):310–5 | Duplicate of study above |
Appendix 3 Quality assessment of included studies
| Study | Patient recruitment | RSV ascertainment method | Measurement methods of subgroups explicit | Clarity of reporting |
|---|---|---|---|---|
| Carbonell-Estrany 200011 | All rehospitalised children | 97% antigen test, 3% culture | Interview at O/P or telephone call | Fair |
| Carbonell-Estrany 200114 | All rehospitalised children | Method unclear, 10% not tested for RSV | Interview at O/P or telephone call | Poor. Very difficult to tell where results were from the different population to Carbonell-Estrany 200011 |
| Eriksson 200215 | From case records, not consecutive? | Nasopharyngeal lavage, antigen detection and ‘virus isolation on the majority of samples’ | From case records only | Poor. Included 149 patients not from the catchment area but not clear which tables this in |
| Figueras-Aloy 200416 | Unclear how cases and controls selected, not consecutive? | Not standardised | From questioning, no further details given | Fair. Includes power calculation |
| Figueras-Aloy 200818 | Unclear how cases and controls selected, not consecutive? | Immunofluorescence assay or viral culture | Risk factors defined, collection at first admission | Good |
| Frogel 200819 | Palivizumab treatment registry co-ordinated by drug company | Virology testing (e.g. rapid antigen detection, viral culture) | From registry data take on enrolment | Fair. No CIs given for ORs, p-values given instead |
| Grimwood 200820 | Unclear, not consecutive? | Nasopharyngeal aspirate, antigen test | During hospitalisation by nurse administered questionnaire | Good |
| Kristensen 20099 | Unclear, not consecutive? | Not given | Unclear | Poor. Tables and text do not link well |
| Law 200421 | Unclear, not consecutive? | Viral culture/rapid test | Interview by trained researchers | Fair |
| Liese 200325 | All neonates eligible enrolled | Clinical diagnosis or antigen test | Questionnaire sent to parents | Good |
| Nielsen 200323 | All children eligible enrolled | Nasopharyngeal suction, antigen test | Not given | Fair |
| Rietveld 20066 | Unclear, not consecutive? | Nasopharyngeal aspirate, viral culture or immunofluorescence assay | From perinatal registry | Poor. Overly confusing explanations |
| Rossi 200724 | Cases and controls, consecutive acute respiratory infections at emergency departments | Nasal secretion, immunoenzymatic test | From ‘osservatorio’ database | Fair |
Glossary
- Chronic lung disease (CLD)
- CLD is defined as oxygen dependency for at least 28 days from birth. It is caused by prolonged supplemental oxygen therapy and ventilation and usually develops in the first 4 weeks after birth, most often affecting children born prematurely. It is caused by the pressure and high concentrations of oxygen which, when prolonged, can cause lung tissue to become inflamed and scarred.
- Confidence interval (CI)
- A measure of the precision of a statistical estimate; quantifies the uncertainty in measurement. Usually reported as 95% CI, i.e. the range of values within which one can be 95% sure that the true values for the whole population lie.
- Credible interval
- An indication of the uncertainty in the true location of a parameter value.
- Discounting
- Discounting refers to the process of adjusting the value of costs or benefits that occur at different points of time in the future, so that they may all be compared as if they had occurred at the same time.
- Incremental cost-effectiveness ratio (ICER)
- An expression of the additional cost of health gain associated with an intervention relative to an appropriate comparator. Expressed as the difference in mean costs (relative to the comparator) divided by the difference in mean health gain.
- Infant
- A child up to 1 year old (up to and including 365 days from birth).
- Meta-analysis
- The statistical pooling of the results of a collection of related individual studies, to increase statistical power and synthesise their findings.
- Quality of life
- A concept incorporating all the factors that might impact on an individual’s life, including factors such as the absence of disease or infirmity and also other factors which might affect an individual's physical, mental and social well-being.
- Quality-adjusted life-year (QALY)
- An index of health gain where survival duration is weighted or adjusted by the patient’s quality of life during the survival period. QALYs have the advantage of incorporating changes in both quantity (mortality) and quality (morbidity) of life.
- Odds
- A ratio of the number of people incurring an event to the number of people who don’t have an event.
- Odds ratio (OR)
- Ratio of odds of a specified characteristic in the treated group to the odds in the control group.
- Risk ratio (RR)
- The ratio of risk in the treated group to the risk in the control group.
List of abbreviations
- AGE
- birth age
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CLD
- chronic lung disease
- CHD
- congenital heart disease
- GA
- gestational age
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- HUI
- Health Utilities Index
- ICER
- incremental cost-effectiveness ratio
- MB
- multiple birth
- NICE
- National Institute for Health and Clinical Excellence
- OC
- overcrowding
- OR
- odds ratio
- PE
- parental education
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- rate ratio
- RSV
- respiratory syncytial virus
- SAS
- siblings at school
- SE
- smoking exposure
All abbreviations that have been used in this report are listed here unless the abbreviation is well known (e.g. NHS), or it has been used only once, or it is a non-standard abbreviation used only in figures/tables/appendices, in which case the abbreviation is defined in the figure legend or in the notes at the end of the table.
Notes
Health Technology Assessment programme
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
Prioritisation Group
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Imti Choonara, Professor in Child Health, Academic Division of Child Health, University of Nottingham
Chair – Pharmaceuticals Panel
-
Dr Bob Coates, Consultant Advisor – Disease Prevention Panel
-
Dr Andrew Cook, Consultant Advisor – Intervention Procedures Panel
-
Dr Peter Davidson, Director of NETSCC, Health Technology Assessment
-
Dr Nick Hicks, Consultant Adviser – Diagnostic Technologies and Screening Panel, Consultant Advisor–Psychological and Community Therapies Panel
-
Ms Susan Hird, Consultant Advisor, External Devices and Physical Therapies Panel
-
Professor Sallie Lamb, Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick
Chair – HTA Clinical Evaluation and Trials Board
-
Professor Jonathan Michaels, Professor of Vascular Surgery, Sheffield Vascular Institute, University of Sheffield
Chair – Interventional Procedures Panel
-
Professor Ruairidh Milne, Director – External Relations
-
Dr John Pounsford, Consultant Physician, Directorate of Medical Services, North Bristol NHS Trust
Chair – External Devices and Physical Therapies Panel
-
Dr Vaughan Thomas, Consultant Advisor – Pharmaceuticals Panel, Clinical
Lead – Clinical Evaluation Trials Prioritisation Group
-
Professor Margaret Thorogood, Professor of Epidemiology, Health Sciences Research Institute, University of Warwick
Chair – Disease Prevention Panel
-
Professor Lindsay Turnbull, Professor of Radiology, Centre for the MR Investigations, University of Hull
Chair – Diagnostic Technologies and Screening Panel
-
Professor Scott Weich, Professor of Psychiatry, Health Sciences Research Institute, University of Warwick
Chair – Psychological and Community Therapies Panel
-
Professor Hywel Williams, Director of Nottingham Clinical Trials Unit, Centre of Evidence-Based Dermatology, University of Nottingham
Chair – HTA Commissioning Board
Deputy HTA Programme Director
HTA Commissioning Board
-
Professor of Dermato-Epidemiology, Centre of Evidence-Based Dermatology, University of Nottingham
-
Professor of General Practice, Department of Primary Health Care, University of Oxford Programme Director,
-
Professor of Clinical Pharmacology, Director, NIHR HTA programme, University of Liverpool
-
Professor Ann Ashburn, Professor of Rehabilitation and Head of Research, Southampton General Hospital
-
Professor Deborah Ashby, Professor of Medical Statistics and Clinical Trials, Queen Mary, Department of Epidemiology and Public Health, Imperial College London
-
Professor Peter Brocklehurst, Director, National Perinatal Epidemiology Unit, University of Oxford
-
Professor John Cairns, Professor of Health Economics, London School of Hygiene and Tropical Medicine
-
Professor Peter Croft, Director of Primary Care Sciences Research Centre, Keele University
-
Professor Jenny Donovan, Professor of Social Medicine, University of Bristol
-
Professor Jonathan Green, Professor and Acting Head of Department, Child and Adolescent Psychiatry, University of Manchester Medical School
-
Professor John W Gregory, Professor in Paediatric Endocrinology, Department of Child Health, Wales School of Medicine, Cardiff University
-
Professor Steve Halligan, Professor of Gastrointestinal Radiology, University College Hospital, London
-
Professor Freddie Hamdy, Professor of Urology, Head of Nuffield Department of Surgery, University of Oxford
-
Professor Allan House, Professor of Liaison Psychiatry, University of Leeds
-
Dr Martin J Landray, Reader in Epidemiology, Honorary Consultant Physician, Clinical Trial Service Unit, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris, Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Professor Stephen Morris, Professor of Health Economics, University College London, Research Department of Epidemiology and Public Health, University College London
-
Professor E Andrea Nelson, Professor of Wound Healing and Director of Research, School of Healthcare, University of Leeds
-
Professor John David Norris Chair in Clinical Trials and Biostatistics, Robertson Centre for Biostatistics, University of Glasgow
-
Dr Rafael Perera, Lecturer in Medical Statisitics, Department of Primary Health Care, University of Oxford
-
Professor James Raftery, Chair of NETSCC and Director of the Wessex Institute, University of Southampton
-
Professor Barney Reeves, Professorial Research Fellow in Health Services Research, Department of Clinical Science, University of Bristol
-
Professor Martin Underwood, Warwick Medical School, University of Warwick
-
Professor Marion Walker, Professor in Stroke Rehabilitation, Associate Director UK Stroke Research Network, University of Nottingham
-
Dr Duncan Young, Senior Clinical Lecturer and Consultant, Nuffield Department of Anaesthetics, University of Oxford
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
HTA Clinical Evaluation and Trials Board
-
Director, Warwick Clinical Trials Unit, Warwick Medical School, University of Warwick and Professor of Rehabilitation, Nuffield Department of Orthopaedic, Rheumatology and Musculoskeletal Sciences, University of Oxford
-
Professor of the Psychology of Health Care, Leeds Institute of Health Sciences, University of Leeds
-
Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Professor Keith Abrams, Professor of Medical Statistics, Department of Health Sciences, University of Leicester
-
Professor Martin Bland, Professor of Health Statistics, Department of Health Sciences, University of York
-
Professor Jane Blazeby, Professor of Surgery and Consultant Upper GI Surgeon, Department of Social Medicine, University of Bristol
-
Professor Julia M Brown, Director, Clinical Trials Research Unit, University of Leeds
-
Professor Alistair Burns, Professor of Old Age Psychiatry, Psychiatry Research Group, School of Community-Based Medicine, The University of Manchester & National Clinical Director for Dementia, Department of Health
-
Dr Jennifer Burr, Director, Centre for Healthcare Randomised trials (CHART), University of Aberdeen
-
Professor Linda Davies, Professor of Health Economics, Health Sciences Research Group, University of Manchester
-
Professor Simon Gilbody, Prof of Psych Medicine and Health Services Research, Department of Health Sciences, University of York
-
Professor Steven Goodacre, Professor and Consultant in Emergency Medicine, School of Health and Related Research, University of Sheffield
-
Professor Dyfrig Hughes, Professor of Pharmacoeconomics, Centre for Economics and Policy in Health, Institute of Medical and Social Care Research, Bangor University
-
Professor Paul Jones, Professor of Respiratory Medicine, Department of Cardiac and Vascular Science, St George‘s Hospital Medical School, University of London
-
Professor Khalid Khan, Professor of Women’s Health and Clinical Epidemiology, Barts and the London School of Medicine, Queen Mary, University of London
-
Professor Richard J McManus, Professor of Primary Care Cardiovascular Research, Primary Care Clinical Sciences Building, University of Birmingham
-
Professor Helen Rodgers, Professor of Stroke Care, Institute for Ageing and Health, Newcastle University
-
Professor Ken Stein, Professor of Public Health, Peninsula Technology Assessment Group, Peninsula College of Medicine and Dentistry, Universities of Exeter and Plymouth
-
Professor Jonathan Sterne, Professor of Medical Statistics and Epidemiology, Department of Social Medicine, University of Bristol
-
Mr Andy Vail, Senior Lecturer, Health Sciences Research Group, University of Manchester
-
Professor Clare Wilkinson, Professor of General Practice and Director of Research North Wales Clinical School, Department of Primary Care and Public Health, Cardiff University
-
Dr Ian B Wilkinson, Senior Lecturer and Honorary Consultant, Clinical Pharmacology Unit, Department of Medicine, University of Cambridge
-
Ms Kate Law, Director of Clinical Trials, Cancer Research UK
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
Diagnostic Technologies and Screening Panel
-
Scientific Director of the Centre for Magnetic Resonance Investigations and YCR Professor of Radiology, Hull Royal Infirmary
-
Professor Judith E Adams, Consultant Radiologist, Manchester Royal Infirmary, Central Manchester & Manchester Children’s University Hospitals NHS Trust, and Professor of Diagnostic Radiology, University of Manchester
-
Mr Angus S Arunkalaivanan, Honorary Senior Lecturer, University of Birmingham and Consultant Urogynaecologist and Obstetrician, City Hospital, Birmingham
-
Dr Stephanie Dancer, Consultant Microbiologist, Hairmyres Hospital, East Kilbride
-
Dr Diane Eccles, Professor of Cancer Genetics, Wessex Clinical Genetics Service, Princess Anne Hospital
-
Dr Trevor Friedman, Consultant Liason Psychiatrist, Brandon Unit, Leicester General Hospital
-
Dr Ron Gray, Consultant, National Perinatal Epidemiology Unit, Institute of Health Sciences, University of Oxford
-
Professor Paul D Griffiths, Professor of Radiology, Academic Unit of Radiology, University of Sheffield
-
Mr Martin Hooper, Service User Representative
-
Professor Anthony Robert Kendrick, Associate Dean for Clinical Research and Professor of Primary Medical Care, University of Southampton
-
Dr Anne Mackie, Director of Programmes, UK National Screening Committee, London
-
Mr David Mathew, Service User Representative
-
Dr Michael Millar, Consultant Senior Lecturer in Microbiology, Department of Pathology & Microbiology, Barts and The London NHS Trust, Royal London Hospital
-
Mrs Una Rennard, Service User Representative
-
Dr Stuart Smellie, Consultant in Clinical Pathology, Bishop Auckland General Hospital
-
Ms Jane Smith, Consultant Ultrasound Practitioner, Leeds Teaching Hospital NHS Trust, Leeds
-
Dr Allison Streetly, Programme Director, NHS Sickle Cell and Thalassaemia Screening Programme, King’s College School of Medicine
-
Dr Alan J Williams, Consultant Physician, General and Respiratory Medicine, The Royal Bournemouth Hospital
-
Dr Tim Elliott, Team Leader, Cancer Screening, Department of Health
-
Dr Catherine Moody, Programme Manager, Medical Research Council
-
Professor Julietta Patrick, Director, NHS Cancer Screening Programme, Sheffield
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Disease Prevention Panel
-
Professor of Epidemiology, University of Warwick Medical School, Coventry
-
Dr Robert Cook, Clinical Programmes Director, Bazian Ltd, London
-
Dr Colin Greaves, Senior Research Fellow, Peninsula Medical School (Primary Care)
-
Mr Michael Head, Service User Representative
-
Professor Cathy Jackson, Professor of Primary Care Medicine, Bute Medical School, University of St Andrews
-
Dr Russell Jago, Senior Lecturer in Exercise, Nutrition and Health, Centre for Sport, Exercise and Health, University of Bristol
-
Dr Julie Mytton, Consultant in Child Public Health, NHS Bristol
-
Professor Irwin Nazareth, Professor of Primary Care and Director, Department of Primary Care and Population Sciences, University College London
-
Dr Richard Richards, Assistant Director of Public Health, Derbyshire Country Primary Care Trust
-
Professor Ian Roberts, Professor of Epidemiology and Public Health, London School of Hygiene & Tropical Medicine
-
Dr Kenneth Robertson, Consultant Paediatrician, Royal Hospital for Sick Children, Glasgow
-
Dr Catherine Swann, Associate Director, Centre for Public Health Excellence, NICE
-
Professor Carol Tannahill, Glasgow Centre for Population Health
-
Mrs Jean Thurston, Service User Representative
-
Professor David Weller, Head, School of Clinical Science and Community Health, University of Edinburgh
-
Ms Christine McGuire, Research & Development, Department of Health
-
Dr Kay Pattison Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
External Devices and Physical Therapies Panel
-
Consultant Physician North Bristol NHS Trust
-
Reader in Wound Healing and Director of Research, University of Leeds
-
Professor Bipin Bhakta, Charterhouse Professor in Rehabilitation Medicine, University of Leeds
-
Mrs Penny Calder, Service User Representative
-
Dr Dawn Carnes, Senior Research Fellow, Barts and the London School of Medicine and Dentistry
-
Dr Emma Clark, Clinician Scientist Fellow & Cons. Rheumatologist, University of Bristol
-
Mrs Anthea De Barton-Watson, Service User Representative
-
Professor Nadine Foster, Professor of Musculoskeletal Health in Primary Care Arthritis Research, Keele University
-
Dr Shaheen Hamdy, Clinical Senior Lecturer and Consultant Physician, University of Manchester
-
Professor Christine Norton, Professor of Clinical Nursing Innovation, Bucks New University and Imperial College Healthcare NHS Trust
-
Dr Lorraine Pinnigton, Associate Professor in Rehabilitation, University of Nottingham
-
Dr Kate Radford, Senior Lecturer (Research), University of Central Lancashire
-
Mr Jim Reece, Service User Representative
-
Professor Maria Stokes, Professor of Neuromusculoskeletal Rehabilitation, University of Southampton
-
Dr Pippa Tyrrell, Senior Lecturer/Consultant, Salford Royal Foundation Hospitals’ Trust and University of Manchester
-
Dr Sarah Tyson, Senior Research Fellow & Associate Head of School, University of Salford
-
Dr Nefyn Williams, Clinical Senior Lecturer, Cardiff University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Interventional Procedures Panel
-
Professor of Vascular Surgery, University of Sheffield
-
Consultant Colorectal Surgeon, Bristol Royal Infirmary
-
Mrs Isabel Boyer, Service User Representative
-
Mr David P Britt, Service User Representative
-
Mr Sankaran ChandraSekharan, Consultant Surgeon, Breast Surgery, Colchester Hospital University NHS Foundation Trust
-
Professor Nicholas Clarke, Consultant Orthopaedic Surgeon, Southampton University Hospitals NHS Trust
-
Ms Leonie Cooke, Service User Representative
-
Mr Seamus Eckford, Consultant in Obstetrics & Gynaecology, North Devon District Hospital
-
Professor David Taggart, Consultant Cardiothoracic Surgeon, John Radcliffe Hospital
-
Professor Sam Eljamel, Consultant Neurosurgeon, Ninewells Hospital and Medical School, Dundee
-
Dr Adele Fielding, Senior Lecturer and Honorary Consultant in Haematology, University College London Medical School
-
Dr Matthew Hatton, Consultant in Clinical Oncology, Sheffield Teaching Hospital Foundation Trust
-
Dr John Holden, General Practitioner, Garswood Surgery, Wigan
-
Professor Nicholas James, Professor of Clinical Oncology, School of Cancer Sciences, University of Birmingham
-
Dr Fiona Lecky, Senior Lecturer/Honorary Consultant in Emergency Medicine, University of Manchester/Salford Royal Hospitals NHS Foundation Trust
-
Dr Nadim Malik, Consultant Cardiologist/ Honorary Lecturer, University of Manchester
-
Mr Hisham Mehanna, Consultant & Honorary Associate Professor, University Hospitals Coventry & Warwickshire NHS Trust
-
Dr Jane Montgomery, Consultant in Anaesthetics and Critical Care, South Devon Healthcare NHS Foundation Trust
-
Professor Jon Moss, Consultant Interventional Radiologist, North Glasgow Hospitals University NHS Trust
-
Dr Simon Padley, Consultant Radiologist, Chelsea & Westminster Hospital
-
Dr Ashish Paul, Medical Director, Bedfordshire PCT
-
Dr Sarah Purdy, Consultant Senior Lecturer, University of Bristol
-
Professor Yit Chiun Yang, Consultant Ophthalmologist, Royal Wolverhampton Hospitals NHS Trust
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Pharmaceuticals Panel
-
Professor in Child Health, University of Nottingham
-
Senior Lecturer in Clinical Pharmacology, University of East Anglia
-
Dr Martin Ashton-Key, Medical Advisor, National Commissioning Group, NHS London
-
Mr John Chapman, Service User Representative
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Peter Elton, Director of Public Health, Bury Primary Care Trust
-
Dr Ben Goldacre, Research Fellow, Division of Psychological Medicine and Psychiatry, King’s College London
-
Dr James Gray, Consultant Microbiologist, Department of Microbiology, Birmingham Children’s Hospital NHS Foundation Trust
-
Ms Kylie Gyertson, Oncology and Haematology Clinical Trials Manager, Guy’s and St Thomas’ NHS Foundation Trust London
-
Dr Jurjees Hasan, Consultant in Medical Oncology, The Christie, Manchester
-
Dr Carl Heneghan Deputy Director Centre for Evidence-Based Medicine and Clinical Lecturer, Department of Primary Health Care, University of Oxford
-
Dr Dyfrig Hughes, Reader in Pharmacoeconomics and Deputy Director, Centre for Economics and Policy in Health, IMSCaR, Bangor University
-
Dr Maria Kouimtzi, Pharmacy and Informatics Director, Global Clinical Solutions, Wiley-Blackwell
-
Professor Femi Oyebode, Consultant Psychiatrist and Head of Department, University of Birmingham
-
Dr Andrew Prentice, Senior Lecturer and Consultant Obstetrician and Gynaecologist, The Rosie Hospital, University of Cambridge
-
Ms Amanda Roberts, Service User Representative
-
Dr Martin Shelly, General Practitioner, Silver Lane Surgery, Leeds
-
Dr Gillian Shepherd, Director, Health and Clinical Excellence, Merck Serono Ltd
-
Mrs Katrina Simister, Assistant Director New Medicines, National Prescribing Centre, Liverpool
-
Professor Donald Singer Professor of Clinical Pharmacology and Therapeutics, Clinical Sciences Research Institute, CSB, University of Warwick Medical School
-
Mr David Symes, Service User Representative
-
Dr Arnold Zermansky, General Practitioner, Senior Research Fellow, Pharmacy Practice and Medicines Management Group, Leeds University
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Mr Simon Reeve, Head of Clinical and Cost-Effectiveness, Medicines, Pharmacy and Industry Group, Department of Health
-
Dr Heike Weber, Programme Manager, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Psychological and Community Therapies Panel
-
Professor of Psychiatry, University of Warwick, Coventry
-
Consultant & University Lecturer in Psychiatry, University of Cambridge
-
Professor Jane Barlow, Professor of Public Health in the Early Years, Health Sciences Research Institute, Warwick Medical School
-
Dr Sabyasachi Bhaumik, Consultant Psychiatrist, Leicestershire Partnership NHS Trust
-
Mrs Val Carlill, Service User Representative
-
Dr Steve Cunningham, Consultant Respiratory Paediatrician, Lothian Health Board
-
Dr Anne Hesketh, Senior Clinical Lecturer in Speech and Language Therapy, University of Manchester
-
Dr Peter Langdon, Senior Clinical Lecturer, School of Medicine, Health Policy and Practice, University of East Anglia
-
Dr Yann Lefeuvre, GP Partner, Burrage Road Surgery, London
-
Dr Jeremy J Murphy, Consultant Physician and Cardiologist, County Durham and Darlington Foundation Trust
-
Dr Richard Neal, Clinical Senior Lecturer in General Practice, Cardiff University
-
Mr John Needham, Service User Representative
-
Ms Mary Nettle, Mental Health User Consultant
-
Professor John Potter, Professor of Ageing and Stroke Medicine, University of East Anglia
-
Dr Greta Rait, Senior Clinical Lecturer and General Practitioner, University College London
-
Dr Paul Ramchandani, Senior Research Fellow/Cons. Child Psychiatrist, University of Oxford
-
Dr Karen Roberts, Nurse/Consultant, Dunston Hill Hospital, Tyne and Wear
-
Dr Karim Saad, Consultant in Old Age Psychiatry, Coventry and Warwickshire Partnership Trust
-
Dr Lesley Stockton, Lecturer, School of Health Sciences, University of Liverpool
-
Dr Simon Wright, GP Partner, Walkden Medical Centre, Manchester
-
Dr Kay Pattison, Senior NIHR Programme Manager, Department of Health
-
Dr Morven Roberts, Clinical Trials Manager, Health Services and Public Health Services Board, Medical Research Council
-
Professor Tom Walley, CBE, Director, NIHR HTA programme, Professor of Clinical Pharmacology, University of Liverpool
-
Dr Ursula Wells, Principal Research Officer, Policy Research Programme, Department of Health
Expert Advisory Network
-
Professor Douglas Altman, Professor of Statistics in Medicine, Centre for Statistics in Medicine, University of Oxford
-
Professor John Bond, Professor of Social Gerontology & Health Services Research, University of Newcastle upon Tyne
-
Professor Andrew Bradbury, Professor of Vascular Surgery, Solihull Hospital, Birmingham
-
Mr Shaun Brogan, Chief Executive, Ridgeway Primary Care Group, Aylesbury
-
Mrs Stella Burnside OBE, Chief Executive, Regulation and Improvement Authority, Belfast
-
Ms Tracy Bury, Project Manager, World Confederation of Physical Therapy, London
-
Professor Iain T Cameron, Professor of Obstetrics and Gynaecology and Head of the School of Medicine, University of Southampton
-
Professor Bruce Campbell, Consultant Vascular & General Surgeon, Royal Devon & Exeter Hospital, Wonford
-
Dr Christine Clark, Medical Writer and Consultant Pharmacist, Rossendale
-
Professor Collette Clifford, Professor of Nursing and Head of Research, The Medical School, University of Birmingham
-
Professor Barry Cookson, Director, Laboratory of Hospital Infection, Public Health Laboratory Service, London
-
Dr Carl Counsell, Clinical Senior Lecturer in Neurology, University of Aberdeen
-
Professor Howard Cuckle, Professor of Reproductive Epidemiology, Department of Paediatrics, Obstetrics & Gynaecology, University of Leeds
-
Professor Carol Dezateux, Professor of Paediatric Epidemiology, Institute of Child Health, London
-
Mr John Dunning, Consultant Cardiothoracic Surgeon, Papworth Hospital NHS Trust, Cambridge
-
Mr Jonothan Earnshaw, Consultant Vascular Surgeon, Gloucestershire Royal Hospital, Gloucester
-
Professor Martin Eccles, Professor of Clinical Effectiveness, Centre for Health Services Research, University of Newcastle upon Tyne
-
Professor Pam Enderby, Dean of Faculty of Medicine, Institute of General Practice and Primary Care, University of Sheffield
-
Professor Gene Feder, Professor of Primary Care Research & Development, Centre for Health Sciences, Barts and The London School of Medicine and Dentistry
-
Mr Leonard R Fenwick, Chief Executive, Freeman Hospital, Newcastle upon Tyne
-
Mrs Gillian Fletcher, Antenatal Teacher and Tutor and President, National Childbirth Trust, Henfield
-
Professor Jayne Franklyn, Professor of Medicine, University of Birmingham
-
Mr Tam Fry, Honorary Chairman, Child Growth Foundation, London
-
Professor Fiona Gilbert, Consultant Radiologist and NCRN Member, University of Aberdeen
-
Professor Paul Gregg, Professor of Orthopaedic Surgical Science, South Tees Hospital NHS Trust
-
Bec Hanley, Co-director, TwoCan Associates, West Sussex
-
Dr Maryann L Hardy, Senior Lecturer, University of Bradford
-
Mrs Sharon Hart, Healthcare Management Consultant, Reading
-
Professor Robert E Hawkins, CRC Professor and Director of Medical Oncology, Christie CRC Research Centre, Christie Hospital NHS Trust, Manchester
-
Professor Richard Hobbs, Head of Department of Primary Care & General Practice, University of Birmingham
-
Professor Alan Horwich, Dean and Section Chairman, The Institute of Cancer Research, London
-
Professor Allen Hutchinson, Director of Public Health and Deputy Dean of ScHARR, University of Sheffield
-
Professor Peter Jones, Professor of Psychiatry, University of Cambridge, Cambridge
-
Professor Stan Kaye, Cancer Research UK Professor of Medical Oncology, Royal Marsden Hospital and Institute of Cancer Research, Surrey
-
Dr Duncan Keeley, General Practitioner (Dr Burch & Ptnrs), The Health Centre, Thame
-
Dr Donna Lamping, Research Degrees Programme Director and Reader in Psychology, Health Services Research Unit, London School of Hygiene and Tropical Medicine, London
-
Professor James Lindesay, Professor of Psychiatry for the Elderly, University of Leicester
-
Professor Julian Little, Professor of Human Genome Epidemiology, University of Ottawa
-
Professor Alistaire McGuire, Professor of Health Economics, London School of Economics
-
Professor Neill McIntosh, Edward Clark Professor of Child Life and Health, University of Edinburgh
-
Professor Rajan Madhok, Consultant in Public Health, South Manchester Primary Care Trust
-
Professor Sir Alexander Markham, Director, Molecular Medicine Unit, St James’s University Hospital, Leeds
-
Dr Peter Moore, Freelance Science Writer, Ashtead
-
Dr Andrew Mortimore, Public Health Director, Southampton City Primary Care Trust
-
Dr Sue Moss, Associate Director, Cancer Screening Evaluation Unit, Institute of Cancer Research, Sutton
-
Professor Miranda Mugford, Professor of Health Economics and Group Co-ordinator, University of East Anglia
-
Professor Jim Neilson, Head of School of Reproductive & Developmental Medicine and Professor of Obstetrics and Gynaecology, University of Liverpool
-
Mrs Julietta Patnick, Director, NHS Cancer Screening Programmes, Sheffield
-
Professor Robert Peveler, Professor of Liaison Psychiatry, Royal South Hants Hospital, Southampton
-
Professor Chris Price, Director of Clinical Research, Bayer Diagnostics Europe, Stoke Poges
-
Professor William Rosenberg, Professor of Hepatology and Consultant Physician, University of Southampton
-
Professor Peter Sandercock, Professor of Medical Neurology, Department of Clinical Neurosciences, University of Edinburgh
-
Dr Philip Shackley, Senior Lecturer in Health Economics, Sheffield Vascular Institute, University of Sheffield
-
Dr Eamonn Sheridan, Consultant in Clinical Genetics, St James’s University Hospital, Leeds
-
Dr Margaret Somerville, Director of Public Health Learning, Peninsula Medical School, University of Plymouth
-
Professor Sarah Stewart-Brown, Professor of Public Health, Division of Health in the Community, University of Warwick, Coventry
-
Dr Nick Summerton, GP Appraiser and Codirector, Research Network, Yorkshire Clinical Consultant, Primary Care and Public Health, University of Oxford
-
Professor Ala Szczepura, Professor of Health Service Research, Centre for Health Services Studies, University of Warwick, Coventry
-
Dr Ross Taylor, Senior Lecturer, University of Aberdeen
-
Dr Richard Tiner, Medical Director, Medical Department, Association of the British Pharmaceutical Industry
-
Mrs Joan Webster, Consumer Member, Southern Derbyshire Community Health Council
-
Professor Martin Whittle, Clinical Co-director, National Co-ordinating Centre for Women’s and Children’s Health, Lymington